User login
Is your patient a candidate for Mohs micrographic surgery?
Mohs micrographic surgery (MMS) is a unique dermatologic surgery technique that allows the dermatologist to fill the concomitant roles of surgeon and pathologist. It is utilized for the extirpation of skin malignancy, with an emphasis on tissue preservation and immediate surgical margin evaluation. In MMS, the Mohs surgeon acts as the surgeon for physical removal of the lesion and the pathologist during evaluation of frozen section margins.1
Primary care providers (PCPs) are on the frontlines of management of cutaneous malignancy. Whether referring to Dermatology for biopsy or performing a biopsy themselves, PCPs can assure optimal treatment outcomes by guiding patients to evidence-based treatments, while still respecting the patient’s wishes. In this evidence-based review of the advantages, improved outcomes, and safety of Mohs surgery for the treatment of common and rare skin neoplasms, we provide our primary care colleagues with information on the indications, process (the order in which steps of the procedure are performed), and techniques used for treating cutaneous malignancies with Mohs surgery.
When is Mohs surgery appropriate?
MMS has typically been reserved for treatment of cutaneous malignancy in cosmetically sensitive areas where tissue preservation is key. In 2012, Connolly et al released appropriate use criteria (AUC) for MMS.2 (See “An app that helps clinicians apply the criteria for Mohs surgery.”) Within the AUC, there are 4 major qualitative and quantitative categories when considering referral for MMS:
- area of the body in which the lesion manifests
- the patient’s medical characteristics
- tumor characteristics
- the size of the lesion to be treated.2
Areas of the body are divided into 3 categories by the AUC according to how challenging tumor extirpation is expected to be and how critical tissue preservation is. Areas termed “H” receive the highest score for appropriate Mohs usage, followed by areas “M” and “L.”
SIDEBAR
An app that helps clinicians apply the criteria for Mohs surgery
“Mohs Surgery Appropriate Use Criteria” is a free and easy-to-use smartphone application to help determine whether Mohs micrographic surgery (MMS) is appropriate for a particular patient. Clinicians can enter the details of a recent skin cancer biopsy along with patient information into the app and it will calculate a score automatically categorized into 1 of 3 categories: “appropriate,” “uncertain,” and “not appropriate” for MMS. The clinician can then talk to the patient about a possible referral to a Mohs surgeon, depending on the appropriateness of the procedure for the patient and their tumor.
Patient medical characteristics that should be taken into account when referring for Mohs surgery are the patient’s immune status, genetic syndromes that may predispose the patient to cutaneous malignancies (eg, xeroderma pigmentosa), history of radiation to the area of involvement, and the patient’s history of aggressive cutaneous malignancies.
Tumor characteristics. The most common malignancies treated with MMS include basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). These malignancies are further delineated through histologic evaluation by a pathologist or dermatopathologist. Aggressive features of a BCC on any area of the body that warrant referral to a Mohs surgeon include morpheaform/fibrosing/sclerosing histologic findings, as well as micronodular architecture and perineural invasion. Concerning histologic SCC findings that warrant Mohs surgery through the AUC include sclerosing, basosquamous, and small cell histology, as well as poorly differentiated and/or undifferentiated SCC.
Melanoma in situ and lentigo maligna, which are variants of melanoma limited to the epidermis without invasion into the underlying dermis, are included within the AUC for MMS. For invasive melanoma (melanoma that has invaded into the dermis or subcutaneous tissue), MMS has been shown to have marginal benefit but currently is not included within the AUC.3
Continue to: Due to excellent margin control...
Due to excellent margin control via immediate microscopic evaluation of surgical margins, MMS is an appropriate treatment choice and indicated for many more uncommon cutaneous malignancies, including sebaceous and mucinous carcinoma, microcystic adnexal carcinoma, Merkel cell carcinoma, leiomyosarcoma, dermatofibrosarcoma protuberans, atypical fibroxanthoma, angiosarcoma, and other more rarely encountered clinical malignancies.2
Tumor size. When considering a referral to MMS for cancer extirpation, the size of the tumor does play a role; however, size depends on the type of tumor as well as the location on the body. In general, most skin cancers of any size on the face, perianal area, genitalia, nipples, hands, feet and ankles, or pretibial surface are appropriate for Mohs surgery. Skin cancers on the trunk and extremities are also appropriate if they are above a certain size specified by the AUC. Tumor type and whether they are recurrences also factor into the equation.
Who will do the procedure?
A recent review showed that PCPs were more likely to refer patients to plastic surgery rather than Mohs surgery for skin cancer removal, especially among younger female patients.4 This is likely because of the perception that plastic surgeons do more complex closures and have more experience removing difficult cancers. Interestingly, this same study showed that Mohs surgeons may actually be doing several-fold more complex closures (flaps and grafts) on the nose and ears than plastic surgeons at similar practice settings.4
Aside from Mohs surgeons doing more closures, perhaps the biggest difference between Mohs surgeons and plastic surgeons is the pathology training of the Mohs surgeon. Mohs surgeons evaluate 100% of the tissue margins at the time of the procedure to both ensure complete tumor removal and to preserve as much tumor-free skin as possible, ultimately resulting in decreased recurrences and smaller scars. In contrast, the plastic surgeon’s rigorous training typically does not include extensive dermatopathology training, particularly the pathology of cutaneous neoplasms. Plastic surgeons will often send pathologic specimens for evaluation, meaning patients have to wait for outside histologic confirmation before their wounds can be closed. Additionally, the histologic evaluation is often not a full-margin assessment, as not all labs are equipped for this technique.
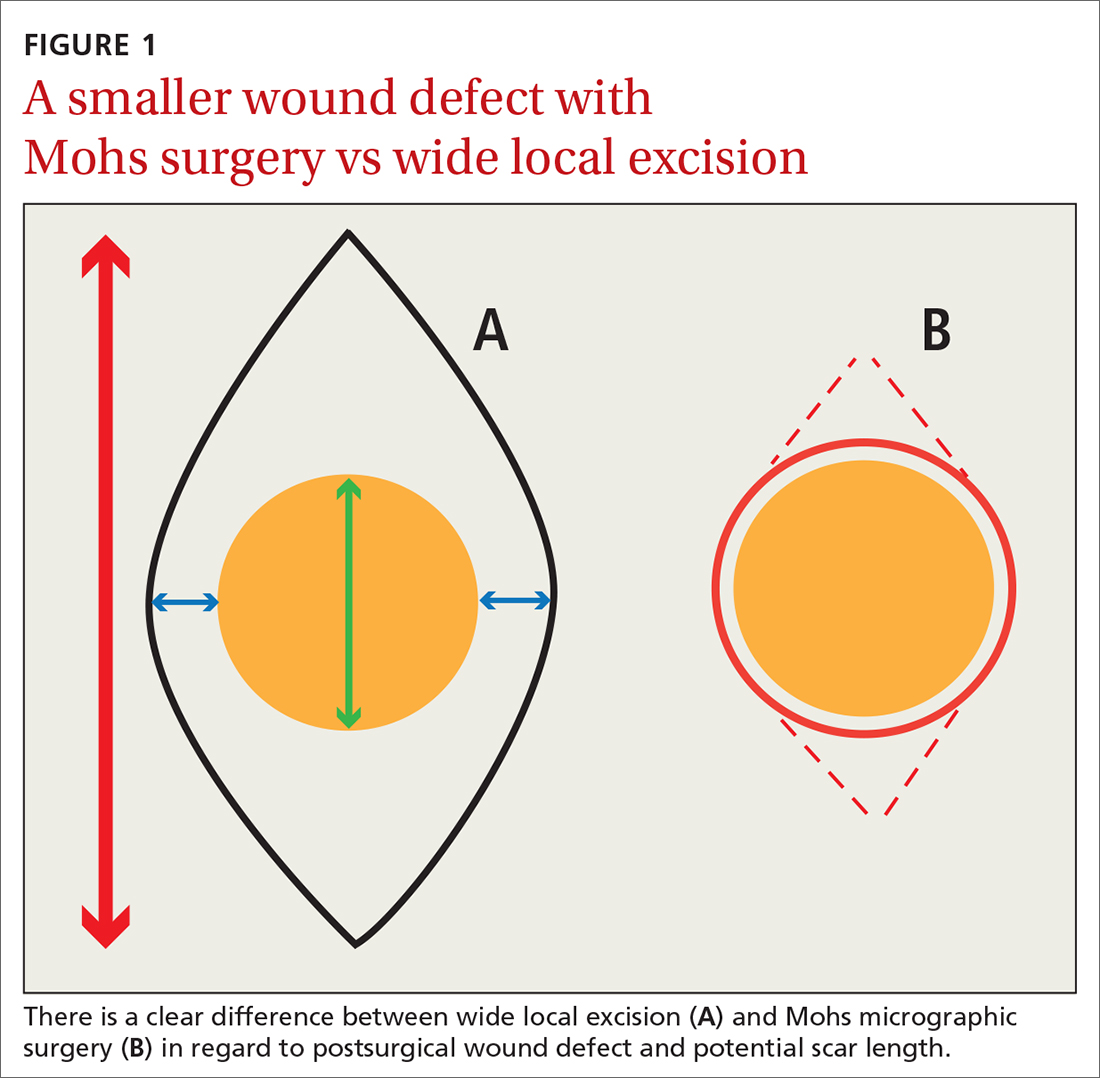
Consider early consultation with a Mohs surgeon for tumor extirpation to keep the defect size as small as possible, as MMS does not require taking margins of healthy surrounding tissue, in contrast to wide local excisions (WLEs; FIGURE 1). A smaller initial incision will result in a smaller scar, which is likely to have better cosmetic outcomes and decreased risk for wound infection.
Continue to: Before consultation...
Before consultation, include a picture of the surgical site with the patient’s referral documentation or have the patient present a photo from his or her phone to the Mohs surgeon. (If a camera or cell phone is not available, triangulation of the site’s location using cosmetic landmarks can be documented in the patient’s chart.)
What the patient can expect during preop visits
During an initial consultation, patients can expect an evaluation by the surgeon that will include more photo taking, a discussion of the surgery, and possibly, performance of an in-clinic biopsy of suspicious lesions. Many practices, including the authors’, use a photo capturing add-on for the EMR in the office.5-7
During the consent process, MMS is described to the patient using lay language and, often, pictorial depictions of the procedure. While explaining that the procedure helps preserve healthy tissue and limit the size of the resulting scar, the surgeon will typically manage the expectations of the patient prior to the first incision. Many clinically small lesions can have significant subclinical extension adjacent to, or on top of, cosmetic landmarks, requiring a flap or graft to close the surgical defect with acceptable cosmetic outcomes.8
One more time. Immediately before surgery, the surgeon will again review the procedure with the patient, using photos of the biopsy site taken during the initial consult, in conjunction with patient verification of the biopsy site, to verify the surgical site and confirm that the patient understands and agrees to the surgery.
A look at how Mohs surgery is performed
MMS typically is performed in the outpatient setting but can also be performed in an operating room or outpatient surgical center. MMS can be performed in a nonsterile procedure room with surgeons and assistants typically utilizing clean, nonsterile gloves, although many Mohs surgeons prefer to perform part, or all, of the technique using sterile gloves.9 A recent systematic review and large meta-analysis showed no significant difference in postsurgical site infections when comparing the use of sterile vs nonsterile gloves.10
Continue to: Prior to initial incision...
Prior to initial incision, the site is marked with a surgical pen and given 1-mm margins around the clinically visualized lesion. The site is then cleansed with an antiseptic, typically a chlorhexidine solution. Local anesthesia is employed, most commonly with a 1:100,000 lidocaine and epinephrine injection. Marking of the tumor prior to numbing is imperative, as the boundaries of the tumor are typically obscured when the local cutaneous vasculature constricts and causes visualized blanching of adjacent skin. Many Mohs surgeons perform a brief curettage of the lesion with a nondisposable, dull curette to better define the tumor edges and to debulk any obvious exophytic tumor noted by the naked eye.
Prior to the first incision, the surgical site is scored in a variety of ways in order to properly orient the tissue after it has been removed from the patient. Mohs surgeons have differing opinions on how to score and/or mark the tissue, but a common practice is to make a nick at the 12 o’clock position. Following removal of the first stage, the nick will be visible on both the extirpated tissue and the tissue just above the surgical defect. This prevents potential confusion regarding orientation during tissue processing.
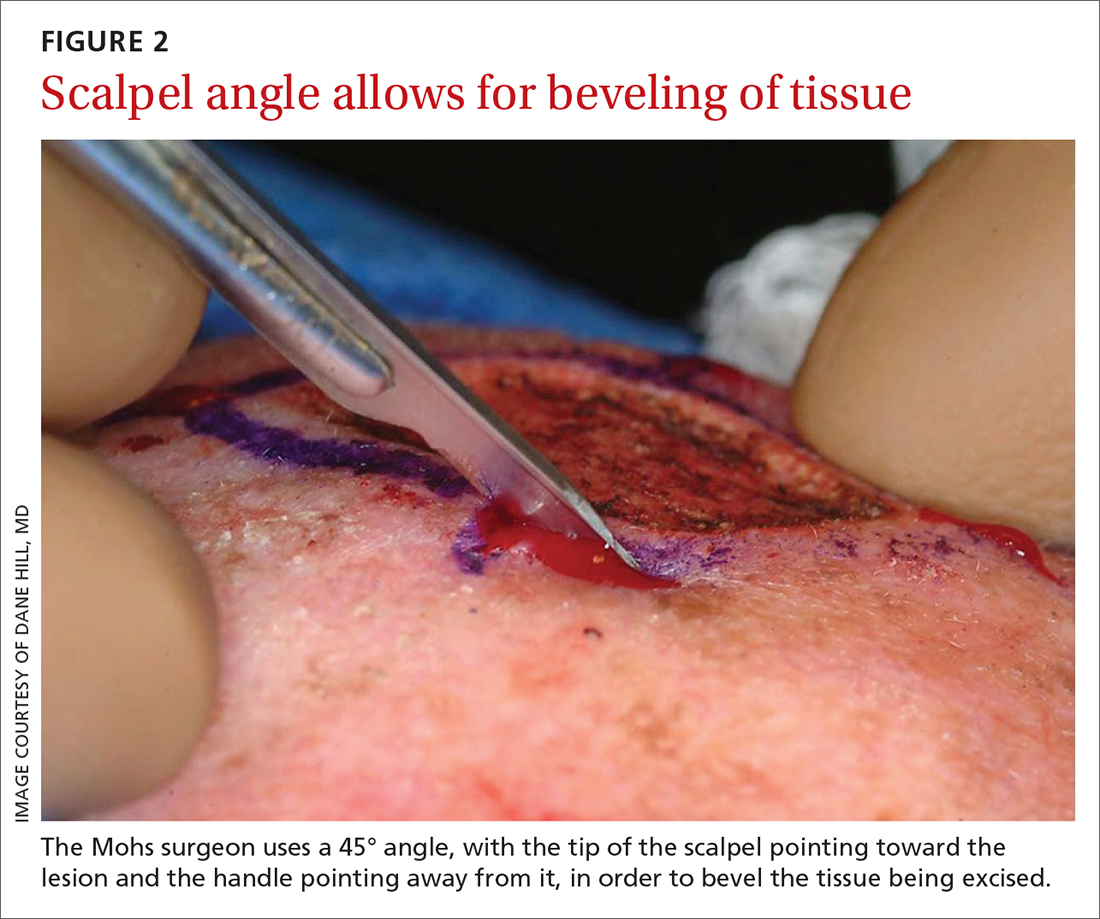
The majority of all WLEs are performed utilizing the scalpel blade at an angle 90° perpendicular to the plane of the skin. In MMS, a signature 45° angle with the tip of the scalpel pointing toward, and the handle pointing away from, the lesion is commonly used in order to bevel the tissue being excised (FIGURE 2). Once the tissue is excised, hemostasis is obtained using electrodessication/electrofulguration or electrocoagulation.
Tissue processing and microscopic evaluation
The technique of beveling allows the epidermis, dermis, and subcutaneous tissue to lie flat on the tissue block, so the Mohs surgeon can evaluate 100% of the excised tissue’s margins. The tissue is transported to a nearby lab for staining and processing. Even if near-perfect beveling is achieved, many stages will require bisecting, quadrissecting, or relaxing cuts in order to allow the margins to lie flat on the tissue block.
Using the scoring system made prior to incision, the tissue is oriented and stained with colored ink. Subsequently, a map is made with sections highlighting the colors used to stain designated areas of the tissue. This step is imperative for orientation during microscopic evaluation. Additionally, the map serves as a guide and log, should a section of the specimen have an involved margin and require another stage.
Continue to: Once fixed to the block...
Once fixed to the block, the tissue is engulfed in appropriate embedding medium and placed within the cryostat. The block is slowly cut to produce several micron-thin wafers of tissue that are then mounted on glass slides and processed with hematoxylin and eosin (H&E) or various stains. The first wafers of tissue that come from the tissue block are those that are closest to the margin that was excised. Thus, 100% of the epidermis and deep margin can be visualized. “Deeper sections” are those that come from deeper cuts within the tissue and are more likely to show the malignant neoplasm.
The evaluation of immediate margins at the very edge of the tissue is in contrast to the technique of “bread-loafing,” which is the standard of evaluating margins after a WLE.11 With this process, the pathologist examines sections that are cut 2- to 4-mm apart. This process only allows the pathologist to examine roughly 1% of the total tissue that was excised, and large variability in cutaneous representation can occur depending on the individual who cuts and processes the tissue.11
Closing the defect
Once the site is deemed clear of residual tumor, the Mohs surgeon approaches the defect and determines the most appropriate way to close the surgical wound. Mohs surgeons are trained to close wounds using a variety of methods, including complex linear closures, flaps, and full-thickness skin grafts. Thoughtful consideration of local anatomy, cosmetic landmarks that may be affected by the closure method, and local tissue laxity are evaluated.
Depending on the location, a secondary intention closure may prove to be just as effective and cosmetically satisfying as a primary intention closure. In light of the many methods of closure, a complex or large surface area defect may better be suited for evaluation and closure by another specialist such as an ENT physician, ophthalmologist, or plastic surgeon.12
Lower recurrence rates for patients who undergo Mohs surgery
As noted earlier, the cutaneous malignancies most commonly treated with MMS are BCCs, followed by SCCs.13 Comparison studies between WLE and MMS show clinically significant differences in terms of recurrence rates between the 2 procedures.
Continue to: For BCCs
For BCCs, recurrence rates for excisions vs MMS are 10% and 1%, respectively.14-16 A randomized trial reviewing 10-year recurrence of primary BCCs on the face showed recurrence rates for MMS of 4.4% compared to 12.2% for WLE.17 This study also showed recurrence rates for recurrent facial BCCs treated with MMS to be 3.9% vs 13.5% for standard WLE.17
SCC. The evidence similarly supports the efficacy of MMS for SCCs. A recent study showed primary T2a tumors had a 1.2% local recurrence rate with Mohs vs a 4% recurrence rate with WLE at an average follow-up of 2.8 years.18 Another study showed that primary tumors that were < 2 cm in diameter had a 5-year cure rate of 99% with Mohs surgery.11
Melanoma in situ. A few studies have shown no clinically significant benefit of MMS compared to WLE when it comes to melanoma in situ.19,20 However, a more recent article by Etzkom et al noted the ability to potentially upstage melanoma in situ and invasive melanoma after reviewing peripheral and deep margins during MMS.21 In this study, the authors uniquely delayed wound closure if upstaging was established and the need for a sentinel lymph node biopsy was warranted. This approach to MMS with delayed closure ultimately paved the way for very low recurrence rates.
CORRESPONDENCE
Andres Garcia, MD, 2612 112th Street, Lubbock, TX 79423; [email protected]
1. Dim-Jamora KC, Perone JB. Management of cutaneous tumors with Mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
2. Ad Hoc Task Force, Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. Published correction appears in J Am Acad Dermatol. 2015;72:748.
3. Cheraghlou S, Christensen S, Agogo G, et al. Comparison of survival after Mohs micrographic surgery vs wide margin excision for early-stage invasive melanoma. JAMA Dermatol. 2019;155:1252-1259.
4. Hill D, Kim K, Mansouri B, et al. Quantity and characteristics of flap or graft repairs for skin cancer on the nose or ears: a comparison between Mohs micrographic surgery and plastic surgery. Cutis. 2019;103:284-287.
5. McGinness JL, Goldstein G. The value of preoperative biopsy-site photography for identifying cutaneous lesions. Dermatol Surg. 2010;36:194-197.
6. Ke M, Moul D, Camouse M, et al. Where is it? The utility of biopsy-site photography. Dermatol Surg. 2010;36:198-202.
7. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies—a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41:499-504
8. Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
9. Rhinehart BM, Murphy Me, Farley MF, et al. Sterile versus nonsterile gloves during Mohs micrographic surgery: infection rate is not affected. Dermatol Surg. 2006;32:170-176.
10. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
11. Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
12. Gladstone HB, Stewart D. An algorithm for the reconstruction of complex facial defects. Skin Therapy Lett. 2007;12:6-9.
13. Robinson JK. Mohs micrographic surgery. Clin Plast Surg. 1993;20:149-156.
14. Swanson NA. Mohs surgery. Technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
15. Robins P. Chemosurgery: my 15 years of experience. J Dermatol Surg Oncol. 1981;7:779-789.
16. Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
17. van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
18. Xiong DD, Beal BT, Varra V, et al. Outcomes in intermediate-risk squamous cell carcinomas treated with Mohs micrographic surgery compared with wide local excision. J Am Acad Dermatol. 2020;82: 1195-1204.
19. Trofymenko O, Bordeaux JS, Zeitouni NC. Melanoma of the face and Mohs micrographic surgery: nationwide mortality data analysis. Dermatol Surg. 2018;44:481-492.
20. Nosrati A, Berliner JG, Goel S, et al. Outcomes of melanoma in situ treated with Mohs micrographic surgery compared with wide local excision. JAMA Dermatol. 2017;153:436-441.
21. Etzkom JR, Sobanko JF, Elenitsas R, et al. Low recurrences for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
Mohs micrographic surgery (MMS) is a unique dermatologic surgery technique that allows the dermatologist to fill the concomitant roles of surgeon and pathologist. It is utilized for the extirpation of skin malignancy, with an emphasis on tissue preservation and immediate surgical margin evaluation. In MMS, the Mohs surgeon acts as the surgeon for physical removal of the lesion and the pathologist during evaluation of frozen section margins.1
Primary care providers (PCPs) are on the frontlines of management of cutaneous malignancy. Whether referring to Dermatology for biopsy or performing a biopsy themselves, PCPs can assure optimal treatment outcomes by guiding patients to evidence-based treatments, while still respecting the patient’s wishes. In this evidence-based review of the advantages, improved outcomes, and safety of Mohs surgery for the treatment of common and rare skin neoplasms, we provide our primary care colleagues with information on the indications, process (the order in which steps of the procedure are performed), and techniques used for treating cutaneous malignancies with Mohs surgery.
When is Mohs surgery appropriate?
MMS has typically been reserved for treatment of cutaneous malignancy in cosmetically sensitive areas where tissue preservation is key. In 2012, Connolly et al released appropriate use criteria (AUC) for MMS.2 (See “An app that helps clinicians apply the criteria for Mohs surgery.”) Within the AUC, there are 4 major qualitative and quantitative categories when considering referral for MMS:
- area of the body in which the lesion manifests
- the patient’s medical characteristics
- tumor characteristics
- the size of the lesion to be treated.2
Areas of the body are divided into 3 categories by the AUC according to how challenging tumor extirpation is expected to be and how critical tissue preservation is. Areas termed “H” receive the highest score for appropriate Mohs usage, followed by areas “M” and “L.”
SIDEBAR
An app that helps clinicians apply the criteria for Mohs surgery
“Mohs Surgery Appropriate Use Criteria” is a free and easy-to-use smartphone application to help determine whether Mohs micrographic surgery (MMS) is appropriate for a particular patient. Clinicians can enter the details of a recent skin cancer biopsy along with patient information into the app and it will calculate a score automatically categorized into 1 of 3 categories: “appropriate,” “uncertain,” and “not appropriate” for MMS. The clinician can then talk to the patient about a possible referral to a Mohs surgeon, depending on the appropriateness of the procedure for the patient and their tumor.
Patient medical characteristics that should be taken into account when referring for Mohs surgery are the patient’s immune status, genetic syndromes that may predispose the patient to cutaneous malignancies (eg, xeroderma pigmentosa), history of radiation to the area of involvement, and the patient’s history of aggressive cutaneous malignancies.
Tumor characteristics. The most common malignancies treated with MMS include basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). These malignancies are further delineated through histologic evaluation by a pathologist or dermatopathologist. Aggressive features of a BCC on any area of the body that warrant referral to a Mohs surgeon include morpheaform/fibrosing/sclerosing histologic findings, as well as micronodular architecture and perineural invasion. Concerning histologic SCC findings that warrant Mohs surgery through the AUC include sclerosing, basosquamous, and small cell histology, as well as poorly differentiated and/or undifferentiated SCC.
Melanoma in situ and lentigo maligna, which are variants of melanoma limited to the epidermis without invasion into the underlying dermis, are included within the AUC for MMS. For invasive melanoma (melanoma that has invaded into the dermis or subcutaneous tissue), MMS has been shown to have marginal benefit but currently is not included within the AUC.3
Continue to: Due to excellent margin control...
Due to excellent margin control via immediate microscopic evaluation of surgical margins, MMS is an appropriate treatment choice and indicated for many more uncommon cutaneous malignancies, including sebaceous and mucinous carcinoma, microcystic adnexal carcinoma, Merkel cell carcinoma, leiomyosarcoma, dermatofibrosarcoma protuberans, atypical fibroxanthoma, angiosarcoma, and other more rarely encountered clinical malignancies.2
Tumor size. When considering a referral to MMS for cancer extirpation, the size of the tumor does play a role; however, size depends on the type of tumor as well as the location on the body. In general, most skin cancers of any size on the face, perianal area, genitalia, nipples, hands, feet and ankles, or pretibial surface are appropriate for Mohs surgery. Skin cancers on the trunk and extremities are also appropriate if they are above a certain size specified by the AUC. Tumor type and whether they are recurrences also factor into the equation.
Who will do the procedure?
A recent review showed that PCPs were more likely to refer patients to plastic surgery rather than Mohs surgery for skin cancer removal, especially among younger female patients.4 This is likely because of the perception that plastic surgeons do more complex closures and have more experience removing difficult cancers. Interestingly, this same study showed that Mohs surgeons may actually be doing several-fold more complex closures (flaps and grafts) on the nose and ears than plastic surgeons at similar practice settings.4
Aside from Mohs surgeons doing more closures, perhaps the biggest difference between Mohs surgeons and plastic surgeons is the pathology training of the Mohs surgeon. Mohs surgeons evaluate 100% of the tissue margins at the time of the procedure to both ensure complete tumor removal and to preserve as much tumor-free skin as possible, ultimately resulting in decreased recurrences and smaller scars. In contrast, the plastic surgeon’s rigorous training typically does not include extensive dermatopathology training, particularly the pathology of cutaneous neoplasms. Plastic surgeons will often send pathologic specimens for evaluation, meaning patients have to wait for outside histologic confirmation before their wounds can be closed. Additionally, the histologic evaluation is often not a full-margin assessment, as not all labs are equipped for this technique.
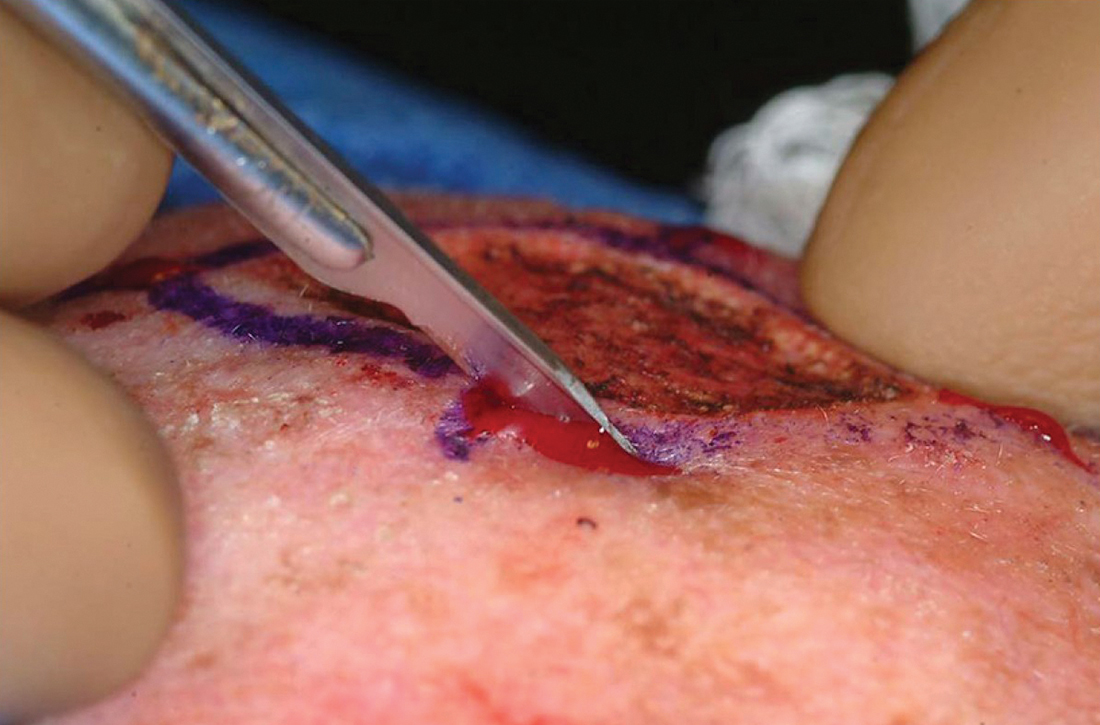
Consider early consultation with a Mohs surgeon for tumor extirpation to keep the defect size as small as possible, as MMS does not require taking margins of healthy surrounding tissue, in contrast to wide local excisions (WLEs; FIGURE 1). A smaller initial incision will result in a smaller scar, which is likely to have better cosmetic outcomes and decreased risk for wound infection.

Continue to: Before consultation...
Before consultation, include a picture of the surgical site with the patient’s referral documentation or have the patient present a photo from his or her phone to the Mohs surgeon. (If a camera or cell phone is not available, triangulation of the site’s location using cosmetic landmarks can be documented in the patient’s chart.)
What the patient can expect during preop visits
During an initial consultation, patients can expect an evaluation by the surgeon that will include more photo taking, a discussion of the surgery, and possibly, performance of an in-clinic biopsy of suspicious lesions. Many practices, including the authors’, use a photo capturing add-on for the EMR in the office.5-7
During the consent process, MMS is described to the patient using lay language and, often, pictorial depictions of the procedure. While explaining that the procedure helps preserve healthy tissue and limit the size of the resulting scar, the surgeon will typically manage the expectations of the patient prior to the first incision. Many clinically small lesions can have significant subclinical extension adjacent to, or on top of, cosmetic landmarks, requiring a flap or graft to close the surgical defect with acceptable cosmetic outcomes.8
One more time. Immediately before surgery, the surgeon will again review the procedure with the patient, using photos of the biopsy site taken during the initial consult, in conjunction with patient verification of the biopsy site, to verify the surgical site and confirm that the patient understands and agrees to the surgery.
A look at how Mohs surgery is performed
MMS typically is performed in the outpatient setting but can also be performed in an operating room or outpatient surgical center. MMS can be performed in a nonsterile procedure room with surgeons and assistants typically utilizing clean, nonsterile gloves, although many Mohs surgeons prefer to perform part, or all, of the technique using sterile gloves.9 A recent systematic review and large meta-analysis showed no significant difference in postsurgical site infections when comparing the use of sterile vs nonsterile gloves.10
Continue to: Prior to initial incision...
Prior to initial incision, the site is marked with a surgical pen and given 1-mm margins around the clinically visualized lesion. The site is then cleansed with an antiseptic, typically a chlorhexidine solution. Local anesthesia is employed, most commonly with a 1:100,000 lidocaine and epinephrine injection. Marking of the tumor prior to numbing is imperative, as the boundaries of the tumor are typically obscured when the local cutaneous vasculature constricts and causes visualized blanching of adjacent skin. Many Mohs surgeons perform a brief curettage of the lesion with a nondisposable, dull curette to better define the tumor edges and to debulk any obvious exophytic tumor noted by the naked eye.
Prior to the first incision, the surgical site is scored in a variety of ways in order to properly orient the tissue after it has been removed from the patient. Mohs surgeons have differing opinions on how to score and/or mark the tissue, but a common practice is to make a nick at the 12 o’clock position. Following removal of the first stage, the nick will be visible on both the extirpated tissue and the tissue just above the surgical defect. This prevents potential confusion regarding orientation during tissue processing.
The majority of all WLEs are performed utilizing the scalpel blade at an angle 90° perpendicular to the plane of the skin. In MMS, a signature 45° angle with the tip of the scalpel pointing toward, and the handle pointing away from, the lesion is commonly used in order to bevel the tissue being excised (FIGURE 2). Once the tissue is excised, hemostasis is obtained using electrodessication/electrofulguration or electrocoagulation.

Tissue processing and microscopic evaluation
The technique of beveling allows the epidermis, dermis, and subcutaneous tissue to lie flat on the tissue block, so the Mohs surgeon can evaluate 100% of the excised tissue’s margins. The tissue is transported to a nearby lab for staining and processing. Even if near-perfect beveling is achieved, many stages will require bisecting, quadrissecting, or relaxing cuts in order to allow the margins to lie flat on the tissue block.
Using the scoring system made prior to incision, the tissue is oriented and stained with colored ink. Subsequently, a map is made with sections highlighting the colors used to stain designated areas of the tissue. This step is imperative for orientation during microscopic evaluation. Additionally, the map serves as a guide and log, should a section of the specimen have an involved margin and require another stage.
Continue to: Once fixed to the block...
Once fixed to the block, the tissue is engulfed in appropriate embedding medium and placed within the cryostat. The block is slowly cut to produce several micron-thin wafers of tissue that are then mounted on glass slides and processed with hematoxylin and eosin (H&E) or various stains. The first wafers of tissue that come from the tissue block are those that are closest to the margin that was excised. Thus, 100% of the epidermis and deep margin can be visualized. “Deeper sections” are those that come from deeper cuts within the tissue and are more likely to show the malignant neoplasm.
The evaluation of immediate margins at the very edge of the tissue is in contrast to the technique of “bread-loafing,” which is the standard of evaluating margins after a WLE.11 With this process, the pathologist examines sections that are cut 2- to 4-mm apart. This process only allows the pathologist to examine roughly 1% of the total tissue that was excised, and large variability in cutaneous representation can occur depending on the individual who cuts and processes the tissue.11
Closing the defect
Once the site is deemed clear of residual tumor, the Mohs surgeon approaches the defect and determines the most appropriate way to close the surgical wound. Mohs surgeons are trained to close wounds using a variety of methods, including complex linear closures, flaps, and full-thickness skin grafts. Thoughtful consideration of local anatomy, cosmetic landmarks that may be affected by the closure method, and local tissue laxity are evaluated.
Depending on the location, a secondary intention closure may prove to be just as effective and cosmetically satisfying as a primary intention closure. In light of the many methods of closure, a complex or large surface area defect may better be suited for evaluation and closure by another specialist such as an ENT physician, ophthalmologist, or plastic surgeon.12
Lower recurrence rates for patients who undergo Mohs surgery
As noted earlier, the cutaneous malignancies most commonly treated with MMS are BCCs, followed by SCCs.13 Comparison studies between WLE and MMS show clinically significant differences in terms of recurrence rates between the 2 procedures.
Continue to: For BCCs
For BCCs, recurrence rates for excisions vs MMS are 10% and 1%, respectively.14-16 A randomized trial reviewing 10-year recurrence of primary BCCs on the face showed recurrence rates for MMS of 4.4% compared to 12.2% for WLE.17 This study also showed recurrence rates for recurrent facial BCCs treated with MMS to be 3.9% vs 13.5% for standard WLE.17
SCC. The evidence similarly supports the efficacy of MMS for SCCs. A recent study showed primary T2a tumors had a 1.2% local recurrence rate with Mohs vs a 4% recurrence rate with WLE at an average follow-up of 2.8 years.18 Another study showed that primary tumors that were < 2 cm in diameter had a 5-year cure rate of 99% with Mohs surgery.11
Melanoma in situ. A few studies have shown no clinically significant benefit of MMS compared to WLE when it comes to melanoma in situ.19,20 However, a more recent article by Etzkom et al noted the ability to potentially upstage melanoma in situ and invasive melanoma after reviewing peripheral and deep margins during MMS.21 In this study, the authors uniquely delayed wound closure if upstaging was established and the need for a sentinel lymph node biopsy was warranted. This approach to MMS with delayed closure ultimately paved the way for very low recurrence rates.
CORRESPONDENCE
Andres Garcia, MD, 2612 112th Street, Lubbock, TX 79423; [email protected]
Mohs micrographic surgery (MMS) is a unique dermatologic surgery technique that allows the dermatologist to fill the concomitant roles of surgeon and pathologist. It is utilized for the extirpation of skin malignancy, with an emphasis on tissue preservation and immediate surgical margin evaluation. In MMS, the Mohs surgeon acts as the surgeon for physical removal of the lesion and the pathologist during evaluation of frozen section margins.1
Primary care providers (PCPs) are on the frontlines of management of cutaneous malignancy. Whether referring to Dermatology for biopsy or performing a biopsy themselves, PCPs can assure optimal treatment outcomes by guiding patients to evidence-based treatments, while still respecting the patient’s wishes. In this evidence-based review of the advantages, improved outcomes, and safety of Mohs surgery for the treatment of common and rare skin neoplasms, we provide our primary care colleagues with information on the indications, process (the order in which steps of the procedure are performed), and techniques used for treating cutaneous malignancies with Mohs surgery.
When is Mohs surgery appropriate?
MMS has typically been reserved for treatment of cutaneous malignancy in cosmetically sensitive areas where tissue preservation is key. In 2012, Connolly et al released appropriate use criteria (AUC) for MMS.2 (See “An app that helps clinicians apply the criteria for Mohs surgery.”) Within the AUC, there are 4 major qualitative and quantitative categories when considering referral for MMS:
- area of the body in which the lesion manifests
- the patient’s medical characteristics
- tumor characteristics
- the size of the lesion to be treated.2
Areas of the body are divided into 3 categories by the AUC according to how challenging tumor extirpation is expected to be and how critical tissue preservation is. Areas termed “H” receive the highest score for appropriate Mohs usage, followed by areas “M” and “L.”
SIDEBAR
An app that helps clinicians apply the criteria for Mohs surgery
“Mohs Surgery Appropriate Use Criteria” is a free and easy-to-use smartphone application to help determine whether Mohs micrographic surgery (MMS) is appropriate for a particular patient. Clinicians can enter the details of a recent skin cancer biopsy along with patient information into the app and it will calculate a score automatically categorized into 1 of 3 categories: “appropriate,” “uncertain,” and “not appropriate” for MMS. The clinician can then talk to the patient about a possible referral to a Mohs surgeon, depending on the appropriateness of the procedure for the patient and their tumor.
Patient medical characteristics that should be taken into account when referring for Mohs surgery are the patient’s immune status, genetic syndromes that may predispose the patient to cutaneous malignancies (eg, xeroderma pigmentosa), history of radiation to the area of involvement, and the patient’s history of aggressive cutaneous malignancies.
Tumor characteristics. The most common malignancies treated with MMS include basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). These malignancies are further delineated through histologic evaluation by a pathologist or dermatopathologist. Aggressive features of a BCC on any area of the body that warrant referral to a Mohs surgeon include morpheaform/fibrosing/sclerosing histologic findings, as well as micronodular architecture and perineural invasion. Concerning histologic SCC findings that warrant Mohs surgery through the AUC include sclerosing, basosquamous, and small cell histology, as well as poorly differentiated and/or undifferentiated SCC.
Melanoma in situ and lentigo maligna, which are variants of melanoma limited to the epidermis without invasion into the underlying dermis, are included within the AUC for MMS. For invasive melanoma (melanoma that has invaded into the dermis or subcutaneous tissue), MMS has been shown to have marginal benefit but currently is not included within the AUC.3
Continue to: Due to excellent margin control...
Due to excellent margin control via immediate microscopic evaluation of surgical margins, MMS is an appropriate treatment choice and indicated for many more uncommon cutaneous malignancies, including sebaceous and mucinous carcinoma, microcystic adnexal carcinoma, Merkel cell carcinoma, leiomyosarcoma, dermatofibrosarcoma protuberans, atypical fibroxanthoma, angiosarcoma, and other more rarely encountered clinical malignancies.2
Tumor size. When considering a referral to MMS for cancer extirpation, the size of the tumor does play a role; however, size depends on the type of tumor as well as the location on the body. In general, most skin cancers of any size on the face, perianal area, genitalia, nipples, hands, feet and ankles, or pretibial surface are appropriate for Mohs surgery. Skin cancers on the trunk and extremities are also appropriate if they are above a certain size specified by the AUC. Tumor type and whether they are recurrences also factor into the equation.
Who will do the procedure?
A recent review showed that PCPs were more likely to refer patients to plastic surgery rather than Mohs surgery for skin cancer removal, especially among younger female patients.4 This is likely because of the perception that plastic surgeons do more complex closures and have more experience removing difficult cancers. Interestingly, this same study showed that Mohs surgeons may actually be doing several-fold more complex closures (flaps and grafts) on the nose and ears than plastic surgeons at similar practice settings.4
Aside from Mohs surgeons doing more closures, perhaps the biggest difference between Mohs surgeons and plastic surgeons is the pathology training of the Mohs surgeon. Mohs surgeons evaluate 100% of the tissue margins at the time of the procedure to both ensure complete tumor removal and to preserve as much tumor-free skin as possible, ultimately resulting in decreased recurrences and smaller scars. In contrast, the plastic surgeon’s rigorous training typically does not include extensive dermatopathology training, particularly the pathology of cutaneous neoplasms. Plastic surgeons will often send pathologic specimens for evaluation, meaning patients have to wait for outside histologic confirmation before their wounds can be closed. Additionally, the histologic evaluation is often not a full-margin assessment, as not all labs are equipped for this technique.
Consider early consultation with a Mohs surgeon for tumor extirpation to keep the defect size as small as possible, as MMS does not require taking margins of healthy surrounding tissue, in contrast to wide local excisions (WLEs; FIGURE 1). A smaller initial incision will result in a smaller scar, which is likely to have better cosmetic outcomes and decreased risk for wound infection.

