User login
Giving Cash to Improve Health
This transcript has been edited for clarity.
It doesn’t really matter what disease you are looking at — cancer, heart disease, dementia, drug abuse, psychiatric disorders. In every case, poverty is associated with worse disease.
But the word “associated” is doing a lot of work there. Many of us feel that poverty itself is causally linked to worse disease outcomes through things like poor access to care and poor access to medicines.
And there is an argument that the arrow goes the other way; perhaps people with worse illness are more likely to be poor because, in this country at least, being sick is incredibly expensive.
Causality is what all medical research is fundamentally about. We want to know if A causes B, because if A causes B, then changing A changes B. If poverty causes bad health outcomes, then alleviating poverty should alleviate bad health outcomes.
But that’s a hard proposition to test. You can’t exactly randomize some people to get extra money and some not to, right? Actually, you can. And in Massachusetts, they did.
What happened in Chelsea, Massachusetts, wasn’t exactly a randomized trial of cash supplementation to avoid bad health outcomes. It was actually a government program instituted during the pandemic. Chelsea has a large immigrant population, many of whom are living in poverty. From April to August 2020, the city ran a food distribution program to aid those in need. But the decision was then made to convert the money spent on that program to cash distributions — free of obligations. Chelsea residents making less than 30% of the median income for the Boston metro area — around $30,000 per family — were invited to enter a lottery. Only one member of any given family could enter. If selected, an individual would receive $200 a month, or $300 for a family of two, or $400 for a family of three or more. These payments went on for about 9 months.
The key thing here is that not everyone won the lottery. The lottery picked winners randomly; 1746 individuals were selected to receive the benefits in the form of a reloadable gift card, and 1134 applied but did not receive any assistance.
This is a perfect natural experiment. As you can see here — and as expected, given that the lottery winners were chosen randomly — winners and losers were similar in terms of age, sex, race, language, income, and more.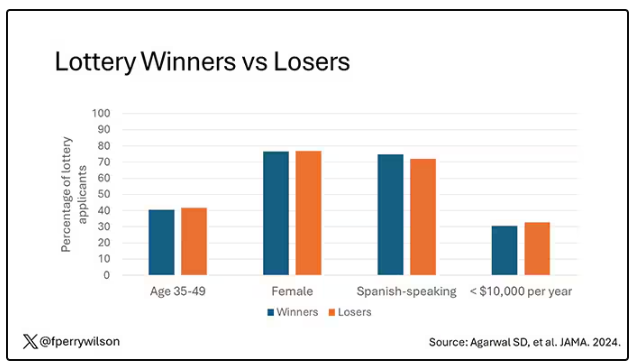
Researchers, led by Sumit Agarwal at the Brigham, leveraged that randomization to ask how these cash benefits would affect healthcare utilization. Their results appeared this week in JAMA.
I know what you’re thinking: Is $400 a month really enough to make a difference? Does $400 a month, less than $5000 a year, really fix poverty? We’ll get to that. But I will point out that the average family income of individuals in this study was about $1400 a month. An extra $400 might not change someone’s life, but it may really make a difference.
The primary outcome of this study was ED visits. There are a few ways this could go. Perhaps the money would lead to improved health and thus fewer ED visits. Or perhaps it would help people get transportation to primary care or other services that would offload the ED. Or maybe it would make things worse. Some folks have suggested that cash payments could increase the use of drugs and alcohol, and lead to more ED visits associated with the complications of using those substances.
Here are the actual data. Per 1000 individuals, there were 217 ED visits in the cash-benefit group, 318 in the no-benefit group. That was a statistically significant finding.
Breaking those ED visits down, you can see that fewer visits resulted in hospital admission, with fewer behavioral health–related visits and — a key finding — fewer visits for substance use disorder. This puts the lie to the idea that cash benefits increase drug use.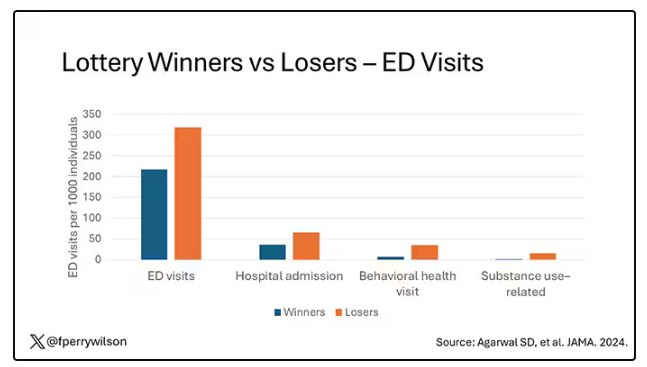
But the authors also looked at other causes of healthcare utilization. Outpatient visits were slightly higher in the cash-benefit group, driven largely by an increase in specialty care visits. The authors note that this is likely due to the fact that reaching a specialist often requires more travel, which can be costly. Indeed, this effect was most pronounced among the people living furthest from a specialty center.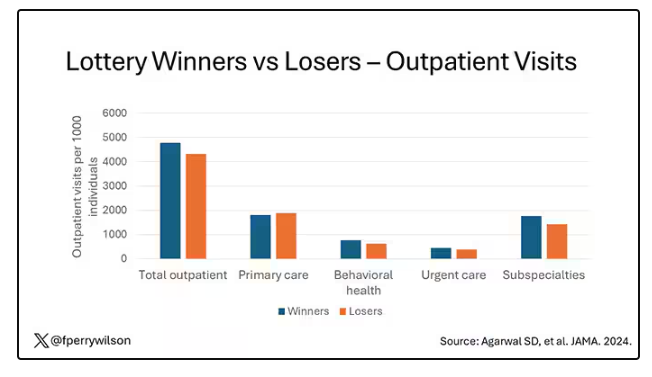
Outside of utilization, the researchers examined a variety of individual health markers — things like blood pressure — to see if the cash benefit had any effect. A bit of caution here because these data were available only among those who interacted with the healthcare system, which may bias the results a bit. Regardless, no major differences were seen in blood pressure, weight, hemoglobin A1c, cholesterol, or COVID vaccination.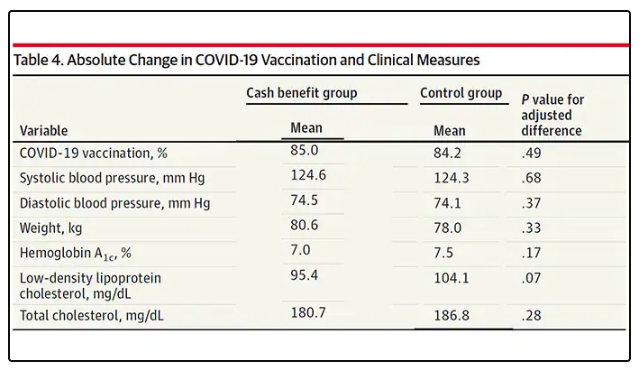
So, it seems that $400 a month doesn’t move the needle too much on risk factors for cardiovascular disease, but the effect on ED visits on their own is fairly impressive.
Is it worth it? The authors did their best to calculate the net effect of this program, accounting for the reduced ED visits and hospitalizations (that’s a big one), but also for the increased number of specialty visits. All told, the program saves about $450 per person in healthcare costs over 9 months. That’s about one seventh of the cost of the overall program.
But remember that they only looked at outcomes for the individual who got the gift cards; it’s likely that there were benefits to their family members as well. And, of course, programs like this can recoup costs indirectly though increases in economic activity, a phenomenon known as the multiplier effect.
I’m not here to tell you whether this program was a good idea; people tend to have quite strong feelings about this sort of thing. But I can tell you what it tells me about healthcare in America. It may not be surprising, but it confirms that access is far from fairly distributed.
I started this story asking about the arrow of causality between poverty and poor health. The truth is, you probably have causality in both directions. 
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It doesn’t really matter what disease you are looking at — cancer, heart disease, dementia, drug abuse, psychiatric disorders. In every case, poverty is associated with worse disease.

But the word “associated” is doing a lot of work there. Many of us feel that poverty itself is causally linked to worse disease outcomes through things like poor access to care and poor access to medicines.
And there is an argument that the arrow goes the other way; perhaps people with worse illness are more likely to be poor because, in this country at least, being sick is incredibly expensive.
Causality is what all medical research is fundamentally about. We want to know if A causes B, because if A causes B, then changing A changes B. If poverty causes bad health outcomes, then alleviating poverty should alleviate bad health outcomes.
But that’s a hard proposition to test. You can’t exactly randomize some people to get extra money and some not to, right? Actually, you can. And in Massachusetts, they did.
What happened in Chelsea, Massachusetts, wasn’t exactly a randomized trial of cash supplementation to avoid bad health outcomes. It was actually a government program instituted during the pandemic. Chelsea has a large immigrant population, many of whom are living in poverty. From April to August 2020, the city ran a food distribution program to aid those in need. But the decision was then made to convert the money spent on that program to cash distributions — free of obligations. Chelsea residents making less than 30% of the median income for the Boston metro area — around $30,000 per family — were invited to enter a lottery. Only one member of any given family could enter. If selected, an individual would receive $200 a month, or $300 for a family of two, or $400 for a family of three or more. These payments went on for about 9 months.
The key thing here is that not everyone won the lottery. The lottery picked winners randomly; 1746 individuals were selected to receive the benefits in the form of a reloadable gift card, and 1134 applied but did not receive any assistance.
This is a perfect natural experiment. As you can see here — and as expected, given that the lottery winners were chosen randomly — winners and losers were similar in terms of age, sex, race, language, income, and more.
Researchers, led by Sumit Agarwal at the Brigham, leveraged that randomization to ask how these cash benefits would affect healthcare utilization. Their results appeared this week in JAMA.
I know what you’re thinking: Is $400 a month really enough to make a difference? Does $400 a month, less than $5000 a year, really fix poverty? We’ll get to that. But I will point out that the average family income of individuals in this study was about $1400 a month. An extra $400 might not change someone’s life, but it may really make a difference.
The primary outcome of this study was ED visits. There are a few ways this could go. Perhaps the money would lead to improved health and thus fewer ED visits. Or perhaps it would help people get transportation to primary care or other services that would offload the ED. Or maybe it would make things worse. Some folks have suggested that cash payments could increase the use of drugs and alcohol, and lead to more ED visits associated with the complications of using those substances.
Here are the actual data. Per 1000 individuals, there were 217 ED visits in the cash-benefit group, 318 in the no-benefit group. That was a statistically significant finding.
Breaking those ED visits down, you can see that fewer visits resulted in hospital admission, with fewer behavioral health–related visits and — a key finding — fewer visits for substance use disorder. This puts the lie to the idea that cash benefits increase drug use.
But the authors also looked at other causes of healthcare utilization. Outpatient visits were slightly higher in the cash-benefit group, driven largely by an increase in specialty care visits. The authors note that this is likely due to the fact that reaching a specialist often requires more travel, which can be costly. Indeed, this effect was most pronounced among the people living furthest from a specialty center.
Outside of utilization, the researchers examined a variety of individual health markers — things like blood pressure — to see if the cash benefit had any effect. A bit of caution here because these data were available only among those who interacted with the healthcare system, which may bias the results a bit. Regardless, no major differences were seen in blood pressure, weight, hemoglobin A1c, cholesterol, or COVID vaccination.
So, it seems that $400 a month doesn’t move the needle too much on risk factors for cardiovascular disease, but the effect on ED visits on their own is fairly impressive.
Is it worth it? The authors did their best to calculate the net effect of this program, accounting for the reduced ED visits and hospitalizations (that’s a big one), but also for the increased number of specialty visits. All told, the program saves about $450 per person in healthcare costs over 9 months. That’s about one seventh of the cost of the overall program.
But remember that they only looked at outcomes for the individual who got the gift cards; it’s likely that there were benefits to their family members as well. And, of course, programs like this can recoup costs indirectly though increases in economic activity, a phenomenon known as the multiplier effect.
I’m not here to tell you whether this program was a good idea; people tend to have quite strong feelings about this sort of thing. But I can tell you what it tells me about healthcare in America. It may not be surprising, but it confirms that access is far from fairly distributed.
I started this story asking about the arrow of causality between poverty and poor health. The truth is, you probably have causality in both directions. 
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It doesn’t really matter what disease you are looking at — cancer, heart disease, dementia, drug abuse, psychiatric disorders. In every case, poverty is associated with worse disease.

But the word “associated” is doing a lot of work there. Many of us feel that poverty itself is causally linked to worse disease outcomes through things like poor access to care and poor access to medicines.
And there is an argument that the arrow goes the other way; perhaps people with worse illness are more likely to be poor because, in this country at least, being sick is incredibly expensive.
Causality is what all medical research is fundamentally about. We want to know if A causes B, because if A causes B, then changing A changes B. If poverty causes bad health outcomes, then alleviating poverty should alleviate bad health outcomes.
But that’s a hard proposition to test. You can’t exactly randomize some people to get extra money and some not to, right? Actually, you can. And in Massachusetts, they did.
What happened in Chelsea, Massachusetts, wasn’t exactly a randomized trial of cash supplementation to avoid bad health outcomes. It was actually a government program instituted during the pandemic. Chelsea has a large immigrant population, many of whom are living in poverty. From April to August 2020, the city ran a food distribution program to aid those in need. But the decision was then made to convert the money spent on that program to cash distributions — free of obligations. Chelsea residents making less than 30% of the median income for the Boston metro area — around $30,000 per family — were invited to enter a lottery. Only one member of any given family could enter. If selected, an individual would receive $200 a month, or $300 for a family of two, or $400 for a family of three or more. These payments went on for about 9 months.
The key thing here is that not everyone won the lottery. The lottery picked winners randomly; 1746 individuals were selected to receive the benefits in the form of a reloadable gift card, and 1134 applied but did not receive any assistance.
This is a perfect natural experiment. As you can see here — and as expected, given that the lottery winners were chosen randomly — winners and losers were similar in terms of age, sex, race, language, income, and more.
Researchers, led by Sumit Agarwal at the Brigham, leveraged that randomization to ask how these cash benefits would affect healthcare utilization. Their results appeared this week in JAMA.
I know what you’re thinking: Is $400 a month really enough to make a difference? Does $400 a month, less than $5000 a year, really fix poverty? We’ll get to that. But I will point out that the average family income of individuals in this study was about $1400 a month. An extra $400 might not change someone’s life, but it may really make a difference.
The primary outcome of this study was ED visits. There are a few ways this could go. Perhaps the money would lead to improved health and thus fewer ED visits. Or perhaps it would help people get transportation to primary care or other services that would offload the ED. Or maybe it would make things worse. Some folks have suggested that cash payments could increase the use of drugs and alcohol, and lead to more ED visits associated with the complications of using those substances.
Here are the actual data. Per 1000 individuals, there were 217 ED visits in the cash-benefit group, 318 in the no-benefit group. That was a statistically significant finding.
Breaking those ED visits down, you can see that fewer visits resulted in hospital admission, with fewer behavioral health–related visits and — a key finding — fewer visits for substance use disorder. This puts the lie to the idea that cash benefits increase drug use.
But the authors also looked at other causes of healthcare utilization. Outpatient visits were slightly higher in the cash-benefit group, driven largely by an increase in specialty care visits. The authors note that this is likely due to the fact that reaching a specialist often requires more travel, which can be costly. Indeed, this effect was most pronounced among the people living furthest from a specialty center.
Outside of utilization, the researchers examined a variety of individual health markers — things like blood pressure — to see if the cash benefit had any effect. A bit of caution here because these data were available only among those who interacted with the healthcare system, which may bias the results a bit. Regardless, no major differences were seen in blood pressure, weight, hemoglobin A1c, cholesterol, or COVID vaccination.
So, it seems that $400 a month doesn’t move the needle too much on risk factors for cardiovascular disease, but the effect on ED visits on their own is fairly impressive.
Is it worth it? The authors did their best to calculate the net effect of this program, accounting for the reduced ED visits and hospitalizations (that’s a big one), but also for the increased number of specialty visits. All told, the program saves about $450 per person in healthcare costs over 9 months. That’s about one seventh of the cost of the overall program.
But remember that they only looked at outcomes for the individual who got the gift cards; it’s likely that there were benefits to their family members as well. And, of course, programs like this can recoup costs indirectly though increases in economic activity, a phenomenon known as the multiplier effect.
I’m not here to tell you whether this program was a good idea; people tend to have quite strong feelings about this sort of thing. But I can tell you what it tells me about healthcare in America. It may not be surprising, but it confirms that access is far from fairly distributed.
I started this story asking about the arrow of causality between poverty and poor health. The truth is, you probably have causality in both directions. 
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Which GI Side Effects Should GLP-1 Prescribers Worry About?
Several recent studies have sought to expound upon what role, if any, GLP-1 RAs may have in increasing the risk for specific gastrointestinal (GI) adverse events.
Herein is a summary of the most current information on this topic, as well as my best guidance for clinicians on integrating it into the clinical care of their patients.
Aspiration Risks
Albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, and tirzepatide are among the class of medications known as GLP-1 RAs. These medications all work by mimicking the action of hormonal incretins, which are released postprandially. Incretins affect the pancreatic glucose-dependent release of insulin, inhibit release of glucagon, stimulate satiety, and reduce gastric emptying. This last effect has raised concerns that patients taking GLP-1 RAs might be at an elevated risk for endoscopy-related aspiration.
In June 2023, the American Society of Anesthesiologists released recommendations asking providers to consider holding back GLP-1 RAs in patients with scheduled elective procedures.
In August 2023, five national GI societies — the American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition — issued their own joint statement on the issue.
In the absence of sufficient evidence, these groups suggested that healthcare providers “exercise best practices when performing endoscopy on these patients on GLP-1 [RAs].” They called for more data and encouraged key stakeholders to work together to develop the necessary evidence to provide guidance for these patients prior to elective endoscopy. A rapid clinical update issued by the American Gastroenterological Association in 2024 was consistent with these earlier multisociety recommendations.
Two studies presented at 2024’s Digestive Disease Week provided additional reassurance that concerns about aspiration with these medications were perhaps unwarranted.
The first (since published in The American Journal of Gastroenterology ) was a case-control study of 16,295 patients undergoing upper endoscopy, among whom 306 were taking GLP-1 RAs. It showed a higher rate of solid gastric residue among those taking GLP-1 RAs compared with controls (14% vs 4%, respectively). Patients who had prolonged fasting and clear liquids for concurrent colonoscopy had lower residue rates (2% vs 11%, respectively). However, there were no recorded incidents of procedural complications or aspiration.
The second was a retrospective cohort study using TriNetX, a federated cloud-based network pulling millions of data points from multiple US healthcare organizations. It found that the incidence of aspiration pneumonitis and emergent intubation during or immediately after esophagogastroduodenoscopy and colonoscopy among those taking GLP-1 RAs was not increased compared with those not taking these medications.
These were followed in June 2024 by a systematic review and meta-analysis published by Hiramoto and colleagues, which included 15 studies. The researchers showed a 36-minute prolongation for solid-food emptying and no delay in liquid emptying for patients taking GLP-1 RAs vs controls. The authors concluded that the minimal delay in solid-food emptying would be offset by standard preprocedural fasting periods.
There is concern that patients with complicated type 2 diabetes may have a bit more of a risk for aspiration. However, this was not supported by an analysis from Barlowe and colleagues, who used a national claims database to identify 15,119 patients with type 2 diabetes on GLP-1 RAs. They found no increased events of pulmonary complications (ie, aspiration, pneumonia, respiratory failure) within 14 days following esophagogastroduodenoscopy. Additional evidence suggests that the risk for aspiration in these patients seems to be offset by prolonged fasting and intake of clear liquids.
Although physicians clearly need to use clinical judgment when performing endoscopic procedures on these patients, the emerging evidence on safety has been encouraging.
Association With GI Adverse Events
A recent retrospective analysis of real-world data from 10,328 new users of GLP-1 RAs with diabetes/obesity reported that the most common GI adverse events in this cohort were abdominal pain (57.6%), constipation (30.4%), diarrhea (32.7%), nausea and vomiting (23.4%), GI bleeding (15.9%), gastroparesis (5.1%), and pancreatitis (3.4%).
Notably, dulaglutide and liraglutide had higher rates of abdominal pain, constipation, diarrhea, and nausea and vomiting than did semaglutide and exenatide. Compared with semaglutide, dulaglutide and liraglutide had slightly higher odds of abdominal pain, gastroparesis, and nausea and vomiting. There were no significant differences between the GLP-1 RAs in the risk for GI bleeding or pancreatitis.
A 2023 report in JAMA observed that the risk for bowel obstruction is also elevated among patients using these agents for weight loss. Possible reasons for this are currently unknown.
Studies are needed to analyze possible variations in safety profiles between GLP-1 RAs to better guide selection of these drugs, particularly in patients with GI risk factors. Furthermore, the causal relationship between GLP-1 RAs with other concomitant medications requires further investigation.
Although relatively infrequent, the risk for GI adverse events should be given special consideration by providers when prescribing them for weight loss, because the risk/benefit ratios may be different from those in patients with diabetes.
A Lack of Hepatic Concerns
GLP-1 RAs have demonstrated a significant impact on body weight and glycemic control, as well as beneficial effects on clinical, biochemical, and histologic markers in patients with metabolic dysfunction–associated steatotic liver disease (MASLD). These favorable changes are evident by reductions in the hepatic cytolysis markers (ie, aspartate aminotransferase and alanine aminotransferase).
GLP-1 RAs may provide a protective function by reducing the accumulation of hepatic triglycerides and expression of several collagen genes. Some preclinical data suggest a risk reduction for progression to hepatocellular carcinoma, and animal studies indicate that complete suppression of hepatic carcinogenesis is achieved with liraglutide.
The most recent assessment of risk reduction for MASLD progression comes from a Scandinavian cohort analysis of national registries. In looking at 91,479 patients using GLP-1 RAs, investigators demonstrated this treatment was associated with a significant reduction in the composite primary endpoint of hepatocellular carcinoma, as well as both compensated and decompensated cirrhosis.
Given the various favorable hepatic effects of GLP-1 RAs, it is likely that the composite benefit on MASLD is multifactorial. The current literature is clear that it is safe to use these agents across the spectrum of MASLD with or without fibrosis, although it must be noted that GLP-1 RAs are not approved by the Food and Drug Administration for this indication.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Several recent studies have sought to expound upon what role, if any, GLP-1 RAs may have in increasing the risk for specific gastrointestinal (GI) adverse events.
Herein is a summary of the most current information on this topic, as well as my best guidance for clinicians on integrating it into the clinical care of their patients.
Aspiration Risks
Albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, and tirzepatide are among the class of medications known as GLP-1 RAs. These medications all work by mimicking the action of hormonal incretins, which are released postprandially. Incretins affect the pancreatic glucose-dependent release of insulin, inhibit release of glucagon, stimulate satiety, and reduce gastric emptying. This last effect has raised concerns that patients taking GLP-1 RAs might be at an elevated risk for endoscopy-related aspiration.
In June 2023, the American Society of Anesthesiologists released recommendations asking providers to consider holding back GLP-1 RAs in patients with scheduled elective procedures.
In August 2023, five national GI societies — the American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition — issued their own joint statement on the issue.
In the absence of sufficient evidence, these groups suggested that healthcare providers “exercise best practices when performing endoscopy on these patients on GLP-1 [RAs].” They called for more data and encouraged key stakeholders to work together to develop the necessary evidence to provide guidance for these patients prior to elective endoscopy. A rapid clinical update issued by the American Gastroenterological Association in 2024 was consistent with these earlier multisociety recommendations.
Two studies presented at 2024’s Digestive Disease Week provided additional reassurance that concerns about aspiration with these medications were perhaps unwarranted.
