User login
The diagnosis and treatment of ureteral injury
A gynecologic surgeon learns very early in his/her career to respect the ureter. Whether from the procedure being performed (endometriosis surgery, hysterectomy, myomectomy for ligamentous fibroids, salpingo-oophorectomy, excision of ovarian remnants, adhesiolysis), blood loss that obscures visualization and must be controlled, or use of energy for cutting, desiccation, and coagulation leading to potential lateral tissue damage, ureteral injury is a well-known complication. Even normal anatomic variations may put some women at greater risk; according to Hurd et al. (Am J Obstet Gynecol. 2001;184:336-9). In a small subset of women, the distance between the cervix and the ureter may be less than 0.5 cm.
As a practicing minimally invasive gynecologic surgeon for the past 30 years, and an early adapter to laparoscopic hysterectomy, I remember quite well the recommendation to always dissect out ureters at time of the procedure. At present, most will agree that selective dissection is safe and thus, more desirable, as bleeding, damage secondary to desiccation, and ureter devascularization with subsequent necrosis are all increased with ureterolysis.
I agree with Dr. Kenton and Dr. Mueller that ureteral stenting has not been shown to significantly decrease ureteral injury rates. Often times, with loss of peristalsis secondary to stent placement, locating the ureter may be even more difficult. Recent advances using lighted stents or indocyanine green, which fluoresces in response to near-infrared laser and can be injected into the ureter via the ureteral catheter tip, are still in the feasibility phase of evaluation and can be costly.
As most urogenital fistulae are secondary to unrecognized injuries at time of surgery, and due to the fact that intraoperative recognition of the injury allows for primary repair, thus, decreasing the rate of secondary surgery and the associated increased morbidity, I recommend cystoscopy to check for ureteral jets (ureteral efflux) be performed when there is concern regarding ureter compromise.
Currently, I utilize a 70° cystoscope to visualize the ureters. While in the past, I have used intravenous indigo carmine, methylene blue, or fluorescein sodium, I currently use Pyridium (phenazopyridine) 200 mg taken by mouth 1 hour prior to the procedure.
Unfortunately, ureteral jetting still may be noted despite partial ligation, laceration, or desiccation of the ureter.
If ureteral injury is not recognized at time of surgery, it can lead to various postoperative symptoms. If there is a ureteral defect, the patient may note profuse wound leakage, increased abdominal fluid, or a urinoma, ileus, fever, peritonitis, or hematuria. With ureteral obstruction, flank or abdominal pain or anuria can be noted; while, with fistula formation, the patient will likely present with urinary incontinence or watery vaginal discharge.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
A gynecologic surgeon learns very early in his/her career to respect the ureter. Whether from the procedure being performed (endometriosis surgery, hysterectomy, myomectomy for ligamentous fibroids, salpingo-oophorectomy, excision of ovarian remnants, adhesiolysis), blood loss that obscures visualization and must be controlled, or use of energy for cutting, desiccation, and coagulation leading to potential lateral tissue damage, ureteral injury is a well-known complication. Even normal anatomic variations may put some women at greater risk; according to Hurd et al. (Am J Obstet Gynecol. 2001;184:336-9). In a small subset of women, the distance between the cervix and the ureter may be less than 0.5 cm.
As a practicing minimally invasive gynecologic surgeon for the past 30 years, and an early adapter to laparoscopic hysterectomy, I remember quite well the recommendation to always dissect out ureters at time of the procedure. At present, most will agree that selective dissection is safe and thus, more desirable, as bleeding, damage secondary to desiccation, and ureter devascularization with subsequent necrosis are all increased with ureterolysis.
I agree with Dr. Kenton and Dr. Mueller that ureteral stenting has not been shown to significantly decrease ureteral injury rates. Often times, with loss of peristalsis secondary to stent placement, locating the ureter may be even more difficult. Recent advances using lighted stents or indocyanine green, which fluoresces in response to near-infrared laser and can be injected into the ureter via the ureteral catheter tip, are still in the feasibility phase of evaluation and can be costly.
As most urogenital fistulae are secondary to unrecognized injuries at time of surgery, and due to the fact that intraoperative recognition of the injury allows for primary repair, thus, decreasing the rate of secondary surgery and the associated increased morbidity, I recommend cystoscopy to check for ureteral jets (ureteral efflux) be performed when there is concern regarding ureter compromise.
Currently, I utilize a 70° cystoscope to visualize the ureters. While in the past, I have used intravenous indigo carmine, methylene blue, or fluorescein sodium, I currently use Pyridium (phenazopyridine) 200 mg taken by mouth 1 hour prior to the procedure.
Unfortunately, ureteral jetting still may be noted despite partial ligation, laceration, or desiccation of the ureter.
If ureteral injury is not recognized at time of surgery, it can lead to various postoperative symptoms. If there is a ureteral defect, the patient may note profuse wound leakage, increased abdominal fluid, or a urinoma, ileus, fever, peritonitis, or hematuria. With ureteral obstruction, flank or abdominal pain or anuria can be noted; while, with fistula formation, the patient will likely present with urinary incontinence or watery vaginal discharge.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
A gynecologic surgeon learns very early in his/her career to respect the ureter. Whether from the procedure being performed (endometriosis surgery, hysterectomy, myomectomy for ligamentous fibroids, salpingo-oophorectomy, excision of ovarian remnants, adhesiolysis), blood loss that obscures visualization and must be controlled, or use of energy for cutting, desiccation, and coagulation leading to potential lateral tissue damage, ureteral injury is a well-known complication. Even normal anatomic variations may put some women at greater risk; according to Hurd et al. (Am J Obstet Gynecol. 2001;184:336-9). In a small subset of women, the distance between the cervix and the ureter may be less than 0.5 cm.
As a practicing minimally invasive gynecologic surgeon for the past 30 years, and an early adapter to laparoscopic hysterectomy, I remember quite well the recommendation to always dissect out ureters at time of the procedure. At present, most will agree that selective dissection is safe and thus, more desirable, as bleeding, damage secondary to desiccation, and ureter devascularization with subsequent necrosis are all increased with ureterolysis.
I agree with Dr. Kenton and Dr. Mueller that ureteral stenting has not been shown to significantly decrease ureteral injury rates. Often times, with loss of peristalsis secondary to stent placement, locating the ureter may be even more difficult. Recent advances using lighted stents or indocyanine green, which fluoresces in response to near-infrared laser and can be injected into the ureter via the ureteral catheter tip, are still in the feasibility phase of evaluation and can be costly.
As most urogenital fistulae are secondary to unrecognized injuries at time of surgery, and due to the fact that intraoperative recognition of the injury allows for primary repair, thus, decreasing the rate of secondary surgery and the associated increased morbidity, I recommend cystoscopy to check for ureteral jets (ureteral efflux) be performed when there is concern regarding ureter compromise.
Currently, I utilize a 70° cystoscope to visualize the ureters. While in the past, I have used intravenous indigo carmine, methylene blue, or fluorescein sodium, I currently use Pyridium (phenazopyridine) 200 mg taken by mouth 1 hour prior to the procedure.
Unfortunately, ureteral jetting still may be noted despite partial ligation, laceration, or desiccation of the ureter.
If ureteral injury is not recognized at time of surgery, it can lead to various postoperative symptoms. If there is a ureteral defect, the patient may note profuse wound leakage, increased abdominal fluid, or a urinoma, ileus, fever, peritonitis, or hematuria. With ureteral obstruction, flank or abdominal pain or anuria can be noted; while, with fistula formation, the patient will likely present with urinary incontinence or watery vaginal discharge.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
The FDA’s novel drugs approved in 2017
Novel drugs are innovative new products that have never before been used in clinical practice. Among the 46 that the Food and Drug Administration approved in 2017, 45 could be used in pregnancy. One, cerliponase alfa (Brineura), is indicated for pediatric patients 3 years of age or older, for treatment of late infantile neuronal ceroid lipofuscinosis type 2. It is doubtful that this drug would be used in pregnancy or during breastfeeding.
With the two exceptions noted below, there are no human pregnancy data for these drugs. It is important to consider that although high molecular weight (MW) drugs (for example, greater than 1,000) probably do not usually cross the placenta in the first half of pregnancy, they may do so in late pregnancy. The cited MWs are shown as the nearest whole number. Animal reproductive data are also cited because, although not definitive, they can provide some measure of the human embryo-fetal risk.
Anti-infectives
Benznidazole (same trade name) (MW 441), given orally, is indicated for pediatric patients aged 2-12 years for treatment of Chagas disease (American trypanosomiasis) caused by Trypanosoma cruzi. However, there are international reports describing its use in pregnancy and breastfeeding. No fetal harm from these exposures were noted. Nevertheless, because of the low MW and the reported animal risk, avoiding the drug during the first half of pregnancy appears to be the best choice. Delafloxacin (Baxdela) (MW 441), a fluoroquinolone antimicrobial given intravenously or orally, is indicated for acute bacterial skin infections. The animal data suggest low risk. However, like other fluoroquinolones, it is contraindicated in pregnancy and should be used only if there are no other alternatives.
Sofosbuvir/velpatasvir /voxilaprevir (Vosevi) (MWs 529, 883, 869), a fixed oral dose combination of three antivirals, is indicated for the treatment of hepatitis C virus infection. The MWs suggest that all three will cross the human placenta. The animal data suggest low risk. Secnidazole (Solosec) (MW 185), given orally, is indicated for the treatment of bacterial vaginosis. It is closely related to metronidazole. No evidence of embryo-fetal toxicity was observed in rats and rabbits, suggesting that the human risk is low. In a report from Brazil, 134 pregnant women with bacterial vaginosis were treated with secnidazole, metronidazole, or tinidazole in the second and third trimesters. Treatment significantly decreased the incidence of premature rupture of membranes, preterm labor, preterm birth, and low birth weight. No fetal harm was reported.
Antineoplastics
[Note: All of the drugs in this category are best avoided, if possible, in pregnancy and breastfeeding.]
Abemaciclib (Verzenio) (MW 507), an oral inhibitor of cyclin-dependent kinases, is indicated for the treatment of breast cancer. The drug is teratogenic in rats. Acalabrutinib (Calquence) (MW 466) is an oral kinase inhibitor indicated for mantle cell lymphoma. The drug had no effect on the rat embryo-fetus but caused decreased fetal body weights and delayed skeletal ossification in rabbits. Avelumab (Bavencio) (MW 147,000) is given intravenously for the treatment of metastatic Merkel cell carcinoma and metastatic urothelial carcinoma. Animal reproduction studies have not been conducted. However, based on its mechanism of action, fetal exposure may increase the risk of developing immune-related disorders or altering the normal immune response.
Brigatinib (Alunbrig) (MW 584) is given orally for the treatment of metastatic non–small-cell lung cancer. In rats, doses less than or slightly above the human exposure caused multiple anomalies in the fetuses of pregnant rats. Copanlisib (Aliqopa) (MW 553) is a kinase inhibitor that is given intravenously for relapsed follicular lymphoma. In rats during organogenesis, doses based on body surface area that were a fraction of the human dose caused embryo-fetal death and fetal defects. Durvalumab (Imfinzi) (MW 146,000), given intravenously, is indicated for the treatment of metastatic urothelial carcinoma and non–small-cell lung cancer. Monkeys given the drug from organogenesis through delivery experienced increased premature birth, fetal loss, and premature neonatal death. Women of reproductive potential should use effective contraception during treatment and for at least 3 months after the last dose.
Enasidenib (Idhifa) (MW 569), given orally, is indicated for the treatment of myeloid leukemia. The drug caused maternal toxicity and adverse embryo-fetal effects (postimplantation loss, resorptions, decrease viable fetuses, lower fetal birth weights, and skeletal variations) in rats and spontaneous abortions in rabbits. Inotuzumab ozogamicin (Besponsa) (MW 160,000), given intravenously, is indicated for relapsed or refractory B-cell precursor acute lymphoblastic leukemia. The drug caused fetal harm in rats but not in rabbits. Midostaurin (Rydapt) (MW 571) is an oral kinase inhibitor indicated for myeloid leukemia. In rats, a dose given during the first week of pregnancy that was a small fraction of the human exposure caused pre- and postimplantation loss. When very small doses were given during organogenesis to rats and rabbits there was significant maternal and fetal toxicity.
Neratinib (Nerlynx) (MW 673) is an oral kinase inhibitor for breast cancer. Although the drug did not cause embryo-fetal toxicity in rats, it did cause this toxicity in rabbits. Doses that resulted in exposures that were less than the human exposure caused maternal toxicity, abortions, and embryo-fetal death. Lower doses caused multiple fetal anomalies. Niraparib (Zejula) (MW 511) is indicated for treatment of epithelial ovarian, fallopian, or peritoneal cancer. Because of the potential human embryo-fetal risk based on its mechanism of action, pregnant animal studies were not conducted. Women with reproductive potential should use effective contraception during treatment and for 6 months after the last dose. Ribociclib (Kisqali) (MW 553) is an oral kinase inhibitor indicated for postmenopausal women with breast cancer. In rats, the drug cause reduced fetal weights and skeletal changes. Increased incidences of fetal abnormalities and lower fetal weights were observed in rabbits.
Cardiovascular
Angiotensin II (Giapreza) (MW 1,046) is a naturally occurring peptide hormone given as an intravenous infusion. It is indicated as a vasoconstrictor to increase blood pressure in adults with septic or other distributive shock. Animal reproduction studies have not been conducted. Because septic or other distributive shock is a medical emergency that can be fatal, the use of this agent in pregnancy should not be withheld.
Central nervous system
Deutetrabenazine (Austedo) (MW 324) is an oral drug indicated for the treatment of chorea associated with Huntington’s disease and for tardive dyskinesia. When given to rats during organogenesis there was no clear effect on embryo-fetal development.
Edaravone (Radicava) (MW 174), given as an intravenous infusion, is indicated for the treatment of amyotrophic lateral sclerosis. Doses that were not maternal toxic did not cause embryo-fetal toxicity in rats and rabbits. However, the no-effect dose for developmental toxicity was less than the recommended human dose. Naldemedine (Symproic) (MW 743) is an opioid antagonist indicated for the treatment of opioid-induced constipation. The drug crosses the human placenta and may precipitate opioid withdrawal in the fetus. The drug caused no embryo-fetal adverse effects, even at high doses, in pregnant rats and rabbits.
Ocrelizumab (Ocrevus) (MW 145,000), an intravenous agent, is used to treat patients with multiple sclerosis. The MW is high but immunoglobulins are known to cross the placenta. When given to monkeys at doses similar to or greater than the human dose, there was increased perinatal mortality, depletion of B-cell populations, and renal, bone marrow, and testicular toxicity in the offspring in the absence of maternal toxicity. Safinamide (Xadago) (MW 399) is an oral drug indicated as adjunctive treatment to levodopa/carbidopa in Parkinson’s disease. In rats, the drug was teratogenic (mainly urogenital defects) at all doses. When it was combined with levodopa/carbidopa or used alone, increased rates of fetal visceral and skeletal defects occurred at all doses studied. In rabbits, given the combination throughout organogenesis, there was an increased incidence of embryo-fetal death and cardiac and skeletal defects. Based on these data, avoiding the drug in pregnancy appears to be the best course.
Valbenazine (Ingrezza) (MW 419) is indicated for the treatment of tardive dyskinesia. The drug caused no malformations in rats and rabbits. However, in rats given the drug during organogenesis through lactation, an increase in the number of stillborn pups and postnatal pup mortalities was observed.
Dermatologic
Brodalumab (Siliq) (MW 144,000), given subcutaneously, is indicated for the treatment of moderate to severe plaque psoriasis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In monkeys, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from mothers given weekly subcutaneous doses of the drug. Dupilumab (Dupixent) (MW 144,000) is given subcutaneously for the treatment of atopic dermatitis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In pregnant monkeys given subcutaneous doses of the drug, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from birth to 6 months of age.
Guselkumab (Tremfya) (MW 143,600) is given subcutaneously for the treatment of moderate to severe plaque psoriasis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In pregnant monkeys given subcutaneous doses of the drug, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from birth to 6 months of age. However, neonatal deaths were observed in three monkeys given six times the maximum recommended human dose.
Endocrine/metabolic
Deflazacort (Emflaza) (MW 442) is an oral corticosteroid prodrug indicated for the treatment of Duchenne muscular dystrophy. The drug is converted in vivo to an active metabolite. The drug readily crosses the placenta. Although animal reproduction studies have not been conducted, such studies with other corticosteroids in various animal species have shown an increased incidence of cleft palate. In some species, there was an increase in embryo-fetal death, intrauterine growth restriction, and constriction of the ductus arteriosus.
Ertugliflozin (Steglatro) (MW 566) is an oral drug indicated to improve glycemic control in adults with type 2 diabetes mellitus. In juvenile rats, doses that were about 13 times the human dose caused increased kidney weight, renal tubule and renal pelvis dilatation, and renal mineralization. These effects occurred during periods of rat renal development that correspond to the late second and third trimester of human renal development, and did not fully reverse within a 1-month recovery period. Etelcalcetide (Parsabiv) (MW 1,048), an intravenous calcium-sensing receptor agonist, is indicated for patients on hemodialysis who have secondary hyperparathyroidism. In rats and rabbits given the drug during organogenesis, there was reduced fetal growth. In rats given the drug during organogenesis through birth and weaning, there was a slight increase in pup mortality, delay in parturition, and transient effects on pup growth, but there were no effects on sexual maturation, neurobehavioral, or reproductive function. Macimorelin (Macrilen) (MW 535) is an oral growth hormone secretagogue receptor agonist. It is indicated for adult growth hormone deficiency. Animal reproduction studies have not been conducted.
