User login
Palmoplantar exacerbation of psoriasis after nivolumab for lung cancer
Nivolumab is a full human immunoglobulin antibody to the programmed cell death 1 (PD-1) immune checkpoint receptor on T cells. This programmed cell death inhibitor is a targeted immunotherapy used to treat patients with melanoma, among other malignancies.1 More recently, nivolumab has been used for advanced non-small-cell lung cancer (NSCLC) after failure of previous chemotherapeutic agents. It was approved by the US Food and Drug Administration for the NSCLC indication in 2015.2
PD-1 inhibitors are efficacious in treating advanced malignancies, although their immune-mediated functions can lead to undesirable side effects. Patients treated with nivolumab have been reported to develop thyroid disease,1,3,4 diabetes,3 hypophysitis,1,3 hypopituitarism,3 and pneumonitis,4,2 as well as other autoimmune conditions.3 Although nivolumab is often used to treat skin diseases such as melanoma, it can have many cutaneous side effects including pruritus,1,3-6 rash,1,3,4,6,7 vitiligo,1,3,7,6 mouth sores,3 injection site reactions,3,6 and alopecia.5 Herein, we describe a patient who was treated with nivolumab and developed an exacerbation of pre-existing psoriasis.
Case presentation and summary
A 57-year-old man with metastatic NSCLC and a history of plaque psoriasis presented to the dermatology clinic for evaluation of new lesions on his palms and soles. The patient had been previously treated with numerous therapies for NSCLC, including chemotherapy and radiation. Previous chemotherapeutic agents included the cisplatin plus etoposide combination, with doxetaxel and pemetrexed. The patient was not able to tolerate the chemotherapy and instead opted for hospice care. After several months, he chose to restart therapy, and was started on the programmed cell death (PD)-1 inhibitor, nivolumab, at a dose of 3 mg/kg for a total of 6 cycles. He received his first dose 5 weeks before his current presentation to the clinic, and his second dose 2 weeks before.
The patient reported a 20-year history of plaque psoriasis, characterized by psoriatic plaques on the elbows and shins and for which he was treated with topical therapies with good effect. Every few months, he would develop one or two small plaques of psoriasis on his palms and soles. The lesions were inconsequential to the patient, as he never experienced more than one or two small palmoplantar lesions at a time. One week after his second cycle of nivolumab, the patient developed an eruption of lesions on his palms and soles. He observed that the lesions seemed to be similar to his previous palmoplantar psoriatic plaques but with significantly greater skin involvement. The patient denied any new-onset joint pain.
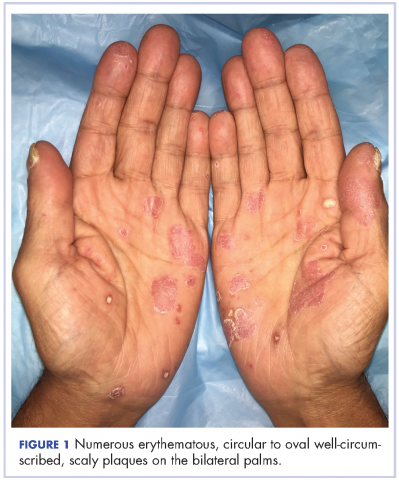
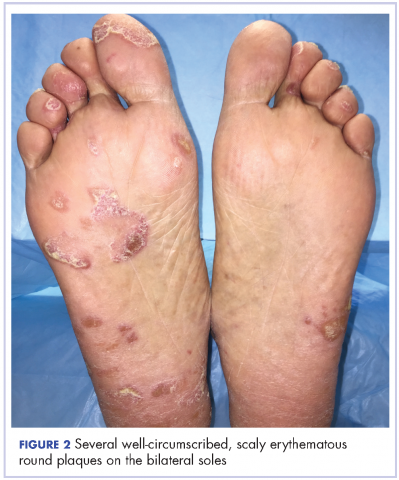
The results of a physical examination revealed a cachectic man in no acute distress, with more than 30 erythematous circular to oval circumscribed plaques with yellow to whitish scales on the bilateral palms (Figure 1) and soles (Figure 2).
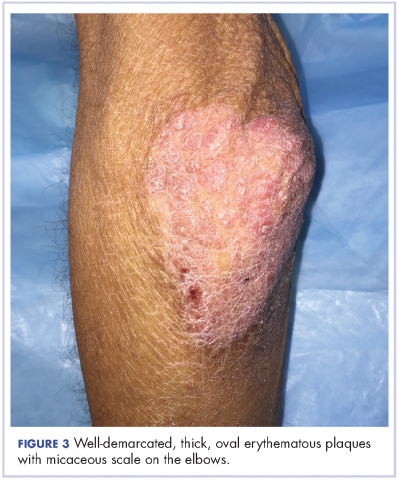
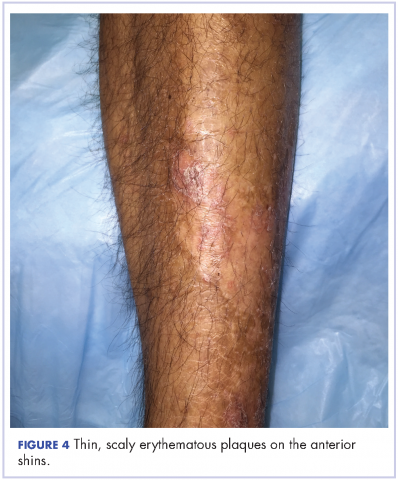
The patient also had well-demarcated, thick oval erythematous plaques with micaceous scales on the bilateral elbows (Figure 3), and thin scaly erythematous plaques on the anterior shins (Figure 4). There were no psoriatic plaques on the remainder of the trunk or extremities. Mucosal surfaces, scalp, and nails were uninvolved.
A clinical diagnosis of exacerbation of pre-existing psoriasis owing to nivolumab therapy was made. The patient was started on clobetasol 0.05% ointment twice daily under occlusion with plastic wrap to the affected areas, and he was continued on nivolumab for his NSCLC.
Discussion
Treatment with nivolumab can lead to a range of autoimmune side effects, and as shown in this case, psoriasis is one of the cutaneous findings that could be exacerbated by treatment with nivolumab. To date, two cases of exacerbation of psoriasis in patients treated with nivolumab for melanoma have been reported in the literature.8,9 In the first case, the patient had well-controlled plaque psoriasis at baseline and he subsequently developed psoriatic plaques on the trunk and extremities after the second infusion of nivolumab for metastatic melanoma. A biopsy showed regular acanthosis with hyperkeratosis and parakeratosis in addition to dilated vessels in the papillary dermis.8 In the second case, the patient had a history of psoriasis vulgaris with no active lesions. Three weeks after his first course of nivolumab for metastatic oral mucosal melanoma, he developed new, well-circumscribed erythematous scaly plaques on the trunk and extremities that were clinically diagnosed as psoriasis.9 In a third case, a patient without a prior history of psoriasis experienced a psoriasiform eruption on the trunk and extremities after the fourth dose of nivolumab for oral mucosal melanoma.10 Thus, our case is the third reported case of exacerbation of preexisting psoriasis in a patient treated with nivolumab. Furthermore, our patient is the first reported case of a patient treated with nivolumab for NSCLC to develop this adverse event. Whereas the previously reported cases were characterized by widespread trunk and extremity involvement, our patient developed focal exacerbation of the palmoplantar areas.
Additional studies are needed to more clearly characterize the specific cutaneous toxicities of nivolumab and to determine if particular skin reactions may indicate a better response to the anticancer agent. Side effects such as psoriasis can often be managed with topical therapies and may not require withdrawal of the medication. We encourage the collaboration of dermatologists and oncologists to enhance the diagnosis and management of these cutaneous side effects in cancer patients.
1. Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of Nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433-440.
2. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004-2012.
3. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
4. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257-265.
5. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384.
6. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311-4318.
7. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber J. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2015.
8. Matsumura N, Ohtsuka M, Kikuchi N, Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm Venereol. 2016;96(2):259-260.
9. Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol. 2016;30(10):e89-e91.
10. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
Nivolumab is a full human immunoglobulin antibody to the programmed cell death 1 (PD-1) immune checkpoint receptor on T cells. This programmed cell death inhibitor is a targeted immunotherapy used to treat patients with melanoma, among other malignancies.1 More recently, nivolumab has been used for advanced non-small-cell lung cancer (NSCLC) after failure of previous chemotherapeutic agents. It was approved by the US Food and Drug Administration for the NSCLC indication in 2015.2
PD-1 inhibitors are efficacious in treating advanced malignancies, although their immune-mediated functions can lead to undesirable side effects. Patients treated with nivolumab have been reported to develop thyroid disease,1,3,4 diabetes,3 hypophysitis,1,3 hypopituitarism,3 and pneumonitis,4,2 as well as other autoimmune conditions.3 Although nivolumab is often used to treat skin diseases such as melanoma, it can have many cutaneous side effects including pruritus,1,3-6 rash,1,3,4,6,7 vitiligo,1,3,7,6 mouth sores,3 injection site reactions,3,6 and alopecia.5 Herein, we describe a patient who was treated with nivolumab and developed an exacerbation of pre-existing psoriasis.
Case presentation and summary
A 57-year-old man with metastatic NSCLC and a history of plaque psoriasis presented to the dermatology clinic for evaluation of new lesions on his palms and soles. The patient had been previously treated with numerous therapies for NSCLC, including chemotherapy and radiation. Previous chemotherapeutic agents included the cisplatin plus etoposide combination, with doxetaxel and pemetrexed. The patient was not able to tolerate the chemotherapy and instead opted for hospice care. After several months, he chose to restart therapy, and was started on the programmed cell death (PD)-1 inhibitor, nivolumab, at a dose of 3 mg/kg for a total of 6 cycles. He received his first dose 5 weeks before his current presentation to the clinic, and his second dose 2 weeks before.
The patient reported a 20-year history of plaque psoriasis, characterized by psoriatic plaques on the elbows and shins and for which he was treated with topical therapies with good effect. Every few months, he would develop one or two small plaques of psoriasis on his palms and soles. The lesions were inconsequential to the patient, as he never experienced more than one or two small palmoplantar lesions at a time. One week after his second cycle of nivolumab, the patient developed an eruption of lesions on his palms and soles. He observed that the lesions seemed to be similar to his previous palmoplantar psoriatic plaques but with significantly greater skin involvement. The patient denied any new-onset joint pain.
The results of a physical examination revealed a cachectic man in no acute distress, with more than 30 erythematous circular to oval circumscribed plaques with yellow to whitish scales on the bilateral palms (Figure 1) and soles (Figure 2).
The patient also had well-demarcated, thick oval erythematous plaques with micaceous scales on the bilateral elbows (Figure 3), and thin scaly erythematous plaques on the anterior shins (Figure 4). There were no psoriatic plaques on the remainder of the trunk or extremities. Mucosal surfaces, scalp, and nails were uninvolved.
A clinical diagnosis of exacerbation of pre-existing psoriasis owing to nivolumab therapy was made. The patient was started on clobetasol 0.05% ointment twice daily under occlusion with plastic wrap to the affected areas, and he was continued on nivolumab for his NSCLC.
Discussion
Treatment with nivolumab can lead to a range of autoimmune side effects, and as shown in this case, psoriasis is one of the cutaneous findings that could be exacerbated by treatment with nivolumab. To date, two cases of exacerbation of psoriasis in patients treated with nivolumab for melanoma have been reported in the literature.8,9 In the first case, the patient had well-controlled plaque psoriasis at baseline and he subsequently developed psoriatic plaques on the trunk and extremities after the second infusion of nivolumab for metastatic melanoma. A biopsy showed regular acanthosis with hyperkeratosis and parakeratosis in addition to dilated vessels in the papillary dermis.8 In the second case, the patient had a history of psoriasis vulgaris with no active lesions. Three weeks after his first course of nivolumab for metastatic oral mucosal melanoma, he developed new, well-circumscribed erythematous scaly plaques on the trunk and extremities that were clinically diagnosed as psoriasis.9 In a third case, a patient without a prior history of psoriasis experienced a psoriasiform eruption on the trunk and extremities after the fourth dose of nivolumab for oral mucosal melanoma.10 Thus, our case is the third reported case of exacerbation of preexisting psoriasis in a patient treated with nivolumab. Furthermore, our patient is the first reported case of a patient treated with nivolumab for NSCLC to develop this adverse event. Whereas the previously reported cases were characterized by widespread trunk and extremity involvement, our patient developed focal exacerbation of the palmoplantar areas.
Additional studies are needed to more clearly characterize the specific cutaneous toxicities of nivolumab and to determine if particular skin reactions may indicate a better response to the anticancer agent. Side effects such as psoriasis can often be managed with topical therapies and may not require withdrawal of the medication. We encourage the collaboration of dermatologists and oncologists to enhance the diagnosis and management of these cutaneous side effects in cancer patients.
Nivolumab is a full human immunoglobulin antibody to the programmed cell death 1 (PD-1) immune checkpoint receptor on T cells. This programmed cell death inhibitor is a targeted immunotherapy used to treat patients with melanoma, among other malignancies.1 More recently, nivolumab has been used for advanced non-small-cell lung cancer (NSCLC) after failure of previous chemotherapeutic agents. It was approved by the US Food and Drug Administration for the NSCLC indication in 2015.2
PD-1 inhibitors are efficacious in treating advanced malignancies, although their immune-mediated functions can lead to undesirable side effects. Patients treated with nivolumab have been reported to develop thyroid disease,1,3,4 diabetes,3 hypophysitis,1,3 hypopituitarism,3 and pneumonitis,4,2 as well as other autoimmune conditions.3 Although nivolumab is often used to treat skin diseases such as melanoma, it can have many cutaneous side effects including pruritus,1,3-6 rash,1,3,4,6,7 vitiligo,1,3,7,6 mouth sores,3 injection site reactions,3,6 and alopecia.5 Herein, we describe a patient who was treated with nivolumab and developed an exacerbation of pre-existing psoriasis.
Case presentation and summary
A 57-year-old man with metastatic NSCLC and a history of plaque psoriasis presented to the dermatology clinic for evaluation of new lesions on his palms and soles. The patient had been previously treated with numerous therapies for NSCLC, including chemotherapy and radiation. Previous chemotherapeutic agents included the cisplatin plus etoposide combination, with doxetaxel and pemetrexed. The patient was not able to tolerate the chemotherapy and instead opted for hospice care. After several months, he chose to restart therapy, and was started on the programmed cell death (PD)-1 inhibitor, nivolumab, at a dose of 3 mg/kg for a total of 6 cycles. He received his first dose 5 weeks before his current presentation to the clinic, and his second dose 2 weeks before.
The patient reported a 20-year history of plaque psoriasis, characterized by psoriatic plaques on the elbows and shins and for which he was treated with topical therapies with good effect. Every few months, he would develop one or two small plaques of psoriasis on his palms and soles. The lesions were inconsequential to the patient, as he never experienced more than one or two small palmoplantar lesions at a time. One week after his second cycle of nivolumab, the patient developed an eruption of lesions on his palms and soles. He observed that the lesions seemed to be similar to his previous palmoplantar psoriatic plaques but with significantly greater skin involvement. The patient denied any new-onset joint pain.
The results of a physical examination revealed a cachectic man in no acute distress, with more than 30 erythematous circular to oval circumscribed plaques with yellow to whitish scales on the bilateral palms (Figure 1) and soles (Figure 2).
The patient also had well-demarcated, thick oval erythematous plaques with micaceous scales on the bilateral elbows (Figure 3), and thin scaly erythematous plaques on the anterior shins (Figure 4). There were no psoriatic plaques on the remainder of the trunk or extremities. Mucosal surfaces, scalp, and nails were uninvolved.
A clinical diagnosis of exacerbation of pre-existing psoriasis owing to nivolumab therapy was made. The patient was started on clobetasol 0.05% ointment twice daily under occlusion with plastic wrap to the affected areas, and he was continued on nivolumab for his NSCLC.
Discussion
Treatment with nivolumab can lead to a range of autoimmune side effects, and as shown in this case, psoriasis is one of the cutaneous findings that could be exacerbated by treatment with nivolumab. To date, two cases of exacerbation of psoriasis in patients treated with nivolumab for melanoma have been reported in the literature.8,9 In the first case, the patient had well-controlled plaque psoriasis at baseline and he subsequently developed psoriatic plaques on the trunk and extremities after the second infusion of nivolumab for metastatic melanoma. A biopsy showed regular acanthosis with hyperkeratosis and parakeratosis in addition to dilated vessels in the papillary dermis.8 In the second case, the patient had a history of psoriasis vulgaris with no active lesions. Three weeks after his first course of nivolumab for metastatic oral mucosal melanoma, he developed new, well-circumscribed erythematous scaly plaques on the trunk and extremities that were clinically diagnosed as psoriasis.9 In a third case, a patient without a prior history of psoriasis experienced a psoriasiform eruption on the trunk and extremities after the fourth dose of nivolumab for oral mucosal melanoma.10 Thus, our case is the third reported case of exacerbation of preexisting psoriasis in a patient treated with nivolumab. Furthermore, our patient is the first reported case of a patient treated with nivolumab for NSCLC to develop this adverse event. Whereas the previously reported cases were characterized by widespread trunk and extremity involvement, our patient developed focal exacerbation of the palmoplantar areas.
Additional studies are needed to more clearly characterize the specific cutaneous toxicities of nivolumab and to determine if particular skin reactions may indicate a better response to the anticancer agent. Side effects such as psoriasis can often be managed with topical therapies and may not require withdrawal of the medication. We encourage the collaboration of dermatologists and oncologists to enhance the diagnosis and management of these cutaneous side effects in cancer patients.
1. Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of Nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433-440.
2. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004-2012.
3. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
4. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257-265.
5. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384.
6. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311-4318.
7. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber J. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2015.
8. Matsumura N, Ohtsuka M, Kikuchi N, Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm Venereol. 2016;96(2):259-260.
9. Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol. 2016;30(10):e89-e91.
10. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
1. Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of Nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433-440.
2. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004-2012.
3. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
4. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257-265.
5. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384.
6. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311-4318.
7. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber J. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2015.
8. Matsumura N, Ohtsuka M, Kikuchi N, Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm Venereol. 2016;96(2):259-260.
9. Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol. 2016;30(10):e89-e91.
10. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
Peripheral Exudative Hemorrhagic Chorioretinopathy in Patients With Nonexudative Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is a common condition that affects the elderly white population. About 6.5% of Americans have been diagnosed with AMD, and 0.8% have received an end-stage AMD diagnosis.1 Exudative AMD is typically more visually debilitating and comprises between 10% and 15% of all AMD cases, with conversion from dry to wet about 10%.1
A thorough examination of the posterior pole is of utmost importance in patients with dry AMD in order to ensure there is no conversion to the exudative form. However, it also is imperative to perform a peripheral evaluation in these patients due to the incidence of peripheral choroidal neovascular membrane (CNVM) and its potential visual significance.
Case Report 1
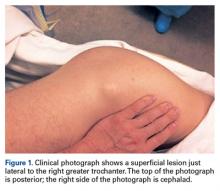
An 80-year-old white male with type 2 diabetes mellitus (DM) without retinopathy, dry AMD, and epiretinal membranes (ERM) in both eyes presented to the eye clinic for a 6-month follow-up. On examination, he had visual acuity (VA) of 20/25 in both eyes and reported no ocular problems. The intraocular pressures were 17 mm Hg in the right eye and 20 mm Hg in the left eye. Slit-lamp examination of the anterior segment of both eyes was significant for 2+ nuclear sclerotic cataracts.
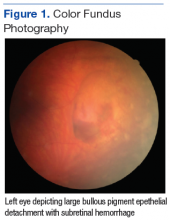
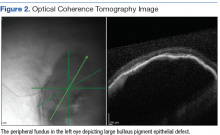
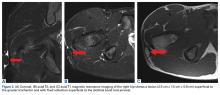
On dilated fundus exam, there were macular drusen and ERM in both eyes; peripherally in the right eye, there was cobblestone degeneration and pigmentary changes. Peripherally in the left eye, there was a large retinal pigment epithelial detachment (PED) with subretinal hemorrhage in the inferior temporal quadrant (Figure 1) along with cobblestone degeneration and pigmentary changes. Peripheral optical coherence tomography (OCT) in the left eye showed a large PED in the location of the hemorrhage (Figure 2).
Case Report 2
An 88-year-old white male presented to the eye clinic reporting blurred vision at distance and dry eyes. The patient’s medical history was remarkable for vascular and heart disease, treated with warfarin. The patient also had insulin controlled DM, with no prior history of retinopathy. His past ocular history included hard drusen in the macula, peripheral drusen, pavingstone degeneration, and a fibrotic scar temporally in the right eye.
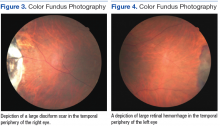
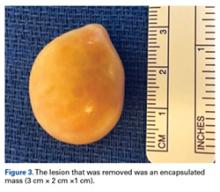
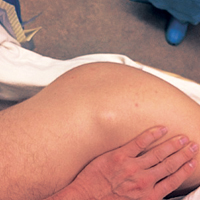
At his annual eye examination, the patient’s vision was correctable to 20/25 in both eyes. His anterior segment slit-lamp exam was remarkable for posterior chamber intraocular lenses, clear and centered in each eye. His posterior pole exam was remarkable for small hard drusen at the macula in both eyes. Peripherally in the right eye, there was a large disciform fibrotic scar temporally (Figure 3) as well as cobblestone degeneration and peripheral drusen. The left eye revealed a large disciform hemorrhage temporally (Figure 4) with cobblestone degeneration and peripheral drusen.
Both patients currently are being closely monitored for any encroachment of the peripheral lesions into the posterior poles.
Discussion
Peripheral exudative hemorrhagic chorioretinopathy (PEHCR), also referred to in the literature as eccentric disciform CNVM, peripheral CNVM, and peripheral age-related degeneration, is a rare condition more prevalent in elderly white females.2-4 Mean age ranges from 70 to 82 years, with bilateral involvement ranging from 18% to 37%.2-4 The mid-periphery or periphery is the most common location for these lesions, more specifically, in the inferior temporal quadrant.2,3,5,6
Age-related macular degeneration is not pathognomonic for PEHCR. Mantel and colleagues reported that 68.9% of the patients in their study had AMD.3 Visual acuity ranges from 20/20 to light perception, dependent upon ocular comorbidities.2,3 As reported by Mantel and colleagues, patients with symptomatic PEHCR commonly experience visual loss, floaters, photopsias, metamorphopsia, and scotoma.3
Peripheral exudative hemorrhagic chorioretinopathy is a hemorrhagic or exudative process that can occur either as an isolated lesion or as multiple lesions that consist of a PED along with hemorrhage, subretinal fluid and/or fibrotic scarring.2-5 Peripheral exudative hemorrhagic chorioretinopathy is not visually significant unless a vitreous hemorrhage is evident or the blood and/or fluid extends to the macular region.2,5
The exact etiology of peripheral CNVM remains unknown; however, ischemia, mechanical forces, and defects in Bruch’s membrane all have been speculated as causative factors.2,3,6 Others have hypothesized that PEHCR is a form of polypoidal choroidal vasculopathy.3,7,8 A rupture in Bruch’s membrane with a vascular complex contributes to the pathophysiology and histology of this condition.3,6
Given the propensity for cardiovascular diseases, such as DM and hypertension, to lead to retinal ischemia, it is important to take a good case history.2,4,6 Additionally, anticoagulants have been shown to exacerbate bleeding.2,5 Due to PEHCR’s location in the periphery, as well as its appearance as an elevated dark mass, it is important to differentiate these lesions from a choroidal melanoma.2,6 Recognition of PEHCR can save the patient from unnecessary treatment with radiation or enucleation.
Peripheral exudative hemorrhagic chorioretinopathy is a self-limiting condition that generally requires close observation only. Long-term follow-up studies show resolution, regression, or stability of the peripheral lesions.4,5,8 If a hemorrhage is present, the blood will resolve and leave a disciform scar with pigmentary changes.2-4 In cases where vision is threatened, CNVM has been treated with photocoagulation, cryopexy, and more recently, intravitreal anti-VEGF injections.4,5,9,10 Given that VEGF is more prevalent in the presence of a choroidal neovascular complex, the goal of anti-VEGF therapy is to prevent the growth of and further damage from these abnormal blood vessels.5
Conclusion
The authors have described 2 cases of asymptomatic PEHCR in elderly white males who are both currently being observed closely. Peripheral exudative hemorrhagic chorioretinopathy is an uncommon finding; therefore, knowledge of this condition also may be rare. Through this article and these cases, the importance of routine peripheral fundus examination to detect PEHCR should be stressed. It also is important to include PEHCR as a differential diagnosis when evaluating a peripheral dark elevated lesion to distinguish from peripheral melanomas and avoid unnecessary treatments. If identified, these lesions often require close observation only, and a retina referral is warranted if there is macular involvement or a rapidly progressive lesion.5
1. Pron G. Optical coherence tomography monitoring strategies for A-VEGF–treated age-related macular degeneration: an evidence-based analysis. Ont Health Technol Assess Ser. 2014;14(10):1–64.
2. Annesley WH Jr. Peripheral exudative hemorrhagic chorioretinopathy. Trans Am Ophthalmol Soc. 1980;78:321-364.
3. Mantel I, Uffer S, Zografos L. Peripheral exudative hemorrhagic chorioretinopathy: a clinical angiographic, and histologic study. Am J Ophthalmol. 2009;148(6):932-938.
4. Pinarci EY, Kilic I, Bayar SA, Sizmaz S, Akkoyun I, Yilmaz G. Clinical characteristics of peripheral exudative hemorrhagic chorioretinopathy and its response to bevacizumab therapy. Eye (Lond). 2013;27(1):111-112.
5. Seibel I, Hager A, Duncker T, et al. Anti-VEGF therapy in symptomatic peripheral exudative hemorrhagic chorioretinopathy (PEHCR) involving the macula. Graefes Arch Clin Exp Ophthalmol. 2016;254(4):653-659.
6. Collaer N, James C. Peripheral exudative and hemorrhagic chorio-retinopathy…the peripheral form of age-related macular degeneration? Report on 2 cases. Bull Soc Belge Ophtalmol. 2007;(305):23-26.
7. Goldman DR, Freund KB, McCannel CA, Sarraf D. Peripheral polypoidal choroidal vasculopathy as a cause of peripheral exudative hemorrhagic chorioretinopathy: A report of 10 eyes. Retina. 2013;33(1):48-55.
8. Mashayekhi A, Shields CL, Shields JA. Peripheral exudative hemorrhagic chorioretinopathy: a variant of polypoidal choroidal vasculopathy? J Ophthalmic Vis Res. 2013;8(3):264-267.
