User login
The professional advancement of drug and device innovation

I often say that there are both “guardrail” days and very good days when it comes to the ins and outs of health care builds and product launches. The process is much like starting down the path of a country road in the middle of a blizzard—unless you have dependable wipers and a good defrost system, that path can get murky very quickly. With this article I hope to offer my counsel to inventors, featuring a few of my prior launches as well as case studies of health care launches I was not involved with, and sharing the lessons learned and hurdles that were overcome. I encourage all entrepreneurs to act on their ideas because, in the world of health care startups, the only failure is not acting on an invention.
Case study 1: Cerezyme
Today, Cerezyme is indicated for patients with Gaucher, which is a lysosomal storage disorder. Cerezyme’s first-generation product, called Ceredase, was a human tissue-derived protein that we extracted from human placentas. At the time, the concept of moving this program forward was denied by the Board of Directors because they said that even if you could collect enough placentas to make the enzyme, it would be too expensive to manufacture. In fact, early scale-up modeling for manufacturing the protein demonstrated that Genzyme would need 4 tons of placentas per Gaucher patient per year.
Gaucher is a severe, early-onset disease that has a significant negative outcome for patients. Patients with Gaucher are in dire need of treatment. Genzyme went forward with the Ceredase program by financing it through the families of patients with the disease, by starting an LLC separate from the business and funding the initial clinical trial and the development of the protein through the families of Gaucher patients. That approach was a successful endeavor. A great example of a creative capital structure to advance a program.
This was in the late 1980s/early 1990s, and at the height of the AIDS challenge. Genzyme based the manufacturing in Lille, France, and we cryopreserved placentas in the United States and Europe and shipped them to Lille to be processed into therapy. Genzyme eventually received approval for Ceredase from the US Food and Drug Administration (FDA) and the European Medicines Agency. At the height of the placenta collection, we were gathering about 10% to 15% of the placentas in the United States and 30% to 40% of the placentas in Europe. Resources supply became an issue until we developed a recombinant form of the protein, accomplished by using a manufacturing system called a CHO cell line.
This is a very good success story: If this invention was not pursued, Gaucher patients would not benefit from the treatment today. In addition, there are a plethora of patients with different lysosomal storage disorders treated with additional proteins that have been aided by us going through the entire development, manufacturing, and global commercialization process. We figured out how to manufacture and deliver the treatment, working through multiple countries’ political systems, and today the therapy is paid for by insurance and government systems on a worldwide basis.
Continue to: Case study 2...
Case study 2: ThinPrep
I like to use the approval of ThinPrep as an example of avoiding a false negative—a stoppage in the development of the product or drug for the wrong reasons. False negatives, in my mind, occur when you are developing a technology and you run into issues during the clinical phase and/or with FDA approval, or with a technical failure or you run out of capital prior to knowing whether or not the innovation actually works. In the case of ThinPrep, a poorly run clinical trial almost resulted in a false negative.
The company at the time was Cytyc, and an initial clinical study presented to the FDA yielded a neutral-negative outcome. The FDA said that there were not enough data to show the differentiation from the current Pap smear standard of care.
The founders of the company at that time had inherited the study protocol from a prior leadership team, so they had to finish the trial with the initial protocol. Given the FDA’s advisement, they developed a new trial. It took the persistence of these two founders, who mortgaged their homes and spent their personal dollars to take this through the next wave of clinical development. In the end it was successful. The revised clinical trial yielded an approval for ThinPrep, which is now considered a standard of care.
The use of ThinPrep reduced cervical cancer deaths by 40% from preapproval. The challenging path from clinical development to eventual commercial launch and physician leadership in advancing patient care makes the story of ThinPrep a great example of not allowing an early false negative of a poorly designed and run clinical trial stop important innovation.
Case study 3: Cologuard
The development of Cologuard is a case study demonstrating that, sometimes, when your first attempt does not work, you need to have the persistence to raise additional capital and/or use a slightly different technical approach. The approval story of Cologuard is important to share because it is an important cancer screening diagnostic, using DNA from stool samples to test for colon cancer, giving access to important colon cancer screening to many patients. Currently, caregivers are only scraping the surface with Cologuard’s ability to screen the population. There are many more patients that need access to the test, and I believe they will get it in the years ahead.
Cologuard went through a first- and second-generational technical failure. They could not get the test’s specificity and sensitivity to be at the level of a screening tool; there were too many false-positive results. With the third iteration came the technical breakthrough, and a very large, expensive study was conducted—one the leadership team was criticized for. However, that study yielded the data that achieved a New England Journal of Medicine article, and reimbursement support across the country. The combination of the right technical team and the right leadership team, who planned a proper commercial launch, with a CEO that supported the extensive clinical study, has resulted in the fourth generation of Cologuard—an important breakthrough offering a very useful new standard of care in colon cancer detection and screening.
Continue to: Pearls for moving your innovations forward...
Pearls for moving your innovations forward
Because of my experience in undergoing health care start-ups, and contributing to several of those advancements of innovation, many inventors approach me for advice on their paths from idea to full-concept company. Here are a few of my lessons learned.
Consider purpose, not financial gain, first and foremost. Financial gain is typically the by-product or outcome of a standard-of-care breakthrough for inventors, but it’s a very hard road. Pursue your invention for advancing patient care and moving a new standard of care forward in health care versus financial gain at the end.
Determine whether your invention is a product or a company, or potentially, not capitalizable at all. Figure this out early. Analyze your idea to make sure it is sound and truly novel. Analyze the competition and to make sure it is sound and truly novel. Analyze the competition and the market dynamics to support a new product. Can the development path be defined very clearly to raise capital? Is your innovation a big enough breakthrough in the market with several current products to actually make a difference in patient outcomes (and eventually achieve product reimbursement)? The creation of a company may be the right strategy if the innovation can support a differentiated enough breakthrough where you can actually support all the infrastructure to build the business. If you find that the market is not there to support and develop your idea to eventual success, backing off early is important to preserve invested capital.
Protect early. Is your invention patentable, or has someone else already thought of the idea? What kind of patent(s) are appropriate? Where, geographically, do you want to protect your invention? Find a good patent attorney in your local area, early in the process, to help you answer all of these critical questions. Patents are expensive to file and maintain, but it is not expensive to do a literature search to find out if your idea is novel. A provisional patent, which would be your first step, is an important cost-effective step.
Capital is out there. If your invention or idea deserves capital, it is available. I will address raising capital in more detail in the next section.
Consider regulatory and manufacturing as achievable hurdles. Inventors often get tripped up here, considering the regulatory hurdles and manufacturing too challenging and abandoning their ideas because the risk is too great. Regulatory and manufacturing are very important aspects of health care standard-of-care builds. Cutting corners is not an option. That said, regulatory and manufacturing should not stop you. Challenges often can be worked through as long as the clinical need is there, and the clinical data support bringing that technology forward.
Consider corporate partnerships. I am a fan of corporate partners. But which ones should you target, and when and why? Corporate partnerships can bring significant capital, which is great, but there is enough investor capital out there that you should not pursue a corporate partner just for capital. The main benefit of a corporate partner is enterprise intellect. They typically know more about the field that you are entering than the investors or a small company leadership team.
Establish and listen to advisors. When thinking about who to trust, research their track record. Advisors who have gone through this process before, and specifically in your product area, are important to have access to.
Persistence is key. I have observed a tremendous “compression of innovation” in the health care areas that I have been involved with—human tissue-derived proteins, robotic surgery, stem cell therapy, and digital health (which is still in its infancy). For each of these breakthrough categories, early on, it appeared that it couldn’t be done. However, after the first 2 or 3 major breakthroughs in each one of these areas, a compression of innovation occurred. For instance, after approximately 15 years of protein development, we came out with the recombinant manufacturing systems for proteins. Very quickly, within 10 years, there were more than 70 proteins on the market. The persistence of the inventors to overcome early obstacles in each of these health care areas was critical to future success in each area.
Continue to: Raising capital...
Raising capital
There are different investors who specialize in different types of investment opportunities. The first phase of raising capital is the seed round—where there is typically early data, or even no data and just a concept. From this seed round forward, there is less risk as you develop your technology; thus, there are different investors that support different stages of development and that specialize in different types of investing. It is important to target the right investors and raise enough capital to be able to go achieve multiple operational milestones. Otherwise, when you go through your first round of capital, or the Series A or B financing rounds, there may not be a set of investors out there to fund the company moving forward. Health care investors will make it known that they invest in certain rounds of capital. You can determine who those investors are by doing a search online.
A mistake health care inventors can make is not taking enough capital from investors, because they are concerned about dilution. I advise investors not to focus on dilution but rather on, how big can you make “the pie” (value of the company) worth? The entire process is about bringing a true product through to a new standard-of-care curve.
Trust is the most important thing to earn with investors, and there is zero tolerance for a lack of trust. Share your vision as the inventor with investors, who want to know where this category could be in the next 5 or 10 years. Clinical data will always win, and health care investors and industry leaders should be focused on executing the most robust clinical data to demonstrate the clearest potential clinical outcome. Investors will follow a good plan that has been developed to achieve FDA approval, successful commercialization or “go to market” launch, and eventual reimbursement to support a true standard-of-care change.
Failure is defined by inaction
The 3 case studies that I have shared were success stories because the ideas and inventions were acted upon. When I was at Genzyme, we built the company up to more than $1 billion in revenue. We commercialized proteins in over 50 countries. Most importantly, many patients benefited from the innovation. If you have an invention and an idea, act on it—and surround yourself with great people in every discipline. Having the right people and team is extremely important. ●

I often say that there are both “guardrail” days and very good days when it comes to the ins and outs of health care builds and product launches. The process is much like starting down the path of a country road in the middle of a blizzard—unless you have dependable wipers and a good defrost system, that path can get murky very quickly. With this article I hope to offer my counsel to inventors, featuring a few of my prior launches as well as case studies of health care launches I was not involved with, and sharing the lessons learned and hurdles that were overcome. I encourage all entrepreneurs to act on their ideas because, in the world of health care startups, the only failure is not acting on an invention.
Case study 1: Cerezyme
Today, Cerezyme is indicated for patients with Gaucher, which is a lysosomal storage disorder. Cerezyme’s first-generation product, called Ceredase, was a human tissue-derived protein that we extracted from human placentas. At the time, the concept of moving this program forward was denied by the Board of Directors because they said that even if you could collect enough placentas to make the enzyme, it would be too expensive to manufacture. In fact, early scale-up modeling for manufacturing the protein demonstrated that Genzyme would need 4 tons of placentas per Gaucher patient per year.
Gaucher is a severe, early-onset disease that has a significant negative outcome for patients. Patients with Gaucher are in dire need of treatment. Genzyme went forward with the Ceredase program by financing it through the families of patients with the disease, by starting an LLC separate from the business and funding the initial clinical trial and the development of the protein through the families of Gaucher patients. That approach was a successful endeavor. A great example of a creative capital structure to advance a program.
This was in the late 1980s/early 1990s, and at the height of the AIDS challenge. Genzyme based the manufacturing in Lille, France, and we cryopreserved placentas in the United States and Europe and shipped them to Lille to be processed into therapy. Genzyme eventually received approval for Ceredase from the US Food and Drug Administration (FDA) and the European Medicines Agency. At the height of the placenta collection, we were gathering about 10% to 15% of the placentas in the United States and 30% to 40% of the placentas in Europe. Resources supply became an issue until we developed a recombinant form of the protein, accomplished by using a manufacturing system called a CHO cell line.
This is a very good success story: If this invention was not pursued, Gaucher patients would not benefit from the treatment today. In addition, there are a plethora of patients with different lysosomal storage disorders treated with additional proteins that have been aided by us going through the entire development, manufacturing, and global commercialization process. We figured out how to manufacture and deliver the treatment, working through multiple countries’ political systems, and today the therapy is paid for by insurance and government systems on a worldwide basis.
Continue to: Case study 2...
Case study 2: ThinPrep
I like to use the approval of ThinPrep as an example of avoiding a false negative—a stoppage in the development of the product or drug for the wrong reasons. False negatives, in my mind, occur when you are developing a technology and you run into issues during the clinical phase and/or with FDA approval, or with a technical failure or you run out of capital prior to knowing whether or not the innovation actually works. In the case of ThinPrep, a poorly run clinical trial almost resulted in a false negative.
The company at the time was Cytyc, and an initial clinical study presented to the FDA yielded a neutral-negative outcome. The FDA said that there were not enough data to show the differentiation from the current Pap smear standard of care.
The founders of the company at that time had inherited the study protocol from a prior leadership team, so they had to finish the trial with the initial protocol. Given the FDA’s advisement, they developed a new trial. It took the persistence of these two founders, who mortgaged their homes and spent their personal dollars to take this through the next wave of clinical development. In the end it was successful. The revised clinical trial yielded an approval for ThinPrep, which is now considered a standard of care.
The use of ThinPrep reduced cervical cancer deaths by 40% from preapproval. The challenging path from clinical development to eventual commercial launch and physician leadership in advancing patient care makes the story of ThinPrep a great example of not allowing an early false negative of a poorly designed and run clinical trial stop important innovation.
Case study 3: Cologuard
The development of Cologuard is a case study demonstrating that, sometimes, when your first attempt does not work, you need to have the persistence to raise additional capital and/or use a slightly different technical approach. The approval story of Cologuard is important to share because it is an important cancer screening diagnostic, using DNA from stool samples to test for colon cancer, giving access to important colon cancer screening to many patients. Currently, caregivers are only scraping the surface with Cologuard’s ability to screen the population. There are many more patients that need access to the test, and I believe they will get it in the years ahead.
Cologuard went through a first- and second-generational technical failure. They could not get the test’s specificity and sensitivity to be at the level of a screening tool; there were too many false-positive results. With the third iteration came the technical breakthrough, and a very large, expensive study was conducted—one the leadership team was criticized for. However, that study yielded the data that achieved a New England Journal of Medicine article, and reimbursement support across the country. The combination of the right technical team and the right leadership team, who planned a proper commercial launch, with a CEO that supported the extensive clinical study, has resulted in the fourth generation of Cologuard—an important breakthrough offering a very useful new standard of care in colon cancer detection and screening.
Continue to: Pearls for moving your innovations forward...
Pearls for moving your innovations forward
Because of my experience in undergoing health care start-ups, and contributing to several of those advancements of innovation, many inventors approach me for advice on their paths from idea to full-concept company. Here are a few of my lessons learned.
Consider purpose, not financial gain, first and foremost. Financial gain is typically the by-product or outcome of a standard-of-care breakthrough for inventors, but it’s a very hard road. Pursue your invention for advancing patient care and moving a new standard of care forward in health care versus financial gain at the end.
Determine whether your invention is a product or a company, or potentially, not capitalizable at all. Figure this out early. Analyze your idea to make sure it is sound and truly novel. Analyze the competition and to make sure it is sound and truly novel. Analyze the competition and the market dynamics to support a new product. Can the development path be defined very clearly to raise capital? Is your innovation a big enough breakthrough in the market with several current products to actually make a difference in patient outcomes (and eventually achieve product reimbursement)? The creation of a company may be the right strategy if the innovation can support a differentiated enough breakthrough where you can actually support all the infrastructure to build the business. If you find that the market is not there to support and develop your idea to eventual success, backing off early is important to preserve invested capital.
Protect early. Is your invention patentable, or has someone else already thought of the idea? What kind of patent(s) are appropriate? Where, geographically, do you want to protect your invention? Find a good patent attorney in your local area, early in the process, to help you answer all of these critical questions. Patents are expensive to file and maintain, but it is not expensive to do a literature search to find out if your idea is novel. A provisional patent, which would be your first step, is an important cost-effective step.
Capital is out there. If your invention or idea deserves capital, it is available. I will address raising capital in more detail in the next section.
Consider regulatory and manufacturing as achievable hurdles. Inventors often get tripped up here, considering the regulatory hurdles and manufacturing too challenging and abandoning their ideas because the risk is too great. Regulatory and manufacturing are very important aspects of health care standard-of-care builds. Cutting corners is not an option. That said, regulatory and manufacturing should not stop you. Challenges often can be worked through as long as the clinical need is there, and the clinical data support bringing that technology forward.
Consider corporate partnerships. I am a fan of corporate partners. But which ones should you target, and when and why? Corporate partnerships can bring significant capital, which is great, but there is enough investor capital out there that you should not pursue a corporate partner just for capital. The main benefit of a corporate partner is enterprise intellect. They typically know more about the field that you are entering than the investors or a small company leadership team.
Establish and listen to advisors. When thinking about who to trust, research their track record. Advisors who have gone through this process before, and specifically in your product area, are important to have access to.
Persistence is key. I have observed a tremendous “compression of innovation” in the health care areas that I have been involved with—human tissue-derived proteins, robotic surgery, stem cell therapy, and digital health (which is still in its infancy). For each of these breakthrough categories, early on, it appeared that it couldn’t be done. However, after the first 2 or 3 major breakthroughs in each one of these areas, a compression of innovation occurred. For instance, after approximately 15 years of protein development, we came out with the recombinant manufacturing systems for proteins. Very quickly, within 10 years, there were more than 70 proteins on the market. The persistence of the inventors to overcome early obstacles in each of these health care areas was critical to future success in each area.
Continue to: Raising capital...
Raising capital
There are different investors who specialize in different types of investment opportunities. The first phase of raising capital is the seed round—where there is typically early data, or even no data and just a concept. From this seed round forward, there is less risk as you develop your technology; thus, there are different investors that support different stages of development and that specialize in different types of investing. It is important to target the right investors and raise enough capital to be able to go achieve multiple operational milestones. Otherwise, when you go through your first round of capital, or the Series A or B financing rounds, there may not be a set of investors out there to fund the company moving forward. Health care investors will make it known that they invest in certain rounds of capital. You can determine who those investors are by doing a search online.
A mistake health care inventors can make is not taking enough capital from investors, because they are concerned about dilution. I advise investors not to focus on dilution but rather on, how big can you make “the pie” (value of the company) worth? The entire process is about bringing a true product through to a new standard-of-care curve.
Trust is the most important thing to earn with investors, and there is zero tolerance for a lack of trust. Share your vision as the inventor with investors, who want to know where this category could be in the next 5 or 10 years. Clinical data will always win, and health care investors and industry leaders should be focused on executing the most robust clinical data to demonstrate the clearest potential clinical outcome. Investors will follow a good plan that has been developed to achieve FDA approval, successful commercialization or “go to market” launch, and eventual reimbursement to support a true standard-of-care change.
Failure is defined by inaction
The 3 case studies that I have shared were success stories because the ideas and inventions were acted upon. When I was at Genzyme, we built the company up to more than $1 billion in revenue. We commercialized proteins in over 50 countries. Most importantly, many patients benefited from the innovation. If you have an invention and an idea, act on it—and surround yourself with great people in every discipline. Having the right people and team is extremely important. ●

I often say that there are both “guardrail” days and very good days when it comes to the ins and outs of health care builds and product launches. The process is much like starting down the path of a country road in the middle of a blizzard—unless you have dependable wipers and a good defrost system, that path can get murky very quickly. With this article I hope to offer my counsel to inventors, featuring a few of my prior launches as well as case studies of health care launches I was not involved with, and sharing the lessons learned and hurdles that were overcome. I encourage all entrepreneurs to act on their ideas because, in the world of health care startups, the only failure is not acting on an invention.
Case study 1: Cerezyme
Today, Cerezyme is indicated for patients with Gaucher, which is a lysosomal storage disorder. Cerezyme’s first-generation product, called Ceredase, was a human tissue-derived protein that we extracted from human placentas. At the time, the concept of moving this program forward was denied by the Board of Directors because they said that even if you could collect enough placentas to make the enzyme, it would be too expensive to manufacture. In fact, early scale-up modeling for manufacturing the protein demonstrated that Genzyme would need 4 tons of placentas per Gaucher patient per year.
Gaucher is a severe, early-onset disease that has a significant negative outcome for patients. Patients with Gaucher are in dire need of treatment. Genzyme went forward with the Ceredase program by financing it through the families of patients with the disease, by starting an LLC separate from the business and funding the initial clinical trial and the development of the protein through the families of Gaucher patients. That approach was a successful endeavor. A great example of a creative capital structure to advance a program.
This was in the late 1980s/early 1990s, and at the height of the AIDS challenge. Genzyme based the manufacturing in Lille, France, and we cryopreserved placentas in the United States and Europe and shipped them to Lille to be processed into therapy. Genzyme eventually received approval for Ceredase from the US Food and Drug Administration (FDA) and the European Medicines Agency. At the height of the placenta collection, we were gathering about 10% to 15% of the placentas in the United States and 30% to 40% of the placentas in Europe. Resources supply became an issue until we developed a recombinant form of the protein, accomplished by using a manufacturing system called a CHO cell line.
This is a very good success story: If this invention was not pursued, Gaucher patients would not benefit from the treatment today. In addition, there are a plethora of patients with different lysosomal storage disorders treated with additional proteins that have been aided by us going through the entire development, manufacturing, and global commercialization process. We figured out how to manufacture and deliver the treatment, working through multiple countries’ political systems, and today the therapy is paid for by insurance and government systems on a worldwide basis.
Continue to: Case study 2...
Case study 2: ThinPrep
I like to use the approval of ThinPrep as an example of avoiding a false negative—a stoppage in the development of the product or drug for the wrong reasons. False negatives, in my mind, occur when you are developing a technology and you run into issues during the clinical phase and/or with FDA approval, or with a technical failure or you run out of capital prior to knowing whether or not the innovation actually works. In the case of ThinPrep, a poorly run clinical trial almost resulted in a false negative.
The company at the time was Cytyc, and an initial clinical study presented to the FDA yielded a neutral-negative outcome. The FDA said that there were not enough data to show the differentiation from the current Pap smear standard of care.
The founders of the company at that time had inherited the study protocol from a prior leadership team, so they had to finish the trial with the initial protocol. Given the FDA’s advisement, they developed a new trial. It took the persistence of these two founders, who mortgaged their homes and spent their personal dollars to take this through the next wave of clinical development. In the end it was successful. The revised clinical trial yielded an approval for ThinPrep, which is now considered a standard of care.
The use of ThinPrep reduced cervical cancer deaths by 40% from preapproval. The challenging path from clinical development to eventual commercial launch and physician leadership in advancing patient care makes the story of ThinPrep a great example of not allowing an early false negative of a poorly designed and run clinical trial stop important innovation.
Case study 3: Cologuard
The development of Cologuard is a case study demonstrating that, sometimes, when your first attempt does not work, you need to have the persistence to raise additional capital and/or use a slightly different technical approach. The approval story of Cologuard is important to share because it is an important cancer screening diagnostic, using DNA from stool samples to test for colon cancer, giving access to important colon cancer screening to many patients. Currently, caregivers are only scraping the surface with Cologuard’s ability to screen the population. There are many more patients that need access to the test, and I believe they will get it in the years ahead.
Cologuard went through a first- and second-generational technical failure. They could not get the test’s specificity and sensitivity to be at the level of a screening tool; there were too many false-positive results. With the third iteration came the technical breakthrough, and a very large, expensive study was conducted—one the leadership team was criticized for. However, that study yielded the data that achieved a New England Journal of Medicine article, and reimbursement support across the country. The combination of the right technical team and the right leadership team, who planned a proper commercial launch, with a CEO that supported the extensive clinical study, has resulted in the fourth generation of Cologuard—an important breakthrough offering a very useful new standard of care in colon cancer detection and screening.
Continue to: Pearls for moving your innovations forward...
Pearls for moving your innovations forward
Because of my experience in undergoing health care start-ups, and contributing to several of those advancements of innovation, many inventors approach me for advice on their paths from idea to full-concept company. Here are a few of my lessons learned.
Consider purpose, not financial gain, first and foremost. Financial gain is typically the by-product or outcome of a standard-of-care breakthrough for inventors, but it’s a very hard road. Pursue your invention for advancing patient care and moving a new standard of care forward in health care versus financial gain at the end.
Determine whether your invention is a product or a company, or potentially, not capitalizable at all. Figure this out early. Analyze your idea to make sure it is sound and truly novel. Analyze the competition and to make sure it is sound and truly novel. Analyze the competition and the market dynamics to support a new product. Can the development path be defined very clearly to raise capital? Is your innovation a big enough breakthrough in the market with several current products to actually make a difference in patient outcomes (and eventually achieve product reimbursement)? The creation of a company may be the right strategy if the innovation can support a differentiated enough breakthrough where you can actually support all the infrastructure to build the business. If you find that the market is not there to support and develop your idea to eventual success, backing off early is important to preserve invested capital.
Protect early. Is your invention patentable, or has someone else already thought of the idea? What kind of patent(s) are appropriate? Where, geographically, do you want to protect your invention? Find a good patent attorney in your local area, early in the process, to help you answer all of these critical questions. Patents are expensive to file and maintain, but it is not expensive to do a literature search to find out if your idea is novel. A provisional patent, which would be your first step, is an important cost-effective step.
Capital is out there. If your invention or idea deserves capital, it is available. I will address raising capital in more detail in the next section.
Consider regulatory and manufacturing as achievable hurdles. Inventors often get tripped up here, considering the regulatory hurdles and manufacturing too challenging and abandoning their ideas because the risk is too great. Regulatory and manufacturing are very important aspects of health care standard-of-care builds. Cutting corners is not an option. That said, regulatory and manufacturing should not stop you. Challenges often can be worked through as long as the clinical need is there, and the clinical data support bringing that technology forward.
Consider corporate partnerships. I am a fan of corporate partners. But which ones should you target, and when and why? Corporate partnerships can bring significant capital, which is great, but there is enough investor capital out there that you should not pursue a corporate partner just for capital. The main benefit of a corporate partner is enterprise intellect. They typically know more about the field that you are entering than the investors or a small company leadership team.
Establish and listen to advisors. When thinking about who to trust, research their track record. Advisors who have gone through this process before, and specifically in your product area, are important to have access to.
Persistence is key. I have observed a tremendous “compression of innovation” in the health care areas that I have been involved with—human tissue-derived proteins, robotic surgery, stem cell therapy, and digital health (which is still in its infancy). For each of these breakthrough categories, early on, it appeared that it couldn’t be done. However, after the first 2 or 3 major breakthroughs in each one of these areas, a compression of innovation occurred. For instance, after approximately 15 years of protein development, we came out with the recombinant manufacturing systems for proteins. Very quickly, within 10 years, there were more than 70 proteins on the market. The persistence of the inventors to overcome early obstacles in each of these health care areas was critical to future success in each area.
Continue to: Raising capital...
Raising capital
There are different investors who specialize in different types of investment opportunities. The first phase of raising capital is the seed round—where there is typically early data, or even no data and just a concept. From this seed round forward, there is less risk as you develop your technology; thus, there are different investors that support different stages of development and that specialize in different types of investing. It is important to target the right investors and raise enough capital to be able to go achieve multiple operational milestones. Otherwise, when you go through your first round of capital, or the Series A or B financing rounds, there may not be a set of investors out there to fund the company moving forward. Health care investors will make it known that they invest in certain rounds of capital. You can determine who those investors are by doing a search online.
A mistake health care inventors can make is not taking enough capital from investors, because they are concerned about dilution. I advise investors not to focus on dilution but rather on, how big can you make “the pie” (value of the company) worth? The entire process is about bringing a true product through to a new standard-of-care curve.
Trust is the most important thing to earn with investors, and there is zero tolerance for a lack of trust. Share your vision as the inventor with investors, who want to know where this category could be in the next 5 or 10 years. Clinical data will always win, and health care investors and industry leaders should be focused on executing the most robust clinical data to demonstrate the clearest potential clinical outcome. Investors will follow a good plan that has been developed to achieve FDA approval, successful commercialization or “go to market” launch, and eventual reimbursement to support a true standard-of-care change.
Failure is defined by inaction
The 3 case studies that I have shared were success stories because the ideas and inventions were acted upon. When I was at Genzyme, we built the company up to more than $1 billion in revenue. We commercialized proteins in over 50 countries. Most importantly, many patients benefited from the innovation. If you have an invention and an idea, act on it—and surround yourself with great people in every discipline. Having the right people and team is extremely important. ●
How ObGyns can best work with radiologists to optimize screening for patients with dense breasts
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
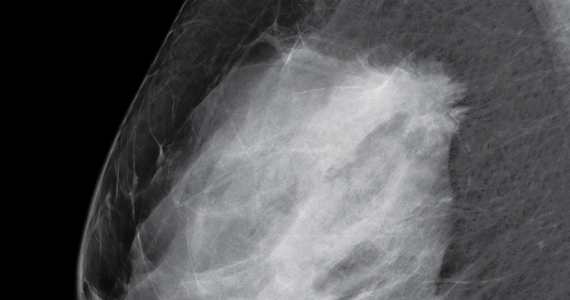
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.
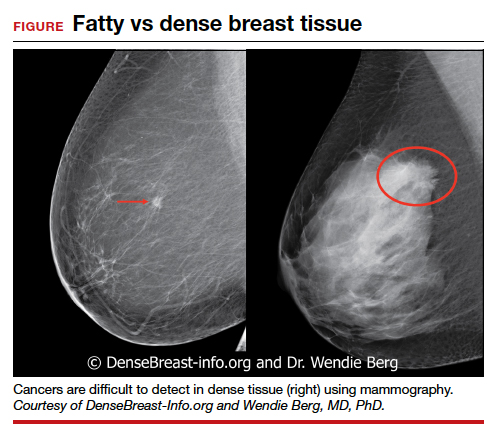
MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.

MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.

MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
Penicillin Allergy Delabeling Can Decrease Antibiotic Resistance, Reduce Costs, and Optimize Patient Outcomes
Antibiotics are one of the most frequently prescribed medications in both inpatient and outpatient settings.1,2 More than 266 million courses of antibiotics are prescribed annually in the outpatient setting; 49.9% of hospitalized patients were prescribed ≥ 1 antibiotic during their hospitalization.1,2 Among all classes of antibiotics, penicillins are prescribed due to their clinical efficacy, cost-effectiveness, and general safety for all ages. Unfortunately, penicillins also are the most common drug allergy listed in medical records. Patients with this allergy are consistently treated with broad-spectrum antibiotics, have more antibiotic resistant infections, incur higher health care costs, and experience more adverse effects (AEs).3,4
Drug allergies are distinguished by different immune mechanisms, including IgE-mediated reaction, T-lymphocyte-mediated mild skin reactions, and severe cutaneous adverse reactions (SCAR), or other systemic immune syndromes, such as hemolytic anemia, nephritis, and rash with eosinophilia.3 Although drug allergies should be a concern, compelling evidence shows that > 90% of patients labeled with a penicillin allergy are not allergic to penicillins (and associated β-lactams).3,4 Although this evidence is growing, clinicians still hesitate to prescribe penicillin, and patients are similarly anxious to take them. This article reviews the health care consequences of penicillin allergy and the application of this information to military medicine and readiness.
Penicillin Allergy Prevalence
Since their approval for public use in 1945, penicillins have been one of the most often prescribed antibiotics due to their clinical efficacy for many types of infections.3 However, 8 to 10% of the US population and up to 15% of hospitalized patients have a documented penicillin allergy, which limits the ability to use these effective antibiotics.3,4 Once a patient is labeled with a penicillin allergy, many clinicians avoid prescribing all β-lactam antibiotics to patients. Clinicians also avoid prescribing cephalosporins due to the concern for potential cross-reactivity (at a rate of about 2%, which is lower than previously reported).3 These reported allergies are often not clear and range from patients avoiding penicillins because their parents exhibited allergies, they had a symptom that was not likely allergic (ie, nausea, headache, itching with no rash), being told by their parents that they had a rash as a child, or experiencing severe anaphylaxis or other systemic reaction.3,4 Despite the high rates of documented penicillin allergy, studies now show that most patients do not have a serious allergy; < 1% of the population has a true immune-mediated penicillin allergy.3,4
Broad-Spectrum Antibiotic Risks
Even though penicillin allergies are often not confirmed, many patients are treated with alternative antibiotics. Unfortunately, most alternative antibiotics are not as effective or as safe as penicillin.3,4 Twenty percent of hospitalized patients will experience an AE related to their antibiotic; 19.3% of emergency department visits for adverse drug reactions (ADRs) are from antibiotics.5,6 Sulfonamides, clindamycin, and quinolones were the antibiotics most commonly associated with AEs.6
In a large database study over a 3-year period, > 400,000 hospitalizations were analyzed in patients matched for admission type, with and without a penicillin allergy in their medical record.7 Those with a documented penicillin allergy had longer hospitalizations; were treated with broad-spectrum antibiotics; and had increased rates of Clostridium difficile (C difficile), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE).7,8 In addition to being first-line treatment for many common infections, penicillins often are used for dental, perinatal, and perioperative prophylaxis.1,3 Nearly 25 million antibiotics are prescribed annually by dentists.1 If a patient has a penicillin allergy listed in their medical record, they will inevitably receive a second- or third-line treatment that is less effective and has higher risks. Common alternative antibiotics include clindamycin, fluoroquinolones, macrolides, and vancomycin.3,7,8
Clindamycin and fluoroquinolones are associated with C difficile infections.9,10 Fluoroquinolones come with a boxed warning for known serious ADRs, including tendon rupture, peripheral neuropathy, central nervous system effects, and are known for causing cardiac reactions such as QT prolongation, life-threatening arrhythmias, and cardiovascular death.11,12 Fluoroquinolones are associated with an increased risk for VRE and MRSA, in more than any other antibiotic classes.3,7,12,13
Macrolides, such as azithromycin and clarithromycin, are another common class of antibiotics used as an alternative for penicillins. Both are used frequently for upper respiratory infections. Known ADRs to macrolides include gastrointestinal adverse effects (AEs) (ie, nausea, vomiting, diarrhea, and abdominal pain), liver toxicity (ie, abnormal liver function tests, hepatitis, and liver failure), and cardiac risks (ie, QT prolongation and sudden death). When compared with amoxicillin, there was an increased risk for cardiovascular mortality in those patients receiving macrolides.14,15
Vancomycin is known for its potential to cause “red man syndrome,” an infusion-related reaction causing redness and itching as well as nephrotoxic and hematologic effects requiring close monitoring.3 Vancomycin is less effective than methicillin in clearing MRSA or other sensitive pathogens; however, vancomycin is used in patients with a penicillin allergy label.16-18 Intrapartum antibiotic use of vancomycin for group B streptococcus infection was associated with clinically significant morbidity and ADRs.19,20 Perioperatively, patients with penicillin allergies developed more surgical site infections due to the use of second-line antibiotics, such as vancomycin or others.21
Cost of Penicillin Allergies
Penicillin allergy plays an important role in rising health care costs. In 2017, health care spending reached 17.9% of the gross domestic product.22 Macy and Contreras demonstrated the significantly higher costs associated with having a reported (and unverified) penicillin allergy in a matched cohort study. Inferred for the extra hospital use, the penicillin allergy group cost the health care system $64,626,630 more than for the group who did not have a penicillin allergy label.7 A subsequent study by Macy and Contreras of both inpatient and outpatient settings showed a potential savings of $2,000 per patient per year in health care expenses with the testing and delabeling of penicillin allergies.23 Use of newer and broad-spectrum antibiotics also are more costly and contribute to higher health care costs.24
When these potential savings are applied to the military insurance population of 9.4 million beneficiaries (TRICARE, including active duty, their dependents, and all retirees participating in the program), the results showed that this could impart a savings of nearly $1.7 billion annually, using the model by Macy and Contreras.23,25,26
Previously with colleagues, I reviewed penicillin’s role in military history, compiled data from relevant studies from military penicillin allergy rates and delabeling efforts, and calculated the potential economic impact of penicillin allergies along with the benefits of testing.26 Calculations were estimated using the TRICARE beneficiary population (9.4 million) × the estimated prevalence (10%) to get an estimate of 940,000 TRICARE patients with penicillin allergy in their medical record.25 If 90% of those patients were delabeled, this would equal 846,000 TRICARE patients. When multiplied by the potential savings of $2,000 per patient per year, the estimated savings would be $1,692,000,000 annually.23,26
Current literature provides compelling evidence that all health care plans should use penicillin allergy testing and delabeling programs.3,23,26 As most patients with a history of penicillin allergy in their medical records do not have a verified allergy, delabeling those who do not have a true allergy will have individual, public health, and cost benefits.3,7,23,26
Antibiotic Stewardship
Antibiotic stewardship programs are now mandated to combat antibiotic resistance.3,27 This program is supported by major medical organizations, including the Centers for Disease Control and Prevention, Society for Healthcare Epidemiology of America, Infectious Disease Society of America, and the American Academy of Allergy Asthma and Immunology.3 Given the role of broad-spectrum antibiotics in antibiotic resistance, penicillin allergy testing and delabeling is an important component of these programs.3
In the US, > 2 million people acquire antibiotic resistant infections annually; 23,000 people die of these infections.27 More than 250,000 illnesses and 14,000 deaths annually are due to C difficile.27 There are many factors contributing to the increase in antibiotic resistance; however, one established and consistent factor is the use of broad-spectrum antibiotics. Further, broad-spectrum antibiotics are often used when first-line agents, such as penicillins, cannot be used due to a reported “allergy.” In addition, there are fewer novel antibiotics being developed, and as they are introduced, pathogens develop resistance to these new agents.27
Military Relevance
Infectious diseases have always accompanied military activity.28-30 Despite preventive programs such as vaccinations, hygiene measures, and prophylactic antibiotics, military personnel are at increased risk for infections due to the military’s mobile nature and crowded living situations.28-30 This situation has operational relevance from basic training, deployments, and combat operations to peacetime activities.
Military recruits are treated routinely with penicillin G benzathine as standard prophylaxis against streptococcal infections.26,30 A recent study by the Marine Corps Recruiting Depot in San Diego, California showed that in a cohort of 402 young healthy male recruits, only 5 (1.5%) had a positive reaction to penicillin testing and challenge over a 21-month period.31 The delabeled other 397 (98.5%) marine recruits were able to receive benzathine penicillin prophylaxis successfully.31 Recruits with a penicillin allergy who had a positive test or were not tested received azithromycin (or erythromycin at some recruit training locations).26,31 Military members may need to operate in remote or austere locations; the ability to use penicillins is important for readiness.
Evaluation and Management of Reported Penicillin Allergy
Verifying penicillin allergies is an important first step in optimizing medical care and decreasing resistance and ADRs.3,4,32,33 Although allergists can provide specialized evaluation, due to the high prevalence of penicillin allergy in the US, all health care team members, including clinicians and pharmacists, should be educated about penicillin allergies and be able to implement evaluations in both inpatient and outpatient settings. Reactions to any of the penicillins should be considered, including the natural penicillins (penicillin V, etc), antistaphylococcal penicillins (dicloxacillin), aminopenicillins (amoxicillin and ampicillin), and extended-spectrum penicillins (piperacillin).3 A thorough history, including the prior reaction (age, type of reaction) and subsequent tolerance are helpful in stratifying patients.3,26
Patient Risk Levels
Based on the clinical history, patients would fall into 4 categories from low risk, medium risk, high risk, to do not test/use.3,32,33 Low-risk patients are those who report mild or nonallergic symptoms (ie, gastrointestinal symptoms, headache, yeast infection, etc), remote cutaneous reactions (> 10 years), or in those with a family history of penicillin allergy.3,32,33 Low-risk patients often can be safely tested with an oral challenge. Although there are different approaches to the oral challenge, a single amoxicillin dose of 250 mg followed by 1 hour of direct monitoring is usually sufficient.3,32,33
Medium-risk patients have a more recent (< 1 year) history of pruritic rashes, urticaria, and/or angioedema without a history of severe or systemic reactions. These patients benefit from negative skin testing prior to an oral challenge, which can be performed by trained clinicians or pharmacists or an allergist. However, due to limited availability of skin testing and the potential for false positive testing with skin tests, a single dose or graded challenge would be a reasonable approach as well.3,32,33
High-risk patients are those with severe symptoms (anaphylaxis), a history of reactions to other β-lactam antibiotics, and/or recurrent reactions to antibiotics. These patients benefit from a formal evaluation by an allergist and skin testing prior to challenge.3,32,33 Testing and/or challenge should not be performed in patients who report a history of severe cutaneous reactions (blistering rash, such as Stevens Johnson syndrome), hemolytic anemia, serum sickness, drug fever, and other organ dysfunction.3,4,31,32
The Figure describes a published questionnaire, personnel, resources, and procedures for penicillin delabeling.26 Although skin testing is reliable in revealing a immunoglobulin E-mediated penicillin allergy, there is potential for false positives.32,33 The oral amoxicillin challenge effectively clears the patient for future penicillin administration.3,32-34 In high-risk patients, desensitization should be considered if penicillins (or cephalosporins) are required as first-line treatment. A test dose (one-tenth dose, higher or lower depending on route of administration, historic reaction, clinical status, and level of certainty of prior reaction) may be considered in low- to moderate-risk patients, depending on the indication for the use of the antibiotics.32
Penicillin evaluation pathways can occur in both inpatient and outpatient settings where antibiotics will be prescribed.32-34 There are several proposed pathways, including a screening questionnaire to determine the penicillin allergy risk.26,32,33 Implementation of perioperative testing has been successful in decreasing the rates of vancomycin use and lessening the morbidity associated with use of second-line antibiotics.35 Many hospitals throughout the country have implemented standardized penicillin delabeling programs.3,32-34
Conclusions
Penicillin allergies are an important barrier to effective antibiotic treatments and are associated with worse outcomes and higher economic costs.3,7,23,26,34 Therefore, in addition to vaccinations, infection control measures, and public health education, penicillin allergy verification and delabeling programs should be a proactive component of military medical readiness and all antibiotic stewardship initiatives in all health care settings.29 Given the many issues and negative impact of having a penicillin allergy label, penicillin delabeling will allow service members to be treated with the necessary antibiotics with fewer adverse complications, and return them to health and readiness for operational duties. In the current standardization of the Defense Health Agency, implementing this program across all services would have significant clinical, public health, and cost benefits for patients, the health care team, taxpayers, and the community at large.
Many patients report an allergy to penicillin, but only a small portion have a current true immune-mediated allergy. Given the clinical, public health, and economic costs associated with a penicillin allergy label, evaluation and clearance of penicillin allergies is a simple method that would improve clinical outcomes, decrease AEs to high-risk alternative broad-spectrum antibiotics, and prevent the spread of antibiotic resistance. In the military, penicillin delabeling improves readiness with optimal antibiotic options and avoidance of unnecessary risks of using alternative antibiotics, expediting return to full duty for military personnel.
1. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions-United States, 2014. https://www.cdc.gov/antibiotic-use/community/pdfs/annual-reportsummary_2014.pdf. Accessed August 15, 2020.
2. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. doi:10.1001/jama.2014.12923
3. Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy. JAMA. 2019;321:188-199. doi:10.1001/jama.2018.19283
4. Har D, Solensky R. Penicillin and beta-lactam hypersensitivity. Immunol Allergy Clin North Am. 2017;37(4):643-662. doi:10.1016/j.iac.2017.07.001
5. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308-1315. doi:10.1001/jamainternmed.2017.1938
6. Shebab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735-743. doi:10.1086/591126
7. Macy E, Contreras R. Healthcare use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
8. Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of methicillin resistant Staphylococcal aureus and Clostridium difficile in patients with a documented penicillin allergy: population-based matched cohort study. BMJ. 2018;361:k2400. doi:10.1136/bmj.k2400
9. Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442-2449. doi:10.1056/NEJMoa051639
10. Pepin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolone as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
11. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infec Dis. 2015;60(4):566-577. doi:10.1093/cid/ciu914
12. Rao GA, Mann JR, Shoaibi A, et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med. 2014;12(2):121-127. doi:10.1370/afm.1601
13. LeBlanc L, Pepin J, Toulouse K, et al. Fluoroquinolone and risk for methicillin-resistant Staphylococcus aureus, Canada. Emerg Infect Dis. 2006;12(9):1398-1405. doi:10.3201/eid1209.060397
14. Schembri S, Williamson PA, Short PM, et al. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. BMJ. 2013;346:f1245. doi:10.1136/bmj.f1235
15. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881-1890. doi:10.1056/NEJMoa1003833
16. McDaniel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61(3):361-367. doi:10.1093/cid/civ308
17. Wong D, Wong T, Romney M, Leung V. Comparison of outcomes in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia who are treated with β-lactam vs vancomycin empiric therapy: a retrospective cohort study. BMC Infect Dis. 2016;16:224. doi:10.1186/s12879-016-1564-5
18. Blumenthal KG, Shenoy ES, Huang M, et al. The impact of reporting a prior penicillin allergy on the treatment of methicillin-sensitivity Staphylococcus aureus bacteremia. PLoS One. 2016;11(7):e0159406. doi:10.1371/journal.pone.0159406
19. Verani JR, McGee L, Schrag SJ; . Prevention of perinatal group B streptococcal disease. MMWR Recomm Rep. 2010;59(RR-10):1-36.
20. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21:16-080. doi:10.7812/TPP/16-080
21. Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66(3):329-336. doi:10.1093/cid/cix794
22. National Health Expenditures 2017 Highlights. Centers for Medicare & Medicaid services. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf. Accessed August 25, 2020.
23. Macy E, Shu YH. The effect of penicillin allergy testing on future health care utilization: a matched cohort study. J Allergy Clin Immunol Pract. 2017;5(3):705-710. doi:10.1016/j.jaip.2017.02.012
24. Picard M, Begin P, Bouchard H, et al. Treatment of patients with a history of penicillin allergy in a large tertiary-care academic hospital. J Allergy Clin Immunol Pract. 2013;1(3):252-257. doi:10.1016/j.jaip.2013.01.006
25. US Department of Defense. Beneficiary population statistics. https://health.mil/I-Am-A/Media/Media-Center/Patient-Population-Statistics. Accessed August 25, 2020.
26. Lee RU, Banks TA, Waibel KH, Rodriguez RG. Penicillin allergy…maybe not? The military relevance for penicillin testing and de-labeling. Mil Med. 2019;184(3-4):e163-e168. doi:10.1093/milmed/usy194
27. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention. https://www.cdc.gov/drugresistance/threat-report-2013/index.html. Accessed May 10, 2019.
28. Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999;5(3):379-385. doi:10.3201/eid0503.990308
29. Beaumier CM, Gomez-Rubio AM, Hotez PJ, Weina PJ. United States military tropical medicine: extraordinary legacy, uncertain future. PLoS Negl Trop Dis. 2013;7(12):e2448. doi:10.1371/journal.pntd.0002448
30. Thomas RJ, Conwill DE, Morton DE, et al. Penicillin prophylaxis for streptococcal infections in the United States Navy and Marine Corps recruit camps, 1951-1985. Rev Infect Dis. 1988;10(1):125-130. doi:10.1093/clinids/10.1.125
31. Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
32. Blumenthal KG, Shenoy ES, Wolfson AR, et al. Addressing inpatient beta-lactam allergies: a multihospital implementation. J Allergy Clin Immunol Pract. 2017;5(3):616-625. doi:10.1016/j.jaip.2017.02.019
33. Kuruvilla M, Shih J, Patel K, Scanlon N. Direct oral amoxicillin challenge without preliminary skin testing in adult patients with allergy and at low risk with reported penicillin allergy. Allergy Asthma Proc. 2019;40(1):57-61. doi:10.2500/aap.2019.40.4184
34. Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
35. Park M, Markus P, Matesic
Antibiotics are one of the most frequently prescribed medications in both inpatient and outpatient settings.1,2 More than 266 million courses of antibiotics are prescribed annually in the outpatient setting; 49.9% of hospitalized patients were prescribed ≥ 1 antibiotic during their hospitalization.1,2 Among all classes of antibiotics, penicillins are prescribed due to their clinical efficacy, cost-effectiveness, and general safety for all ages. Unfortunately, penicillins also are the most common drug allergy listed in medical records. Patients with this allergy are consistently treated with broad-spectrum antibiotics, have more antibiotic resistant infections, incur higher health care costs, and experience more adverse effects (AEs).3,4
Drug allergies are distinguished by different immune mechanisms, including IgE-mediated reaction, T-lymphocyte-mediated mild skin reactions, and severe cutaneous adverse reactions (SCAR), or other systemic immune syndromes, such as hemolytic anemia, nephritis, and rash with eosinophilia.3 Although drug allergies should be a concern, compelling evidence shows that > 90% of patients labeled with a penicillin allergy are not allergic to penicillins (and associated β-lactams).3,4 Although this evidence is growing, clinicians still hesitate to prescribe penicillin, and patients are similarly anxious to take them. This article reviews the health care consequences of penicillin allergy and the application of this information to military medicine and readiness.
Penicillin Allergy Prevalence
Since their approval for public use in 1945, penicillins have been one of the most often prescribed antibiotics due to their clinical efficacy for many types of infections.3 However, 8 to 10% of the US population and up to 15% of hospitalized patients have a documented penicillin allergy, which limits the ability to use these effective antibiotics.3,4 Once a patient is labeled with a penicillin allergy, many clinicians avoid prescribing all β-lactam antibiotics to patients. Clinicians also avoid prescribing cephalosporins due to the concern for potential cross-reactivity (at a rate of about 2%, which is lower than previously reported).3 These reported allergies are often not clear and range from patients avoiding penicillins because their parents exhibited allergies, they had a symptom that was not likely allergic (ie, nausea, headache, itching with no rash), being told by their parents that they had a rash as a child, or experiencing severe anaphylaxis or other systemic reaction.3,4 Despite the high rates of documented penicillin allergy, studies now show that most patients do not have a serious allergy; < 1% of the population has a true immune-mediated penicillin allergy.3,4
Broad-Spectrum Antibiotic Risks
Even though penicillin allergies are often not confirmed, many patients are treated with alternative antibiotics. Unfortunately, most alternative antibiotics are not as effective or as safe as penicillin.3,4 Twenty percent of hospitalized patients will experience an AE related to their antibiotic; 19.3% of emergency department visits for adverse drug reactions (ADRs) are from antibiotics.5,6 Sulfonamides, clindamycin, and quinolones were the antibiotics most commonly associated with AEs.6
In a large database study over a 3-year period, > 400,000 hospitalizations were analyzed in patients matched for admission type, with and without a penicillin allergy in their medical record.7 Those with a documented penicillin allergy had longer hospitalizations; were treated with broad-spectrum antibiotics; and had increased rates of Clostridium difficile (C difficile), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE).7,8 In addition to being first-line treatment for many common infections, penicillins often are used for dental, perinatal, and perioperative prophylaxis.1,3 Nearly 25 million antibiotics are prescribed annually by dentists.1 If a patient has a penicillin allergy listed in their medical record, they will inevitably receive a second- or third-line treatment that is less effective and has higher risks. Common alternative antibiotics include clindamycin, fluoroquinolones, macrolides, and vancomycin.3,7,8
Clindamycin and fluoroquinolones are associated with C difficile infections.9,10 Fluoroquinolones come with a boxed warning for known serious ADRs, including tendon rupture, peripheral neuropathy, central nervous system effects, and are known for causing cardiac reactions such as QT prolongation, life-threatening arrhythmias, and cardiovascular death.11,12 Fluoroquinolones are associated with an increased risk for VRE and MRSA, in more than any other antibiotic classes.3,7,12,13
Macrolides, such as azithromycin and clarithromycin, are another common class of antibiotics used as an alternative for penicillins. Both are used frequently for upper respiratory infections. Known ADRs to macrolides include gastrointestinal adverse effects (AEs) (ie, nausea, vomiting, diarrhea, and abdominal pain), liver toxicity (ie, abnormal liver function tests, hepatitis, and liver failure), and cardiac risks (ie, QT prolongation and sudden death). When compared with amoxicillin, there was an increased risk for cardiovascular mortality in those patients receiving macrolides.14,15
Vancomycin is known for its potential to cause “red man syndrome,” an infusion-related reaction causing redness and itching as well as nephrotoxic and hematologic effects requiring close monitoring.3 Vancomycin is less effective than methicillin in clearing MRSA or other sensitive pathogens; however, vancomycin is used in patients with a penicillin allergy label.16-18 Intrapartum antibiotic use of vancomycin for group B streptococcus infection was associated with clinically significant morbidity and ADRs.19,20 Perioperatively, patients with penicillin allergies developed more surgical site infections due to the use of second-line antibiotics, such as vancomycin or others.21
Cost of Penicillin Allergies
Penicillin allergy plays an important role in rising health care costs. In 2017, health care spending reached 17.9% of the gross domestic product.22 Macy and Contreras demonstrated the significantly higher costs associated with having a reported (and unverified) penicillin allergy in a matched cohort study. Inferred for the extra hospital use, the penicillin allergy group cost the health care system $64,626,630 more than for the group who did not have a penicillin allergy label.7 A subsequent study by Macy and Contreras of both inpatient and outpatient settings showed a potential savings of $2,000 per patient per year in health care expenses with the testing and delabeling of penicillin allergies.23 Use of newer and broad-spectrum antibiotics also are more costly and contribute to higher health care costs.24
When these potential savings are applied to the military insurance population of 9.4 million beneficiaries (TRICARE, including active duty, their dependents, and all retirees participating in the program), the results showed that this could impart a savings of nearly $1.7 billion annually, using the model by Macy and Contreras.23,25,26
Previously with colleagues, I reviewed penicillin’s role in military history, compiled data from relevant studies from military penicillin allergy rates and delabeling efforts, and calculated the potential economic impact of penicillin allergies along with the benefits of testing.26 Calculations were estimated using the TRICARE beneficiary population (9.4 million) × the estimated prevalence (10%) to get an estimate of 940,000 TRICARE patients with penicillin allergy in their medical record.25 If 90% of those patients were delabeled, this would equal 846,000 TRICARE patients. When multiplied by the potential savings of $2,000 per patient per year, the estimated savings would be $1,692,000,000 annually.23,26
Current literature provides compelling evidence that all health care plans should use penicillin allergy testing and delabeling programs.3,23,26 As most patients with a history of penicillin allergy in their medical records do not have a verified allergy, delabeling those who do not have a true allergy will have individual, public health, and cost benefits.3,7,23,26
Antibiotic Stewardship
Antibiotic stewardship programs are now mandated to combat antibiotic resistance.3,27 This program is supported by major medical organizations, including the Centers for Disease Control and Prevention, Society for Healthcare Epidemiology of America, Infectious Disease Society of America, and the American Academy of Allergy Asthma and Immunology.3 Given the role of broad-spectrum antibiotics in antibiotic resistance, penicillin allergy testing and delabeling is an important component of these programs.3
In the US, > 2 million people acquire antibiotic resistant infections annually; 23,000 people die of these infections.27 More than 250,000 illnesses and 14,000 deaths annually are due to C difficile.27 There are many factors contributing to the increase in antibiotic resistance; however, one established and consistent factor is the use of broad-spectrum antibiotics. Further, broad-spectrum antibiotics are often used when first-line agents, such as penicillins, cannot be used due to a reported “allergy.” In addition, there are fewer novel antibiotics being developed, and as they are introduced, pathogens develop resistance to these new agents.27
Military Relevance
Infectious diseases have always accompanied military activity.28-30 Despite preventive programs such as vaccinations, hygiene measures, and prophylactic antibiotics, military personnel are at increased risk for infections due to the military’s mobile nature and crowded living situations.28-30 This situation has operational relevance from basic training, deployments, and combat operations to peacetime activities.
Military recruits are treated routinely with penicillin G benzathine as standard prophylaxis against streptococcal infections.26,30 A recent study by the Marine Corps Recruiting Depot in San Diego, California showed that in a cohort of 402 young healthy male recruits, only 5 (1.5%) had a positive reaction to penicillin testing and challenge over a 21-month period.31 The delabeled other 397 (98.5%) marine recruits were able to receive benzathine penicillin prophylaxis successfully.31 Recruits with a penicillin allergy who had a positive test or were not tested received azithromycin (or erythromycin at some recruit training locations).26,31 Military members may need to operate in remote or austere locations; the ability to use penicillins is important for readiness.
Evaluation and Management of Reported Penicillin Allergy
Verifying penicillin allergies is an important first step in optimizing medical care and decreasing resistance and ADRs.3,4,32,33 Although allergists can provide specialized evaluation, due to the high prevalence of penicillin allergy in the US, all health care team members, including clinicians and pharmacists, should be educated about penicillin allergies and be able to implement evaluations in both inpatient and outpatient settings. Reactions to any of the penicillins should be considered, including the natural penicillins (penicillin V, etc), antistaphylococcal penicillins (dicloxacillin), aminopenicillins (amoxicillin and ampicillin), and extended-spectrum penicillins (piperacillin).3 A thorough history, including the prior reaction (age, type of reaction) and subsequent tolerance are helpful in stratifying patients.3,26
Patient Risk Levels
Based on the clinical history, patients would fall into 4 categories from low risk, medium risk, high risk, to do not test/use.3,32,33 Low-risk patients are those who report mild or nonallergic symptoms (ie, gastrointestinal symptoms, headache, yeast infection, etc), remote cutaneous reactions (> 10 years), or in those with a family history of penicillin allergy.3,32,33 Low-risk patients often can be safely tested with an oral challenge. Although there are different approaches to the oral challenge, a single amoxicillin dose of 250 mg followed by 1 hour of direct monitoring is usually sufficient.3,32,33
Medium-risk patients have a more recent (< 1 year) history of pruritic rashes, urticaria, and/or angioedema without a history of severe or systemic reactions. These patients benefit from negative skin testing prior to an oral challenge, which can be performed by trained clinicians or pharmacists or an allergist. However, due to limited availability of skin testing and the potential for false positive testing with skin tests, a single dose or graded challenge would be a reasonable approach as well.3,32,33
High-risk patients are those with severe symptoms (anaphylaxis), a history of reactions to other β-lactam antibiotics, and/or recurrent reactions to antibiotics. These patients benefit from a formal evaluation by an allergist and skin testing prior to challenge.3,32,33 Testing and/or challenge should not be performed in patients who report a history of severe cutaneous reactions (blistering rash, such as Stevens Johnson syndrome), hemolytic anemia, serum sickness, drug fever, and other organ dysfunction.3,4,31,32
The Figure describes a published questionnaire, personnel, resources, and procedures for penicillin delabeling.26 Although skin testing is reliable in revealing a immunoglobulin E-mediated penicillin allergy, there is potential for false positives.32,33 The oral amoxicillin challenge effectively clears the patient for future penicillin administration.3,32-34 In high-risk patients, desensitization should be considered if penicillins (or cephalosporins) are required as first-line treatment. A test dose (one-tenth dose, higher or lower depending on route of administration, historic reaction, clinical status, and level of certainty of prior reaction) may be considered in low- to moderate-risk patients, depending on the indication for the use of the antibiotics.32
Penicillin evaluation pathways can occur in both inpatient and outpatient settings where antibiotics will be prescribed.32-34 There are several proposed pathways, including a screening questionnaire to determine the penicillin allergy risk.26,32,33 Implementation of perioperative testing has been successful in decreasing the rates of vancomycin use and lessening the morbidity associated with use of second-line antibiotics.35 Many hospitals throughout the country have implemented standardized penicillin delabeling programs.3,32-34
Conclusions
Penicillin allergies are an important barrier to effective antibiotic treatments and are associated with worse outcomes and higher economic costs.3,7,23,26,34 Therefore, in addition to vaccinations, infection control measures, and public health education, penicillin allergy verification and delabeling programs should be a proactive component of military medical readiness and all antibiotic stewardship initiatives in all health care settings.29 Given the many issues and negative impact of having a penicillin allergy label, penicillin delabeling will allow service members to be treated with the necessary antibiotics with fewer adverse complications, and return them to health and readiness for operational duties. In the current standardization of the Defense Health Agency, implementing this program across all services would have significant clinical, public health, and cost benefits for patients, the health care team, taxpayers, and the community at large.
Many patients report an allergy to penicillin, but only a small portion have a current true immune-mediated allergy. Given the clinical, public health, and economic costs associated with a penicillin allergy label, evaluation and clearance of penicillin allergies is a simple method that would improve clinical outcomes, decrease AEs to high-risk alternative broad-spectrum antibiotics, and prevent the spread of antibiotic resistance. In the military, penicillin delabeling improves readiness with optimal antibiotic options and avoidance of unnecessary risks of using alternative antibiotics, expediting return to full duty for military personnel.
Antibiotics are one of the most frequently prescribed medications in both inpatient and outpatient settings.1,2 More than 266 million courses of antibiotics are prescribed annually in the outpatient setting; 49.9% of hospitalized patients were prescribed ≥ 1 antibiotic during their hospitalization.1,2 Among all classes of antibiotics, penicillins are prescribed due to their clinical efficacy, cost-effectiveness, and general safety for all ages. Unfortunately, penicillins also are the most common drug allergy listed in medical records. Patients with this allergy are consistently treated with broad-spectrum antibiotics, have more antibiotic resistant infections, incur higher health care costs, and experience more adverse effects (AEs).3,4
Drug allergies are distinguished by different immune mechanisms, including IgE-mediated reaction, T-lymphocyte-mediated mild skin reactions, and severe cutaneous adverse reactions (SCAR), or other systemic immune syndromes, such as hemolytic anemia, nephritis, and rash with eosinophilia.3 Although drug allergies should be a concern, compelling evidence shows that > 90% of patients labeled with a penicillin allergy are not allergic to penicillins (and associated β-lactams).3,4 Although this evidence is growing, clinicians still hesitate to prescribe penicillin, and patients are similarly anxious to take them. This article reviews the health care consequences of penicillin allergy and the application of this information to military medicine and readiness.
Penicillin Allergy Prevalence
Since their approval for public use in 1945, penicillins have been one of the most often prescribed antibiotics due to their clinical efficacy for many types of infections.3 However, 8 to 10% of the US population and up to 15% of hospitalized patients have a documented penicillin allergy, which limits the ability to use these effective antibiotics.3,4 Once a patient is labeled with a penicillin allergy, many clinicians avoid prescribing all β-lactam antibiotics to patients. Clinicians also avoid prescribing cephalosporins due to the concern for potential cross-reactivity (at a rate of about 2%, which is lower than previously reported).3 These reported allergies are often not clear and range from patients avoiding penicillins because their parents exhibited allergies, they had a symptom that was not likely allergic (ie, nausea, headache, itching with no rash), being told by their parents that they had a rash as a child, or experiencing severe anaphylaxis or other systemic reaction.3,4 Despite the high rates of documented penicillin allergy, studies now show that most patients do not have a serious allergy; < 1% of the population has a true immune-mediated penicillin allergy.3,4
Broad-Spectrum Antibiotic Risks
Even though penicillin allergies are often not confirmed, many patients are treated with alternative antibiotics. Unfortunately, most alternative antibiotics are not as effective or as safe as penicillin.3,4 Twenty percent of hospitalized patients will experience an AE related to their antibiotic; 19.3% of emergency department visits for adverse drug reactions (ADRs) are from antibiotics.5,6 Sulfonamides, clindamycin, and quinolones were the antibiotics most commonly associated with AEs.6
In a large database study over a 3-year period, > 400,000 hospitalizations were analyzed in patients matched for admission type, with and without a penicillin allergy in their medical record.7 Those with a documented penicillin allergy had longer hospitalizations; were treated with broad-spectrum antibiotics; and had increased rates of Clostridium difficile (C difficile), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE).7,8 In addition to being first-line treatment for many common infections, penicillins often are used for dental, perinatal, and perioperative prophylaxis.1,3 Nearly 25 million antibiotics are prescribed annually by dentists.1 If a patient has a penicillin allergy listed in their medical record, they will inevitably receive a second- or third-line treatment that is less effective and has higher risks. Common alternative antibiotics include clindamycin, fluoroquinolones, macrolides, and vancomycin.3,7,8
Clindamycin and fluoroquinolones are associated with C difficile infections.9,10 Fluoroquinolones come with a boxed warning for known serious ADRs, including tendon rupture, peripheral neuropathy, central nervous system effects, and are known for causing cardiac reactions such as QT prolongation, life-threatening arrhythmias, and cardiovascular death.11,12 Fluoroquinolones are associated with an increased risk for VRE and MRSA, in more than any other antibiotic classes.3,7,12,13
Macrolides, such as azithromycin and clarithromycin, are another common class of antibiotics used as an alternative for penicillins. Both are used frequently for upper respiratory infections. Known ADRs to macrolides include gastrointestinal adverse effects (AEs) (ie, nausea, vomiting, diarrhea, and abdominal pain), liver toxicity (ie, abnormal liver function tests, hepatitis, and liver failure), and cardiac risks (ie, QT prolongation and sudden death). When compared with amoxicillin, there was an increased risk for cardiovascular mortality in those patients receiving macrolides.14,15
Vancomycin is known for its potential to cause “red man syndrome,” an infusion-related reaction causing redness and itching as well as nephrotoxic and hematologic effects requiring close monitoring.3 Vancomycin is less effective than methicillin in clearing MRSA or other sensitive pathogens; however, vancomycin is used in patients with a penicillin allergy label.16-18 Intrapartum antibiotic use of vancomycin for group B streptococcus infection was associated with clinically significant morbidity and ADRs.19,20 Perioperatively, patients with penicillin allergies developed more surgical site infections due to the use of second-line antibiotics, such as vancomycin or others.21
Cost of Penicillin Allergies
Penicillin allergy plays an important role in rising health care costs. In 2017, health care spending reached 17.9% of the gross domestic product.22 Macy and Contreras demonstrated the significantly higher costs associated with having a reported (and unverified) penicillin allergy in a matched cohort study. Inferred for the extra hospital use, the penicillin allergy group cost the health care system $64,626,630 more than for the group who did not have a penicillin allergy label.7 A subsequent study by Macy and Contreras of both inpatient and outpatient settings showed a potential savings of $2,000 per patient per year in health care expenses with the testing and delabeling of penicillin allergies.23 Use of newer and broad-spectrum antibiotics also are more costly and contribute to higher health care costs.24
When these potential savings are applied to the military insurance population of 9.4 million beneficiaries (TRICARE, including active duty, their dependents, and all retirees participating in the program), the results showed that this could impart a savings of nearly $1.7 billion annually, using the model by Macy and Contreras.23,25,26
Previously with colleagues, I reviewed penicillin’s role in military history, compiled data from relevant studies from military penicillin allergy rates and delabeling efforts, and calculated the potential economic impact of penicillin allergies along with the benefits of testing.26 Calculations were estimated using the TRICARE beneficiary population (9.4 million) × the estimated prevalence (10%) to get an estimate of 940,000 TRICARE patients with penicillin allergy in their medical record.25 If 90% of those patients were delabeled, this would equal 846,000 TRICARE patients. When multiplied by the potential savings of $2,000 per patient per year, the estimated savings would be $1,692,000,000 annually.23,26
Current literature provides compelling evidence that all health care plans should use penicillin allergy testing and delabeling programs.3,23,26 As most patients with a history of penicillin allergy in their medical records do not have a verified allergy, delabeling those who do not have a true allergy will have individual, public health, and cost benefits.3,7,23,26
Antibiotic Stewardship
Antibiotic stewardship programs are now mandated to combat antibiotic resistance.3,27 This program is supported by major medical organizations, including the Centers for Disease Control and Prevention, Society for Healthcare Epidemiology of America, Infectious Disease Society of America, and the American Academy of Allergy Asthma and Immunology.3 Given the role of broad-spectrum antibiotics in antibiotic resistance, penicillin allergy testing and delabeling is an important component of these programs.3
In the US, > 2 million people acquire antibiotic resistant infections annually; 23,000 people die of these infections.27 More than 250,000 illnesses and 14,000 deaths annually are due to C difficile.27 There are many factors contributing to the increase in antibiotic resistance; however, one established and consistent factor is the use of broad-spectrum antibiotics. Further, broad-spectrum antibiotics are often used when first-line agents, such as penicillins, cannot be used due to a reported “allergy.” In addition, there are fewer novel antibiotics being developed, and as they are introduced, pathogens develop resistance to these new agents.27
Military Relevance
Infectious diseases have always accompanied military activity.28-30 Despite preventive programs such as vaccinations, hygiene measures, and prophylactic antibiotics, military personnel are at increased risk for infections due to the military’s mobile nature and crowded living situations.28-30 This situation has operational relevance from basic training, deployments, and combat operations to peacetime activities.
Military recruits are treated routinely with penicillin G benzathine as standard prophylaxis against streptococcal infections.26,30 A recent study by the Marine Corps Recruiting Depot in San Diego, California showed that in a cohort of 402 young healthy male recruits, only 5 (1.5%) had a positive reaction to penicillin testing and challenge over a 21-month period.31 The delabeled other 397 (98.5%) marine recruits were able to receive benzathine penicillin prophylaxis successfully.31 Recruits with a penicillin allergy who had a positive test or were not tested received azithromycin (or erythromycin at some recruit training locations).26,31 Military members may need to operate in remote or austere locations; the ability to use penicillins is important for readiness.
Evaluation and Management of Reported Penicillin Allergy
Verifying penicillin allergies is an important first step in optimizing medical care and decreasing resistance and ADRs.3,4,32,33 Although allergists can provide specialized evaluation, due to the high prevalence of penicillin allergy in the US, all health care team members, including clinicians and pharmacists, should be educated about penicillin allergies and be able to implement evaluations in both inpatient and outpatient settings. Reactions to any of the penicillins should be considered, including the natural penicillins (penicillin V, etc), antistaphylococcal penicillins (dicloxacillin), aminopenicillins (amoxicillin and ampicillin), and extended-spectrum penicillins (piperacillin).3 A thorough history, including the prior reaction (age, type of reaction) and subsequent tolerance are helpful in stratifying patients.3,26
Patient Risk Levels
Based on the clinical history, patients would fall into 4 categories from low risk, medium risk, high risk, to do not test/use.3,32,33 Low-risk patients are those who report mild or nonallergic symptoms (ie, gastrointestinal symptoms, headache, yeast infection, etc), remote cutaneous reactions (> 10 years), or in those with a family history of penicillin allergy.3,32,33 Low-risk patients often can be safely tested with an oral challenge. Although there are different approaches to the oral challenge, a single amoxicillin dose of 250 mg followed by 1 hour of direct monitoring is usually sufficient.3,32,33
Medium-risk patients have a more recent (< 1 year) history of pruritic rashes, urticaria, and/or angioedema without a history of severe or systemic reactions. These patients benefit from negative skin testing prior to an oral challenge, which can be performed by trained clinicians or pharmacists or an allergist. However, due to limited availability of skin testing and the potential for false positive testing with skin tests, a single dose or graded challenge would be a reasonable approach as well.3,32,33
High-risk patients are those with severe symptoms (anaphylaxis), a history of reactions to other β-lactam antibiotics, and/or recurrent reactions to antibiotics. These patients benefit from a formal evaluation by an allergist and skin testing prior to challenge.3,32,33 Testing and/or challenge should not be performed in patients who report a history of severe cutaneous reactions (blistering rash, such as Stevens Johnson syndrome), hemolytic anemia, serum sickness, drug fever, and other organ dysfunction.3,4,31,32
The Figure describes a published questionnaire, personnel, resources, and procedures for penicillin delabeling.26 Although skin testing is reliable in revealing a immunoglobulin E-mediated penicillin allergy, there is potential for false positives.32,33 The oral amoxicillin challenge effectively clears the patient for future penicillin administration.3,32-34 In high-risk patients, desensitization should be considered if penicillins (or cephalosporins) are required as first-line treatment. A test dose (one-tenth dose, higher or lower depending on route of administration, historic reaction, clinical status, and level of certainty of prior reaction) may be considered in low- to moderate-risk patients, depending on the indication for the use of the antibiotics.32
Penicillin evaluation pathways can occur in both inpatient and outpatient settings where antibiotics will be prescribed.32-34 There are several proposed pathways, including a screening questionnaire to determine the penicillin allergy risk.26,32,33 Implementation of perioperative testing has been successful in decreasing the rates of vancomycin use and lessening the morbidity associated with use of second-line antibiotics.35 Many hospitals throughout the country have implemented standardized penicillin delabeling programs.3,32-34
Conclusions
Penicillin allergies are an important barrier to effective antibiotic treatments and are associated with worse outcomes and higher economic costs.3,7,23,26,34 Therefore, in addition to vaccinations, infection control measures, and public health education, penicillin allergy verification and delabeling programs should be a proactive component of military medical readiness and all antibiotic stewardship initiatives in all health care settings.29 Given the many issues and negative impact of having a penicillin allergy label, penicillin delabeling will allow service members to be treated with the necessary antibiotics with fewer adverse complications, and return them to health and readiness for operational duties. In the current standardization of the Defense Health Agency, implementing this program across all services would have significant clinical, public health, and cost benefits for patients, the health care team, taxpayers, and the community at large.
Many patients report an allergy to penicillin, but only a small portion have a current true immune-mediated allergy. Given the clinical, public health, and economic costs associated with a penicillin allergy label, evaluation and clearance of penicillin allergies is a simple method that would improve clinical outcomes, decrease AEs to high-risk alternative broad-spectrum antibiotics, and prevent the spread of antibiotic resistance. In the military, penicillin delabeling improves readiness with optimal antibiotic options and avoidance of unnecessary risks of using alternative antibiotics, expediting return to full duty for military personnel.
1. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions-United States, 2014. https://www.cdc.gov/antibiotic-use/community/pdfs/annual-reportsummary_2014.pdf. Accessed August 15, 2020.
2. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. doi:10.1001/jama.2014.12923
3. Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy. JAMA. 2019;321:188-199. doi:10.1001/jama.2018.19283
4. Har D, Solensky R. Penicillin and beta-lactam hypersensitivity. Immunol Allergy Clin North Am. 2017;37(4):643-662. doi:10.1016/j.iac.2017.07.001
5. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308-1315. doi:10.1001/jamainternmed.2017.1938
6. Shebab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735-743. doi:10.1086/591126
7. Macy E, Contreras R. Healthcare use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
8. Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of methicillin resistant Staphylococcal aureus and Clostridium difficile in patients with a documented penicillin allergy: population-based matched cohort study. BMJ. 2018;361:k2400. doi:10.1136/bmj.k2400
9. Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442-2449. doi:10.1056/NEJMoa051639
10. Pepin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolone as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
11. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infec Dis. 2015;60(4):566-577. doi:10.1093/cid/ciu914
12. Rao GA, Mann JR, Shoaibi A, et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med. 2014;12(2):121-127. doi:10.1370/afm.1601
13. LeBlanc L, Pepin J, Toulouse K, et al. Fluoroquinolone and risk for methicillin-resistant Staphylococcus aureus, Canada. Emerg Infect Dis. 2006;12(9):1398-1405. doi:10.3201/eid1209.060397
14. Schembri S, Williamson PA, Short PM, et al. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. BMJ. 2013;346:f1245. doi:10.1136/bmj.f1235
15. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881-1890. doi:10.1056/NEJMoa1003833
16. McDaniel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61(3):361-367. doi:10.1093/cid/civ308
17. Wong D, Wong T, Romney M, Leung V. Comparison of outcomes in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia who are treated with β-lactam vs vancomycin empiric therapy: a retrospective cohort study. BMC Infect Dis. 2016;16:224. doi:10.1186/s12879-016-1564-5
18. Blumenthal KG, Shenoy ES, Huang M, et al. The impact of reporting a prior penicillin allergy on the treatment of methicillin-sensitivity Staphylococcus aureus bacteremia. PLoS One. 2016;11(7):e0159406. doi:10.1371/journal.pone.0159406
19. Verani JR, McGee L, Schrag SJ; . Prevention of perinatal group B streptococcal disease. MMWR Recomm Rep. 2010;59(RR-10):1-36.
20. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21:16-080. doi:10.7812/TPP/16-080
21. Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66(3):329-336. doi:10.1093/cid/cix794
22. National Health Expenditures 2017 Highlights. Centers for Medicare & Medicaid services. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf. Accessed August 25, 2020.
23. Macy E, Shu YH. The effect of penicillin allergy testing on future health care utilization: a matched cohort study. J Allergy Clin Immunol Pract. 2017;5(3):705-710. doi:10.1016/j.jaip.2017.02.012
24. Picard M, Begin P, Bouchard H, et al. Treatment of patients with a history of penicillin allergy in a large tertiary-care academic hospital. J Allergy Clin Immunol Pract. 2013;1(3):252-257. doi:10.1016/j.jaip.2013.01.006
25. US Department of Defense. Beneficiary population statistics. https://health.mil/I-Am-A/Media/Media-Center/Patient-Population-Statistics. Accessed August 25, 2020.
26. Lee RU, Banks TA, Waibel KH, Rodriguez RG. Penicillin allergy…maybe not? The military relevance for penicillin testing and de-labeling. Mil Med. 2019;184(3-4):e163-e168. doi:10.1093/milmed/usy194
27. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention. https://www.cdc.gov/drugresistance/threat-report-2013/index.html. Accessed May 10, 2019.
28. Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999;5(3):379-385. doi:10.3201/eid0503.990308
29. Beaumier CM, Gomez-Rubio AM, Hotez PJ, Weina PJ. United States military tropical medicine: extraordinary legacy, uncertain future. PLoS Negl Trop Dis. 2013;7(12):e2448. doi:10.1371/journal.pntd.0002448
30. Thomas RJ, Conwill DE, Morton DE, et al. Penicillin prophylaxis for streptococcal infections in the United States Navy and Marine Corps recruit camps, 1951-1985. Rev Infect Dis. 1988;10(1):125-130. doi:10.1093/clinids/10.1.125
31. Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
32. Blumenthal KG, Shenoy ES, Wolfson AR, et al. Addressing inpatient beta-lactam allergies: a multihospital implementation. J Allergy Clin Immunol Pract. 2017;5(3):616-625. doi:10.1016/j.jaip.2017.02.019
33. Kuruvilla M, Shih J, Patel K, Scanlon N. Direct oral amoxicillin challenge without preliminary skin testing in adult patients with allergy and at low risk with reported penicillin allergy. Allergy Asthma Proc. 2019;40(1):57-61. doi:10.2500/aap.2019.40.4184
34. Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
35. Park M, Markus P, Matesic
1. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions-United States, 2014. https://www.cdc.gov/antibiotic-use/community/pdfs/annual-reportsummary_2014.pdf. Accessed August 15, 2020.
2. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. doi:10.1001/jama.2014.12923
3. Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy. JAMA. 2019;321:188-199. doi:10.1001/jama.2018.19283
4. Har D, Solensky R. Penicillin and beta-lactam hypersensitivity. Immunol Allergy Clin North Am. 2017;37(4):643-662. doi:10.1016/j.iac.2017.07.001
5. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308-1315. doi:10.1001/jamainternmed.2017.1938
6. Shebab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735-743. doi:10.1086/591126
7. Macy E, Contreras R. Healthcare use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
8. Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of methicillin resistant Staphylococcal aureus and Clostridium difficile in patients with a documented penicillin allergy: population-based matched cohort study. BMJ. 2018;361:k2400. doi:10.1136/bmj.k2400
9. Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442-2449. doi:10.1056/NEJMoa051639
10. Pepin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolone as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
11. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infec Dis. 2015;60(4):566-577. doi:10.1093/cid/ciu914
12. Rao GA, Mann JR, Shoaibi A, et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med. 2014;12(2):121-127. doi:10.1370/afm.1601
13. LeBlanc L, Pepin J, Toulouse K, et al. Fluoroquinolone and risk for methicillin-resistant Staphylococcus aureus, Canada. Emerg Infect Dis. 2006;12(9):1398-1405. doi:10.3201/eid1209.060397
14. Schembri S, Williamson PA, Short PM, et al. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. BMJ. 2013;346:f1245. doi:10.1136/bmj.f1235
15. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881-1890. doi:10.1056/NEJMoa1003833
16. McDaniel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61(3):361-367. doi:10.1093/cid/civ308
17. Wong D, Wong T, Romney M, Leung V. Comparison of outcomes in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia who are treated with β-lactam vs vancomycin empiric therapy: a retrospective cohort study. BMC Infect Dis. 2016;16:224. doi:10.1186/s12879-016-1564-5
18. Blumenthal KG, Shenoy ES, Huang M, et al. The impact of reporting a prior penicillin allergy on the treatment of methicillin-sensitivity Staphylococcus aureus bacteremia. PLoS One. 2016;11(7):e0159406. doi:10.1371/journal.pone.0159406
19. Verani JR, McGee L, Schrag SJ; . Prevention of perinatal group B streptococcal disease. MMWR Recomm Rep. 2010;59(RR-10):1-36.
20. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21:16-080. doi:10.7812/TPP/16-080
21. Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66(3):329-336. doi:10.1093/cid/cix794
22. National Health Expenditures 2017 Highlights. Centers for Medicare & Medicaid services. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf. Accessed August 25, 2020.
23. Macy E, Shu YH. The effect of penicillin allergy testing on future health care utilization: a matched cohort study. J Allergy Clin Immunol Pract. 2017;5(3):705-710. doi:10.1016/j.jaip.2017.02.012
24. Picard M, Begin P, Bouchard H, et al. Treatment of patients with a history of penicillin allergy in a large tertiary-care academic hospital. J Allergy Clin Immunol Pract. 2013;1(3):252-257. doi:10.1016/j.jaip.2013.01.006
25. US Department of Defense. Beneficiary population statistics. https://health.mil/I-Am-A/Media/Media-Center/Patient-Population-Statistics. Accessed August 25, 2020.
26. Lee RU, Banks TA, Waibel KH, Rodriguez RG. Penicillin allergy…maybe not? The military relevance for penicillin testing and de-labeling. Mil Med. 2019;184(3-4):e163-e168. doi:10.1093/milmed/usy194
27. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention. https://www.cdc.gov/drugresistance/threat-report-2013/index.html. Accessed May 10, 2019.
28. Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999;5(3):379-385. doi:10.3201/eid0503.990308
29. Beaumier CM, Gomez-Rubio AM, Hotez PJ, Weina PJ. United States military tropical medicine: extraordinary legacy, uncertain future. PLoS Negl Trop Dis. 2013;7(12):e2448. doi:10.1371/journal.pntd.0002448
30. Thomas RJ, Conwill DE, Morton DE, et al. Penicillin prophylaxis for streptococcal infections in the United States Navy and Marine Corps recruit camps, 1951-1985. Rev Infect Dis. 1988;10(1):125-130. doi:10.1093/clinids/10.1.125
31. Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
32. Blumenthal KG, Shenoy ES, Wolfson AR, et al. Addressing inpatient beta-lactam allergies: a multihospital implementation. J Allergy Clin Immunol Pract. 2017;5(3):616-625. doi:10.1016/j.jaip.2017.02.019
33. Kuruvilla M, Shih J, Patel K, Scanlon N. Direct oral amoxicillin challenge without preliminary skin testing in adult patients with allergy and at low risk with reported penicillin allergy. Allergy Asthma Proc. 2019;40(1):57-61. doi:10.2500/aap.2019.40.4184
34. Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
35. Park M, Markus P, Matesic
Serotonin Syndrome/Serotonin Toxicity
Serotonin, or 5-hydroxytryptamine (5-HT), is a chemical neurotransmitter in the central and peripheral nervous systems that was discovered in 1940s. 1 O ne of the most widely studied chemical messengers , serotonin influences many physiologic functions in humans, including regulation of mood, sleep-wake cycle, appetite suppression, memory, emesis, breathing, cognition, blood coagulation, libido, and many other functions. 2 In 1992, Insel and colleagues first document ed the toxic symptoms produced from too much serotonin in the central and peripheral nervous systems , naming it serotonin syndrome. 3,4
Serotonin Syndrome
Experts in the fields of psychiatry, pharmacy, and toxicology refer to these symptoms as serotonin toxicity, because the symptoms result from the toxic effects of too much serotonin.5-9 The term toxicity instead of syndrome “clarifies that it is a form of poisoning, just as lithium toxicity is a form of poisoning.”6 Therefore, serotonin toxicity (ST) can develop with administration of any serotonin-enhancing medication, including therapeutic use, polypharmacy, or accidental/intentional drug overdose.
The incidence of ST has increased over the past decade.5,6,10,11 Several reasons explain this increase: (1) ST mirrors the increase in depression in the US populations10,12,13; (2) There has been an increase in off-label antidepressant prescribing by both primary care and mental health providers14-16; (3) the increased use of illicit drugs13; (4) an increase in suicide attempts with antidepressants17; and (5) increased use of opioids for pain management, including both prescription and illicit use.11,14 This paper reviews the potential lethal combinations of commonly prescribed medications used to treat both veteran and nonveteran patients and includes the latest information on offending medications; a presentation of symptoms from in utero to adult; diagnostic criteria; and recommended treatments.
The Veterans Health Administration (VHA) and non-VHA health care providers can play a key role in identifying and preventing serotonin syndrome/ST by keeping abreast of the latest updates of potentially lethal drug combinations. Commonly prescribed medications with the potential for a reaction include antidepressants, anxiolytics, pain medications, antinausea medications, herbal medications, and over-the-counter (OTC) medications, such as cough suppressants. Patients may be at increased risk for ST due to the growth of polypharmacy management of comorbidities.
Antidepressants
Over the past decade, antidepressant use has increased substantially in the US,United Kingdom, and Canada.14 Also the types of antidepressants prescribed has changed and been replaced with the newer agents. The selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors (SNRIs) have replaced the older tricyclics (TCAs) and monoamine oxidase inhibitors (MAOIs) as first-line treatments for depression due to their improved comparative efficacy, reduced mortality following overdose, adverse effects (AEs) that are more tolerable for most patients, and the SSRIs have no anticholinergic properties (except paroxetine) (Table 1).18
In 2017 the National Institute of Mental Health reported that about 17 million adults and 3 million adolescents (aged 11-18 years) experienced at least 1 episode of major depression.19 About 40% of US veterans will experience depression, which is 3 times higher than the rate of the general US population.12 A random sampling survey conducted of about 17,000 active-duty service members by the US Department of Defense (DoD) from November 2015 to April 2016 revealed 9.4% reported depression.20 Antidepressant usage in the US and among veterans continues to increase.12,16 In 2018, the list of top US prescribed drugs, included sertraline (14th), citalopram (21st), trazodone (24th), and escitalopram (26th).21 Antidepressant prescribing in the US increased 18% from 2012 to 2017.22 This trend also continues within the military with a 40% increase of antidepressant use in the past decade.16
One reason for the increase in antidepressant use is off-label prescribing.14,23 A sampling of about 2 billion psychiatric outpatient visits in a western portion of the US found 12.9% of the prescriptions filled were off-label.15 In Minnesota, off-label prescribing of antidepressants was found to contribute to an increase in drug interactions in elderly nursing home residents.24 An investigation by the Military Times of the military community revealed off-label prescribing occurs not only with antidepressant medications, but also with anticonvulsants, antipsychotics, anti-anxiety drugs, and antiepileptic medications.14
A case report that brought ST to the forefront occurred in the 1980s and involved a college student.25 She was initially diagnosed with the flu. Her symptoms progressed over a 24-hour period despite treatment, leading to seizures, hyperthermia, generalized clonus, muscle rigidity, respiratory failure, and death because of unrecognized ST. Her combination of serotonin-elevating drugs included meperidine, phenelzine, chlorpheniramine, and haldol. On autopsy, there were traces of cocaine found in some of her tissue samples.
Pathophysiology
Tryptophan is a precursor of serotonin and must be ingested from foods, including meats, dairy, fruits, and seeds. About 90% of all serotonin is made in the gastrointestinal epithelium and is the major component of the brain-gut axis.26 Serotonin cannot cross the blood-brain barrier; therefore, it is synthesized and stored in presynaptic terminals around the midline of the brainstem.1,26 Transport of serotonin is provided by serotonin transporter (SERT).1,26,27 Once released, serotonin can either stimulate postsynaptic neuron receptors or is taken up into the presynaptic terminals for reuse. SSRI antidepressants, such as citalopram and paroxetine inhibit the reuptake of serotonin by binding to 2 different sites on the SERT thus allowing more available serotonin to be accessible to other neurons.27 There are 7 families of serotonin receptors, 5-HT1 to 5-HT7 and at least 15 mammalian subtypes.28,29 The majority of these receptors have been implicated in depression or depressive-like behavior as evidenced by the efficacy of increasing extracellular serotonin for the treatment of depression with SSRIs, SNRIs, TCAs, and MAOIs.29 Three of the most studied receptors include 5-HTIA,5-HT1B,and 5-HT2A.
Etiology
Most serotonin-induced drug fatalities occur when combining serotonergic drugs that work through different pathways (Table 2).30 The most toxic combination of serotonin-enhancing drugs includes MAOIs taken with SSRIs or SNRIs, or a combination of 2 MAOIs.5-9
Other potentially lethal combinations may includepolypharmacy with antidepressants, pain medications, OTC medications, and illicit drugs. Linezolid, a new synthetic antimicrobial, is considered to be a weak MAOI. Therefore, prescribing it with other serotonin-elevating agents has been reported to precipitate ST.18
Most cases of ST do not require hospitalization and can be managed by stopping the medication or decreasing the dose. Therapeutic doses of a single drug are highly unlikely to cause toxicity, although there have been reported cases of patients who are sensitive or more susceptible and develop symptoms after administration of a single agent and/or a dosage increase.
Delayed ST reactions have occurred because of a prolonged half-life of a drug, iron deficiency anemia, and coingestion of shorter acting serotonin antagonists.31 Most antidepressants have a short half-life (< 24 hours)except for fluoxetine. A decrease in iron may contribute to ST because iron is needed to process serotonin from tryptophan. An example of 2 shorter-acting serotonin antagonists include cyproheptadine and olanzapine. Cyproheptadine is used in the treatment of ST, and olanzapine is an antipsychotic.
Symptoms
Symptoms of ST range from mild to severe and include a combination of neuromuscular, autonomic, and mental status changes (Table 3).5,10 Mild symptoms of ST can start within 1 to 2 hours after ingesting a medication that increases serotonin to a toxic state unless the drug has a long half-life (eg, fluoxetine). Sometimes mild symptoms of ST can be difficult to distinguish from common drug AEs, flu symptoms, or viruses. Patients taking therapeutic doses of SSRIs can experience serotonin symptoms, such as lower limb hyperreflexia or a few beats of ankle clonus without being toxic. One thing to remember is that not all patients will start with mild symptoms and may present in moderate or severe distress.
Moderate-to-severe ST symptoms require hospitalization, usually in the intensive care unit (ICU). At this stage, clonus progresses from the lower extremities to the upper body and becomes more generalized. Ocular clonus can be continuous, intermittent, or have a ping pong effect (short cycle, periodic, alternating lateral gaze).
Severe ST is life threatening and leads to multiorgan failure within hours if not treated. The patient is intubated to assist with breathing and sedated because excess agitation and muscular tremors can increase temperature, which is already elevated by the time the symptoms reach the severe state. Of note, hyperthermia is due to a noninfectious elevation of body temperature from hypertonicity, agitation, and muscle rigidity.A true core temperature > 105.8°F causes irreversible cell damage, cerebral injury, and death.32,33 The patient can develop seizures and a coma. Multiorgan failure occurs, including rhabdomyolysis, myoglobinuria, renal failure, metabolic acidosis, acute respiratory distress, and disseminated intravascular coagulation.
Diagnosis
The diagnosis of ST is clinical and based on a history of ingesting serotonin-elevating medications and physical findings as per Hunter Serotonin Toxicity Criteria34 (Table 4). An in-depth history needs to include previous and current prescriptions, indications of the prescriptions (eg, therapeutic, increase in dosage, suicide intent), OTC medications, and illicit drug use. Early recognition of symptoms, identification of serotonergic medications, and appropriate resuscitative measures lead to more successful outcomes. A serotonin drug level is ineffective and does not correlate with the dosage since serotonin does not cross the blood-brain barrier.
The type of drug determines the length and response of the episode. The drug(s) elimination half-lives need to be calculated along with the pharmacokinetic or pharmacodynamics; agonist, antagonist, reuptake inhibitor, etc. Many drugs have half-lives of < 24 hours; therefore, reducing or eliminating the offending drug(s) will result in a steady reduction of symptoms.Exceptions include medications with a longer activity, such as the irreversible MAOIs (eg, phenelzine, isocarboxazid) and drugs with a longer half-life, such as fluoxetine. These types of medications may have been stopped weeks earlier and may prolong reduction of symptoms.
When initiating or increasing SSRIs or SNRIs, there are common nontoxic AEs that are not consistent with ST, including anxiety, restlessness, and irritability that may last for 2 weeks. The difference in toxic vs nontoxic reactions are the timing and rapid progression of symptoms. The toxic symptoms will start within hours of ingesting the offending agents(s) and progress rapidly to severe symptoms within 24 hours. Therefore, it is imperative to review AEs with the patient and or caregiver, so they may act as their own advocate and seek immediate assistance.
Differentials
There are symptoms specific to ST that can be used to differentiate it from other conditions. These include hyperthermia, bilateral symmetric clonus (inducible, spontaneous, ocular), and hyperreflexia.These criteria form the basis for Hunter criteria.
Differential diagnoses to consider include neuroleptic malignant syndrome; antidepressant initiation AEs; antidepressant discontinuation syndrome; malignant hyperthermia; anticholinergic toxicity; meningitis/encephalitis; sepsis; drug overdose; alcohol/benzodiazepine withdrawal; and preeclampsia. Neuroleptic malignant syndrome (NMS) is the disorder most often misdiagnosed as ST.Key elements that distinguish ST from NMS include the timing of the clinical course (NMS develops over days to weeks); the medications ingested (NMS from dopaminergic drugs); and the symptoms of NMS (bradyreflexia, bradykinesia, bradyphrenia, and no clonus).According to Gillman, serotonin toxicity is a manifestation of toxicity that is predictable and common with specific drug combinations, while NMS is a “rare idiosyncratic reaction to essentially normal doses and very rarely occurs after overdoses.”35 Preeclampsia is a pregnancy complication that can mimic ST with symptoms of hypertension, clonus, and hyperreflexia. It has been estimated to complicate 2% to 8% of pregnancies and remains a principle cause of maternal and fetal morbidity and mortality.36,37
Treatment
Mild-to-moderate symptoms usually resolve on their own 1 to 3 days after decreasing or stopping the offending drug. The timing will depend on the half-life or active metabolites of the drug. Treatment is largely supportive and may require treatment for control of agitation with benzodiazepines and IV fluids for dehydration/hypotension.14 In cases not responding to supportive care, treatment with oral cyproheptadine is recommended.14
There are other medications that have been used in treatment such as olanzapine, chlorpromazine, propranolol, bromocriptine, dantrolene, droperidol, and haloperidol, but their efficacy is unproven and not recommended.10 Chlorpromazine can cause hypotension and increase hyperthermia. Propranolol has a long duration of action, may cause a prolonged hypotension, and can mask tachycardia that can be used to monitor the effectiveness of treatment.10 Bromocriptine is a serotonin agonist and may exacerbate symptoms. Dantrolene has no effect on survival in animal models.10 Droperidol and haloperidol can worsen hyperthermia by inhibiting sweating.38
Mechanical ventilation should be considered especially if muscle rigidity progresses and depressed respiratory function occurs. If the temperature starts to rise, immediate sedation, paralysis, mechanical ventilation, and cyproheptadine are administered.The overall goal is prevention of hyperthermia, which leads to multiorgan failure. A core temperature of ≥ 104°F is associated with neurologic cell death, and recovery is minimal.32 Consultation with an experienced toxicologist is strongly recommended.Antipyretics should not be used, because elevated temperature is centrally mediated from muscle rigidity. If presentation occurs within 1 hour, activated charcoal can be used for detoxification of potentially lethal amounts.
Warning Label Controversies
In 2006, the US Food and Drug Administration (FDA) issued an advisory warning against concurrently using a tryptan antimigraine drug and serotonin-mediated medications.39 In 2018, a research team conducted a 14-year retrospective analysis on 20,000 patients who were coprescribed a tryptan drug with SSRIs or SNRIs.40 The study reported that the risk of ST was rare and suggested that the FDA reconsider their advisory. There are several other controversial medications with a ST FDA warning label due to their mechanisms of action and inaccurate case reports.41
Human Poisonings
Consistent with the 2017 American Association of Poison Control Centers Toxic Exposure report, antidepressants continue to be in the top 5 substance classes most frequently involved in human exposures.42 Most accidental ingestions of antidepressants occur in toddlers, whereas intentional ingestions are usually done by adolesents.43 Over the past 10 years, antidepressants are the No. 1 fastest growing category of human exposures in all age groups.42
ST in the Pediatric Population
ST in the pediatric population mirrors that in adults.Differences include the inability of the child to report symptoms, lack of clinician awareness, and reluctance of adolescents to disclose recreational drug use.Management is the same as for adults, including discontinuing the offending drug, supportive care, adequate sedation, oxygen, IV fluids, and continuous cardiac monitoring. Sedation is weight based for benzodiazepines. Mild-to-moderate reactions require admission for observation. Severe reactions require admission to the ICU.
There have been at least 4 published case reports of children aged < 6 years with moderate-to-severe ST secondary to acute vilazodone ingestion.44 The dosages included 5.5 to 37 mg/kg. All 4 patients had altered mental status, seizures, hyperthermia, mild clonus, tachycardia, and hypertension. They all survived with intensive care treatment, including intubation, sedation, cyproheptadine in 2 cases, activated charcoal and IV lorazepam in the other cases.
Direk and colleagues reported a case of a 12-year-old girl who was brought to the emergency department by her stepmother for seizurelike activity and was diagnosed with epilepsy and status epilepticus.45 In the pediatric ICU she developed tachycardia, fever, agitation, dilated pupils, tremors, increased deep tendon reflexes, spontaneous clonus, and horizontal ocular movements. A detailed clinical history was retaken and revealed that the child had been prescribed risperidone 1 week before by the psychiatric clinic due to behavioral problems, including stealing money, lying, and running away from home and school. On further investigation, the stepmother was taking clomipramine and discovered 9 missing pills.
Pregnancy and Lactation
The American College of Obstetricians and Gynecologists recommends that clinicians screen patients at least once during the perinatal period for depression and anxiety symptoms, using a standardized, validated tool and complete a full assessment of mood and emotional well-being during the postpartum, including screening for postpartum depression and anxiety with a validated instrument.46 Treatment with antidepressants is controversial. “Current evidence is generally reassuring and indicates that the absolute risks of negative infant outcomes are small except for PNAS [poor neonatal adaptation syndrome], which largely appears to be self-limited.”47 Antidepressants cross the human placenta and fetal blood-brain barrier.48 Several cases of infant toxicity from SSRIs have been reported with citalopram and escitalopram.49,50 Symptoms included severe muscle rigidity, lethargy, tachycardia, QTc prolongation, altered consciousness, hypertonia, and seizures at birth. These mothers had taken an SSRI during pregnancy.
Conclusions
This article highlights some of the latest information on ST. Increased awareness of all clinicians and their patients may help decrease unnecessary comorbidities and death. Early identification of ST symptoms will increase the chances for survival, because of the rapid progression of symptoms within 24 hours. Most fatal reactions occur when combining MAOIs with SSRIs, SNRIs, or another MAOI. Overdose with an SSRI does not progress to the severe symptoms unless combined with another serotonin-elevating medication.
Education of all patients who are prescribed antidepressants must include awareness of the potential for serotonergic drug interactions, particularly from OTC medications, herbal medications, and illicit drugs. The diagnosis of ST is based on clinical findings and there must be a history of ingesting serotonin-elevating drug(s). Hunter Serotonin Toxicity Criteria is the gold standard for diagnosing symptoms along with consulting a toxicologist. Prevention of ST includes informed clinicians, patient education, careful prescribing and monitoring, and avoidance of multidrug regimens.
1. Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176(3):1243-1251.
2. McCorvy JD, Roth BL. Structure and function of serotonin g protein coupled receptors. Pharmacol Ther. 2015;150:129-142. doi:10.1016/j.pharmthera. 2015.01.009 3. Insel TR, Roy BF, Cohen RM, Murphy DL. Possible development of serotonin syndrome in man. Am J Psychiatry. 1982;139(7):954-955. doi:10.1176/ajp.139.7.954
4. Scotton WJ, Hill LJ, Williams AC, Barnes NM. Serotonin syndrome: pathophysiology, clinical features, management, and potential future directions. Int J Tryptophan Res. 2019;12:1-14. doi:10.1177/1178646919873925
5. Buckley N, Dawson AH, Isbister GK. Serotonin syndrome. BMJ. 2014;348:g1626. doi:10.1136/bmj.g1626
6. Gillman KP. Serotonin toxicity: introduction. https://psychotropical.com/serotonin-toxicity-introduction. Published November 13, 2014. Updated July 13, 2019. Accessed August 17, 2020.
7. Foong A-L, Patel T, Kellar J, Grindrod KA. The scoop on serotonin syndrome. Can Pharm J (OTT). 2018;151(4):233-239. doi:10.1177/1715163518779096
8. Foong A-L, Grindrod KA, Patel T, Kellar J. Demystifying serotonin syndrome (or serotonin toxicity). Can Fam Physician. 2018;64(10):720-727.
9. Gillman KP. Serotonin toxicity: summary. https://psychotropical.com/serotonin-toxicity-summary. Published November 13, 2014. Updated January 25, 2018. Accessed August 17, 2020.
10. Boyer EW. Serotonin syndrome (serotonin toxicity). https://www.uptodate.com/contents/serotonin-syndrome-serotonin-toxicity. Updated March 12, 2018. Access December 12, 2019.
11. Wang RZ, Vashistha V, Kaur S, Houchens NW. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817. doi:10.3949/ccjm.83a.15129
12. Walker T. The economic burden of depression among veterans. https://www.managedhealthcareexecutive.com/article/economic-burden-depression-among-veterans. Published November 9, 2018. Accessed August 17, 2020.
13. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2019.
14. Wong J, Motulsky A, Abrahamowicz M, et al. Off label indications for antidepressants in primary care; descriptive study of prescriptions from an indication based electronic prescribing system. BMJ. 2017;356:j603. doi:org/10.1136/bmj.j603
15. Vijay A, Becker JE, Ross JS. Patterns and predictors of off-label prescription of psychiatric drugs. PLOS One. 2018;13(7):e0198363. doi:10.1371/journal.pone.0198363
16. Medicating the military—use of psychiatric drugs has spiked; concerns surface about suicide, other dangers. https://www.militarytimes.com/2013/03/29/medicating-the-military-use-of-psychiatric-drugs-has-spiked-concerns-surface-about-suicide-other-dangers. Published March 29, 2013. Accessed August 17, 2020.
17. Hengartner MP, Plöderl M. Newer-generation antidepressants and suicide risk in randomized controlled trials; a re-analysis of the FDA database [letter]. Psychother Psychosom. 2019:88:247-248. doi:10.1159/000501215
18. Rosebush PI. Serotonin syndrome. https://www.mhaus.org/nmsis/medical-education-programs/serotonin-syndrome. Accessed March 24, 2020.
19. National Institute of Mental Health. Major depression. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed March 24, 2020.
20. Meadows SO, Engel RL, Beckman RL, et al. 2015 health related behaviors survey: mental and emotional health among U.S. active-duty service members. https://www.rand.org/pubs/research_briefs/RB9955z3.html. Published 2018. Accessed August 17, 2020.
21. ClinCalc. The top 300 drugs of 2020. https://clincalc.com/DrugStats/Top300Drugs.aspx. Update February 11, 2017. Accessed March 24, 2020.
22. Corrigan C. Revealed: massive rise in antidepressant prescribing. https://www.rte.ie/news/investigations-unit/2019/0218/1031271-massive-rise-antidepressant-prescribing. Published June 14, 2019. Accessed August 17, 2020.
23. Skånland SS, Cieślar-Pobuda A. Off-label uses of drugs for depression. Eur J Pharmacol. 2019;865: 172732. doi:10.1016/j.ejphar.2019.172732
24. Bobo WV, Grossardt BR, Lapid MI, et al. Frequency and predictors of the potential overprescribing of antidepressants in elderly residents of a geographically defined U.S. population. Pharmacol Res Perspect. 2019;7(1):e00461. doi:10.1002/prp2.461
25. Patel N. Learning lessons. The Libby Zion case revisited. J Am Coll Cardiol. 2014;64(25):2802-2804. doi:10.1016/j.jacc.2014.11.007
26. Jenkins TA, Nguyen JCD, Polglase KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8(1):56. doi:10.3390/nu8010056
27. Coleman JA, Green EM, Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature Int J Sci. 2016;532(7599):334-339. doi:10.1038/nature17629
28. Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. 5-HT(1A) [corrected] receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology (Berl). 2013;231(4):623-636. doi:10.1007/s00213-013-3389-x
29. Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123. doi:10.12688/f1000research.9736.1
30. Francescangeli J, Karamchandani K, Powell M, Bonavia A. The serotonin syndrome: from molecular mechanisms to clinical practice. Int J Mol Sci. 2019;20(9):2288. doi:10.3390/ijms20092288
31. Little K, Lin CM, Reynolds PM. Delayed serotonin syndrome in the setting of a mixed fluoxetine and serotonin antagonist overdose. Am J Case Rep. 2018;19:604-607. doi:10.12659/AJCR.909063
32. Walter EJ, Carraretto M. The neurological and cognitive consequences of hyperthermia. Crit Care. 2016;20:199. doi:10.1186/s13054-016-1376-4

