User login
Ob.gyns. can help end the HIV epidemic
Despite staggering scientific and medical advances, the HIV epidemic in the United States has not changed significantly over the past decade. The estimated incidence of HIV infection has remained stable overall, with between 45,000 and 55,000 new HIV infections diagnosed per year.
This is disheartening because, even without a vaccine, I believe we have the tools today to drive the epidemic down to zero. First of all, we know how to effectively diagnose and treat the infection, and we have evidence that antiretroviral treatment is an effective prevention tool. Secondly, advances in chemoprophylaxis have made pre-exposure prophylaxis a reality.
Ob.gyns. played a central role in one of the greatest successes of the use of antiretroviral drugs: the virtual elimination of mother-to-child transmission of HIV in the United States. Now, by fully utilizing the tools available today, ob.gyns. can play a critical role in ending the epidemic in the United States and beyond.
Tools for diagnosis and treatment
We have so many missed opportunities in fighting the HIV epidemic.
This is evident in data compiled for a model called the “HIV Care Continuum,” or HIV “Cascade of Care.” The model captures the sequential stages of HIV care from diagnosis to suppression of the virus. It was developed in 2011 by Dr. Edward Gardner, an infectious disease/HIV expert at Denver Public Health, and has since been used at the federal, state, and local levels to help identify gaps in HIV services.
Not too long ago, diagnosis was the biggest problem in reducing the public health burden of HIV. Today, the biggest problem is linking and keeping individuals in care. According to the latest analysis by the U.S. Centers for Disease Control and Prevention of the HIV Care Continuum, of the 1.2 million people estimated to be living with HIV in America in 2011, approximately 86% were diagnosed, but only 40% were linked to and stayed in care, 37% were prescribed antiretroviral therapy (ART), and 30% had achieved viral suppression.
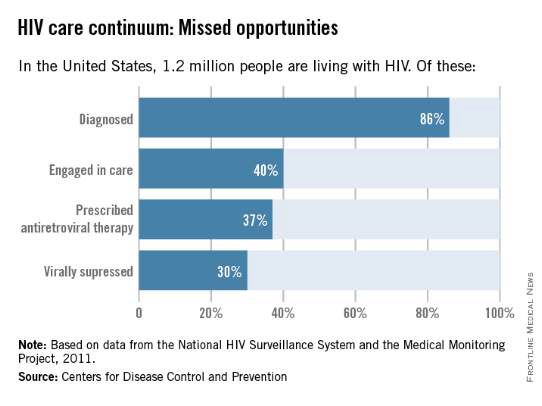
Only 30% of Americans living with HIV infection today are effectively treated, according to these data, even though we have the drugs and drug regimens available to treat everyone effectively.
Other analyses have included an additional stage of being initially linked to care (rather than being linked to care and retained in care). This presentation of the cascade, or continuum, further illuminates the progressive drop-off and that shows why an effective, sustained linkage to care is a critical component to ending the HIV epidemic.
One of these studies – an analysis published in 2013 – showed that approximately 82% of people were diagnosed, 66% were linked to care, 37% were retained in care, 33% were prescribed antiretroviral therapy, and 25% had a suppressed viral load of 200 copies/mL or less (JAMA. Intern. Med. 2013;173:1337-44).
With regard to women specifically, the CDC estimates that one in four people living with HIV infection are women, and that only about half of the women who are diagnosed with the infection are staying in care. Even fewer – 4 in 10 – have viral suppression, according to the CDC.
Expanding the management of HIV in the primary care setting could move us closer to ensuring that everyone in the United States who is infected with HIV is aware of the infection, is committed to treatment, and is virologically suppressed.
Like other primary care physicians, ob.gyns often have some degree of long-term continuity with patients – or the ability to create such continuity – that can be helpful for ensuring treatment compliance.
Ob.gyns also have valuable contact with adolescents, who fare worse throughout the cascade and are significantly more likely than older individuals to have unknown infections. An analysis published in 2014 of data for youth ages 13-29 shows that only 40% of HIV-infected youth were aware of their diagnosis and that an estimated 6% or less of HIV-infected youth were virally suppressed (AIDS. Patient. Care. STDS. 2014;28:128-135).
HIV testing should occur much more frequently than a decade ago, given the move in 2006 by the CDC from targeted risk-based testing to routine opt-out testing for all patients aged 13-64.