Semaglutide (Ozempic) (MW 4,114), given subcutaneously, is a glucagon-like peptide indicated to improve glycemic control in type 2 diabetes mellitus. In rats given the drug during organogenesis, embryo-fetal death, structural defects, and alterations in growth were observed. In rabbits and monkeys given the drug during organogenesis, there were early pregnancy losses and structural abnormalities. In addition, there was marked maternal body weight loss in both animal species. Vestronidase alfa-vjbk (Mepsevii) is given intravenously. It is indicated for the treatment of Mucopolysaccharidosis VII (Sly syndrome). The calculated average MW of each nonglycosylated peptide chain is 72,562. In rats and rabbits given the drug during organogenesis, there was no maternal toxicity or adverse developmental outcomes.
Gastrointestinal
Plecanatide (Trulance) (MW 1,682) is an oral drug indicated for the treatment of constipation. The drug and its active metabolite are negligibly absorbed systemically and fetal exposure to the drug is not expected. In mice and rabbits given the oral drug during organogenesis, no effects on embryo-fetal development were observed. Telotristat ethyl (Xermelo) (MW 754) is an oral drug indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (MW not specified) therapy in adults not controlled by somatostatin analog. When given during organogenesis in rats, there was no effect on embryo-fetal development at doses that were about nine times the recommended human dose. However, an increased incidence of mortality in rat offspring was observed when the drug was given from organogenesis through lactation. During organogenesis in rabbits, the drug had no embryo-fetal effects at doses that were 10 or more times the human dose.
Hematologics
Betrixaban (Bevyxxa) (MW 568) is an oral factor Xa inhibitor indicated for the prophylaxis of venous thromboembolism. The drug was not associated with adverse developmental fetal outcomes in rats and rabbits. However, maternal hemorrhage did occur. In humans, there is an increased risk of hemorrhage during pregnancy and delivery. Emicizumab (Hemlibra) (MW 145,600), given subcutaneously, is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding in patients with hemophilia A with factor VIII inhibitors. Animal reproduction studies have not been conducted. It is a human monoclonal IgG antibody and, though the MW is high, IgG antibodies are known to cross the placenta.
Immunologic
Sarilumab (Kevzara) (MW 150,000) is given subcutaneously. It is indicated for patients with moderate to severe rheumatoid arthritis. Reproduction studies were conducted in pregnant monkeys. There was no evidence of embryo toxicity or fetal malformations. Based on this data, the human pregnancy risk is low.
Ophthalmic
Latanoprostene bunod (Vyzulta) (MW 508) is a prostaglandin analog that is indicated to reduce intraocular pressure. No quantifiable plasma concentrations of latanoprostene bunod were detected in nonpregnant patients. However, very low levels of latanoprost acid (51-59 pg/mL), the active metabolite, were detected with the maximal plasma concentration occurring 5 minutes after administration. When given intravenously to pregnant rabbits, the drug was shown to be abortifacient and teratogenic, but these effects were not observed in pregnant rats. Netarsudil (Rhopressa) (MW 454) is a kinase inhibitor indicated to reduce intraocular pressure in patients with open-angle glaucoma or ocular hypertension. No quantifiable plasma concentrations of netarsudil were detected in 18 subjects. For the active metabolite, a plasma level of 0.11 ng/mL was found in one subject. Intravenous doses to pregnant rats and rabbits during organogenesis did not cause embryo-fetal adverse effects at clinically relevant systemic exposures.
Parathyroid hormone
Abaloparatide (Tymlos) (MW 3,961), given subcutaneously, is a human parathyroid hormone related peptide analog that is indicated for postmenopausal women with osteoporosis at high risk for fracture. Reproduction studies in animals have not been conducted. Because of the indication, it is doubtful if the agent will be used in pregnancy or during breastfeeding.
Respiratory
Benralizumab (Fasenra) (MW 150,000), given subcutaneously, is indicated for the add-on maintenance treatment of severe eosinophilic asthma. It is a human monoclonal IgG antibody and, though the MW is high, IgG antibodies are known to cross the placenta. Studies in monkeys found no evidence of fetal harm with intravenous doses throughout pregnancy that produced exposures up to about 310 times the exposure at the maximum recommended human dose.
The potential adverse effects in an infant when the mother is taking one of the above drugs while breastfeeding will be covered in my next column.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Novel drugs are innovative new products that have never before been used in clinical practice. Among the 46 that the Food and Drug Administration approved in 2017, 45 could be used in pregnancy. One, cerliponase alfa (Brineura), is indicated for pediatric patients 3 years of age or older, for treatment of late infantile neuronal ceroid lipofuscinosis type 2. It is doubtful that this drug would be used in pregnancy or during breastfeeding.
With the two exceptions noted below, there are no human pregnancy data for these drugs. It is important to consider that although high molecular weight (MW) drugs (for example, greater than 1,000) probably do not usually cross the placenta in the first half of pregnancy, they may do so in late pregnancy. The cited MWs are shown as the nearest whole number. Animal reproductive data are also cited because, although not definitive, they can provide some measure of the human embryo-fetal risk.
Anti-infectives
Benznidazole (same trade name) (MW 441), given orally, is indicated for pediatric patients aged 2-12 years for treatment of Chagas disease (American trypanosomiasis) caused by Trypanosoma cruzi. However, there are international reports describing its use in pregnancy and breastfeeding. No fetal harm from these exposures were noted. Nevertheless, because of the low MW and the reported animal risk, avoiding the drug during the first half of pregnancy appears to be the best choice. Delafloxacin (Baxdela) (MW 441), a fluoroquinolone antimicrobial given intravenously or orally, is indicated for acute bacterial skin infections. The animal data suggest low risk. However, like other fluoroquinolones, it is contraindicated in pregnancy and should be used only if there are no other alternatives.
Sofosbuvir/velpatasvir /voxilaprevir (Vosevi) (MWs 529, 883, 869), a fixed oral dose combination of three antivirals, is indicated for the treatment of hepatitis C virus infection. The MWs suggest that all three will cross the human placenta. The animal data suggest low risk. Secnidazole (Solosec) (MW 185), given orally, is indicated for the treatment of bacterial vaginosis. It is closely related to metronidazole. No evidence of embryo-fetal toxicity was observed in rats and rabbits, suggesting that the human risk is low. In a report from Brazil, 134 pregnant women with bacterial vaginosis were treated with secnidazole, metronidazole, or tinidazole in the second and third trimesters. Treatment significantly decreased the incidence of premature rupture of membranes, preterm labor, preterm birth, and low birth weight. No fetal harm was reported.
Antineoplastics
[Note: All of the drugs in this category are best avoided, if possible, in pregnancy and breastfeeding.]
Abemaciclib (Verzenio) (MW 507), an oral inhibitor of cyclin-dependent kinases, is indicated for the treatment of breast cancer. The drug is teratogenic in rats. Acalabrutinib (Calquence) (MW 466) is an oral kinase inhibitor indicated for mantle cell lymphoma. The drug had no effect on the rat embryo-fetus but caused decreased fetal body weights and delayed skeletal ossification in rabbits. Avelumab (Bavencio) (MW 147,000) is given intravenously for the treatment of metastatic Merkel cell carcinoma and metastatic urothelial carcinoma. Animal reproduction studies have not been conducted. However, based on its mechanism of action, fetal exposure may increase the risk of developing immune-related disorders or altering the normal immune response.
Brigatinib (Alunbrig) (MW 584) is given orally for the treatment of metastatic non–small-cell lung cancer. In rats, doses less than or slightly above the human exposure caused multiple anomalies in the fetuses of pregnant rats. Copanlisib (Aliqopa) (MW 553) is a kinase inhibitor that is given intravenously for relapsed follicular lymphoma. In rats during organogenesis, doses based on body surface area that were a fraction of the human dose caused embryo-fetal death and fetal defects. Durvalumab (Imfinzi) (MW 146,000), given intravenously, is indicated for the treatment of metastatic urothelial carcinoma and non–small-cell lung cancer. Monkeys given the drug from organogenesis through delivery experienced increased premature birth, fetal loss, and premature neonatal death. Women of reproductive potential should use effective contraception during treatment and for at least 3 months after the last dose.
Enasidenib (Idhifa) (MW 569), given orally, is indicated for the treatment of myeloid leukemia. The drug caused maternal toxicity and adverse embryo-fetal effects (postimplantation loss, resorptions, decrease viable fetuses, lower fetal birth weights, and skeletal variations) in rats and spontaneous abortions in rabbits. Inotuzumab ozogamicin (Besponsa) (MW 160,000), given intravenously, is indicated for relapsed or refractory B-cell precursor acute lymphoblastic leukemia. The drug caused fetal harm in rats but not in rabbits. Midostaurin (Rydapt) (MW 571) is an oral kinase inhibitor indicated for myeloid leukemia. In rats, a dose given during the first week of pregnancy that was a small fraction of the human exposure caused pre- and postimplantation loss. When very small doses were given during organogenesis to rats and rabbits there was significant maternal and fetal toxicity.
Neratinib (Nerlynx) (MW 673) is an oral kinase inhibitor for breast cancer. Although the drug did not cause embryo-fetal toxicity in rats, it did cause this toxicity in rabbits. Doses that resulted in exposures that were less than the human exposure caused maternal toxicity, abortions, and embryo-fetal death. Lower doses caused multiple fetal anomalies. Niraparib (Zejula) (MW 511) is indicated for treatment of epithelial ovarian, fallopian, or peritoneal cancer. Because of the potential human embryo-fetal risk based on its mechanism of action, pregnant animal studies were not conducted. Women with reproductive potential should use effective contraception during treatment and for 6 months after the last dose. Ribociclib (Kisqali) (MW 553) is an oral kinase inhibitor indicated for postmenopausal women with breast cancer. In rats, the drug cause reduced fetal weights and skeletal changes. Increased incidences of fetal abnormalities and lower fetal weights were observed in rabbits.
Cardiovascular
Angiotensin II (Giapreza) (MW 1,046) is a naturally occurring peptide hormone given as an intravenous infusion. It is indicated as a vasoconstrictor to increase blood pressure in adults with septic or other distributive shock. Animal reproduction studies have not been conducted. Because septic or other distributive shock is a medical emergency that can be fatal, the use of this agent in pregnancy should not be withheld.
Central nervous system
Deutetrabenazine (Austedo) (MW 324) is an oral drug indicated for the treatment of chorea associated with Huntington’s disease and for tardive dyskinesia. When given to rats during organogenesis there was no clear effect on embryo-fetal development.
Edaravone (Radicava) (MW 174), given as an intravenous infusion, is indicated for the treatment of amyotrophic lateral sclerosis. Doses that were not maternal toxic did not cause embryo-fetal toxicity in rats and rabbits. However, the no-effect dose for developmental toxicity was less than the recommended human dose. Naldemedine (Symproic) (MW 743) is an opioid antagonist indicated for the treatment of opioid-induced constipation. The drug crosses the human placenta and may precipitate opioid withdrawal in the fetus. The drug caused no embryo-fetal adverse effects, even at high doses, in pregnant rats and rabbits.
Ocrelizumab (Ocrevus) (MW 145,000), an intravenous agent, is used to treat patients with multiple sclerosis. The MW is high but immunoglobulins are known to cross the placenta. When given to monkeys at doses similar to or greater than the human dose, there was increased perinatal mortality, depletion of B-cell populations, and renal, bone marrow, and testicular toxicity in the offspring in the absence of maternal toxicity. Safinamide (Xadago) (MW 399) is an oral drug indicated as adjunctive treatment to levodopa/carbidopa in Parkinson’s disease. In rats, the drug was teratogenic (mainly urogenital defects) at all doses. When it was combined with levodopa/carbidopa or used alone, increased rates of fetal visceral and skeletal defects occurred at all doses studied. In rabbits, given the combination throughout organogenesis, there was an increased incidence of embryo-fetal death and cardiac and skeletal defects. Based on these data, avoiding the drug in pregnancy appears to be the best course.
Valbenazine (Ingrezza) (MW 419) is indicated for the treatment of tardive dyskinesia. The drug caused no malformations in rats and rabbits. However, in rats given the drug during organogenesis through lactation, an increase in the number of stillborn pups and postnatal pup mortalities was observed.
Dermatologic
Brodalumab (Siliq) (MW 144,000), given subcutaneously, is indicated for the treatment of moderate to severe plaque psoriasis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In monkeys, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from mothers given weekly subcutaneous doses of the drug. Dupilumab (Dupixent) (MW 144,000) is given subcutaneously for the treatment of atopic dermatitis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In pregnant monkeys given subcutaneous doses of the drug, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from birth to 6 months of age.
Guselkumab (Tremfya) (MW 143,600) is given subcutaneously for the treatment of moderate to severe plaque psoriasis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In pregnant monkeys given subcutaneous doses of the drug, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from birth to 6 months of age. However, neonatal deaths were observed in three monkeys given six times the maximum recommended human dose.
Endocrine/metabolic
Deflazacort (Emflaza) (MW 442) is an oral corticosteroid prodrug indicated for the treatment of Duchenne muscular dystrophy. The drug is converted in vivo to an active metabolite. The drug readily crosses the placenta. Although animal reproduction studies have not been conducted, such studies with other corticosteroids in various animal species have shown an increased incidence of cleft palate. In some species, there was an increase in embryo-fetal death, intrauterine growth restriction, and constriction of the ductus arteriosus.
Ertugliflozin (Steglatro) (MW 566) is an oral drug indicated to improve glycemic control in adults with type 2 diabetes mellitus. In juvenile rats, doses that were about 13 times the human dose caused increased kidney weight, renal tubule and renal pelvis dilatation, and renal mineralization. These effects occurred during periods of rat renal development that correspond to the late second and third trimester of human renal development, and did not fully reverse within a 1-month recovery period. Etelcalcetide (Parsabiv) (MW 1,048), an intravenous calcium-sensing receptor agonist, is indicated for patients on hemodialysis who have secondary hyperparathyroidism. In rats and rabbits given the drug during organogenesis, there was reduced fetal growth. In rats given the drug during organogenesis through birth and weaning, there was a slight increase in pup mortality, delay in parturition, and transient effects on pup growth, but there were no effects on sexual maturation, neurobehavioral, or reproductive function. Macimorelin (Macrilen) (MW 535) is an oral growth hormone secretagogue receptor agonist. It is indicated for adult growth hormone deficiency. Animal reproduction studies have not been conducted.
Semaglutide (Ozempic) (MW 4,114), given subcutaneously, is a glucagon-like peptide indicated to improve glycemic control in type 2 diabetes mellitus. In rats given the drug during organogenesis, embryo-fetal death, structural defects, and alterations in growth were observed. In rabbits and monkeys given the drug during organogenesis, there were early pregnancy losses and structural abnormalities. In addition, there was marked maternal body weight loss in both animal species. Vestronidase alfa-vjbk (Mepsevii) is given intravenously. It is indicated for the treatment of Mucopolysaccharidosis VII (Sly syndrome). The calculated average MW of each nonglycosylated peptide chain is 72,562. In rats and rabbits given the drug during organogenesis, there was no maternal toxicity or adverse developmental outcomes.
Gastrointestinal
Plecanatide (Trulance) (MW 1,682) is an oral drug indicated for the treatment of constipation. The drug and its active metabolite are negligibly absorbed systemically and fetal exposure to the drug is not expected. In mice and rabbits given the oral drug during organogenesis, no effects on embryo-fetal development were observed. Telotristat ethyl (Xermelo) (MW 754) is an oral drug indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (MW not specified) therapy in adults not controlled by somatostatin analog. When given during organogenesis in rats, there was no effect on embryo-fetal development at doses that were about nine times the recommended human dose. However, an increased incidence of mortality in rat offspring was observed when the drug was given from organogenesis through lactation. During organogenesis in rabbits, the drug had no embryo-fetal effects at doses that were 10 or more times the human dose.
Hematologics
Betrixaban (Bevyxxa) (MW 568) is an oral factor Xa inhibitor indicated for the prophylaxis of venous thromboembolism. The drug was not associated with adverse developmental fetal outcomes in rats and rabbits. However, maternal hemorrhage did occur. In humans, there is an increased risk of hemorrhage during pregnancy and delivery. Emicizumab (Hemlibra) (MW 145,600), given subcutaneously, is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding in patients with hemophilia A with factor VIII inhibitors. Animal reproduction studies have not been conducted. It is a human monoclonal IgG antibody and, though the MW is high, IgG antibodies are known to cross the placenta.
Immunologic
Sarilumab (Kevzara) (MW 150,000) is given subcutaneously. It is indicated for patients with moderate to severe rheumatoid arthritis. Reproduction studies were conducted in pregnant monkeys. There was no evidence of embryo toxicity or fetal malformations. Based on this data, the human pregnancy risk is low.
Ophthalmic
Latanoprostene bunod (Vyzulta) (MW 508) is a prostaglandin analog that is indicated to reduce intraocular pressure. No quantifiable plasma concentrations of latanoprostene bunod were detected in nonpregnant patients. However, very low levels of latanoprost acid (51-59 pg/mL), the active metabolite, were detected with the maximal plasma concentration occurring 5 minutes after administration. When given intravenously to pregnant rabbits, the drug was shown to be abortifacient and teratogenic, but these effects were not observed in pregnant rats. Netarsudil (Rhopressa) (MW 454) is a kinase inhibitor indicated to reduce intraocular pressure in patients with open-angle glaucoma or ocular hypertension. No quantifiable plasma concentrations of netarsudil were detected in 18 subjects. For the active metabolite, a plasma level of 0.11 ng/mL was found in one subject. Intravenous doses to pregnant rats and rabbits during organogenesis did not cause embryo-fetal adverse effects at clinically relevant systemic exposures.
Parathyroid hormone
Abaloparatide (Tymlos) (MW 3,961), given subcutaneously, is a human parathyroid hormone related peptide analog that is indicated for postmenopausal women with osteoporosis at high risk for fracture. Reproduction studies in animals have not been conducted. Because of the indication, it is doubtful if the agent will be used in pregnancy or during breastfeeding.
