User login
Hepatitis C: How To Fine-Tune Your Approach
› Screen at-risk patients and all those born between 1945 and 1965 for hepatitis C virus (HCV) infection. B
› Screen HCV-positive patients for level of fibrosis and for conditions that may accelerate liver disease, including alcohol use, hepatitis B virus, and human immunodeficiency virus. B
› Continuously monitor patients with chronic HCV for the development of cirrhosis and hepatocellular carcinoma. A
› Refer patients to specialty care for HCV treatment and, if they have cirrhosis, for potential transplant evaluation. C
› Counsel HCV-positive patients about how to avoid transmission to others. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease. Over the next few decades, the number of deaths per year due to complications of HCV such as liver failure and hepatocellular carcinoma (HCC) is predicted to more than triple to 36,000 by 2032.1
Fortunately, major advances in drug therapy have made it possible to cure patients of HCV, and treatment is now less complex, of shorter duration, and better tolerated than it once was. To help family physicians maximize the care they provide to these patients, we’ve summarized screening recommendations from the Centers for Disease Control and Prevention (CDC), innovative alternatives to biopsy for staging liver disease, and counseling points to cover with patients.
A common, usually silent infection with potentially fatal complications
According to the National Health and Nutrition Examination Survey (NHANES), an estimated 2.7 to 3.9 million people in the United States are chronically infected with HCV, about threefourths of whom were born between 1945 and 1965 (the “baby boomer” generation).2 However, by adding “unaccounted groups” (eg, incarcerated, homeless, and active duty military) to these estimates, the number of people with HCV is likely more than 5.2 million.3
HCV is a ribonucleic acid (RNA) virus capable of mutating at a high rate to escape detection and clearance by the host’s immune system.4 Most patients with HCV are asymptomatic during the acute and chronic phases of infection, and may have a silent infection for decades. In fact, 65% to 75% of patients with HCV are unaware of their infection.5
Approximately 20% of chronically infected patients develop cirrhosis after 20 years and, once they do, the annual rate of HCC and liver decompensation is about 5%.6-8 Risk factors for advancement to cirrhosis includes male sex, alcohol consumption, co-infection with human immunodeficiency virus (HIV) or hepatitis B virus (HBV), immunosuppression, having had HCV infection for a long time, becoming infected with HCV after age 40, and not having responded to previous treatment.9
Chronic HCV infection can lead to extrahepatic manifestations such as essential mixed cryoglobulinemia, porphyria cutanea tarda, membranoproliferative glomerulonephritis, lymphoma, and glucose intolerance.10 There is also growing evidence that HCV infection affects cognitive function in the absence of fibrosis and hepatic encephalopathy. Several studies show that HCV-infected patients score poorly on neuropsychological testing for verbal learning, attention, memory, and executive function.11 This may be related to the expression of receptors for HCV by the brain’s microvascular endothelial cells.12
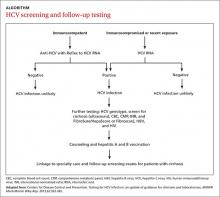
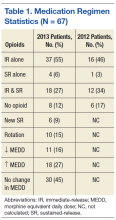
Screening recommendations. Given the high prevalence of HCV infection among baby boomers, the CDC decided in 2012 to recommend one-time HCV screening for all patients born between 1945 and 1965.13 This is in addition to risk-based screening for all patients who have a history of injection drug use, those on long-term hemodialysis or with tattoos obtained in unregulated settings, offspring of HCV-infected mothers, and those with health-care associated exposures (TABLE13). In 2013, the US Preventive Services Task Force upgraded its recommendation to match those of the CDC.14
Despite these recommendations, which are expected to increase detection of HCV among asymptomatic persons who do not know they are infected, there remain significant barriers to HCV testing. These include poor access to primary care and preventive services, lack of knowledge and awareness of the disease among patients and providers, and a lack of studies that support a universal screening approach for HCV.5,15,16 One tool that might help overcome some of these barriers and aid family physicians in the screening process is automatic reminders or standing lab orders for HCV testing in electronic medical records systems.
Screening for HCV can be done using any of the US Food and Drug Administration (FDA)-approved tests for the anti-HCV antibody, which have sensitivities and specificities greater than 99%.17 A positive screening result should be confirmed with an HCV RNA test. However, for practical purposes, ordering the anti-HCV test with reflex to the HCV RNA test decreases the number of blood draws and office visits required of the patient. The reflex confirmation allows the physician to deliver the patient’s full diagnosis and reduces the psychological distress associated with waiting for confirmatory results. The HCV RNA test (alone) should be used, however, in immunocompromised patients, those who may have had exposure to HCV in the past 6 months, and those suspected of having an HCV re-infection after having cleared the virus.18
Look for the evidence of liver disease
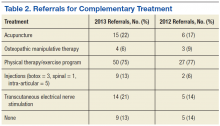
Family physicians should order several additional tests for patients found to have chronic HCV infection before referring such patients to a specialist (ALGORITHM). Work-up should include the complete blood count, HCV genotype (which will help guide treatment), liver function tests, international normalized ratio test, and ultrasound of the liver.18 In addition, all HCV-positive patients should be tested for HIV and HBV, because these co-infections may accelerate liver fibrosis.19,20
All patients with chronic HCV infection should also be screened for the presence of fibrosis and cirrhosis, as this will influence treatment choice and duration. Signs of cirrhosis that may be evident on physical exam include jaundice, spider angiomata, palmar erythema, encephalopathy with asterixis, and fluid overload, especially ascites. Cirrhosis can be classified clinically as compensated (stage 1 with no varices present and stage 2 with varices present) and decompensated (stages 3 and 4), which is defined as cirrhosis with signs of severe portal hypertension (bleeding varices, ascites, hepatic encephalopathy) or liver insufficiency (jaundice).21 Patients with decompensated cirrhosis should be managed by a liver transplant center. For more on cirrhosis, see “Cirrhosis complications: Keeping them under control” (J Fam Pract. 2015;64:338-342).
Several noninvasive alternatives to liver biopsy
Historically, liver biopsy has been the gold standard for staging liver disease. The Metavir scoring system is a histological assessment of the degree of inflammatory activity and the stage of fibrosis.22 The degree of inflammation activity, which is a precursor of fibrosis, is scored from A0 (no activity) to A3 (severe activity). The staging of fibrosis involves a 5-stage scoring system: F0 (chronic hepatitis without fibrosis); F1 (portal fibrosis without septae); F2 (portal fibrosis with rare septae); F3 (many septae without cirrhosis); or F4 (cirrhosis).
That said, noninvasive tests have largely supplanted liver biopsy for fibrosis screening.
For example, the FibroSure test uses the patient’s age, gender, and a combination of 6 serum markers of liver function in a computational algorithm to generate a quantitative indicator of liver fibrosis, with a score of 0.0 to 1.0 that corresponds to the Metavir fibrosis score (F0-F4), and an inflammatory activity score (A0-A3).23 Similarly, HepaScore uses several noninvasive markers to calculate a score from 0.00 to 1.00. A score ≤0.2 accurately excludes significant fibrosis. However, a score of ≥0.55 or higher corresponds to a Metavir score of at least F2, and in such cases further testing would be needed to evaluate for cirrhosis.24
FDA-approved in 2013, transient elastography (FibroScan) is another noninvasive alternative to liver biopsy for determining the stage of liver disease. This bedside test uses ultrasound technology to measure liver stiffness and provides a score ranging from 0 to 75 kPA that correlates with the Metavir score. Although not yet widely available in the United States, FibroScan is becoming increasingly popular as a rapid and noninvasive screening tool for cirrhosis.25
Identifying cirrhosis in patients who have HCV is crucial because such patients need prompt care from a specialist. In addition to receiving HCV treatment, patients with cirrhosis also need regular liver ultrasound exams to screen for HCC (every 6 months) and esophagogastroduodenoscopy to screen for esophageal and gastric varices.26
Advise patients to avoid alcohol, lose weight
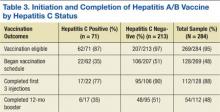
Counsel patients who test positive for HCV infection about making lifestyle changes to avoid further liver damage and transmission of HCV to others. Infectious diseases and hepatology society guidelines recommend vaccination against hepatitis A and B for all HCV-infected patients who are not immune to these viruses because acute co-infection could lead to severe acute liver injury.18,27 Urge all HCV-infected patients to completely abstain from alcohol and, if necessary, refer them to an addiction specialist, because excess alcohol consumption is strongly associated with the development of cirrhosis and HCC.28,29
Comorbid conditions such as metabolic syndrome, obesity, and hyperlipidemia can worsen the prognosis for HCV-infected patients; therefore, intense counseling on weight loss is recommended.30 Statins are safe and beneficial for HCV patients with hypercholesterolemia and compensated cirrhosis.31
Teach patients that the primary mode of transmission of HCV is through infected blood. Sexual transmission of HCV has been well documented in HIV-positive men who have sex with men.32 Although the risk of transmission of HCV among heterosexual couples is extremely low, it is possible, and patients should be counseled accordingly.33 Transmission of HCV from mother to the baby occurs in up to 6% of births and most commonly occurs during delivery.34
Newer treatments are highly effective and well tolderated
HCV treatment has changed dramatically over the past few years. Previous treatments for HCV, particularly those containing interferon, were known for their poor tolerability due to adverse effects and low cure rates. Compared to previous therapies, the new interferon-free direct-acting antiviral (DAA) regimens are not only less complex but also shorter in duration, ranging from 8 to 24 weeks depending on the patient’s viral load, stage of liver disease, and previous treatment experience.18 The specific agents and dosages used in DAA regimens aren’t described here because these regimens are rapidly changing. However, continuously updated treatment recommendations from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America are available at http://www.hcvguidelines.org.
The goal of HCV treatment is cure as evidenced by a sustained virologic response (SVR), which is defined as the absence of HCV RNA 12 weeks or more after completing treatment.35,36 In general, for the most common genotypes of HCV, treatment with a DAA regimen results in a SVR in ≥95% of patients.18 Achieving SVR is associated with a 50% reduction in all-cause mortality, a 90% reduction in liver-associated mortality, and a >70% reduction in the risk of developing HCC.27,37,38 SVR also has been shown to have a significant effect on reducing extrahepatic manifestations of HCV infection, such as cryoglobulinemia and lymphoma.39-41
Current barriers to the newer, highly effective hepatitis C virus (HCV) infection treatments are largely financial. Although insurance companies have been able to negotiate substantial discounts from the high wholesale price of treatment, many insurance programs require prior authorizations and will approve treatment only for patients with advanced liver fibrosis. In our experience, many patients are left to wait for their liver disease to progress before their insurance company will agree to cover treatment.
In addition, many insurance companies have mandated that only subspecialists prescribe these medications. However, infectious diseases and hepatology specialists and their support staffs are often overburdened with paperwork and phone calls related to prior authorizations and justification of treatment, which can add to delays in treatment.
There is already evidence that treatment of all patients with HCV is cost-effective and leads to better healthcare outcomes42 and there are indications that these barriers will decrease over time, with prices already dropping significantly due to increasing competition between drug companies.
The DAAs are well tolerated and have good safety profiles. In phase III clinical trials of today’s most commonly used DAA regimens, the discontinuation rate was <1% in non-cirrhotic patients and 2% in those with cirrhosis.18 The most commonly reported adverse effects were nausea, fatigue, and headache. DAAs may have drug-drug interactions; therefore, careful medication reconciliation should be performed before initiating treatment.18
Prioritizing treatment. Current evidence supports treatment for all patients with HCV except those with a life expectancy of <12 months.18 Evidence indicates that treatment becomes less effective as a patient’s liver injury progresses to cirrhosis. Due to the high cost of available treatments, however, many insurers have imposed strict criteria for coverage. (See “Barriers to HCV Treatment,” above.42)
The highest priority for treatment has been given to patients with advanced liver fibrosis, compensated cirrhosis, those who have received a liver transplant, and those with severe extrahepatic manifestations (eg, mixed cryoglobulinemia and end-organ disease such as nephropathy). Treatment is also prioritized for high-risk populations (eg, patients with HBV and HIV co-infection, diabetes mellitus) and patients who are at high risk of transmitting the virus (eg, individuals who inject drugs or are incarcerated, men who have sex with men, women of childbearing age, hemodialysis patients, and health care professionals who perform exposure-prone procedures).18
While it may eventually become feasible for family physicians to treat HCV-infected patients, the rapid evolution and significant cost of treatment, as well as the challenges in obtaining insurance coverage, have kept HCV treatment largely in the domain of specialists, at least for now. In the interim, family physicians play a crucial role by screening, diagnosing, and counseling patients with this infection, referring them to specialty care, and providing ongoing monitoring for signs of HCC and esophageal and gastric varices.
CORRESPONDENCE
Laura Wangensteen, MD, Department of Family Medicine, Drexel University, 3401 South Market Street #105 A, Philadelphia, PA 19104; [email protected]
1. Rein DB, Wittenborn JS, Weinbaum CM, et al. Forecasting the morbidity and mortality associated with prevalent cases of precirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66-72.
2. Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-714.
3. Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090-1101.
4. Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103-107.
5. Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729-733.
6. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
7. El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83.
8. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68.
9. McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut. 2004;53:318-321.
10. El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-1445.
11. Solinas A, Piras MR, Deplano A. Cognitive dysfunction and hepatitis C virus infection. World J Hepatol. 2015;7:922-925.
12. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142:634-643.e6.
13. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32.
14. US Preventive Services Task Force. Final recommendation statement on hepatitis C screening, June 2013. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-c-screening. Accessed on December 28, 2014.
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-2207.
16. Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754-758.
17. Shivkumar S, Peeling R, Jafari Y, et al. Accuracy of rapid and pointof- care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558-566.
18. American Association for the Study of Liver Diseases; Infectious Diseases Society of America; International Antiviral Society—USA. HCV guidance: Recommendations for testing, managing, and treating hepatitis C. HCV guidelines Web site. Available at: http://www.hcvguidelines.org. Accessed May 25, 2015.
19. Zarski JP, Bohn B, Bastie A, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27-33.
20. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562-569.
21. Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449.
22. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
23. Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896.
24. Becker L, Salameh W, Sferruzza A, et al. Validation of hepascore, compared with simple indices of fibrosis, in patients with chronic hepatitis C virus infection in United States. Clin Gastroenterol Hepatol. 2009;7:696-701.
25. Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16:372.
26. Garcia-Tsao G, Sanyal AJ, Grace ND, et al; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
27. Ghany MG, Strader DB, Thomas DL, et al; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
28. Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717-1722.
29. Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J Gastroenterol. 2009;15:3462-3471.
30. Ortiz V, Berenguer M, Rayón JM, et al. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408-2414.
31. Lewis JH, Mortensen ME, Zweig S, et al; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453-1463.
32. Gamage DG, Read TR, Bradshaw CS, et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis. 2011;11:39.
33. Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881-889.
34. Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223-229.
35. Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593-1601.
36. Thomas AM, Kattakuzhy S, Jones S, et al. SVR durability: HCV patients treated with IFN-free DAA regimens. Presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February, 2015; Seattle, Washington. Abstract 653.
37. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.e1.
38. Russo MW. Antiviral therapy for hepatitis C is associated with improved clinical outcomes in patients with advanced fibrosis. Expert Rev Gastroenterol Hepatol. 2010;4:535-539.
39. Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCVassociated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol. 2013;85:1019-1027.
40. Takahashi K, Nishida N, Kawabata H, et al. Regression of Hodgkin lymphoma in response to antiviral therapy for hepatitis C virus infection. Intern Med. 2012;51:2745-2747.
41. Gisbert JP, García-Buey L, Pajares JM, et al. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther. 2005;21:653-662.
42. Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407-419.
› Screen at-risk patients and all those born between 1945 and 1965 for hepatitis C virus (HCV) infection. B
› Screen HCV-positive patients for level of fibrosis and for conditions that may accelerate liver disease, including alcohol use, hepatitis B virus, and human immunodeficiency virus. B
› Continuously monitor patients with chronic HCV for the development of cirrhosis and hepatocellular carcinoma. A
› Refer patients to specialty care for HCV treatment and, if they have cirrhosis, for potential transplant evaluation. C
› Counsel HCV-positive patients about how to avoid transmission to others. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease. Over the next few decades, the number of deaths per year due to complications of HCV such as liver failure and hepatocellular carcinoma (HCC) is predicted to more than triple to 36,000 by 2032.1
Fortunately, major advances in drug therapy have made it possible to cure patients of HCV, and treatment is now less complex, of shorter duration, and better tolerated than it once was. To help family physicians maximize the care they provide to these patients, we’ve summarized screening recommendations from the Centers for Disease Control and Prevention (CDC), innovative alternatives to biopsy for staging liver disease, and counseling points to cover with patients.
A common, usually silent infection with potentially fatal complications
According to the National Health and Nutrition Examination Survey (NHANES), an estimated 2.7 to 3.9 million people in the United States are chronically infected with HCV, about threefourths of whom were born between 1945 and 1965 (the “baby boomer” generation).2 However, by adding “unaccounted groups” (eg, incarcerated, homeless, and active duty military) to these estimates, the number of people with HCV is likely more than 5.2 million.3
HCV is a ribonucleic acid (RNA) virus capable of mutating at a high rate to escape detection and clearance by the host’s immune system.4 Most patients with HCV are asymptomatic during the acute and chronic phases of infection, and may have a silent infection for decades. In fact, 65% to 75% of patients with HCV are unaware of their infection.5
Approximately 20% of chronically infected patients develop cirrhosis after 20 years and, once they do, the annual rate of HCC and liver decompensation is about 5%.6-8 Risk factors for advancement to cirrhosis includes male sex, alcohol consumption, co-infection with human immunodeficiency virus (HIV) or hepatitis B virus (HBV), immunosuppression, having had HCV infection for a long time, becoming infected with HCV after age 40, and not having responded to previous treatment.9
Chronic HCV infection can lead to extrahepatic manifestations such as essential mixed cryoglobulinemia, porphyria cutanea tarda, membranoproliferative glomerulonephritis, lymphoma, and glucose intolerance.10 There is also growing evidence that HCV infection affects cognitive function in the absence of fibrosis and hepatic encephalopathy. Several studies show that HCV-infected patients score poorly on neuropsychological testing for verbal learning, attention, memory, and executive function.11 This may be related to the expression of receptors for HCV by the brain’s microvascular endothelial cells.12
Screening recommendations. Given the high prevalence of HCV infection among baby boomers, the CDC decided in 2012 to recommend one-time HCV screening for all patients born between 1945 and 1965.13 This is in addition to risk-based screening for all patients who have a history of injection drug use, those on long-term hemodialysis or with tattoos obtained in unregulated settings, offspring of HCV-infected mothers, and those with health-care associated exposures (TABLE13). In 2013, the US Preventive Services Task Force upgraded its recommendation to match those of the CDC.14
Despite these recommendations, which are expected to increase detection of HCV among asymptomatic persons who do not know they are infected, there remain significant barriers to HCV testing. These include poor access to primary care and preventive services, lack of knowledge and awareness of the disease among patients and providers, and a lack of studies that support a universal screening approach for HCV.5,15,16 One tool that might help overcome some of these barriers and aid family physicians in the screening process is automatic reminders or standing lab orders for HCV testing in electronic medical records systems.
Screening for HCV can be done using any of the US Food and Drug Administration (FDA)-approved tests for the anti-HCV antibody, which have sensitivities and specificities greater than 99%.17 A positive screening result should be confirmed with an HCV RNA test. However, for practical purposes, ordering the anti-HCV test with reflex to the HCV RNA test decreases the number of blood draws and office visits required of the patient. The reflex confirmation allows the physician to deliver the patient’s full diagnosis and reduces the psychological distress associated with waiting for confirmatory results. The HCV RNA test (alone) should be used, however, in immunocompromised patients, those who may have had exposure to HCV in the past 6 months, and those suspected of having an HCV re-infection after having cleared the virus.18
Look for the evidence of liver disease
Family physicians should order several additional tests for patients found to have chronic HCV infection before referring such patients to a specialist (ALGORITHM). Work-up should include the complete blood count, HCV genotype (which will help guide treatment), liver function tests, international normalized ratio test, and ultrasound of the liver.18 In addition, all HCV-positive patients should be tested for HIV and HBV, because these co-infections may accelerate liver fibrosis.19,20
All patients with chronic HCV infection should also be screened for the presence of fibrosis and cirrhosis, as this will influence treatment choice and duration. Signs of cirrhosis that may be evident on physical exam include jaundice, spider angiomata, palmar erythema, encephalopathy with asterixis, and fluid overload, especially ascites. Cirrhosis can be classified clinically as compensated (stage 1 with no varices present and stage 2 with varices present) and decompensated (stages 3 and 4), which is defined as cirrhosis with signs of severe portal hypertension (bleeding varices, ascites, hepatic encephalopathy) or liver insufficiency (jaundice).21 Patients with decompensated cirrhosis should be managed by a liver transplant center. For more on cirrhosis, see “Cirrhosis complications: Keeping them under control” (J Fam Pract. 2015;64:338-342).
Several noninvasive alternatives to liver biopsy
Historically, liver biopsy has been the gold standard for staging liver disease. The Metavir scoring system is a histological assessment of the degree of inflammatory activity and the stage of fibrosis.22 The degree of inflammation activity, which is a precursor of fibrosis, is scored from A0 (no activity) to A3 (severe activity). The staging of fibrosis involves a 5-stage scoring system: F0 (chronic hepatitis without fibrosis); F1 (portal fibrosis without septae); F2 (portal fibrosis with rare septae); F3 (many septae without cirrhosis); or F4 (cirrhosis).
That said, noninvasive tests have largely supplanted liver biopsy for fibrosis screening.
For example, the FibroSure test uses the patient’s age, gender, and a combination of 6 serum markers of liver function in a computational algorithm to generate a quantitative indicator of liver fibrosis, with a score of 0.0 to 1.0 that corresponds to the Metavir fibrosis score (F0-F4), and an inflammatory activity score (A0-A3).23 Similarly, HepaScore uses several noninvasive markers to calculate a score from 0.00 to 1.00. A score ≤0.2 accurately excludes significant fibrosis. However, a score of ≥0.55 or higher corresponds to a Metavir score of at least F2, and in such cases further testing would be needed to evaluate for cirrhosis.24
FDA-approved in 2013, transient elastography (FibroScan) is another noninvasive alternative to liver biopsy for determining the stage of liver disease. This bedside test uses ultrasound technology to measure liver stiffness and provides a score ranging from 0 to 75 kPA that correlates with the Metavir score. Although not yet widely available in the United States, FibroScan is becoming increasingly popular as a rapid and noninvasive screening tool for cirrhosis.25
Identifying cirrhosis in patients who have HCV is crucial because such patients need prompt care from a specialist. In addition to receiving HCV treatment, patients with cirrhosis also need regular liver ultrasound exams to screen for HCC (every 6 months) and esophagogastroduodenoscopy to screen for esophageal and gastric varices.26
Advise patients to avoid alcohol, lose weight
Counsel patients who test positive for HCV infection about making lifestyle changes to avoid further liver damage and transmission of HCV to others. Infectious diseases and hepatology society guidelines recommend vaccination against hepatitis A and B for all HCV-infected patients who are not immune to these viruses because acute co-infection could lead to severe acute liver injury.18,27 Urge all HCV-infected patients to completely abstain from alcohol and, if necessary, refer them to an addiction specialist, because excess alcohol consumption is strongly associated with the development of cirrhosis and HCC.28,29
Comorbid conditions such as metabolic syndrome, obesity, and hyperlipidemia can worsen the prognosis for HCV-infected patients; therefore, intense counseling on weight loss is recommended.30 Statins are safe and beneficial for HCV patients with hypercholesterolemia and compensated cirrhosis.31
Teach patients that the primary mode of transmission of HCV is through infected blood. Sexual transmission of HCV has been well documented in HIV-positive men who have sex with men.32 Although the risk of transmission of HCV among heterosexual couples is extremely low, it is possible, and patients should be counseled accordingly.33 Transmission of HCV from mother to the baby occurs in up to 6% of births and most commonly occurs during delivery.34
Newer treatments are highly effective and well tolderated
HCV treatment has changed dramatically over the past few years. Previous treatments for HCV, particularly those containing interferon, were known for their poor tolerability due to adverse effects and low cure rates. Compared to previous therapies, the new interferon-free direct-acting antiviral (DAA) regimens are not only less complex but also shorter in duration, ranging from 8 to 24 weeks depending on the patient’s viral load, stage of liver disease, and previous treatment experience.18 The specific agents and dosages used in DAA regimens aren’t described here because these regimens are rapidly changing. However, continuously updated treatment recommendations from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America are available at http://www.hcvguidelines.org.
The goal of HCV treatment is cure as evidenced by a sustained virologic response (SVR), which is defined as the absence of HCV RNA 12 weeks or more after completing treatment.35,36 In general, for the most common genotypes of HCV, treatment with a DAA regimen results in a SVR in ≥95% of patients.18 Achieving SVR is associated with a 50% reduction in all-cause mortality, a 90% reduction in liver-associated mortality, and a >70% reduction in the risk of developing HCC.27,37,38 SVR also has been shown to have a significant effect on reducing extrahepatic manifestations of HCV infection, such as cryoglobulinemia and lymphoma.39-41
Current barriers to the newer, highly effective hepatitis C virus (HCV) infection treatments are largely financial. Although insurance companies have been able to negotiate substantial discounts from the high wholesale price of treatment, many insurance programs require prior authorizations and will approve treatment only for patients with advanced liver fibrosis. In our experience, many patients are left to wait for their liver disease to progress before their insurance company will agree to cover treatment.
In addition, many insurance companies have mandated that only subspecialists prescribe these medications. However, infectious diseases and hepatology specialists and their support staffs are often overburdened with paperwork and phone calls related to prior authorizations and justification of treatment, which can add to delays in treatment.
There is already evidence that treatment of all patients with HCV is cost-effective and leads to better healthcare outcomes42 and there are indications that these barriers will decrease over time, with prices already dropping significantly due to increasing competition between drug companies.
The DAAs are well tolerated and have good safety profiles. In phase III clinical trials of today’s most commonly used DAA regimens, the discontinuation rate was <1% in non-cirrhotic patients and 2% in those with cirrhosis.18 The most commonly reported adverse effects were nausea, fatigue, and headache. DAAs may have drug-drug interactions; therefore, careful medication reconciliation should be performed before initiating treatment.18
Prioritizing treatment. Current evidence supports treatment for all patients with HCV except those with a life expectancy of <12 months.18 Evidence indicates that treatment becomes less effective as a patient’s liver injury progresses to cirrhosis. Due to the high cost of available treatments, however, many insurers have imposed strict criteria for coverage. (See “Barriers to HCV Treatment,” above.42)
The highest priority for treatment has been given to patients with advanced liver fibrosis, compensated cirrhosis, those who have received a liver transplant, and those with severe extrahepatic manifestations (eg, mixed cryoglobulinemia and end-organ disease such as nephropathy). Treatment is also prioritized for high-risk populations (eg, patients with HBV and HIV co-infection, diabetes mellitus) and patients who are at high risk of transmitting the virus (eg, individuals who inject drugs or are incarcerated, men who have sex with men, women of childbearing age, hemodialysis patients, and health care professionals who perform exposure-prone procedures).18
While it may eventually become feasible for family physicians to treat HCV-infected patients, the rapid evolution and significant cost of treatment, as well as the challenges in obtaining insurance coverage, have kept HCV treatment largely in the domain of specialists, at least for now. In the interim, family physicians play a crucial role by screening, diagnosing, and counseling patients with this infection, referring them to specialty care, and providing ongoing monitoring for signs of HCC and esophageal and gastric varices.
CORRESPONDENCE
Laura Wangensteen, MD, Department of Family Medicine, Drexel University, 3401 South Market Street #105 A, Philadelphia, PA 19104; [email protected]
› Screen at-risk patients and all those born between 1945 and 1965 for hepatitis C virus (HCV) infection. B
› Screen HCV-positive patients for level of fibrosis and for conditions that may accelerate liver disease, including alcohol use, hepatitis B virus, and human immunodeficiency virus. B
› Continuously monitor patients with chronic HCV for the development of cirrhosis and hepatocellular carcinoma. A
› Refer patients to specialty care for HCV treatment and, if they have cirrhosis, for potential transplant evaluation. C
› Counsel HCV-positive patients about how to avoid transmission to others. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease. Over the next few decades, the number of deaths per year due to complications of HCV such as liver failure and hepatocellular carcinoma (HCC) is predicted to more than triple to 36,000 by 2032.1
Fortunately, major advances in drug therapy have made it possible to cure patients of HCV, and treatment is now less complex, of shorter duration, and better tolerated than it once was. To help family physicians maximize the care they provide to these patients, we’ve summarized screening recommendations from the Centers for Disease Control and Prevention (CDC), innovative alternatives to biopsy for staging liver disease, and counseling points to cover with patients.
A common, usually silent infection with potentially fatal complications
According to the National Health and Nutrition Examination Survey (NHANES), an estimated 2.7 to 3.9 million people in the United States are chronically infected with HCV, about threefourths of whom were born between 1945 and 1965 (the “baby boomer” generation).2 However, by adding “unaccounted groups” (eg, incarcerated, homeless, and active duty military) to these estimates, the number of people with HCV is likely more than 5.2 million.3
HCV is a ribonucleic acid (RNA) virus capable of mutating at a high rate to escape detection and clearance by the host’s immune system.4 Most patients with HCV are asymptomatic during the acute and chronic phases of infection, and may have a silent infection for decades. In fact, 65% to 75% of patients with HCV are unaware of their infection.5
Approximately 20% of chronically infected patients develop cirrhosis after 20 years and, once they do, the annual rate of HCC and liver decompensation is about 5%.6-8 Risk factors for advancement to cirrhosis includes male sex, alcohol consumption, co-infection with human immunodeficiency virus (HIV) or hepatitis B virus (HBV), immunosuppression, having had HCV infection for a long time, becoming infected with HCV after age 40, and not having responded to previous treatment.9
Chronic HCV infection can lead to extrahepatic manifestations such as essential mixed cryoglobulinemia, porphyria cutanea tarda, membranoproliferative glomerulonephritis, lymphoma, and glucose intolerance.10 There is also growing evidence that HCV infection affects cognitive function in the absence of fibrosis and hepatic encephalopathy. Several studies show that HCV-infected patients score poorly on neuropsychological testing for verbal learning, attention, memory, and executive function.11 This may be related to the expression of receptors for HCV by the brain’s microvascular endothelial cells.12
Screening recommendations. Given the high prevalence of HCV infection among baby boomers, the CDC decided in 2012 to recommend one-time HCV screening for all patients born between 1945 and 1965.13 This is in addition to risk-based screening for all patients who have a history of injection drug use, those on long-term hemodialysis or with tattoos obtained in unregulated settings, offspring of HCV-infected mothers, and those with health-care associated exposures (TABLE13). In 2013, the US Preventive Services Task Force upgraded its recommendation to match those of the CDC.14
Despite these recommendations, which are expected to increase detection of HCV among asymptomatic persons who do not know they are infected, there remain significant barriers to HCV testing. These include poor access to primary care and preventive services, lack of knowledge and awareness of the disease among patients and providers, and a lack of studies that support a universal screening approach for HCV.5,15,16 One tool that might help overcome some of these barriers and aid family physicians in the screening process is automatic reminders or standing lab orders for HCV testing in electronic medical records systems.
Screening for HCV can be done using any of the US Food and Drug Administration (FDA)-approved tests for the anti-HCV antibody, which have sensitivities and specificities greater than 99%.17 A positive screening result should be confirmed with an HCV RNA test. However, for practical purposes, ordering the anti-HCV test with reflex to the HCV RNA test decreases the number of blood draws and office visits required of the patient. The reflex confirmation allows the physician to deliver the patient’s full diagnosis and reduces the psychological distress associated with waiting for confirmatory results. The HCV RNA test (alone) should be used, however, in immunocompromised patients, those who may have had exposure to HCV in the past 6 months, and those suspected of having an HCV re-infection after having cleared the virus.18
Look for the evidence of liver disease
Family physicians should order several additional tests for patients found to have chronic HCV infection before referring such patients to a specialist (ALGORITHM). Work-up should include the complete blood count, HCV genotype (which will help guide treatment), liver function tests, international normalized ratio test, and ultrasound of the liver.18 In addition, all HCV-positive patients should be tested for HIV and HBV, because these co-infections may accelerate liver fibrosis.19,20
All patients with chronic HCV infection should also be screened for the presence of fibrosis and cirrhosis, as this will influence treatment choice and duration. Signs of cirrhosis that may be evident on physical exam include jaundice, spider angiomata, palmar erythema, encephalopathy with asterixis, and fluid overload, especially ascites. Cirrhosis can be classified clinically as compensated (stage 1 with no varices present and stage 2 with varices present) and decompensated (stages 3 and 4), which is defined as cirrhosis with signs of severe portal hypertension (bleeding varices, ascites, hepatic encephalopathy) or liver insufficiency (jaundice).21 Patients with decompensated cirrhosis should be managed by a liver transplant center. For more on cirrhosis, see “Cirrhosis complications: Keeping them under control” (J Fam Pract. 2015;64:338-342).
Several noninvasive alternatives to liver biopsy
Historically, liver biopsy has been the gold standard for staging liver disease. The Metavir scoring system is a histological assessment of the degree of inflammatory activity and the stage of fibrosis.22 The degree of inflammation activity, which is a precursor of fibrosis, is scored from A0 (no activity) to A3 (severe activity). The staging of fibrosis involves a 5-stage scoring system: F0 (chronic hepatitis without fibrosis); F1 (portal fibrosis without septae); F2 (portal fibrosis with rare septae); F3 (many septae without cirrhosis); or F4 (cirrhosis).
That said, noninvasive tests have largely supplanted liver biopsy for fibrosis screening.
For example, the FibroSure test uses the patient’s age, gender, and a combination of 6 serum markers of liver function in a computational algorithm to generate a quantitative indicator of liver fibrosis, with a score of 0.0 to 1.0 that corresponds to the Metavir fibrosis score (F0-F4), and an inflammatory activity score (A0-A3).23 Similarly, HepaScore uses several noninvasive markers to calculate a score from 0.00 to 1.00. A score ≤0.2 accurately excludes significant fibrosis. However, a score of ≥0.55 or higher corresponds to a Metavir score of at least F2, and in such cases further testing would be needed to evaluate for cirrhosis.24
FDA-approved in 2013, transient elastography (FibroScan) is another noninvasive alternative to liver biopsy for determining the stage of liver disease. This bedside test uses ultrasound technology to measure liver stiffness and provides a score ranging from 0 to 75 kPA that correlates with the Metavir score. Although not yet widely available in the United States, FibroScan is becoming increasingly popular as a rapid and noninvasive screening tool for cirrhosis.25
Identifying cirrhosis in patients who have HCV is crucial because such patients need prompt care from a specialist. In addition to receiving HCV treatment, patients with cirrhosis also need regular liver ultrasound exams to screen for HCC (every 6 months) and esophagogastroduodenoscopy to screen for esophageal and gastric varices.26
Advise patients to avoid alcohol, lose weight
Counsel patients who test positive for HCV infection about making lifestyle changes to avoid further liver damage and transmission of HCV to others. Infectious diseases and hepatology society guidelines recommend vaccination against hepatitis A and B for all HCV-infected patients who are not immune to these viruses because acute co-infection could lead to severe acute liver injury.18,27 Urge all HCV-infected patients to completely abstain from alcohol and, if necessary, refer them to an addiction specialist, because excess alcohol consumption is strongly associated with the development of cirrhosis and HCC.28,29
Comorbid conditions such as metabolic syndrome, obesity, and hyperlipidemia can worsen the prognosis for HCV-infected patients; therefore, intense counseling on weight loss is recommended.30 Statins are safe and beneficial for HCV patients with hypercholesterolemia and compensated cirrhosis.31
Teach patients that the primary mode of transmission of HCV is through infected blood. Sexual transmission of HCV has been well documented in HIV-positive men who have sex with men.32 Although the risk of transmission of HCV among heterosexual couples is extremely low, it is possible, and patients should be counseled accordingly.33 Transmission of HCV from mother to the baby occurs in up to 6% of births and most commonly occurs during delivery.34
Newer treatments are highly effective and well tolderated
HCV treatment has changed dramatically over the past few years. Previous treatments for HCV, particularly those containing interferon, were known for their poor tolerability due to adverse effects and low cure rates. Compared to previous therapies, the new interferon-free direct-acting antiviral (DAA) regimens are not only less complex but also shorter in duration, ranging from 8 to 24 weeks depending on the patient’s viral load, stage of liver disease, and previous treatment experience.18 The specific agents and dosages used in DAA regimens aren’t described here because these regimens are rapidly changing. However, continuously updated treatment recommendations from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America are available at http://www.hcvguidelines.org.
The goal of HCV treatment is cure as evidenced by a sustained virologic response (SVR), which is defined as the absence of HCV RNA 12 weeks or more after completing treatment.35,36 In general, for the most common genotypes of HCV, treatment with a DAA regimen results in a SVR in ≥95% of patients.18 Achieving SVR is associated with a 50% reduction in all-cause mortality, a 90% reduction in liver-associated mortality, and a >70% reduction in the risk of developing HCC.27,37,38 SVR also has been shown to have a significant effect on reducing extrahepatic manifestations of HCV infection, such as cryoglobulinemia and lymphoma.39-41
Current barriers to the newer, highly effective hepatitis C virus (HCV) infection treatments are largely financial. Although insurance companies have been able to negotiate substantial discounts from the high wholesale price of treatment, many insurance programs require prior authorizations and will approve treatment only for patients with advanced liver fibrosis. In our experience, many patients are left to wait for their liver disease to progress before their insurance company will agree to cover treatment.
In addition, many insurance companies have mandated that only subspecialists prescribe these medications. However, infectious diseases and hepatology specialists and their support staffs are often overburdened with paperwork and phone calls related to prior authorizations and justification of treatment, which can add to delays in treatment.
There is already evidence that treatment of all patients with HCV is cost-effective and leads to better healthcare outcomes42 and there are indications that these barriers will decrease over time, with prices already dropping significantly due to increasing competition between drug companies.
The DAAs are well tolerated and have good safety profiles. In phase III clinical trials of today’s most commonly used DAA regimens, the discontinuation rate was <1% in non-cirrhotic patients and 2% in those with cirrhosis.18 The most commonly reported adverse effects were nausea, fatigue, and headache. DAAs may have drug-drug interactions; therefore, careful medication reconciliation should be performed before initiating treatment.18
Prioritizing treatment. Current evidence supports treatment for all patients with HCV except those with a life expectancy of <12 months.18 Evidence indicates that treatment becomes less effective as a patient’s liver injury progresses to cirrhosis. Due to the high cost of available treatments, however, many insurers have imposed strict criteria for coverage. (See “Barriers to HCV Treatment,” above.42)
The highest priority for treatment has been given to patients with advanced liver fibrosis, compensated cirrhosis, those who have received a liver transplant, and those with severe extrahepatic manifestations (eg, mixed cryoglobulinemia and end-organ disease such as nephropathy). Treatment is also prioritized for high-risk populations (eg, patients with HBV and HIV co-infection, diabetes mellitus) and patients who are at high risk of transmitting the virus (eg, individuals who inject drugs or are incarcerated, men who have sex with men, women of childbearing age, hemodialysis patients, and health care professionals who perform exposure-prone procedures).18
While it may eventually become feasible for family physicians to treat HCV-infected patients, the rapid evolution and significant cost of treatment, as well as the challenges in obtaining insurance coverage, have kept HCV treatment largely in the domain of specialists, at least for now. In the interim, family physicians play a crucial role by screening, diagnosing, and counseling patients with this infection, referring them to specialty care, and providing ongoing monitoring for signs of HCC and esophageal and gastric varices.
CORRESPONDENCE
Laura Wangensteen, MD, Department of Family Medicine, Drexel University, 3401 South Market Street #105 A, Philadelphia, PA 19104; [email protected]
1. Rein DB, Wittenborn JS, Weinbaum CM, et al. Forecasting the morbidity and mortality associated with prevalent cases of precirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66-72.
2. Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-714.
3. Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090-1101.
4. Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103-107.
5. Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729-733.
6. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
7. El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83.
8. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68.
9. McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut. 2004;53:318-321.
10. El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-1445.
11. Solinas A, Piras MR, Deplano A. Cognitive dysfunction and hepatitis C virus infection. World J Hepatol. 2015;7:922-925.
12. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142:634-643.e6.
13. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32.
14. US Preventive Services Task Force. Final recommendation statement on hepatitis C screening, June 2013. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-c-screening. Accessed on December 28, 2014.
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-2207.
16. Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754-758.
17. Shivkumar S, Peeling R, Jafari Y, et al. Accuracy of rapid and pointof- care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558-566.
18. American Association for the Study of Liver Diseases; Infectious Diseases Society of America; International Antiviral Society—USA. HCV guidance: Recommendations for testing, managing, and treating hepatitis C. HCV guidelines Web site. Available at: http://www.hcvguidelines.org. Accessed May 25, 2015.
19. Zarski JP, Bohn B, Bastie A, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27-33.
20. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562-569.
21. Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449.
22. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
23. Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896.
24. Becker L, Salameh W, Sferruzza A, et al. Validation of hepascore, compared with simple indices of fibrosis, in patients with chronic hepatitis C virus infection in United States. Clin Gastroenterol Hepatol. 2009;7:696-701.
25. Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16:372.
26. Garcia-Tsao G, Sanyal AJ, Grace ND, et al; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
27. Ghany MG, Strader DB, Thomas DL, et al; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
28. Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717-1722.
29. Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J Gastroenterol. 2009;15:3462-3471.
30. Ortiz V, Berenguer M, Rayón JM, et al. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408-2414.
31. Lewis JH, Mortensen ME, Zweig S, et al; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453-1463.
32. Gamage DG, Read TR, Bradshaw CS, et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis. 2011;11:39.
33. Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881-889.
34. Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223-229.
35. Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593-1601.
36. Thomas AM, Kattakuzhy S, Jones S, et al. SVR durability: HCV patients treated with IFN-free DAA regimens. Presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February, 2015; Seattle, Washington. Abstract 653.
37. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.e1.
38. Russo MW. Antiviral therapy for hepatitis C is associated with improved clinical outcomes in patients with advanced fibrosis. Expert Rev Gastroenterol Hepatol. 2010;4:535-539.
39. Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCVassociated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol. 2013;85:1019-1027.
40. Takahashi K, Nishida N, Kawabata H, et al. Regression of Hodgkin lymphoma in response to antiviral therapy for hepatitis C virus infection. Intern Med. 2012;51:2745-2747.
41. Gisbert JP, García-Buey L, Pajares JM, et al. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther. 2005;21:653-662.
42. Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407-419.
1. Rein DB, Wittenborn JS, Weinbaum CM, et al. Forecasting the morbidity and mortality associated with prevalent cases of precirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66-72.
2. Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-714.
3. Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090-1101.
4. Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103-107.
5. Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729-733.
6. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
7. El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83.
8. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68.
9. McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut. 2004;53:318-321.
10. El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-1445.
11. Solinas A, Piras MR, Deplano A. Cognitive dysfunction and hepatitis C virus infection. World J Hepatol. 2015;7:922-925.
12. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142:634-643.e6.
13. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32.
14. US Preventive Services Task Force. Final recommendation statement on hepatitis C screening, June 2013. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-c-screening. Accessed on December 28, 2014.
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-2207.
16. Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754-758.
17. Shivkumar S, Peeling R, Jafari Y, et al. Accuracy of rapid and pointof- care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558-566.
18. American Association for the Study of Liver Diseases; Infectious Diseases Society of America; International Antiviral Society—USA. HCV guidance: Recommendations for testing, managing, and treating hepatitis C. HCV guidelines Web site. Available at: http://www.hcvguidelines.org. Accessed May 25, 2015.
19. Zarski JP, Bohn B, Bastie A, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27-33.
20. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562-569.
21. Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449.
22. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
23. Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896.
24. Becker L, Salameh W, Sferruzza A, et al. Validation of hepascore, compared with simple indices of fibrosis, in patients with chronic hepatitis C virus infection in United States. Clin Gastroenterol Hepatol. 2009;7:696-701.
25. Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16:372.
26. Garcia-Tsao G, Sanyal AJ, Grace ND, et al; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
27. Ghany MG, Strader DB, Thomas DL, et al; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
28. Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717-1722.
29. Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J Gastroenterol. 2009;15:3462-3471.
30. Ortiz V, Berenguer M, Rayón JM, et al. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408-2414.
31. Lewis JH, Mortensen ME, Zweig S, et al; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453-1463.
32. Gamage DG, Read TR, Bradshaw CS, et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis. 2011;11:39.
33. Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881-889.
34. Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223-229.
35. Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593-1601.
36. Thomas AM, Kattakuzhy S, Jones S, et al. SVR durability: HCV patients treated with IFN-free DAA regimens. Presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February, 2015; Seattle, Washington. Abstract 653.
37. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.e1.
38. Russo MW. Antiviral therapy for hepatitis C is associated with improved clinical outcomes in patients with advanced fibrosis. Expert Rev Gastroenterol Hepatol. 2010;4:535-539.
39. Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCVassociated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol. 2013;85:1019-1027.
40. Takahashi K, Nishida N, Kawabata H, et al. Regression of Hodgkin lymphoma in response to antiviral therapy for hepatitis C virus infection. Intern Med. 2012;51:2745-2747.
41. Gisbert JP, García-Buey L, Pajares JM, et al. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther. 2005;21:653-662.
42. Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407-419.
Perceived Attitudes and Staff Roles of Disaster Management at CBOCs
Recently, the U.S. Department of Homeland Security redefined disasters into 4 types: natural hazards, societal hazards, technologic hazards, and terrorism. The incidence of manmade and natural disasters is on the rise in intensity and frequency globally. Recent events such as tornadoes and hurricanes in the southeastern U.S., tsunamis in Japan, earthquakes in Haiti, wild fires, heat waves, and terrorist attacks like that of September 11, 2001, underscore the urgency of developing and maintaining solid local public health disaster response plans to minimize mortality and morbidity.
The 2010 BP oil spill in the Gulf of Mexico, the largest in history, hurricane Katrina, and the lingering impact of hurricane Sandy on the East Coast further raise concerns about our communities’ ability to handle disasters, especially in the early hours after events, when federally coordinated help is being organized and not yet fully available locally or from other nations.1 The recent fertilizer plant explosion in West Texas, the 2013 Boston marathon bombing, and the Newtown, Connecticut, massacre remind us of the unpredictable nature of both manmade and natural disasters.
Coordinated Response
Regardless of its origin, residents expect a coordinated local response during an emergency, and it is important that government agencies meet this expectation. Fulfilling these expectations, however, takes many partners, and it is important to have a clear idea of who is involved in emergency preparedness (EP) and the response of each partner’s role.
Role of Government
Federal, state, and local governments have a critical role in emergency management (EM). When state government, local government, or an individual entity is overwhelmed with a disaster, the role of the Federal Emergency Management Agency is to provide assistance and resources to cope with the emergency.2 Private industry and traditional disaster relief agencies, such as the American Red Cross and the Adventist Development and Relief Agency, are also involved in response efforts. Recent examples have shown that these partnerships are often overwhelmed with the needs of large regions experiencing limited resources. Therefore, hospitals and local public health departments frequently must carry much of the immediate burden of stabilizing communities and coordinating response with government agencies and local partners.3
Role of Public Health and the CDC
Federal agencies and local public health departments have been given critical roles in planning and responding to disasters. In particular, the PHS focuses on population care and shapes how public health entities should respond to mass casualty events and pandemics, including local response coordination. The CDC is primarily responsible for assisting state and local governments with disaster response and recovery after a large-scale public health emergency.3 The CDC works closely with local public health departments in decision making; tracking the source, spread, and severity of health threats; assessing impacts; educating the public on how to safeguard their health; and implementing measures to protect the public. During a large-scale health emergency, the CDC also maintains and provides resources through the maintenance and distribution of the nation’s Strategic National Stockpile of medications and supplies that may be needed during events such as the recent 2009 H1N1 influenza outbreak or other public health emergencies.3
Role of Local Businesses and Professional Institutions
Nationally, businesses and professional institutions are coming together and organizing in such a way that places them as part of the solution. More specifically, the National Voluntary Organizations Active in Disaster and Community Organizations Active in Disaster have grown exponentially since September 11, 2001.4 These efforts include but are not limited to development of EP plans and the subsequent sharing of those plans, sharing of key assets critical to response activities, development of a community key asset database, and training/exercise participation.
Role of Hospitals
The Hospital Preparedness Program was developed to prepare the nation’s health care system to respond appropriately to mass casualty incidents, whether due to bioterrorism, natural disaster, or other public health emergencies. Health care systems must be able to develop a disaster medical capability that is rapid, flexible, sustainable, integrated, coordinated, and capable of providing appropriate care in the most ethical manner with the resources and capabilities it has at its disposal.3 Although involved as first responders, traditionally, medical care systems, hospitals, physicians, and pharmacists are faced with the dual task of individual patient care and are thus more limited as partners in an overall local response system.
Also vital to this discussion is the reality that hospital emergency departments (EDs) already routinely operate at or above capacity, limiting their ability to prepare for mass casualties due to a public health disaster. Hospitals continue to divert more than half a million ambulances per year due to ED overcrowding.3 How they could step up in a true emergency situation is questionable at best.
Role of First Responders
Individuals who respond immediately are referred to as first responders. First responders come in 2 archetypes: those who are there purely based on unexpected circumstances and take action and those who are trained first responders, such as firefighters, police officers, and emergency medical technicians (EMTs). These first responders are trained to partner with one another. Firefighters primarily handle fire rescue as well as assessing the extent of potential damage to the area. Law enforcement’s responsibility is to restore order after an emergency, whether it is a natural disaster, community disturbance, or outbreak of hazardous chemicals. An EMT’s role is to attend to the immediate medical care of patients who have been injured or become ill during the emergency.5
Related: Disaster Preparedness for Veterans With Dementia and Their Caregivers
There are occasions where other potential incident responders, such as health care professionals, can play a key role and yet are not integrated into the emergency response. The VHA needs to focus on this facet in order to more effectively respond to events that threaten lives, property, and current infrastructure of the veterans it serves.
Role of CBOCs and Private Physician Practices
Community-based outpatient clinics (CBOCs), including outpatient community health centers and private physician practices (PPPs), maintain and improve routine community health but are rarely involved in routine planning for disasters. They are, therefore, typically not open for business or may have limited hours as they recover from the event. This results in patients who do not have access to their primary care providers (PCPs) turning to EDs, which are already at capacity. As a result, in a disaster the costly and overburdened ED functions as the PCP site for even larger populations affected by a disaster, including those who are uninsured.6,7
Kahan and colleagues reported that two-thirds of patients preferred their family doctor or health care authorities as their first choice for care instead of receiving care in the ED.8 Researchers found that 89% of physicians in private practice felt it was their responsibility to treat, for example, patients infected with anthrax.8 Some argue that if PCPs are included in planning and appropriately trained in disaster preparedness, their attitudes and willingness to participate in emergency services would follow.9
Given the many challenges to disaster preparedness, CBOCs could be a critical partner in EM, and interest continues to grow to explore that role. Health professionals in CBOCs who are trained in disaster management (DM) could become active participants in early intervention to initiate the treatment of patients in rescue efforts during a disaster.10 For instance, a CBOC could triage patients in a postdisaster situation, thus limiting the burden on hospital EDs by evaluating populations at risk and providing them with important information when communication is difficult.
This already existing network of community-based triage stations would offer natural locations to assess the health needs of the population and determine their level of appropriate medical care. Additionally, these clinics can ensure continuation of basic services after initial medical care has been completed in the hospital setting.10 Because clinics have not been included in coordinated DM, there is scant literature that addresses their potential role in disaster response. Community-based outpatient clinics and PPPs are untapped resources; however, it is unknown whether medical staff in these medical clinics have the interest, training, knowledge, skills, and resources in DM or whether barriers to providing safe care can be overcome.10
Case Study
The VHA is the largest integrated health care system in the U.S. It is mandated to serve as a backup to the DoD during disasters, and VHA CBOCs can play an important role.11,12 The CBOCs are staffed with a medical director, nurse manager, and other clinical and support staff. As a study population, CBOCs are well suited to examine and explore staff attitudes and roles in DM. To date, no research reports have been found studying EP in CBOCs.
The purpose of this study was to learn how to best integrate the CBOCs into disaster response. This qualitative study aimed to answer 3 questions: (1) How do VA clinic personnel perceive their personal and their clinic’s risk, level of preparedness, role, and knowledge for an active response in a disaster; (2) What do VA clinic personnel perceive they need in order to function in a disaster; and (3) What resources are necessary for clinic staff to function competently in a disaster?
Methods
In this qualitative study, in-depth semistructured key informant (KI) interviews (N = 3) and focus group discussions (N = 20) guided by risk perception theory and the Andersen Behavioral Model of Health Services Use were conducted and analyzed using grounded theory methods to contextualize the potential of local clinics in disaster response.13-15 To optimize breadth of viewpoints on this issue, participants were selected by theoretical sampling methods to explore perceptions of leadership and line staff.
Study Location
Health care providers and support staff from 3 southern California CBOCs that are contracted by the local VA to provide primary care services (ie, internal medicine, geriatrics, women’s health, mental health, and some specialty care services) to veterans were recruited for this study. The CBOCs are generally connected with a VHA local hospital in their region, offer services 5 days a week, and are closed on weekends and federal holidays. Some VA CBOCs participate in telehealth remote services connected to their regional hospital to help manage their patient populations. The CBOCs are managed by a medical director and a clinic manager and report to their respective VISN, and each VISN reports to the VHA Central Office in Washington, DC.13,15 The CBOC staff includes physicians, nurse practitioners, physician assistants, registered nurses (RNs), licensed vocational nurses (LVNs), medical assistants, front office staff, social workers, case managers, counselors, pharmacists, and nonclinical staff.
In this case, the CBOCs are contracted by Loma Linda University Health to manage care of the veterans and agree to care for nonveterans in a disaster. The CBOCs contracted or not all fall under the criteria as set forth in VHA Handbook 1006.1. This handbook criteria indicate that CBOCs must maintain appropriate emergency response capability. Additionally, VHA Handbook 0320.1 states that the CBOC is responsible for developing, implementing, evaluating, and improving a CBOC Comprehensive Emergency Management Program (CEMP) and for participating in the VAMC Emergency Management Committee. The scope of the VISN-wide CEMP integrates VAMC and VISN EM programs to coordinate and enhance operations during planned and unplanned events.
Study Design and Sample
After receiving institutional review board approval, 3 in-depth semi-structured clinic leadership KI interviews and 3 clinic staff (RNs, LVNs, health technicians, and nursing assistants) focus group discussions (N = 20, 1 per CBOC) to follow up on information gleaned from the analyses of the initial KIs were conducted. To provide continuity, all were conducted by the same trained facilitator who used a semistructured KI outline with questions and probes based on the guiding study framework.
Data Collection and Content Analysis
Interviews and focus group discussions were audio recorded and transcribed verbatim and then analyzed using grounded theory methods. Line-by-line coding was done to develop an initial inductive codebook, which was then organized into final codes. Once the codebook was developed, it was applied to all transcripts.
Related: Pre-Storm Dialysis Saves Lives
Transcripts and resulting codes were reviewed 3 times by independent reviewers to validate data, ensure accuracy, and delete any information that might identify participants. Pseudonyms were used to represent the participants by perspective (eg, nurse, MD) to avoid confusion in data analysis. A 4-stage data analysis approach was used: (1) immersion in the raw data by listening to tapes and reading manuscripts and notes in order to list key ideas and recurrent themes using a constant comparison method; (2) indexing by applying the thematic framework systematically to the data using and seeking new, unanticipated emerging codes; (3) arranging the data in codes and concepts/themes that represent the thematic framework of EP in clinics; (4) identifying a thematic framework for EP using codes that identified key issues, concepts, and themes that can be referenced and derived from the text.
Results
The Table describes the 4 primary emerging themes and corresponding quotes: (1) EP barriers, including lack of direction, training, and tools, which would result in negative outcomes; (2) perceived personal and clinic risk for a disaster, including negative outcomes and personal family safety; (3) perceptions of roles and responsibilities in EP, including intent to participate in DM at various staffing levels as well as patient expectations for care; and (4) existing resources that influence EP and the ability to survive a disaster collectively.
Emergency preparedness barriers. Although most respondents realized their potentially critical role in an emergency, they expressed recurrent barrier themes centered on their perceived lack of training, lack of tools to function, and lack of direction to be effective in a disaster response. Lack of knowledge of EP was identified as a great need by multiple participants. One participant stated, “Lack of information is so destructive. If you don’t know how to keep yourself from those things you don’t know…such as in a situation that’s going to be tragic, it is because of a lack of information or a lack of training. And I see that so many times…Mandate that we do our classes, so we know what we’re doing.” Another stated in reference to lack of skills, “I haven’t experienced any drills or anything like that. So I know what is going to happen here.”
Lack of abilities to communicate with key DM players also were identified. For example, “Downed power lines may result in no telephone connection to communicate next steps for critical issues, such as if evacuation of the clinic is required.” Another respondent indicated, “We need backup communication...devices, wind-up radios, or whatever.”
Lack of a clear disaster plan was also identified. Questions arose centered on details—how to actually implement a clinic response plan, including concerns that there were none, as the respondents “had not seen the plan in a couple of years” and were not sure who really was in charge of giving directions. Lack of community/organizational support voiced included aspects such as interdepartmental, facility, and community resource connectedness. There was acknowledgement that department assets should be clearly identified so that resource sharing might be used as part of the plan.
Last, regarding lack of resources, one participant said, “We don’t have the resources. We don’t have gurneys. We don’t have enough wheel chairs….We don’t have a crash cart. We don’t have the triage tarps or whatever for the triage of people; we don’t have any supplies to supply the energy room for diabetics, like what they have in the ER.”
Perceived personal and clinic risk for a disaster. Participants stated they felt at risk for natural disasters, including fire, floods, and earthquakes, but expressed concerns and even more fears about how they would handle a response to bombings, spills of hazardous materials, airplane accidents, and gunfire, which also qualify as disasters but are much harder to prepare for, because they could be so varied. One participated stated, “They are so unpredictable whether it is an earthquake or a fire…they are unpredictable….We see planes that fly close to our window and we wonder about the possibility of a crash—you never know.”
Many staff members expressed fear of what these disasters would mean to them in the clinic and to their patients. Another comment shared was, “I don’t think anybody really thinks about this kind of stuff until it happens and then it is too late…If we had just done this or that or knew how to do this or that then…” The biggest fear expressed was that of a massive earthquake in which there would be power outages and resulting fires, blocked building exits, and no way to get to evacuation areas. Fears expressed included working with people who are dying and trying to get the patients down the stairs and out of the disaster area.
Personal safety in a disaster was also a concern; a nurse stated, “Your personal safety is a priority. Yourself, that is first, if you are not safe, you can’t do any good to anyone else.” Another shared concern was the safety of family members during a disaster and conflicting obligations between duties at work and protecting family members. Participants felt they would want to be at home with their families.
Perceptions of roles and responsibilities in EP. Supervisors of the clinics shared that their primary responsibility is to the staff and their current patients; ensuring their safety was a top priority. Their knowledge, skills, and available resources were crucial to their duties, including establishing methods of communication outside the clinic for advice and direction, such as notifying the power company and other outside agencies of the condition of the clinic. They felt that their duties included making sure generators were working, ensuring telephones and lighting were available, and advising staff when to leave the building. One manager stated that more EP discussions need to happen in order to determine how to react: “...in event of a disaster it is important to control patient flow, staffing the clinic appropriately and managing the employees.” They felt a need to help empower their staff by making sure staff were trained in EP tasks and that they could complete the tasks they were required to perform.
Staff consistently reported that the doctors were in charge of providing direction concerning activities and care of the patients. However, most were able to identify their own role in helping preserve lives and keeping the patients and other staff safe. One nurse stated, “My job would be to evacuate the physicians’ offices, to make sure they are aware of the disaster, get them out safely, put an X on their door, keep the patients calm and guide them out to the designated area, then look out for medics or other help so that they would be directed to the correct locations.” Another staff nurse stated, “My role is to check the bathrooms and then under the direction of the physician assist in the care of patient injuries.”
When asked about the expectations of patients for care during a disaster, staff consistently stated that patients and their families would want to get care and direction from clinic staff who knew them instead of going to the hospital for care. Staff anticipated that patients would be calling the clinic first to discuss their medical problems. One stated, “The veterans would head to us…. We can’t turn them away.” Some staff indicated that some patients might have to go to the ED for care instead of coming to the clinic, because the clinic may not be equipped to respond, noting that “we have to remind [the patients] that in our clinic we have minimal abilities.”
Existing resources. Consistently, the respondents verbalized the importance of acquiring knowledge and skills and using available resources in their disaster plan. They felt that training was critical and that it needed to be simple and uncomplicated. Many felt that they did not have sufficient drills to maintain their knowledge and skills for all types of events. One nursing assistant stated he had extensive training in the military in DM, but clinics did not have sufficient training and were not prepared to handle multiple casualties. Others stated that it would be important for training to be “second nature” so they would not have to think much about it, with everyone pulling together and performing tasks seamlessly. However, some stated that they did not know what to do in an emergency.
Critical resources noted were access to emergency power sources, transistor radios, telephone and communication, 911 services, backup phone services, computers, and text pagers and cell phones so that connections could be made outside the clinic setting. Other critical resources needed included medical supplies and access to food for 1 week.
Finally, teamwork was identified as a critical factor for success. One example involved the clinic responding to a severe snowstorm; the medical director, lead nurse, and support staff agreed to remain on site to assist with any patients who needed help. “We shared our 4-wheel drive trucks to get around, and others called patients, advising them of storm conditions and what to do to maintain care at home and canceled appointments scheduled for that day.” They were very proud of the way they had pooled their resources to support each other and their patients.