33. Platt M, Price T. Heat illness. In: Walls, ed. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Philadelphia, PA: Elsevier; 2018:1755-1764.
34. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642. doi:10.1093/qjmed/hcg109
35. Gillman KP. Serotonin toxicity contrasted with neuroleptic malignant syndrome. https://psychotropical.com/serotonin-syndrome-and-neuroleptic-malignant-syndrome. Published January 1, 2005. Updated November 6, 2017. Accessed August 17, 2020.
36. Asusta HB, Keyser E, Dominguez P, Miller M, Odedokun T. Serotonin syndrome in obstetrics: a case report and review of management. Mil Med. 2018;184(1-2):e284-e286. doi:10.1093/milmed/usy135
37. English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control 2015;8:7-12. doi:10.2147/IBPC.S50641
38. Bruggeman C, O’Day CS. Selective serotonin reuptake inhibitor (SSRI) toxicity. https://pubmed.ncbi.nlm.nih.gov/30521236. Published December 3, 2019. Accessed August 17, 2020.
39. US Food and Drug Administration. Selective serotonin reuptake inhibitors (SSRIs) Information. https://www.fda.gov/drugs/information-drug-class/selective-serotonin-reuptake-inhibitors-ssris-information. Updated December 23, 2014. Accessed March 24, 2020.
40. Orlova Y, Rizzoli P, Loder E. Association of coprescription of triptan antimigraine drugs and selective serotonin reuptake inhibitor or selective norepinephrine reuptake inhibitor antidepressants with serotonin syndrome. JAMA Neurol. 2018;75(5):566-572. doi:10.1001/jamaneurol.2017.5144
41. Gillman KP. Regulatory agencies (WH0, FDA) offer ill-conceived advice about serotonin toxicity (serotonin syndrome) with 5–HT3 antagonist: a worldwide problem. https://psychotropical.com/serotonin-toxicity-and-5-ht3-antagonists. Published November 13, 2014. Updated March 23, 2019. Accessed August 17, 2020.
42. Gummin DD, Mowry JB, Spyker DA, Brooks DE, Osterthaler KM, Banner W. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415. doi:10.1080/15563650.2018.1533727
43. Badawy MK, Maffei FA. Pediatric selective serotonin reuptake inhibitor toxicity. https://emedicine.medscape.com/article/1011436. Updated September 27, 2019. Accessed August 17, 2020.
44. Laliberte B, Kishk OA. Serotonin syndrome in a pediatric patient after vilazodone ingestion. Pediatr Emerg Care. 2018;34(12):e226-e228. doi:10.1097/PEC.0000000000001115
45. Direk MC, Yildirim V, Gϋnes S, Bozlu G, Okuyaz C. Serotonin syndrome after clomipramine overdose in a child. Clin Psychopharmacol Neurosci. 2016;14(4):388-390. doi:10.9758/cpn.2016.14.4.38846. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132(5):e208-e212. doi:10.1097/AOG.0000000000002927
47. Osborne LM, McEvoy K, Payne JL. Antidepressants in pregnancy: balancing needs and risks in clinical practice. Psychiatric Times. 2017;34(4).
48. Stewart D, Vigod S. Antenatal use of antidepressants and risk of teratogenicity and adverse pregnancy outcomes: selective serotonin reuptake inhibitors (SSRIs). https://www.uptodate.com/contents/antenatal-use-of-antidepressants-and-risk-of-teratogenicity-and-adverse-pregnancy-outcomes-selective-serotonin-reuptake-inhibitors-ssris. Accessed March 24, 2020.
49. Degiacomo J, Luedtke S. Neonatal toxicity from escitalopram use in utero: a case report. J Pediatr Pharmacol Ther. 2016;21(6):522-526. doi:10.5863/1551-6776-21.6.522
50. Eleftheriou G, Butera R, Cottini FC, Bonati M, Farina M. Neonatal toxicity following maternal citalopram treatment. Fetal Pediatr Pathol. 2013;32(5):362-356. doi:10.3109/15513815.2013.768743
Serotonin, or 5-hydroxytryptamine (5-HT), is a chemical neurotransmitter in the central and peripheral nervous systems that was discovered in 1940s. 1 O ne of the most widely studied chemical messengers , serotonin influences many physiologic functions in humans, including regulation of mood, sleep-wake cycle, appetite suppression, memory, emesis, breathing, cognition, blood coagulation, libido, and many other functions. 2 In 1992, Insel and colleagues first document ed the toxic symptoms produced from too much serotonin in the central and peripheral nervous systems , naming it serotonin syndrome. 3,4
Serotonin Syndrome
Experts in the fields of psychiatry, pharmacy, and toxicology refer to these symptoms as serotonin toxicity, because the symptoms result from the toxic effects of too much serotonin.5-9 The term toxicity instead of syndrome “clarifies that it is a form of poisoning, just as lithium toxicity is a form of poisoning.”6 Therefore, serotonin toxicity (ST) can develop with administration of any serotonin-enhancing medication, including therapeutic use, polypharmacy, or accidental/intentional drug overdose.
The incidence of ST has increased over the past decade.5,6,10,11 Several reasons explain this increase: (1) ST mirrors the increase in depression in the US populations10,12,13; (2) There has been an increase in off-label antidepressant prescribing by both primary care and mental health providers14-16; (3) the increased use of illicit drugs13; (4) an increase in suicide attempts with antidepressants17; and (5) increased use of opioids for pain management, including both prescription and illicit use.11,14 This paper reviews the potential lethal combinations of commonly prescribed medications used to treat both veteran and nonveteran patients and includes the latest information on offending medications; a presentation of symptoms from in utero to adult; diagnostic criteria; and recommended treatments.
The Veterans Health Administration (VHA) and non-VHA health care providers can play a key role in identifying and preventing serotonin syndrome/ST by keeping abreast of the latest updates of potentially lethal drug combinations. Commonly prescribed medications with the potential for a reaction include antidepressants, anxiolytics, pain medications, antinausea medications, herbal medications, and over-the-counter (OTC) medications, such as cough suppressants. Patients may be at increased risk for ST due to the growth of polypharmacy management of comorbidities.
Antidepressants
Over the past decade, antidepressant use has increased substantially in the US,United Kingdom, and Canada.14 Also the types of antidepressants prescribed has changed and been replaced with the newer agents. The selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors (SNRIs) have replaced the older tricyclics (TCAs) and monoamine oxidase inhibitors (MAOIs) as first-line treatments for depression due to their improved comparative efficacy, reduced mortality following overdose, adverse effects (AEs) that are more tolerable for most patients, and the SSRIs have no anticholinergic properties (except paroxetine) (Table 1).18
In 2017 the National Institute of Mental Health reported that about 17 million adults and 3 million adolescents (aged 11-18 years) experienced at least 1 episode of major depression.19 About 40% of US veterans will experience depression, which is 3 times higher than the rate of the general US population.12 A random sampling survey conducted of about 17,000 active-duty service members by the US Department of Defense (DoD) from November 2015 to April 2016 revealed 9.4% reported depression.20 Antidepressant usage in the US and among veterans continues to increase.12,16 In 2018, the list of top US prescribed drugs, included sertraline (14th), citalopram (21st), trazodone (24th), and escitalopram (26th).21 Antidepressant prescribing in the US increased 18% from 2012 to 2017.22 This trend also continues within the military with a 40% increase of antidepressant use in the past decade.16
One reason for the increase in antidepressant use is off-label prescribing.14,23 A sampling of about 2 billion psychiatric outpatient visits in a western portion of the US found 12.9% of the prescriptions filled were off-label.15 In Minnesota, off-label prescribing of antidepressants was found to contribute to an increase in drug interactions in elderly nursing home residents.24 An investigation by the Military Times of the military community revealed off-label prescribing occurs not only with antidepressant medications, but also with anticonvulsants, antipsychotics, anti-anxiety drugs, and antiepileptic medications.14
A case report that brought ST to the forefront occurred in the 1980s and involved a college student.25 She was initially diagnosed with the flu. Her symptoms progressed over a 24-hour period despite treatment, leading to seizures, hyperthermia, generalized clonus, muscle rigidity, respiratory failure, and death because of unrecognized ST. Her combination of serotonin-elevating drugs included meperidine, phenelzine, chlorpheniramine, and haldol. On autopsy, there were traces of cocaine found in some of her tissue samples.
Pathophysiology
Tryptophan is a precursor of serotonin and must be ingested from foods, including meats, dairy, fruits, and seeds. About 90% of all serotonin is made in the gastrointestinal epithelium and is the major component of the brain-gut axis.26 Serotonin cannot cross the blood-brain barrier; therefore, it is synthesized and stored in presynaptic terminals around the midline of the brainstem.1,26 Transport of serotonin is provided by serotonin transporter (SERT).1,26,27 Once released, serotonin can either stimulate postsynaptic neuron receptors or is taken up into the presynaptic terminals for reuse. SSRI antidepressants, such as citalopram and paroxetine inhibit the reuptake of serotonin by binding to 2 different sites on the SERT thus allowing more available serotonin to be accessible to other neurons.27 There are 7 families of serotonin receptors, 5-HT1 to 5-HT7 and at least 15 mammalian subtypes.28,29 The majority of these receptors have been implicated in depression or depressive-like behavior as evidenced by the efficacy of increasing extracellular serotonin for the treatment of depression with SSRIs, SNRIs, TCAs, and MAOIs.29 Three of the most studied receptors include 5-HTIA,5-HT1B,and 5-HT2A.
Etiology
Most serotonin-induced drug fatalities occur when combining serotonergic drugs that work through different pathways (Table 2).30 The most toxic combination of serotonin-enhancing drugs includes MAOIs taken with SSRIs or SNRIs, or a combination of 2 MAOIs.5-9
Other potentially lethal combinations may includepolypharmacy with antidepressants, pain medications, OTC medications, and illicit drugs. Linezolid, a new synthetic antimicrobial, is considered to be a weak MAOI. Therefore, prescribing it with other serotonin-elevating agents has been reported to precipitate ST.18
Most cases of ST do not require hospitalization and can be managed by stopping the medication or decreasing the dose. Therapeutic doses of a single drug are highly unlikely to cause toxicity, although there have been reported cases of patients who are sensitive or more susceptible and develop symptoms after administration of a single agent and/or a dosage increase.
Delayed ST reactions have occurred because of a prolonged half-life of a drug, iron deficiency anemia, and coingestion of shorter acting serotonin antagonists.31 Most antidepressants have a short half-life (< 24 hours)except for fluoxetine. A decrease in iron may contribute to ST because iron is needed to process serotonin from tryptophan. An example of 2 shorter-acting serotonin antagonists include cyproheptadine and olanzapine. Cyproheptadine is used in the treatment of ST, and olanzapine is an antipsychotic.
Symptoms
Symptoms of ST range from mild to severe and include a combination of neuromuscular, autonomic, and mental status changes (Table 3).5,10 Mild symptoms of ST can start within 1 to 2 hours after ingesting a medication that increases serotonin to a toxic state unless the drug has a long half-life (eg, fluoxetine). Sometimes mild symptoms of ST can be difficult to distinguish from common drug AEs, flu symptoms, or viruses. Patients taking therapeutic doses of SSRIs can experience serotonin symptoms, such as lower limb hyperreflexia or a few beats of ankle clonus without being toxic. One thing to remember is that not all patients will start with mild symptoms and may present in moderate or severe distress.
Moderate-to-severe ST symptoms require hospitalization, usually in the intensive care unit (ICU). At this stage, clonus progresses from the lower extremities to the upper body and becomes more generalized. Ocular clonus can be continuous, intermittent, or have a ping pong effect (short cycle, periodic, alternating lateral gaze).
Severe ST is life threatening and leads to multiorgan failure within hours if not treated. The patient is intubated to assist with breathing and sedated because excess agitation and muscular tremors can increase temperature, which is already elevated by the time the symptoms reach the severe state. Of note, hyperthermia is due to a noninfectious elevation of body temperature from hypertonicity, agitation, and muscle rigidity.A true core temperature > 105.8°F causes irreversible cell damage, cerebral injury, and death.32,33 The patient can develop seizures and a coma. Multiorgan failure occurs, including rhabdomyolysis, myoglobinuria, renal failure, metabolic acidosis, acute respiratory distress, and disseminated intravascular coagulation.
Diagnosis
The diagnosis of ST is clinical and based on a history of ingesting serotonin-elevating medications and physical findings as per Hunter Serotonin Toxicity Criteria34 (Table 4). An in-depth history needs to include previous and current prescriptions, indications of the prescriptions (eg, therapeutic, increase in dosage, suicide intent), OTC medications, and illicit drug use. Early recognition of symptoms, identification of serotonergic medications, and appropriate resuscitative measures lead to more successful outcomes. A serotonin drug level is ineffective and does not correlate with the dosage since serotonin does not cross the blood-brain barrier.
The type of drug determines the length and response of the episode. The drug(s) elimination half-lives need to be calculated along with the pharmacokinetic or pharmacodynamics; agonist, antagonist, reuptake inhibitor, etc. Many drugs have half-lives of < 24 hours; therefore, reducing or eliminating the offending drug(s) will result in a steady reduction of symptoms.Exceptions include medications with a longer activity, such as the irreversible MAOIs (eg, phenelzine, isocarboxazid) and drugs with a longer half-life, such as fluoxetine. These types of medications may have been stopped weeks earlier and may prolong reduction of symptoms.
When initiating or increasing SSRIs or SNRIs, there are common nontoxic AEs that are not consistent with ST, including anxiety, restlessness, and irritability that may last for 2 weeks. The difference in toxic vs nontoxic reactions are the timing and rapid progression of symptoms. The toxic symptoms will start within hours of ingesting the offending agents(s) and progress rapidly to severe symptoms within 24 hours. Therefore, it is imperative to review AEs with the patient and or caregiver, so they may act as their own advocate and seek immediate assistance.
Differentials
There are symptoms specific to ST that can be used to differentiate it from other conditions. These include hyperthermia, bilateral symmetric clonus (inducible, spontaneous, ocular), and hyperreflexia.These criteria form the basis for Hunter criteria.
Differential diagnoses to consider include neuroleptic malignant syndrome; antidepressant initiation AEs; antidepressant discontinuation syndrome; malignant hyperthermia; anticholinergic toxicity; meningitis/encephalitis; sepsis; drug overdose; alcohol/benzodiazepine withdrawal; and preeclampsia. Neuroleptic malignant syndrome (NMS) is the disorder most often misdiagnosed as ST.Key elements that distinguish ST from NMS include the timing of the clinical course (NMS develops over days to weeks); the medications ingested (NMS from dopaminergic drugs); and the symptoms of NMS (bradyreflexia, bradykinesia, bradyphrenia, and no clonus).According to Gillman, serotonin toxicity is a manifestation of toxicity that is predictable and common with specific drug combinations, while NMS is a “rare idiosyncratic reaction to essentially normal doses and very rarely occurs after overdoses.”35 Preeclampsia is a pregnancy complication that can mimic ST with symptoms of hypertension, clonus, and hyperreflexia. It has been estimated to complicate 2% to 8% of pregnancies and remains a principle cause of maternal and fetal morbidity and mortality.36,37
Treatment
Mild-to-moderate symptoms usually resolve on their own 1 to 3 days after decreasing or stopping the offending drug. The timing will depend on the half-life or active metabolites of the drug. Treatment is largely supportive and may require treatment for control of agitation with benzodiazepines and IV fluids for dehydration/hypotension.14 In cases not responding to supportive care, treatment with oral cyproheptadine is recommended.14
There are other medications that have been used in treatment such as olanzapine, chlorpromazine, propranolol, bromocriptine, dantrolene, droperidol, and haloperidol, but their efficacy is unproven and not recommended.10 Chlorpromazine can cause hypotension and increase hyperthermia. Propranolol has a long duration of action, may cause a prolonged hypotension, and can mask tachycardia that can be used to monitor the effectiveness of treatment.10 Bromocriptine is a serotonin agonist and may exacerbate symptoms. Dantrolene has no effect on survival in animal models.10 Droperidol and haloperidol can worsen hyperthermia by inhibiting sweating.38
Mechanical ventilation should be considered especially if muscle rigidity progresses and depressed respiratory function occurs. If the temperature starts to rise, immediate sedation, paralysis, mechanical ventilation, and cyproheptadine are administered.The overall goal is prevention of hyperthermia, which leads to multiorgan failure. A core temperature of ≥ 104°F is associated with neurologic cell death, and recovery is minimal.32 Consultation with an experienced toxicologist is strongly recommended.Antipyretics should not be used, because elevated temperature is centrally mediated from muscle rigidity. If presentation occurs within 1 hour, activated charcoal can be used for detoxification of potentially lethal amounts.
Warning Label Controversies
In 2006, the US Food and Drug Administration (FDA) issued an advisory warning against concurrently using a tryptan antimigraine drug and serotonin-mediated medications.39 In 2018, a research team conducted a 14-year retrospective analysis on 20,000 patients who were coprescribed a tryptan drug with SSRIs or SNRIs.40 The study reported that the risk of ST was rare and suggested that the FDA reconsider their advisory. There are several other controversial medications with a ST FDA warning label due to their mechanisms of action and inaccurate case reports.41
Human Poisonings
Consistent with the 2017 American Association of Poison Control Centers Toxic Exposure report, antidepressants continue to be in the top 5 substance classes most frequently involved in human exposures.42 Most accidental ingestions of antidepressants occur in toddlers, whereas intentional ingestions are usually done by adolesents.43 Over the past 10 years, antidepressants are the No. 1 fastest growing category of human exposures in all age groups.42
ST in the Pediatric Population
ST in the pediatric population mirrors that in adults.Differences include the inability of the child to report symptoms, lack of clinician awareness, and reluctance of adolescents to disclose recreational drug use.Management is the same as for adults, including discontinuing the offending drug, supportive care, adequate sedation, oxygen, IV fluids, and continuous cardiac monitoring. Sedation is weight based for benzodiazepines. Mild-to-moderate reactions require admission for observation. Severe reactions require admission to the ICU.
There have been at least 4 published case reports of children aged < 6 years with moderate-to-severe ST secondary to acute vilazodone ingestion.44 The dosages included 5.5 to 37 mg/kg. All 4 patients had altered mental status, seizures, hyperthermia, mild clonus, tachycardia, and hypertension. They all survived with intensive care treatment, including intubation, sedation, cyproheptadine in 2 cases, activated charcoal and IV lorazepam in the other cases.
Direk and colleagues reported a case of a 12-year-old girl who was brought to the emergency department by her stepmother for seizurelike activity and was diagnosed with epilepsy and status epilepticus.45 In the pediatric ICU she developed tachycardia, fever, agitation, dilated pupils, tremors, increased deep tendon reflexes, spontaneous clonus, and horizontal ocular movements. A detailed clinical history was retaken and revealed that the child had been prescribed risperidone 1 week before by the psychiatric clinic due to behavioral problems, including stealing money, lying, and running away from home and school. On further investigation, the stepmother was taking clomipramine and discovered 9 missing pills.
Pregnancy and Lactation
The American College of Obstetricians and Gynecologists recommends that clinicians screen patients at least once during the perinatal period for depression and anxiety symptoms, using a standardized, validated tool and complete a full assessment of mood and emotional well-being during the postpartum, including screening for postpartum depression and anxiety with a validated instrument.46 Treatment with antidepressants is controversial. “Current evidence is generally reassuring and indicates that the absolute risks of negative infant outcomes are small except for PNAS [poor neonatal adaptation syndrome], which largely appears to be self-limited.”47 Antidepressants cross the human placenta and fetal blood-brain barrier.48 Several cases of infant toxicity from SSRIs have been reported with citalopram and escitalopram.49,50 Symptoms included severe muscle rigidity, lethargy, tachycardia, QTc prolongation, altered consciousness, hypertonia, and seizures at birth. These mothers had taken an SSRI during pregnancy.
Conclusions
This article highlights some of the latest information on ST. Increased awareness of all clinicians and their patients may help decrease unnecessary comorbidities and death. Early identification of ST symptoms will increase the chances for survival, because of the rapid progression of symptoms within 24 hours. Most fatal reactions occur when combining MAOIs with SSRIs, SNRIs, or another MAOI. Overdose with an SSRI does not progress to the severe symptoms unless combined with another serotonin-elevating medication.
Education of all patients who are prescribed antidepressants must include awareness of the potential for serotonergic drug interactions, particularly from OTC medications, herbal medications, and illicit drugs. The diagnosis of ST is based on clinical findings and there must be a history of ingesting serotonin-elevating drug(s). Hunter Serotonin Toxicity Criteria is the gold standard for diagnosing symptoms along with consulting a toxicologist. Prevention of ST includes informed clinicians, patient education, careful prescribing and monitoring, and avoidance of multidrug regimens.
Serotonin, or 5-hydroxytryptamine (5-HT), is a chemical neurotransmitter in the central and peripheral nervous systems that was discovered in 1940s. 1 O ne of the most widely studied chemical messengers , serotonin influences many physiologic functions in humans, including regulation of mood, sleep-wake cycle, appetite suppression, memory, emesis, breathing, cognition, blood coagulation, libido, and many other functions. 2 In 1992, Insel and colleagues first document ed the toxic symptoms produced from too much serotonin in the central and peripheral nervous systems , naming it serotonin syndrome. 3,4
Serotonin Syndrome
Experts in the fields of psychiatry, pharmacy, and toxicology refer to these symptoms as serotonin toxicity, because the symptoms result from the toxic effects of too much serotonin.5-9 The term toxicity instead of syndrome “clarifies that it is a form of poisoning, just as lithium toxicity is a form of poisoning.”6 Therefore, serotonin toxicity (ST) can develop with administration of any serotonin-enhancing medication, including therapeutic use, polypharmacy, or accidental/intentional drug overdose.
The incidence of ST has increased over the past decade.5,6,10,11 Several reasons explain this increase: (1) ST mirrors the increase in depression in the US populations10,12,13; (2) There has been an increase in off-label antidepressant prescribing by both primary care and mental health providers14-16; (3) the increased use of illicit drugs13; (4) an increase in suicide attempts with antidepressants17; and (5) increased use of opioids for pain management, including both prescription and illicit use.11,14 This paper reviews the potential lethal combinations of commonly prescribed medications used to treat both veteran and nonveteran patients and includes the latest information on offending medications; a presentation of symptoms from in utero to adult; diagnostic criteria; and recommended treatments.
The Veterans Health Administration (VHA) and non-VHA health care providers can play a key role in identifying and preventing serotonin syndrome/ST by keeping abreast of the latest updates of potentially lethal drug combinations. Commonly prescribed medications with the potential for a reaction include antidepressants, anxiolytics, pain medications, antinausea medications, herbal medications, and over-the-counter (OTC) medications, such as cough suppressants. Patients may be at increased risk for ST due to the growth of polypharmacy management of comorbidities.
Antidepressants
Over the past decade, antidepressant use has increased substantially in the US,United Kingdom, and Canada.14 Also the types of antidepressants prescribed has changed and been replaced with the newer agents. The selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors (SNRIs) have replaced the older tricyclics (TCAs) and monoamine oxidase inhibitors (MAOIs) as first-line treatments for depression due to their improved comparative efficacy, reduced mortality following overdose, adverse effects (AEs) that are more tolerable for most patients, and the SSRIs have no anticholinergic properties (except paroxetine) (Table 1).18
In 2017 the National Institute of Mental Health reported that about 17 million adults and 3 million adolescents (aged 11-18 years) experienced at least 1 episode of major depression.19 About 40% of US veterans will experience depression, which is 3 times higher than the rate of the general US population.12 A random sampling survey conducted of about 17,000 active-duty service members by the US Department of Defense (DoD) from November 2015 to April 2016 revealed 9.4% reported depression.20 Antidepressant usage in the US and among veterans continues to increase.12,16 In 2018, the list of top US prescribed drugs, included sertraline (14th), citalopram (21st), trazodone (24th), and escitalopram (26th).21 Antidepressant prescribing in the US increased 18% from 2012 to 2017.22 This trend also continues within the military with a 40% increase of antidepressant use in the past decade.16
One reason for the increase in antidepressant use is off-label prescribing.14,23 A sampling of about 2 billion psychiatric outpatient visits in a western portion of the US found 12.9% of the prescriptions filled were off-label.15 In Minnesota, off-label prescribing of antidepressants was found to contribute to an increase in drug interactions in elderly nursing home residents.24 An investigation by the Military Times of the military community revealed off-label prescribing occurs not only with antidepressant medications, but also with anticonvulsants, antipsychotics, anti-anxiety drugs, and antiepileptic medications.14
A case report that brought ST to the forefront occurred in the 1980s and involved a college student.25 She was initially diagnosed with the flu. Her symptoms progressed over a 24-hour period despite treatment, leading to seizures, hyperthermia, generalized clonus, muscle rigidity, respiratory failure, and death because of unrecognized ST. Her combination of serotonin-elevating drugs included meperidine, phenelzine, chlorpheniramine, and haldol. On autopsy, there were traces of cocaine found in some of her tissue samples.
Pathophysiology
Tryptophan is a precursor of serotonin and must be ingested from foods, including meats, dairy, fruits, and seeds. About 90% of all serotonin is made in the gastrointestinal epithelium and is the major component of the brain-gut axis.26 Serotonin cannot cross the blood-brain barrier; therefore, it is synthesized and stored in presynaptic terminals around the midline of the brainstem.1,26 Transport of serotonin is provided by serotonin transporter (SERT).1,26,27 Once released, serotonin can either stimulate postsynaptic neuron receptors or is taken up into the presynaptic terminals for reuse. SSRI antidepressants, such as citalopram and paroxetine inhibit the reuptake of serotonin by binding to 2 different sites on the SERT thus allowing more available serotonin to be accessible to other neurons.27 There are 7 families of serotonin receptors, 5-HT1 to 5-HT7 and at least 15 mammalian subtypes.28,29 The majority of these receptors have been implicated in depression or depressive-like behavior as evidenced by the efficacy of increasing extracellular serotonin for the treatment of depression with SSRIs, SNRIs, TCAs, and MAOIs.29 Three of the most studied receptors include 5-HTIA,5-HT1B,and 5-HT2A.
Etiology
Most serotonin-induced drug fatalities occur when combining serotonergic drugs that work through different pathways (Table 2).30 The most toxic combination of serotonin-enhancing drugs includes MAOIs taken with SSRIs or SNRIs, or a combination of 2 MAOIs.5-9
Other potentially lethal combinations may includepolypharmacy with antidepressants, pain medications, OTC medications, and illicit drugs. Linezolid, a new synthetic antimicrobial, is considered to be a weak MAOI. Therefore, prescribing it with other serotonin-elevating agents has been reported to precipitate ST.18
Most cases of ST do not require hospitalization and can be managed by stopping the medication or decreasing the dose. Therapeutic doses of a single drug are highly unlikely to cause toxicity, although there have been reported cases of patients who are sensitive or more susceptible and develop symptoms after administration of a single agent and/or a dosage increase.
Delayed ST reactions have occurred because of a prolonged half-life of a drug, iron deficiency anemia, and coingestion of shorter acting serotonin antagonists.31 Most antidepressants have a short half-life (< 24 hours)except for fluoxetine. A decrease in iron may contribute to ST because iron is needed to process serotonin from tryptophan. An example of 2 shorter-acting serotonin antagonists include cyproheptadine and olanzapine. Cyproheptadine is used in the treatment of ST, and olanzapine is an antipsychotic.
Symptoms
Symptoms of ST range from mild to severe and include a combination of neuromuscular, autonomic, and mental status changes (Table 3).5,10 Mild symptoms of ST can start within 1 to 2 hours after ingesting a medication that increases serotonin to a toxic state unless the drug has a long half-life (eg, fluoxetine). Sometimes mild symptoms of ST can be difficult to distinguish from common drug AEs, flu symptoms, or viruses. Patients taking therapeutic doses of SSRIs can experience serotonin symptoms, such as lower limb hyperreflexia or a few beats of ankle clonus without being toxic. One thing to remember is that not all patients will start with mild symptoms and may present in moderate or severe distress.
Moderate-to-severe ST symptoms require hospitalization, usually in the intensive care unit (ICU). At this stage, clonus progresses from the lower extremities to the upper body and becomes more generalized. Ocular clonus can be continuous, intermittent, or have a ping pong effect (short cycle, periodic, alternating lateral gaze).
Severe ST is life threatening and leads to multiorgan failure within hours if not treated. The patient is intubated to assist with breathing and sedated because excess agitation and muscular tremors can increase temperature, which is already elevated by the time the symptoms reach the severe state. Of note, hyperthermia is due to a noninfectious elevation of body temperature from hypertonicity, agitation, and muscle rigidity.A true core temperature > 105.8°F causes irreversible cell damage, cerebral injury, and death.32,33 The patient can develop seizures and a coma. Multiorgan failure occurs, including rhabdomyolysis, myoglobinuria, renal failure, metabolic acidosis, acute respiratory distress, and disseminated intravascular coagulation.
Diagnosis
The diagnosis of ST is clinical and based on a history of ingesting serotonin-elevating medications and physical findings as per Hunter Serotonin Toxicity Criteria34 (Table 4). An in-depth history needs to include previous and current prescriptions, indications of the prescriptions (eg, therapeutic, increase in dosage, suicide intent), OTC medications, and illicit drug use. Early recognition of symptoms, identification of serotonergic medications, and appropriate resuscitative measures lead to more successful outcomes. A serotonin drug level is ineffective and does not correlate with the dosage since serotonin does not cross the blood-brain barrier.
The type of drug determines the length and response of the episode. The drug(s) elimination half-lives need to be calculated along with the pharmacokinetic or pharmacodynamics; agonist, antagonist, reuptake inhibitor, etc. Many drugs have half-lives of < 24 hours; therefore, reducing or eliminating the offending drug(s) will result in a steady reduction of symptoms.Exceptions include medications with a longer activity, such as the irreversible MAOIs (eg, phenelzine, isocarboxazid) and drugs with a longer half-life, such as fluoxetine. These types of medications may have been stopped weeks earlier and may prolong reduction of symptoms.
When initiating or increasing SSRIs or SNRIs, there are common nontoxic AEs that are not consistent with ST, including anxiety, restlessness, and irritability that may last for 2 weeks. The difference in toxic vs nontoxic reactions are the timing and rapid progression of symptoms. The toxic symptoms will start within hours of ingesting the offending agents(s) and progress rapidly to severe symptoms within 24 hours. Therefore, it is imperative to review AEs with the patient and or caregiver, so they may act as their own advocate and seek immediate assistance.
Differentials
There are symptoms specific to ST that can be used to differentiate it from other conditions. These include hyperthermia, bilateral symmetric clonus (inducible, spontaneous, ocular), and hyperreflexia.These criteria form the basis for Hunter criteria.
Differential diagnoses to consider include neuroleptic malignant syndrome; antidepressant initiation AEs; antidepressant discontinuation syndrome; malignant hyperthermia; anticholinergic toxicity; meningitis/encephalitis; sepsis; drug overdose; alcohol/benzodiazepine withdrawal; and preeclampsia. Neuroleptic malignant syndrome (NMS) is the disorder most often misdiagnosed as ST.Key elements that distinguish ST from NMS include the timing of the clinical course (NMS develops over days to weeks); the medications ingested (NMS from dopaminergic drugs); and the symptoms of NMS (bradyreflexia, bradykinesia, bradyphrenia, and no clonus).According to Gillman, serotonin toxicity is a manifestation of toxicity that is predictable and common with specific drug combinations, while NMS is a “rare idiosyncratic reaction to essentially normal doses and very rarely occurs after overdoses.”35 Preeclampsia is a pregnancy complication that can mimic ST with symptoms of hypertension, clonus, and hyperreflexia. It has been estimated to complicate 2% to 8% of pregnancies and remains a principle cause of maternal and fetal morbidity and mortality.36,37
Treatment
Mild-to-moderate symptoms usually resolve on their own 1 to 3 days after decreasing or stopping the offending drug. The timing will depend on the half-life or active metabolites of the drug. Treatment is largely supportive and may require treatment for control of agitation with benzodiazepines and IV fluids for dehydration/hypotension.14 In cases not responding to supportive care, treatment with oral cyproheptadine is recommended.14
There are other medications that have been used in treatment such as olanzapine, chlorpromazine, propranolol, bromocriptine, dantrolene, droperidol, and haloperidol, but their efficacy is unproven and not recommended.10 Chlorpromazine can cause hypotension and increase hyperthermia. Propranolol has a long duration of action, may cause a prolonged hypotension, and can mask tachycardia that can be used to monitor the effectiveness of treatment.10 Bromocriptine is a serotonin agonist and may exacerbate symptoms. Dantrolene has no effect on survival in animal models.10 Droperidol and haloperidol can worsen hyperthermia by inhibiting sweating.38
Mechanical ventilation should be considered especially if muscle rigidity progresses and depressed respiratory function occurs. If the temperature starts to rise, immediate sedation, paralysis, mechanical ventilation, and cyproheptadine are administered.The overall goal is prevention of hyperthermia, which leads to multiorgan failure. A core temperature of ≥ 104°F is associated with neurologic cell death, and recovery is minimal.32 Consultation with an experienced toxicologist is strongly recommended.Antipyretics should not be used, because elevated temperature is centrally mediated from muscle rigidity. If presentation occurs within 1 hour, activated charcoal can be used for detoxification of potentially lethal amounts.
Warning Label Controversies
In 2006, the US Food and Drug Administration (FDA) issued an advisory warning against concurrently using a tryptan antimigraine drug and serotonin-mediated medications.39 In 2018, a research team conducted a 14-year retrospective analysis on 20,000 patients who were coprescribed a tryptan drug with SSRIs or SNRIs.40 The study reported that the risk of ST was rare and suggested that the FDA reconsider their advisory. There are several other controversial medications with a ST FDA warning label due to their mechanisms of action and inaccurate case reports.41
Human Poisonings
Consistent with the 2017 American Association of Poison Control Centers Toxic Exposure report, antidepressants continue to be in the top 5 substance classes most frequently involved in human exposures.42 Most accidental ingestions of antidepressants occur in toddlers, whereas intentional ingestions are usually done by adolesents.43 Over the past 10 years, antidepressants are the No. 1 fastest growing category of human exposures in all age groups.42
ST in the Pediatric Population
ST in the pediatric population mirrors that in adults.Differences include the inability of the child to report symptoms, lack of clinician awareness, and reluctance of adolescents to disclose recreational drug use.Management is the same as for adults, including discontinuing the offending drug, supportive care, adequate sedation, oxygen, IV fluids, and continuous cardiac monitoring. Sedation is weight based for benzodiazepines. Mild-to-moderate reactions require admission for observation. Severe reactions require admission to the ICU.
There have been at least 4 published case reports of children aged < 6 years with moderate-to-severe ST secondary to acute vilazodone ingestion.44 The dosages included 5.5 to 37 mg/kg. All 4 patients had altered mental status, seizures, hyperthermia, mild clonus, tachycardia, and hypertension. They all survived with intensive care treatment, including intubation, sedation, cyproheptadine in 2 cases, activated charcoal and IV lorazepam in the other cases.
Direk and colleagues reported a case of a 12-year-old girl who was brought to the emergency department by her stepmother for seizurelike activity and was diagnosed with epilepsy and status epilepticus.45 In the pediatric ICU she developed tachycardia, fever, agitation, dilated pupils, tremors, increased deep tendon reflexes, spontaneous clonus, and horizontal ocular movements. A detailed clinical history was retaken and revealed that the child had been prescribed risperidone 1 week before by the psychiatric clinic due to behavioral problems, including stealing money, lying, and running away from home and school. On further investigation, the stepmother was taking clomipramine and discovered 9 missing pills.
Pregnancy and Lactation
The American College of Obstetricians and Gynecologists recommends that clinicians screen patients at least once during the perinatal period for depression and anxiety symptoms, using a standardized, validated tool and complete a full assessment of mood and emotional well-being during the postpartum, including screening for postpartum depression and anxiety with a validated instrument.46 Treatment with antidepressants is controversial. “Current evidence is generally reassuring and indicates that the absolute risks of negative infant outcomes are small except for PNAS [poor neonatal adaptation syndrome], which largely appears to be self-limited.”47 Antidepressants cross the human placenta and fetal blood-brain barrier.48 Several cases of infant toxicity from SSRIs have been reported with citalopram and escitalopram.49,50 Symptoms included severe muscle rigidity, lethargy, tachycardia, QTc prolongation, altered consciousness, hypertonia, and seizures at birth. These mothers had taken an SSRI during pregnancy.
Conclusions
This article highlights some of the latest information on ST. Increased awareness of all clinicians and their patients may help decrease unnecessary comorbidities and death. Early identification of ST symptoms will increase the chances for survival, because of the rapid progression of symptoms within 24 hours. Most fatal reactions occur when combining MAOIs with SSRIs, SNRIs, or another MAOI. Overdose with an SSRI does not progress to the severe symptoms unless combined with another serotonin-elevating medication.
Education of all patients who are prescribed antidepressants must include awareness of the potential for serotonergic drug interactions, particularly from OTC medications, herbal medications, and illicit drugs. The diagnosis of ST is based on clinical findings and there must be a history of ingesting serotonin-elevating drug(s). Hunter Serotonin Toxicity Criteria is the gold standard for diagnosing symptoms along with consulting a toxicologist. Prevention of ST includes informed clinicians, patient education, careful prescribing and monitoring, and avoidance of multidrug regimens.
1. Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176(3):1243-1251.
2. McCorvy JD, Roth BL. Structure and function of serotonin g protein coupled receptors. Pharmacol Ther. 2015;150:129-142. doi:10.1016/j.pharmthera. 2015.01.009 3. Insel TR, Roy BF, Cohen RM, Murphy DL. Possible development of serotonin syndrome in man. Am J Psychiatry. 1982;139(7):954-955. doi:10.1176/ajp.139.7.954
4. Scotton WJ, Hill LJ, Williams AC, Barnes NM. Serotonin syndrome: pathophysiology, clinical features, management, and potential future directions. Int J Tryptophan Res. 2019;12:1-14. doi:10.1177/1178646919873925
5. Buckley N, Dawson AH, Isbister GK. Serotonin syndrome. BMJ. 2014;348:g1626. doi:10.1136/bmj.g1626
6. Gillman KP. Serotonin toxicity: introduction. https://psychotropical.com/serotonin-toxicity-introduction. Published November 13, 2014. Updated July 13, 2019. Accessed August 17, 2020.
7. Foong A-L, Patel T, Kellar J, Grindrod KA. The scoop on serotonin syndrome. Can Pharm J (OTT). 2018;151(4):233-239. doi:10.1177/1715163518779096
8. Foong A-L, Grindrod KA, Patel T, Kellar J. Demystifying serotonin syndrome (or serotonin toxicity). Can Fam Physician. 2018;64(10):720-727.
9. Gillman KP. Serotonin toxicity: summary. https://psychotropical.com/serotonin-toxicity-summary. Published November 13, 2014. Updated January 25, 2018. Accessed August 17, 2020.
10. Boyer EW. Serotonin syndrome (serotonin toxicity). https://www.uptodate.com/contents/serotonin-syndrome-serotonin-toxicity. Updated March 12, 2018. Access December 12, 2019.
11. Wang RZ, Vashistha V, Kaur S, Houchens NW. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817. doi:10.3949/ccjm.83a.15129
12. Walker T. The economic burden of depression among veterans. https://www.managedhealthcareexecutive.com/article/economic-burden-depression-among-veterans. Published November 9, 2018. Accessed August 17, 2020.
13. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2019.
14. Wong J, Motulsky A, Abrahamowicz M, et al. Off label indications for antidepressants in primary care; descriptive study of prescriptions from an indication based electronic prescribing system. BMJ. 2017;356:j603. doi:org/10.1136/bmj.j603
15. Vijay A, Becker JE, Ross JS. Patterns and predictors of off-label prescription of psychiatric drugs. PLOS One. 2018;13(7):e0198363. doi:10.1371/journal.pone.0198363
16. Medicating the military—use of psychiatric drugs has spiked; concerns surface about suicide, other dangers. https://www.militarytimes.com/2013/03/29/medicating-the-military-use-of-psychiatric-drugs-has-spiked-concerns-surface-about-suicide-other-dangers. Published March 29, 2013. Accessed August 17, 2020.
17. Hengartner MP, Plöderl M. Newer-generation antidepressants and suicide risk in randomized controlled trials; a re-analysis of the FDA database [letter]. Psychother Psychosom. 2019:88:247-248. doi:10.1159/000501215
18. Rosebush PI. Serotonin syndrome. https://www.mhaus.org/nmsis/medical-education-programs/serotonin-syndrome. Accessed March 24, 2020.
19. National Institute of Mental Health. Major depression. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed March 24, 2020.
20. Meadows SO, Engel RL, Beckman RL, et al. 2015 health related behaviors survey: mental and emotional health among U.S. active-duty service members. https://www.rand.org/pubs/research_briefs/RB9955z3.html. Published 2018. Accessed August 17, 2020.
21. ClinCalc. The top 300 drugs of 2020. https://clincalc.com/DrugStats/Top300Drugs.aspx. Update February 11, 2017. Accessed March 24, 2020.
22. Corrigan C. Revealed: massive rise in antidepressant prescribing. https://www.rte.ie/news/investigations-unit/2019/0218/1031271-massive-rise-antidepressant-prescribing. Published June 14, 2019. Accessed August 17, 2020.
23. Skånland SS, Cieślar-Pobuda A. Off-label uses of drugs for depression. Eur J Pharmacol. 2019;865: 172732. doi:10.1016/j.ejphar.2019.172732
24. Bobo WV, Grossardt BR, Lapid MI, et al. Frequency and predictors of the potential overprescribing of antidepressants in elderly residents of a geographically defined U.S. population. Pharmacol Res Perspect. 2019;7(1):e00461. doi:10.1002/prp2.461
25. Patel N. Learning lessons. The Libby Zion case revisited. J Am Coll Cardiol. 2014;64(25):2802-2804. doi:10.1016/j.jacc.2014.11.007
26. Jenkins TA, Nguyen JCD, Polglase KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8(1):56. doi:10.3390/nu8010056
27. Coleman JA, Green EM, Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature Int J Sci. 2016;532(7599):334-339. doi:10.1038/nature17629
28. Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. 5-HT(1A) [corrected] receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology (Berl). 2013;231(4):623-636. doi:10.1007/s00213-013-3389-x
29. Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123. doi:10.12688/f1000research.9736.1
30. Francescangeli J, Karamchandani K, Powell M, Bonavia A. The serotonin syndrome: from molecular mechanisms to clinical practice. Int J Mol Sci. 2019;20(9):2288. doi:10.3390/ijms20092288
31. Little K, Lin CM, Reynolds PM. Delayed serotonin syndrome in the setting of a mixed fluoxetine and serotonin antagonist overdose. Am J Case Rep. 2018;19:604-607. doi:10.12659/AJCR.909063
32. Walter EJ, Carraretto M. The neurological and cognitive consequences of hyperthermia. Crit Care. 2016;20:199. doi:10.1186/s13054-016-1376-4

