User login
VA Ketamine Controversies
To the Editor: We read with interest the editorial on the clinical use of intranasal esketamine in treatment-resistant depression by Editor-in-Chief Cynthia Geppert in the October 2019 issue of Federal Practitioner.1 A recent case report published in your journal illustrated the success of IV ketamine in alleviating refractory chronic pain caused by a rare disease.2 Ketamine has been well established as an appropriate adjuvant as well as an alternative to opioids in attenuating acute postoperative pain and in certain chronic pain syndromes.3 We write out of concern for the rapidity of adoption of intranasal esketamine without considering the merits of IV ketamine.
When adopting new treatments or extending established drugs for newer indications, clinicians must balance beneficence and nonmaleficence. There is an urgent need for better treatment options for depression, suicidality, posttraumatic stress disorder (PTSD), and chronic pain in the veteran population. However, one must proceed with caution before wide adoption of a treatment that lacks real-world data on sustained or long-term benefits.4 Enthusiasm for this drug must also be tempered by the documented adverse effect (AE) of hepatic injury and the lack of data tracking this AE from repeated, long-term use.5 With these considerations in mind, reliable dosing and predictable pharmacokinetics are of great importance.
In addition to outpatient esketamine, outpatient IV administration of racemic ketamine remains an advantageous option with unique benefits compared with esketamine. Pharmacokinetically, IV ketamine is superior to intranasal esketamine. The bioavailability of intranasal esketamine is likely to be variable. A patient with a poor intranasal application or poor absorption might be falsely labeled an esketamine nonresponder. Increasing intranasal esketamine dosage to avoid false nonresponders may place other patients at risk for overdose and undesired AEs, including dysphoria and hallucinations. The variable bioavailability of intranasal ketamine adds complexity to the examination of its clinical effectiveness. IV ketamine should provide a predictable drug level and more reliable data. One might retort that esketamine is not the same as ketamine. True, esketamine is the S-enantiomer of ketamine, whereas ketamine is a racemic mixture of S- and R-ketamine. However, there is no clear evidence of clinically relevant differences between these formulations.5
Psychomimetic effects and cardiovascular changes are the most common short-term AEs resulting from ketamine.5 An IV infusion allows the treating physician to slowly titrate the administered ketamine to reach an effective concentration at the target site. Unlike an all-or-none intranasal administration, an infusion can be stopped at the first appearance of an AE. Psychomimetic effects, such as hallucinations, visual disturbances, and dysphoria are thought to occur in a dose-dependent fashion and remit once a ketamine infusion is stopped.5 Furthermore, cardiovascular AEs, such as hypertension and tachycardia, are commonly in patients with a body mass index > 30, with IV administration on a mg/kg basis. This suggests that calculated ideal body weight is a safer denominator, and reliable dosing is important to mitigating AEs.6
We urge caution with the widespread adoption of intranasal esketamine and suggest the advantages of the IV route, which offers predictability of AEs and titratability of dose. Questions remain regarding the appropriate dose and formulation of ketamine, rate of infusion, and route of administration for chronic pain and psychiatric indications.5,7 It is our responsibility to further study the long-term safety profile of ketamine and determine an appropriate dose of ketamine. The IV route allows many veterans to be helped in a safe and controllable manner.
Eugene Raggi, MD; and Srikantha L. Rao, MD, MS, FAS
1. Geppert CMA. The VA ketamine controversies. Fed Pract. 2019;36(10):446-447.
2. Eliason AH, Seo Y, Murphy D, Beal C. Adiposis dolorosa pain management. Fed Pract. 2019;36(11):530-533.
3. Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241-254.
4. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci. 2019;44(6):384-385.
5. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
6. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
7. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852-e857.
To the Editor: We read with interest the editorial on the clinical use of intranasal esketamine in treatment-resistant depression by Editor-in-Chief Cynthia Geppert in the October 2019 issue of Federal Practitioner.1 A recent case report published in your journal illustrated the success of IV ketamine in alleviating refractory chronic pain caused by a rare disease.2 Ketamine has been well established as an appropriate adjuvant as well as an alternative to opioids in attenuating acute postoperative pain and in certain chronic pain syndromes.3 We write out of concern for the rapidity of adoption of intranasal esketamine without considering the merits of IV ketamine.
When adopting new treatments or extending established drugs for newer indications, clinicians must balance beneficence and nonmaleficence. There is an urgent need for better treatment options for depression, suicidality, posttraumatic stress disorder (PTSD), and chronic pain in the veteran population. However, one must proceed with caution before wide adoption of a treatment that lacks real-world data on sustained or long-term benefits.4 Enthusiasm for this drug must also be tempered by the documented adverse effect (AE) of hepatic injury and the lack of data tracking this AE from repeated, long-term use.5 With these considerations in mind, reliable dosing and predictable pharmacokinetics are of great importance.
In addition to outpatient esketamine, outpatient IV administration of racemic ketamine remains an advantageous option with unique benefits compared with esketamine. Pharmacokinetically, IV ketamine is superior to intranasal esketamine. The bioavailability of intranasal esketamine is likely to be variable. A patient with a poor intranasal application or poor absorption might be falsely labeled an esketamine nonresponder. Increasing intranasal esketamine dosage to avoid false nonresponders may place other patients at risk for overdose and undesired AEs, including dysphoria and hallucinations. The variable bioavailability of intranasal ketamine adds complexity to the examination of its clinical effectiveness. IV ketamine should provide a predictable drug level and more reliable data. One might retort that esketamine is not the same as ketamine. True, esketamine is the S-enantiomer of ketamine, whereas ketamine is a racemic mixture of S- and R-ketamine. However, there is no clear evidence of clinically relevant differences between these formulations.5
Psychomimetic effects and cardiovascular changes are the most common short-term AEs resulting from ketamine.5 An IV infusion allows the treating physician to slowly titrate the administered ketamine to reach an effective concentration at the target site. Unlike an all-or-none intranasal administration, an infusion can be stopped at the first appearance of an AE. Psychomimetic effects, such as hallucinations, visual disturbances, and dysphoria are thought to occur in a dose-dependent fashion and remit once a ketamine infusion is stopped.5 Furthermore, cardiovascular AEs, such as hypertension and tachycardia, are commonly in patients with a body mass index > 30, with IV administration on a mg/kg basis. This suggests that calculated ideal body weight is a safer denominator, and reliable dosing is important to mitigating AEs.6
We urge caution with the widespread adoption of intranasal esketamine and suggest the advantages of the IV route, which offers predictability of AEs and titratability of dose. Questions remain regarding the appropriate dose and formulation of ketamine, rate of infusion, and route of administration for chronic pain and psychiatric indications.5,7 It is our responsibility to further study the long-term safety profile of ketamine and determine an appropriate dose of ketamine. The IV route allows many veterans to be helped in a safe and controllable manner.
Eugene Raggi, MD; and Srikantha L. Rao, MD, MS, FAS
To the Editor: We read with interest the editorial on the clinical use of intranasal esketamine in treatment-resistant depression by Editor-in-Chief Cynthia Geppert in the October 2019 issue of Federal Practitioner.1 A recent case report published in your journal illustrated the success of IV ketamine in alleviating refractory chronic pain caused by a rare disease.2 Ketamine has been well established as an appropriate adjuvant as well as an alternative to opioids in attenuating acute postoperative pain and in certain chronic pain syndromes.3 We write out of concern for the rapidity of adoption of intranasal esketamine without considering the merits of IV ketamine.
When adopting new treatments or extending established drugs for newer indications, clinicians must balance beneficence and nonmaleficence. There is an urgent need for better treatment options for depression, suicidality, posttraumatic stress disorder (PTSD), and chronic pain in the veteran population. However, one must proceed with caution before wide adoption of a treatment that lacks real-world data on sustained or long-term benefits.4 Enthusiasm for this drug must also be tempered by the documented adverse effect (AE) of hepatic injury and the lack of data tracking this AE from repeated, long-term use.5 With these considerations in mind, reliable dosing and predictable pharmacokinetics are of great importance.
In addition to outpatient esketamine, outpatient IV administration of racemic ketamine remains an advantageous option with unique benefits compared with esketamine. Pharmacokinetically, IV ketamine is superior to intranasal esketamine. The bioavailability of intranasal esketamine is likely to be variable. A patient with a poor intranasal application or poor absorption might be falsely labeled an esketamine nonresponder. Increasing intranasal esketamine dosage to avoid false nonresponders may place other patients at risk for overdose and undesired AEs, including dysphoria and hallucinations. The variable bioavailability of intranasal ketamine adds complexity to the examination of its clinical effectiveness. IV ketamine should provide a predictable drug level and more reliable data. One might retort that esketamine is not the same as ketamine. True, esketamine is the S-enantiomer of ketamine, whereas ketamine is a racemic mixture of S- and R-ketamine. However, there is no clear evidence of clinically relevant differences between these formulations.5
Psychomimetic effects and cardiovascular changes are the most common short-term AEs resulting from ketamine.5 An IV infusion allows the treating physician to slowly titrate the administered ketamine to reach an effective concentration at the target site. Unlike an all-or-none intranasal administration, an infusion can be stopped at the first appearance of an AE. Psychomimetic effects, such as hallucinations, visual disturbances, and dysphoria are thought to occur in a dose-dependent fashion and remit once a ketamine infusion is stopped.5 Furthermore, cardiovascular AEs, such as hypertension and tachycardia, are commonly in patients with a body mass index > 30, with IV administration on a mg/kg basis. This suggests that calculated ideal body weight is a safer denominator, and reliable dosing is important to mitigating AEs.6
We urge caution with the widespread adoption of intranasal esketamine and suggest the advantages of the IV route, which offers predictability of AEs and titratability of dose. Questions remain regarding the appropriate dose and formulation of ketamine, rate of infusion, and route of administration for chronic pain and psychiatric indications.5,7 It is our responsibility to further study the long-term safety profile of ketamine and determine an appropriate dose of ketamine. The IV route allows many veterans to be helped in a safe and controllable manner.
Eugene Raggi, MD; and Srikantha L. Rao, MD, MS, FAS
1. Geppert CMA. The VA ketamine controversies. Fed Pract. 2019;36(10):446-447.
2. Eliason AH, Seo Y, Murphy D, Beal C. Adiposis dolorosa pain management. Fed Pract. 2019;36(11):530-533.
3. Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241-254.
4. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci. 2019;44(6):384-385.
5. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
6. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
7. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852-e857.
1. Geppert CMA. The VA ketamine controversies. Fed Pract. 2019;36(10):446-447.
2. Eliason AH, Seo Y, Murphy D, Beal C. Adiposis dolorosa pain management. Fed Pract. 2019;36(11):530-533.
3. Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241-254.
4. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci. 2019;44(6):384-385.
5. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
6. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
7. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852-e857.
The Worst and the Best of 2019
Readers may recall that at the end of each calendar as opposed to fiscal year—I know it is hard to believe time exists outside the Federal system—Federal Practitioner publishes my ethics-focused version of the familiar year-end roundup. This year I am reversing the typical order of most annual rankings by putting the worst first for 2 morally salient reasons.
The first is that, sadly, it is almost always easier to identify multiple incidents that compete ignominiously for the “worst” of federal health care. Even more disappointing, it is comparatively difficult to find stories for the “best” that are of the same scale and scope as the bad news. This is not to say that every day there are not individual narratives of courage and compassion reported in US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA), and hundreds more unsung heroes.
The second reason is that as human beings our psychology is such that we gravitate toward the worst things more powerfully and persistently than we do the best. This is in part why it is more difficult to find uplifting stories and why the demoralizing ones affect us so strongly. In an exhaustive review of the subject, psychologists Roy Baumeister and colleagues conclude that,
When equal measures of good and bad are present, however, the psychological effects of bad ones outweigh those of the good ones. This may in fact be a general principle or law of psychological phenomena, possibly reflecting the innate predispositions of the psyche or at least reflecting the almost inevitable adaptation of each individual to the exigencies of daily life.2
I am thus saving the best for last in the hope that it will be more memorable and impactful than the worst.
Unique to this year’s look-back, both the negative and the positive accounts come from the domain of end-of-life care. And unlike prior reviews where the lack of administrative vigilance and professional competence affected hundreds of patients, families, and staff, each of this year’s incidents involve a single patient.
An incident that occurred in September 2019 at a VA Community Living Center (CLC) in Georgia stood out in infamy apart from all others. It was the report of a veteran in a VA nursing home who had been bitten more than 100 times by ants crawling all over his room. He died shortly afterward. In a scene out of a horror movie tapping into the most primeval human fears, his daughter Laquana Ross described her father, a Vietnam Air Force veteran with cancer, to media and VA officials in graphic terms. “I understand mistakes happen,” she said. “I’ve had ants. But he was bit by ants two days in a row. They feasted on him.”3
In this new era of holding its senior executive service accountable, the outraged chair of the Senate Veterans Affairs Committee demanded that heads roll, and the VA acted rapidly to comply.4 The VA Central Office placed the network director on administrative leave, reassigned the chief medical officer, and initiated quality and safety reviews as well as an administrative investigative board to scrutinize how the parent Atlanta VA medical center managed the situation. In total, 9 officials connected to the incident were placed on leave. The VA apologized, with VA Secretary Robert Wilke zeroing in on the core values involved in the tragedy, “This is about basic humanity and dignity,” he said. “I don’t care what steps were taken to address the issues. We did not treat a vet with the dignity that he and his family deserved.”5 Yet it was the veteran’s daughter, with unbelievable charity, who asked the most crucial question that must be answered within the framework of a just culture if similar tragedies are not to occur in the future, “I know the staff, without a shadow of doubt, respected my dad and even loved him,” Ross said. “But what’s their ability to assess situations and fix things?”3
To begin to give Ms. Ross the answer she deserves, we must understand that the antithesis of love is not hate but indifference; of compassion, it is not cruelty but coldness. A true just culture reserves individual blame for those who have ill-will and adopts a systems perspective of organizational improvement toward most other types of errors.6 This means that the deplorable conditions in the CLC cannot be charged to the failure of a single staff member to fulfil their obligations but to collective collapse at many levels of the organization. Just culture is ethically laudable and far superior to the history in federal service of capricious punishment or institutional apathy that far too often were the default reactions to media exposures or congressional ire. Justice, though necessary, is not sufficient to achieve virtue. Those who work in health care also must be inspired to offer mercy, kindness, and compassion, especially in our most sacred privilege to provide care of the dying.
The best of 2019 illustrates this distinction movingly. This account also involves a Vietnam veteran, this time a Marine also dying of cancer, which happened just about a month after the earlier report. To be transparent it occurred at my home VA medical center in New Mexico. I was peripherally involved in the case as a consultant but had no role in the wondrous things that transpired. The last wish of a patient dying in the hospice unit on campus was to see his beloved dog who had been taken to the local city animal shelter when he was hospitalized because he had no friends or family to look after the companion animal. A social worker on the palliative care team called the animal shelter and explained the patient did not have much time left but wanted to see his dog before he died. Working together with support from facility leadership, shelter workers brought the dog to visit with the patient for an entire day while hospice staff cried with joy and sadness.7
As the epigraph for this editorial from Dame Cicely Saunders, the founder of the modern hospice movement, says, the difference between unspeakable pain and meaningful suffering can be measured in the depth of compassion caregivers show to the dying. It is this quality of mercy that in one case condemns, and in the other praises, us all as health care and administrative professionals in the service of our country. Baumeister and colleagues suggest that the human tendency to magnify the bad and minimize the good in everyday myopia may in a wider vision actually be a reason for hope:
It may be that humans and animals show heightened awareness of and responded more quickly to negative information because it signals a need for change. Hence, the adaptiveness of self-regulation partly lies in the organism’s ability to detect when response modifications are necessary and when they are unnecessary. Moreover, the lessons learned from bad events should ideally be retained permanently so that the same dangers or costs are not encountered repeatedly. Meanwhile, good events (such as those that provide a feeling of satisfaction and contentment) should ideally wear off so that the organism is motivated to continue searching for more and better outcomes.2
Let us all take this lesson into our work in 2020 so that when it comes time to write this column next year in the chilling cold of late autumn there will be more stories of light than darkness from which to choose.
1. Saunders C. The management of patients in the terminal stage. In: Raven R, ed. Cancer, Vol. 6. London: Butterworth and Company; 1960:403-417.
2. Baumeister RF, Bratslavasky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev General Psychol. 2001;5(4);323-370.
3. Knowles H. ‘They feasted on him’: Ants at VA nursing home bite a veteran 100 times before his death, daughter says. Washington Post. September 17, 2019. https://www.washingtonpost.com/health/2019/09/13/they-feasted-him-ants-va-nursing-home-bit-veteran-times-before-his-death-daughter-says. Accessed November 25, 2019.
4. Axelrod T. GOP senator presses VA after veteran reportedly bitten by ants in nursing home. https://thehill.com/homenews/senate/461196-gop-senator-presses-va-after-veteran-reportedly-bitten-by-ants-at-nursing. Published September 12, 2019. Accessed November 25, 2019.
5. Kime P. Nine VA leaders, staff placed on leave amid anti-bite scandal. https://www.military.com/daily-news/2019/09/17/nine-va-leaders-staff-placed-leave-amid-ant-bite-scandal.html. Published September 17, 2019. Accessed November 22, 2019.
6. Sculli GL, Hemphill R. Culture of safety and just culture. https://www.patientsafety.va.gov/docs/joe/just_culture_2013_tagged.pdf. Accessed November 22, 2019.
7. Hughes M. A Vietnam veteran in hospice care got to see his beloved dog one last time. https://www.cnn.com/2019/10/21/us/veteran-dying-wish-dog-trnd/index.html. Published October 21, 2019. Accessed November 22, 2019.
Readers may recall that at the end of each calendar as opposed to fiscal year—I know it is hard to believe time exists outside the Federal system—Federal Practitioner publishes my ethics-focused version of the familiar year-end roundup. This year I am reversing the typical order of most annual rankings by putting the worst first for 2 morally salient reasons.
The first is that, sadly, it is almost always easier to identify multiple incidents that compete ignominiously for the “worst” of federal health care. Even more disappointing, it is comparatively difficult to find stories for the “best” that are of the same scale and scope as the bad news. This is not to say that every day there are not individual narratives of courage and compassion reported in US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA), and hundreds more unsung heroes.
The second reason is that as human beings our psychology is such that we gravitate toward the worst things more powerfully and persistently than we do the best. This is in part why it is more difficult to find uplifting stories and why the demoralizing ones affect us so strongly. In an exhaustive review of the subject, psychologists Roy Baumeister and colleagues conclude that,
When equal measures of good and bad are present, however, the psychological effects of bad ones outweigh those of the good ones. This may in fact be a general principle or law of psychological phenomena, possibly reflecting the innate predispositions of the psyche or at least reflecting the almost inevitable adaptation of each individual to the exigencies of daily life.2
I am thus saving the best for last in the hope that it will be more memorable and impactful than the worst.
Unique to this year’s look-back, both the negative and the positive accounts come from the domain of end-of-life care. And unlike prior reviews where the lack of administrative vigilance and professional competence affected hundreds of patients, families, and staff, each of this year’s incidents involve a single patient.