9. Takayama K, Enoki T, Kojima T, Ishikawa S, Takeuchi M. Treatment of peripheral exudative hemorrhagic chorioretinopathy by intravitreal injections of ranibizumab. Clin Ophthalmol. 2012;6:865-869.
10. Barkmeier AJ, Kadikoy H, Holz ER, Carvounis PE. Regression of serous macular detachment due to peripheral exudative hemorrhagic chorioretinopathy following intravitreal bevacizumab. Eur J Ophthalmol. 2011;21(4):506-508.
Age-related macular degeneration (AMD) is a common condition that affects the elderly white population. About 6.5% of Americans have been diagnosed with AMD, and 0.8% have received an end-stage AMD diagnosis.1 Exudative AMD is typically more visually debilitating and comprises between 10% and 15% of all AMD cases, with conversion from dry to wet about 10%.1
A thorough examination of the posterior pole is of utmost importance in patients with dry AMD in order to ensure there is no conversion to the exudative form. However, it also is imperative to perform a peripheral evaluation in these patients due to the incidence of peripheral choroidal neovascular membrane (CNVM) and its potential visual significance.
Case Report 1
An 80-year-old white male with type 2 diabetes mellitus (DM) without retinopathy, dry AMD, and epiretinal membranes (ERM) in both eyes presented to the eye clinic for a 6-month follow-up. On examination, he had visual acuity (VA) of 20/25 in both eyes and reported no ocular problems. The intraocular pressures were 17 mm Hg in the right eye and 20 mm Hg in the left eye. Slit-lamp examination of the anterior segment of both eyes was significant for 2+ nuclear sclerotic cataracts.
On dilated fundus exam, there were macular drusen and ERM in both eyes; peripherally in the right eye, there was cobblestone degeneration and pigmentary changes. Peripherally in the left eye, there was a large retinal pigment epithelial detachment (PED) with subretinal hemorrhage in the inferior temporal quadrant (Figure 1) along with cobblestone degeneration and pigmentary changes. Peripheral optical coherence tomography (OCT) in the left eye showed a large PED in the location of the hemorrhage (Figure 2).
Case Report 2
An 88-year-old white male presented to the eye clinic reporting blurred vision at distance and dry eyes. The patient’s medical history was remarkable for vascular and heart disease, treated with warfarin. The patient also had insulin controlled DM, with no prior history of retinopathy. His past ocular history included hard drusen in the macula, peripheral drusen, pavingstone degeneration, and a fibrotic scar temporally in the right eye.
At his annual eye examination, the patient’s vision was correctable to 20/25 in both eyes. His anterior segment slit-lamp exam was remarkable for posterior chamber intraocular lenses, clear and centered in each eye. His posterior pole exam was remarkable for small hard drusen at the macula in both eyes. Peripherally in the right eye, there was a large disciform fibrotic scar temporally (Figure 3) as well as cobblestone degeneration and peripheral drusen. The left eye revealed a large disciform hemorrhage temporally (Figure 4) with cobblestone degeneration and peripheral drusen.
Both patients currently are being closely monitored for any encroachment of the peripheral lesions into the posterior poles.
Discussion
Peripheral exudative hemorrhagic chorioretinopathy (PEHCR), also referred to in the literature as eccentric disciform CNVM, peripheral CNVM, and peripheral age-related degeneration, is a rare condition more prevalent in elderly white females.2-4 Mean age ranges from 70 to 82 years, with bilateral involvement ranging from 18% to 37%.2-4 The mid-periphery or periphery is the most common location for these lesions, more specifically, in the inferior temporal quadrant.2,3,5,6
Age-related macular degeneration is not pathognomonic for PEHCR. Mantel and colleagues reported that 68.9% of the patients in their study had AMD.3 Visual acuity ranges from 20/20 to light perception, dependent upon ocular comorbidities.2,3 As reported by Mantel and colleagues, patients with symptomatic PEHCR commonly experience visual loss, floaters, photopsias, metamorphopsia, and scotoma.3
Peripheral exudative hemorrhagic chorioretinopathy is a hemorrhagic or exudative process that can occur either as an isolated lesion or as multiple lesions that consist of a PED along with hemorrhage, subretinal fluid and/or fibrotic scarring.2-5 Peripheral exudative hemorrhagic chorioretinopathy is not visually significant unless a vitreous hemorrhage is evident or the blood and/or fluid extends to the macular region.2,5
The exact etiology of peripheral CNVM remains unknown; however, ischemia, mechanical forces, and defects in Bruch’s membrane all have been speculated as causative factors.2,3,6 Others have hypothesized that PEHCR is a form of polypoidal choroidal vasculopathy.3,7,8 A rupture in Bruch’s membrane with a vascular complex contributes to the pathophysiology and histology of this condition.3,6
Given the propensity for cardiovascular diseases, such as DM and hypertension, to lead to retinal ischemia, it is important to take a good case history.2,4,6 Additionally, anticoagulants have been shown to exacerbate bleeding.2,5 Due to PEHCR’s location in the periphery, as well as its appearance as an elevated dark mass, it is important to differentiate these lesions from a choroidal melanoma.2,6 Recognition of PEHCR can save the patient from unnecessary treatment with radiation or enucleation.
Peripheral exudative hemorrhagic chorioretinopathy is a self-limiting condition that generally requires close observation only. Long-term follow-up studies show resolution, regression, or stability of the peripheral lesions.4,5,8 If a hemorrhage is present, the blood will resolve and leave a disciform scar with pigmentary changes.2-4 In cases where vision is threatened, CNVM has been treated with photocoagulation, cryopexy, and more recently, intravitreal anti-VEGF injections.4,5,9,10 Given that VEGF is more prevalent in the presence of a choroidal neovascular complex, the goal of anti-VEGF therapy is to prevent the growth of and further damage from these abnormal blood vessels.5
Conclusion
The authors have described 2 cases of asymptomatic PEHCR in elderly white males who are both currently being observed closely. Peripheral exudative hemorrhagic chorioretinopathy is an uncommon finding; therefore, knowledge of this condition also may be rare. Through this article and these cases, the importance of routine peripheral fundus examination to detect PEHCR should be stressed. It also is important to include PEHCR as a differential diagnosis when evaluating a peripheral dark elevated lesion to distinguish from peripheral melanomas and avoid unnecessary treatments. If identified, these lesions often require close observation only, and a retina referral is warranted if there is macular involvement or a rapidly progressive lesion.5
Age-related macular degeneration (AMD) is a common condition that affects the elderly white population. About 6.5% of Americans have been diagnosed with AMD, and 0.8% have received an end-stage AMD diagnosis.1 Exudative AMD is typically more visually debilitating and comprises between 10% and 15% of all AMD cases, with conversion from dry to wet about 10%.1
A thorough examination of the posterior pole is of utmost importance in patients with dry AMD in order to ensure there is no conversion to the exudative form. However, it also is imperative to perform a peripheral evaluation in these patients due to the incidence of peripheral choroidal neovascular membrane (CNVM) and its potential visual significance.
Case Report 1
An 80-year-old white male with type 2 diabetes mellitus (DM) without retinopathy, dry AMD, and epiretinal membranes (ERM) in both eyes presented to the eye clinic for a 6-month follow-up. On examination, he had visual acuity (VA) of 20/25 in both eyes and reported no ocular problems. The intraocular pressures were 17 mm Hg in the right eye and 20 mm Hg in the left eye. Slit-lamp examination of the anterior segment of both eyes was significant for 2+ nuclear sclerotic cataracts.
On dilated fundus exam, there were macular drusen and ERM in both eyes; peripherally in the right eye, there was cobblestone degeneration and pigmentary changes. Peripherally in the left eye, there was a large retinal pigment epithelial detachment (PED) with subretinal hemorrhage in the inferior temporal quadrant (Figure 1) along with cobblestone degeneration and pigmentary changes. Peripheral optical coherence tomography (OCT) in the left eye showed a large PED in the location of the hemorrhage (Figure 2).
Case Report 2
An 88-year-old white male presented to the eye clinic reporting blurred vision at distance and dry eyes. The patient’s medical history was remarkable for vascular and heart disease, treated with warfarin. The patient also had insulin controlled DM, with no prior history of retinopathy. His past ocular history included hard drusen in the macula, peripheral drusen, pavingstone degeneration, and a fibrotic scar temporally in the right eye.
At his annual eye examination, the patient’s vision was correctable to 20/25 in both eyes. His anterior segment slit-lamp exam was remarkable for posterior chamber intraocular lenses, clear and centered in each eye. His posterior pole exam was remarkable for small hard drusen at the macula in both eyes. Peripherally in the right eye, there was a large disciform fibrotic scar temporally (Figure 3) as well as cobblestone degeneration and peripheral drusen. The left eye revealed a large disciform hemorrhage temporally (Figure 4) with cobblestone degeneration and peripheral drusen.
Both patients currently are being closely monitored for any encroachment of the peripheral lesions into the posterior poles.
Discussion
Peripheral exudative hemorrhagic chorioretinopathy (PEHCR), also referred to in the literature as eccentric disciform CNVM, peripheral CNVM, and peripheral age-related degeneration, is a rare condition more prevalent in elderly white females.2-4 Mean age ranges from 70 to 82 years, with bilateral involvement ranging from 18% to 37%.2-4 The mid-periphery or periphery is the most common location for these lesions, more specifically, in the inferior temporal quadrant.2,3,5,6
Age-related macular degeneration is not pathognomonic for PEHCR. Mantel and colleagues reported that 68.9% of the patients in their study had AMD.3 Visual acuity ranges from 20/20 to light perception, dependent upon ocular comorbidities.2,3 As reported by Mantel and colleagues, patients with symptomatic PEHCR commonly experience visual loss, floaters, photopsias, metamorphopsia, and scotoma.3
Peripheral exudative hemorrhagic chorioretinopathy is a hemorrhagic or exudative process that can occur either as an isolated lesion or as multiple lesions that consist of a PED along with hemorrhage, subretinal fluid and/or fibrotic scarring.2-5 Peripheral exudative hemorrhagic chorioretinopathy is not visually significant unless a vitreous hemorrhage is evident or the blood and/or fluid extends to the macular region.2,5
The exact etiology of peripheral CNVM remains unknown; however, ischemia, mechanical forces, and defects in Bruch’s membrane all have been speculated as causative factors.2,3,6 Others have hypothesized that PEHCR is a form of polypoidal choroidal vasculopathy.3,7,8 A rupture in Bruch’s membrane with a vascular complex contributes to the pathophysiology and histology of this condition.3,6
Given the propensity for cardiovascular diseases, such as DM and hypertension, to lead to retinal ischemia, it is important to take a good case history.2,4,6 Additionally, anticoagulants have been shown to exacerbate bleeding.2,5 Due to PEHCR’s location in the periphery, as well as its appearance as an elevated dark mass, it is important to differentiate these lesions from a choroidal melanoma.2,6 Recognition of PEHCR can save the patient from unnecessary treatment with radiation or enucleation.
Peripheral exudative hemorrhagic chorioretinopathy is a self-limiting condition that generally requires close observation only. Long-term follow-up studies show resolution, regression, or stability of the peripheral lesions.4,5,8 If a hemorrhage is present, the blood will resolve and leave a disciform scar with pigmentary changes.2-4 In cases where vision is threatened, CNVM has been treated with photocoagulation, cryopexy, and more recently, intravitreal anti-VEGF injections.4,5,9,10 Given that VEGF is more prevalent in the presence of a choroidal neovascular complex, the goal of anti-VEGF therapy is to prevent the growth of and further damage from these abnormal blood vessels.5
Conclusion
The authors have described 2 cases of asymptomatic PEHCR in elderly white males who are both currently being observed closely. Peripheral exudative hemorrhagic chorioretinopathy is an uncommon finding; therefore, knowledge of this condition also may be rare. Through this article and these cases, the importance of routine peripheral fundus examination to detect PEHCR should be stressed. It also is important to include PEHCR as a differential diagnosis when evaluating a peripheral dark elevated lesion to distinguish from peripheral melanomas and avoid unnecessary treatments. If identified, these lesions often require close observation only, and a retina referral is warranted if there is macular involvement or a rapidly progressive lesion.5
1. Pron G. Optical coherence tomography monitoring strategies for A-VEGF–treated age-related macular degeneration: an evidence-based analysis. Ont Health Technol Assess Ser. 2014;14(10):1–64.
2. Annesley WH Jr. Peripheral exudative hemorrhagic chorioretinopathy. Trans Am Ophthalmol Soc. 1980;78:321-364.
3. Mantel I, Uffer S, Zografos L. Peripheral exudative hemorrhagic chorioretinopathy: a clinical angiographic, and histologic study. Am J Ophthalmol. 2009;148(6):932-938.
4. Pinarci EY, Kilic I, Bayar SA, Sizmaz S, Akkoyun I, Yilmaz G. Clinical characteristics of peripheral exudative hemorrhagic chorioretinopathy and its response to bevacizumab therapy. Eye (Lond). 2013;27(1):111-112.
5. Seibel I, Hager A, Duncker T, et al. Anti-VEGF therapy in symptomatic peripheral exudative hemorrhagic chorioretinopathy (PEHCR) involving the macula. Graefes Arch Clin Exp Ophthalmol. 2016;254(4):653-659.
6. Collaer N, James C. Peripheral exudative and hemorrhagic chorio-retinopathy…the peripheral form of age-related macular degeneration? Report on 2 cases. Bull Soc Belge Ophtalmol. 2007;(305):23-26.
7. Goldman DR, Freund KB, McCannel CA, Sarraf D. Peripheral polypoidal choroidal vasculopathy as a cause of peripheral exudative hemorrhagic chorioretinopathy: A report of 10 eyes. Retina. 2013;33(1):48-55.
8. Mashayekhi A, Shields CL, Shields JA. Peripheral exudative hemorrhagic chorioretinopathy: a variant of polypoidal choroidal vasculopathy? J Ophthalmic Vis Res. 2013;8(3):264-267.
9. Takayama K, Enoki T, Kojima T, Ishikawa S, Takeuchi M. Treatment of peripheral exudative hemorrhagic chorioretinopathy by intravitreal injections of ranibizumab. Clin Ophthalmol. 2012;6:865-869.
10. Barkmeier AJ, Kadikoy H, Holz ER, Carvounis PE. Regression of serous macular detachment due to peripheral exudative hemorrhagic chorioretinopathy following intravitreal bevacizumab. Eur J Ophthalmol. 2011;21(4):506-508.
1. Pron G. Optical coherence tomography monitoring strategies for A-VEGF–treated age-related macular degeneration: an evidence-based analysis. Ont Health Technol Assess Ser. 2014;14(10):1–64.
2. Annesley WH Jr. Peripheral exudative hemorrhagic chorioretinopathy. Trans Am Ophthalmol Soc. 1980;78:321-364.
3. Mantel I, Uffer S, Zografos L. Peripheral exudative hemorrhagic chorioretinopathy: a clinical angiographic, and histologic study. Am J Ophthalmol. 2009;148(6):932-938.
4. Pinarci EY, Kilic I, Bayar SA, Sizmaz S, Akkoyun I, Yilmaz G. Clinical characteristics of peripheral exudative hemorrhagic chorioretinopathy and its response to bevacizumab therapy. Eye (Lond). 2013;27(1):111-112.
5. Seibel I, Hager A, Duncker T, et al. Anti-VEGF therapy in symptomatic peripheral exudative hemorrhagic chorioretinopathy (PEHCR) involving the macula. Graefes Arch Clin Exp Ophthalmol. 2016;254(4):653-659.
6. Collaer N, James C. Peripheral exudative and hemorrhagic chorio-retinopathy…the peripheral form of age-related macular degeneration? Report on 2 cases. Bull Soc Belge Ophtalmol. 2007;(305):23-26.
7. Goldman DR, Freund KB, McCannel CA, Sarraf D. Peripheral polypoidal choroidal vasculopathy as a cause of peripheral exudative hemorrhagic chorioretinopathy: A report of 10 eyes. Retina. 2013;33(1):48-55.
8. Mashayekhi A, Shields CL, Shields JA. Peripheral exudative hemorrhagic chorioretinopathy: a variant of polypoidal choroidal vasculopathy? J Ophthalmic Vis Res. 2013;8(3):264-267.
9. Takayama K, Enoki T, Kojima T, Ishikawa S, Takeuchi M. Treatment of peripheral exudative hemorrhagic chorioretinopathy by intravitreal injections of ranibizumab. Clin Ophthalmol. 2012;6:865-869.
10. Barkmeier AJ, Kadikoy H, Holz ER, Carvounis PE. Regression of serous macular detachment due to peripheral exudative hemorrhagic chorioretinopathy following intravitreal bevacizumab. Eur J Ophthalmol. 2011;21(4):506-508.
Acquired Epidermodysplasia Verruciformis Occurring in a Renal Transplant Recipient
Acquired epidermodysplasia verruciformis (EDV) is a rare disorder occurring in patients with depressed cellular immunity, particularly individuals with human immunodeficiency virus (HIV). Rare cases of acquired EDV have been reported in stem cell or solid organ transplant recipients. Weakened cellular immunity predisposes the patient to human papillomavirus (HPV) infections, with 92% of renal transplant recipients developing warts within 5 years posttransplantation.1 Specific EDV-HPV subtypes have been isolated from lesions in several immunosuppressed individuals, with HPV-5 and HPV-8 being the most commonly isolated subtypes.2,3 Herein, we present the clinical findings of a renal transplant recipient who presented for evaluation of multiple skin lesions characteristic of EDV 5 years following transplantation and initiation of immunosuppressive therapy. Additionally, we review the current diagnostic findings, management, and treatment of acquired EDV.
A 44-year-old white woman presented for evaluation of several pruritic cutaneous lesions that had developed on the chest and neck of 1 month’s duration. The patient had been on the immunosuppressant medications cyclosporine and mycophenolate mofetil for more than 5 years following renal transplantation 7 years prior to the current presentation. She also was on low-dose prednisone for chronic systemic lupus erythematosus. Her family history was negative for any pertinent skin conditions.
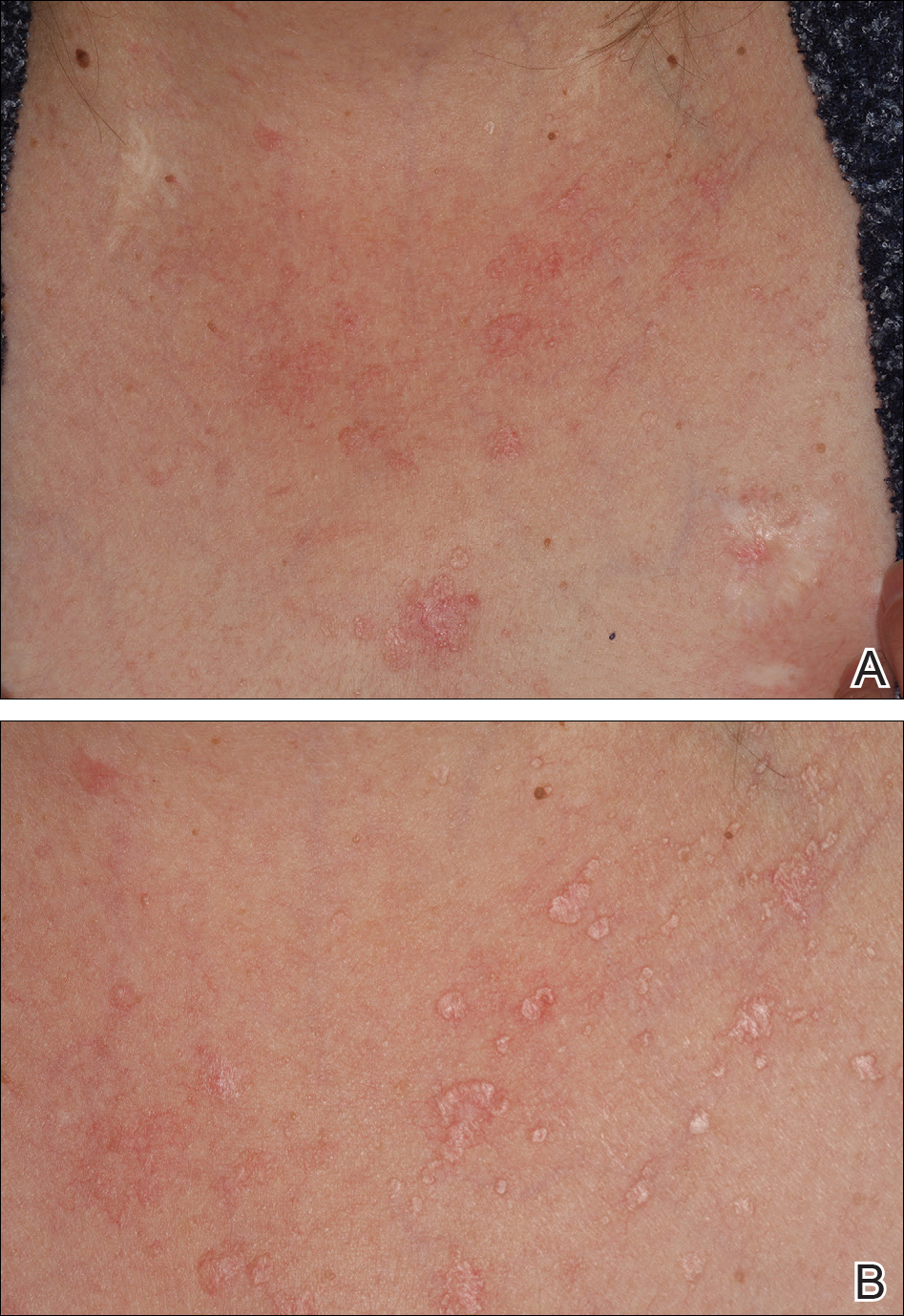
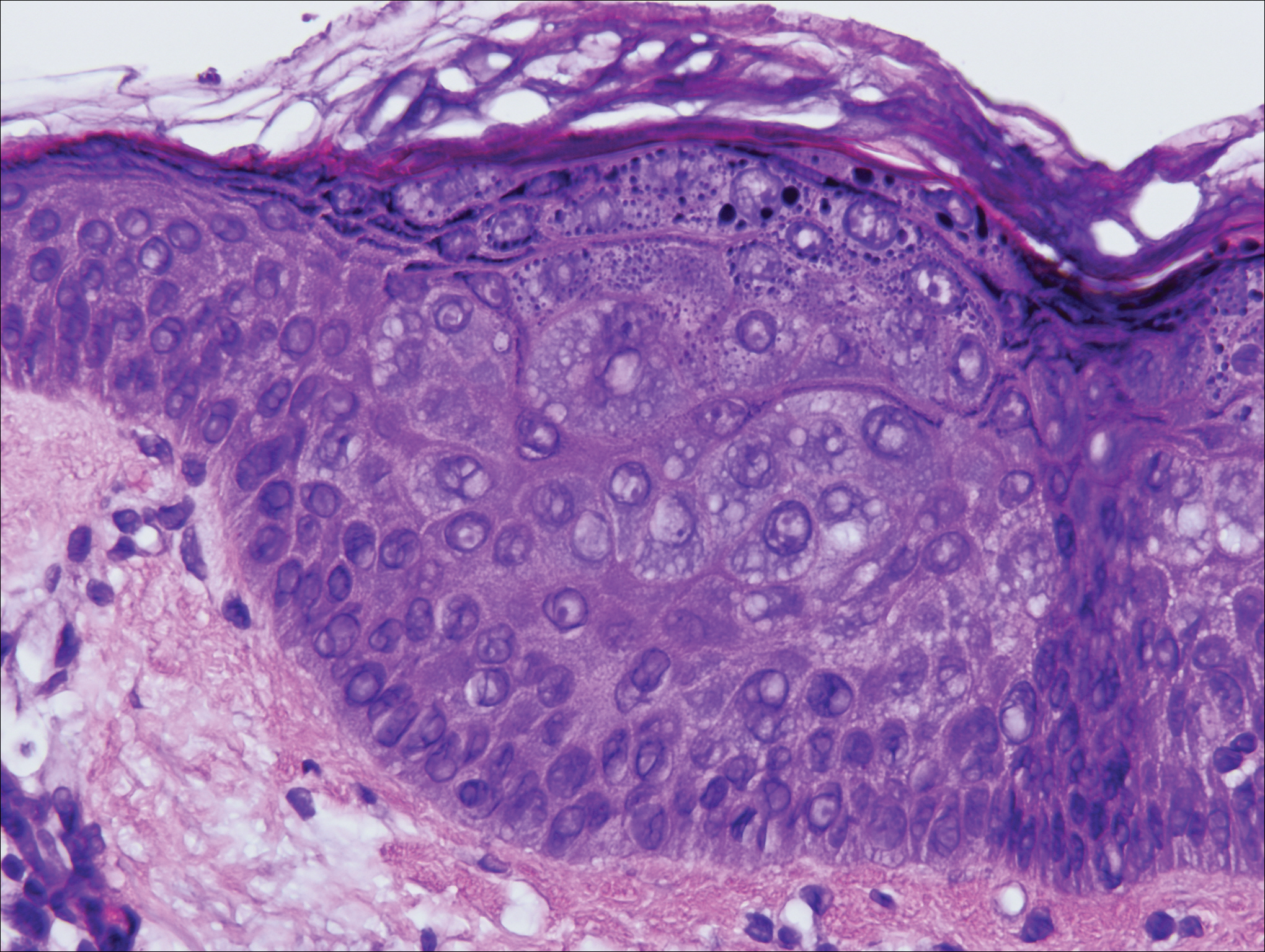
On physical examination the patient exhibited several grouped 0.5-cm, shiny, pink lichenoid macules located on the upper mid chest, anterior neck, and left leg clinically resembling the lesions of pityriasis versicolor (Figure 1). A shave biopsy was taken from one of the newest lesions on the left leg. Histopathology revealed viral epidermal cytopathic changes, blue cytoplasm, and coarse hypergranulosis characteristic of EDV (Figure 2). A diagnosis of acquired EDV was made based on the clinical and histopathologic findings.
The patient’s skin lesions became more widespread despite several different treatment regimens, including cryosurgery; tazarotene cream 0.05% nightly; imiquimod cream 5% once weekly; and intermittent short courses of 5-fluorouracil cream 5%, which provided the best response. At her most recent clinic visit 8 years after initial presentation, she continued to have more widespread lesions on the trunk, arms, and legs, but no evidence of malignant transformation.