33. Platt M, Price T. Heat illness. In: Walls, ed. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Philadelphia, PA: Elsevier; 2018:1755-1764.
34. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642. doi:10.1093/qjmed/hcg109
35. Gillman KP. Serotonin toxicity contrasted with neuroleptic malignant syndrome. https://psychotropical.com/serotonin-syndrome-and-neuroleptic-malignant-syndrome. Published January 1, 2005. Updated November 6, 2017. Accessed August 17, 2020.
36. Asusta HB, Keyser E, Dominguez P, Miller M, Odedokun T. Serotonin syndrome in obstetrics: a case report and review of management. Mil Med. 2018;184(1-2):e284-e286. doi:10.1093/milmed/usy135
37. English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control 2015;8:7-12. doi:10.2147/IBPC.S50641
38. Bruggeman C, O’Day CS. Selective serotonin reuptake inhibitor (SSRI) toxicity. https://pubmed.ncbi.nlm.nih.gov/30521236. Published December 3, 2019. Accessed August 17, 2020.
39. US Food and Drug Administration. Selective serotonin reuptake inhibitors (SSRIs) Information. https://www.fda.gov/drugs/information-drug-class/selective-serotonin-reuptake-inhibitors-ssris-information. Updated December 23, 2014. Accessed March 24, 2020.
40. Orlova Y, Rizzoli P, Loder E. Association of coprescription of triptan antimigraine drugs and selective serotonin reuptake inhibitor or selective norepinephrine reuptake inhibitor antidepressants with serotonin syndrome. JAMA Neurol. 2018;75(5):566-572. doi:10.1001/jamaneurol.2017.5144
41. Gillman KP. Regulatory agencies (WH0, FDA) offer ill-conceived advice about serotonin toxicity (serotonin syndrome) with 5–HT3 antagonist: a worldwide problem. https://psychotropical.com/serotonin-toxicity-and-5-ht3-antagonists. Published November 13, 2014. Updated March 23, 2019. Accessed August 17, 2020.
42. Gummin DD, Mowry JB, Spyker DA, Brooks DE, Osterthaler KM, Banner W. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415. doi:10.1080/15563650.2018.1533727
43. Badawy MK, Maffei FA. Pediatric selective serotonin reuptake inhibitor toxicity. https://emedicine.medscape.com/article/1011436. Updated September 27, 2019. Accessed August 17, 2020.
44. Laliberte B, Kishk OA. Serotonin syndrome in a pediatric patient after vilazodone ingestion. Pediatr Emerg Care. 2018;34(12):e226-e228. doi:10.1097/PEC.0000000000001115
45. Direk MC, Yildirim V, Gϋnes S, Bozlu G, Okuyaz C. Serotonin syndrome after clomipramine overdose in a child. Clin Psychopharmacol Neurosci. 2016;14(4):388-390. doi:10.9758/cpn.2016.14.4.38846. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132(5):e208-e212. doi:10.1097/AOG.0000000000002927
47. Osborne LM, McEvoy K, Payne JL. Antidepressants in pregnancy: balancing needs and risks in clinical practice. Psychiatric Times. 2017;34(4).
48. Stewart D, Vigod S. Antenatal use of antidepressants and risk of teratogenicity and adverse pregnancy outcomes: selective serotonin reuptake inhibitors (SSRIs). https://www.uptodate.com/contents/antenatal-use-of-antidepressants-and-risk-of-teratogenicity-and-adverse-pregnancy-outcomes-selective-serotonin-reuptake-inhibitors-ssris. Accessed March 24, 2020.
49. Degiacomo J, Luedtke S. Neonatal toxicity from escitalopram use in utero: a case report. J Pediatr Pharmacol Ther. 2016;21(6):522-526. doi:10.5863/1551-6776-21.6.522
50. Eleftheriou G, Butera R, Cottini FC, Bonati M, Farina M. Neonatal toxicity following maternal citalopram treatment. Fetal Pediatr Pathol. 2013;32(5):362-356. doi:10.3109/15513815.2013.768743
1. Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176(3):1243-1251.
2. McCorvy JD, Roth BL. Structure and function of serotonin g protein coupled receptors. Pharmacol Ther. 2015;150:129-142. doi:10.1016/j.pharmthera. 2015.01.009 3. Insel TR, Roy BF, Cohen RM, Murphy DL. Possible development of serotonin syndrome in man. Am J Psychiatry. 1982;139(7):954-955. doi:10.1176/ajp.139.7.954
4. Scotton WJ, Hill LJ, Williams AC, Barnes NM. Serotonin syndrome: pathophysiology, clinical features, management, and potential future directions. Int J Tryptophan Res. 2019;12:1-14. doi:10.1177/1178646919873925
5. Buckley N, Dawson AH, Isbister GK. Serotonin syndrome. BMJ. 2014;348:g1626. doi:10.1136/bmj.g1626
6. Gillman KP. Serotonin toxicity: introduction. https://psychotropical.com/serotonin-toxicity-introduction. Published November 13, 2014. Updated July 13, 2019. Accessed August 17, 2020.
7. Foong A-L, Patel T, Kellar J, Grindrod KA. The scoop on serotonin syndrome. Can Pharm J (OTT). 2018;151(4):233-239. doi:10.1177/1715163518779096
8. Foong A-L, Grindrod KA, Patel T, Kellar J. Demystifying serotonin syndrome (or serotonin toxicity). Can Fam Physician. 2018;64(10):720-727.
9. Gillman KP. Serotonin toxicity: summary. https://psychotropical.com/serotonin-toxicity-summary. Published November 13, 2014. Updated January 25, 2018. Accessed August 17, 2020.
10. Boyer EW. Serotonin syndrome (serotonin toxicity). https://www.uptodate.com/contents/serotonin-syndrome-serotonin-toxicity. Updated March 12, 2018. Access December 12, 2019.
11. Wang RZ, Vashistha V, Kaur S, Houchens NW. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817. doi:10.3949/ccjm.83a.15129
12. Walker T. The economic burden of depression among veterans. https://www.managedhealthcareexecutive.com/article/economic-burden-depression-among-veterans. Published November 9, 2018. Accessed August 17, 2020.
13. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2019.
14. Wong J, Motulsky A, Abrahamowicz M, et al. Off label indications for antidepressants in primary care; descriptive study of prescriptions from an indication based electronic prescribing system. BMJ. 2017;356:j603. doi:org/10.1136/bmj.j603
15. Vijay A, Becker JE, Ross JS. Patterns and predictors of off-label prescription of psychiatric drugs. PLOS One. 2018;13(7):e0198363. doi:10.1371/journal.pone.0198363
16. Medicating the military—use of psychiatric drugs has spiked; concerns surface about suicide, other dangers. https://www.militarytimes.com/2013/03/29/medicating-the-military-use-of-psychiatric-drugs-has-spiked-concerns-surface-about-suicide-other-dangers. Published March 29, 2013. Accessed August 17, 2020.
17. Hengartner MP, Plöderl M. Newer-generation antidepressants and suicide risk in randomized controlled trials; a re-analysis of the FDA database [letter]. Psychother Psychosom. 2019:88:247-248. doi:10.1159/000501215
18. Rosebush PI. Serotonin syndrome. https://www.mhaus.org/nmsis/medical-education-programs/serotonin-syndrome. Accessed March 24, 2020.
19. National Institute of Mental Health. Major depression. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed March 24, 2020.
20. Meadows SO, Engel RL, Beckman RL, et al. 2015 health related behaviors survey: mental and emotional health among U.S. active-duty service members. https://www.rand.org/pubs/research_briefs/RB9955z3.html. Published 2018. Accessed August 17, 2020.
21. ClinCalc. The top 300 drugs of 2020. https://clincalc.com/DrugStats/Top300Drugs.aspx. Update February 11, 2017. Accessed March 24, 2020.
22. Corrigan C. Revealed: massive rise in antidepressant prescribing. https://www.rte.ie/news/investigations-unit/2019/0218/1031271-massive-rise-antidepressant-prescribing. Published June 14, 2019. Accessed August 17, 2020.
23. Skånland SS, Cieślar-Pobuda A. Off-label uses of drugs for depression. Eur J Pharmacol. 2019;865: 172732. doi:10.1016/j.ejphar.2019.172732
24. Bobo WV, Grossardt BR, Lapid MI, et al. Frequency and predictors of the potential overprescribing of antidepressants in elderly residents of a geographically defined U.S. population. Pharmacol Res Perspect. 2019;7(1):e00461. doi:10.1002/prp2.461
25. Patel N. Learning lessons. The Libby Zion case revisited. J Am Coll Cardiol. 2014;64(25):2802-2804. doi:10.1016/j.jacc.2014.11.007
26. Jenkins TA, Nguyen JCD, Polglase KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8(1):56. doi:10.3390/nu8010056
27. Coleman JA, Green EM, Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature Int J Sci. 2016;532(7599):334-339. doi:10.1038/nature17629
28. Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. 5-HT(1A) [corrected] receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology (Berl). 2013;231(4):623-636. doi:10.1007/s00213-013-3389-x
29. Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123. doi:10.12688/f1000research.9736.1
30. Francescangeli J, Karamchandani K, Powell M, Bonavia A. The serotonin syndrome: from molecular mechanisms to clinical practice. Int J Mol Sci. 2019;20(9):2288. doi:10.3390/ijms20092288
31. Little K, Lin CM, Reynolds PM. Delayed serotonin syndrome in the setting of a mixed fluoxetine and serotonin antagonist overdose. Am J Case Rep. 2018;19:604-607. doi:10.12659/AJCR.909063
32. Walter EJ, Carraretto M. The neurological and cognitive consequences of hyperthermia. Crit Care. 2016;20:199. doi:10.1186/s13054-016-1376-4

