User login
Addressing posttraumatic stress disorder in children and adolescents
Luke is a 12-year-old who presents for a well-child visit accompanied by his foster mother. He appears more solemn and taciturn than at previous visits. He is not interested in talking about any topics, including things he enjoys. His foster mother states that he has been more irritable, oppositional, and behaviorally dysregulated over the past 2 months. She also notes that his sleep has been poor. He reports this is because of nightmares and trouble falling asleep. Luke states that he will at times remember seeing his mother being struck by his father and – even when he does not want to – will have thoughts about hiding from his dad after being hit. You learn from the foster mother that he has been residing with her for the past 2 months and that he is now in state custody following significant parental home substance use, witnessing domestic violence, and being physically abused by his father.
The above narrative may sound all too familiar to those in pediatric primary care. You may wonder if there is a potential posttraumatic response to the witnessed trauma, but does the patient meet criteria for a trauma-related disorder? If so, what are the best next steps?
Prevalence of posttraumatic stress disorder in the general pediatric population
According to the 2020 National Survey of Children’s Health, approximately 40% of children age 17 and under report experiencing at least one adverse childhood experience. Within the 12-17 age range, it rises to over 50%.1 Adverse childhood experiences (ACEs) are potentially traumatic events and include items such as experiencing violence/abuse/neglect, witnessing violence in the home or community, having a family member attempt or die by suicide, and other adverse household and environmental situations. The accumulation of these ACEs can lead to long-term adverse emotional, physical, and behavioral outcomes.2
However, adverse childhood experiences do not always translate into PTSD. According to one national survey of 13- to 18-year-olds, the lifetime prevalence of PTSD is notably lower than exposure rates to ACEs and is estimated at 5% of adolescents, with higher rates among females (8%) versus males (2.3%).3
There are various risk factors for the development of PTSD that may play a role including genetic vulnerability, length of the trauma (for example, a one-time event versus repeated trauma for years), characteristics specific to the trauma, and the aftermath of the trauma. Again, it is important to note that not all youth exposed to a traumatic event will develop PTSD. Those who do make up a small percentage of at-risk children.4
Diagnosing PTSD in a child or adolescent
For a pediatric patient to be diagnosed with PTSD according to the DSM-5 criteria, they must experience a potentially traumatic event and meet criteria from four categories of symptoms. Trauma is defined as direct or indirect exposure to actual or threatened death, serious injury, or sexual violence. The four symptom categories are re-experiencing, avoidance, hyperarousal, and negative alteration in cognition and mood. The number of symptoms needed from each category varies based on the child’s age, with differing cutoffs based on whether the child is younger or older than 6 years old. Moreover, symptoms must be present for at least 1 month.5
Trauma can be assessed in the office by using a focused interview that includes the full DSM diagnostic criteria. There are additional trauma rating screeners and assessment tools that can be used including the Child PTSD Symptom Scale, Child Trauma Screening Questionnaire, UCLA Posttraumatic Stress Disorder Reaction Index, and the Trauma Symptom Checklist for Children, to name a few. Many of these allow for multiple informants, including the child/adolescent, thereby allowing for varying perspectives regarding trauma reactions.
Treatment options
Familiarity with evidence-based treatment for trauma may be useful to ensure that referral is targeted for the patient/family. There are no Food and Drug Administrations–approved medications for children with PTSD, though medications can be used to target specific PTSD symptoms (e.g. prazosin for trauma-related nightmares) as well as commonly comorbid conditions such as depression. Becoming familiar with the available therapeutic modalities offered in your area is recommended.
Highlighting trauma-focused cognitive behavioral therapy (TF-CBT)
The treatment with the most research evidence for traumatized children is trauma-focused cognitive behavioral therapy (TF-CBT), which is a 12- to 25-session therapeutic intervention for patients 3-18 years old (with some evidence for young adults as well) with PTSD and/or trauma-related behaviors. TF-CBT uses a components-based treatment model encompassed by the PRACTICE acronym/mnemonic.6,7
- P – Psychoeducation and parenting skills.
- R – Relaxation techniques: Focused breathing, progressive muscle relaxation, and teaching the child to control their thoughts (thought stopping).
- A – Affective expression and regulation (feeling identification): To help the child and parent learn to control their emotional reaction to reminders by expanding their emotional vocabulary, enhancing their skills in identification and expression of emotions, and encouraging self-soothing activities
- C – Cognitive coping and processing: Through this component, the child learns to understand the relationships between thoughts, feelings, and behaviors and think in new and healthier ways.
- T – Trauma narrative and processing: Gradual exposure exercises including verbal, written, and/or symbolic recounting of traumatic event(s) so the child learns to be able to discuss the events when they choose to in ways that do not produce overwhelming emotions. Following the completion of the narrative, clients are supported in identifying, challenging, and correcting cognitive distortions and dysfunctional beliefs.
- I – In vivo exposure: Encourage the gradual exposure to innocuous trauma reminders in the child’s environment so the child learns they can control their emotional reactions to things that remind them of the trauma, starting with nonthreatening examples of reminders.
- C – Conjoint parent/child sessions: Sessions generally deal with psycho-education, sharing the trauma narrative, anxiety management, and correction of cognitive distortions. The family works to enhance communication and create opportunities for therapeutic discussion regarding the trauma.
- E – Enhancing personal safety and future growth: Provide training and education with respect to personal safety skills and healthy sexuality and interpersonal relationships; encourage the utilization of skills learned in managing future stressors and/or trauma reminders.
Of note, some elements of this therapy that could possibly be easily incorporated into a primary care office visit include relaxation techniques and focus on coping skills/strategies.
Summary
Children and adolescents often present with trauma-related symptoms to the primary care office. Having increasing familiarity with PTSD diagnostic criteria and treatment modalities will likely lead to increased confidence and comfort recognizing symptoms and when placing a referral. This may also lead to shorter wait times for receiving targeted treatment and ultimately should lead to better outcomes for affected children and families.
Dr. Abdul-Kareem is at the University of Vermont, Burlington.
References
1. National Survey of Children’s Health (2016 - present). https://nschdata.org/browse/survey.
2. Adverse Childhood Experiences (ACEs). Centers for Disease Control and Prevention. https://www.cdc.gov/violenceprevention/aces/index.html].
3. Post-Traumatic Stress Disorder (PTSD). National Institute of Mental Health. https://www.nimh.nih.gov/health/statistics/post-traumatic-stress-disorder-ptsd,
4. Martin A et al. Lewis’s Child and Adolescent Psychiatry (5th edition). Lippincott Williams & Wilkins: Philadelphia, 2017.
5. American Psychiatric Association. Neurodevelopmental disorders. In: DSM-5. 2013.
6. Trauma-Focused Cognitive Behavioral Therapy. The National Child Traumatic Stress Network. https://www.nctsn.org/interventions/trauma-focused-cognitive-behavioral-therapy.
7. Trauma-Focused Cognitive-Behavioral Therapy (TF-CBT). California Evidence-Based Clearinghouse for Child Welfare. https://www.cebc4cw.org/program/trauma-focused-cognitive-behavioral-therapy/.
Luke is a 12-year-old who presents for a well-child visit accompanied by his foster mother. He appears more solemn and taciturn than at previous visits. He is not interested in talking about any topics, including things he enjoys. His foster mother states that he has been more irritable, oppositional, and behaviorally dysregulated over the past 2 months. She also notes that his sleep has been poor. He reports this is because of nightmares and trouble falling asleep. Luke states that he will at times remember seeing his mother being struck by his father and – even when he does not want to – will have thoughts about hiding from his dad after being hit. You learn from the foster mother that he has been residing with her for the past 2 months and that he is now in state custody following significant parental home substance use, witnessing domestic violence, and being physically abused by his father.
The above narrative may sound all too familiar to those in pediatric primary care. You may wonder if there is a potential posttraumatic response to the witnessed trauma, but does the patient meet criteria for a trauma-related disorder? If so, what are the best next steps?
Prevalence of posttraumatic stress disorder in the general pediatric population
According to the 2020 National Survey of Children’s Health, approximately 40% of children age 17 and under report experiencing at least one adverse childhood experience. Within the 12-17 age range, it rises to over 50%.1 Adverse childhood experiences (ACEs) are potentially traumatic events and include items such as experiencing violence/abuse/neglect, witnessing violence in the home or community, having a family member attempt or die by suicide, and other adverse household and environmental situations. The accumulation of these ACEs can lead to long-term adverse emotional, physical, and behavioral outcomes.2
However, adverse childhood experiences do not always translate into PTSD. According to one national survey of 13- to 18-year-olds, the lifetime prevalence of PTSD is notably lower than exposure rates to ACEs and is estimated at 5% of adolescents, with higher rates among females (8%) versus males (2.3%).3
There are various risk factors for the development of PTSD that may play a role including genetic vulnerability, length of the trauma (for example, a one-time event versus repeated trauma for years), characteristics specific to the trauma, and the aftermath of the trauma. Again, it is important to note that not all youth exposed to a traumatic event will develop PTSD. Those who do make up a small percentage of at-risk children.4
Diagnosing PTSD in a child or adolescent
For a pediatric patient to be diagnosed with PTSD according to the DSM-5 criteria, they must experience a potentially traumatic event and meet criteria from four categories of symptoms. Trauma is defined as direct or indirect exposure to actual or threatened death, serious injury, or sexual violence. The four symptom categories are re-experiencing, avoidance, hyperarousal, and negative alteration in cognition and mood. The number of symptoms needed from each category varies based on the child’s age, with differing cutoffs based on whether the child is younger or older than 6 years old. Moreover, symptoms must be present for at least 1 month.5
Trauma can be assessed in the office by using a focused interview that includes the full DSM diagnostic criteria. There are additional trauma rating screeners and assessment tools that can be used including the Child PTSD Symptom Scale, Child Trauma Screening Questionnaire, UCLA Posttraumatic Stress Disorder Reaction Index, and the Trauma Symptom Checklist for Children, to name a few. Many of these allow for multiple informants, including the child/adolescent, thereby allowing for varying perspectives regarding trauma reactions.
Treatment options
Familiarity with evidence-based treatment for trauma may be useful to ensure that referral is targeted for the patient/family. There are no Food and Drug Administrations–approved medications for children with PTSD, though medications can be used to target specific PTSD symptoms (e.g. prazosin for trauma-related nightmares) as well as commonly comorbid conditions such as depression. Becoming familiar with the available therapeutic modalities offered in your area is recommended.
Highlighting trauma-focused cognitive behavioral therapy (TF-CBT)
The treatment with the most research evidence for traumatized children is trauma-focused cognitive behavioral therapy (TF-CBT), which is a 12- to 25-session therapeutic intervention for patients 3-18 years old (with some evidence for young adults as well) with PTSD and/or trauma-related behaviors. TF-CBT uses a components-based treatment model encompassed by the PRACTICE acronym/mnemonic.6,7
- P – Psychoeducation and parenting skills.
- R – Relaxation techniques: Focused breathing, progressive muscle relaxation, and teaching the child to control their thoughts (thought stopping).
- A – Affective expression and regulation (feeling identification): To help the child and parent learn to control their emotional reaction to reminders by expanding their emotional vocabulary, enhancing their skills in identification and expression of emotions, and encouraging self-soothing activities
- C – Cognitive coping and processing: Through this component, the child learns to understand the relationships between thoughts, feelings, and behaviors and think in new and healthier ways.
- T – Trauma narrative and processing: Gradual exposure exercises including verbal, written, and/or symbolic recounting of traumatic event(s) so the child learns to be able to discuss the events when they choose to in ways that do not produce overwhelming emotions. Following the completion of the narrative, clients are supported in identifying, challenging, and correcting cognitive distortions and dysfunctional beliefs.
- I – In vivo exposure: Encourage the gradual exposure to innocuous trauma reminders in the child’s environment so the child learns they can control their emotional reactions to things that remind them of the trauma, starting with nonthreatening examples of reminders.
- C – Conjoint parent/child sessions: Sessions generally deal with psycho-education, sharing the trauma narrative, anxiety management, and correction of cognitive distortions. The family works to enhance communication and create opportunities for therapeutic discussion regarding the trauma.
- E – Enhancing personal safety and future growth: Provide training and education with respect to personal safety skills and healthy sexuality and interpersonal relationships; encourage the utilization of skills learned in managing future stressors and/or trauma reminders.
Of note, some elements of this therapy that could possibly be easily incorporated into a primary care office visit include relaxation techniques and focus on coping skills/strategies.
Summary
Children and adolescents often present with trauma-related symptoms to the primary care office. Having increasing familiarity with PTSD diagnostic criteria and treatment modalities will likely lead to increased confidence and comfort recognizing symptoms and when placing a referral. This may also lead to shorter wait times for receiving targeted treatment and ultimately should lead to better outcomes for affected children and families.
Dr. Abdul-Kareem is at the University of Vermont, Burlington.
References
1. National Survey of Children’s Health (2016 - present). https://nschdata.org/browse/survey.
2. Adverse Childhood Experiences (ACEs). Centers for Disease Control and Prevention. https://www.cdc.gov/violenceprevention/aces/index.html].
3. Post-Traumatic Stress Disorder (PTSD). National Institute of Mental Health. https://www.nimh.nih.gov/health/statistics/post-traumatic-stress-disorder-ptsd,
4. Martin A et al. Lewis’s Child and Adolescent Psychiatry (5th edition). Lippincott Williams & Wilkins: Philadelphia, 2017.
5. American Psychiatric Association. Neurodevelopmental disorders. In: DSM-5. 2013.
6. Trauma-Focused Cognitive Behavioral Therapy. The National Child Traumatic Stress Network. https://www.nctsn.org/interventions/trauma-focused-cognitive-behavioral-therapy.
7. Trauma-Focused Cognitive-Behavioral Therapy (TF-CBT). California Evidence-Based Clearinghouse for Child Welfare. https://www.cebc4cw.org/program/trauma-focused-cognitive-behavioral-therapy/.
Luke is a 12-year-old who presents for a well-child visit accompanied by his foster mother. He appears more solemn and taciturn than at previous visits. He is not interested in talking about any topics, including things he enjoys. His foster mother states that he has been more irritable, oppositional, and behaviorally dysregulated over the past 2 months. She also notes that his sleep has been poor. He reports this is because of nightmares and trouble falling asleep. Luke states that he will at times remember seeing his mother being struck by his father and – even when he does not want to – will have thoughts about hiding from his dad after being hit. You learn from the foster mother that he has been residing with her for the past 2 months and that he is now in state custody following significant parental home substance use, witnessing domestic violence, and being physically abused by his father.
The above narrative may sound all too familiar to those in pediatric primary care. You may wonder if there is a potential posttraumatic response to the witnessed trauma, but does the patient meet criteria for a trauma-related disorder? If so, what are the best next steps?
Prevalence of posttraumatic stress disorder in the general pediatric population
According to the 2020 National Survey of Children’s Health, approximately 40% of children age 17 and under report experiencing at least one adverse childhood experience. Within the 12-17 age range, it rises to over 50%.1 Adverse childhood experiences (ACEs) are potentially traumatic events and include items such as experiencing violence/abuse/neglect, witnessing violence in the home or community, having a family member attempt or die by suicide, and other adverse household and environmental situations. The accumulation of these ACEs can lead to long-term adverse emotional, physical, and behavioral outcomes.2
However, adverse childhood experiences do not always translate into PTSD. According to one national survey of 13- to 18-year-olds, the lifetime prevalence of PTSD is notably lower than exposure rates to ACEs and is estimated at 5% of adolescents, with higher rates among females (8%) versus males (2.3%).3
There are various risk factors for the development of PTSD that may play a role including genetic vulnerability, length of the trauma (for example, a one-time event versus repeated trauma for years), characteristics specific to the trauma, and the aftermath of the trauma. Again, it is important to note that not all youth exposed to a traumatic event will develop PTSD. Those who do make up a small percentage of at-risk children.4
Diagnosing PTSD in a child or adolescent
For a pediatric patient to be diagnosed with PTSD according to the DSM-5 criteria, they must experience a potentially traumatic event and meet criteria from four categories of symptoms. Trauma is defined as direct or indirect exposure to actual or threatened death, serious injury, or sexual violence. The four symptom categories are re-experiencing, avoidance, hyperarousal, and negative alteration in cognition and mood. The number of symptoms needed from each category varies based on the child’s age, with differing cutoffs based on whether the child is younger or older than 6 years old. Moreover, symptoms must be present for at least 1 month.5
Trauma can be assessed in the office by using a focused interview that includes the full DSM diagnostic criteria. There are additional trauma rating screeners and assessment tools that can be used including the Child PTSD Symptom Scale, Child Trauma Screening Questionnaire, UCLA Posttraumatic Stress Disorder Reaction Index, and the Trauma Symptom Checklist for Children, to name a few. Many of these allow for multiple informants, including the child/adolescent, thereby allowing for varying perspectives regarding trauma reactions.
Treatment options
Familiarity with evidence-based treatment for trauma may be useful to ensure that referral is targeted for the patient/family. There are no Food and Drug Administrations–approved medications for children with PTSD, though medications can be used to target specific PTSD symptoms (e.g. prazosin for trauma-related nightmares) as well as commonly comorbid conditions such as depression. Becoming familiar with the available therapeutic modalities offered in your area is recommended.
Highlighting trauma-focused cognitive behavioral therapy (TF-CBT)
The treatment with the most research evidence for traumatized children is trauma-focused cognitive behavioral therapy (TF-CBT), which is a 12- to 25-session therapeutic intervention for patients 3-18 years old (with some evidence for young adults as well) with PTSD and/or trauma-related behaviors. TF-CBT uses a components-based treatment model encompassed by the PRACTICE acronym/mnemonic.6,7
- P – Psychoeducation and parenting skills.
- R – Relaxation techniques: Focused breathing, progressive muscle relaxation, and teaching the child to control their thoughts (thought stopping).
