User login
Gastric Electric Stimulation for Refractory Gastroparesis
From Temple University School of Medicine, Philadelphia, PA.
Abstract
- Objective: To outline the use and utility of gastric electric stimulation (GES) as a therapeutic intervention for gastroparesis.
- Methods: Review of the literature.
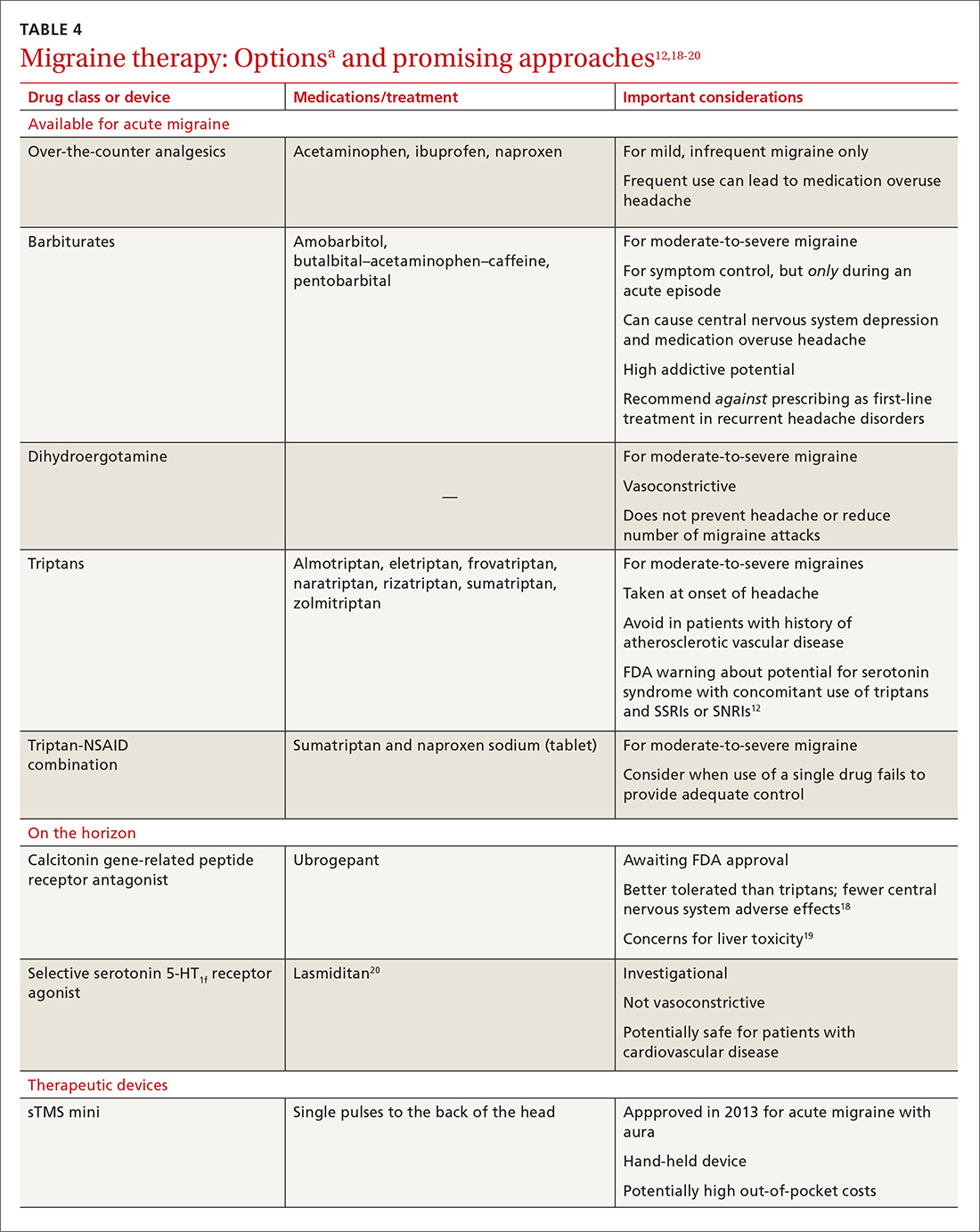
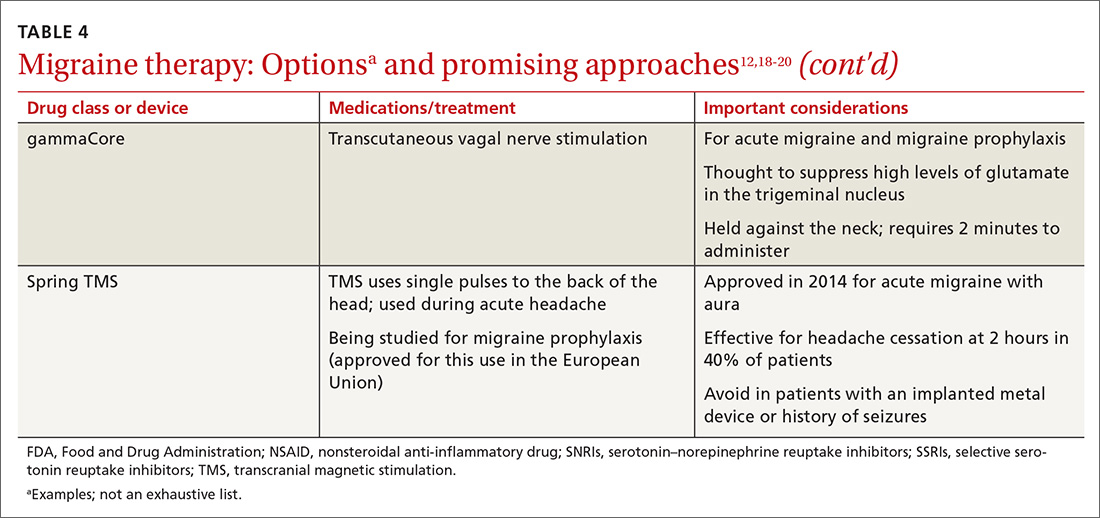
- Results: Gastroparesis is characterized by delayed gastric emptying, with symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Some patients with gastroparesis do not respond to medical intervention, and for these patients surgical intervention may be warranted. GES utilizes high-frequency gastric neurostimulation to facilitate gastric emptying and reduce symptoms of gastroparesis. It is indicated for patients with idiopathic and diabetic gastroparesis who have nausea and vomiting as their primary symptoms and who have not responded to medical therapy. GES has also been used in postsurgical and pediatric gastroparesis patients. Optimizing the outcome of this surgical treatment through proper patient selection and meticulous surgical technique is essential as there are inherent risks to the procedure. Nonblinded studies of GES for medically refractory gastroparesis have demonstrated therapeutic symptomatic benefit, whereas randomized controlled trials have not. New interventions such as pyloromyotomy and pyloroplasty are reasonable alternatives or addendums to GES.
- Conclusion: GES may be considered among the therapies available for treating patients with refractory symptoms of gastroparesis. More studies, specifically those comparing GES, pyloromyotomy, GES combined with pyloromyotomy, and placebo, are needed to help guide therapy selection for refractory gastroparesis.
Keywords: diabetes; gastroparesis; dysmotility; gastric emptying; electric stimulation.
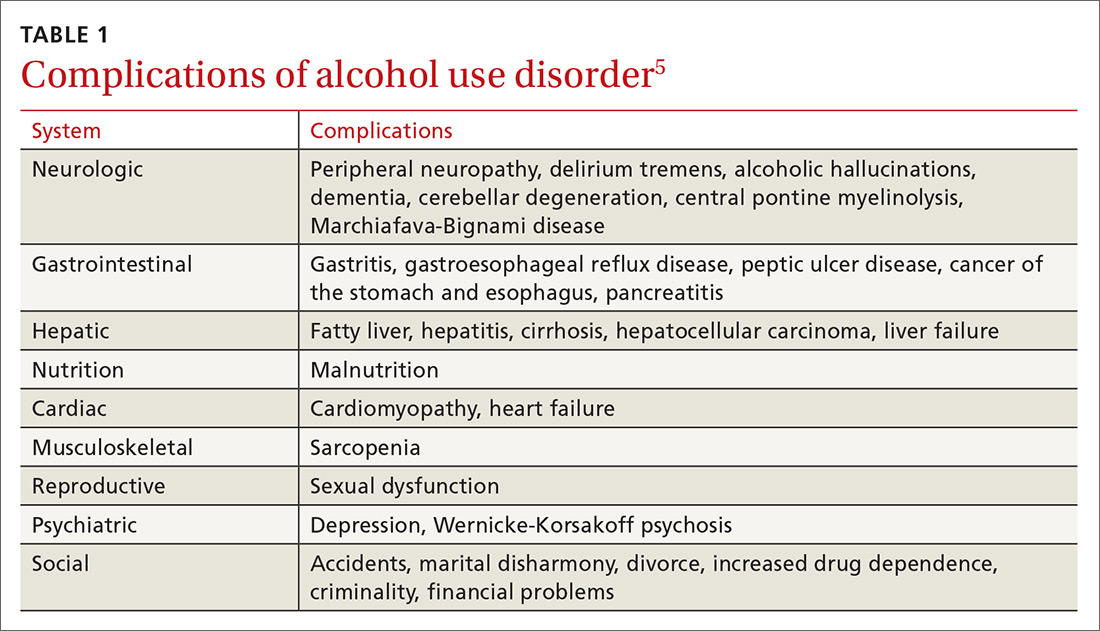
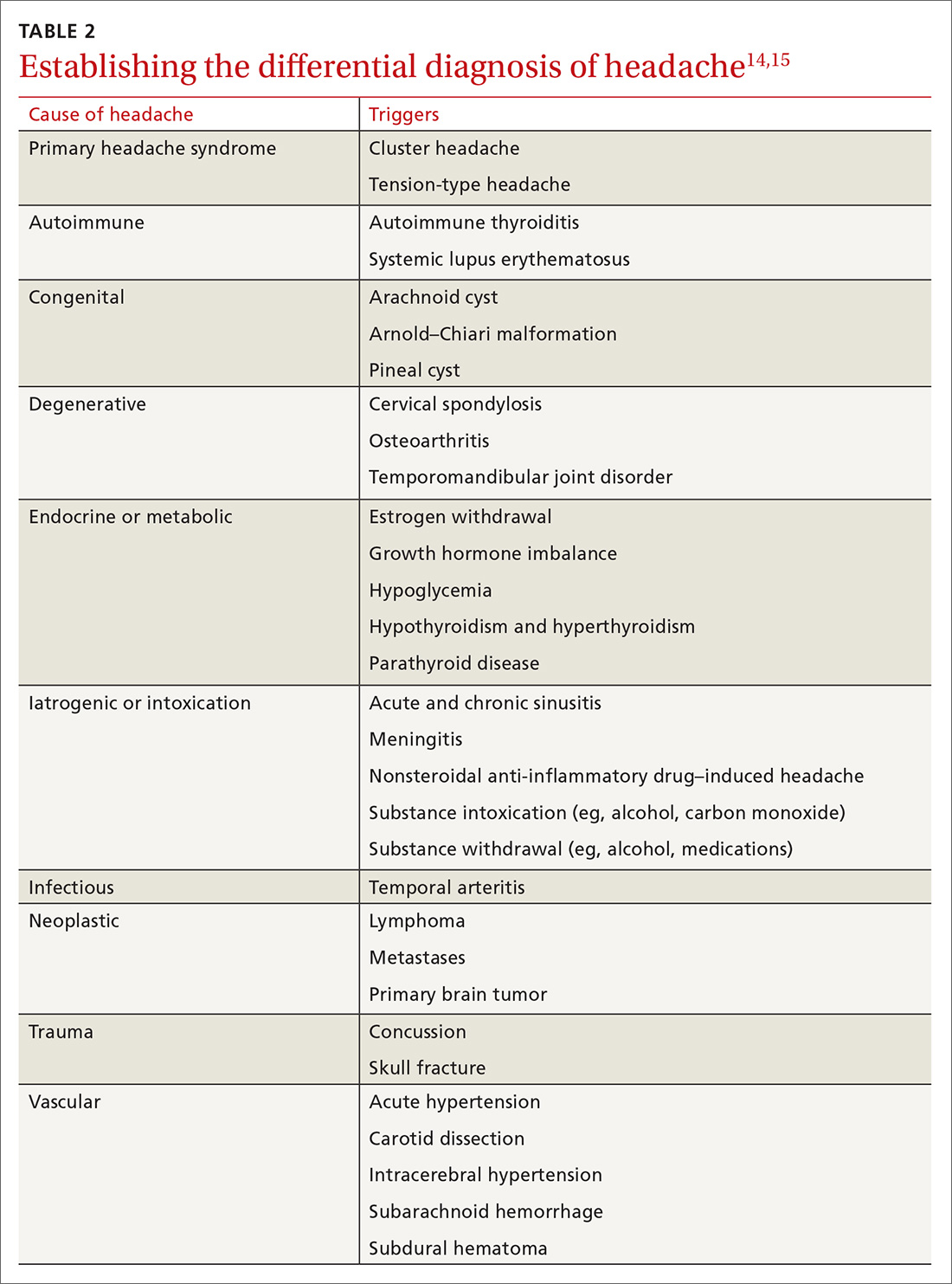
Gastroparesis is a chronic dysmotility disorder characterized by delayed gastric emptying with associated symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Medical treatments for gastroparesis include dietary modifications, glucose control in those with diabetes, prokinetic medications, antiemetic medications, and symptom modulators, but unfortunately patients frequently do not respond to these treatments. In patients refractory to medical therapy, surgical treatments can be considered.
Gastric electric stimulation (GES; Enterra [Medtronic, Minneapolis, MN]) was approved via a Food and Drug Administration (FDA) Humanitarian Use Device (HUD) exemption for the treatment of medically refractory gastroparesis in 2000. Understanding the indications, risks, outcomes, and alternatives to GES is essential to providing appropriate care for patients with medically refractory gastroparesis. This article outlines the use and utility of GES as a therapeutic intervention for gastroparesis.
Types of Gastroparesis
Gastroparesis is a chronic symptomatic disorder of the stomach manifested by delayed gastric emptying without evidence of gastric outlet obstruction or ulceration.1 The pathophysiology of gastroparesis appears to involve abnormalities in functioning of several elements including the autonomic nervous system, especially the vagus nerve, smooth muscle cells, enteric neurons, and interstitial cells of Cajal.
Idiopathic gastroparesis and diabetic gastroparesis are the 2 most common types of gastroparesis.2 Symptomatic delayed gastric emptying with no primary underlying abnormality predisposing to gastroparesis is categorized as idiopathic gastroparesis.3 A small subset of patients with idiopathic gastroparesis report an initial infectious prodrome such as gastroenteritis or respiratory infection. It has been suggested that this postinfectious gastroparesis results from viral injury to the neural innervation of the stomach or the interstitial cells of Cajal in the stomach.4 Viruses that have been implicated in the development of gastroparesis include cytomegalovirus, Epstein-Barr virus, Norwalk virus, rotavirus, herpes zoster, and varicella zoster.5-9
Diabetic gastroparesis is characterized as onset of symptoms of gastroparesis in patients with diabetes, with concomitant delayed gastric emptying. It is often attributed to chronic hyperglycemia-induced damage to the vagus nerve, and is frequently observed in association with other diabetic complications such as neuropathy, retinopathy, and nephropathy.10
Gastroparesis that develops following surgery is classified as postsurgical gastroparesis. In the past, this form of gastroparesis most commonly occurred after ulcer surgery, often performed with vagotomy. These types of surgeries are performed less frequently in the era of proton pump inhibitor therapy and treatments for Helicobacter pylori. Presently, Nissen fundoplication and bariatric surgery are the more common surgical procedures associated with gastroparesis.3 Long-term use of medications that delay gastric emptying, such as opiate narcotic medications, can lead to gastroparesis and represent another form of iatrogenic gastroparesis. Other forms of gastroparesis (atypical gastroparesis) arise due to various underlying etiologies, including neurological disorders (eg, Parkinson disease, multiple sclerosis), metabolic or endocrine conditions (eg, hypothyroidism), autoimmune disorders, connective tissue and collagen vascular disorders (eg, systemic lupus erythematosus, scleroderma, Sjögren syndrome, Ehlers-Danlos syndrome), or eating disorders (eg, anorexia, bulimia).3
Epidemiology
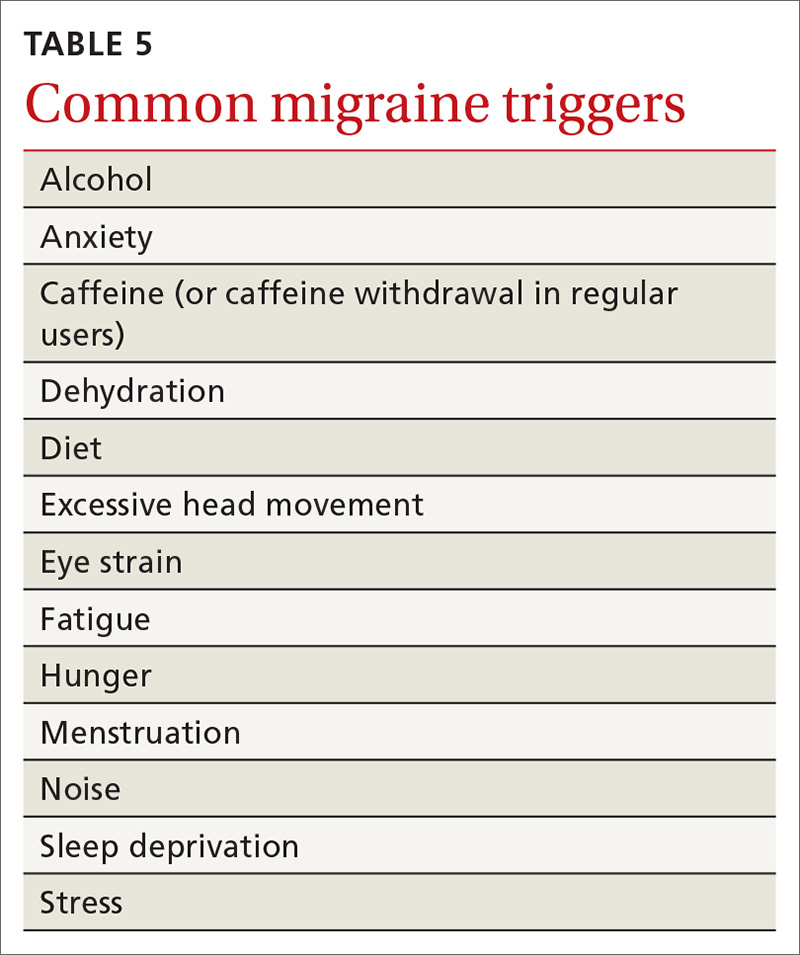
There is a female preponderance in patients with gastroparesis. Data from the Rochester Epidemiology Project, a database of linked medical records for residents of Olmsted County, MN, showed that the age-adjusted prevalence of definite gastroparesis per 100,000 inhabitants was 37.8 for women and 9.6 for men.11 More recent estimates have suggested a much higher prevalence of probable gastroparesis (approximately 1.8%) in the general population using symptoms suggestive of gastroparesis.12 Hospitalization rates for gastroparesis have increased since 2000, which could reflect rising prevalence and/or the effects of heightened awareness about and better identification of gastroparesis.13 This increase may also be due in part to the rising rate of diabetes leading to more cases of diabetic gastroparesis; withdrawal of some gastroparesis treatments from the market (cisapride, tegaserod) leading to hospitalizations for symptoms not adequately being treated; and hospitalizations needed for insertion of the gastric electric stimulator.
Gastroparesis Symptoms
The main symptoms of gastroparesis are early satiety, postprandial fullness, bloating, nausea, and vomiting.14 Nausea (> 90% of patients) and early satiety (60% of patients) are the most common symptoms.15 Abdominal pain is often present in patients with gastroparesis but is usually not the predominant symptom. The pain can be multifactorial, with somatic, visceral, and neuropathic components.16-18 Moderate to severe abdominal pain has been found more often in patients with idiopathic gastroparesis and in association with opiate use.16 Symptoms of gastroparesis may be persistent or present as episodic flares. Due to the symptoms, some patients will experience weight loss and malnutrition and, in severe cases, dehydration.19
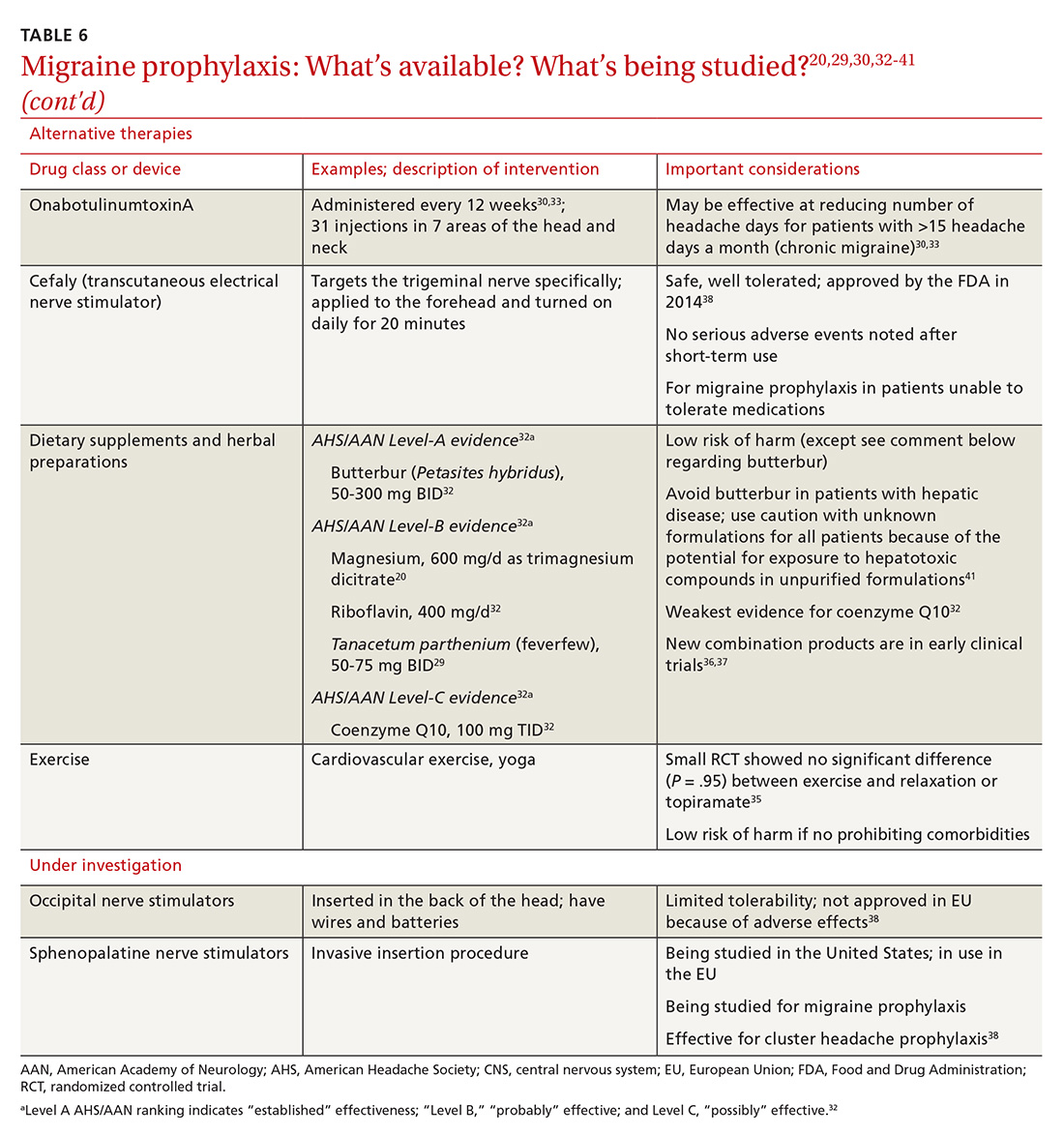
Although the definition of gastroparesis is a delay in gastric emptying along with symptoms, symptoms correlate poorly with the degree of delayed gastric emptying. The symptoms that appear to have the strongest correlation with gastric emptying are nausea, vomiting, early satiety, and postprandial fullness, whereas symptoms such as abdominal pain and bloating have little correlation. Furthermore, improving gastric emptying does not necessarily lead to improved symptoms, and symptom improvement does not always lead to improved gastric emptying times.20 Between 5% and 12% of patients with diabetes report symptoms consistent with gastroparesis, though many of these patients have normal gastric emptying. The symptoms of gastroparesis overlap with those of functional dyspepsia, as both may have motor and sensory alterations.21
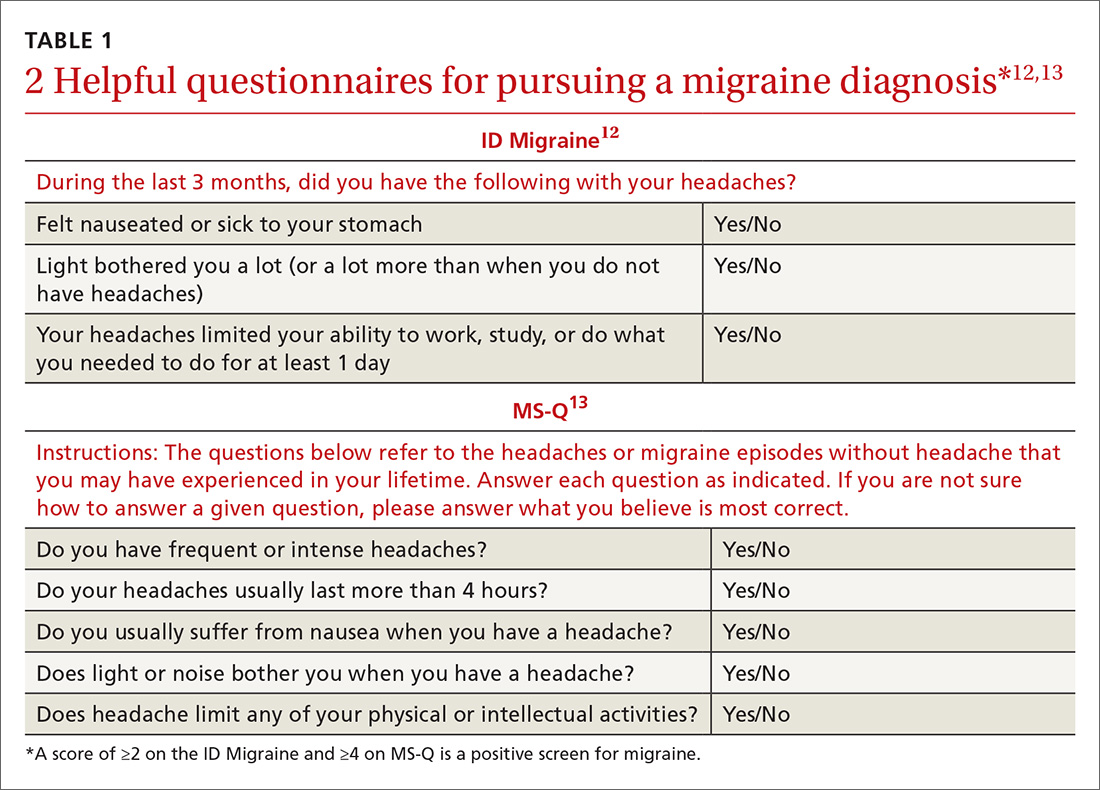
The Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM), is a questionnaire that is commonly used to establish symptom severity in patients with gastroparesis. It is comprised of 3 subscales—nausea and vomiting, postprandial fullness and early satiety, and bloating—which are averaged to provide a total GCSI score. Symptoms over the 2 weeks prior to administration of the questionnaire are assessed and rated from 0 (none) to 5 (very severe).22 Grading the severity of gastroparesis may take into account symptoms, quality of life, and gastric emptying. One commonly used grading system assigns a grade from 1 to 3, with grade 1 being mild gastroparesis, grade 2 being compensated gastroparesis, and grade 3 being gastric failure with refractory symptoms that are uncontrolled.18,23 Quality-of-life surveys also suggest that gastroparesis independent of other factors leads to a worse quality of life.24
Indications for GES
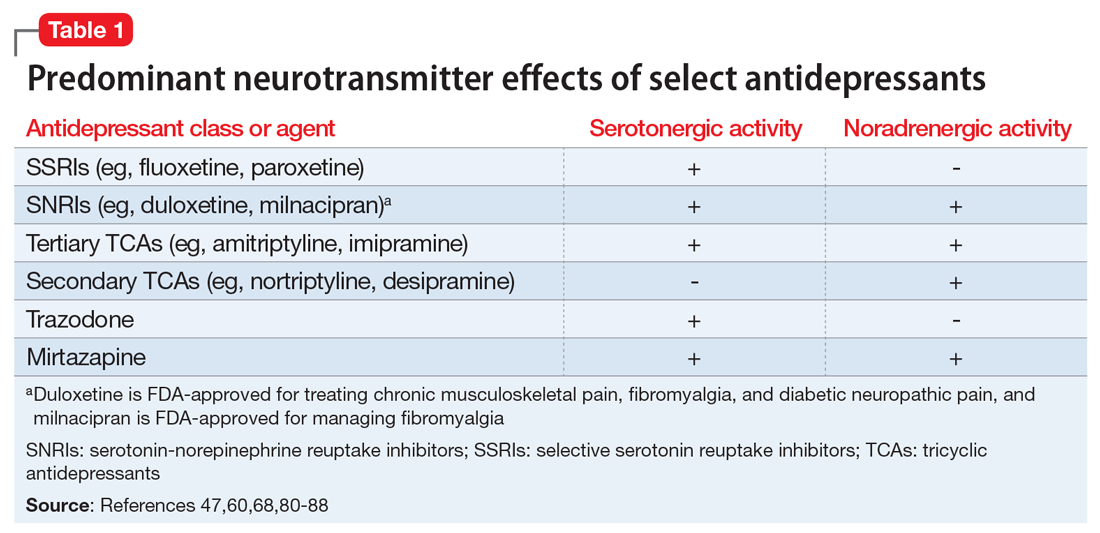
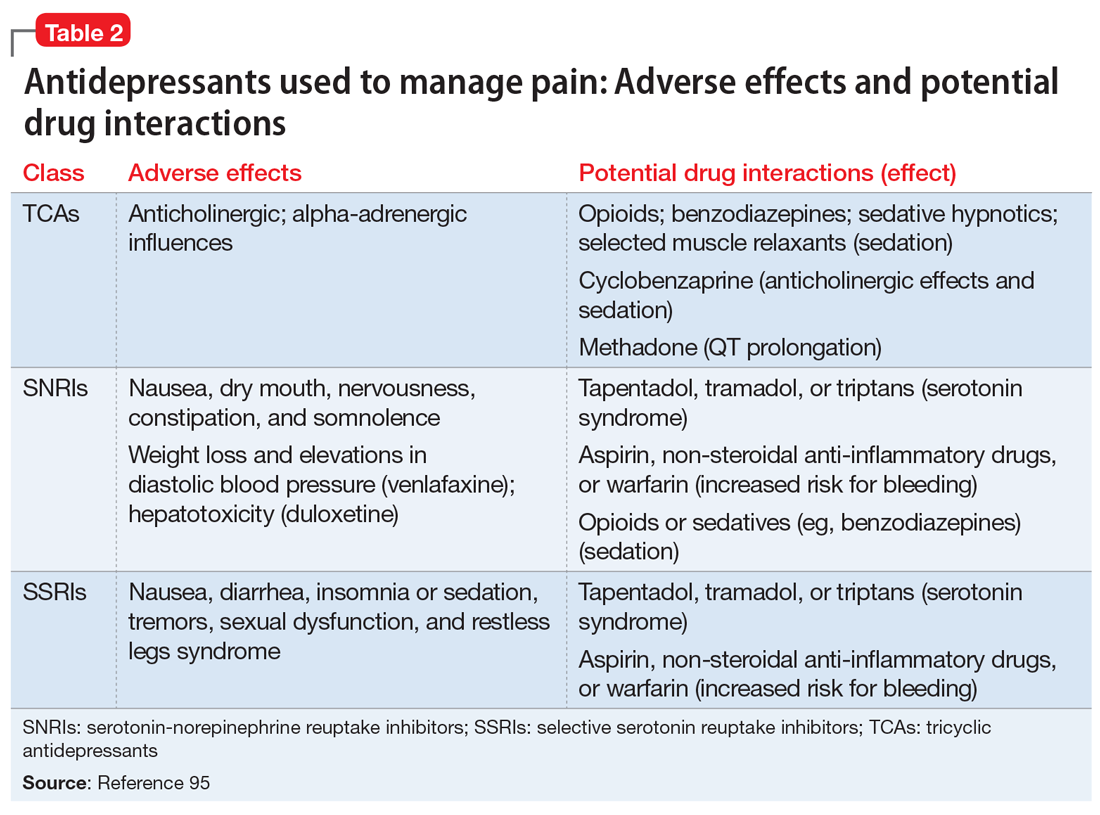
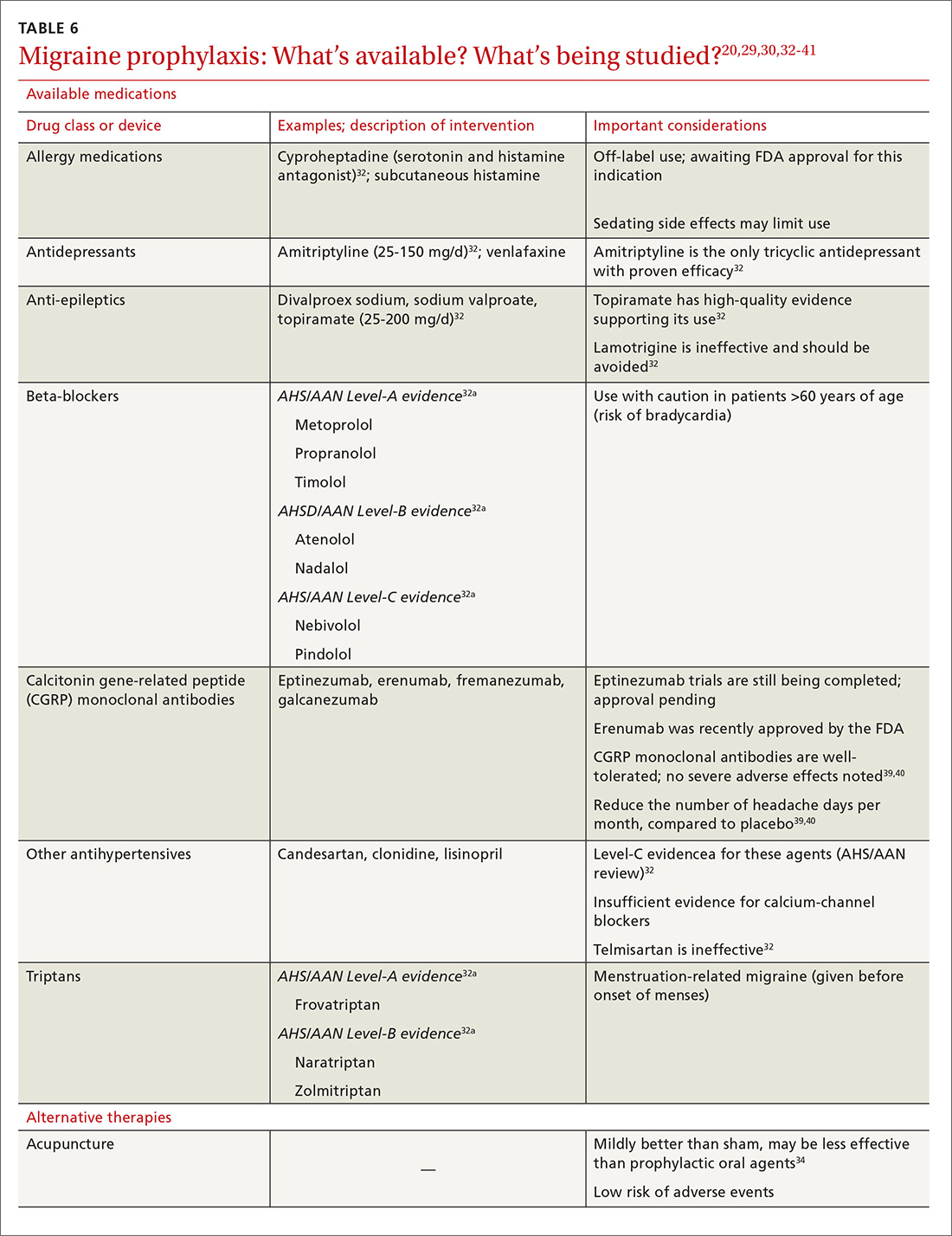
Gastric electric stimulator implantation is a surgical procedure with inherent risks and complications and is reserved for patients with intractable symptoms of gastroparesis who remain symptomatic despite treatment attempts with dietary management, antiemetic agents (eg, compazine, phenergan, and ondansetron), and prokinetic agents (eg, metoclopramide, erythromycin, and domperidone). Symptom modulators such as nortriptyline and mirtazapine are occasionally tried.
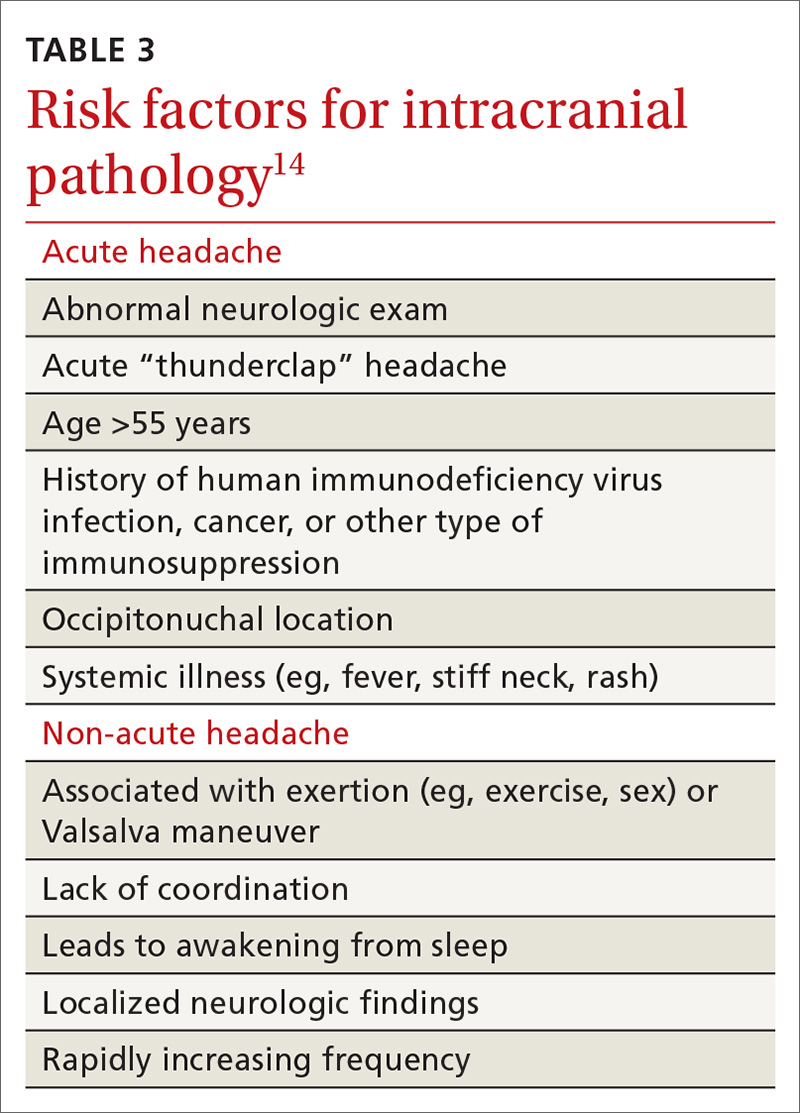
Surgical intervention can be considered upon failure of medical treatment measures. At least a year of documented care provided by a physician specializing in gastroparesis is suggested for surgical consideration. The gastric electric neurostimulator is approved by the FDA as a HUD for the care of patients with idiopathic and diabetic gastroparesis, performed on a compassionate basis. GES implantation requires Institutional Review Board approval at the institution, and patients are required to have documented delayed gastric emptying.
It is important to remember that the GES device is incompatible with magnetic resonance imaging (MRI) and explantation of the device is necessary prior to MRI. As such, in patients with anticipated need of frequent MRI, such as those with multiple sclerosis, serious consideration should be given to alternative strategies prior to focusing on this modality.
Device Placement
GES was devised to improve gastric emptying. The Enterra GES system uses high-frequency, low-energy electric stimulation. An alternative method is true gastric pacing that uses high-energy, low-frequency stimulation to entrain the gastric slow waves and subsequent contractions at 3 cycles per minute (cpm). Gastric pacing has greater energy requirements than GES, which makes the size of the stimulator too large to be practical. In pilot animal studies, GES produced an accelerating effect on gastric emptying, but in human studies GES had an inconsistent effect on gastric emptying. Studies have suggested that GES influences the proximal stomach, with a reduction of gastric tone,25 and also that GES has an afferent modulatory mechanism.26
The Enterra GES is placed surgically under general anesthesia, commonly via laparotomy or minimal access surgical techniques (laparoscopically or robotically assisted). Preoperative intravenous antibiotics are given. The system consists of a pair of electrodes connected to a pulse generator. The 2 stimulation leads are inserted into the gastric muscularis propria 1 cm apart along the greater curvature 10 cm proximal to the pylorus. Upper endoscopy is performed to ensure that the leads do not penetrate through the mucosa into the stomach lumen; if this occurs, repositioning of the lead is necessary. A horizontal incision through the skin is made, and the distal ends of the stimulating wires are tunneled through the abdominal wall and connected to the pulse generator. The impedance (resistance) between the wires is measured to ensure the appropriate range (200-800 Ohms). The neurostimulator with the distal ends of the stimulating wires is then placed into the subcutaneous pocket and sutured to the underlying fascia. The pulse generator delivers a high-frequency, low-energy, 0.1-second train of pulses at a frequency of 12 cpm. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second. The voltage of the stimulations is set to provide a current of 5 milliamps (mA; remembering that voltage = current × resistance).
Patients are often hospitalized with a recovery time of 1 to 3 days. Immediate postoperative care usually includes intravenous fluids, controlling any postoperative ileus, advancing diet, and providing analgesic pain medications. Hospital length of stay can be impacted by surgical technique.25 Patients are seen several weeks after discharge for assessment of the incision and toleration of diet. Medications for gastroparesis that patients were taking prior to the GES implantation are usually continued postoperatively, with a goal of reducing these medications over time. Patients are then followed every 3 to 12 months, depending on their clinical condition.
At follow-up visits, medications are reviewed and new treatments can be added if appropriate. The gastric stimulator is interrogated to determine if changes in resistance occurred; if necessary, minor readjustments can be made to keep the current at desired levels (5 mA). For persistent symptoms with GES treatment, the stimulator parameters can be adjusted after 3 months of follow up, typically first increasing the current from 5 to 7.5 mA and then to 10 mA. After this, the frequency can be increased from 14 Hz to 28 Hz, and then to 55 Hz. Rarely, the ON duration is increased from 0.1 to 1 second. Increasing the ON time can worsen symptoms in some patients, cause abdominal pain, and decrease the battery life from the usual 7 years.
Complications of GES
In an analysis of the Manufacturer and User Facility Device Experience (MAUDE) databank, Bielefeldt identified 1587 reports of adverse effects related to the gastric electric stimulator from January 2001 to October 2015.27 The most common adverse effects are reviewed here.
Skin erosion/wound dehiscence is one of the most common reported complications; it may be related to superficial placement or inadequate securing of the device to the fascia. Abscess can develop postoperatively due to hematogenous seeding or may be a sign of lead erosion into the lumen, tracking along the leads into subcutaneous tissue.28 It is important to warn patients to protect the area over the device from needle injections as this also can lead to hematoma formation and direct contamination of the device. If the device gets infected, it cannot be salvaged and requires explantation. Implantation of a new device can be attempted once all wound issues resolve.
Device migration/flipping most often occurs because the device is inadequately fixed to the underlying fascia, but occasionally it can occur from patients flipping the device around. Flipping can occur due to superficial pocket location within subcutaneous tissue, especially in obese patients. Migration/flipping can lead to prominence of the contour of the device and discomfort, ultimately requiring surgical correction.
Perforation and erosion of the leads. With time, leads can erode into the stomach, although this is rare. Usually erosion is associated with loss of device function. Endoscopy confirms this finding. In rare cases, infection can track proximally along the lead and present as a surgical site infection at the pulse generator. This complication often requires explantation of the neurostimulator leads and pulse generator.
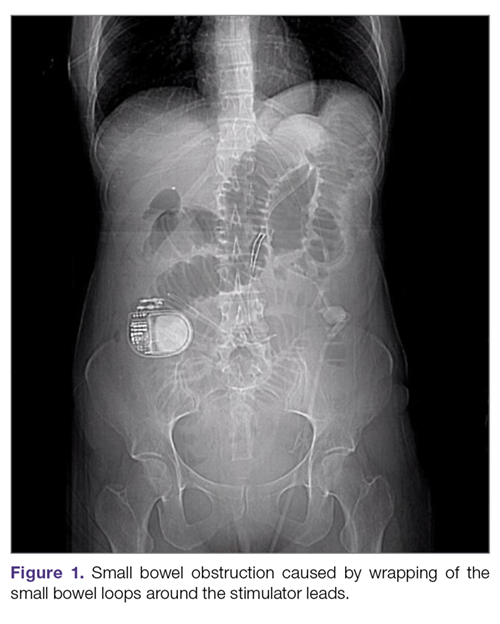
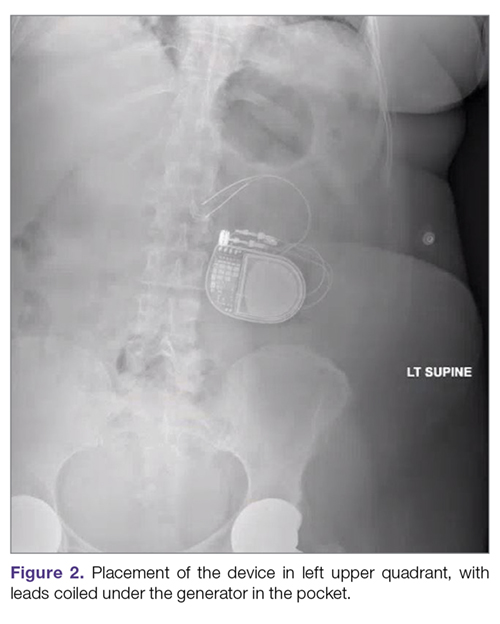
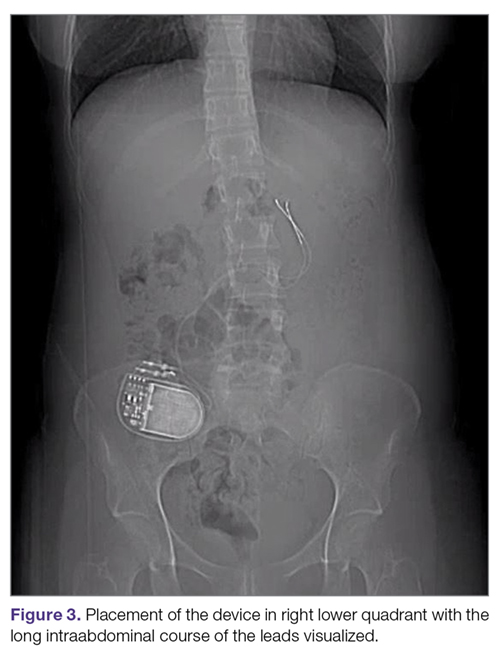
Intestinal obstruction. Although rare, the intestines can get wrapped around the leads of the device, causing different degrees of obstruction (Figure 1). Positioning the device in the left upper quadrant minimizes the intraabdominal length of the leads and pulls them maximally out, coiling under the device (Figure 2). In cases where other locations are used either due to a hostile upper abdominal region (skin infection, presence of gastrostomy or other devices) or surgeon’s preference, the GES device can be implanted in the lower abdomen (Figure 3). In these circumstances, carefully draping the omentum over the bowels might help to prevent this complication. Tacking of the leads to the parietal peritoneum with sutures can also be preventative. In cases of obstruction requiring intervention by laparotomy or minimal access techniques (laparoscopy or robotic assisted surgery), all efforts are made to preserve the neurostimulator leads. In cases that require bowel resection, lead contamination is a serious concern, but lead explantation is not mandatory. Close postoperative monitoring for the development of lead infection is required.
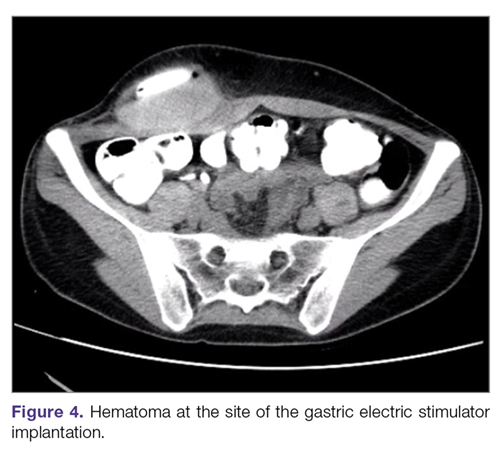
Hematoma and seroma. Postoperative hematomas can occur from inadequate hemostasis, and seromas can occur in the stimulator pocket. Small hematomas may be observed if not complicated (Figure 4). In cases of large hematomas with skin compromise or dehiscence, prompt washout and drainage is required. In ideal cases, the device can be preserved. Relocation to another site might be required if skin necrosis develops. The possibility of device contamination also must be considered; after resolution of wound issues, implantation of a new device may be tried. Seromas at the generator pocket site are a frequent occurrence but are often benign, self-limiting, and generally resolve over 4 to 6 weeks.
Incisional hernia. Hernias can develop after any abdominal surgery and are not unique to GES implantation. Use of minimally invasive technique for the GES implantation minimizes this complication.
Electric shock sensations may occur from breakage of the plastic lining covering the stimulator wires or from fluid buildup around the insertion of the wires into the stimulator. Shocks can also occur due to shortening of the leads on the muscles of the abdominal wall. Patients describe periodic muscle cramps with the frequency of the device (every 5 seconds). To prevent this complication, freshly implanted leads should be covered by an omental flap to isolate them from the abdominal wall. In patients who continue to feel shocks despite all efforts, the possibility of visceral hypersensitivity should be considered. A trial of symptom modulators such as nortriptyline and lowering of the output amperage below the minimal recommended setting of 5 mA can be undertaken. If these interventions do not work, the device must be turned off for a period of time. Occasionally, replacement of the leads or explantation of the device must be considered.
