User login
‘No Pulse’: An MD’s First Night Off in 2 Weeks Turns Grave
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series by this news organization that tells these stories.
It was my first night off after 12 days. It was a Friday night, and I went to a bar in Naples to get a beer with some friends. As it turned out, it wasn’t a night off after all.
As soon as we got inside, we heard over the speaker that they needed medical personnel and to please go to the left side of the bar. I thought it would be syncope or something like that.
I went over there and saw a woman holding up a man. He was basically leaning all over her. The light was low, and the music was pounding. I started to assess him and tried to get him to answer me. No response. I checked for pulses — nothing.
The woman helped me lower him to the floor. I checked again for a pulse. Still nothing. I said, “Call 911,” and started compressions.
The difficult part was the place was completely dark. I knew where his body was on the floor. I could see his chest. But I couldn’t see his face at all.
It was also extremely loud with the music thumping. After a while, they finally shut it off.
Pretty soon, the security personnel from the bar brought me an automated external defibrillator, and it showed the man was having V-fib arrest. I shocked him. Still no pulse. I continued with cardiopulmonary resuscitation (CPR).
I hadn’t noticed, but lots of people were crowding around us. Somebody came up and said, “He’s my friend. He has a 9-year-old daughter. He can’t die. Let me help with the compressions.” I was like, “Go for it.”
The guy started kind of pushing on the man’s abdomen. He had no idea how to do compressions. I said, “Okay, let me take over again.”
Out of the crowd, nobody else volunteered to help. No one asked me, “Hey, what can I do?” Meanwhile, I found out later that someone was filming the whole thing on their phone.
But what the guy said about the man’s young daughter stayed in my brain. I thought, we need to keep going.
I did more compressions and shocked him again. Still no pulse. At that point, the police and emergency medical services showed up. They checked, nothing had changed, so they got him into the ambulance.
I asked one of the paramedics, “Where are you taking him? I can call ahead.”
But he said, “That’s HIPAA. We can’t tell you.” They also wouldn’t let me go with him in the ambulance.
“I have an active Florida license, and I work in the ICU [intensive care unit],” I said.
“No, we need to follow our protocol,” he replied.
I understood that, but I just wanted to help.
It was around 10:30 PM by then, and I was drenched in sweat. I had to go home. The first thing I did after taking a shower was open the computer and check my system. I needed to find out what happened to the guy.
I was looking for admissions, and I didn’t see him. I called the main hospital downtown and the one in North Naples. I couldn’t find him anywhere. I stayed up until almost 1:00 AM checking for his name. At that point I thought, okay, maybe he died.
The next night, Saturday, I was home and got a call from one of my colleagues. “Hey, were you in a bar yesterday? Did you do CPR on somebody?”
“How did you know?” I said.
He said the paramedics had described me — “a tall doctor with glasses who was a nice guy.” It was funny that he knew that was me.
He told me, “The guy’s alive. He’s sick and needs to be put on dialysis, but he’s alive.”
Apparently, the guy had gone to the emergency department at North Naples, and the doctors in the emergency room (ER) worked on him for over an hour. They did continuous CPR and shocked him for close to 40 minutes. They finally got his pulse back, and after that, he was transferred to the main hospital ICU. They didn’t admit him at the ER, which was why I couldn’t find his name.
On Sunday, I was checking my patients’ charts for the ICU that coming week. And there he was. I saw his name and the documentation by the ED that CPR was provided by a critical care doctor in the field. He was still alive. That gave me so much joy.
So, the man I had helped became my patient. When I saw him on Monday, he was intubated and needed dialysis. I finally saw his face and thought, Oh, so that’s what you look like. I hadn’t realized he was only 39 years old.
When he was awake, I explained to him I was the doctor that provided CPR at the bar. He was very grateful, but of course, he didn’t remember anything.
Eventually, I met his daughter, and she just said, “Thank you for allowing me to have my dad.”
The funny part is that he broke his leg. Well, that’s not funny, but no one had any idea how it happened. That was his only complaint. He was asking me, “Doctor, how did you break my leg?”
“Hey, I have no idea how you broke your leg,” I replied. “I was trying to save your life.”
He was in the hospital for almost a month but made a full recovery. The amazing part: After all the evaluations, he has no neurological deficits. He’s back to a normal life now.
They never found a cause for the cardiac arrest. I mean, he had an ejection fraction of 10%. All my money was on something drug related, but that wasn’t the case. They’d done a cardiac cut, and there was no obstruction. They couldn’t find a reason.
We’ve become friends. He still works as a DJ at the bar. He changed his name to “DJ the Survivor” or something like that.
Sometimes, he’ll text me: “Doctor, what are you doing? You want to come down to the bar?”
I’m like, “No. I don’t.”
It’s been more than a year, but I remember every detail. When you go into medicine, you dream that one day you’ll be able to say, “I saved somebody.”
He texted me a year later and told me he’s celebrating two birthdays now. He said, “I’m turning 1 year old today!”
I think about the value of life. How we can take it for granted. We think, I’m young, nothing is going to happen to me. But this guy was 39. He went to work and died that night.
I was able to help bring him back. That makes me thankful for every day.
Jose Valle Giler, MD, is a pulmonary, critical care, and sleep medicine physician at NCH Healthcare System in Naples, Florida.
A version of this article appeared on Medscape.com .
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series by this news organization that tells these stories.
It was my first night off after 12 days. It was a Friday night, and I went to a bar in Naples to get a beer with some friends. As it turned out, it wasn’t a night off after all.
As soon as we got inside, we heard over the speaker that they needed medical personnel and to please go to the left side of the bar. I thought it would be syncope or something like that.
I went over there and saw a woman holding up a man. He was basically leaning all over her. The light was low, and the music was pounding. I started to assess him and tried to get him to answer me. No response. I checked for pulses — nothing.
The woman helped me lower him to the floor. I checked again for a pulse. Still nothing. I said, “Call 911,” and started compressions.
The difficult part was the place was completely dark. I knew where his body was on the floor. I could see his chest. But I couldn’t see his face at all.
It was also extremely loud with the music thumping. After a while, they finally shut it off.
Pretty soon, the security personnel from the bar brought me an automated external defibrillator, and it showed the man was having V-fib arrest. I shocked him. Still no pulse. I continued with cardiopulmonary resuscitation (CPR).
I hadn’t noticed, but lots of people were crowding around us. Somebody came up and said, “He’s my friend. He has a 9-year-old daughter. He can’t die. Let me help with the compressions.” I was like, “Go for it.”
The guy started kind of pushing on the man’s abdomen. He had no idea how to do compressions. I said, “Okay, let me take over again.”
Out of the crowd, nobody else volunteered to help. No one asked me, “Hey, what can I do?” Meanwhile, I found out later that someone was filming the whole thing on their phone.
But what the guy said about the man’s young daughter stayed in my brain. I thought, we need to keep going.
I did more compressions and shocked him again. Still no pulse. At that point, the police and emergency medical services showed up. They checked, nothing had changed, so they got him into the ambulance.
I asked one of the paramedics, “Where are you taking him? I can call ahead.”
But he said, “That’s HIPAA. We can’t tell you.” They also wouldn’t let me go with him in the ambulance.
“I have an active Florida license, and I work in the ICU [intensive care unit],” I said.
“No, we need to follow our protocol,” he replied.
I understood that, but I just wanted to help.
It was around 10:30 PM by then, and I was drenched in sweat. I had to go home. The first thing I did after taking a shower was open the computer and check my system. I needed to find out what happened to the guy.
I was looking for admissions, and I didn’t see him. I called the main hospital downtown and the one in North Naples. I couldn’t find him anywhere. I stayed up until almost 1:00 AM checking for his name. At that point I thought, okay, maybe he died.
The next night, Saturday, I was home and got a call from one of my colleagues. “Hey, were you in a bar yesterday? Did you do CPR on somebody?”
“How did you know?” I said.
He said the paramedics had described me — “a tall doctor with glasses who was a nice guy.” It was funny that he knew that was me.
He told me, “The guy’s alive. He’s sick and needs to be put on dialysis, but he’s alive.”
Apparently, the guy had gone to the emergency department at North Naples, and the doctors in the emergency room (ER) worked on him for over an hour. They did continuous CPR and shocked him for close to 40 minutes. They finally got his pulse back, and after that, he was transferred to the main hospital ICU. They didn’t admit him at the ER, which was why I couldn’t find his name.
On Sunday, I was checking my patients’ charts for the ICU that coming week. And there he was. I saw his name and the documentation by the ED that CPR was provided by a critical care doctor in the field. He was still alive. That gave me so much joy.
So, the man I had helped became my patient. When I saw him on Monday, he was intubated and needed dialysis. I finally saw his face and thought, Oh, so that’s what you look like. I hadn’t realized he was only 39 years old.
When he was awake, I explained to him I was the doctor that provided CPR at the bar. He was very grateful, but of course, he didn’t remember anything.
Eventually, I met his daughter, and she just said, “Thank you for allowing me to have my dad.”
The funny part is that he broke his leg. Well, that’s not funny, but no one had any idea how it happened. That was his only complaint. He was asking me, “Doctor, how did you break my leg?”
“Hey, I have no idea how you broke your leg,” I replied. “I was trying to save your life.”
He was in the hospital for almost a month but made a full recovery. The amazing part: After all the evaluations, he has no neurological deficits. He’s back to a normal life now.
They never found a cause for the cardiac arrest. I mean, he had an ejection fraction of 10%. All my money was on something drug related, but that wasn’t the case. They’d done a cardiac cut, and there was no obstruction. They couldn’t find a reason.
We’ve become friends. He still works as a DJ at the bar. He changed his name to “DJ the Survivor” or something like that.
Sometimes, he’ll text me: “Doctor, what are you doing? You want to come down to the bar?”
I’m like, “No. I don’t.”
It’s been more than a year, but I remember every detail. When you go into medicine, you dream that one day you’ll be able to say, “I saved somebody.”
He texted me a year later and told me he’s celebrating two birthdays now. He said, “I’m turning 1 year old today!”
I think about the value of life. How we can take it for granted. We think, I’m young, nothing is going to happen to me. But this guy was 39. He went to work and died that night.
I was able to help bring him back. That makes me thankful for every day.
Jose Valle Giler, MD, is a pulmonary, critical care, and sleep medicine physician at NCH Healthcare System in Naples, Florida.
A version of this article appeared on Medscape.com .
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series by this news organization that tells these stories.
It was my first night off after 12 days. It was a Friday night, and I went to a bar in Naples to get a beer with some friends. As it turned out, it wasn’t a night off after all.
As soon as we got inside, we heard over the speaker that they needed medical personnel and to please go to the left side of the bar. I thought it would be syncope or something like that.
I went over there and saw a woman holding up a man. He was basically leaning all over her. The light was low, and the music was pounding. I started to assess him and tried to get him to answer me. No response. I checked for pulses — nothing.
The woman helped me lower him to the floor. I checked again for a pulse. Still nothing. I said, “Call 911,” and started compressions.
The difficult part was the place was completely dark. I knew where his body was on the floor. I could see his chest. But I couldn’t see his face at all.
It was also extremely loud with the music thumping. After a while, they finally shut it off.
Pretty soon, the security personnel from the bar brought me an automated external defibrillator, and it showed the man was having V-fib arrest. I shocked him. Still no pulse. I continued with cardiopulmonary resuscitation (CPR).
I hadn’t noticed, but lots of people were crowding around us. Somebody came up and said, “He’s my friend. He has a 9-year-old daughter. He can’t die. Let me help with the compressions.” I was like, “Go for it.”
The guy started kind of pushing on the man’s abdomen. He had no idea how to do compressions. I said, “Okay, let me take over again.”
Out of the crowd, nobody else volunteered to help. No one asked me, “Hey, what can I do?” Meanwhile, I found out later that someone was filming the whole thing on their phone.
But what the guy said about the man’s young daughter stayed in my brain. I thought, we need to keep going.
I did more compressions and shocked him again. Still no pulse. At that point, the police and emergency medical services showed up. They checked, nothing had changed, so they got him into the ambulance.
I asked one of the paramedics, “Where are you taking him? I can call ahead.”
But he said, “That’s HIPAA. We can’t tell you.” They also wouldn’t let me go with him in the ambulance.
“I have an active Florida license, and I work in the ICU [intensive care unit],” I said.
“No, we need to follow our protocol,” he replied.
I understood that, but I just wanted to help.
It was around 10:30 PM by then, and I was drenched in sweat. I had to go home. The first thing I did after taking a shower was open the computer and check my system. I needed to find out what happened to the guy.
I was looking for admissions, and I didn’t see him. I called the main hospital downtown and the one in North Naples. I couldn’t find him anywhere. I stayed up until almost 1:00 AM checking for his name. At that point I thought, okay, maybe he died.
The next night, Saturday, I was home and got a call from one of my colleagues. “Hey, were you in a bar yesterday? Did you do CPR on somebody?”
“How did you know?” I said.
He said the paramedics had described me — “a tall doctor with glasses who was a nice guy.” It was funny that he knew that was me.
He told me, “The guy’s alive. He’s sick and needs to be put on dialysis, but he’s alive.”
Apparently, the guy had gone to the emergency department at North Naples, and the doctors in the emergency room (ER) worked on him for over an hour. They did continuous CPR and shocked him for close to 40 minutes. They finally got his pulse back, and after that, he was transferred to the main hospital ICU. They didn’t admit him at the ER, which was why I couldn’t find his name.
On Sunday, I was checking my patients’ charts for the ICU that coming week. And there he was. I saw his name and the documentation by the ED that CPR was provided by a critical care doctor in the field. He was still alive. That gave me so much joy.
So, the man I had helped became my patient. When I saw him on Monday, he was intubated and needed dialysis. I finally saw his face and thought, Oh, so that’s what you look like. I hadn’t realized he was only 39 years old.
When he was awake, I explained to him I was the doctor that provided CPR at the bar. He was very grateful, but of course, he didn’t remember anything.
Eventually, I met his daughter, and she just said, “Thank you for allowing me to have my dad.”
The funny part is that he broke his leg. Well, that’s not funny, but no one had any idea how it happened. That was his only complaint. He was asking me, “Doctor, how did you break my leg?”
“Hey, I have no idea how you broke your leg,” I replied. “I was trying to save your life.”
He was in the hospital for almost a month but made a full recovery. The amazing part: After all the evaluations, he has no neurological deficits. He’s back to a normal life now.
They never found a cause for the cardiac arrest. I mean, he had an ejection fraction of 10%. All my money was on something drug related, but that wasn’t the case. They’d done a cardiac cut, and there was no obstruction. They couldn’t find a reason.
We’ve become friends. He still works as a DJ at the bar. He changed his name to “DJ the Survivor” or something like that.
Sometimes, he’ll text me: “Doctor, what are you doing? You want to come down to the bar?”
I’m like, “No. I don’t.”
It’s been more than a year, but I remember every detail. When you go into medicine, you dream that one day you’ll be able to say, “I saved somebody.”
He texted me a year later and told me he’s celebrating two birthdays now. He said, “I’m turning 1 year old today!”
I think about the value of life. How we can take it for granted. We think, I’m young, nothing is going to happen to me. But this guy was 39. He went to work and died that night.
I was able to help bring him back. That makes me thankful for every day.
Jose Valle Giler, MD, is a pulmonary, critical care, and sleep medicine physician at NCH Healthcare System in Naples, Florida.
A version of this article appeared on Medscape.com .
