User login
Accelerated Hepatitis A and B Immunization in a Substance Abuse Treatment Program
Homeless individuals and IV drug users are susceptible to hepatitis A, B, and C infections, and co-infection with these diseases may complicate treatment and result in poor medical outcomes.1 Vaccination offers the best protection against hepatitis A and B, particularly among high-risk populations.2,3 Immunization against hepatitis A and B is of even greater importance for patients with hepatitis C, because there is no specific hepatitis C vaccine, and concomitant infections of B with C are damaging to the liver.4
Veterans have a rate of hepatitis C infection that is 3 times that of the general population.5 Some evidence exists that veterans with serious mental illness (SMI) have a higher rate of hepatitis C infection relative to patients without SMI. Co-occurring substance abuse may add another layer of vulnerability to hepatitis C infection, particularly for homeless veterans.5-7
Mental Health and Primary Care Integration
Substance abuse and dual-diagnosis treatment programs (ie, those programs that treat both substance abuse and co-occurring serious mental health problems, such as bipolar disorder, severe major depressive disorder, psychotic disorders, and posttraumatic stress disorder [PTSD]) that have integrated mental health and primary care into their treatment programs may offer a window of opportunity for risk-reducing interventions. These interventions include testing and education of patients regarding infectious diseases, such as viral hepatitis and HIV, and completion of the hepatitis A/B immunization series.
The James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, has demonstrated some limited success in the past with integrating a standard dosing schedule for hepatitis A/B vaccination into its substance abuse treatment program (SATP), though recent evidence points to more promising results using an accelerated regimen as indicated by a high completion rate for hepatitis B vaccination in a methadone clinic.8,9 A relatively low proportion of SATPs in the U.S. provide testing, education, or vaccination for hepatitis A and B, especially considering the public health importance of controlling these diseases in the substance abusing populations.10,11
Related: Combination Pill Approved for HCV
In 1999, a primary care team was added to the alcohol and drug abuse treatment program at JAHVH.In 2005, the nurses in the program began scheduling vaccinations and screening patients for medical and psychiatric issues, pain, hypertension, diabetes, hepatitis C, alcohol use, depression, PTSD, prostate and colorectal cancers.12 Such a multidisciplinary approach provides many treatment advantages for patients and may save lives.13
Even with a multidisciplinary approach, the nurses found it difficult to provide adequate hepatitis A/B immunization within the 3- to 6-week intensive SATP, because standard immunization dosing regimens are spread over 6 months.14 As with all types of immunizations, long dosing schedules may reduce patient adherence and result in inadequate seroprotection.15 Thus, there is a need to provide a completed immunization series in a more expeditious fashion, and an accelerated dosing regimen makes that possible.15,16
Hepatitis A/B Vaccination
Twinrix (GlaxoSmithKline, Brentford, United Kingdom) is a vaccine that provides dual immunization for hepatitis A and B. Whereas the standard vaccination schedule takes 6 months to complete, the accelerated dosing schedule can be used to complete the first 3 doses in less than a month. The accelerated dosing schedule was incorporated into the JAHVH clinic to capture as many patients as possible in the 3- to 6-week time frame: The first dose is administered and followed by a second dose 7 days later. The third dose is administered 21 to 30 days after the first dose. Twelve months after the first dose, a booster dose is given.
After the first 3 accelerated doses, > 98% of patients show a sustained immune response to hepatitis A, and > 63% demonstrate immunity to hepatitis B. If a 12-month booster injection is given, 100% of patients may receive immunity to hepatitis A and > 96% may have immunity to hepatitis B.16 Another study of the combined vaccine showed even greater seroprotection for hepatitis A and B after only 1 month, 100% and 82%, respectively.17
Related: Viral Hepatitis Awareness
This JAHVH retrospective feasibility study describes a risk-reduction program for hepatitis A/B prevention that was implemented within a 3- to 4-week intensive outpatient SATP and a 6-week dual-diagnosis treatment program. The study includes the development and implementation of the program, designed to vaccinate patients using the accelerated Twinrix schedule. To ascertain the feasibility of this vaccination approach, historical medical records were used to describe and examine the vaccination initiation and follow-up rates of the treatment program participants who received the hepatitis A/B immunization series during their intensive SATP.
Study Design
A retrospective review of medical records was conducted for all participants who were admitted to the intensive JAHVH SATP between October 1, 2008, and September 30, 2009. This study was reviewed and approved by the JAHVH research and development committee and its associated University of South Florida institutional review board. Informed consent to participate was not obtained, because the study was retrospective.
Patient Identification and Education
All program participants were offered testing for HIV and hepatitis A, B, and C. Program participants were educated about hepatitis and HIV transmission, as well as about the long-term effects of continued substance abuse on the progression of hepatitis C. Education about hepatitis, HIV, and substance abuse was provided in a group setting by a member of the program’s nursing staff. One-on-one risk education counseling was also provided when requested or otherwise indicated.
Laboratory testing was performed following each participant’s initial physical examination (within 3 to 5 days of program admission), and the nursing staff reviewed the results before vaccination. Explanation of laboratory results and an individualized immunization regimen were provided to each participant. On review of participants’ laboratory results, those with seroconversion of both hepatitis A and B were not given the combined immunization. Participants who had seroconversion of hepatitis A were offered the hepatitis B vaccination series, and vice versa.
Immunization Process
Participants who lacked prior immunization for hepatitis A and B and had no seroconversion of either hepatitis A or B were offered vaccination. Some patients declined vaccination, even though they were eligible. Their reasons were not formally assessed.
Related: Nivolumab Approved for Expanded Indication
Patients who accepted the vaccination were given the accelerated regimen.16 Participants were educated on the importance of compliance with the vaccination series and provided with follow-up immunization dates and a reminder for the 1-year booster vaccine. The immunizations were ordered by the program’s primary care NP and administered by a licensed practical nurse. The nurse who administered the injections took responsibility for scheduling the patients for all their subsequent injections, including the 1-year booster.
Follow-up Care
If the third injection was not completed before discharge, patients were given a follow-up appointment with the nurse if they remained in the JAHVH service area. If they were leaving the area, they were given instructions on how to follow-up at another VA facility to continue their immunization schedule. A note was written in the electronic medical record documenting their abbreviated hepatitis A/B immunization schedule, which could be accessed by other providers at other VA facilities. Patients who did not show up for any follow-up appointments (third injection or the 1-year booster injection) were contacted and reminded about the importance of completing the immunization series and to schedule an appointment.
Statistical Analysis
All data were analyzed using IBM Statistical Package for the Social Sciences (IBM SPSS, Armonk, New York) with a focus on identifying differences between vaccination-eligible patients (n = 269) who did (n = 128) and did not (n = 141) initiate the immunization schedule during the treatment program. Chi-square and Fisher exact tests were used to assess statistical differences in initiation of the immunization schedule related to categoric variables (ie, marital status, race, history of IV drug abuse, cigarette smoking status, housing status, legal status, history of combat, having a psychiatric or medical diagnosis, and program track). Independent sample t tests were used to test for differences between these 2 groups on the continuous variables, including age, number of previous treatment programs, Global Assessment of Functioning score, severity of smoking dependence as measured by the Fagerström Test for Nicotine Dependence, and the Addiction Severity Index scales.18-20
Results
The sample consisted of 284 successive admissions to an intensive outpatient program for veterans with substance use disorders. About one-third of the patients were homeless at the time of admission to the treatment, and 87% required contracted housing while completing treatment for reasons related to lack of housing, transportation, clinical necessity, or a combination of those factors (Table 1). The most common substance problems were alcohol and cocaine dependence, and 21% (n = 59) of the patients acknowledged a history of IV drug use during their initial psychiatric evaluation. Seventy percent were dually diagnosed with some other Axis I disorder, and 40% had a history of serious mental illness. More than one-fourth (n = 77) of the patients admitted to the intensive outpatient SATP were seropositive for hepatitis A, B and/or C, and the most common hepatitis diagnosis was hepatitis C (n = 71).
Accelerated Immunization Regimen
Patients were eligible to receive the accelerated vaccination schedule only if they had no prior immunization for hepatitis A or B and if they had no seroconversion for either hepatitis A or B. Six people had hepatitis B alone, 7 had hepatitis B and C, 1 had hepatitis A and C, and 1 had all 3 (Table 2). Thus, 15 participants were ineligible to receive the accelerated hepatitis A/B immunization. Chi-square, Fisher exact, and independent sample t tests showed that among those who were vaccination-eligible (269), there were no significant differences in any of the demographic or clinical characteristics between those who initiated the vaccination schedule and those who did not. Among those who completed the first 3 vaccine injections, those who received the 1-year booster injection (54) did not differ (on any demographic or clinical variables) from those who did not (58).
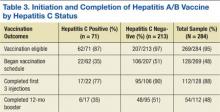
Nearly half (48%) of all the eligible patients admitted to the program began the accelerated immunization schedule for hepatitis A and B. Of those, 88% completed the first 3 injections in the series. Among the patients who received the first 3 injections, 48% received the 1-year booster injection—a 20% completion rate for the vaccination-eligible sample overall (Table 3).
Of the 74 patients who did not complete their vaccinations once initiating the accelerated schedule, the most common reason identified was that the patient moved away (37), or no reason could be identified (33). It was uncommon for a patient not to complete the vaccination schedule because of terminating treatment prematurely (4).
Compared with the vaccine-eligible patients without hepatitis C (207), patients with hepatitis C were less likely to receive any vaccination injections (Table 3). Specifically, 51% of the vaccination-eligible patients who did not have hepatitis C began the vaccination regimen. However, only 22 patients with hepatitis C, or
35% of all vaccination-eligible patients with hepatitis C, began the vaccination regimen. Patients with hepatitis C were also less likely than those without hepatitis C to complete the first 3 injections of the vaccination series once they had initiated it (77%, vs 90%, respectively). This difference continued to be apparent at the time of the 12-month booster injection. Only 35% of vaccine-eligible individuals with hepatitis C received the 12-month booster injection, whereas 51% of vaccination-eligible individuals without hepatitis C received the 12-month booster injection. As with the sample overall, the most common reason patients with hepatitis C did not complete the vaccination regimen was because they moved away (9), followed by no identified reason (5), and premature termination of treatment (2).
Discussion
Individuals abusing alcohol and drugs have an increased vulnerability for infectious diseases, and homeless veterans with substance use disorders may be at a particularly heightened risk.21,22 This study describes a sample of veterans, many were homeless and most were dually diagnosed, in an intensive outpatient SATP that offered an accelerated dosing regimen for hepatitis A and B vaccination. Almost half (48%) of the vaccination-eligible patients began the accelerated regimen for hepatitis A/B vaccination. Moreover, 88% of those who started the vaccination regimen received the first 3 injections of the series, thus possibly conferring substantial immunity to hepatitis A and B and demonstrating the feasibility of an accelerated vaccination schedule in an intensive outpatient SATP.
It is especially important to demonstrate the successful integration of a hepatitis screening and immunization program within a SATP, given that many such programs do not offer screening or immunization for hepatitis, even though substance abusers are disproportionately affected by the disease and contribute greatly to the ongoing hepatitis epidemic.10,11 This study’s results were in line with another study of rapid vaccination for hepatitis B in IV drug users being treated in a methadone clinic, where 83% of the vaccination initiators completed the first 3 injections of the series.9
Unvaccinated Patients
The treatment team in the current study seemed to be less effective at reaching the subset of vaccination-eligible veterans with hepatitis C (almost one-quarter of the sample) in order to administer the accelerated vaccination schedule, as indicated by the lower rate of vaccination initiation as well as a lower rate of completion of the vaccination series among those patients. This replicates a finding from another study that also indicated a low rate of hepatitis A and B vaccination among patients with hepatitis C.23 Only 35% of the vaccination-eligible patients with hepatitis C in the current study initiated the vaccination series, compared with 51% of the patients without hepatitis C. However, the rate of completion of the first 3 injections of the series in the hepatitis C group was respectably high (77%), especially given the high relapse rate and psychosocial instability of individuals with addictive disorders. Initiation seems to be a bigger obstacle than completion of at least the first 3 injections of the vaccination series in both patients with and without hepatitis C.
The study investigators did not formally assess the reasons that more than half the patients in the study did not begin the vaccination series, but anecdotal evidence from the nurses indicated that many patients were afraid of needles. In addition, other patients felt that they simply did not need the vaccination. Some also insisted that they had already had the vaccination despite a blood test showing no evidence for either hepatitis A or B immunization.
Although the nursing team provided group and individual risk-based education as well as information about the effects of continued substance abuse on hepatitis C, it is possible that patients still underestimated their own risk of hepatitis infection and its consequences, or perhaps the information was simply not retained.24
Patient Education
A recent study showed that there is a positive relationship between the amount of hepatitis counseling received and knowledge of hepatitis.25 Possibly, increased intensity of education efforts may make an impact on initiation rates. Encouragingly, there is also evidence that prompting people to predict their future vaccination behavior may increase vaccination initiation rates despite a high-degree of short-term barriers, such as perceived pain or inconvenience.26 A brief intervention to induce people to formulate their future intentions would be relatively easy to incorporate into a vaccination program, and the study team is considering options for this to improve vaccination initiation rates.
Patients can expect to achieve substantial immunity from hepatitis A and, to a lesser degree, hepatitis B after completing the first 3 injections of the series, although the best seroprotection from both is obtained by completing the 12-month booster injection as well.17 Overall, about half of all patients who completed the first 3 injections returned for the booster shot, but only 35% of the patients with hepatitis C did so. The most common known cause of any patient not receiving the booster was movement out of the geographic area. However, much of the time the investigators were unable to determine the reasons patients did not return for the booster shot.
Medication adherence is a difficult problem with vaccination in high-risk samples, although Stitzer and colleagues found a significant improvement in follow-up for a 6-month vaccination protocol by using monetary incentives.27 In addition to ensuring medication adherence, it would also be of value for future immunization efforts to include testing to assess whether seroconversion has occurred once the vaccinations are complete, which is the ultimate measure of the success of a vaccination program. Most patients in the current study did not receive such testing at the completion of their vaccination schedules, and thus, seroconversion rates could not be determined. However, existing studies suggest high rates of seroprotection after the first 3 doses of the combined vaccine.10,17
Limitations
The retrospective nature of the study is its most significant limitation. Any conclusions about the results must be made with caution. However, this design allowed for a naturalistic and potentially generalizable investigation into the application of a vaccination program in a real-world treatment setting. As such, the investigators were able to demonstrate the feasibility of conducting a rapid vaccination program within a 3- to 6-week SATP.
The retrospective nature of the study also limited a full investigation into the reasons behind the lack of vaccination initiation and vaccination noncompletion among the study’s treatment population, especially with regard to the follow-up booster injection. Initial statistical comparisons of initiators and noninitiators and completers and noncompleters showed no significant statistical differences between the groups. Future prospective designs should take into account the need to successfully initiate and complete vaccinations for all eligible patients and include assessment measures to determine the specific reasons that patients did not initiate or complete their vaccinations.
Conclusions
Many patients began and completed the accelerated vaccination schedule for hepatitis A and B in the context of a 3- to 6-week SATP at JAHVH. The overall vaccination rate, including the 12-month booster injection, was one-fifth of the entire vaccination-eligible sample. Additionally, 88% of the vaccination-eligible patients who began the vaccination schedule (or 42% of the whole sample) completed at least the first 3 doses, which may confer substantial immunity from hepatitis A and B. For reasons not entirely clear, a little less than half the vaccination-eligible patients began the vaccination schedule, and only about 50% of those returned to receive their 12-month booster injection. Future prospective studies may be able to determine barriers to both the initiation of and adherence to the vaccination protocol.
The results of this study are also a testament to having primary care nursing staff available and actively involved in the care of patients in a SATP. It seems likely that additional interventions might be needed for outreach to and retention of patients in need of vaccination for hepatitis A and B, and particularly those patients with hepatitis C. It is important to find ways to increase the rates of 12-month booster vaccinations, both for veterans who continue to receive services at JAHVH and for those who transfer care to other VA facilities. Finally, testing to confirm serologic immunity to hepatitis A and hepatitis B would be the next step in the effort to eliminate the risk of hepatitis A and hepatitis B and minimize additional harm for those with hepatitis C in the population receiving treatment for addictive disorders.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Nyamathi A, Liu Y, Marfisee M, et al. Effects of a nurse-managed program on hepatitis A and B vaccine completion among homeless adults. Nurs Res. 2009;58(1):13-22.
2. Center for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2006;55(RR16):1-25.
3. Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2006;55(RR07):1-23.
4. Weltman MD, Brotodihardjo A, Crewe EB, et al. Coinfection with hepatitis B and C or B, C and delta viruses results in severe chronic liver disease and responds poorly to interferon-alpha treatment. J Viral Hepat. 1995;2(1):39-45.
5. Groessl EJ, Weingart KR, Kaplan RM, et al. Living with hepatitis C: qualitative interviews with hepatitis C-infected veterans. J Gen Intern Med. 2008;23(12):1959-1965.
6. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
7. Himeloch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50(1):30-37.
8. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
9. Ramasamy P, Lintzeris N, Sutton Y, Taylor H, Day CA, Haber PS. The outcome of a rapid hepatitis B vaccination programme in a methadone treatment clinic. Addiction. 2010;105(2):329-334.
10. Bini EJ, Kritz S, Brown LS Jr, et al. Hepatitis B virus and hepatitis C virus services offered by substance abuse treatment programs in the United States. J Subst Abuse Treat. 2012;42(4):438-445.
11. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1-39.
12. Francis E, Gonzales-Nolas CL, Markowitz J, Phillips S. Integration of preventive health screening into mental health clinics. Fed Pract. 2008;25(2):39-50.
13. Vreeland B. Bridging the gap between mental and physical health: a multidisciplinary approach. J Clin Psychiatry. 2007;68(suppl 4):26-33.
14. Brim N, Zaller N, Taylor LE, Feller E. Twinrix vaccination schedules among injecting drug users. Expert Opin Biol Ther. 2007;7(3):379-389.
15. Zuckerman J. The place of accelerated schedules for hepatitis A and B vaccinations. Drugs. 2003;63(17):1779-1784.
16. Connor BA, Blatter MM, Beran J, Zou B, Trofa AF. Rapid and sustained immune response against hepatitis A and B achieved with combined vaccine using an accelerated administration schedule. J Travel Med. 2007;14(1):9-15.
17. Nothdurft HD, Dietrich M, Zuckerman JN, et al. A new accelerated vaccination schedule for rapid protection against hepatitis A and B. Vaccine. 2002;20(7-8):1157-1162.
18. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
19. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127.
20. McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199-213.
21. Batki SL, Nathan KI. HIV/AIDS and Hepatitis C. In: Galanter M, Kleber HD, Brady KT, eds. The American Psychiatric Publishing Textbook of Substance Abuse Treatment. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015.
22. Gelberg L, Robertson MJ, Leake B, et al. Hepatitis B among homeless and other impoverished US military veterans in residential care in Los Angeles. Public Health. 2001;115(4):286-291.
23. Felsen UR, Fishbein DA, Litwin AH. Low rates of hepatitis A and B vaccination in patients with chronic hepatitis C at an urban methadone maintenance program. J Addict Dis. 2010;29(4):461-465.
24. Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26(2):136-145.
25. Soto-Salgado M, Suárez E, Ortiz AP, et al. Knowledge of viral hepatitis among Puerto Rican adults: implications for prevention. J Community Health. 2011;36(4):565-573.
26. Cox AD, Cox D, Cyrier R, Graham-Dotson Y, Zimet GD. Can self-prediction overcome barriers to hepatitis B vaccination? A randomized controlled trial. Health Psychol. 2012;31(1):97-105.
27. Stitzer ML, Polk T, Bowles S, Kosten T. Drug users’ adherence to a 6-month vaccination protocol: effects of motivational incentives. Drug Alcohol Depend. 2010;107(1):76-79.
Homeless individuals and IV drug users are susceptible to hepatitis A, B, and C infections, and co-infection with these diseases may complicate treatment and result in poor medical outcomes.1 Vaccination offers the best protection against hepatitis A and B, particularly among high-risk populations.2,3 Immunization against hepatitis A and B is of even greater importance for patients with hepatitis C, because there is no specific hepatitis C vaccine, and concomitant infections of B with C are damaging to the liver.4
Veterans have a rate of hepatitis C infection that is 3 times that of the general population.5 Some evidence exists that veterans with serious mental illness (SMI) have a higher rate of hepatitis C infection relative to patients without SMI. Co-occurring substance abuse may add another layer of vulnerability to hepatitis C infection, particularly for homeless veterans.5-7
Mental Health and Primary Care Integration
Substance abuse and dual-diagnosis treatment programs (ie, those programs that treat both substance abuse and co-occurring serious mental health problems, such as bipolar disorder, severe major depressive disorder, psychotic disorders, and posttraumatic stress disorder [PTSD]) that have integrated mental health and primary care into their treatment programs may offer a window of opportunity for risk-reducing interventions. These interventions include testing and education of patients regarding infectious diseases, such as viral hepatitis and HIV, and completion of the hepatitis A/B immunization series.
The James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, has demonstrated some limited success in the past with integrating a standard dosing schedule for hepatitis A/B vaccination into its substance abuse treatment program (SATP), though recent evidence points to more promising results using an accelerated regimen as indicated by a high completion rate for hepatitis B vaccination in a methadone clinic.8,9 A relatively low proportion of SATPs in the U.S. provide testing, education, or vaccination for hepatitis A and B, especially considering the public health importance of controlling these diseases in the substance abusing populations.10,11
Related: Combination Pill Approved for HCV
In 1999, a primary care team was added to the alcohol and drug abuse treatment program at JAHVH.In 2005, the nurses in the program began scheduling vaccinations and screening patients for medical and psychiatric issues, pain, hypertension, diabetes, hepatitis C, alcohol use, depression, PTSD, prostate and colorectal cancers.12 Such a multidisciplinary approach provides many treatment advantages for patients and may save lives.13
Even with a multidisciplinary approach, the nurses found it difficult to provide adequate hepatitis A/B immunization within the 3- to 6-week intensive SATP, because standard immunization dosing regimens are spread over 6 months.14 As with all types of immunizations, long dosing schedules may reduce patient adherence and result in inadequate seroprotection.15 Thus, there is a need to provide a completed immunization series in a more expeditious fashion, and an accelerated dosing regimen makes that possible.15,16
Hepatitis A/B Vaccination
Twinrix (GlaxoSmithKline, Brentford, United Kingdom) is a vaccine that provides dual immunization for hepatitis A and B. Whereas the standard vaccination schedule takes 6 months to complete, the accelerated dosing schedule can be used to complete the first 3 doses in less than a month. The accelerated dosing schedule was incorporated into the JAHVH clinic to capture as many patients as possible in the 3- to 6-week time frame: The first dose is administered and followed by a second dose 7 days later. The third dose is administered 21 to 30 days after the first dose. Twelve months after the first dose, a booster dose is given.
After the first 3 accelerated doses, > 98% of patients show a sustained immune response to hepatitis A, and > 63% demonstrate immunity to hepatitis B. If a 12-month booster injection is given, 100% of patients may receive immunity to hepatitis A and > 96% may have immunity to hepatitis B.16 Another study of the combined vaccine showed even greater seroprotection for hepatitis A and B after only 1 month, 100% and 82%, respectively.17
Related: Viral Hepatitis Awareness
This JAHVH retrospective feasibility study describes a risk-reduction program for hepatitis A/B prevention that was implemented within a 3- to 4-week intensive outpatient SATP and a 6-week dual-diagnosis treatment program. The study includes the development and implementation of the program, designed to vaccinate patients using the accelerated Twinrix schedule. To ascertain the feasibility of this vaccination approach, historical medical records were used to describe and examine the vaccination initiation and follow-up rates of the treatment program participants who received the hepatitis A/B immunization series during their intensive SATP.
Study Design
A retrospective review of medical records was conducted for all participants who were admitted to the intensive JAHVH SATP between October 1, 2008, and September 30, 2009. This study was reviewed and approved by the JAHVH research and development committee and its associated University of South Florida institutional review board. Informed consent to participate was not obtained, because the study was retrospective.
Patient Identification and Education
All program participants were offered testing for HIV and hepatitis A, B, and C. Program participants were educated about hepatitis and HIV transmission, as well as about the long-term effects of continued substance abuse on the progression of hepatitis C. Education about hepatitis, HIV, and substance abuse was provided in a group setting by a member of the program’s nursing staff. One-on-one risk education counseling was also provided when requested or otherwise indicated.
Laboratory testing was performed following each participant’s initial physical examination (within 3 to 5 days of program admission), and the nursing staff reviewed the results before vaccination. Explanation of laboratory results and an individualized immunization regimen were provided to each participant. On review of participants’ laboratory results, those with seroconversion of both hepatitis A and B were not given the combined immunization. Participants who had seroconversion of hepatitis A were offered the hepatitis B vaccination series, and vice versa.
Immunization Process
Participants who lacked prior immunization for hepatitis A and B and had no seroconversion of either hepatitis A or B were offered vaccination. Some patients declined vaccination, even though they were eligible. Their reasons were not formally assessed.
Related: Nivolumab Approved for Expanded Indication
Patients who accepted the vaccination were given the accelerated regimen.16 Participants were educated on the importance of compliance with the vaccination series and provided with follow-up immunization dates and a reminder for the 1-year booster vaccine. The immunizations were ordered by the program’s primary care NP and administered by a licensed practical nurse. The nurse who administered the injections took responsibility for scheduling the patients for all their subsequent injections, including the 1-year booster.
Follow-up Care
If the third injection was not completed before discharge, patients were given a follow-up appointment with the nurse if they remained in the JAHVH service area. If they were leaving the area, they were given instructions on how to follow-up at another VA facility to continue their immunization schedule. A note was written in the electronic medical record documenting their abbreviated hepatitis A/B immunization schedule, which could be accessed by other providers at other VA facilities. Patients who did not show up for any follow-up appointments (third injection or the 1-year booster injection) were contacted and reminded about the importance of completing the immunization series and to schedule an appointment.
Statistical Analysis
All data were analyzed using IBM Statistical Package for the Social Sciences (IBM SPSS, Armonk, New York) with a focus on identifying differences between vaccination-eligible patients (n = 269) who did (n = 128) and did not (n = 141) initiate the immunization schedule during the treatment program. Chi-square and Fisher exact tests were used to assess statistical differences in initiation of the immunization schedule related to categoric variables (ie, marital status, race, history of IV drug abuse, cigarette smoking status, housing status, legal status, history of combat, having a psychiatric or medical diagnosis, and program track). Independent sample t tests were used to test for differences between these 2 groups on the continuous variables, including age, number of previous treatment programs, Global Assessment of Functioning score, severity of smoking dependence as measured by the Fagerström Test for Nicotine Dependence, and the Addiction Severity Index scales.18-20
Results
The sample consisted of 284 successive admissions to an intensive outpatient program for veterans with substance use disorders. About one-third of the patients were homeless at the time of admission to the treatment, and 87% required contracted housing while completing treatment for reasons related to lack of housing, transportation, clinical necessity, or a combination of those factors (Table 1). The most common substance problems were alcohol and cocaine dependence, and 21% (n = 59) of the patients acknowledged a history of IV drug use during their initial psychiatric evaluation. Seventy percent were dually diagnosed with some other Axis I disorder, and 40% had a history of serious mental illness. More than one-fourth (n = 77) of the patients admitted to the intensive outpatient SATP were seropositive for hepatitis A, B and/or C, and the most common hepatitis diagnosis was hepatitis C (n = 71).
Accelerated Immunization Regimen
Patients were eligible to receive the accelerated vaccination schedule only if they had no prior immunization for hepatitis A or B and if they had no seroconversion for either hepatitis A or B. Six people had hepatitis B alone, 7 had hepatitis B and C, 1 had hepatitis A and C, and 1 had all 3 (Table 2). Thus, 15 participants were ineligible to receive the accelerated hepatitis A/B immunization. Chi-square, Fisher exact, and independent sample t tests showed that among those who were vaccination-eligible (269), there were no significant differences in any of the demographic or clinical characteristics between those who initiated the vaccination schedule and those who did not. Among those who completed the first 3 vaccine injections, those who received the 1-year booster injection (54) did not differ (on any demographic or clinical variables) from those who did not (58).
Nearly half (48%) of all the eligible patients admitted to the program began the accelerated immunization schedule for hepatitis A and B. Of those, 88% completed the first 3 injections in the series. Among the patients who received the first 3 injections, 48% received the 1-year booster injection—a 20% completion rate for the vaccination-eligible sample overall (Table 3).
Of the 74 patients who did not complete their vaccinations once initiating the accelerated schedule, the most common reason identified was that the patient moved away (37), or no reason could be identified (33). It was uncommon for a patient not to complete the vaccination schedule because of terminating treatment prematurely (4).
Compared with the vaccine-eligible patients without hepatitis C (207), patients with hepatitis C were less likely to receive any vaccination injections (Table 3). Specifically, 51% of the vaccination-eligible patients who did not have hepatitis C began the vaccination regimen. However, only 22 patients with hepatitis C, or
35% of all vaccination-eligible patients with hepatitis C, began the vaccination regimen. Patients with hepatitis C were also less likely than those without hepatitis C to complete the first 3 injections of the vaccination series once they had initiated it (77%, vs 90%, respectively). This difference continued to be apparent at the time of the 12-month booster injection. Only 35% of vaccine-eligible individuals with hepatitis C received the 12-month booster injection, whereas 51% of vaccination-eligible individuals without hepatitis C received the 12-month booster injection. As with the sample overall, the most common reason patients with hepatitis C did not complete the vaccination regimen was because they moved away (9), followed by no identified reason (5), and premature termination of treatment (2).
Discussion
Individuals abusing alcohol and drugs have an increased vulnerability for infectious diseases, and homeless veterans with substance use disorders may be at a particularly heightened risk.21,22 This study describes a sample of veterans, many were homeless and most were dually diagnosed, in an intensive outpatient SATP that offered an accelerated dosing regimen for hepatitis A and B vaccination. Almost half (48%) of the vaccination-eligible patients began the accelerated regimen for hepatitis A/B vaccination. Moreover, 88% of those who started the vaccination regimen received the first 3 injections of the series, thus possibly conferring substantial immunity to hepatitis A and B and demonstrating the feasibility of an accelerated vaccination schedule in an intensive outpatient SATP.
It is especially important to demonstrate the successful integration of a hepatitis screening and immunization program within a SATP, given that many such programs do not offer screening or immunization for hepatitis, even though substance abusers are disproportionately affected by the disease and contribute greatly to the ongoing hepatitis epidemic.10,11 This study’s results were in line with another study of rapid vaccination for hepatitis B in IV drug users being treated in a methadone clinic, where 83% of the vaccination initiators completed the first 3 injections of the series.9
Unvaccinated Patients
The treatment team in the current study seemed to be less effective at reaching the subset of vaccination-eligible veterans with hepatitis C (almost one-quarter of the sample) in order to administer the accelerated vaccination schedule, as indicated by the lower rate of vaccination initiation as well as a lower rate of completion of the vaccination series among those patients. This replicates a finding from another study that also indicated a low rate of hepatitis A and B vaccination among patients with hepatitis C.23 Only 35% of the vaccination-eligible patients with hepatitis C in the current study initiated the vaccination series, compared with 51% of the patients without hepatitis C. However, the rate of completion of the first 3 injections of the series in the hepatitis C group was respectably high (77%), especially given the high relapse rate and psychosocial instability of individuals with addictive disorders. Initiation seems to be a bigger obstacle than completion of at least the first 3 injections of the vaccination series in both patients with and without hepatitis C.
The study investigators did not formally assess the reasons that more than half the patients in the study did not begin the vaccination series, but anecdotal evidence from the nurses indicated that many patients were afraid of needles. In addition, other patients felt that they simply did not need the vaccination. Some also insisted that they had already had the vaccination despite a blood test showing no evidence for either hepatitis A or B immunization.
Although the nursing team provided group and individual risk-based education as well as information about the effects of continued substance abuse on hepatitis C, it is possible that patients still underestimated their own risk of hepatitis infection and its consequences, or perhaps the information was simply not retained.24
Patient Education
A recent study showed that there is a positive relationship between the amount of hepatitis counseling received and knowledge of hepatitis.25 Possibly, increased intensity of education efforts may make an impact on initiation rates. Encouragingly, there is also evidence that prompting people to predict their future vaccination behavior may increase vaccination initiation rates despite a high-degree of short-term barriers, such as perceived pain or inconvenience.26 A brief intervention to induce people to formulate their future intentions would be relatively easy to incorporate into a vaccination program, and the study team is considering options for this to improve vaccination initiation rates.
Patients can expect to achieve substantial immunity from hepatitis A and, to a lesser degree, hepatitis B after completing the first 3 injections of the series, although the best seroprotection from both is obtained by completing the 12-month booster injection as well.17 Overall, about half of all patients who completed the first 3 injections returned for the booster shot, but only 35% of the patients with hepatitis C did so. The most common known cause of any patient not receiving the booster was movement out of the geographic area. However, much of the time the investigators were unable to determine the reasons patients did not return for the booster shot.
Medication adherence is a difficult problem with vaccination in high-risk samples, although Stitzer and colleagues found a significant improvement in follow-up for a 6-month vaccination protocol by using monetary incentives.27 In addition to ensuring medication adherence, it would also be of value for future immunization efforts to include testing to assess whether seroconversion has occurred once the vaccinations are complete, which is the ultimate measure of the success of a vaccination program. Most patients in the current study did not receive such testing at the completion of their vaccination schedules, and thus, seroconversion rates could not be determined. However, existing studies suggest high rates of seroprotection after the first 3 doses of the combined vaccine.10,17
Limitations
The retrospective nature of the study is its most significant limitation. Any conclusions about the results must be made with caution. However, this design allowed for a naturalistic and potentially generalizable investigation into the application of a vaccination program in a real-world treatment setting. As such, the investigators were able to demonstrate the feasibility of conducting a rapid vaccination program within a 3- to 6-week SATP.
The retrospective nature of the study also limited a full investigation into the reasons behind the lack of vaccination initiation and vaccination noncompletion among the study’s treatment population, especially with regard to the follow-up booster injection. Initial statistical comparisons of initiators and noninitiators and completers and noncompleters showed no significant statistical differences between the groups. Future prospective designs should take into account the need to successfully initiate and complete vaccinations for all eligible patients and include assessment measures to determine the specific reasons that patients did not initiate or complete their vaccinations.
Conclusions
Many patients began and completed the accelerated vaccination schedule for hepatitis A and B in the context of a 3- to 6-week SATP at JAHVH. The overall vaccination rate, including the 12-month booster injection, was one-fifth of the entire vaccination-eligible sample. Additionally, 88% of the vaccination-eligible patients who began the vaccination schedule (or 42% of the whole sample) completed at least the first 3 doses, which may confer substantial immunity from hepatitis A and B. For reasons not entirely clear, a little less than half the vaccination-eligible patients began the vaccination schedule, and only about 50% of those returned to receive their 12-month booster injection. Future prospective studies may be able to determine barriers to both the initiation of and adherence to the vaccination protocol.
The results of this study are also a testament to having primary care nursing staff available and actively involved in the care of patients in a SATP. It seems likely that additional interventions might be needed for outreach to and retention of patients in need of vaccination for hepatitis A and B, and particularly those patients with hepatitis C. It is important to find ways to increase the rates of 12-month booster vaccinations, both for veterans who continue to receive services at JAHVH and for those who transfer care to other VA facilities. Finally, testing to confirm serologic immunity to hepatitis A and hepatitis B would be the next step in the effort to eliminate the risk of hepatitis A and hepatitis B and minimize additional harm for those with hepatitis C in the population receiving treatment for addictive disorders.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Homeless individuals and IV drug users are susceptible to hepatitis A, B, and C infections, and co-infection with these diseases may complicate treatment and result in poor medical outcomes.1 Vaccination offers the best protection against hepatitis A and B, particularly among high-risk populations.2,3 Immunization against hepatitis A and B is of even greater importance for patients with hepatitis C, because there is no specific hepatitis C vaccine, and concomitant infections of B with C are damaging to the liver.4
Veterans have a rate of hepatitis C infection that is 3 times that of the general population.5 Some evidence exists that veterans with serious mental illness (SMI) have a higher rate of hepatitis C infection relative to patients without SMI. Co-occurring substance abuse may add another layer of vulnerability to hepatitis C infection, particularly for homeless veterans.5-7
Mental Health and Primary Care Integration
Substance abuse and dual-diagnosis treatment programs (ie, those programs that treat both substance abuse and co-occurring serious mental health problems, such as bipolar disorder, severe major depressive disorder, psychotic disorders, and posttraumatic stress disorder [PTSD]) that have integrated mental health and primary care into their treatment programs may offer a window of opportunity for risk-reducing interventions. These interventions include testing and education of patients regarding infectious diseases, such as viral hepatitis and HIV, and completion of the hepatitis A/B immunization series.
The James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, has demonstrated some limited success in the past with integrating a standard dosing schedule for hepatitis A/B vaccination into its substance abuse treatment program (SATP), though recent evidence points to more promising results using an accelerated regimen as indicated by a high completion rate for hepatitis B vaccination in a methadone clinic.8,9 A relatively low proportion of SATPs in the U.S. provide testing, education, or vaccination for hepatitis A and B, especially considering the public health importance of controlling these diseases in the substance abusing populations.10,11
Related: Combination Pill Approved for HCV
In 1999, a primary care team was added to the alcohol and drug abuse treatment program at JAHVH.In 2005, the nurses in the program began scheduling vaccinations and screening patients for medical and psychiatric issues, pain, hypertension, diabetes, hepatitis C, alcohol use, depression, PTSD, prostate and colorectal cancers.12 Such a multidisciplinary approach provides many treatment advantages for patients and may save lives.13
Even with a multidisciplinary approach, the nurses found it difficult to provide adequate hepatitis A/B immunization within the 3- to 6-week intensive SATP, because standard immunization dosing regimens are spread over 6 months.14 As with all types of immunizations, long dosing schedules may reduce patient adherence and result in inadequate seroprotection.15 Thus, there is a need to provide a completed immunization series in a more expeditious fashion, and an accelerated dosing regimen makes that possible.15,16
Hepatitis A/B Vaccination
Twinrix (GlaxoSmithKline, Brentford, United Kingdom) is a vaccine that provides dual immunization for hepatitis A and B. Whereas the standard vaccination schedule takes 6 months to complete, the accelerated dosing schedule can be used to complete the first 3 doses in less than a month. The accelerated dosing schedule was incorporated into the JAHVH clinic to capture as many patients as possible in the 3- to 6-week time frame: The first dose is administered and followed by a second dose 7 days later. The third dose is administered 21 to 30 days after the first dose. Twelve months after the first dose, a booster dose is given.
After the first 3 accelerated doses, > 98% of patients show a sustained immune response to hepatitis A, and > 63% demonstrate immunity to hepatitis B. If a 12-month booster injection is given, 100% of patients may receive immunity to hepatitis A and > 96% may have immunity to hepatitis B.16 Another study of the combined vaccine showed even greater seroprotection for hepatitis A and B after only 1 month, 100% and 82%, respectively.17
Related: Viral Hepatitis Awareness
This JAHVH retrospective feasibility study describes a risk-reduction program for hepatitis A/B prevention that was implemented within a 3- to 4-week intensive outpatient SATP and a 6-week dual-diagnosis treatment program. The study includes the development and implementation of the program, designed to vaccinate patients using the accelerated Twinrix schedule. To ascertain the feasibility of this vaccination approach, historical medical records were used to describe and examine the vaccination initiation and follow-up rates of the treatment program participants who received the hepatitis A/B immunization series during their intensive SATP.
Study Design
A retrospective review of medical records was conducted for all participants who were admitted to the intensive JAHVH SATP between October 1, 2008, and September 30, 2009. This study was reviewed and approved by the JAHVH research and development committee and its associated University of South Florida institutional review board. Informed consent to participate was not obtained, because the study was retrospective.
Patient Identification and Education
All program participants were offered testing for HIV and hepatitis A, B, and C. Program participants were educated about hepatitis and HIV transmission, as well as about the long-term effects of continued substance abuse on the progression of hepatitis C. Education about hepatitis, HIV, and substance abuse was provided in a group setting by a member of the program’s nursing staff. One-on-one risk education counseling was also provided when requested or otherwise indicated.
Laboratory testing was performed following each participant’s initial physical examination (within 3 to 5 days of program admission), and the nursing staff reviewed the results before vaccination. Explanation of laboratory results and an individualized immunization regimen were provided to each participant. On review of participants’ laboratory results, those with seroconversion of both hepatitis A and B were not given the combined immunization. Participants who had seroconversion of hepatitis A were offered the hepatitis B vaccination series, and vice versa.
Immunization Process
Participants who lacked prior immunization for hepatitis A and B and had no seroconversion of either hepatitis A or B were offered vaccination. Some patients declined vaccination, even though they were eligible. Their reasons were not formally assessed.
Related: Nivolumab Approved for Expanded Indication
Patients who accepted the vaccination were given the accelerated regimen.16 Participants were educated on the importance of compliance with the vaccination series and provided with follow-up immunization dates and a reminder for the 1-year booster vaccine. The immunizations were ordered by the program’s primary care NP and administered by a licensed practical nurse. The nurse who administered the injections took responsibility for scheduling the patients for all their subsequent injections, including the 1-year booster.
Follow-up Care
If the third injection was not completed before discharge, patients were given a follow-up appointment with the nurse if they remained in the JAHVH service area. If they were leaving the area, they were given instructions on how to follow-up at another VA facility to continue their immunization schedule. A note was written in the electronic medical record documenting their abbreviated hepatitis A/B immunization schedule, which could be accessed by other providers at other VA facilities. Patients who did not show up for any follow-up appointments (third injection or the 1-year booster injection) were contacted and reminded about the importance of completing the immunization series and to schedule an appointment.
Statistical Analysis
All data were analyzed using IBM Statistical Package for the Social Sciences (IBM SPSS, Armonk, New York) with a focus on identifying differences between vaccination-eligible patients (n = 269) who did (n = 128) and did not (n = 141) initiate the immunization schedule during the treatment program. Chi-square and Fisher exact tests were used to assess statistical differences in initiation of the immunization schedule related to categoric variables (ie, marital status, race, history of IV drug abuse, cigarette smoking status, housing status, legal status, history of combat, having a psychiatric or medical diagnosis, and program track). Independent sample t tests were used to test for differences between these 2 groups on the continuous variables, including age, number of previous treatment programs, Global Assessment of Functioning score, severity of smoking dependence as measured by the Fagerström Test for Nicotine Dependence, and the Addiction Severity Index scales.18-20
Results
The sample consisted of 284 successive admissions to an intensive outpatient program for veterans with substance use disorders. About one-third of the patients were homeless at the time of admission to the treatment, and 87% required contracted housing while completing treatment for reasons related to lack of housing, transportation, clinical necessity, or a combination of those factors (Table 1). The most common substance problems were alcohol and cocaine dependence, and 21% (n = 59) of the patients acknowledged a history of IV drug use during their initial psychiatric evaluation. Seventy percent were dually diagnosed with some other Axis I disorder, and 40% had a history of serious mental illness. More than one-fourth (n = 77) of the patients admitted to the intensive outpatient SATP were seropositive for hepatitis A, B and/or C, and the most common hepatitis diagnosis was hepatitis C (n = 71).
Accelerated Immunization Regimen
Patients were eligible to receive the accelerated vaccination schedule only if they had no prior immunization for hepatitis A or B and if they had no seroconversion for either hepatitis A or B. Six people had hepatitis B alone, 7 had hepatitis B and C, 1 had hepatitis A and C, and 1 had all 3 (Table 2). Thus, 15 participants were ineligible to receive the accelerated hepatitis A/B immunization. Chi-square, Fisher exact, and independent sample t tests showed that among those who were vaccination-eligible (269), there were no significant differences in any of the demographic or clinical characteristics between those who initiated the vaccination schedule and those who did not. Among those who completed the first 3 vaccine injections, those who received the 1-year booster injection (54) did not differ (on any demographic or clinical variables) from those who did not (58).
Nearly half (48%) of all the eligible patients admitted to the program began the accelerated immunization schedule for hepatitis A and B. Of those, 88% completed the first 3 injections in the series. Among the patients who received the first 3 injections, 48% received the 1-year booster injection—a 20% completion rate for the vaccination-eligible sample overall (Table 3).
Of the 74 patients who did not complete their vaccinations once initiating the accelerated schedule, the most common reason identified was that the patient moved away (37), or no reason could be identified (33). It was uncommon for a patient not to complete the vaccination schedule because of terminating treatment prematurely (4).
Compared with the vaccine-eligible patients without hepatitis C (207), patients with hepatitis C were less likely to receive any vaccination injections (Table 3). Specifically, 51% of the vaccination-eligible patients who did not have hepatitis C began the vaccination regimen. However, only 22 patients with hepatitis C, or
35% of all vaccination-eligible patients with hepatitis C, began the vaccination regimen. Patients with hepatitis C were also less likely than those without hepatitis C to complete the first 3 injections of the vaccination series once they had initiated it (77%, vs 90%, respectively). This difference continued to be apparent at the time of the 12-month booster injection. Only 35% of vaccine-eligible individuals with hepatitis C received the 12-month booster injection, whereas 51% of vaccination-eligible individuals without hepatitis C received the 12-month booster injection. As with the sample overall, the most common reason patients with hepatitis C did not complete the vaccination regimen was because they moved away (9), followed by no identified reason (5), and premature termination of treatment (2).
Discussion
Individuals abusing alcohol and drugs have an increased vulnerability for infectious diseases, and homeless veterans with substance use disorders may be at a particularly heightened risk.21,22 This study describes a sample of veterans, many were homeless and most were dually diagnosed, in an intensive outpatient SATP that offered an accelerated dosing regimen for hepatitis A and B vaccination. Almost half (48%) of the vaccination-eligible patients began the accelerated regimen for hepatitis A/B vaccination. Moreover, 88% of those who started the vaccination regimen received the first 3 injections of the series, thus possibly conferring substantial immunity to hepatitis A and B and demonstrating the feasibility of an accelerated vaccination schedule in an intensive outpatient SATP.
It is especially important to demonstrate the successful integration of a hepatitis screening and immunization program within a SATP, given that many such programs do not offer screening or immunization for hepatitis, even though substance abusers are disproportionately affected by the disease and contribute greatly to the ongoing hepatitis epidemic.10,11 This study’s results were in line with another study of rapid vaccination for hepatitis B in IV drug users being treated in a methadone clinic, where 83% of the vaccination initiators completed the first 3 injections of the series.9
Unvaccinated Patients
The treatment team in the current study seemed to be less effective at reaching the subset of vaccination-eligible veterans with hepatitis C (almost one-quarter of the sample) in order to administer the accelerated vaccination schedule, as indicated by the lower rate of vaccination initiation as well as a lower rate of completion of the vaccination series among those patients. This replicates a finding from another study that also indicated a low rate of hepatitis A and B vaccination among patients with hepatitis C.23 Only 35% of the vaccination-eligible patients with hepatitis C in the current study initiated the vaccination series, compared with 51% of the patients without hepatitis C. However, the rate of completion of the first 3 injections of the series in the hepatitis C group was respectably high (77%), especially given the high relapse rate and psychosocial instability of individuals with addictive disorders. Initiation seems to be a bigger obstacle than completion of at least the first 3 injections of the vaccination series in both patients with and without hepatitis C.
The study investigators did not formally assess the reasons that more than half the patients in the study did not begin the vaccination series, but anecdotal evidence from the nurses indicated that many patients were afraid of needles. In addition, other patients felt that they simply did not need the vaccination. Some also insisted that they had already had the vaccination despite a blood test showing no evidence for either hepatitis A or B immunization.
Although the nursing team provided group and individual risk-based education as well as information about the effects of continued substance abuse on hepatitis C, it is possible that patients still underestimated their own risk of hepatitis infection and its consequences, or perhaps the information was simply not retained.24
Patient Education
A recent study showed that there is a positive relationship between the amount of hepatitis counseling received and knowledge of hepatitis.25 Possibly, increased intensity of education efforts may make an impact on initiation rates. Encouragingly, there is also evidence that prompting people to predict their future vaccination behavior may increase vaccination initiation rates despite a high-degree of short-term barriers, such as perceived pain or inconvenience.26 A brief intervention to induce people to formulate their future intentions would be relatively easy to incorporate into a vaccination program, and the study team is considering options for this to improve vaccination initiation rates.
Patients can expect to achieve substantial immunity from hepatitis A and, to a lesser degree, hepatitis B after completing the first 3 injections of the series, although the best seroprotection from both is obtained by completing the 12-month booster injection as well.17 Overall, about half of all patients who completed the first 3 injections returned for the booster shot, but only 35% of the patients with hepatitis C did so. The most common known cause of any patient not receiving the booster was movement out of the geographic area. However, much of the time the investigators were unable to determine the reasons patients did not return for the booster shot.
Medication adherence is a difficult problem with vaccination in high-risk samples, although Stitzer and colleagues found a significant improvement in follow-up for a 6-month vaccination protocol by using monetary incentives.27 In addition to ensuring medication adherence, it would also be of value for future immunization efforts to include testing to assess whether seroconversion has occurred once the vaccinations are complete, which is the ultimate measure of the success of a vaccination program. Most patients in the current study did not receive such testing at the completion of their vaccination schedules, and thus, seroconversion rates could not be determined. However, existing studies suggest high rates of seroprotection after the first 3 doses of the combined vaccine.10,17
Limitations
The retrospective nature of the study is its most significant limitation. Any conclusions about the results must be made with caution. However, this design allowed for a naturalistic and potentially generalizable investigation into the application of a vaccination program in a real-world treatment setting. As such, the investigators were able to demonstrate the feasibility of conducting a rapid vaccination program within a 3- to 6-week SATP.
The retrospective nature of the study also limited a full investigation into the reasons behind the lack of vaccination initiation and vaccination noncompletion among the study’s treatment population, especially with regard to the follow-up booster injection. Initial statistical comparisons of initiators and noninitiators and completers and noncompleters showed no significant statistical differences between the groups. Future prospective designs should take into account the need to successfully initiate and complete vaccinations for all eligible patients and include assessment measures to determine the specific reasons that patients did not initiate or complete their vaccinations.
Conclusions
Many patients began and completed the accelerated vaccination schedule for hepatitis A and B in the context of a 3- to 6-week SATP at JAHVH. The overall vaccination rate, including the 12-month booster injection, was one-fifth of the entire vaccination-eligible sample. Additionally, 88% of the vaccination-eligible patients who began the vaccination schedule (or 42% of the whole sample) completed at least the first 3 doses, which may confer substantial immunity from hepatitis A and B. For reasons not entirely clear, a little less than half the vaccination-eligible patients began the vaccination schedule, and only about 50% of those returned to receive their 12-month booster injection. Future prospective studies may be able to determine barriers to both the initiation of and adherence to the vaccination protocol.
The results of this study are also a testament to having primary care nursing staff available and actively involved in the care of patients in a SATP. It seems likely that additional interventions might be needed for outreach to and retention of patients in need of vaccination for hepatitis A and B, and particularly those patients with hepatitis C. It is important to find ways to increase the rates of 12-month booster vaccinations, both for veterans who continue to receive services at JAHVH and for those who transfer care to other VA facilities. Finally, testing to confirm serologic immunity to hepatitis A and hepatitis B would be the next step in the effort to eliminate the risk of hepatitis A and hepatitis B and minimize additional harm for those with hepatitis C in the population receiving treatment for addictive disorders.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Nyamathi A, Liu Y, Marfisee M, et al. Effects of a nurse-managed program on hepatitis A and B vaccine completion among homeless adults. Nurs Res. 2009;58(1):13-22.
2. Center for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2006;55(RR16):1-25.
3. Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2006;55(RR07):1-23.
4. Weltman MD, Brotodihardjo A, Crewe EB, et al. Coinfection with hepatitis B and C or B, C and delta viruses results in severe chronic liver disease and responds poorly to interferon-alpha treatment. J Viral Hepat. 1995;2(1):39-45.
