User login
Negative home COVID test no ‘free pass’ for kids, study finds
With the country looking increasingly to rapid testing as an off-ramp from the COVID-19 pandemic, a new study shows that the performance of the tests in children falls below standards set by regulatory agencies in the United States and elsewhere for diagnostic accuracy.
Experts said the findings, from a meta-analysis by researchers in the United Kingdom and Germany, underscore that, while a positive result on a rapid test is almost certainly an indicator of infection, negative results often are unreliable and can lead to a false sense of security.
“Real-life performance of current antigen tests for professional use in pediatric populations is below the minimum performance criteria set by WHO, the United States Food and Drug Administration, or the Medicines and Healthcare products Regulatory Agency (U.K.),” according to Naomi Fujita-Rohwerder, PhD, a research associate at the Cologne-based German Institute for Quality and Efficiency in Health Care (IQWiG), and her colleagues, whose study appears in BMJ Evidence-Based Medicine.
The researchers said that the study suggests that performance of rapid testing in a pediatric population is comparable to that in adults. However, they said they could not identify any studies investigating self-testing in children, which also could affect test performance.
Egon Ozer, MD, PhD, director of the center for pathogen genomics and microbial evolution at Northwestern University in Chicago, said the finding that specificity was high but sensitivity was middling “suggests that we should be very careful about interpreting negative antigen test results in children and recognize that there is a fair amount of uncertainty in the tests in this situation.”
Researchers from IQWiG, which examines the advantages and disadvantages of medical interventions, and the University of Manchester (England), conducted the systematic review and meta-analysis, which they described as the first of its kind to evaluate the diagnostic accuracy of rapid point-of-care tests for current SARS-CoV-2 infections in children.
They compiled information from 17 studies with a total 6,355 participants. They compared all antigen tests to reverse-transcription polymerase chain reaction (PCR). The studies compared eight antigen tests from six different brands. The rapid antigen tests, available from pharmacies and online stores, are widely used for self-testing in schools and testing toddlers before kindergarten.
The pooled diagnostic sensitivity of antigen tests was 64.2% and specificity was 99.1%.
Dr. Ozer noted that the analysis “was not able to address important outstanding questions such as the likelihood of transmitting infection with a false-negative antigen test versus a true-negative antigen test or how much repeated testing can increase the sensitivity.”
“In Europe, we don’t know how most tests perform in real life,” Dr. Fujita-Rohwerder said. “And even in countries like the United States, where market access is more stringent, we don’t know whether self-testing performed by children or sample collection in toddlers by laypersons has a significant impact on the diagnostic accuracy. Also, diagnostic accuracy estimates reported in our study may not apply to the current omicron or future variants of SARS-CoV-2 or vaccinated children. Hopefully, these essential gaps in the evidence will get addressed soon.”
Dr. Ozer said one takeaway from this study is negative antigen tests should not be considered a “free pass” in children, especially if the child is symptomatic, has been recently exposed to COVID-19, or is planning to spend time with individuals with conditions that place them at high risk for complications of COVID-19 infection. “In such cases, consider getting PCR testing or at least performing a repeat antigen test 36-48 hours after the first negative,” he said.
Dr. Fujita-Rohwerder said the low diagnostic sensitivity may affect the use of the tests. The gaps in evidence her group found in their study point to research needed to support evidence-based decision-making. “In particular, evidence is needed on real-life performance of tests in schools, self-testing performed by children, and kindergarten, [particularly] sample collection in toddlers by laypersons,” she said.
However, she stressed, testing is only a single measure. “Effectively reducing the spread of SARS-CoV-2 during the current pandemic requires multilayered mitigation measures,” she said. “Rapid testing represents one single layer. It can have its use at the population level, even though the sensitivity of antigen tests is lower than expected. However, antigen-based rapid testing is not a magic bullet: If your kid tests negative, do not disregard other mitigation measures.”
Edward Campbell, PhD, a virologist at Loyola University of Chicago, who serves on the board of LaGrange Elementary School District 102 outside Chicago, said the findings were unsurprising.
“This study generally looks consistent with what is known for adults. These rapid antigen tests are less sensitive than other tests,” said Dr. Campbell, who also runs a testing company for private schools in the Chicago area using reverse transcription-loop-mediated isothermal amplification technology. Even so, he said, “These tests are still effective at identifying people who are infectious to some degree. Never miss an opportunity to test.”
Dr. Fujita-Rohwerder disclosed no relevant financial conflicts of interest. Dr. Campbell owns Safeguard Surveillance.
With the country looking increasingly to rapid testing as an off-ramp from the COVID-19 pandemic, a new study shows that the performance of the tests in children falls below standards set by regulatory agencies in the United States and elsewhere for diagnostic accuracy.
Experts said the findings, from a meta-analysis by researchers in the United Kingdom and Germany, underscore that, while a positive result on a rapid test is almost certainly an indicator of infection, negative results often are unreliable and can lead to a false sense of security.
“Real-life performance of current antigen tests for professional use in pediatric populations is below the minimum performance criteria set by WHO, the United States Food and Drug Administration, or the Medicines and Healthcare products Regulatory Agency (U.K.),” according to Naomi Fujita-Rohwerder, PhD, a research associate at the Cologne-based German Institute for Quality and Efficiency in Health Care (IQWiG), and her colleagues, whose study appears in BMJ Evidence-Based Medicine.
The researchers said that the study suggests that performance of rapid testing in a pediatric population is comparable to that in adults. However, they said they could not identify any studies investigating self-testing in children, which also could affect test performance.
Egon Ozer, MD, PhD, director of the center for pathogen genomics and microbial evolution at Northwestern University in Chicago, said the finding that specificity was high but sensitivity was middling “suggests that we should be very careful about interpreting negative antigen test results in children and recognize that there is a fair amount of uncertainty in the tests in this situation.”
Researchers from IQWiG, which examines the advantages and disadvantages of medical interventions, and the University of Manchester (England), conducted the systematic review and meta-analysis, which they described as the first of its kind to evaluate the diagnostic accuracy of rapid point-of-care tests for current SARS-CoV-2 infections in children.
They compiled information from 17 studies with a total 6,355 participants. They compared all antigen tests to reverse-transcription polymerase chain reaction (PCR). The studies compared eight antigen tests from six different brands. The rapid antigen tests, available from pharmacies and online stores, are widely used for self-testing in schools and testing toddlers before kindergarten.
The pooled diagnostic sensitivity of antigen tests was 64.2% and specificity was 99.1%.
Dr. Ozer noted that the analysis “was not able to address important outstanding questions such as the likelihood of transmitting infection with a false-negative antigen test versus a true-negative antigen test or how much repeated testing can increase the sensitivity.”
“In Europe, we don’t know how most tests perform in real life,” Dr. Fujita-Rohwerder said. “And even in countries like the United States, where market access is more stringent, we don’t know whether self-testing performed by children or sample collection in toddlers by laypersons has a significant impact on the diagnostic accuracy. Also, diagnostic accuracy estimates reported in our study may not apply to the current omicron or future variants of SARS-CoV-2 or vaccinated children. Hopefully, these essential gaps in the evidence will get addressed soon.”
Dr. Ozer said one takeaway from this study is negative antigen tests should not be considered a “free pass” in children, especially if the child is symptomatic, has been recently exposed to COVID-19, or is planning to spend time with individuals with conditions that place them at high risk for complications of COVID-19 infection. “In such cases, consider getting PCR testing or at least performing a repeat antigen test 36-48 hours after the first negative,” he said.
Dr. Fujita-Rohwerder said the low diagnostic sensitivity may affect the use of the tests. The gaps in evidence her group found in their study point to research needed to support evidence-based decision-making. “In particular, evidence is needed on real-life performance of tests in schools, self-testing performed by children, and kindergarten, [particularly] sample collection in toddlers by laypersons,” she said.
However, she stressed, testing is only a single measure. “Effectively reducing the spread of SARS-CoV-2 during the current pandemic requires multilayered mitigation measures,” she said. “Rapid testing represents one single layer. It can have its use at the population level, even though the sensitivity of antigen tests is lower than expected. However, antigen-based rapid testing is not a magic bullet: If your kid tests negative, do not disregard other mitigation measures.”
Edward Campbell, PhD, a virologist at Loyola University of Chicago, who serves on the board of LaGrange Elementary School District 102 outside Chicago, said the findings were unsurprising.
“This study generally looks consistent with what is known for adults. These rapid antigen tests are less sensitive than other tests,” said Dr. Campbell, who also runs a testing company for private schools in the Chicago area using reverse transcription-loop-mediated isothermal amplification technology. Even so, he said, “These tests are still effective at identifying people who are infectious to some degree. Never miss an opportunity to test.”
Dr. Fujita-Rohwerder disclosed no relevant financial conflicts of interest. Dr. Campbell owns Safeguard Surveillance.
With the country looking increasingly to rapid testing as an off-ramp from the COVID-19 pandemic, a new study shows that the performance of the tests in children falls below standards set by regulatory agencies in the United States and elsewhere for diagnostic accuracy.
Experts said the findings, from a meta-analysis by researchers in the United Kingdom and Germany, underscore that, while a positive result on a rapid test is almost certainly an indicator of infection, negative results often are unreliable and can lead to a false sense of security.
“Real-life performance of current antigen tests for professional use in pediatric populations is below the minimum performance criteria set by WHO, the United States Food and Drug Administration, or the Medicines and Healthcare products Regulatory Agency (U.K.),” according to Naomi Fujita-Rohwerder, PhD, a research associate at the Cologne-based German Institute for Quality and Efficiency in Health Care (IQWiG), and her colleagues, whose study appears in BMJ Evidence-Based Medicine.
The researchers said that the study suggests that performance of rapid testing in a pediatric population is comparable to that in adults. However, they said they could not identify any studies investigating self-testing in children, which also could affect test performance.
Egon Ozer, MD, PhD, director of the center for pathogen genomics and microbial evolution at Northwestern University in Chicago, said the finding that specificity was high but sensitivity was middling “suggests that we should be very careful about interpreting negative antigen test results in children and recognize that there is a fair amount of uncertainty in the tests in this situation.”
Researchers from IQWiG, which examines the advantages and disadvantages of medical interventions, and the University of Manchester (England), conducted the systematic review and meta-analysis, which they described as the first of its kind to evaluate the diagnostic accuracy of rapid point-of-care tests for current SARS-CoV-2 infections in children.
They compiled information from 17 studies with a total 6,355 participants. They compared all antigen tests to reverse-transcription polymerase chain reaction (PCR). The studies compared eight antigen tests from six different brands. The rapid antigen tests, available from pharmacies and online stores, are widely used for self-testing in schools and testing toddlers before kindergarten.
The pooled diagnostic sensitivity of antigen tests was 64.2% and specificity was 99.1%.
Dr. Ozer noted that the analysis “was not able to address important outstanding questions such as the likelihood of transmitting infection with a false-negative antigen test versus a true-negative antigen test or how much repeated testing can increase the sensitivity.”
“In Europe, we don’t know how most tests perform in real life,” Dr. Fujita-Rohwerder said. “And even in countries like the United States, where market access is more stringent, we don’t know whether self-testing performed by children or sample collection in toddlers by laypersons has a significant impact on the diagnostic accuracy. Also, diagnostic accuracy estimates reported in our study may not apply to the current omicron or future variants of SARS-CoV-2 or vaccinated children. Hopefully, these essential gaps in the evidence will get addressed soon.”
Dr. Ozer said one takeaway from this study is negative antigen tests should not be considered a “free pass” in children, especially if the child is symptomatic, has been recently exposed to COVID-19, or is planning to spend time with individuals with conditions that place them at high risk for complications of COVID-19 infection. “In such cases, consider getting PCR testing or at least performing a repeat antigen test 36-48 hours after the first negative,” he said.
Dr. Fujita-Rohwerder said the low diagnostic sensitivity may affect the use of the tests. The gaps in evidence her group found in their study point to research needed to support evidence-based decision-making. “In particular, evidence is needed on real-life performance of tests in schools, self-testing performed by children, and kindergarten, [particularly] sample collection in toddlers by laypersons,” she said.
However, she stressed, testing is only a single measure. “Effectively reducing the spread of SARS-CoV-2 during the current pandemic requires multilayered mitigation measures,” she said. “Rapid testing represents one single layer. It can have its use at the population level, even though the sensitivity of antigen tests is lower than expected. However, antigen-based rapid testing is not a magic bullet: If your kid tests negative, do not disregard other mitigation measures.”
Edward Campbell, PhD, a virologist at Loyola University of Chicago, who serves on the board of LaGrange Elementary School District 102 outside Chicago, said the findings were unsurprising.
“This study generally looks consistent with what is known for adults. These rapid antigen tests are less sensitive than other tests,” said Dr. Campbell, who also runs a testing company for private schools in the Chicago area using reverse transcription-loop-mediated isothermal amplification technology. Even so, he said, “These tests are still effective at identifying people who are infectious to some degree. Never miss an opportunity to test.”
Dr. Fujita-Rohwerder disclosed no relevant financial conflicts of interest. Dr. Campbell owns Safeguard Surveillance.
BMJ EVIDENCE-BASED MEDICINE
Docs pen open letter to support Fauci against partisan ‘attacks’
“We deplore the personal attacks on Dr. Fauci. The criticism is inaccurate, unscientific, ill-founded in the facts and, increasingly, motivated by partisan politics,” reads the letter of support, initiated by Ezekiel Emanuel, MD, and signed by almost 300 scientists and public health and medical professionals, including Nobel Laureates, a former Republican senator, and leadership of medical societies and institutions.
Dr. Fauci has led the National Institute for Allergy and Infectious Diseases since 1984 and serves as President Biden’s top medical advisor on the pandemic.
“Dr. Anthony Fauci has served the U.S.A. with wisdom and integrity for nearly 40 years. Through HIV, Ebola, and now COVID, he has unswervingly served the United States guiding the country to very successful outcomes. He has our unreserved respect and trust as a scientist and a national leader,” the letter reads.
Dr. Fauci has repeatedly faced harsh criticism from congressional Republicans, especially Sen. Rand Paul (R-Ky.) and Sen. Roger Marshall (R-Kan.).
At a particularly contentious congressional hearing earlier this week on the federal government’s response to Omicron, Dr. Fauci fought back, telling Sen. Marshall, “You’re so misinformed, it’s extraordinary.”
Dr. Fauci, who has received death threats and harassment of his family, told Sen. Rand that his “completely untrue” statements and rhetoric “kindles the crazies out there.”
‘Sagacious counsel’
The personal attacks on Dr. Fauci are a “distraction from what should be the national focus – working together to finally overcome a pandemic that is killing about 500,000 people a year. We are grateful for Dr. Fauci’s dedication and tireless efforts to help the country through this pandemic and other health crises,” the letter reads.
“Throughout the COVID-19 pandemic, Dr. Fauci has provided the American political leadership and the public with sagacious counsel in these most difficult of times. His advice has been as well informed as data and the rapidly evolving circumstances allowed,” it states.
“Importantly,” Dr. Fauci has given his advice with “humility, being clear about what we know and what is unknown, but requires judgment. He has consistently emphasized the importance of mask-wearing, social distancing, and vaccination. These are standard and necessary public health measures that we all support,” the letter states.
“We are grateful that Dr. Fauci has consistently stated the science in a way that represents the facts as they emerge, without unwarranted speculation.”
“Sadly, in these politically polarized times where misinformation contaminates the United States’ response to the pandemic, routine public health measures have become unnecessarily controversial, undermining the effectiveness of our country’s response,” the letter reads.
