User login
Obstetric anal sphincter injury: How to avoid, how to repair: A literature review
- Avoiding obstetrical injury to the anal sphincter is the single biggest factor in preventing anal incontinence among women (A). Any form of instrument delivery has consistently been noted to increase the risk of obstetric anal sphincter injury and altered fecal continence by between 2- and 7-fold (A).
- Routine episiotomy is not recommended (A). Episiotomy use should be restricted to situations where it directly facilitates an urgent delivery (A). A mediolateral incision, instead of a midline, should be considered for persons at otherwise high risk of obstetric anal sphincter injury (A).
- The internal anal sphincter needs to be separately repaired if torn (A).
- Women with injuries to the internal anal sphincter or rectal mucosa have a worse prognosis for future continence problems (A). All women, particularly those with risk factors for injury, should be surveyed for symptoms of anal incontinence at postpartum follow-up (C).
Do you routinely check with new first-time mothers at a postpartum visit about changes in anal continence? They are at particular risk for obstetric anal sphincter injury and could be too embarrassed to raise the issue.
Sphincter injury following labor is the most common cause of anal incontinence (including flatus) in women, which can severely diminish quality of life and lead to considerable personal and financial costs.1 Endoanal ultrasound can detect these injuries, even in the absence of clinically obvious damage to the anal sphincter (occult obstetric anal sphincter injury).2
In this article, we review measures to reduce the occurrence of obstetric anal sphincter injury, proper primary repair when it does occur, and appropriate long-term follow-up. Women with known obstetric anal sphincter injury must also be counseled about the risk of further damage during a future vaginal delivery.
Injury more common than symptoms would suggest
The conventional definitions of the 4 grades of perineal laceration in the US have been supplemented by more recent modifications included in a recent British Royal College of Obstetricians and Gynaecologists (RCOG) guideline (TABLE 1).3 The definition of third-degree laceration now reflects the various degrees of anal sphincter injury that may occur: partial (3a), full-thickness (3b), external anal sphincter injury, with or without injury to the internal anal sphincter (3c).
The incidence of clinical third- and fourth-degree lacerations varies widely; it is reported at between 0.5% and 3.0% in Europe and between 5.85% and 8.9% in the US.2,4-6 A landmark British paper from 1993 revealed that though only 3% had a clinical third- or fourth-degree perineal laceration, 35% of primiparous women (none of whom had any defect before delivery) had ultrasound evidence of varying degrees of anal sphincter defect at 6 weeks postpartum that persisted at 6 months.2 However, only about a third of these women had symptoms of bowel disturbance during the time of study.
These findings are supported by a meta-analysis in which 70% of women with a documented obstetric anal sphincter injury were asymptomatic.7 This meta-analysis concluded that clinical or occult obstetric anal sphincter injury occurs in 27% of primigravid women, and in 8.5% of multiparous women.
The long-term significance of occult obstetric anal sphincter injury and any relationship with geriatric fecal incontinence is unknown, although 71% of a sample of women with late-onset fecal incontinence were found to have ultrasound evidence of an anal sphincter defect thought to have occurred at a previous vaginal delivery.8 A recent English study9 reveals that when women were carefully re-examined after delivery by a skilled obstetrician looking specifically at the anal sphincter, the prevalence of clinically diagnosed third-degree lacerations rose sharply from the 11% initially diagnosed by the delivering physician or midwife to 24.5%. A subsequent endoanal ultrasound detected only an additional 1.2% (3 injuries, 2 of which were in the internal anal sphincter and therefore clinically undetectable). This strongly suggests that the vast majority of obstetric anal sphincter injuries can be detected clinically by a careful exam and that, when this is done, true occult injuries will be a rare finding.
TABLE 1
Classification of perineal injury9
| INJURY | DEFINITION |
|---|---|
| First degree | Injury confined to vaginal mucosa |
| Second degree | Injury of vaginal mucosa and perineal muscles, but not the anal sphincter |
| Third degree | Injury to the perineum involving the anal sphincter complex (external and internal) |
| 3a | <50% of external sphincter thickness is torn |
| 3b | >50% of external sphincter thickness is torn |
| 3c | Internal sphincter is torn |
| Fourth degree | Injury to external and internal sphincter and rectal mucosa/anal epithelium |
Mechanisms of injury
Maintenance of fecal continence involves the coordinated action of several anatomical and physiological elements (FIGURE 1).10 An intact, innervated anal sphincter complex (both external and internal) is necessary. The sphincter complex can be damaged during childbirth in 3 ways.
Direct mechanical injury. Direct external or internal anal sphincter muscle disruption can occur, as with a clinically obvious third- or fourth-degree perineal laceration or an occult injury subsequently noted on ultrasound.
Neurologic injury. Neuropathy of the pudendal nerve may result from forceps delivery or persistent nerve compression from the fetal head.14 Traction neuropathy may also occur with fetal macrosomia and with prolonged pushing during Stage 2 in successive pregnancies, or with prolonged stretching of the nerve due to persistent poor postpartum pelvic floor tone. Injured nerves often undergo demyelination but usually recover with time.
Combined mechanical and neurologic trauma. Isolated neurologic injury, as described above, is believed to be rare. Neuropathy more commonly accompanies mechanical damage.15
Who is at risk?
Several risk factors are unavoidable. One of these is primiparity, a consistently reported independent variable also associated with other risk factors for obstetric anal sphincter injury, such as instrument delivery (TABLE 2).
TABLE 2
Major risk factors for obstetric anal sphincter injury
| RISK FACTOR | ODDS RATIO |
|---|---|
| Nulliparity (primigravidity) | 3–4 |
| Inherent predisposition: | |
| Short perineal body | 8 |
| Instrumental delivery, overall | 3 |
| Forceps-assisted delivery | 3–7 |
| Vacuum-assisted delivery | 3 |
| Forceps vs vacuum | 2.88* |
| Forceps with midline episiotomy | 25 |
| Prolonged second stage of labor (>1 hour) | 1.5–4 |
| Epidural analgesia | 1.5–3 |
| Intrapartum infant factors: | |
| Birthweight over 4 kg | 2 |
| Persistent occipitoposterior position | 2–3 |
| Episiotomy, mediolateral | 1.4 |
| Episiotomy, midline | 3–5 |
| Previous anal sphincter tear | 4 |
| All variables are statistically significant at P<.05. | |
| *Relative risk of altered fecal symptoms based on RCT findings, vacuum vs forceps.17 Data from randomized controlled trials are lacking for most labor variables. Due to differing methods of analysis (univariate vs regression) and outcome measures, risk ratios reported in the literature vary considerably. This table presents the approximate odds ratios for risk factors that have been reported most consistently from 1 prospective cohort study,16 1 randomized controlled trial,14 and, otherwise higher-quality retrospective analyses.18-23 | |
Preventing obstetric anal sphincter injury
Sphincter injury can occur even when obstetrical management is optimal. Although evidence from RCT data is often lacking, sufficient observational and retrospective data support the following recommendations to reduce the likelihood of injury.
Choose vacuum delivery before forceps
Any form of instrument delivery increases the risk of obstetric anal sphincter injury and altered fecal continence by between 2- and 7-fold.2,16,24 An RCT found clinical third-degree tears in 16% of women with forceps-assisted deliveries, compared with 7% of vacuum-assisted deliveries; the authors concluded that, when circumstances allow, vacuum delivery should be attempted first (acknowledging however that 23% of vacuum deliveries failed and proceeded to a forceps extraction, a sequence associated with increased injury).17 A meta-analysis confirmed that vacuum extraction is preferred when instrumental delivery is necessary (SOR: A).25
When midline episiotomy was performed during instrument delivery, the risk of obstetric anal sphincter injury approximately doubled again, such that, in one study, forceps delivery with episiotomy caused a 25-fold increase in obstetric anal sphincter injury.24
Any steps that may safely reduce the need for instrument delivery should be supported. Toward this end, the Canadian Clinical Practices Obstetrics committee has recommended evidence-based labor interventions such as one-to-one support in labor, the increased use of a partogram in labor and appropriate oxytocin use, all in an effort to reduce needs for operative interventions.26
If episiotomy necessary, mediolateral less risky than midline
Episiotomy was long promoted as a means of preserving the integrity of the perineal musculature and of avoiding damage to the anal sphincter, and it has been practiced routinely by some.27 Strong evidence now indicates that routine episiotomy (midline or mediolateral) is unhelpful and should be abandoned.25,27-29
Observational evidence overwhelmingly shows that midline episiotomy is strongly associated with obstetric anal sphincter injury.19,22,23,30,31 One of the few RCTs comparing midline with mediolateral episiotomy, although flawed in its design, noted that a clinical third-degree laceration occurred as an extension of episiotomy in 11.6% of midline incisions compared with just 2% of mediolateral cuts.32
Another RCT, designed to examine routine versus restrictive episiotomy, noted that all but 1 (98%) of the 47 third- or fourth-degree lacerations in a group of 700 women followed midline episiotomy.29 A retrospective database analysis noted a 6-fold higher risk of third-degree perineal lacerations for women undergoing midline episiotomy compared with mediolateral incision.23 Elsewhere, midline episiotomy was associated with a 5-fold increase in symptoms of fecal incontinence at 3 months postpartum when compared with women with an intact perineum.24
Even when midline episiotomies do not extend into clinical third-degree lacerations, the incidence of resultant postpartum fecal incontinence triples when compared with spontaneous second-degree perineal lacerations.30 The authors postulate that a perineum cut by midline episiotomy allows for more direct contact to occur between the fetal hard parts and the anal sphincter complex during delivery, thereby increasing occult obstetric anal sphincter injury.
Observational data conflict as to whether mediolateral episiotomy contributes to, or protects against, obstetric anal sphincter injury—although the burden of evidence favors it as a risk factor that should be avoided when possible.16,23,33 An angle of mediolateral incision cut closer to 45 degrees from the midline has been associated with less obstetric anal sphincter injury than incisions cut at closer angles to the midline.34
Repairing sphincter injury
Detecting injury in labor
With any severe perineal laceration, closely inspect the external and, if exposed, internal anal sphincter and perform a rectal exam, particularly for women with numerous risk factors (although no good evidence supports the role of the rectal exam in diagnosing obstetric anal sphincter injury). Colorectal surgeons have advocated the use of a muscle stimulator to assist in identifying the ends of the external sphincter, but this has not become common practice.35
Immediate vs delayed repair
It is standard practice to repair a damaged anal sphincter immediately or soon after delivery. However, given that a repair should be well done, and since a short delay does not appear to adversely affect healing, be prepared to wait for assistance for up to 24 hours rather than risk a suboptimal repair.36
Better training is needed
Even among trained obstetricians and obgyn residents, 64% have reported no training or unsatisfactory training in management of obstetric anal sphincter injury; 94% of physicians felt inadequately prepared at the time of their first independent repair of the anal sphincter.37,38 To improve your repair skills in a workshop setting, consult the following sources—www.aafp.org/also.xml in the US, or www.perineum.net in UK.
Analgesia and setting
Adequate analgesia is an essential element in a good repair. Complete relaxation of the anesthetized anal sphincter complex facilitates bringing torn ends of the sphincter together without tension.39 Though theoretically this can be attained with local anesthetic infiltration, RCOG recommends that regional or general anesthesia be considered to provide complete analgesia.37 It is further recommended that repair of the anal sphincter occur in an operating room, given the degree of contamination present in the labor room after delivery and the devastating effects of an infected repair (SOR: C).40
Repair technique
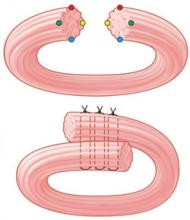
There are 2 commonly used methods of external anal sphincter repair: one, the traditionally taught end-to-end approximation of the cut ends, and the other, overlapping the cut ends of the external sphincter and suturing through the overlapped portions (FIGURE 2).36 Though an RCT from 2000 noted no significant difference in outcomes between these methods,41 other authors have suggested that an overlapping technique is preferred, and it remains the method most often used by colorectal surgeons in elective, secondary anal sphincter repairs.36,39,42
A Cochrane review of which technique is better has been registered in the Clinical Trials Database. General agreement is that closure using interrupted sutures of a monofilament material, such as 2-0 polydioxanone sulfate (PDS), is the preferred closure method for the external sphincter (SOR: C).36,40 It is recommended that a damaged internal sphincter be repaired with a running continuous suture of a material such as 2-0 polyglactin 910 (Vicryl) (SOR: C).36
FIGURE 2
2 methods of anal sphincter repair
Two commonly used methods of external anal sphincter repair are end-to-end approximate of the cut ends (top), and overlapping the cut ends and suturing through the overlapped portions (bottom). (Adapted from Leeman et al, Am Fam Physician 2003.33) ILLUSTRATION BY RICH LaROCCO
Immediate post-repair management
Use a stool softener
It had long been thought that constipation following obstetric anal sphincter injury allowed the sphincter to heal more effectively. However, new evidence from RCTs shows that using a laxative instead of a constipating regimen is more helpful in the immediate postpartum phase.43 Toward this end, use a stool softener, such as lactulose, for 3 to 10 days postpartum for women with obstetric anal sphincter injury.40
Should you prescribe an antibiotic?
Given the devastating effects of post-repair infection, most authorities consider it prudent to prescribe a course of broad-spectrum antibiotics, possibly including metronidazole (SOR: C)37,40 A Cochrane review is registered to further examine this issue. A separate Cochrane review of the use of antibiotics for instrument vaginal delivery concluded that quality data were insufficient to make any recommendations.44
Refer for physical therapy
Some authorities consider an early referral to physical therapy for pelvic floor exercises helpful in the immediate post-partum for all patients with obstetric anal sphincter injury (SOR: C).45
Long-term management
Ask patients about incontinence
Given that some women are too embarrassed to seek assistance, ask those with obstetric anal sphincter injury specific questions about any symptoms of anal incontinence at a follow-up visit, such as the 6-week postpartum visit (SOR: C).37,40 In some practices, all women who have sustained a third- or fourth-degree laceration are routinely scheduled for a 3-month follow-up visit to a dedicated clinic, irrespective of symptoms. Given the prevalence of occult obstetric anal sphincter injury for primigravid women, you may find it best to survey all women postnatally concerning any changes in anal continence. TABLE 3 demonstrates a validated, modified patient survey of anal incontinence.37,40 A score of 6 is often used as a cutoff.
TABLE 3
Fecal Continence Scoring scale
| SYMPTOM |
| 1. Passage of any flatus when socially undesirable |
| 2. Any incontinence of liquid stool |
| 3. Any need to wear a pad because of anal symptoms |
| 4. Any incontinence of solid stool |
| 5. Any fecal urgency (inability to defer defecation for more than 5 minutes) |
| SCALE |
| 0 Never |
| 1 Rarely (<1/month) |
| 2 Sometimes (1/week–1/month |
| 3 Usually (1/day–1/week) |
| 4 Always (>1/day) |
| A score of 0 implies complete continence and a score of 20 complete incontinence. |
| A score of 6 has been suggested as a cut-off to determine need for evaluation. |
| Source: Mahony et al, 2001;43 modified from Jorge and Wexner, 1993.44 |
When additional evaluation is needed
Patients who have symptoms of altered continence at 3 months (or who score above 6 on the Wexner scale) should be seen at a dedicated gynecologic or colorectal surgery clinic,46 where they can receive a more detailed clinical evaluation and undergo anal manometry (during resting and forced squeezing) or endoanal ultrasonography. Some patients respond well to physical therapy, though a few patients ultimately require reconstructive colorectal surgery and temporary colostomy.
Management in a subsequent pregnancy
Women who have had an obstetric anal sphincter injury are at increased risk for repeat injury in a future pregnancy.48 At some units, all such women are routinely offered a prenatal visit at the end of the second trimester to review their symptoms and to evaluate the anal sphincter with manometry or ultrasound. A large prospective study, however, found that recurrence of obstetric anal sphincter injury could not be predicted and that 95% of women with prior injury did not sustain further overt sphincter damage during a subsequent vaginal delivery.49
However, for some women, a repeat anal sphincter laceration could prove devastating. For these women—eg, those with previous severe symptoms that required secondary surgical repair—initiate an in-depth discussion concerning the risks and benefits of elective cesarean delivery versus vaginal delivery.37,40
Historically, fecal incontinence was defined as “the involuntary or inappropriate passage of feces.”10 However, a preferred definition now refers instead to anal incontinence, which is “any involuntary loss of feces or flatus, or urge incontinence, that is adversely affecting a woman’s quality of life.”40 This definition includes urgency of defecation and incontinence of flatus, both of which are much more common symptoms than fecal incontinence.50
Data are lacking on the community prevalence of incontinence, although it is known that women of age 45 experience 8 times the incidence of incontinence as men of the same age and that it increases in prevalence with age.10 A Canadian survey of almost 1000 women at 3 months postpartum revealed that 3.1% admitted to incontinence of feces while 25% admitted to involuntary escape of flatus. The subgroup of women who suffered clinical anal sphincter injury (that is, third- or fourth-degree lacerations) had considerably increased rates of incontinence of feces (7.8%) and of flatus (48%).24 It has been reported that approximately half of women who sustained anal sphincter tears in labor complained of anal, urinary, or perineal symptoms at a mean follow-up of 2.6 years after the injury.51 Most studies agree that many women are embarrassed about symptoms of anal incontinence and are reluctant to self-report them.50
CORRESPONDENCE
David Power, MD, MPH, Department of Family Medicine and Community Health, University of Minnesota, Mayo Mail Code 381, 516 Delaware St SE, Minneapolis, MN 55455. E-mail: [email protected]
1. Mellgren A, Jensen LL, Zetterstrom JP, Wong WD, Hofmeister JH, Lowry AC. Long-term cost of fecal incontinence secondary to obstetric injuries. Dis Colon Rectum 1999;42:857-865.
2. Sultan AH, Kamm MA, Hudson CN, Thomas JM, Bartram CI. Anal-sphincter disruption during vaginal delivery. N Engl J Med 1993;329:1905-1911.
3. Royal College of Obstetricians and Gynaecologists. Methods and Materials used in Perineal Repair. Guideline No. 23. London: RCOG Press; 2004.
4. Fitzpatrick M, Behan M, O’Connell PR, O’Herlihy C. A randomized clinical trial comparing primary overlap with approximation repair of third-degree obstetric tears. Am J Obstet Gynecol 2000;183:1220-1224.
5. Simhan HN, Krohn MA, Heine RP. Obstetric rectal injury: risk factors and the role of physician experience. J Matern Fetal Neonatal Med 2004;16:271-274.
6. Handa VL, Danielsen BH, Gilbert WM. Obstetric anal sphincter lacerations. Obstet Gynecol 2001;98:225-230.
7. Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg 2003;90:1333-1337.
8. Oberwalder M, Dinnewitzer A, Baig MK, et al. The association between late-onset fecal incontinence and obstetric anal sphincter defects. Arch Surg 2004;139:429-432.
9. Andrews V, Sultan AH, Thakar R, Jones PW. Occult anal sphincter injuries-myth or reality? BJOG 2006;113:195-200.
10. Sultan AH, Nugent K. Pathophysiology and nonsurgical treatment of anal incontinence. BJOG 2004;111 (Suppl 1):84-90.
11. Schafer A, Enck P, Furst G, et al. Anatomy of the anal sphincters. Dis Colon Rectum 1994;37:777-781.
12. Mahony R, Daly L, Behan M, Kirwan C, O’Herlihy C, O’Connell R. Internal Anal Sphincter injury predicts continence outcome following obstetric sphincter trauma. AJOG 2004;191:6s1-s89.
13. Bartram CL, Frudringer A. Handbook of Anal Endosonography. Petersfield, UK: Wrightson Biomedical Publishing; 1997.
14. Sultan AH, Kamm MA, Hudson CN. Pudendal nerve damage during labor: prospective study before and after childbirth. BJOG 1994;101:22-28.
15. Snooks SJ, Henry MM, Swash M. Fecal incontinence due to external anal sphincter division in childbirth is associated with damage to the innervation of the pelvic floor musculature: a double pathology. BJOG 1985;92:824-828.
16. Donnelly V, Fynes M, Campbell D, Johnson H, O’Connell PR, O’Herlihy C. Obstetric events leading to anal sphincter damage. Obstet Gynecol 1998;92:955-961.
17. Fitzpatrick M, Behan M, O’Connell PR, O’Herlihy C. Randomized clinical trial to assess anal sphincter function following forceps or vacuum assisted vaginal delivery. BJOG 2003;110:424-429.
18. Deering SH, Carlson N, Stitely M, Allaire AD, Satin AJ. Perineal body length and lacerations at delivery. J Reproductive Medicine 2004;49:306-310.
19. Goldberg J, Hyslop T, Tolosa JE, Sultana C. Racial Differences in severe Perineal lacerations after vaginal delivery. Am J Obstet Gynecol 2003;188:1063-1067.
20. Robinson JN, Norwitz ER, Cohen AP, McElrath TF, Lieberman ES. Epidural analgesia and third- or fourth-degree lacerations in nulliparas. Obstet Gynecol 1999;94:259-262.
21. Carroll TG, Engelken M, Mosier MC, Nazir N. Epidural analgesia and severe perineal laceration in a community-based obstetric practice. J Am Board Fam Prac 2003;16:1-6.
22. Riskin-Mashiah S, O’Brian Smith E, Wilkins IA. Risk factors for severe perineal tear: can we do better? Am J Perinatol 2002;19:225-234.
23. Bodner-Adler B, Bodner K, Kaider A, et al. Risk factors for third-degree perineal tears in vaginal deliveries with an analysis of episiotomy types. J Reprod Med 2001;46:752-756.
24. Eason E, Labrecque M, Marcoux S, Mondor M. Anal incontinence after childbirth. CMAJ 2002;166:326-330.
25. Eason E, Labrecque M, Wells G, Feldman P. Preventing perineal trauma during childbirth: a systematic review. Obstet Gynecol 2000;95:464-471.
26. Cargill YM, MacKinnon CJ, Arsensault MY, et al. Clinical Practice Obstetrics Committee. Guidelines for operative vaginal birth. J Obstet Gynaecol Can 2004;26:747-761.
27. Carroli G, Belizan J. Episiotomy for vaginal birth. Cochrane Database System Rev 1999;(3):CD000081.-
28. Schlomer G, Gross M, Meyer G. Effectiveness of liberal vs. conservative episiotomy in vaginal delivery with reference to preventing urinary and fecal incontinence: a systematic review [in German]. Wien Med Wochenschr 2003;153:269-275.
29. Klein MC, Gauthier RJ, Jorgensen SH, et al. Does episiotomy prevent perineal trauma and pelvic floor relaxation? Online J Curr Clin Trials 1992;Doc No 10.
30. Signorello LB, Harlow BL, Chekos AK, Repke JT. Midline episiotomy and anal incontinence: retrospective cohort study. BMJ 2000;320:86-90.
31. Shiono P, Klebanoff MA, Carey JC. Midline episiotomies: more harm than good? Obstet Gynecol 1990;75:765-770.
32. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. BJOG 1980;87:408-412.
33. Poen AC, Felt-Bersma RJ, Dekker GA, Deville W, Cuesta MA, Meuwissen SG. Third degree obstetric perineal tears: risk factors and the preventive role of mediolateral episiotomy. Br J Obstet Gynaecol 1997;104:563-566.
34. Eogan M, O’Connell R, O’Herlihy C. Does the angle of episiotomy affect the incidence of anal sphincter injury? BJOG 2006;113:190-194.
35. Cook TA, Keane D, Mortensen NJ. Is there a role for the colorectal team in the management of acute severe third-degree vaginal tears? Colorectal Dis 1999;1:263-266.
36. Leeman L, Spearman M, Rogers R. Repair of obstetric perineal lacerations. Am Fam Physician 2003;68:1585-1590.
37. Fernando RJ, Sultan AH, Radley S, Jones PW, Johanson RB. Management of obstetric anal sphincter injury: a systematic review & national practice survey. BMC Health Serv Res 2002;2:9.-
38. Sultan AH, Kamm MA, Hudson CN. Obstetric perineal trauma: an audit of training. J Obstet Gynaecol 1995;15:19-23.
39. Sultan AH, Monga AK, Kumar D, Stanton SL. Primary repair of obstetric anal sphincter rupture using the overlap technique. BJOG 1999;106:318-23.
40. Royal College of Obstetricians and Gynaecologists. Management of third- and fourth-degree perineal tears following vaginal delivery. Guideline No. 29. London: RCOG Press; 2001.
41. Fitzpatrick M, Behan M, O’Connell PR, O’Herlihy C. A randomised clinical trial comparing primary overlap with approximation repair of third degree tears. Am J Obstet Gynecol 2000;183:1220-1224.
42. Kairaluoma MV, Raivio P, Aarnio MT, Kellokumpo IH. Immediate repair of obstetric anal sphincter rupture: medium-term outcome of the overlap technique. Dis Colon Rectum 2004;47:1358-1363.
43. Mahony R, Behan M, O’Herlihy C, O’Connell PR. Randomized, clinical trial of bowel confinement vs. laxative use after primary repair of a third-degree obstetric anal sphincter tear. Dis Colon Rectum 2004;47:12-17.
44. Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam M. Antibiotic prophylaxis for operative vaginal delivery (Cochrane review). In: The Cochrane Library, Issue 2, 2004.
45. Fitzpatrick M, O’Herlihy C. Postpartum anal sphincter dysfunction. Current Obstet Gynaecol 1999;9:210-215.
46. Mahony R, O’Brien C, O’Herlihy C. Evaluation of obstetric anal sphincter injury. Reviews in Gynaecological Practice 2001;1:114-121.
47. Jorge M, Wexner S. Etiology and management of fecal incontinence. Dis Colon Rectum 1993;36:77-97.
48. Fynes M, Donnelly V, Behan M, O’Connell PR, O’Herlihy C. Effect of second vaginal delivery on anorectal physiology and faecal continence: a prospective study. Lancet 1999;354:983-986.
49. Harkin R, Fitzpatrick M, O’Connell PR, O’Herlihy C. Anal sphincter disruption at vaginal deliver: is recurrence predictable? Eur J Obstet Gynaecol 2003;109:149-152.
50. Fitzpatrick M, O’Herlihy C. The effects of labour and delivery on the pelvic floor. Best Pract Res Clin Obstet Gynaecol 2001;15:63-79.
51. Wood J, Amos L, Rieger N. Third degree anal sphincter tears: risk factors and outcome. Aust N Z J Obstet Gynaecol 1998;38:414-417.
- Avoiding obstetrical injury to the anal sphincter is the single biggest factor in preventing anal incontinence among women (A). Any form of instrument delivery has consistently been noted to increase the risk of obstetric anal sphincter injury and altered fecal continence by between 2- and 7-fold (A).
- Routine episiotomy is not recommended (A). Episiotomy use should be restricted to situations where it directly facilitates an urgent delivery (A). A mediolateral incision, instead of a midline, should be considered for persons at otherwise high risk of obstetric anal sphincter injury (A).
- The internal anal sphincter needs to be separately repaired if torn (A).
- Women with injuries to the internal anal sphincter or rectal mucosa have a worse prognosis for future continence problems (A). All women, particularly those with risk factors for injury, should be surveyed for symptoms of anal incontinence at postpartum follow-up (C).
Do you routinely check with new first-time mothers at a postpartum visit about changes in anal continence? They are at particular risk for obstetric anal sphincter injury and could be too embarrassed to raise the issue.
Sphincter injury following labor is the most common cause of anal incontinence (including flatus) in women, which can severely diminish quality of life and lead to considerable personal and financial costs.1 Endoanal ultrasound can detect these injuries, even in the absence of clinically obvious damage to the anal sphincter (occult obstetric anal sphincter injury).2
In this article, we review measures to reduce the occurrence of obstetric anal sphincter injury, proper primary repair when it does occur, and appropriate long-term follow-up. Women with known obstetric anal sphincter injury must also be counseled about the risk of further damage during a future vaginal delivery.
Injury more common than symptoms would suggest
The conventional definitions of the 4 grades of perineal laceration in the US have been supplemented by more recent modifications included in a recent British Royal College of Obstetricians and Gynaecologists (RCOG) guideline (TABLE 1).3 The definition of third-degree laceration now reflects the various degrees of anal sphincter injury that may occur: partial (3a), full-thickness (3b), external anal sphincter injury, with or without injury to the internal anal sphincter (3c).
The incidence of clinical third- and fourth-degree lacerations varies widely; it is reported at between 0.5% and 3.0% in Europe and between 5.85% and 8.9% in the US.2,4-6 A landmark British paper from 1993 revealed that though only 3% had a clinical third- or fourth-degree perineal laceration, 35% of primiparous women (none of whom had any defect before delivery) had ultrasound evidence of varying degrees of anal sphincter defect at 6 weeks postpartum that persisted at 6 months.2 However, only about a third of these women had symptoms of bowel disturbance during the time of study.
These findings are supported by a meta-analysis in which 70% of women with a documented obstetric anal sphincter injury were asymptomatic.7 This meta-analysis concluded that clinical or occult obstetric anal sphincter injury occurs in 27% of primigravid women, and in 8.5% of multiparous women.
The long-term significance of occult obstetric anal sphincter injury and any relationship with geriatric fecal incontinence is unknown, although 71% of a sample of women with late-onset fecal incontinence were found to have ultrasound evidence of an anal sphincter defect thought to have occurred at a previous vaginal delivery.8 A recent English study9 reveals that when women were carefully re-examined after delivery by a skilled obstetrician looking specifically at the anal sphincter, the prevalence of clinically diagnosed third-degree lacerations rose sharply from the 11% initially diagnosed by the delivering physician or midwife to 24.5%. A subsequent endoanal ultrasound detected only an additional 1.2% (3 injuries, 2 of which were in the internal anal sphincter and therefore clinically undetectable). This strongly suggests that the vast majority of obstetric anal sphincter injuries can be detected clinically by a careful exam and that, when this is done, true occult injuries will be a rare finding.
TABLE 1
Classification of perineal injury9
| INJURY | DEFINITION |
|---|---|
| First degree | Injury confined to vaginal mucosa |
| Second degree | Injury of vaginal mucosa and perineal muscles, but not the anal sphincter |
| Third degree | Injury to the perineum involving the anal sphincter complex (external and internal) |
| 3a | <50% of external sphincter thickness is torn |
| 3b | >50% of external sphincter thickness is torn |
| 3c | Internal sphincter is torn |
| Fourth degree | Injury to external and internal sphincter and rectal mucosa/anal epithelium |
Mechanisms of injury
Maintenance of fecal continence involves the coordinated action of several anatomical and physiological elements (FIGURE 1).10 An intact, innervated anal sphincter complex (both external and internal) is necessary. The sphincter complex can be damaged during childbirth in 3 ways.
Direct mechanical injury. Direct external or internal anal sphincter muscle disruption can occur, as with a clinically obvious third- or fourth-degree perineal laceration or an occult injury subsequently noted on ultrasound.
Neurologic injury. Neuropathy of the pudendal nerve may result from forceps delivery or persistent nerve compression from the fetal head.14 Traction neuropathy may also occur with fetal macrosomia and with prolonged pushing during Stage 2 in successive pregnancies, or with prolonged stretching of the nerve due to persistent poor postpartum pelvic floor tone. Injured nerves often undergo demyelination but usually recover with time.
Combined mechanical and neurologic trauma. Isolated neurologic injury, as described above, is believed to be rare. Neuropathy more commonly accompanies mechanical damage.15
Who is at risk?
Several risk factors are unavoidable. One of these is primiparity, a consistently reported independent variable also associated with other risk factors for obstetric anal sphincter injury, such as instrument delivery (TABLE 2).
TABLE 2
Major risk factors for obstetric anal sphincter injury
| RISK FACTOR | ODDS RATIO |
|---|---|
| Nulliparity (primigravidity) | 3–4 |
| Inherent predisposition: | |
| Short perineal body | 8 |
| Instrumental delivery, overall | 3 |
| Forceps-assisted delivery | 3–7 |
| Vacuum-assisted delivery | 3 |
| Forceps vs vacuum | 2.88* |
| Forceps with midline episiotomy | 25 |
| Prolonged second stage of labor (>1 hour) | 1.5–4 |
| Epidural analgesia | 1.5–3 |
| Intrapartum infant factors: | |
| Birthweight over 4 kg | 2 |
| Persistent occipitoposterior position | 2–3 |
| Episiotomy, mediolateral | 1.4 |
| Episiotomy, midline | 3–5 |
| Previous anal sphincter tear | 4 |
| All variables are statistically significant at P<.05. | |
| *Relative risk of altered fecal symptoms based on RCT findings, vacuum vs forceps.17 Data from randomized controlled trials are lacking for most labor variables. Due to differing methods of analysis (univariate vs regression) and outcome measures, risk ratios reported in the literature vary considerably. This table presents the approximate odds ratios for risk factors that have been reported most consistently from 1 prospective cohort study,16 1 randomized controlled trial,14 and, otherwise higher-quality retrospective analyses.18-23 | |
Preventing obstetric anal sphincter injury
Sphincter injury can occur even when obstetrical management is optimal. Although evidence from RCT data is often lacking, sufficient observational and retrospective data support the following recommendations to reduce the likelihood of injury.
Choose vacuum delivery before forceps
Any form of instrument delivery increases the risk of obstetric anal sphincter injury and altered fecal continence by between 2- and 7-fold.2,16,24 An RCT found clinical third-degree tears in 16% of women with forceps-assisted deliveries, compared with 7% of vacuum-assisted deliveries; the authors concluded that, when circumstances allow, vacuum delivery should be attempted first (acknowledging however that 23% of vacuum deliveries failed and proceeded to a forceps extraction, a sequence associated with increased injury).17 A meta-analysis confirmed that vacuum extraction is preferred when instrumental delivery is necessary (SOR: A).25
When midline episiotomy was performed during instrument delivery, the risk of obstetric anal sphincter injury approximately doubled again, such that, in one study, forceps delivery with episiotomy caused a 25-fold increase in obstetric anal sphincter injury.24
Any steps that may safely reduce the need for instrument delivery should be supported. Toward this end, the Canadian Clinical Practices Obstetrics committee has recommended evidence-based labor interventions such as one-to-one support in labor, the increased use of a partogram in labor and appropriate oxytocin use, all in an effort to reduce needs for operative interventions.26
If episiotomy necessary, mediolateral less risky than midline
Episiotomy was long promoted as a means of preserving the integrity of the perineal musculature and of avoiding damage to the anal sphincter, and it has been practiced routinely by some.27 Strong evidence now indicates that routine episiotomy (midline or mediolateral) is unhelpful and should be abandoned.25,27-29
Observational evidence overwhelmingly shows that midline episiotomy is strongly associated with obstetric anal sphincter injury.19,22,23,30,31 One of the few RCTs comparing midline with mediolateral episiotomy, although flawed in its design, noted that a clinical third-degree laceration occurred as an extension of episiotomy in 11.6% of midline incisions compared with just 2% of mediolateral cuts.32
Another RCT, designed to examine routine versus restrictive episiotomy, noted that all but 1 (98%) of the 47 third- or fourth-degree lacerations in a group of 700 women followed midline episiotomy.29 A retrospective database analysis noted a 6-fold higher risk of third-degree perineal lacerations for women undergoing midline episiotomy compared with mediolateral incision.23 Elsewhere, midline episiotomy was associated with a 5-fold increase in symptoms of fecal incontinence at 3 months postpartum when compared with women with an intact perineum.24
Even when midline episiotomies do not extend into clinical third-degree lacerations, the incidence of resultant postpartum fecal incontinence triples when compared with spontaneous second-degree perineal lacerations.30 The authors postulate that a perineum cut by midline episiotomy allows for more direct contact to occur between the fetal hard parts and the anal sphincter complex during delivery, thereby increasing occult obstetric anal sphincter injury.
Observational data conflict as to whether mediolateral episiotomy contributes to, or protects against, obstetric anal sphincter injury—although the burden of evidence favors it as a risk factor that should be avoided when possible.16,23,33 An angle of mediolateral incision cut closer to 45 degrees from the midline has been associated with less obstetric anal sphincter injury than incisions cut at closer angles to the midline.34
Repairing sphincter injury
Detecting injury in labor
With any severe perineal laceration, closely inspect the external and, if exposed, internal anal sphincter and perform a rectal exam, particularly for women with numerous risk factors (although no good evidence supports the role of the rectal exam in diagnosing obstetric anal sphincter injury). Colorectal surgeons have advocated the use of a muscle stimulator to assist in identifying the ends of the external sphincter, but this has not become common practice.35
Immediate vs delayed repair
It is standard practice to repair a damaged anal sphincter immediately or soon after delivery. However, given that a repair should be well done, and since a short delay does not appear to adversely affect healing, be prepared to wait for assistance for up to 24 hours rather than risk a suboptimal repair.36
Better training is needed
Even among trained obstetricians and obgyn residents, 64% have reported no training or unsatisfactory training in management of obstetric anal sphincter injury; 94% of physicians felt inadequately prepared at the time of their first independent repair of the anal sphincter.37,38 To improve your repair skills in a workshop setting, consult the following sources—www.aafp.org/also.xml in the US, or www.perineum.net in UK.
Analgesia and setting
Adequate analgesia is an essential element in a good repair. Complete relaxation of the anesthetized anal sphincter complex facilitates bringing torn ends of the sphincter together without tension.39 Though theoretically this can be attained with local anesthetic infiltration, RCOG recommends that regional or general anesthesia be considered to provide complete analgesia.37 It is further recommended that repair of the anal sphincter occur in an operating room, given the degree of contamination present in the labor room after delivery and the devastating effects of an infected repair (SOR: C).40
Repair technique
There are 2 commonly used methods of external anal sphincter repair: one, the traditionally taught end-to-end approximation of the cut ends, and the other, overlapping the cut ends of the external sphincter and suturing through the overlapped portions (FIGURE 2).36 Though an RCT from 2000 noted no significant difference in outcomes between these methods,41 other authors have suggested that an overlapping technique is preferred, and it remains the method most often used by colorectal surgeons in elective, secondary anal sphincter repairs.36,39,42
A Cochrane review of which technique is better has been registered in the Clinical Trials Database. General agreement is that closure using interrupted sutures of a monofilament material, such as 2-0 polydioxanone sulfate (PDS), is the preferred closure method for the external sphincter (SOR: C).36,40 It is recommended that a damaged internal sphincter be repaired with a running continuous suture of a material such as 2-0 polyglactin 910 (Vicryl) (SOR: C).36
FIGURE 2
2 methods of anal sphincter repair
Two commonly used methods of external anal sphincter repair are end-to-end approximate of the cut ends (top), and overlapping the cut ends and suturing through the overlapped portions (bottom). (Adapted from Leeman et al, Am Fam Physician 2003.33) ILLUSTRATION BY RICH LaROCCO
Immediate post-repair management
Use a stool softener
It had long been thought that constipation following obstetric anal sphincter injury allowed the sphincter to heal more effectively. However, new evidence from RCTs shows that using a laxative instead of a constipating regimen is more helpful in the immediate postpartum phase.43 Toward this end, use a stool softener, such as lactulose, for 3 to 10 days postpartum for women with obstetric anal sphincter injury.40
Should you prescribe an antibiotic?
Given the devastating effects of post-repair infection, most authorities consider it prudent to prescribe a course of broad-spectrum antibiotics, possibly including metronidazole (SOR: C)37,40 A Cochrane review is registered to further examine this issue. A separate Cochrane review of the use of antibiotics for instrument vaginal delivery concluded that quality data were insufficient to make any recommendations.44
Refer for physical therapy
Some authorities consider an early referral to physical therapy for pelvic floor exercises helpful in the immediate post-partum for all patients with obstetric anal sphincter injury (SOR: C).45
Long-term management
Ask patients about incontinence
Given that some women are too embarrassed to seek assistance, ask those with obstetric anal sphincter injury specific questions about any symptoms of anal incontinence at a follow-up visit, such as the 6-week postpartum visit (SOR: C).37,40 In some practices, all women who have sustained a third- or fourth-degree laceration are routinely scheduled for a 3-month follow-up visit to a dedicated clinic, irrespective of symptoms. Given the prevalence of occult obstetric anal sphincter injury for primigravid women, you may find it best to survey all women postnatally concerning any changes in anal continence. TABLE 3 demonstrates a validated, modified patient survey of anal incontinence.37,40 A score of 6 is often used as a cutoff.
TABLE 3
Fecal Continence Scoring scale
| SYMPTOM |
| 1. Passage of any flatus when socially undesirable |
| 2. Any incontinence of liquid stool |
| 3. Any need to wear a pad because of anal symptoms |
| 4. Any incontinence of solid stool |
| 5. Any fecal urgency (inability to defer defecation for more than 5 minutes) |
| SCALE |
| 0 Never |
| 1 Rarely (<1/month) |
| 2 Sometimes (1/week–1/month |
| 3 Usually (1/day–1/week) |
| 4 Always (>1/day) |
| A score of 0 implies complete continence and a score of 20 complete incontinence. |
| A score of 6 has been suggested as a cut-off to determine need for evaluation. |
| Source: Mahony et al, 2001;43 modified from Jorge and Wexner, 1993.44 |
When additional evaluation is needed
Patients who have symptoms of altered continence at 3 months (or who score above 6 on the Wexner scale) should be seen at a dedicated gynecologic or colorectal surgery clinic,46 where they can receive a more detailed clinical evaluation and undergo anal manometry (during resting and forced squeezing) or endoanal ultrasonography. Some patients respond well to physical therapy, though a few patients ultimately require reconstructive colorectal surgery and temporary colostomy.
Management in a subsequent pregnancy
Women who have had an obstetric anal sphincter injury are at increased risk for repeat injury in a future pregnancy.48 At some units, all such women are routinely offered a prenatal visit at the end of the second trimester to review their symptoms and to evaluate the anal sphincter with manometry or ultrasound. A large prospective study, however, found that recurrence of obstetric anal sphincter injury could not be predicted and that 95% of women with prior injury did not sustain further overt sphincter damage during a subsequent vaginal delivery.49
However, for some women, a repeat anal sphincter laceration could prove devastating. For these women—eg, those with previous severe symptoms that required secondary surgical repair—initiate an in-depth discussion concerning the risks and benefits of elective cesarean delivery versus vaginal delivery.37,40
Historically, fecal incontinence was defined as “the involuntary or inappropriate passage of feces.”10 However, a preferred definition now refers instead to anal incontinence, which is “any involuntary loss of feces or flatus, or urge incontinence, that is adversely affecting a woman’s quality of life.”40 This definition includes urgency of defecation and incontinence of flatus, both of which are much more common symptoms than fecal incontinence.50
Data are lacking on the community prevalence of incontinence, although it is known that women of age 45 experience 8 times the incidence of incontinence as men of the same age and that it increases in prevalence with age.10 A Canadian survey of almost 1000 women at 3 months postpartum revealed that 3.1% admitted to incontinence of feces while 25% admitted to involuntary escape of flatus. The subgroup of women who suffered clinical anal sphincter injury (that is, third- or fourth-degree lacerations) had considerably increased rates of incontinence of feces (7.8%) and of flatus (48%).24 It has been reported that approximately half of women who sustained anal sphincter tears in labor complained of anal, urinary, or perineal symptoms at a mean follow-up of 2.6 years after the injury.51 Most studies agree that many women are embarrassed about symptoms of anal incontinence and are reluctant to self-report them.50
CORRESPONDENCE
David Power, MD, MPH, Department of Family Medicine and Community Health, University of Minnesota, Mayo Mail Code 381, 516 Delaware St SE, Minneapolis, MN 55455. E-mail: [email protected]
- Avoiding obstetrical injury to the anal sphincter is the single biggest factor in preventing anal incontinence among women (A). Any form of instrument delivery has consistently been noted to increase the risk of obstetric anal sphincter injury and altered fecal continence by between 2- and 7-fold (A).
- Routine episiotomy is not recommended (A). Episiotomy use should be restricted to situations where it directly facilitates an urgent delivery (A). A mediolateral incision, instead of a midline, should be considered for persons at otherwise high risk of obstetric anal sphincter injury (A).
- The internal anal sphincter needs to be separately repaired if torn (A).
- Women with injuries to the internal anal sphincter or rectal mucosa have a worse prognosis for future continence problems (A). All women, particularly those with risk factors for injury, should be surveyed for symptoms of anal incontinence at postpartum follow-up (C).
Do you routinely check with new first-time mothers at a postpartum visit about changes in anal continence? They are at particular risk for obstetric anal sphincter injury and could be too embarrassed to raise the issue.
Sphincter injury following labor is the most common cause of anal incontinence (including flatus) in women, which can severely diminish quality of life and lead to considerable personal and financial costs.1 Endoanal ultrasound can detect these injuries, even in the absence of clinically obvious damage to the anal sphincter (occult obstetric anal sphincter injury).2
In this article, we review measures to reduce the occurrence of obstetric anal sphincter injury, proper primary repair when it does occur, and appropriate long-term follow-up. Women with known obstetric anal sphincter injury must also be counseled about the risk of further damage during a future vaginal delivery.
Injury more common than symptoms would suggest
The conventional definitions of the 4 grades of perineal laceration in the US have been supplemented by more recent modifications included in a recent British Royal College of Obstetricians and Gynaecologists (RCOG) guideline (TABLE 1).3 The definition of third-degree laceration now reflects the various degrees of anal sphincter injury that may occur: partial (3a), full-thickness (3b), external anal sphincter injury, with or without injury to the internal anal sphincter (3c).
The incidence of clinical third- and fourth-degree lacerations varies widely; it is reported at between 0.5% and 3.0% in Europe and between 5.85% and 8.9% in the US.2,4-6 A landmark British paper from 1993 revealed that though only 3% had a clinical third- or fourth-degree perineal laceration, 35% of primiparous women (none of whom had any defect before delivery) had ultrasound evidence of varying degrees of anal sphincter defect at 6 weeks postpartum that persisted at 6 months.2 However, only about a third of these women had symptoms of bowel disturbance during the time of study.
These findings are supported by a meta-analysis in which 70% of women with a documented obstetric anal sphincter injury were asymptomatic.7 This meta-analysis concluded that clinical or occult obstetric anal sphincter injury occurs in 27% of primigravid women, and in 8.5% of multiparous women.
The long-term significance of occult obstetric anal sphincter injury and any relationship with geriatric fecal incontinence is unknown, although 71% of a sample of women with late-onset fecal incontinence were found to have ultrasound evidence of an anal sphincter defect thought to have occurred at a previous vaginal delivery.8 A recent English study9 reveals that when women were carefully re-examined after delivery by a skilled obstetrician looking specifically at the anal sphincter, the prevalence of clinically diagnosed third-degree lacerations rose sharply from the 11% initially diagnosed by the delivering physician or midwife to 24.5%. A subsequent endoanal ultrasound detected only an additional 1.2% (3 injuries, 2 of which were in the internal anal sphincter and therefore clinically undetectable). This strongly suggests that the vast majority of obstetric anal sphincter injuries can be detected clinically by a careful exam and that, when this is done, true occult injuries will be a rare finding.
TABLE 1
Classification of perineal injury9
| INJURY | DEFINITION |
|---|---|
| First degree | Injury confined to vaginal mucosa |
| Second degree | Injury of vaginal mucosa and perineal muscles, but not the anal sphincter |
| Third degree | Injury to the perineum involving the anal sphincter complex (external and internal) |
| 3a | <50% of external sphincter thickness is torn |
| 3b | >50% of external sphincter thickness is torn |
| 3c | Internal sphincter is torn |
| Fourth degree | Injury to external and internal sphincter and rectal mucosa/anal epithelium |
Mechanisms of injury
Maintenance of fecal continence involves the coordinated action of several anatomical and physiological elements (FIGURE 1).10 An intact, innervated anal sphincter complex (both external and internal) is necessary. The sphincter complex can be damaged during childbirth in 3 ways.
Direct mechanical injury. Direct external or internal anal sphincter muscle disruption can occur, as with a clinically obvious third- or fourth-degree perineal laceration or an occult injury subsequently noted on ultrasound.
Neurologic injury. Neuropathy of the pudendal nerve may result from forceps delivery or persistent nerve compression from the fetal head.14 Traction neuropathy may also occur with fetal macrosomia and with prolonged pushing during Stage 2 in successive pregnancies, or with prolonged stretching of the nerve due to persistent poor postpartum pelvic floor tone. Injured nerves often undergo demyelination but usually recover with time.
Combined mechanical and neurologic trauma. Isolated neurologic injury, as described above, is believed to be rare. Neuropathy more commonly accompanies mechanical damage.15
Who is at risk?
Several risk factors are unavoidable. One of these is primiparity, a consistently reported independent variable also associated with other risk factors for obstetric anal sphincter injury, such as instrument delivery (TABLE 2).
TABLE 2
Major risk factors for obstetric anal sphincter injury
| RISK FACTOR | ODDS RATIO |
|---|---|
| Nulliparity (primigravidity) | 3–4 |
| Inherent predisposition: | |
| Short perineal body | 8 |
| Instrumental delivery, overall | 3 |
| Forceps-assisted delivery | 3–7 |
| Vacuum-assisted delivery | 3 |
| Forceps vs vacuum | 2.88* |
| Forceps with midline episiotomy | 25 |
| Prolonged second stage of labor (>1 hour) | 1.5–4 |
| Epidural analgesia | 1.5–3 |
| Intrapartum infant factors: | |
| Birthweight over 4 kg | 2 |
| Persistent occipitoposterior position | 2–3 |
| Episiotomy, mediolateral | 1.4 |
| Episiotomy, midline | 3–5 |
| Previous anal sphincter tear | 4 |
| All variables are statistically significant at P<.05. | |
| *Relative risk of altered fecal symptoms based on RCT findings, vacuum vs forceps.17 Data from randomized controlled trials are lacking for most labor variables. Due to differing methods of analysis (univariate vs regression) and outcome measures, risk ratios reported in the literature vary considerably. This table presents the approximate odds ratios for risk factors that have been reported most consistently from 1 prospective cohort study,16 1 randomized controlled trial,14 and, otherwise higher-quality retrospective analyses.18-23 | |
Preventing obstetric anal sphincter injury
Sphincter injury can occur even when obstetrical management is optimal. Although evidence from RCT data is often lacking, sufficient observational and retrospective data support the following recommendations to reduce the likelihood of injury.
Choose vacuum delivery before forceps
Any form of instrument delivery increases the risk of obstetric anal sphincter injury and altered fecal continence by between 2- and 7-fold.2,16,24 An RCT found clinical third-degree tears in 16% of women with forceps-assisted deliveries, compared with 7% of vacuum-assisted deliveries; the authors concluded that, when circumstances allow, vacuum delivery should be attempted first (acknowledging however that 23% of vacuum deliveries failed and proceeded to a forceps extraction, a sequence associated with increased injury).17 A meta-analysis confirmed that vacuum extraction is preferred when instrumental delivery is necessary (SOR: A).25
When midline episiotomy was performed during instrument delivery, the risk of obstetric anal sphincter injury approximately doubled again, such that, in one study, forceps delivery with episiotomy caused a 25-fold increase in obstetric anal sphincter injury.24
Any steps that may safely reduce the need for instrument delivery should be supported. Toward this end, the Canadian Clinical Practices Obstetrics committee has recommended evidence-based labor interventions such as one-to-one support in labor, the increased use of a partogram in labor and appropriate oxytocin use, all in an effort to reduce needs for operative interventions.26
If episiotomy necessary, mediolateral less risky than midline
Episiotomy was long promoted as a means of preserving the integrity of the perineal musculature and of avoiding damage to the anal sphincter, and it has been practiced routinely by some.27 Strong evidence now indicates that routine episiotomy (midline or mediolateral) is unhelpful and should be abandoned.25,27-29
Observational evidence overwhelmingly shows that midline episiotomy is strongly associated with obstetric anal sphincter injury.19,22,23,30,31 One of the few RCTs comparing midline with mediolateral episiotomy, although flawed in its design, noted that a clinical third-degree laceration occurred as an extension of episiotomy in 11.6% of midline incisions compared with just 2% of mediolateral cuts.32
Another RCT, designed to examine routine versus restrictive episiotomy, noted that all but 1 (98%) of the 47 third- or fourth-degree lacerations in a group of 700 women followed midline episiotomy.29 A retrospective database analysis noted a 6-fold higher risk of third-degree perineal lacerations for women undergoing midline episiotomy compared with mediolateral incision.23 Elsewhere, midline episiotomy was associated with a 5-fold increase in symptoms of fecal incontinence at 3 months postpartum when compared with women with an intact perineum.24
Even when midline episiotomies do not extend into clinical third-degree lacerations, the incidence of resultant postpartum fecal incontinence triples when compared with spontaneous second-degree perineal lacerations.30 The authors postulate that a perineum cut by midline episiotomy allows for more direct contact to occur between the fetal hard parts and the anal sphincter complex during delivery, thereby increasing occult obstetric anal sphincter injury.
Observational data conflict as to whether mediolateral episiotomy contributes to, or protects against, obstetric anal sphincter injury—although the burden of evidence favors it as a risk factor that should be avoided when possible.16,23,33 An angle of mediolateral incision cut closer to 45 degrees from the midline has been associated with less obstetric anal sphincter injury than incisions cut at closer angles to the midline.34
Repairing sphincter injury
Detecting injury in labor
With any severe perineal laceration, closely inspect the external and, if exposed, internal anal sphincter and perform a rectal exam, particularly for women with numerous risk factors (although no good evidence supports the role of the rectal exam in diagnosing obstetric anal sphincter injury). Colorectal surgeons have advocated the use of a muscle stimulator to assist in identifying the ends of the external sphincter, but this has not become common practice.35
Immediate vs delayed repair
It is standard practice to repair a damaged anal sphincter immediately or soon after delivery. However, given that a repair should be well done, and since a short delay does not appear to adversely affect healing, be prepared to wait for assistance for up to 24 hours rather than risk a suboptimal repair.36
Better training is needed
Even among trained obstetricians and obgyn residents, 64% have reported no training or unsatisfactory training in management of obstetric anal sphincter injury; 94% of physicians felt inadequately prepared at the time of their first independent repair of the anal sphincter.37,38 To improve your repair skills in a workshop setting, consult the following sources—www.aafp.org/also.xml in the US, or www.perineum.net in UK.
Analgesia and setting
Adequate analgesia is an essential element in a good repair. Complete relaxation of the anesthetized anal sphincter complex facilitates bringing torn ends of the sphincter together without tension.39 Though theoretically this can be attained with local anesthetic infiltration, RCOG recommends that regional or general anesthesia be considered to provide complete analgesia.37 It is further recommended that repair of the anal sphincter occur in an operating room, given the degree of contamination present in the labor room after delivery and the devastating effects of an infected repair (SOR: C).40
Repair technique
There are 2 commonly used methods of external anal sphincter repair: one, the traditionally taught end-to-end approximation of the cut ends, and the other, overlapping the cut ends of the external sphincter and suturing through the overlapped portions (FIGURE 2).36 Though an RCT from 2000 noted no significant difference in outcomes between these methods,41 other authors have suggested that an overlapping technique is preferred, and it remains the method most often used by colorectal surgeons in elective, secondary anal sphincter repairs.36,39,42
A Cochrane review of which technique is better has been registered in the Clinical Trials Database. General agreement is that closure using interrupted sutures of a monofilament material, such as 2-0 polydioxanone sulfate (PDS), is the preferred closure method for the external sphincter (SOR: C).36,40 It is recommended that a damaged internal sphincter be repaired with a running continuous suture of a material such as 2-0 polyglactin 910 (Vicryl) (SOR: C).36
FIGURE 2
2 methods of anal sphincter repair
Two commonly used methods of external anal sphincter repair are end-to-end approximate of the cut ends (top), and overlapping the cut ends and suturing through the overlapped portions (bottom). (Adapted from Leeman et al, Am Fam Physician 2003.33) ILLUSTRATION BY RICH LaROCCO
Immediate post-repair management
Use a stool softener
It had long been thought that constipation following obstetric anal sphincter injury allowed the sphincter to heal more effectively. However, new evidence from RCTs shows that using a laxative instead of a constipating regimen is more helpful in the immediate postpartum phase.43 Toward this end, use a stool softener, such as lactulose, for 3 to 10 days postpartum for women with obstetric anal sphincter injury.40
Should you prescribe an antibiotic?
Given the devastating effects of post-repair infection, most authorities consider it prudent to prescribe a course of broad-spectrum antibiotics, possibly including metronidazole (SOR: C)37,40 A Cochrane review is registered to further examine this issue. A separate Cochrane review of the use of antibiotics for instrument vaginal delivery concluded that quality data were insufficient to make any recommendations.44
Refer for physical therapy
Some authorities consider an early referral to physical therapy for pelvic floor exercises helpful in the immediate post-partum for all patients with obstetric anal sphincter injury (SOR: C).45
Long-term management
Ask patients about incontinence
Given that some women are too embarrassed to seek assistance, ask those with obstetric anal sphincter injury specific questions about any symptoms of anal incontinence at a follow-up visit, such as the 6-week postpartum visit (SOR: C).37,40 In some practices, all women who have sustained a third- or fourth-degree laceration are routinely scheduled for a 3-month follow-up visit to a dedicated clinic, irrespective of symptoms. Given the prevalence of occult obstetric anal sphincter injury for primigravid women, you may find it best to survey all women postnatally concerning any changes in anal continence. TABLE 3 demonstrates a validated, modified patient survey of anal incontinence.37,40 A score of 6 is often used as a cutoff.
TABLE 3
Fecal Continence Scoring scale
| SYMPTOM |
| 1. Passage of any flatus when socially undesirable |
| 2. Any incontinence of liquid stool |
| 3. Any need to wear a pad because of anal symptoms |
| 4. Any incontinence of solid stool |
| 5. Any fecal urgency (inability to defer defecation for more than 5 minutes) |
| SCALE |
| 0 Never |
| 1 Rarely (<1/month) |
| 2 Sometimes (1/week–1/month |
| 3 Usually (1/day–1/week) |
| 4 Always (>1/day) |
| A score of 0 implies complete continence and a score of 20 complete incontinence. |
| A score of 6 has been suggested as a cut-off to determine need for evaluation. |
| Source: Mahony et al, 2001;43 modified from Jorge and Wexner, 1993.44 |
When additional evaluation is needed
Patients who have symptoms of altered continence at 3 months (or who score above 6 on the Wexner scale) should be seen at a dedicated gynecologic or colorectal surgery clinic,46 where they can receive a more detailed clinical evaluation and undergo anal manometry (during resting and forced squeezing) or endoanal ultrasonography. Some patients respond well to physical therapy, though a few patients ultimately require reconstructive colorectal surgery and temporary colostomy.
Management in a subsequent pregnancy
Women who have had an obstetric anal sphincter injury are at increased risk for repeat injury in a future pregnancy.48 At some units, all such women are routinely offered a prenatal visit at the end of the second trimester to review their symptoms and to evaluate the anal sphincter with manometry or ultrasound. A large prospective study, however, found that recurrence of obstetric anal sphincter injury could not be predicted and that 95% of women with prior injury did not sustain further overt sphincter damage during a subsequent vaginal delivery.49
However, for some women, a repeat anal sphincter laceration could prove devastating. For these women—eg, those with previous severe symptoms that required secondary surgical repair—initiate an in-depth discussion concerning the risks and benefits of elective cesarean delivery versus vaginal delivery.37,40
Historically, fecal incontinence was defined as “the involuntary or inappropriate passage of feces.”10 However, a preferred definition now refers instead to anal incontinence, which is “any involuntary loss of feces or flatus, or urge incontinence, that is adversely affecting a woman’s quality of life.”40 This definition includes urgency of defecation and incontinence of flatus, both of which are much more common symptoms than fecal incontinence.50
Data are lacking on the community prevalence of incontinence, although it is known that women of age 45 experience 8 times the incidence of incontinence as men of the same age and that it increases in prevalence with age.10 A Canadian survey of almost 1000 women at 3 months postpartum revealed that 3.1% admitted to incontinence of feces while 25% admitted to involuntary escape of flatus. The subgroup of women who suffered clinical anal sphincter injury (that is, third- or fourth-degree lacerations) had considerably increased rates of incontinence of feces (7.8%) and of flatus (48%).24 It has been reported that approximately half of women who sustained anal sphincter tears in labor complained of anal, urinary, or perineal symptoms at a mean follow-up of 2.6 years after the injury.51 Most studies agree that many women are embarrassed about symptoms of anal incontinence and are reluctant to self-report them.50
CORRESPONDENCE
David Power, MD, MPH, Department of Family Medicine and Community Health, University of Minnesota, Mayo Mail Code 381, 516 Delaware St SE, Minneapolis, MN 55455. E-mail: [email protected]
1. Mellgren A, Jensen LL, Zetterstrom JP, Wong WD, Hofmeister JH, Lowry AC. Long-term cost of fecal incontinence secondary to obstetric injuries. Dis Colon Rectum 1999;42:857-865.
2. Sultan AH, Kamm MA, Hudson CN, Thomas JM, Bartram CI. Anal-sphincter disruption during vaginal delivery. N Engl J Med 1993;329:1905-1911.
3. Royal College of Obstetricians and Gynaecologists. Methods and Materials used in Perineal Repair. Guideline No. 23. London: RCOG Press; 2004.
4. Fitzpatrick M, Behan M, O’Connell PR, O’Herlihy C. A randomized clinical trial comparing primary overlap with approximation repair of third-degree obstetric tears. Am J Obstet Gynecol 2000;183:1220-1224.
5. Simhan HN, Krohn MA, Heine RP. Obstetric rectal injury: risk factors and the role of physician experience. J Matern Fetal Neonatal Med 2004;16:271-274.
6. Handa VL, Danielsen BH, Gilbert WM. Obstetric anal sphincter lacerations. Obstet Gynecol 2001;98:225-230.
7. Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg 2003;90:1333-1337.
8. Oberwalder M, Dinnewitzer A, Baig MK, et al. The association between late-onset fecal incontinence and obstetric anal sphincter defects. Arch Surg 2004;139:429-432.
9. Andrews V, Sultan AH, Thakar R, Jones PW. Occult anal sphincter injuries-myth or reality? BJOG 2006;113:195-200.
10. Sultan AH, Nugent K. Pathophysiology and nonsurgical treatment of anal incontinence. BJOG 2004;111 (Suppl 1):84-90.
11. Schafer A, Enck P, Furst G, et al. Anatomy of the anal sphincters. Dis Colon Rectum 1994;37:777-781.
12. Mahony R, Daly L, Behan M, Kirwan C, O’Herlihy C, O’Connell R. Internal Anal Sphincter injury predicts continence outcome following obstetric sphincter trauma. AJOG 2004;191:6s1-s89.
13. Bartram CL, Frudringer A. Handbook of Anal Endosonography. Petersfield, UK: Wrightson Biomedical Publishing; 1997.
14. Sultan AH, Kamm MA, Hudson CN. Pudendal nerve damage during labor: prospective study before and after childbirth. BJOG 1994;101:22-28.
15. Snooks SJ, Henry MM, Swash M. Fecal incontinence due to external anal sphincter division in childbirth is associated with damage to the innervation of the pelvic floor musculature: a double pathology. BJOG 1985;92:824-828.
16. Donnelly V, Fynes M, Campbell D, Johnson H, O’Connell PR, O’Herlihy C. Obstetric events leading to anal sphincter damage. Obstet Gynecol 1998;92:955-961.
17. Fitzpatrick M, Behan M, O’Connell PR, O’Herlihy C. Randomized clinical trial to assess anal sphincter function following forceps or vacuum assisted vaginal delivery. BJOG 2003;110:424-429.
18. Deering SH, Carlson N, Stitely M, Allaire AD, Satin AJ. Perineal body length and lacerations at delivery. J Reproductive Medicine 2004;49:306-310.
19. Goldberg J, Hyslop T, Tolosa JE, Sultana C. Racial Differences in severe Perineal lacerations after vaginal delivery. Am J Obstet Gynecol 2003;188:1063-1067.
20. Robinson JN, Norwitz ER, Cohen AP, McElrath TF, Lieberman ES. Epidural analgesia and third- or fourth-degree lacerations in nulliparas. Obstet Gynecol 1999;94:259-262.
21. Carroll TG, Engelken M, Mosier MC, Nazir N. Epidural analgesia and severe perineal laceration in a community-based obstetric practice. J Am Board Fam Prac 2003;16:1-6.
22. Riskin-Mashiah S, O’Brian Smith E, Wilkins IA. Risk factors for severe perineal tear: can we do better? Am J Perinatol 2002;19:225-234.
23. Bodner-Adler B, Bodner K, Kaider A, et al. Risk factors for third-degree perineal tears in vaginal deliveries with an analysis of episiotomy types. J Reprod Med 2001;46:752-756.
