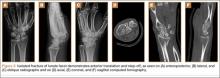
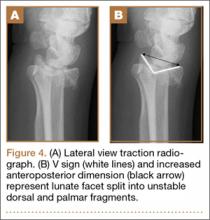
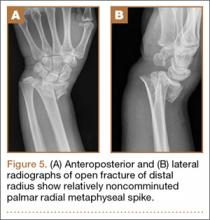
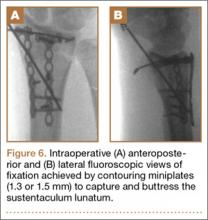
User login
Psychological Distress and Cardiovascular Disease
From the California State University, Long Beach, School of Nursing, Long Beach, CA (Dr. McGuire, Ms. Ahearn), and the University of California, Los Angeles, School of Nursing, Los Angeles, CA (Dr. Doering).
Abstract
- Objective: To review the current literature regarding psychological distress in patients with cardiovascular disease (CVD).
- Methods: Relevant and current (2005–2015) studies were retrieved by a series of searches conducted in the PubMed and PsychINFO databases using Boolean terms/phrases along with manual extraction from the reference lists of pertinent studies. Narrative and tabular summaries of the findings are reported.
- Results: There is a vast literature on psychological distress and CVD. Depression is the most common disorder studied followed by anxiety and post-traumatic stress disorder. Physiologic mechanisms linking psychological distress to CVD are well theorized. Screening for psychological distress in CVD is recommended. Referral and treatment issues need further exploration. Pharmacologic treatment of psychological distress in CVD remains equivocal; however, promising data exists for other therapies such as cognitive behavioral therapy and social support strategies.
- Conclusion: Psychological distress has a significant negative impact on patients with CVD and is underrecognized by health care providers. Primary care providers and cardiovascular specialty providers are called upon to improve their recognition of psychological distress in their patients and assure referrals are made to collaborative care teams for proper diagnosis and treatment.
The association between the heart and the mind has been proposed by scientists since the 17th century. However, it was not until the 1970s that the relationship between cardiovascular disease (CVD) and psychological states came into scientific focus. The study of heart-psyche interactions began with investigations of cardiovascular risk and “type A” personality behaviors (aggressiveness, impatience, a sense of time-urgency, intense achievement drive, seeking recognition) [1,2]. Hundreds of studies generated over the last 10 years have yielded an extensive body of literature regarding this complex interaction.
CVD continues to be the leading cause of death globally. Worldwide and in the United States, CVD accounts for 30% of deaths and more than 2000 deaths per day, respectively [3,4]. Psychological distress (specifically depression) has been reported by the World Health Organization (WHO) as the leading cause of disability in the world [4]. Taken together, CVD and depression constitute an immense health burden and result in poor health status, increased care giver burden [5], increased readmission rates to hospitals, increased utilization of primary care services, poor health compliance [6], decreased health related quality of life [7], and a greater than 2 times increase in mortality [8,9].
Despite its devastating consequences, comorbid CVD and psychological distress remains poorly recognized and treated. In this paper, we present a review of the evidence related to key aspects of psychological distress and CVD (for the purposes of this paper, defined as ischemic heart disease and stroke), and provide information to help improve identification among health care providers. Relevant and current (2005-2015) studies for this review were retrieved by a series of searches conducted in the PubMed and PsychINFO databases using Boolean terms/phrases, along with manual extraction from the reference lists of pertinent studies. Due to the breadth and extent of the literature, a comprehensive review of the literature is beyond the scope of this article. However, the reader will be directed to current systematic reviews, meta-analyses, and recent select research studies sourced for this summary and presented in tabular form.
Mechanisms of Psychological Distress
Psychological Distress Disorders Related to CVD
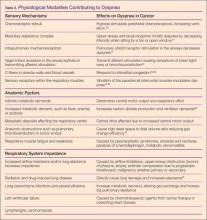
Depression, anxiety, and post-traumatic stress disorder (PTSD) are the 3 most common psychological distress disorders related to CVD [20]. Cardiac disease and depression has been most commonly studied. In stroke, the science is not as well evolved due to greater heterogeneity of study samples and outcome measures.
Depression
Dysphoria (feeling blue), anhedonia (inability to experience joy in otherwise enjoyable activities), insomnia or hypersomnia, fatigue or loss of energy, increased guilt or worthlessness, decreased concentration, appetite change with significant weight loss or gain, psychomotor retardation or agitation, and suicidal ideation are the symptoms of depression [21]. These symptoms exist on a continuum, ranging from mild symptoms with short duration and limited functional impairment to major depression. Importantly, among otherwise healthy individuals, even minor depressive symptoms have been significantly associated with increased incidence of coronary disease [22].
Screening Issues
In recognition of the high prevalence of depression in patients with CHD, an American Heart Association (AHA) science advisory in 2008 recommended routine screening for depression in patients with CHD, with follow-up evaluation for diagnosis and treatment of depression by qualified professionals for positive cases [26]. In 2014, an AHA scientific statement recommended elevating depression to the level of a risk factor in ACS patients [27]. The recommendation for screening was initially met with some concern as being premature [28], when past supporters spoke out against the routine screening of depression in cardiac patients [29]. The dissenting authors claimed that there was a lack of scientific evidence supporting the efficacy of treatment for depression in cardiac patients, and that potential negative effects of routine screening and follow-up treatment were unknown. They argued the following: limited data from randomized controlled trials and/or evidenced-based reviews exist demonstrating improved outcomes in cardiac patients based on screening and referral [30]; antidepressants are not yet recognized to be effective in cardiac populations and there is a lack of evidence related to potential harms [28]; concerns exist about the potential for mass screening to increase health care resource use at the expense of other health care needs [29]; and routine screening may cause unnecessary negative social stigma related to false-positive findings [31].
Although clinical trials of depression treatment in cardiac patients have not demonstrated an increase in survival, treatment has been shown to be effective in reducing depression symptoms, improving patient satisfaction with depression care and improving health related quality of life [32–34]. Further, recent studies described the AHA recommendation as well accepted by cardiac unit staff, not heavily resource intensive, feasible, and accurate [35,36]. Bigger and Glassman [37] published a recent analytical review of the AHA advisory and concluded that the advisory is supported by the literature. A salient point regarding the depression screening debate is that screening without proper follow-up for further diagnosis and potential treatment may be harmful [28,29,31]. Despite concerns of the potential negative impact of depression screening in cardiac patients raised in the literature, the preponderance of the literature indicates that its benefits are likely to outweigh its risks [32,34,36,38–40].
Outcomes of Depression Treatment
Answers to questions about improvement in cardiovascular and all-cause mortality outcomes with depression treatment remain elusive in the literature. However, data show an improvement in depressive symptoms and quality of life for depressed patients receiving some types of treatment [33,41–45]. The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) study was a landmark study of MI patients with a 6-month treatment intervention of cognitive behavioral therapy (CBT) plus pharmacologic intervention if indicated for depression [33]. Patients were followed for an average of 29 months post-MI. A significant improvement was seen in depressive symptoms and social isolation in the treatment group; however, there was no improvement in event-free survival [33]. When outcome measures are restricted to mortality alone, subsequent trials of antidepressant medications for treatment of depression in cardiac patients have shown them to be ineffective [46]. However, CBT and other supportive stress management strategies are effective in decreasing depressive symptoms and improving the quality of life in patients suffering with depression and CVD [46].
Promising results are emerging in the literature as researchers refocus their analysis on subgroups of depressed cardiac patients. In one large study of 442 depressed and 325 non-depressed patients, the number of depressive symptoms after an MI irrespective of the pre-MI depression status was associated with worse cardiac outcomes [47]. For every 1 additional depressive symptom reported 1 year post-MI, patients had a 15% increased risk for a new cardiac event in the next 2.5 years [47]. Another study demonstrated an improvement in depressive symptoms by 75.3% in in post-cardiac surgery patients with low ejection fraction (< 40%) after 8 weeks of nurse-guided CBT and worsening in depressive symptoms by 26.8% in usual care patients. More moderate findings were seen in the those with higher ejection fraction receiving the same CBT intervention for depression [45]. A treatment-resistant depression subgroup analyzed in a recent secondary analysis of the ENRICHD trial showed a twofold increase in mortality when compared to those in the non–treatment-resistant depression group [48]. Since treatment does not work for all patients with depression, including depressed post-MI patients, further evaluation with a focus on those who respond to treatment is needed.
Depression in Stroke
Issues related to treatment of stroke patients parallel those of depressed patients with cardiac disease, as the effect on mortality and survival is unknown. Depression has been reported to go untreated in up to 67.9% of depressed post-stroke patients [54]. In addition, mismatches between antidepressant prescription and those with depression suggested that some patients without depression were being treated for depression while some patients with depression were not being treated [54].
Anxiety
Anxiety disorders create behavioral disturbances of fear and avoidance related to an individual’s propensity to overestimate dangers [21]. Though a number of anxiety disorders are described in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), anxiety as a general constellation of symptoms (afraid, inability to relax, worry about everyday problems, feelings of panic) is described in most of the literature related to CVD [55–57]. To a lesser extent, generalized anxiety disorder (GAD), defined as anxiety symptoms on more days than not and lasting more than 6 months [58,59] has also been studied. Because anxiety can be a component of depression, the 2 are often discussed together. Taken together, in the context of CHD, individuals with both anxiety and depression are at significantly greater risk for death (odds ratio 2.35, 95% confidence interval 1.23-4.47, P =0.01), compared with those without symptoms [60].
Post-Traumatic Stress Disorder
PTSD presents with a heterogeneous cluster of symptoms that are generally described as avoidance, re-experiencing, arousal, and negative cognitions and mood [21]. Previously classified as a anxiety disorder in DSM-IV, PTSD is in a new category of trauma and stress or related disorders in the revised DSM-V [21]. In addition, the temporal component of the symptoms has been changed to a disturbance lasting more than 1 month, without reference to acuity or chronicity.
Implications for Clinical Practice
Despite the extensive body of literature regarding the negative association of psychological distress to health outcomes in CVD patients, there remains a significant practice gap related to screening, referral, and treatment of psychological distress in CVD patients [30,36,66]. Busy clinical practices focused on physical symptoms (which may not be recognized as mental health–related), along with a health system that has historically regarded mental health issues as the sole domain of mental health professionals creates barriers that need to be overcome. Many health care providers are not proactive in screening patients for psychological distress [11]. Additionally, psychological distress is often perceived as a metaphysical, inexact phenomenon, and is not regarded with the same import as physiological indices, such blood pressure and lipid levels [13]. Another significant barrier may be that the importance of psychological distress, especially depression, has been minimized by some investigators and clinicians because of the lack of data that show improvement with treatment of “hard” outcomes, such as mortality. Lastly, no studies have conclusively demonstrated that treating depression in the general population would lower subsequent cardiovascular clinical events, adding to the minimization of the importance of screening, referral, and treatment of psychological distress among clinicians.
The challenges associated with psychological distress and CVD are centered on the perceived role of the health care provider and role of the patient [11,67]. To date, identification of patients with psychological distress in CVD populations has not been considered a part of routine practice, and only a small percent of those identified with psychological distress are treated. In one study, 17.6% of 1181 patients had moderate to severe depression and of those, only 24.5% were recognized as depressed by their health care providers [68]. In a smaller study of 35 patients with depression after an acute MI, only 10% received treatment with antidepressants [66]. Similarly poor treatment rates were seen in a recent study of antidepressant use after stroke and transient ischemic attack (TIA), with 67.9% of stroke patients and 70.0% of TIA patients with persistent depression going untreated [54].
Stress, depression, anxiety, and PTSD are often not self-contained or experienced in isolation. Rather, some or all of these conditions may present as an interconnected phenomenon [69,70]. There are several reasons for this. First, symptom identification relies on self-report and/or observation of the symptoms, which may confound validity and reliability of diagnosis [70]. Second, the conditions share some symptoms, which may complicate diagnosis of a primary condition. In addition, the overlap of somatic, cognitive, and affective symptoms [71] may deter health care providers from using screening tools that have physical symptoms as part of the screening process. In a recent study of depressed cardiac patients, investigators clustered symptoms and demonstrated that cognitive affective symptoms of depression predict depression in patients with heart disease [72]. Certain events, such as stroke, may make screening especially difficult due to the presence of neurological changes that may complicate the screening process [73]. These issues highlight the need for formalized depression screening tools such as the Patient Health Questionnaire 2-item screening tool (PHQ-2) and/or Patient Health Questionnaire nine-item screening tool (PHQ-9) (Appendix), as recommended by the AHA. The PHQs have been validated in stroke patients and cardiac patients and have been found to be accurate, easy to use, and feasible [35,36,73].
Patients look to their providers for information and security during vulnerable times in their life. In a 2014 study regarding the perceptions of psychosocial consequences and access to support after MI, patients reported a high sense of security regarding being able to contact their providers [74]. Providers in both the primary and acute care settings should use these opportunities to assess for psychological distress. Many inpatient health care providers assume that it is the primary care providers’ responsibility to screen for depression [75]; however, screening should be done in all practice settings caring for patients with CVD. Clinical practice environments should develop policies and procedures to define who, when, and how screening for psychological distress is accomplished using currently available brief screening tools.
A plan for insuring that proper referrals are made following screening to ensure accurate diagnosis, effective treatment, and follow-up should be in place. To date, there is ample evidence that patients benefit from screening in the context of an interdisciplinary treatment approach [34]. Collaborative care, utilizing a team of health professionals (physician, case manager trained in working with patients with psychological distress, and mental health specialist) working with the patient, is an ideal model to improve outcomes [44]. A Cochrane review of 79 RCTs with over 24,000 participants compared utilization of a collaborative team with routine care and found decreased depression and anxiety symptoms in patients receiving team care for up 2 years, along with improved medication adherence, improved quality of life, and improved patient satisfaction with care [39].
Conclusion
Psychological distress remains underrecognized in CVD patients. Brief screening tools such as the PHQ-2 and PHQ-9 are available, easy to use, and reliable for use by clinicians to improve case finding. Primary care providers and cardiovascular specialty providers are called upon to improve the recognition of psychological distress in their patients and assure referrals are made to collaborative care teams for proper diagnosis and treatment of mental health issues. Longitudinal studies focused on the impact of primary/secondary/tertiary psychological distress prevention strategies in the general population, as well as those with CVD, are needed to bring the state of the science forward and provide evidence to enhance the care of those with psychological distress and CVD.
Corresponding author: Anthony McGuire, RN, PhD, ACNP-BC, CSULB, School of Nursing, #2 1250 Bellflower Blvd., Long Beach, CA 90804, [email protected].
Financial disclosures: None
Author contributions: conception and design, AWM, EA, LVD; drafting of article, AWM, EA, LVD; critical revision of the article, AWM, EA, LVD; collection and assembly of data, AWM, EA, LVD.
1. Dembroski TM, MacDougall JM, Shields JL, et al. Components of the type A coronary-prone behavior pattern and cardiovascular responses to psychomotor performance challenge. J Behav Med 1978;1:159–76.
2. Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation 2005;111:250–3.
3. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292.
4. Mathers C, Stevens G, Mascarenhas M. Global health risks: Mortality and burden of diesease attributable to select major risks. Geneva, Switzerland: World Health Organization, 2009.
5. Randall G, Molloy GJ, Steptoe A. The impact of an acute cardiac event on the partners of patients: A systematic review. Health Psychol Rev 2009;3:1–84.
6. Kronish IM, Rieckmann N, Halm EA, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med 2006;21:1178–83.
7. Stafford L, Berk M, Reddy P, Jackson HJ. Comorbid depression and health-related quality of life in patients with coronary artery disease. J Psychosom Res 2007;62:401–10.
8. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004;66:802–13.
9. van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004;66:814–22.
10. Doyle F, McGee HM, Conroy RM, Delaney M. What predicts depression in cardiac patients: sociodemographic factors, disease severity or theoretical vulnerabilities? Psychol Health 2011;26:619–34.
11. Figueredo VM. The time has come for physicians to take notice: the impact of psychosocial stressors on the heart. Am J Med 2009;122:704–12.
12. Matthews KA. Matters of the heart: advancing psychological perspectives on cardiovascular diseases. Persp Psychol Sci 2013;8:676–8.
13. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol 2013;9:327–54.
14. Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation 2011;124:346–54.
15. Hamer M, Molloy GJ, Stamatakis E. Psychological distress as a risk factor for cardiovascular events: pathophysiological and behavioral mechanisms. J Am Coll Cardiol 2008;52:2156–62.
16. Richardson S, Shaffer JA, Falzon L, et al. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol 2012;110:1711–6.
17. Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol 2012;9:360–70.
18. Brumby S, Chandrasekara A, McCoombe S, et al. Cardiovascular risk factors and psychological distress in Australian farming communities. Aust J Rural Health 2012;20:131–7.
19. Arnold SV, Smolderen KG, Buchanan DM, et al. Perceived stress in myocardial infarction long-term mortality and health status outcomes. J Am Coll Cardiol 2012;60:1756–63.
20. Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens 2015.
21. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
22. Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med 2006;68:645–50.
23. Niranjan A, Corujo A, Ziegelstein RC, Nwulia E. Depression and heart disease in US adults. Gen Hosp Psychiatry 2012;34:254–61.
24. Seldenrijk A, Vogelzangs N, Batelaan NM, et al. Depression, anxiety and 6-year risk of cardiovascular disease. J Psychosom Res 2015;78:123–9.
25. Ayerbe L, Ayis S, Crichton S, et al. The natural history of depression up to 15 years after stroke: The South London Stroke Register. Stroke 2013.
26. Lichtman JH, Bigger JT Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 2008;118:1768–75.
27. Lichtman JH, Froelicher ES, Blumenthal JA, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350–69.
28. Hasnain M, Vieweg WV, Lesnefsky EJ, Pandurangi AK. Depression screening in patients with coronary heart disease: a critical evaluation of the AHA guidelines. J Psychosom Res 2011;71:6–12.
29. Ziegelstein RC, Thombs BD, Coyne JC, de Jonge P. Routine screening for depression in patients with coronary heart disease never mind. J Am Coll Cardiol 2009;54:886–90.
30. Ziegelstein RC, Kim SY, Kao D, et al. Can doctors and nurses recognize depression in patients hospitalized with an acute myocardial infarction in the absence of formal screening? Psychosom Med 2005;67:393–7.
31. Whooley MA. To screen or not to screen? Depression in patients with cardiovascular disease. J Am Coll Cardiol 2009;54:891–3.
32. Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med 2010;170:600–8.
33. Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106–16.
34. Whooley M, Unutzer J. Interdisciplinary stepped care for depression after acute coronary syndrome. Arch Intern Med 2010;170:585–6.
35. McGuire AW, Eastwood JA, Macabasco-O’Connell A, et al. Depression screening: utility of the patient health questionnaire in patients with acute coronary syndrome. Am J Crit Care 2013;22:12–9.
36. Sowden G, Mastromauro CA, Januzzi JL, et al. Detection of depression in cardiac inpatients: feasibility and results of systematic screening. Am Heart J 2010;159:780–7.
37. Bigger JT, Glassman AH. The American Heart Association science advisory on depression and coronary heart disease: an exploration of the issues raised. Cleve Clin J Med 2010;77 Suppl 3:S12–9.
38. Page KN, Davidson P, Edward KL, et al. Recovering from an acute cardiac event--the relationship between depression and life satisfaction. J Clin Nurs 2010;19:736–43.
39. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10:CD006525.
40. Blumenthal JA, O’Connor C. No laughing matter. J Am Coll Cardiol 2010;55:836.
41. Davidson KW, Korin MR. Depression and cardiovascular disease: selected findings, controversies, and clinical implications from 2009. Cleve Clin J Med 2010;77 Suppl 3:S20–6.
42. Doering LV, McGuire A, Eastwood JA, et al. Cognitive behavioral therapy for depression improves pain and perceived control in cardiac surgery patients. Eur J Cardiovasc Nurs 2015.
43. Freedland KE, Skala JA, Carney RM, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry 2009;66:387–96.
44. Huffman JC, Mastromauro CA, Sowden GL, et al. collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics 2011;52:26–33.
45. Hwang B, Eastwood JA, McGuire A, et al. Cognitive behavioral therapy in depressed cardiac surgery patients: role of ejection fraction. J Cardiovasc Nurs 2015;30:319–24.
46. Mavrides N, Nemeroff C. Treatment of depression in cardiovascular disease. Depression Anxiety 2013;30:328–41.
47. Zuidersma M, Ormel J, Conradi HJ, de Jonge P. An increase in depressive symptoms after myocardial infarction predicts new cardiac events irrespective of depressive symptoms before myocardial infarction. Psychol Med 2012;42:683–93.
48. Banankhah SK, Friedmann E, Thomas S. Effective treatment of depression improves post-myocardial infarction survival. World J Cardiol 2015;7:215–23.
49. Ayerbe L, Ayis S, Crichton S, et al. The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J Neurol Neurosurg Psychiatry 2014;85:514–21.
50. Hama S, Yamashita H, Yamawaki S, Kurisu K. Post-stroke depression and apathy: Interactions between functional recovery, lesion location, and emotional response. Psychogeriatrics 2011;11:68–76.
51. Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovasc Dis 2013;35:23–39.
52. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag 2013;9:483–9.
53.Karamchandani R, Vahidy F, Bajgur S, et al. Early Depression Screening is Feasible in Hospitalized Stroke Patients. Neurology 2014;82(10 Supplement):S62.005.
54. El Husseini N, Goldstein LB, Peterson ED, et al. Depression and antidepressant use after stroke and transient ischemic attack. Stroke 2012;43:1609–16.
55. D’Aniello GE, Scarpina F, Mauro A, Mori I, et al. Characteristics of anxiety and psychological well-being in chronic post-stroke patients. J Neurol Sci 2014;338:191–6.
56. Huffman JC, Smith FA, Blais MA, et al. Anxiety, independent of depressive symptoms, is associated with in-hospital cardiac complications after acute myocardial infarction. J Psychosom Res 2008;65:557–63.
57. Shen B-J, Avivi YE, Todaro JF, et al. Anxiety characteristics independently and prospectively predict myocardial infarction in men: the unique contribution of anxiety among psychologic factors. J Am Coll Cardiol 2008;51:113–9.
58. Butnoriene J, Bunevicius A, Saudargiene A, et al. Metabolic syndrome, major depression, generalized anxiety disorder, and ten-year all-cause and cardiovascular mortality in middle aged and elderly patients. Int J Cardiol 2015;190:360–6.
59. Roest AM, Zuidersma M, de Jonge P. Myocardial infarction and generalised anxiety disorder: 10-year follow-up. Br J Psychiatry 2012;200:324–9.
60. Doering LV, Moser DK, Riegel B, et al. Persistent comorbid symptoms of depression and anxiety predict mortality in heart disease. Int J Cardiol 2010;145:188–92.
61. Edmondson D, Kronish IM, Shaffer JA, et al. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J 2013;166:806–14.
62. Ahmadi N, Hajsadeghi F, Mirshkarlo HB, et al. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol 2011;108:29–33.
63. Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health Psychol 2012;31:194–201.
64. Chung MC, Dennis I, Berger Z, et al. Posttraumatic stress disorder following myocardial infarction: personality, coping, and trauma exposure characteristics. Int J Psychiatry Med 2011;42:393–419.
65. Bluvstein I, Moravchick L, Sheps D, et al. Posttraumatic growth, posttraumatic stress symptoms and mental health among coronary heart disease survivors. J Clin Psychol Med Settings 2013;20:164–72.
66. Huffman JC, Smith FA, Blais MA, et al. Recognition and treatment of depression and anxiety in patients with acute myocardial infarction. Am J Cardiol 2006;98:319–24.
67. Crosson JC, Heisler M, Subramanian U, et al. Physicians’ perceptions of barriers to cardiovascular disease risk factor control among patients with diabetes: results from the translating research into action for diabetes (TRIAD) study. J Am Board Fam Med 2010;23:171–8.
68. Amin AA, Jones AM, Nugent K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J 2006;152:928–34.
69. Chung MC, Berger Z, Jones R, Rudd H. Posttraumatic stress and co-morbidity following myocardial infarction among older patients: the role of coping. Aging Ment Health 2008;12:124–33.
70. Neylon A, Canniffe C, Anand S, et al. A global perspective on psychosocial risk factors for cardiovascular disease. Prog Cardiovasc Dis 2013;55:574–81.
71. Carney RM, Freedland KE. Are somatic symptoms of depression better predictors of cardiac events than cognitive symptoms in coronary heart disease? Psychosom Med 2012;74:33–8.
72. McGuire AW, Eastwood JA, Hays RD, Macabasco-O’Connell A, et al. Depressed or not depressed: untangling symptoms of depression in patients hospitalized with coronary heart disease. Am J Crit Care 2014;23:106–16.
73. Williams LS, Brizendine EJ, Plue L, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke 2005;36:635–8.
74. Junehag L, Asplund K, Svedlund M. A qualitative study: perceptions of the psychosocial consequences and access to support after an acute myocardial infarction. Intensive Crit Care Nurs 2014;30:22–30.
75. Lea P. Factors affecting nurses’ intent to assess for depression in heart failure patients. Dimens Crit Care Nurs 2014;33:320–6.
From the California State University, Long Beach, School of Nursing, Long Beach, CA (Dr. McGuire, Ms. Ahearn), and the University of California, Los Angeles, School of Nursing, Los Angeles, CA (Dr. Doering).
Abstract
- Objective: To review the current literature regarding psychological distress in patients with cardiovascular disease (CVD).
- Methods: Relevant and current (2005–2015) studies were retrieved by a series of searches conducted in the PubMed and PsychINFO databases using Boolean terms/phrases along with manual extraction from the reference lists of pertinent studies. Narrative and tabular summaries of the findings are reported.
- Results: There is a vast literature on psychological distress and CVD. Depression is the most common disorder studied followed by anxiety and post-traumatic stress disorder. Physiologic mechanisms linking psychological distress to CVD are well theorized. Screening for psychological distress in CVD is recommended. Referral and treatment issues need further exploration. Pharmacologic treatment of psychological distress in CVD remains equivocal; however, promising data exists for other therapies such as cognitive behavioral therapy and social support strategies.
- Conclusion: Psychological distress has a significant negative impact on patients with CVD and is underrecognized by health care providers. Primary care providers and cardiovascular specialty providers are called upon to improve their recognition of psychological distress in their patients and assure referrals are made to collaborative care teams for proper diagnosis and treatment.
The association between the heart and the mind has been proposed by scientists since the 17th century. However, it was not until the 1970s that the relationship between cardiovascular disease (CVD) and psychological states came into scientific focus. The study of heart-psyche interactions began with investigations of cardiovascular risk and “type A” personality behaviors (aggressiveness, impatience, a sense of time-urgency, intense achievement drive, seeking recognition) [1,2]. Hundreds of studies generated over the last 10 years have yielded an extensive body of literature regarding this complex interaction.
CVD continues to be the leading cause of death globally. Worldwide and in the United States, CVD accounts for 30% of deaths and more than 2000 deaths per day, respectively [3,4]. Psychological distress (specifically depression) has been reported by the World Health Organization (WHO) as the leading cause of disability in the world [4]. Taken together, CVD and depression constitute an immense health burden and result in poor health status, increased care giver burden [5], increased readmission rates to hospitals, increased utilization of primary care services, poor health compliance [6], decreased health related quality of life [7], and a greater than 2 times increase in mortality [8,9].
Despite its devastating consequences, comorbid CVD and psychological distress remains poorly recognized and treated. In this paper, we present a review of the evidence related to key aspects of psychological distress and CVD (for the purposes of this paper, defined as ischemic heart disease and stroke), and provide information to help improve identification among health care providers. Relevant and current (2005-2015) studies for this review were retrieved by a series of searches conducted in the PubMed and PsychINFO databases using Boolean terms/phrases, along with manual extraction from the reference lists of pertinent studies. Due to the breadth and extent of the literature, a comprehensive review of the literature is beyond the scope of this article. However, the reader will be directed to current systematic reviews, meta-analyses, and recent select research studies sourced for this summary and presented in tabular form.
Mechanisms of Psychological Distress
Psychological Distress Disorders Related to CVD
Depression, anxiety, and post-traumatic stress disorder (PTSD) are the 3 most common psychological distress disorders related to CVD [20]. Cardiac disease and depression has been most commonly studied. In stroke, the science is not as well evolved due to greater heterogeneity of study samples and outcome measures.
Depression
Dysphoria (feeling blue), anhedonia (inability to experience joy in otherwise enjoyable activities), insomnia or hypersomnia, fatigue or loss of energy, increased guilt or worthlessness, decreased concentration, appetite change with significant weight loss or gain, psychomotor retardation or agitation, and suicidal ideation are the symptoms of depression [21]. These symptoms exist on a continuum, ranging from mild symptoms with short duration and limited functional impairment to major depression. Importantly, among otherwise healthy individuals, even minor depressive symptoms have been significantly associated with increased incidence of coronary disease [22].
Screening Issues
In recognition of the high prevalence of depression in patients with CHD, an American Heart Association (AHA) science advisory in 2008 recommended routine screening for depression in patients with CHD, with follow-up evaluation for diagnosis and treatment of depression by qualified professionals for positive cases [26]. In 2014, an AHA scientific statement recommended elevating depression to the level of a risk factor in ACS patients [27]. The recommendation for screening was initially met with some concern as being premature [28], when past supporters spoke out against the routine screening of depression in cardiac patients [29]. The dissenting authors claimed that there was a lack of scientific evidence supporting the efficacy of treatment for depression in cardiac patients, and that potential negative effects of routine screening and follow-up treatment were unknown. They argued the following: limited data from randomized controlled trials and/or evidenced-based reviews exist demonstrating improved outcomes in cardiac patients based on screening and referral [30]; antidepressants are not yet recognized to be effective in cardiac populations and there is a lack of evidence related to potential harms [28]; concerns exist about the potential for mass screening to increase health care resource use at the expense of other health care needs [29]; and routine screening may cause unnecessary negative social stigma related to false-positive findings [31].
Although clinical trials of depression treatment in cardiac patients have not demonstrated an increase in survival, treatment has been shown to be effective in reducing depression symptoms, improving patient satisfaction with depression care and improving health related quality of life [32–34]. Further, recent studies described the AHA recommendation as well accepted by cardiac unit staff, not heavily resource intensive, feasible, and accurate [35,36]. Bigger and Glassman [37] published a recent analytical review of the AHA advisory and concluded that the advisory is supported by the literature. A salient point regarding the depression screening debate is that screening without proper follow-up for further diagnosis and potential treatment may be harmful [28,29,31]. Despite concerns of the potential negative impact of depression screening in cardiac patients raised in the literature, the preponderance of the literature indicates that its benefits are likely to outweigh its risks [32,34,36,38–40].
Outcomes of Depression Treatment
Answers to questions about improvement in cardiovascular and all-cause mortality outcomes with depression treatment remain elusive in the literature. However, data show an improvement in depressive symptoms and quality of life for depressed patients receiving some types of treatment [33,41–45]. The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) study was a landmark study of MI patients with a 6-month treatment intervention of cognitive behavioral therapy (CBT) plus pharmacologic intervention if indicated for depression [33]. Patients were followed for an average of 29 months post-MI. A significant improvement was seen in depressive symptoms and social isolation in the treatment group; however, there was no improvement in event-free survival [33]. When outcome measures are restricted to mortality alone, subsequent trials of antidepressant medications for treatment of depression in cardiac patients have shown them to be ineffective [46]. However, CBT and other supportive stress management strategies are effective in decreasing depressive symptoms and improving the quality of life in patients suffering with depression and CVD [46].
Promising results are emerging in the literature as researchers refocus their analysis on subgroups of depressed cardiac patients. In one large study of 442 depressed and 325 non-depressed patients, the number of depressive symptoms after an MI irrespective of the pre-MI depression status was associated with worse cardiac outcomes [47]. For every 1 additional depressive symptom reported 1 year post-MI, patients had a 15% increased risk for a new cardiac event in the next 2.5 years [47]. Another study demonstrated an improvement in depressive symptoms by 75.3% in in post-cardiac surgery patients with low ejection fraction (< 40%) after 8 weeks of nurse-guided CBT and worsening in depressive symptoms by 26.8% in usual care patients. More moderate findings were seen in the those with higher ejection fraction receiving the same CBT intervention for depression [45]. A treatment-resistant depression subgroup analyzed in a recent secondary analysis of the ENRICHD trial showed a twofold increase in mortality when compared to those in the non–treatment-resistant depression group [48]. Since treatment does not work for all patients with depression, including depressed post-MI patients, further evaluation with a focus on those who respond to treatment is needed.
Depression in Stroke
Issues related to treatment of stroke patients parallel those of depressed patients with cardiac disease, as the effect on mortality and survival is unknown. Depression has been reported to go untreated in up to 67.9% of depressed post-stroke patients [54]. In addition, mismatches between antidepressant prescription and those with depression suggested that some patients without depression were being treated for depression while some patients with depression were not being treated [54].
Anxiety
Anxiety disorders create behavioral disturbances of fear and avoidance related to an individual’s propensity to overestimate dangers [21]. Though a number of anxiety disorders are described in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), anxiety as a general constellation of symptoms (afraid, inability to relax, worry about everyday problems, feelings of panic) is described in most of the literature related to CVD [55–57]. To a lesser extent, generalized anxiety disorder (GAD), defined as anxiety symptoms on more days than not and lasting more than 6 months [58,59] has also been studied. Because anxiety can be a component of depression, the 2 are often discussed together. Taken together, in the context of CHD, individuals with both anxiety and depression are at significantly greater risk for death (odds ratio 2.35, 95% confidence interval 1.23-4.47, P =0.01), compared with those without symptoms [60].
Post-Traumatic Stress Disorder
PTSD presents with a heterogeneous cluster of symptoms that are generally described as avoidance, re-experiencing, arousal, and negative cognitions and mood [21]. Previously classified as a anxiety disorder in DSM-IV, PTSD is in a new category of trauma and stress or related disorders in the revised DSM-V [21]. In addition, the temporal component of the symptoms has been changed to a disturbance lasting more than 1 month, without reference to acuity or chronicity.
Implications for Clinical Practice
Despite the extensive body of literature regarding the negative association of psychological distress to health outcomes in CVD patients, there remains a significant practice gap related to screening, referral, and treatment of psychological distress in CVD patients [30,36,66]. Busy clinical practices focused on physical symptoms (which may not be recognized as mental health–related), along with a health system that has historically regarded mental health issues as the sole domain of mental health professionals creates barriers that need to be overcome. Many health care providers are not proactive in screening patients for psychological distress [11]. Additionally, psychological distress is often perceived as a metaphysical, inexact phenomenon, and is not regarded with the same import as physiological indices, such blood pressure and lipid levels [13]. Another significant barrier may be that the importance of psychological distress, especially depression, has been minimized by some investigators and clinicians because of the lack of data that show improvement with treatment of “hard” outcomes, such as mortality. Lastly, no studies have conclusively demonstrated that treating depression in the general population would lower subsequent cardiovascular clinical events, adding to the minimization of the importance of screening, referral, and treatment of psychological distress among clinicians.
The challenges associated with psychological distress and CVD are centered on the perceived role of the health care provider and role of the patient [11,67]. To date, identification of patients with psychological distress in CVD populations has not been considered a part of routine practice, and only a small percent of those identified with psychological distress are treated. In one study, 17.6% of 1181 patients had moderate to severe depression and of those, only 24.5% were recognized as depressed by their health care providers [68]. In a smaller study of 35 patients with depression after an acute MI, only 10% received treatment with antidepressants [66]. Similarly poor treatment rates were seen in a recent study of antidepressant use after stroke and transient ischemic attack (TIA), with 67.9% of stroke patients and 70.0% of TIA patients with persistent depression going untreated [54].
Stress, depression, anxiety, and PTSD are often not self-contained or experienced in isolation. Rather, some or all of these conditions may present as an interconnected phenomenon [69,70]. There are several reasons for this. First, symptom identification relies on self-report and/or observation of the symptoms, which may confound validity and reliability of diagnosis [70]. Second, the conditions share some symptoms, which may complicate diagnosis of a primary condition. In addition, the overlap of somatic, cognitive, and affective symptoms [71] may deter health care providers from using screening tools that have physical symptoms as part of the screening process. In a recent study of depressed cardiac patients, investigators clustered symptoms and demonstrated that cognitive affective symptoms of depression predict depression in patients with heart disease [72]. Certain events, such as stroke, may make screening especially difficult due to the presence of neurological changes that may complicate the screening process [73]. These issues highlight the need for formalized depression screening tools such as the Patient Health Questionnaire 2-item screening tool (PHQ-2) and/or Patient Health Questionnaire nine-item screening tool (PHQ-9) (Appendix), as recommended by the AHA. The PHQs have been validated in stroke patients and cardiac patients and have been found to be accurate, easy to use, and feasible [35,36,73].
Patients look to their providers for information and security during vulnerable times in their life. In a 2014 study regarding the perceptions of psychosocial consequences and access to support after MI, patients reported a high sense of security regarding being able to contact their providers [74]. Providers in both the primary and acute care settings should use these opportunities to assess for psychological distress. Many inpatient health care providers assume that it is the primary care providers’ responsibility to screen for depression [75]; however, screening should be done in all practice settings caring for patients with CVD. Clinical practice environments should develop policies and procedures to define who, when, and how screening for psychological distress is accomplished using currently available brief screening tools.
A plan for insuring that proper referrals are made following screening to ensure accurate diagnosis, effective treatment, and follow-up should be in place. To date, there is ample evidence that patients benefit from screening in the context of an interdisciplinary treatment approach [34]. Collaborative care, utilizing a team of health professionals (physician, case manager trained in working with patients with psychological distress, and mental health specialist) working with the patient, is an ideal model to improve outcomes [44]. A Cochrane review of 79 RCTs with over 24,000 participants compared utilization of a collaborative team with routine care and found decreased depression and anxiety symptoms in patients receiving team care for up 2 years, along with improved medication adherence, improved quality of life, and improved patient satisfaction with care [39].
Conclusion
Psychological distress remains underrecognized in CVD patients. Brief screening tools such as the PHQ-2 and PHQ-9 are available, easy to use, and reliable for use by clinicians to improve case finding. Primary care providers and cardiovascular specialty providers are called upon to improve the recognition of psychological distress in their patients and assure referrals are made to collaborative care teams for proper diagnosis and treatment of mental health issues. Longitudinal studies focused on the impact of primary/secondary/tertiary psychological distress prevention strategies in the general population, as well as those with CVD, are needed to bring the state of the science forward and provide evidence to enhance the care of those with psychological distress and CVD.
Corresponding author: Anthony McGuire, RN, PhD, ACNP-BC, CSULB, School of Nursing, #2 1250 Bellflower Blvd., Long Beach, CA 90804, [email protected].
Financial disclosures: None
Author contributions: conception and design, AWM, EA, LVD; drafting of article, AWM, EA, LVD; critical revision of the article, AWM, EA, LVD; collection and assembly of data, AWM, EA, LVD.
From the California State University, Long Beach, School of Nursing, Long Beach, CA (Dr. McGuire, Ms. Ahearn), and the University of California, Los Angeles, School of Nursing, Los Angeles, CA (Dr. Doering).
Abstract
- Objective: To review the current literature regarding psychological distress in patients with cardiovascular disease (CVD).
- Methods: Relevant and current (2005–2015) studies were retrieved by a series of searches conducted in the PubMed and PsychINFO databases using Boolean terms/phrases along with manual extraction from the reference lists of pertinent studies. Narrative and tabular summaries of the findings are reported.
- Results: There is a vast literature on psychological distress and CVD. Depression is the most common disorder studied followed by anxiety and post-traumatic stress disorder. Physiologic mechanisms linking psychological distress to CVD are well theorized. Screening for psychological distress in CVD is recommended. Referral and treatment issues need further exploration. Pharmacologic treatment of psychological distress in CVD remains equivocal; however, promising data exists for other therapies such as cognitive behavioral therapy and social support strategies.
- Conclusion: Psychological distress has a significant negative impact on patients with CVD and is underrecognized by health care providers. Primary care providers and cardiovascular specialty providers are called upon to improve their recognition of psychological distress in their patients and assure referrals are made to collaborative care teams for proper diagnosis and treatment.
The association between the heart and the mind has been proposed by scientists since the 17th century. However, it was not until the 1970s that the relationship between cardiovascular disease (CVD) and psychological states came into scientific focus. The study of heart-psyche interactions began with investigations of cardiovascular risk and “type A” personality behaviors (aggressiveness, impatience, a sense of time-urgency, intense achievement drive, seeking recognition) [1,2]. Hundreds of studies generated over the last 10 years have yielded an extensive body of literature regarding this complex interaction.
CVD continues to be the leading cause of death globally. Worldwide and in the United States, CVD accounts for 30% of deaths and more than 2000 deaths per day, respectively [3,4]. Psychological distress (specifically depression) has been reported by the World Health Organization (WHO) as the leading cause of disability in the world [4]. Taken together, CVD and depression constitute an immense health burden and result in poor health status, increased care giver burden [5], increased readmission rates to hospitals, increased utilization of primary care services, poor health compliance [6], decreased health related quality of life [7], and a greater than 2 times increase in mortality [8,9].
Despite its devastating consequences, comorbid CVD and psychological distress remains poorly recognized and treated. In this paper, we present a review of the evidence related to key aspects of psychological distress and CVD (for the purposes of this paper, defined as ischemic heart disease and stroke), and provide information to help improve identification among health care providers. Relevant and current (2005-2015) studies for this review were retrieved by a series of searches conducted in the PubMed and PsychINFO databases using Boolean terms/phrases, along with manual extraction from the reference lists of pertinent studies. Due to the breadth and extent of the literature, a comprehensive review of the literature is beyond the scope of this article. However, the reader will be directed to current systematic reviews, meta-analyses, and recent select research studies sourced for this summary and presented in tabular form.
Mechanisms of Psychological Distress
Psychological Distress Disorders Related to CVD
Depression, anxiety, and post-traumatic stress disorder (PTSD) are the 3 most common psychological distress disorders related to CVD [20]. Cardiac disease and depression has been most commonly studied. In stroke, the science is not as well evolved due to greater heterogeneity of study samples and outcome measures.
Depression
Dysphoria (feeling blue), anhedonia (inability to experience joy in otherwise enjoyable activities), insomnia or hypersomnia, fatigue or loss of energy, increased guilt or worthlessness, decreased concentration, appetite change with significant weight loss or gain, psychomotor retardation or agitation, and suicidal ideation are the symptoms of depression [21]. These symptoms exist on a continuum, ranging from mild symptoms with short duration and limited functional impairment to major depression. Importantly, among otherwise healthy individuals, even minor depressive symptoms have been significantly associated with increased incidence of coronary disease [22].
Screening Issues
In recognition of the high prevalence of depression in patients with CHD, an American Heart Association (AHA) science advisory in 2008 recommended routine screening for depression in patients with CHD, with follow-up evaluation for diagnosis and treatment of depression by qualified professionals for positive cases [26]. In 2014, an AHA scientific statement recommended elevating depression to the level of a risk factor in ACS patients [27]. The recommendation for screening was initially met with some concern as being premature [28], when past supporters spoke out against the routine screening of depression in cardiac patients [29]. The dissenting authors claimed that there was a lack of scientific evidence supporting the efficacy of treatment for depression in cardiac patients, and that potential negative effects of routine screening and follow-up treatment were unknown. They argued the following: limited data from randomized controlled trials and/or evidenced-based reviews exist demonstrating improved outcomes in cardiac patients based on screening and referral [30]; antidepressants are not yet recognized to be effective in cardiac populations and there is a lack of evidence related to potential harms [28]; concerns exist about the potential for mass screening to increase health care resource use at the expense of other health care needs [29]; and routine screening may cause unnecessary negative social stigma related to false-positive findings [31].
Although clinical trials of depression treatment in cardiac patients have not demonstrated an increase in survival, treatment has been shown to be effective in reducing depression symptoms, improving patient satisfaction with depression care and improving health related quality of life [32–34]. Further, recent studies described the AHA recommendation as well accepted by cardiac unit staff, not heavily resource intensive, feasible, and accurate [35,36]. Bigger and Glassman [37] published a recent analytical review of the AHA advisory and concluded that the advisory is supported by the literature. A salient point regarding the depression screening debate is that screening without proper follow-up for further diagnosis and potential treatment may be harmful [28,29,31]. Despite concerns of the potential negative impact of depression screening in cardiac patients raised in the literature, the preponderance of the literature indicates that its benefits are likely to outweigh its risks [32,34,36,38–40].
Outcomes of Depression Treatment
Answers to questions about improvement in cardiovascular and all-cause mortality outcomes with depression treatment remain elusive in the literature. However, data show an improvement in depressive symptoms and quality of life for depressed patients receiving some types of treatment [33,41–45]. The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) study was a landmark study of MI patients with a 6-month treatment intervention of cognitive behavioral therapy (CBT) plus pharmacologic intervention if indicated for depression [33]. Patients were followed for an average of 29 months post-MI. A significant improvement was seen in depressive symptoms and social isolation in the treatment group; however, there was no improvement in event-free survival [33]. When outcome measures are restricted to mortality alone, subsequent trials of antidepressant medications for treatment of depression in cardiac patients have shown them to be ineffective [46]. However, CBT and other supportive stress management strategies are effective in decreasing depressive symptoms and improving the quality of life in patients suffering with depression and CVD [46].
Promising results are emerging in the literature as researchers refocus their analysis on subgroups of depressed cardiac patients. In one large study of 442 depressed and 325 non-depressed patients, the number of depressive symptoms after an MI irrespective of the pre-MI depression status was associated with worse cardiac outcomes [47]. For every 1 additional depressive symptom reported 1 year post-MI, patients had a 15% increased risk for a new cardiac event in the next 2.5 years [47]. Another study demonstrated an improvement in depressive symptoms by 75.3% in in post-cardiac surgery patients with low ejection fraction (< 40%) after 8 weeks of nurse-guided CBT and worsening in depressive symptoms by 26.8% in usual care patients. More moderate findings were seen in the those with higher ejection fraction receiving the same CBT intervention for depression [45]. A treatment-resistant depression subgroup analyzed in a recent secondary analysis of the ENRICHD trial showed a twofold increase in mortality when compared to those in the non–treatment-resistant depression group [48]. Since treatment does not work for all patients with depression, including depressed post-MI patients, further evaluation with a focus on those who respond to treatment is needed.
Depression in Stroke
Issues related to treatment of stroke patients parallel those of depressed patients with cardiac disease, as the effect on mortality and survival is unknown. Depression has been reported to go untreated in up to 67.9% of depressed post-stroke patients [54]. In addition, mismatches between antidepressant prescription and those with depression suggested that some patients without depression were being treated for depression while some patients with depression were not being treated [54].
Anxiety
Anxiety disorders create behavioral disturbances of fear and avoidance related to an individual’s propensity to overestimate dangers [21]. Though a number of anxiety disorders are described in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), anxiety as a general constellation of symptoms (afraid, inability to relax, worry about everyday problems, feelings of panic) is described in most of the literature related to CVD [55–57]. To a lesser extent, generalized anxiety disorder (GAD), defined as anxiety symptoms on more days than not and lasting more than 6 months [58,59] has also been studied. Because anxiety can be a component of depression, the 2 are often discussed together. Taken together, in the context of CHD, individuals with both anxiety and depression are at significantly greater risk for death (odds ratio 2.35, 95% confidence interval 1.23-4.47, P =0.01), compared with those without symptoms [60].
Post-Traumatic Stress Disorder
PTSD presents with a heterogeneous cluster of symptoms that are generally described as avoidance, re-experiencing, arousal, and negative cognitions and mood [21]. Previously classified as a anxiety disorder in DSM-IV, PTSD is in a new category of trauma and stress or related disorders in the revised DSM-V [21]. In addition, the temporal component of the symptoms has been changed to a disturbance lasting more than 1 month, without reference to acuity or chronicity.
Implications for Clinical Practice
Despite the extensive body of literature regarding the negative association of psychological distress to health outcomes in CVD patients, there remains a significant practice gap related to screening, referral, and treatment of psychological distress in CVD patients [30,36,66]. Busy clinical practices focused on physical symptoms (which may not be recognized as mental health–related), along with a health system that has historically regarded mental health issues as the sole domain of mental health professionals creates barriers that need to be overcome. Many health care providers are not proactive in screening patients for psychological distress [11]. Additionally, psychological distress is often perceived as a metaphysical, inexact phenomenon, and is not regarded with the same import as physiological indices, such blood pressure and lipid levels [13]. Another significant barrier may be that the importance of psychological distress, especially depression, has been minimized by some investigators and clinicians because of the lack of data that show improvement with treatment of “hard” outcomes, such as mortality. Lastly, no studies have conclusively demonstrated that treating depression in the general population would lower subsequent cardiovascular clinical events, adding to the minimization of the importance of screening, referral, and treatment of psychological distress among clinicians.
The challenges associated with psychological distress and CVD are centered on the perceived role of the health care provider and role of the patient [11,67]. To date, identification of patients with psychological distress in CVD populations has not been considered a part of routine practice, and only a small percent of those identified with psychological distress are treated. In one study, 17.6% of 1181 patients had moderate to severe depression and of those, only 24.5% were recognized as depressed by their health care providers [68]. In a smaller study of 35 patients with depression after an acute MI, only 10% received treatment with antidepressants [66]. Similarly poor treatment rates were seen in a recent study of antidepressant use after stroke and transient ischemic attack (TIA), with 67.9% of stroke patients and 70.0% of TIA patients with persistent depression going untreated [54].
Stress, depression, anxiety, and PTSD are often not self-contained or experienced in isolation. Rather, some or all of these conditions may present as an interconnected phenomenon [69,70]. There are several reasons for this. First, symptom identification relies on self-report and/or observation of the symptoms, which may confound validity and reliability of diagnosis [70]. Second, the conditions share some symptoms, which may complicate diagnosis of a primary condition. In addition, the overlap of somatic, cognitive, and affective symptoms [71] may deter health care providers from using screening tools that have physical symptoms as part of the screening process. In a recent study of depressed cardiac patients, investigators clustered symptoms and demonstrated that cognitive affective symptoms of depression predict depression in patients with heart disease [72]. Certain events, such as stroke, may make screening especially difficult due to the presence of neurological changes that may complicate the screening process [73]. These issues highlight the need for formalized depression screening tools such as the Patient Health Questionnaire 2-item screening tool (PHQ-2) and/or Patient Health Questionnaire nine-item screening tool (PHQ-9) (Appendix), as recommended by the AHA. The PHQs have been validated in stroke patients and cardiac patients and have been found to be accurate, easy to use, and feasible [35,36,73].
Patients look to their providers for information and security during vulnerable times in their life. In a 2014 study regarding the perceptions of psychosocial consequences and access to support after MI, patients reported a high sense of security regarding being able to contact their providers [74]. Providers in both the primary and acute care settings should use these opportunities to assess for psychological distress. Many inpatient health care providers assume that it is the primary care providers’ responsibility to screen for depression [75]; however, screening should be done in all practice settings caring for patients with CVD. Clinical practice environments should develop policies and procedures to define who, when, and how screening for psychological distress is accomplished using currently available brief screening tools.
A plan for insuring that proper referrals are made following screening to ensure accurate diagnosis, effective treatment, and follow-up should be in place. To date, there is ample evidence that patients benefit from screening in the context of an interdisciplinary treatment approach [34]. Collaborative care, utilizing a team of health professionals (physician, case manager trained in working with patients with psychological distress, and mental health specialist) working with the patient, is an ideal model to improve outcomes [44]. A Cochrane review of 79 RCTs with over 24,000 participants compared utilization of a collaborative team with routine care and found decreased depression and anxiety symptoms in patients receiving team care for up 2 years, along with improved medication adherence, improved quality of life, and improved patient satisfaction with care [39].
Conclusion
Psychological distress remains underrecognized in CVD patients. Brief screening tools such as the PHQ-2 and PHQ-9 are available, easy to use, and reliable for use by clinicians to improve case finding. Primary care providers and cardiovascular specialty providers are called upon to improve the recognition of psychological distress in their patients and assure referrals are made to collaborative care teams for proper diagnosis and treatment of mental health issues. Longitudinal studies focused on the impact of primary/secondary/tertiary psychological distress prevention strategies in the general population, as well as those with CVD, are needed to bring the state of the science forward and provide evidence to enhance the care of those with psychological distress and CVD.
Corresponding author: Anthony McGuire, RN, PhD, ACNP-BC, CSULB, School of Nursing, #2 1250 Bellflower Blvd., Long Beach, CA 90804, [email protected].
Financial disclosures: None
Author contributions: conception and design, AWM, EA, LVD; drafting of article, AWM, EA, LVD; critical revision of the article, AWM, EA, LVD; collection and assembly of data, AWM, EA, LVD.
1. Dembroski TM, MacDougall JM, Shields JL, et al. Components of the type A coronary-prone behavior pattern and cardiovascular responses to psychomotor performance challenge. J Behav Med 1978;1:159–76.
2. Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation 2005;111:250–3.
3. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292.
4. Mathers C, Stevens G, Mascarenhas M. Global health risks: Mortality and burden of diesease attributable to select major risks. Geneva, Switzerland: World Health Organization, 2009.
5. Randall G, Molloy GJ, Steptoe A. The impact of an acute cardiac event on the partners of patients: A systematic review. Health Psychol Rev 2009;3:1–84.
6. Kronish IM, Rieckmann N, Halm EA, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med 2006;21:1178–83.
7. Stafford L, Berk M, Reddy P, Jackson HJ. Comorbid depression and health-related quality of life in patients with coronary artery disease. J Psychosom Res 2007;62:401–10.
8. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004;66:802–13.
9. van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004;66:814–22.
10. Doyle F, McGee HM, Conroy RM, Delaney M. What predicts depression in cardiac patients: sociodemographic factors, disease severity or theoretical vulnerabilities? Psychol Health 2011;26:619–34.
11. Figueredo VM. The time has come for physicians to take notice: the impact of psychosocial stressors on the heart. Am J Med 2009;122:704–12.
12. Matthews KA. Matters of the heart: advancing psychological perspectives on cardiovascular diseases. Persp Psychol Sci 2013;8:676–8.
13. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol 2013;9:327–54.
14. Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation 2011;124:346–54.
15. Hamer M, Molloy GJ, Stamatakis E. Psychological distress as a risk factor for cardiovascular events: pathophysiological and behavioral mechanisms. J Am Coll Cardiol 2008;52:2156–62.
16. Richardson S, Shaffer JA, Falzon L, et al. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol 2012;110:1711–6.
17. Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol 2012;9:360–70.
18. Brumby S, Chandrasekara A, McCoombe S, et al. Cardiovascular risk factors and psychological distress in Australian farming communities. Aust J Rural Health 2012;20:131–7.
19. Arnold SV, Smolderen KG, Buchanan DM, et al. Perceived stress in myocardial infarction long-term mortality and health status outcomes. J Am Coll Cardiol 2012;60:1756–63.
20. Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens 2015.
21. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
22. Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med 2006;68:645–50.
23. Niranjan A, Corujo A, Ziegelstein RC, Nwulia E. Depression and heart disease in US adults. Gen Hosp Psychiatry 2012;34:254–61.
24. Seldenrijk A, Vogelzangs N, Batelaan NM, et al. Depression, anxiety and 6-year risk of cardiovascular disease. J Psychosom Res 2015;78:123–9.
25. Ayerbe L, Ayis S, Crichton S, et al. The natural history of depression up to 15 years after stroke: The South London Stroke Register. Stroke 2013.
26. Lichtman JH, Bigger JT Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 2008;118:1768–75.
27. Lichtman JH, Froelicher ES, Blumenthal JA, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350–69.
28. Hasnain M, Vieweg WV, Lesnefsky EJ, Pandurangi AK. Depression screening in patients with coronary heart disease: a critical evaluation of the AHA guidelines. J Psychosom Res 2011;71:6–12.
29. Ziegelstein RC, Thombs BD, Coyne JC, de Jonge P. Routine screening for depression in patients with coronary heart disease never mind. J Am Coll Cardiol 2009;54:886–90.
30. Ziegelstein RC, Kim SY, Kao D, et al. Can doctors and nurses recognize depression in patients hospitalized with an acute myocardial infarction in the absence of formal screening? Psychosom Med 2005;67:393–7.
31. Whooley MA. To screen or not to screen? Depression in patients with cardiovascular disease. J Am Coll Cardiol 2009;54:891–3.
32. Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med 2010;170:600–8.
33. Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106–16.
34. Whooley M, Unutzer J. Interdisciplinary stepped care for depression after acute coronary syndrome. Arch Intern Med 2010;170:585–6.
35. McGuire AW, Eastwood JA, Macabasco-O’Connell A, et al. Depression screening: utility of the patient health questionnaire in patients with acute coronary syndrome. Am J Crit Care 2013;22:12–9.
36. Sowden G, Mastromauro CA, Januzzi JL, et al. Detection of depression in cardiac inpatients: feasibility and results of systematic screening. Am Heart J 2010;159:780–7.
37. Bigger JT, Glassman AH. The American Heart Association science advisory on depression and coronary heart disease: an exploration of the issues raised. Cleve Clin J Med 2010;77 Suppl 3:S12–9.
38. Page KN, Davidson P, Edward KL, et al. Recovering from an acute cardiac event--the relationship between depression and life satisfaction. J Clin Nurs 2010;19:736–43.
39. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10:CD006525.
40. Blumenthal JA, O’Connor C. No laughing matter. J Am Coll Cardiol 2010;55:836.
41. Davidson KW, Korin MR. Depression and cardiovascular disease: selected findings, controversies, and clinical implications from 2009. Cleve Clin J Med 2010;77 Suppl 3:S20–6.
42. Doering LV, McGuire A, Eastwood JA, et al. Cognitive behavioral therapy for depression improves pain and perceived control in cardiac surgery patients. Eur J Cardiovasc Nurs 2015.
43. Freedland KE, Skala JA, Carney RM, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry 2009;66:387–96.
44. Huffman JC, Mastromauro CA, Sowden GL, et al. collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics 2011;52:26–33.
45. Hwang B, Eastwood JA, McGuire A, et al. Cognitive behavioral therapy in depressed cardiac surgery patients: role of ejection fraction. J Cardiovasc Nurs 2015;30:319–24.
46. Mavrides N, Nemeroff C. Treatment of depression in cardiovascular disease. Depression Anxiety 2013;30:328–41.
47. Zuidersma M, Ormel J, Conradi HJ, de Jonge P. An increase in depressive symptoms after myocardial infarction predicts new cardiac events irrespective of depressive symptoms before myocardial infarction. Psychol Med 2012;42:683–93.
48. Banankhah SK, Friedmann E, Thomas S. Effective treatment of depression improves post-myocardial infarction survival. World J Cardiol 2015;7:215–23.
49. Ayerbe L, Ayis S, Crichton S, et al. The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J Neurol Neurosurg Psychiatry 2014;85:514–21.
50. Hama S, Yamashita H, Yamawaki S, Kurisu K. Post-stroke depression and apathy: Interactions between functional recovery, lesion location, and emotional response. Psychogeriatrics 2011;11:68–76.
51. Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovasc Dis 2013;35:23–39.
52. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag 2013;9:483–9.
53.Karamchandani R, Vahidy F, Bajgur S, et al. Early Depression Screening is Feasible in Hospitalized Stroke Patients. Neurology 2014;82(10 Supplement):S62.005.
54. El Husseini N, Goldstein LB, Peterson ED, et al. Depression and antidepressant use after stroke and transient ischemic attack. Stroke 2012;43:1609–16.
55. D’Aniello GE, Scarpina F, Mauro A, Mori I, et al. Characteristics of anxiety and psychological well-being in chronic post-stroke patients. J Neurol Sci 2014;338:191–6.
56. Huffman JC, Smith FA, Blais MA, et al. Anxiety, independent of depressive symptoms, is associated with in-hospital cardiac complications after acute myocardial infarction. J Psychosom Res 2008;65:557–63.
57. Shen B-J, Avivi YE, Todaro JF, et al. Anxiety characteristics independently and prospectively predict myocardial infarction in men: the unique contribution of anxiety among psychologic factors. J Am Coll Cardiol 2008;51:113–9.
58. Butnoriene J, Bunevicius A, Saudargiene A, et al. Metabolic syndrome, major depression, generalized anxiety disorder, and ten-year all-cause and cardiovascular mortality in middle aged and elderly patients. Int J Cardiol 2015;190:360–6.
59. Roest AM, Zuidersma M, de Jonge P. Myocardial infarction and generalised anxiety disorder: 10-year follow-up. Br J Psychiatry 2012;200:324–9.
60. Doering LV, Moser DK, Riegel B, et al. Persistent comorbid symptoms of depression and anxiety predict mortality in heart disease. Int J Cardiol 2010;145:188–92.
61. Edmondson D, Kronish IM, Shaffer JA, et al. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J 2013;166:806–14.
62. Ahmadi N, Hajsadeghi F, Mirshkarlo HB, et al. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol 2011;108:29–33.
63. Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health Psychol 2012;31:194–201.
64. Chung MC, Dennis I, Berger Z, et al. Posttraumatic stress disorder following myocardial infarction: personality, coping, and trauma exposure characteristics. Int J Psychiatry Med 2011;42:393–419.
65. Bluvstein I, Moravchick L, Sheps D, et al. Posttraumatic growth, posttraumatic stress symptoms and mental health among coronary heart disease survivors. J Clin Psychol Med Settings 2013;20:164–72.
66. Huffman JC, Smith FA, Blais MA, et al. Recognition and treatment of depression and anxiety in patients with acute myocardial infarction. Am J Cardiol 2006;98:319–24.
67. Crosson JC, Heisler M, Subramanian U, et al. Physicians’ perceptions of barriers to cardiovascular disease risk factor control among patients with diabetes: results from the translating research into action for diabetes (TRIAD) study. J Am Board Fam Med 2010;23:171–8.
68. Amin AA, Jones AM, Nugent K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J 2006;152:928–34.
69. Chung MC, Berger Z, Jones R, Rudd H. Posttraumatic stress and co-morbidity following myocardial infarction among older patients: the role of coping. Aging Ment Health 2008;12:124–33.
70. Neylon A, Canniffe C, Anand S, et al. A global perspective on psychosocial risk factors for cardiovascular disease. Prog Cardiovasc Dis 2013;55:574–81.
71. Carney RM, Freedland KE. Are somatic symptoms of depression better predictors of cardiac events than cognitive symptoms in coronary heart disease? Psychosom Med 2012;74:33–8.
72. McGuire AW, Eastwood JA, Hays RD, Macabasco-O’Connell A, et al. Depressed or not depressed: untangling symptoms of depression in patients hospitalized with coronary heart disease. Am J Crit Care 2014;23:106–16.
73. Williams LS, Brizendine EJ, Plue L, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke 2005;36:635–8.
74. Junehag L, Asplund K, Svedlund M. A qualitative study: perceptions of the psychosocial consequences and access to support after an acute myocardial infarction. Intensive Crit Care Nurs 2014;30:22–30.
75. Lea P. Factors affecting nurses’ intent to assess for depression in heart failure patients. Dimens Crit Care Nurs 2014;33:320–6.
1. Dembroski TM, MacDougall JM, Shields JL, et al. Components of the type A coronary-prone behavior pattern and cardiovascular responses to psychomotor performance challenge. J Behav Med 1978;1:159–76.
2. Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation 2005;111:250–3.
3. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292.
4. Mathers C, Stevens G, Mascarenhas M. Global health risks: Mortality and burden of diesease attributable to select major risks. Geneva, Switzerland: World Health Organization, 2009.
5. Randall G, Molloy GJ, Steptoe A. The impact of an acute cardiac event on the partners of patients: A systematic review. Health Psychol Rev 2009;3:1–84.
6. Kronish IM, Rieckmann N, Halm EA, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med 2006;21:1178–83.
7. Stafford L, Berk M, Reddy P, Jackson HJ. Comorbid depression and health-related quality of life in patients with coronary artery disease. J Psychosom Res 2007;62:401–10.
8. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004;66:802–13.
9. van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004;66:814–22.
10. Doyle F, McGee HM, Conroy RM, Delaney M. What predicts depression in cardiac patients: sociodemographic factors, disease severity or theoretical vulnerabilities? Psychol Health 2011;26:619–34.
11. Figueredo VM. The time has come for physicians to take notice: the impact of psychosocial stressors on the heart. Am J Med 2009;122:704–12.
12. Matthews KA. Matters of the heart: advancing psychological perspectives on cardiovascular diseases. Persp Psychol Sci 2013;8:676–8.
13. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol 2013;9:327–54.
14. Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation 2011;124:346–54.
15. Hamer M, Molloy GJ, Stamatakis E. Psychological distress as a risk factor for cardiovascular events: pathophysiological and behavioral mechanisms. J Am Coll Cardiol 2008;52:2156–62.
16. Richardson S, Shaffer JA, Falzon L, et al. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol 2012;110:1711–6.
17. Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol 2012;9:360–70.
18. Brumby S, Chandrasekara A, McCoombe S, et al. Cardiovascular risk factors and psychological distress in Australian farming communities. Aust J Rural Health 2012;20:131–7.
19. Arnold SV, Smolderen KG, Buchanan DM, et al. Perceived stress in myocardial infarction long-term mortality and health status outcomes. J Am Coll Cardiol 2012;60:1756–63.
20. Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens 2015.
21. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
22. Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med 2006;68:645–50.
23. Niranjan A, Corujo A, Ziegelstein RC, Nwulia E. Depression and heart disease in US adults. Gen Hosp Psychiatry 2012;34:254–61.
24. Seldenrijk A, Vogelzangs N, Batelaan NM, et al. Depression, anxiety and 6-year risk of cardiovascular disease. J Psychosom Res 2015;78:123–9.
25. Ayerbe L, Ayis S, Crichton S, et al. The natural history of depression up to 15 years after stroke: The South London Stroke Register. Stroke 2013.
26. Lichtman JH, Bigger JT Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 2008;118:1768–75.
27. Lichtman JH, Froelicher ES, Blumenthal JA, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350–69.
28. Hasnain M, Vieweg WV, Lesnefsky EJ, Pandurangi AK. Depression screening in patients with coronary heart disease: a critical evaluation of the AHA guidelines. J Psychosom Res 2011;71:6–12.
29. Ziegelstein RC, Thombs BD, Coyne JC, de Jonge P. Routine screening for depression in patients with coronary heart disease never mind. J Am Coll Cardiol 2009;54:886–90.
30. Ziegelstein RC, Kim SY, Kao D, et al. Can doctors and nurses recognize depression in patients hospitalized with an acute myocardial infarction in the absence of formal screening? Psychosom Med 2005;67:393–7.
31. Whooley MA. To screen or not to screen? Depression in patients with cardiovascular disease. J Am Coll Cardiol 2009;54:891–3.
32. Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med 2010;170:600–8.
33. Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106–16.
34. Whooley M, Unutzer J. Interdisciplinary stepped care for depression after acute coronary syndrome. Arch Intern Med 2010;170:585–6.
35. McGuire AW, Eastwood JA, Macabasco-O’Connell A, et al. Depression screening: utility of the patient health questionnaire in patients with acute coronary syndrome. Am J Crit Care 2013;22:12–9.
36. Sowden G, Mastromauro CA, Januzzi JL, et al. Detection of depression in cardiac inpatients: feasibility and results of systematic screening. Am Heart J 2010;159:780–7.
37. Bigger JT, Glassman AH. The American Heart Association science advisory on depression and coronary heart disease: an exploration of the issues raised. Cleve Clin J Med 2010;77 Suppl 3:S12–9.
38. Page KN, Davidson P, Edward KL, et al. Recovering from an acute cardiac event--the relationship between depression and life satisfaction. J Clin Nurs 2010;19:736–43.
39. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10:CD006525.
40. Blumenthal JA, O’Connor C. No laughing matter. J Am Coll Cardiol 2010;55:836.
41. Davidson KW, Korin MR. Depression and cardiovascular disease: selected findings, controversies, and clinical implications from 2009. Cleve Clin J Med 2010;77 Suppl 3:S20–6.
42. Doering LV, McGuire A, Eastwood JA, et al. Cognitive behavioral therapy for depression improves pain and perceived control in cardiac surgery patients. Eur J Cardiovasc Nurs 2015.
43. Freedland KE, Skala JA, Carney RM, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry 2009;66:387–96.
44. Huffman JC, Mastromauro CA, Sowden GL, et al. collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics 2011;52:26–33.
45. Hwang B, Eastwood JA, McGuire A, et al. Cognitive behavioral therapy in depressed cardiac surgery patients: role of ejection fraction. J Cardiovasc Nurs 2015;30:319–24.
46. Mavrides N, Nemeroff C. Treatment of depression in cardiovascular disease. Depression Anxiety 2013;30:328–41.
47. Zuidersma M, Ormel J, Conradi HJ, de Jonge P. An increase in depressive symptoms after myocardial infarction predicts new cardiac events irrespective of depressive symptoms before myocardial infarction. Psychol Med 2012;42:683–93.
48. Banankhah SK, Friedmann E, Thomas S. Effective treatment of depression improves post-myocardial infarction survival. World J Cardiol 2015;7:215–23.
49. Ayerbe L, Ayis S, Crichton S, et al. The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J Neurol Neurosurg Psychiatry 2014;85:514–21.
50. Hama S, Yamashita H, Yamawaki S, Kurisu K. Post-stroke depression and apathy: Interactions between functional recovery, lesion location, and emotional response. Psychogeriatrics 2011;11:68–76.
51. Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovasc Dis 2013;35:23–39.
52. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag 2013;9:483–9.
53.Karamchandani R, Vahidy F, Bajgur S, et al. Early Depression Screening is Feasible in Hospitalized Stroke Patients. Neurology 2014;82(10 Supplement):S62.005.
54. El Husseini N, Goldstein LB, Peterson ED, et al. Depression and antidepressant use after stroke and transient ischemic attack. Stroke 2012;43:1609–16.
55. D’Aniello GE, Scarpina F, Mauro A, Mori I, et al. Characteristics of anxiety and psychological well-being in chronic post-stroke patients. J Neurol Sci 2014;338:191–6.
56. Huffman JC, Smith FA, Blais MA, et al. Anxiety, independent of depressive symptoms, is associated with in-hospital cardiac complications after acute myocardial infarction. J Psychosom Res 2008;65:557–63.
57. Shen B-J, Avivi YE, Todaro JF, et al. Anxiety characteristics independently and prospectively predict myocardial infarction in men: the unique contribution of anxiety among psychologic factors. J Am Coll Cardiol 2008;51:113–9.
58. Butnoriene J, Bunevicius A, Saudargiene A, et al. Metabolic syndrome, major depression, generalized anxiety disorder, and ten-year all-cause and cardiovascular mortality in middle aged and elderly patients. Int J Cardiol 2015;190:360–6.
59. Roest AM, Zuidersma M, de Jonge P. Myocardial infarction and generalised anxiety disorder: 10-year follow-up. Br J Psychiatry 2012;200:324–9.
60. Doering LV, Moser DK, Riegel B, et al. Persistent comorbid symptoms of depression and anxiety predict mortality in heart disease. Int J Cardiol 2010;145:188–92.
61. Edmondson D, Kronish IM, Shaffer JA, et al. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J 2013;166:806–14.
62. Ahmadi N, Hajsadeghi F, Mirshkarlo HB, et al. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol 2011;108:29–33.
63. Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health Psychol 2012;31:194–201.
64. Chung MC, Dennis I, Berger Z, et al. Posttraumatic stress disorder following myocardial infarction: personality, coping, and trauma exposure characteristics. Int J Psychiatry Med 2011;42:393–419.
65. Bluvstein I, Moravchick L, Sheps D, et al. Posttraumatic growth, posttraumatic stress symptoms and mental health among coronary heart disease survivors. J Clin Psychol Med Settings 2013;20:164–72.
66. Huffman JC, Smith FA, Blais MA, et al. Recognition and treatment of depression and anxiety in patients with acute myocardial infarction. Am J Cardiol 2006;98:319–24.
67. Crosson JC, Heisler M, Subramanian U, et al. Physicians’ perceptions of barriers to cardiovascular disease risk factor control among patients with diabetes: results from the translating research into action for diabetes (TRIAD) study. J Am Board Fam Med 2010;23:171–8.
68. Amin AA, Jones AM, Nugent K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J 2006;152:928–34.
69. Chung MC, Berger Z, Jones R, Rudd H. Posttraumatic stress and co-morbidity following myocardial infarction among older patients: the role of coping. Aging Ment Health 2008;12:124–33.
70. Neylon A, Canniffe C, Anand S, et al. A global perspective on psychosocial risk factors for cardiovascular disease. Prog Cardiovasc Dis 2013;55:574–81.
71. Carney RM, Freedland KE. Are somatic symptoms of depression better predictors of cardiac events than cognitive symptoms in coronary heart disease? Psychosom Med 2012;74:33–8.
72. McGuire AW, Eastwood JA, Hays RD, Macabasco-O’Connell A, et al. Depressed or not depressed: untangling symptoms of depression in patients hospitalized with coronary heart disease. Am J Crit Care 2014;23:106–16.
73. Williams LS, Brizendine EJ, Plue L, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke 2005;36:635–8.
74. Junehag L, Asplund K, Svedlund M. A qualitative study: perceptions of the psychosocial consequences and access to support after an acute myocardial infarction. Intensive Crit Care Nurs 2014;30:22–30.
75. Lea P. Factors affecting nurses’ intent to assess for depression in heart failure patients. Dimens Crit Care Nurs 2014;33:320–6.
Prostate Cancer in Male Seniors, Part 2: Treatment
This article (part 2 of 2) focuses on the treatment of prostate cancer in seniors. Part 1 provided an overview of prostate cancer epidemiology, pathology, and screening in senior patients.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Recently, the International Society of Geriatric Oncology assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
Note: Page numbers differ between the print issue and digital edition.
This article (part 2 of 2) focuses on the treatment of prostate cancer in seniors. Part 1 provided an overview of prostate cancer epidemiology, pathology, and screening in senior patients.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Recently, the International Society of Geriatric Oncology assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 2 of 2) focuses on the treatment of prostate cancer in seniors. Part 1 provided an overview of prostate cancer epidemiology, pathology, and screening in senior patients.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Recently, the International Society of Geriatric Oncology assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
Note: Page numbers differ between the print issue and digital edition.
Note: Page numbers differ between the print issue and digital edition.
Sustentaculum Lunatum: Appreciation of the Palmar Lunate Facet in Management of Complex Intra-Articular Fractures of the Distal Radius
Fracture of the distal radius is the wrist injury most often encountered by orthopedic and hand surgeons.1 The number of fractures of the distal radius in the United States was estimated to be 640,000 in 2001, and the incidence is increasing.2,3 Recent evidence has shown a substantial increase in treating these fractures with internal rather than closed fixation, even in the elderly.4
Treatment of complex intra-articular fractures of the distal radius requires an accurate diagnosis of the fracture pattern and a thoughtful approach to fixation. Although a majority of the fractures that meet the operative criteria are now treated with various anterior locked-plating techniques with good results, a subset requires more technically demanding fixation approaches, including fragment-specific approaches, dorsal and palmar plating, and combined internal and external fixation.
The sustentaculum lunatum, as we have named the palmar lunate facet, deserves specific attention because of its importance in load transmission across the radiocarpal joint and its key role in restoring the anatomy of the palmar distal radial metaphysis during internal fixation. This fragment in comminuted fractures was first ascribed special importance by Melone5 in his description of common fracture patterns. In the present article, we describe the anatomical characteristics of the sustentaculum lunatum and the clinical relevance of this fragment to management of fractures of the distal radius.
Classification
A variety of classification systems have been proposed to characterize and guide treatment of fractures of the distal radius. The earliest descriptions of fracture patterns were presented by Castaing6 and Frykman7 in the 1960s. The Frykman classification historically has been popular but is limited in accuracy in its characterization of fragments and their displacement and is limited in its ability to guide treatment. The classification system proposed by Melone and colleagues5,8-10 was the first to truly describe fracture of the distal radius fragments in a relevant manner, including their characteristic “4 parts” (Figure 1). The authors emphasized the importance of the “medial complex” as the cornerstone of the radiocarpal and radioulnar joints.
The classification system developed by Müller and colleagues,11 which was adopted by the AO (Arbeitsgemeinschaft für Osteosynthesefragen), might be the most descriptive and informative system, and it is widely used to conduct research and direct treatment. This system classifies fractures into A (extra-articular), B (partial articular), and C (complete articular) types and subclassifies them according to fracture location and comminution. These classifications, along with a conceptualization of the distal forearm as a 3-column structure involving the radial, ulnar, and intermediate columns (including the lunate facet), as proposed by Peine and colleagues,12 gave us a framework for approaching fixation of fractures of the distal radius.
Etymology and Definition
Sustentaculum, from the Latin sustinere, “to support, check, or put off,” and taculum, “receptacle or holding space,” is a fitting description of the most distal portion of the palmar lunate facet, as it supports and holds the carpus, and specifically the lunate, on the radial articular surface. This portion is analogous to the sustentaculum tali, the named portion of the calcaneus that supports and articulates with the middle calcaneal articular surface of the talus13 and provides a reliable fragment for internal fixation of the calcaneus.
Anatomical and Biomechanical Considerations
The distal radial articular surface is composed of distinct scaphoid and lunate facets that articulate with their respective carpal bones. Several studies have characterized the anatomy of the distal radius.14-17 Linscheid14 found that the lunate and scaphoid facets account for 46% and 43% of the contact area across the radiocarpal joint, respectively; this has been corroborated by others.15 A biomechanical study by Genda and Horii18 showed that the majority of stress across the wrist joint was concentrated at the palmar side of the distal radius in the neutral position. Although it is recognized that the scaphoid facet bears most of the load across the wrist in the neutral wrist position, most activities of daily living place the wrist in a slightly extended and ulnarly deviated position. This position results in a shift of the majority of load to the radiolunar articulation, constituting 53% of total force transmission.18 Subchondral bone density analyses have supported this lunate-predominant loading pattern across the radiocarpal articulation in most people.19 This loading pattern is also supported by the observation that failure of fixation and carpal subluxation generally occurs at the radiolunate articulation.
The palmar lip of the distal radius traditionally has been depicted and conceptualized as a flat extension of the metaphysis, leading to the development of implants that are not ideally designed for capturing this area in the fracture setting. A 3-dimensional (3-D) computed tomography (CT) study of the distal radii of healthy volunteers, conducted by Andermahr and colleagues,20 showed that the contour of the palmar lunate facet projects from the palmar cortex of the radius by 3 mm on average and is about 19 mm in width (radial to ulnar dimension) (Figures 2A-2C). In the axial plane, the anterior cortex of the distal radius slopes in a palmar direction, from radial to ulnar. This presents a challenge in attempts to support the entire surface (scaphoid and lunate facets) with a single palmar implant.20-25
A study conducted by Harness and colleagues24 showed that the majority of palmar shear fractures are composed of multiple fragments of the lunar articular facet. Anatomical studies of the distal radiocarpal articulation have also described the ligamentous attachments to the sustentaculum lunatum.26 The short radiolunate ligament, which originates from this fragment and inserts onto the lunate, provides stability to the carpus and, if not adequately fixed, leads to an incompetent restraint to palmar carpal translation. Isolated injuries of the short radiolunate ligament or fractures of the palmar lunate facet have been shown to result in palmar carpal translation.27,28 In addition, attachments of the palmar radioulnar ligament and other more ulnar radiocarpal ligaments act as deforming forces on the palmar lunate facet.24,26
Fracture Pattern Recognition
Although the AO type B palmar shear fracture pattern, also known as the Barton fracture, has classically been recognized as the fracture involving the palmar lunate facet and requiring special attention, many complete articular fractures feature involvement and fragmentation of this portion of the distal radius (Figures 3A-3F).29 In highly comminuted complete articular and palmar shear fracture patterns, the morphology of the sustentaculum lunatum should be appreciated, and its adequate fixation to the radial metaphysis ensured, to prevent loss of reduction.
Visualization of the palmar lunate facet as a distinct fragment might be difficult in cases of highly comminuted fracture patterns. Standard CT or more recently described 3-D CT techniques with subtraction of the carpus might facilitate appreciation of this fragment for preoperative planning of approach and fixation.29,30 Our institutional protocol involves obtaining preoperative traction radiographs of every fracture of the distal radius. These radiographs have reduced the need for CT in understanding the fracture pattern and aid in decision making.31
Besides appreciating the existence of the sustentaculum lunatum fragment, we should recognize that some injury patterns that split the lunate facet into unstable dorsal and palmar fragments might necessitate a separate dorsal approach to reduce and fix the dorsal lunate fragment. Traction radiographs can be especially useful in recognizing these patterns (a V sign is present) (Figures 4A, 4B).
Open Fractures
Highly comminuted fractures of the distal radius presenting with displaced lunate facet fragments can have high-energy mechanisms of injury. Although open fractures of the distal radius are associated with lower risk for infection (compared with open fractures of other long bones), they deserve special attention because of associated tendon and neurovascular injuries. Few studies have specifically assessed open fractures of the distal radius.32-35 Only the study by Rozental and Blazar34 listed associated injuries at the wrist level. The authors identified 4 patients (out of 18) with concomitant flexor tendon or neurovascular injuries that included radial or ulnar artery injury. In our experience, many open fractures of the distal radius are caused by an inside-out mechanism and present with an open wound either over the ulnar styloid or in the area of the ulnar side of the palmar radial metaphysis corresponding to the metaphyseal spike that mates with the sustentaculum lunatum (Figures 5A, 5B). Given these findings, we approach this intermediate column with particular care in cases of open fracture, paying attention to important structures (flexors, neurovascular) and looking for contamination from the environment into the fracture.
Fixation Techniques
The approach to fixation of partial articular palmar shear fractures is fairly straightforward. Buttress plate fixation has been well described and has had reliably good results.36 However, in very distal fracture patterns and in cases in which the palmar lunate facet is fragmented as part of a complete articular fracture, a fragment-specific approach to fixation with or without spanning external fixation often is necessary.37 The unrecognized sustentaculum lunatum fragment in comminuted complete articular fractures can lead to inadequate fixation constructs, resulting in loss of reduction and carpal subluxation in a palmar direction.24,34,38
Our surgical approach uses the standard anterior interval between the radial artery and the flexor carpi radialis, as described by Henry.39 The flexor pollicis longus is retracted ulnarly, revealing the pronator quadratus. We then reflect the pronator quadratus from the distal radial metaphysis until the most proximal and ulnar extent of the fracture is easily visualized. The palmar ulnar metaphyseal cortex that mates with the displaced sustentaculum lunatum is, in our experience, often the least comminuted portion of the metaphysis, thus providing a cortical key for restoration of height and alignment (Figures 5A, 5B). At our institution, fixation typically is achieved by contouring miniplates (1.3 or 1.5 mm) to capture and buttress the sustentaculum lunatum (Figures 6A, 6B). In our experience, the screw lengths in the most distal fixed-angle constructs at the palmar lip are limited to 6 mm or less to avoid penetration of the articular surface, though this has not been previously reported in the literature. After restoring the length and tilt of this intermediate column of the distal radius, we proceed with “rebuilding” the remainder of the fragments to our stabilized initial construct.
Various authors40-43 have described alternative fixation methods for the palmar lunate facet fragment. Jupiter and Marent-Huber42 described 2.4-mm locked-plate fixation with either a standard palmar plate or T- or L-plates for cases in which the palmar lip fragment is very distal and small. In fact, some newer anatomical distal radius implants include features designed to target these fragments (Figures 7A, 7B). An alternative fixation method involves use of a 26-gauge stainless steel wire passed through drill holes in the metaphysis 1 cm proximal to the fracture and then passed through the palmar capsule just distal to the fragment and secured in figure-8 fashion while the fragment is manually held reduced.41 Still others have recommended limited internal fixation of the sustentaculum lunatum through an ulna-sided palmar approach to the distal radius (between the ulnar neurovascular bundle and the flexor tendons) combined with external fixation to restore length and palmar tilt in highly comminuted fractures.40,43
A method involving arthroscopically assisted reduction and fixation of the lunate facet has also been described, though this procedure is technically demanding and has limited indications.44 It uses a Freer elevator passed through the standard 3-4 portal after initial visualization and evacuation of hematoma. The Freer elevator is used to disimpact the sustentaculum lunatum and to elevate it from its depressed position. With the dorsal lunate facet left displaced to facilitate access to the palmar fragment, a nerve hook retractor is used to reduce the palmar facet to the radial styloid, and Kirschner wires are used to achieve interfragmentary fixation. The dorsal lunate fragment is then pieced back to the articular segment, and the entire construct is fixed to the radial metaphysis with additional Kirschner wires.
Discussion
Given the increasing incidence of fractures of the distal radius, internal fixation of these injuries will continue to be relevant. American Academy of Orthopaedic Surgeons guidelines recommend operative fixation for fractures with postreduction radial shortening of more than 3 mm, dorsal tilt of more than 10°, or intra-articular displacement or step-off of more than 2 mm.45 Dr. Eglseder and Dr. Pensy indicate operative treatment of any incongruity of more than 2 mm in a young, active adult with a fracture of the distal radius. For the multifragmentary distal radius being treated operatively, attempts are made to achieve reduction more accurate than this, but formal dorsal exposure or direct visualization of the joint surface via dorsal capsulotomy is carefully chosen based on age, activity level, and bone quality. Recent high-level evidence46 showed that closed treatment of unstable fractures of the distal radius results in good outcomes in the elderly. However, it is important to note that fractures displaced in a palmar direction and palmar shear patterns were excluded from that work. It is widely accepted that palmar carpal translation should be addressed with internal fixation, and specific attention must therefore be paid to the lunate facet as the cornerstone of the distal radius. Furthermore, high-energy comminuted fractures in young patients still necessitate internal fixation of fragments to restore alignment and articular congruity.
Conclusion
The importance of the palmar lunate facet in providing support and restraint to palmar carpal translation and the key role of this facet in restoring the anatomy of the distal radius have been known. This fragment deserves special attention because failure to adequately stabilize it results in loss of fixation and carpal subluxation. Various approaches and fixation techniques have been recommended, including the method we prefer and have described here. Our newly proposed term, sustentaculum lunatum, our review of its structure and function, and our descriptions of fixation techniques are intended to promote awareness of this fragment in the treatment of fractures of the distal radius.
1. Jupiter JB. Fractures of the distal end of the radius. J Bone Joint Surg Am. 1991;73(3):461-469.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113-125.
4. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868-1873.
5. Melone CP Jr. Articular fractures of the distal radius. Orthop Clin North Am. 1984;15(2):217-236.
6. Castaing J. Recent fractures of the lower extremity of the radius in adults [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:581-696.
7. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967;(suppl 108):3+.
8. Isani A, Melone CP Jr. Classification and management of intra-articular fractures of the distal radius. Hand Clin. 1988;4(3):349-360.
9. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
10. Rettig ME, Dassa GL, Raskin KB, Melone CP Jr. Wrist fractures in the athlete: distal radius and carpal fractures. Clin Sports Med. 1998;17(3):469-489.
11. Müller ME, Koch P, Nazarian S, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
12. Peine R, Rikli DA, Hoffmann R, Duda G, Regazzoni P. Comparison of three different plating techniques for the dorsum of the distal radius: a biomechanical study. J Hand Surg Am. 2000;25(1):29-33.
13. Williams PL, Warwick R, Dyson M, Bannister LH, eds. Gray’s Anatomy. 37th ed. New York, NY: Churchill Livingstone; 1989.
14. Linscheid RL. Kinematic considerations of the wrist. Clin Orthop Relat Res. 1986;(202):27-39.
15. Mekhail AO, Ebraheim NA, McCreath WA, Jackson WT, Yeasting RA. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg Am. 1996;21(4):567-573.
16. Schuind FA, Linscheid RL, An KN, Chao EY. A normal data base of posteroanterior roentgenographic measurements of the wrist. J Bone Joint Surg Am. 1992;74(9):1418-1429.
17. Schuind F, Alemzadeh S, Stallenberg B, Burny F. Does the normal contralateral wrist provide the best reference for x-ray film measurements of the pathologic wrist? J Hand Surg Am. 1996;21(1):24-30.
18. Genda E, Horii E. Theoretical stress analysis in wrist joint: neutral position and functional position. J Hand Surg Br. 2000;25(3):292-295.
19. Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck’s disease. J Hand Surg Br. 1997;22(1):16-20.
20. Andermahr J, Lozano-Calderon S, Trafton T, Crisco JJ, Ring D. The volar extension of the lunate facet of the distal radius: a quantitative anatomic study. J Hand Surg Am. 2006;31(6):892-895.
21. Bo WJ, Meschan I, Krueger WA. Basic Atlas of Cross-Sectional Anatomy. Philadelphia, PA: Saunders; 1980.
22. Cahill DR, Orland MJ, Miller GM. Atlas of Human Cross-Sectional Anatomy: With CT and MR Images. 3rd ed. New York, NY: Wiley; 1995.
23. El-Khoury GY, Bergman RA, Montgomery WJ. Sectional Anatomy by MRI. 2nd ed. New York, NY: Churchill Livingstone; 1995.
24. Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004;86(9):1900-1908.
25. Lewis OJ, Hamshere RJ, Bucknill TM. The anatomy of the wrist joint. J Anat. 1970;106(Pt 3):539-552.
26. Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15(6):847-854.
27. Apergis E, Darmanis S, Theodoratos G, Maris J. Beware of the ulno-palmar distal radial fragment. J Hand Surg Br. 2002;27(2):139-145.
28. Chang EY, Chen KC, Meunier MJ, Chung CB. Acute short radiolunate ligament rupture in a rock climber. Skeletal Radiol. 2014;43(2):235-238.
29. Souer JS, Wiggers J, Ring D. Quantitative 3-dimensional computed tomography measurement of volar shearing fractures of the distal radius. J Hand Surg Am. 2011;36(4):599-603.
30. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am. 1994(5);19:720-727.
31. Goldwyn E, Pensy R, O’Toole RV, et al. Do traction radiographs of distal radial fractures influence fracture characterization and treatment? J Bone Joint Surg Am. 2012;94(22):2055-2062.
32. Glueck DA, Charoglu CP, Lawton JN. Factors associated with infection following open distal radius fractures. Hand. 2009;4(3):330-334.
33. Kurylo JC, Axelrad TW, Tornetta P 3rd, Jawa A. Open fractures of the distal radius: the effects of delayed debridement and immediate internal fixation on infection rates and the need for secondary procedures. J Hand Surg Am. 2011;36(7):1131-1134.
34. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
35. Rozental TD, Beredjiklian PK, Steinberg DR, Bozentka DJ. Open fractures of the distal radius. J Hand Surg Am. 2002;27(1):77-85.
36. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
37. Bae DS, Koris MJ. Fragment-specific internal fixation of distal radius fractures. Hand Clin. 2005;21(3):355-362.
38. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
39. Henry AK. Extensile Exposure. 2nd ed. New York, NY: Churchill Livingstone; 1973.
40. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg Am. 1988;13(3):372-377.
41. Chin KR, Jupiter JB. Wire-loop fixation of volar displaced osteochondral fractures of the distal radius. J Hand Surg Am. 1999;24(3):525-533.
42. Jupiter JB, Marent-Huber M; LCP Study Group. Operative management of distal radial fractures with 2.4-millimeter locking plates: a multicenter prospective case series. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1, pt 1):96-106.
43. Ruch DS, Yang C, Smith BP. Results of palmar plating of the lunate facet combined with external fixation for the treatment of high-energy compression fractures of the distal radius. J Orthop Trauma. 2004;18(1):28-33.
44. Wiesler ER, Chloros GD, Lucas RM, Kuzma GR. Arthroscopic management of volar lunate facet fractures of the distal radius. Tech Hand Up Extrem Surg. 2006;10(3):139-144.
45. American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. http://www.aaos.org/research/guidelines/drfguideline.pdf. Accessed August 4, 2015.
46. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153.
Fracture of the distal radius is the wrist injury most often encountered by orthopedic and hand surgeons.1 The number of fractures of the distal radius in the United States was estimated to be 640,000 in 2001, and the incidence is increasing.2,3 Recent evidence has shown a substantial increase in treating these fractures with internal rather than closed fixation, even in the elderly.4
Treatment of complex intra-articular fractures of the distal radius requires an accurate diagnosis of the fracture pattern and a thoughtful approach to fixation. Although a majority of the fractures that meet the operative criteria are now treated with various anterior locked-plating techniques with good results, a subset requires more technically demanding fixation approaches, including fragment-specific approaches, dorsal and palmar plating, and combined internal and external fixation.
The sustentaculum lunatum, as we have named the palmar lunate facet, deserves specific attention because of its importance in load transmission across the radiocarpal joint and its key role in restoring the anatomy of the palmar distal radial metaphysis during internal fixation. This fragment in comminuted fractures was first ascribed special importance by Melone5 in his description of common fracture patterns. In the present article, we describe the anatomical characteristics of the sustentaculum lunatum and the clinical relevance of this fragment to management of fractures of the distal radius.
Classification
A variety of classification systems have been proposed to characterize and guide treatment of fractures of the distal radius. The earliest descriptions of fracture patterns were presented by Castaing6 and Frykman7 in the 1960s. The Frykman classification historically has been popular but is limited in accuracy in its characterization of fragments and their displacement and is limited in its ability to guide treatment. The classification system proposed by Melone and colleagues5,8-10 was the first to truly describe fracture of the distal radius fragments in a relevant manner, including their characteristic “4 parts” (Figure 1). The authors emphasized the importance of the “medial complex” as the cornerstone of the radiocarpal and radioulnar joints.
The classification system developed by Müller and colleagues,11 which was adopted by the AO (Arbeitsgemeinschaft für Osteosynthesefragen), might be the most descriptive and informative system, and it is widely used to conduct research and direct treatment. This system classifies fractures into A (extra-articular), B (partial articular), and C (complete articular) types and subclassifies them according to fracture location and comminution. These classifications, along with a conceptualization of the distal forearm as a 3-column structure involving the radial, ulnar, and intermediate columns (including the lunate facet), as proposed by Peine and colleagues,12 gave us a framework for approaching fixation of fractures of the distal radius.
Etymology and Definition
Sustentaculum, from the Latin sustinere, “to support, check, or put off,” and taculum, “receptacle or holding space,” is a fitting description of the most distal portion of the palmar lunate facet, as it supports and holds the carpus, and specifically the lunate, on the radial articular surface. This portion is analogous to the sustentaculum tali, the named portion of the calcaneus that supports and articulates with the middle calcaneal articular surface of the talus13 and provides a reliable fragment for internal fixation of the calcaneus.
Anatomical and Biomechanical Considerations
The distal radial articular surface is composed of distinct scaphoid and lunate facets that articulate with their respective carpal bones. Several studies have characterized the anatomy of the distal radius.14-17 Linscheid14 found that the lunate and scaphoid facets account for 46% and 43% of the contact area across the radiocarpal joint, respectively; this has been corroborated by others.15 A biomechanical study by Genda and Horii18 showed that the majority of stress across the wrist joint was concentrated at the palmar side of the distal radius in the neutral position. Although it is recognized that the scaphoid facet bears most of the load across the wrist in the neutral wrist position, most activities of daily living place the wrist in a slightly extended and ulnarly deviated position. This position results in a shift of the majority of load to the radiolunar articulation, constituting 53% of total force transmission.18 Subchondral bone density analyses have supported this lunate-predominant loading pattern across the radiocarpal articulation in most people.19 This loading pattern is also supported by the observation that failure of fixation and carpal subluxation generally occurs at the radiolunate articulation.
The palmar lip of the distal radius traditionally has been depicted and conceptualized as a flat extension of the metaphysis, leading to the development of implants that are not ideally designed for capturing this area in the fracture setting. A 3-dimensional (3-D) computed tomography (CT) study of the distal radii of healthy volunteers, conducted by Andermahr and colleagues,20 showed that the contour of the palmar lunate facet projects from the palmar cortex of the radius by 3 mm on average and is about 19 mm in width (radial to ulnar dimension) (Figures 2A-2C). In the axial plane, the anterior cortex of the distal radius slopes in a palmar direction, from radial to ulnar. This presents a challenge in attempts to support the entire surface (scaphoid and lunate facets) with a single palmar implant.20-25
A study conducted by Harness and colleagues24 showed that the majority of palmar shear fractures are composed of multiple fragments of the lunar articular facet. Anatomical studies of the distal radiocarpal articulation have also described the ligamentous attachments to the sustentaculum lunatum.26 The short radiolunate ligament, which originates from this fragment and inserts onto the lunate, provides stability to the carpus and, if not adequately fixed, leads to an incompetent restraint to palmar carpal translation. Isolated injuries of the short radiolunate ligament or fractures of the palmar lunate facet have been shown to result in palmar carpal translation.27,28 In addition, attachments of the palmar radioulnar ligament and other more ulnar radiocarpal ligaments act as deforming forces on the palmar lunate facet.24,26
Fracture Pattern Recognition
Although the AO type B palmar shear fracture pattern, also known as the Barton fracture, has classically been recognized as the fracture involving the palmar lunate facet and requiring special attention, many complete articular fractures feature involvement and fragmentation of this portion of the distal radius (Figures 3A-3F).29 In highly comminuted complete articular and palmar shear fracture patterns, the morphology of the sustentaculum lunatum should be appreciated, and its adequate fixation to the radial metaphysis ensured, to prevent loss of reduction.
Visualization of the palmar lunate facet as a distinct fragment might be difficult in cases of highly comminuted fracture patterns. Standard CT or more recently described 3-D CT techniques with subtraction of the carpus might facilitate appreciation of this fragment for preoperative planning of approach and fixation.29,30 Our institutional protocol involves obtaining preoperative traction radiographs of every fracture of the distal radius. These radiographs have reduced the need for CT in understanding the fracture pattern and aid in decision making.31
Besides appreciating the existence of the sustentaculum lunatum fragment, we should recognize that some injury patterns that split the lunate facet into unstable dorsal and palmar fragments might necessitate a separate dorsal approach to reduce and fix the dorsal lunate fragment. Traction radiographs can be especially useful in recognizing these patterns (a V sign is present) (Figures 4A, 4B).
Open Fractures
Highly comminuted fractures of the distal radius presenting with displaced lunate facet fragments can have high-energy mechanisms of injury. Although open fractures of the distal radius are associated with lower risk for infection (compared with open fractures of other long bones), they deserve special attention because of associated tendon and neurovascular injuries. Few studies have specifically assessed open fractures of the distal radius.32-35 Only the study by Rozental and Blazar34 listed associated injuries at the wrist level. The authors identified 4 patients (out of 18) with concomitant flexor tendon or neurovascular injuries that included radial or ulnar artery injury. In our experience, many open fractures of the distal radius are caused by an inside-out mechanism and present with an open wound either over the ulnar styloid or in the area of the ulnar side of the palmar radial metaphysis corresponding to the metaphyseal spike that mates with the sustentaculum lunatum (Figures 5A, 5B). Given these findings, we approach this intermediate column with particular care in cases of open fracture, paying attention to important structures (flexors, neurovascular) and looking for contamination from the environment into the fracture.
Fixation Techniques
The approach to fixation of partial articular palmar shear fractures is fairly straightforward. Buttress plate fixation has been well described and has had reliably good results.36 However, in very distal fracture patterns and in cases in which the palmar lunate facet is fragmented as part of a complete articular fracture, a fragment-specific approach to fixation with or without spanning external fixation often is necessary.37 The unrecognized sustentaculum lunatum fragment in comminuted complete articular fractures can lead to inadequate fixation constructs, resulting in loss of reduction and carpal subluxation in a palmar direction.24,34,38
Our surgical approach uses the standard anterior interval between the radial artery and the flexor carpi radialis, as described by Henry.39 The flexor pollicis longus is retracted ulnarly, revealing the pronator quadratus. We then reflect the pronator quadratus from the distal radial metaphysis until the most proximal and ulnar extent of the fracture is easily visualized. The palmar ulnar metaphyseal cortex that mates with the displaced sustentaculum lunatum is, in our experience, often the least comminuted portion of the metaphysis, thus providing a cortical key for restoration of height and alignment (Figures 5A, 5B). At our institution, fixation typically is achieved by contouring miniplates (1.3 or 1.5 mm) to capture and buttress the sustentaculum lunatum (Figures 6A, 6B). In our experience, the screw lengths in the most distal fixed-angle constructs at the palmar lip are limited to 6 mm or less to avoid penetration of the articular surface, though this has not been previously reported in the literature. After restoring the length and tilt of this intermediate column of the distal radius, we proceed with “rebuilding” the remainder of the fragments to our stabilized initial construct.
Various authors40-43 have described alternative fixation methods for the palmar lunate facet fragment. Jupiter and Marent-Huber42 described 2.4-mm locked-plate fixation with either a standard palmar plate or T- or L-plates for cases in which the palmar lip fragment is very distal and small. In fact, some newer anatomical distal radius implants include features designed to target these fragments (Figures 7A, 7B). An alternative fixation method involves use of a 26-gauge stainless steel wire passed through drill holes in the metaphysis 1 cm proximal to the fracture and then passed through the palmar capsule just distal to the fragment and secured in figure-8 fashion while the fragment is manually held reduced.41 Still others have recommended limited internal fixation of the sustentaculum lunatum through an ulna-sided palmar approach to the distal radius (between the ulnar neurovascular bundle and the flexor tendons) combined with external fixation to restore length and palmar tilt in highly comminuted fractures.40,43
A method involving arthroscopically assisted reduction and fixation of the lunate facet has also been described, though this procedure is technically demanding and has limited indications.44 It uses a Freer elevator passed through the standard 3-4 portal after initial visualization and evacuation of hematoma. The Freer elevator is used to disimpact the sustentaculum lunatum and to elevate it from its depressed position. With the dorsal lunate facet left displaced to facilitate access to the palmar fragment, a nerve hook retractor is used to reduce the palmar facet to the radial styloid, and Kirschner wires are used to achieve interfragmentary fixation. The dorsal lunate fragment is then pieced back to the articular segment, and the entire construct is fixed to the radial metaphysis with additional Kirschner wires.
Discussion
Given the increasing incidence of fractures of the distal radius, internal fixation of these injuries will continue to be relevant. American Academy of Orthopaedic Surgeons guidelines recommend operative fixation for fractures with postreduction radial shortening of more than 3 mm, dorsal tilt of more than 10°, or intra-articular displacement or step-off of more than 2 mm.45 Dr. Eglseder and Dr. Pensy indicate operative treatment of any incongruity of more than 2 mm in a young, active adult with a fracture of the distal radius. For the multifragmentary distal radius being treated operatively, attempts are made to achieve reduction more accurate than this, but formal dorsal exposure or direct visualization of the joint surface via dorsal capsulotomy is carefully chosen based on age, activity level, and bone quality. Recent high-level evidence46 showed that closed treatment of unstable fractures of the distal radius results in good outcomes in the elderly. However, it is important to note that fractures displaced in a palmar direction and palmar shear patterns were excluded from that work. It is widely accepted that palmar carpal translation should be addressed with internal fixation, and specific attention must therefore be paid to the lunate facet as the cornerstone of the distal radius. Furthermore, high-energy comminuted fractures in young patients still necessitate internal fixation of fragments to restore alignment and articular congruity.
Conclusion
The importance of the palmar lunate facet in providing support and restraint to palmar carpal translation and the key role of this facet in restoring the anatomy of the distal radius have been known. This fragment deserves special attention because failure to adequately stabilize it results in loss of fixation and carpal subluxation. Various approaches and fixation techniques have been recommended, including the method we prefer and have described here. Our newly proposed term, sustentaculum lunatum, our review of its structure and function, and our descriptions of fixation techniques are intended to promote awareness of this fragment in the treatment of fractures of the distal radius.
Fracture of the distal radius is the wrist injury most often encountered by orthopedic and hand surgeons.1 The number of fractures of the distal radius in the United States was estimated to be 640,000 in 2001, and the incidence is increasing.2,3 Recent evidence has shown a substantial increase in treating these fractures with internal rather than closed fixation, even in the elderly.4
Treatment of complex intra-articular fractures of the distal radius requires an accurate diagnosis of the fracture pattern and a thoughtful approach to fixation. Although a majority of the fractures that meet the operative criteria are now treated with various anterior locked-plating techniques with good results, a subset requires more technically demanding fixation approaches, including fragment-specific approaches, dorsal and palmar plating, and combined internal and external fixation.
The sustentaculum lunatum, as we have named the palmar lunate facet, deserves specific attention because of its importance in load transmission across the radiocarpal joint and its key role in restoring the anatomy of the palmar distal radial metaphysis during internal fixation. This fragment in comminuted fractures was first ascribed special importance by Melone5 in his description of common fracture patterns. In the present article, we describe the anatomical characteristics of the sustentaculum lunatum and the clinical relevance of this fragment to management of fractures of the distal radius.
Classification
A variety of classification systems have been proposed to characterize and guide treatment of fractures of the distal radius. The earliest descriptions of fracture patterns were presented by Castaing6 and Frykman7 in the 1960s. The Frykman classification historically has been popular but is limited in accuracy in its characterization of fragments and their displacement and is limited in its ability to guide treatment. The classification system proposed by Melone and colleagues5,8-10 was the first to truly describe fracture of the distal radius fragments in a relevant manner, including their characteristic “4 parts” (Figure 1). The authors emphasized the importance of the “medial complex” as the cornerstone of the radiocarpal and radioulnar joints.
The classification system developed by Müller and colleagues,11 which was adopted by the AO (Arbeitsgemeinschaft für Osteosynthesefragen), might be the most descriptive and informative system, and it is widely used to conduct research and direct treatment. This system classifies fractures into A (extra-articular), B (partial articular), and C (complete articular) types and subclassifies them according to fracture location and comminution. These classifications, along with a conceptualization of the distal forearm as a 3-column structure involving the radial, ulnar, and intermediate columns (including the lunate facet), as proposed by Peine and colleagues,12 gave us a framework for approaching fixation of fractures of the distal radius.
Etymology and Definition
Sustentaculum, from the Latin sustinere, “to support, check, or put off,” and taculum, “receptacle or holding space,” is a fitting description of the most distal portion of the palmar lunate facet, as it supports and holds the carpus, and specifically the lunate, on the radial articular surface. This portion is analogous to the sustentaculum tali, the named portion of the calcaneus that supports and articulates with the middle calcaneal articular surface of the talus13 and provides a reliable fragment for internal fixation of the calcaneus.
Anatomical and Biomechanical Considerations
The distal radial articular surface is composed of distinct scaphoid and lunate facets that articulate with their respective carpal bones. Several studies have characterized the anatomy of the distal radius.14-17 Linscheid14 found that the lunate and scaphoid facets account for 46% and 43% of the contact area across the radiocarpal joint, respectively; this has been corroborated by others.15 A biomechanical study by Genda and Horii18 showed that the majority of stress across the wrist joint was concentrated at the palmar side of the distal radius in the neutral position. Although it is recognized that the scaphoid facet bears most of the load across the wrist in the neutral wrist position, most activities of daily living place the wrist in a slightly extended and ulnarly deviated position. This position results in a shift of the majority of load to the radiolunar articulation, constituting 53% of total force transmission.18 Subchondral bone density analyses have supported this lunate-predominant loading pattern across the radiocarpal articulation in most people.19 This loading pattern is also supported by the observation that failure of fixation and carpal subluxation generally occurs at the radiolunate articulation.
The palmar lip of the distal radius traditionally has been depicted and conceptualized as a flat extension of the metaphysis, leading to the development of implants that are not ideally designed for capturing this area in the fracture setting. A 3-dimensional (3-D) computed tomography (CT) study of the distal radii of healthy volunteers, conducted by Andermahr and colleagues,20 showed that the contour of the palmar lunate facet projects from the palmar cortex of the radius by 3 mm on average and is about 19 mm in width (radial to ulnar dimension) (Figures 2A-2C). In the axial plane, the anterior cortex of the distal radius slopes in a palmar direction, from radial to ulnar. This presents a challenge in attempts to support the entire surface (scaphoid and lunate facets) with a single palmar implant.20-25
A study conducted by Harness and colleagues24 showed that the majority of palmar shear fractures are composed of multiple fragments of the lunar articular facet. Anatomical studies of the distal radiocarpal articulation have also described the ligamentous attachments to the sustentaculum lunatum.26 The short radiolunate ligament, which originates from this fragment and inserts onto the lunate, provides stability to the carpus and, if not adequately fixed, leads to an incompetent restraint to palmar carpal translation. Isolated injuries of the short radiolunate ligament or fractures of the palmar lunate facet have been shown to result in palmar carpal translation.27,28 In addition, attachments of the palmar radioulnar ligament and other more ulnar radiocarpal ligaments act as deforming forces on the palmar lunate facet.24,26
Fracture Pattern Recognition
Although the AO type B palmar shear fracture pattern, also known as the Barton fracture, has classically been recognized as the fracture involving the palmar lunate facet and requiring special attention, many complete articular fractures feature involvement and fragmentation of this portion of the distal radius (Figures 3A-3F).29 In highly comminuted complete articular and palmar shear fracture patterns, the morphology of the sustentaculum lunatum should be appreciated, and its adequate fixation to the radial metaphysis ensured, to prevent loss of reduction.
Visualization of the palmar lunate facet as a distinct fragment might be difficult in cases of highly comminuted fracture patterns. Standard CT or more recently described 3-D CT techniques with subtraction of the carpus might facilitate appreciation of this fragment for preoperative planning of approach and fixation.29,30 Our institutional protocol involves obtaining preoperative traction radiographs of every fracture of the distal radius. These radiographs have reduced the need for CT in understanding the fracture pattern and aid in decision making.31
Besides appreciating the existence of the sustentaculum lunatum fragment, we should recognize that some injury patterns that split the lunate facet into unstable dorsal and palmar fragments might necessitate a separate dorsal approach to reduce and fix the dorsal lunate fragment. Traction radiographs can be especially useful in recognizing these patterns (a V sign is present) (Figures 4A, 4B).
Open Fractures
Highly comminuted fractures of the distal radius presenting with displaced lunate facet fragments can have high-energy mechanisms of injury. Although open fractures of the distal radius are associated with lower risk for infection (compared with open fractures of other long bones), they deserve special attention because of associated tendon and neurovascular injuries. Few studies have specifically assessed open fractures of the distal radius.32-35 Only the study by Rozental and Blazar34 listed associated injuries at the wrist level. The authors identified 4 patients (out of 18) with concomitant flexor tendon or neurovascular injuries that included radial or ulnar artery injury. In our experience, many open fractures of the distal radius are caused by an inside-out mechanism and present with an open wound either over the ulnar styloid or in the area of the ulnar side of the palmar radial metaphysis corresponding to the metaphyseal spike that mates with the sustentaculum lunatum (Figures 5A, 5B). Given these findings, we approach this intermediate column with particular care in cases of open fracture, paying attention to important structures (flexors, neurovascular) and looking for contamination from the environment into the fracture.
Fixation Techniques
The approach to fixation of partial articular palmar shear fractures is fairly straightforward. Buttress plate fixation has been well described and has had reliably good results.36 However, in very distal fracture patterns and in cases in which the palmar lunate facet is fragmented as part of a complete articular fracture, a fragment-specific approach to fixation with or without spanning external fixation often is necessary.37 The unrecognized sustentaculum lunatum fragment in comminuted complete articular fractures can lead to inadequate fixation constructs, resulting in loss of reduction and carpal subluxation in a palmar direction.24,34,38
Our surgical approach uses the standard anterior interval between the radial artery and the flexor carpi radialis, as described by Henry.39 The flexor pollicis longus is retracted ulnarly, revealing the pronator quadratus. We then reflect the pronator quadratus from the distal radial metaphysis until the most proximal and ulnar extent of the fracture is easily visualized. The palmar ulnar metaphyseal cortex that mates with the displaced sustentaculum lunatum is, in our experience, often the least comminuted portion of the metaphysis, thus providing a cortical key for restoration of height and alignment (Figures 5A, 5B). At our institution, fixation typically is achieved by contouring miniplates (1.3 or 1.5 mm) to capture and buttress the sustentaculum lunatum (Figures 6A, 6B). In our experience, the screw lengths in the most distal fixed-angle constructs at the palmar lip are limited to 6 mm or less to avoid penetration of the articular surface, though this has not been previously reported in the literature. After restoring the length and tilt of this intermediate column of the distal radius, we proceed with “rebuilding” the remainder of the fragments to our stabilized initial construct.
Various authors40-43 have described alternative fixation methods for the palmar lunate facet fragment. Jupiter and Marent-Huber42 described 2.4-mm locked-plate fixation with either a standard palmar plate or T- or L-plates for cases in which the palmar lip fragment is very distal and small. In fact, some newer anatomical distal radius implants include features designed to target these fragments (Figures 7A, 7B). An alternative fixation method involves use of a 26-gauge stainless steel wire passed through drill holes in the metaphysis 1 cm proximal to the fracture and then passed through the palmar capsule just distal to the fragment and secured in figure-8 fashion while the fragment is manually held reduced.41 Still others have recommended limited internal fixation of the sustentaculum lunatum through an ulna-sided palmar approach to the distal radius (between the ulnar neurovascular bundle and the flexor tendons) combined with external fixation to restore length and palmar tilt in highly comminuted fractures.40,43
A method involving arthroscopically assisted reduction and fixation of the lunate facet has also been described, though this procedure is technically demanding and has limited indications.44 It uses a Freer elevator passed through the standard 3-4 portal after initial visualization and evacuation of hematoma. The Freer elevator is used to disimpact the sustentaculum lunatum and to elevate it from its depressed position. With the dorsal lunate facet left displaced to facilitate access to the palmar fragment, a nerve hook retractor is used to reduce the palmar facet to the radial styloid, and Kirschner wires are used to achieve interfragmentary fixation. The dorsal lunate fragment is then pieced back to the articular segment, and the entire construct is fixed to the radial metaphysis with additional Kirschner wires.
Discussion
Given the increasing incidence of fractures of the distal radius, internal fixation of these injuries will continue to be relevant. American Academy of Orthopaedic Surgeons guidelines recommend operative fixation for fractures with postreduction radial shortening of more than 3 mm, dorsal tilt of more than 10°, or intra-articular displacement or step-off of more than 2 mm.45 Dr. Eglseder and Dr. Pensy indicate operative treatment of any incongruity of more than 2 mm in a young, active adult with a fracture of the distal radius. For the multifragmentary distal radius being treated operatively, attempts are made to achieve reduction more accurate than this, but formal dorsal exposure or direct visualization of the joint surface via dorsal capsulotomy is carefully chosen based on age, activity level, and bone quality. Recent high-level evidence46 showed that closed treatment of unstable fractures of the distal radius results in good outcomes in the elderly. However, it is important to note that fractures displaced in a palmar direction and palmar shear patterns were excluded from that work. It is widely accepted that palmar carpal translation should be addressed with internal fixation, and specific attention must therefore be paid to the lunate facet as the cornerstone of the distal radius. Furthermore, high-energy comminuted fractures in young patients still necessitate internal fixation of fragments to restore alignment and articular congruity.
Conclusion
The importance of the palmar lunate facet in providing support and restraint to palmar carpal translation and the key role of this facet in restoring the anatomy of the distal radius have been known. This fragment deserves special attention because failure to adequately stabilize it results in loss of fixation and carpal subluxation. Various approaches and fixation techniques have been recommended, including the method we prefer and have described here. Our newly proposed term, sustentaculum lunatum, our review of its structure and function, and our descriptions of fixation techniques are intended to promote awareness of this fragment in the treatment of fractures of the distal radius.
1. Jupiter JB. Fractures of the distal end of the radius. J Bone Joint Surg Am. 1991;73(3):461-469.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113-125.
4. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868-1873.
5. Melone CP Jr. Articular fractures of the distal radius. Orthop Clin North Am. 1984;15(2):217-236.
6. Castaing J. Recent fractures of the lower extremity of the radius in adults [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:581-696.
7. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967;(suppl 108):3+.
8. Isani A, Melone CP Jr. Classification and management of intra-articular fractures of the distal radius. Hand Clin. 1988;4(3):349-360.
9. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
10. Rettig ME, Dassa GL, Raskin KB, Melone CP Jr. Wrist fractures in the athlete: distal radius and carpal fractures. Clin Sports Med. 1998;17(3):469-489.
11. Müller ME, Koch P, Nazarian S, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
12. Peine R, Rikli DA, Hoffmann R, Duda G, Regazzoni P. Comparison of three different plating techniques for the dorsum of the distal radius: a biomechanical study. J Hand Surg Am. 2000;25(1):29-33.
13. Williams PL, Warwick R, Dyson M, Bannister LH, eds. Gray’s Anatomy. 37th ed. New York, NY: Churchill Livingstone; 1989.
14. Linscheid RL. Kinematic considerations of the wrist. Clin Orthop Relat Res. 1986;(202):27-39.
15. Mekhail AO, Ebraheim NA, McCreath WA, Jackson WT, Yeasting RA. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg Am. 1996;21(4):567-573.
16. Schuind FA, Linscheid RL, An KN, Chao EY. A normal data base of posteroanterior roentgenographic measurements of the wrist. J Bone Joint Surg Am. 1992;74(9):1418-1429.
17. Schuind F, Alemzadeh S, Stallenberg B, Burny F. Does the normal contralateral wrist provide the best reference for x-ray film measurements of the pathologic wrist? J Hand Surg Am. 1996;21(1):24-30.
18. Genda E, Horii E. Theoretical stress analysis in wrist joint: neutral position and functional position. J Hand Surg Br. 2000;25(3):292-295.
19. Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck’s disease. J Hand Surg Br. 1997;22(1):16-20.
20. Andermahr J, Lozano-Calderon S, Trafton T, Crisco JJ, Ring D. The volar extension of the lunate facet of the distal radius: a quantitative anatomic study. J Hand Surg Am. 2006;31(6):892-895.
21. Bo WJ, Meschan I, Krueger WA. Basic Atlas of Cross-Sectional Anatomy. Philadelphia, PA: Saunders; 1980.
22. Cahill DR, Orland MJ, Miller GM. Atlas of Human Cross-Sectional Anatomy: With CT and MR Images. 3rd ed. New York, NY: Wiley; 1995.
23. El-Khoury GY, Bergman RA, Montgomery WJ. Sectional Anatomy by MRI. 2nd ed. New York, NY: Churchill Livingstone; 1995.
24. Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004;86(9):1900-1908.
25. Lewis OJ, Hamshere RJ, Bucknill TM. The anatomy of the wrist joint. J Anat. 1970;106(Pt 3):539-552.
26. Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15(6):847-854.
27. Apergis E, Darmanis S, Theodoratos G, Maris J. Beware of the ulno-palmar distal radial fragment. J Hand Surg Br. 2002;27(2):139-145.
28. Chang EY, Chen KC, Meunier MJ, Chung CB. Acute short radiolunate ligament rupture in a rock climber. Skeletal Radiol. 2014;43(2):235-238.
29. Souer JS, Wiggers J, Ring D. Quantitative 3-dimensional computed tomography measurement of volar shearing fractures of the distal radius. J Hand Surg Am. 2011;36(4):599-603.
30. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am. 1994(5);19:720-727.
31. Goldwyn E, Pensy R, O’Toole RV, et al. Do traction radiographs of distal radial fractures influence fracture characterization and treatment? J Bone Joint Surg Am. 2012;94(22):2055-2062.
32. Glueck DA, Charoglu CP, Lawton JN. Factors associated with infection following open distal radius fractures. Hand. 2009;4(3):330-334.
33. Kurylo JC, Axelrad TW, Tornetta P 3rd, Jawa A. Open fractures of the distal radius: the effects of delayed debridement and immediate internal fixation on infection rates and the need for secondary procedures. J Hand Surg Am. 2011;36(7):1131-1134.
34. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
35. Rozental TD, Beredjiklian PK, Steinberg DR, Bozentka DJ. Open fractures of the distal radius. J Hand Surg Am. 2002;27(1):77-85.
36. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
37. Bae DS, Koris MJ. Fragment-specific internal fixation of distal radius fractures. Hand Clin. 2005;21(3):355-362.
38. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
39. Henry AK. Extensile Exposure. 2nd ed. New York, NY: Churchill Livingstone; 1973.
40. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg Am. 1988;13(3):372-377.
41. Chin KR, Jupiter JB. Wire-loop fixation of volar displaced osteochondral fractures of the distal radius. J Hand Surg Am. 1999;24(3):525-533.
42. Jupiter JB, Marent-Huber M; LCP Study Group. Operative management of distal radial fractures with 2.4-millimeter locking plates: a multicenter prospective case series. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1, pt 1):96-106.
43. Ruch DS, Yang C, Smith BP. Results of palmar plating of the lunate facet combined with external fixation for the treatment of high-energy compression fractures of the distal radius. J Orthop Trauma. 2004;18(1):28-33.
44. Wiesler ER, Chloros GD, Lucas RM, Kuzma GR. Arthroscopic management of volar lunate facet fractures of the distal radius. Tech Hand Up Extrem Surg. 2006;10(3):139-144.
45. American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. http://www.aaos.org/research/guidelines/drfguideline.pdf. Accessed August 4, 2015.
46. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153.
1. Jupiter JB. Fractures of the distal end of the radius. J Bone Joint Surg Am. 1991;73(3):461-469.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113-125.
4. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868-1873.
5. Melone CP Jr. Articular fractures of the distal radius. Orthop Clin North Am. 1984;15(2):217-236.
6. Castaing J. Recent fractures of the lower extremity of the radius in adults [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:581-696.
7. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967;(suppl 108):3+.
8. Isani A, Melone CP Jr. Classification and management of intra-articular fractures of the distal radius. Hand Clin. 1988;4(3):349-360.
9. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
10. Rettig ME, Dassa GL, Raskin KB, Melone CP Jr. Wrist fractures in the athlete: distal radius and carpal fractures. Clin Sports Med. 1998;17(3):469-489.
11. Müller ME, Koch P, Nazarian S, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
12. Peine R, Rikli DA, Hoffmann R, Duda G, Regazzoni P. Comparison of three different plating techniques for the dorsum of the distal radius: a biomechanical study. J Hand Surg Am. 2000;25(1):29-33.
13. Williams PL, Warwick R, Dyson M, Bannister LH, eds. Gray’s Anatomy. 37th ed. New York, NY: Churchill Livingstone; 1989.
14. Linscheid RL. Kinematic considerations of the wrist. Clin Orthop Relat Res. 1986;(202):27-39.
15. Mekhail AO, Ebraheim NA, McCreath WA, Jackson WT, Yeasting RA. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg Am. 1996;21(4):567-573.
16. Schuind FA, Linscheid RL, An KN, Chao EY. A normal data base of posteroanterior roentgenographic measurements of the wrist. J Bone Joint Surg Am. 1992;74(9):1418-1429.
17. Schuind F, Alemzadeh S, Stallenberg B, Burny F. Does the normal contralateral wrist provide the best reference for x-ray film measurements of the pathologic wrist? J Hand Surg Am. 1996;21(1):24-30.
18. Genda E, Horii E. Theoretical stress analysis in wrist joint: neutral position and functional position. J Hand Surg Br. 2000;25(3):292-295.
19. Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck’s disease. J Hand Surg Br. 1997;22(1):16-20.
20. Andermahr J, Lozano-Calderon S, Trafton T, Crisco JJ, Ring D. The volar extension of the lunate facet of the distal radius: a quantitative anatomic study. J Hand Surg Am. 2006;31(6):892-895.
21. Bo WJ, Meschan I, Krueger WA. Basic Atlas of Cross-Sectional Anatomy. Philadelphia, PA: Saunders; 1980.
22. Cahill DR, Orland MJ, Miller GM. Atlas of Human Cross-Sectional Anatomy: With CT and MR Images. 3rd ed. New York, NY: Wiley; 1995.
23. El-Khoury GY, Bergman RA, Montgomery WJ. Sectional Anatomy by MRI. 2nd ed. New York, NY: Churchill Livingstone; 1995.
24. Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004;86(9):1900-1908.
25. Lewis OJ, Hamshere RJ, Bucknill TM. The anatomy of the wrist joint. J Anat. 1970;106(Pt 3):539-552.
26. Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15(6):847-854.
27. Apergis E, Darmanis S, Theodoratos G, Maris J. Beware of the ulno-palmar distal radial fragment. J Hand Surg Br. 2002;27(2):139-145.
28. Chang EY, Chen KC, Meunier MJ, Chung CB. Acute short radiolunate ligament rupture in a rock climber. Skeletal Radiol. 2014;43(2):235-238.
29. Souer JS, Wiggers J, Ring D. Quantitative 3-dimensional computed tomography measurement of volar shearing fractures of the distal radius. J Hand Surg Am. 2011;36(4):599-603.
30. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am. 1994(5);19:720-727.
31. Goldwyn E, Pensy R, O’Toole RV, et al. Do traction radiographs of distal radial fractures influence fracture characterization and treatment? J Bone Joint Surg Am. 2012;94(22):2055-2062.
32. Glueck DA, Charoglu CP, Lawton JN. Factors associated with infection following open distal radius fractures. Hand. 2009;4(3):330-334.
33. Kurylo JC, Axelrad TW, Tornetta P 3rd, Jawa A. Open fractures of the distal radius: the effects of delayed debridement and immediate internal fixation on infection rates and the need for secondary procedures. J Hand Surg Am. 2011;36(7):1131-1134.
34. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
35. Rozental TD, Beredjiklian PK, Steinberg DR, Bozentka DJ. Open fractures of the distal radius. J Hand Surg Am. 2002;27(1):77-85.
36. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
37. Bae DS, Koris MJ. Fragment-specific internal fixation of distal radius fractures. Hand Clin. 2005;21(3):355-362.
38. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
39. Henry AK. Extensile Exposure. 2nd ed. New York, NY: Churchill Livingstone; 1973.
40. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg Am. 1988;13(3):372-377.
41. Chin KR, Jupiter JB. Wire-loop fixation of volar displaced osteochondral fractures of the distal radius. J Hand Surg Am. 1999;24(3):525-533.
42. Jupiter JB, Marent-Huber M; LCP Study Group. Operative management of distal radial fractures with 2.4-millimeter locking plates: a multicenter prospective case series. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1, pt 1):96-106.
43. Ruch DS, Yang C, Smith BP. Results of palmar plating of the lunate facet combined with external fixation for the treatment of high-energy compression fractures of the distal radius. J Orthop Trauma. 2004;18(1):28-33.
44. Wiesler ER, Chloros GD, Lucas RM, Kuzma GR. Arthroscopic management of volar lunate facet fractures of the distal radius. Tech Hand Up Extrem Surg. 2006;10(3):139-144.
45. American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. http://www.aaos.org/research/guidelines/drfguideline.pdf. Accessed August 4, 2015.
46. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153.
Perilunate Injuries
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
Oral Lesions You Can’t Afford to Miss

