User login
Early palliative care consultation in the medical ICU
Background: Mortality rates in critically ill patients remain in excess of 20% in many institutions. In the last 2 decades, palliative care has become a core component of ICU care. Current literature recommends a palliative care consult in the ICU setting; however, implementing this recommendation in a meaningful way has been challenging. The purpose of this study is to evaluate whether consulting palliative care in ICU earlier improves patient outcomes.
Study design: Single-center cluster randomized crossover trial.
Setting: Two medical ICUs at Barnes Jewish Hospital, St. Louis.
Synopsis: 199 patients were enrolled using palliative care criteria to identify patients at high risk for morbidity and mortality. In the intervention arm patients received a palliative care consultation from an inter-professional team led by board-certified palliative care providers within 48 hours of ICU admission.
The primary outcome of this study was a change in code status to Do Not Resuscitate/Do Not Intubate (DNR/DNI), which was significantly higher in the intervention group (50.5% vs. 23.4%; P less than .0001). The intervention group also had more hospice discharges, fewer ventilated days, a lower rate of tracheostomy, and fewer hospital readmissions. However, mortality and ICU/hospital length of stay were not significantly different between the two arms. Limitations of this study include using a single academic center and the fact that establishing a DNR/DNI may not measure quality of life or patient/family satisfaction. Further studies are needed to focus on clinical outcomes as well as patient and family satisfaction.
Bottom line: Early goal-directed palliative care consults with experienced clinicians board certified in palliative care influences goals of care, code status, and discharge plans for the critically ill and can improve medical resource utilization.
Citation: Ma J et al. Early palliative care consultation in the medical ICU: A cluster randomized crossover trial. Crit Care Med. 2019 Dec;47: 1707-15.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Mortality rates in critically ill patients remain in excess of 20% in many institutions. In the last 2 decades, palliative care has become a core component of ICU care. Current literature recommends a palliative care consult in the ICU setting; however, implementing this recommendation in a meaningful way has been challenging. The purpose of this study is to evaluate whether consulting palliative care in ICU earlier improves patient outcomes.
Study design: Single-center cluster randomized crossover trial.
Setting: Two medical ICUs at Barnes Jewish Hospital, St. Louis.
Synopsis: 199 patients were enrolled using palliative care criteria to identify patients at high risk for morbidity and mortality. In the intervention arm patients received a palliative care consultation from an inter-professional team led by board-certified palliative care providers within 48 hours of ICU admission.
The primary outcome of this study was a change in code status to Do Not Resuscitate/Do Not Intubate (DNR/DNI), which was significantly higher in the intervention group (50.5% vs. 23.4%; P less than .0001). The intervention group also had more hospice discharges, fewer ventilated days, a lower rate of tracheostomy, and fewer hospital readmissions. However, mortality and ICU/hospital length of stay were not significantly different between the two arms. Limitations of this study include using a single academic center and the fact that establishing a DNR/DNI may not measure quality of life or patient/family satisfaction. Further studies are needed to focus on clinical outcomes as well as patient and family satisfaction.
Bottom line: Early goal-directed palliative care consults with experienced clinicians board certified in palliative care influences goals of care, code status, and discharge plans for the critically ill and can improve medical resource utilization.
Citation: Ma J et al. Early palliative care consultation in the medical ICU: A cluster randomized crossover trial. Crit Care Med. 2019 Dec;47: 1707-15.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Mortality rates in critically ill patients remain in excess of 20% in many institutions. In the last 2 decades, palliative care has become a core component of ICU care. Current literature recommends a palliative care consult in the ICU setting; however, implementing this recommendation in a meaningful way has been challenging. The purpose of this study is to evaluate whether consulting palliative care in ICU earlier improves patient outcomes.
Study design: Single-center cluster randomized crossover trial.
Setting: Two medical ICUs at Barnes Jewish Hospital, St. Louis.
Synopsis: 199 patients were enrolled using palliative care criteria to identify patients at high risk for morbidity and mortality. In the intervention arm patients received a palliative care consultation from an inter-professional team led by board-certified palliative care providers within 48 hours of ICU admission.
The primary outcome of this study was a change in code status to Do Not Resuscitate/Do Not Intubate (DNR/DNI), which was significantly higher in the intervention group (50.5% vs. 23.4%; P less than .0001). The intervention group also had more hospice discharges, fewer ventilated days, a lower rate of tracheostomy, and fewer hospital readmissions. However, mortality and ICU/hospital length of stay were not significantly different between the two arms. Limitations of this study include using a single academic center and the fact that establishing a DNR/DNI may not measure quality of life or patient/family satisfaction. Further studies are needed to focus on clinical outcomes as well as patient and family satisfaction.
Bottom line: Early goal-directed palliative care consults with experienced clinicians board certified in palliative care influences goals of care, code status, and discharge plans for the critically ill and can improve medical resource utilization.
Citation: Ma J et al. Early palliative care consultation in the medical ICU: A cluster randomized crossover trial. Crit Care Med. 2019 Dec;47: 1707-15.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Stroke is ‘not a common complication’ in COVID-19
One study showed a stroke rate of 2.2% among patients with COVID-19 admitted to intensive care in 52 different countries. Another found a stroke rate of 1.48% in patients hospitalized with COVID-19 from 70 different countries. These researchers also found a reduction in stroke presentations and stroke care during the pandemic.
Both studies will be presented at the American Academy of Neurology’s 2021 annual meeting.
“Stroke has been a known serious complication of COVID-19, with some studies reporting a higher-than-expected occurrence, especially in young people,” said coauthor of the intensive care study, Jonathon Fanning, MBBS, PhD, University of Queensland, Brisbane, Australia.
“However, among the sickest of COVID patients – those admitted to an ICU – our research found that stroke was not a common complication and that ischemic stroke did not increase the risk of death,” he added.
Hemorrhagic stroke more common?
In this study, researchers analyzed a database of 2,699 patients who were admitted to the intensive care unit with COVID-19 in 52 countries and found that 59 of these patients (2.2%) subsequently sustained a stroke.
Most of the strokes identified in this cohort were hemorrhagic (46%), with 32% being ischemic and 22% unspecified. Hemorrhagic stroke was associated with a fivefold increased risk for death compared with patients who did not have a stroke. Of those with a hemorrhagic stroke, 72% died, but only 15% died of the stroke. Rather, multiorgan failure was the leading cause of death.
There was no association between ischemic stroke and mortality.
“There is scarce research on new-onset stroke complicating ICU admissions, and many of the limitations of assessing stroke in ICU populations confound the true values and result in variability in reported incidence anywhere from a 1%-4% incidence,” Dr. Fanning said.
He noted that a large Korean study had shown a 1.2% rate of stroke in patients without COVID admitted to non-neurologic ICUs. “In light of this, I think this 2% is higher than we would expect in a general ICU population, but in the context of earlier reports of COVID-19–associated risk for stroke, this figure is actually somewhat reassuring,” Dr. Fanning said.
Asked how this study compared with the large American Heart Association study recently reported that showed an overall rate of ischemic stroke of 0.75%, Dr. Fanning said the two studies reported on different populations, which makes them difficult to compare.
“Our study specifically reports on new-onset stroke complicating ICU admission,” he noted. “The AHA study is a large study of all patients admitted to hospital, but both studies identified less than previous estimates of COVID-related stroke.”
Largest sample to date
The other study, which includes 119,967 COVID-19 hospitalizations and represents the largest sample reporting the concomitant diagnoses of stroke and SARS-CoV-2 infection to date, was presented at the AAN meeting by Thanh N. Nguyen, MD, a professor at Boston University.
This study has also been published online in Neurology, with first author Raul G. Nogueira, MD, Emory University, Atlanta.
In this international observational, retrospective study across 6 continents, 70 countries, and 457 stroke centers, there was a 1.48% stroke rate across 119,967 COVID-19 hospitalizations. SARS-CoV-2 infection was noted in 3.3% (1,722) of all stroke admissions, which numbered 52,026.
The researchers identified stroke diagnoses by the International Classification of Diseases, 10th revision, codes and/or classifications in stroke center databases, and rates of stroke hospitalizations and numbers of patients receiving thrombolysis were compared between the first 4 months of the pandemic (March to June 2020) compared with two control 4-month periods.
Global decline in stroke care during pandemic
Results showed a global decline in the number of stroke patients admitted to the hospital as well as acute stroke treatments, such as thrombolysis, during the first wave of the COVID-19 pandemic. The researchers found that there were 91,373 stroke admissions in the 4 months immediately before the pandemic, compared with 80,894 admissions during the first 4 pandemic months, representing an 11.5% decline.
They also report that 13,334 stroke patients received intravenous thrombolysis in the 4 months preceding the pandemic, compared with 11,570 during the first 4 pandemic months, representing a 13.2% drop.
Interhospital transfers after thrombolysis for a higher level of stroke care decreased from 1,337 before the pandemic to 1,178 during the pandemic, a reduction of 11.9%.
There were greater declines in primary compared with comprehensive stroke centers for stroke hospitalizations (change, –17.3% vs. –10.3%) and for the number of patients receiving thrombolysis (change, –15.5% vs. –12.6%).
The volume of stroke hospitalizations increased by 9.5% in the two later pandemic months (May, June) versus the two earlier months (March, April), with greater recovery in hospitals with lower COVID-19 hospitalization volume, high-volume stroke centers, and comprehensive stroke centers.
Dr. Nguyen suggested that reasons for the reductions in these stroke numbers at the beginning of the pandemic could include a reduction in stroke risk due to a reduction of exposure to other viral infections or patients not presenting to the hospital for fear of contracting the coronavirus.
The higher recovery of stroke volume in high-volume stroke centers and comprehensive stroke centers may represent patients with higher needs – those having more severe strokes – seeking care more frequently than those with milder symptoms, she noted.
“Preserving access to stroke care and emergency stroke care amidst a pandemic is as important as educating patients on the importance of presenting to the hospital in the event of stroke-like symptoms,” Dr. Nguyen concluded.
“We continue to advocate that if a patient has stroke-like symptoms, such as loss of speech, strength, vision, or balance, it is important for the patient to seek medical care as an emergency, as there are treatments that can improve a patient’s ability to recover from disabling stroke in earlier rather than later time windows,” she added.
In the publication, the authors wrote, “Our results concur with other recent reports on the collateral effects of the COVID-19 pandemic on stroke systems of care,” but added that “this is among the first descriptions of the change at a global level, including primary and comprehensive stroke centers.”
They said that hospital access related to high COVID-19 burden was unlikely a factor because the decline was seen in centers with a few or no patients with COVID-19. They suggested that patient fear of contracting coronavirus may have played a role, along with a decrease in presentation of transient ischemic attacks, mild strokes, or moderate strokes, and physical distancing measures may have prevented the timely witnessing of a stroke.
A version of this article first appeared on Medscape.com.
One study showed a stroke rate of 2.2% among patients with COVID-19 admitted to intensive care in 52 different countries. Another found a stroke rate of 1.48% in patients hospitalized with COVID-19 from 70 different countries. These researchers also found a reduction in stroke presentations and stroke care during the pandemic.
Both studies will be presented at the American Academy of Neurology’s 2021 annual meeting.
“Stroke has been a known serious complication of COVID-19, with some studies reporting a higher-than-expected occurrence, especially in young people,” said coauthor of the intensive care study, Jonathon Fanning, MBBS, PhD, University of Queensland, Brisbane, Australia.
“However, among the sickest of COVID patients – those admitted to an ICU – our research found that stroke was not a common complication and that ischemic stroke did not increase the risk of death,” he added.
Hemorrhagic stroke more common?
In this study, researchers analyzed a database of 2,699 patients who were admitted to the intensive care unit with COVID-19 in 52 countries and found that 59 of these patients (2.2%) subsequently sustained a stroke.
Most of the strokes identified in this cohort were hemorrhagic (46%), with 32% being ischemic and 22% unspecified. Hemorrhagic stroke was associated with a fivefold increased risk for death compared with patients who did not have a stroke. Of those with a hemorrhagic stroke, 72% died, but only 15% died of the stroke. Rather, multiorgan failure was the leading cause of death.
There was no association between ischemic stroke and mortality.
“There is scarce research on new-onset stroke complicating ICU admissions, and many of the limitations of assessing stroke in ICU populations confound the true values and result in variability in reported incidence anywhere from a 1%-4% incidence,” Dr. Fanning said.
He noted that a large Korean study had shown a 1.2% rate of stroke in patients without COVID admitted to non-neurologic ICUs. “In light of this, I think this 2% is higher than we would expect in a general ICU population, but in the context of earlier reports of COVID-19–associated risk for stroke, this figure is actually somewhat reassuring,” Dr. Fanning said.
Asked how this study compared with the large American Heart Association study recently reported that showed an overall rate of ischemic stroke of 0.75%, Dr. Fanning said the two studies reported on different populations, which makes them difficult to compare.
“Our study specifically reports on new-onset stroke complicating ICU admission,” he noted. “The AHA study is a large study of all patients admitted to hospital, but both studies identified less than previous estimates of COVID-related stroke.”
Largest sample to date
The other study, which includes 119,967 COVID-19 hospitalizations and represents the largest sample reporting the concomitant diagnoses of stroke and SARS-CoV-2 infection to date, was presented at the AAN meeting by Thanh N. Nguyen, MD, a professor at Boston University.
This study has also been published online in Neurology, with first author Raul G. Nogueira, MD, Emory University, Atlanta.
In this international observational, retrospective study across 6 continents, 70 countries, and 457 stroke centers, there was a 1.48% stroke rate across 119,967 COVID-19 hospitalizations. SARS-CoV-2 infection was noted in 3.3% (1,722) of all stroke admissions, which numbered 52,026.
The researchers identified stroke diagnoses by the International Classification of Diseases, 10th revision, codes and/or classifications in stroke center databases, and rates of stroke hospitalizations and numbers of patients receiving thrombolysis were compared between the first 4 months of the pandemic (March to June 2020) compared with two control 4-month periods.
Global decline in stroke care during pandemic
Results showed a global decline in the number of stroke patients admitted to the hospital as well as acute stroke treatments, such as thrombolysis, during the first wave of the COVID-19 pandemic. The researchers found that there were 91,373 stroke admissions in the 4 months immediately before the pandemic, compared with 80,894 admissions during the first 4 pandemic months, representing an 11.5% decline.
They also report that 13,334 stroke patients received intravenous thrombolysis in the 4 months preceding the pandemic, compared with 11,570 during the first 4 pandemic months, representing a 13.2% drop.
Interhospital transfers after thrombolysis for a higher level of stroke care decreased from 1,337 before the pandemic to 1,178 during the pandemic, a reduction of 11.9%.
