User login
Congratulations to AGA’s New Leaders
Each January, the AGA Nominating Committee meets to complete a very important task — namely, selection of new members of AGA’s Governing Board, pending approval by the membership.
Having served on this committee in the past, I can attest to how challenging a task it is to select these leaders from such a talented and committed pool of candidates, each of whom have served the organization in numerous impactful roles over the course of many years.
This year’s recently announced additions to the Governing Board, who will assume their roles this summer, include Dr. Byron Cryer (incoming Vice President), Dr. Shahnaz Sultan (Clinical Research Councillor), and Dr. Jonathan Rosenberg (Practice Councillor). I have had the pleasure of working with each of them over the years from my very early days at AGA and am confident that AGA will continue to thrive under their leadership. Please join me in congratulating Byron, Shahnaz, and Jonathan on their new roles!
In this month’s issue of GIHN, we highlight a phase 3 RCT from NEJM demonstrating the efficacy of seladelpar, an alternative to ursodeoxycholic acid in patients with PBC with refractory pruritus. From the CGH Practice Management section, Dr. Michelle Kim (Cleveland Clinic) and colleagues provide helpful tips on how to optimize EHR use in GI practice, including by incorporating novel tools based on AI, natural language processing, and speech recognition. In our April Member Spotlight, we are excited to feature gastroenterologist and stand-up comedienne Dr. Shida Haghighat of UCLA, who shares her passion for addressing health disparities and highlights how humor helped her cope with the demands of medical training. We hope you enjoy these, and all the stories included in our April issue.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Each January, the AGA Nominating Committee meets to complete a very important task — namely, selection of new members of AGA’s Governing Board, pending approval by the membership.
Having served on this committee in the past, I can attest to how challenging a task it is to select these leaders from such a talented and committed pool of candidates, each of whom have served the organization in numerous impactful roles over the course of many years.
This year’s recently announced additions to the Governing Board, who will assume their roles this summer, include Dr. Byron Cryer (incoming Vice President), Dr. Shahnaz Sultan (Clinical Research Councillor), and Dr. Jonathan Rosenberg (Practice Councillor). I have had the pleasure of working with each of them over the years from my very early days at AGA and am confident that AGA will continue to thrive under their leadership. Please join me in congratulating Byron, Shahnaz, and Jonathan on their new roles!
In this month’s issue of GIHN, we highlight a phase 3 RCT from NEJM demonstrating the efficacy of seladelpar, an alternative to ursodeoxycholic acid in patients with PBC with refractory pruritus. From the CGH Practice Management section, Dr. Michelle Kim (Cleveland Clinic) and colleagues provide helpful tips on how to optimize EHR use in GI practice, including by incorporating novel tools based on AI, natural language processing, and speech recognition. In our April Member Spotlight, we are excited to feature gastroenterologist and stand-up comedienne Dr. Shida Haghighat of UCLA, who shares her passion for addressing health disparities and highlights how humor helped her cope with the demands of medical training. We hope you enjoy these, and all the stories included in our April issue.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Each January, the AGA Nominating Committee meets to complete a very important task — namely, selection of new members of AGA’s Governing Board, pending approval by the membership.
Having served on this committee in the past, I can attest to how challenging a task it is to select these leaders from such a talented and committed pool of candidates, each of whom have served the organization in numerous impactful roles over the course of many years.
This year’s recently announced additions to the Governing Board, who will assume their roles this summer, include Dr. Byron Cryer (incoming Vice President), Dr. Shahnaz Sultan (Clinical Research Councillor), and Dr. Jonathan Rosenberg (Practice Councillor). I have had the pleasure of working with each of them over the years from my very early days at AGA and am confident that AGA will continue to thrive under their leadership. Please join me in congratulating Byron, Shahnaz, and Jonathan on their new roles!
In this month’s issue of GIHN, we highlight a phase 3 RCT from NEJM demonstrating the efficacy of seladelpar, an alternative to ursodeoxycholic acid in patients with PBC with refractory pruritus. From the CGH Practice Management section, Dr. Michelle Kim (Cleveland Clinic) and colleagues provide helpful tips on how to optimize EHR use in GI practice, including by incorporating novel tools based on AI, natural language processing, and speech recognition. In our April Member Spotlight, we are excited to feature gastroenterologist and stand-up comedienne Dr. Shida Haghighat of UCLA, who shares her passion for addressing health disparities and highlights how humor helped her cope with the demands of medical training. We hope you enjoy these, and all the stories included in our April issue.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).
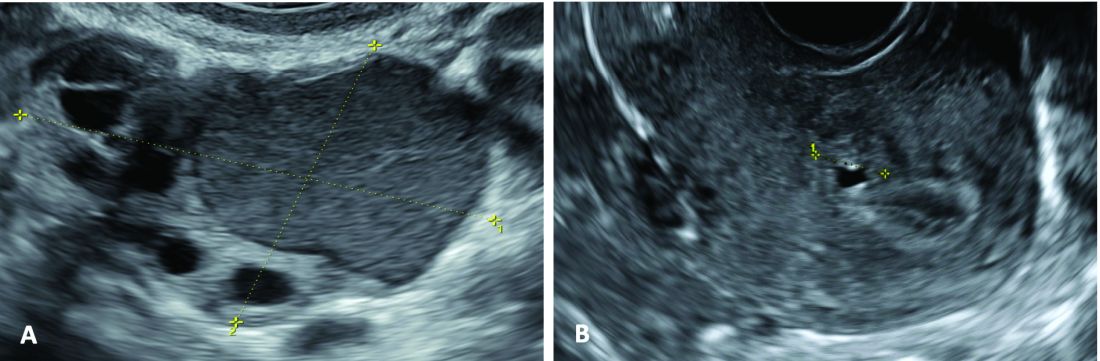
(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).
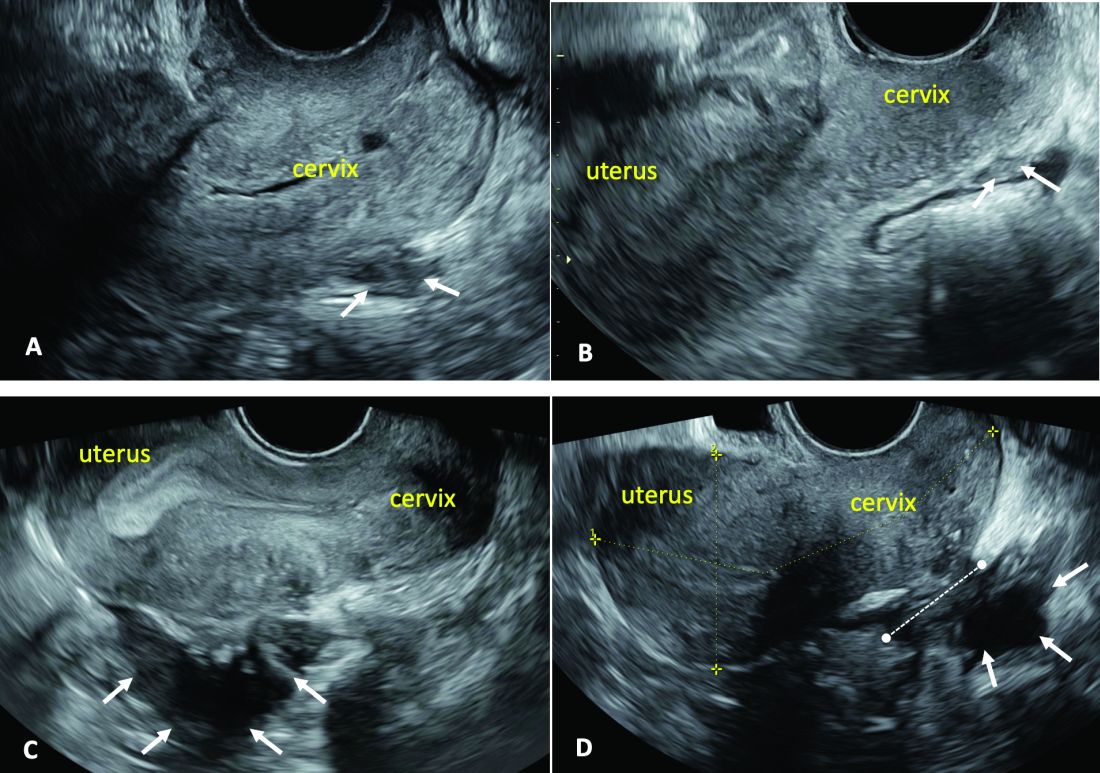
(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).

(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).

(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).

(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).

(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
AI and Suicide Prevention in Primary Care: A Q&A
Primary care physicians play a critical role in identifying patients at risk for serious mental health issues, including suicidality. But the ever-increasing demands on their clinical time can hinder the ability to identify emotional distress in time to intervene. Can artificial intelligence (AI) help?
This news organization spoke with Tom Zaubler, MD, a psychiatrist and chief medical officer of NeuroFlow, about how AI can improve the ability of primary care physicians and other clinicians to screen their patients for suicidal ideation and boost rates of treatment for mental health issues in their patients. This interview has been edited for clarity and length.
Question: How can AI help in suicide prevention and mental health screening in primary care?
Answer: Recent studies have demonstrated the potential of AI in mental health screening and suicide prevention. One method is natural language processing (NLP), which can analyze patients› journal entries for signs of suicidal thoughts or behaviors. This technology has shown promise in detecting suicidal ideation in patients who may not report such thoughts on traditional screening tools like the Patient Health Questionnaire-9 (PHQ-9). AI can be part of an integrated approach to identify and provide support to individuals at risk for suicide or those without a psychiatric history but who may still be at risk.
Q: A recent study by [Maria] Oquendo and colleagues found that one fifth of patients who attempt suicide do not meet the criteria for a mental health disorder.
Improved screening is obviously important, but in some ways it’s not the most important part of the problem. The lack of accessibility to specialized mental health care is a critical obstacle to treating patients with acute psychiatric needs.
How can primary care doctors effectively connect patients with mental health support, given the scarcity of mental health professionals?
A: Primary care doctors can leverage technology to extend mental health support. This includes using platforms for safety screening and providing patients with immediate access to local and national resources and digital interventions. Alerts can be sent to professionals within the practice or employed by technology companies to offer immediate support, including suicide safety planning and counseling. Users can hit a button to “Find a Therapist.” Also, if they acknowledge feelings of self-harm, these keywords are detected within the app by NLP. “Urgent alerts” are then sent to clinicians who are overseeing patient care. If someone is flagged, a social worker or member of a response services team intervenes and calls the person at risk to tailor care. These interventions do not always require a psychiatrist or masters-prepared clinician but can be effectively managed by trained paraprofessionals. These staff members can provide suicide safety planning and lethal-means-restriction counseling, and can assess the need for escalation of care.
Q: How is technology likely to manifest in physician practices in the near future to support mental health care?
A: Automated screening platforms for depression and anxiety, alerts for physicians when patients screen positively, and integration with collaborative care models are a few of the ways technology will become part of clinical practice. Additionally, advanced data analytics and predictive modeling using electronic health records and claims data will help identify high-risk patients. Technologies like voice recognition and machine learning can analyze patient journals and possibly, in the future, social media feeds to detect mental health issues. These technologies aim to extend and augment the capabilities of healthcare practices, improving the identification and management of patients at risk for mental health issues.
Q: Are these technologies as effective in pediatric populations, and are there any specific challenges?
A: Technologies for mental health screening and support are effective in pediatric populations, with certain age-specific considerations and legal restrictions on technology use. For adolescents and older children comfortable with technology, digital tools can significantly impact mental health care. For younger children, technology must facilitate information-gathering from various sources, including parents and teachers. Despite challenges, technology is crucial for early identification and intervention in pediatric mental health, potentially shortening the time to diagnosis and improving outcomes.
The statistics are horrifying. One third of adolescent girls have seriously thought about suicide over the past year; 13% attempt suicide. So there’s a need in the adolescent population and in the preadolescent population, too, because there’s an 8- to 10-year lag between onset of symptoms and diagnosis of mental illness. If we can shorten that lag, you see improved performance in schools; you see decreased truancy; you see greater economic achievement and so on. It makes such a profound difference. Not to mention it saves lives. So, yes, technology is critical in a pediatric population. It exists and it’s happening right now. There are challenges, but the goal can be met.
Q: A 2014 study found that 45% of people who completed suicide visited a primary care physician in the preceding month. And only 23% of people who attempt suicide have not seen a primary care physician within the past year. What does that say about the importance of screening at the primary care level?
A: The fact that a significant percentage of individuals who die by suicide have visited a primary care physician within a month or year prior to their death underscores the critical role of primary care in suicide prevention. This highlights the potential for primary care settings to identify and intervene with individuals at risk for suicide, making the case for the importance of integrating effective mental health screenings and support technologies in primary care practices.
Q: In other words, we’re not talking about a marginal benefit.