Comment
Epidermodysplasia verruciformis was first recognized as an inherited condition, most commonly inherited in an autosomal-dominant fashion; however, X-linked recessive cases have been reported.4,5 Patients with the inherited forms of this condition are prone to recurrent HPV infections secondary to a missense mutation in the epidermodysplasia verruciformis 1 and 2 genes, EVER1 and EVER2, on the EV1 locus located on chromosome 17q25.6 Because of this mutation, the patient’s cellular immunity becomes weakened. Cellular presentation of the EDV-HPV antigen to T lymphocytes becomes impaired, thereby inhibiting the body’s ability to successfully clear itself of the virus.5,6 The most commonly isolated EDV-HPV subtypes are HPV-5 and HPV-8, but HPV types 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, and 50 also have been associated with EDV.1,3,7
Patients who have suppressed cellular immunity, such as transplant recipients on long-term immunosuppressant medications and individuals with HIV, graft-vs-host disease, systemic lupus erythematosus, and hematologic malignancies, are susceptible to EDV, as well as patients with atopic dermatitis being treated with topical calcineurin inhibitors.2,3,8-15 These patients acquire depressed cellular immunity and become increasingly susceptible to infections with the EDV-HPV subtypes. When clinical and histopathologic findings are consistent with EDV, a diagnosis of acquired EDV is given, which was further confirmed in a study conducted by Harwood et al.16 They found immunocompromised patients carry more EDV-HPV subtypes in skin lesions analyzed by polymerase chain reaction than immunocompetent individuals.16 Additionally, there is a positive correlation between the length of immunosuppression and the development of HPV lesions, with a majority of patients developing lesions within 5 years following initial immunosuppression.1,7,10,17
Epidermodysplasia verruciformis commonly presents with multiple hypopigmented to red macules that may coalesce into patches with a fine scale, clinically resembling the lesions of pityriasis versicolor.2,3,8-15 Epidermodysplasia verruciformis also may present as multiple flesh-colored, flat-topped, verrucous papules that clinically resemble the lesions of verruca plana on sun-exposed areas such as the face, arms, and legs.9 The characteristic histopathologic findings are enlarged keratinocytes with perinuclear halos and blue-gray cytoplasm as well as hypergranulosis.18 Immunocompromised hosts infected with EDV-HPV histologically tend to display more severe dysplasia than immunocompetent individuals.19 The differential diagnosis includes pityriasis versicolor, squamous cell carcinoma (SCC), and verruca plana. Tissue cultures and potassium hydroxide scrapings for microorganisms should be negative.
The specific EDV-HPV strains 5, 8, and 41 carry the highest oncogenic potential, with more than 60% of inherited EDV patients developing SCC by the fourth and fifth decades of life.16 Unlike inherited EDV, the clinical course of acquired EDV is less well known; however, UV light is thought to act synergistically with the EDV-HPV in oncogenic transformation of the lesions, as most of the SCCs develop on sun-exposed areas, and darker-skinned patients seem to have a decreased risk for malignant transformation of EDV lesions.4,9,20,21 Preventative measures such as strict sun protection and annual surveillance of lesions can help to prevent oncogenic progression of the lesions; however, several single- and multiple-agent regimens have been used in the treatment of EDV with variable results. Topical imiquimod, 5-fluorouracil, tretinoin, and tazarotene have been used with variable success. Acitretin alone and in combination with interferon alfa-2a also has been used.22,23 Highly active antiretroviral therapy in patients with HIV has effectively decreased the number of lesions in a subset of patients.24 We (anecdotal) and others25 also have had success using photodynamic therapy. Squamous cell carcinoma arising in patients with EDV can be managed by excision or by Mohs micrographic surgery.
Conclusion
We report a rare case of acquired EDV in a solid organ transplant recipient. Epidermodysplasia verruciformis can be acquired in immunosuppressed patients such as ours, and these patients should be followed closely due to the potential for malignant transformation. More studies regarding the anticipated clinical course of skin lesions in patients with acquired EDV are needed to better predict the time frame for malignant transformation.
- Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789.
- Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983;2:422-424.
- Lutzner M, Croissant O, Ducasse MF, et al. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980;75:353-356.
- Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
- Lutzner MA. Epidermodysplasia verruciformis. an autosomal recessive disease characterized by viral warts and skin cancer. a model for viral oncogenesis. Bull Cancer. 1978;65:169-182.
- Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
- Rüdlinger R, Smith IW, Bunney MH, et al. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986;115:681-692.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124-129.
- Tanigaki T, Kanda R, Sato K. Epidermodysplasia verruciformis (L-L, 1922) in a patient with systemic lupus erythematosus. Arch Dermatol Res. 1986;278:247-248.
- Holmes C, Chong AH, Tabrizi SN, et al. Epidermodysplasia verruciformis-like syndrome in association with systemic lupus erythematosus. Australas J Dermatol. 2009;50:44-47.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Fernandez KH, Rady P, Tyring S, et al. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis [published online September 3, 2012]. Pediatr Dermatol. 2014;31:400-402.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:307-311.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297.
- Moloney FJ, Keane S, O’Kelly P, et al. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578.
- Tanigaki T, Endo H. A case of epidermodysplasia verruciformis (Lewandowsky-Lutz, 1922) with skin cancer: histopathology of malignant cutaneous changes. Dermatologica. 1984;169:97-101.
- Morrison C, Eliezri Y, Magro C, et al. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. J Cutan Pathol. 2002;29:480-489.
- Majewski S, Jabło´nska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Gubinelli E, Posteraro P, Cocuroccia B, et al. Epidermodysplasia verruciformis with multiple mucosal carcinomas treated with pegylated interferon alfa and acitretin. J Dermatolog Treat. 2003;14:184-188.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
- Haas N, Fuchs PG, Hermes B, et al. Remission of epidermodysplasia verruciformis-like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus-positive patient. Br J Dermatol. 2001;145:669-670.
- Karrer S, Szeimies RM, Abels C, et al. Epidermo-dysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 1999;140:935-938.
Acquired epidermodysplasia verruciformis (EDV) is a rare disorder occurring in patients with depressed cellular immunity, particularly individuals with human immunodeficiency virus (HIV). Rare cases of acquired EDV have been reported in stem cell or solid organ transplant recipients. Weakened cellular immunity predisposes the patient to human papillomavirus (HPV) infections, with 92% of renal transplant recipients developing warts within 5 years posttransplantation.1 Specific EDV-HPV subtypes have been isolated from lesions in several immunosuppressed individuals, with HPV-5 and HPV-8 being the most commonly isolated subtypes.2,3 Herein, we present the clinical findings of a renal transplant recipient who presented for evaluation of multiple skin lesions characteristic of EDV 5 years following transplantation and initiation of immunosuppressive therapy. Additionally, we review the current diagnostic findings, management, and treatment of acquired EDV.
A 44-year-old white woman presented for evaluation of several pruritic cutaneous lesions that had developed on the chest and neck of 1 month’s duration. The patient had been on the immunosuppressant medications cyclosporine and mycophenolate mofetil for more than 5 years following renal transplantation 7 years prior to the current presentation. She also was on low-dose prednisone for chronic systemic lupus erythematosus. Her family history was negative for any pertinent skin conditions.
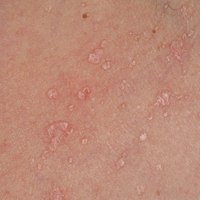
On physical examination the patient exhibited several grouped 0.5-cm, shiny, pink lichenoid macules located on the upper mid chest, anterior neck, and left leg clinically resembling the lesions of pityriasis versicolor (Figure 1). A shave biopsy was taken from one of the newest lesions on the left leg. Histopathology revealed viral epidermal cytopathic changes, blue cytoplasm, and coarse hypergranulosis characteristic of EDV (Figure 2). A diagnosis of acquired EDV was made based on the clinical and histopathologic findings.


The patient’s skin lesions became more widespread despite several different treatment regimens, including cryosurgery; tazarotene cream 0.05% nightly; imiquimod cream 5% once weekly; and intermittent short courses of 5-fluorouracil cream 5%, which provided the best response. At her most recent clinic visit 8 years after initial presentation, she continued to have more widespread lesions on the trunk, arms, and legs, but no evidence of malignant transformation.
Comment
Epidermodysplasia verruciformis was first recognized as an inherited condition, most commonly inherited in an autosomal-dominant fashion; however, X-linked recessive cases have been reported.4,5 Patients with the inherited forms of this condition are prone to recurrent HPV infections secondary to a missense mutation in the epidermodysplasia verruciformis 1 and 2 genes, EVER1 and EVER2, on the EV1 locus located on chromosome 17q25.6 Because of this mutation, the patient’s cellular immunity becomes weakened. Cellular presentation of the EDV-HPV antigen to T lymphocytes becomes impaired, thereby inhibiting the body’s ability to successfully clear itself of the virus.5,6 The most commonly isolated EDV-HPV subtypes are HPV-5 and HPV-8, but HPV types 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, and 50 also have been associated with EDV.1,3,7
Patients who have suppressed cellular immunity, such as transplant recipients on long-term immunosuppressant medications and individuals with HIV, graft-vs-host disease, systemic lupus erythematosus, and hematologic malignancies, are susceptible to EDV, as well as patients with atopic dermatitis being treated with topical calcineurin inhibitors.2,3,8-15 These patients acquire depressed cellular immunity and become increasingly susceptible to infections with the EDV-HPV subtypes. When clinical and histopathologic findings are consistent with EDV, a diagnosis of acquired EDV is given, which was further confirmed in a study conducted by Harwood et al.16 They found immunocompromised patients carry more EDV-HPV subtypes in skin lesions analyzed by polymerase chain reaction than immunocompetent individuals.16 Additionally, there is a positive correlation between the length of immunosuppression and the development of HPV lesions, with a majority of patients developing lesions within 5 years following initial immunosuppression.1,7,10,17
Epidermodysplasia verruciformis commonly presents with multiple hypopigmented to red macules that may coalesce into patches with a fine scale, clinically resembling the lesions of pityriasis versicolor.2,3,8-15 Epidermodysplasia verruciformis also may present as multiple flesh-colored, flat-topped, verrucous papules that clinically resemble the lesions of verruca plana on sun-exposed areas such as the face, arms, and legs.9 The characteristic histopathologic findings are enlarged keratinocytes with perinuclear halos and blue-gray cytoplasm as well as hypergranulosis.18 Immunocompromised hosts infected with EDV-HPV histologically tend to display more severe dysplasia than immunocompetent individuals.19 The differential diagnosis includes pityriasis versicolor, squamous cell carcinoma (SCC), and verruca plana. Tissue cultures and potassium hydroxide scrapings for microorganisms should be negative.
The specific EDV-HPV strains 5, 8, and 41 carry the highest oncogenic potential, with more than 60% of inherited EDV patients developing SCC by the fourth and fifth decades of life.16 Unlike inherited EDV, the clinical course of acquired EDV is less well known; however, UV light is thought to act synergistically with the EDV-HPV in oncogenic transformation of the lesions, as most of the SCCs develop on sun-exposed areas, and darker-skinned patients seem to have a decreased risk for malignant transformation of EDV lesions.4,9,20,21 Preventative measures such as strict sun protection and annual surveillance of lesions can help to prevent oncogenic progression of the lesions; however, several single- and multiple-agent regimens have been used in the treatment of EDV with variable results. Topical imiquimod, 5-fluorouracil, tretinoin, and tazarotene have been used with variable success. Acitretin alone and in combination with interferon alfa-2a also has been used.22,23 Highly active antiretroviral therapy in patients with HIV has effectively decreased the number of lesions in a subset of patients.24 We (anecdotal) and others25 also have had success using photodynamic therapy. Squamous cell carcinoma arising in patients with EDV can be managed by excision or by Mohs micrographic surgery.
Conclusion
We report a rare case of acquired EDV in a solid organ transplant recipient. Epidermodysplasia verruciformis can be acquired in immunosuppressed patients such as ours, and these patients should be followed closely due to the potential for malignant transformation. More studies regarding the anticipated clinical course of skin lesions in patients with acquired EDV are needed to better predict the time frame for malignant transformation.
Acquired epidermodysplasia verruciformis (EDV) is a rare disorder occurring in patients with depressed cellular immunity, particularly individuals with human immunodeficiency virus (HIV). Rare cases of acquired EDV have been reported in stem cell or solid organ transplant recipients. Weakened cellular immunity predisposes the patient to human papillomavirus (HPV) infections, with 92% of renal transplant recipients developing warts within 5 years posttransplantation.1 Specific EDV-HPV subtypes have been isolated from lesions in several immunosuppressed individuals, with HPV-5 and HPV-8 being the most commonly isolated subtypes.2,3 Herein, we present the clinical findings of a renal transplant recipient who presented for evaluation of multiple skin lesions characteristic of EDV 5 years following transplantation and initiation of immunosuppressive therapy. Additionally, we review the current diagnostic findings, management, and treatment of acquired EDV.
A 44-year-old white woman presented for evaluation of several pruritic cutaneous lesions that had developed on the chest and neck of 1 month’s duration. The patient had been on the immunosuppressant medications cyclosporine and mycophenolate mofetil for more than 5 years following renal transplantation 7 years prior to the current presentation. She also was on low-dose prednisone for chronic systemic lupus erythematosus. Her family history was negative for any pertinent skin conditions.
On physical examination the patient exhibited several grouped 0.5-cm, shiny, pink lichenoid macules located on the upper mid chest, anterior neck, and left leg clinically resembling the lesions of pityriasis versicolor (Figure 1). A shave biopsy was taken from one of the newest lesions on the left leg. Histopathology revealed viral epidermal cytopathic changes, blue cytoplasm, and coarse hypergranulosis characteristic of EDV (Figure 2). A diagnosis of acquired EDV was made based on the clinical and histopathologic findings.


The patient’s skin lesions became more widespread despite several different treatment regimens, including cryosurgery; tazarotene cream 0.05% nightly; imiquimod cream 5% once weekly; and intermittent short courses of 5-fluorouracil cream 5%, which provided the best response. At her most recent clinic visit 8 years after initial presentation, she continued to have more widespread lesions on the trunk, arms, and legs, but no evidence of malignant transformation.
Comment
Epidermodysplasia verruciformis was first recognized as an inherited condition, most commonly inherited in an autosomal-dominant fashion; however, X-linked recessive cases have been reported.4,5 Patients with the inherited forms of this condition are prone to recurrent HPV infections secondary to a missense mutation in the epidermodysplasia verruciformis 1 and 2 genes, EVER1 and EVER2, on the EV1 locus located on chromosome 17q25.6 Because of this mutation, the patient’s cellular immunity becomes weakened. Cellular presentation of the EDV-HPV antigen to T lymphocytes becomes impaired, thereby inhibiting the body’s ability to successfully clear itself of the virus.5,6 The most commonly isolated EDV-HPV subtypes are HPV-5 and HPV-8, but HPV types 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, and 50 also have been associated with EDV.1,3,7
Patients who have suppressed cellular immunity, such as transplant recipients on long-term immunosuppressant medications and individuals with HIV, graft-vs-host disease, systemic lupus erythematosus, and hematologic malignancies, are susceptible to EDV, as well as patients with atopic dermatitis being treated with topical calcineurin inhibitors.2,3,8-15 These patients acquire depressed cellular immunity and become increasingly susceptible to infections with the EDV-HPV subtypes. When clinical and histopathologic findings are consistent with EDV, a diagnosis of acquired EDV is given, which was further confirmed in a study conducted by Harwood et al.16 They found immunocompromised patients carry more EDV-HPV subtypes in skin lesions analyzed by polymerase chain reaction than immunocompetent individuals.16 Additionally, there is a positive correlation between the length of immunosuppression and the development of HPV lesions, with a majority of patients developing lesions within 5 years following initial immunosuppression.1,7,10,17
Epidermodysplasia verruciformis commonly presents with multiple hypopigmented to red macules that may coalesce into patches with a fine scale, clinically resembling the lesions of pityriasis versicolor.2,3,8-15 Epidermodysplasia verruciformis also may present as multiple flesh-colored, flat-topped, verrucous papules that clinically resemble the lesions of verruca plana on sun-exposed areas such as the face, arms, and legs.9 The characteristic histopathologic findings are enlarged keratinocytes with perinuclear halos and blue-gray cytoplasm as well as hypergranulosis.18 Immunocompromised hosts infected with EDV-HPV histologically tend to display more severe dysplasia than immunocompetent individuals.19 The differential diagnosis includes pityriasis versicolor, squamous cell carcinoma (SCC), and verruca plana. Tissue cultures and potassium hydroxide scrapings for microorganisms should be negative.
The specific EDV-HPV strains 5, 8, and 41 carry the highest oncogenic potential, with more than 60% of inherited EDV patients developing SCC by the fourth and fifth decades of life.16 Unlike inherited EDV, the clinical course of acquired EDV is less well known; however, UV light is thought to act synergistically with the EDV-HPV in oncogenic transformation of the lesions, as most of the SCCs develop on sun-exposed areas, and darker-skinned patients seem to have a decreased risk for malignant transformation of EDV lesions.4,9,20,21 Preventative measures such as strict sun protection and annual surveillance of lesions can help to prevent oncogenic progression of the lesions; however, several single- and multiple-agent regimens have been used in the treatment of EDV with variable results. Topical imiquimod, 5-fluorouracil, tretinoin, and tazarotene have been used with variable success. Acitretin alone and in combination with interferon alfa-2a also has been used.22,23 Highly active antiretroviral therapy in patients with HIV has effectively decreased the number of lesions in a subset of patients.24 We (anecdotal) and others25 also have had success using photodynamic therapy. Squamous cell carcinoma arising in patients with EDV can be managed by excision or by Mohs micrographic surgery.
Conclusion
We report a rare case of acquired EDV in a solid organ transplant recipient. Epidermodysplasia verruciformis can be acquired in immunosuppressed patients such as ours, and these patients should be followed closely due to the potential for malignant transformation. More studies regarding the anticipated clinical course of skin lesions in patients with acquired EDV are needed to better predict the time frame for malignant transformation.
- Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789.
- Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983;2:422-424.
- Lutzner M, Croissant O, Ducasse MF, et al. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980;75:353-356.
- Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
- Lutzner MA. Epidermodysplasia verruciformis. an autosomal recessive disease characterized by viral warts and skin cancer. a model for viral oncogenesis. Bull Cancer. 1978;65:169-182.
- Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
- Rüdlinger R, Smith IW, Bunney MH, et al. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986;115:681-692.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124-129.
- Tanigaki T, Kanda R, Sato K. Epidermodysplasia verruciformis (L-L, 1922) in a patient with systemic lupus erythematosus. Arch Dermatol Res. 1986;278:247-248.
- Holmes C, Chong AH, Tabrizi SN, et al. Epidermodysplasia verruciformis-like syndrome in association with systemic lupus erythematosus. Australas J Dermatol. 2009;50:44-47.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Fernandez KH, Rady P, Tyring S, et al. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis [published online September 3, 2012]. Pediatr Dermatol. 2014;31:400-402.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:307-311.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297.
- Moloney FJ, Keane S, O’Kelly P, et al. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578.
- Tanigaki T, Endo H. A case of epidermodysplasia verruciformis (Lewandowsky-Lutz, 1922) with skin cancer: histopathology of malignant cutaneous changes. Dermatologica. 1984;169:97-101.
- Morrison C, Eliezri Y, Magro C, et al. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. J Cutan Pathol. 2002;29:480-489.
- Majewski S, Jabło´nska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Gubinelli E, Posteraro P, Cocuroccia B, et al. Epidermodysplasia verruciformis with multiple mucosal carcinomas treated with pegylated interferon alfa and acitretin. J Dermatolog Treat. 2003;14:184-188.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
- Haas N, Fuchs PG, Hermes B, et al. Remission of epidermodysplasia verruciformis-like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus-positive patient. Br J Dermatol. 2001;145:669-670.
- Karrer S, Szeimies RM, Abels C, et al. Epidermo-dysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 1999;140:935-938.
- Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789.
- Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983;2:422-424.
- Lutzner M, Croissant O, Ducasse MF, et al. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980;75:353-356.
- Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
- Lutzner MA. Epidermodysplasia verruciformis. an autosomal recessive disease characterized by viral warts and skin cancer. a model for viral oncogenesis. Bull Cancer. 1978;65:169-182.
- Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
- Rüdlinger R, Smith IW, Bunney MH, et al. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986;115:681-692.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124-129.
- Tanigaki T, Kanda R, Sato K. Epidermodysplasia verruciformis (L-L, 1922) in a patient with systemic lupus erythematosus. Arch Dermatol Res. 1986;278:247-248.
- Holmes C, Chong AH, Tabrizi SN, et al. Epidermodysplasia verruciformis-like syndrome in association with systemic lupus erythematosus. Australas J Dermatol. 2009;50:44-47.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Fernandez KH, Rady P, Tyring S, et al. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis [published online September 3, 2012]. Pediatr Dermatol. 2014;31:400-402.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:307-311.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297.
- Moloney FJ, Keane S, O’Kelly P, et al. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578.
- Tanigaki T, Endo H. A case of epidermodysplasia verruciformis (Lewandowsky-Lutz, 1922) with skin cancer: histopathology of malignant cutaneous changes. Dermatologica. 1984;169:97-101.
- Morrison C, Eliezri Y, Magro C, et al. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. J Cutan Pathol. 2002;29:480-489.
- Majewski S, Jabło´nska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Gubinelli E, Posteraro P, Cocuroccia B, et al. Epidermodysplasia verruciformis with multiple mucosal carcinomas treated with pegylated interferon alfa and acitretin. J Dermatolog Treat. 2003;14:184-188.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
- Haas N, Fuchs PG, Hermes B, et al. Remission of epidermodysplasia verruciformis-like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus-positive patient. Br J Dermatol. 2001;145:669-670.
- Karrer S, Szeimies RM, Abels C, et al. Epidermo-dysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 1999;140:935-938.
Practice Points
- Acquired epidermodysplasia verruciformis (EDV) is a rare complication of iatrogenic immuno-suppression in the setting of solid organ transplantation.
- Patients with EDV should be counseled to avoid exposure to UV radiation to reduce the risk formalignant transformation.
Cutaneous Metastasis of a Pulmonary Carcinoid Tumor
Case Report
A 72-year-old white man with a history of pancreatic adenocarcinoma presented for Mohs micrographic surgery of a basal cell carcinoma on the right helix. On the day of the surgery, the patient reported a new, rapidly growing, exquisitely painful lesion on the cheek of 3 to 4 weeks’ duration. Physical examination revealed a 0.8×0.8×0.8-cm, extremely tender, firm, pink papule on the right preauricular cheek. A horizontal deep shave excision was done and the histopathology was remarkable for neoplastic cells with necrosis in the dermis. We observed dermal cellular infiltrates in the form of sheets and nodules, some showing central necrosis (Figure 1). At higher magnification, a trabecular arrangement of cells was seen. These cells had a moderate amount of cytoplasm with eccentric nuclei and rare nucleoli (Figure 2). Mitotic figures were seen at higher magnification (Figure 3). Immunohistochemistry of the neoplastic cells exhibited similar positive staining for the neuroendocrine markers chromogranin A and synaptophysin (Figure 4). Staining of the neoplastic cells also was positive for thyroid transcription factor 1 (TTF-1) and cancer antigen 19-9. Villin and caudal type homeobox 2 stains were negative. These results were consistent with cutaneous metastasis from a known pulmonary carcinoid tumor.