There were greater declines in primary compared with comprehensive stroke centers for stroke hospitalizations (change, –17.3% vs. –10.3%) and for the number of patients receiving thrombolysis (change, –15.5% vs. –12.6%).
The volume of stroke hospitalizations increased by 9.5% in the two later pandemic months (May, June) versus the two earlier months (March, April), with greater recovery in hospitals with lower COVID-19 hospitalization volume, high-volume stroke centers, and comprehensive stroke centers.
Dr. Nguyen suggested that reasons for the reductions in these stroke numbers at the beginning of the pandemic could include a reduction in stroke risk due to a reduction of exposure to other viral infections or patients not presenting to the hospital for fear of contracting the coronavirus.
The higher recovery of stroke volume in high-volume stroke centers and comprehensive stroke centers may represent patients with higher needs – those having more severe strokes – seeking care more frequently than those with milder symptoms, she noted.
“Preserving access to stroke care and emergency stroke care amidst a pandemic is as important as educating patients on the importance of presenting to the hospital in the event of stroke-like symptoms,” Dr. Nguyen concluded.
“We continue to advocate that if a patient has stroke-like symptoms, such as loss of speech, strength, vision, or balance, it is important for the patient to seek medical care as an emergency, as there are treatments that can improve a patient’s ability to recover from disabling stroke in earlier rather than later time windows,” she added.
In the publication, the authors wrote, “Our results concur with other recent reports on the collateral effects of the COVID-19 pandemic on stroke systems of care,” but added that “this is among the first descriptions of the change at a global level, including primary and comprehensive stroke centers.”
They said that hospital access related to high COVID-19 burden was unlikely a factor because the decline was seen in centers with a few or no patients with COVID-19. They suggested that patient fear of contracting coronavirus may have played a role, along with a decrease in presentation of transient ischemic attacks, mild strokes, or moderate strokes, and physical distancing measures may have prevented the timely witnessing of a stroke.
A version of this article first appeared on Medscape.com.
One study showed a stroke rate of 2.2% among patients with COVID-19 admitted to intensive care in 52 different countries. Another found a stroke rate of 1.48% in patients hospitalized with COVID-19 from 70 different countries. These researchers also found a reduction in stroke presentations and stroke care during the pandemic.
Both studies will be presented at the American Academy of Neurology’s 2021 annual meeting.
“Stroke has been a known serious complication of COVID-19, with some studies reporting a higher-than-expected occurrence, especially in young people,” said coauthor of the intensive care study, Jonathon Fanning, MBBS, PhD, University of Queensland, Brisbane, Australia.
“However, among the sickest of COVID patients – those admitted to an ICU – our research found that stroke was not a common complication and that ischemic stroke did not increase the risk of death,” he added.
Hemorrhagic stroke more common?
In this study, researchers analyzed a database of 2,699 patients who were admitted to the intensive care unit with COVID-19 in 52 countries and found that 59 of these patients (2.2%) subsequently sustained a stroke.
Most of the strokes identified in this cohort were hemorrhagic (46%), with 32% being ischemic and 22% unspecified. Hemorrhagic stroke was associated with a fivefold increased risk for death compared with patients who did not have a stroke. Of those with a hemorrhagic stroke, 72% died, but only 15% died of the stroke. Rather, multiorgan failure was the leading cause of death.
There was no association between ischemic stroke and mortality.
“There is scarce research on new-onset stroke complicating ICU admissions, and many of the limitations of assessing stroke in ICU populations confound the true values and result in variability in reported incidence anywhere from a 1%-4% incidence,” Dr. Fanning said.
He noted that a large Korean study had shown a 1.2% rate of stroke in patients without COVID admitted to non-neurologic ICUs. “In light of this, I think this 2% is higher than we would expect in a general ICU population, but in the context of earlier reports of COVID-19–associated risk for stroke, this figure is actually somewhat reassuring,” Dr. Fanning said.
Asked how this study compared with the large American Heart Association study recently reported that showed an overall rate of ischemic stroke of 0.75%, Dr. Fanning said the two studies reported on different populations, which makes them difficult to compare.
“Our study specifically reports on new-onset stroke complicating ICU admission,” he noted. “The AHA study is a large study of all patients admitted to hospital, but both studies identified less than previous estimates of COVID-related stroke.”
Largest sample to date
The other study, which includes 119,967 COVID-19 hospitalizations and represents the largest sample reporting the concomitant diagnoses of stroke and SARS-CoV-2 infection to date, was presented at the AAN meeting by Thanh N. Nguyen, MD, a professor at Boston University.
This study has also been published online in Neurology, with first author Raul G. Nogueira, MD, Emory University, Atlanta.
In this international observational, retrospective study across 6 continents, 70 countries, and 457 stroke centers, there was a 1.48% stroke rate across 119,967 COVID-19 hospitalizations. SARS-CoV-2 infection was noted in 3.3% (1,722) of all stroke admissions, which numbered 52,026.
The researchers identified stroke diagnoses by the International Classification of Diseases, 10th revision, codes and/or classifications in stroke center databases, and rates of stroke hospitalizations and numbers of patients receiving thrombolysis were compared between the first 4 months of the pandemic (March to June 2020) compared with two control 4-month periods.
Global decline in stroke care during pandemic
Results showed a global decline in the number of stroke patients admitted to the hospital as well as acute stroke treatments, such as thrombolysis, during the first wave of the COVID-19 pandemic. The researchers found that there were 91,373 stroke admissions in the 4 months immediately before the pandemic, compared with 80,894 admissions during the first 4 pandemic months, representing an 11.5% decline.
They also report that 13,334 stroke patients received intravenous thrombolysis in the 4 months preceding the pandemic, compared with 11,570 during the first 4 pandemic months, representing a 13.2% drop.
Interhospital transfers after thrombolysis for a higher level of stroke care decreased from 1,337 before the pandemic to 1,178 during the pandemic, a reduction of 11.9%.
There were greater declines in primary compared with comprehensive stroke centers for stroke hospitalizations (change, –17.3% vs. –10.3%) and for the number of patients receiving thrombolysis (change, –15.5% vs. –12.6%).
The volume of stroke hospitalizations increased by 9.5% in the two later pandemic months (May, June) versus the two earlier months (March, April), with greater recovery in hospitals with lower COVID-19 hospitalization volume, high-volume stroke centers, and comprehensive stroke centers.
Dr. Nguyen suggested that reasons for the reductions in these stroke numbers at the beginning of the pandemic could include a reduction in stroke risk due to a reduction of exposure to other viral infections or patients not presenting to the hospital for fear of contracting the coronavirus.
The higher recovery of stroke volume in high-volume stroke centers and comprehensive stroke centers may represent patients with higher needs – those having more severe strokes – seeking care more frequently than those with milder symptoms, she noted.
“Preserving access to stroke care and emergency stroke care amidst a pandemic is as important as educating patients on the importance of presenting to the hospital in the event of stroke-like symptoms,” Dr. Nguyen concluded.
“We continue to advocate that if a patient has stroke-like symptoms, such as loss of speech, strength, vision, or balance, it is important for the patient to seek medical care as an emergency, as there are treatments that can improve a patient’s ability to recover from disabling stroke in earlier rather than later time windows,” she added.
In the publication, the authors wrote, “Our results concur with other recent reports on the collateral effects of the COVID-19 pandemic on stroke systems of care,” but added that “this is among the first descriptions of the change at a global level, including primary and comprehensive stroke centers.”
They said that hospital access related to high COVID-19 burden was unlikely a factor because the decline was seen in centers with a few or no patients with COVID-19. They suggested that patient fear of contracting coronavirus may have played a role, along with a decrease in presentation of transient ischemic attacks, mild strokes, or moderate strokes, and physical distancing measures may have prevented the timely witnessing of a stroke.
A version of this article first appeared on Medscape.com.
From AAN 2021
Ten reasons airborne transmission of SARS-CoV-2 appears airtight
The scientific evidence for airborne transmission of the SARS-CoV-2 virus from different researchers all point in the same direction – that infectious aerosols are the principal means of person-to-person transmission, according to experts.
Not that it’s without controversy.
The science backing aerosol transmission “is clear-cut, but it is not accepted in many circles,” Trisha Greenhalgh, PhD, said in an interview.
“In particular, some in the evidence-based medicine movement and some infectious diseases clinicians are remarkably resistant to the evidence,” added Dr. Greenhalgh, professor of primary care health sciences at the University of Oxford (England).
“It’s very hard to see why, since the evidence all stacks up,” Dr. Greenhalgh said.
“The scientific evidence on spread from both near-field and far-field aerosols has been clear since early on in the pandemic, but there was resistance to acknowledging this in some circles, including the medical journals,” Joseph G. Allen, DSc, MPH, told this news organization when asked to comment.
“This is the week the dam broke. Three new commentaries came out … in top medical journals – BMJ, The Lancet, JAMA – all making the same point that aerosols are the dominant mode of transmission,” added Dr. Allen, associate professor of exposure assessment science at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Greenhalgh and colleagues point to an increase in COVID-19 cases in the aftermath of so-called “super-spreader” events, spread of SARS-CoV-2 to people across different hotel rooms, and the relatively lower transmission detected after outdoor events.
Top 10 reasons
They outlined 10 scientific reasons backing airborne transmission in a commentary published online April 15 in The Lancet:
- The dominance of airborne transmission is supported by long-range transmission observed at super-spreader events.
- Long-range transmission has been reported among rooms at COVID-19 quarantine hotels, settings where infected people never spent time in the same room.
- Asymptomatic individuals account for an estimated 33%-59% of SARS-CoV-2 transmission, and could be spreading the virus through speaking, which produces thousands of aerosol particles and few large droplets.
- Transmission outdoors and in well-ventilated indoor spaces is lower than in enclosed spaces.
- Nosocomial infections are reported in health care settings where protective measures address large droplets but not aerosols.
- Viable SARS-CoV-2 has been detected in the air of hospital rooms and in the car of an infected person.
- Investigators found SARS-CoV-2 in hospital air filters and building ducts.
- It’s not just humans – infected animals can infect animals in other cages connected only through an air duct.
- No strong evidence refutes airborne transmission, and contact tracing supports secondary transmission in crowded, poorly ventilated indoor spaces.
- Only limited evidence supports other means of SARS-CoV-2 transmission, including through fomites or large droplets.
“We thought we’d summarize [the evidence] to clarify the arguments for and against. We looked hard for evidence against but found none,” Dr. Greenhalgh said.
“Although other routes can contribute, we believe that the airborne route is likely to be dominant,” the authors note.
The evidence on airborne transmission was there very early on but the Centers for Disease Control and Prevention, World Health Organization, and others repeated the message that the primary concern was droplets and fomites.
Response to a review
The top 10 list is also part rebuttal of a systematic review funded by the WHO and published last month that points to inconclusive evidence for airborne transmission. The researchers involved with that review state that “the lack of recoverable viral culture samples of SARS-CoV-2 prevents firm conclusions to be drawn about airborne transmission.”
However, Dr. Greenhalgh and colleagues note that “this conclusion, and the wide circulation of the review’s findings, is concerning because of the public health implications.”
The current authors also argue that enough evidence already exists on airborne transmission. “Policy should change. We don’t need more research on this topic; we need different policy,” Dr. Greenhalgh said. “We need ventilation front and center, air filtration when necessary, and better-fitting masks worn whenever indoors.”
Dr. Allen agreed that guidance hasn’t always kept pace with the science. “With all of the new evidence accumulated on airborne transmission since last winter, there is still widespread confusion in the public about modes of transmission,” he said. Dr. Allen also serves as commissioner of The Lancet COVID-19 Commission and is chair of the commission’s Task Force on Safe Work, Safe Schools, and Safe Travel.
“It was only just last week that CDC pulled back on guidance on ‘deep cleaning’ and in its place correctly said that the risk from touching surfaces is low,” he added. “The science has been clear on this for over a year, but official guidance was only recently updated.”
As a result, many companies and organizations continued to focus on “hygiene theatre,” Dr. Allen said, “wasting resources on overcleaning surfaces. Unbelievably, many schools still close for an entire day each week for deep cleaning and some still quarantine library books. The message that shared air is the problem, not shared surfaces, is a message that still needs to be reinforced.”
The National Institute for Health Research, Economic and Social Research Council, and Wellcome support Dr. Greenhalgh’s research. Dr. Greenhalgh and Dr. Allen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
The scientific evidence for airborne transmission of the SARS-CoV-2 virus from different researchers all point in the same direction – that infectious aerosols are the principal means of person-to-person transmission, according to experts.
Not that it’s without controversy.
The science backing aerosol transmission “is clear-cut, but it is not accepted in many circles,” Trisha Greenhalgh, PhD, said in an interview.
“In particular, some in the evidence-based medicine movement and some infectious diseases clinicians are remarkably resistant to the evidence,” added Dr. Greenhalgh, professor of primary care health sciences at the University of Oxford (England).
“It’s very hard to see why, since the evidence all stacks up,” Dr. Greenhalgh said.
“The scientific evidence on spread from both near-field and far-field aerosols has been clear since early on in the pandemic, but there was resistance to acknowledging this in some circles, including the medical journals,” Joseph G. Allen, DSc, MPH, told this news organization when asked to comment.
“This is the week the dam broke. Three new commentaries came out … in top medical journals – BMJ, The Lancet, JAMA – all making the same point that aerosols are the dominant mode of transmission,” added Dr. Allen, associate professor of exposure assessment science at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Greenhalgh and colleagues point to an increase in COVID-19 cases in the aftermath of so-called “super-spreader” events, spread of SARS-CoV-2 to people across different hotel rooms, and the relatively lower transmission detected after outdoor events.
Top 10 reasons
They outlined 10 scientific reasons backing airborne transmission in a commentary published online April 15 in The Lancet:
- The dominance of airborne transmission is supported by long-range transmission observed at super-spreader events.
- Long-range transmission has been reported among rooms at COVID-19 quarantine hotels, settings where infected people never spent time in the same room.
- Asymptomatic individuals account for an estimated 33%-59% of SARS-CoV-2 transmission, and could be spreading the virus through speaking, which produces thousands of aerosol particles and few large droplets.
- Transmission outdoors and in well-ventilated indoor spaces is lower than in enclosed spaces.
- Nosocomial infections are reported in health care settings where protective measures address large droplets but not aerosols.
- Viable SARS-CoV-2 has been detected in the air of hospital rooms and in the car of an infected person.
- Investigators found SARS-CoV-2 in hospital air filters and building ducts.
- It’s not just humans – infected animals can infect animals in other cages connected only through an air duct.