A: No, the potential benefit is huge. The United States Preventive Services Task Force did not endorse universal screening for suicide in its 2023 recommendations; they felt — and I accept that conclusion — there wasn›t enough evidence [at the time] to really support that recommendation. I think when you talk to a lot of suicide researchers, what you will hear is that providing suicide assessments as far upstream as possible is critical, especially when you start seeing more and more research showing that 20% of the population who die by suicide are not likely to have any psychiatric pathology at all. I believe the evidence base will soon support a recommendation for universal screening for adults. I believe it is especially important to screen for suicidal ideation in kids, given the high rates of suicide in this population.
Dr. Zaubler has disclosed the following relevant financial relationships: chief medical officer, NeuroFlow.
A version of this article appeared on Medscape.com.
Primary care physicians play a critical role in identifying patients at risk for serious mental health issues, including suicidality. But the ever-increasing demands on their clinical time can hinder the ability to identify emotional distress in time to intervene. Can artificial intelligence (AI) help?
This news organization spoke with Tom Zaubler, MD, a psychiatrist and chief medical officer of NeuroFlow, about how AI can improve the ability of primary care physicians and other clinicians to screen their patients for suicidal ideation and boost rates of treatment for mental health issues in their patients. This interview has been edited for clarity and length.
Question: How can AI help in suicide prevention and mental health screening in primary care?
Answer: Recent studies have demonstrated the potential of AI in mental health screening and suicide prevention. One method is natural language processing (NLP), which can analyze patients› journal entries for signs of suicidal thoughts or behaviors. This technology has shown promise in detecting suicidal ideation in patients who may not report such thoughts on traditional screening tools like the Patient Health Questionnaire-9 (PHQ-9). AI can be part of an integrated approach to identify and provide support to individuals at risk for suicide or those without a psychiatric history but who may still be at risk.
Q: A recent study by [Maria] Oquendo and colleagues found that one fifth of patients who attempt suicide do not meet the criteria for a mental health disorder.
Improved screening is obviously important, but in some ways it’s not the most important part of the problem. The lack of accessibility to specialized mental health care is a critical obstacle to treating patients with acute psychiatric needs.
How can primary care doctors effectively connect patients with mental health support, given the scarcity of mental health professionals?
A: Primary care doctors can leverage technology to extend mental health support. This includes using platforms for safety screening and providing patients with immediate access to local and national resources and digital interventions. Alerts can be sent to professionals within the practice or employed by technology companies to offer immediate support, including suicide safety planning and counseling. Users can hit a button to “Find a Therapist.” Also, if they acknowledge feelings of self-harm, these keywords are detected within the app by NLP. “Urgent alerts” are then sent to clinicians who are overseeing patient care. If someone is flagged, a social worker or member of a response services team intervenes and calls the person at risk to tailor care. These interventions do not always require a psychiatrist or masters-prepared clinician but can be effectively managed by trained paraprofessionals. These staff members can provide suicide safety planning and lethal-means-restriction counseling, and can assess the need for escalation of care.
Q: How is technology likely to manifest in physician practices in the near future to support mental health care?
A: Automated screening platforms for depression and anxiety, alerts for physicians when patients screen positively, and integration with collaborative care models are a few of the ways technology will become part of clinical practice. Additionally, advanced data analytics and predictive modeling using electronic health records and claims data will help identify high-risk patients. Technologies like voice recognition and machine learning can analyze patient journals and possibly, in the future, social media feeds to detect mental health issues. These technologies aim to extend and augment the capabilities of healthcare practices, improving the identification and management of patients at risk for mental health issues.
Q: Are these technologies as effective in pediatric populations, and are there any specific challenges?
A: Technologies for mental health screening and support are effective in pediatric populations, with certain age-specific considerations and legal restrictions on technology use. For adolescents and older children comfortable with technology, digital tools can significantly impact mental health care. For younger children, technology must facilitate information-gathering from various sources, including parents and teachers. Despite challenges, technology is crucial for early identification and intervention in pediatric mental health, potentially shortening the time to diagnosis and improving outcomes.
The statistics are horrifying. One third of adolescent girls have seriously thought about suicide over the past year; 13% attempt suicide. So there’s a need in the adolescent population and in the preadolescent population, too, because there’s an 8- to 10-year lag between onset of symptoms and diagnosis of mental illness. If we can shorten that lag, you see improved performance in schools; you see decreased truancy; you see greater economic achievement and so on. It makes such a profound difference. Not to mention it saves lives. So, yes, technology is critical in a pediatric population. It exists and it’s happening right now. There are challenges, but the goal can be met.
Q: A 2014 study found that 45% of people who completed suicide visited a primary care physician in the preceding month. And only 23% of people who attempt suicide have not seen a primary care physician within the past year. What does that say about the importance of screening at the primary care level?
A: The fact that a significant percentage of individuals who die by suicide have visited a primary care physician within a month or year prior to their death underscores the critical role of primary care in suicide prevention. This highlights the potential for primary care settings to identify and intervene with individuals at risk for suicide, making the case for the importance of integrating effective mental health screenings and support technologies in primary care practices.
Q: In other words, we’re not talking about a marginal benefit.
A: No, the potential benefit is huge. The United States Preventive Services Task Force did not endorse universal screening for suicide in its 2023 recommendations; they felt — and I accept that conclusion — there wasn›t enough evidence [at the time] to really support that recommendation. I think when you talk to a lot of suicide researchers, what you will hear is that providing suicide assessments as far upstream as possible is critical, especially when you start seeing more and more research showing that 20% of the population who die by suicide are not likely to have any psychiatric pathology at all. I believe the evidence base will soon support a recommendation for universal screening for adults. I believe it is especially important to screen for suicidal ideation in kids, given the high rates of suicide in this population.
Dr. Zaubler has disclosed the following relevant financial relationships: chief medical officer, NeuroFlow.
A version of this article appeared on Medscape.com.
Primary care physicians play a critical role in identifying patients at risk for serious mental health issues, including suicidality. But the ever-increasing demands on their clinical time can hinder the ability to identify emotional distress in time to intervene. Can artificial intelligence (AI) help?
This news organization spoke with Tom Zaubler, MD, a psychiatrist and chief medical officer of NeuroFlow, about how AI can improve the ability of primary care physicians and other clinicians to screen their patients for suicidal ideation and boost rates of treatment for mental health issues in their patients. This interview has been edited for clarity and length.
Question: How can AI help in suicide prevention and mental health screening in primary care?
Answer: Recent studies have demonstrated the potential of AI in mental health screening and suicide prevention. One method is natural language processing (NLP), which can analyze patients› journal entries for signs of suicidal thoughts or behaviors. This technology has shown promise in detecting suicidal ideation in patients who may not report such thoughts on traditional screening tools like the Patient Health Questionnaire-9 (PHQ-9). AI can be part of an integrated approach to identify and provide support to individuals at risk for suicide or those without a psychiatric history but who may still be at risk.
Q: A recent study by [Maria] Oquendo and colleagues found that one fifth of patients who attempt suicide do not meet the criteria for a mental health disorder.
Improved screening is obviously important, but in some ways it’s not the most important part of the problem. The lack of accessibility to specialized mental health care is a critical obstacle to treating patients with acute psychiatric needs.
How can primary care doctors effectively connect patients with mental health support, given the scarcity of mental health professionals?
A: Primary care doctors can leverage technology to extend mental health support. This includes using platforms for safety screening and providing patients with immediate access to local and national resources and digital interventions. Alerts can be sent to professionals within the practice or employed by technology companies to offer immediate support, including suicide safety planning and counseling. Users can hit a button to “Find a Therapist.” Also, if they acknowledge feelings of self-harm, these keywords are detected within the app by NLP. “Urgent alerts” are then sent to clinicians who are overseeing patient care. If someone is flagged, a social worker or member of a response services team intervenes and calls the person at risk to tailor care. These interventions do not always require a psychiatrist or masters-prepared clinician but can be effectively managed by trained paraprofessionals. These staff members can provide suicide safety planning and lethal-means-restriction counseling, and can assess the need for escalation of care.
Q: How is technology likely to manifest in physician practices in the near future to support mental health care?
A: Automated screening platforms for depression and anxiety, alerts for physicians when patients screen positively, and integration with collaborative care models are a few of the ways technology will become part of clinical practice. Additionally, advanced data analytics and predictive modeling using electronic health records and claims data will help identify high-risk patients. Technologies like voice recognition and machine learning can analyze patient journals and possibly, in the future, social media feeds to detect mental health issues. These technologies aim to extend and augment the capabilities of healthcare practices, improving the identification and management of patients at risk for mental health issues.
Q: Are these technologies as effective in pediatric populations, and are there any specific challenges?
A: Technologies for mental health screening and support are effective in pediatric populations, with certain age-specific considerations and legal restrictions on technology use. For adolescents and older children comfortable with technology, digital tools can significantly impact mental health care. For younger children, technology must facilitate information-gathering from various sources, including parents and teachers. Despite challenges, technology is crucial for early identification and intervention in pediatric mental health, potentially shortening the time to diagnosis and improving outcomes.
The statistics are horrifying. One third of adolescent girls have seriously thought about suicide over the past year; 13% attempt suicide. So there’s a need in the adolescent population and in the preadolescent population, too, because there’s an 8- to 10-year lag between onset of symptoms and diagnosis of mental illness. If we can shorten that lag, you see improved performance in schools; you see decreased truancy; you see greater economic achievement and so on. It makes such a profound difference. Not to mention it saves lives. So, yes, technology is critical in a pediatric population. It exists and it’s happening right now. There are challenges, but the goal can be met.
Q: A 2014 study found that 45% of people who completed suicide visited a primary care physician in the preceding month. And only 23% of people who attempt suicide have not seen a primary care physician within the past year. What does that say about the importance of screening at the primary care level?
A: The fact that a significant percentage of individuals who die by suicide have visited a primary care physician within a month or year prior to their death underscores the critical role of primary care in suicide prevention. This highlights the potential for primary care settings to identify and intervene with individuals at risk for suicide, making the case for the importance of integrating effective mental health screenings and support technologies in primary care practices.
Q: In other words, we’re not talking about a marginal benefit.
A: No, the potential benefit is huge. The United States Preventive Services Task Force did not endorse universal screening for suicide in its 2023 recommendations; they felt — and I accept that conclusion — there wasn›t enough evidence [at the time] to really support that recommendation. I think when you talk to a lot of suicide researchers, what you will hear is that providing suicide assessments as far upstream as possible is critical, especially when you start seeing more and more research showing that 20% of the population who die by suicide are not likely to have any psychiatric pathology at all. I believe the evidence base will soon support a recommendation for universal screening for adults. I believe it is especially important to screen for suicidal ideation in kids, given the high rates of suicide in this population.
Dr. Zaubler has disclosed the following relevant financial relationships: chief medical officer, NeuroFlow.
A version of this article appeared on Medscape.com.
Meditating in the Mundane
I don’t recommend ice baths. Perhaps I should. On my podcast-filled commute, I am reminded for miles of the mental and physical benefits of this revolutionary wellness routine: Cold exposure causes a spike in adrenaline and raises your baseline dopamine, thereby giving you superhuman focus and energy. Goodbye procrastination! Eliminate your ADHD in one icy step! I’m trying to be the fashionable mustached-columnist here so maybe I should get on board.
In fact, a heavyset, similarly-mustached 32-year-old patient just asked if I do ice baths. It was meant as a compliment, I believe. Displaying poise wearing my Chief of Dermatology embroidered white coat in my toddler-art-adorned office, I could hear him thinking: “This doc is legit. On fleek.” (Note, this is an approximation and the patient’s actual thoughts may have varied). We were talking podcasts and he was curious about my daily routine.
Now, ice baths probably do have the benefits that Andrew Huberman, Joe Rogan, and the others have described, I don’t argue. And the experience is oft described as invigorating with a runner’s high-like euphoria that follows a good dunk. I’ve tried it. I would describe it as “very uncomfortable.” To boot, following icy-cold morning showers, I wasn’t any better able to stave off opening my New York Times app on a newsy day. No, cold water isn’t my jams. But then again, I don’t journal like Marcus Aurelius or sleep on a mattress that keeps my body a chill 97 degrees like an inverse sous vide. If I were asked by Huberman in an interview what I do to be mentally strong, I’d answer, “I clean the pool.”
“Here’s how I do it, Dr. Huberman,” I’d say. “First, open the pool cover. Then with a cup with pool water from about 12 inches down, fill these little beakers with water and add a few drops of chemical reagents. Then calculate the ounces of calcium hypochlorite, muriatic acid, and other chemicals to make your pools sparkle. After skimming, take your pool brush and brush the bottom and sides of your pool. Rack your equipment when done and close the cover back up. This exercise takes about 15 minutes.” It’s a mundane task, but ah, there’s the point. Like folding the laundry, weeding the garden, emptying the dishwasher, they can be oh, so gratifying. Each of these has a crisp beginning and end and offer a lovely spot to be present. Let the thoughts flow with each stroke of the brush. Watch the water ripple the surface as you slowly pull the long pole out, dripping 7.4 pH water as you glide it in for the next pass. This is the Benabio secret to success.