Continue to: Before consultation...
Before consultation, include a picture of the surgical site with the patient’s referral documentation or have the patient present a photo from his or her phone to the Mohs surgeon. (If a camera or cell phone is not available, triangulation of the site’s location using cosmetic landmarks can be documented in the patient’s chart.)
What the patient can expect during preop visits
During an initial consultation, patients can expect an evaluation by the surgeon that will include more photo taking, a discussion of the surgery, and possibly, performance of an in-clinic biopsy of suspicious lesions. Many practices, including the authors’, use a photo capturing add-on for the EMR in the office.5-7
During the consent process, MMS is described to the patient using lay language and, often, pictorial depictions of the procedure. While explaining that the procedure helps preserve healthy tissue and limit the size of the resulting scar, the surgeon will typically manage the expectations of the patient prior to the first incision. Many clinically small lesions can have significant subclinical extension adjacent to, or on top of, cosmetic landmarks, requiring a flap or graft to close the surgical defect with acceptable cosmetic outcomes.8
One more time. Immediately before surgery, the surgeon will again review the procedure with the patient, using photos of the biopsy site taken during the initial consult, in conjunction with patient verification of the biopsy site, to verify the surgical site and confirm that the patient understands and agrees to the surgery.
A look at how Mohs surgery is performed
MMS typically is performed in the outpatient setting but can also be performed in an operating room or outpatient surgical center. MMS can be performed in a nonsterile procedure room with surgeons and assistants typically utilizing clean, nonsterile gloves, although many Mohs surgeons prefer to perform part, or all, of the technique using sterile gloves.9 A recent systematic review and large meta-analysis showed no significant difference in postsurgical site infections when comparing the use of sterile vs nonsterile gloves.10
Continue to: Prior to initial incision...
Prior to initial incision, the site is marked with a surgical pen and given 1-mm margins around the clinically visualized lesion. The site is then cleansed with an antiseptic, typically a chlorhexidine solution. Local anesthesia is employed, most commonly with a 1:100,000 lidocaine and epinephrine injection. Marking of the tumor prior to numbing is imperative, as the boundaries of the tumor are typically obscured when the local cutaneous vasculature constricts and causes visualized blanching of adjacent skin. Many Mohs surgeons perform a brief curettage of the lesion with a nondisposable, dull curette to better define the tumor edges and to debulk any obvious exophytic tumor noted by the naked eye.
Prior to the first incision, the surgical site is scored in a variety of ways in order to properly orient the tissue after it has been removed from the patient. Mohs surgeons have differing opinions on how to score and/or mark the tissue, but a common practice is to make a nick at the 12 o’clock position. Following removal of the first stage, the nick will be visible on both the extirpated tissue and the tissue just above the surgical defect. This prevents potential confusion regarding orientation during tissue processing.
The majority of all WLEs are performed utilizing the scalpel blade at an angle 90° perpendicular to the plane of the skin. In MMS, a signature 45° angle with the tip of the scalpel pointing toward, and the handle pointing away from, the lesion is commonly used in order to bevel the tissue being excised (FIGURE 2). Once the tissue is excised, hemostasis is obtained using electrodessication/electrofulguration or electrocoagulation.

Tissue processing and microscopic evaluation
The technique of beveling allows the epidermis, dermis, and subcutaneous tissue to lie flat on the tissue block, so the Mohs surgeon can evaluate 100% of the excised tissue’s margins. The tissue is transported to a nearby lab for staining and processing. Even if near-perfect beveling is achieved, many stages will require bisecting, quadrissecting, or relaxing cuts in order to allow the margins to lie flat on the tissue block.
Using the scoring system made prior to incision, the tissue is oriented and stained with colored ink. Subsequently, a map is made with sections highlighting the colors used to stain designated areas of the tissue. This step is imperative for orientation during microscopic evaluation. Additionally, the map serves as a guide and log, should a section of the specimen have an involved margin and require another stage.
Continue to: Once fixed to the block...
Once fixed to the block, the tissue is engulfed in appropriate embedding medium and placed within the cryostat. The block is slowly cut to produce several micron-thin wafers of tissue that are then mounted on glass slides and processed with hematoxylin and eosin (H&E) or various stains. The first wafers of tissue that come from the tissue block are those that are closest to the margin that was excised. Thus, 100% of the epidermis and deep margin can be visualized. “Deeper sections” are those that come from deeper cuts within the tissue and are more likely to show the malignant neoplasm.
The evaluation of immediate margins at the very edge of the tissue is in contrast to the technique of “bread-loafing,” which is the standard of evaluating margins after a WLE.11 With this process, the pathologist examines sections that are cut 2- to 4-mm apart. This process only allows the pathologist to examine roughly 1% of the total tissue that was excised, and large variability in cutaneous representation can occur depending on the individual who cuts and processes the tissue.11
Closing the defect
Once the site is deemed clear of residual tumor, the Mohs surgeon approaches the defect and determines the most appropriate way to close the surgical wound. Mohs surgeons are trained to close wounds using a variety of methods, including complex linear closures, flaps, and full-thickness skin grafts. Thoughtful consideration of local anatomy, cosmetic landmarks that may be affected by the closure method, and local tissue laxity are evaluated.
Depending on the location, a secondary intention closure may prove to be just as effective and cosmetically satisfying as a primary intention closure. In light of the many methods of closure, a complex or large surface area defect may better be suited for evaluation and closure by another specialist such as an ENT physician, ophthalmologist, or plastic surgeon.12
Lower recurrence rates for patients who undergo Mohs surgery
As noted earlier, the cutaneous malignancies most commonly treated with MMS are BCCs, followed by SCCs.13 Comparison studies between WLE and MMS show clinically significant differences in terms of recurrence rates between the 2 procedures.
Continue to: For BCCs
For BCCs, recurrence rates for excisions vs MMS are 10% and 1%, respectively.14-16 A randomized trial reviewing 10-year recurrence of primary BCCs on the face showed recurrence rates for MMS of 4.4% compared to 12.2% for WLE.17 This study also showed recurrence rates for recurrent facial BCCs treated with MMS to be 3.9% vs 13.5% for standard WLE.17
SCC. The evidence similarly supports the efficacy of MMS for SCCs. A recent study showed primary T2a tumors had a 1.2% local recurrence rate with Mohs vs a 4% recurrence rate with WLE at an average follow-up of 2.8 years.18 Another study showed that primary tumors that were < 2 cm in diameter had a 5-year cure rate of 99% with Mohs surgery.11
Melanoma in situ. A few studies have shown no clinically significant benefit of MMS compared to WLE when it comes to melanoma in situ.19,20 However, a more recent article by Etzkom et al noted the ability to potentially upstage melanoma in situ and invasive melanoma after reviewing peripheral and deep margins during MMS.21 In this study, the authors uniquely delayed wound closure if upstaging was established and the need for a sentinel lymph node biopsy was warranted. This approach to MMS with delayed closure ultimately paved the way for very low recurrence rates.
CORRESPONDENCE
Andres Garcia, MD, 2612 112th Street, Lubbock, TX 79423; [email protected]
1. Dim-Jamora KC, Perone JB. Management of cutaneous tumors with Mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
2. Ad Hoc Task Force, Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. Published correction appears in J Am Acad Dermatol. 2015;72:748.
3. Cheraghlou S, Christensen S, Agogo G, et al. Comparison of survival after Mohs micrographic surgery vs wide margin excision for early-stage invasive melanoma. JAMA Dermatol. 2019;155:1252-1259.
4. Hill D, Kim K, Mansouri B, et al. Quantity and characteristics of flap or graft repairs for skin cancer on the nose or ears: a comparison between Mohs micrographic surgery and plastic surgery. Cutis. 2019;103:284-287.
5. McGinness JL, Goldstein G. The value of preoperative biopsy-site photography for identifying cutaneous lesions. Dermatol Surg. 2010;36:194-197.
6. Ke M, Moul D, Camouse M, et al. Where is it? The utility of biopsy-site photography. Dermatol Surg. 2010;36:198-202.
7. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies—a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41:499-504
8. Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
9. Rhinehart BM, Murphy Me, Farley MF, et al. Sterile versus nonsterile gloves during Mohs micrographic surgery: infection rate is not affected. Dermatol Surg. 2006;32:170-176.
10. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
11. Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
12. Gladstone HB, Stewart D. An algorithm for the reconstruction of complex facial defects. Skin Therapy Lett. 2007;12:6-9.
13. Robinson JK. Mohs micrographic surgery. Clin Plast Surg. 1993;20:149-156.
14. Swanson NA. Mohs surgery. Technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
15. Robins P. Chemosurgery: my 15 years of experience. J Dermatol Surg Oncol. 1981;7:779-789.
16. Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
17. van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
18. Xiong DD, Beal BT, Varra V, et al. Outcomes in intermediate-risk squamous cell carcinomas treated with Mohs micrographic surgery compared with wide local excision. J Am Acad Dermatol. 2020;82: 1195-1204.
19. Trofymenko O, Bordeaux JS, Zeitouni NC. Melanoma of the face and Mohs micrographic surgery: nationwide mortality data analysis. Dermatol Surg. 2018;44:481-492.
20. Nosrati A, Berliner JG, Goel S, et al. Outcomes of melanoma in situ treated with Mohs micrographic surgery compared with wide local excision. JAMA Dermatol. 2017;153:436-441.
21. Etzkom JR, Sobanko JF, Elenitsas R, et al. Low recurrences for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
1. Dim-Jamora KC, Perone JB. Management of cutaneous tumors with Mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
2. Ad Hoc Task Force, Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. Published correction appears in J Am Acad Dermatol. 2015;72:748.
3. Cheraghlou S, Christensen S, Agogo G, et al. Comparison of survival after Mohs micrographic surgery vs wide margin excision for early-stage invasive melanoma. JAMA Dermatol. 2019;155:1252-1259.
4. Hill D, Kim K, Mansouri B, et al. Quantity and characteristics of flap or graft repairs for skin cancer on the nose or ears: a comparison between Mohs micrographic surgery and plastic surgery. Cutis. 2019;103:284-287.
5. McGinness JL, Goldstein G. The value of preoperative biopsy-site photography for identifying cutaneous lesions. Dermatol Surg. 2010;36:194-197.
6. Ke M, Moul D, Camouse M, et al. Where is it? The utility of biopsy-site photography. Dermatol Surg. 2010;36:198-202.
7. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies—a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41:499-504
8. Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
9. Rhinehart BM, Murphy Me, Farley MF, et al. Sterile versus nonsterile gloves during Mohs micrographic surgery: infection rate is not affected. Dermatol Surg. 2006;32:170-176.
10. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
11. Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
12. Gladstone HB, Stewart D. An algorithm for the reconstruction of complex facial defects. Skin Therapy Lett. 2007;12:6-9.
13. Robinson JK. Mohs micrographic surgery. Clin Plast Surg. 1993;20:149-156.
14. Swanson NA. Mohs surgery. Technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
15. Robins P. Chemosurgery: my 15 years of experience. J Dermatol Surg Oncol. 1981;7:779-789.
16. Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
17. van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
18. Xiong DD, Beal BT, Varra V, et al. Outcomes in intermediate-risk squamous cell carcinomas treated with Mohs micrographic surgery compared with wide local excision. J Am Acad Dermatol. 2020;82: 1195-1204.
19. Trofymenko O, Bordeaux JS, Zeitouni NC. Melanoma of the face and Mohs micrographic surgery: nationwide mortality data analysis. Dermatol Surg. 2018;44:481-492.
20. Nosrati A, Berliner JG, Goel S, et al. Outcomes of melanoma in situ treated with Mohs micrographic surgery compared with wide local excision. JAMA Dermatol. 2017;153:436-441.
21. Etzkom JR, Sobanko JF, Elenitsas R, et al. Low recurrences for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
PRACTICE RECOMMENDATIONS
› Consider Mohs surgery for patients who have lesions located mainly in regions of the face that make excision difficult without significant scarring. A
› Consider Mohs surgery for basal cell carcinoma and squamous cell carcinoma that typically involve (but are not necessarily limited to) the face, as the procedure significantly reduces recurrence rates and leads to cure rates of up to 99%. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Managing work disability to help patients return to the job
All clinicians who have patients who are employed play an essential role in work disability programs—whether or not those clinicians have received formal training in occupational health. A study found that primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.1
In this article, we explain why it is important for family physicians to better manage work disability at the point of care, to help patients return to their pre-injury or pre-illness level of activity.
Why managing the duration of work disability matters
Each year, millions of American workers leave their jobs—temporarily or permanently—because of illness, injury, or the effects of a chronic condition.2 It is estimated that 893 million workdays are lost annually due to a new medical problem; an additional 527 million workdays are lost due to the impact of chronic health conditions on the ability to perform at work.3 The great majority of these lost workdays are the result of personal health conditions, not work-related problems; patients must therefore cope with the accompanying disruption of life and work.
Significant injury and illness can create a life crisis, especially when there is uncertainty about future livelihood, such as an income shortfall during a lengthy recovery. Only 40% of the US workforce is covered by a short-term disability insurance program; only 10% of low-wage and low-skill workers have this type of coverage.4 Benefits rarely replace loss of income entirely, and worker compensation insurance programs provide only partial wage replacement.
In short, work disability is destabilizing and can threaten overall well-being.5
Furthermore, the longer a person remains on temporary disability, the more likely that person is to move to a publicly funded disability program or leave the workforce entirely—thus, potentially losing future earnings and self-identity related to being a working member of society.6-8
Most of the annual cost of poor health for US employers derives from medical and wage benefits ($226 billion) and impaired or reduced employee performance ($223 billion).3 In addition, temporarily disabled workers likely account for a disproportionate share of health care costs: A study found that one-half of medical and pharmacy payments were paid out to the one-quarter of employees requiring disability benefits.9
Continue to: Benefits of staying on the job
Benefits of staying on the job. Research shows that there are physical and mental health benefits to remaining at, or returning to, work after an injury or illness.10,11 For example, in a longitudinal cohort of people with low back pain, immediate or early return to work (in 1-7 days) was associated with reduced pain and improved functioning at 3 months.12 Physicians who can guide patients safely back to normal activities, including work, minimize the physical and mental health impact of the injury or illness and avoid chronicity.13
Emphasizing the importance of health, not disease or injury
Health researchers have found that diagnosis, cause, and extent of morbidity do not adequately explain observed variability in the impact of health conditions, utilization of resources, or need for services. A wider view of the functional implications of an injury or illness is therefore required for physicians to effectively recommend disability duration.
The World Health Organization recommends a shift toward a more holistic view of health, impairment, and disability, including an emphasis on functional ability, intrinsic capacity, and environmental context.14 The American Medical Association, American College of Occupational and Environmental Medicine, and Canadian Medical Association emphasize that prolonged absence from one’s normal role can be detrimental to mental, physical, and social well-being.8 These advisory groups recommend that physicians encourage patients who are unable to work to (1) focus on restoring the rhythm of their everyday life in a stepwise fashion and (2) resume their usual responsibilities as soon as possible.
Advising a patient to focus on “what you can do,” not “what you can’t do,” might make all the difference in their return to productivity. Keeping the patient’s—as well as your own—attention focused on the positive process of recovery and documenting evidence of functional progress is an important addition to (or substitute for) detailed inquiries about pain and dysfunction.
Why does duration of disability vary so much from case to case?
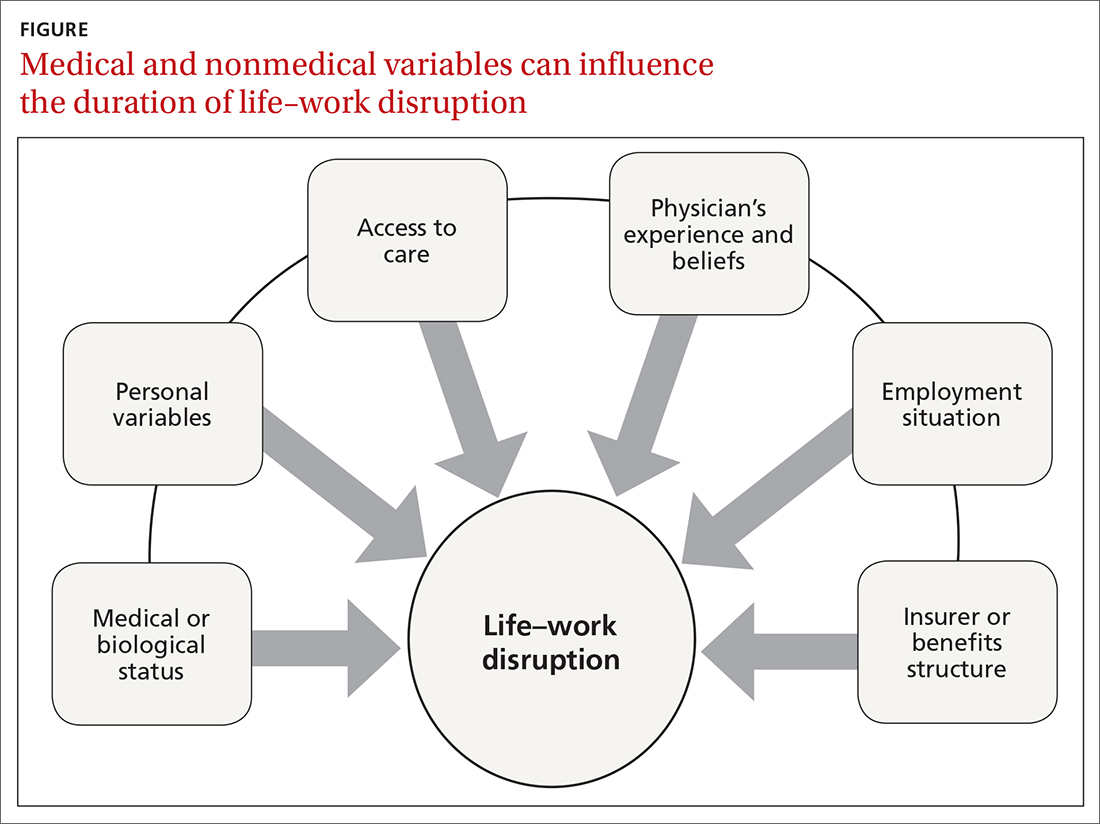
Disability duration is influenced by the individual patient, employer, physician, jurisdiction, insurer or benefits structure, and access to care.15 For you to effectively manage a patient who is out of work for a medical reason, it is important to understand how nonmedical variables often influence the pace of recovery and the timing of return to work (FIGURE).
Continue to: Deficient communication
Deficient communication. Often, employers, insurers, third-party administrators, and clinicians—each a key stakeholder in disability care—are disconnected from one another, resulting in poor communication with the injured worker. Such fragmented communication can delay treatment and recovery.16 Data systems are not designed to measure the duration of disability or provide proactive notification for key stakeholders who might intervene to facilitate a patient’s recovery.
Alternatively, a collaborative approach to disability management has been shown to improve outcomes.17,18 Communication among the various professionals involved can be coordinated and expedited by a case manager or disability manager hired by the medical practice, the employer, or the insurance company.
Psychosocial and economic influences can radically affect the time it takes to return to pre-injury or pre-illness functional status. Demographic variables (age, sex, income, education, and support system) influence how a person responds to a debilitating injury or illness.19 Fear of re-injury, anxiety over the intensity of pain upon movement, worry over dependency on others, and resiliency play an important role when a patient is attempting to return to full activity.20,21
Job satisfaction has been identified as the most significant variable associated with prompt return to work.15 Work has many health-enhancing aspects, including socioeconomic status, psychosocial support, and self-identity22; however, not everyone wants, or feels ready, to go back to work even once they are physically able. Workplace variables, such as the patient–employee’s dislike of the position, coworkers, or manager, have been cited by physicians as leading barriers to returning to work at an appropriate time.23,24
Other external variables. Physicians should formulate activity prescriptions and medical restrictions based on the impact the medical condition has on the usual ability to function, as well as the anticipated impact of specific activities on the body’s natural healing process. However, Rainville and colleagues found that external variables—patient requests, employer characteristics, and jurisdiction issues—considerably influence physicians’ recommendations.20 For example, benefit structure might influence how long a patient wants to remain out of work—thus altering the requests they make to their physician. Jurisdictional characteristics, such as health care systems, state workers’ compensation departments, and payer systems, all influence a patient’s recovery timeline and time away from work.25
Continue to: What does your patient need so that they can recover?
What does your patient need so that they can recover? Individual and systemic factors must be appropriately addressed to minimize the impact that recovery from a disability has on a person’s life. Successful functional recovery enables the person to self-manage symptoms, reduce disruption-associated stress, preserve mental health, and maintain healthy relationships at home and work. An example is the patient who has successfully coped with the entire predicament that their medical condition posed and resumed their usual daily routine and responsibilities at home and at work—albeit sometimes with temporary or permanent modification necessitated by their specific condition.
Strategies that help patients stay at, or return to, their job
Physicians who anticipate, monitor, and actively manage the duration of a work disability can improve patient outcomes by minimizing life disruption, avoiding unnecessary medical care, and shortening the period of absence from work.
Key strategy: Set expectations for functional recovery early in the episode, including a forecast of how long it will take to get life and work back to normal.26,27 This is similar to discussing expectations about pain before surgery, which has been shown to decrease subsequent requests for opioids.28 It is crucial to educate the patient about timelines, define functional outcomes, and encourage them to set goals for recovery.29
Devise an evidence-based treatment plan. A fundamental way to reduce disability duration is to (1) devise a treatment plan that is evidence based and (2) take the most effective route to recovery. Given the pace with which medical research changes the understanding of diseases and treatments, it is essential to rely on up-to-date, comprehensive, independent, and authoritative resources to support your care decisions.
Aligning clinical practice with evidence-based medicine (EBM) is a good way to accomplish that goal. By definition, EBM practice guidelines recommend the safest and most effective treatments after unbiased assessment of the best available research. Increasingly, EBM is adopted to improve clinical and functional outcomes, establish national standards of care, and set criteria to evaluate clinical performance.30
Continue to: Utilize established guidelines
Utilize established guidelines. A tactic that can make it easier to discuss return to work with patients is to rely on an independent and authoritative reference set of codified disability duration guidelines, which, typically, can be searched by diagnosis, procedure, or presenting symptoms. Such guidelines provide a condition-specific expected duration of work disability in the form of number of days, with shortest, typical, and maximum durations for different levels of job demands. If necessary, you can then adjust the guideline’s estimated duration to account for the patient’s age, underlying state of health, comorbidities, and so forth.
The use of disability duration guidelines at the point of care can facilitate the process of setting early and appropriate expectations for a patient’s recovery. If a patient is confrontational in response to your recommendation on the duration of work disability, guidelines can be used to address specific objections and facilitate understanding of functional recovery.
Consider the employer’s needs. To support return-to-work efforts, your guidance about work should consider the employer’s business needs. Employers require that the patient’s abilities, restrictions, and limitations be described in concrete terms because they must decide which specific tasks are unsafe and which ones they can reasonably expect the recovering worker to perform. However, employers often fail to send information to the physician about the patient’s job tasks—such that the clinician must rely on patient self-reporting, which might be inaccurate, incomplete, or biased.15 When a patient needs protection against foreseeable harm, highlight specific activities that are currently unsafe on the recovery timeline.
Employers rely on the physician to (1) estimate what the patient can do and (2) describe work ability in clear, objective terms that both patient and employer can interpret (TABLE). For example, “no heavy lifting” might be hard for an employer to interpret; “may lift 10 pounds from the floor to the waist as many as 12 times an hour” might be applied in a more practical manner to help a patient return to work safely.31 Including specific numbers, rates, and metrics in activity restrictions can also help demonstrate improvement over the course of treatment.
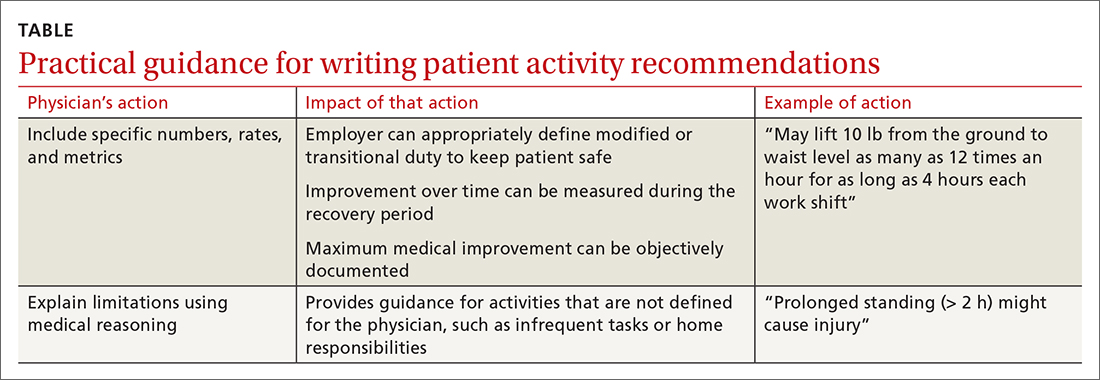
Be clear and specific on work restrictions. During recovery, it is important to tell the patient which temporary work restrictions are intended to prevent further injury or recurrence (prophylactic work restrictions) and which are an estimate of what they are able to do safely at work (capacity-based restrictions). Your written work restrictions form should be kept separate from private medical information because those restrictions will be the basis of subsequent conversations between patient and employer, who should be invited to give feedback if the guidance needs revision or clarification.
Continue to: Employer programs
Employer programs, such as modified duty, transitional duty, or early return to work programs, have been found to resolve claims faster and improve recovery outcomes.10,12 Such programs might also reduce occupational stress and improve productivity when an employee realizes that their functional abilities are matched to realistic job expectations during recovery.16 You can play an important role in empowering your patients to seek out these support programs.
What’s ahead for managing disability durations?
Work disability duration is influenced by the complex mix of biological, psychosocial, and economic variables that we have touched on here. All stakeholders involved in the recovery process should support the patient’s ability to live life with as few restrictions as possible; you play a key role in their recovery by focusing on ability, highlighting remaining capabilities, emphasizing activities that are safe to perform, and encouraging acceptance of, and adaptation to, any irrevocable losses.
This is a holistic approach that might help patients overcome the stress and anxiety associated with major life events arising from illness or injury that trigger disability benefits. Open communication and establishing a shared goal, among all involved, of the best possible outcome increases the likelihood that working patients will return to their familiar life or find another positive path forward.
Using EBM and disability duration guidelines can help decrease the length of life–work disruption by ensuring that patients are given a diagnosis, treated, and managed appropriately.32,33 Although these practices have been adopted by some physicians, health care systems, and insurers, they are not being implemented systematically and are unlikely to become ubiquitous unless they are mandated by payers or by law.
Family physicians are front-line providers for America’s workforce. They are distinctly situated to help patients achieve their best life at home and work. Improving the timeliness and quality of work guidance provided by the physician is an important way to minimize the impact of health problems on working people’s lives and livelihoods—and to help them stay employed.
CORRESPONDENCE
Kerri Wizner, MPH, 10355 Westmoor Drive, Westminster, CO 80021; [email protected].
1. Pransky G, Katz JN, Benjamin K, et al. Improving the physician role in evaluating work ability and managing disability: A survey of primary care practitioners. Disabil Rehabil. 2002;24:867-874. doi: 10.1080/09638280210142176
2. Hollenbeck K. Promoting Retention or Reemployment of Workers After a Significant Injury or Illness. Mathematica Policy Research; October 22, 2015. Accessed June 1, 2021. https://mathematica.org/publications/promoting-retention-or-reemployment-of-workers-after-a-significant-injury-or-illness
3. Poor health costs us employers $530 billion and 1.4 billion work days of absence and impaired performance according to Integrated Benefits Institute. Press release. November 15, 2018. Accessed June 1, 2021. www.ibiweb.org/poor-health-costs-us-employers-530-billion-and-1-4-billion-work-days-of-absence-and-impaired-performance
4. US Bureau of Labor Statistics. Life and disability insurance benefits: How extensive is the employer-provided safety net? BLS looks at life and disability benefits. Program Perspectives. 2010;2:7:1-4. Accessed June 8, 2021. www.bls.gov/opub/btn/archive/program-perspectives-on-life-and-disability-insurance-benefits.pdf
5. Kettlewell N, Morris RW, Ho N, et al. The differential impact of major life events on cognitive and affective wellbeing. SSM Popul Health. 2019;10:100533. doi: 10.1016/j.ssmph.2019.100533
6. Contreary K, Ben-Shalom Y, Gifford B. Using predictive analytics for early identification of short-term disability claimants who exhaust their benefits. J Occup Rehabil. 2018;28:584-596. doi: 10.1007/s10926-018-9815-5
7. Hultin H, Lindholm C, Möller J. Is there an association between long-term sick leave and disability pension and unemployment beyond the effect of health status? – A cohort study. PLoS One. 2012;7:e35614. doi: 10.1371/journal.pone.0035614
8. Canadian Medical Association. CMA policy: The treating physician’s role in helping patients return to work after an illness or injury (update 2013); 2013:1-6. Accessed June 1, 2021. https://policybase.cma.ca/documents/policypdf/PD13-05.pdf
9. Gifford B. Temporarily disabled workers account for a disproportionate share of health care payments. Health Aff (Millwood). 2017;36:245-249. doi:10.1377/hlthaff.2016.1013
10. Rueda S, Chambers L, Wilson M, et al. Association of returning to work with better health in working-aged adults: a systematic review. Am J Public Health. 2012;102:541-556. doi: 10.2105/AJPH.2011.300401
11. Modini M, Joyce S, Mykletun A, et al. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 2016;24:331-336. doi: 10.1177/1039856215618523
12. Shaw WS, Nelson CC, Woiszwillo MJ, et al. Early return to work has benefits for relief of back pain and functional recovery after controlling for multiple confounds. J Occup Environ Med. 2018;60:901-910. doi: 10.1097/JOM.0000000000001380
13. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59:e125-e131. doi: 10.1097/JOM.0000000000001055
14. World Health Organization. Towards a common language for functioning, disability and health. ICF: The International Classification of Functioning, Disability and Health. 2002. Accessed June 2, 2021. www.who.int/classifications/icf/icfbeginnersguide.pdf
15. Talmage JB, Melhorn JM, Hyman MH. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
16. Harrell M. Psychological factors and workforce health. In: Lee LP, Martin DW, Kancelbaum B. Occupational Medicine: A Basic Guide. American College of Occupational and Environmental Medicine; 2019. Accessed June 1, 2021. https://ohguides.acoem.org/07-psychological-factors-and-workforce-health-stress-management
17. Wickizer TM, Franklin GM, Fulton-Kehoe D. Innovations in occupational health care delivery can prevent entry into permanent disability: 8-year follow-up of the Washington State Centers for Occupational Health and Education. Med Care. 2018;56:1018-1023. doi: 10.1097/MLR.0000000000000991
18. Christian J, Wickizer T, Burton K. Implementing a community-focused health & work service. SSDI Solution Initiative, Fiscal Institute of the Committee for a Responsible Federal Budget. May 2019. Accessed June 2, 2021. www.crfb.org/sites/default/files/Implementing_a_Community-Focused_HWS.pdf
19. Macpherson RA, Koehoorn M, Fan J, et al. Do differences in work disability duration between men and women vary by province in Canada? J Occup Rehabil. 2018;29:560-568. doi: 10.1007/s10926-018-9819-1
20. Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine (Phila Pa 1976). 2005;30:2579-2584. doi: 10.1097/01.brs.0000186589.69382.1d
21. Jay K, Thorsen SV, Sundstrup E, et al. Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain. Pain Res Treat. 2018;2018:8347120. doi: 10.1155/2018/8347120
22. Burgard S, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57:10.1177/0002764213487347. doi: 10.1177/0002764213487347
23. Soklaridis S, Tang G, Cartmill C, et al. “Can you go back to work?” Family physicians’ experiences with assessing patients’ functional ability to return to work. Can Fam Physician. 2011;57:202-209.
24. Peters SE, Truong AP, Johnston V. Stakeholders identify similar barriers but different strategies to facilitate return-to-work: a vignette of a worker with an upper extremity condition. Work. 2018;59:401-412. doi: 10.3233/WOR-182692
25. Shraim M, Cifuentes M, Willetts JL, et al. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60:472-483. doi: 10.1002/ajim.22712
26. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91:712-721. doi: 10.2522/ptj.20100280
27. Aasdahl L, Pape K, Jensen C, et al. Associations between the readiness for return to work scale and return to work: a prospective study. J Occup Rehabil. 2018;28:97-106. doi: 10.1007/s10926-017-9705-2
28. Pino C, Covington M. Prescription of opioids for acute pain in opioid naïve patients. UpToDate Web site. February 9, 2021. Accessed June 2, 2021. www.uptodate.com/contents/prescription-of-opioids-for-acute-pain-in-opioid-naive-patients
29. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24:32. doi: 10.1186/s12998-016-0113-z
30. Lewis SJ, Orland BI. The importance and impact of evidence-based medicine. J Manag Care Pharm. 2004;10(5 suppl A):S3-S5. doi: 10.18553/jmcp.2004.10.S5-A.S3
31. Rupe KL. Work restrictions: documenting a patient’s return to work. Nurse Pract. 2010;35:49-53. doi: 10.1097/01.NPR.0000388901.49604.a8
32. Owens JD, Hegmann KT, Thiese MS, et al. Impacts of adherence to evidence-based medicine guidelines for the management of acute low back pain on costs of worker's compensation claims. J Occup Environ Med. 2019;61:445-452. doi: 10.1097/JOM.0000000000001593
33. Gaspar FW, Kownacki R, Zaidel CS, et al. Reducing disability durations and medical costs for patients with a carpal tunnel release surgery through the use of opioid prescribing guidelines. J Occup Environ Med. 2017;59:1180-1187. doi: 10.1097/JOM.0000000000001168
All clinicians who have patients who are employed play an essential role in work disability programs—whether or not those clinicians have received formal training in occupational health. A study found that primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.1
In this article, we explain why it is important for family physicians to better manage work disability at the point of care, to help patients return to their pre-injury or pre-illness level of activity.
Why managing the duration of work disability matters
Each year, millions of American workers leave their jobs—temporarily or permanently—because of illness, injury, or the effects of a chronic condition.2 It is estimated that 893 million workdays are lost annually due to a new medical problem; an additional 527 million workdays are lost due to the impact of chronic health conditions on the ability to perform at work.3 The great majority of these lost workdays are the result of personal health conditions, not work-related problems; patients must therefore cope with the accompanying disruption of life and work.
Significant injury and illness can create a life crisis, especially when there is uncertainty about future livelihood, such as an income shortfall during a lengthy recovery. Only 40% of the US workforce is covered by a short-term disability insurance program; only 10% of low-wage and low-skill workers have this type of coverage.4 Benefits rarely replace loss of income entirely, and worker compensation insurance programs provide only partial wage replacement.
In short, work disability is destabilizing and can threaten overall well-being.5
Furthermore, the longer a person remains on temporary disability, the more likely that person is to move to a publicly funded disability program or leave the workforce entirely—thus, potentially losing future earnings and self-identity related to being a working member of society.6-8
Most of the annual cost of poor health for US employers derives from medical and wage benefits ($226 billion) and impaired or reduced employee performance ($223 billion).3 In addition, temporarily disabled workers likely account for a disproportionate share of health care costs: A study found that one-half of medical and pharmacy payments were paid out to the one-quarter of employees requiring disability benefits.9
Continue to: Benefits of staying on the job
Benefits of staying on the job. Research shows that there are physical and mental health benefits to remaining at, or returning to, work after an injury or illness.10,11 For example, in a longitudinal cohort of people with low back pain, immediate or early return to work (in 1-7 days) was associated with reduced pain and improved functioning at 3 months.12 Physicians who can guide patients safely back to normal activities, including work, minimize the physical and mental health impact of the injury or illness and avoid chronicity.13
Emphasizing the importance of health, not disease or injury
Health researchers have found that diagnosis, cause, and extent of morbidity do not adequately explain observed variability in the impact of health conditions, utilization of resources, or need for services. A wider view of the functional implications of an injury or illness is therefore required for physicians to effectively recommend disability duration.
The World Health Organization recommends a shift toward a more holistic view of health, impairment, and disability, including an emphasis on functional ability, intrinsic capacity, and environmental context.14 The American Medical Association, American College of Occupational and Environmental Medicine, and Canadian Medical Association emphasize that prolonged absence from one’s normal role can be detrimental to mental, physical, and social well-being.8 These advisory groups recommend that physicians encourage patients who are unable to work to (1) focus on restoring the rhythm of their everyday life in a stepwise fashion and (2) resume their usual responsibilities as soon as possible.
Advising a patient to focus on “what you can do,” not “what you can’t do,” might make all the difference in their return to productivity. Keeping the patient’s—as well as your own—attention focused on the positive process of recovery and documenting evidence of functional progress is an important addition to (or substitute for) detailed inquiries about pain and dysfunction.
Why does duration of disability vary so much from case to case?
Disability duration is influenced by the individual patient, employer, physician, jurisdiction, insurer or benefits structure, and access to care.15 For you to effectively manage a patient who is out of work for a medical reason, it is important to understand how nonmedical variables often influence the pace of recovery and the timing of return to work (FIGURE).

Continue to: Deficient communication
Deficient communication. Often, employers, insurers, third-party administrators, and clinicians—each a key stakeholder in disability care—are disconnected from one another, resulting in poor communication with the injured worker. Such fragmented communication can delay treatment and recovery.16 Data systems are not designed to measure the duration of disability or provide proactive notification for key stakeholders who might intervene to facilitate a patient’s recovery.
Alternatively, a collaborative approach to disability management has been shown to improve outcomes.17,18 Communication among the various professionals involved can be coordinated and expedited by a case manager or disability manager hired by the medical practice, the employer, or the insurance company.
Psychosocial and economic influences can radically affect the time it takes to return to pre-injury or pre-illness functional status. Demographic variables (age, sex, income, education, and support system) influence how a person responds to a debilitating injury or illness.19 Fear of re-injury, anxiety over the intensity of pain upon movement, worry over dependency on others, and resiliency play an important role when a patient is attempting to return to full activity.20,21
Job satisfaction has been identified as the most significant variable associated with prompt return to work.15 Work has many health-enhancing aspects, including socioeconomic status, psychosocial support, and self-identity22; however, not everyone wants, or feels ready, to go back to work even once they are physically able. Workplace variables, such as the patient–employee’s dislike of the position, coworkers, or manager, have been cited by physicians as leading barriers to returning to work at an appropriate time.23,24
Other external variables. Physicians should formulate activity prescriptions and medical restrictions based on the impact the medical condition has on the usual ability to function, as well as the anticipated impact of specific activities on the body’s natural healing process. However, Rainville and colleagues found that external variables—patient requests, employer characteristics, and jurisdiction issues—considerably influence physicians’ recommendations.20 For example, benefit structure might influence how long a patient wants to remain out of work—thus altering the requests they make to their physician. Jurisdictional characteristics, such as health care systems, state workers’ compensation departments, and payer systems, all influence a patient’s recovery timeline and time away from work.25
Continue to: What does your patient need so that they can recover?
What does your patient need so that they can recover? Individual and systemic factors must be appropriately addressed to minimize the impact that recovery from a disability has on a person’s life. Successful functional recovery enables the person to self-manage symptoms, reduce disruption-associated stress, preserve mental health, and maintain healthy relationships at home and work. An example is the patient who has successfully coped with the entire predicament that their medical condition posed and resumed their usual daily routine and responsibilities at home and at work—albeit sometimes with temporary or permanent modification necessitated by their specific condition.
Strategies that help patients stay at, or return to, their job
Physicians who anticipate, monitor, and actively manage the duration of a work disability can improve patient outcomes by minimizing life disruption, avoiding unnecessary medical care, and shortening the period of absence from work.
Key strategy: Set expectations for functional recovery early in the episode, including a forecast of how long it will take to get life and work back to normal.26,27 This is similar to discussing expectations about pain before surgery, which has been shown to decrease subsequent requests for opioids.28 It is crucial to educate the patient about timelines, define functional outcomes, and encourage them to set goals for recovery.29
Devise an evidence-based treatment plan. A fundamental way to reduce disability duration is to (1) devise a treatment plan that is evidence based and (2) take the most effective route to recovery. Given the pace with which medical research changes the understanding of diseases and treatments, it is essential to rely on up-to-date, comprehensive, independent, and authoritative resources to support your care decisions.
Aligning clinical practice with evidence-based medicine (EBM) is a good way to accomplish that goal. By definition, EBM practice guidelines recommend the safest and most effective treatments after unbiased assessment of the best available research. Increasingly, EBM is adopted to improve clinical and functional outcomes, establish national standards of care, and set criteria to evaluate clinical performance.30
Continue to: Utilize established guidelines
Utilize established guidelines. A tactic that can make it easier to discuss return to work with patients is to rely on an independent and authoritative reference set of codified disability duration guidelines, which, typically, can be searched by diagnosis, procedure, or presenting symptoms. Such guidelines provide a condition-specific expected duration of work disability in the form of number of days, with shortest, typical, and maximum durations for different levels of job demands. If necessary, you can then adjust the guideline’s estimated duration to account for the patient’s age, underlying state of health, comorbidities, and so forth.
The use of disability duration guidelines at the point of care can facilitate the process of setting early and appropriate expectations for a patient’s recovery. If a patient is confrontational in response to your recommendation on the duration of work disability, guidelines can be used to address specific objections and facilitate understanding of functional recovery.
Consider the employer’s needs. To support return-to-work efforts, your guidance about work should consider the employer’s business needs. Employers require that the patient’s abilities, restrictions, and limitations be described in concrete terms because they must decide which specific tasks are unsafe and which ones they can reasonably expect the recovering worker to perform. However, employers often fail to send information to the physician about the patient’s job tasks—such that the clinician must rely on patient self-reporting, which might be inaccurate, incomplete, or biased.15 When a patient needs protection against foreseeable harm, highlight specific activities that are currently unsafe on the recovery timeline.
Employers rely on the physician to (1) estimate what the patient can do and (2) describe work ability in clear, objective terms that both patient and employer can interpret (TABLE). For example, “no heavy lifting” might be hard for an employer to interpret; “may lift 10 pounds from the floor to the waist as many as 12 times an hour” might be applied in a more practical manner to help a patient return to work safely.31 Including specific numbers, rates, and metrics in activity restrictions can also help demonstrate improvement over the course of treatment.