A version of this article first appeared on Medscape.com.
“We deplore the personal attacks on Dr. Fauci. The criticism is inaccurate, unscientific, ill-founded in the facts and, increasingly, motivated by partisan politics,” reads the letter of support, initiated by Ezekiel Emanuel, MD, and signed by almost 300 scientists and public health and medical professionals, including Nobel Laureates, a former Republican senator, and leadership of medical societies and institutions.
Dr. Fauci has led the National Institute for Allergy and Infectious Diseases since 1984 and serves as President Biden’s top medical advisor on the pandemic.
“Dr. Anthony Fauci has served the U.S.A. with wisdom and integrity for nearly 40 years. Through HIV, Ebola, and now COVID, he has unswervingly served the United States guiding the country to very successful outcomes. He has our unreserved respect and trust as a scientist and a national leader,” the letter reads.
Dr. Fauci has repeatedly faced harsh criticism from congressional Republicans, especially Sen. Rand Paul (R-Ky.) and Sen. Roger Marshall (R-Kan.).
At a particularly contentious congressional hearing earlier this week on the federal government’s response to Omicron, Dr. Fauci fought back, telling Sen. Marshall, “You’re so misinformed, it’s extraordinary.”
Dr. Fauci, who has received death threats and harassment of his family, told Sen. Rand that his “completely untrue” statements and rhetoric “kindles the crazies out there.”
‘Sagacious counsel’
The personal attacks on Dr. Fauci are a “distraction from what should be the national focus – working together to finally overcome a pandemic that is killing about 500,000 people a year. We are grateful for Dr. Fauci’s dedication and tireless efforts to help the country through this pandemic and other health crises,” the letter reads.
“Throughout the COVID-19 pandemic, Dr. Fauci has provided the American political leadership and the public with sagacious counsel in these most difficult of times. His advice has been as well informed as data and the rapidly evolving circumstances allowed,” it states.
“Importantly,” Dr. Fauci has given his advice with “humility, being clear about what we know and what is unknown, but requires judgment. He has consistently emphasized the importance of mask-wearing, social distancing, and vaccination. These are standard and necessary public health measures that we all support,” the letter states.
“We are grateful that Dr. Fauci has consistently stated the science in a way that represents the facts as they emerge, without unwarranted speculation.”
“Sadly, in these politically polarized times where misinformation contaminates the United States’ response to the pandemic, routine public health measures have become unnecessarily controversial, undermining the effectiveness of our country’s response,” the letter reads.
A version of this article first appeared on Medscape.com.
“We deplore the personal attacks on Dr. Fauci. The criticism is inaccurate, unscientific, ill-founded in the facts and, increasingly, motivated by partisan politics,” reads the letter of support, initiated by Ezekiel Emanuel, MD, and signed by almost 300 scientists and public health and medical professionals, including Nobel Laureates, a former Republican senator, and leadership of medical societies and institutions.
Dr. Fauci has led the National Institute for Allergy and Infectious Diseases since 1984 and serves as President Biden’s top medical advisor on the pandemic.
“Dr. Anthony Fauci has served the U.S.A. with wisdom and integrity for nearly 40 years. Through HIV, Ebola, and now COVID, he has unswervingly served the United States guiding the country to very successful outcomes. He has our unreserved respect and trust as a scientist and a national leader,” the letter reads.
Dr. Fauci has repeatedly faced harsh criticism from congressional Republicans, especially Sen. Rand Paul (R-Ky.) and Sen. Roger Marshall (R-Kan.).
At a particularly contentious congressional hearing earlier this week on the federal government’s response to Omicron, Dr. Fauci fought back, telling Sen. Marshall, “You’re so misinformed, it’s extraordinary.”
Dr. Fauci, who has received death threats and harassment of his family, told Sen. Rand that his “completely untrue” statements and rhetoric “kindles the crazies out there.”
‘Sagacious counsel’
The personal attacks on Dr. Fauci are a “distraction from what should be the national focus – working together to finally overcome a pandemic that is killing about 500,000 people a year. We are grateful for Dr. Fauci’s dedication and tireless efforts to help the country through this pandemic and other health crises,” the letter reads.
“Throughout the COVID-19 pandemic, Dr. Fauci has provided the American political leadership and the public with sagacious counsel in these most difficult of times. His advice has been as well informed as data and the rapidly evolving circumstances allowed,” it states.
“Importantly,” Dr. Fauci has given his advice with “humility, being clear about what we know and what is unknown, but requires judgment. He has consistently emphasized the importance of mask-wearing, social distancing, and vaccination. These are standard and necessary public health measures that we all support,” the letter states.
“We are grateful that Dr. Fauci has consistently stated the science in a way that represents the facts as they emerge, without unwarranted speculation.”
“Sadly, in these politically polarized times where misinformation contaminates the United States’ response to the pandemic, routine public health measures have become unnecessarily controversial, undermining the effectiveness of our country’s response,” the letter reads.
A version of this article first appeared on Medscape.com.
More vitamin D not better for reducing cancer or CVD incidence
according to a new randomized controlled study.
In the cohort of nearly 2,500 healthy individuals, the researchers found no differences in cancer or CVD incidence over 5 years between the groups randomly assigned to vitamin D supplementation and to placebo.
The findings, published online Jan. 4, 2022, in the American Journal of Clinical Nutrition, may be influenced by the fact that most participants had sufficient vitamin D levels at baseline, and thus received higher than recommended doses of vitamin D during the study.
“Vitamin D3 supplementation with 1600 or 3200 IU/day for 5 years did not reduce the incidence of major CVD events, any invasive cancer, or mortality among generally healthy and mostly vitamin D sufficient older adults in Finland,” write the authors, led by Jyrki Virtanen, RD, PhD, associate professor of nutrition and public health at University of Eastern Finland, Kuopio.
“The low number of subjects with low vitamin D concentrations was a bit of a surprise for us also, but it likely reflects the quite successful food fortification policy in Finland,” Dr. Virtanen told this news organization.
Prior research has found that vitamin D insufficiency is associated with a higher risk of nearly all diseases. Although the evidence on the benefits of vitamin D supplementation remains more limited, a meta-analysis reported a consistent and significant 13% reduction in cancer mortality in those who received vitamin D supplements.
In this study, Dr. Virtanen and colleagues investigated the effects of vitamin D3 supplementation on cancer and CVD incidence in a cohort of 2,495 healthy participants.
Men 60 years or older and women 65 years or older were randomly assigned to one of three groups: placebo, 40 mcg (1,600 IU) of daily vitamin D3, or 80 mcg (3,200 IU) of daily vitamin D3.
Data collected at baseline and throughout the trial included serum 25(OH)D concentrations, nutrition, sun exposure, medication use, mental health, and other factors that could affect the risk of disease.
The study’s primary endpoints were incident of major CVD and invasive cancer. Secondary endpoints included incidence of myocardial infarction, stroke, and CVD mortality as well as site-specific cancers and cancer death.
Follow-up occurred via annual study questionnaires and national registry data. A representative subcohort of 551 participants had more detailed in-person evaluations. In the sub-cohort, mean serum 25(OH)D concentration was 75 nmol/L (30 ng/mL) at baseline; 9.1% had concentrations less than 50 nmol/L (20 ng/mL) and 50.0% had concentrations of at least 75 nmol/L (30 ng/mL).
The authors identified no major differences between the three arms at baseline, but noted that, compared with the overall study population, those in the subcohort were younger, more likely to use their own vitamin D supplements, and more likely to rate their health as good or excellent.
Among 503 participants that had complete data from baseline, the mean increase in serum 25(OH)D in participants receiving 1,600 IU/day vitamin D3 was 23.4 nmol/L (9.4 ng/mL) and 43.6 nmol/L (17.4 ng/mL) in the arm receiving 3,200 IU/day between baseline and 6 months. The authors observed a small additional increase in levels between the 6-month and 12-month visits, but few changes in vitamin D3 levels in the placebo arm.
At the 5-year follow-up, major CVD events occurred in 4.9% of participants in the placebo arm, 5% in those in the 1,600 IU/d arm (hazard ratio, 0.97), and 4.3% of those in the 3,200 IU/d arm (HR, 0.84; P = .44). Invasive cancer at follow-up was diagnosed in 4.9% of placebo recipients, 5.8% of those on 1,600 IU/d supplementation (HR, 1.14; P = .55), and 4.8% in the 3,200 IU/d group (HR, 0.95; P = .81). No significant differences were observed in the secondary endpoints or in total mortality.
The authors did not conduct a subanalysis in participants who had low 25(OH)D concentrations levels at baseline because “there were too few participants to do any meaningful analyses,” said Dr. Virtanen, who noted that blood samples were available for a representative subgroup of 550 subjects, and only 9% of them had low 25(OH)D concentrations at baseline.
Dr. Virtanen noted that future vitamin D supplementation trials should focus on recruiting participants with low vitamin D status.
The study was supported by funding from the Academy of Finland, University of Eastern Finland, Juho Vainio Foundation, Medicinska Understödsföreningen Liv och Hälsa, Finnish Foundation for Cardiovascular Research, Finnish Diabetes Research Foundation, and Finnish Cultural Foundation. Dr. Virtanen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new randomized controlled study.
In the cohort of nearly 2,500 healthy individuals, the researchers found no differences in cancer or CVD incidence over 5 years between the groups randomly assigned to vitamin D supplementation and to placebo.
The findings, published online Jan. 4, 2022, in the American Journal of Clinical Nutrition, may be influenced by the fact that most participants had sufficient vitamin D levels at baseline, and thus received higher than recommended doses of vitamin D during the study.
“Vitamin D3 supplementation with 1600 or 3200 IU/day for 5 years did not reduce the incidence of major CVD events, any invasive cancer, or mortality among generally healthy and mostly vitamin D sufficient older adults in Finland,” write the authors, led by Jyrki Virtanen, RD, PhD, associate professor of nutrition and public health at University of Eastern Finland, Kuopio.
“The low number of subjects with low vitamin D concentrations was a bit of a surprise for us also, but it likely reflects the quite successful food fortification policy in Finland,” Dr. Virtanen told this news organization.
Prior research has found that vitamin D insufficiency is associated with a higher risk of nearly all diseases. Although the evidence on the benefits of vitamin D supplementation remains more limited, a meta-analysis reported a consistent and significant 13% reduction in cancer mortality in those who received vitamin D supplements.
In this study, Dr. Virtanen and colleagues investigated the effects of vitamin D3 supplementation on cancer and CVD incidence in a cohort of 2,495 healthy participants.
Men 60 years or older and women 65 years or older were randomly assigned to one of three groups: placebo, 40 mcg (1,600 IU) of daily vitamin D3, or 80 mcg (3,200 IU) of daily vitamin D3.
Data collected at baseline and throughout the trial included serum 25(OH)D concentrations, nutrition, sun exposure, medication use, mental health, and other factors that could affect the risk of disease.
The study’s primary endpoints were incident of major CVD and invasive cancer. Secondary endpoints included incidence of myocardial infarction, stroke, and CVD mortality as well as site-specific cancers and cancer death.
Follow-up occurred via annual study questionnaires and national registry data. A representative subcohort of 551 participants had more detailed in-person evaluations. In the sub-cohort, mean serum 25(OH)D concentration was 75 nmol/L (30 ng/mL) at baseline; 9.1% had concentrations less than 50 nmol/L (20 ng/mL) and 50.0% had concentrations of at least 75 nmol/L (30 ng/mL).
The authors identified no major differences between the three arms at baseline, but noted that, compared with the overall study population, those in the subcohort were younger, more likely to use their own vitamin D supplements, and more likely to rate their health as good or excellent.
Among 503 participants that had complete data from baseline, the mean increase in serum 25(OH)D in participants receiving 1,600 IU/day vitamin D3 was 23.4 nmol/L (9.4 ng/mL) and 43.6 nmol/L (17.4 ng/mL) in the arm receiving 3,200 IU/day between baseline and 6 months. The authors observed a small additional increase in levels between the 6-month and 12-month visits, but few changes in vitamin D3 levels in the placebo arm.
At the 5-year follow-up, major CVD events occurred in 4.9% of participants in the placebo arm, 5% in those in the 1,600 IU/d arm (hazard ratio, 0.97), and 4.3% of those in the 3,200 IU/d arm (HR, 0.84; P = .44). Invasive cancer at follow-up was diagnosed in 4.9% of placebo recipients, 5.8% of those on 1,600 IU/d supplementation (HR, 1.14; P = .55), and 4.8% in the 3,200 IU/d group (HR, 0.95; P = .81). No significant differences were observed in the secondary endpoints or in total mortality.
The authors did not conduct a subanalysis in participants who had low 25(OH)D concentrations levels at baseline because “there were too few participants to do any meaningful analyses,” said Dr. Virtanen, who noted that blood samples were available for a representative subgroup of 550 subjects, and only 9% of them had low 25(OH)D concentrations at baseline.
Dr. Virtanen noted that future vitamin D supplementation trials should focus on recruiting participants with low vitamin D status.
The study was supported by funding from the Academy of Finland, University of Eastern Finland, Juho Vainio Foundation, Medicinska Understödsföreningen Liv och Hälsa, Finnish Foundation for Cardiovascular Research, Finnish Diabetes Research Foundation, and Finnish Cultural Foundation. Dr. Virtanen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new randomized controlled study.
In the cohort of nearly 2,500 healthy individuals, the researchers found no differences in cancer or CVD incidence over 5 years between the groups randomly assigned to vitamin D supplementation and to placebo.
The findings, published online Jan. 4, 2022, in the American Journal of Clinical Nutrition, may be influenced by the fact that most participants had sufficient vitamin D levels at baseline, and thus received higher than recommended doses of vitamin D during the study.
“Vitamin D3 supplementation with 1600 or 3200 IU/day for 5 years did not reduce the incidence of major CVD events, any invasive cancer, or mortality among generally healthy and mostly vitamin D sufficient older adults in Finland,” write the authors, led by Jyrki Virtanen, RD, PhD, associate professor of nutrition and public health at University of Eastern Finland, Kuopio.
“The low number of subjects with low vitamin D concentrations was a bit of a surprise for us also, but it likely reflects the quite successful food fortification policy in Finland,” Dr. Virtanen told this news organization.
Prior research has found that vitamin D insufficiency is associated with a higher risk of nearly all diseases. Although the evidence on the benefits of vitamin D supplementation remains more limited, a meta-analysis reported a consistent and significant 13% reduction in cancer mortality in those who received vitamin D supplements.
In this study, Dr. Virtanen and colleagues investigated the effects of vitamin D3 supplementation on cancer and CVD incidence in a cohort of 2,495 healthy participants.
Men 60 years or older and women 65 years or older were randomly assigned to one of three groups: placebo, 40 mcg (1,600 IU) of daily vitamin D3, or 80 mcg (3,200 IU) of daily vitamin D3.
Data collected at baseline and throughout the trial included serum 25(OH)D concentrations, nutrition, sun exposure, medication use, mental health, and other factors that could affect the risk of disease.
The study’s primary endpoints were incident of major CVD and invasive cancer. Secondary endpoints included incidence of myocardial infarction, stroke, and CVD mortality as well as site-specific cancers and cancer death.
Follow-up occurred via annual study questionnaires and national registry data. A representative subcohort of 551 participants had more detailed in-person evaluations. In the sub-cohort, mean serum 25(OH)D concentration was 75 nmol/L (30 ng/mL) at baseline; 9.1% had concentrations less than 50 nmol/L (20 ng/mL) and 50.0% had concentrations of at least 75 nmol/L (30 ng/mL).