Treatment, moreover, has become much simpler in many respects. We have available to us more than 30 different drugs for individualizing therapy and providing treatment that allows patients to live a natural lifetime.
While such a large array of options may require those ob.gyns. who see only a few HIV-infected patients a year to work in consultation with an expert, many of the regimens require only a single, once-a-day pill. And while there was much debate as recently as five years ago about when to start treatment, there now is consensus that treatment should be started immediately after diagnosis (even in pregnant women), rather than waiting for the immune system to show signs of decline.
In fact, there is growing evidence that early treatment is key for both the infected individual and for individuals at risk. In the HIV Prevention Trials Network 052 study of discordant couples, for instance, early antiretroviral therapy in an infected partner not only reduced the number of clinical events; it almost completely blocked sexual transmission of the virus to an HIV-negative partner (N. Engl. J. Med. 2011;365:493-505).
The 052 study was a landmark “treatment as prevention” study. Other research has similarly shown that when the viral load of HIV-infected individuals is significantly reduced, their infectivity is reduced. And on a larger scale, research has shown that when we do this on a population basis, achieving widespread and continual treatment success, we can significantly impact the epidemic. This has been the case with the population of intravenous drug users in Vancouver, where the community viral load was significantly reduced by successful treatment that prevented new infections in this once-high-risk population.
Emerging data suggests that early diagnosis and treatment will likely also impact the likelihood of infected individuals achieving “functional cure.” The issue of functional cure – of achieving viral loads that are so low that drug therapy is no longer needed – has been receiving increasing attention in recent years, with the most promising findings reported thus far involving early treatment.
Tools for preexposure prophylaxis
For many years, we fit HIV care neatly into either the treatment or prevention category. More recently, we have come to appreciate that treatment is prevention, that a comprehensive prevention strategy must include treatment of infected individuals.
On the purely prevention side, it is important to continue educating women about safe sex behaviors. Most new HIV infections in women (84%) result from heterosexual contact, according to the CDC. For those who remain at risk of acquiring HIV despite education and counseling (eg., individuals who continue to engage in high-risk behaviors, or who have an HIV-positive partner), pre-exposure prophylaxis (PrEP) is now a safe and effective tool for preventing transmission. Patients deemed to be at high risk of acquiring HIV need to be made aware of this option.
PrEP originally was recommended only for gay or bisexual men, but in May 2014, the CDC recommended it for all individuals at risk and released the first comprehensive clinical practice guidelines for the prevention tool (www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf).
The PrEP medication, Truvada, is a combination of two drugs (tenovovir and emtricitabine) that, when taken daily on a consistent basis, significantly reduces the risk of getting HIV infection. Several large national and international studies have documented risk reductions of 73% to 92% when the medication was taken every day or almost every day. It is clearly within the purview of any ob.gyn to prescribe, monitor, and manage such prevention therapy.
The availability and relative ease of such a tool, along with advances in treatment and knowledge gained from the HIV Care Continuum, should re-energize ob.gyns. to up the ante in efforts to end the epidemic.
Experience in our clinical program that provides care and treatment to patients in the Baltimore-Washington area has taught us that we do much better when we integrate HIV care within primary care. It’s much more likely that patients will “stay close” with their ob.gyn than to another specialist.
Certainly, HIV infection has its “hot spots” and areas of much lower prevalence, but regardless of where we reside, we must continue to appreciate that the epidemic has had a significant impact on women and that this will persist unless we can all better utilize our available tools, such as early diagnosis and effective treatment that are linked long-term with other primary care physicians.
For women, ob.gyns represent a great resource for our nation to make progress toward President Obama’s National HIV Strategy.
Dr. Redfield reported that he has no disclosures relevant to this Master Class.
Despite staggering scientific and medical advances, the HIV epidemic in the United States has not changed significantly over the past decade. The estimated incidence of HIV infection has remained stable overall, with between 45,000 and 55,000 new HIV infections diagnosed per year.
This is disheartening because, even without a vaccine, I believe we have the tools today to drive the epidemic down to zero. First of all, we know how to effectively diagnose and treat the infection, and we have evidence that antiretroviral treatment is an effective prevention tool. Secondly, advances in chemoprophylaxis have made pre-exposure prophylaxis a reality.