- A – Affective expression and regulation (feeling identification): To help the child and parent learn to control their emotional reaction to reminders by expanding their emotional vocabulary, enhancing their skills in identification and expression of emotions, and encouraging self-soothing activities
- C – Cognitive coping and processing: Through this component, the child learns to understand the relationships between thoughts, feelings, and behaviors and think in new and healthier ways.
- T – Trauma narrative and processing: Gradual exposure exercises including verbal, written, and/or symbolic recounting of traumatic event(s) so the child learns to be able to discuss the events when they choose to in ways that do not produce overwhelming emotions. Following the completion of the narrative, clients are supported in identifying, challenging, and correcting cognitive distortions and dysfunctional beliefs.
- I – In vivo exposure: Encourage the gradual exposure to innocuous trauma reminders in the child’s environment so the child learns they can control their emotional reactions to things that remind them of the trauma, starting with nonthreatening examples of reminders.
- C – Conjoint parent/child sessions: Sessions generally deal with psycho-education, sharing the trauma narrative, anxiety management, and correction of cognitive distortions. The family works to enhance communication and create opportunities for therapeutic discussion regarding the trauma.
- E – Enhancing personal safety and future growth: Provide training and education with respect to personal safety skills and healthy sexuality and interpersonal relationships; encourage the utilization of skills learned in managing future stressors and/or trauma reminders.
Of note, some elements of this therapy that could possibly be easily incorporated into a primary care office visit include relaxation techniques and focus on coping skills/strategies.
Summary
Children and adolescents often present with trauma-related symptoms to the primary care office. Having increasing familiarity with PTSD diagnostic criteria and treatment modalities will likely lead to increased confidence and comfort recognizing symptoms and when placing a referral. This may also lead to shorter wait times for receiving targeted treatment and ultimately should lead to better outcomes for affected children and families.
Dr. Abdul-Kareem is at the University of Vermont, Burlington.
References
1. National Survey of Children’s Health (2016 - present). https://nschdata.org/browse/survey.
2. Adverse Childhood Experiences (ACEs). Centers for Disease Control and Prevention. https://www.cdc.gov/violenceprevention/aces/index.html].
3. Post-Traumatic Stress Disorder (PTSD). National Institute of Mental Health. https://www.nimh.nih.gov/health/statistics/post-traumatic-stress-disorder-ptsd,
4. Martin A et al. Lewis’s Child and Adolescent Psychiatry (5th edition). Lippincott Williams & Wilkins: Philadelphia, 2017.
5. American Psychiatric Association. Neurodevelopmental disorders. In: DSM-5. 2013.
6. Trauma-Focused Cognitive Behavioral Therapy. The National Child Traumatic Stress Network. https://www.nctsn.org/interventions/trauma-focused-cognitive-behavioral-therapy.
7. Trauma-Focused Cognitive-Behavioral Therapy (TF-CBT). California Evidence-Based Clearinghouse for Child Welfare. https://www.cebc4cw.org/program/trauma-focused-cognitive-behavioral-therapy/.
Social media in the lives of adolescents
Adolescence is a time of growing autonomy fueled by puberty, intellectual development, and identity formation. Social media engages adolescents by giving them easy access to (semi) private communication with peers, the ability to safely explore their sexuality, and easily investigate issues of intellectual curiosity, as they move from childhood to older adolescence. Social media facilitates the creation of a teenager’s own world, separate and distinct from adult concern or scrutiny. It is clearly compelling for adolescents, but we are in the early days of understanding the effect of various types of digital activities on the health and well-being of youth. There is evidence that for some, the addictive potential of these applications is potent, exacerbating or triggering mood, anxiety, and eating disorder symptoms. Their drive to explore their identity and relationships and their immature capacity to regulate emotions and behaviors make the risks of overuse substantial. But it would be impossible (and probably socially very costly) to simply avoid social media. So how to discuss its healthy use with your patients and their parents?
The data
Social media are digital communication platforms that allow users to build a public profile and then accumulate a network of followers, and follow other users, based on shared interests. They include FaceBook, Instagram, Snapchat, YouTube, and Twitter. Surveys demonstrated that 90% of U.S. adolescents use social media, with 75% having at least one social media profile and over half visiting social media sites at least once daily. Adolescents spend over 7 hours daily on their phones, not including time devoted to online schoolwork, and 8- to 12-year-olds are not far behind at almost 5 hours of daily phone use. On average, 39% of adolescent screen time is spent on passive consumption, 26% on social media, 25% on interactive activities (browsing the web, interactive video gaming) and 3% on content creation (coding, etc). There was considerable variability in survey results, and differences between genders, with boys engaged in video games almost eight times as often as girls, and girls in social media nearly twice as often as boys.1
The research
There is a growing body of research devoted to understanding the effects of all of this digital activity on youth health and well-being.
A large, longitudinal study of Canadian 13- to 17-year-olds found that time spent on social media or watching television was strongly associated with depressive and anxiety symptoms, with a robust dose-response relationship.2 However, causality is not clear, as anxious, shy, and depressed adolescents may use more social media as a consequence of their mood. Interestingly, there was no such relationship with mood and anxiety symptoms and time spent on video games.3 For youth with depression and anxiety, time spent on social media has been strongly associated with increased levels of self-reported distress, self-injury and suicidality, but again, causality is hard to prove.
One very large study from the United Kingdom (including more than 10,000 participants), demonstrated a strong relationship between time spent on social media and severity of depressive symptoms, with a more pronounced effect in girls than in boys.4 Many more nuanced studies have demonstrated that excessive time spent on social media, the presence of an addictive pattern of use, and the degree to which an adolescent’s sense of well-being is connected to social media are the variables that strongly predict an association with worsening depressive or anxiety symptoms.5
Several studies have demonstrated that low to moderate use of social media, and use to gather information and make plans were associated with better scores of emotional self-regulation and lower rates of depressive symptoms in teens.6 It seems safe to say that social media can be useful and fun, but that too much can be bad for you. So help your adolescent patients to expand their perspective on its use by discussing it with them.
Make them curious about quantity
Most teens feel they do not have enough time for all of the things they need to do, so invite them to play detective by using their phone’s applications that can track their time spent online and in different apps.
Remind them that these apps were designed to be so engaging that for some addiction is a real problem. As with tobacco, addiction is the business model by which these companies earn advertising dollars. Indeed, adolescents are the target demographic, as they are most sensitive to social rewards and are the most valuable audience for advertisers. Engage their natural suspicion of authority by pointing out that with every hour on Insta, someone else is making a lot of money. They get to choose how they want to relax, connect with friends, and explore the world, so help them to be aware of how these apps are designed to keep them from choosing.
Raise awareness of vulnerability
Adolescents who have attention-deficit/hyperactivity disorder already have difficulty with impulse control and with shifting their attention to less engaging activities. Adolescents with anxiety are prone to avoid stressful situations, but still hunger for knowledge and connections. Adolescents with depression are managing low motivation and self-esteem, and the rewards of social media may keep them from exercise and actual social engagement that are critical to their treatment. Youth with eating disorders are especially prone to critical comparison of themselves to others, feeding their distorted body images. Help your patients with these common illnesses to be aware of how social media may make their treatment harder, rather than being the source of relief it may feel like.
Protect their health
For all young people, too much time spent in virtual activities and passive media consumption may not leave enough time to explore potential interests, talents, or relationships. These are important activities throughout life, but they are the central developmental tasks of adolescence. They also need 8-10 hours of sleep nightly and regular exercise. And of course, they have homework! Help them to think about how to use their time wisely to support satisfying relationships and activities, with time for relaxation and good health.
Keep parents in the room for these discussions
State that most of us have difficulty putting down our phones. Children and teens need adults who model striving for balance in all areas of choice. Just as we try to teach them to make good choices about food, getting excellent nutrition while still valuing taste and pleasure, we can talk about how to balance virtual activities with actual activities, work with play, and effort with relaxation. You can help expand your young patients’ self-awareness, acknowledge the fun and utility of their digital time, and enhance their sense of how we must all learn how to put screens down sometimes. In so doing, you can help families to ensure that they are engaging with the digital tools and toys available to all of us in ways that can support their health and well-being.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Geena Davis Institute on Gender and Media. The Common Sense Census: Media Use by Teens and Tweens, 2015.
2. Abi-Jaoude E et al. CMAJ 2020;192(6):E136-41.
3. Boers E et al. Can J Psychiatry. 2020 Mar;65(3):206-8.
4. Kelly Y et al. EClinicalMedicine. 2019 Jan 4;6:59-68.
5. Vidal C et al. Int Rev Psychiatry. 2020 May;32(3):235-53.
6. Coyne SM et al. J Res Adolescence. 2019;29(4):897-907.
Adolescence is a time of growing autonomy fueled by puberty, intellectual development, and identity formation. Social media engages adolescents by giving them easy access to (semi) private communication with peers, the ability to safely explore their sexuality, and easily investigate issues of intellectual curiosity, as they move from childhood to older adolescence. Social media facilitates the creation of a teenager’s own world, separate and distinct from adult concern or scrutiny. It is clearly compelling for adolescents, but we are in the early days of understanding the effect of various types of digital activities on the health and well-being of youth. There is evidence that for some, the addictive potential of these applications is potent, exacerbating or triggering mood, anxiety, and eating disorder symptoms. Their drive to explore their identity and relationships and their immature capacity to regulate emotions and behaviors make the risks of overuse substantial. But it would be impossible (and probably socially very costly) to simply avoid social media. So how to discuss its healthy use with your patients and their parents?
The data
Social media are digital communication platforms that allow users to build a public profile and then accumulate a network of followers, and follow other users, based on shared interests. They include FaceBook, Instagram, Snapchat, YouTube, and Twitter. Surveys demonstrated that 90% of U.S. adolescents use social media, with 75% having at least one social media profile and over half visiting social media sites at least once daily. Adolescents spend over 7 hours daily on their phones, not including time devoted to online schoolwork, and 8- to 12-year-olds are not far behind at almost 5 hours of daily phone use. On average, 39% of adolescent screen time is spent on passive consumption, 26% on social media, 25% on interactive activities (browsing the web, interactive video gaming) and 3% on content creation (coding, etc). There was considerable variability in survey results, and differences between genders, with boys engaged in video games almost eight times as often as girls, and girls in social media nearly twice as often as boys.1
The research
There is a growing body of research devoted to understanding the effects of all of this digital activity on youth health and well-being.
A large, longitudinal study of Canadian 13- to 17-year-olds found that time spent on social media or watching television was strongly associated with depressive and anxiety symptoms, with a robust dose-response relationship.2 However, causality is not clear, as anxious, shy, and depressed adolescents may use more social media as a consequence of their mood. Interestingly, there was no such relationship with mood and anxiety symptoms and time spent on video games.3 For youth with depression and anxiety, time spent on social media has been strongly associated with increased levels of self-reported distress, self-injury and suicidality, but again, causality is hard to prove.
One very large study from the United Kingdom (including more than 10,000 participants), demonstrated a strong relationship between time spent on social media and severity of depressive symptoms, with a more pronounced effect in girls than in boys.4 Many more nuanced studies have demonstrated that excessive time spent on social media, the presence of an addictive pattern of use, and the degree to which an adolescent’s sense of well-being is connected to social media are the variables that strongly predict an association with worsening depressive or anxiety symptoms.5
Several studies have demonstrated that low to moderate use of social media, and use to gather information and make plans were associated with better scores of emotional self-regulation and lower rates of depressive symptoms in teens.6 It seems safe to say that social media can be useful and fun, but that too much can be bad for you. So help your adolescent patients to expand their perspective on its use by discussing it with them.
Make them curious about quantity
Most teens feel they do not have enough time for all of the things they need to do, so invite them to play detective by using their phone’s applications that can track their time spent online and in different apps.
Remind them that these apps were designed to be so engaging that for some addiction is a real problem. As with tobacco, addiction is the business model by which these companies earn advertising dollars. Indeed, adolescents are the target demographic, as they are most sensitive to social rewards and are the most valuable audience for advertisers. Engage their natural suspicion of authority by pointing out that with every hour on Insta, someone else is making a lot of money. They get to choose how they want to relax, connect with friends, and explore the world, so help them to be aware of how these apps are designed to keep them from choosing.
Raise awareness of vulnerability
Adolescents who have attention-deficit/hyperactivity disorder already have difficulty with impulse control and with shifting their attention to less engaging activities. Adolescents with anxiety are prone to avoid stressful situations, but still hunger for knowledge and connections. Adolescents with depression are managing low motivation and self-esteem, and the rewards of social media may keep them from exercise and actual social engagement that are critical to their treatment. Youth with eating disorders are especially prone to critical comparison of themselves to others, feeding their distorted body images. Help your patients with these common illnesses to be aware of how social media may make their treatment harder, rather than being the source of relief it may feel like.
Protect their health
For all young people, too much time spent in virtual activities and passive media consumption may not leave enough time to explore potential interests, talents, or relationships. These are important activities throughout life, but they are the central developmental tasks of adolescence. They also need 8-10 hours of sleep nightly and regular exercise. And of course, they have homework! Help them to think about how to use their time wisely to support satisfying relationships and activities, with time for relaxation and good health.
Keep parents in the room for these discussions
State that most of us have difficulty putting down our phones. Children and teens need adults who model striving for balance in all areas of choice. Just as we try to teach them to make good choices about food, getting excellent nutrition while still valuing taste and pleasure, we can talk about how to balance virtual activities with actual activities, work with play, and effort with relaxation. You can help expand your young patients’ self-awareness, acknowledge the fun and utility of their digital time, and enhance their sense of how we must all learn how to put screens down sometimes. In so doing, you can help families to ensure that they are engaging with the digital tools and toys available to all of us in ways that can support their health and well-being.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Geena Davis Institute on Gender and Media. The Common Sense Census: Media Use by Teens and Tweens, 2015.
2. Abi-Jaoude E et al. CMAJ 2020;192(6):E136-41.
3. Boers E et al. Can J Psychiatry. 2020 Mar;65(3):206-8.
4. Kelly Y et al. EClinicalMedicine. 2019 Jan 4;6:59-68.
5. Vidal C et al. Int Rev Psychiatry. 2020 May;32(3):235-53.
6. Coyne SM et al. J Res Adolescence. 2019;29(4):897-907.
Adolescence is a time of growing autonomy fueled by puberty, intellectual development, and identity formation. Social media engages adolescents by giving them easy access to (semi) private communication with peers, the ability to safely explore their sexuality, and easily investigate issues of intellectual curiosity, as they move from childhood to older adolescence. Social media facilitates the creation of a teenager’s own world, separate and distinct from adult concern or scrutiny. It is clearly compelling for adolescents, but we are in the early days of understanding the effect of various types of digital activities on the health and well-being of youth. There is evidence that for some, the addictive potential of these applications is potent, exacerbating or triggering mood, anxiety, and eating disorder symptoms. Their drive to explore their identity and relationships and their immature capacity to regulate emotions and behaviors make the risks of overuse substantial. But it would be impossible (and probably socially very costly) to simply avoid social media. So how to discuss its healthy use with your patients and their parents?
The data
Social media are digital communication platforms that allow users to build a public profile and then accumulate a network of followers, and follow other users, based on shared interests. They include FaceBook, Instagram, Snapchat, YouTube, and Twitter. Surveys demonstrated that 90% of U.S. adolescents use social media, with 75% having at least one social media profile and over half visiting social media sites at least once daily. Adolescents spend over 7 hours daily on their phones, not including time devoted to online schoolwork, and 8- to 12-year-olds are not far behind at almost 5 hours of daily phone use. On average, 39% of adolescent screen time is spent on passive consumption, 26% on social media, 25% on interactive activities (browsing the web, interactive video gaming) and 3% on content creation (coding, etc). There was considerable variability in survey results, and differences between genders, with boys engaged in video games almost eight times as often as girls, and girls in social media nearly twice as often as boys.1
The research
There is a growing body of research devoted to understanding the effects of all of this digital activity on youth health and well-being.
A large, longitudinal study of Canadian 13- to 17-year-olds found that time spent on social media or watching television was strongly associated with depressive and anxiety symptoms, with a robust dose-response relationship.2 However, causality is not clear, as anxious, shy, and depressed adolescents may use more social media as a consequence of their mood. Interestingly, there was no such relationship with mood and anxiety symptoms and time spent on video games.3 For youth with depression and anxiety, time spent on social media has been strongly associated with increased levels of self-reported distress, self-injury and suicidality, but again, causality is hard to prove.
One very large study from the United Kingdom (including more than 10,000 participants), demonstrated a strong relationship between time spent on social media and severity of depressive symptoms, with a more pronounced effect in girls than in boys.4 Many more nuanced studies have demonstrated that excessive time spent on social media, the presence of an addictive pattern of use, and the degree to which an adolescent’s sense of well-being is connected to social media are the variables that strongly predict an association with worsening depressive or anxiety symptoms.5
Several studies have demonstrated that low to moderate use of social media, and use to gather information and make plans were associated with better scores of emotional self-regulation and lower rates of depressive symptoms in teens.6 It seems safe to say that social media can be useful and fun, but that too much can be bad for you. So help your adolescent patients to expand their perspective on its use by discussing it with them.
Make them curious about quantity
Most teens feel they do not have enough time for all of the things they need to do, so invite them to play detective by using their phone’s applications that can track their time spent online and in different apps.
Remind them that these apps were designed to be so engaging that for some addiction is a real problem. As with tobacco, addiction is the business model by which these companies earn advertising dollars. Indeed, adolescents are the target demographic, as they are most sensitive to social rewards and are the most valuable audience for advertisers. Engage their natural suspicion of authority by pointing out that with every hour on Insta, someone else is making a lot of money. They get to choose how they want to relax, connect with friends, and explore the world, so help them to be aware of how these apps are designed to keep them from choosing.