The authors identified no major differences between the three arms at baseline, but noted that, compared with the overall study population, those in the subcohort were younger, more likely to use their own vitamin D supplements, and more likely to rate their health as good or excellent.
Among 503 participants that had complete data from baseline, the mean increase in serum 25(OH)D in participants receiving 1,600 IU/day vitamin D3 was 23.4 nmol/L (9.4 ng/mL) and 43.6 nmol/L (17.4 ng/mL) in the arm receiving 3,200 IU/day between baseline and 6 months. The authors observed a small additional increase in levels between the 6-month and 12-month visits, but few changes in vitamin D3 levels in the placebo arm.
At the 5-year follow-up, major CVD events occurred in 4.9% of participants in the placebo arm, 5% in those in the 1,600 IU/d arm (hazard ratio, 0.97), and 4.3% of those in the 3,200 IU/d arm (HR, 0.84; P = .44). Invasive cancer at follow-up was diagnosed in 4.9% of placebo recipients, 5.8% of those on 1,600 IU/d supplementation (HR, 1.14; P = .55), and 4.8% in the 3,200 IU/d group (HR, 0.95; P = .81). No significant differences were observed in the secondary endpoints or in total mortality.
The authors did not conduct a subanalysis in participants who had low 25(OH)D concentrations levels at baseline because “there were too few participants to do any meaningful analyses,” said Dr. Virtanen, who noted that blood samples were available for a representative subgroup of 550 subjects, and only 9% of them had low 25(OH)D concentrations at baseline.
Dr. Virtanen noted that future vitamin D supplementation trials should focus on recruiting participants with low vitamin D status.
The study was supported by funding from the Academy of Finland, University of Eastern Finland, Juho Vainio Foundation, Medicinska Understödsföreningen Liv och Hälsa, Finnish Foundation for Cardiovascular Research, Finnish Diabetes Research Foundation, and Finnish Cultural Foundation. Dr. Virtanen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF CLINICAL NUTRITION
What if the National Guard Can’t Help?
What if the National Guard Can’t Help?
In early January, Ohio not only set a state record for COVID-19 hospitalizations—it had the fourth highest rate in the country, with 6,747 hospitalized coronavirus patients on January 10, a 40% increase over the previous 21 days. Most were unvaccinated. To help overwhelmed hospitals cope, Ohio Gov. Mike DeWine turned to the National Guard. Unfortunately, nearly half of the Ohio National Guard also were unvaccinated.
By US Department of Defense (DoD) directive, National Guard members must have a COVID-19 vaccination to be deployed on hospital missions. Thus, in COVID hotspots across the nation, governors are on the horns of a dilemma. They want and need to deploy the National Guard to give medical and nonclinical support but aren’t sure whether they will be able to or, indeed, whether they should.
So far, vaccinated teams are already on the ground in a number of states. In Indiana, where hospitalizations jumped 50% over 2 weeks in December, the National Guard sent 6-person teams, all fully vaccinated. In New Hampshire, 70 guards are being deployed to help hospitals with food service, clerical work, and other nonmedical functions. New York Governor Kathy Hochul has deployed guard members for help to ease the strain on nursing homes. Massachusetts Governor Charlie Baker has activated up to 500 guard members; some will be supporting 55 acute care hospital and 12 ambulance services. In Maine, where cases have peaked, Governor Janet Mills activated guard members to support nursing facilities and administer monoclonal antibodies. The Louisiana National Guard has administered more than 542,000 COVID-19 tests and 206,300 vaccines. As many as 1,000 Maryland Air and Army National Guardsmen are being activated to help with testing and other missions.
However, as in Ohio, other states are facing problematic scenarios. For instance, about 40% of the more than 20,000 Texas National Guard are refusing to get vaccinated, challenging the Biden Administration vaccine requirement for all military.
And a court showdown over federal vaccine mandates, started by Governor Kevin Stitt of Oklahoma and joined by the Republican governors of Wyoming, Iowa, Alaska, Nebraska, and Mississippi, came to a head in December. Last November, Stitt asked Defense Secretary Lloyd Austin to exempt Oklahoma’s National Guard from the vaccine mandate. He claimed the requirement violated the personal freedoms of many Oklahomans and could cause them to “potentially sacrifice their personal beliefs.” But in a memo to the Joint Chiefs chairmen, the service secretaries and the head of the National Guard Bureau, Austin wrote that Pentagon funds could not be used to pay for duties performed under Title 32 for members of the Guard who do not comply with the military’s vaccine requirement. (Title 32 refers to Guard operations under state orders.) Austin also said National Guard members must be vaccinated to participate in drills, training, and other duty conducted under Title 32.
Stitt, maintaining that he is commander in chief of the Oklahoma National Guard as long as it operates under Title 32 orders, put out his own memo stipulating that no Guard member was required to get vaccinated. He also ordered Brig. Gen. Thomas Mancino, newly appointed commander of the Oklahoma National Guard, to not enforce the mandate. Subsequently, Mancino issued a statement pointing out that current state law is limited in protecting troops who opt out of the shot. Moreover, if the Guard were called up under federal orders, he said, he would enforce the mandate. Training events, schools, and mobilizations were going to “eventually force you out of that safe harbor,” he wrote, “…This is reality.”
In late December, a federal judge denied Oklahoma’s motion to enjoin the mandate. The Oklahoma Attorney General’s office responded, “We will not be surprised if the President’s vaccine mandate actually reduces the nation’s military readiness instead of promoting it.”
In a press briefing, Pentagon press secretary John Kirby said, “The Secretary has the authorities he needs to require this vaccine across the force, including the National Guard. …[E]ven when they’re in a Title 32 status.” He added, “It is a lawful order for National Guardsmen to receive the COVID vaccine. It’s a lawful order, and refusing to do that, absent of an improved exemption, puts them in the same potential [position] as active-duty members who refuse the vaccine.” That could mean, for instance, loss of pay and membership in the National Guard.
A core rationale for the mandate, according to Secretary Austin, is the need for military readiness—meaning Guard members must be healthy and fit for duty. And that extends to being healthy and fit for missions like transporting at-risk patients. Ohio National Guard Adjutant General Major General John Harris Jr. said, “I would never put a soldier or airman in harm’s way without the best protection we could put on them—body armor, helmets. And this medical readiness is the exact same thing. We’re putting folks into harm’s way.” He has moved the deadline from the Pentagon’s June 30 date to March 31—a move that boosted the vaccination rate from 53% to 56% in one week.
Ohio Governor DeWine has expressed frustration that almost half of the Ohio Army National Guard personnel can’t be deployed on this mission because they’re unvaccinated. “In some of our testing places, 40 to 50% of the people are testing positive,” he said. “So this is a high-risk operation. You need to be protected. The best way for you to be protected is to get the vaccination.”
As of December 2021, according to the National Guard Bureau, the National Guard as a whole was 66% fully vaccinated. The percentages vary according to service; for instance, nearly 90% of airmen have been vaccinated, compared with only 40% of Army Guardsmen. Among the states challenging the mandate, the vaccinated rates have been moving upward: In Alaska, about 92% of the Air National Guard have been vaccinated—leaving roughly 11,000 troops who had not met the December 2 deadline. In Iowa, as of Nov. 30, 91% of Air National Guard and 80% of Army National Guard members had been vaccinated, but about 9,000 soldiers had been directed to get the vaccination or risk disciplinary action. Almost 2,200 of the more than 2,800-strong Wyoming National Guard (77%) have received at least 1 dose. Nebraska Air National Guard’s force of 1,000 was 94% fully vaccinated as of December 1. (Maj Scott Ingalsbe, public affairs officer, said, “Vaccinations are tied to individual medical readiness. They provide service members with the best protection available so they can perform missions across the globe.”).
In most states, Army National Guard members have until June 30, 2022, to comply. “Our soldiers …have until [the DoD’s deadline], and some of them are just going to wait close to the deadline,” John Goheen of the National Guard Association of the United States said in a discussion on NPR. “That’s human nature.”
Earlier this month, Texas Governor Greg Abbott told National Guard members they can ignore the Pentagon’s COVID-19 vaccine mandate: “President Biden is not your commander-in-chief.” He has also sued the Biden administration over the requirement.
In the meantime, the hospitals at breaking point must hope for the best and take as much help as they can get.
In early January, Ohio not only set a state record for COVID-19 hospitalizations—it had the fourth highest rate in the country, with 6,747 hospitalized coronavirus patients on January 10, a 40% increase over the previous 21 days. Most were unvaccinated. To help overwhelmed hospitals cope, Ohio Gov. Mike DeWine turned to the National Guard. Unfortunately, nearly half of the Ohio National Guard also were unvaccinated.
By US Department of Defense (DoD) directive, National Guard members must have a COVID-19 vaccination to be deployed on hospital missions. Thus, in COVID hotspots across the nation, governors are on the horns of a dilemma. They want and need to deploy the National Guard to give medical and nonclinical support but aren’t sure whether they will be able to or, indeed, whether they should.
So far, vaccinated teams are already on the ground in a number of states. In Indiana, where hospitalizations jumped 50% over 2 weeks in December, the National Guard sent 6-person teams, all fully vaccinated. In New Hampshire, 70 guards are being deployed to help hospitals with food service, clerical work, and other nonmedical functions. New York Governor Kathy Hochul has deployed guard members for help to ease the strain on nursing homes. Massachusetts Governor Charlie Baker has activated up to 500 guard members; some will be supporting 55 acute care hospital and 12 ambulance services. In Maine, where cases have peaked, Governor Janet Mills activated guard members to support nursing facilities and administer monoclonal antibodies. The Louisiana National Guard has administered more than 542,000 COVID-19 tests and 206,300 vaccines. As many as 1,000 Maryland Air and Army National Guardsmen are being activated to help with testing and other missions.
However, as in Ohio, other states are facing problematic scenarios. For instance, about 40% of the more than 20,000 Texas National Guard are refusing to get vaccinated, challenging the Biden Administration vaccine requirement for all military.
And a court showdown over federal vaccine mandates, started by Governor Kevin Stitt of Oklahoma and joined by the Republican governors of Wyoming, Iowa, Alaska, Nebraska, and Mississippi, came to a head in December. Last November, Stitt asked Defense Secretary Lloyd Austin to exempt Oklahoma’s National Guard from the vaccine mandate. He claimed the requirement violated the personal freedoms of many Oklahomans and could cause them to “potentially sacrifice their personal beliefs.” But in a memo to the Joint Chiefs chairmen, the service secretaries and the head of the National Guard Bureau, Austin wrote that Pentagon funds could not be used to pay for duties performed under Title 32 for members of the Guard who do not comply with the military’s vaccine requirement. (Title 32 refers to Guard operations under state orders.) Austin also said National Guard members must be vaccinated to participate in drills, training, and other duty conducted under Title 32.
Stitt, maintaining that he is commander in chief of the Oklahoma National Guard as long as it operates under Title 32 orders, put out his own memo stipulating that no Guard member was required to get vaccinated. He also ordered Brig. Gen. Thomas Mancino, newly appointed commander of the Oklahoma National Guard, to not enforce the mandate. Subsequently, Mancino issued a statement pointing out that current state law is limited in protecting troops who opt out of the shot. Moreover, if the Guard were called up under federal orders, he said, he would enforce the mandate. Training events, schools, and mobilizations were going to “eventually force you out of that safe harbor,” he wrote, “…This is reality.”
In late December, a federal judge denied Oklahoma’s motion to enjoin the mandate. The Oklahoma Attorney General’s office responded, “We will not be surprised if the President’s vaccine mandate actually reduces the nation’s military readiness instead of promoting it.”
In a press briefing, Pentagon press secretary John Kirby said, “The Secretary has the authorities he needs to require this vaccine across the force, including the National Guard. …[E]ven when they’re in a Title 32 status.” He added, “It is a lawful order for National Guardsmen to receive the COVID vaccine. It’s a lawful order, and refusing to do that, absent of an improved exemption, puts them in the same potential [position] as active-duty members who refuse the vaccine.” That could mean, for instance, loss of pay and membership in the National Guard.
A core rationale for the mandate, according to Secretary Austin, is the need for military readiness—meaning Guard members must be healthy and fit for duty. And that extends to being healthy and fit for missions like transporting at-risk patients. Ohio National Guard Adjutant General Major General John Harris Jr. said, “I would never put a soldier or airman in harm’s way without the best protection we could put on them—body armor, helmets. And this medical readiness is the exact same thing. We’re putting folks into harm’s way.” He has moved the deadline from the Pentagon’s June 30 date to March 31—a move that boosted the vaccination rate from 53% to 56% in one week.
Ohio Governor DeWine has expressed frustration that almost half of the Ohio Army National Guard personnel can’t be deployed on this mission because they’re unvaccinated. “In some of our testing places, 40 to 50% of the people are testing positive,” he said. “So this is a high-risk operation. You need to be protected. The best way for you to be protected is to get the vaccination.”
As of December 2021, according to the National Guard Bureau, the National Guard as a whole was 66% fully vaccinated. The percentages vary according to service; for instance, nearly 90% of airmen have been vaccinated, compared with only 40% of Army Guardsmen. Among the states challenging the mandate, the vaccinated rates have been moving upward: In Alaska, about 92% of the Air National Guard have been vaccinated—leaving roughly 11,000 troops who had not met the December 2 deadline. In Iowa, as of Nov. 30, 91% of Air National Guard and 80% of Army National Guard members had been vaccinated, but about 9,000 soldiers had been directed to get the vaccination or risk disciplinary action. Almost 2,200 of the more than 2,800-strong Wyoming National Guard (77%) have received at least 1 dose. Nebraska Air National Guard’s force of 1,000 was 94% fully vaccinated as of December 1. (Maj Scott Ingalsbe, public affairs officer, said, “Vaccinations are tied to individual medical readiness. They provide service members with the best protection available so they can perform missions across the globe.”).
In most states, Army National Guard members have until June 30, 2022, to comply. “Our soldiers …have until [the DoD’s deadline], and some of them are just going to wait close to the deadline,” John Goheen of the National Guard Association of the United States said in a discussion on NPR. “That’s human nature.”
Earlier this month, Texas Governor Greg Abbott told National Guard members they can ignore the Pentagon’s COVID-19 vaccine mandate: “President Biden is not your commander-in-chief.” He has also sued the Biden administration over the requirement.
In the meantime, the hospitals at breaking point must hope for the best and take as much help as they can get.
In early January, Ohio not only set a state record for COVID-19 hospitalizations—it had the fourth highest rate in the country, with 6,747 hospitalized coronavirus patients on January 10, a 40% increase over the previous 21 days. Most were unvaccinated. To help overwhelmed hospitals cope, Ohio Gov. Mike DeWine turned to the National Guard. Unfortunately, nearly half of the Ohio National Guard also were unvaccinated.
By US Department of Defense (DoD) directive, National Guard members must have a COVID-19 vaccination to be deployed on hospital missions. Thus, in COVID hotspots across the nation, governors are on the horns of a dilemma. They want and need to deploy the National Guard to give medical and nonclinical support but aren’t sure whether they will be able to or, indeed, whether they should.
So far, vaccinated teams are already on the ground in a number of states. In Indiana, where hospitalizations jumped 50% over 2 weeks in December, the National Guard sent 6-person teams, all fully vaccinated. In New Hampshire, 70 guards are being deployed to help hospitals with food service, clerical work, and other nonmedical functions. New York Governor Kathy Hochul has deployed guard members for help to ease the strain on nursing homes. Massachusetts Governor Charlie Baker has activated up to 500 guard members; some will be supporting 55 acute care hospital and 12 ambulance services. In Maine, where cases have peaked, Governor Janet Mills activated guard members to support nursing facilities and administer monoclonal antibodies. The Louisiana National Guard has administered more than 542,000 COVID-19 tests and 206,300 vaccines. As many as 1,000 Maryland Air and Army National Guardsmen are being activated to help with testing and other missions.