24. Eason E, Labrecque M, Marcoux S, Mondor M. Anal incontinence after childbirth. CMAJ 2002;166:326-330.
25. Eason E, Labrecque M, Wells G, Feldman P. Preventing perineal trauma during childbirth: a systematic review. Obstet Gynecol 2000;95:464-471.
26. Cargill YM, MacKinnon CJ, Arsensault MY, et al. Clinical Practice Obstetrics Committee. Guidelines for operative vaginal birth. J Obstet Gynaecol Can 2004;26:747-761.
27. Carroli G, Belizan J. Episiotomy for vaginal birth. Cochrane Database System Rev 1999;(3):CD000081.-
28. Schlomer G, Gross M, Meyer G. Effectiveness of liberal vs. conservative episiotomy in vaginal delivery with reference to preventing urinary and fecal incontinence: a systematic review [in German]. Wien Med Wochenschr 2003;153:269-275.
29. Klein MC, Gauthier RJ, Jorgensen SH, et al. Does episiotomy prevent perineal trauma and pelvic floor relaxation? Online J Curr Clin Trials 1992;Doc No 10.
30. Signorello LB, Harlow BL, Chekos AK, Repke JT. Midline episiotomy and anal incontinence: retrospective cohort study. BMJ 2000;320:86-90.
31. Shiono P, Klebanoff MA, Carey JC. Midline episiotomies: more harm than good? Obstet Gynecol 1990;75:765-770.
32. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. BJOG 1980;87:408-412.
33. Poen AC, Felt-Bersma RJ, Dekker GA, Deville W, Cuesta MA, Meuwissen SG. Third degree obstetric perineal tears: risk factors and the preventive role of mediolateral episiotomy. Br J Obstet Gynaecol 1997;104:563-566.
34. Eogan M, O’Connell R, O’Herlihy C. Does the angle of episiotomy affect the incidence of anal sphincter injury? BJOG 2006;113:190-194.
35. Cook TA, Keane D, Mortensen NJ. Is there a role for the colorectal team in the management of acute severe third-degree vaginal tears? Colorectal Dis 1999;1:263-266.
36. Leeman L, Spearman M, Rogers R. Repair of obstetric perineal lacerations. Am Fam Physician 2003;68:1585-1590.
37. Fernando RJ, Sultan AH, Radley S, Jones PW, Johanson RB. Management of obstetric anal sphincter injury: a systematic review & national practice survey. BMC Health Serv Res 2002;2:9.-
38. Sultan AH, Kamm MA, Hudson CN. Obstetric perineal trauma: an audit of training. J Obstet Gynaecol 1995;15:19-23.
39. Sultan AH, Monga AK, Kumar D, Stanton SL. Primary repair of obstetric anal sphincter rupture using the overlap technique. BJOG 1999;106:318-23.
40. Royal College of Obstetricians and Gynaecologists. Management of third- and fourth-degree perineal tears following vaginal delivery. Guideline No. 29. London: RCOG Press; 2001.
41. Fitzpatrick M, Behan M, O’Connell PR, O’Herlihy C. A randomised clinical trial comparing primary overlap with approximation repair of third degree tears. Am J Obstet Gynecol 2000;183:1220-1224.
42. Kairaluoma MV, Raivio P, Aarnio MT, Kellokumpo IH. Immediate repair of obstetric anal sphincter rupture: medium-term outcome of the overlap technique. Dis Colon Rectum 2004;47:1358-1363.
43. Mahony R, Behan M, O’Herlihy C, O’Connell PR. Randomized, clinical trial of bowel confinement vs. laxative use after primary repair of a third-degree obstetric anal sphincter tear. Dis Colon Rectum 2004;47:12-17.
44. Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam M. Antibiotic prophylaxis for operative vaginal delivery (Cochrane review). In: The Cochrane Library, Issue 2, 2004.
45. Fitzpatrick M, O’Herlihy C. Postpartum anal sphincter dysfunction. Current Obstet Gynaecol 1999;9:210-215.
46. Mahony R, O’Brien C, O’Herlihy C. Evaluation of obstetric anal sphincter injury. Reviews in Gynaecological Practice 2001;1:114-121.
47. Jorge M, Wexner S. Etiology and management of fecal incontinence. Dis Colon Rectum 1993;36:77-97.
48. Fynes M, Donnelly V, Behan M, O’Connell PR, O’Herlihy C. Effect of second vaginal delivery on anorectal physiology and faecal continence: a prospective study. Lancet 1999;354:983-986.
49. Harkin R, Fitzpatrick M, O’Connell PR, O’Herlihy C. Anal sphincter disruption at vaginal deliver: is recurrence predictable? Eur J Obstet Gynaecol 2003;109:149-152.
50. Fitzpatrick M, O’Herlihy C. The effects of labour and delivery on the pelvic floor. Best Pract Res Clin Obstet Gynaecol 2001;15:63-79.
51. Wood J, Amos L, Rieger N. Third degree anal sphincter tears: risk factors and outcome. Aust N Z J Obstet Gynaecol 1998;38:414-417.
1. Mellgren A, Jensen LL, Zetterstrom JP, Wong WD, Hofmeister JH, Lowry AC. Long-term cost of fecal incontinence secondary to obstetric injuries. Dis Colon Rectum 1999;42:857-865.
2. Sultan AH, Kamm MA, Hudson CN, Thomas JM, Bartram CI. Anal-sphincter disruption during vaginal delivery. N Engl J Med 1993;329:1905-1911.
3. Royal College of Obstetricians and Gynaecologists. Methods and Materials used in Perineal Repair. Guideline No. 23. London: RCOG Press; 2004.
4. Fitzpatrick M, Behan M, O’Connell PR, O’Herlihy C. A randomized clinical trial comparing primary overlap with approximation repair of third-degree obstetric tears. Am J Obstet Gynecol 2000;183:1220-1224.
5. Simhan HN, Krohn MA, Heine RP. Obstetric rectal injury: risk factors and the role of physician experience. J Matern Fetal Neonatal Med 2004;16:271-274.
6. Handa VL, Danielsen BH, Gilbert WM. Obstetric anal sphincter lacerations. Obstet Gynecol 2001;98:225-230.
7. Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg 2003;90:1333-1337.
8. Oberwalder M, Dinnewitzer A, Baig MK, et al. The association between late-onset fecal incontinence and obstetric anal sphincter defects. Arch Surg 2004;139:429-432.
9. Andrews V, Sultan AH, Thakar R, Jones PW. Occult anal sphincter injuries-myth or reality? BJOG 2006;113:195-200.
10. Sultan AH, Nugent K. Pathophysiology and nonsurgical treatment of anal incontinence. BJOG 2004;111 (Suppl 1):84-90.
11. Schafer A, Enck P, Furst G, et al. Anatomy of the anal sphincters. Dis Colon Rectum 1994;37:777-781.
12. Mahony R, Daly L, Behan M, Kirwan C, O’Herlihy C, O’Connell R. Internal Anal Sphincter injury predicts continence outcome following obstetric sphincter trauma. AJOG 2004;191:6s1-s89.
13. Bartram CL, Frudringer A. Handbook of Anal Endosonography. Petersfield, UK: Wrightson Biomedical Publishing; 1997.
14. Sultan AH, Kamm MA, Hudson CN. Pudendal nerve damage during labor: prospective study before and after childbirth. BJOG 1994;101:22-28.
15. Snooks SJ, Henry MM, Swash M. Fecal incontinence due to external anal sphincter division in childbirth is associated with damage to the innervation of the pelvic floor musculature: a double pathology. BJOG 1985;92:824-828.
16. Donnelly V, Fynes M, Campbell D, Johnson H, O’Connell PR, O’Herlihy C. Obstetric events leading to anal sphincter damage. Obstet Gynecol 1998;92:955-961.
17. Fitzpatrick M, Behan M, O’Connell PR, O’Herlihy C. Randomized clinical trial to assess anal sphincter function following forceps or vacuum assisted vaginal delivery. BJOG 2003;110:424-429.
18. Deering SH, Carlson N, Stitely M, Allaire AD, Satin AJ. Perineal body length and lacerations at delivery. J Reproductive Medicine 2004;49:306-310.
19. Goldberg J, Hyslop T, Tolosa JE, Sultana C. Racial Differences in severe Perineal lacerations after vaginal delivery. Am J Obstet Gynecol 2003;188:1063-1067.
20. Robinson JN, Norwitz ER, Cohen AP, McElrath TF, Lieberman ES. Epidural analgesia and third- or fourth-degree lacerations in nulliparas. Obstet Gynecol 1999;94:259-262.
21. Carroll TG, Engelken M, Mosier MC, Nazir N. Epidural analgesia and severe perineal laceration in a community-based obstetric practice. J Am Board Fam Prac 2003;16:1-6.
22. Riskin-Mashiah S, O’Brian Smith E, Wilkins IA. Risk factors for severe perineal tear: can we do better? Am J Perinatol 2002;19:225-234.
23. Bodner-Adler B, Bodner K, Kaider A, et al. Risk factors for third-degree perineal tears in vaginal deliveries with an analysis of episiotomy types. J Reprod Med 2001;46:752-756.
24. Eason E, Labrecque M, Marcoux S, Mondor M. Anal incontinence after childbirth. CMAJ 2002;166:326-330.
25. Eason E, Labrecque M, Wells G, Feldman P. Preventing perineal trauma during childbirth: a systematic review. Obstet Gynecol 2000;95:464-471.
26. Cargill YM, MacKinnon CJ, Arsensault MY, et al. Clinical Practice Obstetrics Committee. Guidelines for operative vaginal birth. J Obstet Gynaecol Can 2004;26:747-761.
27. Carroli G, Belizan J. Episiotomy for vaginal birth. Cochrane Database System Rev 1999;(3):CD000081.-
28. Schlomer G, Gross M, Meyer G. Effectiveness of liberal vs. conservative episiotomy in vaginal delivery with reference to preventing urinary and fecal incontinence: a systematic review [in German]. Wien Med Wochenschr 2003;153:269-275.
29. Klein MC, Gauthier RJ, Jorgensen SH, et al. Does episiotomy prevent perineal trauma and pelvic floor relaxation? Online J Curr Clin Trials 1992;Doc No 10.
30. Signorello LB, Harlow BL, Chekos AK, Repke JT. Midline episiotomy and anal incontinence: retrospective cohort study. BMJ 2000;320:86-90.
31. Shiono P, Klebanoff MA, Carey JC. Midline episiotomies: more harm than good? Obstet Gynecol 1990;75:765-770.
32. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. BJOG 1980;87:408-412.
33. Poen AC, Felt-Bersma RJ, Dekker GA, Deville W, Cuesta MA, Meuwissen SG. Third degree obstetric perineal tears: risk factors and the preventive role of mediolateral episiotomy. Br J Obstet Gynaecol 1997;104:563-566.
34. Eogan M, O’Connell R, O’Herlihy C. Does the angle of episiotomy affect the incidence of anal sphincter injury? BJOG 2006;113:190-194.
35. Cook TA, Keane D, Mortensen NJ. Is there a role for the colorectal team in the management of acute severe third-degree vaginal tears? Colorectal Dis 1999;1:263-266.
36. Leeman L, Spearman M, Rogers R. Repair of obstetric perineal lacerations. Am Fam Physician 2003;68:1585-1590.
37. Fernando RJ, Sultan AH, Radley S, Jones PW, Johanson RB. Management of obstetric anal sphincter injury: a systematic review & national practice survey. BMC Health Serv Res 2002;2:9.-
38. Sultan AH, Kamm MA, Hudson CN. Obstetric perineal trauma: an audit of training. J Obstet Gynaecol 1995;15:19-23.
39. Sultan AH, Monga AK, Kumar D, Stanton SL. Primary repair of obstetric anal sphincter rupture using the overlap technique. BJOG 1999;106:318-23.
40. Royal College of Obstetricians and Gynaecologists. Management of third- and fourth-degree perineal tears following vaginal delivery. Guideline No. 29. London: RCOG Press; 2001.
41. Fitzpatrick M, Behan M, O’Connell PR, O’Herlihy C. A randomised clinical trial comparing primary overlap with approximation repair of third degree tears. Am J Obstet Gynecol 2000;183:1220-1224.
42. Kairaluoma MV, Raivio P, Aarnio MT, Kellokumpo IH. Immediate repair of obstetric anal sphincter rupture: medium-term outcome of the overlap technique. Dis Colon Rectum 2004;47:1358-1363.
43. Mahony R, Behan M, O’Herlihy C, O’Connell PR. Randomized, clinical trial of bowel confinement vs. laxative use after primary repair of a third-degree obstetric anal sphincter tear. Dis Colon Rectum 2004;47:12-17.
44. Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam M. Antibiotic prophylaxis for operative vaginal delivery (Cochrane review). In: The Cochrane Library, Issue 2, 2004.
45. Fitzpatrick M, O’Herlihy C. Postpartum anal sphincter dysfunction. Current Obstet Gynaecol 1999;9:210-215.
46. Mahony R, O’Brien C, O’Herlihy C. Evaluation of obstetric anal sphincter injury. Reviews in Gynaecological Practice 2001;1:114-121.
47. Jorge M, Wexner S. Etiology and management of fecal incontinence. Dis Colon Rectum 1993;36:77-97.
48. Fynes M, Donnelly V, Behan M, O’Connell PR, O’Herlihy C. Effect of second vaginal delivery on anorectal physiology and faecal continence: a prospective study. Lancet 1999;354:983-986.
49. Harkin R, Fitzpatrick M, O’Connell PR, O’Herlihy C. Anal sphincter disruption at vaginal deliver: is recurrence predictable? Eur J Obstet Gynaecol 2003;109:149-152.
50. Fitzpatrick M, O’Herlihy C. The effects of labour and delivery on the pelvic floor. Best Pract Res Clin Obstet Gynaecol 2001;15:63-79.
51. Wood J, Amos L, Rieger N. Third degree anal sphincter tears: risk factors and outcome. Aust N Z J Obstet Gynaecol 1998;38:414-417.
The liver transplant recipient: What you need to know for long-term care
- In general, long-term treatment of hypertension, diabetes, and obesity after liver transplantation is similar to that for the general population ( C ).
- Measure bone density within the first year after transplantation. Treat osteoporosis with standard agents. Joint replacement surgery appears safe in this group of patients ( B ).
- Resume standard screening for malignancy 2 to 3 years after transplantation, and repeat at intervals similar to that used with the general population. Given the high risk of skin cancer, transplant recipients should wear sunblock (SPF > 40) and have routine dermatologic examinations ( B ).
- Patients should wait at least 2 years before considering pregnancy and use barrier-type methods in this period ( C ).
- Vaccinate patients against hepatitis A and B, influenza, and pneumococcus. Avoid live vaccines ( C ).
Orthotopic liver transplantation (OLT) is the replacement of a whole diseased liver with a healthy donor liver. The number of persons receiving OLT is increasing. Though it is unlikely you will be involved in the care of a patient immediately after OLT, you ’ ll need to know about the complications that occur in this period as they may impact the long-term care of the patient.
Long-term issues — such as cardiovascular disease, bone disease, malignancy, anemia, psychiatric disorders, and financial stressors — put these patients at higher risk for problems more than the average patient. Perhaps the most important task is for you to keep in contact with the transplant center when questions or concerns arise. Over time, you will once again become the primary physician and advocate for these patients.
Complications after transplant (less than 1 year)
Within 1 month post-OLT, the most frequent complications are acute graft rejection, vascular thrombosis, biliary leak or stricture, and infection. Between 1 and 3 months, acute and chronic graft rejection can occur, but medication toxicity and opportunistic infections become more common (TABLE 1). 1
A broad range of infections may develop, including cytomegalovirus, Epstein-Barr virus, herpes simplex virus, varicella zoster virus, adenovirus, tuberculosis, Pneumocystis , toxoplasmosis, Listeria spp, Candida spp, Aspergillus spp, and Cryptococcus spp. During this time, doses of immunosuppressive agents are lowered and corticosteroids are discontinued in many patients.
Once patients are considered stable after OLT, they will likely come under your supervision again. While opportunistic infections, surgical issues, and acute rejection become less common between 3 and 12 months, other complications related to OLT may occur.
Graft reinfection with hepatitis C virus (HCV) is universal, and 50% to 80% patients will develop biopsy-proven hepatitis. 2 Many will require treatment for recurrent HCV to avoid progression to cirrhosis.
Recurrent hepatitis B infection is much less common due to prophylactic therapy with hepatitis B immunoglobulin and antiviral medications, although 10% of transplant recipients will develop hepatitis despite prophylaxis.
Other causes of recurrent liver disease post-OLT include liver injury due to recurrent drug or alcohol abuse, non-alcoholic steatohepatitis, cholestatic and autoimmune liver disease, and liver cancer.
Toxicity due to immunosuppressive medications is also common in this time frame (TABLE 2). 3 Be alert to the potential for hepatotoxicity and drug interactions with any new pharmacologic agent. Other drugs (eg, lipid-lowering agents, antibiotics, antifungals) may cause liver injury on their own and need to be closely monitored.
Lastly, even though patients are at increased risk for such common infections as influenza, pneumonia, and urinary tract infections, opportunistic infections are uncommon in this period. Keep in mind that patients usually develop infections that are community-acquired and not opportunistic, particularly as time goes on.
TABLE 1
Common complications immediately after liver transplantation
| COMPLICATION | SIGNS/SYMPTOMS | LABORATORY TESTS | INITIAL MANAGEMENT |
|---|---|---|---|
| Acute rejection | Usually nonspecific or asymptomatic; low-grade fever, malaise, RUQ pain | Early: high AP, GGT; mild AST/ALT | 1) Doppler U/S: exclude HAT, biliary obstruction |
| Severe: high AST/ALT (usually < 1000) and TB | 2) Liver biopsy | ||
| Biliary obstruction or leak | Nonspecific to cholangitis (high fever, jaundice, sepsis); often no abdominal pain | High TB, AP, GGT | 1) Doppler U/S: exclude HAT, evaluate bile duct dilation |
| Less common: elevations in AST/ALT | 2) T-tube cholangiogram | ||
| 3) ERCP or PTC; surgical revision if failure | |||
| Hepatic artery thrombosis (HAT) | High fever, RUQ pain, jaundice; may progress to liver failure rapidly | High AST/ALT, TB Prolonged INR | 1) Doppler U/S: evaluate artery flow, bile ducts, liver abscess, infarction; if HAT, urgent revascularization |
| 2) Equivocal presentation: arteriography | |||
| Hepatic vein or inferior vena cava obstruction | Hepatomegaly, ascites, lower extremity edema | Nonspecific liver test abnormalities | 1) Doppler U/S |
| 2) If positive or negative+high suspicion, contrast venogram; dilation/stent procedure if stenosis or thrombosis | |||
| Portal vein thrombosis | Hematemesis (variceal bleed), abdominal pain ± ascites | Nonspecific liver test abnormalities; rarely high liver enzymes | 1) Doppler U/S |
| 2) If positive or negative+high suspicion: arteriography with portal venous phase; treat with shunt or retransplantation | |||
| Calcineurin-inhibitor toxicity | Tremor, headache, seizure, gastrointestinal | Elevated creatinine | 1) Drug level and hold if high |
| Hyperkalemia | 2) Replete electrolytes/fluids | ||
| Hypomagnesemia | 3) Review other medications for interactions ( TABLE 2 ) | ||
| Anemia | |||
| ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; ERCP, endoscopic retrograde cholangiopancreatography; GGT, gamma glutamyl-transferase; HAT, hepatic artery thrombosis; INR, international normalized ratio; PTC, percutaneous transhepatic cholangiography, RUQ, right upper quadrant; TB, total bilirubin; U/S, ultrasound | |||
TABLE 2
Immunosuppressive medications and interactions after liver transplantation
| MEDICATION | SIDE EFFECTS | MONITORING | COMMON DRUG INTERACTIONS |
|---|---|---|---|
| Corticosteroids | Weight gain, diabetes, hypertension, high lipids, neurotoxic, cataracts, osteoporosis | Glucose | |
| Blood pressure | |||
| Lipids | |||
| Tacrolimus | Diabetes, hypertension, high lipids, nephrotoxic, neurotoxic, gastrointestinal, high potassium, low magnesium | As above | Increased levels with azole antifungals, macrolide antibiotics, diltiazem, verapamil, danazol, metoclopromide |
| Drug levels | Decreased levels with rifampicin, phenobarbital, phenytoin, carbamazepine, St. John ’ s wort | ||
| Renal function | |||
| Electrolytes | |||
| Cyclosporine | Same as tacrolimus+gingival hyperplasia, hirsutism, rare hepatotoxicity | As tacrolimus | As tacrolimus; increased levels with grapefruit juice and sirolimus |
| Mycophenylate mofetil | Anemia, leukopenia, thrombocytopenia, gastrointestinal | CBC | May increase acyclovir levels Antacids, cholestyramine: lower absorption |
| Azathioprine | Same as mycophenylate+pancreatitis, hepatotoxicity | CBC Liver function tests | Allopurinol, ACE inhibitors, sirolimus: may potentiate marrow toxicity |
| Liver function tests | May lower anticoagulation effect of warfarin | ||
| Sirolimus | Same as mycophenylate+hyperlipidemia, hypertension, hypokalemia, diarrhea | CBC | |
| Lipids | |||
| Abbreviations: ACE, angiotensin-converting enzyme; CBC, complete blood count. | |||
Long-term complications
Cardiovascular disease
Up to 20% of late deaths after OLT are caused by cardiovascular disease. 4 Uncontrollable factors, such as preexisting cardiac disease, male sex, family history of cardiac disease, and advanced age contribute to the incidence of cardiovascular disease. However, a number of potentially controllable factors, such as hypertension, hyperlipidemia, obesity, and diabetes are common after OLT and should be addressed.
Hypertension. Hypertension occurs in 40% to 75% of OLT patients. 5 Causes include calcineurin-inhibitor (cyclosporine, tacrolimus) therapy, high-dose corticosteroids, and renal insufficiency. Calcineurin inhibitors cause renal vasoconstriction, leading to sodium retention and hypertension. Reducing the doses of these medications by the transplant center typically improves blood pressure control.
Treatment of choice for hypertension depends on how recently the transplant was performed. In the first 6 months following the procedure, dihydropyridine calcium-channel blockers (eg, amlodipine) and alpha-blockers are the mainstay of therapy, although peripheral edema and orthostatic hypotension may affect their tolerability. Diuretics can also be used in volume-overloaded patients.
After 6 months, other pharmacologic agents, such as angiotensin-converting enzyme (ACE) inhibitors and beta-blockers, can be administered to patients with stable renal function and without other contraindications (strength of recommendation [SOR]: C ). Long-term management of hypertension does not differ significantly from that in non-transplant patients.
Hyperlipidemia/obesity. Obesity and hyperlipidemia may affect up to half of OLT patients. Factors that contribute to both disorders include immunosuppressive drugs, increased appetite, diabetes, pretransplant hyperlipidemia, and history of cholestatic liver disease.
For hyperlipidemia, lifestyle modifications, such as diet and exercise, are recommended. If these measures are ineffective, statins are first-line agents. Avoid bile acid binding resins, which may interfere with the absorption of all medications. For refractory cases, switching from cyclosporine to tacrolimus under the direction of the transplant center might be indicated.
Treatment of obesity following OLT should also focus on lifestyle changes, as the safety of pharmacotherapy and surgery for obesity is uncertain in these patients.
Glucose intolerance and diabetes. Many patients will have glucose intolerance that resolves after steroid withdrawal. Main risk factors are pre-OLT diabetes, episodes of steroid-resistant rejection, and obesity. Post-OLT onset of diabetes will persist for only a small percentage of patients. 6
More than 56,000 liver transplants have been performed since the United Network for Organ Sharing created a national database for liver transplantation in 1988. In 2002, more than 5000 liver transplants were performed and more than 17,000 patients were on the waiting list for transplantation. Approximately 70% to 80% of these patients will survive to 5 years after transplantation and sustain a high quality of life long-term.
The most common indications for OLT in the US are shown in the FIGURE .7 Cirrhosis due to hepatitis C, chronic alcohol use, and idiopathic/autoimmune causes comprise almost 60% of the indications. Patients who meet minimal listing criteria may be placed on the waiting list for liver transplantation.
On February 27, 2002, a new nationwide system called MELD (Model for End-Stage Liver Disease) was adopted to rank patients on the waiting list based on the severity of liver disease and remove the subjectivity associated with the previous ranking system.8 The MELD score, which ranges from 6 to 40, is a mathematical computation based on the patient ’ s bilirubin, creatinine, and international normalized ratio (INR). Although early in use, the MELD system appears to be a good predictor of the need for transplantation and posttransplantation outcome.
Treatment of post-transplant diabetes is similar to that for any patient. Insulin is often required initially, but with reduction in immunosuppression and corticosteroids, patients can usually be switched to oral agents. Though there is no absolute contraindication to using any antidiabetes medications, most physicians try to avoid those with potential hepatotoxicity, such as the thiazolidinediones (SOR: C).
Weight loss is critical and often improves glucose tolerance. Transplant centers may switch patients from tacrolimus to cyclosporine to control hyperglycemia. Long-term screening for end organ complications (retinopathy, nephropathy, neuropathy) is as important for this population as it is for in non-transplant diabetics.
FIGURE
Indications for liver transplantation in the US
Renal disease
Up to 20% of OLT recipients develop end-stage renal disease, requiring hemodialysis or renal transplantation within 10 years after transplant. 5 If patients have renal dysfunction before OLT, lower-dose calcineurin inhibitors and using alternative immunosuppression post-OLT may improve renal function in the long term. A rise in creatinine in the first year after OLT is a strong risk factor for long-term development of renal insufficiency, while stable creatinine levels at 1 year usually indicate long-term maintenance of renal function.9,10
Closely monitor patients with early renal dysfunction, avoid nephrotoxic agents, and reduce or withdraw calcineurin inhibitors (as directed by the transplant center). Occasionally renal transplantation will be indicated.
Be aware that all OLT recipients need adequate hydration during acute illnesses (influenza, common colds, gastroenteritis), especially if they have renal dysfunction. Potential nephrotoxic agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs), aminoglycoside antibiotics, and intravenous contrast, should be avoided if possible.
Bone disease
Osteoporosis should be screened for and identified before OLT. Contributing factors for bone disease after transplant include preexisting osteoporosis, immobility, vitamin D deficiency, corticosteroid use, and hypogonadism. In the first 6 months after transplant, bone mineral density (BMD) significantly declines, often accelerated by immunosuppressive medications, corticosteroids, and immobility. 11 - 14 After 6 months, BMD increases rapidly and, by 12 months, approaches pre-OLT values. All patients should have bone densitometry performed before OLT or before hospital discharge and receive calcium (1500 mg/d) and vitamin D (800 IU/d) supplementation (SOR: C).
Unless significant risk factors for osteoporosis are present (eg, continued use of corticosteroids, history of bone loss, fracture, or cholestatic liver disease), it is unclear whether low-risk patients should have serial bone densitometry tests performed in the years following OLT. Patients with T-scores ≥ 2 standard deviations below mean should be considered for antiresorptive therapy. Given the recent concerns regarding estrogen use and cardiovascular disease, bisphosphonates and calcitonin are preferred. For patients who develop fractures or avascular necrosis from corticosteroids, joint replacement surgery appears to be safe and effective post-OLT (SOR: B).15,16
Malignancy
Of all of the complications following OLT, malignancy causes the highest morbidity and mortality. The overall incidence of malignancy is between 2.3% and 12.9% and may be up to 5 times higher than in the general population.17,18 The most common malignancies are post-transplant lymphoproliferative disorder (1% – 4.4%) and nonmelanoma skin cancer (0.5% – 4.3%); less common are gastrointestinal (0.4% – 1.0%), genitourinary (0.2% – 2.2%), lung (0.2% – 0.8%), and oropharyngeal (0.4% – 0.8%) malignancies.17 Many patients with small liver cancers (1 lesion < 5 cm or up to 3 lesions each < 3 cm) are receiving transplants and, despite the risk of recurrence post-OLT, have a similar survival rate as patients receiving OLT for other indications.
Though it is clear that OLT recipients are at higher risk than the general population for malignancy, there are no specific guidelines for screening. However, based on the risk of early malignancy, screening should resume within the first 2 to 3 years after OLT (SOR: B).19- 22 Most transplant centers will recommend either performing a more intense screening protocol than for non-transplant patients or individualizing screening protocols for each patient depending on risk factors.
Advise patients who spend time in the sun to wear sun block with a protective factor > 40 and to have routine skin examinations. It is unclear whether colorectal cancer screening in OLT recipients should occur more frequently than the general population. Colorectal adenomas may be more common among OLT recipients than among healthy controls, 23 but until more data are available, screening should mirror that of the general population (SOR: B). Since hepatocellular carcinoma may recur after OLT, transplant centers typically request imaging (computed tomography, ultrasound, magnetic resonance imaging) at regular intervals after OLT.
Use your discretion when screening for other common malignancies, such as breast, cervical, and prostate cancer. It is unclear whether screening specific groups of patients (such as tobacco smokers) for oropharyngeal, lung, and genitourinary cancer will be cost-effective or impact survival.
Anemia
The prevalence of anemia after OLT reportedly is between 4.3% and 28.2%, depending on the population studied and time after transplantation. 24 , 25 Blood loss, sepsis, medications, renal dysfunction, or hypersplenism can contribute to immediate postoperative anemia. Beyond the immediate postoperative period, anemia may be related to different causes (TABLE 3). 26 Medication-induced anemia is usually related to bone marrow suppression, although calcineurin inhibitors may cause microangiopathic hemolysis, hemolyticuremic syndrome, or pure redcell aplasia.
Viral infections often cause anemia in the first 12 weeks after transplantation. Aplastic anemia may be related to parvovirus B19 infection, although it is more commonly seen in patients who undergo liver transplantation for acute liver failure.24,27 Posttransplant lymphoproliferative disorder ranges from polyclonal B-cell hyperplasia (related to Epstein-Barr virus) that responds to reduction in immunosuppression to aggressive lymphoma treated with high dose chemotherapy.
Graft-versus-host disease is a rare but important cause of pancytopenia after OLT and is diagnosed by establishing chimerism, donor and recipient lymphocytes, in the blood and bone marrow; mortality is high.28
Lastly, renal failure and iron deficiency are other common causes of anemia after OLT that warrant investigation. Despite complete evaluation, half of adult patients do not have an identifiable cause of anemia and may respond to a therapeutic trial of erythropoietin (SOR: C).26
TABLE 3
Evaluation of anemia after liver transplantation
| CAUSE | TIME AFTER TRANSPLANT* | EVALUATION |
|---|---|---|
| Medications | ||
| Common : mycophenylate mofetil, azathioprine, sirolimus, tacrolimus, cyclosporine, interferon, ganciclovir | > 2 weeks | Alter immunosuppression, discontinue drug |
| Infrequent : dapsone, furosemide, trimethoprim/sulfamethoxazole | Discontinue drug | |
| Viral Infection | ||
| Parvovivurs B19 | 2 – 6 weeks | IgM titer, B19 DNA |
| Cytomegalovirus | 4 – 12 weeks | Rapid antigen, DNA |
| Epstein-Barr virus | 4 – 12 weeks | IgM titer, DNA |
| Aplastic anemia | 2 – 6 weeks | Bone marrow biopsy |
| Post-transplant lymphoproliferative disorder | > 6 weeks | Hemolysis indices (indirect bilirubin, haptoglobin, Coomb ’ s test), bone marrow biopsy |
| Graft-versus-host disease | 2 – 6 weeks | Demonstrate chimerism |
| Renal insufficiency | ||
| Common : tacrolimus, cyclosporine, diabetes, hypertension | > 2 weeks | Alter immunosuppression, treat diabetes/hypertension |
| Infrequent : HBV/HCV-related glomerulonephritis or cryoglobulinemia | Urinalysis, HBV DNA, HCV RNA, renal ultrasound/biopsy | |
| Iron-deficiency | > 6 weeks | Iron studies, evaluate for chronic blood loss (GI, GU) |
| Unknown cause | > 6 weeks | EPO trial |
| * These values represent the typical interval after transplantation | ||
| EPO, erythropoietin; GI, gastrointestinal; GU, genitourinary; HBV, hepatitis B virus; HCV, hepatitis C virus; IgM, immunoglobulin M. | ||
Psychosocial and socioeconomic concerns
Liver transplantation is a tremendously stressful and life-altering procedure affecting patients and their families. In the initial postoperative period, the stress of the operation and other factors (immunosuppression, infection, prolonged hospital stay) can lead to a variety of psychiatric disorders, such as delirium, anxiety, depression, mania, and psychosis. A multidisciplinary approach, including psychiatry, social work, and nursing care, is required to help the patients and families through this period, as expectations for full recovery may be delayed by psychiatric conditions.
Psychiatric problems
Many transplant recipients have long-term psychiatric problems. Depression and anxiety diminish quality of life, particularly for patients whose transplant was for hepatitis C and those with post-transplant viral recurrence.29,30 Most patients will respond to antidepressants and ongoing psychiatric care. The side-effect profile should be individualized for each patient, keeping in mind the potential interactions with the current medications.
Mania and hypomania, while less common than depression, are often related to higher doses of immunosuppression (eg, corticosteroids). Cyclosporine may increase lithium levels, leading to toxicity.31 Treatment with anticonvulsant medications, such as carbamazepine, may decrease calcineurin-inhibitor levels and should be monitored in coordination with the transplant team. Finally, some patients with encephalopathy prior to OLT have persistent cognitive deficits long after OLT.32
Drug and alcohol recidivism are common post-OLT and typically occurs in about 20% of patients. It is important that active steps are taken to avoid recidivism immediately after OLT. Long-term psychiatric care and continued attendance at support groups help maintain sobriety. The important contributions you can make are maintaining a heightened awareness for recidivism, communicating with patients regularly about drug and alcohol abuse, and providing support and referral services.
Socioeconomic problems
While most transplant recipients maintain a good quality of life, some have long-term socioeconomic problems. One study showed that only one third of OLT recipients returned successfully to work, just slightly higher than the percentage working before OLT.33 The economic situation improved in 11.9% of the recipients, worsened in 33.9%, and stayed the same in 54.2%. Concurrent illness, prolonged inactivity, psychiatric disorders, and the level of physical requirements at work are the main contributing factors to unemployment.
Another major stressor is medical cost. The average cost of immunosuppressive medications alone is $10,000 to $20,000 per year.3 Most of the charges are reimbursable, although this depends on the payer and time from transplantation. Medicare pays for immunosuppressive medications for only 36 months. Beyond that point, patients will require secondary insurance or other assistance. This expenditure is exacerbated by the cost of other medications, clinic visits with the transplant center, family physicians, and specialists, and time away from work.
Although patients are usually well informed of these concerns before OLT, they often do not appreciate the financial magnitude until after OLT. Encourage patients to return to work, stay active physically and mentally, and prepare for these financial considerations.
Sexual issues
Some patients have persistent sexual dysfunction that may have an organic basis (cardiovascular, renal, liver, endocrine) requiring investigation. The safety and efficacy of sildenafil (Viagra) in OLT recipients has not been investigated to date.
However, other patients regain their libido and gonadal function immediately after OLT; pregnancy may occur in this period. Advise patients to wait at least 2 years post-OLT before considering pregnancy (SOR: C). 34 Contraception, preferably barrier-type, should be used during sexual intercourse. Hormonal contraceptives are not contraindicated but should probably not be administered until the patient ’ s transplant status is stable.
If pregnancy does occur, apprise the patient of potential complications and adverse outcomes. Hypertension and preeclampsia are more common in pregnant OLT recipients; life-threatening infections and acute rejection are rare. Fortunately, most patients deliver healthy babies; miscarriages, stillbirths, and malformations are uncommon. An obstetrician specializing in high-risk pregnancy should follow all pregnant OLT recipients.
Vaccination
Vaccination after OLT is controversial. Live vaccines are generally contraindicated post-OLT and their safety in patients with stable graft function and on low levels of immunosuppression is unclear (SOR: C). Patients should receive pneumococcal vaccination, hepatitis A and B vaccination if not already immune, and yearly influenza vaccination (SOR: C). For travel outside of the US or in uncertain situations or exposures, the best reference is the Centers for Disease Control web site: www.cdc.org.
Communication with the transplant center
Direct communication with the patient ’ s transplant center is extremely important. You and the transplant center should determine the most effective way (phone, fax or e-mail) to communicate.
When should you contact the transplant center? First, obtain the center ’ s approval for any new medications that may be used long-term or have the potential for nephrotoxicity, hepatotoxicity, or immunosuppression. Second, notify the transplant center in the event of new signs or symptoms, such as fever, weight loss, abdominal pain, or jaundice. Being cautious by communicating early is often the most prudent course. Third, alert the transplant center of any hospitalizations. Transfer to the transplant center for any transplant-related problem or prolonged hospitalization usually provides the best outcome for the patient.
On the flip side, you are the primary caretaker, and the transplant center should regularly communicate with you regarding general medical concerns and any new diagnoses, interventions, or treatments. The transplant center should also regularly communicate with you regarding general medical concerns and any new diagnoses, interventions, or treatments. A strong, mutual relationship between you and the transplant center will have great impact on the recipient ’ s long-term care.
CORRESPONDENCE
Josh Levitsky, MD, Northwestern Memorial Hospital, 675 North St. Clair St, Suite 15-250, Chicago, IL 60611. E-mail: [email protected]
1. Killenger PG, Clavien PA. Medical Care of the Liver Transplant Patient. 2nd ed. Malden, Mass: Blackwell Science, 2001: pp 183-232.
2. Fukumoto T, Berg T, Ku Y, Bechstein WO, et al. . Viral dynamics of hepatitis C early after orthotopic liver transplantation: Evidence for rapid turnover of serum virions. Hepatology 1996 ;24 :1351 -1354.
3. McCashland TM. Posttransplantation care: role of the primary care physician versus transplant center. Liver Transpl 2001 ;7(Suppl 1) :S2 -S12.
4. Romero M, Parera A, Salcedo M, et al. . Cardiovascular risk factors and late cardiovascular disease in liver transplantation. Transplantation Proc 1999 ;31 :2364 -2365.
5. Gonwa TA. Hypertension and renal dysfunction in long-term liver transplant recipients. Liver Transpl 2001 ;7(Suppl 1) :S22 -S26.
6. Reuben A. Long-term management of the liver transplant patient: diabetes, hyperlipidemia, and obesity. Liver Transpl 2001 ;7(Suppl 1) :S13 -S21.
7. Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transplantation 2004 ;10 :886 -897.
8. Kamath PS, Wiesner RH, Malinchoc M, et al. . A model to predict survival in patients with end-stage liver disease. Hepatology 2001 ;33 :464 -470.
9. Jain A, Reyes J, Kashyap R, et al. . What have we learned about primary liver transplantation under tacrolimus immunosuppression? Long-term follow-up of the first 1000 patients. Ann Surg 1999 ;230 :441 -448.
10. Gonwa TA, Mai ML, Melton LB, et al. . End stage renal disease (ESRD) following orthotopic liver transplantation (OLTX) utilizing calcineurin based immunotherapy: Risk of development and treatment. Transplantation 2001 ;72 :1934 -1939.
11. McDonald JA, Dunstan CR, Dilworth P, et al. . Bone loss after liver transplantation. Hepatology 1991 ;14 :613 -619.
12. Monegal A, Navasa M, Guanabens N, et al. . Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporosis Intl 2001 ;12 :484 -492.
13. Keogh JB, Tsalamandris C, Sewell RB, et al. . Bone loss at the proximal femur and reduced lean mass following liver transplantation: a longitudinal study. Nutrition 1999 ;15 :661 -664.
14. Crosbie OM, Freaney R, McKenna M, et al. . Predicting bone loss following orthotopic liver transplantation. Gut 1999 ;44 :430 -434.
15. Papagelopoulos PJ, Hay JE, Galanis EC, Morrey BF. Total joint arthroplasty in orthotopic liver transplant recipients. J Arthroplasty 1996 ;11 :889 -892.
16. Levitsky J, Te HS, Cohen SM. The safety and outcome of joint replacement surgery in liver transplant recipients. Liver Transpl 2003 ;9 :373 -376.
17. Fung JJ, Jain A, Kwak EJ, et al. . De novo malignancies after liver transplantation: A major cause of late death. Liver Transpl 2001 ;7 :S109 -S118.
18. Frezza EE, Fung JJ, van Thiel DH. Non-lymphoid cancer after liver transplantation. Hepatogastroenterology 1997 ;44 :1172 -1181.
19. Jain AB, Yee LD, Nalesnik MA, et al. . Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using Surveillance Epidemiologic End Result data. Transplantation 1998 ;66 :1193 -1200.
20. Sheil AGR. Malignancy following liver transplantation: A report from the Australian Combined Liver Transplant Registry. Transplant Proc 1995 ;27 :1247. -
21. Sheiner PA, Magliocca JF, Bodian CA, et al. . Long-term medical complications in patients surviving ≥ 5 years after liver transplantation. Transplantation 2000 ;69 :781 -789.
22. Levy M, Backman L, Husberg B, et al. . De novo malignancy following liver transplantation: A single center study. Transplant Proc 1993 ;25 :1397 -1399.
23. Atassi R, Thuluvath PJ. Risk of colorectal adenoma in liver transplant recipients compared to immunocompetent control population undergoing routine screening colonoscopy. J Clin Gastroenterol 2003 ;37 :72 -73.
24. Ndibmie OK, Frezza E, Jordan HA, Koch W, van Thiel DH. Parvovirus B19 in anemia liver transplant recipients. Clin Diagn Lab Immunol 1996 ;3 :756 -760.
25. Misra S, Moore TB, Ament ME, Vargas JH, Busitill RW, McDiarmid SV. Profile of anemia in children after liver transplantation. Transplantation 2000 ;70 :1459 -1463.
26. Maheshwari A, Mishra R, Thuluvath PJ. Post-liver transplant anemia: etiology and management. Liver Transpl 2004 ;10 :165 -173.
27. Tzakis AG, Arditi M, Whittington PF, et al. . Aplastic anemia complicating orthotopic liver transplantation for non-A, non-B hepatitis. N Engl J Med 1988 ;319 :393 -396.
28. Smith DM, Agura E, Netto G, et al. . Liver transplant-associated graft-versus-host disease. Transplantation 2003 ;75 :118 -126.
29. Singh N, Gayowski T, Wagener MM, Marino IR. Vulnerability to psychologic distress and depression in patients with end-stage liver disease due to hepatitis C virus. Clin Transplant 1997 ;11 :406 -411.
30. Paterson DL, Gayowski T, Wannstedt CF, et al. . Quality of life in long-term survivors after liver transplantation: impact of recurrent viral hepatitis C virus hepatitis. Clin Transplant 2000 ;14 :48 -54.
31. Trzepacz PT, DiMartini A, Tringali RD. Psychopharmacologic issues in organ transplantation. Part 2. Psychopharmacologic medications. Psychosomatics 1993 ;34 :290 -298.
32. Mechtcheriakov S, Graziadei IW, Mattedi M, et al. . Incomplete improvement of visuo-motor deficits in patients with minimal hepatic encephalopathy after liver transplantation. Liver Transpl 2004 ;10 :77 -83.
33. Loinaz C, Clemares M, Marqu é s E, et al. . Labor status of 137 patients with liver transplantation. Transplant Proc 1999 ;31 :2470 -2471.
34. Riely CA. Contraception and pregnancy after liver transplantation. Liver Transpl 2001 ;7(Suppl 1) :S74 -S76.
- In general, long-term treatment of hypertension, diabetes, and obesity after liver transplantation is similar to that for the general population ( C ).
- Measure bone density within the first year after transplantation. Treat osteoporosis with standard agents. Joint replacement surgery appears safe in this group of patients ( B ).
- Resume standard screening for malignancy 2 to 3 years after transplantation, and repeat at intervals similar to that used with the general population. Given the high risk of skin cancer, transplant recipients should wear sunblock (SPF > 40) and have routine dermatologic examinations ( B ).
- Patients should wait at least 2 years before considering pregnancy and use barrier-type methods in this period ( C ).
- Vaccinate patients against hepatitis A and B, influenza, and pneumococcus. Avoid live vaccines ( C ).
Orthotopic liver transplantation (OLT) is the replacement of a whole diseased liver with a healthy donor liver. The number of persons receiving OLT is increasing. Though it is unlikely you will be involved in the care of a patient immediately after OLT, you ’ ll need to know about the complications that occur in this period as they may impact the long-term care of the patient.
Long-term issues — such as cardiovascular disease, bone disease, malignancy, anemia, psychiatric disorders, and financial stressors — put these patients at higher risk for problems more than the average patient. Perhaps the most important task is for you to keep in contact with the transplant center when questions or concerns arise. Over time, you will once again become the primary physician and advocate for these patients.
Complications after transplant (less than 1 year)
Within 1 month post-OLT, the most frequent complications are acute graft rejection, vascular thrombosis, biliary leak or stricture, and infection. Between 1 and 3 months, acute and chronic graft rejection can occur, but medication toxicity and opportunistic infections become more common (TABLE 1). 1
A broad range of infections may develop, including cytomegalovirus, Epstein-Barr virus, herpes simplex virus, varicella zoster virus, adenovirus, tuberculosis, Pneumocystis , toxoplasmosis, Listeria spp, Candida spp, Aspergillus spp, and Cryptococcus spp. During this time, doses of immunosuppressive agents are lowered and corticosteroids are discontinued in many patients.
Once patients are considered stable after OLT, they will likely come under your supervision again. While opportunistic infections, surgical issues, and acute rejection become less common between 3 and 12 months, other complications related to OLT may occur.
Graft reinfection with hepatitis C virus (HCV) is universal, and 50% to 80% patients will develop biopsy-proven hepatitis. 2 Many will require treatment for recurrent HCV to avoid progression to cirrhosis.
Recurrent hepatitis B infection is much less common due to prophylactic therapy with hepatitis B immunoglobulin and antiviral medications, although 10% of transplant recipients will develop hepatitis despite prophylaxis.
Other causes of recurrent liver disease post-OLT include liver injury due to recurrent drug or alcohol abuse, non-alcoholic steatohepatitis, cholestatic and autoimmune liver disease, and liver cancer.
Toxicity due to immunosuppressive medications is also common in this time frame (TABLE 2). 3 Be alert to the potential for hepatotoxicity and drug interactions with any new pharmacologic agent. Other drugs (eg, lipid-lowering agents, antibiotics, antifungals) may cause liver injury on their own and need to be closely monitored.
Lastly, even though patients are at increased risk for such common infections as influenza, pneumonia, and urinary tract infections, opportunistic infections are uncommon in this period. Keep in mind that patients usually develop infections that are community-acquired and not opportunistic, particularly as time goes on.
TABLE 1
Common complications immediately after liver transplantation
| COMPLICATION | SIGNS/SYMPTOMS | LABORATORY TESTS | INITIAL MANAGEMENT |
|---|---|---|---|
| Acute rejection | Usually nonspecific or asymptomatic; low-grade fever, malaise, RUQ pain | Early: high AP, GGT; mild AST/ALT | 1) Doppler U/S: exclude HAT, biliary obstruction |
| Severe: high AST/ALT (usually < 1000) and TB | 2) Liver biopsy | ||
| Biliary obstruction or leak | Nonspecific to cholangitis (high fever, jaundice, sepsis); often no abdominal pain | High TB, AP, GGT | 1) Doppler U/S: exclude HAT, evaluate bile duct dilation |
| Less common: elevations in AST/ALT | 2) T-tube cholangiogram | ||
| 3) ERCP or PTC; surgical revision if failure | |||
| Hepatic artery thrombosis (HAT) | High fever, RUQ pain, jaundice; may progress to liver failure rapidly | High AST/ALT, TB Prolonged INR | 1) Doppler U/S: evaluate artery flow, bile ducts, liver abscess, infarction; if HAT, urgent revascularization |
| 2) Equivocal presentation: arteriography | |||
| Hepatic vein or inferior vena cava obstruction | Hepatomegaly, ascites, lower extremity edema | Nonspecific liver test abnormalities | 1) Doppler U/S |
| 2) If positive or negative+high suspicion, contrast venogram; dilation/stent procedure if stenosis or thrombosis | |||
| Portal vein thrombosis | Hematemesis (variceal bleed), abdominal pain ± ascites | Nonspecific liver test abnormalities; rarely high liver enzymes | 1) Doppler U/S |
| 2) If positive or negative+high suspicion: arteriography with portal venous phase; treat with shunt or retransplantation | |||
| Calcineurin-inhibitor toxicity | Tremor, headache, seizure, gastrointestinal | Elevated creatinine | 1) Drug level and hold if high |
| Hyperkalemia | 2) Replete electrolytes/fluids | ||
| Hypomagnesemia | 3) Review other medications for interactions ( TABLE 2 ) | ||
| Anemia | |||
| ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; ERCP, endoscopic retrograde cholangiopancreatography; GGT, gamma glutamyl-transferase; HAT, hepatic artery thrombosis; INR, international normalized ratio; PTC, percutaneous transhepatic cholangiography, RUQ, right upper quadrant; TB, total bilirubin; U/S, ultrasound | |||
TABLE 2
Immunosuppressive medications and interactions after liver transplantation
| MEDICATION | SIDE EFFECTS | MONITORING | COMMON DRUG INTERACTIONS |
|---|---|---|---|
| Corticosteroids | Weight gain, diabetes, hypertension, high lipids, neurotoxic, cataracts, osteoporosis | Glucose | |
| Blood pressure | |||
| Lipids | |||
| Tacrolimus | Diabetes, hypertension, high lipids, nephrotoxic, neurotoxic, gastrointestinal, high potassium, low magnesium | As above | Increased levels with azole antifungals, macrolide antibiotics, diltiazem, verapamil, danazol, metoclopromide |
| Drug levels | Decreased levels with rifampicin, phenobarbital, phenytoin, carbamazepine, St. John ’ s wort | ||
| Renal function | |||
| Electrolytes | |||
| Cyclosporine | Same as tacrolimus+gingival hyperplasia, hirsutism, rare hepatotoxicity | As tacrolimus | As tacrolimus; increased levels with grapefruit juice and sirolimus |
| Mycophenylate mofetil | Anemia, leukopenia, thrombocytopenia, gastrointestinal | CBC | May increase acyclovir levels Antacids, cholestyramine: lower absorption |
| Azathioprine | Same as mycophenylate+pancreatitis, hepatotoxicity | CBC Liver function tests | Allopurinol, ACE inhibitors, sirolimus: may potentiate marrow toxicity |
| Liver function tests | May lower anticoagulation effect of warfarin | ||
| Sirolimus | Same as mycophenylate+hyperlipidemia, hypertension, hypokalemia, diarrhea | CBC | |
| Lipids | |||
| Abbreviations: ACE, angiotensin-converting enzyme; CBC, complete blood count. | |||
Long-term complications
Cardiovascular disease
Up to 20% of late deaths after OLT are caused by cardiovascular disease. 4 Uncontrollable factors, such as preexisting cardiac disease, male sex, family history of cardiac disease, and advanced age contribute to the incidence of cardiovascular disease. However, a number of potentially controllable factors, such as hypertension, hyperlipidemia, obesity, and diabetes are common after OLT and should be addressed.
Hypertension. Hypertension occurs in 40% to 75% of OLT patients. 5 Causes include calcineurin-inhibitor (cyclosporine, tacrolimus) therapy, high-dose corticosteroids, and renal insufficiency. Calcineurin inhibitors cause renal vasoconstriction, leading to sodium retention and hypertension. Reducing the doses of these medications by the transplant center typically improves blood pressure control.
Treatment of choice for hypertension depends on how recently the transplant was performed. In the first 6 months following the procedure, dihydropyridine calcium-channel blockers (eg, amlodipine) and alpha-blockers are the mainstay of therapy, although peripheral edema and orthostatic hypotension may affect their tolerability. Diuretics can also be used in volume-overloaded patients.
After 6 months, other pharmacologic agents, such as angiotensin-converting enzyme (ACE) inhibitors and beta-blockers, can be administered to patients with stable renal function and without other contraindications (strength of recommendation [SOR]: C ). Long-term management of hypertension does not differ significantly from that in non-transplant patients.
Hyperlipidemia/obesity. Obesity and hyperlipidemia may affect up to half of OLT patients. Factors that contribute to both disorders include immunosuppressive drugs, increased appetite, diabetes, pretransplant hyperlipidemia, and history of cholestatic liver disease.
For hyperlipidemia, lifestyle modifications, such as diet and exercise, are recommended. If these measures are ineffective, statins are first-line agents. Avoid bile acid binding resins, which may interfere with the absorption of all medications. For refractory cases, switching from cyclosporine to tacrolimus under the direction of the transplant center might be indicated.
Treatment of obesity following OLT should also focus on lifestyle changes, as the safety of pharmacotherapy and surgery for obesity is uncertain in these patients.
Glucose intolerance and diabetes. Many patients will have glucose intolerance that resolves after steroid withdrawal. Main risk factors are pre-OLT diabetes, episodes of steroid-resistant rejection, and obesity. Post-OLT onset of diabetes will persist for only a small percentage of patients. 6
More than 56,000 liver transplants have been performed since the United Network for Organ Sharing created a national database for liver transplantation in 1988. In 2002, more than 5000 liver transplants were performed and more than 17,000 patients were on the waiting list for transplantation. Approximately 70% to 80% of these patients will survive to 5 years after transplantation and sustain a high quality of life long-term.
The most common indications for OLT in the US are shown in the FIGURE .7 Cirrhosis due to hepatitis C, chronic alcohol use, and idiopathic/autoimmune causes comprise almost 60% of the indications. Patients who meet minimal listing criteria may be placed on the waiting list for liver transplantation.
On February 27, 2002, a new nationwide system called MELD (Model for End-Stage Liver Disease) was adopted to rank patients on the waiting list based on the severity of liver disease and remove the subjectivity associated with the previous ranking system.8 The MELD score, which ranges from 6 to 40, is a mathematical computation based on the patient ’ s bilirubin, creatinine, and international normalized ratio (INR). Although early in use, the MELD system appears to be a good predictor of the need for transplantation and posttransplantation outcome.
Treatment of post-transplant diabetes is similar to that for any patient. Insulin is often required initially, but with reduction in immunosuppression and corticosteroids, patients can usually be switched to oral agents. Though there is no absolute contraindication to using any antidiabetes medications, most physicians try to avoid those with potential hepatotoxicity, such as the thiazolidinediones (SOR: C).
Weight loss is critical and often improves glucose tolerance. Transplant centers may switch patients from tacrolimus to cyclosporine to control hyperglycemia. Long-term screening for end organ complications (retinopathy, nephropathy, neuropathy) is as important for this population as it is for in non-transplant diabetics.
FIGURE
Indications for liver transplantation in the US
Renal disease
Up to 20% of OLT recipients develop end-stage renal disease, requiring hemodialysis or renal transplantation within 10 years after transplant. 5 If patients have renal dysfunction before OLT, lower-dose calcineurin inhibitors and using alternative immunosuppression post-OLT may improve renal function in the long term. A rise in creatinine in the first year after OLT is a strong risk factor for long-term development of renal insufficiency, while stable creatinine levels at 1 year usually indicate long-term maintenance of renal function.9,10
Closely monitor patients with early renal dysfunction, avoid nephrotoxic agents, and reduce or withdraw calcineurin inhibitors (as directed by the transplant center). Occasionally renal transplantation will be indicated.
Be aware that all OLT recipients need adequate hydration during acute illnesses (influenza, common colds, gastroenteritis), especially if they have renal dysfunction. Potential nephrotoxic agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs), aminoglycoside antibiotics, and intravenous contrast, should be avoided if possible.
Bone disease
Osteoporosis should be screened for and identified before OLT. Contributing factors for bone disease after transplant include preexisting osteoporosis, immobility, vitamin D deficiency, corticosteroid use, and hypogonadism. In the first 6 months after transplant, bone mineral density (BMD) significantly declines, often accelerated by immunosuppressive medications, corticosteroids, and immobility. 11 - 14 After 6 months, BMD increases rapidly and, by 12 months, approaches pre-OLT values. All patients should have bone densitometry performed before OLT or before hospital discharge and receive calcium (1500 mg/d) and vitamin D (800 IU/d) supplementation (SOR: C).
Unless significant risk factors for osteoporosis are present (eg, continued use of corticosteroids, history of bone loss, fracture, or cholestatic liver disease), it is unclear whether low-risk patients should have serial bone densitometry tests performed in the years following OLT. Patients with T-scores ≥ 2 standard deviations below mean should be considered for antiresorptive therapy. Given the recent concerns regarding estrogen use and cardiovascular disease, bisphosphonates and calcitonin are preferred. For patients who develop fractures or avascular necrosis from corticosteroids, joint replacement surgery appears to be safe and effective post-OLT (SOR: B).15,16
Malignancy
Of all of the complications following OLT, malignancy causes the highest morbidity and mortality. The overall incidence of malignancy is between 2.3% and 12.9% and may be up to 5 times higher than in the general population.17,18 The most common malignancies are post-transplant lymphoproliferative disorder (1% – 4.4%) and nonmelanoma skin cancer (0.5% – 4.3%); less common are gastrointestinal (0.4% – 1.0%), genitourinary (0.2% – 2.2%), lung (0.2% – 0.8%), and oropharyngeal (0.4% – 0.8%) malignancies.17 Many patients with small liver cancers (1 lesion < 5 cm or up to 3 lesions each < 3 cm) are receiving transplants and, despite the risk of recurrence post-OLT, have a similar survival rate as patients receiving OLT for other indications.
Though it is clear that OLT recipients are at higher risk than the general population for malignancy, there are no specific guidelines for screening. However, based on the risk of early malignancy, screening should resume within the first 2 to 3 years after OLT (SOR: B).19- 22 Most transplant centers will recommend either performing a more intense screening protocol than for non-transplant patients or individualizing screening protocols for each patient depending on risk factors.
Advise patients who spend time in the sun to wear sun block with a protective factor > 40 and to have routine skin examinations. It is unclear whether colorectal cancer screening in OLT recipients should occur more frequently than the general population. Colorectal adenomas may be more common among OLT recipients than among healthy controls, 23 but until more data are available, screening should mirror that of the general population (SOR: B). Since hepatocellular carcinoma may recur after OLT, transplant centers typically request imaging (computed tomography, ultrasound, magnetic resonance imaging) at regular intervals after OLT.
Use your discretion when screening for other common malignancies, such as breast, cervical, and prostate cancer. It is unclear whether screening specific groups of patients (such as tobacco smokers) for oropharyngeal, lung, and genitourinary cancer will be cost-effective or impact survival.
Anemia
The prevalence of anemia after OLT reportedly is between 4.3% and 28.2%, depending on the population studied and time after transplantation. 24 , 25 Blood loss, sepsis, medications, renal dysfunction, or hypersplenism can contribute to immediate postoperative anemia. Beyond the immediate postoperative period, anemia may be related to different causes (TABLE 3). 26 Medication-induced anemia is usually related to bone marrow suppression, although calcineurin inhibitors may cause microangiopathic hemolysis, hemolyticuremic syndrome, or pure redcell aplasia.
Viral infections often cause anemia in the first 12 weeks after transplantation. Aplastic anemia may be related to parvovirus B19 infection, although it is more commonly seen in patients who undergo liver transplantation for acute liver failure.24,27 Posttransplant lymphoproliferative disorder ranges from polyclonal B-cell hyperplasia (related to Epstein-Barr virus) that responds to reduction in immunosuppression to aggressive lymphoma treated with high dose chemotherapy.
Graft-versus-host disease is a rare but important cause of pancytopenia after OLT and is diagnosed by establishing chimerism, donor and recipient lymphocytes, in the blood and bone marrow; mortality is high.28
Lastly, renal failure and iron deficiency are other common causes of anemia after OLT that warrant investigation. Despite complete evaluation, half of adult patients do not have an identifiable cause of anemia and may respond to a therapeutic trial of erythropoietin (SOR: C).26
TABLE 3
Evaluation of anemia after liver transplantation
| CAUSE | TIME AFTER TRANSPLANT* | EVALUATION |
|---|---|---|
| Medications | ||
| Common : mycophenylate mofetil, azathioprine, sirolimus, tacrolimus, cyclosporine, interferon, ganciclovir | > 2 weeks | Alter immunosuppression, discontinue drug |
| Infrequent : dapsone, furosemide, trimethoprim/sulfamethoxazole | Discontinue drug | |
| Viral Infection | ||
| Parvovivurs B19 | 2 – 6 weeks | IgM titer, B19 DNA |
| Cytomegalovirus | 4 – 12 weeks | Rapid antigen, DNA |
| Epstein-Barr virus | 4 – 12 weeks | IgM titer, DNA |
| Aplastic anemia | 2 – 6 weeks | Bone marrow biopsy |
| Post-transplant lymphoproliferative disorder | > 6 weeks | Hemolysis indices (indirect bilirubin, haptoglobin, Coomb ’ s test), bone marrow biopsy |
| Graft-versus-host disease | 2 – 6 weeks | Demonstrate chimerism |
| Renal insufficiency | ||
| Common : tacrolimus, cyclosporine, diabetes, hypertension | > 2 weeks | Alter immunosuppression, treat diabetes/hypertension |
| Infrequent : HBV/HCV-related glomerulonephritis or cryoglobulinemia | Urinalysis, HBV DNA, HCV RNA, renal ultrasound/biopsy | |
| Iron-deficiency | > 6 weeks | Iron studies, evaluate for chronic blood loss (GI, GU) |
| Unknown cause | > 6 weeks | EPO trial |
| * These values represent the typical interval after transplantation | ||
| EPO, erythropoietin; GI, gastrointestinal; GU, genitourinary; HBV, hepatitis B virus; HCV, hepatitis C virus; IgM, immunoglobulin M. | ||
Psychosocial and socioeconomic concerns
Liver transplantation is a tremendously stressful and life-altering procedure affecting patients and their families. In the initial postoperative period, the stress of the operation and other factors (immunosuppression, infection, prolonged hospital stay) can lead to a variety of psychiatric disorders, such as delirium, anxiety, depression, mania, and psychosis. A multidisciplinary approach, including psychiatry, social work, and nursing care, is required to help the patients and families through this period, as expectations for full recovery may be delayed by psychiatric conditions.
Psychiatric problems
Many transplant recipients have long-term psychiatric problems. Depression and anxiety diminish quality of life, particularly for patients whose transplant was for hepatitis C and those with post-transplant viral recurrence.29,30 Most patients will respond to antidepressants and ongoing psychiatric care. The side-effect profile should be individualized for each patient, keeping in mind the potential interactions with the current medications.
Mania and hypomania, while less common than depression, are often related to higher doses of immunosuppression (eg, corticosteroids). Cyclosporine may increase lithium levels, leading to toxicity.31 Treatment with anticonvulsant medications, such as carbamazepine, may decrease calcineurin-inhibitor levels and should be monitored in coordination with the transplant team. Finally, some patients with encephalopathy prior to OLT have persistent cognitive deficits long after OLT.32
Drug and alcohol recidivism are common post-OLT and typically occurs in about 20% of patients. It is important that active steps are taken to avoid recidivism immediately after OLT. Long-term psychiatric care and continued attendance at support groups help maintain sobriety. The important contributions you can make are maintaining a heightened awareness for recidivism, communicating with patients regularly about drug and alcohol abuse, and providing support and referral services.
Socioeconomic problems
While most transplant recipients maintain a good quality of life, some have long-term socioeconomic problems. One study showed that only one third of OLT recipients returned successfully to work, just slightly higher than the percentage working before OLT.33 The economic situation improved in 11.9% of the recipients, worsened in 33.9%, and stayed the same in 54.2%. Concurrent illness, prolonged inactivity, psychiatric disorders, and the level of physical requirements at work are the main contributing factors to unemployment.
Another major stressor is medical cost. The average cost of immunosuppressive medications alone is $10,000 to $20,000 per year.3 Most of the charges are reimbursable, although this depends on the payer and time from transplantation. Medicare pays for immunosuppressive medications for only 36 months. Beyond that point, patients will require secondary insurance or other assistance. This expenditure is exacerbated by the cost of other medications, clinic visits with the transplant center, family physicians, and specialists, and time away from work.
Although patients are usually well informed of these concerns before OLT, they often do not appreciate the financial magnitude until after OLT. Encourage patients to return to work, stay active physically and mentally, and prepare for these financial considerations.
Sexual issues
Some patients have persistent sexual dysfunction that may have an organic basis (cardiovascular, renal, liver, endocrine) requiring investigation. The safety and efficacy of sildenafil (Viagra) in OLT recipients has not been investigated to date.