I hope I’ve not disappointed you with this advice. Much as I’d like to think I’m on trend, I don’t believe self-improvement in the mundane will catch fire like taking magnesium or Wim Hof breathing. I wish it would. A distinction between gardening or pool cleaning or doing laundry and taking ice-baths is that the former aren’t just about you. I’ve got rows of spinach and Swiss chard that depend on me. My self-help is to water them. Feed them. Weed them. Because of me, they are growing deep green and beautiful. Although no one is swimming in our cool pool yet, they will soon. And the water will be sparkly clean, thanks to me. A stack of bright white towels is resting on our bathroom shelf waiting for someone to step out of the shower and need one. I did that.
Speaking of Huberman and the podcast gurus, Arnold Schwarzenegger is making the rounds lately hawking his book, “Be Useful.” It has the usual common sense ideas as most self-help books for the last 100 years.That’s the advice I passed along to my hirsute coming-of-manhood patient. I don’t do ice-baths, but each day I drop in deep on taking care of my patients, providing for my family, refilling the bird feeder in our yard. Why the heck would I sit in a currently 63-degree hot tub when I could be cleaning it? Then everyone is just a little better off, not just me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
I don’t recommend ice baths. Perhaps I should. On my podcast-filled commute, I am reminded for miles of the mental and physical benefits of this revolutionary wellness routine: Cold exposure causes a spike in adrenaline and raises your baseline dopamine, thereby giving you superhuman focus and energy. Goodbye procrastination! Eliminate your ADHD in one icy step! I’m trying to be the fashionable mustached-columnist here so maybe I should get on board.
In fact, a heavyset, similarly-mustached 32-year-old patient just asked if I do ice baths. It was meant as a compliment, I believe. Displaying poise wearing my Chief of Dermatology embroidered white coat in my toddler-art-adorned office, I could hear him thinking: “This doc is legit. On fleek.” (Note, this is an approximation and the patient’s actual thoughts may have varied). We were talking podcasts and he was curious about my daily routine.
Now, ice baths probably do have the benefits that Andrew Huberman, Joe Rogan, and the others have described, I don’t argue. And the experience is oft described as invigorating with a runner’s high-like euphoria that follows a good dunk. I’ve tried it. I would describe it as “very uncomfortable.” To boot, following icy-cold morning showers, I wasn’t any better able to stave off opening my New York Times app on a newsy day. No, cold water isn’t my jams. But then again, I don’t journal like Marcus Aurelius or sleep on a mattress that keeps my body a chill 97 degrees like an inverse sous vide. If I were asked by Huberman in an interview what I do to be mentally strong, I’d answer, “I clean the pool.”
“Here’s how I do it, Dr. Huberman,” I’d say. “First, open the pool cover. Then with a cup with pool water from about 12 inches down, fill these little beakers with water and add a few drops of chemical reagents. Then calculate the ounces of calcium hypochlorite, muriatic acid, and other chemicals to make your pools sparkle. After skimming, take your pool brush and brush the bottom and sides of your pool. Rack your equipment when done and close the cover back up. This exercise takes about 15 minutes.” It’s a mundane task, but ah, there’s the point. Like folding the laundry, weeding the garden, emptying the dishwasher, they can be oh, so gratifying. Each of these has a crisp beginning and end and offer a lovely spot to be present. Let the thoughts flow with each stroke of the brush. Watch the water ripple the surface as you slowly pull the long pole out, dripping 7.4 pH water as you glide it in for the next pass. This is the Benabio secret to success.
I hope I’ve not disappointed you with this advice. Much as I’d like to think I’m on trend, I don’t believe self-improvement in the mundane will catch fire like taking magnesium or Wim Hof breathing. I wish it would. A distinction between gardening or pool cleaning or doing laundry and taking ice-baths is that the former aren’t just about you. I’ve got rows of spinach and Swiss chard that depend on me. My self-help is to water them. Feed them. Weed them. Because of me, they are growing deep green and beautiful. Although no one is swimming in our cool pool yet, they will soon. And the water will be sparkly clean, thanks to me. A stack of bright white towels is resting on our bathroom shelf waiting for someone to step out of the shower and need one. I did that.
Speaking of Huberman and the podcast gurus, Arnold Schwarzenegger is making the rounds lately hawking his book, “Be Useful.” It has the usual common sense ideas as most self-help books for the last 100 years.That’s the advice I passed along to my hirsute coming-of-manhood patient. I don’t do ice-baths, but each day I drop in deep on taking care of my patients, providing for my family, refilling the bird feeder in our yard. Why the heck would I sit in a currently 63-degree hot tub when I could be cleaning it? Then everyone is just a little better off, not just me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
I don’t recommend ice baths. Perhaps I should. On my podcast-filled commute, I am reminded for miles of the mental and physical benefits of this revolutionary wellness routine: Cold exposure causes a spike in adrenaline and raises your baseline dopamine, thereby giving you superhuman focus and energy. Goodbye procrastination! Eliminate your ADHD in one icy step! I’m trying to be the fashionable mustached-columnist here so maybe I should get on board.
In fact, a heavyset, similarly-mustached 32-year-old patient just asked if I do ice baths. It was meant as a compliment, I believe. Displaying poise wearing my Chief of Dermatology embroidered white coat in my toddler-art-adorned office, I could hear him thinking: “This doc is legit. On fleek.” (Note, this is an approximation and the patient’s actual thoughts may have varied). We were talking podcasts and he was curious about my daily routine.
Now, ice baths probably do have the benefits that Andrew Huberman, Joe Rogan, and the others have described, I don’t argue. And the experience is oft described as invigorating with a runner’s high-like euphoria that follows a good dunk. I’ve tried it. I would describe it as “very uncomfortable.” To boot, following icy-cold morning showers, I wasn’t any better able to stave off opening my New York Times app on a newsy day. No, cold water isn’t my jams. But then again, I don’t journal like Marcus Aurelius or sleep on a mattress that keeps my body a chill 97 degrees like an inverse sous vide. If I were asked by Huberman in an interview what I do to be mentally strong, I’d answer, “I clean the pool.”
“Here’s how I do it, Dr. Huberman,” I’d say. “First, open the pool cover. Then with a cup with pool water from about 12 inches down, fill these little beakers with water and add a few drops of chemical reagents. Then calculate the ounces of calcium hypochlorite, muriatic acid, and other chemicals to make your pools sparkle. After skimming, take your pool brush and brush the bottom and sides of your pool. Rack your equipment when done and close the cover back up. This exercise takes about 15 minutes.” It’s a mundane task, but ah, there’s the point. Like folding the laundry, weeding the garden, emptying the dishwasher, they can be oh, so gratifying. Each of these has a crisp beginning and end and offer a lovely spot to be present. Let the thoughts flow with each stroke of the brush. Watch the water ripple the surface as you slowly pull the long pole out, dripping 7.4 pH water as you glide it in for the next pass. This is the Benabio secret to success.
I hope I’ve not disappointed you with this advice. Much as I’d like to think I’m on trend, I don’t believe self-improvement in the mundane will catch fire like taking magnesium or Wim Hof breathing. I wish it would. A distinction between gardening or pool cleaning or doing laundry and taking ice-baths is that the former aren’t just about you. I’ve got rows of spinach and Swiss chard that depend on me. My self-help is to water them. Feed them. Weed them. Because of me, they are growing deep green and beautiful. Although no one is swimming in our cool pool yet, they will soon. And the water will be sparkly clean, thanks to me. A stack of bright white towels is resting on our bathroom shelf waiting for someone to step out of the shower and need one. I did that.
Speaking of Huberman and the podcast gurus, Arnold Schwarzenegger is making the rounds lately hawking his book, “Be Useful.” It has the usual common sense ideas as most self-help books for the last 100 years.That’s the advice I passed along to my hirsute coming-of-manhood patient. I don’t do ice-baths, but each day I drop in deep on taking care of my patients, providing for my family, refilling the bird feeder in our yard. Why the heck would I sit in a currently 63-degree hot tub when I could be cleaning it? Then everyone is just a little better off, not just me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
You Can’t Spell ‘Medicine’ Without D, E, and I
Please note that this is a commentary, an opinion piece: my opinion. The statements here do not necessarily represent those of this news organization or any of the myriad people or institutions that comprise this corner of the human universe.
Some days, speaking as a long-time physician and editor, I wish that there were no such things as race or ethnicity or even geographic origin for that matter. We can’t get away from sex, gender, disability, age, or culture. I’m not sure about religion. I wish people were just people.
But race is deeply embedded in the American experience — an almost invisible but inevitable presence in all of our thoughts and expressions about human activities.
In medical education (for eons it seems) the student has been taught to mention race in the first sentence of a given patient presentation, along with age and sex. In human epidemiologic research, race is almost always a studied variable. In clinical and basic medical research, looking at the impact of race on this, that, or the other is commonplace. “Mixed race not otherwise specified” is ubiquitous in the United States yet blithely ignored by most who tally these statistics. Race is rarely gene-specific. It is more of a social and cultural construct but with plainly visible overt phenotypic markers — an almost infinite mix of daily reality.
Our country, and much of Western civilization in 2024, is based on the principle that all men are created equal, although the originators of that notion were unaware of their own “equity-challenged” situation.
Many organizations, in and out of government, are now understanding, developing, and implementing programs (and thought/language patterns) to socialize diversity, equity, and inclusion (known as DEI) into their culture. It should not be surprising that many who prefer the status quo are not happy with the pressure from this movement and are using whatever methods are available to them to prevent full DEI. Such it always is.
The trusty Copilot from Bing provides these definitions:
- Diversity refers to the presence of variety within the organizational workforce. This includes aspects such as gender, culture, ethnicity, religion, disability, age, and opinion.
- Equity encompasses concepts of fairness and justice. It involves fair compensation, substantive equality, and addressing societal disparities. Equity also considers unique circumstances and adjusts treatment to achieve equal outcomes.
- Inclusion focuses on creating an organizational culture where all employees feel heard, fostering a sense of belonging and integration.
I am more than proud that my old domain of peer-reviewed, primary source, medical (and science) journals is taking a leading role in this noble, necessary, and long overdue movement for medicine.
As the central repository and transmitter of new medical information, including scientific studies, clinical medicine reports, ethics measures, and education, medical journals (including those deemed prestigious) have historically been among the worst offenders in perpetuating non-DEI objectives in their leadership, staffing, focus, instructions for authors, style manuals, and published materials.
This issue came to a head in March 2021 when a JAMA podcast about racism in American medicine was followed by this promotional tweet: “No physician is racist, so how can there be structural racism in health care?”
Reactions and actions were rapid, strong, and decisive. After an interregnum at JAMA, a new editor in chief, Kirsten Bibbins-Domingo, PhD, MD, MAS, was named. She and her large staff of editors and editorial board members from the multijournal JAMA Network joined a worldwide movement of (currently) 56 publishing organizations representing 15,000 journals called the Joint Commitment for Action on Inclusion and Diversity in Publishing.
A recent JAMA editorial with 29 authors describes the entire commitment initiative of publishers-editors. It reports JAMA Network data from 2023 and 2024 from surveys of 455 editors (a 91% response rate) about their own gender (five choices), ethnic origins or geographic ancestry (13 choices), and race (eight choices), demonstrating considerable progress toward DEI goals. The survey’s complex multinational classifications may not jibe with the categorizations used in some countries (too bad that “mixed” is not “mixed in” — a missed opportunity).
This encouraging movement will not fix it all. But when people of certain groups are represented at the table, that point of view is far more likely to make it into the lexicon, language, and omnipresent work products, potentially changing cultural norms. Even the measurement of movement related to disparity in healthcare is marred by frequent variations of data accuracy. More consistency in what to measure can help a lot, and the medical literature can be very influential.
A personal anecdote: When I was a professor at UC Davis in 1978, Allan Bakke, MD, was my student. Some of you will remember the saga of affirmative action on admissions, which was just revisited in the light of a recent decision by the US Supreme Court.
Back in 1978, the dean at UC Davis told me that he kept two file folders on the admission processes in different desk drawers. One categorized all applicants and enrollees by race, and the other did not. Depending on who came to visit and ask questions, he would choose one or the other file to share once he figured out what they were looking for (this is not a joke).
The strength of the current active political pushback against the entire DEI movement has deep roots and should not be underestimated. There will be a lot of to-ing and fro-ing.
French writer Victor Hugo is credited with stating, “There is nothing as powerful as an idea whose time has come.” A majority of Americans, physicians, and other healthcare professionals believe in basic fairness. The time for DEI in all aspects of medicine is now.
Dr. Lundberg, editor in chief of Cancer Commons, disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Please note that this is a commentary, an opinion piece: my opinion. The statements here do not necessarily represent those of this news organization or any of the myriad people or institutions that comprise this corner of the human universe.
Some days, speaking as a long-time physician and editor, I wish that there were no such things as race or ethnicity or even geographic origin for that matter. We can’t get away from sex, gender, disability, age, or culture. I’m not sure about religion. I wish people were just people.
But race is deeply embedded in the American experience — an almost invisible but inevitable presence in all of our thoughts and expressions about human activities.