5. Groessl EJ, Weingart KR, Kaplan RM, et al. Living with hepatitis C: qualitative interviews with hepatitis C-infected veterans. J Gen Intern Med. 2008;23(12):1959-1965.
6. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
7. Himeloch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50(1):30-37.
8. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
9. Ramasamy P, Lintzeris N, Sutton Y, Taylor H, Day CA, Haber PS. The outcome of a rapid hepatitis B vaccination programme in a methadone treatment clinic. Addiction. 2010;105(2):329-334.
10. Bini EJ, Kritz S, Brown LS Jr, et al. Hepatitis B virus and hepatitis C virus services offered by substance abuse treatment programs in the United States. J Subst Abuse Treat. 2012;42(4):438-445.
11. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1-39.
12. Francis E, Gonzales-Nolas CL, Markowitz J, Phillips S. Integration of preventive health screening into mental health clinics. Fed Pract. 2008;25(2):39-50.
13. Vreeland B. Bridging the gap between mental and physical health: a multidisciplinary approach. J Clin Psychiatry. 2007;68(suppl 4):26-33.
14. Brim N, Zaller N, Taylor LE, Feller E. Twinrix vaccination schedules among injecting drug users. Expert Opin Biol Ther. 2007;7(3):379-389.
15. Zuckerman J. The place of accelerated schedules for hepatitis A and B vaccinations. Drugs. 2003;63(17):1779-1784.
16. Connor BA, Blatter MM, Beran J, Zou B, Trofa AF. Rapid and sustained immune response against hepatitis A and B achieved with combined vaccine using an accelerated administration schedule. J Travel Med. 2007;14(1):9-15.
17. Nothdurft HD, Dietrich M, Zuckerman JN, et al. A new accelerated vaccination schedule for rapid protection against hepatitis A and B. Vaccine. 2002;20(7-8):1157-1162.
18. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
19. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127.
20. McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199-213.
21. Batki SL, Nathan KI. HIV/AIDS and Hepatitis C. In: Galanter M, Kleber HD, Brady KT, eds. The American Psychiatric Publishing Textbook of Substance Abuse Treatment. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015.
22. Gelberg L, Robertson MJ, Leake B, et al. Hepatitis B among homeless and other impoverished US military veterans in residential care in Los Angeles. Public Health. 2001;115(4):286-291.
23. Felsen UR, Fishbein DA, Litwin AH. Low rates of hepatitis A and B vaccination in patients with chronic hepatitis C at an urban methadone maintenance program. J Addict Dis. 2010;29(4):461-465.
24. Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26(2):136-145.
25. Soto-Salgado M, Suárez E, Ortiz AP, et al. Knowledge of viral hepatitis among Puerto Rican adults: implications for prevention. J Community Health. 2011;36(4):565-573.
26. Cox AD, Cox D, Cyrier R, Graham-Dotson Y, Zimet GD. Can self-prediction overcome barriers to hepatitis B vaccination? A randomized controlled trial. Health Psychol. 2012;31(1):97-105.
27. Stitzer ML, Polk T, Bowles S, Kosten T. Drug users’ adherence to a 6-month vaccination protocol: effects of motivational incentives. Drug Alcohol Depend. 2010;107(1):76-79.
1. Nyamathi A, Liu Y, Marfisee M, et al. Effects of a nurse-managed program on hepatitis A and B vaccine completion among homeless adults. Nurs Res. 2009;58(1):13-22.
2. Center for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2006;55(RR16):1-25.
3. Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2006;55(RR07):1-23.
4. Weltman MD, Brotodihardjo A, Crewe EB, et al. Coinfection with hepatitis B and C or B, C and delta viruses results in severe chronic liver disease and responds poorly to interferon-alpha treatment. J Viral Hepat. 1995;2(1):39-45.
5. Groessl EJ, Weingart KR, Kaplan RM, et al. Living with hepatitis C: qualitative interviews with hepatitis C-infected veterans. J Gen Intern Med. 2008;23(12):1959-1965.
6. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
7. Himeloch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50(1):30-37.
8. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
9. Ramasamy P, Lintzeris N, Sutton Y, Taylor H, Day CA, Haber PS. The outcome of a rapid hepatitis B vaccination programme in a methadone treatment clinic. Addiction. 2010;105(2):329-334.
10. Bini EJ, Kritz S, Brown LS Jr, et al. Hepatitis B virus and hepatitis C virus services offered by substance abuse treatment programs in the United States. J Subst Abuse Treat. 2012;42(4):438-445.
11. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1-39.
12. Francis E, Gonzales-Nolas CL, Markowitz J, Phillips S. Integration of preventive health screening into mental health clinics. Fed Pract. 2008;25(2):39-50.
13. Vreeland B. Bridging the gap between mental and physical health: a multidisciplinary approach. J Clin Psychiatry. 2007;68(suppl 4):26-33.
14. Brim N, Zaller N, Taylor LE, Feller E. Twinrix vaccination schedules among injecting drug users. Expert Opin Biol Ther. 2007;7(3):379-389.
15. Zuckerman J. The place of accelerated schedules for hepatitis A and B vaccinations. Drugs. 2003;63(17):1779-1784.
16. Connor BA, Blatter MM, Beran J, Zou B, Trofa AF. Rapid and sustained immune response against hepatitis A and B achieved with combined vaccine using an accelerated administration schedule. J Travel Med. 2007;14(1):9-15.
17. Nothdurft HD, Dietrich M, Zuckerman JN, et al. A new accelerated vaccination schedule for rapid protection against hepatitis A and B. Vaccine. 2002;20(7-8):1157-1162.
18. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
19. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127.
20. McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199-213.
21. Batki SL, Nathan KI. HIV/AIDS and Hepatitis C. In: Galanter M, Kleber HD, Brady KT, eds. The American Psychiatric Publishing Textbook of Substance Abuse Treatment. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015.
22. Gelberg L, Robertson MJ, Leake B, et al. Hepatitis B among homeless and other impoverished US military veterans in residential care in Los Angeles. Public Health. 2001;115(4):286-291.
23. Felsen UR, Fishbein DA, Litwin AH. Low rates of hepatitis A and B vaccination in patients with chronic hepatitis C at an urban methadone maintenance program. J Addict Dis. 2010;29(4):461-465.
24. Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26(2):136-145.
25. Soto-Salgado M, Suárez E, Ortiz AP, et al. Knowledge of viral hepatitis among Puerto Rican adults: implications for prevention. J Community Health. 2011;36(4):565-573.
26. Cox AD, Cox D, Cyrier R, Graham-Dotson Y, Zimet GD. Can self-prediction overcome barriers to hepatitis B vaccination? A randomized controlled trial. Health Psychol. 2012;31(1):97-105.
27. Stitzer ML, Polk T, Bowles S, Kosten T. Drug users’ adherence to a 6-month vaccination protocol: effects of motivational incentives. Drug Alcohol Depend. 2010;107(1):76-79.
The Use of Sodium Sulfacetamide in Dermatology
Sodium sulfacetamide has various uses in the field of dermatology due to its anti-inflammatory and antibacterial properties. It has been shown to be effective in the management of a variety of inflammatory facial dermatoses, including papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis. We review the mechanism of action, pharmacology and formulations, clinical uses, and adverse effects of sodium sulfacetamide as a dermatologic treatment.
Mechanism of Action
Sodium sulfacetamide is a sulfonamide-type antibacterial agent. Its mechanism of action is the inhibition of bacterial dihydropteroate synthetase, which prevents the conversion of p-aminobenzoic acid to folic acid. This process causes a bacteriostatic effect on the growth of several gram-negative and gram-positive organisms, including Propionibacterium acnes.1,2
The effectiveness of sodium sulfacetamide is increased when used in combination with sulfur, which has keratolytic, antibacterial, antifungal, and antiparasitic effects. The addition of hydrocortisone has been reported to increase the effectiveness of both agents.3
Pharmacology
Sodium sulfacetamide is highly soluble at the physiologic pH of 7.4, which contributes to its high level of penetration and absorption.4 An in vitro study showed percutaneous absorption of sodium sulfacetamide to be around 4%.5 Sulfonamides are metabolized mainly by the liver and are excreted by the kidneys.
Formulations
The most common concentrations of sodium sulfacetamide and sulfur are 10% and 5%, respectively. A wide variety of sulfacetamide-containing products are available, many of which are marketed to treat specific conditions depending on additional ingredients or the type of delivery system.
Clinical Uses
Topical formulations of sodium sulfacetamide and sulfur have proven to be efficacious in the management of rosacea, with a typical regimen consisting of twice-daily application for 8 weeks.6 The sulfur in the formulation has the additional benefit of targeting Demodex mites, which are implicated as a contributing factor in some cases of rosacea.7 Sodium sulfacetamide 10%–sulfur 5% lotion was more effective in improving the erythema, papulopustules, and overall severity of rosacea as compared to metronidazole gel 0.75%.8 Other studies have reported increased efficacy when sodium sulfacetamide and topical sulfur are used along with metronidazole.9,10
Sodium sulfacetamide also has shown efficacy against acne. Its antibacterial and drying properties have been shown to decrease the number of inflammatory lesions and comedones, and in the treatment of acne vulgaris, no sensitivity reactions have been observed.2 Also, unlike topical antibiotics, cases of P acnes resistance to topical sulfur products have not been widely reported. Studies have demonstrated that twice-daily use of sodium sulfacetamide 10%–sulfur 5% for 12 weeks decreases inflammatory acne lesions by 80.4% to 83%.11,12
Seborrheic dermatitis is a common chronic infection of the skin caused by Malassezia species. One study investigated the use of sodium sulfacetamide ointment and soap to treat seborrheic dermatitis and found that the condition was either improved or completely controlled in 93% (71/76) of cases.4 Sodium sulfacetamide lotion was an effective treatment of seborrheic dermatitis in 89% (54/61) of patients with scalp involvement and 68% (30/44) of patients with glabrous skin involvement.13
Perioral dermatitis is characterized by groups of erythematous papules and pustules localized around the mouth. The use of topical sodium sulfacetamide along with oral tetracyclines has been demonstrated to consistently clear lesions in most patients with perioral dermatitis.14 Sodium sulfacetamide is unique in that it is not associated with the excessive erythema and irritation often found with retinoic acid and benzoyl peroxide.15 Unfortunately, however, there have been no well-controlled trials to compare the efficacy of sodium sulfacetamide to other topical therapies for this condition.
Adverse Effects
Adverse effects from sodium sulfacetamide are rare and generally are limited to cutaneous reactions including dryness, erythema, pruritus, and discomfort.1 Periocular use of sodium sulfacetamide can cause conjunctival irritation. One study reported that 19% (6/31) of patients experienced local reactions but most were considered mild.9 Rare but serious reactions including erythema multiforme and Stevens-Johnson syndrome have been reported from ophthalmic use.16,17
A common limiting factor to sodium sulfacetamide preparations that include elemental sulfur is the offensive smell, which has hindered patient compliance in the past; however, pharmaceutical companies have attempted to create more tolerable products without the odor.10 One study found that the tolerability of a sodium sulfacetamide 10%–sulfur 5% foam using a rinse-off method of application was excellent, with only 33% (8/24) of participants commenting on the smell.18 Another limiting factor of sodium sulfacetamide preparations containing sulfur is orange-brown discoloration when combined with benzoyl peroxide, which does not affect the skin but may stain clothing.19
Sodium sulfacetamide is rendered less effective when combined with silver-containing products.20 No other notable drug interactions are known; however, oral sulfonamides are known to interact with several drugs, including cyclosporine and phenytoin.21,22
Contraindications
Sodium sulfacetamide is contraindicated in patients with known hypersensitivity to sulfonamides, sulfur, or any other component of the preparation. It is a pregnancy category C drug, and pregnant women should only use sodium sulfacetamide if it is the only modality to treat the condition or the benefits outweigh the risks. Although there are no known reports of problems related to topical sodium sulfacetamide during pregnancy, the use of oral sulfonamides during pregnancy can increase the risk for neonatal jaundice.23 Likewise, caution should be exercised in prescribing this product to nursing women, as systemic sulfonamide antibacterials are well known to cause kernicterus in nursing neonates.1
Conclusion
The efficacy and safety of sodium sulfacetamide, used alone or in combination with sulfur, has been demonstrated in the treatment of rosacea, acne, seborrheic dermatitis, and perioral dermatitis. Advances in formulation technology to decrease odor and irritation have allowed for more use of this product. Further studies will help elucidate the role that sodium sulfacetamide should play in the treatment of inflammatory dermatoses in comparison to other available products.
1. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003;4:473-492.
2. Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
3. Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders; 2012:445-459.
4. Duemling WM. Sodium sulfacetamide in topical therapy. AMA Arch Derm Syphilol. 1954;69:75-82.
5. Sodium sulfacetamide. Drugs.com Web site. http://drugs.com/pro/sodium-sulfacetamide.html. Revised December 2012. Accessed June 16, 2015.
6. Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8:79-85.
7. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
8. Lebwohl MG, Medansky RS, Russo CL, et al. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% lotion and metronidazole 0.75% gel in the treatment of rosacea. J Geriatr Dermatol. 1995;3:183-185.
9. Nally JB, Berson DS. Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-26.
10. Pelle MT, Crawford GH, James WD. Rosacea II: therapy. J Am Acad Dermatol. 2004;51:499-512.
11. Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22:301.
12. Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int J Dermatol. 1993;32:365-367.
13. Whelan ST. Sodium sulfacetamide for seborrheic dermatitis. AMA Arch Derm. 1955;71:724.
14. Bendl BJ. Perioral dermatitis: etiology and treatment. Cutis. 1976;17:903-908.
15. Olansky S. Old drug—in a new system—revisited. Cutis. 1977;19:852-854.
16. Genvert GI, Cohen EJ, Donnenfeld ED, et al. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985;99:465-468.
17. Rubin Z. Ophthalmic sulfonamide-induced Stevens-Johnson syndrome. Arch Dermatol. 1977;113:235-236.
18. Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-246.
19. Dubina MI, Fleischer AB. Interaction of topical sulfacetamide and topical dapsone with benzoyl peroxide. Arch Dermatol. 2009;145:1027-1029.
20. Sodium sulfacetamide – sulfacetamide sodium liquid. DailyMed Web site. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0d92c55b-5b54-4f5d-8921-24e4e877ae50. Accessed June 17, 2015.
21. Spes CH, Angermann CE, Stempfle HU, et al. Sulfadiazine therapy for toxoplasmosis in heart transplant recipients decreases cyclosporine concentration. Clin Investig. 1992;70:752-754.
22. Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106-110.
23. Bradley JS, Sauberan JB. Antimicrobial agents. In: Long SS, Pickering LK, Prober CG. Principles and Practices of Pediatric Infectious Diseases. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1453-1483.
Sodium sulfacetamide has various uses in the field of dermatology due to its anti-inflammatory and antibacterial properties. It has been shown to be effective in the management of a variety of inflammatory facial dermatoses, including papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis. We review the mechanism of action, pharmacology and formulations, clinical uses, and adverse effects of sodium sulfacetamide as a dermatologic treatment.
Mechanism of Action
Sodium sulfacetamide is a sulfonamide-type antibacterial agent. Its mechanism of action is the inhibition of bacterial dihydropteroate synthetase, which prevents the conversion of p-aminobenzoic acid to folic acid. This process causes a bacteriostatic effect on the growth of several gram-negative and gram-positive organisms, including Propionibacterium acnes.1,2
The effectiveness of sodium sulfacetamide is increased when used in combination with sulfur, which has keratolytic, antibacterial, antifungal, and antiparasitic effects. The addition of hydrocortisone has been reported to increase the effectiveness of both agents.3
Pharmacology
Sodium sulfacetamide is highly soluble at the physiologic pH of 7.4, which contributes to its high level of penetration and absorption.4 An in vitro study showed percutaneous absorption of sodium sulfacetamide to be around 4%.5 Sulfonamides are metabolized mainly by the liver and are excreted by the kidneys.
Formulations
The most common concentrations of sodium sulfacetamide and sulfur are 10% and 5%, respectively. A wide variety of sulfacetamide-containing products are available, many of which are marketed to treat specific conditions depending on additional ingredients or the type of delivery system.
Clinical Uses
Topical formulations of sodium sulfacetamide and sulfur have proven to be efficacious in the management of rosacea, with a typical regimen consisting of twice-daily application for 8 weeks.6 The sulfur in the formulation has the additional benefit of targeting Demodex mites, which are implicated as a contributing factor in some cases of rosacea.7 Sodium sulfacetamide 10%–sulfur 5% lotion was more effective in improving the erythema, papulopustules, and overall severity of rosacea as compared to metronidazole gel 0.75%.8 Other studies have reported increased efficacy when sodium sulfacetamide and topical sulfur are used along with metronidazole.9,10
Sodium sulfacetamide also has shown efficacy against acne. Its antibacterial and drying properties have been shown to decrease the number of inflammatory lesions and comedones, and in the treatment of acne vulgaris, no sensitivity reactions have been observed.2 Also, unlike topical antibiotics, cases of P acnes resistance to topical sulfur products have not been widely reported. Studies have demonstrated that twice-daily use of sodium sulfacetamide 10%–sulfur 5% for 12 weeks decreases inflammatory acne lesions by 80.4% to 83%.11,12
Seborrheic dermatitis is a common chronic infection of the skin caused by Malassezia species. One study investigated the use of sodium sulfacetamide ointment and soap to treat seborrheic dermatitis and found that the condition was either improved or completely controlled in 93% (71/76) of cases.4 Sodium sulfacetamide lotion was an effective treatment of seborrheic dermatitis in 89% (54/61) of patients with scalp involvement and 68% (30/44) of patients with glabrous skin involvement.13
Perioral dermatitis is characterized by groups of erythematous papules and pustules localized around the mouth. The use of topical sodium sulfacetamide along with oral tetracyclines has been demonstrated to consistently clear lesions in most patients with perioral dermatitis.14 Sodium sulfacetamide is unique in that it is not associated with the excessive erythema and irritation often found with retinoic acid and benzoyl peroxide.15 Unfortunately, however, there have been no well-controlled trials to compare the efficacy of sodium sulfacetamide to other topical therapies for this condition.
Adverse Effects
Adverse effects from sodium sulfacetamide are rare and generally are limited to cutaneous reactions including dryness, erythema, pruritus, and discomfort.1 Periocular use of sodium sulfacetamide can cause conjunctival irritation. One study reported that 19% (6/31) of patients experienced local reactions but most were considered mild.9 Rare but serious reactions including erythema multiforme and Stevens-Johnson syndrome have been reported from ophthalmic use.16,17
A common limiting factor to sodium sulfacetamide preparations that include elemental sulfur is the offensive smell, which has hindered patient compliance in the past; however, pharmaceutical companies have attempted to create more tolerable products without the odor.10 One study found that the tolerability of a sodium sulfacetamide 10%–sulfur 5% foam using a rinse-off method of application was excellent, with only 33% (8/24) of participants commenting on the smell.18 Another limiting factor of sodium sulfacetamide preparations containing sulfur is orange-brown discoloration when combined with benzoyl peroxide, which does not affect the skin but may stain clothing.19
Sodium sulfacetamide is rendered less effective when combined with silver-containing products.20 No other notable drug interactions are known; however, oral sulfonamides are known to interact with several drugs, including cyclosporine and phenytoin.21,22
Contraindications
Sodium sulfacetamide is contraindicated in patients with known hypersensitivity to sulfonamides, sulfur, or any other component of the preparation. It is a pregnancy category C drug, and pregnant women should only use sodium sulfacetamide if it is the only modality to treat the condition or the benefits outweigh the risks. Although there are no known reports of problems related to topical sodium sulfacetamide during pregnancy, the use of oral sulfonamides during pregnancy can increase the risk for neonatal jaundice.23 Likewise, caution should be exercised in prescribing this product to nursing women, as systemic sulfonamide antibacterials are well known to cause kernicterus in nursing neonates.1
Conclusion
The efficacy and safety of sodium sulfacetamide, used alone or in combination with sulfur, has been demonstrated in the treatment of rosacea, acne, seborrheic dermatitis, and perioral dermatitis. Advances in formulation technology to decrease odor and irritation have allowed for more use of this product. Further studies will help elucidate the role that sodium sulfacetamide should play in the treatment of inflammatory dermatoses in comparison to other available products.
Sodium sulfacetamide has various uses in the field of dermatology due to its anti-inflammatory and antibacterial properties. It has been shown to be effective in the management of a variety of inflammatory facial dermatoses, including papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis. We review the mechanism of action, pharmacology and formulations, clinical uses, and adverse effects of sodium sulfacetamide as a dermatologic treatment.
Mechanism of Action
Sodium sulfacetamide is a sulfonamide-type antibacterial agent. Its mechanism of action is the inhibition of bacterial dihydropteroate synthetase, which prevents the conversion of p-aminobenzoic acid to folic acid. This process causes a bacteriostatic effect on the growth of several gram-negative and gram-positive organisms, including Propionibacterium acnes.1,2
The effectiveness of sodium sulfacetamide is increased when used in combination with sulfur, which has keratolytic, antibacterial, antifungal, and antiparasitic effects. The addition of hydrocortisone has been reported to increase the effectiveness of both agents.3
Pharmacology
Sodium sulfacetamide is highly soluble at the physiologic pH of 7.4, which contributes to its high level of penetration and absorption.4 An in vitro study showed percutaneous absorption of sodium sulfacetamide to be around 4%.5 Sulfonamides are metabolized mainly by the liver and are excreted by the kidneys.
Formulations
The most common concentrations of sodium sulfacetamide and sulfur are 10% and 5%, respectively. A wide variety of sulfacetamide-containing products are available, many of which are marketed to treat specific conditions depending on additional ingredients or the type of delivery system.
Clinical Uses
Topical formulations of sodium sulfacetamide and sulfur have proven to be efficacious in the management of rosacea, with a typical regimen consisting of twice-daily application for 8 weeks.6 The sulfur in the formulation has the additional benefit of targeting Demodex mites, which are implicated as a contributing factor in some cases of rosacea.7 Sodium sulfacetamide 10%–sulfur 5% lotion was more effective in improving the erythema, papulopustules, and overall severity of rosacea as compared to metronidazole gel 0.75%.8 Other studies have reported increased efficacy when sodium sulfacetamide and topical sulfur are used along with metronidazole.9,10
Sodium sulfacetamide also has shown efficacy against acne. Its antibacterial and drying properties have been shown to decrease the number of inflammatory lesions and comedones, and in the treatment of acne vulgaris, no sensitivity reactions have been observed.2 Also, unlike topical antibiotics, cases of P acnes resistance to topical sulfur products have not been widely reported. Studies have demonstrated that twice-daily use of sodium sulfacetamide 10%–sulfur 5% for 12 weeks decreases inflammatory acne lesions by 80.4% to 83%.11,12
Seborrheic dermatitis is a common chronic infection of the skin caused by Malassezia species. One study investigated the use of sodium sulfacetamide ointment and soap to treat seborrheic dermatitis and found that the condition was either improved or completely controlled in 93% (71/76) of cases.4 Sodium sulfacetamide lotion was an effective treatment of seborrheic dermatitis in 89% (54/61) of patients with scalp involvement and 68% (30/44) of patients with glabrous skin involvement.13
Perioral dermatitis is characterized by groups of erythematous papules and pustules localized around the mouth. The use of topical sodium sulfacetamide along with oral tetracyclines has been demonstrated to consistently clear lesions in most patients with perioral dermatitis.14 Sodium sulfacetamide is unique in that it is not associated with the excessive erythema and irritation often found with retinoic acid and benzoyl peroxide.15 Unfortunately, however, there have been no well-controlled trials to compare the efficacy of sodium sulfacetamide to other topical therapies for this condition.
Adverse Effects
Adverse effects from sodium sulfacetamide are rare and generally are limited to cutaneous reactions including dryness, erythema, pruritus, and discomfort.1 Periocular use of sodium sulfacetamide can cause conjunctival irritation. One study reported that 19% (6/31) of patients experienced local reactions but most were considered mild.9 Rare but serious reactions including erythema multiforme and Stevens-Johnson syndrome have been reported from ophthalmic use.16,17
A common limiting factor to sodium sulfacetamide preparations that include elemental sulfur is the offensive smell, which has hindered patient compliance in the past; however, pharmaceutical companies have attempted to create more tolerable products without the odor.10 One study found that the tolerability of a sodium sulfacetamide 10%–sulfur 5% foam using a rinse-off method of application was excellent, with only 33% (8/24) of participants commenting on the smell.18 Another limiting factor of sodium sulfacetamide preparations containing sulfur is orange-brown discoloration when combined with benzoyl peroxide, which does not affect the skin but may stain clothing.19
Sodium sulfacetamide is rendered less effective when combined with silver-containing products.20 No other notable drug interactions are known; however, oral sulfonamides are known to interact with several drugs, including cyclosporine and phenytoin.21,22
Contraindications
Sodium sulfacetamide is contraindicated in patients with known hypersensitivity to sulfonamides, sulfur, or any other component of the preparation. It is a pregnancy category C drug, and pregnant women should only use sodium sulfacetamide if it is the only modality to treat the condition or the benefits outweigh the risks. Although there are no known reports of problems related to topical sodium sulfacetamide during pregnancy, the use of oral sulfonamides during pregnancy can increase the risk for neonatal jaundice.23 Likewise, caution should be exercised in prescribing this product to nursing women, as systemic sulfonamide antibacterials are well known to cause kernicterus in nursing neonates.1
Conclusion
The efficacy and safety of sodium sulfacetamide, used alone or in combination with sulfur, has been demonstrated in the treatment of rosacea, acne, seborrheic dermatitis, and perioral dermatitis. Advances in formulation technology to decrease odor and irritation have allowed for more use of this product. Further studies will help elucidate the role that sodium sulfacetamide should play in the treatment of inflammatory dermatoses in comparison to other available products.
1. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003;4:473-492.
2. Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
3. Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders; 2012:445-459.
4. Duemling WM. Sodium sulfacetamide in topical therapy. AMA Arch Derm Syphilol. 1954;69:75-82.
5. Sodium sulfacetamide. Drugs.com Web site. http://drugs.com/pro/sodium-sulfacetamide.html. Revised December 2012. Accessed June 16, 2015.
6. Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8:79-85.
7. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
8. Lebwohl MG, Medansky RS, Russo CL, et al. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% lotion and metronidazole 0.75% gel in the treatment of rosacea. J Geriatr Dermatol. 1995;3:183-185.
9. Nally JB, Berson DS. Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-26.
10. Pelle MT, Crawford GH, James WD. Rosacea II: therapy. J Am Acad Dermatol. 2004;51:499-512.
11. Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22:301.
12. Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int J Dermatol. 1993;32:365-367.
13. Whelan ST. Sodium sulfacetamide for seborrheic dermatitis. AMA Arch Derm. 1955;71:724.
14. Bendl BJ. Perioral dermatitis: etiology and treatment. Cutis. 1976;17:903-908.
15. Olansky S. Old drug—in a new system—revisited. Cutis. 1977;19:852-854.
16. Genvert GI, Cohen EJ, Donnenfeld ED, et al. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985;99:465-468.
17. Rubin Z. Ophthalmic sulfonamide-induced Stevens-Johnson syndrome. Arch Dermatol. 1977;113:235-236.
18. Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-246.
19. Dubina MI, Fleischer AB. Interaction of topical sulfacetamide and topical dapsone with benzoyl peroxide. Arch Dermatol. 2009;145:1027-1029.
20. Sodium sulfacetamide – sulfacetamide sodium liquid. DailyMed Web site. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0d92c55b-5b54-4f5d-8921-24e4e877ae50. Accessed June 17, 2015.
21. Spes CH, Angermann CE, Stempfle HU, et al. Sulfadiazine therapy for toxoplasmosis in heart transplant recipients decreases cyclosporine concentration. Clin Investig. 1992;70:752-754.
22. Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106-110.
23. Bradley JS, Sauberan JB. Antimicrobial agents. In: Long SS, Pickering LK, Prober CG. Principles and Practices of Pediatric Infectious Diseases. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1453-1483.
1. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003;4:473-492.
2. Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
3. Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders; 2012:445-459.
4. Duemling WM. Sodium sulfacetamide in topical therapy. AMA Arch Derm Syphilol. 1954;69:75-82.
5. Sodium sulfacetamide. Drugs.com Web site. http://drugs.com/pro/sodium-sulfacetamide.html. Revised December 2012. Accessed June 16, 2015.
6. Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8:79-85.
7. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
8. Lebwohl MG, Medansky RS, Russo CL, et al. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% lotion and metronidazole 0.75% gel in the treatment of rosacea. J Geriatr Dermatol. 1995;3:183-185.
9. Nally JB, Berson DS. Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-26.
10. Pelle MT, Crawford GH, James WD. Rosacea II: therapy. J Am Acad Dermatol. 2004;51:499-512.
11. Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22:301.
12. Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int J Dermatol. 1993;32:365-367.
13. Whelan ST. Sodium sulfacetamide for seborrheic dermatitis. AMA Arch Derm. 1955;71:724.
14. Bendl BJ. Perioral dermatitis: etiology and treatment. Cutis. 1976;17:903-908.
15. Olansky S. Old drug—in a new system—revisited. Cutis. 1977;19:852-854.
16. Genvert GI, Cohen EJ, Donnenfeld ED, et al. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985;99:465-468.
17. Rubin Z. Ophthalmic sulfonamide-induced Stevens-Johnson syndrome. Arch Dermatol. 1977;113:235-236.
18. Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-246.
19. Dubina MI, Fleischer AB. Interaction of topical sulfacetamide and topical dapsone with benzoyl peroxide. Arch Dermatol. 2009;145:1027-1029.
20. Sodium sulfacetamide – sulfacetamide sodium liquid. DailyMed Web site. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0d92c55b-5b54-4f5d-8921-24e4e877ae50. Accessed June 17, 2015.
21. Spes CH, Angermann CE, Stempfle HU, et al. Sulfadiazine therapy for toxoplasmosis in heart transplant recipients decreases cyclosporine concentration. Clin Investig. 1992;70:752-754.
22. Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106-110.
23. Bradley JS, Sauberan JB. Antimicrobial agents. In: Long SS, Pickering LK, Prober CG. Principles and Practices of Pediatric Infectious Diseases. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1453-1483.
Practice Points
- Sodium sulfacetamide is a useful agent in the management of papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis.
- Adverse effects are rare and generally are limited to dryness, erythema, pruritus, and discomfort.
Genitourinary syndrome of menopause: Current and emerging therapies
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy 
|
FIGURE 2: Atrophic vaginitis 
This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy 

|
FIGURE 2: Atrophic vaginitis 

This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy 

|
FIGURE 2: Atrophic vaginitis 

This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
In this article
- What can we offer our patients?
- Fractional CO2 laser: a study in progress
- Bottom line
The Top 100 Cited Articles in Clinical Orthopedic Sports Medicine
Orthopedics and the sports medicine subspecialty are continually evolving fields that depend on research investigation and publication to further knowledge and advance practice. Research has produced new findings that have changed the way we practice sports medicine. In this review, we identify the most widely referenced sports medicine topics and articles, which we believe by their permeative presence in the literature have made lasting contributions to the field.
Many factors can be used to quantify the influence of an academic article on the practice of medicine. Citation analysis is one method that reflects the impact of a publication on the academic medical community.1-3 Total citations record the number of times a journal article has been credited by another study. Therefore, citation count indirectly highlights the articles that are widespread, relevant, and that form the foundation for other investigations on the topic. Related to the impact of the article is the impact of the journal that published the study. We examined journals by impact factor, a score based on the mean number of citations a published article received during the preceding 2 years.
Similar analyses have been performed of publication history in orthopedics and other medical fields. Investigators have examined which historical articles were the most influential in orthopedics as a whole,4 pediatric orthopedics,5,6 shoulder surgery,7 and arthroscopy.8 This influence has also been studied in general surgery,9 otolaryngology,10 plastic surgery,11 dermatology,12 critical care,13 and other disciplines. To our knowledge, the present study is the first bibliometric analysis of the highest-impact articles in orthopedic sports medicine.
Our goal was to identify the 100 articles that have had the highest impact on the clinical orthopedic sports medicine literature. We hypothesized that the most widely recognized articles would be from the highest-impact journals and may also have earlier publication dates. We describe the topics and objectives of these articles to highlight the sports medicine areas on which most research has focused during the past century.
Materials and Methods
Our bibliometric analysis used the Thomson Reuters Web of Knowledge, which consists of all publications from 1900 to the present. This research modality ranks journal articles by frequency of citation. Similar analyses have identified the most often cited articles in pediatric orthopedics,5 shoulder surgery,7 and arthroscopy.8 In our analysis, we included the top 25 journals by impact factor in the field of sports medicine, as rated by the Journal Citation Reports database. Within the highest-impact journals, we sorted all articles by those most often cited, and read them all to identify which ones discuss conditions commonly encountered in the clinical practice of sports medicine. We focused on clinical articles only and therefore excluded related basic science and cadaveric biomechanical studies. The 100 most cited articles were then further evaluated by primary author, journal of publication, institution, country of origin, year of publication, topic, and total number of citations. One-way analysis of variance (ANOVA) and linear regression analyses were used to determine if publication date correlated with mean number of citations.
Results
Eighty authors wrote the top 100 articles in sports medicine, and each publication garnered several hundred citations, ranging from 229 to 1629 with a mean of 408 (Table 114-113). Most of these articles were written in the past 3 decades, with equal distribution from the 1980s, 1990s, and 2000s (Figure 1A). We ran a linear regression to determine if publication date correlated with higher number of citations by virtue of longer time available for citation. The analysis poorly modeled the variability (R2 = 0.05), revealing no correlation between number of citations and publication date. Further, 1-way ANOVA found no significant difference between the number of citations per decade, F(5, 93) = 1.60, P = .17 (Figure 1B). Despite this finding, the oldest cited article, written by Fairbank39 in 1948, ranked high (position 7). Of these top 100 publications, the most recent, written by Knutsen and colleagues69 in 2007, ranked in the second half at position 66.
Seven journals published the top 100 articles, with the American volume of the Journal of Bone and Joint Surgery publishing nearly half (44%) (Table 2). In second place, with 28 articles, was the American Journal of Sports Medicine, followed by the British volume of the Journal of Bone and Joint Surgery, with 10 articles.
Thirty different topics were investigated in this collection of articles, encompassing nearly every major research area of sports medicine. There was a heavy emphasis on anterior cruciate ligament (ACL) injury and reconstruction, knee rating systems, rotator cuff reconstruction, and chondrocyte transplantation (Table 3).
In several cases, an author contributed more than 1 classic article. In fact, 31 of the top 100 articles were by an individual who had coauthored 2 or more of the publications on this list. The researchers with the largest number of first-authored articles were Noyes88-92 (5 articles), Neer81-84 (4 articles), and Rowe,102-104 Daniel,35-37 Peterson,97-99 and Hewett52-54 (3 articles each) (Table 417,19,21-24,29-31,35-37,42,44,45,52-54,58,61-65,69,70,72,74,80-84,87-92,97-99,101-105,107,109,110,113). Articles from authors with multiple publications had a common topic.
Last, these articles originated from a number of different countries and institutions. Of the 15 source countries (Figure 2), the United States contributed the most (61 articles). Other countries had prominent representation: Sweden and Switzerland (8 each), United Kingdom (5), and Canada, France, and Norway (3 each). These articles originated from 69 universities, hospitals, and clinics; 21 institutions had 2 or more articles (Table 5). The 5 institutions with the highest number of articles were Hospital for Special Surgery, University of Bern, Columbia College of Physicians and Surgeons/Columbia-Presbyterian Medical Center, Cincinnati Sports Medicine and Orthopaedic Center, and Massachusetts General Hospital.
Discussion
Several trends can be ascertained from analyzing the top 100 clinical articles cited in sports medicine. The 5 most frequent topics discussed were ACL injury and reconstruction, knee rating systems for injury and function, rotator cuff reconstruction, chondrocyte transplantation, and femoroacetabular impingement (Table 3). Of those 5 topics, only ACL injury and reconstruction falls within the top 10 most common orthopedic surgical procedures performed in the United States reported by one analysis.114 The most common orthopedic surgical procedure, knee arthroscopy, ranks 10th of all topics covered by the top 100 articles, whereas the second most common procedure, shoulder arthroscopy, was not discussed by any of those 100 articles. Also notable is the high frequency of knee rating system studies, which correlates well with the fact that 4 of the most common orthopedic surgical procedures are knee procedures. The prevalence of rating system articles reflects the importance of and need for accurate methods in the diagnosis of injuries in sports medicine.
The most cited sports medicine article was written by Insall and colleagues62 in 1989, more than 2 decades ago. In this article, “Rationale of the Knee Society Clinical Rating System,” they reported on a rigorous system that rates knee function and ability to walk and climb stairs. The second most cited article, “A Clinical Method of Functional Assessment of the Shoulder,” was written in 1987 by Constant and Murley.32 This article discusses another rating system but offers a functional assessment of the shoulder that is highly reproducible and time-efficient. “Rating Systems in the Evaluation of Knee Ligament Injuries,” the third most cited article, was written in 1985 by Tegner and Lysholm.113 This article details the complexities and variable uses of different knee ligament injury rating systems. These top 3 articles were all published in Clinical Orthopaedics and Related Research. In addition, all 3 discussed rating systems, reinforcing the need for accurate scoring systems to standardize the diagnosis of injury across the field of orthopedics and qualify outcomes after injury.
A number of studies have introduced physical examination findings, clinical tests, and rating systems used in the clinical setting of sports medicine (and named after the contributing authors). For example, the Neer sign82 and the Hawkins-Kennedy test51 are used to determine shoulder impingement. In knee ligament injuries, the Tegner knee activity score113 complements other functional scores (eg, Lysholm knee score74). For grading joint cartilage breakdown, the Outerbridge classification system96 is commonly used. The Fairbank test39 is used to gauge knee instability. In evaluating fatty degeneration of rotator cuff muscles through computed tomography scans, the Goutallier classification47 is used. Other metrics, such as the Knee Injury and Osteoarthritis Outcome Score, introduced by Roos and colleagues,101 measure knee injury and osteoarthritis. In other scenarios, studies have improved on surgical techniques—for example, the Neer open modification84 of the Bankart procedure. Many of these rating systems and named clinical findings are so ingrained in the practice and vernacular of orthopedics that it is possible they are in fact undercited in the literature.
As in other bibliometric analyses, one concession made here was to credit the first author listed for making the primary contribution to an article. As a result of journal variability and inconsistency, we were precluded from analyzing senior authors. When analyzed for authorship at any position, 3 of the top authors (Table 4) showed contributions to additional articles in the top 100 list. Noyes was listed as last author on 2 other articles,52,54 raising his total to 7. Daniel was listed as second author on 1 additional article,105 and Beck was listed as third author on 1 other article,42 raising their totals to 4 and 3, respectively.
A criticism of bibliometric analysis is its use of number of citations as an accurate measure of academic contribution. However, other methods for measuring the productivity and impact of researchers (eg, the recently developed Hirsch Index) have their own drawbacks,115,116 including being able to compare authors only at the same point in their careers and self-citation. It is important to note that our analyses focused strictly on publications related to clinical sports medicine, with the exclusion of basic science and cadaveric biomechanical studies.
Through bibliometric citation analysis, we have identified the authors who have made lasting contributions to the field of sports medicine, and we have highlighted the publications that have been cited by hundreds to thousands of authors. This list identifies trends within the articles that have become “classic,” by nature of their deep permeation into subsequent sports medicine literature, and offers guidance for trainees interested in studying the most high-yield sports medicine literature. Given that 69 institutions in 15 countries conducted these studies, we have also shown that orthopedic research can be readily disseminated internationally. Last, our study provides a thorough overview of the sports medicine literature over the past century and provides a strong framework for future research in our field.
1. Adams AB, Simonson D. Publication, citations, and impact factors of leading investigators in critical care medicine. Respir Care. 2004;49(3):276-281.
2. Bhandari M, Busse J, Devereaux PJ, et al. Factors associated with citation rates in the orthopedic literature. Can J Surg. 2007;50(2):119-123.
3. Cheek J, Garnham B, Quan J. What’s in a number? Issues in providing evidence of impact and quality of research(ers). Qual Health Res. 2006;16(3):423-435.
4. Kelly JC, Glynn RW, O’Briain DE, Felle P, McCabe JP. The 100 classic papers of orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Br. 2010;92(10):1338-1343.
5. Kavanagh RG, Kelly JC, Kelly PM, Moore DP. The 100 classic papers of pediatric orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Am. 2013;95(18):e134.
6. Mehlman CT, Wenger DR. The top 25 at 25: citation classics in the Journal of Pediatric Orthopaedics. J Pediatr Orthop. 2006;26(5):691-694.
7. Namdari S, Baldwin K, Kovatch K, Huffman GR, Glaser D. Fifty most cited articles in orthopedic shoulder surgery. J Shoulder Elbow Surg. 2012;21(12):1796-1802.
8. Cassar Gheiti AJ, Downey RE, Byrne DP, Molony DC, Mulhall KJ. The 25 most cited articles in arthroscopic orthopaedic surgery. Arthroscopy. 2012;28(4):548-564.
9. Paladugu R, Schein M, Gardezi S, Wise L. One hundred citation classics in general surgical journals. World J Surg. 2002;26(9):1099-1105.
10. Fenton JE, Roy D, Hughes JP, Jones AS. A century of citation classics in otolaryngology-head and neck surgery journals. J Laryngol Otol. 2002;116(7):494-498.
11. Loonen MPJ, Hage JJ, Kon M. Plastic surgery classics: characteristics of 50 top-cited articles in four plastic surgery journals since 1946. Plast Reconstr Surg. 2008;121(5):320e-327e.
12. Dubin D, Hafner AW, Arndt KA. Citation classics in clinical dermatologic journals. Citation analysis, biomedical journals, and landmark articles, 1945–1990. Arch Dermatol. 1993;129(9):1121-1129.
13. Baltussen A, Kindler CH. Citation classics in critical care medicine. Intensive Care Med. 2004;30(5):902-910.
14. Aglietti P, Buzzi R, Zaccherotti G, De Biase P. Patellar tendon versus doubled semitendinosus and gracilis tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 1994;22(2):211-218.
15. Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66(5):666-671.
16. Altchek DW, Warren RF, Skyhar MJ, Ortiz G. T-plasty modification of the Bankart procedure for multidirectional instability of the anterior and inferior types. J Bone Joint Surg Am. 1991;73(1):105-112.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701.
19. Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16(2):113-122.
20. Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17(1):1-6.
21. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640-645.
22. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
23. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
24. Bentley G, Biant LC, Carrington RWJ, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223-230.
25. Berchuck M, Andriacchi TP, Bach BR, Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72(6):871-877.
26. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41(6):988-1020.
27. Binkley JM, Stratford PW, Lott SA, Riddle DL, North American Orthopaedic Rehabilitation Research Network. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371-383.
28. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
29. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
30. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
31. Clancy WG Jr, Shelbourne KD, Zoellner GB, Keene JS, Reider B, Rosenberg TD. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J Bone Joint Surg Am. 1983;65(3):310-322.
32. Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
33. Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27(3):444-454.
34. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
35. Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R. Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg Am. 1985;67(5):720-726.
36. Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632-644.
37. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401-407.
38. Ellman H, Hanker G, Bayer M. Repair of the rotator cuff. End-result study of factors influencing reconstruction. J Bone Joint Surg Am. 1986;68(8):1136-1144.
39. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
40. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
41. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
42. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):111-119.
43. Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;(304):43-53.
44. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
45. Gerber C, Krushell RJ. Isolated rupture of the tendon of the subscapularis muscle. Clinical features in 16 cases. J Bone Joint Surg Br. 1991;73(3):389-394.
46. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
47. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78-83.
48. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE Jr. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650.
49. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
50. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
51. Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med. 1980;8(3):151-157.
52. Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699-706.
53. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492-501.
54. Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765-773.
55. Homminga GN, Bulstra SK, Bouwmeester PSM, van der Linden AJ. Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br. 1990;72(6):1003-1007.
56. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185-192.
57. Hovelius L, Augustini BG, Fredin H, Johansson O, Norlin R, Thorling J. Primary anterior dislocation of the shoulder in young patients. A ten-year prospective study. J Bone Joint Surg Am. 1996;78(11):1677-1684.
58. Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58(2):159-172.
59. Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427-436.
60. Iannotti JP, Zlatkin MB, Esterhai JL, Kressel HY, Dalinka MK, Spindler KP. Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg Am. 1991;73(1):17-29.
61. Insall J, Falvo KA, Wise DW. Chondromalacia patellae. A prospective study. J Bone Joint Surg Am. 1976;58(1):1-8.
62. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
63. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600-613.
64. Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132-1145.
65. Ito K, Minka MA 2nd, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect. A MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br. 2001;83(2):171-176.
66. Johnson RJ, Kettelkamp DB, Clark W, Leaverton P. Factors affecting late results after meniscectomy. J Bone Joint Surg Am. 1974;56(3):719-729.
67. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204-208.
68. Jones KG. Reconstruction of the anterior cruciate ligament: a technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
69. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-2112.
70. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86(3):455-464.
71. Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159-163.
72. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
73. Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276-291.
74. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154.
75. Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003-1010.
76. Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991;19(5):478-484.
77. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
78. Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9(3):318-321.
79. McDaniel WJ Jr, Dameron TB Jr. Untreated ruptures of the anterior cruciate ligament. A follow-up study. J Bone Joint Surg Am. 1980;62(5):696-705.
80. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
81. Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54(1):41-50.
82. Neer CS 2nd. Impingement lesions. Clin Orthop Relat Res. 1983;(173):70-77.
83. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
84. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder. A preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
85. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
86. Nistor L. Surgical and non-surgical treatment of Achilles tendon rupture. J Bone Joint Surg Am. 1981;63(3):394-399.
87. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
88. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513-518.
89. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62(5):687-695, 757.
90. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate–deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
91. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate–deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
92. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
93. O’Brien SJ, Warren RF, Pavlov H, Panariello R, Wickiewicz TL. Reconstruction of the chronically insufficient anterior cruciate ligament with the central third of the patellar ligament. J Bone Joint Surg Am. 1991;73(2):278-286.
94. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
95. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
96. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43(4):752-757.
97. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12.
98. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17-24.
99. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-234.
100. Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80(9):1276-1284.
101. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96.
102. Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am. 1956;38(5):957-977.
103. Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am. 1978;60(1):1-16.
104. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
105. Sachs RA, Daniel DM, Stone ML, Garfein RF. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17(6):760-765.
106. Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65(4):456-460.
107. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299.
108. Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders [see comments]. J Bone Joint Surg Am. 1995;77(1):10-15.
109. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85(2):278-286.
110. Solomonow M, Baratta R, Zhou BH, et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15(3):207-213.
111. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484.
112. Tapper EM, Hoover NW. Late results after meniscectomy. J Bone Joint Surg Am. 1969;51(3):517-526.
113. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-49.
114. Garrett WE Jr, Swiontkowski MF, Weinstein JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: Part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
115. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98.
116. Bornmann L, Daniel HD. The state of h index research. Is the h index the ideal way to measure research performance? EMBO Rep. 2009;10(1):2-6.
Orthopedics and the sports medicine subspecialty are continually evolving fields that depend on research investigation and publication to further knowledge and advance practice. Research has produced new findings that have changed the way we practice sports medicine. In this review, we identify the most widely referenced sports medicine topics and articles, which we believe by their permeative presence in the literature have made lasting contributions to the field.
Many factors can be used to quantify the influence of an academic article on the practice of medicine. Citation analysis is one method that reflects the impact of a publication on the academic medical community.1-3 Total citations record the number of times a journal article has been credited by another study. Therefore, citation count indirectly highlights the articles that are widespread, relevant, and that form the foundation for other investigations on the topic. Related to the impact of the article is the impact of the journal that published the study. We examined journals by impact factor, a score based on the mean number of citations a published article received during the preceding 2 years.
Similar analyses have been performed of publication history in orthopedics and other medical fields. Investigators have examined which historical articles were the most influential in orthopedics as a whole,4 pediatric orthopedics,5,6 shoulder surgery,7 and arthroscopy.8 This influence has also been studied in general surgery,9 otolaryngology,10 plastic surgery,11 dermatology,12 critical care,13 and other disciplines. To our knowledge, the present study is the first bibliometric analysis of the highest-impact articles in orthopedic sports medicine.
Our goal was to identify the 100 articles that have had the highest impact on the clinical orthopedic sports medicine literature. We hypothesized that the most widely recognized articles would be from the highest-impact journals and may also have earlier publication dates. We describe the topics and objectives of these articles to highlight the sports medicine areas on which most research has focused during the past century.
Materials and Methods
Our bibliometric analysis used the Thomson Reuters Web of Knowledge, which consists of all publications from 1900 to the present. This research modality ranks journal articles by frequency of citation. Similar analyses have identified the most often cited articles in pediatric orthopedics,5 shoulder surgery,7 and arthroscopy.8 In our analysis, we included the top 25 journals by impact factor in the field of sports medicine, as rated by the Journal Citation Reports database. Within the highest-impact journals, we sorted all articles by those most often cited, and read them all to identify which ones discuss conditions commonly encountered in the clinical practice of sports medicine. We focused on clinical articles only and therefore excluded related basic science and cadaveric biomechanical studies. The 100 most cited articles were then further evaluated by primary author, journal of publication, institution, country of origin, year of publication, topic, and total number of citations. One-way analysis of variance (ANOVA) and linear regression analyses were used to determine if publication date correlated with mean number of citations.
Results
Eighty authors wrote the top 100 articles in sports medicine, and each publication garnered several hundred citations, ranging from 229 to 1629 with a mean of 408 (Table 114-113). Most of these articles were written in the past 3 decades, with equal distribution from the 1980s, 1990s, and 2000s (Figure 1A). We ran a linear regression to determine if publication date correlated with higher number of citations by virtue of longer time available for citation. The analysis poorly modeled the variability (R2 = 0.05), revealing no correlation between number of citations and publication date. Further, 1-way ANOVA found no significant difference between the number of citations per decade, F(5, 93) = 1.60, P = .17 (Figure 1B). Despite this finding, the oldest cited article, written by Fairbank39 in 1948, ranked high (position 7). Of these top 100 publications, the most recent, written by Knutsen and colleagues69 in 2007, ranked in the second half at position 66.
Seven journals published the top 100 articles, with the American volume of the Journal of Bone and Joint Surgery publishing nearly half (44%) (Table 2). In second place, with 28 articles, was the American Journal of Sports Medicine, followed by the British volume of the Journal of Bone and Joint Surgery, with 10 articles.
Thirty different topics were investigated in this collection of articles, encompassing nearly every major research area of sports medicine. There was a heavy emphasis on anterior cruciate ligament (ACL) injury and reconstruction, knee rating systems, rotator cuff reconstruction, and chondrocyte transplantation (Table 3).
In several cases, an author contributed more than 1 classic article. In fact, 31 of the top 100 articles were by an individual who had coauthored 2 or more of the publications on this list. The researchers with the largest number of first-authored articles were Noyes88-92 (5 articles), Neer81-84 (4 articles), and Rowe,102-104 Daniel,35-37 Peterson,97-99 and Hewett52-54 (3 articles each) (Table 417,19,21-24,29-31,35-37,42,44,45,52-54,58,61-65,69,70,72,74,80-84,87-92,97-99,101-105,107,109,110,113). Articles from authors with multiple publications had a common topic.
Last, these articles originated from a number of different countries and institutions. Of the 15 source countries (Figure 2), the United States contributed the most (61 articles). Other countries had prominent representation: Sweden and Switzerland (8 each), United Kingdom (5), and Canada, France, and Norway (3 each). These articles originated from 69 universities, hospitals, and clinics; 21 institutions had 2 or more articles (Table 5). The 5 institutions with the highest number of articles were Hospital for Special Surgery, University of Bern, Columbia College of Physicians and Surgeons/Columbia-Presbyterian Medical Center, Cincinnati Sports Medicine and Orthopaedic Center, and Massachusetts General Hospital.