An incident that occurred in September 2019 at a VA Community Living Center (CLC) in Georgia stood out in infamy apart from all others. It was the report of a veteran in a VA nursing home who had been bitten more than 100 times by ants crawling all over his room. He died shortly afterward. In a scene out of a horror movie tapping into the most primeval human fears, his daughter Laquana Ross described her father, a Vietnam Air Force veteran with cancer, to media and VA officials in graphic terms. “I understand mistakes happen,” she said. “I’ve had ants. But he was bit by ants two days in a row. They feasted on him.”3
In this new era of holding its senior executive service accountable, the outraged chair of the Senate Veterans Affairs Committee demanded that heads roll, and the VA acted rapidly to comply.4 The VA Central Office placed the network director on administrative leave, reassigned the chief medical officer, and initiated quality and safety reviews as well as an administrative investigative board to scrutinize how the parent Atlanta VA medical center managed the situation. In total, 9 officials connected to the incident were placed on leave. The VA apologized, with VA Secretary Robert Wilke zeroing in on the core values involved in the tragedy, “This is about basic humanity and dignity,” he said. “I don’t care what steps were taken to address the issues. We did not treat a vet with the dignity that he and his family deserved.”5 Yet it was the veteran’s daughter, with unbelievable charity, who asked the most crucial question that must be answered within the framework of a just culture if similar tragedies are not to occur in the future, “I know the staff, without a shadow of doubt, respected my dad and even loved him,” Ross said. “But what’s their ability to assess situations and fix things?”3
To begin to give Ms. Ross the answer she deserves, we must understand that the antithesis of love is not hate but indifference; of compassion, it is not cruelty but coldness. A true just culture reserves individual blame for those who have ill-will and adopts a systems perspective of organizational improvement toward most other types of errors.6 This means that the deplorable conditions in the CLC cannot be charged to the failure of a single staff member to fulfil their obligations but to collective collapse at many levels of the organization. Just culture is ethically laudable and far superior to the history in federal service of capricious punishment or institutional apathy that far too often were the default reactions to media exposures or congressional ire. Justice, though necessary, is not sufficient to achieve virtue. Those who work in health care also must be inspired to offer mercy, kindness, and compassion, especially in our most sacred privilege to provide care of the dying.
The best of 2019 illustrates this distinction movingly. This account also involves a Vietnam veteran, this time a Marine also dying of cancer, which happened just about a month after the earlier report. To be transparent it occurred at my home VA medical center in New Mexico. I was peripherally involved in the case as a consultant but had no role in the wondrous things that transpired. The last wish of a patient dying in the hospice unit on campus was to see his beloved dog who had been taken to the local city animal shelter when he was hospitalized because he had no friends or family to look after the companion animal. A social worker on the palliative care team called the animal shelter and explained the patient did not have much time left but wanted to see his dog before he died. Working together with support from facility leadership, shelter workers brought the dog to visit with the patient for an entire day while hospice staff cried with joy and sadness.7
As the epigraph for this editorial from Dame Cicely Saunders, the founder of the modern hospice movement, says, the difference between unspeakable pain and meaningful suffering can be measured in the depth of compassion caregivers show to the dying. It is this quality of mercy that in one case condemns, and in the other praises, us all as health care and administrative professionals in the service of our country. Baumeister and colleagues suggest that the human tendency to magnify the bad and minimize the good in everyday myopia may in a wider vision actually be a reason for hope:
It may be that humans and animals show heightened awareness of and responded more quickly to negative information because it signals a need for change. Hence, the adaptiveness of self-regulation partly lies in the organism’s ability to detect when response modifications are necessary and when they are unnecessary. Moreover, the lessons learned from bad events should ideally be retained permanently so that the same dangers or costs are not encountered repeatedly. Meanwhile, good events (such as those that provide a feeling of satisfaction and contentment) should ideally wear off so that the organism is motivated to continue searching for more and better outcomes.2
Let us all take this lesson into our work in 2020 so that when it comes time to write this column next year in the chilling cold of late autumn there will be more stories of light than darkness from which to choose.
Readers may recall that at the end of each calendar as opposed to fiscal year—I know it is hard to believe time exists outside the Federal system—Federal Practitioner publishes my ethics-focused version of the familiar year-end roundup. This year I am reversing the typical order of most annual rankings by putting the worst first for 2 morally salient reasons.
The first is that, sadly, it is almost always easier to identify multiple incidents that compete ignominiously for the “worst” of federal health care. Even more disappointing, it is comparatively difficult to find stories for the “best” that are of the same scale and scope as the bad news. This is not to say that every day there are not individual narratives of courage and compassion reported in US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA), and hundreds more unsung heroes.
The second reason is that as human beings our psychology is such that we gravitate toward the worst things more powerfully and persistently than we do the best. This is in part why it is more difficult to find uplifting stories and why the demoralizing ones affect us so strongly. In an exhaustive review of the subject, psychologists Roy Baumeister and colleagues conclude that,
When equal measures of good and bad are present, however, the psychological effects of bad ones outweigh those of the good ones. This may in fact be a general principle or law of psychological phenomena, possibly reflecting the innate predispositions of the psyche or at least reflecting the almost inevitable adaptation of each individual to the exigencies of daily life.2
I am thus saving the best for last in the hope that it will be more memorable and impactful than the worst.
Unique to this year’s look-back, both the negative and the positive accounts come from the domain of end-of-life care. And unlike prior reviews where the lack of administrative vigilance and professional competence affected hundreds of patients, families, and staff, each of this year’s incidents involve a single patient.
An incident that occurred in September 2019 at a VA Community Living Center (CLC) in Georgia stood out in infamy apart from all others. It was the report of a veteran in a VA nursing home who had been bitten more than 100 times by ants crawling all over his room. He died shortly afterward. In a scene out of a horror movie tapping into the most primeval human fears, his daughter Laquana Ross described her father, a Vietnam Air Force veteran with cancer, to media and VA officials in graphic terms. “I understand mistakes happen,” she said. “I’ve had ants. But he was bit by ants two days in a row. They feasted on him.”3
In this new era of holding its senior executive service accountable, the outraged chair of the Senate Veterans Affairs Committee demanded that heads roll, and the VA acted rapidly to comply.4 The VA Central Office placed the network director on administrative leave, reassigned the chief medical officer, and initiated quality and safety reviews as well as an administrative investigative board to scrutinize how the parent Atlanta VA medical center managed the situation. In total, 9 officials connected to the incident were placed on leave. The VA apologized, with VA Secretary Robert Wilke zeroing in on the core values involved in the tragedy, “This is about basic humanity and dignity,” he said. “I don’t care what steps were taken to address the issues. We did not treat a vet with the dignity that he and his family deserved.”5 Yet it was the veteran’s daughter, with unbelievable charity, who asked the most crucial question that must be answered within the framework of a just culture if similar tragedies are not to occur in the future, “I know the staff, without a shadow of doubt, respected my dad and even loved him,” Ross said. “But what’s their ability to assess situations and fix things?”3
To begin to give Ms. Ross the answer she deserves, we must understand that the antithesis of love is not hate but indifference; of compassion, it is not cruelty but coldness. A true just culture reserves individual blame for those who have ill-will and adopts a systems perspective of organizational improvement toward most other types of errors.6 This means that the deplorable conditions in the CLC cannot be charged to the failure of a single staff member to fulfil their obligations but to collective collapse at many levels of the organization. Just culture is ethically laudable and far superior to the history in federal service of capricious punishment or institutional apathy that far too often were the default reactions to media exposures or congressional ire. Justice, though necessary, is not sufficient to achieve virtue. Those who work in health care also must be inspired to offer mercy, kindness, and compassion, especially in our most sacred privilege to provide care of the dying.
The best of 2019 illustrates this distinction movingly. This account also involves a Vietnam veteran, this time a Marine also dying of cancer, which happened just about a month after the earlier report. To be transparent it occurred at my home VA medical center in New Mexico. I was peripherally involved in the case as a consultant but had no role in the wondrous things that transpired. The last wish of a patient dying in the hospice unit on campus was to see his beloved dog who had been taken to the local city animal shelter when he was hospitalized because he had no friends or family to look after the companion animal. A social worker on the palliative care team called the animal shelter and explained the patient did not have much time left but wanted to see his dog before he died. Working together with support from facility leadership, shelter workers brought the dog to visit with the patient for an entire day while hospice staff cried with joy and sadness.7
As the epigraph for this editorial from Dame Cicely Saunders, the founder of the modern hospice movement, says, the difference between unspeakable pain and meaningful suffering can be measured in the depth of compassion caregivers show to the dying. It is this quality of mercy that in one case condemns, and in the other praises, us all as health care and administrative professionals in the service of our country. Baumeister and colleagues suggest that the human tendency to magnify the bad and minimize the good in everyday myopia may in a wider vision actually be a reason for hope:
It may be that humans and animals show heightened awareness of and responded more quickly to negative information because it signals a need for change. Hence, the adaptiveness of self-regulation partly lies in the organism’s ability to detect when response modifications are necessary and when they are unnecessary. Moreover, the lessons learned from bad events should ideally be retained permanently so that the same dangers or costs are not encountered repeatedly. Meanwhile, good events (such as those that provide a feeling of satisfaction and contentment) should ideally wear off so that the organism is motivated to continue searching for more and better outcomes.2
Let us all take this lesson into our work in 2020 so that when it comes time to write this column next year in the chilling cold of late autumn there will be more stories of light than darkness from which to choose.
1. Saunders C. The management of patients in the terminal stage. In: Raven R, ed. Cancer, Vol. 6. London: Butterworth and Company; 1960:403-417.
2. Baumeister RF, Bratslavasky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev General Psychol. 2001;5(4);323-370.
3. Knowles H. ‘They feasted on him’: Ants at VA nursing home bite a veteran 100 times before his death, daughter says. Washington Post. September 17, 2019. https://www.washingtonpost.com/health/2019/09/13/they-feasted-him-ants-va-nursing-home-bit-veteran-times-before-his-death-daughter-says. Accessed November 25, 2019.
4. Axelrod T. GOP senator presses VA after veteran reportedly bitten by ants in nursing home. https://thehill.com/homenews/senate/461196-gop-senator-presses-va-after-veteran-reportedly-bitten-by-ants-at-nursing. Published September 12, 2019. Accessed November 25, 2019.
5. Kime P. Nine VA leaders, staff placed on leave amid anti-bite scandal. https://www.military.com/daily-news/2019/09/17/nine-va-leaders-staff-placed-leave-amid-ant-bite-scandal.html. Published September 17, 2019. Accessed November 22, 2019.
6. Sculli GL, Hemphill R. Culture of safety and just culture. https://www.patientsafety.va.gov/docs/joe/just_culture_2013_tagged.pdf. Accessed November 22, 2019.
7. Hughes M. A Vietnam veteran in hospice care got to see his beloved dog one last time. https://www.cnn.com/2019/10/21/us/veteran-dying-wish-dog-trnd/index.html. Published October 21, 2019. Accessed November 22, 2019.
1. Saunders C. The management of patients in the terminal stage. In: Raven R, ed. Cancer, Vol. 6. London: Butterworth and Company; 1960:403-417.
2. Baumeister RF, Bratslavasky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev General Psychol. 2001;5(4);323-370.
3. Knowles H. ‘They feasted on him’: Ants at VA nursing home bite a veteran 100 times before his death, daughter says. Washington Post. September 17, 2019. https://www.washingtonpost.com/health/2019/09/13/they-feasted-him-ants-va-nursing-home-bit-veteran-times-before-his-death-daughter-says. Accessed November 25, 2019.
4. Axelrod T. GOP senator presses VA after veteran reportedly bitten by ants in nursing home. https://thehill.com/homenews/senate/461196-gop-senator-presses-va-after-veteran-reportedly-bitten-by-ants-at-nursing. Published September 12, 2019. Accessed November 25, 2019.
5. Kime P. Nine VA leaders, staff placed on leave amid anti-bite scandal. https://www.military.com/daily-news/2019/09/17/nine-va-leaders-staff-placed-leave-amid-ant-bite-scandal.html. Published September 17, 2019. Accessed November 22, 2019.
6. Sculli GL, Hemphill R. Culture of safety and just culture. https://www.patientsafety.va.gov/docs/joe/just_culture_2013_tagged.pdf. Accessed November 22, 2019.
7. Hughes M. A Vietnam veteran in hospice care got to see his beloved dog one last time. https://www.cnn.com/2019/10/21/us/veteran-dying-wish-dog-trnd/index.html. Published October 21, 2019. Accessed November 22, 2019.
Bariatric surgery should be considered in individuals with class 1 obesity
Mitchel L. Zoler’s article on Abstract A105, presented at Obesity Week 2019, addresses an important health concern and is timely.
Over the past 4 decades we have seen a rise in the prevalence of obesity and associated health complications, not just in the United States but across the world. The incidence of obesity (having a BMI greater than 30) was 35% for women and 31% for men in the United States, and associated deaths and disability were primarily attributed to diabetes and cardiovascular disease resulting from obesity.
This article references the benefits of bariatric/metabolic surgery in individuals with class 1 obesity. In the United States, more than half of those who meet the criteria for obesity come under the class 1 category (BMI, 30-34.9). Those in this class of obesity are at increased risk of developing diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular disease, obstructive sleep apnea, polycystic ovarian syndrome, and bone and joint disorders.
There are several studies that document the significant reduction in incidence of the above cardiometabolic risks with sustained weight loss. Nonsurgical interventions in individuals with class 1 obesity through lifestyle modifications and pharmacotherapy have not demonstrated success in providing persistent weight loss or metabolic benefits. The data presented in this article are of great significance to patients and physicians alike as they highlight the long-term benefits and reversal of metabolic disorders.
Current guidelines for bariatric surgery for individuals with a BMI greater than 35 were published in 1991. Since then several safe surgical options including laparoscopic procedures, sleeve gastrectomy, and adjustable gastric banding have been developed with decreased surgical risks, morbidity, and mortality.
The International Federation for the Surgery of Obesity and Metabolic Disorders, the International Diabetes Federation, and the National Institute for Health and Care Excellence of the United Kingdom, have supported the option of bariatric surgery in class 1 obese individuals with metabolic disorders.
While lifestyle modifications with medications should be the first-line treatment for class 1 obesity, as a primary care physician I believe that, given the major changes in the surgical options, the proven long-term benefits, and the rising incidences of obesity and metabolic syndrome, it is time for the health care community, insurers, patients, and all other stakeholders to consider bariatric surgery in class 1 obese individuals as a potential and viable option.
Noel N. Deep, MD, is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, Wausau, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News.
He made these comments in response to questions from MDedge and had no relevant disclosures.
Mitchel L. Zoler’s article on Abstract A105, presented at Obesity Week 2019, addresses an important health concern and is timely.
Over the past 4 decades we have seen a rise in the prevalence of obesity and associated health complications, not just in the United States but across the world. The incidence of obesity (having a BMI greater than 30) was 35% for women and 31% for men in the United States, and associated deaths and disability were primarily attributed to diabetes and cardiovascular disease resulting from obesity.
This article references the benefits of bariatric/metabolic surgery in individuals with class 1 obesity. In the United States, more than half of those who meet the criteria for obesity come under the class 1 category (BMI, 30-34.9). Those in this class of obesity are at increased risk of developing diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular disease, obstructive sleep apnea, polycystic ovarian syndrome, and bone and joint disorders.
There are several studies that document the significant reduction in incidence of the above cardiometabolic risks with sustained weight loss. Nonsurgical interventions in individuals with class 1 obesity through lifestyle modifications and pharmacotherapy have not demonstrated success in providing persistent weight loss or metabolic benefits. The data presented in this article are of great significance to patients and physicians alike as they highlight the long-term benefits and reversal of metabolic disorders.
Current guidelines for bariatric surgery for individuals with a BMI greater than 35 were published in 1991. Since then several safe surgical options including laparoscopic procedures, sleeve gastrectomy, and adjustable gastric banding have been developed with decreased surgical risks, morbidity, and mortality.
The International Federation for the Surgery of Obesity and Metabolic Disorders, the International Diabetes Federation, and the National Institute for Health and Care Excellence of the United Kingdom, have supported the option of bariatric surgery in class 1 obese individuals with metabolic disorders.
While lifestyle modifications with medications should be the first-line treatment for class 1 obesity, as a primary care physician I believe that, given the major changes in the surgical options, the proven long-term benefits, and the rising incidences of obesity and metabolic syndrome, it is time for the health care community, insurers, patients, and all other stakeholders to consider bariatric surgery in class 1 obese individuals as a potential and viable option.
Noel N. Deep, MD, is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, Wausau, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News.
He made these comments in response to questions from MDedge and had no relevant disclosures.
Mitchel L. Zoler’s article on Abstract A105, presented at Obesity Week 2019, addresses an important health concern and is timely.
Over the past 4 decades we have seen a rise in the prevalence of obesity and associated health complications, not just in the United States but across the world. The incidence of obesity (having a BMI greater than 30) was 35% for women and 31% for men in the United States, and associated deaths and disability were primarily attributed to diabetes and cardiovascular disease resulting from obesity.
This article references the benefits of bariatric/metabolic surgery in individuals with class 1 obesity. In the United States, more than half of those who meet the criteria for obesity come under the class 1 category (BMI, 30-34.9). Those in this class of obesity are at increased risk of developing diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular disease, obstructive sleep apnea, polycystic ovarian syndrome, and bone and joint disorders.
There are several studies that document the significant reduction in incidence of the above cardiometabolic risks with sustained weight loss. Nonsurgical interventions in individuals with class 1 obesity through lifestyle modifications and pharmacotherapy have not demonstrated success in providing persistent weight loss or metabolic benefits. The data presented in this article are of great significance to patients and physicians alike as they highlight the long-term benefits and reversal of metabolic disorders.
Current guidelines for bariatric surgery for individuals with a BMI greater than 35 were published in 1991. Since then several safe surgical options including laparoscopic procedures, sleeve gastrectomy, and adjustable gastric banding have been developed with decreased surgical risks, morbidity, and mortality.
The International Federation for the Surgery of Obesity and Metabolic Disorders, the International Diabetes Federation, and the National Institute for Health and Care Excellence of the United Kingdom, have supported the option of bariatric surgery in class 1 obese individuals with metabolic disorders.
While lifestyle modifications with medications should be the first-line treatment for class 1 obesity, as a primary care physician I believe that, given the major changes in the surgical options, the proven long-term benefits, and the rising incidences of obesity and metabolic syndrome, it is time for the health care community, insurers, patients, and all other stakeholders to consider bariatric surgery in class 1 obese individuals as a potential and viable option.
Noel N. Deep, MD, is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, Wausau, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News.
He made these comments in response to questions from MDedge and had no relevant disclosures.
Progesterone supplementation does not PROLONG pregnancy in women at risk for preterm birth: What do we do now?
Preterm birth (PTB) remains a significant public health concern and a major cause of newborn morbidity and mortality. In the United States, 1 in 10 babies are born preterm (< 37 weeks), and this rate has changed little in 30 years.1
In 2011, the US Food and Drug Administration (FDA) approved progesterone supplementation—specifically, α-hydroxyprogesterone caproate (17P) injection (Makena)—to prevent recurrent PTB in women with a singleton pregnancy at high risk by virtue of a prior spontaneous PTB.2 This was the first-ever FDA-approved drug for PTB prevention, and it was the first drug approved by the FDA for use in pregnancy in more than 15 years. The approval of 17P utilized the FDA's Subpart H Accelerated Approval Pathway, which applies to therapies that: 1) treat serious conditions with unmet need, and 2) demonstrate safety and efficacy on surrogate end points reasonably likely to predict clinical benefit.3
By voting their approval of 17P in 2011, the FDA affirmed that PTB was a serious condition with unmet need, that birth < 37 weeks was an accepted surrogate end point, and that there was compelling evidence of safety and benefit. The compelling evidence presented was a single, randomized, vehicle-controlled clinical trial conducted by the Maternal-Fetal Medicine Units (MFMU) Network, which showed significant reduction in recurrent PTB < 37 weeks (from 54.9% in the placebo group to 36.3% in the 17P group; P<.001; relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81).4
In 2017, the Society for Maternal-Fetal Medicine (SMFM) reaffirmed the use of 17P to prevent recurrent PTB and, that same year, it was estimated that 75% of eligible patients received 17P.5,6 Importantly, Subpart H approval requires one or more follow-up clinical trials confirming safety and efficacy. And the FDA has the right—the responsibility—to revisit approval if such trials are either not performed or are unfavorable.