However, other patients regain their libido and gonadal function immediately after OLT; pregnancy may occur in this period. Advise patients to wait at least 2 years post-OLT before considering pregnancy (SOR: C). 34 Contraception, preferably barrier-type, should be used during sexual intercourse. Hormonal contraceptives are not contraindicated but should probably not be administered until the patient ’ s transplant status is stable.
If pregnancy does occur, apprise the patient of potential complications and adverse outcomes. Hypertension and preeclampsia are more common in pregnant OLT recipients; life-threatening infections and acute rejection are rare. Fortunately, most patients deliver healthy babies; miscarriages, stillbirths, and malformations are uncommon. An obstetrician specializing in high-risk pregnancy should follow all pregnant OLT recipients.
Vaccination
Vaccination after OLT is controversial. Live vaccines are generally contraindicated post-OLT and their safety in patients with stable graft function and on low levels of immunosuppression is unclear (SOR: C). Patients should receive pneumococcal vaccination, hepatitis A and B vaccination if not already immune, and yearly influenza vaccination (SOR: C). For travel outside of the US or in uncertain situations or exposures, the best reference is the Centers for Disease Control web site: www.cdc.org.
Communication with the transplant center
Direct communication with the patient ’ s transplant center is extremely important. You and the transplant center should determine the most effective way (phone, fax or e-mail) to communicate.
When should you contact the transplant center? First, obtain the center ’ s approval for any new medications that may be used long-term or have the potential for nephrotoxicity, hepatotoxicity, or immunosuppression. Second, notify the transplant center in the event of new signs or symptoms, such as fever, weight loss, abdominal pain, or jaundice. Being cautious by communicating early is often the most prudent course. Third, alert the transplant center of any hospitalizations. Transfer to the transplant center for any transplant-related problem or prolonged hospitalization usually provides the best outcome for the patient.
On the flip side, you are the primary caretaker, and the transplant center should regularly communicate with you regarding general medical concerns and any new diagnoses, interventions, or treatments. The transplant center should also regularly communicate with you regarding general medical concerns and any new diagnoses, interventions, or treatments. A strong, mutual relationship between you and the transplant center will have great impact on the recipient ’ s long-term care.
CORRESPONDENCE
Josh Levitsky, MD, Northwestern Memorial Hospital, 675 North St. Clair St, Suite 15-250, Chicago, IL 60611. E-mail: [email protected]
- In general, long-term treatment of hypertension, diabetes, and obesity after liver transplantation is similar to that for the general population ( C ).
- Measure bone density within the first year after transplantation. Treat osteoporosis with standard agents. Joint replacement surgery appears safe in this group of patients ( B ).
- Resume standard screening for malignancy 2 to 3 years after transplantation, and repeat at intervals similar to that used with the general population. Given the high risk of skin cancer, transplant recipients should wear sunblock (SPF > 40) and have routine dermatologic examinations ( B ).
- Patients should wait at least 2 years before considering pregnancy and use barrier-type methods in this period ( C ).
- Vaccinate patients against hepatitis A and B, influenza, and pneumococcus. Avoid live vaccines ( C ).
Orthotopic liver transplantation (OLT) is the replacement of a whole diseased liver with a healthy donor liver. The number of persons receiving OLT is increasing. Though it is unlikely you will be involved in the care of a patient immediately after OLT, you ’ ll need to know about the complications that occur in this period as they may impact the long-term care of the patient.
Long-term issues — such as cardiovascular disease, bone disease, malignancy, anemia, psychiatric disorders, and financial stressors — put these patients at higher risk for problems more than the average patient. Perhaps the most important task is for you to keep in contact with the transplant center when questions or concerns arise. Over time, you will once again become the primary physician and advocate for these patients.
Complications after transplant (less than 1 year)
Within 1 month post-OLT, the most frequent complications are acute graft rejection, vascular thrombosis, biliary leak or stricture, and infection. Between 1 and 3 months, acute and chronic graft rejection can occur, but medication toxicity and opportunistic infections become more common (TABLE 1). 1
A broad range of infections may develop, including cytomegalovirus, Epstein-Barr virus, herpes simplex virus, varicella zoster virus, adenovirus, tuberculosis, Pneumocystis , toxoplasmosis, Listeria spp, Candida spp, Aspergillus spp, and Cryptococcus spp. During this time, doses of immunosuppressive agents are lowered and corticosteroids are discontinued in many patients.
Once patients are considered stable after OLT, they will likely come under your supervision again. While opportunistic infections, surgical issues, and acute rejection become less common between 3 and 12 months, other complications related to OLT may occur.
Graft reinfection with hepatitis C virus (HCV) is universal, and 50% to 80% patients will develop biopsy-proven hepatitis. 2 Many will require treatment for recurrent HCV to avoid progression to cirrhosis.
Recurrent hepatitis B infection is much less common due to prophylactic therapy with hepatitis B immunoglobulin and antiviral medications, although 10% of transplant recipients will develop hepatitis despite prophylaxis.
Other causes of recurrent liver disease post-OLT include liver injury due to recurrent drug or alcohol abuse, non-alcoholic steatohepatitis, cholestatic and autoimmune liver disease, and liver cancer.
Toxicity due to immunosuppressive medications is also common in this time frame (TABLE 2). 3 Be alert to the potential for hepatotoxicity and drug interactions with any new pharmacologic agent. Other drugs (eg, lipid-lowering agents, antibiotics, antifungals) may cause liver injury on their own and need to be closely monitored.
Lastly, even though patients are at increased risk for such common infections as influenza, pneumonia, and urinary tract infections, opportunistic infections are uncommon in this period. Keep in mind that patients usually develop infections that are community-acquired and not opportunistic, particularly as time goes on.
TABLE 1
Common complications immediately after liver transplantation
| COMPLICATION | SIGNS/SYMPTOMS | LABORATORY TESTS | INITIAL MANAGEMENT |
|---|---|---|---|
| Acute rejection | Usually nonspecific or asymptomatic; low-grade fever, malaise, RUQ pain | Early: high AP, GGT; mild AST/ALT | 1) Doppler U/S: exclude HAT, biliary obstruction |
| Severe: high AST/ALT (usually < 1000) and TB | 2) Liver biopsy | ||
| Biliary obstruction or leak | Nonspecific to cholangitis (high fever, jaundice, sepsis); often no abdominal pain | High TB, AP, GGT | 1) Doppler U/S: exclude HAT, evaluate bile duct dilation |
| Less common: elevations in AST/ALT | 2) T-tube cholangiogram | ||
| 3) ERCP or PTC; surgical revision if failure | |||
| Hepatic artery thrombosis (HAT) | High fever, RUQ pain, jaundice; may progress to liver failure rapidly | High AST/ALT, TB Prolonged INR | 1) Doppler U/S: evaluate artery flow, bile ducts, liver abscess, infarction; if HAT, urgent revascularization |
| 2) Equivocal presentation: arteriography | |||
| Hepatic vein or inferior vena cava obstruction | Hepatomegaly, ascites, lower extremity edema | Nonspecific liver test abnormalities | 1) Doppler U/S |
| 2) If positive or negative+high suspicion, contrast venogram; dilation/stent procedure if stenosis or thrombosis | |||
| Portal vein thrombosis | Hematemesis (variceal bleed), abdominal pain ± ascites | Nonspecific liver test abnormalities; rarely high liver enzymes | 1) Doppler U/S |
| 2) If positive or negative+high suspicion: arteriography with portal venous phase; treat with shunt or retransplantation | |||
| Calcineurin-inhibitor toxicity | Tremor, headache, seizure, gastrointestinal | Elevated creatinine | 1) Drug level and hold if high |
| Hyperkalemia | 2) Replete electrolytes/fluids | ||
| Hypomagnesemia | 3) Review other medications for interactions ( TABLE 2 ) | ||
| Anemia | |||
| ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; ERCP, endoscopic retrograde cholangiopancreatography; GGT, gamma glutamyl-transferase; HAT, hepatic artery thrombosis; INR, international normalized ratio; PTC, percutaneous transhepatic cholangiography, RUQ, right upper quadrant; TB, total bilirubin; U/S, ultrasound | |||
TABLE 2
Immunosuppressive medications and interactions after liver transplantation
| MEDICATION | SIDE EFFECTS | MONITORING | COMMON DRUG INTERACTIONS |
|---|---|---|---|
| Corticosteroids | Weight gain, diabetes, hypertension, high lipids, neurotoxic, cataracts, osteoporosis | Glucose | |
| Blood pressure | |||
| Lipids | |||
| Tacrolimus | Diabetes, hypertension, high lipids, nephrotoxic, neurotoxic, gastrointestinal, high potassium, low magnesium | As above | Increased levels with azole antifungals, macrolide antibiotics, diltiazem, verapamil, danazol, metoclopromide |
| Drug levels | Decreased levels with rifampicin, phenobarbital, phenytoin, carbamazepine, St. John ’ s wort | ||
| Renal function | |||
| Electrolytes | |||
| Cyclosporine | Same as tacrolimus+gingival hyperplasia, hirsutism, rare hepatotoxicity | As tacrolimus | As tacrolimus; increased levels with grapefruit juice and sirolimus |
| Mycophenylate mofetil | Anemia, leukopenia, thrombocytopenia, gastrointestinal | CBC | May increase acyclovir levels Antacids, cholestyramine: lower absorption |
| Azathioprine | Same as mycophenylate+pancreatitis, hepatotoxicity | CBC Liver function tests | Allopurinol, ACE inhibitors, sirolimus: may potentiate marrow toxicity |
| Liver function tests | May lower anticoagulation effect of warfarin | ||
| Sirolimus | Same as mycophenylate+hyperlipidemia, hypertension, hypokalemia, diarrhea | CBC | |
| Lipids | |||
| Abbreviations: ACE, angiotensin-converting enzyme; CBC, complete blood count. | |||
Long-term complications
Cardiovascular disease
Up to 20% of late deaths after OLT are caused by cardiovascular disease. 4 Uncontrollable factors, such as preexisting cardiac disease, male sex, family history of cardiac disease, and advanced age contribute to the incidence of cardiovascular disease. However, a number of potentially controllable factors, such as hypertension, hyperlipidemia, obesity, and diabetes are common after OLT and should be addressed.
Hypertension. Hypertension occurs in 40% to 75% of OLT patients. 5 Causes include calcineurin-inhibitor (cyclosporine, tacrolimus) therapy, high-dose corticosteroids, and renal insufficiency. Calcineurin inhibitors cause renal vasoconstriction, leading to sodium retention and hypertension. Reducing the doses of these medications by the transplant center typically improves blood pressure control.
Treatment of choice for hypertension depends on how recently the transplant was performed. In the first 6 months following the procedure, dihydropyridine calcium-channel blockers (eg, amlodipine) and alpha-blockers are the mainstay of therapy, although peripheral edema and orthostatic hypotension may affect their tolerability. Diuretics can also be used in volume-overloaded patients.
After 6 months, other pharmacologic agents, such as angiotensin-converting enzyme (ACE) inhibitors and beta-blockers, can be administered to patients with stable renal function and without other contraindications (strength of recommendation [SOR]: C ). Long-term management of hypertension does not differ significantly from that in non-transplant patients.
Hyperlipidemia/obesity. Obesity and hyperlipidemia may affect up to half of OLT patients. Factors that contribute to both disorders include immunosuppressive drugs, increased appetite, diabetes, pretransplant hyperlipidemia, and history of cholestatic liver disease.
For hyperlipidemia, lifestyle modifications, such as diet and exercise, are recommended. If these measures are ineffective, statins are first-line agents. Avoid bile acid binding resins, which may interfere with the absorption of all medications. For refractory cases, switching from cyclosporine to tacrolimus under the direction of the transplant center might be indicated.
Treatment of obesity following OLT should also focus on lifestyle changes, as the safety of pharmacotherapy and surgery for obesity is uncertain in these patients.
Glucose intolerance and diabetes. Many patients will have glucose intolerance that resolves after steroid withdrawal. Main risk factors are pre-OLT diabetes, episodes of steroid-resistant rejection, and obesity. Post-OLT onset of diabetes will persist for only a small percentage of patients. 6
More than 56,000 liver transplants have been performed since the United Network for Organ Sharing created a national database for liver transplantation in 1988. In 2002, more than 5000 liver transplants were performed and more than 17,000 patients were on the waiting list for transplantation. Approximately 70% to 80% of these patients will survive to 5 years after transplantation and sustain a high quality of life long-term.
The most common indications for OLT in the US are shown in the FIGURE .7 Cirrhosis due to hepatitis C, chronic alcohol use, and idiopathic/autoimmune causes comprise almost 60% of the indications. Patients who meet minimal listing criteria may be placed on the waiting list for liver transplantation.
On February 27, 2002, a new nationwide system called MELD (Model for End-Stage Liver Disease) was adopted to rank patients on the waiting list based on the severity of liver disease and remove the subjectivity associated with the previous ranking system.8 The MELD score, which ranges from 6 to 40, is a mathematical computation based on the patient ’ s bilirubin, creatinine, and international normalized ratio (INR). Although early in use, the MELD system appears to be a good predictor of the need for transplantation and posttransplantation outcome.
Treatment of post-transplant diabetes is similar to that for any patient. Insulin is often required initially, but with reduction in immunosuppression and corticosteroids, patients can usually be switched to oral agents. Though there is no absolute contraindication to using any antidiabetes medications, most physicians try to avoid those with potential hepatotoxicity, such as the thiazolidinediones (SOR: C).
Weight loss is critical and often improves glucose tolerance. Transplant centers may switch patients from tacrolimus to cyclosporine to control hyperglycemia. Long-term screening for end organ complications (retinopathy, nephropathy, neuropathy) is as important for this population as it is for in non-transplant diabetics.
FIGURE
Indications for liver transplantation in the US
Renal disease
Up to 20% of OLT recipients develop end-stage renal disease, requiring hemodialysis or renal transplantation within 10 years after transplant. 5 If patients have renal dysfunction before OLT, lower-dose calcineurin inhibitors and using alternative immunosuppression post-OLT may improve renal function in the long term. A rise in creatinine in the first year after OLT is a strong risk factor for long-term development of renal insufficiency, while stable creatinine levels at 1 year usually indicate long-term maintenance of renal function.9,10
Closely monitor patients with early renal dysfunction, avoid nephrotoxic agents, and reduce or withdraw calcineurin inhibitors (as directed by the transplant center). Occasionally renal transplantation will be indicated.
Be aware that all OLT recipients need adequate hydration during acute illnesses (influenza, common colds, gastroenteritis), especially if they have renal dysfunction. Potential nephrotoxic agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs), aminoglycoside antibiotics, and intravenous contrast, should be avoided if possible.
Bone disease
Osteoporosis should be screened for and identified before OLT. Contributing factors for bone disease after transplant include preexisting osteoporosis, immobility, vitamin D deficiency, corticosteroid use, and hypogonadism. In the first 6 months after transplant, bone mineral density (BMD) significantly declines, often accelerated by immunosuppressive medications, corticosteroids, and immobility. 11 - 14 After 6 months, BMD increases rapidly and, by 12 months, approaches pre-OLT values. All patients should have bone densitometry performed before OLT or before hospital discharge and receive calcium (1500 mg/d) and vitamin D (800 IU/d) supplementation (SOR: C).
Unless significant risk factors for osteoporosis are present (eg, continued use of corticosteroids, history of bone loss, fracture, or cholestatic liver disease), it is unclear whether low-risk patients should have serial bone densitometry tests performed in the years following OLT. Patients with T-scores ≥ 2 standard deviations below mean should be considered for antiresorptive therapy. Given the recent concerns regarding estrogen use and cardiovascular disease, bisphosphonates and calcitonin are preferred. For patients who develop fractures or avascular necrosis from corticosteroids, joint replacement surgery appears to be safe and effective post-OLT (SOR: B).15,16
Malignancy
Of all of the complications following OLT, malignancy causes the highest morbidity and mortality. The overall incidence of malignancy is between 2.3% and 12.9% and may be up to 5 times higher than in the general population.17,18 The most common malignancies are post-transplant lymphoproliferative disorder (1% – 4.4%) and nonmelanoma skin cancer (0.5% – 4.3%); less common are gastrointestinal (0.4% – 1.0%), genitourinary (0.2% – 2.2%), lung (0.2% – 0.8%), and oropharyngeal (0.4% – 0.8%) malignancies.17 Many patients with small liver cancers (1 lesion < 5 cm or up to 3 lesions each < 3 cm) are receiving transplants and, despite the risk of recurrence post-OLT, have a similar survival rate as patients receiving OLT for other indications.
Though it is clear that OLT recipients are at higher risk than the general population for malignancy, there are no specific guidelines for screening. However, based on the risk of early malignancy, screening should resume within the first 2 to 3 years after OLT (SOR: B).19- 22 Most transplant centers will recommend either performing a more intense screening protocol than for non-transplant patients or individualizing screening protocols for each patient depending on risk factors.
Advise patients who spend time in the sun to wear sun block with a protective factor > 40 and to have routine skin examinations. It is unclear whether colorectal cancer screening in OLT recipients should occur more frequently than the general population. Colorectal adenomas may be more common among OLT recipients than among healthy controls, 23 but until more data are available, screening should mirror that of the general population (SOR: B). Since hepatocellular carcinoma may recur after OLT, transplant centers typically request imaging (computed tomography, ultrasound, magnetic resonance imaging) at regular intervals after OLT.
Use your discretion when screening for other common malignancies, such as breast, cervical, and prostate cancer. It is unclear whether screening specific groups of patients (such as tobacco smokers) for oropharyngeal, lung, and genitourinary cancer will be cost-effective or impact survival.
Anemia
The prevalence of anemia after OLT reportedly is between 4.3% and 28.2%, depending on the population studied and time after transplantation. 24 , 25 Blood loss, sepsis, medications, renal dysfunction, or hypersplenism can contribute to immediate postoperative anemia. Beyond the immediate postoperative period, anemia may be related to different causes (TABLE 3). 26 Medication-induced anemia is usually related to bone marrow suppression, although calcineurin inhibitors may cause microangiopathic hemolysis, hemolyticuremic syndrome, or pure redcell aplasia.
Viral infections often cause anemia in the first 12 weeks after transplantation. Aplastic anemia may be related to parvovirus B19 infection, although it is more commonly seen in patients who undergo liver transplantation for acute liver failure.24,27 Posttransplant lymphoproliferative disorder ranges from polyclonal B-cell hyperplasia (related to Epstein-Barr virus) that responds to reduction in immunosuppression to aggressive lymphoma treated with high dose chemotherapy.
Graft-versus-host disease is a rare but important cause of pancytopenia after OLT and is diagnosed by establishing chimerism, donor and recipient lymphocytes, in the blood and bone marrow; mortality is high.28
Lastly, renal failure and iron deficiency are other common causes of anemia after OLT that warrant investigation. Despite complete evaluation, half of adult patients do not have an identifiable cause of anemia and may respond to a therapeutic trial of erythropoietin (SOR: C).26
TABLE 3
Evaluation of anemia after liver transplantation
| CAUSE | TIME AFTER TRANSPLANT* | EVALUATION |
|---|---|---|
| Medications | ||
| Common : mycophenylate mofetil, azathioprine, sirolimus, tacrolimus, cyclosporine, interferon, ganciclovir | > 2 weeks | Alter immunosuppression, discontinue drug |
| Infrequent : dapsone, furosemide, trimethoprim/sulfamethoxazole | Discontinue drug | |
| Viral Infection | ||
| Parvovivurs B19 | 2 – 6 weeks | IgM titer, B19 DNA |
| Cytomegalovirus | 4 – 12 weeks | Rapid antigen, DNA |
| Epstein-Barr virus | 4 – 12 weeks | IgM titer, DNA |
| Aplastic anemia | 2 – 6 weeks | Bone marrow biopsy |
| Post-transplant lymphoproliferative disorder | > 6 weeks | Hemolysis indices (indirect bilirubin, haptoglobin, Coomb ’ s test), bone marrow biopsy |
| Graft-versus-host disease | 2 – 6 weeks | Demonstrate chimerism |
| Renal insufficiency | ||
| Common : tacrolimus, cyclosporine, diabetes, hypertension | > 2 weeks | Alter immunosuppression, treat diabetes/hypertension |
| Infrequent : HBV/HCV-related glomerulonephritis or cryoglobulinemia | Urinalysis, HBV DNA, HCV RNA, renal ultrasound/biopsy | |
| Iron-deficiency | > 6 weeks | Iron studies, evaluate for chronic blood loss (GI, GU) |
| Unknown cause | > 6 weeks | EPO trial |
| * These values represent the typical interval after transplantation | ||
| EPO, erythropoietin; GI, gastrointestinal; GU, genitourinary; HBV, hepatitis B virus; HCV, hepatitis C virus; IgM, immunoglobulin M. | ||
Psychosocial and socioeconomic concerns
Liver transplantation is a tremendously stressful and life-altering procedure affecting patients and their families. In the initial postoperative period, the stress of the operation and other factors (immunosuppression, infection, prolonged hospital stay) can lead to a variety of psychiatric disorders, such as delirium, anxiety, depression, mania, and psychosis. A multidisciplinary approach, including psychiatry, social work, and nursing care, is required to help the patients and families through this period, as expectations for full recovery may be delayed by psychiatric conditions.
Psychiatric problems
Many transplant recipients have long-term psychiatric problems. Depression and anxiety diminish quality of life, particularly for patients whose transplant was for hepatitis C and those with post-transplant viral recurrence.29,30 Most patients will respond to antidepressants and ongoing psychiatric care. The side-effect profile should be individualized for each patient, keeping in mind the potential interactions with the current medications.
Mania and hypomania, while less common than depression, are often related to higher doses of immunosuppression (eg, corticosteroids). Cyclosporine may increase lithium levels, leading to toxicity.31 Treatment with anticonvulsant medications, such as carbamazepine, may decrease calcineurin-inhibitor levels and should be monitored in coordination with the transplant team. Finally, some patients with encephalopathy prior to OLT have persistent cognitive deficits long after OLT.32
Drug and alcohol recidivism are common post-OLT and typically occurs in about 20% of patients. It is important that active steps are taken to avoid recidivism immediately after OLT. Long-term psychiatric care and continued attendance at support groups help maintain sobriety. The important contributions you can make are maintaining a heightened awareness for recidivism, communicating with patients regularly about drug and alcohol abuse, and providing support and referral services.
Socioeconomic problems
While most transplant recipients maintain a good quality of life, some have long-term socioeconomic problems. One study showed that only one third of OLT recipients returned successfully to work, just slightly higher than the percentage working before OLT.33 The economic situation improved in 11.9% of the recipients, worsened in 33.9%, and stayed the same in 54.2%. Concurrent illness, prolonged inactivity, psychiatric disorders, and the level of physical requirements at work are the main contributing factors to unemployment.
Another major stressor is medical cost. The average cost of immunosuppressive medications alone is $10,000 to $20,000 per year.3 Most of the charges are reimbursable, although this depends on the payer and time from transplantation. Medicare pays for immunosuppressive medications for only 36 months. Beyond that point, patients will require secondary insurance or other assistance. This expenditure is exacerbated by the cost of other medications, clinic visits with the transplant center, family physicians, and specialists, and time away from work.
Although patients are usually well informed of these concerns before OLT, they often do not appreciate the financial magnitude until after OLT. Encourage patients to return to work, stay active physically and mentally, and prepare for these financial considerations.
Sexual issues
Some patients have persistent sexual dysfunction that may have an organic basis (cardiovascular, renal, liver, endocrine) requiring investigation. The safety and efficacy of sildenafil (Viagra) in OLT recipients has not been investigated to date.
However, other patients regain their libido and gonadal function immediately after OLT; pregnancy may occur in this period. Advise patients to wait at least 2 years post-OLT before considering pregnancy (SOR: C). 34 Contraception, preferably barrier-type, should be used during sexual intercourse. Hormonal contraceptives are not contraindicated but should probably not be administered until the patient ’ s transplant status is stable.
If pregnancy does occur, apprise the patient of potential complications and adverse outcomes. Hypertension and preeclampsia are more common in pregnant OLT recipients; life-threatening infections and acute rejection are rare. Fortunately, most patients deliver healthy babies; miscarriages, stillbirths, and malformations are uncommon. An obstetrician specializing in high-risk pregnancy should follow all pregnant OLT recipients.
Vaccination
Vaccination after OLT is controversial. Live vaccines are generally contraindicated post-OLT and their safety in patients with stable graft function and on low levels of immunosuppression is unclear (SOR: C). Patients should receive pneumococcal vaccination, hepatitis A and B vaccination if not already immune, and yearly influenza vaccination (SOR: C). For travel outside of the US or in uncertain situations or exposures, the best reference is the Centers for Disease Control web site: www.cdc.org.
Communication with the transplant center
Direct communication with the patient ’ s transplant center is extremely important. You and the transplant center should determine the most effective way (phone, fax or e-mail) to communicate.
When should you contact the transplant center? First, obtain the center ’ s approval for any new medications that may be used long-term or have the potential for nephrotoxicity, hepatotoxicity, or immunosuppression. Second, notify the transplant center in the event of new signs or symptoms, such as fever, weight loss, abdominal pain, or jaundice. Being cautious by communicating early is often the most prudent course. Third, alert the transplant center of any hospitalizations. Transfer to the transplant center for any transplant-related problem or prolonged hospitalization usually provides the best outcome for the patient.
On the flip side, you are the primary caretaker, and the transplant center should regularly communicate with you regarding general medical concerns and any new diagnoses, interventions, or treatments. The transplant center should also regularly communicate with you regarding general medical concerns and any new diagnoses, interventions, or treatments. A strong, mutual relationship between you and the transplant center will have great impact on the recipient ’ s long-term care.
CORRESPONDENCE
Josh Levitsky, MD, Northwestern Memorial Hospital, 675 North St. Clair St, Suite 15-250, Chicago, IL 60611. E-mail: [email protected]
1. Killenger PG, Clavien PA. Medical Care of the Liver Transplant Patient. 2nd ed. Malden, Mass: Blackwell Science, 2001: pp 183-232.
2. Fukumoto T, Berg T, Ku Y, Bechstein WO, et al. . Viral dynamics of hepatitis C early after orthotopic liver transplantation: Evidence for rapid turnover of serum virions. Hepatology 1996 ;24 :1351 -1354.
3. McCashland TM. Posttransplantation care: role of the primary care physician versus transplant center. Liver Transpl 2001 ;7(Suppl 1) :S2 -S12.
4. Romero M, Parera A, Salcedo M, et al. . Cardiovascular risk factors and late cardiovascular disease in liver transplantation. Transplantation Proc 1999 ;31 :2364 -2365.
5. Gonwa TA. Hypertension and renal dysfunction in long-term liver transplant recipients. Liver Transpl 2001 ;7(Suppl 1) :S22 -S26.
6. Reuben A. Long-term management of the liver transplant patient: diabetes, hyperlipidemia, and obesity. Liver Transpl 2001 ;7(Suppl 1) :S13 -S21.
7. Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transplantation 2004 ;10 :886 -897.
8. Kamath PS, Wiesner RH, Malinchoc M, et al. . A model to predict survival in patients with end-stage liver disease. Hepatology 2001 ;33 :464 -470.
9. Jain A, Reyes J, Kashyap R, et al. . What have we learned about primary liver transplantation under tacrolimus immunosuppression? Long-term follow-up of the first 1000 patients. Ann Surg 1999 ;230 :441 -448.
10. Gonwa TA, Mai ML, Melton LB, et al. . End stage renal disease (ESRD) following orthotopic liver transplantation (OLTX) utilizing calcineurin based immunotherapy: Risk of development and treatment. Transplantation 2001 ;72 :1934 -1939.
11. McDonald JA, Dunstan CR, Dilworth P, et al. . Bone loss after liver transplantation. Hepatology 1991 ;14 :613 -619.
12. Monegal A, Navasa M, Guanabens N, et al. . Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporosis Intl 2001 ;12 :484 -492.
13. Keogh JB, Tsalamandris C, Sewell RB, et al. . Bone loss at the proximal femur and reduced lean mass following liver transplantation: a longitudinal study. Nutrition 1999 ;15 :661 -664.
14. Crosbie OM, Freaney R, McKenna M, et al. . Predicting bone loss following orthotopic liver transplantation. Gut 1999 ;44 :430 -434.
15. Papagelopoulos PJ, Hay JE, Galanis EC, Morrey BF. Total joint arthroplasty in orthotopic liver transplant recipients. J Arthroplasty 1996 ;11 :889 -892.
16. Levitsky J, Te HS, Cohen SM. The safety and outcome of joint replacement surgery in liver transplant recipients. Liver Transpl 2003 ;9 :373 -376.
17. Fung JJ, Jain A, Kwak EJ, et al. . De novo malignancies after liver transplantation: A major cause of late death. Liver Transpl 2001 ;7 :S109 -S118.
18. Frezza EE, Fung JJ, van Thiel DH. Non-lymphoid cancer after liver transplantation. Hepatogastroenterology 1997 ;44 :1172 -1181.
19. Jain AB, Yee LD, Nalesnik MA, et al. . Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using Surveillance Epidemiologic End Result data. Transplantation 1998 ;66 :1193 -1200.
20. Sheil AGR. Malignancy following liver transplantation: A report from the Australian Combined Liver Transplant Registry. Transplant Proc 1995 ;27 :1247. -
21. Sheiner PA, Magliocca JF, Bodian CA, et al. . Long-term medical complications in patients surviving ≥ 5 years after liver transplantation. Transplantation 2000 ;69 :781 -789.
22. Levy M, Backman L, Husberg B, et al. . De novo malignancy following liver transplantation: A single center study. Transplant Proc 1993 ;25 :1397 -1399.
23. Atassi R, Thuluvath PJ. Risk of colorectal adenoma in liver transplant recipients compared to immunocompetent control population undergoing routine screening colonoscopy. J Clin Gastroenterol 2003 ;37 :72 -73.
24. Ndibmie OK, Frezza E, Jordan HA, Koch W, van Thiel DH. Parvovirus B19 in anemia liver transplant recipients. Clin Diagn Lab Immunol 1996 ;3 :756 -760.
25. Misra S, Moore TB, Ament ME, Vargas JH, Busitill RW, McDiarmid SV. Profile of anemia in children after liver transplantation. Transplantation 2000 ;70 :1459 -1463.
26. Maheshwari A, Mishra R, Thuluvath PJ. Post-liver transplant anemia: etiology and management. Liver Transpl 2004 ;10 :165 -173.
27. Tzakis AG, Arditi M, Whittington PF, et al. . Aplastic anemia complicating orthotopic liver transplantation for non-A, non-B hepatitis. N Engl J Med 1988 ;319 :393 -396.
28. Smith DM, Agura E, Netto G, et al. . Liver transplant-associated graft-versus-host disease. Transplantation 2003 ;75 :118 -126.
29. Singh N, Gayowski T, Wagener MM, Marino IR. Vulnerability to psychologic distress and depression in patients with end-stage liver disease due to hepatitis C virus. Clin Transplant 1997 ;11 :406 -411.
30. Paterson DL, Gayowski T, Wannstedt CF, et al. . Quality of life in long-term survivors after liver transplantation: impact of recurrent viral hepatitis C virus hepatitis. Clin Transplant 2000 ;14 :48 -54.
31. Trzepacz PT, DiMartini A, Tringali RD. Psychopharmacologic issues in organ transplantation. Part 2. Psychopharmacologic medications. Psychosomatics 1993 ;34 :290 -298.
32. Mechtcheriakov S, Graziadei IW, Mattedi M, et al. . Incomplete improvement of visuo-motor deficits in patients with minimal hepatic encephalopathy after liver transplantation. Liver Transpl 2004 ;10 :77 -83.
33. Loinaz C, Clemares M, Marqu é s E, et al. . Labor status of 137 patients with liver transplantation. Transplant Proc 1999 ;31 :2470 -2471.
34. Riely CA. Contraception and pregnancy after liver transplantation. Liver Transpl 2001 ;7(Suppl 1) :S74 -S76.
1. Killenger PG, Clavien PA. Medical Care of the Liver Transplant Patient. 2nd ed. Malden, Mass: Blackwell Science, 2001: pp 183-232.
2. Fukumoto T, Berg T, Ku Y, Bechstein WO, et al. . Viral dynamics of hepatitis C early after orthotopic liver transplantation: Evidence for rapid turnover of serum virions. Hepatology 1996 ;24 :1351 -1354.
3. McCashland TM. Posttransplantation care: role of the primary care physician versus transplant center. Liver Transpl 2001 ;7(Suppl 1) :S2 -S12.
4. Romero M, Parera A, Salcedo M, et al. . Cardiovascular risk factors and late cardiovascular disease in liver transplantation. Transplantation Proc 1999 ;31 :2364 -2365.
5. Gonwa TA. Hypertension and renal dysfunction in long-term liver transplant recipients. Liver Transpl 2001 ;7(Suppl 1) :S22 -S26.
6. Reuben A. Long-term management of the liver transplant patient: diabetes, hyperlipidemia, and obesity. Liver Transpl 2001 ;7(Suppl 1) :S13 -S21.
7. Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transplantation 2004 ;10 :886 -897.
8. Kamath PS, Wiesner RH, Malinchoc M, et al. . A model to predict survival in patients with end-stage liver disease. Hepatology 2001 ;33 :464 -470.
9. Jain A, Reyes J, Kashyap R, et al. . What have we learned about primary liver transplantation under tacrolimus immunosuppression? Long-term follow-up of the first 1000 patients. Ann Surg 1999 ;230 :441 -448.
10. Gonwa TA, Mai ML, Melton LB, et al. . End stage renal disease (ESRD) following orthotopic liver transplantation (OLTX) utilizing calcineurin based immunotherapy: Risk of development and treatment. Transplantation 2001 ;72 :1934 -1939.
11. McDonald JA, Dunstan CR, Dilworth P, et al. . Bone loss after liver transplantation. Hepatology 1991 ;14 :613 -619.
12. Monegal A, Navasa M, Guanabens N, et al. . Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporosis Intl 2001 ;12 :484 -492.
13. Keogh JB, Tsalamandris C, Sewell RB, et al. . Bone loss at the proximal femur and reduced lean mass following liver transplantation: a longitudinal study. Nutrition 1999 ;15 :661 -664.
14. Crosbie OM, Freaney R, McKenna M, et al. . Predicting bone loss following orthotopic liver transplantation. Gut 1999 ;44 :430 -434.
15. Papagelopoulos PJ, Hay JE, Galanis EC, Morrey BF. Total joint arthroplasty in orthotopic liver transplant recipients. J Arthroplasty 1996 ;11 :889 -892.
16. Levitsky J, Te HS, Cohen SM. The safety and outcome of joint replacement surgery in liver transplant recipients. Liver Transpl 2003 ;9 :373 -376.
17. Fung JJ, Jain A, Kwak EJ, et al. . De novo malignancies after liver transplantation: A major cause of late death. Liver Transpl 2001 ;7 :S109 -S118.
18. Frezza EE, Fung JJ, van Thiel DH. Non-lymphoid cancer after liver transplantation. Hepatogastroenterology 1997 ;44 :1172 -1181.
19. Jain AB, Yee LD, Nalesnik MA, et al. . Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using Surveillance Epidemiologic End Result data. Transplantation 1998 ;66 :1193 -1200.
20. Sheil AGR. Malignancy following liver transplantation: A report from the Australian Combined Liver Transplant Registry. Transplant Proc 1995 ;27 :1247. -
21. Sheiner PA, Magliocca JF, Bodian CA, et al. . Long-term medical complications in patients surviving ≥ 5 years after liver transplantation. Transplantation 2000 ;69 :781 -789.
22. Levy M, Backman L, Husberg B, et al. . De novo malignancy following liver transplantation: A single center study. Transplant Proc 1993 ;25 :1397 -1399.
23. Atassi R, Thuluvath PJ. Risk of colorectal adenoma in liver transplant recipients compared to immunocompetent control population undergoing routine screening colonoscopy. J Clin Gastroenterol 2003 ;37 :72 -73.
24. Ndibmie OK, Frezza E, Jordan HA, Koch W, van Thiel DH. Parvovirus B19 in anemia liver transplant recipients. Clin Diagn Lab Immunol 1996 ;3 :756 -760.
25. Misra S, Moore TB, Ament ME, Vargas JH, Busitill RW, McDiarmid SV. Profile of anemia in children after liver transplantation. Transplantation 2000 ;70 :1459 -1463.
26. Maheshwari A, Mishra R, Thuluvath PJ. Post-liver transplant anemia: etiology and management. Liver Transpl 2004 ;10 :165 -173.
27. Tzakis AG, Arditi M, Whittington PF, et al. . Aplastic anemia complicating orthotopic liver transplantation for non-A, non-B hepatitis. N Engl J Med 1988 ;319 :393 -396.
28. Smith DM, Agura E, Netto G, et al. . Liver transplant-associated graft-versus-host disease. Transplantation 2003 ;75 :118 -126.
29. Singh N, Gayowski T, Wagener MM, Marino IR. Vulnerability to psychologic distress and depression in patients with end-stage liver disease due to hepatitis C virus. Clin Transplant 1997 ;11 :406 -411.
30. Paterson DL, Gayowski T, Wannstedt CF, et al. . Quality of life in long-term survivors after liver transplantation: impact of recurrent viral hepatitis C virus hepatitis. Clin Transplant 2000 ;14 :48 -54.
31. Trzepacz PT, DiMartini A, Tringali RD. Psychopharmacologic issues in organ transplantation. Part 2. Psychopharmacologic medications. Psychosomatics 1993 ;34 :290 -298.
32. Mechtcheriakov S, Graziadei IW, Mattedi M, et al. . Incomplete improvement of visuo-motor deficits in patients with minimal hepatic encephalopathy after liver transplantation. Liver Transpl 2004 ;10 :77 -83.
33. Loinaz C, Clemares M, Marqu é s E, et al. . Labor status of 137 patients with liver transplantation. Transplant Proc 1999 ;31 :2470 -2471.
34. Riely CA. Contraception and pregnancy after liver transplantation. Liver Transpl 2001 ;7(Suppl 1) :S74 -S76.
Is pulse palpation helpful in detecting atrial fibrillation? A systematic review
- Pulse palpation is a good test for ruling out atrial fibrillation (C).
- Patients with an irregular pulse should be followed up with an ECG.
- Background: Atrial fibrillation in the elderly is common and potentially life threatening. The classical sign of atrial fibrillation is an irregularly irregular pulse.
- Objective: The objective of this research was to determine the accuracy of pulse palpation to detect atrial fibrillation.
- Methods: We searched Medline, EMBASE, and the reference lists of review articles for studies that compared pulse palpation with the electrocardiogram (ECG) diagnosis of atrial fibrillation. Two reviewers independently assessed the search results to determine the eligibility of studies, extracted data, and assessed the quality of the studies.
- Results: We identified 3 studies (2385 patients) that compared pulse palpation with ECG. The estimated sensitivity of pulse palpation ranged from 91% to 100%, while specificity ranged from 70% to 77%. Pooled sensitivity was 94% (95% confidence interval [CI], 84%–97%) and pooled specificity was 72% (95% CI, 69%–75%). The pooled positive likelihood ratio was 3.39, while the pooled negative likelihood ratio was 0.10.
- Conclusions: Pulse palpation has a high sensitivity but relatively low specificity for atrial fibrillation. It is therefore useful for ruling out atrial fibrillation. It may also be a useful screen to apply opportunistically for previously undetected atrial fibrillation. Assuming a prevalence of 3% for undetected atrial fibrillation in patients older than 65 years, and given the test’s sensitivity and specificity, opportunistic pulse palpation in this age group would detect an irregular pulse in 30% of screened patients, requiring further testing with ECG. Among screened patients, 0.2% would have atrial fibrillation undetected with pulse palpation.
The objective of this review was to determine how well pulse palpation detects the classical sign of atrial fibrillation—an irregularly irregular pulse—and to estimate the test’s false-positive and false-negative rates. You may want to consider using pulse palpation opportunistically with certain high-risk patients identified in this article.
The scope of the problem. Atrial fibrillation in the elderly is common and potentially life-threatening. Its prevalence increases from 2% in the 65- to 69-year-old age group to 8% among those older than 85 years.1
Atrial fibrillation is an independent risk factor for stroke, increasing a person’s risk 3- to 5-fold. While other major risk factors for stroke, such as hypertension, become less important with age, the risk of stroke from atrial fibrillation increases with age.
Stroke attributable to atrial fibrillation increases from 1.5% among patients 50 to 59 years to 23.5% for those aged 80 to 89.2 Furthermore, stroke in the presence of atrial fibrillation is almost twice as likely to be fatal, results in more functional impairment among survivors, and recurs frequently.3
Stroke due to atrial fibrillation results in considerable mortality and morbidity, but treatment of atrial fibrillation reduces the risk. Warfarin reduces the risk of stroke in non-rheumatic atrial fibrillation by about 70%, an annual absolute risk reduction of 3% (number needed to treat: 30), though the benefits are somewhat offset by the risk of bleeding (annual risk of 0.6%).4
Methods
Search strategy and study selection
We searched the Medline and EMBASE electronic databases from 1966 to June 2005 for all studies comparing pulse palpation with ECG diagnosis. There was no restriction on the language of publication.
The search strategy (see Search strategy and inclusion criteria) included terms for pulse, atrial fibrillation, and a search filter for studies of diagnostic accuracy developed by Van der Weijden et al5 that is highly sensitive and precise.6 We also searched the reference lists of all possibly relevant studies, including review articles. We included all studies that tested patients with both pulse palpation and ECG, and that provided data suitable for calculating sensitivity or specificity (or could be provided by the study authors).
Two reviewers (GC and JD) independently screened the electronic lists of citations. We obtained full-text documents of relevant papers and the 2 reviewers independently reviewed the full-text articles for inclusion according to predetermined criteria (see Search strategy and inclusion criteria). The reviewers agreed on all inclusions.
The authors of this study searched Medline, EMBASE, and the reference lists of review articles for studies using the following criteria.
(pulse) OR (‘Pulse’/all subheadings in MIME,MJ ME))
AND ((atrial fibrillation) OR (‘Atrial-Fibrillation’/all subheadings in MIME,MJME))
AND ((‘sensitivity-and-specificity’/all subheadings in MIME,MJME) OR (sensitivity) OR (specificity) OR (‘Diagnosis-Differential’/all subheadings in MIME,MJME) OR (‘False-Negative-Reactions’/all subheadings in MIME,MJME) OR (‘False-Positive-Reactions’/all subheadings in MIME,MJME) OR (‘Mass-Screening’/all subheadings in MIME,MJME) OR (diagnos*) OR (predictive value*) OR (reference value*) OR (ROC*) OR (Likelihood ratio) OR (monitoring) OR (‘Reference-Values’/all subheadings in MIME,MJME)) OR (atrial fibrillation and (SH=diagnosis))
Two reviewers then independently assessed the search results to determine the eligibility of studies, extracted data, and assessed the quality of the studies.
Inclusion criteria
- Does the study compare pulse palpation to ECG diagnosis of atrial fibrillation?
- Do all the participants receive both tests?
- Are the sensitivity and specificity provided, or calculable from the data provided?
Data extraction and analysis
We assessed study quality with the QUADAS tool.7 Two reviewers assessed study quality and extracted data independently. We calculated the pooled sensitivity and specificity using Metatest (New England Medical Center, Boston, Mass) and the pooled positive and negative likelihood ratios using the Der-Simonian and Laird random effects model of Revman 4.29 (Cochrane Collaboration), which also tests for heterogeneity.
Results
Search results and selection
The search strategy identified 110 citations. Six studies were selected for further assessment for inclusion; 3 met the inclusion criteria and covered 2385 patients (FIGURE). All 3 studies included patients older than 65 years enrolled from general practice clinics, and in each study the pulse was taken by a nurse in the clinic. TABLE 1 summarizes the methodological quality, patient characteristics, and results of the 3 studies.
FIGURE
Studies included in this review
TABLE 1
Detecting atrial fibrillation by pulse palpation: Pertinent studies
| STUDY QUALITY CHARACTERISTICS | MORGAN AND MANT8 | SOMERVILLE ET AL10 | SUDLOW ET AL9 |
| Random or consecutive sampling | Yes | No* | Yes |
| Independent assessment | Yes | Yes | Not stated |
| Blind assessment | Yes | Yes | Not stated |
| Verification by ECG | 100% | 100% | 100% |
| Description of index and reference test | Good | Poor | Poor |
| STUDY CHARACTERISTICS | |||
| Number of patients | 1099 | 86 | 1200 |
| Age | 74.3 (65–100) | 65+ | 65+ |
| Setting | 4 GP practices | Single GP practice | Patients from 9 general practices |
| Location | Southampton, UK | Stoke-on Trent, UK | Southern Northumberland, UK |
| Index test | Radial pulse palpated by a nurse for a minimum of 20 seconds† | Pulse palpated by an experienced nurse.§ Time not specified | Pulse palpated by a nurse. Time not specified |
| Reference test | ECG read by cardiologist | 12-lead ECG read by cardiologist | Limb-lead ECG |
| RESULTS | |||
| Prevalence (%) | 6.1 | 30 | 4.6‡ |
| Sensitivity (%; 95% CI) | 91 (81–96) | 100 (87–100) | 95 (85–98)‡ |
| Specificity (%; 95% CI) | 74 (72–77) | 77 (65–86) | 70 (67–73)‡ |
| Positive likelihood ratio (95% CI) | 3.5 (3.1–4.0) | 4.1 (2.6–6.5) | 3.1 (2.8–3.5) |
| Negative likelihood ratio (95% CI) | 0.12 (0.06–0.27) | 0.024 (0.002–0.38) | 0.09 (0.03–0.25) |
| * Case-control study. | |||
| † Designated as regular or irregular. Irregular pulses were categorized into occasional ectopic, frequent ectopics, or continuously irregular. Patients with any irregularity were considered to have a positive index test. | |||
| § Experienced in community and emergency nursing, as well as taking and interpreting ECGs. | |||
| ‡ Data provided by the authors. | |||
Methodological quality of included studies
The study by Morgan and Mant8 fulfilled all the major quality criteria. The studies by Sudlow et al9 and Somerville et al10 were of moderate quality. Sudlow et al did not report an overall sensitivity or specificity, nor the data for calculating these test attributes. The authors kindly provided us the data from their study to calculate the 2×2 table of results.
Prevalence of atrial fibrillation
We calculated the pooled prevalence of atrial fibrillation, excluding data from Somerville et al, as it was a case-control study. The pooled prevalence of atrial fibrillation in the 2 remaining studies was 5.3%, consistent with previous estimates of atrial fibrillation in general practice settings and data from the Framingham cohort.1,11
Accuracy of pulse palpation for detecting atrial fibrillation
The sensitivities in the 3 studies ranged from 91% to 100% and the specificities ranged from 70% to 77%. The pooled sensitivity was 94% (95% CI, 84%–97%) and the pooled specificity was 72% (95% CI, 69%–75%). Low specificity reflects the pervasiveness of other pulse abnormalities, resulting in false-positive test results for atrial fibrillation (TABLE 2).
The pooled positive likelihood ratio was 3.4 (95% CI, 3.2–3.7), showing that pulse palpation was only moderately helpful for ruling in atrial fibrillation. The pooled negative likelihood ratio was 0.11 (95% CI, 0.06–0.20), showing that when no pulse irregularity is detected, the diagnosis of atrial fibrillation can be excluded with reasonable confidence. The chisquare test for heterogeneity for the positive likelihood ratio was 3.87 (df=2; P=.14) and for the negative likelihood ratio was 1.49 (df=2; P=.49), indicating consistency of results between the studies.
TABLE 2
Causes of an irregular pulse
| Sinus arrhythmia |
| Atrial extrasystoles |
| Ventricular extrasystoles |
| Atrial fibrillation |
| Atrial tachycardia with variable response |
| Second-degree heart block |
| Source: Munro and Campbell, eds, MacLeod’s Clinical Examination, 2000.14 |
Discussion
Though an irregularly irregular pulse is a classical clinical sign, we were able to find only 3 published studies evaluating its accuracy in the detection of atrial fibrillation. Study results showed a relatively high sensitivity but poor specificity.
Each study compared the diagnostic accuracy of pulse palpation by nurses in general practice against the reference test, ECG diagnosis by a cardiologist. No information was available on interobserver reliability of pulse palpation.
Working toward reliable screening. Atrial fibrillation is common, serious, and treatable. The initial clinical manifestation of undetected atrial fibrillation might be a dire consequence of the arrhythmia, such as stroke. If a suitably accurate diagnostic test or series of tests were available, it might be effective and cost-effective to screen for the disorder.12 A randomized controlled trial to evaluate such screening (including a comparison of diagnostic strategies) is currently underway.13
Whom to screen in the meantime. It may be worthwhile considering opportunistic testing for atrial fibrillation particularly in patients with ischemic heart disease or heart failure, who are at particularly high risk. Even among these patients, cases of paroxysmal atrial fibrillation may be missed, whether using pulse palpation or ECG. You may therefore want to repeat the test periodically.
CORRESPONDENCE
Jenny Doust, Level 2 Edith Cavell Building, Royal Brisbane Hospital Complex, Herston, Queensland 4029, Australia. E-mail: [email protected]. Previous presentation of work: GP and PHC RED Research Conference 2-4 June 2004, Brisbane, Australia
1. Wheeldon NM, Tayler DI, Anagnostou E, Cook D, Wales C, Oakley GD. Screening for atrial fibrillation in primary care. Heart 1998;79:50-55.
2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-988.
3. Lin HJ, Wolf PA, Kelly Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27:1760-1764.
4. Segal JB, McNamara RL, Miller MR, et al. Anticoagulants or antiplatelet therapy for non-rheumatic atrial fibrillation and flutter. Cochrane Database of Systematic Reviews 2001, Issue 1.
5. van der Weijden T, IJzermans C, Dinant G, van Duijn N, de Vet R, Buntinx F. Identifying relevant diagnostic studies in MEDLINE. The diagnostic value of the erythrocyte sedimentation rate (ESR) and dipstick as an example. Fam Pract 1997;14:204-208.
6. Doust J, Pietrzak E, Sanders S, Glasziou P. Identifying studies for systematic reviews of diagnostic tests was difficult due to the poor sensitivity and precision of methodologic filters and the lack of information in the abstract. J Clin Epidemiol 2005;58:444-449.
7. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25.-
8. Morgan S, Mant D. Randomised trial of two approaches to screening for atrial fibrillation in UK general practice. Br J Gen Pract 2002;52:373-374,377-380.
9. Sudlow M, Rodgers H, Kenny RA, Thomson R. Identification of patients with atrial fibrillation in general practice: a study of screening methods. BMJ 1998;317:327-328.
10. Somerville S, Somerville J, Croft P, Lewis M. Atrial fibrillation: a comparison of methods to identify cases in general practice. Br J Gen Pract 2000;50:727-729.
11. Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840-844.
12. Sackett D, Haynes R, Guyatt G, Tugwell P. Clinical Epidemiology: A Basic Science for Clinical Medicine. 2nd ed. Boston: Little, Brown and Company; 1991.
13. Swancutt D, Hobbs R, Fitzmaurice D, et al. A randomised controlled trial and cost effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in the over 65s: (SAFE) [ISRCTN19633732]. BMC Cardiovasc Disord 2004;4:12.-
14. Munro JF, Campbell IW, eds. MacLeod’s Clinical Examination. 10th ed. Edinburgh: Churchill Livingston, 2000.
- Pulse palpation is a good test for ruling out atrial fibrillation (C).
- Patients with an irregular pulse should be followed up with an ECG.
- Background: Atrial fibrillation in the elderly is common and potentially life threatening. The classical sign of atrial fibrillation is an irregularly irregular pulse.
- Objective: The objective of this research was to determine the accuracy of pulse palpation to detect atrial fibrillation.
- Methods: We searched Medline, EMBASE, and the reference lists of review articles for studies that compared pulse palpation with the electrocardiogram (ECG) diagnosis of atrial fibrillation. Two reviewers independently assessed the search results to determine the eligibility of studies, extracted data, and assessed the quality of the studies.
- Results: We identified 3 studies (2385 patients) that compared pulse palpation with ECG. The estimated sensitivity of pulse palpation ranged from 91% to 100%, while specificity ranged from 70% to 77%. Pooled sensitivity was 94% (95% confidence interval [CI], 84%–97%) and pooled specificity was 72% (95% CI, 69%–75%). The pooled positive likelihood ratio was 3.39, while the pooled negative likelihood ratio was 0.10.
- Conclusions: Pulse palpation has a high sensitivity but relatively low specificity for atrial fibrillation. It is therefore useful for ruling out atrial fibrillation. It may also be a useful screen to apply opportunistically for previously undetected atrial fibrillation. Assuming a prevalence of 3% for undetected atrial fibrillation in patients older than 65 years, and given the test’s sensitivity and specificity, opportunistic pulse palpation in this age group would detect an irregular pulse in 30% of screened patients, requiring further testing with ECG. Among screened patients, 0.2% would have atrial fibrillation undetected with pulse palpation.
The objective of this review was to determine how well pulse palpation detects the classical sign of atrial fibrillation—an irregularly irregular pulse—and to estimate the test’s false-positive and false-negative rates. You may want to consider using pulse palpation opportunistically with certain high-risk patients identified in this article.
The scope of the problem. Atrial fibrillation in the elderly is common and potentially life-threatening. Its prevalence increases from 2% in the 65- to 69-year-old age group to 8% among those older than 85 years.1
Atrial fibrillation is an independent risk factor for stroke, increasing a person’s risk 3- to 5-fold. While other major risk factors for stroke, such as hypertension, become less important with age, the risk of stroke from atrial fibrillation increases with age.
Stroke attributable to atrial fibrillation increases from 1.5% among patients 50 to 59 years to 23.5% for those aged 80 to 89.2 Furthermore, stroke in the presence of atrial fibrillation is almost twice as likely to be fatal, results in more functional impairment among survivors, and recurs frequently.3
Stroke due to atrial fibrillation results in considerable mortality and morbidity, but treatment of atrial fibrillation reduces the risk. Warfarin reduces the risk of stroke in non-rheumatic atrial fibrillation by about 70%, an annual absolute risk reduction of 3% (number needed to treat: 30), though the benefits are somewhat offset by the risk of bleeding (annual risk of 0.6%).4
Methods
Search strategy and study selection
We searched the Medline and EMBASE electronic databases from 1966 to June 2005 for all studies comparing pulse palpation with ECG diagnosis. There was no restriction on the language of publication.
The search strategy (see Search strategy and inclusion criteria) included terms for pulse, atrial fibrillation, and a search filter for studies of diagnostic accuracy developed by Van der Weijden et al5 that is highly sensitive and precise.6 We also searched the reference lists of all possibly relevant studies, including review articles. We included all studies that tested patients with both pulse palpation and ECG, and that provided data suitable for calculating sensitivity or specificity (or could be provided by the study authors).
Two reviewers (GC and JD) independently screened the electronic lists of citations. We obtained full-text documents of relevant papers and the 2 reviewers independently reviewed the full-text articles for inclusion according to predetermined criteria (see Search strategy and inclusion criteria). The reviewers agreed on all inclusions.
The authors of this study searched Medline, EMBASE, and the reference lists of review articles for studies using the following criteria.
(pulse) OR (‘Pulse’/all subheadings in MIME,MJ ME))
AND ((atrial fibrillation) OR (‘Atrial-Fibrillation’/all subheadings in MIME,MJME))
AND ((‘sensitivity-and-specificity’/all subheadings in MIME,MJME) OR (sensitivity) OR (specificity) OR (‘Diagnosis-Differential’/all subheadings in MIME,MJME) OR (‘False-Negative-Reactions’/all subheadings in MIME,MJME) OR (‘False-Positive-Reactions’/all subheadings in MIME,MJME) OR (‘Mass-Screening’/all subheadings in MIME,MJME) OR (diagnos*) OR (predictive value*) OR (reference value*) OR (ROC*) OR (Likelihood ratio) OR (monitoring) OR (‘Reference-Values’/all subheadings in MIME,MJME)) OR (atrial fibrillation and (SH=diagnosis))
Two reviewers then independently assessed the search results to determine the eligibility of studies, extracted data, and assessed the quality of the studies.
Inclusion criteria
- Does the study compare pulse palpation to ECG diagnosis of atrial fibrillation?
- Do all the participants receive both tests?
- Are the sensitivity and specificity provided, or calculable from the data provided?
Data extraction and analysis
We assessed study quality with the QUADAS tool.7 Two reviewers assessed study quality and extracted data independently. We calculated the pooled sensitivity and specificity using Metatest (New England Medical Center, Boston, Mass) and the pooled positive and negative likelihood ratios using the Der-Simonian and Laird random effects model of Revman 4.29 (Cochrane Collaboration), which also tests for heterogeneity.
Results
Search results and selection
The search strategy identified 110 citations. Six studies were selected for further assessment for inclusion; 3 met the inclusion criteria and covered 2385 patients (FIGURE). All 3 studies included patients older than 65 years enrolled from general practice clinics, and in each study the pulse was taken by a nurse in the clinic. TABLE 1 summarizes the methodological quality, patient characteristics, and results of the 3 studies.
FIGURE
Studies included in this review
TABLE 1
Detecting atrial fibrillation by pulse palpation: Pertinent studies
| STUDY QUALITY CHARACTERISTICS | MORGAN AND MANT8 | SOMERVILLE ET AL10 | SUDLOW ET AL9 |
| Random or consecutive sampling | Yes | No* | Yes |
| Independent assessment | Yes | Yes | Not stated |
| Blind assessment | Yes | Yes | Not stated |
| Verification by ECG | 100% | 100% | 100% |
| Description of index and reference test | Good | Poor | Poor |
| STUDY CHARACTERISTICS | |||
| Number of patients | 1099 | 86 | 1200 |
| Age | 74.3 (65–100) | 65+ | 65+ |
| Setting | 4 GP practices | Single GP practice | Patients from 9 general practices |
| Location | Southampton, UK | Stoke-on Trent, UK | Southern Northumberland, UK |
| Index test | Radial pulse palpated by a nurse for a minimum of 20 seconds† | Pulse palpated by an experienced nurse.§ Time not specified | Pulse palpated by a nurse. Time not specified |
| Reference test | ECG read by cardiologist | 12-lead ECG read by cardiologist | Limb-lead ECG |
| RESULTS | |||
| Prevalence (%) | 6.1 | 30 | 4.6‡ |
| Sensitivity (%; 95% CI) | 91 (81–96) | 100 (87–100) | 95 (85–98)‡ |
| Specificity (%; 95% CI) | 74 (72–77) | 77 (65–86) | 70 (67–73)‡ |
| Positive likelihood ratio (95% CI) | 3.5 (3.1–4.0) | 4.1 (2.6–6.5) | 3.1 (2.8–3.5) |
| Negative likelihood ratio (95% CI) | 0.12 (0.06–0.27) | 0.024 (0.002–0.38) | 0.09 (0.03–0.25) |
| * Case-control study. | |||
| † Designated as regular or irregular. Irregular pulses were categorized into occasional ectopic, frequent ectopics, or continuously irregular. Patients with any irregularity were considered to have a positive index test. | |||
| § Experienced in community and emergency nursing, as well as taking and interpreting ECGs. | |||
| ‡ Data provided by the authors. | |||
Methodological quality of included studies
The study by Morgan and Mant8 fulfilled all the major quality criteria. The studies by Sudlow et al9 and Somerville et al10 were of moderate quality. Sudlow et al did not report an overall sensitivity or specificity, nor the data for calculating these test attributes. The authors kindly provided us the data from their study to calculate the 2×2 table of results.
Prevalence of atrial fibrillation
We calculated the pooled prevalence of atrial fibrillation, excluding data from Somerville et al, as it was a case-control study. The pooled prevalence of atrial fibrillation in the 2 remaining studies was 5.3%, consistent with previous estimates of atrial fibrillation in general practice settings and data from the Framingham cohort.1,11
Accuracy of pulse palpation for detecting atrial fibrillation
The sensitivities in the 3 studies ranged from 91% to 100% and the specificities ranged from 70% to 77%. The pooled sensitivity was 94% (95% CI, 84%–97%) and the pooled specificity was 72% (95% CI, 69%–75%). Low specificity reflects the pervasiveness of other pulse abnormalities, resulting in false-positive test results for atrial fibrillation (TABLE 2).
The pooled positive likelihood ratio was 3.4 (95% CI, 3.2–3.7), showing that pulse palpation was only moderately helpful for ruling in atrial fibrillation. The pooled negative likelihood ratio was 0.11 (95% CI, 0.06–0.20), showing that when no pulse irregularity is detected, the diagnosis of atrial fibrillation can be excluded with reasonable confidence. The chisquare test for heterogeneity for the positive likelihood ratio was 3.87 (df=2; P=.14) and for the negative likelihood ratio was 1.49 (df=2; P=.49), indicating consistency of results between the studies.
TABLE 2
Causes of an irregular pulse
| Sinus arrhythmia |
| Atrial extrasystoles |
| Ventricular extrasystoles |
| Atrial fibrillation |
| Atrial tachycardia with variable response |
| Second-degree heart block |
| Source: Munro and Campbell, eds, MacLeod’s Clinical Examination, 2000.14 |
Discussion
Though an irregularly irregular pulse is a classical clinical sign, we were able to find only 3 published studies evaluating its accuracy in the detection of atrial fibrillation. Study results showed a relatively high sensitivity but poor specificity.
Each study compared the diagnostic accuracy of pulse palpation by nurses in general practice against the reference test, ECG diagnosis by a cardiologist. No information was available on interobserver reliability of pulse palpation.
Working toward reliable screening. Atrial fibrillation is common, serious, and treatable. The initial clinical manifestation of undetected atrial fibrillation might be a dire consequence of the arrhythmia, such as stroke. If a suitably accurate diagnostic test or series of tests were available, it might be effective and cost-effective to screen for the disorder.12 A randomized controlled trial to evaluate such screening (including a comparison of diagnostic strategies) is currently underway.13
Whom to screen in the meantime. It may be worthwhile considering opportunistic testing for atrial fibrillation particularly in patients with ischemic heart disease or heart failure, who are at particularly high risk. Even among these patients, cases of paroxysmal atrial fibrillation may be missed, whether using pulse palpation or ECG. You may therefore want to repeat the test periodically.
CORRESPONDENCE
Jenny Doust, Level 2 Edith Cavell Building, Royal Brisbane Hospital Complex, Herston, Queensland 4029, Australia. E-mail: [email protected]. Previous presentation of work: GP and PHC RED Research Conference 2-4 June 2004, Brisbane, Australia
- Pulse palpation is a good test for ruling out atrial fibrillation (C).
- Patients with an irregular pulse should be followed up with an ECG.
- Background: Atrial fibrillation in the elderly is common and potentially life threatening. The classical sign of atrial fibrillation is an irregularly irregular pulse.
- Objective: The objective of this research was to determine the accuracy of pulse palpation to detect atrial fibrillation.
- Methods: We searched Medline, EMBASE, and the reference lists of review articles for studies that compared pulse palpation with the electrocardiogram (ECG) diagnosis of atrial fibrillation. Two reviewers independently assessed the search results to determine the eligibility of studies, extracted data, and assessed the quality of the studies.
- Results: We identified 3 studies (2385 patients) that compared pulse palpation with ECG. The estimated sensitivity of pulse palpation ranged from 91% to 100%, while specificity ranged from 70% to 77%. Pooled sensitivity was 94% (95% confidence interval [CI], 84%–97%) and pooled specificity was 72% (95% CI, 69%–75%). The pooled positive likelihood ratio was 3.39, while the pooled negative likelihood ratio was 0.10.
- Conclusions: Pulse palpation has a high sensitivity but relatively low specificity for atrial fibrillation. It is therefore useful for ruling out atrial fibrillation. It may also be a useful screen to apply opportunistically for previously undetected atrial fibrillation. Assuming a prevalence of 3% for undetected atrial fibrillation in patients older than 65 years, and given the test’s sensitivity and specificity, opportunistic pulse palpation in this age group would detect an irregular pulse in 30% of screened patients, requiring further testing with ECG. Among screened patients, 0.2% would have atrial fibrillation undetected with pulse palpation.
The objective of this review was to determine how well pulse palpation detects the classical sign of atrial fibrillation—an irregularly irregular pulse—and to estimate the test’s false-positive and false-negative rates. You may want to consider using pulse palpation opportunistically with certain high-risk patients identified in this article.