However, as in Ohio, other states are facing problematic scenarios. For instance, about 40% of the more than 20,000 Texas National Guard are refusing to get vaccinated, challenging the Biden Administration vaccine requirement for all military.
And a court showdown over federal vaccine mandates, started by Governor Kevin Stitt of Oklahoma and joined by the Republican governors of Wyoming, Iowa, Alaska, Nebraska, and Mississippi, came to a head in December. Last November, Stitt asked Defense Secretary Lloyd Austin to exempt Oklahoma’s National Guard from the vaccine mandate. He claimed the requirement violated the personal freedoms of many Oklahomans and could cause them to “potentially sacrifice their personal beliefs.” But in a memo to the Joint Chiefs chairmen, the service secretaries and the head of the National Guard Bureau, Austin wrote that Pentagon funds could not be used to pay for duties performed under Title 32 for members of the Guard who do not comply with the military’s vaccine requirement. (Title 32 refers to Guard operations under state orders.) Austin also said National Guard members must be vaccinated to participate in drills, training, and other duty conducted under Title 32.
Stitt, maintaining that he is commander in chief of the Oklahoma National Guard as long as it operates under Title 32 orders, put out his own memo stipulating that no Guard member was required to get vaccinated. He also ordered Brig. Gen. Thomas Mancino, newly appointed commander of the Oklahoma National Guard, to not enforce the mandate. Subsequently, Mancino issued a statement pointing out that current state law is limited in protecting troops who opt out of the shot. Moreover, if the Guard were called up under federal orders, he said, he would enforce the mandate. Training events, schools, and mobilizations were going to “eventually force you out of that safe harbor,” he wrote, “…This is reality.”
In late December, a federal judge denied Oklahoma’s motion to enjoin the mandate. The Oklahoma Attorney General’s office responded, “We will not be surprised if the President’s vaccine mandate actually reduces the nation’s military readiness instead of promoting it.”
In a press briefing, Pentagon press secretary John Kirby said, “The Secretary has the authorities he needs to require this vaccine across the force, including the National Guard. …[E]ven when they’re in a Title 32 status.” He added, “It is a lawful order for National Guardsmen to receive the COVID vaccine. It’s a lawful order, and refusing to do that, absent of an improved exemption, puts them in the same potential [position] as active-duty members who refuse the vaccine.” That could mean, for instance, loss of pay and membership in the National Guard.
A core rationale for the mandate, according to Secretary Austin, is the need for military readiness—meaning Guard members must be healthy and fit for duty. And that extends to being healthy and fit for missions like transporting at-risk patients. Ohio National Guard Adjutant General Major General John Harris Jr. said, “I would never put a soldier or airman in harm’s way without the best protection we could put on them—body armor, helmets. And this medical readiness is the exact same thing. We’re putting folks into harm’s way.” He has moved the deadline from the Pentagon’s June 30 date to March 31—a move that boosted the vaccination rate from 53% to 56% in one week.
Ohio Governor DeWine has expressed frustration that almost half of the Ohio Army National Guard personnel can’t be deployed on this mission because they’re unvaccinated. “In some of our testing places, 40 to 50% of the people are testing positive,” he said. “So this is a high-risk operation. You need to be protected. The best way for you to be protected is to get the vaccination.”
As of December 2021, according to the National Guard Bureau, the National Guard as a whole was 66% fully vaccinated. The percentages vary according to service; for instance, nearly 90% of airmen have been vaccinated, compared with only 40% of Army Guardsmen. Among the states challenging the mandate, the vaccinated rates have been moving upward: In Alaska, about 92% of the Air National Guard have been vaccinated—leaving roughly 11,000 troops who had not met the December 2 deadline. In Iowa, as of Nov. 30, 91% of Air National Guard and 80% of Army National Guard members had been vaccinated, but about 9,000 soldiers had been directed to get the vaccination or risk disciplinary action. Almost 2,200 of the more than 2,800-strong Wyoming National Guard (77%) have received at least 1 dose. Nebraska Air National Guard’s force of 1,000 was 94% fully vaccinated as of December 1. (Maj Scott Ingalsbe, public affairs officer, said, “Vaccinations are tied to individual medical readiness. They provide service members with the best protection available so they can perform missions across the globe.”).
In most states, Army National Guard members have until June 30, 2022, to comply. “Our soldiers …have until [the DoD’s deadline], and some of them are just going to wait close to the deadline,” John Goheen of the National Guard Association of the United States said in a discussion on NPR. “That’s human nature.”
Earlier this month, Texas Governor Greg Abbott told National Guard members they can ignore the Pentagon’s COVID-19 vaccine mandate: “President Biden is not your commander-in-chief.” He has also sued the Biden administration over the requirement.
In the meantime, the hospitals at breaking point must hope for the best and take as much help as they can get.
What if the National Guard Can’t Help?
What if the National Guard Can’t Help?
COVID-19 vaccination has little impact on menstrual cycle
Women may rest a bit easier thanks to results from a study showing that vaccination against the SARS-CoV-2 virus has almost no impact on a woman’s menstrual cycle. The issue is significant, as regular menstruation is a sign of health and fertility, and fears of disturbances might increase vaccination hesitancy as COVID-19 cases continue to surge.
Alison Edelman, MD, MPH, a professor of obstetrics and gynecology at Oregon Health & Science University, Portland, led a group studying prospective data on almost 24,000 menstrual cycles reported by almost 4,000 U.S. women.
The investigators found that COVID-19 vaccination was associated with a less than 1-day change in cycle length for the menstrual cycles after the first and second inoculations, compared with prevaccine cycles. Vaccination had no effect on the actual number of days menstrual bleeding lasted.
The study looked at the menstrual patterns of women aged 18-45 years with normal cycle lengths of 24-38 days for the three consecutive cycles before the first vaccine dose and for three consecutive postvaccine cycles. The final sample included 2,403 vaccinated and 1,556 unvaccinated individuals.
In vaccinated women, the study initially found a slight average increase in cycle length after dose one of 71% of a day and 91% of a day after dose two. Following adjustments, those increases dropped to 64% of a day after the first dose and 79% of a day after the second dose.
In unvaccinated women, the study looked at six cycles over a similar time period and found no significant changes from baseline.
“Coronavirus disease 2019 vaccination is associated with a small change in cycle length but not menses length,” Dr. Edelman’s group concluded in Obstetrics and Gynecology.
In the rare instance that a woman received two vaccine doses within the same menstrual cycle, the change in length could increase to 2 days. These variations appear to resolve quickly, possibly as soon as the next cycle after vaccination and do not indicate any cause for long-term physical or reproductive health concern, according to the authors.
Reports by women on social media, however, have suggested that postvaccine menstrual disruptions are more common with, for example, heavier and breakthrough bleeding. But it appears such changes are temporary and resolve quickly.
“These findings are reassuring and validating,” Dr. Edelman said in an interview. On a population level, the changes indicate no cause for concern for long-term physical or reproductive health and no reason to avoid vaccination. “On a personal level, people want this information so they know what to expect when they get vaccinated, and not worry about a pregnancy scare or be disappointed if they were trying for pregnancy.”
According to the International Federation of Gynecologists and Obstetricians, variations in cycle length of fewer than 8 days are considered normal, said Christine Metz, PhD, a research biologist and a professor of molecular medicine at the Feinstein Institutes for Medical Research in Manhasset, N.Y. “Thus, the extra 17 hours added to the menstrual cycle length in the vaccination group in this study is well within the ‘normal’ range.”
In a group of about 1,600 menstruating women being studied at Dr. Metz’s center, some have anecdotally reported transient cycle changes post vaccination for COVID-19, including delays in menstruation onset and changes in bleeding patterns.
Exactly how vaccination might alter menstrual cycle length is not known and has not been studied with vaccination against other infections such as influenza and meningococcal disease.
“Many factors are known to affect menstrual cycle length including changes in diet, sleep, and exercise, as well as sickness, travel, and stress,” Dr. Metz said. The COVID-19 vaccines have affected people in different ways, with side effects ranging from injection-site pain to nausea, aches, fever, and fatigue. “Vaccination side effects, particularly if severe, could lead to changes in diet, exercise, and sleep, and feelings of sickness and/or stress.”
These stressors can alter hormone production and stability, as well as the body’s response to hormones such as estrogen, progesterone, follicle-stimulating hormone, luteinizing hormone, and other hormones associated with female reproduction. “Because these hormones regulate the menstrual cycle, variations in these hormones can either shorten or lengthen the cycle,” Dr. Metz explained.
More research needs to be done at the global level, according to the authors. “Questions remain about other possible changes in menstrual cycles, such as menstrual symptoms, unscheduled bleeding, and changes in the quality and quantity of menstrual bleeding.”
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health’s Office of Research on Women’s Health. Dr. Edelman reported support from the American College of Obstetrics and Gynecology, the World Health Organization, Gynuity, and the Karolinska Institute as well as royalties from UpToDate. Other study authors reported similar relationships with not-for-profit and private-sector companies. Three coauthors are employees of Natural Cycles, a fertility tracking device that was used in the study. Dr. Metz disclosed no conflicts of interest with regard to her comments.
Women may rest a bit easier thanks to results from a study showing that vaccination against the SARS-CoV-2 virus has almost no impact on a woman’s menstrual cycle. The issue is significant, as regular menstruation is a sign of health and fertility, and fears of disturbances might increase vaccination hesitancy as COVID-19 cases continue to surge.
Alison Edelman, MD, MPH, a professor of obstetrics and gynecology at Oregon Health & Science University, Portland, led a group studying prospective data on almost 24,000 menstrual cycles reported by almost 4,000 U.S. women.
The investigators found that COVID-19 vaccination was associated with a less than 1-day change in cycle length for the menstrual cycles after the first and second inoculations, compared with prevaccine cycles. Vaccination had no effect on the actual number of days menstrual bleeding lasted.
The study looked at the menstrual patterns of women aged 18-45 years with normal cycle lengths of 24-38 days for the three consecutive cycles before the first vaccine dose and for three consecutive postvaccine cycles. The final sample included 2,403 vaccinated and 1,556 unvaccinated individuals.
In vaccinated women, the study initially found a slight average increase in cycle length after dose one of 71% of a day and 91% of a day after dose two. Following adjustments, those increases dropped to 64% of a day after the first dose and 79% of a day after the second dose.
In unvaccinated women, the study looked at six cycles over a similar time period and found no significant changes from baseline.
“Coronavirus disease 2019 vaccination is associated with a small change in cycle length but not menses length,” Dr. Edelman’s group concluded in Obstetrics and Gynecology.
In the rare instance that a woman received two vaccine doses within the same menstrual cycle, the change in length could increase to 2 days. These variations appear to resolve quickly, possibly as soon as the next cycle after vaccination and do not indicate any cause for long-term physical or reproductive health concern, according to the authors.
Reports by women on social media, however, have suggested that postvaccine menstrual disruptions are more common with, for example, heavier and breakthrough bleeding. But it appears such changes are temporary and resolve quickly.
“These findings are reassuring and validating,” Dr. Edelman said in an interview. On a population level, the changes indicate no cause for concern for long-term physical or reproductive health and no reason to avoid vaccination. “On a personal level, people want this information so they know what to expect when they get vaccinated, and not worry about a pregnancy scare or be disappointed if they were trying for pregnancy.”
According to the International Federation of Gynecologists and Obstetricians, variations in cycle length of fewer than 8 days are considered normal, said Christine Metz, PhD, a research biologist and a professor of molecular medicine at the Feinstein Institutes for Medical Research in Manhasset, N.Y. “Thus, the extra 17 hours added to the menstrual cycle length in the vaccination group in this study is well within the ‘normal’ range.”
In a group of about 1,600 menstruating women being studied at Dr. Metz’s center, some have anecdotally reported transient cycle changes post vaccination for COVID-19, including delays in menstruation onset and changes in bleeding patterns.
Exactly how vaccination might alter menstrual cycle length is not known and has not been studied with vaccination against other infections such as influenza and meningococcal disease.
“Many factors are known to affect menstrual cycle length including changes in diet, sleep, and exercise, as well as sickness, travel, and stress,” Dr. Metz said. The COVID-19 vaccines have affected people in different ways, with side effects ranging from injection-site pain to nausea, aches, fever, and fatigue. “Vaccination side effects, particularly if severe, could lead to changes in diet, exercise, and sleep, and feelings of sickness and/or stress.”
These stressors can alter hormone production and stability, as well as the body’s response to hormones such as estrogen, progesterone, follicle-stimulating hormone, luteinizing hormone, and other hormones associated with female reproduction. “Because these hormones regulate the menstrual cycle, variations in these hormones can either shorten or lengthen the cycle,” Dr. Metz explained.
More research needs to be done at the global level, according to the authors. “Questions remain about other possible changes in menstrual cycles, such as menstrual symptoms, unscheduled bleeding, and changes in the quality and quantity of menstrual bleeding.”
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health’s Office of Research on Women’s Health. Dr. Edelman reported support from the American College of Obstetrics and Gynecology, the World Health Organization, Gynuity, and the Karolinska Institute as well as royalties from UpToDate. Other study authors reported similar relationships with not-for-profit and private-sector companies. Three coauthors are employees of Natural Cycles, a fertility tracking device that was used in the study. Dr. Metz disclosed no conflicts of interest with regard to her comments.
Women may rest a bit easier thanks to results from a study showing that vaccination against the SARS-CoV-2 virus has almost no impact on a woman’s menstrual cycle. The issue is significant, as regular menstruation is a sign of health and fertility, and fears of disturbances might increase vaccination hesitancy as COVID-19 cases continue to surge.
Alison Edelman, MD, MPH, a professor of obstetrics and gynecology at Oregon Health & Science University, Portland, led a group studying prospective data on almost 24,000 menstrual cycles reported by almost 4,000 U.S. women.
The investigators found that COVID-19 vaccination was associated with a less than 1-day change in cycle length for the menstrual cycles after the first and second inoculations, compared with prevaccine cycles. Vaccination had no effect on the actual number of days menstrual bleeding lasted.
The study looked at the menstrual patterns of women aged 18-45 years with normal cycle lengths of 24-38 days for the three consecutive cycles before the first vaccine dose and for three consecutive postvaccine cycles. The final sample included 2,403 vaccinated and 1,556 unvaccinated individuals.
In vaccinated women, the study initially found a slight average increase in cycle length after dose one of 71% of a day and 91% of a day after dose two. Following adjustments, those increases dropped to 64% of a day after the first dose and 79% of a day after the second dose.
In unvaccinated women, the study looked at six cycles over a similar time period and found no significant changes from baseline.
“Coronavirus disease 2019 vaccination is associated with a small change in cycle length but not menses length,” Dr. Edelman’s group concluded in Obstetrics and Gynecology.
In the rare instance that a woman received two vaccine doses within the same menstrual cycle, the change in length could increase to 2 days. These variations appear to resolve quickly, possibly as soon as the next cycle after vaccination and do not indicate any cause for long-term physical or reproductive health concern, according to the authors.
Reports by women on social media, however, have suggested that postvaccine menstrual disruptions are more common with, for example, heavier and breakthrough bleeding. But it appears such changes are temporary and resolve quickly.