The first (since published in The American Journal of Gastroenterology ) was a case-control study of 16,295 patients undergoing upper endoscopy, among whom 306 were taking GLP-1 RAs. It showed a higher rate of solid gastric residue among those taking GLP-1 RAs compared with controls (14% vs 4%, respectively). Patients who had prolonged fasting and clear liquids for concurrent colonoscopy had lower residue rates (2% vs 11%, respectively). However, there were no recorded incidents of procedural complications or aspiration.
The second was a retrospective cohort study using TriNetX, a federated cloud-based network pulling millions of data points from multiple US healthcare organizations. It found that the incidence of aspiration pneumonitis and emergent intubation during or immediately after esophagogastroduodenoscopy and colonoscopy among those taking GLP-1 RAs was not increased compared with those not taking these medications.
These were followed in June 2024 by a systematic review and meta-analysis published by Hiramoto and colleagues, which included 15 studies. The researchers showed a 36-minute prolongation for solid-food emptying and no delay in liquid emptying for patients taking GLP-1 RAs vs controls. The authors concluded that the minimal delay in solid-food emptying would be offset by standard preprocedural fasting periods.
There is concern that patients with complicated type 2 diabetes may have a bit more of a risk for aspiration. However, this was not supported by an analysis from Barlowe and colleagues, who used a national claims database to identify 15,119 patients with type 2 diabetes on GLP-1 RAs. They found no increased events of pulmonary complications (ie, aspiration, pneumonia, respiratory failure) within 14 days following esophagogastroduodenoscopy. Additional evidence suggests that the risk for aspiration in these patients seems to be offset by prolonged fasting and intake of clear liquids.
Although physicians clearly need to use clinical judgment when performing endoscopic procedures on these patients, the emerging evidence on safety has been encouraging.
Association With GI Adverse Events
A recent retrospective analysis of real-world data from 10,328 new users of GLP-1 RAs with diabetes/obesity reported that the most common GI adverse events in this cohort were abdominal pain (57.6%), constipation (30.4%), diarrhea (32.7%), nausea and vomiting (23.4%), GI bleeding (15.9%), gastroparesis (5.1%), and pancreatitis (3.4%).
Notably, dulaglutide and liraglutide had higher rates of abdominal pain, constipation, diarrhea, and nausea and vomiting than did semaglutide and exenatide. Compared with semaglutide, dulaglutide and liraglutide had slightly higher odds of abdominal pain, gastroparesis, and nausea and vomiting. There were no significant differences between the GLP-1 RAs in the risk for GI bleeding or pancreatitis.
A 2023 report in JAMA observed that the risk for bowel obstruction is also elevated among patients using these agents for weight loss. Possible reasons for this are currently unknown.
Studies are needed to analyze possible variations in safety profiles between GLP-1 RAs to better guide selection of these drugs, particularly in patients with GI risk factors. Furthermore, the causal relationship between GLP-1 RAs with other concomitant medications requires further investigation.
Although relatively infrequent, the risk for GI adverse events should be given special consideration by providers when prescribing them for weight loss, because the risk/benefit ratios may be different from those in patients with diabetes.
A Lack of Hepatic Concerns
GLP-1 RAs have demonstrated a significant impact on body weight and glycemic control, as well as beneficial effects on clinical, biochemical, and histologic markers in patients with metabolic dysfunction–associated steatotic liver disease (MASLD). These favorable changes are evident by reductions in the hepatic cytolysis markers (ie, aspartate aminotransferase and alanine aminotransferase).
GLP-1 RAs may provide a protective function by reducing the accumulation of hepatic triglycerides and expression of several collagen genes. Some preclinical data suggest a risk reduction for progression to hepatocellular carcinoma, and animal studies indicate that complete suppression of hepatic carcinogenesis is achieved with liraglutide.
The most recent assessment of risk reduction for MASLD progression comes from a Scandinavian cohort analysis of national registries. In looking at 91,479 patients using GLP-1 RAs, investigators demonstrated this treatment was associated with a significant reduction in the composite primary endpoint of hepatocellular carcinoma, as well as both compensated and decompensated cirrhosis.
Given the various favorable hepatic effects of GLP-1 RAs, it is likely that the composite benefit on MASLD is multifactorial. The current literature is clear that it is safe to use these agents across the spectrum of MASLD with or without fibrosis, although it must be noted that GLP-1 RAs are not approved by the Food and Drug Administration for this indication.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Several recent studies have sought to expound upon what role, if any, GLP-1 RAs may have in increasing the risk for specific gastrointestinal (GI) adverse events.
Herein is a summary of the most current information on this topic, as well as my best guidance for clinicians on integrating it into the clinical care of their patients.
Aspiration Risks
Albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, and tirzepatide are among the class of medications known as GLP-1 RAs. These medications all work by mimicking the action of hormonal incretins, which are released postprandially. Incretins affect the pancreatic glucose-dependent release of insulin, inhibit release of glucagon, stimulate satiety, and reduce gastric emptying. This last effect has raised concerns that patients taking GLP-1 RAs might be at an elevated risk for endoscopy-related aspiration.
In June 2023, the American Society of Anesthesiologists released recommendations asking providers to consider holding back GLP-1 RAs in patients with scheduled elective procedures.
In August 2023, five national GI societies — the American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition — issued their own joint statement on the issue.
In the absence of sufficient evidence, these groups suggested that healthcare providers “exercise best practices when performing endoscopy on these patients on GLP-1 [RAs].” They called for more data and encouraged key stakeholders to work together to develop the necessary evidence to provide guidance for these patients prior to elective endoscopy. A rapid clinical update issued by the American Gastroenterological Association in 2024 was consistent with these earlier multisociety recommendations.
Two studies presented at 2024’s Digestive Disease Week provided additional reassurance that concerns about aspiration with these medications were perhaps unwarranted.
The first (since published in The American Journal of Gastroenterology ) was a case-control study of 16,295 patients undergoing upper endoscopy, among whom 306 were taking GLP-1 RAs. It showed a higher rate of solid gastric residue among those taking GLP-1 RAs compared with controls (14% vs 4%, respectively). Patients who had prolonged fasting and clear liquids for concurrent colonoscopy had lower residue rates (2% vs 11%, respectively). However, there were no recorded incidents of procedural complications or aspiration.
The second was a retrospective cohort study using TriNetX, a federated cloud-based network pulling millions of data points from multiple US healthcare organizations. It found that the incidence of aspiration pneumonitis and emergent intubation during or immediately after esophagogastroduodenoscopy and colonoscopy among those taking GLP-1 RAs was not increased compared with those not taking these medications.
These were followed in June 2024 by a systematic review and meta-analysis published by Hiramoto and colleagues, which included 15 studies. The researchers showed a 36-minute prolongation for solid-food emptying and no delay in liquid emptying for patients taking GLP-1 RAs vs controls. The authors concluded that the minimal delay in solid-food emptying would be offset by standard preprocedural fasting periods.
There is concern that patients with complicated type 2 diabetes may have a bit more of a risk for aspiration. However, this was not supported by an analysis from Barlowe and colleagues, who used a national claims database to identify 15,119 patients with type 2 diabetes on GLP-1 RAs. They found no increased events of pulmonary complications (ie, aspiration, pneumonia, respiratory failure) within 14 days following esophagogastroduodenoscopy. Additional evidence suggests that the risk for aspiration in these patients seems to be offset by prolonged fasting and intake of clear liquids.
Although physicians clearly need to use clinical judgment when performing endoscopic procedures on these patients, the emerging evidence on safety has been encouraging.
Association With GI Adverse Events
A recent retrospective analysis of real-world data from 10,328 new users of GLP-1 RAs with diabetes/obesity reported that the most common GI adverse events in this cohort were abdominal pain (57.6%), constipation (30.4%), diarrhea (32.7%), nausea and vomiting (23.4%), GI bleeding (15.9%), gastroparesis (5.1%), and pancreatitis (3.4%).
Notably, dulaglutide and liraglutide had higher rates of abdominal pain, constipation, diarrhea, and nausea and vomiting than did semaglutide and exenatide. Compared with semaglutide, dulaglutide and liraglutide had slightly higher odds of abdominal pain, gastroparesis, and nausea and vomiting. There were no significant differences between the GLP-1 RAs in the risk for GI bleeding or pancreatitis.
A 2023 report in JAMA observed that the risk for bowel obstruction is also elevated among patients using these agents for weight loss. Possible reasons for this are currently unknown.
Studies are needed to analyze possible variations in safety profiles between GLP-1 RAs to better guide selection of these drugs, particularly in patients with GI risk factors. Furthermore, the causal relationship between GLP-1 RAs with other concomitant medications requires further investigation.
Although relatively infrequent, the risk for GI adverse events should be given special consideration by providers when prescribing them for weight loss, because the risk/benefit ratios may be different from those in patients with diabetes.
A Lack of Hepatic Concerns
GLP-1 RAs have demonstrated a significant impact on body weight and glycemic control, as well as beneficial effects on clinical, biochemical, and histologic markers in patients with metabolic dysfunction–associated steatotic liver disease (MASLD). These favorable changes are evident by reductions in the hepatic cytolysis markers (ie, aspartate aminotransferase and alanine aminotransferase).
GLP-1 RAs may provide a protective function by reducing the accumulation of hepatic triglycerides and expression of several collagen genes. Some preclinical data suggest a risk reduction for progression to hepatocellular carcinoma, and animal studies indicate that complete suppression of hepatic carcinogenesis is achieved with liraglutide.
The most recent assessment of risk reduction for MASLD progression comes from a Scandinavian cohort analysis of national registries. In looking at 91,479 patients using GLP-1 RAs, investigators demonstrated this treatment was associated with a significant reduction in the composite primary endpoint of hepatocellular carcinoma, as well as both compensated and decompensated cirrhosis.
Given the various favorable hepatic effects of GLP-1 RAs, it is likely that the composite benefit on MASLD is multifactorial. The current literature is clear that it is safe to use these agents across the spectrum of MASLD with or without fibrosis, although it must be noted that GLP-1 RAs are not approved by the Food and Drug Administration for this indication.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
For Richer, for Poorer: Low-Carb Diets Work for All Incomes
For 3 years, Ajala Efem’s type 2 diabetes was so poorly controlled that her blood sugar often soared northward of 500 mg/dL despite insulin shots three to five times a day. She would experience dizziness, vomiting, severe headaches, and the neuropathy in her feet made walking painful. She was also — literally — frothing at the mouth. The 47-year-old single mother of two adult children with mental disabilities feared that she would die.
Ms. Efem lives in the South Bronx, which is among the poorest areas of New York City, where the combined rate of prediabetes and diabetes is close to 30%, the highest rate of any borough in the city.
She had to wait 8 months for an appointment with an endocrinologist, but that visit proved to be life-changing. She lost 28 pounds and got off 15 medications in a single month. She did not join a gym or count calories; she simply changed the food she ate and adopted a low-carb diet.
“I went from being sick to feeling so great,” she told her endocrinologist recently: “My feet aren’t hurting; I’m not in pain; I’m eating as much as I want, and I really enjoy my food so much.”
Ms. Efem’s life-changing visit was with Mariela Glandt, MD, at the offices of Essen Health Care. One month earlier, Dr. Glandt’s company, OwnaHealth, was contracted by Essen to conduct a 100-person pilot program for endocrinology patients. Essen is the largest Medicaid provider in New York City, and “they were desperate for an endocrinologist,” said Dr. Glandt, who trained at Columbia University in New York. So she came — all the way from Madrid, Spain. She commutes monthly, staying for a week each visit.
Dr. Glandt keeps up this punishing schedule because, as she explains, “it’s such a high for me to see these incredible transformations.” Her mostly Black and Hispanic patients are poor and lack resources, yet they lose significant amounts of weight, and their health issues resolve.
“Food is medicine” is an idea very much in vogue. The concept was central to the landmark White House Conference on Hunger, Nutrition, and Health in 2022 and is now the focus of a number of a wide range of government programs. Recently, the Senate held a hearing aimed at further expanding food as medicine programs.
Still, only a single randomized controlled clinical trial has been conducted on this nutritional approach, with unexpectedly disappointing results. In the mid-Atlantic region, 456 food-insecure adults with type 2 diabetes were randomly assigned to usual care or the provision of weekly groceries for their entire families for about 1 year. Provisions for a Mediterranean-style diet included whole grains, fruits and vegetables, lean protein, low-fat dairy products, cereal, brown rice, and bread. In addition, participants received dietary consultations. Yet, those who got free food and coaching did not see improvements in their average blood sugar (the study’s primary outcome), and their low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol levels appeared to have worsened.
“To be honest, I was surprised,” the study’s lead author, Joseph Doyle, PhD, professor at the Sloan School of Management at MIT in Cambridge, Massachusetts, told me. “I was hoping we would show improved outcomes, but the way to make progress is to do well-randomized trials to find out what works.”
I was not surprised by these results because a recent rigorous systematic review and meta-analysis in The BMJ did not show a Mediterranean-style diet to be the most effective for glycemic control. And Ms. Efem was not in fact following a Mediterranean-style diet.
Ms. Efem’s low-carb success story is anecdotal, but Dr. Glandt has an established track record from her 9 years’ experience as the medical director of the eponymous diabetes center she founded in Tel Aviv. A recent audit of 344 patients from the center found that after 6 months of following a very low–carbohydrate diet, 96.3% of those with diabetes saw their A1c fall from a median 7.6% to 6.3%. Weight loss was significant, with a median drop of 6.5 kg (14 pounds) for patients with diabetes and 5.7 kg for those with prediabetes. The diet comprises 5%-10% of calories from carbs, but Dr. Glandt does not use numeric targets with her patients.
Blood pressure, triglycerides, and liver enzymes also improved. And though LDL cholesterol went up by 8%, this result may have been offset by an accompanying 13% rise in HDL cholesterol. Of the 78 patients initially on insulin, 62 were able to stop this medication entirely.
Although these results aren’t from a clinical trial, they’re still highly meaningful because the current dietary standard of care for type 2 diabetes can only slow the progression of the disease, not cause remission. Indeed, the idea that type 2 diabetes could be put into remission was not seriously considered by the American Diabetes Association (ADA) until 2009. By 2019, an ADA report concluded that “[r]educing overall carbohydrate intake for individuals with diabetes has demonstrated the most evidence for improving glycemia.” In other words, the best way to improve the key factor in diabetes is to reduce total carbohydrates. Yet, the ADA still advocates filling one quarter of one’s plate with carbohydrate-based foods, an amount that will prevent remission. Given that the ADA’s vision statement is “a life free of diabetes,” it seems negligent not to tell people with a deadly condition that they can reverse this diagnosis.
A 2023 meta-analysis of 42 controlled clinical trials on 4809 patients showed that a very low–carbohydrate ketogenic diet (keto) was “superior” to alternatives for glycemic control. A more recent review of 11 clinical trials found that this diet was equal but not superior to other nutritional approaches in terms of blood sugar control, but this review also concluded that keto led to greater increases in HDL cholesterol and lower triglycerides.
Dr. Glandt’s patients in the Bronx might not seem like obvious low-carb candidates. The diet is considered expensive and difficult to sustain. My interviews with a half dozen patients revealed some of these difficulties, but even for a woman living in a homeless shelter, the obstacles are not insurmountable.
Jerrilyn, who preferred that I use only her first name, lives in a shelter in Queens. While we strolled through a nearby park, she told me about her desire to lose weight and recover from polycystic ovary syndrome, which terrified her because it had caused dramatic hair loss. When she landed in Dr. Glandt’s office at age 28, she weighed 180 pounds.
Less than 5 months later, Jerrilyn had lost 25 pounds, and her period had returned with some regularity. She said she used “food stamps,” known as the Supplemental Nutrition Assistance Program (SNAP), to buy most of her food at local delis because the meals served at the shelter were too heavy in starches. She starts her day with eggs, turkey bacon, and avocado.
“It was hard to give up carbohydrates because in my culture [Latina], we have nothing but carbs: rice, potatoes, yuca,” Jerrilyn shared. She noticed that carbs make her hungrier, but after 3 days of going low-carb, her cravings diminished. “It was like getting over an addiction,” she said.
Jerrilyn told me she’d seen many doctors but none as involved as Dr. Glandt. “It feels awesome to know that I have a lot of really useful information coming from her all the time.” The OwnaHealth app tracks weight, blood pressure, blood sugar, ketones, meals, mood, and cravings. Patients wear continuous glucose monitors and enter other information manually. Ketone bodies are used to measure dietary adherence and are obtained through finger pricks and test strips provided by OwnaHealth. Dr. Glandt gives patients her own food plan, along with free visual guides to low-carbohydrate foods by dietdoctor.com.
Dr. Glandt also sends her patients for regular blood work. She says she does not frequently see a rise in LDL cholesterol, which can sometimes occur on a low-carbohydrate diet. This effect is most common among people who are lean and fit. She says she doesn’t discontinue statins unless cholesterol levels improve significantly.
Samuel Gonzalez, age 56, weighed 275 pounds when he walked into Dr. Glandt’s office this past November. His A1c was 9.2%, but none of his previous doctors had diagnosed him with diabetes. “I was like a walking bag of sugar!” he joked.
A low-carbohydrate diet seemed absurd to a Puerto Rican like himself: “Having coffee without sugar? That’s like sacrilegious in my culture!” exclaimed Mr. Gonzalez. Still, he managed, with SNAP, to cook eggs and bacon for breakfast and some kind of protein for dinner. He keeps lunch light, “like tuna fish,” and finds checking in with the OwnaHealth app to be very helpful. “Every day, I’m on it,” he said. In the past 7 months, he’s lost 50 pounds, normalized his cholesterol and blood pressure levels, and lowered his A1c to 5.5%.
Mr. Gonzalez gets disability payments due to a back injury, and Ms. Efem receives government payments because her husband died serving in the military. Ms. Efem says her new diet challenges her budget, but Mr. Gonzalez says he manages easily.
Mélissa Cruz, a 28-year-old studying to be a nail technician while also doing back office work at a physical therapy practice, says she’s stretched thin. “I end up sad because I can’t put energy into looking up recipes and cooking for me and my boyfriend,” she told me. She’ll often cook rice and plantains for him and meat for herself, but “it’s frustrating when I’m low on funds and can’t figure out what to eat.”
Low-carbohydrate diets have a reputation for being expensive because people often start eating pricier foods, like meat and cheese, to replace cheaper starchy foods such as pasta and rice.
A 2019 cost analysis published in Nutrition & Dietetics compared a low-carbohydrate dietary pattern with the New Zealand government’s recommended guidelines (which are almost identical to those in the United States) and found that it cost only an extra $1.27 in US dollars per person per day. One explanation is that protein and fat are more satiating than carbohydrates, so people who mostly consume these macronutrients often cut back on snacks like packaged chips, crackers, and even fruits. Also, those on a ketogenic diet usually cut down on medications, so the additional $1.27 daily is likely offset by reduced spending at the pharmacy.
It’s not just Bronx residents with low socioeconomic status (SES) who adapt well to low-carbohydrate diets. Among Alabama state employees with diabetes enrolled in a low-carbohydrate dietary program provided by a company called Virta, the low SES population had the best outcomes. Virta also published survey data in 2023 showing that participants in a program with the Veteran’s Administration did not find additional costs to be an obstacle to dietary adherence. In fact, some participants saw cost reductions due to decreased spending on processed snacks and fast foods.
Ms. Cruz told me she struggles financially, yet she’s still lost nearly 30 pounds in 5 months, and her A1c went from 7.1% down to 5.9%, putting her diabetes into remission. Equally motivating for her are the improvements she’s seen in other hormonal issues. Since childhood, she’s had acanthosis, a condition that causes the skin to darken in velvety patches, and more recently, she developed severe hirsutism to the point of growing sideburns. “I had tried going vegan and fasting, but these just weren’t sustainable for me, and I was so overwhelmed with counting calories all the time.” Now, on a low-carbohydrate diet, which doesn’t require calorie counting, she’s finally seeing both these conditions improve significantly.
When I last checked in with Ms. Cruz, she said she had “kind of ghosted” Dr. Glandt due to her work and school constraints, but she hadn’t abandoned the diet. She appreciated, too, that Dr. Glandt had not given up on her and kept calling and messaging. “She’s not at all like a typical doctor who would just tell me to lose weight and shake their head at me,” Ms. Cruz said.
Because Dr. Glandt’s approach is time-intensive and high-touch, it might seem impractical to scale up, but Dr. Glandt’s app uses artificial intelligence to help with communications thus allowing her, with help from part-time health coaches, to care for patients.
This early success in one of the United States’ poorest and sickest neighborhoods should give us hope that type 2 diabetes need not to be a progressive irreversible disease, even among the disadvantaged.
OwnaHealth’s track record, along with that of Virta and other similar low-carbohydrate medical practices also give hope to the food-is-medicine idea. Diabetes can go into remission, and people can be healed, provided that health practitioners prescribe the right foods. And in truth, it’s not a diet. It’s a way of eating that must be maintained. The sustainability of low-carbohydrate diets has been a point of contention, but the Virta trial, with 38% of patients sustaining remission at 2 years, showed that it’s possible. (OwnaHealth, for its part, offers long-term maintenance plans to help patients stay very low-carb permanently.)
Given the tremendous costs and health burden of diabetes, this approach should no doubt be the first line of treatment for doctors and the ADA. The past two decades of clinical trial research have demonstrated that remission of type 2 diabetes is possible through diet alone. It turns out that for metabolic diseases, only certain foods are truly medicine.
Tools and Tips for Clinicians:
- Free two-page keto starter’s guide by OwnaHealth; Dr. Glandt uses this guide with her patients.
- Illustrated low-carb guides by dietdoctor.com
- Free low-carbohydrate starter guide by the Michigan Collaborative for Type 2 Diabetes
- Low-Carb for Any Budget, a free digital booklet by Mark Cucuzzella, MD, and Kristie Sullivan, PhD
- Recipe and meal ideas from Ruled.me, Keto-Mojo.com, and
Dr. Teicholz is the founder of Nutrition Coalition, an independent nonprofit dedicated to ensuring that US dietary guidelines align with current science. She disclosed receiving book royalties from The Big Fat Surprise, and received honorarium not exceeding $2000 for speeches from various sources.
A version of this article appeared on Medscape.com.
For 3 years, Ajala Efem’s type 2 diabetes was so poorly controlled that her blood sugar often soared northward of 500 mg/dL despite insulin shots three to five times a day. She would experience dizziness, vomiting, severe headaches, and the neuropathy in her feet made walking painful. She was also — literally — frothing at the mouth. The 47-year-old single mother of two adult children with mental disabilities feared that she would die.
Ms. Efem lives in the South Bronx, which is among the poorest areas of New York City, where the combined rate of prediabetes and diabetes is close to 30%, the highest rate of any borough in the city.
She had to wait 8 months for an appointment with an endocrinologist, but that visit proved to be life-changing. She lost 28 pounds and got off 15 medications in a single month. She did not join a gym or count calories; she simply changed the food she ate and adopted a low-carb diet.
“I went from being sick to feeling so great,” she told her endocrinologist recently: “My feet aren’t hurting; I’m not in pain; I’m eating as much as I want, and I really enjoy my food so much.”
Ms. Efem’s life-changing visit was with Mariela Glandt, MD, at the offices of Essen Health Care. One month earlier, Dr. Glandt’s company, OwnaHealth, was contracted by Essen to conduct a 100-person pilot program for endocrinology patients. Essen is the largest Medicaid provider in New York City, and “they were desperate for an endocrinologist,” said Dr. Glandt, who trained at Columbia University in New York. So she came — all the way from Madrid, Spain. She commutes monthly, staying for a week each visit.
Dr. Glandt keeps up this punishing schedule because, as she explains, “it’s such a high for me to see these incredible transformations.” Her mostly Black and Hispanic patients are poor and lack resources, yet they lose significant amounts of weight, and their health issues resolve.