- No strong evidence refutes airborne transmission, and contact tracing supports secondary transmission in crowded, poorly ventilated indoor spaces.
- Only limited evidence supports other means of SARS-CoV-2 transmission, including through fomites or large droplets.
“We thought we’d summarize [the evidence] to clarify the arguments for and against. We looked hard for evidence against but found none,” Dr. Greenhalgh said.
“Although other routes can contribute, we believe that the airborne route is likely to be dominant,” the authors note.
The evidence on airborne transmission was there very early on but the Centers for Disease Control and Prevention, World Health Organization, and others repeated the message that the primary concern was droplets and fomites.
Response to a review
The top 10 list is also part rebuttal of a systematic review funded by the WHO and published last month that points to inconclusive evidence for airborne transmission. The researchers involved with that review state that “the lack of recoverable viral culture samples of SARS-CoV-2 prevents firm conclusions to be drawn about airborne transmission.”
However, Dr. Greenhalgh and colleagues note that “this conclusion, and the wide circulation of the review’s findings, is concerning because of the public health implications.”
The current authors also argue that enough evidence already exists on airborne transmission. “Policy should change. We don’t need more research on this topic; we need different policy,” Dr. Greenhalgh said. “We need ventilation front and center, air filtration when necessary, and better-fitting masks worn whenever indoors.”
Dr. Allen agreed that guidance hasn’t always kept pace with the science. “With all of the new evidence accumulated on airborne transmission since last winter, there is still widespread confusion in the public about modes of transmission,” he said. Dr. Allen also serves as commissioner of The Lancet COVID-19 Commission and is chair of the commission’s Task Force on Safe Work, Safe Schools, and Safe Travel.
“It was only just last week that CDC pulled back on guidance on ‘deep cleaning’ and in its place correctly said that the risk from touching surfaces is low,” he added. “The science has been clear on this for over a year, but official guidance was only recently updated.”
As a result, many companies and organizations continued to focus on “hygiene theatre,” Dr. Allen said, “wasting resources on overcleaning surfaces. Unbelievably, many schools still close for an entire day each week for deep cleaning and some still quarantine library books. The message that shared air is the problem, not shared surfaces, is a message that still needs to be reinforced.”
The National Institute for Health Research, Economic and Social Research Council, and Wellcome support Dr. Greenhalgh’s research. Dr. Greenhalgh and Dr. Allen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
The scientific evidence for airborne transmission of the SARS-CoV-2 virus from different researchers all point in the same direction – that infectious aerosols are the principal means of person-to-person transmission, according to experts.
Not that it’s without controversy.
The science backing aerosol transmission “is clear-cut, but it is not accepted in many circles,” Trisha Greenhalgh, PhD, said in an interview.
“In particular, some in the evidence-based medicine movement and some infectious diseases clinicians are remarkably resistant to the evidence,” added Dr. Greenhalgh, professor of primary care health sciences at the University of Oxford (England).
“It’s very hard to see why, since the evidence all stacks up,” Dr. Greenhalgh said.
“The scientific evidence on spread from both near-field and far-field aerosols has been clear since early on in the pandemic, but there was resistance to acknowledging this in some circles, including the medical journals,” Joseph G. Allen, DSc, MPH, told this news organization when asked to comment.
“This is the week the dam broke. Three new commentaries came out … in top medical journals – BMJ, The Lancet, JAMA – all making the same point that aerosols are the dominant mode of transmission,” added Dr. Allen, associate professor of exposure assessment science at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Greenhalgh and colleagues point to an increase in COVID-19 cases in the aftermath of so-called “super-spreader” events, spread of SARS-CoV-2 to people across different hotel rooms, and the relatively lower transmission detected after outdoor events.
Top 10 reasons
They outlined 10 scientific reasons backing airborne transmission in a commentary published online April 15 in The Lancet:
- The dominance of airborne transmission is supported by long-range transmission observed at super-spreader events.
- Long-range transmission has been reported among rooms at COVID-19 quarantine hotels, settings where infected people never spent time in the same room.
- Asymptomatic individuals account for an estimated 33%-59% of SARS-CoV-2 transmission, and could be spreading the virus through speaking, which produces thousands of aerosol particles and few large droplets.
- Transmission outdoors and in well-ventilated indoor spaces is lower than in enclosed spaces.
- Nosocomial infections are reported in health care settings where protective measures address large droplets but not aerosols.
- Viable SARS-CoV-2 has been detected in the air of hospital rooms and in the car of an infected person.
- Investigators found SARS-CoV-2 in hospital air filters and building ducts.
- It’s not just humans – infected animals can infect animals in other cages connected only through an air duct.
- No strong evidence refutes airborne transmission, and contact tracing supports secondary transmission in crowded, poorly ventilated indoor spaces.
- Only limited evidence supports other means of SARS-CoV-2 transmission, including through fomites or large droplets.
“We thought we’d summarize [the evidence] to clarify the arguments for and against. We looked hard for evidence against but found none,” Dr. Greenhalgh said.
“Although other routes can contribute, we believe that the airborne route is likely to be dominant,” the authors note.
The evidence on airborne transmission was there very early on but the Centers for Disease Control and Prevention, World Health Organization, and others repeated the message that the primary concern was droplets and fomites.
Response to a review
The top 10 list is also part rebuttal of a systematic review funded by the WHO and published last month that points to inconclusive evidence for airborne transmission. The researchers involved with that review state that “the lack of recoverable viral culture samples of SARS-CoV-2 prevents firm conclusions to be drawn about airborne transmission.”
However, Dr. Greenhalgh and colleagues note that “this conclusion, and the wide circulation of the review’s findings, is concerning because of the public health implications.”
The current authors also argue that enough evidence already exists on airborne transmission. “Policy should change. We don’t need more research on this topic; we need different policy,” Dr. Greenhalgh said. “We need ventilation front and center, air filtration when necessary, and better-fitting masks worn whenever indoors.”
Dr. Allen agreed that guidance hasn’t always kept pace with the science. “With all of the new evidence accumulated on airborne transmission since last winter, there is still widespread confusion in the public about modes of transmission,” he said. Dr. Allen also serves as commissioner of The Lancet COVID-19 Commission and is chair of the commission’s Task Force on Safe Work, Safe Schools, and Safe Travel.
“It was only just last week that CDC pulled back on guidance on ‘deep cleaning’ and in its place correctly said that the risk from touching surfaces is low,” he added. “The science has been clear on this for over a year, but official guidance was only recently updated.”
As a result, many companies and organizations continued to focus on “hygiene theatre,” Dr. Allen said, “wasting resources on overcleaning surfaces. Unbelievably, many schools still close for an entire day each week for deep cleaning and some still quarantine library books. The message that shared air is the problem, not shared surfaces, is a message that still needs to be reinforced.”
The National Institute for Health Research, Economic and Social Research Council, and Wellcome support Dr. Greenhalgh’s research. Dr. Greenhalgh and Dr. Allen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Correlating hospitalist work schedules with patient outcomes
Background: Studies show better outcomes, decreased length of stay, increased patient satisfaction, improved quality, and decreased readmission rates when hospitalist services are used. This study looks at how hospitalist schedules affect these outcomes.
Study design: Retrospective cohort study.
Setting: 229 hospitals in Texas.
Synopsis: This cohort study used 3 years of Medicare data from 229 hospitals in Texas. It included 114,777 medical admissions of patients with a 3- to 6-day length of stay. The study used the percentage of hospitalist working days that were blocks of 7 days or longer. ICU stays and patients requiring two or more E&M codes were excluded since they are associated with greater illness severity.
The primary outcome was mortality within 30 days of discharge and secondary outcomes were 30-day readmission rates, discharge destination, and 30-day postdischarge costs.
Patients receiving care from hospitalists working several days in a row had better outcomes. It is postulated that continuity of care by one hospitalist is important for several reasons. Most importantly, the development of rapport with patient and family is key to deciding the plan of care and destination post discharge as it is quite challenging to effectively transfer all important information during verbal or written handoffs.
Bottom line: Care provided by hospitalists working more days in a row improved patient outcomes. A variety of hospitalist schedules are being practiced currently; however, these findings must be taken into account as schedules are designed.
Citation: Goodwin JS et al. Association of the work schedules of hospitalists with patient outcomes of hospitalization. JAMA Intern Med. 2020;180(2):215-22. doi: 10.1001/jamainternmed.2019.5193.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Studies show better outcomes, decreased length of stay, increased patient satisfaction, improved quality, and decreased readmission rates when hospitalist services are used. This study looks at how hospitalist schedules affect these outcomes.
Study design: Retrospective cohort study.
Setting: 229 hospitals in Texas.
Synopsis: This cohort study used 3 years of Medicare data from 229 hospitals in Texas. It included 114,777 medical admissions of patients with a 3- to 6-day length of stay. The study used the percentage of hospitalist working days that were blocks of 7 days or longer. ICU stays and patients requiring two or more E&M codes were excluded since they are associated with greater illness severity.
The primary outcome was mortality within 30 days of discharge and secondary outcomes were 30-day readmission rates, discharge destination, and 30-day postdischarge costs.
Patients receiving care from hospitalists working several days in a row had better outcomes. It is postulated that continuity of care by one hospitalist is important for several reasons. Most importantly, the development of rapport with patient and family is key to deciding the plan of care and destination post discharge as it is quite challenging to effectively transfer all important information during verbal or written handoffs.
Bottom line: Care provided by hospitalists working more days in a row improved patient outcomes. A variety of hospitalist schedules are being practiced currently; however, these findings must be taken into account as schedules are designed.
Citation: Goodwin JS et al. Association of the work schedules of hospitalists with patient outcomes of hospitalization. JAMA Intern Med. 2020;180(2):215-22. doi: 10.1001/jamainternmed.2019.5193.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Studies show better outcomes, decreased length of stay, increased patient satisfaction, improved quality, and decreased readmission rates when hospitalist services are used. This study looks at how hospitalist schedules affect these outcomes.
Study design: Retrospective cohort study.
Setting: 229 hospitals in Texas.
Synopsis: This cohort study used 3 years of Medicare data from 229 hospitals in Texas. It included 114,777 medical admissions of patients with a 3- to 6-day length of stay. The study used the percentage of hospitalist working days that were blocks of 7 days or longer. ICU stays and patients requiring two or more E&M codes were excluded since they are associated with greater illness severity.
The primary outcome was mortality within 30 days of discharge and secondary outcomes were 30-day readmission rates, discharge destination, and 30-day postdischarge costs.
Patients receiving care from hospitalists working several days in a row had better outcomes. It is postulated that continuity of care by one hospitalist is important for several reasons. Most importantly, the development of rapport with patient and family is key to deciding the plan of care and destination post discharge as it is quite challenging to effectively transfer all important information during verbal or written handoffs.
Bottom line: Care provided by hospitalists working more days in a row improved patient outcomes. A variety of hospitalist schedules are being practiced currently; however, these findings must be taken into account as schedules are designed.
Citation: Goodwin JS et al. Association of the work schedules of hospitalists with patient outcomes of hospitalization. JAMA Intern Med. 2020;180(2):215-22. doi: 10.1001/jamainternmed.2019.5193.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Children’s share of COVID-19 burden has never been higher
For the first time since the pandemic began, children’s share of weekly COVID-19 cases topped 20% in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
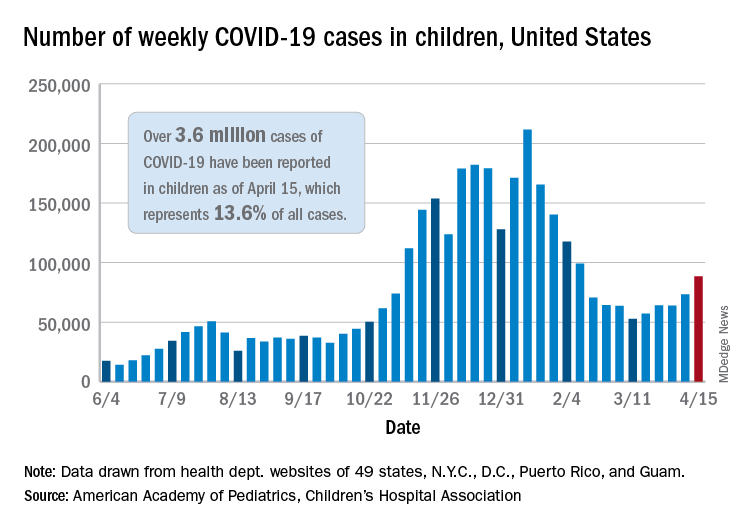
That represented 20.6% of all new cases for the week, eclipsing the previous high of 19.1% recorded just 3 weeks ago, based on data collected by the AAP and CHA from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Cumulative cases of COVID-19 in children exceed 3.6 million in those jurisdictions, which is 13.6% of the total reported among all ages, and the overall rate of coronavirus infection is 4,824 cases per 100,000 children in the population, the AAP and CHA said in their weekly COVID-19 report.
Among the 53 reporting jurisdictions, North Dakota has the highest cumulative rate, 9,167 per 100,000 children, followed by Tennessee (8,580), South Carolina (7,948), South Dakota (7,938), and Connecticut (7,707). Children’s share of cumulative cases is highest in Vermont, at 21.9%, with Alaska next at 20.0% and Wyoming at 19.2%, the AAP and CHA said.
Since the beginning of April, the largest local increases in cases reported came in Michigan (21.6%), Vermont (15.9%), and Maine (15.6%). Nationally, the increase over those same 2 weeks is just under 5%, the two organizations noted.
There were 5 deaths among children during the week of April 9-15, bringing the total to 297, but the recent increases in cases have not affected the long-term trends for serious illness. The death rate for children with COVID-19 has been 0.01% since early November – 43 states, New York City, Puerto Rico, and Guam are reporting such data – and the hospitalization rate has been 0.8% since mid-January in 24 states and New York City, the AAP/CHA data show.
For the first time since the pandemic began, children’s share of weekly COVID-19 cases topped 20% in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That represented 20.6% of all new cases for the week, eclipsing the previous high of 19.1% recorded just 3 weeks ago, based on data collected by the AAP and CHA from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Cumulative cases of COVID-19 in children exceed 3.6 million in those jurisdictions, which is 13.6% of the total reported among all ages, and the overall rate of coronavirus infection is 4,824 cases per 100,000 children in the population, the AAP and CHA said in their weekly COVID-19 report.
Among the 53 reporting jurisdictions, North Dakota has the highest cumulative rate, 9,167 per 100,000 children, followed by Tennessee (8,580), South Carolina (7,948), South Dakota (7,938), and Connecticut (7,707). Children’s share of cumulative cases is highest in Vermont, at 21.9%, with Alaska next at 20.0% and Wyoming at 19.2%, the AAP and CHA said.