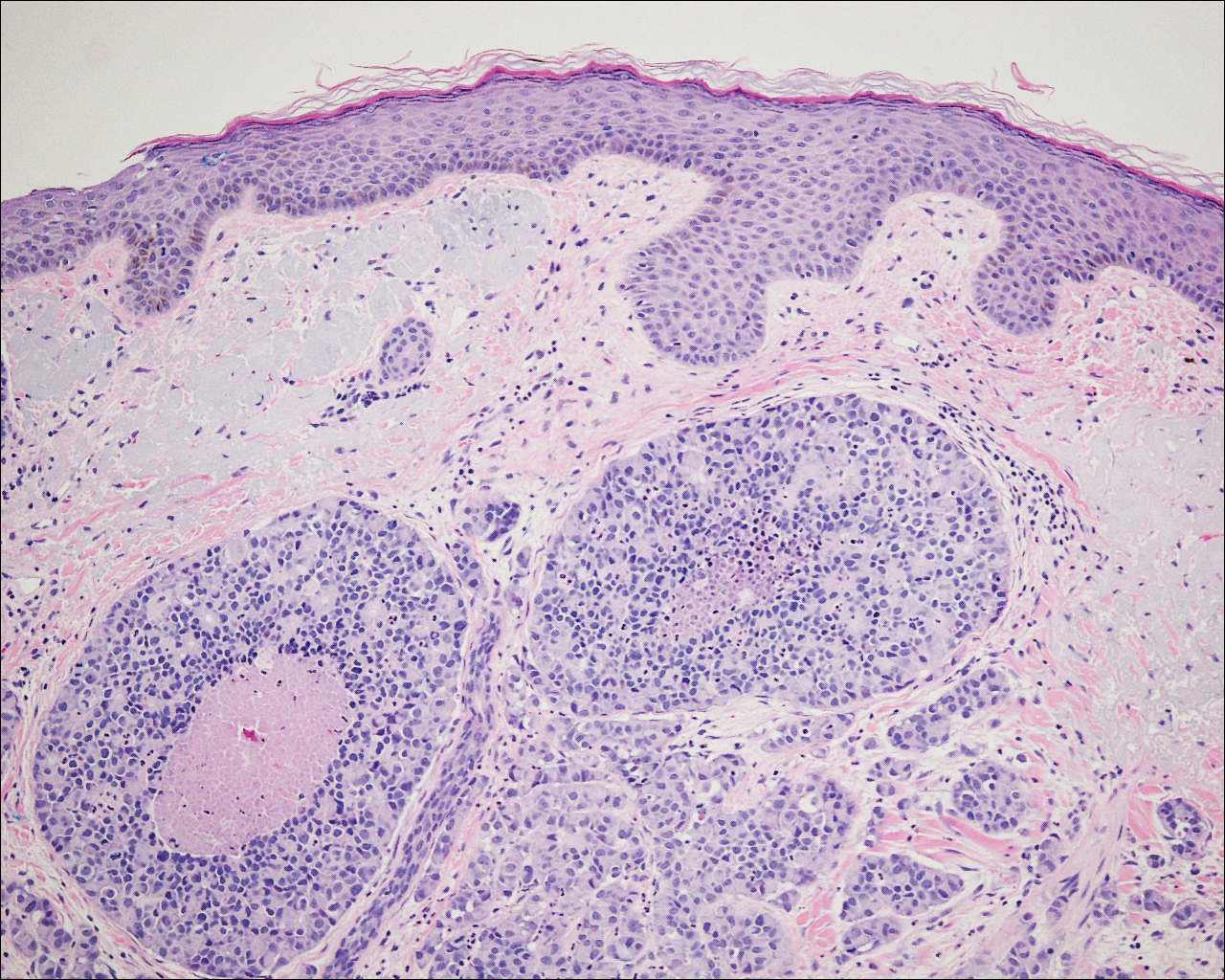
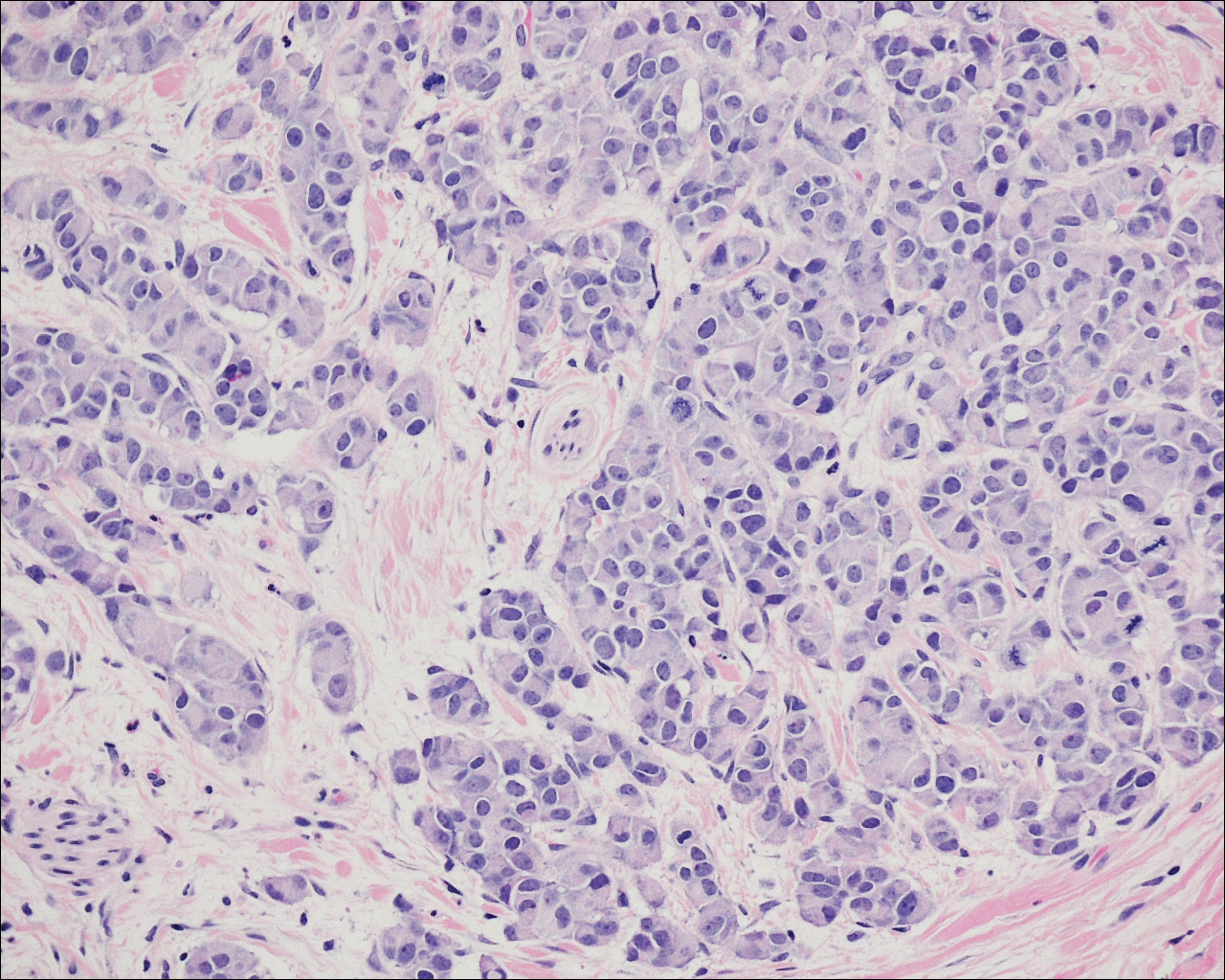
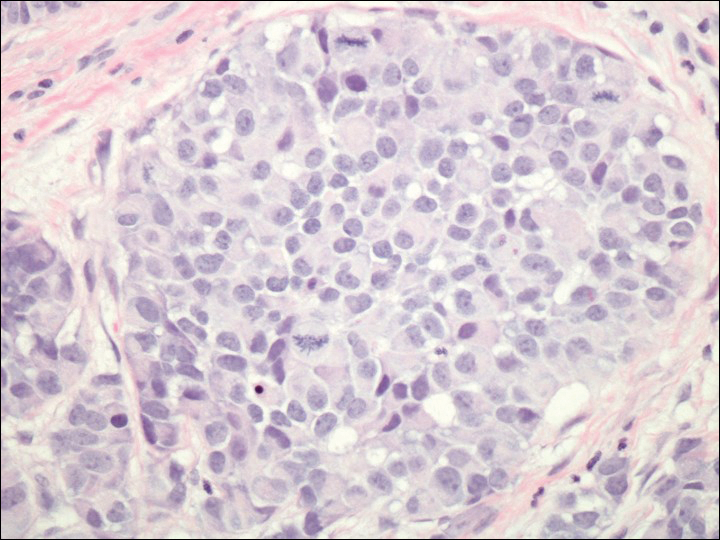
On further review of the patient’s medical history, it was discovered that he had undergone a Whipple procedure with adjuvant chemotherapy and radiation for pancreatic adenocarcinoma approximately 4 years prior to the current presentation. He was then followed by oncology, and 3 years later a chest computed tomography suggested possible disease progression with a new pulmonary metastasis. This pulmonary lesion was biopsied and immunologic staining was consistent with a primary neuroendocrine neoplasm of the lung, a new carcinoid tumor. The tissue was positive for cytokeratin (CK) 7,TTF-1, cancer antigen 19-9, CD56, synaptophysin, and chromogranin A, and was negative for villin and CK20. By the time he was seen in our clinic, several trials of chemotherapy had failed. Serial computed tomography subsequently demonstrated progression of the lung disease and he later developed malignant pleural effusions. Approximately 6 months after the cutaneous carcinoid metastasis was diagnosed, the patient died of respiratory failure.
Comment
Carcinoid tumors are uncommon neoplasms of neuroendocrine origin that generally arise in the gastrointestinal or bronchopulmonary tracts. Metastases from these primary neoplasms more commonly affect the regional lymph nodes or viscera, with rare reports of cutaneous metastases to the skin. The true incidence of carcinoid tumors with metastasis to the skin is unknown because it is limited to single case reports in the literature.
The clinical presentation of cutaneous carcinoid metastases has been reported most commonly as firm papules of varying sizes with no specific site predilection.1 The color of these lesions has ranged from erythematous to violaceous to brown.2 Several of the reported cases were noted to be extremely tender and painful, while other reports of lesions were noted to be asymptomatic or only mildly pruritic.3-7
Carcinoid syndrome is more common with neoplasms present within the gastrointestinal tract, but it also has been reported with large bronchial carcinoid tumors and with metastatic disease.8,9 Paroxysmal flushing is the most prominent cutaneous manifestation of this syndrome, occurring in 75% of patients.10,11 Other common symptoms include patchy cyanosis, telangiectasia, and pellagralike skin lesions.3 Carcinoid syndrome secondary to bronchial adenomas is thought to differ from gastrointestinal carcinoid neoplasms in that it has prolonged flushing (hours to days instead of minutes) and is characterized by marked anxiety, fever, disorientation, sweating, and lacrimation.8,9
Many cases of cutaneous carcinoid metastases have been accompanied by reports of exquisite tenderness,7 similar to our patient. The pathogenesis of the pain in these lesions is still unclear, but several hypotheses have been established. It has been postulated that perineural invasion by the tumor is responsible for the pain; however, this finding has been inconsistent, as neural involvement also has been present in nonpainful lesions.2,5,7,12 Another theory for the pain is that it is secondary to the release of vasoactive substances and peptide hormones from the carcinoid cells, such as kallikrein and serotonin. Lastly, local tissue necrosis and fibrosis also have been suggested as possible etiologies.7
The histology of cutaneous carcinoid metastases typically resembles the primary lesion and may demonstrate fascicles of spindle cells with focal areas of necrosis, mild atypia, and a relatively low mitotic rate.10 Other neoplasms such as Merkel cell carcinoma and carcinoidlike sebaceous carcinoma should be considered in the differential diagnosis. A primary malignant peripheral primitive neuroectodermal tumor or a primary cutaneous carcinoid tumor is less common but should be considered. Differing from carcinoid tumors, Merkel cell carcinomas usually have a higher mitotic rate and positive staining for CK20. The sebaceous neoplasms with a carcinoidlike pattern may appear histologically similar, requiring immunohistochemical evaluation with monoclonal antibodies such as D2-40.13 A diffuse granular cytoplasmic reaction to chromogranin A is characteristic of carcinoid tumors. Synaptophysin and TTF-1 also are positive in carcinoid tumors, with TTF-1 being highly specific for neuroendocrine tumors of the lung.10
Cutaneous metastases of internal malignancies are more common from carcinomas of the lungs, gastrointestinal tract, and breasts.5 Occasionally, the cutaneous metastasis will develop directly over the underlying malignancy. Our case of cutaneous metastasis of a carcinoid tumor presented as an exquisitely tender and painful papule on the cheek. The histology of the lesion was consistent with the known carcinoid tumor of the lung. Because these lesions are extremely uncommon, it is imperative to obtain an accurate clinical history and use the appropriate immunohistochemical panel to correctly diagnose these metastases.
- Blochin E, Stein JA, Wang NS. Atypical carcinoid metastasis to the skin. Am J Dermatopathol. 2010;32:735-739.
- Rodriguez G, Villamizar R. Carcinoid tumor with skin metastasis. Am J Dermatopathol. 1992;14:263-269.
- Archer CB, Rauch HJ, Allen MH, et al. Ultrastructural features of metastatic cutaneous carcinoid. J Cutan Pathol. 1984;11:485-490.
- Archer CB, Wells RS, MacDonald DM. Metastatic cutaneous carcinoid. J Am Acad Dermatol. 1985;13(2, pt 2):363-366.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis:a meta-analysis of data. South Med J. 2003;96:164-167.
- Oleksowicz L, Morris JC, Phelps RG, et al. Pulmonary carcinoid presenting as multiple subcutaneous nodules. Tumori. 1990;76:44-47.
- Zuetenhorst JM, van Velthuysen ML, Rutgers EJ, et al. Pathogenesis and treatment of pain caused by skin metastases in neuroendocrine tumours. Neth J Med. 2002;60:207-211.
- Melmon KL. Kinins: one of the many mediators of the carcinoid spectrum. Gastroenterology. 1968;55:545-548.
- Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical review. Oncologist. 2005;10:123-131.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Braverman IM. Skin manifestations of internal malignancy. Clin Geriatr Med. 2002;18:1-19.
- Santi R, Massi D, Mazzoni F, et al. Skin metastasis from typical carcinoid tumor of the lung. J Cutan Pathol. 2008;35:418-422.
- Kazakov DV, Kutzner H, Rütten A, et al. Carcinoid-like pattern in sebaceous neoplasms. another distinctive, previously unrecognized pattern in extraocular sebaceous carcinoma and sebaceoma. Am J Dermatopathol. 2005;27:195-203.
Case Report
A 72-year-old white man with a history of pancreatic adenocarcinoma presented for Mohs micrographic surgery of a basal cell carcinoma on the right helix. On the day of the surgery, the patient reported a new, rapidly growing, exquisitely painful lesion on the cheek of 3 to 4 weeks’ duration. Physical examination revealed a 0.8×0.8×0.8-cm, extremely tender, firm, pink papule on the right preauricular cheek. A horizontal deep shave excision was done and the histopathology was remarkable for neoplastic cells with necrosis in the dermis. We observed dermal cellular infiltrates in the form of sheets and nodules, some showing central necrosis (Figure 1). At higher magnification, a trabecular arrangement of cells was seen. These cells had a moderate amount of cytoplasm with eccentric nuclei and rare nucleoli (Figure 2). Mitotic figures were seen at higher magnification (Figure 3). Immunohistochemistry of the neoplastic cells exhibited similar positive staining for the neuroendocrine markers chromogranin A and synaptophysin (Figure 4). Staining of the neoplastic cells also was positive for thyroid transcription factor 1 (TTF-1) and cancer antigen 19-9. Villin and caudal type homeobox 2 stains were negative. These results were consistent with cutaneous metastasis from a known pulmonary carcinoid tumor.




On further review of the patient’s medical history, it was discovered that he had undergone a Whipple procedure with adjuvant chemotherapy and radiation for pancreatic adenocarcinoma approximately 4 years prior to the current presentation. He was then followed by oncology, and 3 years later a chest computed tomography suggested possible disease progression with a new pulmonary metastasis. This pulmonary lesion was biopsied and immunologic staining was consistent with a primary neuroendocrine neoplasm of the lung, a new carcinoid tumor. The tissue was positive for cytokeratin (CK) 7,TTF-1, cancer antigen 19-9, CD56, synaptophysin, and chromogranin A, and was negative for villin and CK20. By the time he was seen in our clinic, several trials of chemotherapy had failed. Serial computed tomography subsequently demonstrated progression of the lung disease and he later developed malignant pleural effusions. Approximately 6 months after the cutaneous carcinoid metastasis was diagnosed, the patient died of respiratory failure.
Comment
Carcinoid tumors are uncommon neoplasms of neuroendocrine origin that generally arise in the gastrointestinal or bronchopulmonary tracts. Metastases from these primary neoplasms more commonly affect the regional lymph nodes or viscera, with rare reports of cutaneous metastases to the skin. The true incidence of carcinoid tumors with metastasis to the skin is unknown because it is limited to single case reports in the literature.
The clinical presentation of cutaneous carcinoid metastases has been reported most commonly as firm papules of varying sizes with no specific site predilection.1 The color of these lesions has ranged from erythematous to violaceous to brown.2 Several of the reported cases were noted to be extremely tender and painful, while other reports of lesions were noted to be asymptomatic or only mildly pruritic.3-7
Carcinoid syndrome is more common with neoplasms present within the gastrointestinal tract, but it also has been reported with large bronchial carcinoid tumors and with metastatic disease.8,9 Paroxysmal flushing is the most prominent cutaneous manifestation of this syndrome, occurring in 75% of patients.10,11 Other common symptoms include patchy cyanosis, telangiectasia, and pellagralike skin lesions.3 Carcinoid syndrome secondary to bronchial adenomas is thought to differ from gastrointestinal carcinoid neoplasms in that it has prolonged flushing (hours to days instead of minutes) and is characterized by marked anxiety, fever, disorientation, sweating, and lacrimation.8,9
Many cases of cutaneous carcinoid metastases have been accompanied by reports of exquisite tenderness,7 similar to our patient. The pathogenesis of the pain in these lesions is still unclear, but several hypotheses have been established. It has been postulated that perineural invasion by the tumor is responsible for the pain; however, this finding has been inconsistent, as neural involvement also has been present in nonpainful lesions.2,5,7,12 Another theory for the pain is that it is secondary to the release of vasoactive substances and peptide hormones from the carcinoid cells, such as kallikrein and serotonin. Lastly, local tissue necrosis and fibrosis also have been suggested as possible etiologies.7
The histology of cutaneous carcinoid metastases typically resembles the primary lesion and may demonstrate fascicles of spindle cells with focal areas of necrosis, mild atypia, and a relatively low mitotic rate.10 Other neoplasms such as Merkel cell carcinoma and carcinoidlike sebaceous carcinoma should be considered in the differential diagnosis. A primary malignant peripheral primitive neuroectodermal tumor or a primary cutaneous carcinoid tumor is less common but should be considered. Differing from carcinoid tumors, Merkel cell carcinomas usually have a higher mitotic rate and positive staining for CK20. The sebaceous neoplasms with a carcinoidlike pattern may appear histologically similar, requiring immunohistochemical evaluation with monoclonal antibodies such as D2-40.13 A diffuse granular cytoplasmic reaction to chromogranin A is characteristic of carcinoid tumors. Synaptophysin and TTF-1 also are positive in carcinoid tumors, with TTF-1 being highly specific for neuroendocrine tumors of the lung.10
Cutaneous metastases of internal malignancies are more common from carcinomas of the lungs, gastrointestinal tract, and breasts.5 Occasionally, the cutaneous metastasis will develop directly over the underlying malignancy. Our case of cutaneous metastasis of a carcinoid tumor presented as an exquisitely tender and painful papule on the cheek. The histology of the lesion was consistent with the known carcinoid tumor of the lung. Because these lesions are extremely uncommon, it is imperative to obtain an accurate clinical history and use the appropriate immunohistochemical panel to correctly diagnose these metastases.
Case Report
A 72-year-old white man with a history of pancreatic adenocarcinoma presented for Mohs micrographic surgery of a basal cell carcinoma on the right helix. On the day of the surgery, the patient reported a new, rapidly growing, exquisitely painful lesion on the cheek of 3 to 4 weeks’ duration. Physical examination revealed a 0.8×0.8×0.8-cm, extremely tender, firm, pink papule on the right preauricular cheek. A horizontal deep shave excision was done and the histopathology was remarkable for neoplastic cells with necrosis in the dermis. We observed dermal cellular infiltrates in the form of sheets and nodules, some showing central necrosis (Figure 1). At higher magnification, a trabecular arrangement of cells was seen. These cells had a moderate amount of cytoplasm with eccentric nuclei and rare nucleoli (Figure 2). Mitotic figures were seen at higher magnification (Figure 3). Immunohistochemistry of the neoplastic cells exhibited similar positive staining for the neuroendocrine markers chromogranin A and synaptophysin (Figure 4). Staining of the neoplastic cells also was positive for thyroid transcription factor 1 (TTF-1) and cancer antigen 19-9. Villin and caudal type homeobox 2 stains were negative. These results were consistent with cutaneous metastasis from a known pulmonary carcinoid tumor.




On further review of the patient’s medical history, it was discovered that he had undergone a Whipple procedure with adjuvant chemotherapy and radiation for pancreatic adenocarcinoma approximately 4 years prior to the current presentation. He was then followed by oncology, and 3 years later a chest computed tomography suggested possible disease progression with a new pulmonary metastasis. This pulmonary lesion was biopsied and immunologic staining was consistent with a primary neuroendocrine neoplasm of the lung, a new carcinoid tumor. The tissue was positive for cytokeratin (CK) 7,TTF-1, cancer antigen 19-9, CD56, synaptophysin, and chromogranin A, and was negative for villin and CK20. By the time he was seen in our clinic, several trials of chemotherapy had failed. Serial computed tomography subsequently demonstrated progression of the lung disease and he later developed malignant pleural effusions. Approximately 6 months after the cutaneous carcinoid metastasis was diagnosed, the patient died of respiratory failure.
Comment
Carcinoid tumors are uncommon neoplasms of neuroendocrine origin that generally arise in the gastrointestinal or bronchopulmonary tracts. Metastases from these primary neoplasms more commonly affect the regional lymph nodes or viscera, with rare reports of cutaneous metastases to the skin. The true incidence of carcinoid tumors with metastasis to the skin is unknown because it is limited to single case reports in the literature.
The clinical presentation of cutaneous carcinoid metastases has been reported most commonly as firm papules of varying sizes with no specific site predilection.1 The color of these lesions has ranged from erythematous to violaceous to brown.2 Several of the reported cases were noted to be extremely tender and painful, while other reports of lesions were noted to be asymptomatic or only mildly pruritic.3-7
Carcinoid syndrome is more common with neoplasms present within the gastrointestinal tract, but it also has been reported with large bronchial carcinoid tumors and with metastatic disease.8,9 Paroxysmal flushing is the most prominent cutaneous manifestation of this syndrome, occurring in 75% of patients.10,11 Other common symptoms include patchy cyanosis, telangiectasia, and pellagralike skin lesions.3 Carcinoid syndrome secondary to bronchial adenomas is thought to differ from gastrointestinal carcinoid neoplasms in that it has prolonged flushing (hours to days instead of minutes) and is characterized by marked anxiety, fever, disorientation, sweating, and lacrimation.8,9
Many cases of cutaneous carcinoid metastases have been accompanied by reports of exquisite tenderness,7 similar to our patient. The pathogenesis of the pain in these lesions is still unclear, but several hypotheses have been established. It has been postulated that perineural invasion by the tumor is responsible for the pain; however, this finding has been inconsistent, as neural involvement also has been present in nonpainful lesions.2,5,7,12 Another theory for the pain is that it is secondary to the release of vasoactive substances and peptide hormones from the carcinoid cells, such as kallikrein and serotonin. Lastly, local tissue necrosis and fibrosis also have been suggested as possible etiologies.7
The histology of cutaneous carcinoid metastases typically resembles the primary lesion and may demonstrate fascicles of spindle cells with focal areas of necrosis, mild atypia, and a relatively low mitotic rate.10 Other neoplasms such as Merkel cell carcinoma and carcinoidlike sebaceous carcinoma should be considered in the differential diagnosis. A primary malignant peripheral primitive neuroectodermal tumor or a primary cutaneous carcinoid tumor is less common but should be considered. Differing from carcinoid tumors, Merkel cell carcinomas usually have a higher mitotic rate and positive staining for CK20. The sebaceous neoplasms with a carcinoidlike pattern may appear histologically similar, requiring immunohistochemical evaluation with monoclonal antibodies such as D2-40.13 A diffuse granular cytoplasmic reaction to chromogranin A is characteristic of carcinoid tumors. Synaptophysin and TTF-1 also are positive in carcinoid tumors, with TTF-1 being highly specific for neuroendocrine tumors of the lung.10
Cutaneous metastases of internal malignancies are more common from carcinomas of the lungs, gastrointestinal tract, and breasts.5 Occasionally, the cutaneous metastasis will develop directly over the underlying malignancy. Our case of cutaneous metastasis of a carcinoid tumor presented as an exquisitely tender and painful papule on the cheek. The histology of the lesion was consistent with the known carcinoid tumor of the lung. Because these lesions are extremely uncommon, it is imperative to obtain an accurate clinical history and use the appropriate immunohistochemical panel to correctly diagnose these metastases.
- Blochin E, Stein JA, Wang NS. Atypical carcinoid metastasis to the skin. Am J Dermatopathol. 2010;32:735-739.
- Rodriguez G, Villamizar R. Carcinoid tumor with skin metastasis. Am J Dermatopathol. 1992;14:263-269.
- Archer CB, Rauch HJ, Allen MH, et al. Ultrastructural features of metastatic cutaneous carcinoid. J Cutan Pathol. 1984;11:485-490.
- Archer CB, Wells RS, MacDonald DM. Metastatic cutaneous carcinoid. J Am Acad Dermatol. 1985;13(2, pt 2):363-366.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis:a meta-analysis of data. South Med J. 2003;96:164-167.
- Oleksowicz L, Morris JC, Phelps RG, et al. Pulmonary carcinoid presenting as multiple subcutaneous nodules. Tumori. 1990;76:44-47.
- Zuetenhorst JM, van Velthuysen ML, Rutgers EJ, et al. Pathogenesis and treatment of pain caused by skin metastases in neuroendocrine tumours. Neth J Med. 2002;60:207-211.
- Melmon KL. Kinins: one of the many mediators of the carcinoid spectrum. Gastroenterology. 1968;55:545-548.
- Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical review. Oncologist. 2005;10:123-131.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Braverman IM. Skin manifestations of internal malignancy. Clin Geriatr Med. 2002;18:1-19.
- Santi R, Massi D, Mazzoni F, et al. Skin metastasis from typical carcinoid tumor of the lung. J Cutan Pathol. 2008;35:418-422.
- Kazakov DV, Kutzner H, Rütten A, et al. Carcinoid-like pattern in sebaceous neoplasms. another distinctive, previously unrecognized pattern in extraocular sebaceous carcinoma and sebaceoma. Am J Dermatopathol. 2005;27:195-203.
- Blochin E, Stein JA, Wang NS. Atypical carcinoid metastasis to the skin. Am J Dermatopathol. 2010;32:735-739.
- Rodriguez G, Villamizar R. Carcinoid tumor with skin metastasis. Am J Dermatopathol. 1992;14:263-269.
- Archer CB, Rauch HJ, Allen MH, et al. Ultrastructural features of metastatic cutaneous carcinoid. J Cutan Pathol. 1984;11:485-490.
- Archer CB, Wells RS, MacDonald DM. Metastatic cutaneous carcinoid. J Am Acad Dermatol. 1985;13(2, pt 2):363-366.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis:a meta-analysis of data. South Med J. 2003;96:164-167.
- Oleksowicz L, Morris JC, Phelps RG, et al. Pulmonary carcinoid presenting as multiple subcutaneous nodules. Tumori. 1990;76:44-47.
- Zuetenhorst JM, van Velthuysen ML, Rutgers EJ, et al. Pathogenesis and treatment of pain caused by skin metastases in neuroendocrine tumours. Neth J Med. 2002;60:207-211.
- Melmon KL. Kinins: one of the many mediators of the carcinoid spectrum. Gastroenterology. 1968;55:545-548.
- Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical review. Oncologist. 2005;10:123-131.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Braverman IM. Skin manifestations of internal malignancy. Clin Geriatr Med. 2002;18:1-19.
- Santi R, Massi D, Mazzoni F, et al. Skin metastasis from typical carcinoid tumor of the lung. J Cutan Pathol. 2008;35:418-422.
- Kazakov DV, Kutzner H, Rütten A, et al. Carcinoid-like pattern in sebaceous neoplasms. another distinctive, previously unrecognized pattern in extraocular sebaceous carcinoma and sebaceoma. Am J Dermatopathol. 2005;27:195-203.
Practice Points
- Cutaneous metastases of carcinoid tumors are extremely rare, and clinical presentation can vary. They can present as firm papules ranging in color from pink to brown, can be painful, and could occur at any site.
- It is imperative to obtain an accurate clinical history and use the appropriate immunohistochemical panel to correctly diagnose cutaneous metastases of carcinoid tumors.
- Neoplasms within the gastrointestinal tract commonly present with carcinoid syndrome, but it also has been observed with bronchial carcinoid tumors and with metastatic disease.
Acute Intraprosthetic Dissociation of a Dual-Mobility Hip in the United States
Take-Home Points
- AIPD of DM-THA is defined by dissociation within 1 year of implantation resulting from component impingement or closed reduction maneuvers.
- This is a distinct entity from “late” IPD (>1 year) from implantation as this is associated most often with polyethylene wear, component loosening, and arthrofibrosis.
- A history of DM dislocation followed by subjective “clunking,” instability, and a series of more frequent dislocations should raise concern for AIPD.
- Classic radiographic findings of AIPD include eccentric hip reduction and soft tissue radiolucency (ie, halo sign) from dissociated polyethylene component.