Based on these emerging themes and the inquiry guiding theories, a theoretical framework was proposed on how contributing factors influenced the process by which CBOC staff viewed their roles and the likelihood that they would participate in a disaster plan (Figure). The framework suggests that personal risks and perceived personal and clinic readiness to respond to an emergency were critical barriers to staff willingness to get involved in preparedness, whereas they saw the provision of training and resources as necessary to increase their resilience and ability to function in a disaster.
Clearly addressing barriers through training, planning, ensuring that resources functioned effectively in a disaster, and clarifying roles and responsibilities, combined with promoting personal and clinic readiness facilitated staff EP participation.
Discussion
This qualitative study explored issues surrounding the role of CBOCs in EP and how risk perception and enabling factors contributed to staff intent to participate in DM. As in many qualitative studies, findings were somewhat limited by an overall small sample size (N = 23) across 3 CBOCs in southern California. However, given the lack of available literature, the authors believe that this study helped provide critical insight into CBOC clinic staff’s willingness and readiness to be active in disaster response. The study clearly points to clinic staff’s openness to actively take part in regional disaster response and calls for better and more standardized approaches to EP and DM planning that include local CBOCs. The authors identified factors that contribute to staff intent to participate in DM and the need to reduce barriers that hinder participation.
In general, clinic staff who reported feeling inadequately prepared for disasters (ie, felt more vulnerable) and staff with firsthand disaster experience were more inclined to prepare than were those without experience. Without clearly spelled-out expectations, staff tend to depend and wait on others to lead in a disaster. They noted a desire for better preparation and thus, clarity of roles, need for a reliable method of communication with the outside world during a disaster, and the required equipment and supplies for self-care or care of the patients for ≥ 3 days post disaster. Some indicated that they did not have the resources to provide medical care on the scale that may be required.
Many did not have a clear understanding of an all-hazard approach plan and had not been involved in hazard assessments. Already tightly staffed for personal health care delivery, staff spent minimal time and energy thinking about the risk of a disaster or preparing for one. However, there seemed to be a direct relationship between the attitude of the supervisor and the attitudes of clinic staff to EP. Although these qualitative results are encouraging and point to these clinics as an important undertapped resource for EP, further quantitative studies should expand this inquiry.
Lessons learned from this study include the need to expand qualitative data collection to include a larger sample size to retrieve information that would contribute to a better understanding of how staff view their roles in DM. There are 152 VAMCs and hundreds of associated CBOCs that should be queried as to their EM readiness. Also, replicating this study in non-VHA clinics, such as private CBOCs and PPPs, might bring greater insight into what is needed to involve them in DM plans. Finally, future studies should determine clearer criteria when care can be provided at a clinic and when it would be appropriate for the patient to report at their local ED.
Conclusions and Recommendations
Given the VHA EP mandate, the authors recommend the following steps to address barriers identified in this study: (1) Develop a more structured approach to DM in a CBOC setting to provide staff with a clear understanding of their roles and responsibilities; (2) Conduct a comprehensive assessment of each clinic to determine staff knowledge, skills, and resources required to provide EP and institute a DM training curriculum; (3) Provide clinic leadership with direction on developing a disaster plan as well as how to partner with their primary and local VA health care system, especially onsite physicians, to provide effective DM leadership; (4) Recruit staff into routine drills for natural disasters and expand to an all-hazard approach to manmade disasters to identify gaps in delivering DM in a disaster; (5) Facilitate partnerships and a standardized approach to DM between CBOCs within the VISN by scheduling routine video and teleconferencing, live meetings, and webinars so that procedures and language are clearly understood and communicated between facilities; and (6) Identify key barriers to clinic preparedness by assessing EP elements through mock disaster drills and offer solutions to fill DM gaps.
The authors also recommend that CBOCs should be included in community DM and EP plans in order to understand how to integrate resources in a disaster. Networking, planning, and interdisciplinary staff training between agencies to include CBOCs will bring a wealth of information of what CBOCs require to participate effectively in DM. Lessons learned from these partnerships can provide valuable information to facilitate resource allocation for acute care hospitals, which may be burdened with treating patients with minor medical issues when they should be focusing on providing care to those with catastrophic medical conditions.
Acknowledgments
This study and this material is the result of work supported with resources and the use of facilities at the VA Loma Linda Health Care System. Research in this publication was in part supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under award number P20MD006988.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. McNeill JB, Carafano JJ, Mayer MA, Weitz R. Accepting disaster relief from nations: lessons from Katrina and Gulf oil spill. The Heritage Foundation Website. http://www.heritage.org/research/reports/2011/02/accepting-disaster-relief-from-other-nations-lessons-from-katrina-and-the-gulf-oil-spill. Published February 17, 2011. Accessed July 16, 2015.
2. Haddow GD, Bullock JA. Introduction to Emergency Management. 2nd ed. Burlington, MA: Butterworth-Heinemann; 2006.
3. Institute of Medicine. Medical Surge Capacity: Workshop Summary. Washington, DC: The National Academies Press; 2010.
4. National Voluntary Organizations Active in Disasters. Federal Emergency Management Agency Website. http://www.ready.gov/voluntary-organizations-active-disaster. Updated June 19, 2014. Accessed July 10, 2015.
5. What is the role of police, fire and EMS after a natural disaster strikes? Galls Website. http://gallsblog.com/2011/08/29/what-is-the-role-of-police-fire-and-ems-after-a-natural-disaster-strikes. Published August 29, 2011. Accessed July 14, 2015.
6. Hogan DE, Waeckerle JF, Dire DJ, Lillibridge SR. Emergency department impact of the Oklahoma City terrorist bombing. Ann Emerg Med. 1999;34(2):160-167.
7. Carlson JN, Menegazzi JJ, Callaway CW. Magnitude of national ED visits and resource utilization by the uninsured. Am J Emerg Med. 2013;31(4):722-726.
8. Kahan E, Fogelman Y, Kitai E, Vinker S. Patient and family physician p for care and communication in the eventuality of anthrax terrorism. Fam Pract. 2003;20(4):441-442.
9. Chen FM, Hickner J, Fink KS, Galliher JM, Burstin H. On the front lines: family physicians’ preparedness for bioterrorism. J Fam Pract. 2002;51(9):745-750.
10. Wood K. Community health centers: the untapped resource for public health and medical preparedness. Homeland Secur Aff. 2009;5(8):113.
11. Koenig KL. Homeland security and public health: role of the Department of Veterans Affairs, the US Department of Homeland Security, and implications for the public health community. Prehosp Disaster Med. 2003;18(4):327-333.
12. Panangala SV, Mendez BHP. Veterans Health Administration: Community-Based Outpatient Clinics. Washington, DC: Library of Congress, Congressional Research Service; 2010.
13. Tashakkori A, Teddlie C, eds. Handbook of Mixed Methods in Social & Behavioral Research. Thousand Oaks, CA: Sage Publications, Inc.; 2003.
14. Barnett DJ, Balicer RD, Blodgett DW, et al. Applying risk perception theory to public health workforce preparedness training. J Public Health Manag Pract. 2005;Suppl:33-37.
15. Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36(1):1-10.
Recently, the U.S. Department of Homeland Security redefined disasters into 4 types: natural hazards, societal hazards, technologic hazards, and terrorism. The incidence of manmade and natural disasters is on the rise in intensity and frequency globally. Recent events such as tornadoes and hurricanes in the southeastern U.S., tsunamis in Japan, earthquakes in Haiti, wild fires, heat waves, and terrorist attacks like that of September 11, 2001, underscore the urgency of developing and maintaining solid local public health disaster response plans to minimize mortality and morbidity.
The 2010 BP oil spill in the Gulf of Mexico, the largest in history, hurricane Katrina, and the lingering impact of hurricane Sandy on the East Coast further raise concerns about our communities’ ability to handle disasters, especially in the early hours after events, when federally coordinated help is being organized and not yet fully available locally or from other nations.1 The recent fertilizer plant explosion in West Texas, the 2013 Boston marathon bombing, and the Newtown, Connecticut, massacre remind us of the unpredictable nature of both manmade and natural disasters.
Coordinated Response
Regardless of its origin, residents expect a coordinated local response during an emergency, and it is important that government agencies meet this expectation. Fulfilling these expectations, however, takes many partners, and it is important to have a clear idea of who is involved in emergency preparedness (EP) and the response of each partner’s role.
Role of Government
Federal, state, and local governments have a critical role in emergency management (EM). When state government, local government, or an individual entity is overwhelmed with a disaster, the role of the Federal Emergency Management Agency is to provide assistance and resources to cope with the emergency.2 Private industry and traditional disaster relief agencies, such as the American Red Cross and the Adventist Development and Relief Agency, are also involved in response efforts. Recent examples have shown that these partnerships are often overwhelmed with the needs of large regions experiencing limited resources. Therefore, hospitals and local public health departments frequently must carry much of the immediate burden of stabilizing communities and coordinating response with government agencies and local partners.3
Role of Public Health and the CDC
Federal agencies and local public health departments have been given critical roles in planning and responding to disasters. In particular, the PHS focuses on population care and shapes how public health entities should respond to mass casualty events and pandemics, including local response coordination. The CDC is primarily responsible for assisting state and local governments with disaster response and recovery after a large-scale public health emergency.3 The CDC works closely with local public health departments in decision making; tracking the source, spread, and severity of health threats; assessing impacts; educating the public on how to safeguard their health; and implementing measures to protect the public. During a large-scale health emergency, the CDC also maintains and provides resources through the maintenance and distribution of the nation’s Strategic National Stockpile of medications and supplies that may be needed during events such as the recent 2009 H1N1 influenza outbreak or other public health emergencies.3
Role of Local Businesses and Professional Institutions
Nationally, businesses and professional institutions are coming together and organizing in such a way that places them as part of the solution. More specifically, the National Voluntary Organizations Active in Disaster and Community Organizations Active in Disaster have grown exponentially since September 11, 2001.4 These efforts include but are not limited to development of EP plans and the subsequent sharing of those plans, sharing of key assets critical to response activities, development of a community key asset database, and training/exercise participation.
Role of Hospitals
The Hospital Preparedness Program was developed to prepare the nation’s health care system to respond appropriately to mass casualty incidents, whether due to bioterrorism, natural disaster, or other public health emergencies. Health care systems must be able to develop a disaster medical capability that is rapid, flexible, sustainable, integrated, coordinated, and capable of providing appropriate care in the most ethical manner with the resources and capabilities it has at its disposal.3 Although involved as first responders, traditionally, medical care systems, hospitals, physicians, and pharmacists are faced with the dual task of individual patient care and are thus more limited as partners in an overall local response system.
Also vital to this discussion is the reality that hospital emergency departments (EDs) already routinely operate at or above capacity, limiting their ability to prepare for mass casualties due to a public health disaster. Hospitals continue to divert more than half a million ambulances per year due to ED overcrowding.3 How they could step up in a true emergency situation is questionable at best.
Role of First Responders
Individuals who respond immediately are referred to as first responders. First responders come in 2 archetypes: those who are there purely based on unexpected circumstances and take action and those who are trained first responders, such as firefighters, police officers, and emergency medical technicians (EMTs). These first responders are trained to partner with one another. Firefighters primarily handle fire rescue as well as assessing the extent of potential damage to the area. Law enforcement’s responsibility is to restore order after an emergency, whether it is a natural disaster, community disturbance, or outbreak of hazardous chemicals. An EMT’s role is to attend to the immediate medical care of patients who have been injured or become ill during the emergency.5
Related: Disaster Preparedness for Veterans With Dementia and Their Caregivers
There are occasions where other potential incident responders, such as health care professionals, can play a key role and yet are not integrated into the emergency response. The VHA needs to focus on this facet in order to more effectively respond to events that threaten lives, property, and current infrastructure of the veterans it serves.
Role of CBOCs and Private Physician Practices
Community-based outpatient clinics (CBOCs), including outpatient community health centers and private physician practices (PPPs), maintain and improve routine community health but are rarely involved in routine planning for disasters. They are, therefore, typically not open for business or may have limited hours as they recover from the event. This results in patients who do not have access to their primary care providers (PCPs) turning to EDs, which are already at capacity. As a result, in a disaster the costly and overburdened ED functions as the PCP site for even larger populations affected by a disaster, including those who are uninsured.6,7
Kahan and colleagues reported that two-thirds of patients preferred their family doctor or health care authorities as their first choice for care instead of receiving care in the ED.8 Researchers found that 89% of physicians in private practice felt it was their responsibility to treat, for example, patients infected with anthrax.8 Some argue that if PCPs are included in planning and appropriately trained in disaster preparedness, their attitudes and willingness to participate in emergency services would follow.9
Given the many challenges to disaster preparedness, CBOCs could be a critical partner in EM, and interest continues to grow to explore that role. Health professionals in CBOCs who are trained in disaster management (DM) could become active participants in early intervention to initiate the treatment of patients in rescue efforts during a disaster.10 For instance, a CBOC could triage patients in a postdisaster situation, thus limiting the burden on hospital EDs by evaluating populations at risk and providing them with important information when communication is difficult.
This already existing network of community-based triage stations would offer natural locations to assess the health needs of the population and determine their level of appropriate medical care. Additionally, these clinics can ensure continuation of basic services after initial medical care has been completed in the hospital setting.10 Because clinics have not been included in coordinated DM, there is scant literature that addresses their potential role in disaster response. Community-based outpatient clinics and PPPs are untapped resources; however, it is unknown whether medical staff in these medical clinics have the interest, training, knowledge, skills, and resources in DM or whether barriers to providing safe care can be overcome.10
Case Study
The VHA is the largest integrated health care system in the U.S. It is mandated to serve as a backup to the DoD during disasters, and VHA CBOCs can play an important role.11,12 The CBOCs are staffed with a medical director, nurse manager, and other clinical and support staff. As a study population, CBOCs are well suited to examine and explore staff attitudes and roles in DM. To date, no research reports have been found studying EP in CBOCs.
The purpose of this study was to learn how to best integrate the CBOCs into disaster response. This qualitative study aimed to answer 3 questions: (1) How do VA clinic personnel perceive their personal and their clinic’s risk, level of preparedness, role, and knowledge for an active response in a disaster; (2) What do VA clinic personnel perceive they need in order to function in a disaster; and (3) What resources are necessary for clinic staff to function competently in a disaster?
Methods
In this qualitative study, in-depth semistructured key informant (KI) interviews (N = 3) and focus group discussions (N = 20) guided by risk perception theory and the Andersen Behavioral Model of Health Services Use were conducted and analyzed using grounded theory methods to contextualize the potential of local clinics in disaster response.13-15 To optimize breadth of viewpoints on this issue, participants were selected by theoretical sampling methods to explore perceptions of leadership and line staff.
Study Location
Health care providers and support staff from 3 southern California CBOCs that are contracted by the local VA to provide primary care services (ie, internal medicine, geriatrics, women’s health, mental health, and some specialty care services) to veterans were recruited for this study. The CBOCs are generally connected with a VHA local hospital in their region, offer services 5 days a week, and are closed on weekends and federal holidays. Some VA CBOCs participate in telehealth remote services connected to their regional hospital to help manage their patient populations. The CBOCs are managed by a medical director and a clinic manager and report to their respective VISN, and each VISN reports to the VHA Central Office in Washington, DC.13,15 The CBOC staff includes physicians, nurse practitioners, physician assistants, registered nurses (RNs), licensed vocational nurses (LVNs), medical assistants, front office staff, social workers, case managers, counselors, pharmacists, and nonclinical staff.
In this case, the CBOCs are contracted by Loma Linda University Health to manage care of the veterans and agree to care for nonveterans in a disaster. The CBOCs contracted or not all fall under the criteria as set forth in VHA Handbook 1006.1. This handbook criteria indicate that CBOCs must maintain appropriate emergency response capability. Additionally, VHA Handbook 0320.1 states that the CBOC is responsible for developing, implementing, evaluating, and improving a CBOC Comprehensive Emergency Management Program (CEMP) and for participating in the VAMC Emergency Management Committee. The scope of the VISN-wide CEMP integrates VAMC and VISN EM programs to coordinate and enhance operations during planned and unplanned events.
Study Design and Sample
After receiving institutional review board approval, 3 in-depth semi-structured clinic leadership KI interviews and 3 clinic staff (RNs, LVNs, health technicians, and nursing assistants) focus group discussions (N = 20, 1 per CBOC) to follow up on information gleaned from the analyses of the initial KIs were conducted. To provide continuity, all were conducted by the same trained facilitator who used a semistructured KI outline with questions and probes based on the guiding study framework.
Data Collection and Content Analysis
Interviews and focus group discussions were audio recorded and transcribed verbatim and then analyzed using grounded theory methods. Line-by-line coding was done to develop an initial inductive codebook, which was then organized into final codes. Once the codebook was developed, it was applied to all transcripts.
Related: Pre-Storm Dialysis Saves Lives
Transcripts and resulting codes were reviewed 3 times by independent reviewers to validate data, ensure accuracy, and delete any information that might identify participants. Pseudonyms were used to represent the participants by perspective (eg, nurse, MD) to avoid confusion in data analysis. A 4-stage data analysis approach was used: (1) immersion in the raw data by listening to tapes and reading manuscripts and notes in order to list key ideas and recurrent themes using a constant comparison method; (2) indexing by applying the thematic framework systematically to the data using and seeking new, unanticipated emerging codes; (3) arranging the data in codes and concepts/themes that represent the thematic framework of EP in clinics; (4) identifying a thematic framework for EP using codes that identified key issues, concepts, and themes that can be referenced and derived from the text.
Results
The Table describes the 4 primary emerging themes and corresponding quotes: (1) EP barriers, including lack of direction, training, and tools, which would result in negative outcomes; (2) perceived personal and clinic risk for a disaster, including negative outcomes and personal family safety; (3) perceptions of roles and responsibilities in EP, including intent to participate in DM at various staffing levels as well as patient expectations for care; and (4) existing resources that influence EP and the ability to survive a disaster collectively.
Emergency preparedness barriers. Although most respondents realized their potentially critical role in an emergency, they expressed recurrent barrier themes centered on their perceived lack of training, lack of tools to function, and lack of direction to be effective in a disaster response. Lack of knowledge of EP was identified as a great need by multiple participants. One participant stated, “Lack of information is so destructive. If you don’t know how to keep yourself from those things you don’t know…such as in a situation that’s going to be tragic, it is because of a lack of information or a lack of training. And I see that so many times…Mandate that we do our classes, so we know what we’re doing.” Another stated in reference to lack of skills, “I haven’t experienced any drills or anything like that. So I know what is going to happen here.”
Lack of abilities to communicate with key DM players also were identified. For example, “Downed power lines may result in no telephone connection to communicate next steps for critical issues, such as if evacuation of the clinic is required.” Another respondent indicated, “We need backup communication...devices, wind-up radios, or whatever.”
Lack of a clear disaster plan was also identified. Questions arose centered on details—how to actually implement a clinic response plan, including concerns that there were none, as the respondents “had not seen the plan in a couple of years” and were not sure who really was in charge of giving directions. Lack of community/organizational support voiced included aspects such as interdepartmental, facility, and community resource connectedness. There was acknowledgement that department assets should be clearly identified so that resource sharing might be used as part of the plan.
Last, regarding lack of resources, one participant said, “We don’t have the resources. We don’t have gurneys. We don’t have enough wheel chairs….We don’t have a crash cart. We don’t have the triage tarps or whatever for the triage of people; we don’t have any supplies to supply the energy room for diabetics, like what they have in the ER.”
Perceived personal and clinic risk for a disaster. Participants stated they felt at risk for natural disasters, including fire, floods, and earthquakes, but expressed concerns and even more fears about how they would handle a response to bombings, spills of hazardous materials, airplane accidents, and gunfire, which also qualify as disasters but are much harder to prepare for, because they could be so varied. One participated stated, “They are so unpredictable whether it is an earthquake or a fire…they are unpredictable….We see planes that fly close to our window and we wonder about the possibility of a crash—you never know.”
Many staff members expressed fear of what these disasters would mean to them in the clinic and to their patients. Another comment shared was, “I don’t think anybody really thinks about this kind of stuff until it happens and then it is too late…If we had just done this or that or knew how to do this or that then…” The biggest fear expressed was that of a massive earthquake in which there would be power outages and resulting fires, blocked building exits, and no way to get to evacuation areas. Fears expressed included working with people who are dying and trying to get the patients down the stairs and out of the disaster area.
Personal safety in a disaster was also a concern; a nurse stated, “Your personal safety is a priority. Yourself, that is first, if you are not safe, you can’t do any good to anyone else.” Another shared concern was the safety of family members during a disaster and conflicting obligations between duties at work and protecting family members. Participants felt they would want to be at home with their families.
Perceptions of roles and responsibilities in EP. Supervisors of the clinics shared that their primary responsibility is to the staff and their current patients; ensuring their safety was a top priority. Their knowledge, skills, and available resources were crucial to their duties, including establishing methods of communication outside the clinic for advice and direction, such as notifying the power company and other outside agencies of the condition of the clinic. They felt that their duties included making sure generators were working, ensuring telephones and lighting were available, and advising staff when to leave the building. One manager stated that more EP discussions need to happen in order to determine how to react: “...in event of a disaster it is important to control patient flow, staffing the clinic appropriately and managing the employees.” They felt a need to help empower their staff by making sure staff were trained in EP tasks and that they could complete the tasks they were required to perform.
Staff consistently reported that the doctors were in charge of providing direction concerning activities and care of the patients. However, most were able to identify their own role in helping preserve lives and keeping the patients and other staff safe. One nurse stated, “My job would be to evacuate the physicians’ offices, to make sure they are aware of the disaster, get them out safely, put an X on their door, keep the patients calm and guide them out to the designated area, then look out for medics or other help so that they would be directed to the correct locations.” Another staff nurse stated, “My role is to check the bathrooms and then under the direction of the physician assist in the care of patient injuries.”
When asked about the expectations of patients for care during a disaster, staff consistently stated that patients and their families would want to get care and direction from clinic staff who knew them instead of going to the hospital for care. Staff anticipated that patients would be calling the clinic first to discuss their medical problems. One stated, “The veterans would head to us…. We can’t turn them away.” Some staff indicated that some patients might have to go to the ED for care instead of coming to the clinic, because the clinic may not be equipped to respond, noting that “we have to remind [the patients] that in our clinic we have minimal abilities.”
Existing resources. Consistently, the respondents verbalized the importance of acquiring knowledge and skills and using available resources in their disaster plan. They felt that training was critical and that it needed to be simple and uncomplicated. Many felt that they did not have sufficient drills to maintain their knowledge and skills for all types of events. One nursing assistant stated he had extensive training in the military in DM, but clinics did not have sufficient training and were not prepared to handle multiple casualties. Others stated that it would be important for training to be “second nature” so they would not have to think much about it, with everyone pulling together and performing tasks seamlessly. However, some stated that they did not know what to do in an emergency.
Critical resources noted were access to emergency power sources, transistor radios, telephone and communication, 911 services, backup phone services, computers, and text pagers and cell phones so that connections could be made outside the clinic setting. Other critical resources needed included medical supplies and access to food for 1 week.
Finally, teamwork was identified as a critical factor for success. One example involved the clinic responding to a severe snowstorm; the medical director, lead nurse, and support staff agreed to remain on site to assist with any patients who needed help. “We shared our 4-wheel drive trucks to get around, and others called patients, advising them of storm conditions and what to do to maintain care at home and canceled appointments scheduled for that day.” They were very proud of the way they had pooled their resources to support each other and their patients.
Based on these emerging themes and the inquiry guiding theories, a theoretical framework was proposed on how contributing factors influenced the process by which CBOC staff viewed their roles and the likelihood that they would participate in a disaster plan (Figure). The framework suggests that personal risks and perceived personal and clinic readiness to respond to an emergency were critical barriers to staff willingness to get involved in preparedness, whereas they saw the provision of training and resources as necessary to increase their resilience and ability to function in a disaster.
Clearly addressing barriers through training, planning, ensuring that resources functioned effectively in a disaster, and clarifying roles and responsibilities, combined with promoting personal and clinic readiness facilitated staff EP participation.
Discussion
This qualitative study explored issues surrounding the role of CBOCs in EP and how risk perception and enabling factors contributed to staff intent to participate in DM. As in many qualitative studies, findings were somewhat limited by an overall small sample size (N = 23) across 3 CBOCs in southern California. However, given the lack of available literature, the authors believe that this study helped provide critical insight into CBOC clinic staff’s willingness and readiness to be active in disaster response. The study clearly points to clinic staff’s openness to actively take part in regional disaster response and calls for better and more standardized approaches to EP and DM planning that include local CBOCs. The authors identified factors that contribute to staff intent to participate in DM and the need to reduce barriers that hinder participation.
In general, clinic staff who reported feeling inadequately prepared for disasters (ie, felt more vulnerable) and staff with firsthand disaster experience were more inclined to prepare than were those without experience. Without clearly spelled-out expectations, staff tend to depend and wait on others to lead in a disaster. They noted a desire for better preparation and thus, clarity of roles, need for a reliable method of communication with the outside world during a disaster, and the required equipment and supplies for self-care or care of the patients for ≥ 3 days post disaster. Some indicated that they did not have the resources to provide medical care on the scale that may be required.
Many did not have a clear understanding of an all-hazard approach plan and had not been involved in hazard assessments. Already tightly staffed for personal health care delivery, staff spent minimal time and energy thinking about the risk of a disaster or preparing for one. However, there seemed to be a direct relationship between the attitude of the supervisor and the attitudes of clinic staff to EP. Although these qualitative results are encouraging and point to these clinics as an important undertapped resource for EP, further quantitative studies should expand this inquiry.
Lessons learned from this study include the need to expand qualitative data collection to include a larger sample size to retrieve information that would contribute to a better understanding of how staff view their roles in DM. There are 152 VAMCs and hundreds of associated CBOCs that should be queried as to their EM readiness. Also, replicating this study in non-VHA clinics, such as private CBOCs and PPPs, might bring greater insight into what is needed to involve them in DM plans. Finally, future studies should determine clearer criteria when care can be provided at a clinic and when it would be appropriate for the patient to report at their local ED.
Conclusions and Recommendations
Given the VHA EP mandate, the authors recommend the following steps to address barriers identified in this study: (1) Develop a more structured approach to DM in a CBOC setting to provide staff with a clear understanding of their roles and responsibilities; (2) Conduct a comprehensive assessment of each clinic to determine staff knowledge, skills, and resources required to provide EP and institute a DM training curriculum; (3) Provide clinic leadership with direction on developing a disaster plan as well as how to partner with their primary and local VA health care system, especially onsite physicians, to provide effective DM leadership; (4) Recruit staff into routine drills for natural disasters and expand to an all-hazard approach to manmade disasters to identify gaps in delivering DM in a disaster; (5) Facilitate partnerships and a standardized approach to DM between CBOCs within the VISN by scheduling routine video and teleconferencing, live meetings, and webinars so that procedures and language are clearly understood and communicated between facilities; and (6) Identify key barriers to clinic preparedness by assessing EP elements through mock disaster drills and offer solutions to fill DM gaps.
The authors also recommend that CBOCs should be included in community DM and EP plans in order to understand how to integrate resources in a disaster. Networking, planning, and interdisciplinary staff training between agencies to include CBOCs will bring a wealth of information of what CBOCs require to participate effectively in DM. Lessons learned from these partnerships can provide valuable information to facilitate resource allocation for acute care hospitals, which may be burdened with treating patients with minor medical issues when they should be focusing on providing care to those with catastrophic medical conditions.
Acknowledgments
This study and this material is the result of work supported with resources and the use of facilities at the VA Loma Linda Health Care System. Research in this publication was in part supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under award number P20MD006988.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Recently, the U.S. Department of Homeland Security redefined disasters into 4 types: natural hazards, societal hazards, technologic hazards, and terrorism. The incidence of manmade and natural disasters is on the rise in intensity and frequency globally. Recent events such as tornadoes and hurricanes in the southeastern U.S., tsunamis in Japan, earthquakes in Haiti, wild fires, heat waves, and terrorist attacks like that of September 11, 2001, underscore the urgency of developing and maintaining solid local public health disaster response plans to minimize mortality and morbidity.
The 2010 BP oil spill in the Gulf of Mexico, the largest in history, hurricane Katrina, and the lingering impact of hurricane Sandy on the East Coast further raise concerns about our communities’ ability to handle disasters, especially in the early hours after events, when federally coordinated help is being organized and not yet fully available locally or from other nations.1 The recent fertilizer plant explosion in West Texas, the 2013 Boston marathon bombing, and the Newtown, Connecticut, massacre remind us of the unpredictable nature of both manmade and natural disasters.
Coordinated Response
Regardless of its origin, residents expect a coordinated local response during an emergency, and it is important that government agencies meet this expectation. Fulfilling these expectations, however, takes many partners, and it is important to have a clear idea of who is involved in emergency preparedness (EP) and the response of each partner’s role.
Role of Government
Federal, state, and local governments have a critical role in emergency management (EM). When state government, local government, or an individual entity is overwhelmed with a disaster, the role of the Federal Emergency Management Agency is to provide assistance and resources to cope with the emergency.2 Private industry and traditional disaster relief agencies, such as the American Red Cross and the Adventist Development and Relief Agency, are also involved in response efforts. Recent examples have shown that these partnerships are often overwhelmed with the needs of large regions experiencing limited resources. Therefore, hospitals and local public health departments frequently must carry much of the immediate burden of stabilizing communities and coordinating response with government agencies and local partners.3
Role of Public Health and the CDC
Federal agencies and local public health departments have been given critical roles in planning and responding to disasters. In particular, the PHS focuses on population care and shapes how public health entities should respond to mass casualty events and pandemics, including local response coordination. The CDC is primarily responsible for assisting state and local governments with disaster response and recovery after a large-scale public health emergency.3 The CDC works closely with local public health departments in decision making; tracking the source, spread, and severity of health threats; assessing impacts; educating the public on how to safeguard their health; and implementing measures to protect the public. During a large-scale health emergency, the CDC also maintains and provides resources through the maintenance and distribution of the nation’s Strategic National Stockpile of medications and supplies that may be needed during events such as the recent 2009 H1N1 influenza outbreak or other public health emergencies.3
Role of Local Businesses and Professional Institutions
Nationally, businesses and professional institutions are coming together and organizing in such a way that places them as part of the solution. More specifically, the National Voluntary Organizations Active in Disaster and Community Organizations Active in Disaster have grown exponentially since September 11, 2001.4 These efforts include but are not limited to development of EP plans and the subsequent sharing of those plans, sharing of key assets critical to response activities, development of a community key asset database, and training/exercise participation.
Role of Hospitals
The Hospital Preparedness Program was developed to prepare the nation’s health care system to respond appropriately to mass casualty incidents, whether due to bioterrorism, natural disaster, or other public health emergencies. Health care systems must be able to develop a disaster medical capability that is rapid, flexible, sustainable, integrated, coordinated, and capable of providing appropriate care in the most ethical manner with the resources and capabilities it has at its disposal.3 Although involved as first responders, traditionally, medical care systems, hospitals, physicians, and pharmacists are faced with the dual task of individual patient care and are thus more limited as partners in an overall local response system.
Also vital to this discussion is the reality that hospital emergency departments (EDs) already routinely operate at or above capacity, limiting their ability to prepare for mass casualties due to a public health disaster. Hospitals continue to divert more than half a million ambulances per year due to ED overcrowding.3 How they could step up in a true emergency situation is questionable at best.
Role of First Responders
Individuals who respond immediately are referred to as first responders. First responders come in 2 archetypes: those who are there purely based on unexpected circumstances and take action and those who are trained first responders, such as firefighters, police officers, and emergency medical technicians (EMTs). These first responders are trained to partner with one another. Firefighters primarily handle fire rescue as well as assessing the extent of potential damage to the area. Law enforcement’s responsibility is to restore order after an emergency, whether it is a natural disaster, community disturbance, or outbreak of hazardous chemicals. An EMT’s role is to attend to the immediate medical care of patients who have been injured or become ill during the emergency.5
Related: Disaster Preparedness for Veterans With Dementia and Their Caregivers
There are occasions where other potential incident responders, such as health care professionals, can play a key role and yet are not integrated into the emergency response. The VHA needs to focus on this facet in order to more effectively respond to events that threaten lives, property, and current infrastructure of the veterans it serves.
Role of CBOCs and Private Physician Practices
Community-based outpatient clinics (CBOCs), including outpatient community health centers and private physician practices (PPPs), maintain and improve routine community health but are rarely involved in routine planning for disasters. They are, therefore, typically not open for business or may have limited hours as they recover from the event. This results in patients who do not have access to their primary care providers (PCPs) turning to EDs, which are already at capacity. As a result, in a disaster the costly and overburdened ED functions as the PCP site for even larger populations affected by a disaster, including those who are uninsured.6,7
Kahan and colleagues reported that two-thirds of patients preferred their family doctor or health care authorities as their first choice for care instead of receiving care in the ED.8 Researchers found that 89% of physicians in private practice felt it was their responsibility to treat, for example, patients infected with anthrax.8 Some argue that if PCPs are included in planning and appropriately trained in disaster preparedness, their attitudes and willingness to participate in emergency services would follow.9
Given the many challenges to disaster preparedness, CBOCs could be a critical partner in EM, and interest continues to grow to explore that role. Health professionals in CBOCs who are trained in disaster management (DM) could become active participants in early intervention to initiate the treatment of patients in rescue efforts during a disaster.10 For instance, a CBOC could triage patients in a postdisaster situation, thus limiting the burden on hospital EDs by evaluating populations at risk and providing them with important information when communication is difficult.
This already existing network of community-based triage stations would offer natural locations to assess the health needs of the population and determine their level of appropriate medical care. Additionally, these clinics can ensure continuation of basic services after initial medical care has been completed in the hospital setting.10 Because clinics have not been included in coordinated DM, there is scant literature that addresses their potential role in disaster response. Community-based outpatient clinics and PPPs are untapped resources; however, it is unknown whether medical staff in these medical clinics have the interest, training, knowledge, skills, and resources in DM or whether barriers to providing safe care can be overcome.10
Case Study
The VHA is the largest integrated health care system in the U.S. It is mandated to serve as a backup to the DoD during disasters, and VHA CBOCs can play an important role.11,12 The CBOCs are staffed with a medical director, nurse manager, and other clinical and support staff. As a study population, CBOCs are well suited to examine and explore staff attitudes and roles in DM. To date, no research reports have been found studying EP in CBOCs.
The purpose of this study was to learn how to best integrate the CBOCs into disaster response. This qualitative study aimed to answer 3 questions: (1) How do VA clinic personnel perceive their personal and their clinic’s risk, level of preparedness, role, and knowledge for an active response in a disaster; (2) What do VA clinic personnel perceive they need in order to function in a disaster; and (3) What resources are necessary for clinic staff to function competently in a disaster?
Methods
In this qualitative study, in-depth semistructured key informant (KI) interviews (N = 3) and focus group discussions (N = 20) guided by risk perception theory and the Andersen Behavioral Model of Health Services Use were conducted and analyzed using grounded theory methods to contextualize the potential of local clinics in disaster response.13-15 To optimize breadth of viewpoints on this issue, participants were selected by theoretical sampling methods to explore perceptions of leadership and line staff.
Study Location
Health care providers and support staff from 3 southern California CBOCs that are contracted by the local VA to provide primary care services (ie, internal medicine, geriatrics, women’s health, mental health, and some specialty care services) to veterans were recruited for this study. The CBOCs are generally connected with a VHA local hospital in their region, offer services 5 days a week, and are closed on weekends and federal holidays. Some VA CBOCs participate in telehealth remote services connected to their regional hospital to help manage their patient populations. The CBOCs are managed by a medical director and a clinic manager and report to their respective VISN, and each VISN reports to the VHA Central Office in Washington, DC.13,15 The CBOC staff includes physicians, nurse practitioners, physician assistants, registered nurses (RNs), licensed vocational nurses (LVNs), medical assistants, front office staff, social workers, case managers, counselors, pharmacists, and nonclinical staff.
In this case, the CBOCs are contracted by Loma Linda University Health to manage care of the veterans and agree to care for nonveterans in a disaster. The CBOCs contracted or not all fall under the criteria as set forth in VHA Handbook 1006.1. This handbook criteria indicate that CBOCs must maintain appropriate emergency response capability. Additionally, VHA Handbook 0320.1 states that the CBOC is responsible for developing, implementing, evaluating, and improving a CBOC Comprehensive Emergency Management Program (CEMP) and for participating in the VAMC Emergency Management Committee. The scope of the VISN-wide CEMP integrates VAMC and VISN EM programs to coordinate and enhance operations during planned and unplanned events.
Study Design and Sample
After receiving institutional review board approval, 3 in-depth semi-structured clinic leadership KI interviews and 3 clinic staff (RNs, LVNs, health technicians, and nursing assistants) focus group discussions (N = 20, 1 per CBOC) to follow up on information gleaned from the analyses of the initial KIs were conducted. To provide continuity, all were conducted by the same trained facilitator who used a semistructured KI outline with questions and probes based on the guiding study framework.
Data Collection and Content Analysis
Interviews and focus group discussions were audio recorded and transcribed verbatim and then analyzed using grounded theory methods. Line-by-line coding was done to develop an initial inductive codebook, which was then organized into final codes. Once the codebook was developed, it was applied to all transcripts.
Related: Pre-Storm Dialysis Saves Lives
Transcripts and resulting codes were reviewed 3 times by independent reviewers to validate data, ensure accuracy, and delete any information that might identify participants. Pseudonyms were used to represent the participants by perspective (eg, nurse, MD) to avoid confusion in data analysis. A 4-stage data analysis approach was used: (1) immersion in the raw data by listening to tapes and reading manuscripts and notes in order to list key ideas and recurrent themes using a constant comparison method; (2) indexing by applying the thematic framework systematically to the data using and seeking new, unanticipated emerging codes; (3) arranging the data in codes and concepts/themes that represent the thematic framework of EP in clinics; (4) identifying a thematic framework for EP using codes that identified key issues, concepts, and themes that can be referenced and derived from the text.
Results
The Table describes the 4 primary emerging themes and corresponding quotes: (1) EP barriers, including lack of direction, training, and tools, which would result in negative outcomes; (2) perceived personal and clinic risk for a disaster, including negative outcomes and personal family safety; (3) perceptions of roles and responsibilities in EP, including intent to participate in DM at various staffing levels as well as patient expectations for care; and (4) existing resources that influence EP and the ability to survive a disaster collectively.
Emergency preparedness barriers. Although most respondents realized their potentially critical role in an emergency, they expressed recurrent barrier themes centered on their perceived lack of training, lack of tools to function, and lack of direction to be effective in a disaster response. Lack of knowledge of EP was identified as a great need by multiple participants. One participant stated, “Lack of information is so destructive. If you don’t know how to keep yourself from those things you don’t know…such as in a situation that’s going to be tragic, it is because of a lack of information or a lack of training. And I see that so many times…Mandate that we do our classes, so we know what we’re doing.” Another stated in reference to lack of skills, “I haven’t experienced any drills or anything like that. So I know what is going to happen here.”
Lack of abilities to communicate with key DM players also were identified. For example, “Downed power lines may result in no telephone connection to communicate next steps for critical issues, such as if evacuation of the clinic is required.” Another respondent indicated, “We need backup communication...devices, wind-up radios, or whatever.”
Lack of a clear disaster plan was also identified. Questions arose centered on details—how to actually implement a clinic response plan, including concerns that there were none, as the respondents “had not seen the plan in a couple of years” and were not sure who really was in charge of giving directions. Lack of community/organizational support voiced included aspects such as interdepartmental, facility, and community resource connectedness. There was acknowledgement that department assets should be clearly identified so that resource sharing might be used as part of the plan.
Last, regarding lack of resources, one participant said, “We don’t have the resources. We don’t have gurneys. We don’t have enough wheel chairs….We don’t have a crash cart. We don’t have the triage tarps or whatever for the triage of people; we don’t have any supplies to supply the energy room for diabetics, like what they have in the ER.”
Perceived personal and clinic risk for a disaster. Participants stated they felt at risk for natural disasters, including fire, floods, and earthquakes, but expressed concerns and even more fears about how they would handle a response to bombings, spills of hazardous materials, airplane accidents, and gunfire, which also qualify as disasters but are much harder to prepare for, because they could be so varied. One participated stated, “They are so unpredictable whether it is an earthquake or a fire…they are unpredictable….We see planes that fly close to our window and we wonder about the possibility of a crash—you never know.”
Many staff members expressed fear of what these disasters would mean to them in the clinic and to their patients. Another comment shared was, “I don’t think anybody really thinks about this kind of stuff until it happens and then it is too late…If we had just done this or that or knew how to do this or that then…” The biggest fear expressed was that of a massive earthquake in which there would be power outages and resulting fires, blocked building exits, and no way to get to evacuation areas. Fears expressed included working with people who are dying and trying to get the patients down the stairs and out of the disaster area.
Personal safety in a disaster was also a concern; a nurse stated, “Your personal safety is a priority. Yourself, that is first, if you are not safe, you can’t do any good to anyone else.” Another shared concern was the safety of family members during a disaster and conflicting obligations between duties at work and protecting family members. Participants felt they would want to be at home with their families.
Perceptions of roles and responsibilities in EP. Supervisors of the clinics shared that their primary responsibility is to the staff and their current patients; ensuring their safety was a top priority. Their knowledge, skills, and available resources were crucial to their duties, including establishing methods of communication outside the clinic for advice and direction, such as notifying the power company and other outside agencies of the condition of the clinic. They felt that their duties included making sure generators were working, ensuring telephones and lighting were available, and advising staff when to leave the building. One manager stated that more EP discussions need to happen in order to determine how to react: “...in event of a disaster it is important to control patient flow, staffing the clinic appropriately and managing the employees.” They felt a need to help empower their staff by making sure staff were trained in EP tasks and that they could complete the tasks they were required to perform.
Staff consistently reported that the doctors were in charge of providing direction concerning activities and care of the patients. However, most were able to identify their own role in helping preserve lives and keeping the patients and other staff safe. One nurse stated, “My job would be to evacuate the physicians’ offices, to make sure they are aware of the disaster, get them out safely, put an X on their door, keep the patients calm and guide them out to the designated area, then look out for medics or other help so that they would be directed to the correct locations.” Another staff nurse stated, “My role is to check the bathrooms and then under the direction of the physician assist in the care of patient injuries.”
When asked about the expectations of patients for care during a disaster, staff consistently stated that patients and their families would want to get care and direction from clinic staff who knew them instead of going to the hospital for care. Staff anticipated that patients would be calling the clinic first to discuss their medical problems. One stated, “The veterans would head to us…. We can’t turn them away.” Some staff indicated that some patients might have to go to the ED for care instead of coming to the clinic, because the clinic may not be equipped to respond, noting that “we have to remind [the patients] that in our clinic we have minimal abilities.”
Existing resources. Consistently, the respondents verbalized the importance of acquiring knowledge and skills and using available resources in their disaster plan. They felt that training was critical and that it needed to be simple and uncomplicated. Many felt that they did not have sufficient drills to maintain their knowledge and skills for all types of events. One nursing assistant stated he had extensive training in the military in DM, but clinics did not have sufficient training and were not prepared to handle multiple casualties. Others stated that it would be important for training to be “second nature” so they would not have to think much about it, with everyone pulling together and performing tasks seamlessly. However, some stated that they did not know what to do in an emergency.
Critical resources noted were access to emergency power sources, transistor radios, telephone and communication, 911 services, backup phone services, computers, and text pagers and cell phones so that connections could be made outside the clinic setting. Other critical resources needed included medical supplies and access to food for 1 week.
Finally, teamwork was identified as a critical factor for success. One example involved the clinic responding to a severe snowstorm; the medical director, lead nurse, and support staff agreed to remain on site to assist with any patients who needed help. “We shared our 4-wheel drive trucks to get around, and others called patients, advising them of storm conditions and what to do to maintain care at home and canceled appointments scheduled for that day.” They were very proud of the way they had pooled their resources to support each other and their patients.
Based on these emerging themes and the inquiry guiding theories, a theoretical framework was proposed on how contributing factors influenced the process by which CBOC staff viewed their roles and the likelihood that they would participate in a disaster plan (Figure). The framework suggests that personal risks and perceived personal and clinic readiness to respond to an emergency were critical barriers to staff willingness to get involved in preparedness, whereas they saw the provision of training and resources as necessary to increase their resilience and ability to function in a disaster.
Clearly addressing barriers through training, planning, ensuring that resources functioned effectively in a disaster, and clarifying roles and responsibilities, combined with promoting personal and clinic readiness facilitated staff EP participation.
Discussion
This qualitative study explored issues surrounding the role of CBOCs in EP and how risk perception and enabling factors contributed to staff intent to participate in DM. As in many qualitative studies, findings were somewhat limited by an overall small sample size (N = 23) across 3 CBOCs in southern California. However, given the lack of available literature, the authors believe that this study helped provide critical insight into CBOC clinic staff’s willingness and readiness to be active in disaster response. The study clearly points to clinic staff’s openness to actively take part in regional disaster response and calls for better and more standardized approaches to EP and DM planning that include local CBOCs. The authors identified factors that contribute to staff intent to participate in DM and the need to reduce barriers that hinder participation.
In general, clinic staff who reported feeling inadequately prepared for disasters (ie, felt more vulnerable) and staff with firsthand disaster experience were more inclined to prepare than were those without experience. Without clearly spelled-out expectations, staff tend to depend and wait on others to lead in a disaster. They noted a desire for better preparation and thus, clarity of roles, need for a reliable method of communication with the outside world during a disaster, and the required equipment and supplies for self-care or care of the patients for ≥ 3 days post disaster. Some indicated that they did not have the resources to provide medical care on the scale that may be required.
Many did not have a clear understanding of an all-hazard approach plan and had not been involved in hazard assessments. Already tightly staffed for personal health care delivery, staff spent minimal time and energy thinking about the risk of a disaster or preparing for one. However, there seemed to be a direct relationship between the attitude of the supervisor and the attitudes of clinic staff to EP. Although these qualitative results are encouraging and point to these clinics as an important undertapped resource for EP, further quantitative studies should expand this inquiry.
Lessons learned from this study include the need to expand qualitative data collection to include a larger sample size to retrieve information that would contribute to a better understanding of how staff view their roles in DM. There are 152 VAMCs and hundreds of associated CBOCs that should be queried as to their EM readiness. Also, replicating this study in non-VHA clinics, such as private CBOCs and PPPs, might bring greater insight into what is needed to involve them in DM plans. Finally, future studies should determine clearer criteria when care can be provided at a clinic and when it would be appropriate for the patient to report at their local ED.
Conclusions and Recommendations
Given the VHA EP mandate, the authors recommend the following steps to address barriers identified in this study: (1) Develop a more structured approach to DM in a CBOC setting to provide staff with a clear understanding of their roles and responsibilities; (2) Conduct a comprehensive assessment of each clinic to determine staff knowledge, skills, and resources required to provide EP and institute a DM training curriculum; (3) Provide clinic leadership with direction on developing a disaster plan as well as how to partner with their primary and local VA health care system, especially onsite physicians, to provide effective DM leadership; (4) Recruit staff into routine drills for natural disasters and expand to an all-hazard approach to manmade disasters to identify gaps in delivering DM in a disaster; (5) Facilitate partnerships and a standardized approach to DM between CBOCs within the VISN by scheduling routine video and teleconferencing, live meetings, and webinars so that procedures and language are clearly understood and communicated between facilities; and (6) Identify key barriers to clinic preparedness by assessing EP elements through mock disaster drills and offer solutions to fill DM gaps.
The authors also recommend that CBOCs should be included in community DM and EP plans in order to understand how to integrate resources in a disaster. Networking, planning, and interdisciplinary staff training between agencies to include CBOCs will bring a wealth of information of what CBOCs require to participate effectively in DM. Lessons learned from these partnerships can provide valuable information to facilitate resource allocation for acute care hospitals, which may be burdened with treating patients with minor medical issues when they should be focusing on providing care to those with catastrophic medical conditions.
Acknowledgments
This study and this material is the result of work supported with resources and the use of facilities at the VA Loma Linda Health Care System. Research in this publication was in part supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under award number P20MD006988.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. McNeill JB, Carafano JJ, Mayer MA, Weitz R. Accepting disaster relief from nations: lessons from Katrina and Gulf oil spill. The Heritage Foundation Website. http://www.heritage.org/research/reports/2011/02/accepting-disaster-relief-from-other-nations-lessons-from-katrina-and-the-gulf-oil-spill. Published February 17, 2011. Accessed July 16, 2015.
2. Haddow GD, Bullock JA. Introduction to Emergency Management. 2nd ed. Burlington, MA: Butterworth-Heinemann; 2006.
3. Institute of Medicine. Medical Surge Capacity: Workshop Summary. Washington, DC: The National Academies Press; 2010.
4. National Voluntary Organizations Active in Disasters. Federal Emergency Management Agency Website. http://www.ready.gov/voluntary-organizations-active-disaster. Updated June 19, 2014. Accessed July 10, 2015.
5. What is the role of police, fire and EMS after a natural disaster strikes? Galls Website. http://gallsblog.com/2011/08/29/what-is-the-role-of-police-fire-and-ems-after-a-natural-disaster-strikes. Published August 29, 2011. Accessed July 14, 2015.
6. Hogan DE, Waeckerle JF, Dire DJ, Lillibridge SR. Emergency department impact of the Oklahoma City terrorist bombing. Ann Emerg Med. 1999;34(2):160-167.
7. Carlson JN, Menegazzi JJ, Callaway CW. Magnitude of national ED visits and resource utilization by the uninsured. Am J Emerg Med. 2013;31(4):722-726.
8. Kahan E, Fogelman Y, Kitai E, Vinker S. Patient and family physician p for care and communication in the eventuality of anthrax terrorism. Fam Pract. 2003;20(4):441-442.
9. Chen FM, Hickner J, Fink KS, Galliher JM, Burstin H. On the front lines: family physicians’ preparedness for bioterrorism. J Fam Pract. 2002;51(9):745-750.
10. Wood K. Community health centers: the untapped resource for public health and medical preparedness. Homeland Secur Aff. 2009;5(8):113.
11. Koenig KL. Homeland security and public health: role of the Department of Veterans Affairs, the US Department of Homeland Security, and implications for the public health community. Prehosp Disaster Med. 2003;18(4):327-333.
12. Panangala SV, Mendez BHP. Veterans Health Administration: Community-Based Outpatient Clinics. Washington, DC: Library of Congress, Congressional Research Service; 2010.
13. Tashakkori A, Teddlie C, eds. Handbook of Mixed Methods in Social & Behavioral Research. Thousand Oaks, CA: Sage Publications, Inc.; 2003.
14. Barnett DJ, Balicer RD, Blodgett DW, et al. Applying risk perception theory to public health workforce preparedness training. J Public Health Manag Pract. 2005;Suppl:33-37.
15. Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36(1):1-10.
1. McNeill JB, Carafano JJ, Mayer MA, Weitz R. Accepting disaster relief from nations: lessons from Katrina and Gulf oil spill. The Heritage Foundation Website. http://www.heritage.org/research/reports/2011/02/accepting-disaster-relief-from-other-nations-lessons-from-katrina-and-the-gulf-oil-spill. Published February 17, 2011. Accessed July 16, 2015.
2. Haddow GD, Bullock JA. Introduction to Emergency Management. 2nd ed. Burlington, MA: Butterworth-Heinemann; 2006.
3. Institute of Medicine. Medical Surge Capacity: Workshop Summary. Washington, DC: The National Academies Press; 2010.
4. National Voluntary Organizations Active in Disasters. Federal Emergency Management Agency Website. http://www.ready.gov/voluntary-organizations-active-disaster. Updated June 19, 2014. Accessed July 10, 2015.
5. What is the role of police, fire and EMS after a natural disaster strikes? Galls Website. http://gallsblog.com/2011/08/29/what-is-the-role-of-police-fire-and-ems-after-a-natural-disaster-strikes. Published August 29, 2011. Accessed July 14, 2015.
6. Hogan DE, Waeckerle JF, Dire DJ, Lillibridge SR. Emergency department impact of the Oklahoma City terrorist bombing. Ann Emerg Med. 1999;34(2):160-167.
7. Carlson JN, Menegazzi JJ, Callaway CW. Magnitude of national ED visits and resource utilization by the uninsured. Am J Emerg Med. 2013;31(4):722-726.
8. Kahan E, Fogelman Y, Kitai E, Vinker S. Patient and family physician p for care and communication in the eventuality of anthrax terrorism. Fam Pract. 2003;20(4):441-442.
9. Chen FM, Hickner J, Fink KS, Galliher JM, Burstin H. On the front lines: family physicians’ preparedness for bioterrorism. J Fam Pract. 2002;51(9):745-750.
10. Wood K. Community health centers: the untapped resource for public health and medical preparedness. Homeland Secur Aff. 2009;5(8):113.
11. Koenig KL. Homeland security and public health: role of the Department of Veterans Affairs, the US Department of Homeland Security, and implications for the public health community. Prehosp Disaster Med. 2003;18(4):327-333.
12. Panangala SV, Mendez BHP. Veterans Health Administration: Community-Based Outpatient Clinics. Washington, DC: Library of Congress, Congressional Research Service; 2010.
13. Tashakkori A, Teddlie C, eds. Handbook of Mixed Methods in Social & Behavioral Research. Thousand Oaks, CA: Sage Publications, Inc.; 2003.
14. Barnett DJ, Balicer RD, Blodgett DW, et al. Applying risk perception theory to public health workforce preparedness training. J Public Health Manag Pract. 2005;Suppl:33-37.
15. Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36(1):1-10.
Colonic Dyspnea and the Morgagni Hernia: A Rare Adult Diagnosis
Congenital diaphragmatic hernias (CDHs) occur from a disruption in the muscular formation of the diaphragm, resulting in herniation of abdominal contents into the thoracic cavity. A rare diagnosis, most cases are identified in the pediatric and neonatal populations with an overall historical 50% mortality related to the diagnosis.1 More recent data published in the U.S. and Japan cite an overall survival rate of 67% to 80% secondary to improved understanding of the pathophysiology and subsequent enhancement of neonatal cardiopulmonary support adjuncts.2,3
Bochladek hernias (posterolateral space) are the most common presentation of CDH, accounting for > 90% of cases. First described by the Giovanni Batista Morgagni in On the Seats and Causes of Disease Investigated by Anatomy, the anteromedial sternocostal location is far less common and accounts for only 2% to 3% of cases.4,5 More commonly found on the right side of the diaphragm, despite protection from the liver, the right-sided space has been traditionally referred to as the Morgagni space. A left-sided defect is occasionally called the Larrey gap or space, after Napoleon’s surgeon who described the space as a potential location for pericardial drainage of tamponade.6,7
Related: Colonoscopy Bowel Preparation Instructions
There are a few congenital conditions, such as trisomy 21, Turner syndrome, Prader Willi syndrome, dextrocardia, and Tetralogy of Fallot, that have been associated with Morgagni hernias.7 Pulmonary hypertension and respiratory distress are the most common symptoms for neonatal patients; chest pain, sensations of tightness/fullness, reflux, and transient obstructive symptoms constitute the typical symptoms of adult patients with CDH. In this case study, the authors present a case of adult-onset Morgagni hernia as well as a review of the relevant literature.
Case Report
The patient was a 48-year-old man on active-duty who presented to the Naval Medical Center Portsmouth General Surgery clinic in Virginia with a 4-year history of gastroesophageal reflux-related symptoms. Specifically, he reported epigastric fullness, pyrosis, and discomfort that radiated toward his bilateral lower ribs for the previous 4 years. This discomfort was typically associated with the intake of solid food and was followed a few hours later by a loose bowel movement.
The patient was initially treated with antacids and proton pump inhibitors by his primary care physician, with only minimal relief. He also reported several months of chronic cough as well as intermittent episodes of “gasping air hunger” for about 6 years, which had been incidentally brought up during his separation physical examination. A chest X-ray performed during the workup revealed findings suggesting a right diaphragmatic hernia vs a bronchogenic cyst (Figure 1). A computed tomography (CT) of the thorax demonstrated a 3 x 8-cm hernia through the foramen of Morgagni containing a portion of the transverse colon along with intraperitoneal fat (Figures 2 and 3).
The patient underwent repair of this right Morgagni hernia via a laparoscopic approach. Intraoperative findings confirmed preoperative radiologic studies demonstrating colonic and omental contents within an easily reducible hernia sac (Figures 4 and 5). The hernia sac was left in vivo, and a combined direct hernia repair with mesh reinforcement was performed using Surgimesh XB (BG Medical, Barrington, IL) (Figure 6). The patient remained in the hospital for overnight observation and was discharged on postoperative day 1. The patient has since been seen in follow-up and is doing quite well with complete resolution of his reflux and pulmonary symptoms.
Discussion
A recent review of surgical literature revealed that over a 57-year period, 298 cases of Morgagni hernias have been described in adults.7 Although previous studies have postulated that a majority of adult patients are asymptomatic, more recent retrospective studies have found about a 70% symptomatic rate of patients with Morgagni hernias.7 The natural history of adult presentations lends itself to pulmonary (most common) or chronic upper gastrointestinal symptoms, although an acute presentation with potential volvulus and strangulation of the herniated contents has been described.7
Diagnosis is typically confirmed with a chest X-ray, although the CT scan has become more popular in the era of multimodal imaging.4,7 Multiple methods of repair have been described; however, thoracotomy has been the most widely used approach, and laparoscopy has gained popularity since the early 1990s.7 Mesh has been described in more than 60% of cases, and a laparoscopic repair has proven to have a low (< 5%) complication rate and short hospital stay.8,9 In particular, it has been suggested that a hernia defect larger than 20 to 30 cm2 should be repaired with a prosthetic adjunct, such as polypropylene, polytetrafluoroethylene, and bovine pericardium with a 1.5- to 2.5-cm mesh overlap.7,8
Related: Unusual Congenital Pulmonary Anomaly in an Adult Patient With Dyspnea
There is some controversy about the management of the hernia sac, with about 69% of surgeons choosing not to excise the sac due to concerns of intrathoracic or pericardial injury.7 In a separate study, 36 patients were evaluated retrospectively, and the hernia sac was not resected in any of the patients, with long-term follow-up revealing no evidence of recurrence.6
Conclusion
To allow for early intervention and avoidance of potentially life-threatening volvulus/strangulation, the medical practitioner has to be aware of this rare diagnosis when performing a workup for vague pulmonary and abdominal symptoms as described here. Disagreement exists over the method of repair and management of the hernia sac as well as the need for mesh buttressing of the defect. A well-planned surgical approach individualized to the patient’s anatomy, surgeon’s expertise, and hernia defect size will provide the best possible outcome with a low operative morbidity.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Holcomb GW, Murphy JP. Ashcraft’s Pediatric Surgery. 5th ed. Kansas City: Saunders Elsevier; 2010: 319-320.
2. Haroon, J, Chamberlain RS. An evidence-based review of the current treatment of congenital diaphragmatic hernia. Clin Pediatr (Phila). 2013;52(2):115-124.
3. Nagata K, Usui N, Kanamori Y, et al. The current profile and outcome of congenital diaphragmatic hernia: a nationwide survey in Japan. J Pediatr Surg. 2013;48(4):738-744.
4. Abraham V, Myla Y, Verghese S, Chandran BS. Morgagni-larrey hernia—a review of 20 cases. Indian J Surg. 2012;74(5):391-395.
5. Arora S, Haji A, Ng P. Adult Morgagni hernia: the need for clinical awareness, early diagnosis, and prompt surgical intervention. Ann R Coll Surg Engl. 2008;90(8):694-695.
6. Aghajanzadeh M, Khadem S, Khajeh Jahromi S, Gorabi HE, Ebrahimi H, Maafi AA. Clinical presentation and operative repair of Morgagni hernia. Interact Cardiovasc Thorac Surg. 2012;15(4):608-611.
7. Horton JD, Hofmann LJ, Hetz SP. Presentation and management of Morgagni hernias in adults: a review of 298 cases. Surg Endosc. 2008;22(6):1413-1420.
8. Terrosu G, Brizzolari M, Intini S, Cattin F, Bresadola V, De Anna D. Morgagni hernia: technical variation in the laparoscopic treatment. Ann Ital Chir. 2012;83(5):415-420.
9. Durak E, Gur S, Cokmez A, Atahan K, Zahtz E, Tarcan E. Laparoscopic repair of Morgagni hernia. Hernia. 2007;11(3):265-270.
Congenital diaphragmatic hernias (CDHs) occur from a disruption in the muscular formation of the diaphragm, resulting in herniation of abdominal contents into the thoracic cavity. A rare diagnosis, most cases are identified in the pediatric and neonatal populations with an overall historical 50% mortality related to the diagnosis.1 More recent data published in the U.S. and Japan cite an overall survival rate of 67% to 80% secondary to improved understanding of the pathophysiology and subsequent enhancement of neonatal cardiopulmonary support adjuncts.2,3
Bochladek hernias (posterolateral space) are the most common presentation of CDH, accounting for > 90% of cases. First described by the Giovanni Batista Morgagni in On the Seats and Causes of Disease Investigated by Anatomy, the anteromedial sternocostal location is far less common and accounts for only 2% to 3% of cases.4,5 More commonly found on the right side of the diaphragm, despite protection from the liver, the right-sided space has been traditionally referred to as the Morgagni space. A left-sided defect is occasionally called the Larrey gap or space, after Napoleon’s surgeon who described the space as a potential location for pericardial drainage of tamponade.6,7
Related: Colonoscopy Bowel Preparation Instructions
There are a few congenital conditions, such as trisomy 21, Turner syndrome, Prader Willi syndrome, dextrocardia, and Tetralogy of Fallot, that have been associated with Morgagni hernias.7 Pulmonary hypertension and respiratory distress are the most common symptoms for neonatal patients; chest pain, sensations of tightness/fullness, reflux, and transient obstructive symptoms constitute the typical symptoms of adult patients with CDH. In this case study, the authors present a case of adult-onset Morgagni hernia as well as a review of the relevant literature.