In medical education (for eons it seems) the student has been taught to mention race in the first sentence of a given patient presentation, along with age and sex. In human epidemiologic research, race is almost always a studied variable. In clinical and basic medical research, looking at the impact of race on this, that, or the other is commonplace. “Mixed race not otherwise specified” is ubiquitous in the United States yet blithely ignored by most who tally these statistics. Race is rarely gene-specific. It is more of a social and cultural construct but with plainly visible overt phenotypic markers — an almost infinite mix of daily reality.
Our country, and much of Western civilization in 2024, is based on the principle that all men are created equal, although the originators of that notion were unaware of their own “equity-challenged” situation.
Many organizations, in and out of government, are now understanding, developing, and implementing programs (and thought/language patterns) to socialize diversity, equity, and inclusion (known as DEI) into their culture. It should not be surprising that many who prefer the status quo are not happy with the pressure from this movement and are using whatever methods are available to them to prevent full DEI. Such it always is.
The trusty Copilot from Bing provides these definitions:
- Diversity refers to the presence of variety within the organizational workforce. This includes aspects such as gender, culture, ethnicity, religion, disability, age, and opinion.
- Equity encompasses concepts of fairness and justice. It involves fair compensation, substantive equality, and addressing societal disparities. Equity also considers unique circumstances and adjusts treatment to achieve equal outcomes.
- Inclusion focuses on creating an organizational culture where all employees feel heard, fostering a sense of belonging and integration.
I am more than proud that my old domain of peer-reviewed, primary source, medical (and science) journals is taking a leading role in this noble, necessary, and long overdue movement for medicine.
As the central repository and transmitter of new medical information, including scientific studies, clinical medicine reports, ethics measures, and education, medical journals (including those deemed prestigious) have historically been among the worst offenders in perpetuating non-DEI objectives in their leadership, staffing, focus, instructions for authors, style manuals, and published materials.
This issue came to a head in March 2021 when a JAMA podcast about racism in American medicine was followed by this promotional tweet: “No physician is racist, so how can there be structural racism in health care?”
Reactions and actions were rapid, strong, and decisive. After an interregnum at JAMA, a new editor in chief, Kirsten Bibbins-Domingo, PhD, MD, MAS, was named. She and her large staff of editors and editorial board members from the multijournal JAMA Network joined a worldwide movement of (currently) 56 publishing organizations representing 15,000 journals called the Joint Commitment for Action on Inclusion and Diversity in Publishing.
A recent JAMA editorial with 29 authors describes the entire commitment initiative of publishers-editors. It reports JAMA Network data from 2023 and 2024 from surveys of 455 editors (a 91% response rate) about their own gender (five choices), ethnic origins or geographic ancestry (13 choices), and race (eight choices), demonstrating considerable progress toward DEI goals. The survey’s complex multinational classifications may not jibe with the categorizations used in some countries (too bad that “mixed” is not “mixed in” — a missed opportunity).
This encouraging movement will not fix it all. But when people of certain groups are represented at the table, that point of view is far more likely to make it into the lexicon, language, and omnipresent work products, potentially changing cultural norms. Even the measurement of movement related to disparity in healthcare is marred by frequent variations of data accuracy. More consistency in what to measure can help a lot, and the medical literature can be very influential.
A personal anecdote: When I was a professor at UC Davis in 1978, Allan Bakke, MD, was my student. Some of you will remember the saga of affirmative action on admissions, which was just revisited in the light of a recent decision by the US Supreme Court.
Back in 1978, the dean at UC Davis told me that he kept two file folders on the admission processes in different desk drawers. One categorized all applicants and enrollees by race, and the other did not. Depending on who came to visit and ask questions, he would choose one or the other file to share once he figured out what they were looking for (this is not a joke).
The strength of the current active political pushback against the entire DEI movement has deep roots and should not be underestimated. There will be a lot of to-ing and fro-ing.
French writer Victor Hugo is credited with stating, “There is nothing as powerful as an idea whose time has come.” A majority of Americans, physicians, and other healthcare professionals believe in basic fairness. The time for DEI in all aspects of medicine is now.
Dr. Lundberg, editor in chief of Cancer Commons, disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Please note that this is a commentary, an opinion piece: my opinion. The statements here do not necessarily represent those of this news organization or any of the myriad people or institutions that comprise this corner of the human universe.
Some days, speaking as a long-time physician and editor, I wish that there were no such things as race or ethnicity or even geographic origin for that matter. We can’t get away from sex, gender, disability, age, or culture. I’m not sure about religion. I wish people were just people.
But race is deeply embedded in the American experience — an almost invisible but inevitable presence in all of our thoughts and expressions about human activities.
In medical education (for eons it seems) the student has been taught to mention race in the first sentence of a given patient presentation, along with age and sex. In human epidemiologic research, race is almost always a studied variable. In clinical and basic medical research, looking at the impact of race on this, that, or the other is commonplace. “Mixed race not otherwise specified” is ubiquitous in the United States yet blithely ignored by most who tally these statistics. Race is rarely gene-specific. It is more of a social and cultural construct but with plainly visible overt phenotypic markers — an almost infinite mix of daily reality.
Our country, and much of Western civilization in 2024, is based on the principle that all men are created equal, although the originators of that notion were unaware of their own “equity-challenged” situation.
Many organizations, in and out of government, are now understanding, developing, and implementing programs (and thought/language patterns) to socialize diversity, equity, and inclusion (known as DEI) into their culture. It should not be surprising that many who prefer the status quo are not happy with the pressure from this movement and are using whatever methods are available to them to prevent full DEI. Such it always is.
The trusty Copilot from Bing provides these definitions:
- Diversity refers to the presence of variety within the organizational workforce. This includes aspects such as gender, culture, ethnicity, religion, disability, age, and opinion.
- Equity encompasses concepts of fairness and justice. It involves fair compensation, substantive equality, and addressing societal disparities. Equity also considers unique circumstances and adjusts treatment to achieve equal outcomes.
- Inclusion focuses on creating an organizational culture where all employees feel heard, fostering a sense of belonging and integration.
I am more than proud that my old domain of peer-reviewed, primary source, medical (and science) journals is taking a leading role in this noble, necessary, and long overdue movement for medicine.
As the central repository and transmitter of new medical information, including scientific studies, clinical medicine reports, ethics measures, and education, medical journals (including those deemed prestigious) have historically been among the worst offenders in perpetuating non-DEI objectives in their leadership, staffing, focus, instructions for authors, style manuals, and published materials.
This issue came to a head in March 2021 when a JAMA podcast about racism in American medicine was followed by this promotional tweet: “No physician is racist, so how can there be structural racism in health care?”
Reactions and actions were rapid, strong, and decisive. After an interregnum at JAMA, a new editor in chief, Kirsten Bibbins-Domingo, PhD, MD, MAS, was named. She and her large staff of editors and editorial board members from the multijournal JAMA Network joined a worldwide movement of (currently) 56 publishing organizations representing 15,000 journals called the Joint Commitment for Action on Inclusion and Diversity in Publishing.
A recent JAMA editorial with 29 authors describes the entire commitment initiative of publishers-editors. It reports JAMA Network data from 2023 and 2024 from surveys of 455 editors (a 91% response rate) about their own gender (five choices), ethnic origins or geographic ancestry (13 choices), and race (eight choices), demonstrating considerable progress toward DEI goals. The survey’s complex multinational classifications may not jibe with the categorizations used in some countries (too bad that “mixed” is not “mixed in” — a missed opportunity).
This encouraging movement will not fix it all. But when people of certain groups are represented at the table, that point of view is far more likely to make it into the lexicon, language, and omnipresent work products, potentially changing cultural norms. Even the measurement of movement related to disparity in healthcare is marred by frequent variations of data accuracy. More consistency in what to measure can help a lot, and the medical literature can be very influential.
A personal anecdote: When I was a professor at UC Davis in 1978, Allan Bakke, MD, was my student. Some of you will remember the saga of affirmative action on admissions, which was just revisited in the light of a recent decision by the US Supreme Court.
Back in 1978, the dean at UC Davis told me that he kept two file folders on the admission processes in different desk drawers. One categorized all applicants and enrollees by race, and the other did not. Depending on who came to visit and ask questions, he would choose one or the other file to share once he figured out what they were looking for (this is not a joke).
The strength of the current active political pushback against the entire DEI movement has deep roots and should not be underestimated. There will be a lot of to-ing and fro-ing.
French writer Victor Hugo is credited with stating, “There is nothing as powerful as an idea whose time has come.” A majority of Americans, physicians, and other healthcare professionals believe in basic fairness. The time for DEI in all aspects of medicine is now.
Dr. Lundberg, editor in chief of Cancer Commons, disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Diarrhea in Cancer Therapies — Part 1: Chemotherapeutics
Patients with cancer receiving chemotherapeutics may develop diarrhea, which can be highly distressing. In a recent journal article, oncologist Marcus Hentrich, MD, and gastroenterologist Volker Penndorf, MD, PhD, both of Rotkreuzklinikum in Munich, Germany, explained how affected patients should be treated.
As Dr. Hentrich and Dr. Penndorf explained, classical According to the authors, the risk for toxic mucosal damage (toxic enteritis) increases with the severity and duration of neutropenia. Up to two thirds of patients with neutropenia develop diarrhea, and an infectious cause is rarely identified.
The cytostatic drug irinotecan, which can lead to an acute cholinergic syndrome within 24 hours, is a special case. This syndrome is characterized by watery diarrhea, abdominal cramps, vomiting, sweating, and bradycardia. Additionally, the development of late-onset diarrhea, occurring approximately 3 days after administration, is frequent.
According to the authors, risk factors for toxic enteritis with diarrhea include advanced age, poor performance and nutritional status, simultaneous radiotherapy of the abdomen and pelvis, and preexisting intestinal conditions.
Medication prophylaxis for chemotherapy-induced diarrhea has not been established. An exception is atropine for prophylaxis and treatment of irinotecan-induced cholinergic syndrome.
Indications for diagnostic procedures are outlined in the current German guideline for supportive therapy in patients with cancer.
For diarrhea accompanied by fever, blood cultures are mandatory. A complete blood count provides information on various aspects (leukocytosis as an inflammatory reaction, neutropenia as a marker for infection risk, hemoglobin as a marker for possible hemoconcentration or existing bleeding, and thrombocytopenia as a marker for bleeding tendency). Disproportionate thrombocytopenia may warrant assessment of fragmented cells and enterohemorrhagic Escherichia coli diagnostics.
To assess electrolyte and fluid loss, electrolytes, albumin, and total protein should be measured. The C-reactive protein value may help identify inflammatory conditions. It may also be elevated, however, because of tumor-related factors. Measuring urea and creatinine allows for estimating whether there is already a prerenal impairment of kidney function. Liver function parameters are mandatory for critically ill patients. In patients with hypotension or tachycardia, blood gas analysis and lactate determination are advisable. Among imaging techniques, ultrasound may be helpful. Indications for conventional abdominal x-ray are rare. In the presence of clinical signs of peritoneal irritation (such as guarding and rebound tenderness), a CT scan should be considered to detect further complications (perforation, ileus, enterocolitis, etc.) promptly.
Endoscopic examinations are recommended only in cases of persistent, worsening symptoms, according to the guideline. Colonoscopy is contraindicated in suspected neutropenic enterocolitis (NEC) because of the risk for perforation.
According to Dr. Hentrich and Dr. Penndorf, diarrhea therapy is carried out in stages and depends on the severity and response to each therapy. The Common Terminology Criteria for Adverse Events distinguishes the following severity grades:
- Grade 1: < four stools per day above baseline
- Grade 2: Four to six stools per day above baseline
- Grade 3: ≥ seven stools per day above baseline; fecal incontinence, hospitalization indicated; limited activities of daily living
- Grade 4: Life-threatening consequences, urgent intervention indicated
Therapy for Grades 1-2
The standard therapeutic after excluding infectious causes is loperamide (initially 4 mg orally, followed by 2 mg every 2-4 hours). A daily dose should not exceed 16 mg.
For irinotecan-associated diarrhea, adjunctive administration of budesonide (3 mg orally three times per day) with loperamide was shown to be effective in a small, randomized study (off-label). Another randomized study demonstrated the efficacy of combining loperamide with racecadotril (100 mg orally three times per day for 48 hours).
Therapy for Grade 3 Diarrhea
In severe diarrhea persisting despite loperamide therapy for 24-48 hours, octreotide (100-150 μg subcutaneously three times per day) may be administered (maximum three times 500 μg). Although octreotide is often used successfully for chemotherapy-induced diarrhea, it is not approved for this indication (off-label use).
According to the authors, other therapy options for loperamide-refractory diarrhea include codeine, tincture of opium, budesonide, and racecadotril. Psyllium husk or diphenoxylate plus atropine may also be attempted. In patients with prolonged neutropenia, overdosing of motility inhibitors should be avoided because of the risk for ileus.
The use of probiotics for chemotherapy-induced diarrhea cannot be generally recommended because of insufficient evidence, and cases of probiotic-associated bacteremia and fungemia have been described.