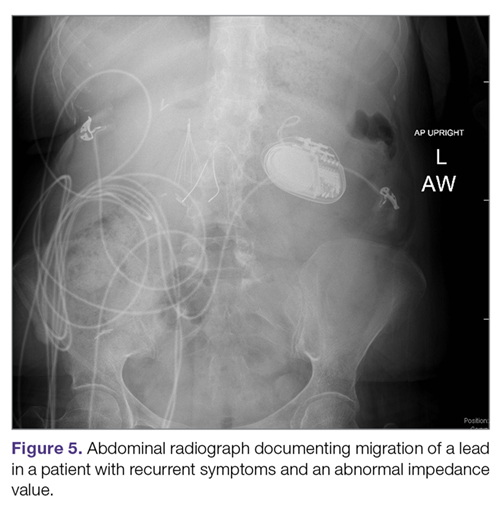
Lack of effect/persistent symptoms. If a patient presents with lack of improvement after device implantation, a thorough workup should be undertaken to ensure that the device is functioning properly. In the case of abnormal impedance values, an abdominal x-ray study can be performed to rule out lead migration (Figure 5). If no abnormalities are detected, the output of the device can be increased. After adjusting device settings, the patient should be assessed for improvement over at least a 1- to 3-month period. One report suggests that in patients not responding to GES, repositioning the location of the stimulator leads on the stomach can be helpful.29
Outcomes of GES
Study results of investigative GES models in animals and select patients were published in 1997.30,31 Following these reports, 2 large multicenter studies were conducted to demonstrate the efficacy of GES for the treatment of refractory gastroparesis. The Gastric Electrical Mechanical Stimulation Study (GEMS) was an open-label, multicenter study of 38 patients who received percutaneous and later permanent GES devices.32 Marked reduction in weekly vomiting and nausea was observed at 4 weeks, with a 90% reduction in nausea and vomiting frequency at 11 months. Following this, a second multicenter study (Worldwide Anti-Vomiting Electrical Stimulation Study [WAVES]) involving a double-blind sham stimulation controlled trial with 33 idiopathic and diabetic gastroparesis patients was performed.33 During the blinded portion of this study, there was a noticeable decrease in vomiting frequency, particularly in the patients with diabetic gastroparesis. Patient preference was for the stimulator ON as compared to OFF. The FDA’s HUD exemption for the Enterra GES device in 2000 was based on these studies.
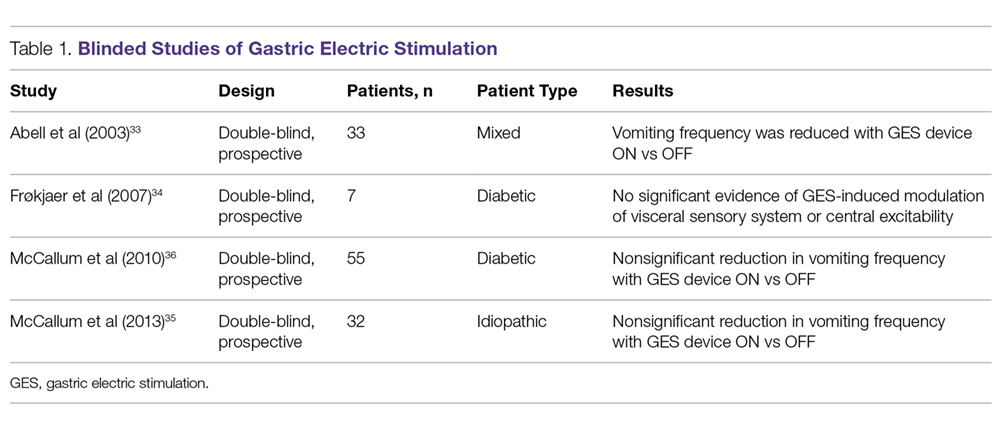
Four independent double-blind studies of GES have been conducted (Table 1).33-37 It has been difficult to demonstrate improvement during the double-blind period with gastric stimulation compared to no stimulation. Despite total symptom severity improvement and individual symptom improvements in these studies, a recent meta-analysis demonstrated a summative insignificant difference between the GES ON versus OFF states.38
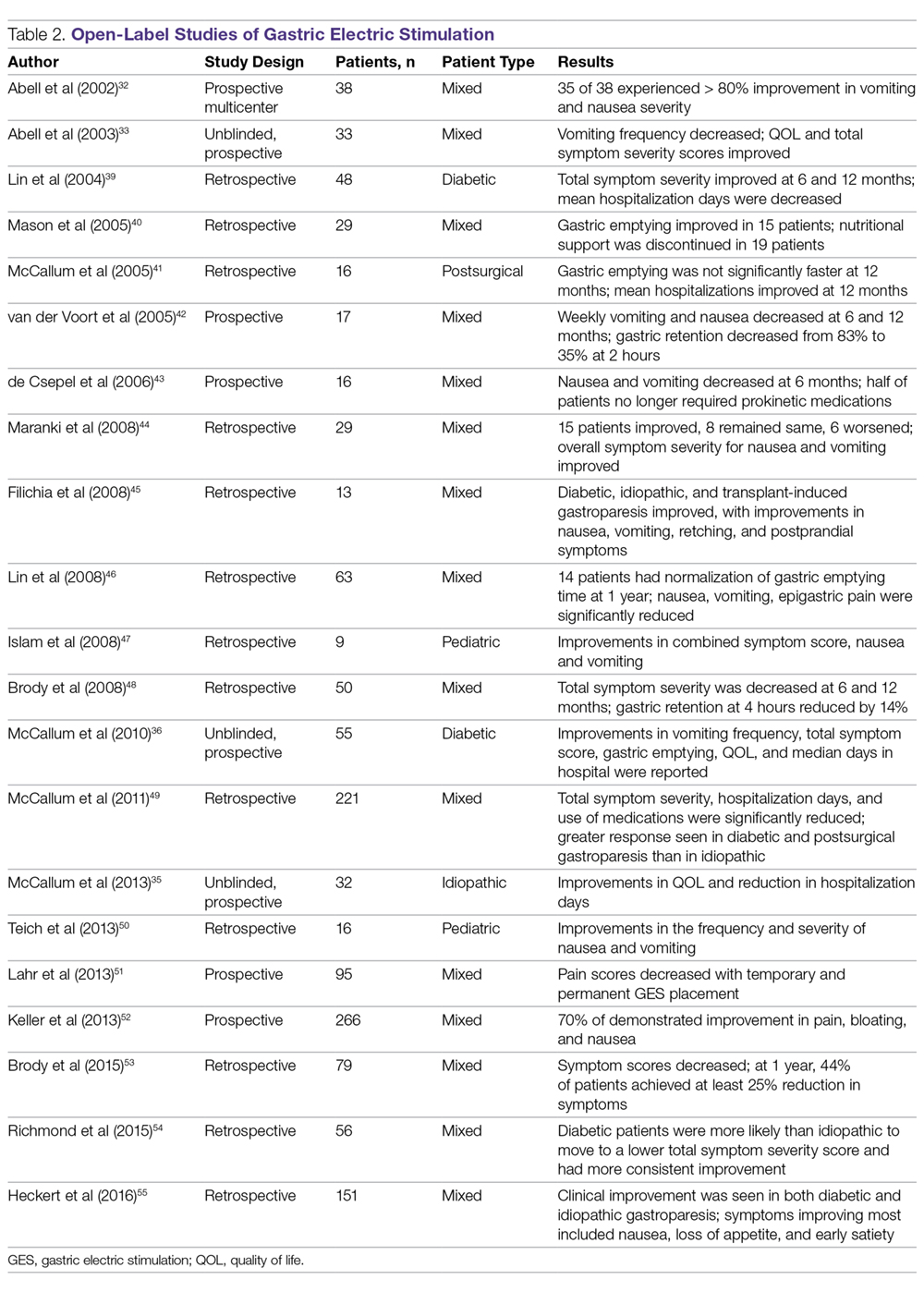
In contrast to the double-blind studies, numerous open-label studies have demonstrated clinical improvements in patients with diabetic and idiopathic gastroparesis (Table 2),32,33,35,36,39-55 leading some to question whether the demonstrable efficacy reflects a placebo effect or regression to the mean. Patients may perceive an operative, aggressive intervention as likely to be effectual in comparison to incremental medication efforts, thus creating a placebo effect. It should also be noted that not all open-label studies have demonstrated improvement with GES. Indeed, Jones et al reported no significant difference in nausea and vomiting at 6-month follow-up, and recommended that physicians exercise caution with GES as a therapeutic strategy given the cost and lack of confirmed demonstrable effect.56 Thus, the clinical successes demonstrated in open-label studies must be weighed not only against the lack of unequivocal improvement, but also against the potential deleterious effects of the surgery.
In an open-label study that employed the GCSI to follow symptoms of gastroparesis, 29 patients underwent GES implantation over an 18-month period, with follow-up in 28 patients.44 GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. The overall GCSI significantly decreased, with improvement in the nausea/vomiting subscore and the post-prandial fullness subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for patients with diabetic versus idiopathic gastroparesis. Patients with the main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not. In this study, 3 clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these 3 factors may allow improved patient selection for GES.
A large prospective study by Heckert et al detailed marked improvements with GES and the patterns of those improvements.55 Nausea, vomiting, loss of appetite, and early satiety improved significantly with stimulator use, with a greater improvement in vomiting in patients with diabetic gastroparesis than in those with the idiopathic form. Although GES improved symptoms in 75% of all patients, patients with diabetes had a post-GES Clinical Patient Grading Assessment score that was statistically higher than the score among patients with idiopathic gastroparesis. This difference is thought to be due to the neuromolecular mechanism of diabetic gastroparesis, where blunting of the enteric nervous system may contribute to symptomatology.
Several studies have demonstrated a clinical response to GES in patients with postsurgical gastroparesis. A study by Oubre et al showed that GES led to weekly vomiting improvements as well as a reduction in total symptom severity score.57 A study by McCallum et al further demonstrated improved symptoms, quality of life, nutritional status, and hospitalization requirements.58 GES has also been shown to improve gastroparesis symptoms in pediatric populations.47,59 Thus, although not a direct indication, GES has been shown to be beneficial in various subtypes of gastroparesis.
Additionally, irrespective of gastroparesis type, the improved symptomatology with GES appears to be durable, with one study showing persistent clinical improvements up to 8 years after device placement.60 The improvements were persistent and incremental. Likewise, McCallum et al showed that continued reductions in total symptom severity scores were evident in all gastroparesis types up to 10 years after stimulator implantation.61 The success of the procedures in part comes from careful selection of patients. Clinical parameters that are associated with favorable clinical response include diabetic gastroparesis subtype, nausea/vomiting predominance, and independence from narcotic analgesics prior to stimulator placement.62
GES has also been noted to improve other patient care metrics besides symptomatology, including nutritional status, reduced need for nutritional supplementation, and improved HbA1c.63-65 Additionally, a study by Cutts et al established that health care resource utilization significantly improved at 12, 24, and 36 months following GES placement, as compared to patients receiving standard medical therapy.66 This decreased resource utilization was also reflected in decreased costs in the GES group compared with the standard care group.
Surgical Alternatives to GES
Pyloric interventions such as pyloroplasty and pyloromyotomy are other surgical treatment modalities offered for gastroparesis. Whereas GES uses neurostimulation to facilitate gastric emptying and potentially improve fundic accommodation, pyloric interventions are intended to increase gastric emptying by reducing outflow resistance from the pyloric sphincter.
Pyloric Interventions
Various studies have shown significant improvements with pyloric interventions, similar to the improvements seen with GES. One such study involving 177 patients demonstrated an 86% improvement in gastric emptying, with symptom severity scores for nausea, vomiting, bloating, abdominal pain, and early satiety decreasing significantly at 3 months following pyloroplasty.67 A significant advantage of pyloric interventions is that pyloromyotomy can be performed endoscopically (gastric peroral endoscopic pyloromyotomy [G-POEM] or peroral pyloromyotomy [POP]), thus minimizing the risks of open surgery. A recent review that included a pooled analysis of 7 studies of G-POEM for gastroparesis demonstrated 100% technical success, with clinical efficacy in 81.5% of the procedures as assessed by the GCSI.68 Additionally, the intraoperative and perioperative complication rates were 6.6% and 7.6%, respectively, suggesting that G-POEM is a safe and clinically beneficial therapeutic option. Few studies comparing the outcomes of pyloric interventions to GES have been performed.
Recently, GES has been combined with pyloric interventions to maximize therapeutic potential. This allows simultaneous neurologic and functional interventions to expedite gastric emptying and improve patient symptomatology. Davis et al demonstrated significant improvement in 21 patients who underwent GES placement and pyloroplasty, with 71% improvement in total symptom severity.69 Notably, dual surgery did not increase the incidence of infection or adverse surgical outcomes. Although this study did not directly compare dual surgery to GES alone, the results are nonetheless favorable. GES provides a strong antiemetic and anti-nausea effect, whereas the pyloromyotomy provides improvement in gastric emptying.
Feeding/Venting Tubes
Feeding jejunostomy tubes and venting gastrostomy tubes can be used alone or in combination with GES. Feeding jejunostomy is performed for malnutrition and weight loss that accompanies the refractory symptoms of early satiety, nausea, and vomiting. Venting gastrostomy tubes allow for removal of retained gastric contents that may cause distension, nausea, and vomiting. Gastrojejunostomy tubes can also be placed endoscopically or by interventional radiology.
Gastrectomy
Gastrectomy can provide therapeutic benefit through elimination of the gastric reservoir function and consequent removal of afferent neural impulses. In select patient populations, outcomes of gastrectomy have compared favorably with those of GES. For example, one study demonstrated favorable outcomes of Roux-en-Y gastrectomy in morbidly obese patients with gastroparesis.70 In another study, favorable outcomes were reported in a cohort of 103 patients, with gastrectomy demonstrating 87% symptom improvement (nausea, vomiting, epigastric pain) compared to just 63% improvement with GES.71 However, the dramatic impact on anatomy and physiology and the invasiveness of the procedure need to be weighed against the therapeutic benefit. For example, in the same study, the 30-day morbidity was 23% for gastrectomy versus just 8% for the GES implant.71
When to Use GES
The gastric electrical neurostimulator (Enterra; Medtronic, Inc.) is approved for treatment of idiopathic and diabetic gastroparesis that is refractory to medical treatment, performed on a compassionate basis. Patients with diabetic gastroparesis respond to GES better than do patients with the idiopathic form. Of the symptoms of gastroparesis, primarily nausea and vomiting improve. Thus, GES favors patients with diabetic gastroparesis who have primarily nausea and vomiting, rather than, for instance, patients with idiopathic gastroparesis who have primarily abdominal pain and may be taking narcotics. Some centers provide GES for postsurgical patients and children with gastroparesis.
The 3 main surgical interventions for medically refractory gastroparesis are GES, pyloric intervention (pyloroplasty or pyloromyotomy), and gastrectomy. Of the 3 interventions, gastrectomy is the most radical given its dramatic effect on anatomy and is thus not preferred. The clinical decision then becomes: GES, pyloric intervention, or both? There are limited data to support a definitive answer to this question.
In a single-center retrospective analysis of prospective data (electronic medical record), Arthur et al compared outcomes of GES patients with medically refractory gastroparesis who received various surgical interventions.72 In total, 33 stimulator, 7 pyloroplasty, 2 gastrectomy, and 16 combined stimulator and pyloroplasty patients were analyzed for postoperative symptom improvement. Pyloroplasty alone demonstrated the least symptom improvement, combination GES and pyloroplasty demonstrated increased improvement, and GES alone demonstrated the most improvement. The results of this study suggest that barring contraindication, placement of a gastric stimulator as the initial treatment is best, with pyloroplasty reserved for patients who do not achieve adequate symptom control. Limitations of the study include its single-center design and low patient numbers for pyloroplasty in isolation.
In contrast, a recent retrospective systematic review synthesized the outcomes of various studies of GES and pyloric interventions for medically refractory gastroparesis.73 A therapeutic effect was found for each surgical intervention, with pyloric surgery patients demonstrating a greater response to intervention than GES patients. Unfortunately, attempts to analyze combination interventions were hindered by a lack of power.
Conclusion
Initial management of gastroparesis is medical (lifestyle and diet changes), with antiemetic and prokinetic agents used in refractory cases. Following failure of this therapy, placement of a GES device is a surgical intervention that has been approved under FDA humanitarian device exemption to help ameliorate symptomatology. Improvement with GES has been demonstrated in nonblinded studies, but the lack of randomized controlled trials demonstrating benefit suggests the possibility of an underlying placebo effect. Additionally, new medical procedures such as G-POEM complicate the decision of which intervention should be attempted first. More studies, specifically comparing GES, pyloric interventions, and combined GES with pyloric intervention to placebo, are needed to fully understand what therapy is best for refractory gastroparesis.
Corresponding author: Henry P. Parkman, MD, Gastroenterology Section, Temple University School of Medicine, 3401 North Broad Street, Philadelphia, PA 19140; [email protected].
Financial disclosures: None.
1. Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
2. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig Dis Sci. 2018;63:2231-2243.
3. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
4. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101-115.
5. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501-1504.
6. Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32:318-322.
7. Meeroff JC, Schreiber DS, Trier JS, Blacklow NR. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370-373.
8. Paliwal M, Prasanna KS, Saraswat VA, et al. Varicella zoster cranial polyneuropathy presenting with dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17:192-194.
9. Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751-754.
10. Kockar MC, Kayahan IK, Bavbek N. Diabetic gastroparesis in association with autonomic neuropathy and microvasculopathy. Acta Med Okayama. 2002;56:237-243.
11. Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233.
12. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34-42.
13. Wang YR, Fisher RS. Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322.
14. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113-133.
15. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
16. Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676-681.
17. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427-438.
18. Jehangir A, Abdallah RT, Parkman HP. Characterizing abdominal pain in patients with gastroparesis into neuropathic and nociceptive components. J Clin Gastroenterol. 2018 May 18. doi: 10.1097/MCG.0000000000001059.
19. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44:1-7.
20. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol. 2018;14:140-145.
21. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972-1978.
22. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670-680.
23. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463.
24. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9-19.
25. Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681-694.
26. Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29-39
27. Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the Manufacturer and User Device Experience (MAUDE) Registry. Auton Neurosci. 2017;202:40-44
28. Liu RC, Sabnis AA, Chand B. Erosion of gastric electrical stimulator electrodes: evaluation, management, and laparoscopic techniques. Surg Laparosc Endosc Percutan Tech. 2007;17:438-441.
29. Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244-249.
30. Familoni BO, Abell TL, Nemoto D, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885-891.
31. Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892-897.
32. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204-212.
33. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428.
34. Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43:1066-1075.
35. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-836.
36. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947-954.
37. Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496-503.
38. Levinthal DJ. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci. 2017;202:45-55.
39. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
40. Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841-846.
41. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
42. van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38-42.
43. de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis. gastric motility restored. Surg Endosc. 2006;20:302-306.
44. Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072-2078.
45. Filichia LA, Cendan CJ. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90-93.
46. Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464-470.
47. Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437-442.
48. Brody F, Vaziri K, Saddler A, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533-538.
49. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
50. Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183.
51. Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457-464.
52. Keller DS, Parkman HP, Boucek DO, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620-626.
53. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
54. Richmond B, Chong B, Modak A, et al. Gastric electrical stimulation for refractory gastroparesis: Predictors of response and redefining a successful outcome. Am Surg. 2015;81:467-471.
55. Heckert J, Sankineni A, Hughes WB, et al. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. 2016;61:168-175.
56. Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578.
57. Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: Long-term outcome. South Med J. 2005;98:693-697.
58. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
59. Islam S, McLaughlin J, Pierson J, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71.
60. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
61. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
62. Maranki J, Lytes V, Meilahn JE, et al. Dig Dis Sci. 2008 53:2072-2078.
63. Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277-281.
64. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
65. Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328-1334.
66. Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35-43.
67. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326-1332.
68. Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis. J Gastroenterol Hepatol. 2018;33:1829-1833.
69. Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222-227.
70. Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683-2689.
71. Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61-66.
72. Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc. 2017;32:977-982.
73. Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J Surg Res. 2018;231:263-269.
From Temple University School of Medicine, Philadelphia, PA.
Abstract
- Objective: To outline the use and utility of gastric electric stimulation (GES) as a therapeutic intervention for gastroparesis.
- Methods: Review of the literature.
- Results: Gastroparesis is characterized by delayed gastric emptying, with symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Some patients with gastroparesis do not respond to medical intervention, and for these patients surgical intervention may be warranted. GES utilizes high-frequency gastric neurostimulation to facilitate gastric emptying and reduce symptoms of gastroparesis. It is indicated for patients with idiopathic and diabetic gastroparesis who have nausea and vomiting as their primary symptoms and who have not responded to medical therapy. GES has also been used in postsurgical and pediatric gastroparesis patients. Optimizing the outcome of this surgical treatment through proper patient selection and meticulous surgical technique is essential as there are inherent risks to the procedure. Nonblinded studies of GES for medically refractory gastroparesis have demonstrated therapeutic symptomatic benefit, whereas randomized controlled trials have not. New interventions such as pyloromyotomy and pyloroplasty are reasonable alternatives or addendums to GES.
- Conclusion: GES may be considered among the therapies available for treating patients with refractory symptoms of gastroparesis. More studies, specifically those comparing GES, pyloromyotomy, GES combined with pyloromyotomy, and placebo, are needed to help guide therapy selection for refractory gastroparesis.
Keywords: diabetes; gastroparesis; dysmotility; gastric emptying; electric stimulation.
Gastroparesis is a chronic dysmotility disorder characterized by delayed gastric emptying with associated symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Medical treatments for gastroparesis include dietary modifications, glucose control in those with diabetes, prokinetic medications, antiemetic medications, and symptom modulators, but unfortunately patients frequently do not respond to these treatments. In patients refractory to medical therapy, surgical treatments can be considered.
Gastric electric stimulation (GES; Enterra [Medtronic, Minneapolis, MN]) was approved via a Food and Drug Administration (FDA) Humanitarian Use Device (HUD) exemption for the treatment of medically refractory gastroparesis in 2000. Understanding the indications, risks, outcomes, and alternatives to GES is essential to providing appropriate care for patients with medically refractory gastroparesis. This article outlines the use and utility of GES as a therapeutic intervention for gastroparesis.
Types of Gastroparesis
Gastroparesis is a chronic symptomatic disorder of the stomach manifested by delayed gastric emptying without evidence of gastric outlet obstruction or ulceration.1 The pathophysiology of gastroparesis appears to involve abnormalities in functioning of several elements including the autonomic nervous system, especially the vagus nerve, smooth muscle cells, enteric neurons, and interstitial cells of Cajal.
Idiopathic gastroparesis and diabetic gastroparesis are the 2 most common types of gastroparesis.2 Symptomatic delayed gastric emptying with no primary underlying abnormality predisposing to gastroparesis is categorized as idiopathic gastroparesis.3 A small subset of patients with idiopathic gastroparesis report an initial infectious prodrome such as gastroenteritis or respiratory infection. It has been suggested that this postinfectious gastroparesis results from viral injury to the neural innervation of the stomach or the interstitial cells of Cajal in the stomach.4 Viruses that have been implicated in the development of gastroparesis include cytomegalovirus, Epstein-Barr virus, Norwalk virus, rotavirus, herpes zoster, and varicella zoster.5-9
Diabetic gastroparesis is characterized as onset of symptoms of gastroparesis in patients with diabetes, with concomitant delayed gastric emptying. It is often attributed to chronic hyperglycemia-induced damage to the vagus nerve, and is frequently observed in association with other diabetic complications such as neuropathy, retinopathy, and nephropathy.10
Gastroparesis that develops following surgery is classified as postsurgical gastroparesis. In the past, this form of gastroparesis most commonly occurred after ulcer surgery, often performed with vagotomy. These types of surgeries are performed less frequently in the era of proton pump inhibitor therapy and treatments for Helicobacter pylori. Presently, Nissen fundoplication and bariatric surgery are the more common surgical procedures associated with gastroparesis.3 Long-term use of medications that delay gastric emptying, such as opiate narcotic medications, can lead to gastroparesis and represent another form of iatrogenic gastroparesis. Other forms of gastroparesis (atypical gastroparesis) arise due to various underlying etiologies, including neurological disorders (eg, Parkinson disease, multiple sclerosis), metabolic or endocrine conditions (eg, hypothyroidism), autoimmune disorders, connective tissue and collagen vascular disorders (eg, systemic lupus erythematosus, scleroderma, Sjögren syndrome, Ehlers-Danlos syndrome), or eating disorders (eg, anorexia, bulimia).3
Epidemiology
There is a female preponderance in patients with gastroparesis. Data from the Rochester Epidemiology Project, a database of linked medical records for residents of Olmsted County, MN, showed that the age-adjusted prevalence of definite gastroparesis per 100,000 inhabitants was 37.8 for women and 9.6 for men.11 More recent estimates have suggested a much higher prevalence of probable gastroparesis (approximately 1.8%) in the general population using symptoms suggestive of gastroparesis.12 Hospitalization rates for gastroparesis have increased since 2000, which could reflect rising prevalence and/or the effects of heightened awareness about and better identification of gastroparesis.13 This increase may also be due in part to the rising rate of diabetes leading to more cases of diabetic gastroparesis; withdrawal of some gastroparesis treatments from the market (cisapride, tegaserod) leading to hospitalizations for symptoms not adequately being treated; and hospitalizations needed for insertion of the gastric electric stimulator.
Gastroparesis Symptoms
The main symptoms of gastroparesis are early satiety, postprandial fullness, bloating, nausea, and vomiting.14 Nausea (> 90% of patients) and early satiety (60% of patients) are the most common symptoms.15 Abdominal pain is often present in patients with gastroparesis but is usually not the predominant symptom. The pain can be multifactorial, with somatic, visceral, and neuropathic components.16-18 Moderate to severe abdominal pain has been found more often in patients with idiopathic gastroparesis and in association with opiate use.16 Symptoms of gastroparesis may be persistent or present as episodic flares. Due to the symptoms, some patients will experience weight loss and malnutrition and, in severe cases, dehydration.19
Although the definition of gastroparesis is a delay in gastric emptying along with symptoms, symptoms correlate poorly with the degree of delayed gastric emptying. The symptoms that appear to have the strongest correlation with gastric emptying are nausea, vomiting, early satiety, and postprandial fullness, whereas symptoms such as abdominal pain and bloating have little correlation. Furthermore, improving gastric emptying does not necessarily lead to improved symptoms, and symptom improvement does not always lead to improved gastric emptying times.20 Between 5% and 12% of patients with diabetes report symptoms consistent with gastroparesis, though many of these patients have normal gastric emptying. The symptoms of gastroparesis overlap with those of functional dyspepsia, as both may have motor and sensory alterations.21
The Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM), is a questionnaire that is commonly used to establish symptom severity in patients with gastroparesis. It is comprised of 3 subscales—nausea and vomiting, postprandial fullness and early satiety, and bloating—which are averaged to provide a total GCSI score. Symptoms over the 2 weeks prior to administration of the questionnaire are assessed and rated from 0 (none) to 5 (very severe).22 Grading the severity of gastroparesis may take into account symptoms, quality of life, and gastric emptying. One commonly used grading system assigns a grade from 1 to 3, with grade 1 being mild gastroparesis, grade 2 being compensated gastroparesis, and grade 3 being gastric failure with refractory symptoms that are uncontrolled.18,23 Quality-of-life surveys also suggest that gastroparesis independent of other factors leads to a worse quality of life.24
Indications for GES
Gastric electric stimulator implantation is a surgical procedure with inherent risks and complications and is reserved for patients with intractable symptoms of gastroparesis who remain symptomatic despite treatment attempts with dietary management, antiemetic agents (eg, compazine, phenergan, and ondansetron), and prokinetic agents (eg, metoclopramide, erythromycin, and domperidone). Symptom modulators such as nortriptyline and mirtazapine are occasionally tried.
Surgical intervention can be considered upon failure of medical treatment measures. At least a year of documented care provided by a physician specializing in gastroparesis is suggested for surgical consideration. The gastric electric neurostimulator is approved by the FDA as a HUD for the care of patients with idiopathic and diabetic gastroparesis, performed on a compassionate basis. GES implantation requires Institutional Review Board approval at the institution, and patients are required to have documented delayed gastric emptying.
It is important to remember that the GES device is incompatible with magnetic resonance imaging (MRI) and explantation of the device is necessary prior to MRI. As such, in patients with anticipated need of frequent MRI, such as those with multiple sclerosis, serious consideration should be given to alternative strategies prior to focusing on this modality.
Device Placement
GES was devised to improve gastric emptying. The Enterra GES system uses high-frequency, low-energy electric stimulation. An alternative method is true gastric pacing that uses high-energy, low-frequency stimulation to entrain the gastric slow waves and subsequent contractions at 3 cycles per minute (cpm). Gastric pacing has greater energy requirements than GES, which makes the size of the stimulator too large to be practical. In pilot animal studies, GES produced an accelerating effect on gastric emptying, but in human studies GES had an inconsistent effect on gastric emptying. Studies have suggested that GES influences the proximal stomach, with a reduction of gastric tone,25 and also that GES has an afferent modulatory mechanism.26
The Enterra GES is placed surgically under general anesthesia, commonly via laparotomy or minimal access surgical techniques (laparoscopically or robotically assisted). Preoperative intravenous antibiotics are given. The system consists of a pair of electrodes connected to a pulse generator. The 2 stimulation leads are inserted into the gastric muscularis propria 1 cm apart along the greater curvature 10 cm proximal to the pylorus. Upper endoscopy is performed to ensure that the leads do not penetrate through the mucosa into the stomach lumen; if this occurs, repositioning of the lead is necessary. A horizontal incision through the skin is made, and the distal ends of the stimulating wires are tunneled through the abdominal wall and connected to the pulse generator. The impedance (resistance) between the wires is measured to ensure the appropriate range (200-800 Ohms). The neurostimulator with the distal ends of the stimulating wires is then placed into the subcutaneous pocket and sutured to the underlying fascia. The pulse generator delivers a high-frequency, low-energy, 0.1-second train of pulses at a frequency of 12 cpm. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second. The voltage of the stimulations is set to provide a current of 5 milliamps (mA; remembering that voltage = current × resistance).
Patients are often hospitalized with a recovery time of 1 to 3 days. Immediate postoperative care usually includes intravenous fluids, controlling any postoperative ileus, advancing diet, and providing analgesic pain medications. Hospital length of stay can be impacted by surgical technique.25 Patients are seen several weeks after discharge for assessment of the incision and toleration of diet. Medications for gastroparesis that patients were taking prior to the GES implantation are usually continued postoperatively, with a goal of reducing these medications over time. Patients are then followed every 3 to 12 months, depending on their clinical condition.
At follow-up visits, medications are reviewed and new treatments can be added if appropriate. The gastric stimulator is interrogated to determine if changes in resistance occurred; if necessary, minor readjustments can be made to keep the current at desired levels (5 mA). For persistent symptoms with GES treatment, the stimulator parameters can be adjusted after 3 months of follow up, typically first increasing the current from 5 to 7.5 mA and then to 10 mA. After this, the frequency can be increased from 14 Hz to 28 Hz, and then to 55 Hz. Rarely, the ON duration is increased from 0.1 to 1 second. Increasing the ON time can worsen symptoms in some patients, cause abdominal pain, and decrease the battery life from the usual 7 years.
Complications of GES
In an analysis of the Manufacturer and User Facility Device Experience (MAUDE) databank, Bielefeldt identified 1587 reports of adverse effects related to the gastric electric stimulator from January 2001 to October 2015.27 The most common adverse effects are reviewed here.
Skin erosion/wound dehiscence is one of the most common reported complications; it may be related to superficial placement or inadequate securing of the device to the fascia. Abscess can develop postoperatively due to hematogenous seeding or may be a sign of lead erosion into the lumen, tracking along the leads into subcutaneous tissue.28 It is important to warn patients to protect the area over the device from needle injections as this also can lead to hematoma formation and direct contamination of the device. If the device gets infected, it cannot be salvaged and requires explantation. Implantation of a new device can be attempted once all wound issues resolve.
Device migration/flipping most often occurs because the device is inadequately fixed to the underlying fascia, but occasionally it can occur from patients flipping the device around. Flipping can occur due to superficial pocket location within subcutaneous tissue, especially in obese patients. Migration/flipping can lead to prominence of the contour of the device and discomfort, ultimately requiring surgical correction.
Perforation and erosion of the leads. With time, leads can erode into the stomach, although this is rare. Usually erosion is associated with loss of device function. Endoscopy confirms this finding. In rare cases, infection can track proximally along the lead and present as a surgical site infection at the pulse generator. This complication often requires explantation of the neurostimulator leads and pulse generator.
Intestinal obstruction. Although rare, the intestines can get wrapped around the leads of the device, causing different degrees of obstruction (Figure 1). Positioning the device in the left upper quadrant minimizes the intraabdominal length of the leads and pulls them maximally out, coiling under the device (Figure 2). In cases where other locations are used either due to a hostile upper abdominal region (skin infection, presence of gastrostomy or other devices) or surgeon’s preference, the GES device can be implanted in the lower abdomen (Figure 3). In these circumstances, carefully draping the omentum over the bowels might help to prevent this complication. Tacking of the leads to the parietal peritoneum with sutures can also be preventative. In cases of obstruction requiring intervention by laparotomy or minimal access techniques (laparoscopy or robotic assisted surgery), all efforts are made to preserve the neurostimulator leads. In cases that require bowel resection, lead contamination is a serious concern, but lead explantation is not mandatory. Close postoperative monitoring for the development of lead infection is required.
Hematoma and seroma. Postoperative hematomas can occur from inadequate hemostasis, and seromas can occur in the stimulator pocket. Small hematomas may be observed if not complicated (Figure 4). In cases of large hematomas with skin compromise or dehiscence, prompt washout and drainage is required. In ideal cases, the device can be preserved. Relocation to another site might be required if skin necrosis develops. The possibility of device contamination also must be considered; after resolution of wound issues, implantation of a new device may be tried. Seromas at the generator pocket site are a frequent occurrence but are often benign, self-limiting, and generally resolve over 4 to 6 weeks.
Incisional hernia. Hernias can develop after any abdominal surgery and are not unique to GES implantation. Use of minimally invasive technique for the GES implantation minimizes this complication.
Electric shock sensations may occur from breakage of the plastic lining covering the stimulator wires or from fluid buildup around the insertion of the wires into the stimulator. Shocks can also occur due to shortening of the leads on the muscles of the abdominal wall. Patients describe periodic muscle cramps with the frequency of the device (every 5 seconds). To prevent this complication, freshly implanted leads should be covered by an omental flap to isolate them from the abdominal wall. In patients who continue to feel shocks despite all efforts, the possibility of visceral hypersensitivity should be considered. A trial of symptom modulators such as nortriptyline and lowering of the output amperage below the minimal recommended setting of 5 mA can be undertaken. If these interventions do not work, the device must be turned off for a period of time. Occasionally, replacement of the leads or explantation of the device must be considered.
Lack of effect/persistent symptoms. If a patient presents with lack of improvement after device implantation, a thorough workup should be undertaken to ensure that the device is functioning properly. In the case of abnormal impedance values, an abdominal x-ray study can be performed to rule out lead migration (Figure 5). If no abnormalities are detected, the output of the device can be increased. After adjusting device settings, the patient should be assessed for improvement over at least a 1- to 3-month period. One report suggests that in patients not responding to GES, repositioning the location of the stimulator leads on the stomach can be helpful.29
Outcomes of GES
Study results of investigative GES models in animals and select patients were published in 1997.30,31 Following these reports, 2 large multicenter studies were conducted to demonstrate the efficacy of GES for the treatment of refractory gastroparesis. The Gastric Electrical Mechanical Stimulation Study (GEMS) was an open-label, multicenter study of 38 patients who received percutaneous and later permanent GES devices.32 Marked reduction in weekly vomiting and nausea was observed at 4 weeks, with a 90% reduction in nausea and vomiting frequency at 11 months. Following this, a second multicenter study (Worldwide Anti-Vomiting Electrical Stimulation Study [WAVES]) involving a double-blind sham stimulation controlled trial with 33 idiopathic and diabetic gastroparesis patients was performed.33 During the blinded portion of this study, there was a noticeable decrease in vomiting frequency, particularly in the patients with diabetic gastroparesis. Patient preference was for the stimulator ON as compared to OFF. The FDA’s HUD exemption for the Enterra GES device in 2000 was based on these studies.
Four independent double-blind studies of GES have been conducted (Table 1).33-37 It has been difficult to demonstrate improvement during the double-blind period with gastric stimulation compared to no stimulation. Despite total symptom severity improvement and individual symptom improvements in these studies, a recent meta-analysis demonstrated a summative insignificant difference between the GES ON versus OFF states.38
In contrast to the double-blind studies, numerous open-label studies have demonstrated clinical improvements in patients with diabetic and idiopathic gastroparesis (Table 2),32,33,35,36,39-55 leading some to question whether the demonstrable efficacy reflects a placebo effect or regression to the mean. Patients may perceive an operative, aggressive intervention as likely to be effectual in comparison to incremental medication efforts, thus creating a placebo effect. It should also be noted that not all open-label studies have demonstrated improvement with GES. Indeed, Jones et al reported no significant difference in nausea and vomiting at 6-month follow-up, and recommended that physicians exercise caution with GES as a therapeutic strategy given the cost and lack of confirmed demonstrable effect.56 Thus, the clinical successes demonstrated in open-label studies must be weighed not only against the lack of unequivocal improvement, but also against the potential deleterious effects of the surgery.
In an open-label study that employed the GCSI to follow symptoms of gastroparesis, 29 patients underwent GES implantation over an 18-month period, with follow-up in 28 patients.44 GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. The overall GCSI significantly decreased, with improvement in the nausea/vomiting subscore and the post-prandial fullness subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for patients with diabetic versus idiopathic gastroparesis. Patients with the main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not. In this study, 3 clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these 3 factors may allow improved patient selection for GES.
A large prospective study by Heckert et al detailed marked improvements with GES and the patterns of those improvements.55 Nausea, vomiting, loss of appetite, and early satiety improved significantly with stimulator use, with a greater improvement in vomiting in patients with diabetic gastroparesis than in those with the idiopathic form. Although GES improved symptoms in 75% of all patients, patients with diabetes had a post-GES Clinical Patient Grading Assessment score that was statistically higher than the score among patients with idiopathic gastroparesis. This difference is thought to be due to the neuromolecular mechanism of diabetic gastroparesis, where blunting of the enteric nervous system may contribute to symptomatology.
Several studies have demonstrated a clinical response to GES in patients with postsurgical gastroparesis. A study by Oubre et al showed that GES led to weekly vomiting improvements as well as a reduction in total symptom severity score.57 A study by McCallum et al further demonstrated improved symptoms, quality of life, nutritional status, and hospitalization requirements.58 GES has also been shown to improve gastroparesis symptoms in pediatric populations.47,59 Thus, although not a direct indication, GES has been shown to be beneficial in various subtypes of gastroparesis.
Additionally, irrespective of gastroparesis type, the improved symptomatology with GES appears to be durable, with one study showing persistent clinical improvements up to 8 years after device placement.60 The improvements were persistent and incremental. Likewise, McCallum et al showed that continued reductions in total symptom severity scores were evident in all gastroparesis types up to 10 years after stimulator implantation.61 The success of the procedures in part comes from careful selection of patients. Clinical parameters that are associated with favorable clinical response include diabetic gastroparesis subtype, nausea/vomiting predominance, and independence from narcotic analgesics prior to stimulator placement.62
GES has also been noted to improve other patient care metrics besides symptomatology, including nutritional status, reduced need for nutritional supplementation, and improved HbA1c.63-65 Additionally, a study by Cutts et al established that health care resource utilization significantly improved at 12, 24, and 36 months following GES placement, as compared to patients receiving standard medical therapy.66 This decreased resource utilization was also reflected in decreased costs in the GES group compared with the standard care group.
Surgical Alternatives to GES
Pyloric interventions such as pyloroplasty and pyloromyotomy are other surgical treatment modalities offered for gastroparesis. Whereas GES uses neurostimulation to facilitate gastric emptying and potentially improve fundic accommodation, pyloric interventions are intended to increase gastric emptying by reducing outflow resistance from the pyloric sphincter.
Pyloric Interventions
Various studies have shown significant improvements with pyloric interventions, similar to the improvements seen with GES. One such study involving 177 patients demonstrated an 86% improvement in gastric emptying, with symptom severity scores for nausea, vomiting, bloating, abdominal pain, and early satiety decreasing significantly at 3 months following pyloroplasty.67 A significant advantage of pyloric interventions is that pyloromyotomy can be performed endoscopically (gastric peroral endoscopic pyloromyotomy [G-POEM] or peroral pyloromyotomy [POP]), thus minimizing the risks of open surgery. A recent review that included a pooled analysis of 7 studies of G-POEM for gastroparesis demonstrated 100% technical success, with clinical efficacy in 81.5% of the procedures as assessed by the GCSI.68 Additionally, the intraoperative and perioperative complication rates were 6.6% and 7.6%, respectively, suggesting that G-POEM is a safe and clinically beneficial therapeutic option. Few studies comparing the outcomes of pyloric interventions to GES have been performed.
Recently, GES has been combined with pyloric interventions to maximize therapeutic potential. This allows simultaneous neurologic and functional interventions to expedite gastric emptying and improve patient symptomatology. Davis et al demonstrated significant improvement in 21 patients who underwent GES placement and pyloroplasty, with 71% improvement in total symptom severity.69 Notably, dual surgery did not increase the incidence of infection or adverse surgical outcomes. Although this study did not directly compare dual surgery to GES alone, the results are nonetheless favorable. GES provides a strong antiemetic and anti-nausea effect, whereas the pyloromyotomy provides improvement in gastric emptying.
Feeding/Venting Tubes
Feeding jejunostomy tubes and venting gastrostomy tubes can be used alone or in combination with GES. Feeding jejunostomy is performed for malnutrition and weight loss that accompanies the refractory symptoms of early satiety, nausea, and vomiting. Venting gastrostomy tubes allow for removal of retained gastric contents that may cause distension, nausea, and vomiting. Gastrojejunostomy tubes can also be placed endoscopically or by interventional radiology.
Gastrectomy
Gastrectomy can provide therapeutic benefit through elimination of the gastric reservoir function and consequent removal of afferent neural impulses. In select patient populations, outcomes of gastrectomy have compared favorably with those of GES. For example, one study demonstrated favorable outcomes of Roux-en-Y gastrectomy in morbidly obese patients with gastroparesis.70 In another study, favorable outcomes were reported in a cohort of 103 patients, with gastrectomy demonstrating 87% symptom improvement (nausea, vomiting, epigastric pain) compared to just 63% improvement with GES.71 However, the dramatic impact on anatomy and physiology and the invasiveness of the procedure need to be weighed against the therapeutic benefit. For example, in the same study, the 30-day morbidity was 23% for gastrectomy versus just 8% for the GES implant.71
When to Use GES
The gastric electrical neurostimulator (Enterra; Medtronic, Inc.) is approved for treatment of idiopathic and diabetic gastroparesis that is refractory to medical treatment, performed on a compassionate basis. Patients with diabetic gastroparesis respond to GES better than do patients with the idiopathic form. Of the symptoms of gastroparesis, primarily nausea and vomiting improve. Thus, GES favors patients with diabetic gastroparesis who have primarily nausea and vomiting, rather than, for instance, patients with idiopathic gastroparesis who have primarily abdominal pain and may be taking narcotics. Some centers provide GES for postsurgical patients and children with gastroparesis.
The 3 main surgical interventions for medically refractory gastroparesis are GES, pyloric intervention (pyloroplasty or pyloromyotomy), and gastrectomy. Of the 3 interventions, gastrectomy is the most radical given its dramatic effect on anatomy and is thus not preferred. The clinical decision then becomes: GES, pyloric intervention, or both? There are limited data to support a definitive answer to this question.
In a single-center retrospective analysis of prospective data (electronic medical record), Arthur et al compared outcomes of GES patients with medically refractory gastroparesis who received various surgical interventions.72 In total, 33 stimulator, 7 pyloroplasty, 2 gastrectomy, and 16 combined stimulator and pyloroplasty patients were analyzed for postoperative symptom improvement. Pyloroplasty alone demonstrated the least symptom improvement, combination GES and pyloroplasty demonstrated increased improvement, and GES alone demonstrated the most improvement. The results of this study suggest that barring contraindication, placement of a gastric stimulator as the initial treatment is best, with pyloroplasty reserved for patients who do not achieve adequate symptom control. Limitations of the study include its single-center design and low patient numbers for pyloroplasty in isolation.
In contrast, a recent retrospective systematic review synthesized the outcomes of various studies of GES and pyloric interventions for medically refractory gastroparesis.73 A therapeutic effect was found for each surgical intervention, with pyloric surgery patients demonstrating a greater response to intervention than GES patients. Unfortunately, attempts to analyze combination interventions were hindered by a lack of power.
Conclusion
Initial management of gastroparesis is medical (lifestyle and diet changes), with antiemetic and prokinetic agents used in refractory cases. Following failure of this therapy, placement of a GES device is a surgical intervention that has been approved under FDA humanitarian device exemption to help ameliorate symptomatology. Improvement with GES has been demonstrated in nonblinded studies, but the lack of randomized controlled trials demonstrating benefit suggests the possibility of an underlying placebo effect. Additionally, new medical procedures such as G-POEM complicate the decision of which intervention should be attempted first. More studies, specifically comparing GES, pyloric interventions, and combined GES with pyloric intervention to placebo, are needed to fully understand what therapy is best for refractory gastroparesis.
Corresponding author: Henry P. Parkman, MD, Gastroenterology Section, Temple University School of Medicine, 3401 North Broad Street, Philadelphia, PA 19140; [email protected].
Financial disclosures: None.
From Temple University School of Medicine, Philadelphia, PA.
Abstract
- Objective: To outline the use and utility of gastric electric stimulation (GES) as a therapeutic intervention for gastroparesis.
- Methods: Review of the literature.
- Results: Gastroparesis is characterized by delayed gastric emptying, with symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Some patients with gastroparesis do not respond to medical intervention, and for these patients surgical intervention may be warranted. GES utilizes high-frequency gastric neurostimulation to facilitate gastric emptying and reduce symptoms of gastroparesis. It is indicated for patients with idiopathic and diabetic gastroparesis who have nausea and vomiting as their primary symptoms and who have not responded to medical therapy. GES has also been used in postsurgical and pediatric gastroparesis patients. Optimizing the outcome of this surgical treatment through proper patient selection and meticulous surgical technique is essential as there are inherent risks to the procedure. Nonblinded studies of GES for medically refractory gastroparesis have demonstrated therapeutic symptomatic benefit, whereas randomized controlled trials have not. New interventions such as pyloromyotomy and pyloroplasty are reasonable alternatives or addendums to GES.
- Conclusion: GES may be considered among the therapies available for treating patients with refractory symptoms of gastroparesis. More studies, specifically those comparing GES, pyloromyotomy, GES combined with pyloromyotomy, and placebo, are needed to help guide therapy selection for refractory gastroparesis.
Keywords: diabetes; gastroparesis; dysmotility; gastric emptying; electric stimulation.
Gastroparesis is a chronic dysmotility disorder characterized by delayed gastric emptying with associated symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Medical treatments for gastroparesis include dietary modifications, glucose control in those with diabetes, prokinetic medications, antiemetic medications, and symptom modulators, but unfortunately patients frequently do not respond to these treatments. In patients refractory to medical therapy, surgical treatments can be considered.
Gastric electric stimulation (GES; Enterra [Medtronic, Minneapolis, MN]) was approved via a Food and Drug Administration (FDA) Humanitarian Use Device (HUD) exemption for the treatment of medically refractory gastroparesis in 2000. Understanding the indications, risks, outcomes, and alternatives to GES is essential to providing appropriate care for patients with medically refractory gastroparesis. This article outlines the use and utility of GES as a therapeutic intervention for gastroparesis.
Types of Gastroparesis
Gastroparesis is a chronic symptomatic disorder of the stomach manifested by delayed gastric emptying without evidence of gastric outlet obstruction or ulceration.1 The pathophysiology of gastroparesis appears to involve abnormalities in functioning of several elements including the autonomic nervous system, especially the vagus nerve, smooth muscle cells, enteric neurons, and interstitial cells of Cajal.
Idiopathic gastroparesis and diabetic gastroparesis are the 2 most common types of gastroparesis.2 Symptomatic delayed gastric emptying with no primary underlying abnormality predisposing to gastroparesis is categorized as idiopathic gastroparesis.3 A small subset of patients with idiopathic gastroparesis report an initial infectious prodrome such as gastroenteritis or respiratory infection. It has been suggested that this postinfectious gastroparesis results from viral injury to the neural innervation of the stomach or the interstitial cells of Cajal in the stomach.4 Viruses that have been implicated in the development of gastroparesis include cytomegalovirus, Epstein-Barr virus, Norwalk virus, rotavirus, herpes zoster, and varicella zoster.5-9
Diabetic gastroparesis is characterized as onset of symptoms of gastroparesis in patients with diabetes, with concomitant delayed gastric emptying. It is often attributed to chronic hyperglycemia-induced damage to the vagus nerve, and is frequently observed in association with other diabetic complications such as neuropathy, retinopathy, and nephropathy.10
Gastroparesis that develops following surgery is classified as postsurgical gastroparesis. In the past, this form of gastroparesis most commonly occurred after ulcer surgery, often performed with vagotomy. These types of surgeries are performed less frequently in the era of proton pump inhibitor therapy and treatments for Helicobacter pylori. Presently, Nissen fundoplication and bariatric surgery are the more common surgical procedures associated with gastroparesis.3 Long-term use of medications that delay gastric emptying, such as opiate narcotic medications, can lead to gastroparesis and represent another form of iatrogenic gastroparesis. Other forms of gastroparesis (atypical gastroparesis) arise due to various underlying etiologies, including neurological disorders (eg, Parkinson disease, multiple sclerosis), metabolic or endocrine conditions (eg, hypothyroidism), autoimmune disorders, connective tissue and collagen vascular disorders (eg, systemic lupus erythematosus, scleroderma, Sjögren syndrome, Ehlers-Danlos syndrome), or eating disorders (eg, anorexia, bulimia).3
Epidemiology
There is a female preponderance in patients with gastroparesis. Data from the Rochester Epidemiology Project, a database of linked medical records for residents of Olmsted County, MN, showed that the age-adjusted prevalence of definite gastroparesis per 100,000 inhabitants was 37.8 for women and 9.6 for men.11 More recent estimates have suggested a much higher prevalence of probable gastroparesis (approximately 1.8%) in the general population using symptoms suggestive of gastroparesis.12 Hospitalization rates for gastroparesis have increased since 2000, which could reflect rising prevalence and/or the effects of heightened awareness about and better identification of gastroparesis.13 This increase may also be due in part to the rising rate of diabetes leading to more cases of diabetic gastroparesis; withdrawal of some gastroparesis treatments from the market (cisapride, tegaserod) leading to hospitalizations for symptoms not adequately being treated; and hospitalizations needed for insertion of the gastric electric stimulator.