Family practice clinicians can play an essential role in managing their patients’ oral health by promptly recognizing and diagnosing conditions that demand further medical attention, including nonodontogenic and odontogenic infections, primary oral mucosal diseases, oral manifestations of systemic disease, and malignancy. Many conditions are amenable to treatment by the primary care provider, while others will require referral to a specialist.
This article and accompanying photo guide describe the types of lesions you may encounter during examinations of the oral cavity and the corresponding diagnoses.
BE VIGILANT FOR NONODONTOGENIC CONDITIONS THAT MAY REQUIRE URGENT TREATMENT There are several uncommon, acute nonodontogenic conditions that affect the oral cavity; when severe, they may require urgent medical attention and possible hospitalization.
Primary herpes simplex virus 1 (HSV-1) infection is generally subclinical, but some patients develop significant oral disease—called primary herpetic gingivostomatitis—that is characterized by painful, diffuse, irregular, croplike ulcerations throughout the oral cavity and lips (see Figure 1).1 The gingiva is nearly universally affected, which distinguishes this condition from erythema multiforme and aphthous stomatitis (described later in this article). The incidence is highest in children, followed by adolescents and young adults.2
Erythema multiforme. This mucocutaneous hypersensitivity reaction can be limited to the oral cavity and lips, without accompanying skin lesions. Flu-like symptoms, including fever and chills, are followed by acute onset of diffuse oral ulcerations that are generally limited to nonkeratinized mucosa and spare the gingiva (see Figure 2).3 Ulceration and crusting of the lips are common.
Aphthous stomatitis. Recurrent aphthous stomatitis (RAS) is a common immune mediated inflammatory condition characterized by “canker sores,” or small round/ovoid ulcers with a well-defined erythematous halo (see Figure 3). Lesions almost exclusively affect nonkeratinized mucosa (and never the lip vermilion) and heal within seven to 10 days, although “major” (> 0.5 cm) lesions may persist much longer (see Figure 4). A herpetiform pattern with multiple coalescing ulcers closely mimics HSV.
Small subsets of patients develop “complex” RAS, which is characterized by continuous and often multiple ulcerations that may extend into the esophagus, with associated chronic pain and compromised intake.2 RAS associated with systemic conditions is reviewed below.
URGENT TREATMENT
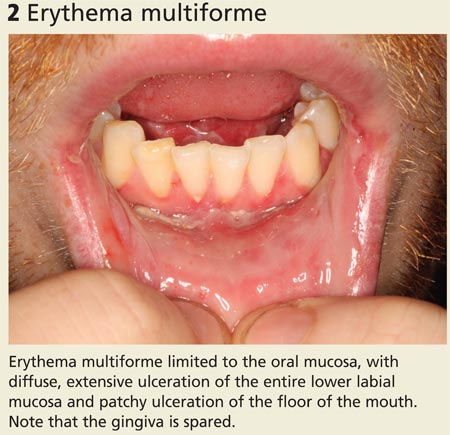
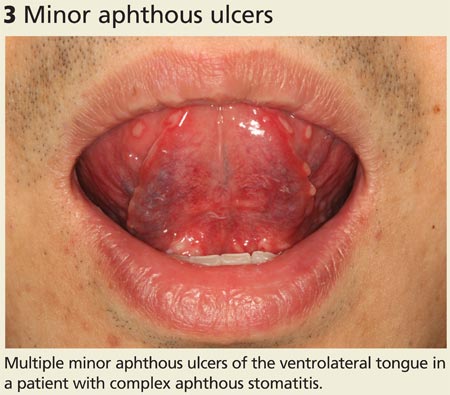
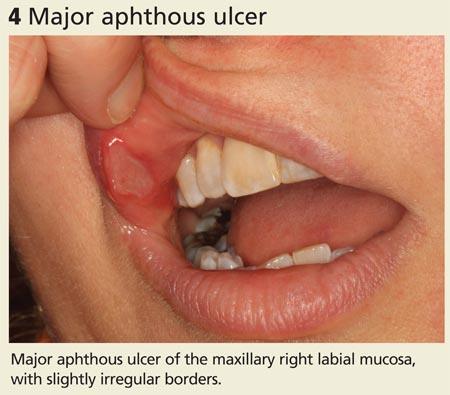
Continue for how to spot signs of common dental diseases >>
HOW TO SPOT SIGNS OF COMMON DENTAL DISEASES
In 2010 in the United States, close to 1.4 million emergency department visits and about $1 billion in hospital charges were due to dental problems.4 Approximately 40% of these visits were made by individuals without insurance.4 Due to a lack of dental insurance, patients may present to a medical professional rather than a dental professional. Additionally, uninsured individuals may neglect their dental problem until it becomes a medical emergency. Family practice clinicians need to recognize dental disease and be able to provide basic management of emergencies.5
Dental abscess. A dental abscess can arise from pulpal infection (due to progression of caries) or periodontal infection (due to progression of periodontal disease). Pain symptoms are variable; however, intense, spontaneous cyclical pain is generally characteristic of a dental abscess of pulpal etiology, whereas a periodontal abscess can have less obvious symptoms. Swelling intraorally or extraorally indicates the spread of a localized infection (see Figure 5).
Severe infection and swelling can limit mouth opening and function and in extreme cases may obstruct swallowing and even breathing (eg, Ludwig’s angina). Affected teeth may or may not demonstrate obvious findings of advanced dental disease, such as gross caries, fracture, heavy calculus deposits, or marked periodontal attachment loss. Oral examination may reveal a parulis (focal erythematous swelling of the adjacent gingiva with a central draining sinus tract) (see Figure 6) and percussion of the affected tooth is generally painful.
Pericoronitis is infection and swelling of the gingival tissues that surround a tooth, typically in association with a partially erupted third molar. Signs and symptoms include pain, discomfort with eating and swallowing, and limited mouth opening. Examination demonstrates gingival inflammation around a tooth, with or without purulence (see Figure 7).
Acute necrotizing ulcerative gingivitis (ANUG) and periodontitis (ANUP) are severe conditions that are typically associated with psychological stress, severe malnutrition, and immunosuppression in patients with preexisting gingivitis or periodontitis.6 ANUG is associated with intense gingival pain, halitosis, generalized erythema, and destruction of the gingival papilla, often with bleeding.7 ANUP is a more advanced condition associated with damage and loss of the periodontium (including bone), often with loose teeth (see Figure 8).8
COMMON DENTAL DISEASES
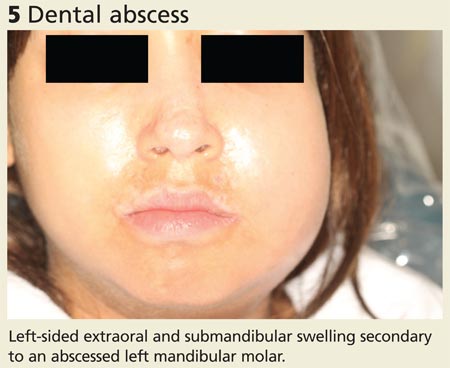
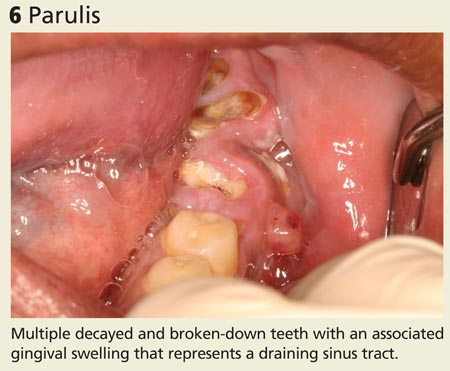
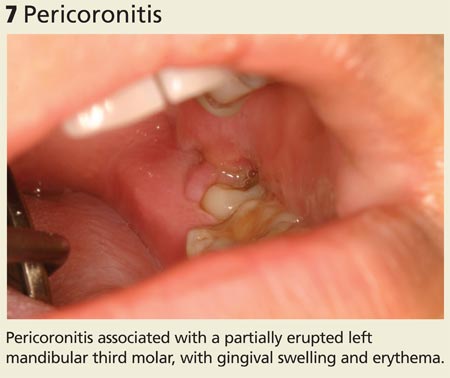
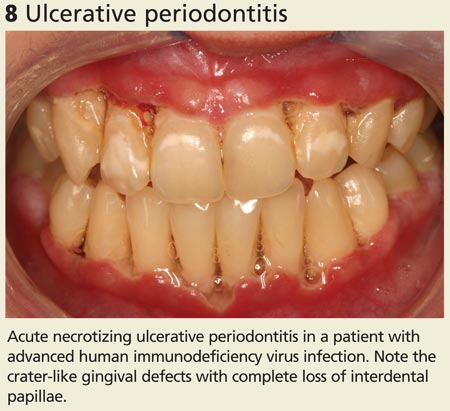
Trauma. Dental trauma can be limited to the teeth and soft tissues, while more severe injuries can also affect the jaw bone.9 Accidental falls, assault, and motor vehicle traffic accidents are the most common causes of facial fractures in the United States and are often associated with dentoalveolar trauma (see Figure 9). The most commonly fractured facial bone is the mandible, characterized by painful opening and closing and an incomplete or altered bite.10
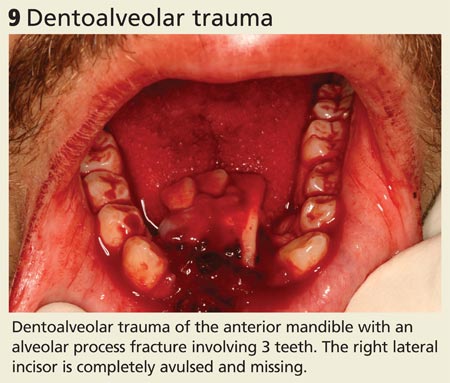
Continue for oral symptoms may be the first sign of systemic disease >>
ORAL SYMPTOMS MAY BE THE FIRST SIGN OF SYSTEMIC DISEASEInflammatory bowel disease. Crohn’s disease may affect the gastrointestinal tract anywhere from the mouth to the anus and may initially present with oral findings that may not correlate with abdominal symptoms. Oral Crohn’s disease may present as mucosal cobblestoning, mucosal tags, deep linear ulcerations, gingival hyperplasia, lip fissuring, aphthous ulcers, and angular cheilitis (see Figures 10 and 11). Other features may include diffuse, painless swelling of the lips and mucosal erythema.
Pyostomatitis vegetans is an uncommon condition typically associated with ulcerative colitis that is characterized by serpentine pustules that coalesce in a “snail track” pattern (see Figure 12).11
Dermatologic/vesiculobullous diseases. Vesiculobullous lesions in the mouth may be seen in pemphigus vulgaris or bullous pemphigoid. Pemphigus vulgaris is an autoimmune intraepithelial blistering disease that often first presents in the oral cavity as flaccid bullae or painful ulcerations, prior to the onset of skin lesions (see Figure 13).
Mucous membrane pemphigoid is an autoimmune subepithelial disease that affects mucous membranes and the skin. Characteristic oral mucosal blisters quickly rupture and form ulcerations, which may occur in the absence of other mucosal involvement (eg, anus, genitalia, nose or throat) (see Figure 14).12
Painful aphthous ulcers are common. When oral ulcerations are diffuse and recurrent, they may be the first sign of Behçet’s disease, a multisystem autoimmune vasculitis.13
Oral lichen planus is a chronic immune-mediated mucocutaneous disease that is often limited to the oral cavity. It presents with characteristic radiating white striations of the buccal mucosa and tongue, often with associated erythema and ulcerations (see Figure 15).14
SIGNS OF SYSTEMIC DISEASE
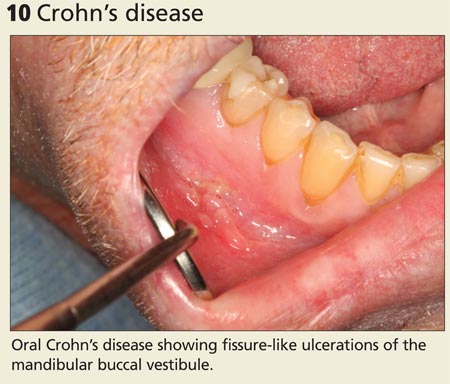