Raise awareness of vulnerability
Adolescents who have attention-deficit/hyperactivity disorder already have difficulty with impulse control and with shifting their attention to less engaging activities. Adolescents with anxiety are prone to avoid stressful situations, but still hunger for knowledge and connections. Adolescents with depression are managing low motivation and self-esteem, and the rewards of social media may keep them from exercise and actual social engagement that are critical to their treatment. Youth with eating disorders are especially prone to critical comparison of themselves to others, feeding their distorted body images. Help your patients with these common illnesses to be aware of how social media may make their treatment harder, rather than being the source of relief it may feel like.
Protect their health
For all young people, too much time spent in virtual activities and passive media consumption may not leave enough time to explore potential interests, talents, or relationships. These are important activities throughout life, but they are the central developmental tasks of adolescence. They also need 8-10 hours of sleep nightly and regular exercise. And of course, they have homework! Help them to think about how to use their time wisely to support satisfying relationships and activities, with time for relaxation and good health.
Keep parents in the room for these discussions
State that most of us have difficulty putting down our phones. Children and teens need adults who model striving for balance in all areas of choice. Just as we try to teach them to make good choices about food, getting excellent nutrition while still valuing taste and pleasure, we can talk about how to balance virtual activities with actual activities, work with play, and effort with relaxation. You can help expand your young patients’ self-awareness, acknowledge the fun and utility of their digital time, and enhance their sense of how we must all learn how to put screens down sometimes. In so doing, you can help families to ensure that they are engaging with the digital tools and toys available to all of us in ways that can support their health and well-being.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Geena Davis Institute on Gender and Media. The Common Sense Census: Media Use by Teens and Tweens, 2015.
2. Abi-Jaoude E et al. CMAJ 2020;192(6):E136-41.
3. Boers E et al. Can J Psychiatry. 2020 Mar;65(3):206-8.
4. Kelly Y et al. EClinicalMedicine. 2019 Jan 4;6:59-68.
5. Vidal C et al. Int Rev Psychiatry. 2020 May;32(3):235-53.
6. Coyne SM et al. J Res Adolescence. 2019;29(4):897-907.
Drugging the undruggable
including 68% of pancreatic tumors and 20% of all non–small cell lung cancers (NSCLC).
We now have a treatment – sotorasib – for patients with locally advanced or metastatic NSCLC that is driven by a KRAS mutation (G12C). And, now, there is a second treatment – adagrasib – under study, which, according to a presentation recently made at the annual meeting of the American Society of Clinical Oncology, looks promising.
Ras is a membrane-bound regulatory protein (G protein) belonging to the family of guanosine triphosphatases (GTPases). Ras functions as a guanosine diphosphate/triphosphate binary switch by cycling between the active GTP-bound and the inactive GDP-bound states in response to extracellular stimuli. The KRAS (G12C) mutation affects the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth, specifically of ERK and MEK signaling pathways.
At the ASCO annual meeting in June, Spira and colleagues reported the results of cohort A of the KRYSTAL-1 study evaluating adagrasib as second-line therapy patients with advanced solid tumors harboring a KRAS (G12C) mutation. Like sotorasib, adagrasib is a KRAS (G12C) inhibitor that irreversibly and selectively binds KRAS (G12C), locking it in its inactive state. In this study, patients had to have failed first-line chemotherapy and immunotherapy with 43% of lung cancer patients responding. The 12-month overall survival (OS) was 51%, median overall survival was 12.6 and median progression-free survival (PFS) was 6.5 months. Twenty-five patients with KRAS (G12C)–mutant NSCLC and active, untreated central nervous system metastases received adagrasib in a phase 1b cohort. The intracranial overall response rate was 31.6% and median intracranial PFS was 4.2 months. Systemic ORR was 35.0% (7/20), the disease control rate was 80.0% (16/20) and median duration of response was 9.6 months. Based on these data, a phase 3 trial evaluating adagrasib monotherapy versus docetaxel in previously treated patients with KRAS (G12C) mutant NSCLC is ongoing.
The Food and Drug Administration approval of sotorasib in 2021 was, in part, based on the results of a single-arm, phase 2, second-line study of patients who had previously received platinum-based chemotherapy and/or immunotherapy. An ORR rate of 37.1% was reported with a median PFS of 6.8 months and median OS of 12.5 months leading to the FDA approval. Responses were observed across the range of baseline PD-L1 expression levels: 48% of PD-L1 negative, 39% with PD-L1 between 1%-49%, and 22% of patients with a PD-L1 of greater than 50% having a response.
The major toxicities observed in these studies were gastrointestinal (diarrhea, nausea, vomiting) and hepatic (elevated liver enzymes). About 97% of patients on adagrasib experienced any treatment-related adverse events, and 43% experienced a grade 3 or 4 treatment-related adverse event leading to dose reduction in 52% of patients, a dose interruption in 61% of patients, and a 7% discontinuation rate. About 70% of patients treated with sotorasib had a treatment-related adverse event of any grade, and 21% reported grade 3 or 4 treatment-related adverse events.
A subgroup in the KRYSTAL-1 trial reported an intracranial ORR of 32% in patients with active, untreated CNS metastases. Median overall survival has not yet reached concordance between systemic and intracranial disease control was 88%. In addition, preliminary data from two patients with untreated CNS metastases from a phase 1b cohort found cerebrospinal fluid concentrations of adagrasib with a mean ratio of unbound brain-to-plasma concentration of 0.47, which is comparable or exceeds values for known CNS-penetrant tyrosine kinase inhibitors.
Unfortunately, KRAS (G12C) is not the only KRAS mutation out there. There are a myriad of others, such as G12V and G12D. Hopefully, we will be seeing more drugs aimed at this set of important mutations. Another question, of course, is when and if these drugs will move to the first-line setting.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
including 68% of pancreatic tumors and 20% of all non–small cell lung cancers (NSCLC).
We now have a treatment – sotorasib – for patients with locally advanced or metastatic NSCLC that is driven by a KRAS mutation (G12C). And, now, there is a second treatment – adagrasib – under study, which, according to a presentation recently made at the annual meeting of the American Society of Clinical Oncology, looks promising.
Ras is a membrane-bound regulatory protein (G protein) belonging to the family of guanosine triphosphatases (GTPases). Ras functions as a guanosine diphosphate/triphosphate binary switch by cycling between the active GTP-bound and the inactive GDP-bound states in response to extracellular stimuli. The KRAS (G12C) mutation affects the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth, specifically of ERK and MEK signaling pathways.
At the ASCO annual meeting in June, Spira and colleagues reported the results of cohort A of the KRYSTAL-1 study evaluating adagrasib as second-line therapy patients with advanced solid tumors harboring a KRAS (G12C) mutation. Like sotorasib, adagrasib is a KRAS (G12C) inhibitor that irreversibly and selectively binds KRAS (G12C), locking it in its inactive state. In this study, patients had to have failed first-line chemotherapy and immunotherapy with 43% of lung cancer patients responding. The 12-month overall survival (OS) was 51%, median overall survival was 12.6 and median progression-free survival (PFS) was 6.5 months. Twenty-five patients with KRAS (G12C)–mutant NSCLC and active, untreated central nervous system metastases received adagrasib in a phase 1b cohort. The intracranial overall response rate was 31.6% and median intracranial PFS was 4.2 months. Systemic ORR was 35.0% (7/20), the disease control rate was 80.0% (16/20) and median duration of response was 9.6 months. Based on these data, a phase 3 trial evaluating adagrasib monotherapy versus docetaxel in previously treated patients with KRAS (G12C) mutant NSCLC is ongoing.
The Food and Drug Administration approval of sotorasib in 2021 was, in part, based on the results of a single-arm, phase 2, second-line study of patients who had previously received platinum-based chemotherapy and/or immunotherapy. An ORR rate of 37.1% was reported with a median PFS of 6.8 months and median OS of 12.5 months leading to the FDA approval. Responses were observed across the range of baseline PD-L1 expression levels: 48% of PD-L1 negative, 39% with PD-L1 between 1%-49%, and 22% of patients with a PD-L1 of greater than 50% having a response.
The major toxicities observed in these studies were gastrointestinal (diarrhea, nausea, vomiting) and hepatic (elevated liver enzymes). About 97% of patients on adagrasib experienced any treatment-related adverse events, and 43% experienced a grade 3 or 4 treatment-related adverse event leading to dose reduction in 52% of patients, a dose interruption in 61% of patients, and a 7% discontinuation rate. About 70% of patients treated with sotorasib had a treatment-related adverse event of any grade, and 21% reported grade 3 or 4 treatment-related adverse events.
A subgroup in the KRYSTAL-1 trial reported an intracranial ORR of 32% in patients with active, untreated CNS metastases. Median overall survival has not yet reached concordance between systemic and intracranial disease control was 88%. In addition, preliminary data from two patients with untreated CNS metastases from a phase 1b cohort found cerebrospinal fluid concentrations of adagrasib with a mean ratio of unbound brain-to-plasma concentration of 0.47, which is comparable or exceeds values for known CNS-penetrant tyrosine kinase inhibitors.
Unfortunately, KRAS (G12C) is not the only KRAS mutation out there. There are a myriad of others, such as G12V and G12D. Hopefully, we will be seeing more drugs aimed at this set of important mutations. Another question, of course, is when and if these drugs will move to the first-line setting.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
including 68% of pancreatic tumors and 20% of all non–small cell lung cancers (NSCLC).
We now have a treatment – sotorasib – for patients with locally advanced or metastatic NSCLC that is driven by a KRAS mutation (G12C). And, now, there is a second treatment – adagrasib – under study, which, according to a presentation recently made at the annual meeting of the American Society of Clinical Oncology, looks promising.
Ras is a membrane-bound regulatory protein (G protein) belonging to the family of guanosine triphosphatases (GTPases). Ras functions as a guanosine diphosphate/triphosphate binary switch by cycling between the active GTP-bound and the inactive GDP-bound states in response to extracellular stimuli. The KRAS (G12C) mutation affects the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth, specifically of ERK and MEK signaling pathways.
At the ASCO annual meeting in June, Spira and colleagues reported the results of cohort A of the KRYSTAL-1 study evaluating adagrasib as second-line therapy patients with advanced solid tumors harboring a KRAS (G12C) mutation. Like sotorasib, adagrasib is a KRAS (G12C) inhibitor that irreversibly and selectively binds KRAS (G12C), locking it in its inactive state. In this study, patients had to have failed first-line chemotherapy and immunotherapy with 43% of lung cancer patients responding. The 12-month overall survival (OS) was 51%, median overall survival was 12.6 and median progression-free survival (PFS) was 6.5 months. Twenty-five patients with KRAS (G12C)–mutant NSCLC and active, untreated central nervous system metastases received adagrasib in a phase 1b cohort. The intracranial overall response rate was 31.6% and median intracranial PFS was 4.2 months. Systemic ORR was 35.0% (7/20), the disease control rate was 80.0% (16/20) and median duration of response was 9.6 months. Based on these data, a phase 3 trial evaluating adagrasib monotherapy versus docetaxel in previously treated patients with KRAS (G12C) mutant NSCLC is ongoing.
The Food and Drug Administration approval of sotorasib in 2021 was, in part, based on the results of a single-arm, phase 2, second-line study of patients who had previously received platinum-based chemotherapy and/or immunotherapy. An ORR rate of 37.1% was reported with a median PFS of 6.8 months and median OS of 12.5 months leading to the FDA approval. Responses were observed across the range of baseline PD-L1 expression levels: 48% of PD-L1 negative, 39% with PD-L1 between 1%-49%, and 22% of patients with a PD-L1 of greater than 50% having a response.
The major toxicities observed in these studies were gastrointestinal (diarrhea, nausea, vomiting) and hepatic (elevated liver enzymes). About 97% of patients on adagrasib experienced any treatment-related adverse events, and 43% experienced a grade 3 or 4 treatment-related adverse event leading to dose reduction in 52% of patients, a dose interruption in 61% of patients, and a 7% discontinuation rate. About 70% of patients treated with sotorasib had a treatment-related adverse event of any grade, and 21% reported grade 3 or 4 treatment-related adverse events.
A subgroup in the KRYSTAL-1 trial reported an intracranial ORR of 32% in patients with active, untreated CNS metastases. Median overall survival has not yet reached concordance between systemic and intracranial disease control was 88%. In addition, preliminary data from two patients with untreated CNS metastases from a phase 1b cohort found cerebrospinal fluid concentrations of adagrasib with a mean ratio of unbound brain-to-plasma concentration of 0.47, which is comparable or exceeds values for known CNS-penetrant tyrosine kinase inhibitors.
Unfortunately, KRAS (G12C) is not the only KRAS mutation out there. There are a myriad of others, such as G12V and G12D. Hopefully, we will be seeing more drugs aimed at this set of important mutations. Another question, of course, is when and if these drugs will move to the first-line setting.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
BA.4 and BA.5 subvariants are more evasive of antibodies, but not of cellular immunity
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
Roe v. Wade overturned: A family medicine resident reacts
I remember how small and shy she looked, curled into herself in her too-large hospital gown. I remember thinking that it was autumn, and she should have been at her first homecoming dance, not sitting in the ER staring mutely at the hospital-issued safety socks on her feet. Her mother, puffy-eyed from crying, was sitting on the bed beside her, stroking her hair.
Together, my patients and I talked about the pregnancy. She told me how scared she was, how she didn’t want to “kill her baby”, but that she also wasn’t sure she could take care of a child. She told me that she was terrified of childbirth, that she didn’t want her friends at school to know and to judge her. We talked about how she was a victim; how she was an innocent child, too. I reassured her, and her mom emphatically agreed – her body was still her own.
The man who hurt her did not take that from her. She could make any choice she wanted, and it would be the right choice.
Eventually, she was able to make a decision which was best for her. I don’t know what became of her, but I hope she is well now, and I hope she’s thriving and happy. I also hope that she doesn’t see the news about Roe v. Wade and feel stripped of her personhood, as many women did.
When I heard about the Supreme Court decision I thought of her, and how important our conversation was to the trajectory of her life. I wondered if across the country these conversations might be silenced, and patients might be left to navigate this important facet of their health alone.
Some version of the conversation I had with my young patient occurs in exam rooms across the country countless times a day. Sometimes these conversations are cut and dry. Other times, they are accompanied by heartbreak and tears.
These conversations are common – one in four women in the United States have had an abortion. I have had many friends who were faced with deciding what to do after an unexpectedly positive pregnancy test. The reasons were different for each person – one was raped at a party, another’s birth control failed, the boyfriend of a third friend wouldn’t wear a condom – but the underlying sentiments were the same for each woman. They thought: “This is a difficult choice, but it’s a choice I’m ready to make. I’m not ready to have a baby at this point in my life.”
My friends talked to their doctors, who assisted them in making an informed choice. Some of them chose abortion. Others chose to deliver their baby. All were helped along in their decision by a physician who was there to support them and assist them in making a well-considered choice for their individual circumstance.
Economic and health consequences of restricting access to abortion
The facts are clear: Nearly half of all pregnancies in American women in 2011 were unplanned, and about 4 in 10 of them ended in an elective abortion, according to the Guttmacher Institute.1 Restricting access to abortions does not stop abortions from happening; it limits the opportunity for women to seek advice from trusted friends and professionals and it reduces access to safe abortions.
The people who will be most harmed by these restrictions are the most socially and economically vulnerable. Wealthy, mobile women with the ability to travel to other states or countries will always be able to access abortion care; low-income, work-tethered women and women with other children to care for at home will struggle to do so.
Denying women abortion services puts them at increased risk for lifelong, multigenerational economic hardship. Women who sought abortions but were unable to obtain them experienced an increase in household poverty which lasted years relative to women who were able to receive an abortion, according to the authors of The Turnaway Study.2 They were less socially, geographically, and economically mobile, and were less likely to go on to receive a higher education.
In a country where citizens do not have paid maternity leave, affordable and accessible childcare services, or universal health care, raising a child is an enormous financial burden. Women who are denied abortions also are much more likely to end up as a single parent, shouldering that burden alone.
Additionally, low socioeconomic status is associated with increased all-cause mortality. People who live in poverty are disproportionately affected by diabetes and other chronic health conditions, and have lower life expectancies overall.
The reversal of Roe v. Wade is not only going to lead directly to patient death by decreasing access to safe abortion, causing women to pursue unsafe alternatives; it will also indirectly result in more women being driven into and remaining in poverty and suffering the health consequences.
In addition to risking a woman’s life medically, pregnancy also significantly increases that individual’s risk of being a victim of intimate partner violence. The number one cause of death in pregnant women is homicide, most often by their sexual partner, said an article published in Nature in 2021.3 Therefore, restricting a woman’s ability to control if and when she has children could put her at risk for death from serious pregnancy-related complications and unsafe abortion consequences and increase her likelihood of dying by domestic violence.
Patient-physicians interactions are changed
As a physician I hope that I am able to convey my intense respect for and support of a woman’s autonomy into every family planning visit I conduct. Unfortunately, this ruling will not only have an immediate impact on the lives of women across the country – it will also alter the way many of us interact with our patients on a day-to-day basis. When patients can report doctors to authorities in some states for offering terminations, and doctors can report patients for seeking them, there will be absolutely no trust in the therapeutic relationship.
With this ruling, the content of private and protected conversations between patients and their physicians will be subject to censure and potentially criminal consequences.
Regardless of where I eventually practice medicine, I should not be in the position of talking to a patient and telling them that they do not have any agency over their body unless they have the money and resources to travel to a state where abortion is legal. I should not have to tell a child that she must carry and birth another child just to appease the often-fickle whims of lawmakers.
The conversation I had with my pediatric patient was important to her health and to her future, and she deserved to have the chance to discuss her feelings with a trusted physician. Every woman has the right to make her own decisions within the sanctity of the exam room, not from the distance of a courtroom.