Since the beginning of April, the largest local increases in cases reported came in Michigan (21.6%), Vermont (15.9%), and Maine (15.6%). Nationally, the increase over those same 2 weeks is just under 5%, the two organizations noted.
There were 5 deaths among children during the week of April 9-15, bringing the total to 297, but the recent increases in cases have not affected the long-term trends for serious illness. The death rate for children with COVID-19 has been 0.01% since early November – 43 states, New York City, Puerto Rico, and Guam are reporting such data – and the hospitalization rate has been 0.8% since mid-January in 24 states and New York City, the AAP/CHA data show.
For the first time since the pandemic began, children’s share of weekly COVID-19 cases topped 20% in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That represented 20.6% of all new cases for the week, eclipsing the previous high of 19.1% recorded just 3 weeks ago, based on data collected by the AAP and CHA from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Cumulative cases of COVID-19 in children exceed 3.6 million in those jurisdictions, which is 13.6% of the total reported among all ages, and the overall rate of coronavirus infection is 4,824 cases per 100,000 children in the population, the AAP and CHA said in their weekly COVID-19 report.
Among the 53 reporting jurisdictions, North Dakota has the highest cumulative rate, 9,167 per 100,000 children, followed by Tennessee (8,580), South Carolina (7,948), South Dakota (7,938), and Connecticut (7,707). Children’s share of cumulative cases is highest in Vermont, at 21.9%, with Alaska next at 20.0% and Wyoming at 19.2%, the AAP and CHA said.
Since the beginning of April, the largest local increases in cases reported came in Michigan (21.6%), Vermont (15.9%), and Maine (15.6%). Nationally, the increase over those same 2 weeks is just under 5%, the two organizations noted.
There were 5 deaths among children during the week of April 9-15, bringing the total to 297, but the recent increases in cases have not affected the long-term trends for serious illness. The death rate for children with COVID-19 has been 0.01% since early November – 43 states, New York City, Puerto Rico, and Guam are reporting such data – and the hospitalization rate has been 0.8% since mid-January in 24 states and New York City, the AAP/CHA data show.
Watch for abnormal movements in hospitalized COVID-19 patients
Myoclonus was diagnosed in about half of hospitalized COVID-19 patients who were evaluated for movement disorders, data from 50 cases show.
Abnormal movements often occur as complications from critical illness, and neurologic consultation can determine whether patients have experienced a seizure or stroke. However, restriction of bedside assessment in the wake of the COVID-19 pandemic increases the risk that abnormal movements will be missed, Jeffrey R. Clark and Eric M. Liotta, MD, of Northwestern University, Chicago, and colleagues wrote.
“Given the limited reports of abnormal movements in hospitalized COVID-19 patients and increased recognition of neurologic manifestations of COVID-19, we sought to examine the frequency and etiology of this finding as an indication of neurologic consultation,” they said.
In a study published in the Journal of the Neurological Sciences, the researchers reviewed data from the first 50 consecutive patients with COVID-19 symptoms who were hospitalized at a single center and underwent neurologic consultation between March 17, 2020, and May 18, 2020.
Overall, 11 patients (22.0%) of patients experienced abnormal movement, and all were admitted to the ICU within 7 days of meeting criteria for severe COVID-19. These patients included nine men and two women with an age range of 36-78 years. The most common comorbidities were obesity, hypertension, diabetes, chronic kidney disease, and coronary artery disease.
Myoclonus (generalized and focal) was the most common abnormal movement, and present in 6 of the 11 patients. Three cases were attributed to high-intensity sedation, and three to toxic-metabolic disturbances. In two patients, abnormal movements were attributed to focal seizures in the setting of encephalopathy, with focal facial twitching. An additional two patients experienced tremors; one showed an acute subdural hemorrhage on CT imaging. The second patient showed no sign of stroke or other abnormality on MRI and the tremor improved during the hospital stay. One patient who experienced abnormal high-amplitude nonrhythmic movements of the lower extremities was diagnosed with serotonin syndrome that resolved after discontinuing high-dose fentanyl.
The study findings were limited by several factors, including the small study population and limited availability of MRI, the researchers noted. Assessing severe COVID-19 cases in the ICU setting presents a challenge because of limited patient participation and the potentially confounding effects of sedation and mechanical ventilation.
However, the researchers said.
“A heightened awareness of abnormal eye movements, or subtle facial tremoring, may be the first steps in recognizing potentially dangerous neurologic manifestations,” and clinicians caring for patients with severe COVID-19 should be able to recognize abnormal movements and seek neurologic consultation when indicated, they emphasized.
The study was supported in part by grants to coauthors Nicholas J. Reish, MD, and Dr. Liotta from the National Institutes of Health. The researchers had no financial conflicts to disclose.
Myoclonus was diagnosed in about half of hospitalized COVID-19 patients who were evaluated for movement disorders, data from 50 cases show.
Abnormal movements often occur as complications from critical illness, and neurologic consultation can determine whether patients have experienced a seizure or stroke. However, restriction of bedside assessment in the wake of the COVID-19 pandemic increases the risk that abnormal movements will be missed, Jeffrey R. Clark and Eric M. Liotta, MD, of Northwestern University, Chicago, and colleagues wrote.
“Given the limited reports of abnormal movements in hospitalized COVID-19 patients and increased recognition of neurologic manifestations of COVID-19, we sought to examine the frequency and etiology of this finding as an indication of neurologic consultation,” they said.
In a study published in the Journal of the Neurological Sciences, the researchers reviewed data from the first 50 consecutive patients with COVID-19 symptoms who were hospitalized at a single center and underwent neurologic consultation between March 17, 2020, and May 18, 2020.
Overall, 11 patients (22.0%) of patients experienced abnormal movement, and all were admitted to the ICU within 7 days of meeting criteria for severe COVID-19. These patients included nine men and two women with an age range of 36-78 years. The most common comorbidities were obesity, hypertension, diabetes, chronic kidney disease, and coronary artery disease.
Myoclonus (generalized and focal) was the most common abnormal movement, and present in 6 of the 11 patients. Three cases were attributed to high-intensity sedation, and three to toxic-metabolic disturbances. In two patients, abnormal movements were attributed to focal seizures in the setting of encephalopathy, with focal facial twitching. An additional two patients experienced tremors; one showed an acute subdural hemorrhage on CT imaging. The second patient showed no sign of stroke or other abnormality on MRI and the tremor improved during the hospital stay. One patient who experienced abnormal high-amplitude nonrhythmic movements of the lower extremities was diagnosed with serotonin syndrome that resolved after discontinuing high-dose fentanyl.
The study findings were limited by several factors, including the small study population and limited availability of MRI, the researchers noted. Assessing severe COVID-19 cases in the ICU setting presents a challenge because of limited patient participation and the potentially confounding effects of sedation and mechanical ventilation.
However, the researchers said.
“A heightened awareness of abnormal eye movements, or subtle facial tremoring, may be the first steps in recognizing potentially dangerous neurologic manifestations,” and clinicians caring for patients with severe COVID-19 should be able to recognize abnormal movements and seek neurologic consultation when indicated, they emphasized.
The study was supported in part by grants to coauthors Nicholas J. Reish, MD, and Dr. Liotta from the National Institutes of Health. The researchers had no financial conflicts to disclose.
Myoclonus was diagnosed in about half of hospitalized COVID-19 patients who were evaluated for movement disorders, data from 50 cases show.
Abnormal movements often occur as complications from critical illness, and neurologic consultation can determine whether patients have experienced a seizure or stroke. However, restriction of bedside assessment in the wake of the COVID-19 pandemic increases the risk that abnormal movements will be missed, Jeffrey R. Clark and Eric M. Liotta, MD, of Northwestern University, Chicago, and colleagues wrote.
“Given the limited reports of abnormal movements in hospitalized COVID-19 patients and increased recognition of neurologic manifestations of COVID-19, we sought to examine the frequency and etiology of this finding as an indication of neurologic consultation,” they said.
In a study published in the Journal of the Neurological Sciences, the researchers reviewed data from the first 50 consecutive patients with COVID-19 symptoms who were hospitalized at a single center and underwent neurologic consultation between March 17, 2020, and May 18, 2020.
Overall, 11 patients (22.0%) of patients experienced abnormal movement, and all were admitted to the ICU within 7 days of meeting criteria for severe COVID-19. These patients included nine men and two women with an age range of 36-78 years. The most common comorbidities were obesity, hypertension, diabetes, chronic kidney disease, and coronary artery disease.
Myoclonus (generalized and focal) was the most common abnormal movement, and present in 6 of the 11 patients. Three cases were attributed to high-intensity sedation, and three to toxic-metabolic disturbances. In two patients, abnormal movements were attributed to focal seizures in the setting of encephalopathy, with focal facial twitching. An additional two patients experienced tremors; one showed an acute subdural hemorrhage on CT imaging. The second patient showed no sign of stroke or other abnormality on MRI and the tremor improved during the hospital stay. One patient who experienced abnormal high-amplitude nonrhythmic movements of the lower extremities was diagnosed with serotonin syndrome that resolved after discontinuing high-dose fentanyl.
The study findings were limited by several factors, including the small study population and limited availability of MRI, the researchers noted. Assessing severe COVID-19 cases in the ICU setting presents a challenge because of limited patient participation and the potentially confounding effects of sedation and mechanical ventilation.
However, the researchers said.
“A heightened awareness of abnormal eye movements, or subtle facial tremoring, may be the first steps in recognizing potentially dangerous neurologic manifestations,” and clinicians caring for patients with severe COVID-19 should be able to recognize abnormal movements and seek neurologic consultation when indicated, they emphasized.
The study was supported in part by grants to coauthors Nicholas J. Reish, MD, and Dr. Liotta from the National Institutes of Health. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF THE NEUROLOGICAL SCIENCES
Pneumonia risk soars in heart failure patients, especially HFpEF
Patients with heart failure get pneumonia at a rate almost three times greater than expected and, once they do get pneumonia, have about a fourfold greater risk of death, investigators for a retrospective analysis of 13,000 patients from two landmark randomized HF trials have found.
The investigators also found that HF patients with preserved ejection fraction (HFpEF) are at the highest risk of developing pneumonia. The findings underscore the importance of patients with HF getting a pneumonia vaccination, they found.
The analysis showed that 6.3% of patients in the PARADIGM-HF trial and 10.6% of those in the PARAGON-HF trial developed pneumonia, reported the study authors, led by John J.V. McMurray, MD, of the British Heart Foundation Cardiovascular Research Center at the University of Glasgow in Scotland (J Am Coll Cardiol. 2021;77:1961-73).
“The main reason for doing this study was the fact that many heart failure patients are not vaccinated, as they should be, against pneumonia – both pneumococcus and influenza vaccination,” Dr. McMurray said in an interview. “We wanted to document the frequency and consequences of pneumonia in patients with heart failure to help highlight this deficiency in care.”
Dr. McMurray said he believes this is the first study to document the incidence of pneumonia and pneumonia-related outcomes according to the two major ejection fraction phenotypes.
PARADIGM-HF and PARAGON-HF
The post hoc analysis consisted of 8,399 patients with HF with reduced ejection fraction (HFrEF) in PARADIGM-HF (Eur J Heart Fail. 2013 Sep;15[9]:1062-73) and 4,796 patients with HFpEF in PARAGON-HF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). The analysis focused on the 528 and 510 patients in each study, respectively, who developed pneumonia. Those rates translated to an incidence rate of 29 per 1,000 patient-years (95% confidence interval, 27-31) in PARADIGM-HF and 39 per 1,000 patient-years (95% CI, 36-42) in PARAGON-HF.
After pneumonia, the risk of death in patients increased substantially. In PARADIGM-HF, the adjusted hazard ratio for the risk of death from any cause after pneumonia was 4.34 (95% CI, 3.73-5.05). In PARAGON-HF, it was 3.76 (95% CI, 3.09-4.58). HF patients who contracted pneumonia also tended to have HF longer than their counterparts who didn’t develop pneumonia, but the frequency of previous hospitalization for HF didn’t vary between the pneumonia and no-pneumonia groups.
Patients who developed pneumonia tended to be older (average age of 66.9 years vs. 64.6 years, P < .001) and male (83.9% vs. 77.8%, P < .001). The mean age of patients in PARADIGM-HF was almost a decade younger than those in PARAGON-HF, 64 vs. 73 years.
Pneumonia patients also had worse Kansas City Cardiomyopathy Questionnaire scores (76 vs. 80 on average), but no difference in New York Heart Association functional class. “In general, patients who developed pneumonia had more symptoms and signs and HF than those who did not develop pneumonia,” Dr. McMurray and colleagues wrote.
Pneumonia patients also had higher rates of chronic obstructive pulmonary disease (26% vs. 12%), diabetes (43% vs. 34%), and atrial fibrillation (46% vs. 36%).
Another reason for conducting the study, Dr. McMurray said, “was the prior findings in patients with coronary disease and acute myocardial infarction that the risk associated with an episode of pneumonia [e.g., in subsequent vascular events and deaths] persisted long after the acute event. We wanted to see if this was also the case for heart failure, and indeed it was.”
For example, the adjusted HR for cardiovascular death or hospitalization in the first month following an episode of pneumonia was 9.48 (range of 6.85-13.12, P < .001), leveling off to 1.59 after 3 months or more.
Vaccination crucial in HF patients
Dr. McMurray noted that this study emphasizes the importance of pneumonia vaccination for patients with HF. “Given that we have so few treatments to offer patients with HFpEF, this makes the potential value of vaccination in these patients all the greater,” he said.
The COVID-19 pandemic, Dr. McMurray said, is a “good reminder of the dangers of a respiratory infection and the importance of vaccination in these patients. COVID-19 has interesting parallels in being a systemic disease and one with postacute, persisting effects.”
The persistent risk for adverse cardiovascular events 3 months and later after pneumonia is a novel finding of the study, wrote Donna Mancini, MD, and Gregory Gibson, MD, in an invited commentary (J Am Coll Cardiol. 2021;77:1974-6). Both are with the Icahn School of Medicine at Mt. Sinai in New York. The post hoc study also “serves as an important reminder” of pneumonia risk in patients with HF, especially during the pandemic, they wrote.
“Although vaccination alone appears unlikely to be a panacea, it is a readily accessible tool for mitigating disease severity and improving outcomes,” Dr. Mancini and Dr. Gibson wrote. “After all, an ounce of prevention is worth a pound of cure.”
Novartis provided funding for the PARADIGM-HF and PARAGON-HF trials, and Dr. McMurray and coauthors disclosed financial relationships with Novartis. Dr. Mancini and Dr. Gibson have no relevant financial relationships to disclose.