Discussion
Several trends can be ascertained from analyzing the top 100 clinical articles cited in sports medicine. The 5 most frequent topics discussed were ACL injury and reconstruction, knee rating systems for injury and function, rotator cuff reconstruction, chondrocyte transplantation, and femoroacetabular impingement (Table 3). Of those 5 topics, only ACL injury and reconstruction falls within the top 10 most common orthopedic surgical procedures performed in the United States reported by one analysis.114 The most common orthopedic surgical procedure, knee arthroscopy, ranks 10th of all topics covered by the top 100 articles, whereas the second most common procedure, shoulder arthroscopy, was not discussed by any of those 100 articles. Also notable is the high frequency of knee rating system studies, which correlates well with the fact that 4 of the most common orthopedic surgical procedures are knee procedures. The prevalence of rating system articles reflects the importance of and need for accurate methods in the diagnosis of injuries in sports medicine.
The most cited sports medicine article was written by Insall and colleagues62 in 1989, more than 2 decades ago. In this article, “Rationale of the Knee Society Clinical Rating System,” they reported on a rigorous system that rates knee function and ability to walk and climb stairs. The second most cited article, “A Clinical Method of Functional Assessment of the Shoulder,” was written in 1987 by Constant and Murley.32 This article discusses another rating system but offers a functional assessment of the shoulder that is highly reproducible and time-efficient. “Rating Systems in the Evaluation of Knee Ligament Injuries,” the third most cited article, was written in 1985 by Tegner and Lysholm.113 This article details the complexities and variable uses of different knee ligament injury rating systems. These top 3 articles were all published in Clinical Orthopaedics and Related Research. In addition, all 3 discussed rating systems, reinforcing the need for accurate scoring systems to standardize the diagnosis of injury across the field of orthopedics and qualify outcomes after injury.
A number of studies have introduced physical examination findings, clinical tests, and rating systems used in the clinical setting of sports medicine (and named after the contributing authors). For example, the Neer sign82 and the Hawkins-Kennedy test51 are used to determine shoulder impingement. In knee ligament injuries, the Tegner knee activity score113 complements other functional scores (eg, Lysholm knee score74). For grading joint cartilage breakdown, the Outerbridge classification system96 is commonly used. The Fairbank test39 is used to gauge knee instability. In evaluating fatty degeneration of rotator cuff muscles through computed tomography scans, the Goutallier classification47 is used. Other metrics, such as the Knee Injury and Osteoarthritis Outcome Score, introduced by Roos and colleagues,101 measure knee injury and osteoarthritis. In other scenarios, studies have improved on surgical techniques—for example, the Neer open modification84 of the Bankart procedure. Many of these rating systems and named clinical findings are so ingrained in the practice and vernacular of orthopedics that it is possible they are in fact undercited in the literature.
As in other bibliometric analyses, one concession made here was to credit the first author listed for making the primary contribution to an article. As a result of journal variability and inconsistency, we were precluded from analyzing senior authors. When analyzed for authorship at any position, 3 of the top authors (Table 4) showed contributions to additional articles in the top 100 list. Noyes was listed as last author on 2 other articles,52,54 raising his total to 7. Daniel was listed as second author on 1 additional article,105 and Beck was listed as third author on 1 other article,42 raising their totals to 4 and 3, respectively.
A criticism of bibliometric analysis is its use of number of citations as an accurate measure of academic contribution. However, other methods for measuring the productivity and impact of researchers (eg, the recently developed Hirsch Index) have their own drawbacks,115,116 including being able to compare authors only at the same point in their careers and self-citation. It is important to note that our analyses focused strictly on publications related to clinical sports medicine, with the exclusion of basic science and cadaveric biomechanical studies.
Through bibliometric citation analysis, we have identified the authors who have made lasting contributions to the field of sports medicine, and we have highlighted the publications that have been cited by hundreds to thousands of authors. This list identifies trends within the articles that have become “classic,” by nature of their deep permeation into subsequent sports medicine literature, and offers guidance for trainees interested in studying the most high-yield sports medicine literature. Given that 69 institutions in 15 countries conducted these studies, we have also shown that orthopedic research can be readily disseminated internationally. Last, our study provides a thorough overview of the sports medicine literature over the past century and provides a strong framework for future research in our field.
Orthopedics and the sports medicine subspecialty are continually evolving fields that depend on research investigation and publication to further knowledge and advance practice. Research has produced new findings that have changed the way we practice sports medicine. In this review, we identify the most widely referenced sports medicine topics and articles, which we believe by their permeative presence in the literature have made lasting contributions to the field.
Many factors can be used to quantify the influence of an academic article on the practice of medicine. Citation analysis is one method that reflects the impact of a publication on the academic medical community.1-3 Total citations record the number of times a journal article has been credited by another study. Therefore, citation count indirectly highlights the articles that are widespread, relevant, and that form the foundation for other investigations on the topic. Related to the impact of the article is the impact of the journal that published the study. We examined journals by impact factor, a score based on the mean number of citations a published article received during the preceding 2 years.
Similar analyses have been performed of publication history in orthopedics and other medical fields. Investigators have examined which historical articles were the most influential in orthopedics as a whole,4 pediatric orthopedics,5,6 shoulder surgery,7 and arthroscopy.8 This influence has also been studied in general surgery,9 otolaryngology,10 plastic surgery,11 dermatology,12 critical care,13 and other disciplines. To our knowledge, the present study is the first bibliometric analysis of the highest-impact articles in orthopedic sports medicine.
Our goal was to identify the 100 articles that have had the highest impact on the clinical orthopedic sports medicine literature. We hypothesized that the most widely recognized articles would be from the highest-impact journals and may also have earlier publication dates. We describe the topics and objectives of these articles to highlight the sports medicine areas on which most research has focused during the past century.
Materials and Methods
Our bibliometric analysis used the Thomson Reuters Web of Knowledge, which consists of all publications from 1900 to the present. This research modality ranks journal articles by frequency of citation. Similar analyses have identified the most often cited articles in pediatric orthopedics,5 shoulder surgery,7 and arthroscopy.8 In our analysis, we included the top 25 journals by impact factor in the field of sports medicine, as rated by the Journal Citation Reports database. Within the highest-impact journals, we sorted all articles by those most often cited, and read them all to identify which ones discuss conditions commonly encountered in the clinical practice of sports medicine. We focused on clinical articles only and therefore excluded related basic science and cadaveric biomechanical studies. The 100 most cited articles were then further evaluated by primary author, journal of publication, institution, country of origin, year of publication, topic, and total number of citations. One-way analysis of variance (ANOVA) and linear regression analyses were used to determine if publication date correlated with mean number of citations.
Results
Eighty authors wrote the top 100 articles in sports medicine, and each publication garnered several hundred citations, ranging from 229 to 1629 with a mean of 408 (Table 114-113). Most of these articles were written in the past 3 decades, with equal distribution from the 1980s, 1990s, and 2000s (Figure 1A). We ran a linear regression to determine if publication date correlated with higher number of citations by virtue of longer time available for citation. The analysis poorly modeled the variability (R2 = 0.05), revealing no correlation between number of citations and publication date. Further, 1-way ANOVA found no significant difference between the number of citations per decade, F(5, 93) = 1.60, P = .17 (Figure 1B). Despite this finding, the oldest cited article, written by Fairbank39 in 1948, ranked high (position 7). Of these top 100 publications, the most recent, written by Knutsen and colleagues69 in 2007, ranked in the second half at position 66.
Seven journals published the top 100 articles, with the American volume of the Journal of Bone and Joint Surgery publishing nearly half (44%) (Table 2). In second place, with 28 articles, was the American Journal of Sports Medicine, followed by the British volume of the Journal of Bone and Joint Surgery, with 10 articles.
Thirty different topics were investigated in this collection of articles, encompassing nearly every major research area of sports medicine. There was a heavy emphasis on anterior cruciate ligament (ACL) injury and reconstruction, knee rating systems, rotator cuff reconstruction, and chondrocyte transplantation (Table 3).
In several cases, an author contributed more than 1 classic article. In fact, 31 of the top 100 articles were by an individual who had coauthored 2 or more of the publications on this list. The researchers with the largest number of first-authored articles were Noyes88-92 (5 articles), Neer81-84 (4 articles), and Rowe,102-104 Daniel,35-37 Peterson,97-99 and Hewett52-54 (3 articles each) (Table 417,19,21-24,29-31,35-37,42,44,45,52-54,58,61-65,69,70,72,74,80-84,87-92,97-99,101-105,107,109,110,113). Articles from authors with multiple publications had a common topic.
Last, these articles originated from a number of different countries and institutions. Of the 15 source countries (Figure 2), the United States contributed the most (61 articles). Other countries had prominent representation: Sweden and Switzerland (8 each), United Kingdom (5), and Canada, France, and Norway (3 each). These articles originated from 69 universities, hospitals, and clinics; 21 institutions had 2 or more articles (Table 5). The 5 institutions with the highest number of articles were Hospital for Special Surgery, University of Bern, Columbia College of Physicians and Surgeons/Columbia-Presbyterian Medical Center, Cincinnati Sports Medicine and Orthopaedic Center, and Massachusetts General Hospital.
Discussion
Several trends can be ascertained from analyzing the top 100 clinical articles cited in sports medicine. The 5 most frequent topics discussed were ACL injury and reconstruction, knee rating systems for injury and function, rotator cuff reconstruction, chondrocyte transplantation, and femoroacetabular impingement (Table 3). Of those 5 topics, only ACL injury and reconstruction falls within the top 10 most common orthopedic surgical procedures performed in the United States reported by one analysis.114 The most common orthopedic surgical procedure, knee arthroscopy, ranks 10th of all topics covered by the top 100 articles, whereas the second most common procedure, shoulder arthroscopy, was not discussed by any of those 100 articles. Also notable is the high frequency of knee rating system studies, which correlates well with the fact that 4 of the most common orthopedic surgical procedures are knee procedures. The prevalence of rating system articles reflects the importance of and need for accurate methods in the diagnosis of injuries in sports medicine.
The most cited sports medicine article was written by Insall and colleagues62 in 1989, more than 2 decades ago. In this article, “Rationale of the Knee Society Clinical Rating System,” they reported on a rigorous system that rates knee function and ability to walk and climb stairs. The second most cited article, “A Clinical Method of Functional Assessment of the Shoulder,” was written in 1987 by Constant and Murley.32 This article discusses another rating system but offers a functional assessment of the shoulder that is highly reproducible and time-efficient. “Rating Systems in the Evaluation of Knee Ligament Injuries,” the third most cited article, was written in 1985 by Tegner and Lysholm.113 This article details the complexities and variable uses of different knee ligament injury rating systems. These top 3 articles were all published in Clinical Orthopaedics and Related Research. In addition, all 3 discussed rating systems, reinforcing the need for accurate scoring systems to standardize the diagnosis of injury across the field of orthopedics and qualify outcomes after injury.
A number of studies have introduced physical examination findings, clinical tests, and rating systems used in the clinical setting of sports medicine (and named after the contributing authors). For example, the Neer sign82 and the Hawkins-Kennedy test51 are used to determine shoulder impingement. In knee ligament injuries, the Tegner knee activity score113 complements other functional scores (eg, Lysholm knee score74). For grading joint cartilage breakdown, the Outerbridge classification system96 is commonly used. The Fairbank test39 is used to gauge knee instability. In evaluating fatty degeneration of rotator cuff muscles through computed tomography scans, the Goutallier classification47 is used. Other metrics, such as the Knee Injury and Osteoarthritis Outcome Score, introduced by Roos and colleagues,101 measure knee injury and osteoarthritis. In other scenarios, studies have improved on surgical techniques—for example, the Neer open modification84 of the Bankart procedure. Many of these rating systems and named clinical findings are so ingrained in the practice and vernacular of orthopedics that it is possible they are in fact undercited in the literature.
As in other bibliometric analyses, one concession made here was to credit the first author listed for making the primary contribution to an article. As a result of journal variability and inconsistency, we were precluded from analyzing senior authors. When analyzed for authorship at any position, 3 of the top authors (Table 4) showed contributions to additional articles in the top 100 list. Noyes was listed as last author on 2 other articles,52,54 raising his total to 7. Daniel was listed as second author on 1 additional article,105 and Beck was listed as third author on 1 other article,42 raising their totals to 4 and 3, respectively.
A criticism of bibliometric analysis is its use of number of citations as an accurate measure of academic contribution. However, other methods for measuring the productivity and impact of researchers (eg, the recently developed Hirsch Index) have their own drawbacks,115,116 including being able to compare authors only at the same point in their careers and self-citation. It is important to note that our analyses focused strictly on publications related to clinical sports medicine, with the exclusion of basic science and cadaveric biomechanical studies.
Through bibliometric citation analysis, we have identified the authors who have made lasting contributions to the field of sports medicine, and we have highlighted the publications that have been cited by hundreds to thousands of authors. This list identifies trends within the articles that have become “classic,” by nature of their deep permeation into subsequent sports medicine literature, and offers guidance for trainees interested in studying the most high-yield sports medicine literature. Given that 69 institutions in 15 countries conducted these studies, we have also shown that orthopedic research can be readily disseminated internationally. Last, our study provides a thorough overview of the sports medicine literature over the past century and provides a strong framework for future research in our field.
1. Adams AB, Simonson D. Publication, citations, and impact factors of leading investigators in critical care medicine. Respir Care. 2004;49(3):276-281.
2. Bhandari M, Busse J, Devereaux PJ, et al. Factors associated with citation rates in the orthopedic literature. Can J Surg. 2007;50(2):119-123.
3. Cheek J, Garnham B, Quan J. What’s in a number? Issues in providing evidence of impact and quality of research(ers). Qual Health Res. 2006;16(3):423-435.
4. Kelly JC, Glynn RW, O’Briain DE, Felle P, McCabe JP. The 100 classic papers of orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Br. 2010;92(10):1338-1343.
5. Kavanagh RG, Kelly JC, Kelly PM, Moore DP. The 100 classic papers of pediatric orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Am. 2013;95(18):e134.
6. Mehlman CT, Wenger DR. The top 25 at 25: citation classics in the Journal of Pediatric Orthopaedics. J Pediatr Orthop. 2006;26(5):691-694.
7. Namdari S, Baldwin K, Kovatch K, Huffman GR, Glaser D. Fifty most cited articles in orthopedic shoulder surgery. J Shoulder Elbow Surg. 2012;21(12):1796-1802.
8. Cassar Gheiti AJ, Downey RE, Byrne DP, Molony DC, Mulhall KJ. The 25 most cited articles in arthroscopic orthopaedic surgery. Arthroscopy. 2012;28(4):548-564.
9. Paladugu R, Schein M, Gardezi S, Wise L. One hundred citation classics in general surgical journals. World J Surg. 2002;26(9):1099-1105.
10. Fenton JE, Roy D, Hughes JP, Jones AS. A century of citation classics in otolaryngology-head and neck surgery journals. J Laryngol Otol. 2002;116(7):494-498.
11. Loonen MPJ, Hage JJ, Kon M. Plastic surgery classics: characteristics of 50 top-cited articles in four plastic surgery journals since 1946. Plast Reconstr Surg. 2008;121(5):320e-327e.
12. Dubin D, Hafner AW, Arndt KA. Citation classics in clinical dermatologic journals. Citation analysis, biomedical journals, and landmark articles, 1945–1990. Arch Dermatol. 1993;129(9):1121-1129.
13. Baltussen A, Kindler CH. Citation classics in critical care medicine. Intensive Care Med. 2004;30(5):902-910.
14. Aglietti P, Buzzi R, Zaccherotti G, De Biase P. Patellar tendon versus doubled semitendinosus and gracilis tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 1994;22(2):211-218.
15. Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66(5):666-671.
16. Altchek DW, Warren RF, Skyhar MJ, Ortiz G. T-plasty modification of the Bankart procedure for multidirectional instability of the anterior and inferior types. J Bone Joint Surg Am. 1991;73(1):105-112.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701.
19. Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16(2):113-122.
20. Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17(1):1-6.
21. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640-645.
22. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
23. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
24. Bentley G, Biant LC, Carrington RWJ, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223-230.
25. Berchuck M, Andriacchi TP, Bach BR, Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72(6):871-877.
26. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41(6):988-1020.
27. Binkley JM, Stratford PW, Lott SA, Riddle DL, North American Orthopaedic Rehabilitation Research Network. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371-383.
28. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
29. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
30. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
31. Clancy WG Jr, Shelbourne KD, Zoellner GB, Keene JS, Reider B, Rosenberg TD. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J Bone Joint Surg Am. 1983;65(3):310-322.
32. Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
33. Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27(3):444-454.
34. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
35. Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R. Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg Am. 1985;67(5):720-726.
36. Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632-644.
37. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401-407.
38. Ellman H, Hanker G, Bayer M. Repair of the rotator cuff. End-result study of factors influencing reconstruction. J Bone Joint Surg Am. 1986;68(8):1136-1144.
39. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
40. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
41. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
42. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):111-119.
43. Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;(304):43-53.
44. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
45. Gerber C, Krushell RJ. Isolated rupture of the tendon of the subscapularis muscle. Clinical features in 16 cases. J Bone Joint Surg Br. 1991;73(3):389-394.
46. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
47. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78-83.
48. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE Jr. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650.
49. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
50. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
51. Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med. 1980;8(3):151-157.
52. Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699-706.
53. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492-501.
54. Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765-773.
55. Homminga GN, Bulstra SK, Bouwmeester PSM, van der Linden AJ. Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br. 1990;72(6):1003-1007.
56. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185-192.
57. Hovelius L, Augustini BG, Fredin H, Johansson O, Norlin R, Thorling J. Primary anterior dislocation of the shoulder in young patients. A ten-year prospective study. J Bone Joint Surg Am. 1996;78(11):1677-1684.
58. Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58(2):159-172.
59. Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427-436.
60. Iannotti JP, Zlatkin MB, Esterhai JL, Kressel HY, Dalinka MK, Spindler KP. Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg Am. 1991;73(1):17-29.
61. Insall J, Falvo KA, Wise DW. Chondromalacia patellae. A prospective study. J Bone Joint Surg Am. 1976;58(1):1-8.
62. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
63. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600-613.
64. Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132-1145.
65. Ito K, Minka MA 2nd, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect. A MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br. 2001;83(2):171-176.
66. Johnson RJ, Kettelkamp DB, Clark W, Leaverton P. Factors affecting late results after meniscectomy. J Bone Joint Surg Am. 1974;56(3):719-729.
67. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204-208.
68. Jones KG. Reconstruction of the anterior cruciate ligament: a technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
69. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-2112.
70. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86(3):455-464.
71. Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159-163.
72. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
73. Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276-291.
74. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154.
75. Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003-1010.
76. Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991;19(5):478-484.
77. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
78. Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9(3):318-321.
79. McDaniel WJ Jr, Dameron TB Jr. Untreated ruptures of the anterior cruciate ligament. A follow-up study. J Bone Joint Surg Am. 1980;62(5):696-705.
80. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
81. Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54(1):41-50.
82. Neer CS 2nd. Impingement lesions. Clin Orthop Relat Res. 1983;(173):70-77.
83. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
84. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder. A preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
85. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
86. Nistor L. Surgical and non-surgical treatment of Achilles tendon rupture. J Bone Joint Surg Am. 1981;63(3):394-399.
87. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
88. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513-518.
89. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62(5):687-695, 757.
90. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate–deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
91. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate–deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
92. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
93. O’Brien SJ, Warren RF, Pavlov H, Panariello R, Wickiewicz TL. Reconstruction of the chronically insufficient anterior cruciate ligament with the central third of the patellar ligament. J Bone Joint Surg Am. 1991;73(2):278-286.
94. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
95. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
96. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43(4):752-757.
97. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12.
98. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17-24.
99. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-234.
100. Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80(9):1276-1284.
101. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96.
102. Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am. 1956;38(5):957-977.
103. Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am. 1978;60(1):1-16.
104. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
105. Sachs RA, Daniel DM, Stone ML, Garfein RF. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17(6):760-765.
106. Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65(4):456-460.
107. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299.
108. Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders [see comments]. J Bone Joint Surg Am. 1995;77(1):10-15.
109. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85(2):278-286.
110. Solomonow M, Baratta R, Zhou BH, et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15(3):207-213.
111. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484.
112. Tapper EM, Hoover NW. Late results after meniscectomy. J Bone Joint Surg Am. 1969;51(3):517-526.
113. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-49.
114. Garrett WE Jr, Swiontkowski MF, Weinstein JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: Part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
115. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98.
116. Bornmann L, Daniel HD. The state of h index research. Is the h index the ideal way to measure research performance? EMBO Rep. 2009;10(1):2-6.
1. Adams AB, Simonson D. Publication, citations, and impact factors of leading investigators in critical care medicine. Respir Care. 2004;49(3):276-281.
2. Bhandari M, Busse J, Devereaux PJ, et al. Factors associated with citation rates in the orthopedic literature. Can J Surg. 2007;50(2):119-123.
3. Cheek J, Garnham B, Quan J. What’s in a number? Issues in providing evidence of impact and quality of research(ers). Qual Health Res. 2006;16(3):423-435.
4. Kelly JC, Glynn RW, O’Briain DE, Felle P, McCabe JP. The 100 classic papers of orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Br. 2010;92(10):1338-1343.
5. Kavanagh RG, Kelly JC, Kelly PM, Moore DP. The 100 classic papers of pediatric orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Am. 2013;95(18):e134.
6. Mehlman CT, Wenger DR. The top 25 at 25: citation classics in the Journal of Pediatric Orthopaedics. J Pediatr Orthop. 2006;26(5):691-694.
7. Namdari S, Baldwin K, Kovatch K, Huffman GR, Glaser D. Fifty most cited articles in orthopedic shoulder surgery. J Shoulder Elbow Surg. 2012;21(12):1796-1802.
8. Cassar Gheiti AJ, Downey RE, Byrne DP, Molony DC, Mulhall KJ. The 25 most cited articles in arthroscopic orthopaedic surgery. Arthroscopy. 2012;28(4):548-564.
9. Paladugu R, Schein M, Gardezi S, Wise L. One hundred citation classics in general surgical journals. World J Surg. 2002;26(9):1099-1105.
10. Fenton JE, Roy D, Hughes JP, Jones AS. A century of citation classics in otolaryngology-head and neck surgery journals. J Laryngol Otol. 2002;116(7):494-498.
11. Loonen MPJ, Hage JJ, Kon M. Plastic surgery classics: characteristics of 50 top-cited articles in four plastic surgery journals since 1946. Plast Reconstr Surg. 2008;121(5):320e-327e.
12. Dubin D, Hafner AW, Arndt KA. Citation classics in clinical dermatologic journals. Citation analysis, biomedical journals, and landmark articles, 1945–1990. Arch Dermatol. 1993;129(9):1121-1129.
13. Baltussen A, Kindler CH. Citation classics in critical care medicine. Intensive Care Med. 2004;30(5):902-910.
14. Aglietti P, Buzzi R, Zaccherotti G, De Biase P. Patellar tendon versus doubled semitendinosus and gracilis tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 1994;22(2):211-218.
15. Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66(5):666-671.
16. Altchek DW, Warren RF, Skyhar MJ, Ortiz G. T-plasty modification of the Bankart procedure for multidirectional instability of the anterior and inferior types. J Bone Joint Surg Am. 1991;73(1):105-112.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701.
19. Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16(2):113-122.
20. Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17(1):1-6.
21. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640-645.
22. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
23. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
24. Bentley G, Biant LC, Carrington RWJ, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223-230.
25. Berchuck M, Andriacchi TP, Bach BR, Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72(6):871-877.
26. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41(6):988-1020.
27. Binkley JM, Stratford PW, Lott SA, Riddle DL, North American Orthopaedic Rehabilitation Research Network. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371-383.
28. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
29. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
30. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
31. Clancy WG Jr, Shelbourne KD, Zoellner GB, Keene JS, Reider B, Rosenberg TD. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J Bone Joint Surg Am. 1983;65(3):310-322.
32. Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
33. Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27(3):444-454.
34. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
35. Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R. Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg Am. 1985;67(5):720-726.
36. Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632-644.
37. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401-407.
38. Ellman H, Hanker G, Bayer M. Repair of the rotator cuff. End-result study of factors influencing reconstruction. J Bone Joint Surg Am. 1986;68(8):1136-1144.
39. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
40. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
41. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
42. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):111-119.
43. Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;(304):43-53.
44. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
45. Gerber C, Krushell RJ. Isolated rupture of the tendon of the subscapularis muscle. Clinical features in 16 cases. J Bone Joint Surg Br. 1991;73(3):389-394.
46. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
47. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78-83.
48. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE Jr. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650.
49. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
50. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
51. Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med. 1980;8(3):151-157.
52. Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699-706.
53. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492-501.
54. Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765-773.
55. Homminga GN, Bulstra SK, Bouwmeester PSM, van der Linden AJ. Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br. 1990;72(6):1003-1007.
56. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185-192.
57. Hovelius L, Augustini BG, Fredin H, Johansson O, Norlin R, Thorling J. Primary anterior dislocation of the shoulder in young patients. A ten-year prospective study. J Bone Joint Surg Am. 1996;78(11):1677-1684.
58. Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58(2):159-172.
59. Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427-436.
60. Iannotti JP, Zlatkin MB, Esterhai JL, Kressel HY, Dalinka MK, Spindler KP. Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg Am. 1991;73(1):17-29.
61. Insall J, Falvo KA, Wise DW. Chondromalacia patellae. A prospective study. J Bone Joint Surg Am. 1976;58(1):1-8.
62. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
63. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600-613.
64. Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132-1145.
65. Ito K, Minka MA 2nd, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect. A MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br. 2001;83(2):171-176.
66. Johnson RJ, Kettelkamp DB, Clark W, Leaverton P. Factors affecting late results after meniscectomy. J Bone Joint Surg Am. 1974;56(3):719-729.
67. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204-208.
68. Jones KG. Reconstruction of the anterior cruciate ligament: a technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
69. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-2112.
70. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86(3):455-464.
71. Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159-163.
72. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
73. Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276-291.
74. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154.
75. Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003-1010.
76. Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991;19(5):478-484.
77. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
78. Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9(3):318-321.
79. McDaniel WJ Jr, Dameron TB Jr. Untreated ruptures of the anterior cruciate ligament. A follow-up study. J Bone Joint Surg Am. 1980;62(5):696-705.
80. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
81. Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54(1):41-50.
82. Neer CS 2nd. Impingement lesions. Clin Orthop Relat Res. 1983;(173):70-77.
83. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
84. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder. A preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
85. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
86. Nistor L. Surgical and non-surgical treatment of Achilles tendon rupture. J Bone Joint Surg Am. 1981;63(3):394-399.
87. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
88. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513-518.
89. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62(5):687-695, 757.
90. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate–deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
91. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate–deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
92. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
93. O’Brien SJ, Warren RF, Pavlov H, Panariello R, Wickiewicz TL. Reconstruction of the chronically insufficient anterior cruciate ligament with the central third of the patellar ligament. J Bone Joint Surg Am. 1991;73(2):278-286.
94. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
95. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
96. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43(4):752-757.
97. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12.
98. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17-24.
99. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-234.
100. Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80(9):1276-1284.
101. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96.
102. Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am. 1956;38(5):957-977.
103. Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am. 1978;60(1):1-16.
104. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
105. Sachs RA, Daniel DM, Stone ML, Garfein RF. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17(6):760-765.
106. Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65(4):456-460.
107. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299.
108. Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders [see comments]. J Bone Joint Surg Am. 1995;77(1):10-15.
109. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85(2):278-286.
110. Solomonow M, Baratta R, Zhou BH, et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15(3):207-213.
111. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484.
112. Tapper EM, Hoover NW. Late results after meniscectomy. J Bone Joint Surg Am. 1969;51(3):517-526.
113. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-49.
114. Garrett WE Jr, Swiontkowski MF, Weinstein JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: Part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
115. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98.
116. Bornmann L, Daniel HD. The state of h index research. Is the h index the ideal way to measure research performance? EMBO Rep. 2009;10(1):2-6.
Addressing the Sexual Health Concerns of Women with Gynecologic Cancer: Guidance for Primary Care Physicians
From the Yale School of Medicine, New Haven, CT.
Abstract
- Objective: To review the sexual health concerns of women with gynecologic cancer and provide guidance for primary care physicians.
- Methods: Review of the literature.
- Results: Issues of sexuality and intimacy are known to significantly impact the quality of life of patients following diagnosis and treatment of gynecologic cancers. At the time of diagnosis, women should be informed of the potential physiologic, hormonal, and psychosocial effects of gynecologic cancer on sexuality. Many providers fail to address these issues given time constraints and patients’ trepidation in alerting their providers to their concerns. While systemic hormone therapy directly addresses these symptoms, its use remains controversial due to potential cancer recurrence risks. Thus, treatment centers around therapeutic alternatives. For vasomotor symptoms, selective serotonin reuptake inhibitors have shown effectiveness and are typically well tolerated, and antiepileptics such as gabapentin have shown promise. There is promising but limited data employing pelvic floor physical therapy as a tool to aid in addressing pelvic floor symptoms. Psychological care and the involvement of the partner are also part of managing the sexual health concerns of these patients.
- Conclusion: Sexual morbidity is a distressing and undertreated problem among gynecologic cancer survivors. Successful treatment requires the provider’s appreciation of the problem and willingness to address it.
Issues of sexuality and intimacy are known to significantly impact the lives of patients following diagnosis and treatment of gynecologic cancers. [1,2] Treatment of gynecologic malignancy is highly dependent on pathology and stage, with some patients receiving small excisional procedures while others subject to extensive surgeries, chemotherapy, and radiation treatment, and it is difficult to predict how each individual’s sexual health will be impacted. However, the evidence suggests that at least half of women treated for gynecologic cancer will experience sexual dysfunction [3]. Although the impact of gynecologic cancer and treatment can be profound, providers often do not address their patients’ sexual concerns [4,5], yet most patients have indicated that they would like these issues to be addressed [6].
Female Sexual Dysfunction
Sexual dysfunction is multifactorial and involves physical, social, and psychological dimensions. It is common in the general population, with rates ranging from 25-63% [7]. The DSM-IV [8] defines female sexual dysfunction as a disturbance in or pain during the sexual response, which can be further classified as hypoactive sexual disorder, orgasmic disorder, sexual pain disorder, or sexual arousal disorder [9]. It should be noted that women who have been treated for gynecologic cancers may have a premorbid history of sexual dysfunction. Assessment of a woman’s sexual function prior to her cancer diagnosis can help establish which sexuality changes are due to the cancer treatment and may allow the provider to tailor interventions accordingly.
Sexual Dysfunction and Quality of Life
As treatments for gynecologic cancers improve, toxicity of treatment decreases, and survival increases, quality of life for survivors has become as become an increasingly important health issue. Several studies have examined patient-reported quality of life in short- and long-term cancer survivors and report overall significant alteration in quality of life over many aspects of health and psychological well-being [10]. It is well established that health-related quality of life and sexual functioning are closely associated [11].
In gynecologic cancer patients, sexual and quality of life outcomes can vary. For example, Kim el al recently compared quality of life and sexual functioning in ovarian cancer survivors with no evidence of disease after primary treatment and a cohort of health women. Sexuality, both in terms of desire, arousal, lubrication, orgasm, satisfaction, and pain and in terms of interest in sex, sexual activity, and enjoyment of sex were similar between the groups; however social functioning deteriorated in cancer patients [12]. Other women report different experiences. Women with a history of vulvar, vaginal, or cervical cancer who may have had extensive pelvic surgery, lymphadenectomy, and radiation treatment to the pelvis typically report greater alterations in quality of life and sexual activity depending on the extent of their treatment [13].
Patients undergoing chemotherapy often report significant changes in quality of life due to the physical symptoms of fatigue, nausea, hair loss, diarrhea, etc. as well the psychological effects of cancer diagnosis, changes in body image, and poor coping [14]. Sexual side effects are common due to alterations in the HPA axis, direct gonadal toxicity and neuropathies [15].
Impact of Gynecologic Cancer on Sexuality
Gynecologic cancer and treatment can alter sexuality through a variety of effects. The impact of anatomical changes may alter a women’s self-esteem, body image, and sense of femininity, resulting in a reluctance to engage in intimate behaviors. Furthermore, if the partnership is disrupted by changes in roles in the household or within the relationship, relationships dynamics may be tested and result in mental distress for the patient, thus effecting sexuality and intimacy.
Surgical or chemical withdrawal of sex hormones, new medications, and postoperative sequelae can contribute to sexual arousal problems. The emotional and psychological components of a cancer diagnosis also can hinder the sexual response [16]. Depression and anxiety, the rates of which increase with cancer diagnosis, can also significantly affect sexual function [17].
Sexual pain is common in this population due to hormonal considerations as well as post-treatment side effects and a frequent cause of sexual dysfunction for these patients. Pelvic radiation may result in adverse physical changes to the vagina including vaginal stenosis, thinning of vaginal mucosa, loss of lubrication, and loss of elasticity [18,19]. A recent review of 20 studies of cervical cancer survivors found that patients were at risk for lack of lubrication and had high rates of dyspareunia [20]. Psychosocial factors have been found to be important predictors of sexual desire and more important than hormones in predicting low sexual desire in middle age [21]. These factors include emotional and physical closeness to the partner, satisfactory communication, and a positive relation to one’s own body [16].
Effects on Relationships and Partners
Women whose sexual capacity is compromised may be worried about their partners’ quality of life and overall well-being. Indeed, the partners of women with gynecologic cancer are also impacted by the changes to sexual function and loss of sexuality and intimacy. Hawkins et al found that cessation or decreased frequency of sex and intimacy was reported in 79% of male partners of women affected by cancer. Among partners of the persons with cancer in this study, changes to sexuality were associated with feelings of self-blame, reflection, sadness, anger and lack of sexual fulfillment [22]. Further, male partners of women diagnosed with gynecological cancer often express conflicting emotional states including feeling worried about their significant other’s health, having the desire to engage in sexual activity, and feeling guilty about wanting to increase sexual intimacy. These feelings, in turn, can lead to resentment and withdrawal from their partner and overall relationship discord [23].
Evidence suggests partners of cancer patients greatly benefit from increased social support even years after an apparent cure [24]. Specifically, male partners who take on a new role as caregiver in the relationship experience difficulties with emotional changes, challenges to their masculinity, and new stressors [25]. These new roles and feelings also contribute to changes in sexuality and intimacy in relationships, making support for partners all the more necessary.
Menopausal Symptoms Following Cancer Treatment
Gynecologic cancer treatment invariably affects the female hormonal balance, sometimes suddenly with surgical excision of the gonads or via radiation treatment or chemotherapy. It is well known that the sudden withdrawal of estrogen and testosterone, especially in the premenopausal postoperative population, can lead to significant acute menopausal symptoms. Given the many emotional and physical issues affecting patients during treatment, it can be difficult to delineate what proportion of sexual problems are caused by or enhanced by vasomotor symptoms, sleep disorders, and vaginal atrophy.
In general, premenopausal women who experience abrupt surgical menopause may often have immediate severe symptoms. Many agree that the younger a woman is when going through this process, the more severe her symptoms may be. Although the average age of endometrial cancer diagnosis is 67, approximately 25% of women who are diagnosed are premenopausal, and 5% of cases occur in women under 40. As the obesity epidemic worsens and more women are exposed to higher levels of estrogen at younger ages, it is expected that the number of premenopausal women who are diagnosed will continue to rise. The average age of diagnosis for ovarian cancer is 63, however, there is a large cohort of women diagnosed with borderline and malignant tumors in the premenopausal period [26]. These patients often experience vasomotor symptoms in the hours to days following surgery.
Interventions for Survivors of Gynecologic Cancer with Sexual Dysfunction
Systemic Hormone Therapy
Systemic hormonal therapy to treat menopausal symptoms remains controversial following the release of findings from the Women’s Health Initiative, which showed a number of adverse effects, including an increased risk of breast cancer in healthy postmenopausal women who received systemic hormonal therapy for menopausal symptoms. While views have changed since that time, providers are often reluctant to prescribe hormonal therapy, and patients are reticent to take it, due to fears of cancer recurrence. However, there is no evidence showing hormones negatively influence survival after treatment for epithelial ovarian, squamous cervical, or vulvar cancer [27]. With the exception of endometrial cancer, there is no biological evidence that HRT may increase recurrence risk [28]. Approach to clinical decision making should be individualized, taking into consideration the patients’ symptoms, quality of life, tumor histology, and overall prognosis.
Cervical cancer, vulvar cancer, and vaginal cancer are not considered hormonally responsive tumors. While the data are limited, a study published in 1987 of cervical cancer survivors treated with systemic hormone replacement therapy showed no increase in relapse rates and showed an increase in quality of life [29]. There are no studies regarding systemic HRT in patients with vulvar or vaginal cancer though it is generally accepted they can be treated with HRT. No significant data exists for cervical adenocarcinoma patients, and most oncologists suggest treating these patients the same as those with endometrial cancer primary.
There is limited data on the use of HRT in women with ovarian cancer but available studies do not show any difference in overall or disease-free survival between HRT groups and controls [27,30–32]. Many studies report a significant increase in quality of life with HRT. A 2013 retrospective chart review of 77 patients with epithelial ovarian cancer who received postoperative HRT showed no significant difference in progression-free survival [33].
The most controversy surrounds the use of HRT in patients with endometrial cancer [34]. The only major data available come from the Gynecologic Oncology Group’s truncated study, which was a large prospective randomized controlled trial. Over 1200 women treated for stage I and II endometrial cancer were enrolled between 1997 and 2003, and before the study was terminated these patients were followed for a median of 3 years following initiation of therapy. The recurrence rate of malignancy was low, 2.1, and an insignificant risk of recurrence of 1.27 was noted between with HRT and placebo groups (80% CI, 0.916-1.77) [35]. Women included in this study were between the ages of 26 and 91, and their indications for therapy included vasomotor symptoms, vaginal atrophy, as well as osteoporosis risk and cardiovascular disease risk. Gynecologist oncologists often use estrogen therapy to treat symptomatic women with early stage endometrial cancer given the very low risk of recurrence.
Tibolone is a synthetic steroid with activity on estrogen and progesterone receptors, and mainly acts as an agonist at estrogen receptors. It is prescribed outside the United States for osteoporosis and is being investigated as treatment for female sexual dysfunction. Efficacy on vasomotor symptoms has been positive thus far. One case-control study confirmed tibolone’s safety for endometrial cancer survivors, with no adverse effects on disease-free or overall survival [36]. One group recently examined tibolone in the setting of breast cancer patients in a prospective randomized controlled trial and reported an increased risk of breast cancer recurrences in women receiving tibolone for HT [37]. That study reported no increase in risk of gynecologic cancers and did report favorable outcomes for patients in terms of osteoporosis and vasomotor symptoms.
Topical Therapy
Women who are concerned about systemic HRT but who have been treated with radiation in addition to their surgery may be interested in topical estrogen therapy. These women may have vaginal stenosis and atrophic symptoms, and for them topical estrogen therapy can be helpful [38]. Many formulations of topical estrogen are available, including creams, tablets, and rings. Using vaginal estrogens and dilators can be useful to help with eventual resumption of sexual activity once healing has taken place and can help to avoid dyspareunia. Combination topical and systemic therapy can also be useful to relieve symptoms. Women concerned about absorption with topical therapy can be advised that while there is some absorption initially, absorption is reduced as atrophy is improved with treatment.
One recent study examined the use of alpha-tocopherol to reduce acute vaginal complications in women with endometrial and cervical cancer undergoing radiation treatment. The treatment group experienced reduced vaginal toxicity and pain, although vaginal secretion was not significantly different in the 2 groups studied [39]. No adverse effects were noted. This compound has not been studied further but may be beneficial in the future.
Some women may prefer to avoid hormones altogether. Over-the-counter vaginal moisturizers and lubricants can be recommended to women to help with intercourse and atrophic symptoms, with or without estrogen therapy.
Physical Therapy
Pelvic floor physical therapy has become an increasingly popular modality for treatment of many aspects pelvic floor dysfunction, the symptoms of which include defecatory dysfunction, constipation, bladder dysfunction, painful urination, dyspareunia, pelvic pain, and low back pain [40]. Pelvic floor physical therapy is well studied and utilized frequently for urinary incontinence and pelvic organ prolapse, but is understudied for sexual dysfunction [41,42].
Promising areas of study include educational sessions, cognitive behavioral therapy, vaginal dilator therapy, pelvic floor muscle strengthening and relaxation techniques with biofeedback, stretching and massage [40]. Biofeedback specifically has been studied in controlled studies for treatment of sexual dysfunction specifically in the setting of vulvar pain syndromes [43]. A small randomized control trial evaluated the use of pelvic floor muscle training in gynecologic cancer survivors and found that the intervention group reported improved sexual function and improved quality of life [44]. Given the few side effects of this therapy, it may be a helpful addition to a multidisciplinary approach to treating pelvic floor dysfunction in gynecologic cancer patients.
For cervical and endometrial cancer patients, vaginal dilators are often prescribed following radiation therapy to minimize vaginal stenosis. Literature suggests that after radiotherapy dilation therapy is effective when used as directed, typically 3 times per week for 10 minutes to mechanically separate the vaginal walls and increase elasticity [45]. Adherence to recommendations for vaginal dilator use is low in this population due to factors such as aversion to the practice and intrusiveness of the mechanism [46,47]. Acknowledgment of apprehension regarding dilator use should be part of the counseling prior to initiation of treatment; interventions designed to educate women about dilator use benefit may increase adherence [48].
Psychological Therapies
Survivors of gynecological cancer with sexual dysfunction may experience the psychological symptoms in the context of the other emotional challenges of a cancer diagnosis [49]. Changes in body image, physical appearance, and feelings of well-being can significantly affect sexuality and intimacy, while anxiety and negative feelings about a diagnosis can cause further sexual dysfunction. Conversely, sexual morbidity in these patients predicts worsening of general psychological symptoms, including depressive symptoms and overall quality of life [13–15].
While there are limited studies directly assessing the effect of psychological therapies for the treatment of sexual dysfunction in this population, the existing literature shows enduring symptomatic improvement following brief interventions. In women for whom emotional distress, depression, and anxiety appear to be a significant aspect of their concern regarding intimacy and sexuality, these interventions can be particularly helpful.
Cognitive behavioral therapy (CBT), which focuses on mindfulness and the relationships between maladaptive thoughts and how they impact behavior, has been shown to have efficacy in treating the broader psycho-social concerns of gynecological cancer patients [50,51]. In a recent study Brotto and colleagues randomized 31 survivors of gynecological cancer with self-reported sexual dysfunction to either three 90-minute CBT sessions or a waitlist control. Patients who underwent the intervention reported significant improvements in sexual arousal and desire both immediately post-therapy and at 6-month follow-up while patients in the waitlist arm experienced no significant changes in symptomatology [52].
Psychoeducational interventions are another promising avenue for addressing sexual dysfunction in this population [53]. In a 2008 therapeutic trial, Brotto and colleagues combined elements of CBT with education in 3 one-hour sessions featuring written materials on sexuality and relationships. The intervention enrolled 22 women with early stage gynecologic cancer who met criteria for female sexual arousal disorder. The psychoeducational intervention was associated with positive effect on sexual desire, arousal, orgasm, satisfaction, sexual distress, depression, and overall well-being [16]. Psychoeducation can also be used to augment other therapeutic modalities. Robinson and colleagues used such an intervention to improve adherence to vaginal dilator use in 32 women with early stage endometrial cancer who were being treated with radiotherapy [48].
Some gynecologic cancer patients may have significant alterations in their anatomy and thus penetrative intercourse may not be possible. In patients even without these physical changes, some may prefer to avoid intercourse due to pain or anxiety. Thus patients can have significant benefits from discussing their concerns with a therapist specializing in these issues as many patients are concerned about their ability to engage in intimate behavior. Therapy can assist with the changing sexual relationship and assist the partners in different ways of engaging in intimate acts. It is important to avoid stressing penetrative intercourse as the goal for sexual function with these or any patients with anxiety relating to their disease as there are many ways to engage in mutually pleasurable experiences for both partners, thus removing anxiety about inability to resume vaginal intercourse post-treatment. Discussing this with patients can be challenging but can often reduce anxiety surrounding body image issues following treatment.
Studies have shown that cancer care providers often do not adequately address sexual concerns [54] but that when these concerns are appropriately managed, patient satisfaction and quality of life significantly improve [55]. Several studies have focused on how providers can incorporate the approaches of CBT and psychoeducation to better address the sexual concerns of patients without requiring external psychiatric care [56,57]. Barbera et al have described a successful model in which multidisciplinary care teams provide education and counseling for gynecological cancer patients in a sexuality clinic [58]. patients had a significant symptom burden, including menopausal symptoms, the effects of radiation therapy, chemotherapy, and surgical operation as well as psychological responses to cancer, and reported high levels of satisfaction with their experience at the clinic.
Involvement of the partner in interventions has not been well studied; however, involving the partner in in psychological therapies to address sexual dysfunction should be beneficial.
Alternative Therapies for Vasomotor Symptoms
Gynecologic cancer patients suffering prominent vasomotor symptoms have limited alternatives to hormone therapy. Clinicians must balance potential medication benefit with potential exacerbation of other medical and psychological issues, including sexual dysfunction.
Antidepressants
The use of SSRIs and SNRIs for vasomotor symptoms was pioneered by medical oncologists for men with hot flashes secondary to GnRH agonist therapy for prostate cancer and women with breast cancer [59,60]. Limited studies have shown that antidepressant medications do not increase cancer recurrence risk in ovarian cancer patients and are relatively well tolerated [61]. However, these medications are known to have partial efficacy in improving vasomotor symptoms and may worsen sexual symptoms, a well-known side effect of antidepressants. There is variation of the reported rates of sexual dysfunction associated with various antidepressants and clinicians may take the likelihood of sexual side effects into account when prescribing SSRIs or SNRIS [62]. More recently developed SSRIS, such as citalopram and its enantiomer escitalopram, have shown significant improvements in vasomotor symptoms and were better tolerated than venlafaxine and fluoxetine [63,64]. Additionally, limited uncontrolled studies of mirtazipine, a structurally unique SSRI, and bupropion, which acts on dopamine and norepinephrine, have shown significant decreases in hot flash symptoms and are less associated with sexual side effects than SSRIS/SNRIS [65,66].
Other Agents
Other pharmaceutical options for menopausal vasomotor symptoms include gabapentin and adrenergic agonists. Gabapentin can yield impressive reductions in vasomotor symptoms. A recent double-blind randomized trial in 50 patients revealed a 60% reduction in hot flashes as 12 weeks and an 80% reduction in self-reported composite symptom scores [67]. However, side effects such as palpitations, edema, and fatigue, lead to high study withdrawal rates and limit its widespread clinical use for this indication [68]. Clonidine has been assessed versus venlafaxine in several clinical trials with breast cancer patients. These trials have shown mixed results, with findings of both inferiority and superiority to venlafaxine, but with consistent significant improvement in symptoms over placebo. Side effects, such insomnia, constipation and dry mouth, occurred but did not lead to higher dropout rates than venlafaxine [69,70].
Long-Term Sexual Outcomes
For women treated for gynecological cancers, alterations in sexual function may persist in the long term. A study following cervical cancer patients managed with radical hysterectomy up to 2 years post treatment showed they had more sexual dysfunction compared with healthy controls, although at rates similar to those who underwent radical hysterectomy for benign disease [71]. A 2007 review of quality of life studies revealed that although ovarian cancer survivors 5 years past diagnosis had excellent overall quality of life, sexual symptoms persisted, with as many as 57% of patients reporting a decline in sexual function due to their cancer [72].
Studies show some differences in outcomes based on treatment modality. A recent review of cervical cancer outcomes revealed that women who received radiotherapy as a component of their treatment have a higher risk of long-term sexual side effects [73]. In contrast, a study assessing endometrial cancer patients 5 years after initial diagnosis between those patients who had received surgery alone and those who had received surgery and vaginal brachytherapy. There was no significant difference in any measures of quality of life and sexual function between these 2 groups [74].
Age appears to play a role in long-term sexual outcomes regardless of diagnosis. Bifulco and colleagues assessed quality of life in survivors of gynecological cancer, comparing women under age 45 to those over 45 after nearly 3 years of survival. After controlling for age and other factors, younger patients were found to have worse sexual activity, including significantly higher rates of poor body image, perceived worse sexual vaginal function, and more severe menopausal symptoms, probably related to the effects of surgical menopause [75].
Despite enduring sexual dysfunction, symptoms tend to improve over time. A cohort study of 103 gynecological cancer patients undergoing radiation therapy were followed for 3 years. Patients were offered standard interventions for sexual dysfunction, including vaginal lubricants, dilators, and menopausal symptomatic therapy, although adherence to these measures was not assessed. Three years after initial therapy, the percentage of sexually active women increased from 21.5% to 44.2% [76]. In the subset of patients who successfully return to sexual activity, outcomes can be comparable to healthy peers. Kim and colleagues compared disease-free sexually active ovarian cancer patients with demographically matched healthy controls on standardized self-report measures. Sexual functioning did not differ between the 2 groups, despite lower social functioning in cancer survivors [12].
Conclusion
Sexuality and intimacy can be greatly affected by the diagnosis and treatment of gynecologic malignancies. It is important to routinely discuss sexuality and sexual functioning with patients from diagnosis onward. Reassuring patients, acknowledging the importance of their concerns, and validating their desire to enjoy improved intimacy should be considered part of the clinician’s role. Valuable information sources that may aid discussions are available on the internet. Oncolink (www.oncolink.org), a large cancer information website maintained by University of Pennsylvania Cancer Center, offers a plethora of information for patients and health care professionals. In addition, the American Cancer Society offers a detailed guide, “Sexuality for the Woman with Cancer” [77]. Treatment is available, and improvement in outcomes is possible. Further prospective studies are needed to clearly delineate risks and benefits of hormone replacement therapy in patients with gynecologic cancers.
Corresponding author: Elena S. Ratner, MD, PO Box 208063, New Haven, CT 06520, [email protected].
1. Juraskova I, Butow P, Robertson R, et al. Post-treatment sexual adjustment following cervical and endometrial cancer: a qualitative insight. Psychooncology 2003;12:267–79.
2. Bodurka DC, Sun CC. Sexual function after gynecologic cancer. Obstet Gynecol Clin North Am 2006;33:621–30, ix.
3. Andersen BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol 1997;65:221.
4. Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J 2009;15:74–7.
5. Stead ML, Brown JM, Fallowfield L, et al. Lack of communication between healthcare professionals and women with ovarian cancer about sexual issues. Br J Cancer 2003;88:666–71.
6. Stead ML, Fallowfield L, Brown JM, et al. Communication about sexual problems and sexual concerns in ovarian cancer: qualitative study. BMJ 2001;323:836–7.
7. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537–44.
8. American Psychiatric Association. Diagnostic and statistical manual of mental health disorders. 4th ed. 2000.
9. Elder JA, Braver Y. Female sexual dysfunction. In: Current clinical medicine. Elsevier; 2010:1222–6.
10. Ferrell BR, Dow KH, Leigh S, et al. Quality of life in long-term cancer survivors. Oncol Nurs Forum 1995;22:915–22.
11. Shamspour N, Assari S, Moghana Lankarani M. Relation between sexuality and health-related quality of life. In: Handbook of disease burdens and quality of life measures. New York: Springer 2010;3457–73.
12. Kim SI, Lee Y, Lim MC, et al. Quality of life and sexuality comparison between sexually active ovarian cancer survivors and healthy women. J Gynecol Oncol 2015;26:148–54.
13. Levin AO, Carpenter KM, Fowler JM, et al. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. Int J Gynecol Cancer 2010;20:461–70.
14. Lutgendorf SK, Anderson B, Rothrock N, et al. Quality of life and mood in women receiving extensive chemotherapy for gynecologic cancer. Cancer 2000;89:1402–11.
15. Tierney DK. Sexuality: a quality-of-life issue for cancer survivors. Semin Oncol Nurs 2008;24:71–9.
16. Brotto LA, Heiman JR, Goff B, et al. A psychoeducational intervention for sexual dysfunction in women with gynecologic cancer. Arch Sex Behav 2008;37:317–29.