A particularly serious complication of intensive chemotherapy associated with diarrhea is NEC. It is characterized by fever, abdominal pain, and diarrhea during severe neutropenia (neutrophil count < 500/μL), the authors explained. NEC occurs predominantly, but not exclusively, after intensive chemotherapy for hematologic malignancies, especially acute leukemias.
More common than NEC and often preceding it is the so-called chemotherapy-associated bowel syndrome. It is characterized by fever ≥ 37.8 °C and abdominal pain or absence of stool for at least 72 hours.
Therapy consists of conservative symptomatic measures such as diet, adequate hydration with electrolyte balance, and analgesia. Due to the high risk for bacteremia, antibiotic therapy is indicated after blood cultures are obtained (piperacillin-tazobactam or a carbapenem). According to the authors, NEC improves in most patients with neutrophil regeneration. Granulocyte colony-stimulating factor therapy appears reasonable in this context, although conclusive studies are lacking. Surgical intervention with removal of necrotic bowel segments may be considered in exceptional cases.
This story was translated from Univadis Germany, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Patients with cancer receiving chemotherapeutics may develop diarrhea, which can be highly distressing. In a recent journal article, oncologist Marcus Hentrich, MD, and gastroenterologist Volker Penndorf, MD, PhD, both of Rotkreuzklinikum in Munich, Germany, explained how affected patients should be treated.
As Dr. Hentrich and Dr. Penndorf explained, classical According to the authors, the risk for toxic mucosal damage (toxic enteritis) increases with the severity and duration of neutropenia. Up to two thirds of patients with neutropenia develop diarrhea, and an infectious cause is rarely identified.
The cytostatic drug irinotecan, which can lead to an acute cholinergic syndrome within 24 hours, is a special case. This syndrome is characterized by watery diarrhea, abdominal cramps, vomiting, sweating, and bradycardia. Additionally, the development of late-onset diarrhea, occurring approximately 3 days after administration, is frequent.
According to the authors, risk factors for toxic enteritis with diarrhea include advanced age, poor performance and nutritional status, simultaneous radiotherapy of the abdomen and pelvis, and preexisting intestinal conditions.
Medication prophylaxis for chemotherapy-induced diarrhea has not been established. An exception is atropine for prophylaxis and treatment of irinotecan-induced cholinergic syndrome.
Indications for diagnostic procedures are outlined in the current German guideline for supportive therapy in patients with cancer.
For diarrhea accompanied by fever, blood cultures are mandatory. A complete blood count provides information on various aspects (leukocytosis as an inflammatory reaction, neutropenia as a marker for infection risk, hemoglobin as a marker for possible hemoconcentration or existing bleeding, and thrombocytopenia as a marker for bleeding tendency). Disproportionate thrombocytopenia may warrant assessment of fragmented cells and enterohemorrhagic Escherichia coli diagnostics.
To assess electrolyte and fluid loss, electrolytes, albumin, and total protein should be measured. The C-reactive protein value may help identify inflammatory conditions. It may also be elevated, however, because of tumor-related factors. Measuring urea and creatinine allows for estimating whether there is already a prerenal impairment of kidney function. Liver function parameters are mandatory for critically ill patients. In patients with hypotension or tachycardia, blood gas analysis and lactate determination are advisable. Among imaging techniques, ultrasound may be helpful. Indications for conventional abdominal x-ray are rare. In the presence of clinical signs of peritoneal irritation (such as guarding and rebound tenderness), a CT scan should be considered to detect further complications (perforation, ileus, enterocolitis, etc.) promptly.
Endoscopic examinations are recommended only in cases of persistent, worsening symptoms, according to the guideline. Colonoscopy is contraindicated in suspected neutropenic enterocolitis (NEC) because of the risk for perforation.
According to Dr. Hentrich and Dr. Penndorf, diarrhea therapy is carried out in stages and depends on the severity and response to each therapy. The Common Terminology Criteria for Adverse Events distinguishes the following severity grades:
- Grade 1: < four stools per day above baseline
- Grade 2: Four to six stools per day above baseline
- Grade 3: ≥ seven stools per day above baseline; fecal incontinence, hospitalization indicated; limited activities of daily living
- Grade 4: Life-threatening consequences, urgent intervention indicated
Therapy for Grades 1-2
The standard therapeutic after excluding infectious causes is loperamide (initially 4 mg orally, followed by 2 mg every 2-4 hours). A daily dose should not exceed 16 mg.
For irinotecan-associated diarrhea, adjunctive administration of budesonide (3 mg orally three times per day) with loperamide was shown to be effective in a small, randomized study (off-label). Another randomized study demonstrated the efficacy of combining loperamide with racecadotril (100 mg orally three times per day for 48 hours).
Therapy for Grade 3 Diarrhea
In severe diarrhea persisting despite loperamide therapy for 24-48 hours, octreotide (100-150 μg subcutaneously three times per day) may be administered (maximum three times 500 μg). Although octreotide is often used successfully for chemotherapy-induced diarrhea, it is not approved for this indication (off-label use).
According to the authors, other therapy options for loperamide-refractory diarrhea include codeine, tincture of opium, budesonide, and racecadotril. Psyllium husk or diphenoxylate plus atropine may also be attempted. In patients with prolonged neutropenia, overdosing of motility inhibitors should be avoided because of the risk for ileus.
The use of probiotics for chemotherapy-induced diarrhea cannot be generally recommended because of insufficient evidence, and cases of probiotic-associated bacteremia and fungemia have been described.
A particularly serious complication of intensive chemotherapy associated with diarrhea is NEC. It is characterized by fever, abdominal pain, and diarrhea during severe neutropenia (neutrophil count < 500/μL), the authors explained. NEC occurs predominantly, but not exclusively, after intensive chemotherapy for hematologic malignancies, especially acute leukemias.
More common than NEC and often preceding it is the so-called chemotherapy-associated bowel syndrome. It is characterized by fever ≥ 37.8 °C and abdominal pain or absence of stool for at least 72 hours.
Therapy consists of conservative symptomatic measures such as diet, adequate hydration with electrolyte balance, and analgesia. Due to the high risk for bacteremia, antibiotic therapy is indicated after blood cultures are obtained (piperacillin-tazobactam or a carbapenem). According to the authors, NEC improves in most patients with neutrophil regeneration. Granulocyte colony-stimulating factor therapy appears reasonable in this context, although conclusive studies are lacking. Surgical intervention with removal of necrotic bowel segments may be considered in exceptional cases.
This story was translated from Univadis Germany, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Patients with cancer receiving chemotherapeutics may develop diarrhea, which can be highly distressing. In a recent journal article, oncologist Marcus Hentrich, MD, and gastroenterologist Volker Penndorf, MD, PhD, both of Rotkreuzklinikum in Munich, Germany, explained how affected patients should be treated.
As Dr. Hentrich and Dr. Penndorf explained, classical According to the authors, the risk for toxic mucosal damage (toxic enteritis) increases with the severity and duration of neutropenia. Up to two thirds of patients with neutropenia develop diarrhea, and an infectious cause is rarely identified.
The cytostatic drug irinotecan, which can lead to an acute cholinergic syndrome within 24 hours, is a special case. This syndrome is characterized by watery diarrhea, abdominal cramps, vomiting, sweating, and bradycardia. Additionally, the development of late-onset diarrhea, occurring approximately 3 days after administration, is frequent.
According to the authors, risk factors for toxic enteritis with diarrhea include advanced age, poor performance and nutritional status, simultaneous radiotherapy of the abdomen and pelvis, and preexisting intestinal conditions.
Medication prophylaxis for chemotherapy-induced diarrhea has not been established. An exception is atropine for prophylaxis and treatment of irinotecan-induced cholinergic syndrome.
Indications for diagnostic procedures are outlined in the current German guideline for supportive therapy in patients with cancer.
For diarrhea accompanied by fever, blood cultures are mandatory. A complete blood count provides information on various aspects (leukocytosis as an inflammatory reaction, neutropenia as a marker for infection risk, hemoglobin as a marker for possible hemoconcentration or existing bleeding, and thrombocytopenia as a marker for bleeding tendency). Disproportionate thrombocytopenia may warrant assessment of fragmented cells and enterohemorrhagic Escherichia coli diagnostics.
To assess electrolyte and fluid loss, electrolytes, albumin, and total protein should be measured. The C-reactive protein value may help identify inflammatory conditions. It may also be elevated, however, because of tumor-related factors. Measuring urea and creatinine allows for estimating whether there is already a prerenal impairment of kidney function. Liver function parameters are mandatory for critically ill patients. In patients with hypotension or tachycardia, blood gas analysis and lactate determination are advisable. Among imaging techniques, ultrasound may be helpful. Indications for conventional abdominal x-ray are rare. In the presence of clinical signs of peritoneal irritation (such as guarding and rebound tenderness), a CT scan should be considered to detect further complications (perforation, ileus, enterocolitis, etc.) promptly.
Endoscopic examinations are recommended only in cases of persistent, worsening symptoms, according to the guideline. Colonoscopy is contraindicated in suspected neutropenic enterocolitis (NEC) because of the risk for perforation.
According to Dr. Hentrich and Dr. Penndorf, diarrhea therapy is carried out in stages and depends on the severity and response to each therapy. The Common Terminology Criteria for Adverse Events distinguishes the following severity grades:
- Grade 1: < four stools per day above baseline
- Grade 2: Four to six stools per day above baseline
- Grade 3: ≥ seven stools per day above baseline; fecal incontinence, hospitalization indicated; limited activities of daily living
- Grade 4: Life-threatening consequences, urgent intervention indicated
Therapy for Grades 1-2
The standard therapeutic after excluding infectious causes is loperamide (initially 4 mg orally, followed by 2 mg every 2-4 hours). A daily dose should not exceed 16 mg.
For irinotecan-associated diarrhea, adjunctive administration of budesonide (3 mg orally three times per day) with loperamide was shown to be effective in a small, randomized study (off-label). Another randomized study demonstrated the efficacy of combining loperamide with racecadotril (100 mg orally three times per day for 48 hours).
Therapy for Grade 3 Diarrhea
In severe diarrhea persisting despite loperamide therapy for 24-48 hours, octreotide (100-150 μg subcutaneously three times per day) may be administered (maximum three times 500 μg). Although octreotide is often used successfully for chemotherapy-induced diarrhea, it is not approved for this indication (off-label use).
According to the authors, other therapy options for loperamide-refractory diarrhea include codeine, tincture of opium, budesonide, and racecadotril. Psyllium husk or diphenoxylate plus atropine may also be attempted. In patients with prolonged neutropenia, overdosing of motility inhibitors should be avoided because of the risk for ileus.
The use of probiotics for chemotherapy-induced diarrhea cannot be generally recommended because of insufficient evidence, and cases of probiotic-associated bacteremia and fungemia have been described.
A particularly serious complication of intensive chemotherapy associated with diarrhea is NEC. It is characterized by fever, abdominal pain, and diarrhea during severe neutropenia (neutrophil count < 500/μL), the authors explained. NEC occurs predominantly, but not exclusively, after intensive chemotherapy for hematologic malignancies, especially acute leukemias.
More common than NEC and often preceding it is the so-called chemotherapy-associated bowel syndrome. It is characterized by fever ≥ 37.8 °C and abdominal pain or absence of stool for at least 72 hours.
Therapy consists of conservative symptomatic measures such as diet, adequate hydration with electrolyte balance, and analgesia. Due to the high risk for bacteremia, antibiotic therapy is indicated after blood cultures are obtained (piperacillin-tazobactam or a carbapenem). According to the authors, NEC improves in most patients with neutrophil regeneration. Granulocyte colony-stimulating factor therapy appears reasonable in this context, although conclusive studies are lacking. Surgical intervention with removal of necrotic bowel segments may be considered in exceptional cases.
This story was translated from Univadis Germany, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The Simple Change That Can Improve Patient Satisfaction
This transcript has been edited for clarity.
Hello. I’m David Kerr, professor of cancer medicine from University of Oxford. I’d like to talk today about how we communicate with patients.
This is current on my mind because on Friday after clinic, I popped around to see a couple of patients who were in our local hospice. They were there for end-of-life care, being wonderfully well looked after. These were patients I have looked after for 3, 4, or 5 years, patients whom I cared for, and patients of whom I was fond. I think that relationship was reciprocated by them.
We know that any effective communication between patients and doctors is absolutely critical and fundamental to the delivery of patient-centered care. It’s really hard to measure and challenging to attain in the dynamic, often noisy environment of a busy ward or even in the relative peace and quiet of a hospice.
We know that specific behavior by doctors can make a real difference to how they’re perceived by the patient, including their communicative skills and so on. I’ve been a doctor for more than 40 years, but sophisticated communicator though I think I am, there I was, standing by the bedside. It’s really interesting and odd, actually, when you stop and think about it.
There’s an increasing body of evidence that suggests that if the physician sits at the patient’s bedside, establishes better, more direct eye-to-eye contact and so on, then the quality of communication and patient satisfaction is improved.