Ob.gyns. played a central role in one of the greatest successes of the use of antiretroviral drugs: the virtual elimination of mother-to-child transmission of HIV in the United States. Now, by fully utilizing the tools available today, ob.gyns. can play a critical role in ending the epidemic in the United States and beyond.
Tools for diagnosis and treatment
We have so many missed opportunities in fighting the HIV epidemic.
This is evident in data compiled for a model called the “HIV Care Continuum,” or HIV “Cascade of Care.” The model captures the sequential stages of HIV care from diagnosis to suppression of the virus. It was developed in 2011 by Dr. Edward Gardner, an infectious disease/HIV expert at Denver Public Health, and has since been used at the federal, state, and local levels to help identify gaps in HIV services.
Not too long ago, diagnosis was the biggest problem in reducing the public health burden of HIV. Today, the biggest problem is linking and keeping individuals in care. According to the latest analysis by the U.S. Centers for Disease Control and Prevention of the HIV Care Continuum, of the 1.2 million people estimated to be living with HIV in America in 2011, approximately 86% were diagnosed, but only 40% were linked to and stayed in care, 37% were prescribed antiretroviral therapy (ART), and 30% had achieved viral suppression.

Only 30% of Americans living with HIV infection today are effectively treated, according to these data, even though we have the drugs and drug regimens available to treat everyone effectively.
Other analyses have included an additional stage of being initially linked to care (rather than being linked to care and retained in care). This presentation of the cascade, or continuum, further illuminates the progressive drop-off and that shows why an effective, sustained linkage to care is a critical component to ending the HIV epidemic.
One of these studies – an analysis published in 2013 – showed that approximately 82% of people were diagnosed, 66% were linked to care, 37% were retained in care, 33% were prescribed antiretroviral therapy, and 25% had a suppressed viral load of 200 copies/mL or less (JAMA. Intern. Med. 2013;173:1337-44).
With regard to women specifically, the CDC estimates that one in four people living with HIV infection are women, and that only about half of the women who are diagnosed with the infection are staying in care. Even fewer – 4 in 10 – have viral suppression, according to the CDC.
Expanding the management of HIV in the primary care setting could move us closer to ensuring that everyone in the United States who is infected with HIV is aware of the infection, is committed to treatment, and is virologically suppressed.
Like other primary care physicians, ob.gyns often have some degree of long-term continuity with patients – or the ability to create such continuity – that can be helpful for ensuring treatment compliance.
Ob.gyns also have valuable contact with adolescents, who fare worse throughout the cascade and are significantly more likely than older individuals to have unknown infections. An analysis published in 2014 of data for youth ages 13-29 shows that only 40% of HIV-infected youth were aware of their diagnosis and that an estimated 6% or less of HIV-infected youth were virally suppressed (AIDS. Patient. Care. STDS. 2014;28:128-135).
HIV testing should occur much more frequently than a decade ago, given the move in 2006 by the CDC from targeted risk-based testing to routine opt-out testing for all patients aged 13-64.
Treatment, moreover, has become much simpler in many respects. We have available to us more than 30 different drugs for individualizing therapy and providing treatment that allows patients to live a natural lifetime.
While such a large array of options may require those ob.gyns. who see only a few HIV-infected patients a year to work in consultation with an expert, many of the regimens require only a single, once-a-day pill. And while there was much debate as recently as five years ago about when to start treatment, there now is consensus that treatment should be started immediately after diagnosis (even in pregnant women), rather than waiting for the immune system to show signs of decline.
In fact, there is growing evidence that early treatment is key for both the infected individual and for individuals at risk. In the HIV Prevention Trials Network 052 study of discordant couples, for instance, early antiretroviral therapy in an infected partner not only reduced the number of clinical events; it almost completely blocked sexual transmission of the virus to an HIV-negative partner (N. Engl. J. Med. 2011;365:493-505).
The 052 study was a landmark “treatment as prevention” study. Other research has similarly shown that when the viral load of HIV-infected individuals is significantly reduced, their infectivity is reduced. And on a larger scale, research has shown that when we do this on a population basis, achieving widespread and continual treatment success, we can significantly impact the epidemic. This has been the case with the population of intravenous drug users in Vancouver, where the community viral load was significantly reduced by successful treatment that prevented new infections in this once-high-risk population.