17. Hollingsworth M, Berman J. The role of androgens in female sexual dysfunction. Sex Reprod Menopause 2006;4:27–32.
18. Brand AH, Bull CA, Cakir B. Vaginal stenosis in patients treated with radiotherapy for carcinoma of the cervix. Int J Gynecol Cancer 2006;16:288–93.
19. Lancaster L. Preventing vaginal stenosis after brachytherapy for gynaecological cancer: an overview of Australian practices. Eur J Oncol Nurs 2004;8:30–9.
20. Ponto JA, Barton D. Husbands’ perspective of living with wives’ ovarian cancer. Psychooncology 2008;17:1225–31.
21. Hartmann U, Philippsohn S, Heiser K, et al. Low sexual desire in midlife and older women: personality factors, psychosocial development, present sexuality. Menopause 2004;11:726–40.
22. Hawkins Y, Ussher J, Gilbert E, et al. Changes in sexuality and intimacy after the diagnosis and treatment of cancer: the experience of partners in a sexual relationship with a person with cancer. Cancer Nurs 2009;32:271–80.
23. Gilbert E, Ussher JM, Hawkins Y. Accounts of disruptions to sexuality following cancer: the perspective of informal carers who are partners of a person with cancer. Health 2009;13:523–41.
24. Hodgkinson K, Butow P, Hunt GE, et al. Life after cancer: couples’ and partners’ psychological adjustment and supportive care needs. Support Care Cancer 2007;15:405–15.
25. Lopez V, Copp G, Molassiotis A. Male caregivers of patients with breast and gynecologic cancer: experiences from caring for their spouses and partners. Cancer Nurs 2012;35:402–10.
26. American Cancer Society. Facts and figures 2015.
27. Michaelson-Cohen R, Beller U. Managing menopausal symptoms after gynecological cancer. Curr Opin Oncol 2009;21:407–11.
28. Biglia N Gadducci A, Ponzone R. Hormone replacement therapy in cancer survivors. Maturitas 2004;48:333–46.
29. Ploch E. Hormonal replacement therapy in patients after cervical cancer treatment. Gynecol Oncol 1987;26:169–77.
30. Guidozzi F, Daponte A. Estrogen replacement therapy for ovarian carcinoma survivors. Cancer 1999;86:1013–8.
31. Creasman WT. Hormone replacement therapy after cancers. Curr Opin Oncol 2005;17:493–9.
32. Bebar S, Ursic-Vrscaj M. Hormone replacement therapy after epithelial ovarian cancer treatment. Eur J Gynaecol Oncol 2000;21:192–6.
33. Wen Y, Huang H, Huang H, et al. The safety of postoperative hormone replacement therapy in epithelial ovarian cancer patients in China. Climacteric 2013;16:673–81.
34. Creasman WT, Henderson D, Hinshaw W, et al. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol 1986;67:326–30.
35. Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2006;24:587–92.
36. Lee K-B, Lee J-M, Lee J-K, et al. Endometrial cancer patients and tibolone: A matched case–control study. Maturitas 2006;55:264–9.
37. Kenemans P, Bundred NJ, Foidart J-M, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol 2009;10:135–46.
38. Al-Baghdadi O, Ewies AAA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 2009;12:91–105.
39. Galuppi A, Perrone AM, La Macchia M, et al. Local α-tocopherol for acute and short-term vaginal toxicity prevention in patients treated with radiotherapy for gynecologic tumors. Int J Gynecol Cancer 2011;21:1708–11.
40. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20.
41. Bø K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol 2012;30:437–43.
42. Dumoulin C, Hay-Smith EJC, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014;5:CD005654.
43. Rosenbaum TY. Pelvic floor involvement in male and female sexual dysfunction and the role of pelvic floor rehabilitation in treatment: A literature review. J Sex Med 2007;4:4–13.
44. Yang EJ, Lim J-Y, Rah UW, et al. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol 2012;125:705–11.
45. Johnson N, Miles TP, Cornes P. Dilating the vagina to prevent damage from radiotherapy: systematic review of the literature. BJOG 2010;117:522–31.
46. Friedman LC, Abdallah R, Schluchter M, et al. Adherence to vaginal dilation following high dose rate brachytherapy for endometrial cancer. Int J Radiat Oncol Biol Phys 2011;80:751–7.
47. Cullen K, Fergus K, DasGupta T, et al. From “sex toy” to intrusive omposition: a qualitative examination of women’s experiences with vaginal dilator use following treatment for gynecological cancer. J Sex Med 2012;9:1162–73.
48. Robinson JW, Faris PD, Scott CB. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynecological carcinoma treated with radiotherapy. Int J Radial Oncol Biol Phys 1999;44:497–506.
49. Stavraka C, Ford A, Ghaem-Maghami S, et al. A study of symptoms described by ovarian cancer survivors. Gynecol Oncol 2012;125:59–64.
50. Moonsammy SH, Guglietti CL, Mina DS, et al. A pilot study of an exercise & cognitive behavioral therapy intervention for epithelial ovarian cancer patients. J Ovarian Res 2013;6:21.
51. Goerling U, Jaeger C, Walz A, et al. The efficacy of short-term psycho-oncological interventions for women with gynaecological cancer: a randomized study. Oncology 2014;87:114–24.
52. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol 2012;125:320–5.
53. Cleary V, McCarthy G, Hegarty J. Development of an educational intervention focused on sexuality for women with gynecological cancer. J Psychosoc Oncol 2012;30:535–55.
54. Miller BE, Pittman B, Strong C. Gynecologic cancer patients’ psychosocial needs and their views on the physician›s role in meeting those needs. Int J Gynecol Cancer 2003;13:111–9.
55. Fang P, Tan K, Grover S, et al. Psychosocial encounters correlates with higher patient-reported functional quality of life in gynecological cancer patients receiving radiotherapy. Radiat Oncol 2015;10:34.
56. Hordern A, Grainger M, Hegarty S, et al. Discussing sexuality in the clinical setting: The impact of a brief training program for oncology health professionals to enhance communication about sexuality. Asia Pac J Clin Oncol 2009;5:270–7.
57. Simonelli LE, Pasipanodya E. Health disparities in unmet support needs of women with gynecologic cancer: an exploratory study. J Psychosoc Oncol 2014;32:727–34.
58. Barbera L, Fitch M, Adams L, et al. Improving care for women after gynecological cancer: the development of a sexuality clinic. Menopause 2011;18:1327–33.
59. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578–83.
60. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000;356:2059–63.
61. Wu C-S, Lu M-L, Liao Y-T, et al. Ovarian cancer and antidepressants. Psychooncology 2015;24:579–84.
62. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol 2009;29:259–66.
63. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011;305:267–74.
64. Reed SD, Guthrie KA, Joffe H, et al. Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2012;119:527–38.
65. Pérez DG, Loprinzi CL, Barton DL, et al. Pilot evaluation of mirtazapine for the treatment of hot flashes. J Support Oncol 2004;2:50–6.
66. Pérez DG, Loprinzi CL, Sloan J, et al. Pilot evaluation of bupropion for the treatment of hot flashes. J Palliat Med 2006;9:631–7.
67. Agarwal N, Singh S, Kriplani A, et al. Evaluation of gabapentin in management of hot flushes in postmenopausal women. Post Reprod Health 2014;20:36–8.
68. Guttuso T Jr, Kurlan R, McDermott MP, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337–45.
69. Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2011;29:3862–8.
70. Loibl S, Schwedler K, von Minckwitz G, et al. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients--a double-blind, randomized study. Ann Oncol 2007;18:689–93.
71. Aerts L, Enzlin P, Verhaeghe J, et al. Long-term sexual functioning in women after surgical treatment of cervical cancer stages IA to IB: a prospective controlled study. Int J Gynecol Cancer 2014;24:1527–34.
72. Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology 2007;16:691–706.
73. Pfaendler KS, Wenzel L, Mechanic MB, et al. Cervical cancer survivorship: long-term quality of life and social support. Clin Ther 2015;37:39–48.
74. Becker M, Malafy T, Bossart M, et al. Quality of life and sexual functioning in endometrial cancer survivors. Gynecol Oncol 2011;121:169–73.
75. Bifulco G, De Rosa N, Tornesello ML, et al. Quality of life, lifestyle behavior and employment experience: a comparison between young and midlife survivors of gynecology early stage cancers. Gynecol Oncol 2012;124:444–51.
76. Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause 2011;18:662–9.
77. American Cancer Society. Sexuality for the woman with cancer. 2013. Available at www.cancer.org/treatment/treatmentsandsideeffects/physicalsideeffects/sexualsideeffectsinwomen/sexualityforthewoman/index.
From the Yale School of Medicine, New Haven, CT.
Abstract
- Objective: To review the sexual health concerns of women with gynecologic cancer and provide guidance for primary care physicians.
- Methods: Review of the literature.
- Results: Issues of sexuality and intimacy are known to significantly impact the quality of life of patients following diagnosis and treatment of gynecologic cancers. At the time of diagnosis, women should be informed of the potential physiologic, hormonal, and psychosocial effects of gynecologic cancer on sexuality. Many providers fail to address these issues given time constraints and patients’ trepidation in alerting their providers to their concerns. While systemic hormone therapy directly addresses these symptoms, its use remains controversial due to potential cancer recurrence risks. Thus, treatment centers around therapeutic alternatives. For vasomotor symptoms, selective serotonin reuptake inhibitors have shown effectiveness and are typically well tolerated, and antiepileptics such as gabapentin have shown promise. There is promising but limited data employing pelvic floor physical therapy as a tool to aid in addressing pelvic floor symptoms. Psychological care and the involvement of the partner are also part of managing the sexual health concerns of these patients.
- Conclusion: Sexual morbidity is a distressing and undertreated problem among gynecologic cancer survivors. Successful treatment requires the provider’s appreciation of the problem and willingness to address it.
Issues of sexuality and intimacy are known to significantly impact the lives of patients following diagnosis and treatment of gynecologic cancers. [1,2] Treatment of gynecologic malignancy is highly dependent on pathology and stage, with some patients receiving small excisional procedures while others subject to extensive surgeries, chemotherapy, and radiation treatment, and it is difficult to predict how each individual’s sexual health will be impacted. However, the evidence suggests that at least half of women treated for gynecologic cancer will experience sexual dysfunction [3]. Although the impact of gynecologic cancer and treatment can be profound, providers often do not address their patients’ sexual concerns [4,5], yet most patients have indicated that they would like these issues to be addressed [6].
Female Sexual Dysfunction
Sexual dysfunction is multifactorial and involves physical, social, and psychological dimensions. It is common in the general population, with rates ranging from 25-63% [7]. The DSM-IV [8] defines female sexual dysfunction as a disturbance in or pain during the sexual response, which can be further classified as hypoactive sexual disorder, orgasmic disorder, sexual pain disorder, or sexual arousal disorder [9]. It should be noted that women who have been treated for gynecologic cancers may have a premorbid history of sexual dysfunction. Assessment of a woman’s sexual function prior to her cancer diagnosis can help establish which sexuality changes are due to the cancer treatment and may allow the provider to tailor interventions accordingly.
Sexual Dysfunction and Quality of Life
As treatments for gynecologic cancers improve, toxicity of treatment decreases, and survival increases, quality of life for survivors has become as become an increasingly important health issue. Several studies have examined patient-reported quality of life in short- and long-term cancer survivors and report overall significant alteration in quality of life over many aspects of health and psychological well-being [10]. It is well established that health-related quality of life and sexual functioning are closely associated [11].
In gynecologic cancer patients, sexual and quality of life outcomes can vary. For example, Kim el al recently compared quality of life and sexual functioning in ovarian cancer survivors with no evidence of disease after primary treatment and a cohort of health women. Sexuality, both in terms of desire, arousal, lubrication, orgasm, satisfaction, and pain and in terms of interest in sex, sexual activity, and enjoyment of sex were similar between the groups; however social functioning deteriorated in cancer patients [12]. Other women report different experiences. Women with a history of vulvar, vaginal, or cervical cancer who may have had extensive pelvic surgery, lymphadenectomy, and radiation treatment to the pelvis typically report greater alterations in quality of life and sexual activity depending on the extent of their treatment [13].
Patients undergoing chemotherapy often report significant changes in quality of life due to the physical symptoms of fatigue, nausea, hair loss, diarrhea, etc. as well the psychological effects of cancer diagnosis, changes in body image, and poor coping [14]. Sexual side effects are common due to alterations in the HPA axis, direct gonadal toxicity and neuropathies [15].
Impact of Gynecologic Cancer on Sexuality
Gynecologic cancer and treatment can alter sexuality through a variety of effects. The impact of anatomical changes may alter a women’s self-esteem, body image, and sense of femininity, resulting in a reluctance to engage in intimate behaviors. Furthermore, if the partnership is disrupted by changes in roles in the household or within the relationship, relationships dynamics may be tested and result in mental distress for the patient, thus effecting sexuality and intimacy.
Surgical or chemical withdrawal of sex hormones, new medications, and postoperative sequelae can contribute to sexual arousal problems. The emotional and psychological components of a cancer diagnosis also can hinder the sexual response [16]. Depression and anxiety, the rates of which increase with cancer diagnosis, can also significantly affect sexual function [17].
Sexual pain is common in this population due to hormonal considerations as well as post-treatment side effects and a frequent cause of sexual dysfunction for these patients. Pelvic radiation may result in adverse physical changes to the vagina including vaginal stenosis, thinning of vaginal mucosa, loss of lubrication, and loss of elasticity [18,19]. A recent review of 20 studies of cervical cancer survivors found that patients were at risk for lack of lubrication and had high rates of dyspareunia [20]. Psychosocial factors have been found to be important predictors of sexual desire and more important than hormones in predicting low sexual desire in middle age [21]. These factors include emotional and physical closeness to the partner, satisfactory communication, and a positive relation to one’s own body [16].
Effects on Relationships and Partners
Women whose sexual capacity is compromised may be worried about their partners’ quality of life and overall well-being. Indeed, the partners of women with gynecologic cancer are also impacted by the changes to sexual function and loss of sexuality and intimacy. Hawkins et al found that cessation or decreased frequency of sex and intimacy was reported in 79% of male partners of women affected by cancer. Among partners of the persons with cancer in this study, changes to sexuality were associated with feelings of self-blame, reflection, sadness, anger and lack of sexual fulfillment [22]. Further, male partners of women diagnosed with gynecological cancer often express conflicting emotional states including feeling worried about their significant other’s health, having the desire to engage in sexual activity, and feeling guilty about wanting to increase sexual intimacy. These feelings, in turn, can lead to resentment and withdrawal from their partner and overall relationship discord [23].
Evidence suggests partners of cancer patients greatly benefit from increased social support even years after an apparent cure [24]. Specifically, male partners who take on a new role as caregiver in the relationship experience difficulties with emotional changes, challenges to their masculinity, and new stressors [25]. These new roles and feelings also contribute to changes in sexuality and intimacy in relationships, making support for partners all the more necessary.
Menopausal Symptoms Following Cancer Treatment
Gynecologic cancer treatment invariably affects the female hormonal balance, sometimes suddenly with surgical excision of the gonads or via radiation treatment or chemotherapy. It is well known that the sudden withdrawal of estrogen and testosterone, especially in the premenopausal postoperative population, can lead to significant acute menopausal symptoms. Given the many emotional and physical issues affecting patients during treatment, it can be difficult to delineate what proportion of sexual problems are caused by or enhanced by vasomotor symptoms, sleep disorders, and vaginal atrophy.
In general, premenopausal women who experience abrupt surgical menopause may often have immediate severe symptoms. Many agree that the younger a woman is when going through this process, the more severe her symptoms may be. Although the average age of endometrial cancer diagnosis is 67, approximately 25% of women who are diagnosed are premenopausal, and 5% of cases occur in women under 40. As the obesity epidemic worsens and more women are exposed to higher levels of estrogen at younger ages, it is expected that the number of premenopausal women who are diagnosed will continue to rise. The average age of diagnosis for ovarian cancer is 63, however, there is a large cohort of women diagnosed with borderline and malignant tumors in the premenopausal period [26]. These patients often experience vasomotor symptoms in the hours to days following surgery.
Interventions for Survivors of Gynecologic Cancer with Sexual Dysfunction
Systemic Hormone Therapy
Systemic hormonal therapy to treat menopausal symptoms remains controversial following the release of findings from the Women’s Health Initiative, which showed a number of adverse effects, including an increased risk of breast cancer in healthy postmenopausal women who received systemic hormonal therapy for menopausal symptoms. While views have changed since that time, providers are often reluctant to prescribe hormonal therapy, and patients are reticent to take it, due to fears of cancer recurrence. However, there is no evidence showing hormones negatively influence survival after treatment for epithelial ovarian, squamous cervical, or vulvar cancer [27]. With the exception of endometrial cancer, there is no biological evidence that HRT may increase recurrence risk [28]. Approach to clinical decision making should be individualized, taking into consideration the patients’ symptoms, quality of life, tumor histology, and overall prognosis.
Cervical cancer, vulvar cancer, and vaginal cancer are not considered hormonally responsive tumors. While the data are limited, a study published in 1987 of cervical cancer survivors treated with systemic hormone replacement therapy showed no increase in relapse rates and showed an increase in quality of life [29]. There are no studies regarding systemic HRT in patients with vulvar or vaginal cancer though it is generally accepted they can be treated with HRT. No significant data exists for cervical adenocarcinoma patients, and most oncologists suggest treating these patients the same as those with endometrial cancer primary.
There is limited data on the use of HRT in women with ovarian cancer but available studies do not show any difference in overall or disease-free survival between HRT groups and controls [27,30–32]. Many studies report a significant increase in quality of life with HRT. A 2013 retrospective chart review of 77 patients with epithelial ovarian cancer who received postoperative HRT showed no significant difference in progression-free survival [33].
The most controversy surrounds the use of HRT in patients with endometrial cancer [34]. The only major data available come from the Gynecologic Oncology Group’s truncated study, which was a large prospective randomized controlled trial. Over 1200 women treated for stage I and II endometrial cancer were enrolled between 1997 and 2003, and before the study was terminated these patients were followed for a median of 3 years following initiation of therapy. The recurrence rate of malignancy was low, 2.1, and an insignificant risk of recurrence of 1.27 was noted between with HRT and placebo groups (80% CI, 0.916-1.77) [35]. Women included in this study were between the ages of 26 and 91, and their indications for therapy included vasomotor symptoms, vaginal atrophy, as well as osteoporosis risk and cardiovascular disease risk. Gynecologist oncologists often use estrogen therapy to treat symptomatic women with early stage endometrial cancer given the very low risk of recurrence.
Tibolone is a synthetic steroid with activity on estrogen and progesterone receptors, and mainly acts as an agonist at estrogen receptors. It is prescribed outside the United States for osteoporosis and is being investigated as treatment for female sexual dysfunction. Efficacy on vasomotor symptoms has been positive thus far. One case-control study confirmed tibolone’s safety for endometrial cancer survivors, with no adverse effects on disease-free or overall survival [36]. One group recently examined tibolone in the setting of breast cancer patients in a prospective randomized controlled trial and reported an increased risk of breast cancer recurrences in women receiving tibolone for HT [37]. That study reported no increase in risk of gynecologic cancers and did report favorable outcomes for patients in terms of osteoporosis and vasomotor symptoms.
Topical Therapy
Women who are concerned about systemic HRT but who have been treated with radiation in addition to their surgery may be interested in topical estrogen therapy. These women may have vaginal stenosis and atrophic symptoms, and for them topical estrogen therapy can be helpful [38]. Many formulations of topical estrogen are available, including creams, tablets, and rings. Using vaginal estrogens and dilators can be useful to help with eventual resumption of sexual activity once healing has taken place and can help to avoid dyspareunia. Combination topical and systemic therapy can also be useful to relieve symptoms. Women concerned about absorption with topical therapy can be advised that while there is some absorption initially, absorption is reduced as atrophy is improved with treatment.
One recent study examined the use of alpha-tocopherol to reduce acute vaginal complications in women with endometrial and cervical cancer undergoing radiation treatment. The treatment group experienced reduced vaginal toxicity and pain, although vaginal secretion was not significantly different in the 2 groups studied [39]. No adverse effects were noted. This compound has not been studied further but may be beneficial in the future.
Some women may prefer to avoid hormones altogether. Over-the-counter vaginal moisturizers and lubricants can be recommended to women to help with intercourse and atrophic symptoms, with or without estrogen therapy.
Physical Therapy
Pelvic floor physical therapy has become an increasingly popular modality for treatment of many aspects pelvic floor dysfunction, the symptoms of which include defecatory dysfunction, constipation, bladder dysfunction, painful urination, dyspareunia, pelvic pain, and low back pain [40]. Pelvic floor physical therapy is well studied and utilized frequently for urinary incontinence and pelvic organ prolapse, but is understudied for sexual dysfunction [41,42].
Promising areas of study include educational sessions, cognitive behavioral therapy, vaginal dilator therapy, pelvic floor muscle strengthening and relaxation techniques with biofeedback, stretching and massage [40]. Biofeedback specifically has been studied in controlled studies for treatment of sexual dysfunction specifically in the setting of vulvar pain syndromes [43]. A small randomized control trial evaluated the use of pelvic floor muscle training in gynecologic cancer survivors and found that the intervention group reported improved sexual function and improved quality of life [44]. Given the few side effects of this therapy, it may be a helpful addition to a multidisciplinary approach to treating pelvic floor dysfunction in gynecologic cancer patients.
For cervical and endometrial cancer patients, vaginal dilators are often prescribed following radiation therapy to minimize vaginal stenosis. Literature suggests that after radiotherapy dilation therapy is effective when used as directed, typically 3 times per week for 10 minutes to mechanically separate the vaginal walls and increase elasticity [45]. Adherence to recommendations for vaginal dilator use is low in this population due to factors such as aversion to the practice and intrusiveness of the mechanism [46,47]. Acknowledgment of apprehension regarding dilator use should be part of the counseling prior to initiation of treatment; interventions designed to educate women about dilator use benefit may increase adherence [48].
Psychological Therapies
Survivors of gynecological cancer with sexual dysfunction may experience the psychological symptoms in the context of the other emotional challenges of a cancer diagnosis [49]. Changes in body image, physical appearance, and feelings of well-being can significantly affect sexuality and intimacy, while anxiety and negative feelings about a diagnosis can cause further sexual dysfunction. Conversely, sexual morbidity in these patients predicts worsening of general psychological symptoms, including depressive symptoms and overall quality of life [13–15].
While there are limited studies directly assessing the effect of psychological therapies for the treatment of sexual dysfunction in this population, the existing literature shows enduring symptomatic improvement following brief interventions. In women for whom emotional distress, depression, and anxiety appear to be a significant aspect of their concern regarding intimacy and sexuality, these interventions can be particularly helpful.
Cognitive behavioral therapy (CBT), which focuses on mindfulness and the relationships between maladaptive thoughts and how they impact behavior, has been shown to have efficacy in treating the broader psycho-social concerns of gynecological cancer patients [50,51]. In a recent study Brotto and colleagues randomized 31 survivors of gynecological cancer with self-reported sexual dysfunction to either three 90-minute CBT sessions or a waitlist control. Patients who underwent the intervention reported significant improvements in sexual arousal and desire both immediately post-therapy and at 6-month follow-up while patients in the waitlist arm experienced no significant changes in symptomatology [52].
Psychoeducational interventions are another promising avenue for addressing sexual dysfunction in this population [53]. In a 2008 therapeutic trial, Brotto and colleagues combined elements of CBT with education in 3 one-hour sessions featuring written materials on sexuality and relationships. The intervention enrolled 22 women with early stage gynecologic cancer who met criteria for female sexual arousal disorder. The psychoeducational intervention was associated with positive effect on sexual desire, arousal, orgasm, satisfaction, sexual distress, depression, and overall well-being [16]. Psychoeducation can also be used to augment other therapeutic modalities. Robinson and colleagues used such an intervention to improve adherence to vaginal dilator use in 32 women with early stage endometrial cancer who were being treated with radiotherapy [48].
Some gynecologic cancer patients may have significant alterations in their anatomy and thus penetrative intercourse may not be possible. In patients even without these physical changes, some may prefer to avoid intercourse due to pain or anxiety. Thus patients can have significant benefits from discussing their concerns with a therapist specializing in these issues as many patients are concerned about their ability to engage in intimate behavior. Therapy can assist with the changing sexual relationship and assist the partners in different ways of engaging in intimate acts. It is important to avoid stressing penetrative intercourse as the goal for sexual function with these or any patients with anxiety relating to their disease as there are many ways to engage in mutually pleasurable experiences for both partners, thus removing anxiety about inability to resume vaginal intercourse post-treatment. Discussing this with patients can be challenging but can often reduce anxiety surrounding body image issues following treatment.
Studies have shown that cancer care providers often do not adequately address sexual concerns [54] but that when these concerns are appropriately managed, patient satisfaction and quality of life significantly improve [55]. Several studies have focused on how providers can incorporate the approaches of CBT and psychoeducation to better address the sexual concerns of patients without requiring external psychiatric care [56,57]. Barbera et al have described a successful model in which multidisciplinary care teams provide education and counseling for gynecological cancer patients in a sexuality clinic [58]. patients had a significant symptom burden, including menopausal symptoms, the effects of radiation therapy, chemotherapy, and surgical operation as well as psychological responses to cancer, and reported high levels of satisfaction with their experience at the clinic.
Involvement of the partner in interventions has not been well studied; however, involving the partner in in psychological therapies to address sexual dysfunction should be beneficial.
Alternative Therapies for Vasomotor Symptoms
Gynecologic cancer patients suffering prominent vasomotor symptoms have limited alternatives to hormone therapy. Clinicians must balance potential medication benefit with potential exacerbation of other medical and psychological issues, including sexual dysfunction.
Antidepressants
The use of SSRIs and SNRIs for vasomotor symptoms was pioneered by medical oncologists for men with hot flashes secondary to GnRH agonist therapy for prostate cancer and women with breast cancer [59,60]. Limited studies have shown that antidepressant medications do not increase cancer recurrence risk in ovarian cancer patients and are relatively well tolerated [61]. However, these medications are known to have partial efficacy in improving vasomotor symptoms and may worsen sexual symptoms, a well-known side effect of antidepressants. There is variation of the reported rates of sexual dysfunction associated with various antidepressants and clinicians may take the likelihood of sexual side effects into account when prescribing SSRIs or SNRIS [62]. More recently developed SSRIS, such as citalopram and its enantiomer escitalopram, have shown significant improvements in vasomotor symptoms and were better tolerated than venlafaxine and fluoxetine [63,64]. Additionally, limited uncontrolled studies of mirtazipine, a structurally unique SSRI, and bupropion, which acts on dopamine and norepinephrine, have shown significant decreases in hot flash symptoms and are less associated with sexual side effects than SSRIS/SNRIS [65,66].
Other Agents
Other pharmaceutical options for menopausal vasomotor symptoms include gabapentin and adrenergic agonists. Gabapentin can yield impressive reductions in vasomotor symptoms. A recent double-blind randomized trial in 50 patients revealed a 60% reduction in hot flashes as 12 weeks and an 80% reduction in self-reported composite symptom scores [67]. However, side effects such as palpitations, edema, and fatigue, lead to high study withdrawal rates and limit its widespread clinical use for this indication [68]. Clonidine has been assessed versus venlafaxine in several clinical trials with breast cancer patients. These trials have shown mixed results, with findings of both inferiority and superiority to venlafaxine, but with consistent significant improvement in symptoms over placebo. Side effects, such insomnia, constipation and dry mouth, occurred but did not lead to higher dropout rates than venlafaxine [69,70].
Long-Term Sexual Outcomes
For women treated for gynecological cancers, alterations in sexual function may persist in the long term. A study following cervical cancer patients managed with radical hysterectomy up to 2 years post treatment showed they had more sexual dysfunction compared with healthy controls, although at rates similar to those who underwent radical hysterectomy for benign disease [71]. A 2007 review of quality of life studies revealed that although ovarian cancer survivors 5 years past diagnosis had excellent overall quality of life, sexual symptoms persisted, with as many as 57% of patients reporting a decline in sexual function due to their cancer [72].
Studies show some differences in outcomes based on treatment modality. A recent review of cervical cancer outcomes revealed that women who received radiotherapy as a component of their treatment have a higher risk of long-term sexual side effects [73]. In contrast, a study assessing endometrial cancer patients 5 years after initial diagnosis between those patients who had received surgery alone and those who had received surgery and vaginal brachytherapy. There was no significant difference in any measures of quality of life and sexual function between these 2 groups [74].
Age appears to play a role in long-term sexual outcomes regardless of diagnosis. Bifulco and colleagues assessed quality of life in survivors of gynecological cancer, comparing women under age 45 to those over 45 after nearly 3 years of survival. After controlling for age and other factors, younger patients were found to have worse sexual activity, including significantly higher rates of poor body image, perceived worse sexual vaginal function, and more severe menopausal symptoms, probably related to the effects of surgical menopause [75].
Despite enduring sexual dysfunction, symptoms tend to improve over time. A cohort study of 103 gynecological cancer patients undergoing radiation therapy were followed for 3 years. Patients were offered standard interventions for sexual dysfunction, including vaginal lubricants, dilators, and menopausal symptomatic therapy, although adherence to these measures was not assessed. Three years after initial therapy, the percentage of sexually active women increased from 21.5% to 44.2% [76]. In the subset of patients who successfully return to sexual activity, outcomes can be comparable to healthy peers. Kim and colleagues compared disease-free sexually active ovarian cancer patients with demographically matched healthy controls on standardized self-report measures. Sexual functioning did not differ between the 2 groups, despite lower social functioning in cancer survivors [12].
Conclusion
Sexuality and intimacy can be greatly affected by the diagnosis and treatment of gynecologic malignancies. It is important to routinely discuss sexuality and sexual functioning with patients from diagnosis onward. Reassuring patients, acknowledging the importance of their concerns, and validating their desire to enjoy improved intimacy should be considered part of the clinician’s role. Valuable information sources that may aid discussions are available on the internet. Oncolink (www.oncolink.org), a large cancer information website maintained by University of Pennsylvania Cancer Center, offers a plethora of information for patients and health care professionals. In addition, the American Cancer Society offers a detailed guide, “Sexuality for the Woman with Cancer” [77]. Treatment is available, and improvement in outcomes is possible. Further prospective studies are needed to clearly delineate risks and benefits of hormone replacement therapy in patients with gynecologic cancers.
Corresponding author: Elena S. Ratner, MD, PO Box 208063, New Haven, CT 06520, [email protected].
From the Yale School of Medicine, New Haven, CT.
Abstract
- Objective: To review the sexual health concerns of women with gynecologic cancer and provide guidance for primary care physicians.
- Methods: Review of the literature.
- Results: Issues of sexuality and intimacy are known to significantly impact the quality of life of patients following diagnosis and treatment of gynecologic cancers. At the time of diagnosis, women should be informed of the potential physiologic, hormonal, and psychosocial effects of gynecologic cancer on sexuality. Many providers fail to address these issues given time constraints and patients’ trepidation in alerting their providers to their concerns. While systemic hormone therapy directly addresses these symptoms, its use remains controversial due to potential cancer recurrence risks. Thus, treatment centers around therapeutic alternatives. For vasomotor symptoms, selective serotonin reuptake inhibitors have shown effectiveness and are typically well tolerated, and antiepileptics such as gabapentin have shown promise. There is promising but limited data employing pelvic floor physical therapy as a tool to aid in addressing pelvic floor symptoms. Psychological care and the involvement of the partner are also part of managing the sexual health concerns of these patients.
- Conclusion: Sexual morbidity is a distressing and undertreated problem among gynecologic cancer survivors. Successful treatment requires the provider’s appreciation of the problem and willingness to address it.
Issues of sexuality and intimacy are known to significantly impact the lives of patients following diagnosis and treatment of gynecologic cancers. [1,2] Treatment of gynecologic malignancy is highly dependent on pathology and stage, with some patients receiving small excisional procedures while others subject to extensive surgeries, chemotherapy, and radiation treatment, and it is difficult to predict how each individual’s sexual health will be impacted. However, the evidence suggests that at least half of women treated for gynecologic cancer will experience sexual dysfunction [3]. Although the impact of gynecologic cancer and treatment can be profound, providers often do not address their patients’ sexual concerns [4,5], yet most patients have indicated that they would like these issues to be addressed [6].
Female Sexual Dysfunction
Sexual dysfunction is multifactorial and involves physical, social, and psychological dimensions. It is common in the general population, with rates ranging from 25-63% [7]. The DSM-IV [8] defines female sexual dysfunction as a disturbance in or pain during the sexual response, which can be further classified as hypoactive sexual disorder, orgasmic disorder, sexual pain disorder, or sexual arousal disorder [9]. It should be noted that women who have been treated for gynecologic cancers may have a premorbid history of sexual dysfunction. Assessment of a woman’s sexual function prior to her cancer diagnosis can help establish which sexuality changes are due to the cancer treatment and may allow the provider to tailor interventions accordingly.
Sexual Dysfunction and Quality of Life
As treatments for gynecologic cancers improve, toxicity of treatment decreases, and survival increases, quality of life for survivors has become as become an increasingly important health issue. Several studies have examined patient-reported quality of life in short- and long-term cancer survivors and report overall significant alteration in quality of life over many aspects of health and psychological well-being [10]. It is well established that health-related quality of life and sexual functioning are closely associated [11].
In gynecologic cancer patients, sexual and quality of life outcomes can vary. For example, Kim el al recently compared quality of life and sexual functioning in ovarian cancer survivors with no evidence of disease after primary treatment and a cohort of health women. Sexuality, both in terms of desire, arousal, lubrication, orgasm, satisfaction, and pain and in terms of interest in sex, sexual activity, and enjoyment of sex were similar between the groups; however social functioning deteriorated in cancer patients [12]. Other women report different experiences. Women with a history of vulvar, vaginal, or cervical cancer who may have had extensive pelvic surgery, lymphadenectomy, and radiation treatment to the pelvis typically report greater alterations in quality of life and sexual activity depending on the extent of their treatment [13].
Patients undergoing chemotherapy often report significant changes in quality of life due to the physical symptoms of fatigue, nausea, hair loss, diarrhea, etc. as well the psychological effects of cancer diagnosis, changes in body image, and poor coping [14]. Sexual side effects are common due to alterations in the HPA axis, direct gonadal toxicity and neuropathies [15].
Impact of Gynecologic Cancer on Sexuality
Gynecologic cancer and treatment can alter sexuality through a variety of effects. The impact of anatomical changes may alter a women’s self-esteem, body image, and sense of femininity, resulting in a reluctance to engage in intimate behaviors. Furthermore, if the partnership is disrupted by changes in roles in the household or within the relationship, relationships dynamics may be tested and result in mental distress for the patient, thus effecting sexuality and intimacy.
Surgical or chemical withdrawal of sex hormones, new medications, and postoperative sequelae can contribute to sexual arousal problems. The emotional and psychological components of a cancer diagnosis also can hinder the sexual response [16]. Depression and anxiety, the rates of which increase with cancer diagnosis, can also significantly affect sexual function [17].
Sexual pain is common in this population due to hormonal considerations as well as post-treatment side effects and a frequent cause of sexual dysfunction for these patients. Pelvic radiation may result in adverse physical changes to the vagina including vaginal stenosis, thinning of vaginal mucosa, loss of lubrication, and loss of elasticity [18,19]. A recent review of 20 studies of cervical cancer survivors found that patients were at risk for lack of lubrication and had high rates of dyspareunia [20]. Psychosocial factors have been found to be important predictors of sexual desire and more important than hormones in predicting low sexual desire in middle age [21]. These factors include emotional and physical closeness to the partner, satisfactory communication, and a positive relation to one’s own body [16].
Effects on Relationships and Partners
Women whose sexual capacity is compromised may be worried about their partners’ quality of life and overall well-being. Indeed, the partners of women with gynecologic cancer are also impacted by the changes to sexual function and loss of sexuality and intimacy. Hawkins et al found that cessation or decreased frequency of sex and intimacy was reported in 79% of male partners of women affected by cancer. Among partners of the persons with cancer in this study, changes to sexuality were associated with feelings of self-blame, reflection, sadness, anger and lack of sexual fulfillment [22]. Further, male partners of women diagnosed with gynecological cancer often express conflicting emotional states including feeling worried about their significant other’s health, having the desire to engage in sexual activity, and feeling guilty about wanting to increase sexual intimacy. These feelings, in turn, can lead to resentment and withdrawal from their partner and overall relationship discord [23].
Evidence suggests partners of cancer patients greatly benefit from increased social support even years after an apparent cure [24]. Specifically, male partners who take on a new role as caregiver in the relationship experience difficulties with emotional changes, challenges to their masculinity, and new stressors [25]. These new roles and feelings also contribute to changes in sexuality and intimacy in relationships, making support for partners all the more necessary.
Menopausal Symptoms Following Cancer Treatment
Gynecologic cancer treatment invariably affects the female hormonal balance, sometimes suddenly with surgical excision of the gonads or via radiation treatment or chemotherapy. It is well known that the sudden withdrawal of estrogen and testosterone, especially in the premenopausal postoperative population, can lead to significant acute menopausal symptoms. Given the many emotional and physical issues affecting patients during treatment, it can be difficult to delineate what proportion of sexual problems are caused by or enhanced by vasomotor symptoms, sleep disorders, and vaginal atrophy.
In general, premenopausal women who experience abrupt surgical menopause may often have immediate severe symptoms. Many agree that the younger a woman is when going through this process, the more severe her symptoms may be. Although the average age of endometrial cancer diagnosis is 67, approximately 25% of women who are diagnosed are premenopausal, and 5% of cases occur in women under 40. As the obesity epidemic worsens and more women are exposed to higher levels of estrogen at younger ages, it is expected that the number of premenopausal women who are diagnosed will continue to rise. The average age of diagnosis for ovarian cancer is 63, however, there is a large cohort of women diagnosed with borderline and malignant tumors in the premenopausal period [26]. These patients often experience vasomotor symptoms in the hours to days following surgery.
Interventions for Survivors of Gynecologic Cancer with Sexual Dysfunction
Systemic Hormone Therapy
Systemic hormonal therapy to treat menopausal symptoms remains controversial following the release of findings from the Women’s Health Initiative, which showed a number of adverse effects, including an increased risk of breast cancer in healthy postmenopausal women who received systemic hormonal therapy for menopausal symptoms. While views have changed since that time, providers are often reluctant to prescribe hormonal therapy, and patients are reticent to take it, due to fears of cancer recurrence. However, there is no evidence showing hormones negatively influence survival after treatment for epithelial ovarian, squamous cervical, or vulvar cancer [27]. With the exception of endometrial cancer, there is no biological evidence that HRT may increase recurrence risk [28]. Approach to clinical decision making should be individualized, taking into consideration the patients’ symptoms, quality of life, tumor histology, and overall prognosis.
Cervical cancer, vulvar cancer, and vaginal cancer are not considered hormonally responsive tumors. While the data are limited, a study published in 1987 of cervical cancer survivors treated with systemic hormone replacement therapy showed no increase in relapse rates and showed an increase in quality of life [29]. There are no studies regarding systemic HRT in patients with vulvar or vaginal cancer though it is generally accepted they can be treated with HRT. No significant data exists for cervical adenocarcinoma patients, and most oncologists suggest treating these patients the same as those with endometrial cancer primary.
There is limited data on the use of HRT in women with ovarian cancer but available studies do not show any difference in overall or disease-free survival between HRT groups and controls [27,30–32]. Many studies report a significant increase in quality of life with HRT. A 2013 retrospective chart review of 77 patients with epithelial ovarian cancer who received postoperative HRT showed no significant difference in progression-free survival [33].
The most controversy surrounds the use of HRT in patients with endometrial cancer [34]. The only major data available come from the Gynecologic Oncology Group’s truncated study, which was a large prospective randomized controlled trial. Over 1200 women treated for stage I and II endometrial cancer were enrolled between 1997 and 2003, and before the study was terminated these patients were followed for a median of 3 years following initiation of therapy. The recurrence rate of malignancy was low, 2.1, and an insignificant risk of recurrence of 1.27 was noted between with HRT and placebo groups (80% CI, 0.916-1.77) [35]. Women included in this study were between the ages of 26 and 91, and their indications for therapy included vasomotor symptoms, vaginal atrophy, as well as osteoporosis risk and cardiovascular disease risk. Gynecologist oncologists often use estrogen therapy to treat symptomatic women with early stage endometrial cancer given the very low risk of recurrence.
Tibolone is a synthetic steroid with activity on estrogen and progesterone receptors, and mainly acts as an agonist at estrogen receptors. It is prescribed outside the United States for osteoporosis and is being investigated as treatment for female sexual dysfunction. Efficacy on vasomotor symptoms has been positive thus far. One case-control study confirmed tibolone’s safety for endometrial cancer survivors, with no adverse effects on disease-free or overall survival [36]. One group recently examined tibolone in the setting of breast cancer patients in a prospective randomized controlled trial and reported an increased risk of breast cancer recurrences in women receiving tibolone for HT [37]. That study reported no increase in risk of gynecologic cancers and did report favorable outcomes for patients in terms of osteoporosis and vasomotor symptoms.
Topical Therapy
Women who are concerned about systemic HRT but who have been treated with radiation in addition to their surgery may be interested in topical estrogen therapy. These women may have vaginal stenosis and atrophic symptoms, and for them topical estrogen therapy can be helpful [38]. Many formulations of topical estrogen are available, including creams, tablets, and rings. Using vaginal estrogens and dilators can be useful to help with eventual resumption of sexual activity once healing has taken place and can help to avoid dyspareunia. Combination topical and systemic therapy can also be useful to relieve symptoms. Women concerned about absorption with topical therapy can be advised that while there is some absorption initially, absorption is reduced as atrophy is improved with treatment.
One recent study examined the use of alpha-tocopherol to reduce acute vaginal complications in women with endometrial and cervical cancer undergoing radiation treatment. The treatment group experienced reduced vaginal toxicity and pain, although vaginal secretion was not significantly different in the 2 groups studied [39]. No adverse effects were noted. This compound has not been studied further but may be beneficial in the future.
Some women may prefer to avoid hormones altogether. Over-the-counter vaginal moisturizers and lubricants can be recommended to women to help with intercourse and atrophic symptoms, with or without estrogen therapy.
Physical Therapy
Pelvic floor physical therapy has become an increasingly popular modality for treatment of many aspects pelvic floor dysfunction, the symptoms of which include defecatory dysfunction, constipation, bladder dysfunction, painful urination, dyspareunia, pelvic pain, and low back pain [40]. Pelvic floor physical therapy is well studied and utilized frequently for urinary incontinence and pelvic organ prolapse, but is understudied for sexual dysfunction [41,42].
Promising areas of study include educational sessions, cognitive behavioral therapy, vaginal dilator therapy, pelvic floor muscle strengthening and relaxation techniques with biofeedback, stretching and massage [40]. Biofeedback specifically has been studied in controlled studies for treatment of sexual dysfunction specifically in the setting of vulvar pain syndromes [43]. A small randomized control trial evaluated the use of pelvic floor muscle training in gynecologic cancer survivors and found that the intervention group reported improved sexual function and improved quality of life [44]. Given the few side effects of this therapy, it may be a helpful addition to a multidisciplinary approach to treating pelvic floor dysfunction in gynecologic cancer patients.
For cervical and endometrial cancer patients, vaginal dilators are often prescribed following radiation therapy to minimize vaginal stenosis. Literature suggests that after radiotherapy dilation therapy is effective when used as directed, typically 3 times per week for 10 minutes to mechanically separate the vaginal walls and increase elasticity [45]. Adherence to recommendations for vaginal dilator use is low in this population due to factors such as aversion to the practice and intrusiveness of the mechanism [46,47]. Acknowledgment of apprehension regarding dilator use should be part of the counseling prior to initiation of treatment; interventions designed to educate women about dilator use benefit may increase adherence [48].
Psychological Therapies
Survivors of gynecological cancer with sexual dysfunction may experience the psychological symptoms in the context of the other emotional challenges of a cancer diagnosis [49]. Changes in body image, physical appearance, and feelings of well-being can significantly affect sexuality and intimacy, while anxiety and negative feelings about a diagnosis can cause further sexual dysfunction. Conversely, sexual morbidity in these patients predicts worsening of general psychological symptoms, including depressive symptoms and overall quality of life [13–15].
While there are limited studies directly assessing the effect of psychological therapies for the treatment of sexual dysfunction in this population, the existing literature shows enduring symptomatic improvement following brief interventions. In women for whom emotional distress, depression, and anxiety appear to be a significant aspect of their concern regarding intimacy and sexuality, these interventions can be particularly helpful.
Cognitive behavioral therapy (CBT), which focuses on mindfulness and the relationships between maladaptive thoughts and how they impact behavior, has been shown to have efficacy in treating the broader psycho-social concerns of gynecological cancer patients [50,51]. In a recent study Brotto and colleagues randomized 31 survivors of gynecological cancer with self-reported sexual dysfunction to either three 90-minute CBT sessions or a waitlist control. Patients who underwent the intervention reported significant improvements in sexual arousal and desire both immediately post-therapy and at 6-month follow-up while patients in the waitlist arm experienced no significant changes in symptomatology [52].
Psychoeducational interventions are another promising avenue for addressing sexual dysfunction in this population [53]. In a 2008 therapeutic trial, Brotto and colleagues combined elements of CBT with education in 3 one-hour sessions featuring written materials on sexuality and relationships. The intervention enrolled 22 women with early stage gynecologic cancer who met criteria for female sexual arousal disorder. The psychoeducational intervention was associated with positive effect on sexual desire, arousal, orgasm, satisfaction, sexual distress, depression, and overall well-being [16]. Psychoeducation can also be used to augment other therapeutic modalities. Robinson and colleagues used such an intervention to improve adherence to vaginal dilator use in 32 women with early stage endometrial cancer who were being treated with radiotherapy [48].
Some gynecologic cancer patients may have significant alterations in their anatomy and thus penetrative intercourse may not be possible. In patients even without these physical changes, some may prefer to avoid intercourse due to pain or anxiety. Thus patients can have significant benefits from discussing their concerns with a therapist specializing in these issues as many patients are concerned about their ability to engage in intimate behavior. Therapy can assist with the changing sexual relationship and assist the partners in different ways of engaging in intimate acts. It is important to avoid stressing penetrative intercourse as the goal for sexual function with these or any patients with anxiety relating to their disease as there are many ways to engage in mutually pleasurable experiences for both partners, thus removing anxiety about inability to resume vaginal intercourse post-treatment. Discussing this with patients can be challenging but can often reduce anxiety surrounding body image issues following treatment.
Studies have shown that cancer care providers often do not adequately address sexual concerns [54] but that when these concerns are appropriately managed, patient satisfaction and quality of life significantly improve [55]. Several studies have focused on how providers can incorporate the approaches of CBT and psychoeducation to better address the sexual concerns of patients without requiring external psychiatric care [56,57]. Barbera et al have described a successful model in which multidisciplinary care teams provide education and counseling for gynecological cancer patients in a sexuality clinic [58]. patients had a significant symptom burden, including menopausal symptoms, the effects of radiation therapy, chemotherapy, and surgical operation as well as psychological responses to cancer, and reported high levels of satisfaction with their experience at the clinic.
Involvement of the partner in interventions has not been well studied; however, involving the partner in in psychological therapies to address sexual dysfunction should be beneficial.
Alternative Therapies for Vasomotor Symptoms
Gynecologic cancer patients suffering prominent vasomotor symptoms have limited alternatives to hormone therapy. Clinicians must balance potential medication benefit with potential exacerbation of other medical and psychological issues, including sexual dysfunction.
Antidepressants
The use of SSRIs and SNRIs for vasomotor symptoms was pioneered by medical oncologists for men with hot flashes secondary to GnRH agonist therapy for prostate cancer and women with breast cancer [59,60]. Limited studies have shown that antidepressant medications do not increase cancer recurrence risk in ovarian cancer patients and are relatively well tolerated [61]. However, these medications are known to have partial efficacy in improving vasomotor symptoms and may worsen sexual symptoms, a well-known side effect of antidepressants. There is variation of the reported rates of sexual dysfunction associated with various antidepressants and clinicians may take the likelihood of sexual side effects into account when prescribing SSRIs or SNRIS [62]. More recently developed SSRIS, such as citalopram and its enantiomer escitalopram, have shown significant improvements in vasomotor symptoms and were better tolerated than venlafaxine and fluoxetine [63,64]. Additionally, limited uncontrolled studies of mirtazipine, a structurally unique SSRI, and bupropion, which acts on dopamine and norepinephrine, have shown significant decreases in hot flash symptoms and are less associated with sexual side effects than SSRIS/SNRIS [65,66].
Other Agents
Other pharmaceutical options for menopausal vasomotor symptoms include gabapentin and adrenergic agonists. Gabapentin can yield impressive reductions in vasomotor symptoms. A recent double-blind randomized trial in 50 patients revealed a 60% reduction in hot flashes as 12 weeks and an 80% reduction in self-reported composite symptom scores [67]. However, side effects such as palpitations, edema, and fatigue, lead to high study withdrawal rates and limit its widespread clinical use for this indication [68]. Clonidine has been assessed versus venlafaxine in several clinical trials with breast cancer patients. These trials have shown mixed results, with findings of both inferiority and superiority to venlafaxine, but with consistent significant improvement in symptoms over placebo. Side effects, such insomnia, constipation and dry mouth, occurred but did not lead to higher dropout rates than venlafaxine [69,70].
Long-Term Sexual Outcomes
For women treated for gynecological cancers, alterations in sexual function may persist in the long term. A study following cervical cancer patients managed with radical hysterectomy up to 2 years post treatment showed they had more sexual dysfunction compared with healthy controls, although at rates similar to those who underwent radical hysterectomy for benign disease [71]. A 2007 review of quality of life studies revealed that although ovarian cancer survivors 5 years past diagnosis had excellent overall quality of life, sexual symptoms persisted, with as many as 57% of patients reporting a decline in sexual function due to their cancer [72].
Studies show some differences in outcomes based on treatment modality. A recent review of cervical cancer outcomes revealed that women who received radiotherapy as a component of their treatment have a higher risk of long-term sexual side effects [73]. In contrast, a study assessing endometrial cancer patients 5 years after initial diagnosis between those patients who had received surgery alone and those who had received surgery and vaginal brachytherapy. There was no significant difference in any measures of quality of life and sexual function between these 2 groups [74].
Age appears to play a role in long-term sexual outcomes regardless of diagnosis. Bifulco and colleagues assessed quality of life in survivors of gynecological cancer, comparing women under age 45 to those over 45 after nearly 3 years of survival. After controlling for age and other factors, younger patients were found to have worse sexual activity, including significantly higher rates of poor body image, perceived worse sexual vaginal function, and more severe menopausal symptoms, probably related to the effects of surgical menopause [75].
Despite enduring sexual dysfunction, symptoms tend to improve over time. A cohort study of 103 gynecological cancer patients undergoing radiation therapy were followed for 3 years. Patients were offered standard interventions for sexual dysfunction, including vaginal lubricants, dilators, and menopausal symptomatic therapy, although adherence to these measures was not assessed. Three years after initial therapy, the percentage of sexually active women increased from 21.5% to 44.2% [76]. In the subset of patients who successfully return to sexual activity, outcomes can be comparable to healthy peers. Kim and colleagues compared disease-free sexually active ovarian cancer patients with demographically matched healthy controls on standardized self-report measures. Sexual functioning did not differ between the 2 groups, despite lower social functioning in cancer survivors [12].