Respiratory
Benralizumab (Fasenra) (MW 150,000), given subcutaneously, is indicated for the add-on maintenance treatment of severe eosinophilic asthma. It is a human monoclonal IgG antibody and, though the MW is high, IgG antibodies are known to cross the placenta. Studies in monkeys found no evidence of fetal harm with intravenous doses throughout pregnancy that produced exposures up to about 310 times the exposure at the maximum recommended human dose.
The potential adverse effects in an infant when the mother is taking one of the above drugs while breastfeeding will be covered in my next column.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Novel drugs are innovative new products that have never before been used in clinical practice. Among the 46 that the Food and Drug Administration approved in 2017, 45 could be used in pregnancy. One, cerliponase alfa (Brineura), is indicated for pediatric patients 3 years of age or older, for treatment of late infantile neuronal ceroid lipofuscinosis type 2. It is doubtful that this drug would be used in pregnancy or during breastfeeding.
With the two exceptions noted below, there are no human pregnancy data for these drugs. It is important to consider that although high molecular weight (MW) drugs (for example, greater than 1,000) probably do not usually cross the placenta in the first half of pregnancy, they may do so in late pregnancy. The cited MWs are shown as the nearest whole number. Animal reproductive data are also cited because, although not definitive, they can provide some measure of the human embryo-fetal risk.
Anti-infectives
Benznidazole (same trade name) (MW 441), given orally, is indicated for pediatric patients aged 2-12 years for treatment of Chagas disease (American trypanosomiasis) caused by Trypanosoma cruzi. However, there are international reports describing its use in pregnancy and breastfeeding. No fetal harm from these exposures were noted. Nevertheless, because of the low MW and the reported animal risk, avoiding the drug during the first half of pregnancy appears to be the best choice. Delafloxacin (Baxdela) (MW 441), a fluoroquinolone antimicrobial given intravenously or orally, is indicated for acute bacterial skin infections. The animal data suggest low risk. However, like other fluoroquinolones, it is contraindicated in pregnancy and should be used only if there are no other alternatives.
Sofosbuvir/velpatasvir /voxilaprevir (Vosevi) (MWs 529, 883, 869), a fixed oral dose combination of three antivirals, is indicated for the treatment of hepatitis C virus infection. The MWs suggest that all three will cross the human placenta. The animal data suggest low risk. Secnidazole (Solosec) (MW 185), given orally, is indicated for the treatment of bacterial vaginosis. It is closely related to metronidazole. No evidence of embryo-fetal toxicity was observed in rats and rabbits, suggesting that the human risk is low. In a report from Brazil, 134 pregnant women with bacterial vaginosis were treated with secnidazole, metronidazole, or tinidazole in the second and third trimesters. Treatment significantly decreased the incidence of premature rupture of membranes, preterm labor, preterm birth, and low birth weight. No fetal harm was reported.
Antineoplastics
[Note: All of the drugs in this category are best avoided, if possible, in pregnancy and breastfeeding.]
Abemaciclib (Verzenio) (MW 507), an oral inhibitor of cyclin-dependent kinases, is indicated for the treatment of breast cancer. The drug is teratogenic in rats. Acalabrutinib (Calquence) (MW 466) is an oral kinase inhibitor indicated for mantle cell lymphoma. The drug had no effect on the rat embryo-fetus but caused decreased fetal body weights and delayed skeletal ossification in rabbits. Avelumab (Bavencio) (MW 147,000) is given intravenously for the treatment of metastatic Merkel cell carcinoma and metastatic urothelial carcinoma. Animal reproduction studies have not been conducted. However, based on its mechanism of action, fetal exposure may increase the risk of developing immune-related disorders or altering the normal immune response.
Brigatinib (Alunbrig) (MW 584) is given orally for the treatment of metastatic non–small-cell lung cancer. In rats, doses less than or slightly above the human exposure caused multiple anomalies in the fetuses of pregnant rats. Copanlisib (Aliqopa) (MW 553) is a kinase inhibitor that is given intravenously for relapsed follicular lymphoma. In rats during organogenesis, doses based on body surface area that were a fraction of the human dose caused embryo-fetal death and fetal defects. Durvalumab (Imfinzi) (MW 146,000), given intravenously, is indicated for the treatment of metastatic urothelial carcinoma and non–small-cell lung cancer. Monkeys given the drug from organogenesis through delivery experienced increased premature birth, fetal loss, and premature neonatal death. Women of reproductive potential should use effective contraception during treatment and for at least 3 months after the last dose.
Enasidenib (Idhifa) (MW 569), given orally, is indicated for the treatment of myeloid leukemia. The drug caused maternal toxicity and adverse embryo-fetal effects (postimplantation loss, resorptions, decrease viable fetuses, lower fetal birth weights, and skeletal variations) in rats and spontaneous abortions in rabbits. Inotuzumab ozogamicin (Besponsa) (MW 160,000), given intravenously, is indicated for relapsed or refractory B-cell precursor acute lymphoblastic leukemia. The drug caused fetal harm in rats but not in rabbits. Midostaurin (Rydapt) (MW 571) is an oral kinase inhibitor indicated for myeloid leukemia. In rats, a dose given during the first week of pregnancy that was a small fraction of the human exposure caused pre- and postimplantation loss. When very small doses were given during organogenesis to rats and rabbits there was significant maternal and fetal toxicity.
Neratinib (Nerlynx) (MW 673) is an oral kinase inhibitor for breast cancer. Although the drug did not cause embryo-fetal toxicity in rats, it did cause this toxicity in rabbits. Doses that resulted in exposures that were less than the human exposure caused maternal toxicity, abortions, and embryo-fetal death. Lower doses caused multiple fetal anomalies. Niraparib (Zejula) (MW 511) is indicated for treatment of epithelial ovarian, fallopian, or peritoneal cancer. Because of the potential human embryo-fetal risk based on its mechanism of action, pregnant animal studies were not conducted. Women with reproductive potential should use effective contraception during treatment and for 6 months after the last dose. Ribociclib (Kisqali) (MW 553) is an oral kinase inhibitor indicated for postmenopausal women with breast cancer. In rats, the drug cause reduced fetal weights and skeletal changes. Increased incidences of fetal abnormalities and lower fetal weights were observed in rabbits.
Cardiovascular
Angiotensin II (Giapreza) (MW 1,046) is a naturally occurring peptide hormone given as an intravenous infusion. It is indicated as a vasoconstrictor to increase blood pressure in adults with septic or other distributive shock. Animal reproduction studies have not been conducted. Because septic or other distributive shock is a medical emergency that can be fatal, the use of this agent in pregnancy should not be withheld.
Central nervous system
Deutetrabenazine (Austedo) (MW 324) is an oral drug indicated for the treatment of chorea associated with Huntington’s disease and for tardive dyskinesia. When given to rats during organogenesis there was no clear effect on embryo-fetal development.
Edaravone (Radicava) (MW 174), given as an intravenous infusion, is indicated for the treatment of amyotrophic lateral sclerosis. Doses that were not maternal toxic did not cause embryo-fetal toxicity in rats and rabbits. However, the no-effect dose for developmental toxicity was less than the recommended human dose. Naldemedine (Symproic) (MW 743) is an opioid antagonist indicated for the treatment of opioid-induced constipation. The drug crosses the human placenta and may precipitate opioid withdrawal in the fetus. The drug caused no embryo-fetal adverse effects, even at high doses, in pregnant rats and rabbits.
Ocrelizumab (Ocrevus) (MW 145,000), an intravenous agent, is used to treat patients with multiple sclerosis. The MW is high but immunoglobulins are known to cross the placenta. When given to monkeys at doses similar to or greater than the human dose, there was increased perinatal mortality, depletion of B-cell populations, and renal, bone marrow, and testicular toxicity in the offspring in the absence of maternal toxicity. Safinamide (Xadago) (MW 399) is an oral drug indicated as adjunctive treatment to levodopa/carbidopa in Parkinson’s disease. In rats, the drug was teratogenic (mainly urogenital defects) at all doses. When it was combined with levodopa/carbidopa or used alone, increased rates of fetal visceral and skeletal defects occurred at all doses studied. In rabbits, given the combination throughout organogenesis, there was an increased incidence of embryo-fetal death and cardiac and skeletal defects. Based on these data, avoiding the drug in pregnancy appears to be the best course.
Valbenazine (Ingrezza) (MW 419) is indicated for the treatment of tardive dyskinesia. The drug caused no malformations in rats and rabbits. However, in rats given the drug during organogenesis through lactation, an increase in the number of stillborn pups and postnatal pup mortalities was observed.
Dermatologic
Brodalumab (Siliq) (MW 144,000), given subcutaneously, is indicated for the treatment of moderate to severe plaque psoriasis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In monkeys, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from mothers given weekly subcutaneous doses of the drug. Dupilumab (Dupixent) (MW 144,000) is given subcutaneously for the treatment of atopic dermatitis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In pregnant monkeys given subcutaneous doses of the drug, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from birth to 6 months of age.
Guselkumab (Tremfya) (MW 143,600) is given subcutaneously for the treatment of moderate to severe plaque psoriasis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In pregnant monkeys given subcutaneous doses of the drug, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from birth to 6 months of age. However, neonatal deaths were observed in three monkeys given six times the maximum recommended human dose.
Endocrine/metabolic
Deflazacort (Emflaza) (MW 442) is an oral corticosteroid prodrug indicated for the treatment of Duchenne muscular dystrophy. The drug is converted in vivo to an active metabolite. The drug readily crosses the placenta. Although animal reproduction studies have not been conducted, such studies with other corticosteroids in various animal species have shown an increased incidence of cleft palate. In some species, there was an increase in embryo-fetal death, intrauterine growth restriction, and constriction of the ductus arteriosus.
Ertugliflozin (Steglatro) (MW 566) is an oral drug indicated to improve glycemic control in adults with type 2 diabetes mellitus. In juvenile rats, doses that were about 13 times the human dose caused increased kidney weight, renal tubule and renal pelvis dilatation, and renal mineralization. These effects occurred during periods of rat renal development that correspond to the late second and third trimester of human renal development, and did not fully reverse within a 1-month recovery period. Etelcalcetide (Parsabiv) (MW 1,048), an intravenous calcium-sensing receptor agonist, is indicated for patients on hemodialysis who have secondary hyperparathyroidism. In rats and rabbits given the drug during organogenesis, there was reduced fetal growth. In rats given the drug during organogenesis through birth and weaning, there was a slight increase in pup mortality, delay in parturition, and transient effects on pup growth, but there were no effects on sexual maturation, neurobehavioral, or reproductive function. Macimorelin (Macrilen) (MW 535) is an oral growth hormone secretagogue receptor agonist. It is indicated for adult growth hormone deficiency. Animal reproduction studies have not been conducted.
Semaglutide (Ozempic) (MW 4,114), given subcutaneously, is a glucagon-like peptide indicated to improve glycemic control in type 2 diabetes mellitus. In rats given the drug during organogenesis, embryo-fetal death, structural defects, and alterations in growth were observed. In rabbits and monkeys given the drug during organogenesis, there were early pregnancy losses and structural abnormalities. In addition, there was marked maternal body weight loss in both animal species. Vestronidase alfa-vjbk (Mepsevii) is given intravenously. It is indicated for the treatment of Mucopolysaccharidosis VII (Sly syndrome). The calculated average MW of each nonglycosylated peptide chain is 72,562. In rats and rabbits given the drug during organogenesis, there was no maternal toxicity or adverse developmental outcomes.
Gastrointestinal
Plecanatide (Trulance) (MW 1,682) is an oral drug indicated for the treatment of constipation. The drug and its active metabolite are negligibly absorbed systemically and fetal exposure to the drug is not expected. In mice and rabbits given the oral drug during organogenesis, no effects on embryo-fetal development were observed. Telotristat ethyl (Xermelo) (MW 754) is an oral drug indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (MW not specified) therapy in adults not controlled by somatostatin analog. When given during organogenesis in rats, there was no effect on embryo-fetal development at doses that were about nine times the recommended human dose. However, an increased incidence of mortality in rat offspring was observed when the drug was given from organogenesis through lactation. During organogenesis in rabbits, the drug had no embryo-fetal effects at doses that were 10 or more times the human dose.
Hematologics
Betrixaban (Bevyxxa) (MW 568) is an oral factor Xa inhibitor indicated for the prophylaxis of venous thromboembolism. The drug was not associated with adverse developmental fetal outcomes in rats and rabbits. However, maternal hemorrhage did occur. In humans, there is an increased risk of hemorrhage during pregnancy and delivery. Emicizumab (Hemlibra) (MW 145,600), given subcutaneously, is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding in patients with hemophilia A with factor VIII inhibitors. Animal reproduction studies have not been conducted. It is a human monoclonal IgG antibody and, though the MW is high, IgG antibodies are known to cross the placenta.
Immunologic
Sarilumab (Kevzara) (MW 150,000) is given subcutaneously. It is indicated for patients with moderate to severe rheumatoid arthritis. Reproduction studies were conducted in pregnant monkeys. There was no evidence of embryo toxicity or fetal malformations. Based on this data, the human pregnancy risk is low.
Ophthalmic
Latanoprostene bunod (Vyzulta) (MW 508) is a prostaglandin analog that is indicated to reduce intraocular pressure. No quantifiable plasma concentrations of latanoprostene bunod were detected in nonpregnant patients. However, very low levels of latanoprost acid (51-59 pg/mL), the active metabolite, were detected with the maximal plasma concentration occurring 5 minutes after administration. When given intravenously to pregnant rabbits, the drug was shown to be abortifacient and teratogenic, but these effects were not observed in pregnant rats. Netarsudil (Rhopressa) (MW 454) is a kinase inhibitor indicated to reduce intraocular pressure in patients with open-angle glaucoma or ocular hypertension. No quantifiable plasma concentrations of netarsudil were detected in 18 subjects. For the active metabolite, a plasma level of 0.11 ng/mL was found in one subject. Intravenous doses to pregnant rats and rabbits during organogenesis did not cause embryo-fetal adverse effects at clinically relevant systemic exposures.
Parathyroid hormone
Abaloparatide (Tymlos) (MW 3,961), given subcutaneously, is a human parathyroid hormone related peptide analog that is indicated for postmenopausal women with osteoporosis at high risk for fracture. Reproduction studies in animals have not been conducted. Because of the indication, it is doubtful if the agent will be used in pregnancy or during breastfeeding.
Respiratory
Benralizumab (Fasenra) (MW 150,000), given subcutaneously, is indicated for the add-on maintenance treatment of severe eosinophilic asthma. It is a human monoclonal IgG antibody and, though the MW is high, IgG antibodies are known to cross the placenta. Studies in monkeys found no evidence of fetal harm with intravenous doses throughout pregnancy that produced exposures up to about 310 times the exposure at the maximum recommended human dose.
The potential adverse effects in an infant when the mother is taking one of the above drugs while breastfeeding will be covered in my next column.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Melanoma in situ: It’s hard to know what you don’t know
The emergency department locum tenens staff recruiter was persuasive. “It’s a quiet little ER where you can study and sleep.” I was board certified in internal medicine and had trained in a busy urban emergency department. This was just the spot to make a little folding money and study for my mock dermatology boards, I thought.
And so, on a Saturday night in rural Texas, after grinding rust out of a pipe fitter’s eye and stitching up two brawlers from the local biker bar, I was faced with treating a comatose kid brought in after a car crash. He had not been wearing a seat belt, and his car had rolled over on his head.
I was way over my skill level, but I was lucky. I was able to stabilize him and, after several long hours, I got him on an emergency helicopter into Dallas.
But the experience changed me. I realized I did not know enough to deal with this case on my own. After making it through that night in the ED, I never put myself in that position again.
I now knew what I did not know.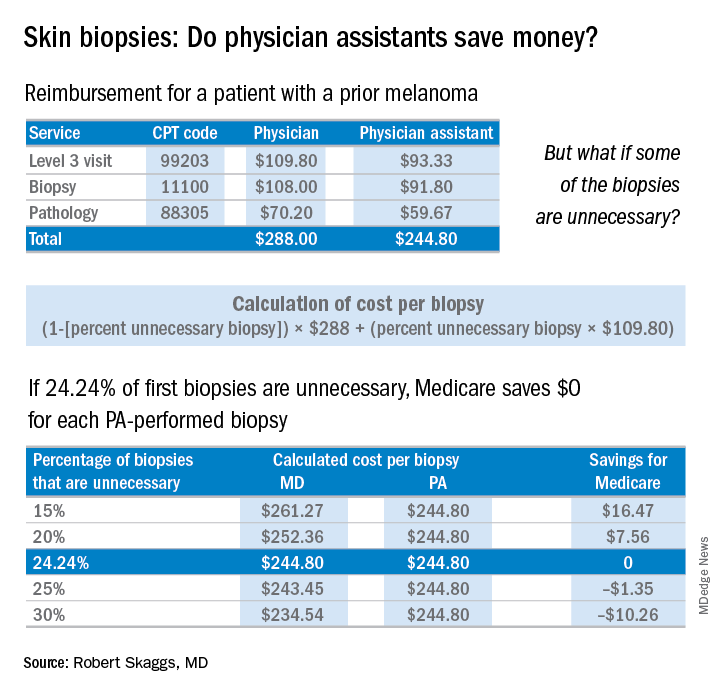
The finding that jumped out to me, though, was that patients screened by a PA were significantly less likely to be diagnosed with melanoma in situ, the stage when melanoma is 100% curable. Yet, those patients screened by PAs underwent a lot more skin biopsies – 36% more skin biopsies per melanoma in situ diagnosed, compared with patients of dermatologists. Interestingly, in the health care system studied, any PA with a question about a patient can ask an attending dermatologist to see the patient. Did that factor account for the diagnostic comparability for nonmelanoma skin cancer and invasive melanoma? Did the PAs not ask for help on the missed melanomas in situ? If so, I believe this may be a situation of PAs not knowing what they didn’t know.
Now a knowledgeable friend of mine thinks this study is biased because 17% more patients with prior melanomas were seen by a dermatologist rather than by a PA. While it’s true that patients with prior melanomas are more likely to develop new melanomas, the counterargument is that the bar for a biopsy in a patient with a prior melanoma is much lower. Patients with a history of melanoma should have more skin biopsies, but the dermatologists in this study still took many fewer biopsies to diagnose melanomas in situ.