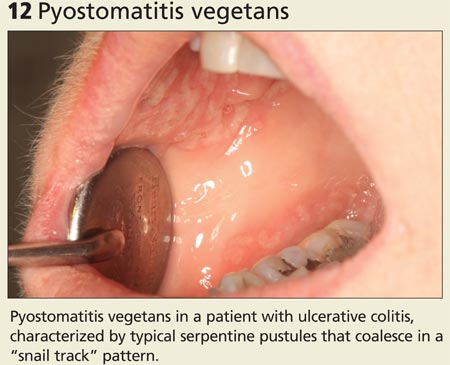

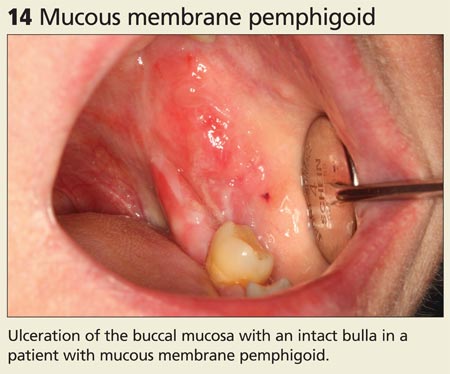
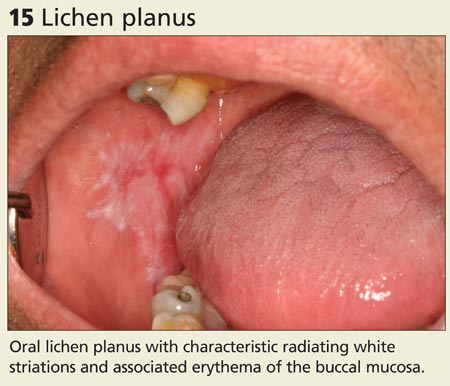
Continue for rheumatologic conditions >>
Rheumatologic conditions. Systemic or discoid lupus erythematosus may present with oral findings that largely resemble those of oral lichen planus (see Figure 16).15 Sjögren’s syndrome is an autoimmune disease with characteristic xerostomia, which can lead to oral discomfort, dysphagia, recurrent candidiasis, and rampant dental caries.
Other conditions to watch for. Erosion of the enamel on the lingual surface of the teeth may be a sign of gastroesophageal reflux disease or bulimia (see Figure 17). Examination of the oral mucosa can reveal typical white plaques of oral candidiasis (see Figure 18), which may be associated with systemic immune suppression as well as salivary gland dysfunction.
SIGNS OF SYSTEMIC DISEASE (cont'd)

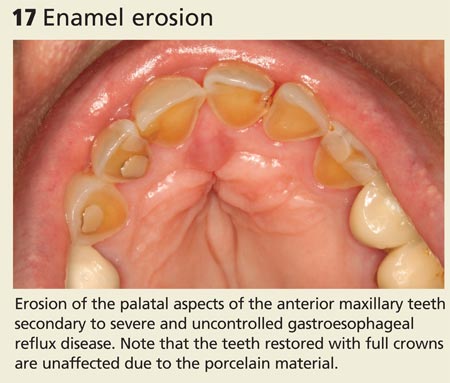
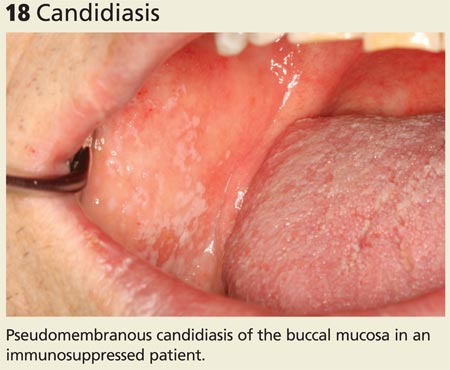
Oral conditions that have been associated with HIV infection include ANUG/ANUP, recurrent candidiasis, and oral hairy leukoplakia (see Figure 19). In the absence of known HIV infection, patients who present with any of these oral conditions should be evaluated for HIV infection.13
Atrophic glossitis may indicate a vitamin B deficiency. Thrombocytopenia and leukemia may present with oral petechiae, purpura, oral hematomas, or hemorrhagic bullae (see Figure 20).13 Painless pseudomembranous mucosal erosions may be a presentation for secondary syphilis.16
SIGNS OF SYSTEMIC DISEASE (cont'd)
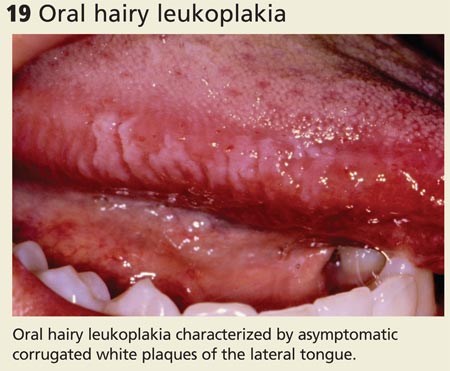
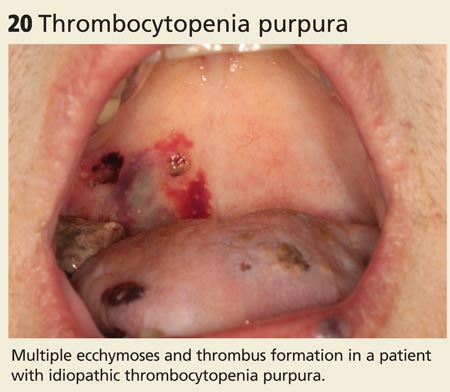
Continue for signs that suggest malignancy >>
LOOK FOR SIGNS THAT SUGGEST MALIGNANCYIn the United States, oral and pharyngeal cancers account for approximately 40,000 cases of cancer and 8,000 deaths each year.17 More than 90% of these are squamous cell carcinomas (SCCs); the remainder are mainly salivary gland tumors, lymphoma, and other infrequent cancers.18
SCC of the oral cavity most commonly occurs on the tongue but can develop in any site, presenting as mucosal ulcers, plaques, or masses that do not heal (see Figure 21). Tobacco and alcohol use are associated with up to 80% of cases of SCC of the head and neck.18 Some oropharyngeal SCCs are associated with human papillomavirus infection type 16.19
Potentially malignant oral lesions include leukoplakia and erythroplakia. Leukoplakia is a white patch or plaque of the oral mucosa that can’t be explained by any other clinical diagnosis (see Figure 22). These lesions are at risk for malignant transformation and may demonstrate dysplasia or frank SCC on biopsy.20 Proliferative verrucous leukoplakia is a unique form of leukoplakia that is characterized by a wrinkled appearance that is often multifocal; the condition is associated with a higher risk for malignant transformation.
Erythroplakia is a red patch that similarly can’t be explained by another diagnosis. It has a very high risk for malignant transformation over time. All potentially malignant oral lesions, including leukoplakia and erythroplakia, require biopsy and careful monitoring.
Non-SCC cancers. Salivary gland tumors are rare and most commonly occur in patients ages 55 to 65. Most neoplasms (70%-85%) occur in the parotid gland, while 8% to 15% develop in the submandibular salivary gland and less than 1% involve the sublingual gland.21 Minor salivary gland tissue, especially in the lips and palate, may also be affected (see Figure 23). Patients present with circumscribed, fixed or movable, painless, soft or firm masses in a salivary gland.
Melanoma should be included in the differential diagnosis of oral pigmented lesions that have any features of cutaneous melanoma, such as asymmetry, irregular borders, or variable or changing color.22
Hematologic malignancies may initially present (or demonstrate evidence of relapse) in the oral cavity. Leukemia typically presents with sheet-like overgrowth and swelling of the gingiva, with associated erythema and bleeding (see Figure 24), whereas lymphoma typically presents as a solitary mass or ulceration. Solid tumors that metastasize to the oral cavity may present with localized unexplained soft or hard tissue growths, with or without associated neurologic symptoms (eg, paresthesia).
MALIGNANCIES
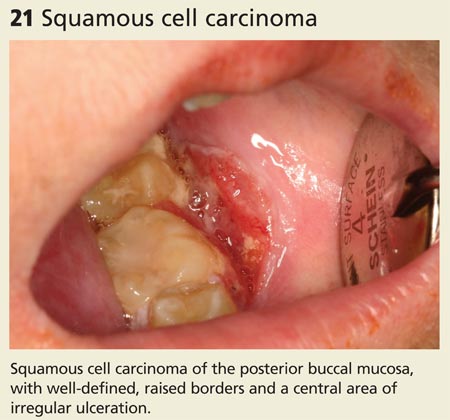
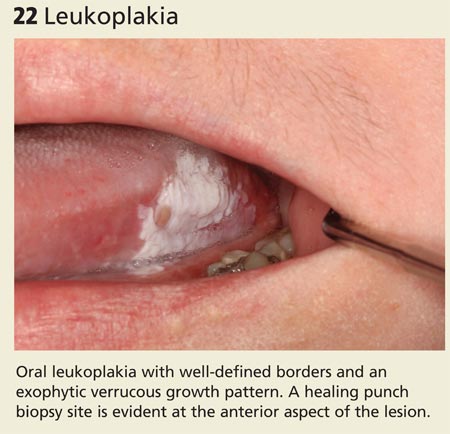
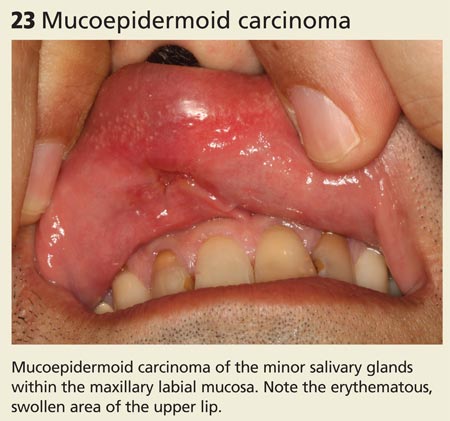
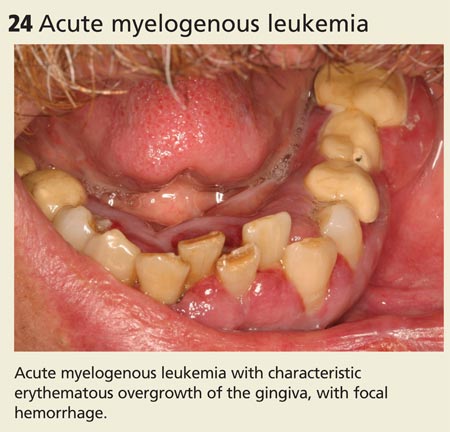
1. Spruance SL. The natural history of recurrent oral-facial herpes simplex virus infection. Semin Dermatol. 1992;11:200-206.
2. Balasubramaniam R, Kuperstein AS, Stoopler ET. Update on oral herpes virus infections. Dent Clin North Am. 2014;58:265-280.
3. Lozada-Nur F, Gorsky M, Silverman S Jr. Oral erythema multiforme: clinical observations and treatment of 95 patients. Oral Surg Oral Med Oral Pathol. 1989;67:36-40.
4. Allareddy V, Rampa S, Lee MK, et al. Hospital-based emergency department visits involving dental conditions: profile and predictors of poor outcomes and resource utilization. J Am Dent Assoc. 2014;145:331-337.
5. Allareddy V, Lin CY, Shah A, et al. Outcomes in patients hospitalized for periapical abscess in the United States: an analysis involving the use of a nationwide inpatient sample. J Am Dent Assoc. 2010;141:1107-1116.
6. Folayan MO. The epidemiology, etiology, and pathophysiology of acute necrotizing ulcerative gingivitis associated with malnutrition. J Contemp Dent Pract. 2004;5:28-41.
7. Atout RN, Todescan S. Managing patients with necrotizing ulcerative gingivitis. J Can Dent Assoc. 2013;79:d46.
8. Todescan S, Nizar R. Managing patients with necrotizing ulcerative periodontitis. J Can Dent Assoc. 2013;79:d44.
9. Allareddy V, Allareddy V, Nalliah RP. Epidemiology of facial fracture injuries. J Oral Maxillofac Surg. 2011;69:2613-2618.
10. Nalliah RP, Allareddy V, Kim MK, et al. Economics of facial fracture reductions in the United States over 12 months. Dent Traumatol. 2013;29:115-120.
11. Padmavathi B, Sharma S, Astekar M, et al. Oral Crohn’s disease. J Oral Maxillofac Pathol. 2014;18(suppl 1):S139-S142.
12. Xu HH, Werth VP, Parisi E, et al. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57:611-630.
13. Chi AC, Neville BW, Krayer JW, et al. Oral manifestations of systemic disease. Am Fam Physician. 2010;82:1381-1388.
14. Lavanya N, Jayanthi P, Rao UK, et al. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127-132.
15. Uva L, Miguel D, Pinheiro C, et al. Cutaneous manifestations of systemic lupus erythematosus. Autoimmune Dis. 2012;2012:834291.
16. Ficarra G, Carlos R. Syphilis: The renaissance of an old disease with oral implications. Head Neck Pathol. 2009;3:195-206.
17. Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-236.
18. Licitra L, Locati LD, Bossi P, et al. Head and neck tumors other than squamous cell carcinoma. Curr Opin Oncol. 2004;16:236-241.
19. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693-703.
20. Silverman S Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation: a follow‐up study of 257 patients. Cancer. 1984;53:563-568.
21. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2807 patients. Head Neck Surg. 1986;8:177-184.22. DeMatos P, Tyler DS, Seigler HF. Malignant melanoma of the mucous membranes: a review of 119 cases. Ann Surg Oncol. 1998;5:733-742.