Conclusion
Sexuality and intimacy can be greatly affected by the diagnosis and treatment of gynecologic malignancies. It is important to routinely discuss sexuality and sexual functioning with patients from diagnosis onward. Reassuring patients, acknowledging the importance of their concerns, and validating their desire to enjoy improved intimacy should be considered part of the clinician’s role. Valuable information sources that may aid discussions are available on the internet. Oncolink (www.oncolink.org), a large cancer information website maintained by University of Pennsylvania Cancer Center, offers a plethora of information for patients and health care professionals. In addition, the American Cancer Society offers a detailed guide, “Sexuality for the Woman with Cancer” [77]. Treatment is available, and improvement in outcomes is possible. Further prospective studies are needed to clearly delineate risks and benefits of hormone replacement therapy in patients with gynecologic cancers.
Corresponding author: Elena S. Ratner, MD, PO Box 208063, New Haven, CT 06520, [email protected].
1. Juraskova I, Butow P, Robertson R, et al. Post-treatment sexual adjustment following cervical and endometrial cancer: a qualitative insight. Psychooncology 2003;12:267–79.
2. Bodurka DC, Sun CC. Sexual function after gynecologic cancer. Obstet Gynecol Clin North Am 2006;33:621–30, ix.
3. Andersen BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol 1997;65:221.
4. Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J 2009;15:74–7.
5. Stead ML, Brown JM, Fallowfield L, et al. Lack of communication between healthcare professionals and women with ovarian cancer about sexual issues. Br J Cancer 2003;88:666–71.
6. Stead ML, Fallowfield L, Brown JM, et al. Communication about sexual problems and sexual concerns in ovarian cancer: qualitative study. BMJ 2001;323:836–7.
7. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537–44.
8. American Psychiatric Association. Diagnostic and statistical manual of mental health disorders. 4th ed. 2000.
9. Elder JA, Braver Y. Female sexual dysfunction. In: Current clinical medicine. Elsevier; 2010:1222–6.
10. Ferrell BR, Dow KH, Leigh S, et al. Quality of life in long-term cancer survivors. Oncol Nurs Forum 1995;22:915–22.
11. Shamspour N, Assari S, Moghana Lankarani M. Relation between sexuality and health-related quality of life. In: Handbook of disease burdens and quality of life measures. New York: Springer 2010;3457–73.
12. Kim SI, Lee Y, Lim MC, et al. Quality of life and sexuality comparison between sexually active ovarian cancer survivors and healthy women. J Gynecol Oncol 2015;26:148–54.
13. Levin AO, Carpenter KM, Fowler JM, et al. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. Int J Gynecol Cancer 2010;20:461–70.
14. Lutgendorf SK, Anderson B, Rothrock N, et al. Quality of life and mood in women receiving extensive chemotherapy for gynecologic cancer. Cancer 2000;89:1402–11.
15. Tierney DK. Sexuality: a quality-of-life issue for cancer survivors. Semin Oncol Nurs 2008;24:71–9.
16. Brotto LA, Heiman JR, Goff B, et al. A psychoeducational intervention for sexual dysfunction in women with gynecologic cancer. Arch Sex Behav 2008;37:317–29.
17. Hollingsworth M, Berman J. The role of androgens in female sexual dysfunction. Sex Reprod Menopause 2006;4:27–32.
18. Brand AH, Bull CA, Cakir B. Vaginal stenosis in patients treated with radiotherapy for carcinoma of the cervix. Int J Gynecol Cancer 2006;16:288–93.
19. Lancaster L. Preventing vaginal stenosis after brachytherapy for gynaecological cancer: an overview of Australian practices. Eur J Oncol Nurs 2004;8:30–9.
20. Ponto JA, Barton D. Husbands’ perspective of living with wives’ ovarian cancer. Psychooncology 2008;17:1225–31.
21. Hartmann U, Philippsohn S, Heiser K, et al. Low sexual desire in midlife and older women: personality factors, psychosocial development, present sexuality. Menopause 2004;11:726–40.
22. Hawkins Y, Ussher J, Gilbert E, et al. Changes in sexuality and intimacy after the diagnosis and treatment of cancer: the experience of partners in a sexual relationship with a person with cancer. Cancer Nurs 2009;32:271–80.
23. Gilbert E, Ussher JM, Hawkins Y. Accounts of disruptions to sexuality following cancer: the perspective of informal carers who are partners of a person with cancer. Health 2009;13:523–41.
24. Hodgkinson K, Butow P, Hunt GE, et al. Life after cancer: couples’ and partners’ psychological adjustment and supportive care needs. Support Care Cancer 2007;15:405–15.
25. Lopez V, Copp G, Molassiotis A. Male caregivers of patients with breast and gynecologic cancer: experiences from caring for their spouses and partners. Cancer Nurs 2012;35:402–10.
26. American Cancer Society. Facts and figures 2015.
27. Michaelson-Cohen R, Beller U. Managing menopausal symptoms after gynecological cancer. Curr Opin Oncol 2009;21:407–11.
28. Biglia N Gadducci A, Ponzone R. Hormone replacement therapy in cancer survivors. Maturitas 2004;48:333–46.
29. Ploch E. Hormonal replacement therapy in patients after cervical cancer treatment. Gynecol Oncol 1987;26:169–77.
30. Guidozzi F, Daponte A. Estrogen replacement therapy for ovarian carcinoma survivors. Cancer 1999;86:1013–8.
31. Creasman WT. Hormone replacement therapy after cancers. Curr Opin Oncol 2005;17:493–9.
32. Bebar S, Ursic-Vrscaj M. Hormone replacement therapy after epithelial ovarian cancer treatment. Eur J Gynaecol Oncol 2000;21:192–6.
33. Wen Y, Huang H, Huang H, et al. The safety of postoperative hormone replacement therapy in epithelial ovarian cancer patients in China. Climacteric 2013;16:673–81.
34. Creasman WT, Henderson D, Hinshaw W, et al. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol 1986;67:326–30.
35. Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2006;24:587–92.
36. Lee K-B, Lee J-M, Lee J-K, et al. Endometrial cancer patients and tibolone: A matched case–control study. Maturitas 2006;55:264–9.
37. Kenemans P, Bundred NJ, Foidart J-M, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol 2009;10:135–46.
38. Al-Baghdadi O, Ewies AAA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 2009;12:91–105.
39. Galuppi A, Perrone AM, La Macchia M, et al. Local α-tocopherol for acute and short-term vaginal toxicity prevention in patients treated with radiotherapy for gynecologic tumors. Int J Gynecol Cancer 2011;21:1708–11.
40. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20.
41. Bø K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol 2012;30:437–43.
42. Dumoulin C, Hay-Smith EJC, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014;5:CD005654.
43. Rosenbaum TY. Pelvic floor involvement in male and female sexual dysfunction and the role of pelvic floor rehabilitation in treatment: A literature review. J Sex Med 2007;4:4–13.
44. Yang EJ, Lim J-Y, Rah UW, et al. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol 2012;125:705–11.
45. Johnson N, Miles TP, Cornes P. Dilating the vagina to prevent damage from radiotherapy: systematic review of the literature. BJOG 2010;117:522–31.
46. Friedman LC, Abdallah R, Schluchter M, et al. Adherence to vaginal dilation following high dose rate brachytherapy for endometrial cancer. Int J Radiat Oncol Biol Phys 2011;80:751–7.
47. Cullen K, Fergus K, DasGupta T, et al. From “sex toy” to intrusive omposition: a qualitative examination of women’s experiences with vaginal dilator use following treatment for gynecological cancer. J Sex Med 2012;9:1162–73.
48. Robinson JW, Faris PD, Scott CB. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynecological carcinoma treated with radiotherapy. Int J Radial Oncol Biol Phys 1999;44:497–506.
49. Stavraka C, Ford A, Ghaem-Maghami S, et al. A study of symptoms described by ovarian cancer survivors. Gynecol Oncol 2012;125:59–64.
50. Moonsammy SH, Guglietti CL, Mina DS, et al. A pilot study of an exercise & cognitive behavioral therapy intervention for epithelial ovarian cancer patients. J Ovarian Res 2013;6:21.
51. Goerling U, Jaeger C, Walz A, et al. The efficacy of short-term psycho-oncological interventions for women with gynaecological cancer: a randomized study. Oncology 2014;87:114–24.
52. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol 2012;125:320–5.
53. Cleary V, McCarthy G, Hegarty J. Development of an educational intervention focused on sexuality for women with gynecological cancer. J Psychosoc Oncol 2012;30:535–55.
54. Miller BE, Pittman B, Strong C. Gynecologic cancer patients’ psychosocial needs and their views on the physician›s role in meeting those needs. Int J Gynecol Cancer 2003;13:111–9.
55. Fang P, Tan K, Grover S, et al. Psychosocial encounters correlates with higher patient-reported functional quality of life in gynecological cancer patients receiving radiotherapy. Radiat Oncol 2015;10:34.
56. Hordern A, Grainger M, Hegarty S, et al. Discussing sexuality in the clinical setting: The impact of a brief training program for oncology health professionals to enhance communication about sexuality. Asia Pac J Clin Oncol 2009;5:270–7.
57. Simonelli LE, Pasipanodya E. Health disparities in unmet support needs of women with gynecologic cancer: an exploratory study. J Psychosoc Oncol 2014;32:727–34.
58. Barbera L, Fitch M, Adams L, et al. Improving care for women after gynecological cancer: the development of a sexuality clinic. Menopause 2011;18:1327–33.
59. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578–83.
60. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000;356:2059–63.
61. Wu C-S, Lu M-L, Liao Y-T, et al. Ovarian cancer and antidepressants. Psychooncology 2015;24:579–84.
62. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol 2009;29:259–66.
63. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011;305:267–74.
64. Reed SD, Guthrie KA, Joffe H, et al. Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2012;119:527–38.
65. Pérez DG, Loprinzi CL, Barton DL, et al. Pilot evaluation of mirtazapine for the treatment of hot flashes. J Support Oncol 2004;2:50–6.
66. Pérez DG, Loprinzi CL, Sloan J, et al. Pilot evaluation of bupropion for the treatment of hot flashes. J Palliat Med 2006;9:631–7.
67. Agarwal N, Singh S, Kriplani A, et al. Evaluation of gabapentin in management of hot flushes in postmenopausal women. Post Reprod Health 2014;20:36–8.
68. Guttuso T Jr, Kurlan R, McDermott MP, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337–45.
69. Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2011;29:3862–8.
70. Loibl S, Schwedler K, von Minckwitz G, et al. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients--a double-blind, randomized study. Ann Oncol 2007;18:689–93.
71. Aerts L, Enzlin P, Verhaeghe J, et al. Long-term sexual functioning in women after surgical treatment of cervical cancer stages IA to IB: a prospective controlled study. Int J Gynecol Cancer 2014;24:1527–34.
72. Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology 2007;16:691–706.
73. Pfaendler KS, Wenzel L, Mechanic MB, et al. Cervical cancer survivorship: long-term quality of life and social support. Clin Ther 2015;37:39–48.
74. Becker M, Malafy T, Bossart M, et al. Quality of life and sexual functioning in endometrial cancer survivors. Gynecol Oncol 2011;121:169–73.
75. Bifulco G, De Rosa N, Tornesello ML, et al. Quality of life, lifestyle behavior and employment experience: a comparison between young and midlife survivors of gynecology early stage cancers. Gynecol Oncol 2012;124:444–51.
76. Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause 2011;18:662–9.
77. American Cancer Society. Sexuality for the woman with cancer. 2013. Available at www.cancer.org/treatment/treatmentsandsideeffects/physicalsideeffects/sexualsideeffectsinwomen/sexualityforthewoman/index.
1. Juraskova I, Butow P, Robertson R, et al. Post-treatment sexual adjustment following cervical and endometrial cancer: a qualitative insight. Psychooncology 2003;12:267–79.
2. Bodurka DC, Sun CC. Sexual function after gynecologic cancer. Obstet Gynecol Clin North Am 2006;33:621–30, ix.
3. Andersen BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol 1997;65:221.
4. Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J 2009;15:74–7.
5. Stead ML, Brown JM, Fallowfield L, et al. Lack of communication between healthcare professionals and women with ovarian cancer about sexual issues. Br J Cancer 2003;88:666–71.
6. Stead ML, Fallowfield L, Brown JM, et al. Communication about sexual problems and sexual concerns in ovarian cancer: qualitative study. BMJ 2001;323:836–7.
7. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537–44.
8. American Psychiatric Association. Diagnostic and statistical manual of mental health disorders. 4th ed. 2000.
9. Elder JA, Braver Y. Female sexual dysfunction. In: Current clinical medicine. Elsevier; 2010:1222–6.
10. Ferrell BR, Dow KH, Leigh S, et al. Quality of life in long-term cancer survivors. Oncol Nurs Forum 1995;22:915–22.
11. Shamspour N, Assari S, Moghana Lankarani M. Relation between sexuality and health-related quality of life. In: Handbook of disease burdens and quality of life measures. New York: Springer 2010;3457–73.
12. Kim SI, Lee Y, Lim MC, et al. Quality of life and sexuality comparison between sexually active ovarian cancer survivors and healthy women. J Gynecol Oncol 2015;26:148–54.
13. Levin AO, Carpenter KM, Fowler JM, et al. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. Int J Gynecol Cancer 2010;20:461–70.
14. Lutgendorf SK, Anderson B, Rothrock N, et al. Quality of life and mood in women receiving extensive chemotherapy for gynecologic cancer. Cancer 2000;89:1402–11.
15. Tierney DK. Sexuality: a quality-of-life issue for cancer survivors. Semin Oncol Nurs 2008;24:71–9.
16. Brotto LA, Heiman JR, Goff B, et al. A psychoeducational intervention for sexual dysfunction in women with gynecologic cancer. Arch Sex Behav 2008;37:317–29.
17. Hollingsworth M, Berman J. The role of androgens in female sexual dysfunction. Sex Reprod Menopause 2006;4:27–32.
18. Brand AH, Bull CA, Cakir B. Vaginal stenosis in patients treated with radiotherapy for carcinoma of the cervix. Int J Gynecol Cancer 2006;16:288–93.
19. Lancaster L. Preventing vaginal stenosis after brachytherapy for gynaecological cancer: an overview of Australian practices. Eur J Oncol Nurs 2004;8:30–9.
20. Ponto JA, Barton D. Husbands’ perspective of living with wives’ ovarian cancer. Psychooncology 2008;17:1225–31.
21. Hartmann U, Philippsohn S, Heiser K, et al. Low sexual desire in midlife and older women: personality factors, psychosocial development, present sexuality. Menopause 2004;11:726–40.
22. Hawkins Y, Ussher J, Gilbert E, et al. Changes in sexuality and intimacy after the diagnosis and treatment of cancer: the experience of partners in a sexual relationship with a person with cancer. Cancer Nurs 2009;32:271–80.
23. Gilbert E, Ussher JM, Hawkins Y. Accounts of disruptions to sexuality following cancer: the perspective of informal carers who are partners of a person with cancer. Health 2009;13:523–41.
24. Hodgkinson K, Butow P, Hunt GE, et al. Life after cancer: couples’ and partners’ psychological adjustment and supportive care needs. Support Care Cancer 2007;15:405–15.
25. Lopez V, Copp G, Molassiotis A. Male caregivers of patients with breast and gynecologic cancer: experiences from caring for their spouses and partners. Cancer Nurs 2012;35:402–10.
26. American Cancer Society. Facts and figures 2015.
27. Michaelson-Cohen R, Beller U. Managing menopausal symptoms after gynecological cancer. Curr Opin Oncol 2009;21:407–11.
28. Biglia N Gadducci A, Ponzone R. Hormone replacement therapy in cancer survivors. Maturitas 2004;48:333–46.
29. Ploch E. Hormonal replacement therapy in patients after cervical cancer treatment. Gynecol Oncol 1987;26:169–77.
30. Guidozzi F, Daponte A. Estrogen replacement therapy for ovarian carcinoma survivors. Cancer 1999;86:1013–8.
31. Creasman WT. Hormone replacement therapy after cancers. Curr Opin Oncol 2005;17:493–9.
32. Bebar S, Ursic-Vrscaj M. Hormone replacement therapy after epithelial ovarian cancer treatment. Eur J Gynaecol Oncol 2000;21:192–6.
33. Wen Y, Huang H, Huang H, et al. The safety of postoperative hormone replacement therapy in epithelial ovarian cancer patients in China. Climacteric 2013;16:673–81.
34. Creasman WT, Henderson D, Hinshaw W, et al. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol 1986;67:326–30.
35. Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2006;24:587–92.
36. Lee K-B, Lee J-M, Lee J-K, et al. Endometrial cancer patients and tibolone: A matched case–control study. Maturitas 2006;55:264–9.
37. Kenemans P, Bundred NJ, Foidart J-M, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol 2009;10:135–46.
38. Al-Baghdadi O, Ewies AAA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 2009;12:91–105.
39. Galuppi A, Perrone AM, La Macchia M, et al. Local α-tocopherol for acute and short-term vaginal toxicity prevention in patients treated with radiotherapy for gynecologic tumors. Int J Gynecol Cancer 2011;21:1708–11.
40. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20.
41. Bø K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol 2012;30:437–43.
42. Dumoulin C, Hay-Smith EJC, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014;5:CD005654.
43. Rosenbaum TY. Pelvic floor involvement in male and female sexual dysfunction and the role of pelvic floor rehabilitation in treatment: A literature review. J Sex Med 2007;4:4–13.
44. Yang EJ, Lim J-Y, Rah UW, et al. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol 2012;125:705–11.
45. Johnson N, Miles TP, Cornes P. Dilating the vagina to prevent damage from radiotherapy: systematic review of the literature. BJOG 2010;117:522–31.
46. Friedman LC, Abdallah R, Schluchter M, et al. Adherence to vaginal dilation following high dose rate brachytherapy for endometrial cancer. Int J Radiat Oncol Biol Phys 2011;80:751–7.
47. Cullen K, Fergus K, DasGupta T, et al. From “sex toy” to intrusive omposition: a qualitative examination of women’s experiences with vaginal dilator use following treatment for gynecological cancer. J Sex Med 2012;9:1162–73.
48. Robinson JW, Faris PD, Scott CB. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynecological carcinoma treated with radiotherapy. Int J Radial Oncol Biol Phys 1999;44:497–506.
49. Stavraka C, Ford A, Ghaem-Maghami S, et al. A study of symptoms described by ovarian cancer survivors. Gynecol Oncol 2012;125:59–64.
50. Moonsammy SH, Guglietti CL, Mina DS, et al. A pilot study of an exercise & cognitive behavioral therapy intervention for epithelial ovarian cancer patients. J Ovarian Res 2013;6:21.
51. Goerling U, Jaeger C, Walz A, et al. The efficacy of short-term psycho-oncological interventions for women with gynaecological cancer: a randomized study. Oncology 2014;87:114–24.
52. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol 2012;125:320–5.
53. Cleary V, McCarthy G, Hegarty J. Development of an educational intervention focused on sexuality for women with gynecological cancer. J Psychosoc Oncol 2012;30:535–55.
54. Miller BE, Pittman B, Strong C. Gynecologic cancer patients’ psychosocial needs and their views on the physician›s role in meeting those needs. Int J Gynecol Cancer 2003;13:111–9.
55. Fang P, Tan K, Grover S, et al. Psychosocial encounters correlates with higher patient-reported functional quality of life in gynecological cancer patients receiving radiotherapy. Radiat Oncol 2015;10:34.
56. Hordern A, Grainger M, Hegarty S, et al. Discussing sexuality in the clinical setting: The impact of a brief training program for oncology health professionals to enhance communication about sexuality. Asia Pac J Clin Oncol 2009;5:270–7.
57. Simonelli LE, Pasipanodya E. Health disparities in unmet support needs of women with gynecologic cancer: an exploratory study. J Psychosoc Oncol 2014;32:727–34.
58. Barbera L, Fitch M, Adams L, et al. Improving care for women after gynecological cancer: the development of a sexuality clinic. Menopause 2011;18:1327–33.
59. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578–83.
60. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000;356:2059–63.
61. Wu C-S, Lu M-L, Liao Y-T, et al. Ovarian cancer and antidepressants. Psychooncology 2015;24:579–84.
62. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol 2009;29:259–66.
63. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011;305:267–74.
64. Reed SD, Guthrie KA, Joffe H, et al. Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2012;119:527–38.
65. Pérez DG, Loprinzi CL, Barton DL, et al. Pilot evaluation of mirtazapine for the treatment of hot flashes. J Support Oncol 2004;2:50–6.
66. Pérez DG, Loprinzi CL, Sloan J, et al. Pilot evaluation of bupropion for the treatment of hot flashes. J Palliat Med 2006;9:631–7.
67. Agarwal N, Singh S, Kriplani A, et al. Evaluation of gabapentin in management of hot flushes in postmenopausal women. Post Reprod Health 2014;20:36–8.
68. Guttuso T Jr, Kurlan R, McDermott MP, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337–45.
69. Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2011;29:3862–8.
70. Loibl S, Schwedler K, von Minckwitz G, et al. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients--a double-blind, randomized study. Ann Oncol 2007;18:689–93.
71. Aerts L, Enzlin P, Verhaeghe J, et al. Long-term sexual functioning in women after surgical treatment of cervical cancer stages IA to IB: a prospective controlled study. Int J Gynecol Cancer 2014;24:1527–34.
72. Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology 2007;16:691–706.
73. Pfaendler KS, Wenzel L, Mechanic MB, et al. Cervical cancer survivorship: long-term quality of life and social support. Clin Ther 2015;37:39–48.
74. Becker M, Malafy T, Bossart M, et al. Quality of life and sexual functioning in endometrial cancer survivors. Gynecol Oncol 2011;121:169–73.
75. Bifulco G, De Rosa N, Tornesello ML, et al. Quality of life, lifestyle behavior and employment experience: a comparison between young and midlife survivors of gynecology early stage cancers. Gynecol Oncol 2012;124:444–51.
76. Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause 2011;18:662–9.
77. American Cancer Society. Sexuality for the woman with cancer. 2013. Available at www.cancer.org/treatment/treatmentsandsideeffects/physicalsideeffects/sexualsideeffectsinwomen/sexualityforthewoman/index.
Allergy & Immunology
DOES PEANUT EXPOSURE INCREASE ALLERGY RISK?
Du Toit G, Roberts G, Sayre PH, et al; LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803-813. doi: 10.1056/NEJMoa1414850.
Among children at high risk for peanut allergy, early introduction of peanuts significantly reduced the risk for allergy, according to a randomized controlled trial of 640 infants with severe eczema, egg allergy, or both.
Participants ages 4 to 11 months who were high risk—based on severe atopy or allergy to eggs—were tested for sensitivity to peanut extract at baseline and then assigned to either avoid or consume peanuts.
At 60 months of age, skin allergy testing was repeated; the resulting prevalence of peanut allergy was as follows
A greater percentage of the consumption group showed an increase in levels of peanut-specific IgG4 antibody; the avoidance group, elevated titers of peanut specific IgE antibody.
COMMENTARY
The prevalence of peanut allergy in the United States has increased from 0.4% in 1997 to more than 2% in 2010.1 In 2000, the American Academy of Pediatrics recommended peanut avoidance until age 3 in children at high risk for atopic disease; then in 2008, based on emerging evidence that early introduction of allergenic foods, including peanuts, may decrease the development of allergy, the recommendations were retracted.2,3 The present study, using a randomized trial design, confirms the paradigm-changing hypothesis that early introduction of allergenic foods decreases the subsequent development of food allergies. The authors of the accompanying editorial state that these data are incontrovertible and that children at high risk for peanut allergy should, under supervision of an allergist, be tested and if skin-prick–negative for peanut allergy, be started on a peanut protein–containing diet.1—NS
1. Gruchalla RS, Sampson HA. Preventing peanut allergy through early consumption ready for prime time? N Engl J Med. 2015;372:875-876.
2. Du Toit G, Katz Y, Sasieni P, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984-991.
3. Katz Y, Rajuan N, Goldberg MR, et al. Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. J Allergy Clin Immunol. 2010;126:77-82.
Continue for Anaphylaxis guideline from AAAAI/ACAAI >>
ANAPHYLAXIS GUIDELINE FROM AAAAI/ACAAI
Campbell RL, Li JT, Nicklas RA, Sadosty AT. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599-608. doi: 10.1016/j.anai.2014.10.007.
The American Academy of Allergy, Asthma & Immunology and the American College of Allergy, Asthma, and Immunology guideline for emergency department diagnosis and treatment of anaphylaxis includes the following recommendations
• Carefully and immediately triage and monitor patients who have signs and symptoms of anaphylaxis in preparation for epinephrine administration.
• First-line treatment for patients experiencing anaphylaxis is epinephrine. It should be administered intramuscularly in the anterolateral thigh immediately after the diagnosis is made. Epinephrine can be administered every 5 to 15 minutes as needed to control symptoms.
• Do not substitute for epinephrine in the treatment of anaphylaxis. Antihistamines and corticosteroids can be administered in conjunction with epinephrine, not in place of it.
• Determine whether the patient has risk factors for severe and potentially fatal anaphylaxis, such as delayed administration of epinephrine, asthma, a history of biphasic reactions, or cardiovascular disease.
• For anaphylaxis patients with bronchospasms, administer a β-agonist.
• Patients should be observed for 4 to 8 hours (longer for those with a history of risk factors for severe anaphylaxis).
• Refer patients to an allergist–immunologist upon discharge.
COMMENTARY
Identification of anaphylaxis requires judgment. Abbreviated criteria for anaphylaxis include essentially two organ systems of involvement: skin manifestations of pruritus, flushing, hives, or angioedema; respiratory manifestations of wheezing or stridor; decreased blood pressure; and GI symptoms of vomiting, cramping abdominal pain, or diarrhea. Quick identification and treatment of anaphylaxis is important, as the median time to respiratory or cardiac arrest in food-induced anaphylaxis is only 30 minutes. All offices should stock epinephrine in an obvious place; you may consider use of a prefilled pen, so it is easy to find and to give the correct dose. —NS
Continue for Updated practice parameter: Diagnosing and treating food allergies >>
UPDATED PRACTICE PARAMETER: DIAGNOSING AND TREATING FOOD ALLERGIES
Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update—2014. J Allergy Clin Immunol. 2014. pii: S0091-6749(14)00672-1. doi: 10.1016/j.jaci.2014.05.013. [Epub ahead of print]
Over the past decade, health care providers have been confronted with a growing number of patients with suspected food allergies, but data supporting an increase in confirmed allergy cases is limited.
The 2014 practice parameter update on food allergies from the American Academy of Allergy, Asthma & Immunology advises clinicians to keep in mind that self-reported food allergy is more common than proven food allergy; that allergy is more common in children and in patients with other atopic conditions; and that the majority of allergic reactions are from peanuts, tree nuts, fish, shellfish, milk, eggs, wheat, soy, and seeds.
The update of the 2006 guidelines includes 64 new summary statements. Highlights include
• Clinicians should advise patients about the risk for cross-reactions from foods similar to their allergens, such as other tree nuts, vertebrate fish, crustaceans, or milk from cows, goats, or other mammals.
• Patients with a seafood allergy should be advised that they are not at increased risk for a reaction to radiocontrast media.
• Patients with food allergies do not need to be concerned about eating genetically modified foods (GMO), due to current FDA screening requirements to rule out allergenicity.
• Patients with chronic idiopathic urticaria or hyperactivity/attention-deficit disorder should not routinely be advised to avoid food additives.
• Patients with asthma should not routinely be advised to avoid sulfates, unless they have had a previous reaction to them.
The guidelines also include a new section on diagnosing and treating non-IgE-mediated food allergies, such as food-protein–induced enterocolitis syndrome, allergic proctocolitis, enteropathy, eosinophilic esophagitis, and gastroenteritis.
COMMENTARY
Food allergies are a commonly encountered and difficult area of practice for those of us in primary care. They can be life threatening; yet many patients who are concerned about the possibility of food allergy do not actually have a food allergy, so making an accurate diagnosis is important. Allergic evaluation starts with a careful history, and then laboratory testing can begin with specific IgE testing to foods suspected to have caused the clinical reaction of concern. The IgE results need to be carefully interpreted in light of the clinical context in which they were ordered. Oral food challenge can be helpful in addition to IgE testing. Consultation with an allergy-immunology specialist is often helpful as well. —NS
DOES PEANUT EXPOSURE INCREASE ALLERGY RISK?
Du Toit G, Roberts G, Sayre PH, et al; LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803-813. doi: 10.1056/NEJMoa1414850.
Among children at high risk for peanut allergy, early introduction of peanuts significantly reduced the risk for allergy, according to a randomized controlled trial of 640 infants with severe eczema, egg allergy, or both.
Participants ages 4 to 11 months who were high risk—based on severe atopy or allergy to eggs—were tested for sensitivity to peanut extract at baseline and then assigned to either avoid or consume peanuts.
At 60 months of age, skin allergy testing was repeated; the resulting prevalence of peanut allergy was as follows
A greater percentage of the consumption group showed an increase in levels of peanut-specific IgG4 antibody; the avoidance group, elevated titers of peanut specific IgE antibody.
COMMENTARY
The prevalence of peanut allergy in the United States has increased from 0.4% in 1997 to more than 2% in 2010.1 In 2000, the American Academy of Pediatrics recommended peanut avoidance until age 3 in children at high risk for atopic disease; then in 2008, based on emerging evidence that early introduction of allergenic foods, including peanuts, may decrease the development of allergy, the recommendations were retracted.2,3 The present study, using a randomized trial design, confirms the paradigm-changing hypothesis that early introduction of allergenic foods decreases the subsequent development of food allergies. The authors of the accompanying editorial state that these data are incontrovertible and that children at high risk for peanut allergy should, under supervision of an allergist, be tested and if skin-prick–negative for peanut allergy, be started on a peanut protein–containing diet.1—NS
1. Gruchalla RS, Sampson HA. Preventing peanut allergy through early consumption ready for prime time? N Engl J Med. 2015;372:875-876.
2. Du Toit G, Katz Y, Sasieni P, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984-991.
3. Katz Y, Rajuan N, Goldberg MR, et al. Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. J Allergy Clin Immunol. 2010;126:77-82.
Continue for Anaphylaxis guideline from AAAAI/ACAAI >>
ANAPHYLAXIS GUIDELINE FROM AAAAI/ACAAI
Campbell RL, Li JT, Nicklas RA, Sadosty AT. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599-608. doi: 10.1016/j.anai.2014.10.007.
The American Academy of Allergy, Asthma & Immunology and the American College of Allergy, Asthma, and Immunology guideline for emergency department diagnosis and treatment of anaphylaxis includes the following recommendations
• Carefully and immediately triage and monitor patients who have signs and symptoms of anaphylaxis in preparation for epinephrine administration.
• First-line treatment for patients experiencing anaphylaxis is epinephrine. It should be administered intramuscularly in the anterolateral thigh immediately after the diagnosis is made. Epinephrine can be administered every 5 to 15 minutes as needed to control symptoms.
• Do not substitute for epinephrine in the treatment of anaphylaxis. Antihistamines and corticosteroids can be administered in conjunction with epinephrine, not in place of it.
• Determine whether the patient has risk factors for severe and potentially fatal anaphylaxis, such as delayed administration of epinephrine, asthma, a history of biphasic reactions, or cardiovascular disease.
• For anaphylaxis patients with bronchospasms, administer a β-agonist.
• Patients should be observed for 4 to 8 hours (longer for those with a history of risk factors for severe anaphylaxis).
• Refer patients to an allergist–immunologist upon discharge.
COMMENTARY
Identification of anaphylaxis requires judgment. Abbreviated criteria for anaphylaxis include essentially two organ systems of involvement: skin manifestations of pruritus, flushing, hives, or angioedema; respiratory manifestations of wheezing or stridor; decreased blood pressure; and GI symptoms of vomiting, cramping abdominal pain, or diarrhea. Quick identification and treatment of anaphylaxis is important, as the median time to respiratory or cardiac arrest in food-induced anaphylaxis is only 30 minutes. All offices should stock epinephrine in an obvious place; you may consider use of a prefilled pen, so it is easy to find and to give the correct dose. —NS
Continue for Updated practice parameter: Diagnosing and treating food allergies >>
UPDATED PRACTICE PARAMETER: DIAGNOSING AND TREATING FOOD ALLERGIES
Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update—2014. J Allergy Clin Immunol. 2014. pii: S0091-6749(14)00672-1. doi: 10.1016/j.jaci.2014.05.013. [Epub ahead of print]
Over the past decade, health care providers have been confronted with a growing number of patients with suspected food allergies, but data supporting an increase in confirmed allergy cases is limited.
The 2014 practice parameter update on food allergies from the American Academy of Allergy, Asthma & Immunology advises clinicians to keep in mind that self-reported food allergy is more common than proven food allergy; that allergy is more common in children and in patients with other atopic conditions; and that the majority of allergic reactions are from peanuts, tree nuts, fish, shellfish, milk, eggs, wheat, soy, and seeds.
The update of the 2006 guidelines includes 64 new summary statements. Highlights include
• Clinicians should advise patients about the risk for cross-reactions from foods similar to their allergens, such as other tree nuts, vertebrate fish, crustaceans, or milk from cows, goats, or other mammals.
• Patients with a seafood allergy should be advised that they are not at increased risk for a reaction to radiocontrast media.
• Patients with food allergies do not need to be concerned about eating genetically modified foods (GMO), due to current FDA screening requirements to rule out allergenicity.
• Patients with chronic idiopathic urticaria or hyperactivity/attention-deficit disorder should not routinely be advised to avoid food additives.
• Patients with asthma should not routinely be advised to avoid sulfates, unless they have had a previous reaction to them.
The guidelines also include a new section on diagnosing and treating non-IgE-mediated food allergies, such as food-protein–induced enterocolitis syndrome, allergic proctocolitis, enteropathy, eosinophilic esophagitis, and gastroenteritis.
COMMENTARY
Food allergies are a commonly encountered and difficult area of practice for those of us in primary care. They can be life threatening; yet many patients who are concerned about the possibility of food allergy do not actually have a food allergy, so making an accurate diagnosis is important. Allergic evaluation starts with a careful history, and then laboratory testing can begin with specific IgE testing to foods suspected to have caused the clinical reaction of concern. The IgE results need to be carefully interpreted in light of the clinical context in which they were ordered. Oral food challenge can be helpful in addition to IgE testing. Consultation with an allergy-immunology specialist is often helpful as well. —NS
DOES PEANUT EXPOSURE INCREASE ALLERGY RISK?
Du Toit G, Roberts G, Sayre PH, et al; LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803-813. doi: 10.1056/NEJMoa1414850.
Among children at high risk for peanut allergy, early introduction of peanuts significantly reduced the risk for allergy, according to a randomized controlled trial of 640 infants with severe eczema, egg allergy, or both.
Participants ages 4 to 11 months who were high risk—based on severe atopy or allergy to eggs—were tested for sensitivity to peanut extract at baseline and then assigned to either avoid or consume peanuts.
At 60 months of age, skin allergy testing was repeated; the resulting prevalence of peanut allergy was as follows
A greater percentage of the consumption group showed an increase in levels of peanut-specific IgG4 antibody; the avoidance group, elevated titers of peanut specific IgE antibody.
COMMENTARY
The prevalence of peanut allergy in the United States has increased from 0.4% in 1997 to more than 2% in 2010.1 In 2000, the American Academy of Pediatrics recommended peanut avoidance until age 3 in children at high risk for atopic disease; then in 2008, based on emerging evidence that early introduction of allergenic foods, including peanuts, may decrease the development of allergy, the recommendations were retracted.2,3 The present study, using a randomized trial design, confirms the paradigm-changing hypothesis that early introduction of allergenic foods decreases the subsequent development of food allergies. The authors of the accompanying editorial state that these data are incontrovertible and that children at high risk for peanut allergy should, under supervision of an allergist, be tested and if skin-prick–negative for peanut allergy, be started on a peanut protein–containing diet.1—NS
1. Gruchalla RS, Sampson HA. Preventing peanut allergy through early consumption ready for prime time? N Engl J Med. 2015;372:875-876.
2. Du Toit G, Katz Y, Sasieni P, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984-991.
3. Katz Y, Rajuan N, Goldberg MR, et al. Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. J Allergy Clin Immunol. 2010;126:77-82.
Continue for Anaphylaxis guideline from AAAAI/ACAAI >>
ANAPHYLAXIS GUIDELINE FROM AAAAI/ACAAI
Campbell RL, Li JT, Nicklas RA, Sadosty AT. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599-608. doi: 10.1016/j.anai.2014.10.007.
The American Academy of Allergy, Asthma & Immunology and the American College of Allergy, Asthma, and Immunology guideline for emergency department diagnosis and treatment of anaphylaxis includes the following recommendations
• Carefully and immediately triage and monitor patients who have signs and symptoms of anaphylaxis in preparation for epinephrine administration.
• First-line treatment for patients experiencing anaphylaxis is epinephrine. It should be administered intramuscularly in the anterolateral thigh immediately after the diagnosis is made. Epinephrine can be administered every 5 to 15 minutes as needed to control symptoms.
• Do not substitute for epinephrine in the treatment of anaphylaxis. Antihistamines and corticosteroids can be administered in conjunction with epinephrine, not in place of it.
• Determine whether the patient has risk factors for severe and potentially fatal anaphylaxis, such as delayed administration of epinephrine, asthma, a history of biphasic reactions, or cardiovascular disease.
• For anaphylaxis patients with bronchospasms, administer a β-agonist.
• Patients should be observed for 4 to 8 hours (longer for those with a history of risk factors for severe anaphylaxis).
• Refer patients to an allergist–immunologist upon discharge.
COMMENTARY
Identification of anaphylaxis requires judgment. Abbreviated criteria for anaphylaxis include essentially two organ systems of involvement: skin manifestations of pruritus, flushing, hives, or angioedema; respiratory manifestations of wheezing or stridor; decreased blood pressure; and GI symptoms of vomiting, cramping abdominal pain, or diarrhea. Quick identification and treatment of anaphylaxis is important, as the median time to respiratory or cardiac arrest in food-induced anaphylaxis is only 30 minutes. All offices should stock epinephrine in an obvious place; you may consider use of a prefilled pen, so it is easy to find and to give the correct dose. —NS
Continue for Updated practice parameter: Diagnosing and treating food allergies >>
UPDATED PRACTICE PARAMETER: DIAGNOSING AND TREATING FOOD ALLERGIES
Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update—2014. J Allergy Clin Immunol. 2014. pii: S0091-6749(14)00672-1. doi: 10.1016/j.jaci.2014.05.013. [Epub ahead of print]
Over the past decade, health care providers have been confronted with a growing number of patients with suspected food allergies, but data supporting an increase in confirmed allergy cases is limited.
The 2014 practice parameter update on food allergies from the American Academy of Allergy, Asthma & Immunology advises clinicians to keep in mind that self-reported food allergy is more common than proven food allergy; that allergy is more common in children and in patients with other atopic conditions; and that the majority of allergic reactions are from peanuts, tree nuts, fish, shellfish, milk, eggs, wheat, soy, and seeds.
The update of the 2006 guidelines includes 64 new summary statements. Highlights include
• Clinicians should advise patients about the risk for cross-reactions from foods similar to their allergens, such as other tree nuts, vertebrate fish, crustaceans, or milk from cows, goats, or other mammals.
• Patients with a seafood allergy should be advised that they are not at increased risk for a reaction to radiocontrast media.
• Patients with food allergies do not need to be concerned about eating genetically modified foods (GMO), due to current FDA screening requirements to rule out allergenicity.
• Patients with chronic idiopathic urticaria or hyperactivity/attention-deficit disorder should not routinely be advised to avoid food additives.
• Patients with asthma should not routinely be advised to avoid sulfates, unless they have had a previous reaction to them.
The guidelines also include a new section on diagnosing and treating non-IgE-mediated food allergies, such as food-protein–induced enterocolitis syndrome, allergic proctocolitis, enteropathy, eosinophilic esophagitis, and gastroenteritis.
COMMENTARY
Food allergies are a commonly encountered and difficult area of practice for those of us in primary care. They can be life threatening; yet many patients who are concerned about the possibility of food allergy do not actually have a food allergy, so making an accurate diagnosis is important. Allergic evaluation starts with a careful history, and then laboratory testing can begin with specific IgE testing to foods suspected to have caused the clinical reaction of concern. The IgE results need to be carefully interpreted in light of the clinical context in which they were ordered. Oral food challenge can be helpful in addition to IgE testing. Consultation with an allergy-immunology specialist is often helpful as well. —NS
Treating Depression: What Works Besides Meds?
› Recommend cognitive behavioral therapy, interpersonal therapy, or problem-solving therapy for the treatment of depression in patients of all ages. A
› Consider prescribing exercise as a stand-alone or adjunctive treatment for patients with depression. B
› Advise patients who ask about omega-3 fatty acid supplements that formulations with a high eicosapentaenoic acid (EPA) to docosahexaenoic acid (DHA) ratio (2:1) may be a useful “add-on” to their current regimen. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Steve J, age 43, comes to your clinic looking uncharacteristically glum. He was recently downsized from his job and misses his former colleagues. His job loss has caused a financial strain for his family, and he admits to crying in the shower when he thinks about how his life has turned out. Mr. J tells you that he’s gotten a part-time job, but he’s already called in sick several times. On those sick days he “stayed in bed all day and slept.” He says that when he does go to work, he rarely interacts with his coworkers and his concentration is poor. He tells you he wakes up early in the morning on most days and cannot return to sleep, despite being “tired all the time.” He denies suicidal ideation. Mr. J has never felt this way before, which is what prompted his visit today, but he thinks it is “weak to take a pill to feel better.”
What nonpharmacologic options can you offer him?
CASE 2 › Kerri S is a 27-year-old mother of 2 who comes to your clinic to establish care. She tells you about a recent recurrence of depressed mood, which she feels is due to the stress of moving to the area. She is experiencing sleep-onset insomnia and concentration lapses. Her appetite is poor (self-reported 8-lb weight loss in 2 months) and she lacks the motivation to engage in her daily activities, saying, “I wouldn’t even get out of bed if my kids didn’t need me.” She notes that she is constantly irritable and has completely lost her sex drive. Unlike her prior depressive episode, she has not had any suicidal thoughts. Mrs. S was previously successfully treated with paroxetine, 20 mg/d, but she is not interested in restarting her medication because she is still breastfeeding her toddler.
Are there evidence-based options for her care that do not include medication?
Major depressive disorder (MDD) is widespread and often disabling, affecting nearly 8% of people ages 12 and older at any given time.1 Thus, it’s crucial to be familiar with the diverse array of evidence-based treatment options from which patients can choose. Although medications are an essential treatment option for patients with severe depression, their value for patients with mild to moderate depression is often limited.2 In addition, when antidepressants aren’t combined with psychosocial interventions, discontinuing them is associated with relapse.3
Fortunately, research has found that certain nonpharmacologic interventions—including psychotherapies, somatic therapies, and dietary supplements—can have either therapeutic or adjunctive benefits for treating depression, and can be provided in ways that are time- and cost-effective. This article reviews the evidence supporting several options in each of these treatment categories.
Evidence backs several types of psychotherapy
Several recent meta-analyses suggest that a variety of psychotherapeutic treatments may hold promise for your patients with depression.4,5 When analyses were limited to larger studies in order to decrease the risk of bias, cognitive behavioral therapy (CBT), interpersonal therapy (IPT), and problem-solving therapy (PST) all resulted in moderate to large improvement in depressive symptoms when compared to wait-list controls.4 These findings were echoed in a recent systematic review/meta-analysis that focused on depressed primary care patients. Linde et al5 found that the number needed to treat (NNT) to achieve one response (≥50% reduction in score on a depression scale) using any type of psychotherapy was 10, and the NNT to achieve one remission (scoring below a predefined score on a depression scale) was 15.
Psychotherapy can be effective when provided in individual and group settings,6 as well as via telephone, the Internet, or software programs.7 (For a list of self-help, computerized, and Internet-based resources, see TABLE W1 below.)
CBT has been studied for several decades and there’s strong evidence for its efficacy.6 Recent investigations have suggested that CBT delivered in less resource-intensive modes (such as via computer program, Internet, telephone, or videoconferencing) can be as effective as face-to-face CBT.6,8 CBT has been shown to be helpful for a wide range of patients,6 improves outcomes over standard primary care treatment,9 and provides a useful adjunct to medication in treatment-resistant severe depression.10
Behavioral activation (BA), which generally is included as a component of CBT, has received support as an independent treatment, and may produce therapeutic results similar to CBT11 and PST (which we’ll discuss in a bit).12 The core components of BA are scheduling pleasant activities and increasing the patient’s positive interactions with his or her environment by decreasing avoidance, withdrawal, and inactivity.11 Compared to CBT, BA is easier for clinicians to learn and incorporate into primary care visits, and it may be especially useful as an adjunctive or first-step intervention in outpatient clinics.11 Like CBT, BA can be effective in diverse patient groups13,14 and can be provided using novel delivery modes, such as via the Internet.15
IPT is a supportive, structured, brief therapy (12-16 visits) that focuses on helping patients identify and solve current situation- and relationship-based problems that stem from or contribute to their depression.16 Enhancing the patient’s interpersonal communication—including improving social skills, assertiveness, and appropriate expression of anger—is typically a component of IPT. Like CBT, IPT has been found to be effective for treating depression when administered in person, in group therapy, or via the phone or Internet, and across a broad age range.17-19
PST involves teaching patients a structured problem-solving process to decrease interpersonal strain and improve positive life experiences.20 Patients are taught to define their problem, generate and evaluate multiple solutions for it, implement a plan for the solution, and evaluate the results. In addition to being used to successfully treat adults,4,5 PST has been adapted effectively to treat adolescents16 and older adults.18
Somatic therapies are also an option
Exercise has long been considered a possible depression treatment due to its activity on endorphin, monoamine, and cortisol levels and via increased social and general activity. A 2013 Cochrane review of 39 randomized control trials (RCTs; N=2326) assessed whether exercise was effective for treating depression in adults.21 Thirty-five trials found a moderate effect size when specifically comparing exercise to no treatment or control interventions. The effect size was reduced, however, when analyses were restricted to trials with the highest methodological quality. There was no statistically significant difference when exercise was compared to pharmacologic treatment or psychotherapy.
Although the amount of research is meager, small but statistically significant improvements have also been found for older adults22 and children/adolescents.23 There is no consensus on the type, frequency, or intensity of exercise needed to achieve benefit. However, because nearly all studies for all age groups have found that exercise has no adverse psychological effects and substantial positive physical effects, exercise should be recommended to all patients with depression unless contraindicated.
Yoga (both exercise-based and meditation-based) has been evaluated both as a sole treatment and as an adjunctive treatment for depression. Several studies have supported the impact of yoga, particularly in pregnant women,24 although the evidence for its efficacy is inconsistent, with yoga frequently failing to improve upon the outcome of waitlist control.25 The evidence for meditation and mindfulness is more consistently positive, with these interventions equaling or exceeding “treatment as usual,” other psychotherapies, and antidepressants in numerous RCTs.25
Electroconvulsive therapy (ECT) has a substantial evidence base supporting its efficacy.26 ECT has been used for decades, although stigma, cardiac and memory risks, and risks of anesthesia often limit its use. Benefits of ECT include a rapid response relative to pharmacotherapy (>50% of patients respond by the end of the first week of ECT)27 and a strong response in older patients.28
In repetitive transcranial magnetic stimulation (rTMS), electromagnetic coils are placed on a patient’s head to deliver electromagnetic pulses that stimulate areas of the brain that regulate mood. Although rTMS is not widely available, a growing body of evidence supports its use for treating depression, including a meta-analysis of 34 RCTs that included 1383 patients.29 A multisite RCT (N=190) that was not industry-funded reported a 15% response rate and 60% maintenance of remission at 3 months (NNT=12).30 Although ECT is more effective than rTMS, rTMS appears useful for treatment-resistant depression, and can be used as an adjunctive treatment.29,31
Dietary supplements may be best used as adjuncts
St. John’s wort (Hypericum perforatum), which contains 2 bioactive ingredients (hyperforin and hypericin), has been effectively used to treat depression.32 A 2008 Cochrane review that was limited to high-quality trials involving patients meeting Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for depression identified 29 trials (N=5489), of which 18 involved comparisons with placebo and 17 with standard antidepressants.33 Patients’ depression was rated mild to moderate in 19 studies and moderate to severe in 9 studies. Trials examined 4 to 12 weeks of treatment with Hypericum extracts. This study (and several published since) provides strong clinical evidence supporting the efficacy of St. John’s wort for mild to moderate depression. There is insufficient evidence for its use for severe major depression.33TABLE 1 contains dosing information for St. John’s wort and other supplements used to treat depression.34-36
S-adenosyl-L-methionine (SAMe). In a 2003 systematic review,37 1600 mg/d of oral SAMe was found to significantly benefit patients with depression in 4 of 5 studies, as did parenteral SAMe (7 of 7 trials). Another review of 48 studies found SAMe was safe and effective for depression.38 SAMe has been proposed for use alone or in combination with an antidepressant.
Folate and folic acid. Low folate levels have been associated with a less robust response to antidepressants in patients with MDD,39 and higher folate levels appear to be associated with better antidepressant response.40 A 2003 Cochrane review suggested folate might have a role in treating depression.39 A 2009 study found folate supplementation could reduce depressive symptoms for patients with normal baseline folate levels as well as those with low levels.41 Although the evidence is equivocal, folate augmentation may enhance antidepressant efficacy or improve response/remission rates.41,42
It seems reasonable to check folate levels in depressed patients, and address deficiencies by instructing patients to increase their dietary intake of folate or to take supplements. Augmenting antidepressants with folate appears to be low-risk and possibly helpful in maintaining remission.
Omega-3 fatty acids. There is substantial evidence that omega-3 fatty acids can prevent and treat depression.43,44 Recent meta-analyses support the use of omega-3 fatty acids as monotherapy and augmentation, but only formulations that contain a high eicosapentaenoic acid (EPA) to docosahexaenoic acid (DHA) ratio (EPA/DHA 2:1).45,46 Omega-3 supplementation has been used with positive results in older adults, children,47 pregnant women,48 and women with postpartum depression.49 Although initial research into omega-3 treatment of depression appears promising, augmentation of standard antidepressant therapy may be a good conservative option.
Use a validated tool to monitor response to treatment
You can enhance outcomes for your patients with depression if you schedule routine follow-up visits with them to gauge adherence to recommendations, monitor response to treatment, and increase the intensity of care when response is inadequate.50 The most important aspect of monitoring response is to use a standardized instrument that quantifies symptoms at every visit.
The Patient Health Questionnaire 9-item depression assessment (PHQ-9)—which is free—has been validated for depression screening and monitoring of treatment response in primary care patients.51 A decrease of 5 points on the PHQ-9 is the minimum considered to be clinically significant.52 Other well-validated, although lengthier, self-report depression assessment and monitoring instruments include the Beck Depression Inventory-revised and the Zung Depression Scale.
CASE 1 › Mr. J is not enjoying his new job or engaging with new coworkers to replace the positive social experiences he had at his previous job. Together, you set a goal of increasing social involvement by having him make plans to see at least one friend per weekend. Because he indicates that he is unlikely to follow through with a therapy referral, you encourage him to try an online CBT program, start an exercise regimen, or take a SAMe supplement. Mr. Jackson agrees to try the CBT and exercise (moderate intensity, 30 minutes 3-4 times per week), but does not want to take SAMe. He agrees to an assessment of his folate levels, which are normal.
Mr. J starts the online CBT program, which reinforces the exercise and social activity prescription you provided. He establishes a regular exercise routine with a good friend. After one month, his mood has started to improve and he has added regular participation in a hobby (woodworking), as well as volunteer work, which he finds fulfilling. You plan to continue monitoring his depression and his adherence to the treatment plan.
CASE 2 › The recent move has decreased Mrs. S’s interactions with family and long-time friends. Because she had previously expressed interest in exercise, you encourage her to join a local “Mommy and Me” exercise and support group for mothers of toddlers. She is willing to participate in psychotherapy, so you provide a referral to a local therapist with expertise in IPT. You also discuss with Mrs. S the possible benefits of omega-3 fatty acid supplementation, which appears to be safe during breastfeeding.34
Mrs. S begins therapy and exercise classes, but can’t motivate herself to continue either of these activities. She becomes discouraged because she’s unable to easily find an omega-3 fatty acid supplement with the ratio you specified (EPA/DHA 2:1). When you see her 2 weeks later, her depression has worsened.