Dr. Persampiere is a resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
References
1. Unintended pregnancy in the United States. Guttmacher Institute. 2019 Jan 9. https://www.guttmacher.org/fact-sheet/unintended-pregnancy-united-states
2. Foster D et al. The harms of denying a woman a wanted abortion - ANSIRH. https://www.ansirh.org/sites/default/files/publications/files/the_harms_of_denying_a_woman_a_wanted_abortion_4-16-2020.pdf
3. Subbaraman N. 2021 Nov 12. Homicide is a top cause of maternal death in the United States. Nature News. https://www.nature.com/articles/d41586-021-03392-8
I remember how small and shy she looked, curled into herself in her too-large hospital gown. I remember thinking that it was autumn, and she should have been at her first homecoming dance, not sitting in the ER staring mutely at the hospital-issued safety socks on her feet. Her mother, puffy-eyed from crying, was sitting on the bed beside her, stroking her hair.
Together, my patients and I talked about the pregnancy. She told me how scared she was, how she didn’t want to “kill her baby”, but that she also wasn’t sure she could take care of a child. She told me that she was terrified of childbirth, that she didn’t want her friends at school to know and to judge her. We talked about how she was a victim; how she was an innocent child, too. I reassured her, and her mom emphatically agreed – her body was still her own.
The man who hurt her did not take that from her. She could make any choice she wanted, and it would be the right choice.
Eventually, she was able to make a decision which was best for her. I don’t know what became of her, but I hope she is well now, and I hope she’s thriving and happy. I also hope that she doesn’t see the news about Roe v. Wade and feel stripped of her personhood, as many women did.
When I heard about the Supreme Court decision I thought of her, and how important our conversation was to the trajectory of her life. I wondered if across the country these conversations might be silenced, and patients might be left to navigate this important facet of their health alone.
Some version of the conversation I had with my young patient occurs in exam rooms across the country countless times a day. Sometimes these conversations are cut and dry. Other times, they are accompanied by heartbreak and tears.
These conversations are common – one in four women in the United States have had an abortion. I have had many friends who were faced with deciding what to do after an unexpectedly positive pregnancy test. The reasons were different for each person – one was raped at a party, another’s birth control failed, the boyfriend of a third friend wouldn’t wear a condom – but the underlying sentiments were the same for each woman. They thought: “This is a difficult choice, but it’s a choice I’m ready to make. I’m not ready to have a baby at this point in my life.”
My friends talked to their doctors, who assisted them in making an informed choice. Some of them chose abortion. Others chose to deliver their baby. All were helped along in their decision by a physician who was there to support them and assist them in making a well-considered choice for their individual circumstance.
Economic and health consequences of restricting access to abortion
The facts are clear: Nearly half of all pregnancies in American women in 2011 were unplanned, and about 4 in 10 of them ended in an elective abortion, according to the Guttmacher Institute.1 Restricting access to abortions does not stop abortions from happening; it limits the opportunity for women to seek advice from trusted friends and professionals and it reduces access to safe abortions.
The people who will be most harmed by these restrictions are the most socially and economically vulnerable. Wealthy, mobile women with the ability to travel to other states or countries will always be able to access abortion care; low-income, work-tethered women and women with other children to care for at home will struggle to do so.
Denying women abortion services puts them at increased risk for lifelong, multigenerational economic hardship. Women who sought abortions but were unable to obtain them experienced an increase in household poverty which lasted years relative to women who were able to receive an abortion, according to the authors of The Turnaway Study.2 They were less socially, geographically, and economically mobile, and were less likely to go on to receive a higher education.
In a country where citizens do not have paid maternity leave, affordable and accessible childcare services, or universal health care, raising a child is an enormous financial burden. Women who are denied abortions also are much more likely to end up as a single parent, shouldering that burden alone.
Additionally, low socioeconomic status is associated with increased all-cause mortality. People who live in poverty are disproportionately affected by diabetes and other chronic health conditions, and have lower life expectancies overall.
The reversal of Roe v. Wade is not only going to lead directly to patient death by decreasing access to safe abortion, causing women to pursue unsafe alternatives; it will also indirectly result in more women being driven into and remaining in poverty and suffering the health consequences.
In addition to risking a woman’s life medically, pregnancy also significantly increases that individual’s risk of being a victim of intimate partner violence. The number one cause of death in pregnant women is homicide, most often by their sexual partner, said an article published in Nature in 2021.3 Therefore, restricting a woman’s ability to control if and when she has children could put her at risk for death from serious pregnancy-related complications and unsafe abortion consequences and increase her likelihood of dying by domestic violence.
Patient-physicians interactions are changed
As a physician I hope that I am able to convey my intense respect for and support of a woman’s autonomy into every family planning visit I conduct. Unfortunately, this ruling will not only have an immediate impact on the lives of women across the country – it will also alter the way many of us interact with our patients on a day-to-day basis. When patients can report doctors to authorities in some states for offering terminations, and doctors can report patients for seeking them, there will be absolutely no trust in the therapeutic relationship.
With this ruling, the content of private and protected conversations between patients and their physicians will be subject to censure and potentially criminal consequences.
Regardless of where I eventually practice medicine, I should not be in the position of talking to a patient and telling them that they do not have any agency over their body unless they have the money and resources to travel to a state where abortion is legal. I should not have to tell a child that she must carry and birth another child just to appease the often-fickle whims of lawmakers.
The conversation I had with my pediatric patient was important to her health and to her future, and she deserved to have the chance to discuss her feelings with a trusted physician. Every woman has the right to make her own decisions within the sanctity of the exam room, not from the distance of a courtroom.
Dr. Persampiere is a resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
References
1. Unintended pregnancy in the United States. Guttmacher Institute. 2019 Jan 9. https://www.guttmacher.org/fact-sheet/unintended-pregnancy-united-states
2. Foster D et al. The harms of denying a woman a wanted abortion - ANSIRH. https://www.ansirh.org/sites/default/files/publications/files/the_harms_of_denying_a_woman_a_wanted_abortion_4-16-2020.pdf
3. Subbaraman N. 2021 Nov 12. Homicide is a top cause of maternal death in the United States. Nature News. https://www.nature.com/articles/d41586-021-03392-8
I remember how small and shy she looked, curled into herself in her too-large hospital gown. I remember thinking that it was autumn, and she should have been at her first homecoming dance, not sitting in the ER staring mutely at the hospital-issued safety socks on her feet. Her mother, puffy-eyed from crying, was sitting on the bed beside her, stroking her hair.
Together, my patients and I talked about the pregnancy. She told me how scared she was, how she didn’t want to “kill her baby”, but that she also wasn’t sure she could take care of a child. She told me that she was terrified of childbirth, that she didn’t want her friends at school to know and to judge her. We talked about how she was a victim; how she was an innocent child, too. I reassured her, and her mom emphatically agreed – her body was still her own.
The man who hurt her did not take that from her. She could make any choice she wanted, and it would be the right choice.
Eventually, she was able to make a decision which was best for her. I don’t know what became of her, but I hope she is well now, and I hope she’s thriving and happy. I also hope that she doesn’t see the news about Roe v. Wade and feel stripped of her personhood, as many women did.
When I heard about the Supreme Court decision I thought of her, and how important our conversation was to the trajectory of her life. I wondered if across the country these conversations might be silenced, and patients might be left to navigate this important facet of their health alone.
Some version of the conversation I had with my young patient occurs in exam rooms across the country countless times a day. Sometimes these conversations are cut and dry. Other times, they are accompanied by heartbreak and tears.
These conversations are common – one in four women in the United States have had an abortion. I have had many friends who were faced with deciding what to do after an unexpectedly positive pregnancy test. The reasons were different for each person – one was raped at a party, another’s birth control failed, the boyfriend of a third friend wouldn’t wear a condom – but the underlying sentiments were the same for each woman. They thought: “This is a difficult choice, but it’s a choice I’m ready to make. I’m not ready to have a baby at this point in my life.”
My friends talked to their doctors, who assisted them in making an informed choice. Some of them chose abortion. Others chose to deliver their baby. All were helped along in their decision by a physician who was there to support them and assist them in making a well-considered choice for their individual circumstance.
Economic and health consequences of restricting access to abortion
The facts are clear: Nearly half of all pregnancies in American women in 2011 were unplanned, and about 4 in 10 of them ended in an elective abortion, according to the Guttmacher Institute.1 Restricting access to abortions does not stop abortions from happening; it limits the opportunity for women to seek advice from trusted friends and professionals and it reduces access to safe abortions.
The people who will be most harmed by these restrictions are the most socially and economically vulnerable. Wealthy, mobile women with the ability to travel to other states or countries will always be able to access abortion care; low-income, work-tethered women and women with other children to care for at home will struggle to do so.
Denying women abortion services puts them at increased risk for lifelong, multigenerational economic hardship. Women who sought abortions but were unable to obtain them experienced an increase in household poverty which lasted years relative to women who were able to receive an abortion, according to the authors of The Turnaway Study.2 They were less socially, geographically, and economically mobile, and were less likely to go on to receive a higher education.
In a country where citizens do not have paid maternity leave, affordable and accessible childcare services, or universal health care, raising a child is an enormous financial burden. Women who are denied abortions also are much more likely to end up as a single parent, shouldering that burden alone.
Additionally, low socioeconomic status is associated with increased all-cause mortality. People who live in poverty are disproportionately affected by diabetes and other chronic health conditions, and have lower life expectancies overall.
The reversal of Roe v. Wade is not only going to lead directly to patient death by decreasing access to safe abortion, causing women to pursue unsafe alternatives; it will also indirectly result in more women being driven into and remaining in poverty and suffering the health consequences.
In addition to risking a woman’s life medically, pregnancy also significantly increases that individual’s risk of being a victim of intimate partner violence. The number one cause of death in pregnant women is homicide, most often by their sexual partner, said an article published in Nature in 2021.3 Therefore, restricting a woman’s ability to control if and when she has children could put her at risk for death from serious pregnancy-related complications and unsafe abortion consequences and increase her likelihood of dying by domestic violence.
Patient-physicians interactions are changed
As a physician I hope that I am able to convey my intense respect for and support of a woman’s autonomy into every family planning visit I conduct. Unfortunately, this ruling will not only have an immediate impact on the lives of women across the country – it will also alter the way many of us interact with our patients on a day-to-day basis. When patients can report doctors to authorities in some states for offering terminations, and doctors can report patients for seeking them, there will be absolutely no trust in the therapeutic relationship.
With this ruling, the content of private and protected conversations between patients and their physicians will be subject to censure and potentially criminal consequences.
Regardless of where I eventually practice medicine, I should not be in the position of talking to a patient and telling them that they do not have any agency over their body unless they have the money and resources to travel to a state where abortion is legal. I should not have to tell a child that she must carry and birth another child just to appease the often-fickle whims of lawmakers.
The conversation I had with my pediatric patient was important to her health and to her future, and she deserved to have the chance to discuss her feelings with a trusted physician. Every woman has the right to make her own decisions within the sanctity of the exam room, not from the distance of a courtroom.
Dr. Persampiere is a resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
References
1. Unintended pregnancy in the United States. Guttmacher Institute. 2019 Jan 9. https://www.guttmacher.org/fact-sheet/unintended-pregnancy-united-states
2. Foster D et al. The harms of denying a woman a wanted abortion - ANSIRH. https://www.ansirh.org/sites/default/files/publications/files/the_harms_of_denying_a_woman_a_wanted_abortion_4-16-2020.pdf
3. Subbaraman N. 2021 Nov 12. Homicide is a top cause of maternal death in the United States. Nature News. https://www.nature.com/articles/d41586-021-03392-8
Many sources of PTSD are cause for concern
A few weeks ago, right after 19 children and two adults were killed by a gunman in Uvalde, Texas, Americans were really on edge. Many people I know became hypervigilant while going about activities previously thought of as routine, such as waiting for a subway or going to a grocery store.
On top of that, we are still facing the ongoing COVID-19 pandemic. Despite vaccines and therapeutics, the United States is still losing more than 300 people each day to the virus. Many people who have tested positive have continued to experience debilitating long-haul symptoms many months after testing negative, and I believe not knowing what your future life will bring from this terrible illness could lead some to posttraumatic stress disorder.
In addition to constant updates about COVID, we are getting almost daily reports about monkeypox. In New York state, medical professionals and institutions receive regular, almost weekly, information about the spread of influenza. But where are the reports and treatment approaches for PTSD, which would not only increase awareness but also lead to more care?
Some might believe that I am obsessed with PTSD, since I’ve written a great deal on the subject, particularly “underdiagnosed” PTSD. The key question I have is:
We know the signs and symptoms of PTSD. They include flashbacks, intrusive recollections, physical distress related to stimuli related to the trauma, insomnia, social isolation, avoidance of certain situations, negative thinking, and hyperarousal – coupled with anxiety and depression. PTSD can be a great masquerader. It can be triggered by many events, large and small, and all too often will masquerade as general anxiety or existential despair and depression. Too often, PTSD is undiagnosed or unrecognized completely. PTSD is also a costly disease that is an enormous economic burden on the U.S. economy.
As clinicians, we must be aware of the more subtle events that may trigger PTSD. We must think beyond ICD codes and DSM criteria and realize that each individual processes an event or a series of events differently. For example, seriously ill people in ICUs or undergoing critical care have been known to experience PTSD well beyond their physical recovery (J Crit Care. 2017 Dec. doi: 10.1016/j.jcrc.2017.06.014). Years after the Sept. 11, 2001, World Trade Center disaster, many are still suffering from PTSD symptoms (Biol Psychiatry. 2020 May 1. doi: 10.1016/j.biopsych.2020.02.817).
Again, in some cases, not knowing what the future may bring regarding life itself can lead to PTSD. I have treated patients who have lost jobs and experienced devastating social and financial losses, which were perceived as a separation from “life as they know it.” These can be precursors to PTSD for some who are sensitive to the disorder.
Intergenerational trauma is also a real phenomenon to which we must be attuned. I have treated two adult children of Holocaust survivors, both born in America well after World War II, who developed PTSD after hearing family recollections over and over about the brutality suffered by relatives, combined with watching films about people sent to concentration camps. Both of those patients self-diagnosed their symptoms as depression. Research shows that Holocaust traumatization can affect three generations (J Anxiety Disord. 2021 Jun. doi: 10.1016/j.janxdis.2021.102401).
In light of the high incidence of traumatic events affecting millions directly, more codified treatment approaches are needed that can be used both for individuals and for those in group settings.
To date, the best treatment rests with cognitive-behavioral therapy (CBT) and guided imagery coupled with relaxation techniques and the various types of in vivo exposure therapy, which I prefer to in vitro or flooding care. In terms of medication management, the U.S. Food and Drug Administration has approved only two antidepressant medications for PTSD, sertraline (Zoloft) and paroxetine (Paxil), although other selective serotonin reuptake inhibitors have been used off- label, and prazosin, a hypertensive medication, has been used off-label for PTSD-related insomnia and nightmares (Prim Care Companion CNS Disord. 2012 Mar 22. doi: 10.4088/PCC.11r01222). Thus, the limited number of choices for medication management means more research is needed so that more medications are developed that are more precisely directed at PTSD treatment.
Implications for society at large
In a recent article published in the Journal of Clinical Psychiatry (2022 Apr 25. doi: 10.4088/JCP.21m14116), authors Lori L. Davis and colleagues point out that the economic burden of PTSD goes beyond health care costs and rivals the costs of other mental illnesses, including depression and anxiety. In the process, Dr. Davis and colleagues note, unemployment caused by job loss, disability, homelessness, substance use, disordered care, as well as premature mortality, all contribute to this severe burden, going beyond PTSD itself.
This study shows that the annual economic burden of PTSD is $232 billion. Most of that burden is attributed to the civilian population, which they suggest to be $189.5 billion, or 82%.
After reading that article, it became clear to me that my “obsession” with PTSD is not really an obsession at all. Rather, it is a true concern that, against the backdrop of long COVID, gun violence, political and economic turmoil, and other factors, it is important that clinicians understand how to recognize and treat PTSD. The stakes have never been higher.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
A few weeks ago, right after 19 children and two adults were killed by a gunman in Uvalde, Texas, Americans were really on edge. Many people I know became hypervigilant while going about activities previously thought of as routine, such as waiting for a subway or going to a grocery store.
On top of that, we are still facing the ongoing COVID-19 pandemic. Despite vaccines and therapeutics, the United States is still losing more than 300 people each day to the virus. Many people who have tested positive have continued to experience debilitating long-haul symptoms many months after testing negative, and I believe not knowing what your future life will bring from this terrible illness could lead some to posttraumatic stress disorder.
In addition to constant updates about COVID, we are getting almost daily reports about monkeypox. In New York state, medical professionals and institutions receive regular, almost weekly, information about the spread of influenza. But where are the reports and treatment approaches for PTSD, which would not only increase awareness but also lead to more care?
Some might believe that I am obsessed with PTSD, since I’ve written a great deal on the subject, particularly “underdiagnosed” PTSD. The key question I have is:
We know the signs and symptoms of PTSD. They include flashbacks, intrusive recollections, physical distress related to stimuli related to the trauma, insomnia, social isolation, avoidance of certain situations, negative thinking, and hyperarousal – coupled with anxiety and depression. PTSD can be a great masquerader. It can be triggered by many events, large and small, and all too often will masquerade as general anxiety or existential despair and depression. Too often, PTSD is undiagnosed or unrecognized completely. PTSD is also a costly disease that is an enormous economic burden on the U.S. economy.
As clinicians, we must be aware of the more subtle events that may trigger PTSD. We must think beyond ICD codes and DSM criteria and realize that each individual processes an event or a series of events differently. For example, seriously ill people in ICUs or undergoing critical care have been known to experience PTSD well beyond their physical recovery (J Crit Care. 2017 Dec. doi: 10.1016/j.jcrc.2017.06.014). Years after the Sept. 11, 2001, World Trade Center disaster, many are still suffering from PTSD symptoms (Biol Psychiatry. 2020 May 1. doi: 10.1016/j.biopsych.2020.02.817).