“Food is medicine” is an idea very much in vogue. The concept was central to the landmark White House Conference on Hunger, Nutrition, and Health in 2022 and is now the focus of a number of a wide range of government programs. Recently, the Senate held a hearing aimed at further expanding food as medicine programs.
Still, only a single randomized controlled clinical trial has been conducted on this nutritional approach, with unexpectedly disappointing results. In the mid-Atlantic region, 456 food-insecure adults with type 2 diabetes were randomly assigned to usual care or the provision of weekly groceries for their entire families for about 1 year. Provisions for a Mediterranean-style diet included whole grains, fruits and vegetables, lean protein, low-fat dairy products, cereal, brown rice, and bread. In addition, participants received dietary consultations. Yet, those who got free food and coaching did not see improvements in their average blood sugar (the study’s primary outcome), and their low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol levels appeared to have worsened.
“To be honest, I was surprised,” the study’s lead author, Joseph Doyle, PhD, professor at the Sloan School of Management at MIT in Cambridge, Massachusetts, told me. “I was hoping we would show improved outcomes, but the way to make progress is to do well-randomized trials to find out what works.”
I was not surprised by these results because a recent rigorous systematic review and meta-analysis in The BMJ did not show a Mediterranean-style diet to be the most effective for glycemic control. And Ms. Efem was not in fact following a Mediterranean-style diet.
Ms. Efem’s low-carb success story is anecdotal, but Dr. Glandt has an established track record from her 9 years’ experience as the medical director of the eponymous diabetes center she founded in Tel Aviv. A recent audit of 344 patients from the center found that after 6 months of following a very low–carbohydrate diet, 96.3% of those with diabetes saw their A1c fall from a median 7.6% to 6.3%. Weight loss was significant, with a median drop of 6.5 kg (14 pounds) for patients with diabetes and 5.7 kg for those with prediabetes. The diet comprises 5%-10% of calories from carbs, but Dr. Glandt does not use numeric targets with her patients.
Blood pressure, triglycerides, and liver enzymes also improved. And though LDL cholesterol went up by 8%, this result may have been offset by an accompanying 13% rise in HDL cholesterol. Of the 78 patients initially on insulin, 62 were able to stop this medication entirely.
Although these results aren’t from a clinical trial, they’re still highly meaningful because the current dietary standard of care for type 2 diabetes can only slow the progression of the disease, not cause remission. Indeed, the idea that type 2 diabetes could be put into remission was not seriously considered by the American Diabetes Association (ADA) until 2009. By 2019, an ADA report concluded that “[r]educing overall carbohydrate intake for individuals with diabetes has demonstrated the most evidence for improving glycemia.” In other words, the best way to improve the key factor in diabetes is to reduce total carbohydrates. Yet, the ADA still advocates filling one quarter of one’s plate with carbohydrate-based foods, an amount that will prevent remission. Given that the ADA’s vision statement is “a life free of diabetes,” it seems negligent not to tell people with a deadly condition that they can reverse this diagnosis.
A 2023 meta-analysis of 42 controlled clinical trials on 4809 patients showed that a very low–carbohydrate ketogenic diet (keto) was “superior” to alternatives for glycemic control. A more recent review of 11 clinical trials found that this diet was equal but not superior to other nutritional approaches in terms of blood sugar control, but this review also concluded that keto led to greater increases in HDL cholesterol and lower triglycerides.
Dr. Glandt’s patients in the Bronx might not seem like obvious low-carb candidates. The diet is considered expensive and difficult to sustain. My interviews with a half dozen patients revealed some of these difficulties, but even for a woman living in a homeless shelter, the obstacles are not insurmountable.
Jerrilyn, who preferred that I use only her first name, lives in a shelter in Queens. While we strolled through a nearby park, she told me about her desire to lose weight and recover from polycystic ovary syndrome, which terrified her because it had caused dramatic hair loss. When she landed in Dr. Glandt’s office at age 28, she weighed 180 pounds.
Less than 5 months later, Jerrilyn had lost 25 pounds, and her period had returned with some regularity. She said she used “food stamps,” known as the Supplemental Nutrition Assistance Program (SNAP), to buy most of her food at local delis because the meals served at the shelter were too heavy in starches. She starts her day with eggs, turkey bacon, and avocado.
“It was hard to give up carbohydrates because in my culture [Latina], we have nothing but carbs: rice, potatoes, yuca,” Jerrilyn shared. She noticed that carbs make her hungrier, but after 3 days of going low-carb, her cravings diminished. “It was like getting over an addiction,” she said.
Jerrilyn told me she’d seen many doctors but none as involved as Dr. Glandt. “It feels awesome to know that I have a lot of really useful information coming from her all the time.” The OwnaHealth app tracks weight, blood pressure, blood sugar, ketones, meals, mood, and cravings. Patients wear continuous glucose monitors and enter other information manually. Ketone bodies are used to measure dietary adherence and are obtained through finger pricks and test strips provided by OwnaHealth. Dr. Glandt gives patients her own food plan, along with free visual guides to low-carbohydrate foods by dietdoctor.com.
Dr. Glandt also sends her patients for regular blood work. She says she does not frequently see a rise in LDL cholesterol, which can sometimes occur on a low-carbohydrate diet. This effect is most common among people who are lean and fit. She says she doesn’t discontinue statins unless cholesterol levels improve significantly.
Samuel Gonzalez, age 56, weighed 275 pounds when he walked into Dr. Glandt’s office this past November. His A1c was 9.2%, but none of his previous doctors had diagnosed him with diabetes. “I was like a walking bag of sugar!” he joked.
A low-carbohydrate diet seemed absurd to a Puerto Rican like himself: “Having coffee without sugar? That’s like sacrilegious in my culture!” exclaimed Mr. Gonzalez. Still, he managed, with SNAP, to cook eggs and bacon for breakfast and some kind of protein for dinner. He keeps lunch light, “like tuna fish,” and finds checking in with the OwnaHealth app to be very helpful. “Every day, I’m on it,” he said. In the past 7 months, he’s lost 50 pounds, normalized his cholesterol and blood pressure levels, and lowered his A1c to 5.5%.
Mr. Gonzalez gets disability payments due to a back injury, and Ms. Efem receives government payments because her husband died serving in the military. Ms. Efem says her new diet challenges her budget, but Mr. Gonzalez says he manages easily.
Mélissa Cruz, a 28-year-old studying to be a nail technician while also doing back office work at a physical therapy practice, says she’s stretched thin. “I end up sad because I can’t put energy into looking up recipes and cooking for me and my boyfriend,” she told me. She’ll often cook rice and plantains for him and meat for herself, but “it’s frustrating when I’m low on funds and can’t figure out what to eat.”
Low-carbohydrate diets have a reputation for being expensive because people often start eating pricier foods, like meat and cheese, to replace cheaper starchy foods such as pasta and rice.
A 2019 cost analysis published in Nutrition & Dietetics compared a low-carbohydrate dietary pattern with the New Zealand government’s recommended guidelines (which are almost identical to those in the United States) and found that it cost only an extra $1.27 in US dollars per person per day. One explanation is that protein and fat are more satiating than carbohydrates, so people who mostly consume these macronutrients often cut back on snacks like packaged chips, crackers, and even fruits. Also, those on a ketogenic diet usually cut down on medications, so the additional $1.27 daily is likely offset by reduced spending at the pharmacy.
It’s not just Bronx residents with low socioeconomic status (SES) who adapt well to low-carbohydrate diets. Among Alabama state employees with diabetes enrolled in a low-carbohydrate dietary program provided by a company called Virta, the low SES population had the best outcomes. Virta also published survey data in 2023 showing that participants in a program with the Veteran’s Administration did not find additional costs to be an obstacle to dietary adherence. In fact, some participants saw cost reductions due to decreased spending on processed snacks and fast foods.
Ms. Cruz told me she struggles financially, yet she’s still lost nearly 30 pounds in 5 months, and her A1c went from 7.1% down to 5.9%, putting her diabetes into remission. Equally motivating for her are the improvements she’s seen in other hormonal issues. Since childhood, she’s had acanthosis, a condition that causes the skin to darken in velvety patches, and more recently, she developed severe hirsutism to the point of growing sideburns. “I had tried going vegan and fasting, but these just weren’t sustainable for me, and I was so overwhelmed with counting calories all the time.” Now, on a low-carbohydrate diet, which doesn’t require calorie counting, she’s finally seeing both these conditions improve significantly.
When I last checked in with Ms. Cruz, she said she had “kind of ghosted” Dr. Glandt due to her work and school constraints, but she hadn’t abandoned the diet. She appreciated, too, that Dr. Glandt had not given up on her and kept calling and messaging. “She’s not at all like a typical doctor who would just tell me to lose weight and shake their head at me,” Ms. Cruz said.
Because Dr. Glandt’s approach is time-intensive and high-touch, it might seem impractical to scale up, but Dr. Glandt’s app uses artificial intelligence to help with communications thus allowing her, with help from part-time health coaches, to care for patients.
This early success in one of the United States’ poorest and sickest neighborhoods should give us hope that type 2 diabetes need not to be a progressive irreversible disease, even among the disadvantaged.
OwnaHealth’s track record, along with that of Virta and other similar low-carbohydrate medical practices also give hope to the food-is-medicine idea. Diabetes can go into remission, and people can be healed, provided that health practitioners prescribe the right foods. And in truth, it’s not a diet. It’s a way of eating that must be maintained. The sustainability of low-carbohydrate diets has been a point of contention, but the Virta trial, with 38% of patients sustaining remission at 2 years, showed that it’s possible. (OwnaHealth, for its part, offers long-term maintenance plans to help patients stay very low-carb permanently.)
Given the tremendous costs and health burden of diabetes, this approach should no doubt be the first line of treatment for doctors and the ADA. The past two decades of clinical trial research have demonstrated that remission of type 2 diabetes is possible through diet alone. It turns out that for metabolic diseases, only certain foods are truly medicine.
Tools and Tips for Clinicians:
- Free two-page keto starter’s guide by OwnaHealth; Dr. Glandt uses this guide with her patients.
- Illustrated low-carb guides by dietdoctor.com
- Free low-carbohydrate starter guide by the Michigan Collaborative for Type 2 Diabetes
- Low-Carb for Any Budget, a free digital booklet by Mark Cucuzzella, MD, and Kristie Sullivan, PhD
- Recipe and meal ideas from Ruled.me, Keto-Mojo.com, and
Dr. Teicholz is the founder of Nutrition Coalition, an independent nonprofit dedicated to ensuring that US dietary guidelines align with current science. She disclosed receiving book royalties from The Big Fat Surprise, and received honorarium not exceeding $2000 for speeches from various sources.
A version of this article appeared on Medscape.com.
For 3 years, Ajala Efem’s type 2 diabetes was so poorly controlled that her blood sugar often soared northward of 500 mg/dL despite insulin shots three to five times a day. She would experience dizziness, vomiting, severe headaches, and the neuropathy in her feet made walking painful. She was also — literally — frothing at the mouth. The 47-year-old single mother of two adult children with mental disabilities feared that she would die.
Ms. Efem lives in the South Bronx, which is among the poorest areas of New York City, where the combined rate of prediabetes and diabetes is close to 30%, the highest rate of any borough in the city.
She had to wait 8 months for an appointment with an endocrinologist, but that visit proved to be life-changing. She lost 28 pounds and got off 15 medications in a single month. She did not join a gym or count calories; she simply changed the food she ate and adopted a low-carb diet.
“I went from being sick to feeling so great,” she told her endocrinologist recently: “My feet aren’t hurting; I’m not in pain; I’m eating as much as I want, and I really enjoy my food so much.”
Ms. Efem’s life-changing visit was with Mariela Glandt, MD, at the offices of Essen Health Care. One month earlier, Dr. Glandt’s company, OwnaHealth, was contracted by Essen to conduct a 100-person pilot program for endocrinology patients. Essen is the largest Medicaid provider in New York City, and “they were desperate for an endocrinologist,” said Dr. Glandt, who trained at Columbia University in New York. So she came — all the way from Madrid, Spain. She commutes monthly, staying for a week each visit.
Dr. Glandt keeps up this punishing schedule because, as she explains, “it’s such a high for me to see these incredible transformations.” Her mostly Black and Hispanic patients are poor and lack resources, yet they lose significant amounts of weight, and their health issues resolve.
“Food is medicine” is an idea very much in vogue. The concept was central to the landmark White House Conference on Hunger, Nutrition, and Health in 2022 and is now the focus of a number of a wide range of government programs. Recently, the Senate held a hearing aimed at further expanding food as medicine programs.
Still, only a single randomized controlled clinical trial has been conducted on this nutritional approach, with unexpectedly disappointing results. In the mid-Atlantic region, 456 food-insecure adults with type 2 diabetes were randomly assigned to usual care or the provision of weekly groceries for their entire families for about 1 year. Provisions for a Mediterranean-style diet included whole grains, fruits and vegetables, lean protein, low-fat dairy products, cereal, brown rice, and bread. In addition, participants received dietary consultations. Yet, those who got free food and coaching did not see improvements in their average blood sugar (the study’s primary outcome), and their low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol levels appeared to have worsened.
“To be honest, I was surprised,” the study’s lead author, Joseph Doyle, PhD, professor at the Sloan School of Management at MIT in Cambridge, Massachusetts, told me. “I was hoping we would show improved outcomes, but the way to make progress is to do well-randomized trials to find out what works.”
I was not surprised by these results because a recent rigorous systematic review and meta-analysis in The BMJ did not show a Mediterranean-style diet to be the most effective for glycemic control. And Ms. Efem was not in fact following a Mediterranean-style diet.
Ms. Efem’s low-carb success story is anecdotal, but Dr. Glandt has an established track record from her 9 years’ experience as the medical director of the eponymous diabetes center she founded in Tel Aviv. A recent audit of 344 patients from the center found that after 6 months of following a very low–carbohydrate diet, 96.3% of those with diabetes saw their A1c fall from a median 7.6% to 6.3%. Weight loss was significant, with a median drop of 6.5 kg (14 pounds) for patients with diabetes and 5.7 kg for those with prediabetes. The diet comprises 5%-10% of calories from carbs, but Dr. Glandt does not use numeric targets with her patients.
Blood pressure, triglycerides, and liver enzymes also improved. And though LDL cholesterol went up by 8%, this result may have been offset by an accompanying 13% rise in HDL cholesterol. Of the 78 patients initially on insulin, 62 were able to stop this medication entirely.
Although these results aren’t from a clinical trial, they’re still highly meaningful because the current dietary standard of care for type 2 diabetes can only slow the progression of the disease, not cause remission. Indeed, the idea that type 2 diabetes could be put into remission was not seriously considered by the American Diabetes Association (ADA) until 2009. By 2019, an ADA report concluded that “[r]educing overall carbohydrate intake for individuals with diabetes has demonstrated the most evidence for improving glycemia.” In other words, the best way to improve the key factor in diabetes is to reduce total carbohydrates. Yet, the ADA still advocates filling one quarter of one’s plate with carbohydrate-based foods, an amount that will prevent remission. Given that the ADA’s vision statement is “a life free of diabetes,” it seems negligent not to tell people with a deadly condition that they can reverse this diagnosis.
A 2023 meta-analysis of 42 controlled clinical trials on 4809 patients showed that a very low–carbohydrate ketogenic diet (keto) was “superior” to alternatives for glycemic control. A more recent review of 11 clinical trials found that this diet was equal but not superior to other nutritional approaches in terms of blood sugar control, but this review also concluded that keto led to greater increases in HDL cholesterol and lower triglycerides.
Dr. Glandt’s patients in the Bronx might not seem like obvious low-carb candidates. The diet is considered expensive and difficult to sustain. My interviews with a half dozen patients revealed some of these difficulties, but even for a woman living in a homeless shelter, the obstacles are not insurmountable.
Jerrilyn, who preferred that I use only her first name, lives in a shelter in Queens. While we strolled through a nearby park, she told me about her desire to lose weight and recover from polycystic ovary syndrome, which terrified her because it had caused dramatic hair loss. When she landed in Dr. Glandt’s office at age 28, she weighed 180 pounds.
Less than 5 months later, Jerrilyn had lost 25 pounds, and her period had returned with some regularity. She said she used “food stamps,” known as the Supplemental Nutrition Assistance Program (SNAP), to buy most of her food at local delis because the meals served at the shelter were too heavy in starches. She starts her day with eggs, turkey bacon, and avocado.
“It was hard to give up carbohydrates because in my culture [Latina], we have nothing but carbs: rice, potatoes, yuca,” Jerrilyn shared. She noticed that carbs make her hungrier, but after 3 days of going low-carb, her cravings diminished. “It was like getting over an addiction,” she said.
Jerrilyn told me she’d seen many doctors but none as involved as Dr. Glandt. “It feels awesome to know that I have a lot of really useful information coming from her all the time.” The OwnaHealth app tracks weight, blood pressure, blood sugar, ketones, meals, mood, and cravings. Patients wear continuous glucose monitors and enter other information manually. Ketone bodies are used to measure dietary adherence and are obtained through finger pricks and test strips provided by OwnaHealth. Dr. Glandt gives patients her own food plan, along with free visual guides to low-carbohydrate foods by dietdoctor.com.
Dr. Glandt also sends her patients for regular blood work. She says she does not frequently see a rise in LDL cholesterol, which can sometimes occur on a low-carbohydrate diet. This effect is most common among people who are lean and fit. She says she doesn’t discontinue statins unless cholesterol levels improve significantly.
Samuel Gonzalez, age 56, weighed 275 pounds when he walked into Dr. Glandt’s office this past November. His A1c was 9.2%, but none of his previous doctors had diagnosed him with diabetes. “I was like a walking bag of sugar!” he joked.
A low-carbohydrate diet seemed absurd to a Puerto Rican like himself: “Having coffee without sugar? That’s like sacrilegious in my culture!” exclaimed Mr. Gonzalez. Still, he managed, with SNAP, to cook eggs and bacon for breakfast and some kind of protein for dinner. He keeps lunch light, “like tuna fish,” and finds checking in with the OwnaHealth app to be very helpful. “Every day, I’m on it,” he said. In the past 7 months, he’s lost 50 pounds, normalized his cholesterol and blood pressure levels, and lowered his A1c to 5.5%.
Mr. Gonzalez gets disability payments due to a back injury, and Ms. Efem receives government payments because her husband died serving in the military. Ms. Efem says her new diet challenges her budget, but Mr. Gonzalez says he manages easily.
Mélissa Cruz, a 28-year-old studying to be a nail technician while also doing back office work at a physical therapy practice, says she’s stretched thin. “I end up sad because I can’t put energy into looking up recipes and cooking for me and my boyfriend,” she told me. She’ll often cook rice and plantains for him and meat for herself, but “it’s frustrating when I’m low on funds and can’t figure out what to eat.”
Low-carbohydrate diets have a reputation for being expensive because people often start eating pricier foods, like meat and cheese, to replace cheaper starchy foods such as pasta and rice.
A 2019 cost analysis published in Nutrition & Dietetics compared a low-carbohydrate dietary pattern with the New Zealand government’s recommended guidelines (which are almost identical to those in the United States) and found that it cost only an extra $1.27 in US dollars per person per day. One explanation is that protein and fat are more satiating than carbohydrates, so people who mostly consume these macronutrients often cut back on snacks like packaged chips, crackers, and even fruits. Also, those on a ketogenic diet usually cut down on medications, so the additional $1.27 daily is likely offset by reduced spending at the pharmacy.
It’s not just Bronx residents with low socioeconomic status (SES) who adapt well to low-carbohydrate diets. Among Alabama state employees with diabetes enrolled in a low-carbohydrate dietary program provided by a company called Virta, the low SES population had the best outcomes. Virta also published survey data in 2023 showing that participants in a program with the Veteran’s Administration did not find additional costs to be an obstacle to dietary adherence. In fact, some participants saw cost reductions due to decreased spending on processed snacks and fast foods.
Ms. Cruz told me she struggles financially, yet she’s still lost nearly 30 pounds in 5 months, and her A1c went from 7.1% down to 5.9%, putting her diabetes into remission. Equally motivating for her are the improvements she’s seen in other hormonal issues. Since childhood, she’s had acanthosis, a condition that causes the skin to darken in velvety patches, and more recently, she developed severe hirsutism to the point of growing sideburns. “I had tried going vegan and fasting, but these just weren’t sustainable for me, and I was so overwhelmed with counting calories all the time.” Now, on a low-carbohydrate diet, which doesn’t require calorie counting, she’s finally seeing both these conditions improve significantly.
When I last checked in with Ms. Cruz, she said she had “kind of ghosted” Dr. Glandt due to her work and school constraints, but she hadn’t abandoned the diet. She appreciated, too, that Dr. Glandt had not given up on her and kept calling and messaging. “She’s not at all like a typical doctor who would just tell me to lose weight and shake their head at me,” Ms. Cruz said.
Because Dr. Glandt’s approach is time-intensive and high-touch, it might seem impractical to scale up, but Dr. Glandt’s app uses artificial intelligence to help with communications thus allowing her, with help from part-time health coaches, to care for patients.
This early success in one of the United States’ poorest and sickest neighborhoods should give us hope that type 2 diabetes need not to be a progressive irreversible disease, even among the disadvantaged.
OwnaHealth’s track record, along with that of Virta and other similar low-carbohydrate medical practices also give hope to the food-is-medicine idea. Diabetes can go into remission, and people can be healed, provided that health practitioners prescribe the right foods. And in truth, it’s not a diet. It’s a way of eating that must be maintained. The sustainability of low-carbohydrate diets has been a point of contention, but the Virta trial, with 38% of patients sustaining remission at 2 years, showed that it’s possible. (OwnaHealth, for its part, offers long-term maintenance plans to help patients stay very low-carb permanently.)
Given the tremendous costs and health burden of diabetes, this approach should no doubt be the first line of treatment for doctors and the ADA. The past two decades of clinical trial research have demonstrated that remission of type 2 diabetes is possible through diet alone. It turns out that for metabolic diseases, only certain foods are truly medicine.
Tools and Tips for Clinicians:
- Free two-page keto starter’s guide by OwnaHealth; Dr. Glandt uses this guide with her patients.
- Illustrated low-carb guides by dietdoctor.com
- Free low-carbohydrate starter guide by the Michigan Collaborative for Type 2 Diabetes
- Low-Carb for Any Budget, a free digital booklet by Mark Cucuzzella, MD, and Kristie Sullivan, PhD
- Recipe and meal ideas from Ruled.me, Keto-Mojo.com, and
Dr. Teicholz is the founder of Nutrition Coalition, an independent nonprofit dedicated to ensuring that US dietary guidelines align with current science. She disclosed receiving book royalties from The Big Fat Surprise, and received honorarium not exceeding $2000 for speeches from various sources.
A version of this article appeared on Medscape.com.
Pulsed Field Ablation for AF: Are US Electrophysiologists Too Easily Impressed?
It dominated 2024’s heart rhythm meetings, and it dominates my private electrophysiologist chat groups. My Google alert for “AF ablation” most often includes notices on PFA and the expansion of the atrial fibrillation ablation market.
Yet, the excitement does not match the empirical data.
Despite having strong brains, electrophysiologists adopt new things as if we were emotional shoppers. Our neighbor buys a sports car and we think we need the same car. Left atrial appendage occlusion and subcutaneous defibrillators were past examples.
The most recent example of soft thinking (especially in the United States) is the enthusiasm and early adoption of first-generation PFA systems for the treatment of AF.
Readers of cardiac news (including some of my patients) might think PFA has solved the AF puzzle. It has not.
A true breakthrough in AF would be to find its cause. PFA is simply another way to destroy (ablate) cardiac myocytes. PFA uses electrical energy (think shocks) to create pores in the cell membranes of myocytes. It’s delivered through various types of catheters.