I picked up on a recent study published just a few days ago in The BMJ; the title of the study is “Effect of Chair Placement on Physicians’ Behavior and Patients’ Satisfaction: Randomized Deception Trial.”
It was done in a single center and there were 125 separate physician interactions. In half of them, the chair in the patient’s room was in its conventional place back against the wall, round a corner, not particularly accessible. The randomization, or the active intervention, if you like, was to have a chair placed less than 3 feet from the patient’s bed and at the patient’s eye level.
What was really interesting was that of these randomized interventions in the setting in which the chair placement was close to the patient’s bed — it was accessible, less than 3 feet — 38 of the 60 physicians sat down in the chair and engaged with the patient from that level.
In the other setting, in which the chair wasn’t immediately adjacent to the bedside (it was back against the wall, out of the way), only in 5 of 60 did the physician retrieve the chair and move it to the right position. Otherwise, they stood and talked to the patient in that way.
The patient satisfaction scores that were measured using a conventional tool were much better for those seated physicians rather than those who stood and towered above.
This is an interesting study with statistically significant findings. It didn’t mean that the physicians who sat spent more time with the patient. It was the same in both settings, at about 10 or 11 minutes. It didn’t alter the physician’s perception of how long they spent with the patient — they guessed it was about 10 minutes, equally on both sides — or indeed the patient’s interpretation of how long the physician stayed.
It wasn’t a temporal thing but just the quality of communication. The patient satisfaction was much better, just simply by sitting at the patient’s bedside and engaging with them. It’s a tiny thing to do that made for a significant qualitative improvement. I’ve learned that lesson. No more towering above. No more standing at the bottom of the patient’s bedside, as I was taught and as I’ve always done.
I’m going to nudge my behavior. I’m going to use the psychology of that small study to nudge myself, the junior doctors that I train, and perhaps even my consultant colleagues, to do the same. It’s a small but effective step forward in improving patient-centered communication.
I’d be delighted to see what you think. How many of you stand? Being old-school, I would have thought that that’s most of us. How many of you make the effort to drag the chair over to sit at the patient’s bedside and to engage more fully? I’d be really interested in any comments that you’ve got.
For the time being, over and out. Ahoy. Thanks for listening.
Dr. Kerr disclosed the following relevant financial relationships Served as a director, officer, partner, employee, advisor, consultant, or trustee for Celleron Therapeutics and Oxford Cancer Biomarkers (board of directors); Afrox (charity; trustee); and GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (consultant). Serve(d) as a speaker or a member of a speakers bureau for Genomic Health and Merck Serono. Received research grant from Roche. Has a 5% or greater equity interest in Celleron Therapeutics and Oxford Cancer Biomarkers.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I’m David Kerr, professor of cancer medicine from University of Oxford. I’d like to talk today about how we communicate with patients.
This is current on my mind because on Friday after clinic, I popped around to see a couple of patients who were in our local hospice. They were there for end-of-life care, being wonderfully well looked after. These were patients I have looked after for 3, 4, or 5 years, patients whom I cared for, and patients of whom I was fond. I think that relationship was reciprocated by them.
We know that any effective communication between patients and doctors is absolutely critical and fundamental to the delivery of patient-centered care. It’s really hard to measure and challenging to attain in the dynamic, often noisy environment of a busy ward or even in the relative peace and quiet of a hospice.
We know that specific behavior by doctors can make a real difference to how they’re perceived by the patient, including their communicative skills and so on. I’ve been a doctor for more than 40 years, but sophisticated communicator though I think I am, there I was, standing by the bedside. It’s really interesting and odd, actually, when you stop and think about it.
There’s an increasing body of evidence that suggests that if the physician sits at the patient’s bedside, establishes better, more direct eye-to-eye contact and so on, then the quality of communication and patient satisfaction is improved.
I picked up on a recent study published just a few days ago in The BMJ; the title of the study is “Effect of Chair Placement on Physicians’ Behavior and Patients’ Satisfaction: Randomized Deception Trial.”
It was done in a single center and there were 125 separate physician interactions. In half of them, the chair in the patient’s room was in its conventional place back against the wall, round a corner, not particularly accessible. The randomization, or the active intervention, if you like, was to have a chair placed less than 3 feet from the patient’s bed and at the patient’s eye level.
What was really interesting was that of these randomized interventions in the setting in which the chair placement was close to the patient’s bed — it was accessible, less than 3 feet — 38 of the 60 physicians sat down in the chair and engaged with the patient from that level.
In the other setting, in which the chair wasn’t immediately adjacent to the bedside (it was back against the wall, out of the way), only in 5 of 60 did the physician retrieve the chair and move it to the right position. Otherwise, they stood and talked to the patient in that way.
The patient satisfaction scores that were measured using a conventional tool were much better for those seated physicians rather than those who stood and towered above.
This is an interesting study with statistically significant findings. It didn’t mean that the physicians who sat spent more time with the patient. It was the same in both settings, at about 10 or 11 minutes. It didn’t alter the physician’s perception of how long they spent with the patient — they guessed it was about 10 minutes, equally on both sides — or indeed the patient’s interpretation of how long the physician stayed.
It wasn’t a temporal thing but just the quality of communication. The patient satisfaction was much better, just simply by sitting at the patient’s bedside and engaging with them. It’s a tiny thing to do that made for a significant qualitative improvement. I’ve learned that lesson. No more towering above. No more standing at the bottom of the patient’s bedside, as I was taught and as I’ve always done.
I’m going to nudge my behavior. I’m going to use the psychology of that small study to nudge myself, the junior doctors that I train, and perhaps even my consultant colleagues, to do the same. It’s a small but effective step forward in improving patient-centered communication.
I’d be delighted to see what you think. How many of you stand? Being old-school, I would have thought that that’s most of us. How many of you make the effort to drag the chair over to sit at the patient’s bedside and to engage more fully? I’d be really interested in any comments that you’ve got.
For the time being, over and out. Ahoy. Thanks for listening.
Dr. Kerr disclosed the following relevant financial relationships Served as a director, officer, partner, employee, advisor, consultant, or trustee for Celleron Therapeutics and Oxford Cancer Biomarkers (board of directors); Afrox (charity; trustee); and GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (consultant). Serve(d) as a speaker or a member of a speakers bureau for Genomic Health and Merck Serono. Received research grant from Roche. Has a 5% or greater equity interest in Celleron Therapeutics and Oxford Cancer Biomarkers.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I’m David Kerr, professor of cancer medicine from University of Oxford. I’d like to talk today about how we communicate with patients.
This is current on my mind because on Friday after clinic, I popped around to see a couple of patients who were in our local hospice. They were there for end-of-life care, being wonderfully well looked after. These were patients I have looked after for 3, 4, or 5 years, patients whom I cared for, and patients of whom I was fond. I think that relationship was reciprocated by them.
We know that any effective communication between patients and doctors is absolutely critical and fundamental to the delivery of patient-centered care. It’s really hard to measure and challenging to attain in the dynamic, often noisy environment of a busy ward or even in the relative peace and quiet of a hospice.
We know that specific behavior by doctors can make a real difference to how they’re perceived by the patient, including their communicative skills and so on. I’ve been a doctor for more than 40 years, but sophisticated communicator though I think I am, there I was, standing by the bedside. It’s really interesting and odd, actually, when you stop and think about it.
There’s an increasing body of evidence that suggests that if the physician sits at the patient’s bedside, establishes better, more direct eye-to-eye contact and so on, then the quality of communication and patient satisfaction is improved.
I picked up on a recent study published just a few days ago in The BMJ; the title of the study is “Effect of Chair Placement on Physicians’ Behavior and Patients’ Satisfaction: Randomized Deception Trial.”
It was done in a single center and there were 125 separate physician interactions. In half of them, the chair in the patient’s room was in its conventional place back against the wall, round a corner, not particularly accessible. The randomization, or the active intervention, if you like, was to have a chair placed less than 3 feet from the patient’s bed and at the patient’s eye level.
What was really interesting was that of these randomized interventions in the setting in which the chair placement was close to the patient’s bed — it was accessible, less than 3 feet — 38 of the 60 physicians sat down in the chair and engaged with the patient from that level.
In the other setting, in which the chair wasn’t immediately adjacent to the bedside (it was back against the wall, out of the way), only in 5 of 60 did the physician retrieve the chair and move it to the right position. Otherwise, they stood and talked to the patient in that way.
The patient satisfaction scores that were measured using a conventional tool were much better for those seated physicians rather than those who stood and towered above.
This is an interesting study with statistically significant findings. It didn’t mean that the physicians who sat spent more time with the patient. It was the same in both settings, at about 10 or 11 minutes. It didn’t alter the physician’s perception of how long they spent with the patient — they guessed it was about 10 minutes, equally on both sides — or indeed the patient’s interpretation of how long the physician stayed.
It wasn’t a temporal thing but just the quality of communication. The patient satisfaction was much better, just simply by sitting at the patient’s bedside and engaging with them. It’s a tiny thing to do that made for a significant qualitative improvement. I’ve learned that lesson. No more towering above. No more standing at the bottom of the patient’s bedside, as I was taught and as I’ve always done.
I’m going to nudge my behavior. I’m going to use the psychology of that small study to nudge myself, the junior doctors that I train, and perhaps even my consultant colleagues, to do the same. It’s a small but effective step forward in improving patient-centered communication.
I’d be delighted to see what you think. How many of you stand? Being old-school, I would have thought that that’s most of us. How many of you make the effort to drag the chair over to sit at the patient’s bedside and to engage more fully? I’d be really interested in any comments that you’ve got.
For the time being, over and out. Ahoy. Thanks for listening.
Dr. Kerr disclosed the following relevant financial relationships Served as a director, officer, partner, employee, advisor, consultant, or trustee for Celleron Therapeutics and Oxford Cancer Biomarkers (board of directors); Afrox (charity; trustee); and GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (consultant). Serve(d) as a speaker or a member of a speakers bureau for Genomic Health and Merck Serono. Received research grant from Roche. Has a 5% or greater equity interest in Celleron Therapeutics and Oxford Cancer Biomarkers.
A version of this article appeared on Medscape.com.
What Do Sex Therapists Do? (Hint: It’s Not What You Think)
This transcript has been edited for clarity.
Rachel S. Rubin, MD: We are here at the Harvard Continuing Medical Education Course in Orlando, Florida. It’s all about testosterone therapy and sexual medicine. I have with me today the wonderful Dr. Marianne Brandon, who is an amazing sex therapist. Could you introduce yourself?
Marianne Brandon, PhD: I am a clinical psychologist and sex therapist. I’ve been in practice for more than 25 years. I’m currently located in Sarasota. I have a Psychology Today blog called The Future of Intimacy, which I have a lot of fun with.
Dr. Rubin: It’s very important, when taking care of patients, that we work in a biopsychosocial model. Yes, we can fix erectile dysfunction. We can help with menopause symptoms and that helps sexual function. But what I find makes my patients able to live their best lives is when they have a team, including a mental health professional — often a sex therapist or a couples’ therapist — where they can learn communication skills. Why is it important for primary care doctors to talk to their patients about sex? My primary care doctor has never asked me about sex.
Dr. Brandon: People have more struggles than you realize. Sexual dysfunction correlates with emotional issues such as depression and anxiety, with medical problems, and with medication use. Chances are that your patients have some kind of sexual concern, even if that’s not to the degree that it would be classified as a sexual dysfunction.
But sexual concerns wreak havoc. Believing they have a sexual problem, they stop touching, they stop relating to their partner. It becomes a really big deal in their lives. If you can open the door for a conversation about sex with your patients, it could do them a great deal of good. It’s also good for the practitioner, because if your patients think they can talk with you about anything, that’s going to establish your relationship with them. Practitioners avoid these conversations because they don’t have the time or the training to offer help.
Dr. Rubin: You don’t have to know all the answers. You just have to show empathy and compassion and say, “I hear you.” That’s the magic in the doctor-patient relationship. We refer patients to specialists when we don’t know what to do. What happens when I send a patient to a sex therapist? Do they watch them have sex? Of course not, but everyone thinks that is what sex therapists do.
Dr. Brandon: Sex therapy is just like any other type of therapy, but we discuss sexual issues. And because just about anything that’s happening in your patient’s life can trickle down into the bedroom, we end up talking about a lot of stuff that’s not directly related to sex but ultimately impacts the patient’s sex life.
Dr. Rubin: It’s true. Most medical conditions that we treat — from diabetes, hypertension, high cholesterol, and obesity to depression and anxiety — are strongly correlated with sexual health. We treat the underlying condition, but our patients don’t care about their A1c levels. They care about the fact that they cannot get aroused; their genitals don’t feel the same way they used to.
Dr. Brandon: I love that point because people make meaning out of their sexual concerns and dysfunction. Suddenly their body isn’t responding the way it used to. They think something’s wrong with them, or maybe they are with the wrong partner. This meaning becomes very powerful in their mind and perpetuates the sexual problem.
Dr. Rubin: First and foremost, we are educators. We can say, “You have pretty out-of-control diabetes,” or, “You’re a smoker, which can affect the health of your genitals. Have you noticed any issues going on there?” If you don’t ask, patients will not bring up their concerns with their doctors.