Family practice clinicians can play an essential role in managing their patients’ oral health by promptly recognizing and diagnosing conditions that demand further medical attention, including nonodontogenic and odontogenic infections, primary oral mucosal diseases, oral manifestations of systemic disease, and malignancy. Many conditions are amenable to treatment by the primary care provider, while others will require referral to a specialist.
This article and accompanying photo guide describe the types of lesions you may encounter during examinations of the oral cavity and the corresponding diagnoses.
BE VIGILANT FOR NONODONTOGENIC CONDITIONS THAT MAY REQUIRE URGENT TREATMENT There are several uncommon, acute nonodontogenic conditions that affect the oral cavity; when severe, they may require urgent medical attention and possible hospitalization.
Primary herpes simplex virus 1 (HSV-1) infection is generally subclinical, but some patients develop significant oral disease—called primary herpetic gingivostomatitis—that is characterized by painful, diffuse, irregular, croplike ulcerations throughout the oral cavity and lips (see Figure 1).1 The gingiva is nearly universally affected, which distinguishes this condition from erythema multiforme and aphthous stomatitis (described later in this article). The incidence is highest in children, followed by adolescents and young adults.2
Erythema multiforme. This mucocutaneous hypersensitivity reaction can be limited to the oral cavity and lips, without accompanying skin lesions. Flu-like symptoms, including fever and chills, are followed by acute onset of diffuse oral ulcerations that are generally limited to nonkeratinized mucosa and spare the gingiva (see Figure 2).3 Ulceration and crusting of the lips are common.
Aphthous stomatitis. Recurrent aphthous stomatitis (RAS) is a common immune mediated inflammatory condition characterized by “canker sores,” or small round/ovoid ulcers with a well-defined erythematous halo (see Figure 3). Lesions almost exclusively affect nonkeratinized mucosa (and never the lip vermilion) and heal within seven to 10 days, although “major” (> 0.5 cm) lesions may persist much longer (see Figure 4). A herpetiform pattern with multiple coalescing ulcers closely mimics HSV.
Small subsets of patients develop “complex” RAS, which is characterized by continuous and often multiple ulcerations that may extend into the esophagus, with associated chronic pain and compromised intake.2 RAS associated with systemic conditions is reviewed below.
URGENT TREATMENT




Continue for how to spot signs of common dental diseases >>
HOW TO SPOT SIGNS OF COMMON DENTAL DISEASES
In 2010 in the United States, close to 1.4 million emergency department visits and about $1 billion in hospital charges were due to dental problems.4 Approximately 40% of these visits were made by individuals without insurance.4 Due to a lack of dental insurance, patients may present to a medical professional rather than a dental professional. Additionally, uninsured individuals may neglect their dental problem until it becomes a medical emergency. Family practice clinicians need to recognize dental disease and be able to provide basic management of emergencies.5
Dental abscess. A dental abscess can arise from pulpal infection (due to progression of caries) or periodontal infection (due to progression of periodontal disease). Pain symptoms are variable; however, intense, spontaneous cyclical pain is generally characteristic of a dental abscess of pulpal etiology, whereas a periodontal abscess can have less obvious symptoms. Swelling intraorally or extraorally indicates the spread of a localized infection (see Figure 5).
Severe infection and swelling can limit mouth opening and function and in extreme cases may obstruct swallowing and even breathing (eg, Ludwig’s angina). Affected teeth may or may not demonstrate obvious findings of advanced dental disease, such as gross caries, fracture, heavy calculus deposits, or marked periodontal attachment loss. Oral examination may reveal a parulis (focal erythematous swelling of the adjacent gingiva with a central draining sinus tract) (see Figure 6) and percussion of the affected tooth is generally painful.
Pericoronitis is infection and swelling of the gingival tissues that surround a tooth, typically in association with a partially erupted third molar. Signs and symptoms include pain, discomfort with eating and swallowing, and limited mouth opening. Examination demonstrates gingival inflammation around a tooth, with or without purulence (see Figure 7).
Acute necrotizing ulcerative gingivitis (ANUG) and periodontitis (ANUP) are severe conditions that are typically associated with psychological stress, severe malnutrition, and immunosuppression in patients with preexisting gingivitis or periodontitis.6 ANUG is associated with intense gingival pain, halitosis, generalized erythema, and destruction of the gingival papilla, often with bleeding.7 ANUP is a more advanced condition associated with damage and loss of the periodontium (including bone), often with loose teeth (see Figure 8).8
COMMON DENTAL DISEASES




Trauma. Dental trauma can be limited to the teeth and soft tissues, while more severe injuries can also affect the jaw bone.9 Accidental falls, assault, and motor vehicle traffic accidents are the most common causes of facial fractures in the United States and are often associated with dentoalveolar trauma (see Figure 9). The most commonly fractured facial bone is the mandible, characterized by painful opening and closing and an incomplete or altered bite.10

Continue for oral symptoms may be the first sign of systemic disease >>
ORAL SYMPTOMS MAY BE THE FIRST SIGN OF SYSTEMIC DISEASEInflammatory bowel disease. Crohn’s disease may affect the gastrointestinal tract anywhere from the mouth to the anus and may initially present with oral findings that may not correlate with abdominal symptoms. Oral Crohn’s disease may present as mucosal cobblestoning, mucosal tags, deep linear ulcerations, gingival hyperplasia, lip fissuring, aphthous ulcers, and angular cheilitis (see Figures 10 and 11). Other features may include diffuse, painless swelling of the lips and mucosal erythema.
Pyostomatitis vegetans is an uncommon condition typically associated with ulcerative colitis that is characterized by serpentine pustules that coalesce in a “snail track” pattern (see Figure 12).11
Dermatologic/vesiculobullous diseases. Vesiculobullous lesions in the mouth may be seen in pemphigus vulgaris or bullous pemphigoid. Pemphigus vulgaris is an autoimmune intraepithelial blistering disease that often first presents in the oral cavity as flaccid bullae or painful ulcerations, prior to the onset of skin lesions (see Figure 13).
Mucous membrane pemphigoid is an autoimmune subepithelial disease that affects mucous membranes and the skin. Characteristic oral mucosal blisters quickly rupture and form ulcerations, which may occur in the absence of other mucosal involvement (eg, anus, genitalia, nose or throat) (see Figure 14).12
Painful aphthous ulcers are common. When oral ulcerations are diffuse and recurrent, they may be the first sign of Behçet’s disease, a multisystem autoimmune vasculitis.13
Oral lichen planus is a chronic immune-mediated mucocutaneous disease that is often limited to the oral cavity. It presents with characteristic radiating white striations of the buccal mucosa and tongue, often with associated erythema and ulcerations (see Figure 15).14
SIGNS OF SYSTEMIC DISEASE






Continue for rheumatologic conditions >>
Rheumatologic conditions. Systemic or discoid lupus erythematosus may present with oral findings that largely resemble those of oral lichen planus (see Figure 16).15 Sjögren’s syndrome is an autoimmune disease with characteristic xerostomia, which can lead to oral discomfort, dysphagia, recurrent candidiasis, and rampant dental caries.
Other conditions to watch for. Erosion of the enamel on the lingual surface of the teeth may be a sign of gastroesophageal reflux disease or bulimia (see Figure 17). Examination of the oral mucosa can reveal typical white plaques of oral candidiasis (see Figure 18), which may be associated with systemic immune suppression as well as salivary gland dysfunction.
SIGNS OF SYSTEMIC DISEASE (cont'd)



Oral conditions that have been associated with HIV infection include ANUG/ANUP, recurrent candidiasis, and oral hairy leukoplakia (see Figure 19). In the absence of known HIV infection, patients who present with any of these oral conditions should be evaluated for HIV infection.13
Atrophic glossitis may indicate a vitamin B deficiency. Thrombocytopenia and leukemia may present with oral petechiae, purpura, oral hematomas, or hemorrhagic bullae (see Figure 20).13 Painless pseudomembranous mucosal erosions may be a presentation for secondary syphilis.16
SIGNS OF SYSTEMIC DISEASE (cont'd)


Continue for signs that suggest malignancy >>
LOOK FOR SIGNS THAT SUGGEST MALIGNANCYIn the United States, oral and pharyngeal cancers account for approximately 40,000 cases of cancer and 8,000 deaths each year.17 More than 90% of these are squamous cell carcinomas (SCCs); the remainder are mainly salivary gland tumors, lymphoma, and other infrequent cancers.18
SCC of the oral cavity most commonly occurs on the tongue but can develop in any site, presenting as mucosal ulcers, plaques, or masses that do not heal (see Figure 21). Tobacco and alcohol use are associated with up to 80% of cases of SCC of the head and neck.18 Some oropharyngeal SCCs are associated with human papillomavirus infection type 16.19
Potentially malignant oral lesions include leukoplakia and erythroplakia. Leukoplakia is a white patch or plaque of the oral mucosa that can’t be explained by any other clinical diagnosis (see Figure 22). These lesions are at risk for malignant transformation and may demonstrate dysplasia or frank SCC on biopsy.20 Proliferative verrucous leukoplakia is a unique form of leukoplakia that is characterized by a wrinkled appearance that is often multifocal; the condition is associated with a higher risk for malignant transformation.
Erythroplakia is a red patch that similarly can’t be explained by another diagnosis. It has a very high risk for malignant transformation over time. All potentially malignant oral lesions, including leukoplakia and erythroplakia, require biopsy and careful monitoring.
Non-SCC cancers. Salivary gland tumors are rare and most commonly occur in patients ages 55 to 65. Most neoplasms (70%-85%) occur in the parotid gland, while 8% to 15% develop in the submandibular salivary gland and less than 1% involve the sublingual gland.21 Minor salivary gland tissue, especially in the lips and palate, may also be affected (see Figure 23). Patients present with circumscribed, fixed or movable, painless, soft or firm masses in a salivary gland.
Melanoma should be included in the differential diagnosis of oral pigmented lesions that have any features of cutaneous melanoma, such as asymmetry, irregular borders, or variable or changing color.22
Hematologic malignancies may initially present (or demonstrate evidence of relapse) in the oral cavity. Leukemia typically presents with sheet-like overgrowth and swelling of the gingiva, with associated erythema and bleeding (see Figure 24), whereas lymphoma typically presents as a solitary mass or ulceration. Solid tumors that metastasize to the oral cavity may present with localized unexplained soft or hard tissue growths, with or without associated neurologic symptoms (eg, paresthesia).
MALIGNANCIES





Family practice clinicians can play an essential role in managing their patients’ oral health by promptly recognizing and diagnosing conditions that demand further medical attention, including nonodontogenic and odontogenic infections, primary oral mucosal diseases, oral manifestations of systemic disease, and malignancy. Many conditions are amenable to treatment by the primary care provider, while others will require referral to a specialist.
This article and accompanying photo guide describe the types of lesions you may encounter during examinations of the oral cavity and the corresponding diagnoses.
BE VIGILANT FOR NONODONTOGENIC CONDITIONS THAT MAY REQUIRE URGENT TREATMENT There are several uncommon, acute nonodontogenic conditions that affect the oral cavity; when severe, they may require urgent medical attention and possible hospitalization.
Primary herpes simplex virus 1 (HSV-1) infection is generally subclinical, but some patients develop significant oral disease—called primary herpetic gingivostomatitis—that is characterized by painful, diffuse, irregular, croplike ulcerations throughout the oral cavity and lips (see Figure 1).1 The gingiva is nearly universally affected, which distinguishes this condition from erythema multiforme and aphthous stomatitis (described later in this article). The incidence is highest in children, followed by adolescents and young adults.2
Erythema multiforme. This mucocutaneous hypersensitivity reaction can be limited to the oral cavity and lips, without accompanying skin lesions. Flu-like symptoms, including fever and chills, are followed by acute onset of diffuse oral ulcerations that are generally limited to nonkeratinized mucosa and spare the gingiva (see Figure 2).3 Ulceration and crusting of the lips are common.
Aphthous stomatitis. Recurrent aphthous stomatitis (RAS) is a common immune mediated inflammatory condition characterized by “canker sores,” or small round/ovoid ulcers with a well-defined erythematous halo (see Figure 3). Lesions almost exclusively affect nonkeratinized mucosa (and never the lip vermilion) and heal within seven to 10 days, although “major” (> 0.5 cm) lesions may persist much longer (see Figure 4). A herpetiform pattern with multiple coalescing ulcers closely mimics HSV.
Small subsets of patients develop “complex” RAS, which is characterized by continuous and often multiple ulcerations that may extend into the esophagus, with associated chronic pain and compromised intake.2 RAS associated with systemic conditions is reviewed below.
URGENT TREATMENT




Continue for how to spot signs of common dental diseases >>
HOW TO SPOT SIGNS OF COMMON DENTAL DISEASES
In 2010 in the United States, close to 1.4 million emergency department visits and about $1 billion in hospital charges were due to dental problems.4 Approximately 40% of these visits were made by individuals without insurance.4 Due to a lack of dental insurance, patients may present to a medical professional rather than a dental professional. Additionally, uninsured individuals may neglect their dental problem until it becomes a medical emergency. Family practice clinicians need to recognize dental disease and be able to provide basic management of emergencies.5
Dental abscess. A dental abscess can arise from pulpal infection (due to progression of caries) or periodontal infection (due to progression of periodontal disease). Pain symptoms are variable; however, intense, spontaneous cyclical pain is generally characteristic of a dental abscess of pulpal etiology, whereas a periodontal abscess can have less obvious symptoms. Swelling intraorally or extraorally indicates the spread of a localized infection (see Figure 5).
Severe infection and swelling can limit mouth opening and function and in extreme cases may obstruct swallowing and even breathing (eg, Ludwig’s angina). Affected teeth may or may not demonstrate obvious findings of advanced dental disease, such as gross caries, fracture, heavy calculus deposits, or marked periodontal attachment loss. Oral examination may reveal a parulis (focal erythematous swelling of the adjacent gingiva with a central draining sinus tract) (see Figure 6) and percussion of the affected tooth is generally painful.
Pericoronitis is infection and swelling of the gingival tissues that surround a tooth, typically in association with a partially erupted third molar. Signs and symptoms include pain, discomfort with eating and swallowing, and limited mouth opening. Examination demonstrates gingival inflammation around a tooth, with or without purulence (see Figure 7).
Acute necrotizing ulcerative gingivitis (ANUG) and periodontitis (ANUP) are severe conditions that are typically associated with psychological stress, severe malnutrition, and immunosuppression in patients with preexisting gingivitis or periodontitis.6 ANUG is associated with intense gingival pain, halitosis, generalized erythema, and destruction of the gingival papilla, often with bleeding.7 ANUP is a more advanced condition associated with damage and loss of the periodontium (including bone), often with loose teeth (see Figure 8).8
COMMON DENTAL DISEASES




Trauma. Dental trauma can be limited to the teeth and soft tissues, while more severe injuries can also affect the jaw bone.9 Accidental falls, assault, and motor vehicle traffic accidents are the most common causes of facial fractures in the United States and are often associated with dentoalveolar trauma (see Figure 9). The most commonly fractured facial bone is the mandible, characterized by painful opening and closing and an incomplete or altered bite.10

Continue for oral symptoms may be the first sign of systemic disease >>
ORAL SYMPTOMS MAY BE THE FIRST SIGN OF SYSTEMIC DISEASEInflammatory bowel disease. Crohn’s disease may affect the gastrointestinal tract anywhere from the mouth to the anus and may initially present with oral findings that may not correlate with abdominal symptoms. Oral Crohn’s disease may present as mucosal cobblestoning, mucosal tags, deep linear ulcerations, gingival hyperplasia, lip fissuring, aphthous ulcers, and angular cheilitis (see Figures 10 and 11). Other features may include diffuse, painless swelling of the lips and mucosal erythema.
Pyostomatitis vegetans is an uncommon condition typically associated with ulcerative colitis that is characterized by serpentine pustules that coalesce in a “snail track” pattern (see Figure 12).11
Dermatologic/vesiculobullous diseases. Vesiculobullous lesions in the mouth may be seen in pemphigus vulgaris or bullous pemphigoid. Pemphigus vulgaris is an autoimmune intraepithelial blistering disease that often first presents in the oral cavity as flaccid bullae or painful ulcerations, prior to the onset of skin lesions (see Figure 13).
Mucous membrane pemphigoid is an autoimmune subepithelial disease that affects mucous membranes and the skin. Characteristic oral mucosal blisters quickly rupture and form ulcerations, which may occur in the absence of other mucosal involvement (eg, anus, genitalia, nose or throat) (see Figure 14).12
Painful aphthous ulcers are common. When oral ulcerations are diffuse and recurrent, they may be the first sign of Behçet’s disease, a multisystem autoimmune vasculitis.13
Oral lichen planus is a chronic immune-mediated mucocutaneous disease that is often limited to the oral cavity. It presents with characteristic radiating white striations of the buccal mucosa and tongue, often with associated erythema and ulcerations (see Figure 15).14
SIGNS OF SYSTEMIC DISEASE






Continue for rheumatologic conditions >>
Rheumatologic conditions. Systemic or discoid lupus erythematosus may present with oral findings that largely resemble those of oral lichen planus (see Figure 16).15 Sjögren’s syndrome is an autoimmune disease with characteristic xerostomia, which can lead to oral discomfort, dysphagia, recurrent candidiasis, and rampant dental caries.
Other conditions to watch for. Erosion of the enamel on the lingual surface of the teeth may be a sign of gastroesophageal reflux disease or bulimia (see Figure 17). Examination of the oral mucosa can reveal typical white plaques of oral candidiasis (see Figure 18), which may be associated with systemic immune suppression as well as salivary gland dysfunction.
SIGNS OF SYSTEMIC DISEASE (cont'd)



Oral conditions that have been associated with HIV infection include ANUG/ANUP, recurrent candidiasis, and oral hairy leukoplakia (see Figure 19). In the absence of known HIV infection, patients who present with any of these oral conditions should be evaluated for HIV infection.13
Atrophic glossitis may indicate a vitamin B deficiency. Thrombocytopenia and leukemia may present with oral petechiae, purpura, oral hematomas, or hemorrhagic bullae (see Figure 20).13 Painless pseudomembranous mucosal erosions may be a presentation for secondary syphilis.16
SIGNS OF SYSTEMIC DISEASE (cont'd)


Continue for signs that suggest malignancy >>
LOOK FOR SIGNS THAT SUGGEST MALIGNANCYIn the United States, oral and pharyngeal cancers account for approximately 40,000 cases of cancer and 8,000 deaths each year.17 More than 90% of these are squamous cell carcinomas (SCCs); the remainder are mainly salivary gland tumors, lymphoma, and other infrequent cancers.18
SCC of the oral cavity most commonly occurs on the tongue but can develop in any site, presenting as mucosal ulcers, plaques, or masses that do not heal (see Figure 21). Tobacco and alcohol use are associated with up to 80% of cases of SCC of the head and neck.18 Some oropharyngeal SCCs are associated with human papillomavirus infection type 16.19
Potentially malignant oral lesions include leukoplakia and erythroplakia. Leukoplakia is a white patch or plaque of the oral mucosa that can’t be explained by any other clinical diagnosis (see Figure 22). These lesions are at risk for malignant transformation and may demonstrate dysplasia or frank SCC on biopsy.20 Proliferative verrucous leukoplakia is a unique form of leukoplakia that is characterized by a wrinkled appearance that is often multifocal; the condition is associated with a higher risk for malignant transformation.
Erythroplakia is a red patch that similarly can’t be explained by another diagnosis. It has a very high risk for malignant transformation over time. All potentially malignant oral lesions, including leukoplakia and erythroplakia, require biopsy and careful monitoring.
Non-SCC cancers. Salivary gland tumors are rare and most commonly occur in patients ages 55 to 65. Most neoplasms (70%-85%) occur in the parotid gland, while 8% to 15% develop in the submandibular salivary gland and less than 1% involve the sublingual gland.21 Minor salivary gland tissue, especially in the lips and palate, may also be affected (see Figure 23). Patients present with circumscribed, fixed or movable, painless, soft or firm masses in a salivary gland.
Melanoma should be included in the differential diagnosis of oral pigmented lesions that have any features of cutaneous melanoma, such as asymmetry, irregular borders, or variable or changing color.22
Hematologic malignancies may initially present (or demonstrate evidence of relapse) in the oral cavity. Leukemia typically presents with sheet-like overgrowth and swelling of the gingiva, with associated erythema and bleeding (see Figure 24), whereas lymphoma typically presents as a solitary mass or ulceration. Solid tumors that metastasize to the oral cavity may present with localized unexplained soft or hard tissue growths, with or without associated neurologic symptoms (eg, paresthesia).
MALIGNANCIES