Be clear and specific on work restrictions. During recovery, it is important to tell the patient which temporary work restrictions are intended to prevent further injury or recurrence (prophylactic work restrictions) and which are an estimate of what they are able to do safely at work (capacity-based restrictions). Your written work restrictions form should be kept separate from private medical information because those restrictions will be the basis of subsequent conversations between patient and employer, who should be invited to give feedback if the guidance needs revision or clarification.
Continue to: Employer programs
Employer programs, such as modified duty, transitional duty, or early return to work programs, have been found to resolve claims faster and improve recovery outcomes.10,12 Such programs might also reduce occupational stress and improve productivity when an employee realizes that their functional abilities are matched to realistic job expectations during recovery.16 You can play an important role in empowering your patients to seek out these support programs.
What’s ahead for managing disability durations?
Work disability duration is influenced by the complex mix of biological, psychosocial, and economic variables that we have touched on here. All stakeholders involved in the recovery process should support the patient’s ability to live life with as few restrictions as possible; you play a key role in their recovery by focusing on ability, highlighting remaining capabilities, emphasizing activities that are safe to perform, and encouraging acceptance of, and adaptation to, any irrevocable losses.
This is a holistic approach that might help patients overcome the stress and anxiety associated with major life events arising from illness or injury that trigger disability benefits. Open communication and establishing a shared goal, among all involved, of the best possible outcome increases the likelihood that working patients will return to their familiar life or find another positive path forward.
Using EBM and disability duration guidelines can help decrease the length of life–work disruption by ensuring that patients are given a diagnosis, treated, and managed appropriately.32,33 Although these practices have been adopted by some physicians, health care systems, and insurers, they are not being implemented systematically and are unlikely to become ubiquitous unless they are mandated by payers or by law.
Family physicians are front-line providers for America’s workforce. They are distinctly situated to help patients achieve their best life at home and work. Improving the timeliness and quality of work guidance provided by the physician is an important way to minimize the impact of health problems on working people’s lives and livelihoods—and to help them stay employed.
CORRESPONDENCE
Kerri Wizner, MPH, 10355 Westmoor Drive, Westminster, CO 80021; [email protected].
All clinicians who have patients who are employed play an essential role in work disability programs—whether or not those clinicians have received formal training in occupational health. A study found that primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.1
In this article, we explain why it is important for family physicians to better manage work disability at the point of care, to help patients return to their pre-injury or pre-illness level of activity.
Why managing the duration of work disability matters
Each year, millions of American workers leave their jobs—temporarily or permanently—because of illness, injury, or the effects of a chronic condition.2 It is estimated that 893 million workdays are lost annually due to a new medical problem; an additional 527 million workdays are lost due to the impact of chronic health conditions on the ability to perform at work.3 The great majority of these lost workdays are the result of personal health conditions, not work-related problems; patients must therefore cope with the accompanying disruption of life and work.
Significant injury and illness can create a life crisis, especially when there is uncertainty about future livelihood, such as an income shortfall during a lengthy recovery. Only 40% of the US workforce is covered by a short-term disability insurance program; only 10% of low-wage and low-skill workers have this type of coverage.4 Benefits rarely replace loss of income entirely, and worker compensation insurance programs provide only partial wage replacement.
In short, work disability is destabilizing and can threaten overall well-being.5
Furthermore, the longer a person remains on temporary disability, the more likely that person is to move to a publicly funded disability program or leave the workforce entirely—thus, potentially losing future earnings and self-identity related to being a working member of society.6-8
Most of the annual cost of poor health for US employers derives from medical and wage benefits ($226 billion) and impaired or reduced employee performance ($223 billion).3 In addition, temporarily disabled workers likely account for a disproportionate share of health care costs: A study found that one-half of medical and pharmacy payments were paid out to the one-quarter of employees requiring disability benefits.9
Continue to: Benefits of staying on the job
Benefits of staying on the job. Research shows that there are physical and mental health benefits to remaining at, or returning to, work after an injury or illness.10,11 For example, in a longitudinal cohort of people with low back pain, immediate or early return to work (in 1-7 days) was associated with reduced pain and improved functioning at 3 months.12 Physicians who can guide patients safely back to normal activities, including work, minimize the physical and mental health impact of the injury or illness and avoid chronicity.13
Emphasizing the importance of health, not disease or injury
Health researchers have found that diagnosis, cause, and extent of morbidity do not adequately explain observed variability in the impact of health conditions, utilization of resources, or need for services. A wider view of the functional implications of an injury or illness is therefore required for physicians to effectively recommend disability duration.
The World Health Organization recommends a shift toward a more holistic view of health, impairment, and disability, including an emphasis on functional ability, intrinsic capacity, and environmental context.14 The American Medical Association, American College of Occupational and Environmental Medicine, and Canadian Medical Association emphasize that prolonged absence from one’s normal role can be detrimental to mental, physical, and social well-being.8 These advisory groups recommend that physicians encourage patients who are unable to work to (1) focus on restoring the rhythm of their everyday life in a stepwise fashion and (2) resume their usual responsibilities as soon as possible.
Advising a patient to focus on “what you can do,” not “what you can’t do,” might make all the difference in their return to productivity. Keeping the patient’s—as well as your own—attention focused on the positive process of recovery and documenting evidence of functional progress is an important addition to (or substitute for) detailed inquiries about pain and dysfunction.
Why does duration of disability vary so much from case to case?
Disability duration is influenced by the individual patient, employer, physician, jurisdiction, insurer or benefits structure, and access to care.15 For you to effectively manage a patient who is out of work for a medical reason, it is important to understand how nonmedical variables often influence the pace of recovery and the timing of return to work (FIGURE).

Continue to: Deficient communication
Deficient communication. Often, employers, insurers, third-party administrators, and clinicians—each a key stakeholder in disability care—are disconnected from one another, resulting in poor communication with the injured worker. Such fragmented communication can delay treatment and recovery.16 Data systems are not designed to measure the duration of disability or provide proactive notification for key stakeholders who might intervene to facilitate a patient’s recovery.
Alternatively, a collaborative approach to disability management has been shown to improve outcomes.17,18 Communication among the various professionals involved can be coordinated and expedited by a case manager or disability manager hired by the medical practice, the employer, or the insurance company.
Psychosocial and economic influences can radically affect the time it takes to return to pre-injury or pre-illness functional status. Demographic variables (age, sex, income, education, and support system) influence how a person responds to a debilitating injury or illness.19 Fear of re-injury, anxiety over the intensity of pain upon movement, worry over dependency on others, and resiliency play an important role when a patient is attempting to return to full activity.20,21
Job satisfaction has been identified as the most significant variable associated with prompt return to work.15 Work has many health-enhancing aspects, including socioeconomic status, psychosocial support, and self-identity22; however, not everyone wants, or feels ready, to go back to work even once they are physically able. Workplace variables, such as the patient–employee’s dislike of the position, coworkers, or manager, have been cited by physicians as leading barriers to returning to work at an appropriate time.23,24
Other external variables. Physicians should formulate activity prescriptions and medical restrictions based on the impact the medical condition has on the usual ability to function, as well as the anticipated impact of specific activities on the body’s natural healing process. However, Rainville and colleagues found that external variables—patient requests, employer characteristics, and jurisdiction issues—considerably influence physicians’ recommendations.20 For example, benefit structure might influence how long a patient wants to remain out of work—thus altering the requests they make to their physician. Jurisdictional characteristics, such as health care systems, state workers’ compensation departments, and payer systems, all influence a patient’s recovery timeline and time away from work.25
Continue to: What does your patient need so that they can recover?
What does your patient need so that they can recover? Individual and systemic factors must be appropriately addressed to minimize the impact that recovery from a disability has on a person’s life. Successful functional recovery enables the person to self-manage symptoms, reduce disruption-associated stress, preserve mental health, and maintain healthy relationships at home and work. An example is the patient who has successfully coped with the entire predicament that their medical condition posed and resumed their usual daily routine and responsibilities at home and at work—albeit sometimes with temporary or permanent modification necessitated by their specific condition.
Strategies that help patients stay at, or return to, their job
Physicians who anticipate, monitor, and actively manage the duration of a work disability can improve patient outcomes by minimizing life disruption, avoiding unnecessary medical care, and shortening the period of absence from work.
Key strategy: Set expectations for functional recovery early in the episode, including a forecast of how long it will take to get life and work back to normal.26,27 This is similar to discussing expectations about pain before surgery, which has been shown to decrease subsequent requests for opioids.28 It is crucial to educate the patient about timelines, define functional outcomes, and encourage them to set goals for recovery.29
Devise an evidence-based treatment plan. A fundamental way to reduce disability duration is to (1) devise a treatment plan that is evidence based and (2) take the most effective route to recovery. Given the pace with which medical research changes the understanding of diseases and treatments, it is essential to rely on up-to-date, comprehensive, independent, and authoritative resources to support your care decisions.
Aligning clinical practice with evidence-based medicine (EBM) is a good way to accomplish that goal. By definition, EBM practice guidelines recommend the safest and most effective treatments after unbiased assessment of the best available research. Increasingly, EBM is adopted to improve clinical and functional outcomes, establish national standards of care, and set criteria to evaluate clinical performance.30
Continue to: Utilize established guidelines
Utilize established guidelines. A tactic that can make it easier to discuss return to work with patients is to rely on an independent and authoritative reference set of codified disability duration guidelines, which, typically, can be searched by diagnosis, procedure, or presenting symptoms. Such guidelines provide a condition-specific expected duration of work disability in the form of number of days, with shortest, typical, and maximum durations for different levels of job demands. If necessary, you can then adjust the guideline’s estimated duration to account for the patient’s age, underlying state of health, comorbidities, and so forth.
The use of disability duration guidelines at the point of care can facilitate the process of setting early and appropriate expectations for a patient’s recovery. If a patient is confrontational in response to your recommendation on the duration of work disability, guidelines can be used to address specific objections and facilitate understanding of functional recovery.
Consider the employer’s needs. To support return-to-work efforts, your guidance about work should consider the employer’s business needs. Employers require that the patient’s abilities, restrictions, and limitations be described in concrete terms because they must decide which specific tasks are unsafe and which ones they can reasonably expect the recovering worker to perform. However, employers often fail to send information to the physician about the patient’s job tasks—such that the clinician must rely on patient self-reporting, which might be inaccurate, incomplete, or biased.15 When a patient needs protection against foreseeable harm, highlight specific activities that are currently unsafe on the recovery timeline.
Employers rely on the physician to (1) estimate what the patient can do and (2) describe work ability in clear, objective terms that both patient and employer can interpret (TABLE). For example, “no heavy lifting” might be hard for an employer to interpret; “may lift 10 pounds from the floor to the waist as many as 12 times an hour” might be applied in a more practical manner to help a patient return to work safely.31 Including specific numbers, rates, and metrics in activity restrictions can also help demonstrate improvement over the course of treatment.

Be clear and specific on work restrictions. During recovery, it is important to tell the patient which temporary work restrictions are intended to prevent further injury or recurrence (prophylactic work restrictions) and which are an estimate of what they are able to do safely at work (capacity-based restrictions). Your written work restrictions form should be kept separate from private medical information because those restrictions will be the basis of subsequent conversations between patient and employer, who should be invited to give feedback if the guidance needs revision or clarification.
Continue to: Employer programs
Employer programs, such as modified duty, transitional duty, or early return to work programs, have been found to resolve claims faster and improve recovery outcomes.10,12 Such programs might also reduce occupational stress and improve productivity when an employee realizes that their functional abilities are matched to realistic job expectations during recovery.16 You can play an important role in empowering your patients to seek out these support programs.
What’s ahead for managing disability durations?
Work disability duration is influenced by the complex mix of biological, psychosocial, and economic variables that we have touched on here. All stakeholders involved in the recovery process should support the patient’s ability to live life with as few restrictions as possible; you play a key role in their recovery by focusing on ability, highlighting remaining capabilities, emphasizing activities that are safe to perform, and encouraging acceptance of, and adaptation to, any irrevocable losses.
This is a holistic approach that might help patients overcome the stress and anxiety associated with major life events arising from illness or injury that trigger disability benefits. Open communication and establishing a shared goal, among all involved, of the best possible outcome increases the likelihood that working patients will return to their familiar life or find another positive path forward.
Using EBM and disability duration guidelines can help decrease the length of life–work disruption by ensuring that patients are given a diagnosis, treated, and managed appropriately.32,33 Although these practices have been adopted by some physicians, health care systems, and insurers, they are not being implemented systematically and are unlikely to become ubiquitous unless they are mandated by payers or by law.
Family physicians are front-line providers for America’s workforce. They are distinctly situated to help patients achieve their best life at home and work. Improving the timeliness and quality of work guidance provided by the physician is an important way to minimize the impact of health problems on working people’s lives and livelihoods—and to help them stay employed.
CORRESPONDENCE
Kerri Wizner, MPH, 10355 Westmoor Drive, Westminster, CO 80021; [email protected].
1. Pransky G, Katz JN, Benjamin K, et al. Improving the physician role in evaluating work ability and managing disability: A survey of primary care practitioners. Disabil Rehabil. 2002;24:867-874. doi: 10.1080/09638280210142176
2. Hollenbeck K. Promoting Retention or Reemployment of Workers After a Significant Injury or Illness. Mathematica Policy Research; October 22, 2015. Accessed June 1, 2021. https://mathematica.org/publications/promoting-retention-or-reemployment-of-workers-after-a-significant-injury-or-illness
3. Poor health costs us employers $530 billion and 1.4 billion work days of absence and impaired performance according to Integrated Benefits Institute. Press release. November 15, 2018. Accessed June 1, 2021. www.ibiweb.org/poor-health-costs-us-employers-530-billion-and-1-4-billion-work-days-of-absence-and-impaired-performance
4. US Bureau of Labor Statistics. Life and disability insurance benefits: How extensive is the employer-provided safety net? BLS looks at life and disability benefits. Program Perspectives. 2010;2:7:1-4. Accessed June 8, 2021. www.bls.gov/opub/btn/archive/program-perspectives-on-life-and-disability-insurance-benefits.pdf
5. Kettlewell N, Morris RW, Ho N, et al. The differential impact of major life events on cognitive and affective wellbeing. SSM Popul Health. 2019;10:100533. doi: 10.1016/j.ssmph.2019.100533
6. Contreary K, Ben-Shalom Y, Gifford B. Using predictive analytics for early identification of short-term disability claimants who exhaust their benefits. J Occup Rehabil. 2018;28:584-596. doi: 10.1007/s10926-018-9815-5
7. Hultin H, Lindholm C, Möller J. Is there an association between long-term sick leave and disability pension and unemployment beyond the effect of health status? – A cohort study. PLoS One. 2012;7:e35614. doi: 10.1371/journal.pone.0035614
8. Canadian Medical Association. CMA policy: The treating physician’s role in helping patients return to work after an illness or injury (update 2013); 2013:1-6. Accessed June 1, 2021. https://policybase.cma.ca/documents/policypdf/PD13-05.pdf
9. Gifford B. Temporarily disabled workers account for a disproportionate share of health care payments. Health Aff (Millwood). 2017;36:245-249. doi:10.1377/hlthaff.2016.1013
10. Rueda S, Chambers L, Wilson M, et al. Association of returning to work with better health in working-aged adults: a systematic review. Am J Public Health. 2012;102:541-556. doi: 10.2105/AJPH.2011.300401
11. Modini M, Joyce S, Mykletun A, et al. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 2016;24:331-336. doi: 10.1177/1039856215618523
12. Shaw WS, Nelson CC, Woiszwillo MJ, et al. Early return to work has benefits for relief of back pain and functional recovery after controlling for multiple confounds. J Occup Environ Med. 2018;60:901-910. doi: 10.1097/JOM.0000000000001380
13. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59:e125-e131. doi: 10.1097/JOM.0000000000001055
14. World Health Organization. Towards a common language for functioning, disability and health. ICF: The International Classification of Functioning, Disability and Health. 2002. Accessed June 2, 2021. www.who.int/classifications/icf/icfbeginnersguide.pdf
15. Talmage JB, Melhorn JM, Hyman MH. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
16. Harrell M. Psychological factors and workforce health. In: Lee LP, Martin DW, Kancelbaum B. Occupational Medicine: A Basic Guide. American College of Occupational and Environmental Medicine; 2019. Accessed June 1, 2021. https://ohguides.acoem.org/07-psychological-factors-and-workforce-health-stress-management
17. Wickizer TM, Franklin GM, Fulton-Kehoe D. Innovations in occupational health care delivery can prevent entry into permanent disability: 8-year follow-up of the Washington State Centers for Occupational Health and Education. Med Care. 2018;56:1018-1023. doi: 10.1097/MLR.0000000000000991
18. Christian J, Wickizer T, Burton K. Implementing a community-focused health & work service. SSDI Solution Initiative, Fiscal Institute of the Committee for a Responsible Federal Budget. May 2019. Accessed June 2, 2021. www.crfb.org/sites/default/files/Implementing_a_Community-Focused_HWS.pdf
19. Macpherson RA, Koehoorn M, Fan J, et al. Do differences in work disability duration between men and women vary by province in Canada? J Occup Rehabil. 2018;29:560-568. doi: 10.1007/s10926-018-9819-1
20. Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine (Phila Pa 1976). 2005;30:2579-2584. doi: 10.1097/01.brs.0000186589.69382.1d
21. Jay K, Thorsen SV, Sundstrup E, et al. Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain. Pain Res Treat. 2018;2018:8347120. doi: 10.1155/2018/8347120
22. Burgard S, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57:10.1177/0002764213487347. doi: 10.1177/0002764213487347
23. Soklaridis S, Tang G, Cartmill C, et al. “Can you go back to work?” Family physicians’ experiences with assessing patients’ functional ability to return to work. Can Fam Physician. 2011;57:202-209.
24. Peters SE, Truong AP, Johnston V. Stakeholders identify similar barriers but different strategies to facilitate return-to-work: a vignette of a worker with an upper extremity condition. Work. 2018;59:401-412. doi: 10.3233/WOR-182692
25. Shraim M, Cifuentes M, Willetts JL, et al. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60:472-483. doi: 10.1002/ajim.22712
26. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91:712-721. doi: 10.2522/ptj.20100280
27. Aasdahl L, Pape K, Jensen C, et al. Associations between the readiness for return to work scale and return to work: a prospective study. J Occup Rehabil. 2018;28:97-106. doi: 10.1007/s10926-017-9705-2
28. Pino C, Covington M. Prescription of opioids for acute pain in opioid naïve patients. UpToDate Web site. February 9, 2021. Accessed June 2, 2021. www.uptodate.com/contents/prescription-of-opioids-for-acute-pain-in-opioid-naive-patients
29. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24:32. doi: 10.1186/s12998-016-0113-z
30. Lewis SJ, Orland BI. The importance and impact of evidence-based medicine. J Manag Care Pharm. 2004;10(5 suppl A):S3-S5. doi: 10.18553/jmcp.2004.10.S5-A.S3
31. Rupe KL. Work restrictions: documenting a patient’s return to work. Nurse Pract. 2010;35:49-53. doi: 10.1097/01.NPR.0000388901.49604.a8
32. Owens JD, Hegmann KT, Thiese MS, et al. Impacts of adherence to evidence-based medicine guidelines for the management of acute low back pain on costs of worker's compensation claims. J Occup Environ Med. 2019;61:445-452. doi: 10.1097/JOM.0000000000001593
33. Gaspar FW, Kownacki R, Zaidel CS, et al. Reducing disability durations and medical costs for patients with a carpal tunnel release surgery through the use of opioid prescribing guidelines. J Occup Environ Med. 2017;59:1180-1187. doi: 10.1097/JOM.0000000000001168
1. Pransky G, Katz JN, Benjamin K, et al. Improving the physician role in evaluating work ability and managing disability: A survey of primary care practitioners. Disabil Rehabil. 2002;24:867-874. doi: 10.1080/09638280210142176
2. Hollenbeck K. Promoting Retention or Reemployment of Workers After a Significant Injury or Illness. Mathematica Policy Research; October 22, 2015. Accessed June 1, 2021. https://mathematica.org/publications/promoting-retention-or-reemployment-of-workers-after-a-significant-injury-or-illness
3. Poor health costs us employers $530 billion and 1.4 billion work days of absence and impaired performance according to Integrated Benefits Institute. Press release. November 15, 2018. Accessed June 1, 2021. www.ibiweb.org/poor-health-costs-us-employers-530-billion-and-1-4-billion-work-days-of-absence-and-impaired-performance
4. US Bureau of Labor Statistics. Life and disability insurance benefits: How extensive is the employer-provided safety net? BLS looks at life and disability benefits. Program Perspectives. 2010;2:7:1-4. Accessed June 8, 2021. www.bls.gov/opub/btn/archive/program-perspectives-on-life-and-disability-insurance-benefits.pdf
5. Kettlewell N, Morris RW, Ho N, et al. The differential impact of major life events on cognitive and affective wellbeing. SSM Popul Health. 2019;10:100533. doi: 10.1016/j.ssmph.2019.100533
6. Contreary K, Ben-Shalom Y, Gifford B. Using predictive analytics for early identification of short-term disability claimants who exhaust their benefits. J Occup Rehabil. 2018;28:584-596. doi: 10.1007/s10926-018-9815-5
7. Hultin H, Lindholm C, Möller J. Is there an association between long-term sick leave and disability pension and unemployment beyond the effect of health status? – A cohort study. PLoS One. 2012;7:e35614. doi: 10.1371/journal.pone.0035614
8. Canadian Medical Association. CMA policy: The treating physician’s role in helping patients return to work after an illness or injury (update 2013); 2013:1-6. Accessed June 1, 2021. https://policybase.cma.ca/documents/policypdf/PD13-05.pdf
9. Gifford B. Temporarily disabled workers account for a disproportionate share of health care payments. Health Aff (Millwood). 2017;36:245-249. doi:10.1377/hlthaff.2016.1013
10. Rueda S, Chambers L, Wilson M, et al. Association of returning to work with better health in working-aged adults: a systematic review. Am J Public Health. 2012;102:541-556. doi: 10.2105/AJPH.2011.300401
11. Modini M, Joyce S, Mykletun A, et al. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 2016;24:331-336. doi: 10.1177/1039856215618523
12. Shaw WS, Nelson CC, Woiszwillo MJ, et al. Early return to work has benefits for relief of back pain and functional recovery after controlling for multiple confounds. J Occup Environ Med. 2018;60:901-910. doi: 10.1097/JOM.0000000000001380
13. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59:e125-e131. doi: 10.1097/JOM.0000000000001055
14. World Health Organization. Towards a common language for functioning, disability and health. ICF: The International Classification of Functioning, Disability and Health. 2002. Accessed June 2, 2021. www.who.int/classifications/icf/icfbeginnersguide.pdf
15. Talmage JB, Melhorn JM, Hyman MH. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
16. Harrell M. Psychological factors and workforce health. In: Lee LP, Martin DW, Kancelbaum B. Occupational Medicine: A Basic Guide. American College of Occupational and Environmental Medicine; 2019. Accessed June 1, 2021. https://ohguides.acoem.org/07-psychological-factors-and-workforce-health-stress-management
17. Wickizer TM, Franklin GM, Fulton-Kehoe D. Innovations in occupational health care delivery can prevent entry into permanent disability: 8-year follow-up of the Washington State Centers for Occupational Health and Education. Med Care. 2018;56:1018-1023. doi: 10.1097/MLR.0000000000000991
18. Christian J, Wickizer T, Burton K. Implementing a community-focused health & work service. SSDI Solution Initiative, Fiscal Institute of the Committee for a Responsible Federal Budget. May 2019. Accessed June 2, 2021. www.crfb.org/sites/default/files/Implementing_a_Community-Focused_HWS.pdf
19. Macpherson RA, Koehoorn M, Fan J, et al. Do differences in work disability duration between men and women vary by province in Canada? J Occup Rehabil. 2018;29:560-568. doi: 10.1007/s10926-018-9819-1
20. Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine (Phila Pa 1976). 2005;30:2579-2584. doi: 10.1097/01.brs.0000186589.69382.1d
21. Jay K, Thorsen SV, Sundstrup E, et al. Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain. Pain Res Treat. 2018;2018:8347120. doi: 10.1155/2018/8347120
22. Burgard S, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57:10.1177/0002764213487347. doi: 10.1177/0002764213487347
23. Soklaridis S, Tang G, Cartmill C, et al. “Can you go back to work?” Family physicians’ experiences with assessing patients’ functional ability to return to work. Can Fam Physician. 2011;57:202-209.
24. Peters SE, Truong AP, Johnston V. Stakeholders identify similar barriers but different strategies to facilitate return-to-work: a vignette of a worker with an upper extremity condition. Work. 2018;59:401-412. doi: 10.3233/WOR-182692
25. Shraim M, Cifuentes M, Willetts JL, et al. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60:472-483. doi: 10.1002/ajim.22712
26. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91:712-721. doi: 10.2522/ptj.20100280
27. Aasdahl L, Pape K, Jensen C, et al. Associations between the readiness for return to work scale and return to work: a prospective study. J Occup Rehabil. 2018;28:97-106. doi: 10.1007/s10926-017-9705-2
28. Pino C, Covington M. Prescription of opioids for acute pain in opioid naïve patients. UpToDate Web site. February 9, 2021. Accessed June 2, 2021. www.uptodate.com/contents/prescription-of-opioids-for-acute-pain-in-opioid-naive-patients
29. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24:32. doi: 10.1186/s12998-016-0113-z
30. Lewis SJ, Orland BI. The importance and impact of evidence-based medicine. J Manag Care Pharm. 2004;10(5 suppl A):S3-S5. doi: 10.18553/jmcp.2004.10.S5-A.S3
31. Rupe KL. Work restrictions: documenting a patient’s return to work. Nurse Pract. 2010;35:49-53. doi: 10.1097/01.NPR.0000388901.49604.a8
32. Owens JD, Hegmann KT, Thiese MS, et al. Impacts of adherence to evidence-based medicine guidelines for the management of acute low back pain on costs of worker's compensation claims. J Occup Environ Med. 2019;61:445-452. doi: 10.1097/JOM.0000000000001593
33. Gaspar FW, Kownacki R, Zaidel CS, et al. Reducing disability durations and medical costs for patients with a carpal tunnel release surgery through the use of opioid prescribing guidelines. J Occup Environ Med. 2017;59:1180-1187. doi: 10.1097/JOM.0000000000001168
PRACTICE RECOMMENDATIONS
› Set appropriate expectations for the patient at the start of any episode of work disability: Estimate the course of functional recovery over time and the total duration of life–work disruption. A
› Include detailed activity prescriptions in the treatment plan, with stepwise progression over time toward full recovery. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Melanoma: An FP’s guide to diagnosis and management
CASE
A 48-year-old man comes to your clinic with a dark nevus on his right upper arm that appeared 2 months earlier. He says that the lesion has continued to grow and has bled (he thought because he initially picked at it). On exam, there is a 7-mm brown papule with 2 black dots and slightly asymmetric borders.
How would you proceed with this patient?
Melanoma is the fifth leading cause of new cancer cases annually, with > 96,000 new cases in 2019.1 Overall, melanoma is more common in men and in Whites, with 48% diagnosed in people ages 55 to 74.1 The past 2 decades have seen numerous developments in the diagnosis, treatment, and surveillance of melanoma. This article covers recommendations, controversies, and issues that require future study. It does not cover uveal or mucosal melanoma.
Evaluating a patient with a new or changing nevus
Known risk factors for melanoma include a changing nevus, indoor tanning, older age, many melanocytic nevi, history of a dysplastic nevus or of blistering sunburns during teen years, red or blonde hair, large congenital nevus, Fitzpatrick skin type I or II, high socioeconomic status, personal or family history of melanoma, and intermittent high-intensity sun exposure.2-3 Presence of 1 or more of these risk factors should lower the threshold for biopsy.
Worrisome physical exam features (FIGURE) are nevus asymmetry, irregular borders, variegated color, and a diameter > 6 mm (the size of a pencil eraser). Inquire as to whether the nevus’ appearance has evolved and if it has bled without trauma. In a patient with multiple nevi, 1 nevus that looks different than the rest (the so-called “ugly duckling”) is concerning. Accuracy of diagnosis is enhanced with dermoscopy. A Cochrane review showed that skilled use of dermoscopy, in addition to inspection with the naked eye, considerably increases the sensitivity and specificity of diagnosing melanoma.4 Yet a 2017 study of 705 US primary care practitioners showed that only 8.3% of them used dermoscopy to evaluate pigmented lesions.5
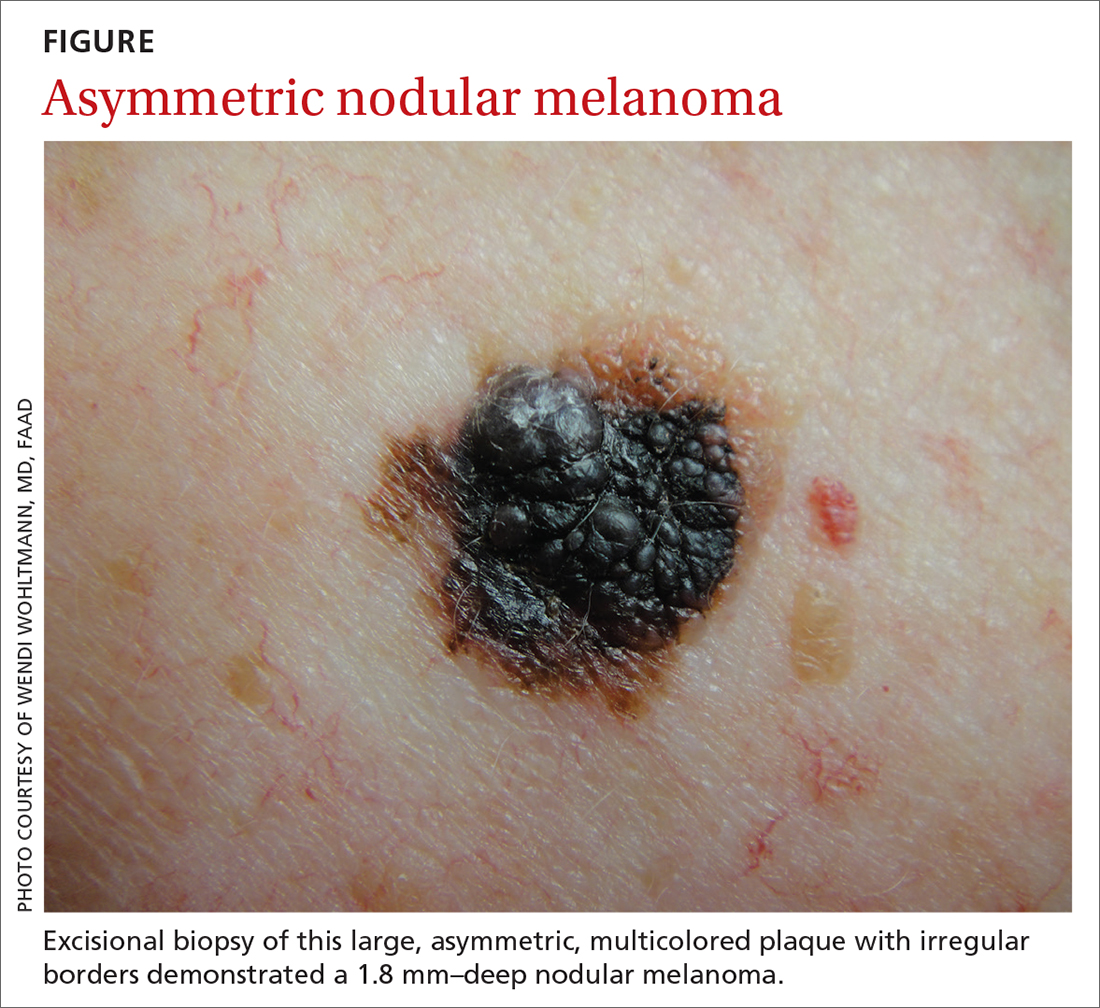
Several published algorithms and checklists can aid clinicians in identifying lesions suggestive of melanoma—eg, ABCDE, CASH, Menzies method, “chaos and clues,” and 2-step and 3- and 7-point checklists.6-10 A simple 3-step algorithm, the TADA (triage amalgamated dermoscopic algorithm) method is available to novice dermoscopy users.11 Experts in pigmented lesions prefer to use pattern analysis, which requires simultaneously assessing multiple lesion patterns that vary according to body site.12,13
Dermoscopic features suggesting melanoma are atypical pigment networks, pseudopods, radial streaking, irregular dots or globules, blue-whitish veil, and granularity or peppering.14 Appropriate and effective use of dermoscopy requires training.15,16 Available methods for learning dermoscopy include online and in-person courses, mentoring by experienced dermoscopists, books and articles, and free apps and online resources.17
Continue to: Perform a skin biopsy, but do this first
Perform a skin biopsy, but do this first
Skin biopsy is the definitive way to diagnose melanoma. Prior to biopsy, take photographs to document the exact location of the lesion and to ensure that the correct area is removed in wide excision (WE). A complete biopsy should include the full depth and breadth of the lesion to ensure there are clinically negative margins. This can be achieved with an elliptical excision (for larger lesions), punch excision (for small lesions), or saucerization (deep shave with 1- to 2-mm peripheral margins, used for intermediate-size lesions).18 Saucerization is distinctly different from a superficial shave biopsy, which is not recommended for lesions with features of melanoma.19
A decision to perform a biopsy on a part of the lesion (partial biopsy) depends on the size of the lesion and its anatomic location, and is best made in agreement with the patient.
If you are untrained or uncomfortable performing the biopsy, contact a dermatologist immediately. In many communities, such referrals are subject to long delays, which further supports the advisability of family physicians doing their own biopsies after photographing the suspicious lesion. Many resources are available to help family physicians learn to do biopsies proficiently (www.mdedge.com/familymedicine/article/164358/oncology/biopsies-skin-cancer-detection-dispelling-myths).19
What to communicate to the pathologist. At a minimum, the biopsy request form should include patient age, sex, biopsy type (punch, excisional, or scoop shave), intention (complete or partial sample), exact site of the biopsy with laterality, and clinical details. These details should include the lesion size and clinical description, the suspected diagnosis, and clinical information, such as whether there is a history of bleeding or changing color, size, or symmetry. In standard biopsy specimens, the pathologist is only examining a portion of the lesion. Communicating clearly to the pathologist may lead to a request for deeper or additional sections or special stains.
If the biopsy results do not match the clinical impression, a phone call to the pathologist is warranted. In addition, evaluation by a dermatopathologist may be merited as pathologic diagnosis of melanoma can be quite challenging. Newer molecular tests, such as fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH), can assist in the histologic evaluation of complex pigmented lesions.
Continue to: CASE
CASE
You perform an elliptical excisional biopsy on your patient. The biopsy report comes back as a nodular malignant melanoma, Breslow depth 2.5 mm without ulceration, and no evidence of lymphovascular invasion or microsatellitosis. The report states that the biopsy margins appear clear of tumor involvement.
Further evaluation when the biopsy result is positive
Key steps in initial patient care include relaying pathology results to the patient, conducting (as needed) a more extensive evaluation, and obtaining appropriate consultation.
Clearly explain the diagnosis and convey an accurate reading of the pathology report. The vital pieces of information in the biopsy report are the Breslow depth and presence of ulceration, as evidence shows these 2 factors to be important independent predictors of outcome.22,23 Also important are the presence of microsatellitosis (essential for staging purposes), pathologic stage, and the status of the peripheral and deep biopsy margins. Review Breslow depth with the patient as this largely dictates treatment options and prognosis.
Evaluate for possible metastatic disease. Obtain a complete history from every patient with cutaneous melanoma, looking for any positive review of systems as a harbinger of metastatic disease. A full-body skin and lymph node exam is vital, given that melanoma can arise anywhere including on the scalp, in the gluteal cleft, and beneath nails. If the lymph node exam is worrisome, conduct an ultrasound exam, even while referring to specialty care. Treating a patient with melanoma requires a multidisciplinary approach that may include dermatologists, surgeons, and oncologists based on the stage of disease. A challenge for family physicians is knowing which consultation to prioritize and how to counsel the patient to schedule these for the most cost-effective and timely evaluation.
Expedite a dermatology consultation. If the melanoma is deep or appears advanced based on size or palpable lymph nodes, contact the dermatologist immediately by phone to set up a rapid referral. Delays in the definitive management of thick melanomas can negatively affect outcome. Paper, facsimile, or electronic referrals can get lost in the system and are not reliable methods for referring patients for a melanoma consultation. One benefit of the family physician performing the initial biopsy is that a confirmed melanoma diagnosis will almost certainly get an expedited dermatology appointment.
Continue to: Wide excision and sentinel node biopsy
Wide excision and sentinel node biopsy
Wide excision of a primary melanoma is standard practice, with evidence favoring the following surgical margins: 0.5 to 1 cm for melanoma in situ, 1 cm for tumors up to 1 mm in thickness, 1 to 2 cm for tumors > 1 to 2 mm thick, and 2 cm for tumors > 2 mm thick.18 WE is often performed by dermatologists for nonulcerated tumors < 0.8 mm thick (T1a) without adverse features. If trained in cutaneous surgery, you can also choose to excise these thin melanomas in your office. Otherwise refer all patients with biopsy-proven melanoma to dermatologists to perform an adequate WE.
Refer patients who have tumors ≥ 0.8 mm thick to the appropriate surgical specialty (surgical oncology, if available) for consultation on sentinel lymph node biopsy. SLNB, when indicated, should be performed prior to WE of the primary tumor, and whenever possible in the same surgical setting, to maximize lymphatic drainage mapping techniques.18 Medical oncology referral, if needed, is usually made after WE.
SLNB remains the standard for lymph node staging. It is controversial mainly in its use for very thin or very thick lesions. Randomized controlled trials, including the Multicenter Selective Lymphadenectomy Trial,24 have shown no difference in melanoma-specific survival for patients with intermediate-thickness melanomas who had undergone SLNB.24
Many professional organizations consider SLNB to be the most significant prognostic indicator of disease recurrence. With a negative SLNB result, the risk of regional node recurrence is 5% or lower.18,25 In addition, sentinel lymph node status is a critical determinant for systemic adjuvant therapy consideration and clinical trial eligibility. For patients who have primary cutaneous melanoma without clinical lymphadenopathy, an online tool is available for patients to use with their physician in predicting the likelihood of SLNB positivity.26
Recommendations for SLNB, supported by multidisciplinary consensus:18
- Do not pursue SLNB for melanoma in situ or most cutaneous melanomas < 0.8 mm without ulceration (T1a). (See TABLE 127)
- Discuss SLNB with patients who have T1a melanoma and additional adverse features: young age, high mitotic rate, lymphovascular invasion, and nevus depth close to 0.8 mm with positive deep biopsy margins.
- Discuss SLNB with patients who have T1b disease (< 0.8 mm with ulceration, or 0.8-1 mm), although rates of SLNB positivity are low.
- Offer SLNB to patients with T2a and higher disease (> 1 mm).18
Continue to: Patients who have...
Patients who have clinical Stage I or II disease (TABLE 127)
Melanoma in women: Considerations to keep in mind
Hormonal influences of pregnancy, lactation, contraception, and menopause introduce special considerations regarding melanoma, which is the most common cancer occurring during pregnancy, accounting for 31% of new malignancies.33 Risk of melanoma lessens, however, for women who first give birth at a younger age or who have had > 5 live births.18,34,35 There is no evidence that nevi darken during pregnancy, although nevi on the breast and abdomen may seem to enlarge due to skin stretching.18 All changing nevi in pregnancy warrant an examination, preferably with dermoscopy, and patients should be offered biopsy if there are any nevus characteristics associated with melanoma.18
The effect of pregnancy on an existing melanoma is not fully understood, but evidence from controlled studies shows no negative effect. Recent working group guidelines advise WE with local anesthesia without delay in pregnant patients.18 Definitive treatment after melanoma diagnosis should take a multidisciplinary approach involving obstetric care coordinated with Dermatology, Surgery, and Medical Oncology.18
Most recommendations on the timing of pregnancy following a melanoma diagnosis have limited evidence. One meta-analysis concluded that pregnancy occurring after successful treatment of melanoma did not change a woman’s prognosis.36 Current guidelines do not recommend delaying future pregnancy if a woman had an early-stage melanoma. For melanomas deemed higher risk, a woman could consider a 2- to 3-year delay in the next planned pregnancy, owing to current data on recurrence rates.18
A systematic review of women who used hormonal contraception or postmenopausal hormone replacement therapy (HRT) showed no associated increased risk of melanoma.35 An additional randomized trial showed no effect of HRT on melanoma risk.37
Continue to: Systemic melanoma treatment and common adverse effects
Systemic melanoma treatment and common adverse effects
Multiple systemic therapies have been approved for the treatment of advanced or unresectable cutaneous melanomas. While these treatments are managed primarily by Oncology in concert with Dermatology, an awareness of the medications’ common dermatologic toxicities is important for the primary care provider. The 2 broad categories of FDA-approved systemic medications for advanced melanoma are mitogen-activated protein kinase (MAPK) inhibitors and immune checkpoint inhibitors, each having its own set of adverse cutaneous effects.
MAPK pathway–targeting drugs include the B-Raf proto-oncogene serine/threonine-kinase inhibitors (BRAFIs) vemurafenib and dabrafenib, and the MAPK inhibitors (MEKIs) trametinib and cobimetinib. The most common adverse skin effects in MAPK pathway–targeting drugs are severe ultraviolet photosensitivity, cutaneous epidermal neoplasms (particularly squamous cell carcinoma, keratoacanthoma-type), thick actinic keratosis, wart-like keratosis, painful palmoplantar keratosis, and dry skin.38 These effects are most commonly seen with BRAFI monotherapy and can be abated with the addition of a MEKI. MEKI therapy can cause acneiform eruptions and paronychia.39 Additional adverse effects include diarrhea, pyrexia, arthralgias, and fatigue for BRAFIs and diarrhea, fatigue, and peripheral edema for MEKIs.40
Immune checkpoint inhibitors include anti-CTLA-4 (ipilimumab), anti-PD-1 (pembrolizumab and nivolumab), and anti-PDL-1 (atezolizumab). Adverse skin effects include morbilliform rash with or without an associated itch, itch with or without an associated rash, vitiligo, and lichenoid skin rashes. PD-1 and PDL-1 inhibitors have been associated with flares or unmasking of atopic dermatitis, psoriasis, sarcoidosis, and autoimmune bullous disease.18 Diarrhea, colitis, hepatitis, elevated liver enzymes, hypophysitis, and thyroiditis are some of the more common noncutaneous adverse effects reported with CTLA-4 inhibitors, while fatigue, diarrhea, nausea, pneumonitis, and thyroid disease are seen with anti-PD-1/PDL-1 therapy.3
A look at the prognosis
For patients diagnosed with primary cutaneous melanoma between 2011 and 2017, the 5-year survival rate for localized disease (Stages I-II) was 99%.1 For regional (Stage III) and distant (Stage IV) disease, the 5-year survival rates were 68% and 30%, respectively.1 With the advent of adjuvant systemic therapy, 5-year overall survival rates for metastatic melanoma have markedly improved from < 10% to up to 40% to 50%.41 The 3-year survival rate for patients with high tumor burden, brain metastasis, and elevated lactate dehydrogenase remains at < 10%.42 Relative survival decreases with increased age, although survival is higher in women than in men.43 Risk of melanoma recurrence after surgical excision is high in patients with stage IIB, IIC, III and IV (resectable) disease. The most important risk factor for recurrence is primary tumor thickness.44 The most common site of first recurrence in stage I-II disease is regional lymph node metastasis (42.8%), closely followed by distant metastasis (37.6%).44
Long-term follow-up and surveillance
Recommendations for long-term care of patients with melanoma have evolved with advances in treatment, prognostication, and imaging. Caring for these patients requires a multidisciplinary approach wherein the family physician provides frontline care and team coordination. Since most recurrences are discovered by the patient or the patient’s family, patient education and self-examination are the cost-effective foundation for recurrence screening. In a trial of patients and partners, a 30-minute structured session on skin examination followed by physician reminders every 4 months increased the detection of melanoma recurrence without significant increases in patient visits.45
Continue to: Patient education should include sun safety...
Patient education should include sun safety (wearing sun-protective clothing, using broad-spectrum sunscreen, and avoiding sun exposure during peak times of the day). The US Preventive Services Task Force (USPSTF) says the level of evidence is insufficient to support routine skin cancer screening in adults.46 However, the USPSTF recommends discussing efforts to minimize UV radiation exposure to prevent skin cancer in fair-skinned individuals 10 to 24 years of age.
Current National Comprehensive Cancer Network (NCCN) guidelines have outlined the follow-up frequency for all melanoma patients. TABLE 232 outlines those recommendations in addition to self-examination and patient education.
Melanoma epidemic or overdiagnosis?
Over the past 2 decades, a marked rise in the incidence of melanoma has been reported in developed countries worldwide, although melanoma mortality rates have not increased as rapidly, with melanoma-specific survival stable in most groups.47-50 Due to conflicting evidence, significant disagreement exists as to whether this is an actual epidemic caused by a true rise in disease burden or is merely an artifact stemming from overdiagnosis.47
Evidence supporting a true melanoma epidemic includes population-based studies demonstrating greater UV radiation–induced carcinogenesis (from the sun and tanning bed use), a larger aging population, and increased incidence regardless of socioeconomic status.47 Those challenging the validity of an epidemic instead attribute the rising incidence to early-detection public awareness campaigns, expanded screenings, improved diagnostic modalities, and increased biopsies. They also credit lower pathologic thresholds that help identify thinner tumors with little to no metastatic potential.48 Additionally, multiple studies report an increased incidence in melanomas of all histologic subtypes and thicknesses, not just thinner, more curable tumors.49,51,52 Although increased screening and biopsies are effective, they alone cannot account for the sharp rise in melanoma cases.47 This “melanoma paradox” of increasing incidence without a parallel increase in mortality remains unsettled.47
CASE
Your patient had Stage IIA disease and a WE was performed with 1-cm margins. Ultrasound of the axilla identified an enlarged node, which was removed and found not to be diseased. He has now returned to have you look at another lesion identified by his spouse. His review of symptoms is negative. His initial melanoma was removed 2 years earlier, and his last dermatology skin exam was 5 months prior. You look at the lesion using a dermatoscope and do not note any worrisome features. You recommend that the patient photograph the area for reexamination and follow-up with his dermatologist next month for a 6-month follow-up.
CORRESPONDENCE
Jessica Servey, MD, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected]
1. NIH. Cancer stat facts: melanoma of the skin. 2018. Accessed May 13, 2021. https://seer.cancer.gov/statfacts/html/melan.html
2. Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
3. Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392:971-984.
4. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018(12):CD011902.
5. Morris JB, Alfonso SV, Hernandez N, et al. Examining the factors associated with past and present dermoscopy use among family physicians. Dermatol Pract Concept. 2017;7:63-70.
6. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
7. Rosendahl C, Cameron A, McColl I, et al. Dermatoscopy in routine practice — “chaos and clues”. Aust Fam Physician. 2012;41:482-487.
8. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy: a new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
9. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
10. Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
11. Rogers T, Marino ML, Dusza SW, et al. A clinical aid for detecting skin cancer: the Triage Amalgamated Dermoscopic Algorithm (TADA). J Am Board Fam Med. 2016;29:694-701.
12. Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679-93.
13. Carli P, Quercioli E, Sestini S, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148:981-984.
14. Yélamos O, Braun RP, Liopyris K, et al. Usefulness of dermoscopy to improve the clinical and histopathologic diagnosis of skin cancers. J Am Acad Dermatol. 2019;80:365-377.
15. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
16. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
17. Usatine RP, Shama LK, Marghoob AA, et al. Dermoscopy in family medicine: a primer. J Fam Pract. 2018;67:E1-E11.
18. Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80:208-250.
19. Seiverling EV, Ahrns HT, Bacik LC, et al. Biopsies for skin cancer detection: dispelling the myths. J Fam Pract. 2018;67:270-274.
20. Martin RCG, Scoggins CR, Ross MI, et al. Is incisional biopsy of melanoma harmful? Am J Surg. 2005;190:913-917.
21. Mir M, Chan CS, Khan F, et al. The rate of melanoma transection with various biopsy techniques and the influence of tumor transection on patient survival. J Am Acad Dermatol. 2013;68:452-458.
22. Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902-908
23. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer 8th ed cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
24. Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599-609.
25. Valsecchi ME, Silbermins D, de Rosa N, et al. Lymphatic mapping and sentinel lymph node biopsy in patients with melanoma: a meta-analysis. J Clin Oncol. 2011;29:1479-1487.
26. Memorial Sloan Kettering Cancer Center. Risk of sentinel lymph node metastasis nomogram. Accessed May 13, 2021. www.mskcc.org/nomograms/melanoma/sentinel_lymph_node_metastasis
27. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, eds. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2017:563-581.
28. Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103:129-142.
29. Tsao H, Feldman M, Fullerton JE, et al. Early detection of asymptomatic pulmonary melanoma metastases by routine chest radiographs is not associated with improved survival. Arch Dermatol. 2004;140:67-70.
30. Wang TS, Johnson TM, Cascade PN, et al. Evaluation of staging chest radiographs and serum lactate dehydrogenase for localized melanoma. J Am Acad Dermatol. 2004;51:399-405.
31. Yancovitz M, Finelt N, Warycha MA, et al. Role of radiologic imaging at the time of initial diagnosis of stage T1b-T3b melanoma. Cancer. 2007; 110:1107-1114.
32. Swetter SM, Thompson JA, Albertini MR, et al. NCCN Guidelines: cutaneous melanoma, version 4.2020. Accessed June 7, 2021. http://medi-guide.meditool.cn/ymtpdf/ACC90A18-6CDF-9443-BF3F-E29394D495E8.pdf
33. Stensheim H, Møller B, van Dijk T, et al. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27:45-51.
34. Lens MB, Rosdahl I, Ahlbom A, et al. Effect of pregnancy on survival in women with cutaneous malignant melanoma. J Clin Oncol. 2004;22:4369-4375.
35. Gandini S, Iodice S, Koomen E, et al. Hormonal and reproductive factors in relation to melanoma in women: current review and meta-analysis. Eur J Cancer. 2011;47:2607-2617.
36. Byrom L, Olsen CM, Knight L, et al. Does pregnancy after a diagnosis of melanoma affect prognosis? Systematic review and meta-analysis. Dermatol Surg. 2015;41:875-882.
37. Tang JY, Spaunhurst KM, Chlebowski RT, et al. Menopausal hormone therapy and risks of melanoma and nonmelanoma skin cancers: women’s health initiative randomized trials. J Natl Cancer Inst. 2011;103:1469-1475.
38. Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA Dermatol. 2015;151:1103-1109.
39. Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part I: inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-218.
40. Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7:122-136.
41. Kandolf Sekulovic L, Peris K, Hauschild A, et al. More than 5000 patients with metastatic melanoma in Europe per year do not have access to recommended first-line innovative treatments. Eur J Cancer. 2017;75:313-322.
42. Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17:1743-1754.
43. Trends in incidence and survival in patients with melanoma, 1974-2013. Am J Cancer Res. 2019;9:1396-1414.
44. Lyth J, Falk M, Maroti M, et al. Prognostic risk factors of first recurrence in patients with primary stages I–II cutaneous malignant melanoma – from the population‐based Swedish melanoma register. J Eur Acad Dermatol Venereol. 2017;31:1468-1474.
45. Robinson JK, Wayne JD, Martini MC, et al. Early detection of new melanomas by patients with melanoma and their partners using a structured skin self-examination skills training intervention: a randomized clinical trial. JAMA Dermatol. 2016;152:979-985.
Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
47. Gardner LJ, Strunck JL, Wu YP, et al. Current controversies in early-stage melanoma: questions on incidence, screening, and histologic regression. J Am Acad Dermatol. 2019;80:1-12.
48. Wei EX, Qureshi AA, Han J, et al. Trends in the diagnosis and clinical features of melanoma in situ (MIS) in US men and women: a prospective, observational study. J Am Acad Dermatol. 2016;75:698-705.
49. Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666-1674.
50. Curchin DJ, Forward E, Dickison P, et al. The acceleration of melanoma in situ: a population-based study of melanoma incidence trends from Victoria, Australia, 1985-2015. J Am Acad Dermatol. 2019;80:1791-1793.
51. Dennis LK. Analysis of the melanoma epidemic, both apparent and real: data from the 1973 through 1994 surveillance, epidemiology, and end results program registry. Arch Dermatol. 1999;135:275-280.
52. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65:S17-S25.
CASE
A 48-year-old man comes to your clinic with a dark nevus on his right upper arm that appeared 2 months earlier. He says that the lesion has continued to grow and has bled (he thought because he initially picked at it). On exam, there is a 7-mm brown papule with 2 black dots and slightly asymmetric borders.
How would you proceed with this patient?
Melanoma is the fifth leading cause of new cancer cases annually, with > 96,000 new cases in 2019.1 Overall, melanoma is more common in men and in Whites, with 48% diagnosed in people ages 55 to 74.1 The past 2 decades have seen numerous developments in the diagnosis, treatment, and surveillance of melanoma. This article covers recommendations, controversies, and issues that require future study. It does not cover uveal or mucosal melanoma.
Evaluating a patient with a new or changing nevus
Known risk factors for melanoma include a changing nevus, indoor tanning, older age, many melanocytic nevi, history of a dysplastic nevus or of blistering sunburns during teen years, red or blonde hair, large congenital nevus, Fitzpatrick skin type I or II, high socioeconomic status, personal or family history of melanoma, and intermittent high-intensity sun exposure.2-3 Presence of 1 or more of these risk factors should lower the threshold for biopsy.
Worrisome physical exam features (FIGURE) are nevus asymmetry, irregular borders, variegated color, and a diameter > 6 mm (the size of a pencil eraser). Inquire as to whether the nevus’ appearance has evolved and if it has bled without trauma. In a patient with multiple nevi, 1 nevus that looks different than the rest (the so-called “ugly duckling”) is concerning. Accuracy of diagnosis is enhanced with dermoscopy. A Cochrane review showed that skilled use of dermoscopy, in addition to inspection with the naked eye, considerably increases the sensitivity and specificity of diagnosing melanoma.4 Yet a 2017 study of 705 US primary care practitioners showed that only 8.3% of them used dermoscopy to evaluate pigmented lesions.5