Why do these findings matter for patients and for the health care system?
PAs billed independently for 12% of skin biopsies (including lip, ear, ear canal, vulva, penis, and eyelid) in Medicare Fee for Service in 2016. Skin biopsies paid for by Medicare have been increasing at a very rapid rate, about twice as fast as the rate reflected in the current skin cancer epidemic.
Every skin biopsy results in a pathology charge, for which Medicare pays about $70. A level 3 new patient visit pays $110. If PAs bill independently, they are paid at 85% of the fee schedule, which often is touted as a great savings. Therefore, if only 24.2% of skin biopsies by PAs were unnecessary, even at a reduced 85% reimbursement, it costs Medicare more than having these visits and biopsies provided by a dermatologist. The cost savings decrease even more with additional skin biopsies, because they pay so little ($33 for a doctor, $28 for a PA), yet the pathology charge is unchanged.
There are other costs beyond monetary ones from unnecessary skin biopsies: scarring, follow-up procedures for uncertain diagnoses such as mild dysplastic nevi, ambiguous results, and emotional angst to patients.
If the results of this large study are to be believed, many melanomas in situ are going to be missed if PAs perform unsupervised skin cancer screenings. This is not a tenable proposition, ethically or legally. Dermatologists and PAs need to work together to ensure this does not happen.
An estimated 2,520 dermatology PAs were practicing in the United States in 2016, based on membership data from the Society of Dermatology PAs (SDPA), according to a research letter published last year (J Am Acad Dermatol. 2017 Jun;76[6]:1200-2). The SDPA, as stated in an SDPA position statement published in the winter 2017 newsletter, hopes to gain access to direct billing to public and private insurers, which would include the Centers for Medicare & Medicaid Services, and for PAs to no longer report to other health care professionals.
Many dermatologists, as well as teaching programs, use PAs to perform skin cancer screenings, sometimes unsupervised, which makes diagnostic accuracy critical. The issues at hand are the safety of patients and the accuracy of diagnosis as well as the costs to the health care system. A team effort, which includes direct supervision, is needed to ensure those issues are addressed.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
The emergency department locum tenens staff recruiter was persuasive. “It’s a quiet little ER where you can study and sleep.” I was board certified in internal medicine and had trained in a busy urban emergency department. This was just the spot to make a little folding money and study for my mock dermatology boards, I thought.
And so, on a Saturday night in rural Texas, after grinding rust out of a pipe fitter’s eye and stitching up two brawlers from the local biker bar, I was faced with treating a comatose kid brought in after a car crash. He had not been wearing a seat belt, and his car had rolled over on his head.
I was way over my skill level, but I was lucky. I was able to stabilize him and, after several long hours, I got him on an emergency helicopter into Dallas.
But the experience changed me. I realized I did not know enough to deal with this case on my own. After making it through that night in the ED, I never put myself in that position again.
I now knew what I did not know.
The finding that jumped out to me, though, was that patients screened by a PA were significantly less likely to be diagnosed with melanoma in situ, the stage when melanoma is 100% curable. Yet, those patients screened by PAs underwent a lot more skin biopsies – 36% more skin biopsies per melanoma in situ diagnosed, compared with patients of dermatologists. Interestingly, in the health care system studied, any PA with a question about a patient can ask an attending dermatologist to see the patient. Did that factor account for the diagnostic comparability for nonmelanoma skin cancer and invasive melanoma? Did the PAs not ask for help on the missed melanomas in situ? If so, I believe this may be a situation of PAs not knowing what they didn’t know.
Now a knowledgeable friend of mine thinks this study is biased because 17% more patients with prior melanomas were seen by a dermatologist rather than by a PA. While it’s true that patients with prior melanomas are more likely to develop new melanomas, the counterargument is that the bar for a biopsy in a patient with a prior melanoma is much lower. Patients with a history of melanoma should have more skin biopsies, but the dermatologists in this study still took many fewer biopsies to diagnose melanomas in situ.
Why do these findings matter for patients and for the health care system?
PAs billed independently for 12% of skin biopsies (including lip, ear, ear canal, vulva, penis, and eyelid) in Medicare Fee for Service in 2016. Skin biopsies paid for by Medicare have been increasing at a very rapid rate, about twice as fast as the rate reflected in the current skin cancer epidemic.
Every skin biopsy results in a pathology charge, for which Medicare pays about $70. A level 3 new patient visit pays $110. If PAs bill independently, they are paid at 85% of the fee schedule, which often is touted as a great savings. Therefore, if only 24.2% of skin biopsies by PAs were unnecessary, even at a reduced 85% reimbursement, it costs Medicare more than having these visits and biopsies provided by a dermatologist. The cost savings decrease even more with additional skin biopsies, because they pay so little ($33 for a doctor, $28 for a PA), yet the pathology charge is unchanged.
There are other costs beyond monetary ones from unnecessary skin biopsies: scarring, follow-up procedures for uncertain diagnoses such as mild dysplastic nevi, ambiguous results, and emotional angst to patients.
If the results of this large study are to be believed, many melanomas in situ are going to be missed if PAs perform unsupervised skin cancer screenings. This is not a tenable proposition, ethically or legally. Dermatologists and PAs need to work together to ensure this does not happen.
An estimated 2,520 dermatology PAs were practicing in the United States in 2016, based on membership data from the Society of Dermatology PAs (SDPA), according to a research letter published last year (J Am Acad Dermatol. 2017 Jun;76[6]:1200-2). The SDPA, as stated in an SDPA position statement published in the winter 2017 newsletter, hopes to gain access to direct billing to public and private insurers, which would include the Centers for Medicare & Medicaid Services, and for PAs to no longer report to other health care professionals.
Many dermatologists, as well as teaching programs, use PAs to perform skin cancer screenings, sometimes unsupervised, which makes diagnostic accuracy critical. The issues at hand are the safety of patients and the accuracy of diagnosis as well as the costs to the health care system. A team effort, which includes direct supervision, is needed to ensure those issues are addressed.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
The emergency department locum tenens staff recruiter was persuasive. “It’s a quiet little ER where you can study and sleep.” I was board certified in internal medicine and had trained in a busy urban emergency department. This was just the spot to make a little folding money and study for my mock dermatology boards, I thought.
And so, on a Saturday night in rural Texas, after grinding rust out of a pipe fitter’s eye and stitching up two brawlers from the local biker bar, I was faced with treating a comatose kid brought in after a car crash. He had not been wearing a seat belt, and his car had rolled over on his head.
I was way over my skill level, but I was lucky. I was able to stabilize him and, after several long hours, I got him on an emergency helicopter into Dallas.
But the experience changed me. I realized I did not know enough to deal with this case on my own. After making it through that night in the ED, I never put myself in that position again.
I now knew what I did not know.
The finding that jumped out to me, though, was that patients screened by a PA were significantly less likely to be diagnosed with melanoma in situ, the stage when melanoma is 100% curable. Yet, those patients screened by PAs underwent a lot more skin biopsies – 36% more skin biopsies per melanoma in situ diagnosed, compared with patients of dermatologists. Interestingly, in the health care system studied, any PA with a question about a patient can ask an attending dermatologist to see the patient. Did that factor account for the diagnostic comparability for nonmelanoma skin cancer and invasive melanoma? Did the PAs not ask for help on the missed melanomas in situ? If so, I believe this may be a situation of PAs not knowing what they didn’t know.
Now a knowledgeable friend of mine thinks this study is biased because 17% more patients with prior melanomas were seen by a dermatologist rather than by a PA. While it’s true that patients with prior melanomas are more likely to develop new melanomas, the counterargument is that the bar for a biopsy in a patient with a prior melanoma is much lower. Patients with a history of melanoma should have more skin biopsies, but the dermatologists in this study still took many fewer biopsies to diagnose melanomas in situ.
Why do these findings matter for patients and for the health care system?
PAs billed independently for 12% of skin biopsies (including lip, ear, ear canal, vulva, penis, and eyelid) in Medicare Fee for Service in 2016. Skin biopsies paid for by Medicare have been increasing at a very rapid rate, about twice as fast as the rate reflected in the current skin cancer epidemic.
Every skin biopsy results in a pathology charge, for which Medicare pays about $70. A level 3 new patient visit pays $110. If PAs bill independently, they are paid at 85% of the fee schedule, which often is touted as a great savings. Therefore, if only 24.2% of skin biopsies by PAs were unnecessary, even at a reduced 85% reimbursement, it costs Medicare more than having these visits and biopsies provided by a dermatologist. The cost savings decrease even more with additional skin biopsies, because they pay so little ($33 for a doctor, $28 for a PA), yet the pathology charge is unchanged.
There are other costs beyond monetary ones from unnecessary skin biopsies: scarring, follow-up procedures for uncertain diagnoses such as mild dysplastic nevi, ambiguous results, and emotional angst to patients.
If the results of this large study are to be believed, many melanomas in situ are going to be missed if PAs perform unsupervised skin cancer screenings. This is not a tenable proposition, ethically or legally. Dermatologists and PAs need to work together to ensure this does not happen.
An estimated 2,520 dermatology PAs were practicing in the United States in 2016, based on membership data from the Society of Dermatology PAs (SDPA), according to a research letter published last year (J Am Acad Dermatol. 2017 Jun;76[6]:1200-2). The SDPA, as stated in an SDPA position statement published in the winter 2017 newsletter, hopes to gain access to direct billing to public and private insurers, which would include the Centers for Medicare & Medicaid Services, and for PAs to no longer report to other health care professionals.
Many dermatologists, as well as teaching programs, use PAs to perform skin cancer screenings, sometimes unsupervised, which makes diagnostic accuracy critical. The issues at hand are the safety of patients and the accuracy of diagnosis as well as the costs to the health care system. A team effort, which includes direct supervision, is needed to ensure those issues are addressed.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Oophorectomy for premenopausal breast cancer
One-quarter of patients with breast cancer are diagnosed at a premenopausal age and these young women may be directed to discuss oophorectomy with their ob.gyn. This may be because of the discovery of a deleterious BRCA gene mutation, which places them at increased risk for ovarian cancer, but oophorectomy may also be a therapeutic option for their breast cancer: 60% of premenopausal breast cancers are hormone receptor–positive. Ovarian ablation has been associated with improved overall survival and disease-free survival among these patients.1
Estrogen is an important promoter of breast cancer and is predominantly derived from ovarian tissue in premenopausal women. However, in postmenopausal women, the majority of estrogen is produced peripherally through the conversion of androgens to estrogen via the enzyme aromatase. Aromatase inhibitors, such as exemestane, anastrazole, and letrazole, are drugs which block this conversion in peripheral tissues. They are contraindicated in premenopausal women with intact ovarian function, because there is a reflex pituitary stimulation of ovarian estrogen release in response to suppression of peripheral conversion of androgens. For such patients, ovarian function must be ablated either with surgery or with gonadotropin-releasing hormone (GnRH) analogues such as leuprorelin and goserelin if aromatase inhibitors are desired.
In these trials, ovarian ablation was achieved either reversibly with GnRH analogues or permanently and irreversibly with oophorectomy. No studies have compared the survival benefit of these two approaches; however, surgical ovarian ablation is immediate, reliable, and has been shown to be the most cost-effective method.4 It is a good option for women who struggle with adherence to repeated appointments for injections. It also substantially reduces the risk for ovarian cancer, which is elevated among this population of patients, even among those without a deleterious BRCA gene mutation.
BRCA populations
For women with BRCA germline mutations and a history of breast cancer, oophorectomy is associated with a 70% risk of all-cause mortality, including a 60% reduction in breast cancer mortality. This effect is inclusive of patients with “triple-negative,” hormone receptor–negative tumors. The positive effect on breast cancer mortality is predominantly seen among BRCA-1 mutation carriers, and if the oophorectomy is performed within 2 years of diagnosis.5
Technique
When performing oophorectomy either for breast cancer or because of a hereditary cancer syndrome such as BRCA mutation, it is important to ensure that the ovarian vessel pedicle is transected at least 2 cm from its insertion in the ovary. This prevents leaving a residual ovarian remnant. In order to do this, it may be necessary to skeletonize the ovarian vessels free from their physiological attachments to the sigmoid colon on the left, and terminal ileum and cecum on the right. It is also important to ensure that the ureter is not invested in this more proximal segment of ovarian vessels. To prevent this, the retroperitoneal space can be opened lateral to and parallel with the ovarian vessels, and the “medial leaf” of the broad ligament swept medially to expose the ureter as it crosses the bifurcation of the external and internal iliac arteries at the pelvic brim. With the ureter in view, a window can then be made in the “medial leaf” above the ureter and below the ovary and ovarian vessels, in doing so creating a skeletonized ovarian vessel segment which can be sealed and cut 2 cm or more from its insertion in the ovary.
The fallopian tubes should be removed with the ovarian specimens, with attention made to removing the fallopian tube at its junction with the uterine cornua. It should be noted that the majority of fallopian tube cancers arise in the fimbriated end of the tube, and cornual tubal malignancies are fairly uncommon.
The decision about whether or not to perform hysterectomy at the time of salpingo-oophorectomy is complex. In patients without hereditary cancer syndromes, such as BRCA or Lynch syndrome, hysterectomy likely offers no benefit to the patient who is undergoing a procedure for the purpose of ovarian ablation. An argument has been made that hysterectomy can eliminate the increased endometrial cancer risk associated with tamoxifen. However, given the previously discussed data, after oophorectomy, aromatase inhibitors are the preferred treatment option, and tamoxifen can be avoided. If a patient has unrelated underlying uterine pathology a hysterectomy might be indicated. Women with BRCA germline mutations, particularly women with BRCA-1 mutations, may be at increased risk for uterine serous carcinoma, and in these patients, hysterectomy at the time of oophorectomy can be discussed and offered, though as yet, it is not a guideline recommendation for all patients.6 Patients who ask to “just take everything out while you are there” without a clear indication for hysterectomy should be counseled that hysterectomy is associated with increased risk, recovery, and cost, compared with bilateral salpingo-oophorectomy. Among patients with elevated surgical risk (such as morbid obesity, known adhesive disease, increased venous thromboembolism risk, diabetes, and so on) it may not always be appropriate to extend the complexity of the procedure given the limited benefit.
Consequences of ovarian ablation
It should be noted that ovarian ablation in the TEXT and SOFT trials was not associated with an increase in overall survival for women with premenopausal breast cancer. Alternatively, large, observational studies such as the Nurses’ Health Study have shown that premenopausal oophorectomy without hormone replacement therapy is associated with increased all-cause mortality. This is primarily driven by the increased cardiopulmonary risk (heart attack and stroke), deaths after osteoporotic hip fractures, and the increased risk for lung and colon cancer.7,8
It is normal for young patients to have heightened concerns regarding their risk of recurrence from their cancer, and less concerned by threats to their health in decades to come. However, it is important to discuss this data with the patient and allow for her to make an informed decision about her immediate versus future risks. If she determines that she is not interested in permanent ovarian ablation with oophorectomy because of either surgical risks, concerns regarding permanent infertility, or increased all-cause mortality, she still has an option for medical ovarian ablation with GnRH analogues in the treatment of her breast cancer.
Hormone replacement therapy postoperatively
Women who undergo oophorectomy for the treatment of breast cancer should not be offered hormone replacement therapy. This is true even for “triple-negative” or hormone receptor–negative breast cancers as there is still some observed benefit of ovarian ablation, and risk from exogenous hormone administration in these women. Alternatively, postoperative hormone replacement therapy remains safe until the age of natural menopause among premenopausal patients with BRCA germline mutations without a preceding breast cancer diagnosis.
Surgical ovarian ablation with bilateral salpingo-oophorectomy is a valuable strategy in the adjuvant therapy of premenopausal breast cancer, particularly among BRCA mutation carriers and women with hormone receptor–positive disease, or among women who find adherence to medical ablation difficult. Patients should be carefully counseled that this may introduce increased long-term cardiovascular risks for them.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1996 Nov 2;348:1189-96.
2. Pagani O et al. N Engl J Med. 2014 Jul 10;371(12):107-18.
3. Francis PA et al. N Engl J Med. 2015 Jan 29;372(5):436-46.
4. Ferrandina G et al. Clin Drug Investig. 2017 Nov;37(11):1093-102.
5. Finch AP et al. J Clin Oncol. 2014 May 20;32(15):1547-53.
6. Shu CA et al. JAMA Oncol. 2016 Nov 1;2(11):1434-40.
7. Parker WH et al. Obstet Gynecol. 2013 Apr;121(4):709-16.
8. Rivera CM et al. Menopause. 2009 Jan-Feb;16:15-23.
One-quarter of patients with breast cancer are diagnosed at a premenopausal age and these young women may be directed to discuss oophorectomy with their ob.gyn. This may be because of the discovery of a deleterious BRCA gene mutation, which places them at increased risk for ovarian cancer, but oophorectomy may also be a therapeutic option for their breast cancer: 60% of premenopausal breast cancers are hormone receptor–positive. Ovarian ablation has been associated with improved overall survival and disease-free survival among these patients.1
Estrogen is an important promoter of breast cancer and is predominantly derived from ovarian tissue in premenopausal women. However, in postmenopausal women, the majority of estrogen is produced peripherally through the conversion of androgens to estrogen via the enzyme aromatase. Aromatase inhibitors, such as exemestane, anastrazole, and letrazole, are drugs which block this conversion in peripheral tissues. They are contraindicated in premenopausal women with intact ovarian function, because there is a reflex pituitary stimulation of ovarian estrogen release in response to suppression of peripheral conversion of androgens. For such patients, ovarian function must be ablated either with surgery or with gonadotropin-releasing hormone (GnRH) analogues such as leuprorelin and goserelin if aromatase inhibitors are desired.