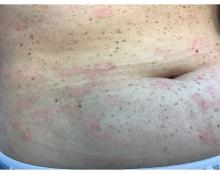
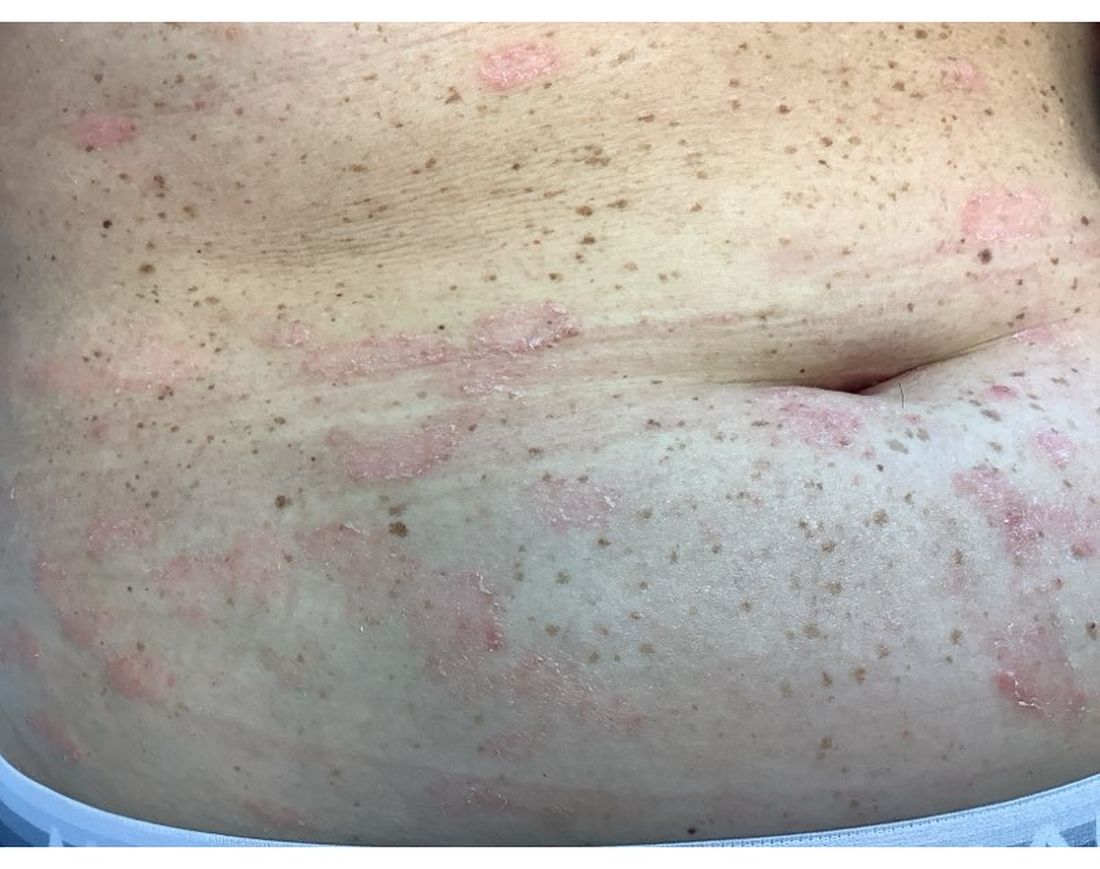
A 30-Year-Old White Female Presented With a 4-Month History of Scaly, Erythematous Patches and Plaques on Her Trunk and Extremities
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Using AI to Transform Diabetic Foot and Limb Preservation
Diabetic foot complications represent a major global health challenge, with a high prevalence among patients with diabetes. A diabetic foot ulcer (DFU) not only affects the patient›s quality of life but also increases the risk for amputation.
Worldwide, a DFU occurs every second, and an amputation occurs every 20 seconds. The limitations of current detection and intervention methods underline the urgent need for innovative solutions.
Recent advances in artificial intelligence (AI) have paved the way for individualized risk prediction models for chronic wound management. These models use deep learning algorithms to analyze clinical data and images, providing personalized treatment plans that may improve healing outcomes and reduce the risk for amputation.
AI-powered tools can also be deployed for the diagnosis of diabetic foot complications. Using image analysis and pattern recognition, AI tools are learning to accurately detect signs of DFUs and other complications, facilitating early and effective intervention. Our group and others have been working not only on imaging devices but also on thermographic tools that — with the help of AI — can create an automated “foot selfie” to predict and prevent problems before they start.
AI’s predictive capabilities are instrumental to its clinical value. By identifying patients at high risk for DFUs, healthcare providers can implement preemptive measures, significantly reducing the likelihood of severe complications.
Although the potential benefits of AI in diabetic foot care are immense, integrating these tools into clinical practice poses challenges. These include ensuring the reliability of AI predictions, addressing data privacy concerns, and training healthcare professionals on the use of AI technologies.
As in so many other areas in our lives, AI holds the promise to revolutionize diabetic foot and limb preservation, offering hope for improved patient outcomes through early detection, precise diagnosis, and personalized care. However, realizing this potential requires ongoing research, development, and collaboration across the medical and technological fields to ensure these innovative solutions can be effectively integrated into standard care practices.
Dr. Armstrong is professor of surgery, Keck School of Medicine of University of Southern California, Los Angeles, California. He has disclosed the following relevant financial relationships: Partially supported by National Institutes of Health; National Institute of Diabetes; Digestive and Kidney Disease Award Number 1R01124789-01A1.
A version of this article first appeared on Medscape.com.
Diabetic foot complications represent a major global health challenge, with a high prevalence among patients with diabetes. A diabetic foot ulcer (DFU) not only affects the patient›s quality of life but also increases the risk for amputation.
Worldwide, a DFU occurs every second, and an amputation occurs every 20 seconds. The limitations of current detection and intervention methods underline the urgent need for innovative solutions.
Recent advances in artificial intelligence (AI) have paved the way for individualized risk prediction models for chronic wound management. These models use deep learning algorithms to analyze clinical data and images, providing personalized treatment plans that may improve healing outcomes and reduce the risk for amputation.
AI-powered tools can also be deployed for the diagnosis of diabetic foot complications. Using image analysis and pattern recognition, AI tools are learning to accurately detect signs of DFUs and other complications, facilitating early and effective intervention. Our group and others have been working not only on imaging devices but also on thermographic tools that — with the help of AI — can create an automated “foot selfie” to predict and prevent problems before they start.
AI’s predictive capabilities are instrumental to its clinical value. By identifying patients at high risk for DFUs, healthcare providers can implement preemptive measures, significantly reducing the likelihood of severe complications.
Although the potential benefits of AI in diabetic foot care are immense, integrating these tools into clinical practice poses challenges. These include ensuring the reliability of AI predictions, addressing data privacy concerns, and training healthcare professionals on the use of AI technologies.
As in so many other areas in our lives, AI holds the promise to revolutionize diabetic foot and limb preservation, offering hope for improved patient outcomes through early detection, precise diagnosis, and personalized care. However, realizing this potential requires ongoing research, development, and collaboration across the medical and technological fields to ensure these innovative solutions can be effectively integrated into standard care practices.
Dr. Armstrong is professor of surgery, Keck School of Medicine of University of Southern California, Los Angeles, California. He has disclosed the following relevant financial relationships: Partially supported by National Institutes of Health; National Institute of Diabetes; Digestive and Kidney Disease Award Number 1R01124789-01A1.
A version of this article first appeared on Medscape.com.
Diabetic foot complications represent a major global health challenge, with a high prevalence among patients with diabetes. A diabetic foot ulcer (DFU) not only affects the patient›s quality of life but also increases the risk for amputation.
Worldwide, a DFU occurs every second, and an amputation occurs every 20 seconds. The limitations of current detection and intervention methods underline the urgent need for innovative solutions.
Recent advances in artificial intelligence (AI) have paved the way for individualized risk prediction models for chronic wound management. These models use deep learning algorithms to analyze clinical data and images, providing personalized treatment plans that may improve healing outcomes and reduce the risk for amputation.
AI-powered tools can also be deployed for the diagnosis of diabetic foot complications. Using image analysis and pattern recognition, AI tools are learning to accurately detect signs of DFUs and other complications, facilitating early and effective intervention. Our group and others have been working not only on imaging devices but also on thermographic tools that — with the help of AI — can create an automated “foot selfie” to predict and prevent problems before they start.
AI’s predictive capabilities are instrumental to its clinical value. By identifying patients at high risk for DFUs, healthcare providers can implement preemptive measures, significantly reducing the likelihood of severe complications.
Although the potential benefits of AI in diabetic foot care are immense, integrating these tools into clinical practice poses challenges. These include ensuring the reliability of AI predictions, addressing data privacy concerns, and training healthcare professionals on the use of AI technologies.
As in so many other areas in our lives, AI holds the promise to revolutionize diabetic foot and limb preservation, offering hope for improved patient outcomes through early detection, precise diagnosis, and personalized care. However, realizing this potential requires ongoing research, development, and collaboration across the medical and technological fields to ensure these innovative solutions can be effectively integrated into standard care practices.
Dr. Armstrong is professor of surgery, Keck School of Medicine of University of Southern California, Los Angeles, California. He has disclosed the following relevant financial relationships: Partially supported by National Institutes of Health; National Institute of Diabetes; Digestive and Kidney Disease Award Number 1R01124789-01A1.
A version of this article first appeared on Medscape.com.
Moral Injury in Health Care: A Unified Definition and its Relationship to Burnout
Moral injury was identified by health care professionals (HCPs) as a driver of occupational distress prior to the COVID-19 pandemic, but the crisis expanded the appeal and investigation of the term.1 HCPs now consider moral injury an essential component of the framework to describe their distress, because using the term burnout alone fails to capture their full experience and has proven resistant to interventions.2 Moral injury goes beyond the transdiagnostic symptoms of exhaustion and cynicism and beyond operational, demand-resource mismatches that characterize burnout. It describes the frustration, anger, and helplessness associated with relational ruptures and the existential threats to a clinician’s professional identity as business interests erode their ability to put their patients’ needs ahead of corporate and health care system obligations.3
Proper characterization of moral injury in health care—separate from the military environments where it originated—is stymied by an ill-defined relationship between 2 definitions of the term and by an unclear relationship between moral injury and the long-standing body of scholarship in burnout. To clarify the concept, inform research agendas, and open avenues for more effective solutions to the crisis of HCP distress, we propose a unified conceptualization of moral injury and its association with burnout in health care.
CONTEXTUAL DISTINCTIONS
It is important to properly distinguish between the original use of moral injury in the military and its expanded use in civilian circumstances. Health care and the military are both professions whereupon donning the “uniform” of a physician—or soldier, sailor, airman, or marine—members must comport with strict expectations of behavior, including the refusal to engage in illegal actions or those contrary to professional ethics. Individuals in both professions acquire a highly specialized body of knowledge and enter an implied contract to provide critical services to society, specifically healing and protection, respectively. Members of both professions are trained to make complex judgments with integrity under conditions of technical and ethical uncertainty, upon which they take highly skilled action. Medical and military professionals must be free to act on their ethical principles, without confounding demands.4 However, the context of each profession’s commitment to society carries different moral implications.
The risk of moral injury is inherent in military service. The military promises protection with an implicit acknowledgment of the need to use lethal force to uphold the agreement. In contrast, HCPs promise healing and care. The military promises to protect our society, with an implicit acknowledgment of the need to use lethal force to uphold the agreement. Some military actions may inflict harm without the hope of benefitting an individual, and are therefore potentially morally injurious. The health care contract with society, promising healing and care, is devoid of inherent moral injury due to harm without potential individual benefit. Therefore, the presence of moral injury in health care settings are warning signs of a dysfunctional environment.
One complex example of the dysfunctional environments is illustrative. The military and health care are among the few industries where supply creates demand. For example, the more bad state actors there are, the more demand for the military. As we have seen since the 1950s, the more technology and therapeutics we create in health care, coupled with a larger share paid for by third parties, the greater the demand for and use of them.5 In a fee for service environment, corporate greed feeds on this reality. In most other environments, more technological and therapeutic options inevitably pit clinicians against multiple other factions: payers, who do not want to underwrite them; patients, who sometimes demand them without justification or later rail against spiraling health care costs; and administrators, especially in capitated systems, who watch their bottom lines erode. The moral injury risk in this instance demands a collective conversation among stakeholders regarding the structural determinants of health—how we choose to distribute limited resources. The intermediary of moral injury is a useful measure of the harm that results from ignoring or avoiding such challenges.
HARMONIZING DEFINITIONS
Moral injury is inherently nuanced. The 2 dominant definitions arise from work with combat veterans and create additional and perhaps unnecessary complexity. Unifying these 2 definitions eliminates inadvertent confusion, preventing the risk of unbridled interdisciplinary investigation which leads to a lack of precision in the meaning of moral injury and other related concepts, such as burnout.6
The first definition was developed by Jonathan Shay in 1994 and outlines 3 necessarycomponents, viewing the violator as a powerholder: (1) betrayal of what is right, (2) by someone who holds legitimate authority, (3) in a high stakes situation.7 Litz and colleagues describe moral injury another way: “Perpetrating, failing to prevent, bearing witness to, or learning about acts that transgress deeply held moral beliefs and expectations.”8 The violator is posited to be either the self or others.
Rather than representing “self” or “other” imposed moral injury, we propose the 2 definitions are related as exposure (ie, the perceived betrayal) and response (ie, the resulting transgression). An individual who experiences a betrayal by a legitimate authority has an opportunity to choose their response. They may acquiesce and transgress their moral beliefs (eg, their oath to provide ethical health care), or they could refuse, by speaking out, or in some way resisting the authority’s betrayal. The case of Ray Brovont is a useful illustration of reconciling the definitions (Box).9
Myriad factors—known as potentially morally injurious events—drive moral injury, such as resource-constrained decision making, witnessing the behaviors of colleagues that violate deeply held moral beliefs, questionable billing practices, and more. Each begins with a betrayal. Spotlighting the betrayal, refusing to perpetuate it, or taking actions toward change, may reduce the risk of experiencing moral injury.9 Conversely, acquiescing and transgressing one’s oath, the profession’s covenant with society, increases the risk of experiencing moral injury.8
Many HCPs believe they are not always free to resist betrayal, fearing retaliation, job loss, blacklisting, or worse. They feel constrained by debt accrued while receiving their education, being their household’s primary earner, community ties, practicing a niche specialty that requires working for a tertiary referral center, or perhaps believing the situation will be the same elsewhere. To not stand up or speak out is to choose complicity with corporate greed that uses HCPs to undermine their professional duties, which significantly increases the risk of experiencing moral injury.
MORAL INJURY AND BURNOUT
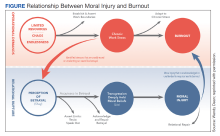
In addition to reconciling the definitions of moral injury, the relationship between moral injury and burnout are still being elucidated. We suggest that moral injury and burnout represent independent and potentially interrelated pathways to distress (Figure). Exposure to chronic, inconsonant, and transactional demands, which things like shorter work hours, better self-care, or improved health system operations might mitigate, manifests as burnout. In contrast, moral injury arises when a superior’s actions or a system’s policies and practices—such as justifiable but unnecessary testing, or referral restrictions to prevent revenue leakage—undermine one’s professional obligations to prioritize the patient’s best interest.
If concerns from HCPs about transactional demands are persistently dismissed, such inaction may be perceived as a betrayal, raising the risk of moral injury. Additionally, the resignation or helplessness of moral injury perceived as inescapable may present with emotional exhaustion, ineffectiveness, and depersonalization, all hallmarks of burnout. Both conditions can mediate and moderate the relationship between triggers for workplace distress and resulting psychological, physical, and existential harm.
CONCLUSIONS
Moral injury is increasingly recognized as a source of distress among HCPs, resulting from structural constraints on their ability to deliver optimal care and their own unwillingness to stand up for their patients, their oaths, and their professions.1 Unlike the military, where moral injury is inherent in the contract with society, moral injury in health care (and the relational rupture it connotes) is a signal of systemic dysfunction, fractured trust, and the need for relational repair.
Health care is at a crossroads, experiencing a workforce retention crisis while simultaneously predicting a significant increase in care needs by Baby Boomers over the next 3 decades.
Health care does not have the luxury of experimenting another 30 years with interventions that have limited impact. We must design a new generation of approaches, shaped by lessons learned from the pandemic while acknowledging that prepandemic standards were already failing the workforce. A unified definition of moral injury must be integrated to frame clinician distress alongside burnout, recentering ethical decision making, rather than profit, at the heart of health care. Harmonizing the definitions of moral injury and clarifying the relationship of moral injury with burnout reduces the need for further reinterpretations, allowing for more robust, easily comparable studies focused on identifying risk factors, as well as rapidly implementing effective mitigation strategies.