- Treating practitioners of AIPD should consider closed reduction with general anesthesia and sedation in the operating room to limit risk of dissociation.
Dual-mobility (DM) components were invented in the 1970s and have been used in primary and revision total hip arthroplasty (THA) in Europe ever since.1 However, DM components are most commonly used in the treatment of recurrent hip instability, and early results have been promising.2 In DM-THAs, a smaller (22-mm or 28-mm) metal femoral head snap-fits into a larger polyethylene ball (inner articulation), which articulates with a highly polished metal shell (outer articulation), which is either implanted directly in the acetabulum or placed in an uncemented acetabular cup. The 2 articulations used in these devices theoretically increase hip range of motion (ROM) and increase the inferior head displacement distance (jump distance) required for dislocation.3
However, this DM articulation with increased ROM may also cause chronic impingement of the femoral component neck or Morse taper against the outer polyethylene bearing, resulting in polyethylene wear and late intraprosthetic dissociation (IPD) (separation of inner articulation between femoral head and polyethylene liner). In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation during the period 1989 to 1997. In 2013, Philippot and colleagues5 reported that 81 of 1960 primary THAs developed IPD a mean of 9 years after implantation. These IPD cases were attributed to polyethylene wear or outer articulation blockage caused by arthrofibrosis or heterotopic ossification. Reports of acute IPD (AIPD), however, are rare. In 2011, Stigbrand and Ullmark6 reported 3 cases in which the DM prosthesis dislocated within 1 year after implantation. It was suggested that the inner metal head dissociated from the larger polyethylene component after attempted closed reduction for dislocation (separation of larger polyethylene component from acetabulum or acetabular liner).
DM components were unavailable to surgeons in the United States until 2011. The first US Food and Drug Administration (FDA)-approved DM device was the MDM (Modular Dual Mobility, Stryker). To our knowledge, 2 cases of AIPD with this prosthesis have been reported.7, 8 As with the cases in Europe, closed reduction was the suspected cause, but there was no explanation for the initial dislocation event.
In this article, we present the case of a nondemented man who developed AIPD of a THA with the MDM component and a 28-mm femoral head with a skirted neck (StelKast). His operative findings suggest a poor head-to-neck ratio caused by a larger diameter femoral neck or a skirted prosthesis, or a forceful reduction maneuver, may predispose DM components to AIPD. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2012, a 63-year-old man with a history of drug abuse underwent left primary THA. Seven posterior dislocations and 3 years later, the acetabular component was revised to the MDM prosthesis; the well-fixed StelKast femoral component was retained (Figure 1).
Within 3 months after revision surgery, the left hip dislocated 3 times in 1 week, when the patient bent over to retrieve an object on the ground. The first 2 dislocations were treated with closed reduction under conscious sedation at an outside emergency department.
With the patient’s erythrocyte sedimentation rate and C-reactive protein level both normal, a second revision was performed. During surgery, the polyethylene head was found beneath the gluteus maximus (Figure 4).
Discussion
Recurrent dislocation and instability accounts for 22.5% of THA revisions in the United States.9 Until 2011, options for managing recurrent dislocation in the United States included modular component exchange, component revision for malposition, and use of constrained components.10
In 1974, Bousquet first reported use of the DM prosthesis in primary THA; the prosthesis allowed increased stability without sacrificing motion or fixation.1 However, longer-term studies of DM components disclosed a new complication, IPD. In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation of the Bousquet prosthesis.
AIPD, which occurs within 1 year after implantation, has been reported much less often than late IPD. Stigbrand and Ullmark6 reported 3 cases of AIPD that developed within 7 months after implantation of Amplitude and Advantage (Zimmer Biomet) DM prostheses.
This unusual complication apparently is not confined to a specific implant or region. Since the MDM component was introduced in the United States, 2 more cases of AIPD have been identified (Table). Banzhof and colleagues7 reported the case of a 68-year-old woman who, 2 months after the MDM was placed for recurrent instability, dislocated the component while rising from a seated position. Her IPD most likely resulted from a closed reduction. The affected hip eventually required closed reduction in the operating room. Postreduction radiographs showed the characteristic eccentric appearance; a halo, also visible in the soft tissues, corresponded with the dissociated radiolucent polyethylene liner. The authors attributed the early failure to an eccentrically seated metal liner that separated the locking mechanism. The MDM component was revised to a conventional THA, with the femoral head upsized and length added.
Ward and colleagues8 reported the case of an 87-year-old woman who had a conventional THA revised to an MDM component for recurrent instability. Two months after surgery, this patient, who had dementia, experienced 2 posterior dislocations while rising from a chair. Closed reduction in the emergency department seemed successful, but later she presented to the surgeon’s office with symptoms of instability and clunking, complaints similar to our patient’s. Radiographs showed an eccentric reduction caused by IPD, and the MDM component was revised to a constrained liner. Adding a MDM component to a retained DePuy (DePuy Synthes) femoral stem and head is considered “off-label use,” which, the authors proposed, may have been related to the AIPD in their patient’s case. However, one manufacturer’s femoral component and head are often mated with another manufacturer’s acetabular component to allow for a less complex revision. Our recommendation for surgeons is that, before proceeding with this treatment option, they investigate each component’s exact dimensions to ensure there are no subtle size differences that could cause problems. For example, a 28-mm head diameter that is actually 28.2 mm may affect mating properties, with the inner polyethylene articulation causing AIPD to develop.
Other cases of earlier IPD have been described, but they do not fit the APID definition given in this article. Riviere and colleagues14 reported the case of a 42-year-old man who, because of a previous adverse reaction to metal debris, underwent revision to a DM polyethylene ball in a retained BHR (Birmingham Hip Resurfacing) acetabular shell (Birmingham Hip, Smith & Nephew). Unfortunately, IPD occurred 14 months after surgery. Banka and colleagues15 reported the case of a 70-year-old woman who underwent revision to a DM cup for recurrent instability, but they did not specify the length of time between implantation and IPD and did not offer an explanation for the complication. Finally, Odland and Sierra16 reported the case of a 77-year-old man, with previous intertrochanteric and pelvic fractures, who underwent revision to a DM cup with retention of a Waldemar femoral component (Waldemar Link). He spontaneously developed IPD with ambulation 2 years after surgery.
Certainly, our patient’s presentation course is similar to other patients’. Within 3 months after revision to the MDM component, his left hip dislocated 3 times in 1 week. We contend his AIPD resulted from closed reduction, with the polyethylene dislodged from the femoral head with contact on the acetabulum. A larger or skirted neck may increase impingement during normal activity and thereby widen the polyethylene opening excessively and/or reduce the polyethylene ball ROM to impinge during the relocation maneuver. In this case, dissociation was noted only after the third dislocation. Pathognomonic eccentric positioning of the head in the acetabulum and, less commonly, the halo sign were evident on postreduction radiographs. Optimal treatment for AIPD of a DM component is controversial. Choices are limited to a constrained liner or, if possible, repeat DM with larger components. For recurrent dislocation, our patient underwent revision to an MDM component, but a femoral head with a skirted neck was used in an attempt to increase soft-tissue tension. During the second revision, minor eccentric wear of the inner articulation of the polyethylene component (consistent with impingement) was noted, and wear was visible on inspection of the outer articulation. We think his AIPD resulted from femoral neck impingement of the skirted head against the polyethylene ball.
AIPD is a discrete entity, with sudden failure of a DM component within 1 year after implantation. AIPD is characterized by dissociation of the femoral head from the inner articulation, resulting from impingement or closed reduction. More studies are needed to determine which patients with DM components are at highest risk and which treatment is most appropriate. We recommend taking extra care when reducing hips with this articulation and adopting a low threshold for general anesthesia use in the presence of paralysis.
Am J Orthop. 2017;46(3):E154-E159. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
2. Lachiewicz PF, Watters TS. The use of dual-mobility components in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(8):481-486.
3. De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187.
4. Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(3):249-255.
5. Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965-970.
6. Stigbrand H, Ullmark G. Component dissociation after closed reduction of dual mobility sockets—a report of three cases. Hip Int. 2011;21(2):263-266.
7. Banzhof JA, Robbins CE, Ven AV, Talmo CT, Bono JV. Femoral head dislodgement complicating use of a dual mobility prosthesis for recurrent instability. J Arthroplasty. 2013;28(3):543.e1-e3.
8. Ward JP, McCardel BR, Hallstrom BR. Complete dissociation of the polyethylene component in a newly available dual-mobility bearing used in total hip arthroplasty: a case report. JBJS Case Connect. 2013;3(3):e94.
9. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
10. Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134-1142.
11. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
12. Lachiewicz PF, Kelley SS. The use of constrained components in total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(4):233-238.
13. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
14. Riviere C, Lavigne M, Alghamdi A, Vendittoli PA. Early failure of metal-on-metal large-diameter head total hip arthroplasty revised with a dual-mobility bearing: a case report. JBJS Case Connect. 2013;3(3):e95.
15. Banka TR, Ast MP, Parks ML. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395-e397.
16. Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):e1124-e1128.
Take-Home Points
- AIPD of DM-THA is defined by dissociation within 1 year of implantation resulting from component impingement or closed reduction maneuvers.
- This is a distinct entity from “late” IPD (>1 year) from implantation as this is associated most often with polyethylene wear, component loosening, and arthrofibrosis.
- A history of DM dislocation followed by subjective “clunking,” instability, and a series of more frequent dislocations should raise concern for AIPD.
- Classic radiographic findings of AIPD include eccentric hip reduction and soft tissue radiolucency (ie, halo sign) from dissociated polyethylene component.
- Treating practitioners of AIPD should consider closed reduction with general anesthesia and sedation in the operating room to limit risk of dissociation.
Dual-mobility (DM) components were invented in the 1970s and have been used in primary and revision total hip arthroplasty (THA) in Europe ever since.1 However, DM components are most commonly used in the treatment of recurrent hip instability, and early results have been promising.2 In DM-THAs, a smaller (22-mm or 28-mm) metal femoral head snap-fits into a larger polyethylene ball (inner articulation), which articulates with a highly polished metal shell (outer articulation), which is either implanted directly in the acetabulum or placed in an uncemented acetabular cup. The 2 articulations used in these devices theoretically increase hip range of motion (ROM) and increase the inferior head displacement distance (jump distance) required for dislocation.3
However, this DM articulation with increased ROM may also cause chronic impingement of the femoral component neck or Morse taper against the outer polyethylene bearing, resulting in polyethylene wear and late intraprosthetic dissociation (IPD) (separation of inner articulation between femoral head and polyethylene liner). In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation during the period 1989 to 1997. In 2013, Philippot and colleagues5 reported that 81 of 1960 primary THAs developed IPD a mean of 9 years after implantation. These IPD cases were attributed to polyethylene wear or outer articulation blockage caused by arthrofibrosis or heterotopic ossification. Reports of acute IPD (AIPD), however, are rare. In 2011, Stigbrand and Ullmark6 reported 3 cases in which the DM prosthesis dislocated within 1 year after implantation. It was suggested that the inner metal head dissociated from the larger polyethylene component after attempted closed reduction for dislocation (separation of larger polyethylene component from acetabulum or acetabular liner).
DM components were unavailable to surgeons in the United States until 2011. The first US Food and Drug Administration (FDA)-approved DM device was the MDM (Modular Dual Mobility, Stryker). To our knowledge, 2 cases of AIPD with this prosthesis have been reported.7, 8 As with the cases in Europe, closed reduction was the suspected cause, but there was no explanation for the initial dislocation event.
In this article, we present the case of a nondemented man who developed AIPD of a THA with the MDM component and a 28-mm femoral head with a skirted neck (StelKast). His operative findings suggest a poor head-to-neck ratio caused by a larger diameter femoral neck or a skirted prosthesis, or a forceful reduction maneuver, may predispose DM components to AIPD. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2012, a 63-year-old man with a history of drug abuse underwent left primary THA. Seven posterior dislocations and 3 years later, the acetabular component was revised to the MDM prosthesis; the well-fixed StelKast femoral component was retained (Figure 1).
Within 3 months after revision surgery, the left hip dislocated 3 times in 1 week, when the patient bent over to retrieve an object on the ground. The first 2 dislocations were treated with closed reduction under conscious sedation at an outside emergency department.
With the patient’s erythrocyte sedimentation rate and C-reactive protein level both normal, a second revision was performed. During surgery, the polyethylene head was found beneath the gluteus maximus (Figure 4).
Discussion
Recurrent dislocation and instability accounts for 22.5% of THA revisions in the United States.9 Until 2011, options for managing recurrent dislocation in the United States included modular component exchange, component revision for malposition, and use of constrained components.10
In 1974, Bousquet first reported use of the DM prosthesis in primary THA; the prosthesis allowed increased stability without sacrificing motion or fixation.1 However, longer-term studies of DM components disclosed a new complication, IPD. In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation of the Bousquet prosthesis.
AIPD, which occurs within 1 year after implantation, has been reported much less often than late IPD. Stigbrand and Ullmark6 reported 3 cases of AIPD that developed within 7 months after implantation of Amplitude and Advantage (Zimmer Biomet) DM prostheses.
This unusual complication apparently is not confined to a specific implant or region. Since the MDM component was introduced in the United States, 2 more cases of AIPD have been identified (Table). Banzhof and colleagues7 reported the case of a 68-year-old woman who, 2 months after the MDM was placed for recurrent instability, dislocated the component while rising from a seated position. Her IPD most likely resulted from a closed reduction. The affected hip eventually required closed reduction in the operating room. Postreduction radiographs showed the characteristic eccentric appearance; a halo, also visible in the soft tissues, corresponded with the dissociated radiolucent polyethylene liner. The authors attributed the early failure to an eccentrically seated metal liner that separated the locking mechanism. The MDM component was revised to a conventional THA, with the femoral head upsized and length added.
Ward and colleagues8 reported the case of an 87-year-old woman who had a conventional THA revised to an MDM component for recurrent instability. Two months after surgery, this patient, who had dementia, experienced 2 posterior dislocations while rising from a chair. Closed reduction in the emergency department seemed successful, but later she presented to the surgeon’s office with symptoms of instability and clunking, complaints similar to our patient’s. Radiographs showed an eccentric reduction caused by IPD, and the MDM component was revised to a constrained liner. Adding a MDM component to a retained DePuy (DePuy Synthes) femoral stem and head is considered “off-label use,” which, the authors proposed, may have been related to the AIPD in their patient’s case. However, one manufacturer’s femoral component and head are often mated with another manufacturer’s acetabular component to allow for a less complex revision. Our recommendation for surgeons is that, before proceeding with this treatment option, they investigate each component’s exact dimensions to ensure there are no subtle size differences that could cause problems. For example, a 28-mm head diameter that is actually 28.2 mm may affect mating properties, with the inner polyethylene articulation causing AIPD to develop.
Other cases of earlier IPD have been described, but they do not fit the APID definition given in this article. Riviere and colleagues14 reported the case of a 42-year-old man who, because of a previous adverse reaction to metal debris, underwent revision to a DM polyethylene ball in a retained BHR (Birmingham Hip Resurfacing) acetabular shell (Birmingham Hip, Smith & Nephew). Unfortunately, IPD occurred 14 months after surgery. Banka and colleagues15 reported the case of a 70-year-old woman who underwent revision to a DM cup for recurrent instability, but they did not specify the length of time between implantation and IPD and did not offer an explanation for the complication. Finally, Odland and Sierra16 reported the case of a 77-year-old man, with previous intertrochanteric and pelvic fractures, who underwent revision to a DM cup with retention of a Waldemar femoral component (Waldemar Link). He spontaneously developed IPD with ambulation 2 years after surgery.
Certainly, our patient’s presentation course is similar to other patients’. Within 3 months after revision to the MDM component, his left hip dislocated 3 times in 1 week. We contend his AIPD resulted from closed reduction, with the polyethylene dislodged from the femoral head with contact on the acetabulum. A larger or skirted neck may increase impingement during normal activity and thereby widen the polyethylene opening excessively and/or reduce the polyethylene ball ROM to impinge during the relocation maneuver. In this case, dissociation was noted only after the third dislocation. Pathognomonic eccentric positioning of the head in the acetabulum and, less commonly, the halo sign were evident on postreduction radiographs. Optimal treatment for AIPD of a DM component is controversial. Choices are limited to a constrained liner or, if possible, repeat DM with larger components. For recurrent dislocation, our patient underwent revision to an MDM component, but a femoral head with a skirted neck was used in an attempt to increase soft-tissue tension. During the second revision, minor eccentric wear of the inner articulation of the polyethylene component (consistent with impingement) was noted, and wear was visible on inspection of the outer articulation. We think his AIPD resulted from femoral neck impingement of the skirted head against the polyethylene ball.
AIPD is a discrete entity, with sudden failure of a DM component within 1 year after implantation. AIPD is characterized by dissociation of the femoral head from the inner articulation, resulting from impingement or closed reduction. More studies are needed to determine which patients with DM components are at highest risk and which treatment is most appropriate. We recommend taking extra care when reducing hips with this articulation and adopting a low threshold for general anesthesia use in the presence of paralysis.
Am J Orthop. 2017;46(3):E154-E159. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- AIPD of DM-THA is defined by dissociation within 1 year of implantation resulting from component impingement or closed reduction maneuvers.
- This is a distinct entity from “late” IPD (>1 year) from implantation as this is associated most often with polyethylene wear, component loosening, and arthrofibrosis.
- A history of DM dislocation followed by subjective “clunking,” instability, and a series of more frequent dislocations should raise concern for AIPD.
- Classic radiographic findings of AIPD include eccentric hip reduction and soft tissue radiolucency (ie, halo sign) from dissociated polyethylene component.
- Treating practitioners of AIPD should consider closed reduction with general anesthesia and sedation in the operating room to limit risk of dissociation.
Dual-mobility (DM) components were invented in the 1970s and have been used in primary and revision total hip arthroplasty (THA) in Europe ever since.1 However, DM components are most commonly used in the treatment of recurrent hip instability, and early results have been promising.2 In DM-THAs, a smaller (22-mm or 28-mm) metal femoral head snap-fits into a larger polyethylene ball (inner articulation), which articulates with a highly polished metal shell (outer articulation), which is either implanted directly in the acetabulum or placed in an uncemented acetabular cup. The 2 articulations used in these devices theoretically increase hip range of motion (ROM) and increase the inferior head displacement distance (jump distance) required for dislocation.3
However, this DM articulation with increased ROM may also cause chronic impingement of the femoral component neck or Morse taper against the outer polyethylene bearing, resulting in polyethylene wear and late intraprosthetic dissociation (IPD) (separation of inner articulation between femoral head and polyethylene liner). In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation during the period 1989 to 1997. In 2013, Philippot and colleagues5 reported that 81 of 1960 primary THAs developed IPD a mean of 9 years after implantation. These IPD cases were attributed to polyethylene wear or outer articulation blockage caused by arthrofibrosis or heterotopic ossification. Reports of acute IPD (AIPD), however, are rare. In 2011, Stigbrand and Ullmark6 reported 3 cases in which the DM prosthesis dislocated within 1 year after implantation. It was suggested that the inner metal head dissociated from the larger polyethylene component after attempted closed reduction for dislocation (separation of larger polyethylene component from acetabulum or acetabular liner).
DM components were unavailable to surgeons in the United States until 2011. The first US Food and Drug Administration (FDA)-approved DM device was the MDM (Modular Dual Mobility, Stryker). To our knowledge, 2 cases of AIPD with this prosthesis have been reported.7, 8 As with the cases in Europe, closed reduction was the suspected cause, but there was no explanation for the initial dislocation event.
In this article, we present the case of a nondemented man who developed AIPD of a THA with the MDM component and a 28-mm femoral head with a skirted neck (StelKast). His operative findings suggest a poor head-to-neck ratio caused by a larger diameter femoral neck or a skirted prosthesis, or a forceful reduction maneuver, may predispose DM components to AIPD. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2012, a 63-year-old man with a history of drug abuse underwent left primary THA. Seven posterior dislocations and 3 years later, the acetabular component was revised to the MDM prosthesis; the well-fixed StelKast femoral component was retained (Figure 1).
Within 3 months after revision surgery, the left hip dislocated 3 times in 1 week, when the patient bent over to retrieve an object on the ground. The first 2 dislocations were treated with closed reduction under conscious sedation at an outside emergency department.
With the patient’s erythrocyte sedimentation rate and C-reactive protein level both normal, a second revision was performed. During surgery, the polyethylene head was found beneath the gluteus maximus (Figure 4).
Discussion
Recurrent dislocation and instability accounts for 22.5% of THA revisions in the United States.9 Until 2011, options for managing recurrent dislocation in the United States included modular component exchange, component revision for malposition, and use of constrained components.10
In 1974, Bousquet first reported use of the DM prosthesis in primary THA; the prosthesis allowed increased stability without sacrificing motion or fixation.1 However, longer-term studies of DM components disclosed a new complication, IPD. In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation of the Bousquet prosthesis.
AIPD, which occurs within 1 year after implantation, has been reported much less often than late IPD. Stigbrand and Ullmark6 reported 3 cases of AIPD that developed within 7 months after implantation of Amplitude and Advantage (Zimmer Biomet) DM prostheses.
This unusual complication apparently is not confined to a specific implant or region. Since the MDM component was introduced in the United States, 2 more cases of AIPD have been identified (Table). Banzhof and colleagues7 reported the case of a 68-year-old woman who, 2 months after the MDM was placed for recurrent instability, dislocated the component while rising from a seated position. Her IPD most likely resulted from a closed reduction. The affected hip eventually required closed reduction in the operating room. Postreduction radiographs showed the characteristic eccentric appearance; a halo, also visible in the soft tissues, corresponded with the dissociated radiolucent polyethylene liner. The authors attributed the early failure to an eccentrically seated metal liner that separated the locking mechanism. The MDM component was revised to a conventional THA, with the femoral head upsized and length added.
Ward and colleagues8 reported the case of an 87-year-old woman who had a conventional THA revised to an MDM component for recurrent instability. Two months after surgery, this patient, who had dementia, experienced 2 posterior dislocations while rising from a chair. Closed reduction in the emergency department seemed successful, but later she presented to the surgeon’s office with symptoms of instability and clunking, complaints similar to our patient’s. Radiographs showed an eccentric reduction caused by IPD, and the MDM component was revised to a constrained liner. Adding a MDM component to a retained DePuy (DePuy Synthes) femoral stem and head is considered “off-label use,” which, the authors proposed, may have been related to the AIPD in their patient’s case. However, one manufacturer’s femoral component and head are often mated with another manufacturer’s acetabular component to allow for a less complex revision. Our recommendation for surgeons is that, before proceeding with this treatment option, they investigate each component’s exact dimensions to ensure there are no subtle size differences that could cause problems. For example, a 28-mm head diameter that is actually 28.2 mm may affect mating properties, with the inner polyethylene articulation causing AIPD to develop.
Other cases of earlier IPD have been described, but they do not fit the APID definition given in this article. Riviere and colleagues14 reported the case of a 42-year-old man who, because of a previous adverse reaction to metal debris, underwent revision to a DM polyethylene ball in a retained BHR (Birmingham Hip Resurfacing) acetabular shell (Birmingham Hip, Smith & Nephew). Unfortunately, IPD occurred 14 months after surgery. Banka and colleagues15 reported the case of a 70-year-old woman who underwent revision to a DM cup for recurrent instability, but they did not specify the length of time between implantation and IPD and did not offer an explanation for the complication. Finally, Odland and Sierra16 reported the case of a 77-year-old man, with previous intertrochanteric and pelvic fractures, who underwent revision to a DM cup with retention of a Waldemar femoral component (Waldemar Link). He spontaneously developed IPD with ambulation 2 years after surgery.
Certainly, our patient’s presentation course is similar to other patients’. Within 3 months after revision to the MDM component, his left hip dislocated 3 times in 1 week. We contend his AIPD resulted from closed reduction, with the polyethylene dislodged from the femoral head with contact on the acetabulum. A larger or skirted neck may increase impingement during normal activity and thereby widen the polyethylene opening excessively and/or reduce the polyethylene ball ROM to impinge during the relocation maneuver. In this case, dissociation was noted only after the third dislocation. Pathognomonic eccentric positioning of the head in the acetabulum and, less commonly, the halo sign were evident on postreduction radiographs. Optimal treatment for AIPD of a DM component is controversial. Choices are limited to a constrained liner or, if possible, repeat DM with larger components. For recurrent dislocation, our patient underwent revision to an MDM component, but a femoral head with a skirted neck was used in an attempt to increase soft-tissue tension. During the second revision, minor eccentric wear of the inner articulation of the polyethylene component (consistent with impingement) was noted, and wear was visible on inspection of the outer articulation. We think his AIPD resulted from femoral neck impingement of the skirted head against the polyethylene ball.
AIPD is a discrete entity, with sudden failure of a DM component within 1 year after implantation. AIPD is characterized by dissociation of the femoral head from the inner articulation, resulting from impingement or closed reduction. More studies are needed to determine which patients with DM components are at highest risk and which treatment is most appropriate. We recommend taking extra care when reducing hips with this articulation and adopting a low threshold for general anesthesia use in the presence of paralysis.
Am J Orthop. 2017;46(3):E154-E159. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
2. Lachiewicz PF, Watters TS. The use of dual-mobility components in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(8):481-486.
3. De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187.
4. Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(3):249-255.
5. Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965-970.
6. Stigbrand H, Ullmark G. Component dissociation after closed reduction of dual mobility sockets—a report of three cases. Hip Int. 2011;21(2):263-266.
7. Banzhof JA, Robbins CE, Ven AV, Talmo CT, Bono JV. Femoral head dislodgement complicating use of a dual mobility prosthesis for recurrent instability. J Arthroplasty. 2013;28(3):543.e1-e3.
8. Ward JP, McCardel BR, Hallstrom BR. Complete dissociation of the polyethylene component in a newly available dual-mobility bearing used in total hip arthroplasty: a case report. JBJS Case Connect. 2013;3(3):e94.
9. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
10. Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134-1142.
11. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
12. Lachiewicz PF, Kelley SS. The use of constrained components in total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(4):233-238.
13. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
14. Riviere C, Lavigne M, Alghamdi A, Vendittoli PA. Early failure of metal-on-metal large-diameter head total hip arthroplasty revised with a dual-mobility bearing: a case report. JBJS Case Connect. 2013;3(3):e95.
15. Banka TR, Ast MP, Parks ML. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395-e397.
16. Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):e1124-e1128.
1. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
2. Lachiewicz PF, Watters TS. The use of dual-mobility components in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(8):481-486.
3. De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187.
4. Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(3):249-255.
5. Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965-970.
6. Stigbrand H, Ullmark G. Component dissociation after closed reduction of dual mobility sockets—a report of three cases. Hip Int. 2011;21(2):263-266.