In these trials, ovarian ablation was achieved either reversibly with GnRH analogues or permanently and irreversibly with oophorectomy. No studies have compared the survival benefit of these two approaches; however, surgical ovarian ablation is immediate, reliable, and has been shown to be the most cost-effective method.4 It is a good option for women who struggle with adherence to repeated appointments for injections. It also substantially reduces the risk for ovarian cancer, which is elevated among this population of patients, even among those without a deleterious BRCA gene mutation.
BRCA populations
For women with BRCA germline mutations and a history of breast cancer, oophorectomy is associated with a 70% risk of all-cause mortality, including a 60% reduction in breast cancer mortality. This effect is inclusive of patients with “triple-negative,” hormone receptor–negative tumors. The positive effect on breast cancer mortality is predominantly seen among BRCA-1 mutation carriers, and if the oophorectomy is performed within 2 years of diagnosis.5
Technique
When performing oophorectomy either for breast cancer or because of a hereditary cancer syndrome such as BRCA mutation, it is important to ensure that the ovarian vessel pedicle is transected at least 2 cm from its insertion in the ovary. This prevents leaving a residual ovarian remnant. In order to do this, it may be necessary to skeletonize the ovarian vessels free from their physiological attachments to the sigmoid colon on the left, and terminal ileum and cecum on the right. It is also important to ensure that the ureter is not invested in this more proximal segment of ovarian vessels. To prevent this, the retroperitoneal space can be opened lateral to and parallel with the ovarian vessels, and the “medial leaf” of the broad ligament swept medially to expose the ureter as it crosses the bifurcation of the external and internal iliac arteries at the pelvic brim. With the ureter in view, a window can then be made in the “medial leaf” above the ureter and below the ovary and ovarian vessels, in doing so creating a skeletonized ovarian vessel segment which can be sealed and cut 2 cm or more from its insertion in the ovary.
The fallopian tubes should be removed with the ovarian specimens, with attention made to removing the fallopian tube at its junction with the uterine cornua. It should be noted that the majority of fallopian tube cancers arise in the fimbriated end of the tube, and cornual tubal malignancies are fairly uncommon.
The decision about whether or not to perform hysterectomy at the time of salpingo-oophorectomy is complex. In patients without hereditary cancer syndromes, such as BRCA or Lynch syndrome, hysterectomy likely offers no benefit to the patient who is undergoing a procedure for the purpose of ovarian ablation. An argument has been made that hysterectomy can eliminate the increased endometrial cancer risk associated with tamoxifen. However, given the previously discussed data, after oophorectomy, aromatase inhibitors are the preferred treatment option, and tamoxifen can be avoided. If a patient has unrelated underlying uterine pathology a hysterectomy might be indicated. Women with BRCA germline mutations, particularly women with BRCA-1 mutations, may be at increased risk for uterine serous carcinoma, and in these patients, hysterectomy at the time of oophorectomy can be discussed and offered, though as yet, it is not a guideline recommendation for all patients.6 Patients who ask to “just take everything out while you are there” without a clear indication for hysterectomy should be counseled that hysterectomy is associated with increased risk, recovery, and cost, compared with bilateral salpingo-oophorectomy. Among patients with elevated surgical risk (such as morbid obesity, known adhesive disease, increased venous thromboembolism risk, diabetes, and so on) it may not always be appropriate to extend the complexity of the procedure given the limited benefit.
Consequences of ovarian ablation
It should be noted that ovarian ablation in the TEXT and SOFT trials was not associated with an increase in overall survival for women with premenopausal breast cancer. Alternatively, large, observational studies such as the Nurses’ Health Study have shown that premenopausal oophorectomy without hormone replacement therapy is associated with increased all-cause mortality. This is primarily driven by the increased cardiopulmonary risk (heart attack and stroke), deaths after osteoporotic hip fractures, and the increased risk for lung and colon cancer.7,8
It is normal for young patients to have heightened concerns regarding their risk of recurrence from their cancer, and less concerned by threats to their health in decades to come. However, it is important to discuss this data with the patient and allow for her to make an informed decision about her immediate versus future risks. If she determines that she is not interested in permanent ovarian ablation with oophorectomy because of either surgical risks, concerns regarding permanent infertility, or increased all-cause mortality, she still has an option for medical ovarian ablation with GnRH analogues in the treatment of her breast cancer.
Hormone replacement therapy postoperatively
Women who undergo oophorectomy for the treatment of breast cancer should not be offered hormone replacement therapy. This is true even for “triple-negative” or hormone receptor–negative breast cancers as there is still some observed benefit of ovarian ablation, and risk from exogenous hormone administration in these women. Alternatively, postoperative hormone replacement therapy remains safe until the age of natural menopause among premenopausal patients with BRCA germline mutations without a preceding breast cancer diagnosis.
Surgical ovarian ablation with bilateral salpingo-oophorectomy is a valuable strategy in the adjuvant therapy of premenopausal breast cancer, particularly among BRCA mutation carriers and women with hormone receptor–positive disease, or among women who find adherence to medical ablation difficult. Patients should be carefully counseled that this may introduce increased long-term cardiovascular risks for them.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1996 Nov 2;348:1189-96.
2. Pagani O et al. N Engl J Med. 2014 Jul 10;371(12):107-18.
3. Francis PA et al. N Engl J Med. 2015 Jan 29;372(5):436-46.
4. Ferrandina G et al. Clin Drug Investig. 2017 Nov;37(11):1093-102.
5. Finch AP et al. J Clin Oncol. 2014 May 20;32(15):1547-53.
6. Shu CA et al. JAMA Oncol. 2016 Nov 1;2(11):1434-40.
7. Parker WH et al. Obstet Gynecol. 2013 Apr;121(4):709-16.
8. Rivera CM et al. Menopause. 2009 Jan-Feb;16:15-23.
One-quarter of patients with breast cancer are diagnosed at a premenopausal age and these young women may be directed to discuss oophorectomy with their ob.gyn. This may be because of the discovery of a deleterious BRCA gene mutation, which places them at increased risk for ovarian cancer, but oophorectomy may also be a therapeutic option for their breast cancer: 60% of premenopausal breast cancers are hormone receptor–positive. Ovarian ablation has been associated with improved overall survival and disease-free survival among these patients.1
Estrogen is an important promoter of breast cancer and is predominantly derived from ovarian tissue in premenopausal women. However, in postmenopausal women, the majority of estrogen is produced peripherally through the conversion of androgens to estrogen via the enzyme aromatase. Aromatase inhibitors, such as exemestane, anastrazole, and letrazole, are drugs which block this conversion in peripheral tissues. They are contraindicated in premenopausal women with intact ovarian function, because there is a reflex pituitary stimulation of ovarian estrogen release in response to suppression of peripheral conversion of androgens. For such patients, ovarian function must be ablated either with surgery or with gonadotropin-releasing hormone (GnRH) analogues such as leuprorelin and goserelin if aromatase inhibitors are desired.
In these trials, ovarian ablation was achieved either reversibly with GnRH analogues or permanently and irreversibly with oophorectomy. No studies have compared the survival benefit of these two approaches; however, surgical ovarian ablation is immediate, reliable, and has been shown to be the most cost-effective method.4 It is a good option for women who struggle with adherence to repeated appointments for injections. It also substantially reduces the risk for ovarian cancer, which is elevated among this population of patients, even among those without a deleterious BRCA gene mutation.
BRCA populations
For women with BRCA germline mutations and a history of breast cancer, oophorectomy is associated with a 70% risk of all-cause mortality, including a 60% reduction in breast cancer mortality. This effect is inclusive of patients with “triple-negative,” hormone receptor–negative tumors. The positive effect on breast cancer mortality is predominantly seen among BRCA-1 mutation carriers, and if the oophorectomy is performed within 2 years of diagnosis.5
Technique
When performing oophorectomy either for breast cancer or because of a hereditary cancer syndrome such as BRCA mutation, it is important to ensure that the ovarian vessel pedicle is transected at least 2 cm from its insertion in the ovary. This prevents leaving a residual ovarian remnant. In order to do this, it may be necessary to skeletonize the ovarian vessels free from their physiological attachments to the sigmoid colon on the left, and terminal ileum and cecum on the right. It is also important to ensure that the ureter is not invested in this more proximal segment of ovarian vessels. To prevent this, the retroperitoneal space can be opened lateral to and parallel with the ovarian vessels, and the “medial leaf” of the broad ligament swept medially to expose the ureter as it crosses the bifurcation of the external and internal iliac arteries at the pelvic brim. With the ureter in view, a window can then be made in the “medial leaf” above the ureter and below the ovary and ovarian vessels, in doing so creating a skeletonized ovarian vessel segment which can be sealed and cut 2 cm or more from its insertion in the ovary.
The fallopian tubes should be removed with the ovarian specimens, with attention made to removing the fallopian tube at its junction with the uterine cornua. It should be noted that the majority of fallopian tube cancers arise in the fimbriated end of the tube, and cornual tubal malignancies are fairly uncommon.
The decision about whether or not to perform hysterectomy at the time of salpingo-oophorectomy is complex. In patients without hereditary cancer syndromes, such as BRCA or Lynch syndrome, hysterectomy likely offers no benefit to the patient who is undergoing a procedure for the purpose of ovarian ablation. An argument has been made that hysterectomy can eliminate the increased endometrial cancer risk associated with tamoxifen. However, given the previously discussed data, after oophorectomy, aromatase inhibitors are the preferred treatment option, and tamoxifen can be avoided. If a patient has unrelated underlying uterine pathology a hysterectomy might be indicated. Women with BRCA germline mutations, particularly women with BRCA-1 mutations, may be at increased risk for uterine serous carcinoma, and in these patients, hysterectomy at the time of oophorectomy can be discussed and offered, though as yet, it is not a guideline recommendation for all patients.6 Patients who ask to “just take everything out while you are there” without a clear indication for hysterectomy should be counseled that hysterectomy is associated with increased risk, recovery, and cost, compared with bilateral salpingo-oophorectomy. Among patients with elevated surgical risk (such as morbid obesity, known adhesive disease, increased venous thromboembolism risk, diabetes, and so on) it may not always be appropriate to extend the complexity of the procedure given the limited benefit.
Consequences of ovarian ablation
It should be noted that ovarian ablation in the TEXT and SOFT trials was not associated with an increase in overall survival for women with premenopausal breast cancer. Alternatively, large, observational studies such as the Nurses’ Health Study have shown that premenopausal oophorectomy without hormone replacement therapy is associated with increased all-cause mortality. This is primarily driven by the increased cardiopulmonary risk (heart attack and stroke), deaths after osteoporotic hip fractures, and the increased risk for lung and colon cancer.7,8
It is normal for young patients to have heightened concerns regarding their risk of recurrence from their cancer, and less concerned by threats to their health in decades to come. However, it is important to discuss this data with the patient and allow for her to make an informed decision about her immediate versus future risks. If she determines that she is not interested in permanent ovarian ablation with oophorectomy because of either surgical risks, concerns regarding permanent infertility, or increased all-cause mortality, she still has an option for medical ovarian ablation with GnRH analogues in the treatment of her breast cancer.
Hormone replacement therapy postoperatively
Women who undergo oophorectomy for the treatment of breast cancer should not be offered hormone replacement therapy. This is true even for “triple-negative” or hormone receptor–negative breast cancers as there is still some observed benefit of ovarian ablation, and risk from exogenous hormone administration in these women. Alternatively, postoperative hormone replacement therapy remains safe until the age of natural menopause among premenopausal patients with BRCA germline mutations without a preceding breast cancer diagnosis.
Surgical ovarian ablation with bilateral salpingo-oophorectomy is a valuable strategy in the adjuvant therapy of premenopausal breast cancer, particularly among BRCA mutation carriers and women with hormone receptor–positive disease, or among women who find adherence to medical ablation difficult. Patients should be carefully counseled that this may introduce increased long-term cardiovascular risks for them.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1996 Nov 2;348:1189-96.
2. Pagani O et al. N Engl J Med. 2014 Jul 10;371(12):107-18.
3. Francis PA et al. N Engl J Med. 2015 Jan 29;372(5):436-46.
4. Ferrandina G et al. Clin Drug Investig. 2017 Nov;37(11):1093-102.
5. Finch AP et al. J Clin Oncol. 2014 May 20;32(15):1547-53.
6. Shu CA et al. JAMA Oncol. 2016 Nov 1;2(11):1434-40.
7. Parker WH et al. Obstet Gynecol. 2013 Apr;121(4):709-16.
8. Rivera CM et al. Menopause. 2009 Jan-Feb;16:15-23.
Be Part of the (Larger) Conversation
Providing free and open access to its high-quality peer-reviewed articles has always been important to Federal Practitioner, but finding them hasn’t always been easy for our readers and researchers. That has now changed. The full text of all
To be sure, Federal Practitioner has always made it easy for print and digital subscribers to find our articles. Print journal subscriptions have been—and will remain—free to the 35,000 subscribers. Furthermore, anyone can access articles online (http://mdedge.com/fedprac), in the Federal Practitioner app, or in our digital edition (http://www.fedprac-digital.com/).
However, until now access beyond our base of loyal readers has been limited. Inclusion in PMC provides a much broader audience for Federal Practitioner authors, because PMC is an integral part of the NLM MEDLINE/PubMed database of 28 million biomedical citations and abstracts from more than 5,000 journals. All PMC articles appear in PubMed searches. On a typical day, about 2.5 million users in the US access PubMed to perform about 3 million searches and access 9 million page views.1
Inclusion also means that Federal Practitioner has passed a rigorous scientific and technical review of its content. Being included in PMC is a recognition of the quality of scholarship the journal publishes and a pledge of our continuing commitment to the highest quality of clinical education and research. Young investigators, clinician-educators, midcareer professionals, and others seeking to launch or enhance an academic career may want to consider or reconsider Federal Practitioner as the destination for manuscript submission.
One of the goals of this journal has been to provide a forum for federal health care providers (HCPs) to discuss and share with other federal colleagues. Federal HCPs from the Military Health System (MHS), Veterans Health Administration (VHA), and Indian Health Service (IHS) have addressed questions in Federal Practitioner that might not be explored elsewhere. Yet something important was missing from those conversations—engagement with the larger public health community. PubMed and PMC enable an ongoing conversation among health care researchers and providers. These are the places where researchers go to understand and respond to the questions that shape their research and clinical care. Now, Federal Practitioner authors can contribute more fully in ongoing debates.
As large integrated health care systems, the VHA, MHS, and IHS confront and address key public health care policy issues. Whether it’s the responsible and safe prescribing of opioids, the resource allocation decisions regarding the treatment of hepatitis C, or addressing suicide risk, the experience of federal HCPs must be a part of the public health debate. Moreover, many Federal Practitioner articles focus not just on preliminary research, but on the practical aspects of implementing patient-centered care. All US HCPs may benefit from hearing about federal providers challenges and success in providing patient-centered care.
Making available the complete text of all the articles furthers the Federal Practitioner mission: to educate federal HCPs and provide a forum for sharing health-care related studies, best practices, guidelines, program profiles, and case studies. We are excited to provide even more benefits for publication in Federal Practitioner. This journal welcomes submissions from new authors, well-traveled scholars, and everyone in between. Come on, join the conversation.
1. Fiorini N, Lipman DJ, Lu Z. Towards PubMed 2.0. eLife. 2017;6:e28801.
Providing free and open access to its high-quality peer-reviewed articles has always been important to Federal Practitioner, but finding them hasn’t always been easy for our readers and researchers. That has now changed. The full text of all
To be sure, Federal Practitioner has always made it easy for print and digital subscribers to find our articles. Print journal subscriptions have been—and will remain—free to the 35,000 subscribers. Furthermore, anyone can access articles online (http://mdedge.com/fedprac), in the Federal Practitioner app, or in our digital edition (http://www.fedprac-digital.com/).
However, until now access beyond our base of loyal readers has been limited. Inclusion in PMC provides a much broader audience for Federal Practitioner authors, because PMC is an integral part of the NLM MEDLINE/PubMed database of 28 million biomedical citations and abstracts from more than 5,000 journals. All PMC articles appear in PubMed searches. On a typical day, about 2.5 million users in the US access PubMed to perform about 3 million searches and access 9 million page views.1
Inclusion also means that Federal Practitioner has passed a rigorous scientific and technical review of its content. Being included in PMC is a recognition of the quality of scholarship the journal publishes and a pledge of our continuing commitment to the highest quality of clinical education and research. Young investigators, clinician-educators, midcareer professionals, and others seeking to launch or enhance an academic career may want to consider or reconsider Federal Practitioner as the destination for manuscript submission.
One of the goals of this journal has been to provide a forum for federal health care providers (HCPs) to discuss and share with other federal colleagues. Federal HCPs from the Military Health System (MHS), Veterans Health Administration (VHA), and Indian Health Service (IHS) have addressed questions in Federal Practitioner that might not be explored elsewhere. Yet something important was missing from those conversations—engagement with the larger public health community. PubMed and PMC enable an ongoing conversation among health care researchers and providers. These are the places where researchers go to understand and respond to the questions that shape their research and clinical care. Now, Federal Practitioner authors can contribute more fully in ongoing debates.
As large integrated health care systems, the VHA, MHS, and IHS confront and address key public health care policy issues. Whether it’s the responsible and safe prescribing of opioids, the resource allocation decisions regarding the treatment of hepatitis C, or addressing suicide risk, the experience of federal HCPs must be a part of the public health debate. Moreover, many Federal Practitioner articles focus not just on preliminary research, but on the practical aspects of implementing patient-centered care. All US HCPs may benefit from hearing about federal providers challenges and success in providing patient-centered care.
Making available the complete text of all the articles furthers the Federal Practitioner mission: to educate federal HCPs and provide a forum for sharing health-care related studies, best practices, guidelines, program profiles, and case studies. We are excited to provide even more benefits for publication in Federal Practitioner. This journal welcomes submissions from new authors, well-traveled scholars, and everyone in between. Come on, join the conversation.