Patients with heart failure get pneumonia at a rate almost three times greater than expected and, once they do get pneumonia, have about a fourfold greater risk of death, investigators for a retrospective analysis of 13,000 patients from two landmark randomized HF trials have found.
The investigators also found that HF patients with preserved ejection fraction (HFpEF) are at the highest risk of developing pneumonia. The findings underscore the importance of patients with HF getting a pneumonia vaccination, they found.
The analysis showed that 6.3% of patients in the PARADIGM-HF trial and 10.6% of those in the PARAGON-HF trial developed pneumonia, reported the study authors, led by John J.V. McMurray, MD, of the British Heart Foundation Cardiovascular Research Center at the University of Glasgow in Scotland (J Am Coll Cardiol. 2021;77:1961-73).
“The main reason for doing this study was the fact that many heart failure patients are not vaccinated, as they should be, against pneumonia – both pneumococcus and influenza vaccination,” Dr. McMurray said in an interview. “We wanted to document the frequency and consequences of pneumonia in patients with heart failure to help highlight this deficiency in care.”
Dr. McMurray said he believes this is the first study to document the incidence of pneumonia and pneumonia-related outcomes according to the two major ejection fraction phenotypes.
PARADIGM-HF and PARAGON-HF
The post hoc analysis consisted of 8,399 patients with HF with reduced ejection fraction (HFrEF) in PARADIGM-HF (Eur J Heart Fail. 2013 Sep;15[9]:1062-73) and 4,796 patients with HFpEF in PARAGON-HF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). The analysis focused on the 528 and 510 patients in each study, respectively, who developed pneumonia. Those rates translated to an incidence rate of 29 per 1,000 patient-years (95% confidence interval, 27-31) in PARADIGM-HF and 39 per 1,000 patient-years (95% CI, 36-42) in PARAGON-HF.
After pneumonia, the risk of death in patients increased substantially. In PARADIGM-HF, the adjusted hazard ratio for the risk of death from any cause after pneumonia was 4.34 (95% CI, 3.73-5.05). In PARAGON-HF, it was 3.76 (95% CI, 3.09-4.58). HF patients who contracted pneumonia also tended to have HF longer than their counterparts who didn’t develop pneumonia, but the frequency of previous hospitalization for HF didn’t vary between the pneumonia and no-pneumonia groups.
Patients who developed pneumonia tended to be older (average age of 66.9 years vs. 64.6 years, P < .001) and male (83.9% vs. 77.8%, P < .001). The mean age of patients in PARADIGM-HF was almost a decade younger than those in PARAGON-HF, 64 vs. 73 years.
Pneumonia patients also had worse Kansas City Cardiomyopathy Questionnaire scores (76 vs. 80 on average), but no difference in New York Heart Association functional class. “In general, patients who developed pneumonia had more symptoms and signs and HF than those who did not develop pneumonia,” Dr. McMurray and colleagues wrote.
Pneumonia patients also had higher rates of chronic obstructive pulmonary disease (26% vs. 12%), diabetes (43% vs. 34%), and atrial fibrillation (46% vs. 36%).
Another reason for conducting the study, Dr. McMurray said, “was the prior findings in patients with coronary disease and acute myocardial infarction that the risk associated with an episode of pneumonia [e.g., in subsequent vascular events and deaths] persisted long after the acute event. We wanted to see if this was also the case for heart failure, and indeed it was.”
For example, the adjusted HR for cardiovascular death or hospitalization in the first month following an episode of pneumonia was 9.48 (range of 6.85-13.12, P < .001), leveling off to 1.59 after 3 months or more.
Vaccination crucial in HF patients
Dr. McMurray noted that this study emphasizes the importance of pneumonia vaccination for patients with HF. “Given that we have so few treatments to offer patients with HFpEF, this makes the potential value of vaccination in these patients all the greater,” he said.
The COVID-19 pandemic, Dr. McMurray said, is a “good reminder of the dangers of a respiratory infection and the importance of vaccination in these patients. COVID-19 has interesting parallels in being a systemic disease and one with postacute, persisting effects.”
The persistent risk for adverse cardiovascular events 3 months and later after pneumonia is a novel finding of the study, wrote Donna Mancini, MD, and Gregory Gibson, MD, in an invited commentary (J Am Coll Cardiol. 2021;77:1974-6). Both are with the Icahn School of Medicine at Mt. Sinai in New York. The post hoc study also “serves as an important reminder” of pneumonia risk in patients with HF, especially during the pandemic, they wrote.
“Although vaccination alone appears unlikely to be a panacea, it is a readily accessible tool for mitigating disease severity and improving outcomes,” Dr. Mancini and Dr. Gibson wrote. “After all, an ounce of prevention is worth a pound of cure.”
Novartis provided funding for the PARADIGM-HF and PARAGON-HF trials, and Dr. McMurray and coauthors disclosed financial relationships with Novartis. Dr. Mancini and Dr. Gibson have no relevant financial relationships to disclose.
Patients with heart failure get pneumonia at a rate almost three times greater than expected and, once they do get pneumonia, have about a fourfold greater risk of death, investigators for a retrospective analysis of 13,000 patients from two landmark randomized HF trials have found.
The investigators also found that HF patients with preserved ejection fraction (HFpEF) are at the highest risk of developing pneumonia. The findings underscore the importance of patients with HF getting a pneumonia vaccination, they found.
The analysis showed that 6.3% of patients in the PARADIGM-HF trial and 10.6% of those in the PARAGON-HF trial developed pneumonia, reported the study authors, led by John J.V. McMurray, MD, of the British Heart Foundation Cardiovascular Research Center at the University of Glasgow in Scotland (J Am Coll Cardiol. 2021;77:1961-73).
“The main reason for doing this study was the fact that many heart failure patients are not vaccinated, as they should be, against pneumonia – both pneumococcus and influenza vaccination,” Dr. McMurray said in an interview. “We wanted to document the frequency and consequences of pneumonia in patients with heart failure to help highlight this deficiency in care.”
Dr. McMurray said he believes this is the first study to document the incidence of pneumonia and pneumonia-related outcomes according to the two major ejection fraction phenotypes.
PARADIGM-HF and PARAGON-HF
The post hoc analysis consisted of 8,399 patients with HF with reduced ejection fraction (HFrEF) in PARADIGM-HF (Eur J Heart Fail. 2013 Sep;15[9]:1062-73) and 4,796 patients with HFpEF in PARAGON-HF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). The analysis focused on the 528 and 510 patients in each study, respectively, who developed pneumonia. Those rates translated to an incidence rate of 29 per 1,000 patient-years (95% confidence interval, 27-31) in PARADIGM-HF and 39 per 1,000 patient-years (95% CI, 36-42) in PARAGON-HF.
After pneumonia, the risk of death in patients increased substantially. In PARADIGM-HF, the adjusted hazard ratio for the risk of death from any cause after pneumonia was 4.34 (95% CI, 3.73-5.05). In PARAGON-HF, it was 3.76 (95% CI, 3.09-4.58). HF patients who contracted pneumonia also tended to have HF longer than their counterparts who didn’t develop pneumonia, but the frequency of previous hospitalization for HF didn’t vary between the pneumonia and no-pneumonia groups.
Patients who developed pneumonia tended to be older (average age of 66.9 years vs. 64.6 years, P < .001) and male (83.9% vs. 77.8%, P < .001). The mean age of patients in PARADIGM-HF was almost a decade younger than those in PARAGON-HF, 64 vs. 73 years.
Pneumonia patients also had worse Kansas City Cardiomyopathy Questionnaire scores (76 vs. 80 on average), but no difference in New York Heart Association functional class. “In general, patients who developed pneumonia had more symptoms and signs and HF than those who did not develop pneumonia,” Dr. McMurray and colleagues wrote.
Pneumonia patients also had higher rates of chronic obstructive pulmonary disease (26% vs. 12%), diabetes (43% vs. 34%), and atrial fibrillation (46% vs. 36%).
Another reason for conducting the study, Dr. McMurray said, “was the prior findings in patients with coronary disease and acute myocardial infarction that the risk associated with an episode of pneumonia [e.g., in subsequent vascular events and deaths] persisted long after the acute event. We wanted to see if this was also the case for heart failure, and indeed it was.”
For example, the adjusted HR for cardiovascular death or hospitalization in the first month following an episode of pneumonia was 9.48 (range of 6.85-13.12, P < .001), leveling off to 1.59 after 3 months or more.
Vaccination crucial in HF patients
Dr. McMurray noted that this study emphasizes the importance of pneumonia vaccination for patients with HF. “Given that we have so few treatments to offer patients with HFpEF, this makes the potential value of vaccination in these patients all the greater,” he said.
The COVID-19 pandemic, Dr. McMurray said, is a “good reminder of the dangers of a respiratory infection and the importance of vaccination in these patients. COVID-19 has interesting parallels in being a systemic disease and one with postacute, persisting effects.”
The persistent risk for adverse cardiovascular events 3 months and later after pneumonia is a novel finding of the study, wrote Donna Mancini, MD, and Gregory Gibson, MD, in an invited commentary (J Am Coll Cardiol. 2021;77:1974-6). Both are with the Icahn School of Medicine at Mt. Sinai in New York. The post hoc study also “serves as an important reminder” of pneumonia risk in patients with HF, especially during the pandemic, they wrote.
“Although vaccination alone appears unlikely to be a panacea, it is a readily accessible tool for mitigating disease severity and improving outcomes,” Dr. Mancini and Dr. Gibson wrote. “After all, an ounce of prevention is worth a pound of cure.”
Novartis provided funding for the PARADIGM-HF and PARAGON-HF trials, and Dr. McMurray and coauthors disclosed financial relationships with Novartis. Dr. Mancini and Dr. Gibson have no relevant financial relationships to disclose.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
How often should we check EKGs in patients starting antipsychotic medications?
Determining relative risk with available data
Case
An 88-year-old woman with history of osteoporosis, hyperlipidemia, and a remote myocardial infarction presents to the ED with altered mental status and agitation. The patient is admitted to the medicine service for further management. Her current medications include a thiazide and a statin. Psychiatry is consulted and recommends administering intravenous haloperidol. A baseline EKG shows a corrected QT interval (QTc) of 486 milliseconds (ms). How often should subsequent EKGs be ordered?
Overview of issue
A prolonged QT interval can predispose a patient to dangerous arrhythmias such as Torsades de pointes (TdP), which results in sudden cardiac death in about 10% of cases.1,2 A prolonged QTc interval can be caused by cardiac, renal, or hepatic dysfunction; congenital Long QT Syndrome (LQTS)2; electrolyte abnormalities; or as a result of many drugs, including most antipsychotic medications such as quetiapine, olanzapine, risperidone and haloperidol.
To diminish risk of TdP while taking these medications, it is necessary to monitor the QTc interval. Before commencing a QT-prolonging medication, it is recommended to get a baseline EKG, then perform EKG monitoring after administering the medication.
According to American Heart Association guidelines, a prolonged QT interval is considered more than 460 ms in women or above 450 ms in men.3 If an abnormal rhythm and/or prolonged QTc is detected via EKG monitoring, then the drug dosage can be changed or an alternative therapy selected.4 However, there are no current guidelines recommending how often EKG monitoring should be performed after a QT-prolonging antipsychotic medication is administered on an inpatient medicine unit. Without guidelines, there is potential for health care providers to under- or over-order EKG monitoring, possibly putting patients at risk of TdP or wasting hospital resources, respectively.
Overview of the data
There are currently no universally accepted guidelines regarding inpatient EKG monitoring for patients started on QTc prolonging antipsychotic medications. A 2018 review of the literature surrounding assessment and management of patients on QTc prolonging medications was performed to analyze the available data and make recommendations; notably the evidence was limited as none of the studies were randomized controlled trials.
The authors recommend assessing the drug for QTc prolonging potential, and if possible, choosing alternative treatment in patients with baseline prolonged QTc. If the QT prolonging medication is the best or only option, then the next step is assessing the patient’s risk for QTc prolongation based on that person’s current condition and medical history.5 They recommend using the QTc prolonging risk point system developed by Tisdale and colleagues, which identified patient risk factors for elevated QTc intervals based on EKG findings in cardiac care units at a large tertiary hospital center.6
Based on the patient’s demographics, current condition, and medication list, the score can be used to stratify patients into low-, medium-, and high-risk categories (see Table 1). 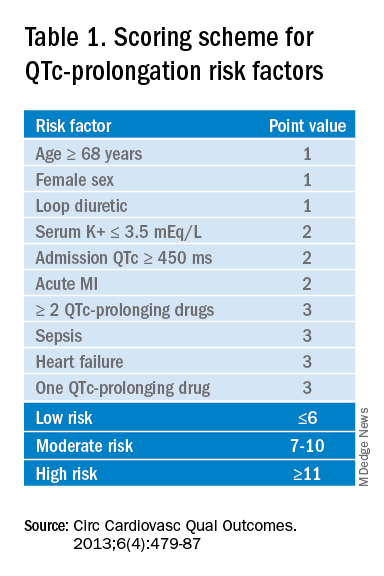
Risk factors include age over 68 years, female sex, prior MI, concurrent use of other QTc prolonging medications, and sepsis, all of which have differing ability to cause QTc prolongation and thus are weighted differentially. This scoring system is helpful in identifying high risk patients; however, the review does not include recommendations for management of these patients beyond removing the offending drug or monitoring EKGs more aggressively in higher risk patients once identified.6
Low-risk patients can be managed expectantly. If the baseline QTc is < 500 ms, then the provider may administer the medication, but should obtain follow-up EKG monitoring to ensure the QTc does not rise above 500 ms; if it does, a management change is necessitated. For moderate- to high-risk patients with a baseline QTc > 500 ms, they recommend not administering the medication and consulting a cardiologist. The review does not provide a recommendation on how often EKG monitoring should be performed after prescribing an antipsychotic medication in an inpatient setting.5
A 2018 review article explored patient risk factors for a prolonged QTc in the setting of prescribing potentially QTc prolonging antipsychotics.7 The authors reiterate that QTc prolonging risk factors are important considerations when prescribing antipsychotics that can lead to adverse events, though they note that much of the literature associating antipsychotics with negative outcomes consists of case reports in which patients had independent risk factors for development of TdP, such as preexisting ventricular arrhythmias.
In addition, the data regarding the risk of each individual antipsychotic agent are not comprehensive. Some medications that have been deemed “QTc prolonging” were identified as such in only a handful of cases where patients had confounding comorbid risk factors. This raises concern that some medications are being unduly stigmatized in situations where there is little chance of TdP. If there is no equivalent or alternate treatment available, this may lead to an antipsychotic medication being held unnecessarily, which may exacerbate the psychiatric illness.