1. Griffin BJ, Weber MC, Hinkson KD, et al. Toward a dimensional contextual model of moral injury: a scoping review on healthcare workers. Curr Treat Options Psych. 2023;10:199-216. doi:10.1007/s40501-023-00296-4
2. National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. The National Academies Press; 2019. doi:10.17226/25521
3. Dean W, Talbot S, Dean A. Reframing clinician distress: moral injury not burnout. Fed Pract. 2019;36(9):400-402.
4. Gardner HE, Schulman LS. The professions in America today: crucial but fragile. Daedalus. 2005;134(3):13-18. doi:10.1162/0011526054622132
5. Fuchs VR. Major trends in the U.S. health economy since 1950. N Engl J Med. 2012;366(11):973-977. doi:10.1056/NEJMp1200478
6. Molendijk T. Warnings against romanticising moral injury. Br J Psychiatry. 2022;220(1):1-3. doi:10.1192/bjp.2021.114
7. Shay J. Moral injury. Psychoanalytic Psychol. 2014;31(2):182-191. doi:10.1037/a0036090
8. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
9. Brovont v KS-I Med. Servs., P.A., 622 SW3d 671 (Mo Ct App 2020).
Moral injury was identified by health care professionals (HCPs) as a driver of occupational distress prior to the COVID-19 pandemic, but the crisis expanded the appeal and investigation of the term.1 HCPs now consider moral injury an essential component of the framework to describe their distress, because using the term burnout alone fails to capture their full experience and has proven resistant to interventions.2 Moral injury goes beyond the transdiagnostic symptoms of exhaustion and cynicism and beyond operational, demand-resource mismatches that characterize burnout. It describes the frustration, anger, and helplessness associated with relational ruptures and the existential threats to a clinician’s professional identity as business interests erode their ability to put their patients’ needs ahead of corporate and health care system obligations.3
Proper characterization of moral injury in health care—separate from the military environments where it originated—is stymied by an ill-defined relationship between 2 definitions of the term and by an unclear relationship between moral injury and the long-standing body of scholarship in burnout. To clarify the concept, inform research agendas, and open avenues for more effective solutions to the crisis of HCP distress, we propose a unified conceptualization of moral injury and its association with burnout in health care.
CONTEXTUAL DISTINCTIONS
It is important to properly distinguish between the original use of moral injury in the military and its expanded use in civilian circumstances. Health care and the military are both professions whereupon donning the “uniform” of a physician—or soldier, sailor, airman, or marine—members must comport with strict expectations of behavior, including the refusal to engage in illegal actions or those contrary to professional ethics. Individuals in both professions acquire a highly specialized body of knowledge and enter an implied contract to provide critical services to society, specifically healing and protection, respectively. Members of both professions are trained to make complex judgments with integrity under conditions of technical and ethical uncertainty, upon which they take highly skilled action. Medical and military professionals must be free to act on their ethical principles, without confounding demands.4 However, the context of each profession’s commitment to society carries different moral implications.
The risk of moral injury is inherent in military service. The military promises protection with an implicit acknowledgment of the need to use lethal force to uphold the agreement. In contrast, HCPs promise healing and care. The military promises to protect our society, with an implicit acknowledgment of the need to use lethal force to uphold the agreement. Some military actions may inflict harm without the hope of benefitting an individual, and are therefore potentially morally injurious. The health care contract with society, promising healing and care, is devoid of inherent moral injury due to harm without potential individual benefit. Therefore, the presence of moral injury in health care settings are warning signs of a dysfunctional environment.
One complex example of the dysfunctional environments is illustrative. The military and health care are among the few industries where supply creates demand. For example, the more bad state actors there are, the more demand for the military. As we have seen since the 1950s, the more technology and therapeutics we create in health care, coupled with a larger share paid for by third parties, the greater the demand for and use of them.5 In a fee for service environment, corporate greed feeds on this reality. In most other environments, more technological and therapeutic options inevitably pit clinicians against multiple other factions: payers, who do not want to underwrite them; patients, who sometimes demand them without justification or later rail against spiraling health care costs; and administrators, especially in capitated systems, who watch their bottom lines erode. The moral injury risk in this instance demands a collective conversation among stakeholders regarding the structural determinants of health—how we choose to distribute limited resources. The intermediary of moral injury is a useful measure of the harm that results from ignoring or avoiding such challenges.
HARMONIZING DEFINITIONS
Moral injury is inherently nuanced. The 2 dominant definitions arise from work with combat veterans and create additional and perhaps unnecessary complexity. Unifying these 2 definitions eliminates inadvertent confusion, preventing the risk of unbridled interdisciplinary investigation which leads to a lack of precision in the meaning of moral injury and other related concepts, such as burnout.6
The first definition was developed by Jonathan Shay in 1994 and outlines 3 necessarycomponents, viewing the violator as a powerholder: (1) betrayal of what is right, (2) by someone who holds legitimate authority, (3) in a high stakes situation.7 Litz and colleagues describe moral injury another way: “Perpetrating, failing to prevent, bearing witness to, or learning about acts that transgress deeply held moral beliefs and expectations.”8 The violator is posited to be either the self or others.
Rather than representing “self” or “other” imposed moral injury, we propose the 2 definitions are related as exposure (ie, the perceived betrayal) and response (ie, the resulting transgression). An individual who experiences a betrayal by a legitimate authority has an opportunity to choose their response. They may acquiesce and transgress their moral beliefs (eg, their oath to provide ethical health care), or they could refuse, by speaking out, or in some way resisting the authority’s betrayal. The case of Ray Brovont is a useful illustration of reconciling the definitions (Box).9
Myriad factors—known as potentially morally injurious events—drive moral injury, such as resource-constrained decision making, witnessing the behaviors of colleagues that violate deeply held moral beliefs, questionable billing practices, and more. Each begins with a betrayal. Spotlighting the betrayal, refusing to perpetuate it, or taking actions toward change, may reduce the risk of experiencing moral injury.9 Conversely, acquiescing and transgressing one’s oath, the profession’s covenant with society, increases the risk of experiencing moral injury.8
Many HCPs believe they are not always free to resist betrayal, fearing retaliation, job loss, blacklisting, or worse. They feel constrained by debt accrued while receiving their education, being their household’s primary earner, community ties, practicing a niche specialty that requires working for a tertiary referral center, or perhaps believing the situation will be the same elsewhere. To not stand up or speak out is to choose complicity with corporate greed that uses HCPs to undermine their professional duties, which significantly increases the risk of experiencing moral injury.
MORAL INJURY AND BURNOUT
In addition to reconciling the definitions of moral injury, the relationship between moral injury and burnout are still being elucidated. We suggest that moral injury and burnout represent independent and potentially interrelated pathways to distress (Figure). Exposure to chronic, inconsonant, and transactional demands, which things like shorter work hours, better self-care, or improved health system operations might mitigate, manifests as burnout. In contrast, moral injury arises when a superior’s actions or a system’s policies and practices—such as justifiable but unnecessary testing, or referral restrictions to prevent revenue leakage—undermine one’s professional obligations to prioritize the patient’s best interest.
If concerns from HCPs about transactional demands are persistently dismissed, such inaction may be perceived as a betrayal, raising the risk of moral injury. Additionally, the resignation or helplessness of moral injury perceived as inescapable may present with emotional exhaustion, ineffectiveness, and depersonalization, all hallmarks of burnout. Both conditions can mediate and moderate the relationship between triggers for workplace distress and resulting psychological, physical, and existential harm.
CONCLUSIONS
Moral injury is increasingly recognized as a source of distress among HCPs, resulting from structural constraints on their ability to deliver optimal care and their own unwillingness to stand up for their patients, their oaths, and their professions.1 Unlike the military, where moral injury is inherent in the contract with society, moral injury in health care (and the relational rupture it connotes) is a signal of systemic dysfunction, fractured trust, and the need for relational repair.
Health care is at a crossroads, experiencing a workforce retention crisis while simultaneously predicting a significant increase in care needs by Baby Boomers over the next 3 decades.
Health care does not have the luxury of experimenting another 30 years with interventions that have limited impact. We must design a new generation of approaches, shaped by lessons learned from the pandemic while acknowledging that prepandemic standards were already failing the workforce. A unified definition of moral injury must be integrated to frame clinician distress alongside burnout, recentering ethical decision making, rather than profit, at the heart of health care. Harmonizing the definitions of moral injury and clarifying the relationship of moral injury with burnout reduces the need for further reinterpretations, allowing for more robust, easily comparable studies focused on identifying risk factors, as well as rapidly implementing effective mitigation strategies.
Moral injury was identified by health care professionals (HCPs) as a driver of occupational distress prior to the COVID-19 pandemic, but the crisis expanded the appeal and investigation of the term.1 HCPs now consider moral injury an essential component of the framework to describe their distress, because using the term burnout alone fails to capture their full experience and has proven resistant to interventions.2 Moral injury goes beyond the transdiagnostic symptoms of exhaustion and cynicism and beyond operational, demand-resource mismatches that characterize burnout. It describes the frustration, anger, and helplessness associated with relational ruptures and the existential threats to a clinician’s professional identity as business interests erode their ability to put their patients’ needs ahead of corporate and health care system obligations.3
Proper characterization of moral injury in health care—separate from the military environments where it originated—is stymied by an ill-defined relationship between 2 definitions of the term and by an unclear relationship between moral injury and the long-standing body of scholarship in burnout. To clarify the concept, inform research agendas, and open avenues for more effective solutions to the crisis of HCP distress, we propose a unified conceptualization of moral injury and its association with burnout in health care.
CONTEXTUAL DISTINCTIONS
It is important to properly distinguish between the original use of moral injury in the military and its expanded use in civilian circumstances. Health care and the military are both professions whereupon donning the “uniform” of a physician—or soldier, sailor, airman, or marine—members must comport with strict expectations of behavior, including the refusal to engage in illegal actions or those contrary to professional ethics. Individuals in both professions acquire a highly specialized body of knowledge and enter an implied contract to provide critical services to society, specifically healing and protection, respectively. Members of both professions are trained to make complex judgments with integrity under conditions of technical and ethical uncertainty, upon which they take highly skilled action. Medical and military professionals must be free to act on their ethical principles, without confounding demands.4 However, the context of each profession’s commitment to society carries different moral implications.
The risk of moral injury is inherent in military service. The military promises protection with an implicit acknowledgment of the need to use lethal force to uphold the agreement. In contrast, HCPs promise healing and care. The military promises to protect our society, with an implicit acknowledgment of the need to use lethal force to uphold the agreement. Some military actions may inflict harm without the hope of benefitting an individual, and are therefore potentially morally injurious. The health care contract with society, promising healing and care, is devoid of inherent moral injury due to harm without potential individual benefit. Therefore, the presence of moral injury in health care settings are warning signs of a dysfunctional environment.
One complex example of the dysfunctional environments is illustrative. The military and health care are among the few industries where supply creates demand. For example, the more bad state actors there are, the more demand for the military. As we have seen since the 1950s, the more technology and therapeutics we create in health care, coupled with a larger share paid for by third parties, the greater the demand for and use of them.5 In a fee for service environment, corporate greed feeds on this reality. In most other environments, more technological and therapeutic options inevitably pit clinicians against multiple other factions: payers, who do not want to underwrite them; patients, who sometimes demand them without justification or later rail against spiraling health care costs; and administrators, especially in capitated systems, who watch their bottom lines erode. The moral injury risk in this instance demands a collective conversation among stakeholders regarding the structural determinants of health—how we choose to distribute limited resources. The intermediary of moral injury is a useful measure of the harm that results from ignoring or avoiding such challenges.
HARMONIZING DEFINITIONS
Moral injury is inherently nuanced. The 2 dominant definitions arise from work with combat veterans and create additional and perhaps unnecessary complexity. Unifying these 2 definitions eliminates inadvertent confusion, preventing the risk of unbridled interdisciplinary investigation which leads to a lack of precision in the meaning of moral injury and other related concepts, such as burnout.6
The first definition was developed by Jonathan Shay in 1994 and outlines 3 necessarycomponents, viewing the violator as a powerholder: (1) betrayal of what is right, (2) by someone who holds legitimate authority, (3) in a high stakes situation.7 Litz and colleagues describe moral injury another way: “Perpetrating, failing to prevent, bearing witness to, or learning about acts that transgress deeply held moral beliefs and expectations.”8 The violator is posited to be either the self or others.
Rather than representing “self” or “other” imposed moral injury, we propose the 2 definitions are related as exposure (ie, the perceived betrayal) and response (ie, the resulting transgression). An individual who experiences a betrayal by a legitimate authority has an opportunity to choose their response. They may acquiesce and transgress their moral beliefs (eg, their oath to provide ethical health care), or they could refuse, by speaking out, or in some way resisting the authority’s betrayal. The case of Ray Brovont is a useful illustration of reconciling the definitions (Box).9
Myriad factors—known as potentially morally injurious events—drive moral injury, such as resource-constrained decision making, witnessing the behaviors of colleagues that violate deeply held moral beliefs, questionable billing practices, and more. Each begins with a betrayal. Spotlighting the betrayal, refusing to perpetuate it, or taking actions toward change, may reduce the risk of experiencing moral injury.9 Conversely, acquiescing and transgressing one’s oath, the profession’s covenant with society, increases the risk of experiencing moral injury.8
Many HCPs believe they are not always free to resist betrayal, fearing retaliation, job loss, blacklisting, or worse. They feel constrained by debt accrued while receiving their education, being their household’s primary earner, community ties, practicing a niche specialty that requires working for a tertiary referral center, or perhaps believing the situation will be the same elsewhere. To not stand up or speak out is to choose complicity with corporate greed that uses HCPs to undermine their professional duties, which significantly increases the risk of experiencing moral injury.
MORAL INJURY AND BURNOUT
In addition to reconciling the definitions of moral injury, the relationship between moral injury and burnout are still being elucidated. We suggest that moral injury and burnout represent independent and potentially interrelated pathways to distress (Figure). Exposure to chronic, inconsonant, and transactional demands, which things like shorter work hours, better self-care, or improved health system operations might mitigate, manifests as burnout. In contrast, moral injury arises when a superior’s actions or a system’s policies and practices—such as justifiable but unnecessary testing, or referral restrictions to prevent revenue leakage—undermine one’s professional obligations to prioritize the patient’s best interest.
If concerns from HCPs about transactional demands are persistently dismissed, such inaction may be perceived as a betrayal, raising the risk of moral injury. Additionally, the resignation or helplessness of moral injury perceived as inescapable may present with emotional exhaustion, ineffectiveness, and depersonalization, all hallmarks of burnout. Both conditions can mediate and moderate the relationship between triggers for workplace distress and resulting psychological, physical, and existential harm.
CONCLUSIONS
Moral injury is increasingly recognized as a source of distress among HCPs, resulting from structural constraints on their ability to deliver optimal care and their own unwillingness to stand up for their patients, their oaths, and their professions.1 Unlike the military, where moral injury is inherent in the contract with society, moral injury in health care (and the relational rupture it connotes) is a signal of systemic dysfunction, fractured trust, and the need for relational repair.
Health care is at a crossroads, experiencing a workforce retention crisis while simultaneously predicting a significant increase in care needs by Baby Boomers over the next 3 decades.
Health care does not have the luxury of experimenting another 30 years with interventions that have limited impact. We must design a new generation of approaches, shaped by lessons learned from the pandemic while acknowledging that prepandemic standards were already failing the workforce. A unified definition of moral injury must be integrated to frame clinician distress alongside burnout, recentering ethical decision making, rather than profit, at the heart of health care. Harmonizing the definitions of moral injury and clarifying the relationship of moral injury with burnout reduces the need for further reinterpretations, allowing for more robust, easily comparable studies focused on identifying risk factors, as well as rapidly implementing effective mitigation strategies.
1. Griffin BJ, Weber MC, Hinkson KD, et al. Toward a dimensional contextual model of moral injury: a scoping review on healthcare workers. Curr Treat Options Psych. 2023;10:199-216. doi:10.1007/s40501-023-00296-4
2. National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. The National Academies Press; 2019. doi:10.17226/25521
3. Dean W, Talbot S, Dean A. Reframing clinician distress: moral injury not burnout. Fed Pract. 2019;36(9):400-402.
4. Gardner HE, Schulman LS. The professions in America today: crucial but fragile. Daedalus. 2005;134(3):13-18. doi:10.1162/0011526054622132
5. Fuchs VR. Major trends in the U.S. health economy since 1950. N Engl J Med. 2012;366(11):973-977. doi:10.1056/NEJMp1200478
6. Molendijk T. Warnings against romanticising moral injury. Br J Psychiatry. 2022;220(1):1-3. doi:10.1192/bjp.2021.114
7. Shay J. Moral injury. Psychoanalytic Psychol. 2014;31(2):182-191. doi:10.1037/a0036090
8. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
9. Brovont v KS-I Med. Servs., P.A., 622 SW3d 671 (Mo Ct App 2020).
1. Griffin BJ, Weber MC, Hinkson KD, et al. Toward a dimensional contextual model of moral injury: a scoping review on healthcare workers. Curr Treat Options Psych. 2023;10:199-216. doi:10.1007/s40501-023-00296-4
2. National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. The National Academies Press; 2019. doi:10.17226/25521
3. Dean W, Talbot S, Dean A. Reframing clinician distress: moral injury not burnout. Fed Pract. 2019;36(9):400-402.
4. Gardner HE, Schulman LS. The professions in America today: crucial but fragile. Daedalus. 2005;134(3):13-18. doi:10.1162/0011526054622132
5. Fuchs VR. Major trends in the U.S. health economy since 1950. N Engl J Med. 2012;366(11):973-977. doi:10.1056/NEJMp1200478
6. Molendijk T. Warnings against romanticising moral injury. Br J Psychiatry. 2022;220(1):1-3. doi:10.1192/bjp.2021.114
7. Shay J. Moral injury. Psychoanalytic Psychol. 2014;31(2):182-191. doi:10.1037/a0036090
8. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
9. Brovont v KS-I Med. Servs., P.A., 622 SW3d 671 (Mo Ct App 2020).
Best Practices for Clinical Image Collection and Utilization in Patients With Skin of Color
Clinical images are integral to dermatologic care, research, and education. Studies have highlighted the underrepresentation of images of skin of color (SOC) in educational materials,1 clinical trials,2 and research publications.3 Recognition of this disparity has ignited a call to action by dermatologists and dermatologic organizations to address the gap by improving the collection and use of SOC images.4 It is critical to remind dermatologists of the importance of properly obtaining informed consent and ensuring images are not used without a patient’s permission, as images in journal articles, conference presentations, and educational materials can be widely distributed and shared. Herein, we summarize current practices of clinical image storage and make general recommendations on how dermatologists can better protect patient privacy. Certain cultural and social factors in patients with SOC should be considered when obtaining informed consent and collecting images.