Again, in some cases, not knowing what the future may bring regarding life itself can lead to PTSD. I have treated patients who have lost jobs and experienced devastating social and financial losses, which were perceived as a separation from “life as they know it.” These can be precursors to PTSD for some who are sensitive to the disorder.
Intergenerational trauma is also a real phenomenon to which we must be attuned. I have treated two adult children of Holocaust survivors, both born in America well after World War II, who developed PTSD after hearing family recollections over and over about the brutality suffered by relatives, combined with watching films about people sent to concentration camps. Both of those patients self-diagnosed their symptoms as depression. Research shows that Holocaust traumatization can affect three generations (J Anxiety Disord. 2021 Jun. doi: 10.1016/j.janxdis.2021.102401).
In light of the high incidence of traumatic events affecting millions directly, more codified treatment approaches are needed that can be used both for individuals and for those in group settings.
To date, the best treatment rests with cognitive-behavioral therapy (CBT) and guided imagery coupled with relaxation techniques and the various types of in vivo exposure therapy, which I prefer to in vitro or flooding care. In terms of medication management, the U.S. Food and Drug Administration has approved only two antidepressant medications for PTSD, sertraline (Zoloft) and paroxetine (Paxil), although other selective serotonin reuptake inhibitors have been used off- label, and prazosin, a hypertensive medication, has been used off-label for PTSD-related insomnia and nightmares (Prim Care Companion CNS Disord. 2012 Mar 22. doi: 10.4088/PCC.11r01222). Thus, the limited number of choices for medication management means more research is needed so that more medications are developed that are more precisely directed at PTSD treatment.
Implications for society at large
In a recent article published in the Journal of Clinical Psychiatry (2022 Apr 25. doi: 10.4088/JCP.21m14116), authors Lori L. Davis and colleagues point out that the economic burden of PTSD goes beyond health care costs and rivals the costs of other mental illnesses, including depression and anxiety. In the process, Dr. Davis and colleagues note, unemployment caused by job loss, disability, homelessness, substance use, disordered care, as well as premature mortality, all contribute to this severe burden, going beyond PTSD itself.
This study shows that the annual economic burden of PTSD is $232 billion. Most of that burden is attributed to the civilian population, which they suggest to be $189.5 billion, or 82%.
After reading that article, it became clear to me that my “obsession” with PTSD is not really an obsession at all. Rather, it is a true concern that, against the backdrop of long COVID, gun violence, political and economic turmoil, and other factors, it is important that clinicians understand how to recognize and treat PTSD. The stakes have never been higher.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
A few weeks ago, right after 19 children and two adults were killed by a gunman in Uvalde, Texas, Americans were really on edge. Many people I know became hypervigilant while going about activities previously thought of as routine, such as waiting for a subway or going to a grocery store.
On top of that, we are still facing the ongoing COVID-19 pandemic. Despite vaccines and therapeutics, the United States is still losing more than 300 people each day to the virus. Many people who have tested positive have continued to experience debilitating long-haul symptoms many months after testing negative, and I believe not knowing what your future life will bring from this terrible illness could lead some to posttraumatic stress disorder.
In addition to constant updates about COVID, we are getting almost daily reports about monkeypox. In New York state, medical professionals and institutions receive regular, almost weekly, information about the spread of influenza. But where are the reports and treatment approaches for PTSD, which would not only increase awareness but also lead to more care?
Some might believe that I am obsessed with PTSD, since I’ve written a great deal on the subject, particularly “underdiagnosed” PTSD. The key question I have is:
We know the signs and symptoms of PTSD. They include flashbacks, intrusive recollections, physical distress related to stimuli related to the trauma, insomnia, social isolation, avoidance of certain situations, negative thinking, and hyperarousal – coupled with anxiety and depression. PTSD can be a great masquerader. It can be triggered by many events, large and small, and all too often will masquerade as general anxiety or existential despair and depression. Too often, PTSD is undiagnosed or unrecognized completely. PTSD is also a costly disease that is an enormous economic burden on the U.S. economy.
As clinicians, we must be aware of the more subtle events that may trigger PTSD. We must think beyond ICD codes and DSM criteria and realize that each individual processes an event or a series of events differently. For example, seriously ill people in ICUs or undergoing critical care have been known to experience PTSD well beyond their physical recovery (J Crit Care. 2017 Dec. doi: 10.1016/j.jcrc.2017.06.014). Years after the Sept. 11, 2001, World Trade Center disaster, many are still suffering from PTSD symptoms (Biol Psychiatry. 2020 May 1. doi: 10.1016/j.biopsych.2020.02.817).
Again, in some cases, not knowing what the future may bring regarding life itself can lead to PTSD. I have treated patients who have lost jobs and experienced devastating social and financial losses, which were perceived as a separation from “life as they know it.” These can be precursors to PTSD for some who are sensitive to the disorder.
Intergenerational trauma is also a real phenomenon to which we must be attuned. I have treated two adult children of Holocaust survivors, both born in America well after World War II, who developed PTSD after hearing family recollections over and over about the brutality suffered by relatives, combined with watching films about people sent to concentration camps. Both of those patients self-diagnosed their symptoms as depression. Research shows that Holocaust traumatization can affect three generations (J Anxiety Disord. 2021 Jun. doi: 10.1016/j.janxdis.2021.102401).
In light of the high incidence of traumatic events affecting millions directly, more codified treatment approaches are needed that can be used both for individuals and for those in group settings.
To date, the best treatment rests with cognitive-behavioral therapy (CBT) and guided imagery coupled with relaxation techniques and the various types of in vivo exposure therapy, which I prefer to in vitro or flooding care. In terms of medication management, the U.S. Food and Drug Administration has approved only two antidepressant medications for PTSD, sertraline (Zoloft) and paroxetine (Paxil), although other selective serotonin reuptake inhibitors have been used off- label, and prazosin, a hypertensive medication, has been used off-label for PTSD-related insomnia and nightmares (Prim Care Companion CNS Disord. 2012 Mar 22. doi: 10.4088/PCC.11r01222). Thus, the limited number of choices for medication management means more research is needed so that more medications are developed that are more precisely directed at PTSD treatment.
Implications for society at large
In a recent article published in the Journal of Clinical Psychiatry (2022 Apr 25. doi: 10.4088/JCP.21m14116), authors Lori L. Davis and colleagues point out that the economic burden of PTSD goes beyond health care costs and rivals the costs of other mental illnesses, including depression and anxiety. In the process, Dr. Davis and colleagues note, unemployment caused by job loss, disability, homelessness, substance use, disordered care, as well as premature mortality, all contribute to this severe burden, going beyond PTSD itself.
This study shows that the annual economic burden of PTSD is $232 billion. Most of that burden is attributed to the civilian population, which they suggest to be $189.5 billion, or 82%.
After reading that article, it became clear to me that my “obsession” with PTSD is not really an obsession at all. Rather, it is a true concern that, against the backdrop of long COVID, gun violence, political and economic turmoil, and other factors, it is important that clinicians understand how to recognize and treat PTSD. The stakes have never been higher.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Attacking childhood anxiety in primary care
Multiple media outlets and numerous children’s professional organizations are discussing the child and adolescent mental health crisis. Finally, society at large seems to be taking notice that our kids are not okay, and that they haven’t been okay for a long time.
Over the past 5-7 years, both in my practice in tertiary children’s hospital emergency departments and in primary care pediatrics, I have seen a disturbing decline in kids’ mental well-being. What can a primary care physician do to make a difference? How do we capitalize on these discussions about mental health and illness now that it is rising to a priority status?
The U.S. Preventive Services Task Force recently drafted a statement of recommendations specifically discussing anxiety in children and adolescents. It shows supporting evidence that there is a moderate benefit to screening children 8-18 years old for anxiety. We know from the 2018-2019 National Survey of Children’s Health that almost 8% of children/adolescents ages 3-17 years old have an anxiety disorder. And among those 13-18 years old, the lifetime prevalence rises to nearly 33%, according to National Institutes of Health statistics.
Childhood anxiety unquestionably increases the chances of persistent anxiety or depression in adulthood. I have followed children who had excessive social anxiety from age 3 or 4 who progressed to generalized anxiety disorder as adolescents, usually when no intervention was done or when the family waited for the child to “outgrow” it. The DSM-5 has six separate categories for anxiety disorders in children and adolescents: generalized anxiety disorder, separation anxiety disorder, specific phobias, social phobia, agoraphobia, and panic disorder. Unfortunately, these illnesses cannot be wished away.
Screening, diagnosis, and follow-up
A few simple screening tools can be used to check for anxiety in children and adolescents. These include SCARED (Screen for Child Anxiety Related Emotional Disorders), GAD-7 (Generalized Anxiety Disorder-7), and/or the PHQ-A (Patient Health Questionnaire for Adolescents). Keep in mind that a screening tool is just that – a screen. Diagnostic confirmation and follow-up are appropriate after a positive screen. I like all of these particular screens as they are easy to administer and can be incorporated into a busy practice without extra training to administer. They are also easy for parents and patients to complete prior to a visit or during a visit.
Ideally, after a positive screen, the next step is to consult a child and adolescent psychiatrist (CAP); however, according to statistics from the American Academy of Child and Adolescent Psychiatry (AACAP), there are only 8,300 CAPs in the United States. The reality is that not a single state in the entire country has a “mostly sufficient supply” of CAP’s (defined as ≥ 47 per 100,000 children). In fact, most have a “severe shortage,” defined as 1-17 per 100,000 children
Adding a child/adolescent therapist is also necessary for patients 8 years old and up, but the harsh truth is that it may take up to several months before the child is seen. If a patient is in a rural or other underserved area, it may be even longer.
So, what does this mean for primary care physicians? When you are faced with a positive screening for childhood anxiety, the next step is “tag, you’re it!” Understandably, this is frightening for many physicians who feel unqualified.
Don’t be afraid! Like the old adage says, a journey of a thousand miles begins with a single step. Starting the conversation with patients and families is foremost. Physicians must be first in line to end the stigma surrounding mental illness, and the easiest way to do that is to start the conversation. Remember that anxiety in kids can present as classic fear or worry, but it also can present as irritability, anger outbursts, and attention issues. There have been so many patients referred to me for “being out of control” or “always angry” or “probable ADHD” who turned out to have significant anxiety.
Part of a routine medical evaluation includes obtaining personal, family, and social history; there should be no difference when considering an anxiety disorder. Obtaining information about family history, personality traits, environmental components, early attachment issues, developmental history, parental style, parental conflict, occupants in the home, any adverse childhood events, and history of child maltreatment is crucial. Assessing other risk factors, including socioeconomic status, race, ethnicity, and gender, is key as well. I have seen families literally breathe a sigh of relief when these questions are asked. Parents feel heard and seen. And, equally significant, so does the child/adolescent.
The ‘Big 4’
An in-depth assessment of patient and family lifestyle factors such as nutrition, sleep, physical activity/exercise, and screen time habits is also basic and essential. This kind of evaluation usually cannot be done in the typical 15-minute visit and often will need to be done over several patient visits. I have had numerous conversations with my patients regarding what I call the “Big 4” – simple but not easy concepts and actions. They include nutrition, sleep, exercise, and screen time. Parents will look at me and say, “I can’t believe I never thought of this!” Some of my favorite moments with patients over the years have involved partnering with the patient and family and encouraging them to do the “simple” but not “easy” things.
Nutrition
Does the child have proper nutrition? That is not meant to be an exercise in labeling foods as “good” or “bad” but meant to confirm whether there is a balance of different foods. It’s also a way of exploring whether there are family meals in the home. Family meals have been shown to have a protective factor for children’s social development and emotional regulation.
Sleep
Review the child’s sleep habits, such as difficulty falling/staying asleep, bedtime routine (soothing, relaxing activities vs. the opposite), nightmares, snoring, nighttime cough, etc. The physical sleeping environment is important as well. Is it quiet? Is it a crowded room?
Exercise
Discuss physical activity with the family. Is there time for the child to play outside without a defined goal? So much of a child’s day is structured, in school or with after-school activities, but can the kid simply be a kid? Does the family take walks together? Is it safe to play outside?
Screen time
Reviewing screen time is important for multiple reasons, especially because the more time spent in front of a TV, computer, or video game, the less time there is to be physically active. Numerous experts, including the American Academy of Pediatrics, recommend limits on screen time for children. For adolescents, there appears to be some evidence that excessive screen time contributes to depression/anxiety.
I am not embarrassed to say that with my own kids I felt so strongly about screen time that we did not own any kind of video games or iPad (that was theirs alone), and they spent the summers until they turned 14 building a two-story bamboo fort in our backyard instead of vegging out in front of the TV or computer. It didn’t hurt them a bit; one is an engineer and the other is in nursing school.
It is easy to see that lifestyle factors can come into play with childhood anxiety and are often ignored in the clinical setting. They do not involve technologically advanced techniques or procedures, which are more likely to be reimbursed. They are straightforward – but not easy – concepts, and require active participation from the patient and family. Some of my most exciting moments with families is when they return for follow up and say, “It worked!”
We need to be as comfortable taking care of a child’s mind and spirit as we are taking care of a child’s physical body. Is this easy in a busy office? No. Is this easy in a 15-minute visit? No. Is this easy with poor reimbursement from insurance companies? No. Is it necessary? Unequivocally YES. Start the conversation.
Tag, you’re it!
Dr. Contrucci is an assistant professor of pediatrics, clinical education department, Philadelphia College of Osteopathic Medicine, Georgia Campus, Suwanee. She disclosed no relevant conflict of interest.
A version of this article first appeared on Medscape.com.
Multiple media outlets and numerous children’s professional organizations are discussing the child and adolescent mental health crisis. Finally, society at large seems to be taking notice that our kids are not okay, and that they haven’t been okay for a long time.
Over the past 5-7 years, both in my practice in tertiary children’s hospital emergency departments and in primary care pediatrics, I have seen a disturbing decline in kids’ mental well-being. What can a primary care physician do to make a difference? How do we capitalize on these discussions about mental health and illness now that it is rising to a priority status?
The U.S. Preventive Services Task Force recently drafted a statement of recommendations specifically discussing anxiety in children and adolescents. It shows supporting evidence that there is a moderate benefit to screening children 8-18 years old for anxiety. We know from the 2018-2019 National Survey of Children’s Health that almost 8% of children/adolescents ages 3-17 years old have an anxiety disorder. And among those 13-18 years old, the lifetime prevalence rises to nearly 33%, according to National Institutes of Health statistics.
Childhood anxiety unquestionably increases the chances of persistent anxiety or depression in adulthood. I have followed children who had excessive social anxiety from age 3 or 4 who progressed to generalized anxiety disorder as adolescents, usually when no intervention was done or when the family waited for the child to “outgrow” it. The DSM-5 has six separate categories for anxiety disorders in children and adolescents: generalized anxiety disorder, separation anxiety disorder, specific phobias, social phobia, agoraphobia, and panic disorder. Unfortunately, these illnesses cannot be wished away.
Screening, diagnosis, and follow-up
A few simple screening tools can be used to check for anxiety in children and adolescents. These include SCARED (Screen for Child Anxiety Related Emotional Disorders), GAD-7 (Generalized Anxiety Disorder-7), and/or the PHQ-A (Patient Health Questionnaire for Adolescents). Keep in mind that a screening tool is just that – a screen. Diagnostic confirmation and follow-up are appropriate after a positive screen. I like all of these particular screens as they are easy to administer and can be incorporated into a busy practice without extra training to administer. They are also easy for parents and patients to complete prior to a visit or during a visit.
Ideally, after a positive screen, the next step is to consult a child and adolescent psychiatrist (CAP); however, according to statistics from the American Academy of Child and Adolescent Psychiatry (AACAP), there are only 8,300 CAPs in the United States. The reality is that not a single state in the entire country has a “mostly sufficient supply” of CAP’s (defined as ≥ 47 per 100,000 children). In fact, most have a “severe shortage,” defined as 1-17 per 100,000 children
Adding a child/adolescent therapist is also necessary for patients 8 years old and up, but the harsh truth is that it may take up to several months before the child is seen. If a patient is in a rural or other underserved area, it may be even longer.
So, what does this mean for primary care physicians? When you are faced with a positive screening for childhood anxiety, the next step is “tag, you’re it!” Understandably, this is frightening for many physicians who feel unqualified.
Don’t be afraid! Like the old adage says, a journey of a thousand miles begins with a single step. Starting the conversation with patients and families is foremost. Physicians must be first in line to end the stigma surrounding mental illness, and the easiest way to do that is to start the conversation. Remember that anxiety in kids can present as classic fear or worry, but it also can present as irritability, anger outbursts, and attention issues. There have been so many patients referred to me for “being out of control” or “always angry” or “probable ADHD” who turned out to have significant anxiety.
Part of a routine medical evaluation includes obtaining personal, family, and social history; there should be no difference when considering an anxiety disorder. Obtaining information about family history, personality traits, environmental components, early attachment issues, developmental history, parental style, parental conflict, occupants in the home, any adverse childhood events, and history of child maltreatment is crucial. Assessing other risk factors, including socioeconomic status, race, ethnicity, and gender, is key as well. I have seen families literally breathe a sigh of relief when these questions are asked. Parents feel heard and seen. And, equally significant, so does the child/adolescent.
The ‘Big 4’
An in-depth assessment of patient and family lifestyle factors such as nutrition, sleep, physical activity/exercise, and screen time habits is also basic and essential. This kind of evaluation usually cannot be done in the typical 15-minute visit and often will need to be done over several patient visits. I have had numerous conversations with my patients regarding what I call the “Big 4” – simple but not easy concepts and actions. They include nutrition, sleep, exercise, and screen time. Parents will look at me and say, “I can’t believe I never thought of this!” Some of my favorite moments with patients over the years have involved partnering with the patient and family and encouraging them to do the “simple” but not “easy” things.