So how do people find a sex therapist?
Dr. Brandon: There are a few fabulous organizations that provide on their websites ways to find a therapist: the American Association of Sex Educators, Counselors and Therapists (AASECT) and Sex Therapy and Research (STAR). Giving patients this information is a huge intervention.
Other places to find a therapist include the International Society for Sexual Medicine, and the International Society for the Study of Women’s Sexual Health.
Since COVID, many therapists have gone virtual. Encourage your patients to look within their states to find options for therapists and psychologists. Recent legislation allows psychologists who have signed up for PSYPACT to practice almost throughout the entire United States. We used to think if we didn’t have a therapist in the community, we couldn’t make a referral. That›s not the case anymore.
Dr. Rubin: All doctors are really sexual medicine doctors. We can change the whole world by giving our patients a better quality of life.
Dr. Rubin, Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, disclosed ties to Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: We are here at the Harvard Continuing Medical Education Course in Orlando, Florida. It’s all about testosterone therapy and sexual medicine. I have with me today the wonderful Dr. Marianne Brandon, who is an amazing sex therapist. Could you introduce yourself?
Marianne Brandon, PhD: I am a clinical psychologist and sex therapist. I’ve been in practice for more than 25 years. I’m currently located in Sarasota. I have a Psychology Today blog called The Future of Intimacy, which I have a lot of fun with.
Dr. Rubin: It’s very important, when taking care of patients, that we work in a biopsychosocial model. Yes, we can fix erectile dysfunction. We can help with menopause symptoms and that helps sexual function. But what I find makes my patients able to live their best lives is when they have a team, including a mental health professional — often a sex therapist or a couples’ therapist — where they can learn communication skills. Why is it important for primary care doctors to talk to their patients about sex? My primary care doctor has never asked me about sex.
Dr. Brandon: People have more struggles than you realize. Sexual dysfunction correlates with emotional issues such as depression and anxiety, with medical problems, and with medication use. Chances are that your patients have some kind of sexual concern, even if that’s not to the degree that it would be classified as a sexual dysfunction.
But sexual concerns wreak havoc. Believing they have a sexual problem, they stop touching, they stop relating to their partner. It becomes a really big deal in their lives. If you can open the door for a conversation about sex with your patients, it could do them a great deal of good. It’s also good for the practitioner, because if your patients think they can talk with you about anything, that’s going to establish your relationship with them. Practitioners avoid these conversations because they don’t have the time or the training to offer help.
Dr. Rubin: You don’t have to know all the answers. You just have to show empathy and compassion and say, “I hear you.” That’s the magic in the doctor-patient relationship. We refer patients to specialists when we don’t know what to do. What happens when I send a patient to a sex therapist? Do they watch them have sex? Of course not, but everyone thinks that is what sex therapists do.
Dr. Brandon: Sex therapy is just like any other type of therapy, but we discuss sexual issues. And because just about anything that’s happening in your patient’s life can trickle down into the bedroom, we end up talking about a lot of stuff that’s not directly related to sex but ultimately impacts the patient’s sex life.
Dr. Rubin: It’s true. Most medical conditions that we treat — from diabetes, hypertension, high cholesterol, and obesity to depression and anxiety — are strongly correlated with sexual health. We treat the underlying condition, but our patients don’t care about their A1c levels. They care about the fact that they cannot get aroused; their genitals don’t feel the same way they used to.
Dr. Brandon: I love that point because people make meaning out of their sexual concerns and dysfunction. Suddenly their body isn’t responding the way it used to. They think something’s wrong with them, or maybe they are with the wrong partner. This meaning becomes very powerful in their mind and perpetuates the sexual problem.
Dr. Rubin: First and foremost, we are educators. We can say, “You have pretty out-of-control diabetes,” or, “You’re a smoker, which can affect the health of your genitals. Have you noticed any issues going on there?” If you don’t ask, patients will not bring up their concerns with their doctors.
So how do people find a sex therapist?
Dr. Brandon: There are a few fabulous organizations that provide on their websites ways to find a therapist: the American Association of Sex Educators, Counselors and Therapists (AASECT) and Sex Therapy and Research (STAR). Giving patients this information is a huge intervention.
Other places to find a therapist include the International Society for Sexual Medicine, and the International Society for the Study of Women’s Sexual Health.
Since COVID, many therapists have gone virtual. Encourage your patients to look within their states to find options for therapists and psychologists. Recent legislation allows psychologists who have signed up for PSYPACT to practice almost throughout the entire United States. We used to think if we didn’t have a therapist in the community, we couldn’t make a referral. That›s not the case anymore.
Dr. Rubin: All doctors are really sexual medicine doctors. We can change the whole world by giving our patients a better quality of life.
Dr. Rubin, Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, disclosed ties to Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: We are here at the Harvard Continuing Medical Education Course in Orlando, Florida. It’s all about testosterone therapy and sexual medicine. I have with me today the wonderful Dr. Marianne Brandon, who is an amazing sex therapist. Could you introduce yourself?
Marianne Brandon, PhD: I am a clinical psychologist and sex therapist. I’ve been in practice for more than 25 years. I’m currently located in Sarasota. I have a Psychology Today blog called The Future of Intimacy, which I have a lot of fun with.
Dr. Rubin: It’s very important, when taking care of patients, that we work in a biopsychosocial model. Yes, we can fix erectile dysfunction. We can help with menopause symptoms and that helps sexual function. But what I find makes my patients able to live their best lives is when they have a team, including a mental health professional — often a sex therapist or a couples’ therapist — where they can learn communication skills. Why is it important for primary care doctors to talk to their patients about sex? My primary care doctor has never asked me about sex.
Dr. Brandon: People have more struggles than you realize. Sexual dysfunction correlates with emotional issues such as depression and anxiety, with medical problems, and with medication use. Chances are that your patients have some kind of sexual concern, even if that’s not to the degree that it would be classified as a sexual dysfunction.
But sexual concerns wreak havoc. Believing they have a sexual problem, they stop touching, they stop relating to their partner. It becomes a really big deal in their lives. If you can open the door for a conversation about sex with your patients, it could do them a great deal of good. It’s also good for the practitioner, because if your patients think they can talk with you about anything, that’s going to establish your relationship with them. Practitioners avoid these conversations because they don’t have the time or the training to offer help.
Dr. Rubin: You don’t have to know all the answers. You just have to show empathy and compassion and say, “I hear you.” That’s the magic in the doctor-patient relationship. We refer patients to specialists when we don’t know what to do. What happens when I send a patient to a sex therapist? Do they watch them have sex? Of course not, but everyone thinks that is what sex therapists do.
Dr. Brandon: Sex therapy is just like any other type of therapy, but we discuss sexual issues. And because just about anything that’s happening in your patient’s life can trickle down into the bedroom, we end up talking about a lot of stuff that’s not directly related to sex but ultimately impacts the patient’s sex life.
Dr. Rubin: It’s true. Most medical conditions that we treat — from diabetes, hypertension, high cholesterol, and obesity to depression and anxiety — are strongly correlated with sexual health. We treat the underlying condition, but our patients don’t care about their A1c levels. They care about the fact that they cannot get aroused; their genitals don’t feel the same way they used to.
Dr. Brandon: I love that point because people make meaning out of their sexual concerns and dysfunction. Suddenly their body isn’t responding the way it used to. They think something’s wrong with them, or maybe they are with the wrong partner. This meaning becomes very powerful in their mind and perpetuates the sexual problem.
Dr. Rubin: First and foremost, we are educators. We can say, “You have pretty out-of-control diabetes,” or, “You’re a smoker, which can affect the health of your genitals. Have you noticed any issues going on there?” If you don’t ask, patients will not bring up their concerns with their doctors.
So how do people find a sex therapist?
Dr. Brandon: There are a few fabulous organizations that provide on their websites ways to find a therapist: the American Association of Sex Educators, Counselors and Therapists (AASECT) and Sex Therapy and Research (STAR). Giving patients this information is a huge intervention.
Other places to find a therapist include the International Society for Sexual Medicine, and the International Society for the Study of Women’s Sexual Health.
Since COVID, many therapists have gone virtual. Encourage your patients to look within their states to find options for therapists and psychologists. Recent legislation allows psychologists who have signed up for PSYPACT to practice almost throughout the entire United States. We used to think if we didn’t have a therapist in the community, we couldn’t make a referral. That›s not the case anymore.
Dr. Rubin: All doctors are really sexual medicine doctors. We can change the whole world by giving our patients a better quality of life.
Dr. Rubin, Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, disclosed ties to Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article appeared on Medscape.com.
New Drug Approvals Are the Wrong Metric for Cancer Policy
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
We Must Learn About Abortion as Primary Care Doctors
“No greater opportunity, responsibility, or obligation can fall to the lot of a human being than to become a physician. In the care of the suffering, [the physician] needs technical skill, scientific knowledge, and human understanding.”1 Internal medicine physicians have risen to this challenge for centuries. Today, it is time for us to use these skills to care for patients who need access to reproductive care — particularly medication abortion. Nationally accredited internal medicine training programs have not been required to provide abortion education, and this may evolve in the future.
However, considering the difficulty in people receiving contraception, the failure rate of contraception, the known risks from pregnancy, the increasing difficulty in accessing abortion, and the recent advocating to protect access to reproductive care by leadership of internal medicine and internal medicine subspecialty societies, we advocate that abortion must become a part of our education and practice.2
Most abortions are performed during the first trimester and can be managed with medications that are very safe.3 In fact, legal medication abortion is so safe that pregnancy in the United States has fourteen times the mortality risk as does legal medication abortion.4 Inability to access an abortion has widely documented negative health effects for women and their children.5,6
Within this context, it is important for internal medicine physicians to understand that the ability to access an abortion is the ability to access a life-saving procedure and there is no medical justification for restricting such a prescription any more than restricting any other standard medical therapy. Furthermore, the recent widespread criminalization of abortion gives new urgency to expanding the pool of physicians who understand this and are trained, able, and willing to prescribe medication abortion.
We understand that reproductive health care may not now be a component of clinical practice for some, but given the heterogeneity of internal medicine, we believe that some knowledge about medical abortion is an essential competency of foundational medical knowledge.7 The heterogeneity of practice in internal medicine lends itself to different levels of knowledge that should be embraced. Because of poor access to abortion, both ambulatory and hospital-based physicians will increasingly be required to care for patients who need abortion for medical or other reasons.
We advocate that all physicians — including those with internal medicine training — should understand counseling about choices and options (including an unbiased discussion of the options to continue or terminate the pregnancy), the safety of medication abortion in contrast to the risks from pregnancy, and where to refer someone seeking an abortion. In addition to this information, primary care physicians with a special interest in women’s health must have basic knowledge about mifepristone and misoprostol and how they work, the benefits and risks of these, and what the pregnant person seeking an abortion will experience.8
Lastly, physicians who wish to provide medication abortion — including in primary care, hospital medicine, and subspecialty care — should receive training and ongoing professional development. Such professional development should include counseling, indications, contraindications, medication regimens, navigating required documentation and reporting, and anticipating possible side effects and complications.
A major challenge to internal medicine and other primary care physicians, subspecialists, and hospitalists addressing abortion is the inadequate training in and knowledge about providing this care. However, the entire spectrum of medical education (undergraduate, graduate, and continuing education) should evolve to address this lack.
Integrating this education into medical conferences and journals is a meaningful start, possibly in partnership with medical societies that have been teaching these skills for decades. Partnering with other specialties can also help us stay current on the local legal landscape and engage in collaborative advocacy.
Specifically, some resources for training can be found at:
- www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2014/11/abortion-training-and-education
- https://prochoice.org/providers/continuing-medical-education/
- www.reproductiveaccess.org/medicationabortion/
Some may have concerns that managing the possible complications of medication abortion is a reason for internal medicine to not be involved in abortion care. However, medication abortions are safe and effective for pregnancy termination and internal medicine physicians can refer patients with complications to peers in gynecology, family medicine, and emergency medicine should complications arise.8 We have managed countless other conditions this way, including most recently during the pandemic.
We live in a country with increasing barriers to care – now with laws in many states that prevent basic health care for women. Internal medicine doctors increasingly may see patients who need care urgently, particularly those who practice in states that neighbor those that prevent this access. We are calling for all who practice internal medicine to educate themselves, optimizing their skills within the full scope of medical practice to provide possibly lifesaving care and thereby address increased needs for medical services.
We must continue to advocate for our patients. The COVID-19 pandemic has reinforced the fact that internal medicine–trained physicians are able to care for conditions that are new and, as a profession, we are capable of rapidly switching practices and learning new modalities of care. It is time for us to extend this competency to care for patients who constitute half the population and are at risk: women.
Dr. Barrett is an internal medicine hospitalist based in Albuquerque, New Mexico; she completed a medical justice in advocacy fellowship in 2022. Dr. Radhakrishnan is an internal medicine physician educator who completed an equity matters fellowship in 2022 and is based in Scottsdale, Arizona. Neither reports conflicts of interest.
