User login
Pregnancy registries are a valuable resource for dermatologists
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of the human pregnancy experience. However, although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of these registries are their prospective nature (enrolled before the outcome is known) and enrollment over a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions). Registries can identify early signals of teratogenicity, but they have several limitations: selection bias that results from voluntary reporting; target populations that are not representative; lost-to-follow-up pregnancies that may have had different outcomes than those with documented outcomes; elective terminations and fetal deaths without birth defects and spontaneous abortions, all of which may lack details; the lack of control groups (with some exceptions); and the publication of results that may be delayed or not be in a peer-reviewed journal. Because the total number of exposed pregnancies is unknown, the data cannot be used to calculate prevalences, but they can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports (reported after outcome is known). Such reports are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. But they may be helpful in detecting unusual patterns of birth defects.
For the following drugs, web addresses can be obtained from the Food and Drug Administration website, List of Pregnancy Exposure Registries.
MotherToBaby
A large registry, the MotherToBaby Organization of Teratology Information Specialists (OTIS) (877-311-8972), involves patients in several different categories and the effects of the drugs on the embryo-fetus: autoimmune diseases (ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma at less than 20 weeks’ gestation; vaccines; and heterozygous or homozygous familial hypercholesterolemia.
For the autoimmune diseases, the drugs and trade names are abatacept (Orencia), adalimumab (Humira), certolizumab pegol (Cimzia), etanercept (Enbrel), infliximab (Remicade), leflunomide (Arava), otezla (Apremilast), teriflunomide (Aubagio), tocilizumab (Actemra), tofacitinib (Xeljanz), and ustekinumab (Stelara).
For the asthma group, the drug being investigated is mepolizumab (Nucala).
Two vaccines – for tetanus, diphtheria, and pertussis (Tdap) and meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135 (Menveo) – are being studied.
The last category is heterozygous or homozygous familial hypercholesterolemia. The two agents in this category are alirocumab (Praluent) and evolocumab (Repatha).
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of the human pregnancy experience. However, although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of these registries are their prospective nature (enrolled before the outcome is known) and enrollment over a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions). Registries can identify early signals of teratogenicity, but they have several limitations: selection bias that results from voluntary reporting; target populations that are not representative; lost-to-follow-up pregnancies that may have had different outcomes than those with documented outcomes; elective terminations and fetal deaths without birth defects and spontaneous abortions, all of which may lack details; the lack of control groups (with some exceptions); and the publication of results that may be delayed or not be in a peer-reviewed journal. Because the total number of exposed pregnancies is unknown, the data cannot be used to calculate prevalences, but they can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports (reported after outcome is known). Such reports are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. But they may be helpful in detecting unusual patterns of birth defects.
For the following drugs, web addresses can be obtained from the Food and Drug Administration website, List of Pregnancy Exposure Registries.
MotherToBaby
A large registry, the MotherToBaby Organization of Teratology Information Specialists (OTIS) (877-311-8972), involves patients in several different categories and the effects of the drugs on the embryo-fetus: autoimmune diseases (ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma at less than 20 weeks’ gestation; vaccines; and heterozygous or homozygous familial hypercholesterolemia.
For the autoimmune diseases, the drugs and trade names are abatacept (Orencia), adalimumab (Humira), certolizumab pegol (Cimzia), etanercept (Enbrel), infliximab (Remicade), leflunomide (Arava), otezla (Apremilast), teriflunomide (Aubagio), tocilizumab (Actemra), tofacitinib (Xeljanz), and ustekinumab (Stelara).
For the asthma group, the drug being investigated is mepolizumab (Nucala).
Two vaccines – for tetanus, diphtheria, and pertussis (Tdap) and meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135 (Menveo) – are being studied.
The last category is heterozygous or homozygous familial hypercholesterolemia. The two agents in this category are alirocumab (Praluent) and evolocumab (Repatha).
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of the human pregnancy experience. However, although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of these registries are their prospective nature (enrolled before the outcome is known) and enrollment over a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions). Registries can identify early signals of teratogenicity, but they have several limitations: selection bias that results from voluntary reporting; target populations that are not representative; lost-to-follow-up pregnancies that may have had different outcomes than those with documented outcomes; elective terminations and fetal deaths without birth defects and spontaneous abortions, all of which may lack details; the lack of control groups (with some exceptions); and the publication of results that may be delayed or not be in a peer-reviewed journal. Because the total number of exposed pregnancies is unknown, the data cannot be used to calculate prevalences, but they can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports (reported after outcome is known). Such reports are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. But they may be helpful in detecting unusual patterns of birth defects.
For the following drugs, web addresses can be obtained from the Food and Drug Administration website, List of Pregnancy Exposure Registries.
MotherToBaby
A large registry, the MotherToBaby Organization of Teratology Information Specialists (OTIS) (877-311-8972), involves patients in several different categories and the effects of the drugs on the embryo-fetus: autoimmune diseases (ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma at less than 20 weeks’ gestation; vaccines; and heterozygous or homozygous familial hypercholesterolemia.
For the autoimmune diseases, the drugs and trade names are abatacept (Orencia), adalimumab (Humira), certolizumab pegol (Cimzia), etanercept (Enbrel), infliximab (Remicade), leflunomide (Arava), otezla (Apremilast), teriflunomide (Aubagio), tocilizumab (Actemra), tofacitinib (Xeljanz), and ustekinumab (Stelara).
For the asthma group, the drug being investigated is mepolizumab (Nucala).
Two vaccines – for tetanus, diphtheria, and pertussis (Tdap) and meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135 (Menveo) – are being studied.
The last category is heterozygous or homozygous familial hypercholesterolemia. The two agents in this category are alirocumab (Praluent) and evolocumab (Repatha).
Physician impairment: A need for prevention
Psychiatry is a field of passion. The reward of experiencing growth and change alongside our patients is what bolsters us through years of difficult training, overnight shifts, endless paperwork, regulatory mandates, and frequent worry about our patients. As physicians, we don’t live for weekends as many other professionals do. To the contrary, we spend them on call, moonlighting, laboring over journal articles, and perfecting lectures.
That passion is what makes us trusted clinicians and experts in our field. It can also make it difficult for us to disconnect from our work, frequently leading to burnout. Physician self-care, support, and professional development are critical topics that modern-day medicine minimizes at the peril of physicians and public health.1
Psychiatry lends itself to a deep and intimate understanding of another human being. The therapist delves into the lives of his or her patients, hears their stories, and holds their secrets. In some cases, we might be the only ones who truly see patients for who they uniquely are, and come to understand them on a deeper level than their closest family and friends. This can be both thrilling and intense. As we delve into the psyche of another individual, contemplate which interpretation we should share, and resonate with our patients, it is easy to become bogged down with our own countertransference, sentiment, and worry, and to become consumed by our work. A professional hazard, some might quip.
Therefore, personal restoration—a tool that keeps our clinical skills sharp—is vitally important to caring for oneself and one’s patient. Surprisingly, this can be neglected until we begin to experience burnout, which over time could transform into impairment, thus endangering ourselves, our patients, and our profession.
Over the past decade, physician impairment has been exhaustively described, researched, and addressed. However, most analyses have focused on identifying impairment, and offering guidance on how to properly report it. How do we shift from managing the crisis to preventing it? To answer this question, this article:
- reviews the dilemma of physician impairment
- explores the duty we have to patients, ourselves, and the profession
- discusses shifting the focus on impairment to prevention through well-being.
Continue to: Dilemma
Dilemma: Vulnerability to impairment
The cornerstone for well-being is a balanced life. No matter how much one loves his or her work, there must be balance between work, relationships, and hobbies. Without that equilibrium, everyone is put at risk.2
Just as our patients, we are not immune to mental illness, cognitive decline, or substance abuse.3 We might even be more susceptible. For many physicians, their identity is intimately tied to their work.4 Dr. Robin Weiss captured that intimate relationship5:
“… [A] therapist may spend hundreds of hours, perhaps more than a thousand, hearing about a patient’s most exalted aspirations and most murderous, hateful fantasies. During this time, the patient may endure excruciating losses, unbearable shame, bitter sadness and great triumphs. You may accompany patients through torturous adolescence into adulthood. Or you may meet them in middle age and be with them as they age and eventually die. You collaborate in a deep process of discovery. Few encounters are this deeply honest, and therefore intimate.”
Given the stories we hear and the resulting intimacy and countertransference that inevitably arise, psychiatrists are even more prone to burnout than other physicians.6 Physician impairment is a public health issue that affects not just physicians but also their families, colleagues, and patients.
“Impairment” for the purpose of this article means a physical, mental, or substance-related disorder that interferes with a physician’s ability to undertake professional activities competently and safely.7 Predisposing factors for physician impairment include an obsessive-compulsive personality type, a family history of mental illness, sensation-seeking behavior, denial of personal problems, perfectionism, and idealism.8,9 Also, work stress becomes a significant factor in already vulnerable physicians, leading to a greater risk for mental illness.10
Continue to: Some warning signs of impairment include...
Some warning signs of impairment include a lack of personal hygiene, emotional lability, sleep deprivation, inattention to our pages or phone calls, and increased professional errors.11 When it comes to addressing such impairment, previous research and literature has focused on how to monitor ourselves and our colleagues; anything less would put the reputation and integrity of the medical profession at risk.3 This has led to a culture of doing nothing but work until things go too far, and then reporting the problems. But what about intervening before things get too far?
Duty: To ourselves, our colleagues, and our patients
There has been much discussion on how to report impaired colleagues, but little on how to help and support ourselves and our colleagues before things escalate into serious problems. And this lack of discussion is at the detriment of individual practitioners, their families, and patients. Physicians-in-training, including psychiatric residents, are at particularly high risk for developing stress-related problems, depression, and substance misuse.12 Occupational demands, self-criticism, and denial of one’s distress are common among physicians, as is self-treatment with drugs and alcohol.13
We all know by now that doctors and physician health programs (PHPs) have a duty to report impaired colleagues who continue to practice despite reasonable offers of assistance. There are an abundance of PHPs that are in place to assist with such situations. The American Medical Association’s official position on reporting impairment is outlined in Policy H-275.952.7 There also is the Federation of State Medical Boards. Its policy states that PHPs have “a primary commitment to [help] state medical boards … protect the public … [These] programs [should] demonstrate an ongoing track of record of ensuring safety to the public and reveal deficiencies if they occur.”14
Legal and ethical issues, however, complicate interventions for colleagues who need assistance.15 Despite the existence of PHPs, it would be much easier—not to mention helpful—to help a colleague by carrying out early interventions.
Discussion: Prevention as a solution
More emphasis should be placed on prevention. That’s where self-care and well-being come into play. Awareness of and sensitivity to physician vulnerability, early detection, and prevention of impairment are important.
Continue to: There has been a paradigm shift in focus...
There has been a paradigm shift in focus across medical boards, professional societies, and medical colleges. They are recognizing that personal well-being can help prevent burnout and, in turn, change the landscape of medicine from endless work to balanced lives that yield more satisfying and joyful work. It is becoming an accepted fact in medicine that well-being is just as important as integrity, professionalism, and patient safety. For example, the American Academy of Medical Colleges (AAMC) issued a statement emphasizing the importance of clinician well-being and dedicated its June 2016 Leadership Forum to a range of topics addressing depression, resilience, burnout, and suicide in academic medicine.16
Anita Everett, MD, put the spotlight on physician well-being during her term as American Psychiatric Association President (2017 to 2018). She formed a specific workgroup on Physician Wellness and Burnout where there is a community focus on prevention and self-care.17 A strong sense of community and purpose is almost always part of the prescription for promoting greater well-being.2
The importance of this issue is also trickling down from policymakers into hospitals and community health centers. Consider an initiative at Minneapolis’s Hennepin County Medical Center. Leaders there created a “reset room” for physicians to quietly decompress. The room is complete with LED lights, flameless candles, a sound machine, comfortable chairs, several plants, and an “in use” sign on the door.18 Other personal strategies to help prevent burnout include making environmental changes, encouraging hobbies, and streamlining burdensome tasks such as paperwork and electronic medical record systems.
As physician health and well-being are finally emerging as a “hot topic,”2 educational and treatment resources are increasingly available for any of us to explore. Consider a simple Google search to look into your State’s PHPs, and get involved in your professional societies to make change.
The culture is starting to shift, and leading by example will be a key to propelling further progress in this area. Model our own self-care for colleagues and patients alike. As Mark Twain said, we might love our work, but we must remember that being solely defined by work comes to the detriment of our health. Maintaining balance is what will allow us to sustain long careers ahead doing what we love.
1. Mahoney, D, Freedy J, Brock C. Improving physician well-being. JAMA Intern Med. 2015;175(4):648-649.
2. Yellowlees P. Addressing physician health and well-being is patient safety issue. Psychiatric News. 2018;53(12):20-21.
3. Mossman D, Farrell HM. Physician impairment: when should you report? Current Psychiatry. 2011;10(9):67-71.
4. Lindeman S, Henriksson M, Isometsä E, et al. Treatment of mental disorders in seven physicians committing suicide. Crisis. 1999;20(2):86-89.
5. Weiss R. How therapists mourn. New York Times. July 4, 2015:SR2.
6. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
7. American Medical Association. Report 2 of the Council on Science and Public Health (A-11). Physician health programs (Reference Committee D). https://www.ama-assn.org/sites/default/files/media-browser/public/about-ama/councils/Council%20Reports/council-on-science-public-health/a11-csaph-physician-health-programs.pdf. Accessed August 6, 2018.
8. Boisaubin EV, Levine RE. Identifying and assisting the impaired physician. Am J Med Sci. 2001;322(1):31-36.
9. Bissel L, Jones RW. The alcoholic physician: a survey. Am J Psychiatry. 1976;133(10):1142-1146.
10. Vaillant GE, Sobowale NC, McArthur C. Some psychologic vulnerabilities of physicians. N Engl J Med. 1972;287(8):372-375.
11. McGovern MP, Agnes DH, Leon S. Characteristics of physicians presenting for assessment at a behavioral health center. J Addict Dis. 2000;19(2):59-73.
12. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
13. Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. JAMA. 2001;286(23):3007-3014.
14. Federation of State Medical Boards of the United States. Policy on physician impairment. http://www.csam-asam.org/pdf/misc/FSMB2011.pdf. Published 2011. Accessed July 15, 2018.
15. Bright RP, Krahn L. Impaired physicians: how to recognize, when to report, and where to refer. Current Psychiatry. 2010;9(6):11-20.
16. Academy of American Colleges. Well-being in academic medicine. https://www.aamc.org/initiatives/462280/well-being-academic-medicine.html. Updated July 9, 2018. Accessed July 17, 2018.
17. American Psychiatric Association. Well-being and burnout. https://www.psychiatry.org/psychiatrists/practice/well-being-and-burnout. Updated February 22, 2018. Accessed July 17, 2018.
18. Parks T. Physicians take to “reset room” to battle burnout. AMA Wire. https://wire.ama-assn.org/practice-management/physicians-take-reset-room-battle-burnout. Published June 8, 2016. Accessed July 18, 2018.
Psychiatry is a field of passion. The reward of experiencing growth and change alongside our patients is what bolsters us through years of difficult training, overnight shifts, endless paperwork, regulatory mandates, and frequent worry about our patients. As physicians, we don’t live for weekends as many other professionals do. To the contrary, we spend them on call, moonlighting, laboring over journal articles, and perfecting lectures.
That passion is what makes us trusted clinicians and experts in our field. It can also make it difficult for us to disconnect from our work, frequently leading to burnout. Physician self-care, support, and professional development are critical topics that modern-day medicine minimizes at the peril of physicians and public health.1
Psychiatry lends itself to a deep and intimate understanding of another human being. The therapist delves into the lives of his or her patients, hears their stories, and holds their secrets. In some cases, we might be the only ones who truly see patients for who they uniquely are, and come to understand them on a deeper level than their closest family and friends. This can be both thrilling and intense. As we delve into the psyche of another individual, contemplate which interpretation we should share, and resonate with our patients, it is easy to become bogged down with our own countertransference, sentiment, and worry, and to become consumed by our work. A professional hazard, some might quip.
Therefore, personal restoration—a tool that keeps our clinical skills sharp—is vitally important to caring for oneself and one’s patient. Surprisingly, this can be neglected until we begin to experience burnout, which over time could transform into impairment, thus endangering ourselves, our patients, and our profession.
Over the past decade, physician impairment has been exhaustively described, researched, and addressed. However, most analyses have focused on identifying impairment, and offering guidance on how to properly report it. How do we shift from managing the crisis to preventing it? To answer this question, this article:
- reviews the dilemma of physician impairment
- explores the duty we have to patients, ourselves, and the profession
- discusses shifting the focus on impairment to prevention through well-being.
Continue to: Dilemma
Dilemma: Vulnerability to impairment
The cornerstone for well-being is a balanced life. No matter how much one loves his or her work, there must be balance between work, relationships, and hobbies. Without that equilibrium, everyone is put at risk.2
Just as our patients, we are not immune to mental illness, cognitive decline, or substance abuse.3 We might even be more susceptible. For many physicians, their identity is intimately tied to their work.4 Dr. Robin Weiss captured that intimate relationship5:
“… [A] therapist may spend hundreds of hours, perhaps more than a thousand, hearing about a patient’s most exalted aspirations and most murderous, hateful fantasies. During this time, the patient may endure excruciating losses, unbearable shame, bitter sadness and great triumphs. You may accompany patients through torturous adolescence into adulthood. Or you may meet them in middle age and be with them as they age and eventually die. You collaborate in a deep process of discovery. Few encounters are this deeply honest, and therefore intimate.”
Given the stories we hear and the resulting intimacy and countertransference that inevitably arise, psychiatrists are even more prone to burnout than other physicians.6 Physician impairment is a public health issue that affects not just physicians but also their families, colleagues, and patients.
“Impairment” for the purpose of this article means a physical, mental, or substance-related disorder that interferes with a physician’s ability to undertake professional activities competently and safely.7 Predisposing factors for physician impairment include an obsessive-compulsive personality type, a family history of mental illness, sensation-seeking behavior, denial of personal problems, perfectionism, and idealism.8,9 Also, work stress becomes a significant factor in already vulnerable physicians, leading to a greater risk for mental illness.10
Continue to: Some warning signs of impairment include...
Some warning signs of impairment include a lack of personal hygiene, emotional lability, sleep deprivation, inattention to our pages or phone calls, and increased professional errors.11 When it comes to addressing such impairment, previous research and literature has focused on how to monitor ourselves and our colleagues; anything less would put the reputation and integrity of the medical profession at risk.3 This has led to a culture of doing nothing but work until things go too far, and then reporting the problems. But what about intervening before things get too far?
Duty: To ourselves, our colleagues, and our patients
There has been much discussion on how to report impaired colleagues, but little on how to help and support ourselves and our colleagues before things escalate into serious problems. And this lack of discussion is at the detriment of individual practitioners, their families, and patients. Physicians-in-training, including psychiatric residents, are at particularly high risk for developing stress-related problems, depression, and substance misuse.12 Occupational demands, self-criticism, and denial of one’s distress are common among physicians, as is self-treatment with drugs and alcohol.13
We all know by now that doctors and physician health programs (PHPs) have a duty to report impaired colleagues who continue to practice despite reasonable offers of assistance. There are an abundance of PHPs that are in place to assist with such situations. The American Medical Association’s official position on reporting impairment is outlined in Policy H-275.952.7 There also is the Federation of State Medical Boards. Its policy states that PHPs have “a primary commitment to [help] state medical boards … protect the public … [These] programs [should] demonstrate an ongoing track of record of ensuring safety to the public and reveal deficiencies if they occur.”14
Legal and ethical issues, however, complicate interventions for colleagues who need assistance.15 Despite the existence of PHPs, it would be much easier—not to mention helpful—to help a colleague by carrying out early interventions.
Discussion: Prevention as a solution
More emphasis should be placed on prevention. That’s where self-care and well-being come into play. Awareness of and sensitivity to physician vulnerability, early detection, and prevention of impairment are important.
Continue to: There has been a paradigm shift in focus...
There has been a paradigm shift in focus across medical boards, professional societies, and medical colleges. They are recognizing that personal well-being can help prevent burnout and, in turn, change the landscape of medicine from endless work to balanced lives that yield more satisfying and joyful work. It is becoming an accepted fact in medicine that well-being is just as important as integrity, professionalism, and patient safety. For example, the American Academy of Medical Colleges (AAMC) issued a statement emphasizing the importance of clinician well-being and dedicated its June 2016 Leadership Forum to a range of topics addressing depression, resilience, burnout, and suicide in academic medicine.16
Anita Everett, MD, put the spotlight on physician well-being during her term as American Psychiatric Association President (2017 to 2018). She formed a specific workgroup on Physician Wellness and Burnout where there is a community focus on prevention and self-care.17 A strong sense of community and purpose is almost always part of the prescription for promoting greater well-being.2
The importance of this issue is also trickling down from policymakers into hospitals and community health centers. Consider an initiative at Minneapolis’s Hennepin County Medical Center. Leaders there created a “reset room” for physicians to quietly decompress. The room is complete with LED lights, flameless candles, a sound machine, comfortable chairs, several plants, and an “in use” sign on the door.18 Other personal strategies to help prevent burnout include making environmental changes, encouraging hobbies, and streamlining burdensome tasks such as paperwork and electronic medical record systems.
As physician health and well-being are finally emerging as a “hot topic,”2 educational and treatment resources are increasingly available for any of us to explore. Consider a simple Google search to look into your State’s PHPs, and get involved in your professional societies to make change.