Several published algorithms and checklists can aid clinicians in identifying lesions suggestive of melanoma—eg, ABCDE, CASH, Menzies method, “chaos and clues,” and 2-step and 3- and 7-point checklists.6-10 A simple 3-step algorithm, the TADA (triage amalgamated dermoscopic algorithm) method is available to novice dermoscopy users.11 Experts in pigmented lesions prefer to use pattern analysis, which requires simultaneously assessing multiple lesion patterns that vary according to body site.12,13
Dermoscopic features suggesting melanoma are atypical pigment networks, pseudopods, radial streaking, irregular dots or globules, blue-whitish veil, and granularity or peppering.14 Appropriate and effective use of dermoscopy requires training.15,16 Available methods for learning dermoscopy include online and in-person courses, mentoring by experienced dermoscopists, books and articles, and free apps and online resources.17
Continue to: Perform a skin biopsy, but do this first
Perform a skin biopsy, but do this first
Skin biopsy is the definitive way to diagnose melanoma. Prior to biopsy, take photographs to document the exact location of the lesion and to ensure that the correct area is removed in wide excision (WE). A complete biopsy should include the full depth and breadth of the lesion to ensure there are clinically negative margins. This can be achieved with an elliptical excision (for larger lesions), punch excision (for small lesions), or saucerization (deep shave with 1- to 2-mm peripheral margins, used for intermediate-size lesions).18 Saucerization is distinctly different from a superficial shave biopsy, which is not recommended for lesions with features of melanoma.19
A decision to perform a biopsy on a part of the lesion (partial biopsy) depends on the size of the lesion and its anatomic location, and is best made in agreement with the patient.
If you are untrained or uncomfortable performing the biopsy, contact a dermatologist immediately. In many communities, such referrals are subject to long delays, which further supports the advisability of family physicians doing their own biopsies after photographing the suspicious lesion. Many resources are available to help family physicians learn to do biopsies proficiently (www.mdedge.com/familymedicine/article/164358/oncology/biopsies-skin-cancer-detection-dispelling-myths).19
What to communicate to the pathologist. At a minimum, the biopsy request form should include patient age, sex, biopsy type (punch, excisional, or scoop shave), intention (complete or partial sample), exact site of the biopsy with laterality, and clinical details. These details should include the lesion size and clinical description, the suspected diagnosis, and clinical information, such as whether there is a history of bleeding or changing color, size, or symmetry. In standard biopsy specimens, the pathologist is only examining a portion of the lesion. Communicating clearly to the pathologist may lead to a request for deeper or additional sections or special stains.
If the biopsy results do not match the clinical impression, a phone call to the pathologist is warranted. In addition, evaluation by a dermatopathologist may be merited as pathologic diagnosis of melanoma can be quite challenging. Newer molecular tests, such as fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH), can assist in the histologic evaluation of complex pigmented lesions.
Continue to: CASE
CASE
You perform an elliptical excisional biopsy on your patient. The biopsy report comes back as a nodular malignant melanoma, Breslow depth 2.5 mm without ulceration, and no evidence of lymphovascular invasion or microsatellitosis. The report states that the biopsy margins appear clear of tumor involvement.
Further evaluation when the biopsy result is positive
Key steps in initial patient care include relaying pathology results to the patient, conducting (as needed) a more extensive evaluation, and obtaining appropriate consultation.
Clearly explain the diagnosis and convey an accurate reading of the pathology report. The vital pieces of information in the biopsy report are the Breslow depth and presence of ulceration, as evidence shows these 2 factors to be important independent predictors of outcome.22,23 Also important are the presence of microsatellitosis (essential for staging purposes), pathologic stage, and the status of the peripheral and deep biopsy margins. Review Breslow depth with the patient as this largely dictates treatment options and prognosis.
Evaluate for possible metastatic disease. Obtain a complete history from every patient with cutaneous melanoma, looking for any positive review of systems as a harbinger of metastatic disease. A full-body skin and lymph node exam is vital, given that melanoma can arise anywhere including on the scalp, in the gluteal cleft, and beneath nails. If the lymph node exam is worrisome, conduct an ultrasound exam, even while referring to specialty care. Treating a patient with melanoma requires a multidisciplinary approach that may include dermatologists, surgeons, and oncologists based on the stage of disease. A challenge for family physicians is knowing which consultation to prioritize and how to counsel the patient to schedule these for the most cost-effective and timely evaluation.
Expedite a dermatology consultation. If the melanoma is deep or appears advanced based on size or palpable lymph nodes, contact the dermatologist immediately by phone to set up a rapid referral. Delays in the definitive management of thick melanomas can negatively affect outcome. Paper, facsimile, or electronic referrals can get lost in the system and are not reliable methods for referring patients for a melanoma consultation. One benefit of the family physician performing the initial biopsy is that a confirmed melanoma diagnosis will almost certainly get an expedited dermatology appointment.
Continue to: Wide excision and sentinel node biopsy
Wide excision and sentinel node biopsy
Wide excision of a primary melanoma is standard practice, with evidence favoring the following surgical margins: 0.5 to 1 cm for melanoma in situ, 1 cm for tumors up to 1 mm in thickness, 1 to 2 cm for tumors > 1 to 2 mm thick, and 2 cm for tumors > 2 mm thick.18 WE is often performed by dermatologists for nonulcerated tumors < 0.8 mm thick (T1a) without adverse features. If trained in cutaneous surgery, you can also choose to excise these thin melanomas in your office. Otherwise refer all patients with biopsy-proven melanoma to dermatologists to perform an adequate WE.
Refer patients who have tumors ≥ 0.8 mm thick to the appropriate surgical specialty (surgical oncology, if available) for consultation on sentinel lymph node biopsy. SLNB, when indicated, should be performed prior to WE of the primary tumor, and whenever possible in the same surgical setting, to maximize lymphatic drainage mapping techniques.18 Medical oncology referral, if needed, is usually made after WE.
SLNB remains the standard for lymph node staging. It is controversial mainly in its use for very thin or very thick lesions. Randomized controlled trials, including the Multicenter Selective Lymphadenectomy Trial,24 have shown no difference in melanoma-specific survival for patients with intermediate-thickness melanomas who had undergone SLNB.24
Many professional organizations consider SLNB to be the most significant prognostic indicator of disease recurrence. With a negative SLNB result, the risk of regional node recurrence is 5% or lower.18,25 In addition, sentinel lymph node status is a critical determinant for systemic adjuvant therapy consideration and clinical trial eligibility. For patients who have primary cutaneous melanoma without clinical lymphadenopathy, an online tool is available for patients to use with their physician in predicting the likelihood of SLNB positivity.26
Recommendations for SLNB, supported by multidisciplinary consensus:18
- Do not pursue SLNB for melanoma in situ or most cutaneous melanomas < 0.8 mm without ulceration (T1a). (See TABLE 127)
- Discuss SLNB with patients who have T1a melanoma and additional adverse features: young age, high mitotic rate, lymphovascular invasion, and nevus depth close to 0.8 mm with positive deep biopsy margins.
- Discuss SLNB with patients who have T1b disease (< 0.8 mm with ulceration, or 0.8-1 mm), although rates of SLNB positivity are low.
- Offer SLNB to patients with T2a and higher disease (> 1 mm).18

Continue to: Patients who have...
Patients who have clinical Stage I or II disease (TABLE 127)
Melanoma in women: Considerations to keep in mind
Hormonal influences of pregnancy, lactation, contraception, and menopause introduce special considerations regarding melanoma, which is the most common cancer occurring during pregnancy, accounting for 31% of new malignancies.33 Risk of melanoma lessens, however, for women who first give birth at a younger age or who have had > 5 live births.18,34,35 There is no evidence that nevi darken during pregnancy, although nevi on the breast and abdomen may seem to enlarge due to skin stretching.18 All changing nevi in pregnancy warrant an examination, preferably with dermoscopy, and patients should be offered biopsy if there are any nevus characteristics associated with melanoma.18
The effect of pregnancy on an existing melanoma is not fully understood, but evidence from controlled studies shows no negative effect. Recent working group guidelines advise WE with local anesthesia without delay in pregnant patients.18 Definitive treatment after melanoma diagnosis should take a multidisciplinary approach involving obstetric care coordinated with Dermatology, Surgery, and Medical Oncology.18
Most recommendations on the timing of pregnancy following a melanoma diagnosis have limited evidence. One meta-analysis concluded that pregnancy occurring after successful treatment of melanoma did not change a woman’s prognosis.36 Current guidelines do not recommend delaying future pregnancy if a woman had an early-stage melanoma. For melanomas deemed higher risk, a woman could consider a 2- to 3-year delay in the next planned pregnancy, owing to current data on recurrence rates.18
A systematic review of women who used hormonal contraception or postmenopausal hormone replacement therapy (HRT) showed no associated increased risk of melanoma.35 An additional randomized trial showed no effect of HRT on melanoma risk.37
Continue to: Systemic melanoma treatment and common adverse effects
Systemic melanoma treatment and common adverse effects
Multiple systemic therapies have been approved for the treatment of advanced or unresectable cutaneous melanomas. While these treatments are managed primarily by Oncology in concert with Dermatology, an awareness of the medications’ common dermatologic toxicities is important for the primary care provider. The 2 broad categories of FDA-approved systemic medications for advanced melanoma are mitogen-activated protein kinase (MAPK) inhibitors and immune checkpoint inhibitors, each having its own set of adverse cutaneous effects.
MAPK pathway–targeting drugs include the B-Raf proto-oncogene serine/threonine-kinase inhibitors (BRAFIs) vemurafenib and dabrafenib, and the MAPK inhibitors (MEKIs) trametinib and cobimetinib. The most common adverse skin effects in MAPK pathway–targeting drugs are severe ultraviolet photosensitivity, cutaneous epidermal neoplasms (particularly squamous cell carcinoma, keratoacanthoma-type), thick actinic keratosis, wart-like keratosis, painful palmoplantar keratosis, and dry skin.38 These effects are most commonly seen with BRAFI monotherapy and can be abated with the addition of a MEKI. MEKI therapy can cause acneiform eruptions and paronychia.39 Additional adverse effects include diarrhea, pyrexia, arthralgias, and fatigue for BRAFIs and diarrhea, fatigue, and peripheral edema for MEKIs.40
Immune checkpoint inhibitors include anti-CTLA-4 (ipilimumab), anti-PD-1 (pembrolizumab and nivolumab), and anti-PDL-1 (atezolizumab). Adverse skin effects include morbilliform rash with or without an associated itch, itch with or without an associated rash, vitiligo, and lichenoid skin rashes. PD-1 and PDL-1 inhibitors have been associated with flares or unmasking of atopic dermatitis, psoriasis, sarcoidosis, and autoimmune bullous disease.18 Diarrhea, colitis, hepatitis, elevated liver enzymes, hypophysitis, and thyroiditis are some of the more common noncutaneous adverse effects reported with CTLA-4 inhibitors, while fatigue, diarrhea, nausea, pneumonitis, and thyroid disease are seen with anti-PD-1/PDL-1 therapy.3
A look at the prognosis
For patients diagnosed with primary cutaneous melanoma between 2011 and 2017, the 5-year survival rate for localized disease (Stages I-II) was 99%.1 For regional (Stage III) and distant (Stage IV) disease, the 5-year survival rates were 68% and 30%, respectively.1 With the advent of adjuvant systemic therapy, 5-year overall survival rates for metastatic melanoma have markedly improved from < 10% to up to 40% to 50%.41 The 3-year survival rate for patients with high tumor burden, brain metastasis, and elevated lactate dehydrogenase remains at < 10%.42 Relative survival decreases with increased age, although survival is higher in women than in men.43 Risk of melanoma recurrence after surgical excision is high in patients with stage IIB, IIC, III and IV (resectable) disease. The most important risk factor for recurrence is primary tumor thickness.44 The most common site of first recurrence in stage I-II disease is regional lymph node metastasis (42.8%), closely followed by distant metastasis (37.6%).44
Long-term follow-up and surveillance
Recommendations for long-term care of patients with melanoma have evolved with advances in treatment, prognostication, and imaging. Caring for these patients requires a multidisciplinary approach wherein the family physician provides frontline care and team coordination. Since most recurrences are discovered by the patient or the patient’s family, patient education and self-examination are the cost-effective foundation for recurrence screening. In a trial of patients and partners, a 30-minute structured session on skin examination followed by physician reminders every 4 months increased the detection of melanoma recurrence without significant increases in patient visits.45
Continue to: Patient education should include sun safety...
Patient education should include sun safety (wearing sun-protective clothing, using broad-spectrum sunscreen, and avoiding sun exposure during peak times of the day). The US Preventive Services Task Force (USPSTF) says the level of evidence is insufficient to support routine skin cancer screening in adults.46 However, the USPSTF recommends discussing efforts to minimize UV radiation exposure to prevent skin cancer in fair-skinned individuals 10 to 24 years of age.
Current National Comprehensive Cancer Network (NCCN) guidelines have outlined the follow-up frequency for all melanoma patients. TABLE 232 outlines those recommendations in addition to self-examination and patient education.

Melanoma epidemic or overdiagnosis?
Over the past 2 decades, a marked rise in the incidence of melanoma has been reported in developed countries worldwide, although melanoma mortality rates have not increased as rapidly, with melanoma-specific survival stable in most groups.47-50 Due to conflicting evidence, significant disagreement exists as to whether this is an actual epidemic caused by a true rise in disease burden or is merely an artifact stemming from overdiagnosis.47
Evidence supporting a true melanoma epidemic includes population-based studies demonstrating greater UV radiation–induced carcinogenesis (from the sun and tanning bed use), a larger aging population, and increased incidence regardless of socioeconomic status.47 Those challenging the validity of an epidemic instead attribute the rising incidence to early-detection public awareness campaigns, expanded screenings, improved diagnostic modalities, and increased biopsies. They also credit lower pathologic thresholds that help identify thinner tumors with little to no metastatic potential.48 Additionally, multiple studies report an increased incidence in melanomas of all histologic subtypes and thicknesses, not just thinner, more curable tumors.49,51,52 Although increased screening and biopsies are effective, they alone cannot account for the sharp rise in melanoma cases.47 This “melanoma paradox” of increasing incidence without a parallel increase in mortality remains unsettled.47
CASE
Your patient had Stage IIA disease and a WE was performed with 1-cm margins. Ultrasound of the axilla identified an enlarged node, which was removed and found not to be diseased. He has now returned to have you look at another lesion identified by his spouse. His review of symptoms is negative. His initial melanoma was removed 2 years earlier, and his last dermatology skin exam was 5 months prior. You look at the lesion using a dermatoscope and do not note any worrisome features. You recommend that the patient photograph the area for reexamination and follow-up with his dermatologist next month for a 6-month follow-up.
CORRESPONDENCE
Jessica Servey, MD, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected]
CASE
A 48-year-old man comes to your clinic with a dark nevus on his right upper arm that appeared 2 months earlier. He says that the lesion has continued to grow and has bled (he thought because he initially picked at it). On exam, there is a 7-mm brown papule with 2 black dots and slightly asymmetric borders.
How would you proceed with this patient?
Melanoma is the fifth leading cause of new cancer cases annually, with > 96,000 new cases in 2019.1 Overall, melanoma is more common in men and in Whites, with 48% diagnosed in people ages 55 to 74.1 The past 2 decades have seen numerous developments in the diagnosis, treatment, and surveillance of melanoma. This article covers recommendations, controversies, and issues that require future study. It does not cover uveal or mucosal melanoma.
Evaluating a patient with a new or changing nevus
Known risk factors for melanoma include a changing nevus, indoor tanning, older age, many melanocytic nevi, history of a dysplastic nevus or of blistering sunburns during teen years, red or blonde hair, large congenital nevus, Fitzpatrick skin type I or II, high socioeconomic status, personal or family history of melanoma, and intermittent high-intensity sun exposure.2-3 Presence of 1 or more of these risk factors should lower the threshold for biopsy.
Worrisome physical exam features (FIGURE) are nevus asymmetry, irregular borders, variegated color, and a diameter > 6 mm (the size of a pencil eraser). Inquire as to whether the nevus’ appearance has evolved and if it has bled without trauma. In a patient with multiple nevi, 1 nevus that looks different than the rest (the so-called “ugly duckling”) is concerning. Accuracy of diagnosis is enhanced with dermoscopy. A Cochrane review showed that skilled use of dermoscopy, in addition to inspection with the naked eye, considerably increases the sensitivity and specificity of diagnosing melanoma.4 Yet a 2017 study of 705 US primary care practitioners showed that only 8.3% of them used dermoscopy to evaluate pigmented lesions.5

Several published algorithms and checklists can aid clinicians in identifying lesions suggestive of melanoma—eg, ABCDE, CASH, Menzies method, “chaos and clues,” and 2-step and 3- and 7-point checklists.6-10 A simple 3-step algorithm, the TADA (triage amalgamated dermoscopic algorithm) method is available to novice dermoscopy users.11 Experts in pigmented lesions prefer to use pattern analysis, which requires simultaneously assessing multiple lesion patterns that vary according to body site.12,13
Dermoscopic features suggesting melanoma are atypical pigment networks, pseudopods, radial streaking, irregular dots or globules, blue-whitish veil, and granularity or peppering.14 Appropriate and effective use of dermoscopy requires training.15,16 Available methods for learning dermoscopy include online and in-person courses, mentoring by experienced dermoscopists, books and articles, and free apps and online resources.17
Continue to: Perform a skin biopsy, but do this first
Perform a skin biopsy, but do this first
Skin biopsy is the definitive way to diagnose melanoma. Prior to biopsy, take photographs to document the exact location of the lesion and to ensure that the correct area is removed in wide excision (WE). A complete biopsy should include the full depth and breadth of the lesion to ensure there are clinically negative margins. This can be achieved with an elliptical excision (for larger lesions), punch excision (for small lesions), or saucerization (deep shave with 1- to 2-mm peripheral margins, used for intermediate-size lesions).18 Saucerization is distinctly different from a superficial shave biopsy, which is not recommended for lesions with features of melanoma.19
A decision to perform a biopsy on a part of the lesion (partial biopsy) depends on the size of the lesion and its anatomic location, and is best made in agreement with the patient.
If you are untrained or uncomfortable performing the biopsy, contact a dermatologist immediately. In many communities, such referrals are subject to long delays, which further supports the advisability of family physicians doing their own biopsies after photographing the suspicious lesion. Many resources are available to help family physicians learn to do biopsies proficiently (www.mdedge.com/familymedicine/article/164358/oncology/biopsies-skin-cancer-detection-dispelling-myths).19
What to communicate to the pathologist. At a minimum, the biopsy request form should include patient age, sex, biopsy type (punch, excisional, or scoop shave), intention (complete or partial sample), exact site of the biopsy with laterality, and clinical details. These details should include the lesion size and clinical description, the suspected diagnosis, and clinical information, such as whether there is a history of bleeding or changing color, size, or symmetry. In standard biopsy specimens, the pathologist is only examining a portion of the lesion. Communicating clearly to the pathologist may lead to a request for deeper or additional sections or special stains.
If the biopsy results do not match the clinical impression, a phone call to the pathologist is warranted. In addition, evaluation by a dermatopathologist may be merited as pathologic diagnosis of melanoma can be quite challenging. Newer molecular tests, such as fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH), can assist in the histologic evaluation of complex pigmented lesions.
Continue to: CASE
CASE
You perform an elliptical excisional biopsy on your patient. The biopsy report comes back as a nodular malignant melanoma, Breslow depth 2.5 mm without ulceration, and no evidence of lymphovascular invasion or microsatellitosis. The report states that the biopsy margins appear clear of tumor involvement.
Further evaluation when the biopsy result is positive
Key steps in initial patient care include relaying pathology results to the patient, conducting (as needed) a more extensive evaluation, and obtaining appropriate consultation.
Clearly explain the diagnosis and convey an accurate reading of the pathology report. The vital pieces of information in the biopsy report are the Breslow depth and presence of ulceration, as evidence shows these 2 factors to be important independent predictors of outcome.22,23 Also important are the presence of microsatellitosis (essential for staging purposes), pathologic stage, and the status of the peripheral and deep biopsy margins. Review Breslow depth with the patient as this largely dictates treatment options and prognosis.
Evaluate for possible metastatic disease. Obtain a complete history from every patient with cutaneous melanoma, looking for any positive review of systems as a harbinger of metastatic disease. A full-body skin and lymph node exam is vital, given that melanoma can arise anywhere including on the scalp, in the gluteal cleft, and beneath nails. If the lymph node exam is worrisome, conduct an ultrasound exam, even while referring to specialty care. Treating a patient with melanoma requires a multidisciplinary approach that may include dermatologists, surgeons, and oncologists based on the stage of disease. A challenge for family physicians is knowing which consultation to prioritize and how to counsel the patient to schedule these for the most cost-effective and timely evaluation.
Expedite a dermatology consultation. If the melanoma is deep or appears advanced based on size or palpable lymph nodes, contact the dermatologist immediately by phone to set up a rapid referral. Delays in the definitive management of thick melanomas can negatively affect outcome. Paper, facsimile, or electronic referrals can get lost in the system and are not reliable methods for referring patients for a melanoma consultation. One benefit of the family physician performing the initial biopsy is that a confirmed melanoma diagnosis will almost certainly get an expedited dermatology appointment.
Continue to: Wide excision and sentinel node biopsy
Wide excision and sentinel node biopsy
Wide excision of a primary melanoma is standard practice, with evidence favoring the following surgical margins: 0.5 to 1 cm for melanoma in situ, 1 cm for tumors up to 1 mm in thickness, 1 to 2 cm for tumors > 1 to 2 mm thick, and 2 cm for tumors > 2 mm thick.18 WE is often performed by dermatologists for nonulcerated tumors < 0.8 mm thick (T1a) without adverse features. If trained in cutaneous surgery, you can also choose to excise these thin melanomas in your office. Otherwise refer all patients with biopsy-proven melanoma to dermatologists to perform an adequate WE.
Refer patients who have tumors ≥ 0.8 mm thick to the appropriate surgical specialty (surgical oncology, if available) for consultation on sentinel lymph node biopsy. SLNB, when indicated, should be performed prior to WE of the primary tumor, and whenever possible in the same surgical setting, to maximize lymphatic drainage mapping techniques.18 Medical oncology referral, if needed, is usually made after WE.
SLNB remains the standard for lymph node staging. It is controversial mainly in its use for very thin or very thick lesions. Randomized controlled trials, including the Multicenter Selective Lymphadenectomy Trial,24 have shown no difference in melanoma-specific survival for patients with intermediate-thickness melanomas who had undergone SLNB.24
Many professional organizations consider SLNB to be the most significant prognostic indicator of disease recurrence. With a negative SLNB result, the risk of regional node recurrence is 5% or lower.18,25 In addition, sentinel lymph node status is a critical determinant for systemic adjuvant therapy consideration and clinical trial eligibility. For patients who have primary cutaneous melanoma without clinical lymphadenopathy, an online tool is available for patients to use with their physician in predicting the likelihood of SLNB positivity.26
Recommendations for SLNB, supported by multidisciplinary consensus:18
- Do not pursue SLNB for melanoma in situ or most cutaneous melanomas < 0.8 mm without ulceration (T1a). (See TABLE 127)
- Discuss SLNB with patients who have T1a melanoma and additional adverse features: young age, high mitotic rate, lymphovascular invasion, and nevus depth close to 0.8 mm with positive deep biopsy margins.
- Discuss SLNB with patients who have T1b disease (< 0.8 mm with ulceration, or 0.8-1 mm), although rates of SLNB positivity are low.
- Offer SLNB to patients with T2a and higher disease (> 1 mm).18

Continue to: Patients who have...
Patients who have clinical Stage I or II disease (TABLE 127)
Melanoma in women: Considerations to keep in mind
Hormonal influences of pregnancy, lactation, contraception, and menopause introduce special considerations regarding melanoma, which is the most common cancer occurring during pregnancy, accounting for 31% of new malignancies.33 Risk of melanoma lessens, however, for women who first give birth at a younger age or who have had > 5 live births.18,34,35 There is no evidence that nevi darken during pregnancy, although nevi on the breast and abdomen may seem to enlarge due to skin stretching.18 All changing nevi in pregnancy warrant an examination, preferably with dermoscopy, and patients should be offered biopsy if there are any nevus characteristics associated with melanoma.18
The effect of pregnancy on an existing melanoma is not fully understood, but evidence from controlled studies shows no negative effect. Recent working group guidelines advise WE with local anesthesia without delay in pregnant patients.18 Definitive treatment after melanoma diagnosis should take a multidisciplinary approach involving obstetric care coordinated with Dermatology, Surgery, and Medical Oncology.18
Most recommendations on the timing of pregnancy following a melanoma diagnosis have limited evidence. One meta-analysis concluded that pregnancy occurring after successful treatment of melanoma did not change a woman’s prognosis.36 Current guidelines do not recommend delaying future pregnancy if a woman had an early-stage melanoma. For melanomas deemed higher risk, a woman could consider a 2- to 3-year delay in the next planned pregnancy, owing to current data on recurrence rates.18
A systematic review of women who used hormonal contraception or postmenopausal hormone replacement therapy (HRT) showed no associated increased risk of melanoma.35 An additional randomized trial showed no effect of HRT on melanoma risk.37
Continue to: Systemic melanoma treatment and common adverse effects
Systemic melanoma treatment and common adverse effects
Multiple systemic therapies have been approved for the treatment of advanced or unresectable cutaneous melanomas. While these treatments are managed primarily by Oncology in concert with Dermatology, an awareness of the medications’ common dermatologic toxicities is important for the primary care provider. The 2 broad categories of FDA-approved systemic medications for advanced melanoma are mitogen-activated protein kinase (MAPK) inhibitors and immune checkpoint inhibitors, each having its own set of adverse cutaneous effects.
MAPK pathway–targeting drugs include the B-Raf proto-oncogene serine/threonine-kinase inhibitors (BRAFIs) vemurafenib and dabrafenib, and the MAPK inhibitors (MEKIs) trametinib and cobimetinib. The most common adverse skin effects in MAPK pathway–targeting drugs are severe ultraviolet photosensitivity, cutaneous epidermal neoplasms (particularly squamous cell carcinoma, keratoacanthoma-type), thick actinic keratosis, wart-like keratosis, painful palmoplantar keratosis, and dry skin.38 These effects are most commonly seen with BRAFI monotherapy and can be abated with the addition of a MEKI. MEKI therapy can cause acneiform eruptions and paronychia.39 Additional adverse effects include diarrhea, pyrexia, arthralgias, and fatigue for BRAFIs and diarrhea, fatigue, and peripheral edema for MEKIs.40
Immune checkpoint inhibitors include anti-CTLA-4 (ipilimumab), anti-PD-1 (pembrolizumab and nivolumab), and anti-PDL-1 (atezolizumab). Adverse skin effects include morbilliform rash with or without an associated itch, itch with or without an associated rash, vitiligo, and lichenoid skin rashes. PD-1 and PDL-1 inhibitors have been associated with flares or unmasking of atopic dermatitis, psoriasis, sarcoidosis, and autoimmune bullous disease.18 Diarrhea, colitis, hepatitis, elevated liver enzymes, hypophysitis, and thyroiditis are some of the more common noncutaneous adverse effects reported with CTLA-4 inhibitors, while fatigue, diarrhea, nausea, pneumonitis, and thyroid disease are seen with anti-PD-1/PDL-1 therapy.3
A look at the prognosis
For patients diagnosed with primary cutaneous melanoma between 2011 and 2017, the 5-year survival rate for localized disease (Stages I-II) was 99%.1 For regional (Stage III) and distant (Stage IV) disease, the 5-year survival rates were 68% and 30%, respectively.1 With the advent of adjuvant systemic therapy, 5-year overall survival rates for metastatic melanoma have markedly improved from < 10% to up to 40% to 50%.41 The 3-year survival rate for patients with high tumor burden, brain metastasis, and elevated lactate dehydrogenase remains at < 10%.42 Relative survival decreases with increased age, although survival is higher in women than in men.43 Risk of melanoma recurrence after surgical excision is high in patients with stage IIB, IIC, III and IV (resectable) disease. The most important risk factor for recurrence is primary tumor thickness.44 The most common site of first recurrence in stage I-II disease is regional lymph node metastasis (42.8%), closely followed by distant metastasis (37.6%).44
Long-term follow-up and surveillance
Recommendations for long-term care of patients with melanoma have evolved with advances in treatment, prognostication, and imaging. Caring for these patients requires a multidisciplinary approach wherein the family physician provides frontline care and team coordination. Since most recurrences are discovered by the patient or the patient’s family, patient education and self-examination are the cost-effective foundation for recurrence screening. In a trial of patients and partners, a 30-minute structured session on skin examination followed by physician reminders every 4 months increased the detection of melanoma recurrence without significant increases in patient visits.45
Continue to: Patient education should include sun safety...
Patient education should include sun safety (wearing sun-protective clothing, using broad-spectrum sunscreen, and avoiding sun exposure during peak times of the day). The US Preventive Services Task Force (USPSTF) says the level of evidence is insufficient to support routine skin cancer screening in adults.46 However, the USPSTF recommends discussing efforts to minimize UV radiation exposure to prevent skin cancer in fair-skinned individuals 10 to 24 years of age.
Current National Comprehensive Cancer Network (NCCN) guidelines have outlined the follow-up frequency for all melanoma patients. TABLE 232 outlines those recommendations in addition to self-examination and patient education.