Because you are concerned her suicidality will return, you revisit the pros and cons of taking an antidepressant. Although small amounts of antidepressants can be passed from mother to infant via breastmilk, the amount varies by specific medication, as do the potential risks. Mrs. S decides to resume taking paroxetine 20 mg/d and eventually, once her motivation improves, she’s able to add psychotherapy and exercise to her maintenance/relapse prevention regimen. After you discuss with her the possibility that B vitamin supplementation may assist in maintenance of remission, she adds L-methylfolate 7.5 mg/day to her regimen.
CORRESPONDENCE
Michele M. Larzelere, PhD; LSUHSC Department of Family Medicine; 200 W. Esplanade Avenue, Suite 409; Kenner, LA 70065; [email protected]
1. Centers for Disease Control and Prevention (CDC). QuickStats: Prevalence of Current Depression Among Persons Aged ≥12 Years, by Age Group and Sex — United States, National Health and Nutrition Examination Survey, 2007–2010. CDC Morbidity and Mortality Weekly Report Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6051a7.htm. Accessed June 11, 2015.
2. Fournier J, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303:47-53.
3. Dobson KS, Hollon SD, Dimidjian S, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol. 2008;76:468-477.
4. Barth J, Munder T, Gerger H, et al. Comparative efficacy of seven psychotherapeutic interventions for patients with depression: A network meta-analysis. PLoS Med. 2013;10:e1001454.
5. Linde K, Sigterman K, Kriston L, et al. Effectiveness of psychological treatments for depressive disorders in primary care: systematic review and meta-analysis. Ann Fam Med. 2015;13:56-68.
6. DeRubeis RJ, Webb CA, Tang TZ, et al. Cognitive therapy. In: Dobson KS, ed. Handbook of Cognitive Behavioral Therapies, 3rd ed. New York, NY: Guilford; 2009:277-316.
7. Andersson G, Cuijpers P. Internet-based and other computerized psychological treatments for adult depression: a meta-analysis. Cogn Behav Ther. 2008;38:196-205.
8. Andersson G, Cuijpers P, Carlbring P, et al. Guided internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: a systematic review and meta-analysis. World Psychiatry. 2014;13:288-295.
9. Twomey C, O’Reilly G, Byrne M. Effectiveness of cognitive behavioral therapy for anxiety and depression in primary care: a meta-analysis. Fam Pract. 2015;32:3-15.
10. Zhou X, Michael K, Liu Y, et al. Systematic review of management for treatment-resistant depression in adolescents. BMC Psychiatry. 2014;14:340.
11. Ekers D, Webster L, Van Straten A, et al. Behavioural activation for depression: An update of meta-analysis of effectiveness and sub group analysis. PLoS One. 2014;9:e100100.
12. Alexopoulos GS, Raue PJ, Kiosses DN, et al. Comparing engage with PST in late-life major depression: A preliminary report. Am J Geriatr Psychiatry. 2015;23:506-513.
13. Soucy Chartier I, Provencher MD. Behavioral activation for depression: Efficacy, effectiveness, and dissemination. J Affect Disord. 2013;145:292-299.
14. McCauley E, Gudmundson G, Schloredt K, et al. The Adolescent Behavior Activation Program: Adapting behavioral activation as a treatment for depression in adolescence. J Clin Child Adolesc Psychol. 2015;1-14. [Epub ahead of print].
15. Carlbring P, Hägglund M, Luthström A, et al. Internet-based behavioral activation and acceptance-based treatment for depression: a randomized controlled trial. J Affect Disord. 2013;148:331-337.
16. Markowitz JC, Weissman MM. Interpersonal psychotherapy: principles and applications. World Psychiatry. 2004; 3:136-139.
17. Kersting A, Kroker K, Schlicht S, et al. Efficacy of a cognitive-behavioral internet-based therapy in parents after the loss of a child during pregnancy: pilot data from a randomized controlled trial. Arch Womens Mental Health. 2011;14:465-477.
18. Francis J, Kumar A. Psychological treatment of late-life depression. Psychiatr Clin North Am. 2013;36:561-575.
19. Picardi A, Gaetano P. Psychotherapy of mood disorders. Clin Pract Epidemiol Ment Health. 2014;10:140-158.
20. Bell AC, D’Zurilla TJ. Problem-solving therapy for depression: a meta-analysis. Clin Psychol Review. 2009;29:348-353.
21. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;9:CD004366
22. Brindle C, Spanjers K, Patel S, et al. Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomized controlled trials. B J Psychiatry. 2012;201:180-185.
23. Brown HE, Pearson N, Braithwaite RE, et al. Physical activity interventions and depression in children and adolescents: a systematic review and meta-analysis. Sports Med. 2013;43:195-206.
24. Gong H, Ni C, Shen X, et al. Yoga for prenatal depression: a systematic review and meta-analysis. BMC Psychiatry. 2015;15:14.
25. D’Silva S, Poscablo C, Habousha R, et al. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407-423.
26. Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357:1939-1945.
27. Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry. 2004;65:485-491.
28. Rhebergen D, Huisman A, Bouckaert F, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. 2015;23:274-282.
29. Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A metaanalysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873-884.
30. George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507-516
31. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham controlled studies. BMC Psychiatry. 2014;14:342.
32. Brown RP, Gerberg PL, Muskin PR. Mood disorders. In: Brown RP, Gerbarg PL, Muskin P. How to Use Herbs, Nutrients and Yoga in Mental Health. New York, NY: WW Norton & Company; 2009.
33. Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;(4):CD000448.
34. Natural Medicines Comprehensive Database. Natural Medicines Comprehensive Database Web site. Available at: http://naturaldatabase.therapeuticresearch.com/home.aspx. Accessed March 1, 2015.
35. Harris WS. Expert opinion: omega-3 fatty acids and bleeding-cause for concern? Am J Cardiol. 2007;99:44C-6C.
36. Freeman MP, Fava M, Lake J, et al. Complementary and alternative medicine in major depressive disorder: the American Psychiatric Association Task Force Report. J Clin Psychiatry. 2010;71:669-681.
37. Papakostas GI, Alpert JE, Fava M. S-adenosyl-methionine in depression: a comprehensive review of the literature. Curr Psychiatry Reports. 2003;5:460-466.
38. Brown RP, Gerbarg PL, Bottiglieri T. S-Adenosylmethionine (SAMe) for depression: biochemical and clinical evidence. Psychiatr Ann. 2002;32:29-44.
39. Taylor MJ, Carney S, Geddes J, et al. Folate for depressive disorders. Cochrane Database Syst Rev. 2003;(2):CD003390.
40. Alpert M, Silva RR, Pouget ER. Prediction of treatment response in geriatric depression from baseline folate level: interaction with an SSRI or a tricyclic antidepressant. J Clin Psychopharmacol. 2003;23:309-313.
41. Fava M, Mischoulon D. Folate in depression: efficacy, safety, differences in formulations, and clinical issues. J Clin Psychiatry. 2009;70(suppl 5):12-17.
42. Almeida OP, Ford AH, Hirani V, et al. B vitamins to enhance treatment response to antidepressants in middle-aged and older adults: results from the B-VITAGE randomised, double-blind, placebo-controlled trial. Br J Psychiatry. 2014;205:450-457.
43. Grosso G, Galvano F, Marventano S, et al. Omega-3 fatty acids and depression: scientific evidence and biological mechanisms. Oxid Med Cell Longev. 2014;2014:313570.
44. Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91:757-770.
45. Grosso G, Pajak A, Marventano S, et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive metaanalysis of randomized clinical trials. PLoS One. 2014;9:e96905.
46. Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012;17:1144-1149.
47. Nemets H, Nemets B, Apter A, et al. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163:1098-1100.
48. Su KP, Huang SY, Chiu TH. Omega-3 fatty acids for major depressive disorder during pregnancy: Results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:644-651.
49. Freeman MP, Davis M, Sinha P, et al. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J Affect Disord. 2008;110:142-148.
50. Mitchell J, Trangle M, Degnan B, et al. Institute for Clinical Systems Improvement (ICSI). Health Care Guideline: Adult depression in primary care. 16th ed. September 2013. Available at: https://www.icsi.org/_asset/fnhdm3/Depr-Interactive0512b.pdf. Accessed June 9, 2015.
51. Kroenke K, Spitzer RL, Williams JBW, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359.
52. Trivedi MH. Tools and strategies for ongoing assessment of depression:
a measurement-based approach to remission. J Clin Psychiatry. 2009;70:26-31.
› Recommend cognitive behavioral therapy, interpersonal therapy, or problem-solving therapy for the treatment of depression in patients of all ages. A
› Consider prescribing exercise as a stand-alone or adjunctive treatment for patients with depression. B
› Advise patients who ask about omega-3 fatty acid supplements that formulations with a high eicosapentaenoic acid (EPA) to docosahexaenoic acid (DHA) ratio (2:1) may be a useful “add-on” to their current regimen. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Steve J, age 43, comes to your clinic looking uncharacteristically glum. He was recently downsized from his job and misses his former colleagues. His job loss has caused a financial strain for his family, and he admits to crying in the shower when he thinks about how his life has turned out. Mr. J tells you that he’s gotten a part-time job, but he’s already called in sick several times. On those sick days he “stayed in bed all day and slept.” He says that when he does go to work, he rarely interacts with his coworkers and his concentration is poor. He tells you he wakes up early in the morning on most days and cannot return to sleep, despite being “tired all the time.” He denies suicidal ideation. Mr. J has never felt this way before, which is what prompted his visit today, but he thinks it is “weak to take a pill to feel better.”
What nonpharmacologic options can you offer him?
CASE 2 › Kerri S is a 27-year-old mother of 2 who comes to your clinic to establish care. She tells you about a recent recurrence of depressed mood, which she feels is due to the stress of moving to the area. She is experiencing sleep-onset insomnia and concentration lapses. Her appetite is poor (self-reported 8-lb weight loss in 2 months) and she lacks the motivation to engage in her daily activities, saying, “I wouldn’t even get out of bed if my kids didn’t need me.” She notes that she is constantly irritable and has completely lost her sex drive. Unlike her prior depressive episode, she has not had any suicidal thoughts. Mrs. S was previously successfully treated with paroxetine, 20 mg/d, but she is not interested in restarting her medication because she is still breastfeeding her toddler.
Are there evidence-based options for her care that do not include medication?
Major depressive disorder (MDD) is widespread and often disabling, affecting nearly 8% of people ages 12 and older at any given time.1 Thus, it’s crucial to be familiar with the diverse array of evidence-based treatment options from which patients can choose. Although medications are an essential treatment option for patients with severe depression, their value for patients with mild to moderate depression is often limited.2 In addition, when antidepressants aren’t combined with psychosocial interventions, discontinuing them is associated with relapse.3
Fortunately, research has found that certain nonpharmacologic interventions—including psychotherapies, somatic therapies, and dietary supplements—can have either therapeutic or adjunctive benefits for treating depression, and can be provided in ways that are time- and cost-effective. This article reviews the evidence supporting several options in each of these treatment categories.
Evidence backs several types of psychotherapy
Several recent meta-analyses suggest that a variety of psychotherapeutic treatments may hold promise for your patients with depression.4,5 When analyses were limited to larger studies in order to decrease the risk of bias, cognitive behavioral therapy (CBT), interpersonal therapy (IPT), and problem-solving therapy (PST) all resulted in moderate to large improvement in depressive symptoms when compared to wait-list controls.4 These findings were echoed in a recent systematic review/meta-analysis that focused on depressed primary care patients. Linde et al5 found that the number needed to treat (NNT) to achieve one response (≥50% reduction in score on a depression scale) using any type of psychotherapy was 10, and the NNT to achieve one remission (scoring below a predefined score on a depression scale) was 15.
Psychotherapy can be effective when provided in individual and group settings,6 as well as via telephone, the Internet, or software programs.7 (For a list of self-help, computerized, and Internet-based resources, see TABLE W1 below.)
CBT has been studied for several decades and there’s strong evidence for its efficacy.6 Recent investigations have suggested that CBT delivered in less resource-intensive modes (such as via computer program, Internet, telephone, or videoconferencing) can be as effective as face-to-face CBT.6,8 CBT has been shown to be helpful for a wide range of patients,6 improves outcomes over standard primary care treatment,9 and provides a useful adjunct to medication in treatment-resistant severe depression.10
Behavioral activation (BA), which generally is included as a component of CBT, has received support as an independent treatment, and may produce therapeutic results similar to CBT11 and PST (which we’ll discuss in a bit).12 The core components of BA are scheduling pleasant activities and increasing the patient’s positive interactions with his or her environment by decreasing avoidance, withdrawal, and inactivity.11 Compared to CBT, BA is easier for clinicians to learn and incorporate into primary care visits, and it may be especially useful as an adjunctive or first-step intervention in outpatient clinics.11 Like CBT, BA can be effective in diverse patient groups13,14 and can be provided using novel delivery modes, such as via the Internet.15
IPT is a supportive, structured, brief therapy (12-16 visits) that focuses on helping patients identify and solve current situation- and relationship-based problems that stem from or contribute to their depression.16 Enhancing the patient’s interpersonal communication—including improving social skills, assertiveness, and appropriate expression of anger—is typically a component of IPT. Like CBT, IPT has been found to be effective for treating depression when administered in person, in group therapy, or via the phone or Internet, and across a broad age range.17-19
PST involves teaching patients a structured problem-solving process to decrease interpersonal strain and improve positive life experiences.20 Patients are taught to define their problem, generate and evaluate multiple solutions for it, implement a plan for the solution, and evaluate the results. In addition to being used to successfully treat adults,4,5 PST has been adapted effectively to treat adolescents16 and older adults.18
Somatic therapies are also an option
Exercise has long been considered a possible depression treatment due to its activity on endorphin, monoamine, and cortisol levels and via increased social and general activity. A 2013 Cochrane review of 39 randomized control trials (RCTs; N=2326) assessed whether exercise was effective for treating depression in adults.21 Thirty-five trials found a moderate effect size when specifically comparing exercise to no treatment or control interventions. The effect size was reduced, however, when analyses were restricted to trials with the highest methodological quality. There was no statistically significant difference when exercise was compared to pharmacologic treatment or psychotherapy.
Although the amount of research is meager, small but statistically significant improvements have also been found for older adults22 and children/adolescents.23 There is no consensus on the type, frequency, or intensity of exercise needed to achieve benefit. However, because nearly all studies for all age groups have found that exercise has no adverse psychological effects and substantial positive physical effects, exercise should be recommended to all patients with depression unless contraindicated.
Yoga (both exercise-based and meditation-based) has been evaluated both as a sole treatment and as an adjunctive treatment for depression. Several studies have supported the impact of yoga, particularly in pregnant women,24 although the evidence for its efficacy is inconsistent, with yoga frequently failing to improve upon the outcome of waitlist control.25 The evidence for meditation and mindfulness is more consistently positive, with these interventions equaling or exceeding “treatment as usual,” other psychotherapies, and antidepressants in numerous RCTs.25
Electroconvulsive therapy (ECT) has a substantial evidence base supporting its efficacy.26 ECT has been used for decades, although stigma, cardiac and memory risks, and risks of anesthesia often limit its use. Benefits of ECT include a rapid response relative to pharmacotherapy (>50% of patients respond by the end of the first week of ECT)27 and a strong response in older patients.28
In repetitive transcranial magnetic stimulation (rTMS), electromagnetic coils are placed on a patient’s head to deliver electromagnetic pulses that stimulate areas of the brain that regulate mood. Although rTMS is not widely available, a growing body of evidence supports its use for treating depression, including a meta-analysis of 34 RCTs that included 1383 patients.29 A multisite RCT (N=190) that was not industry-funded reported a 15% response rate and 60% maintenance of remission at 3 months (NNT=12).30 Although ECT is more effective than rTMS, rTMS appears useful for treatment-resistant depression, and can be used as an adjunctive treatment.29,31
Dietary supplements may be best used as adjuncts
St. John’s wort (Hypericum perforatum), which contains 2 bioactive ingredients (hyperforin and hypericin), has been effectively used to treat depression.32 A 2008 Cochrane review that was limited to high-quality trials involving patients meeting Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for depression identified 29 trials (N=5489), of which 18 involved comparisons with placebo and 17 with standard antidepressants.33 Patients’ depression was rated mild to moderate in 19 studies and moderate to severe in 9 studies. Trials examined 4 to 12 weeks of treatment with Hypericum extracts. This study (and several published since) provides strong clinical evidence supporting the efficacy of St. John’s wort for mild to moderate depression. There is insufficient evidence for its use for severe major depression.33TABLE 1 contains dosing information for St. John’s wort and other supplements used to treat depression.34-36
S-adenosyl-L-methionine (SAMe). In a 2003 systematic review,37 1600 mg/d of oral SAMe was found to significantly benefit patients with depression in 4 of 5 studies, as did parenteral SAMe (7 of 7 trials). Another review of 48 studies found SAMe was safe and effective for depression.38 SAMe has been proposed for use alone or in combination with an antidepressant.
Folate and folic acid. Low folate levels have been associated with a less robust response to antidepressants in patients with MDD,39 and higher folate levels appear to be associated with better antidepressant response.40 A 2003 Cochrane review suggested folate might have a role in treating depression.39 A 2009 study found folate supplementation could reduce depressive symptoms for patients with normal baseline folate levels as well as those with low levels.41 Although the evidence is equivocal, folate augmentation may enhance antidepressant efficacy or improve response/remission rates.41,42
It seems reasonable to check folate levels in depressed patients, and address deficiencies by instructing patients to increase their dietary intake of folate or to take supplements. Augmenting antidepressants with folate appears to be low-risk and possibly helpful in maintaining remission.
Omega-3 fatty acids. There is substantial evidence that omega-3 fatty acids can prevent and treat depression.43,44 Recent meta-analyses support the use of omega-3 fatty acids as monotherapy and augmentation, but only formulations that contain a high eicosapentaenoic acid (EPA) to docosahexaenoic acid (DHA) ratio (EPA/DHA 2:1).45,46 Omega-3 supplementation has been used with positive results in older adults, children,47 pregnant women,48 and women with postpartum depression.49 Although initial research into omega-3 treatment of depression appears promising, augmentation of standard antidepressant therapy may be a good conservative option.
Use a validated tool to monitor response to treatment
You can enhance outcomes for your patients with depression if you schedule routine follow-up visits with them to gauge adherence to recommendations, monitor response to treatment, and increase the intensity of care when response is inadequate.50 The most important aspect of monitoring response is to use a standardized instrument that quantifies symptoms at every visit.
The Patient Health Questionnaire 9-item depression assessment (PHQ-9)—which is free—has been validated for depression screening and monitoring of treatment response in primary care patients.51 A decrease of 5 points on the PHQ-9 is the minimum considered to be clinically significant.52 Other well-validated, although lengthier, self-report depression assessment and monitoring instruments include the Beck Depression Inventory-revised and the Zung Depression Scale.
CASE 1 › Mr. J is not enjoying his new job or engaging with new coworkers to replace the positive social experiences he had at his previous job. Together, you set a goal of increasing social involvement by having him make plans to see at least one friend per weekend. Because he indicates that he is unlikely to follow through with a therapy referral, you encourage him to try an online CBT program, start an exercise regimen, or take a SAMe supplement. Mr. Jackson agrees to try the CBT and exercise (moderate intensity, 30 minutes 3-4 times per week), but does not want to take SAMe. He agrees to an assessment of his folate levels, which are normal.
Mr. J starts the online CBT program, which reinforces the exercise and social activity prescription you provided. He establishes a regular exercise routine with a good friend. After one month, his mood has started to improve and he has added regular participation in a hobby (woodworking), as well as volunteer work, which he finds fulfilling. You plan to continue monitoring his depression and his adherence to the treatment plan.
CASE 2 › The recent move has decreased Mrs. S’s interactions with family and long-time friends. Because she had previously expressed interest in exercise, you encourage her to join a local “Mommy and Me” exercise and support group for mothers of toddlers. She is willing to participate in psychotherapy, so you provide a referral to a local therapist with expertise in IPT. You also discuss with Mrs. S the possible benefits of omega-3 fatty acid supplementation, which appears to be safe during breastfeeding.34
Mrs. S begins therapy and exercise classes, but can’t motivate herself to continue either of these activities. She becomes discouraged because she’s unable to easily find an omega-3 fatty acid supplement with the ratio you specified (EPA/DHA 2:1). When you see her 2 weeks later, her depression has worsened.
Because you are concerned her suicidality will return, you revisit the pros and cons of taking an antidepressant. Although small amounts of antidepressants can be passed from mother to infant via breastmilk, the amount varies by specific medication, as do the potential risks. Mrs. S decides to resume taking paroxetine 20 mg/d and eventually, once her motivation improves, she’s able to add psychotherapy and exercise to her maintenance/relapse prevention regimen. After you discuss with her the possibility that B vitamin supplementation may assist in maintenance of remission, she adds L-methylfolate 7.5 mg/day to her regimen.
CORRESPONDENCE
Michele M. Larzelere, PhD; LSUHSC Department of Family Medicine; 200 W. Esplanade Avenue, Suite 409; Kenner, LA 70065; [email protected]
› Recommend cognitive behavioral therapy, interpersonal therapy, or problem-solving therapy for the treatment of depression in patients of all ages. A
› Consider prescribing exercise as a stand-alone or adjunctive treatment for patients with depression. B
› Advise patients who ask about omega-3 fatty acid supplements that formulations with a high eicosapentaenoic acid (EPA) to docosahexaenoic acid (DHA) ratio (2:1) may be a useful “add-on” to their current regimen. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Steve J, age 43, comes to your clinic looking uncharacteristically glum. He was recently downsized from his job and misses his former colleagues. His job loss has caused a financial strain for his family, and he admits to crying in the shower when he thinks about how his life has turned out. Mr. J tells you that he’s gotten a part-time job, but he’s already called in sick several times. On those sick days he “stayed in bed all day and slept.” He says that when he does go to work, he rarely interacts with his coworkers and his concentration is poor. He tells you he wakes up early in the morning on most days and cannot return to sleep, despite being “tired all the time.” He denies suicidal ideation. Mr. J has never felt this way before, which is what prompted his visit today, but he thinks it is “weak to take a pill to feel better.”
What nonpharmacologic options can you offer him?
CASE 2 › Kerri S is a 27-year-old mother of 2 who comes to your clinic to establish care. She tells you about a recent recurrence of depressed mood, which she feels is due to the stress of moving to the area. She is experiencing sleep-onset insomnia and concentration lapses. Her appetite is poor (self-reported 8-lb weight loss in 2 months) and she lacks the motivation to engage in her daily activities, saying, “I wouldn’t even get out of bed if my kids didn’t need me.” She notes that she is constantly irritable and has completely lost her sex drive. Unlike her prior depressive episode, she has not had any suicidal thoughts. Mrs. S was previously successfully treated with paroxetine, 20 mg/d, but she is not interested in restarting her medication because she is still breastfeeding her toddler.
Are there evidence-based options for her care that do not include medication?
Major depressive disorder (MDD) is widespread and often disabling, affecting nearly 8% of people ages 12 and older at any given time.1 Thus, it’s crucial to be familiar with the diverse array of evidence-based treatment options from which patients can choose. Although medications are an essential treatment option for patients with severe depression, their value for patients with mild to moderate depression is often limited.2 In addition, when antidepressants aren’t combined with psychosocial interventions, discontinuing them is associated with relapse.3
Fortunately, research has found that certain nonpharmacologic interventions—including psychotherapies, somatic therapies, and dietary supplements—can have either therapeutic or adjunctive benefits for treating depression, and can be provided in ways that are time- and cost-effective. This article reviews the evidence supporting several options in each of these treatment categories.
Evidence backs several types of psychotherapy
Several recent meta-analyses suggest that a variety of psychotherapeutic treatments may hold promise for your patients with depression.4,5 When analyses were limited to larger studies in order to decrease the risk of bias, cognitive behavioral therapy (CBT), interpersonal therapy (IPT), and problem-solving therapy (PST) all resulted in moderate to large improvement in depressive symptoms when compared to wait-list controls.4 These findings were echoed in a recent systematic review/meta-analysis that focused on depressed primary care patients. Linde et al5 found that the number needed to treat (NNT) to achieve one response (≥50% reduction in score on a depression scale) using any type of psychotherapy was 10, and the NNT to achieve one remission (scoring below a predefined score on a depression scale) was 15.
Psychotherapy can be effective when provided in individual and group settings,6 as well as via telephone, the Internet, or software programs.7 (For a list of self-help, computerized, and Internet-based resources, see TABLE W1 below.)
CBT has been studied for several decades and there’s strong evidence for its efficacy.6 Recent investigations have suggested that CBT delivered in less resource-intensive modes (such as via computer program, Internet, telephone, or videoconferencing) can be as effective as face-to-face CBT.6,8 CBT has been shown to be helpful for a wide range of patients,6 improves outcomes over standard primary care treatment,9 and provides a useful adjunct to medication in treatment-resistant severe depression.10
Behavioral activation (BA), which generally is included as a component of CBT, has received support as an independent treatment, and may produce therapeutic results similar to CBT11 and PST (which we’ll discuss in a bit).12 The core components of BA are scheduling pleasant activities and increasing the patient’s positive interactions with his or her environment by decreasing avoidance, withdrawal, and inactivity.11 Compared to CBT, BA is easier for clinicians to learn and incorporate into primary care visits, and it may be especially useful as an adjunctive or first-step intervention in outpatient clinics.11 Like CBT, BA can be effective in diverse patient groups13,14 and can be provided using novel delivery modes, such as via the Internet.15
IPT is a supportive, structured, brief therapy (12-16 visits) that focuses on helping patients identify and solve current situation- and relationship-based problems that stem from or contribute to their depression.16 Enhancing the patient’s interpersonal communication—including improving social skills, assertiveness, and appropriate expression of anger—is typically a component of IPT. Like CBT, IPT has been found to be effective for treating depression when administered in person, in group therapy, or via the phone or Internet, and across a broad age range.17-19
PST involves teaching patients a structured problem-solving process to decrease interpersonal strain and improve positive life experiences.20 Patients are taught to define their problem, generate and evaluate multiple solutions for it, implement a plan for the solution, and evaluate the results. In addition to being used to successfully treat adults,4,5 PST has been adapted effectively to treat adolescents16 and older adults.18
Somatic therapies are also an option
Exercise has long been considered a possible depression treatment due to its activity on endorphin, monoamine, and cortisol levels and via increased social and general activity. A 2013 Cochrane review of 39 randomized control trials (RCTs; N=2326) assessed whether exercise was effective for treating depression in adults.21 Thirty-five trials found a moderate effect size when specifically comparing exercise to no treatment or control interventions. The effect size was reduced, however, when analyses were restricted to trials with the highest methodological quality. There was no statistically significant difference when exercise was compared to pharmacologic treatment or psychotherapy.
Although the amount of research is meager, small but statistically significant improvements have also been found for older adults22 and children/adolescents.23 There is no consensus on the type, frequency, or intensity of exercise needed to achieve benefit. However, because nearly all studies for all age groups have found that exercise has no adverse psychological effects and substantial positive physical effects, exercise should be recommended to all patients with depression unless contraindicated.
Yoga (both exercise-based and meditation-based) has been evaluated both as a sole treatment and as an adjunctive treatment for depression. Several studies have supported the impact of yoga, particularly in pregnant women,24 although the evidence for its efficacy is inconsistent, with yoga frequently failing to improve upon the outcome of waitlist control.25 The evidence for meditation and mindfulness is more consistently positive, with these interventions equaling or exceeding “treatment as usual,” other psychotherapies, and antidepressants in numerous RCTs.25
Electroconvulsive therapy (ECT) has a substantial evidence base supporting its efficacy.26 ECT has been used for decades, although stigma, cardiac and memory risks, and risks of anesthesia often limit its use. Benefits of ECT include a rapid response relative to pharmacotherapy (>50% of patients respond by the end of the first week of ECT)27 and a strong response in older patients.28
In repetitive transcranial magnetic stimulation (rTMS), electromagnetic coils are placed on a patient’s head to deliver electromagnetic pulses that stimulate areas of the brain that regulate mood. Although rTMS is not widely available, a growing body of evidence supports its use for treating depression, including a meta-analysis of 34 RCTs that included 1383 patients.29 A multisite RCT (N=190) that was not industry-funded reported a 15% response rate and 60% maintenance of remission at 3 months (NNT=12).30 Although ECT is more effective than rTMS, rTMS appears useful for treatment-resistant depression, and can be used as an adjunctive treatment.29,31
Dietary supplements may be best used as adjuncts
St. John’s wort (Hypericum perforatum), which contains 2 bioactive ingredients (hyperforin and hypericin), has been effectively used to treat depression.32 A 2008 Cochrane review that was limited to high-quality trials involving patients meeting Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for depression identified 29 trials (N=5489), of which 18 involved comparisons with placebo and 17 with standard antidepressants.33 Patients’ depression was rated mild to moderate in 19 studies and moderate to severe in 9 studies. Trials examined 4 to 12 weeks of treatment with Hypericum extracts. This study (and several published since) provides strong clinical evidence supporting the efficacy of St. John’s wort for mild to moderate depression. There is insufficient evidence for its use for severe major depression.33TABLE 1 contains dosing information for St. John’s wort and other supplements used to treat depression.34-36
S-adenosyl-L-methionine (SAMe). In a 2003 systematic review,37 1600 mg/d of oral SAMe was found to significantly benefit patients with depression in 4 of 5 studies, as did parenteral SAMe (7 of 7 trials). Another review of 48 studies found SAMe was safe and effective for depression.38 SAMe has been proposed for use alone or in combination with an antidepressant.
Folate and folic acid. Low folate levels have been associated with a less robust response to antidepressants in patients with MDD,39 and higher folate levels appear to be associated with better antidepressant response.40 A 2003 Cochrane review suggested folate might have a role in treating depression.39 A 2009 study found folate supplementation could reduce depressive symptoms for patients with normal baseline folate levels as well as those with low levels.41 Although the evidence is equivocal, folate augmentation may enhance antidepressant efficacy or improve response/remission rates.41,42
It seems reasonable to check folate levels in depressed patients, and address deficiencies by instructing patients to increase their dietary intake of folate or to take supplements. Augmenting antidepressants with folate appears to be low-risk and possibly helpful in maintaining remission.
Omega-3 fatty acids. There is substantial evidence that omega-3 fatty acids can prevent and treat depression.43,44 Recent meta-analyses support the use of omega-3 fatty acids as monotherapy and augmentation, but only formulations that contain a high eicosapentaenoic acid (EPA) to docosahexaenoic acid (DHA) ratio (EPA/DHA 2:1).45,46 Omega-3 supplementation has been used with positive results in older adults, children,47 pregnant women,48 and women with postpartum depression.49 Although initial research into omega-3 treatment of depression appears promising, augmentation of standard antidepressant therapy may be a good conservative option.
Use a validated tool to monitor response to treatment
You can enhance outcomes for your patients with depression if you schedule routine follow-up visits with them to gauge adherence to recommendations, monitor response to treatment, and increase the intensity of care when response is inadequate.50 The most important aspect of monitoring response is to use a standardized instrument that quantifies symptoms at every visit.
The Patient Health Questionnaire 9-item depression assessment (PHQ-9)—which is free—has been validated for depression screening and monitoring of treatment response in primary care patients.51 A decrease of 5 points on the PHQ-9 is the minimum considered to be clinically significant.52 Other well-validated, although lengthier, self-report depression assessment and monitoring instruments include the Beck Depression Inventory-revised and the Zung Depression Scale.
CASE 1 › Mr. J is not enjoying his new job or engaging with new coworkers to replace the positive social experiences he had at his previous job. Together, you set a goal of increasing social involvement by having him make plans to see at least one friend per weekend. Because he indicates that he is unlikely to follow through with a therapy referral, you encourage him to try an online CBT program, start an exercise regimen, or take a SAMe supplement. Mr. Jackson agrees to try the CBT and exercise (moderate intensity, 30 minutes 3-4 times per week), but does not want to take SAMe. He agrees to an assessment of his folate levels, which are normal.
Mr. J starts the online CBT program, which reinforces the exercise and social activity prescription you provided. He establishes a regular exercise routine with a good friend. After one month, his mood has started to improve and he has added regular participation in a hobby (woodworking), as well as volunteer work, which he finds fulfilling. You plan to continue monitoring his depression and his adherence to the treatment plan.
CASE 2 › The recent move has decreased Mrs. S’s interactions with family and long-time friends. Because she had previously expressed interest in exercise, you encourage her to join a local “Mommy and Me” exercise and support group for mothers of toddlers. She is willing to participate in psychotherapy, so you provide a referral to a local therapist with expertise in IPT. You also discuss with Mrs. S the possible benefits of omega-3 fatty acid supplementation, which appears to be safe during breastfeeding.34
Mrs. S begins therapy and exercise classes, but can’t motivate herself to continue either of these activities. She becomes discouraged because she’s unable to easily find an omega-3 fatty acid supplement with the ratio you specified (EPA/DHA 2:1). When you see her 2 weeks later, her depression has worsened.
Because you are concerned her suicidality will return, you revisit the pros and cons of taking an antidepressant. Although small amounts of antidepressants can be passed from mother to infant via breastmilk, the amount varies by specific medication, as do the potential risks. Mrs. S decides to resume taking paroxetine 20 mg/d and eventually, once her motivation improves, she’s able to add psychotherapy and exercise to her maintenance/relapse prevention regimen. After you discuss with her the possibility that B vitamin supplementation may assist in maintenance of remission, she adds L-methylfolate 7.5 mg/day to her regimen.
CORRESPONDENCE
Michele M. Larzelere, PhD; LSUHSC Department of Family Medicine; 200 W. Esplanade Avenue, Suite 409; Kenner, LA 70065; [email protected]
1. Centers for Disease Control and Prevention (CDC). QuickStats: Prevalence of Current Depression Among Persons Aged ≥12 Years, by Age Group and Sex — United States, National Health and Nutrition Examination Survey, 2007–2010. CDC Morbidity and Mortality Weekly Report Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6051a7.htm. Accessed June 11, 2015.
2. Fournier J, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303:47-53.
3. Dobson KS, Hollon SD, Dimidjian S, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol. 2008;76:468-477.
4. Barth J, Munder T, Gerger H, et al. Comparative efficacy of seven psychotherapeutic interventions for patients with depression: A network meta-analysis. PLoS Med. 2013;10:e1001454.
5. Linde K, Sigterman K, Kriston L, et al. Effectiveness of psychological treatments for depressive disorders in primary care: systematic review and meta-analysis. Ann Fam Med. 2015;13:56-68.
6. DeRubeis RJ, Webb CA, Tang TZ, et al. Cognitive therapy. In: Dobson KS, ed. Handbook of Cognitive Behavioral Therapies, 3rd ed. New York, NY: Guilford; 2009:277-316.
7. Andersson G, Cuijpers P. Internet-based and other computerized psychological treatments for adult depression: a meta-analysis. Cogn Behav Ther. 2008;38:196-205.
8. Andersson G, Cuijpers P, Carlbring P, et al. Guided internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: a systematic review and meta-analysis. World Psychiatry. 2014;13:288-295.
9. Twomey C, O’Reilly G, Byrne M. Effectiveness of cognitive behavioral therapy for anxiety and depression in primary care: a meta-analysis. Fam Pract. 2015;32:3-15.
10. Zhou X, Michael K, Liu Y, et al. Systematic review of management for treatment-resistant depression in adolescents. BMC Psychiatry. 2014;14:340.
11. Ekers D, Webster L, Van Straten A, et al. Behavioural activation for depression: An update of meta-analysis of effectiveness and sub group analysis. PLoS One. 2014;9:e100100.
12. Alexopoulos GS, Raue PJ, Kiosses DN, et al. Comparing engage with PST in late-life major depression: A preliminary report. Am J Geriatr Psychiatry. 2015;23:506-513.
13. Soucy Chartier I, Provencher MD. Behavioral activation for depression: Efficacy, effectiveness, and dissemination. J Affect Disord. 2013;145:292-299.
14. McCauley E, Gudmundson G, Schloredt K, et al. The Adolescent Behavior Activation Program: Adapting behavioral activation as a treatment for depression in adolescence. J Clin Child Adolesc Psychol. 2015;1-14. [Epub ahead of print].
15. Carlbring P, Hägglund M, Luthström A, et al. Internet-based behavioral activation and acceptance-based treatment for depression: a randomized controlled trial. J Affect Disord. 2013;148:331-337.
16. Markowitz JC, Weissman MM. Interpersonal psychotherapy: principles and applications. World Psychiatry. 2004; 3:136-139.
17. Kersting A, Kroker K, Schlicht S, et al. Efficacy of a cognitive-behavioral internet-based therapy in parents after the loss of a child during pregnancy: pilot data from a randomized controlled trial. Arch Womens Mental Health. 2011;14:465-477.
18. Francis J, Kumar A. Psychological treatment of late-life depression. Psychiatr Clin North Am. 2013;36:561-575.
19. Picardi A, Gaetano P. Psychotherapy of mood disorders. Clin Pract Epidemiol Ment Health. 2014;10:140-158.
20. Bell AC, D’Zurilla TJ. Problem-solving therapy for depression: a meta-analysis. Clin Psychol Review. 2009;29:348-353.
21. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;9:CD004366
22. Brindle C, Spanjers K, Patel S, et al. Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomized controlled trials. B J Psychiatry. 2012;201:180-185.
23. Brown HE, Pearson N, Braithwaite RE, et al. Physical activity interventions and depression in children and adolescents: a systematic review and meta-analysis. Sports Med. 2013;43:195-206.
24. Gong H, Ni C, Shen X, et al. Yoga for prenatal depression: a systematic review and meta-analysis. BMC Psychiatry. 2015;15:14.
25. D’Silva S, Poscablo C, Habousha R, et al. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407-423.
26. Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357:1939-1945.
27. Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry. 2004;65:485-491.
28. Rhebergen D, Huisman A, Bouckaert F, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. 2015;23:274-282.
29. Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A metaanalysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873-884.
30. George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507-516
31. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham controlled studies. BMC Psychiatry. 2014;14:342.
32. Brown RP, Gerberg PL, Muskin PR. Mood disorders. In: Brown RP, Gerbarg PL, Muskin P. How to Use Herbs, Nutrients and Yoga in Mental Health. New York, NY: WW Norton & Company; 2009.
33. Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;(4):CD000448.
34. Natural Medicines Comprehensive Database. Natural Medicines Comprehensive Database Web site. Available at: http://naturaldatabase.therapeuticresearch.com/home.aspx. Accessed March 1, 2015.
35. Harris WS. Expert opinion: omega-3 fatty acids and bleeding-cause for concern? Am J Cardiol. 2007;99:44C-6C.
36. Freeman MP, Fava M, Lake J, et al. Complementary and alternative medicine in major depressive disorder: the American Psychiatric Association Task Force Report. J Clin Psychiatry. 2010;71:669-681.
37. Papakostas GI, Alpert JE, Fava M. S-adenosyl-methionine in depression: a comprehensive review of the literature. Curr Psychiatry Reports. 2003;5:460-466.
38. Brown RP, Gerbarg PL, Bottiglieri T. S-Adenosylmethionine (SAMe) for depression: biochemical and clinical evidence. Psychiatr Ann. 2002;32:29-44.
39. Taylor MJ, Carney S, Geddes J, et al. Folate for depressive disorders. Cochrane Database Syst Rev. 2003;(2):CD003390.
40. Alpert M, Silva RR, Pouget ER. Prediction of treatment response in geriatric depression from baseline folate level: interaction with an SSRI or a tricyclic antidepressant. J Clin Psychopharmacol. 2003;23:309-313.
41. Fava M, Mischoulon D. Folate in depression: efficacy, safety, differences in formulations, and clinical issues. J Clin Psychiatry. 2009;70(suppl 5):12-17.
42. Almeida OP, Ford AH, Hirani V, et al. B vitamins to enhance treatment response to antidepressants in middle-aged and older adults: results from the B-VITAGE randomised, double-blind, placebo-controlled trial. Br J Psychiatry. 2014;205:450-457.
43. Grosso G, Galvano F, Marventano S, et al. Omega-3 fatty acids and depression: scientific evidence and biological mechanisms. Oxid Med Cell Longev. 2014;2014:313570.
44. Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91:757-770.
45. Grosso G, Pajak A, Marventano S, et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive metaanalysis of randomized clinical trials. PLoS One. 2014;9:e96905.
46. Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012;17:1144-1149.
47. Nemets H, Nemets B, Apter A, et al. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163:1098-1100.
48. Su KP, Huang SY, Chiu TH. Omega-3 fatty acids for major depressive disorder during pregnancy: Results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:644-651.
49. Freeman MP, Davis M, Sinha P, et al. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J Affect Disord. 2008;110:142-148.
50. Mitchell J, Trangle M, Degnan B, et al. Institute for Clinical Systems Improvement (ICSI). Health Care Guideline: Adult depression in primary care. 16th ed. September 2013. Available at: https://www.icsi.org/_asset/fnhdm3/Depr-Interactive0512b.pdf. Accessed June 9, 2015.
51. Kroenke K, Spitzer RL, Williams JBW, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359.
52. Trivedi MH. Tools and strategies for ongoing assessment of depression:
a measurement-based approach to remission. J Clin Psychiatry. 2009;70:26-31.
1. Centers for Disease Control and Prevention (CDC). QuickStats: Prevalence of Current Depression Among Persons Aged ≥12 Years, by Age Group and Sex — United States, National Health and Nutrition Examination Survey, 2007–2010. CDC Morbidity and Mortality Weekly Report Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6051a7.htm. Accessed June 11, 2015.
2. Fournier J, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303:47-53.
3. Dobson KS, Hollon SD, Dimidjian S, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol. 2008;76:468-477.
4. Barth J, Munder T, Gerger H, et al. Comparative efficacy of seven psychotherapeutic interventions for patients with depression: A network meta-analysis. PLoS Med. 2013;10:e1001454.
5. Linde K, Sigterman K, Kriston L, et al. Effectiveness of psychological treatments for depressive disorders in primary care: systematic review and meta-analysis. Ann Fam Med. 2015;13:56-68.
6. DeRubeis RJ, Webb CA, Tang TZ, et al. Cognitive therapy. In: Dobson KS, ed. Handbook of Cognitive Behavioral Therapies, 3rd ed. New York, NY: Guilford; 2009:277-316.
7. Andersson G, Cuijpers P. Internet-based and other computerized psychological treatments for adult depression: a meta-analysis. Cogn Behav Ther. 2008;38:196-205.
8. Andersson G, Cuijpers P, Carlbring P, et al. Guided internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: a systematic review and meta-analysis. World Psychiatry. 2014;13:288-295.
9. Twomey C, O’Reilly G, Byrne M. Effectiveness of cognitive behavioral therapy for anxiety and depression in primary care: a meta-analysis. Fam Pract. 2015;32:3-15.
10. Zhou X, Michael K, Liu Y, et al. Systematic review of management for treatment-resistant depression in adolescents. BMC Psychiatry. 2014;14:340.
11. Ekers D, Webster L, Van Straten A, et al. Behavioural activation for depression: An update of meta-analysis of effectiveness and sub group analysis. PLoS One. 2014;9:e100100.
12. Alexopoulos GS, Raue PJ, Kiosses DN, et al. Comparing engage with PST in late-life major depression: A preliminary report. Am J Geriatr Psychiatry. 2015;23:506-513.
13. Soucy Chartier I, Provencher MD. Behavioral activation for depression: Efficacy, effectiveness, and dissemination. J Affect Disord. 2013;145:292-299.
14. McCauley E, Gudmundson G, Schloredt K, et al. The Adolescent Behavior Activation Program: Adapting behavioral activation as a treatment for depression in adolescence. J Clin Child Adolesc Psychol. 2015;1-14. [Epub ahead of print].
15. Carlbring P, Hägglund M, Luthström A, et al. Internet-based behavioral activation and acceptance-based treatment for depression: a randomized controlled trial. J Affect Disord. 2013;148:331-337.
16. Markowitz JC, Weissman MM. Interpersonal psychotherapy: principles and applications. World Psychiatry. 2004; 3:136-139.
17. Kersting A, Kroker K, Schlicht S, et al. Efficacy of a cognitive-behavioral internet-based therapy in parents after the loss of a child during pregnancy: pilot data from a randomized controlled trial. Arch Womens Mental Health. 2011;14:465-477.
18. Francis J, Kumar A. Psychological treatment of late-life depression. Psychiatr Clin North Am. 2013;36:561-575.
19. Picardi A, Gaetano P. Psychotherapy of mood disorders. Clin Pract Epidemiol Ment Health. 2014;10:140-158.
20. Bell AC, D’Zurilla TJ. Problem-solving therapy for depression: a meta-analysis. Clin Psychol Review. 2009;29:348-353.
21. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;9:CD004366
22. Brindle C, Spanjers K, Patel S, et al. Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomized controlled trials. B J Psychiatry. 2012;201:180-185.
23. Brown HE, Pearson N, Braithwaite RE, et al. Physical activity interventions and depression in children and adolescents: a systematic review and meta-analysis. Sports Med. 2013;43:195-206.
24. Gong H, Ni C, Shen X, et al. Yoga for prenatal depression: a systematic review and meta-analysis. BMC Psychiatry. 2015;15:14.
25. D’Silva S, Poscablo C, Habousha R, et al. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407-423.
26. Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357:1939-1945.
27. Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry. 2004;65:485-491.
28. Rhebergen D, Huisman A, Bouckaert F, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. 2015;23:274-282.
29. Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A metaanalysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873-884.
30. George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507-516
31. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham controlled studies. BMC Psychiatry. 2014;14:342.
32. Brown RP, Gerberg PL, Muskin PR. Mood disorders. In: Brown RP, Gerbarg PL, Muskin P. How to Use Herbs, Nutrients and Yoga in Mental Health. New York, NY: WW Norton & Company; 2009.
33. Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;(4):CD000448.
34. Natural Medicines Comprehensive Database. Natural Medicines Comprehensive Database Web site. Available at: http://naturaldatabase.therapeuticresearch.com/home.aspx. Accessed March 1, 2015.
35. Harris WS. Expert opinion: omega-3 fatty acids and bleeding-cause for concern? Am J Cardiol. 2007;99:44C-6C.
36. Freeman MP, Fava M, Lake J, et al. Complementary and alternative medicine in major depressive disorder: the American Psychiatric Association Task Force Report. J Clin Psychiatry. 2010;71:669-681.
37. Papakostas GI, Alpert JE, Fava M. S-adenosyl-methionine in depression: a comprehensive review of the literature. Curr Psychiatry Reports. 2003;5:460-466.
38. Brown RP, Gerbarg PL, Bottiglieri T. S-Adenosylmethionine (SAMe) for depression: biochemical and clinical evidence. Psychiatr Ann. 2002;32:29-44.
39. Taylor MJ, Carney S, Geddes J, et al. Folate for depressive disorders. Cochrane Database Syst Rev. 2003;(2):CD003390.
40. Alpert M, Silva RR, Pouget ER. Prediction of treatment response in geriatric depression from baseline folate level: interaction with an SSRI or a tricyclic antidepressant. J Clin Psychopharmacol. 2003;23:309-313.
41. Fava M, Mischoulon D. Folate in depression: efficacy, safety, differences in formulations, and clinical issues. J Clin Psychiatry. 2009;70(suppl 5):12-17.
42. Almeida OP, Ford AH, Hirani V, et al. B vitamins to enhance treatment response to antidepressants in middle-aged and older adults: results from the B-VITAGE randomised, double-blind, placebo-controlled trial. Br J Psychiatry. 2014;205:450-457.
43. Grosso G, Galvano F, Marventano S, et al. Omega-3 fatty acids and depression: scientific evidence and biological mechanisms. Oxid Med Cell Longev. 2014;2014:313570.
44. Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91:757-770.
45. Grosso G, Pajak A, Marventano S, et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive metaanalysis of randomized clinical trials. PLoS One. 2014;9:e96905.
46. Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012;17:1144-1149.
47. Nemets H, Nemets B, Apter A, et al. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163:1098-1100.
48. Su KP, Huang SY, Chiu TH. Omega-3 fatty acids for major depressive disorder during pregnancy: Results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:644-651.
49. Freeman MP, Davis M, Sinha P, et al. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J Affect Disord. 2008;110:142-148.
50. Mitchell J, Trangle M, Degnan B, et al. Institute for Clinical Systems Improvement (ICSI). Health Care Guideline: Adult depression in primary care. 16th ed. September 2013. Available at: https://www.icsi.org/_asset/fnhdm3/Depr-Interactive0512b.pdf. Accessed June 9, 2015.
51. Kroenke K, Spitzer RL, Williams JBW, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359.
52. Trivedi MH. Tools and strategies for ongoing assessment of depression:
a measurement-based approach to remission. J Clin Psychiatry. 2009;70:26-31.
Easy Bruising, Low Platelets, Recent Coldlike Illness
A 6-year-old girl was brought to the emergency department (ED) by her mother after the child bumped her head while playing. While the physician examined the child’s head, the mother remarked that her daughter had recently developed bruises that appeared suddenly and only after minor, if any, known trauma. The ED physician determined that the child’s bump to the head was nothing to worry about, attributed the bruising to the child being a “healthy, active 6-year-old,” and sent her home.
Two days later, the child was brought to our office because the mother was still concerned about her daughter’s easy bruising. The mother pointed out ecchymosis scattered across her daughter’s extremities and torso. The child denied any pain or other complaints, including any active or recurrent bleeding. Upon further questioning, the mother mentioned that her daughter had recovered from a coldlike illness several weeks earlier.
THE DIAGNOSIS
We ordered a complete blood count (CBC) and peripheral smear, which were normal except for the platelet count, which was 7,000/μL (normal, 150,000-450,000/μL). Based on the child’s easy bruising and isolated thrombocytopenia, we diagnosed immune thrombocytopenia, which is also known as idiopathic thrombocytopenic purpura (ITP).
DISCUSSION
In ITP, autoantibodies are directed against platelets, leading to their sequestration and destruction in the spleen and a resultant drop in platelet count.1 Children with ITP typically present between the ages of 2 and 10, with a peak incidence between 2 and 5.2 The incidence is estimated to be as high as 8 per 100,000 children.3 However, this estimate primarily reflects symptomatic children, and the true incidence of childhood ITP may be much higher because asymptomatic children may not be brought in to see a doctor.
For the majority of patients, ITP resolves within three months. However, for 20% to 30% of patients, thrombocytopenia will last beyond six months, with or without treatment.4 In 1% of cases, patients will have a recurrence of ITP.3
In addition to easy bruising, nearly all patients who present with possible ITP will complain of cutaneous bleeding, typically a nose bleed or bleeding in the oral cavity.2 Upon questioning, 60% of patients will report a history of recent infection.4 Not surprisingly, bleeding severity correlates inversely with platelet count; severe bleeding is seen in patients with a platelet count < 10,000/μL.
While rare, the more worrisome complications include intracranial hemorrhage (incidence, 0.1% to 0.8%) and other serious hemorrhages that would require transfusion (estimated incidence, 2.9%).2
Vast differential seen in child bruising
When a child presents with bruising, perform a thorough history, including birth and prenatal course, as well as a physical to exclude other potential causes, such as physical abuse, use of herbal remedies or other natural supplements that may not be disclosed as medication, or even environmental exposure. When bruising is present in a child who has isolated thrombocytopenia, the diagnosis of ITP may be straightforward. However, many conditions share thrombocytopenia in their disease process and should be considered in the differential diagnosis of a child who you suspect may have ITP.
• Suspect physical abuse in a bruised child who does not have thrombocytopenia, whose mood is flat or depressed, or who has experienced recurrent injuries or bruising.