The scope of the problem. Atrial fibrillation in the elderly is common and potentially life-threatening. Its prevalence increases from 2% in the 65- to 69-year-old age group to 8% among those older than 85 years.1
Atrial fibrillation is an independent risk factor for stroke, increasing a person’s risk 3- to 5-fold. While other major risk factors for stroke, such as hypertension, become less important with age, the risk of stroke from atrial fibrillation increases with age.
Stroke attributable to atrial fibrillation increases from 1.5% among patients 50 to 59 years to 23.5% for those aged 80 to 89.2 Furthermore, stroke in the presence of atrial fibrillation is almost twice as likely to be fatal, results in more functional impairment among survivors, and recurs frequently.3
Stroke due to atrial fibrillation results in considerable mortality and morbidity, but treatment of atrial fibrillation reduces the risk. Warfarin reduces the risk of stroke in non-rheumatic atrial fibrillation by about 70%, an annual absolute risk reduction of 3% (number needed to treat: 30), though the benefits are somewhat offset by the risk of bleeding (annual risk of 0.6%).4
Methods
Search strategy and study selection
We searched the Medline and EMBASE electronic databases from 1966 to June 2005 for all studies comparing pulse palpation with ECG diagnosis. There was no restriction on the language of publication.
The search strategy (see Search strategy and inclusion criteria) included terms for pulse, atrial fibrillation, and a search filter for studies of diagnostic accuracy developed by Van der Weijden et al5 that is highly sensitive and precise.6 We also searched the reference lists of all possibly relevant studies, including review articles. We included all studies that tested patients with both pulse palpation and ECG, and that provided data suitable for calculating sensitivity or specificity (or could be provided by the study authors).
Two reviewers (GC and JD) independently screened the electronic lists of citations. We obtained full-text documents of relevant papers and the 2 reviewers independently reviewed the full-text articles for inclusion according to predetermined criteria (see Search strategy and inclusion criteria). The reviewers agreed on all inclusions.
The authors of this study searched Medline, EMBASE, and the reference lists of review articles for studies using the following criteria.
(pulse) OR (‘Pulse’/all subheadings in MIME,MJ ME))
AND ((atrial fibrillation) OR (‘Atrial-Fibrillation’/all subheadings in MIME,MJME))
AND ((‘sensitivity-and-specificity’/all subheadings in MIME,MJME) OR (sensitivity) OR (specificity) OR (‘Diagnosis-Differential’/all subheadings in MIME,MJME) OR (‘False-Negative-Reactions’/all subheadings in MIME,MJME) OR (‘False-Positive-Reactions’/all subheadings in MIME,MJME) OR (‘Mass-Screening’/all subheadings in MIME,MJME) OR (diagnos*) OR (predictive value*) OR (reference value*) OR (ROC*) OR (Likelihood ratio) OR (monitoring) OR (‘Reference-Values’/all subheadings in MIME,MJME)) OR (atrial fibrillation and (SH=diagnosis))
Two reviewers then independently assessed the search results to determine the eligibility of studies, extracted data, and assessed the quality of the studies.
Inclusion criteria
- Does the study compare pulse palpation to ECG diagnosis of atrial fibrillation?
- Do all the participants receive both tests?
- Are the sensitivity and specificity provided, or calculable from the data provided?
Data extraction and analysis
We assessed study quality with the QUADAS tool.7 Two reviewers assessed study quality and extracted data independently. We calculated the pooled sensitivity and specificity using Metatest (New England Medical Center, Boston, Mass) and the pooled positive and negative likelihood ratios using the Der-Simonian and Laird random effects model of Revman 4.29 (Cochrane Collaboration), which also tests for heterogeneity.
Results
Search results and selection
The search strategy identified 110 citations. Six studies were selected for further assessment for inclusion; 3 met the inclusion criteria and covered 2385 patients (FIGURE). All 3 studies included patients older than 65 years enrolled from general practice clinics, and in each study the pulse was taken by a nurse in the clinic. TABLE 1 summarizes the methodological quality, patient characteristics, and results of the 3 studies.
FIGURE
Studies included in this review
TABLE 1
Detecting atrial fibrillation by pulse palpation: Pertinent studies
| STUDY QUALITY CHARACTERISTICS | MORGAN AND MANT8 | SOMERVILLE ET AL10 | SUDLOW ET AL9 |
| Random or consecutive sampling | Yes | No* | Yes |
| Independent assessment | Yes | Yes | Not stated |
| Blind assessment | Yes | Yes | Not stated |
| Verification by ECG | 100% | 100% | 100% |
| Description of index and reference test | Good | Poor | Poor |
| STUDY CHARACTERISTICS | |||
| Number of patients | 1099 | 86 | 1200 |
| Age | 74.3 (65–100) | 65+ | 65+ |
| Setting | 4 GP practices | Single GP practice | Patients from 9 general practices |
| Location | Southampton, UK | Stoke-on Trent, UK | Southern Northumberland, UK |
| Index test | Radial pulse palpated by a nurse for a minimum of 20 seconds† | Pulse palpated by an experienced nurse.§ Time not specified | Pulse palpated by a nurse. Time not specified |
| Reference test | ECG read by cardiologist | 12-lead ECG read by cardiologist | Limb-lead ECG |
| RESULTS | |||
| Prevalence (%) | 6.1 | 30 | 4.6‡ |
| Sensitivity (%; 95% CI) | 91 (81–96) | 100 (87–100) | 95 (85–98)‡ |
| Specificity (%; 95% CI) | 74 (72–77) | 77 (65–86) | 70 (67–73)‡ |
| Positive likelihood ratio (95% CI) | 3.5 (3.1–4.0) | 4.1 (2.6–6.5) | 3.1 (2.8–3.5) |
| Negative likelihood ratio (95% CI) | 0.12 (0.06–0.27) | 0.024 (0.002–0.38) | 0.09 (0.03–0.25) |
| * Case-control study. | |||
| † Designated as regular or irregular. Irregular pulses were categorized into occasional ectopic, frequent ectopics, or continuously irregular. Patients with any irregularity were considered to have a positive index test. | |||
| § Experienced in community and emergency nursing, as well as taking and interpreting ECGs. | |||
| ‡ Data provided by the authors. | |||
Methodological quality of included studies
The study by Morgan and Mant8 fulfilled all the major quality criteria. The studies by Sudlow et al9 and Somerville et al10 were of moderate quality. Sudlow et al did not report an overall sensitivity or specificity, nor the data for calculating these test attributes. The authors kindly provided us the data from their study to calculate the 2×2 table of results.
Prevalence of atrial fibrillation
We calculated the pooled prevalence of atrial fibrillation, excluding data from Somerville et al, as it was a case-control study. The pooled prevalence of atrial fibrillation in the 2 remaining studies was 5.3%, consistent with previous estimates of atrial fibrillation in general practice settings and data from the Framingham cohort.1,11
Accuracy of pulse palpation for detecting atrial fibrillation
The sensitivities in the 3 studies ranged from 91% to 100% and the specificities ranged from 70% to 77%. The pooled sensitivity was 94% (95% CI, 84%–97%) and the pooled specificity was 72% (95% CI, 69%–75%). Low specificity reflects the pervasiveness of other pulse abnormalities, resulting in false-positive test results for atrial fibrillation (TABLE 2).
The pooled positive likelihood ratio was 3.4 (95% CI, 3.2–3.7), showing that pulse palpation was only moderately helpful for ruling in atrial fibrillation. The pooled negative likelihood ratio was 0.11 (95% CI, 0.06–0.20), showing that when no pulse irregularity is detected, the diagnosis of atrial fibrillation can be excluded with reasonable confidence. The chisquare test for heterogeneity for the positive likelihood ratio was 3.87 (df=2; P=.14) and for the negative likelihood ratio was 1.49 (df=2; P=.49), indicating consistency of results between the studies.
TABLE 2
Causes of an irregular pulse
| Sinus arrhythmia |
| Atrial extrasystoles |
| Ventricular extrasystoles |
| Atrial fibrillation |
| Atrial tachycardia with variable response |
| Second-degree heart block |
| Source: Munro and Campbell, eds, MacLeod’s Clinical Examination, 2000.14 |
Discussion
Though an irregularly irregular pulse is a classical clinical sign, we were able to find only 3 published studies evaluating its accuracy in the detection of atrial fibrillation. Study results showed a relatively high sensitivity but poor specificity.
Each study compared the diagnostic accuracy of pulse palpation by nurses in general practice against the reference test, ECG diagnosis by a cardiologist. No information was available on interobserver reliability of pulse palpation.
Working toward reliable screening. Atrial fibrillation is common, serious, and treatable. The initial clinical manifestation of undetected atrial fibrillation might be a dire consequence of the arrhythmia, such as stroke. If a suitably accurate diagnostic test or series of tests were available, it might be effective and cost-effective to screen for the disorder.12 A randomized controlled trial to evaluate such screening (including a comparison of diagnostic strategies) is currently underway.13
Whom to screen in the meantime. It may be worthwhile considering opportunistic testing for atrial fibrillation particularly in patients with ischemic heart disease or heart failure, who are at particularly high risk. Even among these patients, cases of paroxysmal atrial fibrillation may be missed, whether using pulse palpation or ECG. You may therefore want to repeat the test periodically.
CORRESPONDENCE
Jenny Doust, Level 2 Edith Cavell Building, Royal Brisbane Hospital Complex, Herston, Queensland 4029, Australia. E-mail: [email protected]. Previous presentation of work: GP and PHC RED Research Conference 2-4 June 2004, Brisbane, Australia
1. Wheeldon NM, Tayler DI, Anagnostou E, Cook D, Wales C, Oakley GD. Screening for atrial fibrillation in primary care. Heart 1998;79:50-55.
2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-988.
3. Lin HJ, Wolf PA, Kelly Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27:1760-1764.
4. Segal JB, McNamara RL, Miller MR, et al. Anticoagulants or antiplatelet therapy for non-rheumatic atrial fibrillation and flutter. Cochrane Database of Systematic Reviews 2001, Issue 1.
5. van der Weijden T, IJzermans C, Dinant G, van Duijn N, de Vet R, Buntinx F. Identifying relevant diagnostic studies in MEDLINE. The diagnostic value of the erythrocyte sedimentation rate (ESR) and dipstick as an example. Fam Pract 1997;14:204-208.
6. Doust J, Pietrzak E, Sanders S, Glasziou P. Identifying studies for systematic reviews of diagnostic tests was difficult due to the poor sensitivity and precision of methodologic filters and the lack of information in the abstract. J Clin Epidemiol 2005;58:444-449.
7. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25.-
8. Morgan S, Mant D. Randomised trial of two approaches to screening for atrial fibrillation in UK general practice. Br J Gen Pract 2002;52:373-374,377-380.
9. Sudlow M, Rodgers H, Kenny RA, Thomson R. Identification of patients with atrial fibrillation in general practice: a study of screening methods. BMJ 1998;317:327-328.
10. Somerville S, Somerville J, Croft P, Lewis M. Atrial fibrillation: a comparison of methods to identify cases in general practice. Br J Gen Pract 2000;50:727-729.
11. Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840-844.
12. Sackett D, Haynes R, Guyatt G, Tugwell P. Clinical Epidemiology: A Basic Science for Clinical Medicine. 2nd ed. Boston: Little, Brown and Company; 1991.
13. Swancutt D, Hobbs R, Fitzmaurice D, et al. A randomised controlled trial and cost effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in the over 65s: (SAFE) [ISRCTN19633732]. BMC Cardiovasc Disord 2004;4:12.-
14. Munro JF, Campbell IW, eds. MacLeod’s Clinical Examination. 10th ed. Edinburgh: Churchill Livingston, 2000.
1. Wheeldon NM, Tayler DI, Anagnostou E, Cook D, Wales C, Oakley GD. Screening for atrial fibrillation in primary care. Heart 1998;79:50-55.
2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-988.
3. Lin HJ, Wolf PA, Kelly Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27:1760-1764.
4. Segal JB, McNamara RL, Miller MR, et al. Anticoagulants or antiplatelet therapy for non-rheumatic atrial fibrillation and flutter. Cochrane Database of Systematic Reviews 2001, Issue 1.
5. van der Weijden T, IJzermans C, Dinant G, van Duijn N, de Vet R, Buntinx F. Identifying relevant diagnostic studies in MEDLINE. The diagnostic value of the erythrocyte sedimentation rate (ESR) and dipstick as an example. Fam Pract 1997;14:204-208.
6. Doust J, Pietrzak E, Sanders S, Glasziou P. Identifying studies for systematic reviews of diagnostic tests was difficult due to the poor sensitivity and precision of methodologic filters and the lack of information in the abstract. J Clin Epidemiol 2005;58:444-449.
7. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25.-
8. Morgan S, Mant D. Randomised trial of two approaches to screening for atrial fibrillation in UK general practice. Br J Gen Pract 2002;52:373-374,377-380.
9. Sudlow M, Rodgers H, Kenny RA, Thomson R. Identification of patients with atrial fibrillation in general practice: a study of screening methods. BMJ 1998;317:327-328.
10. Somerville S, Somerville J, Croft P, Lewis M. Atrial fibrillation: a comparison of methods to identify cases in general practice. Br J Gen Pract 2000;50:727-729.
11. Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840-844.
12. Sackett D, Haynes R, Guyatt G, Tugwell P. Clinical Epidemiology: A Basic Science for Clinical Medicine. 2nd ed. Boston: Little, Brown and Company; 1991.
13. Swancutt D, Hobbs R, Fitzmaurice D, et al. A randomised controlled trial and cost effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in the over 65s: (SAFE) [ISRCTN19633732]. BMC Cardiovasc Disord 2004;4:12.-
14. Munro JF, Campbell IW, eds. MacLeod’s Clinical Examination. 10th ed. Edinburgh: Churchill Livingston, 2000.
Cephalosporins can be prescribed safely for penicillin-allergic patients
- The widely quoted cross-allergy risk of 10% between penicillin and cephalosporins is a myth (A).
- Cephalothin, cephalexin, cefadroxil, and cefazolin confer an increased risk of allergic reaction among patients with penicillin allergy (B).
- Cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone do not increase risk of an allergic reaction (B).
Undoubtedly you have patients who say they are allergic to penicillin but have difficulty recalling details of the reactions they experienced. To be safe, we often label these patients as penicillin-allergic without further questioning and withhold not only penicillins but cephalosporins due to concerns about potential cross-reactivity and resultant IgE-mediated, type I reactions. But even for patients truly allergic to penicillin, is the concern over cephalosporins justified? It depends on the specific agent. What is certain is that a blanket dismissal of all cephalosporins is unfounded.
The truth about the myth
Despite myriad studies spanning decades and involving varied patient populations, results have not conclusively established that penicillin allergy increases the risk of an allergic reaction to cephalosporins, compared with the incidence of a primary (and unrelated) cephalosporin allergy. Most people produce IgG and IgM antibodies in response to exposure to penicillin1 that may cross-react with cephalosporin antigens.2 The presence of these antibodies does not predict allergic, IgE cross-sensitivity to a cephalosporin. Even penicillin skin testing is generally not predictive of cephalosporin allergy.3
Reliably predicting cross-reactivity
A comprehensive review of the evidence shows that the attributable risk of a cross-reactive allergic reaction varies and is strongest when the chemical side chain of the specific cephalosporin is similar to that of penicillin or amoxicillin.
Administration of cephalothin, cephalexin, cefadroxil, and cefazolin in penicillin-allergic patients is associated with a significant increase in the rate of allergic reactions; whereas administration of cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone is not.
Penicillin skin testing can accurately predict a penicillin-allergic reaction, but is not predictive for cephalosporin allergy unless the side chain of the penicillin or ampicillin testing reagent is similar to the cephalosporin side chain being evaluated. Patients who have a reaction to a penicillin or a cephalosporin that is not IgE mediated and not serious may receive repeated courses of that antibiotic and related antibiotics.
This article provides a comprehensive review of the frequency of allergic cross-reactivity between penicillin/amoxicillin and cephalosporin antibiotics, supporting the recent American Academy of Family Physicians evidence-based clinical practice guideline on treatment of acute otitis media recommending the use of cefuroxime, cefpodoxime, cefdinir, and ceftriaxone cephalosporins for patients allergic to penicillin.
Methods
We searched Medline and EMBASE databases for English-language articles using the keywords cephalosporin, penicillin, allergy, and cross-sensitivity for the years 1960 to 2005. Among 219 articles identified, 101 were included as source material for this review. Articles we excluded were reviews, republication of results, or ones irrelevant to our purpose.
Five articles described the rate of rashes following use of penicillin and cephalosporins,4-8 and 4 articles described rates of anaphylaxis.5,9-11 We included 26 articles for the evidence base evaluating penicillin/amoxicillin cross-allergy.3,12-36 Eleven articles relied on patient history of penicillin/amoxicillin allergy to categorize results and establish reaction rates and relative risks for the penicillin/amoxicillin allergic vs nonallergic when receiving cephalosporins.12-15,17-20,27,28,31 Fourteen articles relied on patient history of penicillin/amoxicillin allergy plus skin testing results to penicillin/amoxicillin to categorize patients.16,21-25,29,30,32-37 One article3 provided data on a subset where penicillin/amoxicillin allergy was established based on history, and a separate subset where penicillin/amoxicillin allergy was established by skin testing. Other articles related to antibiotic chemical structures, animal studies, monoclonal antibody studies, cross-reactive antibody studies, and antibiotic skin testing were also reviewed.
Results
True incidence of reactions to cephalosporins
The most frequent reactions to cephalosporins are non-pruritic, non-urticarial rashes, which occur in 1.0% to 2.8% of patients;4-8 for most, the mechanism is idiopathic and not a contraindication for future use.38 Retrospective studies suggest a 1% to 3% incidence of immune or allergic reactions to cephalosporins independent of any history of penicillin/amoxicillin allergy.31 Anaphylactic reactions from cephalosporins are extremely rare, with the risk estimated at 0.0001% to 0.1%.31,38 A seminal study suggested approximately 0.004% to 0.015% of treatment courses with penicillin results in anaphylaxis.5,9-11 Several studies suggest that cephalosporin-induced anaphylaxis occurs no more frequently among patients with known penicillin allergy than among those without such allergy.23,27,38-41
Determining cross-reactivity
Penicillins and cephalosporins both possess a beta-lactam ring for antimicrobial activity. They differ in that the 5-membered thiazolidine ring of penicillin is replaced in the cephalosporins with a 6-membered dihydrothiazine ring. After degradation, penicillin forms a stable ring, whereas cephalosporins undergo rapid fragmentation of their rings.42 Immunologic cross-reactivity between the penicillin and cephalosporin beta-lactam rings is, therefore, very unlikely—an observation confirmed by monoclonal antibody analysis.43
How the “10% cross-reactivity” myth took hold. When the first-generation cephalosporins cephaloridine and cephalothin were introduced in the 1960s, allergic and anaphylactic reactions were reported in patients with previous allergic reactions to penicillins. Subsequent reports, which attributed up to 10% cross-reactivity between the 2 drug classes, involved these same first-generation cephalosporins plus cephalexin and cefadroxil and a second-generation drug, cefamandole. However, these studies were flawed because the penicillin test compounds had been contaminated with cephalosporins. Until 1982, penicillin was produced commercially using the cephalosporium mold.38
Many recent studies have established that the rate of cross-reactivity between penicillin and cephalosporins has been grossly overestimated. In fact, the rate of cross-reactivity between penicillin/amoxicillin and second- or third-generation cephalosporins is very low and may actually be lower than that between penicillins and other classes of antibiotics.44
The evidence for limited cross-reactivity. A summary of publications evaluating 38,846 children and adults with and without a history of penicillin allergy is presented in TABLE 1. The database included 2435 patients with a history of penicillin allergy and 961 patients with a history of penicillin allergy and positive skin-test results for penicillin or amoxicillin (total penicillin-allergic patients=3396). The allergic reaction rate is compared with 34,047 patients without a history of penicillin allergy and 1403 patients without a history of penicillin allergy and negative skin-test results for penicillin or amoxicillin (total penicillin-nonallergic patients=35,450).
When patients with a positive history of penicillin-allergy received first-generation cephalosporins, which share a chemical side chain similar to penicillin or amoxicillin (cephalothin, cephaloridine, cephalexin, cefadroxil, and cefazolin, plus the early second-generation cephalosporin, cefamandole), they exhibited a significant increased risk of an allergic reaction to the cephalosporin.
Second- and third-generation cephalosporins modified in size and the complexity of their side chains (eg, cefprozil, cefuroxime, ceftazidime, cefpodoxime, and ceftriaxone) were different enough from penicillin and ampicillin that they did not increase risk of allergic cross-reactivity (TABLE 1).
Many other studies have suggested that cross-reactive immune responses to cephalosporins depend on side chain structure;22,23,27,32,37,38,44-49 that is, cephalosporins with a 7-position side chain similar to benzylpenicillin are more likely to cross-react with penicillin (TABLE 2). Cephalosporins that share a similar 7-position or 3-position side chain are more likely to cross-react with each other.
Cephalosporin/penicillin cross-reactivity
Few studies have evaluated whether patients with primary hypersensitivity to cephalosporins will experience cross-reactivity with penicillin. Romano et al49 conducted skin tests and RASTs in patients with immediate allergic reactions to cephalosporins to examine responses to other cephalosporins and to classic penicillin determinants. About 1 in 5 patients allergic to a cephalosporin reacted to penicillin determinants, while most had positive results to other cephalosporins with the same or similar side-chains.
Limitations of skin testing
Penicillin skin testing in patients with a history of penicillin allergy does not reliably predict allergy to a cephalosporin unless the side chain of the penicillin or ampicillin reagent is similar to the cephalosporin side chain being tested.3 The positive and negative predictive values of skin testing results for cephalosporins are not well established; if the haptens that cause cephalosporin allergy were known, cross-reactivity with penicillins could be assessed directly. Cephalosporin skin testing works only for the specific drug and drugs with the same side chains, and can be done only if the drug is available in an IV or IM formulation.
Even a positive result does not guarantee a clinical reaction. When penicillin and cephalosporin skin tests or radioallergosorbent tests (RASTs) are positive, a clinical reaction is observed in only 10% to 60% of patients, depending on the reagent and study.50 For example, among 19 well-characterized patients allergic to penicillin who were studied for their sensitivity to the cephalosporins, cephaloridine and cefamandole (which have identical or very similar side chains to penicillin and were therefore potentially cross-reactive) only 2 (10.5%) reacted to cefamandole, while the other 17 patients tolerated both agents.26 In another study of clinical cross-reactivity between amoxicillin and cefadroxil in patients allergic to amoxicillin with good tolerance of penicillin, only 12% had an immediate allergic reaction to cefadroxil, despite the 2 drugs sharing an identical side chain.33 In a third study, allergenic cross-reactivity with cefadroxil and cefamandole was studied among 21 patients selectively allergic to amoxicillin; 8 (38%) had a positive response to cefadroxil (same side chain) and none to cefamandole (different side chain).32
Discussion
Sensible approach to penicillin-allergic patients
Question patients who report penicillin allergy. In many cases, penicillin may not actually have been taken, or patients may have had non-immunologic adverse events such as vomiting, diarrhea, or nonspecific rash; toxic effects; or contemporaneous side effects inappropriately attributed to the drug. These patients can receive penicillin, amoxicillin, or the cephalosporins.
Without the ability to detect patients with IgE antibody to penicillin prospectively or to distinguish true IgE immunologic reactions from idiopathic reactions in patients receiving cephalosporins, it is impossible to definitely claim that increased immune or IgE-mediated reactions to cephalosporins occur in true penicillinallergic (IgE) patients.
When a cephalosporin is/is not safe for a penicillin-allergic patient. Only IgE-mediated reactions—such as anaphylaxis or hypotension, laryngeal edema, wheezing, angioedema, or urticaria—are likely to become more severe with time. Therefore, with a patient who has had a true IgE-mediated reaction to a penicillin, avoid using cephalosporins with a similar side chain. You may, however, give cephalosporins that have different side chains. Cephalosporins may also be used for patients who have had non-IgE-mediated adverse reactions (“non-type I allergy”)21 to a penicillin, such as a non-pruritic, non-urticarial morbilliform or maculopapular rash.
How prevalent is primary cephalosporin allergy? Even if the patient is not allergic to penicillin, cephalosporins can cause allergic or immune-mediated reactions in approximately 1% to 3% of patients. A patient who had an allergic reaction to a specific cephalosporin probably should not receive that cephalosporin again. The risk of a reaction with a different cephalosporin is very low to nonexistent if the side chains of the 2 drugs are dissimilar.
Bottom line. Penicillin-allergic patients have indeed shown an increased incidence of allergic reactions to cephalothin, cephaloridine, cephalexin, cefadroxil, cefazolin, and cefamandole. However, the risk has been overestimated because most studies reporting this cross-reactivity were flawed (because penicillins were contaminated with cephalosporins) and then failed to account for the fact that penicillin-allergic patients have a 3-fold increased risk of allergic reactions even to nonrelated drugs.51
For patients truly allergic to penicillin, the risk of a reaction from a cephalosporin with side chains that differ from penicillin/amoxicillin (cefuroxime, cefpodoxime, cefdinir, and ceftriaxone, as endorsed by the AAFP) is so low that use is justified and medico-legally defensible by the currently available evidence.
CORRESPONDENCE
Michael E. Pichichero, MD, University of Rochester Medical Center, 601 Elmwood Avenue, Box 672, Rochester, New York 14642. E-mail: [email protected]
1. Levine B. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases of man. N Engl J Med 1966;275:1115-1125.
2. Torres M, Gonzales F, Mayorga C, et al. IgG and IgE antibodies in subjects allergic to penicillins recognize different parts of the penicillin molecule. Int Arch Allergy Immunol 1997;113:342-344.
3. Pichichero M. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005;115:1048-1057.
4. Arndt J, Jick H. Rates of cutaneous reactions to drugs. A report from the Boston Collaborative Drug Surveillance Program. JAMA 1976;235:918-923.
5. Platt R. Adverse effects of third-generation cephalosporins. J Antimicrob Chemother 1982;10(C):135-140.
6. Sanders CV, Greenberg RN, Marier RL. Cefamandole and cefoxitin. Ann Intern Med 1985;103:70-78.
7. Levine LR. Quantitative comparison of adverse reactions to cefaclor vs. amoxicillin in a surveillance study. Pediatr Infect Dis 1985;4:358-361.
8. Norrby S. Side effects of cephalosporins. Drug 1987;34(Suppl 2):105-120.
9. Idsoe O, Guthe T, Wilcox R. Nature and extent of penicillin side-reactions with particular reference to fatalities from anaphylactic shock. Bull WHO 1968;38:159-188.
10. Petz L. Immunologic reactions of humans to cephalosporins. Post Grad Med J 1971;47(Suppl):64-69.
11. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993;270:2456-2463.
12. Walter E, Moelling K, Pavlovic J, et al. Micro-encapsulation of DNA using poly (DL-lactide-co-glycolide): stability issues and release characteristics. J Controlled Release 1999;61:361-374.
13. Weinstein L, Kaplan K, Chang T. Treatment of infections in man with cephalothin. JAMA 1964;189:829-834.
14. Griffith R, Black H. Cephalothin—a new antibiotic. Preliminary clinical and laboratory studies. JAMA 1964;189:823-828.
15. Apicella M, Perkins R, Salsaw S. Cephaloridine treatment of bacterial infections. Am J Med Sci 1966;251:266-276.
16. Assem E, Vickers M. Tests for penicillin allergy in man: The immunological cross-reaction between penicillins and cephalosporins. Immunology 1974;27:255-269.
17. Marks J, Garrett R. Cephalexin in general practice. Post Grad Med J 1970;46(Suppl):113-117.
18. Stewart G. Cross-allergenicity of penicillin G and related substances. Lancet 1962;1:509-510.
19. Dash C. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975;(1 Suppl):107-118.
20. Petz L. Immunologic cross-reactivity between penicillins and cephalosporins. J Infect Dis 1978;137:S74-S79.
21. Thoburn R, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions. IV. The relationship of cephalothin and penicillin allergy. JAMA 1966;198:345-348.
22. Solley G, Gleich G, Van Dellen R. Penicillin allergy: Clincal experience with a battery of skin test reagents. J Allergy Clin Immunol 1982;69:238-244.
23. Warrington R, McPhillipps S. Independent anaphylaxis to cefazolin without allergy to other beta-lactam antibiotics. J Allergy Clin Immunol 1996;98:460-462.
24. Sullivan T, Wedner H, Shatz G, et al. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981;68:171-180.
25. Saxon A. Immediate hypersensitivity reactions to b-lactam antibiotics. Rev Infect Dis 1983;5(Suppl 2):S368-S378.
26. Blanca M, Fernandez J, Miranda A, et al. Cross reactivity between penicillins and cephalosporins: Clinical and immunological studies. J Allergy Clin Immunol 1989;83:381-385.
27. Lin R. A perspective on penicillin allergy. Arch Intern Med 1992;152:930-937.
28. Martin J, Igea J, Fraj J, et al. Allergy to amoxicillin in patients who tolerated benzylpenicillin, aztreonam, and ceftazidime. Clin Infect Dis 1992;14:592-593.
29. Shepherd G, Burton D. Administration of cephalosporin antibiotics to patients with a history of penicillin allergy [abstract]. J Allergy Clin Immunol 1993;91:262.-
30. Audicana M, Bernaola G, Urrutia I, et al. Allergic reactions to betalactams: Studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporins. Allergy 1994;49:108-113.
31. Anne S, Reisman R. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Annals Allergy Asthma Immunology 1995;74:167-170.
32. Miranda A, Blanca M, Vega J, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol 1996;98:671-677.
33. Sastre J, Quijano L, Novalbos A, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy 1996;51:383-386.
34. Pichichero M, Pichichero D. Selecting skin testing reagents to predict amoxicillin and cephalosporin allergy. Pediatr Asthma Allergy Immunol 1997;11:79-93.
35. Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy 2001;31:438-443.
36. Romano A, Gueant-Rodriguez RM, Viola M, et al. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med 2004;141:16-22.
37. Torres M, Blanca M, Garcia J. Evaluation of a large cohort of subjects allergic to penicillins [abstract]. J Allergy Clin Immunol 1995;95:285.-
38. Kelkar P, Li J. Cephalosporin allergy. N Engl J Med 2001;385:804-809.
39. Kabins S, Einstein B, Cohen S. Anaphylactic reaction to an initial dose of sodium cephalothin. JAMA 1965;193:165.-
40. Romano A, Piunti E, De Fronso M, et al. Selective immediate hypersensitivity to ceftriaxone. Allergy 2000;55:418-419.
41. Pumphrey R, Davis S. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet 1999;353:1157-1158.
42. Mayorga C, Torres M, Blanca M. Cephalosporin allergy. N Engl J Med 2002;236:380-381.
43. Mayorga C, Ovispo T, Jimeno L. Epitope mapping of betalactam antibiotics with the use of monoclonal antibodies. Toxicology 1995;97:225-34.
44. Weiss M, Adkinson N. Immediate hypersensitivity reactions to penicillin and related antibiotics. Clin Allergy 1998;18:515-540.
45. Blaiss M, DeShazo R. Drug allergy. Pediatr Clin North Am 1998;35:1131-1147.
46. Baumgart K, Baldo B. Cephalosporin allergy. N Engl J Med 2002;346:380.-
47. James J. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: reliability of examination assessed by skin testing and oral challenge. Pediatrics 1999;104:367.-
48. Pichichero M, Pichichero D. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: Reliability of examination assessed by skin testing and oral challenge. J Pediatr 1998;132:137-143.
49. Romano A, Mayorga C, Torres M, et al. Immediate allergic reactions to cephalosporins: Cross-reactivity and selective responses. J Allergy Clin Immunol 2000;106:1177-1183.
50. Salkind A, Cuddy P, Foxworth J. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001;285:2498-2505.
51. Smith J, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions: II. An evaluation of penicillin allergy. N Engl J Med 1966;274:998-1002.
52. Warrington R, Simons F, Ho H, et al. Diagnosis of penicillin allergy by skin testing: the Manitoba experience. Can Med Assoc J 1978;118:797-791.
53. Saxon A, Beall G, Rohr A. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107:204-215.
- The widely quoted cross-allergy risk of 10% between penicillin and cephalosporins is a myth (A).
- Cephalothin, cephalexin, cefadroxil, and cefazolin confer an increased risk of allergic reaction among patients with penicillin allergy (B).
- Cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone do not increase risk of an allergic reaction (B).
Undoubtedly you have patients who say they are allergic to penicillin but have difficulty recalling details of the reactions they experienced. To be safe, we often label these patients as penicillin-allergic without further questioning and withhold not only penicillins but cephalosporins due to concerns about potential cross-reactivity and resultant IgE-mediated, type I reactions. But even for patients truly allergic to penicillin, is the concern over cephalosporins justified? It depends on the specific agent. What is certain is that a blanket dismissal of all cephalosporins is unfounded.
The truth about the myth
Despite myriad studies spanning decades and involving varied patient populations, results have not conclusively established that penicillin allergy increases the risk of an allergic reaction to cephalosporins, compared with the incidence of a primary (and unrelated) cephalosporin allergy. Most people produce IgG and IgM antibodies in response to exposure to penicillin1 that may cross-react with cephalosporin antigens.2 The presence of these antibodies does not predict allergic, IgE cross-sensitivity to a cephalosporin. Even penicillin skin testing is generally not predictive of cephalosporin allergy.3
Reliably predicting cross-reactivity
A comprehensive review of the evidence shows that the attributable risk of a cross-reactive allergic reaction varies and is strongest when the chemical side chain of the specific cephalosporin is similar to that of penicillin or amoxicillin.
Administration of cephalothin, cephalexin, cefadroxil, and cefazolin in penicillin-allergic patients is associated with a significant increase in the rate of allergic reactions; whereas administration of cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone is not.
Penicillin skin testing can accurately predict a penicillin-allergic reaction, but is not predictive for cephalosporin allergy unless the side chain of the penicillin or ampicillin testing reagent is similar to the cephalosporin side chain being evaluated. Patients who have a reaction to a penicillin or a cephalosporin that is not IgE mediated and not serious may receive repeated courses of that antibiotic and related antibiotics.
This article provides a comprehensive review of the frequency of allergic cross-reactivity between penicillin/amoxicillin and cephalosporin antibiotics, supporting the recent American Academy of Family Physicians evidence-based clinical practice guideline on treatment of acute otitis media recommending the use of cefuroxime, cefpodoxime, cefdinir, and ceftriaxone cephalosporins for patients allergic to penicillin.
Methods
We searched Medline and EMBASE databases for English-language articles using the keywords cephalosporin, penicillin, allergy, and cross-sensitivity for the years 1960 to 2005. Among 219 articles identified, 101 were included as source material for this review. Articles we excluded were reviews, republication of results, or ones irrelevant to our purpose.
Five articles described the rate of rashes following use of penicillin and cephalosporins,4-8 and 4 articles described rates of anaphylaxis.5,9-11 We included 26 articles for the evidence base evaluating penicillin/amoxicillin cross-allergy.3,12-36 Eleven articles relied on patient history of penicillin/amoxicillin allergy to categorize results and establish reaction rates and relative risks for the penicillin/amoxicillin allergic vs nonallergic when receiving cephalosporins.12-15,17-20,27,28,31 Fourteen articles relied on patient history of penicillin/amoxicillin allergy plus skin testing results to penicillin/amoxicillin to categorize patients.16,21-25,29,30,32-37 One article3 provided data on a subset where penicillin/amoxicillin allergy was established based on history, and a separate subset where penicillin/amoxicillin allergy was established by skin testing. Other articles related to antibiotic chemical structures, animal studies, monoclonal antibody studies, cross-reactive antibody studies, and antibiotic skin testing were also reviewed.
Results
True incidence of reactions to cephalosporins
The most frequent reactions to cephalosporins are non-pruritic, non-urticarial rashes, which occur in 1.0% to 2.8% of patients;4-8 for most, the mechanism is idiopathic and not a contraindication for future use.38 Retrospective studies suggest a 1% to 3% incidence of immune or allergic reactions to cephalosporins independent of any history of penicillin/amoxicillin allergy.31 Anaphylactic reactions from cephalosporins are extremely rare, with the risk estimated at 0.0001% to 0.1%.31,38 A seminal study suggested approximately 0.004% to 0.015% of treatment courses with penicillin results in anaphylaxis.5,9-11 Several studies suggest that cephalosporin-induced anaphylaxis occurs no more frequently among patients with known penicillin allergy than among those without such allergy.23,27,38-41
Determining cross-reactivity
Penicillins and cephalosporins both possess a beta-lactam ring for antimicrobial activity. They differ in that the 5-membered thiazolidine ring of penicillin is replaced in the cephalosporins with a 6-membered dihydrothiazine ring. After degradation, penicillin forms a stable ring, whereas cephalosporins undergo rapid fragmentation of their rings.42 Immunologic cross-reactivity between the penicillin and cephalosporin beta-lactam rings is, therefore, very unlikely—an observation confirmed by monoclonal antibody analysis.43
How the “10% cross-reactivity” myth took hold. When the first-generation cephalosporins cephaloridine and cephalothin were introduced in the 1960s, allergic and anaphylactic reactions were reported in patients with previous allergic reactions to penicillins. Subsequent reports, which attributed up to 10% cross-reactivity between the 2 drug classes, involved these same first-generation cephalosporins plus cephalexin and cefadroxil and a second-generation drug, cefamandole. However, these studies were flawed because the penicillin test compounds had been contaminated with cephalosporins. Until 1982, penicillin was produced commercially using the cephalosporium mold.38
Many recent studies have established that the rate of cross-reactivity between penicillin and cephalosporins has been grossly overestimated. In fact, the rate of cross-reactivity between penicillin/amoxicillin and second- or third-generation cephalosporins is very low and may actually be lower than that between penicillins and other classes of antibiotics.44
The evidence for limited cross-reactivity. A summary of publications evaluating 38,846 children and adults with and without a history of penicillin allergy is presented in TABLE 1. The database included 2435 patients with a history of penicillin allergy and 961 patients with a history of penicillin allergy and positive skin-test results for penicillin or amoxicillin (total penicillin-allergic patients=3396). The allergic reaction rate is compared with 34,047 patients without a history of penicillin allergy and 1403 patients without a history of penicillin allergy and negative skin-test results for penicillin or amoxicillin (total penicillin-nonallergic patients=35,450).
When patients with a positive history of penicillin-allergy received first-generation cephalosporins, which share a chemical side chain similar to penicillin or amoxicillin (cephalothin, cephaloridine, cephalexin, cefadroxil, and cefazolin, plus the early second-generation cephalosporin, cefamandole), they exhibited a significant increased risk of an allergic reaction to the cephalosporin.
Second- and third-generation cephalosporins modified in size and the complexity of their side chains (eg, cefprozil, cefuroxime, ceftazidime, cefpodoxime, and ceftriaxone) were different enough from penicillin and ampicillin that they did not increase risk of allergic cross-reactivity (TABLE 1).

Many other studies have suggested that cross-reactive immune responses to cephalosporins depend on side chain structure;22,23,27,32,37,38,44-49 that is, cephalosporins with a 7-position side chain similar to benzylpenicillin are more likely to cross-react with penicillin (TABLE 2). Cephalosporins that share a similar 7-position or 3-position side chain are more likely to cross-react with each other.

Cephalosporin/penicillin cross-reactivity
Few studies have evaluated whether patients with primary hypersensitivity to cephalosporins will experience cross-reactivity with penicillin. Romano et al49 conducted skin tests and RASTs in patients with immediate allergic reactions to cephalosporins to examine responses to other cephalosporins and to classic penicillin determinants. About 1 in 5 patients allergic to a cephalosporin reacted to penicillin determinants, while most had positive results to other cephalosporins with the same or similar side-chains.
Limitations of skin testing
Penicillin skin testing in patients with a history of penicillin allergy does not reliably predict allergy to a cephalosporin unless the side chain of the penicillin or ampicillin reagent is similar to the cephalosporin side chain being tested.3 The positive and negative predictive values of skin testing results for cephalosporins are not well established; if the haptens that cause cephalosporin allergy were known, cross-reactivity with penicillins could be assessed directly. Cephalosporin skin testing works only for the specific drug and drugs with the same side chains, and can be done only if the drug is available in an IV or IM formulation.
Even a positive result does not guarantee a clinical reaction. When penicillin and cephalosporin skin tests or radioallergosorbent tests (RASTs) are positive, a clinical reaction is observed in only 10% to 60% of patients, depending on the reagent and study.50 For example, among 19 well-characterized patients allergic to penicillin who were studied for their sensitivity to the cephalosporins, cephaloridine and cefamandole (which have identical or very similar side chains to penicillin and were therefore potentially cross-reactive) only 2 (10.5%) reacted to cefamandole, while the other 17 patients tolerated both agents.26 In another study of clinical cross-reactivity between amoxicillin and cefadroxil in patients allergic to amoxicillin with good tolerance of penicillin, only 12% had an immediate allergic reaction to cefadroxil, despite the 2 drugs sharing an identical side chain.33 In a third study, allergenic cross-reactivity with cefadroxil and cefamandole was studied among 21 patients selectively allergic to amoxicillin; 8 (38%) had a positive response to cefadroxil (same side chain) and none to cefamandole (different side chain).32
Discussion
Sensible approach to penicillin-allergic patients
Question patients who report penicillin allergy. In many cases, penicillin may not actually have been taken, or patients may have had non-immunologic adverse events such as vomiting, diarrhea, or nonspecific rash; toxic effects; or contemporaneous side effects inappropriately attributed to the drug. These patients can receive penicillin, amoxicillin, or the cephalosporins.
Without the ability to detect patients with IgE antibody to penicillin prospectively or to distinguish true IgE immunologic reactions from idiopathic reactions in patients receiving cephalosporins, it is impossible to definitely claim that increased immune or IgE-mediated reactions to cephalosporins occur in true penicillinallergic (IgE) patients.
When a cephalosporin is/is not safe for a penicillin-allergic patient. Only IgE-mediated reactions—such as anaphylaxis or hypotension, laryngeal edema, wheezing, angioedema, or urticaria—are likely to become more severe with time. Therefore, with a patient who has had a true IgE-mediated reaction to a penicillin, avoid using cephalosporins with a similar side chain. You may, however, give cephalosporins that have different side chains. Cephalosporins may also be used for patients who have had non-IgE-mediated adverse reactions (“non-type I allergy”)21 to a penicillin, such as a non-pruritic, non-urticarial morbilliform or maculopapular rash.
How prevalent is primary cephalosporin allergy? Even if the patient is not allergic to penicillin, cephalosporins can cause allergic or immune-mediated reactions in approximately 1% to 3% of patients. A patient who had an allergic reaction to a specific cephalosporin probably should not receive that cephalosporin again. The risk of a reaction with a different cephalosporin is very low to nonexistent if the side chains of the 2 drugs are dissimilar.
Bottom line. Penicillin-allergic patients have indeed shown an increased incidence of allergic reactions to cephalothin, cephaloridine, cephalexin, cefadroxil, cefazolin, and cefamandole. However, the risk has been overestimated because most studies reporting this cross-reactivity were flawed (because penicillins were contaminated with cephalosporins) and then failed to account for the fact that penicillin-allergic patients have a 3-fold increased risk of allergic reactions even to nonrelated drugs.51
For patients truly allergic to penicillin, the risk of a reaction from a cephalosporin with side chains that differ from penicillin/amoxicillin (cefuroxime, cefpodoxime, cefdinir, and ceftriaxone, as endorsed by the AAFP) is so low that use is justified and medico-legally defensible by the currently available evidence.
CORRESPONDENCE
Michael E. Pichichero, MD, University of Rochester Medical Center, 601 Elmwood Avenue, Box 672, Rochester, New York 14642. E-mail: [email protected]
- The widely quoted cross-allergy risk of 10% between penicillin and cephalosporins is a myth (A).
- Cephalothin, cephalexin, cefadroxil, and cefazolin confer an increased risk of allergic reaction among patients with penicillin allergy (B).
- Cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone do not increase risk of an allergic reaction (B).
Undoubtedly you have patients who say they are allergic to penicillin but have difficulty recalling details of the reactions they experienced. To be safe, we often label these patients as penicillin-allergic without further questioning and withhold not only penicillins but cephalosporins due to concerns about potential cross-reactivity and resultant IgE-mediated, type I reactions. But even for patients truly allergic to penicillin, is the concern over cephalosporins justified? It depends on the specific agent. What is certain is that a blanket dismissal of all cephalosporins is unfounded.
The truth about the myth
Despite myriad studies spanning decades and involving varied patient populations, results have not conclusively established that penicillin allergy increases the risk of an allergic reaction to cephalosporins, compared with the incidence of a primary (and unrelated) cephalosporin allergy. Most people produce IgG and IgM antibodies in response to exposure to penicillin1 that may cross-react with cephalosporin antigens.2 The presence of these antibodies does not predict allergic, IgE cross-sensitivity to a cephalosporin. Even penicillin skin testing is generally not predictive of cephalosporin allergy.3
Reliably predicting cross-reactivity
A comprehensive review of the evidence shows that the attributable risk of a cross-reactive allergic reaction varies and is strongest when the chemical side chain of the specific cephalosporin is similar to that of penicillin or amoxicillin.
Administration of cephalothin, cephalexin, cefadroxil, and cefazolin in penicillin-allergic patients is associated with a significant increase in the rate of allergic reactions; whereas administration of cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone is not.
Penicillin skin testing can accurately predict a penicillin-allergic reaction, but is not predictive for cephalosporin allergy unless the side chain of the penicillin or ampicillin testing reagent is similar to the cephalosporin side chain being evaluated. Patients who have a reaction to a penicillin or a cephalosporin that is not IgE mediated and not serious may receive repeated courses of that antibiotic and related antibiotics.
This article provides a comprehensive review of the frequency of allergic cross-reactivity between penicillin/amoxicillin and cephalosporin antibiotics, supporting the recent American Academy of Family Physicians evidence-based clinical practice guideline on treatment of acute otitis media recommending the use of cefuroxime, cefpodoxime, cefdinir, and ceftriaxone cephalosporins for patients allergic to penicillin.
Methods
We searched Medline and EMBASE databases for English-language articles using the keywords cephalosporin, penicillin, allergy, and cross-sensitivity for the years 1960 to 2005. Among 219 articles identified, 101 were included as source material for this review. Articles we excluded were reviews, republication of results, or ones irrelevant to our purpose.
Five articles described the rate of rashes following use of penicillin and cephalosporins,4-8 and 4 articles described rates of anaphylaxis.5,9-11 We included 26 articles for the evidence base evaluating penicillin/amoxicillin cross-allergy.3,12-36 Eleven articles relied on patient history of penicillin/amoxicillin allergy to categorize results and establish reaction rates and relative risks for the penicillin/amoxicillin allergic vs nonallergic when receiving cephalosporins.12-15,17-20,27,28,31 Fourteen articles relied on patient history of penicillin/amoxicillin allergy plus skin testing results to penicillin/amoxicillin to categorize patients.16,21-25,29,30,32-37 One article3 provided data on a subset where penicillin/amoxicillin allergy was established based on history, and a separate subset where penicillin/amoxicillin allergy was established by skin testing. Other articles related to antibiotic chemical structures, animal studies, monoclonal antibody studies, cross-reactive antibody studies, and antibiotic skin testing were also reviewed.
Results
True incidence of reactions to cephalosporins
The most frequent reactions to cephalosporins are non-pruritic, non-urticarial rashes, which occur in 1.0% to 2.8% of patients;4-8 for most, the mechanism is idiopathic and not a contraindication for future use.38 Retrospective studies suggest a 1% to 3% incidence of immune or allergic reactions to cephalosporins independent of any history of penicillin/amoxicillin allergy.31 Anaphylactic reactions from cephalosporins are extremely rare, with the risk estimated at 0.0001% to 0.1%.31,38 A seminal study suggested approximately 0.004% to 0.015% of treatment courses with penicillin results in anaphylaxis.5,9-11 Several studies suggest that cephalosporin-induced anaphylaxis occurs no more frequently among patients with known penicillin allergy than among those without such allergy.23,27,38-41
Determining cross-reactivity
Penicillins and cephalosporins both possess a beta-lactam ring for antimicrobial activity. They differ in that the 5-membered thiazolidine ring of penicillin is replaced in the cephalosporins with a 6-membered dihydrothiazine ring. After degradation, penicillin forms a stable ring, whereas cephalosporins undergo rapid fragmentation of their rings.42 Immunologic cross-reactivity between the penicillin and cephalosporin beta-lactam rings is, therefore, very unlikely—an observation confirmed by monoclonal antibody analysis.43
How the “10% cross-reactivity” myth took hold. When the first-generation cephalosporins cephaloridine and cephalothin were introduced in the 1960s, allergic and anaphylactic reactions were reported in patients with previous allergic reactions to penicillins. Subsequent reports, which attributed up to 10% cross-reactivity between the 2 drug classes, involved these same first-generation cephalosporins plus cephalexin and cefadroxil and a second-generation drug, cefamandole. However, these studies were flawed because the penicillin test compounds had been contaminated with cephalosporins. Until 1982, penicillin was produced commercially using the cephalosporium mold.38
Many recent studies have established that the rate of cross-reactivity between penicillin and cephalosporins has been grossly overestimated. In fact, the rate of cross-reactivity between penicillin/amoxicillin and second- or third-generation cephalosporins is very low and may actually be lower than that between penicillins and other classes of antibiotics.44
The evidence for limited cross-reactivity. A summary of publications evaluating 38,846 children and adults with and without a history of penicillin allergy is presented in TABLE 1. The database included 2435 patients with a history of penicillin allergy and 961 patients with a history of penicillin allergy and positive skin-test results for penicillin or amoxicillin (total penicillin-allergic patients=3396). The allergic reaction rate is compared with 34,047 patients without a history of penicillin allergy and 1403 patients without a history of penicillin allergy and negative skin-test results for penicillin or amoxicillin (total penicillin-nonallergic patients=35,450).
When patients with a positive history of penicillin-allergy received first-generation cephalosporins, which share a chemical side chain similar to penicillin or amoxicillin (cephalothin, cephaloridine, cephalexin, cefadroxil, and cefazolin, plus the early second-generation cephalosporin, cefamandole), they exhibited a significant increased risk of an allergic reaction to the cephalosporin.
Second- and third-generation cephalosporins modified in size and the complexity of their side chains (eg, cefprozil, cefuroxime, ceftazidime, cefpodoxime, and ceftriaxone) were different enough from penicillin and ampicillin that they did not increase risk of allergic cross-reactivity (TABLE 1).

Many other studies have suggested that cross-reactive immune responses to cephalosporins depend on side chain structure;22,23,27,32,37,38,44-49 that is, cephalosporins with a 7-position side chain similar to benzylpenicillin are more likely to cross-react with penicillin (TABLE 2). Cephalosporins that share a similar 7-position or 3-position side chain are more likely to cross-react with each other.

Cephalosporin/penicillin cross-reactivity
Few studies have evaluated whether patients with primary hypersensitivity to cephalosporins will experience cross-reactivity with penicillin. Romano et al49 conducted skin tests and RASTs in patients with immediate allergic reactions to cephalosporins to examine responses to other cephalosporins and to classic penicillin determinants. About 1 in 5 patients allergic to a cephalosporin reacted to penicillin determinants, while most had positive results to other cephalosporins with the same or similar side-chains.
Limitations of skin testing
Penicillin skin testing in patients with a history of penicillin allergy does not reliably predict allergy to a cephalosporin unless the side chain of the penicillin or ampicillin reagent is similar to the cephalosporin side chain being tested.3 The positive and negative predictive values of skin testing results for cephalosporins are not well established; if the haptens that cause cephalosporin allergy were known, cross-reactivity with penicillins could be assessed directly. Cephalosporin skin testing works only for the specific drug and drugs with the same side chains, and can be done only if the drug is available in an IV or IM formulation.
Even a positive result does not guarantee a clinical reaction. When penicillin and cephalosporin skin tests or radioallergosorbent tests (RASTs) are positive, a clinical reaction is observed in only 10% to 60% of patients, depending on the reagent and study.50 For example, among 19 well-characterized patients allergic to penicillin who were studied for their sensitivity to the cephalosporins, cephaloridine and cefamandole (which have identical or very similar side chains to penicillin and were therefore potentially cross-reactive) only 2 (10.5%) reacted to cefamandole, while the other 17 patients tolerated both agents.26 In another study of clinical cross-reactivity between amoxicillin and cefadroxil in patients allergic to amoxicillin with good tolerance of penicillin, only 12% had an immediate allergic reaction to cefadroxil, despite the 2 drugs sharing an identical side chain.33 In a third study, allergenic cross-reactivity with cefadroxil and cefamandole was studied among 21 patients selectively allergic to amoxicillin; 8 (38%) had a positive response to cefadroxil (same side chain) and none to cefamandole (different side chain).32
Discussion
Sensible approach to penicillin-allergic patients
Question patients who report penicillin allergy. In many cases, penicillin may not actually have been taken, or patients may have had non-immunologic adverse events such as vomiting, diarrhea, or nonspecific rash; toxic effects; or contemporaneous side effects inappropriately attributed to the drug. These patients can receive penicillin, amoxicillin, or the cephalosporins.
Without the ability to detect patients with IgE antibody to penicillin prospectively or to distinguish true IgE immunologic reactions from idiopathic reactions in patients receiving cephalosporins, it is impossible to definitely claim that increased immune or IgE-mediated reactions to cephalosporins occur in true penicillinallergic (IgE) patients.
When a cephalosporin is/is not safe for a penicillin-allergic patient. Only IgE-mediated reactions—such as anaphylaxis or hypotension, laryngeal edema, wheezing, angioedema, or urticaria—are likely to become more severe with time. Therefore, with a patient who has had a true IgE-mediated reaction to a penicillin, avoid using cephalosporins with a similar side chain. You may, however, give cephalosporins that have different side chains. Cephalosporins may also be used for patients who have had non-IgE-mediated adverse reactions (“non-type I allergy”)21 to a penicillin, such as a non-pruritic, non-urticarial morbilliform or maculopapular rash.
How prevalent is primary cephalosporin allergy? Even if the patient is not allergic to penicillin, cephalosporins can cause allergic or immune-mediated reactions in approximately 1% to 3% of patients. A patient who had an allergic reaction to a specific cephalosporin probably should not receive that cephalosporin again. The risk of a reaction with a different cephalosporin is very low to nonexistent if the side chains of the 2 drugs are dissimilar.
Bottom line. Penicillin-allergic patients have indeed shown an increased incidence of allergic reactions to cephalothin, cephaloridine, cephalexin, cefadroxil, cefazolin, and cefamandole. However, the risk has been overestimated because most studies reporting this cross-reactivity were flawed (because penicillins were contaminated with cephalosporins) and then failed to account for the fact that penicillin-allergic patients have a 3-fold increased risk of allergic reactions even to nonrelated drugs.51
For patients truly allergic to penicillin, the risk of a reaction from a cephalosporin with side chains that differ from penicillin/amoxicillin (cefuroxime, cefpodoxime, cefdinir, and ceftriaxone, as endorsed by the AAFP) is so low that use is justified and medico-legally defensible by the currently available evidence.
CORRESPONDENCE
Michael E. Pichichero, MD, University of Rochester Medical Center, 601 Elmwood Avenue, Box 672, Rochester, New York 14642. E-mail: [email protected]
1. Levine B. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases of man. N Engl J Med 1966;275:1115-1125.
2. Torres M, Gonzales F, Mayorga C, et al. IgG and IgE antibodies in subjects allergic to penicillins recognize different parts of the penicillin molecule. Int Arch Allergy Immunol 1997;113:342-344.
3. Pichichero M. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005;115:1048-1057.
4. Arndt J, Jick H. Rates of cutaneous reactions to drugs. A report from the Boston Collaborative Drug Surveillance Program. JAMA 1976;235:918-923.
5. Platt R. Adverse effects of third-generation cephalosporins. J Antimicrob Chemother 1982;10(C):135-140.
6. Sanders CV, Greenberg RN, Marier RL. Cefamandole and cefoxitin. Ann Intern Med 1985;103:70-78.
7. Levine LR. Quantitative comparison of adverse reactions to cefaclor vs. amoxicillin in a surveillance study. Pediatr Infect Dis 1985;4:358-361.
8. Norrby S. Side effects of cephalosporins. Drug 1987;34(Suppl 2):105-120.
9. Idsoe O, Guthe T, Wilcox R. Nature and extent of penicillin side-reactions with particular reference to fatalities from anaphylactic shock. Bull WHO 1968;38:159-188.
10. Petz L. Immunologic reactions of humans to cephalosporins. Post Grad Med J 1971;47(Suppl):64-69.
11. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993;270:2456-2463.
12. Walter E, Moelling K, Pavlovic J, et al. Micro-encapsulation of DNA using poly (DL-lactide-co-glycolide): stability issues and release characteristics. J Controlled Release 1999;61:361-374.
13. Weinstein L, Kaplan K, Chang T. Treatment of infections in man with cephalothin. JAMA 1964;189:829-834.
14. Griffith R, Black H. Cephalothin—a new antibiotic. Preliminary clinical and laboratory studies. JAMA 1964;189:823-828.
15. Apicella M, Perkins R, Salsaw S. Cephaloridine treatment of bacterial infections. Am J Med Sci 1966;251:266-276.
16. Assem E, Vickers M. Tests for penicillin allergy in man: The immunological cross-reaction between penicillins and cephalosporins. Immunology 1974;27:255-269.
17. Marks J, Garrett R. Cephalexin in general practice. Post Grad Med J 1970;46(Suppl):113-117.
18. Stewart G. Cross-allergenicity of penicillin G and related substances. Lancet 1962;1:509-510.
19. Dash C. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975;(1 Suppl):107-118.
20. Petz L. Immunologic cross-reactivity between penicillins and cephalosporins. J Infect Dis 1978;137:S74-S79.
21. Thoburn R, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions. IV. The relationship of cephalothin and penicillin allergy. JAMA 1966;198:345-348.
22. Solley G, Gleich G, Van Dellen R. Penicillin allergy: Clincal experience with a battery of skin test reagents. J Allergy Clin Immunol 1982;69:238-244.
23. Warrington R, McPhillipps S. Independent anaphylaxis to cefazolin without allergy to other beta-lactam antibiotics. J Allergy Clin Immunol 1996;98:460-462.
24. Sullivan T, Wedner H, Shatz G, et al. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981;68:171-180.
25. Saxon A. Immediate hypersensitivity reactions to b-lactam antibiotics. Rev Infect Dis 1983;5(Suppl 2):S368-S378.
26. Blanca M, Fernandez J, Miranda A, et al. Cross reactivity between penicillins and cephalosporins: Clinical and immunological studies. J Allergy Clin Immunol 1989;83:381-385.
27. Lin R. A perspective on penicillin allergy. Arch Intern Med 1992;152:930-937.
28. Martin J, Igea J, Fraj J, et al. Allergy to amoxicillin in patients who tolerated benzylpenicillin, aztreonam, and ceftazidime. Clin Infect Dis 1992;14:592-593.
29. Shepherd G, Burton D. Administration of cephalosporin antibiotics to patients with a history of penicillin allergy [abstract]. J Allergy Clin Immunol 1993;91:262.-
30. Audicana M, Bernaola G, Urrutia I, et al. Allergic reactions to betalactams: Studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporins. Allergy 1994;49:108-113.
31. Anne S, Reisman R. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Annals Allergy Asthma Immunology 1995;74:167-170.
32. Miranda A, Blanca M, Vega J, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol 1996;98:671-677.
33. Sastre J, Quijano L, Novalbos A, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy 1996;51:383-386.
34. Pichichero M, Pichichero D. Selecting skin testing reagents to predict amoxicillin and cephalosporin allergy. Pediatr Asthma Allergy Immunol 1997;11:79-93.
35. Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy 2001;31:438-443.
36. Romano A, Gueant-Rodriguez RM, Viola M, et al. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med 2004;141:16-22.
37. Torres M, Blanca M, Garcia J. Evaluation of a large cohort of subjects allergic to penicillins [abstract]. J Allergy Clin Immunol 1995;95:285.-
38. Kelkar P, Li J. Cephalosporin allergy. N Engl J Med 2001;385:804-809.
39. Kabins S, Einstein B, Cohen S. Anaphylactic reaction to an initial dose of sodium cephalothin. JAMA 1965;193:165.-
40. Romano A, Piunti E, De Fronso M, et al. Selective immediate hypersensitivity to ceftriaxone. Allergy 2000;55:418-419.
41. Pumphrey R, Davis S. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet 1999;353:1157-1158.
42. Mayorga C, Torres M, Blanca M. Cephalosporin allergy. N Engl J Med 2002;236:380-381.
43. Mayorga C, Ovispo T, Jimeno L. Epitope mapping of betalactam antibiotics with the use of monoclonal antibodies. Toxicology 1995;97:225-34.
44. Weiss M, Adkinson N. Immediate hypersensitivity reactions to penicillin and related antibiotics. Clin Allergy 1998;18:515-540.
45. Blaiss M, DeShazo R. Drug allergy. Pediatr Clin North Am 1998;35:1131-1147.
46. Baumgart K, Baldo B. Cephalosporin allergy. N Engl J Med 2002;346:380.-
47. James J. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: reliability of examination assessed by skin testing and oral challenge. Pediatrics 1999;104:367.-
48. Pichichero M, Pichichero D. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: Reliability of examination assessed by skin testing and oral challenge. J Pediatr 1998;132:137-143.
49. Romano A, Mayorga C, Torres M, et al. Immediate allergic reactions to cephalosporins: Cross-reactivity and selective responses. J Allergy Clin Immunol 2000;106:1177-1183.
50. Salkind A, Cuddy P, Foxworth J. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001;285:2498-2505.
51. Smith J, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions: II. An evaluation of penicillin allergy. N Engl J Med 1966;274:998-1002.
52. Warrington R, Simons F, Ho H, et al. Diagnosis of penicillin allergy by skin testing: the Manitoba experience. Can Med Assoc J 1978;118:797-791.
53. Saxon A, Beall G, Rohr A. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107:204-215.
1. Levine B. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases of man. N Engl J Med 1966;275:1115-1125.
2. Torres M, Gonzales F, Mayorga C, et al. IgG and IgE antibodies in subjects allergic to penicillins recognize different parts of the penicillin molecule. Int Arch Allergy Immunol 1997;113:342-344.
3. Pichichero M. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005;115:1048-1057.
4. Arndt J, Jick H. Rates of cutaneous reactions to drugs. A report from the Boston Collaborative Drug Surveillance Program. JAMA 1976;235:918-923.
5. Platt R. Adverse effects of third-generation cephalosporins. J Antimicrob Chemother 1982;10(C):135-140.
6. Sanders CV, Greenberg RN, Marier RL. Cefamandole and cefoxitin. Ann Intern Med 1985;103:70-78.
7. Levine LR. Quantitative comparison of adverse reactions to cefaclor vs. amoxicillin in a surveillance study. Pediatr Infect Dis 1985;4:358-361.
8. Norrby S. Side effects of cephalosporins. Drug 1987;34(Suppl 2):105-120.
9. Idsoe O, Guthe T, Wilcox R. Nature and extent of penicillin side-reactions with particular reference to fatalities from anaphylactic shock. Bull WHO 1968;38:159-188.
10. Petz L. Immunologic reactions of humans to cephalosporins. Post Grad Med J 1971;47(Suppl):64-69.
11. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993;270:2456-2463.
12. Walter E, Moelling K, Pavlovic J, et al. Micro-encapsulation of DNA using poly (DL-lactide-co-glycolide): stability issues and release characteristics. J Controlled Release 1999;61:361-374.
13. Weinstein L, Kaplan K, Chang T. Treatment of infections in man with cephalothin. JAMA 1964;189:829-834.
14. Griffith R, Black H. Cephalothin—a new antibiotic. Preliminary clinical and laboratory studies. JAMA 1964;189:823-828.
15. Apicella M, Perkins R, Salsaw S. Cephaloridine treatment of bacterial infections. Am J Med Sci 1966;251:266-276.
16. Assem E, Vickers M. Tests for penicillin allergy in man: The immunological cross-reaction between penicillins and cephalosporins. Immunology 1974;27:255-269.
17. Marks J, Garrett R. Cephalexin in general practice. Post Grad Med J 1970;46(Suppl):113-117.
18. Stewart G. Cross-allergenicity of penicillin G and related substances. Lancet 1962;1:509-510.
19. Dash C. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975;(1 Suppl):107-118.
20. Petz L. Immunologic cross-reactivity between penicillins and cephalosporins. J Infect Dis 1978;137:S74-S79.
21. Thoburn R, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions. IV. The relationship of cephalothin and penicillin allergy. JAMA 1966;198:345-348.