The authors note that the trend toward ordering baseline EKGs in the inpatient setting following administration of a new antipsychotic may be partly attributed to the ready availability of EKG testing in hospitals. They recommend a baseline EKG to assess the patient’s risk. For most agents, they recommend no further EKG monitoring unless there is a change in patient risk factors. Follow-up EKGs should be done in patients with multiple or significant risk factors to assess their QTc stability. In patients with a QTc > 500 ms on a follow-up EKG, daily monitoring is encouraged alongside reassessment of the current treatment regimen.7
Overall, the current literature suggests that providers should know which antipsychotics carry a risk for QTc prolongation and what other treatment options are available. The risk of QTc prolongation for common antipsychotic agents is provided in Table 2.
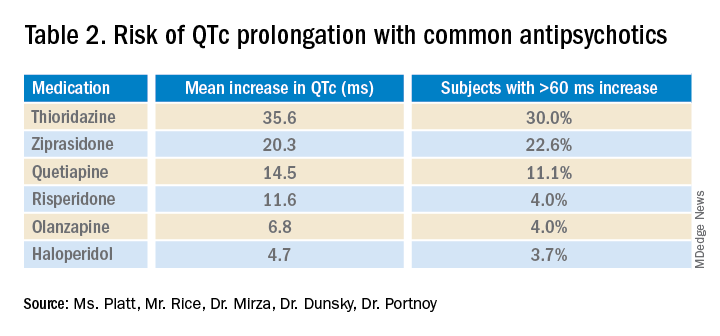
Providers should assess their patients’ risk factors for QTc prolongation and order a baseline EKG to help quantify the cardiac risk associated with prescribing the drug. In patients with many risk factors or complicated medication regimens, a follow-up EKG should be performed to assess the new QTc baseline. If the subsequent QTc is > 500 ms, then an alternative medication should be strongly considered. The majority of patients, however, will not have a clinically significant increase in their QTc, in which case there is no need for a change in medication and monitoring frequency can be deescalated.
Application of data to the case
Our 88-year-old patient has multiple risk factors for a prolonged QTc, and according to the Tisdale scoring system is at moderate risk (7-10 points). Her risk of developing TdP increases with the addition of IV haloperidol to her regimen.
Because of her increased risk, it is reasonable to consider alternative management. If she can cooperate with PO medications, then olanzapine could be given, which has a lesser effect on the QTc interval. If unable to take oral medications, she could be given haloperidol intramuscularly, which causes less QTc prolongation than the IV formulation. If an antipsychotic is administered, she should receive EKG monitoring.
Given the lack of evidence on the optimal monitoring strategy, a protocol should be utilized that balances the ability to capture a clinically meaningful increase in the QTc with appropriate stewardship of resources. Our practice is to initially monitor the EKG every 3 days in moderate- to high-risk patients with baseline QTc < 500 ms. If the QTc remains below 500 ms over three EKGs, then treatment may continue with EKG monitoring weekly while the patient is hospitalized. If the QTc rises above 500 ms, then a management change would be indicated (either dose reduction or a change of agents). If antipsychotic medications are continued, we check the EKG daily while the QTc is >500 ms until there are three unchanged EKGS, and then consider deescalating monitoring to every 3 days.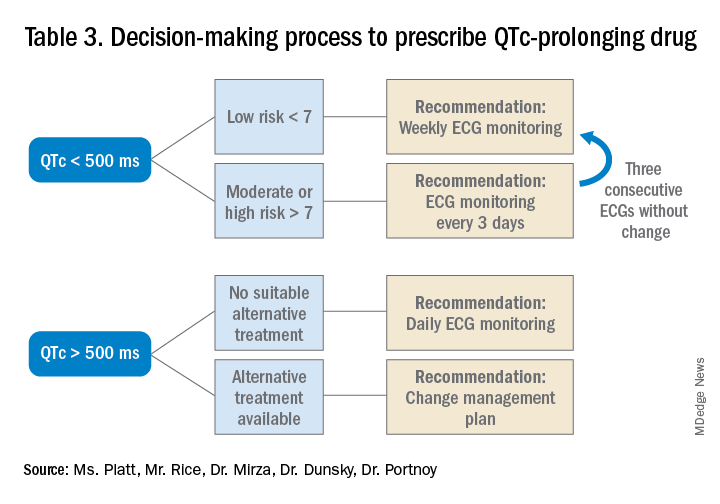
Bottom line
Prior to prescribing, perform a baseline EKG and assess the patient’s risk of QTc prolongation. If the patient is at increased risk, avoid prescribing QTc prolonging medications where alternatives exist. If a QTc prolonging medication is used in a patient with a moderate- to high-risk score, check an EKG every 3 days or daily if the QTc increases to > 500 ms.
Ms. Platt is a medical student at the Icahn School of Medicine at Mount Sinai in New York. Mr. Rice is a medical student at the Icahn School of Medicine. Dr. Mirza is assistant clinical professor of psychiatry at the Icahn School of Medicine. Dr. Dunsky is a cardiologist and assistant professor at the Icahn School of Medicine. Dr. Portnoy is a hospitalist and assistant professor at the Icahn School of Medicine.
References
1. Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Supplements. 2001;3(suppl_K):K70-K80. doi: 10.1016/S1520-765X(01)90009-4.
2. Schwartz PJ, Woosley RL. Predicting the unpredictable: Drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. 2016;67(13):1639-50. doi: 10.1016/j.jacc.2015.12.063.
3. Rautaharju PM et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009 Mar 17;53(11):982-91. doi: 10.1016/j.jacc.2008.12.014.
4. Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
5. Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
6. Tisdale JE et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479-87. doi: 10.1161/CIRCOUTCOMES.113.000152.
7. Beach SR et al. QT prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Key points
- An increased QTc interval can lead to TdP, ventricular fibrillation and cardiac death.
- The relative risk of each antipsychotic medication should be determined based on available data and the Tisdale scoring system can provide a system to assess a patient’s risk of QTc prolongation.
- Low-risk patients with a baseline QTc <500 ms should receive a baseline EKG and inpatient EKG monitoring weekly while moderate- to high-risk patients should receive EKG monitoring every 3 days.
- A QTc > 500 ms suggests the need for a management change (drug discontinuation, dose reduction, or a switch to another agent). If the antipsychotic is absolutely necessary, perform daily EKG monitoring until there are three unchanged EKGs, and then consider deescalating monitoring to every 3 days.
Additional reading
Beach SR et al. QT Prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
Quiz
A 70-year-old male inpatient on furosemide with last known potassium level of 3.3 is going to be started on olanzapine. His baseline EKG has a QTc of 470 ms.
How often should he receive EKG monitoring?
A. Daily
B. Every 3 days
C. Weekly
D. Monthly
Answer (C): He is a low risk patient (6 points: over 70 yrs, loop diuretic, K+< 3.5, QTc > 450 ms), so he should receive weekly EKG monitoring.
Determining relative risk with available data
Determining relative risk with available data
Case
An 88-year-old woman with history of osteoporosis, hyperlipidemia, and a remote myocardial infarction presents to the ED with altered mental status and agitation. The patient is admitted to the medicine service for further management. Her current medications include a thiazide and a statin. Psychiatry is consulted and recommends administering intravenous haloperidol. A baseline EKG shows a corrected QT interval (QTc) of 486 milliseconds (ms). How often should subsequent EKGs be ordered?
Overview of issue
A prolonged QT interval can predispose a patient to dangerous arrhythmias such as Torsades de pointes (TdP), which results in sudden cardiac death in about 10% of cases.1,2 A prolonged QTc interval can be caused by cardiac, renal, or hepatic dysfunction; congenital Long QT Syndrome (LQTS)2; electrolyte abnormalities; or as a result of many drugs, including most antipsychotic medications such as quetiapine, olanzapine, risperidone and haloperidol.
To diminish risk of TdP while taking these medications, it is necessary to monitor the QTc interval. Before commencing a QT-prolonging medication, it is recommended to get a baseline EKG, then perform EKG monitoring after administering the medication.
According to American Heart Association guidelines, a prolonged QT interval is considered more than 460 ms in women or above 450 ms in men.3 If an abnormal rhythm and/or prolonged QTc is detected via EKG monitoring, then the drug dosage can be changed or an alternative therapy selected.4 However, there are no current guidelines recommending how often EKG monitoring should be performed after a QT-prolonging antipsychotic medication is administered on an inpatient medicine unit. Without guidelines, there is potential for health care providers to under- or over-order EKG monitoring, possibly putting patients at risk of TdP or wasting hospital resources, respectively.
Overview of the data
There are currently no universally accepted guidelines regarding inpatient EKG monitoring for patients started on QTc prolonging antipsychotic medications. A 2018 review of the literature surrounding assessment and management of patients on QTc prolonging medications was performed to analyze the available data and make recommendations; notably the evidence was limited as none of the studies were randomized controlled trials.
The authors recommend assessing the drug for QTc prolonging potential, and if possible, choosing alternative treatment in patients with baseline prolonged QTc. If the QT prolonging medication is the best or only option, then the next step is assessing the patient’s risk for QTc prolongation based on that person’s current condition and medical history.5 They recommend using the QTc prolonging risk point system developed by Tisdale and colleagues, which identified patient risk factors for elevated QTc intervals based on EKG findings in cardiac care units at a large tertiary hospital center.6
Based on the patient’s demographics, current condition, and medication list, the score can be used to stratify patients into low-, medium-, and high-risk categories (see Table 1). 
Risk factors include age over 68 years, female sex, prior MI, concurrent use of other QTc prolonging medications, and sepsis, all of which have differing ability to cause QTc prolongation and thus are weighted differentially. This scoring system is helpful in identifying high risk patients; however, the review does not include recommendations for management of these patients beyond removing the offending drug or monitoring EKGs more aggressively in higher risk patients once identified.6
Low-risk patients can be managed expectantly. If the baseline QTc is < 500 ms, then the provider may administer the medication, but should obtain follow-up EKG monitoring to ensure the QTc does not rise above 500 ms; if it does, a management change is necessitated. For moderate- to high-risk patients with a baseline QTc > 500 ms, they recommend not administering the medication and consulting a cardiologist. The review does not provide a recommendation on how often EKG monitoring should be performed after prescribing an antipsychotic medication in an inpatient setting.5
A 2018 review article explored patient risk factors for a prolonged QTc in the setting of prescribing potentially QTc prolonging antipsychotics.7 The authors reiterate that QTc prolonging risk factors are important considerations when prescribing antipsychotics that can lead to adverse events, though they note that much of the literature associating antipsychotics with negative outcomes consists of case reports in which patients had independent risk factors for development of TdP, such as preexisting ventricular arrhythmias.
In addition, the data regarding the risk of each individual antipsychotic agent are not comprehensive. Some medications that have been deemed “QTc prolonging” were identified as such in only a handful of cases where patients had confounding comorbid risk factors. This raises concern that some medications are being unduly stigmatized in situations where there is little chance of TdP. If there is no equivalent or alternate treatment available, this may lead to an antipsychotic medication being held unnecessarily, which may exacerbate the psychiatric illness.
The authors note that the trend toward ordering baseline EKGs in the inpatient setting following administration of a new antipsychotic may be partly attributed to the ready availability of EKG testing in hospitals. They recommend a baseline EKG to assess the patient’s risk. For most agents, they recommend no further EKG monitoring unless there is a change in patient risk factors. Follow-up EKGs should be done in patients with multiple or significant risk factors to assess their QTc stability. In patients with a QTc > 500 ms on a follow-up EKG, daily monitoring is encouraged alongside reassessment of the current treatment regimen.7
Overall, the current literature suggests that providers should know which antipsychotics carry a risk for QTc prolongation and what other treatment options are available. The risk of QTc prolongation for common antipsychotic agents is provided in Table 2.

Providers should assess their patients’ risk factors for QTc prolongation and order a baseline EKG to help quantify the cardiac risk associated with prescribing the drug. In patients with many risk factors or complicated medication regimens, a follow-up EKG should be performed to assess the new QTc baseline. If the subsequent QTc is > 500 ms, then an alternative medication should be strongly considered. The majority of patients, however, will not have a clinically significant increase in their QTc, in which case there is no need for a change in medication and monitoring frequency can be deescalated.
Application of data to the case
Our 88-year-old patient has multiple risk factors for a prolonged QTc, and according to the Tisdale scoring system is at moderate risk (7-10 points). Her risk of developing TdP increases with the addition of IV haloperidol to her regimen.
Because of her increased risk, it is reasonable to consider alternative management. If she can cooperate with PO medications, then olanzapine could be given, which has a lesser effect on the QTc interval. If unable to take oral medications, she could be given haloperidol intramuscularly, which causes less QTc prolongation than the IV formulation. If an antipsychotic is administered, she should receive EKG monitoring.
Given the lack of evidence on the optimal monitoring strategy, a protocol should be utilized that balances the ability to capture a clinically meaningful increase in the QTc with appropriate stewardship of resources. Our practice is to initially monitor the EKG every 3 days in moderate- to high-risk patients with baseline QTc < 500 ms. If the QTc remains below 500 ms over three EKGs, then treatment may continue with EKG monitoring weekly while the patient is hospitalized. If the QTc rises above 500 ms, then a management change would be indicated (either dose reduction or a change of agents). If antipsychotic medications are continued, we check the EKG daily while the QTc is >500 ms until there are three unchanged EKGS, and then consider deescalating monitoring to every 3 days.
Bottom line
Prior to prescribing, perform a baseline EKG and assess the patient’s risk of QTc prolongation. If the patient is at increased risk, avoid prescribing QTc prolonging medications where alternatives exist. If a QTc prolonging medication is used in a patient with a moderate- to high-risk score, check an EKG every 3 days or daily if the QTc increases to > 500 ms.
Ms. Platt is a medical student at the Icahn School of Medicine at Mount Sinai in New York. Mr. Rice is a medical student at the Icahn School of Medicine. Dr. Mirza is assistant clinical professor of psychiatry at the Icahn School of Medicine. Dr. Dunsky is a cardiologist and assistant professor at the Icahn School of Medicine. Dr. Portnoy is a hospitalist and assistant professor at the Icahn School of Medicine.
References
1. Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Supplements. 2001;3(suppl_K):K70-K80. doi: 10.1016/S1520-765X(01)90009-4.
2. Schwartz PJ, Woosley RL. Predicting the unpredictable: Drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. 2016;67(13):1639-50. doi: 10.1016/j.jacc.2015.12.063.