• Leukemia, particularly acute lymphoblastic leukemia (ALL), the predominant leukemia found in children, should be ruled out as well. Symptoms that may distinguish a child with ALL from one with ITP include fever, weight loss, and joint pain, as well as signs such as lymphadenopathy, hepatosplenomegaly, anemia, and leukocytosis. A peripheral smear may be ordered to help confirm or exclude a diagnosis of ALL, should any of the above be present in a child with thrombocytopenia.5 It may show lymphoblasts and/or atypical cells in a patient with ALL.5
• Infections should also be included in a differential when a patient is suspected of having ITP, particularly if he or she has systemic symptoms. Viral infections that may cause thrombocytopenia include mononucleosis, dengue virus, human herpesvirus-6, and HIV.6,7
ITP often follows an infection, and the incidence of ITP may be higher during winter months, when infections are more common. However, infection may not always be the cause of ITP. Sepsis may also lead to thrombocytopenia, but a child with sepsis would present very differently from one who has only ITP. A septic child would present with acute illness and signs and symptoms of severe systemic illness, such as high fever, altered mental status, tachycardia, pallor, diaphoresis, and hypotension.
• Drug-induced thrombocytopenia (DIT) should be considered in any child who is taking or recently took a medication that may cause thrombocytopenia. Medications that can cause thrombocytopenia include heparin, quinine, vancomycin, trimethoprim-sulfamethoxazole, rifampin, carbamazepine, phenytoin, piperacillin, linezolid, and valproic acid.8 The measles, mumps, and rubella vaccine also can cause thrombocytopenia.8 A careful medication history may determine if the child is at risk for DIT.
• To narrow the differential, obtain a CBC and peripheral smear when evaluating a patient you suspect may have ITP5 (strength of recommendation [SOR]: A). A CBC will determine the patient’s platelet count, and a peripheral smear should be obtained to exclude other possible diagnoses.5
If there are any questions regarding the results of a peripheral smear, it may be necessary to perform a bone marrow aspiration. This, however, is not usually necessary in an otherwise typical case of ITP.9 Bone marrow aspiration may, however, be necessary to reevaluate the initial diagnosis for a child who does not respond to treatment for ITP.
Continue for corticosteroids, IVIg are usually effective >>
Corticosteroids, IVIg are usually effective
The first step in treating a patient with ITP is to limit the risk for further injury or bleeding by stopping NSAIDs or ending participation in contact sports2,9 (SOR: C). The next step is to determine if pharmacologic therapy is warranted.
Medication, if necessary, is the mainstay of treatment for patients with ITP, particularly those experiencing significant bleeding.2 Corticosteroids, intravenous (IV) immunoglobulin (IVIg), and IV Rho(D) immune globulin (also known as anti-D) are the medications typically used to treat a child with ITP, depending on availability of the drugs, bleeding or bleeding risk, and convenience of dosing. For example, corticosteroids can be used orally or IV, whereas IVIg and IV Rho(D) may not be readily available in some treatment settings.
Corticosteroids have been shown to more rapidly increase platelet count compared to placebo and appear to have a dose-related effect.10,11 Oral prednisone can be dosed at 1 to 2 mg/kg/d for 14 days and then tapered over the course of one week10,11 or one may prescribe 4 mg/kg/d for four days.10,11 IV methylprednisolone typically is given at 30 mg/kg/d for three to four days.9
IVIg may have greater efficacy than corticosteroids in treating ITP, but it may also cause adverse effects, including nausea, headache, and fever. IVIg can be administered as a single 800 to 1,000 mg/kg dose, or as a daily 400 mg/kg dose form five days; higher doses should be reserved for patients with severe bleeding.12
If ITP persists despite the use of corticosteroids or IVIg, IV Rho(D) Ig may be used in patients with Rho(D)-positive blood at a single dose of 25 to 50 μg/kg, with additional doses administered on separate days as required to elevate platelet count. However, only Rho(D)-positive patients are eligible for anti-D treatment.
The response rates/times and adverse effects of common treatments for ITP are summarized in the Table.9 A small randomized study found that oral methylprednisolone 30 mg/kg/d for three days followed by 20 mg/kg/d for an additional four days was comparable to IVIg 0.4 g/kg/d for five days.11 A different study that compared oral methylprednisolone (30 mg/kg/d or 50 mg/kg/d for seven days) and IVIg (0.5 g/kg/d for five days) found no difference in outcomes among the three treatments.13 One advantage, though, of IVIg is that it can be administered as a single IV dose, rather than multiple doses over several weeks, as is the case with oral prednisone.9,11-13
• Follow platelet counts closely. Patients with ITP should have their platelet counts monitored at least once weekly and as often as twice weekly. The frequency of monitoring may be tapered depending on an individual patient’s response to treatment and the severity of the thrombocytopenia.14
• We referred our patient to a nearby children’s hospital, where a repeat CBC showed her platelets had decreased to 3,000/μL. She received a six-hour infusion of IVIg and was discharged with instructions to have her CBC closely monitored. Her platelets remained stable until four weeks later, when they decreased from 102,000/μL to 71,000/μL. She received a second infusion of IVIg as an outpatient.
Soon after, she presented to our ED with a headache, nausea, and fever of 102°F. CT of her head was normal; a repeat CBC showed no elevation in white blood cells, but her hemoglobin had decreased from 11.9 g/dL to 9.7 g/dL. (Her platelets were 254,000/μL.) The patient’s complaints were likely adverse effects of the IVIg. The CBC abnormalities, fever, headache, and malaise resolved shortly thereafter, and the patient remains asymptomatic with no recurrence of ITP.
Continue for the takeaway >>
THE TAKEAWAY
Suspect ITP in a child who bruises easily and who also has thrombocytopenia. Order a CBC and peripheral blood smear to rule out other potential illnesses. Pharmacotherapy, if needed, typically consists of an oral or IV corticosteroid or IVIg; IV Rho(D) Ig may be used in Rho(D)-positive patients who don’t respond to other treatments. Patients with ITP should have their platelet count monitored at least once weekly until platelets have increased to 150,000/μL or higher. Frequency of monitoring may be reduced as the clinical picture improves and the patient remains stable. More frequent monitoring may be necessary based on severity, complications, and response to treatment.
REFERENCES
1. Johnsen J. Pathogenesis in immune thrombocytopenia: new insights. Hematology Am Soc Hematol Educ Program. 2012;2012:306-312.
2. Kühne T, Buchanan GR, Zimmerman S, et al; Intercontinental Childhood ITP Study Group. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr. 2003;143:605-608.
3. Kurtzberg J, Stockman JA III. Idiopathic autoimmune thrombocytopenic purpura. Adv Pediatr. 1994;41:111-134.
4. Zeller B, Rajantie J, Hedlund-Treutiger I, et al. Childhood idiopathic thrombocytopenic purpura in the Nordic countries: epidemiology and predictors of chronic disease. Acta Paediatr. 2005;94:178-184.
5. Margolin JF, Steuber CP, Poplack DG. Acute lymphoblastic leukemia. In: Pizzo PA, Poplack DG, eds. Principles and Practice of Pediatric Oncology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:317-321.
6. Hashimoto H, Maruyama H, Fujimoto K, et al. Hematologic findings associated with thrombocytopenia during the acute phase of exanthem subitum confirmed by primary human herpesvirus-6 infection. J Pediatr Hematol Oncol. 2002;24:211-214.
7. La Russa VF, Innis BL. Mechanisms of dengue virus-induced bone marrow suppression. Baillieres Clin Haematol. 1995;8:249-270.
8. Aster RH, Curtis BR, McFarland JG, et al. Drug-induced immune thrombocytopenia: pathogenesis, diagnosis, and management. Thromb Haemost. 2009;7:911-918.
9. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168-186.
10. Bellucci S, Charpak Y, Chastang C, et al. Low doses v conventional doses of corticoids in immune thrombocytopenic purpura (ITP): results of a randomized clinical trial in 160 children, 223 adults. Blood. 1988;71:1165-1169.
11. Ozsoylu S, Sayli TR, Oztürk G. Oral megadose methylprednisolone versus intravenous immunoglobulin for acute childhood idiopathic thrombocytopenic purpura. Pediatr Hematol Oncol. 1993;10:317-321.
12. Beck CE, Nathan PC, Parkin PC, et al. Corticosteroids versus intravenous immune globulin for the treatment of acute immune thrombocytopenic purpura in children: a systematic review and meta-analysis of randomized controlled trials. J Pediatr. 2005;147:521-527.
13. Albayrak D, Islek I, Kalaycí AG, et al. Acute immune thrombocytopenic purpura: a comparative study of very high oral doses of methylprednisolone and intravenously administered immune globulin. J Pediatr. 1994;125(6 pt 1):1004-1007.
14. Tarantino MD, Madden RM, Fennewald DL, et al. Treatment of childhood acute immune thrombocytopenic purpura with anti-D immune globulin or pooled immune globulin. J Pediatr. 1999;134:21-26.
A 6-year-old girl was brought to the emergency department (ED) by her mother after the child bumped her head while playing. While the physician examined the child’s head, the mother remarked that her daughter had recently developed bruises that appeared suddenly and only after minor, if any, known trauma. The ED physician determined that the child’s bump to the head was nothing to worry about, attributed the bruising to the child being a “healthy, active 6-year-old,” and sent her home.
Two days later, the child was brought to our office because the mother was still concerned about her daughter’s easy bruising. The mother pointed out ecchymosis scattered across her daughter’s extremities and torso. The child denied any pain or other complaints, including any active or recurrent bleeding. Upon further questioning, the mother mentioned that her daughter had recovered from a coldlike illness several weeks earlier.
THE DIAGNOSIS
We ordered a complete blood count (CBC) and peripheral smear, which were normal except for the platelet count, which was 7,000/μL (normal, 150,000-450,000/μL). Based on the child’s easy bruising and isolated thrombocytopenia, we diagnosed immune thrombocytopenia, which is also known as idiopathic thrombocytopenic purpura (ITP).
DISCUSSION
In ITP, autoantibodies are directed against platelets, leading to their sequestration and destruction in the spleen and a resultant drop in platelet count.1 Children with ITP typically present between the ages of 2 and 10, with a peak incidence between 2 and 5.2 The incidence is estimated to be as high as 8 per 100,000 children.3 However, this estimate primarily reflects symptomatic children, and the true incidence of childhood ITP may be much higher because asymptomatic children may not be brought in to see a doctor.
For the majority of patients, ITP resolves within three months. However, for 20% to 30% of patients, thrombocytopenia will last beyond six months, with or without treatment.4 In 1% of cases, patients will have a recurrence of ITP.3
In addition to easy bruising, nearly all patients who present with possible ITP will complain of cutaneous bleeding, typically a nose bleed or bleeding in the oral cavity.2 Upon questioning, 60% of patients will report a history of recent infection.4 Not surprisingly, bleeding severity correlates inversely with platelet count; severe bleeding is seen in patients with a platelet count < 10,000/μL.
While rare, the more worrisome complications include intracranial hemorrhage (incidence, 0.1% to 0.8%) and other serious hemorrhages that would require transfusion (estimated incidence, 2.9%).2
Vast differential seen in child bruising
When a child presents with bruising, perform a thorough history, including birth and prenatal course, as well as a physical to exclude other potential causes, such as physical abuse, use of herbal remedies or other natural supplements that may not be disclosed as medication, or even environmental exposure. When bruising is present in a child who has isolated thrombocytopenia, the diagnosis of ITP may be straightforward. However, many conditions share thrombocytopenia in their disease process and should be considered in the differential diagnosis of a child who you suspect may have ITP.
• Suspect physical abuse in a bruised child who does not have thrombocytopenia, whose mood is flat or depressed, or who has experienced recurrent injuries or bruising.
• Leukemia, particularly acute lymphoblastic leukemia (ALL), the predominant leukemia found in children, should be ruled out as well. Symptoms that may distinguish a child with ALL from one with ITP include fever, weight loss, and joint pain, as well as signs such as lymphadenopathy, hepatosplenomegaly, anemia, and leukocytosis. A peripheral smear may be ordered to help confirm or exclude a diagnosis of ALL, should any of the above be present in a child with thrombocytopenia.5 It may show lymphoblasts and/or atypical cells in a patient with ALL.5
• Infections should also be included in a differential when a patient is suspected of having ITP, particularly if he or she has systemic symptoms. Viral infections that may cause thrombocytopenia include mononucleosis, dengue virus, human herpesvirus-6, and HIV.6,7
ITP often follows an infection, and the incidence of ITP may be higher during winter months, when infections are more common. However, infection may not always be the cause of ITP. Sepsis may also lead to thrombocytopenia, but a child with sepsis would present very differently from one who has only ITP. A septic child would present with acute illness and signs and symptoms of severe systemic illness, such as high fever, altered mental status, tachycardia, pallor, diaphoresis, and hypotension.
• Drug-induced thrombocytopenia (DIT) should be considered in any child who is taking or recently took a medication that may cause thrombocytopenia. Medications that can cause thrombocytopenia include heparin, quinine, vancomycin, trimethoprim-sulfamethoxazole, rifampin, carbamazepine, phenytoin, piperacillin, linezolid, and valproic acid.8 The measles, mumps, and rubella vaccine also can cause thrombocytopenia.8 A careful medication history may determine if the child is at risk for DIT.
• To narrow the differential, obtain a CBC and peripheral smear when evaluating a patient you suspect may have ITP5 (strength of recommendation [SOR]: A). A CBC will determine the patient’s platelet count, and a peripheral smear should be obtained to exclude other possible diagnoses.5
If there are any questions regarding the results of a peripheral smear, it may be necessary to perform a bone marrow aspiration. This, however, is not usually necessary in an otherwise typical case of ITP.9 Bone marrow aspiration may, however, be necessary to reevaluate the initial diagnosis for a child who does not respond to treatment for ITP.
Continue for corticosteroids, IVIg are usually effective >>
Corticosteroids, IVIg are usually effective
The first step in treating a patient with ITP is to limit the risk for further injury or bleeding by stopping NSAIDs or ending participation in contact sports2,9 (SOR: C). The next step is to determine if pharmacologic therapy is warranted.
Medication, if necessary, is the mainstay of treatment for patients with ITP, particularly those experiencing significant bleeding.2 Corticosteroids, intravenous (IV) immunoglobulin (IVIg), and IV Rho(D) immune globulin (also known as anti-D) are the medications typically used to treat a child with ITP, depending on availability of the drugs, bleeding or bleeding risk, and convenience of dosing. For example, corticosteroids can be used orally or IV, whereas IVIg and IV Rho(D) may not be readily available in some treatment settings.
Corticosteroids have been shown to more rapidly increase platelet count compared to placebo and appear to have a dose-related effect.10,11 Oral prednisone can be dosed at 1 to 2 mg/kg/d for 14 days and then tapered over the course of one week10,11 or one may prescribe 4 mg/kg/d for four days.10,11 IV methylprednisolone typically is given at 30 mg/kg/d for three to four days.9
IVIg may have greater efficacy than corticosteroids in treating ITP, but it may also cause adverse effects, including nausea, headache, and fever. IVIg can be administered as a single 800 to 1,000 mg/kg dose, or as a daily 400 mg/kg dose form five days; higher doses should be reserved for patients with severe bleeding.12
If ITP persists despite the use of corticosteroids or IVIg, IV Rho(D) Ig may be used in patients with Rho(D)-positive blood at a single dose of 25 to 50 μg/kg, with additional doses administered on separate days as required to elevate platelet count. However, only Rho(D)-positive patients are eligible for anti-D treatment.
The response rates/times and adverse effects of common treatments for ITP are summarized in the Table.9 A small randomized study found that oral methylprednisolone 30 mg/kg/d for three days followed by 20 mg/kg/d for an additional four days was comparable to IVIg 0.4 g/kg/d for five days.11 A different study that compared oral methylprednisolone (30 mg/kg/d or 50 mg/kg/d for seven days) and IVIg (0.5 g/kg/d for five days) found no difference in outcomes among the three treatments.13 One advantage, though, of IVIg is that it can be administered as a single IV dose, rather than multiple doses over several weeks, as is the case with oral prednisone.9,11-13
• Follow platelet counts closely. Patients with ITP should have their platelet counts monitored at least once weekly and as often as twice weekly. The frequency of monitoring may be tapered depending on an individual patient’s response to treatment and the severity of the thrombocytopenia.14
• We referred our patient to a nearby children’s hospital, where a repeat CBC showed her platelets had decreased to 3,000/μL. She received a six-hour infusion of IVIg and was discharged with instructions to have her CBC closely monitored. Her platelets remained stable until four weeks later, when they decreased from 102,000/μL to 71,000/μL. She received a second infusion of IVIg as an outpatient.
Soon after, she presented to our ED with a headache, nausea, and fever of 102°F. CT of her head was normal; a repeat CBC showed no elevation in white blood cells, but her hemoglobin had decreased from 11.9 g/dL to 9.7 g/dL. (Her platelets were 254,000/μL.) The patient’s complaints were likely adverse effects of the IVIg. The CBC abnormalities, fever, headache, and malaise resolved shortly thereafter, and the patient remains asymptomatic with no recurrence of ITP.
Continue for the takeaway >>
THE TAKEAWAY
Suspect ITP in a child who bruises easily and who also has thrombocytopenia. Order a CBC and peripheral blood smear to rule out other potential illnesses. Pharmacotherapy, if needed, typically consists of an oral or IV corticosteroid or IVIg; IV Rho(D) Ig may be used in Rho(D)-positive patients who don’t respond to other treatments. Patients with ITP should have their platelet count monitored at least once weekly until platelets have increased to 150,000/μL or higher. Frequency of monitoring may be reduced as the clinical picture improves and the patient remains stable. More frequent monitoring may be necessary based on severity, complications, and response to treatment.
REFERENCES
1. Johnsen J. Pathogenesis in immune thrombocytopenia: new insights. Hematology Am Soc Hematol Educ Program. 2012;2012:306-312.
2. Kühne T, Buchanan GR, Zimmerman S, et al; Intercontinental Childhood ITP Study Group. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr. 2003;143:605-608.
3. Kurtzberg J, Stockman JA III. Idiopathic autoimmune thrombocytopenic purpura. Adv Pediatr. 1994;41:111-134.
4. Zeller B, Rajantie J, Hedlund-Treutiger I, et al. Childhood idiopathic thrombocytopenic purpura in the Nordic countries: epidemiology and predictors of chronic disease. Acta Paediatr. 2005;94:178-184.
5. Margolin JF, Steuber CP, Poplack DG. Acute lymphoblastic leukemia. In: Pizzo PA, Poplack DG, eds. Principles and Practice of Pediatric Oncology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:317-321.
6. Hashimoto H, Maruyama H, Fujimoto K, et al. Hematologic findings associated with thrombocytopenia during the acute phase of exanthem subitum confirmed by primary human herpesvirus-6 infection. J Pediatr Hematol Oncol. 2002;24:211-214.
7. La Russa VF, Innis BL. Mechanisms of dengue virus-induced bone marrow suppression. Baillieres Clin Haematol. 1995;8:249-270.
8. Aster RH, Curtis BR, McFarland JG, et al. Drug-induced immune thrombocytopenia: pathogenesis, diagnosis, and management. Thromb Haemost. 2009;7:911-918.
9. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168-186.
10. Bellucci S, Charpak Y, Chastang C, et al. Low doses v conventional doses of corticoids in immune thrombocytopenic purpura (ITP): results of a randomized clinical trial in 160 children, 223 adults. Blood. 1988;71:1165-1169.
11. Ozsoylu S, Sayli TR, Oztürk G. Oral megadose methylprednisolone versus intravenous immunoglobulin for acute childhood idiopathic thrombocytopenic purpura. Pediatr Hematol Oncol. 1993;10:317-321.
12. Beck CE, Nathan PC, Parkin PC, et al. Corticosteroids versus intravenous immune globulin for the treatment of acute immune thrombocytopenic purpura in children: a systematic review and meta-analysis of randomized controlled trials. J Pediatr. 2005;147:521-527.
13. Albayrak D, Islek I, Kalaycí AG, et al. Acute immune thrombocytopenic purpura: a comparative study of very high oral doses of methylprednisolone and intravenously administered immune globulin. J Pediatr. 1994;125(6 pt 1):1004-1007.
14. Tarantino MD, Madden RM, Fennewald DL, et al. Treatment of childhood acute immune thrombocytopenic purpura with anti-D immune globulin or pooled immune globulin. J Pediatr. 1999;134:21-26.
A 6-year-old girl was brought to the emergency department (ED) by her mother after the child bumped her head while playing. While the physician examined the child’s head, the mother remarked that her daughter had recently developed bruises that appeared suddenly and only after minor, if any, known trauma. The ED physician determined that the child’s bump to the head was nothing to worry about, attributed the bruising to the child being a “healthy, active 6-year-old,” and sent her home.
Two days later, the child was brought to our office because the mother was still concerned about her daughter’s easy bruising. The mother pointed out ecchymosis scattered across her daughter’s extremities and torso. The child denied any pain or other complaints, including any active or recurrent bleeding. Upon further questioning, the mother mentioned that her daughter had recovered from a coldlike illness several weeks earlier.
THE DIAGNOSIS
We ordered a complete blood count (CBC) and peripheral smear, which were normal except for the platelet count, which was 7,000/μL (normal, 150,000-450,000/μL). Based on the child’s easy bruising and isolated thrombocytopenia, we diagnosed immune thrombocytopenia, which is also known as idiopathic thrombocytopenic purpura (ITP).
DISCUSSION
In ITP, autoantibodies are directed against platelets, leading to their sequestration and destruction in the spleen and a resultant drop in platelet count.1 Children with ITP typically present between the ages of 2 and 10, with a peak incidence between 2 and 5.2 The incidence is estimated to be as high as 8 per 100,000 children.3 However, this estimate primarily reflects symptomatic children, and the true incidence of childhood ITP may be much higher because asymptomatic children may not be brought in to see a doctor.
For the majority of patients, ITP resolves within three months. However, for 20% to 30% of patients, thrombocytopenia will last beyond six months, with or without treatment.4 In 1% of cases, patients will have a recurrence of ITP.3
In addition to easy bruising, nearly all patients who present with possible ITP will complain of cutaneous bleeding, typically a nose bleed or bleeding in the oral cavity.2 Upon questioning, 60% of patients will report a history of recent infection.4 Not surprisingly, bleeding severity correlates inversely with platelet count; severe bleeding is seen in patients with a platelet count < 10,000/μL.
While rare, the more worrisome complications include intracranial hemorrhage (incidence, 0.1% to 0.8%) and other serious hemorrhages that would require transfusion (estimated incidence, 2.9%).2
Vast differential seen in child bruising
When a child presents with bruising, perform a thorough history, including birth and prenatal course, as well as a physical to exclude other potential causes, such as physical abuse, use of herbal remedies or other natural supplements that may not be disclosed as medication, or even environmental exposure. When bruising is present in a child who has isolated thrombocytopenia, the diagnosis of ITP may be straightforward. However, many conditions share thrombocytopenia in their disease process and should be considered in the differential diagnosis of a child who you suspect may have ITP.
• Suspect physical abuse in a bruised child who does not have thrombocytopenia, whose mood is flat or depressed, or who has experienced recurrent injuries or bruising.
• Leukemia, particularly acute lymphoblastic leukemia (ALL), the predominant leukemia found in children, should be ruled out as well. Symptoms that may distinguish a child with ALL from one with ITP include fever, weight loss, and joint pain, as well as signs such as lymphadenopathy, hepatosplenomegaly, anemia, and leukocytosis. A peripheral smear may be ordered to help confirm or exclude a diagnosis of ALL, should any of the above be present in a child with thrombocytopenia.5 It may show lymphoblasts and/or atypical cells in a patient with ALL.5
• Infections should also be included in a differential when a patient is suspected of having ITP, particularly if he or she has systemic symptoms. Viral infections that may cause thrombocytopenia include mononucleosis, dengue virus, human herpesvirus-6, and HIV.6,7
ITP often follows an infection, and the incidence of ITP may be higher during winter months, when infections are more common. However, infection may not always be the cause of ITP. Sepsis may also lead to thrombocytopenia, but a child with sepsis would present very differently from one who has only ITP. A septic child would present with acute illness and signs and symptoms of severe systemic illness, such as high fever, altered mental status, tachycardia, pallor, diaphoresis, and hypotension.
• Drug-induced thrombocytopenia (DIT) should be considered in any child who is taking or recently took a medication that may cause thrombocytopenia. Medications that can cause thrombocytopenia include heparin, quinine, vancomycin, trimethoprim-sulfamethoxazole, rifampin, carbamazepine, phenytoin, piperacillin, linezolid, and valproic acid.8 The measles, mumps, and rubella vaccine also can cause thrombocytopenia.8 A careful medication history may determine if the child is at risk for DIT.
• To narrow the differential, obtain a CBC and peripheral smear when evaluating a patient you suspect may have ITP5 (strength of recommendation [SOR]: A). A CBC will determine the patient’s platelet count, and a peripheral smear should be obtained to exclude other possible diagnoses.5
If there are any questions regarding the results of a peripheral smear, it may be necessary to perform a bone marrow aspiration. This, however, is not usually necessary in an otherwise typical case of ITP.9 Bone marrow aspiration may, however, be necessary to reevaluate the initial diagnosis for a child who does not respond to treatment for ITP.
Continue for corticosteroids, IVIg are usually effective >>
Corticosteroids, IVIg are usually effective
The first step in treating a patient with ITP is to limit the risk for further injury or bleeding by stopping NSAIDs or ending participation in contact sports2,9 (SOR: C). The next step is to determine if pharmacologic therapy is warranted.
Medication, if necessary, is the mainstay of treatment for patients with ITP, particularly those experiencing significant bleeding.2 Corticosteroids, intravenous (IV) immunoglobulin (IVIg), and IV Rho(D) immune globulin (also known as anti-D) are the medications typically used to treat a child with ITP, depending on availability of the drugs, bleeding or bleeding risk, and convenience of dosing. For example, corticosteroids can be used orally or IV, whereas IVIg and IV Rho(D) may not be readily available in some treatment settings.
Corticosteroids have been shown to more rapidly increase platelet count compared to placebo and appear to have a dose-related effect.10,11 Oral prednisone can be dosed at 1 to 2 mg/kg/d for 14 days and then tapered over the course of one week10,11 or one may prescribe 4 mg/kg/d for four days.10,11 IV methylprednisolone typically is given at 30 mg/kg/d for three to four days.9
IVIg may have greater efficacy than corticosteroids in treating ITP, but it may also cause adverse effects, including nausea, headache, and fever. IVIg can be administered as a single 800 to 1,000 mg/kg dose, or as a daily 400 mg/kg dose form five days; higher doses should be reserved for patients with severe bleeding.12
If ITP persists despite the use of corticosteroids or IVIg, IV Rho(D) Ig may be used in patients with Rho(D)-positive blood at a single dose of 25 to 50 μg/kg, with additional doses administered on separate days as required to elevate platelet count. However, only Rho(D)-positive patients are eligible for anti-D treatment.
The response rates/times and adverse effects of common treatments for ITP are summarized in the Table.9 A small randomized study found that oral methylprednisolone 30 mg/kg/d for three days followed by 20 mg/kg/d for an additional four days was comparable to IVIg 0.4 g/kg/d for five days.11 A different study that compared oral methylprednisolone (30 mg/kg/d or 50 mg/kg/d for seven days) and IVIg (0.5 g/kg/d for five days) found no difference in outcomes among the three treatments.13 One advantage, though, of IVIg is that it can be administered as a single IV dose, rather than multiple doses over several weeks, as is the case with oral prednisone.9,11-13
• Follow platelet counts closely. Patients with ITP should have their platelet counts monitored at least once weekly and as often as twice weekly. The frequency of monitoring may be tapered depending on an individual patient’s response to treatment and the severity of the thrombocytopenia.14
• We referred our patient to a nearby children’s hospital, where a repeat CBC showed her platelets had decreased to 3,000/μL. She received a six-hour infusion of IVIg and was discharged with instructions to have her CBC closely monitored. Her platelets remained stable until four weeks later, when they decreased from 102,000/μL to 71,000/μL. She received a second infusion of IVIg as an outpatient.
Soon after, she presented to our ED with a headache, nausea, and fever of 102°F. CT of her head was normal; a repeat CBC showed no elevation in white blood cells, but her hemoglobin had decreased from 11.9 g/dL to 9.7 g/dL. (Her platelets were 254,000/μL.) The patient’s complaints were likely adverse effects of the IVIg. The CBC abnormalities, fever, headache, and malaise resolved shortly thereafter, and the patient remains asymptomatic with no recurrence of ITP.
Continue for the takeaway >>
THE TAKEAWAY
Suspect ITP in a child who bruises easily and who also has thrombocytopenia. Order a CBC and peripheral blood smear to rule out other potential illnesses. Pharmacotherapy, if needed, typically consists of an oral or IV corticosteroid or IVIg; IV Rho(D) Ig may be used in Rho(D)-positive patients who don’t respond to other treatments. Patients with ITP should have their platelet count monitored at least once weekly until platelets have increased to 150,000/μL or higher. Frequency of monitoring may be reduced as the clinical picture improves and the patient remains stable. More frequent monitoring may be necessary based on severity, complications, and response to treatment.
REFERENCES
1. Johnsen J. Pathogenesis in immune thrombocytopenia: new insights. Hematology Am Soc Hematol Educ Program. 2012;2012:306-312.
2. Kühne T, Buchanan GR, Zimmerman S, et al; Intercontinental Childhood ITP Study Group. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr. 2003;143:605-608.
3. Kurtzberg J, Stockman JA III. Idiopathic autoimmune thrombocytopenic purpura. Adv Pediatr. 1994;41:111-134.
4. Zeller B, Rajantie J, Hedlund-Treutiger I, et al. Childhood idiopathic thrombocytopenic purpura in the Nordic countries: epidemiology and predictors of chronic disease. Acta Paediatr. 2005;94:178-184.
5. Margolin JF, Steuber CP, Poplack DG. Acute lymphoblastic leukemia. In: Pizzo PA, Poplack DG, eds. Principles and Practice of Pediatric Oncology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:317-321.
6. Hashimoto H, Maruyama H, Fujimoto K, et al. Hematologic findings associated with thrombocytopenia during the acute phase of exanthem subitum confirmed by primary human herpesvirus-6 infection. J Pediatr Hematol Oncol. 2002;24:211-214.
7. La Russa VF, Innis BL. Mechanisms of dengue virus-induced bone marrow suppression. Baillieres Clin Haematol. 1995;8:249-270.
8. Aster RH, Curtis BR, McFarland JG, et al. Drug-induced immune thrombocytopenia: pathogenesis, diagnosis, and management. Thromb Haemost. 2009;7:911-918.
9. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168-186.
10. Bellucci S, Charpak Y, Chastang C, et al. Low doses v conventional doses of corticoids in immune thrombocytopenic purpura (ITP): results of a randomized clinical trial in 160 children, 223 adults. Blood. 1988;71:1165-1169.
11. Ozsoylu S, Sayli TR, Oztürk G. Oral megadose methylprednisolone versus intravenous immunoglobulin for acute childhood idiopathic thrombocytopenic purpura. Pediatr Hematol Oncol. 1993;10:317-321.
12. Beck CE, Nathan PC, Parkin PC, et al. Corticosteroids versus intravenous immune globulin for the treatment of acute immune thrombocytopenic purpura in children: a systematic review and meta-analysis of randomized controlled trials. J Pediatr. 2005;147:521-527.
13. Albayrak D, Islek I, Kalaycí AG, et al. Acute immune thrombocytopenic purpura: a comparative study of very high oral doses of methylprednisolone and intravenously administered immune globulin. J Pediatr. 1994;125(6 pt 1):1004-1007.
14. Tarantino MD, Madden RM, Fennewald DL, et al. Treatment of childhood acute immune thrombocytopenic purpura with anti-D immune globulin or pooled immune globulin. J Pediatr. 1999;134:21-26.
Palliative Medicine in the ED
Overview
Palliative medicine in the ED represents a paradigm shift for the emergency physician (EP)—from identifying and stabilizing acute medical and surgical conditions to providing symptomatic comfort care to a dying patient. When the ED became the “safety net” for patients who have serious, life-limiting illnesses,1-3 it also became the most frequent place where such care is initially sought4—although not considered an ideal place to begin such care.
In one study, approximately 40% of dying patients presented to the ED during their final 2 weeks of life.5 With the ED becoming more recognized as a location for palliative care, the EP plays a key role in the care of these patients. The 2013 Model of the Clinical Practice of Emergency Medicine explicitly lists palliative medicine within the EP’s scope of practice.6 Further support for providing palliative care in emergency medicine includes the cosponsorship of Hospice and Palliative Medicine subspecialty board certification by the American Board of Emergency Medicine in 2008. Finally, palliative care medicine principles have been endorsed in the “Choosing Wisely” initiative of the American College of Emergency Physicians.
Essential Palliative Care Skills
Quest et al7 have identified the following 12 primary palliative care skills in which every EP should be competent:
- Assessment of illness trajectory;
- Determination of prognosis;
- Communication of bad news;
- Interpretation and formation of an advance care plan;
- Allowance of family presence during resuscitation;
- Symptom management (both pain and nonpain);
- Withholding and withdrawal of life-sustaining treatments;
- Management of imminently dying patients;
- Identification and implementation of hospice and palliative care plans;
- Understanding of ethical and legal issues pertinent to end-of-life care;
- Display of spiritual and cultural competency; and
- Management of the dying child.
Although all of the above are important skills, this paper focuses on the symptom management of pain and nonpain (skill 6) in patients presenting to the ED with a life-limiting illness. The evidence base for these treatments is limited due to the many methodological challenges faced when studying symptoms in patients who are at end of life.
Pharmacologic Management of Symptoms
Recent research has found that symptom burden is high at end of life. Despite the increase in attention to these patients and their needs, symptoms including pain, depression, and delirium have repeatedly increased between 1998 and 2010.8 A 2013 study recommended that a minimum of four classes of medications be considered for patients who are at end of life: opioid (for pain); benzodiazepine (for anxiety); antipsychotic (for delirium and nausea); and antimuscarinic (for excessive secretions).9 The role and indications for each of these drug classes will be discussed.
Palliative Care Intervention
Though EPs frequently request specialty and subspecialty consultation for ED patients, they usually do not consider a palliative care medicine consult for the dying patient. Palliative care medicine utilizes an interdisciplinary, collaborative, team-based approach to decrease the pain and suffering of patients with advanced illness.10
Benefits from early palliative care intervention in the ED include improved symptom management, improved patient and family satisfaction, improved outcomes, decreased length of stay, less use of intensive care units, and less costs.4
Pain Management
Pain is one of the most devastating symptoms that a patient can experience, and its management is an integral component of palliative care medicine. Initial evaluation must include appropriate assessment of the pain and its impact on a patient’s function and quality of life.
The general approach to pain management follows the World Health Organization pain ladder. For mild to moderate pain, step 1 begins with acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID), with or without an adjuvant such as an antidepressant or anticonvulsant. If pain persists, step 2 involves the addition of an opioid. For moderate to severe pain, step 3 involves the addition of stronger opioids, such as hydromorphone, morphine, and oxycodone. Typically, a patient with a serious, life-limiting illness who presents to the ED for help will likely require treatment with strong opioids (step 3).
Opioids
In patients requiring step 3 management, opioids are the primary medication used to manage pain. An initial equivalent dose of morphine 5 mg intravenously (IV) is appropriate in an opioid-naïve patient. The adage of “starting low and going slow” is important to follow; however, an important corollary is “…and use enough.” If a patient’s pain is not controlled with initial dosages, additional bolus doses of 50% to 100% increments will be necessary. Because opioids do not have a ceiling effect, it is important to understand that dosages may seem very high for some patients compared to others. In this population, ensuring baseline pain control, with either an oral long-acting formulation or a continuous IV infusion, is important.11
Difficulties clinicians have in determining opioids for the management of pain are multifactorial. One consideration may be the growing public concern for prescription opioid abuse, potentially creating resistance to appropriate use of opioids by clinicians who fear legal or regulatory push back.
General principles in managing severe pain in the opioid-tolerant patient include the following: (1) calculating the morphine equivalent as a daily 24-hour dose; (2) determining the breakthrough dose, which is usually 10% to 15% of the calculated daily dose; (3) titrating doses upward if pain is not controlled, or if more than three breakthrough doses are being required daily; and (4) reducing the calculated conversion dose of a new opioid 25% to 50% when converting to a different opioid.12
The EP is frequently required to convert a patient’s oral opioid analgesic to an equivalent IV dose, and hydromorphone and morphine are the two most commonly used. The Table provides an approximation for this conversion.
Equianalgesic Dosing
Equianalgesic dosing is an important concept in pain management, especially for those patients already receiving opioids. There is great variation in the analgesic potency of the different opioids. The dose at which two opioids provide equivalent pain relief is the equianalgesic dose. Usually, this is standardized to 10 mg of parenteral morphine.13 Unfortunately, it is not uncommon for patients to be undertreated when switched to another opioid.
Nonpain Symptom Management
Nonpain symptoms that all EPs must know how to manage include constipation, dyspnea, nausea/vomiting, the so-called death rattle, and terminal delirium. In one study of reasons for ED visits by palliative care patients, the most common were dyspnea (26%), nausea/vomiting/constipation (17%), and uncontrolled pain (15%).14
Constipation
The most important adverse effect of opioids—one that does not improve or change during treatment—is constipation. Constipation in general—not just associated with opioids—has been ranked as one of the 10 most bothersome symptoms in the palliative care population, leading to discomfort, decreased quality of life, and potential small bowel obstruction or perforation.15 Unless contraindicated, a gastrointestinal stimulant such as senna, or an osmotic laxative such as lactulose, must be prescribed whenever an opioid is initiated. As the author (Galicia-Castillo) often notes, “The hand that writes the prescription for an opioid should be the hand that writes an Rx for a bowel regimen, or it becomes the hand that disimpacts the patient.”
The most recent Cochrane Review for the management of constipation in the palliative care population did not show any differences in the effectiveness among three commonly used laxatives: senna, docusate, and lactulose. This review did not evaluate polyethylene glycol, which is also commonly used.16 The addition of stool softeners, bisacodyl and nightly prune juice can also be helpful.10
Dyspnea
Dyspnea, the subjective feeling of breathing discomfort, is a common end-of-life complaint. Similar to pain, self-report is required for adequate assessment of dyspnea. Treatment recommendations include opioids, anxiolytics, and oxygen therapy.18 Opioids are the most widely studied treatment for dyspnea, demonstrating reduction in breathlessness in patients who have a variety of conditions, such as advanced chronic obstructive pulmonary disease, interstitial lung disease, cancer, and chronic heart failure.19
While many of the benefits of opioids are widely recognized and understood, the manner in which they improve symptoms of dyspnea is less well known. In addition, the evidence of effectiveness is limited to oral or parenteral morphine and fentanyl, and nebulized opioids have not been well studied. Oxygen treatments have been shown to reduce dyspnea in patients who suffer from hypoxemia; however, no benefit was found for patients who had only mild or no hypoxemia. A majority of dying patients did not experience a change in respiratory comfort after their supplemental oxygen was withdrawn. In these cases, when administration of oxygen is unnecessary, it may potentially introduce further discomfort to end-of-life patients by causing nasal dryness and impaired mobility.20
The use of benzodiazepines as the primary medication to manage dyspnea is unfounded, but may provide some benefit when used in conjunction with opioids.11 When indicated, a longer-acting agent (eg, clonazepam, with an initial starting does of 0.25 mg orally every 12 hours) may be used.4
Nausea and Vomiting
Nausea and vomiting have been reported by 16% to 68% of patients who had life-limiting illness, such as cancer, heart failure, renal failure, or acquired immunodeficiency syndrome.21 The etiology of nausea and vomiting is multifactorial in a dying patient. Assessment and treatment has been based on understanding how neurotransmitters are involved in the “emetic pathway,”22 but other pathways, such as a cytokine-mediated model of cancer symptoms, may also be important.23
Nonpharmacologic methods to utilize include avoidance of environmental stimuli, such as fatty, spicy, and salted foods; use of relaxation and distraction; and massage.22 Several medication classes have been utilized to treat nausea and vomiting: prokinetic agents (metoclopramide 10 mg three to four times a day, 30 minutes prior to meals and bedtime); dopamine receptor antagonists (haloperidol 1.5-5 mg two to three times a day); antihistaminic agents (promethazine 25 mg orally or IV every 4-6 hours, with a maximum dose of 100 mg/d); and selective 5 hydroxytryptamine-3 receptor antagonists (ondansetron 4-8 mg once or twice a day). Other agents that have been utilized include corticosteroids, benzodiazepines, octreotide, and cannabinoids.22
Procedures such as percutaneous endoscopic gastrostomy placement, nasogastric tube placement, and stenting may be necessary for patients who have advanced disease caused by a mechanical obstruction.22
Death Rattle
The death rattle occurs when secretions accumulate in the pharynx and/or airways when swallowing and cough mechanisms are no longer intact.24 This phenomenon occurs in 23% to 92% of dying patients.25 Generally, death occurs within 48 hours for about 75% of such patients.26 The noise that results from this process is usually more disturbing for those visiting the patients than to the patient themselves. Conservative measures to employ include placing patients on their sides to facilitate secretion drainage and to minimize upper airway sounds, gentle oral and pharyngeal suctioning, and limiting fluid input.11
One recent study reviewing the use of the anticholinergics atropine, scopolamine, and hyoscine demonstrated similar efficacy among the three drugs. Dosages used in this study included atropine 0.5 mg as a subcutaneous bolus, followed by 3 mg every 24 hours subcutaneously; scopolamine as a 0.25 mg subcutaneous bolus, followed by 1.5 mg every 24 hours IV or by subcutaneous infusion; and hyoscine 20 mg as a subcutaneous bolus followed by 60 mg every 24 hours IV or subcutaneous infusion. Glycopyrrolate is often used in the cognitively intact patient, as it does not cross the blood-brain barrier; however, supply concerns at the time of the study prevented a review of its efficiency.27 All of these medications are also available in oral and transdermal formulations.
Terminal Delirium
Delirium is a common complication for patients nearing the end of life, affecting as many as 88% of dying patients.28 It is characterized by an acute onset of cognitive impairment that may manifest as either a hyperactive or hypoactive state. Causes for terminal delirium are multifactorial. Initially, management should include prevention strategies, such as frequently orientating the patient, maintenance of day-night cycles, provision of adequate sleep, and minimization of sensory overload.11 When pharmacological therapy is required to improve quality of life, a neuroleptic medication, namely haloperidol, should be used initially. The addition of a benzodiazepine may help if the initial treatments are ineffective, or if sedation is desired.28
Summary
Emergency physicians have a unique opportunity to improve the quality of life for patients suffering serious illness, especially those who are actively dying. The management of pain and nonpain symptoms in patients who are at end of life, is a particularly important skill for every EP. If available, a consultation with a palliative care medicine consultant may improve both short- and long-term patient care.
Dr Galicia-Castillo is the Sue Faulkner Scribner professor of geriatrics at the Eastern Virginia Medical School Glennan Center for Geriatrics and Gerontology, and Medical Director for Palliative Care Medicine at Sentara Norfolk General Hospital. Dr Counselman is the distinguished professor and chairman of the department of emergency medicine at Eastern Virginia Medical School, Norfolk; and a physician at Emergency Physicians of Tidewater, Norfolk, Virginia. He is also the associate editor in chief of EMERGENCY MEDICINE editorial board.
- Alsirafy SA, Raheem AA, Al-Zahrani AS, et al. Emergency department visits at the end of life of patients with terminal cancer: pattern, causes, and avoidability. Am J Hosp Palliat Care. 2015:1049909115581819. [Epub ahead of print].
- Grudzen CR, Richardson LD, Morrison M, Cho E, Morrison RS. Palliative care needs of seriously ill, older adults presenting to the emergency department. Acad Emerg Med. 2010;17(11):1253-1257.
- Smith AK, Schonberg MA, Fisher J, et al. Emergency department experiences of acutely symptomatic patients with terminal illness and their family caregivers. J Pain Symptom Manage. 2010;39(6):972-981.
- Mierendorf S, Gidvani V. Palliative care in the emergency department. Perm J. 2014;18(2):77-85.
- Barbera L, Taylor C, Dudgeon D. Why do patients with cancer visit the emergency department near the end of life? CMAJ. 2010;182(6): 563-568.
- Counselman FL, Borenstein MA, Chisholm CD, et al; EM Model Review Task Force; American Board of Emergency Medicine. The 2013 Model of the Clinical Practice of Emergency Medicine. Acad Emerg Med. 2014;21(5):574-598.
- Quest TE, Marco CA, Derse AR. Hospice and palliative medicine: new subspecialty, new opportunities. Ann Emerg Med. 2009;54(1):94-102.
- Singer AE, Meeker D, Teno JM, Lynn J, Lunney JR, Lorenz KA. Symptom trends in the last year of life from 1998 to 2010: a cohort study. Ann Intern Med. 2015;162(3):175-183.
- Lindqvist O, Lundquist G, Dickman A, et al; OPCARE9. Four essential drugs needed for quality care of the dying: a Delphi-study based international expert consensus opinion. J Palliat Med. 2013;16(1):38-43.
- Kandarian B, Morrison RS, Richardson LD, Ortiz J, Grudzen CR. Emergency department-initiated palliative care for advanced cancer patients: protocol for a pilot randomized controlled trial. Trials. 2014;15:251.
- Campbell ML. Caring for dying patients in the intensive care unit: managing pain, dyspnea, anxiety, delirium, and death rattle. AACN Adv Crit Care. 2015;26(2):110-120.
- Lamba S, Quest TE. Hospice care and the emergency department: rules, regulations, and referrals. Ann Emerg Med. 2011;57(3):282-290.
- Shaheen PE, Walsh D, Lasheen W, Davis MP, Lagman RL. Opioid equianalgesic tables: are they all equally dangerous? J Pain Symptom Manage.2009;38(3):409-417.
- Wallace EM, Cooney MC, Walsh J, Conroy M, Twomey F. Why do palliative care patients present to the emergency department? Avoidable or unavoidable? Am J Hosp Palliat Care. 2013;30(3):253-253.
- Erichsén E, Milberg A, Jaarsma T, Friedrichsen MJ. Constipation in specialized palliative care: prevalence, definition, and patient-perceived symptom distress. J Palliat Med. 2015;18(7):585-592.
- Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015;5:CD003448.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- LeGrand SB, Khawam EA, Walsh D, Rivera NI. Opioids, respiratory function, and dyspnea. Am J Hosp Palliat Care. 2003;20(1):57-61.
- Meek PM, Schwartzstein R, Adams L, el al. Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999;159(1):321-340.
- Campbell ML, Yarandi H, Dove-Medows E. Oxygen is nonbeneficial for most patients who are near death. J Pain Symptom Manage. 2013;45(3):517-523.
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31(1):58-69.
- Glare P, Miller J, Nikolova T, Tickoo R. Treating nausea and vomiting in palliative care: a review. Clin Interv Aging. 2011;6:243-259.
- Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919-2925.
- Bennett M, Lucas V, Brennan M, Hughes A, O’Donnell V, Wee B; Association for Palliative Medicine’s Science Committee. Using anti-muscarinic drugs in the management of death rattle: evidence-based guidelines for palliative care. Palliat Med. 2002;16(5):369-374.
- Mercadamte S. Death rattle: critical review and research agenda. Support Care Cancer. 2014;22(2):
- 571-575.
- Wildiers H, Menten J. Death rattle: prevalence, prevention and treatment. J Pain Symptom Manage. 2002;23(4):310-317.
- Wildiers H, Dhaenekint C, Demeulenaere P, et al; Flemish Federation of Palliative Care. Atropine, hyoscine butylbromide, or scopolamine are equally effective for the treatment of death rattle in terminal care. J Pain Symptom Manage. 2009;38(1):124-133.
- Kehl KA. Treatment of terminal restlessness: a review of the evidence. J Pain Palliat Care Pharmacother. 2004;18(1):5-30.
Overview
Palliative medicine in the ED represents a paradigm shift for the emergency physician (EP)—from identifying and stabilizing acute medical and surgical conditions to providing symptomatic comfort care to a dying patient. When the ED became the “safety net” for patients who have serious, life-limiting illnesses,1-3 it also became the most frequent place where such care is initially sought4—although not considered an ideal place to begin such care.
In one study, approximately 40% of dying patients presented to the ED during their final 2 weeks of life.5 With the ED becoming more recognized as a location for palliative care, the EP plays a key role in the care of these patients. The 2013 Model of the Clinical Practice of Emergency Medicine explicitly lists palliative medicine within the EP’s scope of practice.6 Further support for providing palliative care in emergency medicine includes the cosponsorship of Hospice and Palliative Medicine subspecialty board certification by the American Board of Emergency Medicine in 2008. Finally, palliative care medicine principles have been endorsed in the “Choosing Wisely” initiative of the American College of Emergency Physicians.
Essential Palliative Care Skills
Quest et al7 have identified the following 12 primary palliative care skills in which every EP should be competent:
- Assessment of illness trajectory;
- Determination of prognosis;
- Communication of bad news;
- Interpretation and formation of an advance care plan;
- Allowance of family presence during resuscitation;
- Symptom management (both pain and nonpain);
- Withholding and withdrawal of life-sustaining treatments;
- Management of imminently dying patients;
- Identification and implementation of hospice and palliative care plans;
- Understanding of ethical and legal issues pertinent to end-of-life care;
- Display of spiritual and cultural competency; and
- Management of the dying child.
Although all of the above are important skills, this paper focuses on the symptom management of pain and nonpain (skill 6) in patients presenting to the ED with a life-limiting illness. The evidence base for these treatments is limited due to the many methodological challenges faced when studying symptoms in patients who are at end of life.
Pharmacologic Management of Symptoms
Recent research has found that symptom burden is high at end of life. Despite the increase in attention to these patients and their needs, symptoms including pain, depression, and delirium have repeatedly increased between 1998 and 2010.8 A 2013 study recommended that a minimum of four classes of medications be considered for patients who are at end of life: opioid (for pain); benzodiazepine (for anxiety); antipsychotic (for delirium and nausea); and antimuscarinic (for excessive secretions).9 The role and indications for each of these drug classes will be discussed.
Palliative Care Intervention
Though EPs frequently request specialty and subspecialty consultation for ED patients, they usually do not consider a palliative care medicine consult for the dying patient. Palliative care medicine utilizes an interdisciplinary, collaborative, team-based approach to decrease the pain and suffering of patients with advanced illness.10
Benefits from early palliative care intervention in the ED include improved symptom management, improved patient and family satisfaction, improved outcomes, decreased length of stay, less use of intensive care units, and less costs.4
Pain Management
Pain is one of the most devastating symptoms that a patient can experience, and its management is an integral component of palliative care medicine. Initial evaluation must include appropriate assessment of the pain and its impact on a patient’s function and quality of life.
The general approach to pain management follows the World Health Organization pain ladder. For mild to moderate pain, step 1 begins with acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID), with or without an adjuvant such as an antidepressant or anticonvulsant. If pain persists, step 2 involves the addition of an opioid. For moderate to severe pain, step 3 involves the addition of stronger opioids, such as hydromorphone, morphine, and oxycodone. Typically, a patient with a serious, life-limiting illness who presents to the ED for help will likely require treatment with strong opioids (step 3).
Opioids
In patients requiring step 3 management, opioids are the primary medication used to manage pain. An initial equivalent dose of morphine 5 mg intravenously (IV) is appropriate in an opioid-naïve patient. The adage of “starting low and going slow” is important to follow; however, an important corollary is “…and use enough.” If a patient’s pain is not controlled with initial dosages, additional bolus doses of 50% to 100% increments will be necessary. Because opioids do not have a ceiling effect, it is important to understand that dosages may seem very high for some patients compared to others. In this population, ensuring baseline pain control, with either an oral long-acting formulation or a continuous IV infusion, is important.11
Difficulties clinicians have in determining opioids for the management of pain are multifactorial. One consideration may be the growing public concern for prescription opioid abuse, potentially creating resistance to appropriate use of opioids by clinicians who fear legal or regulatory push back.