22. Solley G, Gleich G, Van Dellen R. Penicillin allergy: Clincal experience with a battery of skin test reagents. J Allergy Clin Immunol 1982;69:238-244.
23. Warrington R, McPhillipps S. Independent anaphylaxis to cefazolin without allergy to other beta-lactam antibiotics. J Allergy Clin Immunol 1996;98:460-462.
24. Sullivan T, Wedner H, Shatz G, et al. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981;68:171-180.
25. Saxon A. Immediate hypersensitivity reactions to b-lactam antibiotics. Rev Infect Dis 1983;5(Suppl 2):S368-S378.
26. Blanca M, Fernandez J, Miranda A, et al. Cross reactivity between penicillins and cephalosporins: Clinical and immunological studies. J Allergy Clin Immunol 1989;83:381-385.
27. Lin R. A perspective on penicillin allergy. Arch Intern Med 1992;152:930-937.
28. Martin J, Igea J, Fraj J, et al. Allergy to amoxicillin in patients who tolerated benzylpenicillin, aztreonam, and ceftazidime. Clin Infect Dis 1992;14:592-593.
29. Shepherd G, Burton D. Administration of cephalosporin antibiotics to patients with a history of penicillin allergy [abstract]. J Allergy Clin Immunol 1993;91:262.-
30. Audicana M, Bernaola G, Urrutia I, et al. Allergic reactions to betalactams: Studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporins. Allergy 1994;49:108-113.
31. Anne S, Reisman R. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Annals Allergy Asthma Immunology 1995;74:167-170.
32. Miranda A, Blanca M, Vega J, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol 1996;98:671-677.
33. Sastre J, Quijano L, Novalbos A, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy 1996;51:383-386.
34. Pichichero M, Pichichero D. Selecting skin testing reagents to predict amoxicillin and cephalosporin allergy. Pediatr Asthma Allergy Immunol 1997;11:79-93.
35. Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy 2001;31:438-443.
36. Romano A, Gueant-Rodriguez RM, Viola M, et al. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med 2004;141:16-22.
37. Torres M, Blanca M, Garcia J. Evaluation of a large cohort of subjects allergic to penicillins [abstract]. J Allergy Clin Immunol 1995;95:285.-
38. Kelkar P, Li J. Cephalosporin allergy. N Engl J Med 2001;385:804-809.
39. Kabins S, Einstein B, Cohen S. Anaphylactic reaction to an initial dose of sodium cephalothin. JAMA 1965;193:165.-
40. Romano A, Piunti E, De Fronso M, et al. Selective immediate hypersensitivity to ceftriaxone. Allergy 2000;55:418-419.
41. Pumphrey R, Davis S. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet 1999;353:1157-1158.
42. Mayorga C, Torres M, Blanca M. Cephalosporin allergy. N Engl J Med 2002;236:380-381.
43. Mayorga C, Ovispo T, Jimeno L. Epitope mapping of betalactam antibiotics with the use of monoclonal antibodies. Toxicology 1995;97:225-34.
44. Weiss M, Adkinson N. Immediate hypersensitivity reactions to penicillin and related antibiotics. Clin Allergy 1998;18:515-540.
45. Blaiss M, DeShazo R. Drug allergy. Pediatr Clin North Am 1998;35:1131-1147.
46. Baumgart K, Baldo B. Cephalosporin allergy. N Engl J Med 2002;346:380.-
47. James J. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: reliability of examination assessed by skin testing and oral challenge. Pediatrics 1999;104:367.-
48. Pichichero M, Pichichero D. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: Reliability of examination assessed by skin testing and oral challenge. J Pediatr 1998;132:137-143.
49. Romano A, Mayorga C, Torres M, et al. Immediate allergic reactions to cephalosporins: Cross-reactivity and selective responses. J Allergy Clin Immunol 2000;106:1177-1183.
50. Salkind A, Cuddy P, Foxworth J. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001;285:2498-2505.
51. Smith J, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions: II. An evaluation of penicillin allergy. N Engl J Med 1966;274:998-1002.
52. Warrington R, Simons F, Ho H, et al. Diagnosis of penicillin allergy by skin testing: the Manitoba experience. Can Med Assoc J 1978;118:797-791.
53. Saxon A, Beall G, Rohr A. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107:204-215.
Revisiting spirometry for the diagnosis of COPD
- The primary usefulness of spirometry is in identifying persons who will benefit from pharmacologic treatment to alleviate exacerbations (by confirming bronchodilator responsiveness).
- Reserve spirometry for those with activity-limited respiratory symptoms to help target bronchodilator therapy (most beneficial in those with forced expiratory volume in 1 second (FEV1) 50% or less of predicted value).
- Spirometry paired with clinical examination improves COPD diagnostic accuracy compared with clinical examination alone.
- Spirometry is useful in diagnosing COPD when patients have suggestive symptoms.
- Evidence does not support widespread use of spirometry to…
- –diagnose new cases of COPD in at-risk patients
- –improve smoking cessation rates
- –monitor the clinical course of COPD, or
- –adjust interventions
A Guideline Update1 published in September 2003 summarized the Global Strategy for the management of chronic obstructive pulmonary disease (COPD).2 The evidence in that report recommended against spirometry to “diagnose or assess severity of COPD.” However, the Agency for Healthcare Research and Quality (AHRQ) recently published new evidence3 that supports limited use of spirometry for assessing the condition of COPD patients.
AHRQ’s Minnesota Evidence-Based Practice Center reviewed articles published from 1966–2005. Pertinent studies assessed outcomes for adults in primary care settings who were at risk for COPD according to race, age, gender, tobacco use, symptoms, and spirometric status. Excluded from the review were children, persons with asthma, and those with alpha-1 antitrypsin deficiency. The 169-page report had 82 references. The evidence was not explicitly graded, which made it difficult to interpret the significance of each recommendation.
The authors cautioned against widespread spirometric testing of COPD patients. They cited expense of spirometry, resulting treatment costs, resource utilization expense, and personnel time. There is risk of labeling a large number of individuals as diseased who would not benefit from treatment.
CORRESPONDENCEKeith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Holten K.B. How should we manage an acute exacerbation of COPD? J Fam Pract 2003;52:780-782.
2. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available at: www.goldcopd.com/revised.pdf.
3. Wilt TJ, Niewoehner D, Kim C, et al. Use of spirometry for case finding, diagnosis, and management of chronic obstructive pulmonary disease. Summary, Evidence Report/Technology Assessment: Number 121. AHRQ Publication Number 05-E017-1, August 2005. Rockville, Md: Agency for Healthcare Research and Quality. Available at: www.ahrq.gov/clinic/tp/spirotp.htm.
- The primary usefulness of spirometry is in identifying persons who will benefit from pharmacologic treatment to alleviate exacerbations (by confirming bronchodilator responsiveness).
- Reserve spirometry for those with activity-limited respiratory symptoms to help target bronchodilator therapy (most beneficial in those with forced expiratory volume in 1 second (FEV1) 50% or less of predicted value).
- Spirometry paired with clinical examination improves COPD diagnostic accuracy compared with clinical examination alone.
- Spirometry is useful in diagnosing COPD when patients have suggestive symptoms.
- Evidence does not support widespread use of spirometry to…
- –diagnose new cases of COPD in at-risk patients
- –improve smoking cessation rates
- –monitor the clinical course of COPD, or
- –adjust interventions
A Guideline Update1 published in September 2003 summarized the Global Strategy for the management of chronic obstructive pulmonary disease (COPD).2 The evidence in that report recommended against spirometry to “diagnose or assess severity of COPD.” However, the Agency for Healthcare Research and Quality (AHRQ) recently published new evidence3 that supports limited use of spirometry for assessing the condition of COPD patients.
AHRQ’s Minnesota Evidence-Based Practice Center reviewed articles published from 1966–2005. Pertinent studies assessed outcomes for adults in primary care settings who were at risk for COPD according to race, age, gender, tobacco use, symptoms, and spirometric status. Excluded from the review were children, persons with asthma, and those with alpha-1 antitrypsin deficiency. The 169-page report had 82 references. The evidence was not explicitly graded, which made it difficult to interpret the significance of each recommendation.
The authors cautioned against widespread spirometric testing of COPD patients. They cited expense of spirometry, resulting treatment costs, resource utilization expense, and personnel time. There is risk of labeling a large number of individuals as diseased who would not benefit from treatment.
CORRESPONDENCEKeith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
- The primary usefulness of spirometry is in identifying persons who will benefit from pharmacologic treatment to alleviate exacerbations (by confirming bronchodilator responsiveness).
- Reserve spirometry for those with activity-limited respiratory symptoms to help target bronchodilator therapy (most beneficial in those with forced expiratory volume in 1 second (FEV1) 50% or less of predicted value).
- Spirometry paired with clinical examination improves COPD diagnostic accuracy compared with clinical examination alone.
- Spirometry is useful in diagnosing COPD when patients have suggestive symptoms.
- Evidence does not support widespread use of spirometry to…
- –diagnose new cases of COPD in at-risk patients
- –improve smoking cessation rates
- –monitor the clinical course of COPD, or
- –adjust interventions
A Guideline Update1 published in September 2003 summarized the Global Strategy for the management of chronic obstructive pulmonary disease (COPD).2 The evidence in that report recommended against spirometry to “diagnose or assess severity of COPD.” However, the Agency for Healthcare Research and Quality (AHRQ) recently published new evidence3 that supports limited use of spirometry for assessing the condition of COPD patients.
AHRQ’s Minnesota Evidence-Based Practice Center reviewed articles published from 1966–2005. Pertinent studies assessed outcomes for adults in primary care settings who were at risk for COPD according to race, age, gender, tobacco use, symptoms, and spirometric status. Excluded from the review were children, persons with asthma, and those with alpha-1 antitrypsin deficiency. The 169-page report had 82 references. The evidence was not explicitly graded, which made it difficult to interpret the significance of each recommendation.
The authors cautioned against widespread spirometric testing of COPD patients. They cited expense of spirometry, resulting treatment costs, resource utilization expense, and personnel time. There is risk of labeling a large number of individuals as diseased who would not benefit from treatment.
CORRESPONDENCEKeith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Holten K.B. How should we manage an acute exacerbation of COPD? J Fam Pract 2003;52:780-782.
2. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available at: www.goldcopd.com/revised.pdf.
3. Wilt TJ, Niewoehner D, Kim C, et al. Use of spirometry for case finding, diagnosis, and management of chronic obstructive pulmonary disease. Summary, Evidence Report/Technology Assessment: Number 121. AHRQ Publication Number 05-E017-1, August 2005. Rockville, Md: Agency for Healthcare Research and Quality. Available at: www.ahrq.gov/clinic/tp/spirotp.htm.
1. Holten K.B. How should we manage an acute exacerbation of COPD? J Fam Pract 2003;52:780-782.
2. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available at: www.goldcopd.com/revised.pdf.
3. Wilt TJ, Niewoehner D, Kim C, et al. Use of spirometry for case finding, diagnosis, and management of chronic obstructive pulmonary disease. Summary, Evidence Report/Technology Assessment: Number 121. AHRQ Publication Number 05-E017-1, August 2005. Rockville, Md: Agency for Healthcare Research and Quality. Available at: www.ahrq.gov/clinic/tp/spirotp.htm.
Depression in African Americans: Breaking barriers to detection and treatment
- When evaluating African Americans for depression, look for somatic and neurovegetative symptoms rather than mood or cognitive symptoms (C).
- Education can help patients better tolerate drug regimens and improve chances for treatment success (C).
- Practice improvement efforts can improve health outcomes in depressed African Americans (B).
African Americans depend on quality mental health services in primary care more so than whites, since they are more likely to seek care in this setting. However, accumulating evidence shows that African Americans in primary care settings face disparity in recognition and treatment of depression (see Race and mental health treatment: the divide).1-5 Until now, the specific factors leading to this disparity have not been well described.16
In this article—a systematic review of studies on the evaluation and treatment of depression in African American patients—we draw attention to factors regarding patients, physicians, and clinical settings that raise barriers to diagnosis and treatment. We also recommend strategies to break through these barriers.
Racial disparity in mental health treatment is a difference across racial groups not justified by underlying differences in mental health status or patient treatment preferences. The reasons are numerous. Compared with whites, for example, African Americans are more likely to be poor, uninsured, or have restrictive insurance policies,6,7 and to have limited means of transportation to reach health services.8 But even after controlling for differences in insurance and socioeconomic status, African Americans are less likely than whites to use outpatient mental health services,9,10 and they therefore face disparities in quality of care.11,12 Thus, economic factors and illness severity do not fully account for the observed racial differences in the rate and quality of treatment of mental disorders.
Racial disparities may also emerge in the decisions physicians make in caring for patients. In several clinical contexts, members of minorities have received less and inferior care than white patients.13,14 In one epidemiologic study, African Americans were reported to receive less access to mental health care and to have greater unmet needs for mental health care than whites.11 Because differences in access to care can have consequences for health outcomes, reducing health care disparities is a widely shared goal of clinical and public health care policy.15
Assumptions that mask disparities
Disparities may not be apparent to healthcare providers in part because community-based epidemiologic studies report that African Americans have a lower rate of major depression than whites.5,17-19 In primary care settings specifically, the prevalence of major depression among African Americans appears to be lower than for white or Hispanic Americans, mirroring rates in national and community studies.20 Explanations for this relatively low prevalence of depression have included attributes of the African American experience, such as strong religious beliefs and community ties.
Moreover, according to analyses of data from the National Comorbidity Study, which included an examination of attitudes toward mental health services, African Americans with major depression are more likely to report that they would “definitely go” for mental health services than would whites.21
High-risk populations overlooked. Community-based studies, however, do not fully capture the mental health needs of African Americans, a large number of whom are in high-risk populations excluded from community epidemiologic studies of mental disorders. Studies of these populations show high rates of major depression and other mental disorders, due to factors including alcohol and substance use disorders, poor physical health, and poverty and homelessness.
An unsettling irony. For emotional distress, African Americans are more likely to seek help from primary care clinicians than from specialty mental health providers.22-24 However, in primary care, depression in African Americans is less likely to be detected than it is in whites.3
In a nationally representative sample, African Americans have been found less likely than whites to receive effective care for depression in primary care.12,25,26 In a study of Medicaid recipients who had been diagnosed with major depression, African Americans less often received antidepressant medications than did whites.27 Such disparities in pharmacologic treatments were also found in another study of African American patients in primary care, using data from the National Ambulatory Medical Care Surveys.24
Search methods
Data sources
We systematically searched for and retrieved articles in Medline (January 1966 to December 2004) using the MeSH (medical subject heading) terms African Americans and depressive disorder along with the MesH terms adult or adolescent. We refined our search results using the MeSH terms diagnosis, comorbidity, physician-patient relations, patient satisfaction, and patient acceptance of health care to focus on patient and physician factors.
To search for factors related to practice setting, we refined our initial search strategy with the MeSH terms primary health care, family practice, and health services. We also hand-checked bibliographies to find potential articles.
Study selection and data extraction
Two investigators (AKD, MO) independently reviewed abstracts of the articles retrieved and excluded articles that did not fulfill the criteria to be relevant to the topic of depression in African American patients. Articles were excluded if they did not focus on US populations, African Americans, depressive disorders, and clinical settings, or if they did not report original data. Differences between the 2 reviewers were resolved through consensus after full-text review of the article.
Additional articles were defined by 2 other investigators (HLC, MMW) who provided peer review and identified potential articles in bibliographies. For each article, we classified the barriers described as related to diagnosis, management, or both and to patient, physician, and treatment-setting factors.
Results
The initial search using the MeSH terms African Americans and depressive disorder with adult or adolescent returned 156 articles. We found a total of 73 unique articles by refining our Medline search with additional MeSH terms. Of these publications, 12 fulfilled our criteria as relevant to the topic of diagnosing and managing depression in African American patients.
Our hand-checking of bibliographic references found an additional 12 articles that met our relevancy criteria. We examined each of the 24 articles (TABLE 1) to determine the types of barrier studies and whether the factor was related to the detection of depression, its management, or both.
TABLE 1
Selected studies on managing depression in African Americans
| AUTHOR | SAMPLE (% AFRICAN AMERICAN) | STUDY OUTCOMES | MAJOR FINDINGS |
|---|---|---|---|
| Borowsky et al3 | 19,309 adult primary care patients (13%) | Detection of a mental health problem by internists and family physicians | African American patients who have major depression have a lower rate of being detected than their white counterparts |
| Brown et al28 | 272 depressed adult primary care patients (44%) | Differences in clinical characteristics, health impairments, health beliefs, stressful life events, and social support between African American and white subjects enrolled in a clinical trial of depression | In comparison to white persons, African Americans are more likely to experience somatization, greater health impairment, greater perception of less control of their health status and greater number of stressful life events in the previous 6 months |
| Brown et al29 | 160 depressed adult primary care patients (43%) | Differences in treatment outcomes from a clinical trial of standardized psychotherapy and pharmacotherapy | Both African American and white patients are effectively treated by psychotherapy and antidepressant medications, but African American subjects have poorer outcome in physical functioning |
| Brown et al30 | 865 adult African American persons from a community sample (100%) | Rate of major depression compared with demographic, sociocultural, familial background, and health-related risk factors | The strongest predictors of major depression are self-reported poor or fair physical health and being 20 to 29 years of age |
| Cooper et al31 | 829 depressed adult primary care patients (12%) | Attitudinal measures of acceptability of antidepressant medication and individual counseling | African Americans are less likely than white persons to find antidepressant medication acceptable |
| Cooper-Patrick et al23 | 1816 adult primary care patients (45%) | Patients’ ratings of physicians participatory decision making style | African American patients rate their visits as less participatory than their white counterparts and race-concordant patient-physician relationships are more participatory than race-discordant relationships |
| Cooper-Patrick et al32 | Focus group consisting of 16 patients with a recent episode of depression (50%) | 16 categories of comments regarding treatment of depression were identified from audiotaped discussions | African American patients provided more comments about spirituality and stigma and were less concerned with the relation between physical health and depression than white patients |
| Dunlop et al5 | 7690 subjects aged 54 to 65 years from national probability sample (17%) | Rates of major depression with socio demographic characteristics, self-reported health needs, functional limitations, and economic resources as explanatory variables | African Americans have a greater rate of major depression compared to whites, but have a significantly lower rate after adjustments for health needs (such as comorbid physical problems) and economic access (such as income and health insurance) |
| Fabrega et al33 | 5198 adult community mental health center patients (16%) | Rates of major depression and comorbid psychiatric diagnoses | African Americans have a higher rate of comorbid major depression and substance-use disorders than their white counterparts |
| Ford et al34 | Community-based sample of 7092 adults who had who had a mental health or substance abuse problem and received care in nonpsychiatric settings (29%) | Patient self-reported discussion of a mental health problem or problem with alcohol or drugs | No significance differences between the rates of African Americans and whites discussed emotional or mental health problems with their physicians |
| Grant et al35 | National probability sample of adult non-institutionalized US population (NR) | Rates of major depression and alcohol use disorders stratified by demographic variables | The association between alcohol abuse and major depression was greater among African Americans than among non–African Americans |
| Hanson et al36 | 60 adult patients undergoing psychiatric evaluation (50%) | Congruence between physicians’ and patients’ ratings of depression during an intake assessment | Concordance of symptoms ratings is lower between white psychiatrists and African American patients than with white psychiatrists and white patients |
| McKinlay et al37 | 128 primary care physicians (25%) | Diagnostic assessment of videotaped patient presentations of depression and another medical condition | Demographic attributes of patients did not affect diagnostic accuracy, and white physicians were almost twice as likely as their African American counterparts to diagnose depression correctly |
| Miranda et al38 | 205 adult public-sector gynecologic patients (30%) | Rates of psychiatric disorders along with extent of primary care services and perception of communication with primary care provider | In a largely minority (82%) patient population (with no reported differences among racial/ethnic groups), 1 out of 5 patients endorsed major depression and fewer than half had access to comprehensive primary medical services |
| Miranda et al39 | 1269 adult primary care patients (7%) | Clinical outcome, appropriateness of care, and employment for patients enrolled into a clinical trial of 2 quality improvement interventions with modest adaptation to minority patients | African American patients, in contrast to their white counterparts, experienced a significant decrease in probable depression from baseline |
| O’Malley et al40 | Population-based sample of 1202 women (83%) | Rates of care of depression, extent of primary care services, quality of physician-patient relationship | In a largely African American sample (with no reported differences between racial/ethnic groups), comprehensive primary care delivery (including counseling) and self-reported view of physician as respectful are associated with physician inquiry and treatment of depression |
| Rollman et al41 | 204 adult primary care patients (25%) | Processes of depression care and patient outcome following screening and treatment recommendations to physicians | No differences in treatment patterns and outcomes were found between white and African American patients, except for increased rate of documented counseling by physicians among whites |
| Sleath et al42 | 508 adult outpatients (NR) | Patient expression of symptoms from audiotaped medical encounter and physician perception of health from survey | Among all factors, only patient expression of emotional symptoms among African American patients influenced psychotropic prescribing |
| Steffens et al43 | 113 patients age 60 years or older (11.5%) | Annual rate of enrollment into a geriatric depression research program based on race and ethnicity | Active community outreach and enrollment increased representation of African Americans by threefold over a 2-year period |
| Sussman et al1 | 3004 adult subjects from a community-based sample (38%) | Treatment seeking behavior for subjects who have current depression | African Americans are less likely to seek care for depression than whites, with African Americans reporting greater fear of treatment and of being hospitalized than whites |
| Van Hook44 | 321 adult women in primary care settings (22%) | Rate of major depression and reported barriers to treatment | In a multi-ethnic sample (with no reported differences among racial/ethnic groups), commonly reported barriers to seeking help included stigma and perceived separation of primary care and specialty mental health services |
| Wells et al45 | 9585 adult national survey respondents (28%) | Access to treatment for alcoholism, drug abuse and, mental health care; unmet need for care; satisfaction with care and use of active treatment in the prior year | Among those with perceived need for mental health services, African Americans were more likely to report no access to care than whites and less likely to receive active treatment |
| Wohl et al46 | 20 matched adult depressed African American and white patients (50%) | Symptom pattern and severity of depression | No difference in symptom severity was found between African Americans and whites, and African Americans were more likely to experience diurnal variation in their depression |
Discussion
Barriers in evaluation
Research on depression in African Americans can be analyzed in terms of attributes of the provider, patient, and practice setting.
Provider attributes. The race of the physician has been linked to the diagnosis of depression in African-American patients, but studies of this factor have yielded conflicting results. McKinlay and colleagues used videotaped cases of depressed patients presented to 128 physicians to examine whether diagnosis was affected by physician characteristics (age, gender race, and medical specialty), patient characteristics (age, gender, race, and socioeconomic status), or their combination.37 They found that physician characteristics affected the diagnosis of depression, but that patient attributes did not. In particular, white primary care physicians were twice as likely as African American physicians to diagnose depression. Since approximately 1 in 5 African American patients seeks care from a physician of their own race,47 such differences may influence the overall rate of recognition in primary care. However, data from the Medical Outcome Study, which included demographic characteristics on 349 primary care physicians, did not find that the race of the physician influenced the detection of depression.3
In addition to physician demographic factors, physician communication style may affect ability to diagnose depression. Appropriate diagnosis and treatment of depression depends to a great extent on verbal communications between patient and provider about the nature, extent, severity, and consequences of symptoms. Problems with communication may lead to misunderstandings, misdiagnosis, inappropriate treatments, and premature termination of treatment. An analysis of patient-physician encounters indicates that physicians may be more likely to minimize emotional symptoms of African American than of whites.42 Relative to whites, African Americans are more likely to rate their visits with white physicians as less participatory.
This difference is overcome when African Americans see physicians of their own race.23 In a population-based study among largely low-income African American women, primary care physicians who were rated as showing more respect by the participants were more likely to inquire about depressive symptoms during a clinical visit.40 Thus, despite the fact that African American patients rate their encounters more satisfying and are more likely to disclose their problems to African American physicians, white physicians appear to diagnose depression more commonly in their African American patients. Clearly, more work is needed to clarify this seeming contradiction.
Patient attributes. During the primary care encounter, African Americans are as likely as whites to discuss mental health problems.34 However, African Americans may be more likely to exhibit somatic and neurovegetative symptoms of depression than mood or cognitive symptoms,33,36 which may complicate detection and diagnosis.
A recent national survey suggests that depression in African Americans may be commonly masked by self medication and somatic symptoms and so may pass undetected in primary care.48 In a randomized clinical trial of depression treatment in primary care, depressed African American subjects were more likely than white subjects to have symptoms of poor physical health and pain and to have somatization.29 However, another study found few differences between whites and African Americans in symptom presentation of depression when comorbid disorders and sociodemographic factors were controlled.46
Do primarily somatic presentations of depression reduce physicians’ ability to accurately diagnose major depression in racial and ethnic minorities? This question has not been studied, though coexisting medical problems in primary care populations have been found to impede the diagnosis of depression,49 presumably by competing for the physician’s attention.3,50 In a variety of medical contexts, known medical disorders are associated with under-treatment of unrelated disorders.51
Practice-setting attributes. No studies have examined whether specific practice setting factors, such as insurance coverage, are related to the low detection rate of major depression. Among low-income women of multiethnic backgrounds attending primary care clinics, Van Hook found that perceived separation of primary care services and specialty mental health care was a self-reported barrier to seeking help for depression.44
The Surgeon General’s report documents the overall poor access to general medical services faced by African Americans. They are more likely to receive health care in outpatient hospital and emergency departments, and their mental health services are also characterized by high rates of emergency care. As a result, they are less likely to receive the continuity of treatment provided in primary care, which may allow better detection of depression.15
Barriers to effective management
Research on the potential causes of disparities in treatment for African American patients in primary care has examined a variety of provider, patient, and practice-setting factors.
Provider attributes. Physician communication style may not only influence diagnosis but also a decision to treat. In a study that analyzed audiotaped communication between patients and primary care providers during clinic visits, the tendency of physicians to minimize emotional expression by African Americans, relative to whites, led to lower prescribing of antidepressant medication among African Americans.42 Further research is needed to understand why such communication styles exist between primary care providers and African American patients.
Patient attributes. A number of patient attributes have been examined that may explain the lower rate of mental health services provided in primary care. Significant research has been undertaken on African American attitudes toward and beliefs about mental health treatments. Cooper-Patrick and colleagues conducted focus groups for African American and white patients as well as health care professionals. In questions related to depression and treatment preferences, African American patients expressed more concerns about stigma and spirituality than did white patients.32
In related research, a survey of African American patients recruited from primary care offices indicated they were less likely to find antidepressant medication acceptable than white patients in primary care.31 Such attitudinal differences may explain why African Americans use antidepressants less commonly than whites, even when primary care physicians make similar recommendations for both groups.52 As yet unstudied is the extent to which sensational reports about antidepressant side effects may cause persons already skeptical about the care they receive to discontinue treatment.
Another reason African Americans may avoid or discontinue antidepressant treatment is that they tend to tolerate certain classes of psychotropic medications poorly. Strickland and colleagues found that African Americans are more likely than whites to be “poor metabolizers” of tricyclic antidepressants.53 African Americans treated with tricyclic antidepressants will therefore experience higher plasma levels per dose than whites, and an earlier onset of action. African Americans are also more likely to experience side effects, which may lead to treatment nonadherence.
Few studies have examined the tolerability of newer class of antidepressant medications, such as selective serotonin reuptake inhibitors (SSRIs), for African Americans. More research is required to determine whether antidepressant side effects or other experiences with psychotropic medications are a reason for the lower rates of antidepressant use among African American patients in primary care. Educating patients about antidepressants, their onset of action, and side effects may reduce some of these barriers and prevent early discontinuation of antidepressant therapy.54
Poverty and its associated psychosocial factors may also contribute to the lower quality of mental health care among African Americans. Miranda and colleagues found this to be so for African American women receiving primary care in obstetric-gynecologic clinics.47 They have argued that clinical case management is needed as a component of effective mental health treatment for primary care patients who are poor and likely to face significant negative life events.
Practice-setting attributes. Few practice-setting factors have been examined in relationship to the disparities in depression treatment faced by African Americans. O’Malley and colleagues examined whether primary care physicians who were evaluated as having comprehensive medical services by low-income African American women were more likely to provide treatment for depression.40 Comprehensiveness was determined by the ability to meet all health needs, thoroughness of physical exam, and provision of counseling and screening services. In a survey study, they found that comprehensiveness of medical services was correlated to being asked about and being treated for depression.
Interventions in primary care
Concerted efforts to improve quality of care can reduce the mental health burden of undetected depression for African Americans as well as other ethnic and racial groups. African American and white primary care patients both appear to respond equally to standardized psychotherapy and pharmacotherapy for major depression.29
In a randomized control trial to improve the quality of depression management in primary care, no significant differences existed between white and African American participants in the process of depression care or clinical outcomes, although both groups had less than optimal recovery rates.41 One randomized clinical trial included modified interventions to target the mental health needs of low-income minority primary care patients. The interventions included educating clinicians about depression; teaching nurses to educate, assess, and follow-up depressed patients; and making cognitive behavioral therapy available. Patients and physicians selected the treatment. Modification for minority patients was modest and included translations and cultural training for clinicians.
Using these approaches, African American participants were more likely than whites to have better depression outcome at 6 and 12 months.39 These studies indicate that both general interventions to depression care and small modifications for minority patients can lead to improved health outcomes among African American patients.
It may be unreasonable to believe that simply seeking to improve the primary care assessment and treatment of depression in African Americans will eliminate racial differences in the health outcomes of depressed adults. Reform is also clearly needed in health care financing and in broader social welfare policy as it affects the lives of depressed minority populations. However, primary care providers who are aware of the risk of racial disparities in the recognition and treatment of depression and work to provide treatments that are tailored to the individual’s needs can help to reduce the significant burden of depression (TABLE 2).
TABLE 2
Recommendations for addressing barriers to the detection and treatment of depression in the African American patient
| IMPROVING DIAGNOSIS |
| Assess stigma toward mental health problems for patients suspected to have depression |
| Inquire about patient’s experience of somatic symptoms and their relationship to depression, life stressors, and social conflicts |
| Maintain a respectful, open stance in understanding patient’s style of coping with depressive symptoms, including use of spirituality |
| Evaluate the presence of comorbid mental health problems, such as alcohol abuse, that require different treatment approaches |
| PROVIDING EFFECTIVE MANAGEMENT |
| Determine the patient’s preferences for psychotherapy and pharmacotherapy, and provide treatment referrals for counseling if appropriate and as resources permit |
| Educate patients about antidepressant medications, their onset of action, and side effects |
| At each visit after initiation of depression management, check for regular adherence to pharmacotherapy or to referred psychotherapy |
| Assist patients who cannot maintain regular visits for depression care to find strategies that can overcome social or financial barriers |
Acknowledgments
This review was funded by the Columbia Center for the Health of Urban Minorities (NCMHHD MD000206-019006) (Drs. Olfson and Weissman), an unrestricted grant from Eli Lilly & Company (Dr. Weissman) and a NIMH National Service Research Award Institutional Research Training Grant 5T32MH015144 (Dr. Das).
CORRESPONDENCE
Myrna M. Weissman, Ph.D. Columbia University College of Physicians and Surgeons, 1051 Riverside Drive, New York, NY 10032. E-mail: [email protected]
1. Sussman LK, Robins LN, Earls F. Treatment-seeking for depression by black and white Americans. Soc Sci Med 1987;24:187-196.
2. Vega WA, Kolody B, Aguilar-Gaxiola S, Catalano R. Gaps in service utilization by Mexican Americans with mental health problems. Am J Psychiatry 1999;156:928-934.
3. Borowsky SJ, Rubenstein LV, Meredith LS, Camp P, Jackson-Triche M, Wells KB. Who is at risk of nondetection of mental health problems in primary care? J Gen Intern Med 2000;15:381-388.
4. Fiscella K, Franks P, Doescher MP, Saver BG. Disparities in health care by race, ethnicity and language among the insured: findings from a national sample. Med Care 2002;40:52-59.
5. Dunlop DD, Song J, Lyons JS, Mannheim LM, Chang RW. Racial/ethnic differences in rates of depression among pre-retirement adults. Am J Public Health 2003;93:1945-1952.
6. Phillips KA, Mayer ML, Aday LA. Barriers to care among racial ethnic groups under managed care. Health Aff 2000;19:65-75.
7. Tai-Seale M, Freund D, LoSasso A. Racial disparities in service use among Medicaid beneficiaries after mandatory enrollment in managed care: a difference-in-differences approach. Inquiry 2001;38:49-59.
8. Heckman TG, Somlai AM, Peters J, et al. Barriers to care among persons living with HIV/AIDS in urban and rural areas. AIDS Care 1998;10:365-375.
9. Padgett DK, Patrick C, Burns BJ, Schlesinger HJ. Ethnicity and the use of outpatient mental health services in a national insured population. Am J Public Health 1994;84:222-226.
10. Swartz MS, Wagner HR, Swanson JW, Bruns BJ, Keorge LK, Padgett DK. Comparing use of public and private mental health services; the enduring barriers of race and age. Commun Ment Health J 1998;34:133-144.
11. Wells K, Klap R, Koike A, Sherbourne C. Ethnic disparities in unmet need for alcoholism, drug abuse and mental health care. Am J Psychiatry 2001;158:2027-2032.
12. Wang PS, Berglund P, Kessler RC. Recent care of common mental disorders in the United States. J Gen Intern Med 2000;15:284-292.
13. Bach P, Cramer L, Warren L, Begg C. Racial differences in the treatment of early-stage lung cancer. New Engl J Med 1999;341:1198-1205.
14. Peterson ED, Shaw L, DeLong E, Pryor D, Califf R, Mark D. Racial variation in the use of coronary-revascularization procedures—are the differences real? Do they matter? N Engl J Med 1997;336:480-486.
15. US Department of Health and Human Services. Mental Health: Culture Race, and Ethnicity: A Supplement to Mental Health: A Report of the Surgeon General. Rockville, Md: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, 2001.
16. Areán PA, Alvidrez J. Treating depressive disorders: who responds who does not respond, and who do we need to study? J Fam Pract 2001;50:E2.-
17. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 1994;51:8-19.
18. Zhang AY, Snowden LR. Ethnic characteristics of mental disorders in five US communities. Cult Div Ethnic Minority Psychology 1999;5:134-146.
19. Kessler RC, Berglund P, Demler O, et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095-3105.
20. Olfson M, Shea A, Feder A, et al. Prevalence of anxiety, depression, and substance use disorders in an urban general medicine practice. Arch Fam Med 2000;57:675-682.
21. Diala CD, Muntaner C, Walrath C, Nickerson K, LaVeist T, Leaf P. Racial/ethnic differences in attitudes toward seeking professional mental health services. Am J Public Health 2001;91:805-807.
22. Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA 1999;282:583-589.
23. Gallo JJ, Marino S, Ford D, Anthony J. Filters on the pathway to mental health care II: sociodemographic factors. Psychol Med 1995;25:1149-1160.
24. Snowden LR, Pingitore D. Frequency and scope of mental health delivery service to African Americans in primary care. Ment Health Serv Res 2002;4:123-130.
25. Harman JS, Schulberg HC, Mulsant BH, Reynolds CF. The effect of patient and visit characteristics on diagnosis of depression in primary care. J Fam Pract 2001;50-1068.
26. Young AS, Klap R, Sherbourne CD, Wells KB. The quality of care for depressive and anxiety disorder in the United States. Arch Gen Psychiatry 2001;52:472-478.
27. Melfi CA, Croghan TW, Hanna MP, Robinson RL. Racial variation in antidepressant treatment in a Medicaid population. J Clin Psychiatry 2000;16:16-21.
28. Brown C, Schulberg HC, Madonia MJ. Clinical presentations of major depression by African Americans and Whites in primary medical care practice. J Affect Disord 1996;41:181-191.
29. Brown C, Schulberg HC, Sacco D, Perel JM, Houck PR. Effectiveness of treatments for major depression in primary medical care: a post hoc analysis of outcomes for African Americans and White patients. J Affect Disord 1999;53:185-192.
30. Brown DR, Ahmed F, Gary LE, Milburn NG. Major depression in a community sample of African Americans. Am J Psychiatry 1995;152:373-378.
Cooper LA, Gonzales JJ, Gallo JJ, et al. The acceptability of treatment for depression among African-American, Hispanic, and white primary care patients. Med Care 2003;41:479-489.
32. Cooper-Patrick L, Powe NR, Jenckes MW, Gonzales JJ, Levine DM, Ford DE. Identification of patient attitudes and p regarding treatment of depression. J Gen Intern Med 1997;12:431-438.
33. Fabrega H, Jr, Mezzich J, Ulrich RF. Black-white differences in psychopathology in an urban psychiatric population. Compr Psychiatry 1988;29:285-297.
34. Ford DE, Kamerow DB, Thompson JW. Who talks to physicians about mental health and substance abuse problems? J Gen Intern Med 1988;3:363-369.
35. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend 1995;39:197-206.
36. Hanson B, Klerman GL. Proceedings: Interracial problems in the assessment of clinical depression: concordance differences between white psychiatrists and black and white patients. Psychopharmacol Bull 1974;10:65-67.
37. McKinlay JB, Lin T, Freund K, Moskowitz M. The unexpected influence of physician attributes in clinical decisions: results of an experiment. J Health Soc behavior 2002;43:92-106.
38. Miranda J, Azocar F, Komaromy M, Golding JM. Unmet mental health needs of women in public-sector gynecologic clinics. Am J Obstet Gynecol 1998;178:212-217.
39. Miranda J, Duan N, Sherbourne C, et al. Improving care for minorities: Can quality improvement interventions improve care and outcomes for depressed minorities? results of a randomized, controlled trial. Health Serv Res 2003;613-630.
40. O’Malley AS, Forrest CB, Miranda J. Primary care attributes and care for depression among low-income African American women. Am J Public Health 2003;93:1328-1334.
41. Rollman BL, Hanusa BH, Belnap BH, Gardner W, Cooper LA, Schulberg HC. Race, quality of depression care, and recovery from major depression in a primary care setting. Gen Hosp Psychiatry 2002;24:381-390.
42. Sleath B, Svarstad B, Roter D. Patient race and psychotropic prescribing during medical encounters. Patient Educ Couns 1998;34:227-238.
43. Steffens DC, Artigues DL, Ornstein KA, Krishnan KR. A review of racial differences in geriatric depression: implications for care and clinical research. J Natl Med Assoc 1997;89:731-736.
44. Van Hook MP. Women’s help-seeking patterns for depression. Soc Work Health Care 1999;29:15-34.
45. Wells K, Klap R, Koike A, Sherbourne C. Ethnic disparities in unmet need for alcoholism drug abuse and mental health care. Am J Psychiatry 2001;158:2027-2032.
46. Wohl M, Lesser I, Smith M. Clinical presentations of depression in African Americans and white outpatients. Cult Div Ment Health 1997;3:279-284.
47. LaVeist TA, Carroll T. Race of physician and satisfaction with care among African-American patients. J Natl Med Assoc 2002;94:937-943.
48. Jackson JS. Race and ethnic differences in physical and mental health disparities. University of Michigan Institute for Social Research, Program for Research on Black Americans. Presented at New York State Psychiatric Institute, New York, NY, February 2004.
49. Knauper B, Wittchen HU. Diagnosing major depression in the elderly: evidence for response bias in standardized diagnostic interviews? J Psychiatr Res 1994;28:147-164.
50. Nutting PA, Rost K, Smth J, Weiner JJ, Elliot. Competing demands from physical problems: effect on initiating and completing depression care over 6 months. Arch Fam Med 2000;9:1059-1064.
51. Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med 1998;338:1516-1520.
52. Miranda J, Cooper LA. Disparities in care for depression among primary care patients. J Gen Intern Med 2004;19:120-126.
53. Strickland TL, Stein R, Lin K, Risby E, Fong R. The pharmacologic treatment of anxiety and depression in African Americans. Arch Fam Med 1997;6:371-375.
54. Bull SA, Hu XH, Lee JY, Ming EE, Markson LE, Fireman. Discontinuation of use and switching of antidepressants: influence of patient-physician communication. JAMA 2002;288:1403-1409.
- When evaluating African Americans for depression, look for somatic and neurovegetative symptoms rather than mood or cognitive symptoms (C).
- Education can help patients better tolerate drug regimens and improve chances for treatment success (C).
- Practice improvement efforts can improve health outcomes in depressed African Americans (B).
African Americans depend on quality mental health services in primary care more so than whites, since they are more likely to seek care in this setting. However, accumulating evidence shows that African Americans in primary care settings face disparity in recognition and treatment of depression (see Race and mental health treatment: the divide).1-5 Until now, the specific factors leading to this disparity have not been well described.16
In this article—a systematic review of studies on the evaluation and treatment of depression in African American patients—we draw attention to factors regarding patients, physicians, and clinical settings that raise barriers to diagnosis and treatment. We also recommend strategies to break through these barriers.
Racial disparity in mental health treatment is a difference across racial groups not justified by underlying differences in mental health status or patient treatment preferences. The reasons are numerous. Compared with whites, for example, African Americans are more likely to be poor, uninsured, or have restrictive insurance policies,6,7 and to have limited means of transportation to reach health services.8 But even after controlling for differences in insurance and socioeconomic status, African Americans are less likely than whites to use outpatient mental health services,9,10 and they therefore face disparities in quality of care.11,12 Thus, economic factors and illness severity do not fully account for the observed racial differences in the rate and quality of treatment of mental disorders.
Racial disparities may also emerge in the decisions physicians make in caring for patients. In several clinical contexts, members of minorities have received less and inferior care than white patients.13,14 In one epidemiologic study, African Americans were reported to receive less access to mental health care and to have greater unmet needs for mental health care than whites.11 Because differences in access to care can have consequences for health outcomes, reducing health care disparities is a widely shared goal of clinical and public health care policy.15
Assumptions that mask disparities
Disparities may not be apparent to healthcare providers in part because community-based epidemiologic studies report that African Americans have a lower rate of major depression than whites.5,17-19 In primary care settings specifically, the prevalence of major depression among African Americans appears to be lower than for white or Hispanic Americans, mirroring rates in national and community studies.20 Explanations for this relatively low prevalence of depression have included attributes of the African American experience, such as strong religious beliefs and community ties.
Moreover, according to analyses of data from the National Comorbidity Study, which included an examination of attitudes toward mental health services, African Americans with major depression are more likely to report that they would “definitely go” for mental health services than would whites.21
High-risk populations overlooked. Community-based studies, however, do not fully capture the mental health needs of African Americans, a large number of whom are in high-risk populations excluded from community epidemiologic studies of mental disorders. Studies of these populations show high rates of major depression and other mental disorders, due to factors including alcohol and substance use disorders, poor physical health, and poverty and homelessness.
An unsettling irony. For emotional distress, African Americans are more likely to seek help from primary care clinicians than from specialty mental health providers.22-24 However, in primary care, depression in African Americans is less likely to be detected than it is in whites.3
In a nationally representative sample, African Americans have been found less likely than whites to receive effective care for depression in primary care.12,25,26 In a study of Medicaid recipients who had been diagnosed with major depression, African Americans less often received antidepressant medications than did whites.27 Such disparities in pharmacologic treatments were also found in another study of African American patients in primary care, using data from the National Ambulatory Medical Care Surveys.24
Search methods
Data sources
We systematically searched for and retrieved articles in Medline (January 1966 to December 2004) using the MeSH (medical subject heading) terms African Americans and depressive disorder along with the MesH terms adult or adolescent. We refined our search results using the MeSH terms diagnosis, comorbidity, physician-patient relations, patient satisfaction, and patient acceptance of health care to focus on patient and physician factors.
To search for factors related to practice setting, we refined our initial search strategy with the MeSH terms primary health care, family practice, and health services. We also hand-checked bibliographies to find potential articles.
Study selection and data extraction
Two investigators (AKD, MO) independently reviewed abstracts of the articles retrieved and excluded articles that did not fulfill the criteria to be relevant to the topic of depression in African American patients. Articles were excluded if they did not focus on US populations, African Americans, depressive disorders, and clinical settings, or if they did not report original data. Differences between the 2 reviewers were resolved through consensus after full-text review of the article.
Additional articles were defined by 2 other investigators (HLC, MMW) who provided peer review and identified potential articles in bibliographies. For each article, we classified the barriers described as related to diagnosis, management, or both and to patient, physician, and treatment-setting factors.
Results
The initial search using the MeSH terms African Americans and depressive disorder with adult or adolescent returned 156 articles. We found a total of 73 unique articles by refining our Medline search with additional MeSH terms. Of these publications, 12 fulfilled our criteria as relevant to the topic of diagnosing and managing depression in African American patients.
Our hand-checking of bibliographic references found an additional 12 articles that met our relevancy criteria. We examined each of the 24 articles (TABLE 1) to determine the types of barrier studies and whether the factor was related to the detection of depression, its management, or both.
TABLE 1
Selected studies on managing depression in African Americans
| AUTHOR | SAMPLE (% AFRICAN AMERICAN) | STUDY OUTCOMES | MAJOR FINDINGS |
|---|---|---|---|
| Borowsky et al3 | 19,309 adult primary care patients (13%) | Detection of a mental health problem by internists and family physicians | African American patients who have major depression have a lower rate of being detected than their white counterparts |
| Brown et al28 | 272 depressed adult primary care patients (44%) | Differences in clinical characteristics, health impairments, health beliefs, stressful life events, and social support between African American and white subjects enrolled in a clinical trial of depression | In comparison to white persons, African Americans are more likely to experience somatization, greater health impairment, greater perception of less control of their health status and greater number of stressful life events in the previous 6 months |
| Brown et al29 | 160 depressed adult primary care patients (43%) | Differences in treatment outcomes from a clinical trial of standardized psychotherapy and pharmacotherapy | Both African American and white patients are effectively treated by psychotherapy and antidepressant medications, but African American subjects have poorer outcome in physical functioning |
| Brown et al30 | 865 adult African American persons from a community sample (100%) | Rate of major depression compared with demographic, sociocultural, familial background, and health-related risk factors | The strongest predictors of major depression are self-reported poor or fair physical health and being 20 to 29 years of age |
| Cooper et al31 | 829 depressed adult primary care patients (12%) | Attitudinal measures of acceptability of antidepressant medication and individual counseling | African Americans are less likely than white persons to find antidepressant medication acceptable |
| Cooper-Patrick et al23 | 1816 adult primary care patients (45%) | Patients’ ratings of physicians participatory decision making style | African American patients rate their visits as less participatory than their white counterparts and race-concordant patient-physician relationships are more participatory than race-discordant relationships |
| Cooper-Patrick et al32 | Focus group consisting of 16 patients with a recent episode of depression (50%) | 16 categories of comments regarding treatment of depression were identified from audiotaped discussions | African American patients provided more comments about spirituality and stigma and were less concerned with the relation between physical health and depression than white patients |
| Dunlop et al5 | 7690 subjects aged 54 to 65 years from national probability sample (17%) | Rates of major depression with socio demographic characteristics, self-reported health needs, functional limitations, and economic resources as explanatory variables | African Americans have a greater rate of major depression compared to whites, but have a significantly lower rate after adjustments for health needs (such as comorbid physical problems) and economic access (such as income and health insurance) |
| Fabrega et al33 | 5198 adult community mental health center patients (16%) | Rates of major depression and comorbid psychiatric diagnoses | African Americans have a higher rate of comorbid major depression and substance-use disorders than their white counterparts |
| Ford et al34 | Community-based sample of 7092 adults who had who had a mental health or substance abuse problem and received care in nonpsychiatric settings (29%) | Patient self-reported discussion of a mental health problem or problem with alcohol or drugs | No significance differences between the rates of African Americans and whites discussed emotional or mental health problems with their physicians |
| Grant et al35 | National probability sample of adult non-institutionalized US population (NR) | Rates of major depression and alcohol use disorders stratified by demographic variables | The association between alcohol abuse and major depression was greater among African Americans than among non–African Americans |
| Hanson et al36 | 60 adult patients undergoing psychiatric evaluation (50%) | Congruence between physicians’ and patients’ ratings of depression during an intake assessment | Concordance of symptoms ratings is lower between white psychiatrists and African American patients than with white psychiatrists and white patients |
| McKinlay et al37 | 128 primary care physicians (25%) | Diagnostic assessment of videotaped patient presentations of depression and another medical condition | Demographic attributes of patients did not affect diagnostic accuracy, and white physicians were almost twice as likely as their African American counterparts to diagnose depression correctly |
| Miranda et al38 | 205 adult public-sector gynecologic patients (30%) | Rates of psychiatric disorders along with extent of primary care services and perception of communication with primary care provider | In a largely minority (82%) patient population (with no reported differences among racial/ethnic groups), 1 out of 5 patients endorsed major depression and fewer than half had access to comprehensive primary medical services |
| Miranda et al39 | 1269 adult primary care patients (7%) | Clinical outcome, appropriateness of care, and employment for patients enrolled into a clinical trial of 2 quality improvement interventions with modest adaptation to minority patients | African American patients, in contrast to their white counterparts, experienced a significant decrease in probable depression from baseline |
| O’Malley et al40 | Population-based sample of 1202 women (83%) | Rates of care of depression, extent of primary care services, quality of physician-patient relationship | In a largely African American sample (with no reported differences between racial/ethnic groups), comprehensive primary care delivery (including counseling) and self-reported view of physician as respectful are associated with physician inquiry and treatment of depression |
| Rollman et al41 | 204 adult primary care patients (25%) | Processes of depression care and patient outcome following screening and treatment recommendations to physicians | No differences in treatment patterns and outcomes were found between white and African American patients, except for increased rate of documented counseling by physicians among whites |
| Sleath et al42 | 508 adult outpatients (NR) | Patient expression of symptoms from audiotaped medical encounter and physician perception of health from survey | Among all factors, only patient expression of emotional symptoms among African American patients influenced psychotropic prescribing |
| Steffens et al43 | 113 patients age 60 years or older (11.5%) | Annual rate of enrollment into a geriatric depression research program based on race and ethnicity | Active community outreach and enrollment increased representation of African Americans by threefold over a 2-year period |
| Sussman et al1 | 3004 adult subjects from a community-based sample (38%) | Treatment seeking behavior for subjects who have current depression | African Americans are less likely to seek care for depression than whites, with African Americans reporting greater fear of treatment and of being hospitalized than whites |
| Van Hook44 | 321 adult women in primary care settings (22%) | Rate of major depression and reported barriers to treatment | In a multi-ethnic sample (with no reported differences among racial/ethnic groups), commonly reported barriers to seeking help included stigma and perceived separation of primary care and specialty mental health services |
| Wells et al45 | 9585 adult national survey respondents (28%) | Access to treatment for alcoholism, drug abuse and, mental health care; unmet need for care; satisfaction with care and use of active treatment in the prior year | Among those with perceived need for mental health services, African Americans were more likely to report no access to care than whites and less likely to receive active treatment |
| Wohl et al46 | 20 matched adult depressed African American and white patients (50%) | Symptom pattern and severity of depression | No difference in symptom severity was found between African Americans and whites, and African Americans were more likely to experience diurnal variation in their depression |
Discussion
Barriers in evaluation
Research on depression in African Americans can be analyzed in terms of attributes of the provider, patient, and practice setting.
Provider attributes. The race of the physician has been linked to the diagnosis of depression in African-American patients, but studies of this factor have yielded conflicting results. McKinlay and colleagues used videotaped cases of depressed patients presented to 128 physicians to examine whether diagnosis was affected by physician characteristics (age, gender race, and medical specialty), patient characteristics (age, gender, race, and socioeconomic status), or their combination.37 They found that physician characteristics affected the diagnosis of depression, but that patient attributes did not. In particular, white primary care physicians were twice as likely as African American physicians to diagnose depression. Since approximately 1 in 5 African American patients seeks care from a physician of their own race,47 such differences may influence the overall rate of recognition in primary care. However, data from the Medical Outcome Study, which included demographic characteristics on 349 primary care physicians, did not find that the race of the physician influenced the detection of depression.3
In addition to physician demographic factors, physician communication style may affect ability to diagnose depression. Appropriate diagnosis and treatment of depression depends to a great extent on verbal communications between patient and provider about the nature, extent, severity, and consequences of symptoms. Problems with communication may lead to misunderstandings, misdiagnosis, inappropriate treatments, and premature termination of treatment. An analysis of patient-physician encounters indicates that physicians may be more likely to minimize emotional symptoms of African American than of whites.42 Relative to whites, African Americans are more likely to rate their visits with white physicians as less participatory.
This difference is overcome when African Americans see physicians of their own race.23 In a population-based study among largely low-income African American women, primary care physicians who were rated as showing more respect by the participants were more likely to inquire about depressive symptoms during a clinical visit.40 Thus, despite the fact that African American patients rate their encounters more satisfying and are more likely to disclose their problems to African American physicians, white physicians appear to diagnose depression more commonly in their African American patients. Clearly, more work is needed to clarify this seeming contradiction.
Patient attributes. During the primary care encounter, African Americans are as likely as whites to discuss mental health problems.34 However, African Americans may be more likely to exhibit somatic and neurovegetative symptoms of depression than mood or cognitive symptoms,33,36 which may complicate detection and diagnosis.
A recent national survey suggests that depression in African Americans may be commonly masked by self medication and somatic symptoms and so may pass undetected in primary care.48 In a randomized clinical trial of depression treatment in primary care, depressed African American subjects were more likely than white subjects to have symptoms of poor physical health and pain and to have somatization.29 However, another study found few differences between whites and African Americans in symptom presentation of depression when comorbid disorders and sociodemographic factors were controlled.46
Do primarily somatic presentations of depression reduce physicians’ ability to accurately diagnose major depression in racial and ethnic minorities? This question has not been studied, though coexisting medical problems in primary care populations have been found to impede the diagnosis of depression,49 presumably by competing for the physician’s attention.3,50 In a variety of medical contexts, known medical disorders are associated with under-treatment of unrelated disorders.51
Practice-setting attributes. No studies have examined whether specific practice setting factors, such as insurance coverage, are related to the low detection rate of major depression. Among low-income women of multiethnic backgrounds attending primary care clinics, Van Hook found that perceived separation of primary care services and specialty mental health care was a self-reported barrier to seeking help for depression.44
The Surgeon General’s report documents the overall poor access to general medical services faced by African Americans. They are more likely to receive health care in outpatient hospital and emergency departments, and their mental health services are also characterized by high rates of emergency care. As a result, they are less likely to receive the continuity of treatment provided in primary care, which may allow better detection of depression.15
Barriers to effective management
Research on the potential causes of disparities in treatment for African American patients in primary care has examined a variety of provider, patient, and practice-setting factors.
Provider attributes. Physician communication style may not only influence diagnosis but also a decision to treat. In a study that analyzed audiotaped communication between patients and primary care providers during clinic visits, the tendency of physicians to minimize emotional expression by African Americans, relative to whites, led to lower prescribing of antidepressant medication among African Americans.42 Further research is needed to understand why such communication styles exist between primary care providers and African American patients.
Patient attributes. A number of patient attributes have been examined that may explain the lower rate of mental health services provided in primary care. Significant research has been undertaken on African American attitudes toward and beliefs about mental health treatments. Cooper-Patrick and colleagues conducted focus groups for African American and white patients as well as health care professionals. In questions related to depression and treatment preferences, African American patients expressed more concerns about stigma and spirituality than did white patients.32
In related research, a survey of African American patients recruited from primary care offices indicated they were less likely to find antidepressant medication acceptable than white patients in primary care.31 Such attitudinal differences may explain why African Americans use antidepressants less commonly than whites, even when primary care physicians make similar recommendations for both groups.52 As yet unstudied is the extent to which sensational reports about antidepressant side effects may cause persons already skeptical about the care they receive to discontinue treatment.
Another reason African Americans may avoid or discontinue antidepressant treatment is that they tend to tolerate certain classes of psychotropic medications poorly. Strickland and colleagues found that African Americans are more likely than whites to be “poor metabolizers” of tricyclic antidepressants.53 African Americans treated with tricyclic antidepressants will therefore experience higher plasma levels per dose than whites, and an earlier onset of action. African Americans are also more likely to experience side effects, which may lead to treatment nonadherence.
Few studies have examined the tolerability of newer class of antidepressant medications, such as selective serotonin reuptake inhibitors (SSRIs), for African Americans. More research is required to determine whether antidepressant side effects or other experiences with psychotropic medications are a reason for the lower rates of antidepressant use among African American patients in primary care. Educating patients about antidepressants, their onset of action, and side effects may reduce some of these barriers and prevent early discontinuation of antidepressant therapy.54
Poverty and its associated psychosocial factors may also contribute to the lower quality of mental health care among African Americans. Miranda and colleagues found this to be so for African American women receiving primary care in obstetric-gynecologic clinics.47 They have argued that clinical case management is needed as a component of effective mental health treatment for primary care patients who are poor and likely to face significant negative life events.
Practice-setting attributes. Few practice-setting factors have been examined in relationship to the disparities in depression treatment faced by African Americans. O’Malley and colleagues examined whether primary care physicians who were evaluated as having comprehensive medical services by low-income African American women were more likely to provide treatment for depression.40 Comprehensiveness was determined by the ability to meet all health needs, thoroughness of physical exam, and provision of counseling and screening services. In a survey study, they found that comprehensiveness of medical services was correlated to being asked about and being treated for depression.
Interventions in primary care
Concerted efforts to improve quality of care can reduce the mental health burden of undetected depression for African Americans as well as other ethnic and racial groups. African American and white primary care patients both appear to respond equally to standardized psychotherapy and pharmacotherapy for major depression.29
In a randomized control trial to improve the quality of depression management in primary care, no significant differences existed between white and African American participants in the process of depression care or clinical outcomes, although both groups had less than optimal recovery rates.41 One randomized clinical trial included modified interventions to target the mental health needs of low-income minority primary care patients. The interventions included educating clinicians about depression; teaching nurses to educate, assess, and follow-up depressed patients; and making cognitive behavioral therapy available. Patients and physicians selected the treatment. Modification for minority patients was modest and included translations and cultural training for clinicians.
Using these approaches, African American participants were more likely than whites to have better depression outcome at 6 and 12 months.39 These studies indicate that both general interventions to depression care and small modifications for minority patients can lead to improved health outcomes among African American patients.
It may be unreasonable to believe that simply seeking to improve the primary care assessment and treatment of depression in African Americans will eliminate racial differences in the health outcomes of depressed adults. Reform is also clearly needed in health care financing and in broader social welfare policy as it affects the lives of depressed minority populations. However, primary care providers who are aware of the risk of racial disparities in the recognition and treatment of depression and work to provide treatments that are tailored to the individual’s needs can help to reduce the significant burden of depression (TABLE 2).
TABLE 2
Recommendations for addressing barriers to the detection and treatment of depression in the African American patient
| IMPROVING DIAGNOSIS |
| Assess stigma toward mental health problems for patients suspected to have depression |
| Inquire about patient’s experience of somatic symptoms and their relationship to depression, life stressors, and social conflicts |
| Maintain a respectful, open stance in understanding patient’s style of coping with depressive symptoms, including use of spirituality |
| Evaluate the presence of comorbid mental health problems, such as alcohol abuse, that require different treatment approaches |
| PROVIDING EFFECTIVE MANAGEMENT |
| Determine the patient’s preferences for psychotherapy and pharmacotherapy, and provide treatment referrals for counseling if appropriate and as resources permit |
| Educate patients about antidepressant medications, their onset of action, and side effects |
| At each visit after initiation of depression management, check for regular adherence to pharmacotherapy or to referred psychotherapy |
| Assist patients who cannot maintain regular visits for depression care to find strategies that can overcome social or financial barriers |
Acknowledgments
This review was funded by the Columbia Center for the Health of Urban Minorities (NCMHHD MD000206-019006) (Drs. Olfson and Weissman), an unrestricted grant from Eli Lilly & Company (Dr. Weissman) and a NIMH National Service Research Award Institutional Research Training Grant 5T32MH015144 (Dr. Das).
CORRESPONDENCE
Myrna M. Weissman, Ph.D. Columbia University College of Physicians and Surgeons, 1051 Riverside Drive, New York, NY 10032. E-mail: [email protected]
- When evaluating African Americans for depression, look for somatic and neurovegetative symptoms rather than mood or cognitive symptoms (C).
- Education can help patients better tolerate drug regimens and improve chances for treatment success (C).
- Practice improvement efforts can improve health outcomes in depressed African Americans (B).
African Americans depend on quality mental health services in primary care more so than whites, since they are more likely to seek care in this setting. However, accumulating evidence shows that African Americans in primary care settings face disparity in recognition and treatment of depression (see Race and mental health treatment: the divide).1-5 Until now, the specific factors leading to this disparity have not been well described.16
In this article—a systematic review of studies on the evaluation and treatment of depression in African American patients—we draw attention to factors regarding patients, physicians, and clinical settings that raise barriers to diagnosis and treatment. We also recommend strategies to break through these barriers.
Racial disparity in mental health treatment is a difference across racial groups not justified by underlying differences in mental health status or patient treatment preferences. The reasons are numerous. Compared with whites, for example, African Americans are more likely to be poor, uninsured, or have restrictive insurance policies,6,7 and to have limited means of transportation to reach health services.8 But even after controlling for differences in insurance and socioeconomic status, African Americans are less likely than whites to use outpatient mental health services,9,10 and they therefore face disparities in quality of care.11,12 Thus, economic factors and illness severity do not fully account for the observed racial differences in the rate and quality of treatment of mental disorders.
Racial disparities may also emerge in the decisions physicians make in caring for patients. In several clinical contexts, members of minorities have received less and inferior care than white patients.13,14 In one epidemiologic study, African Americans were reported to receive less access to mental health care and to have greater unmet needs for mental health care than whites.11 Because differences in access to care can have consequences for health outcomes, reducing health care disparities is a widely shared goal of clinical and public health care policy.15
Assumptions that mask disparities
Disparities may not be apparent to healthcare providers in part because community-based epidemiologic studies report that African Americans have a lower rate of major depression than whites.5,17-19 In primary care settings specifically, the prevalence of major depression among African Americans appears to be lower than for white or Hispanic Americans, mirroring rates in national and community studies.20 Explanations for this relatively low prevalence of depression have included attributes of the African American experience, such as strong religious beliefs and community ties.
Moreover, according to analyses of data from the National Comorbidity Study, which included an examination of attitudes toward mental health services, African Americans with major depression are more likely to report that they would “definitely go” for mental health services than would whites.21
High-risk populations overlooked. Community-based studies, however, do not fully capture the mental health needs of African Americans, a large number of whom are in high-risk populations excluded from community epidemiologic studies of mental disorders. Studies of these populations show high rates of major depression and other mental disorders, due to factors including alcohol and substance use disorders, poor physical health, and poverty and homelessness.
An unsettling irony. For emotional distress, African Americans are more likely to seek help from primary care clinicians than from specialty mental health providers.22-24 However, in primary care, depression in African Americans is less likely to be detected than it is in whites.3
In a nationally representative sample, African Americans have been found less likely than whites to receive effective care for depression in primary care.12,25,26 In a study of Medicaid recipients who had been diagnosed with major depression, African Americans less often received antidepressant medications than did whites.27 Such disparities in pharmacologic treatments were also found in another study of African American patients in primary care, using data from the National Ambulatory Medical Care Surveys.24
Search methods
Data sources
We systematically searched for and retrieved articles in Medline (January 1966 to December 2004) using the MeSH (medical subject heading) terms African Americans and depressive disorder along with the MesH terms adult or adolescent. We refined our search results using the MeSH terms diagnosis, comorbidity, physician-patient relations, patient satisfaction, and patient acceptance of health care to focus on patient and physician factors.
To search for factors related to practice setting, we refined our initial search strategy with the MeSH terms primary health care, family practice, and health services. We also hand-checked bibliographies to find potential articles.
Study selection and data extraction
Two investigators (AKD, MO) independently reviewed abstracts of the articles retrieved and excluded articles that did not fulfill the criteria to be relevant to the topic of depression in African American patients. Articles were excluded if they did not focus on US populations, African Americans, depressive disorders, and clinical settings, or if they did not report original data. Differences between the 2 reviewers were resolved through consensus after full-text review of the article.
Additional articles were defined by 2 other investigators (HLC, MMW) who provided peer review and identified potential articles in bibliographies. For each article, we classified the barriers described as related to diagnosis, management, or both and to patient, physician, and treatment-setting factors.
Results
The initial search using the MeSH terms African Americans and depressive disorder with adult or adolescent returned 156 articles. We found a total of 73 unique articles by refining our Medline search with additional MeSH terms. Of these publications, 12 fulfilled our criteria as relevant to the topic of diagnosing and managing depression in African American patients.