3. Rautaharju PM et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009 Mar 17;53(11):982-91. doi: 10.1016/j.jacc.2008.12.014.
4. Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
5. Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
6. Tisdale JE et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479-87. doi: 10.1161/CIRCOUTCOMES.113.000152.
7. Beach SR et al. QT prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Key points
- An increased QTc interval can lead to TdP, ventricular fibrillation and cardiac death.
- The relative risk of each antipsychotic medication should be determined based on available data and the Tisdale scoring system can provide a system to assess a patient’s risk of QTc prolongation.
- Low-risk patients with a baseline QTc <500 ms should receive a baseline EKG and inpatient EKG monitoring weekly while moderate- to high-risk patients should receive EKG monitoring every 3 days.
- A QTc > 500 ms suggests the need for a management change (drug discontinuation, dose reduction, or a switch to another agent). If the antipsychotic is absolutely necessary, perform daily EKG monitoring until there are three unchanged EKGs, and then consider deescalating monitoring to every 3 days.
Additional reading
Beach SR et al. QT Prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
Quiz
A 70-year-old male inpatient on furosemide with last known potassium level of 3.3 is going to be started on olanzapine. His baseline EKG has a QTc of 470 ms.
How often should he receive EKG monitoring?
A. Daily
B. Every 3 days
C. Weekly
D. Monthly
Answer (C): He is a low risk patient (6 points: over 70 yrs, loop diuretic, K+< 3.5, QTc > 450 ms), so he should receive weekly EKG monitoring.
Case
An 88-year-old woman with history of osteoporosis, hyperlipidemia, and a remote myocardial infarction presents to the ED with altered mental status and agitation. The patient is admitted to the medicine service for further management. Her current medications include a thiazide and a statin. Psychiatry is consulted and recommends administering intravenous haloperidol. A baseline EKG shows a corrected QT interval (QTc) of 486 milliseconds (ms). How often should subsequent EKGs be ordered?
Overview of issue
A prolonged QT interval can predispose a patient to dangerous arrhythmias such as Torsades de pointes (TdP), which results in sudden cardiac death in about 10% of cases.1,2 A prolonged QTc interval can be caused by cardiac, renal, or hepatic dysfunction; congenital Long QT Syndrome (LQTS)2; electrolyte abnormalities; or as a result of many drugs, including most antipsychotic medications such as quetiapine, olanzapine, risperidone and haloperidol.
To diminish risk of TdP while taking these medications, it is necessary to monitor the QTc interval. Before commencing a QT-prolonging medication, it is recommended to get a baseline EKG, then perform EKG monitoring after administering the medication.
According to American Heart Association guidelines, a prolonged QT interval is considered more than 460 ms in women or above 450 ms in men.3 If an abnormal rhythm and/or prolonged QTc is detected via EKG monitoring, then the drug dosage can be changed or an alternative therapy selected.4 However, there are no current guidelines recommending how often EKG monitoring should be performed after a QT-prolonging antipsychotic medication is administered on an inpatient medicine unit. Without guidelines, there is potential for health care providers to under- or over-order EKG monitoring, possibly putting patients at risk of TdP or wasting hospital resources, respectively.
Overview of the data
There are currently no universally accepted guidelines regarding inpatient EKG monitoring for patients started on QTc prolonging antipsychotic medications. A 2018 review of the literature surrounding assessment and management of patients on QTc prolonging medications was performed to analyze the available data and make recommendations; notably the evidence was limited as none of the studies were randomized controlled trials.
The authors recommend assessing the drug for QTc prolonging potential, and if possible, choosing alternative treatment in patients with baseline prolonged QTc. If the QT prolonging medication is the best or only option, then the next step is assessing the patient’s risk for QTc prolongation based on that person’s current condition and medical history.5 They recommend using the QTc prolonging risk point system developed by Tisdale and colleagues, which identified patient risk factors for elevated QTc intervals based on EKG findings in cardiac care units at a large tertiary hospital center.6
Based on the patient’s demographics, current condition, and medication list, the score can be used to stratify patients into low-, medium-, and high-risk categories (see Table 1). 
Risk factors include age over 68 years, female sex, prior MI, concurrent use of other QTc prolonging medications, and sepsis, all of which have differing ability to cause QTc prolongation and thus are weighted differentially. This scoring system is helpful in identifying high risk patients; however, the review does not include recommendations for management of these patients beyond removing the offending drug or monitoring EKGs more aggressively in higher risk patients once identified.6
Low-risk patients can be managed expectantly. If the baseline QTc is < 500 ms, then the provider may administer the medication, but should obtain follow-up EKG monitoring to ensure the QTc does not rise above 500 ms; if it does, a management change is necessitated. For moderate- to high-risk patients with a baseline QTc > 500 ms, they recommend not administering the medication and consulting a cardiologist. The review does not provide a recommendation on how often EKG monitoring should be performed after prescribing an antipsychotic medication in an inpatient setting.5
A 2018 review article explored patient risk factors for a prolonged QTc in the setting of prescribing potentially QTc prolonging antipsychotics.7 The authors reiterate that QTc prolonging risk factors are important considerations when prescribing antipsychotics that can lead to adverse events, though they note that much of the literature associating antipsychotics with negative outcomes consists of case reports in which patients had independent risk factors for development of TdP, such as preexisting ventricular arrhythmias.
In addition, the data regarding the risk of each individual antipsychotic agent are not comprehensive. Some medications that have been deemed “QTc prolonging” were identified as such in only a handful of cases where patients had confounding comorbid risk factors. This raises concern that some medications are being unduly stigmatized in situations where there is little chance of TdP. If there is no equivalent or alternate treatment available, this may lead to an antipsychotic medication being held unnecessarily, which may exacerbate the psychiatric illness.
The authors note that the trend toward ordering baseline EKGs in the inpatient setting following administration of a new antipsychotic may be partly attributed to the ready availability of EKG testing in hospitals. They recommend a baseline EKG to assess the patient’s risk. For most agents, they recommend no further EKG monitoring unless there is a change in patient risk factors. Follow-up EKGs should be done in patients with multiple or significant risk factors to assess their QTc stability. In patients with a QTc > 500 ms on a follow-up EKG, daily monitoring is encouraged alongside reassessment of the current treatment regimen.7
Overall, the current literature suggests that providers should know which antipsychotics carry a risk for QTc prolongation and what other treatment options are available. The risk of QTc prolongation for common antipsychotic agents is provided in Table 2.

Providers should assess their patients’ risk factors for QTc prolongation and order a baseline EKG to help quantify the cardiac risk associated with prescribing the drug. In patients with many risk factors or complicated medication regimens, a follow-up EKG should be performed to assess the new QTc baseline. If the subsequent QTc is > 500 ms, then an alternative medication should be strongly considered. The majority of patients, however, will not have a clinically significant increase in their QTc, in which case there is no need for a change in medication and monitoring frequency can be deescalated.
Application of data to the case
Our 88-year-old patient has multiple risk factors for a prolonged QTc, and according to the Tisdale scoring system is at moderate risk (7-10 points). Her risk of developing TdP increases with the addition of IV haloperidol to her regimen.
Because of her increased risk, it is reasonable to consider alternative management. If she can cooperate with PO medications, then olanzapine could be given, which has a lesser effect on the QTc interval. If unable to take oral medications, she could be given haloperidol intramuscularly, which causes less QTc prolongation than the IV formulation. If an antipsychotic is administered, she should receive EKG monitoring.
Given the lack of evidence on the optimal monitoring strategy, a protocol should be utilized that balances the ability to capture a clinically meaningful increase in the QTc with appropriate stewardship of resources. Our practice is to initially monitor the EKG every 3 days in moderate- to high-risk patients with baseline QTc < 500 ms. If the QTc remains below 500 ms over three EKGs, then treatment may continue with EKG monitoring weekly while the patient is hospitalized. If the QTc rises above 500 ms, then a management change would be indicated (either dose reduction or a change of agents). If antipsychotic medications are continued, we check the EKG daily while the QTc is >500 ms until there are three unchanged EKGS, and then consider deescalating monitoring to every 3 days.
Bottom line
Prior to prescribing, perform a baseline EKG and assess the patient’s risk of QTc prolongation. If the patient is at increased risk, avoid prescribing QTc prolonging medications where alternatives exist. If a QTc prolonging medication is used in a patient with a moderate- to high-risk score, check an EKG every 3 days or daily if the QTc increases to > 500 ms.
Ms. Platt is a medical student at the Icahn School of Medicine at Mount Sinai in New York. Mr. Rice is a medical student at the Icahn School of Medicine. Dr. Mirza is assistant clinical professor of psychiatry at the Icahn School of Medicine. Dr. Dunsky is a cardiologist and assistant professor at the Icahn School of Medicine. Dr. Portnoy is a hospitalist and assistant professor at the Icahn School of Medicine.
References
1. Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Supplements. 2001;3(suppl_K):K70-K80. doi: 10.1016/S1520-765X(01)90009-4.
2. Schwartz PJ, Woosley RL. Predicting the unpredictable: Drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. 2016;67(13):1639-50. doi: 10.1016/j.jacc.2015.12.063.
3. Rautaharju PM et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009 Mar 17;53(11):982-91. doi: 10.1016/j.jacc.2008.12.014.
4. Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
5. Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
6. Tisdale JE et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479-87. doi: 10.1161/CIRCOUTCOMES.113.000152.
7. Beach SR et al. QT prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Key points
- An increased QTc interval can lead to TdP, ventricular fibrillation and cardiac death.
- The relative risk of each antipsychotic medication should be determined based on available data and the Tisdale scoring system can provide a system to assess a patient’s risk of QTc prolongation.
- Low-risk patients with a baseline QTc <500 ms should receive a baseline EKG and inpatient EKG monitoring weekly while moderate- to high-risk patients should receive EKG monitoring every 3 days.
- A QTc > 500 ms suggests the need for a management change (drug discontinuation, dose reduction, or a switch to another agent). If the antipsychotic is absolutely necessary, perform daily EKG monitoring until there are three unchanged EKGs, and then consider deescalating monitoring to every 3 days.
Additional reading
Beach SR et al. QT Prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
Quiz
A 70-year-old male inpatient on furosemide with last known potassium level of 3.3 is going to be started on olanzapine. His baseline EKG has a QTc of 470 ms.
How often should he receive EKG monitoring?
A. Daily
B. Every 3 days
C. Weekly
D. Monthly
Answer (C): He is a low risk patient (6 points: over 70 yrs, loop diuretic, K+< 3.5, QTc > 450 ms), so he should receive weekly EKG monitoring.
Navigating challenges in COVID-19 care
Early strategies for adapting to a moving target
During the early months of the COVID-19 pandemic, hospital groups and systems scrambled to create protocols and models to respond to the novel coronavirus. In the pre-pandemic world, hospital groups have traditionally focused on standardizing clinical protocols and care models that rely on evidence-based medical practices or extended experience.
During COVID-19, however, our team at Dell Medical School needed to rapidly and iteratively standardize care based on evolving science, effectively communicate that approach across rotating hospital medicine physicians and residents, and update care models, workflows, and technology every few days. In this article, we review our initial experiences, describe the strategies we employed to respond to these challenges, and reflect on the lessons learned and our proposed strategy moving forward.
Early pandemic challenges
Our initial inpatient strategies focused on containment, infection prevention, and bracing ourselves rather than creating a COVID Center of Excellence (COE). In fact, our hospital network’s initial strategy was to have COVID-19 patients transferred to a different hospital within our network. However, as March progressed, we became the designated COVID-19 hospital in our area’s network because of the increasing volume of patients we saw.
Patients from the surrounding regional hospitals were transferring their COVID-19 patients to us and we quickly saw the wide spectrum of illness, ranging from mild pneumonia to severe disease requiring mechanical ventilation upon admission. All frontline providers felt the stress of needing to find treatment options quickly for our sickest patients. We realized that to provide safe, effective, and high-quality care to COVID-19 patients, we needed to create a sustainable and standardized interdisciplinary approach.
COVID-19 testing was a major challenge when the pandemic hit as testing kits and personal protective equipment were in limited supply. How would we choose who to test or empirically place in COVID-19 isolation? In addition, we faced questions surrounding safe discharge practices, especially for patients who could not self-isolate in their home (if they even had one).
In March, emergency use authorization (EUA) for hydroxychloroquine was granted by the U.S. FDA despite limited data. This resulted in pressure from the public to use this drug in our patients. At the same time, we saw that some patients quickly got better on their own with supportive care. As clinicians striving to practice evidence-based medicine, we certainly did not want to give patients an unproven therapy that could do more harm than good. We also felt the need to respond with statements about what we could do that worked – rather than negotiate about withholding certain treatments featured in the news. Clearly, a “one-size-fits-all” approach to therapeutics was not going to work in treating patients with COVID-19.
We realized we were going to have to learn and adapt together – quickly. It became apparent that we needed to create structures to rapidly adjudicate and integrate emerging science into standardized clinical care delivery.
Solutions in the form of better structures
In response to these challenges, we created early morning meetings or “huddles” among COVID-19 care teams and hospital administration. A designated “COVID ID” physician from Infectious Diseases would meet with hospitalist and critical care teams each morning in our daily huddles to review all newly admitted patients, current hospitalized patients, and patients with pending COVID-19 tests or suspected initial false-negative tests.
Together, and via the newly developed Therapeutics and Informatics Committee, we created early treatment recommendations based upon available evidence, treatment availability, and the patient’s severity of illness. Within the first ten days of admitting our first patient, it had become standard practice to review eligible patients soon after admission for therapies such as convalescent plasma, and, later, remdesivir and steroids.
We codified these consensus recommendations and processes in our Dell Med COVID Manual, a living document that was frequently updated and disseminated to our group. It created a single ‘true north’ of standardized workflows for triage, diagnosis, management, discharge coordination, and end-of-life care. The document allowed for continuous and asynchronous multi-person collaboration and extremely rapid cycles of improvement. Between March and December 2020, this 100-page handbook went through more than 130 iterations.
Strategy for the future
This approach – communicating frequently, adapting on a daily to weekly basis, and continuously scanning the science for opportunities to improve our care delivery – became the foundation of our approach and the Therapeutics and Informatics Committee. Just as importantly, this created a culture of engagement, collaboration, and shared problem-solving that helped us stay organized, keep up to date with the latest science, and innovate rather than panic when faced with ongoing unpredictability and chaos in the early days of the pandemic.
As the pandemic enters into its 13th month, we carry this foundation and our strategies forward. The infrastructure and systems of communication that we have set in place will allow us to be nimble in our response as COVID-19 numbers surge in our region.