The culture is starting to shift, and leading by example will be a key to propelling further progress in this area. Model our own self-care for colleagues and patients alike. As Mark Twain said, we might love our work, but we must remember that being solely defined by work comes to the detriment of our health. Maintaining balance is what will allow us to sustain long careers ahead doing what we love.
Psychiatry is a field of passion. The reward of experiencing growth and change alongside our patients is what bolsters us through years of difficult training, overnight shifts, endless paperwork, regulatory mandates, and frequent worry about our patients. As physicians, we don’t live for weekends as many other professionals do. To the contrary, we spend them on call, moonlighting, laboring over journal articles, and perfecting lectures.
That passion is what makes us trusted clinicians and experts in our field. It can also make it difficult for us to disconnect from our work, frequently leading to burnout. Physician self-care, support, and professional development are critical topics that modern-day medicine minimizes at the peril of physicians and public health.1
Psychiatry lends itself to a deep and intimate understanding of another human being. The therapist delves into the lives of his or her patients, hears their stories, and holds their secrets. In some cases, we might be the only ones who truly see patients for who they uniquely are, and come to understand them on a deeper level than their closest family and friends. This can be both thrilling and intense. As we delve into the psyche of another individual, contemplate which interpretation we should share, and resonate with our patients, it is easy to become bogged down with our own countertransference, sentiment, and worry, and to become consumed by our work. A professional hazard, some might quip.
Therefore, personal restoration—a tool that keeps our clinical skills sharp—is vitally important to caring for oneself and one’s patient. Surprisingly, this can be neglected until we begin to experience burnout, which over time could transform into impairment, thus endangering ourselves, our patients, and our profession.
Over the past decade, physician impairment has been exhaustively described, researched, and addressed. However, most analyses have focused on identifying impairment, and offering guidance on how to properly report it. How do we shift from managing the crisis to preventing it? To answer this question, this article:
- reviews the dilemma of physician impairment
- explores the duty we have to patients, ourselves, and the profession
- discusses shifting the focus on impairment to prevention through well-being.
Continue to: Dilemma
Dilemma: Vulnerability to impairment
The cornerstone for well-being is a balanced life. No matter how much one loves his or her work, there must be balance between work, relationships, and hobbies. Without that equilibrium, everyone is put at risk.2
Just as our patients, we are not immune to mental illness, cognitive decline, or substance abuse.3 We might even be more susceptible. For many physicians, their identity is intimately tied to their work.4 Dr. Robin Weiss captured that intimate relationship5:
“… [A] therapist may spend hundreds of hours, perhaps more than a thousand, hearing about a patient’s most exalted aspirations and most murderous, hateful fantasies. During this time, the patient may endure excruciating losses, unbearable shame, bitter sadness and great triumphs. You may accompany patients through torturous adolescence into adulthood. Or you may meet them in middle age and be with them as they age and eventually die. You collaborate in a deep process of discovery. Few encounters are this deeply honest, and therefore intimate.”
Given the stories we hear and the resulting intimacy and countertransference that inevitably arise, psychiatrists are even more prone to burnout than other physicians.6 Physician impairment is a public health issue that affects not just physicians but also their families, colleagues, and patients.
“Impairment” for the purpose of this article means a physical, mental, or substance-related disorder that interferes with a physician’s ability to undertake professional activities competently and safely.7 Predisposing factors for physician impairment include an obsessive-compulsive personality type, a family history of mental illness, sensation-seeking behavior, denial of personal problems, perfectionism, and idealism.8,9 Also, work stress becomes a significant factor in already vulnerable physicians, leading to a greater risk for mental illness.10
Continue to: Some warning signs of impairment include...
Some warning signs of impairment include a lack of personal hygiene, emotional lability, sleep deprivation, inattention to our pages or phone calls, and increased professional errors.11 When it comes to addressing such impairment, previous research and literature has focused on how to monitor ourselves and our colleagues; anything less would put the reputation and integrity of the medical profession at risk.3 This has led to a culture of doing nothing but work until things go too far, and then reporting the problems. But what about intervening before things get too far?
Duty: To ourselves, our colleagues, and our patients
There has been much discussion on how to report impaired colleagues, but little on how to help and support ourselves and our colleagues before things escalate into serious problems. And this lack of discussion is at the detriment of individual practitioners, their families, and patients. Physicians-in-training, including psychiatric residents, are at particularly high risk for developing stress-related problems, depression, and substance misuse.12 Occupational demands, self-criticism, and denial of one’s distress are common among physicians, as is self-treatment with drugs and alcohol.13
We all know by now that doctors and physician health programs (PHPs) have a duty to report impaired colleagues who continue to practice despite reasonable offers of assistance. There are an abundance of PHPs that are in place to assist with such situations. The American Medical Association’s official position on reporting impairment is outlined in Policy H-275.952.7 There also is the Federation of State Medical Boards. Its policy states that PHPs have “a primary commitment to [help] state medical boards … protect the public … [These] programs [should] demonstrate an ongoing track of record of ensuring safety to the public and reveal deficiencies if they occur.”14
Legal and ethical issues, however, complicate interventions for colleagues who need assistance.15 Despite the existence of PHPs, it would be much easier—not to mention helpful—to help a colleague by carrying out early interventions.
Discussion: Prevention as a solution
More emphasis should be placed on prevention. That’s where self-care and well-being come into play. Awareness of and sensitivity to physician vulnerability, early detection, and prevention of impairment are important.
Continue to: There has been a paradigm shift in focus...
There has been a paradigm shift in focus across medical boards, professional societies, and medical colleges. They are recognizing that personal well-being can help prevent burnout and, in turn, change the landscape of medicine from endless work to balanced lives that yield more satisfying and joyful work. It is becoming an accepted fact in medicine that well-being is just as important as integrity, professionalism, and patient safety. For example, the American Academy of Medical Colleges (AAMC) issued a statement emphasizing the importance of clinician well-being and dedicated its June 2016 Leadership Forum to a range of topics addressing depression, resilience, burnout, and suicide in academic medicine.16
Anita Everett, MD, put the spotlight on physician well-being during her term as American Psychiatric Association President (2017 to 2018). She formed a specific workgroup on Physician Wellness and Burnout where there is a community focus on prevention and self-care.17 A strong sense of community and purpose is almost always part of the prescription for promoting greater well-being.2
The importance of this issue is also trickling down from policymakers into hospitals and community health centers. Consider an initiative at Minneapolis’s Hennepin County Medical Center. Leaders there created a “reset room” for physicians to quietly decompress. The room is complete with LED lights, flameless candles, a sound machine, comfortable chairs, several plants, and an “in use” sign on the door.18 Other personal strategies to help prevent burnout include making environmental changes, encouraging hobbies, and streamlining burdensome tasks such as paperwork and electronic medical record systems.
As physician health and well-being are finally emerging as a “hot topic,”2 educational and treatment resources are increasingly available for any of us to explore. Consider a simple Google search to look into your State’s PHPs, and get involved in your professional societies to make change.
The culture is starting to shift, and leading by example will be a key to propelling further progress in this area. Model our own self-care for colleagues and patients alike. As Mark Twain said, we might love our work, but we must remember that being solely defined by work comes to the detriment of our health. Maintaining balance is what will allow us to sustain long careers ahead doing what we love.
1. Mahoney, D, Freedy J, Brock C. Improving physician well-being. JAMA Intern Med. 2015;175(4):648-649.
2. Yellowlees P. Addressing physician health and well-being is patient safety issue. Psychiatric News. 2018;53(12):20-21.
3. Mossman D, Farrell HM. Physician impairment: when should you report? Current Psychiatry. 2011;10(9):67-71.
4. Lindeman S, Henriksson M, Isometsä E, et al. Treatment of mental disorders in seven physicians committing suicide. Crisis. 1999;20(2):86-89.
5. Weiss R. How therapists mourn. New York Times. July 4, 2015:SR2.
6. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
7. American Medical Association. Report 2 of the Council on Science and Public Health (A-11). Physician health programs (Reference Committee D). https://www.ama-assn.org/sites/default/files/media-browser/public/about-ama/councils/Council%20Reports/council-on-science-public-health/a11-csaph-physician-health-programs.pdf. Accessed August 6, 2018.
8. Boisaubin EV, Levine RE. Identifying and assisting the impaired physician. Am J Med Sci. 2001;322(1):31-36.
9. Bissel L, Jones RW. The alcoholic physician: a survey. Am J Psychiatry. 1976;133(10):1142-1146.
10. Vaillant GE, Sobowale NC, McArthur C. Some psychologic vulnerabilities of physicians. N Engl J Med. 1972;287(8):372-375.
11. McGovern MP, Agnes DH, Leon S. Characteristics of physicians presenting for assessment at a behavioral health center. J Addict Dis. 2000;19(2):59-73.
12. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
13. Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. JAMA. 2001;286(23):3007-3014.
14. Federation of State Medical Boards of the United States. Policy on physician impairment. http://www.csam-asam.org/pdf/misc/FSMB2011.pdf. Published 2011. Accessed July 15, 2018.
15. Bright RP, Krahn L. Impaired physicians: how to recognize, when to report, and where to refer. Current Psychiatry. 2010;9(6):11-20.
16. Academy of American Colleges. Well-being in academic medicine. https://www.aamc.org/initiatives/462280/well-being-academic-medicine.html. Updated July 9, 2018. Accessed July 17, 2018.
17. American Psychiatric Association. Well-being and burnout. https://www.psychiatry.org/psychiatrists/practice/well-being-and-burnout. Updated February 22, 2018. Accessed July 17, 2018.
18. Parks T. Physicians take to “reset room” to battle burnout. AMA Wire. https://wire.ama-assn.org/practice-management/physicians-take-reset-room-battle-burnout. Published June 8, 2016. Accessed July 18, 2018.
1. Mahoney, D, Freedy J, Brock C. Improving physician well-being. JAMA Intern Med. 2015;175(4):648-649.
2. Yellowlees P. Addressing physician health and well-being is patient safety issue. Psychiatric News. 2018;53(12):20-21.
3. Mossman D, Farrell HM. Physician impairment: when should you report? Current Psychiatry. 2011;10(9):67-71.
4. Lindeman S, Henriksson M, Isometsä E, et al. Treatment of mental disorders in seven physicians committing suicide. Crisis. 1999;20(2):86-89.
5. Weiss R. How therapists mourn. New York Times. July 4, 2015:SR2.
6. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
7. American Medical Association. Report 2 of the Council on Science and Public Health (A-11). Physician health programs (Reference Committee D). https://www.ama-assn.org/sites/default/files/media-browser/public/about-ama/councils/Council%20Reports/council-on-science-public-health/a11-csaph-physician-health-programs.pdf. Accessed August 6, 2018.
8. Boisaubin EV, Levine RE. Identifying and assisting the impaired physician. Am J Med Sci. 2001;322(1):31-36.
9. Bissel L, Jones RW. The alcoholic physician: a survey. Am J Psychiatry. 1976;133(10):1142-1146.
10. Vaillant GE, Sobowale NC, McArthur C. Some psychologic vulnerabilities of physicians. N Engl J Med. 1972;287(8):372-375.
11. McGovern MP, Agnes DH, Leon S. Characteristics of physicians presenting for assessment at a behavioral health center. J Addict Dis. 2000;19(2):59-73.
12. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
13. Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. JAMA. 2001;286(23):3007-3014.
14. Federation of State Medical Boards of the United States. Policy on physician impairment. http://www.csam-asam.org/pdf/misc/FSMB2011.pdf. Published 2011. Accessed July 15, 2018.
15. Bright RP, Krahn L. Impaired physicians: how to recognize, when to report, and where to refer. Current Psychiatry. 2010;9(6):11-20.
16. Academy of American Colleges. Well-being in academic medicine. https://www.aamc.org/initiatives/462280/well-being-academic-medicine.html. Updated July 9, 2018. Accessed July 17, 2018.
17. American Psychiatric Association. Well-being and burnout. https://www.psychiatry.org/psychiatrists/practice/well-being-and-burnout. Updated February 22, 2018. Accessed July 17, 2018.
18. Parks T. Physicians take to “reset room” to battle burnout. AMA Wire. https://wire.ama-assn.org/practice-management/physicians-take-reset-room-battle-burnout. Published June 8, 2016. Accessed July 18, 2018.
Pregnancy registries are a valuable resource
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of the human pregnancy experience. However, although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of these registries are their prospective nature (enrolled before the outcome is known) and enrollment over a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions). Registries can identify early signals of teratogenicity, but they have several limitations: selection bias that results from voluntary reporting; target populations that are not representative; lost-to-follow-up pregnancies that may have had different outcomes than those with documented outcomes; elective terminations and fetal deaths without birth defects and spontaneous abortions, all of which may lack details; the lack of control groups (with some exceptions); and the publication of results that may be delayed or not be in a peer-reviewed journal. Because the total number of exposed pregnancies is unknown, the data cannot be used to calculate prevalences, but they can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports (reported after outcome is known). Such reports are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. But they may be helpful in detecting unusual patterns of birth defects.
For the following drugs, web addresses can be obtained from the Food and Drug Administration website, List of Pregnancy Exposure Registries.
MotherToBaby
A large registry, the MotherToBaby Organization of Teratology Information Specialists (OTIS) (877-311-8972), involves patients in several different categories and the effects of the drugs on the embryo-fetus: autoimmune diseases (ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma at less than 20 weeks’ gestation; vaccines; and heterozygous or homozygous familial hypercholesterolemia.
For the autoimmune diseases, the drugs and trade names are abatacept (Orencia), adalimumab (Humira), certolizumab pegol (Cimzia), etanercept (Enbrel), infliximab (Remicade), leflunomide (Arava), otezla (Apremilast), teriflunomide (Aubagio), tocilizumab (Actemra), tofacitinib (Xeljanz), and ustekinumab (Stelara).
For the asthma group, the drug being investigated is mepolizumab (Nucala).
Two vaccines – for tetanus, diphtheria, and pertussis (Tdap) and meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135 (Menveo) – are being studied.
The last category is heterozygous or homozygous familial hypercholesterolemia. The two agents in this category are alirocumab (Praluent) and evolocumab (Repatha).
Other registries
Breast cancer
The Mother Pregnancy Registry, INC Research (800-690-6720), is enrolling breast cancer patients who have been treated during pregnancy with ado-trastuzumab emtansine (Kadcyla), pertuzumab (Perjeta), or trastuzumab (Herceptin).
Epilepsy
The Antiepileptic Drug Pregnancy registry (888-233-2334) is studying eslicarbazepine (Aptiom) and pregabalin (Lyrica).
Fabry disease
The Fabry Registry, Genzyme Corp (617-591-5500) is studying the use in pregnancy of agalsidase beta (Fabrazyme) for Fabry disease.
Fibromyalgia
The Savella Pregnancy Registry (877-643-3010) is looking for patients with fibromyalgia who are being treated with milnacipran (Savella).
Hepatitis B
The Ribavirin Pregnancy Registry, INC Research (800-593-2214) is looking for subjects with hepatitis C who have been treated with ribavirin (Copegus).
Hypercholesterolemia
Lomitapide (Juxtapid) is being studied by the Global Lomitapide Pregnancy Exposure Registry managed by Aegerion (877-902-4099). The drug is used for homozygous familial hypercholesterolemia.
Mucopolysaccharidosis
The Mucopolysaccharidosis I (MPS I) registry, Genzyme (617-591-5500) is studying the use of laronidase (Aldurazyme) for Hurler syndrome, Scheie syndrome, and Hurler-Scheie syndrome.
The use of galsulfase (Naglazyme) for Maroteaux-Lamy syndrome during pregnancy is under study by the Mucopolysaccharidosis VI (MPS VI), clinical surveillance program (BioMarin Pharmaceutical) (415-506-6849 or 415-506-6703).
Multiple sclerosis
Novartis is conducting the Gilenya Pregnancy Registry (877-598-7237) for patients with multiple sclerosis who are taking fingolimod (Gilenya).
Alemtuzumab (Lemtrada), also indicated for multiple sclerosis, is the target agent for the LEMTRADA Pregnancy Exposure Registry (866-758-2990).
Narcolepsy and other sleep disorders
Armodafinil (Nuvigil), used for excessive sleepiness associated with narcolepsy and other sleep disorders, is being studied in the Nuvigil Pregnancy Registry (866-404-4106). A second drug with the same indication and telephone number, modafinil (Provigil), is in the Provigil Pregnancy Registry.
Osteoporosis
Amgen’s Pregnancy Surveillance Program (800-772-6436) is enrolling pregnant subjects with osteoporosis who are being treated with denosumab (Prolia).
Others
Two Merck pregnancy registries (800-986-8999) cover the following conditions: type 2 diabetes sitagliptin+metformin (Janumet) or sitagliptin (Januvia); and migraine headaches rizatriptan (Maxalt).
GlaxoSmithKline is conducting two registries: the Belimumab Pregnancy Registry for patients with systemic lupus erythematosus treated with belimumab (Benlysta) (877-681-6296); and Promacta Pregnancy Registry for women treated for thrombocytopenia with eltrombopag (Promacta) (888-825-5249).
Psychiatric Drugs
The National Pregnancy Registry for Atypical Antipsychotics (866-961-2388) is studying 10 drugs: aripiprazole (Abilify), asenapine (Saphris), clozapine (Clozaril), iloperidone (Fanapt), lurasidone (Latuda), olanzapine (Zyprexa), paliperidone (Invega), quetiapine (Seroquel), risperidone (Risperdal), and ziprasidone (Geodon).
The National Pregnancy Registry for Antidepressants (844-405-6185) is studying amitriptyline (Elavil), amoxapine (Asendin), bupropion (Forfivo XL and Wellbutrin), citalopram (Celexa), clomipramine (Anafranil), desipramine (Norpramin), desvenlafaxine (Prisiq), doxepin (Sinequan), escitalopram (Lexapro), fluvoxamine (Luvox), fluoxetine (Prozac), imipramine (Tofranil), isocarboxazid (Marplan), levomilnacipran (Fetzima), maprotiline (Ludiomil), mirtazapine (Remeron), nefazodone (Serzone), nortriptyline (Pamelor), paroxetine (Paxil), phenelzine (Nardill), protriptyline (Vivactil), selegiline (Emsam), sertraline (Zoloft), tranylcypromine (Pamate), trazodone (Desyrel), trimipramine (Surmontil), venlafaxine (Effexor), and vilazodone (Viibryd).
The National Pregnancy Registry of Psychostimulants (866-961-2388) is studying amphetamine (Adderall), dextroamphetamine (Dexedrine and Focalin), lisdexamfetamine (Vyvanse), methylphenidate (Concerta, Daytrana, Desoxyn, Ritalin), and modafinil (Provigil).
The antidepressant duloxetine (Cymbalta) is being studied by the Cymbalta Pregnancy Registry (866-814-6975).
Transplant patients
Renal transplant patients exposed to mycophenolate (CellCept) can be enrolled in the Transplantation Pregnancy Registry International (877-955-6877) or the Mycophenolate Pregnancy Registry (800-617-8191). The Transplantation Pregnancy Registry International also is enrolling renal transplant patients exposed to belatacept (Nulojix).
Vaccines
A quadrivalent influenza vaccine (Afluria) is being studied by the Seqirus Influenza Vaccine Pregnancy Registry (855-358-8972). A second vaccine for meningococcal disease meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135 (Menactra) is under study by the Menactra Vaccine Pregnancy Registry (800-822-2463). The Bexsero Pregnancy Registry (877-413-4759) is open to patients who have received the meningococcal group B vaccine (Bexsero). The Hepatitis B Vaccine [Recombinant] Adjuvanted Pregnancy Registry, also listed as HEPLISAV-B, is enrolling patients who have received that vaccine (844-443-7734); it is supported by the Dynavax Technologies Corporation.
Because the strength of a registry is based on numbers, health care professionals are encouraged to enroll potential subjects or have their patients call to enroll themselves.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of the human pregnancy experience. However, although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of these registries are their prospective nature (enrolled before the outcome is known) and enrollment over a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions). Registries can identify early signals of teratogenicity, but they have several limitations: selection bias that results from voluntary reporting; target populations that are not representative; lost-to-follow-up pregnancies that may have had different outcomes than those with documented outcomes; elective terminations and fetal deaths without birth defects and spontaneous abortions, all of which may lack details; the lack of control groups (with some exceptions); and the publication of results that may be delayed or not be in a peer-reviewed journal. Because the total number of exposed pregnancies is unknown, the data cannot be used to calculate prevalences, but they can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports (reported after outcome is known). Such reports are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. But they may be helpful in detecting unusual patterns of birth defects.
For the following drugs, web addresses can be obtained from the Food and Drug Administration website, List of Pregnancy Exposure Registries.
MotherToBaby
A large registry, the MotherToBaby Organization of Teratology Information Specialists (OTIS) (877-311-8972), involves patients in several different categories and the effects of the drugs on the embryo-fetus: autoimmune diseases (ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma at less than 20 weeks’ gestation; vaccines; and heterozygous or homozygous familial hypercholesterolemia.
For the autoimmune diseases, the drugs and trade names are abatacept (Orencia), adalimumab (Humira), certolizumab pegol (Cimzia), etanercept (Enbrel), infliximab (Remicade), leflunomide (Arava), otezla (Apremilast), teriflunomide (Aubagio), tocilizumab (Actemra), tofacitinib (Xeljanz), and ustekinumab (Stelara).
For the asthma group, the drug being investigated is mepolizumab (Nucala).
Two vaccines – for tetanus, diphtheria, and pertussis (Tdap) and meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135 (Menveo) – are being studied.
The last category is heterozygous or homozygous familial hypercholesterolemia. The two agents in this category are alirocumab (Praluent) and evolocumab (Repatha).