Providing free and open access to its high-quality peer-reviewed articles has always been important to Federal Practitioner, but finding them hasn’t always been easy for our readers and researchers. That has now changed. The full text of all
To be sure, Federal Practitioner has always made it easy for print and digital subscribers to find our articles. Print journal subscriptions have been—and will remain—free to the 35,000 subscribers. Furthermore, anyone can access articles online (http://mdedge.com/fedprac), in the Federal Practitioner app, or in our digital edition (http://www.fedprac-digital.com/).
However, until now access beyond our base of loyal readers has been limited. Inclusion in PMC provides a much broader audience for Federal Practitioner authors, because PMC is an integral part of the NLM MEDLINE/PubMed database of 28 million biomedical citations and abstracts from more than 5,000 journals. All PMC articles appear in PubMed searches. On a typical day, about 2.5 million users in the US access PubMed to perform about 3 million searches and access 9 million page views.1
Inclusion also means that Federal Practitioner has passed a rigorous scientific and technical review of its content. Being included in PMC is a recognition of the quality of scholarship the journal publishes and a pledge of our continuing commitment to the highest quality of clinical education and research. Young investigators, clinician-educators, midcareer professionals, and others seeking to launch or enhance an academic career may want to consider or reconsider Federal Practitioner as the destination for manuscript submission.
One of the goals of this journal has been to provide a forum for federal health care providers (HCPs) to discuss and share with other federal colleagues. Federal HCPs from the Military Health System (MHS), Veterans Health Administration (VHA), and Indian Health Service (IHS) have addressed questions in Federal Practitioner that might not be explored elsewhere. Yet something important was missing from those conversations—engagement with the larger public health community. PubMed and PMC enable an ongoing conversation among health care researchers and providers. These are the places where researchers go to understand and respond to the questions that shape their research and clinical care. Now, Federal Practitioner authors can contribute more fully in ongoing debates.
As large integrated health care systems, the VHA, MHS, and IHS confront and address key public health care policy issues. Whether it’s the responsible and safe prescribing of opioids, the resource allocation decisions regarding the treatment of hepatitis C, or addressing suicide risk, the experience of federal HCPs must be a part of the public health debate. Moreover, many Federal Practitioner articles focus not just on preliminary research, but on the practical aspects of implementing patient-centered care. All US HCPs may benefit from hearing about federal providers challenges and success in providing patient-centered care.
Making available the complete text of all the articles furthers the Federal Practitioner mission: to educate federal HCPs and provide a forum for sharing health-care related studies, best practices, guidelines, program profiles, and case studies. We are excited to provide even more benefits for publication in Federal Practitioner. This journal welcomes submissions from new authors, well-traveled scholars, and everyone in between. Come on, join the conversation.
1. Fiorini N, Lipman DJ, Lu Z. Towards PubMed 2.0. eLife. 2017;6:e28801.
1. Fiorini N, Lipman DJ, Lu Z. Towards PubMed 2.0. eLife. 2017;6:e28801.
Looking at Ourselves
The genesis of the ongoing debate on healthcare is complex, in part because of the perceived flaws within the proposed systems. Denying the guilt that the medical profession has had is a disingenuous exercise in futility. In fact, orthopedics is possibly the most egregious among the culprits. Such a charge, supportable or not, calls for serious objective criticism.1 No one would dare question the fact that from the orthopedic point of view, the cost of healthcare delivery is an important factor because of the critical level this issue has reached. The use of expensive technology and frequent surgery are of major importance.2,3 However, I submit that it is not the high cost of the technology and surgical procedures per se but their frequent abuse.
As I look at orthopedics in an objective, critical manner, I have the uncomfortable feeling that the profession is rapidly becoming a business where greater and greater profit is its primary raison d’etre. The discipline has lost much of its traditional scientific/biological foundations, and is converting itself into a technical trade heavily controlled in its educational duties and the subsequent conduct of its members by industry. This metamorphous evolution has shown ill effects as demonstrated by the loss of traditional territory to other disciplines and a borderline ridiculous fragmentation into a multitude of subspecialties that contribute to the erosion of the profession. Orthopedics is no longer a solid eclectic body of knowledge and expertise in the care of musculoskeletal conditions, but a fragmented body of techniques independent of each other. This statement is not a criticism of fragmentation per se, because fragmentation in most human endeavors is a natural evolutionary phenomenon that occurs in virtually all walks of life, and to our profession it has brought much progress. My concerns are over the apparent exaggerated degree it has reached.4
The fragmentation and erosion of orthopedics took a relatively short time to occur without any evidence of concern among the orthopedic community, which apparently assumed that the advances made by other disciplines would not compromise the security and independence it had enjoyed for generations. The spectacular advances in joint replacement began to occupy a large segment of orthopedists’ professional time. The attractive reimbursement accompanying these procedures further justified the complacency regarding the shrinking of the discipline, while the previous outsiders expanded their territory. Critical objectivity of this issue is important in the event we decide to address the consequences of further erosion and fragmentation of our profession.
There should be no question that if all, or the overwhelming majority of, orthopedists become subspecialists who take care of only a limited number of pathological conditions, the cost of care will grow exponentially. The poor, regardless of the outcome of any legislation addressing their problems, will suffer most.
In small communities there are not enough patients with conditions requiring subspecialized orthopedic services to satisfy the emotional and economic needs of the fellowship-trained orthopedists. Other physicians and allied health practitioners will fill the void and provide the needed services. However, the facts facing us today suggest that if the current trends continue unabated, orthopedics as a distinct branch of medicine may not survive. Nonetheless, people in need of musculoskeletal care will receive it from a variety of medical and paramedical people, who will gradually develop skills and knowledge in a manner comparable to that possessed today by orthopedists.
Continue to: Of major significance...
Of major significance in the overall issue of critical objectivity is the unquestionable fact that orthopedics voluntarily relegated to the surgical implant industry the control of many of its traditional educational responsibilities to the point where, at present, it is assumed that educational programs cannot be conducted without the financial support of industry. Hundreds, if not thousands, of conferences; grand rounds; local, state, and regional society meetings; the American Academy of Orthopaedic Surgeons’ (AAOS) annual meeting, and its many other educational activities take place with the financial support of industry. Such a dominance has placed the orthopedic profession in a relative subservient position, since the “generosity” provided by industry must be reciprocated. This explains the rapid and overwhelming favorable response given by the practitioners of the medical profession by seeming to accept whatever new products appear on the market. The issue has become even more complicated by the growing acceptance of kickbacks and perks for cooperation with the manufactures, simply for the use of the industrial products and assistance in advertising them.5,6.
I have previously described episodes in which I was personally involved. The one I now describe consisted of a visit by an industrial representative who approached me during my tenure as Chairman of Orthopedics at the University of Southern California.1 He offered me what he called “a very good deal” where I was to be given $200 for every one of his total joint implants I were to use, as well as from those used by any of the orthopedists working at any and all 5 hospitals affiliated with the department. I was to receive a monthly check at home, so no one else would be aware of the transactions. When I asked him what had prompted him to think I was a prostitute, all he could say was, “But Doctor Sarmiento, we do that all the time.”
I am certain that episodes of the nature of my experience occur every day of the week. Had it not been aware of their frequent occurrence, the United States Justice Department’s investigation of the “egregious unethical transgressions” and the “corruption in the relationship between the industry and orthopedics”, and the search for a solution would not have been initiated.6 What can we expect to come out of such investigations? As far as I am concerned, those who may have been accused unfairly, as well as the guilty ones, have probably stated that “all conflicts of interest have been resolved.” I insist that the orthopedic profession, and particularly its representative organizations, should stand up and, as loudly and clearly as possible, protest the despicable practice and bring about a cure for the festering ulcer.
The power of industry in controlling orthopedics has reached a previously unsuspected level. In a commentary I published in the Journal of Bone and Joint Surgery,7 I described an episode where a powerful industrial concern had prevented the release of a book I had written. The product had reached its final form in hardbound and softbound editions in English, as well as in Spanish. After much struggle to find out why at the last minute the marketing had not begun, I learned that an industrial firm had “convinced” the publishers to cancel the release of the book. Though I considered the litigation route, I realized that I did not have the financial resources for such a venture. I have experienced similar problems with other publishers in the past.7 When our representative organizations will seek involvement in this delicate issue and try to bring about a solution is as yet unknown.
A fact influencing the decline of professionalism in our ranks is the now well-known lack of credibility in an increasing number of publications.8 Some respectable journals are making a serious effort to overcome the problem, but they realize how difficult it is to see the truth, since distorting data is not that difficult, and exposing the culprits is even more so. What possible solution can be structured to resolve this embarrassing situation? It is an issue of morality that cannot be legislated; however, it can be positively influenced by the example set for the younger generations, especially by our representative officers. Unfortunately, the latter group has failed to do a very good job, since we frequently observe individuals with obvious conflicts of interest occupying positions of leadership and power.
Continue to: In the United States another move...
In the United States another move, supported by a number of well-qualified authorities in the field of medical economics, advocates the establishment of a system where all physicians would be salaried hospital employees.3 For some time I have felt inclined to support the concept, as I saw it as a logical and practical one. However, my mind has been rapidly changing, particularly after observing the growing number of hospitals employing physicians throughout some regions of the country. I ask myself, how will the number of subspecialists in each hospital be able to enroll? It cannot be open-ended, since the cost of providing a salary, malpractice insurance, secretarial support, vacation time, health insurance, and other benefits can amount to degrees the institutions cannot afford. Another discouraging find was related to the fact that the salaried doctors would receive bonuses according to the amount of work they did. This is a logical and well-intentioned move. However, it would perpetuate one of the major problems besetting the current situation: the rendering of nonessential expensive services and the performance of unnecessary surgery in order to increase the size of the bonus. Would this be a return to the current dilemma?
For the various issues I have identified and for which critical objectivity is necessary, it is essential that our representative organizations assume a leading role, concentrate on fundamental issues, and set aside time-consuming projects of questionable importance. Among those projects I identity 3 important ones: The Joint Replacement Registry, The Orthopedic Guidelines, and Evidence-Based Orthopedics. The Joint Replacement Registry can wait, since its foundations as presented today are weak and based on the illusion that the alleged success of the Scandinavian Registries can be easily duplicated in this country. I envision that 15 years from now the only thing the Registry will give us is millions of pieces of data that were already available through the traditional methods of publications and other means of dissemination of information.9
The recent infatuation with The Orthopedic Guidelines may be a temporary success that will die before they reach maturity. It is a noble effort, since it proposes a mechanism by which to provide “advice” to orthopedic surgeons regarding the degree of benefit that various treatment approaches have to offer. The problem, as I see it, is that soon the “advice” will become, in the eyes of many, “dictates” to be followed. The fear of litigation for not following the “guidelines” could result in deprivation of the independence that is acquired from experience and lessons from others. We are not children in need of instructions regarding behavior. Rather than making changes with new but probably imperfect projects, we should emphasize the credibility of publications and oral presentations. The authors of the Guidelines are appointed individuals who, like all human beings, are afflicted with the innate biases and prejudices that make them see things according to their personal perceptions, and their views are not necessarily representative of ideal situations.10
Evidence-Based Orthopedics is another noble effort to improve matters. I find in this effort the same flaws I have identified with Registries and Guidelines. There is not yet any evidence to suggest that the profession will be helped from the time and expense they require. Any publication should be based on evidence; otherwise it should not be brought to the orthopedic community. Emphasizing credibility is more likely to be beneficial to the profession and to the people we represent. To have 2 different types of articles in our journals where some are based on evidence and others lack evidence does not make sense.
In summary, orthopedics is confronting situations that require critical objectivity as we search for solutions. Some of the situations I have tried to identify may not be “problems” but examples of normal evolution or transient developments that time alone would resolve. On the other hand, some others may be of a serious nature and require our involvement. To ignore them will bring about problems for the next generation who will wonder what precluded us from seeking answers before it was too late.
1. Sarmiento A. Bare Bones: A Surgeon’s Tale. Amherst, NY: Prometheus Books; 2003.
2. Callahan D. Taming the Beloved Beast: How Medical Technology Costs are Destroying Our Health Care System. Princeton, NJ: Princeton University Press; 2009.
3. Relman AS. Doctors as the key to health care reform. N Engl J Med 2009;361(13):1225-1227. doi:10.1056/NEJMp0907925.
4. Sarmiento A. Subspecialization in orthopaedics. Has it been all for the better? J Bone Joint Surg Am. 2003;85-A(2):369-373.
5. Sarmiento A The relationship between orthopaedics and industry must be reformed. Clin Orthop Relat Res. 2003;412:38-44.
6. Five Companies in Hip and Knee Replacement Industry Avoid Prosecution by Agreeing to Compliance Rules and Monitoring. Newark, NJ: US Dept of Justice, US Attorney, District of New Jersey; 2007.
7. Sarmiento A. Infringing on freedom of speech. J Bone Joint Surg Am. 2011;93(2):222. doi:10.2106/JBJS.J.00888.
8. Carr AJ. Which research is to be believed? The ethics of industrial funding of orthopaedic research. J Bone and Joint Surg Br. 2005;87(11):1452-1453.
9. Sarmiento A. Orthopedic registries: second thoughts. Am J Orthop. 2015;44(4):159-160.
10. Sarmiento A. Thoughts on orthopedic guidelines. Am J Orthop. 2010;39(8):373-374.
The genesis of the ongoing debate on healthcare is complex, in part because of the perceived flaws within the proposed systems. Denying the guilt that the medical profession has had is a disingenuous exercise in futility. In fact, orthopedics is possibly the most egregious among the culprits. Such a charge, supportable or not, calls for serious objective criticism.1 No one would dare question the fact that from the orthopedic point of view, the cost of healthcare delivery is an important factor because of the critical level this issue has reached. The use of expensive technology and frequent surgery are of major importance.2,3 However, I submit that it is not the high cost of the technology and surgical procedures per se but their frequent abuse.
As I look at orthopedics in an objective, critical manner, I have the uncomfortable feeling that the profession is rapidly becoming a business where greater and greater profit is its primary raison d’etre. The discipline has lost much of its traditional scientific/biological foundations, and is converting itself into a technical trade heavily controlled in its educational duties and the subsequent conduct of its members by industry. This metamorphous evolution has shown ill effects as demonstrated by the loss of traditional territory to other disciplines and a borderline ridiculous fragmentation into a multitude of subspecialties that contribute to the erosion of the profession. Orthopedics is no longer a solid eclectic body of knowledge and expertise in the care of musculoskeletal conditions, but a fragmented body of techniques independent of each other. This statement is not a criticism of fragmentation per se, because fragmentation in most human endeavors is a natural evolutionary phenomenon that occurs in virtually all walks of life, and to our profession it has brought much progress. My concerns are over the apparent exaggerated degree it has reached.4
The fragmentation and erosion of orthopedics took a relatively short time to occur without any evidence of concern among the orthopedic community, which apparently assumed that the advances made by other disciplines would not compromise the security and independence it had enjoyed for generations. The spectacular advances in joint replacement began to occupy a large segment of orthopedists’ professional time. The attractive reimbursement accompanying these procedures further justified the complacency regarding the shrinking of the discipline, while the previous outsiders expanded their territory. Critical objectivity of this issue is important in the event we decide to address the consequences of further erosion and fragmentation of our profession.
There should be no question that if all, or the overwhelming majority of, orthopedists become subspecialists who take care of only a limited number of pathological conditions, the cost of care will grow exponentially. The poor, regardless of the outcome of any legislation addressing their problems, will suffer most.
In small communities there are not enough patients with conditions requiring subspecialized orthopedic services to satisfy the emotional and economic needs of the fellowship-trained orthopedists. Other physicians and allied health practitioners will fill the void and provide the needed services. However, the facts facing us today suggest that if the current trends continue unabated, orthopedics as a distinct branch of medicine may not survive. Nonetheless, people in need of musculoskeletal care will receive it from a variety of medical and paramedical people, who will gradually develop skills and knowledge in a manner comparable to that possessed today by orthopedists.
Continue to: Of major significance...
Of major significance in the overall issue of critical objectivity is the unquestionable fact that orthopedics voluntarily relegated to the surgical implant industry the control of many of its traditional educational responsibilities to the point where, at present, it is assumed that educational programs cannot be conducted without the financial support of industry. Hundreds, if not thousands, of conferences; grand rounds; local, state, and regional society meetings; the American Academy of Orthopaedic Surgeons’ (AAOS) annual meeting, and its many other educational activities take place with the financial support of industry. Such a dominance has placed the orthopedic profession in a relative subservient position, since the “generosity” provided by industry must be reciprocated. This explains the rapid and overwhelming favorable response given by the practitioners of the medical profession by seeming to accept whatever new products appear on the market. The issue has become even more complicated by the growing acceptance of kickbacks and perks for cooperation with the manufactures, simply for the use of the industrial products and assistance in advertising them.5,6.
I have previously described episodes in which I was personally involved. The one I now describe consisted of a visit by an industrial representative who approached me during my tenure as Chairman of Orthopedics at the University of Southern California.1 He offered me what he called “a very good deal” where I was to be given $200 for every one of his total joint implants I were to use, as well as from those used by any of the orthopedists working at any and all 5 hospitals affiliated with the department. I was to receive a monthly check at home, so no one else would be aware of the transactions. When I asked him what had prompted him to think I was a prostitute, all he could say was, “But Doctor Sarmiento, we do that all the time.”
I am certain that episodes of the nature of my experience occur every day of the week. Had it not been aware of their frequent occurrence, the United States Justice Department’s investigation of the “egregious unethical transgressions” and the “corruption in the relationship between the industry and orthopedics”, and the search for a solution would not have been initiated.6 What can we expect to come out of such investigations? As far as I am concerned, those who may have been accused unfairly, as well as the guilty ones, have probably stated that “all conflicts of interest have been resolved.” I insist that the orthopedic profession, and particularly its representative organizations, should stand up and, as loudly and clearly as possible, protest the despicable practice and bring about a cure for the festering ulcer.