References
1. Harrison’s Principles of Internal Medicine, 20e. Jameson J et al., eds. McGraw Hill; 2018. Accessed Sept. 27, 2023.
2. Serchen J et al. Reproductive Health Policy in the United States: An American College of Physicians Policy Brief. Ann Intern Med.2023;176:364-6. epub 28 Feb. 2023.
3. Jatlaoui TC et al. Abortion Surveillance — United States, 2016. MMWR Surveill Summ 2019;68(No. SS-11):1-41.
4. Raymond EG and Grimes DA. The comparative safety of legal induced abortion and childbirth in the United States. Obstet Gynecol. 2012;119(2 Pt 1):215-9.
5. Ralph LJ et al. Self-reported Physical Health of Women Who Did and Did Not Terminate Pregnancy After Seeking Abortion Services: A Cohort Study. Ann Intern Med.2019;171:238-47. epub 11 June 2019.
6. Gerdts C et al. Side effects, physical health consequences, and mortality associated with abortion and birth after an unwanted pregnancy. Women’s Health Issues 2016;26:55-59.
7. Nobel K et al. Patient-reported experience with discussion of all options during pregnancy options counseling in the US south. Contraception. 2022;106:68-74.
8. Liu N and Ray JG. Short-Term Adverse Outcomes After Mifepristone–Misoprostol Versus Procedural Induced Abortion: A Population-Based Propensity-Weighted Study. Ann Intern Med.2023;176:145-53. epub 3 January 2023.
“No greater opportunity, responsibility, or obligation can fall to the lot of a human being than to become a physician. In the care of the suffering, [the physician] needs technical skill, scientific knowledge, and human understanding.”1 Internal medicine physicians have risen to this challenge for centuries. Today, it is time for us to use these skills to care for patients who need access to reproductive care — particularly medication abortion. Nationally accredited internal medicine training programs have not been required to provide abortion education, and this may evolve in the future.
However, considering the difficulty in people receiving contraception, the failure rate of contraception, the known risks from pregnancy, the increasing difficulty in accessing abortion, and the recent advocating to protect access to reproductive care by leadership of internal medicine and internal medicine subspecialty societies, we advocate that abortion must become a part of our education and practice.2
Most abortions are performed during the first trimester and can be managed with medications that are very safe.3 In fact, legal medication abortion is so safe that pregnancy in the United States has fourteen times the mortality risk as does legal medication abortion.4 Inability to access an abortion has widely documented negative health effects for women and their children.5,6
Within this context, it is important for internal medicine physicians to understand that the ability to access an abortion is the ability to access a life-saving procedure and there is no medical justification for restricting such a prescription any more than restricting any other standard medical therapy. Furthermore, the recent widespread criminalization of abortion gives new urgency to expanding the pool of physicians who understand this and are trained, able, and willing to prescribe medication abortion.
We understand that reproductive health care may not now be a component of clinical practice for some, but given the heterogeneity of internal medicine, we believe that some knowledge about medical abortion is an essential competency of foundational medical knowledge.7 The heterogeneity of practice in internal medicine lends itself to different levels of knowledge that should be embraced. Because of poor access to abortion, both ambulatory and hospital-based physicians will increasingly be required to care for patients who need abortion for medical or other reasons.
We advocate that all physicians — including those with internal medicine training — should understand counseling about choices and options (including an unbiased discussion of the options to continue or terminate the pregnancy), the safety of medication abortion in contrast to the risks from pregnancy, and where to refer someone seeking an abortion. In addition to this information, primary care physicians with a special interest in women’s health must have basic knowledge about mifepristone and misoprostol and how they work, the benefits and risks of these, and what the pregnant person seeking an abortion will experience.8
Lastly, physicians who wish to provide medication abortion — including in primary care, hospital medicine, and subspecialty care — should receive training and ongoing professional development. Such professional development should include counseling, indications, contraindications, medication regimens, navigating required documentation and reporting, and anticipating possible side effects and complications.
A major challenge to internal medicine and other primary care physicians, subspecialists, and hospitalists addressing abortion is the inadequate training in and knowledge about providing this care. However, the entire spectrum of medical education (undergraduate, graduate, and continuing education) should evolve to address this lack.
Integrating this education into medical conferences and journals is a meaningful start, possibly in partnership with medical societies that have been teaching these skills for decades. Partnering with other specialties can also help us stay current on the local legal landscape and engage in collaborative advocacy.
Specifically, some resources for training can be found at:
- www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2014/11/abortion-training-and-education
- https://prochoice.org/providers/continuing-medical-education/
- www.reproductiveaccess.org/medicationabortion/
Some may have concerns that managing the possible complications of medication abortion is a reason for internal medicine to not be involved in abortion care. However, medication abortions are safe and effective for pregnancy termination and internal medicine physicians can refer patients with complications to peers in gynecology, family medicine, and emergency medicine should complications arise.8 We have managed countless other conditions this way, including most recently during the pandemic.
We live in a country with increasing barriers to care – now with laws in many states that prevent basic health care for women. Internal medicine doctors increasingly may see patients who need care urgently, particularly those who practice in states that neighbor those that prevent this access. We are calling for all who practice internal medicine to educate themselves, optimizing their skills within the full scope of medical practice to provide possibly lifesaving care and thereby address increased needs for medical services.
We must continue to advocate for our patients. The COVID-19 pandemic has reinforced the fact that internal medicine–trained physicians are able to care for conditions that are new and, as a profession, we are capable of rapidly switching practices and learning new modalities of care. It is time for us to extend this competency to care for patients who constitute half the population and are at risk: women.
Dr. Barrett is an internal medicine hospitalist based in Albuquerque, New Mexico; she completed a medical justice in advocacy fellowship in 2022. Dr. Radhakrishnan is an internal medicine physician educator who completed an equity matters fellowship in 2022 and is based in Scottsdale, Arizona. Neither reports conflicts of interest.
References
1. Harrison’s Principles of Internal Medicine, 20e. Jameson J et al., eds. McGraw Hill; 2018. Accessed Sept. 27, 2023.
2. Serchen J et al. Reproductive Health Policy in the United States: An American College of Physicians Policy Brief. Ann Intern Med.2023;176:364-6. epub 28 Feb. 2023.
3. Jatlaoui TC et al. Abortion Surveillance — United States, 2016. MMWR Surveill Summ 2019;68(No. SS-11):1-41.
4. Raymond EG and Grimes DA. The comparative safety of legal induced abortion and childbirth in the United States. Obstet Gynecol. 2012;119(2 Pt 1):215-9.
5. Ralph LJ et al. Self-reported Physical Health of Women Who Did and Did Not Terminate Pregnancy After Seeking Abortion Services: A Cohort Study. Ann Intern Med.2019;171:238-47. epub 11 June 2019.
6. Gerdts C et al. Side effects, physical health consequences, and mortality associated with abortion and birth after an unwanted pregnancy. Women’s Health Issues 2016;26:55-59.
7. Nobel K et al. Patient-reported experience with discussion of all options during pregnancy options counseling in the US south. Contraception. 2022;106:68-74.
8. Liu N and Ray JG. Short-Term Adverse Outcomes After Mifepristone–Misoprostol Versus Procedural Induced Abortion: A Population-Based Propensity-Weighted Study. Ann Intern Med.2023;176:145-53. epub 3 January 2023.
“No greater opportunity, responsibility, or obligation can fall to the lot of a human being than to become a physician. In the care of the suffering, [the physician] needs technical skill, scientific knowledge, and human understanding.”1 Internal medicine physicians have risen to this challenge for centuries. Today, it is time for us to use these skills to care for patients who need access to reproductive care — particularly medication abortion. Nationally accredited internal medicine training programs have not been required to provide abortion education, and this may evolve in the future.
However, considering the difficulty in people receiving contraception, the failure rate of contraception, the known risks from pregnancy, the increasing difficulty in accessing abortion, and the recent advocating to protect access to reproductive care by leadership of internal medicine and internal medicine subspecialty societies, we advocate that abortion must become a part of our education and practice.2
Most abortions are performed during the first trimester and can be managed with medications that are very safe.3 In fact, legal medication abortion is so safe that pregnancy in the United States has fourteen times the mortality risk as does legal medication abortion.4 Inability to access an abortion has widely documented negative health effects for women and their children.5,6
Within this context, it is important for internal medicine physicians to understand that the ability to access an abortion is the ability to access a life-saving procedure and there is no medical justification for restricting such a prescription any more than restricting any other standard medical therapy. Furthermore, the recent widespread criminalization of abortion gives new urgency to expanding the pool of physicians who understand this and are trained, able, and willing to prescribe medication abortion.
We understand that reproductive health care may not now be a component of clinical practice for some, but given the heterogeneity of internal medicine, we believe that some knowledge about medical abortion is an essential competency of foundational medical knowledge.7 The heterogeneity of practice in internal medicine lends itself to different levels of knowledge that should be embraced. Because of poor access to abortion, both ambulatory and hospital-based physicians will increasingly be required to care for patients who need abortion for medical or other reasons.
We advocate that all physicians — including those with internal medicine training — should understand counseling about choices and options (including an unbiased discussion of the options to continue or terminate the pregnancy), the safety of medication abortion in contrast to the risks from pregnancy, and where to refer someone seeking an abortion. In addition to this information, primary care physicians with a special interest in women’s health must have basic knowledge about mifepristone and misoprostol and how they work, the benefits and risks of these, and what the pregnant person seeking an abortion will experience.8
Lastly, physicians who wish to provide medication abortion — including in primary care, hospital medicine, and subspecialty care — should receive training and ongoing professional development. Such professional development should include counseling, indications, contraindications, medication regimens, navigating required documentation and reporting, and anticipating possible side effects and complications.
A major challenge to internal medicine and other primary care physicians, subspecialists, and hospitalists addressing abortion is the inadequate training in and knowledge about providing this care. However, the entire spectrum of medical education (undergraduate, graduate, and continuing education) should evolve to address this lack.
Integrating this education into medical conferences and journals is a meaningful start, possibly in partnership with medical societies that have been teaching these skills for decades. Partnering with other specialties can also help us stay current on the local legal landscape and engage in collaborative advocacy.
Specifically, some resources for training can be found at:
- www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2014/11/abortion-training-and-education
- https://prochoice.org/providers/continuing-medical-education/
- www.reproductiveaccess.org/medicationabortion/
Some may have concerns that managing the possible complications of medication abortion is a reason for internal medicine to not be involved in abortion care. However, medication abortions are safe and effective for pregnancy termination and internal medicine physicians can refer patients with complications to peers in gynecology, family medicine, and emergency medicine should complications arise.8 We have managed countless other conditions this way, including most recently during the pandemic.
We live in a country with increasing barriers to care – now with laws in many states that prevent basic health care for women. Internal medicine doctors increasingly may see patients who need care urgently, particularly those who practice in states that neighbor those that prevent this access. We are calling for all who practice internal medicine to educate themselves, optimizing their skills within the full scope of medical practice to provide possibly lifesaving care and thereby address increased needs for medical services.
We must continue to advocate for our patients. The COVID-19 pandemic has reinforced the fact that internal medicine–trained physicians are able to care for conditions that are new and, as a profession, we are capable of rapidly switching practices and learning new modalities of care. It is time for us to extend this competency to care for patients who constitute half the population and are at risk: women.
Dr. Barrett is an internal medicine hospitalist based in Albuquerque, New Mexico; she completed a medical justice in advocacy fellowship in 2022. Dr. Radhakrishnan is an internal medicine physician educator who completed an equity matters fellowship in 2022 and is based in Scottsdale, Arizona. Neither reports conflicts of interest.
References
1. Harrison’s Principles of Internal Medicine, 20e. Jameson J et al., eds. McGraw Hill; 2018. Accessed Sept. 27, 2023.
2. Serchen J et al. Reproductive Health Policy in the United States: An American College of Physicians Policy Brief. Ann Intern Med.2023;176:364-6. epub 28 Feb. 2023.
3. Jatlaoui TC et al. Abortion Surveillance — United States, 2016. MMWR Surveill Summ 2019;68(No. SS-11):1-41.
4. Raymond EG and Grimes DA. The comparative safety of legal induced abortion and childbirth in the United States. Obstet Gynecol. 2012;119(2 Pt 1):215-9.
5. Ralph LJ et al. Self-reported Physical Health of Women Who Did and Did Not Terminate Pregnancy After Seeking Abortion Services: A Cohort Study. Ann Intern Med.2019;171:238-47. epub 11 June 2019.
6. Gerdts C et al. Side effects, physical health consequences, and mortality associated with abortion and birth after an unwanted pregnancy. Women’s Health Issues 2016;26:55-59.
7. Nobel K et al. Patient-reported experience with discussion of all options during pregnancy options counseling in the US south. Contraception. 2022;106:68-74.
8. Liu N and Ray JG. Short-Term Adverse Outcomes After Mifepristone–Misoprostol Versus Procedural Induced Abortion: A Population-Based Propensity-Weighted Study. Ann Intern Med.2023;176:145-53. epub 3 January 2023.