Other registries
Breast cancer
The Mother Pregnancy Registry, INC Research (800-690-6720), is enrolling breast cancer patients who have been treated during pregnancy with ado-trastuzumab emtansine (Kadcyla), pertuzumab (Perjeta), or trastuzumab (Herceptin).
Epilepsy
The Antiepileptic Drug Pregnancy registry (888-233-2334) is studying eslicarbazepine (Aptiom) and pregabalin (Lyrica).
Fabry disease
The Fabry Registry, Genzyme Corp (617-591-5500) is studying the use in pregnancy of agalsidase beta (Fabrazyme) for Fabry disease.
Fibromyalgia
The Savella Pregnancy Registry (877-643-3010) is looking for patients with fibromyalgia who are being treated with milnacipran (Savella).
Hepatitis B
The Ribavirin Pregnancy Registry, INC Research (800-593-2214) is looking for subjects with hepatitis C who have been treated with ribavirin (Copegus).
Hypercholesterolemia
Lomitapide (Juxtapid) is being studied by the Global Lomitapide Pregnancy Exposure Registry managed by Aegerion (877-902-4099). The drug is used for homozygous familial hypercholesterolemia.
Mucopolysaccharidosis
The Mucopolysaccharidosis I (MPS I) registry, Genzyme (617-591-5500) is studying the use of laronidase (Aldurazyme) for Hurler syndrome, Scheie syndrome, and Hurler-Scheie syndrome.
The use of galsulfase (Naglazyme) for Maroteaux-Lamy syndrome during pregnancy is under study by the Mucopolysaccharidosis VI (MPS VI), clinical surveillance program (BioMarin Pharmaceutical) (415-506-6849 or 415-506-6703).
Multiple sclerosis
Novartis is conducting the Gilenya Pregnancy Registry (877-598-7237) for patients with multiple sclerosis who are taking fingolimod (Gilenya).
Alemtuzumab (Lemtrada), also indicated for multiple sclerosis, is the target agent for the LEMTRADA Pregnancy Exposure Registry (866-758-2990).
Narcolepsy and other sleep disorders
Armodafinil (Nuvigil), used for excessive sleepiness associated with narcolepsy and other sleep disorders, is being studied in the Nuvigil Pregnancy Registry (866-404-4106). A second drug with the same indication and telephone number, modafinil (Provigil), is in the Provigil Pregnancy Registry.
Osteoporosis
Amgen’s Pregnancy Surveillance Program (800-772-6436) is enrolling pregnant subjects with osteoporosis who are being treated with denosumab (Prolia).
Others
Two Merck pregnancy registries (800-986-8999) cover the following conditions: type 2 diabetes sitagliptin+metformin (Janumet) or sitagliptin (Januvia); and migraine headaches rizatriptan (Maxalt).
GlaxoSmithKline is conducting two registries: the Belimumab Pregnancy Registry for patients with systemic lupus erythematosus treated with belimumab (Benlysta) (877-681-6296); and Promacta Pregnancy Registry for women treated for thrombocytopenia with eltrombopag (Promacta) (888-825-5249).
Psychiatric Drugs
The National Pregnancy Registry for Atypical Antipsychotics (866-961-2388) is studying 10 drugs: aripiprazole (Abilify), asenapine (Saphris), clozapine (Clozaril), iloperidone (Fanapt), lurasidone (Latuda), olanzapine (Zyprexa), paliperidone (Invega), quetiapine (Seroquel), risperidone (Risperdal), and ziprasidone (Geodon).
The National Pregnancy Registry for Antidepressants (844-405-6185) is studying amitriptyline (Elavil), amoxapine (Asendin), bupropion (Forfivo XL and Wellbutrin), citalopram (Celexa), clomipramine (Anafranil), desipramine (Norpramin), desvenlafaxine (Prisiq), doxepin (Sinequan), escitalopram (Lexapro), fluvoxamine (Luvox), fluoxetine (Prozac), imipramine (Tofranil), isocarboxazid (Marplan), levomilnacipran (Fetzima), maprotiline (Ludiomil), mirtazapine (Remeron), nefazodone (Serzone), nortriptyline (Pamelor), paroxetine (Paxil), phenelzine (Nardill), protriptyline (Vivactil), selegiline (Emsam), sertraline (Zoloft), tranylcypromine (Pamate), trazodone (Desyrel), trimipramine (Surmontil), venlafaxine (Effexor), and vilazodone (Viibryd).
The National Pregnancy Registry of Psychostimulants (866-961-2388) is studying amphetamine (Adderall), dextroamphetamine (Dexedrine and Focalin), lisdexamfetamine (Vyvanse), methylphenidate (Concerta, Daytrana, Desoxyn, Ritalin), and modafinil (Provigil).
The antidepressant duloxetine (Cymbalta) is being studied by the Cymbalta Pregnancy Registry (866-814-6975).
Transplant patients
Renal transplant patients exposed to mycophenolate (CellCept) can be enrolled in the Transplantation Pregnancy Registry International (877-955-6877) or the Mycophenolate Pregnancy Registry (800-617-8191). The Transplantation Pregnancy Registry International also is enrolling renal transplant patients exposed to belatacept (Nulojix).
Vaccines
A quadrivalent influenza vaccine (Afluria) is being studied by the Seqirus Influenza Vaccine Pregnancy Registry (855-358-8972). A second vaccine for meningococcal disease meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135 (Menactra) is under study by the Menactra Vaccine Pregnancy Registry (800-822-2463). The Bexsero Pregnancy Registry (877-413-4759) is open to patients who have received the meningococcal group B vaccine (Bexsero). The Hepatitis B Vaccine [Recombinant] Adjuvanted Pregnancy Registry, also listed as HEPLISAV-B, is enrolling patients who have received that vaccine (844-443-7734); it is supported by the Dynavax Technologies Corporation.
Because the strength of a registry is based on numbers, health care professionals are encouraged to enroll potential subjects or have their patients call to enroll themselves.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of the human pregnancy experience. However, although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of these registries are their prospective nature (enrolled before the outcome is known) and enrollment over a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions). Registries can identify early signals of teratogenicity, but they have several limitations: selection bias that results from voluntary reporting; target populations that are not representative; lost-to-follow-up pregnancies that may have had different outcomes than those with documented outcomes; elective terminations and fetal deaths without birth defects and spontaneous abortions, all of which may lack details; the lack of control groups (with some exceptions); and the publication of results that may be delayed or not be in a peer-reviewed journal. Because the total number of exposed pregnancies is unknown, the data cannot be used to calculate prevalences, but they can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports (reported after outcome is known). Such reports are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. But they may be helpful in detecting unusual patterns of birth defects.
For the following drugs, web addresses can be obtained from the Food and Drug Administration website, List of Pregnancy Exposure Registries.
MotherToBaby
A large registry, the MotherToBaby Organization of Teratology Information Specialists (OTIS) (877-311-8972), involves patients in several different categories and the effects of the drugs on the embryo-fetus: autoimmune diseases (ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma at less than 20 weeks’ gestation; vaccines; and heterozygous or homozygous familial hypercholesterolemia.
For the autoimmune diseases, the drugs and trade names are abatacept (Orencia), adalimumab (Humira), certolizumab pegol (Cimzia), etanercept (Enbrel), infliximab (Remicade), leflunomide (Arava), otezla (Apremilast), teriflunomide (Aubagio), tocilizumab (Actemra), tofacitinib (Xeljanz), and ustekinumab (Stelara).
For the asthma group, the drug being investigated is mepolizumab (Nucala).
Two vaccines – for tetanus, diphtheria, and pertussis (Tdap) and meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135 (Menveo) – are being studied.
The last category is heterozygous or homozygous familial hypercholesterolemia. The two agents in this category are alirocumab (Praluent) and evolocumab (Repatha).
Other registries
Breast cancer
The Mother Pregnancy Registry, INC Research (800-690-6720), is enrolling breast cancer patients who have been treated during pregnancy with ado-trastuzumab emtansine (Kadcyla), pertuzumab (Perjeta), or trastuzumab (Herceptin).
Epilepsy
The Antiepileptic Drug Pregnancy registry (888-233-2334) is studying eslicarbazepine (Aptiom) and pregabalin (Lyrica).
Fabry disease
The Fabry Registry, Genzyme Corp (617-591-5500) is studying the use in pregnancy of agalsidase beta (Fabrazyme) for Fabry disease.
Fibromyalgia
The Savella Pregnancy Registry (877-643-3010) is looking for patients with fibromyalgia who are being treated with milnacipran (Savella).
Hepatitis B
The Ribavirin Pregnancy Registry, INC Research (800-593-2214) is looking for subjects with hepatitis C who have been treated with ribavirin (Copegus).
Hypercholesterolemia
Lomitapide (Juxtapid) is being studied by the Global Lomitapide Pregnancy Exposure Registry managed by Aegerion (877-902-4099). The drug is used for homozygous familial hypercholesterolemia.
Mucopolysaccharidosis
The Mucopolysaccharidosis I (MPS I) registry, Genzyme (617-591-5500) is studying the use of laronidase (Aldurazyme) for Hurler syndrome, Scheie syndrome, and Hurler-Scheie syndrome.
The use of galsulfase (Naglazyme) for Maroteaux-Lamy syndrome during pregnancy is under study by the Mucopolysaccharidosis VI (MPS VI), clinical surveillance program (BioMarin Pharmaceutical) (415-506-6849 or 415-506-6703).
Multiple sclerosis
Novartis is conducting the Gilenya Pregnancy Registry (877-598-7237) for patients with multiple sclerosis who are taking fingolimod (Gilenya).
Alemtuzumab (Lemtrada), also indicated for multiple sclerosis, is the target agent for the LEMTRADA Pregnancy Exposure Registry (866-758-2990).
Narcolepsy and other sleep disorders
Armodafinil (Nuvigil), used for excessive sleepiness associated with narcolepsy and other sleep disorders, is being studied in the Nuvigil Pregnancy Registry (866-404-4106). A second drug with the same indication and telephone number, modafinil (Provigil), is in the Provigil Pregnancy Registry.
Osteoporosis
Amgen’s Pregnancy Surveillance Program (800-772-6436) is enrolling pregnant subjects with osteoporosis who are being treated with denosumab (Prolia).
Others
Two Merck pregnancy registries (800-986-8999) cover the following conditions: type 2 diabetes sitagliptin+metformin (Janumet) or sitagliptin (Januvia); and migraine headaches rizatriptan (Maxalt).
GlaxoSmithKline is conducting two registries: the Belimumab Pregnancy Registry for patients with systemic lupus erythematosus treated with belimumab (Benlysta) (877-681-6296); and Promacta Pregnancy Registry for women treated for thrombocytopenia with eltrombopag (Promacta) (888-825-5249).
Psychiatric Drugs
The National Pregnancy Registry for Atypical Antipsychotics (866-961-2388) is studying 10 drugs: aripiprazole (Abilify), asenapine (Saphris), clozapine (Clozaril), iloperidone (Fanapt), lurasidone (Latuda), olanzapine (Zyprexa), paliperidone (Invega), quetiapine (Seroquel), risperidone (Risperdal), and ziprasidone (Geodon).
The National Pregnancy Registry for Antidepressants (844-405-6185) is studying amitriptyline (Elavil), amoxapine (Asendin), bupropion (Forfivo XL and Wellbutrin), citalopram (Celexa), clomipramine (Anafranil), desipramine (Norpramin), desvenlafaxine (Prisiq), doxepin (Sinequan), escitalopram (Lexapro), fluvoxamine (Luvox), fluoxetine (Prozac), imipramine (Tofranil), isocarboxazid (Marplan), levomilnacipran (Fetzima), maprotiline (Ludiomil), mirtazapine (Remeron), nefazodone (Serzone), nortriptyline (Pamelor), paroxetine (Paxil), phenelzine (Nardill), protriptyline (Vivactil), selegiline (Emsam), sertraline (Zoloft), tranylcypromine (Pamate), trazodone (Desyrel), trimipramine (Surmontil), venlafaxine (Effexor), and vilazodone (Viibryd).
The National Pregnancy Registry of Psychostimulants (866-961-2388) is studying amphetamine (Adderall), dextroamphetamine (Dexedrine and Focalin), lisdexamfetamine (Vyvanse), methylphenidate (Concerta, Daytrana, Desoxyn, Ritalin), and modafinil (Provigil).
The antidepressant duloxetine (Cymbalta) is being studied by the Cymbalta Pregnancy Registry (866-814-6975).
Transplant patients
Renal transplant patients exposed to mycophenolate (CellCept) can be enrolled in the Transplantation Pregnancy Registry International (877-955-6877) or the Mycophenolate Pregnancy Registry (800-617-8191). The Transplantation Pregnancy Registry International also is enrolling renal transplant patients exposed to belatacept (Nulojix).
Vaccines
A quadrivalent influenza vaccine (Afluria) is being studied by the Seqirus Influenza Vaccine Pregnancy Registry (855-358-8972). A second vaccine for meningococcal disease meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135 (Menactra) is under study by the Menactra Vaccine Pregnancy Registry (800-822-2463). The Bexsero Pregnancy Registry (877-413-4759) is open to patients who have received the meningococcal group B vaccine (Bexsero). The Hepatitis B Vaccine [Recombinant] Adjuvanted Pregnancy Registry, also listed as HEPLISAV-B, is enrolling patients who have received that vaccine (844-443-7734); it is supported by the Dynavax Technologies Corporation.
Because the strength of a registry is based on numbers, health care professionals are encouraged to enroll potential subjects or have their patients call to enroll themselves.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Minilaparoscopy is the next step in minimally invasive surgery
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”
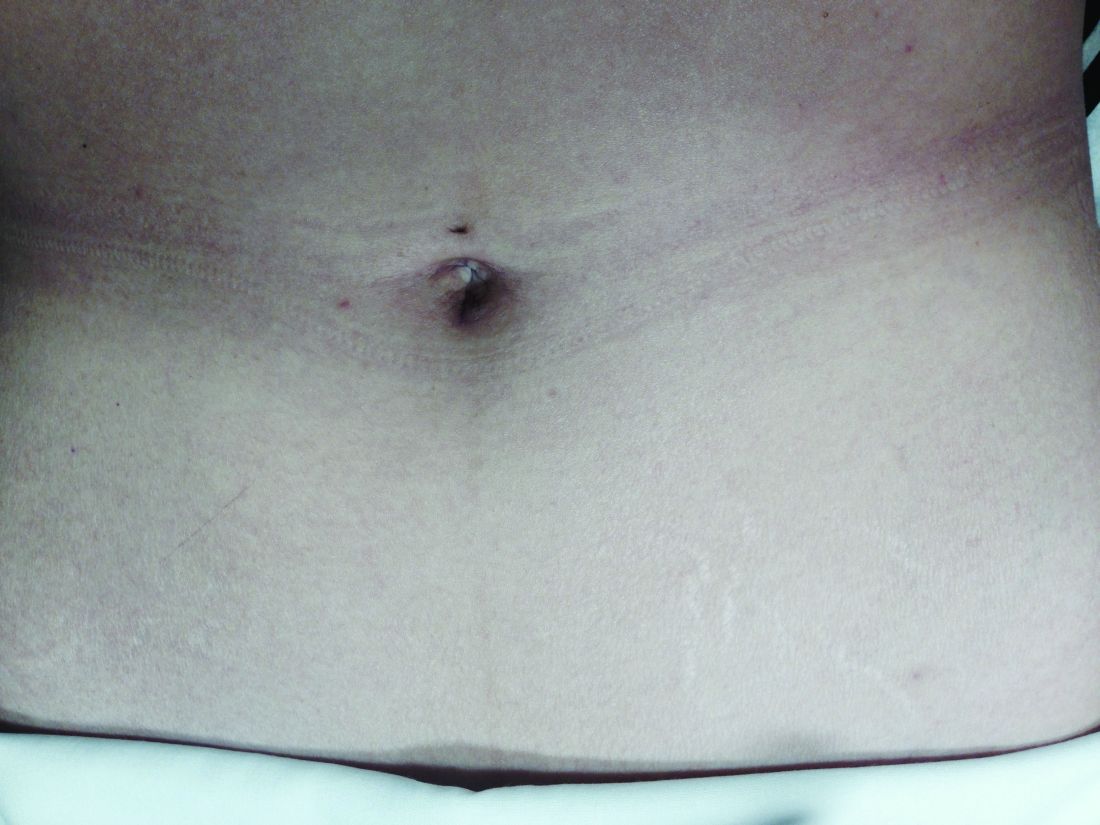
Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”

Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”

Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Full disclosure
I have nothing to disclose.
That is the first line on my second slide in just about every talk I give. I have no financial conflicts of interest. I no longer accept meals from pharmaceutical companies, I no longer conduct pharmaceutical company sponsored research, and I no longer give talks that include honoraria from pharmaceutical companies. I turn down payments from pharmaceutical companies when I participate in drug-monitoring safety boards and advisory committees. I do not have a financial conflict of interest.
Or do I?
In preparing to write this essay, I searched the Open Payments website (www.cms.gov/OpenPayments/index.html) for my name. Open Payments is the product of the Physician Payments Sunshine Act passed in 2010 as part of the Affordable Care Act. The website went live in September 2014 with the intention of making public all payments made to physicians from device and drug makers. I was happy to confirm that I have received no “General Payments,” which are payments for meals, travel, honoraria, consulting, and the like. However, I was surprised to learn that I did receive “Associated Research” payments. According to the website, an Associated Research payment is “funding for a research project or study where the physician is named as a principal investigator.”
I still have a few trials open under my name, but none have accrued a patient in more than 7 years. Nonetheless, I am on record, and publicly so, for accepting an Associated payment for research to the tune of $1,308,360.06.
Upon learning this, my thoughts turned to the New York Times. The Times recently published an expose in cooperation with ProPublica. In it, a prominent cancer researcher at Memorial Sloan Kettering was accused of repeatedly failing to disclose his substantial financial conflicts of interest. The payments creating the conflict were listed on the Open Payments website. Since financial disclosure is almost always required for a manuscript listed in PubMed, a simple comparison of two public websites provided the journalists with nearly all the information they needed to conclude malfeasance in disclosure.
In response, the accused admitted the failure to disclose, but attributed it to an unintentional error. In the frenzy that followed, a man of towering stature, a paragon of cancer research, submitted his resignation. The sequence of events was tragic. Had the payments been for research instead of services rendered would the consequences have been the same?
Most of us believe corporate payments for research are less likely to influence our prescribing and consulting habits than are general payments for entertainment and speaking engagements. I remember receiving my first research grant from the now defunct pharma company Immunex. It was for $10,000 – a paltry sum – but enough for me to set up a clinical trial using Immunex’s drugs. I was flattered, indebted, and conflicted from that point forward. Funded research propels our careers forward. Thinking research payments bias our decision making less than direct payments is naive. Money corrupts, and that is why research dollars need to be disclosed whenever we discuss research at the podium or in print.
With appropriate indignation, I explored the Open Payments website to learn more of my hitherto unknown payment. It was attached to a multicenter, randomized clinical trial for which I served as local principal investigator. The payment was made in January 2017 and our research team cannot verify such a payment was ever received. According to the website, the payment was not disputed. I sought to dispute it.
Our friends at the Centers for Medicare and Medicaid Services do not make filing a dispute easy. I first had to register with my home address and create a new password that, of course, needs to be changed every 60 days. I duly registered and logged into the website as instructed. I followed instructions and filled in data fields for about an additional 10 pages before being informed that I needed to logout, then log back in, to access the Open Payments application. When I did that, I was greeted with instructions to register in the Open Payments system. I then realized that all I had done to that point was register with the CMS.gov portal, not Open Payments. In for a dime, in for a dollar, I registered with Open Payments.
I almost gave up when they asked me to provide a Physician Taxonomy Code. It took me a long time to find it. For those interested, the code for Hematology is 207RH0000X. With that code entered in the right box, I was only two pages away from being registered and ready to open the dispute. Failure hit me like a lake effect snow storm. Despite my diligence, I was not “vetted” and could not file a dispute. I must have done something wrong and cannot seem to investigate the payment further, but I’m sure the New York Times could.
Now, I don’t know if I have anything to disclose or not. I do know that I have to investigate my payment the best I can, that I have to disclose it if it is real, and that I have to check Open Payments every so often to make sure I am not surprised by an investigative journalist’s report in the future. Add these to the pantheon of onerous requirements for a successful academic career.
Many wear their entanglements as a badge of honor on slides highlighting a long list of conflicts. One speaker joked that she had so many conflicts that she had no conflicts. Clearly, much like alarm fatigue, the constant display of financial conflict of interest disclosures rarely raises red flags in an audience of peers. To an audience of interested lay persons, though, those conflicts may be very important and relevant.
It is our duty to accurately account for and report them no matter the difficulty in doing so. Failure to do so can carry tragic consequences.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
I have nothing to disclose.
That is the first line on my second slide in just about every talk I give. I have no financial conflicts of interest. I no longer accept meals from pharmaceutical companies, I no longer conduct pharmaceutical company sponsored research, and I no longer give talks that include honoraria from pharmaceutical companies. I turn down payments from pharmaceutical companies when I participate in drug-monitoring safety boards and advisory committees. I do not have a financial conflict of interest.
Or do I?
In preparing to write this essay, I searched the Open Payments website (www.cms.gov/OpenPayments/index.html) for my name. Open Payments is the product of the Physician Payments Sunshine Act passed in 2010 as part of the Affordable Care Act. The website went live in September 2014 with the intention of making public all payments made to physicians from device and drug makers. I was happy to confirm that I have received no “General Payments,” which are payments for meals, travel, honoraria, consulting, and the like. However, I was surprised to learn that I did receive “Associated Research” payments. According to the website, an Associated Research payment is “funding for a research project or study where the physician is named as a principal investigator.”