Nutrition
Does the child have proper nutrition? That is not meant to be an exercise in labeling foods as “good” or “bad” but meant to confirm whether there is a balance of different foods. It’s also a way of exploring whether there are family meals in the home. Family meals have been shown to have a protective factor for children’s social development and emotional regulation.
Sleep
Review the child’s sleep habits, such as difficulty falling/staying asleep, bedtime routine (soothing, relaxing activities vs. the opposite), nightmares, snoring, nighttime cough, etc. The physical sleeping environment is important as well. Is it quiet? Is it a crowded room?
Exercise
Discuss physical activity with the family. Is there time for the child to play outside without a defined goal? So much of a child’s day is structured, in school or with after-school activities, but can the kid simply be a kid? Does the family take walks together? Is it safe to play outside?
Screen time
Reviewing screen time is important for multiple reasons, especially because the more time spent in front of a TV, computer, or video game, the less time there is to be physically active. Numerous experts, including the American Academy of Pediatrics, recommend limits on screen time for children. For adolescents, there appears to be some evidence that excessive screen time contributes to depression/anxiety.
I am not embarrassed to say that with my own kids I felt so strongly about screen time that we did not own any kind of video games or iPad (that was theirs alone), and they spent the summers until they turned 14 building a two-story bamboo fort in our backyard instead of vegging out in front of the TV or computer. It didn’t hurt them a bit; one is an engineer and the other is in nursing school.
It is easy to see that lifestyle factors can come into play with childhood anxiety and are often ignored in the clinical setting. They do not involve technologically advanced techniques or procedures, which are more likely to be reimbursed. They are straightforward – but not easy – concepts, and require active participation from the patient and family. Some of my most exciting moments with families is when they return for follow up and say, “It worked!”
We need to be as comfortable taking care of a child’s mind and spirit as we are taking care of a child’s physical body. Is this easy in a busy office? No. Is this easy in a 15-minute visit? No. Is this easy with poor reimbursement from insurance companies? No. Is it necessary? Unequivocally YES. Start the conversation.
Tag, you’re it!
Dr. Contrucci is an assistant professor of pediatrics, clinical education department, Philadelphia College of Osteopathic Medicine, Georgia Campus, Suwanee. She disclosed no relevant conflict of interest.
A version of this article first appeared on Medscape.com.
Multiple media outlets and numerous children’s professional organizations are discussing the child and adolescent mental health crisis. Finally, society at large seems to be taking notice that our kids are not okay, and that they haven’t been okay for a long time.
Over the past 5-7 years, both in my practice in tertiary children’s hospital emergency departments and in primary care pediatrics, I have seen a disturbing decline in kids’ mental well-being. What can a primary care physician do to make a difference? How do we capitalize on these discussions about mental health and illness now that it is rising to a priority status?
The U.S. Preventive Services Task Force recently drafted a statement of recommendations specifically discussing anxiety in children and adolescents. It shows supporting evidence that there is a moderate benefit to screening children 8-18 years old for anxiety. We know from the 2018-2019 National Survey of Children’s Health that almost 8% of children/adolescents ages 3-17 years old have an anxiety disorder. And among those 13-18 years old, the lifetime prevalence rises to nearly 33%, according to National Institutes of Health statistics.
Childhood anxiety unquestionably increases the chances of persistent anxiety or depression in adulthood. I have followed children who had excessive social anxiety from age 3 or 4 who progressed to generalized anxiety disorder as adolescents, usually when no intervention was done or when the family waited for the child to “outgrow” it. The DSM-5 has six separate categories for anxiety disorders in children and adolescents: generalized anxiety disorder, separation anxiety disorder, specific phobias, social phobia, agoraphobia, and panic disorder. Unfortunately, these illnesses cannot be wished away.
Screening, diagnosis, and follow-up
A few simple screening tools can be used to check for anxiety in children and adolescents. These include SCARED (Screen for Child Anxiety Related Emotional Disorders), GAD-7 (Generalized Anxiety Disorder-7), and/or the PHQ-A (Patient Health Questionnaire for Adolescents). Keep in mind that a screening tool is just that – a screen. Diagnostic confirmation and follow-up are appropriate after a positive screen. I like all of these particular screens as they are easy to administer and can be incorporated into a busy practice without extra training to administer. They are also easy for parents and patients to complete prior to a visit or during a visit.
Ideally, after a positive screen, the next step is to consult a child and adolescent psychiatrist (CAP); however, according to statistics from the American Academy of Child and Adolescent Psychiatry (AACAP), there are only 8,300 CAPs in the United States. The reality is that not a single state in the entire country has a “mostly sufficient supply” of CAP’s (defined as ≥ 47 per 100,000 children). In fact, most have a “severe shortage,” defined as 1-17 per 100,000 children
Adding a child/adolescent therapist is also necessary for patients 8 years old and up, but the harsh truth is that it may take up to several months before the child is seen. If a patient is in a rural or other underserved area, it may be even longer.
So, what does this mean for primary care physicians? When you are faced with a positive screening for childhood anxiety, the next step is “tag, you’re it!” Understandably, this is frightening for many physicians who feel unqualified.
Don’t be afraid! Like the old adage says, a journey of a thousand miles begins with a single step. Starting the conversation with patients and families is foremost. Physicians must be first in line to end the stigma surrounding mental illness, and the easiest way to do that is to start the conversation. Remember that anxiety in kids can present as classic fear or worry, but it also can present as irritability, anger outbursts, and attention issues. There have been so many patients referred to me for “being out of control” or “always angry” or “probable ADHD” who turned out to have significant anxiety.
Part of a routine medical evaluation includes obtaining personal, family, and social history; there should be no difference when considering an anxiety disorder. Obtaining information about family history, personality traits, environmental components, early attachment issues, developmental history, parental style, parental conflict, occupants in the home, any adverse childhood events, and history of child maltreatment is crucial. Assessing other risk factors, including socioeconomic status, race, ethnicity, and gender, is key as well. I have seen families literally breathe a sigh of relief when these questions are asked. Parents feel heard and seen. And, equally significant, so does the child/adolescent.
The ‘Big 4’
An in-depth assessment of patient and family lifestyle factors such as nutrition, sleep, physical activity/exercise, and screen time habits is also basic and essential. This kind of evaluation usually cannot be done in the typical 15-minute visit and often will need to be done over several patient visits. I have had numerous conversations with my patients regarding what I call the “Big 4” – simple but not easy concepts and actions. They include nutrition, sleep, exercise, and screen time. Parents will look at me and say, “I can’t believe I never thought of this!” Some of my favorite moments with patients over the years have involved partnering with the patient and family and encouraging them to do the “simple” but not “easy” things.
Nutrition
Does the child have proper nutrition? That is not meant to be an exercise in labeling foods as “good” or “bad” but meant to confirm whether there is a balance of different foods. It’s also a way of exploring whether there are family meals in the home. Family meals have been shown to have a protective factor for children’s social development and emotional regulation.
Sleep
Review the child’s sleep habits, such as difficulty falling/staying asleep, bedtime routine (soothing, relaxing activities vs. the opposite), nightmares, snoring, nighttime cough, etc. The physical sleeping environment is important as well. Is it quiet? Is it a crowded room?
Exercise
Discuss physical activity with the family. Is there time for the child to play outside without a defined goal? So much of a child’s day is structured, in school or with after-school activities, but can the kid simply be a kid? Does the family take walks together? Is it safe to play outside?
Screen time
Reviewing screen time is important for multiple reasons, especially because the more time spent in front of a TV, computer, or video game, the less time there is to be physically active. Numerous experts, including the American Academy of Pediatrics, recommend limits on screen time for children. For adolescents, there appears to be some evidence that excessive screen time contributes to depression/anxiety.
I am not embarrassed to say that with my own kids I felt so strongly about screen time that we did not own any kind of video games or iPad (that was theirs alone), and they spent the summers until they turned 14 building a two-story bamboo fort in our backyard instead of vegging out in front of the TV or computer. It didn’t hurt them a bit; one is an engineer and the other is in nursing school.
It is easy to see that lifestyle factors can come into play with childhood anxiety and are often ignored in the clinical setting. They do not involve technologically advanced techniques or procedures, which are more likely to be reimbursed. They are straightforward – but not easy – concepts, and require active participation from the patient and family. Some of my most exciting moments with families is when they return for follow up and say, “It worked!”
We need to be as comfortable taking care of a child’s mind and spirit as we are taking care of a child’s physical body. Is this easy in a busy office? No. Is this easy in a 15-minute visit? No. Is this easy with poor reimbursement from insurance companies? No. Is it necessary? Unequivocally YES. Start the conversation.
Tag, you’re it!
Dr. Contrucci is an assistant professor of pediatrics, clinical education department, Philadelphia College of Osteopathic Medicine, Georgia Campus, Suwanee. She disclosed no relevant conflict of interest.
A version of this article first appeared on Medscape.com.
To vaccinate 6-month- to 5-year-olds against SARS-CoV-2 or not to vaccinate
A family’s decision to vaccinate their child is best made jointly with a trusted medical provider who knows the child and family. The American Academy of Pediatrics created a toolkit with resources for answering questions about the recently authorized SARS-CoV-2 mRNA vaccines (Pfizer and Moderna) for 6-month- to 5-year-olds with science-backed vaccine facts, including links to other useful AAP information websites, talking points, graphics, and videos.1
SARS-CoV-2 seasonality
SARS-CoV-2 is now endemic, not a once-a-year seasonal virus. Seasons (aka surges) will occur whenever a new variant arises (twice yearly since 2020, Omicron BA.4/BA.5 currently), or when enough vaccine holdouts, newborns, and/or those with waning of prior immunity (vaccine or infection induced) accrue.
Emergency use authorization submission data for mRNA vaccine responses in young children2,3
Moderna in 6-month- through 5-year-olds. Two 25-mcg doses given 4-8 weeks apart produced 37.8% (95% confidence interval, 20.9%-51.1%) protection against symptomatic Omicron SARS-CoV-2 infections through 3 months of follow-up. Immunobridging analysis of antibody responses compared to 18- to 25-year-olds (100-mcg doses) showed the children’s responses were noninferior. Thus, the committee inferred that vaccine effectiveness in children should be similar to that in 18- to 25-year-olds. Fever, irritability, or local reaction/pain occurred in two-thirds after the second dose. Grade 3 reactions were noted in less than 5%.
Pfizer in 6-month- through 4-year-olds. Three 3-mcg doses, two doses 3-8 weeks apart and the third dose at least 8 weeks later (median 16 weeks), produced 80.3% (95% CI, 13.9%-96.7%) protection against symptomatic COVID-19 during the 6 weeks after the third dose. Local and systemic reactions occurred in 63.8%; less than 5% had grade 3 reactions (fever in about 3%, irritability in 1.3%, fatigue in 0.8%) mostly after second dose.
Neither duration of follow-up is very long. The Moderna data tell me that a third primary dose would have been better but restarting the trial to evaluate third doses would have delayed Moderna’s EUA another 4-6 months. The three-dose Pfizer data look better but may not have been as good with another 6 weeks of follow-up.
Additional post-EUA data will be collected. Boosters will be needed when immunity from both vaccines wanes (one estimate is about 6 months after the primary series). The Advisory Committee on Immunization Practices noted in their deliberations that vaccine-induced antibody responses are higher and cross-neutralize variants (even Omicron) better than infection-induced immunity.4
Are there downsides to the vaccines? Naysayers question vaccinating children less than 5 years old with reasons containing enough “truth” that they catch people’s attention, for example, “young children don’t get very sick with COVID-19,” “most have been infected already,” “RNA for the spike protein stays in the body for months,” or “myocarditis.” Naysayers can quote references in reputable journals but seem to spin selected data out of context or quote unconfirmed data from the Vaccine Adverse Event Reporting System.
Reasons to vaccinate
- While children have milder disease than adults, mid-June 2022 surveillance indicated 50 hospitalizations and 1 pediatric death each day from SARS-CoV-2.5
- Vaccinating young children endows a foundation of vaccine-induced SARS-CoV-2 immunity that is superior to infection-induced immunity.4
- Long-term effects of large numbers of SARS-CoV-2 particles that enter every organ of a developing child have not been determined.
- Viral loads are lowered by prior vaccine; fewer viral replications lessen chances for newer variants to arise.
- Transmission is less in breakthrough infections than infections in the unvaccinated.
- Thirty percent of 5- to 11-year-olds hospitalized for SARS-CoV-2 had no underlying conditions;6 hospitalization rates in newborn to 4-year-olds have been the highest in the Omicron surge.7
- No myocarditis or pericarditis episodes have been detected in 6-month- to 11-year-old trials.
- The AAP and ACIP recommend the mRNA vaccines.
My thoughts are that SARS-CoV-2 vaccine is just another “routine” childhood vaccine that prepares children for healthier futures, pandemic or not, and the vaccines are as safe as other routine vaccines.
And like other pediatric vaccines, it should be no surprise that boosters will be needed, even if no newer variants than Omicron BA.4/BA.5 arise. But we know newer variants will arise and, similar to influenza vaccine, new formulations, perhaps with multiple SARS-CoV-2 strain antigens, will be needed every year or so. Everyone will get SARS-CoV-2 multiple times in their lives no matter how careful they are. So isn’t it good medical practice to establish early the best available foundation for maintaining lifelong SARS-CoV-2 immunity?
To me it is like pertussis. Most pertussis-infected children are sick enough to be hospitalized; very few die. They are miserable with illnesses that take weeks to months to subside. The worst disease usually occurs in unvaccinated young children or those with underlying conditions. Reactogenicity was reduced with acellular vaccine but resulted in less immunogenicity, so we give boosters at intervals that best match waning immunity. Circulating strains can be different than the vaccine strain, so protection against infection is 80%. Finally, even the safest vaccine may very rarely have sequelae. That is why The National Vaccine Injury Compensation Program was created. Yet the benefit-to-harm ratio for children and society favors universal pertussis vaccine use. And we vaccinate even those who have had pertussis because even infection-based immunity is incomplete and protection wanes. If arguments similar to those by SARS-CoV-2 vaccine naysayers were applied to acellular pertussis vaccine, it seems they would argue against pertussis vaccine for young children.
Another major issue has been “safety concerns” about the vaccines’ small amount of mRNA for the spike protein encased in microscopic lipid bubbles injected in the arm or leg. This mRNA is picked up by human cells, and in the cytoplasm (not the nucleus where our DNA resides) produces a limited supply of spike protein that is then picked up by antigen-presenting cells for short-lived distribution (days to 2 weeks at most) to regional lymph nodes where immune-memory processes are jump-started. Contrast that to even asymptomatic SARS-CoV-2 infection where multibillions of virus particles are produced for up to 14 days with access to every bodily organ that contains ACE-2 receptors (they all do). Each virus particle hijacks a human cell producing thousands of mRNA for spike protein (and multiple other SARS-CoV-2 proteins), eventually releasing multibillions of lipid fragments from the ruptured cell. Comparing the amount of these components in the mRNA vaccines to those from infection is like comparing a campfire to the many-thousand-acre wildfire. So, if one is worried about the effects of spike protein and lipid fragments, the limited localized amounts in mRNA vaccines should make one much less concerned than the enormous amounts circulating throughout the body as a result of a SARS-CoV-2 infection.
My take is that children 6-months to 5-years-old deserve SARS-CoV-2–induced vaccine protection and we can and should strongly recommend it as medical providers and child advocates.
*Dr. Harrison is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. AAP. 2022 Jun 21. As COVID-19 vaccines become available for children ages 6 months to 4 years, AAP urges families to reach out to pediatricians to ask questions and access vaccine. www.aap.org.
2. CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine for children aged 6 months–5 years. www.cdc.gov.
3. CDC. ACIP evidence to recommendations for use of Moderna COVID-19 vaccine in children ages 6 months–5 years and Pfizer-BioNTech COVID-19 vaccine in children ages 6 months–4 years under an emergency use authorization. www.cdc.gov.
4. Tang J et al. Nat Commun. 2022;13:2979.
5. Children and COVID-19: State Data Report. 2022 Jun 30. www.aap.org.
6. Shi DS et al. MMWR Morb Mortal Wkly Rep. 2022;71:574-81.
7. Marks KJ et al. MMWR Morb Mortal Wkly Rep. 2022;71:429-36.
Other good resources for families are https://getvaccineanswers.org/ or www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
*This story was updated on July 19, 2022.
A family’s decision to vaccinate their child is best made jointly with a trusted medical provider who knows the child and family. The American Academy of Pediatrics created a toolkit with resources for answering questions about the recently authorized SARS-CoV-2 mRNA vaccines (Pfizer and Moderna) for 6-month- to 5-year-olds with science-backed vaccine facts, including links to other useful AAP information websites, talking points, graphics, and videos.1
SARS-CoV-2 seasonality
SARS-CoV-2 is now endemic, not a once-a-year seasonal virus. Seasons (aka surges) will occur whenever a new variant arises (twice yearly since 2020, Omicron BA.4/BA.5 currently), or when enough vaccine holdouts, newborns, and/or those with waning of prior immunity (vaccine or infection induced) accrue.
Emergency use authorization submission data for mRNA vaccine responses in young children2,3
Moderna in 6-month- through 5-year-olds. Two 25-mcg doses given 4-8 weeks apart produced 37.8% (95% confidence interval, 20.9%-51.1%) protection against symptomatic Omicron SARS-CoV-2 infections through 3 months of follow-up. Immunobridging analysis of antibody responses compared to 18- to 25-year-olds (100-mcg doses) showed the children’s responses were noninferior. Thus, the committee inferred that vaccine effectiveness in children should be similar to that in 18- to 25-year-olds. Fever, irritability, or local reaction/pain occurred in two-thirds after the second dose. Grade 3 reactions were noted in less than 5%.