The recently published PROLONG study by Blackwell and colleagues is this required postapproval confirmatory trial conducted to verify the clinical benefit of 17P supplementation.7
Continue to: Study design, and stunning results...
Study design, and stunning results
PROLONG (Progestin's Role in Optimizing Neonatal Gestation) was a randomized (2:1), double-blind, vehicle-controlled, multicenter international trial (2009-2018) conducted to assess the safety and efficacy of 17P injection in 1,708 women with a singleton pregnancy and one or more prior spontaneous PTBs.7 Women in the active treatment group (n = 1,130) received weekly intramuscular injections of 17P, while those in the control group (n = 578) received weekly injections of inert oil vehicle.
Results of the trial showed no significant reduction in the co-primary end points, which were PTB < 35 weeks (11.0% in the 17P group vs 11.5% in the placebo group; RR, 0.95; 95% CI, 0.71-1.26) and neonatal morbidity index (5.6% in the 17P group vs 5.0% in the placebo group; RR, 1.12; 95% CI, 0.68-1.61). There was no evidence of benefit for any subpopulation (geographic region, race, or other PTB risk factor). Maternal outcomes also were similar between the groups. No significant safety concerns were identified.
Important differences between MFMU and PROLONG trials
Strengths of the PROLONG trial include its randomized, placebo-controlled design, excellent follow-up rate, and use of a protocol that mirrored that of the MFMU trial. The primary limitation of PROLONG is that participants experienced a lower rate of PTB compared with those in the MFMU trial. The rate of PTB < 37 weeks was 54.9% in the control group of the MFMU trial compared with 21.9% in PROLONG.
Given the low rate of PTB in PROLONG, the study was underpowered for the co-primary outcomes. In addition, lower rates of PTB in PROLONG compared with in the MFMU trial likely reflected different patient populations.8 Moreover, PROLONG was an international trial. Of the 1,708 participants, most were recruited in Russia (36%) and Ukraine (25%); only 23% were from the United States. By contrast, participants in the MFMU trial were recruited from US academic medical centers. Also, participants in the MFMU trial were significantly more likely to have a short cervix, to have a history of more than one PTB, and to be African American.
Discrepant trial results create clinical quandary
In October 2019, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17P. Committee members struggled with the conflicting data between the 2 trials and hesitated to remove a medication whose use has become standard practice. Ultimately, however, it was lack of substantial evidence of effectiveness of 17P that swayed the committee's vote. While the FDA generally follows the recommendation of an advisory committee, it is not bound to do so.
Societies' perspectives
So what are physicians and patients to do? It is possible that a small subgroup of women at extremely high risk for early PTB may benefit from 17P administration. SMFM stated: "...it is reasonable for providers to use 17-OHPC [17P] in women with a profile more representative of the very high-risk population reported in the Meis [MFMU] trial."8 Further, the American College of Obstetricians and Gynecologists (ACOG) stated in a Practice Advisory dated October 25, 2019, that "ACOG is not changing our clinical recommendations at this time... [We] will be reviewing subsequent forthcoming analyses and will issue updated clinical guidance as appropriate."9
Where we stand on 17P use going forward
17P should be available to women who previously may have benefited from its use. However, 17P should not be recommended routinely to prevent recurrent spontaneous PTB in women with one prior PTB and no other risk factors. Of note, the PROLONG trial does not change recommendations for cervical length screening. Women with a history of a prior spontaneous PTB should undergo cervical length screening to identify those individuals who may benefit from an ultrasound-indicated cerclage.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308-1317.
- Makena [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf. Accessed November 10, 2019.
- US Food and Drug Administration. Code of Federal Regulations Title 21. Subpart H--Acceleratedapproval of new drugs for serious or life-threatening illnesses. April 1, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed November 10, 2019.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Society for Maternal-Fetal Medicine Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11-B13.
- Gallagher JR, Gudeman J, Heap K, et al. Understanding if, how, and why women with prior spontaneous preterm births are treated with progestogens: a national survey of obstetrician practice patterns. AJP Rep. 2018;8:e315-e324.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Health%20Advance/journals/ymob/SMFM_Statement_PRO LONG-1572023839767.pdf. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists. Practice advisory: Clinical guidance for integration of the findings of the PROLONG study: progestin's role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing?IsMobileSet=false. Accessed November 10, 2019.
Preterm birth (PTB) remains a significant public health concern and a major cause of newborn morbidity and mortality. In the United States, 1 in 10 babies are born preterm (< 37 weeks), and this rate has changed little in 30 years.1
In 2011, the US Food and Drug Administration (FDA) approved progesterone supplementation—specifically, α-hydroxyprogesterone caproate (17P) injection (Makena)—to prevent recurrent PTB in women with a singleton pregnancy at high risk by virtue of a prior spontaneous PTB.2 This was the first-ever FDA-approved drug for PTB prevention, and it was the first drug approved by the FDA for use in pregnancy in more than 15 years. The approval of 17P utilized the FDA's Subpart H Accelerated Approval Pathway, which applies to therapies that: 1) treat serious conditions with unmet need, and 2) demonstrate safety and efficacy on surrogate end points reasonably likely to predict clinical benefit.3
By voting their approval of 17P in 2011, the FDA affirmed that PTB was a serious condition with unmet need, that birth < 37 weeks was an accepted surrogate end point, and that there was compelling evidence of safety and benefit. The compelling evidence presented was a single, randomized, vehicle-controlled clinical trial conducted by the Maternal-Fetal Medicine Units (MFMU) Network, which showed significant reduction in recurrent PTB < 37 weeks (from 54.9% in the placebo group to 36.3% in the 17P group; P<.001; relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81).4
In 2017, the Society for Maternal-Fetal Medicine (SMFM) reaffirmed the use of 17P to prevent recurrent PTB and, that same year, it was estimated that 75% of eligible patients received 17P.5,6 Importantly, Subpart H approval requires one or more follow-up clinical trials confirming safety and efficacy. And the FDA has the right—the responsibility—to revisit approval if such trials are either not performed or are unfavorable.
The recently published PROLONG study by Blackwell and colleagues is this required postapproval confirmatory trial conducted to verify the clinical benefit of 17P supplementation.7
Continue to: Study design, and stunning results...
Study design, and stunning results
PROLONG (Progestin's Role in Optimizing Neonatal Gestation) was a randomized (2:1), double-blind, vehicle-controlled, multicenter international trial (2009-2018) conducted to assess the safety and efficacy of 17P injection in 1,708 women with a singleton pregnancy and one or more prior spontaneous PTBs.7 Women in the active treatment group (n = 1,130) received weekly intramuscular injections of 17P, while those in the control group (n = 578) received weekly injections of inert oil vehicle.
Results of the trial showed no significant reduction in the co-primary end points, which were PTB < 35 weeks (11.0% in the 17P group vs 11.5% in the placebo group; RR, 0.95; 95% CI, 0.71-1.26) and neonatal morbidity index (5.6% in the 17P group vs 5.0% in the placebo group; RR, 1.12; 95% CI, 0.68-1.61). There was no evidence of benefit for any subpopulation (geographic region, race, or other PTB risk factor). Maternal outcomes also were similar between the groups. No significant safety concerns were identified.
Important differences between MFMU and PROLONG trials
Strengths of the PROLONG trial include its randomized, placebo-controlled design, excellent follow-up rate, and use of a protocol that mirrored that of the MFMU trial. The primary limitation of PROLONG is that participants experienced a lower rate of PTB compared with those in the MFMU trial. The rate of PTB < 37 weeks was 54.9% in the control group of the MFMU trial compared with 21.9% in PROLONG.
Given the low rate of PTB in PROLONG, the study was underpowered for the co-primary outcomes. In addition, lower rates of PTB in PROLONG compared with in the MFMU trial likely reflected different patient populations.8 Moreover, PROLONG was an international trial. Of the 1,708 participants, most were recruited in Russia (36%) and Ukraine (25%); only 23% were from the United States. By contrast, participants in the MFMU trial were recruited from US academic medical centers. Also, participants in the MFMU trial were significantly more likely to have a short cervix, to have a history of more than one PTB, and to be African American.
Discrepant trial results create clinical quandary
In October 2019, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17P. Committee members struggled with the conflicting data between the 2 trials and hesitated to remove a medication whose use has become standard practice. Ultimately, however, it was lack of substantial evidence of effectiveness of 17P that swayed the committee's vote. While the FDA generally follows the recommendation of an advisory committee, it is not bound to do so.
Societies' perspectives
So what are physicians and patients to do? It is possible that a small subgroup of women at extremely high risk for early PTB may benefit from 17P administration. SMFM stated: "...it is reasonable for providers to use 17-OHPC [17P] in women with a profile more representative of the very high-risk population reported in the Meis [MFMU] trial."8 Further, the American College of Obstetricians and Gynecologists (ACOG) stated in a Practice Advisory dated October 25, 2019, that "ACOG is not changing our clinical recommendations at this time... [We] will be reviewing subsequent forthcoming analyses and will issue updated clinical guidance as appropriate."9
Where we stand on 17P use going forward
17P should be available to women who previously may have benefited from its use. However, 17P should not be recommended routinely to prevent recurrent spontaneous PTB in women with one prior PTB and no other risk factors. Of note, the PROLONG trial does not change recommendations for cervical length screening. Women with a history of a prior spontaneous PTB should undergo cervical length screening to identify those individuals who may benefit from an ultrasound-indicated cerclage.
Preterm birth (PTB) remains a significant public health concern and a major cause of newborn morbidity and mortality. In the United States, 1 in 10 babies are born preterm (< 37 weeks), and this rate has changed little in 30 years.1
In 2011, the US Food and Drug Administration (FDA) approved progesterone supplementation—specifically, α-hydroxyprogesterone caproate (17P) injection (Makena)—to prevent recurrent PTB in women with a singleton pregnancy at high risk by virtue of a prior spontaneous PTB.2 This was the first-ever FDA-approved drug for PTB prevention, and it was the first drug approved by the FDA for use in pregnancy in more than 15 years. The approval of 17P utilized the FDA's Subpart H Accelerated Approval Pathway, which applies to therapies that: 1) treat serious conditions with unmet need, and 2) demonstrate safety and efficacy on surrogate end points reasonably likely to predict clinical benefit.3
By voting their approval of 17P in 2011, the FDA affirmed that PTB was a serious condition with unmet need, that birth < 37 weeks was an accepted surrogate end point, and that there was compelling evidence of safety and benefit. The compelling evidence presented was a single, randomized, vehicle-controlled clinical trial conducted by the Maternal-Fetal Medicine Units (MFMU) Network, which showed significant reduction in recurrent PTB < 37 weeks (from 54.9% in the placebo group to 36.3% in the 17P group; P<.001; relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81).4
In 2017, the Society for Maternal-Fetal Medicine (SMFM) reaffirmed the use of 17P to prevent recurrent PTB and, that same year, it was estimated that 75% of eligible patients received 17P.5,6 Importantly, Subpart H approval requires one or more follow-up clinical trials confirming safety and efficacy. And the FDA has the right—the responsibility—to revisit approval if such trials are either not performed or are unfavorable.
The recently published PROLONG study by Blackwell and colleagues is this required postapproval confirmatory trial conducted to verify the clinical benefit of 17P supplementation.7
Continue to: Study design, and stunning results...
Study design, and stunning results
PROLONG (Progestin's Role in Optimizing Neonatal Gestation) was a randomized (2:1), double-blind, vehicle-controlled, multicenter international trial (2009-2018) conducted to assess the safety and efficacy of 17P injection in 1,708 women with a singleton pregnancy and one or more prior spontaneous PTBs.7 Women in the active treatment group (n = 1,130) received weekly intramuscular injections of 17P, while those in the control group (n = 578) received weekly injections of inert oil vehicle.
Results of the trial showed no significant reduction in the co-primary end points, which were PTB < 35 weeks (11.0% in the 17P group vs 11.5% in the placebo group; RR, 0.95; 95% CI, 0.71-1.26) and neonatal morbidity index (5.6% in the 17P group vs 5.0% in the placebo group; RR, 1.12; 95% CI, 0.68-1.61). There was no evidence of benefit for any subpopulation (geographic region, race, or other PTB risk factor). Maternal outcomes also were similar between the groups. No significant safety concerns were identified.
Important differences between MFMU and PROLONG trials
Strengths of the PROLONG trial include its randomized, placebo-controlled design, excellent follow-up rate, and use of a protocol that mirrored that of the MFMU trial. The primary limitation of PROLONG is that participants experienced a lower rate of PTB compared with those in the MFMU trial. The rate of PTB < 37 weeks was 54.9% in the control group of the MFMU trial compared with 21.9% in PROLONG.
Given the low rate of PTB in PROLONG, the study was underpowered for the co-primary outcomes. In addition, lower rates of PTB in PROLONG compared with in the MFMU trial likely reflected different patient populations.8 Moreover, PROLONG was an international trial. Of the 1,708 participants, most were recruited in Russia (36%) and Ukraine (25%); only 23% were from the United States. By contrast, participants in the MFMU trial were recruited from US academic medical centers. Also, participants in the MFMU trial were significantly more likely to have a short cervix, to have a history of more than one PTB, and to be African American.
Discrepant trial results create clinical quandary
In October 2019, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17P. Committee members struggled with the conflicting data between the 2 trials and hesitated to remove a medication whose use has become standard practice. Ultimately, however, it was lack of substantial evidence of effectiveness of 17P that swayed the committee's vote. While the FDA generally follows the recommendation of an advisory committee, it is not bound to do so.
Societies' perspectives
So what are physicians and patients to do? It is possible that a small subgroup of women at extremely high risk for early PTB may benefit from 17P administration. SMFM stated: "...it is reasonable for providers to use 17-OHPC [17P] in women with a profile more representative of the very high-risk population reported in the Meis [MFMU] trial."8 Further, the American College of Obstetricians and Gynecologists (ACOG) stated in a Practice Advisory dated October 25, 2019, that "ACOG is not changing our clinical recommendations at this time... [We] will be reviewing subsequent forthcoming analyses and will issue updated clinical guidance as appropriate."9
Where we stand on 17P use going forward
17P should be available to women who previously may have benefited from its use. However, 17P should not be recommended routinely to prevent recurrent spontaneous PTB in women with one prior PTB and no other risk factors. Of note, the PROLONG trial does not change recommendations for cervical length screening. Women with a history of a prior spontaneous PTB should undergo cervical length screening to identify those individuals who may benefit from an ultrasound-indicated cerclage.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308-1317.
- Makena [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf. Accessed November 10, 2019.
- US Food and Drug Administration. Code of Federal Regulations Title 21. Subpart H--Acceleratedapproval of new drugs for serious or life-threatening illnesses. April 1, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed November 10, 2019.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Society for Maternal-Fetal Medicine Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11-B13.
- Gallagher JR, Gudeman J, Heap K, et al. Understanding if, how, and why women with prior spontaneous preterm births are treated with progestogens: a national survey of obstetrician practice patterns. AJP Rep. 2018;8:e315-e324.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Health%20Advance/journals/ymob/SMFM_Statement_PRO LONG-1572023839767.pdf. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists. Practice advisory: Clinical guidance for integration of the findings of the PROLONG study: progestin's role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing?IsMobileSet=false. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308-1317.
- Makena [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf. Accessed November 10, 2019.
- US Food and Drug Administration. Code of Federal Regulations Title 21. Subpart H--Acceleratedapproval of new drugs for serious or life-threatening illnesses. April 1, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed November 10, 2019.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Society for Maternal-Fetal Medicine Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11-B13.
- Gallagher JR, Gudeman J, Heap K, et al. Understanding if, how, and why women with prior spontaneous preterm births are treated with progestogens: a national survey of obstetrician practice patterns. AJP Rep. 2018;8:e315-e324.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Health%20Advance/journals/ymob/SMFM_Statement_PRO LONG-1572023839767.pdf. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists. Practice advisory: Clinical guidance for integration of the findings of the PROLONG study: progestin's role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing?IsMobileSet=false. Accessed November 10, 2019.
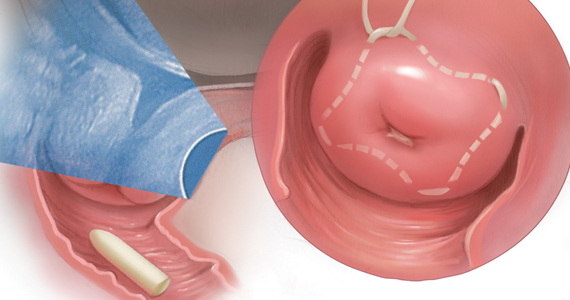
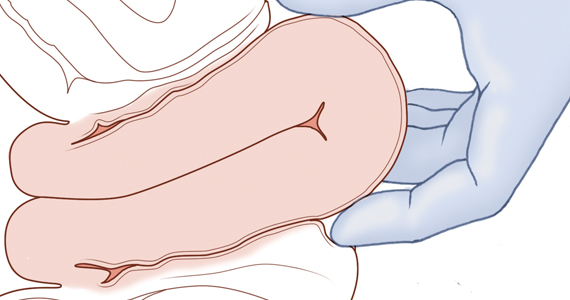
Retained placenta after vaginal birth: How long should you wait to manually remove the placenta?
You have just safely delivered the baby who is quietly resting on her mother’s chest. You begin active management of the third stage of labor, administering oxytocin, performing uterine massage and applying controlled tension on the umbilical cord. There is no evidence of excess postpartum bleeding.
How long will you wait to deliver the placenta?
Active management of the third stage of labor
Most authorities recommend active management of the third stage of labor because active management reduces the risk of maternal hemorrhage >1,000 mL (relative risk [RR], 0.34), postpartum hemoglobin levels < 9 g/dL (RR, 0.50), and maternal blood transfusion (RR, 0.35) compared with expectant management.1
The most important component of active management of the third stage of labor is the administration of a uterotonic after delivery of the newborn. In the United States, oxytocin is the uterotonic most often utilized for the active management of the third stage of labor. Authors of a recent randomized clinical trial reported that intravenous oxytocin is superior to intramuscular oxytocin for reducing postpartum blood loss (385 vs 445 mL), the frequency of blood loss greater than 1,000 mL (4.6% vs 8.1%), and the rate of maternal blood transfusion (1.5% vs 4.4%).2
In addition to administering oxytocin, the active management of the third stage often involves maneuvers to accelerate placental delivery, including the Crede and Brandt-Andrews maneuvers and controlled tension on the umbilical cord. The Crede maneuver, described in 1853, involves placing a hand on the abdominal wall near the uterine fundus and squeezing the uterine fundus between the thumb and fingers.3,4
The Brandt-Andrews maneuver, described in 1933, involves placing a clamp on the umbilical cord close to the vulva.5 The clamp is used to apply judicious tension on the cord with one hand, while the other hand is placed on the mother’s abdomen with the palm and fingers overlying the junction between the uterine corpus and the lower segment. With judicious tension on the cord, the abdominal hand pushes the uterus upward toward the umbilicus. Placental separation is indicated when lengthening of the umbilical cord occurs. The Brandt-Andrews maneuver may be associated with fewer cases of uterine inversion than the Crede maneuver.5-7
Of note, umbilical cord traction has not been demonstrated to reduce the need for blood transfusion or the incidence of postpartum hemorrhage (PPH) >1,000 mL, and it is commonly utilized by obstetricians and midwives.8,9 Hence, in the third stage, the delivering clinician should routinely administer a uterotonic, but use of judicious tension on the cord can be deferred if the woman prefers a noninterventional approach to delivery.