“These findings are reassuring and validating,” Dr. Edelman said in an interview. On a population level, the changes indicate no cause for concern for long-term physical or reproductive health and no reason to avoid vaccination. “On a personal level, people want this information so they know what to expect when they get vaccinated, and not worry about a pregnancy scare or be disappointed if they were trying for pregnancy.”
According to the International Federation of Gynecologists and Obstetricians, variations in cycle length of fewer than 8 days are considered normal, said Christine Metz, PhD, a research biologist and a professor of molecular medicine at the Feinstein Institutes for Medical Research in Manhasset, N.Y. “Thus, the extra 17 hours added to the menstrual cycle length in the vaccination group in this study is well within the ‘normal’ range.”
In a group of about 1,600 menstruating women being studied at Dr. Metz’s center, some have anecdotally reported transient cycle changes post vaccination for COVID-19, including delays in menstruation onset and changes in bleeding patterns.
Exactly how vaccination might alter menstrual cycle length is not known and has not been studied with vaccination against other infections such as influenza and meningococcal disease.
“Many factors are known to affect menstrual cycle length including changes in diet, sleep, and exercise, as well as sickness, travel, and stress,” Dr. Metz said. The COVID-19 vaccines have affected people in different ways, with side effects ranging from injection-site pain to nausea, aches, fever, and fatigue. “Vaccination side effects, particularly if severe, could lead to changes in diet, exercise, and sleep, and feelings of sickness and/or stress.”
These stressors can alter hormone production and stability, as well as the body’s response to hormones such as estrogen, progesterone, follicle-stimulating hormone, luteinizing hormone, and other hormones associated with female reproduction. “Because these hormones regulate the menstrual cycle, variations in these hormones can either shorten or lengthen the cycle,” Dr. Metz explained.
More research needs to be done at the global level, according to the authors. “Questions remain about other possible changes in menstrual cycles, such as menstrual symptoms, unscheduled bleeding, and changes in the quality and quantity of menstrual bleeding.”
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health’s Office of Research on Women’s Health. Dr. Edelman reported support from the American College of Obstetrics and Gynecology, the World Health Organization, Gynuity, and the Karolinska Institute as well as royalties from UpToDate. Other study authors reported similar relationships with not-for-profit and private-sector companies. Three coauthors are employees of Natural Cycles, a fertility tracking device that was used in the study. Dr. Metz disclosed no conflicts of interest with regard to her comments.
FROM OBSTETRICS & GYNECOLOGY
Teledermatology During the COVID-19 Pandemic: Lessons Learned and Future Directions
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
Herpes Zoster Following a Nucleoside-Modified Messenger RNA COVID-19 Vaccine
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.
Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5
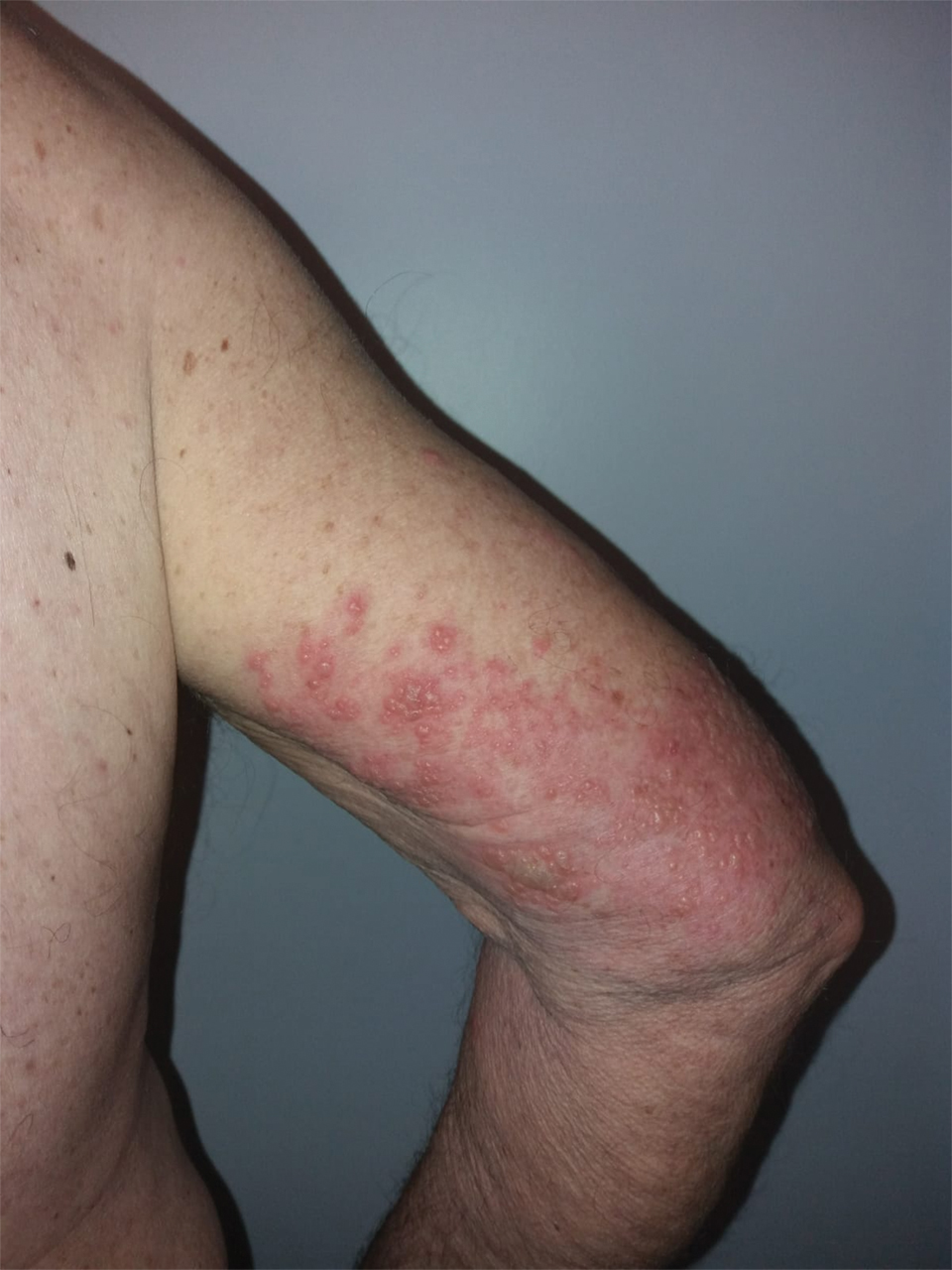
Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9
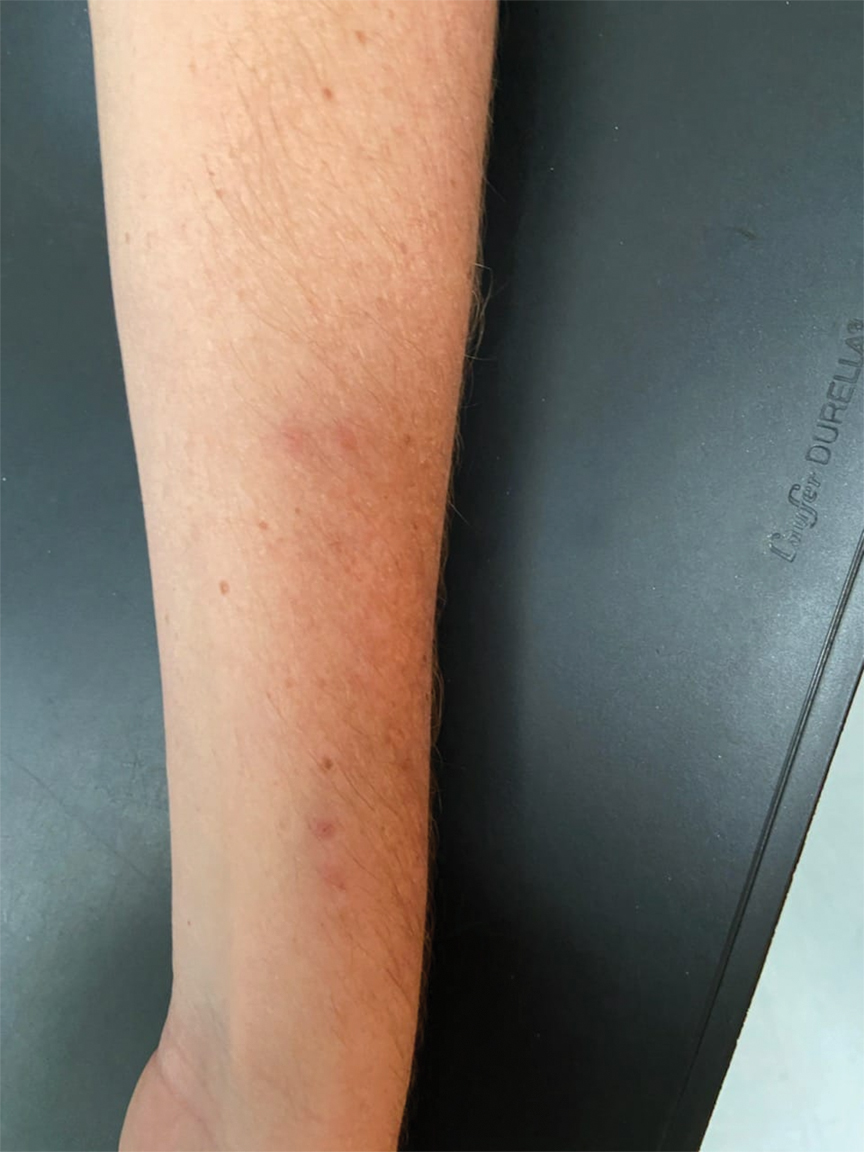
In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.

Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5

Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9

In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.

Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5

Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9

In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
Practice Points
- Herpes zoster (HZ) has been reported following COVID-19 vaccination.
- Postinjection pain is common with COVID-19 vaccination, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay in onset between the injection and the symptoms.
- When indicated, the second vaccine dose should not be avoided in patients who are diagnosed with HZ.
Serious problems rare in ages 5-11 from COVID vaccine
The CDC has released two studies that showed vaccine safety for ages 5-11 and emphasized the importance of vaccinating children against the coronavirus to prevent serious illness and hospitalization.
In one study published in the Morbidity and Mortality Weekly Report, researchers found that serious problems were rare among children who had received the Pfizer vaccine.
In another study, researchers looked at hundreds of pediatric hospitalizations from the summer and found that nearly all of children who developed severe COVID-19 weren’t fully vaccinated.
“This study demonstrates that unvaccinated children hospitalized for COVID-19 could experience severe disease and reinforces the importance of vaccination of all eligible children to provide individual protection and to protect those who are not yet eligible to be vaccinated,” the authors of the second study wrote.
Nearly 9 million doses of the Pfizer vaccine have been given to children aged 5-11 in the United States so far, according to The New York Times. By mid-December, or about 6 weeks after the age group became eligible for vaccination in October, the CDC said it had received very few reports of serious problems.
CDC researchers evaluated reports received from doctors and the public, including survey responses from parents and guardians of about 43,000 children between ages 5 and 11. Many children reported nonserious events such as pain at the injection site, fatigue, or a headache, especially after the second dose.
Among more than 4,100 adverse event reports received in November and December, 100 were for serious events, with the most common being fever or vomiting.
The CDC had received 11 verified reports of myocarditis, or inflammation of the heart muscle, which has been noted as a rare side effect of the vaccine among boys and men between ages 12 and 29. Among those, seven children had already recovered and four were still recovering at the time of the report.
The CDC received reports of two deaths – girls who were aged 5 and 6 – who had chronic medical conditions and were in “fragile health” before their shots. The agency said that no data suggested a “causal association between death and vaccination.”
The CDC also received some reports that children between ages 5 and 11 received the larger vaccine dose meant for older children and adults. Most reports said that the children didn’t experience any problems after an incorrect dose.
In a separate study about pediatric hospitalizations, CDC researchers looked at more than 700 children under age 18 who were hospitalized for COVID-19 in July and August at six children’s hospitals in Arkansas, Florida, Illinois, Louisiana, Texas, and Washington, D.C.
Researchers found that only one of the 272 vaccine-eligible patients between ages 12 and 17 had been fully vaccinated, and 12 were partially vaccinated.
In addition, about two-thirds of the hospitalized children between ages 12 and 17 had an underlying condition, with obesity being the most common. About one-third of children under age 5 had more than one viral infection.
Overall, about 30% of the children had to be treated in intensive care units, and 15% needed invasive medical ventilation, CDC researchers found. Nearly 3% had multisystem inflammatory syndrome in children, or MIS-C, which is a rare but serious inflammatory condition associated with COVID-19.
Among all the children hospitalized with COVID-19, about 1.5% died.
“Few vaccine-eligible patients hospitalized for COVID-19 were vaccinated, highlighting the importance of vaccination for those aged ≥5 years and other prevention strategies to protect children and adolescents from COVID-19, particularly those with underlying medical conditions,” study authors wrote.
A version of this article first appeared on WebMD.com.
The CDC has released two studies that showed vaccine safety for ages 5-11 and emphasized the importance of vaccinating children against the coronavirus to prevent serious illness and hospitalization.
In one study published in the Morbidity and Mortality Weekly Report, researchers found that serious problems were rare among children who had received the Pfizer vaccine.
In another study, researchers looked at hundreds of pediatric hospitalizations from the summer and found that nearly all of children who developed severe COVID-19 weren’t fully vaccinated.
“This study demonstrates that unvaccinated children hospitalized for COVID-19 could experience severe disease and reinforces the importance of vaccination of all eligible children to provide individual protection and to protect those who are not yet eligible to be vaccinated,” the authors of the second study wrote.
Nearly 9 million doses of the Pfizer vaccine have been given to children aged 5-11 in the United States so far, according to The New York Times. By mid-December, or about 6 weeks after the age group became eligible for vaccination in October, the CDC said it had received very few reports of serious problems.
CDC researchers evaluated reports received from doctors and the public, including survey responses from parents and guardians of about 43,000 children between ages 5 and 11. Many children reported nonserious events such as pain at the injection site, fatigue, or a headache, especially after the second dose.
Among more than 4,100 adverse event reports received in November and December, 100 were for serious events, with the most common being fever or vomiting.
The CDC had received 11 verified reports of myocarditis, or inflammation of the heart muscle, which has been noted as a rare side effect of the vaccine among boys and men between ages 12 and 29. Among those, seven children had already recovered and four were still recovering at the time of the report.
The CDC received reports of two deaths – girls who were aged 5 and 6 – who had chronic medical conditions and were in “fragile health” before their shots. The agency said that no data suggested a “causal association between death and vaccination.”
The CDC also received some reports that children between ages 5 and 11 received the larger vaccine dose meant for older children and adults. Most reports said that the children didn’t experience any problems after an incorrect dose.
In a separate study about pediatric hospitalizations, CDC researchers looked at more than 700 children under age 18 who were hospitalized for COVID-19 in July and August at six children’s hospitals in Arkansas, Florida, Illinois, Louisiana, Texas, and Washington, D.C.
Researchers found that only one of the 272 vaccine-eligible patients between ages 12 and 17 had been fully vaccinated, and 12 were partially vaccinated.
In addition, about two-thirds of the hospitalized children between ages 12 and 17 had an underlying condition, with obesity being the most common. About one-third of children under age 5 had more than one viral infection.
Overall, about 30% of the children had to be treated in intensive care units, and 15% needed invasive medical ventilation, CDC researchers found. Nearly 3% had multisystem inflammatory syndrome in children, or MIS-C, which is a rare but serious inflammatory condition associated with COVID-19.