Emerging data suggests that early diagnosis and treatment will likely also impact the likelihood of infected individuals achieving “functional cure.” The issue of functional cure – of achieving viral loads that are so low that drug therapy is no longer needed – has been receiving increasing attention in recent years, with the most promising findings reported thus far involving early treatment.
Tools for preexposure prophylaxis
For many years, we fit HIV care neatly into either the treatment or prevention category. More recently, we have come to appreciate that treatment is prevention, that a comprehensive prevention strategy must include treatment of infected individuals.
On the purely prevention side, it is important to continue educating women about safe sex behaviors. Most new HIV infections in women (84%) result from heterosexual contact, according to the CDC. For those who remain at risk of acquiring HIV despite education and counseling (eg., individuals who continue to engage in high-risk behaviors, or who have an HIV-positive partner), pre-exposure prophylaxis (PrEP) is now a safe and effective tool for preventing transmission. Patients deemed to be at high risk of acquiring HIV need to be made aware of this option.
PrEP originally was recommended only for gay or bisexual men, but in May 2014, the CDC recommended it for all individuals at risk and released the first comprehensive clinical practice guidelines for the prevention tool (www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf).
The PrEP medication, Truvada, is a combination of two drugs (tenovovir and emtricitabine) that, when taken daily on a consistent basis, significantly reduces the risk of getting HIV infection. Several large national and international studies have documented risk reductions of 73% to 92% when the medication was taken every day or almost every day. It is clearly within the purview of any ob.gyn to prescribe, monitor, and manage such prevention therapy.
The availability and relative ease of such a tool, along with advances in treatment and knowledge gained from the HIV Care Continuum, should re-energize ob.gyns. to up the ante in efforts to end the epidemic.
Experience in our clinical program that provides care and treatment to patients in the Baltimore-Washington area has taught us that we do much better when we integrate HIV care within primary care. It’s much more likely that patients will “stay close” with their ob.gyn than to another specialist.
Certainly, HIV infection has its “hot spots” and areas of much lower prevalence, but regardless of where we reside, we must continue to appreciate that the epidemic has had a significant impact on women and that this will persist unless we can all better utilize our available tools, such as early diagnosis and effective treatment that are linked long-term with other primary care physicians.
For women, ob.gyns represent a great resource for our nation to make progress toward President Obama’s National HIV Strategy.
Dr. Redfield reported that he has no disclosures relevant to this Master Class.
Despite staggering scientific and medical advances, the HIV epidemic in the United States has not changed significantly over the past decade. The estimated incidence of HIV infection has remained stable overall, with between 45,000 and 55,000 new HIV infections diagnosed per year.
This is disheartening because, even without a vaccine, I believe we have the tools today to drive the epidemic down to zero. First of all, we know how to effectively diagnose and treat the infection, and we have evidence that antiretroviral treatment is an effective prevention tool. Secondly, advances in chemoprophylaxis have made pre-exposure prophylaxis a reality.
Ob.gyns. played a central role in one of the greatest successes of the use of antiretroviral drugs: the virtual elimination of mother-to-child transmission of HIV in the United States. Now, by fully utilizing the tools available today, ob.gyns. can play a critical role in ending the epidemic in the United States and beyond.
Tools for diagnosis and treatment
We have so many missed opportunities in fighting the HIV epidemic.
This is evident in data compiled for a model called the “HIV Care Continuum,” or HIV “Cascade of Care.” The model captures the sequential stages of HIV care from diagnosis to suppression of the virus. It was developed in 2011 by Dr. Edward Gardner, an infectious disease/HIV expert at Denver Public Health, and has since been used at the federal, state, and local levels to help identify gaps in HIV services.
Not too long ago, diagnosis was the biggest problem in reducing the public health burden of HIV. Today, the biggest problem is linking and keeping individuals in care. According to the latest analysis by the U.S. Centers for Disease Control and Prevention of the HIV Care Continuum, of the 1.2 million people estimated to be living with HIV in America in 2011, approximately 86% were diagnosed, but only 40% were linked to and stayed in care, 37% were prescribed antiretroviral therapy (ART), and 30% had achieved viral suppression.

Only 30% of Americans living with HIV infection today are effectively treated, according to these data, even though we have the drugs and drug regimens available to treat everyone effectively.
Other analyses have included an additional stage of being initially linked to care (rather than being linked to care and retained in care). This presentation of the cascade, or continuum, further illuminates the progressive drop-off and that shows why an effective, sustained linkage to care is a critical component to ending the HIV epidemic.