Melanoma epidemic or overdiagnosis?
Over the past 2 decades, a marked rise in the incidence of melanoma has been reported in developed countries worldwide, although melanoma mortality rates have not increased as rapidly, with melanoma-specific survival stable in most groups.47-50 Due to conflicting evidence, significant disagreement exists as to whether this is an actual epidemic caused by a true rise in disease burden or is merely an artifact stemming from overdiagnosis.47
Evidence supporting a true melanoma epidemic includes population-based studies demonstrating greater UV radiation–induced carcinogenesis (from the sun and tanning bed use), a larger aging population, and increased incidence regardless of socioeconomic status.47 Those challenging the validity of an epidemic instead attribute the rising incidence to early-detection public awareness campaigns, expanded screenings, improved diagnostic modalities, and increased biopsies. They also credit lower pathologic thresholds that help identify thinner tumors with little to no metastatic potential.48 Additionally, multiple studies report an increased incidence in melanomas of all histologic subtypes and thicknesses, not just thinner, more curable tumors.49,51,52 Although increased screening and biopsies are effective, they alone cannot account for the sharp rise in melanoma cases.47 This “melanoma paradox” of increasing incidence without a parallel increase in mortality remains unsettled.47
CASE
Your patient had Stage IIA disease and a WE was performed with 1-cm margins. Ultrasound of the axilla identified an enlarged node, which was removed and found not to be diseased. He has now returned to have you look at another lesion identified by his spouse. His review of symptoms is negative. His initial melanoma was removed 2 years earlier, and his last dermatology skin exam was 5 months prior. You look at the lesion using a dermatoscope and do not note any worrisome features. You recommend that the patient photograph the area for reexamination and follow-up with his dermatologist next month for a 6-month follow-up.
CORRESPONDENCE
Jessica Servey, MD, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected]
1. NIH. Cancer stat facts: melanoma of the skin. 2018. Accessed May 13, 2021. https://seer.cancer.gov/statfacts/html/melan.html
2. Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
3. Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392:971-984.
4. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018(12):CD011902.
5. Morris JB, Alfonso SV, Hernandez N, et al. Examining the factors associated with past and present dermoscopy use among family physicians. Dermatol Pract Concept. 2017;7:63-70.
6. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
7. Rosendahl C, Cameron A, McColl I, et al. Dermatoscopy in routine practice — “chaos and clues”. Aust Fam Physician. 2012;41:482-487.
8. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy: a new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
9. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
10. Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
11. Rogers T, Marino ML, Dusza SW, et al. A clinical aid for detecting skin cancer: the Triage Amalgamated Dermoscopic Algorithm (TADA). J Am Board Fam Med. 2016;29:694-701.
12. Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679-93.
13. Carli P, Quercioli E, Sestini S, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148:981-984.
14. Yélamos O, Braun RP, Liopyris K, et al. Usefulness of dermoscopy to improve the clinical and histopathologic diagnosis of skin cancers. J Am Acad Dermatol. 2019;80:365-377.
15. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
16. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
17. Usatine RP, Shama LK, Marghoob AA, et al. Dermoscopy in family medicine: a primer. J Fam Pract. 2018;67:E1-E11.
18. Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80:208-250.
19. Seiverling EV, Ahrns HT, Bacik LC, et al. Biopsies for skin cancer detection: dispelling the myths. J Fam Pract. 2018;67:270-274.
20. Martin RCG, Scoggins CR, Ross MI, et al. Is incisional biopsy of melanoma harmful? Am J Surg. 2005;190:913-917.
21. Mir M, Chan CS, Khan F, et al. The rate of melanoma transection with various biopsy techniques and the influence of tumor transection on patient survival. J Am Acad Dermatol. 2013;68:452-458.
22. Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902-908
23. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer 8th ed cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
24. Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599-609.
25. Valsecchi ME, Silbermins D, de Rosa N, et al. Lymphatic mapping and sentinel lymph node biopsy in patients with melanoma: a meta-analysis. J Clin Oncol. 2011;29:1479-1487.
26. Memorial Sloan Kettering Cancer Center. Risk of sentinel lymph node metastasis nomogram. Accessed May 13, 2021. www.mskcc.org/nomograms/melanoma/sentinel_lymph_node_metastasis
27. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, eds. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2017:563-581.
28. Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103:129-142.
29. Tsao H, Feldman M, Fullerton JE, et al. Early detection of asymptomatic pulmonary melanoma metastases by routine chest radiographs is not associated with improved survival. Arch Dermatol. 2004;140:67-70.
30. Wang TS, Johnson TM, Cascade PN, et al. Evaluation of staging chest radiographs and serum lactate dehydrogenase for localized melanoma. J Am Acad Dermatol. 2004;51:399-405.
31. Yancovitz M, Finelt N, Warycha MA, et al. Role of radiologic imaging at the time of initial diagnosis of stage T1b-T3b melanoma. Cancer. 2007; 110:1107-1114.
32. Swetter SM, Thompson JA, Albertini MR, et al. NCCN Guidelines: cutaneous melanoma, version 4.2020. Accessed June 7, 2021. http://medi-guide.meditool.cn/ymtpdf/ACC90A18-6CDF-9443-BF3F-E29394D495E8.pdf
33. Stensheim H, Møller B, van Dijk T, et al. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27:45-51.
34. Lens MB, Rosdahl I, Ahlbom A, et al. Effect of pregnancy on survival in women with cutaneous malignant melanoma. J Clin Oncol. 2004;22:4369-4375.
35. Gandini S, Iodice S, Koomen E, et al. Hormonal and reproductive factors in relation to melanoma in women: current review and meta-analysis. Eur J Cancer. 2011;47:2607-2617.
36. Byrom L, Olsen CM, Knight L, et al. Does pregnancy after a diagnosis of melanoma affect prognosis? Systematic review and meta-analysis. Dermatol Surg. 2015;41:875-882.
37. Tang JY, Spaunhurst KM, Chlebowski RT, et al. Menopausal hormone therapy and risks of melanoma and nonmelanoma skin cancers: women’s health initiative randomized trials. J Natl Cancer Inst. 2011;103:1469-1475.
38. Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA Dermatol. 2015;151:1103-1109.
39. Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part I: inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-218.
40. Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7:122-136.
41. Kandolf Sekulovic L, Peris K, Hauschild A, et al. More than 5000 patients with metastatic melanoma in Europe per year do not have access to recommended first-line innovative treatments. Eur J Cancer. 2017;75:313-322.
42. Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17:1743-1754.
43. Trends in incidence and survival in patients with melanoma, 1974-2013. Am J Cancer Res. 2019;9:1396-1414.
44. Lyth J, Falk M, Maroti M, et al. Prognostic risk factors of first recurrence in patients with primary stages I–II cutaneous malignant melanoma – from the population‐based Swedish melanoma register. J Eur Acad Dermatol Venereol. 2017;31:1468-1474.
45. Robinson JK, Wayne JD, Martini MC, et al. Early detection of new melanomas by patients with melanoma and their partners using a structured skin self-examination skills training intervention: a randomized clinical trial. JAMA Dermatol. 2016;152:979-985.
Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
47. Gardner LJ, Strunck JL, Wu YP, et al. Current controversies in early-stage melanoma: questions on incidence, screening, and histologic regression. J Am Acad Dermatol. 2019;80:1-12.
48. Wei EX, Qureshi AA, Han J, et al. Trends in the diagnosis and clinical features of melanoma in situ (MIS) in US men and women: a prospective, observational study. J Am Acad Dermatol. 2016;75:698-705.
49. Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666-1674.
50. Curchin DJ, Forward E, Dickison P, et al. The acceleration of melanoma in situ: a population-based study of melanoma incidence trends from Victoria, Australia, 1985-2015. J Am Acad Dermatol. 2019;80:1791-1793.
51. Dennis LK. Analysis of the melanoma epidemic, both apparent and real: data from the 1973 through 1994 surveillance, epidemiology, and end results program registry. Arch Dermatol. 1999;135:275-280.
52. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65:S17-S25.
1. NIH. Cancer stat facts: melanoma of the skin. 2018. Accessed May 13, 2021. https://seer.cancer.gov/statfacts/html/melan.html
2. Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
3. Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392:971-984.
4. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018(12):CD011902.
5. Morris JB, Alfonso SV, Hernandez N, et al. Examining the factors associated with past and present dermoscopy use among family physicians. Dermatol Pract Concept. 2017;7:63-70.
6. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
7. Rosendahl C, Cameron A, McColl I, et al. Dermatoscopy in routine practice — “chaos and clues”. Aust Fam Physician. 2012;41:482-487.
8. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy: a new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
9. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
10. Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
11. Rogers T, Marino ML, Dusza SW, et al. A clinical aid for detecting skin cancer: the Triage Amalgamated Dermoscopic Algorithm (TADA). J Am Board Fam Med. 2016;29:694-701.
12. Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679-93.
13. Carli P, Quercioli E, Sestini S, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148:981-984.
14. Yélamos O, Braun RP, Liopyris K, et al. Usefulness of dermoscopy to improve the clinical and histopathologic diagnosis of skin cancers. J Am Acad Dermatol. 2019;80:365-377.
15. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
16. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
17. Usatine RP, Shama LK, Marghoob AA, et al. Dermoscopy in family medicine: a primer. J Fam Pract. 2018;67:E1-E11.
18. Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80:208-250.
19. Seiverling EV, Ahrns HT, Bacik LC, et al. Biopsies for skin cancer detection: dispelling the myths. J Fam Pract. 2018;67:270-274.
20. Martin RCG, Scoggins CR, Ross MI, et al. Is incisional biopsy of melanoma harmful? Am J Surg. 2005;190:913-917.
21. Mir M, Chan CS, Khan F, et al. The rate of melanoma transection with various biopsy techniques and the influence of tumor transection on patient survival. J Am Acad Dermatol. 2013;68:452-458.
22. Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902-908
23. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer 8th ed cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
24. Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599-609.
25. Valsecchi ME, Silbermins D, de Rosa N, et al. Lymphatic mapping and sentinel lymph node biopsy in patients with melanoma: a meta-analysis. J Clin Oncol. 2011;29:1479-1487.
26. Memorial Sloan Kettering Cancer Center. Risk of sentinel lymph node metastasis nomogram. Accessed May 13, 2021. www.mskcc.org/nomograms/melanoma/sentinel_lymph_node_metastasis
27. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, eds. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2017:563-581.
28. Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103:129-142.
29. Tsao H, Feldman M, Fullerton JE, et al. Early detection of asymptomatic pulmonary melanoma metastases by routine chest radiographs is not associated with improved survival. Arch Dermatol. 2004;140:67-70.
30. Wang TS, Johnson TM, Cascade PN, et al. Evaluation of staging chest radiographs and serum lactate dehydrogenase for localized melanoma. J Am Acad Dermatol. 2004;51:399-405.
31. Yancovitz M, Finelt N, Warycha MA, et al. Role of radiologic imaging at the time of initial diagnosis of stage T1b-T3b melanoma. Cancer. 2007; 110:1107-1114.
32. Swetter SM, Thompson JA, Albertini MR, et al. NCCN Guidelines: cutaneous melanoma, version 4.2020. Accessed June 7, 2021. http://medi-guide.meditool.cn/ymtpdf/ACC90A18-6CDF-9443-BF3F-E29394D495E8.pdf
33. Stensheim H, Møller B, van Dijk T, et al. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27:45-51.
34. Lens MB, Rosdahl I, Ahlbom A, et al. Effect of pregnancy on survival in women with cutaneous malignant melanoma. J Clin Oncol. 2004;22:4369-4375.
35. Gandini S, Iodice S, Koomen E, et al. Hormonal and reproductive factors in relation to melanoma in women: current review and meta-analysis. Eur J Cancer. 2011;47:2607-2617.
36. Byrom L, Olsen CM, Knight L, et al. Does pregnancy after a diagnosis of melanoma affect prognosis? Systematic review and meta-analysis. Dermatol Surg. 2015;41:875-882.
37. Tang JY, Spaunhurst KM, Chlebowski RT, et al. Menopausal hormone therapy and risks of melanoma and nonmelanoma skin cancers: women’s health initiative randomized trials. J Natl Cancer Inst. 2011;103:1469-1475.
38. Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA Dermatol. 2015;151:1103-1109.
39. Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part I: inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-218.
40. Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7:122-136.
41. Kandolf Sekulovic L, Peris K, Hauschild A, et al. More than 5000 patients with metastatic melanoma in Europe per year do not have access to recommended first-line innovative treatments. Eur J Cancer. 2017;75:313-322.
42. Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17:1743-1754.
43. Trends in incidence and survival in patients with melanoma, 1974-2013. Am J Cancer Res. 2019;9:1396-1414.
44. Lyth J, Falk M, Maroti M, et al. Prognostic risk factors of first recurrence in patients with primary stages I–II cutaneous malignant melanoma – from the population‐based Swedish melanoma register. J Eur Acad Dermatol Venereol. 2017;31:1468-1474.
45. Robinson JK, Wayne JD, Martini MC, et al. Early detection of new melanomas by patients with melanoma and their partners using a structured skin self-examination skills training intervention: a randomized clinical trial. JAMA Dermatol. 2016;152:979-985.
Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
47. Gardner LJ, Strunck JL, Wu YP, et al. Current controversies in early-stage melanoma: questions on incidence, screening, and histologic regression. J Am Acad Dermatol. 2019;80:1-12.
48. Wei EX, Qureshi AA, Han J, et al. Trends in the diagnosis and clinical features of melanoma in situ (MIS) in US men and women: a prospective, observational study. J Am Acad Dermatol. 2016;75:698-705.
49. Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666-1674.
50. Curchin DJ, Forward E, Dickison P, et al. The acceleration of melanoma in situ: a population-based study of melanoma incidence trends from Victoria, Australia, 1985-2015. J Am Acad Dermatol. 2019;80:1791-1793.
51. Dennis LK. Analysis of the melanoma epidemic, both apparent and real: data from the 1973 through 1994 surveillance, epidemiology, and end results program registry. Arch Dermatol. 1999;135:275-280.
52. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65:S17-S25.
PRACTICE RECOMMENDATIONS
› Consider adding dermoscopy to the physical exam to increase sensitivity and specificity in diagnosing melanoma. A
› Perform wide local excision for invasive cutaneous melanoma: 1-cm margin for tumors up to 1 mm thick; 1 to 2 cm for tumors > 1 mm to 2 mm thick; and 2 cm for tumors > 2 mm thick. A
› Do not hesitate to consider, as needed, hormone replacement therapy or hormonal contraception for women with a prior diagnosis of melanoma, as this form of contraception does not confer an increased risk of melanoma. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Transitioning patients with developmental disabilities to adult care
Some adults who have an intellectual or other developmental disability (IDD) require extensive subspecialty care; many, however, depend primarily on their family physician for the bulk of their health care. With that reliance in mind, this article provides (1) an overview of important services that family physicians can provide for their adult patients with IDD and (2) pragmatic clinical suggestions for tailoring that care. Note: We highlight only some high-impact areas of clinical focus; refer to the 2018 Canadian consensus guidelines for a comprehensive approach to optimizing primary care for this population.1
CASE
Laura S, a 24-year-old woman with Down syndrome, is visiting your clinic with her mother to establish care. Ms. S has several medical comorbidities, including type 2 diabetes, hyperlipidemia, repaired congenital heart disease, schizoaffective disorder, and hypothyroidism. She is under the care of multiple specialists, including a cardiologist and an endocrinologist. Her medications include the atypical antipsychotic risperidone, which was prescribed for her through the services of a community mental health center.

Ms. S is due for multiple preventive health screenings. She indicates that she feels nervous today talking about these screenings with a new physician.
First step in care: Proficiency in the lexicon of IDD
Three core concepts of IDD are impairment, disability, and handicap. According to the World Health Organization2:
- impairment “is any loss or abnormality of psychological, physiological, or anatomical structure or function.”
- disability “is any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.”
- handicap therefore “represents socialization of an impairment or disability, and as such it reflects the consequences for the individual—cultural, social, economic, and environmental—that stem from the presence of impairment and disability.”
Essential transition: Pediatric to adult health care
Health care transition (HCT) is the planned process of transferring care from a pediatric to an adult-based health care setting,3 comprising 3 phases:
- preparation
- transfer from pediatric to adult care
- integration into adult-based care.
Two critical components of a smooth HCT include initiating the transition early in adolescence and providing transition-support resources, which are often lacking, even in large, integrated health systems.4 Got Transition, created by the National Alliance to Advance Adolescent Health, outlines core elements of an organized HCT process (www.gottransition.org) specific to young adults with IDD, including young adults with autism spectrum disorder.5,6
Even young people who are served by a family physician and who intend to remain in that family practice as they age into adulthood require HCT services that include6:
- assessment of readiness to transition to adult care
- update of the medical history
- assessment and promotion of self-care skills
- consent discussions and optimized participation in decision-making
- transition of specialty care from pediatric to adult specialists.
Continue to: For an ideal HCT...
For an ideal HCT, full engagement of the patient, the medical home (physicians, nursing staff, and care coordinators), and the patient’s family (including the primary caregiver or guardian) is critical. In addition to preventive care visits and management of chronic disease, additional domains that require explicit attention in transitioning young people with IDD include health insurance, transportation, employment, and postsecondary education.
Young people who have special health care needs and receive high-quality HCT demonstrate improvements in adherence to care, disease-specific measures, quality of life, self-care skills, satisfaction with care, and health care utilization.7TABLE 13 lists resources identified by Berens and colleagues that are helpful in facilitating the transition.
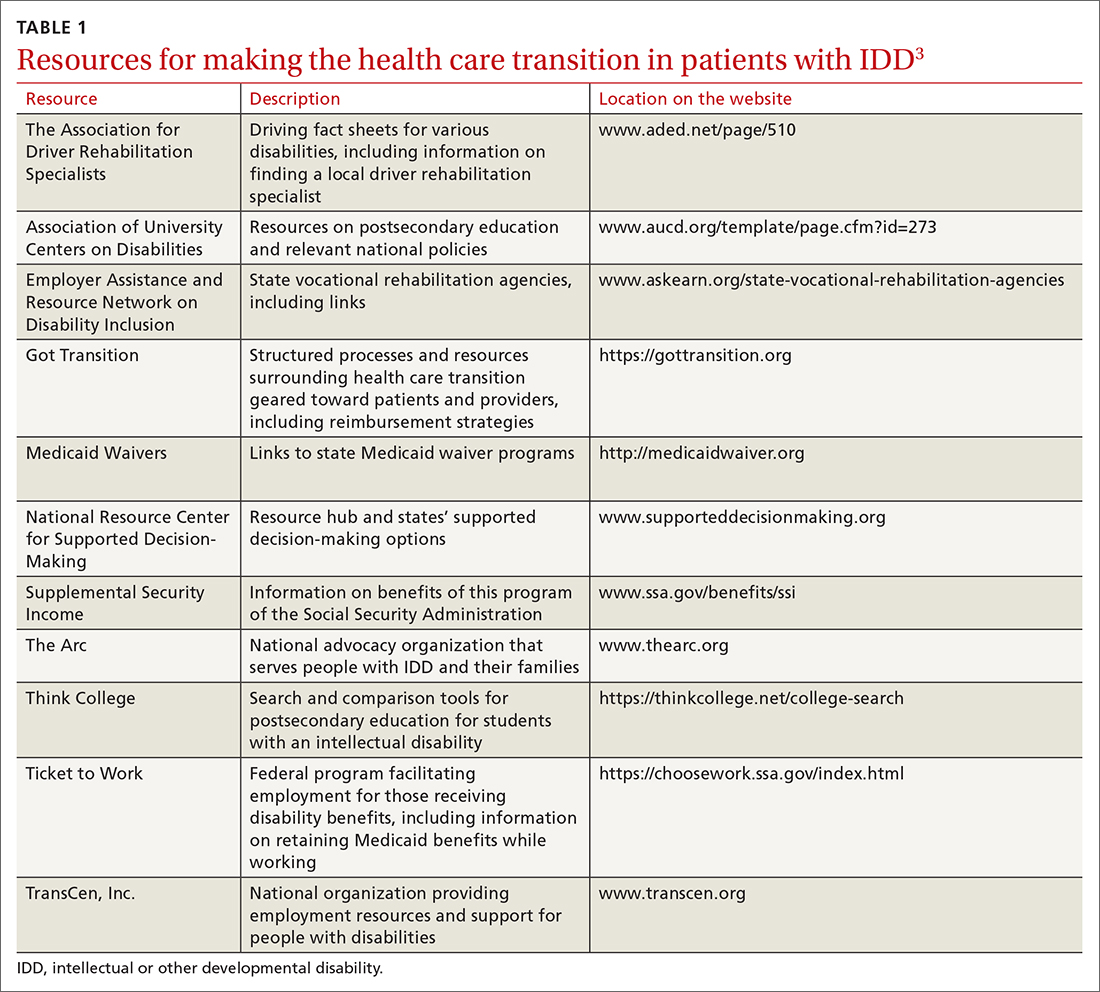
Teach and practice disability etiquette
Societal prejudice harms people with IDD—leading to self-deprecation, alienation from the larger community, and isolation from others with IDD.8 To promote acceptance and inclusivity in residential communities, the workplace, recreational venues, and clinical settings, disability etiquette should be utilized—a set of guidelines on how to interact with patients with IDD. These include speaking to the patient directly, using clear language in an adult voice, and avoiding stereotypes about people with disabilities.9 The entire health care team, including all front-facing staff (receptionists and care and financial coordinators) and clinical staff (physicians, nurses, medical assistants), need to be educated in, and practice, disability etiquette.
Preparing for in-person visits. Pre-visit preparation, ideally by means of dialogue between health care staff and the patient or caregiver (or both), typically by telephone and in advance of the scheduled visit, is often critical for a successful first face-to-face encounter. (See “Pre-visit telephone questionnaire and script for a new adult patient with IDD,” page 287, which we developed for use in our office practice.) Outcomes of the pre-visit preparation should include identifying:
- words or actions that can trigger anxiety or panic
- de-escalation techniques, such as specific calming words and actions
- strategies for optimal communication, physical access, and physical examination.
SIDEBAR
Pre-visit telephone questionnaire and script for a new adult patient with IDD
Introduction
Hello! My name is ______________. I’m a nurse [or medical assistant] from [name of practice]. I understand that [name of patient] is coming to our office for an appointment on [date and time]. I am calling to prepare our health care team to make this first appointment successful for [name of patient] and you.
- How would [name of patient] prefer to be called?
- Who will be accompanying [name of patient] to the appointment? What parts of the appointment will that person remain for?
Describe what to expect, what the patient or caregiver should bring to the appointment, and how long the appointment will last.
- What makes [name of patient] anxious or fearful so that we might avoid doing that? Should we avoid bringing up certain topics? Should we avoid performing any procedures that are customary during a first appointment?
- Does [name of patient] have sensitivities—to light, sound, touch, etc—that we should be aware of?
Offer to have a room ready upon the patient’s arrival if remaining in the waiting area would cause too much anxiety.
- What helps calm [name of patient]? Are there some topics that put [name of patient] at ease?
- How does [name of patient] best communicate?
- Is there anything else the health care team might do to prepare for the appointment?
- Does [name of patient] need personal protective equipment, a wheelchair, oxygen, or other medical equipment upon arrival?
- What would make for a successful first appointment?
- What strategies or techniques have [name of patient’s] providers used in the past that have helped make health care visits successful?
- Is there anything else you want me to know that we haven’t talked about?
- Would it be helpful if I talked with [name of patient] now about their upcoming appointment?
Initial appointments should focus on building trust and rapport with the health care team and desensitizing the patient to the clinical environment.10 Examination techniques used with pediatric patients can be applied to this population: for example, demonstrating an examination maneuver first on the parent or caregiver; beginning the examination with the least invasive or anxiety-provoking components; and stating what you plan to do next—before you do it.
Continue to: Systematic health checks provide great value
Systematic health checks provide great value
A health check is a systematic and comprehensive health assessment that is provided annually to adults with IDD, and includes:
- specific review of signs and symptoms of health conditions that often co-occur in adults with IDD (TABLE 2Calibri11)
- screening for changes in adaptive functioning and secondary disability
- lifestyle counseling
- medication review and counseling
- immunization update
- discussion of caregiver concerns.
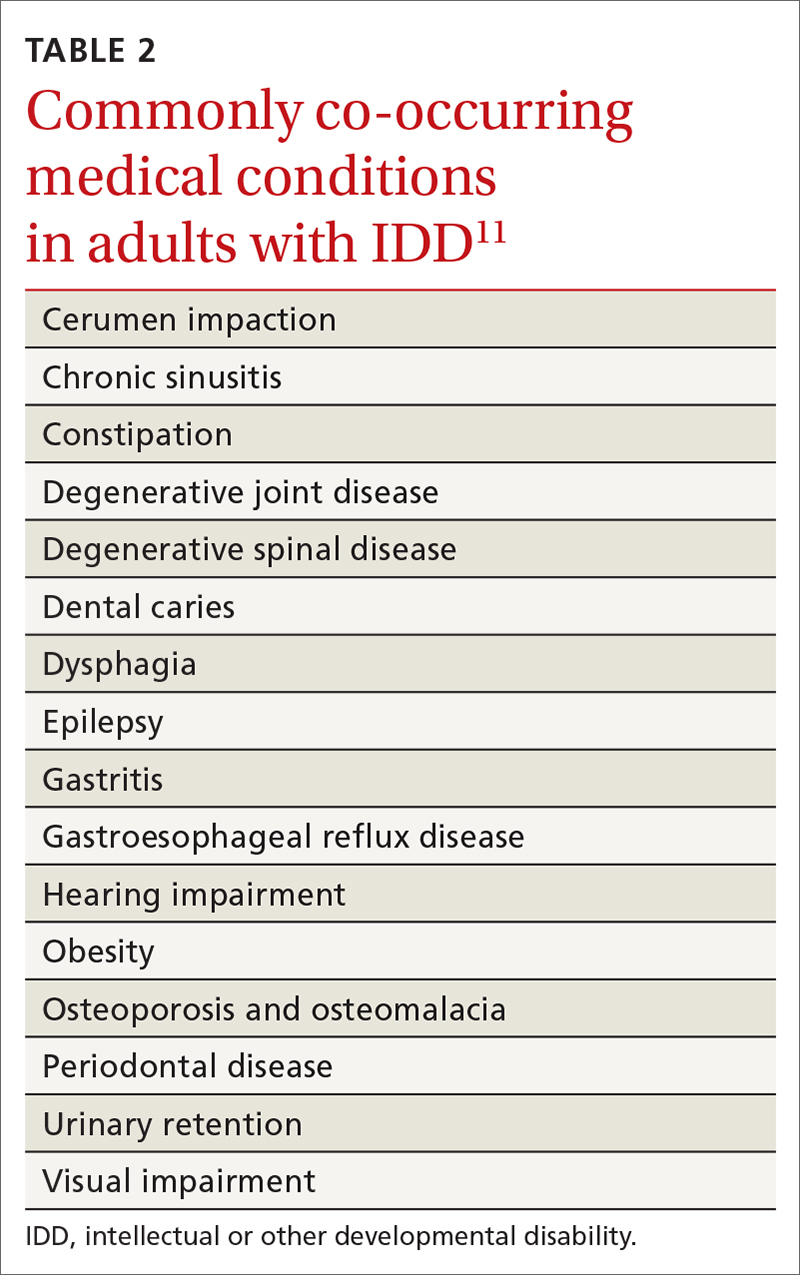
Regarding the last point: Many caregivers are the aging parents of the adult patient with IDD—people who have their own emerging health and support needs. You should initiate conversations about advanced planning for the needs of patients, which often involves engaging siblings and other family members to assume a greater role in caregiving.12
Benefits of the health check. A systematic review of 38 studies, comprising more than 5000 patients with IDD, found that health checks increased the detection of serious conditions, improved screening for sensory impairments, and increased the immunization rate.13 Although many patients with IDD generally understand the need for a periodic health examination, you can enhance their experience by better explaining the rationale for the health check; scheduling sufficient time for the appointment, based on the individual clinical situation; and discussing the value of laboratory testing and referrals to specialists.14
Tailoring preventive care
Many of the preventive services recommendations typically utilized by family physicians, such as guidelines from the US Preventive Services Task Force, have been developed for the general population at average risk of conditions of interest.15 Adults with IDD, depending on the cause of their developmental disability and their behavioral risk profile, might be at significantly higher (or lower) risk of cancer, heart disease, or other conditions than the general population. To address these differences, preventive care guidelines tailored to patients with certain developmental disabilities have been created, including guidelines specific to adults with Down syndrome, fragile X syndrome, Prader-Willi syndrome, Smith-Magenis syndrome, and 22q11.2 deletion (DiGeorge) syndrome.16
Clarifying the molecular genetic etiology of many developmental disabilities has led to more precise understandings about physical and behavioral health issues associated with specific developmental disabilities. For that reason, patients without a known cause for their IDD might benefit from referral to a geneticist—even in early or middle adulthood. Variables generally associated with a higher likelihood of an abnormal genetic test result include17:
- a family history of developmental disability
- a congenital malformation or dysmorphic features
- a dual diagnosis of developmental disability and co-occurring mental illness
- hypotonia
- severe or profound IDD.
Continue to: Successful implementation of preventive health screening tests...
Successful implementation of preventive health screening tests often requires ingenuity and the collective creativity of the patient, family members, staff, and family physician to allay fears and anxieties. Examples: Women who have been advised to undergo screening mammography might feel less anxious by undergoing tandem screening with their sister or mother, and colorectal cancer screening might be more easily accomplished using a fecal DNA test rather than by colonoscopy. Procedural desensitization strategies and preventive care instructional materials targeting people with IDD are posted on YouTube (for example, the “DD CARES Best Practices” series [see www.youtube.com/watch?v=EPJy4zvg4io]) and other websites.
Management of chronic disease
Evidence of health disparities in patients with IDD includes suboptimal management of chronic diseases, such as diabetes18 and hypertension,19 despite contact with a primary care physician. Nonadherence to a medication regimen might be more common in patients who live with their family or in a residential setting where there is a lower degree of supervision—that is, compared to a residence that maintains 24-hour staffing with daily nursing care and supervision. For a patient who is not so closely supervised, reviewing the medication refill history with the pharmacy, or using the so-called brown-bag technique of counting pill bottles brought to appointments, can ensure medication adherence.
CASE
As you interview Ms. S, you note that she is shy, avoids eye contact, and appears generally anxious. You calm her by noticing and complimenting her jewelry and fingernail polish. Ms. S smiles and talks about her favorite polish colors.
Her mother reports that, when Ms. S is stressed, she talks to herself alone in her bedroom. However, you do not observe evidence of schizoaffective disorder, and begin to wonder whether she needs to be taking risperidone.
Essentials of mental health care
It is estimated that one-third of adults with IDD have significant mental and behavioral health care needs.20 Patients with IDD suffer the same psychiatric disorders as the general population; some also engage in problematic behaviors, such as self-injurious actions, physical or verbal aggression (or both), property destruction, and resistance to caregiving assistance.
Continue to: Mental and behavioral health problems...
Mental and behavioral health problems can have a profound impact on the quality of life of patients with IDD, their peers, and their family and other caregivers. If untreated, these problems can lead to premature institutionalization, loss of employment or desired program participation, fractured social relationships, and caregiver withdrawal and burnout.
Initial evaluation of suspected mental and behavioral health problems begins with careful assessment for medical conditions that might be causing pain and distress, stereotypies, and other problematic behaviors. Common sources of pain and discomfort include dental and other oral disease, dysphagia, gastroesophageal reflux disease, gastritis, constipation, allergic disease, headache, musculoskeletal pathology, lower urinary tract disease, and gynecologic disorders.11 Identification and optimal treatment of medical conditions might not eliminate problematic behaviors but often decrease their frequency and intensity.
Psychoactive medications are prescribed for many patients with IDD. Many have behavioral adverse effects, such as akathisia, aggression, and disinhibition—leading to a prescribing cascade of psychoactive medication polypharmacy and escalating dosages.21 Antipsychotic medications are often initiated without a careful diagnosis, explicit outcome targets, or adequate clinical monitoring for effectiveness; in addition, they often lead to insulin resistance, metabolic syndrome, and massive weight gain.21 Even a family physician who is not the prescriber can perform an important advocacy role by critically reviewing psychoactive medications, documenting adverse effects, insisting on a clear therapeutic target, and calling for discontinuation of medications that appear to be ineffective.
Evaluation of mental and behavioral health problems requires a developmental perspective to interpret specific, observable behaviors with a proper clinical lens. For example, many patients with IDD engage in self-talk (soliloquizing) as a means of processing the world around them. This practice might escalate during a time of physical or psychological stress, and the unwary clinician might misinterpret this behavior as psychotic, leading to inappropriate prescribing of antipsychotic medication. Other psychotoform behaviors that, superficially, mimic but are typically not truly psychotic, include talk with or about imaginary friends and repetitive retelling of sometimes elaborate or grandiose tales or assertions. The failure of clinicians to recognize developmentally determined expressions of distress often leads to a misdiagnosis of schizophrenia or other psychotic illness and, consequently, inappropriate psychopharmacotherapy.
Family physicians, familiar with the use of psychiatric scales for diagnosis and treatment monitoring, should use similar scales that have been developed specifically for patients with IDD (TABLE 311). In addition, a psychiatric diagnosis manual, the Diagnostic Manual—Intellectual Disability 2, specific to people with IDD (and analogous to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) provides modification of diagnostic criteria to account for patients who have difficulty articulating their internal emotional state and inner thoughts.22
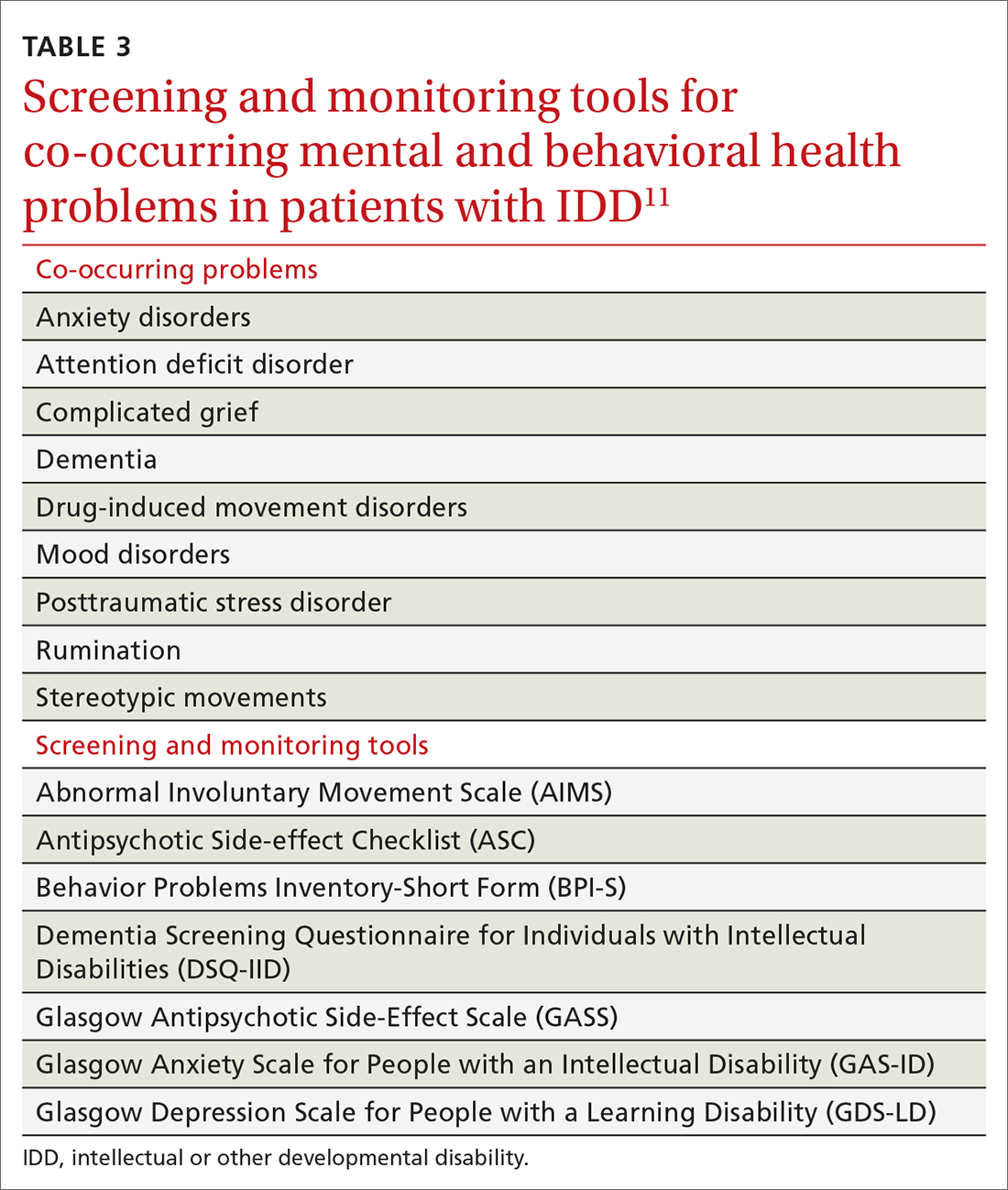
Continue to: Problematic behaviors
Problematic behaviors that are not features of a bona fide psychiatric disorder are often best understood through functional behavioral analysis, which examines antecedents and consequences of problematic behaviors and identifies their predictable outcomes, such as gaining attention, avoiding a task, or securing a desired item. Rather than being given a prescription for psychoactive medication, many adult patients with IDD and problematic behaviors might be best served by having you order consultation with a certified behavior analyst. The analyst will conduct an evaluation and, along with family or residential staff and the patient, craft a behavioral support plan to address core drivers of the undesired behavior. Behavioral support plans might be enriched by multidisciplinary input from a speech and language pathologist, habilitation professionals, occupational and physical therapists, a neuropsychologist, and others.23
Resources to help you address the physical, mental, and behavioral health problems of these patients are available online through Vanderbilt Kennedy Center’s “Toolkit for primary care providers” (https://iddtoolkit.vkcsites.org).
CASE
During your examination, you review Ms. S’s vital signs, including body mass index (BMI). You calculate that she is morbidly obese—BMI, 37—in the setting of a known comorbidity, diabetes.
Ms. S tells you that she is interested in having a healthy lifestyle, but feels frustrated because she does not know how to make the necessary changes. You discuss with her how some medications, including risperidone, can promote weight gain, and that it is important for her mental health provider to carefully reassess whether she needs to continue the drug.
Weight management in a patient population that tends to be sedentary
Patients with IDD are more likely to live a sedentary lifestyle. Compared to adults who do not have IDD, adults with IDD—especially women and patients with Down syndrome—are reported to have a higher prevalence of obesity.24
Continue to: As in the general population...
As in the general population, the greatest success in weight management involves multidisciplinary treatment, including nutritional support, physical activity, behavioral changes, and close follow-up. The importance of such an approach was borne out by the findings of a randomized controlled trial in which a multicomponent intervention—an energy-reduced diet, physical activity, and behavioral sessions—delivered to participants or their caregivers during monthly visits produced clinically meaningful 6-month weight loss.25 Health-promoting behavioral interventions that rely on a dyadic strategy, such as peer health coaches (ie, people with IDD who have been trained as a health coach) or mentors (IDD staff trained as a health coach), might be more successful at changing health behaviors among patients with IDD than traditional office-based, individual patient education and counseling.26
Similarly, undesired weight loss demands careful evaluation and management because such loss can reflect a medically significant condition, such as gastroesophageal reflux, constipation, dysphagia, neglect, and cancer.27
Boosting the amount and effectiveness of physical activity
Young people with IDD participate in physical activity less often than their neurotypical peers; as a result, they tend to be less fit and have a higher prevalence of obesity.28 Based on a meta-analysis, interventions that focus on sport and movement skills training, such as soccer, basketball, and ball-throwing programs, might be more effective than general physical activity programs.28 In addition to year-round sports training and athletic competitions, Special Olympics conducts vital health screenings of athletes and supports community-based initiatives that address bias against patients with IDD, promote inclusion, and foster social relationships (www.specialolympics.org/our-work/inclusive-health?locale=en).
Emphasize regular activity. In adulthood, fewer than 10% of patients with IDD exercise regularly.21 According to the second edition of Physical Activity Guidelines for Americans,29 “all adults, with or without a disability, should get at least 150 minutes of aerobic physical activity a week. Activities can be broken down into smaller amounts, such as about 25 minutes a day every day.”30 Supplementation with muscle-strengthening activities (eg, yoga, weight training, and resistance-band training) provides further health benefit, such as improvement in posture and prevention of future injury.31 An ideal exercise program proposed by Tyler and Baker is based on a daily, “3-2-1” schedule (ie, of every hour of activity, 30 minutes should be of aerobic exercise; 20 minutes, of strength building; and 10 minutes, of flexibility).11 By participating in any type of physical activity, there is potential for considerable health benefit in reducing psychosocial stressors, improving mental health, counteracting metabolic syndromes, and, ultimately, reducing morbidity and mortality related to physical inactivity.
CASE
With permission from Ms. S, you send your progress notes by fax to her mental health provider at the community mental health center and request a call to discuss her case—in particular, to examine potential alternatives to risperidone. With Ms. S’s input, you also co-create an exercise prescription that includes a daily 20-minute walking program with her mother.
At the follow-up visit that is scheduled in 3 months, you anticipate adding a resistance component and balance activity to the exercise prescription to enrich Ms. S’s physical activity regimen.
CORRESPONDENCE
Carl V. Tyler Jr., MD, 14601 Detroit Avenue, Lakewood, OH, 44107; [email protected]
1. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician. 2018;64:254-279.
2. World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. May 1980. Accessed May 27, 2021. https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y
3. Berens J, Wozow C, Peacock C. Transition to adult care. Phys Med Rehabil Clin N Am. 2020;31:159-170. doi:10.1016/j.pmr.2019.09.004
4. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group; Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182-200. doi:10.1542/peds.2011-0969
5. Dressler PB, Nguyen TK, Moody EJ, et al. Use of transition resources by primary care providers for youth with intellectual and developmental disabilities. Intellect Dev Disabil. 2018;56:56-68. doi:10.1352/1934-9556-56.1.56
6. The National Alliance to Advance Adolescent Health. Six Core Elements of Health Care Transition.™ Got Transition website. Accessed May 27, 2021. www.gottransition.org
7. Schmidt A, Ilango SM, McManus MA, et al. Outcomes of pediatric to adult health care transition interventions: an updated systematic review. J Pediatr Nurs. 2020; 51:92-107. doi: 10.1016/j.pedn.2020.01.002
8. Keith JM, Bennetto L, Rogge RD. The relationship between contact and attitudes: reducing prejudice toward individuals with intellectual and developmental disabilities. Res Dev Disabil. 2015;47:14-26. doi:10.1016/j.ridd.2015.07.032
9. United Spinal Association. Disability Etiquette: Tips on Interacting With People With Disabilities. 2015. Accessed June 9, 2021. www.unitedspinal.org/pdf/DisabilityEtiquette.pdf
10. Nathawad R, Hanks C. Optimizing the office visit for adolescents with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:182-189. doi:10.1016/j.cppeds.2017.07.002
11. Tyler CV, Baker S. Intellectual Disabilities at Your Fingertips: A Health Care Resource. High Tide Press; 2009.
12. Williamson HJ, Perkins EA. Family caregivers of adults with intellectual and developmental disabilities: outcomes associated with U.S. services and supports. Intellect Dev Disabil. 2014;52:147-159. doi: 10.1352/1934-9556-52.2.147
13. Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil. 2014;35:2450-2462. doi:10.1016/j.ridd.2014.06.007
14. Perry J, Felce D, Kerr M, et al. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil. 2014;27:200-211. doi: 10.1111/jar.12072
15. Recommendation topics. United States Preventive Services Task Force website. 2020. Accessed May 27, 2021. www.uspreventiveservicestaskforce.org
16. Developmental Disabilities Primary Care Initiative. Tools for the Primary Care of People with Developmental Disabilities. 1st ed. MUMS Guideline Clearinghouse; 2011.
17. Jang W, Kim Y, Han E, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019;39:299-310. doi:10.3343/alm.2019.39.3.299
18. Shireman TI, Reichard A, Nazir N, et al. Quality of diabetes care for adults with developmental disabilities. Disabil Health J. 2010;3:179-185. doi:10.1016/j.dhjo.2009.10.004
19. Cyrus AC, Royer J, Carroll DD, et al. Anti-hypertensive medication use and actors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2019;124:248-262. doi:10.1352/1944-7558-124.3.248
20. IDD/MI diagnosis. National Association for the Dually Diagnosed (NADD) website. 2019. Accessed May 27, 2021. https://thenadd.org/idd-mi-diagnosis
21. Matson JL, Mayville EA, Bielecki J, et al. Reliability of the Matson Evaluation of Drug Side Effects Scale (MEDS). Res Dev Disabil. 1998;19:501-506. doi:10.1016/s0891-4222(98)00021-3
22. Fletcher R, Barnhill J, Cooper SA. (2017). Diagnostic Manual-Intellectual Disability: A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. 2nd ed. National Association for the Dually Diagnosed (NADD); 2017.
23. Marrus N, Hall L. Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am. 2017;26:539-554. doi:10.1016/j.chc.2017.03.001
24. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12;22-7. doi: 10.1002/mrdd.20091
25. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(suppl 1):82-96. doi:10.1111/jar.12348
26. Marks B, Sisirak J, Magallanes R, et al. Effectiveness of a HealthMessages peer-to-peer program for people with intellectual and developmental disabilities. Intellect Dev Disabil. 2019;57:242-258. doi:10.1352/1934-9556-57.3.242
27. Escudé C. Clinical Pearls in IDD Health care. HRS, Inc; 2020.
28. Kapsal NJ, Dicke T, Morin AJS, et al. Effects of physical activity on the physical and psychosocial health of youth with intellectual disabilities: a systematic review and meta-analysis. J Phys Act Health. 2019;16:1187-1195. doi:10.1123/jpah.2018-0675
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. Accessed May 29, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Physical activity for people with disability. September 2020. Accessed May 27, 2021. www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html
31. Introduction to strengthening exercises. National Center on Health, Physical Activity and Disability (NCHPAD). 2020. Accessed May 27, 2021. www.nchpad.org/374/2096/Strengthening~Exercises
Some adults who have an intellectual or other developmental disability (IDD) require extensive subspecialty care; many, however, depend primarily on their family physician for the bulk of their health care. With that reliance in mind, this article provides (1) an overview of important services that family physicians can provide for their adult patients with IDD and (2) pragmatic clinical suggestions for tailoring that care. Note: We highlight only some high-impact areas of clinical focus; refer to the 2018 Canadian consensus guidelines for a comprehensive approach to optimizing primary care for this population.1
CASE
Laura S, a 24-year-old woman with Down syndrome, is visiting your clinic with her mother to establish care. Ms. S has several medical comorbidities, including type 2 diabetes, hyperlipidemia, repaired congenital heart disease, schizoaffective disorder, and hypothyroidism. She is under the care of multiple specialists, including a cardiologist and an endocrinologist. Her medications include the atypical antipsychotic risperidone, which was prescribed for her through the services of a community mental health center.

Ms. S is due for multiple preventive health screenings. She indicates that she feels nervous today talking about these screenings with a new physician.
First step in care: Proficiency in the lexicon of IDD
Three core concepts of IDD are impairment, disability, and handicap. According to the World Health Organization2:
- impairment “is any loss or abnormality of psychological, physiological, or anatomical structure or function.”
- disability “is any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.”
- handicap therefore “represents socialization of an impairment or disability, and as such it reflects the consequences for the individual—cultural, social, economic, and environmental—that stem from the presence of impairment and disability.”
Essential transition: Pediatric to adult health care
Health care transition (HCT) is the planned process of transferring care from a pediatric to an adult-based health care setting,3 comprising 3 phases:
- preparation
- transfer from pediatric to adult care
- integration into adult-based care.
Two critical components of a smooth HCT include initiating the transition early in adolescence and providing transition-support resources, which are often lacking, even in large, integrated health systems.4 Got Transition, created by the National Alliance to Advance Adolescent Health, outlines core elements of an organized HCT process (www.gottransition.org) specific to young adults with IDD, including young adults with autism spectrum disorder.5,6
Even young people who are served by a family physician and who intend to remain in that family practice as they age into adulthood require HCT services that include6:
- assessment of readiness to transition to adult care
- update of the medical history
- assessment and promotion of self-care skills
- consent discussions and optimized participation in decision-making
- transition of specialty care from pediatric to adult specialists.
Continue to: For an ideal HCT...
For an ideal HCT, full engagement of the patient, the medical home (physicians, nursing staff, and care coordinators), and the patient’s family (including the primary caregiver or guardian) is critical. In addition to preventive care visits and management of chronic disease, additional domains that require explicit attention in transitioning young people with IDD include health insurance, transportation, employment, and postsecondary education.
Young people who have special health care needs and receive high-quality HCT demonstrate improvements in adherence to care, disease-specific measures, quality of life, self-care skills, satisfaction with care, and health care utilization.7TABLE 13 lists resources identified by Berens and colleagues that are helpful in facilitating the transition.