Our hand-checking of bibliographic references found an additional 12 articles that met our relevancy criteria. We examined each of the 24 articles (TABLE 1) to determine the types of barrier studies and whether the factor was related to the detection of depression, its management, or both.
TABLE 1
Selected studies on managing depression in African Americans
| AUTHOR | SAMPLE (% AFRICAN AMERICAN) | STUDY OUTCOMES | MAJOR FINDINGS |
|---|---|---|---|
| Borowsky et al3 | 19,309 adult primary care patients (13%) | Detection of a mental health problem by internists and family physicians | African American patients who have major depression have a lower rate of being detected than their white counterparts |
| Brown et al28 | 272 depressed adult primary care patients (44%) | Differences in clinical characteristics, health impairments, health beliefs, stressful life events, and social support between African American and white subjects enrolled in a clinical trial of depression | In comparison to white persons, African Americans are more likely to experience somatization, greater health impairment, greater perception of less control of their health status and greater number of stressful life events in the previous 6 months |
| Brown et al29 | 160 depressed adult primary care patients (43%) | Differences in treatment outcomes from a clinical trial of standardized psychotherapy and pharmacotherapy | Both African American and white patients are effectively treated by psychotherapy and antidepressant medications, but African American subjects have poorer outcome in physical functioning |
| Brown et al30 | 865 adult African American persons from a community sample (100%) | Rate of major depression compared with demographic, sociocultural, familial background, and health-related risk factors | The strongest predictors of major depression are self-reported poor or fair physical health and being 20 to 29 years of age |
| Cooper et al31 | 829 depressed adult primary care patients (12%) | Attitudinal measures of acceptability of antidepressant medication and individual counseling | African Americans are less likely than white persons to find antidepressant medication acceptable |
| Cooper-Patrick et al23 | 1816 adult primary care patients (45%) | Patients’ ratings of physicians participatory decision making style | African American patients rate their visits as less participatory than their white counterparts and race-concordant patient-physician relationships are more participatory than race-discordant relationships |
| Cooper-Patrick et al32 | Focus group consisting of 16 patients with a recent episode of depression (50%) | 16 categories of comments regarding treatment of depression were identified from audiotaped discussions | African American patients provided more comments about spirituality and stigma and were less concerned with the relation between physical health and depression than white patients |
| Dunlop et al5 | 7690 subjects aged 54 to 65 years from national probability sample (17%) | Rates of major depression with socio demographic characteristics, self-reported health needs, functional limitations, and economic resources as explanatory variables | African Americans have a greater rate of major depression compared to whites, but have a significantly lower rate after adjustments for health needs (such as comorbid physical problems) and economic access (such as income and health insurance) |
| Fabrega et al33 | 5198 adult community mental health center patients (16%) | Rates of major depression and comorbid psychiatric diagnoses | African Americans have a higher rate of comorbid major depression and substance-use disorders than their white counterparts |
| Ford et al34 | Community-based sample of 7092 adults who had who had a mental health or substance abuse problem and received care in nonpsychiatric settings (29%) | Patient self-reported discussion of a mental health problem or problem with alcohol or drugs | No significance differences between the rates of African Americans and whites discussed emotional or mental health problems with their physicians |
| Grant et al35 | National probability sample of adult non-institutionalized US population (NR) | Rates of major depression and alcohol use disorders stratified by demographic variables | The association between alcohol abuse and major depression was greater among African Americans than among non–African Americans |
| Hanson et al36 | 60 adult patients undergoing psychiatric evaluation (50%) | Congruence between physicians’ and patients’ ratings of depression during an intake assessment | Concordance of symptoms ratings is lower between white psychiatrists and African American patients than with white psychiatrists and white patients |
| McKinlay et al37 | 128 primary care physicians (25%) | Diagnostic assessment of videotaped patient presentations of depression and another medical condition | Demographic attributes of patients did not affect diagnostic accuracy, and white physicians were almost twice as likely as their African American counterparts to diagnose depression correctly |
| Miranda et al38 | 205 adult public-sector gynecologic patients (30%) | Rates of psychiatric disorders along with extent of primary care services and perception of communication with primary care provider | In a largely minority (82%) patient population (with no reported differences among racial/ethnic groups), 1 out of 5 patients endorsed major depression and fewer than half had access to comprehensive primary medical services |
| Miranda et al39 | 1269 adult primary care patients (7%) | Clinical outcome, appropriateness of care, and employment for patients enrolled into a clinical trial of 2 quality improvement interventions with modest adaptation to minority patients | African American patients, in contrast to their white counterparts, experienced a significant decrease in probable depression from baseline |
| O’Malley et al40 | Population-based sample of 1202 women (83%) | Rates of care of depression, extent of primary care services, quality of physician-patient relationship | In a largely African American sample (with no reported differences between racial/ethnic groups), comprehensive primary care delivery (including counseling) and self-reported view of physician as respectful are associated with physician inquiry and treatment of depression |
| Rollman et al41 | 204 adult primary care patients (25%) | Processes of depression care and patient outcome following screening and treatment recommendations to physicians | No differences in treatment patterns and outcomes were found between white and African American patients, except for increased rate of documented counseling by physicians among whites |
| Sleath et al42 | 508 adult outpatients (NR) | Patient expression of symptoms from audiotaped medical encounter and physician perception of health from survey | Among all factors, only patient expression of emotional symptoms among African American patients influenced psychotropic prescribing |
| Steffens et al43 | 113 patients age 60 years or older (11.5%) | Annual rate of enrollment into a geriatric depression research program based on race and ethnicity | Active community outreach and enrollment increased representation of African Americans by threefold over a 2-year period |
| Sussman et al1 | 3004 adult subjects from a community-based sample (38%) | Treatment seeking behavior for subjects who have current depression | African Americans are less likely to seek care for depression than whites, with African Americans reporting greater fear of treatment and of being hospitalized than whites |
| Van Hook44 | 321 adult women in primary care settings (22%) | Rate of major depression and reported barriers to treatment | In a multi-ethnic sample (with no reported differences among racial/ethnic groups), commonly reported barriers to seeking help included stigma and perceived separation of primary care and specialty mental health services |
| Wells et al45 | 9585 adult national survey respondents (28%) | Access to treatment for alcoholism, drug abuse and, mental health care; unmet need for care; satisfaction with care and use of active treatment in the prior year | Among those with perceived need for mental health services, African Americans were more likely to report no access to care than whites and less likely to receive active treatment |
| Wohl et al46 | 20 matched adult depressed African American and white patients (50%) | Symptom pattern and severity of depression | No difference in symptom severity was found between African Americans and whites, and African Americans were more likely to experience diurnal variation in their depression |
Discussion
Barriers in evaluation
Research on depression in African Americans can be analyzed in terms of attributes of the provider, patient, and practice setting.
Provider attributes. The race of the physician has been linked to the diagnosis of depression in African-American patients, but studies of this factor have yielded conflicting results. McKinlay and colleagues used videotaped cases of depressed patients presented to 128 physicians to examine whether diagnosis was affected by physician characteristics (age, gender race, and medical specialty), patient characteristics (age, gender, race, and socioeconomic status), or their combination.37 They found that physician characteristics affected the diagnosis of depression, but that patient attributes did not. In particular, white primary care physicians were twice as likely as African American physicians to diagnose depression. Since approximately 1 in 5 African American patients seeks care from a physician of their own race,47 such differences may influence the overall rate of recognition in primary care. However, data from the Medical Outcome Study, which included demographic characteristics on 349 primary care physicians, did not find that the race of the physician influenced the detection of depression.3
In addition to physician demographic factors, physician communication style may affect ability to diagnose depression. Appropriate diagnosis and treatment of depression depends to a great extent on verbal communications between patient and provider about the nature, extent, severity, and consequences of symptoms. Problems with communication may lead to misunderstandings, misdiagnosis, inappropriate treatments, and premature termination of treatment. An analysis of patient-physician encounters indicates that physicians may be more likely to minimize emotional symptoms of African American than of whites.42 Relative to whites, African Americans are more likely to rate their visits with white physicians as less participatory.
This difference is overcome when African Americans see physicians of their own race.23 In a population-based study among largely low-income African American women, primary care physicians who were rated as showing more respect by the participants were more likely to inquire about depressive symptoms during a clinical visit.40 Thus, despite the fact that African American patients rate their encounters more satisfying and are more likely to disclose their problems to African American physicians, white physicians appear to diagnose depression more commonly in their African American patients. Clearly, more work is needed to clarify this seeming contradiction.
Patient attributes. During the primary care encounter, African Americans are as likely as whites to discuss mental health problems.34 However, African Americans may be more likely to exhibit somatic and neurovegetative symptoms of depression than mood or cognitive symptoms,33,36 which may complicate detection and diagnosis.
A recent national survey suggests that depression in African Americans may be commonly masked by self medication and somatic symptoms and so may pass undetected in primary care.48 In a randomized clinical trial of depression treatment in primary care, depressed African American subjects were more likely than white subjects to have symptoms of poor physical health and pain and to have somatization.29 However, another study found few differences between whites and African Americans in symptom presentation of depression when comorbid disorders and sociodemographic factors were controlled.46
Do primarily somatic presentations of depression reduce physicians’ ability to accurately diagnose major depression in racial and ethnic minorities? This question has not been studied, though coexisting medical problems in primary care populations have been found to impede the diagnosis of depression,49 presumably by competing for the physician’s attention.3,50 In a variety of medical contexts, known medical disorders are associated with under-treatment of unrelated disorders.51
Practice-setting attributes. No studies have examined whether specific practice setting factors, such as insurance coverage, are related to the low detection rate of major depression. Among low-income women of multiethnic backgrounds attending primary care clinics, Van Hook found that perceived separation of primary care services and specialty mental health care was a self-reported barrier to seeking help for depression.44
The Surgeon General’s report documents the overall poor access to general medical services faced by African Americans. They are more likely to receive health care in outpatient hospital and emergency departments, and their mental health services are also characterized by high rates of emergency care. As a result, they are less likely to receive the continuity of treatment provided in primary care, which may allow better detection of depression.15
Barriers to effective management
Research on the potential causes of disparities in treatment for African American patients in primary care has examined a variety of provider, patient, and practice-setting factors.
Provider attributes. Physician communication style may not only influence diagnosis but also a decision to treat. In a study that analyzed audiotaped communication between patients and primary care providers during clinic visits, the tendency of physicians to minimize emotional expression by African Americans, relative to whites, led to lower prescribing of antidepressant medication among African Americans.42 Further research is needed to understand why such communication styles exist between primary care providers and African American patients.
Patient attributes. A number of patient attributes have been examined that may explain the lower rate of mental health services provided in primary care. Significant research has been undertaken on African American attitudes toward and beliefs about mental health treatments. Cooper-Patrick and colleagues conducted focus groups for African American and white patients as well as health care professionals. In questions related to depression and treatment preferences, African American patients expressed more concerns about stigma and spirituality than did white patients.32
In related research, a survey of African American patients recruited from primary care offices indicated they were less likely to find antidepressant medication acceptable than white patients in primary care.31 Such attitudinal differences may explain why African Americans use antidepressants less commonly than whites, even when primary care physicians make similar recommendations for both groups.52 As yet unstudied is the extent to which sensational reports about antidepressant side effects may cause persons already skeptical about the care they receive to discontinue treatment.
Another reason African Americans may avoid or discontinue antidepressant treatment is that they tend to tolerate certain classes of psychotropic medications poorly. Strickland and colleagues found that African Americans are more likely than whites to be “poor metabolizers” of tricyclic antidepressants.53 African Americans treated with tricyclic antidepressants will therefore experience higher plasma levels per dose than whites, and an earlier onset of action. African Americans are also more likely to experience side effects, which may lead to treatment nonadherence.
Few studies have examined the tolerability of newer class of antidepressant medications, such as selective serotonin reuptake inhibitors (SSRIs), for African Americans. More research is required to determine whether antidepressant side effects or other experiences with psychotropic medications are a reason for the lower rates of antidepressant use among African American patients in primary care. Educating patients about antidepressants, their onset of action, and side effects may reduce some of these barriers and prevent early discontinuation of antidepressant therapy.54
Poverty and its associated psychosocial factors may also contribute to the lower quality of mental health care among African Americans. Miranda and colleagues found this to be so for African American women receiving primary care in obstetric-gynecologic clinics.47 They have argued that clinical case management is needed as a component of effective mental health treatment for primary care patients who are poor and likely to face significant negative life events.
Practice-setting attributes. Few practice-setting factors have been examined in relationship to the disparities in depression treatment faced by African Americans. O’Malley and colleagues examined whether primary care physicians who were evaluated as having comprehensive medical services by low-income African American women were more likely to provide treatment for depression.40 Comprehensiveness was determined by the ability to meet all health needs, thoroughness of physical exam, and provision of counseling and screening services. In a survey study, they found that comprehensiveness of medical services was correlated to being asked about and being treated for depression.
Interventions in primary care
Concerted efforts to improve quality of care can reduce the mental health burden of undetected depression for African Americans as well as other ethnic and racial groups. African American and white primary care patients both appear to respond equally to standardized psychotherapy and pharmacotherapy for major depression.29
In a randomized control trial to improve the quality of depression management in primary care, no significant differences existed between white and African American participants in the process of depression care or clinical outcomes, although both groups had less than optimal recovery rates.41 One randomized clinical trial included modified interventions to target the mental health needs of low-income minority primary care patients. The interventions included educating clinicians about depression; teaching nurses to educate, assess, and follow-up depressed patients; and making cognitive behavioral therapy available. Patients and physicians selected the treatment. Modification for minority patients was modest and included translations and cultural training for clinicians.
Using these approaches, African American participants were more likely than whites to have better depression outcome at 6 and 12 months.39 These studies indicate that both general interventions to depression care and small modifications for minority patients can lead to improved health outcomes among African American patients.
It may be unreasonable to believe that simply seeking to improve the primary care assessment and treatment of depression in African Americans will eliminate racial differences in the health outcomes of depressed adults. Reform is also clearly needed in health care financing and in broader social welfare policy as it affects the lives of depressed minority populations. However, primary care providers who are aware of the risk of racial disparities in the recognition and treatment of depression and work to provide treatments that are tailored to the individual’s needs can help to reduce the significant burden of depression (TABLE 2).
TABLE 2
Recommendations for addressing barriers to the detection and treatment of depression in the African American patient
| IMPROVING DIAGNOSIS |
| Assess stigma toward mental health problems for patients suspected to have depression |
| Inquire about patient’s experience of somatic symptoms and their relationship to depression, life stressors, and social conflicts |
| Maintain a respectful, open stance in understanding patient’s style of coping with depressive symptoms, including use of spirituality |
| Evaluate the presence of comorbid mental health problems, such as alcohol abuse, that require different treatment approaches |
| PROVIDING EFFECTIVE MANAGEMENT |
| Determine the patient’s preferences for psychotherapy and pharmacotherapy, and provide treatment referrals for counseling if appropriate and as resources permit |
| Educate patients about antidepressant medications, their onset of action, and side effects |
| At each visit after initiation of depression management, check for regular adherence to pharmacotherapy or to referred psychotherapy |
| Assist patients who cannot maintain regular visits for depression care to find strategies that can overcome social or financial barriers |
Acknowledgments
This review was funded by the Columbia Center for the Health of Urban Minorities (NCMHHD MD000206-019006) (Drs. Olfson and Weissman), an unrestricted grant from Eli Lilly & Company (Dr. Weissman) and a NIMH National Service Research Award Institutional Research Training Grant 5T32MH015144 (Dr. Das).
CORRESPONDENCE
Myrna M. Weissman, Ph.D. Columbia University College of Physicians and Surgeons, 1051 Riverside Drive, New York, NY 10032. E-mail: [email protected]
1. Sussman LK, Robins LN, Earls F. Treatment-seeking for depression by black and white Americans. Soc Sci Med 1987;24:187-196.
2. Vega WA, Kolody B, Aguilar-Gaxiola S, Catalano R. Gaps in service utilization by Mexican Americans with mental health problems. Am J Psychiatry 1999;156:928-934.
3. Borowsky SJ, Rubenstein LV, Meredith LS, Camp P, Jackson-Triche M, Wells KB. Who is at risk of nondetection of mental health problems in primary care? J Gen Intern Med 2000;15:381-388.
4. Fiscella K, Franks P, Doescher MP, Saver BG. Disparities in health care by race, ethnicity and language among the insured: findings from a national sample. Med Care 2002;40:52-59.
5. Dunlop DD, Song J, Lyons JS, Mannheim LM, Chang RW. Racial/ethnic differences in rates of depression among pre-retirement adults. Am J Public Health 2003;93:1945-1952.
6. Phillips KA, Mayer ML, Aday LA. Barriers to care among racial ethnic groups under managed care. Health Aff 2000;19:65-75.
7. Tai-Seale M, Freund D, LoSasso A. Racial disparities in service use among Medicaid beneficiaries after mandatory enrollment in managed care: a difference-in-differences approach. Inquiry 2001;38:49-59.
8. Heckman TG, Somlai AM, Peters J, et al. Barriers to care among persons living with HIV/AIDS in urban and rural areas. AIDS Care 1998;10:365-375.
9. Padgett DK, Patrick C, Burns BJ, Schlesinger HJ. Ethnicity and the use of outpatient mental health services in a national insured population. Am J Public Health 1994;84:222-226.
10. Swartz MS, Wagner HR, Swanson JW, Bruns BJ, Keorge LK, Padgett DK. Comparing use of public and private mental health services; the enduring barriers of race and age. Commun Ment Health J 1998;34:133-144.
11. Wells K, Klap R, Koike A, Sherbourne C. Ethnic disparities in unmet need for alcoholism, drug abuse and mental health care. Am J Psychiatry 2001;158:2027-2032.
12. Wang PS, Berglund P, Kessler RC. Recent care of common mental disorders in the United States. J Gen Intern Med 2000;15:284-292.
13. Bach P, Cramer L, Warren L, Begg C. Racial differences in the treatment of early-stage lung cancer. New Engl J Med 1999;341:1198-1205.
14. Peterson ED, Shaw L, DeLong E, Pryor D, Califf R, Mark D. Racial variation in the use of coronary-revascularization procedures—are the differences real? Do they matter? N Engl J Med 1997;336:480-486.
15. US Department of Health and Human Services. Mental Health: Culture Race, and Ethnicity: A Supplement to Mental Health: A Report of the Surgeon General. Rockville, Md: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, 2001.
16. Areán PA, Alvidrez J. Treating depressive disorders: who responds who does not respond, and who do we need to study? J Fam Pract 2001;50:E2.-
17. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 1994;51:8-19.
18. Zhang AY, Snowden LR. Ethnic characteristics of mental disorders in five US communities. Cult Div Ethnic Minority Psychology 1999;5:134-146.
19. Kessler RC, Berglund P, Demler O, et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095-3105.
20. Olfson M, Shea A, Feder A, et al. Prevalence of anxiety, depression, and substance use disorders in an urban general medicine practice. Arch Fam Med 2000;57:675-682.
21. Diala CD, Muntaner C, Walrath C, Nickerson K, LaVeist T, Leaf P. Racial/ethnic differences in attitudes toward seeking professional mental health services. Am J Public Health 2001;91:805-807.
22. Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA 1999;282:583-589.
23. Gallo JJ, Marino S, Ford D, Anthony J. Filters on the pathway to mental health care II: sociodemographic factors. Psychol Med 1995;25:1149-1160.
24. Snowden LR, Pingitore D. Frequency and scope of mental health delivery service to African Americans in primary care. Ment Health Serv Res 2002;4:123-130.
25. Harman JS, Schulberg HC, Mulsant BH, Reynolds CF. The effect of patient and visit characteristics on diagnosis of depression in primary care. J Fam Pract 2001;50-1068.
26. Young AS, Klap R, Sherbourne CD, Wells KB. The quality of care for depressive and anxiety disorder in the United States. Arch Gen Psychiatry 2001;52:472-478.
27. Melfi CA, Croghan TW, Hanna MP, Robinson RL. Racial variation in antidepressant treatment in a Medicaid population. J Clin Psychiatry 2000;16:16-21.
28. Brown C, Schulberg HC, Madonia MJ. Clinical presentations of major depression by African Americans and Whites in primary medical care practice. J Affect Disord 1996;41:181-191.
29. Brown C, Schulberg HC, Sacco D, Perel JM, Houck PR. Effectiveness of treatments for major depression in primary medical care: a post hoc analysis of outcomes for African Americans and White patients. J Affect Disord 1999;53:185-192.
30. Brown DR, Ahmed F, Gary LE, Milburn NG. Major depression in a community sample of African Americans. Am J Psychiatry 1995;152:373-378.
Cooper LA, Gonzales JJ, Gallo JJ, et al. The acceptability of treatment for depression among African-American, Hispanic, and white primary care patients. Med Care 2003;41:479-489.
32. Cooper-Patrick L, Powe NR, Jenckes MW, Gonzales JJ, Levine DM, Ford DE. Identification of patient attitudes and p regarding treatment of depression. J Gen Intern Med 1997;12:431-438.
33. Fabrega H, Jr, Mezzich J, Ulrich RF. Black-white differences in psychopathology in an urban psychiatric population. Compr Psychiatry 1988;29:285-297.
34. Ford DE, Kamerow DB, Thompson JW. Who talks to physicians about mental health and substance abuse problems? J Gen Intern Med 1988;3:363-369.
35. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend 1995;39:197-206.
36. Hanson B, Klerman GL. Proceedings: Interracial problems in the assessment of clinical depression: concordance differences between white psychiatrists and black and white patients. Psychopharmacol Bull 1974;10:65-67.
37. McKinlay JB, Lin T, Freund K, Moskowitz M. The unexpected influence of physician attributes in clinical decisions: results of an experiment. J Health Soc behavior 2002;43:92-106.
38. Miranda J, Azocar F, Komaromy M, Golding JM. Unmet mental health needs of women in public-sector gynecologic clinics. Am J Obstet Gynecol 1998;178:212-217.
39. Miranda J, Duan N, Sherbourne C, et al. Improving care for minorities: Can quality improvement interventions improve care and outcomes for depressed minorities? results of a randomized, controlled trial. Health Serv Res 2003;613-630.
40. O’Malley AS, Forrest CB, Miranda J. Primary care attributes and care for depression among low-income African American women. Am J Public Health 2003;93:1328-1334.
41. Rollman BL, Hanusa BH, Belnap BH, Gardner W, Cooper LA, Schulberg HC. Race, quality of depression care, and recovery from major depression in a primary care setting. Gen Hosp Psychiatry 2002;24:381-390.
42. Sleath B, Svarstad B, Roter D. Patient race and psychotropic prescribing during medical encounters. Patient Educ Couns 1998;34:227-238.
43. Steffens DC, Artigues DL, Ornstein KA, Krishnan KR. A review of racial differences in geriatric depression: implications for care and clinical research. J Natl Med Assoc 1997;89:731-736.
44. Van Hook MP. Women’s help-seeking patterns for depression. Soc Work Health Care 1999;29:15-34.
45. Wells K, Klap R, Koike A, Sherbourne C. Ethnic disparities in unmet need for alcoholism drug abuse and mental health care. Am J Psychiatry 2001;158:2027-2032.
46. Wohl M, Lesser I, Smith M. Clinical presentations of depression in African Americans and white outpatients. Cult Div Ment Health 1997;3:279-284.
47. LaVeist TA, Carroll T. Race of physician and satisfaction with care among African-American patients. J Natl Med Assoc 2002;94:937-943.
48. Jackson JS. Race and ethnic differences in physical and mental health disparities. University of Michigan Institute for Social Research, Program for Research on Black Americans. Presented at New York State Psychiatric Institute, New York, NY, February 2004.
49. Knauper B, Wittchen HU. Diagnosing major depression in the elderly: evidence for response bias in standardized diagnostic interviews? J Psychiatr Res 1994;28:147-164.
50. Nutting PA, Rost K, Smth J, Weiner JJ, Elliot. Competing demands from physical problems: effect on initiating and completing depression care over 6 months. Arch Fam Med 2000;9:1059-1064.
51. Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med 1998;338:1516-1520.
52. Miranda J, Cooper LA. Disparities in care for depression among primary care patients. J Gen Intern Med 2004;19:120-126.
53. Strickland TL, Stein R, Lin K, Risby E, Fong R. The pharmacologic treatment of anxiety and depression in African Americans. Arch Fam Med 1997;6:371-375.
54. Bull SA, Hu XH, Lee JY, Ming EE, Markson LE, Fireman. Discontinuation of use and switching of antidepressants: influence of patient-physician communication. JAMA 2002;288:1403-1409.
1. Sussman LK, Robins LN, Earls F. Treatment-seeking for depression by black and white Americans. Soc Sci Med 1987;24:187-196.
2. Vega WA, Kolody B, Aguilar-Gaxiola S, Catalano R. Gaps in service utilization by Mexican Americans with mental health problems. Am J Psychiatry 1999;156:928-934.
3. Borowsky SJ, Rubenstein LV, Meredith LS, Camp P, Jackson-Triche M, Wells KB. Who is at risk of nondetection of mental health problems in primary care? J Gen Intern Med 2000;15:381-388.
4. Fiscella K, Franks P, Doescher MP, Saver BG. Disparities in health care by race, ethnicity and language among the insured: findings from a national sample. Med Care 2002;40:52-59.
5. Dunlop DD, Song J, Lyons JS, Mannheim LM, Chang RW. Racial/ethnic differences in rates of depression among pre-retirement adults. Am J Public Health 2003;93:1945-1952.
6. Phillips KA, Mayer ML, Aday LA. Barriers to care among racial ethnic groups under managed care. Health Aff 2000;19:65-75.
7. Tai-Seale M, Freund D, LoSasso A. Racial disparities in service use among Medicaid beneficiaries after mandatory enrollment in managed care: a difference-in-differences approach. Inquiry 2001;38:49-59.
8. Heckman TG, Somlai AM, Peters J, et al. Barriers to care among persons living with HIV/AIDS in urban and rural areas. AIDS Care 1998;10:365-375.
9. Padgett DK, Patrick C, Burns BJ, Schlesinger HJ. Ethnicity and the use of outpatient mental health services in a national insured population. Am J Public Health 1994;84:222-226.
10. Swartz MS, Wagner HR, Swanson JW, Bruns BJ, Keorge LK, Padgett DK. Comparing use of public and private mental health services; the enduring barriers of race and age. Commun Ment Health J 1998;34:133-144.
11. Wells K, Klap R, Koike A, Sherbourne C. Ethnic disparities in unmet need for alcoholism, drug abuse and mental health care. Am J Psychiatry 2001;158:2027-2032.
12. Wang PS, Berglund P, Kessler RC. Recent care of common mental disorders in the United States. J Gen Intern Med 2000;15:284-292.
13. Bach P, Cramer L, Warren L, Begg C. Racial differences in the treatment of early-stage lung cancer. New Engl J Med 1999;341:1198-1205.
14. Peterson ED, Shaw L, DeLong E, Pryor D, Califf R, Mark D. Racial variation in the use of coronary-revascularization procedures—are the differences real? Do they matter? N Engl J Med 1997;336:480-486.
15. US Department of Health and Human Services. Mental Health: Culture Race, and Ethnicity: A Supplement to Mental Health: A Report of the Surgeon General. Rockville, Md: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, 2001.
16. Areán PA, Alvidrez J. Treating depressive disorders: who responds who does not respond, and who do we need to study? J Fam Pract 2001;50:E2.-
17. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 1994;51:8-19.
18. Zhang AY, Snowden LR. Ethnic characteristics of mental disorders in five US communities. Cult Div Ethnic Minority Psychology 1999;5:134-146.
19. Kessler RC, Berglund P, Demler O, et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095-3105.
20. Olfson M, Shea A, Feder A, et al. Prevalence of anxiety, depression, and substance use disorders in an urban general medicine practice. Arch Fam Med 2000;57:675-682.
21. Diala CD, Muntaner C, Walrath C, Nickerson K, LaVeist T, Leaf P. Racial/ethnic differences in attitudes toward seeking professional mental health services. Am J Public Health 2001;91:805-807.
22. Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA 1999;282:583-589.
23. Gallo JJ, Marino S, Ford D, Anthony J. Filters on the pathway to mental health care II: sociodemographic factors. Psychol Med 1995;25:1149-1160.
24. Snowden LR, Pingitore D. Frequency and scope of mental health delivery service to African Americans in primary care. Ment Health Serv Res 2002;4:123-130.
25. Harman JS, Schulberg HC, Mulsant BH, Reynolds CF. The effect of patient and visit characteristics on diagnosis of depression in primary care. J Fam Pract 2001;50-1068.
26. Young AS, Klap R, Sherbourne CD, Wells KB. The quality of care for depressive and anxiety disorder in the United States. Arch Gen Psychiatry 2001;52:472-478.
27. Melfi CA, Croghan TW, Hanna MP, Robinson RL. Racial variation in antidepressant treatment in a Medicaid population. J Clin Psychiatry 2000;16:16-21.
28. Brown C, Schulberg HC, Madonia MJ. Clinical presentations of major depression by African Americans and Whites in primary medical care practice. J Affect Disord 1996;41:181-191.
29. Brown C, Schulberg HC, Sacco D, Perel JM, Houck PR. Effectiveness of treatments for major depression in primary medical care: a post hoc analysis of outcomes for African Americans and White patients. J Affect Disord 1999;53:185-192.
30. Brown DR, Ahmed F, Gary LE, Milburn NG. Major depression in a community sample of African Americans. Am J Psychiatry 1995;152:373-378.
Cooper LA, Gonzales JJ, Gallo JJ, et al. The acceptability of treatment for depression among African-American, Hispanic, and white primary care patients. Med Care 2003;41:479-489.
32. Cooper-Patrick L, Powe NR, Jenckes MW, Gonzales JJ, Levine DM, Ford DE. Identification of patient attitudes and p regarding treatment of depression. J Gen Intern Med 1997;12:431-438.
33. Fabrega H, Jr, Mezzich J, Ulrich RF. Black-white differences in psychopathology in an urban psychiatric population. Compr Psychiatry 1988;29:285-297.
34. Ford DE, Kamerow DB, Thompson JW. Who talks to physicians about mental health and substance abuse problems? J Gen Intern Med 1988;3:363-369.
35. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend 1995;39:197-206.
36. Hanson B, Klerman GL. Proceedings: Interracial problems in the assessment of clinical depression: concordance differences between white psychiatrists and black and white patients. Psychopharmacol Bull 1974;10:65-67.
37. McKinlay JB, Lin T, Freund K, Moskowitz M. The unexpected influence of physician attributes in clinical decisions: results of an experiment. J Health Soc behavior 2002;43:92-106.
38. Miranda J, Azocar F, Komaromy M, Golding JM. Unmet mental health needs of women in public-sector gynecologic clinics. Am J Obstet Gynecol 1998;178:212-217.
39. Miranda J, Duan N, Sherbourne C, et al. Improving care for minorities: Can quality improvement interventions improve care and outcomes for depressed minorities? results of a randomized, controlled trial. Health Serv Res 2003;613-630.
40. O’Malley AS, Forrest CB, Miranda J. Primary care attributes and care for depression among low-income African American women. Am J Public Health 2003;93:1328-1334.
41. Rollman BL, Hanusa BH, Belnap BH, Gardner W, Cooper LA, Schulberg HC. Race, quality of depression care, and recovery from major depression in a primary care setting. Gen Hosp Psychiatry 2002;24:381-390.
42. Sleath B, Svarstad B, Roter D. Patient race and psychotropic prescribing during medical encounters. Patient Educ Couns 1998;34:227-238.
43. Steffens DC, Artigues DL, Ornstein KA, Krishnan KR. A review of racial differences in geriatric depression: implications for care and clinical research. J Natl Med Assoc 1997;89:731-736.
44. Van Hook MP. Women’s help-seeking patterns for depression. Soc Work Health Care 1999;29:15-34.
45. Wells K, Klap R, Koike A, Sherbourne C. Ethnic disparities in unmet need for alcoholism drug abuse and mental health care. Am J Psychiatry 2001;158:2027-2032.
46. Wohl M, Lesser I, Smith M. Clinical presentations of depression in African Americans and white outpatients. Cult Div Ment Health 1997;3:279-284.
47. LaVeist TA, Carroll T. Race of physician and satisfaction with care among African-American patients. J Natl Med Assoc 2002;94:937-943.
48. Jackson JS. Race and ethnic differences in physical and mental health disparities. University of Michigan Institute for Social Research, Program for Research on Black Americans. Presented at New York State Psychiatric Institute, New York, NY, February 2004.
49. Knauper B, Wittchen HU. Diagnosing major depression in the elderly: evidence for response bias in standardized diagnostic interviews? J Psychiatr Res 1994;28:147-164.
50. Nutting PA, Rost K, Smth J, Weiner JJ, Elliot. Competing demands from physical problems: effect on initiating and completing depression care over 6 months. Arch Fam Med 2000;9:1059-1064.
51. Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med 1998;338:1516-1520.
52. Miranda J, Cooper LA. Disparities in care for depression among primary care patients. J Gen Intern Med 2004;19:120-126.
53. Strickland TL, Stein R, Lin K, Risby E, Fong R. The pharmacologic treatment of anxiety and depression in African Americans. Arch Fam Med 1997;6:371-375.
54. Bull SA, Hu XH, Lee JY, Ming EE, Markson LE, Fireman. Discontinuation of use and switching of antidepressants: influence of patient-physician communication. JAMA 2002;288:1403-1409.
Age-related macular degeneration: Options for earlier detection and improved treatment
- Advanced age, positive family history, smoking, hypertension, and Caucasian ancestry are risk factors consistently associated with age-related macular degeneration (ARMD).
- With older patients, suspect ARMD if vision has decreased or if Amsler grid testing reveals scotoma or metamorphopsia (C).
- Refer any patient with suspected ARMD for an ophthalmology consultation (C).
- Lifestyle modifications, vitamin supplementation, and low-vision aids can slow or mitigate the effects of ARMD (A).
Age-related macular degeneration (ARMD) is no longer the ocular death sentence it once was. Therapies are improving and becoming more widely available.
Though everyone over 40 should have an ophthalmologic exam every year or 2, many patients that may routinely see their primary care physician will not consult an ophthalmologist until a problem manifests (see Demographics of ARMD). With simple, in-office testing described in this article, you can help alert patients to the need for ophthalmologic follow-up.
Age-related macular degeneration (ARMD) is a progressive eye disease that can result in the loss of the center of our field of vision due to damage to the macula, the most important portion of the retina. It is the leading cause of central vision loss in people over the age of 50 years in the United States1 and the resulting functional loss can be devastating. Over a 5-year time span, it is estimated that 1 in 3 people over the age of 70 years will develop signs of ARMD2 and that 315,000 Americans over the age of 74 years will develop signs of advanced ARMD.3 Since the risk of ARMD increases with age,1 the number of patients with this disease will increase as our population grows older.
5 Consistent risk factors
Several large, population-based studies have identified age, family history, smoking, hypertension, and Caucasian ancestry as risk factors consistently associated with ARMD.4 Other risk factors such as atherosclerosis, ultraviolet (UV) exposure, farsightedness, increased body-mass index, and cataracts4-6 have been suggested, but not consistently proven to increase risk.
The risk of developing ARMD increases with age.1 According to the Framingham Eye study, a person between the ages of 65 and 74 has a 6% chance of exhibiting ophthalmoscopic evidence of ARMD. For those 75 or older, the chances are almost 20%.1,7
The Beaver Dam Eye Study and Rotterdam Study looked at families with ARMD and found an odds ratio as high as 10:1 for siblings7,8 and 6:1 for the offspring of ARMD patients.7 Combining these risk factors, a person who lives to be 85 and has a first-degree relative with ARMD has a 48% chance of developing clinically detectable ARMD.7
Smoking and hypertension also correlate strongly with ARMD.4,6 Patients who have ARMD and continue to smoke are 3.6 times more likely to progress to the end stages of the disease with severe functional compromise. Even when patients quit smoking, the risk remains elevated for the next 20 years.9 Studies of ARMD and systemic hypertension show an odds ratio for clinically detectable dry ARMD at 2:1 for patients with a systolic reading of 160 mm Hg or greater, and an odds ratio of 4:1 for wet ARMD if the diastolic reading is greater than 95 mm Hg.10,11
Diagnosing ARMD
Though a dilated fundus exam remains the gold standard for diagnosing ARMD, other in-office diagnostic tools are also available.
Specific clues in the history
In addition to inquiring about a family history of ARMD, ask patients if they have difficulty recognizing faces or if words seem to disappear while reading. ARMD patients may also notice that telephone poles appear crooked.
Testing visual acuity
With the patient wearing a current pair of reading glasses, test one eye at a time in good lighting with a near vision card at 12 to 14 inches from the patient’s face. Though many disorders can cause decreased vision, a reading worse than 20/30 in an elderly patient could indicate undiagnosed ARMD. If the patient has noticed a recent decrease in vision, an immediate ophthalmology referral is indicated. If near vision has gradually worsened, referral is needed but perhaps not as urgently.
Amsler grid test for earlier diagnosis
Amsler grid testing can detect ARMD (as well as other macular diseases) at a much earlier stage than can visual acuity testing alone. The grid (FIGURE 1; for a printable version, go to www.jfponline.com) is read with one eye at a time at a distance of 12 to 14 inches with reading glasses under good lighting conditions. Instruct the patient to focus only on the center dot in the grid and to view the remainder of the grid with peripheral vision and without looking around. If the patient reports that lines are missing (known as a scotoma) or appear wavy (known as metamorphopsia), the test is positive and prompt referral is indicated. The location of the distorted or missing lines corresponds closely with the location of the macular lesion.
FIGURE 1
Amsler grid
Patients with known ARMD can monitor their disease by performing this test once a day. This grid is shown actual size; a printable version is found at www.jfponline.com.
Changes seen on dilated fundus exam
To view the macula, ask the patient to look directly at the light during direct fundoscopy.
Dry ARMD displays drusen and changes in the retinal pigment epithelium. Drusen are discrete, round yellow lesions. Changes in the retinal pigment epithelium may be subtle tiny darkly colored patches in the macula near areas of lighter patches (see How ARMD develops).
Wet ARMD may appear as a dark red or green discoloration in the macula and requires immediate referral.
End-stage ARMD may demonstrate a well-delineated geographic pattern corresponding to retinal atrophy or scarring. Large blood vessels are usually visible in the base of these areas. Any macular lesions found in a patient without a diagnosis of ARMD or other maculopathy must have an ophthalmology referral (TABLE 1).
TABLE 1
Referral guide for age-related macular degeneration
| GROUP 1 | IMMEDIATE REFERRAL (THAT DAY) |
| New onset of decreased vision of unknown cause | |
| New onset of metamorphopsia (crooked telephone poles or new findings on the Amsler grid test) | |
| New onset of a scotoma (losing words while reading) | |
| Retinal hemorrhages | |
| GROUP 2 | PROMPT REFERRAL (WITHIN 1 MONTH) |
| Drusen or retinal pigment epithelium changes seen without a previous diagnosis of ARMD | |
| History of long-standing metamorphopsia or scotoma without a previous diagnosis of ARMD | |
| GROUP 3 | ROUTINE REFERRAL (WITHIN 6 MONTHS) |
| Diagnosis of ARMD, but has not seen an ophthalmologist in more than 1 year | |
| Age over 60, but has not seen an ophthalmologist in more than 2 years | |
Pathologically, ARMD damages the macula in 1 of 2 ways, giving rise to the 2 forms of ARMD, wet and dry. Dry ARMD accounts for 80% of all cases of ARMD.12 In this form, the retinal pigment epithelium (RPE) degenerates as a result of the intracellular deposition of yellow metabolic waste products known as drusen. Drusen are typically accompanied by subtle, progressive changes in the pigmentation of the RPE (FIGURE 2). Without the nutritional support of the RPE, the overlying retina cells slowly atrophy causing a loss of central visual acuity. Wet ARMD is less common, but accounts for 80% of the severe vision loss from ARMD.12 In this form, neovascularization from the arterial system underlying the RPE breaks through the RPE barrier and quickly spreads underneath the retina, causing bleeding and fibrosis which can rapidly destroy the macula (FIGURE 3).
FIGURE 2
Dry ARMD
Dry ARMD demonstrating yellow-white drusen with areas of patchy hyperpigmentation of the retinal pigment epithelium. This patient has 20/30 vision and metamorphopsia on Amsler grid testing.
FIGURE 3
Wet ARMD
The greenish hue indicates bleeding under the retina. The yellow-white lesions represent sub-retinal scarring. This patient has 20/400 vision and a large scotoma on Amsler grid testing.
Treatment for dry macular degeneration
Vitamins confer modest benefit
Some patients with dry ARMD may benefit modestly from certain over-the-counter vitamin supplements. The Age-Related Eye Disease Study found that patients with extensive drusen but no advanced macular degeneration in either eye had an 11% chance of going from legal driving vision to legal blindness within 5 years. However, those who took the once-a-day formulation13 shown in TABLE 2 exhibited a modest drop in risk to 10% over 5 years. More encouraging, patients with advanced macular degeneration in the control eye reduced their chances of legal blindness from 28% to 17% with the supplement (strength of recommendation [SOR]: A14; level of evidence [LOE]: 1, randomized controlled trial [RCT]).
Ten mg of Lutein alone or combined with antioxidants has shown some promise in short-term studies15 (SOR: B; LOE: 2, RCT), but further research will be required.
TABLE 2
Recommended daily vitamin doses for dry ARMD patients
| VITAMIN | DOSE |
|---|---|
| Zinc | 80 mg |
| Vitamin C | 500 mg |
| Vitamin E | 400 IU |
| Beta-carotene | 15 mg |
| Copper | 2 mg |
| The recommended once a day dose from the Age-Related Eye Disease Study. Copper was included in the formulation to prevent a zinc-induced copper deficient anemia.13 It should also be noted that smokers may be at an increased risk of lung cancer from excessive beta-carotene use13 and special formulations for smokers are available. | |
Treatment for wet macular degeneration
Laser treatment most common choice
Though many types of therapy are available, patients with wet ARMD will usually undergo photodynamic therapy or conventional laser therapy.
Photodynamic therapy uses a photosensitizing agent with an affinity for neovascular tissue. After injection into the bloodstream, the agent is light-activated to induce thrombosis of the neovascular vessels.16 The Treatment of Age-Related Macular Degeneration with Photodynamic Therapy study showed that treated patients had a greater chance of retaining their vision compared with untreated patients, and that about 1 in 6 treated patients experienced some improvement in vision (SOR: A; LOE: 1, RCT).17 Unfortunately, recurrence of neovascular tissue is common and retreatment is often necessary.
Conventional thermal laser therapy directly coagulates the neovascular area. The Macular Photocoagulation Study showed that thermal laser therapy reduced the likelihood of losing 3 to 6 lines of vision (for example, going from 20/20 to 20/70) after 2 years of follow-up (SOR: A; LOE: 1, RCT).18 Conventional laser therapy requires retreatment less often than photodynamic therapy. However, it is applicable only to certain subtypes of wet ARMD and may immediately cause vision loss if used too close to the center of the macula.
Surgery usually not recommended
Surgical removal of neovascular tissue in wet ARMD has succeeded anatomically but has not yielded consistent results in protecting vision. Using microsurgical instruments, the surgeon can separate the macula from the retinal pigment epithelium and remove the neovascular tissue through a small incision in the retina. Though the Submacular Surgery Trials did find a subset of patients who benefited from this procedure, overall results did not support the use of this surgery (SOR: A; LOE: 1, RCT).19
Pegaptanib injections show promise
The newest treatment for wet ARMD is pegaptanib (Macugen). Pegaptanib is a macromolecule designed specifically to bind to VEGF165 isoform, thus blocking its angiogenic and permeability enhancing activity. It is injected directly into the vitreous cavity of patients with wet ARMD. Study results have encouragingly shown a significant reduction in moderate and severe visual loss at 12 months of follow-up (SOR: A; LOE: 1, RCT).20
Measures for wet and dry macular degeneration
Smoking cessation most important lifestyle change
Of all the treatment options for patients with wet or dry ARMD, the most effective means of preserving vision is smoking cessation. As mentioned, controlling blood pressure is also an important modifiable risk factor. Lowering cholesterol or decreasing body mass index may help prevent vision loss, but the literature does not consistently support these claims.
Counseling
Instruct patients with ARMD of either type on how to use the Amsler grid at home. They should understand that any changes on the grid should prompt a call to their ophthalmologist.
Patients with dry ARMD should understand that while the disease is progressive, it does so very slowly.
Patients with wet ARMD and those with end-stage dry ARMD should be counseled that, unless another ocular disease is present, they will not go blind. They can maintain normal peripheral vision. If their central vision is 20/200 or worse in the better eye with glasses, they are considered “legally blind” (in most states) and may qualify for certain disability help.
Low-vision aids
Even in the most advanced cases of ARMD, patients must never be told that nothing more can be done. Low-vision devices are available to aid and restore the functional needs of patients. These range from simple handheld magnifiers to closed-circuit television scanners. Inquire of your local ophthalmologist if he or she incorporates low-vision aid training into his practice. If not, it may be necessary to refer to a low-vision clinic at a larger institution.
Acknowledgments
This work was supported in part by unrestricted grants from Research to Prevent Blindness and the Pat & Willard Walker Eye Research Center.
CORRESPONDENCE
Michael N. Wiggins, MD, Jones Eye Institute, University of Arkansas for Medical Sciences, 4301 West Markham St. Slot 523, Little Rock, AR 72205-7199. E-mail: [email protected]
1. American Academy of Ophthalmology. Basic and Clinical Science Course, Section 12, Retina, ed. 2004-2005. San Francisco, Calif: LEO; 2004.
2. Mukesh BN, Dimitrov PN, Leikin S, et al. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology 2004;111:1176-1182.
3. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1997;104:7-21.
4. Klein R, Peto T, Bird A, Vannewkirk M. The epidemiology of age related macular degeneration. Am J Ophthalmol 2004;137:486-495.
5. Tomany S, Cruickshanks K, Klein R, et al. Sunlight and the 10-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Arch Ophthalmol 2004;122:750-757.
6. Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000;107:2224-2232.
7. Klaver C, Wolfs R, Assink J, et al. Genetic risk of age-related maculopathy: Population-based familial aggregation study. Arch Ophthalmol 1998;116:1646-1651.
8. Klein B, Klein R, Lee K, et al. Risk of incident age-related eye diseases in people with an affected sibling: The Beaver Dam Eye Study. Am J Epidemiol 2001;154:207-211.
9. Delcourt C, Diaz J, Ponton-Sanchez A, Papoz L. Smoking and age-related macular degeneration: The POLA Study. Arch Ophthalmol 1998;116:1031-1035.
10. van Leeuwen R, Ikram MK, Vingerling JR, Witteman JC, Hofman A, de Jong PT. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci 2003;44:3771-3777.
11. Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol 2000;118:351-358.
12. Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984;102:1640-1642.
13. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119:1417-1436.
14. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53(2):111-120.
15. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75:216-230.
16. Yanoff M, Duker J. Ophthalmology 2nd ed. St Louis, Mo: Mosby; 2004.
17. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol 1999;117:1329-1345.
18. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol 1993;111:1200-1209.
19. Hawkins BS, Bressler NM, Miskala PH, et al. Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: ophthalmic findings: SST report no. 11. Ophthalmology 2004;111:1967-1980.
20. Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805-2516.
- Advanced age, positive family history, smoking, hypertension, and Caucasian ancestry are risk factors consistently associated with age-related macular degeneration (ARMD).
- With older patients, suspect ARMD if vision has decreased or if Amsler grid testing reveals scotoma or metamorphopsia (C).
- Refer any patient with suspected ARMD for an ophthalmology consultation (C).
- Lifestyle modifications, vitamin supplementation, and low-vision aids can slow or mitigate the effects of ARMD (A).
Age-related macular degeneration (ARMD) is no longer the ocular death sentence it once was. Therapies are improving and becoming more widely available.
Though everyone over 40 should have an ophthalmologic exam every year or 2, many patients that may routinely see their primary care physician will not consult an ophthalmologist until a problem manifests (see Demographics of ARMD). With simple, in-office testing described in this article, you can help alert patients to the need for ophthalmologic follow-up.
Age-related macular degeneration (ARMD) is a progressive eye disease that can result in the loss of the center of our field of vision due to damage to the macula, the most important portion of the retina. It is the leading cause of central vision loss in people over the age of 50 years in the United States1 and the resulting functional loss can be devastating. Over a 5-year time span, it is estimated that 1 in 3 people over the age of 70 years will develop signs of ARMD2 and that 315,000 Americans over the age of 74 years will develop signs of advanced ARMD.3 Since the risk of ARMD increases with age,1 the number of patients with this disease will increase as our population grows older.
5 Consistent risk factors
Several large, population-based studies have identified age, family history, smoking, hypertension, and Caucasian ancestry as risk factors consistently associated with ARMD.4 Other risk factors such as atherosclerosis, ultraviolet (UV) exposure, farsightedness, increased body-mass index, and cataracts4-6 have been suggested, but not consistently proven to increase risk.
The risk of developing ARMD increases with age.1 According to the Framingham Eye study, a person between the ages of 65 and 74 has a 6% chance of exhibiting ophthalmoscopic evidence of ARMD. For those 75 or older, the chances are almost 20%.1,7
The Beaver Dam Eye Study and Rotterdam Study looked at families with ARMD and found an odds ratio as high as 10:1 for siblings7,8 and 6:1 for the offspring of ARMD patients.7 Combining these risk factors, a person who lives to be 85 and has a first-degree relative with ARMD has a 48% chance of developing clinically detectable ARMD.7
Smoking and hypertension also correlate strongly with ARMD.4,6 Patients who have ARMD and continue to smoke are 3.6 times more likely to progress to the end stages of the disease with severe functional compromise. Even when patients quit smoking, the risk remains elevated for the next 20 years.9 Studies of ARMD and systemic hypertension show an odds ratio for clinically detectable dry ARMD at 2:1 for patients with a systolic reading of 160 mm Hg or greater, and an odds ratio of 4:1 for wet ARMD if the diastolic reading is greater than 95 mm Hg.10,11
Diagnosing ARMD
Though a dilated fundus exam remains the gold standard for diagnosing ARMD, other in-office diagnostic tools are also available.
Specific clues in the history
In addition to inquiring about a family history of ARMD, ask patients if they have difficulty recognizing faces or if words seem to disappear while reading. ARMD patients may also notice that telephone poles appear crooked.
Testing visual acuity
With the patient wearing a current pair of reading glasses, test one eye at a time in good lighting with a near vision card at 12 to 14 inches from the patient’s face. Though many disorders can cause decreased vision, a reading worse than 20/30 in an elderly patient could indicate undiagnosed ARMD. If the patient has noticed a recent decrease in vision, an immediate ophthalmology referral is indicated. If near vision has gradually worsened, referral is needed but perhaps not as urgently.
Amsler grid test for earlier diagnosis
Amsler grid testing can detect ARMD (as well as other macular diseases) at a much earlier stage than can visual acuity testing alone. The grid (FIGURE 1; for a printable version, go to www.jfponline.com) is read with one eye at a time at a distance of 12 to 14 inches with reading glasses under good lighting conditions. Instruct the patient to focus only on the center dot in the grid and to view the remainder of the grid with peripheral vision and without looking around. If the patient reports that lines are missing (known as a scotoma) or appear wavy (known as metamorphopsia), the test is positive and prompt referral is indicated. The location of the distorted or missing lines corresponds closely with the location of the macular lesion.
FIGURE 1
Amsler grid
Patients with known ARMD can monitor their disease by performing this test once a day. This grid is shown actual size; a printable version is found at www.jfponline.com.
Changes seen on dilated fundus exam
To view the macula, ask the patient to look directly at the light during direct fundoscopy.
Dry ARMD displays drusen and changes in the retinal pigment epithelium. Drusen are discrete, round yellow lesions. Changes in the retinal pigment epithelium may be subtle tiny darkly colored patches in the macula near areas of lighter patches (see How ARMD develops).
Wet ARMD may appear as a dark red or green discoloration in the macula and requires immediate referral.
End-stage ARMD may demonstrate a well-delineated geographic pattern corresponding to retinal atrophy or scarring. Large blood vessels are usually visible in the base of these areas. Any macular lesions found in a patient without a diagnosis of ARMD or other maculopathy must have an ophthalmology referral (TABLE 1).
TABLE 1
Referral guide for age-related macular degeneration
| GROUP 1 | IMMEDIATE REFERRAL (THAT DAY) |
| New onset of decreased vision of unknown cause | |
| New onset of metamorphopsia (crooked telephone poles or new findings on the Amsler grid test) | |
| New onset of a scotoma (losing words while reading) | |
| Retinal hemorrhages | |
| GROUP 2 | PROMPT REFERRAL (WITHIN 1 MONTH) |
| Drusen or retinal pigment epithelium changes seen without a previous diagnosis of ARMD | |
| History of long-standing metamorphopsia or scotoma without a previous diagnosis of ARMD | |
| GROUP 3 | ROUTINE REFERRAL (WITHIN 6 MONTHS) |
| Diagnosis of ARMD, but has not seen an ophthalmologist in more than 1 year | |
| Age over 60, but has not seen an ophthalmologist in more than 2 years | |
Pathologically, ARMD damages the macula in 1 of 2 ways, giving rise to the 2 forms of ARMD, wet and dry. Dry ARMD accounts for 80% of all cases of ARMD.12 In this form, the retinal pigment epithelium (RPE) degenerates as a result of the intracellular deposition of yellow metabolic waste products known as drusen. Drusen are typically accompanied by subtle, progressive changes in the pigmentation of the RPE (FIGURE 2). Without the nutritional support of the RPE, the overlying retina cells slowly atrophy causing a loss of central visual acuity. Wet ARMD is less common, but accounts for 80% of the severe vision loss from ARMD.12 In this form, neovascularization from the arterial system underlying the RPE breaks through the RPE barrier and quickly spreads underneath the retina, causing bleeding and fibrosis which can rapidly destroy the macula (FIGURE 3).
FIGURE 2
Dry ARMD
Dry ARMD demonstrating yellow-white drusen with areas of patchy hyperpigmentation of the retinal pigment epithelium. This patient has 20/30 vision and metamorphopsia on Amsler grid testing.
FIGURE 3
Wet ARMD
The greenish hue indicates bleeding under the retina. The yellow-white lesions represent sub-retinal scarring. This patient has 20/400 vision and a large scotoma on Amsler grid testing.
Treatment for dry macular degeneration
Vitamins confer modest benefit
Some patients with dry ARMD may benefit modestly from certain over-the-counter vitamin supplements. The Age-Related Eye Disease Study found that patients with extensive drusen but no advanced macular degeneration in either eye had an 11% chance of going from legal driving vision to legal blindness within 5 years. However, those who took the once-a-day formulation13 shown in TABLE 2 exhibited a modest drop in risk to 10% over 5 years. More encouraging, patients with advanced macular degeneration in the control eye reduced their chances of legal blindness from 28% to 17% with the supplement (strength of recommendation [SOR]: A14; level of evidence [LOE]: 1, randomized controlled trial [RCT]).
Ten mg of Lutein alone or combined with antioxidants has shown some promise in short-term studies15 (SOR: B; LOE: 2, RCT), but further research will be required.
TABLE 2
Recommended daily vitamin doses for dry ARMD patients
| VITAMIN | DOSE |
|---|---|
| Zinc | 80 mg |
| Vitamin C | 500 mg |
| Vitamin E | 400 IU |
| Beta-carotene | 15 mg |
| Copper | 2 mg |
| The recommended once a day dose from the Age-Related Eye Disease Study. Copper was included in the formulation to prevent a zinc-induced copper deficient anemia.13 It should also be noted that smokers may be at an increased risk of lung cancer from excessive beta-carotene use13 and special formulations for smokers are available. | |
Treatment for wet macular degeneration
Laser treatment most common choice
Though many types of therapy are available, patients with wet ARMD will usually undergo photodynamic therapy or conventional laser therapy.
Photodynamic therapy uses a photosensitizing agent with an affinity for neovascular tissue. After injection into the bloodstream, the agent is light-activated to induce thrombosis of the neovascular vessels.16 The Treatment of Age-Related Macular Degeneration with Photodynamic Therapy study showed that treated patients had a greater chance of retaining their vision compared with untreated patients, and that about 1 in 6 treated patients experienced some improvement in vision (SOR: A; LOE: 1, RCT).17 Unfortunately, recurrence of neovascular tissue is common and retreatment is often necessary.
Conventional thermal laser therapy directly coagulates the neovascular area. The Macular Photocoagulation Study showed that thermal laser therapy reduced the likelihood of losing 3 to 6 lines of vision (for example, going from 20/20 to 20/70) after 2 years of follow-up (SOR: A; LOE: 1, RCT).18 Conventional laser therapy requires retreatment less often than photodynamic therapy. However, it is applicable only to certain subtypes of wet ARMD and may immediately cause vision loss if used too close to the center of the macula.
Surgery usually not recommended
Surgical removal of neovascular tissue in wet ARMD has succeeded anatomically but has not yielded consistent results in protecting vision. Using microsurgical instruments, the surgeon can separate the macula from the retinal pigment epithelium and remove the neovascular tissue through a small incision in the retina. Though the Submacular Surgery Trials did find a subset of patients who benefited from this procedure, overall results did not support the use of this surgery (SOR: A; LOE: 1, RCT).19
Pegaptanib injections show promise
The newest treatment for wet ARMD is pegaptanib (Macugen). Pegaptanib is a macromolecule designed specifically to bind to VEGF165 isoform, thus blocking its angiogenic and permeability enhancing activity. It is injected directly into the vitreous cavity of patients with wet ARMD. Study results have encouragingly shown a significant reduction in moderate and severe visual loss at 12 months of follow-up (SOR: A; LOE: 1, RCT).20
Measures for wet and dry macular degeneration
Smoking cessation most important lifestyle change
Of all the treatment options for patients with wet or dry ARMD, the most effective means of preserving vision is smoking cessation. As mentioned, controlling blood pressure is also an important modifiable risk factor. Lowering cholesterol or decreasing body mass index may help prevent vision loss, but the literature does not consistently support these claims.
Counseling
Instruct patients with ARMD of either type on how to use the Amsler grid at home. They should understand that any changes on the grid should prompt a call to their ophthalmologist.
Patients with dry ARMD should understand that while the disease is progressive, it does so very slowly.
Patients with wet ARMD and those with end-stage dry ARMD should be counseled that, unless another ocular disease is present, they will not go blind. They can maintain normal peripheral vision. If their central vision is 20/200 or worse in the better eye with glasses, they are considered “legally blind” (in most states) and may qualify for certain disability help.
Low-vision aids
Even in the most advanced cases of ARMD, patients must never be told that nothing more can be done. Low-vision devices are available to aid and restore the functional needs of patients. These range from simple handheld magnifiers to closed-circuit television scanners. Inquire of your local ophthalmologist if he or she incorporates low-vision aid training into his practice. If not, it may be necessary to refer to a low-vision clinic at a larger institution.
Acknowledgments
This work was supported in part by unrestricted grants from Research to Prevent Blindness and the Pat & Willard Walker Eye Research Center.
CORRESPONDENCE
Michael N. Wiggins, MD, Jones Eye Institute, University of Arkansas for Medical Sciences, 4301 West Markham St. Slot 523, Little Rock, AR 72205-7199. E-mail: [email protected]
- Advanced age, positive family history, smoking, hypertension, and Caucasian ancestry are risk factors consistently associated with age-related macular degeneration (ARMD).
- With older patients, suspect ARMD if vision has decreased or if Amsler grid testing reveals scotoma or metamorphopsia (C).
- Refer any patient with suspected ARMD for an ophthalmology consultation (C).
- Lifestyle modifications, vitamin supplementation, and low-vision aids can slow or mitigate the effects of ARMD (A).
Age-related macular degeneration (ARMD) is no longer the ocular death sentence it once was. Therapies are improving and becoming more widely available.
Though everyone over 40 should have an ophthalmologic exam every year or 2, many patients that may routinely see their primary care physician will not consult an ophthalmologist until a problem manifests (see Demographics of ARMD). With simple, in-office testing described in this article, you can help alert patients to the need for ophthalmologic follow-up.
Age-related macular degeneration (ARMD) is a progressive eye disease that can result in the loss of the center of our field of vision due to damage to the macula, the most important portion of the retina. It is the leading cause of central vision loss in people over the age of 50 years in the United States1 and the resulting functional loss can be devastating. Over a 5-year time span, it is estimated that 1 in 3 people over the age of 70 years will develop signs of ARMD2 and that 315,000 Americans over the age of 74 years will develop signs of advanced ARMD.3 Since the risk of ARMD increases with age,1 the number of patients with this disease will increase as our population grows older.
5 Consistent risk factors
Several large, population-based studies have identified age, family history, smoking, hypertension, and Caucasian ancestry as risk factors consistently associated with ARMD.4 Other risk factors such as atherosclerosis, ultraviolet (UV) exposure, farsightedness, increased body-mass index, and cataracts4-6 have been suggested, but not consistently proven to increase risk.
The risk of developing ARMD increases with age.1 According to the Framingham Eye study, a person between the ages of 65 and 74 has a 6% chance of exhibiting ophthalmoscopic evidence of ARMD. For those 75 or older, the chances are almost 20%.1,7
The Beaver Dam Eye Study and Rotterdam Study looked at families with ARMD and found an odds ratio as high as 10:1 for siblings7,8 and 6:1 for the offspring of ARMD patients.7 Combining these risk factors, a person who lives to be 85 and has a first-degree relative with ARMD has a 48% chance of developing clinically detectable ARMD.7
Smoking and hypertension also correlate strongly with ARMD.4,6 Patients who have ARMD and continue to smoke are 3.6 times more likely to progress to the end stages of the disease with severe functional compromise. Even when patients quit smoking, the risk remains elevated for the next 20 years.9 Studies of ARMD and systemic hypertension show an odds ratio for clinically detectable dry ARMD at 2:1 for patients with a systolic reading of 160 mm Hg or greater, and an odds ratio of 4:1 for wet ARMD if the diastolic reading is greater than 95 mm Hg.10,11
Diagnosing ARMD
Though a dilated fundus exam remains the gold standard for diagnosing ARMD, other in-office diagnostic tools are also available.
Specific clues in the history
In addition to inquiring about a family history of ARMD, ask patients if they have difficulty recognizing faces or if words seem to disappear while reading. ARMD patients may also notice that telephone poles appear crooked.
Testing visual acuity
With the patient wearing a current pair of reading glasses, test one eye at a time in good lighting with a near vision card at 12 to 14 inches from the patient’s face. Though many disorders can cause decreased vision, a reading worse than 20/30 in an elderly patient could indicate undiagnosed ARMD. If the patient has noticed a recent decrease in vision, an immediate ophthalmology referral is indicated. If near vision has gradually worsened, referral is needed but perhaps not as urgently.
Amsler grid test for earlier diagnosis
Amsler grid testing can detect ARMD (as well as other macular diseases) at a much earlier stage than can visual acuity testing alone. The grid (FIGURE 1; for a printable version, go to www.jfponline.com) is read with one eye at a time at a distance of 12 to 14 inches with reading glasses under good lighting conditions. Instruct the patient to focus only on the center dot in the grid and to view the remainder of the grid with peripheral vision and without looking around. If the patient reports that lines are missing (known as a scotoma) or appear wavy (known as metamorphopsia), the test is positive and prompt referral is indicated. The location of the distorted or missing lines corresponds closely with the location of the macular lesion.
FIGURE 1
Amsler grid
Patients with known ARMD can monitor their disease by performing this test once a day. This grid is shown actual size; a printable version is found at www.jfponline.com.
Changes seen on dilated fundus exam
To view the macula, ask the patient to look directly at the light during direct fundoscopy.
Dry ARMD displays drusen and changes in the retinal pigment epithelium. Drusen are discrete, round yellow lesions. Changes in the retinal pigment epithelium may be subtle tiny darkly colored patches in the macula near areas of lighter patches (see How ARMD develops).
Wet ARMD may appear as a dark red or green discoloration in the macula and requires immediate referral.
End-stage ARMD may demonstrate a well-delineated geographic pattern corresponding to retinal atrophy or scarring. Large blood vessels are usually visible in the base of these areas. Any macular lesions found in a patient without a diagnosis of ARMD or other maculopathy must have an ophthalmology referral (TABLE 1).