Following a vaginal birth, when should the diagnosis of retained placenta be made?
The historic definition of retained placenta is nonexpulsion of the placenta 30 minutes after delivery of the newborn. However, many observational studies report that, when active management of the third stage is utilized, 90%, 95%, and 99% of placentas deliver by 9 minutes, 13 minutes, and 28 minutes, respectively.10 In addition, many observational studies report that the incidence of PPH increases significantly with longer intervals between birth of the newborn and delivery of the placenta. In one study the rate of blood loss >500 mL was 8.5% when the placenta delivered between 5 and 9 minutes and 35.1% when the placenta delivered ≥30 minutes following birth of the baby.10 In another observational study, compared with women delivering the placenta < 10 minutes after birth, women delivering the placenta ≥30 minutes after birth had a 3-fold increased risk of PPH.11 Similar findings have been reported in other studies.12-14
Continue to: Based on the association between a delay in delivery...
Based on the association between a delay in delivery of the placenta and an increased risk of PPH, some authorities recommend that, in term pregnancy, the diagnosis of retained placenta should be made at 20 minutes following birth and consideration should be given to removing the placenta at this time. For women with effective neuraxial anesthesia, manual removal of the placenta 20 minutes following birth may be the best decision for balancing the benefit of preventing PPH with the risk of unnecessary intervention. For women with no anesthesia, delaying manual removal of the placenta to 30 minutes or more following birth may permit more time for the placenta to deliver prior to performing an intervention that might cause pain, but the delay increases the risk of PPH.
The retained placenta may prevent the uterine muscle from effectively contracting around penetrating veins and arteries, thereby increasing the risk of postpartum hemorrhage. The placenta that has separated from the uterine wall but is trapped inside the uterine cavity can be removed easily with manual extraction. If the placenta is physiologically adherent to the uterine wall, a gentle sweeping motion with an intrauterine hand usually can separate the placenta from the uterus in preparation for manual extraction. However, if a placenta accreta spectrum disorder is contributing to a retained placenta, it may be difficult to separate the densely adherent portion of the uterus from the uterine wall. In the presence of placenta accreta spectrum disorder, vigorous attempts to remove the placenta may precipitate massive bleeding. In some cases, the acchoucheur/midwife may recognize the presence of a focal accreta and cease attempts to remove the placenta in order to organize the personnel and equipment needed to effectively treat a potential case of placenta accreta. In one study, when a placenta accreta was recognized or suspected, immediately ceasing attempts at manually removing the placenta resulted in better case outcomes than continued attempts to remove the placenta.1
Uterine inversion may occur during an attempt to manually remove the placenta. There is universal agreement that once a uterine inversion is recognized it is critically important to immediately restore normal uterine anatomy to avoid massive hemorrhage and maternal shock. The initial management of uterine inversion includes:
- stopping oxytocin infusion
- initiating high volume fluid resuscitation
- considering a dose of a uterine relaxant, such as nitroglycerin or terbutaline
- preparing for blood product replacement.
In my experience, when uterine inversion is immediately recognized and successfully treated, blood product replacement is not usually necessary. However, if uterine inversion has not been immediately recognized or treated, massive hemorrhage and shock may occur.
Two approaches to the vaginal restoration of uterine anatomy involve using the tips of the fingers and palm of the hand to guide the wall of the uterus back to its normal position (FIGURE 1) or to forcefully use a fist to force the uterine wall back to its normal position (FIGURE 2). If these maneuvers are unsuccessful, a laparotomy may be necessary.
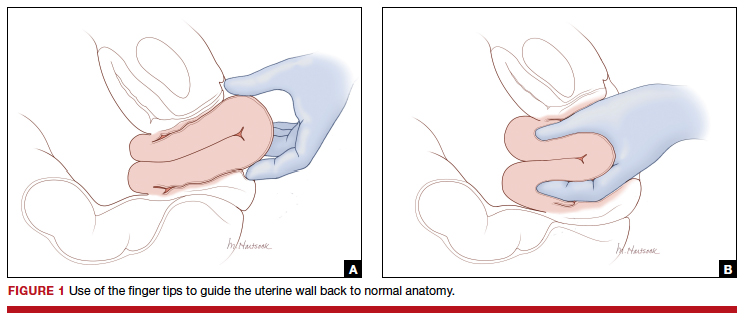
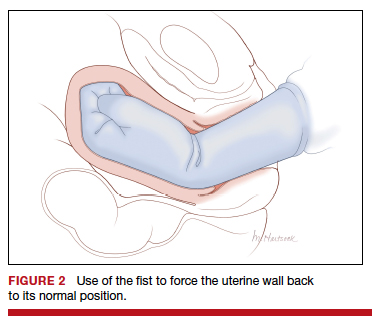
At laparotomy, the Huntington or Haultain procedures may help restore normal uterine anatomy. The Huntington procedure involves using clamps to apply symmetrical tension to the left and right round ligaments and/or uterine serosa to sequentially tease the uterus back to normal anatomy.2,3 The Haultain procedure involves a vertical incision on the posterior wall of the uterus to release the uterine constriction ring that is preventing the return of the uterine fundus to its normal position (FIGURE 3).4,5
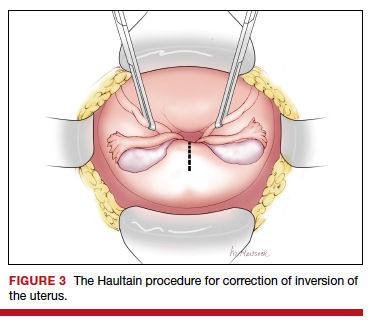
References
- Kayem G, Anselem O, Schmitz T, et al. Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris). 2007;36:680-687.
- Huntington JL. Acute inversion of the uterus. Boston Med Surg J. 1921;184:376-378.
- Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15:34-40.
- Haultain FW. Abdominal hysterotomy for chronic uterine inversion: a record of 3 cases. Proc Roy Soc Med. 1908;1:528-535.
- Easterday CL, Reid DE. Inversion of the puerperal uterus managed by the Haultain technique; A case report. Am J Obstet Gynecol. 1959;78:1224-1226.
Manual extraction of the placenta
Prior to performing manual extraction of the placenta, a decision should be made regarding the approach to anesthesia and perioperative antibiotics. Manual extraction of the placenta is performed by placing one hand on the uterine fundus to stabilize the uterus and using the other hand to follow the umbilical cord into the uterine cavity. The intrauterine hand is used to separate the uterine-placental interface with a gentle sweeping motion. The placental mass is grasped and gently teased through the cervix and vagina. Inspection of the placenta to ensure complete removal is necessary.
An alternative to manual extraction of the placenta is the use of Bierer forceps and ultrasound guidance to tease the placenta through the cervical os. This technique involves the following steps15:
1. use ultrasound to locate the placenta
2. place a ring forceps on the anterior lip of the cervix
3. introduce the Bierer forcep into the uterus
4. use the forceps to grasp the placenta and pull it toward the vagina
5. stop frequently to re-grasp placental tissue that is deeper in the uterine cavity
6. once the placenta is extracted, examine the placenta to ensure complete removal.
Of note when manual extraction is used to deliver a retained placenta, randomized clinical trials report no benefit for the following interventions:
- perioperative antibiotics16
- nitroglycerin to relax the uterus17
- ultrasound to detect retained placental tissue.18
Best timing for manual extraction of the placenta
The timing for the diagnosis of retained placenta, and the risks and benefits of manual extraction would be best evaluated in a large, randomized clinical trial. However, based on observational studies, in a term pregnancy, the diagnosis of retained placenta is best made using a 20-minute interval. In women with effective neuraxial anesthesia, consideration should be given to manual removal of the placenta at that time.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2019;2:CD007412.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomized controlled trial. BMJ. 2018;362:k3546.
- Gülmezoglu AM, Souza JP. The evolving management of the third stage of labour. BJOG. 2009;116(suppl 1):26-28.
- Ebert AD, David M. Meilensteine der Praventionsmedizin. Carl Siegmund Franz Credé (1819-1882), der Credesche Handgriff und die Credesche Augenprophylaxe. Geburtshilfe Frauenheilkd. 2016;76:675-678.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol. 1933;25:662-667.
- Kimbell N. Brandt-Andrews technique of delivery of the placenta. Br Med J. 1958;1:203-204.
- De Lee JB, Greenhill JP. Principles and Practice of Obstetrics. 9th ed. Philadelphia, PA: Saunders; 1947:275.
- Du Y, Ye M, Zheng F. Active management of the third stage of labor with and without controlled cord traction: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2014;93:626-633.
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labor. Cochrane Database Syst Rev. 2015;1:CD008020.
- Frolova AI, Stout MJ, Tuuli MG, et al. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016;127:951-956.
- Shinar S, Schwartz A, Maslovitz S, et al. How long is safe? Setting the cutoff for uncomplicated third stage length: a retrospective case-control study. Birth. 2016;43:36-41.
- Magann EF, Evans S, Chauhan SP, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290-293.
- Cummings K, Doherty DA, Magann EF, et al. Timing of manual placenta removal to prevent postpartum hemorrhage: is it time to act? J Matern Fetal Neonatal Med. 2016;29:3930-3933.
- Rabie NZ, Ounpraseuth S, Hughes D, et al. Association of the length of the third stage of labor and blood loss following vaginal delivery. South Med J. 2018;111:178-182.
- Rosenstein MG, Vargas JE, Drey EA. Ultrasound-guided instrumental removal of the retained placenta after vaginal delivery. Am J Obstet Gynecol. 2014;211:180.e1-e3.
- Chibueze EC, Parsons AJ, Ota E, et al. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;15:313.
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;(11):CD007708.
- Weissback T, Haikin-Herzberger E, Bacci-Hugger K, et al. Immediate postpartum ultrasound evaluation for suspected retained placental tissue in patients undergoing manual removal of placenta. Eur J Obstet Gynecol Reprod Biol. 2015;192:37-40.
You have just safely delivered the baby who is quietly resting on her mother’s chest. You begin active management of the third stage of labor, administering oxytocin, performing uterine massage and applying controlled tension on the umbilical cord. There is no evidence of excess postpartum bleeding.
How long will you wait to deliver the placenta?
Active management of the third stage of labor
Most authorities recommend active management of the third stage of labor because active management reduces the risk of maternal hemorrhage >1,000 mL (relative risk [RR], 0.34), postpartum hemoglobin levels < 9 g/dL (RR, 0.50), and maternal blood transfusion (RR, 0.35) compared with expectant management.1
The most important component of active management of the third stage of labor is the administration of a uterotonic after delivery of the newborn. In the United States, oxytocin is the uterotonic most often utilized for the active management of the third stage of labor. Authors of a recent randomized clinical trial reported that intravenous oxytocin is superior to intramuscular oxytocin for reducing postpartum blood loss (385 vs 445 mL), the frequency of blood loss greater than 1,000 mL (4.6% vs 8.1%), and the rate of maternal blood transfusion (1.5% vs 4.4%).2
In addition to administering oxytocin, the active management of the third stage often involves maneuvers to accelerate placental delivery, including the Crede and Brandt-Andrews maneuvers and controlled tension on the umbilical cord. The Crede maneuver, described in 1853, involves placing a hand on the abdominal wall near the uterine fundus and squeezing the uterine fundus between the thumb and fingers.3,4
The Brandt-Andrews maneuver, described in 1933, involves placing a clamp on the umbilical cord close to the vulva.5 The clamp is used to apply judicious tension on the cord with one hand, while the other hand is placed on the mother’s abdomen with the palm and fingers overlying the junction between the uterine corpus and the lower segment. With judicious tension on the cord, the abdominal hand pushes the uterus upward toward the umbilicus. Placental separation is indicated when lengthening of the umbilical cord occurs. The Brandt-Andrews maneuver may be associated with fewer cases of uterine inversion than the Crede maneuver.5-7
Of note, umbilical cord traction has not been demonstrated to reduce the need for blood transfusion or the incidence of postpartum hemorrhage (PPH) >1,000 mL, and it is commonly utilized by obstetricians and midwives.8,9 Hence, in the third stage, the delivering clinician should routinely administer a uterotonic, but use of judicious tension on the cord can be deferred if the woman prefers a noninterventional approach to delivery.
Following a vaginal birth, when should the diagnosis of retained placenta be made?
The historic definition of retained placenta is nonexpulsion of the placenta 30 minutes after delivery of the newborn. However, many observational studies report that, when active management of the third stage is utilized, 90%, 95%, and 99% of placentas deliver by 9 minutes, 13 minutes, and 28 minutes, respectively.10 In addition, many observational studies report that the incidence of PPH increases significantly with longer intervals between birth of the newborn and delivery of the placenta. In one study the rate of blood loss >500 mL was 8.5% when the placenta delivered between 5 and 9 minutes and 35.1% when the placenta delivered ≥30 minutes following birth of the baby.10 In another observational study, compared with women delivering the placenta < 10 minutes after birth, women delivering the placenta ≥30 minutes after birth had a 3-fold increased risk of PPH.11 Similar findings have been reported in other studies.12-14
Continue to: Based on the association between a delay in delivery...
Based on the association between a delay in delivery of the placenta and an increased risk of PPH, some authorities recommend that, in term pregnancy, the diagnosis of retained placenta should be made at 20 minutes following birth and consideration should be given to removing the placenta at this time. For women with effective neuraxial anesthesia, manual removal of the placenta 20 minutes following birth may be the best decision for balancing the benefit of preventing PPH with the risk of unnecessary intervention. For women with no anesthesia, delaying manual removal of the placenta to 30 minutes or more following birth may permit more time for the placenta to deliver prior to performing an intervention that might cause pain, but the delay increases the risk of PPH.
The retained placenta may prevent the uterine muscle from effectively contracting around penetrating veins and arteries, thereby increasing the risk of postpartum hemorrhage. The placenta that has separated from the uterine wall but is trapped inside the uterine cavity can be removed easily with manual extraction. If the placenta is physiologically adherent to the uterine wall, a gentle sweeping motion with an intrauterine hand usually can separate the placenta from the uterus in preparation for manual extraction. However, if a placenta accreta spectrum disorder is contributing to a retained placenta, it may be difficult to separate the densely adherent portion of the uterus from the uterine wall. In the presence of placenta accreta spectrum disorder, vigorous attempts to remove the placenta may precipitate massive bleeding. In some cases, the acchoucheur/midwife may recognize the presence of a focal accreta and cease attempts to remove the placenta in order to organize the personnel and equipment needed to effectively treat a potential case of placenta accreta. In one study, when a placenta accreta was recognized or suspected, immediately ceasing attempts at manually removing the placenta resulted in better case outcomes than continued attempts to remove the placenta.1
Uterine inversion may occur during an attempt to manually remove the placenta. There is universal agreement that once a uterine inversion is recognized it is critically important to immediately restore normal uterine anatomy to avoid massive hemorrhage and maternal shock. The initial management of uterine inversion includes:
- stopping oxytocin infusion
- initiating high volume fluid resuscitation
- considering a dose of a uterine relaxant, such as nitroglycerin or terbutaline
- preparing for blood product replacement.
In my experience, when uterine inversion is immediately recognized and successfully treated, blood product replacement is not usually necessary. However, if uterine inversion has not been immediately recognized or treated, massive hemorrhage and shock may occur.
Two approaches to the vaginal restoration of uterine anatomy involve using the tips of the fingers and palm of the hand to guide the wall of the uterus back to its normal position (FIGURE 1) or to forcefully use a fist to force the uterine wall back to its normal position (FIGURE 2). If these maneuvers are unsuccessful, a laparotomy may be necessary.


At laparotomy, the Huntington or Haultain procedures may help restore normal uterine anatomy. The Huntington procedure involves using clamps to apply symmetrical tension to the left and right round ligaments and/or uterine serosa to sequentially tease the uterus back to normal anatomy.2,3 The Haultain procedure involves a vertical incision on the posterior wall of the uterus to release the uterine constriction ring that is preventing the return of the uterine fundus to its normal position (FIGURE 3).4,5

References
- Kayem G, Anselem O, Schmitz T, et al. Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris). 2007;36:680-687.
- Huntington JL. Acute inversion of the uterus. Boston Med Surg J. 1921;184:376-378.
- Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15:34-40.
- Haultain FW. Abdominal hysterotomy for chronic uterine inversion: a record of 3 cases. Proc Roy Soc Med. 1908;1:528-535.
- Easterday CL, Reid DE. Inversion of the puerperal uterus managed by the Haultain technique; A case report. Am J Obstet Gynecol. 1959;78:1224-1226.
Manual extraction of the placenta
Prior to performing manual extraction of the placenta, a decision should be made regarding the approach to anesthesia and perioperative antibiotics. Manual extraction of the placenta is performed by placing one hand on the uterine fundus to stabilize the uterus and using the other hand to follow the umbilical cord into the uterine cavity. The intrauterine hand is used to separate the uterine-placental interface with a gentle sweeping motion. The placental mass is grasped and gently teased through the cervix and vagina. Inspection of the placenta to ensure complete removal is necessary.
An alternative to manual extraction of the placenta is the use of Bierer forceps and ultrasound guidance to tease the placenta through the cervical os. This technique involves the following steps15:
1. use ultrasound to locate the placenta
2. place a ring forceps on the anterior lip of the cervix
3. introduce the Bierer forcep into the uterus
4. use the forceps to grasp the placenta and pull it toward the vagina
5. stop frequently to re-grasp placental tissue that is deeper in the uterine cavity
6. once the placenta is extracted, examine the placenta to ensure complete removal.
Of note when manual extraction is used to deliver a retained placenta, randomized clinical trials report no benefit for the following interventions:
- perioperative antibiotics16
- nitroglycerin to relax the uterus17
- ultrasound to detect retained placental tissue.18
Best timing for manual extraction of the placenta
The timing for the diagnosis of retained placenta, and the risks and benefits of manual extraction would be best evaluated in a large, randomized clinical trial. However, based on observational studies, in a term pregnancy, the diagnosis of retained placenta is best made using a 20-minute interval. In women with effective neuraxial anesthesia, consideration should be given to manual removal of the placenta at that time.
You have just safely delivered the baby who is quietly resting on her mother’s chest. You begin active management of the third stage of labor, administering oxytocin, performing uterine massage and applying controlled tension on the umbilical cord. There is no evidence of excess postpartum bleeding.
How long will you wait to deliver the placenta?