Teach and practice disability etiquette
Societal prejudice harms people with IDD—leading to self-deprecation, alienation from the larger community, and isolation from others with IDD.8 To promote acceptance and inclusivity in residential communities, the workplace, recreational venues, and clinical settings, disability etiquette should be utilized—a set of guidelines on how to interact with patients with IDD. These include speaking to the patient directly, using clear language in an adult voice, and avoiding stereotypes about people with disabilities.9 The entire health care team, including all front-facing staff (receptionists and care and financial coordinators) and clinical staff (physicians, nurses, medical assistants), need to be educated in, and practice, disability etiquette.
Preparing for in-person visits. Pre-visit preparation, ideally by means of dialogue between health care staff and the patient or caregiver (or both), typically by telephone and in advance of the scheduled visit, is often critical for a successful first face-to-face encounter. (See “Pre-visit telephone questionnaire and script for a new adult patient with IDD,” page 287, which we developed for use in our office practice.) Outcomes of the pre-visit preparation should include identifying:
- words or actions that can trigger anxiety or panic
- de-escalation techniques, such as specific calming words and actions
- strategies for optimal communication, physical access, and physical examination.
SIDEBAR
Pre-visit telephone questionnaire and script for a new adult patient with IDD
Introduction
Hello! My name is ______________. I’m a nurse [or medical assistant] from [name of practice]. I understand that [name of patient] is coming to our office for an appointment on [date and time]. I am calling to prepare our health care team to make this first appointment successful for [name of patient] and you.
- How would [name of patient] prefer to be called?
- Who will be accompanying [name of patient] to the appointment? What parts of the appointment will that person remain for?
Describe what to expect, what the patient or caregiver should bring to the appointment, and how long the appointment will last.
- What makes [name of patient] anxious or fearful so that we might avoid doing that? Should we avoid bringing up certain topics? Should we avoid performing any procedures that are customary during a first appointment?
- Does [name of patient] have sensitivities—to light, sound, touch, etc—that we should be aware of?
Offer to have a room ready upon the patient’s arrival if remaining in the waiting area would cause too much anxiety.
- What helps calm [name of patient]? Are there some topics that put [name of patient] at ease?
- How does [name of patient] best communicate?
- Is there anything else the health care team might do to prepare for the appointment?
- Does [name of patient] need personal protective equipment, a wheelchair, oxygen, or other medical equipment upon arrival?
- What would make for a successful first appointment?
- What strategies or techniques have [name of patient’s] providers used in the past that have helped make health care visits successful?
- Is there anything else you want me to know that we haven’t talked about?
- Would it be helpful if I talked with [name of patient] now about their upcoming appointment?
Initial appointments should focus on building trust and rapport with the health care team and desensitizing the patient to the clinical environment.10 Examination techniques used with pediatric patients can be applied to this population: for example, demonstrating an examination maneuver first on the parent or caregiver; beginning the examination with the least invasive or anxiety-provoking components; and stating what you plan to do next—before you do it.
Continue to: Systematic health checks provide great value
Systematic health checks provide great value
A health check is a systematic and comprehensive health assessment that is provided annually to adults with IDD, and includes:
- specific review of signs and symptoms of health conditions that often co-occur in adults with IDD (TABLE 2Calibri11)
- screening for changes in adaptive functioning and secondary disability
- lifestyle counseling
- medication review and counseling
- immunization update
- discussion of caregiver concerns.

Regarding the last point: Many caregivers are the aging parents of the adult patient with IDD—people who have their own emerging health and support needs. You should initiate conversations about advanced planning for the needs of patients, which often involves engaging siblings and other family members to assume a greater role in caregiving.12
Benefits of the health check. A systematic review of 38 studies, comprising more than 5000 patients with IDD, found that health checks increased the detection of serious conditions, improved screening for sensory impairments, and increased the immunization rate.13 Although many patients with IDD generally understand the need for a periodic health examination, you can enhance their experience by better explaining the rationale for the health check; scheduling sufficient time for the appointment, based on the individual clinical situation; and discussing the value of laboratory testing and referrals to specialists.14
Tailoring preventive care
Many of the preventive services recommendations typically utilized by family physicians, such as guidelines from the US Preventive Services Task Force, have been developed for the general population at average risk of conditions of interest.15 Adults with IDD, depending on the cause of their developmental disability and their behavioral risk profile, might be at significantly higher (or lower) risk of cancer, heart disease, or other conditions than the general population. To address these differences, preventive care guidelines tailored to patients with certain developmental disabilities have been created, including guidelines specific to adults with Down syndrome, fragile X syndrome, Prader-Willi syndrome, Smith-Magenis syndrome, and 22q11.2 deletion (DiGeorge) syndrome.16
Clarifying the molecular genetic etiology of many developmental disabilities has led to more precise understandings about physical and behavioral health issues associated with specific developmental disabilities. For that reason, patients without a known cause for their IDD might benefit from referral to a geneticist—even in early or middle adulthood. Variables generally associated with a higher likelihood of an abnormal genetic test result include17:
- a family history of developmental disability
- a congenital malformation or dysmorphic features
- a dual diagnosis of developmental disability and co-occurring mental illness
- hypotonia
- severe or profound IDD.
Continue to: Successful implementation of preventive health screening tests...
Successful implementation of preventive health screening tests often requires ingenuity and the collective creativity of the patient, family members, staff, and family physician to allay fears and anxieties. Examples: Women who have been advised to undergo screening mammography might feel less anxious by undergoing tandem screening with their sister or mother, and colorectal cancer screening might be more easily accomplished using a fecal DNA test rather than by colonoscopy. Procedural desensitization strategies and preventive care instructional materials targeting people with IDD are posted on YouTube (for example, the “DD CARES Best Practices” series [see www.youtube.com/watch?v=EPJy4zvg4io]) and other websites.
Management of chronic disease
Evidence of health disparities in patients with IDD includes suboptimal management of chronic diseases, such as diabetes18 and hypertension,19 despite contact with a primary care physician. Nonadherence to a medication regimen might be more common in patients who live with their family or in a residential setting where there is a lower degree of supervision—that is, compared to a residence that maintains 24-hour staffing with daily nursing care and supervision. For a patient who is not so closely supervised, reviewing the medication refill history with the pharmacy, or using the so-called brown-bag technique of counting pill bottles brought to appointments, can ensure medication adherence.
CASE
As you interview Ms. S, you note that she is shy, avoids eye contact, and appears generally anxious. You calm her by noticing and complimenting her jewelry and fingernail polish. Ms. S smiles and talks about her favorite polish colors.
Her mother reports that, when Ms. S is stressed, she talks to herself alone in her bedroom. However, you do not observe evidence of schizoaffective disorder, and begin to wonder whether she needs to be taking risperidone.
Essentials of mental health care
It is estimated that one-third of adults with IDD have significant mental and behavioral health care needs.20 Patients with IDD suffer the same psychiatric disorders as the general population; some also engage in problematic behaviors, such as self-injurious actions, physical or verbal aggression (or both), property destruction, and resistance to caregiving assistance.
Continue to: Mental and behavioral health problems...
Mental and behavioral health problems can have a profound impact on the quality of life of patients with IDD, their peers, and their family and other caregivers. If untreated, these problems can lead to premature institutionalization, loss of employment or desired program participation, fractured social relationships, and caregiver withdrawal and burnout.
Initial evaluation of suspected mental and behavioral health problems begins with careful assessment for medical conditions that might be causing pain and distress, stereotypies, and other problematic behaviors. Common sources of pain and discomfort include dental and other oral disease, dysphagia, gastroesophageal reflux disease, gastritis, constipation, allergic disease, headache, musculoskeletal pathology, lower urinary tract disease, and gynecologic disorders.11 Identification and optimal treatment of medical conditions might not eliminate problematic behaviors but often decrease their frequency and intensity.
Psychoactive medications are prescribed for many patients with IDD. Many have behavioral adverse effects, such as akathisia, aggression, and disinhibition—leading to a prescribing cascade of psychoactive medication polypharmacy and escalating dosages.21 Antipsychotic medications are often initiated without a careful diagnosis, explicit outcome targets, or adequate clinical monitoring for effectiveness; in addition, they often lead to insulin resistance, metabolic syndrome, and massive weight gain.21 Even a family physician who is not the prescriber can perform an important advocacy role by critically reviewing psychoactive medications, documenting adverse effects, insisting on a clear therapeutic target, and calling for discontinuation of medications that appear to be ineffective.
Evaluation of mental and behavioral health problems requires a developmental perspective to interpret specific, observable behaviors with a proper clinical lens. For example, many patients with IDD engage in self-talk (soliloquizing) as a means of processing the world around them. This practice might escalate during a time of physical or psychological stress, and the unwary clinician might misinterpret this behavior as psychotic, leading to inappropriate prescribing of antipsychotic medication. Other psychotoform behaviors that, superficially, mimic but are typically not truly psychotic, include talk with or about imaginary friends and repetitive retelling of sometimes elaborate or grandiose tales or assertions. The failure of clinicians to recognize developmentally determined expressions of distress often leads to a misdiagnosis of schizophrenia or other psychotic illness and, consequently, inappropriate psychopharmacotherapy.
Family physicians, familiar with the use of psychiatric scales for diagnosis and treatment monitoring, should use similar scales that have been developed specifically for patients with IDD (TABLE 311). In addition, a psychiatric diagnosis manual, the Diagnostic Manual—Intellectual Disability 2, specific to people with IDD (and analogous to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) provides modification of diagnostic criteria to account for patients who have difficulty articulating their internal emotional state and inner thoughts.22

Continue to: Problematic behaviors
Problematic behaviors that are not features of a bona fide psychiatric disorder are often best understood through functional behavioral analysis, which examines antecedents and consequences of problematic behaviors and identifies their predictable outcomes, such as gaining attention, avoiding a task, or securing a desired item. Rather than being given a prescription for psychoactive medication, many adult patients with IDD and problematic behaviors might be best served by having you order consultation with a certified behavior analyst. The analyst will conduct an evaluation and, along with family or residential staff and the patient, craft a behavioral support plan to address core drivers of the undesired behavior. Behavioral support plans might be enriched by multidisciplinary input from a speech and language pathologist, habilitation professionals, occupational and physical therapists, a neuropsychologist, and others.23
Resources to help you address the physical, mental, and behavioral health problems of these patients are available online through Vanderbilt Kennedy Center’s “Toolkit for primary care providers” (https://iddtoolkit.vkcsites.org).
CASE
During your examination, you review Ms. S’s vital signs, including body mass index (BMI). You calculate that she is morbidly obese—BMI, 37—in the setting of a known comorbidity, diabetes.
Ms. S tells you that she is interested in having a healthy lifestyle, but feels frustrated because she does not know how to make the necessary changes. You discuss with her how some medications, including risperidone, can promote weight gain, and that it is important for her mental health provider to carefully reassess whether she needs to continue the drug.
Weight management in a patient population that tends to be sedentary
Patients with IDD are more likely to live a sedentary lifestyle. Compared to adults who do not have IDD, adults with IDD—especially women and patients with Down syndrome—are reported to have a higher prevalence of obesity.24
Continue to: As in the general population...
As in the general population, the greatest success in weight management involves multidisciplinary treatment, including nutritional support, physical activity, behavioral changes, and close follow-up. The importance of such an approach was borne out by the findings of a randomized controlled trial in which a multicomponent intervention—an energy-reduced diet, physical activity, and behavioral sessions—delivered to participants or their caregivers during monthly visits produced clinically meaningful 6-month weight loss.25 Health-promoting behavioral interventions that rely on a dyadic strategy, such as peer health coaches (ie, people with IDD who have been trained as a health coach) or mentors (IDD staff trained as a health coach), might be more successful at changing health behaviors among patients with IDD than traditional office-based, individual patient education and counseling.26
Similarly, undesired weight loss demands careful evaluation and management because such loss can reflect a medically significant condition, such as gastroesophageal reflux, constipation, dysphagia, neglect, and cancer.27
Boosting the amount and effectiveness of physical activity
Young people with IDD participate in physical activity less often than their neurotypical peers; as a result, they tend to be less fit and have a higher prevalence of obesity.28 Based on a meta-analysis, interventions that focus on sport and movement skills training, such as soccer, basketball, and ball-throwing programs, might be more effective than general physical activity programs.28 In addition to year-round sports training and athletic competitions, Special Olympics conducts vital health screenings of athletes and supports community-based initiatives that address bias against patients with IDD, promote inclusion, and foster social relationships (www.specialolympics.org/our-work/inclusive-health?locale=en).
Emphasize regular activity. In adulthood, fewer than 10% of patients with IDD exercise regularly.21 According to the second edition of Physical Activity Guidelines for Americans,29 “all adults, with or without a disability, should get at least 150 minutes of aerobic physical activity a week. Activities can be broken down into smaller amounts, such as about 25 minutes a day every day.”30 Supplementation with muscle-strengthening activities (eg, yoga, weight training, and resistance-band training) provides further health benefit, such as improvement in posture and prevention of future injury.31 An ideal exercise program proposed by Tyler and Baker is based on a daily, “3-2-1” schedule (ie, of every hour of activity, 30 minutes should be of aerobic exercise; 20 minutes, of strength building; and 10 minutes, of flexibility).11 By participating in any type of physical activity, there is potential for considerable health benefit in reducing psychosocial stressors, improving mental health, counteracting metabolic syndromes, and, ultimately, reducing morbidity and mortality related to physical inactivity.
CASE
With permission from Ms. S, you send your progress notes by fax to her mental health provider at the community mental health center and request a call to discuss her case—in particular, to examine potential alternatives to risperidone. With Ms. S’s input, you also co-create an exercise prescription that includes a daily 20-minute walking program with her mother.
At the follow-up visit that is scheduled in 3 months, you anticipate adding a resistance component and balance activity to the exercise prescription to enrich Ms. S’s physical activity regimen.
CORRESPONDENCE
Carl V. Tyler Jr., MD, 14601 Detroit Avenue, Lakewood, OH, 44107; [email protected]
Some adults who have an intellectual or other developmental disability (IDD) require extensive subspecialty care; many, however, depend primarily on their family physician for the bulk of their health care. With that reliance in mind, this article provides (1) an overview of important services that family physicians can provide for their adult patients with IDD and (2) pragmatic clinical suggestions for tailoring that care. Note: We highlight only some high-impact areas of clinical focus; refer to the 2018 Canadian consensus guidelines for a comprehensive approach to optimizing primary care for this population.1
CASE
Laura S, a 24-year-old woman with Down syndrome, is visiting your clinic with her mother to establish care. Ms. S has several medical comorbidities, including type 2 diabetes, hyperlipidemia, repaired congenital heart disease, schizoaffective disorder, and hypothyroidism. She is under the care of multiple specialists, including a cardiologist and an endocrinologist. Her medications include the atypical antipsychotic risperidone, which was prescribed for her through the services of a community mental health center.

Ms. S is due for multiple preventive health screenings. She indicates that she feels nervous today talking about these screenings with a new physician.
First step in care: Proficiency in the lexicon of IDD
Three core concepts of IDD are impairment, disability, and handicap. According to the World Health Organization2:
- impairment “is any loss or abnormality of psychological, physiological, or anatomical structure or function.”
- disability “is any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.”
- handicap therefore “represents socialization of an impairment or disability, and as such it reflects the consequences for the individual—cultural, social, economic, and environmental—that stem from the presence of impairment and disability.”
Essential transition: Pediatric to adult health care
Health care transition (HCT) is the planned process of transferring care from a pediatric to an adult-based health care setting,3 comprising 3 phases:
- preparation
- transfer from pediatric to adult care
- integration into adult-based care.
Two critical components of a smooth HCT include initiating the transition early in adolescence and providing transition-support resources, which are often lacking, even in large, integrated health systems.4 Got Transition, created by the National Alliance to Advance Adolescent Health, outlines core elements of an organized HCT process (www.gottransition.org) specific to young adults with IDD, including young adults with autism spectrum disorder.5,6
Even young people who are served by a family physician and who intend to remain in that family practice as they age into adulthood require HCT services that include6:
- assessment of readiness to transition to adult care
- update of the medical history
- assessment and promotion of self-care skills
- consent discussions and optimized participation in decision-making
- transition of specialty care from pediatric to adult specialists.
Continue to: For an ideal HCT...
For an ideal HCT, full engagement of the patient, the medical home (physicians, nursing staff, and care coordinators), and the patient’s family (including the primary caregiver or guardian) is critical. In addition to preventive care visits and management of chronic disease, additional domains that require explicit attention in transitioning young people with IDD include health insurance, transportation, employment, and postsecondary education.
Young people who have special health care needs and receive high-quality HCT demonstrate improvements in adherence to care, disease-specific measures, quality of life, self-care skills, satisfaction with care, and health care utilization.7TABLE 13 lists resources identified by Berens and colleagues that are helpful in facilitating the transition.

Teach and practice disability etiquette
Societal prejudice harms people with IDD—leading to self-deprecation, alienation from the larger community, and isolation from others with IDD.8 To promote acceptance and inclusivity in residential communities, the workplace, recreational venues, and clinical settings, disability etiquette should be utilized—a set of guidelines on how to interact with patients with IDD. These include speaking to the patient directly, using clear language in an adult voice, and avoiding stereotypes about people with disabilities.9 The entire health care team, including all front-facing staff (receptionists and care and financial coordinators) and clinical staff (physicians, nurses, medical assistants), need to be educated in, and practice, disability etiquette.
Preparing for in-person visits. Pre-visit preparation, ideally by means of dialogue between health care staff and the patient or caregiver (or both), typically by telephone and in advance of the scheduled visit, is often critical for a successful first face-to-face encounter. (See “Pre-visit telephone questionnaire and script for a new adult patient with IDD,” page 287, which we developed for use in our office practice.) Outcomes of the pre-visit preparation should include identifying:
- words or actions that can trigger anxiety or panic
- de-escalation techniques, such as specific calming words and actions
- strategies for optimal communication, physical access, and physical examination.
SIDEBAR
Pre-visit telephone questionnaire and script for a new adult patient with IDD
Introduction
Hello! My name is ______________. I’m a nurse [or medical assistant] from [name of practice]. I understand that [name of patient] is coming to our office for an appointment on [date and time]. I am calling to prepare our health care team to make this first appointment successful for [name of patient] and you.
- How would [name of patient] prefer to be called?
- Who will be accompanying [name of patient] to the appointment? What parts of the appointment will that person remain for?
Describe what to expect, what the patient or caregiver should bring to the appointment, and how long the appointment will last.
- What makes [name of patient] anxious or fearful so that we might avoid doing that? Should we avoid bringing up certain topics? Should we avoid performing any procedures that are customary during a first appointment?
- Does [name of patient] have sensitivities—to light, sound, touch, etc—that we should be aware of?
Offer to have a room ready upon the patient’s arrival if remaining in the waiting area would cause too much anxiety.
- What helps calm [name of patient]? Are there some topics that put [name of patient] at ease?
- How does [name of patient] best communicate?
- Is there anything else the health care team might do to prepare for the appointment?
- Does [name of patient] need personal protective equipment, a wheelchair, oxygen, or other medical equipment upon arrival?
- What would make for a successful first appointment?
- What strategies or techniques have [name of patient’s] providers used in the past that have helped make health care visits successful?
- Is there anything else you want me to know that we haven’t talked about?
- Would it be helpful if I talked with [name of patient] now about their upcoming appointment?
Initial appointments should focus on building trust and rapport with the health care team and desensitizing the patient to the clinical environment.10 Examination techniques used with pediatric patients can be applied to this population: for example, demonstrating an examination maneuver first on the parent or caregiver; beginning the examination with the least invasive or anxiety-provoking components; and stating what you plan to do next—before you do it.
Continue to: Systematic health checks provide great value
Systematic health checks provide great value
A health check is a systematic and comprehensive health assessment that is provided annually to adults with IDD, and includes:
- specific review of signs and symptoms of health conditions that often co-occur in adults with IDD (TABLE 2Calibri11)
- screening for changes in adaptive functioning and secondary disability
- lifestyle counseling
- medication review and counseling
- immunization update
- discussion of caregiver concerns.

Regarding the last point: Many caregivers are the aging parents of the adult patient with IDD—people who have their own emerging health and support needs. You should initiate conversations about advanced planning for the needs of patients, which often involves engaging siblings and other family members to assume a greater role in caregiving.12
Benefits of the health check. A systematic review of 38 studies, comprising more than 5000 patients with IDD, found that health checks increased the detection of serious conditions, improved screening for sensory impairments, and increased the immunization rate.13 Although many patients with IDD generally understand the need for a periodic health examination, you can enhance their experience by better explaining the rationale for the health check; scheduling sufficient time for the appointment, based on the individual clinical situation; and discussing the value of laboratory testing and referrals to specialists.14
Tailoring preventive care
Many of the preventive services recommendations typically utilized by family physicians, such as guidelines from the US Preventive Services Task Force, have been developed for the general population at average risk of conditions of interest.15 Adults with IDD, depending on the cause of their developmental disability and their behavioral risk profile, might be at significantly higher (or lower) risk of cancer, heart disease, or other conditions than the general population. To address these differences, preventive care guidelines tailored to patients with certain developmental disabilities have been created, including guidelines specific to adults with Down syndrome, fragile X syndrome, Prader-Willi syndrome, Smith-Magenis syndrome, and 22q11.2 deletion (DiGeorge) syndrome.16
Clarifying the molecular genetic etiology of many developmental disabilities has led to more precise understandings about physical and behavioral health issues associated with specific developmental disabilities. For that reason, patients without a known cause for their IDD might benefit from referral to a geneticist—even in early or middle adulthood. Variables generally associated with a higher likelihood of an abnormal genetic test result include17:
- a family history of developmental disability
- a congenital malformation or dysmorphic features
- a dual diagnosis of developmental disability and co-occurring mental illness
- hypotonia
- severe or profound IDD.
Continue to: Successful implementation of preventive health screening tests...
Successful implementation of preventive health screening tests often requires ingenuity and the collective creativity of the patient, family members, staff, and family physician to allay fears and anxieties. Examples: Women who have been advised to undergo screening mammography might feel less anxious by undergoing tandem screening with their sister or mother, and colorectal cancer screening might be more easily accomplished using a fecal DNA test rather than by colonoscopy. Procedural desensitization strategies and preventive care instructional materials targeting people with IDD are posted on YouTube (for example, the “DD CARES Best Practices” series [see www.youtube.com/watch?v=EPJy4zvg4io]) and other websites.
Management of chronic disease
Evidence of health disparities in patients with IDD includes suboptimal management of chronic diseases, such as diabetes18 and hypertension,19 despite contact with a primary care physician. Nonadherence to a medication regimen might be more common in patients who live with their family or in a residential setting where there is a lower degree of supervision—that is, compared to a residence that maintains 24-hour staffing with daily nursing care and supervision. For a patient who is not so closely supervised, reviewing the medication refill history with the pharmacy, or using the so-called brown-bag technique of counting pill bottles brought to appointments, can ensure medication adherence.
CASE
As you interview Ms. S, you note that she is shy, avoids eye contact, and appears generally anxious. You calm her by noticing and complimenting her jewelry and fingernail polish. Ms. S smiles and talks about her favorite polish colors.
Her mother reports that, when Ms. S is stressed, she talks to herself alone in her bedroom. However, you do not observe evidence of schizoaffective disorder, and begin to wonder whether she needs to be taking risperidone.
Essentials of mental health care
It is estimated that one-third of adults with IDD have significant mental and behavioral health care needs.20 Patients with IDD suffer the same psychiatric disorders as the general population; some also engage in problematic behaviors, such as self-injurious actions, physical or verbal aggression (or both), property destruction, and resistance to caregiving assistance.
Continue to: Mental and behavioral health problems...
Mental and behavioral health problems can have a profound impact on the quality of life of patients with IDD, their peers, and their family and other caregivers. If untreated, these problems can lead to premature institutionalization, loss of employment or desired program participation, fractured social relationships, and caregiver withdrawal and burnout.
Initial evaluation of suspected mental and behavioral health problems begins with careful assessment for medical conditions that might be causing pain and distress, stereotypies, and other problematic behaviors. Common sources of pain and discomfort include dental and other oral disease, dysphagia, gastroesophageal reflux disease, gastritis, constipation, allergic disease, headache, musculoskeletal pathology, lower urinary tract disease, and gynecologic disorders.11 Identification and optimal treatment of medical conditions might not eliminate problematic behaviors but often decrease their frequency and intensity.
Psychoactive medications are prescribed for many patients with IDD. Many have behavioral adverse effects, such as akathisia, aggression, and disinhibition—leading to a prescribing cascade of psychoactive medication polypharmacy and escalating dosages.21 Antipsychotic medications are often initiated without a careful diagnosis, explicit outcome targets, or adequate clinical monitoring for effectiveness; in addition, they often lead to insulin resistance, metabolic syndrome, and massive weight gain.21 Even a family physician who is not the prescriber can perform an important advocacy role by critically reviewing psychoactive medications, documenting adverse effects, insisting on a clear therapeutic target, and calling for discontinuation of medications that appear to be ineffective.
Evaluation of mental and behavioral health problems requires a developmental perspective to interpret specific, observable behaviors with a proper clinical lens. For example, many patients with IDD engage in self-talk (soliloquizing) as a means of processing the world around them. This practice might escalate during a time of physical or psychological stress, and the unwary clinician might misinterpret this behavior as psychotic, leading to inappropriate prescribing of antipsychotic medication. Other psychotoform behaviors that, superficially, mimic but are typically not truly psychotic, include talk with or about imaginary friends and repetitive retelling of sometimes elaborate or grandiose tales or assertions. The failure of clinicians to recognize developmentally determined expressions of distress often leads to a misdiagnosis of schizophrenia or other psychotic illness and, consequently, inappropriate psychopharmacotherapy.
Family physicians, familiar with the use of psychiatric scales for diagnosis and treatment monitoring, should use similar scales that have been developed specifically for patients with IDD (TABLE 311). In addition, a psychiatric diagnosis manual, the Diagnostic Manual—Intellectual Disability 2, specific to people with IDD (and analogous to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) provides modification of diagnostic criteria to account for patients who have difficulty articulating their internal emotional state and inner thoughts.22

Continue to: Problematic behaviors
Problematic behaviors that are not features of a bona fide psychiatric disorder are often best understood through functional behavioral analysis, which examines antecedents and consequences of problematic behaviors and identifies their predictable outcomes, such as gaining attention, avoiding a task, or securing a desired item. Rather than being given a prescription for psychoactive medication, many adult patients with IDD and problematic behaviors might be best served by having you order consultation with a certified behavior analyst. The analyst will conduct an evaluation and, along with family or residential staff and the patient, craft a behavioral support plan to address core drivers of the undesired behavior. Behavioral support plans might be enriched by multidisciplinary input from a speech and language pathologist, habilitation professionals, occupational and physical therapists, a neuropsychologist, and others.23
Resources to help you address the physical, mental, and behavioral health problems of these patients are available online through Vanderbilt Kennedy Center’s “Toolkit for primary care providers” (https://iddtoolkit.vkcsites.org).
CASE
During your examination, you review Ms. S’s vital signs, including body mass index (BMI). You calculate that she is morbidly obese—BMI, 37—in the setting of a known comorbidity, diabetes.
Ms. S tells you that she is interested in having a healthy lifestyle, but feels frustrated because she does not know how to make the necessary changes. You discuss with her how some medications, including risperidone, can promote weight gain, and that it is important for her mental health provider to carefully reassess whether she needs to continue the drug.
Weight management in a patient population that tends to be sedentary
Patients with IDD are more likely to live a sedentary lifestyle. Compared to adults who do not have IDD, adults with IDD—especially women and patients with Down syndrome—are reported to have a higher prevalence of obesity.24
Continue to: As in the general population...
As in the general population, the greatest success in weight management involves multidisciplinary treatment, including nutritional support, physical activity, behavioral changes, and close follow-up. The importance of such an approach was borne out by the findings of a randomized controlled trial in which a multicomponent intervention—an energy-reduced diet, physical activity, and behavioral sessions—delivered to participants or their caregivers during monthly visits produced clinically meaningful 6-month weight loss.25 Health-promoting behavioral interventions that rely on a dyadic strategy, such as peer health coaches (ie, people with IDD who have been trained as a health coach) or mentors (IDD staff trained as a health coach), might be more successful at changing health behaviors among patients with IDD than traditional office-based, individual patient education and counseling.26
Similarly, undesired weight loss demands careful evaluation and management because such loss can reflect a medically significant condition, such as gastroesophageal reflux, constipation, dysphagia, neglect, and cancer.27
Boosting the amount and effectiveness of physical activity
Young people with IDD participate in physical activity less often than their neurotypical peers; as a result, they tend to be less fit and have a higher prevalence of obesity.28 Based on a meta-analysis, interventions that focus on sport and movement skills training, such as soccer, basketball, and ball-throwing programs, might be more effective than general physical activity programs.28 In addition to year-round sports training and athletic competitions, Special Olympics conducts vital health screenings of athletes and supports community-based initiatives that address bias against patients with IDD, promote inclusion, and foster social relationships (www.specialolympics.org/our-work/inclusive-health?locale=en).
Emphasize regular activity. In adulthood, fewer than 10% of patients with IDD exercise regularly.21 According to the second edition of Physical Activity Guidelines for Americans,29 “all adults, with or without a disability, should get at least 150 minutes of aerobic physical activity a week. Activities can be broken down into smaller amounts, such as about 25 minutes a day every day.”30 Supplementation with muscle-strengthening activities (eg, yoga, weight training, and resistance-band training) provides further health benefit, such as improvement in posture and prevention of future injury.31 An ideal exercise program proposed by Tyler and Baker is based on a daily, “3-2-1” schedule (ie, of every hour of activity, 30 minutes should be of aerobic exercise; 20 minutes, of strength building; and 10 minutes, of flexibility).11 By participating in any type of physical activity, there is potential for considerable health benefit in reducing psychosocial stressors, improving mental health, counteracting metabolic syndromes, and, ultimately, reducing morbidity and mortality related to physical inactivity.
CASE
With permission from Ms. S, you send your progress notes by fax to her mental health provider at the community mental health center and request a call to discuss her case—in particular, to examine potential alternatives to risperidone. With Ms. S’s input, you also co-create an exercise prescription that includes a daily 20-minute walking program with her mother.
At the follow-up visit that is scheduled in 3 months, you anticipate adding a resistance component and balance activity to the exercise prescription to enrich Ms. S’s physical activity regimen.
CORRESPONDENCE
Carl V. Tyler Jr., MD, 14601 Detroit Avenue, Lakewood, OH, 44107; [email protected]
1. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician. 2018;64:254-279.
2. World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. May 1980. Accessed May 27, 2021. https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y
3. Berens J, Wozow C, Peacock C. Transition to adult care. Phys Med Rehabil Clin N Am. 2020;31:159-170. doi:10.1016/j.pmr.2019.09.004
4. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group; Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182-200. doi:10.1542/peds.2011-0969
5. Dressler PB, Nguyen TK, Moody EJ, et al. Use of transition resources by primary care providers for youth with intellectual and developmental disabilities. Intellect Dev Disabil. 2018;56:56-68. doi:10.1352/1934-9556-56.1.56
6. The National Alliance to Advance Adolescent Health. Six Core Elements of Health Care Transition.™ Got Transition website. Accessed May 27, 2021. www.gottransition.org
7. Schmidt A, Ilango SM, McManus MA, et al. Outcomes of pediatric to adult health care transition interventions: an updated systematic review. J Pediatr Nurs. 2020; 51:92-107. doi: 10.1016/j.pedn.2020.01.002
8. Keith JM, Bennetto L, Rogge RD. The relationship between contact and attitudes: reducing prejudice toward individuals with intellectual and developmental disabilities. Res Dev Disabil. 2015;47:14-26. doi:10.1016/j.ridd.2015.07.032
9. United Spinal Association. Disability Etiquette: Tips on Interacting With People With Disabilities. 2015. Accessed June 9, 2021. www.unitedspinal.org/pdf/DisabilityEtiquette.pdf
10. Nathawad R, Hanks C. Optimizing the office visit for adolescents with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:182-189. doi:10.1016/j.cppeds.2017.07.002
11. Tyler CV, Baker S. Intellectual Disabilities at Your Fingertips: A Health Care Resource. High Tide Press; 2009.
12. Williamson HJ, Perkins EA. Family caregivers of adults with intellectual and developmental disabilities: outcomes associated with U.S. services and supports. Intellect Dev Disabil. 2014;52:147-159. doi: 10.1352/1934-9556-52.2.147
13. Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil. 2014;35:2450-2462. doi:10.1016/j.ridd.2014.06.007
14. Perry J, Felce D, Kerr M, et al. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil. 2014;27:200-211. doi: 10.1111/jar.12072
15. Recommendation topics. United States Preventive Services Task Force website. 2020. Accessed May 27, 2021. www.uspreventiveservicestaskforce.org
16. Developmental Disabilities Primary Care Initiative. Tools for the Primary Care of People with Developmental Disabilities. 1st ed. MUMS Guideline Clearinghouse; 2011.
17. Jang W, Kim Y, Han E, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019;39:299-310. doi:10.3343/alm.2019.39.3.299
18. Shireman TI, Reichard A, Nazir N, et al. Quality of diabetes care for adults with developmental disabilities. Disabil Health J. 2010;3:179-185. doi:10.1016/j.dhjo.2009.10.004
19. Cyrus AC, Royer J, Carroll DD, et al. Anti-hypertensive medication use and actors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2019;124:248-262. doi:10.1352/1944-7558-124.3.248
20. IDD/MI diagnosis. National Association for the Dually Diagnosed (NADD) website. 2019. Accessed May 27, 2021. https://thenadd.org/idd-mi-diagnosis
21. Matson JL, Mayville EA, Bielecki J, et al. Reliability of the Matson Evaluation of Drug Side Effects Scale (MEDS). Res Dev Disabil. 1998;19:501-506. doi:10.1016/s0891-4222(98)00021-3
22. Fletcher R, Barnhill J, Cooper SA. (2017). Diagnostic Manual-Intellectual Disability: A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. 2nd ed. National Association for the Dually Diagnosed (NADD); 2017.
23. Marrus N, Hall L. Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am. 2017;26:539-554. doi:10.1016/j.chc.2017.03.001
24. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12;22-7. doi: 10.1002/mrdd.20091
25. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(suppl 1):82-96. doi:10.1111/jar.12348
26. Marks B, Sisirak J, Magallanes R, et al. Effectiveness of a HealthMessages peer-to-peer program for people with intellectual and developmental disabilities. Intellect Dev Disabil. 2019;57:242-258. doi:10.1352/1934-9556-57.3.242
27. Escudé C. Clinical Pearls in IDD Health care. HRS, Inc; 2020.
28. Kapsal NJ, Dicke T, Morin AJS, et al. Effects of physical activity on the physical and psychosocial health of youth with intellectual disabilities: a systematic review and meta-analysis. J Phys Act Health. 2019;16:1187-1195. doi:10.1123/jpah.2018-0675
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. Accessed May 29, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Physical activity for people with disability. September 2020. Accessed May 27, 2021. www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html
31. Introduction to strengthening exercises. National Center on Health, Physical Activity and Disability (NCHPAD). 2020. Accessed May 27, 2021. www.nchpad.org/374/2096/Strengthening~Exercises
1. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician. 2018;64:254-279.
2. World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. May 1980. Accessed May 27, 2021. https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y
3. Berens J, Wozow C, Peacock C. Transition to adult care. Phys Med Rehabil Clin N Am. 2020;31:159-170. doi:10.1016/j.pmr.2019.09.004
4. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group; Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182-200. doi:10.1542/peds.2011-0969
5. Dressler PB, Nguyen TK, Moody EJ, et al. Use of transition resources by primary care providers for youth with intellectual and developmental disabilities. Intellect Dev Disabil. 2018;56:56-68. doi:10.1352/1934-9556-56.1.56
6. The National Alliance to Advance Adolescent Health. Six Core Elements of Health Care Transition.™ Got Transition website. Accessed May 27, 2021. www.gottransition.org
7. Schmidt A, Ilango SM, McManus MA, et al. Outcomes of pediatric to adult health care transition interventions: an updated systematic review. J Pediatr Nurs. 2020; 51:92-107. doi: 10.1016/j.pedn.2020.01.002
8. Keith JM, Bennetto L, Rogge RD. The relationship between contact and attitudes: reducing prejudice toward individuals with intellectual and developmental disabilities. Res Dev Disabil. 2015;47:14-26. doi:10.1016/j.ridd.2015.07.032
9. United Spinal Association. Disability Etiquette: Tips on Interacting With People With Disabilities. 2015. Accessed June 9, 2021. www.unitedspinal.org/pdf/DisabilityEtiquette.pdf
10. Nathawad R, Hanks C. Optimizing the office visit for adolescents with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:182-189. doi:10.1016/j.cppeds.2017.07.002
11. Tyler CV, Baker S. Intellectual Disabilities at Your Fingertips: A Health Care Resource. High Tide Press; 2009.
12. Williamson HJ, Perkins EA. Family caregivers of adults with intellectual and developmental disabilities: outcomes associated with U.S. services and supports. Intellect Dev Disabil. 2014;52:147-159. doi: 10.1352/1934-9556-52.2.147
13. Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil. 2014;35:2450-2462. doi:10.1016/j.ridd.2014.06.007
14. Perry J, Felce D, Kerr M, et al. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil. 2014;27:200-211. doi: 10.1111/jar.12072
15. Recommendation topics. United States Preventive Services Task Force website. 2020. Accessed May 27, 2021. www.uspreventiveservicestaskforce.org
16. Developmental Disabilities Primary Care Initiative. Tools for the Primary Care of People with Developmental Disabilities. 1st ed. MUMS Guideline Clearinghouse; 2011.
17. Jang W, Kim Y, Han E, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019;39:299-310. doi:10.3343/alm.2019.39.3.299
18. Shireman TI, Reichard A, Nazir N, et al. Quality of diabetes care for adults with developmental disabilities. Disabil Health J. 2010;3:179-185. doi:10.1016/j.dhjo.2009.10.004
19. Cyrus AC, Royer J, Carroll DD, et al. Anti-hypertensive medication use and actors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2019;124:248-262. doi:10.1352/1944-7558-124.3.248
20. IDD/MI diagnosis. National Association for the Dually Diagnosed (NADD) website. 2019. Accessed May 27, 2021. https://thenadd.org/idd-mi-diagnosis
21. Matson JL, Mayville EA, Bielecki J, et al. Reliability of the Matson Evaluation of Drug Side Effects Scale (MEDS). Res Dev Disabil. 1998;19:501-506. doi:10.1016/s0891-4222(98)00021-3
22. Fletcher R, Barnhill J, Cooper SA. (2017). Diagnostic Manual-Intellectual Disability: A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. 2nd ed. National Association for the Dually Diagnosed (NADD); 2017.
23. Marrus N, Hall L. Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am. 2017;26:539-554. doi:10.1016/j.chc.2017.03.001
24. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12;22-7. doi: 10.1002/mrdd.20091
25. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(suppl 1):82-96. doi:10.1111/jar.12348
26. Marks B, Sisirak J, Magallanes R, et al. Effectiveness of a HealthMessages peer-to-peer program for people with intellectual and developmental disabilities. Intellect Dev Disabil. 2019;57:242-258. doi:10.1352/1934-9556-57.3.242
27. Escudé C. Clinical Pearls in IDD Health care. HRS, Inc; 2020.
28. Kapsal NJ, Dicke T, Morin AJS, et al. Effects of physical activity on the physical and psychosocial health of youth with intellectual disabilities: a systematic review and meta-analysis. J Phys Act Health. 2019;16:1187-1195. doi:10.1123/jpah.2018-0675
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. Accessed May 29, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Physical activity for people with disability. September 2020. Accessed May 27, 2021. www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html
31. Introduction to strengthening exercises. National Center on Health, Physical Activity and Disability (NCHPAD). 2020. Accessed May 27, 2021. www.nchpad.org/374/2096/Strengthening~Exercises
PRACTICE RECOMMENDATIONS
› Provide young people who have an intellectual or other developmental disability (IDD) with a defined, explicit process for making the transition into the adult health care system. A
› Conduct an annual comprehensive, systematic health assessment for patients who have IDD to improve detection of serious conditions and sensory impairments. A
› Encourage young people and adults with IDD to participate in regular physical activity to reduce psychosocial stressors and counteract metabolic syndromes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Osteoporosis management: Use a goal-oriented, individualized approach
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10
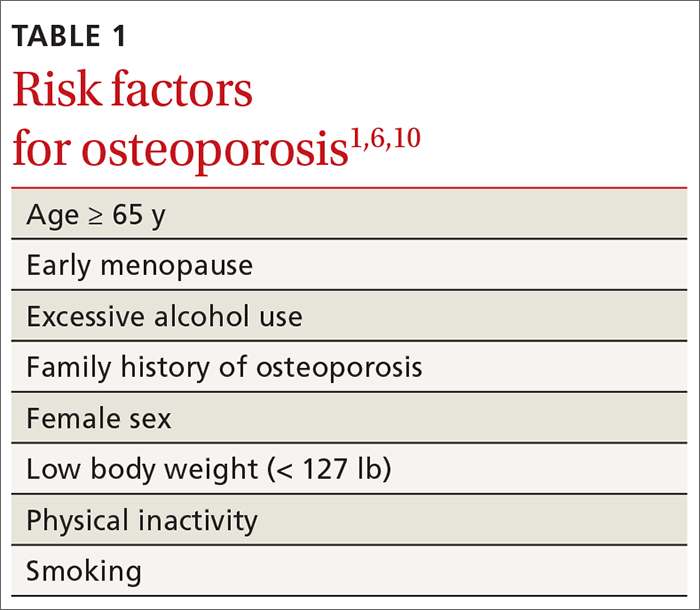
Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)