I still have a few trials open under my name, but none have accrued a patient in more than 7 years. Nonetheless, I am on record, and publicly so, for accepting an Associated payment for research to the tune of $1,308,360.06.
Upon learning this, my thoughts turned to the New York Times. The Times recently published an expose in cooperation with ProPublica. In it, a prominent cancer researcher at Memorial Sloan Kettering was accused of repeatedly failing to disclose his substantial financial conflicts of interest. The payments creating the conflict were listed on the Open Payments website. Since financial disclosure is almost always required for a manuscript listed in PubMed, a simple comparison of two public websites provided the journalists with nearly all the information they needed to conclude malfeasance in disclosure.
In response, the accused admitted the failure to disclose, but attributed it to an unintentional error. In the frenzy that followed, a man of towering stature, a paragon of cancer research, submitted his resignation. The sequence of events was tragic. Had the payments been for research instead of services rendered would the consequences have been the same?
Most of us believe corporate payments for research are less likely to influence our prescribing and consulting habits than are general payments for entertainment and speaking engagements. I remember receiving my first research grant from the now defunct pharma company Immunex. It was for $10,000 – a paltry sum – but enough for me to set up a clinical trial using Immunex’s drugs. I was flattered, indebted, and conflicted from that point forward. Funded research propels our careers forward. Thinking research payments bias our decision making less than direct payments is naive. Money corrupts, and that is why research dollars need to be disclosed whenever we discuss research at the podium or in print.
With appropriate indignation, I explored the Open Payments website to learn more of my hitherto unknown payment. It was attached to a multicenter, randomized clinical trial for which I served as local principal investigator. The payment was made in January 2017 and our research team cannot verify such a payment was ever received. According to the website, the payment was not disputed. I sought to dispute it.
Our friends at the Centers for Medicare and Medicaid Services do not make filing a dispute easy. I first had to register with my home address and create a new password that, of course, needs to be changed every 60 days. I duly registered and logged into the website as instructed. I followed instructions and filled in data fields for about an additional 10 pages before being informed that I needed to logout, then log back in, to access the Open Payments application. When I did that, I was greeted with instructions to register in the Open Payments system. I then realized that all I had done to that point was register with the CMS.gov portal, not Open Payments. In for a dime, in for a dollar, I registered with Open Payments.
I almost gave up when they asked me to provide a Physician Taxonomy Code. It took me a long time to find it. For those interested, the code for Hematology is 207RH0000X. With that code entered in the right box, I was only two pages away from being registered and ready to open the dispute. Failure hit me like a lake effect snow storm. Despite my diligence, I was not “vetted” and could not file a dispute. I must have done something wrong and cannot seem to investigate the payment further, but I’m sure the New York Times could.
Now, I don’t know if I have anything to disclose or not. I do know that I have to investigate my payment the best I can, that I have to disclose it if it is real, and that I have to check Open Payments every so often to make sure I am not surprised by an investigative journalist’s report in the future. Add these to the pantheon of onerous requirements for a successful academic career.
Many wear their entanglements as a badge of honor on slides highlighting a long list of conflicts. One speaker joked that she had so many conflicts that she had no conflicts. Clearly, much like alarm fatigue, the constant display of financial conflict of interest disclosures rarely raises red flags in an audience of peers. To an audience of interested lay persons, though, those conflicts may be very important and relevant.
It is our duty to accurately account for and report them no matter the difficulty in doing so. Failure to do so can carry tragic consequences.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
I have nothing to disclose.
That is the first line on my second slide in just about every talk I give. I have no financial conflicts of interest. I no longer accept meals from pharmaceutical companies, I no longer conduct pharmaceutical company sponsored research, and I no longer give talks that include honoraria from pharmaceutical companies. I turn down payments from pharmaceutical companies when I participate in drug-monitoring safety boards and advisory committees. I do not have a financial conflict of interest.
Or do I?
In preparing to write this essay, I searched the Open Payments website (www.cms.gov/OpenPayments/index.html) for my name. Open Payments is the product of the Physician Payments Sunshine Act passed in 2010 as part of the Affordable Care Act. The website went live in September 2014 with the intention of making public all payments made to physicians from device and drug makers. I was happy to confirm that I have received no “General Payments,” which are payments for meals, travel, honoraria, consulting, and the like. However, I was surprised to learn that I did receive “Associated Research” payments. According to the website, an Associated Research payment is “funding for a research project or study where the physician is named as a principal investigator.”
I still have a few trials open under my name, but none have accrued a patient in more than 7 years. Nonetheless, I am on record, and publicly so, for accepting an Associated payment for research to the tune of $1,308,360.06.
Upon learning this, my thoughts turned to the New York Times. The Times recently published an expose in cooperation with ProPublica. In it, a prominent cancer researcher at Memorial Sloan Kettering was accused of repeatedly failing to disclose his substantial financial conflicts of interest. The payments creating the conflict were listed on the Open Payments website. Since financial disclosure is almost always required for a manuscript listed in PubMed, a simple comparison of two public websites provided the journalists with nearly all the information they needed to conclude malfeasance in disclosure.
In response, the accused admitted the failure to disclose, but attributed it to an unintentional error. In the frenzy that followed, a man of towering stature, a paragon of cancer research, submitted his resignation. The sequence of events was tragic. Had the payments been for research instead of services rendered would the consequences have been the same?
Most of us believe corporate payments for research are less likely to influence our prescribing and consulting habits than are general payments for entertainment and speaking engagements. I remember receiving my first research grant from the now defunct pharma company Immunex. It was for $10,000 – a paltry sum – but enough for me to set up a clinical trial using Immunex’s drugs. I was flattered, indebted, and conflicted from that point forward. Funded research propels our careers forward. Thinking research payments bias our decision making less than direct payments is naive. Money corrupts, and that is why research dollars need to be disclosed whenever we discuss research at the podium or in print.
With appropriate indignation, I explored the Open Payments website to learn more of my hitherto unknown payment. It was attached to a multicenter, randomized clinical trial for which I served as local principal investigator. The payment was made in January 2017 and our research team cannot verify such a payment was ever received. According to the website, the payment was not disputed. I sought to dispute it.
Our friends at the Centers for Medicare and Medicaid Services do not make filing a dispute easy. I first had to register with my home address and create a new password that, of course, needs to be changed every 60 days. I duly registered and logged into the website as instructed. I followed instructions and filled in data fields for about an additional 10 pages before being informed that I needed to logout, then log back in, to access the Open Payments application. When I did that, I was greeted with instructions to register in the Open Payments system. I then realized that all I had done to that point was register with the CMS.gov portal, not Open Payments. In for a dime, in for a dollar, I registered with Open Payments.
I almost gave up when they asked me to provide a Physician Taxonomy Code. It took me a long time to find it. For those interested, the code for Hematology is 207RH0000X. With that code entered in the right box, I was only two pages away from being registered and ready to open the dispute. Failure hit me like a lake effect snow storm. Despite my diligence, I was not “vetted” and could not file a dispute. I must have done something wrong and cannot seem to investigate the payment further, but I’m sure the New York Times could.
Now, I don’t know if I have anything to disclose or not. I do know that I have to investigate my payment the best I can, that I have to disclose it if it is real, and that I have to check Open Payments every so often to make sure I am not surprised by an investigative journalist’s report in the future. Add these to the pantheon of onerous requirements for a successful academic career.
Many wear their entanglements as a badge of honor on slides highlighting a long list of conflicts. One speaker joked that she had so many conflicts that she had no conflicts. Clearly, much like alarm fatigue, the constant display of financial conflict of interest disclosures rarely raises red flags in an audience of peers. To an audience of interested lay persons, though, those conflicts may be very important and relevant.
It is our duty to accurately account for and report them no matter the difficulty in doing so. Failure to do so can carry tragic consequences.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Produce and promises
Most of us are in medicine because we find joy and fulfillment in treating patients. That’s why we signed up for the long educational slog, and why many of us continue to practice medicine long after all the bills have been paid. That is why we all find obstructions between us and our patients so maddening.
I guess Medicare was a great boon for seniors, who found health insurance increasingly more difficult to afford, and for doctors, who now got paid in something other than produce and promises by indigent, elderly patients. The American Medical Association opposed the adoption of Medicare, fearing that it would interfere with the physician-patient relationship. This may sound quaint now, especially at a time when there are calls for Medicare for all. While it is hard to argue against Medicare improving access to health care, the AMA was right about the government’s intrusion into the physician-patient relationship, which has become progressively more intrusive. Medicare has undergone major revisions at least five times; none of these revisions has simplified care. Think about the steadily increasing documentation requirements, audits, inflation-ravaged fee schedules, and MIPS [Merit-Based Incentive Payment System], and MACRA [Medicare Access and CHIP Reauthorization Act of 2015], although the current proposed Medicare rule, with a two-level fee schedule and reduced documentation, claims to eliminate 50 hours of charting per year.
The next big blow was ERISA (the Employee Retirement Income Security Act of 1974), which really did not seem relevant to medical practice at the time. However, embedded in this law was indemnification of insurers from patient lawsuits. Well, OK, insurers don’t practice medicine, right? Fast-forward to today, when critical medical decisions, including which test can be ordered and which drug can be administered, are driven by insurers – who can delay or refuse care and who cannot be legally blamed for the death or harm of the patient. That’s right, step therapy and prior authorizations would not be possible without ERISA.
Of course, absolutely the most onerous intrusion on the physician-patient relationship is the American Recovery and Reinvestment Act of 2014, which mandated electronic health records. I believe this is the major cause of current physician burnout, which has created the worst and most intrusive barrier between physicians and patients to date. Talk about good intentions gone awry!
In addition, now private equity has entered into medicine, in part in response to these issues and intrusions. But has this improved the patient-physician relationship, or just made things worse?
A big selling point of these private equity–backed groups is the central handling of administrative issues, such as billing, coding, compliance, human resources, prior authorizations, as well as other back-office functions. Some groups even claim to improve patient care and value, by instituting quality metrics for care (I would love to see these published). These services all must be paid for, and the logical argument is that pooling these services will result in efficiency and cost less overall.
Maybe so, but private equity creates yet another barrier between the patient and the physician while it eliminate others. These businesses are driven by profit; they are private equity after all. They are a more insidious threat to the physician-patient relationship and the future of medicine than are clumsy laws, since private equity commoditizes patients and their care. .
Any barrier between the patient and the physician is bad, and two or three barriers make things logarithmically worse. No wonder physicians have become cynical and disillusioned. It makes you pause and wonder, how much do we currently pay in time and overhead to navigate these barriers? Maybe we should call it all even. Maybe we would come out ahead if we counted in produce, promises, and unobstructed patient care.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Most of us are in medicine because we find joy and fulfillment in treating patients. That’s why we signed up for the long educational slog, and why many of us continue to practice medicine long after all the bills have been paid. That is why we all find obstructions between us and our patients so maddening.
I guess Medicare was a great boon for seniors, who found health insurance increasingly more difficult to afford, and for doctors, who now got paid in something other than produce and promises by indigent, elderly patients. The American Medical Association opposed the adoption of Medicare, fearing that it would interfere with the physician-patient relationship. This may sound quaint now, especially at a time when there are calls for Medicare for all. While it is hard to argue against Medicare improving access to health care, the AMA was right about the government’s intrusion into the physician-patient relationship, which has become progressively more intrusive. Medicare has undergone major revisions at least five times; none of these revisions has simplified care. Think about the steadily increasing documentation requirements, audits, inflation-ravaged fee schedules, and MIPS [Merit-Based Incentive Payment System], and MACRA [Medicare Access and CHIP Reauthorization Act of 2015], although the current proposed Medicare rule, with a two-level fee schedule and reduced documentation, claims to eliminate 50 hours of charting per year.
The next big blow was ERISA (the Employee Retirement Income Security Act of 1974), which really did not seem relevant to medical practice at the time. However, embedded in this law was indemnification of insurers from patient lawsuits. Well, OK, insurers don’t practice medicine, right? Fast-forward to today, when critical medical decisions, including which test can be ordered and which drug can be administered, are driven by insurers – who can delay or refuse care and who cannot be legally blamed for the death or harm of the patient. That’s right, step therapy and prior authorizations would not be possible without ERISA.
Of course, absolutely the most onerous intrusion on the physician-patient relationship is the American Recovery and Reinvestment Act of 2014, which mandated electronic health records. I believe this is the major cause of current physician burnout, which has created the worst and most intrusive barrier between physicians and patients to date. Talk about good intentions gone awry!
In addition, now private equity has entered into medicine, in part in response to these issues and intrusions. But has this improved the patient-physician relationship, or just made things worse?
A big selling point of these private equity–backed groups is the central handling of administrative issues, such as billing, coding, compliance, human resources, prior authorizations, as well as other back-office functions. Some groups even claim to improve patient care and value, by instituting quality metrics for care (I would love to see these published). These services all must be paid for, and the logical argument is that pooling these services will result in efficiency and cost less overall.
Maybe so, but private equity creates yet another barrier between the patient and the physician while it eliminate others. These businesses are driven by profit; they are private equity after all. They are a more insidious threat to the physician-patient relationship and the future of medicine than are clumsy laws, since private equity commoditizes patients and their care. .
Any barrier between the patient and the physician is bad, and two or three barriers make things logarithmically worse. No wonder physicians have become cynical and disillusioned. It makes you pause and wonder, how much do we currently pay in time and overhead to navigate these barriers? Maybe we should call it all even. Maybe we would come out ahead if we counted in produce, promises, and unobstructed patient care.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Most of us are in medicine because we find joy and fulfillment in treating patients. That’s why we signed up for the long educational slog, and why many of us continue to practice medicine long after all the bills have been paid. That is why we all find obstructions between us and our patients so maddening.
I guess Medicare was a great boon for seniors, who found health insurance increasingly more difficult to afford, and for doctors, who now got paid in something other than produce and promises by indigent, elderly patients. The American Medical Association opposed the adoption of Medicare, fearing that it would interfere with the physician-patient relationship. This may sound quaint now, especially at a time when there are calls for Medicare for all. While it is hard to argue against Medicare improving access to health care, the AMA was right about the government’s intrusion into the physician-patient relationship, which has become progressively more intrusive. Medicare has undergone major revisions at least five times; none of these revisions has simplified care. Think about the steadily increasing documentation requirements, audits, inflation-ravaged fee schedules, and MIPS [Merit-Based Incentive Payment System], and MACRA [Medicare Access and CHIP Reauthorization Act of 2015], although the current proposed Medicare rule, with a two-level fee schedule and reduced documentation, claims to eliminate 50 hours of charting per year.
The next big blow was ERISA (the Employee Retirement Income Security Act of 1974), which really did not seem relevant to medical practice at the time. However, embedded in this law was indemnification of insurers from patient lawsuits. Well, OK, insurers don’t practice medicine, right? Fast-forward to today, when critical medical decisions, including which test can be ordered and which drug can be administered, are driven by insurers – who can delay or refuse care and who cannot be legally blamed for the death or harm of the patient. That’s right, step therapy and prior authorizations would not be possible without ERISA.
Of course, absolutely the most onerous intrusion on the physician-patient relationship is the American Recovery and Reinvestment Act of 2014, which mandated electronic health records. I believe this is the major cause of current physician burnout, which has created the worst and most intrusive barrier between physicians and patients to date. Talk about good intentions gone awry!
In addition, now private equity has entered into medicine, in part in response to these issues and intrusions. But has this improved the patient-physician relationship, or just made things worse?
A big selling point of these private equity–backed groups is the central handling of administrative issues, such as billing, coding, compliance, human resources, prior authorizations, as well as other back-office functions. Some groups even claim to improve patient care and value, by instituting quality metrics for care (I would love to see these published). These services all must be paid for, and the logical argument is that pooling these services will result in efficiency and cost less overall.
Maybe so, but private equity creates yet another barrier between the patient and the physician while it eliminate others. These businesses are driven by profit; they are private equity after all. They are a more insidious threat to the physician-patient relationship and the future of medicine than are clumsy laws, since private equity commoditizes patients and their care. .
Any barrier between the patient and the physician is bad, and two or three barriers make things logarithmically worse. No wonder physicians have become cynical and disillusioned. It makes you pause and wonder, how much do we currently pay in time and overhead to navigate these barriers? Maybe we should call it all even. Maybe we would come out ahead if we counted in produce, promises, and unobstructed patient care.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Vaginal intraepithelial neoplasia: What to do when dysplasia persists after hysterectomy
Vaginal intraepithelial neoplasia (VAIN) is a condition that frequently poses therapeutic dilemmas for gynecologists. VAIN represents dysplastic changes to the epithelium of the vaginal mucosa, and like cervical neoplasia, the extent of disease is characterized as levels I, II, or III dependent upon the depth of involvement in the epithelial layer by dysplastic cells. While VAIN itself typically is asymptomatic and not a harmful condition, it carries a 12% risk of progression to invasive vaginal carcinoma, so accurate identification, thorough treatment, and ongoing surveillance are essential.1
VAIN is associated with high-risk human papillomavirus (HPV) infection, tobacco use, and prior cervical dysplasia. Of women with VAIN, 65% have undergone a prior hysterectomy for cervical dysplasia, which emphasizes the nondefinitive nature of such an intervention.2 These women should be very closely followed for at least 20 years with vaginal cytologic and/or HPV surveillance. High-risk HPV infection is present in 85% of women with VAIN, and the presence of high-risk HPV is a predictor for recurrent VAIN. Recurrent and persistent VAIN also is more common in postmenopausal women and those with multifocal disease.
The most common location for VAIN is at the upper third of the vagina (including the vaginal cuff). It commonly arises within the vaginal fornices, which may be difficult to fully visualize because of their puckered appearance, redundant vaginal tissues, and extensive vaginal rogation.
A diagnosis of VAIN is typically obtained from vaginal cytology which reveals atypical or dysplastic cells. Such a result should prompt the physician to perform vaginal colposcopy and directed biopsies. Comprehensive visualization of the vaginal cuff can be limited in cases where the vaginal fornices are tethered, deeply puckered, or when there is significant mucosal rogation.
The application of 4% acetic acid or Lugol’s iodine are techniques that can enhance the detection of dysplastic vaginal mucosa. Lugol’s iodine selectively stains normal, glycogenated cells, and spares dysplastic glycogen-free cells. The sharp contrast between the brown iodine-stained tissues and the white dysplastic tissues aids in detection of dysplastic areas.
If colposcopic biopsy reveals low grade dysplasia (VAIN I) it does not require intervention, and has a very low rate of conversion to invasive vaginal carcinoma. However moderate- and high-grade vaginal dysplastic lesions should be treated because of the potential for malignant transformation.
Options for treatment of VAIN include topical, ablative, and excisional procedures. Observation also is an option but should be reserved for patients who are closely monitored with repeated colposcopic examinations, and probably should best be reserved for patients with VAIN I or II lesions.
Excisional procedures
The most common excisional procedure employed for VAIN is upper vaginectomy. In this procedure, the surgeon grasps and tents up the vaginal mucosa, incises the mucosa without penetrating the subepithelial tissue layers such as bladder and rectum. The vaginal mucosa then is carefully separated from the underlying endopelvic fascial plane. The specimen should be oriented, ideally on a cork board, with pins or sutures to ascribe margins and borders. Excision is best utilized for women with unifocal disease, or those who fail or do not tolerate ablative or topical interventions.
The most significant risks of excision include the potential for damage to underlying pelvic visceral structures, which is particularly concerning in postmenopausal women with thin vaginal epithelium. Vaginectomy is commonly associated with vaginal shortening or narrowing, which can be deleterious for quality of life. Retrospective series have described a 30% incidence of recurrence after vaginectomy, likely secondary to incomplete excision of all affected tissue.3
Ablation
Ablation of dysplastic foci with a carbon dioxide (CO2) laser is a common method for treatment of VAIN. CO2 laser should ablate tissue to a 1.5 mm minimum depth.3 The benefit of using CO2 laser is its ability to treat multifocal disease in situ without an extensive excisional procedure.
It is technically more straightforward than upper vaginectomy with less blood loss and shorter surgical times, and it can be easily accomplished in an outpatient surgical or office setting. However, one of its greatest limitations is the difficulty in visualizing all lesions and therefore adequately treating all sites. The vaginal rogations also make adequate laser ablation challenging because laser only is able to effectively ablate tissue that is oriented perpendicular to the laser beam.
In addition, there is no pathologic confirmation of adequacy of excision or margin status. These features may contribute to the modestly higher rates of recurrence of dysplasia following laser ablation, compared with vaginectomy.3 It also has been associated with more vaginal scarring than vaginectomy, which can have a negative effect on sexual health.
Topical agents
The most commonly utilized topical therapy for VAIN is the antimetabolite chemotherapeutic agent 5-fluorouracil (5FU). A typical schedule for 5FU treatment is to apply vaginally, at night, once a week for 8 weeks.4 Because it can cause extensive irritation to the vulvar and urethral epithelium, patients are recommended to apply barrier creams or ointments before and following the use of 5FU for several days, wash hands thoroughly after application, and to rinse and shower in the morning after rising. Severe irritation occurs in up to 16% of patients, but in general it is very well tolerated.