Case Report
The patient was a 48-year-old man on active-duty who presented to the Naval Medical Center Portsmouth General Surgery clinic in Virginia with a 4-year history of gastroesophageal reflux-related symptoms. Specifically, he reported epigastric fullness, pyrosis, and discomfort that radiated toward his bilateral lower ribs for the previous 4 years. This discomfort was typically associated with the intake of solid food and was followed a few hours later by a loose bowel movement.
The patient was initially treated with antacids and proton pump inhibitors by his primary care physician, with only minimal relief. He also reported several months of chronic cough as well as intermittent episodes of “gasping air hunger” for about 6 years, which had been incidentally brought up during his separation physical examination. A chest X-ray performed during the workup revealed findings suggesting a right diaphragmatic hernia vs a bronchogenic cyst (Figure 1). A computed tomography (CT) of the thorax demonstrated a 3 x 8-cm hernia through the foramen of Morgagni containing a portion of the transverse colon along with intraperitoneal fat (Figures 2 and 3).
The patient underwent repair of this right Morgagni hernia via a laparoscopic approach. Intraoperative findings confirmed preoperative radiologic studies demonstrating colonic and omental contents within an easily reducible hernia sac (Figures 4 and 5). The hernia sac was left in vivo, and a combined direct hernia repair with mesh reinforcement was performed using Surgimesh XB (BG Medical, Barrington, IL) (Figure 6). The patient remained in the hospital for overnight observation and was discharged on postoperative day 1. The patient has since been seen in follow-up and is doing quite well with complete resolution of his reflux and pulmonary symptoms.
Discussion
A recent review of surgical literature revealed that over a 57-year period, 298 cases of Morgagni hernias have been described in adults.7 Although previous studies have postulated that a majority of adult patients are asymptomatic, more recent retrospective studies have found about a 70% symptomatic rate of patients with Morgagni hernias.7 The natural history of adult presentations lends itself to pulmonary (most common) or chronic upper gastrointestinal symptoms, although an acute presentation with potential volvulus and strangulation of the herniated contents has been described.7
Diagnosis is typically confirmed with a chest X-ray, although the CT scan has become more popular in the era of multimodal imaging.4,7 Multiple methods of repair have been described; however, thoracotomy has been the most widely used approach, and laparoscopy has gained popularity since the early 1990s.7 Mesh has been described in more than 60% of cases, and a laparoscopic repair has proven to have a low (< 5%) complication rate and short hospital stay.8,9 In particular, it has been suggested that a hernia defect larger than 20 to 30 cm2 should be repaired with a prosthetic adjunct, such as polypropylene, polytetrafluoroethylene, and bovine pericardium with a 1.5- to 2.5-cm mesh overlap.7,8
Related: Unusual Congenital Pulmonary Anomaly in an Adult Patient With Dyspnea
There is some controversy about the management of the hernia sac, with about 69% of surgeons choosing not to excise the sac due to concerns of intrathoracic or pericardial injury.7 In a separate study, 36 patients were evaluated retrospectively, and the hernia sac was not resected in any of the patients, with long-term follow-up revealing no evidence of recurrence.6
Conclusion
To allow for early intervention and avoidance of potentially life-threatening volvulus/strangulation, the medical practitioner has to be aware of this rare diagnosis when performing a workup for vague pulmonary and abdominal symptoms as described here. Disagreement exists over the method of repair and management of the hernia sac as well as the need for mesh buttressing of the defect. A well-planned surgical approach individualized to the patient’s anatomy, surgeon’s expertise, and hernia defect size will provide the best possible outcome with a low operative morbidity.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Congenital diaphragmatic hernias (CDHs) occur from a disruption in the muscular formation of the diaphragm, resulting in herniation of abdominal contents into the thoracic cavity. A rare diagnosis, most cases are identified in the pediatric and neonatal populations with an overall historical 50% mortality related to the diagnosis.1 More recent data published in the U.S. and Japan cite an overall survival rate of 67% to 80% secondary to improved understanding of the pathophysiology and subsequent enhancement of neonatal cardiopulmonary support adjuncts.2,3
Bochladek hernias (posterolateral space) are the most common presentation of CDH, accounting for > 90% of cases. First described by the Giovanni Batista Morgagni in On the Seats and Causes of Disease Investigated by Anatomy, the anteromedial sternocostal location is far less common and accounts for only 2% to 3% of cases.4,5 More commonly found on the right side of the diaphragm, despite protection from the liver, the right-sided space has been traditionally referred to as the Morgagni space. A left-sided defect is occasionally called the Larrey gap or space, after Napoleon’s surgeon who described the space as a potential location for pericardial drainage of tamponade.6,7
Related: Colonoscopy Bowel Preparation Instructions
There are a few congenital conditions, such as trisomy 21, Turner syndrome, Prader Willi syndrome, dextrocardia, and Tetralogy of Fallot, that have been associated with Morgagni hernias.7 Pulmonary hypertension and respiratory distress are the most common symptoms for neonatal patients; chest pain, sensations of tightness/fullness, reflux, and transient obstructive symptoms constitute the typical symptoms of adult patients with CDH. In this case study, the authors present a case of adult-onset Morgagni hernia as well as a review of the relevant literature.
Case Report
The patient was a 48-year-old man on active-duty who presented to the Naval Medical Center Portsmouth General Surgery clinic in Virginia with a 4-year history of gastroesophageal reflux-related symptoms. Specifically, he reported epigastric fullness, pyrosis, and discomfort that radiated toward his bilateral lower ribs for the previous 4 years. This discomfort was typically associated with the intake of solid food and was followed a few hours later by a loose bowel movement.
The patient was initially treated with antacids and proton pump inhibitors by his primary care physician, with only minimal relief. He also reported several months of chronic cough as well as intermittent episodes of “gasping air hunger” for about 6 years, which had been incidentally brought up during his separation physical examination. A chest X-ray performed during the workup revealed findings suggesting a right diaphragmatic hernia vs a bronchogenic cyst (Figure 1). A computed tomography (CT) of the thorax demonstrated a 3 x 8-cm hernia through the foramen of Morgagni containing a portion of the transverse colon along with intraperitoneal fat (Figures 2 and 3).
The patient underwent repair of this right Morgagni hernia via a laparoscopic approach. Intraoperative findings confirmed preoperative radiologic studies demonstrating colonic and omental contents within an easily reducible hernia sac (Figures 4 and 5). The hernia sac was left in vivo, and a combined direct hernia repair with mesh reinforcement was performed using Surgimesh XB (BG Medical, Barrington, IL) (Figure 6). The patient remained in the hospital for overnight observation and was discharged on postoperative day 1. The patient has since been seen in follow-up and is doing quite well with complete resolution of his reflux and pulmonary symptoms.
Discussion
A recent review of surgical literature revealed that over a 57-year period, 298 cases of Morgagni hernias have been described in adults.7 Although previous studies have postulated that a majority of adult patients are asymptomatic, more recent retrospective studies have found about a 70% symptomatic rate of patients with Morgagni hernias.7 The natural history of adult presentations lends itself to pulmonary (most common) or chronic upper gastrointestinal symptoms, although an acute presentation with potential volvulus and strangulation of the herniated contents has been described.7
Diagnosis is typically confirmed with a chest X-ray, although the CT scan has become more popular in the era of multimodal imaging.4,7 Multiple methods of repair have been described; however, thoracotomy has been the most widely used approach, and laparoscopy has gained popularity since the early 1990s.7 Mesh has been described in more than 60% of cases, and a laparoscopic repair has proven to have a low (< 5%) complication rate and short hospital stay.8,9 In particular, it has been suggested that a hernia defect larger than 20 to 30 cm2 should be repaired with a prosthetic adjunct, such as polypropylene, polytetrafluoroethylene, and bovine pericardium with a 1.5- to 2.5-cm mesh overlap.7,8
Related: Unusual Congenital Pulmonary Anomaly in an Adult Patient With Dyspnea
There is some controversy about the management of the hernia sac, with about 69% of surgeons choosing not to excise the sac due to concerns of intrathoracic or pericardial injury.7 In a separate study, 36 patients were evaluated retrospectively, and the hernia sac was not resected in any of the patients, with long-term follow-up revealing no evidence of recurrence.6
Conclusion
To allow for early intervention and avoidance of potentially life-threatening volvulus/strangulation, the medical practitioner has to be aware of this rare diagnosis when performing a workup for vague pulmonary and abdominal symptoms as described here. Disagreement exists over the method of repair and management of the hernia sac as well as the need for mesh buttressing of the defect. A well-planned surgical approach individualized to the patient’s anatomy, surgeon’s expertise, and hernia defect size will provide the best possible outcome with a low operative morbidity.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Holcomb GW, Murphy JP. Ashcraft’s Pediatric Surgery. 5th ed. Kansas City: Saunders Elsevier; 2010: 319-320.
2. Haroon, J, Chamberlain RS. An evidence-based review of the current treatment of congenital diaphragmatic hernia. Clin Pediatr (Phila). 2013;52(2):115-124.
3. Nagata K, Usui N, Kanamori Y, et al. The current profile and outcome of congenital diaphragmatic hernia: a nationwide survey in Japan. J Pediatr Surg. 2013;48(4):738-744.
4. Abraham V, Myla Y, Verghese S, Chandran BS. Morgagni-larrey hernia—a review of 20 cases. Indian J Surg. 2012;74(5):391-395.
5. Arora S, Haji A, Ng P. Adult Morgagni hernia: the need for clinical awareness, early diagnosis, and prompt surgical intervention. Ann R Coll Surg Engl. 2008;90(8):694-695.
6. Aghajanzadeh M, Khadem S, Khajeh Jahromi S, Gorabi HE, Ebrahimi H, Maafi AA. Clinical presentation and operative repair of Morgagni hernia. Interact Cardiovasc Thorac Surg. 2012;15(4):608-611.
7. Horton JD, Hofmann LJ, Hetz SP. Presentation and management of Morgagni hernias in adults: a review of 298 cases. Surg Endosc. 2008;22(6):1413-1420.
8. Terrosu G, Brizzolari M, Intini S, Cattin F, Bresadola V, De Anna D. Morgagni hernia: technical variation in the laparoscopic treatment. Ann Ital Chir. 2012;83(5):415-420.
9. Durak E, Gur S, Cokmez A, Atahan K, Zahtz E, Tarcan E. Laparoscopic repair of Morgagni hernia. Hernia. 2007;11(3):265-270.
1. Holcomb GW, Murphy JP. Ashcraft’s Pediatric Surgery. 5th ed. Kansas City: Saunders Elsevier; 2010: 319-320.
2. Haroon, J, Chamberlain RS. An evidence-based review of the current treatment of congenital diaphragmatic hernia. Clin Pediatr (Phila). 2013;52(2):115-124.
3. Nagata K, Usui N, Kanamori Y, et al. The current profile and outcome of congenital diaphragmatic hernia: a nationwide survey in Japan. J Pediatr Surg. 2013;48(4):738-744.
4. Abraham V, Myla Y, Verghese S, Chandran BS. Morgagni-larrey hernia—a review of 20 cases. Indian J Surg. 2012;74(5):391-395.
5. Arora S, Haji A, Ng P. Adult Morgagni hernia: the need for clinical awareness, early diagnosis, and prompt surgical intervention. Ann R Coll Surg Engl. 2008;90(8):694-695.
6. Aghajanzadeh M, Khadem S, Khajeh Jahromi S, Gorabi HE, Ebrahimi H, Maafi AA. Clinical presentation and operative repair of Morgagni hernia. Interact Cardiovasc Thorac Surg. 2012;15(4):608-611.
7. Horton JD, Hofmann LJ, Hetz SP. Presentation and management of Morgagni hernias in adults: a review of 298 cases. Surg Endosc. 2008;22(6):1413-1420.
8. Terrosu G, Brizzolari M, Intini S, Cattin F, Bresadola V, De Anna D. Morgagni hernia: technical variation in the laparoscopic treatment. Ann Ital Chir. 2012;83(5):415-420.
9. Durak E, Gur S, Cokmez A, Atahan K, Zahtz E, Tarcan E. Laparoscopic repair of Morgagni hernia. Hernia. 2007;11(3):265-270.
A Multidisciplinary Chronic Pain Management Clinic in an Indian Health Service Facility
The epidemic of opioid abuse, addiction, and overdose deaths across the U.S. has not forgone the reservations of American Indian/Alaska Native (AI/AN) tribes. Indeed, AI/ANs may be at increased risk for abuse of prescription opioids due to higher rates of reported illicit drug use and misuse of opioids. According to the 2012 National Survey on Drug Use and Mental Health, AI/ANs aged ≥ 12 years had the highest rates of illicit drug use (12.7%) with the national average being only 9.5%.1 In 2009, AI/ANs aged 12 to 17 years were found to have the highest rates of marijuana use (13.8%) and nonmedical prescription drug abuse (6.1%) compared with the overall U.S. averages of 6.9% and 3.3%, respectively, putting them at an increased risk for an opioid overdose.1,2
In 2010, the American Pain Society conducted a survey establishing that about 41% of American adults reported having chronic, recurrent, or long-lasting pain.3 People of AI/AN heritage may experience chronic pain at higher rates, as they were identified as having the greatest incidence rates of low back pain (35%), arthritis (25%), and obesity (40%), which are often significant contributing factors to chronic pain.4-6
These conditions suggest a need for intensified management of chronic pain among IHS patients. The authors’ IHS facility is a closed health-system network where pharmacists are integral components of the health care team throughout the ambulatory care, emergency, and inpatient departments.
Related: Pharmacist Pain E-Consults That Result in a Therapy Change
Given that medications play a central role in the treatment of chronic pain, pharmacists are appropriate leaders for chronic pain management teams. Pharmacists can improve patient outcomes by conducting pain assessments, managing adverse events (AEs), identifying optimal medication choices, determining equianalgesic dosing, and managing care through care protocols.7
The primary objective of the multidisciplinary chronic pain management clinic (MCPMC) is to manage complicated and postsurgical patients, using a multimodal approach. Primary care providers (PCPs), which include physicians, nurse practitioners (NPs), physician assistants (PAs), and pharmacist providers collaborate to meet this goal by minimizing disease progression, preserving activities of daily living (ADL), maintaing employment, preventing an increase in pain, using treatment plans that include pharmacologic, interventional, and complementary components, decreasing emergency department (ED) visits for chronic pain issues, improving pain agreement adherence, managing AEs, performing drug abuse and diversion surveillance, and using sustained-release (SR) opioids when appropriate. Sustained release opioids not only ease dosing schedules and increase adherence, but also improve sleep, functionality, and quality of life (QOL) for chronic pain patients.8
Methods
The MCPMC began enrolling patients in January 2011 and has continued to date. Inclusion criterion is the presence of pain lasting 3 months or more. Exclusionary criteria are the presence of malignant pain, aged < 18 years, pregnancy, unmanaged psychiatric disorders, and a referral not approved by a PCP. Referrals are accepted from providers throughout the facility, including the ED, which then require approval by the PCP before enrollment. The PCP continues to manage these patients through consultations with the MCPMC pharmacists following MCPMC appointments and at separate ambulatory care clinic appointments.
Currently, there are 2 pharmacists practicing in the MCPMC clinic in conjunction with other health care providers, including 5 physical therapists, 1 psychiatrist, 2 clinical social workers, and 15 PCPs, including NPs and PAs. Additionally in 2014, the clinic became a yearlong rotation in the PGY-1 pharmacy practice residency.
Related: Evaluation of Methadone-Induced QTc Prolongation in a Veteran Population
After enrollment, a pharmacist reviews patients’ health records for past pain medications, interventional and complementary treatments, adherence to these treatments, recent ED visits and medications received, urine toxicology results, adherence to pain agreements, and the Arizona Controlled Substances Prescription Monitoring Program Database (ACSPMPD).
During the initial MCPMC appointment, a pain assessment questionnaire (PAQ) is completed with a MCPMC pharmacist. The questionnaire, designed specifically for the MCPMC, consists of a comprehensive pain assessment, including functional status and common comorbidities, such as anxiety, depression, obesity, and insomnia. Patients provide feedback on efficacy of past or current medications, and interventional and complementary treatments if applicable. Patients also rate their satisfaction with health care received and develop goals for their treatment and overall health.
A collaborative treatment plan is then developed with the patient’s PCP. Treatment plans often consist of increasing or starting interventional and complementary treatments, SR opioids, and adjuvant medications. Common adjuvant medications include nonsteroidal anti-inflammatory drugs (NSAIDs), antidepressants, antiepileptics, immunosuppressants, disease-modifying antirheumatic drugs (DMARDs), and topical agents. To maximize benefits of the medications, antidepressants are often prescribed for dual purposes among patients with comorbid conditions, such as anxiety, depression, and insomnia. Among obese patients, weight loss is encouraged, and patients may be referred to dietary counseling and exercise programs. Other intentions of the treatment plans are to decrease breakthrough pain and ED visits while attempting to decrease the use of immediate-release (IR) opioids. Treatment plans are executed in a stepwise approach over multiple MCPMC visits and may be modified throughout the course of the program.
To ensure that medication changes and other issues can be addressed when a prescriber is available, all subsequent visits are scheduled when patients are due for a pain medication refill. The MCPMC pharmacists chose not to pursue prescriptive authority but have privileges to order urine toxicology tests, make nonformulary requests, and refer patients for complementary treatments. Subsequent appointments are commonly scheduled 1 to 4 weeks apart or alternate with PCP appointments.
Related: Multidisciplinary Approach to Back Pain
During each appointment, data are collected to record changes in therapy and pain levels. Questions regarding general health and adherence to pharmacologic, interventional, and complementary treatments, exercise regimens, and specialty referrals are asked of all patients. Additionally, follow-up PAQs are completed every 6 months to track progress in therapy, pain control, treatment plan adherence, and patient satisfaction. To determine pain agreement adherence, the ACSPMPD is reviewed monthly, and urine toxicology tests and pill counts are performed randomly at MCPMC visits.
In October 2013, all PCPs who had patients in the clinic completed a survey to assess their perception of the MCPMC. Questions were related to their satisfaction with the clinic as well as their opinion of patients’ satisfaction. Other questions were related to their view of patient care and outcomes compared with those of the general chronic pain patients at the facility.
Results
As of January 2013, 106 patients had been referred to the MCPMC by 17 PCPs. Thirty-six of these patients were still actively participating in the clinic, while 25 were pending review. Of the remaining 45 patients, 30 were denied initial enrollment, and 15 were disenrolled from the clinic over the previous 2 years. Patients were determined to be inappropriate candidates and not enrolled in the clinic for the following reasons: referral not approved by the PCP, patient refused care, patient had not established care with a PCP, mental health issues, pediatric patient, oncology patient, and death prior to the initial review. Patients were disenrolled from the MCPMC clinic before 2013 for the following reasons: not participating in their treatment plan, illicit drug use, seeking care from other PCPs, suspected diversion, death due to a nonpain-related issue, and remained stable on the medication regimen and were released back to the care of their PCP.
In 2013, there were 47 new referrals to the MCPMC, resulting in a total of 153 referrals since the clinic’s 2011 inception. Over the course of 2013, 31 new patients were enrolled, 32 referrals were denied (15 of which remained from 2012), and 36 patients were disenrolled (Figure 1). At the end of 2013, 31 patients remained active, while 9 referrals remained pending review. A total of 67 patients participated in the MCPMC at some point during 2013 and were included in the data collection. Patients by diagnoses are displayed in Figure 2.
In 2013, patients were scheduled for a total of 337 MCPMC appointments, and 298 (88%) were completed by patients, a 17% increase above 2012. The mean show rate of PCP ambulatory care clinic appointments was about 70%. The completed MCPMC visits for 2013 correlates to about 6.8 MCPMC visits annually per patient. Of the 67 patients included in data collection, the mean total number of months active in the clinic was 12.5. The mean number of months active in the clinic in 2013 was 6.9.
Pain Assessment Questionnaire
In 2013, 27 patients (40%) were enrolled in the clinic for 6 months or more and completed a follow-up PAQ. Throughout 2013, MCPMC patients presented to the ED for care 76 times, which correlates to about 1.8 ED visits annually per patient. MCPMC patients also attended an appointment with their PCP on average 3.7 times per year and provided urine toxicology tests on average 4.3 times per year between MCPMC and PCP visits.
Data collected from follow-up PAQs in January 2014 provided information on the 27 MCPMC patients enrolled in the clinic for 6 months or more. This review indicated alterations in patients’ reported pain levels, functional status, patient satisfaction, and adherence to pain agreements from before and after enrollment in the clinic. Additional information was collected using the electronic health record to reveal the adjustments in treatment plans, including pharmacologic, complementary, and interventional treatments, along with adherence to these treatments.
Patients’ self-reported pain levels at the time of appointment and average pain levels since the previous appointment were documented at each visit for the 27 MCPMC patients. These 2 pain levels were then compared with the levels of the initial assessment and the most recent appointment. Results were inconsistent; however, slight trends were observed with the analysis. The mean change in pain reported at the time of assessment decreased 5.1%. The mean change in average reported pain since the previous appointment also decreased 6.9%. Statistical analysis was performed using the Wilcoxon signed rank test. Both decreases in reported pain were not clinically or statistically significant (P = .21 and P = .17, respectively). Eleven (41%) patients had improvement in average pain, whereas 10 (37%) had no change, and 6 (22%) reported increased average pain levels.
Data on alterations in functional status and ADL were also collected from the 27 MCPMC patients. These patients reported the perceived degree of difficulty, on a scale of 1 to 5, required to complete tasks and get through their day. A rating of 1 represented the ability to complete activities with no difficulty, whereas 5 represented an inability to complete the tasks. For each of the 19 tasks, the differences in scores from the initial to the most recent PAQs were recorded as either a positive or negative alteration for each patient, and the sum of these differences was recorded as an overall positive or negative change in function. A positive change in function indicated an improvement in function, whereas an overall negative change indicated a decrease in ability to complete daily activities.
Twenty-six percent of the 27 pa-tients had a cumulative positive change of up to 5 points, and 19% had a positive change of 6 or more points. Alternatively, 22% of patients had a cumulative negative change of up to 5 points, and 33% of patients had a negative change of 6 points or more. The greatest positive change was 15 points, the greatest negative change was 28 points, and the median change from the initial to the most recent assessments was a negative change of 2 points.
Adjuvant Medications
The pharmacologic component of the treatment plans consisted primarily of optimizing the use of adjuvant medications and SR opioids when appropriate, while minimizing the use of IR opioids and other controlled medications. Of the 67 MCPMC patients in 2013, 55% were on IR opioids alone, a slight increase from 46% in 2012 (Table 1). Eighty-one percent of patients in this group were on ≤ 15 mg of morphine equivalent daily dose (MEDD), which would have required at least a doubling of their dose to initiate the preferred formulary SR opioid, morphine SR tablets. Six percent of patients were on SR opioids alone, also a slight increase from 3% in 2012. Twenty-seven percent of patients were prescribed a combination of IR and SR opioids. Nine percent of patients had been recently transitioned to SR opioids while in the MCPMC, of which 1 patient was prescribed the medication as monotherapy. Twelve percent of patients were not on any opioid therapy throughout 2013.
Opioids were switched to an alternative opioid at some point during the year to minimize tolerance in 15% of patients, of which 9% were IR and 6% were SR opioids. Changes in opioid therapy from the beginning to the end of the year were recorded as a decrease, increase, or no change in MEDD. Doses were decreased for 16%, increased for 27%, and not changed for the remaining 45% of patients. The sum of these changes for the 59 patients on opioids was a decrease of 172 mg MEDD or, on average, a decrease of about 3 mg MEDD per patient. Throughout the year, 36 patients were disenrolled from the clinic, and a total of 941 mg MEDD were discontinued by patients’ PCPs. This resulted in a mean of about 26 mg MEDD discontinued per patient. These statistics demonstrate small trends in decreasing overall MEDD in MCPMC patients.
Adjunctive therapies were often used in 67 MCPMC patients in addition to their opioid medications. If possible, therapies for pain management were chosen to maximize the ability to benefit comorbidities, such as depression, anxiety, and insomnia, while also treating chronic pain. The most frequently prescribed class of medications was antidepressants with 63% of patients prescribed one or more: bupropion, serotonin-norepinephrine reuptake inhibitor, selective-serotonin reuptake inhibitor, and tricyclic antidepressants. The next top 3 medication classes after antidepressants were topical medications (54%), antiepileptics (48%), and muscle relaxers (42%). The single most frequently prescribed adjunctive medication was gabapentin (37%), an antiepileptic.
Complementary Treatments
Complementary treatment referrals were followed throughout 2013 and compared with referrals from 2012 (Table 2). Physical therapy (PT) and exercise programs continued to be the most frequently referred treatment programs within the facility. Fifty-two percent of 67 MCPMC patients did not attend any PT appointments as recommended, of which the majority were required to attend as a component of their pain agreement. Of the remaining patients referred to PT, 48% went to their initial visit, 40% attended a second, and 32% attended 3 or more appointments. Of the group that attended 3 or more appointments, patients completed about 70% of the overall scheduled appointments, which was below the facility averages of 75% in 2012 and 80% in 2013.
Acupuncture, transcutaneous electrical nerve stimulation, and osteopathic manipulative therapy (OMT) were much less frequently suggested treatments, with percentages of patient referrals of 22%, 21%, and 6%, respectively. Sixty percent of patients referred to acupuncture attended the initial visit, 47% attended a second, and 40% attended 3 or more appointments. Of this group that attended at least 3 appointments, patients completed 75% of scheduled appointments, which was also below the facility averages of 86% in 2012 and 81% in 2013. Only 50% of patients referred to OMT attended the initial visit, of which these patients completed 100% of their scheduled appointments. This rate of attendance was above the facility averages of 60% in 2012 and 68% in 2013. Thirteen percent of patients were referred for interventional pain management and completed 1 of 3 types of injections (onabotulinumtoxinA, spinal, or intra-articular). There was a slight decrease in patients without complementary treatment referrals from 14% in 2012 to 13% in 2013.
Adherence
Pain agreement adherence was determined by assessing ED visits, urine toxicology results, and ACSPMPD search results. Sixty-one percent of the 67 MCPMC patients did not seek care in the ED, whereas 12% had 1 visit in 2013. This decrease in frequency of ED visits was significant compared with these same MCPMC patients from prior to participation in the clinic. The mean ED patient visits per year decreased from 5.1 to 1.8.
Urine toxicology tests were completed on 54 of the 67 MCPMC patients in 2013. Overall, urine toxicology reports were determined to be appropriate at the initial review 51% of the time, with 30% of patients having all of their reports completely appropriate. Of the 54 patients, 35% were disenrolled for inappropriate urine toxicology reports for the following reasons: negative for opioids, positive for opioids without a prescription, positive for amphetamines with additional confirmation testing, and positive for barbiturates without a prescription. Six percent of patients were discovered to have trace amphetamine results that were sent out for confirmation, but these reports were found to be negative, thus confirming an initial false-positive result.
Forty-eight percent of MCPMC patients tested negative for opioids at some point during the year when they were expected to have positive results. Of this group, 31% were prescribed morphine; the remaining patients were prescribed synthetic or semisynthetic opioids that are known to cause false-negative results: fentanyl (4%), hydrocodone (50%), and oxycodone (15%).9 Twenty-two percent of patients were disenrolled from the clinic for testing negative for opioids. The reason for disenrollment was often in conjunction with other behaviors that resulted in violations of their pain agreement. The remaining 78% reported running out of pain medications early and remained in the clinic. Two percent of patients were discovered to have a positive opioid result when it was expected to be negative. This group reported finding previously prescribed medications and subsequent results were appropriate, thus they remained in the clinic. Lastly, 2% of patients tested negative for barbiturates when it was expected to be positive. These patients reported running out of pain medication early as well.
The ACSPMPD was also used to assess pain agreement adherencee for all MCPMC patients. Six percent of patients were identified as seeking care from providers outside the IHS facility and receiving prescriptions for opioid medications, thus violating their pain agreements. Seventy-five percent of these patients were disenrolled from the MCPMC for this reason. PCPs referred the other 25% of patients, and the outside prescribers had performed procedures on them. These patients were reminded of their pain agreements, and no further violations were discovered according to the database. Each patient’s status in the MCPMC was evaluated on a case-by-case basis, and often decisions to disenroll or continue treating patients were based on the PCP’s clinical judgment.
Patient satisfaction was measured in the follow-up PAQ by asking 27 patients how they felt about their care, using a typical 5-point Likert scale. The 2 statements were, “I am pleased with the care that I have received for my pain,” and “I believe that I am receiving the best health care available.” Seventy percent of patients answered “strongly agree” or “agree” to the first statement, and 67% of patients answered the same for the second statement. Nineteen percent of patients answered “not sure” to the first statement, and 22% of patients answered the same for the other statement. Eleven percent of patients responded, “disagree” or “strongly disagree” to both statements.
In October 2013, 12 PCPs who had patients in the MCPMC completed an online survey regarding their perception of patient outcomes, time spent providing care to chronic pain patients, comparisons with general chronic pain patients, and satisfaction with the clinic. Most of the PCPs reported they spent 15 to 30 minutes on MCPMC patients compared with 30 to 60 minutes on general chronic pain patients each month. Most of the PCPs stated that they required ambulatory care clinic visits with chronic pain patients every other month, whereas MCPMC patients needed to be seen only quarterly. PCPs agreed that having their patients participate in the MCPMC resulted in better pain control, improved adherence to treatments, increased diversion and abuse surveillance, and better access to pain medications. Eleven of 12 PCPs stated that they were very satisfied with the MCPMC.
Discussion
The ultimate goal for patients of the MCPMC is to minimize disease progression, prevent an increase in pain, and improve adherence to treatment plans, including pharmacologic, interventional, and complementary components. According to the change in reported pain levels from the initial to the most recent assessment, most patients met the goal of preventing an increase in pain. There was a trend toward a decrease in reported pain, though it was not clinically or statistically significant. The follow-up PAQ measured varying changes in functional status and often demonstrated disease stabilization or progression, not improvement among patients. Forty-five percent of patients showed improvements, and 55% reported more difficulty performing daily activities. The median change between all 27 MCPMC patients was an overall decline in function of 2 points. This worsening in function over time would be expected for most of the chronic pain conditions.
In 2013, 9% of patients were initiated on SR opioids, making a clinic total of 33% of patients on SR medications. More than half the patients were on IR opioids as monotherapy, which is not an ideal treatment for chronic pain management. However, 81% of this group was on 15 mg MEDD or less. The use of SR opioids may or may not reduce abuse potential but can improve patient outcomes. Overall, there was an emphasis on using SR opioids when appropriate while continuing to improve patient outcomes. Over 61% of patients remained on the same opioid doses or were decreased over the course of 2013. There was also a significant use of adjuvant medications, primarily antidepressants, antiepileptics, and topical pain relievers. The most frequently prescribed non-opioid medication, excluding NSAIDs, was gabapentin. This medication has abuse potential and was treated as a controlled medication by the MCPMC during this period.
After enrollment in the MCPMC, patients used complementary and interventional treatments more consistently than prior to enrollment in the clinic. Treatments such as injections, acupuncture, OMT, and PT may reduce opioid medication consumption in the long term or slow the progression of disease for most patients. The improvement in QOL and lack of disease progression in these patients is not objectively measurable; however, the summative progress may be subjectively evaluated through reported pain levels and patient satisfaction.
For MCPMC patients who remained in the clinic, PT and acupuncture attendance was 70% and 75%, respectively. Although these were improvements in adherence for many MCPMC patients, the rates were still below the facility average completion rates of 80% and 81%, respectively. It could be argued that patients with acute pain are typically seen in PT for shorter periods and with fewer possibilities of missing appointments. Conversely, the single active MCPMC patient who attended OMT had a 100% completion rate compared with the average facility OMT attendance of 68%.
Other goals of the MCPMC consist of managing AEs, minimizing ED visits, monitoring for drug abuse and diversion, and improving adherence to pain agreements. The substantial 65% decrease in ED visits can be attributed to the patients’ participation in the MCPMC. Before enrollment, many patients would frequent the ED, because their PCP was not available. The cost savings from minimizing ED visits, provider and staff time, and resources is difficult to measure due to low rates of collections from insurance supplemental to IHS insurance yet is a significant benefit to the IHS facility.
Conclusions
Since the implementation of the MCPMC, patient outcomes have improved due to more consistent drug abuse and diversion surveillance of chronic pain patients rather than performing surveillance because of a suspicion of inappropriate medication use. Frequently using the pain agreement and monitoring parameters constructed a more trusting relationship between the PCP and the patient, and identified patients inappropriate for long-term opioid therapy. Identifying these patients was an unintentional, yet positive outcome.
Additionally, PCPs reported spending half the time with MCPMC patients vs general chronic pain patients. Patients who were not compliant with their pain agreements were discontinued from opioid therapy and were disenrolled from the clinic. Patients who have remained active have become more compliant with their pain agreements and treatment plans than they had been before enrollment. The MCPMC has ultimately relieved a significant burden from primary care and ED providers while improving outcomes and satisfaction of chronic pain patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
2. Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-38, HHS Publication No. (SMA) 10-4586. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
3. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey.
J Pain. 2010;11(11):1230-1239.
4. Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine (Phila PA 1976). 2006;31(23):
2724-2727.
5. Bolen J, Schieb L, Hootman JM, et al. Differences in the prevalence and impact of arthritis among racial/ethnic groups in the United States, National Health Interview Survey, 2002, 2003, and 2006. Prev Chronic Dis. 2010;7(3):A64.
6. Schiller JS, Lucas JW, Ward BW, Perogoy JA. Summary health statistics U.S. adults: National Health Interview Survey, 2010. National Center for Health Statistics. Vital Health Stat. 2012;10(252). Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/data/series/sr_10/sr10_252.pdf. Accessed June 26, 2015.
7. Strickland JM, Huskey A, Brushwood DB. Pharmacist-physician collaboration in pain management practice. J Opioid Manag. 2007;3(6):295-301.
8. Rauck RL. What is the case for prescribing long-acting opioids over short-acting opioids for patients with chronic pain? A critical review. Pain Pract. 2009;9(6):468-479.
9. Pesce A, West C, Egan City K, Strickland J. Interpretation of urine drug testing in pain patients. Pain Med. 2012;13(7):868-885.
The epidemic of opioid abuse, addiction, and overdose deaths across the U.S. has not forgone the reservations of American Indian/Alaska Native (AI/AN) tribes. Indeed, AI/ANs may be at increased risk for abuse of prescription opioids due to higher rates of reported illicit drug use and misuse of opioids. According to the 2012 National Survey on Drug Use and Mental Health, AI/ANs aged ≥ 12 years had the highest rates of illicit drug use (12.7%) with the national average being only 9.5%.1 In 2009, AI/ANs aged 12 to 17 years were found to have the highest rates of marijuana use (13.8%) and nonmedical prescription drug abuse (6.1%) compared with the overall U.S. averages of 6.9% and 3.3%, respectively, putting them at an increased risk for an opioid overdose.1,2
In 2010, the American Pain Society conducted a survey establishing that about 41% of American adults reported having chronic, recurrent, or long-lasting pain.3 People of AI/AN heritage may experience chronic pain at higher rates, as they were identified as having the greatest incidence rates of low back pain (35%), arthritis (25%), and obesity (40%), which are often significant contributing factors to chronic pain.4-6
These conditions suggest a need for intensified management of chronic pain among IHS patients. The authors’ IHS facility is a closed health-system network where pharmacists are integral components of the health care team throughout the ambulatory care, emergency, and inpatient departments.
Related: Pharmacist Pain E-Consults That Result in a Therapy Change
Given that medications play a central role in the treatment of chronic pain, pharmacists are appropriate leaders for chronic pain management teams. Pharmacists can improve patient outcomes by conducting pain assessments, managing adverse events (AEs), identifying optimal medication choices, determining equianalgesic dosing, and managing care through care protocols.7
The primary objective of the multidisciplinary chronic pain management clinic (MCPMC) is to manage complicated and postsurgical patients, using a multimodal approach. Primary care providers (PCPs), which include physicians, nurse practitioners (NPs), physician assistants (PAs), and pharmacist providers collaborate to meet this goal by minimizing disease progression, preserving activities of daily living (ADL), maintaing employment, preventing an increase in pain, using treatment plans that include pharmacologic, interventional, and complementary components, decreasing emergency department (ED) visits for chronic pain issues, improving pain agreement adherence, managing AEs, performing drug abuse and diversion surveillance, and using sustained-release (SR) opioids when appropriate. Sustained release opioids not only ease dosing schedules and increase adherence, but also improve sleep, functionality, and quality of life (QOL) for chronic pain patients.8
Methods
The MCPMC began enrolling patients in January 2011 and has continued to date. Inclusion criterion is the presence of pain lasting 3 months or more. Exclusionary criteria are the presence of malignant pain, aged < 18 years, pregnancy, unmanaged psychiatric disorders, and a referral not approved by a PCP. Referrals are accepted from providers throughout the facility, including the ED, which then require approval by the PCP before enrollment. The PCP continues to manage these patients through consultations with the MCPMC pharmacists following MCPMC appointments and at separate ambulatory care clinic appointments.
Currently, there are 2 pharmacists practicing in the MCPMC clinic in conjunction with other health care providers, including 5 physical therapists, 1 psychiatrist, 2 clinical social workers, and 15 PCPs, including NPs and PAs. Additionally in 2014, the clinic became a yearlong rotation in the PGY-1 pharmacy practice residency.
Related: Evaluation of Methadone-Induced QTc Prolongation in a Veteran Population
After enrollment, a pharmacist reviews patients’ health records for past pain medications, interventional and complementary treatments, adherence to these treatments, recent ED visits and medications received, urine toxicology results, adherence to pain agreements, and the Arizona Controlled Substances Prescription Monitoring Program Database (ACSPMPD).
During the initial MCPMC appointment, a pain assessment questionnaire (PAQ) is completed with a MCPMC pharmacist. The questionnaire, designed specifically for the MCPMC, consists of a comprehensive pain assessment, including functional status and common comorbidities, such as anxiety, depression, obesity, and insomnia. Patients provide feedback on efficacy of past or current medications, and interventional and complementary treatments if applicable. Patients also rate their satisfaction with health care received and develop goals for their treatment and overall health.
A collaborative treatment plan is then developed with the patient’s PCP. Treatment plans often consist of increasing or starting interventional and complementary treatments, SR opioids, and adjuvant medications. Common adjuvant medications include nonsteroidal anti-inflammatory drugs (NSAIDs), antidepressants, antiepileptics, immunosuppressants, disease-modifying antirheumatic drugs (DMARDs), and topical agents. To maximize benefits of the medications, antidepressants are often prescribed for dual purposes among patients with comorbid conditions, such as anxiety, depression, and insomnia. Among obese patients, weight loss is encouraged, and patients may be referred to dietary counseling and exercise programs. Other intentions of the treatment plans are to decrease breakthrough pain and ED visits while attempting to decrease the use of immediate-release (IR) opioids. Treatment plans are executed in a stepwise approach over multiple MCPMC visits and may be modified throughout the course of the program.
To ensure that medication changes and other issues can be addressed when a prescriber is available, all subsequent visits are scheduled when patients are due for a pain medication refill. The MCPMC pharmacists chose not to pursue prescriptive authority but have privileges to order urine toxicology tests, make nonformulary requests, and refer patients for complementary treatments. Subsequent appointments are commonly scheduled 1 to 4 weeks apart or alternate with PCP appointments.
Related: Multidisciplinary Approach to Back Pain
During each appointment, data are collected to record changes in therapy and pain levels. Questions regarding general health and adherence to pharmacologic, interventional, and complementary treatments, exercise regimens, and specialty referrals are asked of all patients. Additionally, follow-up PAQs are completed every 6 months to track progress in therapy, pain control, treatment plan adherence, and patient satisfaction. To determine pain agreement adherence, the ACSPMPD is reviewed monthly, and urine toxicology tests and pill counts are performed randomly at MCPMC visits.
In October 2013, all PCPs who had patients in the clinic completed a survey to assess their perception of the MCPMC. Questions were related to their satisfaction with the clinic as well as their opinion of patients’ satisfaction. Other questions were related to their view of patient care and outcomes compared with those of the general chronic pain patients at the facility.
Results
As of January 2013, 106 patients had been referred to the MCPMC by 17 PCPs. Thirty-six of these patients were still actively participating in the clinic, while 25 were pending review. Of the remaining 45 patients, 30 were denied initial enrollment, and 15 were disenrolled from the clinic over the previous 2 years. Patients were determined to be inappropriate candidates and not enrolled in the clinic for the following reasons: referral not approved by the PCP, patient refused care, patient had not established care with a PCP, mental health issues, pediatric patient, oncology patient, and death prior to the initial review. Patients were disenrolled from the MCPMC clinic before 2013 for the following reasons: not participating in their treatment plan, illicit drug use, seeking care from other PCPs, suspected diversion, death due to a nonpain-related issue, and remained stable on the medication regimen and were released back to the care of their PCP.
In 2013, there were 47 new referrals to the MCPMC, resulting in a total of 153 referrals since the clinic’s 2011 inception. Over the course of 2013, 31 new patients were enrolled, 32 referrals were denied (15 of which remained from 2012), and 36 patients were disenrolled (Figure 1). At the end of 2013, 31 patients remained active, while 9 referrals remained pending review. A total of 67 patients participated in the MCPMC at some point during 2013 and were included in the data collection. Patients by diagnoses are displayed in Figure 2.
In 2013, patients were scheduled for a total of 337 MCPMC appointments, and 298 (88%) were completed by patients, a 17% increase above 2012. The mean show rate of PCP ambulatory care clinic appointments was about 70%. The completed MCPMC visits for 2013 correlates to about 6.8 MCPMC visits annually per patient. Of the 67 patients included in data collection, the mean total number of months active in the clinic was 12.5. The mean number of months active in the clinic in 2013 was 6.9.
Pain Assessment Questionnaire
In 2013, 27 patients (40%) were enrolled in the clinic for 6 months or more and completed a follow-up PAQ. Throughout 2013, MCPMC patients presented to the ED for care 76 times, which correlates to about 1.8 ED visits annually per patient. MCPMC patients also attended an appointment with their PCP on average 3.7 times per year and provided urine toxicology tests on average 4.3 times per year between MCPMC and PCP visits.
Data collected from follow-up PAQs in January 2014 provided information on the 27 MCPMC patients enrolled in the clinic for 6 months or more. This review indicated alterations in patients’ reported pain levels, functional status, patient satisfaction, and adherence to pain agreements from before and after enrollment in the clinic. Additional information was collected using the electronic health record to reveal the adjustments in treatment plans, including pharmacologic, complementary, and interventional treatments, along with adherence to these treatments.
Patients’ self-reported pain levels at the time of appointment and average pain levels since the previous appointment were documented at each visit for the 27 MCPMC patients. These 2 pain levels were then compared with the levels of the initial assessment and the most recent appointment. Results were inconsistent; however, slight trends were observed with the analysis. The mean change in pain reported at the time of assessment decreased 5.1%. The mean change in average reported pain since the previous appointment also decreased 6.9%. Statistical analysis was performed using the Wilcoxon signed rank test. Both decreases in reported pain were not clinically or statistically significant (P = .21 and P = .17, respectively). Eleven (41%) patients had improvement in average pain, whereas 10 (37%) had no change, and 6 (22%) reported increased average pain levels.
Data on alterations in functional status and ADL were also collected from the 27 MCPMC patients. These patients reported the perceived degree of difficulty, on a scale of 1 to 5, required to complete tasks and get through their day. A rating of 1 represented the ability to complete activities with no difficulty, whereas 5 represented an inability to complete the tasks. For each of the 19 tasks, the differences in scores from the initial to the most recent PAQs were recorded as either a positive or negative alteration for each patient, and the sum of these differences was recorded as an overall positive or negative change in function. A positive change in function indicated an improvement in function, whereas an overall negative change indicated a decrease in ability to complete daily activities.
Twenty-six percent of the 27 pa-tients had a cumulative positive change of up to 5 points, and 19% had a positive change of 6 or more points. Alternatively, 22% of patients had a cumulative negative change of up to 5 points, and 33% of patients had a negative change of 6 points or more. The greatest positive change was 15 points, the greatest negative change was 28 points, and the median change from the initial to the most recent assessments was a negative change of 2 points.
Adjuvant Medications
The pharmacologic component of the treatment plans consisted primarily of optimizing the use of adjuvant medications and SR opioids when appropriate, while minimizing the use of IR opioids and other controlled medications. Of the 67 MCPMC patients in 2013, 55% were on IR opioids alone, a slight increase from 46% in 2012 (Table 1). Eighty-one percent of patients in this group were on ≤ 15 mg of morphine equivalent daily dose (MEDD), which would have required at least a doubling of their dose to initiate the preferred formulary SR opioid, morphine SR tablets. Six percent of patients were on SR opioids alone, also a slight increase from 3% in 2012. Twenty-seven percent of patients were prescribed a combination of IR and SR opioids. Nine percent of patients had been recently transitioned to SR opioids while in the MCPMC, of which 1 patient was prescribed the medication as monotherapy. Twelve percent of patients were not on any opioid therapy throughout 2013.
Opioids were switched to an alternative opioid at some point during the year to minimize tolerance in 15% of patients, of which 9% were IR and 6% were SR opioids. Changes in opioid therapy from the beginning to the end of the year were recorded as a decrease, increase, or no change in MEDD. Doses were decreased for 16%, increased for 27%, and not changed for the remaining 45% of patients. The sum of these changes for the 59 patients on opioids was a decrease of 172 mg MEDD or, on average, a decrease of about 3 mg MEDD per patient. Throughout the year, 36 patients were disenrolled from the clinic, and a total of 941 mg MEDD were discontinued by patients’ PCPs. This resulted in a mean of about 26 mg MEDD discontinued per patient. These statistics demonstrate small trends in decreasing overall MEDD in MCPMC patients.
Adjunctive therapies were often used in 67 MCPMC patients in addition to their opioid medications. If possible, therapies for pain management were chosen to maximize the ability to benefit comorbidities, such as depression, anxiety, and insomnia, while also treating chronic pain. The most frequently prescribed class of medications was antidepressants with 63% of patients prescribed one or more: bupropion, serotonin-norepinephrine reuptake inhibitor, selective-serotonin reuptake inhibitor, and tricyclic antidepressants. The next top 3 medication classes after antidepressants were topical medications (54%), antiepileptics (48%), and muscle relaxers (42%). The single most frequently prescribed adjunctive medication was gabapentin (37%), an antiepileptic.
Complementary Treatments
Complementary treatment referrals were followed throughout 2013 and compared with referrals from 2012 (Table 2). Physical therapy (PT) and exercise programs continued to be the most frequently referred treatment programs within the facility. Fifty-two percent of 67 MCPMC patients did not attend any PT appointments as recommended, of which the majority were required to attend as a component of their pain agreement. Of the remaining patients referred to PT, 48% went to their initial visit, 40% attended a second, and 32% attended 3 or more appointments. Of the group that attended 3 or more appointments, patients completed about 70% of the overall scheduled appointments, which was below the facility averages of 75% in 2012 and 80% in 2013.
Acupuncture, transcutaneous electrical nerve stimulation, and osteopathic manipulative therapy (OMT) were much less frequently suggested treatments, with percentages of patient referrals of 22%, 21%, and 6%, respectively. Sixty percent of patients referred to acupuncture attended the initial visit, 47% attended a second, and 40% attended 3 or more appointments. Of this group that attended at least 3 appointments, patients completed 75% of scheduled appointments, which was also below the facility averages of 86% in 2012 and 81% in 2013. Only 50% of patients referred to OMT attended the initial visit, of which these patients completed 100% of their scheduled appointments. This rate of attendance was above the facility averages of 60% in 2012 and 68% in 2013. Thirteen percent of patients were referred for interventional pain management and completed 1 of 3 types of injections (onabotulinumtoxinA, spinal, or intra-articular). There was a slight decrease in patients without complementary treatment referrals from 14% in 2012 to 13% in 2013.
Adherence
Pain agreement adherence was determined by assessing ED visits, urine toxicology results, and ACSPMPD search results. Sixty-one percent of the 67 MCPMC patients did not seek care in the ED, whereas 12% had 1 visit in 2013. This decrease in frequency of ED visits was significant compared with these same MCPMC patients from prior to participation in the clinic. The mean ED patient visits per year decreased from 5.1 to 1.8.
Urine toxicology tests were completed on 54 of the 67 MCPMC patients in 2013. Overall, urine toxicology reports were determined to be appropriate at the initial review 51% of the time, with 30% of patients having all of their reports completely appropriate. Of the 54 patients, 35% were disenrolled for inappropriate urine toxicology reports for the following reasons: negative for opioids, positive for opioids without a prescription, positive for amphetamines with additional confirmation testing, and positive for barbiturates without a prescription. Six percent of patients were discovered to have trace amphetamine results that were sent out for confirmation, but these reports were found to be negative, thus confirming an initial false-positive result.
Forty-eight percent of MCPMC patients tested negative for opioids at some point during the year when they were expected to have positive results. Of this group, 31% were prescribed morphine; the remaining patients were prescribed synthetic or semisynthetic opioids that are known to cause false-negative results: fentanyl (4%), hydrocodone (50%), and oxycodone (15%).9 Twenty-two percent of patients were disenrolled from the clinic for testing negative for opioids. The reason for disenrollment was often in conjunction with other behaviors that resulted in violations of their pain agreement. The remaining 78% reported running out of pain medications early and remained in the clinic. Two percent of patients were discovered to have a positive opioid result when it was expected to be negative. This group reported finding previously prescribed medications and subsequent results were appropriate, thus they remained in the clinic. Lastly, 2% of patients tested negative for barbiturates when it was expected to be positive. These patients reported running out of pain medication early as well.
The ACSPMPD was also used to assess pain agreement adherencee for all MCPMC patients. Six percent of patients were identified as seeking care from providers outside the IHS facility and receiving prescriptions for opioid medications, thus violating their pain agreements. Seventy-five percent of these patients were disenrolled from the MCPMC for this reason. PCPs referred the other 25% of patients, and the outside prescribers had performed procedures on them. These patients were reminded of their pain agreements, and no further violations were discovered according to the database. Each patient’s status in the MCPMC was evaluated on a case-by-case basis, and often decisions to disenroll or continue treating patients were based on the PCP’s clinical judgment.
Patient satisfaction was measured in the follow-up PAQ by asking 27 patients how they felt about their care, using a typical 5-point Likert scale. The 2 statements were, “I am pleased with the care that I have received for my pain,” and “I believe that I am receiving the best health care available.” Seventy percent of patients answered “strongly agree” or “agree” to the first statement, and 67% of patients answered the same for the second statement. Nineteen percent of patients answered “not sure” to the first statement, and 22% of patients answered the same for the other statement. Eleven percent of patients responded, “disagree” or “strongly disagree” to both statements.
In October 2013, 12 PCPs who had patients in the MCPMC completed an online survey regarding their perception of patient outcomes, time spent providing care to chronic pain patients, comparisons with general chronic pain patients, and satisfaction with the clinic. Most of the PCPs reported they spent 15 to 30 minutes on MCPMC patients compared with 30 to 60 minutes on general chronic pain patients each month. Most of the PCPs stated that they required ambulatory care clinic visits with chronic pain patients every other month, whereas MCPMC patients needed to be seen only quarterly. PCPs agreed that having their patients participate in the MCPMC resulted in better pain control, improved adherence to treatments, increased diversion and abuse surveillance, and better access to pain medications. Eleven of 12 PCPs stated that they were very satisfied with the MCPMC.
Discussion
The ultimate goal for patients of the MCPMC is to minimize disease progression, prevent an increase in pain, and improve adherence to treatment plans, including pharmacologic, interventional, and complementary components. According to the change in reported pain levels from the initial to the most recent assessment, most patients met the goal of preventing an increase in pain. There was a trend toward a decrease in reported pain, though it was not clinically or statistically significant. The follow-up PAQ measured varying changes in functional status and often demonstrated disease stabilization or progression, not improvement among patients. Forty-five percent of patients showed improvements, and 55% reported more difficulty performing daily activities. The median change between all 27 MCPMC patients was an overall decline in function of 2 points. This worsening in function over time would be expected for most of the chronic pain conditions.
In 2013, 9% of patients were initiated on SR opioids, making a clinic total of 33% of patients on SR medications. More than half the patients were on IR opioids as monotherapy, which is not an ideal treatment for chronic pain management. However, 81% of this group was on 15 mg MEDD or less. The use of SR opioids may or may not reduce abuse potential but can improve patient outcomes. Overall, there was an emphasis on using SR opioids when appropriate while continuing to improve patient outcomes. Over 61% of patients remained on the same opioid doses or were decreased over the course of 2013. There was also a significant use of adjuvant medications, primarily antidepressants, antiepileptics, and topical pain relievers. The most frequently prescribed non-opioid medication, excluding NSAIDs, was gabapentin. This medication has abuse potential and was treated as a controlled medication by the MCPMC during this period.
After enrollment in the MCPMC, patients used complementary and interventional treatments more consistently than prior to enrollment in the clinic. Treatments such as injections, acupuncture, OMT, and PT may reduce opioid medication consumption in the long term or slow the progression of disease for most patients. The improvement in QOL and lack of disease progression in these patients is not objectively measurable; however, the summative progress may be subjectively evaluated through reported pain levels and patient satisfaction.
For MCPMC patients who remained in the clinic, PT and acupuncture attendance was 70% and 75%, respectively. Although these were improvements in adherence for many MCPMC patients, the rates were still below the facility average completion rates of 80% and 81%, respectively. It could be argued that patients with acute pain are typically seen in PT for shorter periods and with fewer possibilities of missing appointments. Conversely, the single active MCPMC patient who attended OMT had a 100% completion rate compared with the average facility OMT attendance of 68%.
Other goals of the MCPMC consist of managing AEs, minimizing ED visits, monitoring for drug abuse and diversion, and improving adherence to pain agreements. The substantial 65% decrease in ED visits can be attributed to the patients’ participation in the MCPMC. Before enrollment, many patients would frequent the ED, because their PCP was not available. The cost savings from minimizing ED visits, provider and staff time, and resources is difficult to measure due to low rates of collections from insurance supplemental to IHS insurance yet is a significant benefit to the IHS facility.
Conclusions
Since the implementation of the MCPMC, patient outcomes have improved due to more consistent drug abuse and diversion surveillance of chronic pain patients rather than performing surveillance because of a suspicion of inappropriate medication use. Frequently using the pain agreement and monitoring parameters constructed a more trusting relationship between the PCP and the patient, and identified patients inappropriate for long-term opioid therapy. Identifying these patients was an unintentional, yet positive outcome.
Additionally, PCPs reported spending half the time with MCPMC patients vs general chronic pain patients. Patients who were not compliant with their pain agreements were discontinued from opioid therapy and were disenrolled from the clinic. Patients who have remained active have become more compliant with their pain agreements and treatment plans than they had been before enrollment. The MCPMC has ultimately relieved a significant burden from primary care and ED providers while improving outcomes and satisfaction of chronic pain patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The epidemic of opioid abuse, addiction, and overdose deaths across the U.S. has not forgone the reservations of American Indian/Alaska Native (AI/AN) tribes. Indeed, AI/ANs may be at increased risk for abuse of prescription opioids due to higher rates of reported illicit drug use and misuse of opioids. According to the 2012 National Survey on Drug Use and Mental Health, AI/ANs aged ≥ 12 years had the highest rates of illicit drug use (12.7%) with the national average being only 9.5%.1 In 2009, AI/ANs aged 12 to 17 years were found to have the highest rates of marijuana use (13.8%) and nonmedical prescription drug abuse (6.1%) compared with the overall U.S. averages of 6.9% and 3.3%, respectively, putting them at an increased risk for an opioid overdose.1,2
In 2010, the American Pain Society conducted a survey establishing that about 41% of American adults reported having chronic, recurrent, or long-lasting pain.3 People of AI/AN heritage may experience chronic pain at higher rates, as they were identified as having the greatest incidence rates of low back pain (35%), arthritis (25%), and obesity (40%), which are often significant contributing factors to chronic pain.4-6
These conditions suggest a need for intensified management of chronic pain among IHS patients. The authors’ IHS facility is a closed health-system network where pharmacists are integral components of the health care team throughout the ambulatory care, emergency, and inpatient departments.
Related: Pharmacist Pain E-Consults That Result in a Therapy Change
Given that medications play a central role in the treatment of chronic pain, pharmacists are appropriate leaders for chronic pain management teams. Pharmacists can improve patient outcomes by conducting pain assessments, managing adverse events (AEs), identifying optimal medication choices, determining equianalgesic dosing, and managing care through care protocols.7
The primary objective of the multidisciplinary chronic pain management clinic (MCPMC) is to manage complicated and postsurgical patients, using a multimodal approach. Primary care providers (PCPs), which include physicians, nurse practitioners (NPs), physician assistants (PAs), and pharmacist providers collaborate to meet this goal by minimizing disease progression, preserving activities of daily living (ADL), maintaing employment, preventing an increase in pain, using treatment plans that include pharmacologic, interventional, and complementary components, decreasing emergency department (ED) visits for chronic pain issues, improving pain agreement adherence, managing AEs, performing drug abuse and diversion surveillance, and using sustained-release (SR) opioids when appropriate. Sustained release opioids not only ease dosing schedules and increase adherence, but also improve sleep, functionality, and quality of life (QOL) for chronic pain patients.8
Methods
The MCPMC began enrolling patients in January 2011 and has continued to date. Inclusion criterion is the presence of pain lasting 3 months or more. Exclusionary criteria are the presence of malignant pain, aged < 18 years, pregnancy, unmanaged psychiatric disorders, and a referral not approved by a PCP. Referrals are accepted from providers throughout the facility, including the ED, which then require approval by the PCP before enrollment. The PCP continues to manage these patients through consultations with the MCPMC pharmacists following MCPMC appointments and at separate ambulatory care clinic appointments.
Currently, there are 2 pharmacists practicing in the MCPMC clinic in conjunction with other health care providers, including 5 physical therapists, 1 psychiatrist, 2 clinical social workers, and 15 PCPs, including NPs and PAs. Additionally in 2014, the clinic became a yearlong rotation in the PGY-1 pharmacy practice residency.
Related: Evaluation of Methadone-Induced QTc Prolongation in a Veteran Population
After enrollment, a pharmacist reviews patients’ health records for past pain medications, interventional and complementary treatments, adherence to these treatments, recent ED visits and medications received, urine toxicology results, adherence to pain agreements, and the Arizona Controlled Substances Prescription Monitoring Program Database (ACSPMPD).
During the initial MCPMC appointment, a pain assessment questionnaire (PAQ) is completed with a MCPMC pharmacist. The questionnaire, designed specifically for the MCPMC, consists of a comprehensive pain assessment, including functional status and common comorbidities, such as anxiety, depression, obesity, and insomnia. Patients provide feedback on efficacy of past or current medications, and interventional and complementary treatments if applicable. Patients also rate their satisfaction with health care received and develop goals for their treatment and overall health.
A collaborative treatment plan is then developed with the patient’s PCP. Treatment plans often consist of increasing or starting interventional and complementary treatments, SR opioids, and adjuvant medications. Common adjuvant medications include nonsteroidal anti-inflammatory drugs (NSAIDs), antidepressants, antiepileptics, immunosuppressants, disease-modifying antirheumatic drugs (DMARDs), and topical agents. To maximize benefits of the medications, antidepressants are often prescribed for dual purposes among patients with comorbid conditions, such as anxiety, depression, and insomnia. Among obese patients, weight loss is encouraged, and patients may be referred to dietary counseling and exercise programs. Other intentions of the treatment plans are to decrease breakthrough pain and ED visits while attempting to decrease the use of immediate-release (IR) opioids. Treatment plans are executed in a stepwise approach over multiple MCPMC visits and may be modified throughout the course of the program.
To ensure that medication changes and other issues can be addressed when a prescriber is available, all subsequent visits are scheduled when patients are due for a pain medication refill. The MCPMC pharmacists chose not to pursue prescriptive authority but have privileges to order urine toxicology tests, make nonformulary requests, and refer patients for complementary treatments. Subsequent appointments are commonly scheduled 1 to 4 weeks apart or alternate with PCP appointments.
Related: Multidisciplinary Approach to Back Pain
During each appointment, data are collected to record changes in therapy and pain levels. Questions regarding general health and adherence to pharmacologic, interventional, and complementary treatments, exercise regimens, and specialty referrals are asked of all patients. Additionally, follow-up PAQs are completed every 6 months to track progress in therapy, pain control, treatment plan adherence, and patient satisfaction. To determine pain agreement adherence, the ACSPMPD is reviewed monthly, and urine toxicology tests and pill counts are performed randomly at MCPMC visits.
In October 2013, all PCPs who had patients in the clinic completed a survey to assess their perception of the MCPMC. Questions were related to their satisfaction with the clinic as well as their opinion of patients’ satisfaction. Other questions were related to their view of patient care and outcomes compared with those of the general chronic pain patients at the facility.
Results
As of January 2013, 106 patients had been referred to the MCPMC by 17 PCPs. Thirty-six of these patients were still actively participating in the clinic, while 25 were pending review. Of the remaining 45 patients, 30 were denied initial enrollment, and 15 were disenrolled from the clinic over the previous 2 years. Patients were determined to be inappropriate candidates and not enrolled in the clinic for the following reasons: referral not approved by the PCP, patient refused care, patient had not established care with a PCP, mental health issues, pediatric patient, oncology patient, and death prior to the initial review. Patients were disenrolled from the MCPMC clinic before 2013 for the following reasons: not participating in their treatment plan, illicit drug use, seeking care from other PCPs, suspected diversion, death due to a nonpain-related issue, and remained stable on the medication regimen and were released back to the care of their PCP.