7. Banzhof JA, Robbins CE, Ven AV, Talmo CT, Bono JV. Femoral head dislodgement complicating use of a dual mobility prosthesis for recurrent instability. J Arthroplasty. 2013;28(3):543.e1-e3.
8. Ward JP, McCardel BR, Hallstrom BR. Complete dissociation of the polyethylene component in a newly available dual-mobility bearing used in total hip arthroplasty: a case report. JBJS Case Connect. 2013;3(3):e94.
9. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
10. Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134-1142.
11. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
12. Lachiewicz PF, Kelley SS. The use of constrained components in total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(4):233-238.
13. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
14. Riviere C, Lavigne M, Alghamdi A, Vendittoli PA. Early failure of metal-on-metal large-diameter head total hip arthroplasty revised with a dual-mobility bearing: a case report. JBJS Case Connect. 2013;3(3):e95.
15. Banka TR, Ast MP, Parks ML. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395-e397.
16. Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):e1124-e1128.
Case Studies in Toxicology: Angioedema Post-tPA: Hemorrhage Is Not the Only Risk Factor
Case
A 49-year-old man with a history of hypertension, for which he was taking aspirin, carvedilol, hydralazine, and nifedipine, presented to the ED with complaints of left-sided weakness that started 3 hours before he came to the ED. Initial vital signs were: blood pressure, 158/90 mm Hg; heart rate, 74 beats/min; respiratory rate, 18 breaths/min; and temperature, 98°F. Oxygen saturation was 100% on room air, and a finger-stick glucose test was 106 mg/dL.
Physical examination revealed slowed speech with mild dysarthria, mild left facial droop, 2/5 strength in all muscle groups in the left upper and lower extremities, and decreased sensation to light touch on the left side. The patient also had left-sided sensory neglect and an abnormal gait, and dragged his left foot on the floor when walking. The rest of his examination was normal.
The stroke team was activated, and the patient was immediately transferred to the ED radiology department for imaging studies. A noncontrast head computed tomography (CT) was negative for any acute intracranial hemorrhage or cerebral edema. A CT angiogram (CTA) also was performed, which revealed atherosclerosis but no arterial occlusion. Based on these findings and the existing protocol, the patient received an intravenous (IV) bolus of tissue plasminogen activator (tPA). Approximately 17 minutes after tPA administration, the patient developed left-sided upper and lower lip swelling. There was no voice change, tongue swelling, or uvular deviation.
What is the differential diagnosis of swelling of the lip?
The differential diagnoses for lip swelling includes trauma, allergic reaction, and angioedema (hereditary, or angiotensin converting enzyme inhibitor [ACEI]-induced). The patient in this case denied any trauma to the lip, and no bleeding was noted from the lip; however, his entire left lip (upper and lower) was swollen. He was not taking any ACEIs or angiotensin-receptor blockers (ARBs). He also denied a family history of angioedema or any prior similar episodes. The patient further denied exposure to any new medications, foods, or other substances and had no respiratory distress, urticaria, or other findings consistent with an allergy.
What are the common adverse effects of tPA?
The only US Food and Drug-approved pharmacological treatment for ischemic stroke is tPA (also known as IV rtPA). Tissue plasminogen activator hydrolyzes plasminogen to plasmin, which exerts a fibrinolytic effect. Based on the ability of tPA to lyse thrombus, it is also a standard therapy for hemodynamically unstable patients with confirmed pulmonary embolism, as well as for patients with myocardial infarction in whom percutaneous intervention is contraindicated or unavailable. Despite the beneficial effects of tPA, significant adverse effects are associated with the drug. For example, thrombolysis may result in conversion of an ischemic stroke into a hemorrhagic event, resulting in generalized bleeding from mucosal surfaces.
The increase in plasmin may play a role in the development of angioedema by activating the kinin pathway, leading to the formation of the vasodilator bradykinin (Figure). Plasmin also activates the complement system and leads to the production of anaphylatoxins C3a, C4a, and C5a, which also cause mast cell degranulation and histamine release.1
When does post-tPA angioedema occur?
In the few published case reports available, tPA-induced angioedema was shown to typically occur in the stroke distribution (which was attributed to the left-sided swelling in this patient).2 Following tPA administration, the onset of angioedema reportedly varies from as early as 10 to 15 minutes from initiation until about 1 hour postinfusion. The short half-life of tPA (approximately 7 minutes)2 limits the outer- time window for the initial development of angioedema, but progression can continue well beyond this timeframe.
What is the treatment for tPA-induced angioedema?
The first priority of acute management of angioedema is discontinuation of the inciting substance, if possible—in this case, the tPA infusion.3 Assessment and maintenance of a patent airway are of utmost concern. Patients with posterior oropharyngeal effects or who are progressing should be admitted to an intensive care unit (ICU) for observation.4-6
Endotracheal Intubation. Providers should have a low threshold for endotracheal intubation, which should ideally be performed in any patient at risk for airway compromise.4 Due to the extensive airway swelling that can occur in the setting of angioedema, airway intervention should optimally be performed by an available clinician with the most skill and experience in this area. It is wise to be prepared to utilize advanced airway techniques, if available, including fiberoptic laryngoscopy or potentially cricothyrotomy.
Histamine Agonists. Standard therapy for patients who develop angioedema should include histamine antagonists, such as diphenhydramine (H1 antagonist) and famotidine (H2 antagonist) along with corticosteroids. Although these therapies are unlikely to be helpful in the treatment of tPA-induced angioedema, the difficulty in excluding allergic angioedema and the low risk of adverse effects associated with these medications support their use.
Fresh Frozen Plasma. Fresh frozen plasma (FFP) should be considered for patients who have a history of hereditary angioedema. Fresh frozen plasma contains enzymes that degrade bradykinin. Although FFP has been used successfully in the treatment of ACEI-induced angioedema, its use (or benefit) in tPA-related cases is not clear.
Icatibant. A selective bradykinin B2-receptor antagonist, icatibant has been used to treat patients with ACEI-induced angioedema because of its effects on bradykinin receptors. Comparison of the efficacy of icatibant to the prevailing treatment strategy of diphenhydramine, famotidine, and methylprednisolone found a shorter time to symptom relief with icatibant.7 However, icatibant is extremely expensive ($23,000/30 mg). As previously mentioned, based on its similar mechanism of action, lower cost, and safety profile, FFP can be given (off label) in this situation.
Case Conclusion
The patient was given diphenhydramine, famotidine, and methylprednisolone, but did not show any improvement. His upper/lower lip swelling continued to worsen, and 30 minutes after the onset of angioedema, he was unable to open his mouth more than 1 cm.
Multiple attempts to perform awake fiberoptic intubation failed due to inadequate sedation; however, intubation was successfully performed following light sedation. The patient self-extubated in the ICU on hospital day 3, and the angioedema had progressively decreased. Angioedema and weakness completely resolved by hospital day 4, and he was discharged home on hospital day 7.
1. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
2. Madden B, Chebl RB. Hemi orolingual angioedema after tPA administration for acute ischemic stroke. West J Emerg Med. 2015;16(1):175-177. doi:10.5811/westjem.2014.12.24210.
3. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
4. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282-286. doi:10.1016/j.amjmed.2007.09.024.
5. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
6. Maertins M, Wold R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278. doi:10.1016/j.amj.2010.12.011.
7. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-425. doi:10.1056/NEJMoa1312524.
Case
A 49-year-old man with a history of hypertension, for which he was taking aspirin, carvedilol, hydralazine, and nifedipine, presented to the ED with complaints of left-sided weakness that started 3 hours before he came to the ED. Initial vital signs were: blood pressure, 158/90 mm Hg; heart rate, 74 beats/min; respiratory rate, 18 breaths/min; and temperature, 98°F. Oxygen saturation was 100% on room air, and a finger-stick glucose test was 106 mg/dL.
Physical examination revealed slowed speech with mild dysarthria, mild left facial droop, 2/5 strength in all muscle groups in the left upper and lower extremities, and decreased sensation to light touch on the left side. The patient also had left-sided sensory neglect and an abnormal gait, and dragged his left foot on the floor when walking. The rest of his examination was normal.
The stroke team was activated, and the patient was immediately transferred to the ED radiology department for imaging studies. A noncontrast head computed tomography (CT) was negative for any acute intracranial hemorrhage or cerebral edema. A CT angiogram (CTA) also was performed, which revealed atherosclerosis but no arterial occlusion. Based on these findings and the existing protocol, the patient received an intravenous (IV) bolus of tissue plasminogen activator (tPA). Approximately 17 minutes after tPA administration, the patient developed left-sided upper and lower lip swelling. There was no voice change, tongue swelling, or uvular deviation.
What is the differential diagnosis of swelling of the lip?
The differential diagnoses for lip swelling includes trauma, allergic reaction, and angioedema (hereditary, or angiotensin converting enzyme inhibitor [ACEI]-induced). The patient in this case denied any trauma to the lip, and no bleeding was noted from the lip; however, his entire left lip (upper and lower) was swollen. He was not taking any ACEIs or angiotensin-receptor blockers (ARBs). He also denied a family history of angioedema or any prior similar episodes. The patient further denied exposure to any new medications, foods, or other substances and had no respiratory distress, urticaria, or other findings consistent with an allergy.
What are the common adverse effects of tPA?
The only US Food and Drug-approved pharmacological treatment for ischemic stroke is tPA (also known as IV rtPA). Tissue plasminogen activator hydrolyzes plasminogen to plasmin, which exerts a fibrinolytic effect. Based on the ability of tPA to lyse thrombus, it is also a standard therapy for hemodynamically unstable patients with confirmed pulmonary embolism, as well as for patients with myocardial infarction in whom percutaneous intervention is contraindicated or unavailable. Despite the beneficial effects of tPA, significant adverse effects are associated with the drug. For example, thrombolysis may result in conversion of an ischemic stroke into a hemorrhagic event, resulting in generalized bleeding from mucosal surfaces.
The increase in plasmin may play a role in the development of angioedema by activating the kinin pathway, leading to the formation of the vasodilator bradykinin (Figure). Plasmin also activates the complement system and leads to the production of anaphylatoxins C3a, C4a, and C5a, which also cause mast cell degranulation and histamine release.1
When does post-tPA angioedema occur?
In the few published case reports available, tPA-induced angioedema was shown to typically occur in the stroke distribution (which was attributed to the left-sided swelling in this patient).2 Following tPA administration, the onset of angioedema reportedly varies from as early as 10 to 15 minutes from initiation until about 1 hour postinfusion. The short half-life of tPA (approximately 7 minutes)2 limits the outer- time window for the initial development of angioedema, but progression can continue well beyond this timeframe.
What is the treatment for tPA-induced angioedema?
The first priority of acute management of angioedema is discontinuation of the inciting substance, if possible—in this case, the tPA infusion.3 Assessment and maintenance of a patent airway are of utmost concern. Patients with posterior oropharyngeal effects or who are progressing should be admitted to an intensive care unit (ICU) for observation.4-6
Endotracheal Intubation. Providers should have a low threshold for endotracheal intubation, which should ideally be performed in any patient at risk for airway compromise.4 Due to the extensive airway swelling that can occur in the setting of angioedema, airway intervention should optimally be performed by an available clinician with the most skill and experience in this area. It is wise to be prepared to utilize advanced airway techniques, if available, including fiberoptic laryngoscopy or potentially cricothyrotomy.
Histamine Agonists. Standard therapy for patients who develop angioedema should include histamine antagonists, such as diphenhydramine (H1 antagonist) and famotidine (H2 antagonist) along with corticosteroids. Although these therapies are unlikely to be helpful in the treatment of tPA-induced angioedema, the difficulty in excluding allergic angioedema and the low risk of adverse effects associated with these medications support their use.
Fresh Frozen Plasma. Fresh frozen plasma (FFP) should be considered for patients who have a history of hereditary angioedema. Fresh frozen plasma contains enzymes that degrade bradykinin. Although FFP has been used successfully in the treatment of ACEI-induced angioedema, its use (or benefit) in tPA-related cases is not clear.
Icatibant. A selective bradykinin B2-receptor antagonist, icatibant has been used to treat patients with ACEI-induced angioedema because of its effects on bradykinin receptors. Comparison of the efficacy of icatibant to the prevailing treatment strategy of diphenhydramine, famotidine, and methylprednisolone found a shorter time to symptom relief with icatibant.7 However, icatibant is extremely expensive ($23,000/30 mg). As previously mentioned, based on its similar mechanism of action, lower cost, and safety profile, FFP can be given (off label) in this situation.
Case Conclusion
The patient was given diphenhydramine, famotidine, and methylprednisolone, but did not show any improvement. His upper/lower lip swelling continued to worsen, and 30 minutes after the onset of angioedema, he was unable to open his mouth more than 1 cm.
Multiple attempts to perform awake fiberoptic intubation failed due to inadequate sedation; however, intubation was successfully performed following light sedation. The patient self-extubated in the ICU on hospital day 3, and the angioedema had progressively decreased. Angioedema and weakness completely resolved by hospital day 4, and he was discharged home on hospital day 7.
Case
A 49-year-old man with a history of hypertension, for which he was taking aspirin, carvedilol, hydralazine, and nifedipine, presented to the ED with complaints of left-sided weakness that started 3 hours before he came to the ED. Initial vital signs were: blood pressure, 158/90 mm Hg; heart rate, 74 beats/min; respiratory rate, 18 breaths/min; and temperature, 98°F. Oxygen saturation was 100% on room air, and a finger-stick glucose test was 106 mg/dL.
Physical examination revealed slowed speech with mild dysarthria, mild left facial droop, 2/5 strength in all muscle groups in the left upper and lower extremities, and decreased sensation to light touch on the left side. The patient also had left-sided sensory neglect and an abnormal gait, and dragged his left foot on the floor when walking. The rest of his examination was normal.
The stroke team was activated, and the patient was immediately transferred to the ED radiology department for imaging studies. A noncontrast head computed tomography (CT) was negative for any acute intracranial hemorrhage or cerebral edema. A CT angiogram (CTA) also was performed, which revealed atherosclerosis but no arterial occlusion. Based on these findings and the existing protocol, the patient received an intravenous (IV) bolus of tissue plasminogen activator (tPA). Approximately 17 minutes after tPA administration, the patient developed left-sided upper and lower lip swelling. There was no voice change, tongue swelling, or uvular deviation.
What is the differential diagnosis of swelling of the lip?
The differential diagnoses for lip swelling includes trauma, allergic reaction, and angioedema (hereditary, or angiotensin converting enzyme inhibitor [ACEI]-induced). The patient in this case denied any trauma to the lip, and no bleeding was noted from the lip; however, his entire left lip (upper and lower) was swollen. He was not taking any ACEIs or angiotensin-receptor blockers (ARBs). He also denied a family history of angioedema or any prior similar episodes. The patient further denied exposure to any new medications, foods, or other substances and had no respiratory distress, urticaria, or other findings consistent with an allergy.
What are the common adverse effects of tPA?
The only US Food and Drug-approved pharmacological treatment for ischemic stroke is tPA (also known as IV rtPA). Tissue plasminogen activator hydrolyzes plasminogen to plasmin, which exerts a fibrinolytic effect. Based on the ability of tPA to lyse thrombus, it is also a standard therapy for hemodynamically unstable patients with confirmed pulmonary embolism, as well as for patients with myocardial infarction in whom percutaneous intervention is contraindicated or unavailable. Despite the beneficial effects of tPA, significant adverse effects are associated with the drug. For example, thrombolysis may result in conversion of an ischemic stroke into a hemorrhagic event, resulting in generalized bleeding from mucosal surfaces.
The increase in plasmin may play a role in the development of angioedema by activating the kinin pathway, leading to the formation of the vasodilator bradykinin (Figure). Plasmin also activates the complement system and leads to the production of anaphylatoxins C3a, C4a, and C5a, which also cause mast cell degranulation and histamine release.1
When does post-tPA angioedema occur?
In the few published case reports available, tPA-induced angioedema was shown to typically occur in the stroke distribution (which was attributed to the left-sided swelling in this patient).2 Following tPA administration, the onset of angioedema reportedly varies from as early as 10 to 15 minutes from initiation until about 1 hour postinfusion. The short half-life of tPA (approximately 7 minutes)2 limits the outer- time window for the initial development of angioedema, but progression can continue well beyond this timeframe.
What is the treatment for tPA-induced angioedema?
The first priority of acute management of angioedema is discontinuation of the inciting substance, if possible—in this case, the tPA infusion.3 Assessment and maintenance of a patent airway are of utmost concern. Patients with posterior oropharyngeal effects or who are progressing should be admitted to an intensive care unit (ICU) for observation.4-6
Endotracheal Intubation. Providers should have a low threshold for endotracheal intubation, which should ideally be performed in any patient at risk for airway compromise.4 Due to the extensive airway swelling that can occur in the setting of angioedema, airway intervention should optimally be performed by an available clinician with the most skill and experience in this area. It is wise to be prepared to utilize advanced airway techniques, if available, including fiberoptic laryngoscopy or potentially cricothyrotomy.
Histamine Agonists. Standard therapy for patients who develop angioedema should include histamine antagonists, such as diphenhydramine (H1 antagonist) and famotidine (H2 antagonist) along with corticosteroids. Although these therapies are unlikely to be helpful in the treatment of tPA-induced angioedema, the difficulty in excluding allergic angioedema and the low risk of adverse effects associated with these medications support their use.
Fresh Frozen Plasma. Fresh frozen plasma (FFP) should be considered for patients who have a history of hereditary angioedema. Fresh frozen plasma contains enzymes that degrade bradykinin. Although FFP has been used successfully in the treatment of ACEI-induced angioedema, its use (or benefit) in tPA-related cases is not clear.
Icatibant. A selective bradykinin B2-receptor antagonist, icatibant has been used to treat patients with ACEI-induced angioedema because of its effects on bradykinin receptors. Comparison of the efficacy of icatibant to the prevailing treatment strategy of diphenhydramine, famotidine, and methylprednisolone found a shorter time to symptom relief with icatibant.7 However, icatibant is extremely expensive ($23,000/30 mg). As previously mentioned, based on its similar mechanism of action, lower cost, and safety profile, FFP can be given (off label) in this situation.
Case Conclusion
The patient was given diphenhydramine, famotidine, and methylprednisolone, but did not show any improvement. His upper/lower lip swelling continued to worsen, and 30 minutes after the onset of angioedema, he was unable to open his mouth more than 1 cm.
Multiple attempts to perform awake fiberoptic intubation failed due to inadequate sedation; however, intubation was successfully performed following light sedation. The patient self-extubated in the ICU on hospital day 3, and the angioedema had progressively decreased. Angioedema and weakness completely resolved by hospital day 4, and he was discharged home on hospital day 7.
1. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
2. Madden B, Chebl RB. Hemi orolingual angioedema after tPA administration for acute ischemic stroke. West J Emerg Med. 2015;16(1):175-177. doi:10.5811/westjem.2014.12.24210.
3. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
4. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282-286. doi:10.1016/j.amjmed.2007.09.024.
5. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
6. Maertins M, Wold R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278. doi:10.1016/j.amj.2010.12.011.
7. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-425. doi:10.1056/NEJMoa1312524.
1. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
2. Madden B, Chebl RB. Hemi orolingual angioedema after tPA administration for acute ischemic stroke. West J Emerg Med. 2015;16(1):175-177. doi:10.5811/westjem.2014.12.24210.
3. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
4. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282-286. doi:10.1016/j.amjmed.2007.09.024.
5. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
6. Maertins M, Wold R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278. doi:10.1016/j.amj.2010.12.011.
7. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-425. doi:10.1056/NEJMoa1312524.
Approach to Management of Giant Basal Cell Carcinomas
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.
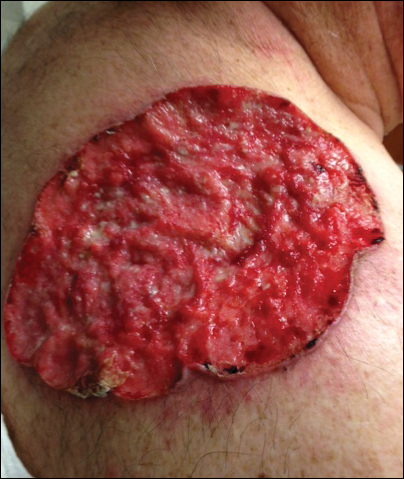
The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.
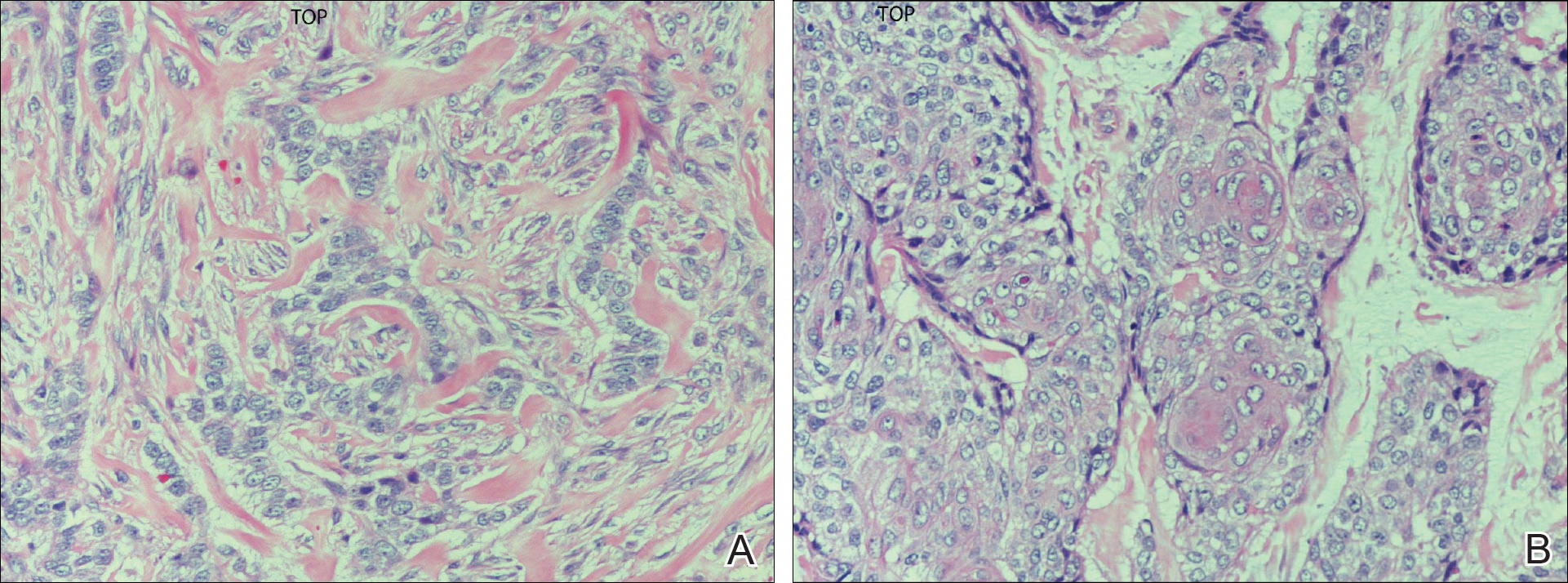
The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.
Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.


The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.

The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.

Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.


The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.

The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.

Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
Practice Points
- Unusually large basal cell carcinomas (BCCs) present a therapeutic challenge.
- A number of therapeutic options exist. Wide excision with margin control and complex reconstruction remains an excellent treatment option for BCC.
Arthroscopic Excision of Bipartite Patella With Preservation of Lateral Retinaculum in an Adolescent Ice Hockey Player
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
Internal Carotid Artery Dissection After Indirect Blunt Cervical Trauma in an Ice Hockey Goaltender
Take-Home Points
- ICA dissections may occur from direct or indirect trauma.
- Symptoms can be mild, including a persistent headache.
- High clinical suspicion is required for diagnosis when symptoms are mild.
- Neuroimaging is required for definitive diagnosis.
- Conservative management with serial imaging can yield successful outcomes.
Cervical artery dissection (CAD) is an uncommon but potentially life-threatening condition that accounts for a high proportion of ischemic strokes in patients under the age of 45 years.1-4 The extracranial internal carotid arteries (ICAs) and vertebral arteries are most commonly involved; dissections can occur after either direct trauma to the neck, or indirect trauma resulting in acute hyperextension or hyperflexion.4-7 ICA dissection can be difficult to diagnose because of the varying symptomatology. Clinical presentation depends on stenosis location, degree of luminal narrowing, and presence or absence of ischemic stroke. Neurologic symptoms may be delayed, and misdiagnosis of an isolated soft-tissue contusion, whiplash, can be made in the setting of indirect cervical trauma.
Although this entity is well described in the literature,2,3,5,8 there are few reported cases of injuries sustained during high-intensity athletic competition. In this case report, we describe the symptoms, physical examination findings, diagnostic imaging results, and treatment of a young male athlete who presented with delayed-onset symptoms of ICA dissection resulting from indirect cervical trauma sustained during an ice hockey game. We discuss the importance of a high level of clinical suspicion in the diagnosis of neck injuries sustained during athletic competition, as well as the need for early vascular imaging for diagnosis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right-handed 32-year-old professional hockey goaltender. Four days before diagnosis, his goaltending mask and attached neck-protector were inadvertently lifted by another player’s stick just as a puck traveling at high speed struck him in the neck, to the right of the larynx, causing acute neck hyperextension. He immediately experienced discomfort and fell to the ice, saying he was “dizzy and light-headed.” Play was stopped, and medical personnel attended to him. His symptoms resolved, and he resumed play without any notable deficits. The next day, he noted discomfort at the impact site, but no additional symptoms, and received a presumptive diagnosis of cervical soft-tissue contusion. Continuing to participate in hockey that day, he did not develop any symptoms other than superficial cervical discomfort. However, the next morning, he presented complaining of severe right frontotemporal headache, which had persisted overnight. Orthopedic examination revealed palpable tenderness over the anterior cervical musculature, including the sternocleidomastoid and strap muscles. There was no appreciable hematoma in the contused area. Cervical range of motion was otherwise preserved. Cervical spine examination, including dermatomal and myotomal examination, was normal, as was cranial nerve examination. However, given the headache intensity and the recency of the injury, the potential for vascular or neurologic injury was considered. A neurology consultation was obtained, and arrangements were made for advanced cross-sectional imaging.
On further evaluation, the patient denied loss of consciousness, seizure, vomiting, amnesia, visual disturbance, language or cognitive impairment, balance or coordination difficulties, or any appreciable face or limb weakness. Review of systems was otherwise negative. Detailed neurologic examination did not reveal any cranial nerve deficits, and pupils were 3 mm, equal, and normally responsive to light and accommodation. Muscular tone and strength were symmetric and full in the upper and lower extremities. Gait, coordination, and response to vibration and temperature sensation were all preserved.