Its virtue is that it is able to conform and travel to all parts of the vaginal mucosa, including those that are poorly visualized within the fornices or vaginal folds. 5FU does not require a hospitalization or surgical procedure, can be applied by the patient at home, and preserves vaginal length and function. In recent reports, 5FU is associated with the lowest rates of recurrence (10%-30%), compared with excision or ablation, and therefore is a very attractive option for primary therapy.3 However, it requires patients to have a degree of comfort with vaginal application of drug and adherence with perineal care strategies to minimize the likelihood of toxicity.
The immune response modifier, imiquimod, that is commonly used in the treatment of vulvar dysplasia also has been described in the treatment of VAIN. It appears to have high rates of clearance (greater than 75%) and be most effective in the treatment of VAIN I.5 It requires application under colposcopic guidance three times a week for 8 weeks, which is a laborious undertaking for both patient and physician. Like 5FU, imiquimod is associated with vulvar and perineal irritation.
Vaginal estrogens are an alternative topical therapy for moderate- and high-grade VAIN and particularly useful for postmenopausal patients. They have been associated with a high rate (up to 90%) of resolution on follow-up vaginal cytology testing and are not associated with toxicities of the above stated therapies.6 Vaginal estrogen can be used alone or in addition to other therapeutic strategies. For example, it can be added to the nontreatment days of 5FU or postoperatively prescribed following laser or excisional procedures.
Radiation
Intracavitary brachytherapy is a technique in which a radiation source is placed within a cylinder or ovoids and placed within the vagina.7 Typically 45 Gy is delivered to a depth 0.5mm below the vaginal mucosal surface (“point z”). Recurrence occurs is approximately 10%-15% of patients, and toxicities can be severe, including vaginal stenosis and ulceration. This aggressive therapy typically is best reserved for cases that are refractory to other therapies. Following radiation, subsequent treatments are more difficult because of radiation-induced changes to the vaginal mucosa that can affect healing.
Vaginal dysplasia is a relatively common sequelae of high-risk HPV, particularly among women who have had a prior hysterectomy for cervical dysplasia. Because of anatomic changes following hysterectomy, adequate visualization and comprehensive vaginal treatment is difficult. Therefore, surgeons should avoid utilization of hysterectomy as a routine strategy to “cure” dysplasia as it may fail to achieve this cure and make subsequent evaluations and treatments of persistent dysplasia more difficult. Women who have had a hysterectomy for dysplasia should be closely followed for several decades, and they should be counseled that they have a persistent risk for vaginal disease. When VAIN develops, clinicians should consider topical therapies as primary treatment options because they may minimize toxicity and have high rates of enduring response.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant conflicts of interest.
References
1. Gynecol Oncol. 2016 Jun;141(3):507-10.
2. Arch Gynecol Obstet. 2016 Feb;293(2):415-9.
3. Anticancer Res. 2013 Jan;33(1):29-38.
4. Obstet Gynecol. 2017 Dec;130(6):1237-43.
5. Eur J Obstet Gynecol Reprod Biol. 2017 Nov;218:129-36.
6. J Low Genit Tract Dis. 2014 Apr;18(2):115-21.
7. Gynecol Oncol. 2007 Jul;106(1):105-11.
Vaginal intraepithelial neoplasia (VAIN) is a condition that frequently poses therapeutic dilemmas for gynecologists. VAIN represents dysplastic changes to the epithelium of the vaginal mucosa, and like cervical neoplasia, the extent of disease is characterized as levels I, II, or III dependent upon the depth of involvement in the epithelial layer by dysplastic cells. While VAIN itself typically is asymptomatic and not a harmful condition, it carries a 12% risk of progression to invasive vaginal carcinoma, so accurate identification, thorough treatment, and ongoing surveillance are essential.1
VAIN is associated with high-risk human papillomavirus (HPV) infection, tobacco use, and prior cervical dysplasia. Of women with VAIN, 65% have undergone a prior hysterectomy for cervical dysplasia, which emphasizes the nondefinitive nature of such an intervention.2 These women should be very closely followed for at least 20 years with vaginal cytologic and/or HPV surveillance. High-risk HPV infection is present in 85% of women with VAIN, and the presence of high-risk HPV is a predictor for recurrent VAIN. Recurrent and persistent VAIN also is more common in postmenopausal women and those with multifocal disease.
The most common location for VAIN is at the upper third of the vagina (including the vaginal cuff). It commonly arises within the vaginal fornices, which may be difficult to fully visualize because of their puckered appearance, redundant vaginal tissues, and extensive vaginal rogation.
A diagnosis of VAIN is typically obtained from vaginal cytology which reveals atypical or dysplastic cells. Such a result should prompt the physician to perform vaginal colposcopy and directed biopsies. Comprehensive visualization of the vaginal cuff can be limited in cases where the vaginal fornices are tethered, deeply puckered, or when there is significant mucosal rogation.
The application of 4% acetic acid or Lugol’s iodine are techniques that can enhance the detection of dysplastic vaginal mucosa. Lugol’s iodine selectively stains normal, glycogenated cells, and spares dysplastic glycogen-free cells. The sharp contrast between the brown iodine-stained tissues and the white dysplastic tissues aids in detection of dysplastic areas.
If colposcopic biopsy reveals low grade dysplasia (VAIN I) it does not require intervention, and has a very low rate of conversion to invasive vaginal carcinoma. However moderate- and high-grade vaginal dysplastic lesions should be treated because of the potential for malignant transformation.
Options for treatment of VAIN include topical, ablative, and excisional procedures. Observation also is an option but should be reserved for patients who are closely monitored with repeated colposcopic examinations, and probably should best be reserved for patients with VAIN I or II lesions.
Excisional procedures
The most common excisional procedure employed for VAIN is upper vaginectomy. In this procedure, the surgeon grasps and tents up the vaginal mucosa, incises the mucosa without penetrating the subepithelial tissue layers such as bladder and rectum. The vaginal mucosa then is carefully separated from the underlying endopelvic fascial plane. The specimen should be oriented, ideally on a cork board, with pins or sutures to ascribe margins and borders. Excision is best utilized for women with unifocal disease, or those who fail or do not tolerate ablative or topical interventions.
The most significant risks of excision include the potential for damage to underlying pelvic visceral structures, which is particularly concerning in postmenopausal women with thin vaginal epithelium. Vaginectomy is commonly associated with vaginal shortening or narrowing, which can be deleterious for quality of life. Retrospective series have described a 30% incidence of recurrence after vaginectomy, likely secondary to incomplete excision of all affected tissue.3
Ablation
Ablation of dysplastic foci with a carbon dioxide (CO2) laser is a common method for treatment of VAIN. CO2 laser should ablate tissue to a 1.5 mm minimum depth.3 The benefit of using CO2 laser is its ability to treat multifocal disease in situ without an extensive excisional procedure.
It is technically more straightforward than upper vaginectomy with less blood loss and shorter surgical times, and it can be easily accomplished in an outpatient surgical or office setting. However, one of its greatest limitations is the difficulty in visualizing all lesions and therefore adequately treating all sites. The vaginal rogations also make adequate laser ablation challenging because laser only is able to effectively ablate tissue that is oriented perpendicular to the laser beam.
In addition, there is no pathologic confirmation of adequacy of excision or margin status. These features may contribute to the modestly higher rates of recurrence of dysplasia following laser ablation, compared with vaginectomy.3 It also has been associated with more vaginal scarring than vaginectomy, which can have a negative effect on sexual health.
Topical agents
The most commonly utilized topical therapy for VAIN is the antimetabolite chemotherapeutic agent 5-fluorouracil (5FU). A typical schedule for 5FU treatment is to apply vaginally, at night, once a week for 8 weeks.4 Because it can cause extensive irritation to the vulvar and urethral epithelium, patients are recommended to apply barrier creams or ointments before and following the use of 5FU for several days, wash hands thoroughly after application, and to rinse and shower in the morning after rising. Severe irritation occurs in up to 16% of patients, but in general it is very well tolerated.

Its virtue is that it is able to conform and travel to all parts of the vaginal mucosa, including those that are poorly visualized within the fornices or vaginal folds. 5FU does not require a hospitalization or surgical procedure, can be applied by the patient at home, and preserves vaginal length and function. In recent reports, 5FU is associated with the lowest rates of recurrence (10%-30%), compared with excision or ablation, and therefore is a very attractive option for primary therapy.3 However, it requires patients to have a degree of comfort with vaginal application of drug and adherence with perineal care strategies to minimize the likelihood of toxicity.
The immune response modifier, imiquimod, that is commonly used in the treatment of vulvar dysplasia also has been described in the treatment of VAIN. It appears to have high rates of clearance (greater than 75%) and be most effective in the treatment of VAIN I.5 It requires application under colposcopic guidance three times a week for 8 weeks, which is a laborious undertaking for both patient and physician. Like 5FU, imiquimod is associated with vulvar and perineal irritation.
Vaginal estrogens are an alternative topical therapy for moderate- and high-grade VAIN and particularly useful for postmenopausal patients. They have been associated with a high rate (up to 90%) of resolution on follow-up vaginal cytology testing and are not associated with toxicities of the above stated therapies.6 Vaginal estrogen can be used alone or in addition to other therapeutic strategies. For example, it can be added to the nontreatment days of 5FU or postoperatively prescribed following laser or excisional procedures.
Radiation
Intracavitary brachytherapy is a technique in which a radiation source is placed within a cylinder or ovoids and placed within the vagina.7 Typically 45 Gy is delivered to a depth 0.5mm below the vaginal mucosal surface (“point z”). Recurrence occurs is approximately 10%-15% of patients, and toxicities can be severe, including vaginal stenosis and ulceration. This aggressive therapy typically is best reserved for cases that are refractory to other therapies. Following radiation, subsequent treatments are more difficult because of radiation-induced changes to the vaginal mucosa that can affect healing.
Vaginal dysplasia is a relatively common sequelae of high-risk HPV, particularly among women who have had a prior hysterectomy for cervical dysplasia. Because of anatomic changes following hysterectomy, adequate visualization and comprehensive vaginal treatment is difficult. Therefore, surgeons should avoid utilization of hysterectomy as a routine strategy to “cure” dysplasia as it may fail to achieve this cure and make subsequent evaluations and treatments of persistent dysplasia more difficult. Women who have had a hysterectomy for dysplasia should be closely followed for several decades, and they should be counseled that they have a persistent risk for vaginal disease. When VAIN develops, clinicians should consider topical therapies as primary treatment options because they may minimize toxicity and have high rates of enduring response.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant conflicts of interest.
References
1. Gynecol Oncol. 2016 Jun;141(3):507-10.
2. Arch Gynecol Obstet. 2016 Feb;293(2):415-9.
3. Anticancer Res. 2013 Jan;33(1):29-38.
4. Obstet Gynecol. 2017 Dec;130(6):1237-43.
5. Eur J Obstet Gynecol Reprod Biol. 2017 Nov;218:129-36.
6. J Low Genit Tract Dis. 2014 Apr;18(2):115-21.
7. Gynecol Oncol. 2007 Jul;106(1):105-11.
Vaginal intraepithelial neoplasia (VAIN) is a condition that frequently poses therapeutic dilemmas for gynecologists. VAIN represents dysplastic changes to the epithelium of the vaginal mucosa, and like cervical neoplasia, the extent of disease is characterized as levels I, II, or III dependent upon the depth of involvement in the epithelial layer by dysplastic cells. While VAIN itself typically is asymptomatic and not a harmful condition, it carries a 12% risk of progression to invasive vaginal carcinoma, so accurate identification, thorough treatment, and ongoing surveillance are essential.1
VAIN is associated with high-risk human papillomavirus (HPV) infection, tobacco use, and prior cervical dysplasia. Of women with VAIN, 65% have undergone a prior hysterectomy for cervical dysplasia, which emphasizes the nondefinitive nature of such an intervention.2 These women should be very closely followed for at least 20 years with vaginal cytologic and/or HPV surveillance. High-risk HPV infection is present in 85% of women with VAIN, and the presence of high-risk HPV is a predictor for recurrent VAIN. Recurrent and persistent VAIN also is more common in postmenopausal women and those with multifocal disease.
The most common location for VAIN is at the upper third of the vagina (including the vaginal cuff). It commonly arises within the vaginal fornices, which may be difficult to fully visualize because of their puckered appearance, redundant vaginal tissues, and extensive vaginal rogation.
A diagnosis of VAIN is typically obtained from vaginal cytology which reveals atypical or dysplastic cells. Such a result should prompt the physician to perform vaginal colposcopy and directed biopsies. Comprehensive visualization of the vaginal cuff can be limited in cases where the vaginal fornices are tethered, deeply puckered, or when there is significant mucosal rogation.
The application of 4% acetic acid or Lugol’s iodine are techniques that can enhance the detection of dysplastic vaginal mucosa. Lugol’s iodine selectively stains normal, glycogenated cells, and spares dysplastic glycogen-free cells. The sharp contrast between the brown iodine-stained tissues and the white dysplastic tissues aids in detection of dysplastic areas.
If colposcopic biopsy reveals low grade dysplasia (VAIN I) it does not require intervention, and has a very low rate of conversion to invasive vaginal carcinoma. However moderate- and high-grade vaginal dysplastic lesions should be treated because of the potential for malignant transformation.
Options for treatment of VAIN include topical, ablative, and excisional procedures. Observation also is an option but should be reserved for patients who are closely monitored with repeated colposcopic examinations, and probably should best be reserved for patients with VAIN I or II lesions.
Excisional procedures
The most common excisional procedure employed for VAIN is upper vaginectomy. In this procedure, the surgeon grasps and tents up the vaginal mucosa, incises the mucosa without penetrating the subepithelial tissue layers such as bladder and rectum. The vaginal mucosa then is carefully separated from the underlying endopelvic fascial plane. The specimen should be oriented, ideally on a cork board, with pins or sutures to ascribe margins and borders. Excision is best utilized for women with unifocal disease, or those who fail or do not tolerate ablative or topical interventions.
The most significant risks of excision include the potential for damage to underlying pelvic visceral structures, which is particularly concerning in postmenopausal women with thin vaginal epithelium. Vaginectomy is commonly associated with vaginal shortening or narrowing, which can be deleterious for quality of life. Retrospective series have described a 30% incidence of recurrence after vaginectomy, likely secondary to incomplete excision of all affected tissue.3
Ablation
Ablation of dysplastic foci with a carbon dioxide (CO2) laser is a common method for treatment of VAIN. CO2 laser should ablate tissue to a 1.5 mm minimum depth.3 The benefit of using CO2 laser is its ability to treat multifocal disease in situ without an extensive excisional procedure.
It is technically more straightforward than upper vaginectomy with less blood loss and shorter surgical times, and it can be easily accomplished in an outpatient surgical or office setting. However, one of its greatest limitations is the difficulty in visualizing all lesions and therefore adequately treating all sites. The vaginal rogations also make adequate laser ablation challenging because laser only is able to effectively ablate tissue that is oriented perpendicular to the laser beam.
In addition, there is no pathologic confirmation of adequacy of excision or margin status. These features may contribute to the modestly higher rates of recurrence of dysplasia following laser ablation, compared with vaginectomy.3 It also has been associated with more vaginal scarring than vaginectomy, which can have a negative effect on sexual health.
Topical agents
The most commonly utilized topical therapy for VAIN is the antimetabolite chemotherapeutic agent 5-fluorouracil (5FU). A typical schedule for 5FU treatment is to apply vaginally, at night, once a week for 8 weeks.4 Because it can cause extensive irritation to the vulvar and urethral epithelium, patients are recommended to apply barrier creams or ointments before and following the use of 5FU for several days, wash hands thoroughly after application, and to rinse and shower in the morning after rising. Severe irritation occurs in up to 16% of patients, but in general it is very well tolerated.

Its virtue is that it is able to conform and travel to all parts of the vaginal mucosa, including those that are poorly visualized within the fornices or vaginal folds. 5FU does not require a hospitalization or surgical procedure, can be applied by the patient at home, and preserves vaginal length and function. In recent reports, 5FU is associated with the lowest rates of recurrence (10%-30%), compared with excision or ablation, and therefore is a very attractive option for primary therapy.3 However, it requires patients to have a degree of comfort with vaginal application of drug and adherence with perineal care strategies to minimize the likelihood of toxicity.
The immune response modifier, imiquimod, that is commonly used in the treatment of vulvar dysplasia also has been described in the treatment of VAIN. It appears to have high rates of clearance (greater than 75%) and be most effective in the treatment of VAIN I.5 It requires application under colposcopic guidance three times a week for 8 weeks, which is a laborious undertaking for both patient and physician. Like 5FU, imiquimod is associated with vulvar and perineal irritation.
Vaginal estrogens are an alternative topical therapy for moderate- and high-grade VAIN and particularly useful for postmenopausal patients. They have been associated with a high rate (up to 90%) of resolution on follow-up vaginal cytology testing and are not associated with toxicities of the above stated therapies.6 Vaginal estrogen can be used alone or in addition to other therapeutic strategies. For example, it can be added to the nontreatment days of 5FU or postoperatively prescribed following laser or excisional procedures.
Radiation
Intracavitary brachytherapy is a technique in which a radiation source is placed within a cylinder or ovoids and placed within the vagina.7 Typically 45 Gy is delivered to a depth 0.5mm below the vaginal mucosal surface (“point z”). Recurrence occurs is approximately 10%-15% of patients, and toxicities can be severe, including vaginal stenosis and ulceration. This aggressive therapy typically is best reserved for cases that are refractory to other therapies. Following radiation, subsequent treatments are more difficult because of radiation-induced changes to the vaginal mucosa that can affect healing.
Vaginal dysplasia is a relatively common sequelae of high-risk HPV, particularly among women who have had a prior hysterectomy for cervical dysplasia. Because of anatomic changes following hysterectomy, adequate visualization and comprehensive vaginal treatment is difficult. Therefore, surgeons should avoid utilization of hysterectomy as a routine strategy to “cure” dysplasia as it may fail to achieve this cure and make subsequent evaluations and treatments of persistent dysplasia more difficult. Women who have had a hysterectomy for dysplasia should be closely followed for several decades, and they should be counseled that they have a persistent risk for vaginal disease. When VAIN develops, clinicians should consider topical therapies as primary treatment options because they may minimize toxicity and have high rates of enduring response.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant conflicts of interest.
References
1. Gynecol Oncol. 2016 Jun;141(3):507-10.
2. Arch Gynecol Obstet. 2016 Feb;293(2):415-9.
3. Anticancer Res. 2013 Jan;33(1):29-38.
4. Obstet Gynecol. 2017 Dec;130(6):1237-43.
5. Eur J Obstet Gynecol Reprod Biol. 2017 Nov;218:129-36.
6. J Low Genit Tract Dis. 2014 Apr;18(2):115-21.
7. Gynecol Oncol. 2007 Jul;106(1):105-11.
Transforming Primary Care Clinical Learning Environments to Optimize Education, Outcomes, and Satisfaction
A broad consensus exists that US health care is now becoming more complex than at any other time in prior decades, potentially contributing to less than optimal outcomes, inadequate or unnecessary care, dissatisfied users, burned-out providers, and excessive costs.1 To reduce health system dysfunction, experts have looked to primary care to improve care continuity, coordination, and quality. The patient-centered medical home was designed to create environments where patients can access skilled professionals for both immediate and long-term needs across the health care spectrum, including nursing, pharmacy, social work, mental health, care coordinators, and educators.2
In 2010, the VHA of the Department of Veterans Affairs (VA) introduced a patient-centered model of primary care known as the patient-aligned care team (PACT). Each enrolled veteran is assigned to a PACT that is staffed by the enrollee’s personal provider, clinical staff, and appropriate professionals who work together to respond to patients in the context of their unique needs. In addition to the primary care provider (physician, physician assistant, or nurse practitioner), a nurse care manager, licensed vocational nurse or medical assistant, and an administrative professional, each PACT team is staffed by pharmacists, social workers, and mental health specialists. An especially important, and possibly unique, aspect of the VA PACT model is the integration of traditional primary care services with mental health access and care. This clinical interprofessional collaboration requires new educational strategies to effectively train a workforce qualified to work in, lead, and improve these settings.3
Although clinical environments are undergoing rapid change, curriculum for the health professions trainees has not adapted as quickly, even though it has been widely recognized that both should evolve concurrently.4 Curriculum emphasizing interprofessional practice, in particular, has been insufficiently implemented in educational settings.5 Static clinical learning environments pose a risk to future systems that will flounder without prepared professionals.6 Professional organizations, consensus groups, and medical education expert recommendations to implement interprofessional training environments have been met with relatively slow uptake in part because the challenges to implementation of scalable platforms for interprofessional clinical education are not trivial.7-9
This issue of Federal Practitioner introduces the first of 5 case studies that describe the implementation of instructional strategies designed and implemented by faculty, staff, and trainees. Each case embodies a unique approach to curriculum design and implementation that illustrates the collaborative innovation required to engage trainees with patients, with one another from differing professions, and with their faculty. The required flattening of the traditional hierarchy of staff in medical settings necessitates modification of clinical faculty and trainees skills. Didactic sessions are limited, and the focus is on experiential teaching and learning.10
As will be seen through the lenses of the cases presented in this series, the investments (including the time line to shift attitudes and change culture) required to achieve measurable outcomes are substantial. These investments not only are monetary, but also include addressing change management, conflict resolution, enhancement of communication skills, employee engagement, and leadership development.
The VA supports a comprehensive health system distributed throughout the nation with more than 1,000 points of care and more than 150 medical centers. Less recognized is that VA is the largest clinical learning platform in the US: More than 120,000 students and trainees enrolled in more than 40 different health professions and disciplines participate in VA clinical training programs annually.11 The VA has incorporated multiple innovative care designs, such as PACTs, along with educational and clinical leadership to create experiential workplace learning environments where structure, processes, and outcomes can be observed, adjusted, measured, and potentially duplicated.