Gastroparesis Symptoms
The main symptoms of gastroparesis are early satiety, postprandial fullness, bloating, nausea, and vomiting.14 Nausea (> 90% of patients) and early satiety (60% of patients) are the most common symptoms.15 Abdominal pain is often present in patients with gastroparesis but is usually not the predominant symptom. The pain can be multifactorial, with somatic, visceral, and neuropathic components.16-18 Moderate to severe abdominal pain has been found more often in patients with idiopathic gastroparesis and in association with opiate use.16 Symptoms of gastroparesis may be persistent or present as episodic flares. Due to the symptoms, some patients will experience weight loss and malnutrition and, in severe cases, dehydration.19
Although the definition of gastroparesis is a delay in gastric emptying along with symptoms, symptoms correlate poorly with the degree of delayed gastric emptying. The symptoms that appear to have the strongest correlation with gastric emptying are nausea, vomiting, early satiety, and postprandial fullness, whereas symptoms such as abdominal pain and bloating have little correlation. Furthermore, improving gastric emptying does not necessarily lead to improved symptoms, and symptom improvement does not always lead to improved gastric emptying times.20 Between 5% and 12% of patients with diabetes report symptoms consistent with gastroparesis, though many of these patients have normal gastric emptying. The symptoms of gastroparesis overlap with those of functional dyspepsia, as both may have motor and sensory alterations.21
The Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM), is a questionnaire that is commonly used to establish symptom severity in patients with gastroparesis. It is comprised of 3 subscales—nausea and vomiting, postprandial fullness and early satiety, and bloating—which are averaged to provide a total GCSI score. Symptoms over the 2 weeks prior to administration of the questionnaire are assessed and rated from 0 (none) to 5 (very severe).22 Grading the severity of gastroparesis may take into account symptoms, quality of life, and gastric emptying. One commonly used grading system assigns a grade from 1 to 3, with grade 1 being mild gastroparesis, grade 2 being compensated gastroparesis, and grade 3 being gastric failure with refractory symptoms that are uncontrolled.18,23 Quality-of-life surveys also suggest that gastroparesis independent of other factors leads to a worse quality of life.24
Indications for GES
Gastric electric stimulator implantation is a surgical procedure with inherent risks and complications and is reserved for patients with intractable symptoms of gastroparesis who remain symptomatic despite treatment attempts with dietary management, antiemetic agents (eg, compazine, phenergan, and ondansetron), and prokinetic agents (eg, metoclopramide, erythromycin, and domperidone). Symptom modulators such as nortriptyline and mirtazapine are occasionally tried.
Surgical intervention can be considered upon failure of medical treatment measures. At least a year of documented care provided by a physician specializing in gastroparesis is suggested for surgical consideration. The gastric electric neurostimulator is approved by the FDA as a HUD for the care of patients with idiopathic and diabetic gastroparesis, performed on a compassionate basis. GES implantation requires Institutional Review Board approval at the institution, and patients are required to have documented delayed gastric emptying.
It is important to remember that the GES device is incompatible with magnetic resonance imaging (MRI) and explantation of the device is necessary prior to MRI. As such, in patients with anticipated need of frequent MRI, such as those with multiple sclerosis, serious consideration should be given to alternative strategies prior to focusing on this modality.
Device Placement
GES was devised to improve gastric emptying. The Enterra GES system uses high-frequency, low-energy electric stimulation. An alternative method is true gastric pacing that uses high-energy, low-frequency stimulation to entrain the gastric slow waves and subsequent contractions at 3 cycles per minute (cpm). Gastric pacing has greater energy requirements than GES, which makes the size of the stimulator too large to be practical. In pilot animal studies, GES produced an accelerating effect on gastric emptying, but in human studies GES had an inconsistent effect on gastric emptying. Studies have suggested that GES influences the proximal stomach, with a reduction of gastric tone,25 and also that GES has an afferent modulatory mechanism.26
The Enterra GES is placed surgically under general anesthesia, commonly via laparotomy or minimal access surgical techniques (laparoscopically or robotically assisted). Preoperative intravenous antibiotics are given. The system consists of a pair of electrodes connected to a pulse generator. The 2 stimulation leads are inserted into the gastric muscularis propria 1 cm apart along the greater curvature 10 cm proximal to the pylorus. Upper endoscopy is performed to ensure that the leads do not penetrate through the mucosa into the stomach lumen; if this occurs, repositioning of the lead is necessary. A horizontal incision through the skin is made, and the distal ends of the stimulating wires are tunneled through the abdominal wall and connected to the pulse generator. The impedance (resistance) between the wires is measured to ensure the appropriate range (200-800 Ohms). The neurostimulator with the distal ends of the stimulating wires is then placed into the subcutaneous pocket and sutured to the underlying fascia. The pulse generator delivers a high-frequency, low-energy, 0.1-second train of pulses at a frequency of 12 cpm. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second. The voltage of the stimulations is set to provide a current of 5 milliamps (mA; remembering that voltage = current × resistance).
Patients are often hospitalized with a recovery time of 1 to 3 days. Immediate postoperative care usually includes intravenous fluids, controlling any postoperative ileus, advancing diet, and providing analgesic pain medications. Hospital length of stay can be impacted by surgical technique.25 Patients are seen several weeks after discharge for assessment of the incision and toleration of diet. Medications for gastroparesis that patients were taking prior to the GES implantation are usually continued postoperatively, with a goal of reducing these medications over time. Patients are then followed every 3 to 12 months, depending on their clinical condition.
At follow-up visits, medications are reviewed and new treatments can be added if appropriate. The gastric stimulator is interrogated to determine if changes in resistance occurred; if necessary, minor readjustments can be made to keep the current at desired levels (5 mA). For persistent symptoms with GES treatment, the stimulator parameters can be adjusted after 3 months of follow up, typically first increasing the current from 5 to 7.5 mA and then to 10 mA. After this, the frequency can be increased from 14 Hz to 28 Hz, and then to 55 Hz. Rarely, the ON duration is increased from 0.1 to 1 second. Increasing the ON time can worsen symptoms in some patients, cause abdominal pain, and decrease the battery life from the usual 7 years.
Complications of GES
In an analysis of the Manufacturer and User Facility Device Experience (MAUDE) databank, Bielefeldt identified 1587 reports of adverse effects related to the gastric electric stimulator from January 2001 to October 2015.27 The most common adverse effects are reviewed here.
Skin erosion/wound dehiscence is one of the most common reported complications; it may be related to superficial placement or inadequate securing of the device to the fascia. Abscess can develop postoperatively due to hematogenous seeding or may be a sign of lead erosion into the lumen, tracking along the leads into subcutaneous tissue.28 It is important to warn patients to protect the area over the device from needle injections as this also can lead to hematoma formation and direct contamination of the device. If the device gets infected, it cannot be salvaged and requires explantation. Implantation of a new device can be attempted once all wound issues resolve.
Device migration/flipping most often occurs because the device is inadequately fixed to the underlying fascia, but occasionally it can occur from patients flipping the device around. Flipping can occur due to superficial pocket location within subcutaneous tissue, especially in obese patients. Migration/flipping can lead to prominence of the contour of the device and discomfort, ultimately requiring surgical correction.
Perforation and erosion of the leads. With time, leads can erode into the stomach, although this is rare. Usually erosion is associated with loss of device function. Endoscopy confirms this finding. In rare cases, infection can track proximally along the lead and present as a surgical site infection at the pulse generator. This complication often requires explantation of the neurostimulator leads and pulse generator.
Intestinal obstruction. Although rare, the intestines can get wrapped around the leads of the device, causing different degrees of obstruction (Figure 1). Positioning the device in the left upper quadrant minimizes the intraabdominal length of the leads and pulls them maximally out, coiling under the device (Figure 2). In cases where other locations are used either due to a hostile upper abdominal region (skin infection, presence of gastrostomy or other devices) or surgeon’s preference, the GES device can be implanted in the lower abdomen (Figure 3). In these circumstances, carefully draping the omentum over the bowels might help to prevent this complication. Tacking of the leads to the parietal peritoneum with sutures can also be preventative. In cases of obstruction requiring intervention by laparotomy or minimal access techniques (laparoscopy or robotic assisted surgery), all efforts are made to preserve the neurostimulator leads. In cases that require bowel resection, lead contamination is a serious concern, but lead explantation is not mandatory. Close postoperative monitoring for the development of lead infection is required.
Hematoma and seroma. Postoperative hematomas can occur from inadequate hemostasis, and seromas can occur in the stimulator pocket. Small hematomas may be observed if not complicated (Figure 4). In cases of large hematomas with skin compromise or dehiscence, prompt washout and drainage is required. In ideal cases, the device can be preserved. Relocation to another site might be required if skin necrosis develops. The possibility of device contamination also must be considered; after resolution of wound issues, implantation of a new device may be tried. Seromas at the generator pocket site are a frequent occurrence but are often benign, self-limiting, and generally resolve over 4 to 6 weeks.
Incisional hernia. Hernias can develop after any abdominal surgery and are not unique to GES implantation. Use of minimally invasive technique for the GES implantation minimizes this complication.
Electric shock sensations may occur from breakage of the plastic lining covering the stimulator wires or from fluid buildup around the insertion of the wires into the stimulator. Shocks can also occur due to shortening of the leads on the muscles of the abdominal wall. Patients describe periodic muscle cramps with the frequency of the device (every 5 seconds). To prevent this complication, freshly implanted leads should be covered by an omental flap to isolate them from the abdominal wall. In patients who continue to feel shocks despite all efforts, the possibility of visceral hypersensitivity should be considered. A trial of symptom modulators such as nortriptyline and lowering of the output amperage below the minimal recommended setting of 5 mA can be undertaken. If these interventions do not work, the device must be turned off for a period of time. Occasionally, replacement of the leads or explantation of the device must be considered.
Lack of effect/persistent symptoms. If a patient presents with lack of improvement after device implantation, a thorough workup should be undertaken to ensure that the device is functioning properly. In the case of abnormal impedance values, an abdominal x-ray study can be performed to rule out lead migration (Figure 5). If no abnormalities are detected, the output of the device can be increased. After adjusting device settings, the patient should be assessed for improvement over at least a 1- to 3-month period. One report suggests that in patients not responding to GES, repositioning the location of the stimulator leads on the stomach can be helpful.29
Outcomes of GES
Study results of investigative GES models in animals and select patients were published in 1997.30,31 Following these reports, 2 large multicenter studies were conducted to demonstrate the efficacy of GES for the treatment of refractory gastroparesis. The Gastric Electrical Mechanical Stimulation Study (GEMS) was an open-label, multicenter study of 38 patients who received percutaneous and later permanent GES devices.32 Marked reduction in weekly vomiting and nausea was observed at 4 weeks, with a 90% reduction in nausea and vomiting frequency at 11 months. Following this, a second multicenter study (Worldwide Anti-Vomiting Electrical Stimulation Study [WAVES]) involving a double-blind sham stimulation controlled trial with 33 idiopathic and diabetic gastroparesis patients was performed.33 During the blinded portion of this study, there was a noticeable decrease in vomiting frequency, particularly in the patients with diabetic gastroparesis. Patient preference was for the stimulator ON as compared to OFF. The FDA’s HUD exemption for the Enterra GES device in 2000 was based on these studies.
Four independent double-blind studies of GES have been conducted (Table 1).33-37 It has been difficult to demonstrate improvement during the double-blind period with gastric stimulation compared to no stimulation. Despite total symptom severity improvement and individual symptom improvements in these studies, a recent meta-analysis demonstrated a summative insignificant difference between the GES ON versus OFF states.38
In contrast to the double-blind studies, numerous open-label studies have demonstrated clinical improvements in patients with diabetic and idiopathic gastroparesis (Table 2),32,33,35,36,39-55 leading some to question whether the demonstrable efficacy reflects a placebo effect or regression to the mean. Patients may perceive an operative, aggressive intervention as likely to be effectual in comparison to incremental medication efforts, thus creating a placebo effect. It should also be noted that not all open-label studies have demonstrated improvement with GES. Indeed, Jones et al reported no significant difference in nausea and vomiting at 6-month follow-up, and recommended that physicians exercise caution with GES as a therapeutic strategy given the cost and lack of confirmed demonstrable effect.56 Thus, the clinical successes demonstrated in open-label studies must be weighed not only against the lack of unequivocal improvement, but also against the potential deleterious effects of the surgery.
In an open-label study that employed the GCSI to follow symptoms of gastroparesis, 29 patients underwent GES implantation over an 18-month period, with follow-up in 28 patients.44 GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. The overall GCSI significantly decreased, with improvement in the nausea/vomiting subscore and the post-prandial fullness subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for patients with diabetic versus idiopathic gastroparesis. Patients with the main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not. In this study, 3 clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these 3 factors may allow improved patient selection for GES.
A large prospective study by Heckert et al detailed marked improvements with GES and the patterns of those improvements.55 Nausea, vomiting, loss of appetite, and early satiety improved significantly with stimulator use, with a greater improvement in vomiting in patients with diabetic gastroparesis than in those with the idiopathic form. Although GES improved symptoms in 75% of all patients, patients with diabetes had a post-GES Clinical Patient Grading Assessment score that was statistically higher than the score among patients with idiopathic gastroparesis. This difference is thought to be due to the neuromolecular mechanism of diabetic gastroparesis, where blunting of the enteric nervous system may contribute to symptomatology.
Several studies have demonstrated a clinical response to GES in patients with postsurgical gastroparesis. A study by Oubre et al showed that GES led to weekly vomiting improvements as well as a reduction in total symptom severity score.57 A study by McCallum et al further demonstrated improved symptoms, quality of life, nutritional status, and hospitalization requirements.58 GES has also been shown to improve gastroparesis symptoms in pediatric populations.47,59 Thus, although not a direct indication, GES has been shown to be beneficial in various subtypes of gastroparesis.
Additionally, irrespective of gastroparesis type, the improved symptomatology with GES appears to be durable, with one study showing persistent clinical improvements up to 8 years after device placement.60 The improvements were persistent and incremental. Likewise, McCallum et al showed that continued reductions in total symptom severity scores were evident in all gastroparesis types up to 10 years after stimulator implantation.61 The success of the procedures in part comes from careful selection of patients. Clinical parameters that are associated with favorable clinical response include diabetic gastroparesis subtype, nausea/vomiting predominance, and independence from narcotic analgesics prior to stimulator placement.62
GES has also been noted to improve other patient care metrics besides symptomatology, including nutritional status, reduced need for nutritional supplementation, and improved HbA1c.63-65 Additionally, a study by Cutts et al established that health care resource utilization significantly improved at 12, 24, and 36 months following GES placement, as compared to patients receiving standard medical therapy.66 This decreased resource utilization was also reflected in decreased costs in the GES group compared with the standard care group.
Surgical Alternatives to GES
Pyloric interventions such as pyloroplasty and pyloromyotomy are other surgical treatment modalities offered for gastroparesis. Whereas GES uses neurostimulation to facilitate gastric emptying and potentially improve fundic accommodation, pyloric interventions are intended to increase gastric emptying by reducing outflow resistance from the pyloric sphincter.
Pyloric Interventions
Various studies have shown significant improvements with pyloric interventions, similar to the improvements seen with GES. One such study involving 177 patients demonstrated an 86% improvement in gastric emptying, with symptom severity scores for nausea, vomiting, bloating, abdominal pain, and early satiety decreasing significantly at 3 months following pyloroplasty.67 A significant advantage of pyloric interventions is that pyloromyotomy can be performed endoscopically (gastric peroral endoscopic pyloromyotomy [G-POEM] or peroral pyloromyotomy [POP]), thus minimizing the risks of open surgery. A recent review that included a pooled analysis of 7 studies of G-POEM for gastroparesis demonstrated 100% technical success, with clinical efficacy in 81.5% of the procedures as assessed by the GCSI.68 Additionally, the intraoperative and perioperative complication rates were 6.6% and 7.6%, respectively, suggesting that G-POEM is a safe and clinically beneficial therapeutic option. Few studies comparing the outcomes of pyloric interventions to GES have been performed.
Recently, GES has been combined with pyloric interventions to maximize therapeutic potential. This allows simultaneous neurologic and functional interventions to expedite gastric emptying and improve patient symptomatology. Davis et al demonstrated significant improvement in 21 patients who underwent GES placement and pyloroplasty, with 71% improvement in total symptom severity.69 Notably, dual surgery did not increase the incidence of infection or adverse surgical outcomes. Although this study did not directly compare dual surgery to GES alone, the results are nonetheless favorable. GES provides a strong antiemetic and anti-nausea effect, whereas the pyloromyotomy provides improvement in gastric emptying.
Feeding/Venting Tubes
Feeding jejunostomy tubes and venting gastrostomy tubes can be used alone or in combination with GES. Feeding jejunostomy is performed for malnutrition and weight loss that accompanies the refractory symptoms of early satiety, nausea, and vomiting. Venting gastrostomy tubes allow for removal of retained gastric contents that may cause distension, nausea, and vomiting. Gastrojejunostomy tubes can also be placed endoscopically or by interventional radiology.
Gastrectomy
Gastrectomy can provide therapeutic benefit through elimination of the gastric reservoir function and consequent removal of afferent neural impulses. In select patient populations, outcomes of gastrectomy have compared favorably with those of GES. For example, one study demonstrated favorable outcomes of Roux-en-Y gastrectomy in morbidly obese patients with gastroparesis.70 In another study, favorable outcomes were reported in a cohort of 103 patients, with gastrectomy demonstrating 87% symptom improvement (nausea, vomiting, epigastric pain) compared to just 63% improvement with GES.71 However, the dramatic impact on anatomy and physiology and the invasiveness of the procedure need to be weighed against the therapeutic benefit. For example, in the same study, the 30-day morbidity was 23% for gastrectomy versus just 8% for the GES implant.71
When to Use GES
The gastric electrical neurostimulator (Enterra; Medtronic, Inc.) is approved for treatment of idiopathic and diabetic gastroparesis that is refractory to medical treatment, performed on a compassionate basis. Patients with diabetic gastroparesis respond to GES better than do patients with the idiopathic form. Of the symptoms of gastroparesis, primarily nausea and vomiting improve. Thus, GES favors patients with diabetic gastroparesis who have primarily nausea and vomiting, rather than, for instance, patients with idiopathic gastroparesis who have primarily abdominal pain and may be taking narcotics. Some centers provide GES for postsurgical patients and children with gastroparesis.
The 3 main surgical interventions for medically refractory gastroparesis are GES, pyloric intervention (pyloroplasty or pyloromyotomy), and gastrectomy. Of the 3 interventions, gastrectomy is the most radical given its dramatic effect on anatomy and is thus not preferred. The clinical decision then becomes: GES, pyloric intervention, or both? There are limited data to support a definitive answer to this question.
In a single-center retrospective analysis of prospective data (electronic medical record), Arthur et al compared outcomes of GES patients with medically refractory gastroparesis who received various surgical interventions.72 In total, 33 stimulator, 7 pyloroplasty, 2 gastrectomy, and 16 combined stimulator and pyloroplasty patients were analyzed for postoperative symptom improvement. Pyloroplasty alone demonstrated the least symptom improvement, combination GES and pyloroplasty demonstrated increased improvement, and GES alone demonstrated the most improvement. The results of this study suggest that barring contraindication, placement of a gastric stimulator as the initial treatment is best, with pyloroplasty reserved for patients who do not achieve adequate symptom control. Limitations of the study include its single-center design and low patient numbers for pyloroplasty in isolation.
In contrast, a recent retrospective systematic review synthesized the outcomes of various studies of GES and pyloric interventions for medically refractory gastroparesis.73 A therapeutic effect was found for each surgical intervention, with pyloric surgery patients demonstrating a greater response to intervention than GES patients. Unfortunately, attempts to analyze combination interventions were hindered by a lack of power.
Conclusion
Initial management of gastroparesis is medical (lifestyle and diet changes), with antiemetic and prokinetic agents used in refractory cases. Following failure of this therapy, placement of a GES device is a surgical intervention that has been approved under FDA humanitarian device exemption to help ameliorate symptomatology. Improvement with GES has been demonstrated in nonblinded studies, but the lack of randomized controlled trials demonstrating benefit suggests the possibility of an underlying placebo effect. Additionally, new medical procedures such as G-POEM complicate the decision of which intervention should be attempted first. More studies, specifically comparing GES, pyloric interventions, and combined GES with pyloric intervention to placebo, are needed to fully understand what therapy is best for refractory gastroparesis.
Corresponding author: Henry P. Parkman, MD, Gastroenterology Section, Temple University School of Medicine, 3401 North Broad Street, Philadelphia, PA 19140; [email protected].
Financial disclosures: None.
1. Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
2. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig Dis Sci. 2018;63:2231-2243.
3. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
4. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101-115.
5. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501-1504.
6. Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32:318-322.
7. Meeroff JC, Schreiber DS, Trier JS, Blacklow NR. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370-373.
8. Paliwal M, Prasanna KS, Saraswat VA, et al. Varicella zoster cranial polyneuropathy presenting with dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17:192-194.
9. Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751-754.
10. Kockar MC, Kayahan IK, Bavbek N. Diabetic gastroparesis in association with autonomic neuropathy and microvasculopathy. Acta Med Okayama. 2002;56:237-243.
11. Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233.
12. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34-42.
13. Wang YR, Fisher RS. Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322.
14. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113-133.
15. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
16. Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676-681.
17. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427-438.
18. Jehangir A, Abdallah RT, Parkman HP. Characterizing abdominal pain in patients with gastroparesis into neuropathic and nociceptive components. J Clin Gastroenterol. 2018 May 18. doi: 10.1097/MCG.0000000000001059.
19. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44:1-7.
20. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol. 2018;14:140-145.
21. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972-1978.
22. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670-680.
23. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463.
24. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9-19.
25. Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681-694.
26. Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29-39
27. Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the Manufacturer and User Device Experience (MAUDE) Registry. Auton Neurosci. 2017;202:40-44
28. Liu RC, Sabnis AA, Chand B. Erosion of gastric electrical stimulator electrodes: evaluation, management, and laparoscopic techniques. Surg Laparosc Endosc Percutan Tech. 2007;17:438-441.
29. Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244-249.
30. Familoni BO, Abell TL, Nemoto D, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885-891.
31. Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892-897.
32. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204-212.
33. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428.
34. Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43:1066-1075.
35. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-836.
36. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947-954.
37. Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496-503.
38. Levinthal DJ. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci. 2017;202:45-55.
39. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
40. Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841-846.
41. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
42. van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38-42.
43. de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis. gastric motility restored. Surg Endosc. 2006;20:302-306.
44. Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072-2078.
45. Filichia LA, Cendan CJ. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90-93.
46. Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464-470.
47. Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437-442.
48. Brody F, Vaziri K, Saddler A, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533-538.
49. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
50. Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183.
51. Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457-464.
52. Keller DS, Parkman HP, Boucek DO, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620-626.
53. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
54. Richmond B, Chong B, Modak A, et al. Gastric electrical stimulation for refractory gastroparesis: Predictors of response and redefining a successful outcome. Am Surg. 2015;81:467-471.
55. Heckert J, Sankineni A, Hughes WB, et al. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. 2016;61:168-175.
56. Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578.
57. Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: Long-term outcome. South Med J. 2005;98:693-697.
58. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
59. Islam S, McLaughlin J, Pierson J, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71.
60. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
61. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
62. Maranki J, Lytes V, Meilahn JE, et al. Dig Dis Sci. 2008 53:2072-2078.
63. Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277-281.
64. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
65. Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328-1334.
66. Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35-43.
67. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326-1332.
68. Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis. J Gastroenterol Hepatol. 2018;33:1829-1833.
69. Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222-227.
70. Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683-2689.
71. Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61-66.
72. Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc. 2017;32:977-982.
73. Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J Surg Res. 2018;231:263-269.
1. Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
2. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig Dis Sci. 2018;63:2231-2243.
3. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
4. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101-115.
5. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501-1504.
6. Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32:318-322.
7. Meeroff JC, Schreiber DS, Trier JS, Blacklow NR. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370-373.
8. Paliwal M, Prasanna KS, Saraswat VA, et al. Varicella zoster cranial polyneuropathy presenting with dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17:192-194.
9. Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751-754.
10. Kockar MC, Kayahan IK, Bavbek N. Diabetic gastroparesis in association with autonomic neuropathy and microvasculopathy. Acta Med Okayama. 2002;56:237-243.
11. Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233.
12. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34-42.
13. Wang YR, Fisher RS. Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322.
14. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113-133.
15. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
16. Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676-681.
17. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427-438.
18. Jehangir A, Abdallah RT, Parkman HP. Characterizing abdominal pain in patients with gastroparesis into neuropathic and nociceptive components. J Clin Gastroenterol. 2018 May 18. doi: 10.1097/MCG.0000000000001059.
19. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44:1-7.
20. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol. 2018;14:140-145.
21. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972-1978.
22. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670-680.
23. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463.
24. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9-19.
25. Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681-694.
26. Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29-39
27. Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the Manufacturer and User Device Experience (MAUDE) Registry. Auton Neurosci. 2017;202:40-44
28. Liu RC, Sabnis AA, Chand B. Erosion of gastric electrical stimulator electrodes: evaluation, management, and laparoscopic techniques. Surg Laparosc Endosc Percutan Tech. 2007;17:438-441.
29. Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244-249.
30. Familoni BO, Abell TL, Nemoto D, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885-891.
31. Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892-897.
32. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204-212.
33. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428.
34. Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43:1066-1075.
35. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-836.
36. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947-954.
37. Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496-503.
38. Levinthal DJ. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci. 2017;202:45-55.
39. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
40. Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841-846.
41. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
42. van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38-42.
43. de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis. gastric motility restored. Surg Endosc. 2006;20:302-306.
44. Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072-2078.
45. Filichia LA, Cendan CJ. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90-93.
46. Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464-470.
47. Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437-442.
48. Brody F, Vaziri K, Saddler A, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533-538.
49. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
50. Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183.
51. Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457-464.
52. Keller DS, Parkman HP, Boucek DO, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620-626.
53. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
54. Richmond B, Chong B, Modak A, et al. Gastric electrical stimulation for refractory gastroparesis: Predictors of response and redefining a successful outcome. Am Surg. 2015;81:467-471.
55. Heckert J, Sankineni A, Hughes WB, et al. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. 2016;61:168-175.
56. Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578.
57. Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: Long-term outcome. South Med J. 2005;98:693-697.
58. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
59. Islam S, McLaughlin J, Pierson J, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71.
60. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
61. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
62. Maranki J, Lytes V, Meilahn JE, et al. Dig Dis Sci. 2008 53:2072-2078.
63. Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277-281.
64. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
65. Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328-1334.
66. Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35-43.
67. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326-1332.
68. Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis. J Gastroenterol Hepatol. 2018;33:1829-1833.
69. Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222-227.
70. Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683-2689.
71. Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61-66.
72. Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc. 2017;32:977-982.
73. Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J Surg Res. 2018;231:263-269.
Antidepressants for chronic pain
Approximately 55 years ago, tricyclic antidepressants (TCAs) began to be used to treat neuropathic pain.1 Eventually, clinical trials emerged suggesting the utility of TCAs for other chronic pain conditions, such as fibromyalgia (FM) and migraine prophylaxis. However, despite TCAs’ effectiveness in mitigating painful conditions, their adverse effects limited their use.
Pharmacologic advancements have led to the development of other antidepressant classes, including selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), and the use of these agents has come to eclipse that of TCAs. In the realm of pain management, such developments have raised the hope of possible alternative co-analgesic agents that could avoid the adverse effects associated with TCAs. Some of these agents have demonstrated efficacy for managing chronic pain states, while others have demonstrated only limited utility.
This article provides a synopsis of systematic reviews and meta-analyses examining the role of antidepressant therapy for managing several chronic pain conditions, including pain associated with neuropathy, FM, headache, and irritable bowel syndrome (IBS). Because the literature base is rapidly evolving, it is necessary to revisit the information gleaned from clinical data with respect to treatment effectiveness, and to clarify how antidepressants might be positioned in the management of chronic pain.
The effectiveness of antidepressants for pain
The pathophysiologic processes that precipitate and maintain chronic pain conditions are complex (Box 12-10). The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects and indirect effects (Box 22,3,8,10,11-33).
Box 1
The pathophysiologic processes precipitating and maintaining chronic pain conditions are complex. Persistent and chronic pain results from changes in sensitivity within both ascending pathways (relaying pain information from the periphery to the spinal cord and brain) and descending pain pathways (functioning to modulate ascending pain information).2,3 Tissue damage or peripheral nerve injury can lead to a cascade of neuroplastic changes within the CNS, resulting in hyperexcitability within the ascending pain pathways.
The descending pain pathways consist of the midbrain periaqueductal gray area (PGA), the rostroventral medulla (RVM), and the dorsolateral pontomesencephalic tegmentum (DLPT). The axons of the RVM (the outflow of which is serotonergic) and DLPT (the outflow of which is noradrenergic) terminate in the dorsal horn of the spinal cord,4 and thereby dampen pain signals arising from the periphery. Diminished output from descending pain pathways can heighten the pain experience. Input from the cortex, hypothalamus, and amygdala (among other structures) converges upon the PGA, RVM and DLPT, and can influence the degree of pain modulation emerging from descending pathways. In this way, thoughts, appraisals, and mood are believed to comprise cognitive and affective modifiers of pain experiences.
Devising effective chronic pain treatment becomes challenging; multimodal treatment approaches often are advocated, including pharmacologic treatment with analgesics in combination with co-analgesic medications such as antidepressants. Although a description of multimodal treatment is beyond the scope of this article, such treatment also would encompass physical therapy, occupational therapy, and psychotherapeutic interventions to augment rehabilitative efforts and the functional capabilities of patients who struggle with persisting pain.
Although the direct pain-mitigating effects of antidepressants are not fully understood, it is believed that augmentation of monoamine neurotransmission from supraspinal nuclei (ie, the RVM and DLPT) modulate pain transmission from the periphery.5,6 In addition, there is evidence that some effects of tricyclic antidepressants can modulate several other functions that impact peripheral and central sensitization.7-10
During the last several decades, antidepressants have been used to address—and have demonstrated clinical utility for—a variety of chronic pain states. However, antidepressants are not a panacea; some chronic pain conditions are more responsive to antidepressants than are others. The chronic painful states most amenable to antidepressants are those that result primarily from a process of neural sensitization, as opposed to acute somatic or visceral nociception. Hence, several meta-analyses and evidence-based reviews have long suggested the usefulness of antidepressants for mitigating pain associated with neuropathy,34,35 FM,36,37 headache,38 and IBS.39,40
Box 2
The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects (impacting neurotransmission of descending pathways independent of influences on mood) and indirect effects (presumably impacting cortical and limbic output to the periaqueductal gray area, the rostroventral medulla, and the dorsolateral pontomesencephalic tegmentum brought about by improvement in mood and/or cognitive appraisals) (Figure2,3,8,10,11,15,20,22,28,29). Support for the direct analgesic effects has been garnered from initial empirical work that demonstrated pain relief among patients with pain who are not depressed. Additionally, among patients who have depression and experience pain, analgesia reportedly often occurs within 2 weeks, which is before antidepressant effects are appreciated,11-15 and, at least for some antidepressants, occurs at doses far lower than those required to produce mood-elevating effects.11,12,16
On the other hand, it is well established that significant comorbidities exist between chronic pain states and psychiatric disorders (eg, depression and somatic symptom and related disorders).17-21 There may be common physiological substrates underlying chronic pain and depression.20,22 There are bidirectional influences of limbic (affective) systems and those CNS structures involved in pain processing and integration. The effects of pain and depression are reciprocal; the presence of one makes the management of the other more challenging.23-27 Mood disturbances can, therefore, impact pain processing by acting as affective and cognitive amplifiers of pain by leading to catastrophizing, pain severity augmentation, poor coping with pain-related stress, etc.28,29 It is plausible that the mood-elevating effects of antidepressants can improve pain by indirect effects, by modulating limbic activity, which in turn, impacts coping, cognitive appraisals of pain, etc.
Patients with somatoform disorders (using pre-DSM-5 terminology) frequently present with chronic pain, often in multiple sites.19 Such patients are characterized by hypervigilance for, and a predisposition to focus on, physical sensations and to appraise these sensations as reflecting a pathological state.30 Neuroimaging studies have begun to identify those neural circuits involved in somatoform disorders, many of which act as cognitive and affective amplifiers of visceral-somatic sensory processing. Many of these neural circuits overlap, and interact with, those involved in pain processing.31 Antidepressants can mitigate the severity of unexplained physical complaints, including pain, among patients who somatize32,33; however, due to the heterogeneity of studies upon which this claim is based, the quality of the evidence is reportedly low.33 There is uncertainty whether, or to what extent, antidepressant benefits among patients who somatize are due to a direct impact on pain modulation, or indirect effects on mood or cognitive appraisals/perceptions.
Despite the uncertainties about the exact mechanisms through which antidepressants exert analgesic effects, antidepressants can be appropriately used to treat patients with selected chronic pain syndromes, regardless of whether or not the patient has a psychiatric comorbidity. For those patients with pain and psychiatric comorbidities, the benefits may be brought about via direct mechanisms, indirect mechanisms, or a combination of both.
Continue to: Neuropathic pain
Neuropathic pain
Several treatment guidelines advocate for the use of antidepressants for neuropathic pain.41-44 For decades, TCAs have been employed off-label to successfully treat many patients with neuropathic pain states. Early investigations suggested that TCAs were robustly efficacious in managing patients with neuropathy.45-48 Calculated number-needed-to-treat (NNT) values for TCAs were quite low (ie, reflecting that few patients would need to be treated to yield a positive response in one patient compared with placebo), and were comparable to, if not slightly better than, the NNTs generated for anticonvulsants and α2-δ ligands, such as gabapentin or pregabalin.45-48
Unfortunately, early studies involving TCAs conducted many years ago do not meet contemporary standards of methodological rigor; they featured relatively small samples of patients assessed for brief post-treatment intervals with variable outcome measures. Thus, the NNT values obtained in meta-analyses based on these studies may overestimate treatment benefits.49 Further, NNT values derived from meta-analyses tended to combine all drugs within a particular antidepressant class (eg, amitriptyline, nortriptyline, desipramine, and imipramine among the TCAs) employed at diverse doses. Taken together, these limitations raise questions about the results of those meta-analyses.
Subsequent meta-analyses, which employed strict criteria to eliminate data from studies with potential sources of bias and used a primary outcome of frequencies of patients reporting at least 30% pain reduction compared with a placebo-controlled sample, suggest that the effectiveness of TCAs as a class for treating neuropathic pain is not as compelling as once was thought. Meta-analyses of studies employing specific TCAs revealed that there was little evidence to support the use of desipramine,50 imipramine,51 or nortriptyline52 in managing diabetic neuropathy or postherpetic neuralgia. Studies evaluating amitriptyline (dose range 12.5 to 150 mg/d), found low-level evidence of effectiveness; the benefit was expected to be present for a small subset (approximately 25%) of patients with neuropathic pain.53
There is moderate-quality evidence that duloxetine (60 to 120 mg/d) can produce a ≥50% improvement in pain severity ratings among patients with diabetic peripheral neuropathy.54 Although head-to-head studies with other antidepressants are limited, it appears that duloxetine and amitriptyline have comparable efficacy, even though the NNTs for amitriptyline were derived from lower-quality studies than those for duloxetine. Duloxetine is the only antidepressant to receive FDA approval for managing diabetic neuropathy. By contrast, studies assessing the utility of venlafaxine in neuropathic pain comprised small samples for brief durations, which limits the ability to draw clear (unbiased) support for its usefulness.55
Given the diversity of pathophysiologic processes underlying the disturbances that cause neuropathic pain disorders, it is unsurprising that the effectiveness of amitriptyline and duloxetine were not generalizable to all neuropathic pain states. Although amitriptyline produced pain-mitigating effects in patients with diabetic neuropathy and post-herpetic neuralgia, and duloxetine mitigated pain among patients with diabetic neuropathy, there was no evidence to suggest their effectiveness in phantom limb pain or human immunodeficiency virus-related and spinal cord-related neuropathies.35,53,54,56-58
Continue to: Fibromyalgia
Fibromyalgia
As with the issues encountered in interpreting the effectiveness of antidepressants in neuropathic pain, interpreting results gleaned from clinical trials of antidepressants for treating FM are fraught with similar difficulties. Although amitriptyline has been a first-line treatment for FM for many years, the evidence upon which such recommendations were based consisted of low-level studies that had a significant potential for bias.59 Large randomized trials would offer more compelling data regarding the efficacy of amitriptyline, but the prohibitive costs of such studies makes it unlikely they will be conducted. Amitriptyline (25 to 50 mg/d) was effective in mitigating FM-related pain in a small percentage of patients studied, with an estimated NNT of 4.59 Adverse effects, often contributing to treatment discontinuation, were encountered more frequently among patients who received amitriptyline compared with placebo.
Selective serotonin reuptake inhibitors failed to demonstrate significant pain relief (estimated NNT of 10), or improvement in fatigue or sleep problems, even though the studies upon which such conclusions were based were low-level studies with a high potential for bias.60 Although SSRIs have limited utility for mitigating pain, they are still quite useful for reducing depression among patients with FM.60
By contrast, the SNRIs duloxetine and milnacipran provided clinically relevant benefit over placebo in the frequency of patients reporting pain relief of ≥30%, as well as patients’ global impression of change.61 These agents, however, failed to provide clinically relevant benefit over placebo in improving health-related quality of life, reducing sleep problems, or improving fatigue. Nonetheless, duloxetine and milnacipran are FDA-approved for managing pain in FM. Studies assessing the efficacy of venlafaxine in the treatment of FM to date have been limited by small sample sizes, inconsistent dosing, lack of a placebo control, and lack of blinding, which limits the ability to clearly delineate the role of venlafaxine in managing FM.62
Mirtazapine (15 to 45 mg/d) showed a clinically relevant benefit compared with placebo for participant-reported pain relief of ≥30% and sleep disturbances. There was no benefit in terms of participant-reported improvement of quality of life, fatigue, or negative mood.63 The evidence was considered to be of low quality overall.
Headache
Amitriptyline has been employed off-label to address headache prophylaxis since 1964.64 Compared with placebo, it is efficacious in ameliorating migraine frequency and intensity as well as the frequency of tension headache.65,66 However, SSRIs and SNRIs (venlafaxine) failed to produce significant reductions in migraine frequency or severity or the frequencies of tension headache when compared with placebo.67,68
Continue to: Irritable bowel syndrome
Irritable bowel syndrome
Early studies addressing antidepressant efficacy in IBS reveal inconsistencies. For example, whereas some suggest that TCAs are effective in mitigating chronic, severe abdominal pain,39,40 others concluded that TCAs failed to demonstrate a significant analgesic benefit.69 A recent meta-analysis that restricted analysis of efficacy to randomized controlled trials (RCTs) with more rigorous methodological adherence found that pain relief in IBS is possible with both TCAs as well as SSRIs. However, adverse effects were more commonly encountered with TCAs than with SSRIs. Some of the inconsistencies in treatment efficacy reported in early studies may be due to variations in responsiveness of subsets of IBS patients. Specifically, the utility of TCAs appears to be best among patients with diarrheal-type (as opposed to constipation-type) IBS, presumably due to TCAs’ anticholinergic effects, whereas SSRIs may provide more of a benefit for patients with predominantly constipation-type IBS.40,70
Other chronic pain conditions
Antidepressants have been used to assist in the management of several other pain conditions, including oral-facial pain, interstitial cystitis, non-cardiac chest pain, and others. The role of antidepressants for such conditions remains unclear due to limitations in the prevailing empirical work, such as few trials, small sample sizes, variations in outcome measures, and insufficient randomization and blinding.71-76 The interpretation of results from systematic reviews and meta-analyses is limited because of these shortcomings.77 Hence, it has not always been possible to determine whether, and to what extent, patients with such conditions may benefit from antidepressants.
Neuromodulatory effects and efficacy for pain
The interplay of norepinephrine (NE) and serotonin (5-HT) neurotransmitter systems and cellular mechanisms involved in the descending modulation of pain pathways is complex. Experimental animal models of pain modulation suggest that 5-HT can both inhibit as well as promote pain perception by different physiological mechanisms, in contrast to NE, which is predominately inhibitory. While 5-HT in the descending modulating system can inhibit pain transmission ascending to the brain from the periphery, it appears that an intact noradrenergic system is necessary for the inhibitory influences of the serotonergic system to be appreciated.16,78,79 Deficiencies in one or both of these neurotransmitter systems may contribute to hyperactive pain processing, and thereby precipitate or maintain chronic pain.
Pain mitigation may be achieved best by enhancing both neurotransmitters simultaneously, less so by enhancing NE alone, and least by enhancing 5-HT alone.6 The ability to impact pain modulation would, therefore, depend on the degree to which an antidepressant capitalizes on both noradrenergic and serotonergic neurotransmission. Antidepressants commonly employed to manage pain are presented in Table 147,60,68,80-88 according to their primary neurotransmitter effects. Thus, the literature summarized above suggests that antidepressants that influence both NE and 5-HT transmission have greater analgesic effects than antidepressants with more specific effects, such as influencing 5-HT reuptake alone.80-85 It is unsurprising, therefore, that the SSRIs have not been demonstrated to be as consistently analgesic.47,60,68,80,86-88
Similarly, pharmacodynamic and pharmacokinetic differences within antidepressant classes may influence analgesic effectiveness. Simultaneous effects on NE and 5-HT are achieved at low doses with duloxetine and milnacipran. By contrast, 5-HT effects predominate at low doses for venlafaxine. To achieve pain-mitigating effects, higher doses of venlafaxine generally are required.89 Therefore, inconsistencies across studies regarding the analgesic benefits of venlafaxine may be attributable to variability in dosing; patients treated with lower doses may not have experienced sufficient NE effects to garner positive results.
Continue to: The differences in analgesic efficacy...
The differences in analgesic efficacy among specific TCAs may be understood in a similar fashion. Specifically, tertiary TCAs (imipramine and amitriptyline) inhibit both 5-HT and NE reuptake.6,90 Secondary amines (desipramine and nortriptyline) predominantly impact NE reuptake, possibly accounting for the lesser pain-mitigating benefit achieved with these agents, such as for treating neuropathic pain. Further, in vivo imipramine and amitriptyline are rapidly metabolized to secondary amines that are potent and selective NE reuptake inhibitors. In this way, the secondary amines may substantially lose the ability to modulate pain transmission because of the loss of concurrent 5-HT influences.90
Clinical pearls
The following practical points can help guide clinicians regarding the usefulness of antidepressants for pain management:
- Antidepressants can alleviate symptoms of depression and pain. The pain-mitigating effects of antidepressants are possible even among chronic pain patients who are not depressed. Antidepressants may confer benefits for chronic pain patients with depression and other comorbid conditions, such as somatic symptom and related disorders.
- Antidepressants are useful for select chronic pain states. Although the noradrenergic and serotonergic antidepressants (SNRIs and, to some extent, amitriptyline) appear to have efficacy for neuropathic pain and FM, the benefits of SSRIs appear to be less robust. On the other hand, SSRIs and TCAs may have potential benefit for patients with IBS. However, the results of meta-analyses are limited in the ability to provide information about which patients will best respond to which specific antidepressant or how well. Future research directed at identifying characteristics that can predict which patients are likely to benefit from one antidepressant vs another would help inform how best to tailor treatment to individual needs.
- The pain-mitigating effects of antidepressants often emerge early in the course of treatment (often before mood-elevating effects are observed). For example, in the case of amitriptyline, pain relief may be possible for some patients at doses generally lower than those required for mood-elevating effects. To date, there is limited information in the literature to determine what constitutes a sufficient duration of treatment, or when treatment should be modified.
- Failure to reduce pain should raise questions about whether the dose should be increased, an alternative agent should be tried, or combinations with other analgesic agents should be considered. Failure to achieve pain-mitigating effects with one antidepressant does not mean failure with others. Hence, failure to achieve desired effects with one agent might warrant an empirical trial with another agent. Presently, too few double-blind RCTs have been conducted to assess the pain-mitigating effects of other antidepressants (eg, bupropion and newer SNRIs such as desvenlafaxine and levomilnacipran). Meta-analysis of the analgesic effectiveness of these agents or comparisons to the efficacy of other antidepressant classes is, therefore, impossible at this time.
Because many chronic pain states are complex, patients will seldom experience clinically relevant benefit from any one intervention.53 The bigger implication for clinical research is to determine whether there is a sequence or combination of medication use that will provide overall better clinical effectiveness.53 Only limited data are available exploring the utility of combining pharmacologic approaches to address pain.91 For example, preliminary evidence suggests that combinations of complementary strategies, such as duloxetine combined with pregabalin, may result in significantly greater numbers of FM patients achieving ≥30% pain reduction compared with monotherapy with either agent alone or placebo.92
- Antidepressant selection may need to be based on medication-related adverse effect profiles and the potential for drug interactions. These factors are useful to consider in delineating multimodal treatment regimens for chronic pain in light of patients’ comorbidities and co-medication regimen. For example, the adverse effects of TCAs (anticholinergic and alpha-adrenergic influences) limit their utility for treating pain. Some of these effects can be more problematic in select populations, such as older adults or those with orthostatic difficulties, among others. TCAs are contraindicated in patients with closed-angle glaucoma, recent myocardial infarction, cardiac arrhythmias, poorly controlled seizures, or severe benign prostatic hypertrophy. Although the pain-mitigating effects of SNRIs have not been demonstrated to significantly exceed those of TCAs,68,93,94 SNRIs would offer an advantage of greater tolerability of adverse effects and relative safety in patients with comorbid medical conditions that would otherwise preclude TCA use. The adverse effects and common drug interactions associated with antidepressants are summarized in Table 295.
Conclusion
Chronic, nonmalignant pain conditions afflict many patients and significantly impair their ability to function. Because of heightened concerns related to the appropriateness of, and restricting inordinate access to, long-term opioid analgesics, clinicians need to explore the usefulness of co-analgesic agents, such as antidepressants. Significant comorbidities exist between psychiatric disorders and chronic pain, and psychiatrists are uniquely positioned to diagnose and treat psychiatric comorbidities, as well as pain, among their patients, especially since they understand the kinetics and dynamics of antidepressants.
Bottom Line
Antidepressants can alleviate symptoms of depression and pain. Noradrenergic and serotonergic antidepressants appear to have efficacy for pain associated with neuropathy and fibromyalgia, while selective serotonin reuptake inhibitors and tricyclic antidepressants may have benefit for patients with irritable bowel syndrome. However, evidence regarding which patients will best respond to which specific antidepressant is limited.
Continue to: Related Resources
Related Resources
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
- Maletic V, Demuri B. Chronic pain and depression: treatment of 2 culprits in common. Current Psychiatry. 2016;15(3):41,47-50,52.
Drug Brand Names
Amitriptyline • Elavil, Endep
Bupropion • Wellbutrin, Zyban
Carisoprodol • Rela, Soma
Cyclobenzaprine • Amrix, Flexeril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Horizant, Neurontin
Imipramine • Tofranil
Levomilnacipran • Fetzima
Methadone • Dolophine, Methadose
Milnacipran • Savella
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Pregabalin • Lyrica, Lyrica CR
Tapentadol • Nucynta
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Warfarin • Coumadin, Jantoven
1. Paoli F, Darcourt G, Cossa P. Preliminary note on the action of imipramine in painful states [in French]. Rev Neurol (Paris). 1960;102:503-504.
2. Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219-245.
3. Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2(2):83-91.
4. Lamont LA, Tranquilli WJ, Grimm KA. Physiology of pain. Vet Clin North Am Small Anim Pract. 2000;30(4):703-728, v.
5. Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, eds. Wall and Melzack’s Textbook of Pain. 5th ed. Burlington, MA: Elsevier Health Sciences; 2005:125-142.
6. Marks DM, Shah MJ, Patkar AA, et al. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol. 2009;7(4):331-336.
7. Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92(2):473-484.
8. Carter GT, Sullivan MD. Antidepressants in pain management. Curr Opin Investig Drugs. 2002;3(3):454-458.
9. Kawasaki Y, Kumamoto E, Furue H, et al. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98(3):682-689.
10. McCleane G. Antidepressants as analgesics. CNS Drugs. 2008;22(2):139-156.
11. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry. 2000;7(5):257-277.
12. Egbunike IG, Chaffee BJ. Antidepressants in the management of chronic pain syndromes. Pharmacotherapy. 1990;10(4):262-270.
13. Fishbain DA. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32(5):305-316.
14. Fishbain DA, Detke MJ, Wernicke J, et al. The relationship between antidepressant and analgesic responses: findings from six placebo-controlled trials assessing the efficacy of duloxetine in patients with major depressive disorder. Curr Med Res Opin. 2008;24(11):3105-3115.
15. Harada E, Tokuoka H, Fujikoshi S, et al. Is duloxetine’s effect on painful physical symptoms in depression an indirect result of improvement of depressive symptoms? Pooled analyses of three randomized controlled trials. Pain. 2016;157(3):577-584.
16. Kehoe WA. Antidepressants for chronic pain: selection and dosing considerations. Am J Pain Med. 1993;3(4):161-165.
17. Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(2):1070-1078.
18. DeVeaugh-Geiss AM, West SL, Miller WC, et al. The adverse effects of comorbid pain on depression outcomes in primary care patients: results from the ARTIST trial. Pain Medicine. 2010;11(5):732-741.
19. Egloff N, Cámara RJ, von Känel R, et al. Hypersensitivity and hyperalgesia in somatoform pain disorders. Gen Hosp Psychiatry. 2014;36(3):284-290.
20. Goesling J, Clauw DW, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psych Reports. 2013;15(12):421.
21. Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-Month longitudinal analysis in primary care. J Pain. 2011;12(9):964-973.
22. Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7(5):403-412.
23. Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17-22.
24. Karp JF, Scott J, Houck P, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591-597.
25. Kroenke K, Shen J, Oxman TE, et al. Impact of pain on the outcomes of depression treatment: results from the RESPECT trial. Pain. 2008;134(1-2):209-215.
26. Mavandadi S, Ten Have TR, Katz IR, et al. Effect of depression treatment on depressive symptoms in older adulthood: the moderating role of pain. J Am Geriatr Soc. 2007;55(2):202-211.
27. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15(8):699-707.
28. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262-268.
29. Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Comorbid painful physical symptoms and depression: Prevalence, work loss, and help seeking. J Affect Disord. 2006;92(2-3):185-193.
30. Nakao M, Barsky AJ. Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosoc Med. 2007;1:17.
31. Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015;27(1):e40-e50.
32. Fishbain DA, Cutler RB, Rosomoff HL, et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom Med. 1998;60(4):503-509.
33. Kleinstäuber M, Witthöft M, Steffanowski A, et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev. 2014;(11):CD010628.
34. Collins SL, Moore RA, McQuay HJ, et al. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20(6):449-458.
35. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry. 2010;81(12):1372-1373.
36. Arnold LM, Keck PE, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41(2):104-113.
37. O’Malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15(9):659-666.
38. Tomkins GE, Jackson JL, O’Malley PG, et al. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med. 2001;111(1):54-63.
39. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108(1):65-72.
40. Lesbros-Pantoflickova D, Michetti P, Fried M et al. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(11-12):1253-1269.
41. Centre for Clinical Practice at NICE (UK). Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. London, UK: National Institute for Health and Care Excellence, (UK); 2013.
42. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(suppl 10):S22-S32.
43. Moulin D, Boulanger A, Clark AJ, et al; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-35.
44. Mu A, Weinberg E, Moulin DE, et al. Pharmacologic management of chronic neuropathic pain: Review of the Canadian Pain Society consensus statement. Can Fam Physician. 2017;63(11):844-852.
45. Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289-305.
46. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164.
47. Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83(3):389-400.
48. Wu CL, Raja SN. An update on the treatment of postherpetic neuralgia. J Pain. 2008;9(suppl 1):S19-S30.
49. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
50. Hearn L, Moore RA, Derry S, et al. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(9):CD011003.
51. Hearn L, Derry S, Phillips T, et al. Imipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(5):CD010769.
52. Derry S, Wiffen PJ, Aldington D, et al. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209.
53. Moore R, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(7):CD008242.
54. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
55. Gallagher HC, Gallagher RM, Butler M, et al. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(8):CD011091.
56. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;10:CD006380.
57. Dinat N, Marinda E, Moch S, et al. Randomized, Double-Blind, Crossover Trial of Amitriptyline for Analgesia in Painful HIV-Associated Sensory Neuropathy. PLoS One. 2015;10(5):e0126297. doi: 10.1371/journal.pone.0126297.eCollection 2015.
58. Mehta S, McIntyre A, Janzen S, et al; Spinal Cord Injury Rehabilitation Evidence Team. Systematic review of pharmacologic treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97(8):1381-1391.e1.
59. Moore RA, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;(12):CD008242..
60. Walitt B, Urrútia G, Nishishinya MB, et al. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
61. Welsch P, Üçeyler N, Klose P, et al. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst Rev. 2018;(2):CD010292.
62. VanderWeide LA, Smith SM, Trinkley KE. A systematic review of the efficacy of venlafaxine for the treatment of fibromyalgia. J Clin Pharm Ther. 2015;40(1):1-6.
63. Welsch P, Bernardy K, Derry S, et al. Mirtazapine for fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(8):CD012708.
64. Lance JW, Curran DA. Treatment of chronic tension headache. Lancet. 1964;283(7345):1236-1239.
65. Jackson JL, William S, Laura S, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222. doi: https://doi.org/10.1136/bmj.c5222
66. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine. 2017;96(22):e6989. doi: 10.1097/MD.0000000000006989.
67. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database Syst Rev. 2015;(4):CD002919.
68. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst Rev. 2015;(5):CD011681.
69. Quartero AO, Meineche-Schmidt V, Muris J, et al. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;(2):CD003460.
70. Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367-378.
71. Coss-Adame E, Erdogan A, Rao SS. Treatment of esophageal (noncardiac) chest pain: an expert review. Clin Gastroenterol Hepatol. 2014;12(8):1224-1245.
72. Kelada E, Jones A. Interstitial cystitis. Arch Gynecol Obstet. 2007;275(4):223-229.
73. Leo RJ, Dewani S. A systematic review of the utility of antidepressant pharmacotherapy in the treatment of vulvodynia pain. J Sex Med. 2013;10(10):2497-2505.
74. McMillan R, Forssell H, Buchanan JA, et al. Interventions for treating burning mouth syndrome. Cochrane Database Syst Rev. 2016;11:CD002779.
75. Patel DN. Inconclusive results of a systematic review of efficacy of antidepressants on orofacial pain disorders. Evid Based Dent. 2013;14(2):55-56.
76. Wang W, Sun YH, Wang YY, et al. Treatment of functional chest pain with antidepressants: a meta-analysis. Pain Physician. 2012;15(2):E131-E142.
77. Lavis JN. How can we support the use of systematic reviews in policymaking? PLoS Med. 2009;6(11):e1000141. doi: 10.1371/journal.pmed.1000141.
78. Sorkin L. Nociceptive transmission within the spinal cord. Mt Sinai J Med. 1991;58(3):208-216.
79. Yokogawa F, Kiuchi Y, Ishikawa Y, et al. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg. 2002;95(1):163-168, table of contents.
80. Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26(1):30-36.
81. Max MB. Treatment of post-herpetic neuralgia: antidepressants. Ann Neurol. 1994;35(suppl):S50-S53.
82. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
83. McQuay HJ, Tramèr M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68(2-3):217-227.
84. Mochizucki D. Serotonin and noradrenaline reuptake inhibitors in animal models of pain. Hum Psychopharmacol Clin Exp. 2004;19(suppl 1):15-19.
85. Sussman N. SNRIs versus SSRIs: mechanisms of action in treating depression and painful physical symptoms. Primary Care Companion J Clin Psychiatry. 2003;5(suppl 7):19-26.
86. Bundeff AW, Woodis CB. Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann Pharmacother. 2014;48(6):777-784.
87. Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12(6):384-389.
88. Xie C, Tang Y, Wang Y, et al. Efficacy and safety of antidepressants for the treatment of irritable bowel syndrome: a meta-analysis. PLoS One. 2015;10(8):e0127815. doi: 10.1371/journal.pone.0127815. eCollection 2015.
89. Zijlstra TR , Barendregt PJ , van de Laar MA. Venlafaxine in fibromyalgia: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2002;46(suppl 9):S105.
90. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25(6):871-880.
91. Thorpe J, Shum B, Moore RA, et al. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(2):CD010585.
92. Gilron I, Chaparro LE, Tu D, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain. 2016;157(7):1532-1540.
93. Häuser W, Petzke F, Üçeyler N, et al. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis. Rheumatology (Oxford). 2011;50(3):532-543.
94. Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy: a systematic review. Clin J Pain. 2016;32(11):1005-1010.
95. Riediger C, Schuster T, Barlinn K, et al. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol. 2017;8:307.
Approximately 55 years ago, tricyclic antidepressants (TCAs) began to be used to treat neuropathic pain.1 Eventually, clinical trials emerged suggesting the utility of TCAs for other chronic pain conditions, such as fibromyalgia (FM) and migraine prophylaxis. However, despite TCAs’ effectiveness in mitigating painful conditions, their adverse effects limited their use.
Pharmacologic advancements have led to the development of other antidepressant classes, including selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), and the use of these agents has come to eclipse that of TCAs. In the realm of pain management, such developments have raised the hope of possible alternative co-analgesic agents that could avoid the adverse effects associated with TCAs. Some of these agents have demonstrated efficacy for managing chronic pain states, while others have demonstrated only limited utility.
This article provides a synopsis of systematic reviews and meta-analyses examining the role of antidepressant therapy for managing several chronic pain conditions, including pain associated with neuropathy, FM, headache, and irritable bowel syndrome (IBS). Because the literature base is rapidly evolving, it is necessary to revisit the information gleaned from clinical data with respect to treatment effectiveness, and to clarify how antidepressants might be positioned in the management of chronic pain.
The effectiveness of antidepressants for pain
The pathophysiologic processes that precipitate and maintain chronic pain conditions are complex (Box 12-10). The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects and indirect effects (Box 22,3,8,10,11-33).
Box 1
The pathophysiologic processes precipitating and maintaining chronic pain conditions are complex. Persistent and chronic pain results from changes in sensitivity within both ascending pathways (relaying pain information from the periphery to the spinal cord and brain) and descending pain pathways (functioning to modulate ascending pain information).2,3 Tissue damage or peripheral nerve injury can lead to a cascade of neuroplastic changes within the CNS, resulting in hyperexcitability within the ascending pain pathways.
The descending pain pathways consist of the midbrain periaqueductal gray area (PGA), the rostroventral medulla (RVM), and the dorsolateral pontomesencephalic tegmentum (DLPT). The axons of the RVM (the outflow of which is serotonergic) and DLPT (the outflow of which is noradrenergic) terminate in the dorsal horn of the spinal cord,4 and thereby dampen pain signals arising from the periphery. Diminished output from descending pain pathways can heighten the pain experience. Input from the cortex, hypothalamus, and amygdala (among other structures) converges upon the PGA, RVM and DLPT, and can influence the degree of pain modulation emerging from descending pathways. In this way, thoughts, appraisals, and mood are believed to comprise cognitive and affective modifiers of pain experiences.
Devising effective chronic pain treatment becomes challenging; multimodal treatment approaches often are advocated, including pharmacologic treatment with analgesics in combination with co-analgesic medications such as antidepressants. Although a description of multimodal treatment is beyond the scope of this article, such treatment also would encompass physical therapy, occupational therapy, and psychotherapeutic interventions to augment rehabilitative efforts and the functional capabilities of patients who struggle with persisting pain.
Although the direct pain-mitigating effects of antidepressants are not fully understood, it is believed that augmentation of monoamine neurotransmission from supraspinal nuclei (ie, the RVM and DLPT) modulate pain transmission from the periphery.5,6 In addition, there is evidence that some effects of tricyclic antidepressants can modulate several other functions that impact peripheral and central sensitization.7-10
During the last several decades, antidepressants have been used to address—and have demonstrated clinical utility for—a variety of chronic pain states. However, antidepressants are not a panacea; some chronic pain conditions are more responsive to antidepressants than are others. The chronic painful states most amenable to antidepressants are those that result primarily from a process of neural sensitization, as opposed to acute somatic or visceral nociception. Hence, several meta-analyses and evidence-based reviews have long suggested the usefulness of antidepressants for mitigating pain associated with neuropathy,34,35 FM,36,37 headache,38 and IBS.39,40
Box 2
The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects (impacting neurotransmission of descending pathways independent of influences on mood) and indirect effects (presumably impacting cortical and limbic output to the periaqueductal gray area, the rostroventral medulla, and the dorsolateral pontomesencephalic tegmentum brought about by improvement in mood and/or cognitive appraisals) (Figure2,3,8,10,11,15,20,22,28,29). Support for the direct analgesic effects has been garnered from initial empirical work that demonstrated pain relief among patients with pain who are not depressed. Additionally, among patients who have depression and experience pain, analgesia reportedly often occurs within 2 weeks, which is before antidepressant effects are appreciated,11-15 and, at least for some antidepressants, occurs at doses far lower than those required to produce mood-elevating effects.11,12,16
On the other hand, it is well established that significant comorbidities exist between chronic pain states and psychiatric disorders (eg, depression and somatic symptom and related disorders).17-21 There may be common physiological substrates underlying chronic pain and depression.20,22 There are bidirectional influences of limbic (affective) systems and those CNS structures involved in pain processing and integration. The effects of pain and depression are reciprocal; the presence of one makes the management of the other more challenging.23-27 Mood disturbances can, therefore, impact pain processing by acting as affective and cognitive amplifiers of pain by leading to catastrophizing, pain severity augmentation, poor coping with pain-related stress, etc.28,29 It is plausible that the mood-elevating effects of antidepressants can improve pain by indirect effects, by modulating limbic activity, which in turn, impacts coping, cognitive appraisals of pain, etc.
Patients with somatoform disorders (using pre-DSM-5 terminology) frequently present with chronic pain, often in multiple sites.19 Such patients are characterized by hypervigilance for, and a predisposition to focus on, physical sensations and to appraise these sensations as reflecting a pathological state.30 Neuroimaging studies have begun to identify those neural circuits involved in somatoform disorders, many of which act as cognitive and affective amplifiers of visceral-somatic sensory processing. Many of these neural circuits overlap, and interact with, those involved in pain processing.31 Antidepressants can mitigate the severity of unexplained physical complaints, including pain, among patients who somatize32,33; however, due to the heterogeneity of studies upon which this claim is based, the quality of the evidence is reportedly low.33 There is uncertainty whether, or to what extent, antidepressant benefits among patients who somatize are due to a direct impact on pain modulation, or indirect effects on mood or cognitive appraisals/perceptions.
Despite the uncertainties about the exact mechanisms through which antidepressants exert analgesic effects, antidepressants can be appropriately used to treat patients with selected chronic pain syndromes, regardless of whether or not the patient has a psychiatric comorbidity. For those patients with pain and psychiatric comorbidities, the benefits may be brought about via direct mechanisms, indirect mechanisms, or a combination of both.

Continue to: Neuropathic pain
Neuropathic pain
Several treatment guidelines advocate for the use of antidepressants for neuropathic pain.41-44 For decades, TCAs have been employed off-label to successfully treat many patients with neuropathic pain states. Early investigations suggested that TCAs were robustly efficacious in managing patients with neuropathy.45-48 Calculated number-needed-to-treat (NNT) values for TCAs were quite low (ie, reflecting that few patients would need to be treated to yield a positive response in one patient compared with placebo), and were comparable to, if not slightly better than, the NNTs generated for anticonvulsants and α2-δ ligands, such as gabapentin or pregabalin.45-48
Unfortunately, early studies involving TCAs conducted many years ago do not meet contemporary standards of methodological rigor; they featured relatively small samples of patients assessed for brief post-treatment intervals with variable outcome measures. Thus, the NNT values obtained in meta-analyses based on these studies may overestimate treatment benefits.49 Further, NNT values derived from meta-analyses tended to combine all drugs within a particular antidepressant class (eg, amitriptyline, nortriptyline, desipramine, and imipramine among the TCAs) employed at diverse doses. Taken together, these limitations raise questions about the results of those meta-analyses.
Subsequent meta-analyses, which employed strict criteria to eliminate data from studies with potential sources of bias and used a primary outcome of frequencies of patients reporting at least 30% pain reduction compared with a placebo-controlled sample, suggest that the effectiveness of TCAs as a class for treating neuropathic pain is not as compelling as once was thought. Meta-analyses of studies employing specific TCAs revealed that there was little evidence to support the use of desipramine,50 imipramine,51 or nortriptyline52 in managing diabetic neuropathy or postherpetic neuralgia. Studies evaluating amitriptyline (dose range 12.5 to 150 mg/d), found low-level evidence of effectiveness; the benefit was expected to be present for a small subset (approximately 25%) of patients with neuropathic pain.53
There is moderate-quality evidence that duloxetine (60 to 120 mg/d) can produce a ≥50% improvement in pain severity ratings among patients with diabetic peripheral neuropathy.54 Although head-to-head studies with other antidepressants are limited, it appears that duloxetine and amitriptyline have comparable efficacy, even though the NNTs for amitriptyline were derived from lower-quality studies than those for duloxetine. Duloxetine is the only antidepressant to receive FDA approval for managing diabetic neuropathy. By contrast, studies assessing the utility of venlafaxine in neuropathic pain comprised small samples for brief durations, which limits the ability to draw clear (unbiased) support for its usefulness.55
Given the diversity of pathophysiologic processes underlying the disturbances that cause neuropathic pain disorders, it is unsurprising that the effectiveness of amitriptyline and duloxetine were not generalizable to all neuropathic pain states. Although amitriptyline produced pain-mitigating effects in patients with diabetic neuropathy and post-herpetic neuralgia, and duloxetine mitigated pain among patients with diabetic neuropathy, there was no evidence to suggest their effectiveness in phantom limb pain or human immunodeficiency virus-related and spinal cord-related neuropathies.35,53,54,56-58
Continue to: Fibromyalgia
Fibromyalgia
As with the issues encountered in interpreting the effectiveness of antidepressants in neuropathic pain, interpreting results gleaned from clinical trials of antidepressants for treating FM are fraught with similar difficulties. Although amitriptyline has been a first-line treatment for FM for many years, the evidence upon which such recommendations were based consisted of low-level studies that had a significant potential for bias.59 Large randomized trials would offer more compelling data regarding the efficacy of amitriptyline, but the prohibitive costs of such studies makes it unlikely they will be conducted. Amitriptyline (25 to 50 mg/d) was effective in mitigating FM-related pain in a small percentage of patients studied, with an estimated NNT of 4.59 Adverse effects, often contributing to treatment discontinuation, were encountered more frequently among patients who received amitriptyline compared with placebo.
Selective serotonin reuptake inhibitors failed to demonstrate significant pain relief (estimated NNT of 10), or improvement in fatigue or sleep problems, even though the studies upon which such conclusions were based were low-level studies with a high potential for bias.60 Although SSRIs have limited utility for mitigating pain, they are still quite useful for reducing depression among patients with FM.60
By contrast, the SNRIs duloxetine and milnacipran provided clinically relevant benefit over placebo in the frequency of patients reporting pain relief of ≥30%, as well as patients’ global impression of change.61 These agents, however, failed to provide clinically relevant benefit over placebo in improving health-related quality of life, reducing sleep problems, or improving fatigue. Nonetheless, duloxetine and milnacipran are FDA-approved for managing pain in FM. Studies assessing the efficacy of venlafaxine in the treatment of FM to date have been limited by small sample sizes, inconsistent dosing, lack of a placebo control, and lack of blinding, which limits the ability to clearly delineate the role of venlafaxine in managing FM.62
Mirtazapine (15 to 45 mg/d) showed a clinically relevant benefit compared with placebo for participant-reported pain relief of ≥30% and sleep disturbances. There was no benefit in terms of participant-reported improvement of quality of life, fatigue, or negative mood.63 The evidence was considered to be of low quality overall.
Headache
Amitriptyline has been employed off-label to address headache prophylaxis since 1964.64 Compared with placebo, it is efficacious in ameliorating migraine frequency and intensity as well as the frequency of tension headache.65,66 However, SSRIs and SNRIs (venlafaxine) failed to produce significant reductions in migraine frequency or severity or the frequencies of tension headache when compared with placebo.67,68
Continue to: Irritable bowel syndrome
Irritable bowel syndrome
Early studies addressing antidepressant efficacy in IBS reveal inconsistencies. For example, whereas some suggest that TCAs are effective in mitigating chronic, severe abdominal pain,39,40 others concluded that TCAs failed to demonstrate a significant analgesic benefit.69 A recent meta-analysis that restricted analysis of efficacy to randomized controlled trials (RCTs) with more rigorous methodological adherence found that pain relief in IBS is possible with both TCAs as well as SSRIs. However, adverse effects were more commonly encountered with TCAs than with SSRIs. Some of the inconsistencies in treatment efficacy reported in early studies may be due to variations in responsiveness of subsets of IBS patients. Specifically, the utility of TCAs appears to be best among patients with diarrheal-type (as opposed to constipation-type) IBS, presumably due to TCAs’ anticholinergic effects, whereas SSRIs may provide more of a benefit for patients with predominantly constipation-type IBS.40,70
Other chronic pain conditions
Antidepressants have been used to assist in the management of several other pain conditions, including oral-facial pain, interstitial cystitis, non-cardiac chest pain, and others. The role of antidepressants for such conditions remains unclear due to limitations in the prevailing empirical work, such as few trials, small sample sizes, variations in outcome measures, and insufficient randomization and blinding.71-76 The interpretation of results from systematic reviews and meta-analyses is limited because of these shortcomings.77 Hence, it has not always been possible to determine whether, and to what extent, patients with such conditions may benefit from antidepressants.
Neuromodulatory effects and efficacy for pain
The interplay of norepinephrine (NE) and serotonin (5-HT) neurotransmitter systems and cellular mechanisms involved in the descending modulation of pain pathways is complex. Experimental animal models of pain modulation suggest that 5-HT can both inhibit as well as promote pain perception by different physiological mechanisms, in contrast to NE, which is predominately inhibitory. While 5-HT in the descending modulating system can inhibit pain transmission ascending to the brain from the periphery, it appears that an intact noradrenergic system is necessary for the inhibitory influences of the serotonergic system to be appreciated.16,78,79 Deficiencies in one or both of these neurotransmitter systems may contribute to hyperactive pain processing, and thereby precipitate or maintain chronic pain.
Pain mitigation may be achieved best by enhancing both neurotransmitters simultaneously, less so by enhancing NE alone, and least by enhancing 5-HT alone.6 The ability to impact pain modulation would, therefore, depend on the degree to which an antidepressant capitalizes on both noradrenergic and serotonergic neurotransmission. Antidepressants commonly employed to manage pain are presented in Table 147,60,68,80-88 according to their primary neurotransmitter effects. Thus, the literature summarized above suggests that antidepressants that influence both NE and 5-HT transmission have greater analgesic effects than antidepressants with more specific effects, such as influencing 5-HT reuptake alone.80-85 It is unsurprising, therefore, that the SSRIs have not been demonstrated to be as consistently analgesic.47,60,68,80,86-88
Similarly, pharmacodynamic and pharmacokinetic differences within antidepressant classes may influence analgesic effectiveness. Simultaneous effects on NE and 5-HT are achieved at low doses with duloxetine and milnacipran. By contrast, 5-HT effects predominate at low doses for venlafaxine. To achieve pain-mitigating effects, higher doses of venlafaxine generally are required.89 Therefore, inconsistencies across studies regarding the analgesic benefits of venlafaxine may be attributable to variability in dosing; patients treated with lower doses may not have experienced sufficient NE effects to garner positive results.
Continue to: The differences in analgesic efficacy...
The differences in analgesic efficacy among specific TCAs may be understood in a similar fashion. Specifically, tertiary TCAs (imipramine and amitriptyline) inhibit both 5-HT and NE reuptake.6,90 Secondary amines (desipramine and nortriptyline) predominantly impact NE reuptake, possibly accounting for the lesser pain-mitigating benefit achieved with these agents, such as for treating neuropathic pain. Further, in vivo imipramine and amitriptyline are rapidly metabolized to secondary amines that are potent and selective NE reuptake inhibitors. In this way, the secondary amines may substantially lose the ability to modulate pain transmission because of the loss of concurrent 5-HT influences.90
Clinical pearls
The following practical points can help guide clinicians regarding the usefulness of antidepressants for pain management:
- Antidepressants can alleviate symptoms of depression and pain. The pain-mitigating effects of antidepressants are possible even among chronic pain patients who are not depressed. Antidepressants may confer benefits for chronic pain patients with depression and other comorbid conditions, such as somatic symptom and related disorders.
- Antidepressants are useful for select chronic pain states. Although the noradrenergic and serotonergic antidepressants (SNRIs and, to some extent, amitriptyline) appear to have efficacy for neuropathic pain and FM, the benefits of SSRIs appear to be less robust. On the other hand, SSRIs and TCAs may have potential benefit for patients with IBS. However, the results of meta-analyses are limited in the ability to provide information about which patients will best respond to which specific antidepressant or how well. Future research directed at identifying characteristics that can predict which patients are likely to benefit from one antidepressant vs another would help inform how best to tailor treatment to individual needs.
- The pain-mitigating effects of antidepressants often emerge early in the course of treatment (often before mood-elevating effects are observed). For example, in the case of amitriptyline, pain relief may be possible for some patients at doses generally lower than those required for mood-elevating effects. To date, there is limited information in the literature to determine what constitutes a sufficient duration of treatment, or when treatment should be modified.
- Failure to reduce pain should raise questions about whether the dose should be increased, an alternative agent should be tried, or combinations with other analgesic agents should be considered. Failure to achieve pain-mitigating effects with one antidepressant does not mean failure with others. Hence, failure to achieve desired effects with one agent might warrant an empirical trial with another agent. Presently, too few double-blind RCTs have been conducted to assess the pain-mitigating effects of other antidepressants (eg, bupropion and newer SNRIs such as desvenlafaxine and levomilnacipran). Meta-analysis of the analgesic effectiveness of these agents or comparisons to the efficacy of other antidepressant classes is, therefore, impossible at this time.
Because many chronic pain states are complex, patients will seldom experience clinically relevant benefit from any one intervention.53 The bigger implication for clinical research is to determine whether there is a sequence or combination of medication use that will provide overall better clinical effectiveness.53 Only limited data are available exploring the utility of combining pharmacologic approaches to address pain.91 For example, preliminary evidence suggests that combinations of complementary strategies, such as duloxetine combined with pregabalin, may result in significantly greater numbers of FM patients achieving ≥30% pain reduction compared with monotherapy with either agent alone or placebo.92
- Antidepressant selection may need to be based on medication-related adverse effect profiles and the potential for drug interactions. These factors are useful to consider in delineating multimodal treatment regimens for chronic pain in light of patients’ comorbidities and co-medication regimen. For example, the adverse effects of TCAs (anticholinergic and alpha-adrenergic influences) limit their utility for treating pain. Some of these effects can be more problematic in select populations, such as older adults or those with orthostatic difficulties, among others. TCAs are contraindicated in patients with closed-angle glaucoma, recent myocardial infarction, cardiac arrhythmias, poorly controlled seizures, or severe benign prostatic hypertrophy. Although the pain-mitigating effects of SNRIs have not been demonstrated to significantly exceed those of TCAs,68,93,94 SNRIs would offer an advantage of greater tolerability of adverse effects and relative safety in patients with comorbid medical conditions that would otherwise preclude TCA use. The adverse effects and common drug interactions associated with antidepressants are summarized in Table 295.
Conclusion
Chronic, nonmalignant pain conditions afflict many patients and significantly impair their ability to function. Because of heightened concerns related to the appropriateness of, and restricting inordinate access to, long-term opioid analgesics, clinicians need to explore the usefulness of co-analgesic agents, such as antidepressants. Significant comorbidities exist between psychiatric disorders and chronic pain, and psychiatrists are uniquely positioned to diagnose and treat psychiatric comorbidities, as well as pain, among their patients, especially since they understand the kinetics and dynamics of antidepressants.
Bottom Line
Antidepressants can alleviate symptoms of depression and pain. Noradrenergic and serotonergic antidepressants appear to have efficacy for pain associated with neuropathy and fibromyalgia, while selective serotonin reuptake inhibitors and tricyclic antidepressants may have benefit for patients with irritable bowel syndrome. However, evidence regarding which patients will best respond to which specific antidepressant is limited.
Continue to: Related Resources
Related Resources
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
- Maletic V, Demuri B. Chronic pain and depression: treatment of 2 culprits in common. Current Psychiatry. 2016;15(3):41,47-50,52.
Drug Brand Names
Amitriptyline • Elavil, Endep
Bupropion • Wellbutrin, Zyban
Carisoprodol • Rela, Soma
Cyclobenzaprine • Amrix, Flexeril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Horizant, Neurontin
Imipramine • Tofranil
Levomilnacipran • Fetzima
Methadone • Dolophine, Methadose
Milnacipran • Savella
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Pregabalin • Lyrica, Lyrica CR
Tapentadol • Nucynta
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Warfarin • Coumadin, Jantoven
Approximately 55 years ago, tricyclic antidepressants (TCAs) began to be used to treat neuropathic pain.1 Eventually, clinical trials emerged suggesting the utility of TCAs for other chronic pain conditions, such as fibromyalgia (FM) and migraine prophylaxis. However, despite TCAs’ effectiveness in mitigating painful conditions, their adverse effects limited their use.
Pharmacologic advancements have led to the development of other antidepressant classes, including selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), and the use of these agents has come to eclipse that of TCAs. In the realm of pain management, such developments have raised the hope of possible alternative co-analgesic agents that could avoid the adverse effects associated with TCAs. Some of these agents have demonstrated efficacy for managing chronic pain states, while others have demonstrated only limited utility.
This article provides a synopsis of systematic reviews and meta-analyses examining the role of antidepressant therapy for managing several chronic pain conditions, including pain associated with neuropathy, FM, headache, and irritable bowel syndrome (IBS). Because the literature base is rapidly evolving, it is necessary to revisit the information gleaned from clinical data with respect to treatment effectiveness, and to clarify how antidepressants might be positioned in the management of chronic pain.
The effectiveness of antidepressants for pain
The pathophysiologic processes that precipitate and maintain chronic pain conditions are complex (Box 12-10). The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects and indirect effects (Box 22,3,8,10,11-33).
Box 1
The pathophysiologic processes precipitating and maintaining chronic pain conditions are complex. Persistent and chronic pain results from changes in sensitivity within both ascending pathways (relaying pain information from the periphery to the spinal cord and brain) and descending pain pathways (functioning to modulate ascending pain information).2,3 Tissue damage or peripheral nerve injury can lead to a cascade of neuroplastic changes within the CNS, resulting in hyperexcitability within the ascending pain pathways.
The descending pain pathways consist of the midbrain periaqueductal gray area (PGA), the rostroventral medulla (RVM), and the dorsolateral pontomesencephalic tegmentum (DLPT). The axons of the RVM (the outflow of which is serotonergic) and DLPT (the outflow of which is noradrenergic) terminate in the dorsal horn of the spinal cord,4 and thereby dampen pain signals arising from the periphery. Diminished output from descending pain pathways can heighten the pain experience. Input from the cortex, hypothalamus, and amygdala (among other structures) converges upon the PGA, RVM and DLPT, and can influence the degree of pain modulation emerging from descending pathways. In this way, thoughts, appraisals, and mood are believed to comprise cognitive and affective modifiers of pain experiences.
Devising effective chronic pain treatment becomes challenging; multimodal treatment approaches often are advocated, including pharmacologic treatment with analgesics in combination with co-analgesic medications such as antidepressants. Although a description of multimodal treatment is beyond the scope of this article, such treatment also would encompass physical therapy, occupational therapy, and psychotherapeutic interventions to augment rehabilitative efforts and the functional capabilities of patients who struggle with persisting pain.
Although the direct pain-mitigating effects of antidepressants are not fully understood, it is believed that augmentation of monoamine neurotransmission from supraspinal nuclei (ie, the RVM and DLPT) modulate pain transmission from the periphery.5,6 In addition, there is evidence that some effects of tricyclic antidepressants can modulate several other functions that impact peripheral and central sensitization.7-10
During the last several decades, antidepressants have been used to address—and have demonstrated clinical utility for—a variety of chronic pain states. However, antidepressants are not a panacea; some chronic pain conditions are more responsive to antidepressants than are others. The chronic painful states most amenable to antidepressants are those that result primarily from a process of neural sensitization, as opposed to acute somatic or visceral nociception. Hence, several meta-analyses and evidence-based reviews have long suggested the usefulness of antidepressants for mitigating pain associated with neuropathy,34,35 FM,36,37 headache,38 and IBS.39,40
Box 2
The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects (impacting neurotransmission of descending pathways independent of influences on mood) and indirect effects (presumably impacting cortical and limbic output to the periaqueductal gray area, the rostroventral medulla, and the dorsolateral pontomesencephalic tegmentum brought about by improvement in mood and/or cognitive appraisals) (Figure2,3,8,10,11,15,20,22,28,29). Support for the direct analgesic effects has been garnered from initial empirical work that demonstrated pain relief among patients with pain who are not depressed. Additionally, among patients who have depression and experience pain, analgesia reportedly often occurs within 2 weeks, which is before antidepressant effects are appreciated,11-15 and, at least for some antidepressants, occurs at doses far lower than those required to produce mood-elevating effects.11,12,16
On the other hand, it is well established that significant comorbidities exist between chronic pain states and psychiatric disorders (eg, depression and somatic symptom and related disorders).17-21 There may be common physiological substrates underlying chronic pain and depression.20,22 There are bidirectional influences of limbic (affective) systems and those CNS structures involved in pain processing and integration. The effects of pain and depression are reciprocal; the presence of one makes the management of the other more challenging.23-27 Mood disturbances can, therefore, impact pain processing by acting as affective and cognitive amplifiers of pain by leading to catastrophizing, pain severity augmentation, poor coping with pain-related stress, etc.28,29 It is plausible that the mood-elevating effects of antidepressants can improve pain by indirect effects, by modulating limbic activity, which in turn, impacts coping, cognitive appraisals of pain, etc.
Patients with somatoform disorders (using pre-DSM-5 terminology) frequently present with chronic pain, often in multiple sites.19 Such patients are characterized by hypervigilance for, and a predisposition to focus on, physical sensations and to appraise these sensations as reflecting a pathological state.30 Neuroimaging studies have begun to identify those neural circuits involved in somatoform disorders, many of which act as cognitive and affective amplifiers of visceral-somatic sensory processing. Many of these neural circuits overlap, and interact with, those involved in pain processing.31 Antidepressants can mitigate the severity of unexplained physical complaints, including pain, among patients who somatize32,33; however, due to the heterogeneity of studies upon which this claim is based, the quality of the evidence is reportedly low.33 There is uncertainty whether, or to what extent, antidepressant benefits among patients who somatize are due to a direct impact on pain modulation, or indirect effects on mood or cognitive appraisals/perceptions.
Despite the uncertainties about the exact mechanisms through which antidepressants exert analgesic effects, antidepressants can be appropriately used to treat patients with selected chronic pain syndromes, regardless of whether or not the patient has a psychiatric comorbidity. For those patients with pain and psychiatric comorbidities, the benefits may be brought about via direct mechanisms, indirect mechanisms, or a combination of both.

Continue to: Neuropathic pain
Neuropathic pain
Several treatment guidelines advocate for the use of antidepressants for neuropathic pain.41-44 For decades, TCAs have been employed off-label to successfully treat many patients with neuropathic pain states. Early investigations suggested that TCAs were robustly efficacious in managing patients with neuropathy.45-48 Calculated number-needed-to-treat (NNT) values for TCAs were quite low (ie, reflecting that few patients would need to be treated to yield a positive response in one patient compared with placebo), and were comparable to, if not slightly better than, the NNTs generated for anticonvulsants and α2-δ ligands, such as gabapentin or pregabalin.45-48
Unfortunately, early studies involving TCAs conducted many years ago do not meet contemporary standards of methodological rigor; they featured relatively small samples of patients assessed for brief post-treatment intervals with variable outcome measures. Thus, the NNT values obtained in meta-analyses based on these studies may overestimate treatment benefits.49 Further, NNT values derived from meta-analyses tended to combine all drugs within a particular antidepressant class (eg, amitriptyline, nortriptyline, desipramine, and imipramine among the TCAs) employed at diverse doses. Taken together, these limitations raise questions about the results of those meta-analyses.
Subsequent meta-analyses, which employed strict criteria to eliminate data from studies with potential sources of bias and used a primary outcome of frequencies of patients reporting at least 30% pain reduction compared with a placebo-controlled sample, suggest that the effectiveness of TCAs as a class for treating neuropathic pain is not as compelling as once was thought. Meta-analyses of studies employing specific TCAs revealed that there was little evidence to support the use of desipramine,50 imipramine,51 or nortriptyline52 in managing diabetic neuropathy or postherpetic neuralgia. Studies evaluating amitriptyline (dose range 12.5 to 150 mg/d), found low-level evidence of effectiveness; the benefit was expected to be present for a small subset (approximately 25%) of patients with neuropathic pain.53
There is moderate-quality evidence that duloxetine (60 to 120 mg/d) can produce a ≥50% improvement in pain severity ratings among patients with diabetic peripheral neuropathy.54 Although head-to-head studies with other antidepressants are limited, it appears that duloxetine and amitriptyline have comparable efficacy, even though the NNTs for amitriptyline were derived from lower-quality studies than those for duloxetine. Duloxetine is the only antidepressant to receive FDA approval for managing diabetic neuropathy. By contrast, studies assessing the utility of venlafaxine in neuropathic pain comprised small samples for brief durations, which limits the ability to draw clear (unbiased) support for its usefulness.55
Given the diversity of pathophysiologic processes underlying the disturbances that cause neuropathic pain disorders, it is unsurprising that the effectiveness of amitriptyline and duloxetine were not generalizable to all neuropathic pain states. Although amitriptyline produced pain-mitigating effects in patients with diabetic neuropathy and post-herpetic neuralgia, and duloxetine mitigated pain among patients with diabetic neuropathy, there was no evidence to suggest their effectiveness in phantom limb pain or human immunodeficiency virus-related and spinal cord-related neuropathies.35,53,54,56-58
Continue to: Fibromyalgia
Fibromyalgia
As with the issues encountered in interpreting the effectiveness of antidepressants in neuropathic pain, interpreting results gleaned from clinical trials of antidepressants for treating FM are fraught with similar difficulties. Although amitriptyline has been a first-line treatment for FM for many years, the evidence upon which such recommendations were based consisted of low-level studies that had a significant potential for bias.59 Large randomized trials would offer more compelling data regarding the efficacy of amitriptyline, but the prohibitive costs of such studies makes it unlikely they will be conducted. Amitriptyline (25 to 50 mg/d) was effective in mitigating FM-related pain in a small percentage of patients studied, with an estimated NNT of 4.59 Adverse effects, often contributing to treatment discontinuation, were encountered more frequently among patients who received amitriptyline compared with placebo.
Selective serotonin reuptake inhibitors failed to demonstrate significant pain relief (estimated NNT of 10), or improvement in fatigue or sleep problems, even though the studies upon which such conclusions were based were low-level studies with a high potential for bias.60 Although SSRIs have limited utility for mitigating pain, they are still quite useful for reducing depression among patients with FM.60
By contrast, the SNRIs duloxetine and milnacipran provided clinically relevant benefit over placebo in the frequency of patients reporting pain relief of ≥30%, as well as patients’ global impression of change.61 These agents, however, failed to provide clinically relevant benefit over placebo in improving health-related quality of life, reducing sleep problems, or improving fatigue. Nonetheless, duloxetine and milnacipran are FDA-approved for managing pain in FM. Studies assessing the efficacy of venlafaxine in the treatment of FM to date have been limited by small sample sizes, inconsistent dosing, lack of a placebo control, and lack of blinding, which limits the ability to clearly delineate the role of venlafaxine in managing FM.62
Mirtazapine (15 to 45 mg/d) showed a clinically relevant benefit compared with placebo for participant-reported pain relief of ≥30% and sleep disturbances. There was no benefit in terms of participant-reported improvement of quality of life, fatigue, or negative mood.63 The evidence was considered to be of low quality overall.
Headache
Amitriptyline has been employed off-label to address headache prophylaxis since 1964.64 Compared with placebo, it is efficacious in ameliorating migraine frequency and intensity as well as the frequency of tension headache.65,66 However, SSRIs and SNRIs (venlafaxine) failed to produce significant reductions in migraine frequency or severity or the frequencies of tension headache when compared with placebo.67,68
Continue to: Irritable bowel syndrome
Irritable bowel syndrome
Early studies addressing antidepressant efficacy in IBS reveal inconsistencies. For example, whereas some suggest that TCAs are effective in mitigating chronic, severe abdominal pain,39,40 others concluded that TCAs failed to demonstrate a significant analgesic benefit.69 A recent meta-analysis that restricted analysis of efficacy to randomized controlled trials (RCTs) with more rigorous methodological adherence found that pain relief in IBS is possible with both TCAs as well as SSRIs. However, adverse effects were more commonly encountered with TCAs than with SSRIs. Some of the inconsistencies in treatment efficacy reported in early studies may be due to variations in responsiveness of subsets of IBS patients. Specifically, the utility of TCAs appears to be best among patients with diarrheal-type (as opposed to constipation-type) IBS, presumably due to TCAs’ anticholinergic effects, whereas SSRIs may provide more of a benefit for patients with predominantly constipation-type IBS.40,70
Other chronic pain conditions
Antidepressants have been used to assist in the management of several other pain conditions, including oral-facial pain, interstitial cystitis, non-cardiac chest pain, and others. The role of antidepressants for such conditions remains unclear due to limitations in the prevailing empirical work, such as few trials, small sample sizes, variations in outcome measures, and insufficient randomization and blinding.71-76 The interpretation of results from systematic reviews and meta-analyses is limited because of these shortcomings.77 Hence, it has not always been possible to determine whether, and to what extent, patients with such conditions may benefit from antidepressants.
Neuromodulatory effects and efficacy for pain
The interplay of norepinephrine (NE) and serotonin (5-HT) neurotransmitter systems and cellular mechanisms involved in the descending modulation of pain pathways is complex. Experimental animal models of pain modulation suggest that 5-HT can both inhibit as well as promote pain perception by different physiological mechanisms, in contrast to NE, which is predominately inhibitory. While 5-HT in the descending modulating system can inhibit pain transmission ascending to the brain from the periphery, it appears that an intact noradrenergic system is necessary for the inhibitory influences of the serotonergic system to be appreciated.16,78,79 Deficiencies in one or both of these neurotransmitter systems may contribute to hyperactive pain processing, and thereby precipitate or maintain chronic pain.
Pain mitigation may be achieved best by enhancing both neurotransmitters simultaneously, less so by enhancing NE alone, and least by enhancing 5-HT alone.6 The ability to impact pain modulation would, therefore, depend on the degree to which an antidepressant capitalizes on both noradrenergic and serotonergic neurotransmission. Antidepressants commonly employed to manage pain are presented in Table 147,60,68,80-88 according to their primary neurotransmitter effects. Thus, the literature summarized above suggests that antidepressants that influence both NE and 5-HT transmission have greater analgesic effects than antidepressants with more specific effects, such as influencing 5-HT reuptake alone.80-85 It is unsurprising, therefore, that the SSRIs have not been demonstrated to be as consistently analgesic.47,60,68,80,86-88
Similarly, pharmacodynamic and pharmacokinetic differences within antidepressant classes may influence analgesic effectiveness. Simultaneous effects on NE and 5-HT are achieved at low doses with duloxetine and milnacipran. By contrast, 5-HT effects predominate at low doses for venlafaxine. To achieve pain-mitigating effects, higher doses of venlafaxine generally are required.89 Therefore, inconsistencies across studies regarding the analgesic benefits of venlafaxine may be attributable to variability in dosing; patients treated with lower doses may not have experienced sufficient NE effects to garner positive results.
Continue to: The differences in analgesic efficacy...
The differences in analgesic efficacy among specific TCAs may be understood in a similar fashion. Specifically, tertiary TCAs (imipramine and amitriptyline) inhibit both 5-HT and NE reuptake.6,90 Secondary amines (desipramine and nortriptyline) predominantly impact NE reuptake, possibly accounting for the lesser pain-mitigating benefit achieved with these agents, such as for treating neuropathic pain. Further, in vivo imipramine and amitriptyline are rapidly metabolized to secondary amines that are potent and selective NE reuptake inhibitors. In this way, the secondary amines may substantially lose the ability to modulate pain transmission because of the loss of concurrent 5-HT influences.90
Clinical pearls
The following practical points can help guide clinicians regarding the usefulness of antidepressants for pain management:
- Antidepressants can alleviate symptoms of depression and pain. The pain-mitigating effects of antidepressants are possible even among chronic pain patients who are not depressed. Antidepressants may confer benefits for chronic pain patients with depression and other comorbid conditions, such as somatic symptom and related disorders.
- Antidepressants are useful for select chronic pain states. Although the noradrenergic and serotonergic antidepressants (SNRIs and, to some extent, amitriptyline) appear to have efficacy for neuropathic pain and FM, the benefits of SSRIs appear to be less robust. On the other hand, SSRIs and TCAs may have potential benefit for patients with IBS. However, the results of meta-analyses are limited in the ability to provide information about which patients will best respond to which specific antidepressant or how well. Future research directed at identifying characteristics that can predict which patients are likely to benefit from one antidepressant vs another would help inform how best to tailor treatment to individual needs.
- The pain-mitigating effects of antidepressants often emerge early in the course of treatment (often before mood-elevating effects are observed). For example, in the case of amitriptyline, pain relief may be possible for some patients at doses generally lower than those required for mood-elevating effects. To date, there is limited information in the literature to determine what constitutes a sufficient duration of treatment, or when treatment should be modified.
- Failure to reduce pain should raise questions about whether the dose should be increased, an alternative agent should be tried, or combinations with other analgesic agents should be considered. Failure to achieve pain-mitigating effects with one antidepressant does not mean failure with others. Hence, failure to achieve desired effects with one agent might warrant an empirical trial with another agent. Presently, too few double-blind RCTs have been conducted to assess the pain-mitigating effects of other antidepressants (eg, bupropion and newer SNRIs such as desvenlafaxine and levomilnacipran). Meta-analysis of the analgesic effectiveness of these agents or comparisons to the efficacy of other antidepressant classes is, therefore, impossible at this time.
Because many chronic pain states are complex, patients will seldom experience clinically relevant benefit from any one intervention.53 The bigger implication for clinical research is to determine whether there is a sequence or combination of medication use that will provide overall better clinical effectiveness.53 Only limited data are available exploring the utility of combining pharmacologic approaches to address pain.91 For example, preliminary evidence suggests that combinations of complementary strategies, such as duloxetine combined with pregabalin, may result in significantly greater numbers of FM patients achieving ≥30% pain reduction compared with monotherapy with either agent alone or placebo.92
- Antidepressant selection may need to be based on medication-related adverse effect profiles and the potential for drug interactions. These factors are useful to consider in delineating multimodal treatment regimens for chronic pain in light of patients’ comorbidities and co-medication regimen. For example, the adverse effects of TCAs (anticholinergic and alpha-adrenergic influences) limit their utility for treating pain. Some of these effects can be more problematic in select populations, such as older adults or those with orthostatic difficulties, among others. TCAs are contraindicated in patients with closed-angle glaucoma, recent myocardial infarction, cardiac arrhythmias, poorly controlled seizures, or severe benign prostatic hypertrophy. Although the pain-mitigating effects of SNRIs have not been demonstrated to significantly exceed those of TCAs,68,93,94 SNRIs would offer an advantage of greater tolerability of adverse effects and relative safety in patients with comorbid medical conditions that would otherwise preclude TCA use. The adverse effects and common drug interactions associated with antidepressants are summarized in Table 295.
Conclusion
Chronic, nonmalignant pain conditions afflict many patients and significantly impair their ability to function. Because of heightened concerns related to the appropriateness of, and restricting inordinate access to, long-term opioid analgesics, clinicians need to explore the usefulness of co-analgesic agents, such as antidepressants. Significant comorbidities exist between psychiatric disorders and chronic pain, and psychiatrists are uniquely positioned to diagnose and treat psychiatric comorbidities, as well as pain, among their patients, especially since they understand the kinetics and dynamics of antidepressants.
Bottom Line
Antidepressants can alleviate symptoms of depression and pain. Noradrenergic and serotonergic antidepressants appear to have efficacy for pain associated with neuropathy and fibromyalgia, while selective serotonin reuptake inhibitors and tricyclic antidepressants may have benefit for patients with irritable bowel syndrome. However, evidence regarding which patients will best respond to which specific antidepressant is limited.
Continue to: Related Resources
Related Resources
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
- Maletic V, Demuri B. Chronic pain and depression: treatment of 2 culprits in common. Current Psychiatry. 2016;15(3):41,47-50,52.
Drug Brand Names
Amitriptyline • Elavil, Endep
Bupropion • Wellbutrin, Zyban
Carisoprodol • Rela, Soma
Cyclobenzaprine • Amrix, Flexeril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Horizant, Neurontin
Imipramine • Tofranil
Levomilnacipran • Fetzima
Methadone • Dolophine, Methadose
Milnacipran • Savella
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Pregabalin • Lyrica, Lyrica CR
Tapentadol • Nucynta
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Warfarin • Coumadin, Jantoven
1. Paoli F, Darcourt G, Cossa P. Preliminary note on the action of imipramine in painful states [in French]. Rev Neurol (Paris). 1960;102:503-504.
2. Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219-245.
3. Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2(2):83-91.
4. Lamont LA, Tranquilli WJ, Grimm KA. Physiology of pain. Vet Clin North Am Small Anim Pract. 2000;30(4):703-728, v.
5. Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, eds. Wall and Melzack’s Textbook of Pain. 5th ed. Burlington, MA: Elsevier Health Sciences; 2005:125-142.
6. Marks DM, Shah MJ, Patkar AA, et al. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol. 2009;7(4):331-336.
7. Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92(2):473-484.
8. Carter GT, Sullivan MD. Antidepressants in pain management. Curr Opin Investig Drugs. 2002;3(3):454-458.
9. Kawasaki Y, Kumamoto E, Furue H, et al. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98(3):682-689.
10. McCleane G. Antidepressants as analgesics. CNS Drugs. 2008;22(2):139-156.
11. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry. 2000;7(5):257-277.
12. Egbunike IG, Chaffee BJ. Antidepressants in the management of chronic pain syndromes. Pharmacotherapy. 1990;10(4):262-270.
13. Fishbain DA. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32(5):305-316.
14. Fishbain DA, Detke MJ, Wernicke J, et al. The relationship between antidepressant and analgesic responses: findings from six placebo-controlled trials assessing the efficacy of duloxetine in patients with major depressive disorder. Curr Med Res Opin. 2008;24(11):3105-3115.
15. Harada E, Tokuoka H, Fujikoshi S, et al. Is duloxetine’s effect on painful physical symptoms in depression an indirect result of improvement of depressive symptoms? Pooled analyses of three randomized controlled trials. Pain. 2016;157(3):577-584.
16. Kehoe WA. Antidepressants for chronic pain: selection and dosing considerations. Am J Pain Med. 1993;3(4):161-165.
17. Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(2):1070-1078.
18. DeVeaugh-Geiss AM, West SL, Miller WC, et al. The adverse effects of comorbid pain on depression outcomes in primary care patients: results from the ARTIST trial. Pain Medicine. 2010;11(5):732-741.
19. Egloff N, Cámara RJ, von Känel R, et al. Hypersensitivity and hyperalgesia in somatoform pain disorders. Gen Hosp Psychiatry. 2014;36(3):284-290.
20. Goesling J, Clauw DW, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psych Reports. 2013;15(12):421.
21. Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-Month longitudinal analysis in primary care. J Pain. 2011;12(9):964-973.
22. Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7(5):403-412.
23. Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17-22.
24. Karp JF, Scott J, Houck P, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591-597.
25. Kroenke K, Shen J, Oxman TE, et al. Impact of pain on the outcomes of depression treatment: results from the RESPECT trial. Pain. 2008;134(1-2):209-215.
26. Mavandadi S, Ten Have TR, Katz IR, et al. Effect of depression treatment on depressive symptoms in older adulthood: the moderating role of pain. J Am Geriatr Soc. 2007;55(2):202-211.
27. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15(8):699-707.
28. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262-268.
29. Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Comorbid painful physical symptoms and depression: Prevalence, work loss, and help seeking. J Affect Disord. 2006;92(2-3):185-193.
30. Nakao M, Barsky AJ. Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosoc Med. 2007;1:17.
31. Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015;27(1):e40-e50.
32. Fishbain DA, Cutler RB, Rosomoff HL, et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom Med. 1998;60(4):503-509.
33. Kleinstäuber M, Witthöft M, Steffanowski A, et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev. 2014;(11):CD010628.
34. Collins SL, Moore RA, McQuay HJ, et al. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20(6):449-458.
35. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry. 2010;81(12):1372-1373.
36. Arnold LM, Keck PE, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41(2):104-113.
37. O’Malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15(9):659-666.
38. Tomkins GE, Jackson JL, O’Malley PG, et al. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med. 2001;111(1):54-63.
39. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108(1):65-72.
40. Lesbros-Pantoflickova D, Michetti P, Fried M et al. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(11-12):1253-1269.
41. Centre for Clinical Practice at NICE (UK). Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. London, UK: National Institute for Health and Care Excellence, (UK); 2013.
42. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(suppl 10):S22-S32.
43. Moulin D, Boulanger A, Clark AJ, et al; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-35.
44. Mu A, Weinberg E, Moulin DE, et al. Pharmacologic management of chronic neuropathic pain: Review of the Canadian Pain Society consensus statement. Can Fam Physician. 2017;63(11):844-852.
45. Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289-305.
46. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164.
47. Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83(3):389-400.
48. Wu CL, Raja SN. An update on the treatment of postherpetic neuralgia. J Pain. 2008;9(suppl 1):S19-S30.
49. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
50. Hearn L, Moore RA, Derry S, et al. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(9):CD011003.
51. Hearn L, Derry S, Phillips T, et al. Imipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(5):CD010769.
52. Derry S, Wiffen PJ, Aldington D, et al. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209.
53. Moore R, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(7):CD008242.
54. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
55. Gallagher HC, Gallagher RM, Butler M, et al. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(8):CD011091.
56. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;10:CD006380.
57. Dinat N, Marinda E, Moch S, et al. Randomized, Double-Blind, Crossover Trial of Amitriptyline for Analgesia in Painful HIV-Associated Sensory Neuropathy. PLoS One. 2015;10(5):e0126297. doi: 10.1371/journal.pone.0126297.eCollection 2015.
58. Mehta S, McIntyre A, Janzen S, et al; Spinal Cord Injury Rehabilitation Evidence Team. Systematic review of pharmacologic treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97(8):1381-1391.e1.
59. Moore RA, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;(12):CD008242..
60. Walitt B, Urrútia G, Nishishinya MB, et al. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
61. Welsch P, Üçeyler N, Klose P, et al. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst Rev. 2018;(2):CD010292.
62. VanderWeide LA, Smith SM, Trinkley KE. A systematic review of the efficacy of venlafaxine for the treatment of fibromyalgia. J Clin Pharm Ther. 2015;40(1):1-6.
63. Welsch P, Bernardy K, Derry S, et al. Mirtazapine for fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(8):CD012708.
64. Lance JW, Curran DA. Treatment of chronic tension headache. Lancet. 1964;283(7345):1236-1239.
65. Jackson JL, William S, Laura S, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222. doi: https://doi.org/10.1136/bmj.c5222
66. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine. 2017;96(22):e6989. doi: 10.1097/MD.0000000000006989.
67. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database Syst Rev. 2015;(4):CD002919.
68. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst Rev. 2015;(5):CD011681.
69. Quartero AO, Meineche-Schmidt V, Muris J, et al. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;(2):CD003460.
70. Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367-378.
71. Coss-Adame E, Erdogan A, Rao SS. Treatment of esophageal (noncardiac) chest pain: an expert review. Clin Gastroenterol Hepatol. 2014;12(8):1224-1245.
72. Kelada E, Jones A. Interstitial cystitis. Arch Gynecol Obstet. 2007;275(4):223-229.
73. Leo RJ, Dewani S. A systematic review of the utility of antidepressant pharmacotherapy in the treatment of vulvodynia pain. J Sex Med. 2013;10(10):2497-2505.
74. McMillan R, Forssell H, Buchanan JA, et al. Interventions for treating burning mouth syndrome. Cochrane Database Syst Rev. 2016;11:CD002779.
75. Patel DN. Inconclusive results of a systematic review of efficacy of antidepressants on orofacial pain disorders. Evid Based Dent. 2013;14(2):55-56.
76. Wang W, Sun YH, Wang YY, et al. Treatment of functional chest pain with antidepressants: a meta-analysis. Pain Physician. 2012;15(2):E131-E142.
77. Lavis JN. How can we support the use of systematic reviews in policymaking? PLoS Med. 2009;6(11):e1000141. doi: 10.1371/journal.pmed.1000141.
78. Sorkin L. Nociceptive transmission within the spinal cord. Mt Sinai J Med. 1991;58(3):208-216.
79. Yokogawa F, Kiuchi Y, Ishikawa Y, et al. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg. 2002;95(1):163-168, table of contents.
80. Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26(1):30-36.
81. Max MB. Treatment of post-herpetic neuralgia: antidepressants. Ann Neurol. 1994;35(suppl):S50-S53.
82. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
83. McQuay HJ, Tramèr M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68(2-3):217-227.
84. Mochizucki D. Serotonin and noradrenaline reuptake inhibitors in animal models of pain. Hum Psychopharmacol Clin Exp. 2004;19(suppl 1):15-19.
85. Sussman N. SNRIs versus SSRIs: mechanisms of action in treating depression and painful physical symptoms. Primary Care Companion J Clin Psychiatry. 2003;5(suppl 7):19-26.
86. Bundeff AW, Woodis CB. Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann Pharmacother. 2014;48(6):777-784.
87. Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12(6):384-389.
88. Xie C, Tang Y, Wang Y, et al. Efficacy and safety of antidepressants for the treatment of irritable bowel syndrome: a meta-analysis. PLoS One. 2015;10(8):e0127815. doi: 10.1371/journal.pone.0127815. eCollection 2015.
89. Zijlstra TR , Barendregt PJ , van de Laar MA. Venlafaxine in fibromyalgia: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2002;46(suppl 9):S105.
90. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25(6):871-880.
91. Thorpe J, Shum B, Moore RA, et al. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(2):CD010585.
92. Gilron I, Chaparro LE, Tu D, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain. 2016;157(7):1532-1540.
93. Häuser W, Petzke F, Üçeyler N, et al. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis. Rheumatology (Oxford). 2011;50(3):532-543.
94. Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy: a systematic review. Clin J Pain. 2016;32(11):1005-1010.
95. Riediger C, Schuster T, Barlinn K, et al. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol. 2017;8:307.
1. Paoli F, Darcourt G, Cossa P. Preliminary note on the action of imipramine in painful states [in French]. Rev Neurol (Paris). 1960;102:503-504.
2. Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219-245.
3. Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2(2):83-91.
4. Lamont LA, Tranquilli WJ, Grimm KA. Physiology of pain. Vet Clin North Am Small Anim Pract. 2000;30(4):703-728, v.
5. Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, eds. Wall and Melzack’s Textbook of Pain. 5th ed. Burlington, MA: Elsevier Health Sciences; 2005:125-142.
6. Marks DM, Shah MJ, Patkar AA, et al. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol. 2009;7(4):331-336.
7. Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92(2):473-484.
8. Carter GT, Sullivan MD. Antidepressants in pain management. Curr Opin Investig Drugs. 2002;3(3):454-458.
9. Kawasaki Y, Kumamoto E, Furue H, et al. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98(3):682-689.
10. McCleane G. Antidepressants as analgesics. CNS Drugs. 2008;22(2):139-156.
11. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry. 2000;7(5):257-277.
12. Egbunike IG, Chaffee BJ. Antidepressants in the management of chronic pain syndromes. Pharmacotherapy. 1990;10(4):262-270.
13. Fishbain DA. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32(5):305-316.
14. Fishbain DA, Detke MJ, Wernicke J, et al. The relationship between antidepressant and analgesic responses: findings from six placebo-controlled trials assessing the efficacy of duloxetine in patients with major depressive disorder. Curr Med Res Opin. 2008;24(11):3105-3115.
15. Harada E, Tokuoka H, Fujikoshi S, et al. Is duloxetine’s effect on painful physical symptoms in depression an indirect result of improvement of depressive symptoms? Pooled analyses of three randomized controlled trials. Pain. 2016;157(3):577-584.
16. Kehoe WA. Antidepressants for chronic pain: selection and dosing considerations. Am J Pain Med. 1993;3(4):161-165.
17. Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(2):1070-1078.
18. DeVeaugh-Geiss AM, West SL, Miller WC, et al. The adverse effects of comorbid pain on depression outcomes in primary care patients: results from the ARTIST trial. Pain Medicine. 2010;11(5):732-741.
19. Egloff N, Cámara RJ, von Känel R, et al. Hypersensitivity and hyperalgesia in somatoform pain disorders. Gen Hosp Psychiatry. 2014;36(3):284-290.
20. Goesling J, Clauw DW, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psych Reports. 2013;15(12):421.
21. Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-Month longitudinal analysis in primary care. J Pain. 2011;12(9):964-973.
22. Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7(5):403-412.
23. Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17-22.
24. Karp JF, Scott J, Houck P, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591-597.
25. Kroenke K, Shen J, Oxman TE, et al. Impact of pain on the outcomes of depression treatment: results from the RESPECT trial. Pain. 2008;134(1-2):209-215.
26. Mavandadi S, Ten Have TR, Katz IR, et al. Effect of depression treatment on depressive symptoms in older adulthood: the moderating role of pain. J Am Geriatr Soc. 2007;55(2):202-211.
27. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15(8):699-707.
28. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262-268.
29. Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Comorbid painful physical symptoms and depression: Prevalence, work loss, and help seeking. J Affect Disord. 2006;92(2-3):185-193.
30. Nakao M, Barsky AJ. Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosoc Med. 2007;1:17.
31. Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015;27(1):e40-e50.
32. Fishbain DA, Cutler RB, Rosomoff HL, et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom Med. 1998;60(4):503-509.
33. Kleinstäuber M, Witthöft M, Steffanowski A, et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev. 2014;(11):CD010628.
34. Collins SL, Moore RA, McQuay HJ, et al. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20(6):449-458.
35. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry. 2010;81(12):1372-1373.
36. Arnold LM, Keck PE, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41(2):104-113.
37. O’Malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15(9):659-666.
38. Tomkins GE, Jackson JL, O’Malley PG, et al. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med. 2001;111(1):54-63.
39. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108(1):65-72.
40. Lesbros-Pantoflickova D, Michetti P, Fried M et al. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(11-12):1253-1269.
41. Centre for Clinical Practice at NICE (UK). Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. London, UK: National Institute for Health and Care Excellence, (UK); 2013.
42. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(suppl 10):S22-S32.
43. Moulin D, Boulanger A, Clark AJ, et al; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-35.
44. Mu A, Weinberg E, Moulin DE, et al. Pharmacologic management of chronic neuropathic pain: Review of the Canadian Pain Society consensus statement. Can Fam Physician. 2017;63(11):844-852.
45. Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289-305.
46. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164.
47. Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83(3):389-400.
48. Wu CL, Raja SN. An update on the treatment of postherpetic neuralgia. J Pain. 2008;9(suppl 1):S19-S30.
49. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
50. Hearn L, Moore RA, Derry S, et al. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(9):CD011003.
51. Hearn L, Derry S, Phillips T, et al. Imipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(5):CD010769.
52. Derry S, Wiffen PJ, Aldington D, et al. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209.
53. Moore R, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(7):CD008242.
54. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
55. Gallagher HC, Gallagher RM, Butler M, et al. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(8):CD011091.
56. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;10:CD006380.
57. Dinat N, Marinda E, Moch S, et al. Randomized, Double-Blind, Crossover Trial of Amitriptyline for Analgesia in Painful HIV-Associated Sensory Neuropathy. PLoS One. 2015;10(5):e0126297. doi: 10.1371/journal.pone.0126297.eCollection 2015.
58. Mehta S, McIntyre A, Janzen S, et al; Spinal Cord Injury Rehabilitation Evidence Team. Systematic review of pharmacologic treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97(8):1381-1391.e1.
59. Moore RA, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;(12):CD008242..
60. Walitt B, Urrútia G, Nishishinya MB, et al. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
61. Welsch P, Üçeyler N, Klose P, et al. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst Rev. 2018;(2):CD010292.
62. VanderWeide LA, Smith SM, Trinkley KE. A systematic review of the efficacy of venlafaxine for the treatment of fibromyalgia. J Clin Pharm Ther. 2015;40(1):1-6.
63. Welsch P, Bernardy K, Derry S, et al. Mirtazapine for fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(8):CD012708.
64. Lance JW, Curran DA. Treatment of chronic tension headache. Lancet. 1964;283(7345):1236-1239.
65. Jackson JL, William S, Laura S, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222. doi: https://doi.org/10.1136/bmj.c5222
66. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine. 2017;96(22):e6989. doi: 10.1097/MD.0000000000006989.
67. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database Syst Rev. 2015;(4):CD002919.
68. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst Rev. 2015;(5):CD011681.
69. Quartero AO, Meineche-Schmidt V, Muris J, et al. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;(2):CD003460.
70. Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367-378.
71. Coss-Adame E, Erdogan A, Rao SS. Treatment of esophageal (noncardiac) chest pain: an expert review. Clin Gastroenterol Hepatol. 2014;12(8):1224-1245.
72. Kelada E, Jones A. Interstitial cystitis. Arch Gynecol Obstet. 2007;275(4):223-229.
73. Leo RJ, Dewani S. A systematic review of the utility of antidepressant pharmacotherapy in the treatment of vulvodynia pain. J Sex Med. 2013;10(10):2497-2505.
74. McMillan R, Forssell H, Buchanan JA, et al. Interventions for treating burning mouth syndrome. Cochrane Database Syst Rev. 2016;11:CD002779.
75. Patel DN. Inconclusive results of a systematic review of efficacy of antidepressants on orofacial pain disorders. Evid Based Dent. 2013;14(2):55-56.
76. Wang W, Sun YH, Wang YY, et al. Treatment of functional chest pain with antidepressants: a meta-analysis. Pain Physician. 2012;15(2):E131-E142.
77. Lavis JN. How can we support the use of systematic reviews in policymaking? PLoS Med. 2009;6(11):e1000141. doi: 10.1371/journal.pmed.1000141.
78. Sorkin L. Nociceptive transmission within the spinal cord. Mt Sinai J Med. 1991;58(3):208-216.
79. Yokogawa F, Kiuchi Y, Ishikawa Y, et al. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg. 2002;95(1):163-168, table of contents.
80. Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26(1):30-36.
81. Max MB. Treatment of post-herpetic neuralgia: antidepressants. Ann Neurol. 1994;35(suppl):S50-S53.
82. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
83. McQuay HJ, Tramèr M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68(2-3):217-227.
84. Mochizucki D. Serotonin and noradrenaline reuptake inhibitors in animal models of pain. Hum Psychopharmacol Clin Exp. 2004;19(suppl 1):15-19.
85. Sussman N. SNRIs versus SSRIs: mechanisms of action in treating depression and painful physical symptoms. Primary Care Companion J Clin Psychiatry. 2003;5(suppl 7):19-26.
86. Bundeff AW, Woodis CB. Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann Pharmacother. 2014;48(6):777-784.
87. Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12(6):384-389.
88. Xie C, Tang Y, Wang Y, et al. Efficacy and safety of antidepressants for the treatment of irritable bowel syndrome: a meta-analysis. PLoS One. 2015;10(8):e0127815. doi: 10.1371/journal.pone.0127815. eCollection 2015.
89. Zijlstra TR , Barendregt PJ , van de Laar MA. Venlafaxine in fibromyalgia: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2002;46(suppl 9):S105.
90. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25(6):871-880.
91. Thorpe J, Shum B, Moore RA, et al. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(2):CD010585.
92. Gilron I, Chaparro LE, Tu D, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain. 2016;157(7):1532-1540.
93. Häuser W, Petzke F, Üçeyler N, et al. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis. Rheumatology (Oxford). 2011;50(3):532-543.
94. Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy: a systematic review. Clin J Pain. 2016;32(11):1005-1010.
95. Riediger C, Schuster T, Barlinn K, et al. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol. 2017;8:307.
Alcohol use disorder: How best to screen and intervene
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12
Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12
Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12
Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
Migraine: Expanding our Tx arsenal
Migraine is a highly disabling primary headache disorder that affects more than 44 million Americans annually.1 The disorder causes pain, photophobia, phonophobia, and nausea that can last for hours, even days. Migraine headaches are 2 times more common in women than in men; although migraine is most common in people 30 to 39 years of age, all ages are affected.2,3 Frequency of migraine headache is variable; chronic migraineurs experience more than 15 headache days a month.
Recent estimates indicate that the cost of acute and chronic migraine headaches reaches approximately $78 million a year in the United States. 4 This high burden of disease has made effective migraine treatment options absolutely essential. Recent advances in our understanding of migraine pathophysiology have led to new therapeutic targets; there are now many novel treatment approaches on the horizon.
In this article, we review the diagnosis and management of migraine in detail. Our emphasis is on evidence-based approaches to acute and prophylactic treatment, including tried-and-true options and newly emerging therapies.
Neuronal dysfunction and a genetic predisposition
Although migraine was once thought to be caused by abnormalities of vasodilation, current research suggests that the disorder has its origins in primary neuronal dysfunction. There appears to be a genetic predisposition toward widespread neuronal hyperexcitability in migraineurs.5 In addition, hypothalamic neurons are thought to initiate migraine by responding to changes in brain homeostasis. Increased parasympathetic tone might activate meningeal pain receptors or lower the threshold for transmitting pain signals from the thalamus to the cortex.6
Prodromal symptoms and aura appear to originate from multiple areas across the brain, including the hypothalamus, cortex, limbic system, and brainstem. This widespread brain involvement might explain why some headache sufferers concurrently experience a variety of symptoms, including fatigue, depression, muscle pain, and an abnormal sensitivity to light, sound, and smell.6,7
Although the exact mechanisms behind each of these symptoms have yet to be defined precisely, waves of neuronal depolarization—known as cortical spreading depression—are suspected to cause migraine aura.8-10 Cortical spreading depression activates the trigeminal pain pathway and leads to the release of pro-inflammatory markers such as calcitonin gene-related protein (CGRP).6 A better understanding of these complex signaling pathways has helped provide potential therapeutic targets for new migraine drugs.
Diagnosis: Close patient inquiry is most helpful
The International Headache Society (IHS) criteria for primary headache disorders serve as the basis for the diagnosis of migraine and its subtypes, which include migraine without aura and migraine with aura. Due to variability of presentation, migraine with aura is further subdivided into migraine with typical aura (with and without headache), migraine with brainstem aura, hemiplegic migraine, and retinal migraine.11
Continue to: How is migraine defined?
How is migraine defined? Simply, migraine is classically defined as a unilateral, pulsating headache of moderate to severe intensity lasting 4 to 72 hours, associated with photophobia and phonophobia or nausea and vomiting, or both.11 Often visual in nature, aura is a set of neurologic symptoms that lasts for minutes and precedes the onset of the headache. The visual aura is often described as a scintillating scotoma that begins near the point of visual fixation and then spreads left or right. Other aura symptoms include tingling or numbness (second most common), speech disturbance (aphasia), motor changes and, in rare cases, a combination of these in succession. By definition, all of these symptoms fully resolve between attacks.11
Validated valuable questionnaires. To help with accurate and timely diagnosis, researchers have developed and validated simplified questionnaires that can be completed independently by patients presenting to primary care (TABLE 112,13):
- ID Migraine is a set of 3 questions that scores positive when a patient endorses at least 2 of the 3 symptoms. 12
- MS-Q is similar to the ID Migraine but includes 5 items. A score of ≥4 is a positive screen. 13
The sensitivity and specificity of MS-Q (0.93 and 0.81, respectively) are slightly higher than those of ID Migraine (0.81 and 0.75).13
Remember POUND. This mnemonic device can also be used during history-taking to aid in diagnostic accuracy. Migraine is highly likely (92%) in patients who endorse 4 of the following 5 symptoms and unlikely (17%) in those who endorse ≤2 symptoms14: Pulsatile quality of headache 4 to 72 hOurs in duration, Unilateral location, Nausea or vomiting, and Disabling intensity.
Differential Dx. Although the differential diagnosis of headache is broad (TABLE 214,15), the history alone can often guide clinicians towards the correct assessment. After taking the initial history (headache onset, location, duration, and associated symptoms), focus your attention on assessing the risk of intracranial pathology. This is best accomplished by assessing specific details of the history (TABLE 314) and findings on physical examination15:
- blood pressure measurement (seated, legs uncrossed, feet flat on the floor; having rested for 5 minutes; arm well supported)
- cranial nerve exam
- extremity strength testing
- eye exam (vision, extra-ocular muscles, visual fields, pupillary reactivity, and funduscopic exam)
- gait (tandem walk)
- reflexes.
Continue to: Further testing needed?
Further testing needed? Neuroimaging should be considered only in patients with an abnormal neurologic exam, atypical headache features, or certain risk factors, such as an immune deficiency. There is no role for electroencephalography or other diagnostic testing in migraine.16
Take a multipronged approach to treatment
As with other complex, chronic conditions, the treatment of migraine should take a multifaceted approach, including management of acute symptoms as well as prevention of future headaches. In 2015, the American Headache Society published a systematic review that specified particular treatment goals for migraine sufferers. 17 These goals include:
- headache reduction
- headache relief
- decreased disability from headache
- elimination of nausea and vomiting
- elimination of photophobia and phonophobia.
Our review, which follows, of therapeutic options focuses on the management of migraine in adults. Approaches in special populations (older adults, pregnant women, and children) are discussed afterward.
Pharmacotherapy for acute migraine
Acute migraine should be treated with an abortive medication at the onset of headache. The immediate goal is to relieve pain within 2 hours and prevent its recurrence within the subsequent 48 hours (TABLE 412,18-20).
In the general population, mild, infrequent migraines can be managed with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).21
Continue to: For moderate-to-severe migraine...
For moderate-to-severe migraine, triptans, which target serotonin receptors, are the drug of choice for most patients.21 Triptans are superior to placebo in achieving a pain-free state at 2 and 24 hours after administration; eletriptan has the most desirable outcome, with 68% of patients pain free at 2 hours and 54% pain free at 24 hours.22 Triptans are available as sublingual tablets and nasal sprays, as well as subcutaneous injections for patients with significant associated nausea and vomiting. Avoid prescribing triptans for patients with known vascular disease (eg, history of stroke, myocardial infarction, peripheral vascular disease, uncontrolled hypertension, or signs and symptoms of these conditions), as well as for patients with severe hepatic impairment.
Importantly, although triptans all have a similar mechanism of action, patients might respond differently to different drugs within the class. If a patient does not get adequate headache relief from an appropriate dosage of a given triptan during a particular migraine episode, a different triptan can be tried during the next migraine.22 Additionally, if a patient experiences an adverse effect from one triptan, this does not necessarily mean that a trial of another triptan at a later time is contraindicated.
For patients who have an incomplete response to migraine treatment or for those with frequent recurrence, the combination formulation of sumatriptan, 85 mg, and naproxen, 500 mg, showed the highest rate of resolution of headache within 2 hours compared with either drug alone.23 A similar result might be found by combining a triptan known to be effective for a patient and an NSAID other than naproxen. If migraine persists despite initial treatment of an attack, a different class of medication should be tried during the course of that attack to attain relief of symptoms of that migraine.21
When a patient is seen in an acute care setting (eg, emergency department, urgent care center) while suffering a migraine, additional treatment options are available. Intravenous (IV) anti-emetics are useful for relieving the pain of migraine and nausea, and can be used in combination with an IV NSAID (eg, ketorolac).21 The most effective anti-emetics are dopamine receptor type-2 blockers, including chlorpromazine, droperidol, metoclopramide, and prochlorperazine, which has the highest level of efficacy.24 Note that these medications do present the risk of a dystonic reaction; diphenhydramine is therefore often used in tandem to mitigate such a response.
Looking ahead. Although triptans are the current first-line therapy for acute migraine, their effectiveness is limited. Only 20% of patients report sustained relief of pain in the 2 to 24 hours after treatment, and the response can vary from episode to episode.25
Continue to: With better understading of the pathophysiology of migraine...
With better understanding of the pathophysiology of migraine, a host of novel anti-migraine drugs are on the horizon.
CGRP receptor antagonists. The neuropeptide CGRP, which mediates central and peripheral nervous system pain signaling, has been noted to be elevated during acute migraine attacks26; clinical trials are therefore underway to evaluate the safety and efficacy of CGRP receptor antagonists.18 These agents appear to be better tolerated than triptans, have fewer vascular and central nervous system adverse effects, and present less of a risk of medication overuse headache.18 Liver toxicity has been seen with some medications in this class and remains an important concern in their development.19
Phase 3 clinical trials for 1 drug in this class, ubrogepant, were completed in late 2017; full analysis of the data is not yet available. Primary outcomes being evaluated include relief of pain at 2 hours and relief from the most bothersome symptoms again at 2 hours.27
Selective serotonin-HT1f receptor agonists, such as lasmiditan, offer another potential approach. Although the exact mechanism of action of these agents is not entirely clear, clinical trials have supported their efficacy and safety.20 Importantly, ongoing trials are specifically targeting patients with known cardiovascular risk factors because they are most likely to benefit from the nonvasoconstrictive mechanism of action.28,29 Adverse effects reported primarily include dizziness, fatigue, and vertigo.
Strategies for managing recurrent episodic migraine
Because of the risk of medication overuse headache with acute treatment, daily preventive therapy for migraine is indicated for any patient with 30 :
- ≥6 headache days a month
- ≥4 headache days a month with some impairment
- ≥3 headache days a month with severe impairment.
Continue to: Treatment begins by having patients identify...
Treatment begins by having patients identify, and then avoid, migraine triggers (TABLE 5). This can be accomplished by having patients keep a headache diary, in which they can enter notations about personal and environmental situations that precede a headache.
For the individual patient, some triggers are modifiable; others are not. Helping a patient develop strategies for coping with triggers, rather than aiming for complete avoidance, might help her (him) manage those that are inescapable (eg stress, menstruation, etc).31 For many patients, however, this is not an adequate intervention and other approaches must be explored. When considering which therapy might be best for a given patient, evaluate her (his) comorbidities and assess that particular treatment for potential secondary benefits and the possibility of adverse effects. Pay attention to the choice of preventive therapy in women who are considering pregnancy because many available treatments are potentially teratogenic.
Oral medications. Oral agents from several classes of drugs can be used for migraine prophylaxis, including anti-epileptics,antidepressants, and antihypertensives (TABLE 620,29,30,32-41). Selected anti-epileptics (divalproex sodium, sodium valproate, topiramate) and beta-blockers (metoprolol, propranolol, and timolol) have the strongest evidence to support their use.32 Overall, regular use of prophylactic medications can reduce headache frequency by 50% for approximately 40% to 45% of patients who take them.29 However, adherence may be limited by adverse effects or perceived lack of efficacy, thus reducing their potential for benefit.42
OnabotulinumtoxinA. In patients with chronic migraine (≥15 headache days a month for at least 3 months) who have failed oral medications, the American Academy of Neurology (AAN) recommends the use of onabotulinumtoxinA.30 The treatment regimen comprises 31 injections at various sites on the head, neck, and shoulders every 3 months.33
A 2010 large randomized controlled trial showed a decrease in the frequency of headache days for patients receiving onabotulinumtoxinA compared to placebo after a 24-week treatment period (7.8 fewer headache days a month, compared to 6.4 fewer in the placebo group).33 A recent systematic review also noted a reduction of 2 headache days a month compared with placebo; the authors cautioned, however, that data with which to evaluate onabotulinumtoxinA in comparison to other prophylactic agents are limited.43
Continue to: In both studies...
In both studies, the risk of adverse drug events due to onabotulinumtoxinA was high and led to a significant rate of discontinuation.33,43 Despite this, onabotulinumtoxinA remains the only Food and Drug Administration (FDA)–approved treatment for chronic migraine, making it reasonable to consider for appropriate patients.
Acupuncture. A 2016 Cochrane review found benefit for patients using acupuncture compared with sham acupuncture.34 When acupuncture was compared with prophylactic agents such as beta-blockers, calcium-channel blockers, and anti-epileptics, however, there was no significant difference between the procedure and pharmacotherapy. Patients willing and able to try acupuncture might see a reduction in the overall number of headaches. Acupuncture has few adverse effects; however, long-term data are lacking.34
Exercise is not supported by robust data for its role as a prophylactic treatment. It is generally considered safe in most populations, however, and can be pursued with little out-of-pocket cost.35
Cognitive behavioral therapy (CBT). The AAN recommends CBT, relaxation therapy, and biofeedback therapy. Accessibility of these services remains limited for many patients, and cost can be prohibitive.16
Supplements used to help prevent migraine include the root of Petasites hybridus (butterbur), magnesium, vitamin B2 (riboflavin), Tanacetum parthenium (feverfew), and coenzyme Q10.16 Although the strength of evidence for these therapies is limited by small trials, their overall risk of adverse effects is low, and they might be easier for patients to obtain than acupuncture or CBT.
Continue to: Butterbur, in particular...
Butterbur, in particular, has been found to be beneficial for migraine prevention in 2 small placebo-controlled trials. In a randomized controlled study of 245 patients P hybridus, (specifically, the German formulation, Petadolex), 75 mg BID, reduced the frequency of migraine attack by 48% at 4 months, compared to placebo (number needed to treat, 5.3).44 No difference was found at lower dosages. The most common reported adverse effect was burping.
Regrettably, unpurified butterbur extract contains pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds. Because of variations in purification in production facilities in the United States, butterbur supplements might not have all of these compounds removed—and so should be used with caution.41
Magnesium. Studies evaluating the use of magnesium have demonstrated varied results; differences in methods and dosing have limited broad application of findings. As with most supplements considered for prophylactic treatment, magnesium dosing is poorly understood, and bioavailability varies in its different forms. Oral supplementation can be given as magnesium dicitrate, 600 mg/d.45
Recently, products containing various combinations of feverfew, coenzyme Q10, riboflavin, magnesium, and other supplements have shown benefit in early clinical trials.36,37
Neural stimulation. Over the past few years, a variety of transcutaneous nerve stimulator devices have gained FDA approval for use in migraine prophylaxis. The long-term safety and efficacy of these devices is not yet well understood, but they appear to provide headache relief in the short term and decrease the frequency of headache.38 Use of the noninvasive stimulators is limited today by high cost and poor coverage by US health care insurers.
Continue to: Newly available medical therapy
Newly available medical therapy. The FDA recently approved erenumab, a fully human monoclonal antibody for prevention of migraine in adults. This is the first drug in the CGRP antagonist class to be approved for this indication. Trials of this once-monthly, self-injectable drug show promising results for patients whose migraines have been refractory to other therapies.
A recent large trial evaluated 955 adults with migraine, randomizing them to receive erenumab, 70 mg; erenumab, 140 mg; or placebo over 28 weeks.39 The groups receiving erenumab had a nearly 2-fold higher odds of having their migraine reduced by 50%, compared with placebo (number needed to treat with the 140-mg dose, 4.27). Similar numbers of participants from all groups discontinued the study.39 Phase 3 trials that are not yet formally published have produced similarly beneficial results.40,46 The FDA has listed injection site reaction and constipation as the most reported adverse effects.40
Three other anti-CGRP antibodies are likely to be approved in the near future: fremanezumab, galcanezumab, and eptinezumab.
The approach to migraine in special populations
Management of acute and chronic migraine in children, pregnant women, and older adults requires special attention: Treatment approaches are different than they are for adults 19 to 65 years of age.
Pediatric patients. Migraine is the most common acute and recurrent headache syndrome in children. Headaches differ from those of adult migraine as a result of variations in brain maturation, plasticity, and cognitive development.47 Migraine attacks are often of shorter duration in children, lasting 1 to 2 hours, but can still be preceded by visual aura.48 Just as with adults, imaging, electroencephalography, lumbar puncture, and routine labs should be considered only if a child has an abnormal neurological exam or other concerning features (TABLE 214,15).
Continue to: The general approach to migraine treatment...
The general approach to migraine treatment in the pediatric population includes education of the child and family about symptom management. Acetaminophen, NSAIDs, and triptans are approved for abortive therapy in children and should be used for acute headache relief in the same way that they are used in adults. Oral rizatriptan, the most well studied triptan in the pediatric population, is approved for use in children as young as 6 years49; the pediatric dosage is 5 mg/d for patients weighing 20 to 39 kg and 10 mg/d for patients weighing more than 40 kg (same as the adult dosage).
Oral almotriptan and zolmitriptan are also approved for use in children 12 to 17 years of age. Usual dosages are: almotriptan, 12.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 25 mg/d); and zolmitriptan, 2.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 10 mg/d).50
For children who are unable to swallow pills or who are vomiting, a non-oral route of administration is preferable. Rizatriptan is available as an orally disintegrating tablet. Zolmitriptan is available in a nasal spray at a dose of 5 mg for children 12 years and older. Sumatriptan is not approved for use in patients younger than 18 years; however, recent studies have shown that it might have good efficacy and tolerability.50
Daily prophylactic treatment for recurrent migraine in the pediatric population is an evolving subject; published guidelines do not exist. It is reasonable to consider treatment using the same guidelines as those in place for adults.51 Topiramate, 1 to 2 mg/kg/d, is the only therapy approved by the FDA for episodic migraine preventive therapy in adolescents.50
Notably, a nonpharmacotherapeutic approach may be more effective for pediatric prevention. In 2017, a large double-blind, placebo-controlled trial investigated the use of amitriptyline, topiramate, and placebo for the treatment of recurrent migraine in children 8 to 17 years of age. An interim analysis of the 328 children enrolled found no significant differences in reduction of headache frequency with treatment compared with placebo over a 24-week period; the trial was stopped early due to futility.52
Continue to: The study did show...
The study did show, however, that reducing migraine triggers provided a high level of benefit to study participants. Stress is one of the most common migraine triggers in children; lack of sleep, exposure to a warm climate, and exposure to video games are also notable triggers.53 CBT may augment the efficacy of standard migraine medications in the pediatric population and may help prevent recurrence of episodes.54
Pregnancy. The treatment of migraine is different in pregnant women than it is in nonpregnant adults because of a concern over adverse effects on fetal development. For acute headache treatment, first-line therapies include trigger avoidance and acetaminophen, 1000 mg (maximum dosage, 4000 mg/d).55 If this is ineffective, a 10-mg dose of metoclopramide, as often as every 6 hours (not an FDA-approved indication), can be considered. During the second trimester, NSAIDs can be considered second-line therapy.
Triptans—specifically, sumatriptan and rizatriptan—can also be considered if first-line therapies fail.56 Triptan-exposed pregnant women with migraine have a rate of congenital malformations, spontaneous abortions, and prematurity that is similar to what is seen in pregnant women with migraine who have not been exposed to triptans. However, when triptan-exposed women are compared with healthy, non-migraine-suffering women, the rate of spontaneous abortion appears to be increased in the triptan-exposed population.57
Ergotamine is contraindicated during pregnancy because of its potential to induce uterine contractions and vasospasm, which can be detrimental to the fetus.56Nonpharmacotherapeutic interventions such as heat, ice, massage, rest, and avoidance of triggers are as successful in the pregnant population as in the nonpregnant population. For migraine prevention, coenzyme Q10, vitamins B2 and B6 (pyridoxine), and oral magnesium can be considered. Feverfew and butterbur should be avoided because of concerns about fetal malformation and preterm labor.58
Older adults. Choosing appropriate migraine therapy for older adults requires special consideration because of changes in drug metabolism and risks associated with drug adverse effects. Additionally, few studies of migraine drugs have included large populations of adults older than 65 years; medications should therefore be prescribed cautiously in this population, with particular attention to drug–drug interactions.
Continue to: Just as for younger adults...
Just as for younger adults, mild symptoms can be managed effectively with acetaminophen. NSAIDs may be used as well, but carry increased risks of gastric bleeding and elevation in blood pressure.59 The use of triptans is acceptable for the appropriate patient, but should be avoided in patients with known vascular disease.60 Antiemetics present an increased risk of extrapyramidal adverse effects in the elderly and should be used with caution at the lowest effective dosage.59 Novel mechanisms of action make some of the newer agents potentially safer for use in older adults when treating acute migraine.
For migraine prevention in older adults, particular attention should be paid to reducing triggers and minimizing polypharmacy.
More and more, successful treatment is within reach
With many clinical trials evaluating novel drugs underway, and additional studies contributing to our understanding of nonpharmacotherapeutic approaches to migraine treatment, improved headache control may become increasingly common over the next few years.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Thomas Jefferson University, 1015 Walnut St, Philadelphia PA 19107; [email protected].
1. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058-1077.
2. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479-484.
5. Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65-80.
6. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosc. 2015;35:6619-6629.
7. Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2013;137(Pt 1):232-241.
8. Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion‐weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25-31.
9. Hadjikhani N, Sanchez Del Rio MS, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692.
10. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Ann Rev Physiol. 2013;75:365-391.
11. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, (beta version). Cephalalgia. 2013;33:629-808.
12. Lipton RB, Dodick D, Sadovsky RE, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology. 2003;61:375-382.
13. Láinez MJ, Domínguez M, Rejas J, et al. Development and validation of the Migraine Screen Questionnaire (MS‐Q). Headache. 2005;45:1328-1338.
14. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
15. Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670-679.
16. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
17. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
18. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887-898.
19. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
20. Färkkilä M, Diener HC, Géraud G, et al; COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405-413.
21. Pringsheim T, Davenport WJ, Marmura MJ, et al. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194-1200.
22. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258-267.
23. Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;(10):CD008541.
24. Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35:271-284.
25. Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633-658.
26. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48-56.
27. A phase 3, multicenter, randomized, double-blind, placebo-controlled single attack study to evaluate the efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/study/NCT02828020. Accessed November 16, 2018.
28. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
29. Diener HC, Charles A, Goadsby PJ, et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-1022.
30. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122.
31. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221-227.
32. Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52:930-945.
33. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
34. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016(6):CD001218.
35. Varkey E, Cider Å, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
36. Guilbot A, Bangratz M, Abdellah SA, et al. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement Altern Med. 2017;17:433.
37. Dalla Volta G, Zavarize P, Ngonga G, et al. Combination of Tanacethum partenium, 5-hydrossitriptophan (5-Http) and magnesium in the prophylaxis of episodic migraine without aura (AURASTOP®) an observational study. Int J Neuro Brain Dis. 2017;4:1-4.
38. Puledda F, Goadsby PJ. An update on non‐pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57:685-691.
39. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132.
40. Reuter U. Efficacy and safety of erenumab in episodic migraine patients with 2-4 prior preventive treatment failures: Results from the Phase 3b LIBERTY study. Abstract 009, AAN 2018 Annual Meeting; April 24, 2018.
41. Diener HC, Freitag FG, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. https://journals.sagepub.com/doi/10.1177/2515816318759304. 2018 May 2. Accessed December 15, 2018.
42. Kingston WS, Halker R. Determinants of suboptimal migraine diagnosis and treatment in the primary care setting. J Clin Outcomes Manag. 2017;24:319-324.
43. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database of Syst Rev. 2018;6:CD011616.
44. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
45. Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence‐based rationale? A systematic review. Headache. 2018;58:199-209.
46. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425-434.
47. Sonal Sekhar M, Sasidharan S, Joseph S, et al. Migraine management: How do the adult and paediatric migraines differ? Saudi Pharm J. 2012;20:1-7.
48. Lewis DW. Pediatric migraine. In: Lewis DW. Clinician’s Manual on Treatment of Pediatric Migraine. London, UK: Springer Healthcare Ltd; 2010:15-26.
49. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized double-blind, placebo controlled trial using a novel adaptive enrichment design. Cephalagia. 2012;32:750-765.
50. Khrizman M, Pakalnis A. Management of pediatric migraine: current therapies. Pediatr Ann. 2018;47:e55-e60.
51. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
52. Powers SW, Coffey CS, Chamberlin LA, et al; CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
53. Neut D, Fily A, Cuvellier JC, et al. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13:61-65.
54. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta‐analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. s2017;57:349-362.
55. Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000;160:3486-3492.
56. Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392-398.
57. Marchenko A, Etwel F, Olutunfesse O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015:55:490-501.
58. Wells RE, Turner DP, Lee M, et al. Managing migraine during pregnancy and lactation. Curr Neurol Neurosci Rep. 2016;16:40.
59. Haan J, Hollander J, Ferrari MD. Migraine in the elderly: a review. Cephalalgia. 2007;27:97-106.
60. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med. 2004;115:39-44,47-50.
Migraine is a highly disabling primary headache disorder that affects more than 44 million Americans annually.1 The disorder causes pain, photophobia, phonophobia, and nausea that can last for hours, even days. Migraine headaches are 2 times more common in women than in men; although migraine is most common in people 30 to 39 years of age, all ages are affected.2,3 Frequency of migraine headache is variable; chronic migraineurs experience more than 15 headache days a month.
Recent estimates indicate that the cost of acute and chronic migraine headaches reaches approximately $78 million a year in the United States. 4 This high burden of disease has made effective migraine treatment options absolutely essential. Recent advances in our understanding of migraine pathophysiology have led to new therapeutic targets; there are now many novel treatment approaches on the horizon.
In this article, we review the diagnosis and management of migraine in detail. Our emphasis is on evidence-based approaches to acute and prophylactic treatment, including tried-and-true options and newly emerging therapies.
Neuronal dysfunction and a genetic predisposition
Although migraine was once thought to be caused by abnormalities of vasodilation, current research suggests that the disorder has its origins in primary neuronal dysfunction. There appears to be a genetic predisposition toward widespread neuronal hyperexcitability in migraineurs.5 In addition, hypothalamic neurons are thought to initiate migraine by responding to changes in brain homeostasis. Increased parasympathetic tone might activate meningeal pain receptors or lower the threshold for transmitting pain signals from the thalamus to the cortex.6
Prodromal symptoms and aura appear to originate from multiple areas across the brain, including the hypothalamus, cortex, limbic system, and brainstem. This widespread brain involvement might explain why some headache sufferers concurrently experience a variety of symptoms, including fatigue, depression, muscle pain, and an abnormal sensitivity to light, sound, and smell.6,7
Although the exact mechanisms behind each of these symptoms have yet to be defined precisely, waves of neuronal depolarization—known as cortical spreading depression—are suspected to cause migraine aura.8-10 Cortical spreading depression activates the trigeminal pain pathway and leads to the release of pro-inflammatory markers such as calcitonin gene-related protein (CGRP).6 A better understanding of these complex signaling pathways has helped provide potential therapeutic targets for new migraine drugs.
Diagnosis: Close patient inquiry is most helpful
The International Headache Society (IHS) criteria for primary headache disorders serve as the basis for the diagnosis of migraine and its subtypes, which include migraine without aura and migraine with aura. Due to variability of presentation, migraine with aura is further subdivided into migraine with typical aura (with and without headache), migraine with brainstem aura, hemiplegic migraine, and retinal migraine.11
Continue to: How is migraine defined?
How is migraine defined? Simply, migraine is classically defined as a unilateral, pulsating headache of moderate to severe intensity lasting 4 to 72 hours, associated with photophobia and phonophobia or nausea and vomiting, or both.11 Often visual in nature, aura is a set of neurologic symptoms that lasts for minutes and precedes the onset of the headache. The visual aura is often described as a scintillating scotoma that begins near the point of visual fixation and then spreads left or right. Other aura symptoms include tingling or numbness (second most common), speech disturbance (aphasia), motor changes and, in rare cases, a combination of these in succession. By definition, all of these symptoms fully resolve between attacks.11
Validated valuable questionnaires. To help with accurate and timely diagnosis, researchers have developed and validated simplified questionnaires that can be completed independently by patients presenting to primary care (TABLE 112,13):
- ID Migraine is a set of 3 questions that scores positive when a patient endorses at least 2 of the 3 symptoms. 12
- MS-Q is similar to the ID Migraine but includes 5 items. A score of ≥4 is a positive screen. 13
The sensitivity and specificity of MS-Q (0.93 and 0.81, respectively) are slightly higher than those of ID Migraine (0.81 and 0.75).13
Remember POUND. This mnemonic device can also be used during history-taking to aid in diagnostic accuracy. Migraine is highly likely (92%) in patients who endorse 4 of the following 5 symptoms and unlikely (17%) in those who endorse ≤2 symptoms14: Pulsatile quality of headache 4 to 72 hOurs in duration, Unilateral location, Nausea or vomiting, and Disabling intensity.
Differential Dx. Although the differential diagnosis of headache is broad (TABLE 214,15), the history alone can often guide clinicians towards the correct assessment. After taking the initial history (headache onset, location, duration, and associated symptoms), focus your attention on assessing the risk of intracranial pathology. This is best accomplished by assessing specific details of the history (TABLE 314) and findings on physical examination15:
- blood pressure measurement (seated, legs uncrossed, feet flat on the floor; having rested for 5 minutes; arm well supported)
- cranial nerve exam
- extremity strength testing
- eye exam (vision, extra-ocular muscles, visual fields, pupillary reactivity, and funduscopic exam)
- gait (tandem walk)
- reflexes.
Continue to: Further testing needed?
Further testing needed? Neuroimaging should be considered only in patients with an abnormal neurologic exam, atypical headache features, or certain risk factors, such as an immune deficiency. There is no role for electroencephalography or other diagnostic testing in migraine.16
Take a multipronged approach to treatment
As with other complex, chronic conditions, the treatment of migraine should take a multifaceted approach, including management of acute symptoms as well as prevention of future headaches. In 2015, the American Headache Society published a systematic review that specified particular treatment goals for migraine sufferers. 17 These goals include:
- headache reduction
- headache relief
- decreased disability from headache
- elimination of nausea and vomiting
- elimination of photophobia and phonophobia.
Our review, which follows, of therapeutic options focuses on the management of migraine in adults. Approaches in special populations (older adults, pregnant women, and children) are discussed afterward.
Pharmacotherapy for acute migraine
Acute migraine should be treated with an abortive medication at the onset of headache. The immediate goal is to relieve pain within 2 hours and prevent its recurrence within the subsequent 48 hours (TABLE 412,18-20).
In the general population, mild, infrequent migraines can be managed with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).21
Continue to: For moderate-to-severe migraine...
For moderate-to-severe migraine, triptans, which target serotonin receptors, are the drug of choice for most patients.21 Triptans are superior to placebo in achieving a pain-free state at 2 and 24 hours after administration; eletriptan has the most desirable outcome, with 68% of patients pain free at 2 hours and 54% pain free at 24 hours.22 Triptans are available as sublingual tablets and nasal sprays, as well as subcutaneous injections for patients with significant associated nausea and vomiting. Avoid prescribing triptans for patients with known vascular disease (eg, history of stroke, myocardial infarction, peripheral vascular disease, uncontrolled hypertension, or signs and symptoms of these conditions), as well as for patients with severe hepatic impairment.
Importantly, although triptans all have a similar mechanism of action, patients might respond differently to different drugs within the class. If a patient does not get adequate headache relief from an appropriate dosage of a given triptan during a particular migraine episode, a different triptan can be tried during the next migraine.22 Additionally, if a patient experiences an adverse effect from one triptan, this does not necessarily mean that a trial of another triptan at a later time is contraindicated.
For patients who have an incomplete response to migraine treatment or for those with frequent recurrence, the combination formulation of sumatriptan, 85 mg, and naproxen, 500 mg, showed the highest rate of resolution of headache within 2 hours compared with either drug alone.23 A similar result might be found by combining a triptan known to be effective for a patient and an NSAID other than naproxen. If migraine persists despite initial treatment of an attack, a different class of medication should be tried during the course of that attack to attain relief of symptoms of that migraine.21
When a patient is seen in an acute care setting (eg, emergency department, urgent care center) while suffering a migraine, additional treatment options are available. Intravenous (IV) anti-emetics are useful for relieving the pain of migraine and nausea, and can be used in combination with an IV NSAID (eg, ketorolac).21 The most effective anti-emetics are dopamine receptor type-2 blockers, including chlorpromazine, droperidol, metoclopramide, and prochlorperazine, which has the highest level of efficacy.24 Note that these medications do present the risk of a dystonic reaction; diphenhydramine is therefore often used in tandem to mitigate such a response.
Looking ahead. Although triptans are the current first-line therapy for acute migraine, their effectiveness is limited. Only 20% of patients report sustained relief of pain in the 2 to 24 hours after treatment, and the response can vary from episode to episode.25
Continue to: With better understading of the pathophysiology of migraine...
With better understanding of the pathophysiology of migraine, a host of novel anti-migraine drugs are on the horizon.
CGRP receptor antagonists. The neuropeptide CGRP, which mediates central and peripheral nervous system pain signaling, has been noted to be elevated during acute migraine attacks26; clinical trials are therefore underway to evaluate the safety and efficacy of CGRP receptor antagonists.18 These agents appear to be better tolerated than triptans, have fewer vascular and central nervous system adverse effects, and present less of a risk of medication overuse headache.18 Liver toxicity has been seen with some medications in this class and remains an important concern in their development.19
Phase 3 clinical trials for 1 drug in this class, ubrogepant, were completed in late 2017; full analysis of the data is not yet available. Primary outcomes being evaluated include relief of pain at 2 hours and relief from the most bothersome symptoms again at 2 hours.27
Selective serotonin-HT1f receptor agonists, such as lasmiditan, offer another potential approach. Although the exact mechanism of action of these agents is not entirely clear, clinical trials have supported their efficacy and safety.20 Importantly, ongoing trials are specifically targeting patients with known cardiovascular risk factors because they are most likely to benefit from the nonvasoconstrictive mechanism of action.28,29 Adverse effects reported primarily include dizziness, fatigue, and vertigo.
Strategies for managing recurrent episodic migraine
Because of the risk of medication overuse headache with acute treatment, daily preventive therapy for migraine is indicated for any patient with 30 :
- ≥6 headache days a month
- ≥4 headache days a month with some impairment
- ≥3 headache days a month with severe impairment.
Continue to: Treatment begins by having patients identify...
Treatment begins by having patients identify, and then avoid, migraine triggers (TABLE 5). This can be accomplished by having patients keep a headache diary, in which they can enter notations about personal and environmental situations that precede a headache.
For the individual patient, some triggers are modifiable; others are not. Helping a patient develop strategies for coping with triggers, rather than aiming for complete avoidance, might help her (him) manage those that are inescapable (eg stress, menstruation, etc).31 For many patients, however, this is not an adequate intervention and other approaches must be explored. When considering which therapy might be best for a given patient, evaluate her (his) comorbidities and assess that particular treatment for potential secondary benefits and the possibility of adverse effects. Pay attention to the choice of preventive therapy in women who are considering pregnancy because many available treatments are potentially teratogenic.
Oral medications. Oral agents from several classes of drugs can be used for migraine prophylaxis, including anti-epileptics,antidepressants, and antihypertensives (TABLE 620,29,30,32-41). Selected anti-epileptics (divalproex sodium, sodium valproate, topiramate) and beta-blockers (metoprolol, propranolol, and timolol) have the strongest evidence to support their use.32 Overall, regular use of prophylactic medications can reduce headache frequency by 50% for approximately 40% to 45% of patients who take them.29 However, adherence may be limited by adverse effects or perceived lack of efficacy, thus reducing their potential for benefit.42
OnabotulinumtoxinA. In patients with chronic migraine (≥15 headache days a month for at least 3 months) who have failed oral medications, the American Academy of Neurology (AAN) recommends the use of onabotulinumtoxinA.30 The treatment regimen comprises 31 injections at various sites on the head, neck, and shoulders every 3 months.33
A 2010 large randomized controlled trial showed a decrease in the frequency of headache days for patients receiving onabotulinumtoxinA compared to placebo after a 24-week treatment period (7.8 fewer headache days a month, compared to 6.4 fewer in the placebo group).33 A recent systematic review also noted a reduction of 2 headache days a month compared with placebo; the authors cautioned, however, that data with which to evaluate onabotulinumtoxinA in comparison to other prophylactic agents are limited.43
Continue to: In both studies...