Pfizer in 6-month- through 4-year-olds. Three 3-mcg doses, two doses 3-8 weeks apart and the third dose at least 8 weeks later (median 16 weeks), produced 80.3% (95% CI, 13.9%-96.7%) protection against symptomatic COVID-19 during the 6 weeks after the third dose. Local and systemic reactions occurred in 63.8%; less than 5% had grade 3 reactions (fever in about 3%, irritability in 1.3%, fatigue in 0.8%) mostly after second dose.
Neither duration of follow-up is very long. The Moderna data tell me that a third primary dose would have been better but restarting the trial to evaluate third doses would have delayed Moderna’s EUA another 4-6 months. The three-dose Pfizer data look better but may not have been as good with another 6 weeks of follow-up.
Additional post-EUA data will be collected. Boosters will be needed when immunity from both vaccines wanes (one estimate is about 6 months after the primary series). The Advisory Committee on Immunization Practices noted in their deliberations that vaccine-induced antibody responses are higher and cross-neutralize variants (even Omicron) better than infection-induced immunity.4
Are there downsides to the vaccines? Naysayers question vaccinating children less than 5 years old with reasons containing enough “truth” that they catch people’s attention, for example, “young children don’t get very sick with COVID-19,” “most have been infected already,” “RNA for the spike protein stays in the body for months,” or “myocarditis.” Naysayers can quote references in reputable journals but seem to spin selected data out of context or quote unconfirmed data from the Vaccine Adverse Event Reporting System.
Reasons to vaccinate
- While children have milder disease than adults, mid-June 2022 surveillance indicated 50 hospitalizations and 1 pediatric death each day from SARS-CoV-2.5
- Vaccinating young children endows a foundation of vaccine-induced SARS-CoV-2 immunity that is superior to infection-induced immunity.4
- Long-term effects of large numbers of SARS-CoV-2 particles that enter every organ of a developing child have not been determined.
- Viral loads are lowered by prior vaccine; fewer viral replications lessen chances for newer variants to arise.
- Transmission is less in breakthrough infections than infections in the unvaccinated.
- Thirty percent of 5- to 11-year-olds hospitalized for SARS-CoV-2 had no underlying conditions;6 hospitalization rates in newborn to 4-year-olds have been the highest in the Omicron surge.7
- No myocarditis or pericarditis episodes have been detected in 6-month- to 11-year-old trials.
- The AAP and ACIP recommend the mRNA vaccines.
My thoughts are that SARS-CoV-2 vaccine is just another “routine” childhood vaccine that prepares children for healthier futures, pandemic or not, and the vaccines are as safe as other routine vaccines.
And like other pediatric vaccines, it should be no surprise that boosters will be needed, even if no newer variants than Omicron BA.4/BA.5 arise. But we know newer variants will arise and, similar to influenza vaccine, new formulations, perhaps with multiple SARS-CoV-2 strain antigens, will be needed every year or so. Everyone will get SARS-CoV-2 multiple times in their lives no matter how careful they are. So isn’t it good medical practice to establish early the best available foundation for maintaining lifelong SARS-CoV-2 immunity?
To me it is like pertussis. Most pertussis-infected children are sick enough to be hospitalized; very few die. They are miserable with illnesses that take weeks to months to subside. The worst disease usually occurs in unvaccinated young children or those with underlying conditions. Reactogenicity was reduced with acellular vaccine but resulted in less immunogenicity, so we give boosters at intervals that best match waning immunity. Circulating strains can be different than the vaccine strain, so protection against infection is 80%. Finally, even the safest vaccine may very rarely have sequelae. That is why The National Vaccine Injury Compensation Program was created. Yet the benefit-to-harm ratio for children and society favors universal pertussis vaccine use. And we vaccinate even those who have had pertussis because even infection-based immunity is incomplete and protection wanes. If arguments similar to those by SARS-CoV-2 vaccine naysayers were applied to acellular pertussis vaccine, it seems they would argue against pertussis vaccine for young children.
Another major issue has been “safety concerns” about the vaccines’ small amount of mRNA for the spike protein encased in microscopic lipid bubbles injected in the arm or leg. This mRNA is picked up by human cells, and in the cytoplasm (not the nucleus where our DNA resides) produces a limited supply of spike protein that is then picked up by antigen-presenting cells for short-lived distribution (days to 2 weeks at most) to regional lymph nodes where immune-memory processes are jump-started. Contrast that to even asymptomatic SARS-CoV-2 infection where multibillions of virus particles are produced for up to 14 days with access to every bodily organ that contains ACE-2 receptors (they all do). Each virus particle hijacks a human cell producing thousands of mRNA for spike protein (and multiple other SARS-CoV-2 proteins), eventually releasing multibillions of lipid fragments from the ruptured cell. Comparing the amount of these components in the mRNA vaccines to those from infection is like comparing a campfire to the many-thousand-acre wildfire. So, if one is worried about the effects of spike protein and lipid fragments, the limited localized amounts in mRNA vaccines should make one much less concerned than the enormous amounts circulating throughout the body as a result of a SARS-CoV-2 infection.
My take is that children 6-months to 5-years-old deserve SARS-CoV-2–induced vaccine protection and we can and should strongly recommend it as medical providers and child advocates.
*Dr. Harrison is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. AAP. 2022 Jun 21. As COVID-19 vaccines become available for children ages 6 months to 4 years, AAP urges families to reach out to pediatricians to ask questions and access vaccine. www.aap.org.
2. CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine for children aged 6 months–5 years. www.cdc.gov.
3. CDC. ACIP evidence to recommendations for use of Moderna COVID-19 vaccine in children ages 6 months–5 years and Pfizer-BioNTech COVID-19 vaccine in children ages 6 months–4 years under an emergency use authorization. www.cdc.gov.
4. Tang J et al. Nat Commun. 2022;13:2979.
5. Children and COVID-19: State Data Report. 2022 Jun 30. www.aap.org.
6. Shi DS et al. MMWR Morb Mortal Wkly Rep. 2022;71:574-81.
7. Marks KJ et al. MMWR Morb Mortal Wkly Rep. 2022;71:429-36.
Other good resources for families are https://getvaccineanswers.org/ or www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
*This story was updated on July 19, 2022.
A family’s decision to vaccinate their child is best made jointly with a trusted medical provider who knows the child and family. The American Academy of Pediatrics created a toolkit with resources for answering questions about the recently authorized SARS-CoV-2 mRNA vaccines (Pfizer and Moderna) for 6-month- to 5-year-olds with science-backed vaccine facts, including links to other useful AAP information websites, talking points, graphics, and videos.1
SARS-CoV-2 seasonality
SARS-CoV-2 is now endemic, not a once-a-year seasonal virus. Seasons (aka surges) will occur whenever a new variant arises (twice yearly since 2020, Omicron BA.4/BA.5 currently), or when enough vaccine holdouts, newborns, and/or those with waning of prior immunity (vaccine or infection induced) accrue.
Emergency use authorization submission data for mRNA vaccine responses in young children2,3
Moderna in 6-month- through 5-year-olds. Two 25-mcg doses given 4-8 weeks apart produced 37.8% (95% confidence interval, 20.9%-51.1%) protection against symptomatic Omicron SARS-CoV-2 infections through 3 months of follow-up. Immunobridging analysis of antibody responses compared to 18- to 25-year-olds (100-mcg doses) showed the children’s responses were noninferior. Thus, the committee inferred that vaccine effectiveness in children should be similar to that in 18- to 25-year-olds. Fever, irritability, or local reaction/pain occurred in two-thirds after the second dose. Grade 3 reactions were noted in less than 5%.
Pfizer in 6-month- through 4-year-olds. Three 3-mcg doses, two doses 3-8 weeks apart and the third dose at least 8 weeks later (median 16 weeks), produced 80.3% (95% CI, 13.9%-96.7%) protection against symptomatic COVID-19 during the 6 weeks after the third dose. Local and systemic reactions occurred in 63.8%; less than 5% had grade 3 reactions (fever in about 3%, irritability in 1.3%, fatigue in 0.8%) mostly after second dose.
Neither duration of follow-up is very long. The Moderna data tell me that a third primary dose would have been better but restarting the trial to evaluate third doses would have delayed Moderna’s EUA another 4-6 months. The three-dose Pfizer data look better but may not have been as good with another 6 weeks of follow-up.
Additional post-EUA data will be collected. Boosters will be needed when immunity from both vaccines wanes (one estimate is about 6 months after the primary series). The Advisory Committee on Immunization Practices noted in their deliberations that vaccine-induced antibody responses are higher and cross-neutralize variants (even Omicron) better than infection-induced immunity.4
Are there downsides to the vaccines? Naysayers question vaccinating children less than 5 years old with reasons containing enough “truth” that they catch people’s attention, for example, “young children don’t get very sick with COVID-19,” “most have been infected already,” “RNA for the spike protein stays in the body for months,” or “myocarditis.” Naysayers can quote references in reputable journals but seem to spin selected data out of context or quote unconfirmed data from the Vaccine Adverse Event Reporting System.
Reasons to vaccinate
- While children have milder disease than adults, mid-June 2022 surveillance indicated 50 hospitalizations and 1 pediatric death each day from SARS-CoV-2.5
- Vaccinating young children endows a foundation of vaccine-induced SARS-CoV-2 immunity that is superior to infection-induced immunity.4
- Long-term effects of large numbers of SARS-CoV-2 particles that enter every organ of a developing child have not been determined.
- Viral loads are lowered by prior vaccine; fewer viral replications lessen chances for newer variants to arise.
- Transmission is less in breakthrough infections than infections in the unvaccinated.
- Thirty percent of 5- to 11-year-olds hospitalized for SARS-CoV-2 had no underlying conditions;6 hospitalization rates in newborn to 4-year-olds have been the highest in the Omicron surge.7
- No myocarditis or pericarditis episodes have been detected in 6-month- to 11-year-old trials.
- The AAP and ACIP recommend the mRNA vaccines.
My thoughts are that SARS-CoV-2 vaccine is just another “routine” childhood vaccine that prepares children for healthier futures, pandemic or not, and the vaccines are as safe as other routine vaccines.
And like other pediatric vaccines, it should be no surprise that boosters will be needed, even if no newer variants than Omicron BA.4/BA.5 arise. But we know newer variants will arise and, similar to influenza vaccine, new formulations, perhaps with multiple SARS-CoV-2 strain antigens, will be needed every year or so. Everyone will get SARS-CoV-2 multiple times in their lives no matter how careful they are. So isn’t it good medical practice to establish early the best available foundation for maintaining lifelong SARS-CoV-2 immunity?
To me it is like pertussis. Most pertussis-infected children are sick enough to be hospitalized; very few die. They are miserable with illnesses that take weeks to months to subside. The worst disease usually occurs in unvaccinated young children or those with underlying conditions. Reactogenicity was reduced with acellular vaccine but resulted in less immunogenicity, so we give boosters at intervals that best match waning immunity. Circulating strains can be different than the vaccine strain, so protection against infection is 80%. Finally, even the safest vaccine may very rarely have sequelae. That is why The National Vaccine Injury Compensation Program was created. Yet the benefit-to-harm ratio for children and society favors universal pertussis vaccine use. And we vaccinate even those who have had pertussis because even infection-based immunity is incomplete and protection wanes. If arguments similar to those by SARS-CoV-2 vaccine naysayers were applied to acellular pertussis vaccine, it seems they would argue against pertussis vaccine for young children.
Another major issue has been “safety concerns” about the vaccines’ small amount of mRNA for the spike protein encased in microscopic lipid bubbles injected in the arm or leg. This mRNA is picked up by human cells, and in the cytoplasm (not the nucleus where our DNA resides) produces a limited supply of spike protein that is then picked up by antigen-presenting cells for short-lived distribution (days to 2 weeks at most) to regional lymph nodes where immune-memory processes are jump-started. Contrast that to even asymptomatic SARS-CoV-2 infection where multibillions of virus particles are produced for up to 14 days with access to every bodily organ that contains ACE-2 receptors (they all do). Each virus particle hijacks a human cell producing thousands of mRNA for spike protein (and multiple other SARS-CoV-2 proteins), eventually releasing multibillions of lipid fragments from the ruptured cell. Comparing the amount of these components in the mRNA vaccines to those from infection is like comparing a campfire to the many-thousand-acre wildfire. So, if one is worried about the effects of spike protein and lipid fragments, the limited localized amounts in mRNA vaccines should make one much less concerned than the enormous amounts circulating throughout the body as a result of a SARS-CoV-2 infection.
My take is that children 6-months to 5-years-old deserve SARS-CoV-2–induced vaccine protection and we can and should strongly recommend it as medical providers and child advocates.
*Dr. Harrison is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. AAP. 2022 Jun 21. As COVID-19 vaccines become available for children ages 6 months to 4 years, AAP urges families to reach out to pediatricians to ask questions and access vaccine. www.aap.org.
2. CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine for children aged 6 months–5 years. www.cdc.gov.
3. CDC. ACIP evidence to recommendations for use of Moderna COVID-19 vaccine in children ages 6 months–5 years and Pfizer-BioNTech COVID-19 vaccine in children ages 6 months–4 years under an emergency use authorization. www.cdc.gov.
4. Tang J et al. Nat Commun. 2022;13:2979.
5. Children and COVID-19: State Data Report. 2022 Jun 30. www.aap.org.
6. Shi DS et al. MMWR Morb Mortal Wkly Rep. 2022;71:574-81.
7. Marks KJ et al. MMWR Morb Mortal Wkly Rep. 2022;71:429-36.
Other good resources for families are https://getvaccineanswers.org/ or www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
*This story was updated on July 19, 2022.
Precocious puberty – how early is too soon?
A 6-year-old girl presents with breast development. Her medical history is unremarkable. The parents are of average height, and the mother reports her thelarche was age 11 years. The girl is at the 97th percentile for her height and 90th percentile for her weight. She has Tanner stage 3 breast development and Tanner stage 2 pubic hair development. She has grown slightly more than 3 inches over the past year. How should she be evaluated and managed (N Engl J Med. 2008;358:2366-77)?
The premature onset of puberty, i.e., precocious puberty (PP), can be an emotionally traumatic event for the child and parents. Over the past century, improvements in public health and nutrition, and, more recently, increased obesity, have been associated with earlier puberty and the dominant factor has been attributed to genetics (Curr Opin Endocrinol Diabetes Obes. 2018;25[1]:49-54). This month’s article will focus on understanding what is considered “early” puberty, evaluating for causes, and managing precocious puberty.
More commonly seen in girls than boys, PP is defined as the onset of secondary sexual characteristics before age 7.5 years in Black and Hispanic girls, and prior to 8 years in White girls, which is 2-2.5 standard deviations below the average age of pubertal onset in healthy children (J Pediatr Adolesc Gynecol. 2019;32:455-9). As a comparison, PP is diagnosed with onset before age 9 years in boys. For White compared with Black girls, the average timing of thelarche is age 10 vs. 9.5 years, peak growth velocity is age 11.5, menarche is age 12.5 vs. 12, while completion of puberty is near age 14.5 vs. 13.5, respectively (J Pediatr. 1985;107:317). Fortunately, most girls with PP have common variants rather than serious pathology.
Classification: Central (CPP) vs. peripheral (PPP)
CPP is gonadotropin dependent, meaning the hypothalamic-pituitary-ovarian axis (HPO) is prematurely activated resulting in the normal progression of puberty.
PPP is gonadotropin independent, caused by sex steroid secretion from any source – ovaries, adrenal gland, exogenous or ectopic production, e.g., germ-cell tumor. This results in a disordered progression of pubertal milestones.
Whereas CPP is typically isosexual development, i.e., consistent with the child’s gender, PPP can be isosexual or contrasexual, e.g., virilization of girls. A third classification is “benign or nonprogressive pubertal variants” manifesting as isolated premature thelarche or adrenarche.
Causes (see table)
CPP. Idiopathic causes account for 80%-90% of presentations in girls and 25%-80% in boys. Remarkably, international and domestic adoption, as well as a family history of PP increases the likelihood of CPP in girls. Other etiologies include CNS lesions, e.g., hamartomas, which are the most common cause of PP in young children. MRI with contrast has been the traditional mode of diagnosis for CNS tumors, yet the yield is dubious in girls above age 6. Genetic causes are found in only a small percentage of PP cases. Rarely, CPP can result from gonadotropin-secreting tumors because of elevated luteinizing hormone levels.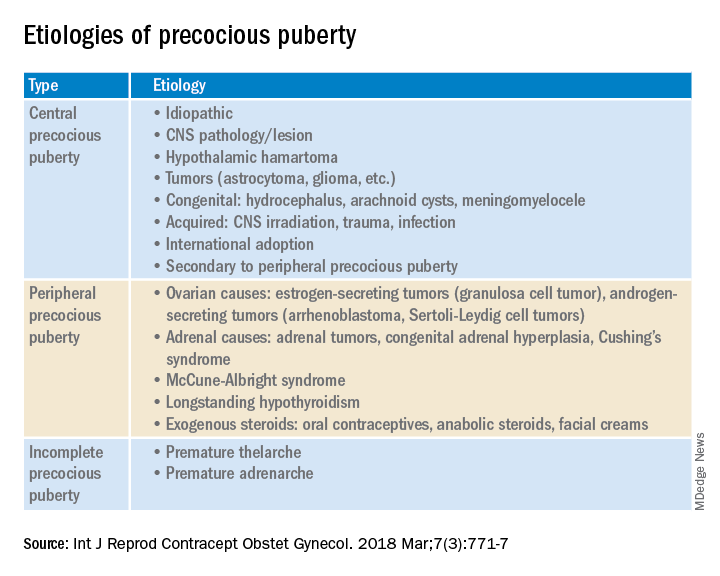
PPP. As a result of sex steroid secretion, peripheral causes of PPP include ovarian cysts and ovarian tumors that increase circulating estradiol, such as granulosa cell tumors, which would cause isosexual PPP and Sertoli-Leydig cell tumors that secrete testosterone, which can result in contrasexual PPP. Mild congenital adrenal hyperplasia can result in PPP with virilization (contrasexual) and markedly advanced bone age.