General principles in managing severe pain in the opioid-tolerant patient include the following: (1) calculating the morphine equivalent as a daily 24-hour dose; (2) determining the breakthrough dose, which is usually 10% to 15% of the calculated daily dose; (3) titrating doses upward if pain is not controlled, or if more than three breakthrough doses are being required daily; and (4) reducing the calculated conversion dose of a new opioid 25% to 50% when converting to a different opioid.12
The EP is frequently required to convert a patient’s oral opioid analgesic to an equivalent IV dose, and hydromorphone and morphine are the two most commonly used. The Table provides an approximation for this conversion.
Equianalgesic Dosing
Equianalgesic dosing is an important concept in pain management, especially for those patients already receiving opioids. There is great variation in the analgesic potency of the different opioids. The dose at which two opioids provide equivalent pain relief is the equianalgesic dose. Usually, this is standardized to 10 mg of parenteral morphine.13 Unfortunately, it is not uncommon for patients to be undertreated when switched to another opioid.
Nonpain Symptom Management
Nonpain symptoms that all EPs must know how to manage include constipation, dyspnea, nausea/vomiting, the so-called death rattle, and terminal delirium. In one study of reasons for ED visits by palliative care patients, the most common were dyspnea (26%), nausea/vomiting/constipation (17%), and uncontrolled pain (15%).14
Constipation
The most important adverse effect of opioids—one that does not improve or change during treatment—is constipation. Constipation in general—not just associated with opioids—has been ranked as one of the 10 most bothersome symptoms in the palliative care population, leading to discomfort, decreased quality of life, and potential small bowel obstruction or perforation.15 Unless contraindicated, a gastrointestinal stimulant such as senna, or an osmotic laxative such as lactulose, must be prescribed whenever an opioid is initiated. As the author (Galicia-Castillo) often notes, “The hand that writes the prescription for an opioid should be the hand that writes an Rx for a bowel regimen, or it becomes the hand that disimpacts the patient.”
The most recent Cochrane Review for the management of constipation in the palliative care population did not show any differences in the effectiveness among three commonly used laxatives: senna, docusate, and lactulose. This review did not evaluate polyethylene glycol, which is also commonly used.16 The addition of stool softeners, bisacodyl and nightly prune juice can also be helpful.10
Dyspnea
Dyspnea, the subjective feeling of breathing discomfort, is a common end-of-life complaint. Similar to pain, self-report is required for adequate assessment of dyspnea. Treatment recommendations include opioids, anxiolytics, and oxygen therapy.18 Opioids are the most widely studied treatment for dyspnea, demonstrating reduction in breathlessness in patients who have a variety of conditions, such as advanced chronic obstructive pulmonary disease, interstitial lung disease, cancer, and chronic heart failure.19
While many of the benefits of opioids are widely recognized and understood, the manner in which they improve symptoms of dyspnea is less well known. In addition, the evidence of effectiveness is limited to oral or parenteral morphine and fentanyl, and nebulized opioids have not been well studied. Oxygen treatments have been shown to reduce dyspnea in patients who suffer from hypoxemia; however, no benefit was found for patients who had only mild or no hypoxemia. A majority of dying patients did not experience a change in respiratory comfort after their supplemental oxygen was withdrawn. In these cases, when administration of oxygen is unnecessary, it may potentially introduce further discomfort to end-of-life patients by causing nasal dryness and impaired mobility.20
The use of benzodiazepines as the primary medication to manage dyspnea is unfounded, but may provide some benefit when used in conjunction with opioids.11 When indicated, a longer-acting agent (eg, clonazepam, with an initial starting does of 0.25 mg orally every 12 hours) may be used.4
Nausea and Vomiting
Nausea and vomiting have been reported by 16% to 68% of patients who had life-limiting illness, such as cancer, heart failure, renal failure, or acquired immunodeficiency syndrome.21 The etiology of nausea and vomiting is multifactorial in a dying patient. Assessment and treatment has been based on understanding how neurotransmitters are involved in the “emetic pathway,”22 but other pathways, such as a cytokine-mediated model of cancer symptoms, may also be important.23
Nonpharmacologic methods to utilize include avoidance of environmental stimuli, such as fatty, spicy, and salted foods; use of relaxation and distraction; and massage.22 Several medication classes have been utilized to treat nausea and vomiting: prokinetic agents (metoclopramide 10 mg three to four times a day, 30 minutes prior to meals and bedtime); dopamine receptor antagonists (haloperidol 1.5-5 mg two to three times a day); antihistaminic agents (promethazine 25 mg orally or IV every 4-6 hours, with a maximum dose of 100 mg/d); and selective 5 hydroxytryptamine-3 receptor antagonists (ondansetron 4-8 mg once or twice a day). Other agents that have been utilized include corticosteroids, benzodiazepines, octreotide, and cannabinoids.22
Procedures such as percutaneous endoscopic gastrostomy placement, nasogastric tube placement, and stenting may be necessary for patients who have advanced disease caused by a mechanical obstruction.22
Death Rattle
The death rattle occurs when secretions accumulate in the pharynx and/or airways when swallowing and cough mechanisms are no longer intact.24 This phenomenon occurs in 23% to 92% of dying patients.25 Generally, death occurs within 48 hours for about 75% of such patients.26 The noise that results from this process is usually more disturbing for those visiting the patients than to the patient themselves. Conservative measures to employ include placing patients on their sides to facilitate secretion drainage and to minimize upper airway sounds, gentle oral and pharyngeal suctioning, and limiting fluid input.11
One recent study reviewing the use of the anticholinergics atropine, scopolamine, and hyoscine demonstrated similar efficacy among the three drugs. Dosages used in this study included atropine 0.5 mg as a subcutaneous bolus, followed by 3 mg every 24 hours subcutaneously; scopolamine as a 0.25 mg subcutaneous bolus, followed by 1.5 mg every 24 hours IV or by subcutaneous infusion; and hyoscine 20 mg as a subcutaneous bolus followed by 60 mg every 24 hours IV or subcutaneous infusion. Glycopyrrolate is often used in the cognitively intact patient, as it does not cross the blood-brain barrier; however, supply concerns at the time of the study prevented a review of its efficiency.27 All of these medications are also available in oral and transdermal formulations.
Terminal Delirium
Delirium is a common complication for patients nearing the end of life, affecting as many as 88% of dying patients.28 It is characterized by an acute onset of cognitive impairment that may manifest as either a hyperactive or hypoactive state. Causes for terminal delirium are multifactorial. Initially, management should include prevention strategies, such as frequently orientating the patient, maintenance of day-night cycles, provision of adequate sleep, and minimization of sensory overload.11 When pharmacological therapy is required to improve quality of life, a neuroleptic medication, namely haloperidol, should be used initially. The addition of a benzodiazepine may help if the initial treatments are ineffective, or if sedation is desired.28
Summary
Emergency physicians have a unique opportunity to improve the quality of life for patients suffering serious illness, especially those who are actively dying. The management of pain and nonpain symptoms in patients who are at end of life, is a particularly important skill for every EP. If available, a consultation with a palliative care medicine consultant may improve both short- and long-term patient care.
Dr Galicia-Castillo is the Sue Faulkner Scribner professor of geriatrics at the Eastern Virginia Medical School Glennan Center for Geriatrics and Gerontology, and Medical Director for Palliative Care Medicine at Sentara Norfolk General Hospital. Dr Counselman is the distinguished professor and chairman of the department of emergency medicine at Eastern Virginia Medical School, Norfolk; and a physician at Emergency Physicians of Tidewater, Norfolk, Virginia. He is also the associate editor in chief of EMERGENCY MEDICINE editorial board.
Overview
Palliative medicine in the ED represents a paradigm shift for the emergency physician (EP)—from identifying and stabilizing acute medical and surgical conditions to providing symptomatic comfort care to a dying patient. When the ED became the “safety net” for patients who have serious, life-limiting illnesses,1-3 it also became the most frequent place where such care is initially sought4—although not considered an ideal place to begin such care.
In one study, approximately 40% of dying patients presented to the ED during their final 2 weeks of life.5 With the ED becoming more recognized as a location for palliative care, the EP plays a key role in the care of these patients. The 2013 Model of the Clinical Practice of Emergency Medicine explicitly lists palliative medicine within the EP’s scope of practice.6 Further support for providing palliative care in emergency medicine includes the cosponsorship of Hospice and Palliative Medicine subspecialty board certification by the American Board of Emergency Medicine in 2008. Finally, palliative care medicine principles have been endorsed in the “Choosing Wisely” initiative of the American College of Emergency Physicians.
Essential Palliative Care Skills
Quest et al7 have identified the following 12 primary palliative care skills in which every EP should be competent:
- Assessment of illness trajectory;
- Determination of prognosis;
- Communication of bad news;
- Interpretation and formation of an advance care plan;
- Allowance of family presence during resuscitation;
- Symptom management (both pain and nonpain);
- Withholding and withdrawal of life-sustaining treatments;
- Management of imminently dying patients;
- Identification and implementation of hospice and palliative care plans;
- Understanding of ethical and legal issues pertinent to end-of-life care;
- Display of spiritual and cultural competency; and
- Management of the dying child.
Although all of the above are important skills, this paper focuses on the symptom management of pain and nonpain (skill 6) in patients presenting to the ED with a life-limiting illness. The evidence base for these treatments is limited due to the many methodological challenges faced when studying symptoms in patients who are at end of life.
Pharmacologic Management of Symptoms
Recent research has found that symptom burden is high at end of life. Despite the increase in attention to these patients and their needs, symptoms including pain, depression, and delirium have repeatedly increased between 1998 and 2010.8 A 2013 study recommended that a minimum of four classes of medications be considered for patients who are at end of life: opioid (for pain); benzodiazepine (for anxiety); antipsychotic (for delirium and nausea); and antimuscarinic (for excessive secretions).9 The role and indications for each of these drug classes will be discussed.
Palliative Care Intervention
Though EPs frequently request specialty and subspecialty consultation for ED patients, they usually do not consider a palliative care medicine consult for the dying patient. Palliative care medicine utilizes an interdisciplinary, collaborative, team-based approach to decrease the pain and suffering of patients with advanced illness.10
Benefits from early palliative care intervention in the ED include improved symptom management, improved patient and family satisfaction, improved outcomes, decreased length of stay, less use of intensive care units, and less costs.4
Pain Management
Pain is one of the most devastating symptoms that a patient can experience, and its management is an integral component of palliative care medicine. Initial evaluation must include appropriate assessment of the pain and its impact on a patient’s function and quality of life.
The general approach to pain management follows the World Health Organization pain ladder. For mild to moderate pain, step 1 begins with acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID), with or without an adjuvant such as an antidepressant or anticonvulsant. If pain persists, step 2 involves the addition of an opioid. For moderate to severe pain, step 3 involves the addition of stronger opioids, such as hydromorphone, morphine, and oxycodone. Typically, a patient with a serious, life-limiting illness who presents to the ED for help will likely require treatment with strong opioids (step 3).
Opioids
In patients requiring step 3 management, opioids are the primary medication used to manage pain. An initial equivalent dose of morphine 5 mg intravenously (IV) is appropriate in an opioid-naïve patient. The adage of “starting low and going slow” is important to follow; however, an important corollary is “…and use enough.” If a patient’s pain is not controlled with initial dosages, additional bolus doses of 50% to 100% increments will be necessary. Because opioids do not have a ceiling effect, it is important to understand that dosages may seem very high for some patients compared to others. In this population, ensuring baseline pain control, with either an oral long-acting formulation or a continuous IV infusion, is important.11
Difficulties clinicians have in determining opioids for the management of pain are multifactorial. One consideration may be the growing public concern for prescription opioid abuse, potentially creating resistance to appropriate use of opioids by clinicians who fear legal or regulatory push back.
General principles in managing severe pain in the opioid-tolerant patient include the following: (1) calculating the morphine equivalent as a daily 24-hour dose; (2) determining the breakthrough dose, which is usually 10% to 15% of the calculated daily dose; (3) titrating doses upward if pain is not controlled, or if more than three breakthrough doses are being required daily; and (4) reducing the calculated conversion dose of a new opioid 25% to 50% when converting to a different opioid.12
The EP is frequently required to convert a patient’s oral opioid analgesic to an equivalent IV dose, and hydromorphone and morphine are the two most commonly used. The Table provides an approximation for this conversion.
Equianalgesic Dosing
Equianalgesic dosing is an important concept in pain management, especially for those patients already receiving opioids. There is great variation in the analgesic potency of the different opioids. The dose at which two opioids provide equivalent pain relief is the equianalgesic dose. Usually, this is standardized to 10 mg of parenteral morphine.13 Unfortunately, it is not uncommon for patients to be undertreated when switched to another opioid.
Nonpain Symptom Management
Nonpain symptoms that all EPs must know how to manage include constipation, dyspnea, nausea/vomiting, the so-called death rattle, and terminal delirium. In one study of reasons for ED visits by palliative care patients, the most common were dyspnea (26%), nausea/vomiting/constipation (17%), and uncontrolled pain (15%).14
Constipation
The most important adverse effect of opioids—one that does not improve or change during treatment—is constipation. Constipation in general—not just associated with opioids—has been ranked as one of the 10 most bothersome symptoms in the palliative care population, leading to discomfort, decreased quality of life, and potential small bowel obstruction or perforation.15 Unless contraindicated, a gastrointestinal stimulant such as senna, or an osmotic laxative such as lactulose, must be prescribed whenever an opioid is initiated. As the author (Galicia-Castillo) often notes, “The hand that writes the prescription for an opioid should be the hand that writes an Rx for a bowel regimen, or it becomes the hand that disimpacts the patient.”
The most recent Cochrane Review for the management of constipation in the palliative care population did not show any differences in the effectiveness among three commonly used laxatives: senna, docusate, and lactulose. This review did not evaluate polyethylene glycol, which is also commonly used.16 The addition of stool softeners, bisacodyl and nightly prune juice can also be helpful.10
Dyspnea
Dyspnea, the subjective feeling of breathing discomfort, is a common end-of-life complaint. Similar to pain, self-report is required for adequate assessment of dyspnea. Treatment recommendations include opioids, anxiolytics, and oxygen therapy.18 Opioids are the most widely studied treatment for dyspnea, demonstrating reduction in breathlessness in patients who have a variety of conditions, such as advanced chronic obstructive pulmonary disease, interstitial lung disease, cancer, and chronic heart failure.19
While many of the benefits of opioids are widely recognized and understood, the manner in which they improve symptoms of dyspnea is less well known. In addition, the evidence of effectiveness is limited to oral or parenteral morphine and fentanyl, and nebulized opioids have not been well studied. Oxygen treatments have been shown to reduce dyspnea in patients who suffer from hypoxemia; however, no benefit was found for patients who had only mild or no hypoxemia. A majority of dying patients did not experience a change in respiratory comfort after their supplemental oxygen was withdrawn. In these cases, when administration of oxygen is unnecessary, it may potentially introduce further discomfort to end-of-life patients by causing nasal dryness and impaired mobility.20
The use of benzodiazepines as the primary medication to manage dyspnea is unfounded, but may provide some benefit when used in conjunction with opioids.11 When indicated, a longer-acting agent (eg, clonazepam, with an initial starting does of 0.25 mg orally every 12 hours) may be used.4
Nausea and Vomiting
Nausea and vomiting have been reported by 16% to 68% of patients who had life-limiting illness, such as cancer, heart failure, renal failure, or acquired immunodeficiency syndrome.21 The etiology of nausea and vomiting is multifactorial in a dying patient. Assessment and treatment has been based on understanding how neurotransmitters are involved in the “emetic pathway,”22 but other pathways, such as a cytokine-mediated model of cancer symptoms, may also be important.23
Nonpharmacologic methods to utilize include avoidance of environmental stimuli, such as fatty, spicy, and salted foods; use of relaxation and distraction; and massage.22 Several medication classes have been utilized to treat nausea and vomiting: prokinetic agents (metoclopramide 10 mg three to four times a day, 30 minutes prior to meals and bedtime); dopamine receptor antagonists (haloperidol 1.5-5 mg two to three times a day); antihistaminic agents (promethazine 25 mg orally or IV every 4-6 hours, with a maximum dose of 100 mg/d); and selective 5 hydroxytryptamine-3 receptor antagonists (ondansetron 4-8 mg once or twice a day). Other agents that have been utilized include corticosteroids, benzodiazepines, octreotide, and cannabinoids.22
Procedures such as percutaneous endoscopic gastrostomy placement, nasogastric tube placement, and stenting may be necessary for patients who have advanced disease caused by a mechanical obstruction.22
Death Rattle
The death rattle occurs when secretions accumulate in the pharynx and/or airways when swallowing and cough mechanisms are no longer intact.24 This phenomenon occurs in 23% to 92% of dying patients.25 Generally, death occurs within 48 hours for about 75% of such patients.26 The noise that results from this process is usually more disturbing for those visiting the patients than to the patient themselves. Conservative measures to employ include placing patients on their sides to facilitate secretion drainage and to minimize upper airway sounds, gentle oral and pharyngeal suctioning, and limiting fluid input.11
One recent study reviewing the use of the anticholinergics atropine, scopolamine, and hyoscine demonstrated similar efficacy among the three drugs. Dosages used in this study included atropine 0.5 mg as a subcutaneous bolus, followed by 3 mg every 24 hours subcutaneously; scopolamine as a 0.25 mg subcutaneous bolus, followed by 1.5 mg every 24 hours IV or by subcutaneous infusion; and hyoscine 20 mg as a subcutaneous bolus followed by 60 mg every 24 hours IV or subcutaneous infusion. Glycopyrrolate is often used in the cognitively intact patient, as it does not cross the blood-brain barrier; however, supply concerns at the time of the study prevented a review of its efficiency.27 All of these medications are also available in oral and transdermal formulations.
Terminal Delirium
Delirium is a common complication for patients nearing the end of life, affecting as many as 88% of dying patients.28 It is characterized by an acute onset of cognitive impairment that may manifest as either a hyperactive or hypoactive state. Causes for terminal delirium are multifactorial. Initially, management should include prevention strategies, such as frequently orientating the patient, maintenance of day-night cycles, provision of adequate sleep, and minimization of sensory overload.11 When pharmacological therapy is required to improve quality of life, a neuroleptic medication, namely haloperidol, should be used initially. The addition of a benzodiazepine may help if the initial treatments are ineffective, or if sedation is desired.28
Summary
Emergency physicians have a unique opportunity to improve the quality of life for patients suffering serious illness, especially those who are actively dying. The management of pain and nonpain symptoms in patients who are at end of life, is a particularly important skill for every EP. If available, a consultation with a palliative care medicine consultant may improve both short- and long-term patient care.
Dr Galicia-Castillo is the Sue Faulkner Scribner professor of geriatrics at the Eastern Virginia Medical School Glennan Center for Geriatrics and Gerontology, and Medical Director for Palliative Care Medicine at Sentara Norfolk General Hospital. Dr Counselman is the distinguished professor and chairman of the department of emergency medicine at Eastern Virginia Medical School, Norfolk; and a physician at Emergency Physicians of Tidewater, Norfolk, Virginia. He is also the associate editor in chief of EMERGENCY MEDICINE editorial board.
- Alsirafy SA, Raheem AA, Al-Zahrani AS, et al. Emergency department visits at the end of life of patients with terminal cancer: pattern, causes, and avoidability. Am J Hosp Palliat Care. 2015:1049909115581819. [Epub ahead of print].
- Grudzen CR, Richardson LD, Morrison M, Cho E, Morrison RS. Palliative care needs of seriously ill, older adults presenting to the emergency department. Acad Emerg Med. 2010;17(11):1253-1257.
- Smith AK, Schonberg MA, Fisher J, et al. Emergency department experiences of acutely symptomatic patients with terminal illness and their family caregivers. J Pain Symptom Manage. 2010;39(6):972-981.
- Mierendorf S, Gidvani V. Palliative care in the emergency department. Perm J. 2014;18(2):77-85.
- Barbera L, Taylor C, Dudgeon D. Why do patients with cancer visit the emergency department near the end of life? CMAJ. 2010;182(6): 563-568.
- Counselman FL, Borenstein MA, Chisholm CD, et al; EM Model Review Task Force; American Board of Emergency Medicine. The 2013 Model of the Clinical Practice of Emergency Medicine. Acad Emerg Med. 2014;21(5):574-598.
- Quest TE, Marco CA, Derse AR. Hospice and palliative medicine: new subspecialty, new opportunities. Ann Emerg Med. 2009;54(1):94-102.
- Singer AE, Meeker D, Teno JM, Lynn J, Lunney JR, Lorenz KA. Symptom trends in the last year of life from 1998 to 2010: a cohort study. Ann Intern Med. 2015;162(3):175-183.
- Lindqvist O, Lundquist G, Dickman A, et al; OPCARE9. Four essential drugs needed for quality care of the dying: a Delphi-study based international expert consensus opinion. J Palliat Med. 2013;16(1):38-43.
- Kandarian B, Morrison RS, Richardson LD, Ortiz J, Grudzen CR. Emergency department-initiated palliative care for advanced cancer patients: protocol for a pilot randomized controlled trial. Trials. 2014;15:251.
- Campbell ML. Caring for dying patients in the intensive care unit: managing pain, dyspnea, anxiety, delirium, and death rattle. AACN Adv Crit Care. 2015;26(2):110-120.
- Lamba S, Quest TE. Hospice care and the emergency department: rules, regulations, and referrals. Ann Emerg Med. 2011;57(3):282-290.
- Shaheen PE, Walsh D, Lasheen W, Davis MP, Lagman RL. Opioid equianalgesic tables: are they all equally dangerous? J Pain Symptom Manage.2009;38(3):409-417.
- Wallace EM, Cooney MC, Walsh J, Conroy M, Twomey F. Why do palliative care patients present to the emergency department? Avoidable or unavoidable? Am J Hosp Palliat Care. 2013;30(3):253-253.
- Erichsén E, Milberg A, Jaarsma T, Friedrichsen MJ. Constipation in specialized palliative care: prevalence, definition, and patient-perceived symptom distress. J Palliat Med. 2015;18(7):585-592.
- Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015;5:CD003448.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- LeGrand SB, Khawam EA, Walsh D, Rivera NI. Opioids, respiratory function, and dyspnea. Am J Hosp Palliat Care. 2003;20(1):57-61.
- Meek PM, Schwartzstein R, Adams L, el al. Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999;159(1):321-340.
- Campbell ML, Yarandi H, Dove-Medows E. Oxygen is nonbeneficial for most patients who are near death. J Pain Symptom Manage. 2013;45(3):517-523.
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31(1):58-69.
- Glare P, Miller J, Nikolova T, Tickoo R. Treating nausea and vomiting in palliative care: a review. Clin Interv Aging. 2011;6:243-259.
- Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919-2925.
- Bennett M, Lucas V, Brennan M, Hughes A, O’Donnell V, Wee B; Association for Palliative Medicine’s Science Committee. Using anti-muscarinic drugs in the management of death rattle: evidence-based guidelines for palliative care. Palliat Med. 2002;16(5):369-374.
- Mercadamte S. Death rattle: critical review and research agenda. Support Care Cancer. 2014;22(2):
- 571-575.
- Wildiers H, Menten J. Death rattle: prevalence, prevention and treatment. J Pain Symptom Manage. 2002;23(4):310-317.
- Wildiers H, Dhaenekint C, Demeulenaere P, et al; Flemish Federation of Palliative Care. Atropine, hyoscine butylbromide, or scopolamine are equally effective for the treatment of death rattle in terminal care. J Pain Symptom Manage. 2009;38(1):124-133.
- Kehl KA. Treatment of terminal restlessness: a review of the evidence. J Pain Palliat Care Pharmacother. 2004;18(1):5-30.
- Alsirafy SA, Raheem AA, Al-Zahrani AS, et al. Emergency department visits at the end of life of patients with terminal cancer: pattern, causes, and avoidability. Am J Hosp Palliat Care. 2015:1049909115581819. [Epub ahead of print].
- Grudzen CR, Richardson LD, Morrison M, Cho E, Morrison RS. Palliative care needs of seriously ill, older adults presenting to the emergency department. Acad Emerg Med. 2010;17(11):1253-1257.
- Smith AK, Schonberg MA, Fisher J, et al. Emergency department experiences of acutely symptomatic patients with terminal illness and their family caregivers. J Pain Symptom Manage. 2010;39(6):972-981.
- Mierendorf S, Gidvani V. Palliative care in the emergency department. Perm J. 2014;18(2):77-85.
- Barbera L, Taylor C, Dudgeon D. Why do patients with cancer visit the emergency department near the end of life? CMAJ. 2010;182(6): 563-568.
- Counselman FL, Borenstein MA, Chisholm CD, et al; EM Model Review Task Force; American Board of Emergency Medicine. The 2013 Model of the Clinical Practice of Emergency Medicine. Acad Emerg Med. 2014;21(5):574-598.
- Quest TE, Marco CA, Derse AR. Hospice and palliative medicine: new subspecialty, new opportunities. Ann Emerg Med. 2009;54(1):94-102.
- Singer AE, Meeker D, Teno JM, Lynn J, Lunney JR, Lorenz KA. Symptom trends in the last year of life from 1998 to 2010: a cohort study. Ann Intern Med. 2015;162(3):175-183.
- Lindqvist O, Lundquist G, Dickman A, et al; OPCARE9. Four essential drugs needed for quality care of the dying: a Delphi-study based international expert consensus opinion. J Palliat Med. 2013;16(1):38-43.
- Kandarian B, Morrison RS, Richardson LD, Ortiz J, Grudzen CR. Emergency department-initiated palliative care for advanced cancer patients: protocol for a pilot randomized controlled trial. Trials. 2014;15:251.
- Campbell ML. Caring for dying patients in the intensive care unit: managing pain, dyspnea, anxiety, delirium, and death rattle. AACN Adv Crit Care. 2015;26(2):110-120.
- Lamba S, Quest TE. Hospice care and the emergency department: rules, regulations, and referrals. Ann Emerg Med. 2011;57(3):282-290.
- Shaheen PE, Walsh D, Lasheen W, Davis MP, Lagman RL. Opioid equianalgesic tables: are they all equally dangerous? J Pain Symptom Manage.2009;38(3):409-417.
- Wallace EM, Cooney MC, Walsh J, Conroy M, Twomey F. Why do palliative care patients present to the emergency department? Avoidable or unavoidable? Am J Hosp Palliat Care. 2013;30(3):253-253.
- Erichsén E, Milberg A, Jaarsma T, Friedrichsen MJ. Constipation in specialized palliative care: prevalence, definition, and patient-perceived symptom distress. J Palliat Med. 2015;18(7):585-592.
- Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015;5:CD003448.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- LeGrand SB, Khawam EA, Walsh D, Rivera NI. Opioids, respiratory function, and dyspnea. Am J Hosp Palliat Care. 2003;20(1):57-61.
- Meek PM, Schwartzstein R, Adams L, el al. Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999;159(1):321-340.
- Campbell ML, Yarandi H, Dove-Medows E. Oxygen is nonbeneficial for most patients who are near death. J Pain Symptom Manage. 2013;45(3):517-523.
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31(1):58-69.
- Glare P, Miller J, Nikolova T, Tickoo R. Treating nausea and vomiting in palliative care: a review. Clin Interv Aging. 2011;6:243-259.
- Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919-2925.
- Bennett M, Lucas V, Brennan M, Hughes A, O’Donnell V, Wee B; Association for Palliative Medicine’s Science Committee. Using anti-muscarinic drugs in the management of death rattle: evidence-based guidelines for palliative care. Palliat Med. 2002;16(5):369-374.
- Mercadamte S. Death rattle: critical review and research agenda. Support Care Cancer. 2014;22(2):
- 571-575.
- Wildiers H, Menten J. Death rattle: prevalence, prevention and treatment. J Pain Symptom Manage. 2002;23(4):310-317.
- Wildiers H, Dhaenekint C, Demeulenaere P, et al; Flemish Federation of Palliative Care. Atropine, hyoscine butylbromide, or scopolamine are equally effective for the treatment of death rattle in terminal care. J Pain Symptom Manage. 2009;38(1):124-133.
- Kehl KA. Treatment of terminal restlessness: a review of the evidence. J Pain Palliat Care Pharmacother. 2004;18(1):5-30.
Case Report: The Hungry, Hungry Haustra: The Case of a Missing Feeding Tube
Introduction
Percutaneous endoscopic gastrostomy (PEG) tubes are a common method employed for long-term feeding in patients who are unable to tolerate oral feedings.1 Though PEG-tube placement is a common, safe, and well-studied practice, there are known complications, including wound infection, dislodgement, and peritonitis.2 Dislodgement and recurrent ED visits are increasingly becoming a burden on both patients and healthcare providers, as up to 12.8% of patients will require ED replacement of a dislodged tube, totaling an estimated $1,200 per visit.3
Newer techniques include Roux-en-Y feeding jejunostomy tubes, which are anticipated to reduce long-term complications.4,5 However, dislodgement, sinus tracts, and superimposed infections still occur, also leading to ED visits.6 Foley catheters are a readily available and low-cost alternative to replace commercial feeding-tubes in the ED, and are commonly used when the original feeding-tube is not suitable for reuse.7 In the following presentation, the authors describe a previously unseen case of a fully intussuscepted Foley catheter though a Roux-en-Y jejunostomy.
Case Report
A 69-year-old man, recently diagnosed with invasive squamous cell carcinoma of the distal esophagus, presented to the ED with a chief complaint of “J-tube fell out.” One month prior to presentation, the patient had undergone a laparoscopic Janeway Roux-en-Y nipple jejunostomy. He had been previously evaluated several times in the ED for a displaced J-tube, and his commercial feeding tube had been replaced with a Foley catheter without incident.
On this visit, the patient’s wife reported that the Foley catheter had become displaced 3 days prior to presentation, and she assumed that the patient had accidentally pulled it out. According to the patient’s wife, he had attempted oral feedings, but had difficulty swallowing as well as coughing episodes.
Upon initial evaluation, the patient complained of diffuse abdominal pain and cramping. He denied any nausea or vomiting, and reported normal bowel movements. The physical examination was remarkable for the following: hypotension (blood pressure, 64/46 mm Hg); heart rate, 94 beats/minute; temperature, 96.4°F; cachexia; a diffusely tender abdomen; and viable stoma on the anterior abdominal wall. Purulent and malodorous drainage was noted at the stoma site. There was no Foley catheter or J-tube in place, and neither the patient nor his wife had brought the dislodged tube to the ED.
A computed tomography scan of the patient’s abdomen and pelvis were ordered with IV and oral contrast. The imaging studies revealed multiple dilated, fluid-filled loops of small bowel, and a Foley catheter proximal to the ileocecal valve, with the balloon still inflated (Figure).
The emergency physician notified the original surgical team of the patient’s status. The surgical team placed a new, 14 French (Fr)-Foley catheter through the stoma, sutured it in place, and admitted the patient to their service. The patient was maintained on IV antibiotics and fluids. As he continued to pass flatus and stool, a diet was advanced through the replacement Foley catheter. The intussuscepted Foley was subsequently passed naturally on day 4 of his hospital admission. The patient unfortunately died several days later of hypoxic respiratory failure, which was not thought to be related to the ingested catheter.
Discussion
Percutaneous Foley catheters, either pre- or postpyloric, have been used for decades as permanent feeding tubes for patients unable to tolerate oral feedings. These catheters are well-known to be inexpensive and safe replacements for commercial gastrostomy tubes.7 However, a number of complications unique to Foley feeding tubes have been described in case reports, including mechanical obstruction leading to pancreatitis, duodenal obstruction, bowel ischemia secondary to balloon overfilling, pyloric obstruction, bowel infection, as well as broken and digested catheters.8-10
Interestingly, despite multiple case reports demonstrating tube migration, prospective studies have shown this to be a relatively uncommon complication.11 In 2012, a patient in Israel ingested a Foley catheter via the gastrostomy stoma, resulting in small bowel obstruction relieved only by enterotomy and removal of the catheter. There have been no previous documented reports of ingested tubes via jejunostomy stoma.12 Significant forces exerted on Foley catheters have been described, resulting in skin necrosis at the hub and stretching of the catheter from the proximal small bowel to the terminal ileum. In this case presentation, bowel peristalsis was able to advance the entire tube through the skin.13
Management of feeding-Foley-catheter complications typically involves deflation of the balloon and removal and replacement of the offending catheter—usually with a smaller sized Foley catheter (eg, 12Fr, 14Fr, 16Fr). Complicated cases with catheter malfunction have been successfully managed endoscopically.14 The patient in this case was likely at higher risk of complication given the abnormally large wound surrounding the stoma and skin breakdown secondary to superimposed infection.
Conclusion
This case highlights the potent peristaltic forces that are exerted upon a feeding Foley catheter and reinforces the importance of proper tube anchorage. Although this patient did well with direct skin suturing of the replacement catheter, previous studies recommend using a plastic retention ring. Placing a mark on the outside of the catheter as a means to continuously visualize its proper anchorage and placement has also been suggested in the literature. Additionally, whenever a patient presents with a displaced feeding tube (Foley catheter or commercial tube), providers should not assume that the tube has been displaced externally and should maintain a low-threshold for advanced imaging and/or endoscopy if the tube cannot otherwise be located.
Dr Lefkove is an attending physician in the department of emergency medicine, DeKalb Medical Center, Atlanta, Georgia. Dr Meloy is an assistant professor of emergency medicine at Emory University School of Medicine, Atlanta, Georgia.
- Vanis N, Saray A, Gornjakovic S, et al. Percutaneous endoscopic gastrostomy (PEG): retrospective analysis of a 7-year clinical experience. Acta Inform Med. 2012;20(4):235-237.
- Schapiro GD, Edmundowicz SA. Complications of percutaneous endoscopic gastrostomy. Gastrointest Endosc Clin N Am. 1996;6(2):409-422.
- Rosenberger LH, Newhook T, Schirmer B, Sawyer RG. Late accidental dislodgement of a percutaneous endoscopic gastrostomy tube: an underestimated burden on patients and the health care system. Surg Endosc. 2011;25(10):3307-3311.
- Neuman HB, Phillips JD. Laparoscopic Roux-en-Y feeding jejunostomy: a new minimally invasive surgical procedure for permanent feeding access in children with gastric dysfunction. J Laparoendosc Adv Surg Tech A. 2005;15(1):71-74.
- Arnal E, Voiglio EJ, Robert M, Schreiber V, Ceruze P, Caillot JL. Laparoscopic Janeway gastrostomy: an advantageous solution for self-sufficient enteral feeding. Ann Chir. 2005;130(10):613-617.
- Maple JT, Petersen BT, Baron TH, Gostout CJ, Wong Kee Song LM, Buttar NS. Direct percutaneous endoscopic jejunostomy: outcomes in 307 consecutive attempts. Am J Gastroenterol. 2005;100(12):2681-2688.
- Kadakia SC, Cassaday M, Shaffer RT. Comparison of Foley catheter as a replacement gastrostomy tube with commercial replacement gastrostomy tube: a prospective randomized trial. Gastrointest Endosc. 1994;40(2 Pt 1):188-193.
- Brauner E, Kluger Y. Gastrostomy tube dislodgment acute pancreatitis. World J Emerg Surg. 2014;9(1):23.
- Hopens T, Schwesinger WH. Complications of tube gastrostomy: radiologic manifestations. South Med J. 1983;76(1):9-11.
- Martel G, Lingas RI, Gutauskas A, Clark HD. Complication of a percutaneous endoscopic gastrostomy tube causing duodenal ischemia. Surg Laparosc Endosc Percutan Tech. 2006;16(6):445-446.
- Kadakia SC, Cassaday M, Shaffer RT. Prospective evaluation of Foley catheter as a replacement gastrostomy tube. Am J Gastroenterol. 1992;87(11):1594-1597.
- Netz U, Perry ZH, Mizrahi S. The lost foley catheter. Am Surg. 2012;78(9):E407-E408.
- Date RS, Das N, Bateson PG. Unusual complications of ballooned feeding tubes. Ir Med J. 2002;95(6):181-182.
- O’Keefe KP, Dula DJ, Varano V. Duodenal obstruction by a nondeflating Foley catheter gastrostomy tube. Ann Emerg Med. 1990;19(12):1454-1457.
Introduction
Percutaneous endoscopic gastrostomy (PEG) tubes are a common method employed for long-term feeding in patients who are unable to tolerate oral feedings.1 Though PEG-tube placement is a common, safe, and well-studied practice, there are known complications, including wound infection, dislodgement, and peritonitis.2 Dislodgement and recurrent ED visits are increasingly becoming a burden on both patients and healthcare providers, as up to 12.8% of patients will require ED replacement of a dislodged tube, totaling an estimated $1,200 per visit.3
Newer techniques include Roux-en-Y feeding jejunostomy tubes, which are anticipated to reduce long-term complications.4,5 However, dislodgement, sinus tracts, and superimposed infections still occur, also leading to ED visits.6 Foley catheters are a readily available and low-cost alternative to replace commercial feeding-tubes in the ED, and are commonly used when the original feeding-tube is not suitable for reuse.7 In the following presentation, the authors describe a previously unseen case of a fully intussuscepted Foley catheter though a Roux-en-Y jejunostomy.
Case Report
A 69-year-old man, recently diagnosed with invasive squamous cell carcinoma of the distal esophagus, presented to the ED with a chief complaint of “J-tube fell out.” One month prior to presentation, the patient had undergone a laparoscopic Janeway Roux-en-Y nipple jejunostomy. He had been previously evaluated several times in the ED for a displaced J-tube, and his commercial feeding tube had been replaced with a Foley catheter without incident.
On this visit, the patient’s wife reported that the Foley catheter had become displaced 3 days prior to presentation, and she assumed that the patient had accidentally pulled it out. According to the patient’s wife, he had attempted oral feedings, but had difficulty swallowing as well as coughing episodes.
Upon initial evaluation, the patient complained of diffuse abdominal pain and cramping. He denied any nausea or vomiting, and reported normal bowel movements. The physical examination was remarkable for the following: hypotension (blood pressure, 64/46 mm Hg); heart rate, 94 beats/minute; temperature, 96.4°F; cachexia; a diffusely tender abdomen; and viable stoma on the anterior abdominal wall. Purulent and malodorous drainage was noted at the stoma site. There was no Foley catheter or J-tube in place, and neither the patient nor his wife had brought the dislodged tube to the ED.
A computed tomography scan of the patient’s abdomen and pelvis were ordered with IV and oral contrast. The imaging studies revealed multiple dilated, fluid-filled loops of small bowel, and a Foley catheter proximal to the ileocecal valve, with the balloon still inflated (Figure).
The emergency physician notified the original surgical team of the patient’s status. The surgical team placed a new, 14 French (Fr)-Foley catheter through the stoma, sutured it in place, and admitted the patient to their service. The patient was maintained on IV antibiotics and fluids. As he continued to pass flatus and stool, a diet was advanced through the replacement Foley catheter. The intussuscepted Foley was subsequently passed naturally on day 4 of his hospital admission. The patient unfortunately died several days later of hypoxic respiratory failure, which was not thought to be related to the ingested catheter.
Discussion
Percutaneous Foley catheters, either pre- or postpyloric, have been used for decades as permanent feeding tubes for patients unable to tolerate oral feedings. These catheters are well-known to be inexpensive and safe replacements for commercial gastrostomy tubes.7 However, a number of complications unique to Foley feeding tubes have been described in case reports, including mechanical obstruction leading to pancreatitis, duodenal obstruction, bowel ischemia secondary to balloon overfilling, pyloric obstruction, bowel infection, as well as broken and digested catheters.8-10
Interestingly, despite multiple case reports demonstrating tube migration, prospective studies have shown this to be a relatively uncommon complication.11 In 2012, a patient in Israel ingested a Foley catheter via the gastrostomy stoma, resulting in small bowel obstruction relieved only by enterotomy and removal of the catheter. There have been no previous documented reports of ingested tubes via jejunostomy stoma.12 Significant forces exerted on Foley catheters have been described, resulting in skin necrosis at the hub and stretching of the catheter from the proximal small bowel to the terminal ileum. In this case presentation, bowel peristalsis was able to advance the entire tube through the skin.13
Management of feeding-Foley-catheter complications typically involves deflation of the balloon and removal and replacement of the offending catheter—usually with a smaller sized Foley catheter (eg, 12Fr, 14Fr, 16Fr). Complicated cases with catheter malfunction have been successfully managed endoscopically.14 The patient in this case was likely at higher risk of complication given the abnormally large wound surrounding the stoma and skin breakdown secondary to superimposed infection.
Conclusion
This case highlights the potent peristaltic forces that are exerted upon a feeding Foley catheter and reinforces the importance of proper tube anchorage. Although this patient did well with direct skin suturing of the replacement catheter, previous studies recommend using a plastic retention ring. Placing a mark on the outside of the catheter as a means to continuously visualize its proper anchorage and placement has also been suggested in the literature. Additionally, whenever a patient presents with a displaced feeding tube (Foley catheter or commercial tube), providers should not assume that the tube has been displaced externally and should maintain a low-threshold for advanced imaging and/or endoscopy if the tube cannot otherwise be located.
Dr Lefkove is an attending physician in the department of emergency medicine, DeKalb Medical Center, Atlanta, Georgia. Dr Meloy is an assistant professor of emergency medicine at Emory University School of Medicine, Atlanta, Georgia.
Introduction
Percutaneous endoscopic gastrostomy (PEG) tubes are a common method employed for long-term feeding in patients who are unable to tolerate oral feedings.1 Though PEG-tube placement is a common, safe, and well-studied practice, there are known complications, including wound infection, dislodgement, and peritonitis.2 Dislodgement and recurrent ED visits are increasingly becoming a burden on both patients and healthcare providers, as up to 12.8% of patients will require ED replacement of a dislodged tube, totaling an estimated $1,200 per visit.3
Newer techniques include Roux-en-Y feeding jejunostomy tubes, which are anticipated to reduce long-term complications.4,5 However, dislodgement, sinus tracts, and superimposed infections still occur, also leading to ED visits.6 Foley catheters are a readily available and low-cost alternative to replace commercial feeding-tubes in the ED, and are commonly used when the original feeding-tube is not suitable for reuse.7 In the following presentation, the authors describe a previously unseen case of a fully intussuscepted Foley catheter though a Roux-en-Y jejunostomy.
Case Report
A 69-year-old man, recently diagnosed with invasive squamous cell carcinoma of the distal esophagus, presented to the ED with a chief complaint of “J-tube fell out.” One month prior to presentation, the patient had undergone a laparoscopic Janeway Roux-en-Y nipple jejunostomy. He had been previously evaluated several times in the ED for a displaced J-tube, and his commercial feeding tube had been replaced with a Foley catheter without incident.
On this visit, the patient’s wife reported that the Foley catheter had become displaced 3 days prior to presentation, and she assumed that the patient had accidentally pulled it out. According to the patient’s wife, he had attempted oral feedings, but had difficulty swallowing as well as coughing episodes.
Upon initial evaluation, the patient complained of diffuse abdominal pain and cramping. He denied any nausea or vomiting, and reported normal bowel movements. The physical examination was remarkable for the following: hypotension (blood pressure, 64/46 mm Hg); heart rate, 94 beats/minute; temperature, 96.4°F; cachexia; a diffusely tender abdomen; and viable stoma on the anterior abdominal wall. Purulent and malodorous drainage was noted at the stoma site. There was no Foley catheter or J-tube in place, and neither the patient nor his wife had brought the dislodged tube to the ED.
A computed tomography scan of the patient’s abdomen and pelvis were ordered with IV and oral contrast. The imaging studies revealed multiple dilated, fluid-filled loops of small bowel, and a Foley catheter proximal to the ileocecal valve, with the balloon still inflated (Figure).
The emergency physician notified the original surgical team of the patient’s status. The surgical team placed a new, 14 French (Fr)-Foley catheter through the stoma, sutured it in place, and admitted the patient to their service. The patient was maintained on IV antibiotics and fluids. As he continued to pass flatus and stool, a diet was advanced through the replacement Foley catheter. The intussuscepted Foley was subsequently passed naturally on day 4 of his hospital admission. The patient unfortunately died several days later of hypoxic respiratory failure, which was not thought to be related to the ingested catheter.
Discussion
Percutaneous Foley catheters, either pre- or postpyloric, have been used for decades as permanent feeding tubes for patients unable to tolerate oral feedings. These catheters are well-known to be inexpensive and safe replacements for commercial gastrostomy tubes.7 However, a number of complications unique to Foley feeding tubes have been described in case reports, including mechanical obstruction leading to pancreatitis, duodenal obstruction, bowel ischemia secondary to balloon overfilling, pyloric obstruction, bowel infection, as well as broken and digested catheters.8-10
Interestingly, despite multiple case reports demonstrating tube migration, prospective studies have shown this to be a relatively uncommon complication.11 In 2012, a patient in Israel ingested a Foley catheter via the gastrostomy stoma, resulting in small bowel obstruction relieved only by enterotomy and removal of the catheter. There have been no previous documented reports of ingested tubes via jejunostomy stoma.12 Significant forces exerted on Foley catheters have been described, resulting in skin necrosis at the hub and stretching of the catheter from the proximal small bowel to the terminal ileum. In this case presentation, bowel peristalsis was able to advance the entire tube through the skin.13
Management of feeding-Foley-catheter complications typically involves deflation of the balloon and removal and replacement of the offending catheter—usually with a smaller sized Foley catheter (eg, 12Fr, 14Fr, 16Fr). Complicated cases with catheter malfunction have been successfully managed endoscopically.14 The patient in this case was likely at higher risk of complication given the abnormally large wound surrounding the stoma and skin breakdown secondary to superimposed infection.
Conclusion
This case highlights the potent peristaltic forces that are exerted upon a feeding Foley catheter and reinforces the importance of proper tube anchorage. Although this patient did well with direct skin suturing of the replacement catheter, previous studies recommend using a plastic retention ring. Placing a mark on the outside of the catheter as a means to continuously visualize its proper anchorage and placement has also been suggested in the literature. Additionally, whenever a patient presents with a displaced feeding tube (Foley catheter or commercial tube), providers should not assume that the tube has been displaced externally and should maintain a low-threshold for advanced imaging and/or endoscopy if the tube cannot otherwise be located.
Dr Lefkove is an attending physician in the department of emergency medicine, DeKalb Medical Center, Atlanta, Georgia. Dr Meloy is an assistant professor of emergency medicine at Emory University School of Medicine, Atlanta, Georgia.
- Vanis N, Saray A, Gornjakovic S, et al. Percutaneous endoscopic gastrostomy (PEG): retrospective analysis of a 7-year clinical experience. Acta Inform Med. 2012;20(4):235-237.
- Schapiro GD, Edmundowicz SA. Complications of percutaneous endoscopic gastrostomy. Gastrointest Endosc Clin N Am. 1996;6(2):409-422.
- Rosenberger LH, Newhook T, Schirmer B, Sawyer RG. Late accidental dislodgement of a percutaneous endoscopic gastrostomy tube: an underestimated burden on patients and the health care system. Surg Endosc. 2011;25(10):3307-3311.
- Neuman HB, Phillips JD. Laparoscopic Roux-en-Y feeding jejunostomy: a new minimally invasive surgical procedure for permanent feeding access in children with gastric dysfunction. J Laparoendosc Adv Surg Tech A. 2005;15(1):71-74.
- Arnal E, Voiglio EJ, Robert M, Schreiber V, Ceruze P, Caillot JL. Laparoscopic Janeway gastrostomy: an advantageous solution for self-sufficient enteral feeding. Ann Chir. 2005;130(10):613-617.
- Maple JT, Petersen BT, Baron TH, Gostout CJ, Wong Kee Song LM, Buttar NS. Direct percutaneous endoscopic jejunostomy: outcomes in 307 consecutive attempts. Am J Gastroenterol. 2005;100(12):2681-2688.
- Kadakia SC, Cassaday M, Shaffer RT. Comparison of Foley catheter as a replacement gastrostomy tube with commercial replacement gastrostomy tube: a prospective randomized trial. Gastrointest Endosc. 1994;40(2 Pt 1):188-193.
- Brauner E, Kluger Y. Gastrostomy tube dislodgment acute pancreatitis. World J Emerg Surg. 2014;9(1):23.
- Hopens T, Schwesinger WH. Complications of tube gastrostomy: radiologic manifestations. South Med J. 1983;76(1):9-11.
- Martel G, Lingas RI, Gutauskas A, Clark HD. Complication of a percutaneous endoscopic gastrostomy tube causing duodenal ischemia. Surg Laparosc Endosc Percutan Tech. 2006;16(6):445-446.
- Kadakia SC, Cassaday M, Shaffer RT. Prospective evaluation of Foley catheter as a replacement gastrostomy tube. Am J Gastroenterol. 1992;87(11):1594-1597.
- Netz U, Perry ZH, Mizrahi S. The lost foley catheter. Am Surg. 2012;78(9):E407-E408.
- Date RS, Das N, Bateson PG. Unusual complications of ballooned feeding tubes. Ir Med J. 2002;95(6):181-182.
- O’Keefe KP, Dula DJ, Varano V. Duodenal obstruction by a nondeflating Foley catheter gastrostomy tube. Ann Emerg Med. 1990;19(12):1454-1457.
- Vanis N, Saray A, Gornjakovic S, et al. Percutaneous endoscopic gastrostomy (PEG): retrospective analysis of a 7-year clinical experience. Acta Inform Med. 2012;20(4):235-237.
- Schapiro GD, Edmundowicz SA. Complications of percutaneous endoscopic gastrostomy. Gastrointest Endosc Clin N Am. 1996;6(2):409-422.
- Rosenberger LH, Newhook T, Schirmer B, Sawyer RG. Late accidental dislodgement of a percutaneous endoscopic gastrostomy tube: an underestimated burden on patients and the health care system. Surg Endosc. 2011;25(10):3307-3311.
- Neuman HB, Phillips JD. Laparoscopic Roux-en-Y feeding jejunostomy: a new minimally invasive surgical procedure for permanent feeding access in children with gastric dysfunction. J Laparoendosc Adv Surg Tech A. 2005;15(1):71-74.
- Arnal E, Voiglio EJ, Robert M, Schreiber V, Ceruze P, Caillot JL. Laparoscopic Janeway gastrostomy: an advantageous solution for self-sufficient enteral feeding. Ann Chir. 2005;130(10):613-617.
- Maple JT, Petersen BT, Baron TH, Gostout CJ, Wong Kee Song LM, Buttar NS. Direct percutaneous endoscopic jejunostomy: outcomes in 307 consecutive attempts. Am J Gastroenterol. 2005;100(12):2681-2688.
- Kadakia SC, Cassaday M, Shaffer RT. Comparison of Foley catheter as a replacement gastrostomy tube with commercial replacement gastrostomy tube: a prospective randomized trial. Gastrointest Endosc. 1994;40(2 Pt 1):188-193.
- Brauner E, Kluger Y. Gastrostomy tube dislodgment acute pancreatitis. World J Emerg Surg. 2014;9(1):23.
- Hopens T, Schwesinger WH. Complications of tube gastrostomy: radiologic manifestations. South Med J. 1983;76(1):9-11.
- Martel G, Lingas RI, Gutauskas A, Clark HD. Complication of a percutaneous endoscopic gastrostomy tube causing duodenal ischemia. Surg Laparosc Endosc Percutan Tech. 2006;16(6):445-446.
- Kadakia SC, Cassaday M, Shaffer RT. Prospective evaluation of Foley catheter as a replacement gastrostomy tube. Am J Gastroenterol. 1992;87(11):1594-1597.
- Netz U, Perry ZH, Mizrahi S. The lost foley catheter. Am Surg. 2012;78(9):E407-E408.
- Date RS, Das N, Bateson PG. Unusual complications of ballooned feeding tubes. Ir Med J. 2002;95(6):181-182.
- O’Keefe KP, Dula DJ, Varano V. Duodenal obstruction by a nondeflating Foley catheter gastrostomy tube. Ann Emerg Med. 1990;19(12):1454-1457.