Active management of the third stage of labor
Most authorities recommend active management of the third stage of labor because active management reduces the risk of maternal hemorrhage >1,000 mL (relative risk [RR], 0.34), postpartum hemoglobin levels < 9 g/dL (RR, 0.50), and maternal blood transfusion (RR, 0.35) compared with expectant management.1
The most important component of active management of the third stage of labor is the administration of a uterotonic after delivery of the newborn. In the United States, oxytocin is the uterotonic most often utilized for the active management of the third stage of labor. Authors of a recent randomized clinical trial reported that intravenous oxytocin is superior to intramuscular oxytocin for reducing postpartum blood loss (385 vs 445 mL), the frequency of blood loss greater than 1,000 mL (4.6% vs 8.1%), and the rate of maternal blood transfusion (1.5% vs 4.4%).2
In addition to administering oxytocin, the active management of the third stage often involves maneuvers to accelerate placental delivery, including the Crede and Brandt-Andrews maneuvers and controlled tension on the umbilical cord. The Crede maneuver, described in 1853, involves placing a hand on the abdominal wall near the uterine fundus and squeezing the uterine fundus between the thumb and fingers.3,4
The Brandt-Andrews maneuver, described in 1933, involves placing a clamp on the umbilical cord close to the vulva.5 The clamp is used to apply judicious tension on the cord with one hand, while the other hand is placed on the mother’s abdomen with the palm and fingers overlying the junction between the uterine corpus and the lower segment. With judicious tension on the cord, the abdominal hand pushes the uterus upward toward the umbilicus. Placental separation is indicated when lengthening of the umbilical cord occurs. The Brandt-Andrews maneuver may be associated with fewer cases of uterine inversion than the Crede maneuver.5-7
Of note, umbilical cord traction has not been demonstrated to reduce the need for blood transfusion or the incidence of postpartum hemorrhage (PPH) >1,000 mL, and it is commonly utilized by obstetricians and midwives.8,9 Hence, in the third stage, the delivering clinician should routinely administer a uterotonic, but use of judicious tension on the cord can be deferred if the woman prefers a noninterventional approach to delivery.
Following a vaginal birth, when should the diagnosis of retained placenta be made?
The historic definition of retained placenta is nonexpulsion of the placenta 30 minutes after delivery of the newborn. However, many observational studies report that, when active management of the third stage is utilized, 90%, 95%, and 99% of placentas deliver by 9 minutes, 13 minutes, and 28 minutes, respectively.10 In addition, many observational studies report that the incidence of PPH increases significantly with longer intervals between birth of the newborn and delivery of the placenta. In one study the rate of blood loss >500 mL was 8.5% when the placenta delivered between 5 and 9 minutes and 35.1% when the placenta delivered ≥30 minutes following birth of the baby.10 In another observational study, compared with women delivering the placenta < 10 minutes after birth, women delivering the placenta ≥30 minutes after birth had a 3-fold increased risk of PPH.11 Similar findings have been reported in other studies.12-14
Continue to: Based on the association between a delay in delivery...
Based on the association between a delay in delivery of the placenta and an increased risk of PPH, some authorities recommend that, in term pregnancy, the diagnosis of retained placenta should be made at 20 minutes following birth and consideration should be given to removing the placenta at this time. For women with effective neuraxial anesthesia, manual removal of the placenta 20 minutes following birth may be the best decision for balancing the benefit of preventing PPH with the risk of unnecessary intervention. For women with no anesthesia, delaying manual removal of the placenta to 30 minutes or more following birth may permit more time for the placenta to deliver prior to performing an intervention that might cause pain, but the delay increases the risk of PPH.
The retained placenta may prevent the uterine muscle from effectively contracting around penetrating veins and arteries, thereby increasing the risk of postpartum hemorrhage. The placenta that has separated from the uterine wall but is trapped inside the uterine cavity can be removed easily with manual extraction. If the placenta is physiologically adherent to the uterine wall, a gentle sweeping motion with an intrauterine hand usually can separate the placenta from the uterus in preparation for manual extraction. However, if a placenta accreta spectrum disorder is contributing to a retained placenta, it may be difficult to separate the densely adherent portion of the uterus from the uterine wall. In the presence of placenta accreta spectrum disorder, vigorous attempts to remove the placenta may precipitate massive bleeding. In some cases, the acchoucheur/midwife may recognize the presence of a focal accreta and cease attempts to remove the placenta in order to organize the personnel and equipment needed to effectively treat a potential case of placenta accreta. In one study, when a placenta accreta was recognized or suspected, immediately ceasing attempts at manually removing the placenta resulted in better case outcomes than continued attempts to remove the placenta.1
Uterine inversion may occur during an attempt to manually remove the placenta. There is universal agreement that once a uterine inversion is recognized it is critically important to immediately restore normal uterine anatomy to avoid massive hemorrhage and maternal shock. The initial management of uterine inversion includes:
- stopping oxytocin infusion
- initiating high volume fluid resuscitation
- considering a dose of a uterine relaxant, such as nitroglycerin or terbutaline
- preparing for blood product replacement.
In my experience, when uterine inversion is immediately recognized and successfully treated, blood product replacement is not usually necessary. However, if uterine inversion has not been immediately recognized or treated, massive hemorrhage and shock may occur.
Two approaches to the vaginal restoration of uterine anatomy involve using the tips of the fingers and palm of the hand to guide the wall of the uterus back to its normal position (FIGURE 1) or to forcefully use a fist to force the uterine wall back to its normal position (FIGURE 2). If these maneuvers are unsuccessful, a laparotomy may be necessary.


At laparotomy, the Huntington or Haultain procedures may help restore normal uterine anatomy. The Huntington procedure involves using clamps to apply symmetrical tension to the left and right round ligaments and/or uterine serosa to sequentially tease the uterus back to normal anatomy.2,3 The Haultain procedure involves a vertical incision on the posterior wall of the uterus to release the uterine constriction ring that is preventing the return of the uterine fundus to its normal position (FIGURE 3).4,5

References
- Kayem G, Anselem O, Schmitz T, et al. Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris). 2007;36:680-687.
- Huntington JL. Acute inversion of the uterus. Boston Med Surg J. 1921;184:376-378.
- Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15:34-40.
- Haultain FW. Abdominal hysterotomy for chronic uterine inversion: a record of 3 cases. Proc Roy Soc Med. 1908;1:528-535.
- Easterday CL, Reid DE. Inversion of the puerperal uterus managed by the Haultain technique; A case report. Am J Obstet Gynecol. 1959;78:1224-1226.
Manual extraction of the placenta
Prior to performing manual extraction of the placenta, a decision should be made regarding the approach to anesthesia and perioperative antibiotics. Manual extraction of the placenta is performed by placing one hand on the uterine fundus to stabilize the uterus and using the other hand to follow the umbilical cord into the uterine cavity. The intrauterine hand is used to separate the uterine-placental interface with a gentle sweeping motion. The placental mass is grasped and gently teased through the cervix and vagina. Inspection of the placenta to ensure complete removal is necessary.
An alternative to manual extraction of the placenta is the use of Bierer forceps and ultrasound guidance to tease the placenta through the cervical os. This technique involves the following steps15:
1. use ultrasound to locate the placenta
2. place a ring forceps on the anterior lip of the cervix
3. introduce the Bierer forcep into the uterus
4. use the forceps to grasp the placenta and pull it toward the vagina
5. stop frequently to re-grasp placental tissue that is deeper in the uterine cavity
6. once the placenta is extracted, examine the placenta to ensure complete removal.
Of note when manual extraction is used to deliver a retained placenta, randomized clinical trials report no benefit for the following interventions:
- perioperative antibiotics16
- nitroglycerin to relax the uterus17
- ultrasound to detect retained placental tissue.18
Best timing for manual extraction of the placenta
The timing for the diagnosis of retained placenta, and the risks and benefits of manual extraction would be best evaluated in a large, randomized clinical trial. However, based on observational studies, in a term pregnancy, the diagnosis of retained placenta is best made using a 20-minute interval. In women with effective neuraxial anesthesia, consideration should be given to manual removal of the placenta at that time.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2019;2:CD007412.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomized controlled trial. BMJ. 2018;362:k3546.
- Gülmezoglu AM, Souza JP. The evolving management of the third stage of labour. BJOG. 2009;116(suppl 1):26-28.
- Ebert AD, David M. Meilensteine der Praventionsmedizin. Carl Siegmund Franz Credé (1819-1882), der Credesche Handgriff und die Credesche Augenprophylaxe. Geburtshilfe Frauenheilkd. 2016;76:675-678.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol. 1933;25:662-667.
- Kimbell N. Brandt-Andrews technique of delivery of the placenta. Br Med J. 1958;1:203-204.
- De Lee JB, Greenhill JP. Principles and Practice of Obstetrics. 9th ed. Philadelphia, PA: Saunders; 1947:275.
- Du Y, Ye M, Zheng F. Active management of the third stage of labor with and without controlled cord traction: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2014;93:626-633.
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labor. Cochrane Database Syst Rev. 2015;1:CD008020.
- Frolova AI, Stout MJ, Tuuli MG, et al. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016;127:951-956.
- Shinar S, Schwartz A, Maslovitz S, et al. How long is safe? Setting the cutoff for uncomplicated third stage length: a retrospective case-control study. Birth. 2016;43:36-41.
- Magann EF, Evans S, Chauhan SP, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290-293.
- Cummings K, Doherty DA, Magann EF, et al. Timing of manual placenta removal to prevent postpartum hemorrhage: is it time to act? J Matern Fetal Neonatal Med. 2016;29:3930-3933.
- Rabie NZ, Ounpraseuth S, Hughes D, et al. Association of the length of the third stage of labor and blood loss following vaginal delivery. South Med J. 2018;111:178-182.
- Rosenstein MG, Vargas JE, Drey EA. Ultrasound-guided instrumental removal of the retained placenta after vaginal delivery. Am J Obstet Gynecol. 2014;211:180.e1-e3.
- Chibueze EC, Parsons AJ, Ota E, et al. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;15:313.
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;(11):CD007708.
- Weissback T, Haikin-Herzberger E, Bacci-Hugger K, et al. Immediate postpartum ultrasound evaluation for suspected retained placental tissue in patients undergoing manual removal of placenta. Eur J Obstet Gynecol Reprod Biol. 2015;192:37-40.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2019;2:CD007412.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomized controlled trial. BMJ. 2018;362:k3546.
- Gülmezoglu AM, Souza JP. The evolving management of the third stage of labour. BJOG. 2009;116(suppl 1):26-28.
- Ebert AD, David M. Meilensteine der Praventionsmedizin. Carl Siegmund Franz Credé (1819-1882), der Credesche Handgriff und die Credesche Augenprophylaxe. Geburtshilfe Frauenheilkd. 2016;76:675-678.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol. 1933;25:662-667.
- Kimbell N. Brandt-Andrews technique of delivery of the placenta. Br Med J. 1958;1:203-204.
- De Lee JB, Greenhill JP. Principles and Practice of Obstetrics. 9th ed. Philadelphia, PA: Saunders; 1947:275.
- Du Y, Ye M, Zheng F. Active management of the third stage of labor with and without controlled cord traction: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2014;93:626-633.
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labor. Cochrane Database Syst Rev. 2015;1:CD008020.
- Frolova AI, Stout MJ, Tuuli MG, et al. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016;127:951-956.
- Shinar S, Schwartz A, Maslovitz S, et al. How long is safe? Setting the cutoff for uncomplicated third stage length: a retrospective case-control study. Birth. 2016;43:36-41.
- Magann EF, Evans S, Chauhan SP, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290-293.
- Cummings K, Doherty DA, Magann EF, et al. Timing of manual placenta removal to prevent postpartum hemorrhage: is it time to act? J Matern Fetal Neonatal Med. 2016;29:3930-3933.
- Rabie NZ, Ounpraseuth S, Hughes D, et al. Association of the length of the third stage of labor and blood loss following vaginal delivery. South Med J. 2018;111:178-182.
- Rosenstein MG, Vargas JE, Drey EA. Ultrasound-guided instrumental removal of the retained placenta after vaginal delivery. Am J Obstet Gynecol. 2014;211:180.e1-e3.
- Chibueze EC, Parsons AJ, Ota E, et al. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;15:313.
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;(11):CD007708.
- Weissback T, Haikin-Herzberger E, Bacci-Hugger K, et al. Immediate postpartum ultrasound evaluation for suspected retained placental tissue in patients undergoing manual removal of placenta. Eur J Obstet Gynecol Reprod Biol. 2015;192:37-40.
The One Step test: The better diagnostic approach for gestational diabetes mellitus
Gestational diabetes mellitus (GDM) generally is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1-14 The best approach and exact criteria to use for GDM screening and diagnosis are under worldwide debate. In TABLE 1 we present just some of the many differing suggestions by varying organizations.2,7-9,11,12,15-17 The American College of Obstetricians and Gynecologists, for instance, suggests a Two Step approach to diagnosis.15 We will make the argument in this article, however, that diagnosis should be defined universally as an abnormal result with the One Step 75-g glucose testing, as adopted by the World Health Organization, International Federation of Gynecology and Obstetrics, and others. Approximately 8% of all pregnancies are complicated by GDM by the One Step test in the United States.18-22 The prevalence may range from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.1,19
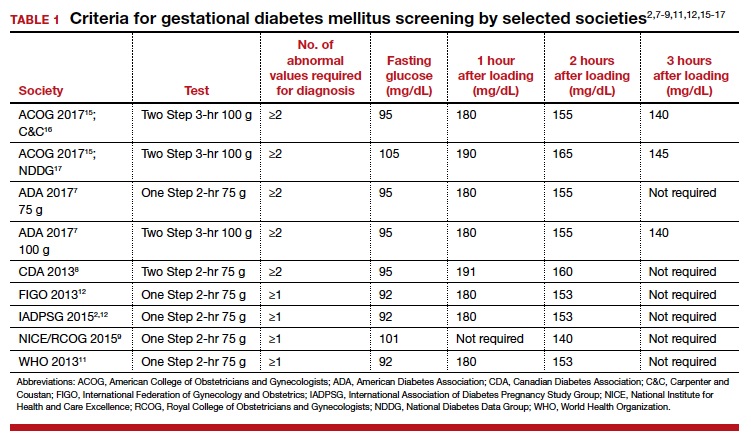
Diagnostic options
Different methods for screening and diagnosis of GDM have been proposed by international societies; there is controversy regarding the diagnosis of GDM by either the One Step or the Two Step approach.6
The One Step approach includes an oral glucose tolerance test with a 75-g glucose load with measurement of plasma glucose concentration at fasting state and 1 hour and 2 hours post–glucose administration. A positive result for the One Step approach is defined as at least 1 measurement higher than 92, 180, or 153 mg/dL at fasting, 1 hour, or 2 hours, respectively.
The Two Step approach includes a nonfasting oral 50-g glucose load, with a glucose blood measurement 1 hour later. A positive screening, defined often as a blood glucose value higher than 135 mg/dL (range, 130 to 140 mg/dL), is followed by a diagnostic test with a 100-g glucose load with measurements at fasting and 1, 2, and 3 hours post–glucose administration. A positive diagnostic test is defined as 2 measurements higher than the target value.
Why we support the One Step test
There are several reasons to prefer the One Step approach for the diagnosis of GDM, compared with the Two Step approach.
Women testing negative for GDM with Two Step still experience complications pregnancy. Women who test positive for GDM with the One Step test, but negative with the Two Step test, despite having therefore a milder degree of glucose intolerance, do have a higher risk of experiencing several complications.23 For the mother, these complications include gestational hypertension, preeclampsia, and cesarean delivery. The baby also can experience problems at birth (TABLE 2).23 Therefore, women who test positive for GDM with the One Step test deserve to be diagnosed with and treated for the condition, as not only are they at risk for these complications but also treatment of the GDM decreases the incidence of these complications.18,19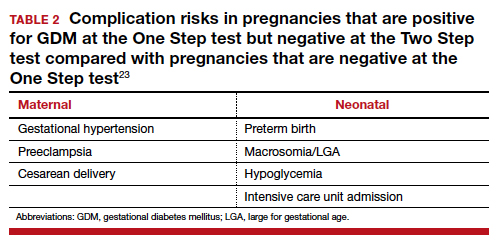
There is indeed an increased GDM diagnosis rate with the One Step (about 8%) compared with the Two Step test (about 4%). Nonetheless, this increase is mild and nonsignificant in the meta-analysis of randomized controlled trials (RCTs),18,19 is less than the 18% difference in diagnosis rate previously hypothesized, is consistent with the increased diabetes/prediabetes rates in the general population, and is linked to the increasing incidence of obesity and insulin resistance.
Overall test adherence is better. Five percent to 15% of patients, depending on the study, are not adherent with taking the second part of the Two Step test. Women indeed prefer the One Step approach; the second step in the Two Step approach may be a burden.
Less costly. The One Step process is cost-effective when postpregnancy diabetes mellitus prevention is considered.
Better maternal and perinatal outcomes. Probably the most important and convincing reason the One Step test should be used is that meta-analysis of the 4 RCTs comparing the approaches (including 2 US trials) shows that diagnosing and treating mild GDM as per the One Step approach, compared with screening and treating using the Two Step approach, is associated with increased incidence of GDM (8% vs 4%) and with better maternal and perinatal outcomes.13,18,19 In fact, the One Step approach is associated with significant reductions in: large for gestational age (56%), admission to neonatal intensive care unit (51%), and neonatal hypoglycemia (48%). Tests of heterogeneity in the meta-analysis and of quality all pointed to better outcomes in the One Step test group.13,19
The need for a second step in the Two Step approach delays diagnosis and treatment. The One Step approach is associated with an increase in GDM test adherence and earlier diagnosis,13 which is another reason for better outcomes with the One Step approach. In the presence of risk factors, such as prior GDM, prior macrosomia, advanced maternal age, multiple gestations, and others, the One Step test should be done at the first prenatal visit.
Continue to: US guidelines should be reconsidered...
US guidelines should be reconsidered
The One Step, 75-g, 2-hour oral glucose tolerance test is universally used to diagnose diabetes mellitus outside of pregnancy. Given our many noted reasons (TABLE 3), we recommend universal screening of GDM by using the One Step approach. It is time, indeed, for the United States to reconsider its guidelines for screening for GDM.
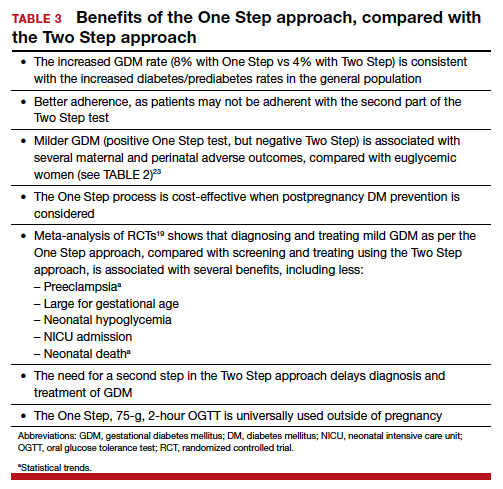
- Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065-1072.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Meltzer SJ, Snyder J, Penrod JR, et al. Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG. 2010;117:407-415.
- Sevket O, Ates S, Uysal O, et al. To evaluate the prevalence and clinical outcomes using a one-step method versus a two-step method to screen gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:36-41.
- Scifres CM, Abebe KZ, Jones KA, et al. Gestational diabetes diagnostic methods (GD2M) pilot randomized trial. Matern Child Health J. 2015;19:1472-1480.
- Farrar D, Duley L, Medley N, et al. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015;1:CD007122.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Diabetes and Pregnancy. Can J Diabetes. 2013;37(suppl 1):S168-S183.
- NICE guideline. Diabetes in pregnancy: management from preconception to the postnatal period. February 2015. https://www.nice.org.uk/guidance/ng3/. Last updated August 2015. Accessed November 18, 2019.