This approach was key for the initial 5 of the current 7 Centers of Excellence in Primary Care Education (CoEPCEs) launched by VA in 2011, and from which the 5 cases in this series have evolved.12 The CoEPCE was developed as a demonstration project to show how to develop the interprofessional primary care curriculum for health professions that the PACT model requires. The CoEPCE, having trained more than 1,000 learners to date, has informed the PACT model to distinguish between PACTs whose mission is to provide clinical care from those that have the additional role of educating health professions trainees. The PACTS with this additional obligation are called interprofessional academic PACTs (iAPACTs). The iAPACTs incorporate features to accommodate clinical teaching and learning, including logistic challenges of scheduling, additional space requirements, faculty assignments, and affiliations with the academic institutions that sponsor the training programs.
Foundational concepts of the CoEPCE include those inherent in primary care, plus interprofessional practice where trainees of multiple professions are integrated into the care model to create a transformed workplace learning environment.13,14 Curricular domains of shared decision making among team members and their veteran patients, interprofessional collaboration, sustained relationships, and performance improvement are all required elements integral to the design and implementation of all CoEPCEs.13 This purposeful design provides clinical and educational infrastructure for interprofessional practice that simultaneously and seamlessly integrates both priorities of transforming clinical care and education.
The vision is to create the clinical learning environments necessary to produce the high-functioning individuals and teams needed to assure beneficial patient care outcomes as well as professional and personal satisfaction within the care team. The goal is to improve the PACT model of care in VA as a vehicle to enhance primary care services, to support changes in policy and practice that improve veterans’ care, safety, experience, health and well-being, and prepare a highly skilled future workforce for VA and for the nation as a whole.15
As all the cases in this series illustrate, the trainees are deeply embedded into clinical care and—very importantly—processes of patient care provision in consideration of all the patients care needs, using a holistic care model. As integrated team members, trainees from multiple professions learn with, from, and about one another as professionals and, as importantly, learn to appreciate the array of skills each brings to patient care, thus transforming their personal as well as professional learning experience. A highly relevant finding is that faculty and leadership—along with the trainees—have also learned, benefited, and transformed their thinking and attitudes, contributing to a cultural shift that is less hierarchical and more inclusive of all team members. A recently released external evaluation of the CoEPCE in their iAPACT environments indicates promising patterns of clinical outcomes with indications of improved staff satisfaction and less burnout. Better understanding of these innovations across and beyond the evaluated sites will be the topic of subsequent inquiries.16
These case studies demonstrate how education can be designed to advance the quality of care and improve the clinical teaching and learning environment, and educational outcomes. These cases are not intended to be recipes but rather exemplify the ingredients required to provide enough information and background to illustrate the transformational process. Superficially the cases may seem simple, but deeper examination reveals the complexity of confronting the challenges of day-to-day clinical work and redesigning both clinical and educational parameters.
These are real cases about real people working hard to revise a fragmented system and build a better future. The true purpose of these case studies is to inspire others to pursue educational modernization and excellence. In fact, there is no other satisfactory choice.
1. Dzau VJ, McClellan MB, McGinnis M, Finkelman EM, eds. Vital Directions for Health and Health Care: An Initiative of the National Academy of Medicine. https://nam.edu/initiatives/vital-directions-for-health-and-health-care. Published 2017. Accessed August 19, 2018.
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Defining the PCMH. https://pcmh.ahrq.gov/page/defining-pcmh. Accessed August 19, 2018.
3. US Department of Veterans Affairs, Patient Care Services. Patient aligned care team (PACT) https://www.patientcare.va.gov/primarycare/PACT.asp. Updated September 22, 2016. Accessed August 19, 2018.
4. Gilman SC, Chokshi DA, Bowen JL, Rugen KW, Cox M. Connecting the dots: health professions education and delivery system redesign. Acad Med. 2014;89(8):1113-1116.
5. Josiah Macy Jr. Foundation. Conference recommendations: transforming patient care: aligning interprofessional education with clinical practice redesign. http://macyfoundation.org/docs/macy_pubs/TransformingPatientCare_ConferenceRec.pdf. Published January 2013. Accessed August 19, 2018.
6. Accreditation Council for Graduate Medical Education. Clinical learning environment review. https://www.acgme.org/What-We-Do/Initiatives/Clinical-Learning-Environment-Review-CLER. Accessed August 19, 2018.
7. Cox M, Cuff P, Brandt B, Reeves S, Zierler B. Measuring the impact of interprofessional education on collaborative practice and patient outcomes. J Interprof Care. 2016;30(1):1-3.
8. National Collaborative for Improving the Clinical Learning Environment. Envisioning the optimal interprofessional clinical learning environment: initial findings from an October 2017 NCICLE symposium. https://storage.googleapis.com/wzukusers/user-27661272documents/5a5e3933a1c1cKVwrfGy/NCICLE%2IP-CLE%20Symposium%20Findings_011218%20update.pdf. Published January 12, 2018. Accessed August 19, 2018.
9. Institute of Medicine of the National Academies. Interprofessional Education for Collaboration: Learning How to Improve Health from Interprofessional Models Across the Continuum of Education to Practice: Workshop Summary. https://doi.org/10.17226/13486. Published 2013. Accessed August 22, 2018.
10. Harada ND, Traylor L, Rugen KW, et al. Interprofessional transformation of clinical education: the first six years of the Veterans Affairs Centers of Excellence in Primary Care Education. J Interprof Care. 2018;20:1-9.
11. US Department of Veterans Affairs, Office of Academic Affiliations. 2017 statistics: health professions trainees. https://www.va.gov/OAA/docs/OAA_Statistics.pdf. Accessed August 19, 2018.
12. US Department of Veterans Affairs, Office of Academic Affiliations. VA centers of excellence in primary care education. https://www.va.gov/oaa/coepce. Updated July 24, 2018. Accessed August 19, 2018.
13. US Department of Veterans Affairs, Office of Academic Affiliations. Academic PACT. https://www.va.gov/oaa/apact. Updated April 3, 2018. Accessed August 19, 2018.
14. US Department of Veterans Affairs, Office of Academic Affiliations. VA academic PACT: a blueprint for primary care redesign in academic practice settings. https://www.va.gov/oaa/docs/VA_Academic_PACT_blueprint.pdf. Published July 29, 2013. Accessed August 19, 2018.
15. US Department of Veterans Affairs, Office of Academic Affiliations. Centers of Excellence in Primary Care Education. Compendium of five case studies: lessons for interprofessional teamwork in education and workplace learning environments 2011-2016. https://www.va.gov/OAA/docs/VACaseStudiesCoEPCE.pdf. Published 2017. Accessed August 19, 2018.
16. US Department of Veterans Affairs, Quality Enhancement Research Initiative. Action-oriented evaluation of interprofessional learning efforts in the CoEPCE and iA-PACT environments. https://www.queri.research.va.gov/about/factsheets/InterProfessional-PEI.pdf. Published June 2018. Accessed August 19, 2018.
A broad consensus exists that US health care is now becoming more complex than at any other time in prior decades, potentially contributing to less than optimal outcomes, inadequate or unnecessary care, dissatisfied users, burned-out providers, and excessive costs.1 To reduce health system dysfunction, experts have looked to primary care to improve care continuity, coordination, and quality. The patient-centered medical home was designed to create environments where patients can access skilled professionals for both immediate and long-term needs across the health care spectrum, including nursing, pharmacy, social work, mental health, care coordinators, and educators.2
In 2010, the VHA of the Department of Veterans Affairs (VA) introduced a patient-centered model of primary care known as the patient-aligned care team (PACT). Each enrolled veteran is assigned to a PACT that is staffed by the enrollee’s personal provider, clinical staff, and appropriate professionals who work together to respond to patients in the context of their unique needs. In addition to the primary care provider (physician, physician assistant, or nurse practitioner), a nurse care manager, licensed vocational nurse or medical assistant, and an administrative professional, each PACT team is staffed by pharmacists, social workers, and mental health specialists. An especially important, and possibly unique, aspect of the VA PACT model is the integration of traditional primary care services with mental health access and care. This clinical interprofessional collaboration requires new educational strategies to effectively train a workforce qualified to work in, lead, and improve these settings.3
Although clinical environments are undergoing rapid change, curriculum for the health professions trainees has not adapted as quickly, even though it has been widely recognized that both should evolve concurrently.4 Curriculum emphasizing interprofessional practice, in particular, has been insufficiently implemented in educational settings.5 Static clinical learning environments pose a risk to future systems that will flounder without prepared professionals.6 Professional organizations, consensus groups, and medical education expert recommendations to implement interprofessional training environments have been met with relatively slow uptake in part because the challenges to implementation of scalable platforms for interprofessional clinical education are not trivial.7-9
This issue of Federal Practitioner introduces the first of 5 case studies that describe the implementation of instructional strategies designed and implemented by faculty, staff, and trainees. Each case embodies a unique approach to curriculum design and implementation that illustrates the collaborative innovation required to engage trainees with patients, with one another from differing professions, and with their faculty. The required flattening of the traditional hierarchy of staff in medical settings necessitates modification of clinical faculty and trainees skills. Didactic sessions are limited, and the focus is on experiential teaching and learning.10
As will be seen through the lenses of the cases presented in this series, the investments (including the time line to shift attitudes and change culture) required to achieve measurable outcomes are substantial. These investments not only are monetary, but also include addressing change management, conflict resolution, enhancement of communication skills, employee engagement, and leadership development.
The VA supports a comprehensive health system distributed throughout the nation with more than 1,000 points of care and more than 150 medical centers. Less recognized is that VA is the largest clinical learning platform in the US: More than 120,000 students and trainees enrolled in more than 40 different health professions and disciplines participate in VA clinical training programs annually.11 The VA has incorporated multiple innovative care designs, such as PACTs, along with educational and clinical leadership to create experiential workplace learning environments where structure, processes, and outcomes can be observed, adjusted, measured, and potentially duplicated.
This approach was key for the initial 5 of the current 7 Centers of Excellence in Primary Care Education (CoEPCEs) launched by VA in 2011, and from which the 5 cases in this series have evolved.12 The CoEPCE was developed as a demonstration project to show how to develop the interprofessional primary care curriculum for health professions that the PACT model requires. The CoEPCE, having trained more than 1,000 learners to date, has informed the PACT model to distinguish between PACTs whose mission is to provide clinical care from those that have the additional role of educating health professions trainees. The PACTS with this additional obligation are called interprofessional academic PACTs (iAPACTs). The iAPACTs incorporate features to accommodate clinical teaching and learning, including logistic challenges of scheduling, additional space requirements, faculty assignments, and affiliations with the academic institutions that sponsor the training programs.
Foundational concepts of the CoEPCE include those inherent in primary care, plus interprofessional practice where trainees of multiple professions are integrated into the care model to create a transformed workplace learning environment.13,14 Curricular domains of shared decision making among team members and their veteran patients, interprofessional collaboration, sustained relationships, and performance improvement are all required elements integral to the design and implementation of all CoEPCEs.13 This purposeful design provides clinical and educational infrastructure for interprofessional practice that simultaneously and seamlessly integrates both priorities of transforming clinical care and education.
The vision is to create the clinical learning environments necessary to produce the high-functioning individuals and teams needed to assure beneficial patient care outcomes as well as professional and personal satisfaction within the care team. The goal is to improve the PACT model of care in VA as a vehicle to enhance primary care services, to support changes in policy and practice that improve veterans’ care, safety, experience, health and well-being, and prepare a highly skilled future workforce for VA and for the nation as a whole.15
As all the cases in this series illustrate, the trainees are deeply embedded into clinical care and—very importantly—processes of patient care provision in consideration of all the patients care needs, using a holistic care model. As integrated team members, trainees from multiple professions learn with, from, and about one another as professionals and, as importantly, learn to appreciate the array of skills each brings to patient care, thus transforming their personal as well as professional learning experience. A highly relevant finding is that faculty and leadership—along with the trainees—have also learned, benefited, and transformed their thinking and attitudes, contributing to a cultural shift that is less hierarchical and more inclusive of all team members. A recently released external evaluation of the CoEPCE in their iAPACT environments indicates promising patterns of clinical outcomes with indications of improved staff satisfaction and less burnout. Better understanding of these innovations across and beyond the evaluated sites will be the topic of subsequent inquiries.16
These case studies demonstrate how education can be designed to advance the quality of care and improve the clinical teaching and learning environment, and educational outcomes. These cases are not intended to be recipes but rather exemplify the ingredients required to provide enough information and background to illustrate the transformational process. Superficially the cases may seem simple, but deeper examination reveals the complexity of confronting the challenges of day-to-day clinical work and redesigning both clinical and educational parameters.
These are real cases about real people working hard to revise a fragmented system and build a better future. The true purpose of these case studies is to inspire others to pursue educational modernization and excellence. In fact, there is no other satisfactory choice.
A broad consensus exists that US health care is now becoming more complex than at any other time in prior decades, potentially contributing to less than optimal outcomes, inadequate or unnecessary care, dissatisfied users, burned-out providers, and excessive costs.1 To reduce health system dysfunction, experts have looked to primary care to improve care continuity, coordination, and quality. The patient-centered medical home was designed to create environments where patients can access skilled professionals for both immediate and long-term needs across the health care spectrum, including nursing, pharmacy, social work, mental health, care coordinators, and educators.2
In 2010, the VHA of the Department of Veterans Affairs (VA) introduced a patient-centered model of primary care known as the patient-aligned care team (PACT). Each enrolled veteran is assigned to a PACT that is staffed by the enrollee’s personal provider, clinical staff, and appropriate professionals who work together to respond to patients in the context of their unique needs. In addition to the primary care provider (physician, physician assistant, or nurse practitioner), a nurse care manager, licensed vocational nurse or medical assistant, and an administrative professional, each PACT team is staffed by pharmacists, social workers, and mental health specialists. An especially important, and possibly unique, aspect of the VA PACT model is the integration of traditional primary care services with mental health access and care. This clinical interprofessional collaboration requires new educational strategies to effectively train a workforce qualified to work in, lead, and improve these settings.3
Although clinical environments are undergoing rapid change, curriculum for the health professions trainees has not adapted as quickly, even though it has been widely recognized that both should evolve concurrently.4 Curriculum emphasizing interprofessional practice, in particular, has been insufficiently implemented in educational settings.5 Static clinical learning environments pose a risk to future systems that will flounder without prepared professionals.6 Professional organizations, consensus groups, and medical education expert recommendations to implement interprofessional training environments have been met with relatively slow uptake in part because the challenges to implementation of scalable platforms for interprofessional clinical education are not trivial.7-9
This issue of Federal Practitioner introduces the first of 5 case studies that describe the implementation of instructional strategies designed and implemented by faculty, staff, and trainees. Each case embodies a unique approach to curriculum design and implementation that illustrates the collaborative innovation required to engage trainees with patients, with one another from differing professions, and with their faculty. The required flattening of the traditional hierarchy of staff in medical settings necessitates modification of clinical faculty and trainees skills. Didactic sessions are limited, and the focus is on experiential teaching and learning.10
As will be seen through the lenses of the cases presented in this series, the investments (including the time line to shift attitudes and change culture) required to achieve measurable outcomes are substantial. These investments not only are monetary, but also include addressing change management, conflict resolution, enhancement of communication skills, employee engagement, and leadership development.
The VA supports a comprehensive health system distributed throughout the nation with more than 1,000 points of care and more than 150 medical centers. Less recognized is that VA is the largest clinical learning platform in the US: More than 120,000 students and trainees enrolled in more than 40 different health professions and disciplines participate in VA clinical training programs annually.11 The VA has incorporated multiple innovative care designs, such as PACTs, along with educational and clinical leadership to create experiential workplace learning environments where structure, processes, and outcomes can be observed, adjusted, measured, and potentially duplicated.
This approach was key for the initial 5 of the current 7 Centers of Excellence in Primary Care Education (CoEPCEs) launched by VA in 2011, and from which the 5 cases in this series have evolved.12 The CoEPCE was developed as a demonstration project to show how to develop the interprofessional primary care curriculum for health professions that the PACT model requires. The CoEPCE, having trained more than 1,000 learners to date, has informed the PACT model to distinguish between PACTs whose mission is to provide clinical care from those that have the additional role of educating health professions trainees. The PACTS with this additional obligation are called interprofessional academic PACTs (iAPACTs). The iAPACTs incorporate features to accommodate clinical teaching and learning, including logistic challenges of scheduling, additional space requirements, faculty assignments, and affiliations with the academic institutions that sponsor the training programs.
Foundational concepts of the CoEPCE include those inherent in primary care, plus interprofessional practice where trainees of multiple professions are integrated into the care model to create a transformed workplace learning environment.13,14 Curricular domains of shared decision making among team members and their veteran patients, interprofessional collaboration, sustained relationships, and performance improvement are all required elements integral to the design and implementation of all CoEPCEs.13 This purposeful design provides clinical and educational infrastructure for interprofessional practice that simultaneously and seamlessly integrates both priorities of transforming clinical care and education.
The vision is to create the clinical learning environments necessary to produce the high-functioning individuals and teams needed to assure beneficial patient care outcomes as well as professional and personal satisfaction within the care team. The goal is to improve the PACT model of care in VA as a vehicle to enhance primary care services, to support changes in policy and practice that improve veterans’ care, safety, experience, health and well-being, and prepare a highly skilled future workforce for VA and for the nation as a whole.15
As all the cases in this series illustrate, the trainees are deeply embedded into clinical care and—very importantly—processes of patient care provision in consideration of all the patients care needs, using a holistic care model. As integrated team members, trainees from multiple professions learn with, from, and about one another as professionals and, as importantly, learn to appreciate the array of skills each brings to patient care, thus transforming their personal as well as professional learning experience. A highly relevant finding is that faculty and leadership—along with the trainees—have also learned, benefited, and transformed their thinking and attitudes, contributing to a cultural shift that is less hierarchical and more inclusive of all team members. A recently released external evaluation of the CoEPCE in their iAPACT environments indicates promising patterns of clinical outcomes with indications of improved staff satisfaction and less burnout. Better understanding of these innovations across and beyond the evaluated sites will be the topic of subsequent inquiries.16
These case studies demonstrate how education can be designed to advance the quality of care and improve the clinical teaching and learning environment, and educational outcomes. These cases are not intended to be recipes but rather exemplify the ingredients required to provide enough information and background to illustrate the transformational process. Superficially the cases may seem simple, but deeper examination reveals the complexity of confronting the challenges of day-to-day clinical work and redesigning both clinical and educational parameters.
These are real cases about real people working hard to revise a fragmented system and build a better future. The true purpose of these case studies is to inspire others to pursue educational modernization and excellence. In fact, there is no other satisfactory choice.
1. Dzau VJ, McClellan MB, McGinnis M, Finkelman EM, eds. Vital Directions for Health and Health Care: An Initiative of the National Academy of Medicine. https://nam.edu/initiatives/vital-directions-for-health-and-health-care. Published 2017. Accessed August 19, 2018.
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Defining the PCMH. https://pcmh.ahrq.gov/page/defining-pcmh. Accessed August 19, 2018.
3. US Department of Veterans Affairs, Patient Care Services. Patient aligned care team (PACT) https://www.patientcare.va.gov/primarycare/PACT.asp. Updated September 22, 2016. Accessed August 19, 2018.
4. Gilman SC, Chokshi DA, Bowen JL, Rugen KW, Cox M. Connecting the dots: health professions education and delivery system redesign. Acad Med. 2014;89(8):1113-1116.
5. Josiah Macy Jr. Foundation. Conference recommendations: transforming patient care: aligning interprofessional education with clinical practice redesign. http://macyfoundation.org/docs/macy_pubs/TransformingPatientCare_ConferenceRec.pdf. Published January 2013. Accessed August 19, 2018.
6. Accreditation Council for Graduate Medical Education. Clinical learning environment review. https://www.acgme.org/What-We-Do/Initiatives/Clinical-Learning-Environment-Review-CLER. Accessed August 19, 2018.
7. Cox M, Cuff P, Brandt B, Reeves S, Zierler B. Measuring the impact of interprofessional education on collaborative practice and patient outcomes. J Interprof Care. 2016;30(1):1-3.
8. National Collaborative for Improving the Clinical Learning Environment. Envisioning the optimal interprofessional clinical learning environment: initial findings from an October 2017 NCICLE symposium. https://storage.googleapis.com/wzukusers/user-27661272documents/5a5e3933a1c1cKVwrfGy/NCICLE%2IP-CLE%20Symposium%20Findings_011218%20update.pdf. Published January 12, 2018. Accessed August 19, 2018.
9. Institute of Medicine of the National Academies. Interprofessional Education for Collaboration: Learning How to Improve Health from Interprofessional Models Across the Continuum of Education to Practice: Workshop Summary. https://doi.org/10.17226/13486. Published 2013. Accessed August 22, 2018.
10. Harada ND, Traylor L, Rugen KW, et al. Interprofessional transformation of clinical education: the first six years of the Veterans Affairs Centers of Excellence in Primary Care Education. J Interprof Care. 2018;20:1-9.
11. US Department of Veterans Affairs, Office of Academic Affiliations. 2017 statistics: health professions trainees. https://www.va.gov/OAA/docs/OAA_Statistics.pdf. Accessed August 19, 2018.
12. US Department of Veterans Affairs, Office of Academic Affiliations. VA centers of excellence in primary care education. https://www.va.gov/oaa/coepce. Updated July 24, 2018. Accessed August 19, 2018.