Among all the children hospitalized with COVID-19, about 1.5% died.
“Few vaccine-eligible patients hospitalized for COVID-19 were vaccinated, highlighting the importance of vaccination for those aged ≥5 years and other prevention strategies to protect children and adolescents from COVID-19, particularly those with underlying medical conditions,” study authors wrote.
A version of this article first appeared on WebMD.com.
The CDC has released two studies that showed vaccine safety for ages 5-11 and emphasized the importance of vaccinating children against the coronavirus to prevent serious illness and hospitalization.
In one study published in the Morbidity and Mortality Weekly Report, researchers found that serious problems were rare among children who had received the Pfizer vaccine.
In another study, researchers looked at hundreds of pediatric hospitalizations from the summer and found that nearly all of children who developed severe COVID-19 weren’t fully vaccinated.
“This study demonstrates that unvaccinated children hospitalized for COVID-19 could experience severe disease and reinforces the importance of vaccination of all eligible children to provide individual protection and to protect those who are not yet eligible to be vaccinated,” the authors of the second study wrote.
Nearly 9 million doses of the Pfizer vaccine have been given to children aged 5-11 in the United States so far, according to The New York Times. By mid-December, or about 6 weeks after the age group became eligible for vaccination in October, the CDC said it had received very few reports of serious problems.
CDC researchers evaluated reports received from doctors and the public, including survey responses from parents and guardians of about 43,000 children between ages 5 and 11. Many children reported nonserious events such as pain at the injection site, fatigue, or a headache, especially after the second dose.
Among more than 4,100 adverse event reports received in November and December, 100 were for serious events, with the most common being fever or vomiting.
The CDC had received 11 verified reports of myocarditis, or inflammation of the heart muscle, which has been noted as a rare side effect of the vaccine among boys and men between ages 12 and 29. Among those, seven children had already recovered and four were still recovering at the time of the report.
The CDC received reports of two deaths – girls who were aged 5 and 6 – who had chronic medical conditions and were in “fragile health” before their shots. The agency said that no data suggested a “causal association between death and vaccination.”
The CDC also received some reports that children between ages 5 and 11 received the larger vaccine dose meant for older children and adults. Most reports said that the children didn’t experience any problems after an incorrect dose.
In a separate study about pediatric hospitalizations, CDC researchers looked at more than 700 children under age 18 who were hospitalized for COVID-19 in July and August at six children’s hospitals in Arkansas, Florida, Illinois, Louisiana, Texas, and Washington, D.C.
Researchers found that only one of the 272 vaccine-eligible patients between ages 12 and 17 had been fully vaccinated, and 12 were partially vaccinated.
In addition, about two-thirds of the hospitalized children between ages 12 and 17 had an underlying condition, with obesity being the most common. About one-third of children under age 5 had more than one viral infection.
Overall, about 30% of the children had to be treated in intensive care units, and 15% needed invasive medical ventilation, CDC researchers found. Nearly 3% had multisystem inflammatory syndrome in children, or MIS-C, which is a rare but serious inflammatory condition associated with COVID-19.
Among all the children hospitalized with COVID-19, about 1.5% died.
“Few vaccine-eligible patients hospitalized for COVID-19 were vaccinated, highlighting the importance of vaccination for those aged ≥5 years and other prevention strategies to protect children and adolescents from COVID-19, particularly those with underlying medical conditions,” study authors wrote.
A version of this article first appeared on WebMD.com.
Pandemic poses short- and long-term risks to babies, especially boys
The pandemic has created a hostile environment for pregnant people and their babies.
Stress levels among expectant mothers have soared. Pregnant women with COVID are 5 times as likely as uninfected pregnant people to require intensive care and 22 times as likely to die. Infected moms are four times as likely to have a stillborn child.
Yet some of the pandemic’s greatest threats to infants’ health may not be apparent for years or even decades.
That’s because babies of COVID-infected moms are 60% more likely to be born very prematurely, which increases the danger of infant mortality and long-term disabilities such as cerebral palsy, asthma, and hearing loss, as well as a child’s risk of adult disease, including depression, anxiety, heart disease, and kidney disease.
Studies have linked fever and infection during pregnancy to developmental and psychiatric conditions such as autism, depression, and schizophrenia.
“Some of these conditions do not show up until middle childhood or early adult life, but they have their origins in fetal life,” said Evdokia Anagnostou, MD, a child neurologist at Holland Bloorview Kids Rehabilitation Hospital and a pediatrics professor at the University of Toronto.
For fetuses exposed to COVID, the greatest danger is usually not the coronavirus itself, but the mother’s immune system.
Both severe COVID infections and the strain of the pandemic can expose fetuses to harmful inflammation, which can occur when a mother’s immune system is fighting a virus or when stress hormones send nonstop alarm signals.
Prenatal inflammation “changes the way the brain develops and, depending on the timing of the infection, it can change the way the heart or kidneys develop,” Dr. Anagnostou said.
Although health officials have strongly recommended COVID vaccines for pregnant people, only 35% are fully vaccinated.
At least 150,000 pregnant women have been diagnosed with COVID; more than 25,000 of them have been hospitalized, and 249 have died, according to the Centers for Disease Control and Prevention.
Although most babies will be fine, even a small increase in the percentage of children with special medical or educational needs could have a large effect on the population, given the huge number of COVID infections, Dr. Anagnostou said.
“If someone has a baby who is doing well, that is what they should focus on,” Dr. Anagnostou said. “But from a public health point of view, we need to follow women who experienced severe COVID and their babies to understand the impact.”
Learning from history
Researchers in the United States and other countries are already studying “the COVID generation” to see whether these children have more health issues than those conceived or born before 2020.
Previous crises have shown that the challenges fetuses face in the womb – such as maternal infections, hunger, stress, and hormone-disrupting chemicals – can leave a lasting imprint on their health, as well as that of their children and grandchildren, said Frederick Kaskel, MD, director of pediatric nephrology at the Children’s Hospital at Montefiore, New York.
People whose mothers were pregnant during surges in the 1918 influenza pandemic, for example, had poorer health throughout their lives, compared with Americans born at other times, said John McCarthy, who is a medical student at Albert Einstein College of Medicine, New York, and cowrote a recent review in JAMA Pediatrics with Dr. Kaskel.
Researchers don’t know exactly which moms were infected with pandemic flu, Mr. McCarthy said. But women who were pregnant during major surges – when infection was widespread – had children with higher rates of heart disease or diabetes. These children were also less successful in school, less economically productive, and more likely to live with a disability.
Because organ systems develop during different periods of pregnancy, fetuses exposed during the first trimester may face different risks than those exposed toward the end of pregnancy, Mr. McCarthy said. For example, people born in the fall of 1918 were 50% more likely than others to develop kidney disease; that may reflect an exposure to the pandemic in the third trimester, while the kidneys were still developing.
Nearly 2 years into the COVID pandemic, researchers have begun to publish preliminary observations of infants exposed to COVID infections and stress before birth.
Although Dr. Anagnostou noted that it’s too early to reach definitive conclusions, “there is evidence that babies born to moms with severe COVID infections have changes to their immune system,” she said. “It’s enough to make us worry a little bit.”
Damaging a fetal security system
The good news about the coronavirus is that it seldom crosses the placenta, the organ tasked with protecting a developing fetus from infections and providing it with oxygen. So moms with COVID rarely give the virus to their children before birth.
That’s important, because some viruses that directly infect the fetus – such as Zika – can cause devastating birth defects, said Karin Nielsen-Saines, MD, a specialist in pediatric infectious diseases at University of California, Los Angeles.
But studies also suggest that inflammation from a mother’s COVID infection can injure the placenta, said Jeffery Goldstein, MD, an assistant professor of pathology at Northwestern University, Chicago. In a study published in American Journal of Clinical Pathology , Dr. Goldstein and his coauthors found that placentas from COVID-infected moms had more abnormal blood vessels than placentas from patients without COVID, making it harder for them to deliver sufficient oxygen to the fetus.
Placental damage can also lead to preeclampsia, a serious complication of pregnancy that can cause a mother’s blood pressure to spike.
Preeclampsia occurs when blood vessels in the placenta don’t develop or function properly, forcing the mother’s heart to work harder to get blood to the fetus, which may not receive enough oxygen and nutrients. Preeclampsia also predisposes women to heart attacks and strokes later in life.
Rewiring the immune system
In some cases, COVID also appears to rewire a baby’s immune response, Dr. Nielsen-Saines said.
In an October study in the journal Cell Reports Medicine, Dr. Nielsen-Saines and her coauthors found that infants born to people with severe COVID infections had a different mix of immune cells and proteins than other babies. None of the newborns tested positive for the coronavirus.
The immune changes are concerning, Dr. Nielsen-Saines said, because this pattern of immune cells and proteins has previously been found in infants with respiratory problems and in some cases poor neurodevelopment.
Notably, all the babies in her study appear healthy, said Dr. Nielsen-Saines, who plans to follow them for 3 years to see whether these early signals translate into developmental delays, such as problems talking, walking, or interacting with others.
“How big of a difference does any of this make in the baby?” asked Dr. Anagnostou. “We won’t know for a few years. All we can do is try to be as prepared as possible.”
Increasing the risk for boys
Boys could face higher risks from COVID, even before birth.
Males are generally more vulnerable than females as fetuses and newborns; they’re more likely to be born prematurely and to die as infants. Preterm boys also have a higher risk of disability and death.
But coronavirus infection poses special dangers, said Sabra Klein, PhD, a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore.
That’s because boys are disproportionately affected by conditions linked to maternal infections. Boys are four times as likely as girls to be diagnosed with autism or attention-deficit/hyperactivity disorder, for example, while men are 75% more likely than women to develop schizophrenia.
Scientists don’t fully understand why boys appear more fragile in the womb, although testosterone – which can dampen immune response – may play a role, said Kristina Adams Waldorf, MD, a professor of obstetrics and gynecology at the University of Washington.
Men generally mount weaker immune responses than women and more often develop severe COVID infections. Recent research suggests boys with COVID are more likely than girls to become seriously ill or develop a rare inflammatory condition called multisystem inflammatory syndrome.
New research on COVID could help illuminate this vulnerability.
In a study published in October, researchers found that the sex of a fetus influences the way its placenta responds to COVID, as well as how its mother’s immune system responds.
Pregnant people infected with COVID made fewer antibodies against the coronavirus if they were carrying male fetuses than if they were carrying females. Mothers also transferred fewer antibodies to boys than to girls, said Andrea Edlow, MD, senior author of the study and a maternal-fetal medicine specialist at Massachusetts General Hospital, Boston.
When examining the placentas of male fetuses after delivery, researchers found changes that could leave boys less protected against damaging inflammation.
The sex of a fetus can influence its mother’s response to other illnesses, as well.
For example, research shows that pregnant women with asthma have worse symptoms if they’re carrying a female. Women carrying males are slightly more likely to develop gestational diabetes.
Dr. Edlow said her findings raise questions about the “cross talk” between mother and baby. “The mom’s immune system is sensing there is a male fetus,” Dr. Edlow said. “And the fetus is actively communicating with the mom’s immune system.”
Boosting toxic stress
Rates of depression and stress among pregnant women have increased dramatically during the pandemic.
That’s concerning because chronic stress can lead to inflammation, affecting the babies of both infected and uninfected women, Dr. Anagnostou said.
Studies consistently show that infants born to mothers who experience significant stress during pregnancy have higher rates of short- and long-term health damage – including heart defects and obesity – than babies born to women with less stress.
“We know that inflammation directly influences the way a baby’s brain develops,” said Elinor Sullivan, PhD, an associate professor in psychiatry at Oregon Health & Science University, Portland.
Lockdowns, travel restrictions and physical distancing left many pregnant women without the support of family and friends. The stress of losing a loved one, a job, or a home further heightens the risks to moms and babies, said Dr. Sullivan, who is following children born during the pandemic for 5 years.
In research that has not yet been published, Dr. Sullivan found that babies of women who were pregnant during the pandemic showed more sadness and negative emotions in the first year of life, compared with infants of women who were pregnant before the pandemic.
The findings show the importance of helping and protecting pregnant people before and after delivery, said Dr. Sullivan, who conducted a separate study that found women who received more social support were less depressed.
Italian researchers are also studying the effect of maternal stress on infants’ behavior, as well as the way their genes are regulated.
Although stress-related inflammation doesn’t alter the structure of a baby’s genes, it can influence whether they’re turned on and off, said Livio Provenzi, PhD, a psychologist at the C. Mondino National Institute of Neurology Foundation in Pavia, Italy.
In Dr. Provenzi’s study of 163 mother-baby pairs he found differences in how genes that regulate the stress response were activated. Genes that help people respond to stress were more likely to be turned off in babies whose moms reported the most stress during pregnancy. The same moms also reported that their babies cried more and were fussier when they were 3 months old.
Researchers usually prefer to make in-person observations of babies as they interact with their mothers, Dr. Provenzi said. But because of the pandemic, Dr. Provenzi asked mothers to fill out questionnaires about infant behavior. He plans to observe mothers and babies in person when the children are 12 months old.
While vaccinating pregnant people is the best way to protect them and their fetuses from the virus, Dr. Anagnostou said, society needs to do more to preserve expectant mothers’ mental health.
“We can’t escape the fact that we’ve lived through 2 years of a pandemic,” Dr. Anagnostou said. “But we can think about opportunities for reducing the risk.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The pandemic has created a hostile environment for pregnant people and their babies.
Stress levels among expectant mothers have soared. Pregnant women with COVID are 5 times as likely as uninfected pregnant people to require intensive care and 22 times as likely to die. Infected moms are four times as likely to have a stillborn child.
Yet some of the pandemic’s greatest threats to infants’ health may not be apparent for years or even decades.
That’s because babies of COVID-infected moms are 60% more likely to be born very prematurely, which increases the danger of infant mortality and long-term disabilities such as cerebral palsy, asthma, and hearing loss, as well as a child’s risk of adult disease, including depression, anxiety, heart disease, and kidney disease.
Studies have linked fever and infection during pregnancy to developmental and psychiatric conditions such as autism, depression, and schizophrenia.
“Some of these conditions do not show up until middle childhood or early adult life, but they have their origins in fetal life,” said Evdokia Anagnostou, MD, a child neurologist at Holland Bloorview Kids Rehabilitation Hospital and a pediatrics professor at the University of Toronto.
For fetuses exposed to COVID, the greatest danger is usually not the coronavirus itself, but the mother’s immune system.
Both severe COVID infections and the strain of the pandemic can expose fetuses to harmful inflammation, which can occur when a mother’s immune system is fighting a virus or when stress hormones send nonstop alarm signals.
Prenatal inflammation “changes the way the brain develops and, depending on the timing of the infection, it can change the way the heart or kidneys develop,” Dr. Anagnostou said.
Although health officials have strongly recommended COVID vaccines for pregnant people, only 35% are fully vaccinated.
At least 150,000 pregnant women have been diagnosed with COVID; more than 25,000 of them have been hospitalized, and 249 have died, according to the Centers for Disease Control and Prevention.
Although most babies will be fine, even a small increase in the percentage of children with special medical or educational needs could have a large effect on the population, given the huge number of COVID infections, Dr. Anagnostou said.
“If someone has a baby who is doing well, that is what they should focus on,” Dr. Anagnostou said. “But from a public health point of view, we need to follow women who experienced severe COVID and their babies to understand the impact.”