One of these studies – an analysis published in 2013 – showed that approximately 82% of people were diagnosed, 66% were linked to care, 37% were retained in care, 33% were prescribed antiretroviral therapy, and 25% had a suppressed viral load of 200 copies/mL or less (JAMA. Intern. Med. 2013;173:1337-44).
With regard to women specifically, the CDC estimates that one in four people living with HIV infection are women, and that only about half of the women who are diagnosed with the infection are staying in care. Even fewer – 4 in 10 – have viral suppression, according to the CDC.
Expanding the management of HIV in the primary care setting could move us closer to ensuring that everyone in the United States who is infected with HIV is aware of the infection, is committed to treatment, and is virologically suppressed.
Like other primary care physicians, ob.gyns often have some degree of long-term continuity with patients – or the ability to create such continuity – that can be helpful for ensuring treatment compliance.
Ob.gyns also have valuable contact with adolescents, who fare worse throughout the cascade and are significantly more likely than older individuals to have unknown infections. An analysis published in 2014 of data for youth ages 13-29 shows that only 40% of HIV-infected youth were aware of their diagnosis and that an estimated 6% or less of HIV-infected youth were virally suppressed (AIDS. Patient. Care. STDS. 2014;28:128-135).
HIV testing should occur much more frequently than a decade ago, given the move in 2006 by the CDC from targeted risk-based testing to routine opt-out testing for all patients aged 13-64.
Treatment, moreover, has become much simpler in many respects. We have available to us more than 30 different drugs for individualizing therapy and providing treatment that allows patients to live a natural lifetime.
While such a large array of options may require those ob.gyns. who see only a few HIV-infected patients a year to work in consultation with an expert, many of the regimens require only a single, once-a-day pill. And while there was much debate as recently as five years ago about when to start treatment, there now is consensus that treatment should be started immediately after diagnosis (even in pregnant women), rather than waiting for the immune system to show signs of decline.
In fact, there is growing evidence that early treatment is key for both the infected individual and for individuals at risk. In the HIV Prevention Trials Network 052 study of discordant couples, for instance, early antiretroviral therapy in an infected partner not only reduced the number of clinical events; it almost completely blocked sexual transmission of the virus to an HIV-negative partner (N. Engl. J. Med. 2011;365:493-505).
The 052 study was a landmark “treatment as prevention” study. Other research has similarly shown that when the viral load of HIV-infected individuals is significantly reduced, their infectivity is reduced. And on a larger scale, research has shown that when we do this on a population basis, achieving widespread and continual treatment success, we can significantly impact the epidemic. This has been the case with the population of intravenous drug users in Vancouver, where the community viral load was significantly reduced by successful treatment that prevented new infections in this once-high-risk population.
Emerging data suggests that early diagnosis and treatment will likely also impact the likelihood of infected individuals achieving “functional cure.” The issue of functional cure – of achieving viral loads that are so low that drug therapy is no longer needed – has been receiving increasing attention in recent years, with the most promising findings reported thus far involving early treatment.
Tools for preexposure prophylaxis
For many years, we fit HIV care neatly into either the treatment or prevention category. More recently, we have come to appreciate that treatment is prevention, that a comprehensive prevention strategy must include treatment of infected individuals.
On the purely prevention side, it is important to continue educating women about safe sex behaviors. Most new HIV infections in women (84%) result from heterosexual contact, according to the CDC. For those who remain at risk of acquiring HIV despite education and counseling (eg., individuals who continue to engage in high-risk behaviors, or who have an HIV-positive partner), pre-exposure prophylaxis (PrEP) is now a safe and effective tool for preventing transmission. Patients deemed to be at high risk of acquiring HIV need to be made aware of this option.
PrEP originally was recommended only for gay or bisexual men, but in May 2014, the CDC recommended it for all individuals at risk and released the first comprehensive clinical practice guidelines for the prevention tool (www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf).
The PrEP medication, Truvada, is a combination of two drugs (tenovovir and emtricitabine) that, when taken daily on a consistent basis, significantly reduces the risk of getting HIV infection. Several large national and international studies have documented risk reductions of 73% to 92% when the medication was taken every day or almost every day. It is clearly within the purview of any ob.gyn to prescribe, monitor, and manage such prevention therapy.