In both studies, the risk of adverse drug events due to onabotulinumtoxinA was high and led to a significant rate of discontinuation.33,43 Despite this, onabotulinumtoxinA remains the only Food and Drug Administration (FDA)–approved treatment for chronic migraine, making it reasonable to consider for appropriate patients.
Acupuncture. A 2016 Cochrane review found benefit for patients using acupuncture compared with sham acupuncture.34 When acupuncture was compared with prophylactic agents such as beta-blockers, calcium-channel blockers, and anti-epileptics, however, there was no significant difference between the procedure and pharmacotherapy. Patients willing and able to try acupuncture might see a reduction in the overall number of headaches. Acupuncture has few adverse effects; however, long-term data are lacking.34
Exercise is not supported by robust data for its role as a prophylactic treatment. It is generally considered safe in most populations, however, and can be pursued with little out-of-pocket cost.35
Cognitive behavioral therapy (CBT). The AAN recommends CBT, relaxation therapy, and biofeedback therapy. Accessibility of these services remains limited for many patients, and cost can be prohibitive.16
Supplements used to help prevent migraine include the root of Petasites hybridus (butterbur), magnesium, vitamin B2 (riboflavin), Tanacetum parthenium (feverfew), and coenzyme Q10.16 Although the strength of evidence for these therapies is limited by small trials, their overall risk of adverse effects is low, and they might be easier for patients to obtain than acupuncture or CBT.
Continue to: Butterbur, in particular...
Butterbur, in particular, has been found to be beneficial for migraine prevention in 2 small placebo-controlled trials. In a randomized controlled study of 245 patients P hybridus, (specifically, the German formulation, Petadolex), 75 mg BID, reduced the frequency of migraine attack by 48% at 4 months, compared to placebo (number needed to treat, 5.3).44 No difference was found at lower dosages. The most common reported adverse effect was burping.
Regrettably, unpurified butterbur extract contains pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds. Because of variations in purification in production facilities in the United States, butterbur supplements might not have all of these compounds removed—and so should be used with caution.41
Magnesium. Studies evaluating the use of magnesium have demonstrated varied results; differences in methods and dosing have limited broad application of findings. As with most supplements considered for prophylactic treatment, magnesium dosing is poorly understood, and bioavailability varies in its different forms. Oral supplementation can be given as magnesium dicitrate, 600 mg/d.45
Recently, products containing various combinations of feverfew, coenzyme Q10, riboflavin, magnesium, and other supplements have shown benefit in early clinical trials.36,37
Neural stimulation. Over the past few years, a variety of transcutaneous nerve stimulator devices have gained FDA approval for use in migraine prophylaxis. The long-term safety and efficacy of these devices is not yet well understood, but they appear to provide headache relief in the short term and decrease the frequency of headache.38 Use of the noninvasive stimulators is limited today by high cost and poor coverage by US health care insurers.
Continue to: Newly available medical therapy
Newly available medical therapy. The FDA recently approved erenumab, a fully human monoclonal antibody for prevention of migraine in adults. This is the first drug in the CGRP antagonist class to be approved for this indication. Trials of this once-monthly, self-injectable drug show promising results for patients whose migraines have been refractory to other therapies.
A recent large trial evaluated 955 adults with migraine, randomizing them to receive erenumab, 70 mg; erenumab, 140 mg; or placebo over 28 weeks.39 The groups receiving erenumab had a nearly 2-fold higher odds of having their migraine reduced by 50%, compared with placebo (number needed to treat with the 140-mg dose, 4.27). Similar numbers of participants from all groups discontinued the study.39 Phase 3 trials that are not yet formally published have produced similarly beneficial results.40,46 The FDA has listed injection site reaction and constipation as the most reported adverse effects.40
Three other anti-CGRP antibodies are likely to be approved in the near future: fremanezumab, galcanezumab, and eptinezumab.
The approach to migraine in special populations
Management of acute and chronic migraine in children, pregnant women, and older adults requires special attention: Treatment approaches are different than they are for adults 19 to 65 years of age.
Pediatric patients. Migraine is the most common acute and recurrent headache syndrome in children. Headaches differ from those of adult migraine as a result of variations in brain maturation, plasticity, and cognitive development.47 Migraine attacks are often of shorter duration in children, lasting 1 to 2 hours, but can still be preceded by visual aura.48 Just as with adults, imaging, electroencephalography, lumbar puncture, and routine labs should be considered only if a child has an abnormal neurological exam or other concerning features (TABLE 214,15).
Continue to: The general approach to migraine treatment...
The general approach to migraine treatment in the pediatric population includes education of the child and family about symptom management. Acetaminophen, NSAIDs, and triptans are approved for abortive therapy in children and should be used for acute headache relief in the same way that they are used in adults. Oral rizatriptan, the most well studied triptan in the pediatric population, is approved for use in children as young as 6 years49; the pediatric dosage is 5 mg/d for patients weighing 20 to 39 kg and 10 mg/d for patients weighing more than 40 kg (same as the adult dosage).
Oral almotriptan and zolmitriptan are also approved for use in children 12 to 17 years of age. Usual dosages are: almotriptan, 12.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 25 mg/d); and zolmitriptan, 2.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 10 mg/d).50
For children who are unable to swallow pills or who are vomiting, a non-oral route of administration is preferable. Rizatriptan is available as an orally disintegrating tablet. Zolmitriptan is available in a nasal spray at a dose of 5 mg for children 12 years and older. Sumatriptan is not approved for use in patients younger than 18 years; however, recent studies have shown that it might have good efficacy and tolerability.50
Daily prophylactic treatment for recurrent migraine in the pediatric population is an evolving subject; published guidelines do not exist. It is reasonable to consider treatment using the same guidelines as those in place for adults.51 Topiramate, 1 to 2 mg/kg/d, is the only therapy approved by the FDA for episodic migraine preventive therapy in adolescents.50
Notably, a nonpharmacotherapeutic approach may be more effective for pediatric prevention. In 2017, a large double-blind, placebo-controlled trial investigated the use of amitriptyline, topiramate, and placebo for the treatment of recurrent migraine in children 8 to 17 years of age. An interim analysis of the 328 children enrolled found no significant differences in reduction of headache frequency with treatment compared with placebo over a 24-week period; the trial was stopped early due to futility.52
Continue to: The study did show...
The study did show, however, that reducing migraine triggers provided a high level of benefit to study participants. Stress is one of the most common migraine triggers in children; lack of sleep, exposure to a warm climate, and exposure to video games are also notable triggers.53 CBT may augment the efficacy of standard migraine medications in the pediatric population and may help prevent recurrence of episodes.54
Pregnancy. The treatment of migraine is different in pregnant women than it is in nonpregnant adults because of a concern over adverse effects on fetal development. For acute headache treatment, first-line therapies include trigger avoidance and acetaminophen, 1000 mg (maximum dosage, 4000 mg/d).55 If this is ineffective, a 10-mg dose of metoclopramide, as often as every 6 hours (not an FDA-approved indication), can be considered. During the second trimester, NSAIDs can be considered second-line therapy.
Triptans—specifically, sumatriptan and rizatriptan—can also be considered if first-line therapies fail.56 Triptan-exposed pregnant women with migraine have a rate of congenital malformations, spontaneous abortions, and prematurity that is similar to what is seen in pregnant women with migraine who have not been exposed to triptans. However, when triptan-exposed women are compared with healthy, non-migraine-suffering women, the rate of spontaneous abortion appears to be increased in the triptan-exposed population.57
Ergotamine is contraindicated during pregnancy because of its potential to induce uterine contractions and vasospasm, which can be detrimental to the fetus.56Nonpharmacotherapeutic interventions such as heat, ice, massage, rest, and avoidance of triggers are as successful in the pregnant population as in the nonpregnant population. For migraine prevention, coenzyme Q10, vitamins B2 and B6 (pyridoxine), and oral magnesium can be considered. Feverfew and butterbur should be avoided because of concerns about fetal malformation and preterm labor.58
Older adults. Choosing appropriate migraine therapy for older adults requires special consideration because of changes in drug metabolism and risks associated with drug adverse effects. Additionally, few studies of migraine drugs have included large populations of adults older than 65 years; medications should therefore be prescribed cautiously in this population, with particular attention to drug–drug interactions.
Continue to: Just as for younger adults...
Just as for younger adults, mild symptoms can be managed effectively with acetaminophen. NSAIDs may be used as well, but carry increased risks of gastric bleeding and elevation in blood pressure.59 The use of triptans is acceptable for the appropriate patient, but should be avoided in patients with known vascular disease.60 Antiemetics present an increased risk of extrapyramidal adverse effects in the elderly and should be used with caution at the lowest effective dosage.59 Novel mechanisms of action make some of the newer agents potentially safer for use in older adults when treating acute migraine.
For migraine prevention in older adults, particular attention should be paid to reducing triggers and minimizing polypharmacy.
More and more, successful treatment is within reach
With many clinical trials evaluating novel drugs underway, and additional studies contributing to our understanding of nonpharmacotherapeutic approaches to migraine treatment, improved headache control may become increasingly common over the next few years.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Thomas Jefferson University, 1015 Walnut St, Philadelphia PA 19107; [email protected].
Migraine is a highly disabling primary headache disorder that affects more than 44 million Americans annually.1 The disorder causes pain, photophobia, phonophobia, and nausea that can last for hours, even days. Migraine headaches are 2 times more common in women than in men; although migraine is most common in people 30 to 39 years of age, all ages are affected.2,3 Frequency of migraine headache is variable; chronic migraineurs experience more than 15 headache days a month.
Recent estimates indicate that the cost of acute and chronic migraine headaches reaches approximately $78 million a year in the United States. 4 This high burden of disease has made effective migraine treatment options absolutely essential. Recent advances in our understanding of migraine pathophysiology have led to new therapeutic targets; there are now many novel treatment approaches on the horizon.
In this article, we review the diagnosis and management of migraine in detail. Our emphasis is on evidence-based approaches to acute and prophylactic treatment, including tried-and-true options and newly emerging therapies.
Neuronal dysfunction and a genetic predisposition
Although migraine was once thought to be caused by abnormalities of vasodilation, current research suggests that the disorder has its origins in primary neuronal dysfunction. There appears to be a genetic predisposition toward widespread neuronal hyperexcitability in migraineurs.5 In addition, hypothalamic neurons are thought to initiate migraine by responding to changes in brain homeostasis. Increased parasympathetic tone might activate meningeal pain receptors or lower the threshold for transmitting pain signals from the thalamus to the cortex.6
Prodromal symptoms and aura appear to originate from multiple areas across the brain, including the hypothalamus, cortex, limbic system, and brainstem. This widespread brain involvement might explain why some headache sufferers concurrently experience a variety of symptoms, including fatigue, depression, muscle pain, and an abnormal sensitivity to light, sound, and smell.6,7
Although the exact mechanisms behind each of these symptoms have yet to be defined precisely, waves of neuronal depolarization—known as cortical spreading depression—are suspected to cause migraine aura.8-10 Cortical spreading depression activates the trigeminal pain pathway and leads to the release of pro-inflammatory markers such as calcitonin gene-related protein (CGRP).6 A better understanding of these complex signaling pathways has helped provide potential therapeutic targets for new migraine drugs.
Diagnosis: Close patient inquiry is most helpful
The International Headache Society (IHS) criteria for primary headache disorders serve as the basis for the diagnosis of migraine and its subtypes, which include migraine without aura and migraine with aura. Due to variability of presentation, migraine with aura is further subdivided into migraine with typical aura (with and without headache), migraine with brainstem aura, hemiplegic migraine, and retinal migraine.11
Continue to: How is migraine defined?
How is migraine defined? Simply, migraine is classically defined as a unilateral, pulsating headache of moderate to severe intensity lasting 4 to 72 hours, associated with photophobia and phonophobia or nausea and vomiting, or both.11 Often visual in nature, aura is a set of neurologic symptoms that lasts for minutes and precedes the onset of the headache. The visual aura is often described as a scintillating scotoma that begins near the point of visual fixation and then spreads left or right. Other aura symptoms include tingling or numbness (second most common), speech disturbance (aphasia), motor changes and, in rare cases, a combination of these in succession. By definition, all of these symptoms fully resolve between attacks.11
Validated valuable questionnaires. To help with accurate and timely diagnosis, researchers have developed and validated simplified questionnaires that can be completed independently by patients presenting to primary care (TABLE 112,13):
- ID Migraine is a set of 3 questions that scores positive when a patient endorses at least 2 of the 3 symptoms. 12
- MS-Q is similar to the ID Migraine but includes 5 items. A score of ≥4 is a positive screen. 13
The sensitivity and specificity of MS-Q (0.93 and 0.81, respectively) are slightly higher than those of ID Migraine (0.81 and 0.75).13
Remember POUND. This mnemonic device can also be used during history-taking to aid in diagnostic accuracy. Migraine is highly likely (92%) in patients who endorse 4 of the following 5 symptoms and unlikely (17%) in those who endorse ≤2 symptoms14: Pulsatile quality of headache 4 to 72 hOurs in duration, Unilateral location, Nausea or vomiting, and Disabling intensity.
Differential Dx. Although the differential diagnosis of headache is broad (TABLE 214,15), the history alone can often guide clinicians towards the correct assessment. After taking the initial history (headache onset, location, duration, and associated symptoms), focus your attention on assessing the risk of intracranial pathology. This is best accomplished by assessing specific details of the history (TABLE 314) and findings on physical examination15:
- blood pressure measurement (seated, legs uncrossed, feet flat on the floor; having rested for 5 minutes; arm well supported)
- cranial nerve exam
- extremity strength testing
- eye exam (vision, extra-ocular muscles, visual fields, pupillary reactivity, and funduscopic exam)
- gait (tandem walk)
- reflexes.
Continue to: Further testing needed?
Further testing needed? Neuroimaging should be considered only in patients with an abnormal neurologic exam, atypical headache features, or certain risk factors, such as an immune deficiency. There is no role for electroencephalography or other diagnostic testing in migraine.16
Take a multipronged approach to treatment
As with other complex, chronic conditions, the treatment of migraine should take a multifaceted approach, including management of acute symptoms as well as prevention of future headaches. In 2015, the American Headache Society published a systematic review that specified particular treatment goals for migraine sufferers. 17 These goals include:
- headache reduction
- headache relief
- decreased disability from headache
- elimination of nausea and vomiting
- elimination of photophobia and phonophobia.
Our review, which follows, of therapeutic options focuses on the management of migraine in adults. Approaches in special populations (older adults, pregnant women, and children) are discussed afterward.
Pharmacotherapy for acute migraine
Acute migraine should be treated with an abortive medication at the onset of headache. The immediate goal is to relieve pain within 2 hours and prevent its recurrence within the subsequent 48 hours (TABLE 412,18-20).
In the general population, mild, infrequent migraines can be managed with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).21
Continue to: For moderate-to-severe migraine...
For moderate-to-severe migraine, triptans, which target serotonin receptors, are the drug of choice for most patients.21 Triptans are superior to placebo in achieving a pain-free state at 2 and 24 hours after administration; eletriptan has the most desirable outcome, with 68% of patients pain free at 2 hours and 54% pain free at 24 hours.22 Triptans are available as sublingual tablets and nasal sprays, as well as subcutaneous injections for patients with significant associated nausea and vomiting. Avoid prescribing triptans for patients with known vascular disease (eg, history of stroke, myocardial infarction, peripheral vascular disease, uncontrolled hypertension, or signs and symptoms of these conditions), as well as for patients with severe hepatic impairment.
Importantly, although triptans all have a similar mechanism of action, patients might respond differently to different drugs within the class. If a patient does not get adequate headache relief from an appropriate dosage of a given triptan during a particular migraine episode, a different triptan can be tried during the next migraine.22 Additionally, if a patient experiences an adverse effect from one triptan, this does not necessarily mean that a trial of another triptan at a later time is contraindicated.
For patients who have an incomplete response to migraine treatment or for those with frequent recurrence, the combination formulation of sumatriptan, 85 mg, and naproxen, 500 mg, showed the highest rate of resolution of headache within 2 hours compared with either drug alone.23 A similar result might be found by combining a triptan known to be effective for a patient and an NSAID other than naproxen. If migraine persists despite initial treatment of an attack, a different class of medication should be tried during the course of that attack to attain relief of symptoms of that migraine.21
When a patient is seen in an acute care setting (eg, emergency department, urgent care center) while suffering a migraine, additional treatment options are available. Intravenous (IV) anti-emetics are useful for relieving the pain of migraine and nausea, and can be used in combination with an IV NSAID (eg, ketorolac).21 The most effective anti-emetics are dopamine receptor type-2 blockers, including chlorpromazine, droperidol, metoclopramide, and prochlorperazine, which has the highest level of efficacy.24 Note that these medications do present the risk of a dystonic reaction; diphenhydramine is therefore often used in tandem to mitigate such a response.
Looking ahead. Although triptans are the current first-line therapy for acute migraine, their effectiveness is limited. Only 20% of patients report sustained relief of pain in the 2 to 24 hours after treatment, and the response can vary from episode to episode.25
Continue to: With better understading of the pathophysiology of migraine...
With better understanding of the pathophysiology of migraine, a host of novel anti-migraine drugs are on the horizon.
CGRP receptor antagonists. The neuropeptide CGRP, which mediates central and peripheral nervous system pain signaling, has been noted to be elevated during acute migraine attacks26; clinical trials are therefore underway to evaluate the safety and efficacy of CGRP receptor antagonists.18 These agents appear to be better tolerated than triptans, have fewer vascular and central nervous system adverse effects, and present less of a risk of medication overuse headache.18 Liver toxicity has been seen with some medications in this class and remains an important concern in their development.19
Phase 3 clinical trials for 1 drug in this class, ubrogepant, were completed in late 2017; full analysis of the data is not yet available. Primary outcomes being evaluated include relief of pain at 2 hours and relief from the most bothersome symptoms again at 2 hours.27
Selective serotonin-HT1f receptor agonists, such as lasmiditan, offer another potential approach. Although the exact mechanism of action of these agents is not entirely clear, clinical trials have supported their efficacy and safety.20 Importantly, ongoing trials are specifically targeting patients with known cardiovascular risk factors because they are most likely to benefit from the nonvasoconstrictive mechanism of action.28,29 Adverse effects reported primarily include dizziness, fatigue, and vertigo.
Strategies for managing recurrent episodic migraine
Because of the risk of medication overuse headache with acute treatment, daily preventive therapy for migraine is indicated for any patient with 30 :
- ≥6 headache days a month
- ≥4 headache days a month with some impairment
- ≥3 headache days a month with severe impairment.
Continue to: Treatment begins by having patients identify...
Treatment begins by having patients identify, and then avoid, migraine triggers (TABLE 5). This can be accomplished by having patients keep a headache diary, in which they can enter notations about personal and environmental situations that precede a headache.
For the individual patient, some triggers are modifiable; others are not. Helping a patient develop strategies for coping with triggers, rather than aiming for complete avoidance, might help her (him) manage those that are inescapable (eg stress, menstruation, etc).31 For many patients, however, this is not an adequate intervention and other approaches must be explored. When considering which therapy might be best for a given patient, evaluate her (his) comorbidities and assess that particular treatment for potential secondary benefits and the possibility of adverse effects. Pay attention to the choice of preventive therapy in women who are considering pregnancy because many available treatments are potentially teratogenic.
Oral medications. Oral agents from several classes of drugs can be used for migraine prophylaxis, including anti-epileptics,antidepressants, and antihypertensives (TABLE 620,29,30,32-41). Selected anti-epileptics (divalproex sodium, sodium valproate, topiramate) and beta-blockers (metoprolol, propranolol, and timolol) have the strongest evidence to support their use.32 Overall, regular use of prophylactic medications can reduce headache frequency by 50% for approximately 40% to 45% of patients who take them.29 However, adherence may be limited by adverse effects or perceived lack of efficacy, thus reducing their potential for benefit.42
OnabotulinumtoxinA. In patients with chronic migraine (≥15 headache days a month for at least 3 months) who have failed oral medications, the American Academy of Neurology (AAN) recommends the use of onabotulinumtoxinA.30 The treatment regimen comprises 31 injections at various sites on the head, neck, and shoulders every 3 months.33
A 2010 large randomized controlled trial showed a decrease in the frequency of headache days for patients receiving onabotulinumtoxinA compared to placebo after a 24-week treatment period (7.8 fewer headache days a month, compared to 6.4 fewer in the placebo group).33 A recent systematic review also noted a reduction of 2 headache days a month compared with placebo; the authors cautioned, however, that data with which to evaluate onabotulinumtoxinA in comparison to other prophylactic agents are limited.43
Continue to: In both studies...
In both studies, the risk of adverse drug events due to onabotulinumtoxinA was high and led to a significant rate of discontinuation.33,43 Despite this, onabotulinumtoxinA remains the only Food and Drug Administration (FDA)–approved treatment for chronic migraine, making it reasonable to consider for appropriate patients.
Acupuncture. A 2016 Cochrane review found benefit for patients using acupuncture compared with sham acupuncture.34 When acupuncture was compared with prophylactic agents such as beta-blockers, calcium-channel blockers, and anti-epileptics, however, there was no significant difference between the procedure and pharmacotherapy. Patients willing and able to try acupuncture might see a reduction in the overall number of headaches. Acupuncture has few adverse effects; however, long-term data are lacking.34
Exercise is not supported by robust data for its role as a prophylactic treatment. It is generally considered safe in most populations, however, and can be pursued with little out-of-pocket cost.35
Cognitive behavioral therapy (CBT). The AAN recommends CBT, relaxation therapy, and biofeedback therapy. Accessibility of these services remains limited for many patients, and cost can be prohibitive.16
Supplements used to help prevent migraine include the root of Petasites hybridus (butterbur), magnesium, vitamin B2 (riboflavin), Tanacetum parthenium (feverfew), and coenzyme Q10.16 Although the strength of evidence for these therapies is limited by small trials, their overall risk of adverse effects is low, and they might be easier for patients to obtain than acupuncture or CBT.
Continue to: Butterbur, in particular...
Butterbur, in particular, has been found to be beneficial for migraine prevention in 2 small placebo-controlled trials. In a randomized controlled study of 245 patients P hybridus, (specifically, the German formulation, Petadolex), 75 mg BID, reduced the frequency of migraine attack by 48% at 4 months, compared to placebo (number needed to treat, 5.3).44 No difference was found at lower dosages. The most common reported adverse effect was burping.
Regrettably, unpurified butterbur extract contains pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds. Because of variations in purification in production facilities in the United States, butterbur supplements might not have all of these compounds removed—and so should be used with caution.41
Magnesium. Studies evaluating the use of magnesium have demonstrated varied results; differences in methods and dosing have limited broad application of findings. As with most supplements considered for prophylactic treatment, magnesium dosing is poorly understood, and bioavailability varies in its different forms. Oral supplementation can be given as magnesium dicitrate, 600 mg/d.45
Recently, products containing various combinations of feverfew, coenzyme Q10, riboflavin, magnesium, and other supplements have shown benefit in early clinical trials.36,37
Neural stimulation. Over the past few years, a variety of transcutaneous nerve stimulator devices have gained FDA approval for use in migraine prophylaxis. The long-term safety and efficacy of these devices is not yet well understood, but they appear to provide headache relief in the short term and decrease the frequency of headache.38 Use of the noninvasive stimulators is limited today by high cost and poor coverage by US health care insurers.
Continue to: Newly available medical therapy
Newly available medical therapy. The FDA recently approved erenumab, a fully human monoclonal antibody for prevention of migraine in adults. This is the first drug in the CGRP antagonist class to be approved for this indication. Trials of this once-monthly, self-injectable drug show promising results for patients whose migraines have been refractory to other therapies.
A recent large trial evaluated 955 adults with migraine, randomizing them to receive erenumab, 70 mg; erenumab, 140 mg; or placebo over 28 weeks.39 The groups receiving erenumab had a nearly 2-fold higher odds of having their migraine reduced by 50%, compared with placebo (number needed to treat with the 140-mg dose, 4.27). Similar numbers of participants from all groups discontinued the study.39 Phase 3 trials that are not yet formally published have produced similarly beneficial results.40,46 The FDA has listed injection site reaction and constipation as the most reported adverse effects.40
Three other anti-CGRP antibodies are likely to be approved in the near future: fremanezumab, galcanezumab, and eptinezumab.
The approach to migraine in special populations
Management of acute and chronic migraine in children, pregnant women, and older adults requires special attention: Treatment approaches are different than they are for adults 19 to 65 years of age.
Pediatric patients. Migraine is the most common acute and recurrent headache syndrome in children. Headaches differ from those of adult migraine as a result of variations in brain maturation, plasticity, and cognitive development.47 Migraine attacks are often of shorter duration in children, lasting 1 to 2 hours, but can still be preceded by visual aura.48 Just as with adults, imaging, electroencephalography, lumbar puncture, and routine labs should be considered only if a child has an abnormal neurological exam or other concerning features (TABLE 214,15).
Continue to: The general approach to migraine treatment...
The general approach to migraine treatment in the pediatric population includes education of the child and family about symptom management. Acetaminophen, NSAIDs, and triptans are approved for abortive therapy in children and should be used for acute headache relief in the same way that they are used in adults. Oral rizatriptan, the most well studied triptan in the pediatric population, is approved for use in children as young as 6 years49; the pediatric dosage is 5 mg/d for patients weighing 20 to 39 kg and 10 mg/d for patients weighing more than 40 kg (same as the adult dosage).
Oral almotriptan and zolmitriptan are also approved for use in children 12 to 17 years of age. Usual dosages are: almotriptan, 12.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 25 mg/d); and zolmitriptan, 2.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 10 mg/d).50
For children who are unable to swallow pills or who are vomiting, a non-oral route of administration is preferable. Rizatriptan is available as an orally disintegrating tablet. Zolmitriptan is available in a nasal spray at a dose of 5 mg for children 12 years and older. Sumatriptan is not approved for use in patients younger than 18 years; however, recent studies have shown that it might have good efficacy and tolerability.50
Daily prophylactic treatment for recurrent migraine in the pediatric population is an evolving subject; published guidelines do not exist. It is reasonable to consider treatment using the same guidelines as those in place for adults.51 Topiramate, 1 to 2 mg/kg/d, is the only therapy approved by the FDA for episodic migraine preventive therapy in adolescents.50
Notably, a nonpharmacotherapeutic approach may be more effective for pediatric prevention. In 2017, a large double-blind, placebo-controlled trial investigated the use of amitriptyline, topiramate, and placebo for the treatment of recurrent migraine in children 8 to 17 years of age. An interim analysis of the 328 children enrolled found no significant differences in reduction of headache frequency with treatment compared with placebo over a 24-week period; the trial was stopped early due to futility.52
Continue to: The study did show...
The study did show, however, that reducing migraine triggers provided a high level of benefit to study participants. Stress is one of the most common migraine triggers in children; lack of sleep, exposure to a warm climate, and exposure to video games are also notable triggers.53 CBT may augment the efficacy of standard migraine medications in the pediatric population and may help prevent recurrence of episodes.54
Pregnancy. The treatment of migraine is different in pregnant women than it is in nonpregnant adults because of a concern over adverse effects on fetal development. For acute headache treatment, first-line therapies include trigger avoidance and acetaminophen, 1000 mg (maximum dosage, 4000 mg/d).55 If this is ineffective, a 10-mg dose of metoclopramide, as often as every 6 hours (not an FDA-approved indication), can be considered. During the second trimester, NSAIDs can be considered second-line therapy.
Triptans—specifically, sumatriptan and rizatriptan—can also be considered if first-line therapies fail.56 Triptan-exposed pregnant women with migraine have a rate of congenital malformations, spontaneous abortions, and prematurity that is similar to what is seen in pregnant women with migraine who have not been exposed to triptans. However, when triptan-exposed women are compared with healthy, non-migraine-suffering women, the rate of spontaneous abortion appears to be increased in the triptan-exposed population.57
Ergotamine is contraindicated during pregnancy because of its potential to induce uterine contractions and vasospasm, which can be detrimental to the fetus.56Nonpharmacotherapeutic interventions such as heat, ice, massage, rest, and avoidance of triggers are as successful in the pregnant population as in the nonpregnant population. For migraine prevention, coenzyme Q10, vitamins B2 and B6 (pyridoxine), and oral magnesium can be considered. Feverfew and butterbur should be avoided because of concerns about fetal malformation and preterm labor.58
Older adults. Choosing appropriate migraine therapy for older adults requires special consideration because of changes in drug metabolism and risks associated with drug adverse effects. Additionally, few studies of migraine drugs have included large populations of adults older than 65 years; medications should therefore be prescribed cautiously in this population, with particular attention to drug–drug interactions.
Continue to: Just as for younger adults...
Just as for younger adults, mild symptoms can be managed effectively with acetaminophen. NSAIDs may be used as well, but carry increased risks of gastric bleeding and elevation in blood pressure.59 The use of triptans is acceptable for the appropriate patient, but should be avoided in patients with known vascular disease.60 Antiemetics present an increased risk of extrapyramidal adverse effects in the elderly and should be used with caution at the lowest effective dosage.59 Novel mechanisms of action make some of the newer agents potentially safer for use in older adults when treating acute migraine.
For migraine prevention in older adults, particular attention should be paid to reducing triggers and minimizing polypharmacy.
More and more, successful treatment is within reach
With many clinical trials evaluating novel drugs underway, and additional studies contributing to our understanding of nonpharmacotherapeutic approaches to migraine treatment, improved headache control may become increasingly common over the next few years.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Thomas Jefferson University, 1015 Walnut St, Philadelphia PA 19107; [email protected].
1. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058-1077.
2. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479-484.
5. Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65-80.
6. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosc. 2015;35:6619-6629.
7. Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2013;137(Pt 1):232-241.
8. Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion‐weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25-31.
9. Hadjikhani N, Sanchez Del Rio MS, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692.
10. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Ann Rev Physiol. 2013;75:365-391.
11. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, (beta version). Cephalalgia. 2013;33:629-808.
12. Lipton RB, Dodick D, Sadovsky RE, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology. 2003;61:375-382.
13. Láinez MJ, Domínguez M, Rejas J, et al. Development and validation of the Migraine Screen Questionnaire (MS‐Q). Headache. 2005;45:1328-1338.
14. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
15. Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670-679.
16. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
17. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
18. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887-898.
19. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
20. Färkkilä M, Diener HC, Géraud G, et al; COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405-413.
21. Pringsheim T, Davenport WJ, Marmura MJ, et al. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194-1200.
22. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258-267.
23. Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;(10):CD008541.
24. Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35:271-284.
25. Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633-658.
26. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48-56.
27. A phase 3, multicenter, randomized, double-blind, placebo-controlled single attack study to evaluate the efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/study/NCT02828020. Accessed November 16, 2018.
28. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
29. Diener HC, Charles A, Goadsby PJ, et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-1022.
30. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122.
31. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221-227.
32. Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52:930-945.
33. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
34. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016(6):CD001218.
35. Varkey E, Cider Å, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
36. Guilbot A, Bangratz M, Abdellah SA, et al. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement Altern Med. 2017;17:433.
37. Dalla Volta G, Zavarize P, Ngonga G, et al. Combination of Tanacethum partenium, 5-hydrossitriptophan (5-Http) and magnesium in the prophylaxis of episodic migraine without aura (AURASTOP®) an observational study. Int J Neuro Brain Dis. 2017;4:1-4.
38. Puledda F, Goadsby PJ. An update on non‐pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57:685-691.
39. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132.
40. Reuter U. Efficacy and safety of erenumab in episodic migraine patients with 2-4 prior preventive treatment failures: Results from the Phase 3b LIBERTY study. Abstract 009, AAN 2018 Annual Meeting; April 24, 2018.
41. Diener HC, Freitag FG, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. https://journals.sagepub.com/doi/10.1177/2515816318759304. 2018 May 2. Accessed December 15, 2018.
42. Kingston WS, Halker R. Determinants of suboptimal migraine diagnosis and treatment in the primary care setting. J Clin Outcomes Manag. 2017;24:319-324.
43. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database of Syst Rev. 2018;6:CD011616.
44. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
45. Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence‐based rationale? A systematic review. Headache. 2018;58:199-209.
46. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425-434.
47. Sonal Sekhar M, Sasidharan S, Joseph S, et al. Migraine management: How do the adult and paediatric migraines differ? Saudi Pharm J. 2012;20:1-7.
48. Lewis DW. Pediatric migraine. In: Lewis DW. Clinician’s Manual on Treatment of Pediatric Migraine. London, UK: Springer Healthcare Ltd; 2010:15-26.
49. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized double-blind, placebo controlled trial using a novel adaptive enrichment design. Cephalagia. 2012;32:750-765.
50. Khrizman M, Pakalnis A. Management of pediatric migraine: current therapies. Pediatr Ann. 2018;47:e55-e60.
51. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
52. Powers SW, Coffey CS, Chamberlin LA, et al; CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
53. Neut D, Fily A, Cuvellier JC, et al. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13:61-65.
54. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta‐analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. s2017;57:349-362.
55. Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000;160:3486-3492.
56. Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392-398.
57. Marchenko A, Etwel F, Olutunfesse O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015:55:490-501.
58. Wells RE, Turner DP, Lee M, et al. Managing migraine during pregnancy and lactation. Curr Neurol Neurosci Rep. 2016;16:40.
59. Haan J, Hollander J, Ferrari MD. Migraine in the elderly: a review. Cephalalgia. 2007;27:97-106.
60. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med. 2004;115:39-44,47-50.
1. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058-1077.
2. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479-484.
5. Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65-80.
6. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosc. 2015;35:6619-6629.
7. Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2013;137(Pt 1):232-241.
8. Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion‐weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25-31.
9. Hadjikhani N, Sanchez Del Rio MS, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692.
10. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Ann Rev Physiol. 2013;75:365-391.
11. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, (beta version). Cephalalgia. 2013;33:629-808.
12. Lipton RB, Dodick D, Sadovsky RE, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology. 2003;61:375-382.
13. Láinez MJ, Domínguez M, Rejas J, et al. Development and validation of the Migraine Screen Questionnaire (MS‐Q). Headache. 2005;45:1328-1338.
14. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
15. Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670-679.
16. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
17. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
18. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887-898.
19. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
20. Färkkilä M, Diener HC, Géraud G, et al; COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405-413.
21. Pringsheim T, Davenport WJ, Marmura MJ, et al. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194-1200.
22. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258-267.
23. Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;(10):CD008541.
24. Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35:271-284.
25. Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633-658.
26. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48-56.
27. A phase 3, multicenter, randomized, double-blind, placebo-controlled single attack study to evaluate the efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/study/NCT02828020. Accessed November 16, 2018.
28. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
29. Diener HC, Charles A, Goadsby PJ, et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-1022.
30. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122.
31. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221-227.
32. Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52:930-945.
33. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
34. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016(6):CD001218.
35. Varkey E, Cider Å, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
36. Guilbot A, Bangratz M, Abdellah SA, et al. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement Altern Med. 2017;17:433.
37. Dalla Volta G, Zavarize P, Ngonga G, et al. Combination of Tanacethum partenium, 5-hydrossitriptophan (5-Http) and magnesium in the prophylaxis of episodic migraine without aura (AURASTOP®) an observational study. Int J Neuro Brain Dis. 2017;4:1-4.
38. Puledda F, Goadsby PJ. An update on non‐pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57:685-691.
39. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132.
40. Reuter U. Efficacy and safety of erenumab in episodic migraine patients with 2-4 prior preventive treatment failures: Results from the Phase 3b LIBERTY study. Abstract 009, AAN 2018 Annual Meeting; April 24, 2018.
41. Diener HC, Freitag FG, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. https://journals.sagepub.com/doi/10.1177/2515816318759304. 2018 May 2. Accessed December 15, 2018.
42. Kingston WS, Halker R. Determinants of suboptimal migraine diagnosis and treatment in the primary care setting. J Clin Outcomes Manag. 2017;24:319-324.
43. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database of Syst Rev. 2018;6:CD011616.
44. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
45. Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence‐based rationale? A systematic review. Headache. 2018;58:199-209.
46. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425-434.
47. Sonal Sekhar M, Sasidharan S, Joseph S, et al. Migraine management: How do the adult and paediatric migraines differ? Saudi Pharm J. 2012;20:1-7.
48. Lewis DW. Pediatric migraine. In: Lewis DW. Clinician’s Manual on Treatment of Pediatric Migraine. London, UK: Springer Healthcare Ltd; 2010:15-26.
49. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized double-blind, placebo controlled trial using a novel adaptive enrichment design. Cephalagia. 2012;32:750-765.
50. Khrizman M, Pakalnis A. Management of pediatric migraine: current therapies. Pediatr Ann. 2018;47:e55-e60.
51. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
52. Powers SW, Coffey CS, Chamberlin LA, et al; CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
53. Neut D, Fily A, Cuvellier JC, et al. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13:61-65.
54. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta‐analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. s2017;57:349-362.
55. Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000;160:3486-3492.
56. Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392-398.
57. Marchenko A, Etwel F, Olutunfesse O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015:55:490-501.
58. Wells RE, Turner DP, Lee M, et al. Managing migraine during pregnancy and lactation. Curr Neurol Neurosci Rep. 2016;16:40.
59. Haan J, Hollander J, Ferrari MD. Migraine in the elderly: a review. Cephalalgia. 2007;27:97-106.
60. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med. 2004;115:39-44,47-50.
PRACTICE RECOMMENDATIONS
› Offer treatment with a triptan to adult patients with moderate-to-severe episodic migraine. A
› Consider prescribing topiramate, divalproex sodium, metoprolol, propranolol, or the herbal, Petasites hybridum, for the prevention of recurrent episodic migraine that has not responded to a reduction in headache triggers. A
› Add onabotulinumtoxinA injection to your therapeutic toolbox as an effective preventive treatment for chronic migraine (≥15 headache days a month for 3 months). B
› Recommend magnesium and feverfew as adjunctive preventive treatments for migraine. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Aplastic Anemia: Current Treatment
Aplastic anemia is a rare hematologic disorder marked by pancytopenia and a hypocellular marrow. Aplastic anemia results from either inherited or acquired causes, and the treatment approach varies significantly between the 2 causes. This article reviews the treatment of inherited and acquired forms of aplastic anemia. The approach to evaluation and diagnosis of aplastic anemia is reviewed in a separate article.
Inherited Aplastic Anemia
First-line treatment options for patients with inherited marrow failure syndromes (IMFS) are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect.1 Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with bone marrow demonstrating lower rates of acute GVHD than a peripheral blood stem cell source.2-4 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.5,6 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival2,5,7 compared to cyclophosphamide conditioning, which was historically used in matched related donors.6,8 The addition of fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.7,9 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data is limited.5
For patients presenting with acute myeloid leukemia (AML) or a high-risk myelodysplastic syndrome (MDS) who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggests that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased overall survival (OS) despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.10,11 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan is being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.12
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.13,14 Transfusion support is often required to prevent complications associated with thrombocytopenia and anemia; all blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.15 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an absolute neutrophil count [ANC] < 500 cells/µL).16-19 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study by Tichelli and colleagues evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.20 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006).This difference was largely driven by a decrease in infectious episodes in patients with very severe aplastic anemia (VSAA) treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).20
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.21 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.19,22
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.18,19 This appears to be changing with time. Valdez and colleagues demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.22 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.19 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with severe aplastic anema (SAA) or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).17,23 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.17 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues demonstrated that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level: 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).24 Approximately 25% of patients in this trial demonstrated an increase in creatinine, with patients taking concomitant cyclosporine more affected than those on chelation therapy alone.24 For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.15
Approach to Therapy
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.
Matched Sibling Donor Transplant
Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years of age, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).25 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and matched unrelated donor HSCT (38% and 65%).25 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.26
Current conditioning regimens typically use a combination of cyclophosphamide and ATG27,28 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.9 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (RR, 0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.29 In addition, Kim et al evaluated their experience with patients older than 40 years of age receiving matched related donors, finding comparable outcomes in those aged 41 to 50 years compared to younger patients. Outcomes did decline in those over the age of 50 years.30 Long-term data for matched related donor transplant for aplastic anemia shows excellent long-term outcomes, with minimal chronic GVHD and good performance status.31 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and matched unrelated donor (MUD) transplants for pediatric patients32,33 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.34,35
Immunosuppressive Therapy
For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.36 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.16,36,37
Anti-thymocyte globulin. Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.38 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).16 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS: 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Cyclosporine A. Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.39 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.39
The combination of the ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.37 In this study patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.40 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.41 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.42
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.42,43 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.42 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Immunosuppressive therapy plus eltrombopag. Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.44 When given at a dose of 150 mg daily in patients aged 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.44 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.45 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.44 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.46 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.4,22 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant
For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-V GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Additional benefits seen in this analysis included improved survival when transplanted under age 20 years (84% versus 72%), when transplanted within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and when the donor and recipient were cytomegalovirus-negative compared to other combinations (82% versus 76%).47 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.4,43 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).33 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients included underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.4 A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.33 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical paroxysmal nocturnal hemoglobinuria, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.48
With continued improvement of less toxic and more immunomodulating conditioning regimens, utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.49 However, there is still a large population of patients without matched sibling or unrelated donor options. In an effort to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant paroxysmal nocturnal hemoglobinuria, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.50
Summary
Aplastic anemia is a rare but potentially life-threatening disorder characterized by pancytopenia and a marked reduction in the hematopoietic stem cell compartment. Treatment should be instituted as soon as the dignosis of aplastic anemia is established. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
1. Peffault De Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience.” Blood. 2013;122:4279-4286.
2. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
3. Eapen M, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
4. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
5. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes.” Bone Marrow Transplant. 2015;50:1168-1172.
6. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
7. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
8 Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
9. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
10. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-200.
11. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
12. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
13. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
14. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
15. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
16. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Eng J Med. 2011;365:430-438.
17. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
18. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Sem Hematol. 2009;46:269-276.
19. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
20. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
21. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
22. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
23. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
24. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2554.
25. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-8.
26. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
27. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
28. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. ASH Education Program Book. 2013;2013:82-86.
29. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
30. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
31. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
32. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
33. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br. J Haematol. 2015;151:585-594.
34. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
35. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
36. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
37. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al, German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
38. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
39. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
40. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
41. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
42. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
43. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
44. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
45. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
46. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018 Oct 11. doi: 10.1002/cncr.31658.
47. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
48. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. 2018Oct 17. doi: 10.1002/ajh.25314.
49. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
50. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
Aplastic anemia is a rare hematologic disorder marked by pancytopenia and a hypocellular marrow. Aplastic anemia results from either inherited or acquired causes, and the treatment approach varies significantly between the 2 causes. This article reviews the treatment of inherited and acquired forms of aplastic anemia. The approach to evaluation and diagnosis of aplastic anemia is reviewed in a separate article.
Inherited Aplastic Anemia
First-line treatment options for patients with inherited marrow failure syndromes (IMFS) are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect.1 Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with bone marrow demonstrating lower rates of acute GVHD than a peripheral blood stem cell source.2-4 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.5,6 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival2,5,7 compared to cyclophosphamide conditioning, which was historically used in matched related donors.6,8 The addition of fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.7,9 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data is limited.5
For patients presenting with acute myeloid leukemia (AML) or a high-risk myelodysplastic syndrome (MDS) who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggests that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased overall survival (OS) despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.10,11 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan is being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.12
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.13,14 Transfusion support is often required to prevent complications associated with thrombocytopenia and anemia; all blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.15 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an absolute neutrophil count [ANC] < 500 cells/µL).16-19 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study by Tichelli and colleagues evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.20 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006).This difference was largely driven by a decrease in infectious episodes in patients with very severe aplastic anemia (VSAA) treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).20
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.21 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.19,22
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.18,19 This appears to be changing with time. Valdez and colleagues demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.22 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.19 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with severe aplastic anema (SAA) or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).17,23 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.17 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues demonstrated that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level: 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).24 Approximately 25% of patients in this trial demonstrated an increase in creatinine, with patients taking concomitant cyclosporine more affected than those on chelation therapy alone.24 For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.15
Approach to Therapy
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.


Matched Sibling Donor Transplant
Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years of age, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).25 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and matched unrelated donor HSCT (38% and 65%).25 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.26
Current conditioning regimens typically use a combination of cyclophosphamide and ATG27,28 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.9 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (RR, 0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.29 In addition, Kim et al evaluated their experience with patients older than 40 years of age receiving matched related donors, finding comparable outcomes in those aged 41 to 50 years compared to younger patients. Outcomes did decline in those over the age of 50 years.30 Long-term data for matched related donor transplant for aplastic anemia shows excellent long-term outcomes, with minimal chronic GVHD and good performance status.31 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and matched unrelated donor (MUD) transplants for pediatric patients32,33 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.34,35
Immunosuppressive Therapy
For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.36 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.16,36,37
Anti-thymocyte globulin. Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.38 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).16 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS: 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Cyclosporine A. Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.39 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.39
The combination of the ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.37 In this study patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.40 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.41 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.42
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.42,43 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.42 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Immunosuppressive therapy plus eltrombopag. Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.44 When given at a dose of 150 mg daily in patients aged 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.44 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.45 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.44 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.46 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.4,22 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant
For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-V GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Additional benefits seen in this analysis included improved survival when transplanted under age 20 years (84% versus 72%), when transplanted within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and when the donor and recipient were cytomegalovirus-negative compared to other combinations (82% versus 76%).47 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.4,43 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).33 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients included underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.4 A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.33 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical paroxysmal nocturnal hemoglobinuria, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.48
With continued improvement of less toxic and more immunomodulating conditioning regimens, utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.49 However, there is still a large population of patients without matched sibling or unrelated donor options. In an effort to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant paroxysmal nocturnal hemoglobinuria, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.50
Summary
Aplastic anemia is a rare but potentially life-threatening disorder characterized by pancytopenia and a marked reduction in the hematopoietic stem cell compartment. Treatment should be instituted as soon as the dignosis of aplastic anemia is established. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
Aplastic anemia is a rare hematologic disorder marked by pancytopenia and a hypocellular marrow. Aplastic anemia results from either inherited or acquired causes, and the treatment approach varies significantly between the 2 causes. This article reviews the treatment of inherited and acquired forms of aplastic anemia. The approach to evaluation and diagnosis of aplastic anemia is reviewed in a separate article.
Inherited Aplastic Anemia
First-line treatment options for patients with inherited marrow failure syndromes (IMFS) are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect.1 Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with bone marrow demonstrating lower rates of acute GVHD than a peripheral blood stem cell source.2-4 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.5,6 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival2,5,7 compared to cyclophosphamide conditioning, which was historically used in matched related donors.6,8 The addition of fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.7,9 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data is limited.5
For patients presenting with acute myeloid leukemia (AML) or a high-risk myelodysplastic syndrome (MDS) who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggests that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased overall survival (OS) despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.10,11 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan is being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.12
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.13,14 Transfusion support is often required to prevent complications associated with thrombocytopenia and anemia; all blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.15 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an absolute neutrophil count [ANC] < 500 cells/µL).16-19 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study by Tichelli and colleagues evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.20 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006).This difference was largely driven by a decrease in infectious episodes in patients with very severe aplastic anemia (VSAA) treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).20
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.21 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.19,22
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.18,19 This appears to be changing with time. Valdez and colleagues demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.22 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.19 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with severe aplastic anema (SAA) or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).17,23 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.17 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues demonstrated that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level: 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).24 Approximately 25% of patients in this trial demonstrated an increase in creatinine, with patients taking concomitant cyclosporine more affected than those on chelation therapy alone.24 For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.15
Approach to Therapy
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.


Matched Sibling Donor Transplant
Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years of age, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).25 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and matched unrelated donor HSCT (38% and 65%).25 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.26
Current conditioning regimens typically use a combination of cyclophosphamide and ATG27,28 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.9 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (RR, 0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.29 In addition, Kim et al evaluated their experience with patients older than 40 years of age receiving matched related donors, finding comparable outcomes in those aged 41 to 50 years compared to younger patients. Outcomes did decline in those over the age of 50 years.30 Long-term data for matched related donor transplant for aplastic anemia shows excellent long-term outcomes, with minimal chronic GVHD and good performance status.31 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and matched unrelated donor (MUD) transplants for pediatric patients32,33 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.34,35
Immunosuppressive Therapy
For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.36 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.16,36,37
Anti-thymocyte globulin. Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.38 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).16 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS: 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Cyclosporine A. Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.39 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.39
The combination of the ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.37 In this study patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.40 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.41 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.42
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.42,43 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.42 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Immunosuppressive therapy plus eltrombopag. Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.44 When given at a dose of 150 mg daily in patients aged 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.44 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.45 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.44 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.46 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.4,22 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant
For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-V GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Additional benefits seen in this analysis included improved survival when transplanted under age 20 years (84% versus 72%), when transplanted within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and when the donor and recipient were cytomegalovirus-negative compared to other combinations (82% versus 76%).47 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.4,43 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).33 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients included underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.4 A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.33 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical paroxysmal nocturnal hemoglobinuria, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.48
With continued improvement of less toxic and more immunomodulating conditioning regimens, utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.49 However, there is still a large population of patients without matched sibling or unrelated donor options. In an effort to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant paroxysmal nocturnal hemoglobinuria, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.50
Summary
Aplastic anemia is a rare but potentially life-threatening disorder characterized by pancytopenia and a marked reduction in the hematopoietic stem cell compartment. Treatment should be instituted as soon as the dignosis of aplastic anemia is established. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
1. Peffault De Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience.” Blood. 2013;122:4279-4286.
2. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
3. Eapen M, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
4. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
5. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes.” Bone Marrow Transplant. 2015;50:1168-1172.
6. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
7. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
8 Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
9. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
10. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-200.
11. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
12. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
13. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
14. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
15. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
16. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Eng J Med. 2011;365:430-438.
17. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
18. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Sem Hematol. 2009;46:269-276.
19. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
20. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
21. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
22. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
23. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
24. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2554.
25. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-8.
26. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
27. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
28. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. ASH Education Program Book. 2013;2013:82-86.
29. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
30. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
31. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
32. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
33. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br. J Haematol. 2015;151:585-594.
34. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
35. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
36. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
37. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al, German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
38. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
39. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
40. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
41. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
42. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
43. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
44. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
45. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
46. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018 Oct 11. doi: 10.1002/cncr.31658.
47. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
48. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. 2018Oct 17. doi: 10.1002/ajh.25314.
49. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
50. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
1. Peffault De Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience.” Blood. 2013;122:4279-4286.
2. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
3. Eapen M, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
4. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
5. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes.” Bone Marrow Transplant. 2015;50:1168-1172.
6. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
7. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
8 Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
9. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
10. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-200.
11. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
12. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
13. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
14. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
15. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
16. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Eng J Med. 2011;365:430-438.
17. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
18. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Sem Hematol. 2009;46:269-276.
19. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
20. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
21. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
22. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
23. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
24. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2554.
25. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-8.
26. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
27. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
28. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. ASH Education Program Book. 2013;2013:82-86.
29. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
30. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
31. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
32. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
33. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br. J Haematol. 2015;151:585-594.
34. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
35. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
36. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
37. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al, German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
38. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
39. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
40. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
41. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
42. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
43. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
44. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
45. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
46. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018 Oct 11. doi: 10.1002/cncr.31658.
47. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
48. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. 2018Oct 17. doi: 10.1002/ajh.25314.
49. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
50. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
Aplastic Anemia: Evaluation and Diagnosis
Aplastic anemia is a clinical and pathological entity of bone marrow failure that causes progressive loss of hematopoietic progenitor stem cells (HPSC), resulting in pancytopenia.1 Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to having life-threatening neutropenic infections or bleeding. Aplastic anemia results from either inherited or acquired causes, and the pathophysiology and treatment approach vary significantly between these 2 causes. Therefore, recognition of inherited marrow failure diseases, such as Fanconi anemia and telomere biology disorders, is critical to establish
Epidemiology
Aplastic anemia is a rare disorder, with an incidence of approximately 1.5 to 7 cases per million individuals per year.2,3 A recent Scandinavian study reported that the incidence of aplastic anemia among the Swedish population is 2.3 cases per million individuals per year, with a median age at diagnosis of 60 years and a slight female predominance (52% versus 48%, respectively).2 This data is congruent with prior observations made in Barcelona, where the incidence was 2.34 cases per million individuals per year, albeit with a slightly higher incidence in males compared to females (2.54 versus 2.16, respectively).4 The incidence of aplastic anemia varies globally, with a disproportionate increase in incidence seen among Asian populations, with rates as high as 8.8 per million individuals per year.3-5 This variation in incidence in Asia versus other countries has not been well explained. There appears to be a bimodal distribution, with incidence peaks seen in young adults and in older adults.2,3,6
Pathophysiology
Acquired Aplastic Anemia
The leading hypothesis as to the cause of most cases of acquired aplastic anemia is that a dysregulated immune system destroys hematopoietic progenitor cells. Inciting etiologies implicated in the development of acquired aplastic anemia include pregnancy, infection, medications, and exposure to certain chemicals, such as benzene.1,7 The historical understanding of acquired aplastic anemia implicates cytotoxic T-lymphocyte–mediated destruction of CD34+ hematopoietic stem cells.1,8,9 This hypothesis served as the basis for treatment of acquired aplastic anemia with immunosuppressive therapy, predominantly anti-thymocyte globulin (ATG) combined with cyclosporine A.1,8 More recent work has focused on cytokine interactions, particularly the suppressive role of interferon (IFN)-γ on hematopoietic stem cells independent of T-lymphocyte–mediated hematopoietic destruction, which has been demonstrated in a murine model.8 The interaction of IFN-γ with the hematopoietic stem cells pool is dynamic. IFN-γ levels are elevated during an acute inflammatory response such as a viral infection, providing further basis for the immune-mediated nature of the acquired disease.10 Specifically, in vitro studies suggest the effects of IFN-γ on HPSC may be secondary to interruption of thrombopoietin and its respective signaling pathways, which play a key role in hematopoietic stem cell renewal.11 Eltrombopag, a thrombopoietin receptor antagonist, has shown promise in the treatment of refractory aplastic anemia, with studies indicating that its effectiveness is independent of IFN-γ levels.11,12
Inherited Aplastic Anemia
The inherited marrow failure syndromes (IMFSs) are a group of disorders characterized by cellular maintenance and repair defects, leading to cytopenias, increased cancer risk, structural defects, and risk of end organ damage, such as liver cirrhosis and pulmonary fibrosis.13-15 The most common diseases include Fanconi anemia, dyskeratosis congenita/telomere biology disorders, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, but with the advent of whole exome sequencing new syndromes continue to be discovered. While classically these disorders present in children, adult presentations of these syndromes are now commonplace. Broadly, the pathophysiology of inherited aplastic anemia relates to the defective hematopoietic progenitor cells and an accelerated decline of the hematopoietic stem cell compartment.
The most common IMFS, Fanconi anemia and telomere biology disorders, are associated with numerous mutations in DNA damage repair pathways and telomere maintenance pathways. TERT, DKC, and TERC mutations are most commonly associated with dyskeratosis congenita, but may also be found infrequently in patients with aplastic anemia presenting at an older age in the absence of the classic phenotypical features.1,16,17 The recognition of an underlying genetic disorder or telomere biology disorder leading to constitutional aplastic anemia is significant, as these conditions are associated not only with marrow failure, but also endocrinopathies, organ fibrosis, and solid organ malignancies.13-15 In particular, mutations in the TERT and TERC genes have been associated with dyskeratosis congenita as well as pulmonary fibrosis and cirrhosis.18,19 The implications of early diagnosis of an IMFS lie in the approach to treatment and prognosis.
Clonal Disorders and Secondary Malignancies
Myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML) are 2 clonal disorders that may arise from a background of aplastic anemia.9,20,21 Hypoplastic MDS can be difficult to differentiate from aplastic anemia at diagnosis based on morphology alone, although recent work has demonstrated that molecular testing for somatic mutations in ASXL1, DNMT3A, and BCOR can aid in differentiating a subset of aplastic anemia patients who are more likely to progress to MDS.21 Clonal populations of cells harboring 6p uniparental disomy are seen in more than 10% of patients with aplastic anemia on cytogenetic analysis, which can help differentiate the diseases.9 Yoshizato and colleagues found lower rates of ASXL1 and DNMT3A mutations in patients with aplastic anemia as compared with patients with MDS or AML. In this study, patients with aplastic anemia had higher rates of mutations in PIGA (reflecting the increased paroxysmal nocturnal hemoglobinuria [PNH] clonality seen in aplastic anemia) and BCOR.9 Mutations were also found in genes commonly mutated in MDS and AML, including TET2, RUNX1, TP53, and JAK2, albeit at lower frequencies.9 These mutations as a whole have not predicted response to therapy or prognosis. However, when performing survival analysis in patients with specific mutations, those commonly encountered in MDS/AML (ASXL1, DNMT3A, TP53, RUNX1, CSMD1) are associated with faster progression to overt MDS/AML and decreased overall survival (OS),20,21 suggesting these mutations may represent early clonality that can lead to clonal evolution and the development of secondary malignancies. Conversely, mutations in BCOR and BCORL appear to identify patients who may have a favorable outcome in response to immunosuppressive therapy and, similar to patients with PIGA mutations, improved OS.9
Paroxysmal Nocturnal Hemoglobinuria
In addition to having an increased risk of myelodysplasia and malignancy due to the development of a dominant pre-malignant clone, patients with aplastic anemia often harbor progenitor cell clones associated with PNH.1,17 PNH clones have been identified in more than 50% of patients with aplastic anemia.22,23 PNH represents a clonal disorder of hematopoiesis in which cells harbor X-linked somatic mutations in the PIGA gene; this gene encodes a protein responsible for the synthesis of glycosylphosphatidylinositol (GPI) anchors on the cell surface.22,24 The lack of these cell surface proteins, specifically CD55 (also known as decay accelerating factor) and CD59 (also known as membrane inhibitor of reactive lysis), predisposes red cells to increased complement-mediated lysis.25 The exact mechanism for the development of these clones in patients with aplastic anemia is not fully understood. Current theories hypothesize that these clones are protected from the immune-mediated destruction of normal hematopoietic stem cells due to the absence of the cell surface proteins.1,20 The role of these clones over time in patients with aplastic anemia is less clear, though recent work demonstrated that despite differences in clonality over the disease course, aplastic anemia patients with small PNH clones are less likely to develop overt hemolysis and larger PNH clones compared to patients harboring larger (≥ 50%) PNH clones at diagnosis.23,26,27 Additionally, PNH clones in patients with aplastic anemia infrequently become clinically significant.27 It should be noted that these conditions exist along a continuum; that is, patients with aplastic anemia may develop PNH clones, while conversely patients with PNH may develop aplastic anemia.20 Patients with PNH clones should be followed via peripheral blood flow cytometry in addition to complete blood count to track clonal stability and identify clinically significant PNH among aplastic anemia patients.28
Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying, pulmonary, renal, and liver disease, and blood disorders.
Patients with an IMFS, (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, in patients with IMFS, classic phenotypical findings may be lacking in up to 30% to 40% of patients.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
Differential Diagnosis
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7

Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 The typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35
In addition to peripheral blood findings, bone marrow biopsy is essential for the diagnosis, and should demonstrate a markedly hypocellular marrow (cellularity < 25%), occasionally with an increase in T lymphocytes.7,39 Because marrow cellularity varies with age and can be challenging to assess, additional biopsies may be needed to confirm the diagnosis.29 A 1- to 2-cm core biopsy is necessary to confirm hypocellularity, as small areas of residual hematopoiesis may be present and obscure the diagnosis.35
Excluding Hypocellular MDS and IMFS
A diagnostic challenge is the exclusion of hypocellular MDS, especially in the older adult presenting with aplastic anemia, as patients with aplastic anemia may have some degree of erythroid dysplasia on bone marrow morphology.36 The presence of a PNH clone on flow cytometry can aid in diagnosing aplastic anemia and excluding MDS,34 although PNH clones can be present in refractory anemia MDS. Patients with aplastic anemia have a lower ratio of CD34+ cells compared to those with hypoplastic MDS, with one study demonstrating a mean CD34+ percentage of < 0.5% in aplastic anemia versus 3.7% in hypoplastic MDS.40 Cytogenetic and molecular testing can also aid in making this distinction by identifying mutations commonly implicated in MDS.7 The presence of monosomy 7 (-7) in aplastic anemia patients is associated with a poor overall prognosis.34,41
Peripheral blood screening using chromosome breakage analysis (done using either mitomycin C or diepoxybutane as in vitro DNA-crosslinking agents) and telomere length testing (of peripheral blood leukocytes) is necessary to exclude the main IMFS, Fanconi anemia and telomere biology disorders, respectively. Ruling out these conditions is imperative, as the approach to treatment varies significantly between IMFS and aplastic anemia. Patients with shortened telomeres should undergo genetic screening for mutations in the telomere maintenance genes to evaluate the underlying defect leading to shortened telomeres. Patients with increased peripheral blood breakage should have genetic testing to detect mutations associated with Fanconi anemia.
Classification
Once the diagnosis of aplastic anemia has been made, the patient should be classified according to the severity of their disease. Disease severity is determined based on peripheral blood ANC:34 non-severe aplastic anemia (NSAA), ANC > 500 polymorphonuclear neutrophils (PMNs)/µL; severe aplastic anemia (SAA), 200–500 PMNs/µL; and very severe (VSAA), 0–200 PMNs/µL.4,34 Disease classification is important, as VSAA is associated with a decreased OS compared to SAA.2 Disease classification may affect treatment decisions, as patients with NSAA may be observed for a short period of time, while conversely patients with SAA have a worse prognosis with delays in therapy.42-44
Summary
Aplastic anemia is a rare but potentially life-threatening disorder characterized by pancytopenia and a marked reduction in the hematopoietic stem cell compartment. It can be acquired or associated with an IMFS, and the treatment and prognosis vary dramatically between these 2 etiologies. Work-up and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia. After aplastic anemia has been diagnosed, the patient should be classified according to the severity of their disease based on peripheral blood ANC.
1. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519.
2. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
3. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718-1721.
4. Montané E, Ibanez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica. 2008;93:518-523.
5. Ohta A, Nagai M, Nishina M, et al. Incidence of aplastic anemia in Japan: analysis of data from a nationwide registration system. Int J Epidemiol. 2015; 44(suppl_1):i178.
6. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
7. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
8. Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoiesis stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699-3708.
9. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35-47.
10. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479-2486.
11. Cheng H, Cheruku PS, Alvarado L, et al. Interferon-γ perturbs key signaling pathways induced by thrombopoietin, but not eltrombopag, in human hematopoietic stem/progenitor cells. Blood. 2016;128:3870.
12. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
13. Townsley DM, Dumitriu B, Young NS, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922-1931.
14. Feurstein S, Drazer MW, Godley LA. Genetic predisposition to leukemia and other hematologic malignancies. Sem Oncol. 2016;43:598-608.
15. Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775-2783.
16. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16:S119-125.
17. Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446-4455.
18. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
19. Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Resp J. 2016;48:1721-1731.
20. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337-347.
21. Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood. 2014; 124:2698-2704.
22. Mukhina GL, Buckley JT, Barber JP, et al. Multilineage glycosylphosphatidylinositol anchor‐deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476-482.
23. Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45.
24. Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-5340.
25. Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190-198.
26. Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308-1314.
27. Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-1080.
28. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709.
29. Guinan EC. Diagnosis and management of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:76-81.
30. Giampietro PF, Verlander PC, Davis JG, Auerbach AD. Diagnosis of Fanconi anemia in patients without congenital malformations: an international Fanconi Anemia Registry Study. Am J Med Genetics. 1997;68:58-61.
31. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4-10.
32. Giampietro PF, Davis JG, Adler-Brecher B, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116-1120.
33. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
34. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
35. DeZern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol. 2011;4:221-230.
36. Tichelli A, Gratwohl A, Nissen C, et al. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood. 1992;80:337-345.
37. Camitta BM, Storb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645-652.
38. Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988:70:177-182.
39. Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136-2141.
40. Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458-462.
41. Maciejewski JP, Risitano AM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129-3135.
42. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-8.
43. Passweg JR, Socié G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858-864.
44. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95:2119-2125.
Aplastic anemia is a clinical and pathological entity of bone marrow failure that causes progressive loss of hematopoietic progenitor stem cells (HPSC), resulting in pancytopenia.1 Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to having life-threatening neutropenic infections or bleeding. Aplastic anemia results from either inherited or acquired causes, and the pathophysiology and treatment approach vary significantly between these 2 causes. Therefore, recognition of inherited marrow failure diseases, such as Fanconi anemia and telomere biology disorders, is critical to establish
Epidemiology
Aplastic anemia is a rare disorder, with an incidence of approximately 1.5 to 7 cases per million individuals per year.2,3 A recent Scandinavian study reported that the incidence of aplastic anemia among the Swedish population is 2.3 cases per million individuals per year, with a median age at diagnosis of 60 years and a slight female predominance (52% versus 48%, respectively).2 This data is congruent with prior observations made in Barcelona, where the incidence was 2.34 cases per million individuals per year, albeit with a slightly higher incidence in males compared to females (2.54 versus 2.16, respectively).4 The incidence of aplastic anemia varies globally, with a disproportionate increase in incidence seen among Asian populations, with rates as high as 8.8 per million individuals per year.3-5 This variation in incidence in Asia versus other countries has not been well explained. There appears to be a bimodal distribution, with incidence peaks seen in young adults and in older adults.2,3,6
Pathophysiology
Acquired Aplastic Anemia
The leading hypothesis as to the cause of most cases of acquired aplastic anemia is that a dysregulated immune system destroys hematopoietic progenitor cells. Inciting etiologies implicated in the development of acquired aplastic anemia include pregnancy, infection, medications, and exposure to certain chemicals, such as benzene.1,7 The historical understanding of acquired aplastic anemia implicates cytotoxic T-lymphocyte–mediated destruction of CD34+ hematopoietic stem cells.1,8,9 This hypothesis served as the basis for treatment of acquired aplastic anemia with immunosuppressive therapy, predominantly anti-thymocyte globulin (ATG) combined with cyclosporine A.1,8 More recent work has focused on cytokine interactions, particularly the suppressive role of interferon (IFN)-γ on hematopoietic stem cells independent of T-lymphocyte–mediated hematopoietic destruction, which has been demonstrated in a murine model.8 The interaction of IFN-γ with the hematopoietic stem cells pool is dynamic. IFN-γ levels are elevated during an acute inflammatory response such as a viral infection, providing further basis for the immune-mediated nature of the acquired disease.10 Specifically, in vitro studies suggest the effects of IFN-γ on HPSC may be secondary to interruption of thrombopoietin and its respective signaling pathways, which play a key role in hematopoietic stem cell renewal.11 Eltrombopag, a thrombopoietin receptor antagonist, has shown promise in the treatment of refractory aplastic anemia, with studies indicating that its effectiveness is independent of IFN-γ levels.11,12
Inherited Aplastic Anemia
The inherited marrow failure syndromes (IMFSs) are a group of disorders characterized by cellular maintenance and repair defects, leading to cytopenias, increased cancer risk, structural defects, and risk of end organ damage, such as liver cirrhosis and pulmonary fibrosis.13-15 The most common diseases include Fanconi anemia, dyskeratosis congenita/telomere biology disorders, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, but with the advent of whole exome sequencing new syndromes continue to be discovered. While classically these disorders present in children, adult presentations of these syndromes are now commonplace. Broadly, the pathophysiology of inherited aplastic anemia relates to the defective hematopoietic progenitor cells and an accelerated decline of the hematopoietic stem cell compartment.
The most common IMFS, Fanconi anemia and telomere biology disorders, are associated with numerous mutations in DNA damage repair pathways and telomere maintenance pathways. TERT, DKC, and TERC mutations are most commonly associated with dyskeratosis congenita, but may also be found infrequently in patients with aplastic anemia presenting at an older age in the absence of the classic phenotypical features.1,16,17 The recognition of an underlying genetic disorder or telomere biology disorder leading to constitutional aplastic anemia is significant, as these conditions are associated not only with marrow failure, but also endocrinopathies, organ fibrosis, and solid organ malignancies.13-15 In particular, mutations in the TERT and TERC genes have been associated with dyskeratosis congenita as well as pulmonary fibrosis and cirrhosis.18,19 The implications of early diagnosis of an IMFS lie in the approach to treatment and prognosis.
Clonal Disorders and Secondary Malignancies
Myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML) are 2 clonal disorders that may arise from a background of aplastic anemia.9,20,21 Hypoplastic MDS can be difficult to differentiate from aplastic anemia at diagnosis based on morphology alone, although recent work has demonstrated that molecular testing for somatic mutations in ASXL1, DNMT3A, and BCOR can aid in differentiating a subset of aplastic anemia patients who are more likely to progress to MDS.21 Clonal populations of cells harboring 6p uniparental disomy are seen in more than 10% of patients with aplastic anemia on cytogenetic analysis, which can help differentiate the diseases.9 Yoshizato and colleagues found lower rates of ASXL1 and DNMT3A mutations in patients with aplastic anemia as compared with patients with MDS or AML. In this study, patients with aplastic anemia had higher rates of mutations in PIGA (reflecting the increased paroxysmal nocturnal hemoglobinuria [PNH] clonality seen in aplastic anemia) and BCOR.9 Mutations were also found in genes commonly mutated in MDS and AML, including TET2, RUNX1, TP53, and JAK2, albeit at lower frequencies.9 These mutations as a whole have not predicted response to therapy or prognosis. However, when performing survival analysis in patients with specific mutations, those commonly encountered in MDS/AML (ASXL1, DNMT3A, TP53, RUNX1, CSMD1) are associated with faster progression to overt MDS/AML and decreased overall survival (OS),20,21 suggesting these mutations may represent early clonality that can lead to clonal evolution and the development of secondary malignancies. Conversely, mutations in BCOR and BCORL appear to identify patients who may have a favorable outcome in response to immunosuppressive therapy and, similar to patients with PIGA mutations, improved OS.9
Paroxysmal Nocturnal Hemoglobinuria
In addition to having an increased risk of myelodysplasia and malignancy due to the development of a dominant pre-malignant clone, patients with aplastic anemia often harbor progenitor cell clones associated with PNH.1,17 PNH clones have been identified in more than 50% of patients with aplastic anemia.22,23 PNH represents a clonal disorder of hematopoiesis in which cells harbor X-linked somatic mutations in the PIGA gene; this gene encodes a protein responsible for the synthesis of glycosylphosphatidylinositol (GPI) anchors on the cell surface.22,24 The lack of these cell surface proteins, specifically CD55 (also known as decay accelerating factor) and CD59 (also known as membrane inhibitor of reactive lysis), predisposes red cells to increased complement-mediated lysis.25 The exact mechanism for the development of these clones in patients with aplastic anemia is not fully understood. Current theories hypothesize that these clones are protected from the immune-mediated destruction of normal hematopoietic stem cells due to the absence of the cell surface proteins.1,20 The role of these clones over time in patients with aplastic anemia is less clear, though recent work demonstrated that despite differences in clonality over the disease course, aplastic anemia patients with small PNH clones are less likely to develop overt hemolysis and larger PNH clones compared to patients harboring larger (≥ 50%) PNH clones at diagnosis.23,26,27 Additionally, PNH clones in patients with aplastic anemia infrequently become clinically significant.27 It should be noted that these conditions exist along a continuum; that is, patients with aplastic anemia may develop PNH clones, while conversely patients with PNH may develop aplastic anemia.20 Patients with PNH clones should be followed via peripheral blood flow cytometry in addition to complete blood count to track clonal stability and identify clinically significant PNH among aplastic anemia patients.28
Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying, pulmonary, renal, and liver disease, and blood disorders.
Patients with an IMFS, (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, in patients with IMFS, classic phenotypical findings may be lacking in up to 30% to 40% of patients.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
Differential Diagnosis
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7

Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 The typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35
In addition to peripheral blood findings, bone marrow biopsy is essential for the diagnosis, and should demonstrate a markedly hypocellular marrow (cellularity < 25%), occasionally with an increase in T lymphocytes.7,39 Because marrow cellularity varies with age and can be challenging to assess, additional biopsies may be needed to confirm the diagnosis.29 A 1- to 2-cm core biopsy is necessary to confirm hypocellularity, as small areas of residual hematopoiesis may be present and obscure the diagnosis.35
Excluding Hypocellular MDS and IMFS
A diagnostic challenge is the exclusion of hypocellular MDS, especially in the older adult presenting with aplastic anemia, as patients with aplastic anemia may have some degree of erythroid dysplasia on bone marrow morphology.36 The presence of a PNH clone on flow cytometry can aid in diagnosing aplastic anemia and excluding MDS,34 although PNH clones can be present in refractory anemia MDS. Patients with aplastic anemia have a lower ratio of CD34+ cells compared to those with hypoplastic MDS, with one study demonstrating a mean CD34+ percentage of < 0.5% in aplastic anemia versus 3.7% in hypoplastic MDS.40 Cytogenetic and molecular testing can also aid in making this distinction by identifying mutations commonly implicated in MDS.7 The presence of monosomy 7 (-7) in aplastic anemia patients is associated with a poor overall prognosis.34,41
Peripheral blood screening using chromosome breakage analysis (done using either mitomycin C or diepoxybutane as in vitro DNA-crosslinking agents) and telomere length testing (of peripheral blood leukocytes) is necessary to exclude the main IMFS, Fanconi anemia and telomere biology disorders, respectively. Ruling out these conditions is imperative, as the approach to treatment varies significantly between IMFS and aplastic anemia. Patients with shortened telomeres should undergo genetic screening for mutations in the telomere maintenance genes to evaluate the underlying defect leading to shortened telomeres. Patients with increased peripheral blood breakage should have genetic testing to detect mutations associated with Fanconi anemia.
Classification
Once the diagnosis of aplastic anemia has been made, the patient should be classified according to the severity of their disease. Disease severity is determined based on peripheral blood ANC:34 non-severe aplastic anemia (NSAA), ANC > 500 polymorphonuclear neutrophils (PMNs)/µL; severe aplastic anemia (SAA), 200–500 PMNs/µL; and very severe (VSAA), 0–200 PMNs/µL.4,34 Disease classification is important, as VSAA is associated with a decreased OS compared to SAA.2 Disease classification may affect treatment decisions, as patients with NSAA may be observed for a short period of time, while conversely patients with SAA have a worse prognosis with delays in therapy.42-44
Summary
Aplastic anemia is a rare but potentially life-threatening disorder characterized by pancytopenia and a marked reduction in the hematopoietic stem cell compartment. It can be acquired or associated with an IMFS, and the treatment and prognosis vary dramatically between these 2 etiologies. Work-up and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia. After aplastic anemia has been diagnosed, the patient should be classified according to the severity of their disease based on peripheral blood ANC.
Aplastic anemia is a clinical and pathological entity of bone marrow failure that causes progressive loss of hematopoietic progenitor stem cells (HPSC), resulting in pancytopenia.1 Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to having life-threatening neutropenic infections or bleeding. Aplastic anemia results from either inherited or acquired causes, and the pathophysiology and treatment approach vary significantly between these 2 causes. Therefore, recognition of inherited marrow failure diseases, such as Fanconi anemia and telomere biology disorders, is critical to establish
Epidemiology
Aplastic anemia is a rare disorder, with an incidence of approximately 1.5 to 7 cases per million individuals per year.2,3 A recent Scandinavian study reported that the incidence of aplastic anemia among the Swedish population is 2.3 cases per million individuals per year, with a median age at diagnosis of 60 years and a slight female predominance (52% versus 48%, respectively).2 This data is congruent with prior observations made in Barcelona, where the incidence was 2.34 cases per million individuals per year, albeit with a slightly higher incidence in males compared to females (2.54 versus 2.16, respectively).4 The incidence of aplastic anemia varies globally, with a disproportionate increase in incidence seen among Asian populations, with rates as high as 8.8 per million individuals per year.3-5 This variation in incidence in Asia versus other countries has not been well explained. There appears to be a bimodal distribution, with incidence peaks seen in young adults and in older adults.2,3,6
Pathophysiology
Acquired Aplastic Anemia
The leading hypothesis as to the cause of most cases of acquired aplastic anemia is that a dysregulated immune system destroys hematopoietic progenitor cells. Inciting etiologies implicated in the development of acquired aplastic anemia include pregnancy, infection, medications, and exposure to certain chemicals, such as benzene.1,7 The historical understanding of acquired aplastic anemia implicates cytotoxic T-lymphocyte–mediated destruction of CD34+ hematopoietic stem cells.1,8,9 This hypothesis served as the basis for treatment of acquired aplastic anemia with immunosuppressive therapy, predominantly anti-thymocyte globulin (ATG) combined with cyclosporine A.1,8 More recent work has focused on cytokine interactions, particularly the suppressive role of interferon (IFN)-γ on hematopoietic stem cells independent of T-lymphocyte–mediated hematopoietic destruction, which has been demonstrated in a murine model.8 The interaction of IFN-γ with the hematopoietic stem cells pool is dynamic. IFN-γ levels are elevated during an acute inflammatory response such as a viral infection, providing further basis for the immune-mediated nature of the acquired disease.10 Specifically, in vitro studies suggest the effects of IFN-γ on HPSC may be secondary to interruption of thrombopoietin and its respective signaling pathways, which play a key role in hematopoietic stem cell renewal.11 Eltrombopag, a thrombopoietin receptor antagonist, has shown promise in the treatment of refractory aplastic anemia, with studies indicating that its effectiveness is independent of IFN-γ levels.11,12
Inherited Aplastic Anemia
The inherited marrow failure syndromes (IMFSs) are a group of disorders characterized by cellular maintenance and repair defects, leading to cytopenias, increased cancer risk, structural defects, and risk of end organ damage, such as liver cirrhosis and pulmonary fibrosis.13-15 The most common diseases include Fanconi anemia, dyskeratosis congenita/telomere biology disorders, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, but with the advent of whole exome sequencing new syndromes continue to be discovered. While classically these disorders present in children, adult presentations of these syndromes are now commonplace. Broadly, the pathophysiology of inherited aplastic anemia relates to the defective hematopoietic progenitor cells and an accelerated decline of the hematopoietic stem cell compartment.
The most common IMFS, Fanconi anemia and telomere biology disorders, are associated with numerous mutations in DNA damage repair pathways and telomere maintenance pathways. TERT, DKC, and TERC mutations are most commonly associated with dyskeratosis congenita, but may also be found infrequently in patients with aplastic anemia presenting at an older age in the absence of the classic phenotypical features.1,16,17 The recognition of an underlying genetic disorder or telomere biology disorder leading to constitutional aplastic anemia is significant, as these conditions are associated not only with marrow failure, but also endocrinopathies, organ fibrosis, and solid organ malignancies.13-15 In particular, mutations in the TERT and TERC genes have been associated with dyskeratosis congenita as well as pulmonary fibrosis and cirrhosis.18,19 The implications of early diagnosis of an IMFS lie in the approach to treatment and prognosis.
Clonal Disorders and Secondary Malignancies
Myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML) are 2 clonal disorders that may arise from a background of aplastic anemia.9,20,21 Hypoplastic MDS can be difficult to differentiate from aplastic anemia at diagnosis based on morphology alone, although recent work has demonstrated that molecular testing for somatic mutations in ASXL1, DNMT3A, and BCOR can aid in differentiating a subset of aplastic anemia patients who are more likely to progress to MDS.21 Clonal populations of cells harboring 6p uniparental disomy are seen in more than 10% of patients with aplastic anemia on cytogenetic analysis, which can help differentiate the diseases.9 Yoshizato and colleagues found lower rates of ASXL1 and DNMT3A mutations in patients with aplastic anemia as compared with patients with MDS or AML. In this study, patients with aplastic anemia had higher rates of mutations in PIGA (reflecting the increased paroxysmal nocturnal hemoglobinuria [PNH] clonality seen in aplastic anemia) and BCOR.9 Mutations were also found in genes commonly mutated in MDS and AML, including TET2, RUNX1, TP53, and JAK2, albeit at lower frequencies.9 These mutations as a whole have not predicted response to therapy or prognosis. However, when performing survival analysis in patients with specific mutations, those commonly encountered in MDS/AML (ASXL1, DNMT3A, TP53, RUNX1, CSMD1) are associated with faster progression to overt MDS/AML and decreased overall survival (OS),20,21 suggesting these mutations may represent early clonality that can lead to clonal evolution and the development of secondary malignancies. Conversely, mutations in BCOR and BCORL appear to identify patients who may have a favorable outcome in response to immunosuppressive therapy and, similar to patients with PIGA mutations, improved OS.9
Paroxysmal Nocturnal Hemoglobinuria
In addition to having an increased risk of myelodysplasia and malignancy due to the development of a dominant pre-malignant clone, patients with aplastic anemia often harbor progenitor cell clones associated with PNH.1,17 PNH clones have been identified in more than 50% of patients with aplastic anemia.22,23 PNH represents a clonal disorder of hematopoiesis in which cells harbor X-linked somatic mutations in the PIGA gene; this gene encodes a protein responsible for the synthesis of glycosylphosphatidylinositol (GPI) anchors on the cell surface.22,24 The lack of these cell surface proteins, specifically CD55 (also known as decay accelerating factor) and CD59 (also known as membrane inhibitor of reactive lysis), predisposes red cells to increased complement-mediated lysis.25 The exact mechanism for the development of these clones in patients with aplastic anemia is not fully understood. Current theories hypothesize that these clones are protected from the immune-mediated destruction of normal hematopoietic stem cells due to the absence of the cell surface proteins.1,20 The role of these clones over time in patients with aplastic anemia is less clear, though recent work demonstrated that despite differences in clonality over the disease course, aplastic anemia patients with small PNH clones are less likely to develop overt hemolysis and larger PNH clones compared to patients harboring larger (≥ 50%) PNH clones at diagnosis.23,26,27 Additionally, PNH clones in patients with aplastic anemia infrequently become clinically significant.27 It should be noted that these conditions exist along a continuum; that is, patients with aplastic anemia may develop PNH clones, while conversely patients with PNH may develop aplastic anemia.20 Patients with PNH clones should be followed via peripheral blood flow cytometry in addition to complete blood count to track clonal stability and identify clinically significant PNH among aplastic anemia patients.28
Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying, pulmonary, renal, and liver disease, and blood disorders.
Patients with an IMFS, (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, in patients with IMFS, classic phenotypical findings may be lacking in up to 30% to 40% of patients.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
Differential Diagnosis
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7

Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 The typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35
In addition to peripheral blood findings, bone marrow biopsy is essential for the diagnosis, and should demonstrate a markedly hypocellular marrow (cellularity < 25%), occasionally with an increase in T lymphocytes.7,39 Because marrow cellularity varies with age and can be challenging to assess, additional biopsies may be needed to confirm the diagnosis.29 A 1- to 2-cm core biopsy is necessary to confirm hypocellularity, as small areas of residual hematopoiesis may be present and obscure the diagnosis.35
Excluding Hypocellular MDS and IMFS
A diagnostic challenge is the exclusion of hypocellular MDS, especially in the older adult presenting with aplastic anemia, as patients with aplastic anemia may have some degree of erythroid dysplasia on bone marrow morphology.36 The presence of a PNH clone on flow cytometry can aid in diagnosing aplastic anemia and excluding MDS,34 although PNH clones can be present in refractory anemia MDS. Patients with aplastic anemia have a lower ratio of CD34+ cells compared to those with hypoplastic MDS, with one study demonstrating a mean CD34+ percentage of < 0.5% in aplastic anemia versus 3.7% in hypoplastic MDS.40 Cytogenetic and molecular testing can also aid in making this distinction by identifying mutations commonly implicated in MDS.7 The presence of monosomy 7 (-7) in aplastic anemia patients is associated with a poor overall prognosis.34,41
Peripheral blood screening using chromosome breakage analysis (done using either mitomycin C or diepoxybutane as in vitro DNA-crosslinking agents) and telomere length testing (of peripheral blood leukocytes) is necessary to exclude the main IMFS, Fanconi anemia and telomere biology disorders, respectively. Ruling out these conditions is imperative, as the approach to treatment varies significantly between IMFS and aplastic anemia. Patients with shortened telomeres should undergo genetic screening for mutations in the telomere maintenance genes to evaluate the underlying defect leading to shortened telomeres. Patients with increased peripheral blood breakage should have genetic testing to detect mutations associated with Fanconi anemia.
Classification
Once the diagnosis of aplastic anemia has been made, the patient should be classified according to the severity of their disease. Disease severity is determined based on peripheral blood ANC:34 non-severe aplastic anemia (NSAA), ANC > 500 polymorphonuclear neutrophils (PMNs)/µL; severe aplastic anemia (SAA), 200–500 PMNs/µL; and very severe (VSAA), 0–200 PMNs/µL.4,34 Disease classification is important, as VSAA is associated with a decreased OS compared to SAA.2 Disease classification may affect treatment decisions, as patients with NSAA may be observed for a short period of time, while conversely patients with SAA have a worse prognosis with delays in therapy.42-44
Summary
Aplastic anemia is a rare but potentially life-threatening disorder characterized by pancytopenia and a marked reduction in the hematopoietic stem cell compartment. It can be acquired or associated with an IMFS, and the treatment and prognosis vary dramatically between these 2 etiologies. Work-up and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia. After aplastic anemia has been diagnosed, the patient should be classified according to the severity of their disease based on peripheral blood ANC.
1. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519.
2. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
3. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718-1721.
4. Montané E, Ibanez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica. 2008;93:518-523.
5. Ohta A, Nagai M, Nishina M, et al. Incidence of aplastic anemia in Japan: analysis of data from a nationwide registration system. Int J Epidemiol. 2015; 44(suppl_1):i178.
6. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
7. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
8. Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoiesis stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699-3708.
9. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35-47.
10. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479-2486.
11. Cheng H, Cheruku PS, Alvarado L, et al. Interferon-γ perturbs key signaling pathways induced by thrombopoietin, but not eltrombopag, in human hematopoietic stem/progenitor cells. Blood. 2016;128:3870.
12. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
13. Townsley DM, Dumitriu B, Young NS, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922-1931.
14. Feurstein S, Drazer MW, Godley LA. Genetic predisposition to leukemia and other hematologic malignancies. Sem Oncol. 2016;43:598-608.
15. Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775-2783.
16. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16:S119-125.
17. Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446-4455.
18. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
19. Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Resp J. 2016;48:1721-1731.
20. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337-347.
21. Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood. 2014; 124:2698-2704.
22. Mukhina GL, Buckley JT, Barber JP, et al. Multilineage glycosylphosphatidylinositol anchor‐deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476-482.
23. Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45.
24. Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-5340.
25. Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190-198.
26. Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308-1314.
27. Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-1080.
28. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709.
29. Guinan EC. Diagnosis and management of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:76-81.
30. Giampietro PF, Verlander PC, Davis JG, Auerbach AD. Diagnosis of Fanconi anemia in patients without congenital malformations: an international Fanconi Anemia Registry Study. Am J Med Genetics. 1997;68:58-61.
31. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4-10.
32. Giampietro PF, Davis JG, Adler-Brecher B, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116-1120.
33. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
34. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
35. DeZern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol. 2011;4:221-230.
36. Tichelli A, Gratwohl A, Nissen C, et al. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood. 1992;80:337-345.
37. Camitta BM, Storb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645-652.
38. Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988:70:177-182.
39. Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136-2141.
40. Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458-462.
41. Maciejewski JP, Risitano AM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129-3135.
42. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-8.
43. Passweg JR, Socié G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858-864.
44. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95:2119-2125.
1. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519.
2. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
3. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718-1721.
4. Montané E, Ibanez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica. 2008;93:518-523.
5. Ohta A, Nagai M, Nishina M, et al. Incidence of aplastic anemia in Japan: analysis of data from a nationwide registration system. Int J Epidemiol. 2015; 44(suppl_1):i178.
6. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
7. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
8. Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoiesis stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699-3708.
9. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35-47.
10. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479-2486.
11. Cheng H, Cheruku PS, Alvarado L, et al. Interferon-γ perturbs key signaling pathways induced by thrombopoietin, but not eltrombopag, in human hematopoietic stem/progenitor cells. Blood. 2016;128:3870.
12. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
13. Townsley DM, Dumitriu B, Young NS, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922-1931.
14. Feurstein S, Drazer MW, Godley LA. Genetic predisposition to leukemia and other hematologic malignancies. Sem Oncol. 2016;43:598-608.
15. Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775-2783.
16. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16:S119-125.
17. Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446-4455.
18. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
19. Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Resp J. 2016;48:1721-1731.
20. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337-347.
21. Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood. 2014; 124:2698-2704.
22. Mukhina GL, Buckley JT, Barber JP, et al. Multilineage glycosylphosphatidylinositol anchor‐deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476-482.
23. Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45.
24. Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-5340.
25. Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190-198.
26. Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308-1314.
27. Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-1080.
28. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709.
29. Guinan EC. Diagnosis and management of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:76-81.
30. Giampietro PF, Verlander PC, Davis JG, Auerbach AD. Diagnosis of Fanconi anemia in patients without congenital malformations: an international Fanconi Anemia Registry Study. Am J Med Genetics. 1997;68:58-61.
31. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4-10.
32. Giampietro PF, Davis JG, Adler-Brecher B, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116-1120.
33. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
34. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
35. DeZern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol. 2011;4:221-230.
36. Tichelli A, Gratwohl A, Nissen C, et al. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood. 1992;80:337-345.
37. Camitta BM, Storb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645-652.
38. Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988:70:177-182.
39. Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136-2141.
40. Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458-462.
41. Maciejewski JP, Risitano AM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129-3135.
42. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-8.
43. Passweg JR, Socié G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858-864.
44. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95:2119-2125.
2019 Update on Obstetrics
The past year was an exciting one in obstetrics. The landmark ARRIVE trial presented at the Society for Maternal-Fetal Medicine’s (SMFM) annual meeting and subsequently published in the New England Journal of Medicine contradicted a long-held belief about the safety of elective labor induction. In a large randomized trial, Cahill and colleagues took a controversial but practical clinical question about second-stage labor management and answered it for the practicing obstetrician in the trenches. Finally, the American College of Obstetricians and Gynecologists (ACOG) placed new emphasis on the oft overlooked but increasingly more complicated postpartum period, offering guidance to support improving care for women in this transitional period.
Ultimately, this was the year of the patient, as research, clinical guidelines, and education focused on how to achieve the best in safety and quality of care for delivery planning, the delivery itself, and the so-called fourth trimester.
ARRIVE: Labor induction at 39 weeks reduces CD rate with no difference in perinatal death or serious outcomes
Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
The term "elective induction of labor" has long had a negative connotation because of its association with increased CD rates and adverse perinatal outcomes. This view was based on results from older observational studies that compared outcomes for labor induction with those of spontaneous labor. In more recent observational studies that more appropriately compared labor induction with expectant management, however, elective induction of labor appears to be associated with similar CD rates and perinatal outcomes.
To test the hypothesis that elective induction would have a lower risk for perinatal death or severe neonatal complications than expectant management in low-risk nulliparous women, Grobman and colleagues conducted A Randomized Trial of Induction Versus Expectant Management (ARRIVE).1
Study population, timing of delivery, and trial outcomes
This randomized controlled trial included 6,106 women at 41 US centers in the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Study participants were low-risk nulliparous women with a singleton vertex fetus who were randomly assigned to induction of labor at 39 to 39 4/7 weeks (n = 3,062) or expectant management (n = 3,044) until 40 5/7 to 42 2/7 weeks.
"Low risk" was defined as having no maternal or fetal indication for delivery prior to 40 5/7 weeks. Reliable gestational dating was required.
While no specific protocol for induction of labor management was required, there were 2 requests: 1) Cervical ripening was requested for an unfavorable cervix (63% of participants had a modified Bishop score <5), and 2) a duration of at least 12 hours after cervical ripening, rupture of membranes, and use of uterine stimulant was requested before performing a CD for "failed induction" (if medically appropriate).
The primary outcome was a composite of perinatal death or serious neonatal complications. The main secondary outcome was CD.
Potentially game-changing findings
The investigators found that there was no statistically significant difference between the elective induction and expectant management groups for the primary composite perinatal outcome (4.3% vs 5.4%; P = .049, with P<.046 prespecified for significance). In addition, the rate of CD was significantly lower in the labor induction group than in the expectant management group (18.6% vs 22.2%; P<.001).
Other significant findings in secondary outcomes included the following:
- Hypertensive disorders of pregnancy were significantly lower in the labor induction group compared with the expectant management group (9.1% vs 14.1%; P<.001).
- The labor induction group had a longer length of stay in the labor and delivery unit but a shorter postpartum hospital stay.
- The labor induction group reported less pain and more control during labor.
Results refute negative notion of elective labor induction
The authors concluded that in a low-risk nulliparous patient population, elective induction of labor at 39 weeks does not increase the risk for adverse perinatal outcomes and decreases the rate of CD and hypertensive disorders of pregnancy. Additionally, they noted that induction at 39 weeks should not be avoided with the goal of preventing CD, as even women with an unfavorable cervix had a lower rate of CD in the induction group compared with the expectant management group.
After publication of the ARRIVE trial findings, both ACOG and SMFM released statements supporting elective labor induction at or beyond 39 weeks’ gestation in low-risk nulliparous women with good gestational dating.2,3 They cited the following as important issues: adherence to the trial inclusion criteria except for research purposes, shared decision-making with the patient, consideration of the logistics and impact on the health care facility, and the yet unknown impact on cost. Finally, it should be a priority to avoid the primary CD for a failed induction by allowing a longer latent phase of labor, as long as maternal and fetal conditions allow. In my practice, I actively offer induction of labor to most of my patients at 39 weeks after a discussion of the risks and benefits.
Continue to: Immediate pushing in second stage...
Immediate pushing in second stage offers benefits and is preferable to delayed pushing
Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
In a randomized trial of 2,414 women, Cahill and colleagues sought to answer a seemingly simple question: What is the best timing for pushing during the second stage of labor--immediate or delayed?
Practical management of the second stage of labor (defined as complete cervical dilation to the delivery of the infant) varies by provider and setting, and previous data on pushing efforts are conflicting. Delayed pushing, or "laboring down," has been suggested to allow passive fetal rotation and to conserve maternal energy for pushing. Older studies have shown that delayed pushing decreases the rate of operative delivery. More recent study data have not demonstrated a difference between immediate and delayed pushing techniques on vaginal delivery rates and have noted that increased maternal and neonatal morbidities are associated with a longer second stage of labor.
The recent trial by Cahill and colleagues was designed to determine the effect of these 2 techniques on spontaneous vaginal delivery rates and on maternal and neonatal morbidities.4
Large study population
This randomized pragmatic trial was conducted at 6 centers in the United States. Study participants (2,404 women completed the study) were nulliparous women at 37 or more weeks' gestation with neuraxial anesthesia who were randomly assigned at complete cervical dilation either to immediate pushing (n = 1,200) or to delayed pushing, that is, instructed to wait 60 minutes before starting to push (n = 1,204). The obstetric provider determined the rest of the labor management.
The primary outcome was the rate of spontaneous vaginal delivery. Secondary outcomes included duration of the second stage of labor, duration of active pushing, operative vaginal delivery, CD, and several maternal assessments (postpartum hemorrhage, chorioamnionitis, endometritis, and perineal lacerations).
Both groups had similar vaginal delivery rates, differences in some measures
There was no difference in the primary outcome between the 2 groups: The spontaneous vaginal delivery rate was 85.9% (n = 1,031) in the immediate pushing group and 86.5% (n = 1,041) in the delayed pushing group (P = .67).
Analysis of secondary outcomes revealed several significant differences:
- decreased total time for the second stage of labor in the immediate pushing group compared with the delayed pushing group (102.4 vs 134.2 minutes) but longer active pushing time (83.7 vs 74.5 minutes)
- a lower rate of postpartum hemorrhage, chorioamnionitis in the second stage, neonatal acidemia, and suspected neonatal sepsis in the immediate pushing group
- a higher rate of third-degree perineal lacerations in the immediate pushing group.
No difference was found between groups in rates of operative vaginal deliveries, CDs, endometritis, overall perineal lacerations, or spontaneous vaginal delivery by fetal station or occiput position.
Authors' takeaway
The authors concluded that since delayed pushing does not increase spontaneous vaginal delivery rates and increases the duration of the second stage of labor and both maternal and neonatal morbidity, immediate pushing may be preferred in this patient population.
After reviewing the available literature in light of this study’s findings, ACOG released a practice advisory in October 2018 stating that “it is reasonable to choose immediate over delayed pushing in nulliparous patients with neuraxial anesthesia.”5 Nulliparous patients with neuraxial anesthesia should be counseled that delayed pushing does not increase the rate of spontaneous vaginal birth and may increase both maternal and neonatal complications. As this may be a practice change for many obstetrics units, the obstetric nursing department should be included in this education and counseling. In my practice, I would recommend immediate pushing, but it is important to include both the patient and her nurse in the discussion.
ACOG aims to optimize postpartum care
American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
In May 2018, ACOG released "Optimizing postpartum care," a committee opinion that proposes a new model of comprehensive postpartum care focused on improving both short- and long-term health outcomes for women and infants. (This replaces the June 2016 committee opinion No. 666.) Described as "the fourth trimester," the postpartum period is a critical transitional period in which both pregnancy-related and pre-existing conditions may affect maternal, neonatal, and family status; half of pregnancy-related maternal deaths occur during the postpartum period.6
The postpartum visit: Often a lost opportunity
ACOG cites that up to 40% of women in the United States do not attend their postpartum visit.6 Many aspects of the postpartum visit, including follow-up for chronic diseases, mental health screening, and contraceptive counseling, provide opportunities for acute intervention as well as establishment of healthy behaviors. Some studies have shown that postpartum depression, breastfeeding, and patient satisfaction outcomes improve as a result of postpartum engagement.
Continue to: ACOG's recommendations...
ACOG's recommendations
Ongoing process. ACOG's first proposed change concerns the structure of the postpartum visit itself, which traditionally has been a single visit with a provider at approximately 6 weeks postpartum. Postpartum care plans actually should be started before birth, during regular prenatal care, and adjusted in the hospital as needed so that the provider can educate patients about the issues they may face and resources they may need during this time. This prenatal preparation hopefully will encourage more patients to attend their postpartum visits.
Increased provider contact. Another proposed change is that after delivery, the patient should have contact with a provider within the first 3 weeks postpartum. For high-risk patients, this may involve an in-person clinic visit as soon as 3 to 10 days postpartum (for hypertensive disorders of pregnancy) or at 1 to 2 weeks (for postpartum depression screening, incision checks, and lactation issues). For lower-risk patients, a phone call may be appropriate and/or preferred. Ongoing follow-up for all patients before the final postpartum visit should be individualized.
Postpartum visit and care transition. ACOG recommends a comprehensive postpartum visit at 4 to 12 weeks to fully evaluate the woman's physical, social, and psychologic well-being and to serve as a transition from pregnancy care to well-woman care. This is a large order and includes evaluation of the following:
- mood and emotional well-being
- infant care and feeding
- sexuality, contraception, and birth spacing
- sleep and fatigue
- physical recovery from birth
- chronic disease management and transition to primary care provider
- health maintenance
- review of labor and delivery course if needed
- review of risks and recommendations for future pregnancies.
After these components are addressed, it is expected that the patient will be transitioned to a primary care provider (who may continue to be the ObGyn, as appropriate) to coordinate her future care in the primary medical home.
Useful resource for adopting new paradigm
ACOG's recommendations are somewhat daunting, and these changes will require education and resources, a significant increase in obstetric provider time and effort, and consideration of policy change regarding such issues as parental leave and postpartum care reimbursement. As a start, ACOG has developed an online aid for health care providers called "Postpartum toolkit" (https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit), which provides education and resources for all steps in the process and can be individualized for each practice and patient.7
Postpartum care should be seen as an ongoing process to address both short- and long-term health outcomes for the patient, her newborn, and their family. This process should begin with planning in the antenatal period, continue with close individualized follow-up within the first 3 weeks of birth, and conclude with a comprehensive postpartum evaluation and transition to well-woman care. Shifting the paradigm of postpartum care will take considerable commitment and resources on the part of obstetric providers and their practices. In my practice, we routinely see hypertensive patients within the first week postpartum and patients at risk for postpartum depression within the first 2 weeks in our clinics. We have a standard 6-week postpartum visit for all patients as well. Going forward, we need to further determine how and when we can implement ACOG’s extensive new recommendations for optimizing postpartum care.
- Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists. Practice advisory: clinical guidance for integration of the findings of the ARRIVE trial: Labor induction versus expectant management in low-risk nulliparous women. August 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Clinical-guidance-for-integration-of-the-findings-of-The-ARRIVE-Trial. Accessed November 25, 2018.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2018.08.009. In press.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
- American College of Obstetricians and Gynecologists. Practice advisory: immediate versus delayed pushing in nulliparous women receiving neuraxial analgesia. October 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Immediate-vs-delayed-pushing-in-nulliparous-women-receiving-neuraxial-analgesia. Accessed November 25, 2018.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
- American College of Obstetricians and Gynecologists. ACOG Postpartum toolkit. https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit. Accessed November 25, 2018.
The past year was an exciting one in obstetrics. The landmark ARRIVE trial presented at the Society for Maternal-Fetal Medicine’s (SMFM) annual meeting and subsequently published in the New England Journal of Medicine contradicted a long-held belief about the safety of elective labor induction. In a large randomized trial, Cahill and colleagues took a controversial but practical clinical question about second-stage labor management and answered it for the practicing obstetrician in the trenches. Finally, the American College of Obstetricians and Gynecologists (ACOG) placed new emphasis on the oft overlooked but increasingly more complicated postpartum period, offering guidance to support improving care for women in this transitional period.
Ultimately, this was the year of the patient, as research, clinical guidelines, and education focused on how to achieve the best in safety and quality of care for delivery planning, the delivery itself, and the so-called fourth trimester.