In 2013, there were 47 new referrals to the MCPMC, resulting in a total of 153 referrals since the clinic’s 2011 inception. Over the course of 2013, 31 new patients were enrolled, 32 referrals were denied (15 of which remained from 2012), and 36 patients were disenrolled (Figure 1). At the end of 2013, 31 patients remained active, while 9 referrals remained pending review. A total of 67 patients participated in the MCPMC at some point during 2013 and were included in the data collection. Patients by diagnoses are displayed in Figure 2.
In 2013, patients were scheduled for a total of 337 MCPMC appointments, and 298 (88%) were completed by patients, a 17% increase above 2012. The mean show rate of PCP ambulatory care clinic appointments was about 70%. The completed MCPMC visits for 2013 correlates to about 6.8 MCPMC visits annually per patient. Of the 67 patients included in data collection, the mean total number of months active in the clinic was 12.5. The mean number of months active in the clinic in 2013 was 6.9.
Pain Assessment Questionnaire
In 2013, 27 patients (40%) were enrolled in the clinic for 6 months or more and completed a follow-up PAQ. Throughout 2013, MCPMC patients presented to the ED for care 76 times, which correlates to about 1.8 ED visits annually per patient. MCPMC patients also attended an appointment with their PCP on average 3.7 times per year and provided urine toxicology tests on average 4.3 times per year between MCPMC and PCP visits.
Data collected from follow-up PAQs in January 2014 provided information on the 27 MCPMC patients enrolled in the clinic for 6 months or more. This review indicated alterations in patients’ reported pain levels, functional status, patient satisfaction, and adherence to pain agreements from before and after enrollment in the clinic. Additional information was collected using the electronic health record to reveal the adjustments in treatment plans, including pharmacologic, complementary, and interventional treatments, along with adherence to these treatments.
Patients’ self-reported pain levels at the time of appointment and average pain levels since the previous appointment were documented at each visit for the 27 MCPMC patients. These 2 pain levels were then compared with the levels of the initial assessment and the most recent appointment. Results were inconsistent; however, slight trends were observed with the analysis. The mean change in pain reported at the time of assessment decreased 5.1%. The mean change in average reported pain since the previous appointment also decreased 6.9%. Statistical analysis was performed using the Wilcoxon signed rank test. Both decreases in reported pain were not clinically or statistically significant (P = .21 and P = .17, respectively). Eleven (41%) patients had improvement in average pain, whereas 10 (37%) had no change, and 6 (22%) reported increased average pain levels.
Data on alterations in functional status and ADL were also collected from the 27 MCPMC patients. These patients reported the perceived degree of difficulty, on a scale of 1 to 5, required to complete tasks and get through their day. A rating of 1 represented the ability to complete activities with no difficulty, whereas 5 represented an inability to complete the tasks. For each of the 19 tasks, the differences in scores from the initial to the most recent PAQs were recorded as either a positive or negative alteration for each patient, and the sum of these differences was recorded as an overall positive or negative change in function. A positive change in function indicated an improvement in function, whereas an overall negative change indicated a decrease in ability to complete daily activities.
Twenty-six percent of the 27 pa-tients had a cumulative positive change of up to 5 points, and 19% had a positive change of 6 or more points. Alternatively, 22% of patients had a cumulative negative change of up to 5 points, and 33% of patients had a negative change of 6 points or more. The greatest positive change was 15 points, the greatest negative change was 28 points, and the median change from the initial to the most recent assessments was a negative change of 2 points.
Adjuvant Medications
The pharmacologic component of the treatment plans consisted primarily of optimizing the use of adjuvant medications and SR opioids when appropriate, while minimizing the use of IR opioids and other controlled medications. Of the 67 MCPMC patients in 2013, 55% were on IR opioids alone, a slight increase from 46% in 2012 (Table 1). Eighty-one percent of patients in this group were on ≤ 15 mg of morphine equivalent daily dose (MEDD), which would have required at least a doubling of their dose to initiate the preferred formulary SR opioid, morphine SR tablets. Six percent of patients were on SR opioids alone, also a slight increase from 3% in 2012. Twenty-seven percent of patients were prescribed a combination of IR and SR opioids. Nine percent of patients had been recently transitioned to SR opioids while in the MCPMC, of which 1 patient was prescribed the medication as monotherapy. Twelve percent of patients were not on any opioid therapy throughout 2013.
Opioids were switched to an alternative opioid at some point during the year to minimize tolerance in 15% of patients, of which 9% were IR and 6% were SR opioids. Changes in opioid therapy from the beginning to the end of the year were recorded as a decrease, increase, or no change in MEDD. Doses were decreased for 16%, increased for 27%, and not changed for the remaining 45% of patients. The sum of these changes for the 59 patients on opioids was a decrease of 172 mg MEDD or, on average, a decrease of about 3 mg MEDD per patient. Throughout the year, 36 patients were disenrolled from the clinic, and a total of 941 mg MEDD were discontinued by patients’ PCPs. This resulted in a mean of about 26 mg MEDD discontinued per patient. These statistics demonstrate small trends in decreasing overall MEDD in MCPMC patients.
Adjunctive therapies were often used in 67 MCPMC patients in addition to their opioid medications. If possible, therapies for pain management were chosen to maximize the ability to benefit comorbidities, such as depression, anxiety, and insomnia, while also treating chronic pain. The most frequently prescribed class of medications was antidepressants with 63% of patients prescribed one or more: bupropion, serotonin-norepinephrine reuptake inhibitor, selective-serotonin reuptake inhibitor, and tricyclic antidepressants. The next top 3 medication classes after antidepressants were topical medications (54%), antiepileptics (48%), and muscle relaxers (42%). The single most frequently prescribed adjunctive medication was gabapentin (37%), an antiepileptic.
Complementary Treatments
Complementary treatment referrals were followed throughout 2013 and compared with referrals from 2012 (Table 2). Physical therapy (PT) and exercise programs continued to be the most frequently referred treatment programs within the facility. Fifty-two percent of 67 MCPMC patients did not attend any PT appointments as recommended, of which the majority were required to attend as a component of their pain agreement. Of the remaining patients referred to PT, 48% went to their initial visit, 40% attended a second, and 32% attended 3 or more appointments. Of the group that attended 3 or more appointments, patients completed about 70% of the overall scheduled appointments, which was below the facility averages of 75% in 2012 and 80% in 2013.
Acupuncture, transcutaneous electrical nerve stimulation, and osteopathic manipulative therapy (OMT) were much less frequently suggested treatments, with percentages of patient referrals of 22%, 21%, and 6%, respectively. Sixty percent of patients referred to acupuncture attended the initial visit, 47% attended a second, and 40% attended 3 or more appointments. Of this group that attended at least 3 appointments, patients completed 75% of scheduled appointments, which was also below the facility averages of 86% in 2012 and 81% in 2013. Only 50% of patients referred to OMT attended the initial visit, of which these patients completed 100% of their scheduled appointments. This rate of attendance was above the facility averages of 60% in 2012 and 68% in 2013. Thirteen percent of patients were referred for interventional pain management and completed 1 of 3 types of injections (onabotulinumtoxinA, spinal, or intra-articular). There was a slight decrease in patients without complementary treatment referrals from 14% in 2012 to 13% in 2013.
Adherence
Pain agreement adherence was determined by assessing ED visits, urine toxicology results, and ACSPMPD search results. Sixty-one percent of the 67 MCPMC patients did not seek care in the ED, whereas 12% had 1 visit in 2013. This decrease in frequency of ED visits was significant compared with these same MCPMC patients from prior to participation in the clinic. The mean ED patient visits per year decreased from 5.1 to 1.8.
Urine toxicology tests were completed on 54 of the 67 MCPMC patients in 2013. Overall, urine toxicology reports were determined to be appropriate at the initial review 51% of the time, with 30% of patients having all of their reports completely appropriate. Of the 54 patients, 35% were disenrolled for inappropriate urine toxicology reports for the following reasons: negative for opioids, positive for opioids without a prescription, positive for amphetamines with additional confirmation testing, and positive for barbiturates without a prescription. Six percent of patients were discovered to have trace amphetamine results that were sent out for confirmation, but these reports were found to be negative, thus confirming an initial false-positive result.
Forty-eight percent of MCPMC patients tested negative for opioids at some point during the year when they were expected to have positive results. Of this group, 31% were prescribed morphine; the remaining patients were prescribed synthetic or semisynthetic opioids that are known to cause false-negative results: fentanyl (4%), hydrocodone (50%), and oxycodone (15%).9 Twenty-two percent of patients were disenrolled from the clinic for testing negative for opioids. The reason for disenrollment was often in conjunction with other behaviors that resulted in violations of their pain agreement. The remaining 78% reported running out of pain medications early and remained in the clinic. Two percent of patients were discovered to have a positive opioid result when it was expected to be negative. This group reported finding previously prescribed medications and subsequent results were appropriate, thus they remained in the clinic. Lastly, 2% of patients tested negative for barbiturates when it was expected to be positive. These patients reported running out of pain medication early as well.
The ACSPMPD was also used to assess pain agreement adherencee for all MCPMC patients. Six percent of patients were identified as seeking care from providers outside the IHS facility and receiving prescriptions for opioid medications, thus violating their pain agreements. Seventy-five percent of these patients were disenrolled from the MCPMC for this reason. PCPs referred the other 25% of patients, and the outside prescribers had performed procedures on them. These patients were reminded of their pain agreements, and no further violations were discovered according to the database. Each patient’s status in the MCPMC was evaluated on a case-by-case basis, and often decisions to disenroll or continue treating patients were based on the PCP’s clinical judgment.
Patient satisfaction was measured in the follow-up PAQ by asking 27 patients how they felt about their care, using a typical 5-point Likert scale. The 2 statements were, “I am pleased with the care that I have received for my pain,” and “I believe that I am receiving the best health care available.” Seventy percent of patients answered “strongly agree” or “agree” to the first statement, and 67% of patients answered the same for the second statement. Nineteen percent of patients answered “not sure” to the first statement, and 22% of patients answered the same for the other statement. Eleven percent of patients responded, “disagree” or “strongly disagree” to both statements.
In October 2013, 12 PCPs who had patients in the MCPMC completed an online survey regarding their perception of patient outcomes, time spent providing care to chronic pain patients, comparisons with general chronic pain patients, and satisfaction with the clinic. Most of the PCPs reported they spent 15 to 30 minutes on MCPMC patients compared with 30 to 60 minutes on general chronic pain patients each month. Most of the PCPs stated that they required ambulatory care clinic visits with chronic pain patients every other month, whereas MCPMC patients needed to be seen only quarterly. PCPs agreed that having their patients participate in the MCPMC resulted in better pain control, improved adherence to treatments, increased diversion and abuse surveillance, and better access to pain medications. Eleven of 12 PCPs stated that they were very satisfied with the MCPMC.
Discussion
The ultimate goal for patients of the MCPMC is to minimize disease progression, prevent an increase in pain, and improve adherence to treatment plans, including pharmacologic, interventional, and complementary components. According to the change in reported pain levels from the initial to the most recent assessment, most patients met the goal of preventing an increase in pain. There was a trend toward a decrease in reported pain, though it was not clinically or statistically significant. The follow-up PAQ measured varying changes in functional status and often demonstrated disease stabilization or progression, not improvement among patients. Forty-five percent of patients showed improvements, and 55% reported more difficulty performing daily activities. The median change between all 27 MCPMC patients was an overall decline in function of 2 points. This worsening in function over time would be expected for most of the chronic pain conditions.
In 2013, 9% of patients were initiated on SR opioids, making a clinic total of 33% of patients on SR medications. More than half the patients were on IR opioids as monotherapy, which is not an ideal treatment for chronic pain management. However, 81% of this group was on 15 mg MEDD or less. The use of SR opioids may or may not reduce abuse potential but can improve patient outcomes. Overall, there was an emphasis on using SR opioids when appropriate while continuing to improve patient outcomes. Over 61% of patients remained on the same opioid doses or were decreased over the course of 2013. There was also a significant use of adjuvant medications, primarily antidepressants, antiepileptics, and topical pain relievers. The most frequently prescribed non-opioid medication, excluding NSAIDs, was gabapentin. This medication has abuse potential and was treated as a controlled medication by the MCPMC during this period.
After enrollment in the MCPMC, patients used complementary and interventional treatments more consistently than prior to enrollment in the clinic. Treatments such as injections, acupuncture, OMT, and PT may reduce opioid medication consumption in the long term or slow the progression of disease for most patients. The improvement in QOL and lack of disease progression in these patients is not objectively measurable; however, the summative progress may be subjectively evaluated through reported pain levels and patient satisfaction.
For MCPMC patients who remained in the clinic, PT and acupuncture attendance was 70% and 75%, respectively. Although these were improvements in adherence for many MCPMC patients, the rates were still below the facility average completion rates of 80% and 81%, respectively. It could be argued that patients with acute pain are typically seen in PT for shorter periods and with fewer possibilities of missing appointments. Conversely, the single active MCPMC patient who attended OMT had a 100% completion rate compared with the average facility OMT attendance of 68%.
Other goals of the MCPMC consist of managing AEs, minimizing ED visits, monitoring for drug abuse and diversion, and improving adherence to pain agreements. The substantial 65% decrease in ED visits can be attributed to the patients’ participation in the MCPMC. Before enrollment, many patients would frequent the ED, because their PCP was not available. The cost savings from minimizing ED visits, provider and staff time, and resources is difficult to measure due to low rates of collections from insurance supplemental to IHS insurance yet is a significant benefit to the IHS facility.
Conclusions
Since the implementation of the MCPMC, patient outcomes have improved due to more consistent drug abuse and diversion surveillance of chronic pain patients rather than performing surveillance because of a suspicion of inappropriate medication use. Frequently using the pain agreement and monitoring parameters constructed a more trusting relationship between the PCP and the patient, and identified patients inappropriate for long-term opioid therapy. Identifying these patients was an unintentional, yet positive outcome.
Additionally, PCPs reported spending half the time with MCPMC patients vs general chronic pain patients. Patients who were not compliant with their pain agreements were discontinued from opioid therapy and were disenrolled from the clinic. Patients who have remained active have become more compliant with their pain agreements and treatment plans than they had been before enrollment. The MCPMC has ultimately relieved a significant burden from primary care and ED providers while improving outcomes and satisfaction of chronic pain patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
2. Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-38, HHS Publication No. (SMA) 10-4586. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
3. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey.
J Pain. 2010;11(11):1230-1239.
4. Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine (Phila PA 1976). 2006;31(23):
2724-2727.
5. Bolen J, Schieb L, Hootman JM, et al. Differences in the prevalence and impact of arthritis among racial/ethnic groups in the United States, National Health Interview Survey, 2002, 2003, and 2006. Prev Chronic Dis. 2010;7(3):A64.
6. Schiller JS, Lucas JW, Ward BW, Perogoy JA. Summary health statistics U.S. adults: National Health Interview Survey, 2010. National Center for Health Statistics. Vital Health Stat. 2012;10(252). Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/data/series/sr_10/sr10_252.pdf. Accessed June 26, 2015.
7. Strickland JM, Huskey A, Brushwood DB. Pharmacist-physician collaboration in pain management practice. J Opioid Manag. 2007;3(6):295-301.
8. Rauck RL. What is the case for prescribing long-acting opioids over short-acting opioids for patients with chronic pain? A critical review. Pain Pract. 2009;9(6):468-479.
9. Pesce A, West C, Egan City K, Strickland J. Interpretation of urine drug testing in pain patients. Pain Med. 2012;13(7):868-885.
1. Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
2. Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-38, HHS Publication No. (SMA) 10-4586. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
3. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey.
J Pain. 2010;11(11):1230-1239.
4. Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine (Phila PA 1976). 2006;31(23):
2724-2727.
5. Bolen J, Schieb L, Hootman JM, et al. Differences in the prevalence and impact of arthritis among racial/ethnic groups in the United States, National Health Interview Survey, 2002, 2003, and 2006. Prev Chronic Dis. 2010;7(3):A64.
6. Schiller JS, Lucas JW, Ward BW, Perogoy JA. Summary health statistics U.S. adults: National Health Interview Survey, 2010. National Center for Health Statistics. Vital Health Stat. 2012;10(252). Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/data/series/sr_10/sr10_252.pdf. Accessed June 26, 2015.
7. Strickland JM, Huskey A, Brushwood DB. Pharmacist-physician collaboration in pain management practice. J Opioid Manag. 2007;3(6):295-301.
8. Rauck RL. What is the case for prescribing long-acting opioids over short-acting opioids for patients with chronic pain? A critical review. Pain Pract. 2009;9(6):468-479.
9. Pesce A, West C, Egan City K, Strickland J. Interpretation of urine drug testing in pain patients. Pain Med. 2012;13(7):868-885.
A Qualitative Study of Treating Dual-Use Patients Across Health Care Systems
The VHA assigns all enrolled veterans to a primary care provider (PCP). However, almost 80% of veterans enrolled in VHA have another form of health care coverage, including Medicare, Medicaid, private insurance, and TRICARE for Life program.1 Consequently, veterans may choose to use more than 1 health care system to manage their health care needs.
Studies based on merged VHA and Medicare claims data have demonstrated substantial dual use by VHA enrollees with Medicare. Petersen and colleagues reported that about 80% of VHA enrollees with Medicare chose to use services in both systems and that greater distance to VHA facilities and lower priority level for VHA care predicted lower VHA reliance.2 Among those aged < 65 years who had Medicare due to disability, 58% weredual users. These dual users relied more on private sector care for many health conditions, with the notable exception of substance abuse and mental health disorders, for which reliance on VHA care was greater.2 Another study found that over half of VHA enrollees assigned to a PCP at a community-based outpatient clinic (CBOC) received some or all of their care outside VHA and that reliance on VHA outpatient care declined over the 4-year study period.3
Related: Mutual Alignment Trumps Merger for Joint VA/DoD Health Care Programs
This use of multiple health care providers (HCPs), facilities, and modalities is often described as dual use or comanagement. Dual use in the case of veterans refers to use of both VHA and non-VHA health care, whereas comanagement implies an expectation of shared decision making and open communication between VHA and non-VHA providers. In addition to VHA PCPs, rural veterans frequently receive care from local, non-VHA HCPs in the community where they live. As health care in the U.S. evolves and patients have increasing choices through the Affordable Care Act (ACA), the challenge of comanagement for patients receiving care in multiple systems is likely to increase both within and outside VHA.
This study was part of a qualitative rural health needs assessment designed to ascertain the issues facing rural veterans and their providers in the upper Midwest.4 The objective was to examine VHA primary care clinic staff perspectives on dual users, perceived barriers that inhibit comanagement, and factors that contribute to the need for dual use in rural areas.
Methods
A qualitative study design with in-person interviews was used to elicit the perspective of VHA clinic staff on the current and ideal states of comanagement. Clinics were selected using a stratified purposeful sample of 15 urban and rural primary care clinics at VHA CBOCs and VAMCs in 8 Midwestern states (Illinois, Iowa, Minnesota, Nebraska, North Dakota, South Dakota, Wisconsin, and Wyoming). The stratification criteria included (1) urban and rural; (2) geographic coverage of VISN 23; and (3) VHA-managed and contract clinics, resulting in a purposeful sample of 2 urban VAMC clinics, 3 urban CBOCs, 7 rural VHA-managed CBOCs, and 3 rural contract CBOCs. The distance from the CBOC to the closest VAMC ranged from 32 to 242 miles.
Related: VA Relaxes Rules for Choice Program
Interview guides were developed and tested by the research team for comprehension, length, and timing prior to data collection and iteratively revised as analysis evolved and new topics emerged. Clinic staff were asked about their perceptions of rural veteran use of VHA care; barriers and facilitators to accessing care; and their personal experience working within VHA. Several questions focused on dual use and why rural veterans use multiple health care systems, their perspectives of dual use, their expectations of patients’ role(s) in health care coordination, and the perceived barriers that inhibit comanagement. Interviewers used comanagement and dual use interchangeably to discuss patients with multiple care providers, allowing interviewees to use their preferred terminology; assigned meanings were probed for clarification but not corrected by interviewers.
Between June and October 2009, teams of 2 to 3 researchers visited 15 clinics for 1 to 2 business days each. Researchers conducted interviews with a convenience sample of clinical staff. Consent forms and an explanation of the study were distributed, and those electing to participate voluntarily came to a designated room to complete an interview. All interviews were audio recorded for accuracy.
Interview recordings were transcribed verbatim and reviewed for accuracy. Prior to coding, transcripts were imported into a qualitative data management software program. A codebook, including a priori research hypotheses and de novo themes, was developed based on a systematic review of a randomly selected subset of interview transcripts.5 Four coders were responsible for coding all transcripts and validating coding through tests of agreement at predetermined intervals.
Regular meetings were conducted with coders and the lead qualitative investigator to discuss disagreements, clarify code definitions, or add new codes as needed. As codes were added, previous transcripts were coded/recoded for content related to the new codes. An audit trail was maintained, and iterative mediation of codes continued throughout the process. The final codebook contained 42 thematic codes, which reached saturation or data redundancy.6 Detailed analysis of the codes dual use, distance, and care coordination were used to inform this study.
Results
Among the 15 sites, 64 in-depth individual interviews were conducted, ranging from 5 to 53 minutes (average 26 minutes). Clinic staff demographic characteristics are depicted in the Table. Analysis of data captured in the codes dual use, distance, and care coordination resulted in notable concentration in 4 thematic areas: (1) clinic staff perceptions of the influence of access, convenience, and distance on dual use for rural patients; (2) communication and patient’s role in comanagement; (3) rules and regulations related to comanagement from the VHA perspective; and (4) barriers to comanagement and recommendations for education.
Influence of Access, Convenience, and Distance
Access to health care was central to the discussion of dual use and comanagement by clinic staff. Convenience was identified as the primary reason for rural patients’ use of non-VHA services, as many rural patients must travel outside their local community to access VHA care. Thus, dual use was most often noted for services typically available in patients’ local communities, especially management of chronic conditions.
The CBOCs provide important services for primary care and management of chronic conditions but are not available in all communities and may have limited hours/days that do not fit with patients’ schedules. The CBOCs are often unable to provide needed services, including but not limited to emergency care, diagnostic tests, physical and occupational therapy, and other specialty care services. As one VHA provider put it, “The biggest factor for [dual use] is availability, access, convenience.… It’s a lot more convenient to go to the hospital down the street than it is to go 120 miles to [the VAMC], or for some guys who live 30, 40 minutes the other side of here it becomes 150, 160-mile one-way trip.”
Related to access, distance and transportation barriers were identified by clinic staff as obstacles to care for rural patients. Despite efforts to offset the expense of travel through reimbursement to qualified veterans and coordinated van transport with Veterans Service Organizations, travel costs—both time and money—were seen as significant barriers to accessing VHA care, as was an inability to travel for those who are ill or frail and elderly. “We send people … in the van and for the most part that works, but eventually it gets expensive, or you’ve got somebody with chronic pain that can’t tolerate the van ride for 2 hours,” one interviewee
reported.
According to clinic staff, dual-use patients also rely on non-VHA providers in particular for urgent or emergency care, while relying on VHA primary care for reduced-cost medications, diagnostic testing, chronic disease management, or annual exams. When asked why rural patients may choose to see more than 1 provider, VHA providers responded. “[It’s] more convenient to have a local doctor just in case something went wrong and they need to see a doctor right away. So distance to this clinic would be the number one reason.” Another reported, “If it’s once or twice a year routine appointments they’ll come here, but… they’d rather go to a walk-in clinic nearby than spend so much [money] on gas.”
Communication and Patients’ Role
Communication between VHA and non-VHA providers is a necessary element of comanagement. Although phone calls or faxing patient medical records are available options, clinic staff reported it was more common to encounter patients hand carrying their records between providers. For dual-use patients, clinic staff indicated it was often unclear who was responsible for relaying information between providers. There is often ambiguity about who will (and should) fulfill this role and not enough time to adequately address or clarify how this is done. Some clinic staff believed that acting as the main conduits of information placed an undue burden on the patients, particularly asking them to be able to accurately relay medical information about tests or prescriptions that they may not fully understand. Others said that it was primarily the patients’ responsibility to give relevant information about their care to all their providers, because of VHA regulations and patient privacy laws. “[The] patient should tell the primary doctor to send them [medical records] because we can’t get the medical records without the patient’s permission,” said one provider.
Another provider utilized the nursing staff to call patients after their appointments to remind them to give their medical records to their non-VHA provider. The data suggest that responsibility for maintaining communication between providers ultimately falls on the patient. From the perspective of a nurse practitioner, “We just keep trying to educate the community…. I’ve been told that if the patient wants that privilege of using the VA for a pharmacy for an outside provider that we’re glad to do that. But it is their responsibility to communicate with their [non-VHA] physician. I think we just need to keep educating the patients.”
Rules and Regulations
VHA policies governing prescriptions, hospitalizations at outside facilities, and release of patient information regulate, and in some cases hinder, information flow between VHA and non-VHA providers. Many patients use VHA to obtain medications for lower out-of-pocket costs. This contributes to the number of dual-use patients in VHA and results in several challenges for VHA providers trying to manage patients’ prescriptions. For example, patients will ask to fill a prescription at a VHA pharmacy from their non-VHA providers; however, VHA pharmacies can only fill prescriptions from VHA providers.
Many VHA providers are willing to rewrite these prescriptions, but they may need to see the patient before adding or changing the prescription and require documentation to address contraindications, adverse reactions and/or therapeutic failure, and associated risks before making the authorization. VHA providers noted that because the VHA formulary does not contain all medications, non-VHA providers are often unfamiliar with the VHA National Formulary specifics and will write prescriptions for nonformulary medications, which require a nonformulary request from a VHA provider.
Clinic staff also mentioned difficulty in obtaining records from non-VHA providers. This can be particularly problematic if the patient lives a distance away from a VHA facility and does not have the necessary authorization to share records on file.
Barriers and Education Recommendations
Clinic staff identified coordination of care for dual-use patients as a barrier to providing care. Specifically, providers identified coordination as complicated by communication difficulties, inefficient medical record exchange, short staffing in VHA clinics, duplication of diagnostic services, and non-VHA providers’ lack of understanding regarding the services that VHA provides. Specific to rural clinics, comanagement was reportedly hindered by limitations in technology (eg, consistent Internet access), access to routine diagnostic services, and lack of relationships with non-VHA providers. Providers most frequently reported that the critical piece missing in comanagement is a relationship—and implied communication—between VHA clinics and non-VHA community clinics. The concept of a relationship between providers is evoked as a critical element to comanaging dual-use patients; however, clinic staff had a difficult time articulating what that relationship would actually look like if put into practice.
Related: Patients Benefit From ICU Telemedicine
In spite of the numerous barriers identified by clinic staff, the recommendation for education to improve comanagement was consistent across study sites and clinic staff roles. Education was proposed for patients and non-VHA providers as the best intervention. In response to a question about ideas and recommendations to improve comanagement, clinic staff drew on varied experiences. To illustrate this theme, a provider gave this example of dual-use patients seeking prescription medication from VHA and its impact on comanagement: “I would [recommend] an outreach program to community resources and [non-VHA] providers. To let them know more about how the VA works and the resources that are available, and how specifically to coordinate care through the VA, would be a significant benefit.… If the [non-VHA] providers knew how to—who to—talk to, what information the VA needs, for example, for medication changes, it would help the patients make it work…without having to overburden the patients with having to physically hand carry their blood test results, or their notes, discharge summaries, procedure notes.”
Along with providing outreach and education on working with the VHA, clinic staff addressed the need to educate patients more effectively, because they are seen as central to the information exchange. There is motivation on the part of patients to learn the system. “Just making sure that the patients realize that they need to tell their local providers to send us the records and make sure that there is an exchange going on consistently,” explained a case manager. “If the patient wants to get those medications that are costly, then they figure out pretty quick what they have to have, what they need to send to us.” The need for education is an ongoing process; who is responsible for this continues to be a point of debate.
Discussion
In order to better understand comanagement of dual-use patients, this study focused on the experiences and perceptions of staff at VHA primary care clinics in the upper Midwest. The data indicated that:
- VHA clinical staff perceive the primary reason patients choose to seek non-VHA care is because of access, convenience, and
distance - In order for comanagement to occur, communication and information exchange—currently facilitated largely by patients—needs to improve
- Education of patients and their non-VHA providers is recommended, to increase understanding of rules and regulations tied to exchange of patient information across health care systems
- Education may facilitate communication, develop relationships, and overcome barriers to information exchange
Distance to health care and perceived convenience were clearly seen by clinic staff as the driving factors behind their patients’ dual use. In the authors’ prior work, interviews with veterans and their VA providers supported this assertion as well; however, it was also found that distance must not be understood in isolation of other contingencies, such as urgency of need.4
Clinic staff identified institutional and individual barriers that lead to miscommunication and confusion on the part of patients and reported misunderstandings with non-VHA providers, including 3 potential barriers to comanagement. These included (1) inconsistent communication and flow of information between VHA and non-VHA providers; (2) uncertainty about who will (and should) be responsible for information flow between providers; and (3) VHA and federal regulations over patient privacy. Throughout the interviews, access to less expensive prescription medications in VHA was considered an additional driver of dual use. According to clinic staff interviewed, education of patients and non-VHA providers could facilitate efficient and safe comanagement for dual-use patients.7
This study suggests both advantages and disadvantages for patients choosing to use multiple health care systems from the perspective of the clinic staff. The primary advantage is better overall health care access, especially for rural patients and those with longer travel times to VHA facilities. The primary disadvantage of dual use is discontinuity of care between multiple care sites. Specifically, this study identified concerns regarding poor communication between providers and transfer of patient medical records. An underlying theme was a concern for quality of care and patient safety, which are recognized by others in the literature as potential consequences of inadequate comanagement.8-12
If there is one aspect of co-management for dual-use patients to target, this study’s findings point to developing strategies to improve communication between providers caring for dual-use patients and, more specifically, cultivating relationships that are currently underdeveloped. This will necessitate a clearer articulation of what constitutes a relationship between comanaging providers and is a direction for further research that would have applicability beyond VHA to any comanagement of patients using multiple health care systems.
There are 3 simultaneous, yet unrelated, factors that may contribute to increasing dual use. First is the rise in VHA eligible veterans from Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn.13,14 All returning veterans who meet minimal requirements are eligible for 5 years of VHA health care. A large proportion of these individuals are in the Reserve and National Guard, most of whom have nonmilitary jobs that may provide employer-based health insurance. Thus, these veterans have a greater opportunity for dual use. Second, with the aging cohort of Vietnam-era veterans, a greater proportion is becoming Medicare eligible. Third, with the recent passing of the ACA, more patients, including veterans, may choose to purchase insurance through ACA health exchanges. Taken individually or collectively, these factors will likely have effects reaching beyond VHA, especially when veterans receiving care in non-VHA health care systems engage in dual use.3,13,15,16
Limitations
This study has a number of limitations. First, it was limited to VHA facilities located in the upper Midwest, which may limit generalizability to other parts of the country. The convenience sample of clinic staff at VHA clinics may not represent the full range of perspectives among HCPs generally. This study did not interview clinic staff in non-VHA clinics, although this has been the focus of other studies.17,18 Although dual use also applies to specialty care and related access issues in rural areas, this was not a focus of this study. Last, the data were collected in 2009, prior to the implementation of the patient-aligned care team (PACT) model and prior to the recently revealed issues regarding patient wait times for VHA care. Thus, perceptions may have changed, and additional study is needed.
Conclusions
The results of this study support prior assumptions of barriers to care, but also introduce previously unreported challenges. Dual use is perceived to have both positive and negative impacts, but for the positives to outweigh the negatives, thoughtful comanagement is critical. This may be particularly so in rural areas where dual use is encouraged as a way to overcome distance and increase convenience in accessing care.
As demonstrated by recent events, there are still VHA health care access issues for veterans. Recently, VA leadership and the U.S. Congress proposed that veterans have greater access to community providers as well as VHA in order to overcome delays in care.19 As this option is explored and put into practice, it is more important than ever to consider the need for care coordination and management of dual-use patients, to ensure good communication and care that is timely, safe, and high quality.
Few models exist in which 2 PCPs coordinate across health care systems, and greater understanding of this dual use is needed. This information is important in designing interventions to improve care coordination across systems to ensure continuity of care, patient safety, and patient satisfaction. Although some work has been done to examine the perspectives of non-VA PCPs, little is known about VHA provider perspectives on rural veteran dual use.17,18 This study explores VHA provider perspectives and identifies areas where interventions to improve care coordination across systems might be targeted.
Next steps for intervention studies would be to improve communication and develop educational tools to aid in the coordination of care between VHA and non-VHA providers. A recent example of this is the Co-Management Toolkit developed by the Veterans Rural Health Resource Center-Central Region, which provides information on VHA policies and targets non-VHA providers.20 Although VHA perceptions of comanageing dual-use patients were the target, a similar study of non-VHA providers is important to understand this complex and multifaceted dynamic. Additional work is needed to measure the impact of dual use on clinical outcomes, patient safety and quality, and efficient use of resources, as these are understudied. As dual use continues and potentially increases with the ACA and changing health care in the U.S., it is important to understand the management of patients using multiple health care systems. This is salient as primary care adopts the PACT model and to inform interventions to improve quality and safety while eliminating duplicative health care and costs.
Acknowledgements
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Rural Health, Veterans Rural Health Resource Center-Central Region (VRHRC-CR) and the VA Health Services Research and Development (HSR&D) Service, the Comprehensive Access and Delivery Research and Evaluation (CADRE) Center at the Iowa City VA Health Care System, and Center to Improve Veteran Involvement in Care (CIVIC) at VA Portland Health Care System. Dr. Reisinger was supported by a Research Career Development Award from the Health Services Research and Development Service, Department of Veterans Affairs (CD1 08-013-1).
We would like to thank all health care providers who graciously agreed to participate in this study and VRHRC-CR staff, in particular Monica Paez for assistance on this manuscript.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Department of Veterans Affairs Office of Rural Health, VHA. Veterans Rural Health: Perspectives and Opportunities. Rockville, MD: Booz Allen Hamilton; 2008. http://www.ruralhealth.va.gov/docs/PAO-final-report-0208.pdf. Accessed July 6, 2015.
2. Petersen LA, Byrne MM, Daw CN, Hasche J, Reis B, Pietz K. Relationship between clinical conditions and use of Veterans Affairs health care among Medicare‐enrolled veterans. Health Serv Res. 2010;45(3):762-791.
3. Liu CF, Chapko M, Bryson CL, et al. Use of outpatient care in Veterans Health Administration and Medicare among veterans receiving primary care in community-based and hospital outpatient clinics. Health Serv Res. 2010;45(5 pt 1):1268-1286.
4. Buzza C, Ono SS, Turvey C, et al. Distance is relative: unpacking a principal barrier in rural healthcare. J Gen Intern Med. 2011;26(suppl 2):648-654.
5. Bernard HR, Ryan GW. Analyzing Qualitative Data: Systematic Approaches. Los Angeles, CA: SAGE; 2010.
6. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82.
7. Kramer BJ, Vivrette RL, Satter DE, Jouldjian S, McDonald LR. Dual use of Veterans Health Administration and Indian Health Service: healthcare provider and patient perspectives. J Gen Intern Med. 2009;24(6):758-764.
8. Ajmera M, Wilkins TL, Sambamoorthi U. Dual Medicare and Veteran Health Administration use and ambulatory care sensitive hospitalizations. J Gen Intern Med. 2011;26(suppl 2):669-675.
9. Jia H, Zheng Y, Reker DM, et al. Multiple system utilization and mortality for veterans with stroke. Stroke. 2007;38(2):355-360.
10. Trivedi AN, Grebla RC, Jiang L, Yoon J, Mor V, Kizer KW. Duplicate federal payments for dual enrollees in Medicare Advantage plans and the Veterans Affairs health care system. JAMA. 2012;308(1):67-72.
11. Kaboli PJ, Shivapour DM, Henderson MS, Ishani A, Charlton ME. The impact of primary care dual-management on quality of care. J Prim Care Community Health. 2012;3(1):11-16.
12. Wolinsky FD, Miller TR, An H, Brezinski PR, Vaughn TE, Rosenthal GE. Dual use of Medicare and the Veterans Health Administration: are there adverse health outcomes? BMC Health Serv Res. 2006;6:131.
13. Liu CF, Bryson CL, Burgess JF Jr, Sharp N, Perkins M, Maciejewski ML. Use of outpatient care in VA and Medicare among disability-eligible and age-eligible veteran patients. BMC Health Serv Res. 2012;12:51.
14. Miller EA, Intrator O. Veterans use of non-VHA services: implications for policy and planning. Soc Work Public Health. 2012;27(4):379-391.
15. Bachman SS, Gonyea JG. Improving health care delivery to aging adults with disabilities: social work with dual eligibles in a climate of health care reform. J Gerontol Soc Work. 2012;55(2):191-207.
16. Kizer KW. Veterans and the Affordable Care Act. JAMA. 2012;307(8):789-790.
17. Lampman MA, Mueller KJ. Experiences of rural non-VA providers in treating dual care veterans and the development of electronic health information exchange networks between the two systems. J Rural Soc Sci. 2011;26(3):201-219.
18. Gaglioti A, Cozad A, Wittrock S, et al. Non-VA primary care providers’ perspectives on comanagement for rural veterans. Mil Med. 2014;179(11):1236-1243.
19. U.S. Department of Veterans Affairs. Acting Secretary Gibson outlines problems, actions taken, and budget resources needed to ensure access to care. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/pressrel/pressrelease.cfm?id=2586. Published July 16, 2014. Accessed July 6, 2015.
20. Office of Rural Health Central Region. Co-managed care toolkit. U.S. Department of Veterans Affairs Website. http://www.ruralhealth.va.gov/resource-centers/central/comanagement-toolkit.asp. Updated June 3, 2015. Accessed July 6, 2015.
The VHA assigns all enrolled veterans to a primary care provider (PCP). However, almost 80% of veterans enrolled in VHA have another form of health care coverage, including Medicare, Medicaid, private insurance, and TRICARE for Life program.1 Consequently, veterans may choose to use more than 1 health care system to manage their health care needs.
Studies based on merged VHA and Medicare claims data have demonstrated substantial dual use by VHA enrollees with Medicare. Petersen and colleagues reported that about 80% of VHA enrollees with Medicare chose to use services in both systems and that greater distance to VHA facilities and lower priority level for VHA care predicted lower VHA reliance.2 Among those aged < 65 years who had Medicare due to disability, 58% weredual users. These dual users relied more on private sector care for many health conditions, with the notable exception of substance abuse and mental health disorders, for which reliance on VHA care was greater.2 Another study found that over half of VHA enrollees assigned to a PCP at a community-based outpatient clinic (CBOC) received some or all of their care outside VHA and that reliance on VHA outpatient care declined over the 4-year study period.3
Related: Mutual Alignment Trumps Merger for Joint VA/DoD Health Care Programs
This use of multiple health care providers (HCPs), facilities, and modalities is often described as dual use or comanagement. Dual use in the case of veterans refers to use of both VHA and non-VHA health care, whereas comanagement implies an expectation of shared decision making and open communication between VHA and non-VHA providers. In addition to VHA PCPs, rural veterans frequently receive care from local, non-VHA HCPs in the community where they live. As health care in the U.S. evolves and patients have increasing choices through the Affordable Care Act (ACA), the challenge of comanagement for patients receiving care in multiple systems is likely to increase both within and outside VHA.
This study was part of a qualitative rural health needs assessment designed to ascertain the issues facing rural veterans and their providers in the upper Midwest.4 The objective was to examine VHA primary care clinic staff perspectives on dual users, perceived barriers that inhibit comanagement, and factors that contribute to the need for dual use in rural areas.
Methods
A qualitative study design with in-person interviews was used to elicit the perspective of VHA clinic staff on the current and ideal states of comanagement. Clinics were selected using a stratified purposeful sample of 15 urban and rural primary care clinics at VHA CBOCs and VAMCs in 8 Midwestern states (Illinois, Iowa, Minnesota, Nebraska, North Dakota, South Dakota, Wisconsin, and Wyoming). The stratification criteria included (1) urban and rural; (2) geographic coverage of VISN 23; and (3) VHA-managed and contract clinics, resulting in a purposeful sample of 2 urban VAMC clinics, 3 urban CBOCs, 7 rural VHA-managed CBOCs, and 3 rural contract CBOCs. The distance from the CBOC to the closest VAMC ranged from 32 to 242 miles.
Related: VA Relaxes Rules for Choice Program
Interview guides were developed and tested by the research team for comprehension, length, and timing prior to data collection and iteratively revised as analysis evolved and new topics emerged. Clinic staff were asked about their perceptions of rural veteran use of VHA care; barriers and facilitators to accessing care; and their personal experience working within VHA. Several questions focused on dual use and why rural veterans use multiple health care systems, their perspectives of dual use, their expectations of patients’ role(s) in health care coordination, and the perceived barriers that inhibit comanagement. Interviewers used comanagement and dual use interchangeably to discuss patients with multiple care providers, allowing interviewees to use their preferred terminology; assigned meanings were probed for clarification but not corrected by interviewers.
Between June and October 2009, teams of 2 to 3 researchers visited 15 clinics for 1 to 2 business days each. Researchers conducted interviews with a convenience sample of clinical staff. Consent forms and an explanation of the study were distributed, and those electing to participate voluntarily came to a designated room to complete an interview. All interviews were audio recorded for accuracy.
Interview recordings were transcribed verbatim and reviewed for accuracy. Prior to coding, transcripts were imported into a qualitative data management software program. A codebook, including a priori research hypotheses and de novo themes, was developed based on a systematic review of a randomly selected subset of interview transcripts.5 Four coders were responsible for coding all transcripts and validating coding through tests of agreement at predetermined intervals.
Regular meetings were conducted with coders and the lead qualitative investigator to discuss disagreements, clarify code definitions, or add new codes as needed. As codes were added, previous transcripts were coded/recoded for content related to the new codes. An audit trail was maintained, and iterative mediation of codes continued throughout the process. The final codebook contained 42 thematic codes, which reached saturation or data redundancy.6 Detailed analysis of the codes dual use, distance, and care coordination were used to inform this study.
Results
Among the 15 sites, 64 in-depth individual interviews were conducted, ranging from 5 to 53 minutes (average 26 minutes). Clinic staff demographic characteristics are depicted in the Table. Analysis of data captured in the codes dual use, distance, and care coordination resulted in notable concentration in 4 thematic areas: (1) clinic staff perceptions of the influence of access, convenience, and distance on dual use for rural patients; (2) communication and patient’s role in comanagement; (3) rules and regulations related to comanagement from the VHA perspective; and (4) barriers to comanagement and recommendations for education.
Influence of Access, Convenience, and Distance
Access to health care was central to the discussion of dual use and comanagement by clinic staff. Convenience was identified as the primary reason for rural patients’ use of non-VHA services, as many rural patients must travel outside their local community to access VHA care. Thus, dual use was most often noted for services typically available in patients’ local communities, especially management of chronic conditions.
The CBOCs provide important services for primary care and management of chronic conditions but are not available in all communities and may have limited hours/days that do not fit with patients’ schedules. The CBOCs are often unable to provide needed services, including but not limited to emergency care, diagnostic tests, physical and occupational therapy, and other specialty care services. As one VHA provider put it, “The biggest factor for [dual use] is availability, access, convenience.… It’s a lot more convenient to go to the hospital down the street than it is to go 120 miles to [the VAMC], or for some guys who live 30, 40 minutes the other side of here it becomes 150, 160-mile one-way trip.”
Related to access, distance and transportation barriers were identified by clinic staff as obstacles to care for rural patients. Despite efforts to offset the expense of travel through reimbursement to qualified veterans and coordinated van transport with Veterans Service Organizations, travel costs—both time and money—were seen as significant barriers to accessing VHA care, as was an inability to travel for those who are ill or frail and elderly. “We send people … in the van and for the most part that works, but eventually it gets expensive, or you’ve got somebody with chronic pain that can’t tolerate the van ride for 2 hours,” one interviewee
reported.
According to clinic staff, dual-use patients also rely on non-VHA providers in particular for urgent or emergency care, while relying on VHA primary care for reduced-cost medications, diagnostic testing, chronic disease management, or annual exams. When asked why rural patients may choose to see more than 1 provider, VHA providers responded. “[It’s] more convenient to have a local doctor just in case something went wrong and they need to see a doctor right away. So distance to this clinic would be the number one reason.” Another reported, “If it’s once or twice a year routine appointments they’ll come here, but… they’d rather go to a walk-in clinic nearby than spend so much [money] on gas.”
Communication and Patients’ Role
Communication between VHA and non-VHA providers is a necessary element of comanagement. Although phone calls or faxing patient medical records are available options, clinic staff reported it was more common to encounter patients hand carrying their records between providers. For dual-use patients, clinic staff indicated it was often unclear who was responsible for relaying information between providers. There is often ambiguity about who will (and should) fulfill this role and not enough time to adequately address or clarify how this is done. Some clinic staff believed that acting as the main conduits of information placed an undue burden on the patients, particularly asking them to be able to accurately relay medical information about tests or prescriptions that they may not fully understand. Others said that it was primarily the patients’ responsibility to give relevant information about their care to all their providers, because of VHA regulations and patient privacy laws. “[The] patient should tell the primary doctor to send them [medical records] because we can’t get the medical records without the patient’s permission,” said one provider.
Another provider utilized the nursing staff to call patients after their appointments to remind them to give their medical records to their non-VHA provider. The data suggest that responsibility for maintaining communication between providers ultimately falls on the patient. From the perspective of a nurse practitioner, “We just keep trying to educate the community…. I’ve been told that if the patient wants that privilege of using the VA for a pharmacy for an outside provider that we’re glad to do that. But it is their responsibility to communicate with their [non-VHA] physician. I think we just need to keep educating the patients.”
Rules and Regulations
VHA policies governing prescriptions, hospitalizations at outside facilities, and release of patient information regulate, and in some cases hinder, information flow between VHA and non-VHA providers. Many patients use VHA to obtain medications for lower out-of-pocket costs. This contributes to the number of dual-use patients in VHA and results in several challenges for VHA providers trying to manage patients’ prescriptions. For example, patients will ask to fill a prescription at a VHA pharmacy from their non-VHA providers; however, VHA pharmacies can only fill prescriptions from VHA providers.
Many VHA providers are willing to rewrite these prescriptions, but they may need to see the patient before adding or changing the prescription and require documentation to address contraindications, adverse reactions and/or therapeutic failure, and associated risks before making the authorization. VHA providers noted that because the VHA formulary does not contain all medications, non-VHA providers are often unfamiliar with the VHA National Formulary specifics and will write prescriptions for nonformulary medications, which require a nonformulary request from a VHA provider.
Clinic staff also mentioned difficulty in obtaining records from non-VHA providers. This can be particularly problematic if the patient lives a distance away from a VHA facility and does not have the necessary authorization to share records on file.
Barriers and Education Recommendations
Clinic staff identified coordination of care for dual-use patients as a barrier to providing care. Specifically, providers identified coordination as complicated by communication difficulties, inefficient medical record exchange, short staffing in VHA clinics, duplication of diagnostic services, and non-VHA providers’ lack of understanding regarding the services that VHA provides. Specific to rural clinics, comanagement was reportedly hindered by limitations in technology (eg, consistent Internet access), access to routine diagnostic services, and lack of relationships with non-VHA providers. Providers most frequently reported that the critical piece missing in comanagement is a relationship—and implied communication—between VHA clinics and non-VHA community clinics. The concept of a relationship between providers is evoked as a critical element to comanaging dual-use patients; however, clinic staff had a difficult time articulating what that relationship would actually look like if put into practice.
Related: Patients Benefit From ICU Telemedicine
In spite of the numerous barriers identified by clinic staff, the recommendation for education to improve comanagement was consistent across study sites and clinic staff roles. Education was proposed for patients and non-VHA providers as the best intervention. In response to a question about ideas and recommendations to improve comanagement, clinic staff drew on varied experiences. To illustrate this theme, a provider gave this example of dual-use patients seeking prescription medication from VHA and its impact on comanagement: “I would [recommend] an outreach program to community resources and [non-VHA] providers. To let them know more about how the VA works and the resources that are available, and how specifically to coordinate care through the VA, would be a significant benefit.… If the [non-VHA] providers knew how to—who to—talk to, what information the VA needs, for example, for medication changes, it would help the patients make it work…without having to overburden the patients with having to physically hand carry their blood test results, or their notes, discharge summaries, procedure notes.”
Along with providing outreach and education on working with the VHA, clinic staff addressed the need to educate patients more effectively, because they are seen as central to the information exchange. There is motivation on the part of patients to learn the system. “Just making sure that the patients realize that they need to tell their local providers to send us the records and make sure that there is an exchange going on consistently,” explained a case manager. “If the patient wants to get those medications that are costly, then they figure out pretty quick what they have to have, what they need to send to us.” The need for education is an ongoing process; who is responsible for this continues to be a point of debate.
Discussion
In order to better understand comanagement of dual-use patients, this study focused on the experiences and perceptions of staff at VHA primary care clinics in the upper Midwest. The data indicated that:
- VHA clinical staff perceive the primary reason patients choose to seek non-VHA care is because of access, convenience, and
distance - In order for comanagement to occur, communication and information exchange—currently facilitated largely by patients—needs to improve
- Education of patients and their non-VHA providers is recommended, to increase understanding of rules and regulations tied to exchange of patient information across health care systems
- Education may facilitate communication, develop relationships, and overcome barriers to information exchange
Distance to health care and perceived convenience were clearly seen by clinic staff as the driving factors behind their patients’ dual use. In the authors’ prior work, interviews with veterans and their VA providers supported this assertion as well; however, it was also found that distance must not be understood in isolation of other contingencies, such as urgency of need.4
Clinic staff identified institutional and individual barriers that lead to miscommunication and confusion on the part of patients and reported misunderstandings with non-VHA providers, including 3 potential barriers to comanagement. These included (1) inconsistent communication and flow of information between VHA and non-VHA providers; (2) uncertainty about who will (and should) be responsible for information flow between providers; and (3) VHA and federal regulations over patient privacy. Throughout the interviews, access to less expensive prescription medications in VHA was considered an additional driver of dual use. According to clinic staff interviewed, education of patients and non-VHA providers could facilitate efficient and safe comanagement for dual-use patients.7
This study suggests both advantages and disadvantages for patients choosing to use multiple health care systems from the perspective of the clinic staff. The primary advantage is better overall health care access, especially for rural patients and those with longer travel times to VHA facilities. The primary disadvantage of dual use is discontinuity of care between multiple care sites. Specifically, this study identified concerns regarding poor communication between providers and transfer of patient medical records. An underlying theme was a concern for quality of care and patient safety, which are recognized by others in the literature as potential consequences of inadequate comanagement.8-12
If there is one aspect of co-management for dual-use patients to target, this study’s findings point to developing strategies to improve communication between providers caring for dual-use patients and, more specifically, cultivating relationships that are currently underdeveloped. This will necessitate a clearer articulation of what constitutes a relationship between comanaging providers and is a direction for further research that would have applicability beyond VHA to any comanagement of patients using multiple health care systems.
There are 3 simultaneous, yet unrelated, factors that may contribute to increasing dual use. First is the rise in VHA eligible veterans from Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn.13,14 All returning veterans who meet minimal requirements are eligible for 5 years of VHA health care. A large proportion of these individuals are in the Reserve and National Guard, most of whom have nonmilitary jobs that may provide employer-based health insurance. Thus, these veterans have a greater opportunity for dual use. Second, with the aging cohort of Vietnam-era veterans, a greater proportion is becoming Medicare eligible. Third, with the recent passing of the ACA, more patients, including veterans, may choose to purchase insurance through ACA health exchanges. Taken individually or collectively, these factors will likely have effects reaching beyond VHA, especially when veterans receiving care in non-VHA health care systems engage in dual use.3,13,15,16
Limitations
This study has a number of limitations. First, it was limited to VHA facilities located in the upper Midwest, which may limit generalizability to other parts of the country. The convenience sample of clinic staff at VHA clinics may not represent the full range of perspectives among HCPs generally. This study did not interview clinic staff in non-VHA clinics, although this has been the focus of other studies.17,18 Although dual use also applies to specialty care and related access issues in rural areas, this was not a focus of this study. Last, the data were collected in 2009, prior to the implementation of the patient-aligned care team (PACT) model and prior to the recently revealed issues regarding patient wait times for VHA care. Thus, perceptions may have changed, and additional study is needed.
Conclusions
The results of this study support prior assumptions of barriers to care, but also introduce previously unreported challenges. Dual use is perceived to have both positive and negative impacts, but for the positives to outweigh the negatives, thoughtful comanagement is critical. This may be particularly so in rural areas where dual use is encouraged as a way to overcome distance and increase convenience in accessing care.
As demonstrated by recent events, there are still VHA health care access issues for veterans. Recently, VA leadership and the U.S. Congress proposed that veterans have greater access to community providers as well as VHA in order to overcome delays in care.19 As this option is explored and put into practice, it is more important than ever to consider the need for care coordination and management of dual-use patients, to ensure good communication and care that is timely, safe, and high quality.
Few models exist in which 2 PCPs coordinate across health care systems, and greater understanding of this dual use is needed. This information is important in designing interventions to improve care coordination across systems to ensure continuity of care, patient safety, and patient satisfaction. Although some work has been done to examine the perspectives of non-VA PCPs, little is known about VHA provider perspectives on rural veteran dual use.17,18 This study explores VHA provider perspectives and identifies areas where interventions to improve care coordination across systems might be targeted.
Next steps for intervention studies would be to improve communication and develop educational tools to aid in the coordination of care between VHA and non-VHA providers. A recent example of this is the Co-Management Toolkit developed by the Veterans Rural Health Resource Center-Central Region, which provides information on VHA policies and targets non-VHA providers.20 Although VHA perceptions of comanageing dual-use patients were the target, a similar study of non-VHA providers is important to understand this complex and multifaceted dynamic. Additional work is needed to measure the impact of dual use on clinical outcomes, patient safety and quality, and efficient use of resources, as these are understudied. As dual use continues and potentially increases with the ACA and changing health care in the U.S., it is important to understand the management of patients using multiple health care systems. This is salient as primary care adopts the PACT model and to inform interventions to improve quality and safety while eliminating duplicative health care and costs.
Acknowledgements
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Rural Health, Veterans Rural Health Resource Center-Central Region (VRHRC-CR) and the VA Health Services Research and Development (HSR&D) Service, the Comprehensive Access and Delivery Research and Evaluation (CADRE) Center at the Iowa City VA Health Care System, and Center to Improve Veteran Involvement in Care (CIVIC) at VA Portland Health Care System. Dr. Reisinger was supported by a Research Career Development Award from the Health Services Research and Development Service, Department of Veterans Affairs (CD1 08-013-1).
We would like to thank all health care providers who graciously agreed to participate in this study and VRHRC-CR staff, in particular Monica Paez for assistance on this manuscript.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The VHA assigns all enrolled veterans to a primary care provider (PCP). However, almost 80% of veterans enrolled in VHA have another form of health care coverage, including Medicare, Medicaid, private insurance, and TRICARE for Life program.1 Consequently, veterans may choose to use more than 1 health care system to manage their health care needs.
Studies based on merged VHA and Medicare claims data have demonstrated substantial dual use by VHA enrollees with Medicare. Petersen and colleagues reported that about 80% of VHA enrollees with Medicare chose to use services in both systems and that greater distance to VHA facilities and lower priority level for VHA care predicted lower VHA reliance.2 Among those aged < 65 years who had Medicare due to disability, 58% weredual users. These dual users relied more on private sector care for many health conditions, with the notable exception of substance abuse and mental health disorders, for which reliance on VHA care was greater.2 Another study found that over half of VHA enrollees assigned to a PCP at a community-based outpatient clinic (CBOC) received some or all of their care outside VHA and that reliance on VHA outpatient care declined over the 4-year study period.3
Related: Mutual Alignment Trumps Merger for Joint VA/DoD Health Care Programs
This use of multiple health care providers (HCPs), facilities, and modalities is often described as dual use or comanagement. Dual use in the case of veterans refers to use of both VHA and non-VHA health care, whereas comanagement implies an expectation of shared decision making and open communication between VHA and non-VHA providers. In addition to VHA PCPs, rural veterans frequently receive care from local, non-VHA HCPs in the community where they live. As health care in the U.S. evolves and patients have increasing choices through the Affordable Care Act (ACA), the challenge of comanagement for patients receiving care in multiple systems is likely to increase both within and outside VHA.
This study was part of a qualitative rural health needs assessment designed to ascertain the issues facing rural veterans and their providers in the upper Midwest.4 The objective was to examine VHA primary care clinic staff perspectives on dual users, perceived barriers that inhibit comanagement, and factors that contribute to the need for dual use in rural areas.
Methods
A qualitative study design with in-person interviews was used to elicit the perspective of VHA clinic staff on the current and ideal states of comanagement. Clinics were selected using a stratified purposeful sample of 15 urban and rural primary care clinics at VHA CBOCs and VAMCs in 8 Midwestern states (Illinois, Iowa, Minnesota, Nebraska, North Dakota, South Dakota, Wisconsin, and Wyoming). The stratification criteria included (1) urban and rural; (2) geographic coverage of VISN 23; and (3) VHA-managed and contract clinics, resulting in a purposeful sample of 2 urban VAMC clinics, 3 urban CBOCs, 7 rural VHA-managed CBOCs, and 3 rural contract CBOCs. The distance from the CBOC to the closest VAMC ranged from 32 to 242 miles.
Related: VA Relaxes Rules for Choice Program
Interview guides were developed and tested by the research team for comprehension, length, and timing prior to data collection and iteratively revised as analysis evolved and new topics emerged. Clinic staff were asked about their perceptions of rural veteran use of VHA care; barriers and facilitators to accessing care; and their personal experience working within VHA. Several questions focused on dual use and why rural veterans use multiple health care systems, their perspectives of dual use, their expectations of patients’ role(s) in health care coordination, and the perceived barriers that inhibit comanagement. Interviewers used comanagement and dual use interchangeably to discuss patients with multiple care providers, allowing interviewees to use their preferred terminology; assigned meanings were probed for clarification but not corrected by interviewers.
Between June and October 2009, teams of 2 to 3 researchers visited 15 clinics for 1 to 2 business days each. Researchers conducted interviews with a convenience sample of clinical staff. Consent forms and an explanation of the study were distributed, and those electing to participate voluntarily came to a designated room to complete an interview. All interviews were audio recorded for accuracy.
Interview recordings were transcribed verbatim and reviewed for accuracy. Prior to coding, transcripts were imported into a qualitative data management software program. A codebook, including a priori research hypotheses and de novo themes, was developed based on a systematic review of a randomly selected subset of interview transcripts.5 Four coders were responsible for coding all transcripts and validating coding through tests of agreement at predetermined intervals.
Regular meetings were conducted with coders and the lead qualitative investigator to discuss disagreements, clarify code definitions, or add new codes as needed. As codes were added, previous transcripts were coded/recoded for content related to the new codes. An audit trail was maintained, and iterative mediation of codes continued throughout the process. The final codebook contained 42 thematic codes, which reached saturation or data redundancy.6 Detailed analysis of the codes dual use, distance, and care coordination were used to inform this study.
Results
Among the 15 sites, 64 in-depth individual interviews were conducted, ranging from 5 to 53 minutes (average 26 minutes). Clinic staff demographic characteristics are depicted in the Table. Analysis of data captured in the codes dual use, distance, and care coordination resulted in notable concentration in 4 thematic areas: (1) clinic staff perceptions of the influence of access, convenience, and distance on dual use for rural patients; (2) communication and patient’s role in comanagement; (3) rules and regulations related to comanagement from the VHA perspective; and (4) barriers to comanagement and recommendations for education.
Influence of Access, Convenience, and Distance
Access to health care was central to the discussion of dual use and comanagement by clinic staff. Convenience was identified as the primary reason for rural patients’ use of non-VHA services, as many rural patients must travel outside their local community to access VHA care. Thus, dual use was most often noted for services typically available in patients’ local communities, especially management of chronic conditions.
The CBOCs provide important services for primary care and management of chronic conditions but are not available in all communities and may have limited hours/days that do not fit with patients’ schedules. The CBOCs are often unable to provide needed services, including but not limited to emergency care, diagnostic tests, physical and occupational therapy, and other specialty care services. As one VHA provider put it, “The biggest factor for [dual use] is availability, access, convenience.… It’s a lot more convenient to go to the hospital down the street than it is to go 120 miles to [the VAMC], or for some guys who live 30, 40 minutes the other side of here it becomes 150, 160-mile one-way trip.”
Related to access, distance and transportation barriers were identified by clinic staff as obstacles to care for rural patients. Despite efforts to offset the expense of travel through reimbursement to qualified veterans and coordinated van transport with Veterans Service Organizations, travel costs—both time and money—were seen as significant barriers to accessing VHA care, as was an inability to travel for those who are ill or frail and elderly. “We send people … in the van and for the most part that works, but eventually it gets expensive, or you’ve got somebody with chronic pain that can’t tolerate the van ride for 2 hours,” one interviewee
reported.
According to clinic staff, dual-use patients also rely on non-VHA providers in particular for urgent or emergency care, while relying on VHA primary care for reduced-cost medications, diagnostic testing, chronic disease management, or annual exams. When asked why rural patients may choose to see more than 1 provider, VHA providers responded. “[It’s] more convenient to have a local doctor just in case something went wrong and they need to see a doctor right away. So distance to this clinic would be the number one reason.” Another reported, “If it’s once or twice a year routine appointments they’ll come here, but… they’d rather go to a walk-in clinic nearby than spend so much [money] on gas.”
Communication and Patients’ Role
Communication between VHA and non-VHA providers is a necessary element of comanagement. Although phone calls or faxing patient medical records are available options, clinic staff reported it was more common to encounter patients hand carrying their records between providers. For dual-use patients, clinic staff indicated it was often unclear who was responsible for relaying information between providers. There is often ambiguity about who will (and should) fulfill this role and not enough time to adequately address or clarify how this is done. Some clinic staff believed that acting as the main conduits of information placed an undue burden on the patients, particularly asking them to be able to accurately relay medical information about tests or prescriptions that they may not fully understand. Others said that it was primarily the patients’ responsibility to give relevant information about their care to all their providers, because of VHA regulations and patient privacy laws. “[The] patient should tell the primary doctor to send them [medical records] because we can’t get the medical records without the patient’s permission,” said one provider.