The main theoretical advantage of PFA is cardioselectivity, which is possible because myocytes have lower thresholds for irreversible electroporation than surrounding tissues. The dose of electrical energy that ablates cardiac tissue does not affect surrounding tissues. Cardioselectivity decreases the chance of the most feared complication of standard AF ablation, thermal damage to the esophagus, which is often fatal. The esophagus lies immediately behind the posterior wall of the left atrium and can be inadvertently injured during thermal ablation.
The challenge in assessing this potential advantage is that thermal esophageal damage is, thankfully, exceedingly rare. Its incidence is in the range of 1 in 10,000 AF ablations. But it might be even lower than that in contemporary practice, because knowledge of esophageal injury has led to innovations that probably have reduced its incidence even further.
Proponents of PFA would rightly point to the fact that not having to worry about esophageal injury allows operators to add posterior wall ablation to the normal pulmonary vein isolation lesion set. This ability, they would argue, is likely to improve AF ablation outcomes. The problem is that the strongest and most recent trial of posterior wall isolation (with radiofrequency ablation) did not show better outcomes. A more recent observational analysis also showed no benefit to posterior wall isolation (using PFA) over pulmonary vein isolation alone.
What About PFA Efficacy?
I’ve long spoken and written about the lack of progress in AF ablation. In 1998, the first report on ablation of AF showed a 62% arrhythmia-free rate. Two decades later, in the carefully chosen labs treating patients in the CABANA trial, arrhythmia-free rates after AF ablation remain unchanged. We have improved our speed and ability to isolate pulmonary veins, but this has not increased our success in eliminating AF. The reason, I believe, is that we have made little to no progress in understanding the pathophysiology of AF.
The Food and Drug Administration regulatory trial called ADVENT randomly assigned more than 600 patients to thermal ablation or PFA, and the primary endpoint of ablation success was nearly identical. Single-center studies, observational registries, and single-arm studies have all shown similar efficacy of PFA and thermal ablation.
Proponents of PFA might argue that these early studies used first-generation PFA systems, and iteration will lead to better efficacy. Perhaps, but we’ve had 20 years of iteration of thermal ablation, and its efficacy has not budged.
What About PFA Safety?
In the ADVENT randomized trial, safety results were similar, though the one death, caused by cardiac perforation and tamponade, occurred in the PFA arm. In the MANIFEST-17K multinational survey of PFA ablation, safety events were in the range reported with thermal ablation. PFA still involves placing catheters in the heart, and complications such as tamponade, stroke, and vascular damage occur.
The large MANIFEST-17K survey also exposed two PFA-specific complications: coronary artery spasm, which can occur when PFA is delivered close to coronary arteries; and hemolysis-related kidney failure — severe enough to require dialysis in five patients. Supporters of PFA speculate that hemolysis occurs because electrical energy within the atrium can shred red blood cells. Their solution is to strive for good contact and use hydration. The irony of this latter fix is that one of the best advances in thermal ablation has been catheters that deliver less fluid and less need for diuresis after the procedure.
No PFA study has shown a decreased incidence of thermal damage to the esophagus with PFA ablation. Of course, this is because it is such a low-incidence event.
One of my concerns with PFA is brain safety. PFA creates substantial microbubbles in the left atrium, which can then travel north to the brain. In a small series from ADVENT, three patients had brain lesions after PFA vs none with thermal ablation. PFA proponents wrote that brain safety was important to study, but few patients have been systematically studied with brain MRI scans. Asymptomatic brain lesions have been noted after many arterial procedures. The clinical significance of these is not known. As a new technology, and one that creates substantial microbubbles in the left atrium, I agree with the PFA proponents that brain safety should be thoroughly studied — before widespread adoption.
What About Speed and Cost?
Observational studies from European labs report fast procedure times. I have seen PFA procedures in Europe; they’re fast — typically under an hour. A standard thermal ablation takes me about 60-70 minutes.
I am not sure that US operators can duplicate European procedural times. In the ADVENT regulatory trial, the mean procedure time was 105 minutes and that was in experienced US centers. While this still represents early experience with PFA, the culture of US AF ablation entails far more mapping and extra catheters than I have seen used in European labs.
Cost is a major issue. It’s hard to sort out exact costs in the United States, but a PFA catheter costs approximately threefold more than a standard ablation catheter. A recent study from Liverpool, England, found that PFA ablation was faster but more expensive than standard thermal ablation because of higher PFA equipment prices. For better or worse, US patients are not directly affected by the higher procedural costs. But the fact remains that PFA adds more costs to the healthcare system.
What Drives the Enthusiasm for First-Generation PFA?
So why all the enthusiasm? It’s surely not the empirical data. Evidence thus far shows no obvious advantage in safety or efficacy. European use of PFA does seem to reduce procedure time. But in many electrophysiology labs in the United States, the rate-limiting step for AF ablation is not time in the lab but having enough staff to turn rooms around.
The main factor driving early acceptance of PFA relates to basic human nature. It is the fear of missing out. Marketing works on consumers, and it surely works on doctors. Companies that make PFA systems sponsor key opinion leaders to discuss PFA. These companies have beautiful booths in the expo of our meetings; they host dinners and talks. When a hospital in a city does PFA, the other hospitals feel the urge to keep up. It’s hard to be a Top Person in electrophysiology and not be a PFA user.
One of my favorite comments came from a key opinion leader. He told me that he advised his administration to buy a PFA system, promote that they have it, and keep it in the closet until better systems are released.
Iteration in the medical device field is tricky. There are negatives to being too harsh on first-generation systems. Early cardiac resynchronization tools, for instance, were horrible. Now CRT is transformative in selected patients with heart failure.
It’s possible (but not certain) that electrical ablative therapy will iterate and surpass thermal ablation in the future. Maybe.
But for now, the enthusiasm for PFA far outstrips its evidence. Until better evidence emerges, I will be a slow adopter. And I hope that our field gathers evidence before widespread adoption makes it impossible to do proper studies.
Dr. Mandrola, clinical electrophysiologist, Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
It dominated 2024’s heart rhythm meetings, and it dominates my private electrophysiologist chat groups. My Google alert for “AF ablation” most often includes notices on PFA and the expansion of the atrial fibrillation ablation market.
Yet, the excitement does not match the empirical data.
Despite having strong brains, electrophysiologists adopt new things as if we were emotional shoppers. Our neighbor buys a sports car and we think we need the same car. Left atrial appendage occlusion and subcutaneous defibrillators were past examples.
The most recent example of soft thinking (especially in the United States) is the enthusiasm and early adoption of first-generation PFA systems for the treatment of AF.
Readers of cardiac news (including some of my patients) might think PFA has solved the AF puzzle. It has not.
A true breakthrough in AF would be to find its cause. PFA is simply another way to destroy (ablate) cardiac myocytes. PFA uses electrical energy (think shocks) to create pores in the cell membranes of myocytes. It’s delivered through various types of catheters.
The main theoretical advantage of PFA is cardioselectivity, which is possible because myocytes have lower thresholds for irreversible electroporation than surrounding tissues. The dose of electrical energy that ablates cardiac tissue does not affect surrounding tissues. Cardioselectivity decreases the chance of the most feared complication of standard AF ablation, thermal damage to the esophagus, which is often fatal. The esophagus lies immediately behind the posterior wall of the left atrium and can be inadvertently injured during thermal ablation.
The challenge in assessing this potential advantage is that thermal esophageal damage is, thankfully, exceedingly rare. Its incidence is in the range of 1 in 10,000 AF ablations. But it might be even lower than that in contemporary practice, because knowledge of esophageal injury has led to innovations that probably have reduced its incidence even further.
Proponents of PFA would rightly point to the fact that not having to worry about esophageal injury allows operators to add posterior wall ablation to the normal pulmonary vein isolation lesion set. This ability, they would argue, is likely to improve AF ablation outcomes. The problem is that the strongest and most recent trial of posterior wall isolation (with radiofrequency ablation) did not show better outcomes. A more recent observational analysis also showed no benefit to posterior wall isolation (using PFA) over pulmonary vein isolation alone.
What About PFA Efficacy?
I’ve long spoken and written about the lack of progress in AF ablation. In 1998, the first report on ablation of AF showed a 62% arrhythmia-free rate. Two decades later, in the carefully chosen labs treating patients in the CABANA trial, arrhythmia-free rates after AF ablation remain unchanged. We have improved our speed and ability to isolate pulmonary veins, but this has not increased our success in eliminating AF. The reason, I believe, is that we have made little to no progress in understanding the pathophysiology of AF.
The Food and Drug Administration regulatory trial called ADVENT randomly assigned more than 600 patients to thermal ablation or PFA, and the primary endpoint of ablation success was nearly identical. Single-center studies, observational registries, and single-arm studies have all shown similar efficacy of PFA and thermal ablation.
Proponents of PFA might argue that these early studies used first-generation PFA systems, and iteration will lead to better efficacy. Perhaps, but we’ve had 20 years of iteration of thermal ablation, and its efficacy has not budged.
What About PFA Safety?
In the ADVENT randomized trial, safety results were similar, though the one death, caused by cardiac perforation and tamponade, occurred in the PFA arm. In the MANIFEST-17K multinational survey of PFA ablation, safety events were in the range reported with thermal ablation. PFA still involves placing catheters in the heart, and complications such as tamponade, stroke, and vascular damage occur.
The large MANIFEST-17K survey also exposed two PFA-specific complications: coronary artery spasm, which can occur when PFA is delivered close to coronary arteries; and hemolysis-related kidney failure — severe enough to require dialysis in five patients. Supporters of PFA speculate that hemolysis occurs because electrical energy within the atrium can shred red blood cells. Their solution is to strive for good contact and use hydration. The irony of this latter fix is that one of the best advances in thermal ablation has been catheters that deliver less fluid and less need for diuresis after the procedure.
No PFA study has shown a decreased incidence of thermal damage to the esophagus with PFA ablation. Of course, this is because it is such a low-incidence event.
One of my concerns with PFA is brain safety. PFA creates substantial microbubbles in the left atrium, which can then travel north to the brain. In a small series from ADVENT, three patients had brain lesions after PFA vs none with thermal ablation. PFA proponents wrote that brain safety was important to study, but few patients have been systematically studied with brain MRI scans. Asymptomatic brain lesions have been noted after many arterial procedures. The clinical significance of these is not known. As a new technology, and one that creates substantial microbubbles in the left atrium, I agree with the PFA proponents that brain safety should be thoroughly studied — before widespread adoption.
What About Speed and Cost?
Observational studies from European labs report fast procedure times. I have seen PFA procedures in Europe; they’re fast — typically under an hour. A standard thermal ablation takes me about 60-70 minutes.
I am not sure that US operators can duplicate European procedural times. In the ADVENT regulatory trial, the mean procedure time was 105 minutes and that was in experienced US centers. While this still represents early experience with PFA, the culture of US AF ablation entails far more mapping and extra catheters than I have seen used in European labs.
Cost is a major issue. It’s hard to sort out exact costs in the United States, but a PFA catheter costs approximately threefold more than a standard ablation catheter. A recent study from Liverpool, England, found that PFA ablation was faster but more expensive than standard thermal ablation because of higher PFA equipment prices. For better or worse, US patients are not directly affected by the higher procedural costs. But the fact remains that PFA adds more costs to the healthcare system.
What Drives the Enthusiasm for First-Generation PFA?
So why all the enthusiasm? It’s surely not the empirical data. Evidence thus far shows no obvious advantage in safety or efficacy. European use of PFA does seem to reduce procedure time. But in many electrophysiology labs in the United States, the rate-limiting step for AF ablation is not time in the lab but having enough staff to turn rooms around.
The main factor driving early acceptance of PFA relates to basic human nature. It is the fear of missing out. Marketing works on consumers, and it surely works on doctors. Companies that make PFA systems sponsor key opinion leaders to discuss PFA. These companies have beautiful booths in the expo of our meetings; they host dinners and talks. When a hospital in a city does PFA, the other hospitals feel the urge to keep up. It’s hard to be a Top Person in electrophysiology and not be a PFA user.
One of my favorite comments came from a key opinion leader. He told me that he advised his administration to buy a PFA system, promote that they have it, and keep it in the closet until better systems are released.
Iteration in the medical device field is tricky. There are negatives to being too harsh on first-generation systems. Early cardiac resynchronization tools, for instance, were horrible. Now CRT is transformative in selected patients with heart failure.
It’s possible (but not certain) that electrical ablative therapy will iterate and surpass thermal ablation in the future. Maybe.
But for now, the enthusiasm for PFA far outstrips its evidence. Until better evidence emerges, I will be a slow adopter. And I hope that our field gathers evidence before widespread adoption makes it impossible to do proper studies.
Dr. Mandrola, clinical electrophysiologist, Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
It dominated 2024’s heart rhythm meetings, and it dominates my private electrophysiologist chat groups. My Google alert for “AF ablation” most often includes notices on PFA and the expansion of the atrial fibrillation ablation market.
Yet, the excitement does not match the empirical data.
Despite having strong brains, electrophysiologists adopt new things as if we were emotional shoppers. Our neighbor buys a sports car and we think we need the same car. Left atrial appendage occlusion and subcutaneous defibrillators were past examples.
The most recent example of soft thinking (especially in the United States) is the enthusiasm and early adoption of first-generation PFA systems for the treatment of AF.
Readers of cardiac news (including some of my patients) might think PFA has solved the AF puzzle. It has not.
A true breakthrough in AF would be to find its cause. PFA is simply another way to destroy (ablate) cardiac myocytes. PFA uses electrical energy (think shocks) to create pores in the cell membranes of myocytes. It’s delivered through various types of catheters.
The main theoretical advantage of PFA is cardioselectivity, which is possible because myocytes have lower thresholds for irreversible electroporation than surrounding tissues. The dose of electrical energy that ablates cardiac tissue does not affect surrounding tissues. Cardioselectivity decreases the chance of the most feared complication of standard AF ablation, thermal damage to the esophagus, which is often fatal. The esophagus lies immediately behind the posterior wall of the left atrium and can be inadvertently injured during thermal ablation.
The challenge in assessing this potential advantage is that thermal esophageal damage is, thankfully, exceedingly rare. Its incidence is in the range of 1 in 10,000 AF ablations. But it might be even lower than that in contemporary practice, because knowledge of esophageal injury has led to innovations that probably have reduced its incidence even further.
Proponents of PFA would rightly point to the fact that not having to worry about esophageal injury allows operators to add posterior wall ablation to the normal pulmonary vein isolation lesion set. This ability, they would argue, is likely to improve AF ablation outcomes. The problem is that the strongest and most recent trial of posterior wall isolation (with radiofrequency ablation) did not show better outcomes. A more recent observational analysis also showed no benefit to posterior wall isolation (using PFA) over pulmonary vein isolation alone.
What About PFA Efficacy?
I’ve long spoken and written about the lack of progress in AF ablation. In 1998, the first report on ablation of AF showed a 62% arrhythmia-free rate. Two decades later, in the carefully chosen labs treating patients in the CABANA trial, arrhythmia-free rates after AF ablation remain unchanged. We have improved our speed and ability to isolate pulmonary veins, but this has not increased our success in eliminating AF. The reason, I believe, is that we have made little to no progress in understanding the pathophysiology of AF.
The Food and Drug Administration regulatory trial called ADVENT randomly assigned more than 600 patients to thermal ablation or PFA, and the primary endpoint of ablation success was nearly identical. Single-center studies, observational registries, and single-arm studies have all shown similar efficacy of PFA and thermal ablation.
Proponents of PFA might argue that these early studies used first-generation PFA systems, and iteration will lead to better efficacy. Perhaps, but we’ve had 20 years of iteration of thermal ablation, and its efficacy has not budged.
What About PFA Safety?
In the ADVENT randomized trial, safety results were similar, though the one death, caused by cardiac perforation and tamponade, occurred in the PFA arm. In the MANIFEST-17K multinational survey of PFA ablation, safety events were in the range reported with thermal ablation. PFA still involves placing catheters in the heart, and complications such as tamponade, stroke, and vascular damage occur.
The large MANIFEST-17K survey also exposed two PFA-specific complications: coronary artery spasm, which can occur when PFA is delivered close to coronary arteries; and hemolysis-related kidney failure — severe enough to require dialysis in five patients. Supporters of PFA speculate that hemolysis occurs because electrical energy within the atrium can shred red blood cells. Their solution is to strive for good contact and use hydration. The irony of this latter fix is that one of the best advances in thermal ablation has been catheters that deliver less fluid and less need for diuresis after the procedure.
No PFA study has shown a decreased incidence of thermal damage to the esophagus with PFA ablation. Of course, this is because it is such a low-incidence event.
One of my concerns with PFA is brain safety. PFA creates substantial microbubbles in the left atrium, which can then travel north to the brain. In a small series from ADVENT, three patients had brain lesions after PFA vs none with thermal ablation. PFA proponents wrote that brain safety was important to study, but few patients have been systematically studied with brain MRI scans. Asymptomatic brain lesions have been noted after many arterial procedures. The clinical significance of these is not known. As a new technology, and one that creates substantial microbubbles in the left atrium, I agree with the PFA proponents that brain safety should be thoroughly studied — before widespread adoption.
What About Speed and Cost?
Observational studies from European labs report fast procedure times. I have seen PFA procedures in Europe; they’re fast — typically under an hour. A standard thermal ablation takes me about 60-70 minutes.
I am not sure that US operators can duplicate European procedural times. In the ADVENT regulatory trial, the mean procedure time was 105 minutes and that was in experienced US centers. While this still represents early experience with PFA, the culture of US AF ablation entails far more mapping and extra catheters than I have seen used in European labs.
Cost is a major issue. It’s hard to sort out exact costs in the United States, but a PFA catheter costs approximately threefold more than a standard ablation catheter. A recent study from Liverpool, England, found that PFA ablation was faster but more expensive than standard thermal ablation because of higher PFA equipment prices. For better or worse, US patients are not directly affected by the higher procedural costs. But the fact remains that PFA adds more costs to the healthcare system.
What Drives the Enthusiasm for First-Generation PFA?
So why all the enthusiasm? It’s surely not the empirical data. Evidence thus far shows no obvious advantage in safety or efficacy. European use of PFA does seem to reduce procedure time. But in many electrophysiology labs in the United States, the rate-limiting step for AF ablation is not time in the lab but having enough staff to turn rooms around.
The main factor driving early acceptance of PFA relates to basic human nature. It is the fear of missing out. Marketing works on consumers, and it surely works on doctors. Companies that make PFA systems sponsor key opinion leaders to discuss PFA. These companies have beautiful booths in the expo of our meetings; they host dinners and talks. When a hospital in a city does PFA, the other hospitals feel the urge to keep up. It’s hard to be a Top Person in electrophysiology and not be a PFA user.
One of my favorite comments came from a key opinion leader. He told me that he advised his administration to buy a PFA system, promote that they have it, and keep it in the closet until better systems are released.
Iteration in the medical device field is tricky. There are negatives to being too harsh on first-generation systems. Early cardiac resynchronization tools, for instance, were horrible. Now CRT is transformative in selected patients with heart failure.
It’s possible (but not certain) that electrical ablative therapy will iterate and surpass thermal ablation in the future. Maybe.
But for now, the enthusiasm for PFA far outstrips its evidence. Until better evidence emerges, I will be a slow adopter. And I hope that our field gathers evidence before widespread adoption makes it impossible to do proper studies.
Dr. Mandrola, clinical electrophysiologist, Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Primary Care Internal Medicine Is Dead
Editor’s Note: This piece was originally published in Dr. Glasser’s bimonthly column in The Jolt, a nonprofit online news organization based in Olympia, Washington. She was inspired to write her story after meeting Christine Laine, MD, one of three female physician presenters at the Sommer Lectures in Portland, Oregon, in May 2024. The article has been edited lightly from the original.
Primary care internal medicine — the medical field I chose, loved, and practiced for four decades — is dead.
The grief and shock I feel about this is personal and transpersonal. The loss of internists (internal medicine physicians) practicing primary care is a major loss to us all.
From the 1970s to roughly 2020, there were three groups of primary care physicians: family practice, pediatricians, and internists. In their 3-year residencies (after 4 years of medical school), pediatricians trained to care for children and adolescents; internists for adults; and FPs for children, adults, and women and pregnancy. Family practitioners are the most general of the generalists, whereas the others’ training involves comprehensive care of complex patients in their age groups.
How and when the field of primary care internal medicine flourished is my story.
I was one of those kids who was hyperfocused on science, math, and the human body. By the end of high school, I was considering medicine for my career.
To learn more, I volunteered at the local hospital. In my typical style, I requested not to be one of those candy stripers serving drinks on the wards. Instead, they put me in the emergency department, where I would transport patients and clean the stretchers. There I was free to watch whatever was going on if I did not interfere with the staff. On my first shift, a 20-year-old drowning victim arrived by ambulance. I watched the entire unsuccessful resuscitation and as shocked and saddened as I was, I knew (in the way only a headstrong 18-year-old can) that medicine was for me.
It was a fortuitous time to graduate as a female pre-med student.
In 1975, our country was in the midst of the women’s movement and a national effort to train primary care physicians. I was accepted to my state medical school. The University of Massachusetts Medical School had been established a few years earlier, with its main purpose to train primary care physicians and spread them around the state (especially out of the Boston metropolitan area). The curriculum was designed to expose students to primary care from year one. I was assigned to shadow a general practice physician in inner-city Springfield who saw over 50 patients a day! The patients knew they could see and afford him, so they crammed into his waiting room until their name was called in order of their arrival. No appointments necessary. His chart notes were a few scribbled sentences. I didn’t see myself in that practice exactly, but his work ethic and dedication inspired me.
Over half of our graduating class chose to train in primary care specialties, and most stayed in-state. It turned out to be a good bet on the part of the government of Massachusetts.
When I applied for residency in 1980, several internal medicine programs had a focus on primary care, which was my goal. I matched at Providence St. Vincent Hospital in Portland, Oregon, and moved across the country to the Pacific Northwest, never to look back. There, my attendings were doctors like I wanted to be: primary care internists in the community, not in academia. It was the perfect choice and an excellent training program.
In 1984, I hung out my private practice internal medicine shingle in Hillsboro, Oregon, across the street from the community hospital. My primary care internal medicine colleagues and I shared weekend calls and admitted and cared for our patients in the hospital, and when they were discharged. That is now called “continuity of care.” It was a time when we ate in the doctors’ lounge together, met in hallways, and informally consulted each other about our patients. These were called “curbside consults.” They were invaluable to our ability to provide comprehensive care to our patients in primary care, led to fewer specialty referrals, and were free. That would now be called interprofessional communication and collegiality.
“Burnout” was not a word you heard. We were busy and happy doing what we had spent 12 years of our precious youth to prepare for.
What did internists offer to primary care? That also is part of my story.
When I moved to Olympia, I took a position in the women’s health clinic at the American Lake Veterans Administration Medical Center.
We were a small group: two family practice doctors, three nurse practitioners, and me, the only internist. Many of our patients were sick and complex. Two of the nurse practitioners (NPs) asked me to take their most complicated patients. Being comfortable with complexity as an internist, I said yes.
One of the NPs was inappropriately hired, as she had experience in women’s health. She came to me freaked out: “Oh my God, I have no idea how to manage COPD!” The other wanted simpler patients. I don’t blame them for the patient transfers. NPs typically have 3 years of training before they practice, in contrast to primary care physicians’ 8.
Guess who made friends with the custodian, staying until 8 p.m. most evenings, and who left by 5:30 p.m.
What was I doing in those extra hours? I was trudging through clerical, yet important, tasks my medical assistant and transcriptionist used to do in private practice. In the 30 minutes allotted for the patient, I needed to focus entirely on them and their multiple complex medical problems.
What is lost with the death of primary care internal medicine?