McCune-Albright syndrome is rare and presents with the classic triad of PPP, skin pigmentation called café-au-lait, and fibrous dysplasia of bone. The pathophysiology of McCune-Albright syndrome is autoactivation of the G-protein leading to activation of ovarian tissue that results in formation of large ovarian cysts and extreme elevations in serum estradiol as well as the potential production of other hormones, e.g., thyrotoxicosis, excess growth hormone (acromegaly), and Cushing syndrome.
Premature thelarche. Premature thelarche typically occurs in girls between the ages of 1 and 3 years and is limited to breast enlargement. While no cause has been determined, the plausible explanations include partial activation of the HPO axis, endocrine-disrupting chemicals (EDCs), or a genetic origin. A small percentage of these girls progress to CPP.
EDCs have been considered as potential influencers of early puberty, but no consensus has been established. (Examples of EDCs in the environment include air, soil, or water supply along with food sources, personal care products, and manufactured products that can affect the endocrine system.)
Premature adenarche. Premature adrenarche presents with adult body odor and/or body hair (pubic and/or axillary) in girls who have an elevated body mass index, most commonly at the ages of 6-7 years. The presumed mechanism is normal maturation of the adrenal gland with resultant elevation of circulating androgens. Bone age may be mildly accelerated and DHEAS is prematurely elevated for age. These girls appear to be at increased risk for polycystic ovary syndrome.
Evaluation
The initial step in the evaluation of PP is to determine whether the cause is CPP or PPP; the latter includes distinguishing isosexual from contrasexual development. A thorough history (growth, headaches, behavior or visual change, seizures, abdominal pain), physical exam, including Tanner staging, and bone age is required. However, with isolated premature thelarche or adrenarche, a bone age may not be necessary, as initial close clinical observation for pubertal progression is likely sufficient.
For CPP, the diagnosis is based on serum LH, whether random levels or elevations follow GnRH stimulation. Puberty milestones progress normally although adrenarche is not consistently apparent. For girls younger than age 6, a brain MRI is recommended but not in asymptomatic older girls with CPP. LH and FSH along with estradiol or testosterone, the latter especially in boys, are the first line of serum testing. Serum TSH is recommended for suspicion of primary hypothyroidism. In girls with premature adrenarche, a bone age, testosterone, DHEAS, and 17-OHP to rule out adrenal hyperplasia should be obtained. Pelvic ultrasound may be a useful adjunct to assess uterine volume and/or ovarian cysts/tumors.
Rapidity of onset can also lead the evaluation since a normal growth chart and skeletal maturation suggests a benign pubertal variant whereas a more rapid rate can signal CPP or PPP. Of note, health care providers should ensure prescription, over-the-counter oral or topical sources of hormones, and EDCs are ruled out.
Consequences
An association between childhood sexual abuse and earlier pubertal onset has been cited. These girls may be at increased risk for psychosocial difficulties, menstrual and fertility problems, and even reproductive cancers because of prolonged exposure to sex hormones (J Adolesc Health. 2016;60[1]:65-71).
Treatment
The mainstay of CPP treatment is maximizing adult height, typically through the use of a GnRH agonist for HPO suppression from pituitary downregulation. For girls above age 8 years, attempts at improving adult height have not shown a benefit.
In girls with PPP, treatment is directed at the prevailing pathology. Interestingly, early PPP can activate the HPO axis thereby converting to “secondary” CPP. In PPP, McCune-Albright syndrome treatment targets reducing circulating estrogens through letrozole or tamoxifen as well as addressing other autoactivated hormone production. Ovarian and adrenal tumors, albeit rare, can cause PP; therefore, surgical excision is the goal of treatment.
PP should be approached with equal concerns about the physical and emotional effects while including the family to help them understand the pathophysiology and psychosocial risks.
Dr. Mark P. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A 6-year-old girl presents with breast development. Her medical history is unremarkable. The parents are of average height, and the mother reports her thelarche was age 11 years. The girl is at the 97th percentile for her height and 90th percentile for her weight. She has Tanner stage 3 breast development and Tanner stage 2 pubic hair development. She has grown slightly more than 3 inches over the past year. How should she be evaluated and managed (N Engl J Med. 2008;358:2366-77)?
The premature onset of puberty, i.e., precocious puberty (PP), can be an emotionally traumatic event for the child and parents. Over the past century, improvements in public health and nutrition, and, more recently, increased obesity, have been associated with earlier puberty and the dominant factor has been attributed to genetics (Curr Opin Endocrinol Diabetes Obes. 2018;25[1]:49-54). This month’s article will focus on understanding what is considered “early” puberty, evaluating for causes, and managing precocious puberty.
More commonly seen in girls than boys, PP is defined as the onset of secondary sexual characteristics before age 7.5 years in Black and Hispanic girls, and prior to 8 years in White girls, which is 2-2.5 standard deviations below the average age of pubertal onset in healthy children (J Pediatr Adolesc Gynecol. 2019;32:455-9). As a comparison, PP is diagnosed with onset before age 9 years in boys. For White compared with Black girls, the average timing of thelarche is age 10 vs. 9.5 years, peak growth velocity is age 11.5, menarche is age 12.5 vs. 12, while completion of puberty is near age 14.5 vs. 13.5, respectively (J Pediatr. 1985;107:317). Fortunately, most girls with PP have common variants rather than serious pathology.
Classification: Central (CPP) vs. peripheral (PPP)
CPP is gonadotropin dependent, meaning the hypothalamic-pituitary-ovarian axis (HPO) is prematurely activated resulting in the normal progression of puberty.
PPP is gonadotropin independent, caused by sex steroid secretion from any source – ovaries, adrenal gland, exogenous or ectopic production, e.g., germ-cell tumor. This results in a disordered progression of pubertal milestones.
Whereas CPP is typically isosexual development, i.e., consistent with the child’s gender, PPP can be isosexual or contrasexual, e.g., virilization of girls. A third classification is “benign or nonprogressive pubertal variants” manifesting as isolated premature thelarche or adrenarche.
Causes (see table)
CPP. Idiopathic causes account for 80%-90% of presentations in girls and 25%-80% in boys. Remarkably, international and domestic adoption, as well as a family history of PP increases the likelihood of CPP in girls. Other etiologies include CNS lesions, e.g., hamartomas, which are the most common cause of PP in young children. MRI with contrast has been the traditional mode of diagnosis for CNS tumors, yet the yield is dubious in girls above age 6. Genetic causes are found in only a small percentage of PP cases. Rarely, CPP can result from gonadotropin-secreting tumors because of elevated luteinizing hormone levels.
PPP. As a result of sex steroid secretion, peripheral causes of PPP include ovarian cysts and ovarian tumors that increase circulating estradiol, such as granulosa cell tumors, which would cause isosexual PPP and Sertoli-Leydig cell tumors that secrete testosterone, which can result in contrasexual PPP. Mild congenital adrenal hyperplasia can result in PPP with virilization (contrasexual) and markedly advanced bone age.
McCune-Albright syndrome is rare and presents with the classic triad of PPP, skin pigmentation called café-au-lait, and fibrous dysplasia of bone. The pathophysiology of McCune-Albright syndrome is autoactivation of the G-protein leading to activation of ovarian tissue that results in formation of large ovarian cysts and extreme elevations in serum estradiol as well as the potential production of other hormones, e.g., thyrotoxicosis, excess growth hormone (acromegaly), and Cushing syndrome.
Premature thelarche. Premature thelarche typically occurs in girls between the ages of 1 and 3 years and is limited to breast enlargement. While no cause has been determined, the plausible explanations include partial activation of the HPO axis, endocrine-disrupting chemicals (EDCs), or a genetic origin. A small percentage of these girls progress to CPP.
EDCs have been considered as potential influencers of early puberty, but no consensus has been established. (Examples of EDCs in the environment include air, soil, or water supply along with food sources, personal care products, and manufactured products that can affect the endocrine system.)
Premature adenarche. Premature adrenarche presents with adult body odor and/or body hair (pubic and/or axillary) in girls who have an elevated body mass index, most commonly at the ages of 6-7 years. The presumed mechanism is normal maturation of the adrenal gland with resultant elevation of circulating androgens. Bone age may be mildly accelerated and DHEAS is prematurely elevated for age. These girls appear to be at increased risk for polycystic ovary syndrome.
Evaluation
The initial step in the evaluation of PP is to determine whether the cause is CPP or PPP; the latter includes distinguishing isosexual from contrasexual development. A thorough history (growth, headaches, behavior or visual change, seizures, abdominal pain), physical exam, including Tanner staging, and bone age is required. However, with isolated premature thelarche or adrenarche, a bone age may not be necessary, as initial close clinical observation for pubertal progression is likely sufficient.
For CPP, the diagnosis is based on serum LH, whether random levels or elevations follow GnRH stimulation. Puberty milestones progress normally although adrenarche is not consistently apparent. For girls younger than age 6, a brain MRI is recommended but not in asymptomatic older girls with CPP. LH and FSH along with estradiol or testosterone, the latter especially in boys, are the first line of serum testing. Serum TSH is recommended for suspicion of primary hypothyroidism. In girls with premature adrenarche, a bone age, testosterone, DHEAS, and 17-OHP to rule out adrenal hyperplasia should be obtained. Pelvic ultrasound may be a useful adjunct to assess uterine volume and/or ovarian cysts/tumors.
Rapidity of onset can also lead the evaluation since a normal growth chart and skeletal maturation suggests a benign pubertal variant whereas a more rapid rate can signal CPP or PPP. Of note, health care providers should ensure prescription, over-the-counter oral or topical sources of hormones, and EDCs are ruled out.
Consequences
An association between childhood sexual abuse and earlier pubertal onset has been cited. These girls may be at increased risk for psychosocial difficulties, menstrual and fertility problems, and even reproductive cancers because of prolonged exposure to sex hormones (J Adolesc Health. 2016;60[1]:65-71).
Treatment
The mainstay of CPP treatment is maximizing adult height, typically through the use of a GnRH agonist for HPO suppression from pituitary downregulation. For girls above age 8 years, attempts at improving adult height have not shown a benefit.
In girls with PPP, treatment is directed at the prevailing pathology. Interestingly, early PPP can activate the HPO axis thereby converting to “secondary” CPP. In PPP, McCune-Albright syndrome treatment targets reducing circulating estrogens through letrozole or tamoxifen as well as addressing other autoactivated hormone production. Ovarian and adrenal tumors, albeit rare, can cause PP; therefore, surgical excision is the goal of treatment.
PP should be approached with equal concerns about the physical and emotional effects while including the family to help them understand the pathophysiology and psychosocial risks.
Dr. Mark P. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A 6-year-old girl presents with breast development. Her medical history is unremarkable. The parents are of average height, and the mother reports her thelarche was age 11 years. The girl is at the 97th percentile for her height and 90th percentile for her weight. She has Tanner stage 3 breast development and Tanner stage 2 pubic hair development. She has grown slightly more than 3 inches over the past year. How should she be evaluated and managed (N Engl J Med. 2008;358:2366-77)?
The premature onset of puberty, i.e., precocious puberty (PP), can be an emotionally traumatic event for the child and parents. Over the past century, improvements in public health and nutrition, and, more recently, increased obesity, have been associated with earlier puberty and the dominant factor has been attributed to genetics (Curr Opin Endocrinol Diabetes Obes. 2018;25[1]:49-54). This month’s article will focus on understanding what is considered “early” puberty, evaluating for causes, and managing precocious puberty.
More commonly seen in girls than boys, PP is defined as the onset of secondary sexual characteristics before age 7.5 years in Black and Hispanic girls, and prior to 8 years in White girls, which is 2-2.5 standard deviations below the average age of pubertal onset in healthy children (J Pediatr Adolesc Gynecol. 2019;32:455-9). As a comparison, PP is diagnosed with onset before age 9 years in boys. For White compared with Black girls, the average timing of thelarche is age 10 vs. 9.5 years, peak growth velocity is age 11.5, menarche is age 12.5 vs. 12, while completion of puberty is near age 14.5 vs. 13.5, respectively (J Pediatr. 1985;107:317). Fortunately, most girls with PP have common variants rather than serious pathology.
Classification: Central (CPP) vs. peripheral (PPP)
CPP is gonadotropin dependent, meaning the hypothalamic-pituitary-ovarian axis (HPO) is prematurely activated resulting in the normal progression of puberty.
PPP is gonadotropin independent, caused by sex steroid secretion from any source – ovaries, adrenal gland, exogenous or ectopic production, e.g., germ-cell tumor. This results in a disordered progression of pubertal milestones.
Whereas CPP is typically isosexual development, i.e., consistent with the child’s gender, PPP can be isosexual or contrasexual, e.g., virilization of girls. A third classification is “benign or nonprogressive pubertal variants” manifesting as isolated premature thelarche or adrenarche.
Causes (see table)
CPP. Idiopathic causes account for 80%-90% of presentations in girls and 25%-80% in boys. Remarkably, international and domestic adoption, as well as a family history of PP increases the likelihood of CPP in girls. Other etiologies include CNS lesions, e.g., hamartomas, which are the most common cause of PP in young children. MRI with contrast has been the traditional mode of diagnosis for CNS tumors, yet the yield is dubious in girls above age 6. Genetic causes are found in only a small percentage of PP cases. Rarely, CPP can result from gonadotropin-secreting tumors because of elevated luteinizing hormone levels.
PPP. As a result of sex steroid secretion, peripheral causes of PPP include ovarian cysts and ovarian tumors that increase circulating estradiol, such as granulosa cell tumors, which would cause isosexual PPP and Sertoli-Leydig cell tumors that secrete testosterone, which can result in contrasexual PPP. Mild congenital adrenal hyperplasia can result in PPP with virilization (contrasexual) and markedly advanced bone age.
McCune-Albright syndrome is rare and presents with the classic triad of PPP, skin pigmentation called café-au-lait, and fibrous dysplasia of bone. The pathophysiology of McCune-Albright syndrome is autoactivation of the G-protein leading to activation of ovarian tissue that results in formation of large ovarian cysts and extreme elevations in serum estradiol as well as the potential production of other hormones, e.g., thyrotoxicosis, excess growth hormone (acromegaly), and Cushing syndrome.
Premature thelarche. Premature thelarche typically occurs in girls between the ages of 1 and 3 years and is limited to breast enlargement. While no cause has been determined, the plausible explanations include partial activation of the HPO axis, endocrine-disrupting chemicals (EDCs), or a genetic origin. A small percentage of these girls progress to CPP.
EDCs have been considered as potential influencers of early puberty, but no consensus has been established. (Examples of EDCs in the environment include air, soil, or water supply along with food sources, personal care products, and manufactured products that can affect the endocrine system.)
Premature adenarche. Premature adrenarche presents with adult body odor and/or body hair (pubic and/or axillary) in girls who have an elevated body mass index, most commonly at the ages of 6-7 years. The presumed mechanism is normal maturation of the adrenal gland with resultant elevation of circulating androgens. Bone age may be mildly accelerated and DHEAS is prematurely elevated for age. These girls appear to be at increased risk for polycystic ovary syndrome.
Evaluation
The initial step in the evaluation of PP is to determine whether the cause is CPP or PPP; the latter includes distinguishing isosexual from contrasexual development. A thorough history (growth, headaches, behavior or visual change, seizures, abdominal pain), physical exam, including Tanner staging, and bone age is required. However, with isolated premature thelarche or adrenarche, a bone age may not be necessary, as initial close clinical observation for pubertal progression is likely sufficient.
For CPP, the diagnosis is based on serum LH, whether random levels or elevations follow GnRH stimulation. Puberty milestones progress normally although adrenarche is not consistently apparent. For girls younger than age 6, a brain MRI is recommended but not in asymptomatic older girls with CPP. LH and FSH along with estradiol or testosterone, the latter especially in boys, are the first line of serum testing. Serum TSH is recommended for suspicion of primary hypothyroidism. In girls with premature adrenarche, a bone age, testosterone, DHEAS, and 17-OHP to rule out adrenal hyperplasia should be obtained. Pelvic ultrasound may be a useful adjunct to assess uterine volume and/or ovarian cysts/tumors.
Rapidity of onset can also lead the evaluation since a normal growth chart and skeletal maturation suggests a benign pubertal variant whereas a more rapid rate can signal CPP or PPP. Of note, health care providers should ensure prescription, over-the-counter oral or topical sources of hormones, and EDCs are ruled out.
Consequences
An association between childhood sexual abuse and earlier pubertal onset has been cited. These girls may be at increased risk for psychosocial difficulties, menstrual and fertility problems, and even reproductive cancers because of prolonged exposure to sex hormones (J Adolesc Health. 2016;60[1]:65-71).
Treatment
The mainstay of CPP treatment is maximizing adult height, typically through the use of a GnRH agonist for HPO suppression from pituitary downregulation. For girls above age 8 years, attempts at improving adult height have not shown a benefit.
In girls with PPP, treatment is directed at the prevailing pathology. Interestingly, early PPP can activate the HPO axis thereby converting to “secondary” CPP. In PPP, McCune-Albright syndrome treatment targets reducing circulating estrogens through letrozole or tamoxifen as well as addressing other autoactivated hormone production. Ovarian and adrenal tumors, albeit rare, can cause PP; therefore, surgical excision is the goal of treatment.
PP should be approached with equal concerns about the physical and emotional effects while including the family to help them understand the pathophysiology and psychosocial risks.
Dr. Mark P. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.