33. Platt M, Price T. Heat illness. In: Walls, ed. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Philadelphia, PA: Elsevier; 2018:1755-1764.
34. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642. doi:10.1093/qjmed/hcg109
35. Gillman KP. Serotonin toxicity contrasted with neuroleptic malignant syndrome. https://psychotropical.com/serotonin-syndrome-and-neuroleptic-malignant-syndrome. Published January 1, 2005. Updated November 6, 2017. Accessed August 17, 2020.
36. Asusta HB, Keyser E, Dominguez P, Miller M, Odedokun T. Serotonin syndrome in obstetrics: a case report and review of management. Mil Med. 2018;184(1-2):e284-e286. doi:10.1093/milmed/usy135
37. English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control 2015;8:7-12. doi:10.2147/IBPC.S50641
38. Bruggeman C, O’Day CS. Selective serotonin reuptake inhibitor (SSRI) toxicity. https://pubmed.ncbi.nlm.nih.gov/30521236. Published December 3, 2019. Accessed August 17, 2020.
39. US Food and Drug Administration. Selective serotonin reuptake inhibitors (SSRIs) Information. https://www.fda.gov/drugs/information-drug-class/selective-serotonin-reuptake-inhibitors-ssris-information. Updated December 23, 2014. Accessed March 24, 2020.
40. Orlova Y, Rizzoli P, Loder E. Association of coprescription of triptan antimigraine drugs and selective serotonin reuptake inhibitor or selective norepinephrine reuptake inhibitor antidepressants with serotonin syndrome. JAMA Neurol. 2018;75(5):566-572. doi:10.1001/jamaneurol.2017.5144
41. Gillman KP. Regulatory agencies (WH0, FDA) offer ill-conceived advice about serotonin toxicity (serotonin syndrome) with 5–HT3 antagonist: a worldwide problem. https://psychotropical.com/serotonin-toxicity-and-5-ht3-antagonists. Published November 13, 2014. Updated March 23, 2019. Accessed August 17, 2020.
42. Gummin DD, Mowry JB, Spyker DA, Brooks DE, Osterthaler KM, Banner W. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415. doi:10.1080/15563650.2018.1533727
43. Badawy MK, Maffei FA. Pediatric selective serotonin reuptake inhibitor toxicity. https://emedicine.medscape.com/article/1011436. Updated September 27, 2019. Accessed August 17, 2020.
44. Laliberte B, Kishk OA. Serotonin syndrome in a pediatric patient after vilazodone ingestion. Pediatr Emerg Care. 2018;34(12):e226-e228. doi:10.1097/PEC.0000000000001115
45. Direk MC, Yildirim V, Gϋnes S, Bozlu G, Okuyaz C. Serotonin syndrome after clomipramine overdose in a child. Clin Psychopharmacol Neurosci. 2016;14(4):388-390. doi:10.9758/cpn.2016.14.4.38846. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132(5):e208-e212. doi:10.1097/AOG.0000000000002927
47. Osborne LM, McEvoy K, Payne JL. Antidepressants in pregnancy: balancing needs and risks in clinical practice. Psychiatric Times. 2017;34(4).
48. Stewart D, Vigod S. Antenatal use of antidepressants and risk of teratogenicity and adverse pregnancy outcomes: selective serotonin reuptake inhibitors (SSRIs). https://www.uptodate.com/contents/antenatal-use-of-antidepressants-and-risk-of-teratogenicity-and-adverse-pregnancy-outcomes-selective-serotonin-reuptake-inhibitors-ssris. Accessed March 24, 2020.
49. Degiacomo J, Luedtke S. Neonatal toxicity from escitalopram use in utero: a case report. J Pediatr Pharmacol Ther. 2016;21(6):522-526. doi:10.5863/1551-6776-21.6.522
50. Eleftheriou G, Butera R, Cottini FC, Bonati M, Farina M. Neonatal toxicity following maternal citalopram treatment. Fetal Pediatr Pathol. 2013;32(5):362-356. doi:10.3109/15513815.2013.768743
Update on Pediatric Atopic Dermatitis
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that occurs most frequently in children but also affects many adolescents and adults. There has been a tremendous evolution of knowledge in AD, with insights into pathogenesis, epidemiology, impact of disease, and new therapies. A variety of studies examine the epidemiology of AD and associated comorbidities. The broad developments in disease state research are reflected in new publication numbers of AD citations on PubMed. A PubMed search of articles indexed for MEDLINE at the end of 2010 using the term atopic dermatitis would have shown 965 citations during the preceding 1-year period. In the 1-year period of June 2019 to June 2020, there were more than 2000 articles. The large body of research includes work of great significance in pediatric AD, and in this article we review recent findings that are important in understanding the progress being made in the field.
Epidemiology and Comorbidities
The epidemiology of AD has evolved over the last few decades, with emerging trends and novel insights into the burden of disease.1 In a recent cross-sectional study on the epidemiology of AD in children aged 6 to 11 years, the 1-year diagnosed AD prevalence estimates worldwide included the following: United States, 10.0%; Canada, 13.3%; the EU5 Countries, 15.5%; Japan, 10.3%; and all countries studied, 12.2%.2 Another recent paper that analyzed data from the Fragile Families and Child Wellbeing Study showed that the prevalence and persistence of AD in urban US children was 15.0%.3Although pediatric AD may spontaneously remit over time, disease continuing into adolescence and adulthood is common. Paternoster et al4 studied the longitudinal course of AD in children from 2 birth cohort prospective studies, showing distinct AD phenotypes having differing course trajectories over time. Disease subsets included patients with early-onset-persistent and early-onset-late-resolving disease.4 Whether phenotyping or subgroup analysis can be used to predict disease course or risk for development of comorbidities is unknown, but it is interesting to consider how such work could influence tailoring of specific therapies to early disease presentation.
Atopic dermatitis poses a serious public health burden owing to its high prevalence, considerable morbidity and disability, increased health care utilization, and cost of care.1 Recent studies have found notably higher rates of multiple medical and mental health comorbidities in both children and adults with AD, including infections, atopic comorbidities (eg, allergic rhinitis, asthma, food allergies), eye diseases (eg, keratitis, conjunctivitis, keratoconus), and possible cardiovascular diseases and autoimmune disorders.1,5-9 Allergic comorbidities are quite common in pediatric AD patients.10 In a recent study examining the efficacy and safety of dupilumab monotherapy in 251 adolescents with moderate to severe inadequately controlled AD, most had comorbid type 2 diseases including asthma (53.6%), food allergies (60.8%), and allergic rhinitis (65.6%).11
Quality of Life/Life Impact of AD
Pediatric AD has a major impact on the quality of life of patients and their families.12 The well-being and development of children are strongly influenced by the physical and psychosocial health of parents/guardians. Two studies by Ramirez and colleagues13,14 published in 2019 examined sleep disturbances and exhaustion in mothers of children with AD. Data for the studies came from the Avon Longitudinal Study of Parents and Children. Children with active AD reported worse sleep quality than those without AD, with nearly 50% higher odds of sleep-quality disturbances. Analysis of the cohort data from 11,649 mother-child pairs who were followed up with a time-varying measure of child AD activity and severity as well as self-reported maternal sleep measures repeated at multiple time points for children aged 6 months to 11 years showed that mothers of children with AD reported difficulty falling asleep, subjectively insufficient sleep, and daytime exhaustion throughout the first 11 years of childhood.13,14 These data suggest that sleep disturbance may be a family affair.
A cross-sectional, real-world study on the burden of AD in children aged 6 to 11 years assessed by self-report demonstrated a substantial and multidimensional impact of AD, including itch, sleep disturbance, skin pain, and health-related quality-of-life impact, as well as comorbidities and school productivity losses. The burden associated with AD was remarkable and increased with disease severity.15
Drucker et al16 completed a comprehensive literature review on the burden of AD, summarized as a report for the National Eczema Association. Quality-of-life impact on pediatric patients included high rates of emotional distress; social isolation; depression; limitations in activities due to lesions with fear of triggers; and behavioral problems such as irritability, crying, and sleep disturbance resulting in difficulty performing at school.16 The psychological impact on children as well as emotional and behavioral difficulties may impact the ability for parents/guardians to implement treatment plans.17
There is a striking association between mental health disorders and AD in the US pediatric population, with a clear dose-dependent relationship that has been observed between the prevalence of a mental health disorder and the reported severity of the skin disease. Data suggest children with AD may be at increased risk for developing mental health disorders. The National Survey of Children’s Health found statistically significant increases in the likelihood of attention deficit hyperactivity disorder (odds ratio [OR], 1.87), depression (OR, 1.81), anxiety (OR, 1.77), conduct disorder (OR, 1.87), and autism (OR, 3.04).6
Evolving Practices and Therapies
Bathing Practices
There has long been much controversy regarding best bathing habits for patients with AD. In a 2009 study, cutaneous hydration was quantified after various bathing and moisturizing regimens.18 The study showed clear benefits of emollient application on skin hydration, either after bathing or without bathing. Bathing followed by emollient applications did not decrease skin hydration in contrast to bathing without emollient application.18
There are limited studies evaluating bathing frequency in pediatric patients, and many families receive conflicting information regarding best practice. In one study that surveyed 354 parents, more than 75% of parents/guardians who had seen multiple providers for their child’s AD reported a substantial amount of confusion and frustration from conflicting advice on bathing frequency.19 Cardona et al20 undertook a randomized clinical trial of frequent bathing and moisturizing vs less-frequent bathing and moisturizing in pediatric patients with AD aged 6 months to 11 years. Patients were divided into 2 groups: 1 being bathed twice daily with immediate moisturizer application and the other being bathed twice weekly followed by moisturization, then a switch to the other method. Patients used standardized topical corticosteroids (TCSs) in both groups. There were significant improvements in scoring AD and other objective measures during the frequent bathing time period vs infrequent bathing; in the group that bathed more frequently, SCORAD (SCORing Atopic Dermatitis) decreased by 21.2 compared with the group that bathed less frequently (95% confidence interval, 14.9-27.6; P<.0001). These findings suggest that more-frequent bathing with immediate moisturization is superior as an acute treatment intervention for improving AD disease severity in comparison to less-frequent bathing with immediate moisturization.20
Expanding Treatment Options
Topical Phosphodiesterase Inhibitors
There are several new and evolving topical therapies in AD. Crisaborole ointment 2% is a steroid-free phosphodiesterase inhibitor approved in 2016 by the US Food and Drug Administration (FDA) for mild to moderate AD in patients aged 2 years and older. A recent multicenter, open-label, single-arm study in 137 infants (CrisADe CARE 1) evaluated the pharmacokinetics and efficacy of crisaborole ointment 2% applied twice daily for 4 weeks in pediatric patients aged 3 months to less than 24 months of age with mild to moderate AD.21 The study had 2 cohorts: one with a minimum of 5% body surface area involvement and another (the pharmacokinetic cohort) with a minimum of 35% body surface area involvement. Both cohorts demonstrated similar efficacy data. From baseline to day 29, the mean percentage change in eczema area and severity index (EASI) score was −57.5%, and an investigator global assessment (IGA) score of clear or almost clear with at least a 2-grade improvement was achieved in 30.2% of patients. Crisaborole systemic exposures in infants were comparable with those in patients aged 2 years or older. Patients tolerated crisaborole well, with a 4% rate of burning, which was similar to other studies in children and adults but perhaps lower than seen in clinical practice. Pharmacokinetic studies did not show any remarkable noticeable concern with accumulation of propylene glycol absorption.21
Based on the CrisADe CARE 1 study data, in March 2020 the FDA extended the indication of crisaborole ointment 2% from a prior lower age limit of 24 months to approval for use in treating mild to moderate AD in children as young as 3 months, making it the first nonsteroidal topical anti-inflammatory medication to be approved in children younger than 2 years in the United States.
Evolving Topical Therapies
Topical Janus Kinase Inhibitors
Ruxolitinib is a potent inhibitor of Janus kinase 1 (JAK-1) and Janus kinase 2 (JAK-2) and has been developed in topical formulations. In recent phase 3 clinical trials of patients with AD aged 12 years and older with mild to moderate disease (TRuE-AD1 and TRuE-AD2), more than half of the patients treated with either ruxolitinib cream in a 0.75% or 1.5% concentration reached EASI-75 after 8 weeks of treatment.22 Additionally, more patients treated with topical ruxolitinib reached an IGA score of clear to almost clear than patients treated with vehicle at the end of treatment. Thus far, it appears to be very well tolerated, significantly decreases EASI score (P<.0001), and improves overall pruritus.22
Delgocitinib is a topical pan-JAK inhibitor that blocks several cytokine-signaling cascade pathways. It was first developed and approved in Japan in an ointment formulation for use in patients with AD aged 16 years and older.23 The efficacy and safety profile of delgocitinib is currently being evaluated in pediatric patients with AD in Japan. In a recent phase 2 clinical study of 103 Japanese patients aged 2 to 15 years with moderate to severe AD, patients were randomized to receive either delgocitinib ointment in 0.25% or 0.5% concentrations or vehicle ointment twice daily for 4 weeks. The proportion of patients with a modified EASI-75 score was 38.2% (13/34) in the 0.25% group and 50.0% (17/34) in the 0.5% group vs 8.6% (3/35) in the placebo group. More patients treated with delgocitinib ointment received an IGA score of clear or almost clear than patients treated with vehicle at the end of treatment. Overall, both delgocitinib groups demonstrated superior improvement in clinical symptoms and signs without notable side effects.24
Tapinarof
Tapinarof is a topical therapeutic aryl hydrocarbon receptor agonist. In a recent phase 2 randomized study of 2 concentrations and 2 frequencies of tapinarof cream vs vehicle in 247 randomized patients aged 12 to 65 years with moderate to severe disease, tapinarof demonstrated greater success with both concentrations than vehicle at all visits beyond week 2.25 Additionally, in patients treated with tapinarof cream 1%, nearly 50% reached an IGA score of clear to almost clear with at least a 2-grade improvement. More than 50% of patients achieved EASI-75 improvement at 12 weeks of treatment with tapinarof cream 1% used daily. These findings suggest that tapinarof may be an efficacious and well-tolerated treatment for both adolescents and adults with AD; however, large confirmation trials are needed to further investigate.25
Systemic Treatments
Oral JAK Inhibitors
Some of the most exciting novel therapies include several oral JAK inhibitors that target different combinations of kinases and have been shown to decrease AD severity and symptoms. Some of these agents have indications in other disease states, such as baricitinib and upadacitinib, which are both FDA approved for the treatment of rheumatoid arthritis, whereas others, such as abrocitinib, have been studied specifically for AD.
Although some agents have only been studied in adults to date, others have included adolescents in their core studies, such as abrocitinib, which received Breakthrough Therapy designation from the FDA for the treatment of patients with moderate to severe AD in February 2018. In recent phase 3 trials of patients aged 12 years and older with moderate to severe AD (JADE MONO-1 and JADE MONO-2), both doses of abrocitinib improved the IGA and EASI-75 outcomes compared with placebo.26 Additional studies will be conducted to further investigate the relative efficacy and safety in patients younger than 18 years.
Biologics
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling without suppressing the immune system. It is approved for use in patients aged 12 years and older with moderate to severe asthma and in adults with chronic rhinosinusitis with nasal polyposis. It is the first biologic to show positive results in the moderate to severe pediatric AD population. There are now extended data available exhibiting sustained benefit in adolescent patients who were continued on dupilumab therapy, evidenced by further improvement in EASI scores at the 1-year mark.27
Recently, dupilumab received approval for use in patients aged 6 to 11 years, making it the first biologic for AD to be approved for use in patients younger than 12 years. The expedited FDA approval was based on the phase 3 results in which the efficacy and safety of dupilumab combined with TCSs were compared to TCSs alone (N=367).28 In this trial, more than twice as many children achieved clear or almost clear skin and more than 4 times as many achieved itch reduction with dupilumab plus TCSs than with TCSs alone. Three-quarters of patients receiving dupilumab at the subsequently approved dosing achieved at least a 75% improvement in overall disease.28 An additional study is being conducted that includes pediatric patients aged 6 months to younger than 6 years (ClinicalTrials.gov Identifier NCT03346434).
Future Directions in Pediatric AD
Our review summarizes only some of the agents under clinical investigation for use in pediatric AD. Early treatment to establish excellent long-term disease control with aggressive topical regimens or with systemic agents may alter the course of AD and influence the development of comorbidities, though this has not yet been shown in clinical studies. The long-term impact of early treatment, along with many other intriguing issues, will be studied more in the near future.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paper presented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1.
- Paternoster L, Savenije OEM, Heron J, et al. IJ Allergy Clin Immunol. 2018;141:964-971.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Yaghmaie P, Koudelka CW, Simpson Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
- Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468.
- al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.
- Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074-1081.
- Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821-838.
- Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis.
- Quality of life in families with children with atopic dermatitis. Pediatr Dermatol. 2016;33:28-32.
- Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556-563.
- Association of atopic dermatitis with sleep quality in children.
- Weidinger S, Simpson EL, Eckert L, et al. The patient-reported disease burden in pediatric patients with atopic dermatitis: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paperpresented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag. 2018;11:289-298.
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Kempe E, Jain N, Cardona I. Bathing frequency recommendations for pediatric atopic dermatitis: are we adding to parental frustration? Ann Allergy Asthma Immunol. 2013;111:298‐299.
- Cardona ID, Kempe EE, Lary C, et al. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8:1014‐1021.
- Gower , Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275-284.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment atopic dermatitis: results from two phase 3, randomized, double-blind studies. Presented at: 2nd Annual Revolutionizing Atopic Dermatitis Conference; April 5, 2020; Chicago, IL.
- Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609‐615.
- Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575‐1583.
- Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89‐98.e3.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85‐96.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published online June 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.054.
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that occurs most frequently in children but also affects many adolescents and adults. There has been a tremendous evolution of knowledge in AD, with insights into pathogenesis, epidemiology, impact of disease, and new therapies. A variety of studies examine the epidemiology of AD and associated comorbidities. The broad developments in disease state research are reflected in new publication numbers of AD citations on PubMed. A PubMed search of articles indexed for MEDLINE at the end of 2010 using the term atopic dermatitis would have shown 965 citations during the preceding 1-year period. In the 1-year period of June 2019 to June 2020, there were more than 2000 articles. The large body of research includes work of great significance in pediatric AD, and in this article we review recent findings that are important in understanding the progress being made in the field.
Epidemiology and Comorbidities
The epidemiology of AD has evolved over the last few decades, with emerging trends and novel insights into the burden of disease.1 In a recent cross-sectional study on the epidemiology of AD in children aged 6 to 11 years, the 1-year diagnosed AD prevalence estimates worldwide included the following: United States, 10.0%; Canada, 13.3%; the EU5 Countries, 15.5%; Japan, 10.3%; and all countries studied, 12.2%.2 Another recent paper that analyzed data from the Fragile Families and Child Wellbeing Study showed that the prevalence and persistence of AD in urban US children was 15.0%.3Although pediatric AD may spontaneously remit over time, disease continuing into adolescence and adulthood is common. Paternoster et al4 studied the longitudinal course of AD in children from 2 birth cohort prospective studies, showing distinct AD phenotypes having differing course trajectories over time. Disease subsets included patients with early-onset-persistent and early-onset-late-resolving disease.4 Whether phenotyping or subgroup analysis can be used to predict disease course or risk for development of comorbidities is unknown, but it is interesting to consider how such work could influence tailoring of specific therapies to early disease presentation.
Atopic dermatitis poses a serious public health burden owing to its high prevalence, considerable morbidity and disability, increased health care utilization, and cost of care.1 Recent studies have found notably higher rates of multiple medical and mental health comorbidities in both children and adults with AD, including infections, atopic comorbidities (eg, allergic rhinitis, asthma, food allergies), eye diseases (eg, keratitis, conjunctivitis, keratoconus), and possible cardiovascular diseases and autoimmune disorders.1,5-9 Allergic comorbidities are quite common in pediatric AD patients.10 In a recent study examining the efficacy and safety of dupilumab monotherapy in 251 adolescents with moderate to severe inadequately controlled AD, most had comorbid type 2 diseases including asthma (53.6%), food allergies (60.8%), and allergic rhinitis (65.6%).11
Quality of Life/Life Impact of AD
Pediatric AD has a major impact on the quality of life of patients and their families.12 The well-being and development of children are strongly influenced by the physical and psychosocial health of parents/guardians. Two studies by Ramirez and colleagues13,14 published in 2019 examined sleep disturbances and exhaustion in mothers of children with AD. Data for the studies came from the Avon Longitudinal Study of Parents and Children. Children with active AD reported worse sleep quality than those without AD, with nearly 50% higher odds of sleep-quality disturbances. Analysis of the cohort data from 11,649 mother-child pairs who were followed up with a time-varying measure of child AD activity and severity as well as self-reported maternal sleep measures repeated at multiple time points for children aged 6 months to 11 years showed that mothers of children with AD reported difficulty falling asleep, subjectively insufficient sleep, and daytime exhaustion throughout the first 11 years of childhood.13,14 These data suggest that sleep disturbance may be a family affair.
A cross-sectional, real-world study on the burden of AD in children aged 6 to 11 years assessed by self-report demonstrated a substantial and multidimensional impact of AD, including itch, sleep disturbance, skin pain, and health-related quality-of-life impact, as well as comorbidities and school productivity losses. The burden associated with AD was remarkable and increased with disease severity.15
Drucker et al16 completed a comprehensive literature review on the burden of AD, summarized as a report for the National Eczema Association. Quality-of-life impact on pediatric patients included high rates of emotional distress; social isolation; depression; limitations in activities due to lesions with fear of triggers; and behavioral problems such as irritability, crying, and sleep disturbance resulting in difficulty performing at school.16 The psychological impact on children as well as emotional and behavioral difficulties may impact the ability for parents/guardians to implement treatment plans.17
There is a striking association between mental health disorders and AD in the US pediatric population, with a clear dose-dependent relationship that has been observed between the prevalence of a mental health disorder and the reported severity of the skin disease. Data suggest children with AD may be at increased risk for developing mental health disorders. The National Survey of Children’s Health found statistically significant increases in the likelihood of attention deficit hyperactivity disorder (odds ratio [OR], 1.87), depression (OR, 1.81), anxiety (OR, 1.77), conduct disorder (OR, 1.87), and autism (OR, 3.04).6
Evolving Practices and Therapies
Bathing Practices
There has long been much controversy regarding best bathing habits for patients with AD. In a 2009 study, cutaneous hydration was quantified after various bathing and moisturizing regimens.18 The study showed clear benefits of emollient application on skin hydration, either after bathing or without bathing. Bathing followed by emollient applications did not decrease skin hydration in contrast to bathing without emollient application.18
There are limited studies evaluating bathing frequency in pediatric patients, and many families receive conflicting information regarding best practice. In one study that surveyed 354 parents, more than 75% of parents/guardians who had seen multiple providers for their child’s AD reported a substantial amount of confusion and frustration from conflicting advice on bathing frequency.19 Cardona et al20 undertook a randomized clinical trial of frequent bathing and moisturizing vs less-frequent bathing and moisturizing in pediatric patients with AD aged 6 months to 11 years. Patients were divided into 2 groups: 1 being bathed twice daily with immediate moisturizer application and the other being bathed twice weekly followed by moisturization, then a switch to the other method. Patients used standardized topical corticosteroids (TCSs) in both groups. There were significant improvements in scoring AD and other objective measures during the frequent bathing time period vs infrequent bathing; in the group that bathed more frequently, SCORAD (SCORing Atopic Dermatitis) decreased by 21.2 compared with the group that bathed less frequently (95% confidence interval, 14.9-27.6; P<.0001). These findings suggest that more-frequent bathing with immediate moisturization is superior as an acute treatment intervention for improving AD disease severity in comparison to less-frequent bathing with immediate moisturization.20
Expanding Treatment Options
Topical Phosphodiesterase Inhibitors
There are several new and evolving topical therapies in AD. Crisaborole ointment 2% is a steroid-free phosphodiesterase inhibitor approved in 2016 by the US Food and Drug Administration (FDA) for mild to moderate AD in patients aged 2 years and older. A recent multicenter, open-label, single-arm study in 137 infants (CrisADe CARE 1) evaluated the pharmacokinetics and efficacy of crisaborole ointment 2% applied twice daily for 4 weeks in pediatric patients aged 3 months to less than 24 months of age with mild to moderate AD.21 The study had 2 cohorts: one with a minimum of 5% body surface area involvement and another (the pharmacokinetic cohort) with a minimum of 35% body surface area involvement. Both cohorts demonstrated similar efficacy data. From baseline to day 29, the mean percentage change in eczema area and severity index (EASI) score was −57.5%, and an investigator global assessment (IGA) score of clear or almost clear with at least a 2-grade improvement was achieved in 30.2% of patients. Crisaborole systemic exposures in infants were comparable with those in patients aged 2 years or older. Patients tolerated crisaborole well, with a 4% rate of burning, which was similar to other studies in children and adults but perhaps lower than seen in clinical practice. Pharmacokinetic studies did not show any remarkable noticeable concern with accumulation of propylene glycol absorption.21
Based on the CrisADe CARE 1 study data, in March 2020 the FDA extended the indication of crisaborole ointment 2% from a prior lower age limit of 24 months to approval for use in treating mild to moderate AD in children as young as 3 months, making it the first nonsteroidal topical anti-inflammatory medication to be approved in children younger than 2 years in the United States.
Evolving Topical Therapies
Topical Janus Kinase Inhibitors
Ruxolitinib is a potent inhibitor of Janus kinase 1 (JAK-1) and Janus kinase 2 (JAK-2) and has been developed in topical formulations. In recent phase 3 clinical trials of patients with AD aged 12 years and older with mild to moderate disease (TRuE-AD1 and TRuE-AD2), more than half of the patients treated with either ruxolitinib cream in a 0.75% or 1.5% concentration reached EASI-75 after 8 weeks of treatment.22 Additionally, more patients treated with topical ruxolitinib reached an IGA score of clear to almost clear than patients treated with vehicle at the end of treatment. Thus far, it appears to be very well tolerated, significantly decreases EASI score (P<.0001), and improves overall pruritus.22
Delgocitinib is a topical pan-JAK inhibitor that blocks several cytokine-signaling cascade pathways. It was first developed and approved in Japan in an ointment formulation for use in patients with AD aged 16 years and older.23 The efficacy and safety profile of delgocitinib is currently being evaluated in pediatric patients with AD in Japan. In a recent phase 2 clinical study of 103 Japanese patients aged 2 to 15 years with moderate to severe AD, patients were randomized to receive either delgocitinib ointment in 0.25% or 0.5% concentrations or vehicle ointment twice daily for 4 weeks. The proportion of patients with a modified EASI-75 score was 38.2% (13/34) in the 0.25% group and 50.0% (17/34) in the 0.5% group vs 8.6% (3/35) in the placebo group. More patients treated with delgocitinib ointment received an IGA score of clear or almost clear than patients treated with vehicle at the end of treatment. Overall, both delgocitinib groups demonstrated superior improvement in clinical symptoms and signs without notable side effects.24
Tapinarof
Tapinarof is a topical therapeutic aryl hydrocarbon receptor agonist. In a recent phase 2 randomized study of 2 concentrations and 2 frequencies of tapinarof cream vs vehicle in 247 randomized patients aged 12 to 65 years with moderate to severe disease, tapinarof demonstrated greater success with both concentrations than vehicle at all visits beyond week 2.25 Additionally, in patients treated with tapinarof cream 1%, nearly 50% reached an IGA score of clear to almost clear with at least a 2-grade improvement. More than 50% of patients achieved EASI-75 improvement at 12 weeks of treatment with tapinarof cream 1% used daily. These findings suggest that tapinarof may be an efficacious and well-tolerated treatment for both adolescents and adults with AD; however, large confirmation trials are needed to further investigate.25
Systemic Treatments
Oral JAK Inhibitors
Some of the most exciting novel therapies include several oral JAK inhibitors that target different combinations of kinases and have been shown to decrease AD severity and symptoms. Some of these agents have indications in other disease states, such as baricitinib and upadacitinib, which are both FDA approved for the treatment of rheumatoid arthritis, whereas others, such as abrocitinib, have been studied specifically for AD.
Although some agents have only been studied in adults to date, others have included adolescents in their core studies, such as abrocitinib, which received Breakthrough Therapy designation from the FDA for the treatment of patients with moderate to severe AD in February 2018. In recent phase 3 trials of patients aged 12 years and older with moderate to severe AD (JADE MONO-1 and JADE MONO-2), both doses of abrocitinib improved the IGA and EASI-75 outcomes compared with placebo.26 Additional studies will be conducted to further investigate the relative efficacy and safety in patients younger than 18 years.
Biologics
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling without suppressing the immune system. It is approved for use in patients aged 12 years and older with moderate to severe asthma and in adults with chronic rhinosinusitis with nasal polyposis. It is the first biologic to show positive results in the moderate to severe pediatric AD population. There are now extended data available exhibiting sustained benefit in adolescent patients who were continued on dupilumab therapy, evidenced by further improvement in EASI scores at the 1-year mark.27
Recently, dupilumab received approval for use in patients aged 6 to 11 years, making it the first biologic for AD to be approved for use in patients younger than 12 years. The expedited FDA approval was based on the phase 3 results in which the efficacy and safety of dupilumab combined with TCSs were compared to TCSs alone (N=367).28 In this trial, more than twice as many children achieved clear or almost clear skin and more than 4 times as many achieved itch reduction with dupilumab plus TCSs than with TCSs alone. Three-quarters of patients receiving dupilumab at the subsequently approved dosing achieved at least a 75% improvement in overall disease.28 An additional study is being conducted that includes pediatric patients aged 6 months to younger than 6 years (ClinicalTrials.gov Identifier NCT03346434).
Future Directions in Pediatric AD
Our review summarizes only some of the agents under clinical investigation for use in pediatric AD. Early treatment to establish excellent long-term disease control with aggressive topical regimens or with systemic agents may alter the course of AD and influence the development of comorbidities, though this has not yet been shown in clinical studies. The long-term impact of early treatment, along with many other intriguing issues, will be studied more in the near future.
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that occurs most frequently in children but also affects many adolescents and adults. There has been a tremendous evolution of knowledge in AD, with insights into pathogenesis, epidemiology, impact of disease, and new therapies. A variety of studies examine the epidemiology of AD and associated comorbidities. The broad developments in disease state research are reflected in new publication numbers of AD citations on PubMed. A PubMed search of articles indexed for MEDLINE at the end of 2010 using the term atopic dermatitis would have shown 965 citations during the preceding 1-year period. In the 1-year period of June 2019 to June 2020, there were more than 2000 articles. The large body of research includes work of great significance in pediatric AD, and in this article we review recent findings that are important in understanding the progress being made in the field.
Epidemiology and Comorbidities
The epidemiology of AD has evolved over the last few decades, with emerging trends and novel insights into the burden of disease.1 In a recent cross-sectional study on the epidemiology of AD in children aged 6 to 11 years, the 1-year diagnosed AD prevalence estimates worldwide included the following: United States, 10.0%; Canada, 13.3%; the EU5 Countries, 15.5%; Japan, 10.3%; and all countries studied, 12.2%.2 Another recent paper that analyzed data from the Fragile Families and Child Wellbeing Study showed that the prevalence and persistence of AD in urban US children was 15.0%.3Although pediatric AD may spontaneously remit over time, disease continuing into adolescence and adulthood is common. Paternoster et al4 studied the longitudinal course of AD in children from 2 birth cohort prospective studies, showing distinct AD phenotypes having differing course trajectories over time. Disease subsets included patients with early-onset-persistent and early-onset-late-resolving disease.4 Whether phenotyping or subgroup analysis can be used to predict disease course or risk for development of comorbidities is unknown, but it is interesting to consider how such work could influence tailoring of specific therapies to early disease presentation.
Atopic dermatitis poses a serious public health burden owing to its high prevalence, considerable morbidity and disability, increased health care utilization, and cost of care.1 Recent studies have found notably higher rates of multiple medical and mental health comorbidities in both children and adults with AD, including infections, atopic comorbidities (eg, allergic rhinitis, asthma, food allergies), eye diseases (eg, keratitis, conjunctivitis, keratoconus), and possible cardiovascular diseases and autoimmune disorders.1,5-9 Allergic comorbidities are quite common in pediatric AD patients.10 In a recent study examining the efficacy and safety of dupilumab monotherapy in 251 adolescents with moderate to severe inadequately controlled AD, most had comorbid type 2 diseases including asthma (53.6%), food allergies (60.8%), and allergic rhinitis (65.6%).11
Quality of Life/Life Impact of AD
Pediatric AD has a major impact on the quality of life of patients and their families.12 The well-being and development of children are strongly influenced by the physical and psychosocial health of parents/guardians. Two studies by Ramirez and colleagues13,14 published in 2019 examined sleep disturbances and exhaustion in mothers of children with AD. Data for the studies came from the Avon Longitudinal Study of Parents and Children. Children with active AD reported worse sleep quality than those without AD, with nearly 50% higher odds of sleep-quality disturbances. Analysis of the cohort data from 11,649 mother-child pairs who were followed up with a time-varying measure of child AD activity and severity as well as self-reported maternal sleep measures repeated at multiple time points for children aged 6 months to 11 years showed that mothers of children with AD reported difficulty falling asleep, subjectively insufficient sleep, and daytime exhaustion throughout the first 11 years of childhood.13,14 These data suggest that sleep disturbance may be a family affair.
A cross-sectional, real-world study on the burden of AD in children aged 6 to 11 years assessed by self-report demonstrated a substantial and multidimensional impact of AD, including itch, sleep disturbance, skin pain, and health-related quality-of-life impact, as well as comorbidities and school productivity losses. The burden associated with AD was remarkable and increased with disease severity.15
Drucker et al16 completed a comprehensive literature review on the burden of AD, summarized as a report for the National Eczema Association. Quality-of-life impact on pediatric patients included high rates of emotional distress; social isolation; depression; limitations in activities due to lesions with fear of triggers; and behavioral problems such as irritability, crying, and sleep disturbance resulting in difficulty performing at school.16 The psychological impact on children as well as emotional and behavioral difficulties may impact the ability for parents/guardians to implement treatment plans.17
There is a striking association between mental health disorders and AD in the US pediatric population, with a clear dose-dependent relationship that has been observed between the prevalence of a mental health disorder and the reported severity of the skin disease. Data suggest children with AD may be at increased risk for developing mental health disorders. The National Survey of Children’s Health found statistically significant increases in the likelihood of attention deficit hyperactivity disorder (odds ratio [OR], 1.87), depression (OR, 1.81), anxiety (OR, 1.77), conduct disorder (OR, 1.87), and autism (OR, 3.04).6
Evolving Practices and Therapies
Bathing Practices
There has long been much controversy regarding best bathing habits for patients with AD. In a 2009 study, cutaneous hydration was quantified after various bathing and moisturizing regimens.18 The study showed clear benefits of emollient application on skin hydration, either after bathing or without bathing. Bathing followed by emollient applications did not decrease skin hydration in contrast to bathing without emollient application.18
There are limited studies evaluating bathing frequency in pediatric patients, and many families receive conflicting information regarding best practice. In one study that surveyed 354 parents, more than 75% of parents/guardians who had seen multiple providers for their child’s AD reported a substantial amount of confusion and frustration from conflicting advice on bathing frequency.19 Cardona et al20 undertook a randomized clinical trial of frequent bathing and moisturizing vs less-frequent bathing and moisturizing in pediatric patients with AD aged 6 months to 11 years. Patients were divided into 2 groups: 1 being bathed twice daily with immediate moisturizer application and the other being bathed twice weekly followed by moisturization, then a switch to the other method. Patients used standardized topical corticosteroids (TCSs) in both groups. There were significant improvements in scoring AD and other objective measures during the frequent bathing time period vs infrequent bathing; in the group that bathed more frequently, SCORAD (SCORing Atopic Dermatitis) decreased by 21.2 compared with the group that bathed less frequently (95% confidence interval, 14.9-27.6; P<.0001). These findings suggest that more-frequent bathing with immediate moisturization is superior as an acute treatment intervention for improving AD disease severity in comparison to less-frequent bathing with immediate moisturization.20
Expanding Treatment Options
Topical Phosphodiesterase Inhibitors
There are several new and evolving topical therapies in AD. Crisaborole ointment 2% is a steroid-free phosphodiesterase inhibitor approved in 2016 by the US Food and Drug Administration (FDA) for mild to moderate AD in patients aged 2 years and older. A recent multicenter, open-label, single-arm study in 137 infants (CrisADe CARE 1) evaluated the pharmacokinetics and efficacy of crisaborole ointment 2% applied twice daily for 4 weeks in pediatric patients aged 3 months to less than 24 months of age with mild to moderate AD.21 The study had 2 cohorts: one with a minimum of 5% body surface area involvement and another (the pharmacokinetic cohort) with a minimum of 35% body surface area involvement. Both cohorts demonstrated similar efficacy data. From baseline to day 29, the mean percentage change in eczema area and severity index (EASI) score was −57.5%, and an investigator global assessment (IGA) score of clear or almost clear with at least a 2-grade improvement was achieved in 30.2% of patients. Crisaborole systemic exposures in infants were comparable with those in patients aged 2 years or older. Patients tolerated crisaborole well, with a 4% rate of burning, which was similar to other studies in children and adults but perhaps lower than seen in clinical practice. Pharmacokinetic studies did not show any remarkable noticeable concern with accumulation of propylene glycol absorption.21
Based on the CrisADe CARE 1 study data, in March 2020 the FDA extended the indication of crisaborole ointment 2% from a prior lower age limit of 24 months to approval for use in treating mild to moderate AD in children as young as 3 months, making it the first nonsteroidal topical anti-inflammatory medication to be approved in children younger than 2 years in the United States.
Evolving Topical Therapies
Topical Janus Kinase Inhibitors
Ruxolitinib is a potent inhibitor of Janus kinase 1 (JAK-1) and Janus kinase 2 (JAK-2) and has been developed in topical formulations. In recent phase 3 clinical trials of patients with AD aged 12 years and older with mild to moderate disease (TRuE-AD1 and TRuE-AD2), more than half of the patients treated with either ruxolitinib cream in a 0.75% or 1.5% concentration reached EASI-75 after 8 weeks of treatment.22 Additionally, more patients treated with topical ruxolitinib reached an IGA score of clear to almost clear than patients treated with vehicle at the end of treatment. Thus far, it appears to be very well tolerated, significantly decreases EASI score (P<.0001), and improves overall pruritus.22
Delgocitinib is a topical pan-JAK inhibitor that blocks several cytokine-signaling cascade pathways. It was first developed and approved in Japan in an ointment formulation for use in patients with AD aged 16 years and older.23 The efficacy and safety profile of delgocitinib is currently being evaluated in pediatric patients with AD in Japan. In a recent phase 2 clinical study of 103 Japanese patients aged 2 to 15 years with moderate to severe AD, patients were randomized to receive either delgocitinib ointment in 0.25% or 0.5% concentrations or vehicle ointment twice daily for 4 weeks. The proportion of patients with a modified EASI-75 score was 38.2% (13/34) in the 0.25% group and 50.0% (17/34) in the 0.5% group vs 8.6% (3/35) in the placebo group. More patients treated with delgocitinib ointment received an IGA score of clear or almost clear than patients treated with vehicle at the end of treatment. Overall, both delgocitinib groups demonstrated superior improvement in clinical symptoms and signs without notable side effects.24
Tapinarof
Tapinarof is a topical therapeutic aryl hydrocarbon receptor agonist. In a recent phase 2 randomized study of 2 concentrations and 2 frequencies of tapinarof cream vs vehicle in 247 randomized patients aged 12 to 65 years with moderate to severe disease, tapinarof demonstrated greater success with both concentrations than vehicle at all visits beyond week 2.25 Additionally, in patients treated with tapinarof cream 1%, nearly 50% reached an IGA score of clear to almost clear with at least a 2-grade improvement. More than 50% of patients achieved EASI-75 improvement at 12 weeks of treatment with tapinarof cream 1% used daily. These findings suggest that tapinarof may be an efficacious and well-tolerated treatment for both adolescents and adults with AD; however, large confirmation trials are needed to further investigate.25
Systemic Treatments
Oral JAK Inhibitors
Some of the most exciting novel therapies include several oral JAK inhibitors that target different combinations of kinases and have been shown to decrease AD severity and symptoms. Some of these agents have indications in other disease states, such as baricitinib and upadacitinib, which are both FDA approved for the treatment of rheumatoid arthritis, whereas others, such as abrocitinib, have been studied specifically for AD.
Although some agents have only been studied in adults to date, others have included adolescents in their core studies, such as abrocitinib, which received Breakthrough Therapy designation from the FDA for the treatment of patients with moderate to severe AD in February 2018. In recent phase 3 trials of patients aged 12 years and older with moderate to severe AD (JADE MONO-1 and JADE MONO-2), both doses of abrocitinib improved the IGA and EASI-75 outcomes compared with placebo.26 Additional studies will be conducted to further investigate the relative efficacy and safety in patients younger than 18 years.
Biologics
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling without suppressing the immune system. It is approved for use in patients aged 12 years and older with moderate to severe asthma and in adults with chronic rhinosinusitis with nasal polyposis. It is the first biologic to show positive results in the moderate to severe pediatric AD population. There are now extended data available exhibiting sustained benefit in adolescent patients who were continued on dupilumab therapy, evidenced by further improvement in EASI scores at the 1-year mark.27
Recently, dupilumab received approval for use in patients aged 6 to 11 years, making it the first biologic for AD to be approved for use in patients younger than 12 years. The expedited FDA approval was based on the phase 3 results in which the efficacy and safety of dupilumab combined with TCSs were compared to TCSs alone (N=367).28 In this trial, more than twice as many children achieved clear or almost clear skin and more than 4 times as many achieved itch reduction with dupilumab plus TCSs than with TCSs alone. Three-quarters of patients receiving dupilumab at the subsequently approved dosing achieved at least a 75% improvement in overall disease.28 An additional study is being conducted that includes pediatric patients aged 6 months to younger than 6 years (ClinicalTrials.gov Identifier NCT03346434).
Future Directions in Pediatric AD
Our review summarizes only some of the agents under clinical investigation for use in pediatric AD. Early treatment to establish excellent long-term disease control with aggressive topical regimens or with systemic agents may alter the course of AD and influence the development of comorbidities, though this has not yet been shown in clinical studies. The long-term impact of early treatment, along with many other intriguing issues, will be studied more in the near future.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paper presented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1.
- Paternoster L, Savenije OEM, Heron J, et al. IJ Allergy Clin Immunol. 2018;141:964-971.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Yaghmaie P, Koudelka CW, Simpson Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
- Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468.
- al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.
- Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074-1081.
- Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821-838.
- Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis.
- Quality of life in families with children with atopic dermatitis. Pediatr Dermatol. 2016;33:28-32.
- Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556-563.
- Association of atopic dermatitis with sleep quality in children.
- Weidinger S, Simpson EL, Eckert L, et al. The patient-reported disease burden in pediatric patients with atopic dermatitis: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paperpresented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag. 2018;11:289-298.
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Kempe E, Jain N, Cardona I. Bathing frequency recommendations for pediatric atopic dermatitis: are we adding to parental frustration? Ann Allergy Asthma Immunol. 2013;111:298‐299.
- Cardona ID, Kempe EE, Lary C, et al. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8:1014‐1021.
- Gower , Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275-284.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment atopic dermatitis: results from two phase 3, randomized, double-blind studies. Presented at: 2nd Annual Revolutionizing Atopic Dermatitis Conference; April 5, 2020; Chicago, IL.
- Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609‐615.
- Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575‐1583.
- Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89‐98.e3.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85‐96.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published online June 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.054.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paper presented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1.
- Paternoster L, Savenije OEM, Heron J, et al. IJ Allergy Clin Immunol. 2018;141:964-971.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Yaghmaie P, Koudelka CW, Simpson Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
- Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468.
- al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.
- Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074-1081.
- Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821-838.
- Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis.
- Quality of life in families with children with atopic dermatitis. Pediatr Dermatol. 2016;33:28-32.
- Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556-563.
- Association of atopic dermatitis with sleep quality in children.
- Weidinger S, Simpson EL, Eckert L, et al. The patient-reported disease burden in pediatric patients with atopic dermatitis: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paperpresented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag. 2018;11:289-298.
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Kempe E, Jain N, Cardona I. Bathing frequency recommendations for pediatric atopic dermatitis: are we adding to parental frustration? Ann Allergy Asthma Immunol. 2013;111:298‐299.
- Cardona ID, Kempe EE, Lary C, et al. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8:1014‐1021.
- Gower , Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275-284.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment atopic dermatitis: results from two phase 3, randomized, double-blind studies. Presented at: 2nd Annual Revolutionizing Atopic Dermatitis Conference; April 5, 2020; Chicago, IL.
- Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609‐615.
- Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575‐1583.
- Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89‐98.e3.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85‐96.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published online June 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.054.
Practice Points
- There has been tremendous growth in our understanding of atopic dermatitis, with further insight into epidemiology, the impact on quality of life of affected individuals and their families, best bathing practices, and expanding treatment options.
- There are several novel topical and systemic agents recently approved and in late-stage clinical development programs that are evolving therapeutic approaches to pediatric disease.
How to build your identity as a physician online
To have a thriving business in today’s world, a functioning website is crucial to the overall business health. For a medical practice in general, and for its physicians specifically, it is one of the first steps for maintaining a practice. But to grow that practice, it is crucial to take the steps beyond just having a website. Growth requires website optimization for search engines, an expanding referral base, and the knowledge to use web tools and social media at your disposal to promote the practice and its physicians. In this roundtable, several marketing experts and web-savvy physicians discuss using available tools to best position and grow a practice.
Choosing a web upgrade
Patrick J. Culligan, MD: Peter, can you start us off by describing your relationship with Heather, and how your practice benefitted from her expertise?
Peter M. Lotze, MD: Sure. I am a urogynecologist in the competitive market of pelvic reconstructive surgery in Houston, Texas. Within that market, my main approach was to reach out to other physicians to refer patients to my practice. It generally would work, but took increasingly greater amounts of time to call these physicians up, write them letters, and maintain relationships. I felt that the large, national practice group that I am in did not have a significant web presence optimized to promote my practice, which makes it difficult for patients seeking your services to find you in their search for a doctor. It is helpful for patients to be able to understand from your website who you are, what you do, and what their experience may be like.
Glaring to me was that a web search specific for me or things that I do, would not produce our company’s results until page 2 or more on Google. This can be devastating for a practice because most people don’t go past the first page, and you can end up with fewer self-referrals, which should be a significant portion of new patients to your practice. I knew I needed guidance; I knew of Heather’s expertise given her exceptional past work building marketing strategies.
Digital go-tos for marketing
Heather Schueppert: Yes, I was pleased to work with Dr. Lotze, and at the time was a marketing consultant for practices such as his. But gone are the days of printed material—brochures, pamphlets, or even billboards—to effectively promote a business, or in this case, a practice. What still withstands the test of time, however, as the number 1 marketing referral source is word of mouth—from your trusted friend, family member, or coworker.
It is now proven that the number 2 most trusted form of advertising, the most persuasive and the most motivating, is online marketing.1 It is the “digital word of mouth”—the review. Patients are actively online, and a strong digital presence is critical to provide that direct value to retain and grow your patient base.
Continue to: Foundations of private practice reach out...
Foundations of private practice reach out
There are 3 important areas that I consider the foundation of any private practice marketing strategy (TABLE). First is an updated website that is search engine optimized (SEO). You can’t just set it and forget it, it needs to be an updated website. The algorithms for search engines are changing constantly to try to make it as fair and relevant as possible for patients or consumers to find the businesses they are searching for online.