Another provider utilized the nursing staff to call patients after their appointments to remind them to give their medical records to their non-VHA provider. The data suggest that responsibility for maintaining communication between providers ultimately falls on the patient. From the perspective of a nurse practitioner, “We just keep trying to educate the community…. I’ve been told that if the patient wants that privilege of using the VA for a pharmacy for an outside provider that we’re glad to do that. But it is their responsibility to communicate with their [non-VHA] physician. I think we just need to keep educating the patients.”
Rules and Regulations
VHA policies governing prescriptions, hospitalizations at outside facilities, and release of patient information regulate, and in some cases hinder, information flow between VHA and non-VHA providers. Many patients use VHA to obtain medications for lower out-of-pocket costs. This contributes to the number of dual-use patients in VHA and results in several challenges for VHA providers trying to manage patients’ prescriptions. For example, patients will ask to fill a prescription at a VHA pharmacy from their non-VHA providers; however, VHA pharmacies can only fill prescriptions from VHA providers.
Many VHA providers are willing to rewrite these prescriptions, but they may need to see the patient before adding or changing the prescription and require documentation to address contraindications, adverse reactions and/or therapeutic failure, and associated risks before making the authorization. VHA providers noted that because the VHA formulary does not contain all medications, non-VHA providers are often unfamiliar with the VHA National Formulary specifics and will write prescriptions for nonformulary medications, which require a nonformulary request from a VHA provider.
Clinic staff also mentioned difficulty in obtaining records from non-VHA providers. This can be particularly problematic if the patient lives a distance away from a VHA facility and does not have the necessary authorization to share records on file.
Barriers and Education Recommendations
Clinic staff identified coordination of care for dual-use patients as a barrier to providing care. Specifically, providers identified coordination as complicated by communication difficulties, inefficient medical record exchange, short staffing in VHA clinics, duplication of diagnostic services, and non-VHA providers’ lack of understanding regarding the services that VHA provides. Specific to rural clinics, comanagement was reportedly hindered by limitations in technology (eg, consistent Internet access), access to routine diagnostic services, and lack of relationships with non-VHA providers. Providers most frequently reported that the critical piece missing in comanagement is a relationship—and implied communication—between VHA clinics and non-VHA community clinics. The concept of a relationship between providers is evoked as a critical element to comanaging dual-use patients; however, clinic staff had a difficult time articulating what that relationship would actually look like if put into practice.
Related: Patients Benefit From ICU Telemedicine
In spite of the numerous barriers identified by clinic staff, the recommendation for education to improve comanagement was consistent across study sites and clinic staff roles. Education was proposed for patients and non-VHA providers as the best intervention. In response to a question about ideas and recommendations to improve comanagement, clinic staff drew on varied experiences. To illustrate this theme, a provider gave this example of dual-use patients seeking prescription medication from VHA and its impact on comanagement: “I would [recommend] an outreach program to community resources and [non-VHA] providers. To let them know more about how the VA works and the resources that are available, and how specifically to coordinate care through the VA, would be a significant benefit.… If the [non-VHA] providers knew how to—who to—talk to, what information the VA needs, for example, for medication changes, it would help the patients make it work…without having to overburden the patients with having to physically hand carry their blood test results, or their notes, discharge summaries, procedure notes.”
Along with providing outreach and education on working with the VHA, clinic staff addressed the need to educate patients more effectively, because they are seen as central to the information exchange. There is motivation on the part of patients to learn the system. “Just making sure that the patients realize that they need to tell their local providers to send us the records and make sure that there is an exchange going on consistently,” explained a case manager. “If the patient wants to get those medications that are costly, then they figure out pretty quick what they have to have, what they need to send to us.” The need for education is an ongoing process; who is responsible for this continues to be a point of debate.
Discussion
In order to better understand comanagement of dual-use patients, this study focused on the experiences and perceptions of staff at VHA primary care clinics in the upper Midwest. The data indicated that:
- VHA clinical staff perceive the primary reason patients choose to seek non-VHA care is because of access, convenience, and
distance - In order for comanagement to occur, communication and information exchange—currently facilitated largely by patients—needs to improve
- Education of patients and their non-VHA providers is recommended, to increase understanding of rules and regulations tied to exchange of patient information across health care systems
- Education may facilitate communication, develop relationships, and overcome barriers to information exchange
Distance to health care and perceived convenience were clearly seen by clinic staff as the driving factors behind their patients’ dual use. In the authors’ prior work, interviews with veterans and their VA providers supported this assertion as well; however, it was also found that distance must not be understood in isolation of other contingencies, such as urgency of need.4
Clinic staff identified institutional and individual barriers that lead to miscommunication and confusion on the part of patients and reported misunderstandings with non-VHA providers, including 3 potential barriers to comanagement. These included (1) inconsistent communication and flow of information between VHA and non-VHA providers; (2) uncertainty about who will (and should) be responsible for information flow between providers; and (3) VHA and federal regulations over patient privacy. Throughout the interviews, access to less expensive prescription medications in VHA was considered an additional driver of dual use. According to clinic staff interviewed, education of patients and non-VHA providers could facilitate efficient and safe comanagement for dual-use patients.7
This study suggests both advantages and disadvantages for patients choosing to use multiple health care systems from the perspective of the clinic staff. The primary advantage is better overall health care access, especially for rural patients and those with longer travel times to VHA facilities. The primary disadvantage of dual use is discontinuity of care between multiple care sites. Specifically, this study identified concerns regarding poor communication between providers and transfer of patient medical records. An underlying theme was a concern for quality of care and patient safety, which are recognized by others in the literature as potential consequences of inadequate comanagement.8-12
If there is one aspect of co-management for dual-use patients to target, this study’s findings point to developing strategies to improve communication between providers caring for dual-use patients and, more specifically, cultivating relationships that are currently underdeveloped. This will necessitate a clearer articulation of what constitutes a relationship between comanaging providers and is a direction for further research that would have applicability beyond VHA to any comanagement of patients using multiple health care systems.
There are 3 simultaneous, yet unrelated, factors that may contribute to increasing dual use. First is the rise in VHA eligible veterans from Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn.13,14 All returning veterans who meet minimal requirements are eligible for 5 years of VHA health care. A large proportion of these individuals are in the Reserve and National Guard, most of whom have nonmilitary jobs that may provide employer-based health insurance. Thus, these veterans have a greater opportunity for dual use. Second, with the aging cohort of Vietnam-era veterans, a greater proportion is becoming Medicare eligible. Third, with the recent passing of the ACA, more patients, including veterans, may choose to purchase insurance through ACA health exchanges. Taken individually or collectively, these factors will likely have effects reaching beyond VHA, especially when veterans receiving care in non-VHA health care systems engage in dual use.3,13,15,16
Limitations
This study has a number of limitations. First, it was limited to VHA facilities located in the upper Midwest, which may limit generalizability to other parts of the country. The convenience sample of clinic staff at VHA clinics may not represent the full range of perspectives among HCPs generally. This study did not interview clinic staff in non-VHA clinics, although this has been the focus of other studies.17,18 Although dual use also applies to specialty care and related access issues in rural areas, this was not a focus of this study. Last, the data were collected in 2009, prior to the implementation of the patient-aligned care team (PACT) model and prior to the recently revealed issues regarding patient wait times for VHA care. Thus, perceptions may have changed, and additional study is needed.
Conclusions
The results of this study support prior assumptions of barriers to care, but also introduce previously unreported challenges. Dual use is perceived to have both positive and negative impacts, but for the positives to outweigh the negatives, thoughtful comanagement is critical. This may be particularly so in rural areas where dual use is encouraged as a way to overcome distance and increase convenience in accessing care.
As demonstrated by recent events, there are still VHA health care access issues for veterans. Recently, VA leadership and the U.S. Congress proposed that veterans have greater access to community providers as well as VHA in order to overcome delays in care.19 As this option is explored and put into practice, it is more important than ever to consider the need for care coordination and management of dual-use patients, to ensure good communication and care that is timely, safe, and high quality.
Few models exist in which 2 PCPs coordinate across health care systems, and greater understanding of this dual use is needed. This information is important in designing interventions to improve care coordination across systems to ensure continuity of care, patient safety, and patient satisfaction. Although some work has been done to examine the perspectives of non-VA PCPs, little is known about VHA provider perspectives on rural veteran dual use.17,18 This study explores VHA provider perspectives and identifies areas where interventions to improve care coordination across systems might be targeted.
Next steps for intervention studies would be to improve communication and develop educational tools to aid in the coordination of care between VHA and non-VHA providers. A recent example of this is the Co-Management Toolkit developed by the Veterans Rural Health Resource Center-Central Region, which provides information on VHA policies and targets non-VHA providers.20 Although VHA perceptions of comanageing dual-use patients were the target, a similar study of non-VHA providers is important to understand this complex and multifaceted dynamic. Additional work is needed to measure the impact of dual use on clinical outcomes, patient safety and quality, and efficient use of resources, as these are understudied. As dual use continues and potentially increases with the ACA and changing health care in the U.S., it is important to understand the management of patients using multiple health care systems. This is salient as primary care adopts the PACT model and to inform interventions to improve quality and safety while eliminating duplicative health care and costs.
Acknowledgements
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Rural Health, Veterans Rural Health Resource Center-Central Region (VRHRC-CR) and the VA Health Services Research and Development (HSR&D) Service, the Comprehensive Access and Delivery Research and Evaluation (CADRE) Center at the Iowa City VA Health Care System, and Center to Improve Veteran Involvement in Care (CIVIC) at VA Portland Health Care System. Dr. Reisinger was supported by a Research Career Development Award from the Health Services Research and Development Service, Department of Veterans Affairs (CD1 08-013-1).
We would like to thank all health care providers who graciously agreed to participate in this study and VRHRC-CR staff, in particular Monica Paez for assistance on this manuscript.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Department of Veterans Affairs Office of Rural Health, VHA. Veterans Rural Health: Perspectives and Opportunities. Rockville, MD: Booz Allen Hamilton; 2008. http://www.ruralhealth.va.gov/docs/PAO-final-report-0208.pdf. Accessed July 6, 2015.
2. Petersen LA, Byrne MM, Daw CN, Hasche J, Reis B, Pietz K. Relationship between clinical conditions and use of Veterans Affairs health care among Medicare‐enrolled veterans. Health Serv Res. 2010;45(3):762-791.
3. Liu CF, Chapko M, Bryson CL, et al. Use of outpatient care in Veterans Health Administration and Medicare among veterans receiving primary care in community-based and hospital outpatient clinics. Health Serv Res. 2010;45(5 pt 1):1268-1286.
4. Buzza C, Ono SS, Turvey C, et al. Distance is relative: unpacking a principal barrier in rural healthcare. J Gen Intern Med. 2011;26(suppl 2):648-654.
5. Bernard HR, Ryan GW. Analyzing Qualitative Data: Systematic Approaches. Los Angeles, CA: SAGE; 2010.
6. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82.
7. Kramer BJ, Vivrette RL, Satter DE, Jouldjian S, McDonald LR. Dual use of Veterans Health Administration and Indian Health Service: healthcare provider and patient perspectives. J Gen Intern Med. 2009;24(6):758-764.
8. Ajmera M, Wilkins TL, Sambamoorthi U. Dual Medicare and Veteran Health Administration use and ambulatory care sensitive hospitalizations. J Gen Intern Med. 2011;26(suppl 2):669-675.
9. Jia H, Zheng Y, Reker DM, et al. Multiple system utilization and mortality for veterans with stroke. Stroke. 2007;38(2):355-360.
10. Trivedi AN, Grebla RC, Jiang L, Yoon J, Mor V, Kizer KW. Duplicate federal payments for dual enrollees in Medicare Advantage plans and the Veterans Affairs health care system. JAMA. 2012;308(1):67-72.
11. Kaboli PJ, Shivapour DM, Henderson MS, Ishani A, Charlton ME. The impact of primary care dual-management on quality of care. J Prim Care Community Health. 2012;3(1):11-16.
12. Wolinsky FD, Miller TR, An H, Brezinski PR, Vaughn TE, Rosenthal GE. Dual use of Medicare and the Veterans Health Administration: are there adverse health outcomes? BMC Health Serv Res. 2006;6:131.
13. Liu CF, Bryson CL, Burgess JF Jr, Sharp N, Perkins M, Maciejewski ML. Use of outpatient care in VA and Medicare among disability-eligible and age-eligible veteran patients. BMC Health Serv Res. 2012;12:51.
14. Miller EA, Intrator O. Veterans use of non-VHA services: implications for policy and planning. Soc Work Public Health. 2012;27(4):379-391.
15. Bachman SS, Gonyea JG. Improving health care delivery to aging adults with disabilities: social work with dual eligibles in a climate of health care reform. J Gerontol Soc Work. 2012;55(2):191-207.
16. Kizer KW. Veterans and the Affordable Care Act. JAMA. 2012;307(8):789-790.
17. Lampman MA, Mueller KJ. Experiences of rural non-VA providers in treating dual care veterans and the development of electronic health information exchange networks between the two systems. J Rural Soc Sci. 2011;26(3):201-219.
18. Gaglioti A, Cozad A, Wittrock S, et al. Non-VA primary care providers’ perspectives on comanagement for rural veterans. Mil Med. 2014;179(11):1236-1243.
19. U.S. Department of Veterans Affairs. Acting Secretary Gibson outlines problems, actions taken, and budget resources needed to ensure access to care. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/pressrel/pressrelease.cfm?id=2586. Published July 16, 2014. Accessed July 6, 2015.
20. Office of Rural Health Central Region. Co-managed care toolkit. U.S. Department of Veterans Affairs Website. http://www.ruralhealth.va.gov/resource-centers/central/comanagement-toolkit.asp. Updated June 3, 2015. Accessed July 6, 2015.
1. Department of Veterans Affairs Office of Rural Health, VHA. Veterans Rural Health: Perspectives and Opportunities. Rockville, MD: Booz Allen Hamilton; 2008. http://www.ruralhealth.va.gov/docs/PAO-final-report-0208.pdf. Accessed July 6, 2015.
2. Petersen LA, Byrne MM, Daw CN, Hasche J, Reis B, Pietz K. Relationship between clinical conditions and use of Veterans Affairs health care among Medicare‐enrolled veterans. Health Serv Res. 2010;45(3):762-791.
3. Liu CF, Chapko M, Bryson CL, et al. Use of outpatient care in Veterans Health Administration and Medicare among veterans receiving primary care in community-based and hospital outpatient clinics. Health Serv Res. 2010;45(5 pt 1):1268-1286.
4. Buzza C, Ono SS, Turvey C, et al. Distance is relative: unpacking a principal barrier in rural healthcare. J Gen Intern Med. 2011;26(suppl 2):648-654.
5. Bernard HR, Ryan GW. Analyzing Qualitative Data: Systematic Approaches. Los Angeles, CA: SAGE; 2010.
6. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82.
7. Kramer BJ, Vivrette RL, Satter DE, Jouldjian S, McDonald LR. Dual use of Veterans Health Administration and Indian Health Service: healthcare provider and patient perspectives. J Gen Intern Med. 2009;24(6):758-764.
8. Ajmera M, Wilkins TL, Sambamoorthi U. Dual Medicare and Veteran Health Administration use and ambulatory care sensitive hospitalizations. J Gen Intern Med. 2011;26(suppl 2):669-675.
9. Jia H, Zheng Y, Reker DM, et al. Multiple system utilization and mortality for veterans with stroke. Stroke. 2007;38(2):355-360.
10. Trivedi AN, Grebla RC, Jiang L, Yoon J, Mor V, Kizer KW. Duplicate federal payments for dual enrollees in Medicare Advantage plans and the Veterans Affairs health care system. JAMA. 2012;308(1):67-72.
11. Kaboli PJ, Shivapour DM, Henderson MS, Ishani A, Charlton ME. The impact of primary care dual-management on quality of care. J Prim Care Community Health. 2012;3(1):11-16.
12. Wolinsky FD, Miller TR, An H, Brezinski PR, Vaughn TE, Rosenthal GE. Dual use of Medicare and the Veterans Health Administration: are there adverse health outcomes? BMC Health Serv Res. 2006;6:131.
13. Liu CF, Bryson CL, Burgess JF Jr, Sharp N, Perkins M, Maciejewski ML. Use of outpatient care in VA and Medicare among disability-eligible and age-eligible veteran patients. BMC Health Serv Res. 2012;12:51.
14. Miller EA, Intrator O. Veterans use of non-VHA services: implications for policy and planning. Soc Work Public Health. 2012;27(4):379-391.
15. Bachman SS, Gonyea JG. Improving health care delivery to aging adults with disabilities: social work with dual eligibles in a climate of health care reform. J Gerontol Soc Work. 2012;55(2):191-207.
16. Kizer KW. Veterans and the Affordable Care Act. JAMA. 2012;307(8):789-790.
17. Lampman MA, Mueller KJ. Experiences of rural non-VA providers in treating dual care veterans and the development of electronic health information exchange networks between the two systems. J Rural Soc Sci. 2011;26(3):201-219.
18. Gaglioti A, Cozad A, Wittrock S, et al. Non-VA primary care providers’ perspectives on comanagement for rural veterans. Mil Med. 2014;179(11):1236-1243.
19. U.S. Department of Veterans Affairs. Acting Secretary Gibson outlines problems, actions taken, and budget resources needed to ensure access to care. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/pressrel/pressrelease.cfm?id=2586. Published July 16, 2014. Accessed July 6, 2015.
20. Office of Rural Health Central Region. Co-managed care toolkit. U.S. Department of Veterans Affairs Website. http://www.ruralhealth.va.gov/resource-centers/central/comanagement-toolkit.asp. Updated June 3, 2015. Accessed July 6, 2015.
Accelerated Hepatitis A and B Immunization in a Substance Abuse Treatment Program
Homeless individuals and IV drug users are susceptible to hepatitis A, B, and C infections, and co-infection with these diseases may complicate treatment and result in poor medical outcomes.1 Vaccination offers the best protection against hepatitis A and B, particularly among high-risk populations.2,3 Immunization against hepatitis A and B is of even greater importance for patients with hepatitis C, because there is no specific hepatitis C vaccine, and concomitant infections of B with C are damaging to the liver.4
Veterans have a rate of hepatitis C infection that is 3 times that of the general population.5 Some evidence exists that veterans with serious mental illness (SMI) have a higher rate of hepatitis C infection relative to patients without SMI. Co-occurring substance abuse may add another layer of vulnerability to hepatitis C infection, particularly for homeless veterans.5-7
Mental Health and Primary Care Integration
Substance abuse and dual-diagnosis treatment programs (ie, those programs that treat both substance abuse and co-occurring serious mental health problems, such as bipolar disorder, severe major depressive disorder, psychotic disorders, and posttraumatic stress disorder [PTSD]) that have integrated mental health and primary care into their treatment programs may offer a window of opportunity for risk-reducing interventions. These interventions include testing and education of patients regarding infectious diseases, such as viral hepatitis and HIV, and completion of the hepatitis A/B immunization series.
The James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, has demonstrated some limited success in the past with integrating a standard dosing schedule for hepatitis A/B vaccination into its substance abuse treatment program (SATP), though recent evidence points to more promising results using an accelerated regimen as indicated by a high completion rate for hepatitis B vaccination in a methadone clinic.8,9 A relatively low proportion of SATPs in the U.S. provide testing, education, or vaccination for hepatitis A and B, especially considering the public health importance of controlling these diseases in the substance abusing populations.10,11
Related: Combination Pill Approved for HCV
In 1999, a primary care team was added to the alcohol and drug abuse treatment program at JAHVH.In 2005, the nurses in the program began scheduling vaccinations and screening patients for medical and psychiatric issues, pain, hypertension, diabetes, hepatitis C, alcohol use, depression, PTSD, prostate and colorectal cancers.12 Such a multidisciplinary approach provides many treatment advantages for patients and may save lives.13
Even with a multidisciplinary approach, the nurses found it difficult to provide adequate hepatitis A/B immunization within the 3- to 6-week intensive SATP, because standard immunization dosing regimens are spread over 6 months.14 As with all types of immunizations, long dosing schedules may reduce patient adherence and result in inadequate seroprotection.15 Thus, there is a need to provide a completed immunization series in a more expeditious fashion, and an accelerated dosing regimen makes that possible.15,16
Hepatitis A/B Vaccination
Twinrix (GlaxoSmithKline, Brentford, United Kingdom) is a vaccine that provides dual immunization for hepatitis A and B. Whereas the standard vaccination schedule takes 6 months to complete, the accelerated dosing schedule can be used to complete the first 3 doses in less than a month. The accelerated dosing schedule was incorporated into the JAHVH clinic to capture as many patients as possible in the 3- to 6-week time frame: The first dose is administered and followed by a second dose 7 days later. The third dose is administered 21 to 30 days after the first dose. Twelve months after the first dose, a booster dose is given.
After the first 3 accelerated doses, > 98% of patients show a sustained immune response to hepatitis A, and > 63% demonstrate immunity to hepatitis B. If a 12-month booster injection is given, 100% of patients may receive immunity to hepatitis A and > 96% may have immunity to hepatitis B.16 Another study of the combined vaccine showed even greater seroprotection for hepatitis A and B after only 1 month, 100% and 82%, respectively.17
Related: Viral Hepatitis Awareness
This JAHVH retrospective feasibility study describes a risk-reduction program for hepatitis A/B prevention that was implemented within a 3- to 4-week intensive outpatient SATP and a 6-week dual-diagnosis treatment program. The study includes the development and implementation of the program, designed to vaccinate patients using the accelerated Twinrix schedule. To ascertain the feasibility of this vaccination approach, historical medical records were used to describe and examine the vaccination initiation and follow-up rates of the treatment program participants who received the hepatitis A/B immunization series during their intensive SATP.
Study Design
A retrospective review of medical records was conducted for all participants who were admitted to the intensive JAHVH SATP between October 1, 2008, and September 30, 2009. This study was reviewed and approved by the JAHVH research and development committee and its associated University of South Florida institutional review board. Informed consent to participate was not obtained, because the study was retrospective.
Patient Identification and Education
All program participants were offered testing for HIV and hepatitis A, B, and C. Program participants were educated about hepatitis and HIV transmission, as well as about the long-term effects of continued substance abuse on the progression of hepatitis C. Education about hepatitis, HIV, and substance abuse was provided in a group setting by a member of the program’s nursing staff. One-on-one risk education counseling was also provided when requested or otherwise indicated.
Laboratory testing was performed following each participant’s initial physical examination (within 3 to 5 days of program admission), and the nursing staff reviewed the results before vaccination. Explanation of laboratory results and an individualized immunization regimen were provided to each participant. On review of participants’ laboratory results, those with seroconversion of both hepatitis A and B were not given the combined immunization. Participants who had seroconversion of hepatitis A were offered the hepatitis B vaccination series, and vice versa.
Immunization Process
Participants who lacked prior immunization for hepatitis A and B and had no seroconversion of either hepatitis A or B were offered vaccination. Some patients declined vaccination, even though they were eligible. Their reasons were not formally assessed.
Related: Nivolumab Approved for Expanded Indication
Patients who accepted the vaccination were given the accelerated regimen.16 Participants were educated on the importance of compliance with the vaccination series and provided with follow-up immunization dates and a reminder for the 1-year booster vaccine. The immunizations were ordered by the program’s primary care NP and administered by a licensed practical nurse. The nurse who administered the injections took responsibility for scheduling the patients for all their subsequent injections, including the 1-year booster.
Follow-up Care
If the third injection was not completed before discharge, patients were given a follow-up appointment with the nurse if they remained in the JAHVH service area. If they were leaving the area, they were given instructions on how to follow-up at another VA facility to continue their immunization schedule. A note was written in the electronic medical record documenting their abbreviated hepatitis A/B immunization schedule, which could be accessed by other providers at other VA facilities. Patients who did not show up for any follow-up appointments (third injection or the 1-year booster injection) were contacted and reminded about the importance of completing the immunization series and to schedule an appointment.
Statistical Analysis
All data were analyzed using IBM Statistical Package for the Social Sciences (IBM SPSS, Armonk, New York) with a focus on identifying differences between vaccination-eligible patients (n = 269) who did (n = 128) and did not (n = 141) initiate the immunization schedule during the treatment program. Chi-square and Fisher exact tests were used to assess statistical differences in initiation of the immunization schedule related to categoric variables (ie, marital status, race, history of IV drug abuse, cigarette smoking status, housing status, legal status, history of combat, having a psychiatric or medical diagnosis, and program track). Independent sample t tests were used to test for differences between these 2 groups on the continuous variables, including age, number of previous treatment programs, Global Assessment of Functioning score, severity of smoking dependence as measured by the Fagerström Test for Nicotine Dependence, and the Addiction Severity Index scales.18-20
Results
The sample consisted of 284 successive admissions to an intensive outpatient program for veterans with substance use disorders. About one-third of the patients were homeless at the time of admission to the treatment, and 87% required contracted housing while completing treatment for reasons related to lack of housing, transportation, clinical necessity, or a combination of those factors (Table 1). The most common substance problems were alcohol and cocaine dependence, and 21% (n = 59) of the patients acknowledged a history of IV drug use during their initial psychiatric evaluation. Seventy percent were dually diagnosed with some other Axis I disorder, and 40% had a history of serious mental illness. More than one-fourth (n = 77) of the patients admitted to the intensive outpatient SATP were seropositive for hepatitis A, B and/or C, and the most common hepatitis diagnosis was hepatitis C (n = 71).
Accelerated Immunization Regimen
Patients were eligible to receive the accelerated vaccination schedule only if they had no prior immunization for hepatitis A or B and if they had no seroconversion for either hepatitis A or B. Six people had hepatitis B alone, 7 had hepatitis B and C, 1 had hepatitis A and C, and 1 had all 3 (Table 2). Thus, 15 participants were ineligible to receive the accelerated hepatitis A/B immunization. Chi-square, Fisher exact, and independent sample t tests showed that among those who were vaccination-eligible (269), there were no significant differences in any of the demographic or clinical characteristics between those who initiated the vaccination schedule and those who did not. Among those who completed the first 3 vaccine injections, those who received the 1-year booster injection (54) did not differ (on any demographic or clinical variables) from those who did not (58).
Nearly half (48%) of all the eligible patients admitted to the program began the accelerated immunization schedule for hepatitis A and B. Of those, 88% completed the first 3 injections in the series. Among the patients who received the first 3 injections, 48% received the 1-year booster injection—a 20% completion rate for the vaccination-eligible sample overall (Table 3).
Of the 74 patients who did not complete their vaccinations once initiating the accelerated schedule, the most common reason identified was that the patient moved away (37), or no reason could be identified (33). It was uncommon for a patient not to complete the vaccination schedule because of terminating treatment prematurely (4).
Compared with the vaccine-eligible patients without hepatitis C (207), patients with hepatitis C were less likely to receive any vaccination injections (Table 3). Specifically, 51% of the vaccination-eligible patients who did not have hepatitis C began the vaccination regimen. However, only 22 patients with hepatitis C, or
35% of all vaccination-eligible patients with hepatitis C, began the vaccination regimen. Patients with hepatitis C were also less likely than those without hepatitis C to complete the first 3 injections of the vaccination series once they had initiated it (77%, vs 90%, respectively). This difference continued to be apparent at the time of the 12-month booster injection. Only 35% of vaccine-eligible individuals with hepatitis C received the 12-month booster injection, whereas 51% of vaccination-eligible individuals without hepatitis C received the 12-month booster injection. As with the sample overall, the most common reason patients with hepatitis C did not complete the vaccination regimen was because they moved away (9), followed by no identified reason (5), and premature termination of treatment (2).
Discussion
Individuals abusing alcohol and drugs have an increased vulnerability for infectious diseases, and homeless veterans with substance use disorders may be at a particularly heightened risk.21,22 This study describes a sample of veterans, many were homeless and most were dually diagnosed, in an intensive outpatient SATP that offered an accelerated dosing regimen for hepatitis A and B vaccination. Almost half (48%) of the vaccination-eligible patients began the accelerated regimen for hepatitis A/B vaccination. Moreover, 88% of those who started the vaccination regimen received the first 3 injections of the series, thus possibly conferring substantial immunity to hepatitis A and B and demonstrating the feasibility of an accelerated vaccination schedule in an intensive outpatient SATP.
It is especially important to demonstrate the successful integration of a hepatitis screening and immunization program within a SATP, given that many such programs do not offer screening or immunization for hepatitis, even though substance abusers are disproportionately affected by the disease and contribute greatly to the ongoing hepatitis epidemic.10,11 This study’s results were in line with another study of rapid vaccination for hepatitis B in IV drug users being treated in a methadone clinic, where 83% of the vaccination initiators completed the first 3 injections of the series.9
Unvaccinated Patients
The treatment team in the current study seemed to be less effective at reaching the subset of vaccination-eligible veterans with hepatitis C (almost one-quarter of the sample) in order to administer the accelerated vaccination schedule, as indicated by the lower rate of vaccination initiation as well as a lower rate of completion of the vaccination series among those patients. This replicates a finding from another study that also indicated a low rate of hepatitis A and B vaccination among patients with hepatitis C.23 Only 35% of the vaccination-eligible patients with hepatitis C in the current study initiated the vaccination series, compared with 51% of the patients without hepatitis C. However, the rate of completion of the first 3 injections of the series in the hepatitis C group was respectably high (77%), especially given the high relapse rate and psychosocial instability of individuals with addictive disorders. Initiation seems to be a bigger obstacle than completion of at least the first 3 injections of the vaccination series in both patients with and without hepatitis C.
The study investigators did not formally assess the reasons that more than half the patients in the study did not begin the vaccination series, but anecdotal evidence from the nurses indicated that many patients were afraid of needles. In addition, other patients felt that they simply did not need the vaccination. Some also insisted that they had already had the vaccination despite a blood test showing no evidence for either hepatitis A or B immunization.
Although the nursing team provided group and individual risk-based education as well as information about the effects of continued substance abuse on hepatitis C, it is possible that patients still underestimated their own risk of hepatitis infection and its consequences, or perhaps the information was simply not retained.24
Patient Education
A recent study showed that there is a positive relationship between the amount of hepatitis counseling received and knowledge of hepatitis.25 Possibly, increased intensity of education efforts may make an impact on initiation rates. Encouragingly, there is also evidence that prompting people to predict their future vaccination behavior may increase vaccination initiation rates despite a high-degree of short-term barriers, such as perceived pain or inconvenience.26 A brief intervention to induce people to formulate their future intentions would be relatively easy to incorporate into a vaccination program, and the study team is considering options for this to improve vaccination initiation rates.
Patients can expect to achieve substantial immunity from hepatitis A and, to a lesser degree, hepatitis B after completing the first 3 injections of the series, although the best seroprotection from both is obtained by completing the 12-month booster injection as well.17 Overall, about half of all patients who completed the first 3 injections returned for the booster shot, but only 35% of the patients with hepatitis C did so. The most common known cause of any patient not receiving the booster was movement out of the geographic area. However, much of the time the investigators were unable to determine the reasons patients did not return for the booster shot.
Medication adherence is a difficult problem with vaccination in high-risk samples, although Stitzer and colleagues found a significant improvement in follow-up for a 6-month vaccination protocol by using monetary incentives.27 In addition to ensuring medication adherence, it would also be of value for future immunization efforts to include testing to assess whether seroconversion has occurred once the vaccinations are complete, which is the ultimate measure of the success of a vaccination program. Most patients in the current study did not receive such testing at the completion of their vaccination schedules, and thus, seroconversion rates could not be determined. However, existing studies suggest high rates of seroprotection after the first 3 doses of the combined vaccine.10,17
Limitations
The retrospective nature of the study is its most significant limitation. Any conclusions about the results must be made with caution. However, this design allowed for a naturalistic and potentially generalizable investigation into the application of a vaccination program in a real-world treatment setting. As such, the investigators were able to demonstrate the feasibility of conducting a rapid vaccination program within a 3- to 6-week SATP.
The retrospective nature of the study also limited a full investigation into the reasons behind the lack of vaccination initiation and vaccination noncompletion among the study’s treatment population, especially with regard to the follow-up booster injection. Initial statistical comparisons of initiators and noninitiators and completers and noncompleters showed no significant statistical differences between the groups. Future prospective designs should take into account the need to successfully initiate and complete vaccinations for all eligible patients and include assessment measures to determine the specific reasons that patients did not initiate or complete their vaccinations.
Conclusions
Many patients began and completed the accelerated vaccination schedule for hepatitis A and B in the context of a 3- to 6-week SATP at JAHVH. The overall vaccination rate, including the 12-month booster injection, was one-fifth of the entire vaccination-eligible sample. Additionally, 88% of the vaccination-eligible patients who began the vaccination schedule (or 42% of the whole sample) completed at least the first 3 doses, which may confer substantial immunity from hepatitis A and B. For reasons not entirely clear, a little less than half the vaccination-eligible patients began the vaccination schedule, and only about 50% of those returned to receive their 12-month booster injection. Future prospective studies may be able to determine barriers to both the initiation of and adherence to the vaccination protocol.
The results of this study are also a testament to having primary care nursing staff available and actively involved in the care of patients in a SATP. It seems likely that additional interventions might be needed for outreach to and retention of patients in need of vaccination for hepatitis A and B, and particularly those patients with hepatitis C. It is important to find ways to increase the rates of 12-month booster vaccinations, both for veterans who continue to receive services at JAHVH and for those who transfer care to other VA facilities. Finally, testing to confirm serologic immunity to hepatitis A and hepatitis B would be the next step in the effort to eliminate the risk of hepatitis A and hepatitis B and minimize additional harm for those with hepatitis C in the population receiving treatment for addictive disorders.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Nyamathi A, Liu Y, Marfisee M, et al. Effects of a nurse-managed program on hepatitis A and B vaccine completion among homeless adults. Nurs Res. 2009;58(1):13-22.
2. Center for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2006;55(RR16):1-25.
3. Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2006;55(RR07):1-23.
4. Weltman MD, Brotodihardjo A, Crewe EB, et al. Coinfection with hepatitis B and C or B, C and delta viruses results in severe chronic liver disease and responds poorly to interferon-alpha treatment. J Viral Hepat. 1995;2(1):39-45.
5. Groessl EJ, Weingart KR, Kaplan RM, et al. Living with hepatitis C: qualitative interviews with hepatitis C-infected veterans. J Gen Intern Med. 2008;23(12):1959-1965.
6. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
7. Himeloch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50(1):30-37.
8. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
9. Ramasamy P, Lintzeris N, Sutton Y, Taylor H, Day CA, Haber PS. The outcome of a rapid hepatitis B vaccination programme in a methadone treatment clinic. Addiction. 2010;105(2):329-334.
10. Bini EJ, Kritz S, Brown LS Jr, et al. Hepatitis B virus and hepatitis C virus services offered by substance abuse treatment programs in the United States. J Subst Abuse Treat. 2012;42(4):438-445.
11. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1-39.
12. Francis E, Gonzales-Nolas CL, Markowitz J, Phillips S. Integration of preventive health screening into mental health clinics. Fed Pract. 2008;25(2):39-50.
13. Vreeland B. Bridging the gap between mental and physical health: a multidisciplinary approach. J Clin Psychiatry. 2007;68(suppl 4):26-33.
14. Brim N, Zaller N, Taylor LE, Feller E. Twinrix vaccination schedules among injecting drug users. Expert Opin Biol Ther. 2007;7(3):379-389.
15. Zuckerman J. The place of accelerated schedules for hepatitis A and B vaccinations. Drugs. 2003;63(17):1779-1784.
16. Connor BA, Blatter MM, Beran J, Zou B, Trofa AF. Rapid and sustained immune response against hepatitis A and B achieved with combined vaccine using an accelerated administration schedule. J Travel Med. 2007;14(1):9-15.
17. Nothdurft HD, Dietrich M, Zuckerman JN, et al. A new accelerated vaccination schedule for rapid protection against hepatitis A and B. Vaccine. 2002;20(7-8):1157-1162.
18. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
19. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127.
20. McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199-213.
21. Batki SL, Nathan KI. HIV/AIDS and Hepatitis C. In: Galanter M, Kleber HD, Brady KT, eds. The American Psychiatric Publishing Textbook of Substance Abuse Treatment. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015.
22. Gelberg L, Robertson MJ, Leake B, et al. Hepatitis B among homeless and other impoverished US military veterans in residential care in Los Angeles. Public Health. 2001;115(4):286-291.
23. Felsen UR, Fishbein DA, Litwin AH. Low rates of hepatitis A and B vaccination in patients with chronic hepatitis C at an urban methadone maintenance program. J Addict Dis. 2010;29(4):461-465.
24. Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26(2):136-145.
25. Soto-Salgado M, Suárez E, Ortiz AP, et al. Knowledge of viral hepatitis among Puerto Rican adults: implications for prevention. J Community Health. 2011;36(4):565-573.
26. Cox AD, Cox D, Cyrier R, Graham-Dotson Y, Zimet GD. Can self-prediction overcome barriers to hepatitis B vaccination? A randomized controlled trial. Health Psychol. 2012;31(1):97-105.
27. Stitzer ML, Polk T, Bowles S, Kosten T. Drug users’ adherence to a 6-month vaccination protocol: effects of motivational incentives. Drug Alcohol Depend. 2010;107(1):76-79.
Homeless individuals and IV drug users are susceptible to hepatitis A, B, and C infections, and co-infection with these diseases may complicate treatment and result in poor medical outcomes.1 Vaccination offers the best protection against hepatitis A and B, particularly among high-risk populations.2,3 Immunization against hepatitis A and B is of even greater importance for patients with hepatitis C, because there is no specific hepatitis C vaccine, and concomitant infections of B with C are damaging to the liver.4
Veterans have a rate of hepatitis C infection that is 3 times that of the general population.5 Some evidence exists that veterans with serious mental illness (SMI) have a higher rate of hepatitis C infection relative to patients without SMI. Co-occurring substance abuse may add another layer of vulnerability to hepatitis C infection, particularly for homeless veterans.5-7
Mental Health and Primary Care Integration
Substance abuse and dual-diagnosis treatment programs (ie, those programs that treat both substance abuse and co-occurring serious mental health problems, such as bipolar disorder, severe major depressive disorder, psychotic disorders, and posttraumatic stress disorder [PTSD]) that have integrated mental health and primary care into their treatment programs may offer a window of opportunity for risk-reducing interventions. These interventions include testing and education of patients regarding infectious diseases, such as viral hepatitis and HIV, and completion of the hepatitis A/B immunization series.
The James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, has demonstrated some limited success in the past with integrating a standard dosing schedule for hepatitis A/B vaccination into its substance abuse treatment program (SATP), though recent evidence points to more promising results using an accelerated regimen as indicated by a high completion rate for hepatitis B vaccination in a methadone clinic.8,9 A relatively low proportion of SATPs in the U.S. provide testing, education, or vaccination for hepatitis A and B, especially considering the public health importance of controlling these diseases in the substance abusing populations.10,11
Related: Combination Pill Approved for HCV
In 1999, a primary care team was added to the alcohol and drug abuse treatment program at JAHVH.In 2005, the nurses in the program began scheduling vaccinations and screening patients for medical and psychiatric issues, pain, hypertension, diabetes, hepatitis C, alcohol use, depression, PTSD, prostate and colorectal cancers.12 Such a multidisciplinary approach provides many treatment advantages for patients and may save lives.13
Even with a multidisciplinary approach, the nurses found it difficult to provide adequate hepatitis A/B immunization within the 3- to 6-week intensive SATP, because standard immunization dosing regimens are spread over 6 months.14 As with all types of immunizations, long dosing schedules may reduce patient adherence and result in inadequate seroprotection.15 Thus, there is a need to provide a completed immunization series in a more expeditious fashion, and an accelerated dosing regimen makes that possible.15,16
Hepatitis A/B Vaccination
Twinrix (GlaxoSmithKline, Brentford, United Kingdom) is a vaccine that provides dual immunization for hepatitis A and B. Whereas the standard vaccination schedule takes 6 months to complete, the accelerated dosing schedule can be used to complete the first 3 doses in less than a month. The accelerated dosing schedule was incorporated into the JAHVH clinic to capture as many patients as possible in the 3- to 6-week time frame: The first dose is administered and followed by a second dose 7 days later. The third dose is administered 21 to 30 days after the first dose. Twelve months after the first dose, a booster dose is given.
After the first 3 accelerated doses, > 98% of patients show a sustained immune response to hepatitis A, and > 63% demonstrate immunity to hepatitis B. If a 12-month booster injection is given, 100% of patients may receive immunity to hepatitis A and > 96% may have immunity to hepatitis B.16 Another study of the combined vaccine showed even greater seroprotection for hepatitis A and B after only 1 month, 100% and 82%, respectively.17
Related: Viral Hepatitis Awareness
This JAHVH retrospective feasibility study describes a risk-reduction program for hepatitis A/B prevention that was implemented within a 3- to 4-week intensive outpatient SATP and a 6-week dual-diagnosis treatment program. The study includes the development and implementation of the program, designed to vaccinate patients using the accelerated Twinrix schedule. To ascertain the feasibility of this vaccination approach, historical medical records were used to describe and examine the vaccination initiation and follow-up rates of the treatment program participants who received the hepatitis A/B immunization series during their intensive SATP.
Study Design
A retrospective review of medical records was conducted for all participants who were admitted to the intensive JAHVH SATP between October 1, 2008, and September 30, 2009. This study was reviewed and approved by the JAHVH research and development committee and its associated University of South Florida institutional review board. Informed consent to participate was not obtained, because the study was retrospective.
Patient Identification and Education
All program participants were offered testing for HIV and hepatitis A, B, and C. Program participants were educated about hepatitis and HIV transmission, as well as about the long-term effects of continued substance abuse on the progression of hepatitis C. Education about hepatitis, HIV, and substance abuse was provided in a group setting by a member of the program’s nursing staff. One-on-one risk education counseling was also provided when requested or otherwise indicated.
Laboratory testing was performed following each participant’s initial physical examination (within 3 to 5 days of program admission), and the nursing staff reviewed the results before vaccination. Explanation of laboratory results and an individualized immunization regimen were provided to each participant. On review of participants’ laboratory results, those with seroconversion of both hepatitis A and B were not given the combined immunization. Participants who had seroconversion of hepatitis A were offered the hepatitis B vaccination series, and vice versa.
Immunization Process
Participants who lacked prior immunization for hepatitis A and B and had no seroconversion of either hepatitis A or B were offered vaccination. Some patients declined vaccination, even though they were eligible. Their reasons were not formally assessed.
Related: Nivolumab Approved for Expanded Indication
Patients who accepted the vaccination were given the accelerated regimen.16 Participants were educated on the importance of compliance with the vaccination series and provided with follow-up immunization dates and a reminder for the 1-year booster vaccine. The immunizations were ordered by the program’s primary care NP and administered by a licensed practical nurse. The nurse who administered the injections took responsibility for scheduling the patients for all their subsequent injections, including the 1-year booster.
Follow-up Care
If the third injection was not completed before discharge, patients were given a follow-up appointment with the nurse if they remained in the JAHVH service area. If they were leaving the area, they were given instructions on how to follow-up at another VA facility to continue their immunization schedule. A note was written in the electronic medical record documenting their abbreviated hepatitis A/B immunization schedule, which could be accessed by other providers at other VA facilities. Patients who did not show up for any follow-up appointments (third injection or the 1-year booster injection) were contacted and reminded about the importance of completing the immunization series and to schedule an appointment.
Statistical Analysis
All data were analyzed using IBM Statistical Package for the Social Sciences (IBM SPSS, Armonk, New York) with a focus on identifying differences between vaccination-eligible patients (n = 269) who did (n = 128) and did not (n = 141) initiate the immunization schedule during the treatment program. Chi-square and Fisher exact tests were used to assess statistical differences in initiation of the immunization schedule related to categoric variables (ie, marital status, race, history of IV drug abuse, cigarette smoking status, housing status, legal status, history of combat, having a psychiatric or medical diagnosis, and program track). Independent sample t tests were used to test for differences between these 2 groups on the continuous variables, including age, number of previous treatment programs, Global Assessment of Functioning score, severity of smoking dependence as measured by the Fagerström Test for Nicotine Dependence, and the Addiction Severity Index scales.18-20
Results
The sample consisted of 284 successive admissions to an intensive outpatient program for veterans with substance use disorders. About one-third of the patients were homeless at the time of admission to the treatment, and 87% required contracted housing while completing treatment for reasons related to lack of housing, transportation, clinical necessity, or a combination of those factors (Table 1). The most common substance problems were alcohol and cocaine dependence, and 21% (n = 59) of the patients acknowledged a history of IV drug use during their initial psychiatric evaluation. Seventy percent were dually diagnosed with some other Axis I disorder, and 40% had a history of serious mental illness. More than one-fourth (n = 77) of the patients admitted to the intensive outpatient SATP were seropositive for hepatitis A, B and/or C, and the most common hepatitis diagnosis was hepatitis C (n = 71).
Accelerated Immunization Regimen
Patients were eligible to receive the accelerated vaccination schedule only if they had no prior immunization for hepatitis A or B and if they had no seroconversion for either hepatitis A or B. Six people had hepatitis B alone, 7 had hepatitis B and C, 1 had hepatitis A and C, and 1 had all 3 (Table 2). Thus, 15 participants were ineligible to receive the accelerated hepatitis A/B immunization. Chi-square, Fisher exact, and independent sample t tests showed that among those who were vaccination-eligible (269), there were no significant differences in any of the demographic or clinical characteristics between those who initiated the vaccination schedule and those who did not. Among those who completed the first 3 vaccine injections, those who received the 1-year booster injection (54) did not differ (on any demographic or clinical variables) from those who did not (58).
Nearly half (48%) of all the eligible patients admitted to the program began the accelerated immunization schedule for hepatitis A and B. Of those, 88% completed the first 3 injections in the series. Among the patients who received the first 3 injections, 48% received the 1-year booster injection—a 20% completion rate for the vaccination-eligible sample overall (Table 3).
Of the 74 patients who did not complete their vaccinations once initiating the accelerated schedule, the most common reason identified was that the patient moved away (37), or no reason could be identified (33). It was uncommon for a patient not to complete the vaccination schedule because of terminating treatment prematurely (4).
Compared with the vaccine-eligible patients without hepatitis C (207), patients with hepatitis C were less likely to receive any vaccination injections (Table 3). Specifically, 51% of the vaccination-eligible patients who did not have hepatitis C began the vaccination regimen. However, only 22 patients with hepatitis C, or
35% of all vaccination-eligible patients with hepatitis C, began the vaccination regimen. Patients with hepatitis C were also less likely than those without hepatitis C to complete the first 3 injections of the vaccination series once they had initiated it (77%, vs 90%, respectively). This difference continued to be apparent at the time of the 12-month booster injection. Only 35% of vaccine-eligible individuals with hepatitis C received the 12-month booster injection, whereas 51% of vaccination-eligible individuals without hepatitis C received the 12-month booster injection. As with the sample overall, the most common reason patients with hepatitis C did not complete the vaccination regimen was because they moved away (9), followed by no identified reason (5), and premature termination of treatment (2).
Discussion
Individuals abusing alcohol and drugs have an increased vulnerability for infectious diseases, and homeless veterans with substance use disorders may be at a particularly heightened risk.21,22 This study describes a sample of veterans, many were homeless and most were dually diagnosed, in an intensive outpatient SATP that offered an accelerated dosing regimen for hepatitis A and B vaccination. Almost half (48%) of the vaccination-eligible patients began the accelerated regimen for hepatitis A/B vaccination. Moreover, 88% of those who started the vaccination regimen received the first 3 injections of the series, thus possibly conferring substantial immunity to hepatitis A and B and demonstrating the feasibility of an accelerated vaccination schedule in an intensive outpatient SATP.
It is especially important to demonstrate the successful integration of a hepatitis screening and immunization program within a SATP, given that many such programs do not offer screening or immunization for hepatitis, even though substance abusers are disproportionately affected by the disease and contribute greatly to the ongoing hepatitis epidemic.10,11 This study’s results were in line with another study of rapid vaccination for hepatitis B in IV drug users being treated in a methadone clinic, where 83% of the vaccination initiators completed the first 3 injections of the series.9
Unvaccinated Patients
The treatment team in the current study seemed to be less effective at reaching the subset of vaccination-eligible veterans with hepatitis C (almost one-quarter of the sample) in order to administer the accelerated vaccination schedule, as indicated by the lower rate of vaccination initiation as well as a lower rate of completion of the vaccination series among those patients. This replicates a finding from another study that also indicated a low rate of hepatitis A and B vaccination among patients with hepatitis C.23 Only 35% of the vaccination-eligible patients with hepatitis C in the current study initiated the vaccination series, compared with 51% of the patients without hepatitis C. However, the rate of completion of the first 3 injections of the series in the hepatitis C group was respectably high (77%), especially given the high relapse rate and psychosocial instability of individuals with addictive disorders. Initiation seems to be a bigger obstacle than completion of at least the first 3 injections of the vaccination series in both patients with and without hepatitis C.
The study investigators did not formally assess the reasons that more than half the patients in the study did not begin the vaccination series, but anecdotal evidence from the nurses indicated that many patients were afraid of needles. In addition, other patients felt that they simply did not need the vaccination. Some also insisted that they had already had the vaccination despite a blood test showing no evidence for either hepatitis A or B immunization.
Although the nursing team provided group and individual risk-based education as well as information about the effects of continued substance abuse on hepatitis C, it is possible that patients still underestimated their own risk of hepatitis infection and its consequences, or perhaps the information was simply not retained.24
Patient Education
A recent study showed that there is a positive relationship between the amount of hepatitis counseling received and knowledge of hepatitis.25 Possibly, increased intensity of education efforts may make an impact on initiation rates. Encouragingly, there is also evidence that prompting people to predict their future vaccination behavior may increase vaccination initiation rates despite a high-degree of short-term barriers, such as perceived pain or inconvenience.26 A brief intervention to induce people to formulate their future intentions would be relatively easy to incorporate into a vaccination program, and the study team is considering options for this to improve vaccination initiation rates.
Patients can expect to achieve substantial immunity from hepatitis A and, to a lesser degree, hepatitis B after completing the first 3 injections of the series, although the best seroprotection from both is obtained by completing the 12-month booster injection as well.17 Overall, about half of all patients who completed the first 3 injections returned for the booster shot, but only 35% of the patients with hepatitis C did so. The most common known cause of any patient not receiving the booster was movement out of the geographic area. However, much of the time the investigators were unable to determine the reasons patients did not return for the booster shot.
Medication adherence is a difficult problem with vaccination in high-risk samples, although Stitzer and colleagues found a significant improvement in follow-up for a 6-month vaccination protocol by using monetary incentives.27 In addition to ensuring medication adherence, it would also be of value for future immunization efforts to include testing to assess whether seroconversion has occurred once the vaccinations are complete, which is the ultimate measure of the success of a vaccination program. Most patients in the current study did not receive such testing at the completion of their vaccination schedules, and thus, seroconversion rates could not be determined. However, existing studies suggest high rates of seroprotection after the first 3 doses of the combined vaccine.10,17
Limitations
The retrospective nature of the study is its most significant limitation. Any conclusions about the results must be made with caution. However, this design allowed for a naturalistic and potentially generalizable investigation into the application of a vaccination program in a real-world treatment setting. As such, the investigators were able to demonstrate the feasibility of conducting a rapid vaccination program within a 3- to 6-week SATP.
The retrospective nature of the study also limited a full investigation into the reasons behind the lack of vaccination initiation and vaccination noncompletion among the study’s treatment population, especially with regard to the follow-up booster injection. Initial statistical comparisons of initiators and noninitiators and completers and noncompleters showed no significant statistical differences between the groups. Future prospective designs should take into account the need to successfully initiate and complete vaccinations for all eligible patients and include assessment measures to determine the specific reasons that patients did not initiate or complete their vaccinations.
Conclusions
Many patients began and completed the accelerated vaccination schedule for hepatitis A and B in the context of a 3- to 6-week SATP at JAHVH. The overall vaccination rate, including the 12-month booster injection, was one-fifth of the entire vaccination-eligible sample. Additionally, 88% of the vaccination-eligible patients who began the vaccination schedule (or 42% of the whole sample) completed at least the first 3 doses, which may confer substantial immunity from hepatitis A and B. For reasons not entirely clear, a little less than half the vaccination-eligible patients began the vaccination schedule, and only about 50% of those returned to receive their 12-month booster injection. Future prospective studies may be able to determine barriers to both the initiation of and adherence to the vaccination protocol.
The results of this study are also a testament to having primary care nursing staff available and actively involved in the care of patients in a SATP. It seems likely that additional interventions might be needed for outreach to and retention of patients in need of vaccination for hepatitis A and B, and particularly those patients with hepatitis C. It is important to find ways to increase the rates of 12-month booster vaccinations, both for veterans who continue to receive services at JAHVH and for those who transfer care to other VA facilities. Finally, testing to confirm serologic immunity to hepatitis A and hepatitis B would be the next step in the effort to eliminate the risk of hepatitis A and hepatitis B and minimize additional harm for those with hepatitis C in the population receiving treatment for addictive disorders.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Homeless individuals and IV drug users are susceptible to hepatitis A, B, and C infections, and co-infection with these diseases may complicate treatment and result in poor medical outcomes.1 Vaccination offers the best protection against hepatitis A and B, particularly among high-risk populations.2,3 Immunization against hepatitis A and B is of even greater importance for patients with hepatitis C, because there is no specific hepatitis C vaccine, and concomitant infections of B with C are damaging to the liver.4
Veterans have a rate of hepatitis C infection that is 3 times that of the general population.5 Some evidence exists that veterans with serious mental illness (SMI) have a higher rate of hepatitis C infection relative to patients without SMI. Co-occurring substance abuse may add another layer of vulnerability to hepatitis C infection, particularly for homeless veterans.5-7
Mental Health and Primary Care Integration
Substance abuse and dual-diagnosis treatment programs (ie, those programs that treat both substance abuse and co-occurring serious mental health problems, such as bipolar disorder, severe major depressive disorder, psychotic disorders, and posttraumatic stress disorder [PTSD]) that have integrated mental health and primary care into their treatment programs may offer a window of opportunity for risk-reducing interventions. These interventions include testing and education of patients regarding infectious diseases, such as viral hepatitis and HIV, and completion of the hepatitis A/B immunization series.
The James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, has demonstrated some limited success in the past with integrating a standard dosing schedule for hepatitis A/B vaccination into its substance abuse treatment program (SATP), though recent evidence points to more promising results using an accelerated regimen as indicated by a high completion rate for hepatitis B vaccination in a methadone clinic.8,9 A relatively low proportion of SATPs in the U.S. provide testing, education, or vaccination for hepatitis A and B, especially considering the public health importance of controlling these diseases in the substance abusing populations.10,11
Related: Combination Pill Approved for HCV
In 1999, a primary care team was added to the alcohol and drug abuse treatment program at JAHVH.In 2005, the nurses in the program began scheduling vaccinations and screening patients for medical and psychiatric issues, pain, hypertension, diabetes, hepatitis C, alcohol use, depression, PTSD, prostate and colorectal cancers.12 Such a multidisciplinary approach provides many treatment advantages for patients and may save lives.13
Even with a multidisciplinary approach, the nurses found it difficult to provide adequate hepatitis A/B immunization within the 3- to 6-week intensive SATP, because standard immunization dosing regimens are spread over 6 months.14 As with all types of immunizations, long dosing schedules may reduce patient adherence and result in inadequate seroprotection.15 Thus, there is a need to provide a completed immunization series in a more expeditious fashion, and an accelerated dosing regimen makes that possible.15,16
Hepatitis A/B Vaccination
Twinrix (GlaxoSmithKline, Brentford, United Kingdom) is a vaccine that provides dual immunization for hepatitis A and B. Whereas the standard vaccination schedule takes 6 months to complete, the accelerated dosing schedule can be used to complete the first 3 doses in less than a month. The accelerated dosing schedule was incorporated into the JAHVH clinic to capture as many patients as possible in the 3- to 6-week time frame: The first dose is administered and followed by a second dose 7 days later. The third dose is administered 21 to 30 days after the first dose. Twelve months after the first dose, a booster dose is given.
After the first 3 accelerated doses, > 98% of patients show a sustained immune response to hepatitis A, and > 63% demonstrate immunity to hepatitis B. If a 12-month booster injection is given, 100% of patients may receive immunity to hepatitis A and > 96% may have immunity to hepatitis B.16 Another study of the combined vaccine showed even greater seroprotection for hepatitis A and B after only 1 month, 100% and 82%, respectively.17
Related: Viral Hepatitis Awareness
This JAHVH retrospective feasibility study describes a risk-reduction program for hepatitis A/B prevention that was implemented within a 3- to 4-week intensive outpatient SATP and a 6-week dual-diagnosis treatment program. The study includes the development and implementation of the program, designed to vaccinate patients using the accelerated Twinrix schedule. To ascertain the feasibility of this vaccination approach, historical medical records were used to describe and examine the vaccination initiation and follow-up rates of the treatment program participants who received the hepatitis A/B immunization series during their intensive SATP.
Study Design
A retrospective review of medical records was conducted for all participants who were admitted to the intensive JAHVH SATP between October 1, 2008, and September 30, 2009. This study was reviewed and approved by the JAHVH research and development committee and its associated University of South Florida institutional review board. Informed consent to participate was not obtained, because the study was retrospective.
Patient Identification and Education
All program participants were offered testing for HIV and hepatitis A, B, and C. Program participants were educated about hepatitis and HIV transmission, as well as about the long-term effects of continued substance abuse on the progression of hepatitis C. Education about hepatitis, HIV, and substance abuse was provided in a group setting by a member of the program’s nursing staff. One-on-one risk education counseling was also provided when requested or otherwise indicated.
Laboratory testing was performed following each participant’s initial physical examination (within 3 to 5 days of program admission), and the nursing staff reviewed the results before vaccination. Explanation of laboratory results and an individualized immunization regimen were provided to each participant. On review of participants’ laboratory results, those with seroconversion of both hepatitis A and B were not given the combined immunization. Participants who had seroconversion of hepatitis A were offered the hepatitis B vaccination series, and vice versa.
Immunization Process
Participants who lacked prior immunization for hepatitis A and B and had no seroconversion of either hepatitis A or B were offered vaccination. Some patients declined vaccination, even though they were eligible. Their reasons were not formally assessed.
Related: Nivolumab Approved for Expanded Indication
Patients who accepted the vaccination were given the accelerated regimen.16 Participants were educated on the importance of compliance with the vaccination series and provided with follow-up immunization dates and a reminder for the 1-year booster vaccine. The immunizations were ordered by the program’s primary care NP and administered by a licensed practical nurse. The nurse who administered the injections took responsibility for scheduling the patients for all their subsequent injections, including the 1-year booster.
Follow-up Care
If the third injection was not completed before discharge, patients were given a follow-up appointment with the nurse if they remained in the JAHVH service area. If they were leaving the area, they were given instructions on how to follow-up at another VA facility to continue their immunization schedule. A note was written in the electronic medical record documenting their abbreviated hepatitis A/B immunization schedule, which could be accessed by other providers at other VA facilities. Patients who did not show up for any follow-up appointments (third injection or the 1-year booster injection) were contacted and reminded about the importance of completing the immunization series and to schedule an appointment.
Statistical Analysis
All data were analyzed using IBM Statistical Package for the Social Sciences (IBM SPSS, Armonk, New York) with a focus on identifying differences between vaccination-eligible patients (n = 269) who did (n = 128) and did not (n = 141) initiate the immunization schedule during the treatment program. Chi-square and Fisher exact tests were used to assess statistical differences in initiation of the immunization schedule related to categoric variables (ie, marital status, race, history of IV drug abuse, cigarette smoking status, housing status, legal status, history of combat, having a psychiatric or medical diagnosis, and program track). Independent sample t tests were used to test for differences between these 2 groups on the continuous variables, including age, number of previous treatment programs, Global Assessment of Functioning score, severity of smoking dependence as measured by the Fagerström Test for Nicotine Dependence, and the Addiction Severity Index scales.18-20
Results
The sample consisted of 284 successive admissions to an intensive outpatient program for veterans with substance use disorders. About one-third of the patients were homeless at the time of admission to the treatment, and 87% required contracted housing while completing treatment for reasons related to lack of housing, transportation, clinical necessity, or a combination of those factors (Table 1). The most common substance problems were alcohol and cocaine dependence, and 21% (n = 59) of the patients acknowledged a history of IV drug use during their initial psychiatric evaluation. Seventy percent were dually diagnosed with some other Axis I disorder, and 40% had a history of serious mental illness. More than one-fourth (n = 77) of the patients admitted to the intensive outpatient SATP were seropositive for hepatitis A, B and/or C, and the most common hepatitis diagnosis was hepatitis C (n = 71).
Accelerated Immunization Regimen
Patients were eligible to receive the accelerated vaccination schedule only if they had no prior immunization for hepatitis A or B and if they had no seroconversion for either hepatitis A or B. Six people had hepatitis B alone, 7 had hepatitis B and C, 1 had hepatitis A and C, and 1 had all 3 (Table 2). Thus, 15 participants were ineligible to receive the accelerated hepatitis A/B immunization. Chi-square, Fisher exact, and independent sample t tests showed that among those who were vaccination-eligible (269), there were no significant differences in any of the demographic or clinical characteristics between those who initiated the vaccination schedule and those who did not. Among those who completed the first 3 vaccine injections, those who received the 1-year booster injection (54) did not differ (on any demographic or clinical variables) from those who did not (58).
Nearly half (48%) of all the eligible patients admitted to the program began the accelerated immunization schedule for hepatitis A and B. Of those, 88% completed the first 3 injections in the series. Among the patients who received the first 3 injections, 48% received the 1-year booster injection—a 20% completion rate for the vaccination-eligible sample overall (Table 3).