- WHO 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. From http://apps.who.int/iris/bitstream/10665/66040/1/WHO
_NCD_NCS_99.2.pdf. Accessed November 18, 2019. - World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. http://apps.who.int/iris/bitstream/10665/85975/1/WHO_
NMH_MND_13.2_eng.pdf. Accessed November 18, 2019. - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211.
- Berghella V, Caissutti C, Saccone G, et al. The One Step approach for diagnosing gestational diabetes is associated with better perinatal outcomes than using the Two Step approach: evidence of randomized clinical trials. Am J Obstet Gynecol. 2019;220:562-564.
- Berghella V, Caissutti C, Saccone G, et al. One-Step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2019;133:383.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17-e31.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2018:1-9.
- Saccone G, Caissutti C, Khalifeh A, et al. One step versus two step approach for gestational diabetes screening: systematic review and meta-analysis of the randomized trials. J Matern Fetal Neonatal Med. 2019;32:1547-1555.
- Khalifeh A, Eckler R, Felder L, et al. One-step versus two-step diagnostic testing for gestational diabetes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-6.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2905-2914.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: which should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:235-247.
- Caissutti C, Khalifeh A, Saccone G, et al. Are women positive for the One Step but negative for the Two Step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018;97:122-134.
Gestational diabetes mellitus (GDM) generally is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1-14 The best approach and exact criteria to use for GDM screening and diagnosis are under worldwide debate. In TABLE 1 we present just some of the many differing suggestions by varying organizations.2,7-9,11,12,15-17 The American College of Obstetricians and Gynecologists, for instance, suggests a Two Step approach to diagnosis.15 We will make the argument in this article, however, that diagnosis should be defined universally as an abnormal result with the One Step 75-g glucose testing, as adopted by the World Health Organization, International Federation of Gynecology and Obstetrics, and others. Approximately 8% of all pregnancies are complicated by GDM by the One Step test in the United States.18-22 The prevalence may range from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.1,19

Diagnostic options
Different methods for screening and diagnosis of GDM have been proposed by international societies; there is controversy regarding the diagnosis of GDM by either the One Step or the Two Step approach.6
The One Step approach includes an oral glucose tolerance test with a 75-g glucose load with measurement of plasma glucose concentration at fasting state and 1 hour and 2 hours post–glucose administration. A positive result for the One Step approach is defined as at least 1 measurement higher than 92, 180, or 153 mg/dL at fasting, 1 hour, or 2 hours, respectively.
The Two Step approach includes a nonfasting oral 50-g glucose load, with a glucose blood measurement 1 hour later. A positive screening, defined often as a blood glucose value higher than 135 mg/dL (range, 130 to 140 mg/dL), is followed by a diagnostic test with a 100-g glucose load with measurements at fasting and 1, 2, and 3 hours post–glucose administration. A positive diagnostic test is defined as 2 measurements higher than the target value.
Why we support the One Step test
There are several reasons to prefer the One Step approach for the diagnosis of GDM, compared with the Two Step approach.
Women testing negative for GDM with Two Step still experience complications pregnancy. Women who test positive for GDM with the One Step test, but negative with the Two Step test, despite having therefore a milder degree of glucose intolerance, do have a higher risk of experiencing several complications.23 For the mother, these complications include gestational hypertension, preeclampsia, and cesarean delivery. The baby also can experience problems at birth (TABLE 2).23 Therefore, women who test positive for GDM with the One Step test deserve to be diagnosed with and treated for the condition, as not only are they at risk for these complications but also treatment of the GDM decreases the incidence of these complications.18,19
There is indeed an increased GDM diagnosis rate with the One Step (about 8%) compared with the Two Step test (about 4%). Nonetheless, this increase is mild and nonsignificant in the meta-analysis of randomized controlled trials (RCTs),18,19 is less than the 18% difference in diagnosis rate previously hypothesized, is consistent with the increased diabetes/prediabetes rates in the general population, and is linked to the increasing incidence of obesity and insulin resistance.
Overall test adherence is better. Five percent to 15% of patients, depending on the study, are not adherent with taking the second part of the Two Step test. Women indeed prefer the One Step approach; the second step in the Two Step approach may be a burden.
Less costly. The One Step process is cost-effective when postpregnancy diabetes mellitus prevention is considered.
Better maternal and perinatal outcomes. Probably the most important and convincing reason the One Step test should be used is that meta-analysis of the 4 RCTs comparing the approaches (including 2 US trials) shows that diagnosing and treating mild GDM as per the One Step approach, compared with screening and treating using the Two Step approach, is associated with increased incidence of GDM (8% vs 4%) and with better maternal and perinatal outcomes.13,18,19 In fact, the One Step approach is associated with significant reductions in: large for gestational age (56%), admission to neonatal intensive care unit (51%), and neonatal hypoglycemia (48%). Tests of heterogeneity in the meta-analysis and of quality all pointed to better outcomes in the One Step test group.13,19
The need for a second step in the Two Step approach delays diagnosis and treatment. The One Step approach is associated with an increase in GDM test adherence and earlier diagnosis,13 which is another reason for better outcomes with the One Step approach. In the presence of risk factors, such as prior GDM, prior macrosomia, advanced maternal age, multiple gestations, and others, the One Step test should be done at the first prenatal visit.
Continue to: US guidelines should be reconsidered...
US guidelines should be reconsidered
The One Step, 75-g, 2-hour oral glucose tolerance test is universally used to diagnose diabetes mellitus outside of pregnancy. Given our many noted reasons (TABLE 3), we recommend universal screening of GDM by using the One Step approach. It is time, indeed, for the United States to reconsider its guidelines for screening for GDM.

Gestational diabetes mellitus (GDM) generally is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1-14 The best approach and exact criteria to use for GDM screening and diagnosis are under worldwide debate. In TABLE 1 we present just some of the many differing suggestions by varying organizations.2,7-9,11,12,15-17 The American College of Obstetricians and Gynecologists, for instance, suggests a Two Step approach to diagnosis.15 We will make the argument in this article, however, that diagnosis should be defined universally as an abnormal result with the One Step 75-g glucose testing, as adopted by the World Health Organization, International Federation of Gynecology and Obstetrics, and others. Approximately 8% of all pregnancies are complicated by GDM by the One Step test in the United States.18-22 The prevalence may range from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.1,19

Diagnostic options
Different methods for screening and diagnosis of GDM have been proposed by international societies; there is controversy regarding the diagnosis of GDM by either the One Step or the Two Step approach.6
The One Step approach includes an oral glucose tolerance test with a 75-g glucose load with measurement of plasma glucose concentration at fasting state and 1 hour and 2 hours post–glucose administration. A positive result for the One Step approach is defined as at least 1 measurement higher than 92, 180, or 153 mg/dL at fasting, 1 hour, or 2 hours, respectively.
The Two Step approach includes a nonfasting oral 50-g glucose load, with a glucose blood measurement 1 hour later. A positive screening, defined often as a blood glucose value higher than 135 mg/dL (range, 130 to 140 mg/dL), is followed by a diagnostic test with a 100-g glucose load with measurements at fasting and 1, 2, and 3 hours post–glucose administration. A positive diagnostic test is defined as 2 measurements higher than the target value.
Why we support the One Step test
There are several reasons to prefer the One Step approach for the diagnosis of GDM, compared with the Two Step approach.
Women testing negative for GDM with Two Step still experience complications pregnancy. Women who test positive for GDM with the One Step test, but negative with the Two Step test, despite having therefore a milder degree of glucose intolerance, do have a higher risk of experiencing several complications.23 For the mother, these complications include gestational hypertension, preeclampsia, and cesarean delivery. The baby also can experience problems at birth (TABLE 2).23 Therefore, women who test positive for GDM with the One Step test deserve to be diagnosed with and treated for the condition, as not only are they at risk for these complications but also treatment of the GDM decreases the incidence of these complications.18,19
There is indeed an increased GDM diagnosis rate with the One Step (about 8%) compared with the Two Step test (about 4%). Nonetheless, this increase is mild and nonsignificant in the meta-analysis of randomized controlled trials (RCTs),18,19 is less than the 18% difference in diagnosis rate previously hypothesized, is consistent with the increased diabetes/prediabetes rates in the general population, and is linked to the increasing incidence of obesity and insulin resistance.
Overall test adherence is better. Five percent to 15% of patients, depending on the study, are not adherent with taking the second part of the Two Step test. Women indeed prefer the One Step approach; the second step in the Two Step approach may be a burden.
Less costly. The One Step process is cost-effective when postpregnancy diabetes mellitus prevention is considered.
Better maternal and perinatal outcomes. Probably the most important and convincing reason the One Step test should be used is that meta-analysis of the 4 RCTs comparing the approaches (including 2 US trials) shows that diagnosing and treating mild GDM as per the One Step approach, compared with screening and treating using the Two Step approach, is associated with increased incidence of GDM (8% vs 4%) and with better maternal and perinatal outcomes.13,18,19 In fact, the One Step approach is associated with significant reductions in: large for gestational age (56%), admission to neonatal intensive care unit (51%), and neonatal hypoglycemia (48%). Tests of heterogeneity in the meta-analysis and of quality all pointed to better outcomes in the One Step test group.13,19
The need for a second step in the Two Step approach delays diagnosis and treatment. The One Step approach is associated with an increase in GDM test adherence and earlier diagnosis,13 which is another reason for better outcomes with the One Step approach. In the presence of risk factors, such as prior GDM, prior macrosomia, advanced maternal age, multiple gestations, and others, the One Step test should be done at the first prenatal visit.
Continue to: US guidelines should be reconsidered...
US guidelines should be reconsidered
The One Step, 75-g, 2-hour oral glucose tolerance test is universally used to diagnose diabetes mellitus outside of pregnancy. Given our many noted reasons (TABLE 3), we recommend universal screening of GDM by using the One Step approach. It is time, indeed, for the United States to reconsider its guidelines for screening for GDM.

- Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065-1072.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Meltzer SJ, Snyder J, Penrod JR, et al. Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG. 2010;117:407-415.
- Sevket O, Ates S, Uysal O, et al. To evaluate the prevalence and clinical outcomes using a one-step method versus a two-step method to screen gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:36-41.
- Scifres CM, Abebe KZ, Jones KA, et al. Gestational diabetes diagnostic methods (GD2M) pilot randomized trial. Matern Child Health J. 2015;19:1472-1480.
- Farrar D, Duley L, Medley N, et al. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015;1:CD007122.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Diabetes and Pregnancy. Can J Diabetes. 2013;37(suppl 1):S168-S183.
- NICE guideline. Diabetes in pregnancy: management from preconception to the postnatal period. February 2015. https://www.nice.org.uk/guidance/ng3/. Last updated August 2015. Accessed November 18, 2019.
- WHO 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. From http://apps.who.int/iris/bitstream/10665/66040/1/WHO
_NCD_NCS_99.2.pdf. Accessed November 18, 2019. - World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. http://apps.who.int/iris/bitstream/10665/85975/1/WHO_
NMH_MND_13.2_eng.pdf. Accessed November 18, 2019. - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211.
- Berghella V, Caissutti C, Saccone G, et al. The One Step approach for diagnosing gestational diabetes is associated with better perinatal outcomes than using the Two Step approach: evidence of randomized clinical trials. Am J Obstet Gynecol. 2019;220:562-564.
- Berghella V, Caissutti C, Saccone G, et al. One-Step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2019;133:383.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17-e31.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2018:1-9.
- Saccone G, Caissutti C, Khalifeh A, et al. One step versus two step approach for gestational diabetes screening: systematic review and meta-analysis of the randomized trials. J Matern Fetal Neonatal Med. 2019;32:1547-1555.
- Khalifeh A, Eckler R, Felder L, et al. One-step versus two-step diagnostic testing for gestational diabetes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-6.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2905-2914.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: which should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:235-247.
- Caissutti C, Khalifeh A, Saccone G, et al. Are women positive for the One Step but negative for the Two Step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018;97:122-134.
- Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065-1072.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Meltzer SJ, Snyder J, Penrod JR, et al. Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG. 2010;117:407-415.
- Sevket O, Ates S, Uysal O, et al. To evaluate the prevalence and clinical outcomes using a one-step method versus a two-step method to screen gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:36-41.
- Scifres CM, Abebe KZ, Jones KA, et al. Gestational diabetes diagnostic methods (GD2M) pilot randomized trial. Matern Child Health J. 2015;19:1472-1480.
- Farrar D, Duley L, Medley N, et al. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015;1:CD007122.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Diabetes and Pregnancy. Can J Diabetes. 2013;37(suppl 1):S168-S183.
- NICE guideline. Diabetes in pregnancy: management from preconception to the postnatal period. February 2015. https://www.nice.org.uk/guidance/ng3/. Last updated August 2015. Accessed November 18, 2019.
- WHO 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. From http://apps.who.int/iris/bitstream/10665/66040/1/WHO
_NCD_NCS_99.2.pdf. Accessed November 18, 2019. - World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. http://apps.who.int/iris/bitstream/10665/85975/1/WHO_
NMH_MND_13.2_eng.pdf. Accessed November 18, 2019. - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211.
- Berghella V, Caissutti C, Saccone G, et al. The One Step approach for diagnosing gestational diabetes is associated with better perinatal outcomes than using the Two Step approach: evidence of randomized clinical trials. Am J Obstet Gynecol. 2019;220:562-564.
- Berghella V, Caissutti C, Saccone G, et al. One-Step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2019;133:383.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17-e31.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2018:1-9.
- Saccone G, Caissutti C, Khalifeh A, et al. One step versus two step approach for gestational diabetes screening: systematic review and meta-analysis of the randomized trials. J Matern Fetal Neonatal Med. 2019;32:1547-1555.
- Khalifeh A, Eckler R, Felder L, et al. One-step versus two-step diagnostic testing for gestational diabetes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-6.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2905-2914.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: which should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:235-247.
- Caissutti C, Khalifeh A, Saccone G, et al. Are women positive for the One Step but negative for the Two Step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018;97:122-134.
Presence
The plan was in motion before I got on the plane.
When your leukemia came back suddenly 3 years after your stem cell transplant, it was devastating. But we had a plan. Your cancer developed a new mutation we could target with a chemotherapy drug. If we got you into a second remission, we could consolidate it by infusing more of your donor’s stem cells.
We met in the hospital, but I was adamant to “keep” you as my patient when you got to the clinic. I made swaps to see you, to get the continuity I value so much but often lose as a fellow, rotating from clinic to hospital to clinic.
I was grateful to see how well you dealt with the chemotherapy. Typically it’s a tough regimen, but you hardly had side effects. Between visits, I would check your blood counts on my phone, watching your blast count fall and your normal blood cells rise. Watching the cancer disappear.
Your lumbar punctures were negative, negative, negative – my favorite word, I told you. I was involved in long email threads coordinating the timing of your stem cell infusion with the remission we were achieving.
We were on our way.
One day, your lumbar puncture came back with a few “atypical” cells. I called the pathologist, and upon further review they were convinced the cells were reactive, not cancer. The next lumbar puncture was normal, but it was hard to ignore.
“Are you worried?” I asked my attending in clinic.
“I’m always worried,” she said. Neither of us truly believed the leukemia was back, but with the odds against us, we pored over every detail, always on the alert for a clue to an outcome we feared.
By now, the stem cell infusion was all set up. The donor was ready; so was the medical team; so were you. It was exciting. I thought of how a different attending described his interest in leukemia: There’s a subset you get to cure. Yes, you were going to be one of them.
Your big day coincided with a vacation I had scheduled months before. I was sorry I would be missing the actual moment, but happy I would come back to good news.
I left my coat and badge at the hospital, packed my bags, and got on the plane. I refrained from immediately checking your blood counts on my phone as soon as we landed. That night, jet lagged, I let myself look before I go to sleep. Relief. Your numbers still looked good.
Every day, I explored. My Internet was spotty during my travels, and when I would I finally get service I would peek at your latest blood tests.
Day 1. Cooled lava canyons. Black sand beaches. Circulating blast count: 0%.
Day 2: Glacier tour. A national park. Geysers. Blast count: 2%.
Day 3: We drive along the shore to see a famous waterfall, where you can climb a set of winding stairs to the top.
I check my phone before we start the climb. No service.
And so we begin. The wind cuts as I count steps. 403, 404, 405 … and 406. We are there. The air is thin, the world quiet. My nose is running from the cold.
We hike a bit, and I glance down again. Still no signal. It’s probably for the best. The scenery is spectacular.
Two miles later, I get service. I open the blood work first. Circulating blast count: 5%. But the other counts are okay. It could still be reactive, I say to myself, though on a deeper level I think of my attending’s words: I’m always worried. The stem cell infusion is scheduled for tomorrow.
I hear the rush of the water hitting the rocks below. Icicles form to our left. Sheep graze on our right. I appreciate the feeling of my muscles aching as we climb, higher and higher, a reminder of where I am and my place in it.
At the very top, we pause to take photos. And I get a signal again. I open the bone marrow biopsy report and skim the pathologist’s words. My eyes glue on the summary: 80% blasts, compatible with relapsed leukemia.
I let out an audible gasp.
Do you know? How will they tell you? I am painfully aware of the distance between us, in so many ways.
I want to be present. And soon I will be back, and I will be visiting in the hospital, and we will be having hard conversations and thinking about hard decisions.
But I’m not there right now. Someone else is. Here, now, I realize what I cannot do. The best way I can be present for you later is to be present where I am now.
I stuff my phone in my backpack and zip it closed. I step carefully forward on the rocks, slippery from the rain. My nose is running again, but not from the cold.
“What do you think?” my partner asks.
“The views are incredible,” I say.
Minor details of this story were changed to protect privacy.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
The plan was in motion before I got on the plane.
When your leukemia came back suddenly 3 years after your stem cell transplant, it was devastating. But we had a plan. Your cancer developed a new mutation we could target with a chemotherapy drug. If we got you into a second remission, we could consolidate it by infusing more of your donor’s stem cells.
We met in the hospital, but I was adamant to “keep” you as my patient when you got to the clinic. I made swaps to see you, to get the continuity I value so much but often lose as a fellow, rotating from clinic to hospital to clinic.
I was grateful to see how well you dealt with the chemotherapy. Typically it’s a tough regimen, but you hardly had side effects. Between visits, I would check your blood counts on my phone, watching your blast count fall and your normal blood cells rise. Watching the cancer disappear.
Your lumbar punctures were negative, negative, negative – my favorite word, I told you. I was involved in long email threads coordinating the timing of your stem cell infusion with the remission we were achieving.
We were on our way.
One day, your lumbar puncture came back with a few “atypical” cells. I called the pathologist, and upon further review they were convinced the cells were reactive, not cancer. The next lumbar puncture was normal, but it was hard to ignore.
“Are you worried?” I asked my attending in clinic.
“I’m always worried,” she said. Neither of us truly believed the leukemia was back, but with the odds against us, we pored over every detail, always on the alert for a clue to an outcome we feared.
By now, the stem cell infusion was all set up. The donor was ready; so was the medical team; so were you. It was exciting. I thought of how a different attending described his interest in leukemia: There’s a subset you get to cure. Yes, you were going to be one of them.
Your big day coincided with a vacation I had scheduled months before. I was sorry I would be missing the actual moment, but happy I would come back to good news.
I left my coat and badge at the hospital, packed my bags, and got on the plane. I refrained from immediately checking your blood counts on my phone as soon as we landed. That night, jet lagged, I let myself look before I go to sleep. Relief. Your numbers still looked good.