TABLE 1
Referral guide for age-related macular degeneration
| GROUP 1 | IMMEDIATE REFERRAL (THAT DAY) |
| New onset of decreased vision of unknown cause | |
| New onset of metamorphopsia (crooked telephone poles or new findings on the Amsler grid test) | |
| New onset of a scotoma (losing words while reading) | |
| Retinal hemorrhages | |
| GROUP 2 | PROMPT REFERRAL (WITHIN 1 MONTH) |
| Drusen or retinal pigment epithelium changes seen without a previous diagnosis of ARMD | |
| History of long-standing metamorphopsia or scotoma without a previous diagnosis of ARMD | |
| GROUP 3 | ROUTINE REFERRAL (WITHIN 6 MONTHS) |
| Diagnosis of ARMD, but has not seen an ophthalmologist in more than 1 year | |
| Age over 60, but has not seen an ophthalmologist in more than 2 years | |
Pathologically, ARMD damages the macula in 1 of 2 ways, giving rise to the 2 forms of ARMD, wet and dry. Dry ARMD accounts for 80% of all cases of ARMD.12 In this form, the retinal pigment epithelium (RPE) degenerates as a result of the intracellular deposition of yellow metabolic waste products known as drusen. Drusen are typically accompanied by subtle, progressive changes in the pigmentation of the RPE (FIGURE 2). Without the nutritional support of the RPE, the overlying retina cells slowly atrophy causing a loss of central visual acuity. Wet ARMD is less common, but accounts for 80% of the severe vision loss from ARMD.12 In this form, neovascularization from the arterial system underlying the RPE breaks through the RPE barrier and quickly spreads underneath the retina, causing bleeding and fibrosis which can rapidly destroy the macula (FIGURE 3).
FIGURE 2
Dry ARMD
Dry ARMD demonstrating yellow-white drusen with areas of patchy hyperpigmentation of the retinal pigment epithelium. This patient has 20/30 vision and metamorphopsia on Amsler grid testing.
FIGURE 3
Wet ARMD
The greenish hue indicates bleeding under the retina. The yellow-white lesions represent sub-retinal scarring. This patient has 20/400 vision and a large scotoma on Amsler grid testing.
Treatment for dry macular degeneration
Vitamins confer modest benefit
Some patients with dry ARMD may benefit modestly from certain over-the-counter vitamin supplements. The Age-Related Eye Disease Study found that patients with extensive drusen but no advanced macular degeneration in either eye had an 11% chance of going from legal driving vision to legal blindness within 5 years. However, those who took the once-a-day formulation13 shown in TABLE 2 exhibited a modest drop in risk to 10% over 5 years. More encouraging, patients with advanced macular degeneration in the control eye reduced their chances of legal blindness from 28% to 17% with the supplement (strength of recommendation [SOR]: A14; level of evidence [LOE]: 1, randomized controlled trial [RCT]).
Ten mg of Lutein alone or combined with antioxidants has shown some promise in short-term studies15 (SOR: B; LOE: 2, RCT), but further research will be required.
TABLE 2
Recommended daily vitamin doses for dry ARMD patients
| VITAMIN | DOSE |
|---|---|
| Zinc | 80 mg |
| Vitamin C | 500 mg |
| Vitamin E | 400 IU |
| Beta-carotene | 15 mg |
| Copper | 2 mg |
| The recommended once a day dose from the Age-Related Eye Disease Study. Copper was included in the formulation to prevent a zinc-induced copper deficient anemia.13 It should also be noted that smokers may be at an increased risk of lung cancer from excessive beta-carotene use13 and special formulations for smokers are available. | |
Treatment for wet macular degeneration
Laser treatment most common choice
Though many types of therapy are available, patients with wet ARMD will usually undergo photodynamic therapy or conventional laser therapy.
Photodynamic therapy uses a photosensitizing agent with an affinity for neovascular tissue. After injection into the bloodstream, the agent is light-activated to induce thrombosis of the neovascular vessels.16 The Treatment of Age-Related Macular Degeneration with Photodynamic Therapy study showed that treated patients had a greater chance of retaining their vision compared with untreated patients, and that about 1 in 6 treated patients experienced some improvement in vision (SOR: A; LOE: 1, RCT).17 Unfortunately, recurrence of neovascular tissue is common and retreatment is often necessary.
Conventional thermal laser therapy directly coagulates the neovascular area. The Macular Photocoagulation Study showed that thermal laser therapy reduced the likelihood of losing 3 to 6 lines of vision (for example, going from 20/20 to 20/70) after 2 years of follow-up (SOR: A; LOE: 1, RCT).18 Conventional laser therapy requires retreatment less often than photodynamic therapy. However, it is applicable only to certain subtypes of wet ARMD and may immediately cause vision loss if used too close to the center of the macula.
Surgery usually not recommended
Surgical removal of neovascular tissue in wet ARMD has succeeded anatomically but has not yielded consistent results in protecting vision. Using microsurgical instruments, the surgeon can separate the macula from the retinal pigment epithelium and remove the neovascular tissue through a small incision in the retina. Though the Submacular Surgery Trials did find a subset of patients who benefited from this procedure, overall results did not support the use of this surgery (SOR: A; LOE: 1, RCT).19
Pegaptanib injections show promise
The newest treatment for wet ARMD is pegaptanib (Macugen). Pegaptanib is a macromolecule designed specifically to bind to VEGF165 isoform, thus blocking its angiogenic and permeability enhancing activity. It is injected directly into the vitreous cavity of patients with wet ARMD. Study results have encouragingly shown a significant reduction in moderate and severe visual loss at 12 months of follow-up (SOR: A; LOE: 1, RCT).20
Measures for wet and dry macular degeneration
Smoking cessation most important lifestyle change
Of all the treatment options for patients with wet or dry ARMD, the most effective means of preserving vision is smoking cessation. As mentioned, controlling blood pressure is also an important modifiable risk factor. Lowering cholesterol or decreasing body mass index may help prevent vision loss, but the literature does not consistently support these claims.
Counseling
Instruct patients with ARMD of either type on how to use the Amsler grid at home. They should understand that any changes on the grid should prompt a call to their ophthalmologist.
Patients with dry ARMD should understand that while the disease is progressive, it does so very slowly.
Patients with wet ARMD and those with end-stage dry ARMD should be counseled that, unless another ocular disease is present, they will not go blind. They can maintain normal peripheral vision. If their central vision is 20/200 or worse in the better eye with glasses, they are considered “legally blind” (in most states) and may qualify for certain disability help.
Low-vision aids
Even in the most advanced cases of ARMD, patients must never be told that nothing more can be done. Low-vision devices are available to aid and restore the functional needs of patients. These range from simple handheld magnifiers to closed-circuit television scanners. Inquire of your local ophthalmologist if he or she incorporates low-vision aid training into his practice. If not, it may be necessary to refer to a low-vision clinic at a larger institution.
Acknowledgments
This work was supported in part by unrestricted grants from Research to Prevent Blindness and the Pat & Willard Walker Eye Research Center.
CORRESPONDENCE
Michael N. Wiggins, MD, Jones Eye Institute, University of Arkansas for Medical Sciences, 4301 West Markham St. Slot 523, Little Rock, AR 72205-7199. E-mail: [email protected]
1. American Academy of Ophthalmology. Basic and Clinical Science Course, Section 12, Retina, ed. 2004-2005. San Francisco, Calif: LEO; 2004.
2. Mukesh BN, Dimitrov PN, Leikin S, et al. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology 2004;111:1176-1182.
3. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1997;104:7-21.
4. Klein R, Peto T, Bird A, Vannewkirk M. The epidemiology of age related macular degeneration. Am J Ophthalmol 2004;137:486-495.
5. Tomany S, Cruickshanks K, Klein R, et al. Sunlight and the 10-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Arch Ophthalmol 2004;122:750-757.
6. Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000;107:2224-2232.
7. Klaver C, Wolfs R, Assink J, et al. Genetic risk of age-related maculopathy: Population-based familial aggregation study. Arch Ophthalmol 1998;116:1646-1651.
8. Klein B, Klein R, Lee K, et al. Risk of incident age-related eye diseases in people with an affected sibling: The Beaver Dam Eye Study. Am J Epidemiol 2001;154:207-211.
9. Delcourt C, Diaz J, Ponton-Sanchez A, Papoz L. Smoking and age-related macular degeneration: The POLA Study. Arch Ophthalmol 1998;116:1031-1035.
10. van Leeuwen R, Ikram MK, Vingerling JR, Witteman JC, Hofman A, de Jong PT. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci 2003;44:3771-3777.
11. Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol 2000;118:351-358.
12. Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984;102:1640-1642.
13. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119:1417-1436.
14. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53(2):111-120.
15. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75:216-230.
16. Yanoff M, Duker J. Ophthalmology 2nd ed. St Louis, Mo: Mosby; 2004.
17. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol 1999;117:1329-1345.
18. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol 1993;111:1200-1209.
19. Hawkins BS, Bressler NM, Miskala PH, et al. Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: ophthalmic findings: SST report no. 11. Ophthalmology 2004;111:1967-1980.
20. Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805-2516.
1. American Academy of Ophthalmology. Basic and Clinical Science Course, Section 12, Retina, ed. 2004-2005. San Francisco, Calif: LEO; 2004.
2. Mukesh BN, Dimitrov PN, Leikin S, et al. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology 2004;111:1176-1182.
3. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1997;104:7-21.
4. Klein R, Peto T, Bird A, Vannewkirk M. The epidemiology of age related macular degeneration. Am J Ophthalmol 2004;137:486-495.
5. Tomany S, Cruickshanks K, Klein R, et al. Sunlight and the 10-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Arch Ophthalmol 2004;122:750-757.
6. Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000;107:2224-2232.
7. Klaver C, Wolfs R, Assink J, et al. Genetic risk of age-related maculopathy: Population-based familial aggregation study. Arch Ophthalmol 1998;116:1646-1651.
8. Klein B, Klein R, Lee K, et al. Risk of incident age-related eye diseases in people with an affected sibling: The Beaver Dam Eye Study. Am J Epidemiol 2001;154:207-211.
9. Delcourt C, Diaz J, Ponton-Sanchez A, Papoz L. Smoking and age-related macular degeneration: The POLA Study. Arch Ophthalmol 1998;116:1031-1035.
10. van Leeuwen R, Ikram MK, Vingerling JR, Witteman JC, Hofman A, de Jong PT. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci 2003;44:3771-3777.
11. Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol 2000;118:351-358.
12. Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984;102:1640-1642.
13. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119:1417-1436.
14. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53(2):111-120.
15. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75:216-230.
16. Yanoff M, Duker J. Ophthalmology 2nd ed. St Louis, Mo: Mosby; 2004.
17. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol 1999;117:1329-1345.
18. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol 1993;111:1200-1209.
19. Hawkins BS, Bressler NM, Miskala PH, et al. Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: ophthalmic findings: SST report no. 11. Ophthalmology 2004;111:1967-1980.
20. Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805-2516.
How should we evaluate and treat ADHD in children and adolescents?
- Who should be screened for ADHD?
- Are laboratory studies helpful?
- What are the most effective medications for treatment?
- How effective is behavior modification for treatment?
These questions are answered in the graded recommendations at right (page 1059), derived from an evidence-based clinical practice guideline developed at Cincinnati Children’s Hospital Medical Center. The target populations are children between the ages of 5 and 18 years with symptoms of attention deficit/hyperactivity disorder (ADHD), excluding those with autism, mental retardation, or another mental disorder. Guideline objectives were to improve diagnostic accuracy, treatment outcomes, and patient/parent satisfaction.
The evidence categories for this guideline are assessment/diagnosis, management, referral, and education. Adolescents have been included, as 45% to 85% of children diagnosed with ADHD in elementary school continue to manifest core symptoms and have social and educational dysfunction in middle and high school. The rating scheme is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
ADHD is one of the most common chronic behavioral disorders encountered by primary care physicians. It was first described by Dr Heinrich Hoffman in 1845. ADHD becomes apparent in some children during preschool and early school years. It is hard for these children to control their behavior or pay attention. Between 3% and 5% of children are thought to have ADHD—approximately 2 million children in the US. In a classroom of 25 to 30 children, 1 will likely have ADHD.2 Data collected by the guideline developers identified ADHD ranging from 3.5% to 9.2% at their center, reflecting national rates. A bibliography of 145 references accompanies this guideline. The guideline is strengthened by an algorithm for outpatient evaluation and management, educational resources for families, and cost-effectiveness analysis.
Guideline development and evidence review
The guideline was formulated by an interdisciplinary working group. Evidence was selected by searching Medline, EmBase, and Cochrane databases. A critical appraisal was performed and recommendations were graded. The guideline was released in April 2004.
Source for this guideline
Cincinnati Children’s Hospital Medical Center. Evidence-Based Clinical Practice Guideline for Outpatient Evaluation and Management of Attention Deficit/Hyperactivity Disorder. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2004 Apr 30. 23 pp.
GRADE A RECOMMENDATIONS
- Use comprehensive screening forms (eg, Vanderbilt ADHD Screening Tool) to evaluate for symptoms, impairment, and comorbidity.
- Diagnostic studies, including serum lead level, serum thyroid testing, computerized performance tests, and EEG studies are not recommended.
- Combining medication and behavior therapy has been more effective than using medication alone.
- Choose stimulants as first-line medication due to high efficacy and safety for periods as long as 24 months.
- Tricyclic antidepressants are effective in treating ADHD symptoms.
GRADE B RECOMMENDATIONS
- Test for ADHD in any child aged 5 to 18 years who exhibits inattention, impulsivity, academic underachievement, or behavior problems.
- Satisfying the DSM-IV criteria is required for the diagnosis of ADHD.
- Screen all ADHD patients for comorbid psychological, developmental, and psychiatric problems.
- Clonidine may be an effective adjunct to stimulant therapy.
- Mild growth suppression can occur in children ages 7 to 10 years who were treated with stimulants for longer than 2 years.
GRADE C RECOMMENDATIONS
- For a treatment plan that includes behavior therapy, use a group treatment setting for 1 to 6 months.
- Alternative therapies—including vitamins, herbs, vision therapy, and food supplements—do not appear to be beneficial.
- Communicate actively and directly with schools for treatment monitoring and follow-up.
Other guidelines on ADHD in children
Diagnosis and management of ADHD in primary care
This 2005 guideline is comprehensive, but recommendations are not individually graded in the summary. It covers DSM-IV criteria for diagnosis, screening for other primary conditions and comorbidities for patients newly diagnosed, the use of first-line medications through a systematic, uniform approach, and emphasizes a multimodality approach in treatment planning for children.
Source. Institute for Clinical Systems Improvement (ICSI). Diagnosis and Management of Attention Deficit Hyperactivity Disorder in Primary Care for School Age Children and Adolescents. Bloomington, Minn: ICSI; 2005 Jan. 69 pp. [131 references]
Diagnosis and evaluation of a child with ADHD
This 2000 guideline contains an algorithm, patient information, and links to the National Initiative for Children’s Healthcare Quality (NICHQ) ADHD Practitioners’ Toolkits.
Source. American Academy of Pediatrics. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics 2000; 105:1158–1170. [60 references]
Treatment of ADHD
This 2002 guideline is a thorough review of medications, but needs to be updated to include recent drug therapy advances.
Source. American Academy of Pediatrics. Clinical practice guidelines: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics 2001; 108:1033–1044. [62 references]
CORRESPONDENCE
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
2. National Institute of Mental Health, National Institute of Health. Attention Deficit Hyperactivity Disorder. 2003. Available at: www.nimh.nih.gov/publicat/adhd.cfm.
- Who should be screened for ADHD?
- Are laboratory studies helpful?
- What are the most effective medications for treatment?
- How effective is behavior modification for treatment?
These questions are answered in the graded recommendations at right (page 1059), derived from an evidence-based clinical practice guideline developed at Cincinnati Children’s Hospital Medical Center. The target populations are children between the ages of 5 and 18 years with symptoms of attention deficit/hyperactivity disorder (ADHD), excluding those with autism, mental retardation, or another mental disorder. Guideline objectives were to improve diagnostic accuracy, treatment outcomes, and patient/parent satisfaction.
The evidence categories for this guideline are assessment/diagnosis, management, referral, and education. Adolescents have been included, as 45% to 85% of children diagnosed with ADHD in elementary school continue to manifest core symptoms and have social and educational dysfunction in middle and high school. The rating scheme is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
ADHD is one of the most common chronic behavioral disorders encountered by primary care physicians. It was first described by Dr Heinrich Hoffman in 1845. ADHD becomes apparent in some children during preschool and early school years. It is hard for these children to control their behavior or pay attention. Between 3% and 5% of children are thought to have ADHD—approximately 2 million children in the US. In a classroom of 25 to 30 children, 1 will likely have ADHD.2 Data collected by the guideline developers identified ADHD ranging from 3.5% to 9.2% at their center, reflecting national rates. A bibliography of 145 references accompanies this guideline. The guideline is strengthened by an algorithm for outpatient evaluation and management, educational resources for families, and cost-effectiveness analysis.
Guideline development and evidence review
The guideline was formulated by an interdisciplinary working group. Evidence was selected by searching Medline, EmBase, and Cochrane databases. A critical appraisal was performed and recommendations were graded. The guideline was released in April 2004.
Source for this guideline
Cincinnati Children’s Hospital Medical Center. Evidence-Based Clinical Practice Guideline for Outpatient Evaluation and Management of Attention Deficit/Hyperactivity Disorder. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2004 Apr 30. 23 pp.
GRADE A RECOMMENDATIONS
- Use comprehensive screening forms (eg, Vanderbilt ADHD Screening Tool) to evaluate for symptoms, impairment, and comorbidity.
- Diagnostic studies, including serum lead level, serum thyroid testing, computerized performance tests, and EEG studies are not recommended.
- Combining medication and behavior therapy has been more effective than using medication alone.
- Choose stimulants as first-line medication due to high efficacy and safety for periods as long as 24 months.
- Tricyclic antidepressants are effective in treating ADHD symptoms.
GRADE B RECOMMENDATIONS
- Test for ADHD in any child aged 5 to 18 years who exhibits inattention, impulsivity, academic underachievement, or behavior problems.
- Satisfying the DSM-IV criteria is required for the diagnosis of ADHD.
- Screen all ADHD patients for comorbid psychological, developmental, and psychiatric problems.
- Clonidine may be an effective adjunct to stimulant therapy.
- Mild growth suppression can occur in children ages 7 to 10 years who were treated with stimulants for longer than 2 years.
GRADE C RECOMMENDATIONS
- For a treatment plan that includes behavior therapy, use a group treatment setting for 1 to 6 months.
- Alternative therapies—including vitamins, herbs, vision therapy, and food supplements—do not appear to be beneficial.
- Communicate actively and directly with schools for treatment monitoring and follow-up.
Other guidelines on ADHD in children
Diagnosis and management of ADHD in primary care
This 2005 guideline is comprehensive, but recommendations are not individually graded in the summary. It covers DSM-IV criteria for diagnosis, screening for other primary conditions and comorbidities for patients newly diagnosed, the use of first-line medications through a systematic, uniform approach, and emphasizes a multimodality approach in treatment planning for children.
Source. Institute for Clinical Systems Improvement (ICSI). Diagnosis and Management of Attention Deficit Hyperactivity Disorder in Primary Care for School Age Children and Adolescents. Bloomington, Minn: ICSI; 2005 Jan. 69 pp. [131 references]
Diagnosis and evaluation of a child with ADHD
This 2000 guideline contains an algorithm, patient information, and links to the National Initiative for Children’s Healthcare Quality (NICHQ) ADHD Practitioners’ Toolkits.
Source. American Academy of Pediatrics. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics 2000; 105:1158–1170. [60 references]
Treatment of ADHD
This 2002 guideline is a thorough review of medications, but needs to be updated to include recent drug therapy advances.
Source. American Academy of Pediatrics. Clinical practice guidelines: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics 2001; 108:1033–1044. [62 references]
CORRESPONDENCE
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
- Who should be screened for ADHD?
- Are laboratory studies helpful?
- What are the most effective medications for treatment?
- How effective is behavior modification for treatment?
These questions are answered in the graded recommendations at right (page 1059), derived from an evidence-based clinical practice guideline developed at Cincinnati Children’s Hospital Medical Center. The target populations are children between the ages of 5 and 18 years with symptoms of attention deficit/hyperactivity disorder (ADHD), excluding those with autism, mental retardation, or another mental disorder. Guideline objectives were to improve diagnostic accuracy, treatment outcomes, and patient/parent satisfaction.
The evidence categories for this guideline are assessment/diagnosis, management, referral, and education. Adolescents have been included, as 45% to 85% of children diagnosed with ADHD in elementary school continue to manifest core symptoms and have social and educational dysfunction in middle and high school. The rating scheme is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
ADHD is one of the most common chronic behavioral disorders encountered by primary care physicians. It was first described by Dr Heinrich Hoffman in 1845. ADHD becomes apparent in some children during preschool and early school years. It is hard for these children to control their behavior or pay attention. Between 3% and 5% of children are thought to have ADHD—approximately 2 million children in the US. In a classroom of 25 to 30 children, 1 will likely have ADHD.2 Data collected by the guideline developers identified ADHD ranging from 3.5% to 9.2% at their center, reflecting national rates. A bibliography of 145 references accompanies this guideline. The guideline is strengthened by an algorithm for outpatient evaluation and management, educational resources for families, and cost-effectiveness analysis.
Guideline development and evidence review
The guideline was formulated by an interdisciplinary working group. Evidence was selected by searching Medline, EmBase, and Cochrane databases. A critical appraisal was performed and recommendations were graded. The guideline was released in April 2004.
Source for this guideline
Cincinnati Children’s Hospital Medical Center. Evidence-Based Clinical Practice Guideline for Outpatient Evaluation and Management of Attention Deficit/Hyperactivity Disorder. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2004 Apr 30. 23 pp.
GRADE A RECOMMENDATIONS
- Use comprehensive screening forms (eg, Vanderbilt ADHD Screening Tool) to evaluate for symptoms, impairment, and comorbidity.
- Diagnostic studies, including serum lead level, serum thyroid testing, computerized performance tests, and EEG studies are not recommended.
- Combining medication and behavior therapy has been more effective than using medication alone.
- Choose stimulants as first-line medication due to high efficacy and safety for periods as long as 24 months.
- Tricyclic antidepressants are effective in treating ADHD symptoms.
GRADE B RECOMMENDATIONS
- Test for ADHD in any child aged 5 to 18 years who exhibits inattention, impulsivity, academic underachievement, or behavior problems.
- Satisfying the DSM-IV criteria is required for the diagnosis of ADHD.
- Screen all ADHD patients for comorbid psychological, developmental, and psychiatric problems.
- Clonidine may be an effective adjunct to stimulant therapy.
- Mild growth suppression can occur in children ages 7 to 10 years who were treated with stimulants for longer than 2 years.
GRADE C RECOMMENDATIONS
- For a treatment plan that includes behavior therapy, use a group treatment setting for 1 to 6 months.
- Alternative therapies—including vitamins, herbs, vision therapy, and food supplements—do not appear to be beneficial.
- Communicate actively and directly with schools for treatment monitoring and follow-up.
Other guidelines on ADHD in children
Diagnosis and management of ADHD in primary care
This 2005 guideline is comprehensive, but recommendations are not individually graded in the summary. It covers DSM-IV criteria for diagnosis, screening for other primary conditions and comorbidities for patients newly diagnosed, the use of first-line medications through a systematic, uniform approach, and emphasizes a multimodality approach in treatment planning for children.
Source. Institute for Clinical Systems Improvement (ICSI). Diagnosis and Management of Attention Deficit Hyperactivity Disorder in Primary Care for School Age Children and Adolescents. Bloomington, Minn: ICSI; 2005 Jan. 69 pp. [131 references]
Diagnosis and evaluation of a child with ADHD
This 2000 guideline contains an algorithm, patient information, and links to the National Initiative for Children’s Healthcare Quality (NICHQ) ADHD Practitioners’ Toolkits.
Source. American Academy of Pediatrics. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics 2000; 105:1158–1170. [60 references]
Treatment of ADHD
This 2002 guideline is a thorough review of medications, but needs to be updated to include recent drug therapy advances.
Source. American Academy of Pediatrics. Clinical practice guidelines: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics 2001; 108:1033–1044. [62 references]
CORRESPONDENCE
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
2. National Institute of Mental Health, National Institute of Health. Attention Deficit Hyperactivity Disorder. 2003. Available at: www.nimh.nih.gov/publicat/adhd.cfm.
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
2. National Institute of Mental Health, National Institute of Health. Attention Deficit Hyperactivity Disorder. 2003. Available at: www.nimh.nih.gov/publicat/adhd.cfm.
Naturopathic medicine: What can patients expect?
- Patients who inquire about naturopathy will want to know that clinical tools typically include nutrition evaluation and dietary revision, counseling for lifestyle modification, botanical medicine, homeopathy, physical medicine, and mind-body therapies.
- Advise patients who wish to seek naturopathic care to contact the state licensing authority to learn the scope of naturopathic practice allowed in their local area.
What can patients expect when they seek a naturopathic approach to disease management? A case presented in this article illustrates the applications of naturopathy in practice (see Naturopathic approach to one patient’s case: A summary).
Naturopathic physicians (NDs) diagnose and treat conditions typically seen in a “first contact” setting. They are not trained in the advanced use of highly technical conventional therapies for life-threatening diseases. Rather, they focus primarily on health issues encountered in out-patient ambulatory care settings (see Naturopathic training).
Though some tools of naturopathic practice differ from those of conventional practice, the goals of naturopathic medicine parallel those of family medicine in providing for and maintaining the well-being of both the patient and the healthcare system as a whole.
Collaboration is growing between conventional and naturopathic communities to examine the safety and efficacy of naturopathic medicine in preventing and managing a broad range of common conditions, and to determine whether availability of naturopathic services will improve patient health in a cost-effective manner.
Patient encounter
Martha S., a 39-year-old Asian-American
Presenting complaint
Has not felt well since onset of light-headedness, fatigue, muscle pain, and lassitude 4 years earlier/muscle tightness or tension and achiness come and go, often relieved by chiropractic treatment/some fuzzy cognition/dry gritty feeling in back of eyes/decreased libido/intermittent heart palpitations/sadness, easy weeping in conjunction with menses, lessens somewhat with St John’s Wort/disturbed, unrefreshing sleep 4 out of 7 days/body pain worse on waking
History
Three normal births and 3 spontaneous abortions with anticardiolipin antibodies that resolved after pregnancy/2 D&Cs, no other surgery/incidental finding of partially empty sella tursica on MS MRI investigation
Extensive specialty workups since 2001 have ruled out disease/internist who coordinated specialty consultations favors diagnosis of generalized anxiety disorder/some improvement with low doses Celexa, but unacceptable side effects (same with Effexor)/has tried amitriptyline/husband travels and she is uncomfortable with a “drugged sleep” when alone with the children
Fell off horse as teenager/no other physical injury or significant viral or bacterial illness/job exposes her to adversarial atmosphere and also requires occasional extensive hours at keyboard/infertility issues; experience of foreign adoption was a prolonged stressor
Family
Maternal aunt and grandmother had breast cancer age 50+; both survivors/paternal grandmother had stroke/father had postoperative DVT
Social
1–2 glasses wine/week; recreational drug use over 10 years in past/no regular exercise/attorney for city/married 11 years; husband 48, Euro/American, smokes, on anti-HTN medication/adopted sibs from Korea 1 year ago, 5-yr-old girl, 2-year-old boy, some malnutrition, parasites, now recovered
Exam
Patient is pleasant, articulate/no active disease/5’8”, 128#, BP 128/62, P 82/findings normal for HEENT, neck, chest, heart, abdomen, extremities, neurology, and skin/tender trigger points at bilateral trapezoids, paraspinal to subscapular, upper third gluteal and at hips
Lab
extensive records provided, essentially normal, none since 11/2003
Medications
None now
Allergies
Sensitive to drug side effects, but no known drug allergies
Management plan discussed with patient
In absence of other underlying disease, would like to treat you for fibromyalgia syndrome from long-term professional and personal stress/will work to recover your system from the physiologic effects of tension, worry, and hard work over past decade/if progress unsatisfactory after 3 months, we will revisit the diagnosis
Plan is to restore-rejuvenate your body, which knows how to right itself/think 6 months to a year for full recovery, after which you will have new knowledge of yourself and tools to maintain your well-being/details of the plan will shift as you recover and learn to use developing self knowledge to protect yourself during new challenges/flexibility and resilience are key and develop continually from self awareness/note what works for you and what doesn’t
Diet Goal
Hypoallergenic, whole-foods; small, frequent meals/adjust eicosanoid balance to increase systemic circulation, musculoskeletal flexibility, and cellular repair (patient given background article)/decrease production of pain-signaling chemistry, swelling that presses on nerves and creates the sensations of pain and stiffness; avoid sweets and refined carbohydrates, in order to maintain steady blood sugar levels
Use serotype diet (diet printed for patient) for the next 6 weeks/stick with best foods; dip into OK foods as little as possible/whet appetite for best food by “selfishly” focusing on your recovery/invite family to share meals, but primary purpose is your recovery; this can be hard for a mom to pull off; please invite your husband to call me if I can help him understand how he can enable you accomplish goal
Possible further steps
(see online version for details of action steps):
- Support/restore digestive tract: May not be making optimal gastric acid and other digestive factors as a result of long term stress stealing circulation away from those tissues that produce it.
- Eliminate simple sugars and refined flour products: Will help reduce pain/simple sugar creates hypoglycemic episode that can be experienced as nameless anxiety, weakness, fatigue, and dizziness/stable blood sugar essential for sense of well-being/eat pears, berries, or nuts if you need dessert.
- Exercise: Aerobic exercise 45 to 60 minutes, 3 or more times/week
- Sleep: Melatonin 250 μg to 500 mcg 30 or so minutes before bedtime/Deeper, assisted sleep will help, and you can adjust dosages to keep head clear in morning.
- Fundamental supplementation: For general well being, including fish oils, vitamins, and minerals.
- Adrenal recovery formula: We can presume your endocrine system has been affected by perceptions of threat (anxiety as related to the mystery of your physical pains) as well as long-term pain/recommend adrenal function test, to more closely determine optimal timing and doses of raw material that supports adrenal function
- Massage/body work
- Generalized anxiety disorder: your internist is convinced of this diagnosis; let’s discuss.
Outcome
Over 2 months: Sleep improved; trigger point pain diminished in upper body (by 30%), in gluteal and hips (80%)/able to manage diet “70% to 80% of the time”/exercise 3 to 5 times weekly, less when husband travels
Vacation interlude: Treatment plan jettisoned for vacation/return of rheumatic symptoms; dizziness and lassitude, however, continued to improve
Next 2 months: Continued improvement, with trigger point pain flaring only on long drives
Naturopathic physicians graduate from 1 of 6 naturopathic medical schools accredited by the Council on Naturopathic Medical Education (CNME) (TABLE 1). The CNME is a member of the Association of Specialized and Professional Accreditors recognized by the US Department of Education. Each school in the United States is also accredited by, or has candidacy status through, the specific regional agencies responsible for overseeing postsecondary institutions of higher learning.
Requirements for admission. The goal of naturopathic medical education is to prepare clinicians for the challenges of general practice, with a foundation in current medical science as well as traditional naturopathic theory. Candidates for admission to naturopathic medical school must earn a baccalaureate degree (or equivalent) prior to matriculation, including standard premedical undergraduate courses.
Naturopathic curricula. Subjects include inorganic and organic chemistry, physics, general biology and psychology. Other coursework is comparable to that of allopathic and osteopathic medical schools (TABLE 2). While the first 2 years of education combine courses in naturopathic principles with basic and diagnostic sciences, third and fourth year students focus on clinical practice, receiving training at naturopathic primary care outpatient clinics as well as conventional medical facilities (TABLE 3). Academic faculty at such institutions include NDs, PhDs, MDs, DOs, and other allied health professionals.
For information on postgraduate residencies, research, and collaborative opportunities for NDs, please see APPENDIX I. For additional information on naturopathic licensure, please see APPENDIX II.
Practice principles of naturopathic medicine
Naturopathic medical practice is based upon the premise that it is intrinsic to the nature of living organisms to heal. The naturopathic physician understands illness to be a disruption of normal orderly function. Healing therefore is the process by which living systems return to a resilient equilibrium, either unassisted or with the therapeutic support of the practitioner.
Western medicine rarely takes into consideration the inherent organizing forces underlying known physiologic processes such as metabolism or tissue repair. Naturopathic medicine calls this primary principle the vis medicatrix naturae, or the healing power of nature.
Another principle fundamental to the can complement customary clinical practice restoration of health is the understanding that any intervention employed should not further disrupt a system attempting to regain homeostasis. This is expressed as primum non nocere, the imperative to first choose interventions which do the least harm.
When confronted with an ill patient, the naturopathic physician seeks to understand the totality of fundamental causes disrupting the patient’s optimal equilibrium. In order to remove the cause of the illness (tolle causum), one must treat the whole person.
In pursuit of removing or moderating the insults and stressors that result in harm to the patient, the doctor becomes teacher (docere) and engages the patient in the essential responsibilities of self-care. Participation in the restoration of personal wellbeing prepares the patient to behave proactively in the future, when mutual efforts at prevention predominate in the physician-patient relationship.1
Although these practice principles form the foundation of the naturopathic approach to health and healthcare, the philosophic and conceptual approaches of conventional medical theory apply as well, including complexity science, quantum physics, medical ecology, public health, energy medicine, and the biological basis of healing. The above principles do not replace the foundation of biological pathology, but offer the practitioner an expanded perspective when treating each individual patient. Naturopathic medicine ascribes to a therapeutic hierarchy that integrates the full spectrum of modern biomedicine in a continuum that includes mental, emotional and spiritual therapies, as appropriate to each patient’s needs.2 Applied in this context, biomedical interventions are highly valued as both diagnostic and therapeutic approaches.
Clinical approach to patients
The ultimate goal of each clinical assessment is to obtain an in-depth understanding of the patient’s underlying condition (including his or her experience) and to effectively communicate relevant information to other healthcare providers participating in the patient’s care.
Essential to a comprehensive evaluation is the extended interview, which ranges from 60 to 90 minutes for new patients. Follow up visits range between 30 and 60 minutes. A standard review of systems is supplemented with patient-generated reports of daily activities, such as dietary habits, physical activity, and psychological issues (see Naturopathic approach to one patient’s case). NDs perform physical examinations appropriate to the patient’s presenting complaint and health history, and employ conventional laboratory and diagnostic imaging services as needed. Clinical evaluation is patient-centered and addresses a full range of factors that influence health as well as illness, generating a problem-oriented patient record based on International Classification of Diseases (ICD-9) criteria.
Modalities most often used in naturopathic practice include clinical nutrition and dietary revision, counseling for lifestyle modification, botanical medicine, homeopathy, physical medicine, and mind-body therapies.
Scope of practice. Depending on local licensure statutes, naturopathic physicians may be fully recognized as primary healthcare providers.3 Prescriptive authority varies, as do provisions for other procedures commonly associated with general medical practice.4
Details of the scope of naturopathic practice in each licensed jurisdiction can be accessed by contacting local licensing authorities, usually via a state or provincial agency website. In the majority of jurisdictions, licensed NDs are responsible for all public health regulations including reportable diseases and immunizations. Most ND practice acts require annual continuing education credits to maintain practice privileges.
Interdisciplinary collaboration. NDs are trained to recognize serious and life-threatening situations and to identify conditions outside of the scope of their professional or legal limitations. Appropriate referral mechanisms are indoctrinated during educational and clinical training. NDs often work with conventionally trained family practice physicians, internists, and specialists in co-managing patients, participating in decisions regarding referral for evaluation and treatment by other allopathic and complementary/alternative medicine practitioners.
Safety and effectiveness of naturopathic medicine
Naturopathic practice is distinguished by treatments individualized to a patient’s physical condition and environmental circumstances, requiring combination therapies adjusted over time as guided by patient response. Documenting the safety and efficacy of naturopathic interventions presents significant challenges—eg, the limitations of the reductionistic approach of allopathic research models when applied to complex interventions and inadequacy of available funding sources. Research on the clinical and quality of life outcomes, particularly evaluation of the actual, complex whole practice as opposed to single agent or specific modalities, is relatively scant.5
Responding to this challenge, in 2002 the NIH funded more than 1200 scientists and physicians from both conventional and naturopathic academic and professional communities in a 2-year effort to design the Naturopathic Medical Research Agenda (NMRA). Guided by the NMRA process, the research departments of naturopathic academic centers are systematically developing the collaborative infrastructure required to examine the theory and practice of naturopathic medicine.6 The recently inaugurated International Journal of Naturopathic Medicine (available at www.intjnm.org) provides access to naturopathic-specific, peer-reviewed research.
Documentation of safety is as relevant as documentation of efficacy. As the potential for harm does exist within the naturopathic scope of practice,7 licensure in the US requires that adverse medical events be reported to the federally mandated National Practitioner Databank.8 The disciplinary records of naturopathic licensing boards provide scrutiny of practices regulated in those jurisdictions as well as documentation of specific offenses: over a 10-year period (1992–2002), 173 complaints were filed with state licensing boards from a total of 1805 licensees. During this period, 31 disciplinary actions were initiated, ranging from probation to fines or censure.9
Safety and efficacy are also of concern to third-party payers. More than 70 companies, trade unions, and state organizations offer health plans that cover naturopathic medical services,10-11 requiring utilization reviews incorporating documented patient outcomes. As NDs are increasingly covered as specialists and primary care providers under reimbursement plans of corporations such as Microsoft and Boeing, the credentialing processes required by their insurers (such as Blue Cross and Blue Shield, Kaiser Permanente, Connecticare, Oxford, and Healthnet) result in formal analyses of safety and efficacy of practice. Malpractice insurance industry data also verify incidents of harm that may occur related to naturopathic practice.
To access more in-depth information, including how to identify licensed NDs in a particular geographic area, contact the American Association of Naturopathic Physicians at www.naturopathic.org.
For information on naturopathic medical education, particularly the advanced standing programs available to degreed professionals, contact the American Association of Naturopathic Medical Colleges at www.aanmc.org.
An additional resource for degreed medical professionals interested in naturopathic professional practice is the website for the North American Board of Naturopathic Examiners at www.nabne.org.
The advent of integrated care has resulted in staff privileges granted to NDs at approximately 20 conventional hospitals and numerous integrated clinics. As a result, efficacy of peer review is strengthened as payers elect naturopathic medical directors to peer advisory committees charged with formulating reimbursement and case management policies.12
CORRESPONDING AUTHOR
Nancy Dunne, ND, Bitterroot Natural Medicine, 200 East Pine St., Missoula, MT 59802. E-mail: [email protected]
1. Snider P, Zeff J. Report of the Select Committee on the Definition of Naturopathic Medicine. Washington, DC: AANP; 1988.
2. Micozzi M. Fundamentals of Complementary and Alternative Medicine. 2nd ed. Philadelphia, Pa: Churchill-Livingstone; 2001;181-183.
3. Kim L, Mills E. Physicians’ Information and Education Resource (PIER). Naturopathic Medicine, American College of Physicians; American Society of Internal Medicine July 1 2003.
4. Hough H, Dower C, O’Neill E. Profile of a Profession: Naturopathic Practice. San Francisco, Calif: Center for the Health Professions, University of California, San Francisco; 2001;27.-
5. Standish LJ, Calabrese C, Snider P. The Naturopathic Medical Research Agenda: The Future and Foundation of Naturopathic Medical Science. Kenmore, Wash: Bastyr University Press; 2005;10.-
6. Calabrese C, Reilly P, Lukaczer D. Position paper on Naturopathic Research, American Association of Naturopathic Physicians, 2003. Available at: http://www.naturopathic.org/positions/research_position_paper.html. Accessed on January 2, 2005.
7. Myers SP, Cheras PA. The other side of the coin: Safety of complementary and alternative medicine. Med J Aust. 2004;181:222-225.
8. Healthcare Integrity and Protection Databank National Practitioner Databank. Available at: http://www.npdb-hipdb.com/hipdb.html. Accessed on February 1, 2004.
9. Snider P, Cutler S. Naturopathic Profession Research Documentation. Kenmore, Wash: Bastyr University Press, 2002.
10. Quinn S, et al. Naturopathic Medicine: Primary Care for the Twenty-First Century. Washington, DC: AANP; 2003;12.-
11. Lafferty WE, Bellas A, Baden A, Tyree PT, Standish LJ, Patterson R. The use of complementary and alternative medical providers by insured cancer patients in Washington state. Cancer 2004;100:1522-1530.
12. Pizzorno J, Snider P. Fundamentals of Complementary and Alternative Medicine. Ed.M. Micozzi. Philadelphia, Pa: Churchill-Livingstone; 1996;173.
- Patients who inquire about naturopathy will want to know that clinical tools typically include nutrition evaluation and dietary revision, counseling for lifestyle modification, botanical medicine, homeopathy, physical medicine, and mind-body therapies.
- Advise patients who wish to seek naturopathic care to contact the state licensing authority to learn the scope of naturopathic practice allowed in their local area.
What can patients expect when they seek a naturopathic approach to disease management? A case presented in this article illustrates the applications of naturopathy in practice (see Naturopathic approach to one patient’s case: A summary).
Naturopathic physicians (NDs) diagnose and treat conditions typically seen in a “first contact” setting. They are not trained in the advanced use of highly technical conventional therapies for life-threatening diseases. Rather, they focus primarily on health issues encountered in out-patient ambulatory care settings (see Naturopathic training).
Though some tools of naturopathic practice differ from those of conventional practice, the goals of naturopathic medicine parallel those of family medicine in providing for and maintaining the well-being of both the patient and the healthcare system as a whole.
Collaboration is growing between conventional and naturopathic communities to examine the safety and efficacy of naturopathic medicine in preventing and managing a broad range of common conditions, and to determine whether availability of naturopathic services will improve patient health in a cost-effective manner.
Patient encounter
Martha S., a 39-year-old Asian-American
Presenting complaint
Has not felt well since onset of light-headedness, fatigue, muscle pain, and lassitude 4 years earlier/muscle tightness or tension and achiness come and go, often relieved by chiropractic treatment/some fuzzy cognition/dry gritty feeling in back of eyes/decreased libido/intermittent heart palpitations/sadness, easy weeping in conjunction with menses, lessens somewhat with St John’s Wort/disturbed, unrefreshing sleep 4 out of 7 days/body pain worse on waking
History
Three normal births and 3 spontaneous abortions with anticardiolipin antibodies that resolved after pregnancy/2 D&Cs, no other surgery/incidental finding of partially empty sella tursica on MS MRI investigation
Extensive specialty workups since 2001 have ruled out disease/internist who coordinated specialty consultations favors diagnosis of generalized anxiety disorder/some improvement with low doses Celexa, but unacceptable side effects (same with Effexor)/has tried amitriptyline/husband travels and she is uncomfortable with a “drugged sleep” when alone with the children
Fell off horse as teenager/no other physical injury or significant viral or bacterial illness/job exposes her to adversarial atmosphere and also requires occasional extensive hours at keyboard/infertility issues; experience of foreign adoption was a prolonged stressor
Family
Maternal aunt and grandmother had breast cancer age 50+; both survivors/paternal grandmother had stroke/father had postoperative DVT
Social
1–2 glasses wine/week; recreational drug use over 10 years in past/no regular exercise/attorney for city/married 11 years; husband 48, Euro/American, smokes, on anti-HTN medication/adopted sibs from Korea 1 year ago, 5-yr-old girl, 2-year-old boy, some malnutrition, parasites, now recovered
Exam
Patient is pleasant, articulate/no active disease/5’8”, 128#, BP 128/62, P 82/findings normal for HEENT, neck, chest, heart, abdomen, extremities, neurology, and skin/tender trigger points at bilateral trapezoids, paraspinal to subscapular, upper third gluteal and at hips
Lab
extensive records provided, essentially normal, none since 11/2003
Medications
None now
Allergies
Sensitive to drug side effects, but no known drug allergies
Management plan discussed with patient
In absence of other underlying disease, would like to treat you for fibromyalgia syndrome from long-term professional and personal stress/will work to recover your system from the physiologic effects of tension, worry, and hard work over past decade/if progress unsatisfactory after 3 months, we will revisit the diagnosis
Plan is to restore-rejuvenate your body, which knows how to right itself/think 6 months to a year for full recovery, after which you will have new knowledge of yourself and tools to maintain your well-being/details of the plan will shift as you recover and learn to use developing self knowledge to protect yourself during new challenges/flexibility and resilience are key and develop continually from self awareness/note what works for you and what doesn’t
Diet Goal
Hypoallergenic, whole-foods; small, frequent meals/adjust eicosanoid balance to increase systemic circulation, musculoskeletal flexibility, and cellular repair (patient given background article)/decrease production of pain-signaling chemistry, swelling that presses on nerves and creates the sensations of pain and stiffness; avoid sweets and refined carbohydrates, in order to maintain steady blood sugar levels
Use serotype diet (diet printed for patient) for the next 6 weeks/stick with best foods; dip into OK foods as little as possible/whet appetite for best food by “selfishly” focusing on your recovery/invite family to share meals, but primary purpose is your recovery; this can be hard for a mom to pull off; please invite your husband to call me if I can help him understand how he can enable you accomplish goal
Possible further steps
(see online version for details of action steps):
- Support/restore digestive tract: May not be making optimal gastric acid and other digestive factors as a result of long term stress stealing circulation away from those tissues that produce it.
- Eliminate simple sugars and refined flour products: Will help reduce pain/simple sugar creates hypoglycemic episode that can be experienced as nameless anxiety, weakness, fatigue, and dizziness/stable blood sugar essential for sense of well-being/eat pears, berries, or nuts if you need dessert.
- Exercise: Aerobic exercise 45 to 60 minutes, 3 or more times/week
- Sleep: Melatonin 250 μg to 500 mcg 30 or so minutes before bedtime/Deeper, assisted sleep will help, and you can adjust dosages to keep head clear in morning.
- Fundamental supplementation: For general well being, including fish oils, vitamins, and minerals.
- Adrenal recovery formula: We can presume your endocrine system has been affected by perceptions of threat (anxiety as related to the mystery of your physical pains) as well as long-term pain/recommend adrenal function test, to more closely determine optimal timing and doses of raw material that supports adrenal function
- Massage/body work
- Generalized anxiety disorder: your internist is convinced of this diagnosis; let’s discuss.
Outcome
Over 2 months: Sleep improved; trigger point pain diminished in upper body (by 30%), in gluteal and hips (80%)/able to manage diet “70% to 80% of the time”/exercise 3 to 5 times weekly, less when husband travels
Vacation interlude: Treatment plan jettisoned for vacation/return of rheumatic symptoms; dizziness and lassitude, however, continued to improve
Next 2 months: Continued improvement, with trigger point pain flaring only on long drives
Naturopathic physicians graduate from 1 of 6 naturopathic medical schools accredited by the Council on Naturopathic Medical Education (CNME) (TABLE 1). The CNME is a member of the Association of Specialized and Professional Accreditors recognized by the US Department of Education. Each school in the United States is also accredited by, or has candidacy status through, the specific regional agencies responsible for overseeing postsecondary institutions of higher learning.
Requirements for admission. The goal of naturopathic medical education is to prepare clinicians for the challenges of general practice, with a foundation in current medical science as well as traditional naturopathic theory. Candidates for admission to naturopathic medical school must earn a baccalaureate degree (or equivalent) prior to matriculation, including standard premedical undergraduate courses.
Naturopathic curricula. Subjects include inorganic and organic chemistry, physics, general biology and psychology. Other coursework is comparable to that of allopathic and osteopathic medical schools (TABLE 2). While the first 2 years of education combine courses in naturopathic principles with basic and diagnostic sciences, third and fourth year students focus on clinical practice, receiving training at naturopathic primary care outpatient clinics as well as conventional medical facilities (TABLE 3). Academic faculty at such institutions include NDs, PhDs, MDs, DOs, and other allied health professionals.
For information on postgraduate residencies, research, and collaborative opportunities for NDs, please see APPENDIX I. For additional information on naturopathic licensure, please see APPENDIX II.
Practice principles of naturopathic medicine
Naturopathic medical practice is based upon the premise that it is intrinsic to the nature of living organisms to heal. The naturopathic physician understands illness to be a disruption of normal orderly function. Healing therefore is the process by which living systems return to a resilient equilibrium, either unassisted or with the therapeutic support of the practitioner.
Western medicine rarely takes into consideration the inherent organizing forces underlying known physiologic processes such as metabolism or tissue repair. Naturopathic medicine calls this primary principle the vis medicatrix naturae, or the healing power of nature.
Another principle fundamental to the can complement customary clinical practice restoration of health is the understanding that any intervention employed should not further disrupt a system attempting to regain homeostasis. This is expressed as primum non nocere, the imperative to first choose interventions which do the least harm.
When confronted with an ill patient, the naturopathic physician seeks to understand the totality of fundamental causes disrupting the patient’s optimal equilibrium. In order to remove the cause of the illness (tolle causum), one must treat the whole person.
In pursuit of removing or moderating the insults and stressors that result in harm to the patient, the doctor becomes teacher (docere) and engages the patient in the essential responsibilities of self-care. Participation in the restoration of personal wellbeing prepares the patient to behave proactively in the future, when mutual efforts at prevention predominate in the physician-patient relationship.1
Although these practice principles form the foundation of the naturopathic approach to health and healthcare, the philosophic and conceptual approaches of conventional medical theory apply as well, including complexity science, quantum physics, medical ecology, public health, energy medicine, and the biological basis of healing. The above principles do not replace the foundation of biological pathology, but offer the practitioner an expanded perspective when treating each individual patient. Naturopathic medicine ascribes to a therapeutic hierarchy that integrates the full spectrum of modern biomedicine in a continuum that includes mental, emotional and spiritual therapies, as appropriate to each patient’s needs.2 Applied in this context, biomedical interventions are highly valued as both diagnostic and therapeutic approaches.
Clinical approach to patients
The ultimate goal of each clinical assessment is to obtain an in-depth understanding of the patient’s underlying condition (including his or her experience) and to effectively communicate relevant information to other healthcare providers participating in the patient’s care.
Essential to a comprehensive evaluation is the extended interview, which ranges from 60 to 90 minutes for new patients. Follow up visits range between 30 and 60 minutes. A standard review of systems is supplemented with patient-generated reports of daily activities, such as dietary habits, physical activity, and psychological issues (see Naturopathic approach to one patient’s case). NDs perform physical examinations appropriate to the patient’s presenting complaint and health history, and employ conventional laboratory and diagnostic imaging services as needed. Clinical evaluation is patient-centered and addresses a full range of factors that influence health as well as illness, generating a problem-oriented patient record based on International Classification of Diseases (ICD-9) criteria.
Modalities most often used in naturopathic practice include clinical nutrition and dietary revision, counseling for lifestyle modification, botanical medicine, homeopathy, physical medicine, and mind-body therapies.
Scope of practice. Depending on local licensure statutes, naturopathic physicians may be fully recognized as primary healthcare providers.3 Prescriptive authority varies, as do provisions for other procedures commonly associated with general medical practice.4
Details of the scope of naturopathic practice in each licensed jurisdiction can be accessed by contacting local licensing authorities, usually via a state or provincial agency website. In the majority of jurisdictions, licensed NDs are responsible for all public health regulations including reportable diseases and immunizations. Most ND practice acts require annual continuing education credits to maintain practice privileges.
Interdisciplinary collaboration. NDs are trained to recognize serious and life-threatening situations and to identify conditions outside of the scope of their professional or legal limitations. Appropriate referral mechanisms are indoctrinated during educational and clinical training. NDs often work with conventionally trained family practice physicians, internists, and specialists in co-managing patients, participating in decisions regarding referral for evaluation and treatment by other allopathic and complementary/alternative medicine practitioners.
Safety and effectiveness of naturopathic medicine
Naturopathic practice is distinguished by treatments individualized to a patient’s physical condition and environmental circumstances, requiring combination therapies adjusted over time as guided by patient response. Documenting the safety and efficacy of naturopathic interventions presents significant challenges—eg, the limitations of the reductionistic approach of allopathic research models when applied to complex interventions and inadequacy of available funding sources. Research on the clinical and quality of life outcomes, particularly evaluation of the actual, complex whole practice as opposed to single agent or specific modalities, is relatively scant.5
Responding to this challenge, in 2002 the NIH funded more than 1200 scientists and physicians from both conventional and naturopathic academic and professional communities in a 2-year effort to design the Naturopathic Medical Research Agenda (NMRA). Guided by the NMRA process, the research departments of naturopathic academic centers are systematically developing the collaborative infrastructure required to examine the theory and practice of naturopathic medicine.6 The recently inaugurated International Journal of Naturopathic Medicine (available at www.intjnm.org) provides access to naturopathic-specific, peer-reviewed research.
Documentation of safety is as relevant as documentation of efficacy. As the potential for harm does exist within the naturopathic scope of practice,7 licensure in the US requires that adverse medical events be reported to the federally mandated National Practitioner Databank.8 The disciplinary records of naturopathic licensing boards provide scrutiny of practices regulated in those jurisdictions as well as documentation of specific offenses: over a 10-year period (1992–2002), 173 complaints were filed with state licensing boards from a total of 1805 licensees. During this period, 31 disciplinary actions were initiated, ranging from probation to fines or censure.9
Safety and efficacy are also of concern to third-party payers. More than 70 companies, trade unions, and state organizations offer health plans that cover naturopathic medical services,10-11 requiring utilization reviews incorporating documented patient outcomes. As NDs are increasingly covered as specialists and primary care providers under reimbursement plans of corporations such as Microsoft and Boeing, the credentialing processes required by their insurers (such as Blue Cross and Blue Shield, Kaiser Permanente, Connecticare, Oxford, and Healthnet) result in formal analyses of safety and efficacy of practice. Malpractice insurance industry data also verify incidents of harm that may occur related to naturopathic practice.
To access more in-depth information, including how to identify licensed NDs in a particular geographic area, contact the American Association of Naturopathic Physicians at www.naturopathic.org.
For information on naturopathic medical education, particularly the advanced standing programs available to degreed professionals, contact the American Association of Naturopathic Medical Colleges at www.aanmc.org.
An additional resource for degreed medical professionals interested in naturopathic professional practice is the website for the North American Board of Naturopathic Examiners at www.nabne.org.
The advent of integrated care has resulted in staff privileges granted to NDs at approximately 20 conventional hospitals and numerous integrated clinics. As a result, efficacy of peer review is strengthened as payers elect naturopathic medical directors to peer advisory committees charged with formulating reimbursement and case management policies.12
CORRESPONDING AUTHOR
Nancy Dunne, ND, Bitterroot Natural Medicine, 200 East Pine St., Missoula, MT 59802. E-mail: [email protected]
- Patients who inquire about naturopathy will want to know that clinical tools typically include nutrition evaluation and dietary revision, counseling for lifestyle modification, botanical medicine, homeopathy, physical medicine, and mind-body therapies.
- Advise patients who wish to seek naturopathic care to contact the state licensing authority to learn the scope of naturopathic practice allowed in their local area.
What can patients expect when they seek a naturopathic approach to disease management? A case presented in this article illustrates the applications of naturopathy in practice (see Naturopathic approach to one patient’s case: A summary).
Naturopathic physicians (NDs) diagnose and treat conditions typically seen in a “first contact” setting. They are not trained in the advanced use of highly technical conventional therapies for life-threatening diseases. Rather, they focus primarily on health issues encountered in out-patient ambulatory care settings (see Naturopathic training).
Though some tools of naturopathic practice differ from those of conventional practice, the goals of naturopathic medicine parallel those of family medicine in providing for and maintaining the well-being of both the patient and the healthcare system as a whole.
Collaboration is growing between conventional and naturopathic communities to examine the safety and efficacy of naturopathic medicine in preventing and managing a broad range of common conditions, and to determine whether availability of naturopathic services will improve patient health in a cost-effective manner.
Patient encounter
Martha S., a 39-year-old Asian-American
Presenting complaint
Has not felt well since onset of light-headedness, fatigue, muscle pain, and lassitude 4 years earlier/muscle tightness or tension and achiness come and go, often relieved by chiropractic treatment/some fuzzy cognition/dry gritty feeling in back of eyes/decreased libido/intermittent heart palpitations/sadness, easy weeping in conjunction with menses, lessens somewhat with St John’s Wort/disturbed, unrefreshing sleep 4 out of 7 days/body pain worse on waking
History
Three normal births and 3 spontaneous abortions with anticardiolipin antibodies that resolved after pregnancy/2 D&Cs, no other surgery/incidental finding of partially empty sella tursica on MS MRI investigation
Extensive specialty workups since 2001 have ruled out disease/internist who coordinated specialty consultations favors diagnosis of generalized anxiety disorder/some improvement with low doses Celexa, but unacceptable side effects (same with Effexor)/has tried amitriptyline/husband travels and she is uncomfortable with a “drugged sleep” when alone with the children
Fell off horse as teenager/no other physical injury or significant viral or bacterial illness/job exposes her to adversarial atmosphere and also requires occasional extensive hours at keyboard/infertility issues; experience of foreign adoption was a prolonged stressor
Family
Maternal aunt and grandmother had breast cancer age 50+; both survivors/paternal grandmother had stroke/father had postoperative DVT
Social
1–2 glasses wine/week; recreational drug use over 10 years in past/no regular exercise/attorney for city/married 11 years; husband 48, Euro/American, smokes, on anti-HTN medication/adopted sibs from Korea 1 year ago, 5-yr-old girl, 2-year-old boy, some malnutrition, parasites, now recovered
Exam
Patient is pleasant, articulate/no active disease/5’8”, 128#, BP 128/62, P 82/findings normal for HEENT, neck, chest, heart, abdomen, extremities, neurology, and skin/tender trigger points at bilateral trapezoids, paraspinal to subscapular, upper third gluteal and at hips
Lab
extensive records provided, essentially normal, none since 11/2003
Medications
None now
Allergies
Sensitive to drug side effects, but no known drug allergies
Management plan discussed with patient
In absence of other underlying disease, would like to treat you for fibromyalgia syndrome from long-term professional and personal stress/will work to recover your system from the physiologic effects of tension, worry, and hard work over past decade/if progress unsatisfactory after 3 months, we will revisit the diagnosis
Plan is to restore-rejuvenate your body, which knows how to right itself/think 6 months to a year for full recovery, after which you will have new knowledge of yourself and tools to maintain your well-being/details of the plan will shift as you recover and learn to use developing self knowledge to protect yourself during new challenges/flexibility and resilience are key and develop continually from self awareness/note what works for you and what doesn’t
Diet Goal
Hypoallergenic, whole-foods; small, frequent meals/adjust eicosanoid balance to increase systemic circulation, musculoskeletal flexibility, and cellular repair (patient given background article)/decrease production of pain-signaling chemistry, swelling that presses on nerves and creates the sensations of pain and stiffness; avoid sweets and refined carbohydrates, in order to maintain steady blood sugar levels
Use serotype diet (diet printed for patient) for the next 6 weeks/stick with best foods; dip into OK foods as little as possible/whet appetite for best food by “selfishly” focusing on your recovery/invite family to share meals, but primary purpose is your recovery; this can be hard for a mom to pull off; please invite your husband to call me if I can help him understand how he can enable you accomplish goal
Possible further steps
(see online version for details of action steps):
- Support/restore digestive tract: May not be making optimal gastric acid and other digestive factors as a result of long term stress stealing circulation away from those tissues that produce it.
- Eliminate simple sugars and refined flour products: Will help reduce pain/simple sugar creates hypoglycemic episode that can be experienced as nameless anxiety, weakness, fatigue, and dizziness/stable blood sugar essential for sense of well-being/eat pears, berries, or nuts if you need dessert.
- Exercise: Aerobic exercise 45 to 60 minutes, 3 or more times/week
- Sleep: Melatonin 250 μg to 500 mcg 30 or so minutes before bedtime/Deeper, assisted sleep will help, and you can adjust dosages to keep head clear in morning.
- Fundamental supplementation: For general well being, including fish oils, vitamins, and minerals.
- Adrenal recovery formula: We can presume your endocrine system has been affected by perceptions of threat (anxiety as related to the mystery of your physical pains) as well as long-term pain/recommend adrenal function test, to more closely determine optimal timing and doses of raw material that supports adrenal function
- Massage/body work
- Generalized anxiety disorder: your internist is convinced of this diagnosis; let’s discuss.
Outcome
Over 2 months: Sleep improved; trigger point pain diminished in upper body (by 30%), in gluteal and hips (80%)/able to manage diet “70% to 80% of the time”/exercise 3 to 5 times weekly, less when husband travels
Vacation interlude: Treatment plan jettisoned for vacation/return of rheumatic symptoms; dizziness and lassitude, however, continued to improve
Next 2 months: Continued improvement, with trigger point pain flaring only on long drives
Naturopathic physicians graduate from 1 of 6 naturopathic medical schools accredited by the Council on Naturopathic Medical Education (CNME) (TABLE 1). The CNME is a member of the Association of Specialized and Professional Accreditors recognized by the US Department of Education. Each school in the United States is also accredited by, or has candidacy status through, the specific regional agencies responsible for overseeing postsecondary institutions of higher learning.
Requirements for admission. The goal of naturopathic medical education is to prepare clinicians for the challenges of general practice, with a foundation in current medical science as well as traditional naturopathic theory. Candidates for admission to naturopathic medical school must earn a baccalaureate degree (or equivalent) prior to matriculation, including standard premedical undergraduate courses.
Naturopathic curricula. Subjects include inorganic and organic chemistry, physics, general biology and psychology. Other coursework is comparable to that of allopathic and osteopathic medical schools (TABLE 2). While the first 2 years of education combine courses in naturopathic principles with basic and diagnostic sciences, third and fourth year students focus on clinical practice, receiving training at naturopathic primary care outpatient clinics as well as conventional medical facilities (TABLE 3). Academic faculty at such institutions include NDs, PhDs, MDs, DOs, and other allied health professionals.
For information on postgraduate residencies, research, and collaborative opportunities for NDs, please see APPENDIX I. For additional information on naturopathic licensure, please see APPENDIX II.
Practice principles of naturopathic medicine
Naturopathic medical practice is based upon the premise that it is intrinsic to the nature of living organisms to heal. The naturopathic physician understands illness to be a disruption of normal orderly function. Healing therefore is the process by which living systems return to a resilient equilibrium, either unassisted or with the therapeutic support of the practitioner.
Western medicine rarely takes into consideration the inherent organizing forces underlying known physiologic processes such as metabolism or tissue repair. Naturopathic medicine calls this primary principle the vis medicatrix naturae, or the healing power of nature.
Another principle fundamental to the can complement customary clinical practice restoration of health is the understanding that any intervention employed should not further disrupt a system attempting to regain homeostasis. This is expressed as primum non nocere, the imperative to first choose interventions which do the least harm.
When confronted with an ill patient, the naturopathic physician seeks to understand the totality of fundamental causes disrupting the patient’s optimal equilibrium. In order to remove the cause of the illness (tolle causum), one must treat the whole person.
In pursuit of removing or moderating the insults and stressors that result in harm to the patient, the doctor becomes teacher (docere) and engages the patient in the essential responsibilities of self-care. Participation in the restoration of personal wellbeing prepares the patient to behave proactively in the future, when mutual efforts at prevention predominate in the physician-patient relationship.1
Although these practice principles form the foundation of the naturopathic approach to health and healthcare, the philosophic and conceptual approaches of conventional medical theory apply as well, including complexity science, quantum physics, medical ecology, public health, energy medicine, and the biological basis of healing. The above principles do not replace the foundation of biological pathology, but offer the practitioner an expanded perspective when treating each individual patient. Naturopathic medicine ascribes to a therapeutic hierarchy that integrates the full spectrum of modern biomedicine in a continuum that includes mental, emotional and spiritual therapies, as appropriate to each patient’s needs.2 Applied in this context, biomedical interventions are highly valued as both diagnostic and therapeutic approaches.
Clinical approach to patients
The ultimate goal of each clinical assessment is to obtain an in-depth understanding of the patient’s underlying condition (including his or her experience) and to effectively communicate relevant information to other healthcare providers participating in the patient’s care.
Essential to a comprehensive evaluation is the extended interview, which ranges from 60 to 90 minutes for new patients. Follow up visits range between 30 and 60 minutes. A standard review of systems is supplemented with patient-generated reports of daily activities, such as dietary habits, physical activity, and psychological issues (see Naturopathic approach to one patient’s case). NDs perform physical examinations appropriate to the patient’s presenting complaint and health history, and employ conventional laboratory and diagnostic imaging services as needed. Clinical evaluation is patient-centered and addresses a full range of factors that influence health as well as illness, generating a problem-oriented patient record based on International Classification of Diseases (ICD-9) criteria.