Of the 74 patients who did not complete their vaccinations once initiating the accelerated schedule, the most common reason identified was that the patient moved away (37), or no reason could be identified (33). It was uncommon for a patient not to complete the vaccination schedule because of terminating treatment prematurely (4).
Compared with the vaccine-eligible patients without hepatitis C (207), patients with hepatitis C were less likely to receive any vaccination injections (Table 3). Specifically, 51% of the vaccination-eligible patients who did not have hepatitis C began the vaccination regimen. However, only 22 patients with hepatitis C, or
35% of all vaccination-eligible patients with hepatitis C, began the vaccination regimen. Patients with hepatitis C were also less likely than those without hepatitis C to complete the first 3 injections of the vaccination series once they had initiated it (77%, vs 90%, respectively). This difference continued to be apparent at the time of the 12-month booster injection. Only 35% of vaccine-eligible individuals with hepatitis C received the 12-month booster injection, whereas 51% of vaccination-eligible individuals without hepatitis C received the 12-month booster injection. As with the sample overall, the most common reason patients with hepatitis C did not complete the vaccination regimen was because they moved away (9), followed by no identified reason (5), and premature termination of treatment (2).
Discussion
Individuals abusing alcohol and drugs have an increased vulnerability for infectious diseases, and homeless veterans with substance use disorders may be at a particularly heightened risk.21,22 This study describes a sample of veterans, many were homeless and most were dually diagnosed, in an intensive outpatient SATP that offered an accelerated dosing regimen for hepatitis A and B vaccination. Almost half (48%) of the vaccination-eligible patients began the accelerated regimen for hepatitis A/B vaccination. Moreover, 88% of those who started the vaccination regimen received the first 3 injections of the series, thus possibly conferring substantial immunity to hepatitis A and B and demonstrating the feasibility of an accelerated vaccination schedule in an intensive outpatient SATP.
It is especially important to demonstrate the successful integration of a hepatitis screening and immunization program within a SATP, given that many such programs do not offer screening or immunization for hepatitis, even though substance abusers are disproportionately affected by the disease and contribute greatly to the ongoing hepatitis epidemic.10,11 This study’s results were in line with another study of rapid vaccination for hepatitis B in IV drug users being treated in a methadone clinic, where 83% of the vaccination initiators completed the first 3 injections of the series.9
Unvaccinated Patients
The treatment team in the current study seemed to be less effective at reaching the subset of vaccination-eligible veterans with hepatitis C (almost one-quarter of the sample) in order to administer the accelerated vaccination schedule, as indicated by the lower rate of vaccination initiation as well as a lower rate of completion of the vaccination series among those patients. This replicates a finding from another study that also indicated a low rate of hepatitis A and B vaccination among patients with hepatitis C.23 Only 35% of the vaccination-eligible patients with hepatitis C in the current study initiated the vaccination series, compared with 51% of the patients without hepatitis C. However, the rate of completion of the first 3 injections of the series in the hepatitis C group was respectably high (77%), especially given the high relapse rate and psychosocial instability of individuals with addictive disorders. Initiation seems to be a bigger obstacle than completion of at least the first 3 injections of the vaccination series in both patients with and without hepatitis C.
The study investigators did not formally assess the reasons that more than half the patients in the study did not begin the vaccination series, but anecdotal evidence from the nurses indicated that many patients were afraid of needles. In addition, other patients felt that they simply did not need the vaccination. Some also insisted that they had already had the vaccination despite a blood test showing no evidence for either hepatitis A or B immunization.
Although the nursing team provided group and individual risk-based education as well as information about the effects of continued substance abuse on hepatitis C, it is possible that patients still underestimated their own risk of hepatitis infection and its consequences, or perhaps the information was simply not retained.24
Patient Education
A recent study showed that there is a positive relationship between the amount of hepatitis counseling received and knowledge of hepatitis.25 Possibly, increased intensity of education efforts may make an impact on initiation rates. Encouragingly, there is also evidence that prompting people to predict their future vaccination behavior may increase vaccination initiation rates despite a high-degree of short-term barriers, such as perceived pain or inconvenience.26 A brief intervention to induce people to formulate their future intentions would be relatively easy to incorporate into a vaccination program, and the study team is considering options for this to improve vaccination initiation rates.
Patients can expect to achieve substantial immunity from hepatitis A and, to a lesser degree, hepatitis B after completing the first 3 injections of the series, although the best seroprotection from both is obtained by completing the 12-month booster injection as well.17 Overall, about half of all patients who completed the first 3 injections returned for the booster shot, but only 35% of the patients with hepatitis C did so. The most common known cause of any patient not receiving the booster was movement out of the geographic area. However, much of the time the investigators were unable to determine the reasons patients did not return for the booster shot.
Medication adherence is a difficult problem with vaccination in high-risk samples, although Stitzer and colleagues found a significant improvement in follow-up for a 6-month vaccination protocol by using monetary incentives.27 In addition to ensuring medication adherence, it would also be of value for future immunization efforts to include testing to assess whether seroconversion has occurred once the vaccinations are complete, which is the ultimate measure of the success of a vaccination program. Most patients in the current study did not receive such testing at the completion of their vaccination schedules, and thus, seroconversion rates could not be determined. However, existing studies suggest high rates of seroprotection after the first 3 doses of the combined vaccine.10,17
Limitations
The retrospective nature of the study is its most significant limitation. Any conclusions about the results must be made with caution. However, this design allowed for a naturalistic and potentially generalizable investigation into the application of a vaccination program in a real-world treatment setting. As such, the investigators were able to demonstrate the feasibility of conducting a rapid vaccination program within a 3- to 6-week SATP.
The retrospective nature of the study also limited a full investigation into the reasons behind the lack of vaccination initiation and vaccination noncompletion among the study’s treatment population, especially with regard to the follow-up booster injection. Initial statistical comparisons of initiators and noninitiators and completers and noncompleters showed no significant statistical differences between the groups. Future prospective designs should take into account the need to successfully initiate and complete vaccinations for all eligible patients and include assessment measures to determine the specific reasons that patients did not initiate or complete their vaccinations.
Conclusions
Many patients began and completed the accelerated vaccination schedule for hepatitis A and B in the context of a 3- to 6-week SATP at JAHVH. The overall vaccination rate, including the 12-month booster injection, was one-fifth of the entire vaccination-eligible sample. Additionally, 88% of the vaccination-eligible patients who began the vaccination schedule (or 42% of the whole sample) completed at least the first 3 doses, which may confer substantial immunity from hepatitis A and B. For reasons not entirely clear, a little less than half the vaccination-eligible patients began the vaccination schedule, and only about 50% of those returned to receive their 12-month booster injection. Future prospective studies may be able to determine barriers to both the initiation of and adherence to the vaccination protocol.
The results of this study are also a testament to having primary care nursing staff available and actively involved in the care of patients in a SATP. It seems likely that additional interventions might be needed for outreach to and retention of patients in need of vaccination for hepatitis A and B, and particularly those patients with hepatitis C. It is important to find ways to increase the rates of 12-month booster vaccinations, both for veterans who continue to receive services at JAHVH and for those who transfer care to other VA facilities. Finally, testing to confirm serologic immunity to hepatitis A and hepatitis B would be the next step in the effort to eliminate the risk of hepatitis A and hepatitis B and minimize additional harm for those with hepatitis C in the population receiving treatment for addictive disorders.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Nyamathi A, Liu Y, Marfisee M, et al. Effects of a nurse-managed program on hepatitis A and B vaccine completion among homeless adults. Nurs Res. 2009;58(1):13-22.
2. Center for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2006;55(RR16):1-25.
3. Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2006;55(RR07):1-23.
4. Weltman MD, Brotodihardjo A, Crewe EB, et al. Coinfection with hepatitis B and C or B, C and delta viruses results in severe chronic liver disease and responds poorly to interferon-alpha treatment. J Viral Hepat. 1995;2(1):39-45.
5. Groessl EJ, Weingart KR, Kaplan RM, et al. Living with hepatitis C: qualitative interviews with hepatitis C-infected veterans. J Gen Intern Med. 2008;23(12):1959-1965.
6. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
7. Himeloch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50(1):30-37.
8. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
9. Ramasamy P, Lintzeris N, Sutton Y, Taylor H, Day CA, Haber PS. The outcome of a rapid hepatitis B vaccination programme in a methadone treatment clinic. Addiction. 2010;105(2):329-334.
10. Bini EJ, Kritz S, Brown LS Jr, et al. Hepatitis B virus and hepatitis C virus services offered by substance abuse treatment programs in the United States. J Subst Abuse Treat. 2012;42(4):438-445.
11. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1-39.
12. Francis E, Gonzales-Nolas CL, Markowitz J, Phillips S. Integration of preventive health screening into mental health clinics. Fed Pract. 2008;25(2):39-50.
13. Vreeland B. Bridging the gap between mental and physical health: a multidisciplinary approach. J Clin Psychiatry. 2007;68(suppl 4):26-33.
14. Brim N, Zaller N, Taylor LE, Feller E. Twinrix vaccination schedules among injecting drug users. Expert Opin Biol Ther. 2007;7(3):379-389.
15. Zuckerman J. The place of accelerated schedules for hepatitis A and B vaccinations. Drugs. 2003;63(17):1779-1784.
16. Connor BA, Blatter MM, Beran J, Zou B, Trofa AF. Rapid and sustained immune response against hepatitis A and B achieved with combined vaccine using an accelerated administration schedule. J Travel Med. 2007;14(1):9-15.
17. Nothdurft HD, Dietrich M, Zuckerman JN, et al. A new accelerated vaccination schedule for rapid protection against hepatitis A and B. Vaccine. 2002;20(7-8):1157-1162.
18. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
19. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127.
20. McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199-213.
21. Batki SL, Nathan KI. HIV/AIDS and Hepatitis C. In: Galanter M, Kleber HD, Brady KT, eds. The American Psychiatric Publishing Textbook of Substance Abuse Treatment. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015.
22. Gelberg L, Robertson MJ, Leake B, et al. Hepatitis B among homeless and other impoverished US military veterans in residential care in Los Angeles. Public Health. 2001;115(4):286-291.
23. Felsen UR, Fishbein DA, Litwin AH. Low rates of hepatitis A and B vaccination in patients with chronic hepatitis C at an urban methadone maintenance program. J Addict Dis. 2010;29(4):461-465.
24. Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26(2):136-145.
25. Soto-Salgado M, Suárez E, Ortiz AP, et al. Knowledge of viral hepatitis among Puerto Rican adults: implications for prevention. J Community Health. 2011;36(4):565-573.
26. Cox AD, Cox D, Cyrier R, Graham-Dotson Y, Zimet GD. Can self-prediction overcome barriers to hepatitis B vaccination? A randomized controlled trial. Health Psychol. 2012;31(1):97-105.
27. Stitzer ML, Polk T, Bowles S, Kosten T. Drug users’ adherence to a 6-month vaccination protocol: effects of motivational incentives. Drug Alcohol Depend. 2010;107(1):76-79.
1. Nyamathi A, Liu Y, Marfisee M, et al. Effects of a nurse-managed program on hepatitis A and B vaccine completion among homeless adults. Nurs Res. 2009;58(1):13-22.
2. Center for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2006;55(RR16):1-25.
3. Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2006;55(RR07):1-23.
4. Weltman MD, Brotodihardjo A, Crewe EB, et al. Coinfection with hepatitis B and C or B, C and delta viruses results in severe chronic liver disease and responds poorly to interferon-alpha treatment. J Viral Hepat. 1995;2(1):39-45.
5. Groessl EJ, Weingart KR, Kaplan RM, et al. Living with hepatitis C: qualitative interviews with hepatitis C-infected veterans. J Gen Intern Med. 2008;23(12):1959-1965.
6. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
7. Himeloch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50(1):30-37.
8. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
9. Ramasamy P, Lintzeris N, Sutton Y, Taylor H, Day CA, Haber PS. The outcome of a rapid hepatitis B vaccination programme in a methadone treatment clinic. Addiction. 2010;105(2):329-334.
10. Bini EJ, Kritz S, Brown LS Jr, et al. Hepatitis B virus and hepatitis C virus services offered by substance abuse treatment programs in the United States. J Subst Abuse Treat. 2012;42(4):438-445.
11. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1-39.
12. Francis E, Gonzales-Nolas CL, Markowitz J, Phillips S. Integration of preventive health screening into mental health clinics. Fed Pract. 2008;25(2):39-50.
13. Vreeland B. Bridging the gap between mental and physical health: a multidisciplinary approach. J Clin Psychiatry. 2007;68(suppl 4):26-33.
14. Brim N, Zaller N, Taylor LE, Feller E. Twinrix vaccination schedules among injecting drug users. Expert Opin Biol Ther. 2007;7(3):379-389.
15. Zuckerman J. The place of accelerated schedules for hepatitis A and B vaccinations. Drugs. 2003;63(17):1779-1784.
16. Connor BA, Blatter MM, Beran J, Zou B, Trofa AF. Rapid and sustained immune response against hepatitis A and B achieved with combined vaccine using an accelerated administration schedule. J Travel Med. 2007;14(1):9-15.
17. Nothdurft HD, Dietrich M, Zuckerman JN, et al. A new accelerated vaccination schedule for rapid protection against hepatitis A and B. Vaccine. 2002;20(7-8):1157-1162.
18. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
19. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127.
20. McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199-213.
21. Batki SL, Nathan KI. HIV/AIDS and Hepatitis C. In: Galanter M, Kleber HD, Brady KT, eds. The American Psychiatric Publishing Textbook of Substance Abuse Treatment. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015.
22. Gelberg L, Robertson MJ, Leake B, et al. Hepatitis B among homeless and other impoverished US military veterans in residential care in Los Angeles. Public Health. 2001;115(4):286-291.
23. Felsen UR, Fishbein DA, Litwin AH. Low rates of hepatitis A and B vaccination in patients with chronic hepatitis C at an urban methadone maintenance program. J Addict Dis. 2010;29(4):461-465.
24. Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26(2):136-145.
25. Soto-Salgado M, Suárez E, Ortiz AP, et al. Knowledge of viral hepatitis among Puerto Rican adults: implications for prevention. J Community Health. 2011;36(4):565-573.
26. Cox AD, Cox D, Cyrier R, Graham-Dotson Y, Zimet GD. Can self-prediction overcome barriers to hepatitis B vaccination? A randomized controlled trial. Health Psychol. 2012;31(1):97-105.
27. Stitzer ML, Polk T, Bowles S, Kosten T. Drug users’ adherence to a 6-month vaccination protocol: effects of motivational incentives. Drug Alcohol Depend. 2010;107(1):76-79.
The Use of Sodium Sulfacetamide in Dermatology
Sodium sulfacetamide has various uses in the field of dermatology due to its anti-inflammatory and antibacterial properties. It has been shown to be effective in the management of a variety of inflammatory facial dermatoses, including papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis. We review the mechanism of action, pharmacology and formulations, clinical uses, and adverse effects of sodium sulfacetamide as a dermatologic treatment.
Mechanism of Action
Sodium sulfacetamide is a sulfonamide-type antibacterial agent. Its mechanism of action is the inhibition of bacterial dihydropteroate synthetase, which prevents the conversion of p-aminobenzoic acid to folic acid. This process causes a bacteriostatic effect on the growth of several gram-negative and gram-positive organisms, including Propionibacterium acnes.1,2
The effectiveness of sodium sulfacetamide is increased when used in combination with sulfur, which has keratolytic, antibacterial, antifungal, and antiparasitic effects. The addition of hydrocortisone has been reported to increase the effectiveness of both agents.3
Pharmacology
Sodium sulfacetamide is highly soluble at the physiologic pH of 7.4, which contributes to its high level of penetration and absorption.4 An in vitro study showed percutaneous absorption of sodium sulfacetamide to be around 4%.5 Sulfonamides are metabolized mainly by the liver and are excreted by the kidneys.
Formulations
The most common concentrations of sodium sulfacetamide and sulfur are 10% and 5%, respectively. A wide variety of sulfacetamide-containing products are available, many of which are marketed to treat specific conditions depending on additional ingredients or the type of delivery system.
Clinical Uses
Topical formulations of sodium sulfacetamide and sulfur have proven to be efficacious in the management of rosacea, with a typical regimen consisting of twice-daily application for 8 weeks.6 The sulfur in the formulation has the additional benefit of targeting Demodex mites, which are implicated as a contributing factor in some cases of rosacea.7 Sodium sulfacetamide 10%–sulfur 5% lotion was more effective in improving the erythema, papulopustules, and overall severity of rosacea as compared to metronidazole gel 0.75%.8 Other studies have reported increased efficacy when sodium sulfacetamide and topical sulfur are used along with metronidazole.9,10
Sodium sulfacetamide also has shown efficacy against acne. Its antibacterial and drying properties have been shown to decrease the number of inflammatory lesions and comedones, and in the treatment of acne vulgaris, no sensitivity reactions have been observed.2 Also, unlike topical antibiotics, cases of P acnes resistance to topical sulfur products have not been widely reported. Studies have demonstrated that twice-daily use of sodium sulfacetamide 10%–sulfur 5% for 12 weeks decreases inflammatory acne lesions by 80.4% to 83%.11,12
Seborrheic dermatitis is a common chronic infection of the skin caused by Malassezia species. One study investigated the use of sodium sulfacetamide ointment and soap to treat seborrheic dermatitis and found that the condition was either improved or completely controlled in 93% (71/76) of cases.4 Sodium sulfacetamide lotion was an effective treatment of seborrheic dermatitis in 89% (54/61) of patients with scalp involvement and 68% (30/44) of patients with glabrous skin involvement.13
Perioral dermatitis is characterized by groups of erythematous papules and pustules localized around the mouth. The use of topical sodium sulfacetamide along with oral tetracyclines has been demonstrated to consistently clear lesions in most patients with perioral dermatitis.14 Sodium sulfacetamide is unique in that it is not associated with the excessive erythema and irritation often found with retinoic acid and benzoyl peroxide.15 Unfortunately, however, there have been no well-controlled trials to compare the efficacy of sodium sulfacetamide to other topical therapies for this condition.
Adverse Effects
Adverse effects from sodium sulfacetamide are rare and generally are limited to cutaneous reactions including dryness, erythema, pruritus, and discomfort.1 Periocular use of sodium sulfacetamide can cause conjunctival irritation. One study reported that 19% (6/31) of patients experienced local reactions but most were considered mild.9 Rare but serious reactions including erythema multiforme and Stevens-Johnson syndrome have been reported from ophthalmic use.16,17
A common limiting factor to sodium sulfacetamide preparations that include elemental sulfur is the offensive smell, which has hindered patient compliance in the past; however, pharmaceutical companies have attempted to create more tolerable products without the odor.10 One study found that the tolerability of a sodium sulfacetamide 10%–sulfur 5% foam using a rinse-off method of application was excellent, with only 33% (8/24) of participants commenting on the smell.18 Another limiting factor of sodium sulfacetamide preparations containing sulfur is orange-brown discoloration when combined with benzoyl peroxide, which does not affect the skin but may stain clothing.19
Sodium sulfacetamide is rendered less effective when combined with silver-containing products.20 No other notable drug interactions are known; however, oral sulfonamides are known to interact with several drugs, including cyclosporine and phenytoin.21,22
Contraindications
Sodium sulfacetamide is contraindicated in patients with known hypersensitivity to sulfonamides, sulfur, or any other component of the preparation. It is a pregnancy category C drug, and pregnant women should only use sodium sulfacetamide if it is the only modality to treat the condition or the benefits outweigh the risks. Although there are no known reports of problems related to topical sodium sulfacetamide during pregnancy, the use of oral sulfonamides during pregnancy can increase the risk for neonatal jaundice.23 Likewise, caution should be exercised in prescribing this product to nursing women, as systemic sulfonamide antibacterials are well known to cause kernicterus in nursing neonates.1
Conclusion
The efficacy and safety of sodium sulfacetamide, used alone or in combination with sulfur, has been demonstrated in the treatment of rosacea, acne, seborrheic dermatitis, and perioral dermatitis. Advances in formulation technology to decrease odor and irritation have allowed for more use of this product. Further studies will help elucidate the role that sodium sulfacetamide should play in the treatment of inflammatory dermatoses in comparison to other available products.
1. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003;4:473-492.
2. Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
3. Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders; 2012:445-459.
4. Duemling WM. Sodium sulfacetamide in topical therapy. AMA Arch Derm Syphilol. 1954;69:75-82.
5. Sodium sulfacetamide. Drugs.com Web site. http://drugs.com/pro/sodium-sulfacetamide.html. Revised December 2012. Accessed June 16, 2015.
6. Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8:79-85.
7. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
8. Lebwohl MG, Medansky RS, Russo CL, et al. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% lotion and metronidazole 0.75% gel in the treatment of rosacea. J Geriatr Dermatol. 1995;3:183-185.
9. Nally JB, Berson DS. Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-26.
10. Pelle MT, Crawford GH, James WD. Rosacea II: therapy. J Am Acad Dermatol. 2004;51:499-512.
11. Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22:301.
12. Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int J Dermatol. 1993;32:365-367.
13. Whelan ST. Sodium sulfacetamide for seborrheic dermatitis. AMA Arch Derm. 1955;71:724.
14. Bendl BJ. Perioral dermatitis: etiology and treatment. Cutis. 1976;17:903-908.
15. Olansky S. Old drug—in a new system—revisited. Cutis. 1977;19:852-854.
16. Genvert GI, Cohen EJ, Donnenfeld ED, et al. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985;99:465-468.
17. Rubin Z. Ophthalmic sulfonamide-induced Stevens-Johnson syndrome. Arch Dermatol. 1977;113:235-236.
18. Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-246.
19. Dubina MI, Fleischer AB. Interaction of topical sulfacetamide and topical dapsone with benzoyl peroxide. Arch Dermatol. 2009;145:1027-1029.
20. Sodium sulfacetamide – sulfacetamide sodium liquid. DailyMed Web site. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0d92c55b-5b54-4f5d-8921-24e4e877ae50. Accessed June 17, 2015.
21. Spes CH, Angermann CE, Stempfle HU, et al. Sulfadiazine therapy for toxoplasmosis in heart transplant recipients decreases cyclosporine concentration. Clin Investig. 1992;70:752-754.
22. Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106-110.
23. Bradley JS, Sauberan JB. Antimicrobial agents. In: Long SS, Pickering LK, Prober CG. Principles and Practices of Pediatric Infectious Diseases. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1453-1483.
Sodium sulfacetamide has various uses in the field of dermatology due to its anti-inflammatory and antibacterial properties. It has been shown to be effective in the management of a variety of inflammatory facial dermatoses, including papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis. We review the mechanism of action, pharmacology and formulations, clinical uses, and adverse effects of sodium sulfacetamide as a dermatologic treatment.
Mechanism of Action
Sodium sulfacetamide is a sulfonamide-type antibacterial agent. Its mechanism of action is the inhibition of bacterial dihydropteroate synthetase, which prevents the conversion of p-aminobenzoic acid to folic acid. This process causes a bacteriostatic effect on the growth of several gram-negative and gram-positive organisms, including Propionibacterium acnes.1,2
The effectiveness of sodium sulfacetamide is increased when used in combination with sulfur, which has keratolytic, antibacterial, antifungal, and antiparasitic effects. The addition of hydrocortisone has been reported to increase the effectiveness of both agents.3
Pharmacology
Sodium sulfacetamide is highly soluble at the physiologic pH of 7.4, which contributes to its high level of penetration and absorption.4 An in vitro study showed percutaneous absorption of sodium sulfacetamide to be around 4%.5 Sulfonamides are metabolized mainly by the liver and are excreted by the kidneys.
Formulations
The most common concentrations of sodium sulfacetamide and sulfur are 10% and 5%, respectively. A wide variety of sulfacetamide-containing products are available, many of which are marketed to treat specific conditions depending on additional ingredients or the type of delivery system.
Clinical Uses
Topical formulations of sodium sulfacetamide and sulfur have proven to be efficacious in the management of rosacea, with a typical regimen consisting of twice-daily application for 8 weeks.6 The sulfur in the formulation has the additional benefit of targeting Demodex mites, which are implicated as a contributing factor in some cases of rosacea.7 Sodium sulfacetamide 10%–sulfur 5% lotion was more effective in improving the erythema, papulopustules, and overall severity of rosacea as compared to metronidazole gel 0.75%.8 Other studies have reported increased efficacy when sodium sulfacetamide and topical sulfur are used along with metronidazole.9,10
Sodium sulfacetamide also has shown efficacy against acne. Its antibacterial and drying properties have been shown to decrease the number of inflammatory lesions and comedones, and in the treatment of acne vulgaris, no sensitivity reactions have been observed.2 Also, unlike topical antibiotics, cases of P acnes resistance to topical sulfur products have not been widely reported. Studies have demonstrated that twice-daily use of sodium sulfacetamide 10%–sulfur 5% for 12 weeks decreases inflammatory acne lesions by 80.4% to 83%.11,12
Seborrheic dermatitis is a common chronic infection of the skin caused by Malassezia species. One study investigated the use of sodium sulfacetamide ointment and soap to treat seborrheic dermatitis and found that the condition was either improved or completely controlled in 93% (71/76) of cases.4 Sodium sulfacetamide lotion was an effective treatment of seborrheic dermatitis in 89% (54/61) of patients with scalp involvement and 68% (30/44) of patients with glabrous skin involvement.13
Perioral dermatitis is characterized by groups of erythematous papules and pustules localized around the mouth. The use of topical sodium sulfacetamide along with oral tetracyclines has been demonstrated to consistently clear lesions in most patients with perioral dermatitis.14 Sodium sulfacetamide is unique in that it is not associated with the excessive erythema and irritation often found with retinoic acid and benzoyl peroxide.15 Unfortunately, however, there have been no well-controlled trials to compare the efficacy of sodium sulfacetamide to other topical therapies for this condition.
Adverse Effects
Adverse effects from sodium sulfacetamide are rare and generally are limited to cutaneous reactions including dryness, erythema, pruritus, and discomfort.1 Periocular use of sodium sulfacetamide can cause conjunctival irritation. One study reported that 19% (6/31) of patients experienced local reactions but most were considered mild.9 Rare but serious reactions including erythema multiforme and Stevens-Johnson syndrome have been reported from ophthalmic use.16,17
A common limiting factor to sodium sulfacetamide preparations that include elemental sulfur is the offensive smell, which has hindered patient compliance in the past; however, pharmaceutical companies have attempted to create more tolerable products without the odor.10 One study found that the tolerability of a sodium sulfacetamide 10%–sulfur 5% foam using a rinse-off method of application was excellent, with only 33% (8/24) of participants commenting on the smell.18 Another limiting factor of sodium sulfacetamide preparations containing sulfur is orange-brown discoloration when combined with benzoyl peroxide, which does not affect the skin but may stain clothing.19
Sodium sulfacetamide is rendered less effective when combined with silver-containing products.20 No other notable drug interactions are known; however, oral sulfonamides are known to interact with several drugs, including cyclosporine and phenytoin.21,22
Contraindications
Sodium sulfacetamide is contraindicated in patients with known hypersensitivity to sulfonamides, sulfur, or any other component of the preparation. It is a pregnancy category C drug, and pregnant women should only use sodium sulfacetamide if it is the only modality to treat the condition or the benefits outweigh the risks. Although there are no known reports of problems related to topical sodium sulfacetamide during pregnancy, the use of oral sulfonamides during pregnancy can increase the risk for neonatal jaundice.23 Likewise, caution should be exercised in prescribing this product to nursing women, as systemic sulfonamide antibacterials are well known to cause kernicterus in nursing neonates.1
Conclusion
The efficacy and safety of sodium sulfacetamide, used alone or in combination with sulfur, has been demonstrated in the treatment of rosacea, acne, seborrheic dermatitis, and perioral dermatitis. Advances in formulation technology to decrease odor and irritation have allowed for more use of this product. Further studies will help elucidate the role that sodium sulfacetamide should play in the treatment of inflammatory dermatoses in comparison to other available products.
Sodium sulfacetamide has various uses in the field of dermatology due to its anti-inflammatory and antibacterial properties. It has been shown to be effective in the management of a variety of inflammatory facial dermatoses, including papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis. We review the mechanism of action, pharmacology and formulations, clinical uses, and adverse effects of sodium sulfacetamide as a dermatologic treatment.
Mechanism of Action
Sodium sulfacetamide is a sulfonamide-type antibacterial agent. Its mechanism of action is the inhibition of bacterial dihydropteroate synthetase, which prevents the conversion of p-aminobenzoic acid to folic acid. This process causes a bacteriostatic effect on the growth of several gram-negative and gram-positive organisms, including Propionibacterium acnes.1,2
The effectiveness of sodium sulfacetamide is increased when used in combination with sulfur, which has keratolytic, antibacterial, antifungal, and antiparasitic effects. The addition of hydrocortisone has been reported to increase the effectiveness of both agents.3
Pharmacology
Sodium sulfacetamide is highly soluble at the physiologic pH of 7.4, which contributes to its high level of penetration and absorption.4 An in vitro study showed percutaneous absorption of sodium sulfacetamide to be around 4%.5 Sulfonamides are metabolized mainly by the liver and are excreted by the kidneys.
Formulations
The most common concentrations of sodium sulfacetamide and sulfur are 10% and 5%, respectively. A wide variety of sulfacetamide-containing products are available, many of which are marketed to treat specific conditions depending on additional ingredients or the type of delivery system.
Clinical Uses
Topical formulations of sodium sulfacetamide and sulfur have proven to be efficacious in the management of rosacea, with a typical regimen consisting of twice-daily application for 8 weeks.6 The sulfur in the formulation has the additional benefit of targeting Demodex mites, which are implicated as a contributing factor in some cases of rosacea.7 Sodium sulfacetamide 10%–sulfur 5% lotion was more effective in improving the erythema, papulopustules, and overall severity of rosacea as compared to metronidazole gel 0.75%.8 Other studies have reported increased efficacy when sodium sulfacetamide and topical sulfur are used along with metronidazole.9,10
Sodium sulfacetamide also has shown efficacy against acne. Its antibacterial and drying properties have been shown to decrease the number of inflammatory lesions and comedones, and in the treatment of acne vulgaris, no sensitivity reactions have been observed.2 Also, unlike topical antibiotics, cases of P acnes resistance to topical sulfur products have not been widely reported. Studies have demonstrated that twice-daily use of sodium sulfacetamide 10%–sulfur 5% for 12 weeks decreases inflammatory acne lesions by 80.4% to 83%.11,12
Seborrheic dermatitis is a common chronic infection of the skin caused by Malassezia species. One study investigated the use of sodium sulfacetamide ointment and soap to treat seborrheic dermatitis and found that the condition was either improved or completely controlled in 93% (71/76) of cases.4 Sodium sulfacetamide lotion was an effective treatment of seborrheic dermatitis in 89% (54/61) of patients with scalp involvement and 68% (30/44) of patients with glabrous skin involvement.13
Perioral dermatitis is characterized by groups of erythematous papules and pustules localized around the mouth. The use of topical sodium sulfacetamide along with oral tetracyclines has been demonstrated to consistently clear lesions in most patients with perioral dermatitis.14 Sodium sulfacetamide is unique in that it is not associated with the excessive erythema and irritation often found with retinoic acid and benzoyl peroxide.15 Unfortunately, however, there have been no well-controlled trials to compare the efficacy of sodium sulfacetamide to other topical therapies for this condition.
Adverse Effects
Adverse effects from sodium sulfacetamide are rare and generally are limited to cutaneous reactions including dryness, erythema, pruritus, and discomfort.1 Periocular use of sodium sulfacetamide can cause conjunctival irritation. One study reported that 19% (6/31) of patients experienced local reactions but most were considered mild.9 Rare but serious reactions including erythema multiforme and Stevens-Johnson syndrome have been reported from ophthalmic use.16,17
A common limiting factor to sodium sulfacetamide preparations that include elemental sulfur is the offensive smell, which has hindered patient compliance in the past; however, pharmaceutical companies have attempted to create more tolerable products without the odor.10 One study found that the tolerability of a sodium sulfacetamide 10%–sulfur 5% foam using a rinse-off method of application was excellent, with only 33% (8/24) of participants commenting on the smell.18 Another limiting factor of sodium sulfacetamide preparations containing sulfur is orange-brown discoloration when combined with benzoyl peroxide, which does not affect the skin but may stain clothing.19
Sodium sulfacetamide is rendered less effective when combined with silver-containing products.20 No other notable drug interactions are known; however, oral sulfonamides are known to interact with several drugs, including cyclosporine and phenytoin.21,22
Contraindications
Sodium sulfacetamide is contraindicated in patients with known hypersensitivity to sulfonamides, sulfur, or any other component of the preparation. It is a pregnancy category C drug, and pregnant women should only use sodium sulfacetamide if it is the only modality to treat the condition or the benefits outweigh the risks. Although there are no known reports of problems related to topical sodium sulfacetamide during pregnancy, the use of oral sulfonamides during pregnancy can increase the risk for neonatal jaundice.23 Likewise, caution should be exercised in prescribing this product to nursing women, as systemic sulfonamide antibacterials are well known to cause kernicterus in nursing neonates.1
Conclusion
The efficacy and safety of sodium sulfacetamide, used alone or in combination with sulfur, has been demonstrated in the treatment of rosacea, acne, seborrheic dermatitis, and perioral dermatitis. Advances in formulation technology to decrease odor and irritation have allowed for more use of this product. Further studies will help elucidate the role that sodium sulfacetamide should play in the treatment of inflammatory dermatoses in comparison to other available products.
1. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003;4:473-492.
2. Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
3. Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders; 2012:445-459.
4. Duemling WM. Sodium sulfacetamide in topical therapy. AMA Arch Derm Syphilol. 1954;69:75-82.
5. Sodium sulfacetamide. Drugs.com Web site. http://drugs.com/pro/sodium-sulfacetamide.html. Revised December 2012. Accessed June 16, 2015.
6. Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8:79-85.
7. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
8. Lebwohl MG, Medansky RS, Russo CL, et al. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% lotion and metronidazole 0.75% gel in the treatment of rosacea. J Geriatr Dermatol. 1995;3:183-185.
9. Nally JB, Berson DS. Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-26.
10. Pelle MT, Crawford GH, James WD. Rosacea II: therapy. J Am Acad Dermatol. 2004;51:499-512.
11. Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22:301.
12. Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int J Dermatol. 1993;32:365-367.
13. Whelan ST. Sodium sulfacetamide for seborrheic dermatitis. AMA Arch Derm. 1955;71:724.
14. Bendl BJ. Perioral dermatitis: etiology and treatment. Cutis. 1976;17:903-908.
15. Olansky S. Old drug—in a new system—revisited. Cutis. 1977;19:852-854.
16. Genvert GI, Cohen EJ, Donnenfeld ED, et al. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985;99:465-468.
17. Rubin Z. Ophthalmic sulfonamide-induced Stevens-Johnson syndrome. Arch Dermatol. 1977;113:235-236.
18. Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-246.
19. Dubina MI, Fleischer AB. Interaction of topical sulfacetamide and topical dapsone with benzoyl peroxide. Arch Dermatol. 2009;145:1027-1029.
20. Sodium sulfacetamide – sulfacetamide sodium liquid. DailyMed Web site. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0d92c55b-5b54-4f5d-8921-24e4e877ae50. Accessed June 17, 2015.
21. Spes CH, Angermann CE, Stempfle HU, et al. Sulfadiazine therapy for toxoplasmosis in heart transplant recipients decreases cyclosporine concentration. Clin Investig. 1992;70:752-754.
22. Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106-110.
23. Bradley JS, Sauberan JB. Antimicrobial agents. In: Long SS, Pickering LK, Prober CG. Principles and Practices of Pediatric Infectious Diseases. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1453-1483.
1. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003;4:473-492.
2. Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
3. Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders; 2012:445-459.
4. Duemling WM. Sodium sulfacetamide in topical therapy. AMA Arch Derm Syphilol. 1954;69:75-82.
5. Sodium sulfacetamide. Drugs.com Web site. http://drugs.com/pro/sodium-sulfacetamide.html. Revised December 2012. Accessed June 16, 2015.
6. Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8:79-85.
7. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
8. Lebwohl MG, Medansky RS, Russo CL, et al. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% lotion and metronidazole 0.75% gel in the treatment of rosacea. J Geriatr Dermatol. 1995;3:183-185.
9. Nally JB, Berson DS. Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-26.
10. Pelle MT, Crawford GH, James WD. Rosacea II: therapy. J Am Acad Dermatol. 2004;51:499-512.
11. Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22:301.
12. Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int J Dermatol. 1993;32:365-367.
13. Whelan ST. Sodium sulfacetamide for seborrheic dermatitis. AMA Arch Derm. 1955;71:724.
14. Bendl BJ. Perioral dermatitis: etiology and treatment. Cutis. 1976;17:903-908.
15. Olansky S. Old drug—in a new system—revisited. Cutis. 1977;19:852-854.
16. Genvert GI, Cohen EJ, Donnenfeld ED, et al. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985;99:465-468.
17. Rubin Z. Ophthalmic sulfonamide-induced Stevens-Johnson syndrome. Arch Dermatol. 1977;113:235-236.
18. Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-246.
19. Dubina MI, Fleischer AB. Interaction of topical sulfacetamide and topical dapsone with benzoyl peroxide. Arch Dermatol. 2009;145:1027-1029.
20. Sodium sulfacetamide – sulfacetamide sodium liquid. DailyMed Web site. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0d92c55b-5b54-4f5d-8921-24e4e877ae50. Accessed June 17, 2015.
21. Spes CH, Angermann CE, Stempfle HU, et al. Sulfadiazine therapy for toxoplasmosis in heart transplant recipients decreases cyclosporine concentration. Clin Investig. 1992;70:752-754.
22. Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106-110.
23. Bradley JS, Sauberan JB. Antimicrobial agents. In: Long SS, Pickering LK, Prober CG. Principles and Practices of Pediatric Infectious Diseases. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1453-1483.
Practice Points
- Sodium sulfacetamide is a useful agent in the management of papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis.
- Adverse effects are rare and generally are limited to dryness, erythema, pruritus, and discomfort.
Genitourinary syndrome of menopause: Current and emerging therapies
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy 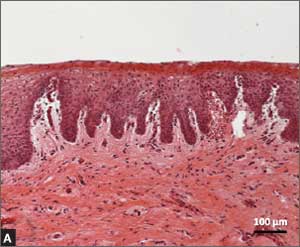 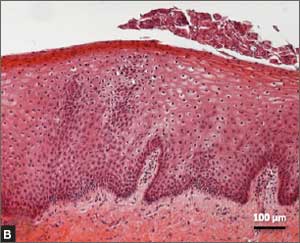 |
FIGURE 2: Atrophic vaginitis 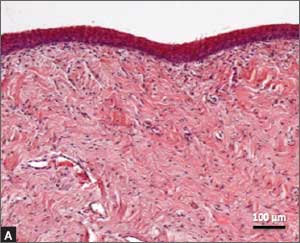  This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy   |
FIGURE 2: Atrophic vaginitis   This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy   |
FIGURE 2: Atrophic vaginitis   This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
In this article
- What can we offer our patients?
- Fractional CO2 laser: a study in progress
- Bottom line
The Top 100 Cited Articles in Clinical Orthopedic Sports Medicine
Orthopedics and the sports medicine subspecialty are continually evolving fields that depend on research investigation and publication to further knowledge and advance practice. Research has produced new findings that have changed the way we practice sports medicine. In this review, we identify the most widely referenced sports medicine topics and articles, which we believe by their permeative presence in the literature have made lasting contributions to the field.
Many factors can be used to quantify the influence of an academic article on the practice of medicine. Citation analysis is one method that reflects the impact of a publication on the academic medical community.1-3 Total citations record the number of times a journal article has been credited by another study. Therefore, citation count indirectly highlights the articles that are widespread, relevant, and that form the foundation for other investigations on the topic. Related to the impact of the article is the impact of the journal that published the study. We examined journals by impact factor, a score based on the mean number of citations a published article received during the preceding 2 years.
Similar analyses have been performed of publication history in orthopedics and other medical fields. Investigators have examined which historical articles were the most influential in orthopedics as a whole,4 pediatric orthopedics,5,6 shoulder surgery,7 and arthroscopy.8 This influence has also been studied in general surgery,9 otolaryngology,10 plastic surgery,11 dermatology,12 critical care,13 and other disciplines. To our knowledge, the present study is the first bibliometric analysis of the highest-impact articles in orthopedic sports medicine.
Our goal was to identify the 100 articles that have had the highest impact on the clinical orthopedic sports medicine literature. We hypothesized that the most widely recognized articles would be from the highest-impact journals and may also have earlier publication dates. We describe the topics and objectives of these articles to highlight the sports medicine areas on which most research has focused during the past century.
Materials and Methods
Our bibliometric analysis used the Thomson Reuters Web of Knowledge, which consists of all publications from 1900 to the present. This research modality ranks journal articles by frequency of citation. Similar analyses have identified the most often cited articles in pediatric orthopedics,5 shoulder surgery,7 and arthroscopy.8 In our analysis, we included the top 25 journals by impact factor in the field of sports medicine, as rated by the Journal Citation Reports database. Within the highest-impact journals, we sorted all articles by those most often cited, and read them all to identify which ones discuss conditions commonly encountered in the clinical practice of sports medicine. We focused on clinical articles only and therefore excluded related basic science and cadaveric biomechanical studies. The 100 most cited articles were then further evaluated by primary author, journal of publication, institution, country of origin, year of publication, topic, and total number of citations. One-way analysis of variance (ANOVA) and linear regression analyses were used to determine if publication date correlated with mean number of citations.
Results
Eighty authors wrote the top 100 articles in sports medicine, and each publication garnered several hundred citations, ranging from 229 to 1629 with a mean of 408 (Table 114-113). Most of these articles were written in the past 3 decades, with equal distribution from the 1980s, 1990s, and 2000s (Figure 1A). We ran a linear regression to determine if publication date correlated with higher number of citations by virtue of longer time available for citation. The analysis poorly modeled the variability (R2 = 0.05), revealing no correlation between number of citations and publication date. Further, 1-way ANOVA found no significant difference between the number of citations per decade, F(5, 93) = 1.60, P = .17 (Figure 1B). Despite this finding, the oldest cited article, written by Fairbank39 in 1948, ranked high (position 7). Of these top 100 publications, the most recent, written by Knutsen and colleagues69 in 2007, ranked in the second half at position 66.
Seven journals published the top 100 articles, with the American volume of the Journal of Bone and Joint Surgery publishing nearly half (44%) (Table 2). In second place, with 28 articles, was the American Journal of Sports Medicine, followed by the British volume of the Journal of Bone and Joint Surgery, with 10 articles.
Thirty different topics were investigated in this collection of articles, encompassing nearly every major research area of sports medicine. There was a heavy emphasis on anterior cruciate ligament (ACL) injury and reconstruction, knee rating systems, rotator cuff reconstruction, and chondrocyte transplantation (Table 3).
In several cases, an author contributed more than 1 classic article. In fact, 31 of the top 100 articles were by an individual who had coauthored 2 or more of the publications on this list. The researchers with the largest number of first-authored articles were Noyes88-92 (5 articles), Neer81-84 (4 articles), and Rowe,102-104 Daniel,35-37 Peterson,97-99 and Hewett52-54 (3 articles each) (Table 417,19,21-24,29-31,35-37,42,44,45,52-54,58,61-65,69,70,72,74,80-84,87-92,97-99,101-105,107,109,110,113). Articles from authors with multiple publications had a common topic.
Last, these articles originated from a number of different countries and institutions. Of the 15 source countries (Figure 2), the United States contributed the most (61 articles). Other countries had prominent representation: Sweden and Switzerland (8 each), United Kingdom (5), and Canada, France, and Norway (3 each). These articles originated from 69 universities, hospitals, and clinics; 21 institutions had 2 or more articles (Table 5). The 5 institutions with the highest number of articles were Hospital for Special Surgery, University of Bern, Columbia College of Physicians and Surgeons/Columbia-Presbyterian Medical Center, Cincinnati Sports Medicine and Orthopaedic Center, and Massachusetts General Hospital.
Discussion
Several trends can be ascertained from analyzing the top 100 clinical articles cited in sports medicine. The 5 most frequent topics discussed were ACL injury and reconstruction, knee rating systems for injury and function, rotator cuff reconstruction, chondrocyte transplantation, and femoroacetabular impingement (Table 3). Of those 5 topics, only ACL injury and reconstruction falls within the top 10 most common orthopedic surgical procedures performed in the United States reported by one analysis.114 The most common orthopedic surgical procedure, knee arthroscopy, ranks 10th of all topics covered by the top 100 articles, whereas the second most common procedure, shoulder arthroscopy, was not discussed by any of those 100 articles. Also notable is the high frequency of knee rating system studies, which correlates well with the fact that 4 of the most common orthopedic surgical procedures are knee procedures. The prevalence of rating system articles reflects the importance of and need for accurate methods in the diagnosis of injuries in sports medicine.
The most cited sports medicine article was written by Insall and colleagues62 in 1989, more than 2 decades ago. In this article, “Rationale of the Knee Society Clinical Rating System,” they reported on a rigorous system that rates knee function and ability to walk and climb stairs. The second most cited article, “A Clinical Method of Functional Assessment of the Shoulder,” was written in 1987 by Constant and Murley.32 This article discusses another rating system but offers a functional assessment of the shoulder that is highly reproducible and time-efficient. “Rating Systems in the Evaluation of Knee Ligament Injuries,” the third most cited article, was written in 1985 by Tegner and Lysholm.113 This article details the complexities and variable uses of different knee ligament injury rating systems. These top 3 articles were all published in Clinical Orthopaedics and Related Research. In addition, all 3 discussed rating systems, reinforcing the need for accurate scoring systems to standardize the diagnosis of injury across the field of orthopedics and qualify outcomes after injury.
A number of studies have introduced physical examination findings, clinical tests, and rating systems used in the clinical setting of sports medicine (and named after the contributing authors). For example, the Neer sign82 and the Hawkins-Kennedy test51 are used to determine shoulder impingement. In knee ligament injuries, the Tegner knee activity score113 complements other functional scores (eg, Lysholm knee score74). For grading joint cartilage breakdown, the Outerbridge classification system96 is commonly used. The Fairbank test39 is used to gauge knee instability. In evaluating fatty degeneration of rotator cuff muscles through computed tomography scans, the Goutallier classification47 is used. Other metrics, such as the Knee Injury and Osteoarthritis Outcome Score, introduced by Roos and colleagues,101 measure knee injury and osteoarthritis. In other scenarios, studies have improved on surgical techniques—for example, the Neer open modification84 of the Bankart procedure. Many of these rating systems and named clinical findings are so ingrained in the practice and vernacular of orthopedics that it is possible they are in fact undercited in the literature.
As in other bibliometric analyses, one concession made here was to credit the first author listed for making the primary contribution to an article. As a result of journal variability and inconsistency, we were precluded from analyzing senior authors. When analyzed for authorship at any position, 3 of the top authors (Table 4) showed contributions to additional articles in the top 100 list. Noyes was listed as last author on 2 other articles,52,54 raising his total to 7. Daniel was listed as second author on 1 additional article,105 and Beck was listed as third author on 1 other article,42 raising their totals to 4 and 3, respectively.
A criticism of bibliometric analysis is its use of number of citations as an accurate measure of academic contribution. However, other methods for measuring the productivity and impact of researchers (eg, the recently developed Hirsch Index) have their own drawbacks,115,116 including being able to compare authors only at the same point in their careers and self-citation. It is important to note that our analyses focused strictly on publications related to clinical sports medicine, with the exclusion of basic science and cadaveric biomechanical studies.
Through bibliometric citation analysis, we have identified the authors who have made lasting contributions to the field of sports medicine, and we have highlighted the publications that have been cited by hundreds to thousands of authors. This list identifies trends within the articles that have become “classic,” by nature of their deep permeation into subsequent sports medicine literature, and offers guidance for trainees interested in studying the most high-yield sports medicine literature. Given that 69 institutions in 15 countries conducted these studies, we have also shown that orthopedic research can be readily disseminated internationally. Last, our study provides a thorough overview of the sports medicine literature over the past century and provides a strong framework for future research in our field.
1. Adams AB, Simonson D. Publication, citations, and impact factors of leading investigators in critical care medicine. Respir Care. 2004;49(3):276-281.
2. Bhandari M, Busse J, Devereaux PJ, et al. Factors associated with citation rates in the orthopedic literature. Can J Surg. 2007;50(2):119-123.
3. Cheek J, Garnham B, Quan J. What’s in a number? Issues in providing evidence of impact and quality of research(ers). Qual Health Res. 2006;16(3):423-435.
4. Kelly JC, Glynn RW, O’Briain DE, Felle P, McCabe JP. The 100 classic papers of orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Br. 2010;92(10):1338-1343.
5. Kavanagh RG, Kelly JC, Kelly PM, Moore DP. The 100 classic papers of pediatric orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Am. 2013;95(18):e134.
6. Mehlman CT, Wenger DR. The top 25 at 25: citation classics in the Journal of Pediatric Orthopaedics. J Pediatr Orthop. 2006;26(5):691-694.
7. Namdari S, Baldwin K, Kovatch K, Huffman GR, Glaser D. Fifty most cited articles in orthopedic shoulder surgery. J Shoulder Elbow Surg. 2012;21(12):1796-1802.
8. Cassar Gheiti AJ, Downey RE, Byrne DP, Molony DC, Mulhall KJ. The 25 most cited articles in arthroscopic orthopaedic surgery. Arthroscopy. 2012;28(4):548-564.
9. Paladugu R, Schein M, Gardezi S, Wise L. One hundred citation classics in general surgical journals. World J Surg. 2002;26(9):1099-1105.
10. Fenton JE, Roy D, Hughes JP, Jones AS. A century of citation classics in otolaryngology-head and neck surgery journals. J Laryngol Otol. 2002;116(7):494-498.
11. Loonen MPJ, Hage JJ, Kon M. Plastic surgery classics: characteristics of 50 top-cited articles in four plastic surgery journals since 1946. Plast Reconstr Surg. 2008;121(5):320e-327e.
12. Dubin D, Hafner AW, Arndt KA. Citation classics in clinical dermatologic journals. Citation analysis, biomedical journals, and landmark articles, 1945–1990. Arch Dermatol. 1993;129(9):1121-1129.
13. Baltussen A, Kindler CH. Citation classics in critical care medicine. Intensive Care Med. 2004;30(5):902-910.
14. Aglietti P, Buzzi R, Zaccherotti G, De Biase P. Patellar tendon versus doubled semitendinosus and gracilis tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 1994;22(2):211-218.
15. Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66(5):666-671.
16. Altchek DW, Warren RF, Skyhar MJ, Ortiz G. T-plasty modification of the Bankart procedure for multidirectional instability of the anterior and inferior types. J Bone Joint Surg Am. 1991;73(1):105-112.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701.
19. Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16(2):113-122.
20. Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17(1):1-6.
21. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640-645.
22. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
23. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
24. Bentley G, Biant LC, Carrington RWJ, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223-230.
25. Berchuck M, Andriacchi TP, Bach BR, Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72(6):871-877.
26. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41(6):988-1020.
27. Binkley JM, Stratford PW, Lott SA, Riddle DL, North American Orthopaedic Rehabilitation Research Network. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371-383.
28. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
29. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
30. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
31. Clancy WG Jr, Shelbourne KD, Zoellner GB, Keene JS, Reider B, Rosenberg TD. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J Bone Joint Surg Am. 1983;65(3):310-322.
32. Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
33. Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27(3):444-454.
34. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
35. Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R. Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg Am. 1985;67(5):720-726.
36. Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632-644.
37. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401-407.
38. Ellman H, Hanker G, Bayer M. Repair of the rotator cuff. End-result study of factors influencing reconstruction. J Bone Joint Surg Am. 1986;68(8):1136-1144.
39. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
40. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
41. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
42. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):111-119.
43. Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;(304):43-53.
44. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
45. Gerber C, Krushell RJ. Isolated rupture of the tendon of the subscapularis muscle. Clinical features in 16 cases. J Bone Joint Surg Br. 1991;73(3):389-394.
46. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
47. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78-83.
48. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE Jr. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650.
49. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
50. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
51. Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med. 1980;8(3):151-157.
52. Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699-706.
53. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492-501.
54. Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765-773.
55. Homminga GN, Bulstra SK, Bouwmeester PSM, van der Linden AJ. Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br. 1990;72(6):1003-1007.
56. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185-192.
57. Hovelius L, Augustini BG, Fredin H, Johansson O, Norlin R, Thorling J. Primary anterior dislocation of the shoulder in young patients. A ten-year prospective study. J Bone Joint Surg Am. 1996;78(11):1677-1684.
58. Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58(2):159-172.
59. Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427-436.
60. Iannotti JP, Zlatkin MB, Esterhai JL, Kressel HY, Dalinka MK, Spindler KP. Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg Am. 1991;73(1):17-29.
61. Insall J, Falvo KA, Wise DW. Chondromalacia patellae. A prospective study. J Bone Joint Surg Am. 1976;58(1):1-8.
62. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
63. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600-613.
64. Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132-1145.
65. Ito K, Minka MA 2nd, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect. A MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br. 2001;83(2):171-176.
66. Johnson RJ, Kettelkamp DB, Clark W, Leaverton P. Factors affecting late results after meniscectomy. J Bone Joint Surg Am. 1974;56(3):719-729.
67. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204-208.
68. Jones KG. Reconstruction of the anterior cruciate ligament: a technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
69. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-2112.
70. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86(3):455-464.
71. Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159-163.
72. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
73. Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276-291.
74. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154.
75. Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003-1010.
76. Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991;19(5):478-484.
77. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
78. Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9(3):318-321.
79. McDaniel WJ Jr, Dameron TB Jr. Untreated ruptures of the anterior cruciate ligament. A follow-up study. J Bone Joint Surg Am. 1980;62(5):696-705.
80. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
81. Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54(1):41-50.
82. Neer CS 2nd. Impingement lesions. Clin Orthop Relat Res. 1983;(173):70-77.
83. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
84. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder. A preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
85. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
86. Nistor L. Surgical and non-surgical treatment of Achilles tendon rupture. J Bone Joint Surg Am. 1981;63(3):394-399.
87. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
88. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513-518.
89. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62(5):687-695, 757.
90. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate–deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
91. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate–deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
92. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
93. O’Brien SJ, Warren RF, Pavlov H, Panariello R, Wickiewicz TL. Reconstruction of the chronically insufficient anterior cruciate ligament with the central third of the patellar ligament. J Bone Joint Surg Am. 1991;73(2):278-286.
94. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
95. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
96. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43(4):752-757.
97. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12.
98. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17-24.
99. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-234.
100. Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80(9):1276-1284.
101. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96.
102. Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am. 1956;38(5):957-977.
103. Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am. 1978;60(1):1-16.
104. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
105. Sachs RA, Daniel DM, Stone ML, Garfein RF. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17(6):760-765.
106. Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65(4):456-460.
107. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299.
108. Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders [see comments]. J Bone Joint Surg Am. 1995;77(1):10-15.
109. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85(2):278-286.
110. Solomonow M, Baratta R, Zhou BH, et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15(3):207-213.
111. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484.
112. Tapper EM, Hoover NW. Late results after meniscectomy. J Bone Joint Surg Am. 1969;51(3):517-526.
113. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-49.
114. Garrett WE Jr, Swiontkowski MF, Weinstein JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: Part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
115. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98.
116. Bornmann L, Daniel HD. The state of h index research. Is the h index the ideal way to measure research performance? EMBO Rep. 2009;10(1):2-6.
Orthopedics and the sports medicine subspecialty are continually evolving fields that depend on research investigation and publication to further knowledge and advance practice. Research has produced new findings that have changed the way we practice sports medicine. In this review, we identify the most widely referenced sports medicine topics and articles, which we believe by their permeative presence in the literature have made lasting contributions to the field.
Many factors can be used to quantify the influence of an academic article on the practice of medicine. Citation analysis is one method that reflects the impact of a publication on the academic medical community.1-3 Total citations record the number of times a journal article has been credited by another study. Therefore, citation count indirectly highlights the articles that are widespread, relevant, and that form the foundation for other investigations on the topic. Related to the impact of the article is the impact of the journal that published the study. We examined journals by impact factor, a score based on the mean number of citations a published article received during the preceding 2 years.
Similar analyses have been performed of publication history in orthopedics and other medical fields. Investigators have examined which historical articles were the most influential in orthopedics as a whole,4 pediatric orthopedics,5,6 shoulder surgery,7 and arthroscopy.8 This influence has also been studied in general surgery,9 otolaryngology,10 plastic surgery,11 dermatology,12 critical care,13 and other disciplines. To our knowledge, the present study is the first bibliometric analysis of the highest-impact articles in orthopedic sports medicine.
Our goal was to identify the 100 articles that have had the highest impact on the clinical orthopedic sports medicine literature. We hypothesized that the most widely recognized articles would be from the highest-impact journals and may also have earlier publication dates. We describe the topics and objectives of these articles to highlight the sports medicine areas on which most research has focused during the past century.
Materials and Methods
Our bibliometric analysis used the Thomson Reuters Web of Knowledge, which consists of all publications from 1900 to the present. This research modality ranks journal articles by frequency of citation. Similar analyses have identified the most often cited articles in pediatric orthopedics,5 shoulder surgery,7 and arthroscopy.8 In our analysis, we included the top 25 journals by impact factor in the field of sports medicine, as rated by the Journal Citation Reports database. Within the highest-impact journals, we sorted all articles by those most often cited, and read them all to identify which ones discuss conditions commonly encountered in the clinical practice of sports medicine. We focused on clinical articles only and therefore excluded related basic science and cadaveric biomechanical studies. The 100 most cited articles were then further evaluated by primary author, journal of publication, institution, country of origin, year of publication, topic, and total number of citations. One-way analysis of variance (ANOVA) and linear regression analyses were used to determine if publication date correlated with mean number of citations.
Results
Eighty authors wrote the top 100 articles in sports medicine, and each publication garnered several hundred citations, ranging from 229 to 1629 with a mean of 408 (Table 114-113). Most of these articles were written in the past 3 decades, with equal distribution from the 1980s, 1990s, and 2000s (Figure 1A). We ran a linear regression to determine if publication date correlated with higher number of citations by virtue of longer time available for citation. The analysis poorly modeled the variability (R2 = 0.05), revealing no correlation between number of citations and publication date. Further, 1-way ANOVA found no significant difference between the number of citations per decade, F(5, 93) = 1.60, P = .17 (Figure 1B). Despite this finding, the oldest cited article, written by Fairbank39 in 1948, ranked high (position 7). Of these top 100 publications, the most recent, written by Knutsen and colleagues69 in 2007, ranked in the second half at position 66.
Seven journals published the top 100 articles, with the American volume of the Journal of Bone and Joint Surgery publishing nearly half (44%) (Table 2). In second place, with 28 articles, was the American Journal of Sports Medicine, followed by the British volume of the Journal of Bone and Joint Surgery, with 10 articles.
Thirty different topics were investigated in this collection of articles, encompassing nearly every major research area of sports medicine. There was a heavy emphasis on anterior cruciate ligament (ACL) injury and reconstruction, knee rating systems, rotator cuff reconstruction, and chondrocyte transplantation (Table 3).
In several cases, an author contributed more than 1 classic article. In fact, 31 of the top 100 articles were by an individual who had coauthored 2 or more of the publications on this list. The researchers with the largest number of first-authored articles were Noyes88-92 (5 articles), Neer81-84 (4 articles), and Rowe,102-104 Daniel,35-37 Peterson,97-99 and Hewett52-54 (3 articles each) (Table 417,19,21-24,29-31,35-37,42,44,45,52-54,58,61-65,69,70,72,74,80-84,87-92,97-99,101-105,107,109,110,113). Articles from authors with multiple publications had a common topic.
Last, these articles originated from a number of different countries and institutions. Of the 15 source countries (Figure 2), the United States contributed the most (61 articles). Other countries had prominent representation: Sweden and Switzerland (8 each), United Kingdom (5), and Canada, France, and Norway (3 each). These articles originated from 69 universities, hospitals, and clinics; 21 institutions had 2 or more articles (Table 5). The 5 institutions with the highest number of articles were Hospital for Special Surgery, University of Bern, Columbia College of Physicians and Surgeons/Columbia-Presbyterian Medical Center, Cincinnati Sports Medicine and Orthopaedic Center, and Massachusetts General Hospital.
Discussion
Several trends can be ascertained from analyzing the top 100 clinical articles cited in sports medicine. The 5 most frequent topics discussed were ACL injury and reconstruction, knee rating systems for injury and function, rotator cuff reconstruction, chondrocyte transplantation, and femoroacetabular impingement (Table 3). Of those 5 topics, only ACL injury and reconstruction falls within the top 10 most common orthopedic surgical procedures performed in the United States reported by one analysis.114 The most common orthopedic surgical procedure, knee arthroscopy, ranks 10th of all topics covered by the top 100 articles, whereas the second most common procedure, shoulder arthroscopy, was not discussed by any of those 100 articles. Also notable is the high frequency of knee rating system studies, which correlates well with the fact that 4 of the most common orthopedic surgical procedures are knee procedures. The prevalence of rating system articles reflects the importance of and need for accurate methods in the diagnosis of injuries in sports medicine.
The most cited sports medicine article was written by Insall and colleagues62 in 1989, more than 2 decades ago. In this article, “Rationale of the Knee Society Clinical Rating System,” they reported on a rigorous system that rates knee function and ability to walk and climb stairs. The second most cited article, “A Clinical Method of Functional Assessment of the Shoulder,” was written in 1987 by Constant and Murley.32 This article discusses another rating system but offers a functional assessment of the shoulder that is highly reproducible and time-efficient. “Rating Systems in the Evaluation of Knee Ligament Injuries,” the third most cited article, was written in 1985 by Tegner and Lysholm.113 This article details the complexities and variable uses of different knee ligament injury rating systems. These top 3 articles were all published in Clinical Orthopaedics and Related Research. In addition, all 3 discussed rating systems, reinforcing the need for accurate scoring systems to standardize the diagnosis of injury across the field of orthopedics and qualify outcomes after injury.