At the recent Sommer Memorial Lectures in Portland, Steven D. Freer, MD, the current director of the residency program where I trained, has not had a single of his eight annual internal medicine graduates choose primary care in several years. Half (two of four) of those in my year did: One went to Tillamook, an underserved area on the Oregon coast, and I to Hillsboro.
Why are they not choosing primary care? As when the University of Massachusetts Medical School was established, a shortage of primary care physicians persists and probably is more severe than it was in the 1970s. Massachusetts was proactive. We are already years behind catching up. The shortage is no longer in rural areas alone.
Christine Laine, MD, who is editor in chief of Annals of Internal Medicine and spoke at the Sommer Memorial Lectures, lives in Philadelphia. Even there, she has lost her own primary care internal medicine physician and cannot find another primary care physician (much less an internist) for herself.
Washington State, where I live, scores a D grade for our primary care staffing statewide.
Is there hope for the future of primary care in general? Or for the restoration of primary care internal medicine?
Maybe. I was relieved to hear from Dr. Freer and Dr. Laine that efforts are beginning to revive the field.
Just like internists’ patients, the potential restoration of the field will be complex and multilayered. It will require new laws, policies, residency programs, and incentives for students, including debt reduction. Administrative burdens will need to be reduced; de-corporatization and restoring healthcare leadership to those with in-depth medical training will need to be a part of the solution as well.
Let’s all hope the new resuscitation efforts will be successful for the field of primary care in general and primary care internal medicine specifically. It will be good for healthcare and for your patients!
Many work for large systems in which they feel powerless to effect change.
Dr. Glasser is a retired internal medicine physician in Olympia, Washington. She can be reached at [email protected].
A version of this article appeared on Medscape.com.
Editor’s Note: This piece was originally published in Dr. Glasser’s bimonthly column in The Jolt, a nonprofit online news organization based in Olympia, Washington. She was inspired to write her story after meeting Christine Laine, MD, one of three female physician presenters at the Sommer Lectures in Portland, Oregon, in May 2024. The article has been edited lightly from the original.
Primary care internal medicine — the medical field I chose, loved, and practiced for four decades — is dead.
The grief and shock I feel about this is personal and transpersonal. The loss of internists (internal medicine physicians) practicing primary care is a major loss to us all.
From the 1970s to roughly 2020, there were three groups of primary care physicians: family practice, pediatricians, and internists. In their 3-year residencies (after 4 years of medical school), pediatricians trained to care for children and adolescents; internists for adults; and FPs for children, adults, and women and pregnancy. Family practitioners are the most general of the generalists, whereas the others’ training involves comprehensive care of complex patients in their age groups.
How and when the field of primary care internal medicine flourished is my story.
I was one of those kids who was hyperfocused on science, math, and the human body. By the end of high school, I was considering medicine for my career.
To learn more, I volunteered at the local hospital. In my typical style, I requested not to be one of those candy stripers serving drinks on the wards. Instead, they put me in the emergency department, where I would transport patients and clean the stretchers. There I was free to watch whatever was going on if I did not interfere with the staff. On my first shift, a 20-year-old drowning victim arrived by ambulance. I watched the entire unsuccessful resuscitation and as shocked and saddened as I was, I knew (in the way only a headstrong 18-year-old can) that medicine was for me.
It was a fortuitous time to graduate as a female pre-med student.
In 1975, our country was in the midst of the women’s movement and a national effort to train primary care physicians. I was accepted to my state medical school. The University of Massachusetts Medical School had been established a few years earlier, with its main purpose to train primary care physicians and spread them around the state (especially out of the Boston metropolitan area). The curriculum was designed to expose students to primary care from year one. I was assigned to shadow a general practice physician in inner-city Springfield who saw over 50 patients a day! The patients knew they could see and afford him, so they crammed into his waiting room until their name was called in order of their arrival. No appointments necessary. His chart notes were a few scribbled sentences. I didn’t see myself in that practice exactly, but his work ethic and dedication inspired me.
Over half of our graduating class chose to train in primary care specialties, and most stayed in-state. It turned out to be a good bet on the part of the government of Massachusetts.
When I applied for residency in 1980, several internal medicine programs had a focus on primary care, which was my goal. I matched at Providence St. Vincent Hospital in Portland, Oregon, and moved across the country to the Pacific Northwest, never to look back. There, my attendings were doctors like I wanted to be: primary care internists in the community, not in academia. It was the perfect choice and an excellent training program.
In 1984, I hung out my private practice internal medicine shingle in Hillsboro, Oregon, across the street from the community hospital. My primary care internal medicine colleagues and I shared weekend calls and admitted and cared for our patients in the hospital, and when they were discharged. That is now called “continuity of care.” It was a time when we ate in the doctors’ lounge together, met in hallways, and informally consulted each other about our patients. These were called “curbside consults.” They were invaluable to our ability to provide comprehensive care to our patients in primary care, led to fewer specialty referrals, and were free. That would now be called interprofessional communication and collegiality.
“Burnout” was not a word you heard. We were busy and happy doing what we had spent 12 years of our precious youth to prepare for.
What did internists offer to primary care? That also is part of my story.
When I moved to Olympia, I took a position in the women’s health clinic at the American Lake Veterans Administration Medical Center.
We were a small group: two family practice doctors, three nurse practitioners, and me, the only internist. Many of our patients were sick and complex. Two of the nurse practitioners (NPs) asked me to take their most complicated patients. Being comfortable with complexity as an internist, I said yes.
One of the NPs was inappropriately hired, as she had experience in women’s health. She came to me freaked out: “Oh my God, I have no idea how to manage COPD!” The other wanted simpler patients. I don’t blame them for the patient transfers. NPs typically have 3 years of training before they practice, in contrast to primary care physicians’ 8.
Guess who made friends with the custodian, staying until 8 p.m. most evenings, and who left by 5:30 p.m.
What was I doing in those extra hours? I was trudging through clerical, yet important, tasks my medical assistant and transcriptionist used to do in private practice. In the 30 minutes allotted for the patient, I needed to focus entirely on them and their multiple complex medical problems.
What is lost with the death of primary care internal medicine?
At the recent Sommer Memorial Lectures in Portland, Steven D. Freer, MD, the current director of the residency program where I trained, has not had a single of his eight annual internal medicine graduates choose primary care in several years. Half (two of four) of those in my year did: One went to Tillamook, an underserved area on the Oregon coast, and I to Hillsboro.
Why are they not choosing primary care? As when the University of Massachusetts Medical School was established, a shortage of primary care physicians persists and probably is more severe than it was in the 1970s. Massachusetts was proactive. We are already years behind catching up. The shortage is no longer in rural areas alone.
Christine Laine, MD, who is editor in chief of Annals of Internal Medicine and spoke at the Sommer Memorial Lectures, lives in Philadelphia. Even there, she has lost her own primary care internal medicine physician and cannot find another primary care physician (much less an internist) for herself.
Washington State, where I live, scores a D grade for our primary care staffing statewide.
Is there hope for the future of primary care in general? Or for the restoration of primary care internal medicine?
Maybe. I was relieved to hear from Dr. Freer and Dr. Laine that efforts are beginning to revive the field.
Just like internists’ patients, the potential restoration of the field will be complex and multilayered. It will require new laws, policies, residency programs, and incentives for students, including debt reduction. Administrative burdens will need to be reduced; de-corporatization and restoring healthcare leadership to those with in-depth medical training will need to be a part of the solution as well.
Let’s all hope the new resuscitation efforts will be successful for the field of primary care in general and primary care internal medicine specifically. It will be good for healthcare and for your patients!
Many work for large systems in which they feel powerless to effect change.
Dr. Glasser is a retired internal medicine physician in Olympia, Washington. She can be reached at [email protected].
A version of this article appeared on Medscape.com.
Editor’s Note: This piece was originally published in Dr. Glasser’s bimonthly column in The Jolt, a nonprofit online news organization based in Olympia, Washington. She was inspired to write her story after meeting Christine Laine, MD, one of three female physician presenters at the Sommer Lectures in Portland, Oregon, in May 2024. The article has been edited lightly from the original.
Primary care internal medicine — the medical field I chose, loved, and practiced for four decades — is dead.
The grief and shock I feel about this is personal and transpersonal. The loss of internists (internal medicine physicians) practicing primary care is a major loss to us all.
From the 1970s to roughly 2020, there were three groups of primary care physicians: family practice, pediatricians, and internists. In their 3-year residencies (after 4 years of medical school), pediatricians trained to care for children and adolescents; internists for adults; and FPs for children, adults, and women and pregnancy. Family practitioners are the most general of the generalists, whereas the others’ training involves comprehensive care of complex patients in their age groups.
How and when the field of primary care internal medicine flourished is my story.
I was one of those kids who was hyperfocused on science, math, and the human body. By the end of high school, I was considering medicine for my career.
To learn more, I volunteered at the local hospital. In my typical style, I requested not to be one of those candy stripers serving drinks on the wards. Instead, they put me in the emergency department, where I would transport patients and clean the stretchers. There I was free to watch whatever was going on if I did not interfere with the staff. On my first shift, a 20-year-old drowning victim arrived by ambulance. I watched the entire unsuccessful resuscitation and as shocked and saddened as I was, I knew (in the way only a headstrong 18-year-old can) that medicine was for me.
It was a fortuitous time to graduate as a female pre-med student.
In 1975, our country was in the midst of the women’s movement and a national effort to train primary care physicians. I was accepted to my state medical school. The University of Massachusetts Medical School had been established a few years earlier, with its main purpose to train primary care physicians and spread them around the state (especially out of the Boston metropolitan area). The curriculum was designed to expose students to primary care from year one. I was assigned to shadow a general practice physician in inner-city Springfield who saw over 50 patients a day! The patients knew they could see and afford him, so they crammed into his waiting room until their name was called in order of their arrival. No appointments necessary. His chart notes were a few scribbled sentences. I didn’t see myself in that practice exactly, but his work ethic and dedication inspired me.
Over half of our graduating class chose to train in primary care specialties, and most stayed in-state. It turned out to be a good bet on the part of the government of Massachusetts.
When I applied for residency in 1980, several internal medicine programs had a focus on primary care, which was my goal. I matched at Providence St. Vincent Hospital in Portland, Oregon, and moved across the country to the Pacific Northwest, never to look back. There, my attendings were doctors like I wanted to be: primary care internists in the community, not in academia. It was the perfect choice and an excellent training program.
In 1984, I hung out my private practice internal medicine shingle in Hillsboro, Oregon, across the street from the community hospital. My primary care internal medicine colleagues and I shared weekend calls and admitted and cared for our patients in the hospital, and when they were discharged. That is now called “continuity of care.” It was a time when we ate in the doctors’ lounge together, met in hallways, and informally consulted each other about our patients. These were called “curbside consults.” They were invaluable to our ability to provide comprehensive care to our patients in primary care, led to fewer specialty referrals, and were free. That would now be called interprofessional communication and collegiality.
“Burnout” was not a word you heard. We were busy and happy doing what we had spent 12 years of our precious youth to prepare for.
What did internists offer to primary care? That also is part of my story.
When I moved to Olympia, I took a position in the women’s health clinic at the American Lake Veterans Administration Medical Center.
We were a small group: two family practice doctors, three nurse practitioners, and me, the only internist. Many of our patients were sick and complex. Two of the nurse practitioners (NPs) asked me to take their most complicated patients. Being comfortable with complexity as an internist, I said yes.
One of the NPs was inappropriately hired, as she had experience in women’s health. She came to me freaked out: “Oh my God, I have no idea how to manage COPD!” The other wanted simpler patients. I don’t blame them for the patient transfers. NPs typically have 3 years of training before they practice, in contrast to primary care physicians’ 8.
Guess who made friends with the custodian, staying until 8 p.m. most evenings, and who left by 5:30 p.m.
What was I doing in those extra hours? I was trudging through clerical, yet important, tasks my medical assistant and transcriptionist used to do in private practice. In the 30 minutes allotted for the patient, I needed to focus entirely on them and their multiple complex medical problems.
What is lost with the death of primary care internal medicine?
At the recent Sommer Memorial Lectures in Portland, Steven D. Freer, MD, the current director of the residency program where I trained, has not had a single of his eight annual internal medicine graduates choose primary care in several years. Half (two of four) of those in my year did: One went to Tillamook, an underserved area on the Oregon coast, and I to Hillsboro.
Why are they not choosing primary care? As when the University of Massachusetts Medical School was established, a shortage of primary care physicians persists and probably is more severe than it was in the 1970s. Massachusetts was proactive. We are already years behind catching up. The shortage is no longer in rural areas alone.
Christine Laine, MD, who is editor in chief of Annals of Internal Medicine and spoke at the Sommer Memorial Lectures, lives in Philadelphia. Even there, she has lost her own primary care internal medicine physician and cannot find another primary care physician (much less an internist) for herself.
Washington State, where I live, scores a D grade for our primary care staffing statewide.
Is there hope for the future of primary care in general? Or for the restoration of primary care internal medicine?
Maybe. I was relieved to hear from Dr. Freer and Dr. Laine that efforts are beginning to revive the field.
Just like internists’ patients, the potential restoration of the field will be complex and multilayered. It will require new laws, policies, residency programs, and incentives for students, including debt reduction. Administrative burdens will need to be reduced; de-corporatization and restoring healthcare leadership to those with in-depth medical training will need to be a part of the solution as well.
Let’s all hope the new resuscitation efforts will be successful for the field of primary care in general and primary care internal medicine specifically. It will be good for healthcare and for your patients!
Many work for large systems in which they feel powerless to effect change.
Dr. Glasser is a retired internal medicine physician in Olympia, Washington. She can be reached at [email protected].
A version of this article appeared on Medscape.com.
Can Diabetes Lead to a False-Positive Alcohol Test?
This transcript has been edited for clarity.
I’m going to tell you the story of two patients with diabetes who had false-positive alcohol tests.
The first patient is a patient of mine with type 1 diabetes. He was in a car accident. He hit the car in front of him that hit the car in front of them. Because the cars were quite damaged, the police were summoned.
At the scene, he had a breathalyzer test. He flunked the breathalyzer test, and he was charged with a DUI. The woman in the middle car got out of her car and said her neck hurt. This then rose this to the level of a DUI with injury to one of the other people, and my patient was charged with a felony. He was taken to jail.
He told them at the scene and at the jail that he had type 1 diabetes. The reason this is so important is because type 1 diabetes can cause a false-positive breathalyzer test. In particular, this patient of mine had not been eating all day long. He’d been getting his basal insulin through the pump, but he had not given any bolus doses of insulin. He was actually quite ketotic.
When he was put in jail, they took away his cell phone so he could no longer see his glucose levels, and they took away the controller for his Omnipod system. He basically had no way to give bolus doses of insulin. Fortunately, the Omnipod system lasted for a day and a half just by giving him basal. The jail physicians did not give him insulin until he’d been in jail for 3 days.
This is someone with type 1 diabetes, and their protocol for insulin has something to do with high glucose levels and giving something like a sliding scale of insulin. They were not really prepared for managing somebody with type 1 diabetes who was on an automated insulin delivery system.
I, along with my patient’s parents, worked very hard to get the jail doctors to finally give him Lantus. Inherent in all of this, it made me aware of a number of different issues. The first is that breathalyzer tests can be falsely positive in people with type 1 diabetes if they are ketotic; therefore, people with type 1 diabetes should ask for a blood test to test their alcohol levels if they think it could be a false positive.
Second, we need to actually figure out a way to help people with type 1 diabetes who happen to be in jail or in prison because if they don’t have access to a smartphone, they’re not going to be able to run their devices. We need to make sure that devices have receivers that can be used, particularly continuous glucose monitors (CGMs), because CGM is the standard of care for patients and should be so for people who are incarcerated.
The second case is much shorter and isn’t mine, but it was a letter in The New England Journal of Medicine about a man who was on probation, who was having urine tests to show that he had not been consuming alcohol. He was started on empagliflozin, which, interestingly, made his urine test become falsely positive.
Why? Well, it’s thought it’s because it caused fermentation of the sugar with the bacteria that was in his urine because the people who were processing the sample hadn’t done it correctly, and they kept it out at room temperature for a prolonged period of time before testing it. The urine samples should be kept refrigerated to prevent this from happening.
These are two people who had false-positive tests because they had diabetes. I think it’s important that we realize that this can happen, and we need to help our patients deal with these situations.
Dr. Peters is professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles. She reported conflicts of interest with Abbott Diabetes Care, Becton Dickinson, Boehringer Ingelheim, Eli Lilly, Lexicon Pharmaceuticals, Livongo, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to tell you the story of two patients with diabetes who had false-positive alcohol tests.
The first patient is a patient of mine with type 1 diabetes. He was in a car accident. He hit the car in front of him that hit the car in front of them. Because the cars were quite damaged, the police were summoned.
At the scene, he had a breathalyzer test. He flunked the breathalyzer test, and he was charged with a DUI. The woman in the middle car got out of her car and said her neck hurt. This then rose this to the level of a DUI with injury to one of the other people, and my patient was charged with a felony. He was taken to jail.
He told them at the scene and at the jail that he had type 1 diabetes. The reason this is so important is because type 1 diabetes can cause a false-positive breathalyzer test. In particular, this patient of mine had not been eating all day long. He’d been getting his basal insulin through the pump, but he had not given any bolus doses of insulin. He was actually quite ketotic.
When he was put in jail, they took away his cell phone so he could no longer see his glucose levels, and they took away the controller for his Omnipod system. He basically had no way to give bolus doses of insulin. Fortunately, the Omnipod system lasted for a day and a half just by giving him basal. The jail physicians did not give him insulin until he’d been in jail for 3 days.
This is someone with type 1 diabetes, and their protocol for insulin has something to do with high glucose levels and giving something like a sliding scale of insulin. They were not really prepared for managing somebody with type 1 diabetes who was on an automated insulin delivery system.
I, along with my patient’s parents, worked very hard to get the jail doctors to finally give him Lantus. Inherent in all of this, it made me aware of a number of different issues. The first is that breathalyzer tests can be falsely positive in people with type 1 diabetes if they are ketotic; therefore, people with type 1 diabetes should ask for a blood test to test their alcohol levels if they think it could be a false positive.
Second, we need to actually figure out a way to help people with type 1 diabetes who happen to be in jail or in prison because if they don’t have access to a smartphone, they’re not going to be able to run their devices. We need to make sure that devices have receivers that can be used, particularly continuous glucose monitors (CGMs), because CGM is the standard of care for patients and should be so for people who are incarcerated.
The second case is much shorter and isn’t mine, but it was a letter in The New England Journal of Medicine about a man who was on probation, who was having urine tests to show that he had not been consuming alcohol. He was started on empagliflozin, which, interestingly, made his urine test become falsely positive.
Why? Well, it’s thought it’s because it caused fermentation of the sugar with the bacteria that was in his urine because the people who were processing the sample hadn’t done it correctly, and they kept it out at room temperature for a prolonged period of time before testing it. The urine samples should be kept refrigerated to prevent this from happening.
These are two people who had false-positive tests because they had diabetes. I think it’s important that we realize that this can happen, and we need to help our patients deal with these situations.
Dr. Peters is professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles. She reported conflicts of interest with Abbott Diabetes Care, Becton Dickinson, Boehringer Ingelheim, Eli Lilly, Lexicon Pharmaceuticals, Livongo, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to tell you the story of two patients with diabetes who had false-positive alcohol tests.
The first patient is a patient of mine with type 1 diabetes. He was in a car accident. He hit the car in front of him that hit the car in front of them. Because the cars were quite damaged, the police were summoned.
At the scene, he had a breathalyzer test. He flunked the breathalyzer test, and he was charged with a DUI. The woman in the middle car got out of her car and said her neck hurt. This then rose this to the level of a DUI with injury to one of the other people, and my patient was charged with a felony. He was taken to jail.
He told them at the scene and at the jail that he had type 1 diabetes. The reason this is so important is because type 1 diabetes can cause a false-positive breathalyzer test. In particular, this patient of mine had not been eating all day long. He’d been getting his basal insulin through the pump, but he had not given any bolus doses of insulin. He was actually quite ketotic.
When he was put in jail, they took away his cell phone so he could no longer see his glucose levels, and they took away the controller for his Omnipod system. He basically had no way to give bolus doses of insulin. Fortunately, the Omnipod system lasted for a day and a half just by giving him basal. The jail physicians did not give him insulin until he’d been in jail for 3 days.
This is someone with type 1 diabetes, and their protocol for insulin has something to do with high glucose levels and giving something like a sliding scale of insulin. They were not really prepared for managing somebody with type 1 diabetes who was on an automated insulin delivery system.
I, along with my patient’s parents, worked very hard to get the jail doctors to finally give him Lantus. Inherent in all of this, it made me aware of a number of different issues. The first is that breathalyzer tests can be falsely positive in people with type 1 diabetes if they are ketotic; therefore, people with type 1 diabetes should ask for a blood test to test their alcohol levels if they think it could be a false positive.
Second, we need to actually figure out a way to help people with type 1 diabetes who happen to be in jail or in prison because if they don’t have access to a smartphone, they’re not going to be able to run their devices. We need to make sure that devices have receivers that can be used, particularly continuous glucose monitors (CGMs), because CGM is the standard of care for patients and should be so for people who are incarcerated.
The second case is much shorter and isn’t mine, but it was a letter in The New England Journal of Medicine about a man who was on probation, who was having urine tests to show that he had not been consuming alcohol. He was started on empagliflozin, which, interestingly, made his urine test become falsely positive.
Why? Well, it’s thought it’s because it caused fermentation of the sugar with the bacteria that was in his urine because the people who were processing the sample hadn’t done it correctly, and they kept it out at room temperature for a prolonged period of time before testing it. The urine samples should be kept refrigerated to prevent this from happening.
These are two people who had false-positive tests because they had diabetes. I think it’s important that we realize that this can happen, and we need to help our patients deal with these situations.
Dr. Peters is professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles. She reported conflicts of interest with Abbott Diabetes Care, Becton Dickinson, Boehringer Ingelheim, Eli Lilly, Lexicon Pharmaceuticals, Livongo, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
New Mid-Year Vaccine Recommendations From ACIP
This transcript has been edited for clarity.
ACIP, the CDC’s Advisory Committee on Immunization Practices, met for 3 days in June. New vaccines and new recommendations for respiratory syncytial virus (RSV), flu, COVID, and a new pneumococcal vaccine were revealed.
RSV Protection
We’ll begin with RSV vaccines for adults aged 60 or older. For this group, shared clinical decision-making is out; it no longer applies. New, more specific recommendations from ACIP for RSV vaccines are both age based and risk based. The age-based recommendation applies to those aged 75 or older, who should receive a single RSV vaccine dose. If they have already received a dose under the old recommendation, they don’t need another one, at least for now.
The risk-based recommendation applies to adults from age 60 up to 75, but only for those with risk factors for severe RSV. These risk factors include lung disease, heart disease, immunocompromise, diabetes, obesity with a BMI of 40 or more, neurologic conditions, neuromuscular conditions, chronic kidney disease, liver disorders, hematologic disorders, frailty, and living in a nursing home or other long-term care facility. Those aged 60-75 with these risk factors should receive the RSV vaccine, and those without them should not receive it. The best time to get the RSV vaccine is late summer, but early fall administration with other adult vaccines is allowed and is acceptable.
Vaccine safety concerns were top of mind as ACIP members began their deliberations. Possible safety concerns for RSV vaccines have been detected for Guillain-Barré syndrome, atrial fibrillation, and idiopathic thrombocytopenic purpura. Safety surveillance updates are still interim and inconclusive. These signals still need further study and clarification.