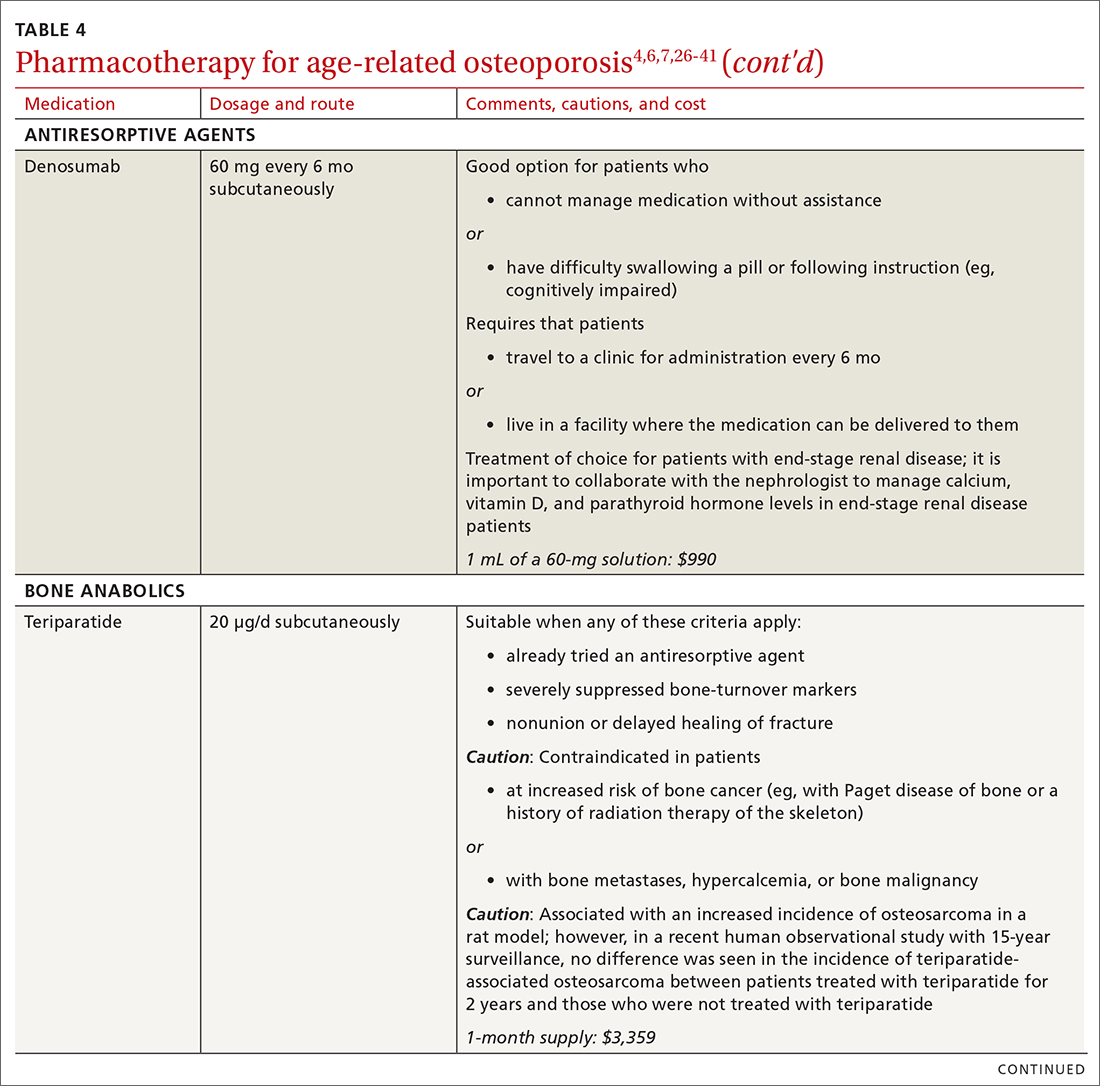
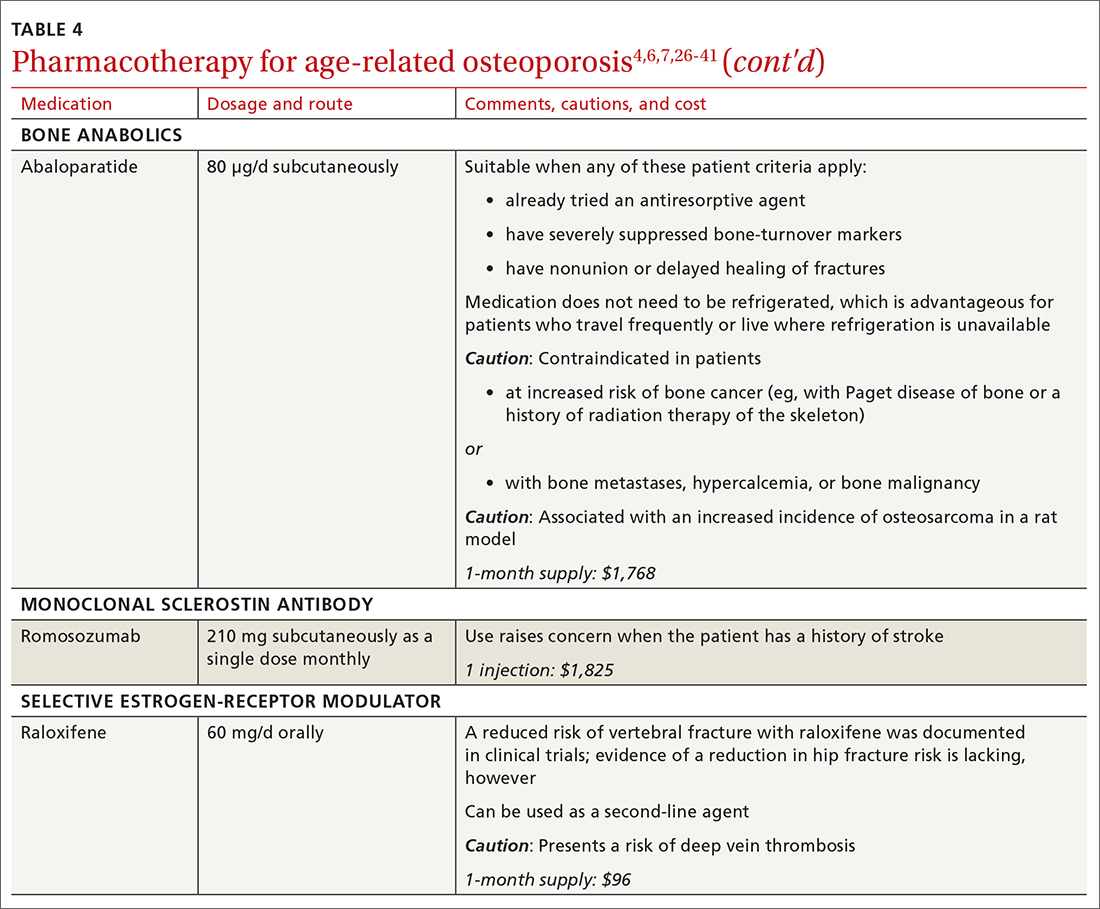
❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10

Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)



❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10

Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)



❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
PRACTICE RECOMMENDATIONS
❯ Consider screening for osteoporosis, using bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA), in all postmenopausal women ≥ 65 years and in women < 65 years at high risk of osteoporosis.
❯ Consider screening in men ≥ 70 years and in younger men at high risk of fracture.
❯ Use the trabecular bone score with DXA BMD to screen patients at high risk of fracture who have a normal BMD—eg, patients with type 2 diabetes or ankylosing spondylitis.
❯ Offer individualized pharmacotherapy to older patients with a diagnosis of osteoporosis and to those at high risk of fracture.
A guide to the Tx of cellulitis and other soft-tissue infections
Skin and soft-tissue infections, frequently encountered in primary care, range from the uncomplicated erysipelas to the life-threatening necrotizing fasciitis. This review draws from the latest evidence and guidelines to help guide the care you provide to patients with cellulitis, orbital cellulitis, erysipelas, folliculitis, furuncles, carbuncles, abscesses, and necrotizing fasciitis.
Cellulitis
Cellulitis, an infection of the deep dermal and subcutaneous layers of the skin, has become increasingly common in recent years, with both incidence and hospitalization rates rising.1 Cellulitis occurs when pathogens enter the dermis through breaks in the skin barrier due to cutaneous fungal infections, trauma, pressure sores, venous stasis, or inflammation. The diagnosis is often made clinically based on characteristic skin findings—classically an acute, poorly demarcated area of erythema, warmth, swelling, and tenderness. Lymphangitic streaking and local lymphadenopathy may also be present. Infection often occurs on an extremity (although it can be found on other areas of the body) and is usually unilateral. Fever may or may not be present.2
Likely responsible microorganisms. Staphylococcus aureus and Group A streptococci (often Streptococcus pyogenes) are common culprits. One systematic review that examined cultures taken of intact skin in cellulitis patients found S aureus to be about twice as common as S pyogenes, with both bacteria accounting for a little more than 70% of cases. Of the remaining positive cultures, the most common organisms were alpha-hemolytic streptococcus, group B streptococcus, Pseudomonas aeruginosa, Clostridium perfringens, Escherichia coli, Pasteurella multocida, and Proteus mirabilis.3 Similarly, a systematic review of bacteremia in patients with cellulitis and erysipelas found that S pyogenes, other beta-hemolytic strep, and S aureus account for about 70% of cases (although S aureus was responsible for just 14%), with the remainder of cases caused by gram-negative organisms such as E coli and P aeruginosa.4
Treatment considerations. Strict treatment guidelines for cellulitis are lacking, but general consensus encourages the use of antibiotics and occasionally surgery. For mild and moderate cases of cellulitis, prescribe oral and parenteral antibiotics to cover for streptococci and methicillin-susceptible S aureus, respectively. Expand coverage to include vancomycin if nasal colonization shows methicillin-resistant S aureus (MRSA) or if you otherwise suspect prior MRSA exposure. Expanded coverage will also be needed if there is severe nonpurulent infection associated with penetrating trauma or a history of intravenous drug use, or the patient meets criteria for systemic inflammatory response syndrome. If patients are severely compromised (eg, neutropenic), it is reasonable to further add broad-spectrum coverage (eg, intravenous piperacillin-tazobactam or carbapenem). Typical duration of treatment is 5 to 7 days, although this should be extended if there is no clinical improvement.
Generally, cellulitis can be managed in the outpatient setting, although hospitalization is recommended if there are concerns for deep or necrotizing infection, if patients are nonadherent to therapy or are immunocompromised, or if outpatient therapy has failed.5 Furthermore, in an observational study of 606 adult patients, prior episodes of cellulitis, venous insufficiency, and immunosuppression were all independently associated with poorer clinical outcomes.2 Also treat underlying predisposing factors such as edema, obesity, eczema, venous insufficiency, and toe web abnormalities such as fissures, scaling, or maceration.5 Consider the use of prophylactic antibiotics for patients who have had 3 to 4 episodes of cellulitis despite attempts to treat predisposing conditions. Prophylactic antibiotic regimens include penicillin or erythromycin orally and penicillin G benzathine intramuscularly.5 Antibiotic regimens are summarized in the TABLE.5
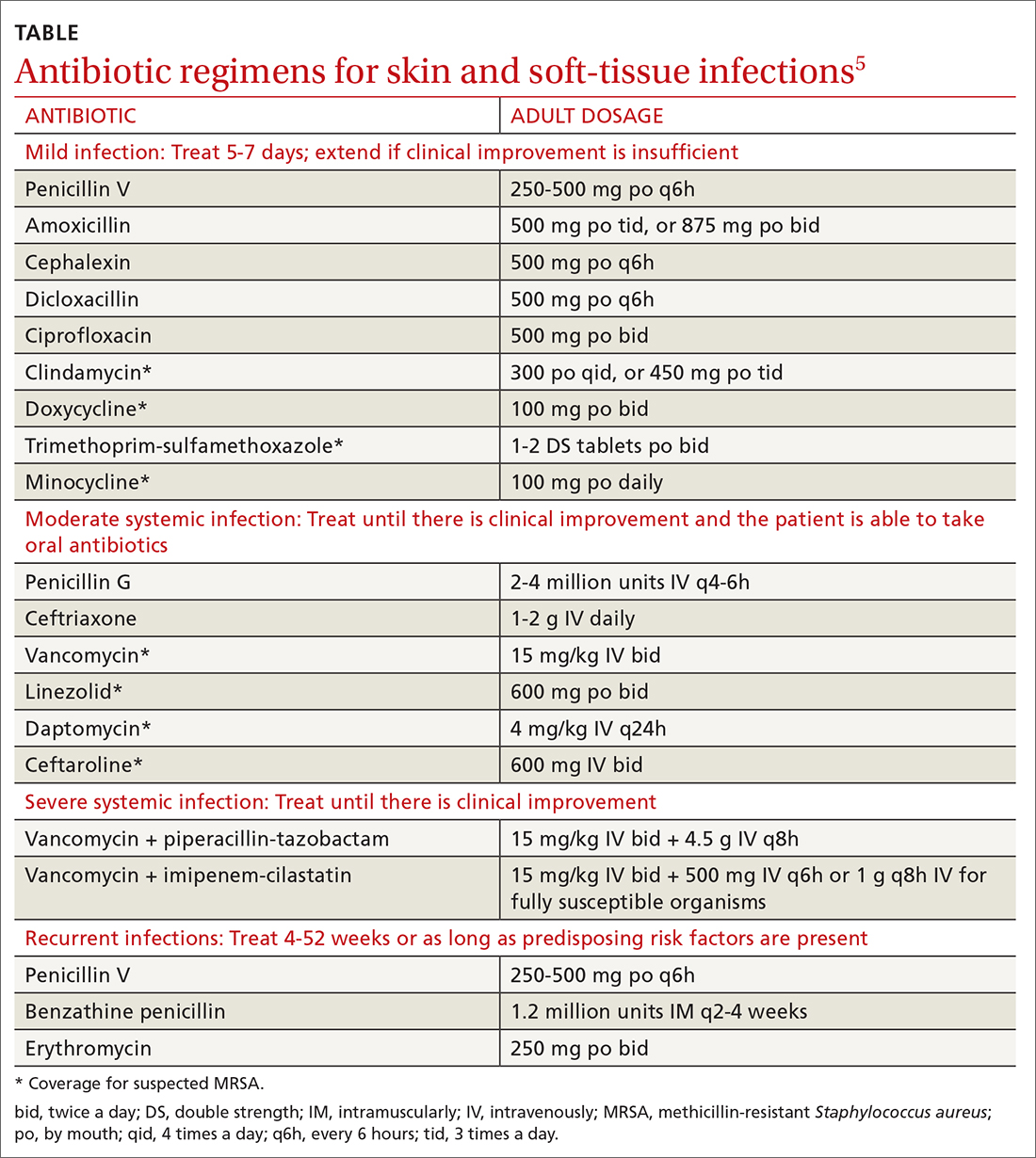
Orbital cellulitis
Orbital cellulitis is an infection of the tissues posterior to the orbital septum.6,7 Periorbital, or preseptal, cellulitis occurs anterior to the orbital septum and is the more common of the 2 infections—84% compared with 16% for orbital cellulitis.6 However, orbital cellulitis, which affects mainly children at a median age of 7 years,6 must be detected and treated early due to the potential for serious complications such as cavernous sinus thrombosis, meningitis, intracranial abscess, and vision loss.7 Chemosis (conjunctival edema) and diplopia are more commonly associated with orbital cellulitis and are seldom seen with preseptal cellulitis.
Predominant causative organisms are S pneumoniae, Moraxella catarrhalis, non-typeable Haemophilus influenzae, and group A streptococcus. The most common mechanism of infection is tracking from periorbital structures (eg, paranasal and ethmoid sinusitis). Other causes include orbital trauma/fracture, periorbital surgery, and bacterial endocarditis. Clinically, patients present with limited ocular motility and proptosis associated with inflamed conjunctiva, orbital pain, headache, malaise, fever, eyelid edema, and possible decrease in visual acuity. The diagnosis is often made clinically and confirmed with orbital computed tomography (CT) with contrast, which can assist in ruling out intracranial involvement such as abscess.
Continue to: Antibiotic therapy
Antibiotic therapy, generally administered intravenously, is recommended for at least 3 days or until orbital symptoms begin to resolve. Choose antibiotics effective against sinusitis-related pathogens (eg, S pneumoniae, H influenzae, M catarrhalis), S aureus, and anaerobes.8 For instance, a regimen may include vancomycin for MRSA coverage, a third-generation cephalosporin, or metronidazole for anaerobic coverage if there is concern about intracranial involvement. Surgical intervention is often reserved for patients with inadequate response to antibiotic therapy, necessitating biopsy for pathogen identification, as well as drainage of large abscesses refractory to antibiotics.
Erysipelas
Erysipelas, a related yet distinct form of cellulitis, is a bacterial infection of the superficial dermis and hypodermis and is commonly caused by group A streptococcus.5,9 Other less common organisms include S aureus, P aeruginosa, and enterobacteria. Erysipelas predominantly affects the lower extremities unilaterally (~90%); the arms and the face are the next most common locations. In addition to the rapid onset of well-demarcated erythema, pain, and swelling, patients may have fever and regional lymphadenopathy. Risk factors include portal of entry (eg, tinea pedis, ulceration), lymphedema, and diabetes. Complications of erysipelas include bullae from edema, abscess formation, and, rarely, bacteremia.
Antibiotic treatment regimens include penicillin G, macrolides (reserved for those with penicillin allergies), fluoroquinolones, and cephalosporins, with duration of treatment ranging from 10 to 14 days depending on infection severity. Fever, pain, and erythema generally improve within 48 to 72 hours of antibiotic therapy. If there is no improvement, consider alternative diagnoses, such as necrotizing fasciitis. Recurrence rates following the initial episode of erysipelas are estimated at 10% of patients at 6 months and 30% at 3 years.10
Folliculitis
Inflammation of hair follicles is characterized by superficial inflammation with the development of perifollicular papules or pustules on an erythematous base.11,12 Folliculitis most commonly affects the face, scalp, thighs, buttocks, axillae, and inguinal areas.13 It may be caused by infection, an inflammatory reaction, or physical injury. Diagnosis is typically based on the patient’s history and physical examination.
Bacteria are the most common cause of infection, although fungi, viruses, and other entities can cause folliculitis. S aureus (methicillin sensitive or methicillin resistant) is the most common pathogen; in the past, superficial pustular folliculitis attributed to S aureus was referred to as Bockhart impetigo. Folliculitis secondary to P aeruginosa, often seen after exposure to contaminated water or hot tubs, is frequently referred to as “hot tub folliculitis.” Malassezia, a reported cause of fungal folliculitis, tends to occur in adolescents of either sex and men with high sebum production, is common in tropical climates, and can be associated with HIV or immunosuppression.11,12,14
Continue to: Differential diagnosis...
Differential diagnosis of folliculitis includes pseudofolliculitis barbae, eosinophilic folliculitis, keratosis pilaris, acne vulgaris, candidiasis, contact dermatitis, impetigo, and miliaria.13 Pseudofolliculitis barbae is an inflammatory reaction to shaving, more commonly seen in darkly pigmented skin. Pseudofolliculitis develops when the hair shaft penetrates the wall of the follicle or directly enters the epidermis.
Initial treatment for mild disease includes the elimination of predisposing factors such as occlusion, moisture, and abrasion. The area should be kept clean and dry, avoiding friction. For localized disease, prescribe topical clindamycin, mupirocin ointment, or benzoyl peroxide. If symptoms fail to respond, prescribe a 7-day course of antibiotic that targets methicillin-sensitive S aureus—eg, cephalexin or dicloxacillin. Also consider doxycycline, which has anti-inflammatory effects and is effective against MRSA. For refractory lesions, trimethoprim-sulfamethoxazole, clindamycin, or minocycline may be useful. If you suspect pseudomonas, consider giving ciprofloxacin for 10 to 14 days for persistent lesions or if the patient is immunocompromised.13,15 Consider obtaining bacterial, fungal, or viral cultures for lesions that fail to respond to initial treatment.
Furuncles/carbuncles/abscesses
A furuncle, commonly referred to as a boil, is an infected hair follicle that becomes enclosed, creating a collection of pus. A carbuncle is a collection of furuncles that converge and drain through a single opening. An abscess is a localized collection of pus arising from within the dermis that can extend within deeper tissues.5 Furuncles, carbuncles, and abscesses are managed similarly with drainage and consideration for MRSA risk factors.
S aureus is the most common cause of these infections; 59% of skin abscesses are due to community-acquired MRSA.16 Anaerobes may contribute to the polymicrobial flora of skin abscesses.17 Risk factors for MRSA infection include a history of previous MRSA infection, diabetes, dialysis or renal failure, placement of an indwelling catheter or medical device, injection drug use, incarceration, close contact with a person with known MRSA infection or colonization, long-term care residence, hospitalization or surgery within the past 12 months, and high prevalence of MRSA in the community.5
Ultrasound improves diagnostic accuracy. One study showed that when a clinical exam alone was inconclusive in evaluating skin and soft-tissue infections in children and adolescents, an ultrasound-assisted examination improved diagnostic accuracy.18 Sensitivity of the clinical examination was 43.7%, compared with 77.6% for the clinical examination plus ultrasound.18
Continue to: Incision and drainage first
Incision and drainage first. Ultrasound-guided needle aspiration, however, has not improved treatment efficacy compared with incision and drainage,19 the mainstay approach for abscesses.17 The procedure to drain a furuncle, carbuncle, or abscess should include the expression of all purulent material and the removal of all loculations if possible. Wound culture is recommended during incision and drainage per current guidelines.5 Simple dry dressings are convenient and effective, although some wounds may require packing. Tap water (that is potable) is suitable for wound cleansing. However, there is no strong evidence that irrigating wounds increases healing or reduces infection.20
Routine use of antibiotics is not recommended for simple cutaneous abscesses.5,17,21 Evidence has been conflicting regarding empiric antibiotic coverage of MRSA following incision and drainage.22-25 Guidelines recommend considering the initiation of antibiotics if there are multiple abscesses, gangrene, surrounding cellulitis, or systemic signs of infection, or if the host is immunocompromised.5
If MRSA is suspected, recommended antibiotic coverage includes trimethoprim-sulfamethoxazole, clindamycin, doxycycline, or minocycline.5 If MRSA is identified, treatment options include dicloxacillin or cephalexin. For severe infections persisting after incision and drainage, in addition to oral antibiotic therapy, consider intravenous antibiotic options for MRSA: cefazolin, clindamycin, linezolid, nafcillin, telavancin, or vancomycin.5
Necrotizing fasciitis
Necrotizing fasciitis is a rare but potentially deadly infection of the skin and soft tissue. It progresses rapidly and spreads along fascial planes, leading to the necrosis of the superficial fascia. The infection often is more extensive than is indicated by superficial signs. Prompt diagnosis is imperative as necrotizing fasciitis is a surgical emergency.5,26 In the United States, 500 to 1500 cases of necrotizing fasciitis occur each year.27 Risk factors for necrotizing fasciitis include diabetes, peripheral vascular disease, malignancy, obesity, cirrhosis, renal failure, injection drug use, chronic corticosteroid therapy, alcohol abuse, malnutrition, and iatrogenic immunosuppression.26,28
Necrotizing fasciitis may be polymicrobial or monomicrobial. Polymicrobial infection, also referred to as type I, is often due to multiple bacteria that originate from the bowel flora, typically including a mix of anaerobic and aerobic organisms. On average, there can be 5 infecting organisms identified per wound, although in some cases up to 15 organisms have been identified in a single wound.5 Type I infection is often associated with tissue injury, abscess, or abdominal surgery. The majority of cases of necrotizing fasciitis are polymicrobial.27,28
Continue to: Monomicrobial infection...
Monomicrobial infection, also referred to as type II, is often due to group A streptococcus, S aureus, vibrio spp, Aeromonas hyrophilio, or an anaerobic streptococci like peptostreptococcus spp. Typically monomicrobial infections, which account for 20% to 30% of cases of necrotizing fasciitis, are community acquired.5,26,29,30
Clinical presentation. In the early stages of disease, patients commonly complain of flu-like symptoms and extreme pain that is out of proportion to findings on the exam. Additional warning signs include fevers and other symptoms of toxicity such as tachycardia, hypotension, nausea, vomiting, and diarrhea. Later in the course, symptoms may localize to the affected area and include erythema, tense swelling, development of blisters or bullae, blackish blue discoloration of the skin, severe pain, and loss of sensation. In some cases involving gas-forming bacteria, tissue crepitus may be noted on exam.5,27-31
Rely on clinical judgment to hasten surgical intervention. Laboratory or imaging findings may augment clinical judgment. But if you suspect necrotizing fasciitis, obtaining blood tests and imaging should not delay surgery. Blood tests that may aid in the diagnosis of necrotizing fasciitis include a complete blood count with differential; coagulation studies; a comprehensive metabolic panel; assays of lactate, C-reactive protein (CRP), and creatinine kinase; and blood cultures. Most often, patients with necrotizing fasciitis will have leukocytosis or leukopenia, evidence of hemolysis, thrombocytopenia, acute renal failure, and significantly elevated CRP.
On any imaging modality, indications of necrotizing fasciitis are inflammatory infiltration of the deep fascia on the affected side that is absent on the contralateral side, and the presence of subcutaneous air (which is a specific but rare finding). Imaging modalities may include CT or magnetic resonance imaging. A definitive diagnosis can only be made with surgical exploration of the involved area. Definitive microbiologic diagnosis will require culture of organisms from affected tissue or blood.5,26,30,31
First address any hemodynamic instability (hypotension is frequently encountered), followed by urgent surgical exploration, debridement of the wound, and antimicrobial therapy. Antibiotic treatment should align with probable pathogens and treatment should be continued until repeated surgical debridement is no longer necessary, clinical improvement is evident, and 48 to 72 hours have passed since defervescence. A reasonable initial empiric regimen in adults would include an agent that is effective against group A streptococcus, gram-negative pathogens, and anaerobes, such as a carbapenem or a beta-lactam-beta-lactamase inhibitor such as piperacillin-tazobactam. Additionally, include an agent that targets MRSA, such as vancomycin, linezolid, or clindamycin.5
CORRESPONDENCE
Karl T. Clebak, MD, Department of Family and Community Medicine Residency Program, Penn State Health M.S. Hershey Medical Center, 500 University Drive, H154/C1613, Hershey, PA 17033; [email protected]
1. Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
2. Collazos J, de la Fuente B, García A, et al. Cellulitis in adult patients: a large, multicenter, observational, prospective study of 606 episodes and analysis of the factors related to the response to treatment. PLoS One. 2018;13:e0204036.
3. Chira S, Miller LG. Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138:313-317.
4. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64:148-155.
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
6. Jain A, Rubin PA. Orbital cellulitis in children. Int Ophthalmol Clin. 2001;41:71-86.
7. Seltz LB, Smith J, Durairaj VD, et al. Microbiology and antibiotic management of orbital cellulitis. Pediatrics. 2011;127:e566-e572.
8. Nageswaran S, Woods CR, Benjamin DK, et al. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25:695-699.
9. Bonnetblanc J-M, Bédane C. Erysipelas. Am J Clin Dermatol. 2003;4:157-163.
10. Jorup-Rönström C, Britton S. Recurrent erysipelas: predisposing factors and costs of prophylaxis. Infection. 1987;15:105-106.
11. Clebak KT, Malone MA. Skin Infections. Prim Care. 2018;45:433-454.
12. Luelmo-Aguilar J, Santandreu MS. Folliculitis: recognition and management. Am J Clin Dermatol. 2004;5:301-310.
13. Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
14. Akaza N, Akamatsu H, Sasaki Y, et al. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009;47:618-624.
15. Berger RS, Seifert MR. Whirlpool folliculitis: a review of its cause, treatment, and prevention. Cutis. 1990;45:97-98.
16. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-1444.
17. Meislin HW, Lerner SA, Graves MH, et al. Cutaneous abscesses: anaerobic and aerobic bacteriology and outpatient management. Ann Intern Med. 1977;87:145-149.
18. Marin JR, Dean AJ, Bilker WB, et al. Emergency ultrasound-assisted examination of skin and soft tissue infections in the pediatric emergency department. Acad Emerg Med. 2013;20:545-553.
19. Gaspari RJ, Resop D, Mendoza M, et al. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2011;57:483-491.
20. Fernandez R, Griffiths R, Ussia C. Water for wound cleansing. Cochrane Database Syst Rev. 2002: CD003861.
21. Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15-19.
22. Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374:823-832.
23. Korownyk C, Allan GM. Evidence-based approach to abscess management. Can Fam Physician. 2007;53:1680-1684.
24. Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56:283-287.
25. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
26. Hunter J, Quarterman C, Waseem M, et al. Diagnosis and management of necrotizing fasciitis. Br J Hosp Med. 2011;72:391-395.
27. Hussein QA, Anaya DA. Necrotizing soft tissue infections. Crit Care Clin. 2013;29:795-806.
28. Puvanendran R, Huey JCM, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55:981-987.
29. Raven MC, Billings JC, Goldfrank LR, et al. Medicaid patients at high risk for frequent hospital admission: real-time identification and remediable risks. J Urban Health. 2009;86:230-241.
30. Ustin JS, Malangoni MA. Necrotizing soft-tissue infections: Crit Care Med. 2011;39:2156-2162.
31. Bystritsky R, Chambers H. Cellulitis and soft tissue infections. Ann Intern Med. 2018;168:ITC17- ITC32.
Skin and soft-tissue infections, frequently encountered in primary care, range from the uncomplicated erysipelas to the life-threatening necrotizing fasciitis. This review draws from the latest evidence and guidelines to help guide the care you provide to patients with cellulitis, orbital cellulitis, erysipelas, folliculitis, furuncles, carbuncles, abscesses, and necrotizing fasciitis.
Cellulitis
Cellulitis, an infection of the deep dermal and subcutaneous layers of the skin, has become increasingly common in recent years, with both incidence and hospitalization rates rising.1 Cellulitis occurs when pathogens enter the dermis through breaks in the skin barrier due to cutaneous fungal infections, trauma, pressure sores, venous stasis, or inflammation. The diagnosis is often made clinically based on characteristic skin findings—classically an acute, poorly demarcated area of erythema, warmth, swelling, and tenderness. Lymphangitic streaking and local lymphadenopathy may also be present. Infection often occurs on an extremity (although it can be found on other areas of the body) and is usually unilateral. Fever may or may not be present.2
Likely responsible microorganisms. Staphylococcus aureus and Group A streptococci (often Streptococcus pyogenes) are common culprits. One systematic review that examined cultures taken of intact skin in cellulitis patients found S aureus to be about twice as common as S pyogenes, with both bacteria accounting for a little more than 70% of cases. Of the remaining positive cultures, the most common organisms were alpha-hemolytic streptococcus, group B streptococcus, Pseudomonas aeruginosa, Clostridium perfringens, Escherichia coli, Pasteurella multocida, and Proteus mirabilis.3 Similarly, a systematic review of bacteremia in patients with cellulitis and erysipelas found that S pyogenes, other beta-hemolytic strep, and S aureus account for about 70% of cases (although S aureus was responsible for just 14%), with the remainder of cases caused by gram-negative organisms such as E coli and P aeruginosa.4
Treatment considerations. Strict treatment guidelines for cellulitis are lacking, but general consensus encourages the use of antibiotics and occasionally surgery. For mild and moderate cases of cellulitis, prescribe oral and parenteral antibiotics to cover for streptococci and methicillin-susceptible S aureus, respectively. Expand coverage to include vancomycin if nasal colonization shows methicillin-resistant S aureus (MRSA) or if you otherwise suspect prior MRSA exposure. Expanded coverage will also be needed if there is severe nonpurulent infection associated with penetrating trauma or a history of intravenous drug use, or the patient meets criteria for systemic inflammatory response syndrome. If patients are severely compromised (eg, neutropenic), it is reasonable to further add broad-spectrum coverage (eg, intravenous piperacillin-tazobactam or carbapenem). Typical duration of treatment is 5 to 7 days, although this should be extended if there is no clinical improvement.
Generally, cellulitis can be managed in the outpatient setting, although hospitalization is recommended if there are concerns for deep or necrotizing infection, if patients are nonadherent to therapy or are immunocompromised, or if outpatient therapy has failed.5 Furthermore, in an observational study of 606 adult patients, prior episodes of cellulitis, venous insufficiency, and immunosuppression were all independently associated with poorer clinical outcomes.2 Also treat underlying predisposing factors such as edema, obesity, eczema, venous insufficiency, and toe web abnormalities such as fissures, scaling, or maceration.5 Consider the use of prophylactic antibiotics for patients who have had 3 to 4 episodes of cellulitis despite attempts to treat predisposing conditions. Prophylactic antibiotic regimens include penicillin or erythromycin orally and penicillin G benzathine intramuscularly.5 Antibiotic regimens are summarized in the TABLE.5

Orbital cellulitis
Orbital cellulitis is an infection of the tissues posterior to the orbital septum.6,7 Periorbital, or preseptal, cellulitis occurs anterior to the orbital septum and is the more common of the 2 infections—84% compared with 16% for orbital cellulitis.6 However, orbital cellulitis, which affects mainly children at a median age of 7 years,6 must be detected and treated early due to the potential for serious complications such as cavernous sinus thrombosis, meningitis, intracranial abscess, and vision loss.7 Chemosis (conjunctival edema) and diplopia are more commonly associated with orbital cellulitis and are seldom seen with preseptal cellulitis.
Predominant causative organisms are S pneumoniae, Moraxella catarrhalis, non-typeable Haemophilus influenzae, and group A streptococcus. The most common mechanism of infection is tracking from periorbital structures (eg, paranasal and ethmoid sinusitis). Other causes include orbital trauma/fracture, periorbital surgery, and bacterial endocarditis. Clinically, patients present with limited ocular motility and proptosis associated with inflamed conjunctiva, orbital pain, headache, malaise, fever, eyelid edema, and possible decrease in visual acuity. The diagnosis is often made clinically and confirmed with orbital computed tomography (CT) with contrast, which can assist in ruling out intracranial involvement such as abscess.
Continue to: Antibiotic therapy
Antibiotic therapy, generally administered intravenously, is recommended for at least 3 days or until orbital symptoms begin to resolve. Choose antibiotics effective against sinusitis-related pathogens (eg, S pneumoniae, H influenzae, M catarrhalis), S aureus, and anaerobes.8 For instance, a regimen may include vancomycin for MRSA coverage, a third-generation cephalosporin, or metronidazole for anaerobic coverage if there is concern about intracranial involvement. Surgical intervention is often reserved for patients with inadequate response to antibiotic therapy, necessitating biopsy for pathogen identification, as well as drainage of large abscesses refractory to antibiotics.
Erysipelas
Erysipelas, a related yet distinct form of cellulitis, is a bacterial infection of the superficial dermis and hypodermis and is commonly caused by group A streptococcus.5,9 Other less common organisms include S aureus, P aeruginosa, and enterobacteria. Erysipelas predominantly affects the lower extremities unilaterally (~90%); the arms and the face are the next most common locations. In addition to the rapid onset of well-demarcated erythema, pain, and swelling, patients may have fever and regional lymphadenopathy. Risk factors include portal of entry (eg, tinea pedis, ulceration), lymphedema, and diabetes. Complications of erysipelas include bullae from edema, abscess formation, and, rarely, bacteremia.
Antibiotic treatment regimens include penicillin G, macrolides (reserved for those with penicillin allergies), fluoroquinolones, and cephalosporins, with duration of treatment ranging from 10 to 14 days depending on infection severity. Fever, pain, and erythema generally improve within 48 to 72 hours of antibiotic therapy. If there is no improvement, consider alternative diagnoses, such as necrotizing fasciitis. Recurrence rates following the initial episode of erysipelas are estimated at 10% of patients at 6 months and 30% at 3 years.10
Folliculitis
Inflammation of hair follicles is characterized by superficial inflammation with the development of perifollicular papules or pustules on an erythematous base.11,12 Folliculitis most commonly affects the face, scalp, thighs, buttocks, axillae, and inguinal areas.13 It may be caused by infection, an inflammatory reaction, or physical injury. Diagnosis is typically based on the patient’s history and physical examination.
Bacteria are the most common cause of infection, although fungi, viruses, and other entities can cause folliculitis. S aureus (methicillin sensitive or methicillin resistant) is the most common pathogen; in the past, superficial pustular folliculitis attributed to S aureus was referred to as Bockhart impetigo. Folliculitis secondary to P aeruginosa, often seen after exposure to contaminated water or hot tubs, is frequently referred to as “hot tub folliculitis.” Malassezia, a reported cause of fungal folliculitis, tends to occur in adolescents of either sex and men with high sebum production, is common in tropical climates, and can be associated with HIV or immunosuppression.11,12,14
Continue to: Differential diagnosis...
Differential diagnosis of folliculitis includes pseudofolliculitis barbae, eosinophilic folliculitis, keratosis pilaris, acne vulgaris, candidiasis, contact dermatitis, impetigo, and miliaria.13 Pseudofolliculitis barbae is an inflammatory reaction to shaving, more commonly seen in darkly pigmented skin. Pseudofolliculitis develops when the hair shaft penetrates the wall of the follicle or directly enters the epidermis.
Initial treatment for mild disease includes the elimination of predisposing factors such as occlusion, moisture, and abrasion. The area should be kept clean and dry, avoiding friction. For localized disease, prescribe topical clindamycin, mupirocin ointment, or benzoyl peroxide. If symptoms fail to respond, prescribe a 7-day course of antibiotic that targets methicillin-sensitive S aureus—eg, cephalexin or dicloxacillin. Also consider doxycycline, which has anti-inflammatory effects and is effective against MRSA. For refractory lesions, trimethoprim-sulfamethoxazole, clindamycin, or minocycline may be useful. If you suspect pseudomonas, consider giving ciprofloxacin for 10 to 14 days for persistent lesions or if the patient is immunocompromised.13,15 Consider obtaining bacterial, fungal, or viral cultures for lesions that fail to respond to initial treatment.
Furuncles/carbuncles/abscesses
A furuncle, commonly referred to as a boil, is an infected hair follicle that becomes enclosed, creating a collection of pus. A carbuncle is a collection of furuncles that converge and drain through a single opening. An abscess is a localized collection of pus arising from within the dermis that can extend within deeper tissues.5 Furuncles, carbuncles, and abscesses are managed similarly with drainage and consideration for MRSA risk factors.
S aureus is the most common cause of these infections; 59% of skin abscesses are due to community-acquired MRSA.16 Anaerobes may contribute to the polymicrobial flora of skin abscesses.17 Risk factors for MRSA infection include a history of previous MRSA infection, diabetes, dialysis or renal failure, placement of an indwelling catheter or medical device, injection drug use, incarceration, close contact with a person with known MRSA infection or colonization, long-term care residence, hospitalization or surgery within the past 12 months, and high prevalence of MRSA in the community.5
Ultrasound improves diagnostic accuracy. One study showed that when a clinical exam alone was inconclusive in evaluating skin and soft-tissue infections in children and adolescents, an ultrasound-assisted examination improved diagnostic accuracy.18 Sensitivity of the clinical examination was 43.7%, compared with 77.6% for the clinical examination plus ultrasound.18
Continue to: Incision and drainage first
Incision and drainage first. Ultrasound-guided needle aspiration, however, has not improved treatment efficacy compared with incision and drainage,19 the mainstay approach for abscesses.17 The procedure to drain a furuncle, carbuncle, or abscess should include the expression of all purulent material and the removal of all loculations if possible. Wound culture is recommended during incision and drainage per current guidelines.5 Simple dry dressings are convenient and effective, although some wounds may require packing. Tap water (that is potable) is suitable for wound cleansing. However, there is no strong evidence that irrigating wounds increases healing or reduces infection.20
Routine use of antibiotics is not recommended for simple cutaneous abscesses.5,17,21 Evidence has been conflicting regarding empiric antibiotic coverage of MRSA following incision and drainage.22-25 Guidelines recommend considering the initiation of antibiotics if there are multiple abscesses, gangrene, surrounding cellulitis, or systemic signs of infection, or if the host is immunocompromised.5
If MRSA is suspected, recommended antibiotic coverage includes trimethoprim-sulfamethoxazole, clindamycin, doxycycline, or minocycline.5 If MRSA is identified, treatment options include dicloxacillin or cephalexin. For severe infections persisting after incision and drainage, in addition to oral antibiotic therapy, consider intravenous antibiotic options for MRSA: cefazolin, clindamycin, linezolid, nafcillin, telavancin, or vancomycin.5
Necrotizing fasciitis
Necrotizing fasciitis is a rare but potentially deadly infection of the skin and soft tissue. It progresses rapidly and spreads along fascial planes, leading to the necrosis of the superficial fascia. The infection often is more extensive than is indicated by superficial signs. Prompt diagnosis is imperative as necrotizing fasciitis is a surgical emergency.5,26 In the United States, 500 to 1500 cases of necrotizing fasciitis occur each year.27 Risk factors for necrotizing fasciitis include diabetes, peripheral vascular disease, malignancy, obesity, cirrhosis, renal failure, injection drug use, chronic corticosteroid therapy, alcohol abuse, malnutrition, and iatrogenic immunosuppression.26,28
Necrotizing fasciitis may be polymicrobial or monomicrobial. Polymicrobial infection, also referred to as type I, is often due to multiple bacteria that originate from the bowel flora, typically including a mix of anaerobic and aerobic organisms. On average, there can be 5 infecting organisms identified per wound, although in some cases up to 15 organisms have been identified in a single wound.5 Type I infection is often associated with tissue injury, abscess, or abdominal surgery. The majority of cases of necrotizing fasciitis are polymicrobial.27,28
Continue to: Monomicrobial infection...
Monomicrobial infection, also referred to as type II, is often due to group A streptococcus, S aureus, vibrio spp, Aeromonas hyrophilio, or an anaerobic streptococci like peptostreptococcus spp. Typically monomicrobial infections, which account for 20% to 30% of cases of necrotizing fasciitis, are community acquired.5,26,29,30
Clinical presentation. In the early stages of disease, patients commonly complain of flu-like symptoms and extreme pain that is out of proportion to findings on the exam. Additional warning signs include fevers and other symptoms of toxicity such as tachycardia, hypotension, nausea, vomiting, and diarrhea. Later in the course, symptoms may localize to the affected area and include erythema, tense swelling, development of blisters or bullae, blackish blue discoloration of the skin, severe pain, and loss of sensation. In some cases involving gas-forming bacteria, tissue crepitus may be noted on exam.5,27-31
Rely on clinical judgment to hasten surgical intervention. Laboratory or imaging findings may augment clinical judgment. But if you suspect necrotizing fasciitis, obtaining blood tests and imaging should not delay surgery. Blood tests that may aid in the diagnosis of necrotizing fasciitis include a complete blood count with differential; coagulation studies; a comprehensive metabolic panel; assays of lactate, C-reactive protein (CRP), and creatinine kinase; and blood cultures. Most often, patients with necrotizing fasciitis will have leukocytosis or leukopenia, evidence of hemolysis, thrombocytopenia, acute renal failure, and significantly elevated CRP.
On any imaging modality, indications of necrotizing fasciitis are inflammatory infiltration of the deep fascia on the affected side that is absent on the contralateral side, and the presence of subcutaneous air (which is a specific but rare finding). Imaging modalities may include CT or magnetic resonance imaging. A definitive diagnosis can only be made with surgical exploration of the involved area. Definitive microbiologic diagnosis will require culture of organisms from affected tissue or blood.5,26,30,31
First address any hemodynamic instability (hypotension is frequently encountered), followed by urgent surgical exploration, debridement of the wound, and antimicrobial therapy. Antibiotic treatment should align with probable pathogens and treatment should be continued until repeated surgical debridement is no longer necessary, clinical improvement is evident, and 48 to 72 hours have passed since defervescence. A reasonable initial empiric regimen in adults would include an agent that is effective against group A streptococcus, gram-negative pathogens, and anaerobes, such as a carbapenem or a beta-lactam-beta-lactamase inhibitor such as piperacillin-tazobactam. Additionally, include an agent that targets MRSA, such as vancomycin, linezolid, or clindamycin.5
CORRESPONDENCE
Karl T. Clebak, MD, Department of Family and Community Medicine Residency Program, Penn State Health M.S. Hershey Medical Center, 500 University Drive, H154/C1613, Hershey, PA 17033; [email protected]
Skin and soft-tissue infections, frequently encountered in primary care, range from the uncomplicated erysipelas to the life-threatening necrotizing fasciitis. This review draws from the latest evidence and guidelines to help guide the care you provide to patients with cellulitis, orbital cellulitis, erysipelas, folliculitis, furuncles, carbuncles, abscesses, and necrotizing fasciitis.
Cellulitis
Cellulitis, an infection of the deep dermal and subcutaneous layers of the skin, has become increasingly common in recent years, with both incidence and hospitalization rates rising.1 Cellulitis occurs when pathogens enter the dermis through breaks in the skin barrier due to cutaneous fungal infections, trauma, pressure sores, venous stasis, or inflammation. The diagnosis is often made clinically based on characteristic skin findings—classically an acute, poorly demarcated area of erythema, warmth, swelling, and tenderness. Lymphangitic streaking and local lymphadenopathy may also be present. Infection often occurs on an extremity (although it can be found on other areas of the body) and is usually unilateral. Fever may or may not be present.2
Likely responsible microorganisms. Staphylococcus aureus and Group A streptococci (often Streptococcus pyogenes) are common culprits. One systematic review that examined cultures taken of intact skin in cellulitis patients found S aureus to be about twice as common as S pyogenes, with both bacteria accounting for a little more than 70% of cases. Of the remaining positive cultures, the most common organisms were alpha-hemolytic streptococcus, group B streptococcus, Pseudomonas aeruginosa, Clostridium perfringens, Escherichia coli, Pasteurella multocida, and Proteus mirabilis.3 Similarly, a systematic review of bacteremia in patients with cellulitis and erysipelas found that S pyogenes, other beta-hemolytic strep, and S aureus account for about 70% of cases (although S aureus was responsible for just 14%), with the remainder of cases caused by gram-negative organisms such as E coli and P aeruginosa.4
Treatment considerations. Strict treatment guidelines for cellulitis are lacking, but general consensus encourages the use of antibiotics and occasionally surgery. For mild and moderate cases of cellulitis, prescribe oral and parenteral antibiotics to cover for streptococci and methicillin-susceptible S aureus, respectively. Expand coverage to include vancomycin if nasal colonization shows methicillin-resistant S aureus (MRSA) or if you otherwise suspect prior MRSA exposure. Expanded coverage will also be needed if there is severe nonpurulent infection associated with penetrating trauma or a history of intravenous drug use, or the patient meets criteria for systemic inflammatory response syndrome. If patients are severely compromised (eg, neutropenic), it is reasonable to further add broad-spectrum coverage (eg, intravenous piperacillin-tazobactam or carbapenem). Typical duration of treatment is 5 to 7 days, although this should be extended if there is no clinical improvement.
Generally, cellulitis can be managed in the outpatient setting, although hospitalization is recommended if there are concerns for deep or necrotizing infection, if patients are nonadherent to therapy or are immunocompromised, or if outpatient therapy has failed.5 Furthermore, in an observational study of 606 adult patients, prior episodes of cellulitis, venous insufficiency, and immunosuppression were all independently associated with poorer clinical outcomes.2 Also treat underlying predisposing factors such as edema, obesity, eczema, venous insufficiency, and toe web abnormalities such as fissures, scaling, or maceration.5 Consider the use of prophylactic antibiotics for patients who have had 3 to 4 episodes of cellulitis despite attempts to treat predisposing conditions. Prophylactic antibiotic regimens include penicillin or erythromycin orally and penicillin G benzathine intramuscularly.5 Antibiotic regimens are summarized in the TABLE.5