The power of industry in controlling orthopedics has reached a previously unsuspected level. In a commentary I published in the Journal of Bone and Joint Surgery,7 I described an episode where a powerful industrial concern had prevented the release of a book I had written. The product had reached its final form in hardbound and softbound editions in English, as well as in Spanish. After much struggle to find out why at the last minute the marketing had not begun, I learned that an industrial firm had “convinced” the publishers to cancel the release of the book. Though I considered the litigation route, I realized that I did not have the financial resources for such a venture. I have experienced similar problems with other publishers in the past.7 When our representative organizations will seek involvement in this delicate issue and try to bring about a solution is as yet unknown.
A fact influencing the decline of professionalism in our ranks is the now well-known lack of credibility in an increasing number of publications.8 Some respectable journals are making a serious effort to overcome the problem, but they realize how difficult it is to see the truth, since distorting data is not that difficult, and exposing the culprits is even more so. What possible solution can be structured to resolve this embarrassing situation? It is an issue of morality that cannot be legislated; however, it can be positively influenced by the example set for the younger generations, especially by our representative officers. Unfortunately, the latter group has failed to do a very good job, since we frequently observe individuals with obvious conflicts of interest occupying positions of leadership and power.
Continue to: In the United States another move...
In the United States another move, supported by a number of well-qualified authorities in the field of medical economics, advocates the establishment of a system where all physicians would be salaried hospital employees.3 For some time I have felt inclined to support the concept, as I saw it as a logical and practical one. However, my mind has been rapidly changing, particularly after observing the growing number of hospitals employing physicians throughout some regions of the country. I ask myself, how will the number of subspecialists in each hospital be able to enroll? It cannot be open-ended, since the cost of providing a salary, malpractice insurance, secretarial support, vacation time, health insurance, and other benefits can amount to degrees the institutions cannot afford. Another discouraging find was related to the fact that the salaried doctors would receive bonuses according to the amount of work they did. This is a logical and well-intentioned move. However, it would perpetuate one of the major problems besetting the current situation: the rendering of nonessential expensive services and the performance of unnecessary surgery in order to increase the size of the bonus. Would this be a return to the current dilemma?
For the various issues I have identified and for which critical objectivity is necessary, it is essential that our representative organizations assume a leading role, concentrate on fundamental issues, and set aside time-consuming projects of questionable importance. Among those projects I identity 3 important ones: The Joint Replacement Registry, The Orthopedic Guidelines, and Evidence-Based Orthopedics. The Joint Replacement Registry can wait, since its foundations as presented today are weak and based on the illusion that the alleged success of the Scandinavian Registries can be easily duplicated in this country. I envision that 15 years from now the only thing the Registry will give us is millions of pieces of data that were already available through the traditional methods of publications and other means of dissemination of information.9
The recent infatuation with The Orthopedic Guidelines may be a temporary success that will die before they reach maturity. It is a noble effort, since it proposes a mechanism by which to provide “advice” to orthopedic surgeons regarding the degree of benefit that various treatment approaches have to offer. The problem, as I see it, is that soon the “advice” will become, in the eyes of many, “dictates” to be followed. The fear of litigation for not following the “guidelines” could result in deprivation of the independence that is acquired from experience and lessons from others. We are not children in need of instructions regarding behavior. Rather than making changes with new but probably imperfect projects, we should emphasize the credibility of publications and oral presentations. The authors of the Guidelines are appointed individuals who, like all human beings, are afflicted with the innate biases and prejudices that make them see things according to their personal perceptions, and their views are not necessarily representative of ideal situations.10
Evidence-Based Orthopedics is another noble effort to improve matters. I find in this effort the same flaws I have identified with Registries and Guidelines. There is not yet any evidence to suggest that the profession will be helped from the time and expense they require. Any publication should be based on evidence; otherwise it should not be brought to the orthopedic community. Emphasizing credibility is more likely to be beneficial to the profession and to the people we represent. To have 2 different types of articles in our journals where some are based on evidence and others lack evidence does not make sense.
In summary, orthopedics is confronting situations that require critical objectivity as we search for solutions. Some of the situations I have tried to identify may not be “problems” but examples of normal evolution or transient developments that time alone would resolve. On the other hand, some others may be of a serious nature and require our involvement. To ignore them will bring about problems for the next generation who will wonder what precluded us from seeking answers before it was too late.
The genesis of the ongoing debate on healthcare is complex, in part because of the perceived flaws within the proposed systems. Denying the guilt that the medical profession has had is a disingenuous exercise in futility. In fact, orthopedics is possibly the most egregious among the culprits. Such a charge, supportable or not, calls for serious objective criticism.1 No one would dare question the fact that from the orthopedic point of view, the cost of healthcare delivery is an important factor because of the critical level this issue has reached. The use of expensive technology and frequent surgery are of major importance.2,3 However, I submit that it is not the high cost of the technology and surgical procedures per se but their frequent abuse.
As I look at orthopedics in an objective, critical manner, I have the uncomfortable feeling that the profession is rapidly becoming a business where greater and greater profit is its primary raison d’etre. The discipline has lost much of its traditional scientific/biological foundations, and is converting itself into a technical trade heavily controlled in its educational duties and the subsequent conduct of its members by industry. This metamorphous evolution has shown ill effects as demonstrated by the loss of traditional territory to other disciplines and a borderline ridiculous fragmentation into a multitude of subspecialties that contribute to the erosion of the profession. Orthopedics is no longer a solid eclectic body of knowledge and expertise in the care of musculoskeletal conditions, but a fragmented body of techniques independent of each other. This statement is not a criticism of fragmentation per se, because fragmentation in most human endeavors is a natural evolutionary phenomenon that occurs in virtually all walks of life, and to our profession it has brought much progress. My concerns are over the apparent exaggerated degree it has reached.4
The fragmentation and erosion of orthopedics took a relatively short time to occur without any evidence of concern among the orthopedic community, which apparently assumed that the advances made by other disciplines would not compromise the security and independence it had enjoyed for generations. The spectacular advances in joint replacement began to occupy a large segment of orthopedists’ professional time. The attractive reimbursement accompanying these procedures further justified the complacency regarding the shrinking of the discipline, while the previous outsiders expanded their territory. Critical objectivity of this issue is important in the event we decide to address the consequences of further erosion and fragmentation of our profession.
There should be no question that if all, or the overwhelming majority of, orthopedists become subspecialists who take care of only a limited number of pathological conditions, the cost of care will grow exponentially. The poor, regardless of the outcome of any legislation addressing their problems, will suffer most.
In small communities there are not enough patients with conditions requiring subspecialized orthopedic services to satisfy the emotional and economic needs of the fellowship-trained orthopedists. Other physicians and allied health practitioners will fill the void and provide the needed services. However, the facts facing us today suggest that if the current trends continue unabated, orthopedics as a distinct branch of medicine may not survive. Nonetheless, people in need of musculoskeletal care will receive it from a variety of medical and paramedical people, who will gradually develop skills and knowledge in a manner comparable to that possessed today by orthopedists.
Continue to: Of major significance...
Of major significance in the overall issue of critical objectivity is the unquestionable fact that orthopedics voluntarily relegated to the surgical implant industry the control of many of its traditional educational responsibilities to the point where, at present, it is assumed that educational programs cannot be conducted without the financial support of industry. Hundreds, if not thousands, of conferences; grand rounds; local, state, and regional society meetings; the American Academy of Orthopaedic Surgeons’ (AAOS) annual meeting, and its many other educational activities take place with the financial support of industry. Such a dominance has placed the orthopedic profession in a relative subservient position, since the “generosity” provided by industry must be reciprocated. This explains the rapid and overwhelming favorable response given by the practitioners of the medical profession by seeming to accept whatever new products appear on the market. The issue has become even more complicated by the growing acceptance of kickbacks and perks for cooperation with the manufactures, simply for the use of the industrial products and assistance in advertising them.5,6.
I have previously described episodes in which I was personally involved. The one I now describe consisted of a visit by an industrial representative who approached me during my tenure as Chairman of Orthopedics at the University of Southern California.1 He offered me what he called “a very good deal” where I was to be given $200 for every one of his total joint implants I were to use, as well as from those used by any of the orthopedists working at any and all 5 hospitals affiliated with the department. I was to receive a monthly check at home, so no one else would be aware of the transactions. When I asked him what had prompted him to think I was a prostitute, all he could say was, “But Doctor Sarmiento, we do that all the time.”
I am certain that episodes of the nature of my experience occur every day of the week. Had it not been aware of their frequent occurrence, the United States Justice Department’s investigation of the “egregious unethical transgressions” and the “corruption in the relationship between the industry and orthopedics”, and the search for a solution would not have been initiated.6 What can we expect to come out of such investigations? As far as I am concerned, those who may have been accused unfairly, as well as the guilty ones, have probably stated that “all conflicts of interest have been resolved.” I insist that the orthopedic profession, and particularly its representative organizations, should stand up and, as loudly and clearly as possible, protest the despicable practice and bring about a cure for the festering ulcer.
The power of industry in controlling orthopedics has reached a previously unsuspected level. In a commentary I published in the Journal of Bone and Joint Surgery,7 I described an episode where a powerful industrial concern had prevented the release of a book I had written. The product had reached its final form in hardbound and softbound editions in English, as well as in Spanish. After much struggle to find out why at the last minute the marketing had not begun, I learned that an industrial firm had “convinced” the publishers to cancel the release of the book. Though I considered the litigation route, I realized that I did not have the financial resources for such a venture. I have experienced similar problems with other publishers in the past.7 When our representative organizations will seek involvement in this delicate issue and try to bring about a solution is as yet unknown.
A fact influencing the decline of professionalism in our ranks is the now well-known lack of credibility in an increasing number of publications.8 Some respectable journals are making a serious effort to overcome the problem, but they realize how difficult it is to see the truth, since distorting data is not that difficult, and exposing the culprits is even more so. What possible solution can be structured to resolve this embarrassing situation? It is an issue of morality that cannot be legislated; however, it can be positively influenced by the example set for the younger generations, especially by our representative officers. Unfortunately, the latter group has failed to do a very good job, since we frequently observe individuals with obvious conflicts of interest occupying positions of leadership and power.
Continue to: In the United States another move...
In the United States another move, supported by a number of well-qualified authorities in the field of medical economics, advocates the establishment of a system where all physicians would be salaried hospital employees.3 For some time I have felt inclined to support the concept, as I saw it as a logical and practical one. However, my mind has been rapidly changing, particularly after observing the growing number of hospitals employing physicians throughout some regions of the country. I ask myself, how will the number of subspecialists in each hospital be able to enroll? It cannot be open-ended, since the cost of providing a salary, malpractice insurance, secretarial support, vacation time, health insurance, and other benefits can amount to degrees the institutions cannot afford. Another discouraging find was related to the fact that the salaried doctors would receive bonuses according to the amount of work they did. This is a logical and well-intentioned move. However, it would perpetuate one of the major problems besetting the current situation: the rendering of nonessential expensive services and the performance of unnecessary surgery in order to increase the size of the bonus. Would this be a return to the current dilemma?
For the various issues I have identified and for which critical objectivity is necessary, it is essential that our representative organizations assume a leading role, concentrate on fundamental issues, and set aside time-consuming projects of questionable importance. Among those projects I identity 3 important ones: The Joint Replacement Registry, The Orthopedic Guidelines, and Evidence-Based Orthopedics. The Joint Replacement Registry can wait, since its foundations as presented today are weak and based on the illusion that the alleged success of the Scandinavian Registries can be easily duplicated in this country. I envision that 15 years from now the only thing the Registry will give us is millions of pieces of data that were already available through the traditional methods of publications and other means of dissemination of information.9
The recent infatuation with The Orthopedic Guidelines may be a temporary success that will die before they reach maturity. It is a noble effort, since it proposes a mechanism by which to provide “advice” to orthopedic surgeons regarding the degree of benefit that various treatment approaches have to offer. The problem, as I see it, is that soon the “advice” will become, in the eyes of many, “dictates” to be followed. The fear of litigation for not following the “guidelines” could result in deprivation of the independence that is acquired from experience and lessons from others. We are not children in need of instructions regarding behavior. Rather than making changes with new but probably imperfect projects, we should emphasize the credibility of publications and oral presentations. The authors of the Guidelines are appointed individuals who, like all human beings, are afflicted with the innate biases and prejudices that make them see things according to their personal perceptions, and their views are not necessarily representative of ideal situations.10
Evidence-Based Orthopedics is another noble effort to improve matters. I find in this effort the same flaws I have identified with Registries and Guidelines. There is not yet any evidence to suggest that the profession will be helped from the time and expense they require. Any publication should be based on evidence; otherwise it should not be brought to the orthopedic community. Emphasizing credibility is more likely to be beneficial to the profession and to the people we represent. To have 2 different types of articles in our journals where some are based on evidence and others lack evidence does not make sense.
In summary, orthopedics is confronting situations that require critical objectivity as we search for solutions. Some of the situations I have tried to identify may not be “problems” but examples of normal evolution or transient developments that time alone would resolve. On the other hand, some others may be of a serious nature and require our involvement. To ignore them will bring about problems for the next generation who will wonder what precluded us from seeking answers before it was too late.
1. Sarmiento A. Bare Bones: A Surgeon’s Tale. Amherst, NY: Prometheus Books; 2003.
2. Callahan D. Taming the Beloved Beast: How Medical Technology Costs are Destroying Our Health Care System. Princeton, NJ: Princeton University Press; 2009.
3. Relman AS. Doctors as the key to health care reform. N Engl J Med 2009;361(13):1225-1227. doi:10.1056/NEJMp0907925.
4. Sarmiento A. Subspecialization in orthopaedics. Has it been all for the better? J Bone Joint Surg Am. 2003;85-A(2):369-373.
5. Sarmiento A The relationship between orthopaedics and industry must be reformed. Clin Orthop Relat Res. 2003;412:38-44.
6. Five Companies in Hip and Knee Replacement Industry Avoid Prosecution by Agreeing to Compliance Rules and Monitoring. Newark, NJ: US Dept of Justice, US Attorney, District of New Jersey; 2007.
7. Sarmiento A. Infringing on freedom of speech. J Bone Joint Surg Am. 2011;93(2):222. doi:10.2106/JBJS.J.00888.
8. Carr AJ. Which research is to be believed? The ethics of industrial funding of orthopaedic research. J Bone and Joint Surg Br. 2005;87(11):1452-1453.
9. Sarmiento A. Orthopedic registries: second thoughts. Am J Orthop. 2015;44(4):159-160.
10. Sarmiento A. Thoughts on orthopedic guidelines. Am J Orthop. 2010;39(8):373-374.
1. Sarmiento A. Bare Bones: A Surgeon’s Tale. Amherst, NY: Prometheus Books; 2003.
2. Callahan D. Taming the Beloved Beast: How Medical Technology Costs are Destroying Our Health Care System. Princeton, NJ: Princeton University Press; 2009.
3. Relman AS. Doctors as the key to health care reform. N Engl J Med 2009;361(13):1225-1227. doi:10.1056/NEJMp0907925.
4. Sarmiento A. Subspecialization in orthopaedics. Has it been all for the better? J Bone Joint Surg Am. 2003;85-A(2):369-373.
5. Sarmiento A The relationship between orthopaedics and industry must be reformed. Clin Orthop Relat Res. 2003;412:38-44.
6. Five Companies in Hip and Knee Replacement Industry Avoid Prosecution by Agreeing to Compliance Rules and Monitoring. Newark, NJ: US Dept of Justice, US Attorney, District of New Jersey; 2007.
7. Sarmiento A. Infringing on freedom of speech. J Bone Joint Surg Am. 2011;93(2):222. doi:10.2106/JBJS.J.00888.
8. Carr AJ. Which research is to be believed? The ethics of industrial funding of orthopaedic research. J Bone and Joint Surg Br. 2005;87(11):1452-1453.
9. Sarmiento A. Orthopedic registries: second thoughts. Am J Orthop. 2015;44(4):159-160.
10. Sarmiento A. Thoughts on orthopedic guidelines. Am J Orthop. 2010;39(8):373-374.
Collagen drinks – do they really work?
The question is, do they really do anything? Previously, most collagen supplements in the beauty industry came in the form of a topical cream or an injectable, with collagen being the main filler of choice before hyaluronic acid fillers became available. Today, collagen supplementation in the form of oral pills and drinks is rampant. These drinks and “vitamins” are purported to improve skin and provide a more youthful appearance, both from an immediate and preventative standpoint. Some of the drinks come from companies in Japan and beyond. According to market forecasts, the collagen supplement industry is anticipated to be worth $6.63 billion by 2025, up from $3.71 billion in 2016. An email advertisement this month from NewBeauty magazine claims one brand of collagen supplementation “with grape seed extract [as] an effective collagen drink for the skin.” Each 1.7 oz. bottle contains 13,000 mg of marine hydrolyzed collagen with six antiaging ingredients that – the ad claims – will help visibly transform your skin to a fuller, firmer, younger look in as soon as 21 days.
Diet absolutely plays a role in our overall health and skin appearance. But can these concentrated collagen drinks provide an increased benefit?
We know from prior experience with injecting collagen in the lips – namely from bovine (such as Zyderm and Zyplast) or human-derived (such as Cosmoderm and CosmoPlast) sources – that it provided beautiful and often natural-appearing results, which, however, did not last. If longevity is an issue with collagen injections, assuming proper absorption from the gastrointestinal tract and subsequent integration into skin, how long should we expect the results from drinking collagen to last in skin, if any? If it does work and is something that improves skin when used on a continuous basis, is there an endpoint at which the benefit is maximized or where an excess of collagen could be detrimental?
Collagen disorders are those where there is inflammation or deficiency in collagen. Could supplementation improve these diseases? Or could supplementation exacerbate or bring on these disorders if consumed in excess? In collagen vascular diseases, such as scleroderma, where apparent autoimmune inflammation of collagen occurs, would supplementation exacerbate the disease by bringing about more collagen to attack, or would it improve the condition by providing new collagen where there may be a defect? Would it help in conditions of collagen deficiency, such as osteogenesis imperfecta?