Magnetic resonance imaging of the head and neck was normal, but magnetic resonance angiography (MRA) of the neck showed a 1-cm-long region of the ICA, before piercing the petrous bone, with evidence of dissection.
Given the normal neurologic examination, and no evidence of brain infarction or other neurovascular complications, the acute ICA dissection was managed with antiplatelet therapy using aspirin (325 mg/d). In addition, the patient was advised to refrain from strenuous physical activity and to present to the hospital immediately if symptoms worsened or any neurologic impairment developed. Follow-up and repeat MRA were planned to monitor healing progression.
Two weeks after injury, the patient returned for follow-up. His headache and neck pain had resolved. Physical examination findings were unchanged, and there were no notable neurologic deficits. Repeat MRA findings were essentially unchanged, except for slightly increased luminal stenosis, exceeding 50% (Figure 2), attributable to intramural hematoma formation.
At 6-week follow-up, the patient had no clinical symptoms and no recurrence of headaches.
Discussion
In cases of direct (blunt) or indirect cervical trauma, CAD should be considered, as it carries a risk of potentially debilitating ischemic stroke in otherwise healthy young patients. Fortunately, CAD is rare; its annual incidence is 1 in 100,000, occurring in 0.08% to 1.2% of blunt trauma cases.9
As symptoms of ICA dissection can vary depending on stenosis severity, diagnosis can be challenging. The classically associated triad of symptoms includes unilateral head, facial, or neck pain accompanied by partial Horner syndrome with progression to cerebral or retinal ischemia. However, these symptoms occur in less than a third of patients with ICA dissection.2 Neck pain may occur secondary to blunt cervical trauma, consistent with a cervical soft-tissue contusion; however, it may have more severe implications and should be carefully monitored, particularly if accompanied by additional symptoms, such as headache. Headaches, which are present in 44% to 69% of patients, are often unilateral and constant. Either headache or neck pain in isolation is relatively uncommon, occurring in <10% of cases,2 though retrospective reviews of delayed-onset ICA dissection found atypical headache or neck pain in 100% of patients,11 indicating that persistent symptoms should be further evaluated.
More commonly, patients present with neurologic symptoms, particularly Horner syndrome, which is caused by the disruption of the sympathetic nerve fibers adjacent to the ICA, resulting in ipsilateral ptosis and miosis. In addition, patients may present with cranial nerve palsies, most commonly involving cranial nerve XII (the hypoglossal nerve), resulting in tongue weakness and abnormal taste. These and other neurologic findings associated with retinal or cerebral ischemia should raise clinical suspicion for the injury and prompt computed tomography or MRA evaluation.
MRA has largely replaced conventional angiography for the diagnosis of CAD. As MRA is noninvasive, it allows for improved visualization of luminal narrowing and for evaluation of the arterial wall and intramural hematoma.2 Because of the potential for devastating sequelae with missed or delayed diagnosis, several authors have become proponents of early aggressive screening for detection of these injuries.9 Postdiagnostic treatment depends on the presence of neurologic symptoms. Management is directed toward limiting neurologic deficits; anticoagulant or antiplatelet agents are used to prevent thromboembolic events. A randomized controlled trial and other studies have failed to find any appreciable difference in subsequent rates of stroke or associated complications with use of either class of medication.8,12 Conventionally, treatment is continued for 3 to 6 months, depending on clinical resolution. Endovascular or surgical intervention typically is reserved for extreme luminal narrowing, conditions that are preventing anticoagulation, an expanding area of dissection with a persistent pseudoaneurysm, and cases of failed medical management with subsequent ischemic stroke.2The literature includes several case reports involving indirect trauma in recreational athletes. First, a 31-year-old woman sustained an ICA dissection secondary to a head injury that occurred during a soccer match; she presented with headache, altered sense of taste, and objective findings of ptosis and miosis consistent with Horner syndrome.13 Second, a 39-year-old man had an ICA dissection after a snowboarding fall that caused neck hyperextension; he presented with periocular headache, ptosis, and miosis.6 Third, 3 people who participated in CrossFit training sustained ICA dissection.7 They presented with varying degrees of neurologic symptoms: ptosis and miosis; right-side upper extremity ataxia; and visual distortion and receptive aphasia. Our patient’s ICA dissection resulted from indirect trauma that caused sudden hyperextension and lateral flexion in response to contact from a hockey puck. However, his case is unique in that symptoms onset was delayed, and there were no associated neurologic findings on clinical presentation. His case should raise awareness of this potential diagnosis, even in the absence of overt neurologic findings. In addition, the patient’s return to sport at 8 weeks was facilitated by full clinical resolution of symptoms and thorough radiographic documentation of improved intramural narrowing. Finally, to our knowledge this is the first report of this injury in a professional athlete.
Conclusion
We have reported the case of a 32-year-old professional hockey goaltender who presented with isolated, persistent, worsening headache of delayed onset after ICA dissection. The ICA dissection resulted from indirect trauma, with reaction to a puck causing acute hyperextension and rotational injury. To our knowledge, this is the first report of a case of ICA dissection in an athlete, lacking neurologic examination findings that could aid in the diagnosis. The index of suspicion for CAD should be high after direct or indirect cervical trauma when patients present with unilateral neck pain or headache, even in the absence of neurologic findings, as stroke is a catastrophic but preventable complication.
Am J Orthop. 2017;46(3):E139-E143. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Mohan IV. Current optimal assessment and management of carotid and vertebral spontaneous and traumatic dissection. Angiology. 2014;65(4):274-283.
2. Patel RR, Adam R, Maldjian C, Lincoln CM, Yuen A, Arneja A. Cervical carotid artery dissection: current review of diagnosis and treatment. Cardiol Rev. 2012;20(3):145-152.
3. Biller J, Sacco RL, Albuquerque FC, et al; American Heart Association Stroke Council. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(10):3155-3174.
4. Fukunaga N, Hanaoka M, Sato K. Asymptomatic common carotid artery dissection caused by blunt injury. Emerg Med J. 2011;28(1):50.
5. Chen J, Zhou X, Li C, Cheung BM. Risk of stroke due to spontaneous cervical artery dissection. Intern Med. 2013;52(19):2237-2240.
6. Kalantzis G, Georgalas I, Chang BY, Ong C, El-Hindy N. An unusual case of traumatic internal carotid artery dissection during snowboarding. J Sports Sci Med. 2014;13(2):451-453.
7. Lu A, Shen P, Lee P, et al. CrossFit-related cervical internal carotid artery dissection. Emerg Radiol. 2015;22(4):449-452.
8. CADISS Trial Investigators, Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
9. van Wessem KJ, Meijer JM, Leenen LP, van der Worp HB, Moll FL, de Borst GJ. Blunt traumatic carotid artery dissection still a pitfall? The rationale for aggressive screening. Eur J Trauma Emerg Surg. 2011;37(2):147-154.
10. Haneline M, Triano J. Cervical artery dissection. A comparison of highly dynamic mechanisms: manipulation versus motor vehicle collision. J Manipulative Physiol Ther. 2005;28(1):57-63.
11. Thomas LC, Rivett DA, Attia JR, Levi C. Risk factors and clinical presentation of cervical arterial dissection: preliminary results of a prospective case-control study. J Orthop Sports Phys Ther. 2015;45(7):503-511.
12. Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2010;(10):CD000255.
13. Creavin ST, Rice CM, Pollentine A, Cowburn P. Carotid artery dissection presenting with isolated headache and Horner syndrome after minor head injury. Am J Emerg Med. 2012;30(9):2103.e5-e7.
Take-Home Points
- ICA dissections may occur from direct or indirect trauma.
- Symptoms can be mild, including a persistent headache.
- High clinical suspicion is required for diagnosis when symptoms are mild.
- Neuroimaging is required for definitive diagnosis.
- Conservative management with serial imaging can yield successful outcomes.
Cervical artery dissection (CAD) is an uncommon but potentially life-threatening condition that accounts for a high proportion of ischemic strokes in patients under the age of 45 years.1-4 The extracranial internal carotid arteries (ICAs) and vertebral arteries are most commonly involved; dissections can occur after either direct trauma to the neck, or indirect trauma resulting in acute hyperextension or hyperflexion.4-7 ICA dissection can be difficult to diagnose because of the varying symptomatology. Clinical presentation depends on stenosis location, degree of luminal narrowing, and presence or absence of ischemic stroke. Neurologic symptoms may be delayed, and misdiagnosis of an isolated soft-tissue contusion, whiplash, can be made in the setting of indirect cervical trauma.
Although this entity is well described in the literature,2,3,5,8 there are few reported cases of injuries sustained during high-intensity athletic competition. In this case report, we describe the symptoms, physical examination findings, diagnostic imaging results, and treatment of a young male athlete who presented with delayed-onset symptoms of ICA dissection resulting from indirect cervical trauma sustained during an ice hockey game. We discuss the importance of a high level of clinical suspicion in the diagnosis of neck injuries sustained during athletic competition, as well as the need for early vascular imaging for diagnosis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right-handed 32-year-old professional hockey goaltender. Four days before diagnosis, his goaltending mask and attached neck-protector were inadvertently lifted by another player’s stick just as a puck traveling at high speed struck him in the neck, to the right of the larynx, causing acute neck hyperextension. He immediately experienced discomfort and fell to the ice, saying he was “dizzy and light-headed.” Play was stopped, and medical personnel attended to him. His symptoms resolved, and he resumed play without any notable deficits. The next day, he noted discomfort at the impact site, but no additional symptoms, and received a presumptive diagnosis of cervical soft-tissue contusion. Continuing to participate in hockey that day, he did not develop any symptoms other than superficial cervical discomfort. However, the next morning, he presented complaining of severe right frontotemporal headache, which had persisted overnight. Orthopedic examination revealed palpable tenderness over the anterior cervical musculature, including the sternocleidomastoid and strap muscles. There was no appreciable hematoma in the contused area. Cervical range of motion was otherwise preserved. Cervical spine examination, including dermatomal and myotomal examination, was normal, as was cranial nerve examination. However, given the headache intensity and the recency of the injury, the potential for vascular or neurologic injury was considered. A neurology consultation was obtained, and arrangements were made for advanced cross-sectional imaging.
On further evaluation, the patient denied loss of consciousness, seizure, vomiting, amnesia, visual disturbance, language or cognitive impairment, balance or coordination difficulties, or any appreciable face or limb weakness. Review of systems was otherwise negative. Detailed neurologic examination did not reveal any cranial nerve deficits, and pupils were 3 mm, equal, and normally responsive to light and accommodation. Muscular tone and strength were symmetric and full in the upper and lower extremities. Gait, coordination, and response to vibration and temperature sensation were all preserved.
Magnetic resonance imaging of the head and neck was normal, but magnetic resonance angiography (MRA) of the neck showed a 1-cm-long region of the ICA, before piercing the petrous bone, with evidence of dissection.
Given the normal neurologic examination, and no evidence of brain infarction or other neurovascular complications, the acute ICA dissection was managed with antiplatelet therapy using aspirin (325 mg/d). In addition, the patient was advised to refrain from strenuous physical activity and to present to the hospital immediately if symptoms worsened or any neurologic impairment developed. Follow-up and repeat MRA were planned to monitor healing progression.
Two weeks after injury, the patient returned for follow-up. His headache and neck pain had resolved. Physical examination findings were unchanged, and there were no notable neurologic deficits. Repeat MRA findings were essentially unchanged, except for slightly increased luminal stenosis, exceeding 50% (Figure 2), attributable to intramural hematoma formation.
At 6-week follow-up, the patient had no clinical symptoms and no recurrence of headaches.
Discussion
In cases of direct (blunt) or indirect cervical trauma, CAD should be considered, as it carries a risk of potentially debilitating ischemic stroke in otherwise healthy young patients. Fortunately, CAD is rare; its annual incidence is 1 in 100,000, occurring in 0.08% to 1.2% of blunt trauma cases.9
As symptoms of ICA dissection can vary depending on stenosis severity, diagnosis can be challenging. The classically associated triad of symptoms includes unilateral head, facial, or neck pain accompanied by partial Horner syndrome with progression to cerebral or retinal ischemia. However, these symptoms occur in less than a third of patients with ICA dissection.2 Neck pain may occur secondary to blunt cervical trauma, consistent with a cervical soft-tissue contusion; however, it may have more severe implications and should be carefully monitored, particularly if accompanied by additional symptoms, such as headache. Headaches, which are present in 44% to 69% of patients, are often unilateral and constant. Either headache or neck pain in isolation is relatively uncommon, occurring in <10% of cases,2 though retrospective reviews of delayed-onset ICA dissection found atypical headache or neck pain in 100% of patients,11 indicating that persistent symptoms should be further evaluated.
More commonly, patients present with neurologic symptoms, particularly Horner syndrome, which is caused by the disruption of the sympathetic nerve fibers adjacent to the ICA, resulting in ipsilateral ptosis and miosis. In addition, patients may present with cranial nerve palsies, most commonly involving cranial nerve XII (the hypoglossal nerve), resulting in tongue weakness and abnormal taste. These and other neurologic findings associated with retinal or cerebral ischemia should raise clinical suspicion for the injury and prompt computed tomography or MRA evaluation.
MRA has largely replaced conventional angiography for the diagnosis of CAD. As MRA is noninvasive, it allows for improved visualization of luminal narrowing and for evaluation of the arterial wall and intramural hematoma.2 Because of the potential for devastating sequelae with missed or delayed diagnosis, several authors have become proponents of early aggressive screening for detection of these injuries.9 Postdiagnostic treatment depends on the presence of neurologic symptoms. Management is directed toward limiting neurologic deficits; anticoagulant or antiplatelet agents are used to prevent thromboembolic events. A randomized controlled trial and other studies have failed to find any appreciable difference in subsequent rates of stroke or associated complications with use of either class of medication.8,12 Conventionally, treatment is continued for 3 to 6 months, depending on clinical resolution. Endovascular or surgical intervention typically is reserved for extreme luminal narrowing, conditions that are preventing anticoagulation, an expanding area of dissection with a persistent pseudoaneurysm, and cases of failed medical management with subsequent ischemic stroke.2The literature includes several case reports involving indirect trauma in recreational athletes. First, a 31-year-old woman sustained an ICA dissection secondary to a head injury that occurred during a soccer match; she presented with headache, altered sense of taste, and objective findings of ptosis and miosis consistent with Horner syndrome.13 Second, a 39-year-old man had an ICA dissection after a snowboarding fall that caused neck hyperextension; he presented with periocular headache, ptosis, and miosis.6 Third, 3 people who participated in CrossFit training sustained ICA dissection.7 They presented with varying degrees of neurologic symptoms: ptosis and miosis; right-side upper extremity ataxia; and visual distortion and receptive aphasia. Our patient’s ICA dissection resulted from indirect trauma that caused sudden hyperextension and lateral flexion in response to contact from a hockey puck. However, his case is unique in that symptoms onset was delayed, and there were no associated neurologic findings on clinical presentation. His case should raise awareness of this potential diagnosis, even in the absence of overt neurologic findings. In addition, the patient’s return to sport at 8 weeks was facilitated by full clinical resolution of symptoms and thorough radiographic documentation of improved intramural narrowing. Finally, to our knowledge this is the first report of this injury in a professional athlete.
Conclusion
We have reported the case of a 32-year-old professional hockey goaltender who presented with isolated, persistent, worsening headache of delayed onset after ICA dissection. The ICA dissection resulted from indirect trauma, with reaction to a puck causing acute hyperextension and rotational injury. To our knowledge, this is the first report of a case of ICA dissection in an athlete, lacking neurologic examination findings that could aid in the diagnosis. The index of suspicion for CAD should be high after direct or indirect cervical trauma when patients present with unilateral neck pain or headache, even in the absence of neurologic findings, as stroke is a catastrophic but preventable complication.
Am J Orthop. 2017;46(3):E139-E143. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- ICA dissections may occur from direct or indirect trauma.
- Symptoms can be mild, including a persistent headache.
- High clinical suspicion is required for diagnosis when symptoms are mild.
- Neuroimaging is required for definitive diagnosis.
- Conservative management with serial imaging can yield successful outcomes.
Cervical artery dissection (CAD) is an uncommon but potentially life-threatening condition that accounts for a high proportion of ischemic strokes in patients under the age of 45 years.1-4 The extracranial internal carotid arteries (ICAs) and vertebral arteries are most commonly involved; dissections can occur after either direct trauma to the neck, or indirect trauma resulting in acute hyperextension or hyperflexion.4-7 ICA dissection can be difficult to diagnose because of the varying symptomatology. Clinical presentation depends on stenosis location, degree of luminal narrowing, and presence or absence of ischemic stroke. Neurologic symptoms may be delayed, and misdiagnosis of an isolated soft-tissue contusion, whiplash, can be made in the setting of indirect cervical trauma.
Although this entity is well described in the literature,2,3,5,8 there are few reported cases of injuries sustained during high-intensity athletic competition. In this case report, we describe the symptoms, physical examination findings, diagnostic imaging results, and treatment of a young male athlete who presented with delayed-onset symptoms of ICA dissection resulting from indirect cervical trauma sustained during an ice hockey game. We discuss the importance of a high level of clinical suspicion in the diagnosis of neck injuries sustained during athletic competition, as well as the need for early vascular imaging for diagnosis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right-handed 32-year-old professional hockey goaltender. Four days before diagnosis, his goaltending mask and attached neck-protector were inadvertently lifted by another player’s stick just as a puck traveling at high speed struck him in the neck, to the right of the larynx, causing acute neck hyperextension. He immediately experienced discomfort and fell to the ice, saying he was “dizzy and light-headed.” Play was stopped, and medical personnel attended to him. His symptoms resolved, and he resumed play without any notable deficits. The next day, he noted discomfort at the impact site, but no additional symptoms, and received a presumptive diagnosis of cervical soft-tissue contusion. Continuing to participate in hockey that day, he did not develop any symptoms other than superficial cervical discomfort. However, the next morning, he presented complaining of severe right frontotemporal headache, which had persisted overnight. Orthopedic examination revealed palpable tenderness over the anterior cervical musculature, including the sternocleidomastoid and strap muscles. There was no appreciable hematoma in the contused area. Cervical range of motion was otherwise preserved. Cervical spine examination, including dermatomal and myotomal examination, was normal, as was cranial nerve examination. However, given the headache intensity and the recency of the injury, the potential for vascular or neurologic injury was considered. A neurology consultation was obtained, and arrangements were made for advanced cross-sectional imaging.
On further evaluation, the patient denied loss of consciousness, seizure, vomiting, amnesia, visual disturbance, language or cognitive impairment, balance or coordination difficulties, or any appreciable face or limb weakness. Review of systems was otherwise negative. Detailed neurologic examination did not reveal any cranial nerve deficits, and pupils were 3 mm, equal, and normally responsive to light and accommodation. Muscular tone and strength were symmetric and full in the upper and lower extremities. Gait, coordination, and response to vibration and temperature sensation were all preserved.
Magnetic resonance imaging of the head and neck was normal, but magnetic resonance angiography (MRA) of the neck showed a 1-cm-long region of the ICA, before piercing the petrous bone, with evidence of dissection.
Given the normal neurologic examination, and no evidence of brain infarction or other neurovascular complications, the acute ICA dissection was managed with antiplatelet therapy using aspirin (325 mg/d). In addition, the patient was advised to refrain from strenuous physical activity and to present to the hospital immediately if symptoms worsened or any neurologic impairment developed. Follow-up and repeat MRA were planned to monitor healing progression.
Two weeks after injury, the patient returned for follow-up. His headache and neck pain had resolved. Physical examination findings were unchanged, and there were no notable neurologic deficits. Repeat MRA findings were essentially unchanged, except for slightly increased luminal stenosis, exceeding 50% (Figure 2), attributable to intramural hematoma formation.
At 6-week follow-up, the patient had no clinical symptoms and no recurrence of headaches.
Discussion
In cases of direct (blunt) or indirect cervical trauma, CAD should be considered, as it carries a risk of potentially debilitating ischemic stroke in otherwise healthy young patients. Fortunately, CAD is rare; its annual incidence is 1 in 100,000, occurring in 0.08% to 1.2% of blunt trauma cases.9
As symptoms of ICA dissection can vary depending on stenosis severity, diagnosis can be challenging. The classically associated triad of symptoms includes unilateral head, facial, or neck pain accompanied by partial Horner syndrome with progression to cerebral or retinal ischemia. However, these symptoms occur in less than a third of patients with ICA dissection.2 Neck pain may occur secondary to blunt cervical trauma, consistent with a cervical soft-tissue contusion; however, it may have more severe implications and should be carefully monitored, particularly if accompanied by additional symptoms, such as headache. Headaches, which are present in 44% to 69% of patients, are often unilateral and constant. Either headache or neck pain in isolation is relatively uncommon, occurring in <10% of cases,2 though retrospective reviews of delayed-onset ICA dissection found atypical headache or neck pain in 100% of patients,11 indicating that persistent symptoms should be further evaluated.
More commonly, patients present with neurologic symptoms, particularly Horner syndrome, which is caused by the disruption of the sympathetic nerve fibers adjacent to the ICA, resulting in ipsilateral ptosis and miosis. In addition, patients may present with cranial nerve palsies, most commonly involving cranial nerve XII (the hypoglossal nerve), resulting in tongue weakness and abnormal taste. These and other neurologic findings associated with retinal or cerebral ischemia should raise clinical suspicion for the injury and prompt computed tomography or MRA evaluation.
MRA has largely replaced conventional angiography for the diagnosis of CAD. As MRA is noninvasive, it allows for improved visualization of luminal narrowing and for evaluation of the arterial wall and intramural hematoma.2 Because of the potential for devastating sequelae with missed or delayed diagnosis, several authors have become proponents of early aggressive screening for detection of these injuries.9 Postdiagnostic treatment depends on the presence of neurologic symptoms. Management is directed toward limiting neurologic deficits; anticoagulant or antiplatelet agents are used to prevent thromboembolic events. A randomized controlled trial and other studies have failed to find any appreciable difference in subsequent rates of stroke or associated complications with use of either class of medication.8,12 Conventionally, treatment is continued for 3 to 6 months, depending on clinical resolution. Endovascular or surgical intervention typically is reserved for extreme luminal narrowing, conditions that are preventing anticoagulation, an expanding area of dissection with a persistent pseudoaneurysm, and cases of failed medical management with subsequent ischemic stroke.2The literature includes several case reports involving indirect trauma in recreational athletes. First, a 31-year-old woman sustained an ICA dissection secondary to a head injury that occurred during a soccer match; she presented with headache, altered sense of taste, and objective findings of ptosis and miosis consistent with Horner syndrome.13 Second, a 39-year-old man had an ICA dissection after a snowboarding fall that caused neck hyperextension; he presented with periocular headache, ptosis, and miosis.6 Third, 3 people who participated in CrossFit training sustained ICA dissection.7 They presented with varying degrees of neurologic symptoms: ptosis and miosis; right-side upper extremity ataxia; and visual distortion and receptive aphasia. Our patient’s ICA dissection resulted from indirect trauma that caused sudden hyperextension and lateral flexion in response to contact from a hockey puck. However, his case is unique in that symptoms onset was delayed, and there were no associated neurologic findings on clinical presentation. His case should raise awareness of this potential diagnosis, even in the absence of overt neurologic findings. In addition, the patient’s return to sport at 8 weeks was facilitated by full clinical resolution of symptoms and thorough radiographic documentation of improved intramural narrowing. Finally, to our knowledge this is the first report of this injury in a professional athlete.
Conclusion
We have reported the case of a 32-year-old professional hockey goaltender who presented with isolated, persistent, worsening headache of delayed onset after ICA dissection. The ICA dissection resulted from indirect trauma, with reaction to a puck causing acute hyperextension and rotational injury. To our knowledge, this is the first report of a case of ICA dissection in an athlete, lacking neurologic examination findings that could aid in the diagnosis. The index of suspicion for CAD should be high after direct or indirect cervical trauma when patients present with unilateral neck pain or headache, even in the absence of neurologic findings, as stroke is a catastrophic but preventable complication.
Am J Orthop. 2017;46(3):E139-E143. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Mohan IV. Current optimal assessment and management of carotid and vertebral spontaneous and traumatic dissection. Angiology. 2014;65(4):274-283.
2. Patel RR, Adam R, Maldjian C, Lincoln CM, Yuen A, Arneja A. Cervical carotid artery dissection: current review of diagnosis and treatment. Cardiol Rev. 2012;20(3):145-152.
3. Biller J, Sacco RL, Albuquerque FC, et al; American Heart Association Stroke Council. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(10):3155-3174.
4. Fukunaga N, Hanaoka M, Sato K. Asymptomatic common carotid artery dissection caused by blunt injury. Emerg Med J. 2011;28(1):50.
5. Chen J, Zhou X, Li C, Cheung BM. Risk of stroke due to spontaneous cervical artery dissection. Intern Med. 2013;52(19):2237-2240.
6. Kalantzis G, Georgalas I, Chang BY, Ong C, El-Hindy N. An unusual case of traumatic internal carotid artery dissection during snowboarding. J Sports Sci Med. 2014;13(2):451-453.
7. Lu A, Shen P, Lee P, et al. CrossFit-related cervical internal carotid artery dissection. Emerg Radiol. 2015;22(4):449-452.
8. CADISS Trial Investigators, Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
9. van Wessem KJ, Meijer JM, Leenen LP, van der Worp HB, Moll FL, de Borst GJ. Blunt traumatic carotid artery dissection still a pitfall? The rationale for aggressive screening. Eur J Trauma Emerg Surg. 2011;37(2):147-154.
10. Haneline M, Triano J. Cervical artery dissection. A comparison of highly dynamic mechanisms: manipulation versus motor vehicle collision. J Manipulative Physiol Ther. 2005;28(1):57-63.
11. Thomas LC, Rivett DA, Attia JR, Levi C. Risk factors and clinical presentation of cervical arterial dissection: preliminary results of a prospective case-control study. J Orthop Sports Phys Ther. 2015;45(7):503-511.
12. Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2010;(10):CD000255.
13. Creavin ST, Rice CM, Pollentine A, Cowburn P. Carotid artery dissection presenting with isolated headache and Horner syndrome after minor head injury. Am J Emerg Med. 2012;30(9):2103.e5-e7.
1. Mohan IV. Current optimal assessment and management of carotid and vertebral spontaneous and traumatic dissection. Angiology. 2014;65(4):274-283.
2. Patel RR, Adam R, Maldjian C, Lincoln CM, Yuen A, Arneja A. Cervical carotid artery dissection: current review of diagnosis and treatment. Cardiol Rev. 2012;20(3):145-152.
3. Biller J, Sacco RL, Albuquerque FC, et al; American Heart Association Stroke Council. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(10):3155-3174.