Orbital cellulitis
Orbital cellulitis is an infection of the tissues posterior to the orbital septum.6,7 Periorbital, or preseptal, cellulitis occurs anterior to the orbital septum and is the more common of the 2 infections—84% compared with 16% for orbital cellulitis.6 However, orbital cellulitis, which affects mainly children at a median age of 7 years,6 must be detected and treated early due to the potential for serious complications such as cavernous sinus thrombosis, meningitis, intracranial abscess, and vision loss.7 Chemosis (conjunctival edema) and diplopia are more commonly associated with orbital cellulitis and are seldom seen with preseptal cellulitis.
Predominant causative organisms are S pneumoniae, Moraxella catarrhalis, non-typeable Haemophilus influenzae, and group A streptococcus. The most common mechanism of infection is tracking from periorbital structures (eg, paranasal and ethmoid sinusitis). Other causes include orbital trauma/fracture, periorbital surgery, and bacterial endocarditis. Clinically, patients present with limited ocular motility and proptosis associated with inflamed conjunctiva, orbital pain, headache, malaise, fever, eyelid edema, and possible decrease in visual acuity. The diagnosis is often made clinically and confirmed with orbital computed tomography (CT) with contrast, which can assist in ruling out intracranial involvement such as abscess.
Continue to: Antibiotic therapy
Antibiotic therapy, generally administered intravenously, is recommended for at least 3 days or until orbital symptoms begin to resolve. Choose antibiotics effective against sinusitis-related pathogens (eg, S pneumoniae, H influenzae, M catarrhalis), S aureus, and anaerobes.8 For instance, a regimen may include vancomycin for MRSA coverage, a third-generation cephalosporin, or metronidazole for anaerobic coverage if there is concern about intracranial involvement. Surgical intervention is often reserved for patients with inadequate response to antibiotic therapy, necessitating biopsy for pathogen identification, as well as drainage of large abscesses refractory to antibiotics.
Erysipelas
Erysipelas, a related yet distinct form of cellulitis, is a bacterial infection of the superficial dermis and hypodermis and is commonly caused by group A streptococcus.5,9 Other less common organisms include S aureus, P aeruginosa, and enterobacteria. Erysipelas predominantly affects the lower extremities unilaterally (~90%); the arms and the face are the next most common locations. In addition to the rapid onset of well-demarcated erythema, pain, and swelling, patients may have fever and regional lymphadenopathy. Risk factors include portal of entry (eg, tinea pedis, ulceration), lymphedema, and diabetes. Complications of erysipelas include bullae from edema, abscess formation, and, rarely, bacteremia.
Antibiotic treatment regimens include penicillin G, macrolides (reserved for those with penicillin allergies), fluoroquinolones, and cephalosporins, with duration of treatment ranging from 10 to 14 days depending on infection severity. Fever, pain, and erythema generally improve within 48 to 72 hours of antibiotic therapy. If there is no improvement, consider alternative diagnoses, such as necrotizing fasciitis. Recurrence rates following the initial episode of erysipelas are estimated at 10% of patients at 6 months and 30% at 3 years.10
Folliculitis
Inflammation of hair follicles is characterized by superficial inflammation with the development of perifollicular papules or pustules on an erythematous base.11,12 Folliculitis most commonly affects the face, scalp, thighs, buttocks, axillae, and inguinal areas.13 It may be caused by infection, an inflammatory reaction, or physical injury. Diagnosis is typically based on the patient’s history and physical examination.
Bacteria are the most common cause of infection, although fungi, viruses, and other entities can cause folliculitis. S aureus (methicillin sensitive or methicillin resistant) is the most common pathogen; in the past, superficial pustular folliculitis attributed to S aureus was referred to as Bockhart impetigo. Folliculitis secondary to P aeruginosa, often seen after exposure to contaminated water or hot tubs, is frequently referred to as “hot tub folliculitis.” Malassezia, a reported cause of fungal folliculitis, tends to occur in adolescents of either sex and men with high sebum production, is common in tropical climates, and can be associated with HIV or immunosuppression.11,12,14
Continue to: Differential diagnosis...
Differential diagnosis of folliculitis includes pseudofolliculitis barbae, eosinophilic folliculitis, keratosis pilaris, acne vulgaris, candidiasis, contact dermatitis, impetigo, and miliaria.13 Pseudofolliculitis barbae is an inflammatory reaction to shaving, more commonly seen in darkly pigmented skin. Pseudofolliculitis develops when the hair shaft penetrates the wall of the follicle or directly enters the epidermis.
Initial treatment for mild disease includes the elimination of predisposing factors such as occlusion, moisture, and abrasion. The area should be kept clean and dry, avoiding friction. For localized disease, prescribe topical clindamycin, mupirocin ointment, or benzoyl peroxide. If symptoms fail to respond, prescribe a 7-day course of antibiotic that targets methicillin-sensitive S aureus—eg, cephalexin or dicloxacillin. Also consider doxycycline, which has anti-inflammatory effects and is effective against MRSA. For refractory lesions, trimethoprim-sulfamethoxazole, clindamycin, or minocycline may be useful. If you suspect pseudomonas, consider giving ciprofloxacin for 10 to 14 days for persistent lesions or if the patient is immunocompromised.13,15 Consider obtaining bacterial, fungal, or viral cultures for lesions that fail to respond to initial treatment.
Furuncles/carbuncles/abscesses
A furuncle, commonly referred to as a boil, is an infected hair follicle that becomes enclosed, creating a collection of pus. A carbuncle is a collection of furuncles that converge and drain through a single opening. An abscess is a localized collection of pus arising from within the dermis that can extend within deeper tissues.5 Furuncles, carbuncles, and abscesses are managed similarly with drainage and consideration for MRSA risk factors.
S aureus is the most common cause of these infections; 59% of skin abscesses are due to community-acquired MRSA.16 Anaerobes may contribute to the polymicrobial flora of skin abscesses.17 Risk factors for MRSA infection include a history of previous MRSA infection, diabetes, dialysis or renal failure, placement of an indwelling catheter or medical device, injection drug use, incarceration, close contact with a person with known MRSA infection or colonization, long-term care residence, hospitalization or surgery within the past 12 months, and high prevalence of MRSA in the community.5
Ultrasound improves diagnostic accuracy. One study showed that when a clinical exam alone was inconclusive in evaluating skin and soft-tissue infections in children and adolescents, an ultrasound-assisted examination improved diagnostic accuracy.18 Sensitivity of the clinical examination was 43.7%, compared with 77.6% for the clinical examination plus ultrasound.18
Continue to: Incision and drainage first
Incision and drainage first. Ultrasound-guided needle aspiration, however, has not improved treatment efficacy compared with incision and drainage,19 the mainstay approach for abscesses.17 The procedure to drain a furuncle, carbuncle, or abscess should include the expression of all purulent material and the removal of all loculations if possible. Wound culture is recommended during incision and drainage per current guidelines.5 Simple dry dressings are convenient and effective, although some wounds may require packing. Tap water (that is potable) is suitable for wound cleansing. However, there is no strong evidence that irrigating wounds increases healing or reduces infection.20
Routine use of antibiotics is not recommended for simple cutaneous abscesses.5,17,21 Evidence has been conflicting regarding empiric antibiotic coverage of MRSA following incision and drainage.22-25 Guidelines recommend considering the initiation of antibiotics if there are multiple abscesses, gangrene, surrounding cellulitis, or systemic signs of infection, or if the host is immunocompromised.5
If MRSA is suspected, recommended antibiotic coverage includes trimethoprim-sulfamethoxazole, clindamycin, doxycycline, or minocycline.5 If MRSA is identified, treatment options include dicloxacillin or cephalexin. For severe infections persisting after incision and drainage, in addition to oral antibiotic therapy, consider intravenous antibiotic options for MRSA: cefazolin, clindamycin, linezolid, nafcillin, telavancin, or vancomycin.5
Necrotizing fasciitis
Necrotizing fasciitis is a rare but potentially deadly infection of the skin and soft tissue. It progresses rapidly and spreads along fascial planes, leading to the necrosis of the superficial fascia. The infection often is more extensive than is indicated by superficial signs. Prompt diagnosis is imperative as necrotizing fasciitis is a surgical emergency.5,26 In the United States, 500 to 1500 cases of necrotizing fasciitis occur each year.27 Risk factors for necrotizing fasciitis include diabetes, peripheral vascular disease, malignancy, obesity, cirrhosis, renal failure, injection drug use, chronic corticosteroid therapy, alcohol abuse, malnutrition, and iatrogenic immunosuppression.26,28
Necrotizing fasciitis may be polymicrobial or monomicrobial. Polymicrobial infection, also referred to as type I, is often due to multiple bacteria that originate from the bowel flora, typically including a mix of anaerobic and aerobic organisms. On average, there can be 5 infecting organisms identified per wound, although in some cases up to 15 organisms have been identified in a single wound.5 Type I infection is often associated with tissue injury, abscess, or abdominal surgery. The majority of cases of necrotizing fasciitis are polymicrobial.27,28
Continue to: Monomicrobial infection...
Monomicrobial infection, also referred to as type II, is often due to group A streptococcus, S aureus, vibrio spp, Aeromonas hyrophilio, or an anaerobic streptococci like peptostreptococcus spp. Typically monomicrobial infections, which account for 20% to 30% of cases of necrotizing fasciitis, are community acquired.5,26,29,30
Clinical presentation. In the early stages of disease, patients commonly complain of flu-like symptoms and extreme pain that is out of proportion to findings on the exam. Additional warning signs include fevers and other symptoms of toxicity such as tachycardia, hypotension, nausea, vomiting, and diarrhea. Later in the course, symptoms may localize to the affected area and include erythema, tense swelling, development of blisters or bullae, blackish blue discoloration of the skin, severe pain, and loss of sensation. In some cases involving gas-forming bacteria, tissue crepitus may be noted on exam.5,27-31
Rely on clinical judgment to hasten surgical intervention. Laboratory or imaging findings may augment clinical judgment. But if you suspect necrotizing fasciitis, obtaining blood tests and imaging should not delay surgery. Blood tests that may aid in the diagnosis of necrotizing fasciitis include a complete blood count with differential; coagulation studies; a comprehensive metabolic panel; assays of lactate, C-reactive protein (CRP), and creatinine kinase; and blood cultures. Most often, patients with necrotizing fasciitis will have leukocytosis or leukopenia, evidence of hemolysis, thrombocytopenia, acute renal failure, and significantly elevated CRP.
On any imaging modality, indications of necrotizing fasciitis are inflammatory infiltration of the deep fascia on the affected side that is absent on the contralateral side, and the presence of subcutaneous air (which is a specific but rare finding). Imaging modalities may include CT or magnetic resonance imaging. A definitive diagnosis can only be made with surgical exploration of the involved area. Definitive microbiologic diagnosis will require culture of organisms from affected tissue or blood.5,26,30,31
First address any hemodynamic instability (hypotension is frequently encountered), followed by urgent surgical exploration, debridement of the wound, and antimicrobial therapy. Antibiotic treatment should align with probable pathogens and treatment should be continued until repeated surgical debridement is no longer necessary, clinical improvement is evident, and 48 to 72 hours have passed since defervescence. A reasonable initial empiric regimen in adults would include an agent that is effective against group A streptococcus, gram-negative pathogens, and anaerobes, such as a carbapenem or a beta-lactam-beta-lactamase inhibitor such as piperacillin-tazobactam. Additionally, include an agent that targets MRSA, such as vancomycin, linezolid, or clindamycin.5
CORRESPONDENCE
Karl T. Clebak, MD, Department of Family and Community Medicine Residency Program, Penn State Health M.S. Hershey Medical Center, 500 University Drive, H154/C1613, Hershey, PA 17033; [email protected]
1. Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
2. Collazos J, de la Fuente B, García A, et al. Cellulitis in adult patients: a large, multicenter, observational, prospective study of 606 episodes and analysis of the factors related to the response to treatment. PLoS One. 2018;13:e0204036.
3. Chira S, Miller LG. Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138:313-317.
4. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64:148-155.
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
6. Jain A, Rubin PA. Orbital cellulitis in children. Int Ophthalmol Clin. 2001;41:71-86.
7. Seltz LB, Smith J, Durairaj VD, et al. Microbiology and antibiotic management of orbital cellulitis. Pediatrics. 2011;127:e566-e572.
8. Nageswaran S, Woods CR, Benjamin DK, et al. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25:695-699.
9. Bonnetblanc J-M, Bédane C. Erysipelas. Am J Clin Dermatol. 2003;4:157-163.
10. Jorup-Rönström C, Britton S. Recurrent erysipelas: predisposing factors and costs of prophylaxis. Infection. 1987;15:105-106.
11. Clebak KT, Malone MA. Skin Infections. Prim Care. 2018;45:433-454.
12. Luelmo-Aguilar J, Santandreu MS. Folliculitis: recognition and management. Am J Clin Dermatol. 2004;5:301-310.
13. Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
14. Akaza N, Akamatsu H, Sasaki Y, et al. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009;47:618-624.
15. Berger RS, Seifert MR. Whirlpool folliculitis: a review of its cause, treatment, and prevention. Cutis. 1990;45:97-98.
16. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-1444.
17. Meislin HW, Lerner SA, Graves MH, et al. Cutaneous abscesses: anaerobic and aerobic bacteriology and outpatient management. Ann Intern Med. 1977;87:145-149.
18. Marin JR, Dean AJ, Bilker WB, et al. Emergency ultrasound-assisted examination of skin and soft tissue infections in the pediatric emergency department. Acad Emerg Med. 2013;20:545-553.
19. Gaspari RJ, Resop D, Mendoza M, et al. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2011;57:483-491.
20. Fernandez R, Griffiths R, Ussia C. Water for wound cleansing. Cochrane Database Syst Rev. 2002: CD003861.
21. Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15-19.
22. Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374:823-832.
23. Korownyk C, Allan GM. Evidence-based approach to abscess management. Can Fam Physician. 2007;53:1680-1684.
24. Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56:283-287.
25. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
26. Hunter J, Quarterman C, Waseem M, et al. Diagnosis and management of necrotizing fasciitis. Br J Hosp Med. 2011;72:391-395.
27. Hussein QA, Anaya DA. Necrotizing soft tissue infections. Crit Care Clin. 2013;29:795-806.
28. Puvanendran R, Huey JCM, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55:981-987.
29. Raven MC, Billings JC, Goldfrank LR, et al. Medicaid patients at high risk for frequent hospital admission: real-time identification and remediable risks. J Urban Health. 2009;86:230-241.
30. Ustin JS, Malangoni MA. Necrotizing soft-tissue infections: Crit Care Med. 2011;39:2156-2162.
31. Bystritsky R, Chambers H. Cellulitis and soft tissue infections. Ann Intern Med. 2018;168:ITC17- ITC32.
1. Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
2. Collazos J, de la Fuente B, García A, et al. Cellulitis in adult patients: a large, multicenter, observational, prospective study of 606 episodes and analysis of the factors related to the response to treatment. PLoS One. 2018;13:e0204036.
3. Chira S, Miller LG. Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138:313-317.
4. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64:148-155.
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
6. Jain A, Rubin PA. Orbital cellulitis in children. Int Ophthalmol Clin. 2001;41:71-86.
7. Seltz LB, Smith J, Durairaj VD, et al. Microbiology and antibiotic management of orbital cellulitis. Pediatrics. 2011;127:e566-e572.
8. Nageswaran S, Woods CR, Benjamin DK, et al. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25:695-699.
9. Bonnetblanc J-M, Bédane C. Erysipelas. Am J Clin Dermatol. 2003;4:157-163.
10. Jorup-Rönström C, Britton S. Recurrent erysipelas: predisposing factors and costs of prophylaxis. Infection. 1987;15:105-106.
11. Clebak KT, Malone MA. Skin Infections. Prim Care. 2018;45:433-454.
12. Luelmo-Aguilar J, Santandreu MS. Folliculitis: recognition and management. Am J Clin Dermatol. 2004;5:301-310.
13. Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
14. Akaza N, Akamatsu H, Sasaki Y, et al. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009;47:618-624.
15. Berger RS, Seifert MR. Whirlpool folliculitis: a review of its cause, treatment, and prevention. Cutis. 1990;45:97-98.
16. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-1444.
17. Meislin HW, Lerner SA, Graves MH, et al. Cutaneous abscesses: anaerobic and aerobic bacteriology and outpatient management. Ann Intern Med. 1977;87:145-149.
18. Marin JR, Dean AJ, Bilker WB, et al. Emergency ultrasound-assisted examination of skin and soft tissue infections in the pediatric emergency department. Acad Emerg Med. 2013;20:545-553.
19. Gaspari RJ, Resop D, Mendoza M, et al. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2011;57:483-491.
20. Fernandez R, Griffiths R, Ussia C. Water for wound cleansing. Cochrane Database Syst Rev. 2002: CD003861.
21. Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15-19.
22. Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374:823-832.
23. Korownyk C, Allan GM. Evidence-based approach to abscess management. Can Fam Physician. 2007;53:1680-1684.
24. Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56:283-287.
25. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
26. Hunter J, Quarterman C, Waseem M, et al. Diagnosis and management of necrotizing fasciitis. Br J Hosp Med. 2011;72:391-395.
27. Hussein QA, Anaya DA. Necrotizing soft tissue infections. Crit Care Clin. 2013;29:795-806.
28. Puvanendran R, Huey JCM, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55:981-987.
29. Raven MC, Billings JC, Goldfrank LR, et al. Medicaid patients at high risk for frequent hospital admission: real-time identification and remediable risks. J Urban Health. 2009;86:230-241.
30. Ustin JS, Malangoni MA. Necrotizing soft-tissue infections: Crit Care Med. 2011;39:2156-2162.
31. Bystritsky R, Chambers H. Cellulitis and soft tissue infections. Ann Intern Med. 2018;168:ITC17- ITC32.
PRACTICE RECOMMENDATIONS
› Start trimethoprim-sulfamethoxazole, clindamycin, doxycycline, minocycline, or a third- or fourth-generation fluoroquinolone for patients with cellulitis likely caused by community acquired methicillin-resistant Staphylococcus aureus (MRSA). A
› Consider culturing for MRSA and treating with oral doxycycline or trimethoprim-sulfamethoxazole for resistant cases of folliculitis. C
› Perform complete surgical debridement promptly if necrotizing fasciitis is suspected. C
› Prescribe broad-spectrum antibiotics for necrotizing fasciitis, covering both anaerobes and aerobes including MRSA. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Getting hypertension under control in the youngest of patients
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7
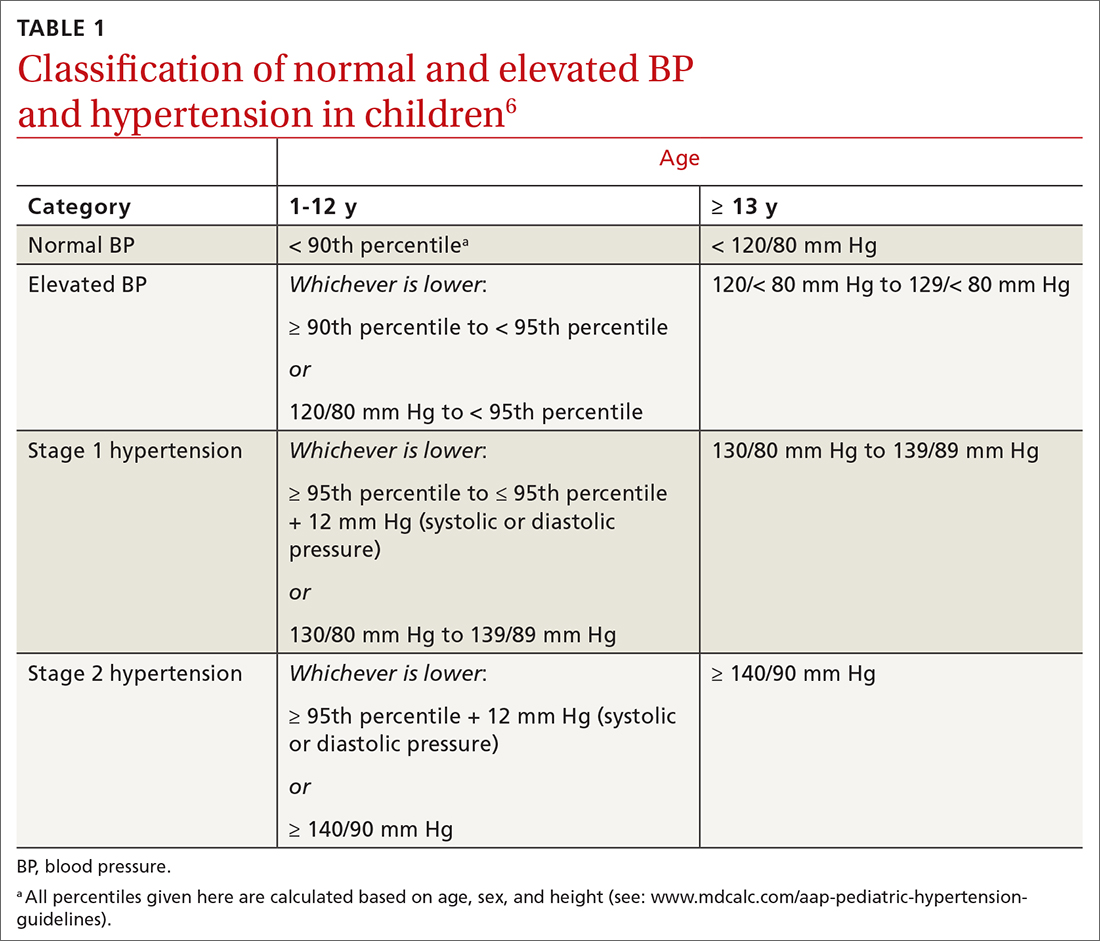
As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.
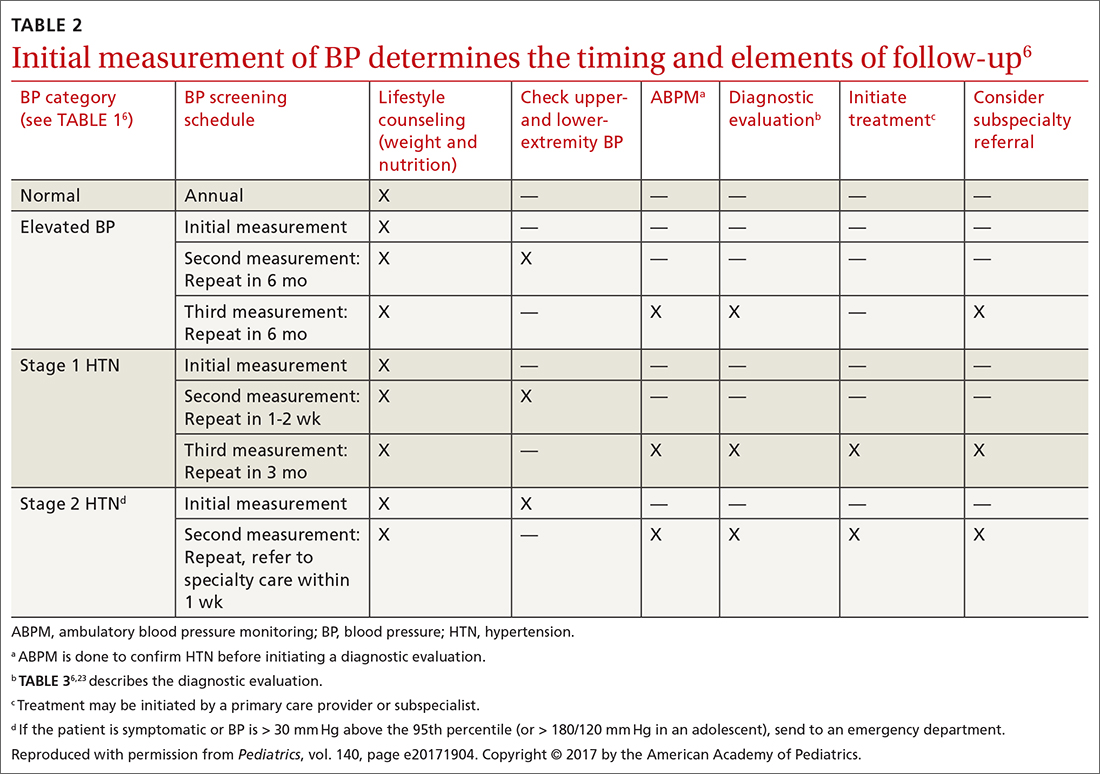
ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.
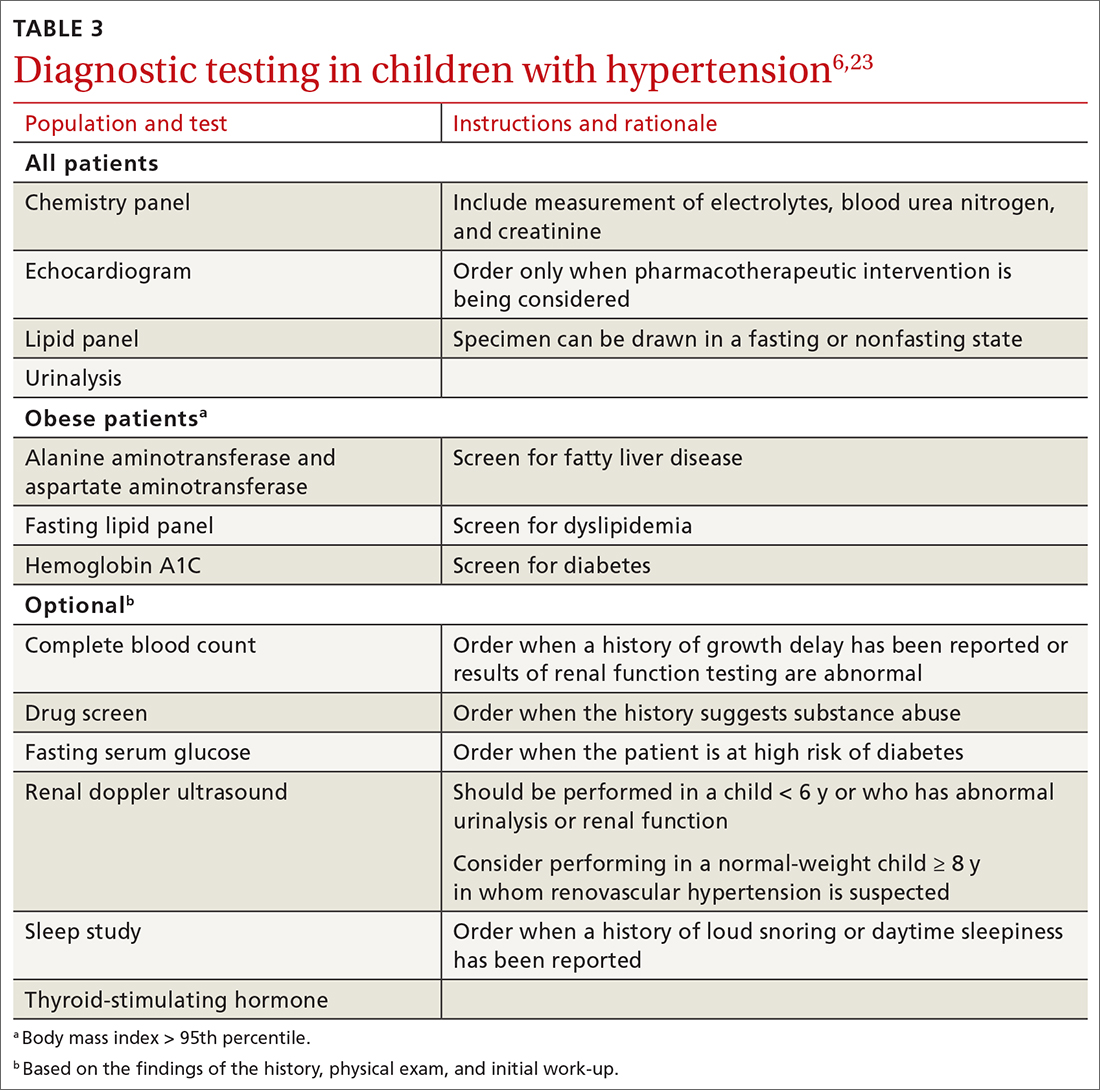
TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.
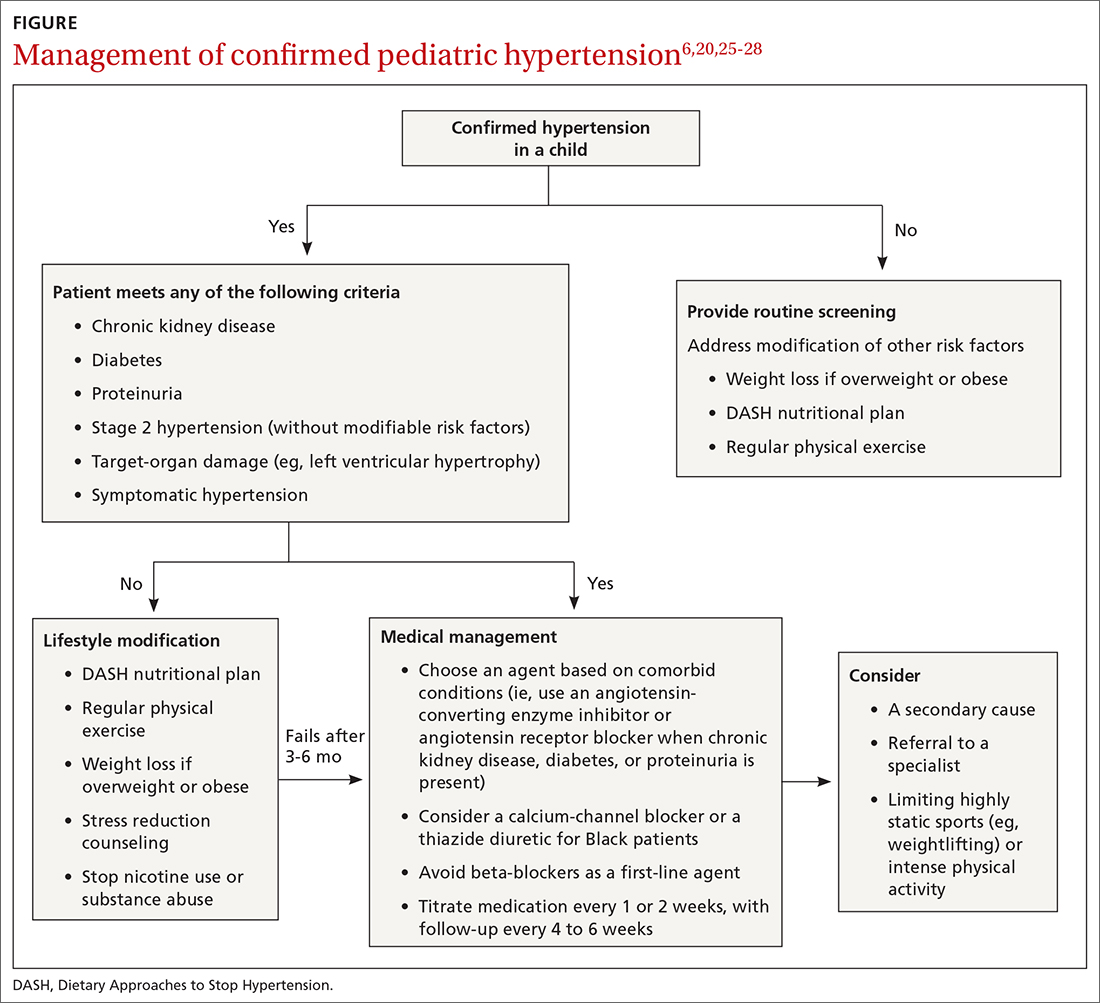
Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.

ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.

TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.

Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.

ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.

TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.

Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
PRACTICE RECOMMENDATIONS
› Measure the blood pressure (BP) of all children 3 years and older annually; those who have a specific comorbid condition (eg, obesity, diabetes, renal disease, or an aortic-arch abnormality) or who are taking medication known to elevate BP should have their BP checked at every health care visit. C
› Encourage lifestyle modification as the initial treatment for elevated BP or hypertension in children. A
› Utilize pharmacotherapy for (1) children with stage 1 hypertension who have failed to meet BP goals after 3 to 6 months of lifestyle modification and (2) children with stage 2 hypertension who do not have a modifiable risk factor, such as obesity. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A guide to diagnosing and managing ascites in cirrhosis
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8
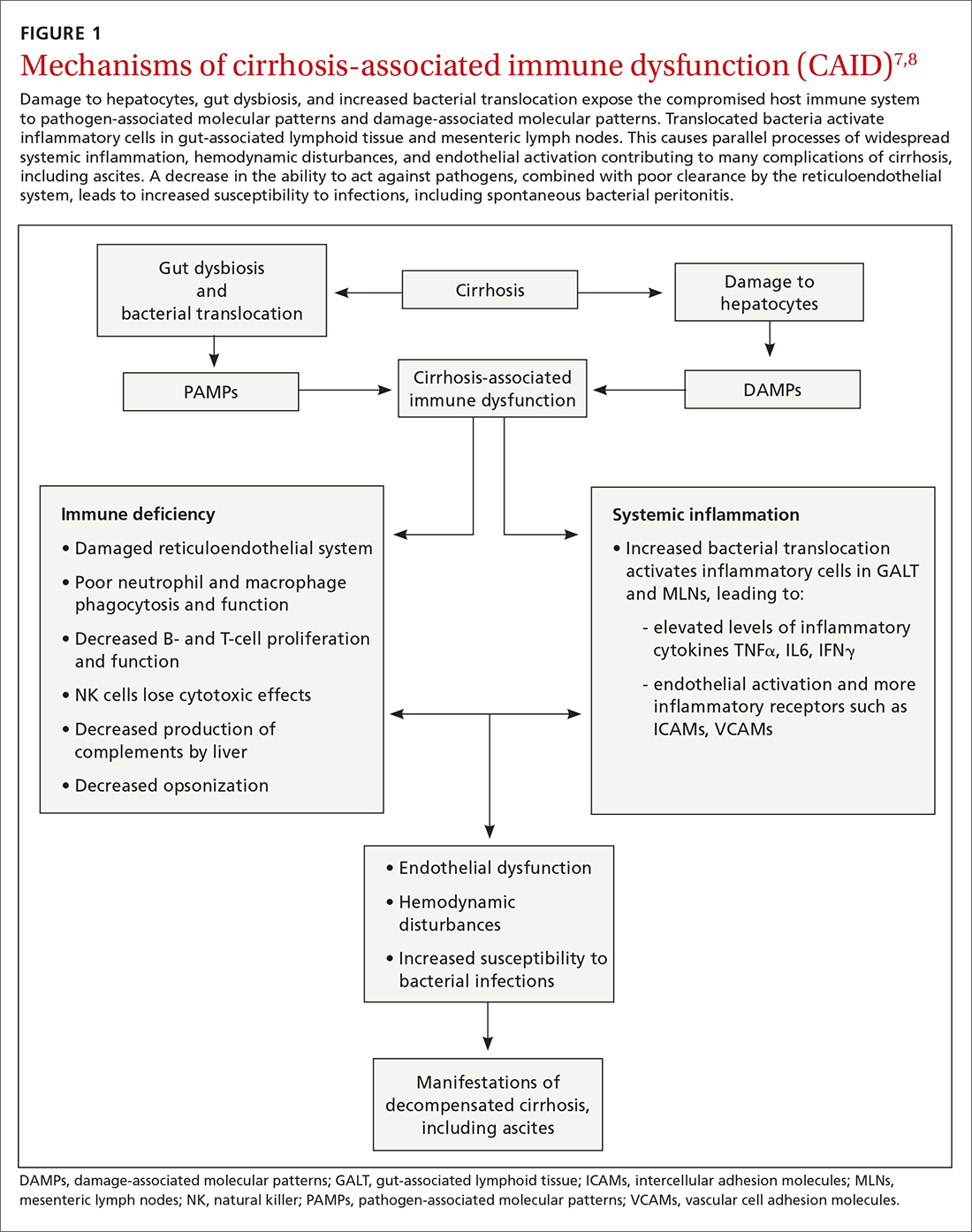
Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).

History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29
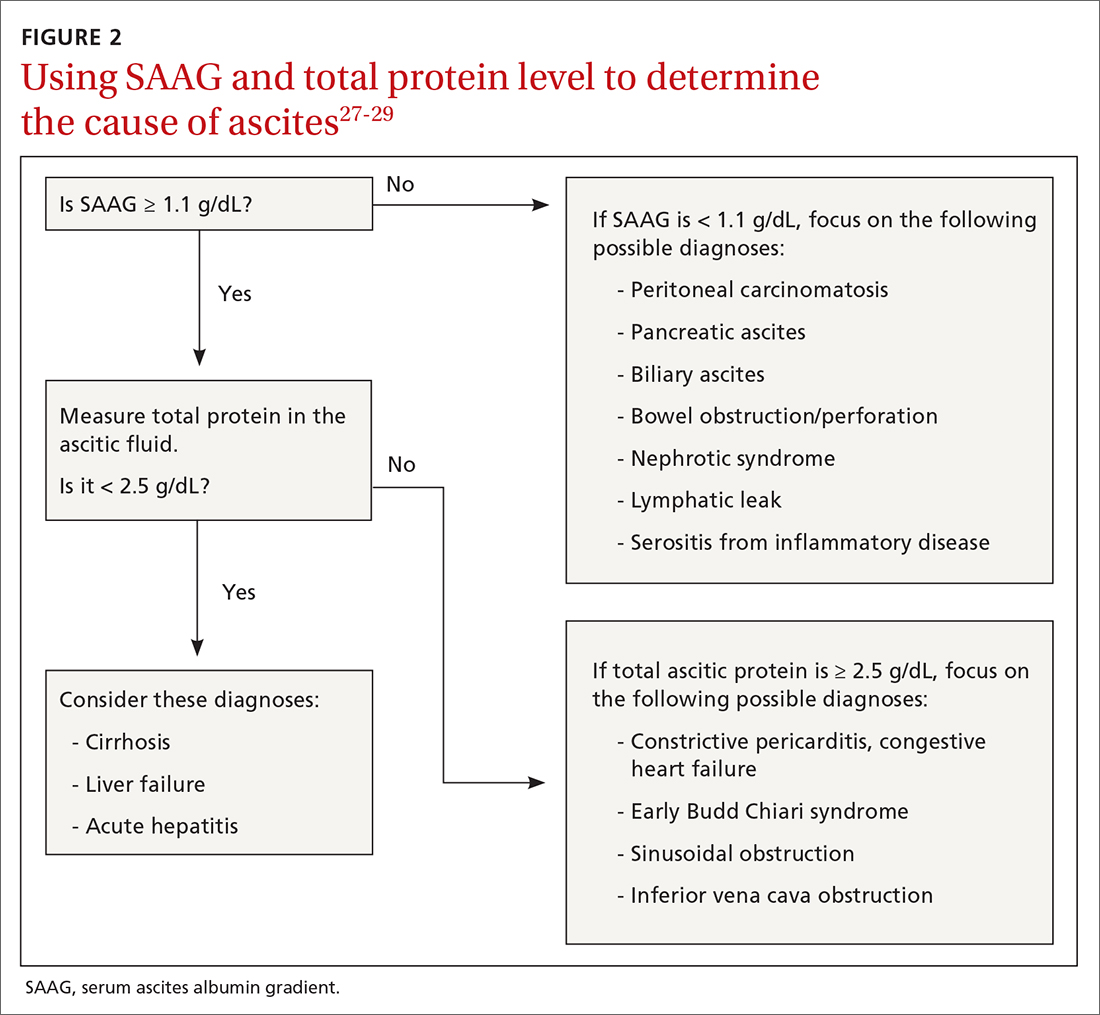
Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8

Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).

History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29

Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8

Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).

History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29

Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
PRACTICE RECOMMENDATIONS
› Calculate the serum ascites albumin gradient and measure the total ascites protein level to distinguish cirrhotic ascites from that caused by heart failure or other disorders. C
› Recommend sodium restriction of 4.9-6.9 g for patients with established ascites secondary to cirrhosis. C
› Avoid giving angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs in cirrhosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series