Clinical Image Acquisition
Consenting procedures are crucial components of proper image usage. However, current consenting practices are inconsistent across various platforms, including academic journals, websites, printed text, social media, and educational presentations.5
Current regulations for use of patient health information in the United States are governed by the Health Insurance Portability and Accountability Act (HIPAA)of 1996. Although this act explicitly prohibits use of “full face photographic images and any comparable images” without consent from the patient or the patient’s representative, there is less restriction regarding the use of deidentified images.6 Some clinicians or researchers may consider using a black bar or a masking technique over the eyes or face, but this is not always a sufficient method of anonymizing an image.
One study investigating the different requirements listed by the top 20 dermatology journals (as determined by the Google Scholar h5-index) found that while 95% (19/20) of journals stated that written or signed consent or permission was a requirement for use of patient images, only 20% (4/20) instructed authors to inform the patient or the patient’s representative that images may become available on the internet.5 Once an article is accepted for publication by a medical journal, it eventually may be accessible online; however, patients may not be aware of this factor, which is particularly concerning for those with SOC due to the increased demand for diverse dermatologic resources and images as well as the highly digitalized manner in which we access and share media.
Furthermore, cultural and social factors exist that present challenges to informed decision-making during the consenting process for certain SOC populations such as a lack of trust in the medical and scientific research community, inadequate comprehension of the consent material, health illiteracy, language barriers, or use of complex terminology in consent documentation.7,8 Studies also have shown that patients in ethnic minority groups have greater barriers to health literacy compared to other patient groups, and patients with limited health literacy are less likely to ask questions during their medical visits.9,10 Therefore, when obtaining informed consent for images, it is important that measures are taken to ensure that the patient has full knowledge and understanding of what the consent covers, including the extent to which the images will be used and/or shared and whether the patient’s confidentiality and/or anonymity are at risk.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Encourage influential dermatology organizations such as the American Academy of Dermatology to establish standardized consenting procedures for image acquisition and use, including requirements to provide (a) written consent for all patient images and (b) specific details as to where and how the image may be used and/or shared.
2. Ensure that consent terminology is presented at a sixth-grade reading level or below, minimize the use of medical jargon and complex terms, and provide consent documentation in the patient’s preferred language.
3. Allow patients to take the consent document home so they can have additional time to comprehensively review the material or have it reviewed by family or friends.
4. Employ strategies such as teach-back methods and encourage questions to maximize the level of understanding during the consent process.
Clinical Image Storage
Clinical image storage procedures can have an impact on a patient’s health information remaining anonymous and confidential. In a survey evaluating medical photography use among 153 US board-certified dermatologists, 69.1% of respondents reported emailing or texting images between patients and colleagues. Additionally, 30.3% (46/152) reported having patient photographs stored on their personal phone at the time of the survey, and 39.1% (18/46) of those individuals had images that showed identifiable features, such as the patient’s face or a tattoo.11
Although most providers state that their devices are password protected, it cannot be guaranteed that the device and consequently the images remain secure and inaccessible to unauthorized individuals. As sharing and viewing images continue to play an essential role in assessing disease state, progression, treatment response, and inclusion in research, we must establish and encourage clear guidelines for the storage and retention of such images.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Store clinical images exclusively on password-protected devices and in password-protected files.
2. Use work-related cameras or electronic devices rather than personal devices, unless the personal device is being used to upload directly into the patient’s medical record. In such cases, use a HIPAA-compliant electronic medical record mobile application that does not store images on the application or the device itself.
3. Avoid using text-messaging systems or unencrypted email to share identifying images without clear patient consent.
Clinical Image Use
Once a thorough consenting process has been completed, it is crucial that the use and distribution of the clinical image are in accordance with the terms specified in the original consent. With the current state of technologic advancement, widespread social media usage, and constant sharing of information, adherence to these terms can be challenging. For example, an image initially intended for use in an educational presentation at a professional conference can be shared on social media if an audience member captures a photo of it. In another example, a patient may consent to their image being shown on a dermatologic website but that image can be duplicated and shared on other unauthorized sites and locations. This situation can be particularly distressing to patients whose image may include all or most of their face, an intimate area, or other physical features that they did not wish to share widely.
Individuals identifying as Black/African American, Latino/Hispanic, or Asian have been shown to express less comfort with providing permission for images of a nonidentifiable sensitive area to be taken (or obtained) or for use for teaching irrespective of identifiability compared to their White counterparts,12 which may be due to the aforementioned lack of trust in medical providers and the health care system in general, both of which may contribute to concerns with how a clinical image is used and/or shared. Although consent from a patient or the patient’s representative can be granted, we must ensure that the use of these images adheres to the patient’s initial agreement. Ultimately, medical providers, researchers, and other parties involved in acquiring or sharing patient images have both an ethical and legal responsibility to ensure that anonymity, privacy, and confidentiality are preserved to the greatest extent possible.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Display a message on websites containing patient images stating that the sharing of the images outside the established guidelines and intended use is prohibited.
2. Place a watermark on images to discourage unauthorized duplication.
3. Issue explicit instructions to audiences prohibiting the copying or reproducing of any patient images during teaching events or presentations.
Final Thoughts
The use of clinical images is an essential component of dermatologic care, education, and research. Due to the higher demand for diverse and representative images and the dearth of images in the medical literature, many SOC images have been widely disseminated and utilized by dermatologists, raising concerns of the adequacy of informed consent for the storage and use of such material. Therefore, dermatologists should implement streamlined guidelines and consent procedures to ensure a patient’s informed consent is provided with full knowledge of how and where their images might be used and shared. Additional efforts should be made to protect patients’ privacy and unauthorized use of their images. Furthermore, we encourage our leading dermatology organizations to develop expert consensus on best practices for appropriate clinical image consent, storage, and use.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198. doi:10.1001/jamadermatol.2016.4129
- Marroquin NA, Carboni A, Zueger M, et al. Skin of color representation trends in JAAD case reports 2015-2021: content analysis. JMIR Dermatol. 2023;6:e40816. doi:10.2196/40816
- Kim Y, Miller JJ, Hollins LC. Skin of color matters: a call to action. J Am Acad Dermatol. 2021;84:E273-E274. doi:10.1016/j.jaad.2020.11.026
- Nanda JK, Marchetti MA. Consent and deidentification of patient images in dermatology journals: observational study. JMIR Dermatol. 2022;5:E37398. doi:10.2196/37398
- US Department of Health and Human Services. Summary of the HIPAA privacy rule. Updated October 19, 2022. Accessed March 15, 2024. https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html
- Quinn SC, Garza MA, Butler J, et al. Improving informed consent with minority participants: results from researcher and community surveys. J Empir Res Hum Res Ethics. 2012;7:44-55. doi:10.1525/jer.2012.7.5.44
- Hadden KB, Prince LY, Moore TD, et al. Improving readability of informed consents for research at an academic medical institution. J Clin Transl Sci. 2017;1:361-365. doi:10.1017/cts.2017.312
- Muvuka B, Combs RM, Ayangeakaa SD, et al. Health literacy in African-American communities: barriers and strategies. Health Lit Res Pract. 2020;4:E138-E143. doi:10.3928/24748307-20200617-01
- Menendez ME, van Hoorn BT, Mackert M, et al. Patients with limited health literacy ask fewer questions during office visits with hand surgeons. Clin Orthop Relat Res. 2017;475:1291-1297. doi:10.1007/s11999-016-5140-5
- Milam EC, Leger MC. Use of medical photography among dermatologists: a nationwide online survey study. J Eur Acad Dermatol Venereol. 2018;32:1804-1809. doi:10.1111/jdv.14839
- Leger MC, Wu T, Haimovic A, et al. Patient perspectives on medical photography in dermatology. Dermatol Surg. 2014;40:1028-1037. doi:10.1097/01.DSS.0000452632.22081.79
Clinical images are integral to dermatologic care, research, and education. Studies have highlighted the underrepresentation of images of skin of color (SOC) in educational materials,1 clinical trials,2 and research publications.3 Recognition of this disparity has ignited a call to action by dermatologists and dermatologic organizations to address the gap by improving the collection and use of SOC images.4 It is critical to remind dermatologists of the importance of properly obtaining informed consent and ensuring images are not used without a patient’s permission, as images in journal articles, conference presentations, and educational materials can be widely distributed and shared. Herein, we summarize current practices of clinical image storage and make general recommendations on how dermatologists can better protect patient privacy. Certain cultural and social factors in patients with SOC should be considered when obtaining informed consent and collecting images.
Clinical Image Acquisition
Consenting procedures are crucial components of proper image usage. However, current consenting practices are inconsistent across various platforms, including academic journals, websites, printed text, social media, and educational presentations.5
Current regulations for use of patient health information in the United States are governed by the Health Insurance Portability and Accountability Act (HIPAA)of 1996. Although this act explicitly prohibits use of “full face photographic images and any comparable images” without consent from the patient or the patient’s representative, there is less restriction regarding the use of deidentified images.6 Some clinicians or researchers may consider using a black bar or a masking technique over the eyes or face, but this is not always a sufficient method of anonymizing an image.
One study investigating the different requirements listed by the top 20 dermatology journals (as determined by the Google Scholar h5-index) found that while 95% (19/20) of journals stated that written or signed consent or permission was a requirement for use of patient images, only 20% (4/20) instructed authors to inform the patient or the patient’s representative that images may become available on the internet.5 Once an article is accepted for publication by a medical journal, it eventually may be accessible online; however, patients may not be aware of this factor, which is particularly concerning for those with SOC due to the increased demand for diverse dermatologic resources and images as well as the highly digitalized manner in which we access and share media.
Furthermore, cultural and social factors exist that present challenges to informed decision-making during the consenting process for certain SOC populations such as a lack of trust in the medical and scientific research community, inadequate comprehension of the consent material, health illiteracy, language barriers, or use of complex terminology in consent documentation.7,8 Studies also have shown that patients in ethnic minority groups have greater barriers to health literacy compared to other patient groups, and patients with limited health literacy are less likely to ask questions during their medical visits.9,10 Therefore, when obtaining informed consent for images, it is important that measures are taken to ensure that the patient has full knowledge and understanding of what the consent covers, including the extent to which the images will be used and/or shared and whether the patient’s confidentiality and/or anonymity are at risk.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Encourage influential dermatology organizations such as the American Academy of Dermatology to establish standardized consenting procedures for image acquisition and use, including requirements to provide (a) written consent for all patient images and (b) specific details as to where and how the image may be used and/or shared.
2. Ensure that consent terminology is presented at a sixth-grade reading level or below, minimize the use of medical jargon and complex terms, and provide consent documentation in the patient’s preferred language.
3. Allow patients to take the consent document home so they can have additional time to comprehensively review the material or have it reviewed by family or friends.
4. Employ strategies such as teach-back methods and encourage questions to maximize the level of understanding during the consent process.
Clinical Image Storage
Clinical image storage procedures can have an impact on a patient’s health information remaining anonymous and confidential. In a survey evaluating medical photography use among 153 US board-certified dermatologists, 69.1% of respondents reported emailing or texting images between patients and colleagues. Additionally, 30.3% (46/152) reported having patient photographs stored on their personal phone at the time of the survey, and 39.1% (18/46) of those individuals had images that showed identifiable features, such as the patient’s face or a tattoo.11
Although most providers state that their devices are password protected, it cannot be guaranteed that the device and consequently the images remain secure and inaccessible to unauthorized individuals. As sharing and viewing images continue to play an essential role in assessing disease state, progression, treatment response, and inclusion in research, we must establish and encourage clear guidelines for the storage and retention of such images.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Store clinical images exclusively on password-protected devices and in password-protected files.
2. Use work-related cameras or electronic devices rather than personal devices, unless the personal device is being used to upload directly into the patient’s medical record. In such cases, use a HIPAA-compliant electronic medical record mobile application that does not store images on the application or the device itself.
3. Avoid using text-messaging systems or unencrypted email to share identifying images without clear patient consent.
Clinical Image Use
Once a thorough consenting process has been completed, it is crucial that the use and distribution of the clinical image are in accordance with the terms specified in the original consent. With the current state of technologic advancement, widespread social media usage, and constant sharing of information, adherence to these terms can be challenging. For example, an image initially intended for use in an educational presentation at a professional conference can be shared on social media if an audience member captures a photo of it. In another example, a patient may consent to their image being shown on a dermatologic website but that image can be duplicated and shared on other unauthorized sites and locations. This situation can be particularly distressing to patients whose image may include all or most of their face, an intimate area, or other physical features that they did not wish to share widely.
Individuals identifying as Black/African American, Latino/Hispanic, or Asian have been shown to express less comfort with providing permission for images of a nonidentifiable sensitive area to be taken (or obtained) or for use for teaching irrespective of identifiability compared to their White counterparts,12 which may be due to the aforementioned lack of trust in medical providers and the health care system in general, both of which may contribute to concerns with how a clinical image is used and/or shared. Although consent from a patient or the patient’s representative can be granted, we must ensure that the use of these images adheres to the patient’s initial agreement. Ultimately, medical providers, researchers, and other parties involved in acquiring or sharing patient images have both an ethical and legal responsibility to ensure that anonymity, privacy, and confidentiality are preserved to the greatest extent possible.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Display a message on websites containing patient images stating that the sharing of the images outside the established guidelines and intended use is prohibited.
2. Place a watermark on images to discourage unauthorized duplication.
3. Issue explicit instructions to audiences prohibiting the copying or reproducing of any patient images during teaching events or presentations.
Final Thoughts
The use of clinical images is an essential component of dermatologic care, education, and research. Due to the higher demand for diverse and representative images and the dearth of images in the medical literature, many SOC images have been widely disseminated and utilized by dermatologists, raising concerns of the adequacy of informed consent for the storage and use of such material. Therefore, dermatologists should implement streamlined guidelines and consent procedures to ensure a patient’s informed consent is provided with full knowledge of how and where their images might be used and shared. Additional efforts should be made to protect patients’ privacy and unauthorized use of their images. Furthermore, we encourage our leading dermatology organizations to develop expert consensus on best practices for appropriate clinical image consent, storage, and use.
Clinical images are integral to dermatologic care, research, and education. Studies have highlighted the underrepresentation of images of skin of color (SOC) in educational materials,1 clinical trials,2 and research publications.3 Recognition of this disparity has ignited a call to action by dermatologists and dermatologic organizations to address the gap by improving the collection and use of SOC images.4 It is critical to remind dermatologists of the importance of properly obtaining informed consent and ensuring images are not used without a patient’s permission, as images in journal articles, conference presentations, and educational materials can be widely distributed and shared. Herein, we summarize current practices of clinical image storage and make general recommendations on how dermatologists can better protect patient privacy. Certain cultural and social factors in patients with SOC should be considered when obtaining informed consent and collecting images.
Clinical Image Acquisition
Consenting procedures are crucial components of proper image usage. However, current consenting practices are inconsistent across various platforms, including academic journals, websites, printed text, social media, and educational presentations.5
Current regulations for use of patient health information in the United States are governed by the Health Insurance Portability and Accountability Act (HIPAA)of 1996. Although this act explicitly prohibits use of “full face photographic images and any comparable images” without consent from the patient or the patient’s representative, there is less restriction regarding the use of deidentified images.6 Some clinicians or researchers may consider using a black bar or a masking technique over the eyes or face, but this is not always a sufficient method of anonymizing an image.