1. Spruance SL. The natural history of recurrent oral-facial herpes simplex virus infection. Semin Dermatol. 1992;11:200-206.
2. Balasubramaniam R, Kuperstein AS, Stoopler ET. Update on oral herpes virus infections. Dent Clin North Am. 2014;58:265-280.
3. Lozada-Nur F, Gorsky M, Silverman S Jr. Oral erythema multiforme: clinical observations and treatment of 95 patients. Oral Surg Oral Med Oral Pathol. 1989;67:36-40.
4. Allareddy V, Rampa S, Lee MK, et al. Hospital-based emergency department visits involving dental conditions: profile and predictors of poor outcomes and resource utilization. J Am Dent Assoc. 2014;145:331-337.
5. Allareddy V, Lin CY, Shah A, et al. Outcomes in patients hospitalized for periapical abscess in the United States: an analysis involving the use of a nationwide inpatient sample. J Am Dent Assoc. 2010;141:1107-1116.
6. Folayan MO. The epidemiology, etiology, and pathophysiology of acute necrotizing ulcerative gingivitis associated with malnutrition. J Contemp Dent Pract. 2004;5:28-41.
7. Atout RN, Todescan S. Managing patients with necrotizing ulcerative gingivitis. J Can Dent Assoc. 2013;79:d46.
8. Todescan S, Nizar R. Managing patients with necrotizing ulcerative periodontitis. J Can Dent Assoc. 2013;79:d44.
9. Allareddy V, Allareddy V, Nalliah RP. Epidemiology of facial fracture injuries. J Oral Maxillofac Surg. 2011;69:2613-2618.
10. Nalliah RP, Allareddy V, Kim MK, et al. Economics of facial fracture reductions in the United States over 12 months. Dent Traumatol. 2013;29:115-120.
11. Padmavathi B, Sharma S, Astekar M, et al. Oral Crohn’s disease. J Oral Maxillofac Pathol. 2014;18(suppl 1):S139-S142.
12. Xu HH, Werth VP, Parisi E, et al. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57:611-630.
13. Chi AC, Neville BW, Krayer JW, et al. Oral manifestations of systemic disease. Am Fam Physician. 2010;82:1381-1388.
14. Lavanya N, Jayanthi P, Rao UK, et al. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127-132.
15. Uva L, Miguel D, Pinheiro C, et al. Cutaneous manifestations of systemic lupus erythematosus. Autoimmune Dis. 2012;2012:834291.
16. Ficarra G, Carlos R. Syphilis: The renaissance of an old disease with oral implications. Head Neck Pathol. 2009;3:195-206.
17. Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-236.
18. Licitra L, Locati LD, Bossi P, et al. Head and neck tumors other than squamous cell carcinoma. Curr Opin Oncol. 2004;16:236-241.
19. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693-703.
20. Silverman S Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation: a follow‐up study of 257 patients. Cancer. 1984;53:563-568.
21. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2807 patients. Head Neck Surg. 1986;8:177-184.22. DeMatos P, Tyler DS, Seigler HF. Malignant melanoma of the mucous membranes: a review of 119 cases. Ann Surg Oncol. 1998;5:733-742.
1. Spruance SL. The natural history of recurrent oral-facial herpes simplex virus infection. Semin Dermatol. 1992;11:200-206.
2. Balasubramaniam R, Kuperstein AS, Stoopler ET. Update on oral herpes virus infections. Dent Clin North Am. 2014;58:265-280.
3. Lozada-Nur F, Gorsky M, Silverman S Jr. Oral erythema multiforme: clinical observations and treatment of 95 patients. Oral Surg Oral Med Oral Pathol. 1989;67:36-40.
4. Allareddy V, Rampa S, Lee MK, et al. Hospital-based emergency department visits involving dental conditions: profile and predictors of poor outcomes and resource utilization. J Am Dent Assoc. 2014;145:331-337.
5. Allareddy V, Lin CY, Shah A, et al. Outcomes in patients hospitalized for periapical abscess in the United States: an analysis involving the use of a nationwide inpatient sample. J Am Dent Assoc. 2010;141:1107-1116.
6. Folayan MO. The epidemiology, etiology, and pathophysiology of acute necrotizing ulcerative gingivitis associated with malnutrition. J Contemp Dent Pract. 2004;5:28-41.
7. Atout RN, Todescan S. Managing patients with necrotizing ulcerative gingivitis. J Can Dent Assoc. 2013;79:d46.
8. Todescan S, Nizar R. Managing patients with necrotizing ulcerative periodontitis. J Can Dent Assoc. 2013;79:d44.
9. Allareddy V, Allareddy V, Nalliah RP. Epidemiology of facial fracture injuries. J Oral Maxillofac Surg. 2011;69:2613-2618.
10. Nalliah RP, Allareddy V, Kim MK, et al. Economics of facial fracture reductions in the United States over 12 months. Dent Traumatol. 2013;29:115-120.
11. Padmavathi B, Sharma S, Astekar M, et al. Oral Crohn’s disease. J Oral Maxillofac Pathol. 2014;18(suppl 1):S139-S142.
12. Xu HH, Werth VP, Parisi E, et al. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57:611-630.
13. Chi AC, Neville BW, Krayer JW, et al. Oral manifestations of systemic disease. Am Fam Physician. 2010;82:1381-1388.
14. Lavanya N, Jayanthi P, Rao UK, et al. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127-132.
15. Uva L, Miguel D, Pinheiro C, et al. Cutaneous manifestations of systemic lupus erythematosus. Autoimmune Dis. 2012;2012:834291.
16. Ficarra G, Carlos R. Syphilis: The renaissance of an old disease with oral implications. Head Neck Pathol. 2009;3:195-206.
17. Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-236.
18. Licitra L, Locati LD, Bossi P, et al. Head and neck tumors other than squamous cell carcinoma. Curr Opin Oncol. 2004;16:236-241.
19. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693-703.
20. Silverman S Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation: a follow‐up study of 257 patients. Cancer. 1984;53:563-568.
21. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2807 patients. Head Neck Surg. 1986;8:177-184.22. DeMatos P, Tyler DS, Seigler HF. Malignant melanoma of the mucous membranes: a review of 119 cases. Ann Surg Oncol. 1998;5:733-742.
Osteoporosis: What About Men?
› Order dual-energy x-ray absorptiometry of the spine and hip for men who are at increased risk for osteoporosis and candidates for pharmacotherapy. C
› Prescribe bisphosphonates for men with osteoporosis to reduce the risk of vertebral fractures. A
› Advise men who have, or are at risk for, osteoporosis to consume 1000 to 1200 mg of calcium and 600 to 800 IU of vitamin D daily. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With older women in the United States about 4 times more likely than their male counterparts to develop osteoporosis,1,2 physicians often fail to screen for—or to treat—low bone mass in men. There are plenty of reasons why they should.
First and foremost: Osteoporosis is a leading cause of morbidity and mortality in the elderly.3 An estimated 8.8 million American men suffer from osteoporosis or osteopenia.3 And, although only about 20% of osteoporosis patients are male, men sustain between 30% and 40% of osteoporotic fractures.1,2 What’s more, hip fracture in men has a mortality rate of up to 37.5%—2 to 3 times higher than that of women with hip fracture.4,5
Clearly, then, it is crucial to be aware of the risks of osteoporosis faced by both men and women as they age. Here’s a look at what to consider, when to screen, and how to treat male patients who have, or are at risk for, osteoporosis.
Which men are at risk?
The incidence of fractures secondary to osteoporosis varies with race/ethnicity and geography. The highest rates worldwide occur in Scandinavia and among Caucasians in the United States; black, Asian, and Hispanic populations have the lowest rates.6,7 As with women, the risk of osteoporotic fracture in men increases with age. However, the peak incidence of fracture occurs about 10 years later in men than in women, starting at about age 70.8 Approximately 13% of white men older than 50 years will experience at least one osteoporotic fracture.9
There are 2 main types of osteoporosis: primary and secondary. Up to 40% of osteoporosis in men is primary,4 with bone loss due either to age (senile osteoporosis) or to an unknown cause (idiopathic osteoporosis).10 For men 70 years or older, osteoporosis is assumed to be age related. Idiopathic osteoporosis is diagnosed only in men younger than 70 who have no obvious secondary cause.10 There are numerous secondary causes, however, and most men with bone loss have at least one.4
Common secondary causes: Lifestyle, medical conditions, and meds
The most common causes of secondary osteoporosis in men are exposure to glucocorticoids, primary or secondary hypogonadism (low testosterone), diabetes, alcohol abuse, smoking, gastrointestinal (GI) disease, hypercalciuria, low body weight (body mass index <20 kg/m2), and immobility (TABLE 1).4,5,8,10
Chronic use of corticosteroids, often used to treat chronic obstructive pulmonary disease (COPD), asthma, and rheumatoid arthritis, directly affects the bone, decreasing skeletal muscle, increasing immobility, and reducing intestinal absorption of calcium as well as serum testosterone levels.10 Men with androgen deficiency (which may be due to androgen deprivation therapy to treat prostate cancer) or chronic use of opioids are also at increased risk.4,5,10-12
Diagnostic screening and criteria
The World Health Organization has established diagnostic criteria for osteoporosis using bone mineral density (BMD), reported as both T-scores and Z-scores as measured on dual-energy x-ray absorptiometry (DEXA) scan.13 The T-score represents the number of standard deviations above or below the mean BMD for young adults, matched for sex and race, but not age. It classifies individuals into 3 categories: normal; low (osteopenia), with a T-score between -1 and -2.5; and osteoporosis (T-score ≤-2.5).4,14 The Z-score indicates the number of standard deviations above or below the mean for age, as well as sex and race. A Z-score of ≤-2.0 is below the expected range, indicating an increased likelihood of a secondary form of osteoporosis.14
Which men to screen?
The US Preventive Services Task Force has concluded that evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis in men. It therefore makes no recommendation to screen men who don't have evidence of previous fractures or secondary causes of osteoporosis.15
Other organizations agree that there is insufficient evidence to recommend routine screening for men without known osteoporotic fractures or secondary causes for osteoporosis. There are, however, some guidelines that are useful in clinical practice.
The Endocrine Society, American College of Physicians (ACP), and National Osteoporosis Foundation (NOF) recommend screening men ages 70 years or older, and men ages 50 to 69 who have risk factors for fracture and/or a history of fracture sustained after age 50.5,16,17 (See “Did you know?”)1,2,4,5,9-12,16,17 Prior to screening, it is important to do a complete medical history and physical examination.
Screening considerations. The Endocrine Society, ACP, and NOF recommend a DEXA scan of the spine and hip for men who are at increased risk for osteoporosis and have no contraindications to drug therapy.5,16,17 In patients who have degenerative changes of the spine and hip that would likely obscure DEXA outcomes, a scan of the radius may provide a more accurate assessment of bone status. Men receiving androgen deprivation therapy for prostate cancer will have a greater decline of bone density in the radius than in the hip or spine and are therefore ideal candidates for DEXA of the forearm, as well.5,11 Keep in mind, however, that no studies have looked at how well, or whether, men with osteoporosis measured only in the radius respond to treatment.5
A DEXA scan is not always widely available, nor is it a perfect predictor of fracture risk. In addition, it is not always cost effective. For some patients, the use of a validated clinical predictive tool is preferable as an initial option.
The Male Osteoporosis Risk Estimation Score (MORES) uses age, weight, and history of COPD to identify men 60 years or older who are at risk for osteoporosis (TABLE 2).18 The score can be easily calculated during a clinical encounter and is beneficial for identifying men who should be referred for DEXA scan. A score of ≥6 has been found to yield an overall sensitivity of 0.93 (95% confidence interval [CI], 0.85-0.97) and a specificity of 0.59 (95% CI, 0.56-0.62), with a number needed to screen to prevent one additional hip fracture of 279.18
The Osteoporosis Self-assessment Tool (OST) (http://depts.washington.edu/osteoed/tools.php?type=ost) is a calculated value that uses age and weight to determine an individual’s risk for osteoporosis (risk score=weight [in kg] – age [in years]/5).16,19 Although there is not a defined value to determine a positive OST risk score, scores of -1 to 3 have been used in a variety of studies.16 In a study of 181 American men, the OST predicted osteoporosis with a sensitivity of 93% and a specificity of 66% when using a cutoff score of 3.20
Treating men at risk
Pharmacologic therapy is recommended for men at an increased risk for fracture. This includes men who have had a hip or vertebral fracture without major trauma, as well as those who have not had such a fracture but have a BMD of the spine, femoral neck, and/or total hip of ≤-2.5.5,17 This standard also applies to the radius when used as an alternative site.
The International Society for Clinical Densitometry and International Osteoporosis Foundation endorse the use of the Fracture Risk Assessment Tool (FRAX). Available at http://shef.ac.uk/FRAX/tool.aspx?country=9, FRAX is a computer-based calculator that uses risk factors and BMD of the femoral neck to estimate an individual’s 10-year fracture probability.21 Men who are 50 years or older, have a T-score between -1.0 and -2.5 in the spine, femoral neck, or total hip, and a 10-year risk of ≥20% of developing any fracture or ≥3% of developing a hip fracture based on FRAX, should be offered pharmacotherapy.5,17
Bisphosphonates are first-line therapy
Although oral bisphosphonates are first-line therapy for men who meet these criteria,4 pharmacotherapy should be individualized based on factors such as fracture history, severity of osteoporosis, comorbidities (eg, peptic ulcer disease, malignancy, renal disease, or malabsorption), and cost (TABLE 3).22,23
Alendronate once weekly has been proven to increase BMD and to reduce the risk of fracture in men.24,25 A randomized, placebo-controlled trial of 241 men with osteoporosis found that alendronate increased BMD by 7.1% (±0.3) at the lumbar spine, 2.5% (±0.4) at the femoral neck, and 2% (±0.2) for the total body. Those in the placebo group had a 1.8% (±0.5) increase in BMD of the lumbar spine, with no significant change in femoral neck or total-body BMD—and a higher incidence of vertebral fractures (7.1% vs. 0.8% for those on alendronate; P=.02).24
Risedronate once daily has also been proven to increase BMD in the lumbar spine and hip, with a reduction in vertebral fractures.26 Another investigation—a 2-year, multicenter double-blind placebo-controlled study of 284 men with osteoporosis—found that risedronate given once a week increased BMD in the spine and hip, but did not reduce the incidence of either vertebral or nonvertebral fractures.27
Both alendronate and risedronate are effective for secondary causes of bone loss, such as corticosteroid use, androgen deprivation therapy/hypogonadism, and rheumatologic conditions.28 Oral bisphosphonates may cause GI irritation, however. Abdominal pain associated with alendronate use is between 1% and 7%, vs 2% to 12% for risedronate.23 Neither medication is recommended for use in patients with an estimated glomerular filtration rate <35 mL/min.23 There is no clearly established duration of therapy for men.
Zoledronic acid infusions, given intravenously (IV) once a year, are available for men who cannot tolerate oral bisphosphonates. In a multicenter double-blind, placebocontrolled trial, zoledronic acid was found to reduce the risk of vertebral fractures in men with primary or hypogonadism-associated osteoporosis by 67% (1.6% vertebral fractures in the treatment group after 24 months vs 4.9% with placebo).29 Given within 90 days of a hip fracture repair, zoledronic acid was associated with both a reduction in the rate of new fractures and an increased survival rate.30
Adverse effects of zoledronic acid include diffuse bone pain (3%-9%), fever (9%-22%) and flu-like symptoms (1%-11%). Osteonecrosis of the jaw has been reported in <1% of patients.23
Recombinant human parathyroid hormone stimulates bone growth
Teriparatide, administered subcutaneously (SC) once a day, directly stimulates bone formation. In a randomized placebo controlled trial of 437 men with a T-score of -2, teriparatide was found to increase BMD at the spine and femoral neck. Participants were randomized to receive teriparatide (20 or 40 mcg/d) or placebo. Those who received teriparatide had a doserelated increase in BMD from baseline at the spine (5.9% with 20 mcg and 9% with 40 mcg) and femoral neck (1.5% and 2.9%, respectively) compared with the placebo group.31 Teriparatide was shown to reduce vertebral fractures by 51% compared with placebo in a randomized study of 355 men with osteoporosis.32
Teriparatide is indicated for men with severe osteoporosis and those for whom bisphosphonate treatment has been unsuccessful. Its use is limited to 2 years due to a dose-dependent risk of osteosarcoma. Teriparatide is contraindicated in patients with skeletal metastasis and has been associated with transient hypercalcemia 4 to 6 hours after administration.23 Its use in combination with bisphosphonates is not recommended due to the lack of proven benefit, risk of adverse effects, and associated cost.5
Testosterone boosts bone density
Testosterone therapy is recommended for men with low levels of testosterone (<200 ng/dL), high risk for fracture, and contraindications to pharmacologic agents approved for the treatment of osteoporosis.5 Supplementation of testosterone to restore correct physiologic levels will decrease bone turnover and increase bone density.33 In a meta-analysis of 8 trials with a total of 365 participants, testosterone administered intramuscularly was found to increase lumbar BMD by 8% compared with placebo. The effect on fractures is not known.12
• Although US women are 4 times more likely than men to suffer from osteoporosis, men incur between 30% and 40% of osteoporotic fractures.
• Men who sustain hip fractures have a mortality rate of up to 37.5%—2 to 3 times that of women with hip fractures.
• Men treated with androgen deprivation therapy face an increased risk of osteoporosis.
• About 13% of white men older than 50 years will experience at least one osteoporotic fracture in their lifetime.
• The Endocrine Society, American College of Physicians, and National Osteoporosis Foundation recommend screening all men ages 70 years or older—and younger men with risk factors for fracture and/or a history of fracture after age 50—for osteoporosis.
Monoclonal antibody reduces fracture risk
Denosumab, a monoclonal antibody that prevents osteoclast formation leading to decreased bone resorption, is administered SC every 6 months.23 In a placebo-controlled trial of 242 men with low bone mass, denosumab increased BMD at the lumbar spine (5.7%), total hip (2.4%), femoral neck (2.1%), trochanter (3.1%), and one-third radius (0.6%) compared with placebo after one year.34 In men receiving androgen deprivation therapy for nonmetastatic prostate cancer, denosumab has been shown to increase BMD and reduce the incidence of vertebral fractures.35
Adverse effects include hypocalcemia, hypophosphatemia, fatigue, and back pain.23 No data exist on the ability of denosumab to reduce fracture risk in men without androgen deprivation.
Calcium and vitamin D for men at risk
Men who are at risk for or have osteoporosis should consume 1000 mg to 1200 mg of calcium per day. Ideally, this should come through dietary sources, but calcium supplementation may be added when diet is inadequate.5 The Institute of Medicine recommends a calcium intake of 1000 mg/d for men ages 51 to 70 years and 1200 mg/d for men ages 70 and older.36
Men with vitamin D levels below 30 ng/mL should receive vitamin D supplementation to attain blood 25(OH) D levels of at least 30 ng/mL.5 The Institute of Medicine recommends a daily intake of 600 international units (IU) of vitamin D for men ages 51 to 70 and 800 IU for men 70 and older.36 A recent Cochrane review on vitamin D and vitamin D analogues concluded that vitamin D alone was unlikely to prevent fractures in older people; when taken with calcium, however, it may have a preventive effect.37
Counseling and follow-up
Lifestyle modification is an important means of primary prevention for osteoporosis. Advise men at risk for osteoporosis to limit alcohol consumption to 2 drinks daily.4,5,8,10 Tell those who smoke that doing so increases their risk for osteoporotic fracture and refer them for smoking cessation counseling. Emphasize that weight-bearing exercise can improve BMD and should be done at least 3 days per week.4,5,8,10 It is important, too, to do a medication review to look for drug-drug interactions and to discuss fall prevention strategies, such as gait training and an environmental assessment and removal of fall hazards.
The evidence for monitoring treatment using BMD is not very strong.5,14 However, the Endocrine Society recommends that response to treatment be monitored using DEXA scans every one to 2 years, with reduced frequency once the BMD has stabilized.5 Any patient found to have a decrease in BMD after treatment is initiated should undergo further evaluation to determine the cause of the decline.
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic Division of Regional Medicine, 742 Marsh Landing Parkway, Jacksonville Beach, FL 32250; [email protected]
1. Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
2. Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
3. Gennari L, Bilezikian JP. Osteoporosis in men. Endocrinol Metab Clin North Am. 2007;36:399-419.
4. Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358:1474-1482.
5. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822.
6. Memon A, Pospula WM, Tantawy AY, et al. Incidence of hip fracture in Kuwait. Int J Epidemiol. 1998;27:860-865.
7. Maggi S, Kelsey JL, Litvak J, et al. Incidence of hip fractures in the elderly: a cross-national analysis. Osteoporos Int. 1991;1:232-241.
8. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82:503-508.
9. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16 (Suppl 2):S3-S7.
10. National Institutes of Health. NIH osteoporosis and related bone diseases national resource center. Osteoporosis in men. January 2012. National Institutes of Health Web site. Available at: http://www.niams.nih.gov/health_info/bone/osteoporosis/men.asp. Accessed April 22, 2015.
11. Bruder JM, Ma JZ, Basler JW, et al. Prevalence of osteopenia and osteoporosis by central and peripheral bone mineral density in men with prostate cancer during androgen-deprivation therapy. Urology. 2006;67:152-155.
12. Tracz MJ, Sideras K, Boloña ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91:2011-2016.
13. World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level. Summary meeting report. Geneva, Switzerland: World Health Organization. 2007. Available at: http://who.int/chp/topics/Osteoporosis.pdf. Accessed April 22, 2015.
14. The International Society for Clinical Densitometry. 2007 official positions & pediatric official positions of The International Society for Clinical Densitometry. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/wp-content/uploads/2012/10/ISCD2007OfficialPositions-Combined-AdultandPediatric.pdf. Accessed August 11, 2015.
15. U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154:356-364.
16. Qaseem A, Snow V, Shekelle P, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680-684.
17. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation Web site. Washington, DC: 2014. Available at: http://nof.org/files/nof/public/content/file/2791/upload/919.pdf. Accessed April 22, 2015.
18. Shepherd AJ, Cass AR, Carlson CA, et al. Development and internal validation of the male osteoporosis risk estimation score. Ann Fam Med. 2007;5:540-546.
19. Lynn HS, Woo J, Leung PC, et al; Osteoporotic Fractures in Men (MrOS) Study. An evaluation of osteoporosis screening tools for the osteoporotic fractures in men (MrOS) study. Osteoporos Int. 2008;19:1087-1092.
20. Adler RA, Tran MT, Petkov VI. Performance of the osteoporosis self-assessment screening tool for osteoporosis in American men. Mayo Clin Proc. 2003;78:723-727.
21. International Osteoporosis Foundation, The International Society for Clinical Densitometry. 2010 Official Positions on FRAX®. International Osteoporosis Foundation Web site. Available at: http://www.iofbonehealth.org/sites/default/files/PDFs/2010_Official_%20Positions_%20ISCD-IOF_%20FRAX.pdf. Accessed March 21, 2015.
22. Epocrates essentials. Epocrates Web site. Available at: www.epocrates.com. Accessed April 17, 2015.
23. American Pharmacist Association. Drug information handbook: a comprehensive resource for all clinicians and healthcare professionals. 21st ed. Alphen aan den Rijn, The Netherlands: Lexi-Comp, Inc. Wolters Kluwer; 2012-2013.
24. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604-610.
25. Ringe JD, Dorst A, Faber H, et al. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004;24:110-113.
26. Ringe JD, Faber H, Farahmand P, et al. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26:427-431.
27. Boonen S, Orwoll ES, Wenderoth D, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebocontrolled, double-blind, multicenter study. J Bone Miner Res. 2009;24:719-725.
28. Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441-464.
29. Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367:1714-1723.
30. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
31. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9-17.
32. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510-516.
33. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966-1972.
34. Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97:3161-3169.
35. Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745-755.
36. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Institute of Medicine Web site. Available at: http://www.iom.edu/reports/2010/dietary-reference-intakes-for-calcium-and-vitamin-d.aspx. Accessed April 10, 2015.
37. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
› Order dual-energy x-ray absorptiometry of the spine and hip for men who are at increased risk for osteoporosis and candidates for pharmacotherapy. C
› Prescribe bisphosphonates for men with osteoporosis to reduce the risk of vertebral fractures. A
› Advise men who have, or are at risk for, osteoporosis to consume 1000 to 1200 mg of calcium and 600 to 800 IU of vitamin D daily. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With older women in the United States about 4 times more likely than their male counterparts to develop osteoporosis,1,2 physicians often fail to screen for—or to treat—low bone mass in men. There are plenty of reasons why they should.
First and foremost: Osteoporosis is a leading cause of morbidity and mortality in the elderly.3 An estimated 8.8 million American men suffer from osteoporosis or osteopenia.3 And, although only about 20% of osteoporosis patients are male, men sustain between 30% and 40% of osteoporotic fractures.1,2 What’s more, hip fracture in men has a mortality rate of up to 37.5%—2 to 3 times higher than that of women with hip fracture.4,5
Clearly, then, it is crucial to be aware of the risks of osteoporosis faced by both men and women as they age. Here’s a look at what to consider, when to screen, and how to treat male patients who have, or are at risk for, osteoporosis.
Which men are at risk?
The incidence of fractures secondary to osteoporosis varies with race/ethnicity and geography. The highest rates worldwide occur in Scandinavia and among Caucasians in the United States; black, Asian, and Hispanic populations have the lowest rates.6,7 As with women, the risk of osteoporotic fracture in men increases with age. However, the peak incidence of fracture occurs about 10 years later in men than in women, starting at about age 70.8 Approximately 13% of white men older than 50 years will experience at least one osteoporotic fracture.9
There are 2 main types of osteoporosis: primary and secondary. Up to 40% of osteoporosis in men is primary,4 with bone loss due either to age (senile osteoporosis) or to an unknown cause (idiopathic osteoporosis).10 For men 70 years or older, osteoporosis is assumed to be age related. Idiopathic osteoporosis is diagnosed only in men younger than 70 who have no obvious secondary cause.10 There are numerous secondary causes, however, and most men with bone loss have at least one.4
Common secondary causes: Lifestyle, medical conditions, and meds
The most common causes of secondary osteoporosis in men are exposure to glucocorticoids, primary or secondary hypogonadism (low testosterone), diabetes, alcohol abuse, smoking, gastrointestinal (GI) disease, hypercalciuria, low body weight (body mass index <20 kg/m2), and immobility (TABLE 1).4,5,8,10
Chronic use of corticosteroids, often used to treat chronic obstructive pulmonary disease (COPD), asthma, and rheumatoid arthritis, directly affects the bone, decreasing skeletal muscle, increasing immobility, and reducing intestinal absorption of calcium as well as serum testosterone levels.10 Men with androgen deficiency (which may be due to androgen deprivation therapy to treat prostate cancer) or chronic use of opioids are also at increased risk.4,5,10-12
Diagnostic screening and criteria
The World Health Organization has established diagnostic criteria for osteoporosis using bone mineral density (BMD), reported as both T-scores and Z-scores as measured on dual-energy x-ray absorptiometry (DEXA) scan.13 The T-score represents the number of standard deviations above or below the mean BMD for young adults, matched for sex and race, but not age. It classifies individuals into 3 categories: normal; low (osteopenia), with a T-score between -1 and -2.5; and osteoporosis (T-score ≤-2.5).4,14 The Z-score indicates the number of standard deviations above or below the mean for age, as well as sex and race. A Z-score of ≤-2.0 is below the expected range, indicating an increased likelihood of a secondary form of osteoporosis.14
Which men to screen?
The US Preventive Services Task Force has concluded that evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis in men. It therefore makes no recommendation to screen men who don't have evidence of previous fractures or secondary causes of osteoporosis.15
Other organizations agree that there is insufficient evidence to recommend routine screening for men without known osteoporotic fractures or secondary causes for osteoporosis. There are, however, some guidelines that are useful in clinical practice.
The Endocrine Society, American College of Physicians (ACP), and National Osteoporosis Foundation (NOF) recommend screening men ages 70 years or older, and men ages 50 to 69 who have risk factors for fracture and/or a history of fracture sustained after age 50.5,16,17 (See “Did you know?”)1,2,4,5,9-12,16,17 Prior to screening, it is important to do a complete medical history and physical examination.
Screening considerations. The Endocrine Society, ACP, and NOF recommend a DEXA scan of the spine and hip for men who are at increased risk for osteoporosis and have no contraindications to drug therapy.5,16,17 In patients who have degenerative changes of the spine and hip that would likely obscure DEXA outcomes, a scan of the radius may provide a more accurate assessment of bone status. Men receiving androgen deprivation therapy for prostate cancer will have a greater decline of bone density in the radius than in the hip or spine and are therefore ideal candidates for DEXA of the forearm, as well.5,11 Keep in mind, however, that no studies have looked at how well, or whether, men with osteoporosis measured only in the radius respond to treatment.5
A DEXA scan is not always widely available, nor is it a perfect predictor of fracture risk. In addition, it is not always cost effective. For some patients, the use of a validated clinical predictive tool is preferable as an initial option.
The Male Osteoporosis Risk Estimation Score (MORES) uses age, weight, and history of COPD to identify men 60 years or older who are at risk for osteoporosis (TABLE 2).18 The score can be easily calculated during a clinical encounter and is beneficial for identifying men who should be referred for DEXA scan. A score of ≥6 has been found to yield an overall sensitivity of 0.93 (95% confidence interval [CI], 0.85-0.97) and a specificity of 0.59 (95% CI, 0.56-0.62), with a number needed to screen to prevent one additional hip fracture of 279.18
The Osteoporosis Self-assessment Tool (OST) (http://depts.washington.edu/osteoed/tools.php?type=ost) is a calculated value that uses age and weight to determine an individual’s risk for osteoporosis (risk score=weight [in kg] – age [in years]/5).16,19 Although there is not a defined value to determine a positive OST risk score, scores of -1 to 3 have been used in a variety of studies.16 In a study of 181 American men, the OST predicted osteoporosis with a sensitivity of 93% and a specificity of 66% when using a cutoff score of 3.20
Treating men at risk
Pharmacologic therapy is recommended for men at an increased risk for fracture. This includes men who have had a hip or vertebral fracture without major trauma, as well as those who have not had such a fracture but have a BMD of the spine, femoral neck, and/or total hip of ≤-2.5.5,17 This standard also applies to the radius when used as an alternative site.
The International Society for Clinical Densitometry and International Osteoporosis Foundation endorse the use of the Fracture Risk Assessment Tool (FRAX). Available at http://shef.ac.uk/FRAX/tool.aspx?country=9, FRAX is a computer-based calculator that uses risk factors and BMD of the femoral neck to estimate an individual’s 10-year fracture probability.21 Men who are 50 years or older, have a T-score between -1.0 and -2.5 in the spine, femoral neck, or total hip, and a 10-year risk of ≥20% of developing any fracture or ≥3% of developing a hip fracture based on FRAX, should be offered pharmacotherapy.5,17
Bisphosphonates are first-line therapy
Although oral bisphosphonates are first-line therapy for men who meet these criteria,4 pharmacotherapy should be individualized based on factors such as fracture history, severity of osteoporosis, comorbidities (eg, peptic ulcer disease, malignancy, renal disease, or malabsorption), and cost (TABLE 3).22,23
Alendronate once weekly has been proven to increase BMD and to reduce the risk of fracture in men.24,25 A randomized, placebo-controlled trial of 241 men with osteoporosis found that alendronate increased BMD by 7.1% (±0.3) at the lumbar spine, 2.5% (±0.4) at the femoral neck, and 2% (±0.2) for the total body. Those in the placebo group had a 1.8% (±0.5) increase in BMD of the lumbar spine, with no significant change in femoral neck or total-body BMD—and a higher incidence of vertebral fractures (7.1% vs. 0.8% for those on alendronate; P=.02).24
Risedronate once daily has also been proven to increase BMD in the lumbar spine and hip, with a reduction in vertebral fractures.26 Another investigation—a 2-year, multicenter double-blind placebo-controlled study of 284 men with osteoporosis—found that risedronate given once a week increased BMD in the spine and hip, but did not reduce the incidence of either vertebral or nonvertebral fractures.27
Both alendronate and risedronate are effective for secondary causes of bone loss, such as corticosteroid use, androgen deprivation therapy/hypogonadism, and rheumatologic conditions.28 Oral bisphosphonates may cause GI irritation, however. Abdominal pain associated with alendronate use is between 1% and 7%, vs 2% to 12% for risedronate.23 Neither medication is recommended for use in patients with an estimated glomerular filtration rate <35 mL/min.23 There is no clearly established duration of therapy for men.
Zoledronic acid infusions, given intravenously (IV) once a year, are available for men who cannot tolerate oral bisphosphonates. In a multicenter double-blind, placebocontrolled trial, zoledronic acid was found to reduce the risk of vertebral fractures in men with primary or hypogonadism-associated osteoporosis by 67% (1.6% vertebral fractures in the treatment group after 24 months vs 4.9% with placebo).29 Given within 90 days of a hip fracture repair, zoledronic acid was associated with both a reduction in the rate of new fractures and an increased survival rate.30
Adverse effects of zoledronic acid include diffuse bone pain (3%-9%), fever (9%-22%) and flu-like symptoms (1%-11%). Osteonecrosis of the jaw has been reported in <1% of patients.23
Recombinant human parathyroid hormone stimulates bone growth
Teriparatide, administered subcutaneously (SC) once a day, directly stimulates bone formation. In a randomized placebo controlled trial of 437 men with a T-score of -2, teriparatide was found to increase BMD at the spine and femoral neck. Participants were randomized to receive teriparatide (20 or 40 mcg/d) or placebo. Those who received teriparatide had a doserelated increase in BMD from baseline at the spine (5.9% with 20 mcg and 9% with 40 mcg) and femoral neck (1.5% and 2.9%, respectively) compared with the placebo group.31 Teriparatide was shown to reduce vertebral fractures by 51% compared with placebo in a randomized study of 355 men with osteoporosis.32
Teriparatide is indicated for men with severe osteoporosis and those for whom bisphosphonate treatment has been unsuccessful. Its use is limited to 2 years due to a dose-dependent risk of osteosarcoma. Teriparatide is contraindicated in patients with skeletal metastasis and has been associated with transient hypercalcemia 4 to 6 hours after administration.23 Its use in combination with bisphosphonates is not recommended due to the lack of proven benefit, risk of adverse effects, and associated cost.5
Testosterone boosts bone density
Testosterone therapy is recommended for men with low levels of testosterone (<200 ng/dL), high risk for fracture, and contraindications to pharmacologic agents approved for the treatment of osteoporosis.5 Supplementation of testosterone to restore correct physiologic levels will decrease bone turnover and increase bone density.33 In a meta-analysis of 8 trials with a total of 365 participants, testosterone administered intramuscularly was found to increase lumbar BMD by 8% compared with placebo. The effect on fractures is not known.12
• Although US women are 4 times more likely than men to suffer from osteoporosis, men incur between 30% and 40% of osteoporotic fractures.
• Men who sustain hip fractures have a mortality rate of up to 37.5%—2 to 3 times that of women with hip fractures.
• Men treated with androgen deprivation therapy face an increased risk of osteoporosis.
• About 13% of white men older than 50 years will experience at least one osteoporotic fracture in their lifetime.
• The Endocrine Society, American College of Physicians, and National Osteoporosis Foundation recommend screening all men ages 70 years or older—and younger men with risk factors for fracture and/or a history of fracture after age 50—for osteoporosis.
Monoclonal antibody reduces fracture risk
Denosumab, a monoclonal antibody that prevents osteoclast formation leading to decreased bone resorption, is administered SC every 6 months.23 In a placebo-controlled trial of 242 men with low bone mass, denosumab increased BMD at the lumbar spine (5.7%), total hip (2.4%), femoral neck (2.1%), trochanter (3.1%), and one-third radius (0.6%) compared with placebo after one year.34 In men receiving androgen deprivation therapy for nonmetastatic prostate cancer, denosumab has been shown to increase BMD and reduce the incidence of vertebral fractures.35
Adverse effects include hypocalcemia, hypophosphatemia, fatigue, and back pain.23 No data exist on the ability of denosumab to reduce fracture risk in men without androgen deprivation.
Calcium and vitamin D for men at risk
Men who are at risk for or have osteoporosis should consume 1000 mg to 1200 mg of calcium per day. Ideally, this should come through dietary sources, but calcium supplementation may be added when diet is inadequate.5 The Institute of Medicine recommends a calcium intake of 1000 mg/d for men ages 51 to 70 years and 1200 mg/d for men ages 70 and older.36
Men with vitamin D levels below 30 ng/mL should receive vitamin D supplementation to attain blood 25(OH) D levels of at least 30 ng/mL.5 The Institute of Medicine recommends a daily intake of 600 international units (IU) of vitamin D for men ages 51 to 70 and 800 IU for men 70 and older.36 A recent Cochrane review on vitamin D and vitamin D analogues concluded that vitamin D alone was unlikely to prevent fractures in older people; when taken with calcium, however, it may have a preventive effect.37
Counseling and follow-up
Lifestyle modification is an important means of primary prevention for osteoporosis. Advise men at risk for osteoporosis to limit alcohol consumption to 2 drinks daily.4,5,8,10 Tell those who smoke that doing so increases their risk for osteoporotic fracture and refer them for smoking cessation counseling. Emphasize that weight-bearing exercise can improve BMD and should be done at least 3 days per week.4,5,8,10 It is important, too, to do a medication review to look for drug-drug interactions and to discuss fall prevention strategies, such as gait training and an environmental assessment and removal of fall hazards.
The evidence for monitoring treatment using BMD is not very strong.5,14 However, the Endocrine Society recommends that response to treatment be monitored using DEXA scans every one to 2 years, with reduced frequency once the BMD has stabilized.5 Any patient found to have a decrease in BMD after treatment is initiated should undergo further evaluation to determine the cause of the decline.
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic Division of Regional Medicine, 742 Marsh Landing Parkway, Jacksonville Beach, FL 32250; [email protected]
› Order dual-energy x-ray absorptiometry of the spine and hip for men who are at increased risk for osteoporosis and candidates for pharmacotherapy. C
› Prescribe bisphosphonates for men with osteoporosis to reduce the risk of vertebral fractures. A
› Advise men who have, or are at risk for, osteoporosis to consume 1000 to 1200 mg of calcium and 600 to 800 IU of vitamin D daily. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With older women in the United States about 4 times more likely than their male counterparts to develop osteoporosis,1,2 physicians often fail to screen for—or to treat—low bone mass in men. There are plenty of reasons why they should.
First and foremost: Osteoporosis is a leading cause of morbidity and mortality in the elderly.3 An estimated 8.8 million American men suffer from osteoporosis or osteopenia.3 And, although only about 20% of osteoporosis patients are male, men sustain between 30% and 40% of osteoporotic fractures.1,2 What’s more, hip fracture in men has a mortality rate of up to 37.5%—2 to 3 times higher than that of women with hip fracture.4,5
Clearly, then, it is crucial to be aware of the risks of osteoporosis faced by both men and women as they age. Here’s a look at what to consider, when to screen, and how to treat male patients who have, or are at risk for, osteoporosis.
Which men are at risk?
The incidence of fractures secondary to osteoporosis varies with race/ethnicity and geography. The highest rates worldwide occur in Scandinavia and among Caucasians in the United States; black, Asian, and Hispanic populations have the lowest rates.6,7 As with women, the risk of osteoporotic fracture in men increases with age. However, the peak incidence of fracture occurs about 10 years later in men than in women, starting at about age 70.8 Approximately 13% of white men older than 50 years will experience at least one osteoporotic fracture.9
There are 2 main types of osteoporosis: primary and secondary. Up to 40% of osteoporosis in men is primary,4 with bone loss due either to age (senile osteoporosis) or to an unknown cause (idiopathic osteoporosis).10 For men 70 years or older, osteoporosis is assumed to be age related. Idiopathic osteoporosis is diagnosed only in men younger than 70 who have no obvious secondary cause.10 There are numerous secondary causes, however, and most men with bone loss have at least one.4
Common secondary causes: Lifestyle, medical conditions, and meds
The most common causes of secondary osteoporosis in men are exposure to glucocorticoids, primary or secondary hypogonadism (low testosterone), diabetes, alcohol abuse, smoking, gastrointestinal (GI) disease, hypercalciuria, low body weight (body mass index <20 kg/m2), and immobility (TABLE 1).4,5,8,10
Chronic use of corticosteroids, often used to treat chronic obstructive pulmonary disease (COPD), asthma, and rheumatoid arthritis, directly affects the bone, decreasing skeletal muscle, increasing immobility, and reducing intestinal absorption of calcium as well as serum testosterone levels.10 Men with androgen deficiency (which may be due to androgen deprivation therapy to treat prostate cancer) or chronic use of opioids are also at increased risk.4,5,10-12
Diagnostic screening and criteria
The World Health Organization has established diagnostic criteria for osteoporosis using bone mineral density (BMD), reported as both T-scores and Z-scores as measured on dual-energy x-ray absorptiometry (DEXA) scan.13 The T-score represents the number of standard deviations above or below the mean BMD for young adults, matched for sex and race, but not age. It classifies individuals into 3 categories: normal; low (osteopenia), with a T-score between -1 and -2.5; and osteoporosis (T-score ≤-2.5).4,14 The Z-score indicates the number of standard deviations above or below the mean for age, as well as sex and race. A Z-score of ≤-2.0 is below the expected range, indicating an increased likelihood of a secondary form of osteoporosis.14
Which men to screen?
The US Preventive Services Task Force has concluded that evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis in men. It therefore makes no recommendation to screen men who don't have evidence of previous fractures or secondary causes of osteoporosis.15
Other organizations agree that there is insufficient evidence to recommend routine screening for men without known osteoporotic fractures or secondary causes for osteoporosis. There are, however, some guidelines that are useful in clinical practice.
The Endocrine Society, American College of Physicians (ACP), and National Osteoporosis Foundation (NOF) recommend screening men ages 70 years or older, and men ages 50 to 69 who have risk factors for fracture and/or a history of fracture sustained after age 50.5,16,17 (See “Did you know?”)1,2,4,5,9-12,16,17 Prior to screening, it is important to do a complete medical history and physical examination.
Screening considerations. The Endocrine Society, ACP, and NOF recommend a DEXA scan of the spine and hip for men who are at increased risk for osteoporosis and have no contraindications to drug therapy.5,16,17 In patients who have degenerative changes of the spine and hip that would likely obscure DEXA outcomes, a scan of the radius may provide a more accurate assessment of bone status. Men receiving androgen deprivation therapy for prostate cancer will have a greater decline of bone density in the radius than in the hip or spine and are therefore ideal candidates for DEXA of the forearm, as well.5,11 Keep in mind, however, that no studies have looked at how well, or whether, men with osteoporosis measured only in the radius respond to treatment.5
A DEXA scan is not always widely available, nor is it a perfect predictor of fracture risk. In addition, it is not always cost effective. For some patients, the use of a validated clinical predictive tool is preferable as an initial option.
The Male Osteoporosis Risk Estimation Score (MORES) uses age, weight, and history of COPD to identify men 60 years or older who are at risk for osteoporosis (TABLE 2).18 The score can be easily calculated during a clinical encounter and is beneficial for identifying men who should be referred for DEXA scan. A score of ≥6 has been found to yield an overall sensitivity of 0.93 (95% confidence interval [CI], 0.85-0.97) and a specificity of 0.59 (95% CI, 0.56-0.62), with a number needed to screen to prevent one additional hip fracture of 279.18
The Osteoporosis Self-assessment Tool (OST) (http://depts.washington.edu/osteoed/tools.php?type=ost) is a calculated value that uses age and weight to determine an individual’s risk for osteoporosis (risk score=weight [in kg] – age [in years]/5).16,19 Although there is not a defined value to determine a positive OST risk score, scores of -1 to 3 have been used in a variety of studies.16 In a study of 181 American men, the OST predicted osteoporosis with a sensitivity of 93% and a specificity of 66% when using a cutoff score of 3.20
Treating men at risk
Pharmacologic therapy is recommended for men at an increased risk for fracture. This includes men who have had a hip or vertebral fracture without major trauma, as well as those who have not had such a fracture but have a BMD of the spine, femoral neck, and/or total hip of ≤-2.5.5,17 This standard also applies to the radius when used as an alternative site.
The International Society for Clinical Densitometry and International Osteoporosis Foundation endorse the use of the Fracture Risk Assessment Tool (FRAX). Available at http://shef.ac.uk/FRAX/tool.aspx?country=9, FRAX is a computer-based calculator that uses risk factors and BMD of the femoral neck to estimate an individual’s 10-year fracture probability.21 Men who are 50 years or older, have a T-score between -1.0 and -2.5 in the spine, femoral neck, or total hip, and a 10-year risk of ≥20% of developing any fracture or ≥3% of developing a hip fracture based on FRAX, should be offered pharmacotherapy.5,17
Bisphosphonates are first-line therapy
Although oral bisphosphonates are first-line therapy for men who meet these criteria,4 pharmacotherapy should be individualized based on factors such as fracture history, severity of osteoporosis, comorbidities (eg, peptic ulcer disease, malignancy, renal disease, or malabsorption), and cost (TABLE 3).22,23
Alendronate once weekly has been proven to increase BMD and to reduce the risk of fracture in men.24,25 A randomized, placebo-controlled trial of 241 men with osteoporosis found that alendronate increased BMD by 7.1% (±0.3) at the lumbar spine, 2.5% (±0.4) at the femoral neck, and 2% (±0.2) for the total body. Those in the placebo group had a 1.8% (±0.5) increase in BMD of the lumbar spine, with no significant change in femoral neck or total-body BMD—and a higher incidence of vertebral fractures (7.1% vs. 0.8% for those on alendronate; P=.02).24
Risedronate once daily has also been proven to increase BMD in the lumbar spine and hip, with a reduction in vertebral fractures.26 Another investigation—a 2-year, multicenter double-blind placebo-controlled study of 284 men with osteoporosis—found that risedronate given once a week increased BMD in the spine and hip, but did not reduce the incidence of either vertebral or nonvertebral fractures.27
Both alendronate and risedronate are effective for secondary causes of bone loss, such as corticosteroid use, androgen deprivation therapy/hypogonadism, and rheumatologic conditions.28 Oral bisphosphonates may cause GI irritation, however. Abdominal pain associated with alendronate use is between 1% and 7%, vs 2% to 12% for risedronate.23 Neither medication is recommended for use in patients with an estimated glomerular filtration rate <35 mL/min.23 There is no clearly established duration of therapy for men.
Zoledronic acid infusions, given intravenously (IV) once a year, are available for men who cannot tolerate oral bisphosphonates. In a multicenter double-blind, placebocontrolled trial, zoledronic acid was found to reduce the risk of vertebral fractures in men with primary or hypogonadism-associated osteoporosis by 67% (1.6% vertebral fractures in the treatment group after 24 months vs 4.9% with placebo).29 Given within 90 days of a hip fracture repair, zoledronic acid was associated with both a reduction in the rate of new fractures and an increased survival rate.30
Adverse effects of zoledronic acid include diffuse bone pain (3%-9%), fever (9%-22%) and flu-like symptoms (1%-11%). Osteonecrosis of the jaw has been reported in <1% of patients.23
Recombinant human parathyroid hormone stimulates bone growth
Teriparatide, administered subcutaneously (SC) once a day, directly stimulates bone formation. In a randomized placebo controlled trial of 437 men with a T-score of -2, teriparatide was found to increase BMD at the spine and femoral neck. Participants were randomized to receive teriparatide (20 or 40 mcg/d) or placebo. Those who received teriparatide had a doserelated increase in BMD from baseline at the spine (5.9% with 20 mcg and 9% with 40 mcg) and femoral neck (1.5% and 2.9%, respectively) compared with the placebo group.31 Teriparatide was shown to reduce vertebral fractures by 51% compared with placebo in a randomized study of 355 men with osteoporosis.32
Teriparatide is indicated for men with severe osteoporosis and those for whom bisphosphonate treatment has been unsuccessful. Its use is limited to 2 years due to a dose-dependent risk of osteosarcoma. Teriparatide is contraindicated in patients with skeletal metastasis and has been associated with transient hypercalcemia 4 to 6 hours after administration.23 Its use in combination with bisphosphonates is not recommended due to the lack of proven benefit, risk of adverse effects, and associated cost.5
Testosterone boosts bone density
Testosterone therapy is recommended for men with low levels of testosterone (<200 ng/dL), high risk for fracture, and contraindications to pharmacologic agents approved for the treatment of osteoporosis.5 Supplementation of testosterone to restore correct physiologic levels will decrease bone turnover and increase bone density.33 In a meta-analysis of 8 trials with a total of 365 participants, testosterone administered intramuscularly was found to increase lumbar BMD by 8% compared with placebo. The effect on fractures is not known.12
• Although US women are 4 times more likely than men to suffer from osteoporosis, men incur between 30% and 40% of osteoporotic fractures.
• Men who sustain hip fractures have a mortality rate of up to 37.5%—2 to 3 times that of women with hip fractures.
• Men treated with androgen deprivation therapy face an increased risk of osteoporosis.
• About 13% of white men older than 50 years will experience at least one osteoporotic fracture in their lifetime.
• The Endocrine Society, American College of Physicians, and National Osteoporosis Foundation recommend screening all men ages 70 years or older—and younger men with risk factors for fracture and/or a history of fracture after age 50—for osteoporosis.
Monoclonal antibody reduces fracture risk
Denosumab, a monoclonal antibody that prevents osteoclast formation leading to decreased bone resorption, is administered SC every 6 months.23 In a placebo-controlled trial of 242 men with low bone mass, denosumab increased BMD at the lumbar spine (5.7%), total hip (2.4%), femoral neck (2.1%), trochanter (3.1%), and one-third radius (0.6%) compared with placebo after one year.34 In men receiving androgen deprivation therapy for nonmetastatic prostate cancer, denosumab has been shown to increase BMD and reduce the incidence of vertebral fractures.35
Adverse effects include hypocalcemia, hypophosphatemia, fatigue, and back pain.23 No data exist on the ability of denosumab to reduce fracture risk in men without androgen deprivation.
Calcium and vitamin D for men at risk
Men who are at risk for or have osteoporosis should consume 1000 mg to 1200 mg of calcium per day. Ideally, this should come through dietary sources, but calcium supplementation may be added when diet is inadequate.5 The Institute of Medicine recommends a calcium intake of 1000 mg/d for men ages 51 to 70 years and 1200 mg/d for men ages 70 and older.36
Men with vitamin D levels below 30 ng/mL should receive vitamin D supplementation to attain blood 25(OH) D levels of at least 30 ng/mL.5 The Institute of Medicine recommends a daily intake of 600 international units (IU) of vitamin D for men ages 51 to 70 and 800 IU for men 70 and older.36 A recent Cochrane review on vitamin D and vitamin D analogues concluded that vitamin D alone was unlikely to prevent fractures in older people; when taken with calcium, however, it may have a preventive effect.37
Counseling and follow-up
Lifestyle modification is an important means of primary prevention for osteoporosis. Advise men at risk for osteoporosis to limit alcohol consumption to 2 drinks daily.4,5,8,10 Tell those who smoke that doing so increases their risk for osteoporotic fracture and refer them for smoking cessation counseling. Emphasize that weight-bearing exercise can improve BMD and should be done at least 3 days per week.4,5,8,10 It is important, too, to do a medication review to look for drug-drug interactions and to discuss fall prevention strategies, such as gait training and an environmental assessment and removal of fall hazards.
The evidence for monitoring treatment using BMD is not very strong.5,14 However, the Endocrine Society recommends that response to treatment be monitored using DEXA scans every one to 2 years, with reduced frequency once the BMD has stabilized.5 Any patient found to have a decrease in BMD after treatment is initiated should undergo further evaluation to determine the cause of the decline.
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic Division of Regional Medicine, 742 Marsh Landing Parkway, Jacksonville Beach, FL 32250; [email protected]
1. Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
2. Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
3. Gennari L, Bilezikian JP. Osteoporosis in men. Endocrinol Metab Clin North Am. 2007;36:399-419.
4. Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358:1474-1482.
5. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822.
6. Memon A, Pospula WM, Tantawy AY, et al. Incidence of hip fracture in Kuwait. Int J Epidemiol. 1998;27:860-865.
7. Maggi S, Kelsey JL, Litvak J, et al. Incidence of hip fractures in the elderly: a cross-national analysis. Osteoporos Int. 1991;1:232-241.
8. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82:503-508.
9. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16 (Suppl 2):S3-S7.
10. National Institutes of Health. NIH osteoporosis and related bone diseases national resource center. Osteoporosis in men. January 2012. National Institutes of Health Web site. Available at: http://www.niams.nih.gov/health_info/bone/osteoporosis/men.asp. Accessed April 22, 2015.
11. Bruder JM, Ma JZ, Basler JW, et al. Prevalence of osteopenia and osteoporosis by central and peripheral bone mineral density in men with prostate cancer during androgen-deprivation therapy. Urology. 2006;67:152-155.
12. Tracz MJ, Sideras K, Boloña ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91:2011-2016.
13. World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level. Summary meeting report. Geneva, Switzerland: World Health Organization. 2007. Available at: http://who.int/chp/topics/Osteoporosis.pdf. Accessed April 22, 2015.
14. The International Society for Clinical Densitometry. 2007 official positions & pediatric official positions of The International Society for Clinical Densitometry. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/wp-content/uploads/2012/10/ISCD2007OfficialPositions-Combined-AdultandPediatric.pdf. Accessed August 11, 2015.
15. U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154:356-364.
16. Qaseem A, Snow V, Shekelle P, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680-684.
17. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation Web site. Washington, DC: 2014. Available at: http://nof.org/files/nof/public/content/file/2791/upload/919.pdf. Accessed April 22, 2015.
18. Shepherd AJ, Cass AR, Carlson CA, et al. Development and internal validation of the male osteoporosis risk estimation score. Ann Fam Med. 2007;5:540-546.
19. Lynn HS, Woo J, Leung PC, et al; Osteoporotic Fractures in Men (MrOS) Study. An evaluation of osteoporosis screening tools for the osteoporotic fractures in men (MrOS) study. Osteoporos Int. 2008;19:1087-1092.
20. Adler RA, Tran MT, Petkov VI. Performance of the osteoporosis self-assessment screening tool for osteoporosis in American men. Mayo Clin Proc. 2003;78:723-727.
21. International Osteoporosis Foundation, The International Society for Clinical Densitometry. 2010 Official Positions on FRAX®. International Osteoporosis Foundation Web site. Available at: http://www.iofbonehealth.org/sites/default/files/PDFs/2010_Official_%20Positions_%20ISCD-IOF_%20FRAX.pdf. Accessed March 21, 2015.
22. Epocrates essentials. Epocrates Web site. Available at: www.epocrates.com. Accessed April 17, 2015.
23. American Pharmacist Association. Drug information handbook: a comprehensive resource for all clinicians and healthcare professionals. 21st ed. Alphen aan den Rijn, The Netherlands: Lexi-Comp, Inc. Wolters Kluwer; 2012-2013.
24. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604-610.
25. Ringe JD, Dorst A, Faber H, et al. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004;24:110-113.
26. Ringe JD, Faber H, Farahmand P, et al. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26:427-431.
27. Boonen S, Orwoll ES, Wenderoth D, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebocontrolled, double-blind, multicenter study. J Bone Miner Res. 2009;24:719-725.
28. Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441-464.
29. Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367:1714-1723.
30. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
31. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9-17.
32. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510-516.
33. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966-1972.
34. Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97:3161-3169.
35. Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745-755.
36. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Institute of Medicine Web site. Available at: http://www.iom.edu/reports/2010/dietary-reference-intakes-for-calcium-and-vitamin-d.aspx. Accessed April 10, 2015.
37. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
1. Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
2. Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
3. Gennari L, Bilezikian JP. Osteoporosis in men. Endocrinol Metab Clin North Am. 2007;36:399-419.
4. Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358:1474-1482.
5. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822.
6. Memon A, Pospula WM, Tantawy AY, et al. Incidence of hip fracture in Kuwait. Int J Epidemiol. 1998;27:860-865.
7. Maggi S, Kelsey JL, Litvak J, et al. Incidence of hip fractures in the elderly: a cross-national analysis. Osteoporos Int. 1991;1:232-241.
8. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82:503-508.
9. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16 (Suppl 2):S3-S7.
10. National Institutes of Health. NIH osteoporosis and related bone diseases national resource center. Osteoporosis in men. January 2012. National Institutes of Health Web site. Available at: http://www.niams.nih.gov/health_info/bone/osteoporosis/men.asp. Accessed April 22, 2015.
11. Bruder JM, Ma JZ, Basler JW, et al. Prevalence of osteopenia and osteoporosis by central and peripheral bone mineral density in men with prostate cancer during androgen-deprivation therapy. Urology. 2006;67:152-155.
12. Tracz MJ, Sideras K, Boloña ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91:2011-2016.
13. World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level. Summary meeting report. Geneva, Switzerland: World Health Organization. 2007. Available at: http://who.int/chp/topics/Osteoporosis.pdf. Accessed April 22, 2015.
14. The International Society for Clinical Densitometry. 2007 official positions & pediatric official positions of The International Society for Clinical Densitometry. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/wp-content/uploads/2012/10/ISCD2007OfficialPositions-Combined-AdultandPediatric.pdf. Accessed August 11, 2015.
15. U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154:356-364.
16. Qaseem A, Snow V, Shekelle P, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680-684.
17. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation Web site. Washington, DC: 2014. Available at: http://nof.org/files/nof/public/content/file/2791/upload/919.pdf. Accessed April 22, 2015.
18. Shepherd AJ, Cass AR, Carlson CA, et al. Development and internal validation of the male osteoporosis risk estimation score. Ann Fam Med. 2007;5:540-546.
19. Lynn HS, Woo J, Leung PC, et al; Osteoporotic Fractures in Men (MrOS) Study. An evaluation of osteoporosis screening tools for the osteoporotic fractures in men (MrOS) study. Osteoporos Int. 2008;19:1087-1092.
20. Adler RA, Tran MT, Petkov VI. Performance of the osteoporosis self-assessment screening tool for osteoporosis in American men. Mayo Clin Proc. 2003;78:723-727.
21. International Osteoporosis Foundation, The International Society for Clinical Densitometry. 2010 Official Positions on FRAX®. International Osteoporosis Foundation Web site. Available at: http://www.iofbonehealth.org/sites/default/files/PDFs/2010_Official_%20Positions_%20ISCD-IOF_%20FRAX.pdf. Accessed March 21, 2015.
22. Epocrates essentials. Epocrates Web site. Available at: www.epocrates.com. Accessed April 17, 2015.
23. American Pharmacist Association. Drug information handbook: a comprehensive resource for all clinicians and healthcare professionals. 21st ed. Alphen aan den Rijn, The Netherlands: Lexi-Comp, Inc. Wolters Kluwer; 2012-2013.
24. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604-610.
25. Ringe JD, Dorst A, Faber H, et al. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004;24:110-113.
26. Ringe JD, Faber H, Farahmand P, et al. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26:427-431.
27. Boonen S, Orwoll ES, Wenderoth D, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebocontrolled, double-blind, multicenter study. J Bone Miner Res. 2009;24:719-725.
28. Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441-464.
29. Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367:1714-1723.
30. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
31. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9-17.
32. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510-516.
33. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966-1972.
34. Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97:3161-3169.
35. Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745-755.
36. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Institute of Medicine Web site. Available at: http://www.iom.edu/reports/2010/dietary-reference-intakes-for-calcium-and-vitamin-d.aspx. Accessed April 10, 2015.
37. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
Management of Locally Advanced Rectal Adenocarcinoma
Colorectal cancers are among the most common cancers worldwide, and there is a high mortality rate for advanced-stage disease. Approximately 132,000 new cases of colorectal cancer will be diagnosed in the United States in 2015, and approximately 40,000 of these cases will be primary rectal cancers. The incidence and mortality rates have been steadily declining over the past two decades, largely through advances in screening and improvements in treatment. However, rectal cancer remains a significant cause of morbidity and mortality in the United States and worldwide.
To read the full article in PDF:
Colorectal cancers are among the most common cancers worldwide, and there is a high mortality rate for advanced-stage disease. Approximately 132,000 new cases of colorectal cancer will be diagnosed in the United States in 2015, and approximately 40,000 of these cases will be primary rectal cancers. The incidence and mortality rates have been steadily declining over the past two decades, largely through advances in screening and improvements in treatment. However, rectal cancer remains a significant cause of morbidity and mortality in the United States and worldwide.
To read the full article in PDF:
Colorectal cancers are among the most common cancers worldwide, and there is a high mortality rate for advanced-stage disease. Approximately 132,000 new cases of colorectal cancer will be diagnosed in the United States in 2015, and approximately 40,000 of these cases will be primary rectal cancers. The incidence and mortality rates have been steadily declining over the past two decades, largely through advances in screening and improvements in treatment. However, rectal cancer remains a significant cause of morbidity and mortality in the United States and worldwide.
To read the full article in PDF:
Dyspnea in Malignancy
Overview
Dyspnea, the subjective inability to breathe comfortably, is a common symptom, accounting for 3 to 4 million ED visits annually.1,2 Dyspnea can be acute, subacute, or chronic, with chronic dyspnea defined as the presence of symptoms for more than 1 to 2 months, and subacute dyspnea as symptoms lasting for hours to days.3,4
The reported prevalence of dyspnea based on etiology presenting to the ED is as follows: malignancy (16% to 77%), chronic heart failure (18% to 88%), chronic obstructive pulmonary disease (56% to 98%), or renal disease (11% to 82%).5 Irrespective of time course and etiology, dyspnea can be a debilitating symptom affecting up to 50% of patients admitted to acute, tertiary care hospitals, and a quarter of patients seeking care in the ambulatory setting.6-13
Although dyspnea is common in patients with cancer, it is often underreported.14-19 This article reviews the current information available regarding the epidemiology, pathophysiology, assessment, and treatment of dyspnea in the oncologic patient population. By providing insight into the unique nature of dyspnea for oncologic patients, this article illustrates why dyspnea is an important symptom to recognize, address, and treat.
Epidemiology, Background, and Recognition
Dyspnea has a significant impact on the quality of life for oncologic patients and often intensifies near the time of death. At one regional oncology center, dyspnea was present in 49% of general cancer patients.20 The percentage and severity of dyspnea increases as a patient’s cancer progresses. The National Hospice Study found that dyspnea occurred in 70% of 1,754 terminally ill cancer patients studied during their last 6 weeks of life; more than 28% of these patients graded the distress that they felt from dyspnea as moderate to severe. The incidence of dyspnea was exceeded only by the incidences of pain and anorexia.21
A study conducted by Roberts et al22 showed that dyspnea is a very common, undertreated, and underrecognized condition. In 62% of patients reporting dyspnea, the symptoms had been present for greater than 3 months before patients received medical or nursing intervention. In the inpatient hospital setting, 77% of patients claimed on interview to experience dyspnea, but only 39% of cases were actually reported by nursing staff. In addition, Reddy et al23 found that dyspnea can negatively influence activities of daily living in oncology patients. Moreover, as the patients’ dyspnea intensified, their ability to perform activities of daily living was increasingly affected.23,24
Differential Diagnosis
Studies demonstrate the presence of dyspnea in approximately 20% to 40% of patients at the time of diagnosis of advanced disease, with the percentage increasing to around 70% in the last 6 weeks of life.25-27 Symptom grading is at least moderate in more than 28% of terminally ill cancer patients.21 Although common in oncology patients, dyspnea is difficult to diagnose because it is seldom explained by a single, specific organic etiology.24,25,28 Extrinsic modulators that are difficult to quantify, such as psychological state, cultural background, and life experiences, can also attenuate the perception of dyspnea.18,25
The cause of dyspnea in cancer patients can be broken down into four categories to aid in the differential diagnosis as described by Dudgeon et al29 in 2001 (Table 1). The four categories consist of dyspnea as being directly due to the malignancy itself; indirectly due to the malignancy; a side effect of cancer treatment; or due to underlying lung and cardiac disease unrelated to the malignancy itself.21,29
Etiology
Dyspnea Directly Due to Cancer. Dudgeon et al29 reported that the top three malignancies that directly caused dyspnea are lung, head and neck cancer, and malignancies of genitourinary origin.29 Reuben21 showed that 75% of patients with tumors involving the lung reported dyspnea at some point. Metastasis to the mediastinum, especially to the ribs, was associated with higher levels of dyspnea when compared to metastasis to lung.29
Dyspnea Indirectly Due to Cancer. Dyspnea due to indirect causes such as anemia, pneumonia, and pulmonary emboli may be easier to rectify once diagnosed.
Dyspnea Due to Cancer Treatment. Treatment-related causes of dyspnea include the side effects of surgery and lung irradiation. Patients with a history of pneumonectomy, lobectomy, or pleurodesis report higher levels of dyspnea as do patients with a history of thoracic irradiation. Radiation pneumonitis can occur 6 to 12 weeks following treatment, and radiation fibrosis can occur 6 to 12 months following treatment.29
Dyspnea Unrelated to Cancer. Other medical conditions that must be considered when diagnosing dyspnea include chronic obstructive pulmonary disease, asthma, and congestive heart failure. Psychological distress—described as anxiety and depression in the cancer population—may also cause the sensation of dyspnea.25
Dyspnea can be constant or occur as breakthrough episodes. In the study by Reddy et al,23 39% of patients had constant dyspnea and 20% experienced breakthrough episodes. Breakthrough dyspnea is shorter in duration, lasting less than 5 minutes. Breakthrough dyspnea occurs approximately 5 to 6 times a day and is predominant with 80% of patients who are symptom-free between episodes. As opiates are the drug of choice for dyspnea treatment, differentiating the time course of dyspnea is important in terms of treatment strategy and response. For example, treating breakthrough dyspnea with standard opiate medications might not work given that symptom onset and resolution might occur even before the medication’s onset of action.
Pathophysiology and Psychology
The cause of dyspnea in an oncology patient can be physiological and/or psychological in nature, with more numerous and unique presentations as compared to dyspnea associated with cardiac or lung disease. Chemoreceptor stimulation, mechanical stimuli originating in the lung and chest wall receptors, and neuroventilatory effects all contribute to dyspnea.12,16 Physiological modalities contributing to dyspnea are outlined in Table 2.
Psychological Contributions to the Etiology of Dyspnea
Dyspnea is subjective and biopsychosocial factors play a large role in an oncologic patient’s self-report of this condition. Multiple studies demonstrate that objective signs may not match the patient’s perception of dyspnea.18,21,25 Other studies report a correlation between psychological distress and worsened perception of dyspnea. Psychological distress is often measured by anxiety and depression and is also augmented by the presence of pain.
Assessment
Dyspnea is more challenging to assess in the ED than the laboratory setting36 as the condition is multidimensional in nature, often characterized by three factors consisting of breathing effort, chest tightness, and air hunger.37,38 Since these are subjective symptoms, assessment is difficult if a patient has delirium or other symptoms that alter the ability to provide a coherent response. Also, the subjective nature of dyspnea can lead to bias during symptom measurement and management.39
Research has documented factors such as lung involvement, anxiety, and maximum inspiratory pressure as influential to the perceived intensity of dyspnea for oncologic patients.40 Therefore, the sensation of dyspnea is multifactorial and includes physiological and psychological components for cancer patients.40 Subjective sensation does not always correlate with physiological measurements, adding to the difficulty of objective assessments.
Tanaka et al39 describe six criteria that must be met when attempting to create a scale to measure dyspnea in oncology patients. The Cancer Dyspnea Scale (CDS) is a self-rating scale that has been found to be acceptable and practicable in the clinical setting. The 12-item scale was originally conducted in Japanese and has been validated when translated into Swedish (CDS-S) and English (CDS-E) versions.39,41 Uronis et al41 further tested a reduced Cancer Dyspnea Scale that dropped three items from the original CDS scale (r-CDS-E). According to Uronis, the CDS-E better measures global dyspnea whereas the r-CDS-E can be used to measure effects of an intervention on dyspnea. One of the limitations of the CDS is that the scale was only validated in patients with lung cancer.
Since a universally useful scale has yet to be validated, the findings of a comprehensive history and thorough physical examination are the most important considerations when assessing oncologic patients for dyspnea.18,42 The history and physical examination must assess both physical factors and psychological factors. Physical factors include symptom quality and associated symptoms, provoking and relieving factors, previous treatments and response to treatments, and past medical history. Psychological factors include the patient’s emotional status at onset; as symptoms progress, the EP must assess whether perception of dyspnea is related to emotions such as anxiety and fear.
The longitudinal progression of symptoms must be considered. Currow et al43 found that at days 10 and 3 before death, dyspnea increased in oncologic patients but remained unchanged in patients with a noncancer diagnosis. Thus, the longitudinal progression of dyspnea differs depending on the underlying condition. Nearing death, patients without cancer experience a sustained period of symptoms whereas patients with cancer frequently experience both increased symptom prevalence and intensity.44
Diagnostic testing may also help to identify treatable causes. Chest imaging can be performed by radiography and computed tomography. Complete blood counts and chemistry panels can assess for anemia and electrolyte abnormalities. Maximal inspiratory pressure (MIP), a measure that tests diaphragm and inspiratory muscle strength, is also helpful if no apparent cause is found using other diagnostic modalities.1
Treatment
Treatment of dyspnea in malignancy is uniquely challenging. Ideally, treatment should focus on correcting an underlying cause, but for malignancy, the cause is often not reversible. Therefore, dyspnea treatment often consists of palliative management to control the sensation of symptom burden.45 Terminal cancer patients oftentimes require hospitalization and sedation to adequately manage their symptoms.45
The American Thoracic Society has grouped dyspnea therapy into the following four categories: reducing ventilator demand, decreasing ventilator impedance, improving respiratory muscle strength, or altering central perception.1
Reducing Ventilator Demand
Reducing ventilator demand can be accomplished by decreasing metabolic load or altering central respiratory drive. Decreasing metabolic load by strengthening respiratory muscles helps to get rid of lactic acid accumulation.46 Exercise also helps to prevent overall sensations of fatigue.47,48
Central respiratory drive can be altered in two ways, either with pharmacotherapy or supplemental oxygen. Pharmacotherapy aids focus on either oxygenation or the removal of carbon dioxide. Studies show that opioids, although only with small statistical significance, can be beneficial in treating dyspnea. Morphine is currently the mainstay of pharmacotherapy, but studies have yet to identify the best standard for dosing or optimal route for administration. In 1990, Bruera et al49 found that morphine improved dyspnea without negative impact on respiratory rate, oxygen saturation, or expiratory carbon dioxide.
Benzodiazepines help manage dyspnea by mitigating associated anxiety. Benzodiazepines have yet to be proven beneficial in a study with high statistical significance; however, reassurance and patient education that dyspnea is a common symptom can help allay anxiety. Patients living at home should have strong family support and reassurance that someone will be available to assist if an episode of dyspnea occurs.50
Supplemental oxygen can decrease the sensation of dyspnea by altering central respiratory drive. Oxygen should be given at the lowest effective dose and administered with humidified air to prevent desiccation of the respiratory tract. In 1993, Bruera et al51 showed that oxygen decreases dyspnea in hypoxic cancer patients.
In 1996, a double-blind crossover trial of 14 oncologic patients by Dudgeon et al42 indicated that oxygen helps decrease the sensation of dyspnea, respiratory rate, and breathing effort when compared to air. Improvement in carbon dioxide elimination with the use of breathing techniques such as diaphragmatic breathing and pursed lip breathing are also methods to decrease dyspnea sensation through alteration of the central respiratory drive. These breath-retraining techniques promote relaxed and gentle breathing, help minimize the work of breathing, and promote a sensation of well-being.44,52
Decreasing Ventilator Impedance
Ventilator impedance can result from bronchospasm, airway obstruction, effusions, or increased secretions. Treat airway obstruction from extrinsic compression or an endobronchial tumor with procedures such as balloon bronchoplasty, tracheobronchial stent placement, and brachytherapy. Pleural effusions can be treated using repeat thoracentesis or a thoracentesis with the placement of a chronic indwelling catheter; long-term control can be achieved using procedures such as pleurodesis and a thoracotomy with decortication. Manage secretions by using anticholinergic agents (eg, scopolamine) along with physical suctioning. Corticosteroids also help to reduce ventilator impedance by reducing tumor edema, lymphangitis, and bronchospasm.44 Unfortunately, these treatments are not without side effects and additional risks such as bleeding, infection, and pneumothorax.53
Improving Respiratory Muscle Strength
Maximum inspiratory pressure measures respiratory muscle strength. Normal values of negative pressure are usually greater than –50 cm H2O, and the average MIP found in cancer patients is only –16 cm H2O. Any level below –25 cm H2O is associated with severe respiratory muscle impairment. Maintaining adequate nutrition is important to help improve respiratory muscle strength.
Altering Central Perception
Limited trials suggest altering perception with the use of acupuncture and guided imagery may be of benefit. Morphine and other opiates also help to decrease oxygen requirements by altering central perception.49 Relaxation methods are most effective after a patient has gained the ability to alter and control sensation with breathing techniques.
Dyspnea is a chief complaint reported by the oncologic patients presenting to the ED for evaluation. Despite its prevalence in this patient population, diagnosis can be difficult since it is rarely the result of a single etiology. Consequently, dyspnea is often underdiagnosed and undertreated in the cancer population. Moreover, this condition is a composite of manifestations that are unique to each patient and his or her corresponding disease. These composite manifestations differ in timing and severity and require targeted interventions. Dyspnea that is primarily physiological in nature can be managed by reversing the causative mechanisms through treatments including oxygen therapy and antibiotics. Psychological components of dyspnea can be modified with interventions for anxiety and depression. Dyspnea is a complex composite of problems that deserves more attention in the literature and in practice.
Dr Wattana is an assistant professor in the department of emergency medicine, division of internal medicine at The University of Texas MD Anderson Cancer Center, Houston. Dr Miller is an associate professor in the department of emergency medicine, division of internal medicine at The University of Texas MD Anderson Cancer Center, Houston.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data. 2007;(386):1-32.
- Ekström MP, Abernethy AP, Currow DC. The management of chronic breathlessness in patients with advanced and terminal illness. BMJ. 2015;349:g7617.
- Karnani NG, Reisfield GM, Wilson GR. Evaluation of chronic dyspnea. Am Fam Physician. 2005;71(8):1529-1537.
- Moens K, Higginson IJ, Harding R; EURO IMPACT. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage. 2014;48(4):660-677.
- Peters SP. When the chief complaint is (or should be) dyspnea in adults. J Allergy Clin Immunol Pract. 2013;1(2):129-136.
- Campbell ML. Dyspnea prevalence, trajectories, and measurement in critical care and at life’s end. Curr Opin Support Palliat Care. 2012;6(2):168-171.
- Argulian E, Agarwal V, Bangalore S, et al. Meta-analysis of prognostic implications of dyspnea versus chest pain in patients referred for stress testing. Am J Cardiol. 2014;113(3):559-564.
- van Mourik Y, Rutten FH, Moons KG, Bertens LC, Hoes AW, Reitsma JB. Prevalence and underlying causes of dyspnoea in older people: a systematic review. Age Ageing. 2014;43(3):319-326.
- Scano G, Gigliotti F, Stendardi L, Gagliardi E. Dyspnea and emotional states in health and disease. Respir Med. 2013;107(5):649-655.
- Murakami J, Ueda K, Sano F, Hayashi M, Tanaka N, Hamano K. Prediction of postoperative dyspnea and chronic respiratory failure. J Surg Res. 2015;195(1):303-310.
- Manning HL, Mahler DA. Pathophysiology of dyspnea. Monaldi Arch Chest Dis. 2001;56(4):325-330.
- Robinson LM. A Healthy 22-year-old woman with new onset dyspnea. J Emerg Nurs. 2010;36(2):146-147.
- Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncol. 2012;51(8):996-1008.
- Guirimand F, Sahut d’izarn M, Laporte L, Francillard M, Richard JF, Aegerter P. Sequential occurrence of dyspnea at the end of life in palliative care, according to the underlying cancer. Cancer Med. 2015;4(4):532-539.
- Fishbein D, Kearon C, Killian KJ. An approach to dyspnea in cancer patients. J Pain Symptom Manage. 1989;4(2):76-81.
- Ost DE, Ernst A, Grosu HB, et al; AQuIRE Brochoscopy Registry. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest. 2015;147(5):1282-1298.
- Ripamonti C, Bruera E. Dyspnea: pathophysiology and assessment. J Pain Symptom Manage. 1997;13(4):220-232.
- Petersen S, Ritz T. The role of fearful beliefs in the relationship between situational self-awareness and report of breathing-related sensations. Br J Health Psychol. 2011;16(Pt 2):359-372.
- Dudgeon DJ, Kristjanson L, Sloan JA, Lertzman M, Clement K. Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage. 2001;21(2):95-102.
- Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest. 1986;89(2):234-236.
- Roberts DK, Thorne SE, Pearson C. The experience of dyspnea in late-stage cancer. Patients’ and nurses’ perspectives. Cancer Nurs. 1993;16(4):310-320.
- Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12(1):29-36.
- Hayen A, Herigstad M, Pattinson KT. Understanding dyspnea as a complex individual experience. Maturitas. 2013;76(1):45-50.
- Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2000;19(5):357-362.
- Ripamonti C. Management of dyspnea in advanced cancer patients. Support Care Cancer. 1999;7(4):
- 233-243.
- Hui D, Morgado M, Vidal M, et al. Dyspnea in hospitalized advanced cancer patients: subjective and physiologic correlates. J Palliat Med. 2013;16(3):
- 274-280.
- Ripamonti C, Fulfaro F, Bruera E. Dyspnoea in patients with advanced cancer: incidence, causes and treatments. Cancer Treat Rev. 1998;24(1):69-80.
- Dudgeon DJ, Lertzman M, Askew GR. Physiological changes and clinical correlations of dyspnea in cancer outpatients. J Pain Symptom Manage. 2001;21(5):373-379.
- Swinburn CR, Wakefield JM, Jones PW. Relationship between ventilation and breathlessness during exercise in chronic obstructive airways disease is not altered by prevention of hypoxaemia. Clin Sci (Lond). 1984;67(5):515-519.
- Rock LK, Schwartzstein RM. Mechanisms of dyspnea in chronic lung disease. Curr Opin Support Palliat Care. 2007;1(2):102-108.
- Moy ML, Woodrow Weiss J, Sparrow D, Israel E, Schwartzstein RM. Quality of dyspnea in bronchoconstriction differs from external resistive loads. Am J Respir Crit Care Med. 2000;162(2 Pt 1):451-455.
- Nakayama H, Shibuya M, Yamada M, Suzuki H, Arakawa M, Homma I. In-phase chest wall vibration decreases dyspnea during arm elevation in chronic obstructive pulmonary disease patients. Intern Med. 1998;37(10):831-835.
- Killian KJ, Jones NL. Mechanisms of exertional dyspnea. Clin Chest Med. 1994;15(2):247-257
- Gross NJ. Chronic obstructive pulmonary disease. Current concepts and therapeutic approaches. Chest. 1990;97(2 Suppl):19S-23S.
- Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009;167(1):53-60.
- Petersen S, Orth B, Ritz T. Awareness of breathing: the structure of language descriptors of respiratory sensations. Health Psychol. 2008;27(1):122-127.
- Parshall MB, Meek PM, Sklar D, Alcock J, Bittner P. Test-retest reliability of multidimensional dyspnea profile recall ratings in the emergency department: a prospective, longitudinal study. BMC Emerg Med. 2012;12:6.
- Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: a multidimensional, brief, self-rating scale. Br J Cancer. 2000;82(4):800-805.
- Booth S. The management of dyspnoea in advanced cancer. Hosp Med. 1998;59(5):348-349.
- Uronis HE, Shelby RA, Currow DC, et al. Assessment of the psychometric properties of an English version of the cancer dyspnea scale in people with advanced lung cancer. J Pain Symptom Manage. 2012;44(5):741-749.
- Dudgeon DJ, Rosenthal S. Management of dyspnea and cough in patients with cancer. Hematol Oncol Clin North Am. 1996;10(1):157-171.
- Currow DC, Smith J, Davidson PM, Newton PJ, Agar MR, Abernethy AP. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage. 2010;39(4):680.
- Acheson A, MacCormack D. Dyspnea and the cancer patient--an overview. Can Oncol Nurs J. 1997;7(4):209-213.
- Dudgeon DJ, Lertzman M. Dyspnea in the advanced cancer patient. J Pain Symptom Manage. 1998;16(4):212-219.
- Corner J, Plant H, A’Hern R, Bailey C. Non-pharmacological intervention for breathlessness in lung cancer. Palliat Med. 1996;10(4):299-305.
- LeGrand SB, Walsh D. Palliative management of dyspnea in advanced cancer. Curr Opin Oncol. 1999;11(4):250-254.
- LeGrand SB. Dyspnea: the continuing challenge of palliative management. Curr Opin Oncol. 2002;14(4):394-398.
- Bruera E, Macmillan K, Pither J, MacDonald RN. Effects of morphine on the dyspnea of terminal cancer patients. J Pain Symptom Manage. 1990;5(6):341-344.
- Held JL. Cancer care. Managing shortness of breath. Nursing. 1994;24(7):31.
- Bruera E, de Stoutz N, Velasco-Leiva A, Schoeller T, Hanson J. Effects of oxygen on dyspnoea in hypoxaemic terminal-cancer patients. Lancet. 1993;342(8862):13-14.
- Bailey C. Nursing as therapy in the management of breathlessness in lung cancer. Eur. J. Cancer Care (Engl.). 1995;4(4):184-190.
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage. 2012;(2):301-306.
Overview
Dyspnea, the subjective inability to breathe comfortably, is a common symptom, accounting for 3 to 4 million ED visits annually.1,2 Dyspnea can be acute, subacute, or chronic, with chronic dyspnea defined as the presence of symptoms for more than 1 to 2 months, and subacute dyspnea as symptoms lasting for hours to days.3,4
The reported prevalence of dyspnea based on etiology presenting to the ED is as follows: malignancy (16% to 77%), chronic heart failure (18% to 88%), chronic obstructive pulmonary disease (56% to 98%), or renal disease (11% to 82%).5 Irrespective of time course and etiology, dyspnea can be a debilitating symptom affecting up to 50% of patients admitted to acute, tertiary care hospitals, and a quarter of patients seeking care in the ambulatory setting.6-13
Although dyspnea is common in patients with cancer, it is often underreported.14-19 This article reviews the current information available regarding the epidemiology, pathophysiology, assessment, and treatment of dyspnea in the oncologic patient population. By providing insight into the unique nature of dyspnea for oncologic patients, this article illustrates why dyspnea is an important symptom to recognize, address, and treat.
Epidemiology, Background, and Recognition
Dyspnea has a significant impact on the quality of life for oncologic patients and often intensifies near the time of death. At one regional oncology center, dyspnea was present in 49% of general cancer patients.20 The percentage and severity of dyspnea increases as a patient’s cancer progresses. The National Hospice Study found that dyspnea occurred in 70% of 1,754 terminally ill cancer patients studied during their last 6 weeks of life; more than 28% of these patients graded the distress that they felt from dyspnea as moderate to severe. The incidence of dyspnea was exceeded only by the incidences of pain and anorexia.21
A study conducted by Roberts et al22 showed that dyspnea is a very common, undertreated, and underrecognized condition. In 62% of patients reporting dyspnea, the symptoms had been present for greater than 3 months before patients received medical or nursing intervention. In the inpatient hospital setting, 77% of patients claimed on interview to experience dyspnea, but only 39% of cases were actually reported by nursing staff. In addition, Reddy et al23 found that dyspnea can negatively influence activities of daily living in oncology patients. Moreover, as the patients’ dyspnea intensified, their ability to perform activities of daily living was increasingly affected.23,24
Differential Diagnosis
Studies demonstrate the presence of dyspnea in approximately 20% to 40% of patients at the time of diagnosis of advanced disease, with the percentage increasing to around 70% in the last 6 weeks of life.25-27 Symptom grading is at least moderate in more than 28% of terminally ill cancer patients.21 Although common in oncology patients, dyspnea is difficult to diagnose because it is seldom explained by a single, specific organic etiology.24,25,28 Extrinsic modulators that are difficult to quantify, such as psychological state, cultural background, and life experiences, can also attenuate the perception of dyspnea.18,25
The cause of dyspnea in cancer patients can be broken down into four categories to aid in the differential diagnosis as described by Dudgeon et al29 in 2001 (Table 1). The four categories consist of dyspnea as being directly due to the malignancy itself; indirectly due to the malignancy; a side effect of cancer treatment; or due to underlying lung and cardiac disease unrelated to the malignancy itself.21,29
Etiology
Dyspnea Directly Due to Cancer. Dudgeon et al29 reported that the top three malignancies that directly caused dyspnea are lung, head and neck cancer, and malignancies of genitourinary origin.29 Reuben21 showed that 75% of patients with tumors involving the lung reported dyspnea at some point. Metastasis to the mediastinum, especially to the ribs, was associated with higher levels of dyspnea when compared to metastasis to lung.29
Dyspnea Indirectly Due to Cancer. Dyspnea due to indirect causes such as anemia, pneumonia, and pulmonary emboli may be easier to rectify once diagnosed.
Dyspnea Due to Cancer Treatment. Treatment-related causes of dyspnea include the side effects of surgery and lung irradiation. Patients with a history of pneumonectomy, lobectomy, or pleurodesis report higher levels of dyspnea as do patients with a history of thoracic irradiation. Radiation pneumonitis can occur 6 to 12 weeks following treatment, and radiation fibrosis can occur 6 to 12 months following treatment.29
Dyspnea Unrelated to Cancer. Other medical conditions that must be considered when diagnosing dyspnea include chronic obstructive pulmonary disease, asthma, and congestive heart failure. Psychological distress—described as anxiety and depression in the cancer population—may also cause the sensation of dyspnea.25
Dyspnea can be constant or occur as breakthrough episodes. In the study by Reddy et al,23 39% of patients had constant dyspnea and 20% experienced breakthrough episodes. Breakthrough dyspnea is shorter in duration, lasting less than 5 minutes. Breakthrough dyspnea occurs approximately 5 to 6 times a day and is predominant with 80% of patients who are symptom-free between episodes. As opiates are the drug of choice for dyspnea treatment, differentiating the time course of dyspnea is important in terms of treatment strategy and response. For example, treating breakthrough dyspnea with standard opiate medications might not work given that symptom onset and resolution might occur even before the medication’s onset of action.
Pathophysiology and Psychology
The cause of dyspnea in an oncology patient can be physiological and/or psychological in nature, with more numerous and unique presentations as compared to dyspnea associated with cardiac or lung disease. Chemoreceptor stimulation, mechanical stimuli originating in the lung and chest wall receptors, and neuroventilatory effects all contribute to dyspnea.12,16 Physiological modalities contributing to dyspnea are outlined in Table 2.
Psychological Contributions to the Etiology of Dyspnea
Dyspnea is subjective and biopsychosocial factors play a large role in an oncologic patient’s self-report of this condition. Multiple studies demonstrate that objective signs may not match the patient’s perception of dyspnea.18,21,25 Other studies report a correlation between psychological distress and worsened perception of dyspnea. Psychological distress is often measured by anxiety and depression and is also augmented by the presence of pain.
Assessment
Dyspnea is more challenging to assess in the ED than the laboratory setting36 as the condition is multidimensional in nature, often characterized by three factors consisting of breathing effort, chest tightness, and air hunger.37,38 Since these are subjective symptoms, assessment is difficult if a patient has delirium or other symptoms that alter the ability to provide a coherent response. Also, the subjective nature of dyspnea can lead to bias during symptom measurement and management.39
Research has documented factors such as lung involvement, anxiety, and maximum inspiratory pressure as influential to the perceived intensity of dyspnea for oncologic patients.40 Therefore, the sensation of dyspnea is multifactorial and includes physiological and psychological components for cancer patients.40 Subjective sensation does not always correlate with physiological measurements, adding to the difficulty of objective assessments.
Tanaka et al39 describe six criteria that must be met when attempting to create a scale to measure dyspnea in oncology patients. The Cancer Dyspnea Scale (CDS) is a self-rating scale that has been found to be acceptable and practicable in the clinical setting. The 12-item scale was originally conducted in Japanese and has been validated when translated into Swedish (CDS-S) and English (CDS-E) versions.39,41 Uronis et al41 further tested a reduced Cancer Dyspnea Scale that dropped three items from the original CDS scale (r-CDS-E). According to Uronis, the CDS-E better measures global dyspnea whereas the r-CDS-E can be used to measure effects of an intervention on dyspnea. One of the limitations of the CDS is that the scale was only validated in patients with lung cancer.
Since a universally useful scale has yet to be validated, the findings of a comprehensive history and thorough physical examination are the most important considerations when assessing oncologic patients for dyspnea.18,42 The history and physical examination must assess both physical factors and psychological factors. Physical factors include symptom quality and associated symptoms, provoking and relieving factors, previous treatments and response to treatments, and past medical history. Psychological factors include the patient’s emotional status at onset; as symptoms progress, the EP must assess whether perception of dyspnea is related to emotions such as anxiety and fear.
The longitudinal progression of symptoms must be considered. Currow et al43 found that at days 10 and 3 before death, dyspnea increased in oncologic patients but remained unchanged in patients with a noncancer diagnosis. Thus, the longitudinal progression of dyspnea differs depending on the underlying condition. Nearing death, patients without cancer experience a sustained period of symptoms whereas patients with cancer frequently experience both increased symptom prevalence and intensity.44
Diagnostic testing may also help to identify treatable causes. Chest imaging can be performed by radiography and computed tomography. Complete blood counts and chemistry panels can assess for anemia and electrolyte abnormalities. Maximal inspiratory pressure (MIP), a measure that tests diaphragm and inspiratory muscle strength, is also helpful if no apparent cause is found using other diagnostic modalities.1
Treatment
Treatment of dyspnea in malignancy is uniquely challenging. Ideally, treatment should focus on correcting an underlying cause, but for malignancy, the cause is often not reversible. Therefore, dyspnea treatment often consists of palliative management to control the sensation of symptom burden.45 Terminal cancer patients oftentimes require hospitalization and sedation to adequately manage their symptoms.45
The American Thoracic Society has grouped dyspnea therapy into the following four categories: reducing ventilator demand, decreasing ventilator impedance, improving respiratory muscle strength, or altering central perception.1
Reducing Ventilator Demand
Reducing ventilator demand can be accomplished by decreasing metabolic load or altering central respiratory drive. Decreasing metabolic load by strengthening respiratory muscles helps to get rid of lactic acid accumulation.46 Exercise also helps to prevent overall sensations of fatigue.47,48
Central respiratory drive can be altered in two ways, either with pharmacotherapy or supplemental oxygen. Pharmacotherapy aids focus on either oxygenation or the removal of carbon dioxide. Studies show that opioids, although only with small statistical significance, can be beneficial in treating dyspnea. Morphine is currently the mainstay of pharmacotherapy, but studies have yet to identify the best standard for dosing or optimal route for administration. In 1990, Bruera et al49 found that morphine improved dyspnea without negative impact on respiratory rate, oxygen saturation, or expiratory carbon dioxide.
Benzodiazepines help manage dyspnea by mitigating associated anxiety. Benzodiazepines have yet to be proven beneficial in a study with high statistical significance; however, reassurance and patient education that dyspnea is a common symptom can help allay anxiety. Patients living at home should have strong family support and reassurance that someone will be available to assist if an episode of dyspnea occurs.50
Supplemental oxygen can decrease the sensation of dyspnea by altering central respiratory drive. Oxygen should be given at the lowest effective dose and administered with humidified air to prevent desiccation of the respiratory tract. In 1993, Bruera et al51 showed that oxygen decreases dyspnea in hypoxic cancer patients.
In 1996, a double-blind crossover trial of 14 oncologic patients by Dudgeon et al42 indicated that oxygen helps decrease the sensation of dyspnea, respiratory rate, and breathing effort when compared to air. Improvement in carbon dioxide elimination with the use of breathing techniques such as diaphragmatic breathing and pursed lip breathing are also methods to decrease dyspnea sensation through alteration of the central respiratory drive. These breath-retraining techniques promote relaxed and gentle breathing, help minimize the work of breathing, and promote a sensation of well-being.44,52
Decreasing Ventilator Impedance
Ventilator impedance can result from bronchospasm, airway obstruction, effusions, or increased secretions. Treat airway obstruction from extrinsic compression or an endobronchial tumor with procedures such as balloon bronchoplasty, tracheobronchial stent placement, and brachytherapy. Pleural effusions can be treated using repeat thoracentesis or a thoracentesis with the placement of a chronic indwelling catheter; long-term control can be achieved using procedures such as pleurodesis and a thoracotomy with decortication. Manage secretions by using anticholinergic agents (eg, scopolamine) along with physical suctioning. Corticosteroids also help to reduce ventilator impedance by reducing tumor edema, lymphangitis, and bronchospasm.44 Unfortunately, these treatments are not without side effects and additional risks such as bleeding, infection, and pneumothorax.53
Improving Respiratory Muscle Strength
Maximum inspiratory pressure measures respiratory muscle strength. Normal values of negative pressure are usually greater than –50 cm H2O, and the average MIP found in cancer patients is only –16 cm H2O. Any level below –25 cm H2O is associated with severe respiratory muscle impairment. Maintaining adequate nutrition is important to help improve respiratory muscle strength.
Altering Central Perception
Limited trials suggest altering perception with the use of acupuncture and guided imagery may be of benefit. Morphine and other opiates also help to decrease oxygen requirements by altering central perception.49 Relaxation methods are most effective after a patient has gained the ability to alter and control sensation with breathing techniques.
Dyspnea is a chief complaint reported by the oncologic patients presenting to the ED for evaluation. Despite its prevalence in this patient population, diagnosis can be difficult since it is rarely the result of a single etiology. Consequently, dyspnea is often underdiagnosed and undertreated in the cancer population. Moreover, this condition is a composite of manifestations that are unique to each patient and his or her corresponding disease. These composite manifestations differ in timing and severity and require targeted interventions. Dyspnea that is primarily physiological in nature can be managed by reversing the causative mechanisms through treatments including oxygen therapy and antibiotics. Psychological components of dyspnea can be modified with interventions for anxiety and depression. Dyspnea is a complex composite of problems that deserves more attention in the literature and in practice.
Dr Wattana is an assistant professor in the department of emergency medicine, division of internal medicine at The University of Texas MD Anderson Cancer Center, Houston. Dr Miller is an associate professor in the department of emergency medicine, division of internal medicine at The University of Texas MD Anderson Cancer Center, Houston.
Overview
Dyspnea, the subjective inability to breathe comfortably, is a common symptom, accounting for 3 to 4 million ED visits annually.1,2 Dyspnea can be acute, subacute, or chronic, with chronic dyspnea defined as the presence of symptoms for more than 1 to 2 months, and subacute dyspnea as symptoms lasting for hours to days.3,4
The reported prevalence of dyspnea based on etiology presenting to the ED is as follows: malignancy (16% to 77%), chronic heart failure (18% to 88%), chronic obstructive pulmonary disease (56% to 98%), or renal disease (11% to 82%).5 Irrespective of time course and etiology, dyspnea can be a debilitating symptom affecting up to 50% of patients admitted to acute, tertiary care hospitals, and a quarter of patients seeking care in the ambulatory setting.6-13
Although dyspnea is common in patients with cancer, it is often underreported.14-19 This article reviews the current information available regarding the epidemiology, pathophysiology, assessment, and treatment of dyspnea in the oncologic patient population. By providing insight into the unique nature of dyspnea for oncologic patients, this article illustrates why dyspnea is an important symptom to recognize, address, and treat.
Epidemiology, Background, and Recognition
Dyspnea has a significant impact on the quality of life for oncologic patients and often intensifies near the time of death. At one regional oncology center, dyspnea was present in 49% of general cancer patients.20 The percentage and severity of dyspnea increases as a patient’s cancer progresses. The National Hospice Study found that dyspnea occurred in 70% of 1,754 terminally ill cancer patients studied during their last 6 weeks of life; more than 28% of these patients graded the distress that they felt from dyspnea as moderate to severe. The incidence of dyspnea was exceeded only by the incidences of pain and anorexia.21
A study conducted by Roberts et al22 showed that dyspnea is a very common, undertreated, and underrecognized condition. In 62% of patients reporting dyspnea, the symptoms had been present for greater than 3 months before patients received medical or nursing intervention. In the inpatient hospital setting, 77% of patients claimed on interview to experience dyspnea, but only 39% of cases were actually reported by nursing staff. In addition, Reddy et al23 found that dyspnea can negatively influence activities of daily living in oncology patients. Moreover, as the patients’ dyspnea intensified, their ability to perform activities of daily living was increasingly affected.23,24
Differential Diagnosis
Studies demonstrate the presence of dyspnea in approximately 20% to 40% of patients at the time of diagnosis of advanced disease, with the percentage increasing to around 70% in the last 6 weeks of life.25-27 Symptom grading is at least moderate in more than 28% of terminally ill cancer patients.21 Although common in oncology patients, dyspnea is difficult to diagnose because it is seldom explained by a single, specific organic etiology.24,25,28 Extrinsic modulators that are difficult to quantify, such as psychological state, cultural background, and life experiences, can also attenuate the perception of dyspnea.18,25
The cause of dyspnea in cancer patients can be broken down into four categories to aid in the differential diagnosis as described by Dudgeon et al29 in 2001 (Table 1). The four categories consist of dyspnea as being directly due to the malignancy itself; indirectly due to the malignancy; a side effect of cancer treatment; or due to underlying lung and cardiac disease unrelated to the malignancy itself.21,29
Etiology
Dyspnea Directly Due to Cancer. Dudgeon et al29 reported that the top three malignancies that directly caused dyspnea are lung, head and neck cancer, and malignancies of genitourinary origin.29 Reuben21 showed that 75% of patients with tumors involving the lung reported dyspnea at some point. Metastasis to the mediastinum, especially to the ribs, was associated with higher levels of dyspnea when compared to metastasis to lung.29
Dyspnea Indirectly Due to Cancer. Dyspnea due to indirect causes such as anemia, pneumonia, and pulmonary emboli may be easier to rectify once diagnosed.
Dyspnea Due to Cancer Treatment. Treatment-related causes of dyspnea include the side effects of surgery and lung irradiation. Patients with a history of pneumonectomy, lobectomy, or pleurodesis report higher levels of dyspnea as do patients with a history of thoracic irradiation. Radiation pneumonitis can occur 6 to 12 weeks following treatment, and radiation fibrosis can occur 6 to 12 months following treatment.29
Dyspnea Unrelated to Cancer. Other medical conditions that must be considered when diagnosing dyspnea include chronic obstructive pulmonary disease, asthma, and congestive heart failure. Psychological distress—described as anxiety and depression in the cancer population—may also cause the sensation of dyspnea.25
Dyspnea can be constant or occur as breakthrough episodes. In the study by Reddy et al,23 39% of patients had constant dyspnea and 20% experienced breakthrough episodes. Breakthrough dyspnea is shorter in duration, lasting less than 5 minutes. Breakthrough dyspnea occurs approximately 5 to 6 times a day and is predominant with 80% of patients who are symptom-free between episodes. As opiates are the drug of choice for dyspnea treatment, differentiating the time course of dyspnea is important in terms of treatment strategy and response. For example, treating breakthrough dyspnea with standard opiate medications might not work given that symptom onset and resolution might occur even before the medication’s onset of action.
Pathophysiology and Psychology
The cause of dyspnea in an oncology patient can be physiological and/or psychological in nature, with more numerous and unique presentations as compared to dyspnea associated with cardiac or lung disease. Chemoreceptor stimulation, mechanical stimuli originating in the lung and chest wall receptors, and neuroventilatory effects all contribute to dyspnea.12,16 Physiological modalities contributing to dyspnea are outlined in Table 2.
Psychological Contributions to the Etiology of Dyspnea
Dyspnea is subjective and biopsychosocial factors play a large role in an oncologic patient’s self-report of this condition. Multiple studies demonstrate that objective signs may not match the patient’s perception of dyspnea.18,21,25 Other studies report a correlation between psychological distress and worsened perception of dyspnea. Psychological distress is often measured by anxiety and depression and is also augmented by the presence of pain.
Assessment
Dyspnea is more challenging to assess in the ED than the laboratory setting36 as the condition is multidimensional in nature, often characterized by three factors consisting of breathing effort, chest tightness, and air hunger.37,38 Since these are subjective symptoms, assessment is difficult if a patient has delirium or other symptoms that alter the ability to provide a coherent response. Also, the subjective nature of dyspnea can lead to bias during symptom measurement and management.39
Research has documented factors such as lung involvement, anxiety, and maximum inspiratory pressure as influential to the perceived intensity of dyspnea for oncologic patients.40 Therefore, the sensation of dyspnea is multifactorial and includes physiological and psychological components for cancer patients.40 Subjective sensation does not always correlate with physiological measurements, adding to the difficulty of objective assessments.
Tanaka et al39 describe six criteria that must be met when attempting to create a scale to measure dyspnea in oncology patients. The Cancer Dyspnea Scale (CDS) is a self-rating scale that has been found to be acceptable and practicable in the clinical setting. The 12-item scale was originally conducted in Japanese and has been validated when translated into Swedish (CDS-S) and English (CDS-E) versions.39,41 Uronis et al41 further tested a reduced Cancer Dyspnea Scale that dropped three items from the original CDS scale (r-CDS-E). According to Uronis, the CDS-E better measures global dyspnea whereas the r-CDS-E can be used to measure effects of an intervention on dyspnea. One of the limitations of the CDS is that the scale was only validated in patients with lung cancer.
Since a universally useful scale has yet to be validated, the findings of a comprehensive history and thorough physical examination are the most important considerations when assessing oncologic patients for dyspnea.18,42 The history and physical examination must assess both physical factors and psychological factors. Physical factors include symptom quality and associated symptoms, provoking and relieving factors, previous treatments and response to treatments, and past medical history. Psychological factors include the patient’s emotional status at onset; as symptoms progress, the EP must assess whether perception of dyspnea is related to emotions such as anxiety and fear.
The longitudinal progression of symptoms must be considered. Currow et al43 found that at days 10 and 3 before death, dyspnea increased in oncologic patients but remained unchanged in patients with a noncancer diagnosis. Thus, the longitudinal progression of dyspnea differs depending on the underlying condition. Nearing death, patients without cancer experience a sustained period of symptoms whereas patients with cancer frequently experience both increased symptom prevalence and intensity.44
Diagnostic testing may also help to identify treatable causes. Chest imaging can be performed by radiography and computed tomography. Complete blood counts and chemistry panels can assess for anemia and electrolyte abnormalities. Maximal inspiratory pressure (MIP), a measure that tests diaphragm and inspiratory muscle strength, is also helpful if no apparent cause is found using other diagnostic modalities.1
Treatment
Treatment of dyspnea in malignancy is uniquely challenging. Ideally, treatment should focus on correcting an underlying cause, but for malignancy, the cause is often not reversible. Therefore, dyspnea treatment often consists of palliative management to control the sensation of symptom burden.45 Terminal cancer patients oftentimes require hospitalization and sedation to adequately manage their symptoms.45
The American Thoracic Society has grouped dyspnea therapy into the following four categories: reducing ventilator demand, decreasing ventilator impedance, improving respiratory muscle strength, or altering central perception.1
Reducing Ventilator Demand
Reducing ventilator demand can be accomplished by decreasing metabolic load or altering central respiratory drive. Decreasing metabolic load by strengthening respiratory muscles helps to get rid of lactic acid accumulation.46 Exercise also helps to prevent overall sensations of fatigue.47,48
Central respiratory drive can be altered in two ways, either with pharmacotherapy or supplemental oxygen. Pharmacotherapy aids focus on either oxygenation or the removal of carbon dioxide. Studies show that opioids, although only with small statistical significance, can be beneficial in treating dyspnea. Morphine is currently the mainstay of pharmacotherapy, but studies have yet to identify the best standard for dosing or optimal route for administration. In 1990, Bruera et al49 found that morphine improved dyspnea without negative impact on respiratory rate, oxygen saturation, or expiratory carbon dioxide.
Benzodiazepines help manage dyspnea by mitigating associated anxiety. Benzodiazepines have yet to be proven beneficial in a study with high statistical significance; however, reassurance and patient education that dyspnea is a common symptom can help allay anxiety. Patients living at home should have strong family support and reassurance that someone will be available to assist if an episode of dyspnea occurs.50
Supplemental oxygen can decrease the sensation of dyspnea by altering central respiratory drive. Oxygen should be given at the lowest effective dose and administered with humidified air to prevent desiccation of the respiratory tract. In 1993, Bruera et al51 showed that oxygen decreases dyspnea in hypoxic cancer patients.
In 1996, a double-blind crossover trial of 14 oncologic patients by Dudgeon et al42 indicated that oxygen helps decrease the sensation of dyspnea, respiratory rate, and breathing effort when compared to air. Improvement in carbon dioxide elimination with the use of breathing techniques such as diaphragmatic breathing and pursed lip breathing are also methods to decrease dyspnea sensation through alteration of the central respiratory drive. These breath-retraining techniques promote relaxed and gentle breathing, help minimize the work of breathing, and promote a sensation of well-being.44,52
Decreasing Ventilator Impedance
Ventilator impedance can result from bronchospasm, airway obstruction, effusions, or increased secretions. Treat airway obstruction from extrinsic compression or an endobronchial tumor with procedures such as balloon bronchoplasty, tracheobronchial stent placement, and brachytherapy. Pleural effusions can be treated using repeat thoracentesis or a thoracentesis with the placement of a chronic indwelling catheter; long-term control can be achieved using procedures such as pleurodesis and a thoracotomy with decortication. Manage secretions by using anticholinergic agents (eg, scopolamine) along with physical suctioning. Corticosteroids also help to reduce ventilator impedance by reducing tumor edema, lymphangitis, and bronchospasm.44 Unfortunately, these treatments are not without side effects and additional risks such as bleeding, infection, and pneumothorax.53
Improving Respiratory Muscle Strength
Maximum inspiratory pressure measures respiratory muscle strength. Normal values of negative pressure are usually greater than –50 cm H2O, and the average MIP found in cancer patients is only –16 cm H2O. Any level below –25 cm H2O is associated with severe respiratory muscle impairment. Maintaining adequate nutrition is important to help improve respiratory muscle strength.
Altering Central Perception
Limited trials suggest altering perception with the use of acupuncture and guided imagery may be of benefit. Morphine and other opiates also help to decrease oxygen requirements by altering central perception.49 Relaxation methods are most effective after a patient has gained the ability to alter and control sensation with breathing techniques.
Dyspnea is a chief complaint reported by the oncologic patients presenting to the ED for evaluation. Despite its prevalence in this patient population, diagnosis can be difficult since it is rarely the result of a single etiology. Consequently, dyspnea is often underdiagnosed and undertreated in the cancer population. Moreover, this condition is a composite of manifestations that are unique to each patient and his or her corresponding disease. These composite manifestations differ in timing and severity and require targeted interventions. Dyspnea that is primarily physiological in nature can be managed by reversing the causative mechanisms through treatments including oxygen therapy and antibiotics. Psychological components of dyspnea can be modified with interventions for anxiety and depression. Dyspnea is a complex composite of problems that deserves more attention in the literature and in practice.
Dr Wattana is an assistant professor in the department of emergency medicine, division of internal medicine at The University of Texas MD Anderson Cancer Center, Houston. Dr Miller is an associate professor in the department of emergency medicine, division of internal medicine at The University of Texas MD Anderson Cancer Center, Houston.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data. 2007;(386):1-32.
- Ekström MP, Abernethy AP, Currow DC. The management of chronic breathlessness in patients with advanced and terminal illness. BMJ. 2015;349:g7617.
- Karnani NG, Reisfield GM, Wilson GR. Evaluation of chronic dyspnea. Am Fam Physician. 2005;71(8):1529-1537.
- Moens K, Higginson IJ, Harding R; EURO IMPACT. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage. 2014;48(4):660-677.
- Peters SP. When the chief complaint is (or should be) dyspnea in adults. J Allergy Clin Immunol Pract. 2013;1(2):129-136.
- Campbell ML. Dyspnea prevalence, trajectories, and measurement in critical care and at life’s end. Curr Opin Support Palliat Care. 2012;6(2):168-171.
- Argulian E, Agarwal V, Bangalore S, et al. Meta-analysis of prognostic implications of dyspnea versus chest pain in patients referred for stress testing. Am J Cardiol. 2014;113(3):559-564.
- van Mourik Y, Rutten FH, Moons KG, Bertens LC, Hoes AW, Reitsma JB. Prevalence and underlying causes of dyspnoea in older people: a systematic review. Age Ageing. 2014;43(3):319-326.
- Scano G, Gigliotti F, Stendardi L, Gagliardi E. Dyspnea and emotional states in health and disease. Respir Med. 2013;107(5):649-655.
- Murakami J, Ueda K, Sano F, Hayashi M, Tanaka N, Hamano K. Prediction of postoperative dyspnea and chronic respiratory failure. J Surg Res. 2015;195(1):303-310.
- Manning HL, Mahler DA. Pathophysiology of dyspnea. Monaldi Arch Chest Dis. 2001;56(4):325-330.
- Robinson LM. A Healthy 22-year-old woman with new onset dyspnea. J Emerg Nurs. 2010;36(2):146-147.
- Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncol. 2012;51(8):996-1008.
- Guirimand F, Sahut d’izarn M, Laporte L, Francillard M, Richard JF, Aegerter P. Sequential occurrence of dyspnea at the end of life in palliative care, according to the underlying cancer. Cancer Med. 2015;4(4):532-539.
- Fishbein D, Kearon C, Killian KJ. An approach to dyspnea in cancer patients. J Pain Symptom Manage. 1989;4(2):76-81.
- Ost DE, Ernst A, Grosu HB, et al; AQuIRE Brochoscopy Registry. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest. 2015;147(5):1282-1298.
- Ripamonti C, Bruera E. Dyspnea: pathophysiology and assessment. J Pain Symptom Manage. 1997;13(4):220-232.
- Petersen S, Ritz T. The role of fearful beliefs in the relationship between situational self-awareness and report of breathing-related sensations. Br J Health Psychol. 2011;16(Pt 2):359-372.
- Dudgeon DJ, Kristjanson L, Sloan JA, Lertzman M, Clement K. Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage. 2001;21(2):95-102.
- Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest. 1986;89(2):234-236.
- Roberts DK, Thorne SE, Pearson C. The experience of dyspnea in late-stage cancer. Patients’ and nurses’ perspectives. Cancer Nurs. 1993;16(4):310-320.
- Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12(1):29-36.
- Hayen A, Herigstad M, Pattinson KT. Understanding dyspnea as a complex individual experience. Maturitas. 2013;76(1):45-50.
- Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2000;19(5):357-362.
- Ripamonti C. Management of dyspnea in advanced cancer patients. Support Care Cancer. 1999;7(4):
- 233-243.
- Hui D, Morgado M, Vidal M, et al. Dyspnea in hospitalized advanced cancer patients: subjective and physiologic correlates. J Palliat Med. 2013;16(3):
- 274-280.
- Ripamonti C, Fulfaro F, Bruera E. Dyspnoea in patients with advanced cancer: incidence, causes and treatments. Cancer Treat Rev. 1998;24(1):69-80.
- Dudgeon DJ, Lertzman M, Askew GR. Physiological changes and clinical correlations of dyspnea in cancer outpatients. J Pain Symptom Manage. 2001;21(5):373-379.
- Swinburn CR, Wakefield JM, Jones PW. Relationship between ventilation and breathlessness during exercise in chronic obstructive airways disease is not altered by prevention of hypoxaemia. Clin Sci (Lond). 1984;67(5):515-519.
- Rock LK, Schwartzstein RM. Mechanisms of dyspnea in chronic lung disease. Curr Opin Support Palliat Care. 2007;1(2):102-108.
- Moy ML, Woodrow Weiss J, Sparrow D, Israel E, Schwartzstein RM. Quality of dyspnea in bronchoconstriction differs from external resistive loads. Am J Respir Crit Care Med. 2000;162(2 Pt 1):451-455.
- Nakayama H, Shibuya M, Yamada M, Suzuki H, Arakawa M, Homma I. In-phase chest wall vibration decreases dyspnea during arm elevation in chronic obstructive pulmonary disease patients. Intern Med. 1998;37(10):831-835.
- Killian KJ, Jones NL. Mechanisms of exertional dyspnea. Clin Chest Med. 1994;15(2):247-257
- Gross NJ. Chronic obstructive pulmonary disease. Current concepts and therapeutic approaches. Chest. 1990;97(2 Suppl):19S-23S.
- Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009;167(1):53-60.
- Petersen S, Orth B, Ritz T. Awareness of breathing: the structure of language descriptors of respiratory sensations. Health Psychol. 2008;27(1):122-127.
- Parshall MB, Meek PM, Sklar D, Alcock J, Bittner P. Test-retest reliability of multidimensional dyspnea profile recall ratings in the emergency department: a prospective, longitudinal study. BMC Emerg Med. 2012;12:6.
- Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: a multidimensional, brief, self-rating scale. Br J Cancer. 2000;82(4):800-805.
- Booth S. The management of dyspnoea in advanced cancer. Hosp Med. 1998;59(5):348-349.
- Uronis HE, Shelby RA, Currow DC, et al. Assessment of the psychometric properties of an English version of the cancer dyspnea scale in people with advanced lung cancer. J Pain Symptom Manage. 2012;44(5):741-749.
- Dudgeon DJ, Rosenthal S. Management of dyspnea and cough in patients with cancer. Hematol Oncol Clin North Am. 1996;10(1):157-171.
- Currow DC, Smith J, Davidson PM, Newton PJ, Agar MR, Abernethy AP. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage. 2010;39(4):680.
- Acheson A, MacCormack D. Dyspnea and the cancer patient--an overview. Can Oncol Nurs J. 1997;7(4):209-213.
- Dudgeon DJ, Lertzman M. Dyspnea in the advanced cancer patient. J Pain Symptom Manage. 1998;16(4):212-219.
- Corner J, Plant H, A’Hern R, Bailey C. Non-pharmacological intervention for breathlessness in lung cancer. Palliat Med. 1996;10(4):299-305.
- LeGrand SB, Walsh D. Palliative management of dyspnea in advanced cancer. Curr Opin Oncol. 1999;11(4):250-254.
- LeGrand SB. Dyspnea: the continuing challenge of palliative management. Curr Opin Oncol. 2002;14(4):394-398.
- Bruera E, Macmillan K, Pither J, MacDonald RN. Effects of morphine on the dyspnea of terminal cancer patients. J Pain Symptom Manage. 1990;5(6):341-344.
- Held JL. Cancer care. Managing shortness of breath. Nursing. 1994;24(7):31.
- Bruera E, de Stoutz N, Velasco-Leiva A, Schoeller T, Hanson J. Effects of oxygen on dyspnoea in hypoxaemic terminal-cancer patients. Lancet. 1993;342(8862):13-14.
- Bailey C. Nursing as therapy in the management of breathlessness in lung cancer. Eur. J. Cancer Care (Engl.). 1995;4(4):184-190.
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage. 2012;(2):301-306.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data. 2007;(386):1-32.
- Ekström MP, Abernethy AP, Currow DC. The management of chronic breathlessness in patients with advanced and terminal illness. BMJ. 2015;349:g7617.
- Karnani NG, Reisfield GM, Wilson GR. Evaluation of chronic dyspnea. Am Fam Physician. 2005;71(8):1529-1537.
- Moens K, Higginson IJ, Harding R; EURO IMPACT. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage. 2014;48(4):660-677.
- Peters SP. When the chief complaint is (or should be) dyspnea in adults. J Allergy Clin Immunol Pract. 2013;1(2):129-136.
- Campbell ML. Dyspnea prevalence, trajectories, and measurement in critical care and at life’s end. Curr Opin Support Palliat Care. 2012;6(2):168-171.
- Argulian E, Agarwal V, Bangalore S, et al. Meta-analysis of prognostic implications of dyspnea versus chest pain in patients referred for stress testing. Am J Cardiol. 2014;113(3):559-564.
- van Mourik Y, Rutten FH, Moons KG, Bertens LC, Hoes AW, Reitsma JB. Prevalence and underlying causes of dyspnoea in older people: a systematic review. Age Ageing. 2014;43(3):319-326.
- Scano G, Gigliotti F, Stendardi L, Gagliardi E. Dyspnea and emotional states in health and disease. Respir Med. 2013;107(5):649-655.
- Murakami J, Ueda K, Sano F, Hayashi M, Tanaka N, Hamano K. Prediction of postoperative dyspnea and chronic respiratory failure. J Surg Res. 2015;195(1):303-310.
- Manning HL, Mahler DA. Pathophysiology of dyspnea. Monaldi Arch Chest Dis. 2001;56(4):325-330.
- Robinson LM. A Healthy 22-year-old woman with new onset dyspnea. J Emerg Nurs. 2010;36(2):146-147.
- Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncol. 2012;51(8):996-1008.
- Guirimand F, Sahut d’izarn M, Laporte L, Francillard M, Richard JF, Aegerter P. Sequential occurrence of dyspnea at the end of life in palliative care, according to the underlying cancer. Cancer Med. 2015;4(4):532-539.
- Fishbein D, Kearon C, Killian KJ. An approach to dyspnea in cancer patients. J Pain Symptom Manage. 1989;4(2):76-81.
- Ost DE, Ernst A, Grosu HB, et al; AQuIRE Brochoscopy Registry. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest. 2015;147(5):1282-1298.
- Ripamonti C, Bruera E. Dyspnea: pathophysiology and assessment. J Pain Symptom Manage. 1997;13(4):220-232.
- Petersen S, Ritz T. The role of fearful beliefs in the relationship between situational self-awareness and report of breathing-related sensations. Br J Health Psychol. 2011;16(Pt 2):359-372.
- Dudgeon DJ, Kristjanson L, Sloan JA, Lertzman M, Clement K. Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage. 2001;21(2):95-102.
- Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest. 1986;89(2):234-236.
- Roberts DK, Thorne SE, Pearson C. The experience of dyspnea in late-stage cancer. Patients’ and nurses’ perspectives. Cancer Nurs. 1993;16(4):310-320.
- Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12(1):29-36.
- Hayen A, Herigstad M, Pattinson KT. Understanding dyspnea as a complex individual experience. Maturitas. 2013;76(1):45-50.
- Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2000;19(5):357-362.
- Ripamonti C. Management of dyspnea in advanced cancer patients. Support Care Cancer. 1999;7(4):
- 233-243.
- Hui D, Morgado M, Vidal M, et al. Dyspnea in hospitalized advanced cancer patients: subjective and physiologic correlates. J Palliat Med. 2013;16(3):
- 274-280.
- Ripamonti C, Fulfaro F, Bruera E. Dyspnoea in patients with advanced cancer: incidence, causes and treatments. Cancer Treat Rev. 1998;24(1):69-80.
- Dudgeon DJ, Lertzman M, Askew GR. Physiological changes and clinical correlations of dyspnea in cancer outpatients. J Pain Symptom Manage. 2001;21(5):373-379.
- Swinburn CR, Wakefield JM, Jones PW. Relationship between ventilation and breathlessness during exercise in chronic obstructive airways disease is not altered by prevention of hypoxaemia. Clin Sci (Lond). 1984;67(5):515-519.
- Rock LK, Schwartzstein RM. Mechanisms of dyspnea in chronic lung disease. Curr Opin Support Palliat Care. 2007;1(2):102-108.
- Moy ML, Woodrow Weiss J, Sparrow D, Israel E, Schwartzstein RM. Quality of dyspnea in bronchoconstriction differs from external resistive loads. Am J Respir Crit Care Med. 2000;162(2 Pt 1):451-455.
- Nakayama H, Shibuya M, Yamada M, Suzuki H, Arakawa M, Homma I. In-phase chest wall vibration decreases dyspnea during arm elevation in chronic obstructive pulmonary disease patients. Intern Med. 1998;37(10):831-835.
- Killian KJ, Jones NL. Mechanisms of exertional dyspnea. Clin Chest Med. 1994;15(2):247-257
- Gross NJ. Chronic obstructive pulmonary disease. Current concepts and therapeutic approaches. Chest. 1990;97(2 Suppl):19S-23S.
- Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009;167(1):53-60.
- Petersen S, Orth B, Ritz T. Awareness of breathing: the structure of language descriptors of respiratory sensations. Health Psychol. 2008;27(1):122-127.
- Parshall MB, Meek PM, Sklar D, Alcock J, Bittner P. Test-retest reliability of multidimensional dyspnea profile recall ratings in the emergency department: a prospective, longitudinal study. BMC Emerg Med. 2012;12:6.
- Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: a multidimensional, brief, self-rating scale. Br J Cancer. 2000;82(4):800-805.
- Booth S. The management of dyspnoea in advanced cancer. Hosp Med. 1998;59(5):348-349.
- Uronis HE, Shelby RA, Currow DC, et al. Assessment of the psychometric properties of an English version of the cancer dyspnea scale in people with advanced lung cancer. J Pain Symptom Manage. 2012;44(5):741-749.
- Dudgeon DJ, Rosenthal S. Management of dyspnea and cough in patients with cancer. Hematol Oncol Clin North Am. 1996;10(1):157-171.
- Currow DC, Smith J, Davidson PM, Newton PJ, Agar MR, Abernethy AP. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage. 2010;39(4):680.
- Acheson A, MacCormack D. Dyspnea and the cancer patient--an overview. Can Oncol Nurs J. 1997;7(4):209-213.
- Dudgeon DJ, Lertzman M. Dyspnea in the advanced cancer patient. J Pain Symptom Manage. 1998;16(4):212-219.
- Corner J, Plant H, A’Hern R, Bailey C. Non-pharmacological intervention for breathlessness in lung cancer. Palliat Med. 1996;10(4):299-305.
- LeGrand SB, Walsh D. Palliative management of dyspnea in advanced cancer. Curr Opin Oncol. 1999;11(4):250-254.
- LeGrand SB. Dyspnea: the continuing challenge of palliative management. Curr Opin Oncol. 2002;14(4):394-398.
- Bruera E, Macmillan K, Pither J, MacDonald RN. Effects of morphine on the dyspnea of terminal cancer patients. J Pain Symptom Manage. 1990;5(6):341-344.
- Held JL. Cancer care. Managing shortness of breath. Nursing. 1994;24(7):31.
- Bruera E, de Stoutz N, Velasco-Leiva A, Schoeller T, Hanson J. Effects of oxygen on dyspnoea in hypoxaemic terminal-cancer patients. Lancet. 1993;342(8862):13-14.
- Bailey C. Nursing as therapy in the management of breathlessness in lung cancer. Eur. J. Cancer Care (Engl.). 1995;4(4):184-190.
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage. 2012;(2):301-306.
Case Report: A Bittersweet Death
Case
A 32-year-old Hispanic man presented to the ED with complications associated with diabetes mellitus (DM), the symptoms of which started approximately 3 days prior to arrival. The patient reported feelings of fatigue, dry mouth, increased thirst, and frequent urination. He denied sweating, nausea, chest pain, shortness of breath, diarrhea, or blood in his urine; he also denied blurry vision or dizziness.
During history intake, the patient informed the emergency physician (EP) that he had been diagnosed with DM and hyperglycemia earlier that day by his primary care physician, who had immediately referred the patient to the ED for urgent management. The patient’s own medical history was noncontributory; however, his father’s history was notable for DM and chronic renal failure. The patient further stated that he was not on any medications. Regarding his social history, he denied cigarette smoking and noted only occasional alcohol consumption.
The patient’s vital signs on presentation were: blood pressure (BP), 116/74 mm Hg; heart rate, 113 beats/minute; respiratory rate, 26 breaths/minute; and temperature, 97.8°F. Oxygen saturation was 97% on room air. On physical examination, the patient was severely anxious, with tachycardia and respiratory distress. He was obese, with a body mass index of 30.9 kg/m2 (height, 5 feet, 4 inches; weight, 180 lb).
The patient was started on an intravenous (IV) bolus of 0.9% normal saline (2 L at 20 mL/kg). After a consultation with endocrinology, he was then given a maintenance dose of normal saline IV at 250 cc/h and an IV insulin drip at 0.1 U/kg/h following a bolus of 8 units of insulin IV. His glucose levels were carefully monitored via hourly finger-stick glucose testing.
Although the patient’s condition stabilized, he collapsed while walking to the bathroom. He had agonal respirations and no pulse. Resuscitation efforts were started with bag-valve-mask ventilation, along with emergent advanced cardiac life support (ACLS) treatment, the protocol of which included epinephrine administration (x2) IV push 5 minutes apart, 2 ampules of sodium bicarbonate (50 mEq each) IV push, and calcium gluconate 10% (x1) 10 mL (1 g) IV push. A pulse was re-established, and the patient was intubated.
The patient was diagnosed with diabetic ketoacidosis (DKA) and admitted to the intensive care unit where repeat laboratory evaluation was ordered. Additional pharmacological management included IV administration of dopamine, norepinephrine, phenylephrine, vasopressin, antibiotics (azithromycin, meropenem, and vancomycin), pantoprazole, and subcutaneous heparin.
During treatment, the patient coded a second time and was revived according to ACLS protocols. Shortly thereafter, he coded a third time, but resuscitation efforts failed. Pathology reported no biological cause of death, and the coroner closed the case as death due to DM-related complications.
Diabetic Ketoacidosis
Diabetic ketoacidosis is a major complication of DM.4 Although the condition usually occurs in type 1 DM, it can also develop in type 2 DM. Diabetic ketoacidosis may be an inciting event leading to the eventual diagnosis of DM, but can also develop during a concurrent illness such as a urinary tract infection or an eating disorder.5 Risk factors for DKA include patients with type 1 or type 2 DM, a family history of DM, obesity, and nonwhite patients whose ethnic background places them at increased risk.6 Hispanic, black, and African American patients are at a greater risk of developing DKA and are more likely to develop “ketosis-prone” type 2 DM.7
Patients who do not fit into the definitive categories of type 1 or 2 DM can be classified under ketosis-prone DM.7,8 Diabetic ketoacidosis acts as the inciting event for the disease and evolves into severe β-cell dysfunction, hence blurring the lines between the archetypal DM categories. Fifty percent of ketosis-prone DM patients are A-β+ (absent autoantibodies, present β-cell function), which indicates that the dysfunction can be partially reversed. Reversal of the condition is largely based on long-term β-cell reserves, which are dependent on tight glycemic control and insulin dependence. Higher incidences of the A-β+ variant of ketosis-prone diabetes are seen in the male population and are often unprovoked.9-11
Diabetic ketoacidosis is the result of either a decrease or absence of insulin in the body (Table 2).4 Without insulin modulating exogenous glucose intake and endogenous glucose production (via glucagon, glycogenolysis, and gluconeogenesis), high levels of glucose are found in the circulation, leading to prominent hyperglycemia (>250 mg/dL or >13.8 mmol/L).6 This environment causes the body to switch from carbohydrate metabolism to fatty acid metabolism. As a result, acidic ketone bodies such β-hydroxybutyrate and acetoacetate are produced. These physiological changes in the body cause the signs and symptoms typically found in DKA.
Signs and Symptoms
Over a period of 24 hours, symptoms such as nausea, vomiting, increased thirst, and polyuria develop due to dehydration caused by osmotic diuresis and glucosuria.5 Patients may also present with hypotension and tachycardia. Confusion, deep gasping breaths or Kussmaul respirations, and metabolic acidosis result from hyperventilation and failure to compensate for the increased serum concentration of ketone bodies. Ketone production leads to a fruit-like odor in the patient’s breath and ketonuria in the urinalysis. In DKA, laboratory values will indicate metabolic acidosis and abnormal serum electrolytes. In both DM and DKA, increased urea and creatinine due to dehydration, increased ketones, and the presence of diabetic nephropathy are useful indicators of impaired kidney function.12
Management and Treatment
Diabetic ketoacidosis can be managed and reversed, especially when recognized and treated early.6,13 Dehydration in DKA can be corrected with IV fluid replacement. Normal saline (0.9%) can be started at 15 to 20 mL/kg/h or 1 L/h. As the patient’s vital signs stabilize, IV fluids can be titrated to a lower dose of 250 to 500 mL/h. Monitoring BP and electrolytes are key at this point as alterations in sodium levels and glucose levels may require switching to half-normal saline and/or dextrose.
The hyperglycemic state of patients with DKA is managed by IV insulin. An initial bolus of 0.1 U/kg/h can be given, but should only be administered when potassium levels are greater than 3.3 mmol/L.14 If adequate perfusion can be maintained, then 0.14 U/kg/h can be used instead of a bolus. Glucose levels must be monitored; once the levels decrease to approximately 200 mg/dL, the infusion rate of insulin should be titrated down to 0.05 to 0.1 U/kg/h. Dextrose is then added to maintain glucose levels at approximately 150 to 200 mg/dL.
Electrolytes, especially potassium, must be monitored closely in patients with DKA. Insulin leads to the shift of potassium into cells. The lack of insulin keeps potassium in the extracellular space. Due to osmotic diuresis, potassium is lost in the urine, leading to hypokalemia. Potassium levels in patients with DKA should be maintained at a level between 4 to 5 mmol/L. Patients with potassium levels between 3.3 to 5.2 mmol/L can be started on IV potassium between 20 to 30 mmol/h. If the patient is severely hypokalemic (<3.3 mmol/L), insulin should be withheld, and only IV potassium should be given at a rate of 20 to 30 mmol/h.
Bicarbonate levels can also be managed as acidosis can lead to both neurological and cardiac complications. If the patient’s pH is less than 6.9, the American Diabetes Association recommends starting 100 mmol of sodium bicarbonate in 400 mL sterile water (in addition to potassium chloride at 200 mL/h) for 2 hours. Dosing should be repeated every 2 hours until the patient’s pH is greater than 6.9.
In uncomplicated cases of DKA, the condition is resolved when a patient’s pH is greater than 7.3; glucose level is less than 200 mg/dL; and bicarbonate level is greater than or equal to 18 mmol/L. After patients become hemodynamically stable, they can be discharged and managed at home with a combination of intermediate- or long-acting insulin as well as short- or rapid-acting insulin.
Complications and Mortality
Diabetic ketoacidosis can cause sudden and fluctuating changes in the body. Therefore, it is very important to monitor a patient’s laboratory values very carefully and frequently to avoid any pitfalls. Since patients can present with hyponatremia due to the osmotic draw of glucose in the blood,13 sodium levels may have to be corrected. The corrected serum sodium can be calculated by adding 1.6 mmol/L for every 100 mg/dL of glucose (when finger-stick readings are above 200 mg/dL).15 Patients with DKA can also present with leukocytosis (even in the absence of infection) and hypertriglyceridemia (due to impaired lipoprotein lipase).15 Serum creatine may be elevated due to blood acetoacetate levels.15
Interestingly, there are other acute conditions that can mimic DKA.15 For example, chronic ethanol abuse can lead to ketoacidosis. Unlike DKA, however, alcoholic ketoacidosis does not have profound hyperglycemia, which can help differentiate the two during initial assessment.
Complications due to DKA can arise comprising the patient’s health, including hypoglycemia, hypokalemia, rhabdomyolysis, acute renal failure, pulmonary edema, and shock.16 Cerebral edema is seen in up to 1% of DKA patients,15 the cause of which may be due to the severity of the acidosis, high glucose levels, and rapid hydration. Even when cerebral edema is reduced, patients are often neurologically impaired. Mortality rates from DKA deaths due to cerebral edema can be as high as 24%.13 In the United States, over 100,000 patients with DM per year are admitted to the hospital for DKA, and 9% of patients with DM suffer from DKA-related complications postdischarge.15 With current treatment protocols, mortality rates for DKA-associated deaths are now down to 1%.6,15
Diabetes ketoacidosis-related deaths are usually the result of the following: a triad of DKA symptoms (hyperglycemia, hyperketonemia, and metabolic acidosis), another underlying comorbid condition (eg, myocardial infarction, sepsis, acute respiratory distress syndrome), or the release of biological markers (ie, catecholamines).14,15,17 Thus, as previously stated, the management of potassium levels is important as both hyperkalemia and hypokalemia can lead to fatal arrthythmias.15
Direct mortality from DKA has dropped significantly over the past 20 years, from 8% to less than 1%.6 The US Centers for Disease Control and Prevention has observed a downward trend in death and estimates that 2,417 patients died in 2009 due to DKA,18 and recent postmortem studies have revealed new insights into DKA-related deaths.19 Blood and vitreous acetone concentrations are strong indicators for predeath hyperglycemia and ketosis (if there are no underlying comorbid and/or pharmacological provocations). Blood acetone levels greater than 0.01 g/dL antemortem are suggestive of DKA. It is recommended that these tests should be performed in sudden deaths which have no biological or anatomical cause of death. Postmortem diagnosis of DKA is made with the following criteria: history of DM, increased vitreous glucose concentrations, and elevated blood/vitreous/urine acetone concentrations (>200 mg/dL). If results of the abovementioned parameters are inconclusive, measurement of lactic acid postmortem is thought to further support a diagnosis of DKA.19
Patient Counseling and Education
Approximately 33% of patients whose death was associated with DKA had no personal history of DM.19 This statistic emphasizes the importance of taking a thorough history, physical examination, blood glucose evaluation, and educating patients about the signs and symptoms of DM and DKA.
Patient counseling and education are important, especially in patients whose racial/ethnic background places them at increased risk of developing DM (eg, patients of black or African American, American Indian, Alaskan Native, Asian American, Hispanic, Native Hawaiian, or Pacific Islander descent).20,21 Strategies for preventive management include advocating regular glucose monitoring as well as dietary and lifestyle modifications. In patients with DM, successful management of the condition and its comorbidities can help prevent DKA and associated mortality.
Conclusion
As this case demonstrates, despite prompt diagnosis and management, patients with DKA—especially those with uncontrolled, undiagnosed, or advanced DM—are associated with fatal outcomes. In many cases, however, DKA can be successfully managed and reversed, especially when the condition is recognized early. Management includes not only IV therapy to adjust fluid and insulin levels, but also restoring electrolyte balance (especially potassium and bicarbonate). Frequent and careful evaluation of laboratory values is vital to the successful treatment of DKA, as there are numerous pitfalls and complications that the emergency physician can encounter. Patients who either have or are at an increased risk of developing DM or DKA may benefit from preventive measures, including regular glucose monitoring and appropriate diet and lifestyle modifications.
Mr Hassan-Ali is a fourth-year medical student at Windsor University School of Medicine, St Kitts, West Indies. Dr Raziuddin is an internist and an emergency medicine physician at Weiss Memorial, Thorek Memorial, and Westlake Hospitals, Chicago, Illinois.
- Kitabchi AH, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153.
- Farinda A. Lab values, normal adult: laboratory reference ranges in healthy adults. 2015. Medscape Web site. http://emedicine.medscape.com/article/2172316-overview. Updated May 14, 2014. Accessed August 14, 2015.
- Young D. Implementation of SI units for clinical laboratory data. Ann Intern Med. 1987;106(1):114-129.
- Maitra A. The endocrine system. In: Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed. New York, NY: Elsevier Saunders; 2015:1105-1120.
- Powers AC. Diabetes mellitus: management and therapies. In: Kasper DL, Fauci AS, Longo DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY; McGraw-Hill Medical Publishing Division; 2015:2407-2422.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343.
- Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350-357.
- Umpierrez G, Smiley D, Gosmanov A, Thomason D. Ketosis-prone type 2 diabetes: effect of hyperglycemia on beta-cell function and skeletal muscle insulin signaling. Endocr Pract. 2007;13(3):283-290.
- Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645-653.
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes. 1995;44(7):790-795.
- Piñero-Piloña A, Raskin P. Idiopathic type 1 diabetes. J Diabetes Complications. 2001;15(6):328-335.
- Kemperman FA, Weber JA, Gorgels J, van Zanten AP, Krediet RT, Arisz L. The influence of ketoacids on plasma creatinine assays in diabetic ketoacidosis. J Intern Med. 2000;248(6):511-517.
- Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician. 2013;87(5):337-346.
- Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physician. 2005;71(9):1705-1714.
- Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar ayndrome. Diabetes Spectrum. 2002;15(1):28-36.
- Wolfsdorf J, Glaser N, Sperling MA; American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150-1159.
- Rosenbloom AL. Sudden death of a young woman attributed to diabetic ketoacidosis. J Forensic Leg Med. 2013;20(8):1063-1065.
- Centers for Disease Control and Prevention. Number of deaths for hyperglycemic crises as underlying cause, United States, 1980-2009. http://www.cdc.gov/diabetes/statistics/mortalitydka/fnumberofdka.htm. Updated November 19, 2013. Accessed August 14, 2015.
- Ali Z, Levine B, Ripple M, Fowler DR. Diabetic ketoacidosis: a silent death. Am J Forensic Med Pathol. 2012;33(3):189-193.
- US Department of Health and Human Services Office of Minority Health. Diabetes and Hispanic Americans. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=63. Updated June 15, 2013. Accessed August 14, 2015.
- US Department of Health and Human Services Office of Minority Health. Profile: Native Hawaiian/Other Pacific Islanders. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=65. Updated January 15, 2015. Accessed August 14, 2015.
Case
A 32-year-old Hispanic man presented to the ED with complications associated with diabetes mellitus (DM), the symptoms of which started approximately 3 days prior to arrival. The patient reported feelings of fatigue, dry mouth, increased thirst, and frequent urination. He denied sweating, nausea, chest pain, shortness of breath, diarrhea, or blood in his urine; he also denied blurry vision or dizziness.
During history intake, the patient informed the emergency physician (EP) that he had been diagnosed with DM and hyperglycemia earlier that day by his primary care physician, who had immediately referred the patient to the ED for urgent management. The patient’s own medical history was noncontributory; however, his father’s history was notable for DM and chronic renal failure. The patient further stated that he was not on any medications. Regarding his social history, he denied cigarette smoking and noted only occasional alcohol consumption.
The patient’s vital signs on presentation were: blood pressure (BP), 116/74 mm Hg; heart rate, 113 beats/minute; respiratory rate, 26 breaths/minute; and temperature, 97.8°F. Oxygen saturation was 97% on room air. On physical examination, the patient was severely anxious, with tachycardia and respiratory distress. He was obese, with a body mass index of 30.9 kg/m2 (height, 5 feet, 4 inches; weight, 180 lb).
The patient was started on an intravenous (IV) bolus of 0.9% normal saline (2 L at 20 mL/kg). After a consultation with endocrinology, he was then given a maintenance dose of normal saline IV at 250 cc/h and an IV insulin drip at 0.1 U/kg/h following a bolus of 8 units of insulin IV. His glucose levels were carefully monitored via hourly finger-stick glucose testing.
Although the patient’s condition stabilized, he collapsed while walking to the bathroom. He had agonal respirations and no pulse. Resuscitation efforts were started with bag-valve-mask ventilation, along with emergent advanced cardiac life support (ACLS) treatment, the protocol of which included epinephrine administration (x2) IV push 5 minutes apart, 2 ampules of sodium bicarbonate (50 mEq each) IV push, and calcium gluconate 10% (x1) 10 mL (1 g) IV push. A pulse was re-established, and the patient was intubated.
The patient was diagnosed with diabetic ketoacidosis (DKA) and admitted to the intensive care unit where repeat laboratory evaluation was ordered. Additional pharmacological management included IV administration of dopamine, norepinephrine, phenylephrine, vasopressin, antibiotics (azithromycin, meropenem, and vancomycin), pantoprazole, and subcutaneous heparin.
During treatment, the patient coded a second time and was revived according to ACLS protocols. Shortly thereafter, he coded a third time, but resuscitation efforts failed. Pathology reported no biological cause of death, and the coroner closed the case as death due to DM-related complications.
Diabetic Ketoacidosis
Diabetic ketoacidosis is a major complication of DM.4 Although the condition usually occurs in type 1 DM, it can also develop in type 2 DM. Diabetic ketoacidosis may be an inciting event leading to the eventual diagnosis of DM, but can also develop during a concurrent illness such as a urinary tract infection or an eating disorder.5 Risk factors for DKA include patients with type 1 or type 2 DM, a family history of DM, obesity, and nonwhite patients whose ethnic background places them at increased risk.6 Hispanic, black, and African American patients are at a greater risk of developing DKA and are more likely to develop “ketosis-prone” type 2 DM.7
Patients who do not fit into the definitive categories of type 1 or 2 DM can be classified under ketosis-prone DM.7,8 Diabetic ketoacidosis acts as the inciting event for the disease and evolves into severe β-cell dysfunction, hence blurring the lines between the archetypal DM categories. Fifty percent of ketosis-prone DM patients are A-β+ (absent autoantibodies, present β-cell function), which indicates that the dysfunction can be partially reversed. Reversal of the condition is largely based on long-term β-cell reserves, which are dependent on tight glycemic control and insulin dependence. Higher incidences of the A-β+ variant of ketosis-prone diabetes are seen in the male population and are often unprovoked.9-11
Diabetic ketoacidosis is the result of either a decrease or absence of insulin in the body (Table 2).4 Without insulin modulating exogenous glucose intake and endogenous glucose production (via glucagon, glycogenolysis, and gluconeogenesis), high levels of glucose are found in the circulation, leading to prominent hyperglycemia (>250 mg/dL or >13.8 mmol/L).6 This environment causes the body to switch from carbohydrate metabolism to fatty acid metabolism. As a result, acidic ketone bodies such β-hydroxybutyrate and acetoacetate are produced. These physiological changes in the body cause the signs and symptoms typically found in DKA.
Signs and Symptoms
Over a period of 24 hours, symptoms such as nausea, vomiting, increased thirst, and polyuria develop due to dehydration caused by osmotic diuresis and glucosuria.5 Patients may also present with hypotension and tachycardia. Confusion, deep gasping breaths or Kussmaul respirations, and metabolic acidosis result from hyperventilation and failure to compensate for the increased serum concentration of ketone bodies. Ketone production leads to a fruit-like odor in the patient’s breath and ketonuria in the urinalysis. In DKA, laboratory values will indicate metabolic acidosis and abnormal serum electrolytes. In both DM and DKA, increased urea and creatinine due to dehydration, increased ketones, and the presence of diabetic nephropathy are useful indicators of impaired kidney function.12
Management and Treatment
Diabetic ketoacidosis can be managed and reversed, especially when recognized and treated early.6,13 Dehydration in DKA can be corrected with IV fluid replacement. Normal saline (0.9%) can be started at 15 to 20 mL/kg/h or 1 L/h. As the patient’s vital signs stabilize, IV fluids can be titrated to a lower dose of 250 to 500 mL/h. Monitoring BP and electrolytes are key at this point as alterations in sodium levels and glucose levels may require switching to half-normal saline and/or dextrose.
The hyperglycemic state of patients with DKA is managed by IV insulin. An initial bolus of 0.1 U/kg/h can be given, but should only be administered when potassium levels are greater than 3.3 mmol/L.14 If adequate perfusion can be maintained, then 0.14 U/kg/h can be used instead of a bolus. Glucose levels must be monitored; once the levels decrease to approximately 200 mg/dL, the infusion rate of insulin should be titrated down to 0.05 to 0.1 U/kg/h. Dextrose is then added to maintain glucose levels at approximately 150 to 200 mg/dL.
Electrolytes, especially potassium, must be monitored closely in patients with DKA. Insulin leads to the shift of potassium into cells. The lack of insulin keeps potassium in the extracellular space. Due to osmotic diuresis, potassium is lost in the urine, leading to hypokalemia. Potassium levels in patients with DKA should be maintained at a level between 4 to 5 mmol/L. Patients with potassium levels between 3.3 to 5.2 mmol/L can be started on IV potassium between 20 to 30 mmol/h. If the patient is severely hypokalemic (<3.3 mmol/L), insulin should be withheld, and only IV potassium should be given at a rate of 20 to 30 mmol/h.
Bicarbonate levels can also be managed as acidosis can lead to both neurological and cardiac complications. If the patient’s pH is less than 6.9, the American Diabetes Association recommends starting 100 mmol of sodium bicarbonate in 400 mL sterile water (in addition to potassium chloride at 200 mL/h) for 2 hours. Dosing should be repeated every 2 hours until the patient’s pH is greater than 6.9.
In uncomplicated cases of DKA, the condition is resolved when a patient’s pH is greater than 7.3; glucose level is less than 200 mg/dL; and bicarbonate level is greater than or equal to 18 mmol/L. After patients become hemodynamically stable, they can be discharged and managed at home with a combination of intermediate- or long-acting insulin as well as short- or rapid-acting insulin.
Complications and Mortality
Diabetic ketoacidosis can cause sudden and fluctuating changes in the body. Therefore, it is very important to monitor a patient’s laboratory values very carefully and frequently to avoid any pitfalls. Since patients can present with hyponatremia due to the osmotic draw of glucose in the blood,13 sodium levels may have to be corrected. The corrected serum sodium can be calculated by adding 1.6 mmol/L for every 100 mg/dL of glucose (when finger-stick readings are above 200 mg/dL).15 Patients with DKA can also present with leukocytosis (even in the absence of infection) and hypertriglyceridemia (due to impaired lipoprotein lipase).15 Serum creatine may be elevated due to blood acetoacetate levels.15
Interestingly, there are other acute conditions that can mimic DKA.15 For example, chronic ethanol abuse can lead to ketoacidosis. Unlike DKA, however, alcoholic ketoacidosis does not have profound hyperglycemia, which can help differentiate the two during initial assessment.
Complications due to DKA can arise comprising the patient’s health, including hypoglycemia, hypokalemia, rhabdomyolysis, acute renal failure, pulmonary edema, and shock.16 Cerebral edema is seen in up to 1% of DKA patients,15 the cause of which may be due to the severity of the acidosis, high glucose levels, and rapid hydration. Even when cerebral edema is reduced, patients are often neurologically impaired. Mortality rates from DKA deaths due to cerebral edema can be as high as 24%.13 In the United States, over 100,000 patients with DM per year are admitted to the hospital for DKA, and 9% of patients with DM suffer from DKA-related complications postdischarge.15 With current treatment protocols, mortality rates for DKA-associated deaths are now down to 1%.6,15
Diabetes ketoacidosis-related deaths are usually the result of the following: a triad of DKA symptoms (hyperglycemia, hyperketonemia, and metabolic acidosis), another underlying comorbid condition (eg, myocardial infarction, sepsis, acute respiratory distress syndrome), or the release of biological markers (ie, catecholamines).14,15,17 Thus, as previously stated, the management of potassium levels is important as both hyperkalemia and hypokalemia can lead to fatal arrthythmias.15
Direct mortality from DKA has dropped significantly over the past 20 years, from 8% to less than 1%.6 The US Centers for Disease Control and Prevention has observed a downward trend in death and estimates that 2,417 patients died in 2009 due to DKA,18 and recent postmortem studies have revealed new insights into DKA-related deaths.19 Blood and vitreous acetone concentrations are strong indicators for predeath hyperglycemia and ketosis (if there are no underlying comorbid and/or pharmacological provocations). Blood acetone levels greater than 0.01 g/dL antemortem are suggestive of DKA. It is recommended that these tests should be performed in sudden deaths which have no biological or anatomical cause of death. Postmortem diagnosis of DKA is made with the following criteria: history of DM, increased vitreous glucose concentrations, and elevated blood/vitreous/urine acetone concentrations (>200 mg/dL). If results of the abovementioned parameters are inconclusive, measurement of lactic acid postmortem is thought to further support a diagnosis of DKA.19
Patient Counseling and Education
Approximately 33% of patients whose death was associated with DKA had no personal history of DM.19 This statistic emphasizes the importance of taking a thorough history, physical examination, blood glucose evaluation, and educating patients about the signs and symptoms of DM and DKA.
Patient counseling and education are important, especially in patients whose racial/ethnic background places them at increased risk of developing DM (eg, patients of black or African American, American Indian, Alaskan Native, Asian American, Hispanic, Native Hawaiian, or Pacific Islander descent).20,21 Strategies for preventive management include advocating regular glucose monitoring as well as dietary and lifestyle modifications. In patients with DM, successful management of the condition and its comorbidities can help prevent DKA and associated mortality.
Conclusion
As this case demonstrates, despite prompt diagnosis and management, patients with DKA—especially those with uncontrolled, undiagnosed, or advanced DM—are associated with fatal outcomes. In many cases, however, DKA can be successfully managed and reversed, especially when the condition is recognized early. Management includes not only IV therapy to adjust fluid and insulin levels, but also restoring electrolyte balance (especially potassium and bicarbonate). Frequent and careful evaluation of laboratory values is vital to the successful treatment of DKA, as there are numerous pitfalls and complications that the emergency physician can encounter. Patients who either have or are at an increased risk of developing DM or DKA may benefit from preventive measures, including regular glucose monitoring and appropriate diet and lifestyle modifications.
Mr Hassan-Ali is a fourth-year medical student at Windsor University School of Medicine, St Kitts, West Indies. Dr Raziuddin is an internist and an emergency medicine physician at Weiss Memorial, Thorek Memorial, and Westlake Hospitals, Chicago, Illinois.
Case
A 32-year-old Hispanic man presented to the ED with complications associated with diabetes mellitus (DM), the symptoms of which started approximately 3 days prior to arrival. The patient reported feelings of fatigue, dry mouth, increased thirst, and frequent urination. He denied sweating, nausea, chest pain, shortness of breath, diarrhea, or blood in his urine; he also denied blurry vision or dizziness.
During history intake, the patient informed the emergency physician (EP) that he had been diagnosed with DM and hyperglycemia earlier that day by his primary care physician, who had immediately referred the patient to the ED for urgent management. The patient’s own medical history was noncontributory; however, his father’s history was notable for DM and chronic renal failure. The patient further stated that he was not on any medications. Regarding his social history, he denied cigarette smoking and noted only occasional alcohol consumption.
The patient’s vital signs on presentation were: blood pressure (BP), 116/74 mm Hg; heart rate, 113 beats/minute; respiratory rate, 26 breaths/minute; and temperature, 97.8°F. Oxygen saturation was 97% on room air. On physical examination, the patient was severely anxious, with tachycardia and respiratory distress. He was obese, with a body mass index of 30.9 kg/m2 (height, 5 feet, 4 inches; weight, 180 lb).
The patient was started on an intravenous (IV) bolus of 0.9% normal saline (2 L at 20 mL/kg). After a consultation with endocrinology, he was then given a maintenance dose of normal saline IV at 250 cc/h and an IV insulin drip at 0.1 U/kg/h following a bolus of 8 units of insulin IV. His glucose levels were carefully monitored via hourly finger-stick glucose testing.
Although the patient’s condition stabilized, he collapsed while walking to the bathroom. He had agonal respirations and no pulse. Resuscitation efforts were started with bag-valve-mask ventilation, along with emergent advanced cardiac life support (ACLS) treatment, the protocol of which included epinephrine administration (x2) IV push 5 minutes apart, 2 ampules of sodium bicarbonate (50 mEq each) IV push, and calcium gluconate 10% (x1) 10 mL (1 g) IV push. A pulse was re-established, and the patient was intubated.
The patient was diagnosed with diabetic ketoacidosis (DKA) and admitted to the intensive care unit where repeat laboratory evaluation was ordered. Additional pharmacological management included IV administration of dopamine, norepinephrine, phenylephrine, vasopressin, antibiotics (azithromycin, meropenem, and vancomycin), pantoprazole, and subcutaneous heparin.
During treatment, the patient coded a second time and was revived according to ACLS protocols. Shortly thereafter, he coded a third time, but resuscitation efforts failed. Pathology reported no biological cause of death, and the coroner closed the case as death due to DM-related complications.
Diabetic Ketoacidosis
Diabetic ketoacidosis is a major complication of DM.4 Although the condition usually occurs in type 1 DM, it can also develop in type 2 DM. Diabetic ketoacidosis may be an inciting event leading to the eventual diagnosis of DM, but can also develop during a concurrent illness such as a urinary tract infection or an eating disorder.5 Risk factors for DKA include patients with type 1 or type 2 DM, a family history of DM, obesity, and nonwhite patients whose ethnic background places them at increased risk.6 Hispanic, black, and African American patients are at a greater risk of developing DKA and are more likely to develop “ketosis-prone” type 2 DM.7
Patients who do not fit into the definitive categories of type 1 or 2 DM can be classified under ketosis-prone DM.7,8 Diabetic ketoacidosis acts as the inciting event for the disease and evolves into severe β-cell dysfunction, hence blurring the lines between the archetypal DM categories. Fifty percent of ketosis-prone DM patients are A-β+ (absent autoantibodies, present β-cell function), which indicates that the dysfunction can be partially reversed. Reversal of the condition is largely based on long-term β-cell reserves, which are dependent on tight glycemic control and insulin dependence. Higher incidences of the A-β+ variant of ketosis-prone diabetes are seen in the male population and are often unprovoked.9-11
Diabetic ketoacidosis is the result of either a decrease or absence of insulin in the body (Table 2).4 Without insulin modulating exogenous glucose intake and endogenous glucose production (via glucagon, glycogenolysis, and gluconeogenesis), high levels of glucose are found in the circulation, leading to prominent hyperglycemia (>250 mg/dL or >13.8 mmol/L).6 This environment causes the body to switch from carbohydrate metabolism to fatty acid metabolism. As a result, acidic ketone bodies such β-hydroxybutyrate and acetoacetate are produced. These physiological changes in the body cause the signs and symptoms typically found in DKA.
Signs and Symptoms
Over a period of 24 hours, symptoms such as nausea, vomiting, increased thirst, and polyuria develop due to dehydration caused by osmotic diuresis and glucosuria.5 Patients may also present with hypotension and tachycardia. Confusion, deep gasping breaths or Kussmaul respirations, and metabolic acidosis result from hyperventilation and failure to compensate for the increased serum concentration of ketone bodies. Ketone production leads to a fruit-like odor in the patient’s breath and ketonuria in the urinalysis. In DKA, laboratory values will indicate metabolic acidosis and abnormal serum electrolytes. In both DM and DKA, increased urea and creatinine due to dehydration, increased ketones, and the presence of diabetic nephropathy are useful indicators of impaired kidney function.12
Management and Treatment
Diabetic ketoacidosis can be managed and reversed, especially when recognized and treated early.6,13 Dehydration in DKA can be corrected with IV fluid replacement. Normal saline (0.9%) can be started at 15 to 20 mL/kg/h or 1 L/h. As the patient’s vital signs stabilize, IV fluids can be titrated to a lower dose of 250 to 500 mL/h. Monitoring BP and electrolytes are key at this point as alterations in sodium levels and glucose levels may require switching to half-normal saline and/or dextrose.
The hyperglycemic state of patients with DKA is managed by IV insulin. An initial bolus of 0.1 U/kg/h can be given, but should only be administered when potassium levels are greater than 3.3 mmol/L.14 If adequate perfusion can be maintained, then 0.14 U/kg/h can be used instead of a bolus. Glucose levels must be monitored; once the levels decrease to approximately 200 mg/dL, the infusion rate of insulin should be titrated down to 0.05 to 0.1 U/kg/h. Dextrose is then added to maintain glucose levels at approximately 150 to 200 mg/dL.
Electrolytes, especially potassium, must be monitored closely in patients with DKA. Insulin leads to the shift of potassium into cells. The lack of insulin keeps potassium in the extracellular space. Due to osmotic diuresis, potassium is lost in the urine, leading to hypokalemia. Potassium levels in patients with DKA should be maintained at a level between 4 to 5 mmol/L. Patients with potassium levels between 3.3 to 5.2 mmol/L can be started on IV potassium between 20 to 30 mmol/h. If the patient is severely hypokalemic (<3.3 mmol/L), insulin should be withheld, and only IV potassium should be given at a rate of 20 to 30 mmol/h.
Bicarbonate levels can also be managed as acidosis can lead to both neurological and cardiac complications. If the patient’s pH is less than 6.9, the American Diabetes Association recommends starting 100 mmol of sodium bicarbonate in 400 mL sterile water (in addition to potassium chloride at 200 mL/h) for 2 hours. Dosing should be repeated every 2 hours until the patient’s pH is greater than 6.9.
In uncomplicated cases of DKA, the condition is resolved when a patient’s pH is greater than 7.3; glucose level is less than 200 mg/dL; and bicarbonate level is greater than or equal to 18 mmol/L. After patients become hemodynamically stable, they can be discharged and managed at home with a combination of intermediate- or long-acting insulin as well as short- or rapid-acting insulin.
Complications and Mortality
Diabetic ketoacidosis can cause sudden and fluctuating changes in the body. Therefore, it is very important to monitor a patient’s laboratory values very carefully and frequently to avoid any pitfalls. Since patients can present with hyponatremia due to the osmotic draw of glucose in the blood,13 sodium levels may have to be corrected. The corrected serum sodium can be calculated by adding 1.6 mmol/L for every 100 mg/dL of glucose (when finger-stick readings are above 200 mg/dL).15 Patients with DKA can also present with leukocytosis (even in the absence of infection) and hypertriglyceridemia (due to impaired lipoprotein lipase).15 Serum creatine may be elevated due to blood acetoacetate levels.15
Interestingly, there are other acute conditions that can mimic DKA.15 For example, chronic ethanol abuse can lead to ketoacidosis. Unlike DKA, however, alcoholic ketoacidosis does not have profound hyperglycemia, which can help differentiate the two during initial assessment.
Complications due to DKA can arise comprising the patient’s health, including hypoglycemia, hypokalemia, rhabdomyolysis, acute renal failure, pulmonary edema, and shock.16 Cerebral edema is seen in up to 1% of DKA patients,15 the cause of which may be due to the severity of the acidosis, high glucose levels, and rapid hydration. Even when cerebral edema is reduced, patients are often neurologically impaired. Mortality rates from DKA deaths due to cerebral edema can be as high as 24%.13 In the United States, over 100,000 patients with DM per year are admitted to the hospital for DKA, and 9% of patients with DM suffer from DKA-related complications postdischarge.15 With current treatment protocols, mortality rates for DKA-associated deaths are now down to 1%.6,15
Diabetes ketoacidosis-related deaths are usually the result of the following: a triad of DKA symptoms (hyperglycemia, hyperketonemia, and metabolic acidosis), another underlying comorbid condition (eg, myocardial infarction, sepsis, acute respiratory distress syndrome), or the release of biological markers (ie, catecholamines).14,15,17 Thus, as previously stated, the management of potassium levels is important as both hyperkalemia and hypokalemia can lead to fatal arrthythmias.15
Direct mortality from DKA has dropped significantly over the past 20 years, from 8% to less than 1%.6 The US Centers for Disease Control and Prevention has observed a downward trend in death and estimates that 2,417 patients died in 2009 due to DKA,18 and recent postmortem studies have revealed new insights into DKA-related deaths.19 Blood and vitreous acetone concentrations are strong indicators for predeath hyperglycemia and ketosis (if there are no underlying comorbid and/or pharmacological provocations). Blood acetone levels greater than 0.01 g/dL antemortem are suggestive of DKA. It is recommended that these tests should be performed in sudden deaths which have no biological or anatomical cause of death. Postmortem diagnosis of DKA is made with the following criteria: history of DM, increased vitreous glucose concentrations, and elevated blood/vitreous/urine acetone concentrations (>200 mg/dL). If results of the abovementioned parameters are inconclusive, measurement of lactic acid postmortem is thought to further support a diagnosis of DKA.19
Patient Counseling and Education
Approximately 33% of patients whose death was associated with DKA had no personal history of DM.19 This statistic emphasizes the importance of taking a thorough history, physical examination, blood glucose evaluation, and educating patients about the signs and symptoms of DM and DKA.
Patient counseling and education are important, especially in patients whose racial/ethnic background places them at increased risk of developing DM (eg, patients of black or African American, American Indian, Alaskan Native, Asian American, Hispanic, Native Hawaiian, or Pacific Islander descent).20,21 Strategies for preventive management include advocating regular glucose monitoring as well as dietary and lifestyle modifications. In patients with DM, successful management of the condition and its comorbidities can help prevent DKA and associated mortality.
Conclusion
As this case demonstrates, despite prompt diagnosis and management, patients with DKA—especially those with uncontrolled, undiagnosed, or advanced DM—are associated with fatal outcomes. In many cases, however, DKA can be successfully managed and reversed, especially when the condition is recognized early. Management includes not only IV therapy to adjust fluid and insulin levels, but also restoring electrolyte balance (especially potassium and bicarbonate). Frequent and careful evaluation of laboratory values is vital to the successful treatment of DKA, as there are numerous pitfalls and complications that the emergency physician can encounter. Patients who either have or are at an increased risk of developing DM or DKA may benefit from preventive measures, including regular glucose monitoring and appropriate diet and lifestyle modifications.
Mr Hassan-Ali is a fourth-year medical student at Windsor University School of Medicine, St Kitts, West Indies. Dr Raziuddin is an internist and an emergency medicine physician at Weiss Memorial, Thorek Memorial, and Westlake Hospitals, Chicago, Illinois.
- Kitabchi AH, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153.
- Farinda A. Lab values, normal adult: laboratory reference ranges in healthy adults. 2015. Medscape Web site. http://emedicine.medscape.com/article/2172316-overview. Updated May 14, 2014. Accessed August 14, 2015.
- Young D. Implementation of SI units for clinical laboratory data. Ann Intern Med. 1987;106(1):114-129.
- Maitra A. The endocrine system. In: Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed. New York, NY: Elsevier Saunders; 2015:1105-1120.
- Powers AC. Diabetes mellitus: management and therapies. In: Kasper DL, Fauci AS, Longo DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY; McGraw-Hill Medical Publishing Division; 2015:2407-2422.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343.
- Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350-357.
- Umpierrez G, Smiley D, Gosmanov A, Thomason D. Ketosis-prone type 2 diabetes: effect of hyperglycemia on beta-cell function and skeletal muscle insulin signaling. Endocr Pract. 2007;13(3):283-290.
- Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645-653.
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes. 1995;44(7):790-795.
- Piñero-Piloña A, Raskin P. Idiopathic type 1 diabetes. J Diabetes Complications. 2001;15(6):328-335.
- Kemperman FA, Weber JA, Gorgels J, van Zanten AP, Krediet RT, Arisz L. The influence of ketoacids on plasma creatinine assays in diabetic ketoacidosis. J Intern Med. 2000;248(6):511-517.
- Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician. 2013;87(5):337-346.
- Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physician. 2005;71(9):1705-1714.
- Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar ayndrome. Diabetes Spectrum. 2002;15(1):28-36.
- Wolfsdorf J, Glaser N, Sperling MA; American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150-1159.
- Rosenbloom AL. Sudden death of a young woman attributed to diabetic ketoacidosis. J Forensic Leg Med. 2013;20(8):1063-1065.
- Centers for Disease Control and Prevention. Number of deaths for hyperglycemic crises as underlying cause, United States, 1980-2009. http://www.cdc.gov/diabetes/statistics/mortalitydka/fnumberofdka.htm. Updated November 19, 2013. Accessed August 14, 2015.
- Ali Z, Levine B, Ripple M, Fowler DR. Diabetic ketoacidosis: a silent death. Am J Forensic Med Pathol. 2012;33(3):189-193.
- US Department of Health and Human Services Office of Minority Health. Diabetes and Hispanic Americans. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=63. Updated June 15, 2013. Accessed August 14, 2015.
- US Department of Health and Human Services Office of Minority Health. Profile: Native Hawaiian/Other Pacific Islanders. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=65. Updated January 15, 2015. Accessed August 14, 2015.
- Kitabchi AH, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153.
- Farinda A. Lab values, normal adult: laboratory reference ranges in healthy adults. 2015. Medscape Web site. http://emedicine.medscape.com/article/2172316-overview. Updated May 14, 2014. Accessed August 14, 2015.
- Young D. Implementation of SI units for clinical laboratory data. Ann Intern Med. 1987;106(1):114-129.
- Maitra A. The endocrine system. In: Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed. New York, NY: Elsevier Saunders; 2015:1105-1120.
- Powers AC. Diabetes mellitus: management and therapies. In: Kasper DL, Fauci AS, Longo DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY; McGraw-Hill Medical Publishing Division; 2015:2407-2422.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343.
- Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350-357.
- Umpierrez G, Smiley D, Gosmanov A, Thomason D. Ketosis-prone type 2 diabetes: effect of hyperglycemia on beta-cell function and skeletal muscle insulin signaling. Endocr Pract. 2007;13(3):283-290.
- Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645-653.
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes. 1995;44(7):790-795.
- Piñero-Piloña A, Raskin P. Idiopathic type 1 diabetes. J Diabetes Complications. 2001;15(6):328-335.
- Kemperman FA, Weber JA, Gorgels J, van Zanten AP, Krediet RT, Arisz L. The influence of ketoacids on plasma creatinine assays in diabetic ketoacidosis. J Intern Med. 2000;248(6):511-517.
- Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician. 2013;87(5):337-346.
- Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physician. 2005;71(9):1705-1714.
- Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar ayndrome. Diabetes Spectrum. 2002;15(1):28-36.
- Wolfsdorf J, Glaser N, Sperling MA; American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150-1159.
- Rosenbloom AL. Sudden death of a young woman attributed to diabetic ketoacidosis. J Forensic Leg Med. 2013;20(8):1063-1065.
- Centers for Disease Control and Prevention. Number of deaths for hyperglycemic crises as underlying cause, United States, 1980-2009. http://www.cdc.gov/diabetes/statistics/mortalitydka/fnumberofdka.htm. Updated November 19, 2013. Accessed August 14, 2015.
- Ali Z, Levine B, Ripple M, Fowler DR. Diabetic ketoacidosis: a silent death. Am J Forensic Med Pathol. 2012;33(3):189-193.
- US Department of Health and Human Services Office of Minority Health. Diabetes and Hispanic Americans. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=63. Updated June 15, 2013. Accessed August 14, 2015.
- US Department of Health and Human Services Office of Minority Health. Profile: Native Hawaiian/Other Pacific Islanders. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=65. Updated January 15, 2015. Accessed August 14, 2015.
Causes and Rates of Unplanned Readmissions After Elective Primary Total Joint Arthroplasty: A Systematic Review and Meta-Analysis
Total joint arthroplasty (TJA) is a clinically effective, cost-effective treatment for symptomatic arthritis.1,2 After TJA, patients report reduced pain, restored range of motion, high satisfaction, and ability to return to a more active lifestyle.3-7 The number of total hip arthroplasties (THAs) performed in the United States is expected to reach 572,000 by 2030, a 174% increase, and the number of total knee arthroplasties (TKAs) 3.5 million, nearly a 7-fold increase.8,9 Since 2005, the cost of THA has risen more than 4 times, to $13.43 billion, and the cost of TKA has risen more than 5 times, to $40.8 billion.8,9 Given the demand and price tag, TJA is the single largest cost in the Medicare budget.10
Given its potential to improve care and reduce costs, reducing readmission rates in the surgical setting is a priority for physicians and policymakers.11 Readmissions for TJA are highly scrutinized as a performance indicator—the Centers for Medicare & Medicaid Services (CMS) started including them in its readmissions penalty program in 2013—and were recently validated as a measure of surgical quality.12-14 Accurate assessments of readmissions after TJA are unclear, with rates ranging from 1% to 8.5% between 7 and 90 days after surgery.2,15-17 The early success of TJA as an elective (and more frequently outpatient) procedure has paradoxically translated to less tolerance for readmissions. Post-TJA complications resulting in readmission are subject to financial penalties, and there is an implicit judgment of inadequate surgical management.12
Not only is the readmission rate poorly characterized, but there is no consensus on the leading reasons for readmissions after primary elective unilateral TJAs. The range of rates, reasons, and follow-up periods reported in the literature is wide.18,19 CMS plans to monitor readmissions over 7 to 90 days after surgery (the period depends on the complication), whereas a significant portion of the orthopedic literature documents 90-day rates.19 In 2012, the Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation prepared for CMS a comprehensive report identifying rates of post-TJA complications and readmissions.20 The report, however, is limited to US hospitals and Medicare patients and therefore may overstate the rates, given this population’s documented comorbidities and the reimbursement variations between Medicare and commercial insurance.21 Lack of consensus on readmissions after primary elective unilateral TJAs requires that we synthesize available data to answer several questions: What is the overall readmission rate 30 and 90 days after TJA? What are the primary reasons for readmission 30 and 90 days after TJA? What are the cause-specific readmission rates? We performed a systematic review and a meta-analysis to answer these questions and to add clarity to the literature in order to help guide policy.
Materials and Methods
We performed a systematic review in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.22 Two reviewers independently completed structured searches of the Medline and Cochrane Central Register of Controlled Trials databases. Search terms were: (total hip replacement OR hip arthroplasty OR total hip arthroplasty OR total knee replacement OR knee arthroplasty OR total knee arthroplasty) AND (readmission OR complication OR discharge). They updated the search June 1, 2013. Four limits were applied: publication between January 1, 1982 and December 12, 2012; human subjects only; age 19+ years; and English-language articles. Study eligibility was determined by using standardized criteria as defined by the inclusion and exclusion criteria described in 3 stages: title review, abstract review, and full-article review. The reviewers also performed ancestry searches, including searches for major review articles and bibliographies of all retrieved studies, to identify additional studies not identified in the keyword searches. Discrepancies were resolved by author consensus.
Inclusion criteria were original studies that presented level I to III evidence and that were identified in structured online searches; published in English between January 1, 1982 and December 31, 2012; involved patients older than 19 years; and reported both readmission rates and reasons at follow-up 30 or 90 days after elective primary unilateral TJA, regardless of indication. Exclusion criteria were studies that reported data from hip fracture, knee fracture, and pelvis fracture cases; those that reported data from hemiarthroplasty, Birmingham hip resurfacing procedures, other resurfacing procedures, simultaneous bilateral hip or knee arthroplasties, unicompartmental knee arthroplasty, patellofemoral arthroplasty, metastatic or bone cancer, or revision hip or knee arthroplasty; those that did not report extractable reasons for readmission; those that reported complications but did not specify readmission rates; and those that reported readmission data only from after the 90-day follow-up window. In cases in which multiple studies reported data from the same patient population, only the largest or most recent report was used.
Two reviewers extracted the quantitative data from eligible studies. The 2 primary outcomes of interests were all-cause readmission rates, and reasons for readmission 30 and 90 days after TJA. Other extracted data were evidence level; publication journal, year, and country; data source (academic institution, Medicare); study design; number of patients; patient characteristics; surgical approach; follow-up period; overall readmission rate; anticoagulant use; tourniquet use; and compression stocking use. In addition, all post-TJA readmissions were assumed to be unplanned, except for staged sequential bilateral arthroplasty for osteoarthritis (excluded from analysis).
Readmission reasons were divided into 4 major categories as defined by the literature and the authors: thromboembolic disease, joint-specific reasons, surgical site infection, and surgical sequelae. The diagnoses in these categories are listed in Table 1. Other extracted reasons were cardiac dysrhythmia and pneumonia.
In cases in which there were at least 2 comparable studies, a meta-analysis was performed to obtain pooled estimates of the proportion of patients readmitted at 30 or 90 days. We calculated a Higgins I2 measure for between-study heterogeneity and random-effects analysis, using the method of DerSimonian and Laird23 if I2 was greater than 0.5. Pooled estimates were obtained for both overall and cause-specific reasons for readmission for all reasons reported in at least 3 studies. Small-study or publication bias was assessed using funnel plot asymmetry when at least 5 studies were analyzed as recommended.24 The meta-analytic findings for both overall and cause-specific readmission are presented as pooled proportions with 95% confidence intervals (CIs). All meta-analyses were performed using Stata 10.0.
Results
Fifteen unique TJA studies (12 THA, 10 TKA) met the criteria for the meta-analysis.20,25-38Figure 1 depicts the PRISMA flowchart for study identification.22
Of the 12 studies eligible for the THA analysis (Table 2), 6 were conducted in the United States,20,26,27,30,33,34 5 in Europe,25,28,29,32,35 and 1 in Canada.31 Seven of the 12 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (2 reported rates at both follow-ups). We analyzed a total of 113,396 patients at the 30-day window and 192,380 patients at the 90-day window. Mean age was 74.2 years. The included studies were variable and sparse in their reporting of specific characteristics (Table 3).
Of the 10 studies (2 prospective, 8 retrospective) eligible for the TKA analysis (Table 4), 6 were conducted in the United States,20,26,27,34,36,37 3 in Europe,25,29,35 and 1 in Asia.38 Four of the 10 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (1 reported rates at both follow-ups).27 We analyzed a total of 3,278,635 patients at the 30-day window and 272,419 patients at the 90-day window. Mean age was 74.3 years. The included studies were quite variable and sparse in their reporting of specific characteristics (Table 5).
We performed random-effects meta-analyses of all unplanned readmissions at both 30 and 90 days (all I2s > 0.5). Among 5 THA studies that reported overall rates at 30 days,20,27,28,32,33 the estimated overall unplanned rate among the 120,272 index surgeries was 5.6% (95% CI, 3.2%-8.0%). Among 5 THA studies that reported overall rates at 90 days,20,25-27,31 the estimated overall unplanned rate among the 192,380 index surgeries was 7.7% (95% CI, 3.2%-12.2%) (I2 = 1.00). Among 3 TKA studies that reported overall rates at 30 days,27,37,38 the estimated overall unplanned rate among the 3,278,635 index surgeries was 3.3% (95% CI, 0.7%-5.9%). Among 5 TKA studies that reported overall rates at 90 days,20,25-27,36 the estimated overall unplanned rate among the 272,419 index surgeries was 9.7% (95% CI, 7.1%-12.4%) (I2 = 0.97).
30-Day Readmission Rates
The most common reason for readmission 30 days after THA discharge was joint-specific. This reason accounted for 39.3% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.2% (95% CI, 0.0%-4.6%; P < .001; I2 = 1.00) among 4 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 0.8%-2.5%; P < .001; I2 = 0.95) and thromboembolic disease (1.5%; 95% CI, 1.0%-1.9%; P < .001; I2 = 0.95). See Figure 2 for 30-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.1%; P < .001; I2 = 0.94). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported in only 1 study each.
The most common reason for readmission 30 days after TKA discharge was surgical site infection. This reason accounted for 12.1% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.4% (95% CI, 0.3%-0.6%; P < .001; I2 = 0.61) among 3 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.3% of the time. Joint-specific reasons were reported in 2 studies (95% CI, 0.0%-0.8%; P = .259; I2 = 0.94). Thromboembolic disease was reported in 4 studies (95% CI, 0.0%-0.7%; P = .067; I2 = 0.98) (Figure 3). Only these 3 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and “sequelae” were reported in only 1 study each.
90-Day Readmission Rates
Consistent with the 30-day THA results, the most common reason for readmission 90 days after THA discharge was joint-specific. This reason accounted for 31.2% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.4% (95% CI, 0.0%-4.9%; P < .001; I2 = 1.00) among 5 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 1.0%-2.2%; P < .003; I2 = 0.83) and thromboembolic disease (1.0%; 95% CI, 0.7%-1.4%; P < .001; I2 = 0.97). See Figure 4 for 90-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.0%; P < .001; I2 = 0.99). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported by only 1 study each.
Consistent with the 30-day TKA results, the most common reason for readmission 90 days after TKA discharge was surgical site infection. This reason accounted for 9.3% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.9% (95% CI, 0.4%-1.4%; P < .001; I2 = 0.93) among 5 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.7% of the time. Joint-specific reasons were reported in 5 studies (95% CI, 0.2%-1.1%; P =.003; I2 = 0.94). Thromboembolic disease was reported in 7 studies (95% CI, 0.3%-1.1%; P < .001; I2 = 0.97) (Figure 5). Bleeding was reported in 3 studies, with a pooled rate of 0.4% (95% CI, 0.0%-0.9%; P = .128; I2 = 0.83). Cardiac dysrhythmia was reported in 2 studies, with an estimated pooled rate of 0.3% (95% CI, 0.2%-0.5%; P < .001). Only these 5 reasons could be pooled, as pneumonia and “sequelae” were reported in only 1 study each.
Discussion
This study is the first systematic review and meta-analysis of the literature to identify overall and cause-specific readmission rates after TJA.
For THA, 30- and 90-day readmission rates were 5.6% and 7.7%, respectively. Joint-specific causes were the most common reason for readmission at both 30 and 90 days after THA. For TKA, 30- and 90-day rates were 3.3% and 9.7%, respectively. Surgical site infection was the most common reason for readmission at both 30 and 90 days after TKA.
Hospital readmissions are an important area of scrutiny for Medicare and the health care systems broadly. Readmissions after surgery are deemed quality indicators potentially suggesting incomplete management of active issues and inadequate preparation for discharge.39 Unplanned readmissions also place a significant economic burden on Medicare: $17.5 billion in 2010.40 Given their association with quality of overall surgical care, improved readmission rates have the potential to improve the standard of care and reduce costs.