The availability and relative ease of such a tool, along with advances in treatment and knowledge gained from the HIV Care Continuum, should re-energize ob.gyns. to up the ante in efforts to end the epidemic.
Experience in our clinical program that provides care and treatment to patients in the Baltimore-Washington area has taught us that we do much better when we integrate HIV care within primary care. It’s much more likely that patients will “stay close” with their ob.gyn than to another specialist.
Certainly, HIV infection has its “hot spots” and areas of much lower prevalence, but regardless of where we reside, we must continue to appreciate that the epidemic has had a significant impact on women and that this will persist unless we can all better utilize our available tools, such as early diagnosis and effective treatment that are linked long-term with other primary care physicians.
For women, ob.gyns represent a great resource for our nation to make progress toward President Obama’s National HIV Strategy.
Dr. Redfield reported that he has no disclosures relevant to this Master Class.
HIV treatment adherence still a challenge
It’s hard to believe that it was 30 years ago that HIV was discovered as the cause of AIDS by Dr. Robert Gallo and Dr. Luc Montagnier. Since then, the medical community has focused on preventing and eradicating the virus and its transmission. Despite the advent of highly efficacious antiretroviral therapy, and education efforts to prevent transmission, the disease continues to cause significant morbidity and mortality.
Surveillance data from the Centers for Disease Control and Prevention have indicated that screening and prevention efforts led to a decline in perinatally acquired HIV and AIDS by 80% and 93%, respectively. However, we still have far to go.
The CDC estimated that in 2010 more than 1 million people over age 13 were living with HIV, and approximately 50,000 new cases of HIV occur each year in the United States.
President Obama’s National HIV/AIDS Strategy for the United States, released in 2010, set ambitious goals for eradicating the disease in our country. We can only hope to achieve the President’s aims if the fight against the disease is taken up by all health care professionals, on multiple fronts, and throughout the many stages of a patient’s health.
In a 2011 Master Class, we addressed the importance of ob.gyns. testing nonpregnant women for HIV, as well as employing HIV prevention strategies to keep our female patients healthy, and prevent potential mother-to-baby transmission of the virus. Although transmission has decreased significantly, helping patients follow their treatment regimens remains a major barrier to eradicating the disease.
Ob.gyns. may be the only physicians who many women see throughout their lives. Therefore, we have a unique opportunity to educate our patients about seeking appropriate care and the need for adhering to treatment regimens.
Our guest author this month is Dr. Robert R. Redfield Jr., a distinguished professor in the department of medicine at the University of Maryland, Baltimore, and associate director of the university’s Institute of Human Virology, with clinical and research programs in virtually all countries in the continent of Africa. Dr. Redfield will discuss the role that physicians can play in terms of linking patients to care as a means of treating those with HIV and reducing the burden of disease. Dr. Redfield’s expertise in the area of novel therapeutics for the treatment of the virus, and his clinical experience in treating patients, provides a unique perspective into this important public health issue.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
It’s hard to believe that it was 30 years ago that HIV was discovered as the cause of AIDS by Dr. Robert Gallo and Dr. Luc Montagnier. Since then, the medical community has focused on preventing and eradicating the virus and its transmission. Despite the advent of highly efficacious antiretroviral therapy, and education efforts to prevent transmission, the disease continues to cause significant morbidity and mortality.
Surveillance data from the Centers for Disease Control and Prevention have indicated that screening and prevention efforts led to a decline in perinatally acquired HIV and AIDS by 80% and 93%, respectively. However, we still have far to go.
The CDC estimated that in 2010 more than 1 million people over age 13 were living with HIV, and approximately 50,000 new cases of HIV occur each year in the United States.
President Obama’s National HIV/AIDS Strategy for the United States, released in 2010, set ambitious goals for eradicating the disease in our country. We can only hope to achieve the President’s aims if the fight against the disease is taken up by all health care professionals, on multiple fronts, and throughout the many stages of a patient’s health.
In a 2011 Master Class, we addressed the importance of ob.gyns. testing nonpregnant women for HIV, as well as employing HIV prevention strategies to keep our female patients healthy, and prevent potential mother-to-baby transmission of the virus. Although transmission has decreased significantly, helping patients follow their treatment regimens remains a major barrier to eradicating the disease.