One study investigating the different requirements listed by the top 20 dermatology journals (as determined by the Google Scholar h5-index) found that while 95% (19/20) of journals stated that written or signed consent or permission was a requirement for use of patient images, only 20% (4/20) instructed authors to inform the patient or the patient’s representative that images may become available on the internet.5 Once an article is accepted for publication by a medical journal, it eventually may be accessible online; however, patients may not be aware of this factor, which is particularly concerning for those with SOC due to the increased demand for diverse dermatologic resources and images as well as the highly digitalized manner in which we access and share media.
Furthermore, cultural and social factors exist that present challenges to informed decision-making during the consenting process for certain SOC populations such as a lack of trust in the medical and scientific research community, inadequate comprehension of the consent material, health illiteracy, language barriers, or use of complex terminology in consent documentation.7,8 Studies also have shown that patients in ethnic minority groups have greater barriers to health literacy compared to other patient groups, and patients with limited health literacy are less likely to ask questions during their medical visits.9,10 Therefore, when obtaining informed consent for images, it is important that measures are taken to ensure that the patient has full knowledge and understanding of what the consent covers, including the extent to which the images will be used and/or shared and whether the patient’s confidentiality and/or anonymity are at risk.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Encourage influential dermatology organizations such as the American Academy of Dermatology to establish standardized consenting procedures for image acquisition and use, including requirements to provide (a) written consent for all patient images and (b) specific details as to where and how the image may be used and/or shared.
2. Ensure that consent terminology is presented at a sixth-grade reading level or below, minimize the use of medical jargon and complex terms, and provide consent documentation in the patient’s preferred language.
3. Allow patients to take the consent document home so they can have additional time to comprehensively review the material or have it reviewed by family or friends.
4. Employ strategies such as teach-back methods and encourage questions to maximize the level of understanding during the consent process.
Clinical Image Storage
Clinical image storage procedures can have an impact on a patient’s health information remaining anonymous and confidential. In a survey evaluating medical photography use among 153 US board-certified dermatologists, 69.1% of respondents reported emailing or texting images between patients and colleagues. Additionally, 30.3% (46/152) reported having patient photographs stored on their personal phone at the time of the survey, and 39.1% (18/46) of those individuals had images that showed identifiable features, such as the patient’s face or a tattoo.11
Although most providers state that their devices are password protected, it cannot be guaranteed that the device and consequently the images remain secure and inaccessible to unauthorized individuals. As sharing and viewing images continue to play an essential role in assessing disease state, progression, treatment response, and inclusion in research, we must establish and encourage clear guidelines for the storage and retention of such images.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Store clinical images exclusively on password-protected devices and in password-protected files.
2. Use work-related cameras or electronic devices rather than personal devices, unless the personal device is being used to upload directly into the patient’s medical record. In such cases, use a HIPAA-compliant electronic medical record mobile application that does not store images on the application or the device itself.
3. Avoid using text-messaging systems or unencrypted email to share identifying images without clear patient consent.
Clinical Image Use
Once a thorough consenting process has been completed, it is crucial that the use and distribution of the clinical image are in accordance with the terms specified in the original consent. With the current state of technologic advancement, widespread social media usage, and constant sharing of information, adherence to these terms can be challenging. For example, an image initially intended for use in an educational presentation at a professional conference can be shared on social media if an audience member captures a photo of it. In another example, a patient may consent to their image being shown on a dermatologic website but that image can be duplicated and shared on other unauthorized sites and locations. This situation can be particularly distressing to patients whose image may include all or most of their face, an intimate area, or other physical features that they did not wish to share widely.
Individuals identifying as Black/African American, Latino/Hispanic, or Asian have been shown to express less comfort with providing permission for images of a nonidentifiable sensitive area to be taken (or obtained) or for use for teaching irrespective of identifiability compared to their White counterparts,12 which may be due to the aforementioned lack of trust in medical providers and the health care system in general, both of which may contribute to concerns with how a clinical image is used and/or shared. Although consent from a patient or the patient’s representative can be granted, we must ensure that the use of these images adheres to the patient’s initial agreement. Ultimately, medical providers, researchers, and other parties involved in acquiring or sharing patient images have both an ethical and legal responsibility to ensure that anonymity, privacy, and confidentiality are preserved to the greatest extent possible.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Display a message on websites containing patient images stating that the sharing of the images outside the established guidelines and intended use is prohibited.
2. Place a watermark on images to discourage unauthorized duplication.
3. Issue explicit instructions to audiences prohibiting the copying or reproducing of any patient images during teaching events or presentations.
Final Thoughts
The use of clinical images is an essential component of dermatologic care, education, and research. Due to the higher demand for diverse and representative images and the dearth of images in the medical literature, many SOC images have been widely disseminated and utilized by dermatologists, raising concerns of the adequacy of informed consent for the storage and use of such material. Therefore, dermatologists should implement streamlined guidelines and consent procedures to ensure a patient’s informed consent is provided with full knowledge of how and where their images might be used and shared. Additional efforts should be made to protect patients’ privacy and unauthorized use of their images. Furthermore, we encourage our leading dermatology organizations to develop expert consensus on best practices for appropriate clinical image consent, storage, and use.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198. doi:10.1001/jamadermatol.2016.4129
- Marroquin NA, Carboni A, Zueger M, et al. Skin of color representation trends in JAAD case reports 2015-2021: content analysis. JMIR Dermatol. 2023;6:e40816. doi:10.2196/40816
- Kim Y, Miller JJ, Hollins LC. Skin of color matters: a call to action. J Am Acad Dermatol. 2021;84:E273-E274. doi:10.1016/j.jaad.2020.11.026
- Nanda JK, Marchetti MA. Consent and deidentification of patient images in dermatology journals: observational study. JMIR Dermatol. 2022;5:E37398. doi:10.2196/37398
- US Department of Health and Human Services. Summary of the HIPAA privacy rule. Updated October 19, 2022. Accessed March 15, 2024. https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html
- Quinn SC, Garza MA, Butler J, et al. Improving informed consent with minority participants: results from researcher and community surveys. J Empir Res Hum Res Ethics. 2012;7:44-55. doi:10.1525/jer.2012.7.5.44
- Hadden KB, Prince LY, Moore TD, et al. Improving readability of informed consents for research at an academic medical institution. J Clin Transl Sci. 2017;1:361-365. doi:10.1017/cts.2017.312
- Muvuka B, Combs RM, Ayangeakaa SD, et al. Health literacy in African-American communities: barriers and strategies. Health Lit Res Pract. 2020;4:E138-E143. doi:10.3928/24748307-20200617-01
- Menendez ME, van Hoorn BT, Mackert M, et al. Patients with limited health literacy ask fewer questions during office visits with hand surgeons. Clin Orthop Relat Res. 2017;475:1291-1297. doi:10.1007/s11999-016-5140-5
- Milam EC, Leger MC. Use of medical photography among dermatologists: a nationwide online survey study. J Eur Acad Dermatol Venereol. 2018;32:1804-1809. doi:10.1111/jdv.14839
- Leger MC, Wu T, Haimovic A, et al. Patient perspectives on medical photography in dermatology. Dermatol Surg. 2014;40:1028-1037. doi:10.1097/01.DSS.0000452632.22081.79
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198. doi:10.1001/jamadermatol.2016.4129
- Marroquin NA, Carboni A, Zueger M, et al. Skin of color representation trends in JAAD case reports 2015-2021: content analysis. JMIR Dermatol. 2023;6:e40816. doi:10.2196/40816
- Kim Y, Miller JJ, Hollins LC. Skin of color matters: a call to action. J Am Acad Dermatol. 2021;84:E273-E274. doi:10.1016/j.jaad.2020.11.026
- Nanda JK, Marchetti MA. Consent and deidentification of patient images in dermatology journals: observational study. JMIR Dermatol. 2022;5:E37398. doi:10.2196/37398
- US Department of Health and Human Services. Summary of the HIPAA privacy rule. Updated October 19, 2022. Accessed March 15, 2024. https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html
- Quinn SC, Garza MA, Butler J, et al. Improving informed consent with minority participants: results from researcher and community surveys. J Empir Res Hum Res Ethics. 2012;7:44-55. doi:10.1525/jer.2012.7.5.44
- Hadden KB, Prince LY, Moore TD, et al. Improving readability of informed consents for research at an academic medical institution. J Clin Transl Sci. 2017;1:361-365. doi:10.1017/cts.2017.312
- Muvuka B, Combs RM, Ayangeakaa SD, et al. Health literacy in African-American communities: barriers and strategies. Health Lit Res Pract. 2020;4:E138-E143. doi:10.3928/24748307-20200617-01
- Menendez ME, van Hoorn BT, Mackert M, et al. Patients with limited health literacy ask fewer questions during office visits with hand surgeons. Clin Orthop Relat Res. 2017;475:1291-1297. doi:10.1007/s11999-016-5140-5
- Milam EC, Leger MC. Use of medical photography among dermatologists: a nationwide online survey study. J Eur Acad Dermatol Venereol. 2018;32:1804-1809. doi:10.1111/jdv.14839
- Leger MC, Wu T, Haimovic A, et al. Patient perspectives on medical photography in dermatology. Dermatol Surg. 2014;40:1028-1037. doi:10.1097/01.DSS.0000452632.22081.79
How to Cure Hedonic Eating?
Logan is a 62-year-old woman who has reached the pinnacle of professional success. She started a $50 million consumer products company and, after selling it, managed to develop another successful brand. She is healthy and happily married, with four adult children. And yet, despite all her achievements and stable family life, Logan was always bothered by her inability to lose weight.
Despite peddling in beauty, she felt perpetually overweight and, frankly, unattractive. She has no family history of obesity, drinks minimal alcohol, and follows an (allegedly) healthy diet. Logan had tried “everything” to lose weight — human growth hormone injections (not prescribed by me), Ozempic-like medications, Belviq, etc. — all to no avail.
Here’s the catch: After she finished with her busy days of meetings and spreadsheets, Logan sat down to read through countless emails and rewarded herself with all her favorite foods. Without realizing it, she often doubled her daily caloric intake in one sitting. She wasn’t hungry in these moments, rather just a little worn out and perhaps a little careless. She then proceeded to email her doctor (me) to report on this endless cycle of unwanted behavior.
In January 2024, a novel study from Turkey examined the relationship between hedonic eating, self-condemnation, and self-esteem. Surprising to no one, the study determined that higher hedonic hunger scores were associated with lower self-esteem and an increased propensity to self-stigmatize.
Oprah could have handily predicted this conclusion. Many years ago, she described food as a fake friend: Perhaps you’ve had a long and difficult day. While you’re busy eating your feelings, the heaping plate of pasta feels like your best buddy in the world. However, the moment the plate is empty, you realize that you feel worse than before. Not only do you have to unbutton your new jeans, but you also realize that you have just lost your ability to self-regulate.
While the positive association between hedonic eating and low self-esteem may seem self-evident, the solution is less obvious. Mindfulness is one possible approach to this issue. Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally” and has existed for thousands of years. Mindful eating, in particular, involves paying close attention to our food choices and how they affect our emotions, and typically includes some combination of:
- Slowing down eating/chewing thoroughly
- Eliminating distractions such as TV, computers, and phones — perhaps even eating in silence
- Eating only until physically satiated
- Distinguishing between true hunger and cravings
- Noticing the texture, flavors, and smell of food
- Paying attention to the effect of food on your mood
- Appreciating food
In our society, where processed food is so readily available and stress is so ubiquitous, eating can become a hedonic and fast-paced activity. Our brains don’t have time to process our bodies’ signals of fullness and, as a result, we often ingest many more calories than we need for a healthy lifestyle.
If mindless eating is part of the problem, mindful eating is part of the solution. Indeed, a meta-review of 10 scientific studies showed that mindful eating is as effective as conventional weight loss programs in regard to body mass index and waist circumference. On the basis of these studies — as well as some good old-fashioned common sense — intuitive eating is an important component of sustainable weight reduction.
Eventually, I convinced Logan to meet up with the psychologist in our group who specializes in emotional eating. Through weekly cognitive-behavioral therapy sessions, Logan was able to understand the impetus behind her self-defeating behavior and has finally been able to reverse some of her lifelong habits. Once she started practicing mindful eating, I was able to introduce Ozempic, and now Logan is happily shedding several pounds a week.
Dr. Messer has disclosed no relevant financial relationships.
Dr. Messer is clinical assistant professor, Mount Sinai School of Medicine and associate professor, Hofstra School of Medicine, both in New York City.
A version of this article first appeared on Medscape.com.
Logan is a 62-year-old woman who has reached the pinnacle of professional success. She started a $50 million consumer products company and, after selling it, managed to develop another successful brand. She is healthy and happily married, with four adult children. And yet, despite all her achievements and stable family life, Logan was always bothered by her inability to lose weight.
Despite peddling in beauty, she felt perpetually overweight and, frankly, unattractive. She has no family history of obesity, drinks minimal alcohol, and follows an (allegedly) healthy diet. Logan had tried “everything” to lose weight — human growth hormone injections (not prescribed by me), Ozempic-like medications, Belviq, etc. — all to no avail.
Here’s the catch: After she finished with her busy days of meetings and spreadsheets, Logan sat down to read through countless emails and rewarded herself with all her favorite foods. Without realizing it, she often doubled her daily caloric intake in one sitting. She wasn’t hungry in these moments, rather just a little worn out and perhaps a little careless. She then proceeded to email her doctor (me) to report on this endless cycle of unwanted behavior.
In January 2024, a novel study from Turkey examined the relationship between hedonic eating, self-condemnation, and self-esteem. Surprising to no one, the study determined that higher hedonic hunger scores were associated with lower self-esteem and an increased propensity to self-stigmatize.
Oprah could have handily predicted this conclusion. Many years ago, she described food as a fake friend: Perhaps you’ve had a long and difficult day. While you’re busy eating your feelings, the heaping plate of pasta feels like your best buddy in the world. However, the moment the plate is empty, you realize that you feel worse than before. Not only do you have to unbutton your new jeans, but you also realize that you have just lost your ability to self-regulate.
While the positive association between hedonic eating and low self-esteem may seem self-evident, the solution is less obvious. Mindfulness is one possible approach to this issue. Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally” and has existed for thousands of years. Mindful eating, in particular, involves paying close attention to our food choices and how they affect our emotions, and typically includes some combination of:
- Slowing down eating/chewing thoroughly
- Eliminating distractions such as TV, computers, and phones — perhaps even eating in silence
- Eating only until physically satiated
- Distinguishing between true hunger and cravings
- Noticing the texture, flavors, and smell of food
- Paying attention to the effect of food on your mood
- Appreciating food
In our society, where processed food is so readily available and stress is so ubiquitous, eating can become a hedonic and fast-paced activity. Our brains don’t have time to process our bodies’ signals of fullness and, as a result, we often ingest many more calories than we need for a healthy lifestyle.
If mindless eating is part of the problem, mindful eating is part of the solution. Indeed, a meta-review of 10 scientific studies showed that mindful eating is as effective as conventional weight loss programs in regard to body mass index and waist circumference. On the basis of these studies — as well as some good old-fashioned common sense — intuitive eating is an important component of sustainable weight reduction.
Eventually, I convinced Logan to meet up with the psychologist in our group who specializes in emotional eating. Through weekly cognitive-behavioral therapy sessions, Logan was able to understand the impetus behind her self-defeating behavior and has finally been able to reverse some of her lifelong habits. Once she started practicing mindful eating, I was able to introduce Ozempic, and now Logan is happily shedding several pounds a week.
Dr. Messer has disclosed no relevant financial relationships.
Dr. Messer is clinical assistant professor, Mount Sinai School of Medicine and associate professor, Hofstra School of Medicine, both in New York City.
A version of this article first appeared on Medscape.com.
Logan is a 62-year-old woman who has reached the pinnacle of professional success. She started a $50 million consumer products company and, after selling it, managed to develop another successful brand. She is healthy and happily married, with four adult children. And yet, despite all her achievements and stable family life, Logan was always bothered by her inability to lose weight.
Despite peddling in beauty, she felt perpetually overweight and, frankly, unattractive. She has no family history of obesity, drinks minimal alcohol, and follows an (allegedly) healthy diet. Logan had tried “everything” to lose weight — human growth hormone injections (not prescribed by me), Ozempic-like medications, Belviq, etc. — all to no avail.
Here’s the catch: After she finished with her busy days of meetings and spreadsheets, Logan sat down to read through countless emails and rewarded herself with all her favorite foods. Without realizing it, she often doubled her daily caloric intake in one sitting. She wasn’t hungry in these moments, rather just a little worn out and perhaps a little careless. She then proceeded to email her doctor (me) to report on this endless cycle of unwanted behavior.