A number of studies have introduced physical examination findings, clinical tests, and rating systems used in the clinical setting of sports medicine (and named after the contributing authors). For example, the Neer sign82 and the Hawkins-Kennedy test51 are used to determine shoulder impingement. In knee ligament injuries, the Tegner knee activity score113 complements other functional scores (eg, Lysholm knee score74). For grading joint cartilage breakdown, the Outerbridge classification system96 is commonly used. The Fairbank test39 is used to gauge knee instability. In evaluating fatty degeneration of rotator cuff muscles through computed tomography scans, the Goutallier classification47 is used. Other metrics, such as the Knee Injury and Osteoarthritis Outcome Score, introduced by Roos and colleagues,101 measure knee injury and osteoarthritis. In other scenarios, studies have improved on surgical techniques—for example, the Neer open modification84 of the Bankart procedure. Many of these rating systems and named clinical findings are so ingrained in the practice and vernacular of orthopedics that it is possible they are in fact undercited in the literature.
As in other bibliometric analyses, one concession made here was to credit the first author listed for making the primary contribution to an article. As a result of journal variability and inconsistency, we were precluded from analyzing senior authors. When analyzed for authorship at any position, 3 of the top authors (Table 4) showed contributions to additional articles in the top 100 list. Noyes was listed as last author on 2 other articles,52,54 raising his total to 7. Daniel was listed as second author on 1 additional article,105 and Beck was listed as third author on 1 other article,42 raising their totals to 4 and 3, respectively.
A criticism of bibliometric analysis is its use of number of citations as an accurate measure of academic contribution. However, other methods for measuring the productivity and impact of researchers (eg, the recently developed Hirsch Index) have their own drawbacks,115,116 including being able to compare authors only at the same point in their careers and self-citation. It is important to note that our analyses focused strictly on publications related to clinical sports medicine, with the exclusion of basic science and cadaveric biomechanical studies.
Through bibliometric citation analysis, we have identified the authors who have made lasting contributions to the field of sports medicine, and we have highlighted the publications that have been cited by hundreds to thousands of authors. This list identifies trends within the articles that have become “classic,” by nature of their deep permeation into subsequent sports medicine literature, and offers guidance for trainees interested in studying the most high-yield sports medicine literature. Given that 69 institutions in 15 countries conducted these studies, we have also shown that orthopedic research can be readily disseminated internationally. Last, our study provides a thorough overview of the sports medicine literature over the past century and provides a strong framework for future research in our field.
Orthopedics and the sports medicine subspecialty are continually evolving fields that depend on research investigation and publication to further knowledge and advance practice. Research has produced new findings that have changed the way we practice sports medicine. In this review, we identify the most widely referenced sports medicine topics and articles, which we believe by their permeative presence in the literature have made lasting contributions to the field.
Many factors can be used to quantify the influence of an academic article on the practice of medicine. Citation analysis is one method that reflects the impact of a publication on the academic medical community.1-3 Total citations record the number of times a journal article has been credited by another study. Therefore, citation count indirectly highlights the articles that are widespread, relevant, and that form the foundation for other investigations on the topic. Related to the impact of the article is the impact of the journal that published the study. We examined journals by impact factor, a score based on the mean number of citations a published article received during the preceding 2 years.
Similar analyses have been performed of publication history in orthopedics and other medical fields. Investigators have examined which historical articles were the most influential in orthopedics as a whole,4 pediatric orthopedics,5,6 shoulder surgery,7 and arthroscopy.8 This influence has also been studied in general surgery,9 otolaryngology,10 plastic surgery,11 dermatology,12 critical care,13 and other disciplines. To our knowledge, the present study is the first bibliometric analysis of the highest-impact articles in orthopedic sports medicine.
Our goal was to identify the 100 articles that have had the highest impact on the clinical orthopedic sports medicine literature. We hypothesized that the most widely recognized articles would be from the highest-impact journals and may also have earlier publication dates. We describe the topics and objectives of these articles to highlight the sports medicine areas on which most research has focused during the past century.
Materials and Methods
Our bibliometric analysis used the Thomson Reuters Web of Knowledge, which consists of all publications from 1900 to the present. This research modality ranks journal articles by frequency of citation. Similar analyses have identified the most often cited articles in pediatric orthopedics,5 shoulder surgery,7 and arthroscopy.8 In our analysis, we included the top 25 journals by impact factor in the field of sports medicine, as rated by the Journal Citation Reports database. Within the highest-impact journals, we sorted all articles by those most often cited, and read them all to identify which ones discuss conditions commonly encountered in the clinical practice of sports medicine. We focused on clinical articles only and therefore excluded related basic science and cadaveric biomechanical studies. The 100 most cited articles were then further evaluated by primary author, journal of publication, institution, country of origin, year of publication, topic, and total number of citations. One-way analysis of variance (ANOVA) and linear regression analyses were used to determine if publication date correlated with mean number of citations.
Results
Eighty authors wrote the top 100 articles in sports medicine, and each publication garnered several hundred citations, ranging from 229 to 1629 with a mean of 408 (Table 114-113). Most of these articles were written in the past 3 decades, with equal distribution from the 1980s, 1990s, and 2000s (Figure 1A). We ran a linear regression to determine if publication date correlated with higher number of citations by virtue of longer time available for citation. The analysis poorly modeled the variability (R2 = 0.05), revealing no correlation between number of citations and publication date. Further, 1-way ANOVA found no significant difference between the number of citations per decade, F(5, 93) = 1.60, P = .17 (Figure 1B). Despite this finding, the oldest cited article, written by Fairbank39 in 1948, ranked high (position 7). Of these top 100 publications, the most recent, written by Knutsen and colleagues69 in 2007, ranked in the second half at position 66.
Seven journals published the top 100 articles, with the American volume of the Journal of Bone and Joint Surgery publishing nearly half (44%) (Table 2). In second place, with 28 articles, was the American Journal of Sports Medicine, followed by the British volume of the Journal of Bone and Joint Surgery, with 10 articles.
Thirty different topics were investigated in this collection of articles, encompassing nearly every major research area of sports medicine. There was a heavy emphasis on anterior cruciate ligament (ACL) injury and reconstruction, knee rating systems, rotator cuff reconstruction, and chondrocyte transplantation (Table 3).
In several cases, an author contributed more than 1 classic article. In fact, 31 of the top 100 articles were by an individual who had coauthored 2 or more of the publications on this list. The researchers with the largest number of first-authored articles were Noyes88-92 (5 articles), Neer81-84 (4 articles), and Rowe,102-104 Daniel,35-37 Peterson,97-99 and Hewett52-54 (3 articles each) (Table 417,19,21-24,29-31,35-37,42,44,45,52-54,58,61-65,69,70,72,74,80-84,87-92,97-99,101-105,107,109,110,113). Articles from authors with multiple publications had a common topic.
Last, these articles originated from a number of different countries and institutions. Of the 15 source countries (Figure 2), the United States contributed the most (61 articles). Other countries had prominent representation: Sweden and Switzerland (8 each), United Kingdom (5), and Canada, France, and Norway (3 each). These articles originated from 69 universities, hospitals, and clinics; 21 institutions had 2 or more articles (Table 5). The 5 institutions with the highest number of articles were Hospital for Special Surgery, University of Bern, Columbia College of Physicians and Surgeons/Columbia-Presbyterian Medical Center, Cincinnati Sports Medicine and Orthopaedic Center, and Massachusetts General Hospital.
Discussion
Several trends can be ascertained from analyzing the top 100 clinical articles cited in sports medicine. The 5 most frequent topics discussed were ACL injury and reconstruction, knee rating systems for injury and function, rotator cuff reconstruction, chondrocyte transplantation, and femoroacetabular impingement (Table 3). Of those 5 topics, only ACL injury and reconstruction falls within the top 10 most common orthopedic surgical procedures performed in the United States reported by one analysis.114 The most common orthopedic surgical procedure, knee arthroscopy, ranks 10th of all topics covered by the top 100 articles, whereas the second most common procedure, shoulder arthroscopy, was not discussed by any of those 100 articles. Also notable is the high frequency of knee rating system studies, which correlates well with the fact that 4 of the most common orthopedic surgical procedures are knee procedures. The prevalence of rating system articles reflects the importance of and need for accurate methods in the diagnosis of injuries in sports medicine.
The most cited sports medicine article was written by Insall and colleagues62 in 1989, more than 2 decades ago. In this article, “Rationale of the Knee Society Clinical Rating System,” they reported on a rigorous system that rates knee function and ability to walk and climb stairs. The second most cited article, “A Clinical Method of Functional Assessment of the Shoulder,” was written in 1987 by Constant and Murley.32 This article discusses another rating system but offers a functional assessment of the shoulder that is highly reproducible and time-efficient. “Rating Systems in the Evaluation of Knee Ligament Injuries,” the third most cited article, was written in 1985 by Tegner and Lysholm.113 This article details the complexities and variable uses of different knee ligament injury rating systems. These top 3 articles were all published in Clinical Orthopaedics and Related Research. In addition, all 3 discussed rating systems, reinforcing the need for accurate scoring systems to standardize the diagnosis of injury across the field of orthopedics and qualify outcomes after injury.
A number of studies have introduced physical examination findings, clinical tests, and rating systems used in the clinical setting of sports medicine (and named after the contributing authors). For example, the Neer sign82 and the Hawkins-Kennedy test51 are used to determine shoulder impingement. In knee ligament injuries, the Tegner knee activity score113 complements other functional scores (eg, Lysholm knee score74). For grading joint cartilage breakdown, the Outerbridge classification system96 is commonly used. The Fairbank test39 is used to gauge knee instability. In evaluating fatty degeneration of rotator cuff muscles through computed tomography scans, the Goutallier classification47 is used. Other metrics, such as the Knee Injury and Osteoarthritis Outcome Score, introduced by Roos and colleagues,101 measure knee injury and osteoarthritis. In other scenarios, studies have improved on surgical techniques—for example, the Neer open modification84 of the Bankart procedure. Many of these rating systems and named clinical findings are so ingrained in the practice and vernacular of orthopedics that it is possible they are in fact undercited in the literature.
As in other bibliometric analyses, one concession made here was to credit the first author listed for making the primary contribution to an article. As a result of journal variability and inconsistency, we were precluded from analyzing senior authors. When analyzed for authorship at any position, 3 of the top authors (Table 4) showed contributions to additional articles in the top 100 list. Noyes was listed as last author on 2 other articles,52,54 raising his total to 7. Daniel was listed as second author on 1 additional article,105 and Beck was listed as third author on 1 other article,42 raising their totals to 4 and 3, respectively.
A criticism of bibliometric analysis is its use of number of citations as an accurate measure of academic contribution. However, other methods for measuring the productivity and impact of researchers (eg, the recently developed Hirsch Index) have their own drawbacks,115,116 including being able to compare authors only at the same point in their careers and self-citation. It is important to note that our analyses focused strictly on publications related to clinical sports medicine, with the exclusion of basic science and cadaveric biomechanical studies.
Through bibliometric citation analysis, we have identified the authors who have made lasting contributions to the field of sports medicine, and we have highlighted the publications that have been cited by hundreds to thousands of authors. This list identifies trends within the articles that have become “classic,” by nature of their deep permeation into subsequent sports medicine literature, and offers guidance for trainees interested in studying the most high-yield sports medicine literature. Given that 69 institutions in 15 countries conducted these studies, we have also shown that orthopedic research can be readily disseminated internationally. Last, our study provides a thorough overview of the sports medicine literature over the past century and provides a strong framework for future research in our field.
1. Adams AB, Simonson D. Publication, citations, and impact factors of leading investigators in critical care medicine. Respir Care. 2004;49(3):276-281.
2. Bhandari M, Busse J, Devereaux PJ, et al. Factors associated with citation rates in the orthopedic literature. Can J Surg. 2007;50(2):119-123.
3. Cheek J, Garnham B, Quan J. What’s in a number? Issues in providing evidence of impact and quality of research(ers). Qual Health Res. 2006;16(3):423-435.
4. Kelly JC, Glynn RW, O’Briain DE, Felle P, McCabe JP. The 100 classic papers of orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Br. 2010;92(10):1338-1343.
5. Kavanagh RG, Kelly JC, Kelly PM, Moore DP. The 100 classic papers of pediatric orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Am. 2013;95(18):e134.
6. Mehlman CT, Wenger DR. The top 25 at 25: citation classics in the Journal of Pediatric Orthopaedics. J Pediatr Orthop. 2006;26(5):691-694.
7. Namdari S, Baldwin K, Kovatch K, Huffman GR, Glaser D. Fifty most cited articles in orthopedic shoulder surgery. J Shoulder Elbow Surg. 2012;21(12):1796-1802.
8. Cassar Gheiti AJ, Downey RE, Byrne DP, Molony DC, Mulhall KJ. The 25 most cited articles in arthroscopic orthopaedic surgery. Arthroscopy. 2012;28(4):548-564.
9. Paladugu R, Schein M, Gardezi S, Wise L. One hundred citation classics in general surgical journals. World J Surg. 2002;26(9):1099-1105.
10. Fenton JE, Roy D, Hughes JP, Jones AS. A century of citation classics in otolaryngology-head and neck surgery journals. J Laryngol Otol. 2002;116(7):494-498.
11. Loonen MPJ, Hage JJ, Kon M. Plastic surgery classics: characteristics of 50 top-cited articles in four plastic surgery journals since 1946. Plast Reconstr Surg. 2008;121(5):320e-327e.
12. Dubin D, Hafner AW, Arndt KA. Citation classics in clinical dermatologic journals. Citation analysis, biomedical journals, and landmark articles, 1945–1990. Arch Dermatol. 1993;129(9):1121-1129.
13. Baltussen A, Kindler CH. Citation classics in critical care medicine. Intensive Care Med. 2004;30(5):902-910.
14. Aglietti P, Buzzi R, Zaccherotti G, De Biase P. Patellar tendon versus doubled semitendinosus and gracilis tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 1994;22(2):211-218.
15. Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66(5):666-671.
16. Altchek DW, Warren RF, Skyhar MJ, Ortiz G. T-plasty modification of the Bankart procedure for multidirectional instability of the anterior and inferior types. J Bone Joint Surg Am. 1991;73(1):105-112.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701.
19. Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16(2):113-122.
20. Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17(1):1-6.
21. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640-645.
22. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
23. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
24. Bentley G, Biant LC, Carrington RWJ, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223-230.
25. Berchuck M, Andriacchi TP, Bach BR, Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72(6):871-877.
26. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41(6):988-1020.
27. Binkley JM, Stratford PW, Lott SA, Riddle DL, North American Orthopaedic Rehabilitation Research Network. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371-383.
28. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
29. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
30. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
31. Clancy WG Jr, Shelbourne KD, Zoellner GB, Keene JS, Reider B, Rosenberg TD. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J Bone Joint Surg Am. 1983;65(3):310-322.
32. Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
33. Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27(3):444-454.
34. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
35. Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R. Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg Am. 1985;67(5):720-726.
36. Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632-644.
37. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401-407.
38. Ellman H, Hanker G, Bayer M. Repair of the rotator cuff. End-result study of factors influencing reconstruction. J Bone Joint Surg Am. 1986;68(8):1136-1144.
39. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
40. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
41. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
42. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):111-119.
43. Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;(304):43-53.
44. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
45. Gerber C, Krushell RJ. Isolated rupture of the tendon of the subscapularis muscle. Clinical features in 16 cases. J Bone Joint Surg Br. 1991;73(3):389-394.
46. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
47. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78-83.
48. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE Jr. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650.
49. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
50. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
51. Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med. 1980;8(3):151-157.
52. Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699-706.
53. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492-501.
54. Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765-773.
55. Homminga GN, Bulstra SK, Bouwmeester PSM, van der Linden AJ. Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br. 1990;72(6):1003-1007.
56. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185-192.
57. Hovelius L, Augustini BG, Fredin H, Johansson O, Norlin R, Thorling J. Primary anterior dislocation of the shoulder in young patients. A ten-year prospective study. J Bone Joint Surg Am. 1996;78(11):1677-1684.
58. Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58(2):159-172.
59. Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427-436.
60. Iannotti JP, Zlatkin MB, Esterhai JL, Kressel HY, Dalinka MK, Spindler KP. Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg Am. 1991;73(1):17-29.
61. Insall J, Falvo KA, Wise DW. Chondromalacia patellae. A prospective study. J Bone Joint Surg Am. 1976;58(1):1-8.
62. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
63. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600-613.
64. Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132-1145.
65. Ito K, Minka MA 2nd, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect. A MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br. 2001;83(2):171-176.
66. Johnson RJ, Kettelkamp DB, Clark W, Leaverton P. Factors affecting late results after meniscectomy. J Bone Joint Surg Am. 1974;56(3):719-729.
67. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204-208.
68. Jones KG. Reconstruction of the anterior cruciate ligament: a technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
69. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-2112.
70. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86(3):455-464.
71. Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159-163.
72. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
73. Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276-291.
74. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154.
75. Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003-1010.
76. Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991;19(5):478-484.
77. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
78. Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9(3):318-321.
79. McDaniel WJ Jr, Dameron TB Jr. Untreated ruptures of the anterior cruciate ligament. A follow-up study. J Bone Joint Surg Am. 1980;62(5):696-705.
80. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
81. Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54(1):41-50.
82. Neer CS 2nd. Impingement lesions. Clin Orthop Relat Res. 1983;(173):70-77.
83. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
84. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder. A preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
85. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
86. Nistor L. Surgical and non-surgical treatment of Achilles tendon rupture. J Bone Joint Surg Am. 1981;63(3):394-399.
87. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
88. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513-518.
89. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62(5):687-695, 757.
90. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate–deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
91. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate–deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
92. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
93. O’Brien SJ, Warren RF, Pavlov H, Panariello R, Wickiewicz TL. Reconstruction of the chronically insufficient anterior cruciate ligament with the central third of the patellar ligament. J Bone Joint Surg Am. 1991;73(2):278-286.
94. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
95. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
96. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43(4):752-757.
97. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12.
98. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17-24.
99. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-234.
100. Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80(9):1276-1284.
101. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96.
102. Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am. 1956;38(5):957-977.
103. Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am. 1978;60(1):1-16.
104. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
105. Sachs RA, Daniel DM, Stone ML, Garfein RF. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17(6):760-765.
106. Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65(4):456-460.
107. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299.
108. Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders [see comments]. J Bone Joint Surg Am. 1995;77(1):10-15.
109. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85(2):278-286.
110. Solomonow M, Baratta R, Zhou BH, et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15(3):207-213.
111. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484.
112. Tapper EM, Hoover NW. Late results after meniscectomy. J Bone Joint Surg Am. 1969;51(3):517-526.
113. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-49.
114. Garrett WE Jr, Swiontkowski MF, Weinstein JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: Part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
115. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98.
116. Bornmann L, Daniel HD. The state of h index research. Is the h index the ideal way to measure research performance? EMBO Rep. 2009;10(1):2-6.
1. Adams AB, Simonson D. Publication, citations, and impact factors of leading investigators in critical care medicine. Respir Care. 2004;49(3):276-281.
2. Bhandari M, Busse J, Devereaux PJ, et al. Factors associated with citation rates in the orthopedic literature. Can J Surg. 2007;50(2):119-123.
3. Cheek J, Garnham B, Quan J. What’s in a number? Issues in providing evidence of impact and quality of research(ers). Qual Health Res. 2006;16(3):423-435.
4. Kelly JC, Glynn RW, O’Briain DE, Felle P, McCabe JP. The 100 classic papers of orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Br. 2010;92(10):1338-1343.
5. Kavanagh RG, Kelly JC, Kelly PM, Moore DP. The 100 classic papers of pediatric orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Am. 2013;95(18):e134.
6. Mehlman CT, Wenger DR. The top 25 at 25: citation classics in the Journal of Pediatric Orthopaedics. J Pediatr Orthop. 2006;26(5):691-694.
7. Namdari S, Baldwin K, Kovatch K, Huffman GR, Glaser D. Fifty most cited articles in orthopedic shoulder surgery. J Shoulder Elbow Surg. 2012;21(12):1796-1802.
8. Cassar Gheiti AJ, Downey RE, Byrne DP, Molony DC, Mulhall KJ. The 25 most cited articles in arthroscopic orthopaedic surgery. Arthroscopy. 2012;28(4):548-564.
9. Paladugu R, Schein M, Gardezi S, Wise L. One hundred citation classics in general surgical journals. World J Surg. 2002;26(9):1099-1105.
10. Fenton JE, Roy D, Hughes JP, Jones AS. A century of citation classics in otolaryngology-head and neck surgery journals. J Laryngol Otol. 2002;116(7):494-498.
11. Loonen MPJ, Hage JJ, Kon M. Plastic surgery classics: characteristics of 50 top-cited articles in four plastic surgery journals since 1946. Plast Reconstr Surg. 2008;121(5):320e-327e.
12. Dubin D, Hafner AW, Arndt KA. Citation classics in clinical dermatologic journals. Citation analysis, biomedical journals, and landmark articles, 1945–1990. Arch Dermatol. 1993;129(9):1121-1129.
13. Baltussen A, Kindler CH. Citation classics in critical care medicine. Intensive Care Med. 2004;30(5):902-910.
14. Aglietti P, Buzzi R, Zaccherotti G, De Biase P. Patellar tendon versus doubled semitendinosus and gracilis tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 1994;22(2):211-218.
15. Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66(5):666-671.
16. Altchek DW, Warren RF, Skyhar MJ, Ortiz G. T-plasty modification of the Bankart procedure for multidirectional instability of the anterior and inferior types. J Bone Joint Surg Am. 1991;73(1):105-112.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701.
19. Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16(2):113-122.
20. Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17(1):1-6.
21. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640-645.
22. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
23. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
24. Bentley G, Biant LC, Carrington RWJ, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223-230.
25. Berchuck M, Andriacchi TP, Bach BR, Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72(6):871-877.
26. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41(6):988-1020.
27. Binkley JM, Stratford PW, Lott SA, Riddle DL, North American Orthopaedic Rehabilitation Research Network. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371-383.
28. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
29. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
30. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
31. Clancy WG Jr, Shelbourne KD, Zoellner GB, Keene JS, Reider B, Rosenberg TD. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J Bone Joint Surg Am. 1983;65(3):310-322.
32. Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
33. Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27(3):444-454.
34. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
35. Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R. Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg Am. 1985;67(5):720-726.
36. Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632-644.
37. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401-407.
38. Ellman H, Hanker G, Bayer M. Repair of the rotator cuff. End-result study of factors influencing reconstruction. J Bone Joint Surg Am. 1986;68(8):1136-1144.
39. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
40. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
41. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
42. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):111-119.
43. Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;(304):43-53.
44. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
45. Gerber C, Krushell RJ. Isolated rupture of the tendon of the subscapularis muscle. Clinical features in 16 cases. J Bone Joint Surg Br. 1991;73(3):389-394.
46. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
47. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78-83.
48. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE Jr. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650.
49. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
50. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
51. Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med. 1980;8(3):151-157.
52. Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699-706.
53. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492-501.
54. Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765-773.
55. Homminga GN, Bulstra SK, Bouwmeester PSM, van der Linden AJ. Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br. 1990;72(6):1003-1007.
56. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185-192.
57. Hovelius L, Augustini BG, Fredin H, Johansson O, Norlin R, Thorling J. Primary anterior dislocation of the shoulder in young patients. A ten-year prospective study. J Bone Joint Surg Am. 1996;78(11):1677-1684.
58. Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58(2):159-172.
59. Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427-436.
60. Iannotti JP, Zlatkin MB, Esterhai JL, Kressel HY, Dalinka MK, Spindler KP. Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg Am. 1991;73(1):17-29.
61. Insall J, Falvo KA, Wise DW. Chondromalacia patellae. A prospective study. J Bone Joint Surg Am. 1976;58(1):1-8.
62. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
63. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600-613.
64. Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132-1145.
65. Ito K, Minka MA 2nd, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect. A MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br. 2001;83(2):171-176.
66. Johnson RJ, Kettelkamp DB, Clark W, Leaverton P. Factors affecting late results after meniscectomy. J Bone Joint Surg Am. 1974;56(3):719-729.
67. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204-208.
68. Jones KG. Reconstruction of the anterior cruciate ligament: a technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
69. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-2112.
70. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86(3):455-464.
71. Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159-163.
72. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
73. Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276-291.
74. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154.
75. Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003-1010.
76. Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991;19(5):478-484.
77. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
78. Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9(3):318-321.
79. McDaniel WJ Jr, Dameron TB Jr. Untreated ruptures of the anterior cruciate ligament. A follow-up study. J Bone Joint Surg Am. 1980;62(5):696-705.
80. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
81. Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54(1):41-50.
82. Neer CS 2nd. Impingement lesions. Clin Orthop Relat Res. 1983;(173):70-77.
83. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
84. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder. A preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
85. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
86. Nistor L. Surgical and non-surgical treatment of Achilles tendon rupture. J Bone Joint Surg Am. 1981;63(3):394-399.
87. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
88. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513-518.
89. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62(5):687-695, 757.
90. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate–deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
91. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate–deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
92. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
93. O’Brien SJ, Warren RF, Pavlov H, Panariello R, Wickiewicz TL. Reconstruction of the chronically insufficient anterior cruciate ligament with the central third of the patellar ligament. J Bone Joint Surg Am. 1991;73(2):278-286.
94. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
95. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
96. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43(4):752-757.
97. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12.
98. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17-24.
99. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-234.
100. Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80(9):1276-1284.
101. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96.
102. Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am. 1956;38(5):957-977.
103. Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am. 1978;60(1):1-16.
104. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
105. Sachs RA, Daniel DM, Stone ML, Garfein RF. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17(6):760-765.
106. Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65(4):456-460.
107. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299.
108. Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders [see comments]. J Bone Joint Surg Am. 1995;77(1):10-15.
109. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85(2):278-286.
110. Solomonow M, Baratta R, Zhou BH, et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15(3):207-213.
111. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484.
112. Tapper EM, Hoover NW. Late results after meniscectomy. J Bone Joint Surg Am. 1969;51(3):517-526.
113. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-49.
114. Garrett WE Jr, Swiontkowski MF, Weinstein JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: Part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
115. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98.
116. Bornmann L, Daniel HD. The state of h index research. Is the h index the ideal way to measure research performance? EMBO Rep. 2009;10(1):2-6.
Addressing the Sexual Health Concerns of Women with Gynecologic Cancer: Guidance for Primary Care Physicians
From the Yale School of Medicine, New Haven, CT.
Abstract
- Objective: To review the sexual health concerns of women with gynecologic cancer and provide guidance for primary care physicians.
- Methods: Review of the literature.
- Results: Issues of sexuality and intimacy are known to significantly impact the quality of life of patients following diagnosis and treatment of gynecologic cancers. At the time of diagnosis, women should be informed of the potential physiologic, hormonal, and psychosocial effects of gynecologic cancer on sexuality. Many providers fail to address these issues given time constraints and patients’ trepidation in alerting their providers to their concerns. While systemic hormone therapy directly addresses these symptoms, its use remains controversial due to potential cancer recurrence risks. Thus, treatment centers around therapeutic alternatives. For vasomotor symptoms, selective serotonin reuptake inhibitors have shown effectiveness and are typically well tolerated, and antiepileptics such as gabapentin have shown promise. There is promising but limited data employing pelvic floor physical therapy as a tool to aid in addressing pelvic floor symptoms. Psychological care and the involvement of the partner are also part of managing the sexual health concerns of these patients.
- Conclusion: Sexual morbidity is a distressing and undertreated problem among gynecologic cancer survivors. Successful treatment requires the provider’s appreciation of the problem and willingness to address it.
Issues of sexuality and intimacy are known to significantly impact the lives of patients following diagnosis and treatment of gynecologic cancers. [1,2] Treatment of gynecologic malignancy is highly dependent on pathology and stage, with some patients receiving small excisional procedures while others subject to extensive surgeries, chemotherapy, and radiation treatment, and it is difficult to predict how each individual’s sexual health will be impacted. However, the evidence suggests that at least half of women treated for gynecologic cancer will experience sexual dysfunction [3]. Although the impact of gynecologic cancer and treatment can be profound, providers often do not address their patients’ sexual concerns [4,5], yet most patients have indicated that they would like these issues to be addressed [6].
Female Sexual Dysfunction
Sexual dysfunction is multifactorial and involves physical, social, and psychological dimensions. It is common in the general population, with rates ranging from 25-63% [7]. The DSM-IV [8] defines female sexual dysfunction as a disturbance in or pain during the sexual response, which can be further classified as hypoactive sexual disorder, orgasmic disorder, sexual pain disorder, or sexual arousal disorder [9]. It should be noted that women who have been treated for gynecologic cancers may have a premorbid history of sexual dysfunction. Assessment of a woman’s sexual function prior to her cancer diagnosis can help establish which sexuality changes are due to the cancer treatment and may allow the provider to tailor interventions accordingly.
Sexual Dysfunction and Quality of Life
As treatments for gynecologic cancers improve, toxicity of treatment decreases, and survival increases, quality of life for survivors has become as become an increasingly important health issue. Several studies have examined patient-reported quality of life in short- and long-term cancer survivors and report overall significant alteration in quality of life over many aspects of health and psychological well-being [10]. It is well established that health-related quality of life and sexual functioning are closely associated [11].
In gynecologic cancer patients, sexual and quality of life outcomes can vary. For example, Kim el al recently compared quality of life and sexual functioning in ovarian cancer survivors with no evidence of disease after primary treatment and a cohort of health women. Sexuality, both in terms of desire, arousal, lubrication, orgasm, satisfaction, and pain and in terms of interest in sex, sexual activity, and enjoyment of sex were similar between the groups; however social functioning deteriorated in cancer patients [12]. Other women report different experiences. Women with a history of vulvar, vaginal, or cervical cancer who may have had extensive pelvic surgery, lymphadenectomy, and radiation treatment to the pelvis typically report greater alterations in quality of life and sexual activity depending on the extent of their treatment [13].
Patients undergoing chemotherapy often report significant changes in quality of life due to the physical symptoms of fatigue, nausea, hair loss, diarrhea, etc. as well the psychological effects of cancer diagnosis, changes in body image, and poor coping [14]. Sexual side effects are common due to alterations in the HPA axis, direct gonadal toxicity and neuropathies [15].
Impact of Gynecologic Cancer on Sexuality
Gynecologic cancer and treatment can alter sexuality through a variety of effects. The impact of anatomical changes may alter a women’s self-esteem, body image, and sense of femininity, resulting in a reluctance to engage in intimate behaviors. Furthermore, if the partnership is disrupted by changes in roles in the household or within the relationship, relationships dynamics may be tested and result in mental distress for the patient, thus effecting sexuality and intimacy.
Surgical or chemical withdrawal of sex hormones, new medications, and postoperative sequelae can contribute to sexual arousal problems. The emotional and psychological components of a cancer diagnosis also can hinder the sexual response [16]. Depression and anxiety, the rates of which increase with cancer diagnosis, can also significantly affect sexual function [17].
Sexual pain is common in this population due to hormonal considerations as well as post-treatment side effects and a frequent cause of sexual dysfunction for these patients. Pelvic radiation may result in adverse physical changes to the vagina including vaginal stenosis, thinning of vaginal mucosa, loss of lubrication, and loss of elasticity [18,19]. A recent review of 20 studies of cervical cancer survivors found that patients were at risk for lack of lubrication and had high rates of dyspareunia [20]. Psychosocial factors have been found to be important predictors of sexual desire and more important than hormones in predicting low sexual desire in middle age [21]. These factors include emotional and physical closeness to the partner, satisfactory communication, and a positive relation to one’s own body [16].
Effects on Relationships and Partners
Women whose sexual capacity is compromised may be worried about their partners’ quality of life and overall well-being. Indeed, the partners of women with gynecologic cancer are also impacted by the changes to sexual function and loss of sexuality and intimacy. Hawkins et al found that cessation or decreased frequency of sex and intimacy was reported in 79% of male partners of women affected by cancer. Among partners of the persons with cancer in this study, changes to sexuality were associated with feelings of self-blame, reflection, sadness, anger and lack of sexual fulfillment [22]. Further, male partners of women diagnosed with gynecological cancer often express conflicting emotional states including feeling worried about their significant other’s health, having the desire to engage in sexual activity, and feeling guilty about wanting to increase sexual intimacy. These feelings, in turn, can lead to resentment and withdrawal from their partner and overall relationship discord [23].
Evidence suggests partners of cancer patients greatly benefit from increased social support even years after an apparent cure [24]. Specifically, male partners who take on a new role as caregiver in the relationship experience difficulties with emotional changes, challenges to their masculinity, and new stressors [25]. These new roles and feelings also contribute to changes in sexuality and intimacy in relationships, making support for partners all the more necessary.
Menopausal Symptoms Following Cancer Treatment
Gynecologic cancer treatment invariably affects the female hormonal balance, sometimes suddenly with surgical excision of the gonads or via radiation treatment or chemotherapy. It is well known that the sudden withdrawal of estrogen and testosterone, especially in the premenopausal postoperative population, can lead to significant acute menopausal symptoms. Given the many emotional and physical issues affecting patients during treatment, it can be difficult to delineate what proportion of sexual problems are caused by or enhanced by vasomotor symptoms, sleep disorders, and vaginal atrophy.
In general, premenopausal women who experience abrupt surgical menopause may often have immediate severe symptoms. Many agree that the younger a woman is when going through this process, the more severe her symptoms may be. Although the average age of endometrial cancer diagnosis is 67, approximately 25% of women who are diagnosed are premenopausal, and 5% of cases occur in women under 40. As the obesity epidemic worsens and more women are exposed to higher levels of estrogen at younger ages, it is expected that the number of premenopausal women who are diagnosed will continue to rise. The average age of diagnosis for ovarian cancer is 63, however, there is a large cohort of women diagnosed with borderline and malignant tumors in the premenopausal period [26]. These patients often experience vasomotor symptoms in the hours to days following surgery.
Interventions for Survivors of Gynecologic Cancer with Sexual Dysfunction
Systemic Hormone Therapy
Systemic hormonal therapy to treat menopausal symptoms remains controversial following the release of findings from the Women’s Health Initiative, which showed a number of adverse effects, including an increased risk of breast cancer in healthy postmenopausal women who received systemic hormonal therapy for menopausal symptoms. While views have changed since that time, providers are often reluctant to prescribe hormonal therapy, and patients are reticent to take it, due to fears of cancer recurrence. However, there is no evidence showing hormones negatively influence survival after treatment for epithelial ovarian, squamous cervical, or vulvar cancer [27]. With the exception of endometrial cancer, there is no biological evidence that HRT may increase recurrence risk [28]. Approach to clinical decision making should be individualized, taking into consideration the patients’ symptoms, quality of life, tumor histology, and overall prognosis.
Cervical cancer, vulvar cancer, and vaginal cancer are not considered hormonally responsive tumors. While the data are limited, a study published in 1987 of cervical cancer survivors treated with systemic hormone replacement therapy showed no increase in relapse rates and showed an increase in quality of life [29]. There are no studies regarding systemic HRT in patients with vulvar or vaginal cancer though it is generally accepted they can be treated with HRT. No significant data exists for cervical adenocarcinoma patients, and most oncologists suggest treating these patients the same as those with endometrial cancer primary.
There is limited data on the use of HRT in women with ovarian cancer but available studies do not show any difference in overall or disease-free survival between HRT groups and controls [27,30–32]. Many studies report a significant increase in quality of life with HRT. A 2013 retrospective chart review of 77 patients with epithelial ovarian cancer who received postoperative HRT showed no significant difference in progression-free survival [33].
The most controversy surrounds the use of HRT in patients with endometrial cancer [34]. The only major data available come from the Gynecologic Oncology Group’s truncated study, which was a large prospective randomized controlled trial. Over 1200 women treated for stage I and II endometrial cancer were enrolled between 1997 and 2003, and before the study was terminated these patients were followed for a median of 3 years following initiation of therapy. The recurrence rate of malignancy was low, 2.1, and an insignificant risk of recurrence of 1.27 was noted between with HRT and placebo groups (80% CI, 0.916-1.77) [35]. Women included in this study were between the ages of 26 and 91, and their indications for therapy included vasomotor symptoms, vaginal atrophy, as well as osteoporosis risk and cardiovascular disease risk. Gynecologist oncologists often use estrogen therapy to treat symptomatic women with early stage endometrial cancer given the very low risk of recurrence.
Tibolone is a synthetic steroid with activity on estrogen and progesterone receptors, and mainly acts as an agonist at estrogen receptors. It is prescribed outside the United States for osteoporosis and is being investigated as treatment for female sexual dysfunction. Efficacy on vasomotor symptoms has been positive thus far. One case-control study confirmed tibolone’s safety for endometrial cancer survivors, with no adverse effects on disease-free or overall survival [36]. One group recently examined tibolone in the setting of breast cancer patients in a prospective randomized controlled trial and reported an increased risk of breast cancer recurrences in women receiving tibolone for HT [37]. That study reported no increase in risk of gynecologic cancers and did report favorable outcomes for patients in terms of osteoporosis and vasomotor symptoms.
Topical Therapy
Women who are concerned about systemic HRT but who have been treated with radiation in addition to their surgery may be interested in topical estrogen therapy. These women may have vaginal stenosis and atrophic symptoms, and for them topical estrogen therapy can be helpful [38]. Many formulations of topical estrogen are available, including creams, tablets, and rings. Using vaginal estrogens and dilators can be useful to help with eventual resumption of sexual activity once healing has taken place and can help to avoid dyspareunia. Combination topical and systemic therapy can also be useful to relieve symptoms. Women concerned about absorption with topical therapy can be advised that while there is some absorption initially, absorption is reduced as atrophy is improved with treatment.
One recent study examined the use of alpha-tocopherol to reduce acute vaginal complications in women with endometrial and cervical cancer undergoing radiation treatment. The treatment group experienced reduced vaginal toxicity and pain, although vaginal secretion was not significantly different in the 2 groups studied [39]. No adverse effects were noted. This compound has not been studied further but may be beneficial in the future.
Some women may prefer to avoid hormones altogether. Over-the-counter vaginal moisturizers and lubricants can be recommended to women to help with intercourse and atrophic symptoms, with or without estrogen therapy.
Physical Therapy
Pelvic floor physical therapy has become an increasingly popular modality for treatment of many aspects pelvic floor dysfunction, the symptoms of which include defecatory dysfunction, constipation, bladder dysfunction, painful urination, dyspareunia, pelvic pain, and low back pain [40]. Pelvic floor physical therapy is well studied and utilized frequently for urinary incontinence and pelvic organ prolapse, but is understudied for sexual dysfunction [41,42].
Promising areas of study include educational sessions, cognitive behavioral therapy, vaginal dilator therapy, pelvic floor muscle strengthening and relaxation techniques with biofeedback, stretching and massage [40]. Biofeedback specifically has been studied in controlled studies for treatment of sexual dysfunction specifically in the setting of vulvar pain syndromes [43]. A small randomized control trial evaluated the use of pelvic floor muscle training in gynecologic cancer survivors and found that the intervention group reported improved sexual function and improved quality of life [44]. Given the few side effects of this therapy, it may be a helpful addition to a multidisciplinary approach to treating pelvic floor dysfunction in gynecologic cancer patients.
For cervical and endometrial cancer patients, vaginal dilators are often prescribed following radiation therapy to minimize vaginal stenosis. Literature suggests that after radiotherapy dilation therapy is effective when used as directed, typically 3 times per week for 10 minutes to mechanically separate the vaginal walls and increase elasticity [45]. Adherence to recommendations for vaginal dilator use is low in this population due to factors such as aversion to the practice and intrusiveness of the mechanism [46,47]. Acknowledgment of apprehension regarding dilator use should be part of the counseling prior to initiation of treatment; interventions designed to educate women about dilator use benefit may increase adherence [48].
Psychological Therapies
Survivors of gynecological cancer with sexual dysfunction may experience the psychological symptoms in the context of the other emotional challenges of a cancer diagnosis [49]. Changes in body image, physical appearance, and feelings of well-being can significantly affect sexuality and intimacy, while anxiety and negative feelings about a diagnosis can cause further sexual dysfunction. Conversely, sexual morbidity in these patients predicts worsening of general psychological symptoms, including depressive symptoms and overall quality of life [13–15].
While there are limited studies directly assessing the effect of psychological therapies for the treatment of sexual dysfunction in this population, the existing literature shows enduring symptomatic improvement following brief interventions. In women for whom emotional distress, depression, and anxiety appear to be a significant aspect of their concern regarding intimacy and sexuality, these interventions can be particularly helpful.
Cognitive behavioral therapy (CBT), which focuses on mindfulness and the relationships between maladaptive thoughts and how they impact behavior, has been shown to have efficacy in treating the broader psycho-social concerns of gynecological cancer patients [50,51]. In a recent study Brotto and colleagues randomized 31 survivors of gynecological cancer with self-reported sexual dysfunction to either three 90-minute CBT sessions or a waitlist control. Patients who underwent the intervention reported significant improvements in sexual arousal and desire both immediately post-therapy and at 6-month follow-up while patients in the waitlist arm experienced no significant changes in symptomatology [52].
Psychoeducational interventions are another promising avenue for addressing sexual dysfunction in this population [53]. In a 2008 therapeutic trial, Brotto and colleagues combined elements of CBT with education in 3 one-hour sessions featuring written materials on sexuality and relationships. The intervention enrolled 22 women with early stage gynecologic cancer who met criteria for female sexual arousal disorder. The psychoeducational intervention was associated with positive effect on sexual desire, arousal, orgasm, satisfaction, sexual distress, depression, and overall well-being [16]. Psychoeducation can also be used to augment other therapeutic modalities. Robinson and colleagues used such an intervention to improve adherence to vaginal dilator use in 32 women with early stage endometrial cancer who were being treated with radiotherapy [48].
Some gynecologic cancer patients may have significant alterations in their anatomy and thus penetrative intercourse may not be possible. In patients even without these physical changes, some may prefer to avoid intercourse due to pain or anxiety. Thus patients can have significant benefits from discussing their concerns with a therapist specializing in these issues as many patients are concerned about their ability to engage in intimate behavior. Therapy can assist with the changing sexual relationship and assist the partners in different ways of engaging in intimate acts. It is important to avoid stressing penetrative intercourse as the goal for sexual function with these or any patients with anxiety relating to their disease as there are many ways to engage in mutually pleasurable experiences for both partners, thus removing anxiety about inability to resume vaginal intercourse post-treatment. Discussing this with patients can be challenging but can often reduce anxiety surrounding body image issues following treatment.
Studies have shown that cancer care providers often do not adequately address sexual concerns [54] but that when these concerns are appropriately managed, patient satisfaction and quality of life significantly improve [55]. Several studies have focused on how providers can incorporate the approaches of CBT and psychoeducation to better address the sexual concerns of patients without requiring external psychiatric care [56,57]. Barbera et al have described a successful model in which multidisciplinary care teams provide education and counseling for gynecological cancer patients in a sexuality clinic [58]. patients had a significant symptom burden, including menopausal symptoms, the effects of radiation therapy, chemotherapy, and surgical operation as well as psychological responses to cancer, and reported high levels of satisfaction with their experience at the clinic.
Involvement of the partner in interventions has not been well studied; however, involving the partner in in psychological therapies to address sexual dysfunction should be beneficial.
Alternative Therapies for Vasomotor Symptoms
Gynecologic cancer patients suffering prominent vasomotor symptoms have limited alternatives to hormone therapy. Clinicians must balance potential medication benefit with potential exacerbation of other medical and psychological issues, including sexual dysfunction.
Antidepressants
The use of SSRIs and SNRIs for vasomotor symptoms was pioneered by medical oncologists for men with hot flashes secondary to GnRH agonist therapy for prostate cancer and women with breast cancer [59,60]. Limited studies have shown that antidepressant medications do not increase cancer recurrence risk in ovarian cancer patients and are relatively well tolerated [61]. However, these medications are known to have partial efficacy in improving vasomotor symptoms and may worsen sexual symptoms, a well-known side effect of antidepressants. There is variation of the reported rates of sexual dysfunction associated with various antidepressants and clinicians may take the likelihood of sexual side effects into account when prescribing SSRIs or SNRIS [62]. More recently developed SSRIS, such as citalopram and its enantiomer escitalopram, have shown significant improvements in vasomotor symptoms and were better tolerated than venlafaxine and fluoxetine [63,64]. Additionally, limited uncontrolled studies of mirtazipine, a structurally unique SSRI, and bupropion, which acts on dopamine and norepinephrine, have shown significant decreases in hot flash symptoms and are less associated with sexual side effects than SSRIS/SNRIS [65,66].
Other Agents
Other pharmaceutical options for menopausal vasomotor symptoms include gabapentin and adrenergic agonists. Gabapentin can yield impressive reductions in vasomotor symptoms. A recent double-blind randomized trial in 50 patients revealed a 60% reduction in hot flashes as 12 weeks and an 80% reduction in self-reported composite symptom scores [67]. However, side effects such as palpitations, edema, and fatigue, lead to high study withdrawal rates and limit its widespread clinical use for this indication [68]. Clonidine has been assessed versus venlafaxine in several clinical trials with breast cancer patients. These trials have shown mixed results, with findings of both inferiority and superiority to venlafaxine, but with consistent significant improvement in symptoms over placebo. Side effects, such insomnia, constipation and dry mouth, occurred but did not lead to higher dropout rates than venlafaxine [69,70].
Long-Term Sexual Outcomes
For women treated for gynecological cancers, alterations in sexual function may persist in the long term. A study following cervical cancer patients managed with radical hysterectomy up to 2 years post treatment showed they had more sexual dysfunction compared with healthy controls, although at rates similar to those who underwent radical hysterectomy for benign disease [71]. A 2007 review of quality of life studies revealed that although ovarian cancer survivors 5 years past diagnosis had excellent overall quality of life, sexual symptoms persisted, with as many as 57% of patients reporting a decline in sexual function due to their cancer [72].
Studies show some differences in outcomes based on treatment modality. A recent review of cervical cancer outcomes revealed that women who received radiotherapy as a component of their treatment have a higher risk of long-term sexual side effects [73]. In contrast, a study assessing endometrial cancer patients 5 years after initial diagnosis between those patients who had received surgery alone and those who had received surgery and vaginal brachytherapy. There was no significant difference in any measures of quality of life and sexual function between these 2 groups [74].
Age appears to play a role in long-term sexual outcomes regardless of diagnosis. Bifulco and colleagues assessed quality of life in survivors of gynecological cancer, comparing women under age 45 to those over 45 after nearly 3 years of survival. After controlling for age and other factors, younger patients were found to have worse sexual activity, including significantly higher rates of poor body image, perceived worse sexual vaginal function, and more severe menopausal symptoms, probably related to the effects of surgical menopause [75].
Despite enduring sexual dysfunction, symptoms tend to improve over time. A cohort study of 103 gynecological cancer patients undergoing radiation therapy were followed for 3 years. Patients were offered standard interventions for sexual dysfunction, including vaginal lubricants, dilators, and menopausal symptomatic therapy, although adherence to these measures was not assessed. Three years after initial therapy, the percentage of sexually active women increased from 21.5% to 44.2% [76]. In the subset of patients who successfully return to sexual activity, outcomes can be comparable to healthy peers. Kim and colleagues compared disease-free sexually active ovarian cancer patients with demographically matched healthy controls on standardized self-report measures. Sexual functioning did not differ between the 2 groups, despite lower social functioning in cancer survivors [12].
Conclusion
Sexuality and intimacy can be greatly affected by the diagnosis and treatment of gynecologic malignancies. It is important to routinely discuss sexuality and sexual functioning with patients from diagnosis onward. Reassuring patients, acknowledging the importance of their concerns, and validating their desire to enjoy improved intimacy should be considered part of the clinician’s role. Valuable information sources that may aid discussions are available on the internet. Oncolink (www.oncolink.org), a large cancer information website maintained by University of Pennsylvania Cancer Center, offers a plethora of information for patients and health care professionals. In addition, the American Cancer Society offers a detailed guide, “Sexuality for the Woman with Cancer” [77]. Treatment is available, and improvement in outcomes is possible. Further prospective studies are needed to clearly delineate risks and benefits of hormone replacement therapy in patients with gynecologic cancers.
Corresponding author: Elena S. Ratner, MD, PO Box 208063, New Haven, CT 06520, [email protected].
1. Juraskova I, Butow P, Robertson R, et al. Post-treatment sexual adjustment following cervical and endometrial cancer: a qualitative insight. Psychooncology 2003;12:267–79.
2. Bodurka DC, Sun CC. Sexual function after gynecologic cancer. Obstet Gynecol Clin North Am 2006;33:621–30, ix.
3. Andersen BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol 1997;65:221.
4. Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J 2009;15:74–7.
5. Stead ML, Brown JM, Fallowfield L, et al. Lack of communication between healthcare professionals and women with ovarian cancer about sexual issues. Br J Cancer 2003;88:666–71.
6. Stead ML, Fallowfield L, Brown JM, et al. Communication about sexual problems and sexual concerns in ovarian cancer: qualitative study. BMJ 2001;323:836–7.
7. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537–44.
8. American Psychiatric Association. Diagnostic and statistical manual of mental health disorders. 4th ed. 2000.
9. Elder JA, Braver Y. Female sexual dysfunction. In: Current clinical medicine. Elsevier; 2010:1222–6.
10. Ferrell BR, Dow KH, Leigh S, et al. Quality of life in long-term cancer survivors. Oncol Nurs Forum 1995;22:915–22.
11. Shamspour N, Assari S, Moghana Lankarani M. Relation between sexuality and health-related quality of life. In: Handbook of disease burdens and quality of life measures. New York: Springer 2010;3457–73.
12. Kim SI, Lee Y, Lim MC, et al. Quality of life and sexuality comparison between sexually active ovarian cancer survivors and healthy women. J Gynecol Oncol 2015;26:148–54.
13. Levin AO, Carpenter KM, Fowler JM, et al. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. Int J Gynecol Cancer 2010;20:461–70.
14. Lutgendorf SK, Anderson B, Rothrock N, et al. Quality of life and mood in women receiving extensive chemotherapy for gynecologic cancer. Cancer 2000;89:1402–11.
15. Tierney DK. Sexuality: a quality-of-life issue for cancer survivors. Semin Oncol Nurs 2008;24:71–9.
16. Brotto LA, Heiman JR, Goff B, et al. A psychoeducational intervention for sexual dysfunction in women with gynecologic cancer. Arch Sex Behav 2008;37:317–29.
17. Hollingsworth M, Berman J. The role of androgens in female sexual dysfunction. Sex Reprod Menopause 2006;4:27–32.
18. Brand AH, Bull CA, Cakir B. Vaginal stenosis in patients treated with radiotherapy for carcinoma of the cervix. Int J Gynecol Cancer 2006;16:288–93.
19. Lancaster L. Preventing vaginal stenosis after brachytherapy for gynaecological cancer: an overview of Australian practices. Eur J Oncol Nurs 2004;8:30–9.
20. Ponto JA, Barton D. Husbands’ perspective of living with wives’ ovarian cancer. Psychooncology 2008;17:1225–31.
21. Hartmann U, Philippsohn S, Heiser K, et al. Low sexual desire in midlife and older women: personality factors, psychosocial development, present sexuality. Menopause 2004;11:726–40.
22. Hawkins Y, Ussher J, Gilbert E, et al. Changes in sexuality and intimacy after the diagnosis and treatment of cancer: the experience of partners in a sexual relationship with a person with cancer. Cancer Nurs 2009;32:271–80.
23. Gilbert E, Ussher JM, Hawkins Y. Accounts of disruptions to sexuality following cancer: the perspective of informal carers who are partners of a person with cancer. Health 2009;13:523–41.
24. Hodgkinson K, Butow P, Hunt GE, et al. Life after cancer: couples’ and partners’ psychological adjustment and supportive care needs. Support Care Cancer 2007;15:405–15.
25. Lopez V, Copp G, Molassiotis A. Male caregivers of patients with breast and gynecologic cancer: experiences from caring for their spouses and partners. Cancer Nurs 2012;35:402–10.
26. American Cancer Society. Facts and figures 2015.
27. Michaelson-Cohen R, Beller U. Managing menopausal symptoms after gynecological cancer. Curr Opin Oncol 2009;21:407–11.
28. Biglia N Gadducci A, Ponzone R. Hormone replacement therapy in cancer survivors. Maturitas 2004;48:333–46.
29. Ploch E. Hormonal replacement therapy in patients after cervical cancer treatment. Gynecol Oncol 1987;26:169–77.
30. Guidozzi F, Daponte A. Estrogen replacement therapy for ovarian carcinoma survivors. Cancer 1999;86:1013–8.
31. Creasman WT. Hormone replacement therapy after cancers. Curr Opin Oncol 2005;17:493–9.
32. Bebar S, Ursic-Vrscaj M. Hormone replacement therapy after epithelial ovarian cancer treatment. Eur J Gynaecol Oncol 2000;21:192–6.
33. Wen Y, Huang H, Huang H, et al. The safety of postoperative hormone replacement therapy in epithelial ovarian cancer patients in China. Climacteric 2013;16:673–81.
34. Creasman WT, Henderson D, Hinshaw W, et al. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol 1986;67:326–30.
35. Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2006;24:587–92.
36. Lee K-B, Lee J-M, Lee J-K, et al. Endometrial cancer patients and tibolone: A matched case–control study. Maturitas 2006;55:264–9.
37. Kenemans P, Bundred NJ, Foidart J-M, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol 2009;10:135–46.
38. Al-Baghdadi O, Ewies AAA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 2009;12:91–105.
39. Galuppi A, Perrone AM, La Macchia M, et al. Local α-tocopherol for acute and short-term vaginal toxicity prevention in patients treated with radiotherapy for gynecologic tumors. Int J Gynecol Cancer 2011;21:1708–11.
40. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20.
41. Bø K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol 2012;30:437–43.
42. Dumoulin C, Hay-Smith EJC, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014;5:CD005654.
43. Rosenbaum TY. Pelvic floor involvement in male and female sexual dysfunction and the role of pelvic floor rehabilitation in treatment: A literature review. J Sex Med 2007;4:4–13.
44. Yang EJ, Lim J-Y, Rah UW, et al. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol 2012;125:705–11.
45. Johnson N, Miles TP, Cornes P. Dilating the vagina to prevent damage from radiotherapy: systematic review of the literature. BJOG 2010;117:522–31.
46. Friedman LC, Abdallah R, Schluchter M, et al. Adherence to vaginal dilation following high dose rate brachytherapy for endometrial cancer. Int J Radiat Oncol Biol Phys 2011;80:751–7.
47. Cullen K, Fergus K, DasGupta T, et al. From “sex toy” to intrusive omposition: a qualitative examination of women’s experiences with vaginal dilator use following treatment for gynecological cancer. J Sex Med 2012;9:1162–73.
48. Robinson JW, Faris PD, Scott CB. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynecological carcinoma treated with radiotherapy. Int J Radial Oncol Biol Phys 1999;44:497–506.
49. Stavraka C, Ford A, Ghaem-Maghami S, et al. A study of symptoms described by ovarian cancer survivors. Gynecol Oncol 2012;125:59–64.
50. Moonsammy SH, Guglietti CL, Mina DS, et al. A pilot study of an exercise & cognitive behavioral therapy intervention for epithelial ovarian cancer patients. J Ovarian Res 2013;6:21.
51. Goerling U, Jaeger C, Walz A, et al. The efficacy of short-term psycho-oncological interventions for women with gynaecological cancer: a randomized study. Oncology 2014;87:114–24.
52. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol 2012;125:320–5.
53. Cleary V, McCarthy G, Hegarty J. Development of an educational intervention focused on sexuality for women with gynecological cancer. J Psychosoc Oncol 2012;30:535–55.
54. Miller BE, Pittman B, Strong C. Gynecologic cancer patients’ psychosocial needs and their views on the physician›s role in meeting those needs. Int J Gynecol Cancer 2003;13:111–9.
55. Fang P, Tan K, Grover S, et al. Psychosocial encounters correlates with higher patient-reported functional quality of life in gynecological cancer patients receiving radiotherapy. Radiat Oncol 2015;10:34.
56. Hordern A, Grainger M, Hegarty S, et al. Discussing sexuality in the clinical setting: The impact of a brief training program for oncology health professionals to enhance communication about sexuality. Asia Pac J Clin Oncol 2009;5:270–7.
57. Simonelli LE, Pasipanodya E. Health disparities in unmet support needs of women with gynecologic cancer: an exploratory study. J Psychosoc Oncol 2014;32:727–34.
58. Barbera L, Fitch M, Adams L, et al. Improving care for women after gynecological cancer: the development of a sexuality clinic. Menopause 2011;18:1327–33.
59. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578–83.
60. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000;356:2059–63.
61. Wu C-S, Lu M-L, Liao Y-T, et al. Ovarian cancer and antidepressants. Psychooncology 2015;24:579–84.
62. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol 2009;29:259–66.
63. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011;305:267–74.
64. Reed SD, Guthrie KA, Joffe H, et al. Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2012;119:527–38.
65. Pérez DG, Loprinzi CL, Barton DL, et al. Pilot evaluation of mirtazapine for the treatment of hot flashes. J Support Oncol 2004;2:50–6.
66. Pérez DG, Loprinzi CL, Sloan J, et al. Pilot evaluation of bupropion for the treatment of hot flashes. J Palliat Med 2006;9:631–7.
67. Agarwal N, Singh S, Kriplani A, et al. Evaluation of gabapentin in management of hot flushes in postmenopausal women. Post Reprod Health 2014;20:36–8.
68. Guttuso T Jr, Kurlan R, McDermott MP, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337–45.
69. Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2011;29:3862–8.
70. Loibl S, Schwedler K, von Minckwitz G, et al. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients--a double-blind, randomized study. Ann Oncol 2007;18:689–93.
71. Aerts L, Enzlin P, Verhaeghe J, et al. Long-term sexual functioning in women after surgical treatment of cervical cancer stages IA to IB: a prospective controlled study. Int J Gynecol Cancer 2014;24:1527–34.
72. Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology 2007;16:691–706.
73. Pfaendler KS, Wenzel L, Mechanic MB, et al. Cervical cancer survivorship: long-term quality of life and social support. Clin Ther 2015;37:39–48.
74. Becker M, Malafy T, Bossart M, et al. Quality of life and sexual functioning in endometrial cancer survivors. Gynecol Oncol 2011;121:169–73.
75. Bifulco G, De Rosa N, Tornesello ML, et al. Quality of life, lifestyle behavior and employment experience: a comparison between young and midlife survivors of gynecology early stage cancers. Gynecol Oncol 2012;124:444–51.
76. Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause 2011;18:662–9.
77. American Cancer Society. Sexuality for the woman with cancer. 2013. Available at www.cancer.org/treatment/treatmentsandsideeffects/physicalsideeffects/sexualsideeffectsinwomen/sexualityforthewoman/index.
From the Yale School of Medicine, New Haven, CT.
Abstract
- Objective: To review the sexual health concerns of women with gynecologic cancer and provide guidance for primary care physicians.
- Methods: Review of the literature.
- Results: Issues of sexuality and intimacy are known to significantly impact the quality of life of patients following diagnosis and treatment of gynecologic cancers. At the time of diagnosis, women should be informed of the potential physiologic, hormonal, and psychosocial effects of gynecologic cancer on sexuality. Many providers fail to address these issues given time constraints and patients’ trepidation in alerting their providers to their concerns. While systemic hormone therapy directly addresses these symptoms, its use remains controversial due to potential cancer recurrence risks. Thus, treatment centers around therapeutic alternatives. For vasomotor symptoms, selective serotonin reuptake inhibitors have shown effectiveness and are typically well tolerated, and antiepileptics such as gabapentin have shown promise. There is promising but limited data employing pelvic floor physical therapy as a tool to aid in addressing pelvic floor symptoms. Psychological care and the involvement of the partner are also part of managing the sexual health concerns of these patients.
- Conclusion: Sexual morbidity is a distressing and undertreated problem among gynecologic cancer survivors. Successful treatment requires the provider’s appreciation of the problem and willingness to address it.
Issues of sexuality and intimacy are known to significantly impact the lives of patients following diagnosis and treatment of gynecologic cancers. [1,2] Treatment of gynecologic malignancy is highly dependent on pathology and stage, with some patients receiving small excisional procedures while others subject to extensive surgeries, chemotherapy, and radiation treatment, and it is difficult to predict how each individual’s sexual health will be impacted. However, the evidence suggests that at least half of women treated for gynecologic cancer will experience sexual dysfunction [3]. Although the impact of gynecologic cancer and treatment can be profound, providers often do not address their patients’ sexual concerns [4,5], yet most patients have indicated that they would like these issues to be addressed [6].
Female Sexual Dysfunction
Sexual dysfunction is multifactorial and involves physical, social, and psychological dimensions. It is common in the general population, with rates ranging from 25-63% [7]. The DSM-IV [8] defines female sexual dysfunction as a disturbance in or pain during the sexual response, which can be further classified as hypoactive sexual disorder, orgasmic disorder, sexual pain disorder, or sexual arousal disorder [9]. It should be noted that women who have been treated for gynecologic cancers may have a premorbid history of sexual dysfunction. Assessment of a woman’s sexual function prior to her cancer diagnosis can help establish which sexuality changes are due to the cancer treatment and may allow the provider to tailor interventions accordingly.
Sexual Dysfunction and Quality of Life
As treatments for gynecologic cancers improve, toxicity of treatment decreases, and survival increases, quality of life for survivors has become as become an increasingly important health issue. Several studies have examined patient-reported quality of life in short- and long-term cancer survivors and report overall significant alteration in quality of life over many aspects of health and psychological well-being [10]. It is well established that health-related quality of life and sexual functioning are closely associated [11].