Learning from history
Researchers in the United States and other countries are already studying “the COVID generation” to see whether these children have more health issues than those conceived or born before 2020.
Previous crises have shown that the challenges fetuses face in the womb – such as maternal infections, hunger, stress, and hormone-disrupting chemicals – can leave a lasting imprint on their health, as well as that of their children and grandchildren, said Frederick Kaskel, MD, director of pediatric nephrology at the Children’s Hospital at Montefiore, New York.
People whose mothers were pregnant during surges in the 1918 influenza pandemic, for example, had poorer health throughout their lives, compared with Americans born at other times, said John McCarthy, who is a medical student at Albert Einstein College of Medicine, New York, and cowrote a recent review in JAMA Pediatrics with Dr. Kaskel.
Researchers don’t know exactly which moms were infected with pandemic flu, Mr. McCarthy said. But women who were pregnant during major surges – when infection was widespread – had children with higher rates of heart disease or diabetes. These children were also less successful in school, less economically productive, and more likely to live with a disability.
Because organ systems develop during different periods of pregnancy, fetuses exposed during the first trimester may face different risks than those exposed toward the end of pregnancy, Mr. McCarthy said. For example, people born in the fall of 1918 were 50% more likely than others to develop kidney disease; that may reflect an exposure to the pandemic in the third trimester, while the kidneys were still developing.
Nearly 2 years into the COVID pandemic, researchers have begun to publish preliminary observations of infants exposed to COVID infections and stress before birth.
Although Dr. Anagnostou noted that it’s too early to reach definitive conclusions, “there is evidence that babies born to moms with severe COVID infections have changes to their immune system,” she said. “It’s enough to make us worry a little bit.”
Damaging a fetal security system
The good news about the coronavirus is that it seldom crosses the placenta, the organ tasked with protecting a developing fetus from infections and providing it with oxygen. So moms with COVID rarely give the virus to their children before birth.
That’s important, because some viruses that directly infect the fetus – such as Zika – can cause devastating birth defects, said Karin Nielsen-Saines, MD, a specialist in pediatric infectious diseases at University of California, Los Angeles.
But studies also suggest that inflammation from a mother’s COVID infection can injure the placenta, said Jeffery Goldstein, MD, an assistant professor of pathology at Northwestern University, Chicago. In a study published in American Journal of Clinical Pathology , Dr. Goldstein and his coauthors found that placentas from COVID-infected moms had more abnormal blood vessels than placentas from patients without COVID, making it harder for them to deliver sufficient oxygen to the fetus.
Placental damage can also lead to preeclampsia, a serious complication of pregnancy that can cause a mother’s blood pressure to spike.
Preeclampsia occurs when blood vessels in the placenta don’t develop or function properly, forcing the mother’s heart to work harder to get blood to the fetus, which may not receive enough oxygen and nutrients. Preeclampsia also predisposes women to heart attacks and strokes later in life.
Rewiring the immune system
In some cases, COVID also appears to rewire a baby’s immune response, Dr. Nielsen-Saines said.
In an October study in the journal Cell Reports Medicine, Dr. Nielsen-Saines and her coauthors found that infants born to people with severe COVID infections had a different mix of immune cells and proteins than other babies. None of the newborns tested positive for the coronavirus.
The immune changes are concerning, Dr. Nielsen-Saines said, because this pattern of immune cells and proteins has previously been found in infants with respiratory problems and in some cases poor neurodevelopment.
Notably, all the babies in her study appear healthy, said Dr. Nielsen-Saines, who plans to follow them for 3 years to see whether these early signals translate into developmental delays, such as problems talking, walking, or interacting with others.
“How big of a difference does any of this make in the baby?” asked Dr. Anagnostou. “We won’t know for a few years. All we can do is try to be as prepared as possible.”
Increasing the risk for boys
Boys could face higher risks from COVID, even before birth.
Males are generally more vulnerable than females as fetuses and newborns; they’re more likely to be born prematurely and to die as infants. Preterm boys also have a higher risk of disability and death.
But coronavirus infection poses special dangers, said Sabra Klein, PhD, a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore.
That’s because boys are disproportionately affected by conditions linked to maternal infections. Boys are four times as likely as girls to be diagnosed with autism or attention-deficit/hyperactivity disorder, for example, while men are 75% more likely than women to develop schizophrenia.
Scientists don’t fully understand why boys appear more fragile in the womb, although testosterone – which can dampen immune response – may play a role, said Kristina Adams Waldorf, MD, a professor of obstetrics and gynecology at the University of Washington.
Men generally mount weaker immune responses than women and more often develop severe COVID infections. Recent research suggests boys with COVID are more likely than girls to become seriously ill or develop a rare inflammatory condition called multisystem inflammatory syndrome.
New research on COVID could help illuminate this vulnerability.
In a study published in October, researchers found that the sex of a fetus influences the way its placenta responds to COVID, as well as how its mother’s immune system responds.
Pregnant people infected with COVID made fewer antibodies against the coronavirus if they were carrying male fetuses than if they were carrying females. Mothers also transferred fewer antibodies to boys than to girls, said Andrea Edlow, MD, senior author of the study and a maternal-fetal medicine specialist at Massachusetts General Hospital, Boston.
When examining the placentas of male fetuses after delivery, researchers found changes that could leave boys less protected against damaging inflammation.
The sex of a fetus can influence its mother’s response to other illnesses, as well.
For example, research shows that pregnant women with asthma have worse symptoms if they’re carrying a female. Women carrying males are slightly more likely to develop gestational diabetes.
Dr. Edlow said her findings raise questions about the “cross talk” between mother and baby. “The mom’s immune system is sensing there is a male fetus,” Dr. Edlow said. “And the fetus is actively communicating with the mom’s immune system.”
Boosting toxic stress
Rates of depression and stress among pregnant women have increased dramatically during the pandemic.
That’s concerning because chronic stress can lead to inflammation, affecting the babies of both infected and uninfected women, Dr. Anagnostou said.
Studies consistently show that infants born to mothers who experience significant stress during pregnancy have higher rates of short- and long-term health damage – including heart defects and obesity – than babies born to women with less stress.
“We know that inflammation directly influences the way a baby’s brain develops,” said Elinor Sullivan, PhD, an associate professor in psychiatry at Oregon Health & Science University, Portland.
Lockdowns, travel restrictions and physical distancing left many pregnant women without the support of family and friends. The stress of losing a loved one, a job, or a home further heightens the risks to moms and babies, said Dr. Sullivan, who is following children born during the pandemic for 5 years.
In research that has not yet been published, Dr. Sullivan found that babies of women who were pregnant during the pandemic showed more sadness and negative emotions in the first year of life, compared with infants of women who were pregnant before the pandemic.
The findings show the importance of helping and protecting pregnant people before and after delivery, said Dr. Sullivan, who conducted a separate study that found women who received more social support were less depressed.
Italian researchers are also studying the effect of maternal stress on infants’ behavior, as well as the way their genes are regulated.
Although stress-related inflammation doesn’t alter the structure of a baby’s genes, it can influence whether they’re turned on and off, said Livio Provenzi, PhD, a psychologist at the C. Mondino National Institute of Neurology Foundation in Pavia, Italy.
In Dr. Provenzi’s study of 163 mother-baby pairs he found differences in how genes that regulate the stress response were activated. Genes that help people respond to stress were more likely to be turned off in babies whose moms reported the most stress during pregnancy. The same moms also reported that their babies cried more and were fussier when they were 3 months old.
Researchers usually prefer to make in-person observations of babies as they interact with their mothers, Dr. Provenzi said. But because of the pandemic, Dr. Provenzi asked mothers to fill out questionnaires about infant behavior. He plans to observe mothers and babies in person when the children are 12 months old.
While vaccinating pregnant people is the best way to protect them and their fetuses from the virus, Dr. Anagnostou said, society needs to do more to preserve expectant mothers’ mental health.
“We can’t escape the fact that we’ve lived through 2 years of a pandemic,” Dr. Anagnostou said. “But we can think about opportunities for reducing the risk.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The pandemic has created a hostile environment for pregnant people and their babies.
Stress levels among expectant mothers have soared. Pregnant women with COVID are 5 times as likely as uninfected pregnant people to require intensive care and 22 times as likely to die. Infected moms are four times as likely to have a stillborn child.
Yet some of the pandemic’s greatest threats to infants’ health may not be apparent for years or even decades.
That’s because babies of COVID-infected moms are 60% more likely to be born very prematurely, which increases the danger of infant mortality and long-term disabilities such as cerebral palsy, asthma, and hearing loss, as well as a child’s risk of adult disease, including depression, anxiety, heart disease, and kidney disease.
Studies have linked fever and infection during pregnancy to developmental and psychiatric conditions such as autism, depression, and schizophrenia.
“Some of these conditions do not show up until middle childhood or early adult life, but they have their origins in fetal life,” said Evdokia Anagnostou, MD, a child neurologist at Holland Bloorview Kids Rehabilitation Hospital and a pediatrics professor at the University of Toronto.
For fetuses exposed to COVID, the greatest danger is usually not the coronavirus itself, but the mother’s immune system.
Both severe COVID infections and the strain of the pandemic can expose fetuses to harmful inflammation, which can occur when a mother’s immune system is fighting a virus or when stress hormones send nonstop alarm signals.
Prenatal inflammation “changes the way the brain develops and, depending on the timing of the infection, it can change the way the heart or kidneys develop,” Dr. Anagnostou said.
Although health officials have strongly recommended COVID vaccines for pregnant people, only 35% are fully vaccinated.
At least 150,000 pregnant women have been diagnosed with COVID; more than 25,000 of them have been hospitalized, and 249 have died, according to the Centers for Disease Control and Prevention.
Although most babies will be fine, even a small increase in the percentage of children with special medical or educational needs could have a large effect on the population, given the huge number of COVID infections, Dr. Anagnostou said.
“If someone has a baby who is doing well, that is what they should focus on,” Dr. Anagnostou said. “But from a public health point of view, we need to follow women who experienced severe COVID and their babies to understand the impact.”
Learning from history
Researchers in the United States and other countries are already studying “the COVID generation” to see whether these children have more health issues than those conceived or born before 2020.
Previous crises have shown that the challenges fetuses face in the womb – such as maternal infections, hunger, stress, and hormone-disrupting chemicals – can leave a lasting imprint on their health, as well as that of their children and grandchildren, said Frederick Kaskel, MD, director of pediatric nephrology at the Children’s Hospital at Montefiore, New York.
People whose mothers were pregnant during surges in the 1918 influenza pandemic, for example, had poorer health throughout their lives, compared with Americans born at other times, said John McCarthy, who is a medical student at Albert Einstein College of Medicine, New York, and cowrote a recent review in JAMA Pediatrics with Dr. Kaskel.
Researchers don’t know exactly which moms were infected with pandemic flu, Mr. McCarthy said. But women who were pregnant during major surges – when infection was widespread – had children with higher rates of heart disease or diabetes. These children were also less successful in school, less economically productive, and more likely to live with a disability.
Because organ systems develop during different periods of pregnancy, fetuses exposed during the first trimester may face different risks than those exposed toward the end of pregnancy, Mr. McCarthy said. For example, people born in the fall of 1918 were 50% more likely than others to develop kidney disease; that may reflect an exposure to the pandemic in the third trimester, while the kidneys were still developing.
Nearly 2 years into the COVID pandemic, researchers have begun to publish preliminary observations of infants exposed to COVID infections and stress before birth.
Although Dr. Anagnostou noted that it’s too early to reach definitive conclusions, “there is evidence that babies born to moms with severe COVID infections have changes to their immune system,” she said. “It’s enough to make us worry a little bit.”
Damaging a fetal security system
The good news about the coronavirus is that it seldom crosses the placenta, the organ tasked with protecting a developing fetus from infections and providing it with oxygen. So moms with COVID rarely give the virus to their children before birth.
That’s important, because some viruses that directly infect the fetus – such as Zika – can cause devastating birth defects, said Karin Nielsen-Saines, MD, a specialist in pediatric infectious diseases at University of California, Los Angeles.
But studies also suggest that inflammation from a mother’s COVID infection can injure the placenta, said Jeffery Goldstein, MD, an assistant professor of pathology at Northwestern University, Chicago. In a study published in American Journal of Clinical Pathology , Dr. Goldstein and his coauthors found that placentas from COVID-infected moms had more abnormal blood vessels than placentas from patients without COVID, making it harder for them to deliver sufficient oxygen to the fetus.
Placental damage can also lead to preeclampsia, a serious complication of pregnancy that can cause a mother’s blood pressure to spike.
Preeclampsia occurs when blood vessels in the placenta don’t develop or function properly, forcing the mother’s heart to work harder to get blood to the fetus, which may not receive enough oxygen and nutrients. Preeclampsia also predisposes women to heart attacks and strokes later in life.
Rewiring the immune system
In some cases, COVID also appears to rewire a baby’s immune response, Dr. Nielsen-Saines said.
In an October study in the journal Cell Reports Medicine, Dr. Nielsen-Saines and her coauthors found that infants born to people with severe COVID infections had a different mix of immune cells and proteins than other babies. None of the newborns tested positive for the coronavirus.
The immune changes are concerning, Dr. Nielsen-Saines said, because this pattern of immune cells and proteins has previously been found in infants with respiratory problems and in some cases poor neurodevelopment.
Notably, all the babies in her study appear healthy, said Dr. Nielsen-Saines, who plans to follow them for 3 years to see whether these early signals translate into developmental delays, such as problems talking, walking, or interacting with others.
“How big of a difference does any of this make in the baby?” asked Dr. Anagnostou. “We won’t know for a few years. All we can do is try to be as prepared as possible.”
Increasing the risk for boys
Boys could face higher risks from COVID, even before birth.
Males are generally more vulnerable than females as fetuses and newborns; they’re more likely to be born prematurely and to die as infants. Preterm boys also have a higher risk of disability and death.
But coronavirus infection poses special dangers, said Sabra Klein, PhD, a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore.
That’s because boys are disproportionately affected by conditions linked to maternal infections. Boys are four times as likely as girls to be diagnosed with autism or attention-deficit/hyperactivity disorder, for example, while men are 75% more likely than women to develop schizophrenia.
Scientists don’t fully understand why boys appear more fragile in the womb, although testosterone – which can dampen immune response – may play a role, said Kristina Adams Waldorf, MD, a professor of obstetrics and gynecology at the University of Washington.
Men generally mount weaker immune responses than women and more often develop severe COVID infections. Recent research suggests boys with COVID are more likely than girls to become seriously ill or develop a rare inflammatory condition called multisystem inflammatory syndrome.
New research on COVID could help illuminate this vulnerability.
In a study published in October, researchers found that the sex of a fetus influences the way its placenta responds to COVID, as well as how its mother’s immune system responds.
Pregnant people infected with COVID made fewer antibodies against the coronavirus if they were carrying male fetuses than if they were carrying females. Mothers also transferred fewer antibodies to boys than to girls, said Andrea Edlow, MD, senior author of the study and a maternal-fetal medicine specialist at Massachusetts General Hospital, Boston.
When examining the placentas of male fetuses after delivery, researchers found changes that could leave boys less protected against damaging inflammation.
The sex of a fetus can influence its mother’s response to other illnesses, as well.
For example, research shows that pregnant women with asthma have worse symptoms if they’re carrying a female. Women carrying males are slightly more likely to develop gestational diabetes.
Dr. Edlow said her findings raise questions about the “cross talk” between mother and baby. “The mom’s immune system is sensing there is a male fetus,” Dr. Edlow said. “And the fetus is actively communicating with the mom’s immune system.”