The second area is review management, and for a physician, or even a care center, to do this on your own is a daunting task. It is a critical component, however, to making sure that your reputation out there, that online word of mouth, is as high a star rating as possible.
The third component is local search, which is basically a form of SEO that helps businesses show up in relevant local searches. We are all familiar with the search, “find a restaurant near me,” anything that pushes those search engines to find something local.
Those are what I call the effective triad: that updated website, the review management, and the local search, and all of these are tied together. I think Dr. Lotze and his practice did these effectively well, and I believe that he achieved his goals for the longer term.
Review/reputation management
Dr. Culligan: Brad, is there something that doctors may not know about Healthgrades, and are there opportunities to take full advantage of this physician-rating site?
Brad Bowman, MD: I agree with everything that Dr. Lotze and Heather have said. Start with yourself—what is it that you want to be, the one thing you want to stand for? Get your own marketing, your website right, then, the point is, once you do all that and you are number 1 in SEO, you are still only going to get about 25% of the people looking for you by name to come to your website. The other 75% are going to look at all the other different sites that are out there to provide information to consumers. So the question becomes what do you do with all these other third-party sites? Healthgrades is the most comprehensive and has the highest traffic of the third-party “find a doctor” sites. In 2020, half of all Americans who go to a doctor will use Healthgrades at some point to help select and connect with that doctor.
Physicians have their focus on the quality of the care they provide. Patients, however, focus on the quality of the entire health care experience. Did I get better? How long did I have to wait? Was the office staff helpful? Scarily enough, we still spend more time shopping for a refrigerator or mattress than we do shopping for a doctor. We still tend to think that all doctors are the same. It is the reality of how we have been trained by our insurance companies and by the health care system. That is why getting your marketing right and getting what is it that you want to be known for out there is important, so that you can get the types of patients you want.
Listings management is very important. Make sure that you are findable everywhere. There are services that will do this: Doctor.com, Reputation.com, and many others. They can help you make sure you get all your basic materials right: addresses, phone numbers, your picture. Because 75% of people are going to end up on third-party websites, if your phone number is wrong there, you could lose that patient.
Then the second piece of working with third-party sites is reputation management. Physician reviews are not a bad thing, they are the new word of mouth, as Heather pointed out. Most (80%) of the reviews are going to be positive. The others will be negative, and that is okay. It is important that you get at least 1 or 2 reviews on all the different sites. We know from Healthgrades.com that going from zero reviews to 1 review will increase your call volume by 60%. If you have the choice between 2 physicians and one practice looks like people have been there before, you will go to that one.
You can learn from reviews as well, consumers provide valid feedback. Best practice is to respond to every positive and negative review. Thank them, indicate that you have listened to them, and address any concerns as necessary.
Continue to: Dr. Lotze...
Dr. Lotze: As an example, one of the paramount things that Heather introduced me to was the third party I use to run my website. That company sends a HIPAA-compliant review out to each patient we have seen that day and gives them the opportunity to rate our services and leave comments. If a patient brings up a concern, we can respond immediately, which is important. Patients appreciate feeling that they have been heard. Typically, communicating with a patient will turn the 3-star review into a 5-star as she follows up with the practice.
Ms. Schueppert: Timeliness is important. And just to mention, there certainly is a time commitment to this (and it is a marathon versus a sprint) and there is some financial investment to get it going, but it could truly be detrimental to a practice if you decide not to do anything at all.
Dr. Bowman: Agencies can really help with the time commitment.
Handling bad reviews
Dr. Culligan: What about that person who seems to have it out for you, perhaps giving you multiple bad reviews?
Dr. Bowman: I have seen this before. At Healthgrades, we recently analyzed 8.4 million patient reviews to see what people wrote about.2 The first thing they will talk about is quality of care as they see it. Did I get better or not? You can’t “fix” every patient; there will be some that you cannot help. The next thing patients comment on is bedside manner. With negative reviews, you will see more comments about the office staff.2
A single negative review actually helps make the positive ones look more credible. But if you do believe someone is trolling you, we can flag it and will investigate to the best of our ability. (Different sites likely have different editorial policies.) For example, we look at the IP addresses of all reviews, and if multiple reviews are coming from the same location, we would only let one through, overwriting the previous review from that address.
Patients just want to be heard. We have seen people change their views, based on how their review is handled and responded to.
Dr. Lotze: Is there a response by the physician that you think tends to work better in terms of resolving the issue that can minimize a perceived caustic reaction to a patient’s criticism?
Dr. Bowman: First, just like with any stressful situation, take a deep breath and respond when you feel like you can be constructive. When you do respond, be gracious. Thank them for their feedback. Make sure you reference something about their concern: “I understand that you had to wait longer than you would have liked.” Acknowledge the problem they reference, and then just apologize: “I’m sorry we didn’t meet your expectations.” Then, if they waited too long for example, “We have a new system where no one should have to wait more than 30 minutes….” You can respond privately or publically. Generally, public responses are better as it shows other consumers that you are willing to listen and consider their point of view.
Continue to: The next phase at Healthgrades...
The next phase at Healthgrades
Dr. Culligan: Do you see changes to the way physician-rating sites are working now? Are we going to stay status quo over the next 10 years, or do you see frontiers in how your site is going to develop?
Dr. Bowman: For Healthgrades, we rely on quantitative and objective measures, not just the qualitative. We are investing heavily right now in trying to help consumers understand what are the relative volumes of different procedures or different patient types that each individual doctor sees. Orthopedics is an easy example—if you have a knee problem, you want to go to someone who specializes in knees. Our job is to help consumers easily identify, “This is a shoulder doctor, this is a knee doctor, and this is why that matters.”
In the meantime, as a physician, you can always go into our site and state your care philosophy, identifying what is the sort of patient that you like to treat. Transparency is good for everyone, and especially physicians. It helps the right patient show up for you, and it helps you do a better job providing referrals.
Social media: Avoid pitfalls, and use it to your benefit
Dr. Lotze: Branding was one of the things that I was confused about, and Heather really helped me out. As physicians, we put ourselves out there on our websites, which we try to make professional sources of information for patients. But patients often want to see what else they can find out about us, including Healthgrades and social media. I think the thing that is important to know with social media is that it is a place where people learn about you as a person. Your social media should be another avenue of promotion. Whether it is your personal or professional Facebook page, people are going to see those sites. You have an opportunity to promote yourself as a good physician and a good person with a wholesome practice that you want people to come to. If a physician is posting questionable things about themselves on any kind of social media, it could be perceived as inappropriate by the patient. That can impact how patients think of you as a person, and how they are going to grade you. If people lose sight of who you are due to a questionable social media posting, everything else (SEO, the website) can be for naught.
Dr. Culligan: What are the most important social media tools to invest your time in?
Ms. Schueppert: Before anybody jumps into social media, I firmly recommend that you make sure your local search and your Google 3-pack is set up—which is basically a method Google uses to display the top 3 results on its listings page. Then make sure you have a review management system in place. Make sure you have that updated website. Those are the foundational elements. Once you have that going, social media is the next added layer to that digital presence.
I usually recommend LinkedIn. It is huge because you are staying in contact with your colleagues, that business-to-business type of connection. It remains a way for physicians to set themselves up as experts in their level of specialty.
From there, it’s either Instagram or Facebook. If you are serving more of the younger generations, the millennials and younger, then Instagram is the way to go. If you are focusing on your 40+, 50+, they are going to be far more on Facebook.
Continue to: Dr. Lotze...
Dr. Lotze: For me, a Facebook page was a great place to start. The cost of those Google ads—the first things we see at the top of a Google search in their own separate box—is significant. If a practice has that kind of money to invest, great; it is an instant way to be first on the page during a search. But there are more cost-effective ways of doing that, especially as you are getting your name out. Facebook provides, at a smaller cost, promotion of whatever it is that you are seeking to promote. You can find people within a certain zip code, for instance, and use a Facebook ad campaign that can drive people to your Facebook page—which should have both routinely updated new posts and a link to your website. The posts should be interesting topics relevant to the patients you wish to treat (avoiding personal stories or controversial discussions). You can put a post together, or you can have a third-party service do this. People who follow your page will get reminders of you and your practice with each new post. As your page followers increase, your Facebook rank will improve, and your page will more likely be discovered by Facebook searches for your services. With an added link to your office practice website, those patients go straight to your site without getting lost in the noise of Google search results.
For Instagram, a short video or an interesting picture, along with a brief statement, are the essentials. You can add a single link. Marketing here is by direct messaging or having patients going to your website through a link. Instagram, like Facebook, offers analytics to help show you what your audience likes to read about, improving the quality of your posts and increasing number of followers.
YouTube is the number 2 search engine behind Google. A Google search for your field of medicine may be filled with pages of competitors. However, YouTube has a much lower volume of competing practices, making it easier for patients to find you. The only downside to YouTube is that it will list your video along with other competing videos, which can draw attention away from your practice.
If you want to promote your website or practice with video, using a company such as Vimeo is a better choice compared with YouTube, as YouTube gets credit for video views—which improves YouTube’s SEO and not your own website. Vimeo allows for your website to get credit each time the video is watched. Regardless of where you place your videos, make them short and to the point, with links to your website. Videos only need to be long enough to get your message across and stimulate interest in your practice.
If you can have a blog on your website, it also will help with SEO. What a search engine like Google wants to see is that a patient is on your web page and looking at something for at least 60 seconds. If so, the website is deemed to have information that is relevant, improving your SEO ranking.
Finally, Twitter also can be used for getting messaging out and for branding. The problem with it is that many people go to Twitter to follow a Hollywood celebrity, a sports star, or are looking for mass communication. There is less interest on Twitter for physician outreach.
Continue to: Measuring ROI...
Measuring ROI
Dr. Culligan: What’s the best way to track your return on investment?
Dr. Lotze: First for me was to find out what didn’t work in the office and fix that before really promoting my practice. It’s about the global experience for a patient, as Brad mentioned. As a marketing expert, Heather met with me to understand my goals. She then called my office as a patient to set up an appointment and went through that entire office experience. We identified issues needing improvement.
The next step was to develop a working relationship with my webmaster—someone who can help manage Internet image and SEO. Together, you will develop goals for what the SEO should promote specific to your practice. Once a good SEO program is in place, your website’s ranking will go up—although it can take a minimum of 6 months to see a significant increase. To help understand your website’s performance, your webmaster should provide you with reports on your site’s analytics.
As you go through this process, it is great to have a marketing expert to be the point person. You will work closely together for a while, but eventually you can back off over time. The time and expense you invest on the front end have huge rewards on the back end. Currently, I still spend a reasonable amount of money every month. I have a high self-referral base because of these efforts, however, which results in more patient surgeries and easily covers my expenses. It is money well invested. My website traffic increased by 268% over 2 years (FIGURE). I’ll propose that currently more than half of my patients are self-referrals due to online marketing.
Ms. Schueppert: The only thing I would add is training your front staff. They are checking people in, taking appointments, checking your patients out. Have them be mindful that there are campaigns going on, whether it is a social media push, or a new video that went on the website. They can ask, “How did you hear about us?” when a new patient calls.
Dr. Bowman: Unless you are a large university hospital, where the analytics get significantly more advanced in terms of measuring return on investment (ROI), I think you should just be looking at your schedule and looking at your monthly billings and seeing how they change over time. You can calculate how much a new patient is worth because you can figure out how many patients you have and how much you bill and what your profits are.
Dr. Culligan: For those of us who are hospital employees, you can try to convince the hospital that you can do a detailed ROI analysis, or you can just look at it like (say it’s $3,000 per month), how many surgeries does this project have to generate before the hospital makes that back? The answer is a fraction of 1 case.
Thank you to all of you for your expertise on this roundtable.
- Anderson A. Online reviews vs. word of mouth: Which one is more important. https://www.revlocal.com/blog/reviewandreputationmanagement/onlinereviewsvswordofmouthwhichoneismoreimportant. Accessed July 17, 2020.
- 2020 Patient sentiment report. Healthgrades; Medical Group Management Association. https://www.healthgrades.com/content /patientsentimentreport. Accessed July 17, 2020
To have a thriving business in today’s world, a functioning website is crucial to the overall business health. For a medical practice in general, and for its physicians specifically, it is one of the first steps for maintaining a practice. But to grow that practice, it is crucial to take the steps beyond just having a website. Growth requires website optimization for search engines, an expanding referral base, and the knowledge to use web tools and social media at your disposal to promote the practice and its physicians. In this roundtable, several marketing experts and web-savvy physicians discuss using available tools to best position and grow a practice.
Choosing a web upgrade
Patrick J. Culligan, MD: Peter, can you start us off by describing your relationship with Heather, and how your practice benefitted from her expertise?
Peter M. Lotze, MD: Sure. I am a urogynecologist in the competitive market of pelvic reconstructive surgery in Houston, Texas. Within that market, my main approach was to reach out to other physicians to refer patients to my practice. It generally would work, but took increasingly greater amounts of time to call these physicians up, write them letters, and maintain relationships. I felt that the large, national practice group that I am in did not have a significant web presence optimized to promote my practice, which makes it difficult for patients seeking your services to find you in their search for a doctor. It is helpful for patients to be able to understand from your website who you are, what you do, and what their experience may be like.
Glaring to me was that a web search specific for me or things that I do, would not produce our company’s results until page 2 or more on Google. This can be devastating for a practice because most people don’t go past the first page, and you can end up with fewer self-referrals, which should be a significant portion of new patients to your practice. I knew I needed guidance; I knew of Heather’s expertise given her exceptional past work building marketing strategies.
Digital go-tos for marketing
Heather Schueppert: Yes, I was pleased to work with Dr. Lotze, and at the time was a marketing consultant for practices such as his. But gone are the days of printed material—brochures, pamphlets, or even billboards—to effectively promote a business, or in this case, a practice. What still withstands the test of time, however, as the number 1 marketing referral source is word of mouth—from your trusted friend, family member, or coworker.
It is now proven that the number 2 most trusted form of advertising, the most persuasive and the most motivating, is online marketing.1 It is the “digital word of mouth”—the review. Patients are actively online, and a strong digital presence is critical to provide that direct value to retain and grow your patient base.
Continue to: Foundations of private practice reach out...
Foundations of private practice reach out
There are 3 important areas that I consider the foundation of any private practice marketing strategy (TABLE). First is an updated website that is search engine optimized (SEO). You can’t just set it and forget it, it needs to be an updated website. The algorithms for search engines are changing constantly to try to make it as fair and relevant as possible for patients or consumers to find the businesses they are searching for online.

The second area is review management, and for a physician, or even a care center, to do this on your own is a daunting task. It is a critical component, however, to making sure that your reputation out there, that online word of mouth, is as high a star rating as possible.
The third component is local search, which is basically a form of SEO that helps businesses show up in relevant local searches. We are all familiar with the search, “find a restaurant near me,” anything that pushes those search engines to find something local.
Those are what I call the effective triad: that updated website, the review management, and the local search, and all of these are tied together. I think Dr. Lotze and his practice did these effectively well, and I believe that he achieved his goals for the longer term.
Review/reputation management
Dr. Culligan: Brad, is there something that doctors may not know about Healthgrades, and are there opportunities to take full advantage of this physician-rating site?
Brad Bowman, MD: I agree with everything that Dr. Lotze and Heather have said. Start with yourself—what is it that you want to be, the one thing you want to stand for? Get your own marketing, your website right, then, the point is, once you do all that and you are number 1 in SEO, you are still only going to get about 25% of the people looking for you by name to come to your website. The other 75% are going to look at all the other different sites that are out there to provide information to consumers. So the question becomes what do you do with all these other third-party sites? Healthgrades is the most comprehensive and has the highest traffic of the third-party “find a doctor” sites. In 2020, half of all Americans who go to a doctor will use Healthgrades at some point to help select and connect with that doctor.
Physicians have their focus on the quality of the care they provide. Patients, however, focus on the quality of the entire health care experience. Did I get better? How long did I have to wait? Was the office staff helpful? Scarily enough, we still spend more time shopping for a refrigerator or mattress than we do shopping for a doctor. We still tend to think that all doctors are the same. It is the reality of how we have been trained by our insurance companies and by the health care system. That is why getting your marketing right and getting what is it that you want to be known for out there is important, so that you can get the types of patients you want.
Listings management is very important. Make sure that you are findable everywhere. There are services that will do this: Doctor.com, Reputation.com, and many others. They can help you make sure you get all your basic materials right: addresses, phone numbers, your picture. Because 75% of people are going to end up on third-party websites, if your phone number is wrong there, you could lose that patient.
Then the second piece of working with third-party sites is reputation management. Physician reviews are not a bad thing, they are the new word of mouth, as Heather pointed out. Most (80%) of the reviews are going to be positive. The others will be negative, and that is okay. It is important that you get at least 1 or 2 reviews on all the different sites. We know from Healthgrades.com that going from zero reviews to 1 review will increase your call volume by 60%. If you have the choice between 2 physicians and one practice looks like people have been there before, you will go to that one.
You can learn from reviews as well, consumers provide valid feedback. Best practice is to respond to every positive and negative review. Thank them, indicate that you have listened to them, and address any concerns as necessary.
Continue to: Dr. Lotze...
Dr. Lotze: As an example, one of the paramount things that Heather introduced me to was the third party I use to run my website. That company sends a HIPAA-compliant review out to each patient we have seen that day and gives them the opportunity to rate our services and leave comments. If a patient brings up a concern, we can respond immediately, which is important. Patients appreciate feeling that they have been heard. Typically, communicating with a patient will turn the 3-star review into a 5-star as she follows up with the practice.
Ms. Schueppert: Timeliness is important. And just to mention, there certainly is a time commitment to this (and it is a marathon versus a sprint) and there is some financial investment to get it going, but it could truly be detrimental to a practice if you decide not to do anything at all.
Dr. Bowman: Agencies can really help with the time commitment.
Handling bad reviews
Dr. Culligan: What about that person who seems to have it out for you, perhaps giving you multiple bad reviews?
Dr. Bowman: I have seen this before. At Healthgrades, we recently analyzed 8.4 million patient reviews to see what people wrote about.2 The first thing they will talk about is quality of care as they see it. Did I get better or not? You can’t “fix” every patient; there will be some that you cannot help. The next thing patients comment on is bedside manner. With negative reviews, you will see more comments about the office staff.2
A single negative review actually helps make the positive ones look more credible. But if you do believe someone is trolling you, we can flag it and will investigate to the best of our ability. (Different sites likely have different editorial policies.) For example, we look at the IP addresses of all reviews, and if multiple reviews are coming from the same location, we would only let one through, overwriting the previous review from that address.
Patients just want to be heard. We have seen people change their views, based on how their review is handled and responded to.
Dr. Lotze: Is there a response by the physician that you think tends to work better in terms of resolving the issue that can minimize a perceived caustic reaction to a patient’s criticism?
Dr. Bowman: First, just like with any stressful situation, take a deep breath and respond when you feel like you can be constructive. When you do respond, be gracious. Thank them for their feedback. Make sure you reference something about their concern: “I understand that you had to wait longer than you would have liked.” Acknowledge the problem they reference, and then just apologize: “I’m sorry we didn’t meet your expectations.” Then, if they waited too long for example, “We have a new system where no one should have to wait more than 30 minutes….” You can respond privately or publically. Generally, public responses are better as it shows other consumers that you are willing to listen and consider their point of view.
Continue to: The next phase at Healthgrades...
The next phase at Healthgrades
Dr. Culligan: Do you see changes to the way physician-rating sites are working now? Are we going to stay status quo over the next 10 years, or do you see frontiers in how your site is going to develop?
Dr. Bowman: For Healthgrades, we rely on quantitative and objective measures, not just the qualitative. We are investing heavily right now in trying to help consumers understand what are the relative volumes of different procedures or different patient types that each individual doctor sees. Orthopedics is an easy example—if you have a knee problem, you want to go to someone who specializes in knees. Our job is to help consumers easily identify, “This is a shoulder doctor, this is a knee doctor, and this is why that matters.”
In the meantime, as a physician, you can always go into our site and state your care philosophy, identifying what is the sort of patient that you like to treat. Transparency is good for everyone, and especially physicians. It helps the right patient show up for you, and it helps you do a better job providing referrals.
Social media: Avoid pitfalls, and use it to your benefit
Dr. Lotze: Branding was one of the things that I was confused about, and Heather really helped me out. As physicians, we put ourselves out there on our websites, which we try to make professional sources of information for patients. But patients often want to see what else they can find out about us, including Healthgrades and social media. I think the thing that is important to know with social media is that it is a place where people learn about you as a person. Your social media should be another avenue of promotion. Whether it is your personal or professional Facebook page, people are going to see those sites. You have an opportunity to promote yourself as a good physician and a good person with a wholesome practice that you want people to come to. If a physician is posting questionable things about themselves on any kind of social media, it could be perceived as inappropriate by the patient. That can impact how patients think of you as a person, and how they are going to grade you. If people lose sight of who you are due to a questionable social media posting, everything else (SEO, the website) can be for naught.
Dr. Culligan: What are the most important social media tools to invest your time in?
Ms. Schueppert: Before anybody jumps into social media, I firmly recommend that you make sure your local search and your Google 3-pack is set up—which is basically a method Google uses to display the top 3 results on its listings page. Then make sure you have a review management system in place. Make sure you have that updated website. Those are the foundational elements. Once you have that going, social media is the next added layer to that digital presence.
I usually recommend LinkedIn. It is huge because you are staying in contact with your colleagues, that business-to-business type of connection. It remains a way for physicians to set themselves up as experts in their level of specialty.
From there, it’s either Instagram or Facebook. If you are serving more of the younger generations, the millennials and younger, then Instagram is the way to go. If you are focusing on your 40+, 50+, they are going to be far more on Facebook.
Continue to: Dr. Lotze...
Dr. Lotze: For me, a Facebook page was a great place to start. The cost of those Google ads—the first things we see at the top of a Google search in their own separate box—is significant. If a practice has that kind of money to invest, great; it is an instant way to be first on the page during a search. But there are more cost-effective ways of doing that, especially as you are getting your name out. Facebook provides, at a smaller cost, promotion of whatever it is that you are seeking to promote. You can find people within a certain zip code, for instance, and use a Facebook ad campaign that can drive people to your Facebook page—which should have both routinely updated new posts and a link to your website. The posts should be interesting topics relevant to the patients you wish to treat (avoiding personal stories or controversial discussions). You can put a post together, or you can have a third-party service do this. People who follow your page will get reminders of you and your practice with each new post. As your page followers increase, your Facebook rank will improve, and your page will more likely be discovered by Facebook searches for your services. With an added link to your office practice website, those patients go straight to your site without getting lost in the noise of Google search results.
For Instagram, a short video or an interesting picture, along with a brief statement, are the essentials. You can add a single link. Marketing here is by direct messaging or having patients going to your website through a link. Instagram, like Facebook, offers analytics to help show you what your audience likes to read about, improving the quality of your posts and increasing number of followers.
YouTube is the number 2 search engine behind Google. A Google search for your field of medicine may be filled with pages of competitors. However, YouTube has a much lower volume of competing practices, making it easier for patients to find you. The only downside to YouTube is that it will list your video along with other competing videos, which can draw attention away from your practice.
If you want to promote your website or practice with video, using a company such as Vimeo is a better choice compared with YouTube, as YouTube gets credit for video views—which improves YouTube’s SEO and not your own website. Vimeo allows for your website to get credit each time the video is watched. Regardless of where you place your videos, make them short and to the point, with links to your website. Videos only need to be long enough to get your message across and stimulate interest in your practice.
If you can have a blog on your website, it also will help with SEO. What a search engine like Google wants to see is that a patient is on your web page and looking at something for at least 60 seconds. If so, the website is deemed to have information that is relevant, improving your SEO ranking.
Finally, Twitter also can be used for getting messaging out and for branding. The problem with it is that many people go to Twitter to follow a Hollywood celebrity, a sports star, or are looking for mass communication. There is less interest on Twitter for physician outreach.
Continue to: Measuring ROI...
Measuring ROI
Dr. Culligan: What’s the best way to track your return on investment?
Dr. Lotze: First for me was to find out what didn’t work in the office and fix that before really promoting my practice. It’s about the global experience for a patient, as Brad mentioned. As a marketing expert, Heather met with me to understand my goals. She then called my office as a patient to set up an appointment and went through that entire office experience. We identified issues needing improvement.
The next step was to develop a working relationship with my webmaster—someone who can help manage Internet image and SEO. Together, you will develop goals for what the SEO should promote specific to your practice. Once a good SEO program is in place, your website’s ranking will go up—although it can take a minimum of 6 months to see a significant increase. To help understand your website’s performance, your webmaster should provide you with reports on your site’s analytics.
As you go through this process, it is great to have a marketing expert to be the point person. You will work closely together for a while, but eventually you can back off over time. The time and expense you invest on the front end have huge rewards on the back end. Currently, I still spend a reasonable amount of money every month. I have a high self-referral base because of these efforts, however, which results in more patient surgeries and easily covers my expenses. It is money well invested. My website traffic increased by 268% over 2 years (FIGURE). I’ll propose that currently more than half of my patients are self-referrals due to online marketing.