Many questions about collagen drinks and supplementation remain to be answered. Photoprotection from an early age and a healthy diet that supports production of our bodies’ own natural collagen are the best measures for skin health. With the surplus of collagen drinks and supplements now on the market, objective studies should be conducted and are warranted to answer these question for ourselves and our patients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
The question is, do they really do anything? Previously, most collagen supplements in the beauty industry came in the form of a topical cream or an injectable, with collagen being the main filler of choice before hyaluronic acid fillers became available. Today, collagen supplementation in the form of oral pills and drinks is rampant. These drinks and “vitamins” are purported to improve skin and provide a more youthful appearance, both from an immediate and preventative standpoint. Some of the drinks come from companies in Japan and beyond. According to market forecasts, the collagen supplement industry is anticipated to be worth $6.63 billion by 2025, up from $3.71 billion in 2016. An email advertisement this month from NewBeauty magazine claims one brand of collagen supplementation “with grape seed extract [as] an effective collagen drink for the skin.” Each 1.7 oz. bottle contains 13,000 mg of marine hydrolyzed collagen with six antiaging ingredients that – the ad claims – will help visibly transform your skin to a fuller, firmer, younger look in as soon as 21 days.
Diet absolutely plays a role in our overall health and skin appearance. But can these concentrated collagen drinks provide an increased benefit?
We know from prior experience with injecting collagen in the lips – namely from bovine (such as Zyderm and Zyplast) or human-derived (such as Cosmoderm and CosmoPlast) sources – that it provided beautiful and often natural-appearing results, which, however, did not last. If longevity is an issue with collagen injections, assuming proper absorption from the gastrointestinal tract and subsequent integration into skin, how long should we expect the results from drinking collagen to last in skin, if any? If it does work and is something that improves skin when used on a continuous basis, is there an endpoint at which the benefit is maximized or where an excess of collagen could be detrimental?
Collagen disorders are those where there is inflammation or deficiency in collagen. Could supplementation improve these diseases? Or could supplementation exacerbate or bring on these disorders if consumed in excess? In collagen vascular diseases, such as scleroderma, where apparent autoimmune inflammation of collagen occurs, would supplementation exacerbate the disease by bringing about more collagen to attack, or would it improve the condition by providing new collagen where there may be a defect? Would it help in conditions of collagen deficiency, such as osteogenesis imperfecta?
Many questions about collagen drinks and supplementation remain to be answered. Photoprotection from an early age and a healthy diet that supports production of our bodies’ own natural collagen are the best measures for skin health. With the surplus of collagen drinks and supplements now on the market, objective studies should be conducted and are warranted to answer these question for ourselves and our patients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
The question is, do they really do anything? Previously, most collagen supplements in the beauty industry came in the form of a topical cream or an injectable, with collagen being the main filler of choice before hyaluronic acid fillers became available. Today, collagen supplementation in the form of oral pills and drinks is rampant. These drinks and “vitamins” are purported to improve skin and provide a more youthful appearance, both from an immediate and preventative standpoint. Some of the drinks come from companies in Japan and beyond. According to market forecasts, the collagen supplement industry is anticipated to be worth $6.63 billion by 2025, up from $3.71 billion in 2016. An email advertisement this month from NewBeauty magazine claims one brand of collagen supplementation “with grape seed extract [as] an effective collagen drink for the skin.” Each 1.7 oz. bottle contains 13,000 mg of marine hydrolyzed collagen with six antiaging ingredients that – the ad claims – will help visibly transform your skin to a fuller, firmer, younger look in as soon as 21 days.
Diet absolutely plays a role in our overall health and skin appearance. But can these concentrated collagen drinks provide an increased benefit?
We know from prior experience with injecting collagen in the lips – namely from bovine (such as Zyderm and Zyplast) or human-derived (such as Cosmoderm and CosmoPlast) sources – that it provided beautiful and often natural-appearing results, which, however, did not last. If longevity is an issue with collagen injections, assuming proper absorption from the gastrointestinal tract and subsequent integration into skin, how long should we expect the results from drinking collagen to last in skin, if any? If it does work and is something that improves skin when used on a continuous basis, is there an endpoint at which the benefit is maximized or where an excess of collagen could be detrimental?
Collagen disorders are those where there is inflammation or deficiency in collagen. Could supplementation improve these diseases? Or could supplementation exacerbate or bring on these disorders if consumed in excess? In collagen vascular diseases, such as scleroderma, where apparent autoimmune inflammation of collagen occurs, would supplementation exacerbate the disease by bringing about more collagen to attack, or would it improve the condition by providing new collagen where there may be a defect? Would it help in conditions of collagen deficiency, such as osteogenesis imperfecta?
Many questions about collagen drinks and supplementation remain to be answered. Photoprotection from an early age and a healthy diet that supports production of our bodies’ own natural collagen are the best measures for skin health. With the surplus of collagen drinks and supplements now on the market, objective studies should be conducted and are warranted to answer these question for ourselves and our patients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
The double-edged sword
Veterinarians and farmers have known it for decades. If you give a herd or flock antibiotics, its members grow better and have a better survival rate than an equivalent group of unmedicated animals. The economic benefits of administering antibiotics are so great that until very recently the practice has been the norm. However, the “everything organic” movement has begun to turn the tide as more consumers have become aware of the hazards inherent in the agricultural use of antibiotics.
Following this conservative and prudent party line can be difficult, and few of us can claim to have never sinned and written a less-than-defensible prescription for an antibiotic. However, for physicians who work in places where the mortality rate for children under age 5 years can be as high as 25%, the temptation to treat the entire population with an antibiotic must be very real.
When decreased early-childhood mortality was observed in several populations that had been given prophylactic azithromycin for trachoma, a group of scientists from the University of California, San Francisco, were prompted to take a longer look at the phenomenon (“Azithromycin to Reduce Childhood Mortality in Sub-Saharan Africa,” N Engl J Med. 2018 Apr 26;378[17]:1583-92). Almost 200,000 children aged 1 month to 5 years in Niger, Malawi, and Tanzania were enrolled in the study. Half received a single dose of azithromycin every 6 months for 2 years. Overall, the mortality rate was 14% lower in the experimental group (P less than .001) and 25% lower in the children aged 1-5 months. Most of the effect was observed in Niger where only one in four children live until their fifth birthday.
Like any good experiment, this study raises more questions than it answers. Will the emergence of antibiotic resistance make broader application of the strategy impractical? Keenan et al. refer to previous trachoma treatment programs in which resistance occurred but seemed to recede when the programs were halted. What conditions were being treated successfully but blindly? Respiratory disease, diarrhea illness, and malaria are most prevalent and are the likely suspects. The authors acknowledge that more studies need to be done.
And of course, we must remember that, when it comes to antibiotic resistance, ultimately we are all neighbors.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Veterinarians and farmers have known it for decades. If you give a herd or flock antibiotics, its members grow better and have a better survival rate than an equivalent group of unmedicated animals. The economic benefits of administering antibiotics are so great that until very recently the practice has been the norm. However, the “everything organic” movement has begun to turn the tide as more consumers have become aware of the hazards inherent in the agricultural use of antibiotics.
Following this conservative and prudent party line can be difficult, and few of us can claim to have never sinned and written a less-than-defensible prescription for an antibiotic. However, for physicians who work in places where the mortality rate for children under age 5 years can be as high as 25%, the temptation to treat the entire population with an antibiotic must be very real.
When decreased early-childhood mortality was observed in several populations that had been given prophylactic azithromycin for trachoma, a group of scientists from the University of California, San Francisco, were prompted to take a longer look at the phenomenon (“Azithromycin to Reduce Childhood Mortality in Sub-Saharan Africa,” N Engl J Med. 2018 Apr 26;378[17]:1583-92). Almost 200,000 children aged 1 month to 5 years in Niger, Malawi, and Tanzania were enrolled in the study. Half received a single dose of azithromycin every 6 months for 2 years. Overall, the mortality rate was 14% lower in the experimental group (P less than .001) and 25% lower in the children aged 1-5 months. Most of the effect was observed in Niger where only one in four children live until their fifth birthday.
Like any good experiment, this study raises more questions than it answers. Will the emergence of antibiotic resistance make broader application of the strategy impractical? Keenan et al. refer to previous trachoma treatment programs in which resistance occurred but seemed to recede when the programs were halted. What conditions were being treated successfully but blindly? Respiratory disease, diarrhea illness, and malaria are most prevalent and are the likely suspects. The authors acknowledge that more studies need to be done.
And of course, we must remember that, when it comes to antibiotic resistance, ultimately we are all neighbors.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Veterinarians and farmers have known it for decades. If you give a herd or flock antibiotics, its members grow better and have a better survival rate than an equivalent group of unmedicated animals. The economic benefits of administering antibiotics are so great that until very recently the practice has been the norm. However, the “everything organic” movement has begun to turn the tide as more consumers have become aware of the hazards inherent in the agricultural use of antibiotics.
Following this conservative and prudent party line can be difficult, and few of us can claim to have never sinned and written a less-than-defensible prescription for an antibiotic. However, for physicians who work in places where the mortality rate for children under age 5 years can be as high as 25%, the temptation to treat the entire population with an antibiotic must be very real.
When decreased early-childhood mortality was observed in several populations that had been given prophylactic azithromycin for trachoma, a group of scientists from the University of California, San Francisco, were prompted to take a longer look at the phenomenon (“Azithromycin to Reduce Childhood Mortality in Sub-Saharan Africa,” N Engl J Med. 2018 Apr 26;378[17]:1583-92). Almost 200,000 children aged 1 month to 5 years in Niger, Malawi, and Tanzania were enrolled in the study. Half received a single dose of azithromycin every 6 months for 2 years. Overall, the mortality rate was 14% lower in the experimental group (P less than .001) and 25% lower in the children aged 1-5 months. Most of the effect was observed in Niger where only one in four children live until their fifth birthday.
Like any good experiment, this study raises more questions than it answers. Will the emergence of antibiotic resistance make broader application of the strategy impractical? Keenan et al. refer to previous trachoma treatment programs in which resistance occurred but seemed to recede when the programs were halted. What conditions were being treated successfully but blindly? Respiratory disease, diarrhea illness, and malaria are most prevalent and are the likely suspects. The authors acknowledge that more studies need to be done.
And of course, we must remember that, when it comes to antibiotic resistance, ultimately we are all neighbors.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
No-shows
When a patient fails to show up for his appointment, your reaction may run the gamut from elation to anger or land somewhere on the spectrum between concern and self-doubt. If you are overbooked and running behind with a waiting room that looks like a bus station at rush hour, an unexpectedly unfilled appointment slot can provide a much needed but all too brief respite. However, if the patient who no-shows is someone whom you have been worried about, you may wonder if he has slipped further into a debilitating depression. Or maybe he found a physician that he prefers?
If you keep your finger on the economic pulse of your practice, you know that the empty slot created when a patient no-shows is valuable time that is not generating any income. Your practice administrator may have sent a practice-wide email expressing concern about what she feels is an unacceptably high and economically unsustainable no-show rate. She already may have replaced your antiquated system using postcards and personal phone call reminders with preprogrammed emails and robo-calls.
If despite these high tech targeted reminders your no-show rate continues to be unacceptably high, the problem may be with how and when your office schedules appointments. When a parent or older patient calls with what she feels is an urgent or time-sensitive complaint, is she offered an appointment that satisfies her sense of urgency? She may agree to make an appointment but as soon as she hangs up may begin searching for another source of care and neglect to cancel the appointment with you when she finds a more timely response.
On the other hand, the patient’s problem may have resolved itself. With this in mind, I asked our receptionists to not make next-day appointments for a child with ear pain if for whatever reason the child was unable to come in for a same-day appointment. I knew from experience that ear pain often resolved and appointments weren’t kept or parents would cancel at the last minute. However, we guaranteed that if the child’s pain persisted we would see them immediately in the morning.
You may be muttering to yourself that you can’t possibly give every patient an appointment as soon as they would like to be seen. True. But aren’t there some patients who could be well served by a quick same-day appointment to allay their fear and sketch out a starting point for diagnosis and management at a later visit? A skillful and calming appointment secretary or nurse may be able to provide the same level of reassurance. But sometimes a short office visit is a more effective and efficient way to depressurize the situation and avoid a longer appointment that has a high likelihood of being no-showed or canceled.
Finally, are you or other members of your group in the habit of making follow-up appointments for problems that probably don’t require follow up? Most patients have an excellent sense when a follow-up appointment is unnecessary and are likely to cancel at the last minute or no-show. They may have had more than one experience in which they took off time from work and traveled 20 miles for a 3-minute visit that didn’t seem worth the effort. A quick phone call or two from you or your staff may be a better way to make sure things are going in the right direction and avoid the cost and frustration of a no-show.
The bottom line is that no-shows happen but when appointments are thoughtfully made the patients are more likely to keep them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When a patient fails to show up for his appointment, your reaction may run the gamut from elation to anger or land somewhere on the spectrum between concern and self-doubt. If you are overbooked and running behind with a waiting room that looks like a bus station at rush hour, an unexpectedly unfilled appointment slot can provide a much needed but all too brief respite. However, if the patient who no-shows is someone whom you have been worried about, you may wonder if he has slipped further into a debilitating depression. Or maybe he found a physician that he prefers?
If you keep your finger on the economic pulse of your practice, you know that the empty slot created when a patient no-shows is valuable time that is not generating any income. Your practice administrator may have sent a practice-wide email expressing concern about what she feels is an unacceptably high and economically unsustainable no-show rate. She already may have replaced your antiquated system using postcards and personal phone call reminders with preprogrammed emails and robo-calls.
If despite these high tech targeted reminders your no-show rate continues to be unacceptably high, the problem may be with how and when your office schedules appointments. When a parent or older patient calls with what she feels is an urgent or time-sensitive complaint, is she offered an appointment that satisfies her sense of urgency? She may agree to make an appointment but as soon as she hangs up may begin searching for another source of care and neglect to cancel the appointment with you when she finds a more timely response.
On the other hand, the patient’s problem may have resolved itself. With this in mind, I asked our receptionists to not make next-day appointments for a child with ear pain if for whatever reason the child was unable to come in for a same-day appointment. I knew from experience that ear pain often resolved and appointments weren’t kept or parents would cancel at the last minute. However, we guaranteed that if the child’s pain persisted we would see them immediately in the morning.
You may be muttering to yourself that you can’t possibly give every patient an appointment as soon as they would like to be seen. True. But aren’t there some patients who could be well served by a quick same-day appointment to allay their fear and sketch out a starting point for diagnosis and management at a later visit? A skillful and calming appointment secretary or nurse may be able to provide the same level of reassurance. But sometimes a short office visit is a more effective and efficient way to depressurize the situation and avoid a longer appointment that has a high likelihood of being no-showed or canceled.
Finally, are you or other members of your group in the habit of making follow-up appointments for problems that probably don’t require follow up? Most patients have an excellent sense when a follow-up appointment is unnecessary and are likely to cancel at the last minute or no-show. They may have had more than one experience in which they took off time from work and traveled 20 miles for a 3-minute visit that didn’t seem worth the effort. A quick phone call or two from you or your staff may be a better way to make sure things are going in the right direction and avoid the cost and frustration of a no-show.
The bottom line is that no-shows happen but when appointments are thoughtfully made the patients are more likely to keep them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When a patient fails to show up for his appointment, your reaction may run the gamut from elation to anger or land somewhere on the spectrum between concern and self-doubt. If you are overbooked and running behind with a waiting room that looks like a bus station at rush hour, an unexpectedly unfilled appointment slot can provide a much needed but all too brief respite. However, if the patient who no-shows is someone whom you have been worried about, you may wonder if he has slipped further into a debilitating depression. Or maybe he found a physician that he prefers?
If you keep your finger on the economic pulse of your practice, you know that the empty slot created when a patient no-shows is valuable time that is not generating any income. Your practice administrator may have sent a practice-wide email expressing concern about what she feels is an unacceptably high and economically unsustainable no-show rate. She already may have replaced your antiquated system using postcards and personal phone call reminders with preprogrammed emails and robo-calls.
If despite these high tech targeted reminders your no-show rate continues to be unacceptably high, the problem may be with how and when your office schedules appointments. When a parent or older patient calls with what she feels is an urgent or time-sensitive complaint, is she offered an appointment that satisfies her sense of urgency? She may agree to make an appointment but as soon as she hangs up may begin searching for another source of care and neglect to cancel the appointment with you when she finds a more timely response.
On the other hand, the patient’s problem may have resolved itself. With this in mind, I asked our receptionists to not make next-day appointments for a child with ear pain if for whatever reason the child was unable to come in for a same-day appointment. I knew from experience that ear pain often resolved and appointments weren’t kept or parents would cancel at the last minute. However, we guaranteed that if the child’s pain persisted we would see them immediately in the morning.
You may be muttering to yourself that you can’t possibly give every patient an appointment as soon as they would like to be seen. True. But aren’t there some patients who could be well served by a quick same-day appointment to allay their fear and sketch out a starting point for diagnosis and management at a later visit? A skillful and calming appointment secretary or nurse may be able to provide the same level of reassurance. But sometimes a short office visit is a more effective and efficient way to depressurize the situation and avoid a longer appointment that has a high likelihood of being no-showed or canceled.
Finally, are you or other members of your group in the habit of making follow-up appointments for problems that probably don’t require follow up? Most patients have an excellent sense when a follow-up appointment is unnecessary and are likely to cancel at the last minute or no-show. They may have had more than one experience in which they took off time from work and traveled 20 miles for a 3-minute visit that didn’t seem worth the effort. A quick phone call or two from you or your staff may be a better way to make sure things are going in the right direction and avoid the cost and frustration of a no-show.
The bottom line is that no-shows happen but when appointments are thoughtfully made the patients are more likely to keep them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
