In January 2024, a novel study from Turkey examined the relationship between hedonic eating, self-condemnation, and self-esteem. Surprising to no one, the study determined that higher hedonic hunger scores were associated with lower self-esteem and an increased propensity to self-stigmatize.
Oprah could have handily predicted this conclusion. Many years ago, she described food as a fake friend: Perhaps you’ve had a long and difficult day. While you’re busy eating your feelings, the heaping plate of pasta feels like your best buddy in the world. However, the moment the plate is empty, you realize that you feel worse than before. Not only do you have to unbutton your new jeans, but you also realize that you have just lost your ability to self-regulate.
While the positive association between hedonic eating and low self-esteem may seem self-evident, the solution is less obvious. Mindfulness is one possible approach to this issue. Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally” and has existed for thousands of years. Mindful eating, in particular, involves paying close attention to our food choices and how they affect our emotions, and typically includes some combination of:
- Slowing down eating/chewing thoroughly
- Eliminating distractions such as TV, computers, and phones — perhaps even eating in silence
- Eating only until physically satiated
- Distinguishing between true hunger and cravings
- Noticing the texture, flavors, and smell of food
- Paying attention to the effect of food on your mood
- Appreciating food
In our society, where processed food is so readily available and stress is so ubiquitous, eating can become a hedonic and fast-paced activity. Our brains don’t have time to process our bodies’ signals of fullness and, as a result, we often ingest many more calories than we need for a healthy lifestyle.
If mindless eating is part of the problem, mindful eating is part of the solution. Indeed, a meta-review of 10 scientific studies showed that mindful eating is as effective as conventional weight loss programs in regard to body mass index and waist circumference. On the basis of these studies — as well as some good old-fashioned common sense — intuitive eating is an important component of sustainable weight reduction.
Eventually, I convinced Logan to meet up with the psychologist in our group who specializes in emotional eating. Through weekly cognitive-behavioral therapy sessions, Logan was able to understand the impetus behind her self-defeating behavior and has finally been able to reverse some of her lifelong habits. Once she started practicing mindful eating, I was able to introduce Ozempic, and now Logan is happily shedding several pounds a week.
Dr. Messer has disclosed no relevant financial relationships.
Dr. Messer is clinical assistant professor, Mount Sinai School of Medicine and associate professor, Hofstra School of Medicine, both in New York City.
A version of this article first appeared on Medscape.com.
Why We Need to Know About Our Patients’ History of Trauma
This case is a little out of the ordinary, but we would love to find out how readers would handle it.
Diana is a 51-year-old woman with a history of depression, obesity, hypertension, type 2 diabetes, and coronary artery disease. She has come in for a routine visit for her chronic illnesses. She seems very distant and has a flat affect during the initial interview. When you ask about any recent stressful events, she begins crying and explains that her daughter was just deported, leaving behind a child and boyfriend.
Their country of origin suffers from chronic instability and violence. Diana’s father was murdered there, and Diana was the victim of sexual assault. “I escaped when I was 18, and I tried to never look back. Until now.” Diana is very worried about her daughter’s return to that country. “I don’t want her to have to endure what I have endured.”
You spend some time discussing the patient’s mental health burden and identify a counselor and online resources that might help. You wonder if Diana’s adverse childhood experiences (ACEs) might have contributed to some of her physical illnesses.
ACEs and Adult Health
One of the most pronounced and straightforward links is that between ACEs and depression. In the Southern Community Cohort Study of more than 38,200 US adults, the highest odds ratio between ACEs and chronic disease was for depression. Persons who reported more than three ACEs had about a twofold increase in the risk for depression compared with persons without ACEs. There was a monotonic increase in the risk for depression and other chronic illnesses as the burden of ACEs increased.
In another study from the United Kingdom, each additional ACE was associated with a significant 11% increase in the risk for incident diabetes during adulthood. Researchers found that both depression symptoms and cardiometabolic dysfunction mediated the effects of ACEs in promoting higher rates of diabetes.
Depression and diabetes are significant risk factors for coronary artery disease, so it is not surprising that ACEs are also associated with a higher risk for coronary events. A review by Godoy and colleagues described how ACEs promote neuroendocrine, autonomic, and inflammatory dysfunction, which in turn leads to higher rates of traditional cardiovascular risk factors such as diabetes and obesity. Ultimately, the presence of four or more ACEs is associated with more than a twofold higher risk for cardiovascular disease compared with no ACEs.
Many of the pathologic processes that promote cardiovascular disease also increase the risk for dementia. Could the reach of ACEs span decades to promote a higher risk for dementia among older adults? A study by Yuan and colleagues of 7222 Chinese adults suggests that the answer is yes. This study divided the cohort into persons with a history of no ACEs, household dysfunction during childhood, or mistreatment during childhood. Child mistreatment was associated with higher rates of diabetes, depression, and cardiovascular disease, as well as an odds ratio of 1.37 (95% CI, 1.12 to 1.68) for cognitive impairment.
The magnitude of the effects ACEs can have on well-being is reinforced by epidemiologic data surrounding ACEs. According to the US Centers for Disease Control and Prevention (CDC), 64% of US adults report at least one ACE and 17% experienced at least four ACEs. Risk factors for ACEs include being female, American Indian or Alaska Native, or unemployed.
How do we reduce the impact of ACEs? Prevention is key. The CDC estimates that nearly 2 million cases of adult heart disease and more than 20 million cases of adult depression could be avoided if ACEs were eliminated.
But what is the best means to pragmatically reduce ACEs in our current practice models? How do we discover a history of ACEs in patients, and what are the best practices in managing persons with a positive history? We will cover these critical subjects in a future article, but for now, please provide your own comments and pearls regarding the prevention and management of ACEs.
Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, disclosed ties with GlaxoSmithKline and Johnson and Johnson. Ms. Hurtado, MD candidate, University of California, Irvine School of Medicine, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This case is a little out of the ordinary, but we would love to find out how readers would handle it.
Diana is a 51-year-old woman with a history of depression, obesity, hypertension, type 2 diabetes, and coronary artery disease. She has come in for a routine visit for her chronic illnesses. She seems very distant and has a flat affect during the initial interview. When you ask about any recent stressful events, she begins crying and explains that her daughter was just deported, leaving behind a child and boyfriend.
Their country of origin suffers from chronic instability and violence. Diana’s father was murdered there, and Diana was the victim of sexual assault. “I escaped when I was 18, and I tried to never look back. Until now.” Diana is very worried about her daughter’s return to that country. “I don’t want her to have to endure what I have endured.”
You spend some time discussing the patient’s mental health burden and identify a counselor and online resources that might help. You wonder if Diana’s adverse childhood experiences (ACEs) might have contributed to some of her physical illnesses.
ACEs and Adult Health
One of the most pronounced and straightforward links is that between ACEs and depression. In the Southern Community Cohort Study of more than 38,200 US adults, the highest odds ratio between ACEs and chronic disease was for depression. Persons who reported more than three ACEs had about a twofold increase in the risk for depression compared with persons without ACEs. There was a monotonic increase in the risk for depression and other chronic illnesses as the burden of ACEs increased.
In another study from the United Kingdom, each additional ACE was associated with a significant 11% increase in the risk for incident diabetes during adulthood. Researchers found that both depression symptoms and cardiometabolic dysfunction mediated the effects of ACEs in promoting higher rates of diabetes.
Depression and diabetes are significant risk factors for coronary artery disease, so it is not surprising that ACEs are also associated with a higher risk for coronary events. A review by Godoy and colleagues described how ACEs promote neuroendocrine, autonomic, and inflammatory dysfunction, which in turn leads to higher rates of traditional cardiovascular risk factors such as diabetes and obesity. Ultimately, the presence of four or more ACEs is associated with more than a twofold higher risk for cardiovascular disease compared with no ACEs.
Many of the pathologic processes that promote cardiovascular disease also increase the risk for dementia. Could the reach of ACEs span decades to promote a higher risk for dementia among older adults? A study by Yuan and colleagues of 7222 Chinese adults suggests that the answer is yes. This study divided the cohort into persons with a history of no ACEs, household dysfunction during childhood, or mistreatment during childhood. Child mistreatment was associated with higher rates of diabetes, depression, and cardiovascular disease, as well as an odds ratio of 1.37 (95% CI, 1.12 to 1.68) for cognitive impairment.
The magnitude of the effects ACEs can have on well-being is reinforced by epidemiologic data surrounding ACEs. According to the US Centers for Disease Control and Prevention (CDC), 64% of US adults report at least one ACE and 17% experienced at least four ACEs. Risk factors for ACEs include being female, American Indian or Alaska Native, or unemployed.
How do we reduce the impact of ACEs? Prevention is key. The CDC estimates that nearly 2 million cases of adult heart disease and more than 20 million cases of adult depression could be avoided if ACEs were eliminated.
But what is the best means to pragmatically reduce ACEs in our current practice models? How do we discover a history of ACEs in patients, and what are the best practices in managing persons with a positive history? We will cover these critical subjects in a future article, but for now, please provide your own comments and pearls regarding the prevention and management of ACEs.
Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, disclosed ties with GlaxoSmithKline and Johnson and Johnson. Ms. Hurtado, MD candidate, University of California, Irvine School of Medicine, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This case is a little out of the ordinary, but we would love to find out how readers would handle it.
Diana is a 51-year-old woman with a history of depression, obesity, hypertension, type 2 diabetes, and coronary artery disease. She has come in for a routine visit for her chronic illnesses. She seems very distant and has a flat affect during the initial interview. When you ask about any recent stressful events, she begins crying and explains that her daughter was just deported, leaving behind a child and boyfriend.
Their country of origin suffers from chronic instability and violence. Diana’s father was murdered there, and Diana was the victim of sexual assault. “I escaped when I was 18, and I tried to never look back. Until now.” Diana is very worried about her daughter’s return to that country. “I don’t want her to have to endure what I have endured.”
You spend some time discussing the patient’s mental health burden and identify a counselor and online resources that might help. You wonder if Diana’s adverse childhood experiences (ACEs) might have contributed to some of her physical illnesses.
ACEs and Adult Health
One of the most pronounced and straightforward links is that between ACEs and depression. In the Southern Community Cohort Study of more than 38,200 US adults, the highest odds ratio between ACEs and chronic disease was for depression. Persons who reported more than three ACEs had about a twofold increase in the risk for depression compared with persons without ACEs. There was a monotonic increase in the risk for depression and other chronic illnesses as the burden of ACEs increased.
In another study from the United Kingdom, each additional ACE was associated with a significant 11% increase in the risk for incident diabetes during adulthood. Researchers found that both depression symptoms and cardiometabolic dysfunction mediated the effects of ACEs in promoting higher rates of diabetes.
Depression and diabetes are significant risk factors for coronary artery disease, so it is not surprising that ACEs are also associated with a higher risk for coronary events. A review by Godoy and colleagues described how ACEs promote neuroendocrine, autonomic, and inflammatory dysfunction, which in turn leads to higher rates of traditional cardiovascular risk factors such as diabetes and obesity. Ultimately, the presence of four or more ACEs is associated with more than a twofold higher risk for cardiovascular disease compared with no ACEs.
Many of the pathologic processes that promote cardiovascular disease also increase the risk for dementia. Could the reach of ACEs span decades to promote a higher risk for dementia among older adults? A study by Yuan and colleagues of 7222 Chinese adults suggests that the answer is yes. This study divided the cohort into persons with a history of no ACEs, household dysfunction during childhood, or mistreatment during childhood. Child mistreatment was associated with higher rates of diabetes, depression, and cardiovascular disease, as well as an odds ratio of 1.37 (95% CI, 1.12 to 1.68) for cognitive impairment.
The magnitude of the effects ACEs can have on well-being is reinforced by epidemiologic data surrounding ACEs. According to the US Centers for Disease Control and Prevention (CDC), 64% of US adults report at least one ACE and 17% experienced at least four ACEs. Risk factors for ACEs include being female, American Indian or Alaska Native, or unemployed.
How do we reduce the impact of ACEs? Prevention is key. The CDC estimates that nearly 2 million cases of adult heart disease and more than 20 million cases of adult depression could be avoided if ACEs were eliminated.
But what is the best means to pragmatically reduce ACEs in our current practice models? How do we discover a history of ACEs in patients, and what are the best practices in managing persons with a positive history? We will cover these critical subjects in a future article, but for now, please provide your own comments and pearls regarding the prevention and management of ACEs.
Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, disclosed ties with GlaxoSmithKline and Johnson and Johnson. Ms. Hurtado, MD candidate, University of California, Irvine School of Medicine, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Do New Antiobesity Meds Still Require Lifestyle Management?
Is lifestyle counseling needed with the more effective second-generation nutrient-stimulated, hormone-based medications like semaglutide and tirzepatide?
If so, how intensive does the counseling need to be, and what components should be emphasized?
These are the clinical practice questions at the top of mind for healthcare professionals and researchers who provide care to patients who have overweight and/or obesity.
This is what we know. Lifestyle management is considered foundational in the care of patients with obesity.
Because obesity is fundamentally a disease of energy dysregulation, counseling has traditionally focused on dietary caloric reduction, increased physical activity, and strategies to adapt new cognitive and lifestyle behaviors.
On the basis of trial results from the Diabetes Prevention Program and the Look AHEAD studies, provision of intensive behavioral therapy (IBT) is recommended for treatment of obesity by the Centers for Medicare & Medicaid Services and by the US Preventive Services Task Force (Moyer VA; US Preventive Services Task Force).
IBT is commonly defined as consisting of 12-26 comprehensive and multicomponent sessions over the course of a year.
Reaffirming the primacy of lifestyle management, all antiobesity medications are approved by the US Food and Drug Administration as an adjunct to a reduced-calorie diet and increased physical activity.
The beneficial effect of combining IBT with earlier-generation medications like naltrexone/bupropion or liraglutide demonstrated that more participants in the trials achieved ≥ 10% weight loss with IBT compared with those taking the medication without IBT: 38.4% vs 20% for naltrexone/bupropion and 46% vs 33% for liraglutide.
Although there aren’t trial data for other first-generation medications like phentermine, orlistat, or phentermine/topiramate, it is assumed that patients taking these medications would also achieve greater weight loss when combined with IBT.
The obesity pharmacotherapy landscape was upended, however, with the approval of semaglutide (Wegovy), a glucagon-like peptide-1 (GLP-1) receptor agonist, in 2021; and tirzepatide (Zepbound), a GLP-1 and glucose-dependent insulinotropic polypeptide dual receptor agonist, in 2023.
These highly effective medications harness the effect of naturally occurring incretin hormones that reduce appetite through direct and indirect effects on the brain. Although the study designs differed between the STEP 1 and STEP 3 trials, the addition of IBT to semaglutide increased mean percent weight loss from 15% to 16% after 68 weeks of treatment (Wilding JPH et al; Wadden TA).
Comparable benefits from the STEP 3 and SURMOUNT-1 trials of adding IBT to tirzepatide at the maximal tolerated dose increased mean percent weight loss from 21% to 24% after 72 weeks (Wadden TA; Jastreboff AM). Though multicomponent IBT appears to provide greater weight loss when used with nutrient-stimulated hormone-based therapeutics, the additional benefit may be less when compared with first-generation medications.
So, how should we view the role and importance of lifestyle management when a patient is taking a second-generation medication? We need to shift the focus from prescribing a calorie-reduced diet to counseling for healthy eating patterns.
Because the second-generation drugs are more biologically effective in suppressing appetite (ie, reducing hunger, food noise, and cravings, and increasing satiation and satiety), it is easier for patients to reduce their food intake without a sense of deprivation. Furthermore, many patients express less desire to consume savory, sweet, and other enticing foods.
Patients should be encouraged to optimize the quality of their diet, prioritizing lean protein sources with meals and snacks; increasing fruits, vegetables, fiber, and complex carbohydrates; and keeping well hydrated. Because of the risk of developing micronutrient deficiencies while consuming a low-calorie diet — most notably calcium, iron, and vitamin D — patients may be advised to take a daily multivitamin supplement. Dietary counseling should be introduced when patients start pharmacotherapy, and if needed, referral to a registered dietitian nutritionist may be helpful in making these changes.
Additional counseling tips to mitigate the gastrointestinal side effects of these drugs that most commonly occur during the early dose-escalation phase include eating slowly; choosing smaller portion sizes; stopping eating when full; not skipping meals; and avoiding fatty, fried, and greasy foods. These dietary changes are particularly important over the first days after patients take the injection.
The increased weight loss achieved also raises concerns about the need to maintain lean body mass and the importance of physical activity and exercise counseling. All weight loss interventions, including dietary restriction, pharmacotherapy, or bariatric surgery, result in loss of fat mass and lean body mass.