Ms. Schueppert: The only thing I would add is training your front staff. They are checking people in, taking appointments, checking your patients out. Have them be mindful that there are campaigns going on, whether it is a social media push, or a new video that went on the website. They can ask, “How did you hear about us?” when a new patient calls.
Dr. Bowman: Unless you are a large university hospital, where the analytics get significantly more advanced in terms of measuring return on investment (ROI), I think you should just be looking at your schedule and looking at your monthly billings and seeing how they change over time. You can calculate how much a new patient is worth because you can figure out how many patients you have and how much you bill and what your profits are.
Dr. Culligan: For those of us who are hospital employees, you can try to convince the hospital that you can do a detailed ROI analysis, or you can just look at it like (say it’s $3,000 per month), how many surgeries does this project have to generate before the hospital makes that back? The answer is a fraction of 1 case.
Thank you to all of you for your expertise on this roundtable.
To have a thriving business in today’s world, a functioning website is crucial to the overall business health. For a medical practice in general, and for its physicians specifically, it is one of the first steps for maintaining a practice. But to grow that practice, it is crucial to take the steps beyond just having a website. Growth requires website optimization for search engines, an expanding referral base, and the knowledge to use web tools and social media at your disposal to promote the practice and its physicians. In this roundtable, several marketing experts and web-savvy physicians discuss using available tools to best position and grow a practice.
Choosing a web upgrade
Patrick J. Culligan, MD: Peter, can you start us off by describing your relationship with Heather, and how your practice benefitted from her expertise?
Peter M. Lotze, MD: Sure. I am a urogynecologist in the competitive market of pelvic reconstructive surgery in Houston, Texas. Within that market, my main approach was to reach out to other physicians to refer patients to my practice. It generally would work, but took increasingly greater amounts of time to call these physicians up, write them letters, and maintain relationships. I felt that the large, national practice group that I am in did not have a significant web presence optimized to promote my practice, which makes it difficult for patients seeking your services to find you in their search for a doctor. It is helpful for patients to be able to understand from your website who you are, what you do, and what their experience may be like.
Glaring to me was that a web search specific for me or things that I do, would not produce our company’s results until page 2 or more on Google. This can be devastating for a practice because most people don’t go past the first page, and you can end up with fewer self-referrals, which should be a significant portion of new patients to your practice. I knew I needed guidance; I knew of Heather’s expertise given her exceptional past work building marketing strategies.
Digital go-tos for marketing
Heather Schueppert: Yes, I was pleased to work with Dr. Lotze, and at the time was a marketing consultant for practices such as his. But gone are the days of printed material—brochures, pamphlets, or even billboards—to effectively promote a business, or in this case, a practice. What still withstands the test of time, however, as the number 1 marketing referral source is word of mouth—from your trusted friend, family member, or coworker.
It is now proven that the number 2 most trusted form of advertising, the most persuasive and the most motivating, is online marketing.1 It is the “digital word of mouth”—the review. Patients are actively online, and a strong digital presence is critical to provide that direct value to retain and grow your patient base.
Continue to: Foundations of private practice reach out...
Foundations of private practice reach out
There are 3 important areas that I consider the foundation of any private practice marketing strategy (TABLE). First is an updated website that is search engine optimized (SEO). You can’t just set it and forget it, it needs to be an updated website. The algorithms for search engines are changing constantly to try to make it as fair and relevant as possible for patients or consumers to find the businesses they are searching for online.

The second area is review management, and for a physician, or even a care center, to do this on your own is a daunting task. It is a critical component, however, to making sure that your reputation out there, that online word of mouth, is as high a star rating as possible.
The third component is local search, which is basically a form of SEO that helps businesses show up in relevant local searches. We are all familiar with the search, “find a restaurant near me,” anything that pushes those search engines to find something local.
Those are what I call the effective triad: that updated website, the review management, and the local search, and all of these are tied together. I think Dr. Lotze and his practice did these effectively well, and I believe that he achieved his goals for the longer term.
Review/reputation management
Dr. Culligan: Brad, is there something that doctors may not know about Healthgrades, and are there opportunities to take full advantage of this physician-rating site?
Brad Bowman, MD: I agree with everything that Dr. Lotze and Heather have said. Start with yourself—what is it that you want to be, the one thing you want to stand for? Get your own marketing, your website right, then, the point is, once you do all that and you are number 1 in SEO, you are still only going to get about 25% of the people looking for you by name to come to your website. The other 75% are going to look at all the other different sites that are out there to provide information to consumers. So the question becomes what do you do with all these other third-party sites? Healthgrades is the most comprehensive and has the highest traffic of the third-party “find a doctor” sites. In 2020, half of all Americans who go to a doctor will use Healthgrades at some point to help select and connect with that doctor.
Physicians have their focus on the quality of the care they provide. Patients, however, focus on the quality of the entire health care experience. Did I get better? How long did I have to wait? Was the office staff helpful? Scarily enough, we still spend more time shopping for a refrigerator or mattress than we do shopping for a doctor. We still tend to think that all doctors are the same. It is the reality of how we have been trained by our insurance companies and by the health care system. That is why getting your marketing right and getting what is it that you want to be known for out there is important, so that you can get the types of patients you want.
Listings management is very important. Make sure that you are findable everywhere. There are services that will do this: Doctor.com, Reputation.com, and many others. They can help you make sure you get all your basic materials right: addresses, phone numbers, your picture. Because 75% of people are going to end up on third-party websites, if your phone number is wrong there, you could lose that patient.
Then the second piece of working with third-party sites is reputation management. Physician reviews are not a bad thing, they are the new word of mouth, as Heather pointed out. Most (80%) of the reviews are going to be positive. The others will be negative, and that is okay. It is important that you get at least 1 or 2 reviews on all the different sites. We know from Healthgrades.com that going from zero reviews to 1 review will increase your call volume by 60%. If you have the choice between 2 physicians and one practice looks like people have been there before, you will go to that one.
You can learn from reviews as well, consumers provide valid feedback. Best practice is to respond to every positive and negative review. Thank them, indicate that you have listened to them, and address any concerns as necessary.
Continue to: Dr. Lotze...
Dr. Lotze: As an example, one of the paramount things that Heather introduced me to was the third party I use to run my website. That company sends a HIPAA-compliant review out to each patient we have seen that day and gives them the opportunity to rate our services and leave comments. If a patient brings up a concern, we can respond immediately, which is important. Patients appreciate feeling that they have been heard. Typically, communicating with a patient will turn the 3-star review into a 5-star as she follows up with the practice.
Ms. Schueppert: Timeliness is important. And just to mention, there certainly is a time commitment to this (and it is a marathon versus a sprint) and there is some financial investment to get it going, but it could truly be detrimental to a practice if you decide not to do anything at all.
Dr. Bowman: Agencies can really help with the time commitment.
Handling bad reviews
Dr. Culligan: What about that person who seems to have it out for you, perhaps giving you multiple bad reviews?
Dr. Bowman: I have seen this before. At Healthgrades, we recently analyzed 8.4 million patient reviews to see what people wrote about.2 The first thing they will talk about is quality of care as they see it. Did I get better or not? You can’t “fix” every patient; there will be some that you cannot help. The next thing patients comment on is bedside manner. With negative reviews, you will see more comments about the office staff.2
A single negative review actually helps make the positive ones look more credible. But if you do believe someone is trolling you, we can flag it and will investigate to the best of our ability. (Different sites likely have different editorial policies.) For example, we look at the IP addresses of all reviews, and if multiple reviews are coming from the same location, we would only let one through, overwriting the previous review from that address.
Patients just want to be heard. We have seen people change their views, based on how their review is handled and responded to.
Dr. Lotze: Is there a response by the physician that you think tends to work better in terms of resolving the issue that can minimize a perceived caustic reaction to a patient’s criticism?
Dr. Bowman: First, just like with any stressful situation, take a deep breath and respond when you feel like you can be constructive. When you do respond, be gracious. Thank them for their feedback. Make sure you reference something about their concern: “I understand that you had to wait longer than you would have liked.” Acknowledge the problem they reference, and then just apologize: “I’m sorry we didn’t meet your expectations.” Then, if they waited too long for example, “We have a new system where no one should have to wait more than 30 minutes….” You can respond privately or publically. Generally, public responses are better as it shows other consumers that you are willing to listen and consider their point of view.
Continue to: The next phase at Healthgrades...
The next phase at Healthgrades
Dr. Culligan: Do you see changes to the way physician-rating sites are working now? Are we going to stay status quo over the next 10 years, or do you see frontiers in how your site is going to develop?
Dr. Bowman: For Healthgrades, we rely on quantitative and objective measures, not just the qualitative. We are investing heavily right now in trying to help consumers understand what are the relative volumes of different procedures or different patient types that each individual doctor sees. Orthopedics is an easy example—if you have a knee problem, you want to go to someone who specializes in knees. Our job is to help consumers easily identify, “This is a shoulder doctor, this is a knee doctor, and this is why that matters.”
In the meantime, as a physician, you can always go into our site and state your care philosophy, identifying what is the sort of patient that you like to treat. Transparency is good for everyone, and especially physicians. It helps the right patient show up for you, and it helps you do a better job providing referrals.
Social media: Avoid pitfalls, and use it to your benefit
Dr. Lotze: Branding was one of the things that I was confused about, and Heather really helped me out. As physicians, we put ourselves out there on our websites, which we try to make professional sources of information for patients. But patients often want to see what else they can find out about us, including Healthgrades and social media. I think the thing that is important to know with social media is that it is a place where people learn about you as a person. Your social media should be another avenue of promotion. Whether it is your personal or professional Facebook page, people are going to see those sites. You have an opportunity to promote yourself as a good physician and a good person with a wholesome practice that you want people to come to. If a physician is posting questionable things about themselves on any kind of social media, it could be perceived as inappropriate by the patient. That can impact how patients think of you as a person, and how they are going to grade you. If people lose sight of who you are due to a questionable social media posting, everything else (SEO, the website) can be for naught.
Dr. Culligan: What are the most important social media tools to invest your time in?
Ms. Schueppert: Before anybody jumps into social media, I firmly recommend that you make sure your local search and your Google 3-pack is set up—which is basically a method Google uses to display the top 3 results on its listings page. Then make sure you have a review management system in place. Make sure you have that updated website. Those are the foundational elements. Once you have that going, social media is the next added layer to that digital presence.
I usually recommend LinkedIn. It is huge because you are staying in contact with your colleagues, that business-to-business type of connection. It remains a way for physicians to set themselves up as experts in their level of specialty.
From there, it’s either Instagram or Facebook. If you are serving more of the younger generations, the millennials and younger, then Instagram is the way to go. If you are focusing on your 40+, 50+, they are going to be far more on Facebook.
Continue to: Dr. Lotze...
Dr. Lotze: For me, a Facebook page was a great place to start. The cost of those Google ads—the first things we see at the top of a Google search in their own separate box—is significant. If a practice has that kind of money to invest, great; it is an instant way to be first on the page during a search. But there are more cost-effective ways of doing that, especially as you are getting your name out. Facebook provides, at a smaller cost, promotion of whatever it is that you are seeking to promote. You can find people within a certain zip code, for instance, and use a Facebook ad campaign that can drive people to your Facebook page—which should have both routinely updated new posts and a link to your website. The posts should be interesting topics relevant to the patients you wish to treat (avoiding personal stories or controversial discussions). You can put a post together, or you can have a third-party service do this. People who follow your page will get reminders of you and your practice with each new post. As your page followers increase, your Facebook rank will improve, and your page will more likely be discovered by Facebook searches for your services. With an added link to your office practice website, those patients go straight to your site without getting lost in the noise of Google search results.
For Instagram, a short video or an interesting picture, along with a brief statement, are the essentials. You can add a single link. Marketing here is by direct messaging or having patients going to your website through a link. Instagram, like Facebook, offers analytics to help show you what your audience likes to read about, improving the quality of your posts and increasing number of followers.
YouTube is the number 2 search engine behind Google. A Google search for your field of medicine may be filled with pages of competitors. However, YouTube has a much lower volume of competing practices, making it easier for patients to find you. The only downside to YouTube is that it will list your video along with other competing videos, which can draw attention away from your practice.
If you want to promote your website or practice with video, using a company such as Vimeo is a better choice compared with YouTube, as YouTube gets credit for video views—which improves YouTube’s SEO and not your own website. Vimeo allows for your website to get credit each time the video is watched. Regardless of where you place your videos, make them short and to the point, with links to your website. Videos only need to be long enough to get your message across and stimulate interest in your practice.
If you can have a blog on your website, it also will help with SEO. What a search engine like Google wants to see is that a patient is on your web page and looking at something for at least 60 seconds. If so, the website is deemed to have information that is relevant, improving your SEO ranking.
Finally, Twitter also can be used for getting messaging out and for branding. The problem with it is that many people go to Twitter to follow a Hollywood celebrity, a sports star, or are looking for mass communication. There is less interest on Twitter for physician outreach.
Continue to: Measuring ROI...
Measuring ROI
Dr. Culligan: What’s the best way to track your return on investment?
Dr. Lotze: First for me was to find out what didn’t work in the office and fix that before really promoting my practice. It’s about the global experience for a patient, as Brad mentioned. As a marketing expert, Heather met with me to understand my goals. She then called my office as a patient to set up an appointment and went through that entire office experience. We identified issues needing improvement.
The next step was to develop a working relationship with my webmaster—someone who can help manage Internet image and SEO. Together, you will develop goals for what the SEO should promote specific to your practice. Once a good SEO program is in place, your website’s ranking will go up—although it can take a minimum of 6 months to see a significant increase. To help understand your website’s performance, your webmaster should provide you with reports on your site’s analytics.
As you go through this process, it is great to have a marketing expert to be the point person. You will work closely together for a while, but eventually you can back off over time. The time and expense you invest on the front end have huge rewards on the back end. Currently, I still spend a reasonable amount of money every month. I have a high self-referral base because of these efforts, however, which results in more patient surgeries and easily covers my expenses. It is money well invested. My website traffic increased by 268% over 2 years (FIGURE). I’ll propose that currently more than half of my patients are self-referrals due to online marketing.

Ms. Schueppert: The only thing I would add is training your front staff. They are checking people in, taking appointments, checking your patients out. Have them be mindful that there are campaigns going on, whether it is a social media push, or a new video that went on the website. They can ask, “How did you hear about us?” when a new patient calls.
Dr. Bowman: Unless you are a large university hospital, where the analytics get significantly more advanced in terms of measuring return on investment (ROI), I think you should just be looking at your schedule and looking at your monthly billings and seeing how they change over time. You can calculate how much a new patient is worth because you can figure out how many patients you have and how much you bill and what your profits are.
Dr. Culligan: For those of us who are hospital employees, you can try to convince the hospital that you can do a detailed ROI analysis, or you can just look at it like (say it’s $3,000 per month), how many surgeries does this project have to generate before the hospital makes that back? The answer is a fraction of 1 case.
Thank you to all of you for your expertise on this roundtable.
- Anderson A. Online reviews vs. word of mouth: Which one is more important. https://www.revlocal.com/blog/reviewandreputationmanagement/onlinereviewsvswordofmouthwhichoneismoreimportant. Accessed July 17, 2020.
- 2020 Patient sentiment report. Healthgrades; Medical Group Management Association. https://www.healthgrades.com/content /patientsentimentreport. Accessed July 17, 2020
- Anderson A. Online reviews vs. word of mouth: Which one is more important. https://www.revlocal.com/blog/reviewandreputationmanagement/onlinereviewsvswordofmouthwhichoneismoreimportant. Accessed July 17, 2020.
- 2020 Patient sentiment report. Healthgrades; Medical Group Management Association. https://www.healthgrades.com/content /patientsentimentreport. Accessed July 17, 2020
Hysteroscopy and COVID-19: Have recommended techniques changed due to the pandemic?
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
2020 Update on pelvic floor dysfunction
Postoperative voiding dysfunction refers to the acute inability to spontaneously and adequately empty the bladder after surgery. Postoperative voiding dysfunction occurs in 21% to 42% of pelvic reconstructive surgeries, as well as 7% to 21% of benign gynecologic surgeries.1-4 While much of its peril lies in patient discomfort or dissatisfaction with temporary bladder drainage, serious consequences of the disorder include bladder overdistension injury with inadequate drainage and urinary tract infection (UTI) associated with prolonged catheterization.4-6
Although transient postoperative voiding dysfunction is associated with anti-incontinence surgery, tricyclic antidepressant use, diabetes, preoperative voiding dysfunction, and postoperative narcotic use, it also may occur in patients without risk factors.4,7,8 Thus, all gynecologic surgeons should be prepared to assess and manage the patient with postoperative voiding dysfunction.
Diagnosis of postoperative voiding dysfunction can be approached in myriad ways, including spontaneous (or natural) bladder filling or bladder backfill followed by spontaneous void. When compared with spontaneous void trials, backfill-assisted void trial is associated with improved accuracy in predicting voiding dysfunction in patients who undergo urogynecologic surgery, leading to widespread adoption of the procedure following pelvic reconstructive surgeries.9,10
Criteria for “passing” a void trial may include the patient’s subjective feeling of having emptied her bladder; having a near-baseline force of stream; or commonly by objective parameters of voided volume and postvoid residual (PVR), assessed via catheterization or bladder scan.3,6,10 Completing a postoperative void trial typically requires significant nursing effort because of the technical demands of backfilling the bladder, obtaining the voided volume and PVR, or assessing subjective emptying.
Management of postoperative voiding dysfunction typically consists of continuous drainage with a transurethral catheter or clean intermittent self-catheterization (CISC). Patients discharged home with a bladder drainage method also may be prescribed various medications, such as antibiotics, anticholinergics, and bladder analgesics, which often depends on provider practice.
Given the minimal universal guidance available for gynecologic surgeons on postoperative voiding dysfunction, we review several articles that contribute new evidence on the assessment and management of this condition.
Continue to: How can we efficiently approach the postoperative void trial for pelvic floor surgery?
How can we efficiently approach the postoperative void trial for pelvic floor surgery?
Chao L, Mansuria S. Postoperative bladder filling after outpatient laparoscopic hysterectomy and time to discharge: a randomized controlled trial. Obstet Gynecol. 2019;133:879-887.
Despite efforts to implement and promote enhanced recovery after surgery pathways, waiting for spontaneous void can be a barrier to efficient same-day discharge. Chao and Mansuria conducted a randomized controlled trial (RCT) to determine whether backfilling the bladder intraoperatively, compared with spontaneous (physiologic) filling, would reduce time to discharge in patients undergoing total laparoscopic hysterectomy (TLH) or supracervical hysterectomy (SCH).
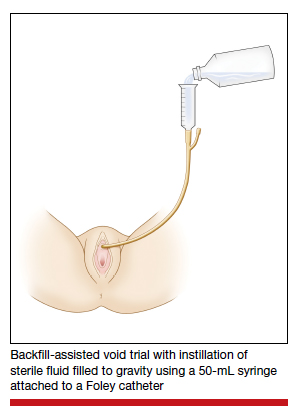
Study details

Women undergoing TLH or laparoscopic SCH for benign indications were randomly assigned to undergo either a backfill-assisted void trial in the operating room with 200 mL of sterile normal saline (n = 75) or Foley catheter removal with spontaneous fill in the postanesthesia care unit (PACU) (n = 78).
For both groups, the maximum time allowed for spontaneous void was 5 hours. A successful void trial was defined as a voided volume of at least 200 mL. If a patient was unable to void at least 200 mL, a bladder scan was performed, and the patient was considered to have failed the void trial if a PVR of 200 mL or greater was noted. If the PVR was less than 200 mL, the patient was given an additional 1 hour to spontaneously void 200 mL by 6 hours after the surgery. Patients who failed the void trial were discharged home with a transurethral catheter.
The primary outcome was time to discharge, and the sample size (153 participants included in the analysis) allowed 80% power to detect a 30-minute difference in time to discharge. Participant baseline characteristics, concomitant procedures, and indication for hysterectomy were similar for both groups.
Results. The mean time to discharge was 273.4 minutes for the backfill-assisted void trial group and 283.2 minutes for the spontaneous fill group, a difference of 9.8 minutes that was not statistically significant (P = .45).
Although it was not a primary outcome, time to spontaneous void was 24.9 minutes shorter in the backfill group (P = .04). Rates of postoperative voiding dysfunction did not differ between the 2 groups (6.7% for the backfill group and 12.8% for the spontaneous fill group; P = .2). There were no significant differences in emergency department visits, UTI rates, or readmissions.
Bladder backfill is safe, simple, and may reduce time to spontaneous void
Strengths of the study included its prospective randomized design, blinded outcome assessors, and diversity in benign gynecologic surgeries performed. Although this study found a reduced time to spontaneous void in the backfill group, it was not powered to assess this difference, limiting ability to draw conclusions from those data. Data on postoperative nausea and pain scores also were not collected, which likely influenced the overall time to discharge.
Void trial completion is one of many criteria to fulfill prior to patient discharge, and a reduced time to first void may not decrease the overall length of PACU stay if other factors, such as nausea or pain, are not controlled. Nonetheless, backfilling the bladder intraoperatively is a safe alternative that may decrease the time to first spontaneous void, and it is a relatively simple alteration in the surgical workflow that could significantly lessen PACU nursing demands.
Backfilling the bladder in the operating room prior to catheter discontinuation can reduce time to first spontaneous void, but not the overall time to discharge.
Continue to: Algorithm assesses need for PVR, although further study required...
Algorithm assesses need for PVR, although further study required
Meekins AR, Siddiqui N, Amundsen CL, et al. Improving postoperative efficiency: an algorithm for expedited void trials after urogynecologic surgery. South Med J. 2017;110:785-790.
To determine ways to further maximize postoperative efficiency, Meekins and colleagues sought to determine whether certain voided volumes during backfill-assisted void trials could obviate the need for PVR assessment.
Void trial results calculated to develop algorithm
The study was a secondary analysis of a previously conducted RCT that assessed antibiotics for the prevention of UTI after urogynecologic surgery. Void trials from the parent RCT were performed via the backfill-assisted method in which the bladder was backfilled in the PACU with 300 mL of normal saline or until the patient reported urgency to void, after which the catheter was removed and the patient was prompted to void immediately.
Postvoid residual levels were assessed via ultrasonography or catheterization. A void trial was considered to be passed when a PVR was less than 100 mL or less than 50% of the total bladder volume, with a minimum voided volume of 200 mL.
In the follow-up study, the authors analyzed the void trial results of 255 women of the original 264 in the parent RCT. A total of 69% of patients passed their void trial. The authors assessed the optimal positive predictive value (PPV) and negative predictive value (NPV) combinations, which were then used to create lower and upper voided volume thresholds that would best predict a failed or passed trial, thus obviating PVR measurement.
Results. When patients voided less than 100 mL, the NPV was 96.7% (meaning that they had a 96.7% chance of failing the void trial). When patients voided 200 mL or more, the PPV was 97% (meaning that they had a 97% chance of passing the void trial). Receiver operating characteristic analysis confirmed that voided volume alone was an excellent predictor of final void trial results, with area under the curve of 0.97. The authors estimated that applying this algorithm to their study population would have eliminated the need for assessing PVR in 85% of patients. Ultimately, they proposed the algorithm shown in TABLE 1.
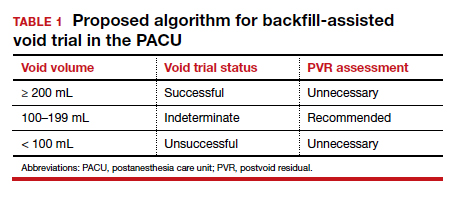
A potential alternative for assessing PVR
This study's strengths include the use of prospectively and systematically collected void trial data in a large patient population undergoing various urogynecologic procedures. By contrast, the generalizability of the results is limited regarding other void trial methods, such as spontaneous filling and void, as well as populations outside of the studied institution.
With the algorithm, the authors estimated that the majority of postoperative patients would no longer require a PVR assessment in the PACU. This could have beneficial downstream implications, including decreasing the nursing workload, reducing total time in the PACU, and minimizing patient discomfort with PVR assessment.
While further studies are needed to validate the proposed algorithm in larger populations, this study provides evidence of an efficient alternative to the traditional approach to PVR assessment in the PACU.
Application of the algorithm proposed by the study investigators has the potential to eliminate the need for a PVR assessment in most patients following a backfill-assisted void trial.
Continue to: An alternative to Foley use if a patient does not know CISC...
An alternative to Foley use if a patient does not know CISC
Boyd SS, O'Sullivan DM, Tunitsky-Bitton E. A comparison of two methods of catheter management after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:1037-1045.
The traditional indwelling catheter as a postoperative bladder drainage method has a number of drawbacks, including an increased rate of UTI, patient discomfort, and potential limitations in mobility due to the presence of a drainage bag.5
Boyd and colleagues reported on a variation of traditional transurethral catheterization that hypothetically allows for improved mobility. With this method, the transurethral catheter is occluded with a plastic plug that is intermittently plugged and unplugged (plug-unplug method) for bladder drainage. To test whether activity levels are improved with the plug-unplug method versus the continuous drainage approach, the authors conducted an RCT in women undergoing pelvic reconstructive surgery to compare the plug-unplug method with transurethral catheterization (with a continuous drainage bag) and a reference group of freely voiding women.
Study particulars and outcomes
The trial's primary outcome was the patients' activity score as measured by the Activity Assessment Scale (AAS) at 5 to 7 days postoperatively. Because of the theoretically increased risk of a UTI with opening and closing a closed drainage system, secondary outcomes included the UTI rate, the time to pass an outpatient void trial, postoperative pain, patient satisfaction, and catheter effect. To detect an effect size of 0.33 in the primary outcome between the 3 groups, 90 participants were needed along with a difference in proportions of 0.3 between the catheterized and noncatheterized groups.
The participants were randomly assigned 1:1 preoperatively to the continuous drainage or plug-unplug method. All patients underwent a backfill-assisted void trial prior to hospital discharge; the first 30 randomly assigned patients to pass their void trial comprised the reference group. Patients in the plug-unplug arm were instructed to uncap the plastic plug to drain their bladder when they felt the urge to void or at least every 4 hours. All catheterized patients were provided with a large drainage bag for gravity-based drainage for overnight use.
Participants who were discharged home with a catheter underwent an outpatient void trial between postoperative days 5 and 7. A urinalysis was performed at that time and a urine culture was done if a patient reported UTI symptoms. All patients underwent routine follow-up until they passed the office void trial.
Results. Ninety-three women were included in the primary analysis. There were no differences in baseline characteristics between groups. No difference was detected in activity by AAS scores between all 3 groups (scores: plug-unplug, 70.3; continuous drainage, 67.7; reference arm, 79.4; P = .09). The 2 treatment arms had no overall difference in culture-positive UTI (plug-unplug, 68.8%; continuous drainage, 48.4%; P = .625). No significant difference was found in the percentage of patients who passed their initial outpatient void trial (plug-unplug, 71.9%, vs continuous drainage, 58.1%; P = .25) (TABLE 2).
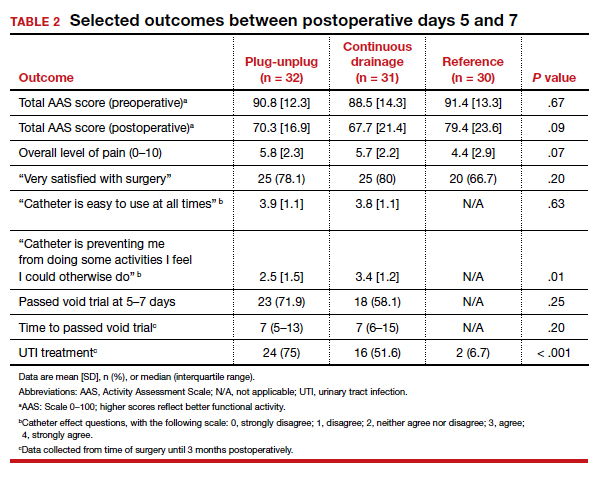
Catheter impact on postoperative activity considered
Strengths of the study include the prospective randomized design, the inclusion of a noncatheterized reference arm, and use of a validated questionnaire to assess activity. The study was limited, however, by the inability to blind patients to treatment and the lack of power to assess other important outcomes, such as UTI rates.
Although the authors did not find a difference in activity scores between the 2 catheterization methods, no significant difference was found between the catheterized and noncatheterized groups, which suggests that catheters in general may not significantly impact postoperative activity. The theoretical concern that opening and closing a transurethral drainage system would increase UTI rates was not substantiated, although the study was not powered specifically for this outcome.
Ultimately, the plug-unplug method may be a safe alternative for patients who desire to avoid attachment to a drainage bag postoperatively.
Based on the results of an RCT that compared 2 methods of catheter management after pelvic reconstructive surgery, the plug-unplug catheterization method may be an acceptable alternative to traditional catheterization.
- Bladder backfill in the operating room followed by spontaneous void in the postanesthesia care unit (PACU) is a safe and efficient way to assess for postoperative voiding dysfunction.
- Voids of 200 mL or more (following a 300-mL backfill) may not require a PACU postvoid residual assessment.
- Postoperative activity does not appear to be impacted by the presence of an indwelling catheter.
Continue to: Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Lavelle ES, Alam P, Meister M, et al. Antibiotic prophylaxis during catheter-managed postoperative urinary retention after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:727-735.
Limited high-quality evidence supports the use of prophylactic antibiotics during catheterization following prolapse or incontinence surgery, and the Infectious Disease Society of America cautions against routine antibiotic prophylaxis for those requiring catheterization.11
Lavelle and colleagues conducted a multicenter RCT to determine whether nitrofurantoin is more effective than placebo in decreasing UTIs among patients with postoperative voiding dysfunction following surgery for prolapse or incontinence.
Focus of the study
The investigators conducted a double-blind RCT at 5 academic sites that included women with postoperative voiding dysfunction who required catheter management (transurethral indwelling catheter or CISC). Voiding dysfunction was diagnosed by backfill or spontaneous fill void trial and was defined as a PVR of greater than 100 mL. Women were randomly assigned 1:1 to nitrofurantoin 100 mg or placebo taken daily during catheter use. Catheter use was discontinued once an outpatient void trial confirmed efficient voiding.
The primary outcome was symptomatic culture-confirmed UTI within 6 weeks of surgery. Secondary outcomes included frequency of urine cultures with nitrofurantoin-resistant or intermediate-sensitivity isolates and adverse symptoms possibly related to nitrofurantoin. The authors calculated that 154 participants would provide 80% power to detect a decrease in UTI incidence from 33% to 13%, allowing for 10% dropout.
A total of 151 women were randomly assigned and included in the intention-to-treat analysis. There were no differences in baseline characteristics. The median duration of catheter use was 4 days (interquartile range, 3-7).
Results. Overall, 13 women in the nitrofurantoin group and 13 in the placebo group experienced the primary outcome of UTI within 6 weeks postoperatively (17.3% nitrofurantoin vs 17.1% placebo; P = .97; relative risk [RR], 1.01; 95% confidence interval [CI], 0.50-2.04). The number needed to treat with nitrofurantoin to prevent 1 UTI was 500. A subanalysis found no difference in UTI incidence stratified by CISC versus indwelling catheter.
Urine cultures were obtained for 94.5% of all patients reporting UTI symptoms. Four isolates of the 13 cultures in the nitrofurantoin group (30.8%) and 3 in the placebo group (21.4%) showed nitrofurantoin resistance (P = .58). The rate of endorsing at least 1 adverse symptom attributable to nitrofurantoin was similar between groups (68.0% vs 60.5%, respectively; P = .34).
Study strong points and limitations
This study's randomized, placebo-controlled design and multicenter recruitment increase the generalizability of the results. An additional strength is that the authors chose a clinically relevant definition of UTI. The study was likely underpowered, however, to detect differences in secondary outcomes, such as nitrofurantoin resistance. We cannot conclude on the role of antibiotics for patients who require more prolonged catheterization.
Notably, a similar RCT by Dieter and colleagues of 159 patients undergoing daily nitrofurantoin versus placebo during CISC or transurethral catheterization failed to detect a difference in the rate of UTI treatment up to 3 weeks postoperatively with nitrofurantoin prophylaxis.12
Ultimately, the study by Lavelle and colleagues contributes to a growing body of evidence that supports the avoidance of antibiotic prophylaxis during short-term postoperative catheterization.
Nitrofurantoin prophylaxis did not reduce the incidence of postoperative UTI in patients with catheter-managed postoperative voiding dysfunction.
- Prophylactic antibiotics are not necessary for short-term catheterization in postoperative patients.
- Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24:1843-1852.
- Yune JJ, Cheng JW, Wagner H, et al. Postoperative urinary retention after pelvic organ prolapse repair: vaginal versus robotic transabdominal approach. Neurourol Urodyn. 2018;37:1794-1800.
- Ghezzi F, Cromi A, Uccella S, et al. Immediate Foley removal after laparoscopic and vaginal hysterectomy: determinants of postoperative urinary retention. J Minim Invasive Gynecol. 2007;14:706-711.
- Smorgick N, DeLancey J, Patzkowsky K, et al. Risk factors for postoperative urinary retention after laparoscopic and robotic hysterectomy for benign indications. Obstet Gynecol. 2012;120:581-586.
- Dieter AA, Amundsen CL, Visco AG, et al. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18:175-178.
- Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597.e1-e9.
- Kandadai P, Saini J, Patterson D, et al. Urinary retention after hysterectomy and postoperative analgesic use. Female Pelvic Med Reconstr Surg. 2015;21:257-262.
- Liang CC, Lee CL, Chang TC, et al. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy--a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:295-300.
- Foster RT Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627.e1-e4.
- Geller EJ, Hankins KJ, Parnell BA, et al. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118:637-642.
Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Disease Society of America. Clin Infect Dis. 2010;50:625-663.
Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96-103.
Postoperative voiding dysfunction refers to the acute inability to spontaneously and adequately empty the bladder after surgery. Postoperative voiding dysfunction occurs in 21% to 42% of pelvic reconstructive surgeries, as well as 7% to 21% of benign gynecologic surgeries.1-4 While much of its peril lies in patient discomfort or dissatisfaction with temporary bladder drainage, serious consequences of the disorder include bladder overdistension injury with inadequate drainage and urinary tract infection (UTI) associated with prolonged catheterization.4-6
Although transient postoperative voiding dysfunction is associated with anti-incontinence surgery, tricyclic antidepressant use, diabetes, preoperative voiding dysfunction, and postoperative narcotic use, it also may occur in patients without risk factors.4,7,8 Thus, all gynecologic surgeons should be prepared to assess and manage the patient with postoperative voiding dysfunction.
Diagnosis of postoperative voiding dysfunction can be approached in myriad ways, including spontaneous (or natural) bladder filling or bladder backfill followed by spontaneous void. When compared with spontaneous void trials, backfill-assisted void trial is associated with improved accuracy in predicting voiding dysfunction in patients who undergo urogynecologic surgery, leading to widespread adoption of the procedure following pelvic reconstructive surgeries.9,10
Criteria for “passing” a void trial may include the patient’s subjective feeling of having emptied her bladder; having a near-baseline force of stream; or commonly by objective parameters of voided volume and postvoid residual (PVR), assessed via catheterization or bladder scan.3,6,10 Completing a postoperative void trial typically requires significant nursing effort because of the technical demands of backfilling the bladder, obtaining the voided volume and PVR, or assessing subjective emptying.
Management of postoperative voiding dysfunction typically consists of continuous drainage with a transurethral catheter or clean intermittent self-catheterization (CISC). Patients discharged home with a bladder drainage method also may be prescribed various medications, such as antibiotics, anticholinergics, and bladder analgesics, which often depends on provider practice.
Given the minimal universal guidance available for gynecologic surgeons on postoperative voiding dysfunction, we review several articles that contribute new evidence on the assessment and management of this condition.
Continue to: How can we efficiently approach the postoperative void trial for pelvic floor surgery?
How can we efficiently approach the postoperative void trial for pelvic floor surgery?
Chao L, Mansuria S. Postoperative bladder filling after outpatient laparoscopic hysterectomy and time to discharge: a randomized controlled trial. Obstet Gynecol. 2019;133:879-887.
Despite efforts to implement and promote enhanced recovery after surgery pathways, waiting for spontaneous void can be a barrier to efficient same-day discharge. Chao and Mansuria conducted a randomized controlled trial (RCT) to determine whether backfilling the bladder intraoperatively, compared with spontaneous (physiologic) filling, would reduce time to discharge in patients undergoing total laparoscopic hysterectomy (TLH) or supracervical hysterectomy (SCH).