Modalities most often used in naturopathic practice include clinical nutrition and dietary revision, counseling for lifestyle modification, botanical medicine, homeopathy, physical medicine, and mind-body therapies.
Scope of practice. Depending on local licensure statutes, naturopathic physicians may be fully recognized as primary healthcare providers.3 Prescriptive authority varies, as do provisions for other procedures commonly associated with general medical practice.4
Details of the scope of naturopathic practice in each licensed jurisdiction can be accessed by contacting local licensing authorities, usually via a state or provincial agency website. In the majority of jurisdictions, licensed NDs are responsible for all public health regulations including reportable diseases and immunizations. Most ND practice acts require annual continuing education credits to maintain practice privileges.
Interdisciplinary collaboration. NDs are trained to recognize serious and life-threatening situations and to identify conditions outside of the scope of their professional or legal limitations. Appropriate referral mechanisms are indoctrinated during educational and clinical training. NDs often work with conventionally trained family practice physicians, internists, and specialists in co-managing patients, participating in decisions regarding referral for evaluation and treatment by other allopathic and complementary/alternative medicine practitioners.
Safety and effectiveness of naturopathic medicine
Naturopathic practice is distinguished by treatments individualized to a patient’s physical condition and environmental circumstances, requiring combination therapies adjusted over time as guided by patient response. Documenting the safety and efficacy of naturopathic interventions presents significant challenges—eg, the limitations of the reductionistic approach of allopathic research models when applied to complex interventions and inadequacy of available funding sources. Research on the clinical and quality of life outcomes, particularly evaluation of the actual, complex whole practice as opposed to single agent or specific modalities, is relatively scant.5
Responding to this challenge, in 2002 the NIH funded more than 1200 scientists and physicians from both conventional and naturopathic academic and professional communities in a 2-year effort to design the Naturopathic Medical Research Agenda (NMRA). Guided by the NMRA process, the research departments of naturopathic academic centers are systematically developing the collaborative infrastructure required to examine the theory and practice of naturopathic medicine.6 The recently inaugurated International Journal of Naturopathic Medicine (available at www.intjnm.org) provides access to naturopathic-specific, peer-reviewed research.
Documentation of safety is as relevant as documentation of efficacy. As the potential for harm does exist within the naturopathic scope of practice,7 licensure in the US requires that adverse medical events be reported to the federally mandated National Practitioner Databank.8 The disciplinary records of naturopathic licensing boards provide scrutiny of practices regulated in those jurisdictions as well as documentation of specific offenses: over a 10-year period (1992–2002), 173 complaints were filed with state licensing boards from a total of 1805 licensees. During this period, 31 disciplinary actions were initiated, ranging from probation to fines or censure.9
Safety and efficacy are also of concern to third-party payers. More than 70 companies, trade unions, and state organizations offer health plans that cover naturopathic medical services,10-11 requiring utilization reviews incorporating documented patient outcomes. As NDs are increasingly covered as specialists and primary care providers under reimbursement plans of corporations such as Microsoft and Boeing, the credentialing processes required by their insurers (such as Blue Cross and Blue Shield, Kaiser Permanente, Connecticare, Oxford, and Healthnet) result in formal analyses of safety and efficacy of practice. Malpractice insurance industry data also verify incidents of harm that may occur related to naturopathic practice.
To access more in-depth information, including how to identify licensed NDs in a particular geographic area, contact the American Association of Naturopathic Physicians at www.naturopathic.org.
For information on naturopathic medical education, particularly the advanced standing programs available to degreed professionals, contact the American Association of Naturopathic Medical Colleges at www.aanmc.org.
An additional resource for degreed medical professionals interested in naturopathic professional practice is the website for the North American Board of Naturopathic Examiners at www.nabne.org.
The advent of integrated care has resulted in staff privileges granted to NDs at approximately 20 conventional hospitals and numerous integrated clinics. As a result, efficacy of peer review is strengthened as payers elect naturopathic medical directors to peer advisory committees charged with formulating reimbursement and case management policies.12
CORRESPONDING AUTHOR
Nancy Dunne, ND, Bitterroot Natural Medicine, 200 East Pine St., Missoula, MT 59802. E-mail: [email protected]
1. Snider P, Zeff J. Report of the Select Committee on the Definition of Naturopathic Medicine. Washington, DC: AANP; 1988.
2. Micozzi M. Fundamentals of Complementary and Alternative Medicine. 2nd ed. Philadelphia, Pa: Churchill-Livingstone; 2001;181-183.
3. Kim L, Mills E. Physicians’ Information and Education Resource (PIER). Naturopathic Medicine, American College of Physicians; American Society of Internal Medicine July 1 2003.
4. Hough H, Dower C, O’Neill E. Profile of a Profession: Naturopathic Practice. San Francisco, Calif: Center for the Health Professions, University of California, San Francisco; 2001;27.-
5. Standish LJ, Calabrese C, Snider P. The Naturopathic Medical Research Agenda: The Future and Foundation of Naturopathic Medical Science. Kenmore, Wash: Bastyr University Press; 2005;10.-
6. Calabrese C, Reilly P, Lukaczer D. Position paper on Naturopathic Research, American Association of Naturopathic Physicians, 2003. Available at: http://www.naturopathic.org/positions/research_position_paper.html. Accessed on January 2, 2005.
7. Myers SP, Cheras PA. The other side of the coin: Safety of complementary and alternative medicine. Med J Aust. 2004;181:222-225.
8. Healthcare Integrity and Protection Databank National Practitioner Databank. Available at: http://www.npdb-hipdb.com/hipdb.html. Accessed on February 1, 2004.
9. Snider P, Cutler S. Naturopathic Profession Research Documentation. Kenmore, Wash: Bastyr University Press, 2002.
10. Quinn S, et al. Naturopathic Medicine: Primary Care for the Twenty-First Century. Washington, DC: AANP; 2003;12.-
11. Lafferty WE, Bellas A, Baden A, Tyree PT, Standish LJ, Patterson R. The use of complementary and alternative medical providers by insured cancer patients in Washington state. Cancer 2004;100:1522-1530.
12. Pizzorno J, Snider P. Fundamentals of Complementary and Alternative Medicine. Ed.M. Micozzi. Philadelphia, Pa: Churchill-Livingstone; 1996;173.
1. Snider P, Zeff J. Report of the Select Committee on the Definition of Naturopathic Medicine. Washington, DC: AANP; 1988.
2. Micozzi M. Fundamentals of Complementary and Alternative Medicine. 2nd ed. Philadelphia, Pa: Churchill-Livingstone; 2001;181-183.
3. Kim L, Mills E. Physicians’ Information and Education Resource (PIER). Naturopathic Medicine, American College of Physicians; American Society of Internal Medicine July 1 2003.
4. Hough H, Dower C, O’Neill E. Profile of a Profession: Naturopathic Practice. San Francisco, Calif: Center for the Health Professions, University of California, San Francisco; 2001;27.-
5. Standish LJ, Calabrese C, Snider P. The Naturopathic Medical Research Agenda: The Future and Foundation of Naturopathic Medical Science. Kenmore, Wash: Bastyr University Press; 2005;10.-
6. Calabrese C, Reilly P, Lukaczer D. Position paper on Naturopathic Research, American Association of Naturopathic Physicians, 2003. Available at: http://www.naturopathic.org/positions/research_position_paper.html. Accessed on January 2, 2005.
7. Myers SP, Cheras PA. The other side of the coin: Safety of complementary and alternative medicine. Med J Aust. 2004;181:222-225.
8. Healthcare Integrity and Protection Databank National Practitioner Databank. Available at: http://www.npdb-hipdb.com/hipdb.html. Accessed on February 1, 2004.
9. Snider P, Cutler S. Naturopathic Profession Research Documentation. Kenmore, Wash: Bastyr University Press, 2002.
10. Quinn S, et al. Naturopathic Medicine: Primary Care for the Twenty-First Century. Washington, DC: AANP; 2003;12.-
11. Lafferty WE, Bellas A, Baden A, Tyree PT, Standish LJ, Patterson R. The use of complementary and alternative medical providers by insured cancer patients in Washington state. Cancer 2004;100:1522-1530.
12. Pizzorno J, Snider P. Fundamentals of Complementary and Alternative Medicine. Ed.M. Micozzi. Philadelphia, Pa: Churchill-Livingstone; 1996;173.
Practical strategy for detecting and relieving cluster headaches
- Learn to recognize the distinctive pattern of cluster headaches that in most cases, even if some symptoms suggest migraine, will readily reveal this disorder (C).
- 100% oxygen by face mask and injected sumatriptan (Imitrex) are effective choices to terminate an acute attack (B).
- Verapamil (Calan) or corticosteroids abort cluster cycles and prevent further attacks (B).
You interview a “migraine” sufferer who hopes to find the relief that has so far eluded him. During the history taking, he reports experiencing the premonitory aura typical of migraine as well as photophobia and occasionally nausea. One description, though, raises a question about the cause of his headaches: he says the intense pain recurs at roughly the same time of day and lasts for about 45 minutes; he is unable to concentrate on anything but the pain and he paces ceaselessly until it abates. The odds now favor a diagnosis of cluster headache.
Given that symptoms of migraine and cluster headaches overlap, and that migraine is more prevalent than cluster headache, recognizing the latter requires sensitivity to its key attributes described in this article.
Careful selection of therapeutic agents will hasten resolution of acute pain and prevent recurrences. Some drugs may be combined for quicker onset of action.
Telltale characteristics of cluster headaches
As shown in the opening example, cluster headache is diagnosed primarily by history (see Source of cluster headaches).
Recognize the pattern. Its most striking feature is the unmistakable pattern of repeated bouts of pain that “cluster” at the same time of the day or night.
Each attack is extremely intense and brief, typically lasting 15 to 180 minutes.
For patients with the episodic variety of cluster headaches, the pattern of repeated headaches may last days to weeks, then resolve spontaneously. The relatively few patients with the chronic form of the disorder experience an unending cycle of daily or near daily headaches.
Attacks may occur during the day or night, and often wake the patient from sleep.1
Pain is always unilateral. Location of the pain varies among patients but is usually within the distribution of the trigeminal nerve. Almost all patients (92%) have retro-orbital pain, and most (70%) also have pain in the temporal region. Pain is present in the upper teeth, jaw, forehead, or cheek half of the time. Less common sites of pain are the ears, lower jaw, neck and shoulder.1
Look for associated findings. Pain is accompanied by signs and symptoms of ipsilateral autonomic dysfunction. Lacrimation on the affected side is the most common associated feature. Rhinorrhea or a blocked nasal passage, red eye, and swelling or pallor of the forehead or cheek are often found bilaterally but are clinically dominant on the symptomatic side.
Restlessness occurs during an attack and the patient often prefers to pace about, in striking contrast to the migraine sufferer, who avoids activity so as not to exacerbate the pain. Half of cluster headache sufferers experience nausea, photophobia, or phonophobia during attacks. A smaller number (14%) report an aura similar to that of a migraine. Ninety percent of cluster headache patients who drink alcohol say it triggers headache while they are in the midst of a cluster cycle.1
Delay in diagnosis common. The 2004 revision of the International Classification of Headache Disorders, reflects our improved understanding of and ability to identify these disorders.4 The mean time to diagnosis of cluster headache has decreased from 22 years in the 1960s to 2.6 years in the 1990s, reflecting much better recognition of the syndrome.5 Nevertheless most patients consult three primary care physicians before a diagnosis is made. The time between the first episode and diagnosis ranged from 1 week to 48 years (median 3 years) in one recent study.6
Overlap of migraine and cluster symptoms may lead to misdiagnosis. Factors contributing to diagnostic delay include photophobia or phonophobia, nausea, an episodic attack pattern and a younger age at onset (P<.01). Correct diagnosis is further complicated in that 26% of cluster headache suffers also report a history of migraine headaches.1 The Key differentiating factor between the two headaches types is the predictable pattern of repeated, intense, brief head pain.
Rare underlying causes. A very few patients with headaches have brain tumors. Headache is present in 50% to 60% of newly diagnosed brain tumors, but is usually accompanied by other signs or symptoms. It is the only presenting symptom in approximately 8% of cases. Most headaches due to tumors are clinically similar to tension headache (77%), and some mimic migraine (9%). Rapidly growing tumors are more likely to be associated with constant unremitting headache. Rarely brain tumors may produce pain syndromes similar to cluster headache.7 Other causes of secondary cluster headache include infections, vascular abnormalities, and head trauma.
A new subclassification of primary headache, trigeminal autonomic cephalgia, incorporates cluster headache with several other rarer types of headache that can be difficult to distinguish from primary cluster headache.8 Differentiation is important because the non-cluster types respond dramatically to indomethacin, whereas cluster headaches do not (TABLE 1).9
TABLE 1
Source of cluster headaches
| FEATURE | CLUSTER | MIGRAINE | PAROXYSMAL HEMICRANIA | SUNCT* | HEMICRANIA CONTINUA |
|---|---|---|---|---|---|
| Duration | 15–180 min | 4–72 hrs | 2–30 min | 5–240 sec | Continuous |
| Autonomic dysfunction | Yes | Unusual | Yes | Yes | Sometimes |
| Pain quality | Sharp, boring | Often pulsatile | Stabbing or Stabbing | Stabbing or pulsatile | Stabbing or pulsatile |
| Severity | Severe | Mod–severe | Severe | Severe | Mod–severe |
| Frequency | Predictable | Varies | >5/day | 3–200/day | Continuous |
| Laterality | Unilateral | Varies | Unilateral | Unilateral | Unilateral |
| Response to Indomethacin | Not usually | Not usually | Always | Always | Always |
| *SUNCT, short-lasting neuralgiform headache attacks with conjunctival injection and tearing. | |||||
| Source: Lipton et al, Neurology 2004.4 | |||||
The 2 goals of treatment
Terminating acute headache is the first goal; shortening or aborting the cluster cycle is the second.
Research in the treatment of cluster headaches has been hampered by the relative infrequency of the condition, the short duration of each episode, and a robust placebo response.10 Much of the available evidence for the efficacy of various treatments comes from small controlled studies and case series.
Episodic cluster headaches respond much more readily to therapy than do chronic cluster headaches. Remember that no single intervention will work for every patient, and that some options are highly effective only for a small percentage of patients.
Of the drugs discussed in this section, injectable sumatriptan for acute attacks and oral verapamil for prophylaxis of attacks have the best evidence of efficacy based on controlled clinical trials.11
Terminating acute headaches
100% oxygen. One hundred percent oxygen, delivered by face mask at high flow rates, has been shown to reduce the severity of cluster headaches or terminate acute attacks (TABLE 2). Absence of side effects is the real advantage of this therapy; the major drawback is the lack of portability of an adequate oxygen supply (strength of recommendation [SOR]: B). 22
Triptans. Subcutaneous sumatriptan has been shown to provide relief for 88% of users (number needed to treat [NNT]=2.1), and its effectiveness seems not to wane with repeated use. The preferred dose is 6 mg; higher doses have been studied and are no more effective (SOR: A).23 Intranasal sumatriptan is also effective, although less so than the parenteral form (NNT=3.2).13 Oral zolmitriptan (Zomig) has shown benefit in approximately 60% of cases with both the 5- and 10-mg doses in episodic cluster headaches (NNT=5.6), but is no more effective than placebo in chronic cluster patients.15
Side effects. Most patients report side effects with triptans,24 the most common being “atypical sensations” such as tingling, heat, pressure, tightness, numbness, or flushing. Dizziness and sedation can also occur, and, with injectable sumatriptan, reactions at the injection site are common. Before prescribing injectable sumatriptan, supervise administration of the first dose.
Caveats. Triptans are contraindicated for patients with vascular disease (coronary artery disease, stroke, peripheral vascular disease), renal, or liver dysfunction. Triptans should be used with caution by persons with multiple risk factors for coronary disease. They cannot be used in combination with other triptans or within 24 hours of the use of dihydroergotamine (DHE 45).25
Dihydroergotamine. DHE can be used to terminate acute attacks using intravenous, subcutaneous, or intramuscular routes of administration (SOR: B). The usual dose is 1 mg, and many clinicians administer 10 mg of metoclopramide (Reglan) simultaneously to counter nausea (SOR: C). Complete familiarity with the proper use and potential adverse effects of injectable DHE is critical before using it in the outpatient setting.
Like triptans, DHE is contraindicated for those with vascular disease or severe liver or kidney impairment. Side effects include numbness or tingling in the extremities, muscle cramps, palpitations, and pain or tightness in the chest. Pleural and retroperitoneal fibrosis has occurred following prolonged daily use of ergots, and the use of DHE in patients with unrecognized coronary artery disease has caused death.
DHE levels are elevated by concurrent use of cytochrome P450 3A4 inhibitors such as macrolide antibiotics, protease inhibitors, ketoconazole, and itraconazole.26
Other abortive agents. There is little evidence for the use of other abortive agents. This poses a significant problem for the patient with cluster headaches who cannot take vasoconstrictors. A study of 5 patients showed olanzapine (Zyprexa), 2.5 to 10 mg, is a potentially effective abortive agent,27 and a larger study showed that octreotide (Sandostatin), 100 μg subcutaneously, relieved 52% of cluster headaches (NNT=6.3).16 Intranasal lidocaine has been shown to provide relief for 55% of migraine headaches, and some recommend its use in cluster headache.28
Prevention and interruption of the cluster cycle
More important than aborting the acute headache is ending the cluster episode.
Verapamil. Ample evidence supports the effectiveness of verapamil (Calan) for this purpose (NNT=1.2).17 Larger doses than are typical for hypertensive therapy may be required.
Cluster headache, the most severe primary headache, is rare compared with other types of headache. Thus, despite severe head pain, the diagnosis may be overlooked.6 Studies in which the diagnosis was clinically confirmed reveal a prevalence ranging from 56 to 381 cases per 100,000 people.29 Cluster differs from migraine in that men are affected more commonly than women. Once believed to have a gender differential of 6:1, the ratio is now reported at 3.7:1.6
Age of onset also contrasts with migraine. Cluster headaches typically begin at around 30 years of age with a range of 20 to 50 years, but rarely as old as 80 years. Women are more likely than men to have onset in later years.
Most patients are smokers or former smokers (74%), but cessation of smoking does not appear to modify the pattern of headaches.
Inheritance plays a greater role than previously realized, suggesting a genetic cause. First-degree relatives have a 5- to 18-fold higher risk for cluster headache than the general population. Second-degree relatives have a 1- to 3-fold higher risk. The mode of inheritance is likely autosomal dominant with low penetrance in some families, and multifactoral inheritance or autosomal recessive in other families.29
A regimen of 40 mg in the morning, 80 mg at noon, and 80 mg at bedtime, allowing patients to titrate doses up by 40 mg on alternate days, relieved 94% of episodic cluster headaches and 55% of chronic cases (SOR: B).30 Most patients need 200 to 480 mg/d to achieve success, but some require up to 960 mg/d.30
If a patient is asymptomatic but has a history consistent with cluster headaches, and if your examination reveals no other cause of headache, a trial of verapamil is warranted to abort the cluster cycle or prevent additional cycles.
Other agents. Corticosteroids may act faster than verapamil, and the two can be used in combination (SOR: C). A typical regimen of prednisone starts with 40 mg/d and tapers over 3 weeks.28
Lithium (Lithobid, Eskalith) is effective, but acts slowly and causes more side effects than other agents.20
Other agents that have shown efficacy in small studies are gabapentin, baclofen, clonidine, twice daily eletriptan, and topiramate.31-34
Sodium valproate (Depakote) is also used prophylactically for both cluster and migraine headaches. One small study demonstrated efficacy, but a larger trial failed to show benefit due to a unexpectedly high placebo response rate.35,36
When to refer
Treatment for most cluster headache sufferers is adequately handled in the primary care setting. When medical therapy fails, consider referral to a headache specialist, particularly for those with chronic cluster headaches. In some cases, sympathetic nerve blockade might be a worthwhile consideration.37 Unfortunately, a small subset of patients will not find relief regardless of the regimen employed.
TABLE 2
Selected cluster headache trials
| DRUG | RESPONSE | # OF PTS | NNT |
|---|---|---|---|
| Sumatriptan 6 mg subcut. vs placebo12 | 74% response @ 15 min 26% placebo response | 39 | 2.1 |
| Sumatriptan nasal vs placebo13 | 57% response @ 30 min 26% placebo response | 118 | 3.2 |
| Sumatriptan subcut. vs sumatriptan nasal14 | 94% response to injection @ 15 min 13% response to nasal | 49 | 1.2 |
| Zolmitriptan 10 mg orally vs placebo15 | 47% response @ 30 min 29% placebo response | 124 | 5.6 |
| Octreotide 100 μg subcut. vs placebo16 | 52% response @ 30 min 36% placebo response | 46 | 6.3 |
| Verapamil 120 mg orally 3× daily vs placebo17 | 80% response in 2nd week | 15 each arm | 1.2 |
| Oxygen 100%18 | 75% with significant pain relief within 15 min | 52 | — |
| Dihydroergotamine IV19 | 73% relief refractory episodic cluster | 60 | — |
| 63% relief refractory chronic cluster | 37 | — | |
| Lithium 900 mg daily vs Verapamil 360 mg daily20 | Lithium: 37% improve in 1st week Verapamil: 58% improve in 1st week | 24 | 4.8 |
| Eletriptan 40 mg twice daily×6 days21 | 40% fewer attacks | 16 | — |
CORRESPONDENCE
Stephen Adams, MD, 1100 East Third Street, Chattanooga TN 37403. E-mail: [email protected]
1. Bahra A, May A, Goadsby PJ. Cluster headache: a prospective clinical study with diagnostic implications. Neurology 2002;58:354-361.
2. D’Andrea G, Terrazzino S, et al. Elevated levels of circulating trace amines in primary headaches. Neurology 2004;62:1701-1705.
3. Cohen AS, Goadsby PJ. Functional neuroimaging of primary headache disorders. Curr Neurol Neurosci Rep 2004;4:105-110.
4. Lipton RB, Bigal ME, Steiner TJ, Silberstein SD, Olesen J. Classification of primary headaches. Neurology 2004;63:427-435.
5. Bahra A, Goadsby PJ. Diagnostic delays and mis-management in cluster headache. Acta Neurol Scand 2004;109:175-179.
6. van Vliet JA, Eekers PJ, Haan J, Ferrari MD. Dutch RUSSH Study Group. Features involved in the diagnostic delay of cluster headache. J Neurol Neurosurg Psychiatry 2003;74:1123-1125.
7. Purdy RA, Kirby S. Headaches and brain tumors. Neurol Clin 2004;22:39-53.
8. Carter DM. Cluster headache mimics. Curr Pain Headache Rep 2004;8:133-139.
9. May A. Headaches with (ipsilateral) autonomic symptoms. J Neurol 2003;250:1273-1278.
10. Nilsson Remahl AI, Laudon Meyer E, Cordonnier C, et al. Placebo response in cluster headache trials: a review. Cephalalgia 2003;23:504-510.
11. Moore K. Cluster headache: the challenge of clinical trials. Curr Pain Headache Rep 2002;6:52-56.
12. Treatment of acute cluster headache with sumatriptan. The Sumatriptan Cluster Headache Study Group. N Engl J Med 1991;325:322-326.
13. van Vliet JA, Bahra A, Martin V, et al. Intranasal sumatriptan in cluster headache: randomized placebo controlled double-blind study. Neurology 2003;60:630-633.
14. Hardebo JE, Dahlof C. Sumatriptan nasal spray (20 mg/dose) in the acute treatment of cluster headache. Cephalalgia 1998;18:487-489.
15. Bahra A, Gawel MJ, Hardebo JE, Millson D, Breen SA, Goadsby PJ. Oral zolmitriptan is effective in the acute treatment of cluster headache. Neurology 2000;54:1832-1839.
16. Matharu MS, Levy MJ, Meeran K, Goadsby PJ. Subcutaneous octreotide in cluster headache: randomized placebo-controlled double-blind crossover study. Ann Neurol 2004;56:488-494.
17. Leone M, D’Amico D, Frediani F, et al. Verapamil in the prophylaxis of episodic cluster headache: a double bline study versus placebo. Neurology 2000;54:1382-1385.
18. Kudrow L. Response of cluster headache attacks to oxygen inhalation. Headache 1981;21:1-4.
19. Magnoux E, Zlotnik G. Outpatient intravenous dihydroergotamine for refractory cluster headache. Headache 2004;44:249-255.
20. Bussone G, Leone M, Peccarisi C, et al. Double blind comparison of lithium and verapamil in cluster headache prophylaxis. Headache 1990;30:411-417.
21. Zebenholzer K, Wober C, Vigl M, Wessely P. Eletriptan for the short-term prophylaxis of cluster headache. Headache 2004;44:361-364.
22. Fogan L. Treatment of cluster headache. A double-blind comparison of oxygen v air inhalation. Arch Neurol 1985;42:362-363.
23. Ekbom K, Monstad I, Prusinski A, Cole JA, Pilgrim AJ, Noronha D. Subcutaneous sumatriptan in the acute treatment of cluster headache: a dose comparison study. The Sumatriptan Cluster Headache Study Group. Acta Neurol Scand 1993;88:63-69.
24. Gobel H, Lindner V, Heinze A, Ribbat M, Deuschl G. Acute therapy for cluster headache with sumatriptan: findings of a one-year long-term study. Neurology 1998;51:908-911.
25. Physicians’ Desk Reference. 57th ed. Montvale, NJ: Thomson PDR; 2003;1544.-
26. DHE 45 package insert; Novartis Pharmaceuticals AG. 2002.
27. Rozen TD. Olanzapine as an abortive agent for cluster headache. Headache 2001;41:813-816.
28. Freitag FG. Cluster headache. Primary Care; Clinics in Office Practice 2004;31(no 2), June.
29. Russell MB. Epidemiology and genetics of cluster headache. Lancet Neurol 2004;3:279-283.
30. Blau JN, Engel HO. Individualizing treatment with verapamil for cluster headache patients. Headache 2004;44:1013-1018.
31. Leandri M, Luzzani M, Cruccu G, Gottlieb A. Drug-resistant cluster headache responding to gabapentin: a pilot study. Cephalalgia 2001;21:744-746.
32. Hering-Hanit R, Gadoth N. The use of baclofen in cluster headache. Curr Pain Headache Rep 2001;5:79-82.
33. D’Andrea G, Perini F, Granella F, Cananzi A, Sergi A. Efficacy of transdermal clonidine in short-term treatment of cluster headache: a pilot study. Cephalalgia 1995;15:430-433.
34. Lainez MJ, Pascual J, Pascual AM, Santonja JM, Ponz A, Salvador A. Topiramate in the prophylactic treatment of cluster headache. Headache 2003;43:784-789.
35. Hering R, Kuritzky A. Sodium valproate in the treatment of cluster headache: an open clinical trial. Cephalalgia 1989;9:195-198.
36. El Amrani M, Massiou H, Bousser MG. A negative trial of sodium valproate in cluster headache: methodological issues. Cephalalgia 2002;22:205-208.
37. Albertyn J, Barry R, Odendall CL. Cluster headache and the sympathetic nerve. Headache 2004;44:183-185.
- Learn to recognize the distinctive pattern of cluster headaches that in most cases, even if some symptoms suggest migraine, will readily reveal this disorder (C).
- 100% oxygen by face mask and injected sumatriptan (Imitrex) are effective choices to terminate an acute attack (B).
- Verapamil (Calan) or corticosteroids abort cluster cycles and prevent further attacks (B).
You interview a “migraine” sufferer who hopes to find the relief that has so far eluded him. During the history taking, he reports experiencing the premonitory aura typical of migraine as well as photophobia and occasionally nausea. One description, though, raises a question about the cause of his headaches: he says the intense pain recurs at roughly the same time of day and lasts for about 45 minutes; he is unable to concentrate on anything but the pain and he paces ceaselessly until it abates. The odds now favor a diagnosis of cluster headache.
Given that symptoms of migraine and cluster headaches overlap, and that migraine is more prevalent than cluster headache, recognizing the latter requires sensitivity to its key attributes described in this article.
Careful selection of therapeutic agents will hasten resolution of acute pain and prevent recurrences. Some drugs may be combined for quicker onset of action.
Telltale characteristics of cluster headaches
As shown in the opening example, cluster headache is diagnosed primarily by history (see Source of cluster headaches).
Recognize the pattern. Its most striking feature is the unmistakable pattern of repeated bouts of pain that “cluster” at the same time of the day or night.
Each attack is extremely intense and brief, typically lasting 15 to 180 minutes.
For patients with the episodic variety of cluster headaches, the pattern of repeated headaches may last days to weeks, then resolve spontaneously. The relatively few patients with the chronic form of the disorder experience an unending cycle of daily or near daily headaches.
Attacks may occur during the day or night, and often wake the patient from sleep.1
Pain is always unilateral. Location of the pain varies among patients but is usually within the distribution of the trigeminal nerve. Almost all patients (92%) have retro-orbital pain, and most (70%) also have pain in the temporal region. Pain is present in the upper teeth, jaw, forehead, or cheek half of the time. Less common sites of pain are the ears, lower jaw, neck and shoulder.1
Look for associated findings. Pain is accompanied by signs and symptoms of ipsilateral autonomic dysfunction. Lacrimation on the affected side is the most common associated feature. Rhinorrhea or a blocked nasal passage, red eye, and swelling or pallor of the forehead or cheek are often found bilaterally but are clinically dominant on the symptomatic side.
Restlessness occurs during an attack and the patient often prefers to pace about, in striking contrast to the migraine sufferer, who avoids activity so as not to exacerbate the pain. Half of cluster headache sufferers experience nausea, photophobia, or phonophobia during attacks. A smaller number (14%) report an aura similar to that of a migraine. Ninety percent of cluster headache patients who drink alcohol say it triggers headache while they are in the midst of a cluster cycle.1
Delay in diagnosis common. The 2004 revision of the International Classification of Headache Disorders, reflects our improved understanding of and ability to identify these disorders.4 The mean time to diagnosis of cluster headache has decreased from 22 years in the 1960s to 2.6 years in the 1990s, reflecting much better recognition of the syndrome.5 Nevertheless most patients consult three primary care physicians before a diagnosis is made. The time between the first episode and diagnosis ranged from 1 week to 48 years (median 3 years) in one recent study.6
Overlap of migraine and cluster symptoms may lead to misdiagnosis. Factors contributing to diagnostic delay include photophobia or phonophobia, nausea, an episodic attack pattern and a younger age at onset (P<.01). Correct diagnosis is further complicated in that 26% of cluster headache suffers also report a history of migraine headaches.1 The Key differentiating factor between the two headaches types is the predictable pattern of repeated, intense, brief head pain.
Rare underlying causes. A very few patients with headaches have brain tumors. Headache is present in 50% to 60% of newly diagnosed brain tumors, but is usually accompanied by other signs or symptoms. It is the only presenting symptom in approximately 8% of cases. Most headaches due to tumors are clinically similar to tension headache (77%), and some mimic migraine (9%). Rapidly growing tumors are more likely to be associated with constant unremitting headache. Rarely brain tumors may produce pain syndromes similar to cluster headache.7 Other causes of secondary cluster headache include infections, vascular abnormalities, and head trauma.
A new subclassification of primary headache, trigeminal autonomic cephalgia, incorporates cluster headache with several other rarer types of headache that can be difficult to distinguish from primary cluster headache.8 Differentiation is important because the non-cluster types respond dramatically to indomethacin, whereas cluster headaches do not (TABLE 1).9
TABLE 1
Source of cluster headaches
| FEATURE | CLUSTER | MIGRAINE | PAROXYSMAL HEMICRANIA | SUNCT* | HEMICRANIA CONTINUA |
|---|---|---|---|---|---|
| Duration | 15–180 min | 4–72 hrs | 2–30 min | 5–240 sec | Continuous |
| Autonomic dysfunction | Yes | Unusual | Yes | Yes | Sometimes |
| Pain quality | Sharp, boring | Often pulsatile | Stabbing or Stabbing | Stabbing or pulsatile | Stabbing or pulsatile |
| Severity | Severe | Mod–severe | Severe | Severe | Mod–severe |
| Frequency | Predictable | Varies | >5/day | 3–200/day | Continuous |
| Laterality | Unilateral | Varies | Unilateral | Unilateral | Unilateral |
| Response to Indomethacin | Not usually | Not usually | Always | Always | Always |
| *SUNCT, short-lasting neuralgiform headache attacks with conjunctival injection and tearing. | |||||
| Source: Lipton et al, Neurology 2004.4 | |||||
The 2 goals of treatment
Terminating acute headache is the first goal; shortening or aborting the cluster cycle is the second.
Research in the treatment of cluster headaches has been hampered by the relative infrequency of the condition, the short duration of each episode, and a robust placebo response.10 Much of the available evidence for the efficacy of various treatments comes from small controlled studies and case series.
Episodic cluster headaches respond much more readily to therapy than do chronic cluster headaches. Remember that no single intervention will work for every patient, and that some options are highly effective only for a small percentage of patients.
Of the drugs discussed in this section, injectable sumatriptan for acute attacks and oral verapamil for prophylaxis of attacks have the best evidence of efficacy based on controlled clinical trials.11
Terminating acute headaches
100% oxygen. One hundred percent oxygen, delivered by face mask at high flow rates, has been shown to reduce the severity of cluster headaches or terminate acute attacks (TABLE 2). Absence of side effects is the real advantage of this therapy; the major drawback is the lack of portability of an adequate oxygen supply (strength of recommendation [SOR]: B). 22
Triptans. Subcutaneous sumatriptan has been shown to provide relief for 88% of users (number needed to treat [NNT]=2.1), and its effectiveness seems not to wane with repeated use. The preferred dose is 6 mg; higher doses have been studied and are no more effective (SOR: A).23 Intranasal sumatriptan is also effective, although less so than the parenteral form (NNT=3.2).13 Oral zolmitriptan (Zomig) has shown benefit in approximately 60% of cases with both the 5- and 10-mg doses in episodic cluster headaches (NNT=5.6), but is no more effective than placebo in chronic cluster patients.15
Side effects. Most patients report side effects with triptans,24 the most common being “atypical sensations” such as tingling, heat, pressure, tightness, numbness, or flushing. Dizziness and sedation can also occur, and, with injectable sumatriptan, reactions at the injection site are common. Before prescribing injectable sumatriptan, supervise administration of the first dose.
Caveats. Triptans are contraindicated for patients with vascular disease (coronary artery disease, stroke, peripheral vascular disease), renal, or liver dysfunction. Triptans should be used with caution by persons with multiple risk factors for coronary disease. They cannot be used in combination with other triptans or within 24 hours of the use of dihydroergotamine (DHE 45).25
Dihydroergotamine. DHE can be used to terminate acute attacks using intravenous, subcutaneous, or intramuscular routes of administration (SOR: B). The usual dose is 1 mg, and many clinicians administer 10 mg of metoclopramide (Reglan) simultaneously to counter nausea (SOR: C). Complete familiarity with the proper use and potential adverse effects of injectable DHE is critical before using it in the outpatient setting.
Like triptans, DHE is contraindicated for those with vascular disease or severe liver or kidney impairment. Side effects include numbness or tingling in the extremities, muscle cramps, palpitations, and pain or tightness in the chest. Pleural and retroperitoneal fibrosis has occurred following prolonged daily use of ergots, and the use of DHE in patients with unrecognized coronary artery disease has caused death.
DHE levels are elevated by concurrent use of cytochrome P450 3A4 inhibitors such as macrolide antibiotics, protease inhibitors, ketoconazole, and itraconazole.26
Other abortive agents. There is little evidence for the use of other abortive agents. This poses a significant problem for the patient with cluster headaches who cannot take vasoconstrictors. A study of 5 patients showed olanzapine (Zyprexa), 2.5 to 10 mg, is a potentially effective abortive agent,27 and a larger study showed that octreotide (Sandostatin), 100 μg subcutaneously, relieved 52% of cluster headaches (NNT=6.3).16 Intranasal lidocaine has been shown to provide relief for 55% of migraine headaches, and some recommend its use in cluster headache.28
Prevention and interruption of the cluster cycle
More important than aborting the acute headache is ending the cluster episode.
Verapamil. Ample evidence supports the effectiveness of verapamil (Calan) for this purpose (NNT=1.2).17 Larger doses than are typical for hypertensive therapy may be required.
Cluster headache, the most severe primary headache, is rare compared with other types of headache. Thus, despite severe head pain, the diagnosis may be overlooked.6 Studies in which the diagnosis was clinically confirmed reveal a prevalence ranging from 56 to 381 cases per 100,000 people.29 Cluster differs from migraine in that men are affected more commonly than women. Once believed to have a gender differential of 6:1, the ratio is now reported at 3.7:1.6
Age of onset also contrasts with migraine. Cluster headaches typically begin at around 30 years of age with a range of 20 to 50 years, but rarely as old as 80 years. Women are more likely than men to have onset in later years.
Most patients are smokers or former smokers (74%), but cessation of smoking does not appear to modify the pattern of headaches.
Inheritance plays a greater role than previously realized, suggesting a genetic cause. First-degree relatives have a 5- to 18-fold higher risk for cluster headache than the general population. Second-degree relatives have a 1- to 3-fold higher risk. The mode of inheritance is likely autosomal dominant with low penetrance in some families, and multifactoral inheritance or autosomal recessive in other families.29
A regimen of 40 mg in the morning, 80 mg at noon, and 80 mg at bedtime, allowing patients to titrate doses up by 40 mg on alternate days, relieved 94% of episodic cluster headaches and 55% of chronic cases (SOR: B).30 Most patients need 200 to 480 mg/d to achieve success, but some require up to 960 mg/d.30
If a patient is asymptomatic but has a history consistent with cluster headaches, and if your examination reveals no other cause of headache, a trial of verapamil is warranted to abort the cluster cycle or prevent additional cycles.
Other agents. Corticosteroids may act faster than verapamil, and the two can be used in combination (SOR: C). A typical regimen of prednisone starts with 40 mg/d and tapers over 3 weeks.28
Lithium (Lithobid, Eskalith) is effective, but acts slowly and causes more side effects than other agents.20
Other agents that have shown efficacy in small studies are gabapentin, baclofen, clonidine, twice daily eletriptan, and topiramate.31-34
Sodium valproate (Depakote) is also used prophylactically for both cluster and migraine headaches. One small study demonstrated efficacy, but a larger trial failed to show benefit due to a unexpectedly high placebo response rate.35,36
When to refer
Treatment for most cluster headache sufferers is adequately handled in the primary care setting. When medical therapy fails, consider referral to a headache specialist, particularly for those with chronic cluster headaches. In some cases, sympathetic nerve blockade might be a worthwhile consideration.37 Unfortunately, a small subset of patients will not find relief regardless of the regimen employed.
TABLE 2
Selected cluster headache trials
| DRUG | RESPONSE | # OF PTS | NNT |
|---|---|---|---|
| Sumatriptan 6 mg subcut. vs placebo12 | 74% response @ 15 min 26% placebo response | 39 | 2.1 |
| Sumatriptan nasal vs placebo13 | 57% response @ 30 min 26% placebo response | 118 | 3.2 |
| Sumatriptan subcut. vs sumatriptan nasal14 | 94% response to injection @ 15 min 13% response to nasal | 49 | 1.2 |
| Zolmitriptan 10 mg orally vs placebo15 | 47% response @ 30 min 29% placebo response | 124 | 5.6 |
| Octreotide 100 μg subcut. vs placebo16 | 52% response @ 30 min 36% placebo response | 46 | 6.3 |
| Verapamil 120 mg orally 3× daily vs placebo17 | 80% response in 2nd week | 15 each arm | 1.2 |
| Oxygen 100%18 | 75% with significant pain relief within 15 min | 52 | — |
| Dihydroergotamine IV19 | 73% relief refractory episodic cluster | 60 | — |
| 63% relief refractory chronic cluster | 37 | — | |
| Lithium 900 mg daily vs Verapamil 360 mg daily20 | Lithium: 37% improve in 1st week Verapamil: 58% improve in 1st week | 24 | 4.8 |
| Eletriptan 40 mg twice daily×6 days21 | 40% fewer attacks | 16 | — |
CORRESPONDENCE
Stephen Adams, MD, 1100 East Third Street, Chattanooga TN 37403. E-mail: [email protected]
- Learn to recognize the distinctive pattern of cluster headaches that in most cases, even if some symptoms suggest migraine, will readily reveal this disorder (C).
- 100% oxygen by face mask and injected sumatriptan (Imitrex) are effective choices to terminate an acute attack (B).
- Verapamil (Calan) or corticosteroids abort cluster cycles and prevent further attacks (B).
You interview a “migraine” sufferer who hopes to find the relief that has so far eluded him. During the history taking, he reports experiencing the premonitory aura typical of migraine as well as photophobia and occasionally nausea. One description, though, raises a question about the cause of his headaches: he says the intense pain recurs at roughly the same time of day and lasts for about 45 minutes; he is unable to concentrate on anything but the pain and he paces ceaselessly until it abates. The odds now favor a diagnosis of cluster headache.
Given that symptoms of migraine and cluster headaches overlap, and that migraine is more prevalent than cluster headache, recognizing the latter requires sensitivity to its key attributes described in this article.
Careful selection of therapeutic agents will hasten resolution of acute pain and prevent recurrences. Some drugs may be combined for quicker onset of action.
Telltale characteristics of cluster headaches
As shown in the opening example, cluster headache is diagnosed primarily by history (see Source of cluster headaches).
Recognize the pattern. Its most striking feature is the unmistakable pattern of repeated bouts of pain that “cluster” at the same time of the day or night.
Each attack is extremely intense and brief, typically lasting 15 to 180 minutes.
For patients with the episodic variety of cluster headaches, the pattern of repeated headaches may last days to weeks, then resolve spontaneously. The relatively few patients with the chronic form of the disorder experience an unending cycle of daily or near daily headaches.
Attacks may occur during the day or night, and often wake the patient from sleep.1
Pain is always unilateral. Location of the pain varies among patients but is usually within the distribution of the trigeminal nerve. Almost all patients (92%) have retro-orbital pain, and most (70%) also have pain in the temporal region. Pain is present in the upper teeth, jaw, forehead, or cheek half of the time. Less common sites of pain are the ears, lower jaw, neck and shoulder.1
Look for associated findings. Pain is accompanied by signs and symptoms of ipsilateral autonomic dysfunction. Lacrimation on the affected side is the most common associated feature. Rhinorrhea or a blocked nasal passage, red eye, and swelling or pallor of the forehead or cheek are often found bilaterally but are clinically dominant on the symptomatic side.
Restlessness occurs during an attack and the patient often prefers to pace about, in striking contrast to the migraine sufferer, who avoids activity so as not to exacerbate the pain. Half of cluster headache sufferers experience nausea, photophobia, or phonophobia during attacks. A smaller number (14%) report an aura similar to that of a migraine. Ninety percent of cluster headache patients who drink alcohol say it triggers headache while they are in the midst of a cluster cycle.1
Delay in diagnosis common. The 2004 revision of the International Classification of Headache Disorders, reflects our improved understanding of and ability to identify these disorders.4 The mean time to diagnosis of cluster headache has decreased from 22 years in the 1960s to 2.6 years in the 1990s, reflecting much better recognition of the syndrome.5 Nevertheless most patients consult three primary care physicians before a diagnosis is made. The time between the first episode and diagnosis ranged from 1 week to 48 years (median 3 years) in one recent study.6
Overlap of migraine and cluster symptoms may lead to misdiagnosis. Factors contributing to diagnostic delay include photophobia or phonophobia, nausea, an episodic attack pattern and a younger age at onset (P<.01). Correct diagnosis is further complicated in that 26% of cluster headache suffers also report a history of migraine headaches.1 The Key differentiating factor between the two headaches types is the predictable pattern of repeated, intense, brief head pain.
Rare underlying causes. A very few patients with headaches have brain tumors. Headache is present in 50% to 60% of newly diagnosed brain tumors, but is usually accompanied by other signs or symptoms. It is the only presenting symptom in approximately 8% of cases. Most headaches due to tumors are clinically similar to tension headache (77%), and some mimic migraine (9%). Rapidly growing tumors are more likely to be associated with constant unremitting headache. Rarely brain tumors may produce pain syndromes similar to cluster headache.7 Other causes of secondary cluster headache include infections, vascular abnormalities, and head trauma.
A new subclassification of primary headache, trigeminal autonomic cephalgia, incorporates cluster headache with several other rarer types of headache that can be difficult to distinguish from primary cluster headache.8 Differentiation is important because the non-cluster types respond dramatically to indomethacin, whereas cluster headaches do not (TABLE 1).9
TABLE 1
Source of cluster headaches
| FEATURE | CLUSTER | MIGRAINE | PAROXYSMAL HEMICRANIA | SUNCT* | HEMICRANIA CONTINUA |
|---|---|---|---|---|---|
| Duration | 15–180 min | 4–72 hrs | 2–30 min | 5–240 sec | Continuous |
| Autonomic dysfunction | Yes | Unusual | Yes | Yes | Sometimes |
| Pain quality | Sharp, boring | Often pulsatile | Stabbing or Stabbing | Stabbing or pulsatile | Stabbing or pulsatile |
| Severity | Severe | Mod–severe | Severe | Severe | Mod–severe |
| Frequency | Predictable | Varies | >5/day | 3–200/day | Continuous |
| Laterality | Unilateral | Varies | Unilateral | Unilateral | Unilateral |
| Response to Indomethacin | Not usually | Not usually | Always | Always | Always |
| *SUNCT, short-lasting neuralgiform headache attacks with conjunctival injection and tearing. | |||||
| Source: Lipton et al, Neurology 2004.4 | |||||
The 2 goals of treatment
Terminating acute headache is the first goal; shortening or aborting the cluster cycle is the second.
Research in the treatment of cluster headaches has been hampered by the relative infrequency of the condition, the short duration of each episode, and a robust placebo response.10 Much of the available evidence for the efficacy of various treatments comes from small controlled studies and case series.
Episodic cluster headaches respond much more readily to therapy than do chronic cluster headaches. Remember that no single intervention will work for every patient, and that some options are highly effective only for a small percentage of patients.
Of the drugs discussed in this section, injectable sumatriptan for acute attacks and oral verapamil for prophylaxis of attacks have the best evidence of efficacy based on controlled clinical trials.11
Terminating acute headaches
100% oxygen. One hundred percent oxygen, delivered by face mask at high flow rates, has been shown to reduce the severity of cluster headaches or terminate acute attacks (TABLE 2). Absence of side effects is the real advantage of this therapy; the major drawback is the lack of portability of an adequate oxygen supply (strength of recommendation [SOR]: B). 22
Triptans. Subcutaneous sumatriptan has been shown to provide relief for 88% of users (number needed to treat [NNT]=2.1), and its effectiveness seems not to wane with repeated use. The preferred dose is 6 mg; higher doses have been studied and are no more effective (SOR: A).23 Intranasal sumatriptan is also effective, although less so than the parenteral form (NNT=3.2).13 Oral zolmitriptan (Zomig) has shown benefit in approximately 60% of cases with both the 5- and 10-mg doses in episodic cluster headaches (NNT=5.6), but is no more effective than placebo in chronic cluster patients.15
Side effects. Most patients report side effects with triptans,24 the most common being “atypical sensations” such as tingling, heat, pressure, tightness, numbness, or flushing. Dizziness and sedation can also occur, and, with injectable sumatriptan, reactions at the injection site are common. Before prescribing injectable sumatriptan, supervise administration of the first dose.
Caveats. Triptans are contraindicated for patients with vascular disease (coronary artery disease, stroke, peripheral vascular disease), renal, or liver dysfunction. Triptans should be used with caution by persons with multiple risk factors for coronary disease. They cannot be used in combination with other triptans or within 24 hours of the use of dihydroergotamine (DHE 45).25
Dihydroergotamine. DHE can be used to terminate acute attacks using intravenous, subcutaneous, or intramuscular routes of administration (SOR: B). The usual dose is 1 mg, and many clinicians administer 10 mg of metoclopramide (Reglan) simultaneously to counter nausea (SOR: C). Complete familiarity with the proper use and potential adverse effects of injectable DHE is critical before using it in the outpatient setting.
Like triptans, DHE is contraindicated for those with vascular disease or severe liver or kidney impairment. Side effects include numbness or tingling in the extremities, muscle cramps, palpitations, and pain or tightness in the chest. Pleural and retroperitoneal fibrosis has occurred following prolonged daily use of ergots, and the use of DHE in patients with unrecognized coronary artery disease has caused death.
DHE levels are elevated by concurrent use of cytochrome P450 3A4 inhibitors such as macrolide antibiotics, protease inhibitors, ketoconazole, and itraconazole.26
Other abortive agents. There is little evidence for the use of other abortive agents. This poses a significant problem for the patient with cluster headaches who cannot take vasoconstrictors. A study of 5 patients showed olanzapine (Zyprexa), 2.5 to 10 mg, is a potentially effective abortive agent,27 and a larger study showed that octreotide (Sandostatin), 100 μg subcutaneously, relieved 52% of cluster headaches (NNT=6.3).16 Intranasal lidocaine has been shown to provide relief for 55% of migraine headaches, and some recommend its use in cluster headache.28
Prevention and interruption of the cluster cycle
More important than aborting the acute headache is ending the cluster episode.
Verapamil. Ample evidence supports the effectiveness of verapamil (Calan) for this purpose (NNT=1.2).17 Larger doses than are typical for hypertensive therapy may be required.
Cluster headache, the most severe primary headache, is rare compared with other types of headache. Thus, despite severe head pain, the diagnosis may be overlooked.6 Studies in which the diagnosis was clinically confirmed reveal a prevalence ranging from 56 to 381 cases per 100,000 people.29 Cluster differs from migraine in that men are affected more commonly than women. Once believed to have a gender differential of 6:1, the ratio is now reported at 3.7:1.6
Age of onset also contrasts with migraine. Cluster headaches typically begin at around 30 years of age with a range of 20 to 50 years, but rarely as old as 80 years. Women are more likely than men to have onset in later years.
Most patients are smokers or former smokers (74%), but cessation of smoking does not appear to modify the pattern of headaches.
Inheritance plays a greater role than previously realized, suggesting a genetic cause. First-degree relatives have a 5- to 18-fold higher risk for cluster headache than the general population. Second-degree relatives have a 1- to 3-fold higher risk. The mode of inheritance is likely autosomal dominant with low penetrance in some families, and multifactoral inheritance or autosomal recessive in other families.29
A regimen of 40 mg in the morning, 80 mg at noon, and 80 mg at bedtime, allowing patients to titrate doses up by 40 mg on alternate days, relieved 94% of episodic cluster headaches and 55% of chronic cases (SOR: B).30 Most patients need 200 to 480 mg/d to achieve success, but some require up to 960 mg/d.30
If a patient is asymptomatic but has a history consistent with cluster headaches, and if your examination reveals no other cause of headache, a trial of verapamil is warranted to abort the cluster cycle or prevent additional cycles.
Other agents. Corticosteroids may act faster than verapamil, and the two can be used in combination (SOR: C). A typical regimen of prednisone starts with 40 mg/d and tapers over 3 weeks.28
Lithium (Lithobid, Eskalith) is effective, but acts slowly and causes more side effects than other agents.20
Other agents that have shown efficacy in small studies are gabapentin, baclofen, clonidine, twice daily eletriptan, and topiramate.31-34
Sodium valproate (Depakote) is also used prophylactically for both cluster and migraine headaches. One small study demonstrated efficacy, but a larger trial failed to show benefit due to a unexpectedly high placebo response rate.35,36
When to refer
Treatment for most cluster headache sufferers is adequately handled in the primary care setting. When medical therapy fails, consider referral to a headache specialist, particularly for those with chronic cluster headaches. In some cases, sympathetic nerve blockade might be a worthwhile consideration.37 Unfortunately, a small subset of patients will not find relief regardless of the regimen employed.
TABLE 2
Selected cluster headache trials
| DRUG | RESPONSE | # OF PTS | NNT |
|---|---|---|---|
| Sumatriptan 6 mg subcut. vs placebo12 | 74% response @ 15 min 26% placebo response | 39 | 2.1 |
| Sumatriptan nasal vs placebo13 | 57% response @ 30 min 26% placebo response | 118 | 3.2 |
| Sumatriptan subcut. vs sumatriptan nasal14 | 94% response to injection @ 15 min 13% response to nasal | 49 | 1.2 |
| Zolmitriptan 10 mg orally vs placebo15 | 47% response @ 30 min 29% placebo response | 124 | 5.6 |
| Octreotide 100 μg subcut. vs placebo16 | 52% response @ 30 min 36% placebo response | 46 | 6.3 |
| Verapamil 120 mg orally 3× daily vs placebo17 | 80% response in 2nd week | 15 each arm | 1.2 |
| Oxygen 100%18 | 75% with significant pain relief within 15 min | 52 | — |
| Dihydroergotamine IV19 | 73% relief refractory episodic cluster | 60 | — |
| 63% relief refractory chronic cluster | 37 | — | |
| Lithium 900 mg daily vs Verapamil 360 mg daily20 | Lithium: 37% improve in 1st week Verapamil: 58% improve in 1st week | 24 | 4.8 |
| Eletriptan 40 mg twice daily×6 days21 | 40% fewer attacks | 16 | — |
CORRESPONDENCE
Stephen Adams, MD, 1100 East Third Street, Chattanooga TN 37403. E-mail: [email protected]
1. Bahra A, May A, Goadsby PJ. Cluster headache: a prospective clinical study with diagnostic implications. Neurology 2002;58:354-361.
2. D’Andrea G, Terrazzino S, et al. Elevated levels of circulating trace amines in primary headaches. Neurology 2004;62:1701-1705.
3. Cohen AS, Goadsby PJ. Functional neuroimaging of primary headache disorders. Curr Neurol Neurosci Rep 2004;4:105-110.
4. Lipton RB, Bigal ME, Steiner TJ, Silberstein SD, Olesen J. Classification of primary headaches. Neurology 2004;63:427-435.
5. Bahra A, Goadsby PJ. Diagnostic delays and mis-management in cluster headache. Acta Neurol Scand 2004;109:175-179.
6. van Vliet JA, Eekers PJ, Haan J, Ferrari MD. Dutch RUSSH Study Group. Features involved in the diagnostic delay of cluster headache. J Neurol Neurosurg Psychiatry 2003;74:1123-1125.
7. Purdy RA, Kirby S. Headaches and brain tumors. Neurol Clin 2004;22:39-53.
8. Carter DM. Cluster headache mimics. Curr Pain Headache Rep 2004;8:133-139.
9. May A. Headaches with (ipsilateral) autonomic symptoms. J Neurol 2003;250:1273-1278.
10. Nilsson Remahl AI, Laudon Meyer E, Cordonnier C, et al. Placebo response in cluster headache trials: a review. Cephalalgia 2003;23:504-510.
11. Moore K. Cluster headache: the challenge of clinical trials. Curr Pain Headache Rep 2002;6:52-56.
12. Treatment of acute cluster headache with sumatriptan. The Sumatriptan Cluster Headache Study Group. N Engl J Med 1991;325:322-326.
13. van Vliet JA, Bahra A, Martin V, et al. Intranasal sumatriptan in cluster headache: randomized placebo controlled double-blind study. Neurology 2003;60:630-633.
14. Hardebo JE, Dahlof C. Sumatriptan nasal spray (20 mg/dose) in the acute treatment of cluster headache. Cephalalgia 1998;18:487-489.
15. Bahra A, Gawel MJ, Hardebo JE, Millson D, Breen SA, Goadsby PJ. Oral zolmitriptan is effective in the acute treatment of cluster headache. Neurology 2000;54:1832-1839.
16. Matharu MS, Levy MJ, Meeran K, Goadsby PJ. Subcutaneous octreotide in cluster headache: randomized placebo-controlled double-blind crossover study. Ann Neurol 2004;56:488-494.
17. Leone M, D’Amico D, Frediani F, et al. Verapamil in the prophylaxis of episodic cluster headache: a double bline study versus placebo. Neurology 2000;54:1382-1385.
18. Kudrow L. Response of cluster headache attacks to oxygen inhalation. Headache 1981;21:1-4.
19. Magnoux E, Zlotnik G. Outpatient intravenous dihydroergotamine for refractory cluster headache. Headache 2004;44:249-255.
20. Bussone G, Leone M, Peccarisi C, et al. Double blind comparison of lithium and verapamil in cluster headache prophylaxis. Headache 1990;30:411-417.
21. Zebenholzer K, Wober C, Vigl M, Wessely P. Eletriptan for the short-term prophylaxis of cluster headache. Headache 2004;44:361-364.
22. Fogan L. Treatment of cluster headache. A double-blind comparison of oxygen v air inhalation. Arch Neurol 1985;42:362-363.
23. Ekbom K, Monstad I, Prusinski A, Cole JA, Pilgrim AJ, Noronha D. Subcutaneous sumatriptan in the acute treatment of cluster headache: a dose comparison study. The Sumatriptan Cluster Headache Study Group. Acta Neurol Scand 1993;88:63-69.
24. Gobel H, Lindner V, Heinze A, Ribbat M, Deuschl G. Acute therapy for cluster headache with sumatriptan: findings of a one-year long-term study. Neurology 1998;51:908-911.
25. Physicians’ Desk Reference. 57th ed. Montvale, NJ: Thomson PDR; 2003;1544.-
26. DHE 45 package insert; Novartis Pharmaceuticals AG. 2002.
27. Rozen TD. Olanzapine as an abortive agent for cluster headache. Headache 2001;41:813-816.
28. Freitag FG. Cluster headache. Primary Care; Clinics in Office Practice 2004;31(no 2), June.
29. Russell MB. Epidemiology and genetics of cluster headache. Lancet Neurol 2004;3:279-283.
30. Blau JN, Engel HO. Individualizing treatment with verapamil for cluster headache patients. Headache 2004;44:1013-1018.
31. Leandri M, Luzzani M, Cruccu G, Gottlieb A. Drug-resistant cluster headache responding to gabapentin: a pilot study. Cephalalgia 2001;21:744-746.
32. Hering-Hanit R, Gadoth N. The use of baclofen in cluster headache. Curr Pain Headache Rep 2001;5:79-82.
33. D’Andrea G, Perini F, Granella F, Cananzi A, Sergi A. Efficacy of transdermal clonidine in short-term treatment of cluster headache: a pilot study. Cephalalgia 1995;15:430-433.
34. Lainez MJ, Pascual J, Pascual AM, Santonja JM, Ponz A, Salvador A. Topiramate in the prophylactic treatment of cluster headache. Headache 2003;43:784-789.
35. Hering R, Kuritzky A. Sodium valproate in the treatment of cluster headache: an open clinical trial. Cephalalgia 1989;9:195-198.
36. El Amrani M, Massiou H, Bousser MG. A negative trial of sodium valproate in cluster headache: methodological issues. Cephalalgia 2002;22:205-208.
37. Albertyn J, Barry R, Odendall CL. Cluster headache and the sympathetic nerve. Headache 2004;44:183-185.
1. Bahra A, May A, Goadsby PJ. Cluster headache: a prospective clinical study with diagnostic implications. Neurology 2002;58:354-361.
2. D’Andrea G, Terrazzino S, et al. Elevated levels of circulating trace amines in primary headaches. Neurology 2004;62:1701-1705.
3. Cohen AS, Goadsby PJ. Functional neuroimaging of primary headache disorders. Curr Neurol Neurosci Rep 2004;4:105-110.
4. Lipton RB, Bigal ME, Steiner TJ, Silberstein SD, Olesen J. Classification of primary headaches. Neurology 2004;63:427-435.
5. Bahra A, Goadsby PJ. Diagnostic delays and mis-management in cluster headache. Acta Neurol Scand 2004;109:175-179.
6. van Vliet JA, Eekers PJ, Haan J, Ferrari MD. Dutch RUSSH Study Group. Features involved in the diagnostic delay of cluster headache. J Neurol Neurosurg Psychiatry 2003;74:1123-1125.
7. Purdy RA, Kirby S. Headaches and brain tumors. Neurol Clin 2004;22:39-53.
8. Carter DM. Cluster headache mimics. Curr Pain Headache Rep 2004;8:133-139.
9. May A. Headaches with (ipsilateral) autonomic symptoms. J Neurol 2003;250:1273-1278.
10. Nilsson Remahl AI, Laudon Meyer E, Cordonnier C, et al. Placebo response in cluster headache trials: a review. Cephalalgia 2003;23:504-510.
11. Moore K. Cluster headache: the challenge of clinical trials. Curr Pain Headache Rep 2002;6:52-56.
12. Treatment of acute cluster headache with sumatriptan. The Sumatriptan Cluster Headache Study Group. N Engl J Med 1991;325:322-326.
13. van Vliet JA, Bahra A, Martin V, et al. Intranasal sumatriptan in cluster headache: randomized placebo controlled double-blind study. Neurology 2003;60:630-633.
14. Hardebo JE, Dahlof C. Sumatriptan nasal spray (20 mg/dose) in the acute treatment of cluster headache. Cephalalgia 1998;18:487-489.
15. Bahra A, Gawel MJ, Hardebo JE, Millson D, Breen SA, Goadsby PJ. Oral zolmitriptan is effective in the acute treatment of cluster headache. Neurology 2000;54:1832-1839.
16. Matharu MS, Levy MJ, Meeran K, Goadsby PJ. Subcutaneous octreotide in cluster headache: randomized placebo-controlled double-blind crossover study. Ann Neurol 2004;56:488-494.
17. Leone M, D’Amico D, Frediani F, et al. Verapamil in the prophylaxis of episodic cluster headache: a double bline study versus placebo. Neurology 2000;54:1382-1385.
18. Kudrow L. Response of cluster headache attacks to oxygen inhalation. Headache 1981;21:1-4.
19. Magnoux E, Zlotnik G. Outpatient intravenous dihydroergotamine for refractory cluster headache. Headache 2004;44:249-255.
20. Bussone G, Leone M, Peccarisi C, et al. Double blind comparison of lithium and verapamil in cluster headache prophylaxis. Headache 1990;30:411-417.
21. Zebenholzer K, Wober C, Vigl M, Wessely P. Eletriptan for the short-term prophylaxis of cluster headache. Headache 2004;44:361-364.
22. Fogan L. Treatment of cluster headache. A double-blind comparison of oxygen v air inhalation. Arch Neurol 1985;42:362-363.
23. Ekbom K, Monstad I, Prusinski A, Cole JA, Pilgrim AJ, Noronha D. Subcutaneous sumatriptan in the acute treatment of cluster headache: a dose comparison study. The Sumatriptan Cluster Headache Study Group. Acta Neurol Scand 1993;88:63-69.
24. Gobel H, Lindner V, Heinze A, Ribbat M, Deuschl G. Acute therapy for cluster headache with sumatriptan: findings of a one-year long-term study. Neurology 1998;51:908-911.
25. Physicians’ Desk Reference. 57th ed. Montvale, NJ: Thomson PDR; 2003;1544.-
26. DHE 45 package insert; Novartis Pharmaceuticals AG. 2002.
27. Rozen TD. Olanzapine as an abortive agent for cluster headache. Headache 2001;41:813-816.
28. Freitag FG. Cluster headache. Primary Care; Clinics in Office Practice 2004;31(no 2), June.
29. Russell MB. Epidemiology and genetics of cluster headache. Lancet Neurol 2004;3:279-283.
30. Blau JN, Engel HO. Individualizing treatment with verapamil for cluster headache patients. Headache 2004;44:1013-1018.
31. Leandri M, Luzzani M, Cruccu G, Gottlieb A. Drug-resistant cluster headache responding to gabapentin: a pilot study. Cephalalgia 2001;21:744-746.
32. Hering-Hanit R, Gadoth N. The use of baclofen in cluster headache. Curr Pain Headache Rep 2001;5:79-82.
33. D’Andrea G, Perini F, Granella F, Cananzi A, Sergi A. Efficacy of transdermal clonidine in short-term treatment of cluster headache: a pilot study. Cephalalgia 1995;15:430-433.
34. Lainez MJ, Pascual J, Pascual AM, Santonja JM, Ponz A, Salvador A. Topiramate in the prophylactic treatment of cluster headache. Headache 2003;43:784-789.
35. Hering R, Kuritzky A. Sodium valproate in the treatment of cluster headache: an open clinical trial. Cephalalgia 1989;9:195-198.
36. El Amrani M, Massiou H, Bousser MG. A negative trial of sodium valproate in cluster headache: methodological issues. Cephalalgia 2002;22:205-208.
37. Albertyn J, Barry R, Odendall CL. Cluster headache and the sympathetic nerve. Headache 2004;44:183-185.