4. Fukunaga N, Hanaoka M, Sato K. Asymptomatic common carotid artery dissection caused by blunt injury. Emerg Med J. 2011;28(1):50.
5. Chen J, Zhou X, Li C, Cheung BM. Risk of stroke due to spontaneous cervical artery dissection. Intern Med. 2013;52(19):2237-2240.
6. Kalantzis G, Georgalas I, Chang BY, Ong C, El-Hindy N. An unusual case of traumatic internal carotid artery dissection during snowboarding. J Sports Sci Med. 2014;13(2):451-453.
7. Lu A, Shen P, Lee P, et al. CrossFit-related cervical internal carotid artery dissection. Emerg Radiol. 2015;22(4):449-452.
8. CADISS Trial Investigators, Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
9. van Wessem KJ, Meijer JM, Leenen LP, van der Worp HB, Moll FL, de Borst GJ. Blunt traumatic carotid artery dissection still a pitfall? The rationale for aggressive screening. Eur J Trauma Emerg Surg. 2011;37(2):147-154.
10. Haneline M, Triano J. Cervical artery dissection. A comparison of highly dynamic mechanisms: manipulation versus motor vehicle collision. J Manipulative Physiol Ther. 2005;28(1):57-63.
11. Thomas LC, Rivett DA, Attia JR, Levi C. Risk factors and clinical presentation of cervical arterial dissection: preliminary results of a prospective case-control study. J Orthop Sports Phys Ther. 2015;45(7):503-511.
12. Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2010;(10):CD000255.
13. Creavin ST, Rice CM, Pollentine A, Cowburn P. Carotid artery dissection presenting with isolated headache and Horner syndrome after minor head injury. Am J Emerg Med. 2012;30(9):2103.e5-e7.
Encapsulated Fat Necrosis Lesion Caused by Morel-Lavallée Lesion in a Professional Ice Hockey Player
Take-Home Points
- ML lesions usually occur with high-energy injuries and have been reported in wrestlers, football players, and other athlete populations.
- Encapsulated fat necrosis lesions are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions.
- Encapsulated fat necrosis lesions are rare; only 65 have been reported.
- Encapsulated fat necrosis lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.
- Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
What would become known as the Morel-Lavallée (ML) lesion was first reported in 1853 by French physician Maurice Morel-Lavallée. He described a proximal thigh soft-tissue injury that resulted in a hemolymphatic collection between superficial fascial planes. Deforming forces of pressure and shear result in an internal degloving injury in which subcutaneous tissue is stripped from the fascia and replaced with a hematoma or, less commonly, necrotic fat.1-4 The injury can take several weeks to heal. Up to one-third of such injuries are initially missed because of the initial ecchymosis covering the injured area.5
ML lesions usually occur with high-energy injuries and have been reported in wrestlers,6 football players,7-9 and other athlete populations. ML lesions usually occur about the knee, the site of the sheer mechanism in these athletes’ sports. Tejwani and colleagues9 reported on 24 National Football League (NFL) players (27 knees). These elite athletes typically were able to return to practice and game play long before complete resolution of their lesions.
Nodular cystic fat necrosis was first described by Przyjemski and Schuster10 in 1977. The terms encapsulated fat necrosis lesions and mobile encapsulated lipomas11 were introduced later. Clinically, these entities usually present as lesions on the lower limbs of young men and middle-aged women and can range in size from 1 mm to 35 mm. Most of these lesions are mobile.11 They are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions. Trauma accounts for the usual occurrence in the lower extremities, though only 40% of patients recall a precipitating event.12 Histologically, these lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.13In this article, we report the case of a professional ice hockey player who presented with an ML lesion of the hip and then developed a symptomatic encapsulated fat necrosis lesion that required surgical removal. To our knowledge, this is the first reported case of an encapsulated fat necrosis lesion caused by an ML lesion in an athlete. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 21-year-old professional hockey player presented with a history of pain from a mass on his right hip. He first noticed the lesion, just lateral to the greater trochanter, about 3 years earlier. The mass appeared after he sustained a shearing-type injury to the lateral aspect of the hip. At the time, there was significant swelling along the lateral aspect, with ecchymosis that resolved over 2 months. The mass, diagnosed as an ML lesion, resolved with nonoperative treatment. However, in the area where the swelling had occurred, a hard mobile mass remained. At times, this mass became painful when direct pressure was applied, as when he hit the boards while playing hockey, or when he lay on his right side or used a roller in the training room. He rated the pain as a 4 on a 1-to-10 scale and said the mass was mobile and had not changed in size or consistency.
Physical examination revealed a palpable mass over the lateral aspect of the hip, over the greater trochanter. The mass, about 3 cm in diameter (Figure 1), was mobile in a subcutaneous pocket, consistent with an old ML lesion.
Options discussed with the patient included use of ice, activity modification, and use of protective padded equipment. As the patient had tried these treatments before and was still intermittently having pain with direct pressure, he asked for surgical removal of the mass.
For the surgery, the patient was positioned in the lateral decubitus position with his right hip facing up. The right hip and thigh were prepared and draped in sterile fashion. An incision 4 cm in length was made directly over the mass, along the lateral aspect of the hip, over the greater trochanter. The incision was taken through skin and subcutaneous tissue down to the deep fascia. The fascia was incised longitudinally in line with the overlying skin incision. As soon as the incision was made through the fascia, the mass was easily seen. The 3-cm × 2-cm × 1-cm mass was free, not attached to any underlying soft tissue (Figure 3).
Discussion
We have described a case of symptomatic encapsulated fat necrosis lesion caused by an ML lesion in a professional hockey player. The ML lesion had resolved with nonoperative treatment (compression), but a subcutaneous pocket remained at the lesion site. Given the patient’s lesion site and occupation as a hockey player, pain with direct pressure on this lesion was a concern.
Long-standing ML lesions have 3 common patterns on MRI.14 A central region, encapsulated partially or completely by a peripheral ring of fibrous tissue or hemosiderin, shows signal properties consistent with a seroma, a homogeneous hemorrhagic collection, or a heterogeneous hemorrhagic collection. In our patient’s case, MRI was used to characterize the mobile mass for operative planning. Although thin strands or lobules of fat have been found within ML lesions, this case was the first to demonstrate a sequestered mass of necrotic fat.
Most football players who develop ML lesions on their knees do not wear kneepads.7-9 Of the 24 NFL players in the study by Tejwani and colleagues,9 52% were successfully treated with compression wrap, cryotherapy, and motion exercises. The rest, however, were treated with aspiration, and 11% underwent doxycycline sclerodesis for recurrent fluid collection. After treatment, all of their players were able to return to football. Their outcomes are consistent with that of our patient, who was treated with compression wrap and returned to hockey without any other intervention.
After our patient’s ML lesion resolved, he developed an encapsulated fat necrosis lesion from the disruption of the blood supply in the subcutaneous pocket. Encapsulated fat necrosis lesions are rare; only 65 have been reported.13,15 Clinically, these lesions are single or multiple pale-yellow encapsulated nodes.13 Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
The literature includes 1 report of an adolescent football player who developed multiple encapsulated fat necrosis lesions 4 months after landing on another player’s cleats.15 The patient, who was having pain with direct pressure during squatting and kneeling, elected to have the lesions surgically removed. These lesions are rare and usually asymptomatic,11 but our patient had his lesion surgically removed to address the pain induced by the direct impacts that came with playing professional hockey. Surgical removal is the treatment for symptomatic encapsulated fat necrosis lesions. Other than 1 case of recurrence after excision,16 these lesions have an excellent prognosis.
Conclusion
Our patient, a professional hockey player, underwent successful surgical removal of a symptomatic encapsulated fat necrosis lesion that had developed from an ML lesion.
Am J Orthop. 2017;46(3):E144-E147. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Aguiar RO, Viegas FC, Fernandez RY, Trudell D, Haghighi P, Resnick D. The prepatellar bursa: cadaveric investigation of regional anatomy with MRI after sonographically guided bursography. AJR Am J Roentgenol. 2007;188(4):W355-W358.
2. Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallée lesion. J Trauma. 1997;42(6):1046-1051.
3. Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853-855.
4. Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin North Am. 2005;13(4):775-782.
5. Dye SF, Campagna-Pinto D, Dye CC, Shifflett S, Eiman T. Soft-tissue anatomy anterior to the human patella. J Bone Joint Surg Am. 2003;85(6):1012-1017.
6. Northam MC, Gaskin CM. Presumed prepatellar fibrosis in collegiate wrestlers: imaging findings and clinical correlation. Skeletal Radiol. 2015;44(2):271-277.
7. Anakwenze OA, Trivedi V, Goodman AM, Ganley TJ. Concealed degloving injury (the Morel-Lavallée lesion) in childhood sports: a case report. J Bone Joint Surg Am. 2011;93(24):e148.
8. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-Lavallée lesion in a professional American football player. Am J Orthop. 2010;39(3):144-147.
9. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the National Football League. Am J Sports Med. 2007;35(7):1162-1167.
10. Przyjemski CJ, Schuster SR. Nodular-cystic fat necrosis. J Pediatr. 1977;91(4):605-607.
11. Kiryu H, Rikihisa W, Furue M. Encapsulated fat necrosis—a clinicopathological study of 8 cases and a literature review. J Cutan Pathol. 2000;27(1):19-23.
12. Santos-Juanes J, Coto P, Galache C, Sánchez del Rio J, Soto de Delás J. Encapsulated fat necrosis: a form of traumatic panniculitis. J Eur Acad Dermatol Venereol. 2007;21(3):405-406.
13. Sempau L, Sambucetty PS, Garcia JL, Sixto BG, Morán AG, Prieto MA. Mobile encapsulated lipoma. Int J Dermatol. 2012;51(4):448-450.
14. Mellado JM, Pérez del Palomar L, Díaz L, Ramos A, Saurí A. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol. 2004;182(5):1289-1294.
15. Sole JS, Wisniewski SJ, Dahm DL, Bond J, Smith J. Posttraumatic fat necrosis presenting as prepatellar loose bodies in an adolescent football player. PM R. 2014;6(8):749-752.
16. Felipo F, Vaquero M, del Agua C. Pseudotumoral encapsulated fat necrosis with diffuse pseudomembranous degeneration. J Cutan Pathol. 2004;31(8):565-567.
Take-Home Points
- ML lesions usually occur with high-energy injuries and have been reported in wrestlers, football players, and other athlete populations.
- Encapsulated fat necrosis lesions are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions.
- Encapsulated fat necrosis lesions are rare; only 65 have been reported.
- Encapsulated fat necrosis lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.
- Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
What would become known as the Morel-Lavallée (ML) lesion was first reported in 1853 by French physician Maurice Morel-Lavallée. He described a proximal thigh soft-tissue injury that resulted in a hemolymphatic collection between superficial fascial planes. Deforming forces of pressure and shear result in an internal degloving injury in which subcutaneous tissue is stripped from the fascia and replaced with a hematoma or, less commonly, necrotic fat.1-4 The injury can take several weeks to heal. Up to one-third of such injuries are initially missed because of the initial ecchymosis covering the injured area.5
ML lesions usually occur with high-energy injuries and have been reported in wrestlers,6 football players,7-9 and other athlete populations. ML lesions usually occur about the knee, the site of the sheer mechanism in these athletes’ sports. Tejwani and colleagues9 reported on 24 National Football League (NFL) players (27 knees). These elite athletes typically were able to return to practice and game play long before complete resolution of their lesions.
Nodular cystic fat necrosis was first described by Przyjemski and Schuster10 in 1977. The terms encapsulated fat necrosis lesions and mobile encapsulated lipomas11 were introduced later. Clinically, these entities usually present as lesions on the lower limbs of young men and middle-aged women and can range in size from 1 mm to 35 mm. Most of these lesions are mobile.11 They are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions. Trauma accounts for the usual occurrence in the lower extremities, though only 40% of patients recall a precipitating event.12 Histologically, these lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.13In this article, we report the case of a professional ice hockey player who presented with an ML lesion of the hip and then developed a symptomatic encapsulated fat necrosis lesion that required surgical removal. To our knowledge, this is the first reported case of an encapsulated fat necrosis lesion caused by an ML lesion in an athlete. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 21-year-old professional hockey player presented with a history of pain from a mass on his right hip. He first noticed the lesion, just lateral to the greater trochanter, about 3 years earlier. The mass appeared after he sustained a shearing-type injury to the lateral aspect of the hip. At the time, there was significant swelling along the lateral aspect, with ecchymosis that resolved over 2 months. The mass, diagnosed as an ML lesion, resolved with nonoperative treatment. However, in the area where the swelling had occurred, a hard mobile mass remained. At times, this mass became painful when direct pressure was applied, as when he hit the boards while playing hockey, or when he lay on his right side or used a roller in the training room. He rated the pain as a 4 on a 1-to-10 scale and said the mass was mobile and had not changed in size or consistency.
Physical examination revealed a palpable mass over the lateral aspect of the hip, over the greater trochanter. The mass, about 3 cm in diameter (Figure 1), was mobile in a subcutaneous pocket, consistent with an old ML lesion.
Options discussed with the patient included use of ice, activity modification, and use of protective padded equipment. As the patient had tried these treatments before and was still intermittently having pain with direct pressure, he asked for surgical removal of the mass.
For the surgery, the patient was positioned in the lateral decubitus position with his right hip facing up. The right hip and thigh were prepared and draped in sterile fashion. An incision 4 cm in length was made directly over the mass, along the lateral aspect of the hip, over the greater trochanter. The incision was taken through skin and subcutaneous tissue down to the deep fascia. The fascia was incised longitudinally in line with the overlying skin incision. As soon as the incision was made through the fascia, the mass was easily seen. The 3-cm × 2-cm × 1-cm mass was free, not attached to any underlying soft tissue (Figure 3).
Discussion
We have described a case of symptomatic encapsulated fat necrosis lesion caused by an ML lesion in a professional hockey player. The ML lesion had resolved with nonoperative treatment (compression), but a subcutaneous pocket remained at the lesion site. Given the patient’s lesion site and occupation as a hockey player, pain with direct pressure on this lesion was a concern.
Long-standing ML lesions have 3 common patterns on MRI.14 A central region, encapsulated partially or completely by a peripheral ring of fibrous tissue or hemosiderin, shows signal properties consistent with a seroma, a homogeneous hemorrhagic collection, or a heterogeneous hemorrhagic collection. In our patient’s case, MRI was used to characterize the mobile mass for operative planning. Although thin strands or lobules of fat have been found within ML lesions, this case was the first to demonstrate a sequestered mass of necrotic fat.
Most football players who develop ML lesions on their knees do not wear kneepads.7-9 Of the 24 NFL players in the study by Tejwani and colleagues,9 52% were successfully treated with compression wrap, cryotherapy, and motion exercises. The rest, however, were treated with aspiration, and 11% underwent doxycycline sclerodesis for recurrent fluid collection. After treatment, all of their players were able to return to football. Their outcomes are consistent with that of our patient, who was treated with compression wrap and returned to hockey without any other intervention.
After our patient’s ML lesion resolved, he developed an encapsulated fat necrosis lesion from the disruption of the blood supply in the subcutaneous pocket. Encapsulated fat necrosis lesions are rare; only 65 have been reported.13,15 Clinically, these lesions are single or multiple pale-yellow encapsulated nodes.13 Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
The literature includes 1 report of an adolescent football player who developed multiple encapsulated fat necrosis lesions 4 months after landing on another player’s cleats.15 The patient, who was having pain with direct pressure during squatting and kneeling, elected to have the lesions surgically removed. These lesions are rare and usually asymptomatic,11 but our patient had his lesion surgically removed to address the pain induced by the direct impacts that came with playing professional hockey. Surgical removal is the treatment for symptomatic encapsulated fat necrosis lesions. Other than 1 case of recurrence after excision,16 these lesions have an excellent prognosis.
Conclusion
Our patient, a professional hockey player, underwent successful surgical removal of a symptomatic encapsulated fat necrosis lesion that had developed from an ML lesion.
Am J Orthop. 2017;46(3):E144-E147. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- ML lesions usually occur with high-energy injuries and have been reported in wrestlers, football players, and other athlete populations.
- Encapsulated fat necrosis lesions are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions.
- Encapsulated fat necrosis lesions are rare; only 65 have been reported.
- Encapsulated fat necrosis lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.
- Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
What would become known as the Morel-Lavallée (ML) lesion was first reported in 1853 by French physician Maurice Morel-Lavallée. He described a proximal thigh soft-tissue injury that resulted in a hemolymphatic collection between superficial fascial planes. Deforming forces of pressure and shear result in an internal degloving injury in which subcutaneous tissue is stripped from the fascia and replaced with a hematoma or, less commonly, necrotic fat.1-4 The injury can take several weeks to heal. Up to one-third of such injuries are initially missed because of the initial ecchymosis covering the injured area.5
ML lesions usually occur with high-energy injuries and have been reported in wrestlers,6 football players,7-9 and other athlete populations. ML lesions usually occur about the knee, the site of the sheer mechanism in these athletes’ sports. Tejwani and colleagues9 reported on 24 National Football League (NFL) players (27 knees). These elite athletes typically were able to return to practice and game play long before complete resolution of their lesions.
Nodular cystic fat necrosis was first described by Przyjemski and Schuster10 in 1977. The terms encapsulated fat necrosis lesions and mobile encapsulated lipomas11 were introduced later. Clinically, these entities usually present as lesions on the lower limbs of young men and middle-aged women and can range in size from 1 mm to 35 mm. Most of these lesions are mobile.11 They are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions. Trauma accounts for the usual occurrence in the lower extremities, though only 40% of patients recall a precipitating event.12 Histologically, these lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.13In this article, we report the case of a professional ice hockey player who presented with an ML lesion of the hip and then developed a symptomatic encapsulated fat necrosis lesion that required surgical removal. To our knowledge, this is the first reported case of an encapsulated fat necrosis lesion caused by an ML lesion in an athlete. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 21-year-old professional hockey player presented with a history of pain from a mass on his right hip. He first noticed the lesion, just lateral to the greater trochanter, about 3 years earlier. The mass appeared after he sustained a shearing-type injury to the lateral aspect of the hip. At the time, there was significant swelling along the lateral aspect, with ecchymosis that resolved over 2 months. The mass, diagnosed as an ML lesion, resolved with nonoperative treatment. However, in the area where the swelling had occurred, a hard mobile mass remained. At times, this mass became painful when direct pressure was applied, as when he hit the boards while playing hockey, or when he lay on his right side or used a roller in the training room. He rated the pain as a 4 on a 1-to-10 scale and said the mass was mobile and had not changed in size or consistency.
Physical examination revealed a palpable mass over the lateral aspect of the hip, over the greater trochanter. The mass, about 3 cm in diameter (Figure 1), was mobile in a subcutaneous pocket, consistent with an old ML lesion.
Options discussed with the patient included use of ice, activity modification, and use of protective padded equipment. As the patient had tried these treatments before and was still intermittently having pain with direct pressure, he asked for surgical removal of the mass.
For the surgery, the patient was positioned in the lateral decubitus position with his right hip facing up. The right hip and thigh were prepared and draped in sterile fashion. An incision 4 cm in length was made directly over the mass, along the lateral aspect of the hip, over the greater trochanter. The incision was taken through skin and subcutaneous tissue down to the deep fascia. The fascia was incised longitudinally in line with the overlying skin incision. As soon as the incision was made through the fascia, the mass was easily seen. The 3-cm × 2-cm × 1-cm mass was free, not attached to any underlying soft tissue (Figure 3).
Discussion
We have described a case of symptomatic encapsulated fat necrosis lesion caused by an ML lesion in a professional hockey player. The ML lesion had resolved with nonoperative treatment (compression), but a subcutaneous pocket remained at the lesion site. Given the patient’s lesion site and occupation as a hockey player, pain with direct pressure on this lesion was a concern.
Long-standing ML lesions have 3 common patterns on MRI.14 A central region, encapsulated partially or completely by a peripheral ring of fibrous tissue or hemosiderin, shows signal properties consistent with a seroma, a homogeneous hemorrhagic collection, or a heterogeneous hemorrhagic collection. In our patient’s case, MRI was used to characterize the mobile mass for operative planning. Although thin strands or lobules of fat have been found within ML lesions, this case was the first to demonstrate a sequestered mass of necrotic fat.
Most football players who develop ML lesions on their knees do not wear kneepads.7-9 Of the 24 NFL players in the study by Tejwani and colleagues,9 52% were successfully treated with compression wrap, cryotherapy, and motion exercises. The rest, however, were treated with aspiration, and 11% underwent doxycycline sclerodesis for recurrent fluid collection. After treatment, all of their players were able to return to football. Their outcomes are consistent with that of our patient, who was treated with compression wrap and returned to hockey without any other intervention.
After our patient’s ML lesion resolved, he developed an encapsulated fat necrosis lesion from the disruption of the blood supply in the subcutaneous pocket. Encapsulated fat necrosis lesions are rare; only 65 have been reported.13,15 Clinically, these lesions are single or multiple pale-yellow encapsulated nodes.13 Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
The literature includes 1 report of an adolescent football player who developed multiple encapsulated fat necrosis lesions 4 months after landing on another player’s cleats.15 The patient, who was having pain with direct pressure during squatting and kneeling, elected to have the lesions surgically removed. These lesions are rare and usually asymptomatic,11 but our patient had his lesion surgically removed to address the pain induced by the direct impacts that came with playing professional hockey. Surgical removal is the treatment for symptomatic encapsulated fat necrosis lesions. Other than 1 case of recurrence after excision,16 these lesions have an excellent prognosis.
Conclusion
Our patient, a professional hockey player, underwent successful surgical removal of a symptomatic encapsulated fat necrosis lesion that had developed from an ML lesion.
Am J Orthop. 2017;46(3):E144-E147. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Aguiar RO, Viegas FC, Fernandez RY, Trudell D, Haghighi P, Resnick D. The prepatellar bursa: cadaveric investigation of regional anatomy with MRI after sonographically guided bursography. AJR Am J Roentgenol. 2007;188(4):W355-W358.
2. Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallée lesion. J Trauma. 1997;42(6):1046-1051.
3. Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853-855.
4. Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin North Am. 2005;13(4):775-782.
5. Dye SF, Campagna-Pinto D, Dye CC, Shifflett S, Eiman T. Soft-tissue anatomy anterior to the human patella. J Bone Joint Surg Am. 2003;85(6):1012-1017.
6. Northam MC, Gaskin CM. Presumed prepatellar fibrosis in collegiate wrestlers: imaging findings and clinical correlation. Skeletal Radiol. 2015;44(2):271-277.
7. Anakwenze OA, Trivedi V, Goodman AM, Ganley TJ. Concealed degloving injury (the Morel-Lavallée lesion) in childhood sports: a case report. J Bone Joint Surg Am. 2011;93(24):e148.
8. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-Lavallée lesion in a professional American football player. Am J Orthop. 2010;39(3):144-147.
9. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the National Football League. Am J Sports Med. 2007;35(7):1162-1167.
10. Przyjemski CJ, Schuster SR. Nodular-cystic fat necrosis. J Pediatr. 1977;91(4):605-607.
11. Kiryu H, Rikihisa W, Furue M. Encapsulated fat necrosis—a clinicopathological study of 8 cases and a literature review. J Cutan Pathol. 2000;27(1):19-23.
12. Santos-Juanes J, Coto P, Galache C, Sánchez del Rio J, Soto de Delás J. Encapsulated fat necrosis: a form of traumatic panniculitis. J Eur Acad Dermatol Venereol. 2007;21(3):405-406.
13. Sempau L, Sambucetty PS, Garcia JL, Sixto BG, Morán AG, Prieto MA. Mobile encapsulated lipoma. Int J Dermatol. 2012;51(4):448-450.
14. Mellado JM, Pérez del Palomar L, Díaz L, Ramos A, Saurí A. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol. 2004;182(5):1289-1294.
15. Sole JS, Wisniewski SJ, Dahm DL, Bond J, Smith J. Posttraumatic fat necrosis presenting as prepatellar loose bodies in an adolescent football player. PM R. 2014;6(8):749-752.
16. Felipo F, Vaquero M, del Agua C. Pseudotumoral encapsulated fat necrosis with diffuse pseudomembranous degeneration. J Cutan Pathol. 2004;31(8):565-567.
1. Aguiar RO, Viegas FC, Fernandez RY, Trudell D, Haghighi P, Resnick D. The prepatellar bursa: cadaveric investigation of regional anatomy with MRI after sonographically guided bursography. AJR Am J Roentgenol. 2007;188(4):W355-W358.
2. Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallée lesion. J Trauma. 1997;42(6):1046-1051.
3. Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853-855.
4. Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin North Am. 2005;13(4):775-782.
5. Dye SF, Campagna-Pinto D, Dye CC, Shifflett S, Eiman T. Soft-tissue anatomy anterior to the human patella. J Bone Joint Surg Am. 2003;85(6):1012-1017.
6. Northam MC, Gaskin CM. Presumed prepatellar fibrosis in collegiate wrestlers: imaging findings and clinical correlation. Skeletal Radiol. 2015;44(2):271-277.
7. Anakwenze OA, Trivedi V, Goodman AM, Ganley TJ. Concealed degloving injury (the Morel-Lavallée lesion) in childhood sports: a case report. J Bone Joint Surg Am. 2011;93(24):e148.
8. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-Lavallée lesion in a professional American football player. Am J Orthop. 2010;39(3):144-147.
9. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the National Football League. Am J Sports Med. 2007;35(7):1162-1167.
10. Przyjemski CJ, Schuster SR. Nodular-cystic fat necrosis. J Pediatr. 1977;91(4):605-607.
11. Kiryu H, Rikihisa W, Furue M. Encapsulated fat necrosis—a clinicopathological study of 8 cases and a literature review. J Cutan Pathol. 2000;27(1):19-23.
12. Santos-Juanes J, Coto P, Galache C, Sánchez del Rio J, Soto de Delás J. Encapsulated fat necrosis: a form of traumatic panniculitis. J Eur Acad Dermatol Venereol. 2007;21(3):405-406.
13. Sempau L, Sambucetty PS, Garcia JL, Sixto BG, Morán AG, Prieto MA. Mobile encapsulated lipoma. Int J Dermatol. 2012;51(4):448-450.
14. Mellado JM, Pérez del Palomar L, Díaz L, Ramos A, Saurí A. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol. 2004;182(5):1289-1294.
15. Sole JS, Wisniewski SJ, Dahm DL, Bond J, Smith J. Posttraumatic fat necrosis presenting as prepatellar loose bodies in an adolescent football player. PM R. 2014;6(8):749-752.
16. Felipo F, Vaquero M, del Agua C. Pseudotumoral encapsulated fat necrosis with diffuse pseudomembranous degeneration. J Cutan Pathol. 2004;31(8):565-567.