The goal of lifestyle counseling is to minimize and preserve muscle mass (a component of lean body mass) which is needed for optimal health, mobility, daily function, and quality of life. Counseling should incorporate both aerobic and resistance training. Aerobic exercise (eg, brisk walking, jogging, dancing, elliptical machine, and cycling) improves cardiovascular fitness, metabolic health, and energy expenditure. Resistance (strength) training (eg, weightlifting, resistance bands, and circuit training) lessens the loss of muscle mass, enhances functional strength and mobility, and improves bone density (Gorgojo-Martinez JJ et al; Oppert JM et al).
Robust physical activity has also been shown to be a predictor of weight loss maintenance. A recently published randomized placebo-controlled trial demonstrated the benefit of supervised exercise in maintaining body weight and lean body mass after discontinuing 52 weeks of liraglutide treatment compared with no exercise.
Rather than minimizing the provision of lifestyle management, using highly effective second-generation therapeutics redirects the focus on how patients with obesity can strive to achieve a healthy and productive life.
A version of this article first appeared on Medscape.com.
Is lifestyle counseling needed with the more effective second-generation nutrient-stimulated, hormone-based medications like semaglutide and tirzepatide?
If so, how intensive does the counseling need to be, and what components should be emphasized?
These are the clinical practice questions at the top of mind for healthcare professionals and researchers who provide care to patients who have overweight and/or obesity.
This is what we know. Lifestyle management is considered foundational in the care of patients with obesity.
Because obesity is fundamentally a disease of energy dysregulation, counseling has traditionally focused on dietary caloric reduction, increased physical activity, and strategies to adapt new cognitive and lifestyle behaviors.
On the basis of trial results from the Diabetes Prevention Program and the Look AHEAD studies, provision of intensive behavioral therapy (IBT) is recommended for treatment of obesity by the Centers for Medicare & Medicaid Services and by the US Preventive Services Task Force (Moyer VA; US Preventive Services Task Force).
IBT is commonly defined as consisting of 12-26 comprehensive and multicomponent sessions over the course of a year.
Reaffirming the primacy of lifestyle management, all antiobesity medications are approved by the US Food and Drug Administration as an adjunct to a reduced-calorie diet and increased physical activity.
The beneficial effect of combining IBT with earlier-generation medications like naltrexone/bupropion or liraglutide demonstrated that more participants in the trials achieved ≥ 10% weight loss with IBT compared with those taking the medication without IBT: 38.4% vs 20% for naltrexone/bupropion and 46% vs 33% for liraglutide.
Although there aren’t trial data for other first-generation medications like phentermine, orlistat, or phentermine/topiramate, it is assumed that patients taking these medications would also achieve greater weight loss when combined with IBT.
The obesity pharmacotherapy landscape was upended, however, with the approval of semaglutide (Wegovy), a glucagon-like peptide-1 (GLP-1) receptor agonist, in 2021; and tirzepatide (Zepbound), a GLP-1 and glucose-dependent insulinotropic polypeptide dual receptor agonist, in 2023.
These highly effective medications harness the effect of naturally occurring incretin hormones that reduce appetite through direct and indirect effects on the brain. Although the study designs differed between the STEP 1 and STEP 3 trials, the addition of IBT to semaglutide increased mean percent weight loss from 15% to 16% after 68 weeks of treatment (Wilding JPH et al; Wadden TA).
Comparable benefits from the STEP 3 and SURMOUNT-1 trials of adding IBT to tirzepatide at the maximal tolerated dose increased mean percent weight loss from 21% to 24% after 72 weeks (Wadden TA; Jastreboff AM). Though multicomponent IBT appears to provide greater weight loss when used with nutrient-stimulated hormone-based therapeutics, the additional benefit may be less when compared with first-generation medications.
So, how should we view the role and importance of lifestyle management when a patient is taking a second-generation medication? We need to shift the focus from prescribing a calorie-reduced diet to counseling for healthy eating patterns.
Because the second-generation drugs are more biologically effective in suppressing appetite (ie, reducing hunger, food noise, and cravings, and increasing satiation and satiety), it is easier for patients to reduce their food intake without a sense of deprivation. Furthermore, many patients express less desire to consume savory, sweet, and other enticing foods.
Patients should be encouraged to optimize the quality of their diet, prioritizing lean protein sources with meals and snacks; increasing fruits, vegetables, fiber, and complex carbohydrates; and keeping well hydrated. Because of the risk of developing micronutrient deficiencies while consuming a low-calorie diet — most notably calcium, iron, and vitamin D — patients may be advised to take a daily multivitamin supplement. Dietary counseling should be introduced when patients start pharmacotherapy, and if needed, referral to a registered dietitian nutritionist may be helpful in making these changes.
Additional counseling tips to mitigate the gastrointestinal side effects of these drugs that most commonly occur during the early dose-escalation phase include eating slowly; choosing smaller portion sizes; stopping eating when full; not skipping meals; and avoiding fatty, fried, and greasy foods. These dietary changes are particularly important over the first days after patients take the injection.
The increased weight loss achieved also raises concerns about the need to maintain lean body mass and the importance of physical activity and exercise counseling. All weight loss interventions, including dietary restriction, pharmacotherapy, or bariatric surgery, result in loss of fat mass and lean body mass.
The goal of lifestyle counseling is to minimize and preserve muscle mass (a component of lean body mass) which is needed for optimal health, mobility, daily function, and quality of life. Counseling should incorporate both aerobic and resistance training. Aerobic exercise (eg, brisk walking, jogging, dancing, elliptical machine, and cycling) improves cardiovascular fitness, metabolic health, and energy expenditure. Resistance (strength) training (eg, weightlifting, resistance bands, and circuit training) lessens the loss of muscle mass, enhances functional strength and mobility, and improves bone density (Gorgojo-Martinez JJ et al; Oppert JM et al).
Robust physical activity has also been shown to be a predictor of weight loss maintenance. A recently published randomized placebo-controlled trial demonstrated the benefit of supervised exercise in maintaining body weight and lean body mass after discontinuing 52 weeks of liraglutide treatment compared with no exercise.
Rather than minimizing the provision of lifestyle management, using highly effective second-generation therapeutics redirects the focus on how patients with obesity can strive to achieve a healthy and productive life.
A version of this article first appeared on Medscape.com.
Is lifestyle counseling needed with the more effective second-generation nutrient-stimulated, hormone-based medications like semaglutide and tirzepatide?
If so, how intensive does the counseling need to be, and what components should be emphasized?
These are the clinical practice questions at the top of mind for healthcare professionals and researchers who provide care to patients who have overweight and/or obesity.
This is what we know. Lifestyle management is considered foundational in the care of patients with obesity.
Because obesity is fundamentally a disease of energy dysregulation, counseling has traditionally focused on dietary caloric reduction, increased physical activity, and strategies to adapt new cognitive and lifestyle behaviors.
On the basis of trial results from the Diabetes Prevention Program and the Look AHEAD studies, provision of intensive behavioral therapy (IBT) is recommended for treatment of obesity by the Centers for Medicare & Medicaid Services and by the US Preventive Services Task Force (Moyer VA; US Preventive Services Task Force).
IBT is commonly defined as consisting of 12-26 comprehensive and multicomponent sessions over the course of a year.
Reaffirming the primacy of lifestyle management, all antiobesity medications are approved by the US Food and Drug Administration as an adjunct to a reduced-calorie diet and increased physical activity.
The beneficial effect of combining IBT with earlier-generation medications like naltrexone/bupropion or liraglutide demonstrated that more participants in the trials achieved ≥ 10% weight loss with IBT compared with those taking the medication without IBT: 38.4% vs 20% for naltrexone/bupropion and 46% vs 33% for liraglutide.
Although there aren’t trial data for other first-generation medications like phentermine, orlistat, or phentermine/topiramate, it is assumed that patients taking these medications would also achieve greater weight loss when combined with IBT.
The obesity pharmacotherapy landscape was upended, however, with the approval of semaglutide (Wegovy), a glucagon-like peptide-1 (GLP-1) receptor agonist, in 2021; and tirzepatide (Zepbound), a GLP-1 and glucose-dependent insulinotropic polypeptide dual receptor agonist, in 2023.
These highly effective medications harness the effect of naturally occurring incretin hormones that reduce appetite through direct and indirect effects on the brain. Although the study designs differed between the STEP 1 and STEP 3 trials, the addition of IBT to semaglutide increased mean percent weight loss from 15% to 16% after 68 weeks of treatment (Wilding JPH et al; Wadden TA).
Comparable benefits from the STEP 3 and SURMOUNT-1 trials of adding IBT to tirzepatide at the maximal tolerated dose increased mean percent weight loss from 21% to 24% after 72 weeks (Wadden TA; Jastreboff AM). Though multicomponent IBT appears to provide greater weight loss when used with nutrient-stimulated hormone-based therapeutics, the additional benefit may be less when compared with first-generation medications.
So, how should we view the role and importance of lifestyle management when a patient is taking a second-generation medication? We need to shift the focus from prescribing a calorie-reduced diet to counseling for healthy eating patterns.
Because the second-generation drugs are more biologically effective in suppressing appetite (ie, reducing hunger, food noise, and cravings, and increasing satiation and satiety), it is easier for patients to reduce their food intake without a sense of deprivation. Furthermore, many patients express less desire to consume savory, sweet, and other enticing foods.
Patients should be encouraged to optimize the quality of their diet, prioritizing lean protein sources with meals and snacks; increasing fruits, vegetables, fiber, and complex carbohydrates; and keeping well hydrated. Because of the risk of developing micronutrient deficiencies while consuming a low-calorie diet — most notably calcium, iron, and vitamin D — patients may be advised to take a daily multivitamin supplement. Dietary counseling should be introduced when patients start pharmacotherapy, and if needed, referral to a registered dietitian nutritionist may be helpful in making these changes.
Additional counseling tips to mitigate the gastrointestinal side effects of these drugs that most commonly occur during the early dose-escalation phase include eating slowly; choosing smaller portion sizes; stopping eating when full; not skipping meals; and avoiding fatty, fried, and greasy foods. These dietary changes are particularly important over the first days after patients take the injection.
The increased weight loss achieved also raises concerns about the need to maintain lean body mass and the importance of physical activity and exercise counseling. All weight loss interventions, including dietary restriction, pharmacotherapy, or bariatric surgery, result in loss of fat mass and lean body mass.
The goal of lifestyle counseling is to minimize and preserve muscle mass (a component of lean body mass) which is needed for optimal health, mobility, daily function, and quality of life. Counseling should incorporate both aerobic and resistance training. Aerobic exercise (eg, brisk walking, jogging, dancing, elliptical machine, and cycling) improves cardiovascular fitness, metabolic health, and energy expenditure. Resistance (strength) training (eg, weightlifting, resistance bands, and circuit training) lessens the loss of muscle mass, enhances functional strength and mobility, and improves bone density (Gorgojo-Martinez JJ et al; Oppert JM et al).
Robust physical activity has also been shown to be a predictor of weight loss maintenance. A recently published randomized placebo-controlled trial demonstrated the benefit of supervised exercise in maintaining body weight and lean body mass after discontinuing 52 weeks of liraglutide treatment compared with no exercise.
Rather than minimizing the provision of lifestyle management, using highly effective second-generation therapeutics redirects the focus on how patients with obesity can strive to achieve a healthy and productive life.
A version of this article first appeared on Medscape.com.
Frozen Embryos: Legally Children? The End of IVF, Says Ethicist
This transcript has been edited for clarity.
I think we’re all aware that Alabama has put itself and the rest of the country into a moral bind when it comes to abortion and the status of human embryos. Back on February 16, 2024, the Alabama Supreme Court rendered a decision in a case called LePage v. Center for Reproductive Medicine, in which the court said that cryopreserved embryos in frozen nitrogen were legally equivalent to children.
They basically said they’re granted the same rights, meaning you certainly can’t destroy them. You certainly could not be in a situation where somebody said, “I’m going to not use them,” because once you create them, you seem to have some duty to make sure they end up in an environment where they can become full-fledged adults.
This decision that embryos in frozen nitrogen — but literally embryos anywhere — are the equivalent of full-bore children put Alabama in a terrible situation if you were a person or a couple seeking in vitro fertilization (IVF).
IVF requires the creation of many eggs. Women have to undergo drug treatment so that they superovulate. It’s too expensive to just go one egg at a time, egg procurement costs too much, and a cycle of IVF could cost as much as $15,000. There are some people who don’t make many eggs, so you want to get as many as you can.
When you get them, you freeze them, as happened in this Alabama case. By the way, what triggered the court case was that somebody in the lab dropped the tray with embryos in it, and they were basically accused not just of a mistake but of murder.
It’s pretty serious when you see this decision and you realize that if you make a multitude of embryos and then you had a child after two tries, but you have six more, you can’t destroy them. What are you going to do with them? Will they be under the governance of the utility company? What’s going to happen?
Many women in Alabama were outraged by the court’s opinion because they want to do IVF. In fact, politically, proponents of thinking that life begins at conception — or fetal personhood as it’s called, and the view that human embryos are children from the minute of conception — were stuck. It’s hard to argue that IVF is not pro-life. It’s hard to argue that people who desperately want to have children should find it difficult to use the technique.
The state has tried to pass a law that exempts IVF clinics from liability if they’re trying to use human embryos to make babies. I do not think this will stand. The court decision is fundamentally wrong, in part because human embryos are not children. They are potential children. They are possible children, but outside of implantation in the environment of a woman’s uterus, they’ll never become anything.
In fact, the court decision is a version of what used to be called preformationism, which sees a tiny baby inside a human embryo. That’s not true. We know today that you’ve got sets of genes that need messages from the mom in order to begin the process of division and development. It isn’t just expanding a tiny, miniature baby into a full-bore baby, as the court in Alabama seems to think.
I think you’re going to find that other states may be influenced to try to mimic the Alabama court decision, but if they do so, it’s going to mean ultimately the end — regardless of what Alabama legislature tried to do — of IVF.
That has a political consequence that I don’t think can be sustained by proponents of fetal personhood or embryo personhood. There is just too much momentum to support the use of IVF to try to create life to make that a politically viable situation.
Alabama may have its court ruling, but I think it’s going to have to pass legislation that overrules the view that embryos are children, not just trying to exempt IVF from the consequences of that view, if we’re going to see IVF possible in that state or anywhere else in the United States.
Dr. Caplan, director, division of medical ethics, New York University Langone Medical Center, New York, has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). He is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I think we’re all aware that Alabama has put itself and the rest of the country into a moral bind when it comes to abortion and the status of human embryos. Back on February 16, 2024, the Alabama Supreme Court rendered a decision in a case called LePage v. Center for Reproductive Medicine, in which the court said that cryopreserved embryos in frozen nitrogen were legally equivalent to children.
They basically said they’re granted the same rights, meaning you certainly can’t destroy them. You certainly could not be in a situation where somebody said, “I’m going to not use them,” because once you create them, you seem to have some duty to make sure they end up in an environment where they can become full-fledged adults.
This decision that embryos in frozen nitrogen — but literally embryos anywhere — are the equivalent of full-bore children put Alabama in a terrible situation if you were a person or a couple seeking in vitro fertilization (IVF).
IVF requires the creation of many eggs. Women have to undergo drug treatment so that they superovulate. It’s too expensive to just go one egg at a time, egg procurement costs too much, and a cycle of IVF could cost as much as $15,000. There are some people who don’t make many eggs, so you want to get as many as you can.
When you get them, you freeze them, as happened in this Alabama case. By the way, what triggered the court case was that somebody in the lab dropped the tray with embryos in it, and they were basically accused not just of a mistake but of murder.
It’s pretty serious when you see this decision and you realize that if you make a multitude of embryos and then you had a child after two tries, but you have six more, you can’t destroy them. What are you going to do with them? Will they be under the governance of the utility company? What’s going to happen?
Many women in Alabama were outraged by the court’s opinion because they want to do IVF. In fact, politically, proponents of thinking that life begins at conception — or fetal personhood as it’s called, and the view that human embryos are children from the minute of conception — were stuck. It’s hard to argue that IVF is not pro-life. It’s hard to argue that people who desperately want to have children should find it difficult to use the technique.
The state has tried to pass a law that exempts IVF clinics from liability if they’re trying to use human embryos to make babies. I do not think this will stand. The court decision is fundamentally wrong, in part because human embryos are not children. They are potential children. They are possible children, but outside of implantation in the environment of a woman’s uterus, they’ll never become anything.