ARRIVE: Labor induction at 39 weeks reduces CD rate with no difference in perinatal death or serious outcomes
Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
The term "elective induction of labor" has long had a negative connotation because of its association with increased CD rates and adverse perinatal outcomes. This view was based on results from older observational studies that compared outcomes for labor induction with those of spontaneous labor. In more recent observational studies that more appropriately compared labor induction with expectant management, however, elective induction of labor appears to be associated with similar CD rates and perinatal outcomes.
To test the hypothesis that elective induction would have a lower risk for perinatal death or severe neonatal complications than expectant management in low-risk nulliparous women, Grobman and colleagues conducted A Randomized Trial of Induction Versus Expectant Management (ARRIVE).1

Study population, timing of delivery, and trial outcomes
This randomized controlled trial included 6,106 women at 41 US centers in the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Study participants were low-risk nulliparous women with a singleton vertex fetus who were randomly assigned to induction of labor at 39 to 39 4/7 weeks (n = 3,062) or expectant management (n = 3,044) until 40 5/7 to 42 2/7 weeks.
"Low risk" was defined as having no maternal or fetal indication for delivery prior to 40 5/7 weeks. Reliable gestational dating was required.
While no specific protocol for induction of labor management was required, there were 2 requests: 1) Cervical ripening was requested for an unfavorable cervix (63% of participants had a modified Bishop score <5), and 2) a duration of at least 12 hours after cervical ripening, rupture of membranes, and use of uterine stimulant was requested before performing a CD for "failed induction" (if medically appropriate).
The primary outcome was a composite of perinatal death or serious neonatal complications. The main secondary outcome was CD.
Potentially game-changing findings
The investigators found that there was no statistically significant difference between the elective induction and expectant management groups for the primary composite perinatal outcome (4.3% vs 5.4%; P = .049, with P<.046 prespecified for significance). In addition, the rate of CD was significantly lower in the labor induction group than in the expectant management group (18.6% vs 22.2%; P<.001).
Other significant findings in secondary outcomes included the following:
- Hypertensive disorders of pregnancy were significantly lower in the labor induction group compared with the expectant management group (9.1% vs 14.1%; P<.001).
- The labor induction group had a longer length of stay in the labor and delivery unit but a shorter postpartum hospital stay.
- The labor induction group reported less pain and more control during labor.
Results refute negative notion of elective labor induction
The authors concluded that in a low-risk nulliparous patient population, elective induction of labor at 39 weeks does not increase the risk for adverse perinatal outcomes and decreases the rate of CD and hypertensive disorders of pregnancy. Additionally, they noted that induction at 39 weeks should not be avoided with the goal of preventing CD, as even women with an unfavorable cervix had a lower rate of CD in the induction group compared with the expectant management group.
After publication of the ARRIVE trial findings, both ACOG and SMFM released statements supporting elective labor induction at or beyond 39 weeks’ gestation in low-risk nulliparous women with good gestational dating.2,3 They cited the following as important issues: adherence to the trial inclusion criteria except for research purposes, shared decision-making with the patient, consideration of the logistics and impact on the health care facility, and the yet unknown impact on cost. Finally, it should be a priority to avoid the primary CD for a failed induction by allowing a longer latent phase of labor, as long as maternal and fetal conditions allow. In my practice, I actively offer induction of labor to most of my patients at 39 weeks after a discussion of the risks and benefits.
Continue to: Immediate pushing in second stage...
Immediate pushing in second stage offers benefits and is preferable to delayed pushing
Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
In a randomized trial of 2,414 women, Cahill and colleagues sought to answer a seemingly simple question: What is the best timing for pushing during the second stage of labor--immediate or delayed?
Practical management of the second stage of labor (defined as complete cervical dilation to the delivery of the infant) varies by provider and setting, and previous data on pushing efforts are conflicting. Delayed pushing, or "laboring down," has been suggested to allow passive fetal rotation and to conserve maternal energy for pushing. Older studies have shown that delayed pushing decreases the rate of operative delivery. More recent study data have not demonstrated a difference between immediate and delayed pushing techniques on vaginal delivery rates and have noted that increased maternal and neonatal morbidities are associated with a longer second stage of labor.
The recent trial by Cahill and colleagues was designed to determine the effect of these 2 techniques on spontaneous vaginal delivery rates and on maternal and neonatal morbidities.4
Large study population
This randomized pragmatic trial was conducted at 6 centers in the United States. Study participants (2,404 women completed the study) were nulliparous women at 37 or more weeks' gestation with neuraxial anesthesia who were randomly assigned at complete cervical dilation either to immediate pushing (n = 1,200) or to delayed pushing, that is, instructed to wait 60 minutes before starting to push (n = 1,204). The obstetric provider determined the rest of the labor management.
The primary outcome was the rate of spontaneous vaginal delivery. Secondary outcomes included duration of the second stage of labor, duration of active pushing, operative vaginal delivery, CD, and several maternal assessments (postpartum hemorrhage, chorioamnionitis, endometritis, and perineal lacerations).
Both groups had similar vaginal delivery rates, differences in some measures
There was no difference in the primary outcome between the 2 groups: The spontaneous vaginal delivery rate was 85.9% (n = 1,031) in the immediate pushing group and 86.5% (n = 1,041) in the delayed pushing group (P = .67).
Analysis of secondary outcomes revealed several significant differences:
- decreased total time for the second stage of labor in the immediate pushing group compared with the delayed pushing group (102.4 vs 134.2 minutes) but longer active pushing time (83.7 vs 74.5 minutes)
- a lower rate of postpartum hemorrhage, chorioamnionitis in the second stage, neonatal acidemia, and suspected neonatal sepsis in the immediate pushing group
- a higher rate of third-degree perineal lacerations in the immediate pushing group.
No difference was found between groups in rates of operative vaginal deliveries, CDs, endometritis, overall perineal lacerations, or spontaneous vaginal delivery by fetal station or occiput position.
Authors' takeaway
The authors concluded that since delayed pushing does not increase spontaneous vaginal delivery rates and increases the duration of the second stage of labor and both maternal and neonatal morbidity, immediate pushing may be preferred in this patient population.
After reviewing the available literature in light of this study’s findings, ACOG released a practice advisory in October 2018 stating that “it is reasonable to choose immediate over delayed pushing in nulliparous patients with neuraxial anesthesia.”5 Nulliparous patients with neuraxial anesthesia should be counseled that delayed pushing does not increase the rate of spontaneous vaginal birth and may increase both maternal and neonatal complications. As this may be a practice change for many obstetrics units, the obstetric nursing department should be included in this education and counseling. In my practice, I would recommend immediate pushing, but it is important to include both the patient and her nurse in the discussion.
ACOG aims to optimize postpartum care
American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
In May 2018, ACOG released "Optimizing postpartum care," a committee opinion that proposes a new model of comprehensive postpartum care focused on improving both short- and long-term health outcomes for women and infants. (This replaces the June 2016 committee opinion No. 666.) Described as "the fourth trimester," the postpartum period is a critical transitional period in which both pregnancy-related and pre-existing conditions may affect maternal, neonatal, and family status; half of pregnancy-related maternal deaths occur during the postpartum period.6
The postpartum visit: Often a lost opportunity
ACOG cites that up to 40% of women in the United States do not attend their postpartum visit.6 Many aspects of the postpartum visit, including follow-up for chronic diseases, mental health screening, and contraceptive counseling, provide opportunities for acute intervention as well as establishment of healthy behaviors. Some studies have shown that postpartum depression, breastfeeding, and patient satisfaction outcomes improve as a result of postpartum engagement.
Continue to: ACOG's recommendations...
ACOG's recommendations
Ongoing process. ACOG's first proposed change concerns the structure of the postpartum visit itself, which traditionally has been a single visit with a provider at approximately 6 weeks postpartum. Postpartum care plans actually should be started before birth, during regular prenatal care, and adjusted in the hospital as needed so that the provider can educate patients about the issues they may face and resources they may need during this time. This prenatal preparation hopefully will encourage more patients to attend their postpartum visits.
Increased provider contact. Another proposed change is that after delivery, the patient should have contact with a provider within the first 3 weeks postpartum. For high-risk patients, this may involve an in-person clinic visit as soon as 3 to 10 days postpartum (for hypertensive disorders of pregnancy) or at 1 to 2 weeks (for postpartum depression screening, incision checks, and lactation issues). For lower-risk patients, a phone call may be appropriate and/or preferred. Ongoing follow-up for all patients before the final postpartum visit should be individualized.
Postpartum visit and care transition. ACOG recommends a comprehensive postpartum visit at 4 to 12 weeks to fully evaluate the woman's physical, social, and psychologic well-being and to serve as a transition from pregnancy care to well-woman care. This is a large order and includes evaluation of the following:
- mood and emotional well-being
- infant care and feeding
- sexuality, contraception, and birth spacing
- sleep and fatigue
- physical recovery from birth
- chronic disease management and transition to primary care provider
- health maintenance
- review of labor and delivery course if needed
- review of risks and recommendations for future pregnancies.
After these components are addressed, it is expected that the patient will be transitioned to a primary care provider (who may continue to be the ObGyn, as appropriate) to coordinate her future care in the primary medical home.
Useful resource for adopting new paradigm
ACOG's recommendations are somewhat daunting, and these changes will require education and resources, a significant increase in obstetric provider time and effort, and consideration of policy change regarding such issues as parental leave and postpartum care reimbursement. As a start, ACOG has developed an online aid for health care providers called "Postpartum toolkit" (https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit), which provides education and resources for all steps in the process and can be individualized for each practice and patient.7
Postpartum care should be seen as an ongoing process to address both short- and long-term health outcomes for the patient, her newborn, and their family. This process should begin with planning in the antenatal period, continue with close individualized follow-up within the first 3 weeks of birth, and conclude with a comprehensive postpartum evaluation and transition to well-woman care. Shifting the paradigm of postpartum care will take considerable commitment and resources on the part of obstetric providers and their practices. In my practice, we routinely see hypertensive patients within the first week postpartum and patients at risk for postpartum depression within the first 2 weeks in our clinics. We have a standard 6-week postpartum visit for all patients as well. Going forward, we need to further determine how and when we can implement ACOG’s extensive new recommendations for optimizing postpartum care.
The past year was an exciting one in obstetrics. The landmark ARRIVE trial presented at the Society for Maternal-Fetal Medicine’s (SMFM) annual meeting and subsequently published in the New England Journal of Medicine contradicted a long-held belief about the safety of elective labor induction. In a large randomized trial, Cahill and colleagues took a controversial but practical clinical question about second-stage labor management and answered it for the practicing obstetrician in the trenches. Finally, the American College of Obstetricians and Gynecologists (ACOG) placed new emphasis on the oft overlooked but increasingly more complicated postpartum period, offering guidance to support improving care for women in this transitional period.
Ultimately, this was the year of the patient, as research, clinical guidelines, and education focused on how to achieve the best in safety and quality of care for delivery planning, the delivery itself, and the so-called fourth trimester.
ARRIVE: Labor induction at 39 weeks reduces CD rate with no difference in perinatal death or serious outcomes
Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
The term "elective induction of labor" has long had a negative connotation because of its association with increased CD rates and adverse perinatal outcomes. This view was based on results from older observational studies that compared outcomes for labor induction with those of spontaneous labor. In more recent observational studies that more appropriately compared labor induction with expectant management, however, elective induction of labor appears to be associated with similar CD rates and perinatal outcomes.
To test the hypothesis that elective induction would have a lower risk for perinatal death or severe neonatal complications than expectant management in low-risk nulliparous women, Grobman and colleagues conducted A Randomized Trial of Induction Versus Expectant Management (ARRIVE).1

Study population, timing of delivery, and trial outcomes
This randomized controlled trial included 6,106 women at 41 US centers in the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Study participants were low-risk nulliparous women with a singleton vertex fetus who were randomly assigned to induction of labor at 39 to 39 4/7 weeks (n = 3,062) or expectant management (n = 3,044) until 40 5/7 to 42 2/7 weeks.
"Low risk" was defined as having no maternal or fetal indication for delivery prior to 40 5/7 weeks. Reliable gestational dating was required.
While no specific protocol for induction of labor management was required, there were 2 requests: 1) Cervical ripening was requested for an unfavorable cervix (63% of participants had a modified Bishop score <5), and 2) a duration of at least 12 hours after cervical ripening, rupture of membranes, and use of uterine stimulant was requested before performing a CD for "failed induction" (if medically appropriate).
The primary outcome was a composite of perinatal death or serious neonatal complications. The main secondary outcome was CD.
Potentially game-changing findings
The investigators found that there was no statistically significant difference between the elective induction and expectant management groups for the primary composite perinatal outcome (4.3% vs 5.4%; P = .049, with P<.046 prespecified for significance). In addition, the rate of CD was significantly lower in the labor induction group than in the expectant management group (18.6% vs 22.2%; P<.001).
Other significant findings in secondary outcomes included the following:
- Hypertensive disorders of pregnancy were significantly lower in the labor induction group compared with the expectant management group (9.1% vs 14.1%; P<.001).
- The labor induction group had a longer length of stay in the labor and delivery unit but a shorter postpartum hospital stay.
- The labor induction group reported less pain and more control during labor.
Results refute negative notion of elective labor induction
The authors concluded that in a low-risk nulliparous patient population, elective induction of labor at 39 weeks does not increase the risk for adverse perinatal outcomes and decreases the rate of CD and hypertensive disorders of pregnancy. Additionally, they noted that induction at 39 weeks should not be avoided with the goal of preventing CD, as even women with an unfavorable cervix had a lower rate of CD in the induction group compared with the expectant management group.
After publication of the ARRIVE trial findings, both ACOG and SMFM released statements supporting elective labor induction at or beyond 39 weeks’ gestation in low-risk nulliparous women with good gestational dating.2,3 They cited the following as important issues: adherence to the trial inclusion criteria except for research purposes, shared decision-making with the patient, consideration of the logistics and impact on the health care facility, and the yet unknown impact on cost. Finally, it should be a priority to avoid the primary CD for a failed induction by allowing a longer latent phase of labor, as long as maternal and fetal conditions allow. In my practice, I actively offer induction of labor to most of my patients at 39 weeks after a discussion of the risks and benefits.
Continue to: Immediate pushing in second stage...
Immediate pushing in second stage offers benefits and is preferable to delayed pushing
Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
In a randomized trial of 2,414 women, Cahill and colleagues sought to answer a seemingly simple question: What is the best timing for pushing during the second stage of labor--immediate or delayed?
Practical management of the second stage of labor (defined as complete cervical dilation to the delivery of the infant) varies by provider and setting, and previous data on pushing efforts are conflicting. Delayed pushing, or "laboring down," has been suggested to allow passive fetal rotation and to conserve maternal energy for pushing. Older studies have shown that delayed pushing decreases the rate of operative delivery. More recent study data have not demonstrated a difference between immediate and delayed pushing techniques on vaginal delivery rates and have noted that increased maternal and neonatal morbidities are associated with a longer second stage of labor.
The recent trial by Cahill and colleagues was designed to determine the effect of these 2 techniques on spontaneous vaginal delivery rates and on maternal and neonatal morbidities.4
Large study population
This randomized pragmatic trial was conducted at 6 centers in the United States. Study participants (2,404 women completed the study) were nulliparous women at 37 or more weeks' gestation with neuraxial anesthesia who were randomly assigned at complete cervical dilation either to immediate pushing (n = 1,200) or to delayed pushing, that is, instructed to wait 60 minutes before starting to push (n = 1,204). The obstetric provider determined the rest of the labor management.
The primary outcome was the rate of spontaneous vaginal delivery. Secondary outcomes included duration of the second stage of labor, duration of active pushing, operative vaginal delivery, CD, and several maternal assessments (postpartum hemorrhage, chorioamnionitis, endometritis, and perineal lacerations).
Both groups had similar vaginal delivery rates, differences in some measures
There was no difference in the primary outcome between the 2 groups: The spontaneous vaginal delivery rate was 85.9% (n = 1,031) in the immediate pushing group and 86.5% (n = 1,041) in the delayed pushing group (P = .67).
Analysis of secondary outcomes revealed several significant differences:
- decreased total time for the second stage of labor in the immediate pushing group compared with the delayed pushing group (102.4 vs 134.2 minutes) but longer active pushing time (83.7 vs 74.5 minutes)
- a lower rate of postpartum hemorrhage, chorioamnionitis in the second stage, neonatal acidemia, and suspected neonatal sepsis in the immediate pushing group
- a higher rate of third-degree perineal lacerations in the immediate pushing group.
No difference was found between groups in rates of operative vaginal deliveries, CDs, endometritis, overall perineal lacerations, or spontaneous vaginal delivery by fetal station or occiput position.
Authors' takeaway
The authors concluded that since delayed pushing does not increase spontaneous vaginal delivery rates and increases the duration of the second stage of labor and both maternal and neonatal morbidity, immediate pushing may be preferred in this patient population.
After reviewing the available literature in light of this study’s findings, ACOG released a practice advisory in October 2018 stating that “it is reasonable to choose immediate over delayed pushing in nulliparous patients with neuraxial anesthesia.”5 Nulliparous patients with neuraxial anesthesia should be counseled that delayed pushing does not increase the rate of spontaneous vaginal birth and may increase both maternal and neonatal complications. As this may be a practice change for many obstetrics units, the obstetric nursing department should be included in this education and counseling. In my practice, I would recommend immediate pushing, but it is important to include both the patient and her nurse in the discussion.
ACOG aims to optimize postpartum care
American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
In May 2018, ACOG released "Optimizing postpartum care," a committee opinion that proposes a new model of comprehensive postpartum care focused on improving both short- and long-term health outcomes for women and infants. (This replaces the June 2016 committee opinion No. 666.) Described as "the fourth trimester," the postpartum period is a critical transitional period in which both pregnancy-related and pre-existing conditions may affect maternal, neonatal, and family status; half of pregnancy-related maternal deaths occur during the postpartum period.6
The postpartum visit: Often a lost opportunity
ACOG cites that up to 40% of women in the United States do not attend their postpartum visit.6 Many aspects of the postpartum visit, including follow-up for chronic diseases, mental health screening, and contraceptive counseling, provide opportunities for acute intervention as well as establishment of healthy behaviors. Some studies have shown that postpartum depression, breastfeeding, and patient satisfaction outcomes improve as a result of postpartum engagement.
Continue to: ACOG's recommendations...
ACOG's recommendations
Ongoing process. ACOG's first proposed change concerns the structure of the postpartum visit itself, which traditionally has been a single visit with a provider at approximately 6 weeks postpartum. Postpartum care plans actually should be started before birth, during regular prenatal care, and adjusted in the hospital as needed so that the provider can educate patients about the issues they may face and resources they may need during this time. This prenatal preparation hopefully will encourage more patients to attend their postpartum visits.
Increased provider contact. Another proposed change is that after delivery, the patient should have contact with a provider within the first 3 weeks postpartum. For high-risk patients, this may involve an in-person clinic visit as soon as 3 to 10 days postpartum (for hypertensive disorders of pregnancy) or at 1 to 2 weeks (for postpartum depression screening, incision checks, and lactation issues). For lower-risk patients, a phone call may be appropriate and/or preferred. Ongoing follow-up for all patients before the final postpartum visit should be individualized.
Postpartum visit and care transition. ACOG recommends a comprehensive postpartum visit at 4 to 12 weeks to fully evaluate the woman's physical, social, and psychologic well-being and to serve as a transition from pregnancy care to well-woman care. This is a large order and includes evaluation of the following:
- mood and emotional well-being
- infant care and feeding
- sexuality, contraception, and birth spacing
- sleep and fatigue
- physical recovery from birth
- chronic disease management and transition to primary care provider
- health maintenance
- review of labor and delivery course if needed
- review of risks and recommendations for future pregnancies.
After these components are addressed, it is expected that the patient will be transitioned to a primary care provider (who may continue to be the ObGyn, as appropriate) to coordinate her future care in the primary medical home.
Useful resource for adopting new paradigm
ACOG's recommendations are somewhat daunting, and these changes will require education and resources, a significant increase in obstetric provider time and effort, and consideration of policy change regarding such issues as parental leave and postpartum care reimbursement. As a start, ACOG has developed an online aid for health care providers called "Postpartum toolkit" (https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit), which provides education and resources for all steps in the process and can be individualized for each practice and patient.7
Postpartum care should be seen as an ongoing process to address both short- and long-term health outcomes for the patient, her newborn, and their family. This process should begin with planning in the antenatal period, continue with close individualized follow-up within the first 3 weeks of birth, and conclude with a comprehensive postpartum evaluation and transition to well-woman care. Shifting the paradigm of postpartum care will take considerable commitment and resources on the part of obstetric providers and their practices. In my practice, we routinely see hypertensive patients within the first week postpartum and patients at risk for postpartum depression within the first 2 weeks in our clinics. We have a standard 6-week postpartum visit for all patients as well. Going forward, we need to further determine how and when we can implement ACOG’s extensive new recommendations for optimizing postpartum care.
- Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists. Practice advisory: clinical guidance for integration of the findings of the ARRIVE trial: Labor induction versus expectant management in low-risk nulliparous women. August 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Clinical-guidance-for-integration-of-the-findings-of-The-ARRIVE-Trial. Accessed November 25, 2018.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2018.08.009. In press.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
- American College of Obstetricians and Gynecologists. Practice advisory: immediate versus delayed pushing in nulliparous women receiving neuraxial analgesia. October 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Immediate-vs-delayed-pushing-in-nulliparous-women-receiving-neuraxial-analgesia. Accessed November 25, 2018.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
- American College of Obstetricians and Gynecologists. ACOG Postpartum toolkit. https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit. Accessed November 25, 2018.
- Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists. Practice advisory: clinical guidance for integration of the findings of the ARRIVE trial: Labor induction versus expectant management in low-risk nulliparous women. August 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Clinical-guidance-for-integration-of-the-findings-of-The-ARRIVE-Trial. Accessed November 25, 2018.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2018.08.009. In press.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
- American College of Obstetricians and Gynecologists. Practice advisory: immediate versus delayed pushing in nulliparous women receiving neuraxial analgesia. October 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Immediate-vs-delayed-pushing-in-nulliparous-women-receiving-neuraxial-analgesia. Accessed November 25, 2018.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
- American College of Obstetricians and Gynecologists. ACOG Postpartum toolkit. https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit. Accessed November 25, 2018.
Health care costs matter to patients, and we can do something about it
CASE 1 Huge out-of-pocket cost makes patient forego treatment
Ms. M. is a 28-year-old patient who recently posted this on her Facebook page: “I went to the drugstore this morning to pick up a prescription, and as the pharmacist handed it to me she said, ‘That will be $180.00.’ And that’s after insurance coverage! Wow! I think I’ll pass!”
Our patients probably experience this type of situation more commonly than we know.
CASE 2 Catastrophic medical costs bankrupt family
A middle-class couple who had college degrees and full-time jobs with health insurance had twins at 24 weeks’ gestation. They accrued $450,000 in medical debt after exceeding the $2 million cap of their insurance policy. Having premature twins cost them everything. They liquidated their retirement and savings accounts, sold everything they had, and still ended up filing for bankruptcy.1
Costs indeed matter to patients, and we have a professional responsibility to help our patients navigate the murky waters of health care so that they can maintain financial as well as physical health.
Rising costs, lower yield,and opportunities for change
Rising health care costs are unsustainable for both our patients and our society. Although the United States spends more on health care than any other developed country, our health outcomes are actually worse—ranking at or near the bottom in both prevalence and mortality for multiple diseases, risk factors, and injuries.2
Of the 171 countries included in a study by the United Nations Maternal Mortality Estimation Inter-Agency Group, the United States was 1 of 13 countries that had an increasing maternal mortality and the only developed nation with an increasing maternal mortality rate.3 This tells us that, as our country spends more on health care, our patients’ health is not improving. For individuals, medical bills are now the leading cause of personal bankruptcy in the United States, even for those who are insured.4
ObGyns play an important leadership role in the practice of cost-conscious health care, as 25% of hospitalizations in the United States are pregnancy related.5,6 In addition, the wide scope of ObGyn practice reaches beyond pregnancy-related conditions and provides multiple opportunities to decrease the use of unnecessary tests and treatments.
The good news is that approximately 30% of health care costs are wasted on unnecessary care that could be eliminated without decreasing the quality of care.7
High-value change #1: Eliminate use of expensive products
Embarking on a high-value care improvement project, experts at Greenville Health System examined the cost of different topical pain medications for perineal pain after a vaginal delivery. They found that Epifoam (hydrocortisone acetate/pramoxine hydrochloride) was ordered 2,287 times over the course of a year.
The study intervention consisted of an educational grand rounds and discussion of a Cochrane review, which concluded there was no difference in pain relief with topical anesthetics compared with placebo.8 Less expensive options for pain relief were discussed, and the department agreed to remove Epifoam as a standing order.
After the intervention, Epifoam was ordered 228 times, a 90% reduction. Over the period of a year, this translated to a cost savings of $92,655 for the hospital, with reduced charges passed on to patients.9 Thus, a seemingly small individual cost ($45.00 per can of Epifoam) can add up to a substantial sum in a large health care system.
Similarly, practitioners were educated about options for cervical ripening and were given information on the cost and efficacy of various cervical ripening agents. A Cochrane review found that oral misoprostol is as effective as vaginal misoprostol and results in fewer cesarean deliveries than vaginal dinoprostone (Cervidil).10 Practitioners were asked to consider making the transition to oral misoprostol. This action resulted in a 50.5% decrease in Cervidil use, from 384 to 194 cases, producing a cost savings of $66,500. The following year, the department removed Cervidil from the formulary as a high-value decision.9
Both of these examples illustrate what a value-minded department can accomplish by implementing performance improvement projects that focus on high-value care.
Continue to: High-value change #2: Stop ordering unnecessary lab work...
High-value change #2: Stop ordering unnecessary lab work
Another high-value change to consider: Examine each laboratory test order to understand if the test results will really alter the care of a patient. Providers vary, and ordering lab tests to “make sure” can add up as financial expense.
Best practices from the American College of Obstetricians and Gynecologists (ACOG) and other professional societies can help guide decision-making as we order lab tests. Think twice, for example, about whether every evaluation for preeclampsia requires a uric acid test, since ACOG does not endorse that as part of the diagnostic criteria. While a single uric acid test costs only $8.00 to $38.00 (according to Healthcare Bluebook), testing uric acid in many patients over the course of a year can add up to significant dollars.11
High-value change #3: Consider care redesign
In addition to seeking opportunities to use more cost-effective products and reduce the use of unnecessary tests, “care redesign” is an innovative way to provide high-quality care (and increased patient satisfaction) at a lower cost for both the health care system and the patient. A prime example of care redesign is using telehealth to enhance prenatal care.
Several health systems around the country are piloting and implementing remote blood pressure monitoring, app-based prenatal education, and telehealth visits to enhance prenatal care.12,13 Use of a home blood pressure monitor can reduce in-person visits for low-risk prenatal care and open up access for other patients. Additionally, allowing the patient to participate in her own care at home or work can eliminate drives to and waits in the office and reduce absence from work because of a doctor visit.
A systematic review of more than 60,000 women showed that low-risk women who attend 5 to 9 prenatal visits have the same outcomes as women who attend the standard schedule of 13 to 15 visits.14 Although patient satisfaction was higher with more visits, when a bidirectional app or a telehealth visit is offered as an option, then patient satisfaction is equivalent to that in the standard schedule group.12 So why not expand the choice for patients?
The challenge of teaching high-value care: Medical education responds
In a 2010 article in the New England Journal of Medicine, Dr. Molly Cooke commented on medical education’s responsibility regarding cost consciousness in patient care, and she highlighted the importance of teaching medical students and residents about considering cost in treating patients.15 Similarly, the Accreditation Council for Graduate Medical Education asks residents to consider cost and stewardship of medical resources as one of its system-based practice competencies.16 In 2012, the Choosing Wisely campaign, initiated by the American Board of Internal Medicine Foundation, asked specialty society members to identify tests or procedures commonly used in their field whose necessity should be questioned and discussed.17 ACOG and other women’s health specialty societies participate in this campaign.
From an educational standpoint, ACOG’s Council on Resident Education in Obstetrics and Gynecology has developed a curriculum resource, “Cases in High Value Care,” that can be used by any women’s health department to start the conversation on high-value care.18 The web program encourages medical students and residents to submit clinical vignettes that demonstrate examples of low- and high-value care. These cases can be used for discussion and debate and can serve as high-value care performance improvement projects in your own department.
Other useful publications are available outside the ObGyn specialty. Consider the Society of Hospital Medicine’s article series in the Journal of Hospital Medicine, “Choosing Wisely: Things We Do for No Reason”and “Choosing Wisely: Next Steps in Improving Healthcare Value.”19 The former focuses on discussing practices (tests, procedures, supplies, and prescriptions) that may be poorly supported by evidence or are part of standard practice even though other less expensive, higher-value alternatives may be available. The latter highlights perspective pieces that describe health care value initiatives relating to the practice of hospital medicine.
Continue to: The bottom line...
The bottom line
ObGyns and other health care providers are concerned about providing high-value care to patients and are working toward improving performance in this area. We really do care about the health care–related financial burdens that confront Ms. M., the premature twins’ parents, and all our patients.
- Sinconis J. Bankrupted by giving birth: having premature twins cost me everything. The Guardian. January 17, 2018. https://www.theguardian.com/us-news/commentisfree/2018/jan/16/bankrupted-by-giving-birth-having-premature-twins-cost-me-everything. Accessed December 20, 2018.
- Woolf SH, Aron LY. The US health disadvantage relative to other high-income countries: findings from a National Research Council/Institute of Medicine report. JAMA. 2013;309:771-772.
- Ozimek JA, Kilpatrick SJ. Maternal mortality in the twenty-first century. Obstet Gynecol Clin North Am. 2018;45:175-186.
- Himmelstein DU, Thorne D, Warren E, et al. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med. 2009;122:741-746.
- Healthy babies healthy business. March of Dimes website. http://www.marchofdimes.org/hbhb/index.asp. Accessed December 20, 2018.
- Werner EF. Cost matters. Obstet Gynecol. 2014;123:919-920.
- Institute of Medicine (US) Roundtable on Evidence-Based Medicine; Yong PL, Saunders RS, Olsen LA, eds. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: National Academies Press; 2010.
- Hedayati H, Parsons J, Crowther CA. Topically applied anaesthetics for treating perineal pain after childbirth. Cochrane Database Syst Rev. 2005;2:CD004223.
- Demosthenes LD, Lane AS, Blackhurst DW. Implementing high-value care. South Med J. 2015;108:645-648.
- Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database Syst Rev. 2014;6:CD001338.
- Lane A. Preeclampsia evaluation. American College of Obstetricians and Gynecologists website. https://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/Cases-in-High-Value-Care/Example-2. Published July 14, 2015. Accessed July 10, 2018.
- Clark EN. Evidence-based prenatal care. University of Utah Health website. https://physicians.utah.edu/echo/pdfs/2018-06-29_evidence-based_prenatal_care.pdf. Accessed August 6, 2018.
- Marko KI, Krapf JM, Meltzer AC, et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200.
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2010;10:CD000934.
- Cooke M. Cost consciousness in patient care—what is medical education’s responsibility? N Engl J Med. 2010;362:1253-1255.
- Accreditation Council for Graduate Medical Education. ACGME Common program requirements (residency).https://www.acgme.org/Portals/0/PFAssets/Program Requirements/CPRs_2017-07-01.pdf. Accessed December 19, 2018.
- Choosing Wisely. American Board of Internal Medicine Foundation website. http://www.choosingwisely.org/. Accessed August 7, 2018.
- American College of Obstetricians and Gynecologists Council on Resident Education in Obstetrics and Gynecology. Cases in high value care. https://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/Cases-in-High-Value-Care. Accessed August 8, 2018.
- Journal of Hospital Medicine website. https://www.journalofhospitalmedicine.com/jhospmed/page/author-guidelines. Accessed August 8, 2018.
CASE 1 Huge out-of-pocket cost makes patient forego treatment
Ms. M. is a 28-year-old patient who recently posted this on her Facebook page: “I went to the drugstore this morning to pick up a prescription, and as the pharmacist handed it to me she said, ‘That will be $180.00.’ And that’s after insurance coverage! Wow! I think I’ll pass!”
Our patients probably experience this type of situation more commonly than we know.
CASE 2 Catastrophic medical costs bankrupt family
A middle-class couple who had college degrees and full-time jobs with health insurance had twins at 24 weeks’ gestation. They accrued $450,000 in medical debt after exceeding the $2 million cap of their insurance policy. Having premature twins cost them everything. They liquidated their retirement and savings accounts, sold everything they had, and still ended up filing for bankruptcy.1
Costs indeed matter to patients, and we have a professional responsibility to help our patients navigate the murky waters of health care so that they can maintain financial as well as physical health.
Rising costs, lower yield,and opportunities for change
Rising health care costs are unsustainable for both our patients and our society. Although the United States spends more on health care than any other developed country, our health outcomes are actually worse—ranking at or near the bottom in both prevalence and mortality for multiple diseases, risk factors, and injuries.2
Of the 171 countries included in a study by the United Nations Maternal Mortality Estimation Inter-Agency Group, the United States was 1 of 13 countries that had an increasing maternal mortality and the only developed nation with an increasing maternal mortality rate.3 This tells us that, as our country spends more on health care, our patients’ health is not improving. For individuals, medical bills are now the leading cause of personal bankruptcy in the United States, even for those who are insured.4
ObGyns play an important leadership role in the practice of cost-conscious health care, as 25% of hospitalizations in the United States are pregnancy related.5,6 In addition, the wide scope of ObGyn practice reaches beyond pregnancy-related conditions and provides multiple opportunities to decrease the use of unnecessary tests and treatments.
The good news is that approximately 30% of health care costs are wasted on unnecessary care that could be eliminated without decreasing the quality of care.7
High-value change #1: Eliminate use of expensive products
Embarking on a high-value care improvement project, experts at Greenville Health System examined the cost of different topical pain medications for perineal pain after a vaginal delivery. They found that Epifoam (hydrocortisone acetate/pramoxine hydrochloride) was ordered 2,287 times over the course of a year.
The study intervention consisted of an educational grand rounds and discussion of a Cochrane review, which concluded there was no difference in pain relief with topical anesthetics compared with placebo.8 Less expensive options for pain relief were discussed, and the department agreed to remove Epifoam as a standing order.
After the intervention, Epifoam was ordered 228 times, a 90% reduction. Over the period of a year, this translated to a cost savings of $92,655 for the hospital, with reduced charges passed on to patients.9 Thus, a seemingly small individual cost ($45.00 per can of Epifoam) can add up to a substantial sum in a large health care system.

Similarly, practitioners were educated about options for cervical ripening and were given information on the cost and efficacy of various cervical ripening agents. A Cochrane review found that oral misoprostol is as effective as vaginal misoprostol and results in fewer cesarean deliveries than vaginal dinoprostone (Cervidil).10 Practitioners were asked to consider making the transition to oral misoprostol. This action resulted in a 50.5% decrease in Cervidil use, from 384 to 194 cases, producing a cost savings of $66,500. The following year, the department removed Cervidil from the formulary as a high-value decision.9
Both of these examples illustrate what a value-minded department can accomplish by implementing performance improvement projects that focus on high-value care.
Continue to: High-value change #2: Stop ordering unnecessary lab work...
High-value change #2: Stop ordering unnecessary lab work
Another high-value change to consider: Examine each laboratory test order to understand if the test results will really alter the care of a patient. Providers vary, and ordering lab tests to “make sure” can add up as financial expense.
Best practices from the American College of Obstetricians and Gynecologists (ACOG) and other professional societies can help guide decision-making as we order lab tests. Think twice, for example, about whether every evaluation for preeclampsia requires a uric acid test, since ACOG does not endorse that as part of the diagnostic criteria. While a single uric acid test costs only $8.00 to $38.00 (according to Healthcare Bluebook), testing uric acid in many patients over the course of a year can add up to significant dollars.11
High-value change #3: Consider care redesign
In addition to seeking opportunities to use more cost-effective products and reduce the use of unnecessary tests, “care redesign” is an innovative way to provide high-quality care (and increased patient satisfaction) at a lower cost for both the health care system and the patient. A prime example of care redesign is using telehealth to enhance prenatal care.
Several health systems around the country are piloting and implementing remote blood pressure monitoring, app-based prenatal education, and telehealth visits to enhance prenatal care.12,13 Use of a home blood pressure monitor can reduce in-person visits for low-risk prenatal care and open up access for other patients. Additionally, allowing the patient to participate in her own care at home or work can eliminate drives to and waits in the office and reduce absence from work because of a doctor visit.
A systematic review of more than 60,000 women showed that low-risk women who attend 5 to 9 prenatal visits have the same outcomes as women who attend the standard schedule of 13 to 15 visits.14 Although patient satisfaction was higher with more visits, when a bidirectional app or a telehealth visit is offered as an option, then patient satisfaction is equivalent to that in the standard schedule group.12 So why not expand the choice for patients?
The challenge of teaching high-value care: Medical education responds
In a 2010 article in the New England Journal of Medicine, Dr. Molly Cooke commented on medical education’s responsibility regarding cost consciousness in patient care, and she highlighted the importance of teaching medical students and residents about considering cost in treating patients.15 Similarly, the Accreditation Council for Graduate Medical Education asks residents to consider cost and stewardship of medical resources as one of its system-based practice competencies.16 In 2012, the Choosing Wisely campaign, initiated by the American Board of Internal Medicine Foundation, asked specialty society members to identify tests or procedures commonly used in their field whose necessity should be questioned and discussed.17 ACOG and other women’s health specialty societies participate in this campaign.
From an educational standpoint, ACOG’s Council on Resident Education in Obstetrics and Gynecology has developed a curriculum resource, “Cases in High Value Care,” that can be used by any women’s health department to start the conversation on high-value care.18 The web program encourages medical students and residents to submit clinical vignettes that demonstrate examples of low- and high-value care. These cases can be used for discussion and debate and can serve as high-value care performance improvement projects in your own department.
Other useful publications are available outside the ObGyn specialty. Consider the Society of Hospital Medicine’s article series in the Journal of Hospital Medicine, “Choosing Wisely: Things We Do for No Reason”and “Choosing Wisely: Next Steps in Improving Healthcare Value.”19 The former focuses on discussing practices (tests, procedures, supplies, and prescriptions) that may be poorly supported by evidence or are part of standard practice even though other less expensive, higher-value alternatives may be available. The latter highlights perspective pieces that describe health care value initiatives relating to the practice of hospital medicine.
Continue to: The bottom line...
The bottom line
ObGyns and other health care providers are concerned about providing high-value care to patients and are working toward improving performance in this area. We really do care about the health care–related financial burdens that confront Ms. M., the premature twins’ parents, and all our patients.
CASE 1 Huge out-of-pocket cost makes patient forego treatment
Ms. M. is a 28-year-old patient who recently posted this on her Facebook page: “I went to the drugstore this morning to pick up a prescription, and as the pharmacist handed it to me she said, ‘That will be $180.00.’ And that’s after insurance coverage! Wow! I think I’ll pass!”
Our patients probably experience this type of situation more commonly than we know.
CASE 2 Catastrophic medical costs bankrupt family
A middle-class couple who had college degrees and full-time jobs with health insurance had twins at 24 weeks’ gestation. They accrued $450,000 in medical debt after exceeding the $2 million cap of their insurance policy. Having premature twins cost them everything. They liquidated their retirement and savings accounts, sold everything they had, and still ended up filing for bankruptcy.1
Costs indeed matter to patients, and we have a professional responsibility to help our patients navigate the murky waters of health care so that they can maintain financial as well as physical health.
Rising costs, lower yield,and opportunities for change
Rising health care costs are unsustainable for both our patients and our society. Although the United States spends more on health care than any other developed country, our health outcomes are actually worse—ranking at or near the bottom in both prevalence and mortality for multiple diseases, risk factors, and injuries.2
Of the 171 countries included in a study by the United Nations Maternal Mortality Estimation Inter-Agency Group, the United States was 1 of 13 countries that had an increasing maternal mortality and the only developed nation with an increasing maternal mortality rate.3 This tells us that, as our country spends more on health care, our patients’ health is not improving. For individuals, medical bills are now the leading cause of personal bankruptcy in the United States, even for those who are insured.4
ObGyns play an important leadership role in the practice of cost-conscious health care, as 25% of hospitalizations in the United States are pregnancy related.5,6 In addition, the wide scope of ObGyn practice reaches beyond pregnancy-related conditions and provides multiple opportunities to decrease the use of unnecessary tests and treatments.
The good news is that approximately 30% of health care costs are wasted on unnecessary care that could be eliminated without decreasing the quality of care.7
High-value change #1: Eliminate use of expensive products
Embarking on a high-value care improvement project, experts at Greenville Health System examined the cost of different topical pain medications for perineal pain after a vaginal delivery. They found that Epifoam (hydrocortisone acetate/pramoxine hydrochloride) was ordered 2,287 times over the course of a year.
The study intervention consisted of an educational grand rounds and discussion of a Cochrane review, which concluded there was no difference in pain relief with topical anesthetics compared with placebo.8 Less expensive options for pain relief were discussed, and the department agreed to remove Epifoam as a standing order.
After the intervention, Epifoam was ordered 228 times, a 90% reduction. Over the period of a year, this translated to a cost savings of $92,655 for the hospital, with reduced charges passed on to patients.9 Thus, a seemingly small individual cost ($45.00 per can of Epifoam) can add up to a substantial sum in a large health care system.

Similarly, practitioners were educated about options for cervical ripening and were given information on the cost and efficacy of various cervical ripening agents. A Cochrane review found that oral misoprostol is as effective as vaginal misoprostol and results in fewer cesarean deliveries than vaginal dinoprostone (Cervidil).10 Practitioners were asked to consider making the transition to oral misoprostol. This action resulted in a 50.5% decrease in Cervidil use, from 384 to 194 cases, producing a cost savings of $66,500. The following year, the department removed Cervidil from the formulary as a high-value decision.9
Both of these examples illustrate what a value-minded department can accomplish by implementing performance improvement projects that focus on high-value care.
Continue to: High-value change #2: Stop ordering unnecessary lab work...
High-value change #2: Stop ordering unnecessary lab work
Another high-value change to consider: Examine each laboratory test order to understand if the test results will really alter the care of a patient. Providers vary, and ordering lab tests to “make sure” can add up as financial expense.
Best practices from the American College of Obstetricians and Gynecologists (ACOG) and other professional societies can help guide decision-making as we order lab tests. Think twice, for example, about whether every evaluation for preeclampsia requires a uric acid test, since ACOG does not endorse that as part of the diagnostic criteria. While a single uric acid test costs only $8.00 to $38.00 (according to Healthcare Bluebook), testing uric acid in many patients over the course of a year can add up to significant dollars.11
High-value change #3: Consider care redesign
In addition to seeking opportunities to use more cost-effective products and reduce the use of unnecessary tests, “care redesign” is an innovative way to provide high-quality care (and increased patient satisfaction) at a lower cost for both the health care system and the patient. A prime example of care redesign is using telehealth to enhance prenatal care.
Several health systems around the country are piloting and implementing remote blood pressure monitoring, app-based prenatal education, and telehealth visits to enhance prenatal care.12,13 Use of a home blood pressure monitor can reduce in-person visits for low-risk prenatal care and open up access for other patients. Additionally, allowing the patient to participate in her own care at home or work can eliminate drives to and waits in the office and reduce absence from work because of a doctor visit.
A systematic review of more than 60,000 women showed that low-risk women who attend 5 to 9 prenatal visits have the same outcomes as women who attend the standard schedule of 13 to 15 visits.14 Although patient satisfaction was higher with more visits, when a bidirectional app or a telehealth visit is offered as an option, then patient satisfaction is equivalent to that in the standard schedule group.12 So why not expand the choice for patients?
The challenge of teaching high-value care: Medical education responds
In a 2010 article in the New England Journal of Medicine, Dr. Molly Cooke commented on medical education’s responsibility regarding cost consciousness in patient care, and she highlighted the importance of teaching medical students and residents about considering cost in treating patients.15 Similarly, the Accreditation Council for Graduate Medical Education asks residents to consider cost and stewardship of medical resources as one of its system-based practice competencies.16 In 2012, the Choosing Wisely campaign, initiated by the American Board of Internal Medicine Foundation, asked specialty society members to identify tests or procedures commonly used in their field whose necessity should be questioned and discussed.17 ACOG and other women’s health specialty societies participate in this campaign.
From an educational standpoint, ACOG’s Council on Resident Education in Obstetrics and Gynecology has developed a curriculum resource, “Cases in High Value Care,” that can be used by any women’s health department to start the conversation on high-value care.18 The web program encourages medical students and residents to submit clinical vignettes that demonstrate examples of low- and high-value care. These cases can be used for discussion and debate and can serve as high-value care performance improvement projects in your own department.
Other useful publications are available outside the ObGyn specialty. Consider the Society of Hospital Medicine’s article series in the Journal of Hospital Medicine, “Choosing Wisely: Things We Do for No Reason”and “Choosing Wisely: Next Steps in Improving Healthcare Value.”19 The former focuses on discussing practices (tests, procedures, supplies, and prescriptions) that may be poorly supported by evidence or are part of standard practice even though other less expensive, higher-value alternatives may be available. The latter highlights perspective pieces that describe health care value initiatives relating to the practice of hospital medicine.
Continue to: The bottom line...
The bottom line
ObGyns and other health care providers are concerned about providing high-value care to patients and are working toward improving performance in this area. We really do care about the health care–related financial burdens that confront Ms. M., the premature twins’ parents, and all our patients.
- Sinconis J. Bankrupted by giving birth: having premature twins cost me everything. The Guardian. January 17, 2018. https://www.theguardian.com/us-news/commentisfree/2018/jan/16/bankrupted-by-giving-birth-having-premature-twins-cost-me-everything. Accessed December 20, 2018.
- Woolf SH, Aron LY. The US health disadvantage relative to other high-income countries: findings from a National Research Council/Institute of Medicine report. JAMA. 2013;309:771-772.
- Ozimek JA, Kilpatrick SJ. Maternal mortality in the twenty-first century. Obstet Gynecol Clin North Am. 2018;45:175-186.
- Himmelstein DU, Thorne D, Warren E, et al. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med. 2009;122:741-746.
- Healthy babies healthy business. March of Dimes website. http://www.marchofdimes.org/hbhb/index.asp. Accessed December 20, 2018.
- Werner EF. Cost matters. Obstet Gynecol. 2014;123:919-920.
- Institute of Medicine (US) Roundtable on Evidence-Based Medicine; Yong PL, Saunders RS, Olsen LA, eds. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: National Academies Press; 2010.
- Hedayati H, Parsons J, Crowther CA. Topically applied anaesthetics for treating perineal pain after childbirth. Cochrane Database Syst Rev. 2005;2:CD004223.
- Demosthenes LD, Lane AS, Blackhurst DW. Implementing high-value care. South Med J. 2015;108:645-648.
- Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database Syst Rev. 2014;6:CD001338.
- Lane A. Preeclampsia evaluation. American College of Obstetricians and Gynecologists website. https://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/Cases-in-High-Value-Care/Example-2. Published July 14, 2015. Accessed July 10, 2018.
- Clark EN. Evidence-based prenatal care. University of Utah Health website. https://physicians.utah.edu/echo/pdfs/2018-06-29_evidence-based_prenatal_care.pdf. Accessed August 6, 2018.
- Marko KI, Krapf JM, Meltzer AC, et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200.
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2010;10:CD000934.
- Cooke M. Cost consciousness in patient care—what is medical education’s responsibility? N Engl J Med. 2010;362:1253-1255.
- Accreditation Council for Graduate Medical Education. ACGME Common program requirements (residency).https://www.acgme.org/Portals/0/PFAssets/Program Requirements/CPRs_2017-07-01.pdf. Accessed December 19, 2018.
- Choosing Wisely. American Board of Internal Medicine Foundation website. http://www.choosingwisely.org/. Accessed August 7, 2018.
- American College of Obstetricians and Gynecologists Council on Resident Education in Obstetrics and Gynecology. Cases in high value care. https://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/Cases-in-High-Value-Care. Accessed August 8, 2018.
- Journal of Hospital Medicine website. https://www.journalofhospitalmedicine.com/jhospmed/page/author-guidelines. Accessed August 8, 2018.
- Sinconis J. Bankrupted by giving birth: having premature twins cost me everything. The Guardian. January 17, 2018. https://www.theguardian.com/us-news/commentisfree/2018/jan/16/bankrupted-by-giving-birth-having-premature-twins-cost-me-everything. Accessed December 20, 2018.
- Woolf SH, Aron LY. The US health disadvantage relative to other high-income countries: findings from a National Research Council/Institute of Medicine report. JAMA. 2013;309:771-772.
- Ozimek JA, Kilpatrick SJ. Maternal mortality in the twenty-first century. Obstet Gynecol Clin North Am. 2018;45:175-186.
- Himmelstein DU, Thorne D, Warren E, et al. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med. 2009;122:741-746.
- Healthy babies healthy business. March of Dimes website. http://www.marchofdimes.org/hbhb/index.asp. Accessed December 20, 2018.
- Werner EF. Cost matters. Obstet Gynecol. 2014;123:919-920.
- Institute of Medicine (US) Roundtable on Evidence-Based Medicine; Yong PL, Saunders RS, Olsen LA, eds. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: National Academies Press; 2010.
- Hedayati H, Parsons J, Crowther CA. Topically applied anaesthetics for treating perineal pain after childbirth. Cochrane Database Syst Rev. 2005;2:CD004223.
- Demosthenes LD, Lane AS, Blackhurst DW. Implementing high-value care. South Med J. 2015;108:645-648.
- Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database Syst Rev. 2014;6:CD001338.
- Lane A. Preeclampsia evaluation. American College of Obstetricians and Gynecologists website. https://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/Cases-in-High-Value-Care/Example-2. Published July 14, 2015. Accessed July 10, 2018.
- Clark EN. Evidence-based prenatal care. University of Utah Health website. https://physicians.utah.edu/echo/pdfs/2018-06-29_evidence-based_prenatal_care.pdf. Accessed August 6, 2018.
- Marko KI, Krapf JM, Meltzer AC, et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200.
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2010;10:CD000934.
- Cooke M. Cost consciousness in patient care—what is medical education’s responsibility? N Engl J Med. 2010;362:1253-1255.
- Accreditation Council for Graduate Medical Education. ACGME Common program requirements (residency).https://www.acgme.org/Portals/0/PFAssets/Program Requirements/CPRs_2017-07-01.pdf. Accessed December 19, 2018.
- Choosing Wisely. American Board of Internal Medicine Foundation website. http://www.choosingwisely.org/. Accessed August 7, 2018.
- American College of Obstetricians and Gynecologists Council on Resident Education in Obstetrics and Gynecology. Cases in high value care. https://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/Cases-in-High-Value-Care. Accessed August 8, 2018.
- Journal of Hospital Medicine website. https://www.journalofhospitalmedicine.com/jhospmed/page/author-guidelines. Accessed August 8, 2018.
Managing menopausal vasomotor and genitourinary symptoms after breast cancer
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.
The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.

The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.

The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.