In gynecologic cancer patients, sexual and quality of life outcomes can vary. For example, Kim el al recently compared quality of life and sexual functioning in ovarian cancer survivors with no evidence of disease after primary treatment and a cohort of health women. Sexuality, both in terms of desire, arousal, lubrication, orgasm, satisfaction, and pain and in terms of interest in sex, sexual activity, and enjoyment of sex were similar between the groups; however social functioning deteriorated in cancer patients [12]. Other women report different experiences. Women with a history of vulvar, vaginal, or cervical cancer who may have had extensive pelvic surgery, lymphadenectomy, and radiation treatment to the pelvis typically report greater alterations in quality of life and sexual activity depending on the extent of their treatment [13].
Patients undergoing chemotherapy often report significant changes in quality of life due to the physical symptoms of fatigue, nausea, hair loss, diarrhea, etc. as well the psychological effects of cancer diagnosis, changes in body image, and poor coping [14]. Sexual side effects are common due to alterations in the HPA axis, direct gonadal toxicity and neuropathies [15].
Impact of Gynecologic Cancer on Sexuality
Gynecologic cancer and treatment can alter sexuality through a variety of effects. The impact of anatomical changes may alter a women’s self-esteem, body image, and sense of femininity, resulting in a reluctance to engage in intimate behaviors. Furthermore, if the partnership is disrupted by changes in roles in the household or within the relationship, relationships dynamics may be tested and result in mental distress for the patient, thus effecting sexuality and intimacy.
Surgical or chemical withdrawal of sex hormones, new medications, and postoperative sequelae can contribute to sexual arousal problems. The emotional and psychological components of a cancer diagnosis also can hinder the sexual response [16]. Depression and anxiety, the rates of which increase with cancer diagnosis, can also significantly affect sexual function [17].
Sexual pain is common in this population due to hormonal considerations as well as post-treatment side effects and a frequent cause of sexual dysfunction for these patients. Pelvic radiation may result in adverse physical changes to the vagina including vaginal stenosis, thinning of vaginal mucosa, loss of lubrication, and loss of elasticity [18,19]. A recent review of 20 studies of cervical cancer survivors found that patients were at risk for lack of lubrication and had high rates of dyspareunia [20]. Psychosocial factors have been found to be important predictors of sexual desire and more important than hormones in predicting low sexual desire in middle age [21]. These factors include emotional and physical closeness to the partner, satisfactory communication, and a positive relation to one’s own body [16].
Effects on Relationships and Partners
Women whose sexual capacity is compromised may be worried about their partners’ quality of life and overall well-being. Indeed, the partners of women with gynecologic cancer are also impacted by the changes to sexual function and loss of sexuality and intimacy. Hawkins et al found that cessation or decreased frequency of sex and intimacy was reported in 79% of male partners of women affected by cancer. Among partners of the persons with cancer in this study, changes to sexuality were associated with feelings of self-blame, reflection, sadness, anger and lack of sexual fulfillment [22]. Further, male partners of women diagnosed with gynecological cancer often express conflicting emotional states including feeling worried about their significant other’s health, having the desire to engage in sexual activity, and feeling guilty about wanting to increase sexual intimacy. These feelings, in turn, can lead to resentment and withdrawal from their partner and overall relationship discord [23].
Evidence suggests partners of cancer patients greatly benefit from increased social support even years after an apparent cure [24]. Specifically, male partners who take on a new role as caregiver in the relationship experience difficulties with emotional changes, challenges to their masculinity, and new stressors [25]. These new roles and feelings also contribute to changes in sexuality and intimacy in relationships, making support for partners all the more necessary.
Menopausal Symptoms Following Cancer Treatment
Gynecologic cancer treatment invariably affects the female hormonal balance, sometimes suddenly with surgical excision of the gonads or via radiation treatment or chemotherapy. It is well known that the sudden withdrawal of estrogen and testosterone, especially in the premenopausal postoperative population, can lead to significant acute menopausal symptoms. Given the many emotional and physical issues affecting patients during treatment, it can be difficult to delineate what proportion of sexual problems are caused by or enhanced by vasomotor symptoms, sleep disorders, and vaginal atrophy.
In general, premenopausal women who experience abrupt surgical menopause may often have immediate severe symptoms. Many agree that the younger a woman is when going through this process, the more severe her symptoms may be. Although the average age of endometrial cancer diagnosis is 67, approximately 25% of women who are diagnosed are premenopausal, and 5% of cases occur in women under 40. As the obesity epidemic worsens and more women are exposed to higher levels of estrogen at younger ages, it is expected that the number of premenopausal women who are diagnosed will continue to rise. The average age of diagnosis for ovarian cancer is 63, however, there is a large cohort of women diagnosed with borderline and malignant tumors in the premenopausal period [26]. These patients often experience vasomotor symptoms in the hours to days following surgery.
Interventions for Survivors of Gynecologic Cancer with Sexual Dysfunction
Systemic Hormone Therapy
Systemic hormonal therapy to treat menopausal symptoms remains controversial following the release of findings from the Women’s Health Initiative, which showed a number of adverse effects, including an increased risk of breast cancer in healthy postmenopausal women who received systemic hormonal therapy for menopausal symptoms. While views have changed since that time, providers are often reluctant to prescribe hormonal therapy, and patients are reticent to take it, due to fears of cancer recurrence. However, there is no evidence showing hormones negatively influence survival after treatment for epithelial ovarian, squamous cervical, or vulvar cancer [27]. With the exception of endometrial cancer, there is no biological evidence that HRT may increase recurrence risk [28]. Approach to clinical decision making should be individualized, taking into consideration the patients’ symptoms, quality of life, tumor histology, and overall prognosis.
Cervical cancer, vulvar cancer, and vaginal cancer are not considered hormonally responsive tumors. While the data are limited, a study published in 1987 of cervical cancer survivors treated with systemic hormone replacement therapy showed no increase in relapse rates and showed an increase in quality of life [29]. There are no studies regarding systemic HRT in patients with vulvar or vaginal cancer though it is generally accepted they can be treated with HRT. No significant data exists for cervical adenocarcinoma patients, and most oncologists suggest treating these patients the same as those with endometrial cancer primary.
There is limited data on the use of HRT in women with ovarian cancer but available studies do not show any difference in overall or disease-free survival between HRT groups and controls [27,30–32]. Many studies report a significant increase in quality of life with HRT. A 2013 retrospective chart review of 77 patients with epithelial ovarian cancer who received postoperative HRT showed no significant difference in progression-free survival [33].
The most controversy surrounds the use of HRT in patients with endometrial cancer [34]. The only major data available come from the Gynecologic Oncology Group’s truncated study, which was a large prospective randomized controlled trial. Over 1200 women treated for stage I and II endometrial cancer were enrolled between 1997 and 2003, and before the study was terminated these patients were followed for a median of 3 years following initiation of therapy. The recurrence rate of malignancy was low, 2.1, and an insignificant risk of recurrence of 1.27 was noted between with HRT and placebo groups (80% CI, 0.916-1.77) [35]. Women included in this study were between the ages of 26 and 91, and their indications for therapy included vasomotor symptoms, vaginal atrophy, as well as osteoporosis risk and cardiovascular disease risk. Gynecologist oncologists often use estrogen therapy to treat symptomatic women with early stage endometrial cancer given the very low risk of recurrence.
Tibolone is a synthetic steroid with activity on estrogen and progesterone receptors, and mainly acts as an agonist at estrogen receptors. It is prescribed outside the United States for osteoporosis and is being investigated as treatment for female sexual dysfunction. Efficacy on vasomotor symptoms has been positive thus far. One case-control study confirmed tibolone’s safety for endometrial cancer survivors, with no adverse effects on disease-free or overall survival [36]. One group recently examined tibolone in the setting of breast cancer patients in a prospective randomized controlled trial and reported an increased risk of breast cancer recurrences in women receiving tibolone for HT [37]. That study reported no increase in risk of gynecologic cancers and did report favorable outcomes for patients in terms of osteoporosis and vasomotor symptoms.
Topical Therapy
Women who are concerned about systemic HRT but who have been treated with radiation in addition to their surgery may be interested in topical estrogen therapy. These women may have vaginal stenosis and atrophic symptoms, and for them topical estrogen therapy can be helpful [38]. Many formulations of topical estrogen are available, including creams, tablets, and rings. Using vaginal estrogens and dilators can be useful to help with eventual resumption of sexual activity once healing has taken place and can help to avoid dyspareunia. Combination topical and systemic therapy can also be useful to relieve symptoms. Women concerned about absorption with topical therapy can be advised that while there is some absorption initially, absorption is reduced as atrophy is improved with treatment.
One recent study examined the use of alpha-tocopherol to reduce acute vaginal complications in women with endometrial and cervical cancer undergoing radiation treatment. The treatment group experienced reduced vaginal toxicity and pain, although vaginal secretion was not significantly different in the 2 groups studied [39]. No adverse effects were noted. This compound has not been studied further but may be beneficial in the future.
Some women may prefer to avoid hormones altogether. Over-the-counter vaginal moisturizers and lubricants can be recommended to women to help with intercourse and atrophic symptoms, with or without estrogen therapy.
Physical Therapy
Pelvic floor physical therapy has become an increasingly popular modality for treatment of many aspects pelvic floor dysfunction, the symptoms of which include defecatory dysfunction, constipation, bladder dysfunction, painful urination, dyspareunia, pelvic pain, and low back pain [40]. Pelvic floor physical therapy is well studied and utilized frequently for urinary incontinence and pelvic organ prolapse, but is understudied for sexual dysfunction [41,42].
Promising areas of study include educational sessions, cognitive behavioral therapy, vaginal dilator therapy, pelvic floor muscle strengthening and relaxation techniques with biofeedback, stretching and massage [40]. Biofeedback specifically has been studied in controlled studies for treatment of sexual dysfunction specifically in the setting of vulvar pain syndromes [43]. A small randomized control trial evaluated the use of pelvic floor muscle training in gynecologic cancer survivors and found that the intervention group reported improved sexual function and improved quality of life [44]. Given the few side effects of this therapy, it may be a helpful addition to a multidisciplinary approach to treating pelvic floor dysfunction in gynecologic cancer patients.
For cervical and endometrial cancer patients, vaginal dilators are often prescribed following radiation therapy to minimize vaginal stenosis. Literature suggests that after radiotherapy dilation therapy is effective when used as directed, typically 3 times per week for 10 minutes to mechanically separate the vaginal walls and increase elasticity [45]. Adherence to recommendations for vaginal dilator use is low in this population due to factors such as aversion to the practice and intrusiveness of the mechanism [46,47]. Acknowledgment of apprehension regarding dilator use should be part of the counseling prior to initiation of treatment; interventions designed to educate women about dilator use benefit may increase adherence [48].
Psychological Therapies
Survivors of gynecological cancer with sexual dysfunction may experience the psychological symptoms in the context of the other emotional challenges of a cancer diagnosis [49]. Changes in body image, physical appearance, and feelings of well-being can significantly affect sexuality and intimacy, while anxiety and negative feelings about a diagnosis can cause further sexual dysfunction. Conversely, sexual morbidity in these patients predicts worsening of general psychological symptoms, including depressive symptoms and overall quality of life [13–15].
While there are limited studies directly assessing the effect of psychological therapies for the treatment of sexual dysfunction in this population, the existing literature shows enduring symptomatic improvement following brief interventions. In women for whom emotional distress, depression, and anxiety appear to be a significant aspect of their concern regarding intimacy and sexuality, these interventions can be particularly helpful.
Cognitive behavioral therapy (CBT), which focuses on mindfulness and the relationships between maladaptive thoughts and how they impact behavior, has been shown to have efficacy in treating the broader psycho-social concerns of gynecological cancer patients [50,51]. In a recent study Brotto and colleagues randomized 31 survivors of gynecological cancer with self-reported sexual dysfunction to either three 90-minute CBT sessions or a waitlist control. Patients who underwent the intervention reported significant improvements in sexual arousal and desire both immediately post-therapy and at 6-month follow-up while patients in the waitlist arm experienced no significant changes in symptomatology [52].
Psychoeducational interventions are another promising avenue for addressing sexual dysfunction in this population [53]. In a 2008 therapeutic trial, Brotto and colleagues combined elements of CBT with education in 3 one-hour sessions featuring written materials on sexuality and relationships. The intervention enrolled 22 women with early stage gynecologic cancer who met criteria for female sexual arousal disorder. The psychoeducational intervention was associated with positive effect on sexual desire, arousal, orgasm, satisfaction, sexual distress, depression, and overall well-being [16]. Psychoeducation can also be used to augment other therapeutic modalities. Robinson and colleagues used such an intervention to improve adherence to vaginal dilator use in 32 women with early stage endometrial cancer who were being treated with radiotherapy [48].
Some gynecologic cancer patients may have significant alterations in their anatomy and thus penetrative intercourse may not be possible. In patients even without these physical changes, some may prefer to avoid intercourse due to pain or anxiety. Thus patients can have significant benefits from discussing their concerns with a therapist specializing in these issues as many patients are concerned about their ability to engage in intimate behavior. Therapy can assist with the changing sexual relationship and assist the partners in different ways of engaging in intimate acts. It is important to avoid stressing penetrative intercourse as the goal for sexual function with these or any patients with anxiety relating to their disease as there are many ways to engage in mutually pleasurable experiences for both partners, thus removing anxiety about inability to resume vaginal intercourse post-treatment. Discussing this with patients can be challenging but can often reduce anxiety surrounding body image issues following treatment.
Studies have shown that cancer care providers often do not adequately address sexual concerns [54] but that when these concerns are appropriately managed, patient satisfaction and quality of life significantly improve [55]. Several studies have focused on how providers can incorporate the approaches of CBT and psychoeducation to better address the sexual concerns of patients without requiring external psychiatric care [56,57]. Barbera et al have described a successful model in which multidisciplinary care teams provide education and counseling for gynecological cancer patients in a sexuality clinic [58]. patients had a significant symptom burden, including menopausal symptoms, the effects of radiation therapy, chemotherapy, and surgical operation as well as psychological responses to cancer, and reported high levels of satisfaction with their experience at the clinic.
Involvement of the partner in interventions has not been well studied; however, involving the partner in in psychological therapies to address sexual dysfunction should be beneficial.
Alternative Therapies for Vasomotor Symptoms
Gynecologic cancer patients suffering prominent vasomotor symptoms have limited alternatives to hormone therapy. Clinicians must balance potential medication benefit with potential exacerbation of other medical and psychological issues, including sexual dysfunction.
Antidepressants
The use of SSRIs and SNRIs for vasomotor symptoms was pioneered by medical oncologists for men with hot flashes secondary to GnRH agonist therapy for prostate cancer and women with breast cancer [59,60]. Limited studies have shown that antidepressant medications do not increase cancer recurrence risk in ovarian cancer patients and are relatively well tolerated [61]. However, these medications are known to have partial efficacy in improving vasomotor symptoms and may worsen sexual symptoms, a well-known side effect of antidepressants. There is variation of the reported rates of sexual dysfunction associated with various antidepressants and clinicians may take the likelihood of sexual side effects into account when prescribing SSRIs or SNRIS [62]. More recently developed SSRIS, such as citalopram and its enantiomer escitalopram, have shown significant improvements in vasomotor symptoms and were better tolerated than venlafaxine and fluoxetine [63,64]. Additionally, limited uncontrolled studies of mirtazipine, a structurally unique SSRI, and bupropion, which acts on dopamine and norepinephrine, have shown significant decreases in hot flash symptoms and are less associated with sexual side effects than SSRIS/SNRIS [65,66].
Other Agents
Other pharmaceutical options for menopausal vasomotor symptoms include gabapentin and adrenergic agonists. Gabapentin can yield impressive reductions in vasomotor symptoms. A recent double-blind randomized trial in 50 patients revealed a 60% reduction in hot flashes as 12 weeks and an 80% reduction in self-reported composite symptom scores [67]. However, side effects such as palpitations, edema, and fatigue, lead to high study withdrawal rates and limit its widespread clinical use for this indication [68]. Clonidine has been assessed versus venlafaxine in several clinical trials with breast cancer patients. These trials have shown mixed results, with findings of both inferiority and superiority to venlafaxine, but with consistent significant improvement in symptoms over placebo. Side effects, such insomnia, constipation and dry mouth, occurred but did not lead to higher dropout rates than venlafaxine [69,70].
Long-Term Sexual Outcomes
For women treated for gynecological cancers, alterations in sexual function may persist in the long term. A study following cervical cancer patients managed with radical hysterectomy up to 2 years post treatment showed they had more sexual dysfunction compared with healthy controls, although at rates similar to those who underwent radical hysterectomy for benign disease [71]. A 2007 review of quality of life studies revealed that although ovarian cancer survivors 5 years past diagnosis had excellent overall quality of life, sexual symptoms persisted, with as many as 57% of patients reporting a decline in sexual function due to their cancer [72].
Studies show some differences in outcomes based on treatment modality. A recent review of cervical cancer outcomes revealed that women who received radiotherapy as a component of their treatment have a higher risk of long-term sexual side effects [73]. In contrast, a study assessing endometrial cancer patients 5 years after initial diagnosis between those patients who had received surgery alone and those who had received surgery and vaginal brachytherapy. There was no significant difference in any measures of quality of life and sexual function between these 2 groups [74].
Age appears to play a role in long-term sexual outcomes regardless of diagnosis. Bifulco and colleagues assessed quality of life in survivors of gynecological cancer, comparing women under age 45 to those over 45 after nearly 3 years of survival. After controlling for age and other factors, younger patients were found to have worse sexual activity, including significantly higher rates of poor body image, perceived worse sexual vaginal function, and more severe menopausal symptoms, probably related to the effects of surgical menopause [75].
Despite enduring sexual dysfunction, symptoms tend to improve over time. A cohort study of 103 gynecological cancer patients undergoing radiation therapy were followed for 3 years. Patients were offered standard interventions for sexual dysfunction, including vaginal lubricants, dilators, and menopausal symptomatic therapy, although adherence to these measures was not assessed. Three years after initial therapy, the percentage of sexually active women increased from 21.5% to 44.2% [76]. In the subset of patients who successfully return to sexual activity, outcomes can be comparable to healthy peers. Kim and colleagues compared disease-free sexually active ovarian cancer patients with demographically matched healthy controls on standardized self-report measures. Sexual functioning did not differ between the 2 groups, despite lower social functioning in cancer survivors [12].
Conclusion
Sexuality and intimacy can be greatly affected by the diagnosis and treatment of gynecologic malignancies. It is important to routinely discuss sexuality and sexual functioning with patients from diagnosis onward. Reassuring patients, acknowledging the importance of their concerns, and validating their desire to enjoy improved intimacy should be considered part of the clinician’s role. Valuable information sources that may aid discussions are available on the internet. Oncolink (www.oncolink.org), a large cancer information website maintained by University of Pennsylvania Cancer Center, offers a plethora of information for patients and health care professionals. In addition, the American Cancer Society offers a detailed guide, “Sexuality for the Woman with Cancer” [77]. Treatment is available, and improvement in outcomes is possible. Further prospective studies are needed to clearly delineate risks and benefits of hormone replacement therapy in patients with gynecologic cancers.
Corresponding author: Elena S. Ratner, MD, PO Box 208063, New Haven, CT 06520, [email protected].
From the Yale School of Medicine, New Haven, CT.
Abstract
- Objective: To review the sexual health concerns of women with gynecologic cancer and provide guidance for primary care physicians.
- Methods: Review of the literature.
- Results: Issues of sexuality and intimacy are known to significantly impact the quality of life of patients following diagnosis and treatment of gynecologic cancers. At the time of diagnosis, women should be informed of the potential physiologic, hormonal, and psychosocial effects of gynecologic cancer on sexuality. Many providers fail to address these issues given time constraints and patients’ trepidation in alerting their providers to their concerns. While systemic hormone therapy directly addresses these symptoms, its use remains controversial due to potential cancer recurrence risks. Thus, treatment centers around therapeutic alternatives. For vasomotor symptoms, selective serotonin reuptake inhibitors have shown effectiveness and are typically well tolerated, and antiepileptics such as gabapentin have shown promise. There is promising but limited data employing pelvic floor physical therapy as a tool to aid in addressing pelvic floor symptoms. Psychological care and the involvement of the partner are also part of managing the sexual health concerns of these patients.
- Conclusion: Sexual morbidity is a distressing and undertreated problem among gynecologic cancer survivors. Successful treatment requires the provider’s appreciation of the problem and willingness to address it.
Issues of sexuality and intimacy are known to significantly impact the lives of patients following diagnosis and treatment of gynecologic cancers. [1,2] Treatment of gynecologic malignancy is highly dependent on pathology and stage, with some patients receiving small excisional procedures while others subject to extensive surgeries, chemotherapy, and radiation treatment, and it is difficult to predict how each individual’s sexual health will be impacted. However, the evidence suggests that at least half of women treated for gynecologic cancer will experience sexual dysfunction [3]. Although the impact of gynecologic cancer and treatment can be profound, providers often do not address their patients’ sexual concerns [4,5], yet most patients have indicated that they would like these issues to be addressed [6].
Female Sexual Dysfunction
Sexual dysfunction is multifactorial and involves physical, social, and psychological dimensions. It is common in the general population, with rates ranging from 25-63% [7]. The DSM-IV [8] defines female sexual dysfunction as a disturbance in or pain during the sexual response, which can be further classified as hypoactive sexual disorder, orgasmic disorder, sexual pain disorder, or sexual arousal disorder [9]. It should be noted that women who have been treated for gynecologic cancers may have a premorbid history of sexual dysfunction. Assessment of a woman’s sexual function prior to her cancer diagnosis can help establish which sexuality changes are due to the cancer treatment and may allow the provider to tailor interventions accordingly.
Sexual Dysfunction and Quality of Life
As treatments for gynecologic cancers improve, toxicity of treatment decreases, and survival increases, quality of life for survivors has become as become an increasingly important health issue. Several studies have examined patient-reported quality of life in short- and long-term cancer survivors and report overall significant alteration in quality of life over many aspects of health and psychological well-being [10]. It is well established that health-related quality of life and sexual functioning are closely associated [11].
In gynecologic cancer patients, sexual and quality of life outcomes can vary. For example, Kim el al recently compared quality of life and sexual functioning in ovarian cancer survivors with no evidence of disease after primary treatment and a cohort of health women. Sexuality, both in terms of desire, arousal, lubrication, orgasm, satisfaction, and pain and in terms of interest in sex, sexual activity, and enjoyment of sex were similar between the groups; however social functioning deteriorated in cancer patients [12]. Other women report different experiences. Women with a history of vulvar, vaginal, or cervical cancer who may have had extensive pelvic surgery, lymphadenectomy, and radiation treatment to the pelvis typically report greater alterations in quality of life and sexual activity depending on the extent of their treatment [13].
Patients undergoing chemotherapy often report significant changes in quality of life due to the physical symptoms of fatigue, nausea, hair loss, diarrhea, etc. as well the psychological effects of cancer diagnosis, changes in body image, and poor coping [14]. Sexual side effects are common due to alterations in the HPA axis, direct gonadal toxicity and neuropathies [15].
Impact of Gynecologic Cancer on Sexuality
Gynecologic cancer and treatment can alter sexuality through a variety of effects. The impact of anatomical changes may alter a women’s self-esteem, body image, and sense of femininity, resulting in a reluctance to engage in intimate behaviors. Furthermore, if the partnership is disrupted by changes in roles in the household or within the relationship, relationships dynamics may be tested and result in mental distress for the patient, thus effecting sexuality and intimacy.
Surgical or chemical withdrawal of sex hormones, new medications, and postoperative sequelae can contribute to sexual arousal problems. The emotional and psychological components of a cancer diagnosis also can hinder the sexual response [16]. Depression and anxiety, the rates of which increase with cancer diagnosis, can also significantly affect sexual function [17].
Sexual pain is common in this population due to hormonal considerations as well as post-treatment side effects and a frequent cause of sexual dysfunction for these patients. Pelvic radiation may result in adverse physical changes to the vagina including vaginal stenosis, thinning of vaginal mucosa, loss of lubrication, and loss of elasticity [18,19]. A recent review of 20 studies of cervical cancer survivors found that patients were at risk for lack of lubrication and had high rates of dyspareunia [20]. Psychosocial factors have been found to be important predictors of sexual desire and more important than hormones in predicting low sexual desire in middle age [21]. These factors include emotional and physical closeness to the partner, satisfactory communication, and a positive relation to one’s own body [16].
Effects on Relationships and Partners
Women whose sexual capacity is compromised may be worried about their partners’ quality of life and overall well-being. Indeed, the partners of women with gynecologic cancer are also impacted by the changes to sexual function and loss of sexuality and intimacy. Hawkins et al found that cessation or decreased frequency of sex and intimacy was reported in 79% of male partners of women affected by cancer. Among partners of the persons with cancer in this study, changes to sexuality were associated with feelings of self-blame, reflection, sadness, anger and lack of sexual fulfillment [22]. Further, male partners of women diagnosed with gynecological cancer often express conflicting emotional states including feeling worried about their significant other’s health, having the desire to engage in sexual activity, and feeling guilty about wanting to increase sexual intimacy. These feelings, in turn, can lead to resentment and withdrawal from their partner and overall relationship discord [23].
Evidence suggests partners of cancer patients greatly benefit from increased social support even years after an apparent cure [24]. Specifically, male partners who take on a new role as caregiver in the relationship experience difficulties with emotional changes, challenges to their masculinity, and new stressors [25]. These new roles and feelings also contribute to changes in sexuality and intimacy in relationships, making support for partners all the more necessary.
Menopausal Symptoms Following Cancer Treatment
Gynecologic cancer treatment invariably affects the female hormonal balance, sometimes suddenly with surgical excision of the gonads or via radiation treatment or chemotherapy. It is well known that the sudden withdrawal of estrogen and testosterone, especially in the premenopausal postoperative population, can lead to significant acute menopausal symptoms. Given the many emotional and physical issues affecting patients during treatment, it can be difficult to delineate what proportion of sexual problems are caused by or enhanced by vasomotor symptoms, sleep disorders, and vaginal atrophy.
In general, premenopausal women who experience abrupt surgical menopause may often have immediate severe symptoms. Many agree that the younger a woman is when going through this process, the more severe her symptoms may be. Although the average age of endometrial cancer diagnosis is 67, approximately 25% of women who are diagnosed are premenopausal, and 5% of cases occur in women under 40. As the obesity epidemic worsens and more women are exposed to higher levels of estrogen at younger ages, it is expected that the number of premenopausal women who are diagnosed will continue to rise. The average age of diagnosis for ovarian cancer is 63, however, there is a large cohort of women diagnosed with borderline and malignant tumors in the premenopausal period [26]. These patients often experience vasomotor symptoms in the hours to days following surgery.
Interventions for Survivors of Gynecologic Cancer with Sexual Dysfunction
Systemic Hormone Therapy
Systemic hormonal therapy to treat menopausal symptoms remains controversial following the release of findings from the Women’s Health Initiative, which showed a number of adverse effects, including an increased risk of breast cancer in healthy postmenopausal women who received systemic hormonal therapy for menopausal symptoms. While views have changed since that time, providers are often reluctant to prescribe hormonal therapy, and patients are reticent to take it, due to fears of cancer recurrence. However, there is no evidence showing hormones negatively influence survival after treatment for epithelial ovarian, squamous cervical, or vulvar cancer [27]. With the exception of endometrial cancer, there is no biological evidence that HRT may increase recurrence risk [28]. Approach to clinical decision making should be individualized, taking into consideration the patients’ symptoms, quality of life, tumor histology, and overall prognosis.
Cervical cancer, vulvar cancer, and vaginal cancer are not considered hormonally responsive tumors. While the data are limited, a study published in 1987 of cervical cancer survivors treated with systemic hormone replacement therapy showed no increase in relapse rates and showed an increase in quality of life [29]. There are no studies regarding systemic HRT in patients with vulvar or vaginal cancer though it is generally accepted they can be treated with HRT. No significant data exists for cervical adenocarcinoma patients, and most oncologists suggest treating these patients the same as those with endometrial cancer primary.
There is limited data on the use of HRT in women with ovarian cancer but available studies do not show any difference in overall or disease-free survival between HRT groups and controls [27,30–32]. Many studies report a significant increase in quality of life with HRT. A 2013 retrospective chart review of 77 patients with epithelial ovarian cancer who received postoperative HRT showed no significant difference in progression-free survival [33].
The most controversy surrounds the use of HRT in patients with endometrial cancer [34]. The only major data available come from the Gynecologic Oncology Group’s truncated study, which was a large prospective randomized controlled trial. Over 1200 women treated for stage I and II endometrial cancer were enrolled between 1997 and 2003, and before the study was terminated these patients were followed for a median of 3 years following initiation of therapy. The recurrence rate of malignancy was low, 2.1, and an insignificant risk of recurrence of 1.27 was noted between with HRT and placebo groups (80% CI, 0.916-1.77) [35]. Women included in this study were between the ages of 26 and 91, and their indications for therapy included vasomotor symptoms, vaginal atrophy, as well as osteoporosis risk and cardiovascular disease risk. Gynecologist oncologists often use estrogen therapy to treat symptomatic women with early stage endometrial cancer given the very low risk of recurrence.
Tibolone is a synthetic steroid with activity on estrogen and progesterone receptors, and mainly acts as an agonist at estrogen receptors. It is prescribed outside the United States for osteoporosis and is being investigated as treatment for female sexual dysfunction. Efficacy on vasomotor symptoms has been positive thus far. One case-control study confirmed tibolone’s safety for endometrial cancer survivors, with no adverse effects on disease-free or overall survival [36]. One group recently examined tibolone in the setting of breast cancer patients in a prospective randomized controlled trial and reported an increased risk of breast cancer recurrences in women receiving tibolone for HT [37]. That study reported no increase in risk of gynecologic cancers and did report favorable outcomes for patients in terms of osteoporosis and vasomotor symptoms.
Topical Therapy
Women who are concerned about systemic HRT but who have been treated with radiation in addition to their surgery may be interested in topical estrogen therapy. These women may have vaginal stenosis and atrophic symptoms, and for them topical estrogen therapy can be helpful [38]. Many formulations of topical estrogen are available, including creams, tablets, and rings. Using vaginal estrogens and dilators can be useful to help with eventual resumption of sexual activity once healing has taken place and can help to avoid dyspareunia. Combination topical and systemic therapy can also be useful to relieve symptoms. Women concerned about absorption with topical therapy can be advised that while there is some absorption initially, absorption is reduced as atrophy is improved with treatment.
One recent study examined the use of alpha-tocopherol to reduce acute vaginal complications in women with endometrial and cervical cancer undergoing radiation treatment. The treatment group experienced reduced vaginal toxicity and pain, although vaginal secretion was not significantly different in the 2 groups studied [39]. No adverse effects were noted. This compound has not been studied further but may be beneficial in the future.
Some women may prefer to avoid hormones altogether. Over-the-counter vaginal moisturizers and lubricants can be recommended to women to help with intercourse and atrophic symptoms, with or without estrogen therapy.
Physical Therapy
Pelvic floor physical therapy has become an increasingly popular modality for treatment of many aspects pelvic floor dysfunction, the symptoms of which include defecatory dysfunction, constipation, bladder dysfunction, painful urination, dyspareunia, pelvic pain, and low back pain [40]. Pelvic floor physical therapy is well studied and utilized frequently for urinary incontinence and pelvic organ prolapse, but is understudied for sexual dysfunction [41,42].
Promising areas of study include educational sessions, cognitive behavioral therapy, vaginal dilator therapy, pelvic floor muscle strengthening and relaxation techniques with biofeedback, stretching and massage [40]. Biofeedback specifically has been studied in controlled studies for treatment of sexual dysfunction specifically in the setting of vulvar pain syndromes [43]. A small randomized control trial evaluated the use of pelvic floor muscle training in gynecologic cancer survivors and found that the intervention group reported improved sexual function and improved quality of life [44]. Given the few side effects of this therapy, it may be a helpful addition to a multidisciplinary approach to treating pelvic floor dysfunction in gynecologic cancer patients.
For cervical and endometrial cancer patients, vaginal dilators are often prescribed following radiation therapy to minimize vaginal stenosis. Literature suggests that after radiotherapy dilation therapy is effective when used as directed, typically 3 times per week for 10 minutes to mechanically separate the vaginal walls and increase elasticity [45]. Adherence to recommendations for vaginal dilator use is low in this population due to factors such as aversion to the practice and intrusiveness of the mechanism [46,47]. Acknowledgment of apprehension regarding dilator use should be part of the counseling prior to initiation of treatment; interventions designed to educate women about dilator use benefit may increase adherence [48].
Psychological Therapies
Survivors of gynecological cancer with sexual dysfunction may experience the psychological symptoms in the context of the other emotional challenges of a cancer diagnosis [49]. Changes in body image, physical appearance, and feelings of well-being can significantly affect sexuality and intimacy, while anxiety and negative feelings about a diagnosis can cause further sexual dysfunction. Conversely, sexual morbidity in these patients predicts worsening of general psychological symptoms, including depressive symptoms and overall quality of life [13–15].
While there are limited studies directly assessing the effect of psychological therapies for the treatment of sexual dysfunction in this population, the existing literature shows enduring symptomatic improvement following brief interventions. In women for whom emotional distress, depression, and anxiety appear to be a significant aspect of their concern regarding intimacy and sexuality, these interventions can be particularly helpful.
Cognitive behavioral therapy (CBT), which focuses on mindfulness and the relationships between maladaptive thoughts and how they impact behavior, has been shown to have efficacy in treating the broader psycho-social concerns of gynecological cancer patients [50,51]. In a recent study Brotto and colleagues randomized 31 survivors of gynecological cancer with self-reported sexual dysfunction to either three 90-minute CBT sessions or a waitlist control. Patients who underwent the intervention reported significant improvements in sexual arousal and desire both immediately post-therapy and at 6-month follow-up while patients in the waitlist arm experienced no significant changes in symptomatology [52].
Psychoeducational interventions are another promising avenue for addressing sexual dysfunction in this population [53]. In a 2008 therapeutic trial, Brotto and colleagues combined elements of CBT with education in 3 one-hour sessions featuring written materials on sexuality and relationships. The intervention enrolled 22 women with early stage gynecologic cancer who met criteria for female sexual arousal disorder. The psychoeducational intervention was associated with positive effect on sexual desire, arousal, orgasm, satisfaction, sexual distress, depression, and overall well-being [16]. Psychoeducation can also be used to augment other therapeutic modalities. Robinson and colleagues used such an intervention to improve adherence to vaginal dilator use in 32 women with early stage endometrial cancer who were being treated with radiotherapy [48].
Some gynecologic cancer patients may have significant alterations in their anatomy and thus penetrative intercourse may not be possible. In patients even without these physical changes, some may prefer to avoid intercourse due to pain or anxiety. Thus patients can have significant benefits from discussing their concerns with a therapist specializing in these issues as many patients are concerned about their ability to engage in intimate behavior. Therapy can assist with the changing sexual relationship and assist the partners in different ways of engaging in intimate acts. It is important to avoid stressing penetrative intercourse as the goal for sexual function with these or any patients with anxiety relating to their disease as there are many ways to engage in mutually pleasurable experiences for both partners, thus removing anxiety about inability to resume vaginal intercourse post-treatment. Discussing this with patients can be challenging but can often reduce anxiety surrounding body image issues following treatment.
Studies have shown that cancer care providers often do not adequately address sexual concerns [54] but that when these concerns are appropriately managed, patient satisfaction and quality of life significantly improve [55]. Several studies have focused on how providers can incorporate the approaches of CBT and psychoeducation to better address the sexual concerns of patients without requiring external psychiatric care [56,57]. Barbera et al have described a successful model in which multidisciplinary care teams provide education and counseling for gynecological cancer patients in a sexuality clinic [58]. patients had a significant symptom burden, including menopausal symptoms, the effects of radiation therapy, chemotherapy, and surgical operation as well as psychological responses to cancer, and reported high levels of satisfaction with their experience at the clinic.
Involvement of the partner in interventions has not been well studied; however, involving the partner in in psychological therapies to address sexual dysfunction should be beneficial.
Alternative Therapies for Vasomotor Symptoms
Gynecologic cancer patients suffering prominent vasomotor symptoms have limited alternatives to hormone therapy. Clinicians must balance potential medication benefit with potential exacerbation of other medical and psychological issues, including sexual dysfunction.
Antidepressants
The use of SSRIs and SNRIs for vasomotor symptoms was pioneered by medical oncologists for men with hot flashes secondary to GnRH agonist therapy for prostate cancer and women with breast cancer [59,60]. Limited studies have shown that antidepressant medications do not increase cancer recurrence risk in ovarian cancer patients and are relatively well tolerated [61]. However, these medications are known to have partial efficacy in improving vasomotor symptoms and may worsen sexual symptoms, a well-known side effect of antidepressants. There is variation of the reported rates of sexual dysfunction associated with various antidepressants and clinicians may take the likelihood of sexual side effects into account when prescribing SSRIs or SNRIS [62]. More recently developed SSRIS, such as citalopram and its enantiomer escitalopram, have shown significant improvements in vasomotor symptoms and were better tolerated than venlafaxine and fluoxetine [63,64]. Additionally, limited uncontrolled studies of mirtazipine, a structurally unique SSRI, and bupropion, which acts on dopamine and norepinephrine, have shown significant decreases in hot flash symptoms and are less associated with sexual side effects than SSRIS/SNRIS [65,66].
Other Agents
Other pharmaceutical options for menopausal vasomotor symptoms include gabapentin and adrenergic agonists. Gabapentin can yield impressive reductions in vasomotor symptoms. A recent double-blind randomized trial in 50 patients revealed a 60% reduction in hot flashes as 12 weeks and an 80% reduction in self-reported composite symptom scores [67]. However, side effects such as palpitations, edema, and fatigue, lead to high study withdrawal rates and limit its widespread clinical use for this indication [68]. Clonidine has been assessed versus venlafaxine in several clinical trials with breast cancer patients. These trials have shown mixed results, with findings of both inferiority and superiority to venlafaxine, but with consistent significant improvement in symptoms over placebo. Side effects, such insomnia, constipation and dry mouth, occurred but did not lead to higher dropout rates than venlafaxine [69,70].
Long-Term Sexual Outcomes
For women treated for gynecological cancers, alterations in sexual function may persist in the long term. A study following cervical cancer patients managed with radical hysterectomy up to 2 years post treatment showed they had more sexual dysfunction compared with healthy controls, although at rates similar to those who underwent radical hysterectomy for benign disease [71]. A 2007 review of quality of life studies revealed that although ovarian cancer survivors 5 years past diagnosis had excellent overall quality of life, sexual symptoms persisted, with as many as 57% of patients reporting a decline in sexual function due to their cancer [72].
Studies show some differences in outcomes based on treatment modality. A recent review of cervical cancer outcomes revealed that women who received radiotherapy as a component of their treatment have a higher risk of long-term sexual side effects [73]. In contrast, a study assessing endometrial cancer patients 5 years after initial diagnosis between those patients who had received surgery alone and those who had received surgery and vaginal brachytherapy. There was no significant difference in any measures of quality of life and sexual function between these 2 groups [74].
Age appears to play a role in long-term sexual outcomes regardless of diagnosis. Bifulco and colleagues assessed quality of life in survivors of gynecological cancer, comparing women under age 45 to those over 45 after nearly 3 years of survival. After controlling for age and other factors, younger patients were found to have worse sexual activity, including significantly higher rates of poor body image, perceived worse sexual vaginal function, and more severe menopausal symptoms, probably related to the effects of surgical menopause [75].
Despite enduring sexual dysfunction, symptoms tend to improve over time. A cohort study of 103 gynecological cancer patients undergoing radiation therapy were followed for 3 years. Patients were offered standard interventions for sexual dysfunction, including vaginal lubricants, dilators, and menopausal symptomatic therapy, although adherence to these measures was not assessed. Three years after initial therapy, the percentage of sexually active women increased from 21.5% to 44.2% [76]. In the subset of patients who successfully return to sexual activity, outcomes can be comparable to healthy peers. Kim and colleagues compared disease-free sexually active ovarian cancer patients with demographically matched healthy controls on standardized self-report measures. Sexual functioning did not differ between the 2 groups, despite lower social functioning in cancer survivors [12].
Conclusion
Sexuality and intimacy can be greatly affected by the diagnosis and treatment of gynecologic malignancies. It is important to routinely discuss sexuality and sexual functioning with patients from diagnosis onward. Reassuring patients, acknowledging the importance of their concerns, and validating their desire to enjoy improved intimacy should be considered part of the clinician’s role. Valuable information sources that may aid discussions are available on the internet. Oncolink (www.oncolink.org), a large cancer information website maintained by University of Pennsylvania Cancer Center, offers a plethora of information for patients and health care professionals. In addition, the American Cancer Society offers a detailed guide, “Sexuality for the Woman with Cancer” [77]. Treatment is available, and improvement in outcomes is possible. Further prospective studies are needed to clearly delineate risks and benefits of hormone replacement therapy in patients with gynecologic cancers.
Corresponding author: Elena S. Ratner, MD, PO Box 208063, New Haven, CT 06520, [email protected].
1. Juraskova I, Butow P, Robertson R, et al. Post-treatment sexual adjustment following cervical and endometrial cancer: a qualitative insight. Psychooncology 2003;12:267–79.
2. Bodurka DC, Sun CC. Sexual function after gynecologic cancer. Obstet Gynecol Clin North Am 2006;33:621–30, ix.
3. Andersen BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol 1997;65:221.
4. Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J 2009;15:74–7.
5. Stead ML, Brown JM, Fallowfield L, et al. Lack of communication between healthcare professionals and women with ovarian cancer about sexual issues. Br J Cancer 2003;88:666–71.
6. Stead ML, Fallowfield L, Brown JM, et al. Communication about sexual problems and sexual concerns in ovarian cancer: qualitative study. BMJ 2001;323:836–7.
7. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537–44.
8. American Psychiatric Association. Diagnostic and statistical manual of mental health disorders. 4th ed. 2000.
9. Elder JA, Braver Y. Female sexual dysfunction. In: Current clinical medicine. Elsevier; 2010:1222–6.
10. Ferrell BR, Dow KH, Leigh S, et al. Quality of life in long-term cancer survivors. Oncol Nurs Forum 1995;22:915–22.
11. Shamspour N, Assari S, Moghana Lankarani M. Relation between sexuality and health-related quality of life. In: Handbook of disease burdens and quality of life measures. New York: Springer 2010;3457–73.
12. Kim SI, Lee Y, Lim MC, et al. Quality of life and sexuality comparison between sexually active ovarian cancer survivors and healthy women. J Gynecol Oncol 2015;26:148–54.
13. Levin AO, Carpenter KM, Fowler JM, et al. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. Int J Gynecol Cancer 2010;20:461–70.
14. Lutgendorf SK, Anderson B, Rothrock N, et al. Quality of life and mood in women receiving extensive chemotherapy for gynecologic cancer. Cancer 2000;89:1402–11.
15. Tierney DK. Sexuality: a quality-of-life issue for cancer survivors. Semin Oncol Nurs 2008;24:71–9.
16. Brotto LA, Heiman JR, Goff B, et al. A psychoeducational intervention for sexual dysfunction in women with gynecologic cancer. Arch Sex Behav 2008;37:317–29.
17. Hollingsworth M, Berman J. The role of androgens in female sexual dysfunction. Sex Reprod Menopause 2006;4:27–32.
18. Brand AH, Bull CA, Cakir B. Vaginal stenosis in patients treated with radiotherapy for carcinoma of the cervix. Int J Gynecol Cancer 2006;16:288–93.
19. Lancaster L. Preventing vaginal stenosis after brachytherapy for gynaecological cancer: an overview of Australian practices. Eur J Oncol Nurs 2004;8:30–9.
20. Ponto JA, Barton D. Husbands’ perspective of living with wives’ ovarian cancer. Psychooncology 2008;17:1225–31.
21. Hartmann U, Philippsohn S, Heiser K, et al. Low sexual desire in midlife and older women: personality factors, psychosocial development, present sexuality. Menopause 2004;11:726–40.
22. Hawkins Y, Ussher J, Gilbert E, et al. Changes in sexuality and intimacy after the diagnosis and treatment of cancer: the experience of partners in a sexual relationship with a person with cancer. Cancer Nurs 2009;32:271–80.
23. Gilbert E, Ussher JM, Hawkins Y. Accounts of disruptions to sexuality following cancer: the perspective of informal carers who are partners of a person with cancer. Health 2009;13:523–41.
24. Hodgkinson K, Butow P, Hunt GE, et al. Life after cancer: couples’ and partners’ psychological adjustment and supportive care needs. Support Care Cancer 2007;15:405–15.
25. Lopez V, Copp G, Molassiotis A. Male caregivers of patients with breast and gynecologic cancer: experiences from caring for their spouses and partners. Cancer Nurs 2012;35:402–10.
26. American Cancer Society. Facts and figures 2015.
27. Michaelson-Cohen R, Beller U. Managing menopausal symptoms after gynecological cancer. Curr Opin Oncol 2009;21:407–11.
28. Biglia N Gadducci A, Ponzone R. Hormone replacement therapy in cancer survivors. Maturitas 2004;48:333–46.
29. Ploch E. Hormonal replacement therapy in patients after cervical cancer treatment. Gynecol Oncol 1987;26:169–77.
30. Guidozzi F, Daponte A. Estrogen replacement therapy for ovarian carcinoma survivors. Cancer 1999;86:1013–8.
31. Creasman WT. Hormone replacement therapy after cancers. Curr Opin Oncol 2005;17:493–9.
32. Bebar S, Ursic-Vrscaj M. Hormone replacement therapy after epithelial ovarian cancer treatment. Eur J Gynaecol Oncol 2000;21:192–6.
33. Wen Y, Huang H, Huang H, et al. The safety of postoperative hormone replacement therapy in epithelial ovarian cancer patients in China. Climacteric 2013;16:673–81.
34. Creasman WT, Henderson D, Hinshaw W, et al. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol 1986;67:326–30.
35. Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2006;24:587–92.
36. Lee K-B, Lee J-M, Lee J-K, et al. Endometrial cancer patients and tibolone: A matched case–control study. Maturitas 2006;55:264–9.
37. Kenemans P, Bundred NJ, Foidart J-M, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol 2009;10:135–46.
38. Al-Baghdadi O, Ewies AAA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 2009;12:91–105.
39. Galuppi A, Perrone AM, La Macchia M, et al. Local α-tocopherol for acute and short-term vaginal toxicity prevention in patients treated with radiotherapy for gynecologic tumors. Int J Gynecol Cancer 2011;21:1708–11.
40. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20.
41. Bø K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol 2012;30:437–43.
42. Dumoulin C, Hay-Smith EJC, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014;5:CD005654.
43. Rosenbaum TY. Pelvic floor involvement in male and female sexual dysfunction and the role of pelvic floor rehabilitation in treatment: A literature review. J Sex Med 2007;4:4–13.
44. Yang EJ, Lim J-Y, Rah UW, et al. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol 2012;125:705–11.
45. Johnson N, Miles TP, Cornes P. Dilating the vagina to prevent damage from radiotherapy: systematic review of the literature. BJOG 2010;117:522–31.
46. Friedman LC, Abdallah R, Schluchter M, et al. Adherence to vaginal dilation following high dose rate brachytherapy for endometrial cancer. Int J Radiat Oncol Biol Phys 2011;80:751–7.
47. Cullen K, Fergus K, DasGupta T, et al. From “sex toy” to intrusive omposition: a qualitative examination of women’s experiences with vaginal dilator use following treatment for gynecological cancer. J Sex Med 2012;9:1162–73.
48. Robinson JW, Faris PD, Scott CB. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynecological carcinoma treated with radiotherapy. Int J Radial Oncol Biol Phys 1999;44:497–506.
49. Stavraka C, Ford A, Ghaem-Maghami S, et al. A study of symptoms described by ovarian cancer survivors. Gynecol Oncol 2012;125:59–64.
50. Moonsammy SH, Guglietti CL, Mina DS, et al. A pilot study of an exercise & cognitive behavioral therapy intervention for epithelial ovarian cancer patients. J Ovarian Res 2013;6:21.
51. Goerling U, Jaeger C, Walz A, et al. The efficacy of short-term psycho-oncological interventions for women with gynaecological cancer: a randomized study. Oncology 2014;87:114–24.
52. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol 2012;125:320–5.
53. Cleary V, McCarthy G, Hegarty J. Development of an educational intervention focused on sexuality for women with gynecological cancer. J Psychosoc Oncol 2012;30:535–55.
54. Miller BE, Pittman B, Strong C. Gynecologic cancer patients’ psychosocial needs and their views on the physician›s role in meeting those needs. Int J Gynecol Cancer 2003;13:111–9.
55. Fang P, Tan K, Grover S, et al. Psychosocial encounters correlates with higher patient-reported functional quality of life in gynecological cancer patients receiving radiotherapy. Radiat Oncol 2015;10:34.
56. Hordern A, Grainger M, Hegarty S, et al. Discussing sexuality in the clinical setting: The impact of a brief training program for oncology health professionals to enhance communication about sexuality. Asia Pac J Clin Oncol 2009;5:270–7.
57. Simonelli LE, Pasipanodya E. Health disparities in unmet support needs of women with gynecologic cancer: an exploratory study. J Psychosoc Oncol 2014;32:727–34.
58. Barbera L, Fitch M, Adams L, et al. Improving care for women after gynecological cancer: the development of a sexuality clinic. Menopause 2011;18:1327–33.
59. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578–83.
60. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000;356:2059–63.
61. Wu C-S, Lu M-L, Liao Y-T, et al. Ovarian cancer and antidepressants. Psychooncology 2015;24:579–84.
62. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol 2009;29:259–66.
63. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011;305:267–74.
64. Reed SD, Guthrie KA, Joffe H, et al. Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2012;119:527–38.
65. Pérez DG, Loprinzi CL, Barton DL, et al. Pilot evaluation of mirtazapine for the treatment of hot flashes. J Support Oncol 2004;2:50–6.
66. Pérez DG, Loprinzi CL, Sloan J, et al. Pilot evaluation of bupropion for the treatment of hot flashes. J Palliat Med 2006;9:631–7.
67. Agarwal N, Singh S, Kriplani A, et al. Evaluation of gabapentin in management of hot flushes in postmenopausal women. Post Reprod Health 2014;20:36–8.
68. Guttuso T Jr, Kurlan R, McDermott MP, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337–45.
69. Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2011;29:3862–8.
70. Loibl S, Schwedler K, von Minckwitz G, et al. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients--a double-blind, randomized study. Ann Oncol 2007;18:689–93.
71. Aerts L, Enzlin P, Verhaeghe J, et al. Long-term sexual functioning in women after surgical treatment of cervical cancer stages IA to IB: a prospective controlled study. Int J Gynecol Cancer 2014;24:1527–34.
72. Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology 2007;16:691–706.
73. Pfaendler KS, Wenzel L, Mechanic MB, et al. Cervical cancer survivorship: long-term quality of life and social support. Clin Ther 2015;37:39–48.
74. Becker M, Malafy T, Bossart M, et al. Quality of life and sexual functioning in endometrial cancer survivors. Gynecol Oncol 2011;121:169–73.
75. Bifulco G, De Rosa N, Tornesello ML, et al. Quality of life, lifestyle behavior and employment experience: a comparison between young and midlife survivors of gynecology early stage cancers. Gynecol Oncol 2012;124:444–51.
76. Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause 2011;18:662–9.
77. American Cancer Society. Sexuality for the woman with cancer. 2013. Available at www.cancer.org/treatment/treatmentsandsideeffects/physicalsideeffects/sexualsideeffectsinwomen/sexualityforthewoman/index.
1. Juraskova I, Butow P, Robertson R, et al. Post-treatment sexual adjustment following cervical and endometrial cancer: a qualitative insight. Psychooncology 2003;12:267–79.
2. Bodurka DC, Sun CC. Sexual function after gynecologic cancer. Obstet Gynecol Clin North Am 2006;33:621–30, ix.
3. Andersen BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol 1997;65:221.
4. Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J 2009;15:74–7.
5. Stead ML, Brown JM, Fallowfield L, et al. Lack of communication between healthcare professionals and women with ovarian cancer about sexual issues. Br J Cancer 2003;88:666–71.
6. Stead ML, Fallowfield L, Brown JM, et al. Communication about sexual problems and sexual concerns in ovarian cancer: qualitative study. BMJ 2001;323:836–7.
7. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537–44.
8. American Psychiatric Association. Diagnostic and statistical manual of mental health disorders. 4th ed. 2000.
9. Elder JA, Braver Y. Female sexual dysfunction. In: Current clinical medicine. Elsevier; 2010:1222–6.
10. Ferrell BR, Dow KH, Leigh S, et al. Quality of life in long-term cancer survivors. Oncol Nurs Forum 1995;22:915–22.
11. Shamspour N, Assari S, Moghana Lankarani M. Relation between sexuality and health-related quality of life. In: Handbook of disease burdens and quality of life measures. New York: Springer 2010;3457–73.
12. Kim SI, Lee Y, Lim MC, et al. Quality of life and sexuality comparison between sexually active ovarian cancer survivors and healthy women. J Gynecol Oncol 2015;26:148–54.
13. Levin AO, Carpenter KM, Fowler JM, et al. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. Int J Gynecol Cancer 2010;20:461–70.
14. Lutgendorf SK, Anderson B, Rothrock N, et al. Quality of life and mood in women receiving extensive chemotherapy for gynecologic cancer. Cancer 2000;89:1402–11.
15. Tierney DK. Sexuality: a quality-of-life issue for cancer survivors. Semin Oncol Nurs 2008;24:71–9.
16. Brotto LA, Heiman JR, Goff B, et al. A psychoeducational intervention for sexual dysfunction in women with gynecologic cancer. Arch Sex Behav 2008;37:317–29.
17. Hollingsworth M, Berman J. The role of androgens in female sexual dysfunction. Sex Reprod Menopause 2006;4:27–32.
18. Brand AH, Bull CA, Cakir B. Vaginal stenosis in patients treated with radiotherapy for carcinoma of the cervix. Int J Gynecol Cancer 2006;16:288–93.
19. Lancaster L. Preventing vaginal stenosis after brachytherapy for gynaecological cancer: an overview of Australian practices. Eur J Oncol Nurs 2004;8:30–9.
20. Ponto JA, Barton D. Husbands’ perspective of living with wives’ ovarian cancer. Psychooncology 2008;17:1225–31.
21. Hartmann U, Philippsohn S, Heiser K, et al. Low sexual desire in midlife and older women: personality factors, psychosocial development, present sexuality. Menopause 2004;11:726–40.
22. Hawkins Y, Ussher J, Gilbert E, et al. Changes in sexuality and intimacy after the diagnosis and treatment of cancer: the experience of partners in a sexual relationship with a person with cancer. Cancer Nurs 2009;32:271–80.
23. Gilbert E, Ussher JM, Hawkins Y. Accounts of disruptions to sexuality following cancer: the perspective of informal carers who are partners of a person with cancer. Health 2009;13:523–41.
24. Hodgkinson K, Butow P, Hunt GE, et al. Life after cancer: couples’ and partners’ psychological adjustment and supportive care needs. Support Care Cancer 2007;15:405–15.
25. Lopez V, Copp G, Molassiotis A. Male caregivers of patients with breast and gynecologic cancer: experiences from caring for their spouses and partners. Cancer Nurs 2012;35:402–10.
26. American Cancer Society. Facts and figures 2015.
27. Michaelson-Cohen R, Beller U. Managing menopausal symptoms after gynecological cancer. Curr Opin Oncol 2009;21:407–11.
28. Biglia N Gadducci A, Ponzone R. Hormone replacement therapy in cancer survivors. Maturitas 2004;48:333–46.
29. Ploch E. Hormonal replacement therapy in patients after cervical cancer treatment. Gynecol Oncol 1987;26:169–77.
30. Guidozzi F, Daponte A. Estrogen replacement therapy for ovarian carcinoma survivors. Cancer 1999;86:1013–8.
31. Creasman WT. Hormone replacement therapy after cancers. Curr Opin Oncol 2005;17:493–9.
32. Bebar S, Ursic-Vrscaj M. Hormone replacement therapy after epithelial ovarian cancer treatment. Eur J Gynaecol Oncol 2000;21:192–6.
33. Wen Y, Huang H, Huang H, et al. The safety of postoperative hormone replacement therapy in epithelial ovarian cancer patients in China. Climacteric 2013;16:673–81.
34. Creasman WT, Henderson D, Hinshaw W, et al. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol 1986;67:326–30.
35. Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2006;24:587–92.
36. Lee K-B, Lee J-M, Lee J-K, et al. Endometrial cancer patients and tibolone: A matched case–control study. Maturitas 2006;55:264–9.
37. Kenemans P, Bundred NJ, Foidart J-M, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol 2009;10:135–46.
38. Al-Baghdadi O, Ewies AAA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 2009;12:91–105.
39. Galuppi A, Perrone AM, La Macchia M, et al. Local α-tocopherol for acute and short-term vaginal toxicity prevention in patients treated with radiotherapy for gynecologic tumors. Int J Gynecol Cancer 2011;21:1708–11.
40. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20.
41. Bø K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol 2012;30:437–43.
42. Dumoulin C, Hay-Smith EJC, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014;5:CD005654.
43. Rosenbaum TY. Pelvic floor involvement in male and female sexual dysfunction and the role of pelvic floor rehabilitation in treatment: A literature review. J Sex Med 2007;4:4–13.
44. Yang EJ, Lim J-Y, Rah UW, et al. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol 2012;125:705–11.
45. Johnson N, Miles TP, Cornes P. Dilating the vagina to prevent damage from radiotherapy: systematic review of the literature. BJOG 2010;117:522–31.
46. Friedman LC, Abdallah R, Schluchter M, et al. Adherence to vaginal dilation following high dose rate brachytherapy for endometrial cancer. Int J Radiat Oncol Biol Phys 2011;80:751–7.
47. Cullen K, Fergus K, DasGupta T, et al. From “sex toy” to intrusive omposition: a qualitative examination of women’s experiences with vaginal dilator use following treatment for gynecological cancer. J Sex Med 2012;9:1162–73.
48. Robinson JW, Faris PD, Scott CB. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynecological carcinoma treated with radiotherapy. Int J Radial Oncol Biol Phys 1999;44:497–506.
49. Stavraka C, Ford A, Ghaem-Maghami S, et al. A study of symptoms described by ovarian cancer survivors. Gynecol Oncol 2012;125:59–64.
50. Moonsammy SH, Guglietti CL, Mina DS, et al. A pilot study of an exercise & cognitive behavioral therapy intervention for epithelial ovarian cancer patients. J Ovarian Res 2013;6:21.
51. Goerling U, Jaeger C, Walz A, et al. The efficacy of short-term psycho-oncological interventions for women with gynaecological cancer: a randomized study. Oncology 2014;87:114–24.
52. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol 2012;125:320–5.
53. Cleary V, McCarthy G, Hegarty J. Development of an educational intervention focused on sexuality for women with gynecological cancer. J Psychosoc Oncol 2012;30:535–55.
54. Miller BE, Pittman B, Strong C. Gynecologic cancer patients’ psychosocial needs and their views on the physician›s role in meeting those needs. Int J Gynecol Cancer 2003;13:111–9.
55. Fang P, Tan K, Grover S, et al. Psychosocial encounters correlates with higher patient-reported functional quality of life in gynecological cancer patients receiving radiotherapy. Radiat Oncol 2015;10:34.
56. Hordern A, Grainger M, Hegarty S, et al. Discussing sexuality in the clinical setting: The impact of a brief training program for oncology health professionals to enhance communication about sexuality. Asia Pac J Clin Oncol 2009;5:270–7.
57. Simonelli LE, Pasipanodya E. Health disparities in unmet support needs of women with gynecologic cancer: an exploratory study. J Psychosoc Oncol 2014;32:727–34.
58. Barbera L, Fitch M, Adams L, et al. Improving care for women after gynecological cancer: the development of a sexuality clinic. Menopause 2011;18:1327–33.
59. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578–83.
60. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000;356:2059–63.
61. Wu C-S, Lu M-L, Liao Y-T, et al. Ovarian cancer and antidepressants. Psychooncology 2015;24:579–84.
62. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol 2009;29:259–66.
63. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011;305:267–74.
64. Reed SD, Guthrie KA, Joffe H, et al. Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2012;119:527–38.
65. Pérez DG, Loprinzi CL, Barton DL, et al. Pilot evaluation of mirtazapine for the treatment of hot flashes. J Support Oncol 2004;2:50–6.
66. Pérez DG, Loprinzi CL, Sloan J, et al. Pilot evaluation of bupropion for the treatment of hot flashes. J Palliat Med 2006;9:631–7.
67. Agarwal N, Singh S, Kriplani A, et al. Evaluation of gabapentin in management of hot flushes in postmenopausal women. Post Reprod Health 2014;20:36–8.
68. Guttuso T Jr, Kurlan R, McDermott MP, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337–45.
69. Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2011;29:3862–8.
70. Loibl S, Schwedler K, von Minckwitz G, et al. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients--a double-blind, randomized study. Ann Oncol 2007;18:689–93.
71. Aerts L, Enzlin P, Verhaeghe J, et al. Long-term sexual functioning in women after surgical treatment of cervical cancer stages IA to IB: a prospective controlled study. Int J Gynecol Cancer 2014;24:1527–34.
72. Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology 2007;16:691–706.
73. Pfaendler KS, Wenzel L, Mechanic MB, et al. Cervical cancer survivorship: long-term quality of life and social support. Clin Ther 2015;37:39–48.
74. Becker M, Malafy T, Bossart M, et al. Quality of life and sexual functioning in endometrial cancer survivors. Gynecol Oncol 2011;121:169–73.
75. Bifulco G, De Rosa N, Tornesello ML, et al. Quality of life, lifestyle behavior and employment experience: a comparison between young and midlife survivors of gynecology early stage cancers. Gynecol Oncol 2012;124:444–51.
76. Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause 2011;18:662–9.
77. American Cancer Society. Sexuality for the woman with cancer. 2013. Available at www.cancer.org/treatment/treatmentsandsideeffects/physicalsideeffects/sexualsideeffectsinwomen/sexualityforthewoman/index.