In fact, the court decision is a version of what used to be called preformationism, which sees a tiny baby inside a human embryo. That’s not true. We know today that you’ve got sets of genes that need messages from the mom in order to begin the process of division and development. It isn’t just expanding a tiny, miniature baby into a full-bore baby, as the court in Alabama seems to think.
I think you’re going to find that other states may be influenced to try to mimic the Alabama court decision, but if they do so, it’s going to mean ultimately the end — regardless of what Alabama legislature tried to do — of IVF.
That has a political consequence that I don’t think can be sustained by proponents of fetal personhood or embryo personhood. There is just too much momentum to support the use of IVF to try to create life to make that a politically viable situation.
Alabama may have its court ruling, but I think it’s going to have to pass legislation that overrules the view that embryos are children, not just trying to exempt IVF from the consequences of that view, if we’re going to see IVF possible in that state or anywhere else in the United States.
Dr. Caplan, director, division of medical ethics, New York University Langone Medical Center, New York, has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). He is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I think we’re all aware that Alabama has put itself and the rest of the country into a moral bind when it comes to abortion and the status of human embryos. Back on February 16, 2024, the Alabama Supreme Court rendered a decision in a case called LePage v. Center for Reproductive Medicine, in which the court said that cryopreserved embryos in frozen nitrogen were legally equivalent to children.
They basically said they’re granted the same rights, meaning you certainly can’t destroy them. You certainly could not be in a situation where somebody said, “I’m going to not use them,” because once you create them, you seem to have some duty to make sure they end up in an environment where they can become full-fledged adults.
This decision that embryos in frozen nitrogen — but literally embryos anywhere — are the equivalent of full-bore children put Alabama in a terrible situation if you were a person or a couple seeking in vitro fertilization (IVF).
IVF requires the creation of many eggs. Women have to undergo drug treatment so that they superovulate. It’s too expensive to just go one egg at a time, egg procurement costs too much, and a cycle of IVF could cost as much as $15,000. There are some people who don’t make many eggs, so you want to get as many as you can.
When you get them, you freeze them, as happened in this Alabama case. By the way, what triggered the court case was that somebody in the lab dropped the tray with embryos in it, and they were basically accused not just of a mistake but of murder.
It’s pretty serious when you see this decision and you realize that if you make a multitude of embryos and then you had a child after two tries, but you have six more, you can’t destroy them. What are you going to do with them? Will they be under the governance of the utility company? What’s going to happen?
Many women in Alabama were outraged by the court’s opinion because they want to do IVF. In fact, politically, proponents of thinking that life begins at conception — or fetal personhood as it’s called, and the view that human embryos are children from the minute of conception — were stuck. It’s hard to argue that IVF is not pro-life. It’s hard to argue that people who desperately want to have children should find it difficult to use the technique.
The state has tried to pass a law that exempts IVF clinics from liability if they’re trying to use human embryos to make babies. I do not think this will stand. The court decision is fundamentally wrong, in part because human embryos are not children. They are potential children. They are possible children, but outside of implantation in the environment of a woman’s uterus, they’ll never become anything.
In fact, the court decision is a version of what used to be called preformationism, which sees a tiny baby inside a human embryo. That’s not true. We know today that you’ve got sets of genes that need messages from the mom in order to begin the process of division and development. It isn’t just expanding a tiny, miniature baby into a full-bore baby, as the court in Alabama seems to think.
I think you’re going to find that other states may be influenced to try to mimic the Alabama court decision, but if they do so, it’s going to mean ultimately the end — regardless of what Alabama legislature tried to do — of IVF.
That has a political consequence that I don’t think can be sustained by proponents of fetal personhood or embryo personhood. There is just too much momentum to support the use of IVF to try to create life to make that a politically viable situation.
Alabama may have its court ruling, but I think it’s going to have to pass legislation that overrules the view that embryos are children, not just trying to exempt IVF from the consequences of that view, if we’re going to see IVF possible in that state or anywhere else in the United States.
Dr. Caplan, director, division of medical ethics, New York University Langone Medical Center, New York, has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). He is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
A Banned Chemical That Is Still Causing Cancer
This transcript has been edited for clarity.
These types of stories usually end with a call for regulation — to ban said chemical or substance, or to regulate it — but in this case, that has already happened. This new carcinogen I’m telling you about is actually an old chemical. And it has not been manufactured or legally imported in the US since 2013.
So, why bother? Because in this case, the chemical — or, really, a group of chemicals called polybrominated diphenyl ethers (PBDEs) — are still around: in our soil, in our food, and in our blood.
PBDEs are a group of compounds that confer flame-retardant properties to plastics, and they were used extensively in the latter part of the 20th century in electronic enclosures, business equipment, and foam cushioning in upholstery.
But there was a problem. They don’t chemically bond to plastics; they are just sort of mixed in, which means they can leach out. They are hydrophobic, meaning they don’t get washed out of soil, and, when ingested or inhaled by humans, they dissolve in our fat stores, making it difficult for our normal excretory systems to excrete them.
PBDEs biomagnify. Small animals can take them up from contaminated soil or water, and those animals are eaten by larger animals, which accumulate higher concentrations of the chemicals. This bioaccumulation increases as you move up the food web until you get to an apex predator — like you and me.
This is true of lots of chemicals, of course. The concern arises when these chemicals are toxic. To date, the toxicity data for PBDEs were pretty limited. There were some animal studies where rats were exposed to extremely high doses and they developed liver lesions — but I am always very wary of extrapolating high-dose rat toxicity studies to humans. There was also some suggestion that the chemicals could be endocrine disruptors, affecting breast and thyroid tissue.
What about cancer? In 2016, the International Agency for Research on Cancer concluded there was “inadequate evidence in humans for the carcinogencity of” PBDEs.
In the same report, though, they suggested PBDEs are “probably carcinogenic to humans” based on mechanistic studies.
In other words, we can’t prove they’re cancerous — but come on, they probably are.
Finally, we have some evidence that really pushes us toward the carcinogenic conclusion, in the form of this study, appearing in JAMA Network Open. It’s a nice bit of epidemiology leveraging the population-based National Health and Nutrition Examination Survey (NHANES).
Researchers measured PBDE levels in blood samples from 1100 people enrolled in NHANES in 2003 and 2004 and linked them to death records collected over the next 20 years or so.
The first thing to note is that the researchers were able to measure PBDEs in the blood samples. They were in there. They were detectable. And they were variable. Dividing the 1100 participants into low, medium, and high PBDE tertiles, you can see a nearly 10-fold difference across the population.
Importantly, not many baseline variables correlated with PBDE levels. People in the highest group were a bit younger but had a fairly similar sex distribution, race, ethnicity, education, income, physical activity, smoking status, and body mass index.
This is not a randomized trial, of course — but at least based on these data, exposure levels do seem fairly random, which is what you would expect from an environmental toxin that percolates up through the food chain. They are often somewhat indiscriminate.
This similarity in baseline characteristics between people with low or high blood levels of PBDE also allows us to make some stronger inferences about the observed outcomes. Let’s take a look at them.
After adjustment for baseline factors, individuals in the highest PBDE group had a 43% higher rate of death from any cause over the follow-up period. This was not enough to achieve statistical significance, but it was close.
But the key finding is deaths due to cancer. After adjustment, cancer deaths occurred four times as frequently among those in the high PBDE group, and that is a statistically significant difference.
To be fair, cancer deaths were rare in this cohort. The vast majority of people did not die of anything during the follow-up period regardless of PBDE level. But the data are strongly suggestive of the carcinogenicity of these chemicals.
I should also point out that the researchers are linking the PBDE level at a single time point to all these future events. If PBDE levels remain relatively stable within an individual over time, that’s fine, but if they tend to vary with intake of different foods for example, this would not be captured and would actually lead to an underestimation of the cancer risk.
The researchers also didn’t have granular enough data to determine the type of cancer, but they do show that rates are similar between men and women, which might point away from the more sex-specific cancer etiologies. Clearly, some more work is needed.
Of course, I started this piece by telling you that these chemicals are already pretty much banned in the United States. What are we supposed to do about these findings? Studies have examined the primary ongoing sources of PBDE in our environment and it seems like most of our exposure will be coming from the food we eat due to that biomagnification thing: high-fat fish, meat and dairy products, and fish oil supplements. It may be worth some investigation into the relative adulteration of these products with this new old carcinogen.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
These types of stories usually end with a call for regulation — to ban said chemical or substance, or to regulate it — but in this case, that has already happened. This new carcinogen I’m telling you about is actually an old chemical. And it has not been manufactured or legally imported in the US since 2013.
So, why bother? Because in this case, the chemical — or, really, a group of chemicals called polybrominated diphenyl ethers (PBDEs) — are still around: in our soil, in our food, and in our blood.
PBDEs are a group of compounds that confer flame-retardant properties to plastics, and they were used extensively in the latter part of the 20th century in electronic enclosures, business equipment, and foam cushioning in upholstery.
But there was a problem. They don’t chemically bond to plastics; they are just sort of mixed in, which means they can leach out. They are hydrophobic, meaning they don’t get washed out of soil, and, when ingested or inhaled by humans, they dissolve in our fat stores, making it difficult for our normal excretory systems to excrete them.
PBDEs biomagnify. Small animals can take them up from contaminated soil or water, and those animals are eaten by larger animals, which accumulate higher concentrations of the chemicals. This bioaccumulation increases as you move up the food web until you get to an apex predator — like you and me.
This is true of lots of chemicals, of course. The concern arises when these chemicals are toxic. To date, the toxicity data for PBDEs were pretty limited. There were some animal studies where rats were exposed to extremely high doses and they developed liver lesions — but I am always very wary of extrapolating high-dose rat toxicity studies to humans. There was also some suggestion that the chemicals could be endocrine disruptors, affecting breast and thyroid tissue.
What about cancer? In 2016, the International Agency for Research on Cancer concluded there was “inadequate evidence in humans for the carcinogencity of” PBDEs.
In the same report, though, they suggested PBDEs are “probably carcinogenic to humans” based on mechanistic studies.
In other words, we can’t prove they’re cancerous — but come on, they probably are.
Finally, we have some evidence that really pushes us toward the carcinogenic conclusion, in the form of this study, appearing in JAMA Network Open. It’s a nice bit of epidemiology leveraging the population-based National Health and Nutrition Examination Survey (NHANES).
Researchers measured PBDE levels in blood samples from 1100 people enrolled in NHANES in 2003 and 2004 and linked them to death records collected over the next 20 years or so.
The first thing to note is that the researchers were able to measure PBDEs in the blood samples. They were in there. They were detectable. And they were variable. Dividing the 1100 participants into low, medium, and high PBDE tertiles, you can see a nearly 10-fold difference across the population.
Importantly, not many baseline variables correlated with PBDE levels. People in the highest group were a bit younger but had a fairly similar sex distribution, race, ethnicity, education, income, physical activity, smoking status, and body mass index.
This is not a randomized trial, of course — but at least based on these data, exposure levels do seem fairly random, which is what you would expect from an environmental toxin that percolates up through the food chain. They are often somewhat indiscriminate.
This similarity in baseline characteristics between people with low or high blood levels of PBDE also allows us to make some stronger inferences about the observed outcomes. Let’s take a look at them.
After adjustment for baseline factors, individuals in the highest PBDE group had a 43% higher rate of death from any cause over the follow-up period. This was not enough to achieve statistical significance, but it was close.
But the key finding is deaths due to cancer. After adjustment, cancer deaths occurred four times as frequently among those in the high PBDE group, and that is a statistically significant difference.
To be fair, cancer deaths were rare in this cohort. The vast majority of people did not die of anything during the follow-up period regardless of PBDE level. But the data are strongly suggestive of the carcinogenicity of these chemicals.
I should also point out that the researchers are linking the PBDE level at a single time point to all these future events. If PBDE levels remain relatively stable within an individual over time, that’s fine, but if they tend to vary with intake of different foods for example, this would not be captured and would actually lead to an underestimation of the cancer risk.
The researchers also didn’t have granular enough data to determine the type of cancer, but they do show that rates are similar between men and women, which might point away from the more sex-specific cancer etiologies. Clearly, some more work is needed.
Of course, I started this piece by telling you that these chemicals are already pretty much banned in the United States. What are we supposed to do about these findings? Studies have examined the primary ongoing sources of PBDE in our environment and it seems like most of our exposure will be coming from the food we eat due to that biomagnification thing: high-fat fish, meat and dairy products, and fish oil supplements. It may be worth some investigation into the relative adulteration of these products with this new old carcinogen.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
These types of stories usually end with a call for regulation — to ban said chemical or substance, or to regulate it — but in this case, that has already happened. This new carcinogen I’m telling you about is actually an old chemical. And it has not been manufactured or legally imported in the US since 2013.
So, why bother? Because in this case, the chemical — or, really, a group of chemicals called polybrominated diphenyl ethers (PBDEs) — are still around: in our soil, in our food, and in our blood.
PBDEs are a group of compounds that confer flame-retardant properties to plastics, and they were used extensively in the latter part of the 20th century in electronic enclosures, business equipment, and foam cushioning in upholstery.
But there was a problem. They don’t chemically bond to plastics; they are just sort of mixed in, which means they can leach out. They are hydrophobic, meaning they don’t get washed out of soil, and, when ingested or inhaled by humans, they dissolve in our fat stores, making it difficult for our normal excretory systems to excrete them.
PBDEs biomagnify. Small animals can take them up from contaminated soil or water, and those animals are eaten by larger animals, which accumulate higher concentrations of the chemicals. This bioaccumulation increases as you move up the food web until you get to an apex predator — like you and me.
This is true of lots of chemicals, of course. The concern arises when these chemicals are toxic. To date, the toxicity data for PBDEs were pretty limited. There were some animal studies where rats were exposed to extremely high doses and they developed liver lesions — but I am always very wary of extrapolating high-dose rat toxicity studies to humans. There was also some suggestion that the chemicals could be endocrine disruptors, affecting breast and thyroid tissue.
What about cancer? In 2016, the International Agency for Research on Cancer concluded there was “inadequate evidence in humans for the carcinogencity of” PBDEs.
In the same report, though, they suggested PBDEs are “probably carcinogenic to humans” based on mechanistic studies.
In other words, we can’t prove they’re cancerous — but come on, they probably are.
Finally, we have some evidence that really pushes us toward the carcinogenic conclusion, in the form of this study, appearing in JAMA Network Open. It’s a nice bit of epidemiology leveraging the population-based National Health and Nutrition Examination Survey (NHANES).
Researchers measured PBDE levels in blood samples from 1100 people enrolled in NHANES in 2003 and 2004 and linked them to death records collected over the next 20 years or so.
The first thing to note is that the researchers were able to measure PBDEs in the blood samples. They were in there. They were detectable. And they were variable. Dividing the 1100 participants into low, medium, and high PBDE tertiles, you can see a nearly 10-fold difference across the population.
Importantly, not many baseline variables correlated with PBDE levels. People in the highest group were a bit younger but had a fairly similar sex distribution, race, ethnicity, education, income, physical activity, smoking status, and body mass index.
This is not a randomized trial, of course — but at least based on these data, exposure levels do seem fairly random, which is what you would expect from an environmental toxin that percolates up through the food chain. They are often somewhat indiscriminate.
This similarity in baseline characteristics between people with low or high blood levels of PBDE also allows us to make some stronger inferences about the observed outcomes. Let’s take a look at them.
After adjustment for baseline factors, individuals in the highest PBDE group had a 43% higher rate of death from any cause over the follow-up period. This was not enough to achieve statistical significance, but it was close.
But the key finding is deaths due to cancer. After adjustment, cancer deaths occurred four times as frequently among those in the high PBDE group, and that is a statistically significant difference.
To be fair, cancer deaths were rare in this cohort. The vast majority of people did not die of anything during the follow-up period regardless of PBDE level. But the data are strongly suggestive of the carcinogenicity of these chemicals.
I should also point out that the researchers are linking the PBDE level at a single time point to all these future events. If PBDE levels remain relatively stable within an individual over time, that’s fine, but if they tend to vary with intake of different foods for example, this would not be captured and would actually lead to an underestimation of the cancer risk.
The researchers also didn’t have granular enough data to determine the type of cancer, but they do show that rates are similar between men and women, which might point away from the more sex-specific cancer etiologies. Clearly, some more work is needed.
Of course, I started this piece by telling you that these chemicals are already pretty much banned in the United States. What are we supposed to do about these findings? Studies have examined the primary ongoing sources of PBDE in our environment and it seems like most of our exposure will be coming from the food we eat due to that biomagnification thing: high-fat fish, meat and dairy products, and fish oil supplements. It may be worth some investigation into the relative adulteration of these products with this new old carcinogen.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.