Study details

Women undergoing TLH or laparoscopic SCH for benign indications were randomly assigned to undergo either a backfill-assisted void trial in the operating room with 200 mL of sterile normal saline (n = 75) or Foley catheter removal with spontaneous fill in the postanesthesia care unit (PACU) (n = 78).
For both groups, the maximum time allowed for spontaneous void was 5 hours. A successful void trial was defined as a voided volume of at least 200 mL. If a patient was unable to void at least 200 mL, a bladder scan was performed, and the patient was considered to have failed the void trial if a PVR of 200 mL or greater was noted. If the PVR was less than 200 mL, the patient was given an additional 1 hour to spontaneously void 200 mL by 6 hours after the surgery. Patients who failed the void trial were discharged home with a transurethral catheter.
The primary outcome was time to discharge, and the sample size (153 participants included in the analysis) allowed 80% power to detect a 30-minute difference in time to discharge. Participant baseline characteristics, concomitant procedures, and indication for hysterectomy were similar for both groups.
Results. The mean time to discharge was 273.4 minutes for the backfill-assisted void trial group and 283.2 minutes for the spontaneous fill group, a difference of 9.8 minutes that was not statistically significant (P = .45).
Although it was not a primary outcome, time to spontaneous void was 24.9 minutes shorter in the backfill group (P = .04). Rates of postoperative voiding dysfunction did not differ between the 2 groups (6.7% for the backfill group and 12.8% for the spontaneous fill group; P = .2). There were no significant differences in emergency department visits, UTI rates, or readmissions.
Bladder backfill is safe, simple, and may reduce time to spontaneous void
Strengths of the study included its prospective randomized design, blinded outcome assessors, and diversity in benign gynecologic surgeries performed. Although this study found a reduced time to spontaneous void in the backfill group, it was not powered to assess this difference, limiting ability to draw conclusions from those data. Data on postoperative nausea and pain scores also were not collected, which likely influenced the overall time to discharge.
Void trial completion is one of many criteria to fulfill prior to patient discharge, and a reduced time to first void may not decrease the overall length of PACU stay if other factors, such as nausea or pain, are not controlled. Nonetheless, backfilling the bladder intraoperatively is a safe alternative that may decrease the time to first spontaneous void, and it is a relatively simple alteration in the surgical workflow that could significantly lessen PACU nursing demands.
Backfilling the bladder in the operating room prior to catheter discontinuation can reduce time to first spontaneous void, but not the overall time to discharge.
Continue to: Algorithm assesses need for PVR, although further study required...
Algorithm assesses need for PVR, although further study required
Meekins AR, Siddiqui N, Amundsen CL, et al. Improving postoperative efficiency: an algorithm for expedited void trials after urogynecologic surgery. South Med J. 2017;110:785-790.
To determine ways to further maximize postoperative efficiency, Meekins and colleagues sought to determine whether certain voided volumes during backfill-assisted void trials could obviate the need for PVR assessment.
Void trial results calculated to develop algorithm
The study was a secondary analysis of a previously conducted RCT that assessed antibiotics for the prevention of UTI after urogynecologic surgery. Void trials from the parent RCT were performed via the backfill-assisted method in which the bladder was backfilled in the PACU with 300 mL of normal saline or until the patient reported urgency to void, after which the catheter was removed and the patient was prompted to void immediately.
Postvoid residual levels were assessed via ultrasonography or catheterization. A void trial was considered to be passed when a PVR was less than 100 mL or less than 50% of the total bladder volume, with a minimum voided volume of 200 mL.
In the follow-up study, the authors analyzed the void trial results of 255 women of the original 264 in the parent RCT. A total of 69% of patients passed their void trial. The authors assessed the optimal positive predictive value (PPV) and negative predictive value (NPV) combinations, which were then used to create lower and upper voided volume thresholds that would best predict a failed or passed trial, thus obviating PVR measurement.
Results. When patients voided less than 100 mL, the NPV was 96.7% (meaning that they had a 96.7% chance of failing the void trial). When patients voided 200 mL or more, the PPV was 97% (meaning that they had a 97% chance of passing the void trial). Receiver operating characteristic analysis confirmed that voided volume alone was an excellent predictor of final void trial results, with area under the curve of 0.97. The authors estimated that applying this algorithm to their study population would have eliminated the need for assessing PVR in 85% of patients. Ultimately, they proposed the algorithm shown in TABLE 1.

A potential alternative for assessing PVR
This study's strengths include the use of prospectively and systematically collected void trial data in a large patient population undergoing various urogynecologic procedures. By contrast, the generalizability of the results is limited regarding other void trial methods, such as spontaneous filling and void, as well as populations outside of the studied institution.
With the algorithm, the authors estimated that the majority of postoperative patients would no longer require a PVR assessment in the PACU. This could have beneficial downstream implications, including decreasing the nursing workload, reducing total time in the PACU, and minimizing patient discomfort with PVR assessment.
While further studies are needed to validate the proposed algorithm in larger populations, this study provides evidence of an efficient alternative to the traditional approach to PVR assessment in the PACU.
Application of the algorithm proposed by the study investigators has the potential to eliminate the need for a PVR assessment in most patients following a backfill-assisted void trial.
Continue to: An alternative to Foley use if a patient does not know CISC...
An alternative to Foley use if a patient does not know CISC
Boyd SS, O'Sullivan DM, Tunitsky-Bitton E. A comparison of two methods of catheter management after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:1037-1045.
The traditional indwelling catheter as a postoperative bladder drainage method has a number of drawbacks, including an increased rate of UTI, patient discomfort, and potential limitations in mobility due to the presence of a drainage bag.5
Boyd and colleagues reported on a variation of traditional transurethral catheterization that hypothetically allows for improved mobility. With this method, the transurethral catheter is occluded with a plastic plug that is intermittently plugged and unplugged (plug-unplug method) for bladder drainage. To test whether activity levels are improved with the plug-unplug method versus the continuous drainage approach, the authors conducted an RCT in women undergoing pelvic reconstructive surgery to compare the plug-unplug method with transurethral catheterization (with a continuous drainage bag) and a reference group of freely voiding women.
Study particulars and outcomes
The trial's primary outcome was the patients' activity score as measured by the Activity Assessment Scale (AAS) at 5 to 7 days postoperatively. Because of the theoretically increased risk of a UTI with opening and closing a closed drainage system, secondary outcomes included the UTI rate, the time to pass an outpatient void trial, postoperative pain, patient satisfaction, and catheter effect. To detect an effect size of 0.33 in the primary outcome between the 3 groups, 90 participants were needed along with a difference in proportions of 0.3 between the catheterized and noncatheterized groups.
The participants were randomly assigned 1:1 preoperatively to the continuous drainage or plug-unplug method. All patients underwent a backfill-assisted void trial prior to hospital discharge; the first 30 randomly assigned patients to pass their void trial comprised the reference group. Patients in the plug-unplug arm were instructed to uncap the plastic plug to drain their bladder when they felt the urge to void or at least every 4 hours. All catheterized patients were provided with a large drainage bag for gravity-based drainage for overnight use.
Participants who were discharged home with a catheter underwent an outpatient void trial between postoperative days 5 and 7. A urinalysis was performed at that time and a urine culture was done if a patient reported UTI symptoms. All patients underwent routine follow-up until they passed the office void trial.
Results. Ninety-three women were included in the primary analysis. There were no differences in baseline characteristics between groups. No difference was detected in activity by AAS scores between all 3 groups (scores: plug-unplug, 70.3; continuous drainage, 67.7; reference arm, 79.4; P = .09). The 2 treatment arms had no overall difference in culture-positive UTI (plug-unplug, 68.8%; continuous drainage, 48.4%; P = .625). No significant difference was found in the percentage of patients who passed their initial outpatient void trial (plug-unplug, 71.9%, vs continuous drainage, 58.1%; P = .25) (TABLE 2).

Catheter impact on postoperative activity considered
Strengths of the study include the prospective randomized design, the inclusion of a noncatheterized reference arm, and use of a validated questionnaire to assess activity. The study was limited, however, by the inability to blind patients to treatment and the lack of power to assess other important outcomes, such as UTI rates.
Although the authors did not find a difference in activity scores between the 2 catheterization methods, no significant difference was found between the catheterized and noncatheterized groups, which suggests that catheters in general may not significantly impact postoperative activity. The theoretical concern that opening and closing a transurethral drainage system would increase UTI rates was not substantiated, although the study was not powered specifically for this outcome.
Ultimately, the plug-unplug method may be a safe alternative for patients who desire to avoid attachment to a drainage bag postoperatively.
Based on the results of an RCT that compared 2 methods of catheter management after pelvic reconstructive surgery, the plug-unplug catheterization method may be an acceptable alternative to traditional catheterization.
- Bladder backfill in the operating room followed by spontaneous void in the postanesthesia care unit (PACU) is a safe and efficient way to assess for postoperative voiding dysfunction.
- Voids of 200 mL or more (following a 300-mL backfill) may not require a PACU postvoid residual assessment.
- Postoperative activity does not appear to be impacted by the presence of an indwelling catheter.
Continue to: Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Lavelle ES, Alam P, Meister M, et al. Antibiotic prophylaxis during catheter-managed postoperative urinary retention after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:727-735.
Limited high-quality evidence supports the use of prophylactic antibiotics during catheterization following prolapse or incontinence surgery, and the Infectious Disease Society of America cautions against routine antibiotic prophylaxis for those requiring catheterization.11
Lavelle and colleagues conducted a multicenter RCT to determine whether nitrofurantoin is more effective than placebo in decreasing UTIs among patients with postoperative voiding dysfunction following surgery for prolapse or incontinence.
Focus of the study
The investigators conducted a double-blind RCT at 5 academic sites that included women with postoperative voiding dysfunction who required catheter management (transurethral indwelling catheter or CISC). Voiding dysfunction was diagnosed by backfill or spontaneous fill void trial and was defined as a PVR of greater than 100 mL. Women were randomly assigned 1:1 to nitrofurantoin 100 mg or placebo taken daily during catheter use. Catheter use was discontinued once an outpatient void trial confirmed efficient voiding.
The primary outcome was symptomatic culture-confirmed UTI within 6 weeks of surgery. Secondary outcomes included frequency of urine cultures with nitrofurantoin-resistant or intermediate-sensitivity isolates and adverse symptoms possibly related to nitrofurantoin. The authors calculated that 154 participants would provide 80% power to detect a decrease in UTI incidence from 33% to 13%, allowing for 10% dropout.
A total of 151 women were randomly assigned and included in the intention-to-treat analysis. There were no differences in baseline characteristics. The median duration of catheter use was 4 days (interquartile range, 3-7).
Results. Overall, 13 women in the nitrofurantoin group and 13 in the placebo group experienced the primary outcome of UTI within 6 weeks postoperatively (17.3% nitrofurantoin vs 17.1% placebo; P = .97; relative risk [RR], 1.01; 95% confidence interval [CI], 0.50-2.04). The number needed to treat with nitrofurantoin to prevent 1 UTI was 500. A subanalysis found no difference in UTI incidence stratified by CISC versus indwelling catheter.
Urine cultures were obtained for 94.5% of all patients reporting UTI symptoms. Four isolates of the 13 cultures in the nitrofurantoin group (30.8%) and 3 in the placebo group (21.4%) showed nitrofurantoin resistance (P = .58). The rate of endorsing at least 1 adverse symptom attributable to nitrofurantoin was similar between groups (68.0% vs 60.5%, respectively; P = .34).
Study strong points and limitations
This study's randomized, placebo-controlled design and multicenter recruitment increase the generalizability of the results. An additional strength is that the authors chose a clinically relevant definition of UTI. The study was likely underpowered, however, to detect differences in secondary outcomes, such as nitrofurantoin resistance. We cannot conclude on the role of antibiotics for patients who require more prolonged catheterization.
Notably, a similar RCT by Dieter and colleagues of 159 patients undergoing daily nitrofurantoin versus placebo during CISC or transurethral catheterization failed to detect a difference in the rate of UTI treatment up to 3 weeks postoperatively with nitrofurantoin prophylaxis.12
Ultimately, the study by Lavelle and colleagues contributes to a growing body of evidence that supports the avoidance of antibiotic prophylaxis during short-term postoperative catheterization.
Nitrofurantoin prophylaxis did not reduce the incidence of postoperative UTI in patients with catheter-managed postoperative voiding dysfunction.
- Prophylactic antibiotics are not necessary for short-term catheterization in postoperative patients.
Postoperative voiding dysfunction refers to the acute inability to spontaneously and adequately empty the bladder after surgery. Postoperative voiding dysfunction occurs in 21% to 42% of pelvic reconstructive surgeries, as well as 7% to 21% of benign gynecologic surgeries.1-4 While much of its peril lies in patient discomfort or dissatisfaction with temporary bladder drainage, serious consequences of the disorder include bladder overdistension injury with inadequate drainage and urinary tract infection (UTI) associated with prolonged catheterization.4-6
Although transient postoperative voiding dysfunction is associated with anti-incontinence surgery, tricyclic antidepressant use, diabetes, preoperative voiding dysfunction, and postoperative narcotic use, it also may occur in patients without risk factors.4,7,8 Thus, all gynecologic surgeons should be prepared to assess and manage the patient with postoperative voiding dysfunction.
Diagnosis of postoperative voiding dysfunction can be approached in myriad ways, including spontaneous (or natural) bladder filling or bladder backfill followed by spontaneous void. When compared with spontaneous void trials, backfill-assisted void trial is associated with improved accuracy in predicting voiding dysfunction in patients who undergo urogynecologic surgery, leading to widespread adoption of the procedure following pelvic reconstructive surgeries.9,10
Criteria for “passing” a void trial may include the patient’s subjective feeling of having emptied her bladder; having a near-baseline force of stream; or commonly by objective parameters of voided volume and postvoid residual (PVR), assessed via catheterization or bladder scan.3,6,10 Completing a postoperative void trial typically requires significant nursing effort because of the technical demands of backfilling the bladder, obtaining the voided volume and PVR, or assessing subjective emptying.
Management of postoperative voiding dysfunction typically consists of continuous drainage with a transurethral catheter or clean intermittent self-catheterization (CISC). Patients discharged home with a bladder drainage method also may be prescribed various medications, such as antibiotics, anticholinergics, and bladder analgesics, which often depends on provider practice.
Given the minimal universal guidance available for gynecologic surgeons on postoperative voiding dysfunction, we review several articles that contribute new evidence on the assessment and management of this condition.
Continue to: How can we efficiently approach the postoperative void trial for pelvic floor surgery?
How can we efficiently approach the postoperative void trial for pelvic floor surgery?
Chao L, Mansuria S. Postoperative bladder filling after outpatient laparoscopic hysterectomy and time to discharge: a randomized controlled trial. Obstet Gynecol. 2019;133:879-887.
Despite efforts to implement and promote enhanced recovery after surgery pathways, waiting for spontaneous void can be a barrier to efficient same-day discharge. Chao and Mansuria conducted a randomized controlled trial (RCT) to determine whether backfilling the bladder intraoperatively, compared with spontaneous (physiologic) filling, would reduce time to discharge in patients undergoing total laparoscopic hysterectomy (TLH) or supracervical hysterectomy (SCH).

Study details

Women undergoing TLH or laparoscopic SCH for benign indications were randomly assigned to undergo either a backfill-assisted void trial in the operating room with 200 mL of sterile normal saline (n = 75) or Foley catheter removal with spontaneous fill in the postanesthesia care unit (PACU) (n = 78).
For both groups, the maximum time allowed for spontaneous void was 5 hours. A successful void trial was defined as a voided volume of at least 200 mL. If a patient was unable to void at least 200 mL, a bladder scan was performed, and the patient was considered to have failed the void trial if a PVR of 200 mL or greater was noted. If the PVR was less than 200 mL, the patient was given an additional 1 hour to spontaneously void 200 mL by 6 hours after the surgery. Patients who failed the void trial were discharged home with a transurethral catheter.
The primary outcome was time to discharge, and the sample size (153 participants included in the analysis) allowed 80% power to detect a 30-minute difference in time to discharge. Participant baseline characteristics, concomitant procedures, and indication for hysterectomy were similar for both groups.
Results. The mean time to discharge was 273.4 minutes for the backfill-assisted void trial group and 283.2 minutes for the spontaneous fill group, a difference of 9.8 minutes that was not statistically significant (P = .45).
Although it was not a primary outcome, time to spontaneous void was 24.9 minutes shorter in the backfill group (P = .04). Rates of postoperative voiding dysfunction did not differ between the 2 groups (6.7% for the backfill group and 12.8% for the spontaneous fill group; P = .2). There were no significant differences in emergency department visits, UTI rates, or readmissions.
Bladder backfill is safe, simple, and may reduce time to spontaneous void
Strengths of the study included its prospective randomized design, blinded outcome assessors, and diversity in benign gynecologic surgeries performed. Although this study found a reduced time to spontaneous void in the backfill group, it was not powered to assess this difference, limiting ability to draw conclusions from those data. Data on postoperative nausea and pain scores also were not collected, which likely influenced the overall time to discharge.
Void trial completion is one of many criteria to fulfill prior to patient discharge, and a reduced time to first void may not decrease the overall length of PACU stay if other factors, such as nausea or pain, are not controlled. Nonetheless, backfilling the bladder intraoperatively is a safe alternative that may decrease the time to first spontaneous void, and it is a relatively simple alteration in the surgical workflow that could significantly lessen PACU nursing demands.
Backfilling the bladder in the operating room prior to catheter discontinuation can reduce time to first spontaneous void, but not the overall time to discharge.
Continue to: Algorithm assesses need for PVR, although further study required...
Algorithm assesses need for PVR, although further study required
Meekins AR, Siddiqui N, Amundsen CL, et al. Improving postoperative efficiency: an algorithm for expedited void trials after urogynecologic surgery. South Med J. 2017;110:785-790.
To determine ways to further maximize postoperative efficiency, Meekins and colleagues sought to determine whether certain voided volumes during backfill-assisted void trials could obviate the need for PVR assessment.
Void trial results calculated to develop algorithm
The study was a secondary analysis of a previously conducted RCT that assessed antibiotics for the prevention of UTI after urogynecologic surgery. Void trials from the parent RCT were performed via the backfill-assisted method in which the bladder was backfilled in the PACU with 300 mL of normal saline or until the patient reported urgency to void, after which the catheter was removed and the patient was prompted to void immediately.
Postvoid residual levels were assessed via ultrasonography or catheterization. A void trial was considered to be passed when a PVR was less than 100 mL or less than 50% of the total bladder volume, with a minimum voided volume of 200 mL.
In the follow-up study, the authors analyzed the void trial results of 255 women of the original 264 in the parent RCT. A total of 69% of patients passed their void trial. The authors assessed the optimal positive predictive value (PPV) and negative predictive value (NPV) combinations, which were then used to create lower and upper voided volume thresholds that would best predict a failed or passed trial, thus obviating PVR measurement.
Results. When patients voided less than 100 mL, the NPV was 96.7% (meaning that they had a 96.7% chance of failing the void trial). When patients voided 200 mL or more, the PPV was 97% (meaning that they had a 97% chance of passing the void trial). Receiver operating characteristic analysis confirmed that voided volume alone was an excellent predictor of final void trial results, with area under the curve of 0.97. The authors estimated that applying this algorithm to their study population would have eliminated the need for assessing PVR in 85% of patients. Ultimately, they proposed the algorithm shown in TABLE 1.

A potential alternative for assessing PVR
This study's strengths include the use of prospectively and systematically collected void trial data in a large patient population undergoing various urogynecologic procedures. By contrast, the generalizability of the results is limited regarding other void trial methods, such as spontaneous filling and void, as well as populations outside of the studied institution.
With the algorithm, the authors estimated that the majority of postoperative patients would no longer require a PVR assessment in the PACU. This could have beneficial downstream implications, including decreasing the nursing workload, reducing total time in the PACU, and minimizing patient discomfort with PVR assessment.
While further studies are needed to validate the proposed algorithm in larger populations, this study provides evidence of an efficient alternative to the traditional approach to PVR assessment in the PACU.
Application of the algorithm proposed by the study investigators has the potential to eliminate the need for a PVR assessment in most patients following a backfill-assisted void trial.
Continue to: An alternative to Foley use if a patient does not know CISC...
An alternative to Foley use if a patient does not know CISC
Boyd SS, O'Sullivan DM, Tunitsky-Bitton E. A comparison of two methods of catheter management after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:1037-1045.
The traditional indwelling catheter as a postoperative bladder drainage method has a number of drawbacks, including an increased rate of UTI, patient discomfort, and potential limitations in mobility due to the presence of a drainage bag.5
Boyd and colleagues reported on a variation of traditional transurethral catheterization that hypothetically allows for improved mobility. With this method, the transurethral catheter is occluded with a plastic plug that is intermittently plugged and unplugged (plug-unplug method) for bladder drainage. To test whether activity levels are improved with the plug-unplug method versus the continuous drainage approach, the authors conducted an RCT in women undergoing pelvic reconstructive surgery to compare the plug-unplug method with transurethral catheterization (with a continuous drainage bag) and a reference group of freely voiding women.
Study particulars and outcomes
The trial's primary outcome was the patients' activity score as measured by the Activity Assessment Scale (AAS) at 5 to 7 days postoperatively. Because of the theoretically increased risk of a UTI with opening and closing a closed drainage system, secondary outcomes included the UTI rate, the time to pass an outpatient void trial, postoperative pain, patient satisfaction, and catheter effect. To detect an effect size of 0.33 in the primary outcome between the 3 groups, 90 participants were needed along with a difference in proportions of 0.3 between the catheterized and noncatheterized groups.
The participants were randomly assigned 1:1 preoperatively to the continuous drainage or plug-unplug method. All patients underwent a backfill-assisted void trial prior to hospital discharge; the first 30 randomly assigned patients to pass their void trial comprised the reference group. Patients in the plug-unplug arm were instructed to uncap the plastic plug to drain their bladder when they felt the urge to void or at least every 4 hours. All catheterized patients were provided with a large drainage bag for gravity-based drainage for overnight use.
Participants who were discharged home with a catheter underwent an outpatient void trial between postoperative days 5 and 7. A urinalysis was performed at that time and a urine culture was done if a patient reported UTI symptoms. All patients underwent routine follow-up until they passed the office void trial.
Results. Ninety-three women were included in the primary analysis. There were no differences in baseline characteristics between groups. No difference was detected in activity by AAS scores between all 3 groups (scores: plug-unplug, 70.3; continuous drainage, 67.7; reference arm, 79.4; P = .09). The 2 treatment arms had no overall difference in culture-positive UTI (plug-unplug, 68.8%; continuous drainage, 48.4%; P = .625). No significant difference was found in the percentage of patients who passed their initial outpatient void trial (plug-unplug, 71.9%, vs continuous drainage, 58.1%; P = .25) (TABLE 2).

Catheter impact on postoperative activity considered
Strengths of the study include the prospective randomized design, the inclusion of a noncatheterized reference arm, and use of a validated questionnaire to assess activity. The study was limited, however, by the inability to blind patients to treatment and the lack of power to assess other important outcomes, such as UTI rates.
Although the authors did not find a difference in activity scores between the 2 catheterization methods, no significant difference was found between the catheterized and noncatheterized groups, which suggests that catheters in general may not significantly impact postoperative activity. The theoretical concern that opening and closing a transurethral drainage system would increase UTI rates was not substantiated, although the study was not powered specifically for this outcome.
Ultimately, the plug-unplug method may be a safe alternative for patients who desire to avoid attachment to a drainage bag postoperatively.
Based on the results of an RCT that compared 2 methods of catheter management after pelvic reconstructive surgery, the plug-unplug catheterization method may be an acceptable alternative to traditional catheterization.
- Bladder backfill in the operating room followed by spontaneous void in the postanesthesia care unit (PACU) is a safe and efficient way to assess for postoperative voiding dysfunction.
- Voids of 200 mL or more (following a 300-mL backfill) may not require a PACU postvoid residual assessment.
- Postoperative activity does not appear to be impacted by the presence of an indwelling catheter.
Continue to: Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Lavelle ES, Alam P, Meister M, et al. Antibiotic prophylaxis during catheter-managed postoperative urinary retention after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:727-735.
Limited high-quality evidence supports the use of prophylactic antibiotics during catheterization following prolapse or incontinence surgery, and the Infectious Disease Society of America cautions against routine antibiotic prophylaxis for those requiring catheterization.11
Lavelle and colleagues conducted a multicenter RCT to determine whether nitrofurantoin is more effective than placebo in decreasing UTIs among patients with postoperative voiding dysfunction following surgery for prolapse or incontinence.
Focus of the study
The investigators conducted a double-blind RCT at 5 academic sites that included women with postoperative voiding dysfunction who required catheter management (transurethral indwelling catheter or CISC). Voiding dysfunction was diagnosed by backfill or spontaneous fill void trial and was defined as a PVR of greater than 100 mL. Women were randomly assigned 1:1 to nitrofurantoin 100 mg or placebo taken daily during catheter use. Catheter use was discontinued once an outpatient void trial confirmed efficient voiding.
The primary outcome was symptomatic culture-confirmed UTI within 6 weeks of surgery. Secondary outcomes included frequency of urine cultures with nitrofurantoin-resistant or intermediate-sensitivity isolates and adverse symptoms possibly related to nitrofurantoin. The authors calculated that 154 participants would provide 80% power to detect a decrease in UTI incidence from 33% to 13%, allowing for 10% dropout.
A total of 151 women were randomly assigned and included in the intention-to-treat analysis. There were no differences in baseline characteristics. The median duration of catheter use was 4 days (interquartile range, 3-7).
Results. Overall, 13 women in the nitrofurantoin group and 13 in the placebo group experienced the primary outcome of UTI within 6 weeks postoperatively (17.3% nitrofurantoin vs 17.1% placebo; P = .97; relative risk [RR], 1.01; 95% confidence interval [CI], 0.50-2.04). The number needed to treat with nitrofurantoin to prevent 1 UTI was 500. A subanalysis found no difference in UTI incidence stratified by CISC versus indwelling catheter.
Urine cultures were obtained for 94.5% of all patients reporting UTI symptoms. Four isolates of the 13 cultures in the nitrofurantoin group (30.8%) and 3 in the placebo group (21.4%) showed nitrofurantoin resistance (P = .58). The rate of endorsing at least 1 adverse symptom attributable to nitrofurantoin was similar between groups (68.0% vs 60.5%, respectively; P = .34).
Study strong points and limitations
This study's randomized, placebo-controlled design and multicenter recruitment increase the generalizability of the results. An additional strength is that the authors chose a clinically relevant definition of UTI. The study was likely underpowered, however, to detect differences in secondary outcomes, such as nitrofurantoin resistance. We cannot conclude on the role of antibiotics for patients who require more prolonged catheterization.
Notably, a similar RCT by Dieter and colleagues of 159 patients undergoing daily nitrofurantoin versus placebo during CISC or transurethral catheterization failed to detect a difference in the rate of UTI treatment up to 3 weeks postoperatively with nitrofurantoin prophylaxis.12
Ultimately, the study by Lavelle and colleagues contributes to a growing body of evidence that supports the avoidance of antibiotic prophylaxis during short-term postoperative catheterization.
Nitrofurantoin prophylaxis did not reduce the incidence of postoperative UTI in patients with catheter-managed postoperative voiding dysfunction.
- Prophylactic antibiotics are not necessary for short-term catheterization in postoperative patients.
- Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24:1843-1852.
- Yune JJ, Cheng JW, Wagner H, et al. Postoperative urinary retention after pelvic organ prolapse repair: vaginal versus robotic transabdominal approach. Neurourol Urodyn. 2018;37:1794-1800.
- Ghezzi F, Cromi A, Uccella S, et al. Immediate Foley removal after laparoscopic and vaginal hysterectomy: determinants of postoperative urinary retention. J Minim Invasive Gynecol. 2007;14:706-711.
- Smorgick N, DeLancey J, Patzkowsky K, et al. Risk factors for postoperative urinary retention after laparoscopic and robotic hysterectomy for benign indications. Obstet Gynecol. 2012;120:581-586.
- Dieter AA, Amundsen CL, Visco AG, et al. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18:175-178.
- Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597.e1-e9.
- Kandadai P, Saini J, Patterson D, et al. Urinary retention after hysterectomy and postoperative analgesic use. Female Pelvic Med Reconstr Surg. 2015;21:257-262.
- Liang CC, Lee CL, Chang TC, et al. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy--a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:295-300.
- Foster RT Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627.e1-e4.
- Geller EJ, Hankins KJ, Parnell BA, et al. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118:637-642.
Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Disease Society of America. Clin Infect Dis. 2010;50:625-663.
Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96-103.
- Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24:1843-1852.
- Yune JJ, Cheng JW, Wagner H, et al. Postoperative urinary retention after pelvic organ prolapse repair: vaginal versus robotic transabdominal approach. Neurourol Urodyn. 2018;37:1794-1800.
- Ghezzi F, Cromi A, Uccella S, et al. Immediate Foley removal after laparoscopic and vaginal hysterectomy: determinants of postoperative urinary retention. J Minim Invasive Gynecol. 2007;14:706-711.
- Smorgick N, DeLancey J, Patzkowsky K, et al. Risk factors for postoperative urinary retention after laparoscopic and robotic hysterectomy for benign indications. Obstet Gynecol. 2012;120:581-586.
- Dieter AA, Amundsen CL, Visco AG, et al. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18:175-178.
- Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597.e1-e9.
- Kandadai P, Saini J, Patterson D, et al. Urinary retention after hysterectomy and postoperative analgesic use. Female Pelvic Med Reconstr Surg. 2015;21:257-262.
- Liang CC, Lee CL, Chang TC, et al. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy--a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:295-300.
- Foster RT Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627.e1-e4.
- Geller EJ, Hankins KJ, Parnell BA, et al. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118:637-642.
Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Disease Society of America. Clin Infect Dis. 2010;50:625-663.
Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96-103.
VA-Radiation Oncology Quality Surveillance Program: Enhancing Quality Measure Data Capture, Measuring Quality Benchmarks and Ensuring Long Term Sustainability of Quality Improvements in Community Care
INTRODUCTION: Delivery of high-quality cancer care improves oncologic outcomes, including survival and quality of life. The VA National Radiation Oncology (NROP) established the VA Radiation Oncology Quality Surveillance Program (VAROQS) which has developed clinical quality measures (QM) as a measure of quality indices in radiation oncology. We sought to measure quality in community care, assess barriers to data capture, and develop solutions to ensure long term sustainability of continuous quality improvement for veterans that receive dual care, both within the VA and in non-VA community care (NVCC).
METHODS: From 2016-2018, the VA-ROQS project randomly selected three Veterans Integrated Service Networks (VISNs) for quality analysis using established QM for prostate cancer, specifically, 6, 16, and 22. NROP manually abstracted data for QM treated in NVCC QMs, which was compared to the performance of the VA QM in the same VISN as well as for all VISNs in the VA.
RESULTS: Out of the 723 NVCC cases that were examined, none were fully evaluable for all 25 Prostate quality metrics. QM was able to be assessed in only 28% of NVCC patients (n=208) reviewed. Only 12/25 (48%) of all Prostate QM were able to be compared between VA and NVCC. Out of the 12 available Prostate QM, 9 were performance, 2 were surveillance, while 1 was an aspirational measure. The overall > 75% pass rate of all the expected performance QM measures for the VA was 13/14 (92%). For NVCC, of the available expected QM for comparison, 8 of which were high potential impact, only 1/9 (11%) QM received a >75% pass rate in all three NVCC VISNs. When examining the 8 high potential impact QM, the VA had a 100% pass rate.
CONCLUSIONS: There are challenges to obtaining data to perform QM assessment from community care. For cases where QM performance could be assessed, VA care outperformed non-VA care. VA-ROQS program is an ongoing quality improvement initiative and in order to ensure that quality is comprehensively collected for NVCC, we propose a web-based portal that will enable providers to directly upload anonymized treatment information and the DICOM treatment plan.
INTRODUCTION: Delivery of high-quality cancer care improves oncologic outcomes, including survival and quality of life. The VA National Radiation Oncology (NROP) established the VA Radiation Oncology Quality Surveillance Program (VAROQS) which has developed clinical quality measures (QM) as a measure of quality indices in radiation oncology. We sought to measure quality in community care, assess barriers to data capture, and develop solutions to ensure long term sustainability of continuous quality improvement for veterans that receive dual care, both within the VA and in non-VA community care (NVCC).
METHODS: From 2016-2018, the VA-ROQS project randomly selected three Veterans Integrated Service Networks (VISNs) for quality analysis using established QM for prostate cancer, specifically, 6, 16, and 22. NROP manually abstracted data for QM treated in NVCC QMs, which was compared to the performance of the VA QM in the same VISN as well as for all VISNs in the VA.
RESULTS: Out of the 723 NVCC cases that were examined, none were fully evaluable for all 25 Prostate quality metrics. QM was able to be assessed in only 28% of NVCC patients (n=208) reviewed. Only 12/25 (48%) of all Prostate QM were able to be compared between VA and NVCC. Out of the 12 available Prostate QM, 9 were performance, 2 were surveillance, while 1 was an aspirational measure. The overall > 75% pass rate of all the expected performance QM measures for the VA was 13/14 (92%). For NVCC, of the available expected QM for comparison, 8 of which were high potential impact, only 1/9 (11%) QM received a >75% pass rate in all three NVCC VISNs. When examining the 8 high potential impact QM, the VA had a 100% pass rate.
CONCLUSIONS: There are challenges to obtaining data to perform QM assessment from community care. For cases where QM performance could be assessed, VA care outperformed non-VA care. VA-ROQS program is an ongoing quality improvement initiative and in order to ensure that quality is comprehensively collected for NVCC, we propose a web-based portal that will enable providers to directly upload anonymized treatment information and the DICOM treatment plan.
INTRODUCTION: Delivery of high-quality cancer care improves oncologic outcomes, including survival and quality of life. The VA National Radiation Oncology (NROP) established the VA Radiation Oncology Quality Surveillance Program (VAROQS) which has developed clinical quality measures (QM) as a measure of quality indices in radiation oncology. We sought to measure quality in community care, assess barriers to data capture, and develop solutions to ensure long term sustainability of continuous quality improvement for veterans that receive dual care, both within the VA and in non-VA community care (NVCC).
METHODS: From 2016-2018, the VA-ROQS project randomly selected three Veterans Integrated Service Networks (VISNs) for quality analysis using established QM for prostate cancer, specifically, 6, 16, and 22. NROP manually abstracted data for QM treated in NVCC QMs, which was compared to the performance of the VA QM in the same VISN as well as for all VISNs in the VA.
RESULTS: Out of the 723 NVCC cases that were examined, none were fully evaluable for all 25 Prostate quality metrics. QM was able to be assessed in only 28% of NVCC patients (n=208) reviewed. Only 12/25 (48%) of all Prostate QM were able to be compared between VA and NVCC. Out of the 12 available Prostate QM, 9 were performance, 2 were surveillance, while 1 was an aspirational measure. The overall > 75% pass rate of all the expected performance QM measures for the VA was 13/14 (92%). For NVCC, of the available expected QM for comparison, 8 of which were high potential impact, only 1/9 (11%) QM received a >75% pass rate in all three NVCC VISNs. When examining the 8 high potential impact QM, the VA had a 100% pass rate.
CONCLUSIONS: There are challenges to obtaining data to perform QM assessment from community care. For cases where QM performance could be assessed, VA care outperformed non-VA care. VA-ROQS program is an ongoing quality improvement initiative and in order to ensure that quality is comprehensively collected for NVCC, we propose a web-based portal that will enable providers to directly upload anonymized treatment information and the DICOM treatment plan.