Every day, I explored. My Internet was spotty during my travels, and when I would I finally get service I would peek at your latest blood tests.
Day 1. Cooled lava canyons. Black sand beaches. Circulating blast count: 0%.
Day 2: Glacier tour. A national park. Geysers. Blast count: 2%.
Day 3: We drive along the shore to see a famous waterfall, where you can climb a set of winding stairs to the top.
I check my phone before we start the climb. No service.
And so we begin. The wind cuts as I count steps. 403, 404, 405 … and 406. We are there. The air is thin, the world quiet. My nose is running from the cold.
We hike a bit, and I glance down again. Still no signal. It’s probably for the best. The scenery is spectacular.
Two miles later, I get service. I open the blood work first. Circulating blast count: 5%. But the other counts are okay. It could still be reactive, I say to myself, though on a deeper level I think of my attending’s words: I’m always worried. The stem cell infusion is scheduled for tomorrow.
I hear the rush of the water hitting the rocks below. Icicles form to our left. Sheep graze on our right. I appreciate the feeling of my muscles aching as we climb, higher and higher, a reminder of where I am and my place in it.
At the very top, we pause to take photos. And I get a signal again. I open the bone marrow biopsy report and skim the pathologist’s words. My eyes glue on the summary: 80% blasts, compatible with relapsed leukemia.
I let out an audible gasp.
Do you know? How will they tell you? I am painfully aware of the distance between us, in so many ways.
I want to be present. And soon I will be back, and I will be visiting in the hospital, and we will be having hard conversations and thinking about hard decisions.
But I’m not there right now. Someone else is. Here, now, I realize what I cannot do. The best way I can be present for you later is to be present where I am now.
I stuff my phone in my backpack and zip it closed. I step carefully forward on the rocks, slippery from the rain. My nose is running again, but not from the cold.
“What do you think?” my partner asks.
“The views are incredible,” I say.
Minor details of this story were changed to protect privacy.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
The plan was in motion before I got on the plane.
When your leukemia came back suddenly 3 years after your stem cell transplant, it was devastating. But we had a plan. Your cancer developed a new mutation we could target with a chemotherapy drug. If we got you into a second remission, we could consolidate it by infusing more of your donor’s stem cells.
We met in the hospital, but I was adamant to “keep” you as my patient when you got to the clinic. I made swaps to see you, to get the continuity I value so much but often lose as a fellow, rotating from clinic to hospital to clinic.
I was grateful to see how well you dealt with the chemotherapy. Typically it’s a tough regimen, but you hardly had side effects. Between visits, I would check your blood counts on my phone, watching your blast count fall and your normal blood cells rise. Watching the cancer disappear.
Your lumbar punctures were negative, negative, negative – my favorite word, I told you. I was involved in long email threads coordinating the timing of your stem cell infusion with the remission we were achieving.
We were on our way.
One day, your lumbar puncture came back with a few “atypical” cells. I called the pathologist, and upon further review they were convinced the cells were reactive, not cancer. The next lumbar puncture was normal, but it was hard to ignore.
“Are you worried?” I asked my attending in clinic.
“I’m always worried,” she said. Neither of us truly believed the leukemia was back, but with the odds against us, we pored over every detail, always on the alert for a clue to an outcome we feared.
By now, the stem cell infusion was all set up. The donor was ready; so was the medical team; so were you. It was exciting. I thought of how a different attending described his interest in leukemia: There’s a subset you get to cure. Yes, you were going to be one of them.
Your big day coincided with a vacation I had scheduled months before. I was sorry I would be missing the actual moment, but happy I would come back to good news.
I left my coat and badge at the hospital, packed my bags, and got on the plane. I refrained from immediately checking your blood counts on my phone as soon as we landed. That night, jet lagged, I let myself look before I go to sleep. Relief. Your numbers still looked good.
Every day, I explored. My Internet was spotty during my travels, and when I would I finally get service I would peek at your latest blood tests.
Day 1. Cooled lava canyons. Black sand beaches. Circulating blast count: 0%.
Day 2: Glacier tour. A national park. Geysers. Blast count: 2%.
Day 3: We drive along the shore to see a famous waterfall, where you can climb a set of winding stairs to the top.
I check my phone before we start the climb. No service.
And so we begin. The wind cuts as I count steps. 403, 404, 405 … and 406. We are there. The air is thin, the world quiet. My nose is running from the cold.
We hike a bit, and I glance down again. Still no signal. It’s probably for the best. The scenery is spectacular.
Two miles later, I get service. I open the blood work first. Circulating blast count: 5%. But the other counts are okay. It could still be reactive, I say to myself, though on a deeper level I think of my attending’s words: I’m always worried. The stem cell infusion is scheduled for tomorrow.
I hear the rush of the water hitting the rocks below. Icicles form to our left. Sheep graze on our right. I appreciate the feeling of my muscles aching as we climb, higher and higher, a reminder of where I am and my place in it.
At the very top, we pause to take photos. And I get a signal again. I open the bone marrow biopsy report and skim the pathologist’s words. My eyes glue on the summary: 80% blasts, compatible with relapsed leukemia.
I let out an audible gasp.
Do you know? How will they tell you? I am painfully aware of the distance between us, in so many ways.
I want to be present. And soon I will be back, and I will be visiting in the hospital, and we will be having hard conversations and thinking about hard decisions.
But I’m not there right now. Someone else is. Here, now, I realize what I cannot do. The best way I can be present for you later is to be present where I am now.
I stuff my phone in my backpack and zip it closed. I step carefully forward on the rocks, slippery from the rain. My nose is running again, but not from the cold.
“What do you think?” my partner asks.
“The views are incredible,” I say.
Minor details of this story were changed to protect privacy.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
How to respond to flu vaccine doubters
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
Neuroimaging in psychiatry: Potentials and pitfalls
Advances in neuroimaging over the past 25 years have allowed for an increasingly sophisticated understanding of the structural and functional brain abnormalities associated with psychiatric disease.1 It has been postulated that a better understanding of aberrant brain circuitry in psychiatric illness will be critical for transforming the diagnosis and treatment of these illnesses.2 In fact, in 2008, the National Institute of Mental Health launched the Research Domain Criteria project to reformulate psychiatric diagnosis based on biologic underpinnings.3
In the midst of these scientific advances and the increased availability of neuroimaging, some private clinics have begun to offer routine brain scans as part of a comprehensive psychiatric evaluation.4-7 These clinics suggest that single-photon emission computed tomography (SPECT) of the brain can provide objective, reliable psychiatric diagnoses. Unfortunately, using SPECT for psychiatric diagnosis lacks empirical support and carries risks, including exposing patients to radioisotopes and detracting from empirically validated treatments.8 Nonetheless, given the current diagnostic challenges in psychiatry, it is understandable that patients, parents, and clinicians alike have reported high receptivity to the use of neuroimaging for psychiatric diagnosis and treatment planning.9
While neuroimaging is central to the search for improved understanding of the biologic foundations of mental illness, progress in identifying biomarkers has been disappointing. There are currently no neuroimaging biomarkers that can reliably distinguish patients from controls, and no empirical evidence supports the use of neuroimaging in diagnosing psychiatric conditions.10 The current standard of clinical care is to use neuroimaging to diagnose neurologic diseases that are masquerading as psychiatric disorders. However, given the rapid advances and availability of this technology, determining if and when neuroimaging is clinically indicated will likely soon become increasingly complex. Prior to the widespread availability of this technology, it is worth considering the potential advantages and pitfalls to the adoption of neuroimaging in psychiatry. In this article, we:
- outline arguments that support the use of neuroimaging in psychiatry, and some of the limitations
- discuss special considerations for patients with first-episode psychosis (FEP) and forensic psychiatry
- suggest guidelines for best-practice models based on the current evidence.
Advantages of widespread use of neuroimaging in psychiatry
Currently, neuroimaging is used in psychiatry to rule out neurologic disorders such as seizures, tumors, or infectious illness that might be causing psychiatric symptoms. If neuroimaging were routinely used for this purpose, one theoretical advantage would be increased neurologic diagnostic accuracy. Furthermore, increased adoption of neuroimaging may eventually help broaden the phenotype of neurologic disorders. In other words, psychiatric symptoms may be more common in neurologic disorders than we currently recognize. A second advantage might be that early and definitive exclusion of a structural neurologic disorder may help patients and families more readily accept a psychiatric diagnosis and appropriate treatment.
In the future, if biomarkers of psychiatric illness are discerned, using neuroimaging for diagnosis, assessment, and treatment planning may help increase objectivity and reduce the stigma associated with mental illness. Currently, psychiatric diagnoses are based on emotional and behavioral self-report and clinical observations. It is not uncommon for patients to receive different diagnoses and even conflicting recommendations from different clinicians. Tools that aid objective diagnosis will likely improve the reliability of the diagnosis and help in assessing treatment response. Also, concrete biomarkers that respond to treatment may help align psychiatric disorders with other medical illnesses, thereby decreasing stigma.
Cautions against routine neuroimaging
There are several potential pitfalls to the routine use of neuroimaging in psychiatry. First, clinical psychiatry is centered on clinical acumen and the doctor–patient relationship. Many psychiatric clinicians are not accustomed to using lab measures or tests to support the diagnostic process or treatment planning. Psychiatrists may be resistant to technologies that threaten clinical acumen, the power of the therapeutic relationship, and the value of getting to know patients over time.11 Overreliance on neuroimaging for psychiatric diagnosis also carries the risk of becoming overly reductionistic. This approach may overemphasize the biologic aspects of mental illness, while excluding social and psychological factors that may be responsive to treatment.
Second, the widespread use of neuroimaging is likely to result in many incidental findings. This is especially relevant because abnormality does not establish causality. Incidental findings may cause unnecessary anxiety for patients and families, particularly if there are minimal treatment options.
Continue to: Third, it remains unclear...
Third, it remains unclear whether widespread neuroimaging in psychiatry will be cost-effective. Unless imaging results are tied to effective treatments, neuroimaging is unlikely to result in cost savings. Presently, patients who can afford out-of-pocket care might be able to access neuroimaging. If neuroimaging were shown to improve clinical outcomes but remains costly, this unequal distribution of resources would create an ethical quandary.
Finally, neuroimaging is complex and almost certainly not as objective as one might hope. Interpreting images will require specialized knowledge and skills that are beyond those of currently certified general psychiatrists.12 Because there is a great deal of overlap in brain anomalies across psychiatric illnesses, it is unclear whether using neuroimaging for diagnostic purposes will eclipse a thorough clinical assessment. For example, the amygdala and insula show activation across a range of anxiety disorders. Abnormal amygdala activation has also been reported in depression, bipolar disorder, schizophrenia, and psychopathy.13
In addition, psychiatric comorbidity is common. It is unclear how much neuroimaging will add diagnostically when a patient presents with multiple psychiatric disorders. Comorbidity of psychiatric and neurologic disorders also is common. A neurologic illness that is detectable by structural neuroimaging does not necessarily exclude the presence of a psychiatric disorder. This poses yet another challenge to developing reliable, valid neuroimaging techniques for clinical use.
Areas of controversy
First-episode psychosis. Current practice guidelines for neuroimaging in patients with FEP are inconsistent. The Canadian Choosing Wisely Guidelines recommend against routinely ordering neuroimaging in first-episode psychoses in the absence of signs or symptoms that suggest intracranial pathology.14 Similarly, the American Psychiatric Association’s Practice Guideline for the Treatment of Patients with Schizophrenia recommends ordering neuroimaging in patients for whom the clinical picture is unclear or when examination reveals abnormal findings.15 In contrast, the Australian Clinical Guidelines for Early Psychosis recommend that all patients with FEP receive brain MRI.16 Freudenreich et al17 describe 2 philosophies regarding the initial medical workup of FEP: (1) a comprehensive medical workup requires extensive testing, and (2) in their natural histories, most illnesses eventually declare themselves.
Despite this inconsistency, the overall evidence does not seem to support routine brain imaging for patients with FEP in the absence of neurologic or cognitive impairment. A systematic review of 16 studies assessing the clinical utility of structural neuroimaging in FEP found that there was “insufficient evidence to suggest that brain imaging should be routinely ordered for patients presenting with first-episode psychosis without associated neurological or cognitive impairment.”18
Continue to: Forensic psychiatry
Forensic psychiatry. Two academic disciplines—neuroethics and neurolaw—attempt to study how medications and neuroimaging could impact forensic psychiatry.19 And in this golden age of neuroscience, psychiatrists specializing in forensics may be increasingly asked to opine on brain scans. This requires specific thoughtfulness and attention because forensic psychiatrists must “distinguish neuroscience from neuro-nonsense.”20 These specialists will need to consider the Daubert standard, which resulted from the 1993 case Daubert v Merrell Dow Pharmaceuticals, Inc.21 In this case, the US Supreme Court ruled that evidence must be “‘generally accepted’ as reliable in the relevant scientific community” to be admissible. According to the Daubert standard, “evidentiary reliability” is based on scientific validity.21
How should we use neuroimaging?
While neuroimaging is a quickly evolving research tool, empirical support for its clinical use remains limited. The hope is that future neuroimaging research will yield biomarker profiles for mental illness, identification of risk factors, and predictors of vulnerability and treatment response, which will allow for more targeted treatments.1
The current standard of clinical care for using neuroimaging in psychiatry is to diagnose neurologic diseases. Although there are no consensus guidelines for when to order imaging, it is reasonable to consider imaging when a patient has22:
- abrupt onset of symptoms
- change in level of consciousness
- deficits in neurologic or cognitive examination
- a history of head trauma (with loss of consciousness), whole-brain radiation, neurologic comorbidities, or cancer
- late onset of symptoms (age >50)
- atypical presentation of psychiatric illness.
1. Silbersweig DA, Rauch SL. Neuroimaging in psychiatry: a quarter century of progress. Harv Rev Psychiatry. 2017;25(5):195-197.
2. Insel TR, Wang PS. Rethinking mental illness. JAMA. 2010;303(19):1970-1971.
3. Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66(11):988-989.
4. Cyranoski D. Neuroscience: thought experiment. Nature. 2011;469:148-149.
5. Amen Clinics. https://www.amenclinics.com/. Accessed October 22, 2019.
6. Pathfinder Brain SPECT Imaging. https://pathfinder.md/. Accessed October 22, 2019.
7. DrSpectScan. http://www.drspectscan.org/. Accessed October 22, 2019.
8. Adinoff B, Devous M. Scientifically unfounded claims in diagnosing and treating patients. Am J Psychiatry. 2010;167(5):598.
9. Borgelt EL, Buchman DZ, Illes J. Neuroimaging in mental health care: voices in translation. Front Hum Neurosci. 2012;6:293.
10. Linden DEJ. The challenges and promise of neuroimaging in psychiatry. Neuron. 2012;73(1):8-22.
11. Macqueen GM. Will there be a role for neuroimaging in clinical psychiatry? J Psychiatry Neurosci. 2010;35(5):291-293.
12. Boyce AC. Neuroimaging in psychiatry: evaluating the ethical consequences for patient care. Bioethics. 2009;23(6):349-359.
13. Farah MJ, Gillihan SJ. Diagnostic brain imaging in psychiatry: current uses and future prospects. Virtual Mentor. 2012;14(6):464-471.
14. Canadian Academy of Child and Adolescent Psychiatry, et al. Thirteen things physicians and patients should question. Choosing Wisely Canada. https://choosingwiselycanada.org/wp-content/uploads/2017/02/Psychiatry.pdf. Updated June 2017. Accessed October 22, 2019.
15. Lehman AF, Lieberman JA, Dixon LB, et al; Work Group on Schizophrenia. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
16. Australian Clinical Guidelines for Early Psychosis. 2nd edition. The National Centre of Excellence in Youth Mental Health. https://www.orygen.org.au/Campus/Expert-Network/Resources/Free/Clinical-Practice/Australian-Clinical-Guidelines-for-Early-Psychosis/Australian-Clinical-Guidelines-for-Early-Psychosis.aspx?ext=. Updated 2016. Accessed October 22, 2019.
17. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
18. Forbes M, Stefler D, Velakoulis D, et al. The clinical utility of structural neuroimaging in first-episode psychosis: a systematic review. Aust N Z J Psychiatry. 2019:000486741984803. doi: 10.1177/0004867419848035.
19. Aggarwal N. Neuroimaging, culture, and forensic psychiatry. J Am Acad Psychiatry Law. 2009;37(2):239-244
20. Choi O. What neuroscience can and cannot answer. J Am Acad Psychiatry Law. 2017;45(3):278-285.
21. Daubert v Merrell Dow Pharmaceuticals, Inc. 509 US 579 (1993).
22. Camprodon JA, Stern TA. Selecting neuroimaging techniques: a review for the clinician. Prim Care Companion CNS Disord. 2013;15(4):PCC.12f01490. doi: 10.4088/PCC.12f01490.
Advances in neuroimaging over the past 25 years have allowed for an increasingly sophisticated understanding of the structural and functional brain abnormalities associated with psychiatric disease.1 It has been postulated that a better understanding of aberrant brain circuitry in psychiatric illness will be critical for transforming the diagnosis and treatment of these illnesses.2 In fact, in 2008, the National Institute of Mental Health launched the Research Domain Criteria project to reformulate psychiatric diagnosis based on biologic underpinnings.3
In the midst of these scientific advances and the increased availability of neuroimaging, some private clinics have begun to offer routine brain scans as part of a comprehensive psychiatric evaluation.4-7 These clinics suggest that single-photon emission computed tomography (SPECT) of the brain can provide objective, reliable psychiatric diagnoses. Unfortunately, using SPECT for psychiatric diagnosis lacks empirical support and carries risks, including exposing patients to radioisotopes and detracting from empirically validated treatments.8 Nonetheless, given the current diagnostic challenges in psychiatry, it is understandable that patients, parents, and clinicians alike have reported high receptivity to the use of neuroimaging for psychiatric diagnosis and treatment planning.9
While neuroimaging is central to the search for improved understanding of the biologic foundations of mental illness, progress in identifying biomarkers has been disappointing. There are currently no neuroimaging biomarkers that can reliably distinguish patients from controls, and no empirical evidence supports the use of neuroimaging in diagnosing psychiatric conditions.10 The current standard of clinical care is to use neuroimaging to diagnose neurologic diseases that are masquerading as psychiatric disorders. However, given the rapid advances and availability of this technology, determining if and when neuroimaging is clinically indicated will likely soon become increasingly complex. Prior to the widespread availability of this technology, it is worth considering the potential advantages and pitfalls to the adoption of neuroimaging in psychiatry. In this article, we:
- outline arguments that support the use of neuroimaging in psychiatry, and some of the limitations
- discuss special considerations for patients with first-episode psychosis (FEP) and forensic psychiatry
- suggest guidelines for best-practice models based on the current evidence.
Advantages of widespread use of neuroimaging in psychiatry
Currently, neuroimaging is used in psychiatry to rule out neurologic disorders such as seizures, tumors, or infectious illness that might be causing psychiatric symptoms. If neuroimaging were routinely used for this purpose, one theoretical advantage would be increased neurologic diagnostic accuracy. Furthermore, increased adoption of neuroimaging may eventually help broaden the phenotype of neurologic disorders. In other words, psychiatric symptoms may be more common in neurologic disorders than we currently recognize. A second advantage might be that early and definitive exclusion of a structural neurologic disorder may help patients and families more readily accept a psychiatric diagnosis and appropriate treatment.