Dr. Gandhi is an assistant professor in the department of internal medicine at Dell Medical School, University of Texas, Austin. Dr. Mondy is chief of the division of infectious disease and associate professor in the department of internal medicine at Dell Medical School. Dr. Busch and Dr. Brode are assistant professors in the department of internal medicine at Dell Medical School. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
Early strategies for adapting to a moving target
Early strategies for adapting to a moving target
During the early months of the COVID-19 pandemic, hospital groups and systems scrambled to create protocols and models to respond to the novel coronavirus. In the pre-pandemic world, hospital groups have traditionally focused on standardizing clinical protocols and care models that rely on evidence-based medical practices or extended experience.
During COVID-19, however, our team at Dell Medical School needed to rapidly and iteratively standardize care based on evolving science, effectively communicate that approach across rotating hospital medicine physicians and residents, and update care models, workflows, and technology every few days. In this article, we review our initial experiences, describe the strategies we employed to respond to these challenges, and reflect on the lessons learned and our proposed strategy moving forward.
Early pandemic challenges
Our initial inpatient strategies focused on containment, infection prevention, and bracing ourselves rather than creating a COVID Center of Excellence (COE). In fact, our hospital network’s initial strategy was to have COVID-19 patients transferred to a different hospital within our network. However, as March progressed, we became the designated COVID-19 hospital in our area’s network because of the increasing volume of patients we saw.
Patients from the surrounding regional hospitals were transferring their COVID-19 patients to us and we quickly saw the wide spectrum of illness, ranging from mild pneumonia to severe disease requiring mechanical ventilation upon admission. All frontline providers felt the stress of needing to find treatment options quickly for our sickest patients. We realized that to provide safe, effective, and high-quality care to COVID-19 patients, we needed to create a sustainable and standardized interdisciplinary approach.
COVID-19 testing was a major challenge when the pandemic hit as testing kits and personal protective equipment were in limited supply. How would we choose who to test or empirically place in COVID-19 isolation? In addition, we faced questions surrounding safe discharge practices, especially for patients who could not self-isolate in their home (if they even had one).
In March, emergency use authorization (EUA) for hydroxychloroquine was granted by the U.S. FDA despite limited data. This resulted in pressure from the public to use this drug in our patients. At the same time, we saw that some patients quickly got better on their own with supportive care. As clinicians striving to practice evidence-based medicine, we certainly did not want to give patients an unproven therapy that could do more harm than good. We also felt the need to respond with statements about what we could do that worked – rather than negotiate about withholding certain treatments featured in the news. Clearly, a “one-size-fits-all” approach to therapeutics was not going to work in treating patients with COVID-19.
We realized we were going to have to learn and adapt together – quickly. It became apparent that we needed to create structures to rapidly adjudicate and integrate emerging science into standardized clinical care delivery.
Solutions in the form of better structures
In response to these challenges, we created early morning meetings or “huddles” among COVID-19 care teams and hospital administration. A designated “COVID ID” physician from Infectious Diseases would meet with hospitalist and critical care teams each morning in our daily huddles to review all newly admitted patients, current hospitalized patients, and patients with pending COVID-19 tests or suspected initial false-negative tests.
Together, and via the newly developed Therapeutics and Informatics Committee, we created early treatment recommendations based upon available evidence, treatment availability, and the patient’s severity of illness. Within the first ten days of admitting our first patient, it had become standard practice to review eligible patients soon after admission for therapies such as convalescent plasma, and, later, remdesivir and steroids.
We codified these consensus recommendations and processes in our Dell Med COVID Manual, a living document that was frequently updated and disseminated to our group. It created a single ‘true north’ of standardized workflows for triage, diagnosis, management, discharge coordination, and end-of-life care. The document allowed for continuous and asynchronous multi-person collaboration and extremely rapid cycles of improvement. Between March and December 2020, this 100-page handbook went through more than 130 iterations.
Strategy for the future
This approach – communicating frequently, adapting on a daily to weekly basis, and continuously scanning the science for opportunities to improve our care delivery – became the foundation of our approach and the Therapeutics and Informatics Committee. Just as importantly, this created a culture of engagement, collaboration, and shared problem-solving that helped us stay organized, keep up to date with the latest science, and innovate rather than panic when faced with ongoing unpredictability and chaos in the early days of the pandemic.
As the pandemic enters into its 13th month, we carry this foundation and our strategies forward. The infrastructure and systems of communication that we have set in place will allow us to be nimble in our response as COVID-19 numbers surge in our region.
Dr. Gandhi is an assistant professor in the department of internal medicine at Dell Medical School, University of Texas, Austin. Dr. Mondy is chief of the division of infectious disease and associate professor in the department of internal medicine at Dell Medical School. Dr. Busch and Dr. Brode are assistant professors in the department of internal medicine at Dell Medical School. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
During the early months of the COVID-19 pandemic, hospital groups and systems scrambled to create protocols and models to respond to the novel coronavirus. In the pre-pandemic world, hospital groups have traditionally focused on standardizing clinical protocols and care models that rely on evidence-based medical practices or extended experience.
During COVID-19, however, our team at Dell Medical School needed to rapidly and iteratively standardize care based on evolving science, effectively communicate that approach across rotating hospital medicine physicians and residents, and update care models, workflows, and technology every few days. In this article, we review our initial experiences, describe the strategies we employed to respond to these challenges, and reflect on the lessons learned and our proposed strategy moving forward.
Early pandemic challenges
Our initial inpatient strategies focused on containment, infection prevention, and bracing ourselves rather than creating a COVID Center of Excellence (COE). In fact, our hospital network’s initial strategy was to have COVID-19 patients transferred to a different hospital within our network. However, as March progressed, we became the designated COVID-19 hospital in our area’s network because of the increasing volume of patients we saw.
Patients from the surrounding regional hospitals were transferring their COVID-19 patients to us and we quickly saw the wide spectrum of illness, ranging from mild pneumonia to severe disease requiring mechanical ventilation upon admission. All frontline providers felt the stress of needing to find treatment options quickly for our sickest patients. We realized that to provide safe, effective, and high-quality care to COVID-19 patients, we needed to create a sustainable and standardized interdisciplinary approach.
COVID-19 testing was a major challenge when the pandemic hit as testing kits and personal protective equipment were in limited supply. How would we choose who to test or empirically place in COVID-19 isolation? In addition, we faced questions surrounding safe discharge practices, especially for patients who could not self-isolate in their home (if they even had one).
In March, emergency use authorization (EUA) for hydroxychloroquine was granted by the U.S. FDA despite limited data. This resulted in pressure from the public to use this drug in our patients. At the same time, we saw that some patients quickly got better on their own with supportive care. As clinicians striving to practice evidence-based medicine, we certainly did not want to give patients an unproven therapy that could do more harm than good. We also felt the need to respond with statements about what we could do that worked – rather than negotiate about withholding certain treatments featured in the news. Clearly, a “one-size-fits-all” approach to therapeutics was not going to work in treating patients with COVID-19.
We realized we were going to have to learn and adapt together – quickly. It became apparent that we needed to create structures to rapidly adjudicate and integrate emerging science into standardized clinical care delivery.
Solutions in the form of better structures
In response to these challenges, we created early morning meetings or “huddles” among COVID-19 care teams and hospital administration. A designated “COVID ID” physician from Infectious Diseases would meet with hospitalist and critical care teams each morning in our daily huddles to review all newly admitted patients, current hospitalized patients, and patients with pending COVID-19 tests or suspected initial false-negative tests.
Together, and via the newly developed Therapeutics and Informatics Committee, we created early treatment recommendations based upon available evidence, treatment availability, and the patient’s severity of illness. Within the first ten days of admitting our first patient, it had become standard practice to review eligible patients soon after admission for therapies such as convalescent plasma, and, later, remdesivir and steroids.
We codified these consensus recommendations and processes in our Dell Med COVID Manual, a living document that was frequently updated and disseminated to our group. It created a single ‘true north’ of standardized workflows for triage, diagnosis, management, discharge coordination, and end-of-life care. The document allowed for continuous and asynchronous multi-person collaboration and extremely rapid cycles of improvement. Between March and December 2020, this 100-page handbook went through more than 130 iterations.
Strategy for the future
This approach – communicating frequently, adapting on a daily to weekly basis, and continuously scanning the science for opportunities to improve our care delivery – became the foundation of our approach and the Therapeutics and Informatics Committee. Just as importantly, this created a culture of engagement, collaboration, and shared problem-solving that helped us stay organized, keep up to date with the latest science, and innovate rather than panic when faced with ongoing unpredictability and chaos in the early days of the pandemic.
As the pandemic enters into its 13th month, we carry this foundation and our strategies forward. The infrastructure and systems of communication that we have set in place will allow us to be nimble in our response as COVID-19 numbers surge in our region.
Dr. Gandhi is an assistant professor in the department of internal medicine at Dell Medical School, University of Texas, Austin. Dr. Mondy is chief of the division of infectious disease and associate professor in the department of internal medicine at Dell Medical School. Dr. Busch and Dr. Brode are assistant professors in the department of internal medicine at Dell Medical School. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
Blacks and Hispanics have higher inpatient use for mycosis fungoides
according to an analysis of the 2012-2017 National Inpatient Sample (NIS).
The findings are consistent with prior studies implicating earlier and more severe disease in Black and Hispanic patients, and reinforce the importance of accurate diagnosis and early treatment.
Dermatologists should maintain “a higher index of suspicion for MF in patients with skin of color, as early diagnosis may help mitigate the downstream costs of management,” Justin Choi, BA, a medical student at the University of Illinois at Chicago, said at the annual Skin of Color Society symposium.
Mr. Choi and coinvestigators, led by Shawn Kwatra, MD, of Johns Hopkins University, Baltimore, identified hospital admissions for MF in the NIS for 10,790 White patients, 4,020 Black patients, and 1,615 Hispanic patients over the 5-year period. The inpatient prevalence of MF – the most common variant of primary cutaneous T-cell lymphoma – was highest in these groups.
Black and Hispanic patients who were hospitalized for MF were significantly younger than White patients, with a mean age of 51.7 years and 48.5 years, respectively, compared with 59.9 years (P < .001 in each case). They also had longer lengths of stay: 8.34 days on average for Black patients and 8.88 for Hispanic patients, compared with 6.66 days for White patients (P < .001 and P = .001, respectively).
Hispanic patients accrued the highest costs of care (a mean of $107,242 vs. $64,049, P =.003) and underwent more procedures (a mean of 2.43 vs. 1.93, P = .004) than White patients. Black patients similarly had higher costs associated with their hospital stay (a mean of $75,053 vs. $64,049, P =.042).
In a multivariate linear regression adjusted for age, sex and insurance type, Black race remained significantly associated with a longer LOS than White race, and Hispanic ethnicity with a longer LOS, increased costs, and more procedures than White race.
The NIS is a publicly available, all-payer inpatient care database developed for the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.
Mr. Choi is a dermatology research fellow working under the guidance of Dr. Kwatra.
according to an analysis of the 2012-2017 National Inpatient Sample (NIS).
The findings are consistent with prior studies implicating earlier and more severe disease in Black and Hispanic patients, and reinforce the importance of accurate diagnosis and early treatment.
Dermatologists should maintain “a higher index of suspicion for MF in patients with skin of color, as early diagnosis may help mitigate the downstream costs of management,” Justin Choi, BA, a medical student at the University of Illinois at Chicago, said at the annual Skin of Color Society symposium.
Mr. Choi and coinvestigators, led by Shawn Kwatra, MD, of Johns Hopkins University, Baltimore, identified hospital admissions for MF in the NIS for 10,790 White patients, 4,020 Black patients, and 1,615 Hispanic patients over the 5-year period. The inpatient prevalence of MF – the most common variant of primary cutaneous T-cell lymphoma – was highest in these groups.
Black and Hispanic patients who were hospitalized for MF were significantly younger than White patients, with a mean age of 51.7 years and 48.5 years, respectively, compared with 59.9 years (P < .001 in each case). They also had longer lengths of stay: 8.34 days on average for Black patients and 8.88 for Hispanic patients, compared with 6.66 days for White patients (P < .001 and P = .001, respectively).
Hispanic patients accrued the highest costs of care (a mean of $107,242 vs. $64,049, P =.003) and underwent more procedures (a mean of 2.43 vs. 1.93, P = .004) than White patients. Black patients similarly had higher costs associated with their hospital stay (a mean of $75,053 vs. $64,049, P =.042).
In a multivariate linear regression adjusted for age, sex and insurance type, Black race remained significantly associated with a longer LOS than White race, and Hispanic ethnicity with a longer LOS, increased costs, and more procedures than White race.
The NIS is a publicly available, all-payer inpatient care database developed for the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.
Mr. Choi is a dermatology research fellow working under the guidance of Dr. Kwatra.
according to an analysis of the 2012-2017 National Inpatient Sample (NIS).
The findings are consistent with prior studies implicating earlier and more severe disease in Black and Hispanic patients, and reinforce the importance of accurate diagnosis and early treatment.
Dermatologists should maintain “a higher index of suspicion for MF in patients with skin of color, as early diagnosis may help mitigate the downstream costs of management,” Justin Choi, BA, a medical student at the University of Illinois at Chicago, said at the annual Skin of Color Society symposium.
Mr. Choi and coinvestigators, led by Shawn Kwatra, MD, of Johns Hopkins University, Baltimore, identified hospital admissions for MF in the NIS for 10,790 White patients, 4,020 Black patients, and 1,615 Hispanic patients over the 5-year period. The inpatient prevalence of MF – the most common variant of primary cutaneous T-cell lymphoma – was highest in these groups.
Black and Hispanic patients who were hospitalized for MF were significantly younger than White patients, with a mean age of 51.7 years and 48.5 years, respectively, compared with 59.9 years (P < .001 in each case). They also had longer lengths of stay: 8.34 days on average for Black patients and 8.88 for Hispanic patients, compared with 6.66 days for White patients (P < .001 and P = .001, respectively).
Hispanic patients accrued the highest costs of care (a mean of $107,242 vs. $64,049, P =.003) and underwent more procedures (a mean of 2.43 vs. 1.93, P = .004) than White patients. Black patients similarly had higher costs associated with their hospital stay (a mean of $75,053 vs. $64,049, P =.042).
In a multivariate linear regression adjusted for age, sex and insurance type, Black race remained significantly associated with a longer LOS than White race, and Hispanic ethnicity with a longer LOS, increased costs, and more procedures than White race.
The NIS is a publicly available, all-payer inpatient care database developed for the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.
Mr. Choi is a dermatology research fellow working under the guidance of Dr. Kwatra.
FROM SOC SOCIETY 2021