13. US Department of Veterans Affairs, Office of Academic Affiliations. Academic PACT. https://www.va.gov/oaa/apact. Updated April 3, 2018. Accessed August 19, 2018.
14. US Department of Veterans Affairs, Office of Academic Affiliations. VA academic PACT: a blueprint for primary care redesign in academic practice settings. https://www.va.gov/oaa/docs/VA_Academic_PACT_blueprint.pdf. Published July 29, 2013. Accessed August 19, 2018.
15. US Department of Veterans Affairs, Office of Academic Affiliations. Centers of Excellence in Primary Care Education. Compendium of five case studies: lessons for interprofessional teamwork in education and workplace learning environments 2011-2016. https://www.va.gov/OAA/docs/VACaseStudiesCoEPCE.pdf. Published 2017. Accessed August 19, 2018.
16. US Department of Veterans Affairs, Quality Enhancement Research Initiative. Action-oriented evaluation of interprofessional learning efforts in the CoEPCE and iA-PACT environments. https://www.queri.research.va.gov/about/factsheets/InterProfessional-PEI.pdf. Published June 2018. Accessed August 19, 2018.
1. Dzau VJ, McClellan MB, McGinnis M, Finkelman EM, eds. Vital Directions for Health and Health Care: An Initiative of the National Academy of Medicine. https://nam.edu/initiatives/vital-directions-for-health-and-health-care. Published 2017. Accessed August 19, 2018.
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Defining the PCMH. https://pcmh.ahrq.gov/page/defining-pcmh. Accessed August 19, 2018.
3. US Department of Veterans Affairs, Patient Care Services. Patient aligned care team (PACT) https://www.patientcare.va.gov/primarycare/PACT.asp. Updated September 22, 2016. Accessed August 19, 2018.
4. Gilman SC, Chokshi DA, Bowen JL, Rugen KW, Cox M. Connecting the dots: health professions education and delivery system redesign. Acad Med. 2014;89(8):1113-1116.
5. Josiah Macy Jr. Foundation. Conference recommendations: transforming patient care: aligning interprofessional education with clinical practice redesign. http://macyfoundation.org/docs/macy_pubs/TransformingPatientCare_ConferenceRec.pdf. Published January 2013. Accessed August 19, 2018.
6. Accreditation Council for Graduate Medical Education. Clinical learning environment review. https://www.acgme.org/What-We-Do/Initiatives/Clinical-Learning-Environment-Review-CLER. Accessed August 19, 2018.
7. Cox M, Cuff P, Brandt B, Reeves S, Zierler B. Measuring the impact of interprofessional education on collaborative practice and patient outcomes. J Interprof Care. 2016;30(1):1-3.
8. National Collaborative for Improving the Clinical Learning Environment. Envisioning the optimal interprofessional clinical learning environment: initial findings from an October 2017 NCICLE symposium. https://storage.googleapis.com/wzukusers/user-27661272documents/5a5e3933a1c1cKVwrfGy/NCICLE%2IP-CLE%20Symposium%20Findings_011218%20update.pdf. Published January 12, 2018. Accessed August 19, 2018.
9. Institute of Medicine of the National Academies. Interprofessional Education for Collaboration: Learning How to Improve Health from Interprofessional Models Across the Continuum of Education to Practice: Workshop Summary. https://doi.org/10.17226/13486. Published 2013. Accessed August 22, 2018.
10. Harada ND, Traylor L, Rugen KW, et al. Interprofessional transformation of clinical education: the first six years of the Veterans Affairs Centers of Excellence in Primary Care Education. J Interprof Care. 2018;20:1-9.
11. US Department of Veterans Affairs, Office of Academic Affiliations. 2017 statistics: health professions trainees. https://www.va.gov/OAA/docs/OAA_Statistics.pdf. Accessed August 19, 2018.
12. US Department of Veterans Affairs, Office of Academic Affiliations. VA centers of excellence in primary care education. https://www.va.gov/oaa/coepce. Updated July 24, 2018. Accessed August 19, 2018.
13. US Department of Veterans Affairs, Office of Academic Affiliations. Academic PACT. https://www.va.gov/oaa/apact. Updated April 3, 2018. Accessed August 19, 2018.
14. US Department of Veterans Affairs, Office of Academic Affiliations. VA academic PACT: a blueprint for primary care redesign in academic practice settings. https://www.va.gov/oaa/docs/VA_Academic_PACT_blueprint.pdf. Published July 29, 2013. Accessed August 19, 2018.
15. US Department of Veterans Affairs, Office of Academic Affiliations. Centers of Excellence in Primary Care Education. Compendium of five case studies: lessons for interprofessional teamwork in education and workplace learning environments 2011-2016. https://www.va.gov/OAA/docs/VACaseStudiesCoEPCE.pdf. Published 2017. Accessed August 19, 2018.
16. US Department of Veterans Affairs, Quality Enhancement Research Initiative. Action-oriented evaluation of interprofessional learning efforts in the CoEPCE and iA-PACT environments. https://www.queri.research.va.gov/about/factsheets/InterProfessional-PEI.pdf. Published June 2018. Accessed August 19, 2018.
Are Antipsychotic Medications Safe During Pregnancy?
Psychiatric illnesses are prevalent in about 25% of the US adult population.1 Approximately 21% to 33% of women are prescribed antipsychotic drugs during pregnancies,and about 50% experience a relapse of symptoms related to mental illness.2,3 In 2015, the Pregnancy and Lactation Labeling Rule removed the A, B, C, D, and X categories for medications prescribed during gestation. Labels now include more information and detail about these pharmaceuticals regarding potential risks to a mother and fetus.4 As with all other pharmacotherapies during pregnancy, teratogenicity and medicinal adverse effects (AEs) must be balanced against the risk of nonpharmacotherapy.
Medications
Antipsychotic medications often are prescribed to treat people with a wide range of psychiatric conditions, including schizophrenia, bipolar disorder, depression, anxiety, and personality disorders.5 Commonly, second-generation antipsychotic medications are selected for pregnant women. Olanzapine, haloperidol, risperidone, and quetiapine freely pass through the placenta.5 During gestation, when an antipsychotic agent is strongly indicated, it is prudent to select one of the second-generation versions or haloperidol.
Risks vs Benefits
Physicians should always consider the risk-to-benefit ratio of these medicines for both the pregnant woman and the fetus.6 The National Pregnancy Registry for Atypical Antipsychotics was established to evaluate the safety and efficacy of these drugs during pregnancy and the postpartum period.4,6
Even during the first trimester of pregnancy most antipsychotic medications prescribed to women, are documented to cause few major fetal malformations.7 Research during 487 pregnancies revealed that the risk of a malformed infant previously exposed in utero to antipsychotic drugs was 1.4%, compared with 1.1% for those not exposed.6 Risperidone, however is an exception, because evidence has shown that it results in more cardiac malformations and congenital anomalies than do other medications.7
Complications
Maternal complications, such as increased weight gain, gestational diabetes mellitus, hypertension, and venous thromboembolism, are reported in pregnant women prescribed antipsychotic medications.3 Sudden discontinuation of these drugs might interfere with activities of daily living, allow more psychotic symptoms in the mother, impair prenatal self-care, and increase the risk for suicide or infanticide.8 Fetal complications might include prematurity, intrauterine growth retardation, distress, suboptimal birth weights, low Apgar scores, neonatal hypoglycemia, and congenital defects. Stillbirths can occur as well.9
Neonates exposed to antipsychotic medications in utero can experience withdrawal symptoms after delivery. They might exhibit agitation, feeding disorders, hypotonia, hypertonia, respiratory distress, somnolence, and tremor.10 Extrapyramidal symptoms, such as abnormal movements, restlessness, stiffness, and tremors, may occur more often when prescribing first-generation rather than with second-generation antipsychotic drugs.11 These clinical manifestations occur from a few hours after birth to 1 month later. The management of withdrawal symptoms is not clear, though symptomatic intervention is recommended.11
However, studies have shown that documented AEs are not significantly increased in the patients or infants exposed to antipsychotic medications compared with those of a control group.7 Furthermore, pregnant women with mental illness who remain untreated or who discontinue these drugs during a gestation evidence increased maternal morbidity12;they also exhibit more complications, such as placental abnormalities, antepartum hemorrhage, or preeclampsia.6 Hence, when medications are indicated, physicians should encourage patients to continue taking these medications after being educated about the risks and benefits of pharmacotherapy.6
Conclusions
The advantages of prescribing antipsychotic drugs during pregnancy include better psychiatric, obstetric, and neonatal health. Although antipsychotic medications continue to be safe during pregnancy, only necessary prescribing of indicated antipsychotic medicine and maintaining the safest possible therapeutic profile is an optimal approach to treat pregnant women requiring these medications.12 The efficacy of these medications also depends on an individual assessment of the patient’s health and lifestyle. When obtaining a patient history, physicians should include a review of smoking, alcohol consumption, substance abuse, and prior and/or concomitant use of other medications. Demographics, medical comorbidities, and psychiatric illnesses have a role in the clinical outcome.13 Physicians also should consider dosage, timing, and duration of medication exposure.
A baby born with birth defects can be devastating to the mother and is always balanced against the risk of less intervention. Apart from guiding patients regarding antipsychotic medication intake, pregnant women should be educated about regular prenatal checkups, taking vitamins and other supplements, monitoring for gestational diabetes mellitus, a proper diet, and exercise. Physicians and their patients should always minimize exposure to smoking or drugs and medications, especially polypharmacy.13 A higher level of prenatal care is advised whenever a physician suspects complications, including a referral to a maternal-fetal specialist.
1. Centers for Disease Control and Prevention. CDC report: mental illness surveillance among adults in the United States. https://www.cdc.gov/mentalhealthsurveillance/fact_sheet.html. Archived document. Updated December 2, 2011. Accessed April 10, 2018.
2. L evenson JL, ed . The American Psychiatric Publishing Textbook of Psychosomatic Medicine: Psychiatric Care of the Medically Ill. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2011 .
3. Kulkarni J, Worsley R, Gilbert H, et al. A prospective cohort study of antipsychotic medications in pregnancy: the first 147 pregnancies and 100 one year old babies. PLoS One. 2014;9(5):e94788.
4. US Food and Drug Administration. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. https://www.fda.gov/downloads/aboutfda/reportsmanualsforms/reports/economicanalyses/ucm427798.pdf. Accessed April 10, 2018.
5. Ennis ZN, Damkier P. Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations. A systematic review. Basic Clin Pharmacol Toxicol. 2015;116(4):315-320.
6. Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry. 2016:173(3):263-270.
7. Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938-946.
8. Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf. 2014;5(2):100-109.
9 . Crawford MB, DeLisi LE. Issues related to sex differences in antipsychotic treatment. Curr Opin Psychiatry. 2016;29(3):211-217.
10. Chisolm MS, Payne JL. Management of psychotropic drugs during pregnancy. BMJ. 2016;352:h5918.
11. US Food and Drug Administration. FDA drug safety communication: antipsychotic drug labels updated on use during pregnancy and risk of abnormal muscle movements and withdrawal symptoms in newborns. https://www.fda.gov/Drugs/DrugSafety/ucm243903.htm. Updated August 4, 2017. Accessed April 10, 2018.
12 . Tosato S, Albert U, Tomassi S, et al. A systematized review of atypical antipsychotics in pregnant women: balancing between risks of untreated illness and risks of drug-related adverse effects. J Clin Psychiatry. 2017;78(5):e477-e489.
13. Petersen I, Sammon CJ, McCrea RL, et al. Risks associated with antipsychotic treatment in pregnancy: comparative cohort studies based on electronic health records. Schizophr Res. 2016; 76(2-3):349-356.
Psychiatric illnesses are prevalent in about 25% of the US adult population.1 Approximately 21% to 33% of women are prescribed antipsychotic drugs during pregnancies,and about 50% experience a relapse of symptoms related to mental illness.2,3 In 2015, the Pregnancy and Lactation Labeling Rule removed the A, B, C, D, and X categories for medications prescribed during gestation. Labels now include more information and detail about these pharmaceuticals regarding potential risks to a mother and fetus.4 As with all other pharmacotherapies during pregnancy, teratogenicity and medicinal adverse effects (AEs) must be balanced against the risk of nonpharmacotherapy.
Medications
Antipsychotic medications often are prescribed to treat people with a wide range of psychiatric conditions, including schizophrenia, bipolar disorder, depression, anxiety, and personality disorders.5 Commonly, second-generation antipsychotic medications are selected for pregnant women. Olanzapine, haloperidol, risperidone, and quetiapine freely pass through the placenta.5 During gestation, when an antipsychotic agent is strongly indicated, it is prudent to select one of the second-generation versions or haloperidol.
Risks vs Benefits
Physicians should always consider the risk-to-benefit ratio of these medicines for both the pregnant woman and the fetus.6 The National Pregnancy Registry for Atypical Antipsychotics was established to evaluate the safety and efficacy of these drugs during pregnancy and the postpartum period.4,6
Even during the first trimester of pregnancy most antipsychotic medications prescribed to women, are documented to cause few major fetal malformations.7 Research during 487 pregnancies revealed that the risk of a malformed infant previously exposed in utero to antipsychotic drugs was 1.4%, compared with 1.1% for those not exposed.6 Risperidone, however is an exception, because evidence has shown that it results in more cardiac malformations and congenital anomalies than do other medications.7
Complications
Maternal complications, such as increased weight gain, gestational diabetes mellitus, hypertension, and venous thromboembolism, are reported in pregnant women prescribed antipsychotic medications.3 Sudden discontinuation of these drugs might interfere with activities of daily living, allow more psychotic symptoms in the mother, impair prenatal self-care, and increase the risk for suicide or infanticide.8 Fetal complications might include prematurity, intrauterine growth retardation, distress, suboptimal birth weights, low Apgar scores, neonatal hypoglycemia, and congenital defects. Stillbirths can occur as well.9
Neonates exposed to antipsychotic medications in utero can experience withdrawal symptoms after delivery. They might exhibit agitation, feeding disorders, hypotonia, hypertonia, respiratory distress, somnolence, and tremor.10 Extrapyramidal symptoms, such as abnormal movements, restlessness, stiffness, and tremors, may occur more often when prescribing first-generation rather than with second-generation antipsychotic drugs.11 These clinical manifestations occur from a few hours after birth to 1 month later. The management of withdrawal symptoms is not clear, though symptomatic intervention is recommended.11
However, studies have shown that documented AEs are not significantly increased in the patients or infants exposed to antipsychotic medications compared with those of a control group.7 Furthermore, pregnant women with mental illness who remain untreated or who discontinue these drugs during a gestation evidence increased maternal morbidity12;they also exhibit more complications, such as placental abnormalities, antepartum hemorrhage, or preeclampsia.6 Hence, when medications are indicated, physicians should encourage patients to continue taking these medications after being educated about the risks and benefits of pharmacotherapy.6
Conclusions
The advantages of prescribing antipsychotic drugs during pregnancy include better psychiatric, obstetric, and neonatal health. Although antipsychotic medications continue to be safe during pregnancy, only necessary prescribing of indicated antipsychotic medicine and maintaining the safest possible therapeutic profile is an optimal approach to treat pregnant women requiring these medications.12 The efficacy of these medications also depends on an individual assessment of the patient’s health and lifestyle. When obtaining a patient history, physicians should include a review of smoking, alcohol consumption, substance abuse, and prior and/or concomitant use of other medications. Demographics, medical comorbidities, and psychiatric illnesses have a role in the clinical outcome.13 Physicians also should consider dosage, timing, and duration of medication exposure.
A baby born with birth defects can be devastating to the mother and is always balanced against the risk of less intervention. Apart from guiding patients regarding antipsychotic medication intake, pregnant women should be educated about regular prenatal checkups, taking vitamins and other supplements, monitoring for gestational diabetes mellitus, a proper diet, and exercise. Physicians and their patients should always minimize exposure to smoking or drugs and medications, especially polypharmacy.13 A higher level of prenatal care is advised whenever a physician suspects complications, including a referral to a maternal-fetal specialist.
Psychiatric illnesses are prevalent in about 25% of the US adult population.1 Approximately 21% to 33% of women are prescribed antipsychotic drugs during pregnancies,and about 50% experience a relapse of symptoms related to mental illness.2,3 In 2015, the Pregnancy and Lactation Labeling Rule removed the A, B, C, D, and X categories for medications prescribed during gestation. Labels now include more information and detail about these pharmaceuticals regarding potential risks to a mother and fetus.4 As with all other pharmacotherapies during pregnancy, teratogenicity and medicinal adverse effects (AEs) must be balanced against the risk of nonpharmacotherapy.
Medications
Antipsychotic medications often are prescribed to treat people with a wide range of psychiatric conditions, including schizophrenia, bipolar disorder, depression, anxiety, and personality disorders.5 Commonly, second-generation antipsychotic medications are selected for pregnant women. Olanzapine, haloperidol, risperidone, and quetiapine freely pass through the placenta.5 During gestation, when an antipsychotic agent is strongly indicated, it is prudent to select one of the second-generation versions or haloperidol.
Risks vs Benefits
Physicians should always consider the risk-to-benefit ratio of these medicines for both the pregnant woman and the fetus.6 The National Pregnancy Registry for Atypical Antipsychotics was established to evaluate the safety and efficacy of these drugs during pregnancy and the postpartum period.4,6
Even during the first trimester of pregnancy most antipsychotic medications prescribed to women, are documented to cause few major fetal malformations.7 Research during 487 pregnancies revealed that the risk of a malformed infant previously exposed in utero to antipsychotic drugs was 1.4%, compared with 1.1% for those not exposed.6 Risperidone, however is an exception, because evidence has shown that it results in more cardiac malformations and congenital anomalies than do other medications.7
Complications
Maternal complications, such as increased weight gain, gestational diabetes mellitus, hypertension, and venous thromboembolism, are reported in pregnant women prescribed antipsychotic medications.3 Sudden discontinuation of these drugs might interfere with activities of daily living, allow more psychotic symptoms in the mother, impair prenatal self-care, and increase the risk for suicide or infanticide.8 Fetal complications might include prematurity, intrauterine growth retardation, distress, suboptimal birth weights, low Apgar scores, neonatal hypoglycemia, and congenital defects. Stillbirths can occur as well.9
Neonates exposed to antipsychotic medications in utero can experience withdrawal symptoms after delivery. They might exhibit agitation, feeding disorders, hypotonia, hypertonia, respiratory distress, somnolence, and tremor.10 Extrapyramidal symptoms, such as abnormal movements, restlessness, stiffness, and tremors, may occur more often when prescribing first-generation rather than with second-generation antipsychotic drugs.11 These clinical manifestations occur from a few hours after birth to 1 month later. The management of withdrawal symptoms is not clear, though symptomatic intervention is recommended.11
However, studies have shown that documented AEs are not significantly increased in the patients or infants exposed to antipsychotic medications compared with those of a control group.7 Furthermore, pregnant women with mental illness who remain untreated or who discontinue these drugs during a gestation evidence increased maternal morbidity12;they also exhibit more complications, such as placental abnormalities, antepartum hemorrhage, or preeclampsia.6 Hence, when medications are indicated, physicians should encourage patients to continue taking these medications after being educated about the risks and benefits of pharmacotherapy.6
Conclusions
The advantages of prescribing antipsychotic drugs during pregnancy include better psychiatric, obstetric, and neonatal health. Although antipsychotic medications continue to be safe during pregnancy, only necessary prescribing of indicated antipsychotic medicine and maintaining the safest possible therapeutic profile is an optimal approach to treat pregnant women requiring these medications.12 The efficacy of these medications also depends on an individual assessment of the patient’s health and lifestyle. When obtaining a patient history, physicians should include a review of smoking, alcohol consumption, substance abuse, and prior and/or concomitant use of other medications. Demographics, medical comorbidities, and psychiatric illnesses have a role in the clinical outcome.13 Physicians also should consider dosage, timing, and duration of medication exposure.
A baby born with birth defects can be devastating to the mother and is always balanced against the risk of less intervention. Apart from guiding patients regarding antipsychotic medication intake, pregnant women should be educated about regular prenatal checkups, taking vitamins and other supplements, monitoring for gestational diabetes mellitus, a proper diet, and exercise. Physicians and their patients should always minimize exposure to smoking or drugs and medications, especially polypharmacy.13 A higher level of prenatal care is advised whenever a physician suspects complications, including a referral to a maternal-fetal specialist.
1. Centers for Disease Control and Prevention. CDC report: mental illness surveillance among adults in the United States. https://www.cdc.gov/mentalhealthsurveillance/fact_sheet.html. Archived document. Updated December 2, 2011. Accessed April 10, 2018.
2. L evenson JL, ed . The American Psychiatric Publishing Textbook of Psychosomatic Medicine: Psychiatric Care of the Medically Ill. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2011 .
3. Kulkarni J, Worsley R, Gilbert H, et al. A prospective cohort study of antipsychotic medications in pregnancy: the first 147 pregnancies and 100 one year old babies. PLoS One. 2014;9(5):e94788.
4. US Food and Drug Administration. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. https://www.fda.gov/downloads/aboutfda/reportsmanualsforms/reports/economicanalyses/ucm427798.pdf. Accessed April 10, 2018.
5. Ennis ZN, Damkier P. Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations. A systematic review. Basic Clin Pharmacol Toxicol. 2015;116(4):315-320.
6. Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry. 2016:173(3):263-270.
7. Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938-946.
8. Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf. 2014;5(2):100-109.
9 . Crawford MB, DeLisi LE. Issues related to sex differences in antipsychotic treatment. Curr Opin Psychiatry. 2016;29(3):211-217.