In the future, if biomarkers of psychiatric illness are discerned, using neuroimaging for diagnosis, assessment, and treatment planning may help increase objectivity and reduce the stigma associated with mental illness. Currently, psychiatric diagnoses are based on emotional and behavioral self-report and clinical observations. It is not uncommon for patients to receive different diagnoses and even conflicting recommendations from different clinicians. Tools that aid objective diagnosis will likely improve the reliability of the diagnosis and help in assessing treatment response. Also, concrete biomarkers that respond to treatment may help align psychiatric disorders with other medical illnesses, thereby decreasing stigma.
Cautions against routine neuroimaging
There are several potential pitfalls to the routine use of neuroimaging in psychiatry. First, clinical psychiatry is centered on clinical acumen and the doctor–patient relationship. Many psychiatric clinicians are not accustomed to using lab measures or tests to support the diagnostic process or treatment planning. Psychiatrists may be resistant to technologies that threaten clinical acumen, the power of the therapeutic relationship, and the value of getting to know patients over time.11 Overreliance on neuroimaging for psychiatric diagnosis also carries the risk of becoming overly reductionistic. This approach may overemphasize the biologic aspects of mental illness, while excluding social and psychological factors that may be responsive to treatment.
Second, the widespread use of neuroimaging is likely to result in many incidental findings. This is especially relevant because abnormality does not establish causality. Incidental findings may cause unnecessary anxiety for patients and families, particularly if there are minimal treatment options.
Continue to: Third, it remains unclear...
Third, it remains unclear whether widespread neuroimaging in psychiatry will be cost-effective. Unless imaging results are tied to effective treatments, neuroimaging is unlikely to result in cost savings. Presently, patients who can afford out-of-pocket care might be able to access neuroimaging. If neuroimaging were shown to improve clinical outcomes but remains costly, this unequal distribution of resources would create an ethical quandary.
Finally, neuroimaging is complex and almost certainly not as objective as one might hope. Interpreting images will require specialized knowledge and skills that are beyond those of currently certified general psychiatrists.12 Because there is a great deal of overlap in brain anomalies across psychiatric illnesses, it is unclear whether using neuroimaging for diagnostic purposes will eclipse a thorough clinical assessment. For example, the amygdala and insula show activation across a range of anxiety disorders. Abnormal amygdala activation has also been reported in depression, bipolar disorder, schizophrenia, and psychopathy.13
In addition, psychiatric comorbidity is common. It is unclear how much neuroimaging will add diagnostically when a patient presents with multiple psychiatric disorders. Comorbidity of psychiatric and neurologic disorders also is common. A neurologic illness that is detectable by structural neuroimaging does not necessarily exclude the presence of a psychiatric disorder. This poses yet another challenge to developing reliable, valid neuroimaging techniques for clinical use.
Areas of controversy
First-episode psychosis. Current practice guidelines for neuroimaging in patients with FEP are inconsistent. The Canadian Choosing Wisely Guidelines recommend against routinely ordering neuroimaging in first-episode psychoses in the absence of signs or symptoms that suggest intracranial pathology.14 Similarly, the American Psychiatric Association’s Practice Guideline for the Treatment of Patients with Schizophrenia recommends ordering neuroimaging in patients for whom the clinical picture is unclear or when examination reveals abnormal findings.15 In contrast, the Australian Clinical Guidelines for Early Psychosis recommend that all patients with FEP receive brain MRI.16 Freudenreich et al17 describe 2 philosophies regarding the initial medical workup of FEP: (1) a comprehensive medical workup requires extensive testing, and (2) in their natural histories, most illnesses eventually declare themselves.
Despite this inconsistency, the overall evidence does not seem to support routine brain imaging for patients with FEP in the absence of neurologic or cognitive impairment. A systematic review of 16 studies assessing the clinical utility of structural neuroimaging in FEP found that there was “insufficient evidence to suggest that brain imaging should be routinely ordered for patients presenting with first-episode psychosis without associated neurological or cognitive impairment.”18
Continue to: Forensic psychiatry
Forensic psychiatry. Two academic disciplines—neuroethics and neurolaw—attempt to study how medications and neuroimaging could impact forensic psychiatry.19 And in this golden age of neuroscience, psychiatrists specializing in forensics may be increasingly asked to opine on brain scans. This requires specific thoughtfulness and attention because forensic psychiatrists must “distinguish neuroscience from neuro-nonsense.”20 These specialists will need to consider the Daubert standard, which resulted from the 1993 case Daubert v Merrell Dow Pharmaceuticals, Inc.21 In this case, the US Supreme Court ruled that evidence must be “‘generally accepted’ as reliable in the relevant scientific community” to be admissible. According to the Daubert standard, “evidentiary reliability” is based on scientific validity.21
How should we use neuroimaging?
While neuroimaging is a quickly evolving research tool, empirical support for its clinical use remains limited. The hope is that future neuroimaging research will yield biomarker profiles for mental illness, identification of risk factors, and predictors of vulnerability and treatment response, which will allow for more targeted treatments.1
The current standard of clinical care for using neuroimaging in psychiatry is to diagnose neurologic diseases. Although there are no consensus guidelines for when to order imaging, it is reasonable to consider imaging when a patient has22:
- abrupt onset of symptoms
- change in level of consciousness
- deficits in neurologic or cognitive examination
- a history of head trauma (with loss of consciousness), whole-brain radiation, neurologic comorbidities, or cancer
- late onset of symptoms (age >50)
- atypical presentation of psychiatric illness.
Advances in neuroimaging over the past 25 years have allowed for an increasingly sophisticated understanding of the structural and functional brain abnormalities associated with psychiatric disease.1 It has been postulated that a better understanding of aberrant brain circuitry in psychiatric illness will be critical for transforming the diagnosis and treatment of these illnesses.2 In fact, in 2008, the National Institute of Mental Health launched the Research Domain Criteria project to reformulate psychiatric diagnosis based on biologic underpinnings.3
In the midst of these scientific advances and the increased availability of neuroimaging, some private clinics have begun to offer routine brain scans as part of a comprehensive psychiatric evaluation.4-7 These clinics suggest that single-photon emission computed tomography (SPECT) of the brain can provide objective, reliable psychiatric diagnoses. Unfortunately, using SPECT for psychiatric diagnosis lacks empirical support and carries risks, including exposing patients to radioisotopes and detracting from empirically validated treatments.8 Nonetheless, given the current diagnostic challenges in psychiatry, it is understandable that patients, parents, and clinicians alike have reported high receptivity to the use of neuroimaging for psychiatric diagnosis and treatment planning.9
While neuroimaging is central to the search for improved understanding of the biologic foundations of mental illness, progress in identifying biomarkers has been disappointing. There are currently no neuroimaging biomarkers that can reliably distinguish patients from controls, and no empirical evidence supports the use of neuroimaging in diagnosing psychiatric conditions.10 The current standard of clinical care is to use neuroimaging to diagnose neurologic diseases that are masquerading as psychiatric disorders. However, given the rapid advances and availability of this technology, determining if and when neuroimaging is clinically indicated will likely soon become increasingly complex. Prior to the widespread availability of this technology, it is worth considering the potential advantages and pitfalls to the adoption of neuroimaging in psychiatry. In this article, we:
- outline arguments that support the use of neuroimaging in psychiatry, and some of the limitations
- discuss special considerations for patients with first-episode psychosis (FEP) and forensic psychiatry
- suggest guidelines for best-practice models based on the current evidence.
Advantages of widespread use of neuroimaging in psychiatry
Currently, neuroimaging is used in psychiatry to rule out neurologic disorders such as seizures, tumors, or infectious illness that might be causing psychiatric symptoms. If neuroimaging were routinely used for this purpose, one theoretical advantage would be increased neurologic diagnostic accuracy. Furthermore, increased adoption of neuroimaging may eventually help broaden the phenotype of neurologic disorders. In other words, psychiatric symptoms may be more common in neurologic disorders than we currently recognize. A second advantage might be that early and definitive exclusion of a structural neurologic disorder may help patients and families more readily accept a psychiatric diagnosis and appropriate treatment.
In the future, if biomarkers of psychiatric illness are discerned, using neuroimaging for diagnosis, assessment, and treatment planning may help increase objectivity and reduce the stigma associated with mental illness. Currently, psychiatric diagnoses are based on emotional and behavioral self-report and clinical observations. It is not uncommon for patients to receive different diagnoses and even conflicting recommendations from different clinicians. Tools that aid objective diagnosis will likely improve the reliability of the diagnosis and help in assessing treatment response. Also, concrete biomarkers that respond to treatment may help align psychiatric disorders with other medical illnesses, thereby decreasing stigma.
Cautions against routine neuroimaging
There are several potential pitfalls to the routine use of neuroimaging in psychiatry. First, clinical psychiatry is centered on clinical acumen and the doctor–patient relationship. Many psychiatric clinicians are not accustomed to using lab measures or tests to support the diagnostic process or treatment planning. Psychiatrists may be resistant to technologies that threaten clinical acumen, the power of the therapeutic relationship, and the value of getting to know patients over time.11 Overreliance on neuroimaging for psychiatric diagnosis also carries the risk of becoming overly reductionistic. This approach may overemphasize the biologic aspects of mental illness, while excluding social and psychological factors that may be responsive to treatment.
Second, the widespread use of neuroimaging is likely to result in many incidental findings. This is especially relevant because abnormality does not establish causality. Incidental findings may cause unnecessary anxiety for patients and families, particularly if there are minimal treatment options.
Continue to: Third, it remains unclear...
Third, it remains unclear whether widespread neuroimaging in psychiatry will be cost-effective. Unless imaging results are tied to effective treatments, neuroimaging is unlikely to result in cost savings. Presently, patients who can afford out-of-pocket care might be able to access neuroimaging. If neuroimaging were shown to improve clinical outcomes but remains costly, this unequal distribution of resources would create an ethical quandary.
Finally, neuroimaging is complex and almost certainly not as objective as one might hope. Interpreting images will require specialized knowledge and skills that are beyond those of currently certified general psychiatrists.12 Because there is a great deal of overlap in brain anomalies across psychiatric illnesses, it is unclear whether using neuroimaging for diagnostic purposes will eclipse a thorough clinical assessment. For example, the amygdala and insula show activation across a range of anxiety disorders. Abnormal amygdala activation has also been reported in depression, bipolar disorder, schizophrenia, and psychopathy.13
In addition, psychiatric comorbidity is common. It is unclear how much neuroimaging will add diagnostically when a patient presents with multiple psychiatric disorders. Comorbidity of psychiatric and neurologic disorders also is common. A neurologic illness that is detectable by structural neuroimaging does not necessarily exclude the presence of a psychiatric disorder. This poses yet another challenge to developing reliable, valid neuroimaging techniques for clinical use.
Areas of controversy
First-episode psychosis. Current practice guidelines for neuroimaging in patients with FEP are inconsistent. The Canadian Choosing Wisely Guidelines recommend against routinely ordering neuroimaging in first-episode psychoses in the absence of signs or symptoms that suggest intracranial pathology.14 Similarly, the American Psychiatric Association’s Practice Guideline for the Treatment of Patients with Schizophrenia recommends ordering neuroimaging in patients for whom the clinical picture is unclear or when examination reveals abnormal findings.15 In contrast, the Australian Clinical Guidelines for Early Psychosis recommend that all patients with FEP receive brain MRI.16 Freudenreich et al17 describe 2 philosophies regarding the initial medical workup of FEP: (1) a comprehensive medical workup requires extensive testing, and (2) in their natural histories, most illnesses eventually declare themselves.
Despite this inconsistency, the overall evidence does not seem to support routine brain imaging for patients with FEP in the absence of neurologic or cognitive impairment. A systematic review of 16 studies assessing the clinical utility of structural neuroimaging in FEP found that there was “insufficient evidence to suggest that brain imaging should be routinely ordered for patients presenting with first-episode psychosis without associated neurological or cognitive impairment.”18
Continue to: Forensic psychiatry
Forensic psychiatry. Two academic disciplines—neuroethics and neurolaw—attempt to study how medications and neuroimaging could impact forensic psychiatry.19 And in this golden age of neuroscience, psychiatrists specializing in forensics may be increasingly asked to opine on brain scans. This requires specific thoughtfulness and attention because forensic psychiatrists must “distinguish neuroscience from neuro-nonsense.”20 These specialists will need to consider the Daubert standard, which resulted from the 1993 case Daubert v Merrell Dow Pharmaceuticals, Inc.21 In this case, the US Supreme Court ruled that evidence must be “‘generally accepted’ as reliable in the relevant scientific community” to be admissible. According to the Daubert standard, “evidentiary reliability” is based on scientific validity.21
How should we use neuroimaging?
While neuroimaging is a quickly evolving research tool, empirical support for its clinical use remains limited. The hope is that future neuroimaging research will yield biomarker profiles for mental illness, identification of risk factors, and predictors of vulnerability and treatment response, which will allow for more targeted treatments.1
The current standard of clinical care for using neuroimaging in psychiatry is to diagnose neurologic diseases. Although there are no consensus guidelines for when to order imaging, it is reasonable to consider imaging when a patient has22:
- abrupt onset of symptoms
- change in level of consciousness
- deficits in neurologic or cognitive examination
- a history of head trauma (with loss of consciousness), whole-brain radiation, neurologic comorbidities, or cancer
- late onset of symptoms (age >50)
- atypical presentation of psychiatric illness.
1. Silbersweig DA, Rauch SL. Neuroimaging in psychiatry: a quarter century of progress. Harv Rev Psychiatry. 2017;25(5):195-197.
2. Insel TR, Wang PS. Rethinking mental illness. JAMA. 2010;303(19):1970-1971.
3. Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66(11):988-989.
4. Cyranoski D. Neuroscience: thought experiment. Nature. 2011;469:148-149.
5. Amen Clinics. https://www.amenclinics.com/. Accessed October 22, 2019.
6. Pathfinder Brain SPECT Imaging. https://pathfinder.md/. Accessed October 22, 2019.
7. DrSpectScan. http://www.drspectscan.org/. Accessed October 22, 2019.
8. Adinoff B, Devous M. Scientifically unfounded claims in diagnosing and treating patients. Am J Psychiatry. 2010;167(5):598.
9. Borgelt EL, Buchman DZ, Illes J. Neuroimaging in mental health care: voices in translation. Front Hum Neurosci. 2012;6:293.
10. Linden DEJ. The challenges and promise of neuroimaging in psychiatry. Neuron. 2012;73(1):8-22.
11. Macqueen GM. Will there be a role for neuroimaging in clinical psychiatry? J Psychiatry Neurosci. 2010;35(5):291-293.
12. Boyce AC. Neuroimaging in psychiatry: evaluating the ethical consequences for patient care. Bioethics. 2009;23(6):349-359.
13. Farah MJ, Gillihan SJ. Diagnostic brain imaging in psychiatry: current uses and future prospects. Virtual Mentor. 2012;14(6):464-471.
14. Canadian Academy of Child and Adolescent Psychiatry, et al. Thirteen things physicians and patients should question. Choosing Wisely Canada. https://choosingwiselycanada.org/wp-content/uploads/2017/02/Psychiatry.pdf. Updated June 2017. Accessed October 22, 2019.
15. Lehman AF, Lieberman JA, Dixon LB, et al; Work Group on Schizophrenia. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
16. Australian Clinical Guidelines for Early Psychosis. 2nd edition. The National Centre of Excellence in Youth Mental Health. https://www.orygen.org.au/Campus/Expert-Network/Resources/Free/Clinical-Practice/Australian-Clinical-Guidelines-for-Early-Psychosis/Australian-Clinical-Guidelines-for-Early-Psychosis.aspx?ext=. Updated 2016. Accessed October 22, 2019.
17. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
18. Forbes M, Stefler D, Velakoulis D, et al. The clinical utility of structural neuroimaging in first-episode psychosis: a systematic review. Aust N Z J Psychiatry. 2019:000486741984803. doi: 10.1177/0004867419848035.
19. Aggarwal N. Neuroimaging, culture, and forensic psychiatry. J Am Acad Psychiatry Law. 2009;37(2):239-244
20. Choi O. What neuroscience can and cannot answer. J Am Acad Psychiatry Law. 2017;45(3):278-285.
21. Daubert v Merrell Dow Pharmaceuticals, Inc. 509 US 579 (1993).
22. Camprodon JA, Stern TA. Selecting neuroimaging techniques: a review for the clinician. Prim Care Companion CNS Disord. 2013;15(4):PCC.12f01490. doi: 10.4088/PCC.12f01490.
1. Silbersweig DA, Rauch SL. Neuroimaging in psychiatry: a quarter century of progress. Harv Rev Psychiatry. 2017;25(5):195-197.
2. Insel TR, Wang PS. Rethinking mental illness. JAMA. 2010;303(19):1970-1971.
3. Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66(11):988-989.
4. Cyranoski D. Neuroscience: thought experiment. Nature. 2011;469:148-149.
5. Amen Clinics. https://www.amenclinics.com/. Accessed October 22, 2019.
6. Pathfinder Brain SPECT Imaging. https://pathfinder.md/. Accessed October 22, 2019.
7. DrSpectScan. http://www.drspectscan.org/. Accessed October 22, 2019.
8. Adinoff B, Devous M. Scientifically unfounded claims in diagnosing and treating patients. Am J Psychiatry. 2010;167(5):598.
9. Borgelt EL, Buchman DZ, Illes J. Neuroimaging in mental health care: voices in translation. Front Hum Neurosci. 2012;6:293.
10. Linden DEJ. The challenges and promise of neuroimaging in psychiatry. Neuron. 2012;73(1):8-22.
11. Macqueen GM. Will there be a role for neuroimaging in clinical psychiatry? J Psychiatry Neurosci. 2010;35(5):291-293.
12. Boyce AC. Neuroimaging in psychiatry: evaluating the ethical consequences for patient care. Bioethics. 2009;23(6):349-359.
13. Farah MJ, Gillihan SJ. Diagnostic brain imaging in psychiatry: current uses and future prospects. Virtual Mentor. 2012;14(6):464-471.
14. Canadian Academy of Child and Adolescent Psychiatry, et al. Thirteen things physicians and patients should question. Choosing Wisely Canada. https://choosingwiselycanada.org/wp-content/uploads/2017/02/Psychiatry.pdf. Updated June 2017. Accessed October 22, 2019.
15. Lehman AF, Lieberman JA, Dixon LB, et al; Work Group on Schizophrenia. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
16. Australian Clinical Guidelines for Early Psychosis. 2nd edition. The National Centre of Excellence in Youth Mental Health. https://www.orygen.org.au/Campus/Expert-Network/Resources/Free/Clinical-Practice/Australian-Clinical-Guidelines-for-Early-Psychosis/Australian-Clinical-Guidelines-for-Early-Psychosis.aspx?ext=. Updated 2016. Accessed October 22, 2019.
17. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
18. Forbes M, Stefler D, Velakoulis D, et al. The clinical utility of structural neuroimaging in first-episode psychosis: a systematic review. Aust N Z J Psychiatry. 2019:000486741984803. doi: 10.1177/0004867419848035.
19. Aggarwal N. Neuroimaging, culture, and forensic psychiatry. J Am Acad Psychiatry Law. 2009;37(2):239-244
20. Choi O. What neuroscience can and cannot answer. J Am Acad Psychiatry Law. 2017;45(3):278-285.
21. Daubert v Merrell Dow Pharmaceuticals, Inc. 509 US 579 (1993).
22. Camprodon JA, Stern TA. Selecting neuroimaging techniques: a review for the clinician. Prim Care Companion CNS Disord. 2013;15(4):PCC.12f01490. doi: 10.4088/PCC.12f01490.