Two RSV vaccines have been on the market: one by Pfizer, called Abrysvo, which does not contain an adjuvant; and another one by GSK, called Arexvy, which does contain an adjuvant. With the recent FDA approval of Moderna’s new mRNA RSV vaccine, mRESVIA, there are now three RSV vaccines licensed for those 60 or older. Arexvy is now FDA approved for adults in their 50s. That just happened in early June, but ACIP doesn’t currently recommend it for this fifty-something age group, even for those at high risk for severe RSV disease. This may change with greater clarification of potential vaccine safety concerns.
There is also news about protecting babies from RSV. RSV is the most common cause of hospitalization for infants in the United States, and most hospitalizations for RSV are in healthy, full-term infants. We now have two ways to protect babies: a dose of RSV vaccine given to mom, or a dose of the long-acting monoclonal antibody nirsevimab given to the baby. ACIP clarified that those who received a dose of maternal RSV vaccine during a previous pregnancy are not recommended to receive additional doses during future pregnancies, but infants born to those who were vaccinated for RSV during a prior pregnancy can receive nirsevimab, which is recommended for infants up to 8 months of age during their first RSV season, and for high-risk infants and toddlers aged 8-19 months during their second RSV season.
Last RSV season, supplies of nirsevimab were limited and doses had to be prioritized. No supply problems are anticipated for the upcoming season. A study published in March showed that nirsevimab was 90% effective at preventing RSV-associated hospitalization for infants in their first RSV season.
COVID
Here’s what’s new for COVID vaccines. A new-formula COVID vaccine will be ready for fall. ACIP voted unanimously to recommend a dose of the updated 2024-2025 COVID vaccine for everyone aged 6 months or older. This is a universal recommendation, just like the one we have for flu. But understand that even though COVID has waned, it’s still more deadly than flu. Most Americans now have some immunity against COVID, but this immunity wanes with time, and it also wanes as the virus keeps changing. These updated vaccines provide an incremental boost to our immunity for the new formula for fall. FDA has directed manufacturers to use a monovalent JN.1 lineage formula, with a preference for the KP.2 strain.
Older adults (aged 75 or older) and children under 6 months old are hit hardest by COVID. The littlest ones are too young to be vaccinated, but they can get protection from maternal vaccination. The uptake for last year’s COVID vaccine has been disappointing. Only 22.5% of adults and 14% of children received a dose of the updated shot. Focus-group discussions highlight the importance of a physician recommendation. Adults and children who receive a healthcare provider’s recommendation to get the COVID vaccine are more likely to get vaccinated.
Pneumococcal Vaccines
On June 17, 2024, a new pneumococcal vaccine, PCV21, was FDA approved for those aged 18 or older under an accelerated-approval pathway. ACIP voted to keep it simple and recommends PCV21 as an option for adults aged 19 or older who currently have an indication to receive a dose of PCV. This new PCV21 vaccine is indicated for prevention of both invasive pneumococcal disease (IPD) and pneumococcal pneumonia. Its brand name is Capvaxive and it’s made by Merck. IPD includes bacteremia, pneumonia, pneumococcal bacteremia, and meningitis.
There are two basic types of pneumococcal vaccines: polysaccharide vaccines (PPSV), which do not produce memory B cells; and PCV conjugate vaccines, which do trigger memory B-cell production and therefore induce greater long-term immunity. PCV21 covers 11 unique serotypes not in PCV20. This is important because many cases of adult disease are caused by subtypes not covered by other FDA-approved pneumococcal vaccines. PCV21 has greater coverage of the serotypes that cause invasive disease in adults as compared with PCV20. PCV20 covers up to 58% of those strains, while PCV21 covers up to 84% of strains responsible for invasive disease in adults. But there’s one serotype missing in PCV21, which may limit the groups who receive it. PCV21 does not cover serotype 4, a major cause of IPD in certain populations. Adults experiencing homelessness are 100-300 times more likely to develop IPD due to serotype 4. So are adults in Alaska, especially Alaska Natives. They have an 88-fold increase in serotype 4 invasive disease. Serotype 4 is covered by other pneumococcal vaccines, so for these patients, PCV20 is likely a better high-valent conjugate vaccine option than PCV21.
Flu Vaccines
What’s new for flu? Everyone aged 6 months or older needs a seasonal flu vaccination every year. That’s not new, but there are two new things coming this fall: (1) The seasonal flu vaccine is going trivalent. FDA has removed the Yamagata flu B strain because it no longer appears to be circulating. (2) ACIP made a special off-label recommendation to boost flu protection for solid organ transplant recipients ages 18-64 who are on immunosuppressive medications. These high-risk patients now have the off-label option of receiving one of the higher-dose flu vaccines, including high-dose and adjuvanted flu vaccines, which are FDA approved only for those 65 or older.
Sandra Adamson Fryhofer, Adjunct Clinical Associate Professor of Medicine, Emory University School of Medicine, Atlanta, Georgia, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for American Medical Association; Medical Association of Atlanta; ACIP liaison. Received income in an amount equal to or greater than $250 from American College of Physicians; Medscape; American Medical Association.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
ACIP, the CDC’s Advisory Committee on Immunization Practices, met for 3 days in June. New vaccines and new recommendations for respiratory syncytial virus (RSV), flu, COVID, and a new pneumococcal vaccine were revealed.
RSV Protection
We’ll begin with RSV vaccines for adults aged 60 or older. For this group, shared clinical decision-making is out; it no longer applies. New, more specific recommendations from ACIP for RSV vaccines are both age based and risk based. The age-based recommendation applies to those aged 75 or older, who should receive a single RSV vaccine dose. If they have already received a dose under the old recommendation, they don’t need another one, at least for now.
The risk-based recommendation applies to adults from age 60 up to 75, but only for those with risk factors for severe RSV. These risk factors include lung disease, heart disease, immunocompromise, diabetes, obesity with a BMI of 40 or more, neurologic conditions, neuromuscular conditions, chronic kidney disease, liver disorders, hematologic disorders, frailty, and living in a nursing home or other long-term care facility. Those aged 60-75 with these risk factors should receive the RSV vaccine, and those without them should not receive it. The best time to get the RSV vaccine is late summer, but early fall administration with other adult vaccines is allowed and is acceptable.
Vaccine safety concerns were top of mind as ACIP members began their deliberations. Possible safety concerns for RSV vaccines have been detected for Guillain-Barré syndrome, atrial fibrillation, and idiopathic thrombocytopenic purpura. Safety surveillance updates are still interim and inconclusive. These signals still need further study and clarification.
Two RSV vaccines have been on the market: one by Pfizer, called Abrysvo, which does not contain an adjuvant; and another one by GSK, called Arexvy, which does contain an adjuvant. With the recent FDA approval of Moderna’s new mRNA RSV vaccine, mRESVIA, there are now three RSV vaccines licensed for those 60 or older. Arexvy is now FDA approved for adults in their 50s. That just happened in early June, but ACIP doesn’t currently recommend it for this fifty-something age group, even for those at high risk for severe RSV disease. This may change with greater clarification of potential vaccine safety concerns.
There is also news about protecting babies from RSV. RSV is the most common cause of hospitalization for infants in the United States, and most hospitalizations for RSV are in healthy, full-term infants. We now have two ways to protect babies: a dose of RSV vaccine given to mom, or a dose of the long-acting monoclonal antibody nirsevimab given to the baby. ACIP clarified that those who received a dose of maternal RSV vaccine during a previous pregnancy are not recommended to receive additional doses during future pregnancies, but infants born to those who were vaccinated for RSV during a prior pregnancy can receive nirsevimab, which is recommended for infants up to 8 months of age during their first RSV season, and for high-risk infants and toddlers aged 8-19 months during their second RSV season.
Last RSV season, supplies of nirsevimab were limited and doses had to be prioritized. No supply problems are anticipated for the upcoming season. A study published in March showed that nirsevimab was 90% effective at preventing RSV-associated hospitalization for infants in their first RSV season.
COVID
Here’s what’s new for COVID vaccines. A new-formula COVID vaccine will be ready for fall. ACIP voted unanimously to recommend a dose of the updated 2024-2025 COVID vaccine for everyone aged 6 months or older. This is a universal recommendation, just like the one we have for flu. But understand that even though COVID has waned, it’s still more deadly than flu. Most Americans now have some immunity against COVID, but this immunity wanes with time, and it also wanes as the virus keeps changing. These updated vaccines provide an incremental boost to our immunity for the new formula for fall. FDA has directed manufacturers to use a monovalent JN.1 lineage formula, with a preference for the KP.2 strain.
Older adults (aged 75 or older) and children under 6 months old are hit hardest by COVID. The littlest ones are too young to be vaccinated, but they can get protection from maternal vaccination. The uptake for last year’s COVID vaccine has been disappointing. Only 22.5% of adults and 14% of children received a dose of the updated shot. Focus-group discussions highlight the importance of a physician recommendation. Adults and children who receive a healthcare provider’s recommendation to get the COVID vaccine are more likely to get vaccinated.
Pneumococcal Vaccines
On June 17, 2024, a new pneumococcal vaccine, PCV21, was FDA approved for those aged 18 or older under an accelerated-approval pathway. ACIP voted to keep it simple and recommends PCV21 as an option for adults aged 19 or older who currently have an indication to receive a dose of PCV. This new PCV21 vaccine is indicated for prevention of both invasive pneumococcal disease (IPD) and pneumococcal pneumonia. Its brand name is Capvaxive and it’s made by Merck. IPD includes bacteremia, pneumonia, pneumococcal bacteremia, and meningitis.
There are two basic types of pneumococcal vaccines: polysaccharide vaccines (PPSV), which do not produce memory B cells; and PCV conjugate vaccines, which do trigger memory B-cell production and therefore induce greater long-term immunity. PCV21 covers 11 unique serotypes not in PCV20. This is important because many cases of adult disease are caused by subtypes not covered by other FDA-approved pneumococcal vaccines. PCV21 has greater coverage of the serotypes that cause invasive disease in adults as compared with PCV20. PCV20 covers up to 58% of those strains, while PCV21 covers up to 84% of strains responsible for invasive disease in adults. But there’s one serotype missing in PCV21, which may limit the groups who receive it. PCV21 does not cover serotype 4, a major cause of IPD in certain populations. Adults experiencing homelessness are 100-300 times more likely to develop IPD due to serotype 4. So are adults in Alaska, especially Alaska Natives. They have an 88-fold increase in serotype 4 invasive disease. Serotype 4 is covered by other pneumococcal vaccines, so for these patients, PCV20 is likely a better high-valent conjugate vaccine option than PCV21.
Flu Vaccines
What’s new for flu? Everyone aged 6 months or older needs a seasonal flu vaccination every year. That’s not new, but there are two new things coming this fall: (1) The seasonal flu vaccine is going trivalent. FDA has removed the Yamagata flu B strain because it no longer appears to be circulating. (2) ACIP made a special off-label recommendation to boost flu protection for solid organ transplant recipients ages 18-64 who are on immunosuppressive medications. These high-risk patients now have the off-label option of receiving one of the higher-dose flu vaccines, including high-dose and adjuvanted flu vaccines, which are FDA approved only for those 65 or older.
Sandra Adamson Fryhofer, Adjunct Clinical Associate Professor of Medicine, Emory University School of Medicine, Atlanta, Georgia, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for American Medical Association; Medical Association of Atlanta; ACIP liaison. Received income in an amount equal to or greater than $250 from American College of Physicians; Medscape; American Medical Association.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
ACIP, the CDC’s Advisory Committee on Immunization Practices, met for 3 days in June. New vaccines and new recommendations for respiratory syncytial virus (RSV), flu, COVID, and a new pneumococcal vaccine were revealed.
RSV Protection
We’ll begin with RSV vaccines for adults aged 60 or older. For this group, shared clinical decision-making is out; it no longer applies. New, more specific recommendations from ACIP for RSV vaccines are both age based and risk based. The age-based recommendation applies to those aged 75 or older, who should receive a single RSV vaccine dose. If they have already received a dose under the old recommendation, they don’t need another one, at least for now.
The risk-based recommendation applies to adults from age 60 up to 75, but only for those with risk factors for severe RSV. These risk factors include lung disease, heart disease, immunocompromise, diabetes, obesity with a BMI of 40 or more, neurologic conditions, neuromuscular conditions, chronic kidney disease, liver disorders, hematologic disorders, frailty, and living in a nursing home or other long-term care facility. Those aged 60-75 with these risk factors should receive the RSV vaccine, and those without them should not receive it. The best time to get the RSV vaccine is late summer, but early fall administration with other adult vaccines is allowed and is acceptable.
Vaccine safety concerns were top of mind as ACIP members began their deliberations. Possible safety concerns for RSV vaccines have been detected for Guillain-Barré syndrome, atrial fibrillation, and idiopathic thrombocytopenic purpura. Safety surveillance updates are still interim and inconclusive. These signals still need further study and clarification.
Two RSV vaccines have been on the market: one by Pfizer, called Abrysvo, which does not contain an adjuvant; and another one by GSK, called Arexvy, which does contain an adjuvant. With the recent FDA approval of Moderna’s new mRNA RSV vaccine, mRESVIA, there are now three RSV vaccines licensed for those 60 or older. Arexvy is now FDA approved for adults in their 50s. That just happened in early June, but ACIP doesn’t currently recommend it for this fifty-something age group, even for those at high risk for severe RSV disease. This may change with greater clarification of potential vaccine safety concerns.
There is also news about protecting babies from RSV. RSV is the most common cause of hospitalization for infants in the United States, and most hospitalizations for RSV are in healthy, full-term infants. We now have two ways to protect babies: a dose of RSV vaccine given to mom, or a dose of the long-acting monoclonal antibody nirsevimab given to the baby. ACIP clarified that those who received a dose of maternal RSV vaccine during a previous pregnancy are not recommended to receive additional doses during future pregnancies, but infants born to those who were vaccinated for RSV during a prior pregnancy can receive nirsevimab, which is recommended for infants up to 8 months of age during their first RSV season, and for high-risk infants and toddlers aged 8-19 months during their second RSV season.
Last RSV season, supplies of nirsevimab were limited and doses had to be prioritized. No supply problems are anticipated for the upcoming season. A study published in March showed that nirsevimab was 90% effective at preventing RSV-associated hospitalization for infants in their first RSV season.
COVID
Here’s what’s new for COVID vaccines. A new-formula COVID vaccine will be ready for fall. ACIP voted unanimously to recommend a dose of the updated 2024-2025 COVID vaccine for everyone aged 6 months or older. This is a universal recommendation, just like the one we have for flu. But understand that even though COVID has waned, it’s still more deadly than flu. Most Americans now have some immunity against COVID, but this immunity wanes with time, and it also wanes as the virus keeps changing. These updated vaccines provide an incremental boost to our immunity for the new formula for fall. FDA has directed manufacturers to use a monovalent JN.1 lineage formula, with a preference for the KP.2 strain.
Older adults (aged 75 or older) and children under 6 months old are hit hardest by COVID. The littlest ones are too young to be vaccinated, but they can get protection from maternal vaccination. The uptake for last year’s COVID vaccine has been disappointing. Only 22.5% of adults and 14% of children received a dose of the updated shot. Focus-group discussions highlight the importance of a physician recommendation. Adults and children who receive a healthcare provider’s recommendation to get the COVID vaccine are more likely to get vaccinated.
Pneumococcal Vaccines
On June 17, 2024, a new pneumococcal vaccine, PCV21, was FDA approved for those aged 18 or older under an accelerated-approval pathway. ACIP voted to keep it simple and recommends PCV21 as an option for adults aged 19 or older who currently have an indication to receive a dose of PCV. This new PCV21 vaccine is indicated for prevention of both invasive pneumococcal disease (IPD) and pneumococcal pneumonia. Its brand name is Capvaxive and it’s made by Merck. IPD includes bacteremia, pneumonia, pneumococcal bacteremia, and meningitis.
There are two basic types of pneumococcal vaccines: polysaccharide vaccines (PPSV), which do not produce memory B cells; and PCV conjugate vaccines, which do trigger memory B-cell production and therefore induce greater long-term immunity. PCV21 covers 11 unique serotypes not in PCV20. This is important because many cases of adult disease are caused by subtypes not covered by other FDA-approved pneumococcal vaccines. PCV21 has greater coverage of the serotypes that cause invasive disease in adults as compared with PCV20. PCV20 covers up to 58% of those strains, while PCV21 covers up to 84% of strains responsible for invasive disease in adults. But there’s one serotype missing in PCV21, which may limit the groups who receive it. PCV21 does not cover serotype 4, a major cause of IPD in certain populations. Adults experiencing homelessness are 100-300 times more likely to develop IPD due to serotype 4. So are adults in Alaska, especially Alaska Natives. They have an 88-fold increase in serotype 4 invasive disease. Serotype 4 is covered by other pneumococcal vaccines, so for these patients, PCV20 is likely a better high-valent conjugate vaccine option than PCV21.
Flu Vaccines
What’s new for flu? Everyone aged 6 months or older needs a seasonal flu vaccination every year. That’s not new, but there are two new things coming this fall: (1) The seasonal flu vaccine is going trivalent. FDA has removed the Yamagata flu B strain because it no longer appears to be circulating. (2) ACIP made a special off-label recommendation to boost flu protection for solid organ transplant recipients ages 18-64 who are on immunosuppressive medications. These high-risk patients now have the off-label option of receiving one of the higher-dose flu vaccines, including high-dose and adjuvanted flu vaccines, which are FDA approved only for those 65 or older.
Sandra Adamson Fryhofer, Adjunct Clinical Associate Professor of Medicine, Emory University School of Medicine, Atlanta, Georgia, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for American Medical Association; Medical Association of Atlanta; ACIP liaison. Received income in an amount equal to or greater than $250 from American College of Physicians; Medscape; American Medical Association.
A version of this article first appeared on Medscape.com.
Expanding Use of GLP-1 RAs for Weight Management
To discuss issues related to counseling patients about weight loss with glucagon-like peptide 1 receptor agonists (GLP-1 RAs), I recently posted a case from my own practice. This was a 44-year-old woman with hyperlipidemia, hypertension, and obesity who wanted to try to lose weight with a GLP-1 RA, having been unsuccessful in maintaining a normal weight with lifestyle change alone.
I am very happy to see a high number of favorable responses to this article, and I also recognize that it was very focused on GLP-1 RA therapy while not addressing the multivariate treatment of obesity.
A healthy lifestyle remains foundational for the management of obesity, and clinicians should guide patients to make constructive choices regarding their diet, physical activity, mental health, and sleep. However, like for our patient introduced in that article, lifestyle changes are rarely sufficient to obtain a goal of sustained weight loss that promotes better health outcomes. A meta-analysis of clinical trials testing lifestyle interventions to lose weight among adults with overweight and obesity found that the relative reduction in body weight in the intervention vs control cohorts was −3.63 kg at 1 year and −2.45 kg at 3 years. More intensive programs with at least 28 interventions per year were associated with slightly more weight loss than less intensive programs.
That is why clinicians and patients have been reaching for effective pharmacotherapy to create better outcomes among adults with obesity. In a national survey of 1479 US adults, 12% reported having used a GLP-1 RA. Diabetes was the most common indication (43%), followed by heart disease (26%) and overweight/obesity (22%).
The high cost of GLP-1 RA therapy was a major barrier to even wider use. Some 54% of participants said that it was difficult to afford GLP-1 RA therapy, and an additional 22% found it very difficult to pay for the drugs. Having health insurance did not alter these figures substantially.
While cost and access remain some of the greatest challenges with the use of GLP-1 RAs, there is hope for change there. In March 2024, the US Food and Drug Administration approved semaglutide to reduce the risk for cardiovascular events among patients with overweight and obesity and existing cardiovascular disease. It appears that Medicare will cover semaglutide for that indication, which bucks a trend of more than 20 years during which Medicare Part D would not cover pharmacotherapy for weight loss.
There is bipartisan support in the US Congress to further increase coverage of GLP-1 RAs for obesity, which makes sense. GLP-1 RAs are associated with greater average weight loss than either lifestyle interventions alone or that associated with previous anti-obesity medications. While there are no safety data for these drugs stretching back for 50 or 100 years, clinicians should bear in mind that exenatide was approved for the management of type 2 diabetes in 2005. So, we are approaching two decades of practical experience with these drugs, and it appears clear that the benefits of GLP-1 RAs outweigh any known harms. For the right patient, and with the right kind of guidance by clinicians, GLP-1 RA therapy can have a profound effect on individual and public health.
Dr. Vega, health sciences clinical professor, Family Medicine, University of California, Irvine, disclosed ties with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
To discuss issues related to counseling patients about weight loss with glucagon-like peptide 1 receptor agonists (GLP-1 RAs), I recently posted a case from my own practice. This was a 44-year-old woman with hyperlipidemia, hypertension, and obesity who wanted to try to lose weight with a GLP-1 RA, having been unsuccessful in maintaining a normal weight with lifestyle change alone.
I am very happy to see a high number of favorable responses to this article, and I also recognize that it was very focused on GLP-1 RA therapy while not addressing the multivariate treatment of obesity.
A healthy lifestyle remains foundational for the management of obesity, and clinicians should guide patients to make constructive choices regarding their diet, physical activity, mental health, and sleep. However, like for our patient introduced in that article, lifestyle changes are rarely sufficient to obtain a goal of sustained weight loss that promotes better health outcomes. A meta-analysis of clinical trials testing lifestyle interventions to lose weight among adults with overweight and obesity found that the relative reduction in body weight in the intervention vs control cohorts was −3.63 kg at 1 year and −2.45 kg at 3 years. More intensive programs with at least 28 interventions per year were associated with slightly more weight loss than less intensive programs.
That is why clinicians and patients have been reaching for effective pharmacotherapy to create better outcomes among adults with obesity. In a national survey of 1479 US adults, 12% reported having used a GLP-1 RA. Diabetes was the most common indication (43%), followed by heart disease (26%) and overweight/obesity (22%).
The high cost of GLP-1 RA therapy was a major barrier to even wider use. Some 54% of participants said that it was difficult to afford GLP-1 RA therapy, and an additional 22% found it very difficult to pay for the drugs. Having health insurance did not alter these figures substantially.
While cost and access remain some of the greatest challenges with the use of GLP-1 RAs, there is hope for change there. In March 2024, the US Food and Drug Administration approved semaglutide to reduce the risk for cardiovascular events among patients with overweight and obesity and existing cardiovascular disease. It appears that Medicare will cover semaglutide for that indication, which bucks a trend of more than 20 years during which Medicare Part D would not cover pharmacotherapy for weight loss.
There is bipartisan support in the US Congress to further increase coverage of GLP-1 RAs for obesity, which makes sense. GLP-1 RAs are associated with greater average weight loss than either lifestyle interventions alone or that associated with previous anti-obesity medications. While there are no safety data for these drugs stretching back for 50 or 100 years, clinicians should bear in mind that exenatide was approved for the management of type 2 diabetes in 2005. So, we are approaching two decades of practical experience with these drugs, and it appears clear that the benefits of GLP-1 RAs outweigh any known harms. For the right patient, and with the right kind of guidance by clinicians, GLP-1 RA therapy can have a profound effect on individual and public health.
Dr. Vega, health sciences clinical professor, Family Medicine, University of California, Irvine, disclosed ties with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
To discuss issues related to counseling patients about weight loss with glucagon-like peptide 1 receptor agonists (GLP-1 RAs), I recently posted a case from my own practice. This was a 44-year-old woman with hyperlipidemia, hypertension, and obesity who wanted to try to lose weight with a GLP-1 RA, having been unsuccessful in maintaining a normal weight with lifestyle change alone.
I am very happy to see a high number of favorable responses to this article, and I also recognize that it was very focused on GLP-1 RA therapy while not addressing the multivariate treatment of obesity.