10. Chisolm MS, Payne JL. Management of psychotropic drugs during pregnancy. BMJ. 2016;352:h5918.
11. US Food and Drug Administration. FDA drug safety communication: antipsychotic drug labels updated on use during pregnancy and risk of abnormal muscle movements and withdrawal symptoms in newborns. https://www.fda.gov/Drugs/DrugSafety/ucm243903.htm. Updated August 4, 2017. Accessed April 10, 2018.
12 . Tosato S, Albert U, Tomassi S, et al. A systematized review of atypical antipsychotics in pregnant women: balancing between risks of untreated illness and risks of drug-related adverse effects. J Clin Psychiatry. 2017;78(5):e477-e489.
13. Petersen I, Sammon CJ, McCrea RL, et al. Risks associated with antipsychotic treatment in pregnancy: comparative cohort studies based on electronic health records. Schizophr Res. 2016; 76(2-3):349-356.
1. Centers for Disease Control and Prevention. CDC report: mental illness surveillance among adults in the United States. https://www.cdc.gov/mentalhealthsurveillance/fact_sheet.html. Archived document. Updated December 2, 2011. Accessed April 10, 2018.
2. L evenson JL, ed . The American Psychiatric Publishing Textbook of Psychosomatic Medicine: Psychiatric Care of the Medically Ill. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2011 .
3. Kulkarni J, Worsley R, Gilbert H, et al. A prospective cohort study of antipsychotic medications in pregnancy: the first 147 pregnancies and 100 one year old babies. PLoS One. 2014;9(5):e94788.
4. US Food and Drug Administration. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. https://www.fda.gov/downloads/aboutfda/reportsmanualsforms/reports/economicanalyses/ucm427798.pdf. Accessed April 10, 2018.
5. Ennis ZN, Damkier P. Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations. A systematic review. Basic Clin Pharmacol Toxicol. 2015;116(4):315-320.
6. Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry. 2016:173(3):263-270.
7. Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938-946.
8. Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf. 2014;5(2):100-109.
9 . Crawford MB, DeLisi LE. Issues related to sex differences in antipsychotic treatment. Curr Opin Psychiatry. 2016;29(3):211-217.
10. Chisolm MS, Payne JL. Management of psychotropic drugs during pregnancy. BMJ. 2016;352:h5918.
11. US Food and Drug Administration. FDA drug safety communication: antipsychotic drug labels updated on use during pregnancy and risk of abnormal muscle movements and withdrawal symptoms in newborns. https://www.fda.gov/Drugs/DrugSafety/ucm243903.htm. Updated August 4, 2017. Accessed April 10, 2018.
12 . Tosato S, Albert U, Tomassi S, et al. A systematized review of atypical antipsychotics in pregnant women: balancing between risks of untreated illness and risks of drug-related adverse effects. J Clin Psychiatry. 2017;78(5):e477-e489.
13. Petersen I, Sammon CJ, McCrea RL, et al. Risks associated with antipsychotic treatment in pregnancy: comparative cohort studies based on electronic health records. Schizophr Res. 2016; 76(2-3):349-356.
Johnson v. Monsanto: Roundup and product liability
QUESTION: A groundskeeper alleges he developed terminal lymphoma from the use of the weed killer Roundup. A homeowner injures his leg while using a lawnmower. One thousand litigants allege Lipitor caused them to develop diabetes. A patient sues his doctor for a serious allergic reaction to an antibiotic.
Which of the following statements is incorrect?
A. These are all examples of product liability.
B. If successful, the groundskeeper may be awarded hundreds of millions of dollars in damages.
C. The homeowner has a cause of action against the manufacturer, even if it’s a borrowed lawnmower from a neighbor.
D. It is now harder to include multiple plaintiffs in a class-action lawsuit.
E. The “learned intermediary doctrine” immunizes the drug manufacturer from liability for a patient’s allergic reaction.
ANSWER: A. Johnson v. Monsanto was a recent case in San Francisco that resulted in a verdict for the plaintiff to the tune of $289 million.1 DeWayne Johnson, a 46-year-old groundskeeper, had developed non-Hodgkin lymphoma after using the weed killer, Roundup, to treat the school grounds, sometimes spraying the herbicide for several hours a day. Mr. Johnson alleged that Roundup’s active ingredient, glyphosate, is a known carcinogen, and that Monsanto, its manufacturer, failed to provide appropriate warning regarding this dangerous product.
The judge in the case allowed into evidence internal emails and experts’ warnings, as well as a critical 2015 position paper of the World Health Organization’s international agency for research on cancer, which classified glyphosate as “probably carcinogenic to humans.”2 Yet the herbicide, used widely in households and in commerce, is registered in 130 countries and approved for use on more than 100 crops. It was the first such case involving Roundup.
At trial, the jury unanimously found that Roundup was a substantial contributing factor in causing Mr. Johnson’s malignancy, that Monsanto failed to warn him of its health hazards (marketing “defect”), and that it knew or should have known that its product was unreasonably dangerous. The main portion, $250 million (of the $289 million award), was awarded as punitive damages.
Tort damages are of two types: compensatory and punitive. The former is to compensate the victim for past and future losses such as wages and medical expenses, pain and suffering, and/or emotional distress. On the other hand, punitive damages, also called exemplary damages, are awarded where there is a reckless, willful, or wanton disregard of the obvious risk of harm.
The case is currently under appeal. Meanwhile, another Roundup trial will soon take place in St. Louis, and the company is facing a class-action suit in U.S. district court in San Francisco, as well as several thousand claims in state courts throughout the country.
Johnson v. Monsanto is a typical product liability action. According to Section 102(2) of the Uniform Product Liability Act, product liability includes “all claims or action brought for personal injury, death, or property damage caused by the manufacture, design, formula, preparation, assembly, installation, testing, warnings, instructions, marketing, packaging, or labeling of any product.”
There are basically three legal theories in a product liability claim: negligence, breach of warranty, and strict product liability. The latter is the most favored by plaintiffs, as there is no need to prove fault or warranty.
In a seminal case in 1963, William Greenman was injured when he used a power tool that was given to him as a gift.3 He sued the manufacturer, although there was no direct contract of warranty between him and the manufacturer, as he did not make the purchase himself.
The California Supreme Court went beyond the law of contracts and negligence by introducing the notion of strict liability, which centers on whether a product is defective and unreasonably dangerous. It holds that a professional supplier who sells a product that is both defective and unreasonably dangerous is strictly liable to foreseeable plaintiffs.
“Defective” is usually defined as product quality that is less than what a reasonable consumer expects. It can be a design, manufacturing, or marketing defect, the latter instance typically showing up as a failure to warn. “Unreasonably dangerous” is a conclusion that the risks that result from its condition outweigh the product’s advantages.
Product liability lawsuits commonly involve pharmaceutical products and medical devices. Recent examples are suits against Pfizer over Lipitor’s alleged role as a cause of diabetes and against Johnson & Johnson over its talcum products purportedly causing ovarian cancer.4
When the same product injures multiple plaintiffs, they may band together to file a common legal action against the manufacturer. This is called a class-action suit, and will proceed if it is certified to satisfy four prerequisites: numerosity, commonality, typicality, and adequacy.5 A class-action lawsuit, governed by Rule 23 of the Federal Rules of Civil Procedure, describes a legal cause of action where a representative plaintiff asserts claims on behalf of a large class of similarly injured members, who then give up their rights to pursue an individual lawsuit. It confers several advantages upon the plaintiffs, including the potential of higher damages.
A recent U.S. Supreme Court decision has, however, put a damper on class-action suits by tightening the jurisdictional requirement.6
The case involved Bristol-Myers Squibb, which was sued in California by several hundred individuals from 33 states for injuries from the platelet inhibitor Plavix (clopidogrel). The issue was whether non–California residents could sue in that state for injuries incurred elsewhere.
In a 8-1 decision, the U.S. Supreme Court held that California courts did not have specific jurisdiction to hear the claims of nonresidents without identifying an adequate link between the state and the nonresidents’ claims, as they weren’t prescribed Plavix in the state, didn’t buy or take the drug there, and weren’t injured by the drug there.
Finally, note that, should a doctor fail to warn an injured patient of a known medication risk, the patient may have a claim against the doctor – but usually not against the drug manufacturer. This is termed the “learned-intermediary doctrine.” The justification is that manufacturers can reasonably rely on the treating doctor to warn of adverse effects, which are disclosed to the profession through its sales reps and in its package insert and the Physician’s Desk Reference. The treating doctor, in turn, is expected to use his or her professional judgment to adequately warn the patient. It is simply not feasible for the manufacturer to directly warn every patient without usurping the doctor-patient relationship.
Such lawsuits fall in the common category of medical negligence and lack of informed consent, and are not considered a product liability action.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. DeWayne Johnson v. Monsanto Co., Superior Court of California, County of San Francisco, Case No. CGC-16-550128, June 18, 2018.
2. The Monsanto Papers: Roundup (Glyphosate) Cancer Case Key Documents & Analysis. Available at usrtk.org.
3. Greenman v. Yuba Power Products Inc., 377 P.2d 897 (Cal. 1963).
4. “Defective and unreasonably dangerous,” Internal Medicine News, Nov. 4, 2014.
5. “Class-action lawsuits,” Internal Medicine News, April 1, 2015.
6. Bristol-Myers Squibb Co. v. Superior Court of California, 582 U.S. ____ (2017).
QUESTION: A groundskeeper alleges he developed terminal lymphoma from the use of the weed killer Roundup. A homeowner injures his leg while using a lawnmower. One thousand litigants allege Lipitor caused them to develop diabetes. A patient sues his doctor for a serious allergic reaction to an antibiotic.
Which of the following statements is incorrect?
A. These are all examples of product liability.
B. If successful, the groundskeeper may be awarded hundreds of millions of dollars in damages.
C. The homeowner has a cause of action against the manufacturer, even if it’s a borrowed lawnmower from a neighbor.
D. It is now harder to include multiple plaintiffs in a class-action lawsuit.
E. The “learned intermediary doctrine” immunizes the drug manufacturer from liability for a patient’s allergic reaction.
ANSWER: A. Johnson v. Monsanto was a recent case in San Francisco that resulted in a verdict for the plaintiff to the tune of $289 million.1 DeWayne Johnson, a 46-year-old groundskeeper, had developed non-Hodgkin lymphoma after using the weed killer, Roundup, to treat the school grounds, sometimes spraying the herbicide for several hours a day. Mr. Johnson alleged that Roundup’s active ingredient, glyphosate, is a known carcinogen, and that Monsanto, its manufacturer, failed to provide appropriate warning regarding this dangerous product.
The judge in the case allowed into evidence internal emails and experts’ warnings, as well as a critical 2015 position paper of the World Health Organization’s international agency for research on cancer, which classified glyphosate as “probably carcinogenic to humans.”2 Yet the herbicide, used widely in households and in commerce, is registered in 130 countries and approved for use on more than 100 crops. It was the first such case involving Roundup.
At trial, the jury unanimously found that Roundup was a substantial contributing factor in causing Mr. Johnson’s malignancy, that Monsanto failed to warn him of its health hazards (marketing “defect”), and that it knew or should have known that its product was unreasonably dangerous. The main portion, $250 million (of the $289 million award), was awarded as punitive damages.
Tort damages are of two types: compensatory and punitive. The former is to compensate the victim for past and future losses such as wages and medical expenses, pain and suffering, and/or emotional distress. On the other hand, punitive damages, also called exemplary damages, are awarded where there is a reckless, willful, or wanton disregard of the obvious risk of harm.
The case is currently under appeal. Meanwhile, another Roundup trial will soon take place in St. Louis, and the company is facing a class-action suit in U.S. district court in San Francisco, as well as several thousand claims in state courts throughout the country.
Johnson v. Monsanto is a typical product liability action. According to Section 102(2) of the Uniform Product Liability Act, product liability includes “all claims or action brought for personal injury, death, or property damage caused by the manufacture, design, formula, preparation, assembly, installation, testing, warnings, instructions, marketing, packaging, or labeling of any product.”
There are basically three legal theories in a product liability claim: negligence, breach of warranty, and strict product liability. The latter is the most favored by plaintiffs, as there is no need to prove fault or warranty.
In a seminal case in 1963, William Greenman was injured when he used a power tool that was given to him as a gift.3 He sued the manufacturer, although there was no direct contract of warranty between him and the manufacturer, as he did not make the purchase himself.
The California Supreme Court went beyond the law of contracts and negligence by introducing the notion of strict liability, which centers on whether a product is defective and unreasonably dangerous. It holds that a professional supplier who sells a product that is both defective and unreasonably dangerous is strictly liable to foreseeable plaintiffs.
“Defective” is usually defined as product quality that is less than what a reasonable consumer expects. It can be a design, manufacturing, or marketing defect, the latter instance typically showing up as a failure to warn. “Unreasonably dangerous” is a conclusion that the risks that result from its condition outweigh the product’s advantages.
Product liability lawsuits commonly involve pharmaceutical products and medical devices. Recent examples are suits against Pfizer over Lipitor’s alleged role as a cause of diabetes and against Johnson & Johnson over its talcum products purportedly causing ovarian cancer.4
When the same product injures multiple plaintiffs, they may band together to file a common legal action against the manufacturer. This is called a class-action suit, and will proceed if it is certified to satisfy four prerequisites: numerosity, commonality, typicality, and adequacy.5 A class-action lawsuit, governed by Rule 23 of the Federal Rules of Civil Procedure, describes a legal cause of action where a representative plaintiff asserts claims on behalf of a large class of similarly injured members, who then give up their rights to pursue an individual lawsuit. It confers several advantages upon the plaintiffs, including the potential of higher damages.
A recent U.S. Supreme Court decision has, however, put a damper on class-action suits by tightening the jurisdictional requirement.6
The case involved Bristol-Myers Squibb, which was sued in California by several hundred individuals from 33 states for injuries from the platelet inhibitor Plavix (clopidogrel). The issue was whether non–California residents could sue in that state for injuries incurred elsewhere.
In a 8-1 decision, the U.S. Supreme Court held that California courts did not have specific jurisdiction to hear the claims of nonresidents without identifying an adequate link between the state and the nonresidents’ claims, as they weren’t prescribed Plavix in the state, didn’t buy or take the drug there, and weren’t injured by the drug there.
Finally, note that, should a doctor fail to warn an injured patient of a known medication risk, the patient may have a claim against the doctor – but usually not against the drug manufacturer. This is termed the “learned-intermediary doctrine.” The justification is that manufacturers can reasonably rely on the treating doctor to warn of adverse effects, which are disclosed to the profession through its sales reps and in its package insert and the Physician’s Desk Reference. The treating doctor, in turn, is expected to use his or her professional judgment to adequately warn the patient. It is simply not feasible for the manufacturer to directly warn every patient without usurping the doctor-patient relationship.
Such lawsuits fall in the common category of medical negligence and lack of informed consent, and are not considered a product liability action.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. DeWayne Johnson v. Monsanto Co., Superior Court of California, County of San Francisco, Case No. CGC-16-550128, June 18, 2018.
2. The Monsanto Papers: Roundup (Glyphosate) Cancer Case Key Documents & Analysis. Available at usrtk.org.
3. Greenman v. Yuba Power Products Inc., 377 P.2d 897 (Cal. 1963).
4. “Defective and unreasonably dangerous,” Internal Medicine News, Nov. 4, 2014.
5. “Class-action lawsuits,” Internal Medicine News, April 1, 2015.
6. Bristol-Myers Squibb Co. v. Superior Court of California, 582 U.S. ____ (2017).
QUESTION: A groundskeeper alleges he developed terminal lymphoma from the use of the weed killer Roundup. A homeowner injures his leg while using a lawnmower. One thousand litigants allege Lipitor caused them to develop diabetes. A patient sues his doctor for a serious allergic reaction to an antibiotic.
Which of the following statements is incorrect?
A. These are all examples of product liability.
B. If successful, the groundskeeper may be awarded hundreds of millions of dollars in damages.
C. The homeowner has a cause of action against the manufacturer, even if it’s a borrowed lawnmower from a neighbor.
D. It is now harder to include multiple plaintiffs in a class-action lawsuit.
E. The “learned intermediary doctrine” immunizes the drug manufacturer from liability for a patient’s allergic reaction.
ANSWER: A. Johnson v. Monsanto was a recent case in San Francisco that resulted in a verdict for the plaintiff to the tune of $289 million.1 DeWayne Johnson, a 46-year-old groundskeeper, had developed non-Hodgkin lymphoma after using the weed killer, Roundup, to treat the school grounds, sometimes spraying the herbicide for several hours a day. Mr. Johnson alleged that Roundup’s active ingredient, glyphosate, is a known carcinogen, and that Monsanto, its manufacturer, failed to provide appropriate warning regarding this dangerous product.
The judge in the case allowed into evidence internal emails and experts’ warnings, as well as a critical 2015 position paper of the World Health Organization’s international agency for research on cancer, which classified glyphosate as “probably carcinogenic to humans.”2 Yet the herbicide, used widely in households and in commerce, is registered in 130 countries and approved for use on more than 100 crops. It was the first such case involving Roundup.
At trial, the jury unanimously found that Roundup was a substantial contributing factor in causing Mr. Johnson’s malignancy, that Monsanto failed to warn him of its health hazards (marketing “defect”), and that it knew or should have known that its product was unreasonably dangerous. The main portion, $250 million (of the $289 million award), was awarded as punitive damages.
Tort damages are of two types: compensatory and punitive. The former is to compensate the victim for past and future losses such as wages and medical expenses, pain and suffering, and/or emotional distress. On the other hand, punitive damages, also called exemplary damages, are awarded where there is a reckless, willful, or wanton disregard of the obvious risk of harm.
The case is currently under appeal. Meanwhile, another Roundup trial will soon take place in St. Louis, and the company is facing a class-action suit in U.S. district court in San Francisco, as well as several thousand claims in state courts throughout the country.
Johnson v. Monsanto is a typical product liability action. According to Section 102(2) of the Uniform Product Liability Act, product liability includes “all claims or action brought for personal injury, death, or property damage caused by the manufacture, design, formula, preparation, assembly, installation, testing, warnings, instructions, marketing, packaging, or labeling of any product.”
There are basically three legal theories in a product liability claim: negligence, breach of warranty, and strict product liability. The latter is the most favored by plaintiffs, as there is no need to prove fault or warranty.
In a seminal case in 1963, William Greenman was injured when he used a power tool that was given to him as a gift.3 He sued the manufacturer, although there was no direct contract of warranty between him and the manufacturer, as he did not make the purchase himself.
The California Supreme Court went beyond the law of contracts and negligence by introducing the notion of strict liability, which centers on whether a product is defective and unreasonably dangerous. It holds that a professional supplier who sells a product that is both defective and unreasonably dangerous is strictly liable to foreseeable plaintiffs.
“Defective” is usually defined as product quality that is less than what a reasonable consumer expects. It can be a design, manufacturing, or marketing defect, the latter instance typically showing up as a failure to warn. “Unreasonably dangerous” is a conclusion that the risks that result from its condition outweigh the product’s advantages.
Product liability lawsuits commonly involve pharmaceutical products and medical devices. Recent examples are suits against Pfizer over Lipitor’s alleged role as a cause of diabetes and against Johnson & Johnson over its talcum products purportedly causing ovarian cancer.4
When the same product injures multiple plaintiffs, they may band together to file a common legal action against the manufacturer. This is called a class-action suit, and will proceed if it is certified to satisfy four prerequisites: numerosity, commonality, typicality, and adequacy.5 A class-action lawsuit, governed by Rule 23 of the Federal Rules of Civil Procedure, describes a legal cause of action where a representative plaintiff asserts claims on behalf of a large class of similarly injured members, who then give up their rights to pursue an individual lawsuit. It confers several advantages upon the plaintiffs, including the potential of higher damages.
A recent U.S. Supreme Court decision has, however, put a damper on class-action suits by tightening the jurisdictional requirement.6
The case involved Bristol-Myers Squibb, which was sued in California by several hundred individuals from 33 states for injuries from the platelet inhibitor Plavix (clopidogrel). The issue was whether non–California residents could sue in that state for injuries incurred elsewhere.
In a 8-1 decision, the U.S. Supreme Court held that California courts did not have specific jurisdiction to hear the claims of nonresidents without identifying an adequate link between the state and the nonresidents’ claims, as they weren’t prescribed Plavix in the state, didn’t buy or take the drug there, and weren’t injured by the drug there.
Finally, note that, should a doctor fail to warn an injured patient of a known medication risk, the patient may have a claim against the doctor – but usually not against the drug manufacturer. This is termed the “learned-intermediary doctrine.” The justification is that manufacturers can reasonably rely on the treating doctor to warn of adverse effects, which are disclosed to the profession through its sales reps and in its package insert and the Physician’s Desk Reference. The treating doctor, in turn, is expected to use his or her professional judgment to adequately warn the patient. It is simply not feasible for the manufacturer to directly warn every patient without usurping the doctor-patient relationship.
Such lawsuits fall in the common category of medical negligence and lack of informed consent, and are not considered a product liability action.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. DeWayne Johnson v. Monsanto Co., Superior Court of California, County of San Francisco, Case No. CGC-16-550128, June 18, 2018.
2. The Monsanto Papers: Roundup (Glyphosate) Cancer Case Key Documents & Analysis. Available at usrtk.org.
3. Greenman v. Yuba Power Products Inc., 377 P.2d 897 (Cal. 1963).
4. “Defective and unreasonably dangerous,” Internal Medicine News, Nov. 4, 2014.
5. “Class-action lawsuits,” Internal Medicine News, April 1, 2015.
6. Bristol-Myers Squibb Co. v. Superior Court of California, 582 U.S. ____ (2017).