Boosting toxic stress
Rates of depression and stress among pregnant women have increased dramatically during the pandemic.
That’s concerning because chronic stress can lead to inflammation, affecting the babies of both infected and uninfected women, Dr. Anagnostou said.
Studies consistently show that infants born to mothers who experience significant stress during pregnancy have higher rates of short- and long-term health damage – including heart defects and obesity – than babies born to women with less stress.
“We know that inflammation directly influences the way a baby’s brain develops,” said Elinor Sullivan, PhD, an associate professor in psychiatry at Oregon Health & Science University, Portland.
Lockdowns, travel restrictions and physical distancing left many pregnant women without the support of family and friends. The stress of losing a loved one, a job, or a home further heightens the risks to moms and babies, said Dr. Sullivan, who is following children born during the pandemic for 5 years.
In research that has not yet been published, Dr. Sullivan found that babies of women who were pregnant during the pandemic showed more sadness and negative emotions in the first year of life, compared with infants of women who were pregnant before the pandemic.
The findings show the importance of helping and protecting pregnant people before and after delivery, said Dr. Sullivan, who conducted a separate study that found women who received more social support were less depressed.
Italian researchers are also studying the effect of maternal stress on infants’ behavior, as well as the way their genes are regulated.
Although stress-related inflammation doesn’t alter the structure of a baby’s genes, it can influence whether they’re turned on and off, said Livio Provenzi, PhD, a psychologist at the C. Mondino National Institute of Neurology Foundation in Pavia, Italy.
In Dr. Provenzi’s study of 163 mother-baby pairs he found differences in how genes that regulate the stress response were activated. Genes that help people respond to stress were more likely to be turned off in babies whose moms reported the most stress during pregnancy. The same moms also reported that their babies cried more and were fussier when they were 3 months old.
Researchers usually prefer to make in-person observations of babies as they interact with their mothers, Dr. Provenzi said. But because of the pandemic, Dr. Provenzi asked mothers to fill out questionnaires about infant behavior. He plans to observe mothers and babies in person when the children are 12 months old.
While vaccinating pregnant people is the best way to protect them and their fetuses from the virus, Dr. Anagnostou said, society needs to do more to preserve expectant mothers’ mental health.
“We can’t escape the fact that we’ve lived through 2 years of a pandemic,” Dr. Anagnostou said. “But we can think about opportunities for reducing the risk.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
NHL: As a second-line treatment in phase 3 trial, tisa-cel disappoints
according to results of a randomized, phase 3 trial.
The chimeric antigen receptor (CAR) T-cell therapy did not improve event-free survival (EFS) in this phase 3 BELINDA study, potentially because of study design decisions or imbalances in relevant patient characteristics, according to the study investigators.
Despite the negative result, insights from this study will inform the development of future clinical trials of CAR T-cell therapy, said BELINDA investigator Michael R. Bishop, MD, of the David and Etta Jonas Center for Cellular Therapy, University of Chicago.
Findings of BELINDA, presented at the annual meeting of the American Society of Hematology, stand in contrast to two other high-profile CAR T-cell therapy studies also presented at the meeting. Those other studies demonstrated significant improvements in EFS in the second-line treatment of large B-cell lymphomas.
“All of us are excited to see that the other two trials were positive, and we were hoping that ours would be as well, but there are significant differences in the trial design,” Dr. Bishop said in a press conference held at the ASH meeting.
Tisagenlecleucel (tisa-cel), an anti-CD19 CAR T-cell therapy, is already approved by the Food and Drug Administration for the treatment of patients with relapsed or refractory large B-cell lymphomas after at least two other lines of systemic therapy.
The aim of the pivotal phase 3, randomized, multicenter BELINDA study was to evaluate tisa-cel earlier in the course of treatment for patients with more aggressive disease, according to Dr. Bishop.
About two-thirds of non-Hodgkin lymphoma patients will be cured with first-line treatment. However, very poor outcomes are seen among patients with disease that does not respond to the initial treatment or that reoccurs shortly afterward, Dr. Bishop said.
The standard of care approach for those patients is second-line therapy, he noted, usually with combination chemoimmunotherapy, followed by autologous stem cell transplant if the disease responds to chemotherapy.
“Unfortunately, only a minority of those patients will be found to have chemotherapy-sensitive disease and be able to go on to autologous stem cell transplantation,” Dr. Bishop said. “And even in that subgroup of patients, the outcomes are relatively poor.”
Accordingly, the phase 3 BELINDA study enrolled patients with aggressive non-Hodgkin lymphomas that either did not respond to first-line treatment or that reoccurred within 12 months.
The primary endpoint of the study was EFS, defined as the time from randomization to either stable or progressive disease at or after a week 12 assessment or to any-cause death at any time.
While that primary endpoint was not met for tisa-cel versus standard of care therapy, two other randomized, phase 3 studies presented at the ASH meeting did demonstrate that CAR T-cell therapy extended EFS when given as a second-line lymphoma treatment.
In the randomized, phase 3 ZUMA-7 trial, axicabtagene ciloleucel (axi-cel) significantly improved EFS versus standard of care in the treatment of patients with large B-cell lymphoma refractory to or relapsed within 12 months of adequate first-line therapy, according to investigators.
Similarly, the investigators said that treatment with lisocabtagene maraleucel (liso-cel) led to a significant improvement in EFS in TRANSFORM, a randomized, phase 3 clinical trial that enrolled patients with large B-cell lymphoma that was refractory to first-line therapy or else relapsed within 12 months of that treatment.
“It’s very possible that either or both the patient characteristics and the study design is what led to the difference in the top-line study results,” lymphoma specialist Andrew M. Evens, DO, said in an interview.
There were substantial differences between the studies in terms of what was allowed as optional bridging therapy and salvage therapy, according to Dr. Evens, associate director for clinical services and director of the lymphoma program at Rutgers Cancer Institute in New Brunswick, N.J.
“In ZUMA-7, they only allowed steroids as bridging therapy,” said Dr. Evens, who was not an investigator on any of the three second-line CAR T-cell studies.
In the BELINDA study, optional platinum-based chemotherapy bridging treatment allowed in one arm of the study could have potentially delayed tisa-cel infusion until after the week 6 assessment, study investigators reported in their ASH meeting abstract.
Differences in lymphodepleting therapy prior to CAR T-cell therapy could have also played a role. According to Dr. Bishop, the total doses of cyclophosphamide and fludarabine in BELINDA were 900 mg/m2 and 75 mg/m2, respectively, while in the other two trials, doses were 1,500 mg/m2 and 90 mg/m2, respectively.
Lymphodepleting chemotherapy is “extremely important” in the success of CAR T-cell therapeutic approaches, he noted at the press conference.
Dr. Bishop reported receiving consultancy fees from Arcellx, Autolus Therapeutics, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis. He also reported research funding from Bristol-Myers Squibb and Kite/Gilead.
according to results of a randomized, phase 3 trial.
The chimeric antigen receptor (CAR) T-cell therapy did not improve event-free survival (EFS) in this phase 3 BELINDA study, potentially because of study design decisions or imbalances in relevant patient characteristics, according to the study investigators.
Despite the negative result, insights from this study will inform the development of future clinical trials of CAR T-cell therapy, said BELINDA investigator Michael R. Bishop, MD, of the David and Etta Jonas Center for Cellular Therapy, University of Chicago.
Findings of BELINDA, presented at the annual meeting of the American Society of Hematology, stand in contrast to two other high-profile CAR T-cell therapy studies also presented at the meeting. Those other studies demonstrated significant improvements in EFS in the second-line treatment of large B-cell lymphomas.
“All of us are excited to see that the other two trials were positive, and we were hoping that ours would be as well, but there are significant differences in the trial design,” Dr. Bishop said in a press conference held at the ASH meeting.
Tisagenlecleucel (tisa-cel), an anti-CD19 CAR T-cell therapy, is already approved by the Food and Drug Administration for the treatment of patients with relapsed or refractory large B-cell lymphomas after at least two other lines of systemic therapy.
The aim of the pivotal phase 3, randomized, multicenter BELINDA study was to evaluate tisa-cel earlier in the course of treatment for patients with more aggressive disease, according to Dr. Bishop.
About two-thirds of non-Hodgkin lymphoma patients will be cured with first-line treatment. However, very poor outcomes are seen among patients with disease that does not respond to the initial treatment or that reoccurs shortly afterward, Dr. Bishop said.
The standard of care approach for those patients is second-line therapy, he noted, usually with combination chemoimmunotherapy, followed by autologous stem cell transplant if the disease responds to chemotherapy.
“Unfortunately, only a minority of those patients will be found to have chemotherapy-sensitive disease and be able to go on to autologous stem cell transplantation,” Dr. Bishop said. “And even in that subgroup of patients, the outcomes are relatively poor.”
Accordingly, the phase 3 BELINDA study enrolled patients with aggressive non-Hodgkin lymphomas that either did not respond to first-line treatment or that reoccurred within 12 months.
The primary endpoint of the study was EFS, defined as the time from randomization to either stable or progressive disease at or after a week 12 assessment or to any-cause death at any time.
While that primary endpoint was not met for tisa-cel versus standard of care therapy, two other randomized, phase 3 studies presented at the ASH meeting did demonstrate that CAR T-cell therapy extended EFS when given as a second-line lymphoma treatment.
In the randomized, phase 3 ZUMA-7 trial, axicabtagene ciloleucel (axi-cel) significantly improved EFS versus standard of care in the treatment of patients with large B-cell lymphoma refractory to or relapsed within 12 months of adequate first-line therapy, according to investigators.
Similarly, the investigators said that treatment with lisocabtagene maraleucel (liso-cel) led to a significant improvement in EFS in TRANSFORM, a randomized, phase 3 clinical trial that enrolled patients with large B-cell lymphoma that was refractory to first-line therapy or else relapsed within 12 months of that treatment.
“It’s very possible that either or both the patient characteristics and the study design is what led to the difference in the top-line study results,” lymphoma specialist Andrew M. Evens, DO, said in an interview.
There were substantial differences between the studies in terms of what was allowed as optional bridging therapy and salvage therapy, according to Dr. Evens, associate director for clinical services and director of the lymphoma program at Rutgers Cancer Institute in New Brunswick, N.J.
“In ZUMA-7, they only allowed steroids as bridging therapy,” said Dr. Evens, who was not an investigator on any of the three second-line CAR T-cell studies.
In the BELINDA study, optional platinum-based chemotherapy bridging treatment allowed in one arm of the study could have potentially delayed tisa-cel infusion until after the week 6 assessment, study investigators reported in their ASH meeting abstract.
Differences in lymphodepleting therapy prior to CAR T-cell therapy could have also played a role. According to Dr. Bishop, the total doses of cyclophosphamide and fludarabine in BELINDA were 900 mg/m2 and 75 mg/m2, respectively, while in the other two trials, doses were 1,500 mg/m2 and 90 mg/m2, respectively.
Lymphodepleting chemotherapy is “extremely important” in the success of CAR T-cell therapeutic approaches, he noted at the press conference.
Dr. Bishop reported receiving consultancy fees from Arcellx, Autolus Therapeutics, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis. He also reported research funding from Bristol-Myers Squibb and Kite/Gilead.
according to results of a randomized, phase 3 trial.
The chimeric antigen receptor (CAR) T-cell therapy did not improve event-free survival (EFS) in this phase 3 BELINDA study, potentially because of study design decisions or imbalances in relevant patient characteristics, according to the study investigators.
Despite the negative result, insights from this study will inform the development of future clinical trials of CAR T-cell therapy, said BELINDA investigator Michael R. Bishop, MD, of the David and Etta Jonas Center for Cellular Therapy, University of Chicago.
Findings of BELINDA, presented at the annual meeting of the American Society of Hematology, stand in contrast to two other high-profile CAR T-cell therapy studies also presented at the meeting. Those other studies demonstrated significant improvements in EFS in the second-line treatment of large B-cell lymphomas.
“All of us are excited to see that the other two trials were positive, and we were hoping that ours would be as well, but there are significant differences in the trial design,” Dr. Bishop said in a press conference held at the ASH meeting.
Tisagenlecleucel (tisa-cel), an anti-CD19 CAR T-cell therapy, is already approved by the Food and Drug Administration for the treatment of patients with relapsed or refractory large B-cell lymphomas after at least two other lines of systemic therapy.
The aim of the pivotal phase 3, randomized, multicenter BELINDA study was to evaluate tisa-cel earlier in the course of treatment for patients with more aggressive disease, according to Dr. Bishop.
About two-thirds of non-Hodgkin lymphoma patients will be cured with first-line treatment. However, very poor outcomes are seen among patients with disease that does not respond to the initial treatment or that reoccurs shortly afterward, Dr. Bishop said.
The standard of care approach for those patients is second-line therapy, he noted, usually with combination chemoimmunotherapy, followed by autologous stem cell transplant if the disease responds to chemotherapy.
“Unfortunately, only a minority of those patients will be found to have chemotherapy-sensitive disease and be able to go on to autologous stem cell transplantation,” Dr. Bishop said. “And even in that subgroup of patients, the outcomes are relatively poor.”
Accordingly, the phase 3 BELINDA study enrolled patients with aggressive non-Hodgkin lymphomas that either did not respond to first-line treatment or that reoccurred within 12 months.
The primary endpoint of the study was EFS, defined as the time from randomization to either stable or progressive disease at or after a week 12 assessment or to any-cause death at any time.
While that primary endpoint was not met for tisa-cel versus standard of care therapy, two other randomized, phase 3 studies presented at the ASH meeting did demonstrate that CAR T-cell therapy extended EFS when given as a second-line lymphoma treatment.
In the randomized, phase 3 ZUMA-7 trial, axicabtagene ciloleucel (axi-cel) significantly improved EFS versus standard of care in the treatment of patients with large B-cell lymphoma refractory to or relapsed within 12 months of adequate first-line therapy, according to investigators.
Similarly, the investigators said that treatment with lisocabtagene maraleucel (liso-cel) led to a significant improvement in EFS in TRANSFORM, a randomized, phase 3 clinical trial that enrolled patients with large B-cell lymphoma that was refractory to first-line therapy or else relapsed within 12 months of that treatment.
“It’s very possible that either or both the patient characteristics and the study design is what led to the difference in the top-line study results,” lymphoma specialist Andrew M. Evens, DO, said in an interview.
There were substantial differences between the studies in terms of what was allowed as optional bridging therapy and salvage therapy, according to Dr. Evens, associate director for clinical services and director of the lymphoma program at Rutgers Cancer Institute in New Brunswick, N.J.
“In ZUMA-7, they only allowed steroids as bridging therapy,” said Dr. Evens, who was not an investigator on any of the three second-line CAR T-cell studies.
In the BELINDA study, optional platinum-based chemotherapy bridging treatment allowed in one arm of the study could have potentially delayed tisa-cel infusion until after the week 6 assessment, study investigators reported in their ASH meeting abstract.
Differences in lymphodepleting therapy prior to CAR T-cell therapy could have also played a role. According to Dr. Bishop, the total doses of cyclophosphamide and fludarabine in BELINDA were 900 mg/m2 and 75 mg/m2, respectively, while in the other two trials, doses were 1,500 mg/m2 and 90 mg/m2, respectively.
Lymphodepleting chemotherapy is “extremely important” in the success of CAR T-cell therapeutic approaches, he noted at the press conference.
Dr. Bishop reported receiving consultancy fees from Arcellx, Autolus Therapeutics, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis. He also reported research funding from Bristol-Myers Squibb and Kite/Gilead.
FROM ASH 2021