Higher readmission rates will significantly affect hospitals as CMS shifts to bundling payments for acute-care episodes, such as TJA.41-43 Further, private and public health care payers are increasingly using unplanned 30- and 90-day readmission rates as a marker of quality of care. However, there is little agreement about readmission rates and reasons, let alone what follow-up window should be used to define orthopedic readmissions. One study involving the MEDPAR (Medicare Provider Analysis and Review) database found that a common reason for readmission after major hip or knee surgery was “aftercare” for surgical sequelae (10.3%)15; another study found a 15% increase in post-THA hospitalizations, most commonly for a mechanical complication (joint-related).44 There are no prior complete systematic reviews or meta-analyses of overall rates of readmissions after primary unilateral TJAs, or of the reasons for these readmissions. The closest such report, the Yale report to CMS, was skewed to a proportion of US hospitals treating a population prone to significant comorbidities.20
Although the strength of this study lies in its rigorous identification and extraction of data, notable clarifications must be made when synthesizing the information. First, the definitions of various thromboembolic events varied greatly. Some studies reported deep vein thrombosis (DVT) and pulmonary embolism (PE) separately, whereas others reported only DVT or only PE. Some studies reported rates of readmission for “thromboembolic disorder,” and one25 reported rates for DVT, PE, and thromboembolic disorder. To pool these related events, we created a composite definition that included DVT, PE, and thromboembolic disorders, which we termed thromboembolic disease. We also created a composite measure for joint-specific reasons for readmission. This category included joint infection that definitely required reentry into the joint, but using this category may have led to underestimation of surgical site infection rates, which were defined separately. Third, there was significant variation in documentation of surgical site infection among the studies included in this review. Some studies specified superficial wounds, whereas others did not categorize complications as superficial, deep, or intracapsular, which would qualify as a “joint-specific” cause. Despite this variation, surgical site infection after TJA was found to be the most common reason for readmission.
Our systematic review and meta-analysis were limited, as any others are, by the quality of studies investigated. Few studies reported cause-specific rates and reasons for readmission. Given the small sample, formal tests for small-study or publication bias could not be performed. Some studies included tremendous amounts of data, and International Classification of Diseases, Ninth Revision (ICD-9) codes were used without physician review of readmission diagnoses. In the absence of oversight, many readmissions could have been misinterpreted and incorrectly logged, or simply miscoded. Saucedo and colleagues27,45 found that readmission diagnostic codes were often unverified. Numerous other studies corroborated this lack of correlation with physician-derived readmission diagnoses in just 25% of cases.46-54 Another study limitation is the unknown number of patients who had TJA but presented and were subsequently readmitted to a different hospital. Last, as this review included patients who had surgery performed within a 30-year period, it could not address the shifts in postoperative management that occurred in that time, particularly with respect to anticoagulation. This limitation was partially addressed in THA by dividing final studies into 3 decades. Of these studies, only 1 was from the first decade, 3 were from the second, and the rest were from the third. Of the 3 from the second decade, only the study by Warwick and colleagues29 (1995) explicitly did not use anticoagulation, but compression stockings were used, and consequently there was a 4.0% rate of readmission for thromboembolic disease alone, compared with the study by White and colleagues34 (1998), which explicitly used anticoagulation and boasted a 1.7% rate of readmission for thromboembolic disease. This isolated comparison illustrates the effect of routine anticoagulation and the changes in surgical standards over the 3 decades.
The numbers from this systematic review and meta-analysis represent an international benchmark for TJA as a procedure. Knowing the top reasons for readmission will lead to more focus on joint-related and medical issues (surgical site infection, thromboembolic disease) before discharge to avoid readmission after elective unilateral primary TJA. Although readmission rates have received attention in the United States as a primary means of combating soaring health care costs, knowing the rates for a common procedure applies broadly as an indicator for standard of care worldwide, according to the World Health Organization.55 This study is the first systematic review and meta-analysis of documented readmission rates and reasons for readmission to identify overall and cause-specific rates after TJA. The hope is that our findings will add clarity to the literature and help guide the decisions of physicians and policymakers.
Conclusion
Readmission rates are an increasingly important metric in the United States and around the world, yet there is no consensus regarding overall readmission rates and reasons for readmission after primary unilateral TJAs. Our systematic review and meta-analysis of the literature found overall unplanned readmission rates of 5.6% (30 days) and 7.7% (90 days) for THA and 3.3% (30 days) and 9.7% (90 days) for TKA. At both 30 and 90 days, the most common readmission reasons were joint-specific (THA) and surgical site infection (TKA). New investigations should be directed toward developing countermeasures to lower the rates of readmission.
1. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
2. Cram P, Lu X, Kaboli PJ, et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2001. JAMA. 2011;305(15):1560-1567.
3. de Vries LM, Sturkenboom MC, Verhaar JA, Kingma JH, Stricker BH. Complications after hip arthroplasty and the association with hospital procedure volume. Acta Orthop. 2011;82(5):545-552.
4. Mariconda M, Galasso O, Costa GG, Recano P, Cerbasi S. Quality of life and functionality after total hip arthroplasty: a long-term follow-up study. BMC Musculoskelet Disord. 2011;12:222.
5. Zmistowski B, Restrepo C, Hess J, Adibi D, Cangoz S, Parvizi J. Unplanned readmission after total joint arthroplasty: rates, reasons, and risk factors. J Bone Joint Surg Am. 2013;95(20):1869-1876.
6. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533.
7. Mancuso CA, Salvati EA, Johanson NA, Peterson MG, Charlson ME. Patients’ expectations and satisfaction with total hip arthroplasty. J Arthroplasty. 1997;12(4):387-396.
8. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
9. Kurtz SM, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Bozic KJ, Rubash HE, Sculco TP, Berry DJ. An analysis of Medicare payment policy for total joint arthroplasty. J Arthroplasty. 2008;23(6 suppl 1):133-138.
11. Li LT, Mills WL, White DL, et al. Causes and prevalence of unplanned readmissions after colorectal surgery: a systematic review and meta-analysis. J Am Geriatr Soc. 2013;61(7):1175-1181.
12. Readmissions Reduction Program. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed July 27, 2015.
13. Tsai TC, Joynt KE, Orav J, Gawande AA, Jha AK. Variation in surgical readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134-1142.
14. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program [published correction appears in N Engl J Med. 2011;364(16):1582]. N Engl J Med. 2009;360(14):1418-1428.
15. Zmistowski B, Hozack WJ, Parvizi J. Readmission rates after total hip arthroplasty. JAMA. 2011;306(8):825.
16. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
17. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
18. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369.
19. Atkinson JG. Flaws in the Medicare readmission penalty. N Engl J Med. 2012;367(21):2056-2057.
20. Grosso LM, Curtis JP, Lin Z, et al. Hospital-level Risk-Standardized Complication Rate Following Elective Primary Total Hip Arthroplasty (THA) And/Or Total Knee Arthroplasty (TKA): Measure Methodology Report. Report prepared for Centers for Medicare & Medicaid Services. QualityNet website. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1228772504368. Submitted June 25, 2012. Accessed August 4, 2015.
21. Robinson JC. Analysis of Medicare and commercial insurer–paid total knee replacement reveals opportunities for cost reduction. Health Care Incentives Improvement Institute website. http://www.hci3.org/sites/default/files/files/HCI-2012-IssueBrief-L6-2.pdf. Published 2012. Accessed July 27, 2015.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
24. Higgins JP, Thompson SG. Quantifying heterogeniety in a meta-analysis. Stat Med. 2002;21(11):1539-1558.
25. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
26. Keeney JA, Adelani MA, Nunley RM, Clohisy JC, Barrack RL. Assessing readmission databases: how reliable is the information? J Arthroplasty. 2012;27(8 suppl):72-76.e1-e2.
27. Saucedo JM, Marecek GS, Wanke TR, Lee J, Stulberg SD, Puri L. Understanding readmissions after primary total hip and knee arthroplasty: who’s at risk? J Arthroplasty. 2014;29(2):256-260.
28. Seagroatt V, Tan HS, Goldacre M, Bulstrode C, Nugent I, Gill L. Elective total hip replacement: incidence, emergency readmission rate, and postoperative mortality. BMJ. 1991;303(6815):1431-1435.
29. Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement. A series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br. 1995;77(1):6-10.
30. Kreder HJ, Deyo RA, Koepsell T, Swiontkowski MF, Kreuter W. Relationship between the volume of total hip replacements performed by providers and the rates of postoperative complications in the state of Washington. J Bone Joint Surg Am. 1997;79(4):485-494.
31. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
32. Cullen C, Johnson DS, Cook G. Re-admission rates within 28 days of total hip replacement. Ann R Coll Surg Engl. 2006;88(5):475-478.
33. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
34. White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158(14):1525-1531.
35. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
36. Berger RA, Kusuma SK, Sanders SA, Thill ES, Sporer SM. The feasibility and perioperative complications of outpatient knee arthroplasty. Clin Orthop Relat Res. 2009;467(6):1443-1449.
37. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
38. Seah VW, Singh G, Yang KY, Yeo SJ, Lo NN, Seow KH. Thirty-day mortality and morbidity after total knee arthroplasty. Ann Acad Med Singapore. 2007;36(12):1010-1012.
39. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
40. The Revolving Door: A Report on U.S. Hospital Readmissions. An Analysis of Medicare Data by the Dartmouth Atlas Project. Stories From Patients and Health Care Providers by PerryUndem Research & Communication. Robert Wood Johnson Foundation. http://www.rwjf.org/content/dam/farm/reports/reports/2013/rwjf404178. Published February 2013. Accessed July 27, 2015.
41. Riggs RV, Roberts PS, Aronow H, Younan T. Joint replacement and hip fracture readmission rates: impact of discharge destination. PM R. 2010;2(9):806-810.
42. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
43. McCormack R, Michels R, Ramos N, Hutzler L, Slover JD, Bosco JA. Thirty-day readmission rates as a measure of quality: causes of readmission after orthopedic surgeries and accuracy of administrative data. J Healthc Manag. 2013;58(1):64-76.
44. Bohm ER, Dunbar MJ, Frood JJ, Johnson TM, Morris KA. Rehospitalizations, early revisions, infections, and hospital resource use in the first year after hip and knee arthroplasties. J Arthroplasty. 2012;27(2)232-237.
45. Saucedo J, Marecek GS, Lee J, Huminiak L, Stulberg SD, Puri L. How accurately are we coding readmission diagnoses after total joint arthroplasty? J Arthroplasty. 2013;28(7):1076-1079.
46. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
47. Bozic KJ, Chiu VW, Takemoto SK, et al. The validity of using administrative claims data in total joint arthroplasty outcomes research. J Arthroplasty. 2010;25(6 suppl):58-61.
48. Cram P, Ibrahim SA, Lu X, Wolf BR. Impact of alternative coding schemes on incidence rates of key complications after total hip arthroplasty: a risk-adjusted analysis of a national data set. Geriatr Orthop Surg Rehabil. 2012;3(1):17-26.
49. Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981.
50. Cima RR, Lackore KA, Nehring SA, et al. How best to measure surgical quality? Comparison of the Agency for Healthcare Research and Quality Patient Safety Indicators (AHRQ-PSI) and the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) postoperative adverse events at a single institution. Surgery. 2011;150(5):943-949.
51. Steinberg SM, Popa MR, Michalek JA, Bethel MJ, Ellison EC. Comparison of risk adjustment methodologies in surgical quality improvement. Surgery. 2008;144(4):662-667.
52. Baron JA, Barrett J, Katz JN, Liang MH. Total hip arthroplasty: use and select complications in the US Medicare population. Am J Public Health. 1996;86(1):70-72.
53. HCUPnet. Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov. Accessed July 27, 2015.
54. Singh JA. Epidemiology of knee and hip arthroplasty: a systematic review. Open Orthop J. 2011;5:80-85.
55. Parker SG. Do Current Discharge Arrangements From Inpatient Hospital Care for the Elderly Reduce Readmission Rates, the Length of Inpatient Stay or Mortality, or Improve Health Status? Health Evidence Network report. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2005. http://www.euro.who.int/__data/assets/pdf_file/0006/74670/E87542.pdf. Accessed July 27, 2015.
Total joint arthroplasty (TJA) is a clinically effective, cost-effective treatment for symptomatic arthritis.1,2 After TJA, patients report reduced pain, restored range of motion, high satisfaction, and ability to return to a more active lifestyle.3-7 The number of total hip arthroplasties (THAs) performed in the United States is expected to reach 572,000 by 2030, a 174% increase, and the number of total knee arthroplasties (TKAs) 3.5 million, nearly a 7-fold increase.8,9 Since 2005, the cost of THA has risen more than 4 times, to $13.43 billion, and the cost of TKA has risen more than 5 times, to $40.8 billion.8,9 Given the demand and price tag, TJA is the single largest cost in the Medicare budget.10
Given its potential to improve care and reduce costs, reducing readmission rates in the surgical setting is a priority for physicians and policymakers.11 Readmissions for TJA are highly scrutinized as a performance indicator—the Centers for Medicare & Medicaid Services (CMS) started including them in its readmissions penalty program in 2013—and were recently validated as a measure of surgical quality.12-14 Accurate assessments of readmissions after TJA are unclear, with rates ranging from 1% to 8.5% between 7 and 90 days after surgery.2,15-17 The early success of TJA as an elective (and more frequently outpatient) procedure has paradoxically translated to less tolerance for readmissions. Post-TJA complications resulting in readmission are subject to financial penalties, and there is an implicit judgment of inadequate surgical management.12
Not only is the readmission rate poorly characterized, but there is no consensus on the leading reasons for readmissions after primary elective unilateral TJAs. The range of rates, reasons, and follow-up periods reported in the literature is wide.18,19 CMS plans to monitor readmissions over 7 to 90 days after surgery (the period depends on the complication), whereas a significant portion of the orthopedic literature documents 90-day rates.19 In 2012, the Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation prepared for CMS a comprehensive report identifying rates of post-TJA complications and readmissions.20 The report, however, is limited to US hospitals and Medicare patients and therefore may overstate the rates, given this population’s documented comorbidities and the reimbursement variations between Medicare and commercial insurance.21 Lack of consensus on readmissions after primary elective unilateral TJAs requires that we synthesize available data to answer several questions: What is the overall readmission rate 30 and 90 days after TJA? What are the primary reasons for readmission 30 and 90 days after TJA? What are the cause-specific readmission rates? We performed a systematic review and a meta-analysis to answer these questions and to add clarity to the literature in order to help guide policy.
Materials and Methods
We performed a systematic review in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.22 Two reviewers independently completed structured searches of the Medline and Cochrane Central Register of Controlled Trials databases. Search terms were: (total hip replacement OR hip arthroplasty OR total hip arthroplasty OR total knee replacement OR knee arthroplasty OR total knee arthroplasty) AND (readmission OR complication OR discharge). They updated the search June 1, 2013. Four limits were applied: publication between January 1, 1982 and December 12, 2012; human subjects only; age 19+ years; and English-language articles. Study eligibility was determined by using standardized criteria as defined by the inclusion and exclusion criteria described in 3 stages: title review, abstract review, and full-article review. The reviewers also performed ancestry searches, including searches for major review articles and bibliographies of all retrieved studies, to identify additional studies not identified in the keyword searches. Discrepancies were resolved by author consensus.
Inclusion criteria were original studies that presented level I to III evidence and that were identified in structured online searches; published in English between January 1, 1982 and December 31, 2012; involved patients older than 19 years; and reported both readmission rates and reasons at follow-up 30 or 90 days after elective primary unilateral TJA, regardless of indication. Exclusion criteria were studies that reported data from hip fracture, knee fracture, and pelvis fracture cases; those that reported data from hemiarthroplasty, Birmingham hip resurfacing procedures, other resurfacing procedures, simultaneous bilateral hip or knee arthroplasties, unicompartmental knee arthroplasty, patellofemoral arthroplasty, metastatic or bone cancer, or revision hip or knee arthroplasty; those that did not report extractable reasons for readmission; those that reported complications but did not specify readmission rates; and those that reported readmission data only from after the 90-day follow-up window. In cases in which multiple studies reported data from the same patient population, only the largest or most recent report was used.
Two reviewers extracted the quantitative data from eligible studies. The 2 primary outcomes of interests were all-cause readmission rates, and reasons for readmission 30 and 90 days after TJA. Other extracted data were evidence level; publication journal, year, and country; data source (academic institution, Medicare); study design; number of patients; patient characteristics; surgical approach; follow-up period; overall readmission rate; anticoagulant use; tourniquet use; and compression stocking use. In addition, all post-TJA readmissions were assumed to be unplanned, except for staged sequential bilateral arthroplasty for osteoarthritis (excluded from analysis).
Readmission reasons were divided into 4 major categories as defined by the literature and the authors: thromboembolic disease, joint-specific reasons, surgical site infection, and surgical sequelae. The diagnoses in these categories are listed in Table 1. Other extracted reasons were cardiac dysrhythmia and pneumonia.
In cases in which there were at least 2 comparable studies, a meta-analysis was performed to obtain pooled estimates of the proportion of patients readmitted at 30 or 90 days. We calculated a Higgins I2 measure for between-study heterogeneity and random-effects analysis, using the method of DerSimonian and Laird23 if I2 was greater than 0.5. Pooled estimates were obtained for both overall and cause-specific reasons for readmission for all reasons reported in at least 3 studies. Small-study or publication bias was assessed using funnel plot asymmetry when at least 5 studies were analyzed as recommended.24 The meta-analytic findings for both overall and cause-specific readmission are presented as pooled proportions with 95% confidence intervals (CIs). All meta-analyses were performed using Stata 10.0.
Results
Fifteen unique TJA studies (12 THA, 10 TKA) met the criteria for the meta-analysis.20,25-38Figure 1 depicts the PRISMA flowchart for study identification.22
Of the 12 studies eligible for the THA analysis (Table 2), 6 were conducted in the United States,20,26,27,30,33,34 5 in Europe,25,28,29,32,35 and 1 in Canada.31 Seven of the 12 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (2 reported rates at both follow-ups). We analyzed a total of 113,396 patients at the 30-day window and 192,380 patients at the 90-day window. Mean age was 74.2 years. The included studies were variable and sparse in their reporting of specific characteristics (Table 3).
Of the 10 studies (2 prospective, 8 retrospective) eligible for the TKA analysis (Table 4), 6 were conducted in the United States,20,26,27,34,36,37 3 in Europe,25,29,35 and 1 in Asia.38 Four of the 10 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (1 reported rates at both follow-ups).27 We analyzed a total of 3,278,635 patients at the 30-day window and 272,419 patients at the 90-day window. Mean age was 74.3 years. The included studies were quite variable and sparse in their reporting of specific characteristics (Table 5).
We performed random-effects meta-analyses of all unplanned readmissions at both 30 and 90 days (all I2s > 0.5). Among 5 THA studies that reported overall rates at 30 days,20,27,28,32,33 the estimated overall unplanned rate among the 120,272 index surgeries was 5.6% (95% CI, 3.2%-8.0%). Among 5 THA studies that reported overall rates at 90 days,20,25-27,31 the estimated overall unplanned rate among the 192,380 index surgeries was 7.7% (95% CI, 3.2%-12.2%) (I2 = 1.00). Among 3 TKA studies that reported overall rates at 30 days,27,37,38 the estimated overall unplanned rate among the 3,278,635 index surgeries was 3.3% (95% CI, 0.7%-5.9%). Among 5 TKA studies that reported overall rates at 90 days,20,25-27,36 the estimated overall unplanned rate among the 272,419 index surgeries was 9.7% (95% CI, 7.1%-12.4%) (I2 = 0.97).
30-Day Readmission Rates
The most common reason for readmission 30 days after THA discharge was joint-specific. This reason accounted for 39.3% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.2% (95% CI, 0.0%-4.6%; P < .001; I2 = 1.00) among 4 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 0.8%-2.5%; P < .001; I2 = 0.95) and thromboembolic disease (1.5%; 95% CI, 1.0%-1.9%; P < .001; I2 = 0.95). See Figure 2 for 30-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.1%; P < .001; I2 = 0.94). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported in only 1 study each.
The most common reason for readmission 30 days after TKA discharge was surgical site infection. This reason accounted for 12.1% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.4% (95% CI, 0.3%-0.6%; P < .001; I2 = 0.61) among 3 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.3% of the time. Joint-specific reasons were reported in 2 studies (95% CI, 0.0%-0.8%; P = .259; I2 = 0.94). Thromboembolic disease was reported in 4 studies (95% CI, 0.0%-0.7%; P = .067; I2 = 0.98) (Figure 3). Only these 3 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and “sequelae” were reported in only 1 study each.
90-Day Readmission Rates
Consistent with the 30-day THA results, the most common reason for readmission 90 days after THA discharge was joint-specific. This reason accounted for 31.2% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.4% (95% CI, 0.0%-4.9%; P < .001; I2 = 1.00) among 5 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 1.0%-2.2%; P < .003; I2 = 0.83) and thromboembolic disease (1.0%; 95% CI, 0.7%-1.4%; P < .001; I2 = 0.97). See Figure 4 for 90-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.0%; P < .001; I2 = 0.99). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported by only 1 study each.
Consistent with the 30-day TKA results, the most common reason for readmission 90 days after TKA discharge was surgical site infection. This reason accounted for 9.3% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.9% (95% CI, 0.4%-1.4%; P < .001; I2 = 0.93) among 5 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.7% of the time. Joint-specific reasons were reported in 5 studies (95% CI, 0.2%-1.1%; P =.003; I2 = 0.94). Thromboembolic disease was reported in 7 studies (95% CI, 0.3%-1.1%; P < .001; I2 = 0.97) (Figure 5). Bleeding was reported in 3 studies, with a pooled rate of 0.4% (95% CI, 0.0%-0.9%; P = .128; I2 = 0.83). Cardiac dysrhythmia was reported in 2 studies, with an estimated pooled rate of 0.3% (95% CI, 0.2%-0.5%; P < .001). Only these 5 reasons could be pooled, as pneumonia and “sequelae” were reported in only 1 study each.
Discussion
This study is the first systematic review and meta-analysis of the literature to identify overall and cause-specific readmission rates after TJA.
For THA, 30- and 90-day readmission rates were 5.6% and 7.7%, respectively. Joint-specific causes were the most common reason for readmission at both 30 and 90 days after THA. For TKA, 30- and 90-day rates were 3.3% and 9.7%, respectively. Surgical site infection was the most common reason for readmission at both 30 and 90 days after TKA.
Hospital readmissions are an important area of scrutiny for Medicare and the health care systems broadly. Readmissions after surgery are deemed quality indicators potentially suggesting incomplete management of active issues and inadequate preparation for discharge.39 Unplanned readmissions also place a significant economic burden on Medicare: $17.5 billion in 2010.40 Given their association with quality of overall surgical care, improved readmission rates have the potential to improve the standard of care and reduce costs.
Higher readmission rates will significantly affect hospitals as CMS shifts to bundling payments for acute-care episodes, such as TJA.41-43 Further, private and public health care payers are increasingly using unplanned 30- and 90-day readmission rates as a marker of quality of care. However, there is little agreement about readmission rates and reasons, let alone what follow-up window should be used to define orthopedic readmissions. One study involving the MEDPAR (Medicare Provider Analysis and Review) database found that a common reason for readmission after major hip or knee surgery was “aftercare” for surgical sequelae (10.3%)15; another study found a 15% increase in post-THA hospitalizations, most commonly for a mechanical complication (joint-related).44 There are no prior complete systematic reviews or meta-analyses of overall rates of readmissions after primary unilateral TJAs, or of the reasons for these readmissions. The closest such report, the Yale report to CMS, was skewed to a proportion of US hospitals treating a population prone to significant comorbidities.20
Although the strength of this study lies in its rigorous identification and extraction of data, notable clarifications must be made when synthesizing the information. First, the definitions of various thromboembolic events varied greatly. Some studies reported deep vein thrombosis (DVT) and pulmonary embolism (PE) separately, whereas others reported only DVT or only PE. Some studies reported rates of readmission for “thromboembolic disorder,” and one25 reported rates for DVT, PE, and thromboembolic disorder. To pool these related events, we created a composite definition that included DVT, PE, and thromboembolic disorders, which we termed thromboembolic disease. We also created a composite measure for joint-specific reasons for readmission. This category included joint infection that definitely required reentry into the joint, but using this category may have led to underestimation of surgical site infection rates, which were defined separately. Third, there was significant variation in documentation of surgical site infection among the studies included in this review. Some studies specified superficial wounds, whereas others did not categorize complications as superficial, deep, or intracapsular, which would qualify as a “joint-specific” cause. Despite this variation, surgical site infection after TJA was found to be the most common reason for readmission.
Our systematic review and meta-analysis were limited, as any others are, by the quality of studies investigated. Few studies reported cause-specific rates and reasons for readmission. Given the small sample, formal tests for small-study or publication bias could not be performed. Some studies included tremendous amounts of data, and International Classification of Diseases, Ninth Revision (ICD-9) codes were used without physician review of readmission diagnoses. In the absence of oversight, many readmissions could have been misinterpreted and incorrectly logged, or simply miscoded. Saucedo and colleagues27,45 found that readmission diagnostic codes were often unverified. Numerous other studies corroborated this lack of correlation with physician-derived readmission diagnoses in just 25% of cases.46-54 Another study limitation is the unknown number of patients who had TJA but presented and were subsequently readmitted to a different hospital. Last, as this review included patients who had surgery performed within a 30-year period, it could not address the shifts in postoperative management that occurred in that time, particularly with respect to anticoagulation. This limitation was partially addressed in THA by dividing final studies into 3 decades. Of these studies, only 1 was from the first decade, 3 were from the second, and the rest were from the third. Of the 3 from the second decade, only the study by Warwick and colleagues29 (1995) explicitly did not use anticoagulation, but compression stockings were used, and consequently there was a 4.0% rate of readmission for thromboembolic disease alone, compared with the study by White and colleagues34 (1998), which explicitly used anticoagulation and boasted a 1.7% rate of readmission for thromboembolic disease. This isolated comparison illustrates the effect of routine anticoagulation and the changes in surgical standards over the 3 decades.
The numbers from this systematic review and meta-analysis represent an international benchmark for TJA as a procedure. Knowing the top reasons for readmission will lead to more focus on joint-related and medical issues (surgical site infection, thromboembolic disease) before discharge to avoid readmission after elective unilateral primary TJA. Although readmission rates have received attention in the United States as a primary means of combating soaring health care costs, knowing the rates for a common procedure applies broadly as an indicator for standard of care worldwide, according to the World Health Organization.55 This study is the first systematic review and meta-analysis of documented readmission rates and reasons for readmission to identify overall and cause-specific rates after TJA. The hope is that our findings will add clarity to the literature and help guide the decisions of physicians and policymakers.
Conclusion
Readmission rates are an increasingly important metric in the United States and around the world, yet there is no consensus regarding overall readmission rates and reasons for readmission after primary unilateral TJAs. Our systematic review and meta-analysis of the literature found overall unplanned readmission rates of 5.6% (30 days) and 7.7% (90 days) for THA and 3.3% (30 days) and 9.7% (90 days) for TKA. At both 30 and 90 days, the most common readmission reasons were joint-specific (THA) and surgical site infection (TKA). New investigations should be directed toward developing countermeasures to lower the rates of readmission.
Total joint arthroplasty (TJA) is a clinically effective, cost-effective treatment for symptomatic arthritis.1,2 After TJA, patients report reduced pain, restored range of motion, high satisfaction, and ability to return to a more active lifestyle.3-7 The number of total hip arthroplasties (THAs) performed in the United States is expected to reach 572,000 by 2030, a 174% increase, and the number of total knee arthroplasties (TKAs) 3.5 million, nearly a 7-fold increase.8,9 Since 2005, the cost of THA has risen more than 4 times, to $13.43 billion, and the cost of TKA has risen more than 5 times, to $40.8 billion.8,9 Given the demand and price tag, TJA is the single largest cost in the Medicare budget.10
Given its potential to improve care and reduce costs, reducing readmission rates in the surgical setting is a priority for physicians and policymakers.11 Readmissions for TJA are highly scrutinized as a performance indicator—the Centers for Medicare & Medicaid Services (CMS) started including them in its readmissions penalty program in 2013—and were recently validated as a measure of surgical quality.12-14 Accurate assessments of readmissions after TJA are unclear, with rates ranging from 1% to 8.5% between 7 and 90 days after surgery.2,15-17 The early success of TJA as an elective (and more frequently outpatient) procedure has paradoxically translated to less tolerance for readmissions. Post-TJA complications resulting in readmission are subject to financial penalties, and there is an implicit judgment of inadequate surgical management.12
Not only is the readmission rate poorly characterized, but there is no consensus on the leading reasons for readmissions after primary elective unilateral TJAs. The range of rates, reasons, and follow-up periods reported in the literature is wide.18,19 CMS plans to monitor readmissions over 7 to 90 days after surgery (the period depends on the complication), whereas a significant portion of the orthopedic literature documents 90-day rates.19 In 2012, the Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation prepared for CMS a comprehensive report identifying rates of post-TJA complications and readmissions.20 The report, however, is limited to US hospitals and Medicare patients and therefore may overstate the rates, given this population’s documented comorbidities and the reimbursement variations between Medicare and commercial insurance.21 Lack of consensus on readmissions after primary elective unilateral TJAs requires that we synthesize available data to answer several questions: What is the overall readmission rate 30 and 90 days after TJA? What are the primary reasons for readmission 30 and 90 days after TJA? What are the cause-specific readmission rates? We performed a systematic review and a meta-analysis to answer these questions and to add clarity to the literature in order to help guide policy.
Materials and Methods
We performed a systematic review in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.22 Two reviewers independently completed structured searches of the Medline and Cochrane Central Register of Controlled Trials databases. Search terms were: (total hip replacement OR hip arthroplasty OR total hip arthroplasty OR total knee replacement OR knee arthroplasty OR total knee arthroplasty) AND (readmission OR complication OR discharge). They updated the search June 1, 2013. Four limits were applied: publication between January 1, 1982 and December 12, 2012; human subjects only; age 19+ years; and English-language articles. Study eligibility was determined by using standardized criteria as defined by the inclusion and exclusion criteria described in 3 stages: title review, abstract review, and full-article review. The reviewers also performed ancestry searches, including searches for major review articles and bibliographies of all retrieved studies, to identify additional studies not identified in the keyword searches. Discrepancies were resolved by author consensus.
Inclusion criteria were original studies that presented level I to III evidence and that were identified in structured online searches; published in English between January 1, 1982 and December 31, 2012; involved patients older than 19 years; and reported both readmission rates and reasons at follow-up 30 or 90 days after elective primary unilateral TJA, regardless of indication. Exclusion criteria were studies that reported data from hip fracture, knee fracture, and pelvis fracture cases; those that reported data from hemiarthroplasty, Birmingham hip resurfacing procedures, other resurfacing procedures, simultaneous bilateral hip or knee arthroplasties, unicompartmental knee arthroplasty, patellofemoral arthroplasty, metastatic or bone cancer, or revision hip or knee arthroplasty; those that did not report extractable reasons for readmission; those that reported complications but did not specify readmission rates; and those that reported readmission data only from after the 90-day follow-up window. In cases in which multiple studies reported data from the same patient population, only the largest or most recent report was used.
Two reviewers extracted the quantitative data from eligible studies. The 2 primary outcomes of interests were all-cause readmission rates, and reasons for readmission 30 and 90 days after TJA. Other extracted data were evidence level; publication journal, year, and country; data source (academic institution, Medicare); study design; number of patients; patient characteristics; surgical approach; follow-up period; overall readmission rate; anticoagulant use; tourniquet use; and compression stocking use. In addition, all post-TJA readmissions were assumed to be unplanned, except for staged sequential bilateral arthroplasty for osteoarthritis (excluded from analysis).
Readmission reasons were divided into 4 major categories as defined by the literature and the authors: thromboembolic disease, joint-specific reasons, surgical site infection, and surgical sequelae. The diagnoses in these categories are listed in Table 1. Other extracted reasons were cardiac dysrhythmia and pneumonia.
In cases in which there were at least 2 comparable studies, a meta-analysis was performed to obtain pooled estimates of the proportion of patients readmitted at 30 or 90 days. We calculated a Higgins I2 measure for between-study heterogeneity and random-effects analysis, using the method of DerSimonian and Laird23 if I2 was greater than 0.5. Pooled estimates were obtained for both overall and cause-specific reasons for readmission for all reasons reported in at least 3 studies. Small-study or publication bias was assessed using funnel plot asymmetry when at least 5 studies were analyzed as recommended.24 The meta-analytic findings for both overall and cause-specific readmission are presented as pooled proportions with 95% confidence intervals (CIs). All meta-analyses were performed using Stata 10.0.
Results
Fifteen unique TJA studies (12 THA, 10 TKA) met the criteria for the meta-analysis.20,25-38Figure 1 depicts the PRISMA flowchart for study identification.22
Of the 12 studies eligible for the THA analysis (Table 2), 6 were conducted in the United States,20,26,27,30,33,34 5 in Europe,25,28,29,32,35 and 1 in Canada.31 Seven of the 12 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (2 reported rates at both follow-ups). We analyzed a total of 113,396 patients at the 30-day window and 192,380 patients at the 90-day window. Mean age was 74.2 years. The included studies were variable and sparse in their reporting of specific characteristics (Table 3).
Of the 10 studies (2 prospective, 8 retrospective) eligible for the TKA analysis (Table 4), 6 were conducted in the United States,20,26,27,34,36,37 3 in Europe,25,29,35 and 1 in Asia.38 Four of the 10 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (1 reported rates at both follow-ups).27 We analyzed a total of 3,278,635 patients at the 30-day window and 272,419 patients at the 90-day window. Mean age was 74.3 years. The included studies were quite variable and sparse in their reporting of specific characteristics (Table 5).
We performed random-effects meta-analyses of all unplanned readmissions at both 30 and 90 days (all I2s > 0.5). Among 5 THA studies that reported overall rates at 30 days,20,27,28,32,33 the estimated overall unplanned rate among the 120,272 index surgeries was 5.6% (95% CI, 3.2%-8.0%). Among 5 THA studies that reported overall rates at 90 days,20,25-27,31 the estimated overall unplanned rate among the 192,380 index surgeries was 7.7% (95% CI, 3.2%-12.2%) (I2 = 1.00). Among 3 TKA studies that reported overall rates at 30 days,27,37,38 the estimated overall unplanned rate among the 3,278,635 index surgeries was 3.3% (95% CI, 0.7%-5.9%). Among 5 TKA studies that reported overall rates at 90 days,20,25-27,36 the estimated overall unplanned rate among the 272,419 index surgeries was 9.7% (95% CI, 7.1%-12.4%) (I2 = 0.97).
30-Day Readmission Rates
The most common reason for readmission 30 days after THA discharge was joint-specific. This reason accounted for 39.3% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.2% (95% CI, 0.0%-4.6%; P < .001; I2 = 1.00) among 4 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 0.8%-2.5%; P < .001; I2 = 0.95) and thromboembolic disease (1.5%; 95% CI, 1.0%-1.9%; P < .001; I2 = 0.95). See Figure 2 for 30-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.1%; P < .001; I2 = 0.94). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported in only 1 study each.
The most common reason for readmission 30 days after TKA discharge was surgical site infection. This reason accounted for 12.1% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.4% (95% CI, 0.3%-0.6%; P < .001; I2 = 0.61) among 3 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.3% of the time. Joint-specific reasons were reported in 2 studies (95% CI, 0.0%-0.8%; P = .259; I2 = 0.94). Thromboembolic disease was reported in 4 studies (95% CI, 0.0%-0.7%; P = .067; I2 = 0.98) (Figure 3). Only these 3 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and “sequelae” were reported in only 1 study each.
90-Day Readmission Rates
Consistent with the 30-day THA results, the most common reason for readmission 90 days after THA discharge was joint-specific. This reason accounted for 31.2% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.4% (95% CI, 0.0%-4.9%; P < .001; I2 = 1.00) among 5 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 1.0%-2.2%; P < .003; I2 = 0.83) and thromboembolic disease (1.0%; 95% CI, 0.7%-1.4%; P < .001; I2 = 0.97). See Figure 4 for 90-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.0%; P < .001; I2 = 0.99). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported by only 1 study each.
Consistent with the 30-day TKA results, the most common reason for readmission 90 days after TKA discharge was surgical site infection. This reason accounted for 9.3% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.9% (95% CI, 0.4%-1.4%; P < .001; I2 = 0.93) among 5 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.7% of the time. Joint-specific reasons were reported in 5 studies (95% CI, 0.2%-1.1%; P =.003; I2 = 0.94). Thromboembolic disease was reported in 7 studies (95% CI, 0.3%-1.1%; P < .001; I2 = 0.97) (Figure 5). Bleeding was reported in 3 studies, with a pooled rate of 0.4% (95% CI, 0.0%-0.9%; P = .128; I2 = 0.83). Cardiac dysrhythmia was reported in 2 studies, with an estimated pooled rate of 0.3% (95% CI, 0.2%-0.5%; P < .001). Only these 5 reasons could be pooled, as pneumonia and “sequelae” were reported in only 1 study each.
Discussion
This study is the first systematic review and meta-analysis of the literature to identify overall and cause-specific readmission rates after TJA.
For THA, 30- and 90-day readmission rates were 5.6% and 7.7%, respectively. Joint-specific causes were the most common reason for readmission at both 30 and 90 days after THA. For TKA, 30- and 90-day rates were 3.3% and 9.7%, respectively. Surgical site infection was the most common reason for readmission at both 30 and 90 days after TKA.
Hospital readmissions are an important area of scrutiny for Medicare and the health care systems broadly. Readmissions after surgery are deemed quality indicators potentially suggesting incomplete management of active issues and inadequate preparation for discharge.39 Unplanned readmissions also place a significant economic burden on Medicare: $17.5 billion in 2010.40 Given their association with quality of overall surgical care, improved readmission rates have the potential to improve the standard of care and reduce costs.
Higher readmission rates will significantly affect hospitals as CMS shifts to bundling payments for acute-care episodes, such as TJA.41-43 Further, private and public health care payers are increasingly using unplanned 30- and 90-day readmission rates as a marker of quality of care. However, there is little agreement about readmission rates and reasons, let alone what follow-up window should be used to define orthopedic readmissions. One study involving the MEDPAR (Medicare Provider Analysis and Review) database found that a common reason for readmission after major hip or knee surgery was “aftercare” for surgical sequelae (10.3%)15; another study found a 15% increase in post-THA hospitalizations, most commonly for a mechanical complication (joint-related).44 There are no prior complete systematic reviews or meta-analyses of overall rates of readmissions after primary unilateral TJAs, or of the reasons for these readmissions. The closest such report, the Yale report to CMS, was skewed to a proportion of US hospitals treating a population prone to significant comorbidities.20
Although the strength of this study lies in its rigorous identification and extraction of data, notable clarifications must be made when synthesizing the information. First, the definitions of various thromboembolic events varied greatly. Some studies reported deep vein thrombosis (DVT) and pulmonary embolism (PE) separately, whereas others reported only DVT or only PE. Some studies reported rates of readmission for “thromboembolic disorder,” and one25 reported rates for DVT, PE, and thromboembolic disorder. To pool these related events, we created a composite definition that included DVT, PE, and thromboembolic disorders, which we termed thromboembolic disease. We also created a composite measure for joint-specific reasons for readmission. This category included joint infection that definitely required reentry into the joint, but using this category may have led to underestimation of surgical site infection rates, which were defined separately. Third, there was significant variation in documentation of surgical site infection among the studies included in this review. Some studies specified superficial wounds, whereas others did not categorize complications as superficial, deep, or intracapsular, which would qualify as a “joint-specific” cause. Despite this variation, surgical site infection after TJA was found to be the most common reason for readmission.
Our systematic review and meta-analysis were limited, as any others are, by the quality of studies investigated. Few studies reported cause-specific rates and reasons for readmission. Given the small sample, formal tests for small-study or publication bias could not be performed. Some studies included tremendous amounts of data, and International Classification of Diseases, Ninth Revision (ICD-9) codes were used without physician review of readmission diagnoses. In the absence of oversight, many readmissions could have been misinterpreted and incorrectly logged, or simply miscoded. Saucedo and colleagues27,45 found that readmission diagnostic codes were often unverified. Numerous other studies corroborated this lack of correlation with physician-derived readmission diagnoses in just 25% of cases.46-54 Another study limitation is the unknown number of patients who had TJA but presented and were subsequently readmitted to a different hospital. Last, as this review included patients who had surgery performed within a 30-year period, it could not address the shifts in postoperative management that occurred in that time, particularly with respect to anticoagulation. This limitation was partially addressed in THA by dividing final studies into 3 decades. Of these studies, only 1 was from the first decade, 3 were from the second, and the rest were from the third. Of the 3 from the second decade, only the study by Warwick and colleagues29 (1995) explicitly did not use anticoagulation, but compression stockings were used, and consequently there was a 4.0% rate of readmission for thromboembolic disease alone, compared with the study by White and colleagues34 (1998), which explicitly used anticoagulation and boasted a 1.7% rate of readmission for thromboembolic disease. This isolated comparison illustrates the effect of routine anticoagulation and the changes in surgical standards over the 3 decades.
The numbers from this systematic review and meta-analysis represent an international benchmark for TJA as a procedure. Knowing the top reasons for readmission will lead to more focus on joint-related and medical issues (surgical site infection, thromboembolic disease) before discharge to avoid readmission after elective unilateral primary TJA. Although readmission rates have received attention in the United States as a primary means of combating soaring health care costs, knowing the rates for a common procedure applies broadly as an indicator for standard of care worldwide, according to the World Health Organization.55 This study is the first systematic review and meta-analysis of documented readmission rates and reasons for readmission to identify overall and cause-specific rates after TJA. The hope is that our findings will add clarity to the literature and help guide the decisions of physicians and policymakers.
Conclusion
Readmission rates are an increasingly important metric in the United States and around the world, yet there is no consensus regarding overall readmission rates and reasons for readmission after primary unilateral TJAs. Our systematic review and meta-analysis of the literature found overall unplanned readmission rates of 5.6% (30 days) and 7.7% (90 days) for THA and 3.3% (30 days) and 9.7% (90 days) for TKA. At both 30 and 90 days, the most common readmission reasons were joint-specific (THA) and surgical site infection (TKA). New investigations should be directed toward developing countermeasures to lower the rates of readmission.
1. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
2. Cram P, Lu X, Kaboli PJ, et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2001. JAMA. 2011;305(15):1560-1567.
3. de Vries LM, Sturkenboom MC, Verhaar JA, Kingma JH, Stricker BH. Complications after hip arthroplasty and the association with hospital procedure volume. Acta Orthop. 2011;82(5):545-552.
4. Mariconda M, Galasso O, Costa GG, Recano P, Cerbasi S. Quality of life and functionality after total hip arthroplasty: a long-term follow-up study. BMC Musculoskelet Disord. 2011;12:222.
5. Zmistowski B, Restrepo C, Hess J, Adibi D, Cangoz S, Parvizi J. Unplanned readmission after total joint arthroplasty: rates, reasons, and risk factors. J Bone Joint Surg Am. 2013;95(20):1869-1876.
6. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533.
7. Mancuso CA, Salvati EA, Johanson NA, Peterson MG, Charlson ME. Patients’ expectations and satisfaction with total hip arthroplasty. J Arthroplasty. 1997;12(4):387-396.
8. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
9. Kurtz SM, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Bozic KJ, Rubash HE, Sculco TP, Berry DJ. An analysis of Medicare payment policy for total joint arthroplasty. J Arthroplasty. 2008;23(6 suppl 1):133-138.
11. Li LT, Mills WL, White DL, et al. Causes and prevalence of unplanned readmissions after colorectal surgery: a systematic review and meta-analysis. J Am Geriatr Soc. 2013;61(7):1175-1181.
12. Readmissions Reduction Program. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed July 27, 2015.
13. Tsai TC, Joynt KE, Orav J, Gawande AA, Jha AK. Variation in surgical readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134-1142.
14. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program [published correction appears in N Engl J Med. 2011;364(16):1582]. N Engl J Med. 2009;360(14):1418-1428.
15. Zmistowski B, Hozack WJ, Parvizi J. Readmission rates after total hip arthroplasty. JAMA. 2011;306(8):825.
16. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
17. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
18. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369.
19. Atkinson JG. Flaws in the Medicare readmission penalty. N Engl J Med. 2012;367(21):2056-2057.
20. Grosso LM, Curtis JP, Lin Z, et al. Hospital-level Risk-Standardized Complication Rate Following Elective Primary Total Hip Arthroplasty (THA) And/Or Total Knee Arthroplasty (TKA): Measure Methodology Report. Report prepared for Centers for Medicare & Medicaid Services. QualityNet website. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1228772504368. Submitted June 25, 2012. Accessed August 4, 2015.
21. Robinson JC. Analysis of Medicare and commercial insurer–paid total knee replacement reveals opportunities for cost reduction. Health Care Incentives Improvement Institute website. http://www.hci3.org/sites/default/files/files/HCI-2012-IssueBrief-L6-2.pdf. Published 2012. Accessed July 27, 2015.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
24. Higgins JP, Thompson SG. Quantifying heterogeniety in a meta-analysis. Stat Med. 2002;21(11):1539-1558.
25. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
26. Keeney JA, Adelani MA, Nunley RM, Clohisy JC, Barrack RL. Assessing readmission databases: how reliable is the information? J Arthroplasty. 2012;27(8 suppl):72-76.e1-e2.
27. Saucedo JM, Marecek GS, Wanke TR, Lee J, Stulberg SD, Puri L. Understanding readmissions after primary total hip and knee arthroplasty: who’s at risk? J Arthroplasty. 2014;29(2):256-260.
28. Seagroatt V, Tan HS, Goldacre M, Bulstrode C, Nugent I, Gill L. Elective total hip replacement: incidence, emergency readmission rate, and postoperative mortality. BMJ. 1991;303(6815):1431-1435.
29. Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement. A series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br. 1995;77(1):6-10.
30. Kreder HJ, Deyo RA, Koepsell T, Swiontkowski MF, Kreuter W. Relationship between the volume of total hip replacements performed by providers and the rates of postoperative complications in the state of Washington. J Bone Joint Surg Am. 1997;79(4):485-494.
31. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
32. Cullen C, Johnson DS, Cook G. Re-admission rates within 28 days of total hip replacement. Ann R Coll Surg Engl. 2006;88(5):475-478.
33. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
34. White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158(14):1525-1531.
35. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
36. Berger RA, Kusuma SK, Sanders SA, Thill ES, Sporer SM. The feasibility and perioperative complications of outpatient knee arthroplasty. Clin Orthop Relat Res. 2009;467(6):1443-1449.
37. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
38. Seah VW, Singh G, Yang KY, Yeo SJ, Lo NN, Seow KH. Thirty-day mortality and morbidity after total knee arthroplasty. Ann Acad Med Singapore. 2007;36(12):1010-1012.
39. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
40. The Revolving Door: A Report on U.S. Hospital Readmissions. An Analysis of Medicare Data by the Dartmouth Atlas Project. Stories From Patients and Health Care Providers by PerryUndem Research & Communication. Robert Wood Johnson Foundation. http://www.rwjf.org/content/dam/farm/reports/reports/2013/rwjf404178. Published February 2013. Accessed July 27, 2015.
41. Riggs RV, Roberts PS, Aronow H, Younan T. Joint replacement and hip fracture readmission rates: impact of discharge destination. PM R. 2010;2(9):806-810.
42. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
43. McCormack R, Michels R, Ramos N, Hutzler L, Slover JD, Bosco JA. Thirty-day readmission rates as a measure of quality: causes of readmission after orthopedic surgeries and accuracy of administrative data. J Healthc Manag. 2013;58(1):64-76.
44. Bohm ER, Dunbar MJ, Frood JJ, Johnson TM, Morris KA. Rehospitalizations, early revisions, infections, and hospital resource use in the first year after hip and knee arthroplasties. J Arthroplasty. 2012;27(2)232-237.
45. Saucedo J, Marecek GS, Lee J, Huminiak L, Stulberg SD, Puri L. How accurately are we coding readmission diagnoses after total joint arthroplasty? J Arthroplasty. 2013;28(7):1076-1079.
46. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
47. Bozic KJ, Chiu VW, Takemoto SK, et al. The validity of using administrative claims data in total joint arthroplasty outcomes research. J Arthroplasty. 2010;25(6 suppl):58-61.
48. Cram P, Ibrahim SA, Lu X, Wolf BR. Impact of alternative coding schemes on incidence rates of key complications after total hip arthroplasty: a risk-adjusted analysis of a national data set. Geriatr Orthop Surg Rehabil. 2012;3(1):17-26.
49. Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981.
50. Cima RR, Lackore KA, Nehring SA, et al. How best to measure surgical quality? Comparison of the Agency for Healthcare Research and Quality Patient Safety Indicators (AHRQ-PSI) and the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) postoperative adverse events at a single institution. Surgery. 2011;150(5):943-949.
51. Steinberg SM, Popa MR, Michalek JA, Bethel MJ, Ellison EC. Comparison of risk adjustment methodologies in surgical quality improvement. Surgery. 2008;144(4):662-667.
52. Baron JA, Barrett J, Katz JN, Liang MH. Total hip arthroplasty: use and select complications in the US Medicare population. Am J Public Health. 1996;86(1):70-72.
53. HCUPnet. Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov. Accessed July 27, 2015.
54. Singh JA. Epidemiology of knee and hip arthroplasty: a systematic review. Open Orthop J. 2011;5:80-85.
55. Parker SG. Do Current Discharge Arrangements From Inpatient Hospital Care for the Elderly Reduce Readmission Rates, the Length of Inpatient Stay or Mortality, or Improve Health Status? Health Evidence Network report. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2005. http://www.euro.who.int/__data/assets/pdf_file/0006/74670/E87542.pdf. Accessed July 27, 2015.
1. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
2. Cram P, Lu X, Kaboli PJ, et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2001. JAMA. 2011;305(15):1560-1567.
3. de Vries LM, Sturkenboom MC, Verhaar JA, Kingma JH, Stricker BH. Complications after hip arthroplasty and the association with hospital procedure volume. Acta Orthop. 2011;82(5):545-552.
4. Mariconda M, Galasso O, Costa GG, Recano P, Cerbasi S. Quality of life and functionality after total hip arthroplasty: a long-term follow-up study. BMC Musculoskelet Disord. 2011;12:222.
5. Zmistowski B, Restrepo C, Hess J, Adibi D, Cangoz S, Parvizi J. Unplanned readmission after total joint arthroplasty: rates, reasons, and risk factors. J Bone Joint Surg Am. 2013;95(20):1869-1876.
6. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533.
7. Mancuso CA, Salvati EA, Johanson NA, Peterson MG, Charlson ME. Patients’ expectations and satisfaction with total hip arthroplasty. J Arthroplasty. 1997;12(4):387-396.
8. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
9. Kurtz SM, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Bozic KJ, Rubash HE, Sculco TP, Berry DJ. An analysis of Medicare payment policy for total joint arthroplasty. J Arthroplasty. 2008;23(6 suppl 1):133-138.
11. Li LT, Mills WL, White DL, et al. Causes and prevalence of unplanned readmissions after colorectal surgery: a systematic review and meta-analysis. J Am Geriatr Soc. 2013;61(7):1175-1181.
12. Readmissions Reduction Program. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed July 27, 2015.
13. Tsai TC, Joynt KE, Orav J, Gawande AA, Jha AK. Variation in surgical readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134-1142.
14. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program [published correction appears in N Engl J Med. 2011;364(16):1582]. N Engl J Med. 2009;360(14):1418-1428.
15. Zmistowski B, Hozack WJ, Parvizi J. Readmission rates after total hip arthroplasty. JAMA. 2011;306(8):825.
16. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
17. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
18. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369.
19. Atkinson JG. Flaws in the Medicare readmission penalty. N Engl J Med. 2012;367(21):2056-2057.
20. Grosso LM, Curtis JP, Lin Z, et al. Hospital-level Risk-Standardized Complication Rate Following Elective Primary Total Hip Arthroplasty (THA) And/Or Total Knee Arthroplasty (TKA): Measure Methodology Report. Report prepared for Centers for Medicare & Medicaid Services. QualityNet website. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1228772504368. Submitted June 25, 2012. Accessed August 4, 2015.
21. Robinson JC. Analysis of Medicare and commercial insurer–paid total knee replacement reveals opportunities for cost reduction. Health Care Incentives Improvement Institute website. http://www.hci3.org/sites/default/files/files/HCI-2012-IssueBrief-L6-2.pdf. Published 2012. Accessed July 27, 2015.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
24. Higgins JP, Thompson SG. Quantifying heterogeniety in a meta-analysis. Stat Med. 2002;21(11):1539-1558.
25. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
26. Keeney JA, Adelani MA, Nunley RM, Clohisy JC, Barrack RL. Assessing readmission databases: how reliable is the information? J Arthroplasty. 2012;27(8 suppl):72-76.e1-e2.
27. Saucedo JM, Marecek GS, Wanke TR, Lee J, Stulberg SD, Puri L. Understanding readmissions after primary total hip and knee arthroplasty: who’s at risk? J Arthroplasty. 2014;29(2):256-260.
28. Seagroatt V, Tan HS, Goldacre M, Bulstrode C, Nugent I, Gill L. Elective total hip replacement: incidence, emergency readmission rate, and postoperative mortality. BMJ. 1991;303(6815):1431-1435.
29. Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement. A series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br. 1995;77(1):6-10.
30. Kreder HJ, Deyo RA, Koepsell T, Swiontkowski MF, Kreuter W. Relationship between the volume of total hip replacements performed by providers and the rates of postoperative complications in the state of Washington. J Bone Joint Surg Am. 1997;79(4):485-494.
31. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
32. Cullen C, Johnson DS, Cook G. Re-admission rates within 28 days of total hip replacement. Ann R Coll Surg Engl. 2006;88(5):475-478.
33. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
34. White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158(14):1525-1531.
35. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
36. Berger RA, Kusuma SK, Sanders SA, Thill ES, Sporer SM. The feasibility and perioperative complications of outpatient knee arthroplasty. Clin Orthop Relat Res. 2009;467(6):1443-1449.
37. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
38. Seah VW, Singh G, Yang KY, Yeo SJ, Lo NN, Seow KH. Thirty-day mortality and morbidity after total knee arthroplasty. Ann Acad Med Singapore. 2007;36(12):1010-1012.
39. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
40. The Revolving Door: A Report on U.S. Hospital Readmissions. An Analysis of Medicare Data by the Dartmouth Atlas Project. Stories From Patients and Health Care Providers by PerryUndem Research & Communication. Robert Wood Johnson Foundation. http://www.rwjf.org/content/dam/farm/reports/reports/2013/rwjf404178. Published February 2013. Accessed July 27, 2015.
41. Riggs RV, Roberts PS, Aronow H, Younan T. Joint replacement and hip fracture readmission rates: impact of discharge destination. PM R. 2010;2(9):806-810.
42. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
43. McCormack R, Michels R, Ramos N, Hutzler L, Slover JD, Bosco JA. Thirty-day readmission rates as a measure of quality: causes of readmission after orthopedic surgeries and accuracy of administrative data. J Healthc Manag. 2013;58(1):64-76.
44. Bohm ER, Dunbar MJ, Frood JJ, Johnson TM, Morris KA. Rehospitalizations, early revisions, infections, and hospital resource use in the first year after hip and knee arthroplasties. J Arthroplasty. 2012;27(2)232-237.
45. Saucedo J, Marecek GS, Lee J, Huminiak L, Stulberg SD, Puri L. How accurately are we coding readmission diagnoses after total joint arthroplasty? J Arthroplasty. 2013;28(7):1076-1079.
46. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
47. Bozic KJ, Chiu VW, Takemoto SK, et al. The validity of using administrative claims data in total joint arthroplasty outcomes research. J Arthroplasty. 2010;25(6 suppl):58-61.
48. Cram P, Ibrahim SA, Lu X, Wolf BR. Impact of alternative coding schemes on incidence rates of key complications after total hip arthroplasty: a risk-adjusted analysis of a national data set. Geriatr Orthop Surg Rehabil. 2012;3(1):17-26.
49. Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981.
50. Cima RR, Lackore KA, Nehring SA, et al. How best to measure surgical quality? Comparison of the Agency for Healthcare Research and Quality Patient Safety Indicators (AHRQ-PSI) and the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) postoperative adverse events at a single institution. Surgery. 2011;150(5):943-949.
51. Steinberg SM, Popa MR, Michalek JA, Bethel MJ, Ellison EC. Comparison of risk adjustment methodologies in surgical quality improvement. Surgery. 2008;144(4):662-667.
52. Baron JA, Barrett J, Katz JN, Liang MH. Total hip arthroplasty: use and select complications in the US Medicare population. Am J Public Health. 1996;86(1):70-72.
53. HCUPnet. Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov. Accessed July 27, 2015.
54. Singh JA. Epidemiology of knee and hip arthroplasty: a systematic review. Open Orthop J. 2011;5:80-85.
55. Parker SG. Do Current Discharge Arrangements From Inpatient Hospital Care for the Elderly Reduce Readmission Rates, the Length of Inpatient Stay or Mortality, or Improve Health Status? Health Evidence Network report. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2005. http://www.euro.who.int/__data/assets/pdf_file/0006/74670/E87542.pdf. Accessed July 27, 2015.