A healthy lifestyle remains foundational for the management of obesity, and clinicians should guide patients to make constructive choices regarding their diet, physical activity, mental health, and sleep. However, like for our patient introduced in that article, lifestyle changes are rarely sufficient to obtain a goal of sustained weight loss that promotes better health outcomes. A meta-analysis of clinical trials testing lifestyle interventions to lose weight among adults with overweight and obesity found that the relative reduction in body weight in the intervention vs control cohorts was −3.63 kg at 1 year and −2.45 kg at 3 years. More intensive programs with at least 28 interventions per year were associated with slightly more weight loss than less intensive programs.
That is why clinicians and patients have been reaching for effective pharmacotherapy to create better outcomes among adults with obesity. In a national survey of 1479 US adults, 12% reported having used a GLP-1 RA. Diabetes was the most common indication (43%), followed by heart disease (26%) and overweight/obesity (22%).
The high cost of GLP-1 RA therapy was a major barrier to even wider use. Some 54% of participants said that it was difficult to afford GLP-1 RA therapy, and an additional 22% found it very difficult to pay for the drugs. Having health insurance did not alter these figures substantially.
While cost and access remain some of the greatest challenges with the use of GLP-1 RAs, there is hope for change there. In March 2024, the US Food and Drug Administration approved semaglutide to reduce the risk for cardiovascular events among patients with overweight and obesity and existing cardiovascular disease. It appears that Medicare will cover semaglutide for that indication, which bucks a trend of more than 20 years during which Medicare Part D would not cover pharmacotherapy for weight loss.
There is bipartisan support in the US Congress to further increase coverage of GLP-1 RAs for obesity, which makes sense. GLP-1 RAs are associated with greater average weight loss than either lifestyle interventions alone or that associated with previous anti-obesity medications. While there are no safety data for these drugs stretching back for 50 or 100 years, clinicians should bear in mind that exenatide was approved for the management of type 2 diabetes in 2005. So, we are approaching two decades of practical experience with these drugs, and it appears clear that the benefits of GLP-1 RAs outweigh any known harms. For the right patient, and with the right kind of guidance by clinicians, GLP-1 RA therapy can have a profound effect on individual and public health.
Dr. Vega, health sciences clinical professor, Family Medicine, University of California, Irvine, disclosed ties with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
New Vitamin D Recs: Testing, Supplementing, Dosing
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I’m going to talk about the Endocrine Society Guideline on Vitamin D. The question of who and when to test for vitamin D, and when to prescribe vitamin D, comes up frequently. There have been a lot of studies, and many people I know have opinions about this, but I haven’t seen a lot of clear, evidence-based guidance. This much-needed guideline provides guidance, though I’m not sure that everyone is going to be happy with the recommendations. That said, the society did conduct a comprehensive assessment and systematic review of the evidence that was impressive and well done. For our discussion, I will focus on the recommendations for nonpregnant adults.
The assumption for all of the recommendations is that these are for individuals who are already getting the Institute of Medicine’s recommended amount of vitamin D, which is 600 IU daily for those 50-70 years of age and 800 IU daily for those above 80 years.
For adults aged 18-74 years, who do not have prediabetes, the guidelines suggest against routinely testing for vitamin D deficiency and recommend against routine supplementation. For the older part of this cohort, adults aged 50-74 years, there is abundant randomized trial evidence showing little to no significant differences with vitamin D supplementation on outcomes of fracture, cancer, cardiovascular disease, kidney stones, or mortality. While supplementation is safe, there does not appear to be any benefit to routine supplementation or testing. It is important to note that the trials were done in populations that were meeting the daily recommended intake of vitamin D and who did not have low vitamin D levels at baseline, so individuals who may not be meeting the recommended daily intake though their diet or through sun exposure may consider vitamin D supplementation.
For adults with prediabetes, vitamin D supplementation is recommended to reduce the risk for progression from prediabetes to diabetes. This is about 1 in 3 adults in the United States. A number of trials have looked at vitamin D supplementation for adults with prediabetes in addition to lifestyle modification (diet and exercise). Vitamin D decreases the risk for progression from prediabetes to diabetes by approximately 10%-15%. The effect may be greater in those who are over age 60 and who have lower initial vitamin D levels.
Vitamin D in older adults (aged 75 or older) has a separate recommendation. In this age group, low vitamin D levels are common, with up to 20% of older adults having low levels. The guidelines suggest against testing vitamin D in adults aged 75 or over and recommend empiric vitamin D supplementation for all adults aged 75 or older. While observational studies have shown a relationship between low vitamin D levels in this age group and adverse outcomes, including falls, fractures, and respiratory infections, evidence from randomized placebo-controlled trials of vitamin D supplementation have been inconsistent in regard to benefit. That said, a meta-analysis has shown that vitamin D supplementation lowers mortality compared with placebo, with a relative risk of 0.96 (confidence interval, 0.93-1.00). There was no difference in effect according to setting (community vs nursing home), vitamin D dosage, or baseline vitamin D level.
There appeared to be a benefit of low-dose vitamin D supplementation on fall risk, with possibly greater fall risk when high-dose supplementation was used. No significant effect on fracture rate was seen with vitamin D supplementation alone, although there was a decrease in fractures when vitamin D was combined with calcium. In these studies, the median dose of calcium was 1000 mg per day.
Based on the probability of a “slight decrease in all-cause mortality” and its safety, as well as possible benefit to decrease falls, the recommendation is for supplementation for all adults aged 75 or older. Since there was not a consistent difference by vitamin D level, testing is not necessary.
Let’s now discuss dosage. The guidelines recommend daily lower-dose vitamin D over nondaily higher-dose vitamin D. Unfortunately, the guideline does not specify a specific dose of vitamin D. The supplementation dose used in trials of adults aged 75 or older ranged from 400 to 3333 IU daily, with an average dose of 900 IU daily, so it seems to me that a dose of 1000-2000 IU daily is a reasonable choice for older adults. In the prediabetes trials, a higher average dose was used, with a mean of 3500 IU daily, so a higher dose might make sense in this group.
Dr. Skolnik, is a professor in the Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania. He disclosed ties with AstraZeneca, Bayer, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, and Merck.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I’m going to talk about the Endocrine Society Guideline on Vitamin D. The question of who and when to test for vitamin D, and when to prescribe vitamin D, comes up frequently. There have been a lot of studies, and many people I know have opinions about this, but I haven’t seen a lot of clear, evidence-based guidance. This much-needed guideline provides guidance, though I’m not sure that everyone is going to be happy with the recommendations. That said, the society did conduct a comprehensive assessment and systematic review of the evidence that was impressive and well done. For our discussion, I will focus on the recommendations for nonpregnant adults.
The assumption for all of the recommendations is that these are for individuals who are already getting the Institute of Medicine’s recommended amount of vitamin D, which is 600 IU daily for those 50-70 years of age and 800 IU daily for those above 80 years.
For adults aged 18-74 years, who do not have prediabetes, the guidelines suggest against routinely testing for vitamin D deficiency and recommend against routine supplementation. For the older part of this cohort, adults aged 50-74 years, there is abundant randomized trial evidence showing little to no significant differences with vitamin D supplementation on outcomes of fracture, cancer, cardiovascular disease, kidney stones, or mortality. While supplementation is safe, there does not appear to be any benefit to routine supplementation or testing. It is important to note that the trials were done in populations that were meeting the daily recommended intake of vitamin D and who did not have low vitamin D levels at baseline, so individuals who may not be meeting the recommended daily intake though their diet or through sun exposure may consider vitamin D supplementation.
For adults with prediabetes, vitamin D supplementation is recommended to reduce the risk for progression from prediabetes to diabetes. This is about 1 in 3 adults in the United States. A number of trials have looked at vitamin D supplementation for adults with prediabetes in addition to lifestyle modification (diet and exercise). Vitamin D decreases the risk for progression from prediabetes to diabetes by approximately 10%-15%. The effect may be greater in those who are over age 60 and who have lower initial vitamin D levels.
Vitamin D in older adults (aged 75 or older) has a separate recommendation. In this age group, low vitamin D levels are common, with up to 20% of older adults having low levels. The guidelines suggest against testing vitamin D in adults aged 75 or over and recommend empiric vitamin D supplementation for all adults aged 75 or older. While observational studies have shown a relationship between low vitamin D levels in this age group and adverse outcomes, including falls, fractures, and respiratory infections, evidence from randomized placebo-controlled trials of vitamin D supplementation have been inconsistent in regard to benefit. That said, a meta-analysis has shown that vitamin D supplementation lowers mortality compared with placebo, with a relative risk of 0.96 (confidence interval, 0.93-1.00). There was no difference in effect according to setting (community vs nursing home), vitamin D dosage, or baseline vitamin D level.
There appeared to be a benefit of low-dose vitamin D supplementation on fall risk, with possibly greater fall risk when high-dose supplementation was used. No significant effect on fracture rate was seen with vitamin D supplementation alone, although there was a decrease in fractures when vitamin D was combined with calcium. In these studies, the median dose of calcium was 1000 mg per day.
Based on the probability of a “slight decrease in all-cause mortality” and its safety, as well as possible benefit to decrease falls, the recommendation is for supplementation for all adults aged 75 or older. Since there was not a consistent difference by vitamin D level, testing is not necessary.
Let’s now discuss dosage. The guidelines recommend daily lower-dose vitamin D over nondaily higher-dose vitamin D. Unfortunately, the guideline does not specify a specific dose of vitamin D. The supplementation dose used in trials of adults aged 75 or older ranged from 400 to 3333 IU daily, with an average dose of 900 IU daily, so it seems to me that a dose of 1000-2000 IU daily is a reasonable choice for older adults. In the prediabetes trials, a higher average dose was used, with a mean of 3500 IU daily, so a higher dose might make sense in this group.
Dr. Skolnik, is a professor in the Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania. He disclosed ties with AstraZeneca, Bayer, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, and Merck.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I’m going to talk about the Endocrine Society Guideline on Vitamin D. The question of who and when to test for vitamin D, and when to prescribe vitamin D, comes up frequently. There have been a lot of studies, and many people I know have opinions about this, but I haven’t seen a lot of clear, evidence-based guidance. This much-needed guideline provides guidance, though I’m not sure that everyone is going to be happy with the recommendations. That said, the society did conduct a comprehensive assessment and systematic review of the evidence that was impressive and well done. For our discussion, I will focus on the recommendations for nonpregnant adults.
The assumption for all of the recommendations is that these are for individuals who are already getting the Institute of Medicine’s recommended amount of vitamin D, which is 600 IU daily for those 50-70 years of age and 800 IU daily for those above 80 years.
For adults aged 18-74 years, who do not have prediabetes, the guidelines suggest against routinely testing for vitamin D deficiency and recommend against routine supplementation. For the older part of this cohort, adults aged 50-74 years, there is abundant randomized trial evidence showing little to no significant differences with vitamin D supplementation on outcomes of fracture, cancer, cardiovascular disease, kidney stones, or mortality. While supplementation is safe, there does not appear to be any benefit to routine supplementation or testing. It is important to note that the trials were done in populations that were meeting the daily recommended intake of vitamin D and who did not have low vitamin D levels at baseline, so individuals who may not be meeting the recommended daily intake though their diet or through sun exposure may consider vitamin D supplementation.
For adults with prediabetes, vitamin D supplementation is recommended to reduce the risk for progression from prediabetes to diabetes. This is about 1 in 3 adults in the United States. A number of trials have looked at vitamin D supplementation for adults with prediabetes in addition to lifestyle modification (diet and exercise). Vitamin D decreases the risk for progression from prediabetes to diabetes by approximately 10%-15%. The effect may be greater in those who are over age 60 and who have lower initial vitamin D levels.
Vitamin D in older adults (aged 75 or older) has a separate recommendation. In this age group, low vitamin D levels are common, with up to 20% of older adults having low levels. The guidelines suggest against testing vitamin D in adults aged 75 or over and recommend empiric vitamin D supplementation for all adults aged 75 or older. While observational studies have shown a relationship between low vitamin D levels in this age group and adverse outcomes, including falls, fractures, and respiratory infections, evidence from randomized placebo-controlled trials of vitamin D supplementation have been inconsistent in regard to benefit. That said, a meta-analysis has shown that vitamin D supplementation lowers mortality compared with placebo, with a relative risk of 0.96 (confidence interval, 0.93-1.00). There was no difference in effect according to setting (community vs nursing home), vitamin D dosage, or baseline vitamin D level.
There appeared to be a benefit of low-dose vitamin D supplementation on fall risk, with possibly greater fall risk when high-dose supplementation was used. No significant effect on fracture rate was seen with vitamin D supplementation alone, although there was a decrease in fractures when vitamin D was combined with calcium. In these studies, the median dose of calcium was 1000 mg per day.
Based on the probability of a “slight decrease in all-cause mortality” and its safety, as well as possible benefit to decrease falls, the recommendation is for supplementation for all adults aged 75 or older. Since there was not a consistent difference by vitamin D level, testing is not necessary.
Let’s now discuss dosage. The guidelines recommend daily lower-dose vitamin D over nondaily higher-dose vitamin D. Unfortunately, the guideline does not specify a specific dose of vitamin D. The supplementation dose used in trials of adults aged 75 or older ranged from 400 to 3333 IU daily, with an average dose of 900 IU daily, so it seems to me that a dose of 1000-2000 IU daily is a reasonable choice for older adults. In the prediabetes trials, a higher average dose was used, with a mean of 3500 IU daily, so a higher dose might make sense in this group.
Dr. Skolnik, is a professor in the Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania. He disclosed ties with AstraZeneca, Bayer, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, and Merck.
A version of this article first appeared on Medscape.com.
Managing Cancer in Pregnancy: Improvements and Considerations
Introduction: Tremendous Progress on Cancer Extends to Cancer in Pregnancy
The biomedical research enterprise that took shape in the United States after World War II has had numerous positive effects, including significant progress made during the past 75-plus years in the diagnosis, prevention, and treatment of cancer.
President Franklin D. Roosevelt’s 1944 request of Dr. Vannevar Bush, director of the then Office of Scientific Research and Development, to organize a program that would advance and apply scientific knowledge for times of peace — just as it been advanced and applied in times of war — culminated in a historic report, Science – The Endless Frontier. Presented in 1945 to President Harry S. Truman, this report helped fuel decades of broad, bold, and coordinated government-sponsored biomedical research aimed at addressing disease and improving the health of the American people (National Science Foundation, 1945).
Discoveries made from research in basic and translational sciences deepened our knowledge of the cellular and molecular underpinnings of cancer, leading to advances in chemotherapy, radiotherapy, and other treatment approaches as well as continual refinements in their application. Similarly, our diagnostic armamentarium has significantly improved.
As a result, we have reduced both the incidence and mortality of cancer. Today, some cancers can be prevented. Others can be reversed or put in remission. Granted, progress has been variable, with some cancers such as ovarian cancer still having relatively low survival rates. Much more needs to be done. Overall, however, the positive effects of the U.S. biomedical research enterprise on cancer are evident. According to the National Cancer Institute’s most recent report on the status of cancer, death rates from cancer fell 1.9% per year on average in females from 2015 to 2019 (Cancer. 2022 Oct 22. doi: 10.1002/cncr.34479).
It is not only patients whose cancer occurs outside of pregnancy who have benefited. When treatment is appropriately selected and timing considerations are made, patients whose cancer is diagnosed during pregnancy — and their children — can have good outcomes.
To explain how the management of cancer in pregnancy has improved, we have invited Gautam G. Rao, MD, gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to write this installment of the Master Class in Obstetrics. As Dr. Rao explains, radiation is not as dangerous to the fetus as once thought, and the safety of many chemotherapeutic regimens in pregnancy has been documented. Obstetricians can and should counsel patients, he explains, about the likelihood of good maternal and fetal outcomes.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Managing Cancer in Pregnancy
Cancer can cause fear and distress for any patient, but when cancer is diagnosed during pregnancy, an expectant mother fears not only for her own health but for the health of her unborn child. Fortunately, ob.gyn.s and multidisciplinary teams have good reason to reassure patients about the likelihood of good outcomes.
Cancer treatment in pregnancy has improved with advancements in imaging and chemotherapy, and while maternal and fetal outcomes of prenatal cancer treatment are not well reported, evidence acquired in recent years from case series and retrospective studies shows that most imaging studies and procedural diagnostic tests – and many treatments – can be performed safely in pregnancy.
Decades ago, we avoided CT scans during pregnancy because of concerns about radiation exposure to the fetus, leaving some patients without an accurate staging of their cancer. Today, we have evidence that a CT scan is generally safe in pregnancy. Similarly, the safety of many chemotherapeutic regimens in pregnancy has been documented in recent decades,and the use of chemotherapy during pregnancy has increased progressively. Radiation is also commonly utilized in the management of cancers that may occur during pregnancy, such as breast cancer.1
Considerations of timing are often central to decision-making; chemotherapy and radiotherapy are generally avoided in the first trimester to prevent structural fetal anomalies, for instance, and delaying cancer treatment is often warranted when the patient is a few weeks away from delivery. On occasion, iatrogenic preterm birth is considered when the risks to the mother of delaying a necessary cancer treatment outweigh the risks to the fetus of prematurity.1
Pregnancy termination is rarely indicated, however, and information gathered over the past 2 decades suggests that fetal and placental metastases are rare.1 There is broad agreement that prenatal treatment of cancer in pregnancy should adhere as much as possible to protocols and guidelines for nonpregnant patients and that treatment delays driven by fear of fetal anomalies and miscarriage are unnecessary.
Cancer Incidence, Use of Diagnostic Imaging
Data on the incidence of cancer in pregnancy comes from population-based cancer registries, and unfortunately, these data are not standardized and are often incomplete. Many studies include cancer diagnosed up to 1 year after pregnancy, and some include preinvasive disease. Estimates therefore vary considerably (see Table 1 for a sampling of estimates incidences.)
It has been reported, and often cited in the literature, that invasive malignancy complicates one in 1,000 pregnancies and that the incidence of cancer in pregnancy (invasive and noninvasive malignancies) has been rising over time.8 Increasing maternal age is believed to be playing a role in this rise; as women delay childbearing, they enter the age range in which some cancers become more common. Additionally, improvements in screening and diagnostics have led to earlier cancer detection. The incidence of ovarian neoplasms found during pregnancy has increased, for instance, with the routine use of diagnostic ultrasound in pregnancy.1
Among the studies showing an increased incidence of pregnancy-associated cancer is a population-based study in Australia, which found that from 1994 to 2007 the crude incidence of pregnancy-associated cancer increased from 112.3 to 191.5 per 100,000 pregnancies (P < .001).9 A cohort study in the United States documented an increase in incidence from 75.0 per 100,000 pregnancies in 2002 to 138.5 per 100,000 pregnancies in 2012.10
Overall, the literature shows us that the skin, cervix, and breast are also common sites for malignancy during pregnancy.1 According to a 2022 review, breast cancer during pregnancy is less often hormone receptor–positive and more frequently triple negative compared with age-matched controls.11 The frequencies of other pregnancy-associated cancers appear overall to be similar to that of cancer occurring in all women across their reproductive years.1
Too often, diagnosis is delayed because cancer symptoms can be masked by or can mimic normal physiological changes in pregnancy. For instance, breast cancer can be difficult to diagnose during pregnancy and lactation due to anatomic changes in the breast parenchyma. Several studies published in the 1990s showed that breast cancer presents at a more advanced stage in pregnant patients than in nonpregnant patients because of this delay.1 Skin changes suggestive of melanoma can be attributed to hyperpigmentation of pregnancy, for instance. Several observational studies have suggested that thicker melanomas found in pregnancy may be because of delayed diagnosis.8
It is important that we thoroughly investigate signs and symptoms suggestive of a malignancy and not automatically attribute these symptoms to the pregnancy itself. Cervical biopsy of a mass or lesion suspicious for cervical cancer can be done safely during pregnancy and should not be delayed or deferred.
Fetal radiation exposure from radiologic examinations has long been a concern, but we know today that while the imaging modality should be chosen to minimize fetal radiation exposure, CT scans and even PET scans should be performed if these exams are deemed best for evaluation. Embryonic exposure to a dose of less than 50 mGy is rarely if at all associated with fetal malformations or miscarriage and a radiation dose of 100 mGy may be considered a floor for consideration of therapeutic termination of pregnancy.1,8
CT exams are associated with a fetal dose far less than 50 mGy (see Table 2 for radiation doses).
Magnetic resonance imaging with a magnet strength of 3 Tesla or less in any trimester is not associated with an increased risk of harm to the fetus or in early childhood, but the contrast agent gadolinium should be avoided in pregnancy as it has been associated with an increased risk of stillbirth, neonatal death, and childhood inflammatory, rheumatologic, and infiltrative skin lesions.1,8,12
Chemotherapy, Surgery, and Radiation in Pregnancy
The management of cancer during pregnancy requires a multidisciplinary team including medical, gynecologic, or radiation oncologists, and maternal-fetal medicine specialists (Figure 1). Prematurity and low birth weight are frequent complications for fetuses exposed to chemotherapy, although there is some uncertainty as to whether the treatment is causative. However, congenital anomalies no longer are a major concern, provided that drugs are appropriately selected and that fetal exposure occurs during the second or third trimester.
For instance, alkylating agents including cisplatin (an important drug in the management of gynecologic malignancies) have been associated with congenital anomalies in the first trimester but not in the second and third trimesters, and a variety of antimetabolites — excluding methotrexate and aminopterin — similarly have been shown to be relatively safe when used after the first trimester.1
Small studies have shown no long-term effects of chemotherapy exposure on postnatal growth and long-term neurologic/neurocognitive function,1 but this is an area that needs more research.
Also in need of investigation is the safety of newer agents in pregnancy. Data are limited on the use of new targeted treatments, monoclonal antibodies, and immunotherapies in pregnancy and their effects on the fetus, with current knowledge coming mainly from single case reports.13
Until more is learned — a challenge given that pregnant women are generally excluded from clinical trials — management teams are generally postponing use of these therapies until after delivery. Considering the pace of new developments revolutionizing cancer treatment, this topic will likely get more complex and confusing before we begin acquiring sufficient knowledge.
The timing of surgery for malignancy in pregnancy is similarly based on the balance of maternal and fetal risks, including the risk of maternal disease progression, the risk of preterm delivery, and the prevention of fetal metastases. In general, the safest time is the second trimester.
Maternal surgery in the third trimester may be associated with a risk of premature labor and altered uteroplacental perfusion. A 2005 systematic review of 12,452 women who underwent nonobstetric surgery during pregnancy provides some reassurance, however; compared with the general obstetric population, there was no increase in the rate of miscarriage or major birth defects.14
Radiotherapy used to be contraindicated in pregnancy but many experts today believe it can be safely utilized provided the uterus is out of field and is protected from scattered radiation. The head, neck, and breast, for instance, can be treated with newer radiotherapies, including stereotactic ablative radiation therapy.8 Patients with advanced cervical cancer often receive chemotherapy during pregnancy to slow metastatic growth followed by definitive treatment with postpartum radiation or surgery.
More research is needed, but available data on maternal outcomes are encouraging. For instance, there appear to be no significant differences in short- and long-term complications or survival between women who are pregnant and nonpregnant when treated for invasive cervical cancer.8 Similarly, while earlier studies of breast cancer diagnosed during pregnancy suggested a poor prognosis, data now show similar prognoses for pregnant and nonpregnant patients when controlled for stage.1
Dr. Rao is a gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported no relevant disclosures.
References
1. Rao GG. Chapter 42. Clinical Obstetrics: The Fetus & Mother, 4th ed. Reece EA et al. (eds): 2021.
2. Bannister-Tyrrell M et al. Aust N Z J Obstet Gynaecol. 2014;55:116-122.
3. Oehler MK et al. Aust N Z J Obstet Gynaecol. 2003;43(6):414-420.
4. Ruiz R et al. Breast. 2017;35:136-141. doi: 10.1016/j.breast.2017.07.008.
5. Nolan S et al. Am J Obstet Gynecol. 2019;220(1):S480. doi: 10.1016/j.ajog.2018.11.752.
6. El-Messidi A et al. J Perinat Med. 2015;43(6):683-688. doi: 10.1515/jpm-2014-0133.
7. Pellino G et al. Eur J Gastroenterol Hepatol. 2017;29(7):743-753. doi: 10.1097/MEG.0000000000000863.
8. Eastwood-Wilshere N et al. Asia-Pac J Clin Oncol. 2019;15:296-308.
9. Lee YY et al. BJOG. 2012;119(13):1572-1582.
10. Cottreau CM et al. J Womens Health (Larchmt). 2019 Feb;28(2):250-257.
11. Boere I et al. Best Pract Res Clin Obstet Gynaecol. 2022;82:46-59.
12. Ray JG et al. JAMA 2016;316(9):952-961.
13. Schwab R et al. Cancers. (Basel) 2021;13(12):3048.
14. Cohen-Kerem et al. Am J Surg. 2005;190(3):467-473.
Introduction: Tremendous Progress on Cancer Extends to Cancer in Pregnancy
The biomedical research enterprise that took shape in the United States after World War II has had numerous positive effects, including significant progress made during the past 75-plus years in the diagnosis, prevention, and treatment of cancer.
President Franklin D. Roosevelt’s 1944 request of Dr. Vannevar Bush, director of the then Office of Scientific Research and Development, to organize a program that would advance and apply scientific knowledge for times of peace — just as it been advanced and applied in times of war — culminated in a historic report, Science – The Endless Frontier. Presented in 1945 to President Harry S. Truman, this report helped fuel decades of broad, bold, and coordinated government-sponsored biomedical research aimed at addressing disease and improving the health of the American people (National Science Foundation, 1945).
Discoveries made from research in basic and translational sciences deepened our knowledge of the cellular and molecular underpinnings of cancer, leading to advances in chemotherapy, radiotherapy, and other treatment approaches as well as continual refinements in their application. Similarly, our diagnostic armamentarium has significantly improved.
As a result, we have reduced both the incidence and mortality of cancer. Today, some cancers can be prevented. Others can be reversed or put in remission. Granted, progress has been variable, with some cancers such as ovarian cancer still having relatively low survival rates. Much more needs to be done. Overall, however, the positive effects of the U.S. biomedical research enterprise on cancer are evident. According to the National Cancer Institute’s most recent report on the status of cancer, death rates from cancer fell 1.9% per year on average in females from 2015 to 2019 (Cancer. 2022 Oct 22. doi: 10.1002/cncr.34479).
It is not only patients whose cancer occurs outside of pregnancy who have benefited. When treatment is appropriately selected and timing considerations are made, patients whose cancer is diagnosed during pregnancy — and their children — can have good outcomes.
To explain how the management of cancer in pregnancy has improved, we have invited Gautam G. Rao, MD, gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to write this installment of the Master Class in Obstetrics. As Dr. Rao explains, radiation is not as dangerous to the fetus as once thought, and the safety of many chemotherapeutic regimens in pregnancy has been documented. Obstetricians can and should counsel patients, he explains, about the likelihood of good maternal and fetal outcomes.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Managing Cancer in Pregnancy
Cancer can cause fear and distress for any patient, but when cancer is diagnosed during pregnancy, an expectant mother fears not only for her own health but for the health of her unborn child. Fortunately, ob.gyn.s and multidisciplinary teams have good reason to reassure patients about the likelihood of good outcomes.
Cancer treatment in pregnancy has improved with advancements in imaging and chemotherapy, and while maternal and fetal outcomes of prenatal cancer treatment are not well reported, evidence acquired in recent years from case series and retrospective studies shows that most imaging studies and procedural diagnostic tests – and many treatments – can be performed safely in pregnancy.
Decades ago, we avoided CT scans during pregnancy because of concerns about radiation exposure to the fetus, leaving some patients without an accurate staging of their cancer. Today, we have evidence that a CT scan is generally safe in pregnancy. Similarly, the safety of many chemotherapeutic regimens in pregnancy has been documented in recent decades,and the use of chemotherapy during pregnancy has increased progressively. Radiation is also commonly utilized in the management of cancers that may occur during pregnancy, such as breast cancer.1
Considerations of timing are often central to decision-making; chemotherapy and radiotherapy are generally avoided in the first trimester to prevent structural fetal anomalies, for instance, and delaying cancer treatment is often warranted when the patient is a few weeks away from delivery. On occasion, iatrogenic preterm birth is considered when the risks to the mother of delaying a necessary cancer treatment outweigh the risks to the fetus of prematurity.1
Pregnancy termination is rarely indicated, however, and information gathered over the past 2 decades suggests that fetal and placental metastases are rare.1 There is broad agreement that prenatal treatment of cancer in pregnancy should adhere as much as possible to protocols and guidelines for nonpregnant patients and that treatment delays driven by fear of fetal anomalies and miscarriage are unnecessary.
Cancer Incidence, Use of Diagnostic Imaging
Data on the incidence of cancer in pregnancy comes from population-based cancer registries, and unfortunately, these data are not standardized and are often incomplete. Many studies include cancer diagnosed up to 1 year after pregnancy, and some include preinvasive disease. Estimates therefore vary considerably (see Table 1 for a sampling of estimates incidences.)
It has been reported, and often cited in the literature, that invasive malignancy complicates one in 1,000 pregnancies and that the incidence of cancer in pregnancy (invasive and noninvasive malignancies) has been rising over time.8 Increasing maternal age is believed to be playing a role in this rise; as women delay childbearing, they enter the age range in which some cancers become more common. Additionally, improvements in screening and diagnostics have led to earlier cancer detection. The incidence of ovarian neoplasms found during pregnancy has increased, for instance, with the routine use of diagnostic ultrasound in pregnancy.1
Among the studies showing an increased incidence of pregnancy-associated cancer is a population-based study in Australia, which found that from 1994 to 2007 the crude incidence of pregnancy-associated cancer increased from 112.3 to 191.5 per 100,000 pregnancies (P < .001).9 A cohort study in the United States documented an increase in incidence from 75.0 per 100,000 pregnancies in 2002 to 138.5 per 100,000 pregnancies in 2012.10
Overall, the literature shows us that the skin, cervix, and breast are also common sites for malignancy during pregnancy.1 According to a 2022 review, breast cancer during pregnancy is less often hormone receptor–positive and more frequently triple negative compared with age-matched controls.11 The frequencies of other pregnancy-associated cancers appear overall to be similar to that of cancer occurring in all women across their reproductive years.1
Too often, diagnosis is delayed because cancer symptoms can be masked by or can mimic normal physiological changes in pregnancy. For instance, breast cancer can be difficult to diagnose during pregnancy and lactation due to anatomic changes in the breast parenchyma. Several studies published in the 1990s showed that breast cancer presents at a more advanced stage in pregnant patients than in nonpregnant patients because of this delay.1 Skin changes suggestive of melanoma can be attributed to hyperpigmentation of pregnancy, for instance. Several observational studies have suggested that thicker melanomas found in pregnancy may be because of delayed diagnosis.8
It is important that we thoroughly investigate signs and symptoms suggestive of a malignancy and not automatically attribute these symptoms to the pregnancy itself. Cervical biopsy of a mass or lesion suspicious for cervical cancer can be done safely during pregnancy and should not be delayed or deferred.
Fetal radiation exposure from radiologic examinations has long been a concern, but we know today that while the imaging modality should be chosen to minimize fetal radiation exposure, CT scans and even PET scans should be performed if these exams are deemed best for evaluation. Embryonic exposure to a dose of less than 50 mGy is rarely if at all associated with fetal malformations or miscarriage and a radiation dose of 100 mGy may be considered a floor for consideration of therapeutic termination of pregnancy.1,8
CT exams are associated with a fetal dose far less than 50 mGy (see Table 2 for radiation doses).
Magnetic resonance imaging with a magnet strength of 3 Tesla or less in any trimester is not associated with an increased risk of harm to the fetus or in early childhood, but the contrast agent gadolinium should be avoided in pregnancy as it has been associated with an increased risk of stillbirth, neonatal death, and childhood inflammatory, rheumatologic, and infiltrative skin lesions.1,8,12
Chemotherapy, Surgery, and Radiation in Pregnancy
The management of cancer during pregnancy requires a multidisciplinary team including medical, gynecologic, or radiation oncologists, and maternal-fetal medicine specialists (Figure 1). Prematurity and low birth weight are frequent complications for fetuses exposed to chemotherapy, although there is some uncertainty as to whether the treatment is causative. However, congenital anomalies no longer are a major concern, provided that drugs are appropriately selected and that fetal exposure occurs during the second or third trimester.
For instance, alkylating agents including cisplatin (an important drug in the management of gynecologic malignancies) have been associated with congenital anomalies in the first trimester but not in the second and third trimesters, and a variety of antimetabolites — excluding methotrexate and aminopterin — similarly have been shown to be relatively safe when used after the first trimester.1
Small studies have shown no long-term effects of chemotherapy exposure on postnatal growth and long-term neurologic/neurocognitive function,1 but this is an area that needs more research.
Also in need of investigation is the safety of newer agents in pregnancy. Data are limited on the use of new targeted treatments, monoclonal antibodies, and immunotherapies in pregnancy and their effects on the fetus, with current knowledge coming mainly from single case reports.13
Until more is learned — a challenge given that pregnant women are generally excluded from clinical trials — management teams are generally postponing use of these therapies until after delivery. Considering the pace of new developments revolutionizing cancer treatment, this topic will likely get more complex and confusing before we begin acquiring sufficient knowledge.
The timing of surgery for malignancy in pregnancy is similarly based on the balance of maternal and fetal risks, including the risk of maternal disease progression, the risk of preterm delivery, and the prevention of fetal metastases. In general, the safest time is the second trimester.
Maternal surgery in the third trimester may be associated with a risk of premature labor and altered uteroplacental perfusion. A 2005 systematic review of 12,452 women who underwent nonobstetric surgery during pregnancy provides some reassurance, however; compared with the general obstetric population, there was no increase in the rate of miscarriage or major birth defects.14
Radiotherapy used to be contraindicated in pregnancy but many experts today believe it can be safely utilized provided the uterus is out of field and is protected from scattered radiation. The head, neck, and breast, for instance, can be treated with newer radiotherapies, including stereotactic ablative radiation therapy.8 Patients with advanced cervical cancer often receive chemotherapy during pregnancy to slow metastatic growth followed by definitive treatment with postpartum radiation or surgery.
More research is needed, but available data on maternal outcomes are encouraging. For instance, there appear to be no significant differences in short- and long-term complications or survival between women who are pregnant and nonpregnant when treated for invasive cervical cancer.8 Similarly, while earlier studies of breast cancer diagnosed during pregnancy suggested a poor prognosis, data now show similar prognoses for pregnant and nonpregnant patients when controlled for stage.1
Dr. Rao is a gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported no relevant disclosures.
References
1. Rao GG. Chapter 42. Clinical Obstetrics: The Fetus & Mother, 4th ed. Reece EA et al. (eds): 2021.
2. Bannister-Tyrrell M et al. Aust N Z J Obstet Gynaecol. 2014;55:116-122.
3. Oehler MK et al. Aust N Z J Obstet Gynaecol. 2003;43(6):414-420.
4. Ruiz R et al. Breast. 2017;35:136-141. doi: 10.1016/j.breast.2017.07.008.
5. Nolan S et al. Am J Obstet Gynecol. 2019;220(1):S480. doi: 10.1016/j.ajog.2018.11.752.
6. El-Messidi A et al. J Perinat Med. 2015;43(6):683-688. doi: 10.1515/jpm-2014-0133.
7. Pellino G et al. Eur J Gastroenterol Hepatol. 2017;29(7):743-753. doi: 10.1097/MEG.0000000000000863.
8. Eastwood-Wilshere N et al. Asia-Pac J Clin Oncol. 2019;15:296-308.
9. Lee YY et al. BJOG. 2012;119(13):1572-1582.
10. Cottreau CM et al. J Womens Health (Larchmt). 2019 Feb;28(2):250-257.
11. Boere I et al. Best Pract Res Clin Obstet Gynaecol. 2022;82:46-59.
12. Ray JG et al. JAMA 2016;316(9):952-961.
13. Schwab R et al. Cancers. (Basel) 2021;13(12):3048.
14. Cohen-Kerem et al. Am J Surg. 2005;190(3):467-473.
Introduction: Tremendous Progress on Cancer Extends to Cancer in Pregnancy
The biomedical research enterprise that took shape in the United States after World War II has had numerous positive effects, including significant progress made during the past 75-plus years in the diagnosis, prevention, and treatment of cancer.
President Franklin D. Roosevelt’s 1944 request of Dr. Vannevar Bush, director of the then Office of Scientific Research and Development, to organize a program that would advance and apply scientific knowledge for times of peace — just as it been advanced and applied in times of war — culminated in a historic report, Science – The Endless Frontier. Presented in 1945 to President Harry S. Truman, this report helped fuel decades of broad, bold, and coordinated government-sponsored biomedical research aimed at addressing disease and improving the health of the American people (National Science Foundation, 1945).
Discoveries made from research in basic and translational sciences deepened our knowledge of the cellular and molecular underpinnings of cancer, leading to advances in chemotherapy, radiotherapy, and other treatment approaches as well as continual refinements in their application. Similarly, our diagnostic armamentarium has significantly improved.
As a result, we have reduced both the incidence and mortality of cancer. Today, some cancers can be prevented. Others can be reversed or put in remission. Granted, progress has been variable, with some cancers such as ovarian cancer still having relatively low survival rates. Much more needs to be done. Overall, however, the positive effects of the U.S. biomedical research enterprise on cancer are evident. According to the National Cancer Institute’s most recent report on the status of cancer, death rates from cancer fell 1.9% per year on average in females from 2015 to 2019 (Cancer. 2022 Oct 22. doi: 10.1002/cncr.34479).
It is not only patients whose cancer occurs outside of pregnancy who have benefited. When treatment is appropriately selected and timing considerations are made, patients whose cancer is diagnosed during pregnancy — and their children — can have good outcomes.
To explain how the management of cancer in pregnancy has improved, we have invited Gautam G. Rao, MD, gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to write this installment of the Master Class in Obstetrics. As Dr. Rao explains, radiation is not as dangerous to the fetus as once thought, and the safety of many chemotherapeutic regimens in pregnancy has been documented. Obstetricians can and should counsel patients, he explains, about the likelihood of good maternal and fetal outcomes.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Managing Cancer in Pregnancy
Cancer can cause fear and distress for any patient, but when cancer is diagnosed during pregnancy, an expectant mother fears not only for her own health but for the health of her unborn child. Fortunately, ob.gyn.s and multidisciplinary teams have good reason to reassure patients about the likelihood of good outcomes.
Cancer treatment in pregnancy has improved with advancements in imaging and chemotherapy, and while maternal and fetal outcomes of prenatal cancer treatment are not well reported, evidence acquired in recent years from case series and retrospective studies shows that most imaging studies and procedural diagnostic tests – and many treatments – can be performed safely in pregnancy.
Decades ago, we avoided CT scans during pregnancy because of concerns about radiation exposure to the fetus, leaving some patients without an accurate staging of their cancer. Today, we have evidence that a CT scan is generally safe in pregnancy. Similarly, the safety of many chemotherapeutic regimens in pregnancy has been documented in recent decades,and the use of chemotherapy during pregnancy has increased progressively. Radiation is also commonly utilized in the management of cancers that may occur during pregnancy, such as breast cancer.1
Considerations of timing are often central to decision-making; chemotherapy and radiotherapy are generally avoided in the first trimester to prevent structural fetal anomalies, for instance, and delaying cancer treatment is often warranted when the patient is a few weeks away from delivery. On occasion, iatrogenic preterm birth is considered when the risks to the mother of delaying a necessary cancer treatment outweigh the risks to the fetus of prematurity.1
Pregnancy termination is rarely indicated, however, and information gathered over the past 2 decades suggests that fetal and placental metastases are rare.1 There is broad agreement that prenatal treatment of cancer in pregnancy should adhere as much as possible to protocols and guidelines for nonpregnant patients and that treatment delays driven by fear of fetal anomalies and miscarriage are unnecessary.
Cancer Incidence, Use of Diagnostic Imaging
Data on the incidence of cancer in pregnancy comes from population-based cancer registries, and unfortunately, these data are not standardized and are often incomplete. Many studies include cancer diagnosed up to 1 year after pregnancy, and some include preinvasive disease. Estimates therefore vary considerably (see Table 1 for a sampling of estimates incidences.)
It has been reported, and often cited in the literature, that invasive malignancy complicates one in 1,000 pregnancies and that the incidence of cancer in pregnancy (invasive and noninvasive malignancies) has been rising over time.8 Increasing maternal age is believed to be playing a role in this rise; as women delay childbearing, they enter the age range in which some cancers become more common. Additionally, improvements in screening and diagnostics have led to earlier cancer detection. The incidence of ovarian neoplasms found during pregnancy has increased, for instance, with the routine use of diagnostic ultrasound in pregnancy.1
Among the studies showing an increased incidence of pregnancy-associated cancer is a population-based study in Australia, which found that from 1994 to 2007 the crude incidence of pregnancy-associated cancer increased from 112.3 to 191.5 per 100,000 pregnancies (P < .001).9 A cohort study in the United States documented an increase in incidence from 75.0 per 100,000 pregnancies in 2002 to 138.5 per 100,000 pregnancies in 2012.10
Overall, the literature shows us that the skin, cervix, and breast are also common sites for malignancy during pregnancy.1 According to a 2022 review, breast cancer during pregnancy is less often hormone receptor–positive and more frequently triple negative compared with age-matched controls.11 The frequencies of other pregnancy-associated cancers appear overall to be similar to that of cancer occurring in all women across their reproductive years.1
Too often, diagnosis is delayed because cancer symptoms can be masked by or can mimic normal physiological changes in pregnancy. For instance, breast cancer can be difficult to diagnose during pregnancy and lactation due to anatomic changes in the breast parenchyma. Several studies published in the 1990s showed that breast cancer presents at a more advanced stage in pregnant patients than in nonpregnant patients because of this delay.1 Skin changes suggestive of melanoma can be attributed to hyperpigmentation of pregnancy, for instance. Several observational studies have suggested that thicker melanomas found in pregnancy may be because of delayed diagnosis.8
It is important that we thoroughly investigate signs and symptoms suggestive of a malignancy and not automatically attribute these symptoms to the pregnancy itself. Cervical biopsy of a mass or lesion suspicious for cervical cancer can be done safely during pregnancy and should not be delayed or deferred.
Fetal radiation exposure from radiologic examinations has long been a concern, but we know today that while the imaging modality should be chosen to minimize fetal radiation exposure, CT scans and even PET scans should be performed if these exams are deemed best for evaluation. Embryonic exposure to a dose of less than 50 mGy is rarely if at all associated with fetal malformations or miscarriage and a radiation dose of 100 mGy may be considered a floor for consideration of therapeutic termination of pregnancy.1,8
CT exams are associated with a fetal dose far less than 50 mGy (see Table 2 for radiation doses).
Magnetic resonance imaging with a magnet strength of 3 Tesla or less in any trimester is not associated with an increased risk of harm to the fetus or in early childhood, but the contrast agent gadolinium should be avoided in pregnancy as it has been associated with an increased risk of stillbirth, neonatal death, and childhood inflammatory, rheumatologic, and infiltrative skin lesions.1,8,12
Chemotherapy, Surgery, and Radiation in Pregnancy
The management of cancer during pregnancy requires a multidisciplinary team including medical, gynecologic, or radiation oncologists, and maternal-fetal medicine specialists (Figure 1). Prematurity and low birth weight are frequent complications for fetuses exposed to chemotherapy, although there is some uncertainty as to whether the treatment is causative. However, congenital anomalies no longer are a major concern, provided that drugs are appropriately selected and that fetal exposure occurs during the second or third trimester.
For instance, alkylating agents including cisplatin (an important drug in the management of gynecologic malignancies) have been associated with congenital anomalies in the first trimester but not in the second and third trimesters, and a variety of antimetabolites — excluding methotrexate and aminopterin — similarly have been shown to be relatively safe when used after the first trimester.1
Small studies have shown no long-term effects of chemotherapy exposure on postnatal growth and long-term neurologic/neurocognitive function,1 but this is an area that needs more research.
Also in need of investigation is the safety of newer agents in pregnancy. Data are limited on the use of new targeted treatments, monoclonal antibodies, and immunotherapies in pregnancy and their effects on the fetus, with current knowledge coming mainly from single case reports.13
Until more is learned — a challenge given that pregnant women are generally excluded from clinical trials — management teams are generally postponing use of these therapies until after delivery. Considering the pace of new developments revolutionizing cancer treatment, this topic will likely get more complex and confusing before we begin acquiring sufficient knowledge.
The timing of surgery for malignancy in pregnancy is similarly based on the balance of maternal and fetal risks, including the risk of maternal disease progression, the risk of preterm delivery, and the prevention of fetal metastases. In general, the safest time is the second trimester.
Maternal surgery in the third trimester may be associated with a risk of premature labor and altered uteroplacental perfusion. A 2005 systematic review of 12,452 women who underwent nonobstetric surgery during pregnancy provides some reassurance, however; compared with the general obstetric population, there was no increase in the rate of miscarriage or major birth defects.14
Radiotherapy used to be contraindicated in pregnancy but many experts today believe it can be safely utilized provided the uterus is out of field and is protected from scattered radiation. The head, neck, and breast, for instance, can be treated with newer radiotherapies, including stereotactic ablative radiation therapy.8 Patients with advanced cervical cancer often receive chemotherapy during pregnancy to slow metastatic growth followed by definitive treatment with postpartum radiation or surgery.
More research is needed, but available data on maternal outcomes are encouraging. For instance, there appear to be no significant differences in short- and long-term complications or survival between women who are pregnant and nonpregnant when treated for invasive cervical cancer.8 Similarly, while earlier studies of breast cancer diagnosed during pregnancy suggested a poor prognosis, data now show similar prognoses for pregnant and nonpregnant patients when controlled for stage.1
Dr. Rao is a gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported no relevant disclosures.
References
1. Rao GG. Chapter 42. Clinical Obstetrics: The Fetus & Mother, 4th ed. Reece EA et al. (eds): 2021.
2. Bannister-Tyrrell M et al. Aust N Z J Obstet Gynaecol. 2014;55:116-122.
3. Oehler MK et al. Aust N Z J Obstet Gynaecol. 2003;43(6):414-420.
4. Ruiz R et al. Breast. 2017;35:136-141. doi: 10.1016/j.breast.2017.07.008.
5. Nolan S et al. Am J Obstet Gynecol. 2019;220(1):S480. doi: 10.1016/j.ajog.2018.11.752.
6. El-Messidi A et al. J Perinat Med. 2015;43(6):683-688. doi: 10.1515/jpm-2014-0133.
7. Pellino G et al. Eur J Gastroenterol Hepatol. 2017;29(7):743-753. doi: 10.1097/MEG.0000000000000863.
8. Eastwood-Wilshere N et al. Asia-Pac J Clin Oncol. 2019;15:296-308.
9. Lee YY et al. BJOG. 2012;119(13):1572-1582.
10. Cottreau CM et al. J Womens Health (Larchmt). 2019 Feb;28(2):250-257.
11. Boere I et al. Best Pract Res Clin Obstet Gynaecol. 2022;82:46-59.
12. Ray JG et al. JAMA 2016;316(9):952-961.
13. Schwab R et al. Cancers. (Basel) 2021;13(12):3048.
14. Cohen-Kerem et al. Am J Surg. 2005;190(3):467-473.