Ob.gyns. may be the only physicians who many women see throughout their lives. Therefore, we have a unique opportunity to educate our patients about seeking appropriate care and the need for adhering to treatment regimens.
Our guest author this month is Dr. Robert R. Redfield Jr., a distinguished professor in the department of medicine at the University of Maryland, Baltimore, and associate director of the university’s Institute of Human Virology, with clinical and research programs in virtually all countries in the continent of Africa. Dr. Redfield will discuss the role that physicians can play in terms of linking patients to care as a means of treating those with HIV and reducing the burden of disease. Dr. Redfield’s expertise in the area of novel therapeutics for the treatment of the virus, and his clinical experience in treating patients, provides a unique perspective into this important public health issue.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
It’s hard to believe that it was 30 years ago that HIV was discovered as the cause of AIDS by Dr. Robert Gallo and Dr. Luc Montagnier. Since then, the medical community has focused on preventing and eradicating the virus and its transmission. Despite the advent of highly efficacious antiretroviral therapy, and education efforts to prevent transmission, the disease continues to cause significant morbidity and mortality.
Surveillance data from the Centers for Disease Control and Prevention have indicated that screening and prevention efforts led to a decline in perinatally acquired HIV and AIDS by 80% and 93%, respectively. However, we still have far to go.
The CDC estimated that in 2010 more than 1 million people over age 13 were living with HIV, and approximately 50,000 new cases of HIV occur each year in the United States.
President Obama’s National HIV/AIDS Strategy for the United States, released in 2010, set ambitious goals for eradicating the disease in our country. We can only hope to achieve the President’s aims if the fight against the disease is taken up by all health care professionals, on multiple fronts, and throughout the many stages of a patient’s health.
In a 2011 Master Class, we addressed the importance of ob.gyns. testing nonpregnant women for HIV, as well as employing HIV prevention strategies to keep our female patients healthy, and prevent potential mother-to-baby transmission of the virus. Although transmission has decreased significantly, helping patients follow their treatment regimens remains a major barrier to eradicating the disease.
Ob.gyns. may be the only physicians who many women see throughout their lives. Therefore, we have a unique opportunity to educate our patients about seeking appropriate care and the need for adhering to treatment regimens.
Our guest author this month is Dr. Robert R. Redfield Jr., a distinguished professor in the department of medicine at the University of Maryland, Baltimore, and associate director of the university’s Institute of Human Virology, with clinical and research programs in virtually all countries in the continent of Africa. Dr. Redfield will discuss the role that physicians can play in terms of linking patients to care as a means of treating those with HIV and reducing the burden of disease. Dr. Redfield’s expertise in the area of novel therapeutics for the treatment of the virus, and his clinical experience in treating patients, provides a unique perspective into this important public health issue.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Selling the better mousetrap
Despite all the hoopla about Ebola and measles this winter, the most common reason for admitting an infant or young child to the hospital continues to be bronchiolitis. Yet clinical practice guidelines for diagnosing and treating this common infection have not been incorporated into clinical practice.
The use of over-the-counter cold medications to treat upper respiratory infections in young children was shown by meta-analysis in the mid-1990’s to be ineffective, but that use continued until the Food and Drug Administration mandated revisions to packaging in 2008. Antibiotics have been commonly prescribed to treat the ear infections and sinusitis that frequently occur with bronchiolitis. But over the past 20 years, the use of antibiotics has become less prevalent. I date that trend to the work of Dr. Jack Paradise, professor emeritus of pediatrics at the University of Pittsburgh, and Dr. Ellen Wald, now chair of pediatrics at the University of Wisconsin, Madison, in the mid-1990’s. RespiGam was approved in 1996, then supplanted with palivizumab, as a medication to reduce the burden of respiratory syncytial virus disease. In the summer of 2014, an updated analysis of the costs, risks, and benefits of RSV prophylaxis led to new recommendations that curtailed the indications for that treatment (Pediatrics 2014:134;415-20). What do these trends have in common? The time frame.
It is often cited that it takes 17 years for new evidence to be assimilated into clinical practice (J.R. Soc. Med. 2011;104:510-20). An Institute of Medicine report in 2001, “Crossing the Quality Chasm,” emphasized the importance of becoming more efficient at making progress. Those recommendations themselves are now 14 years old, and I’m not expecting a revolution in human behavior within the next 3 years.
In the new clinical practice guideline issued by the American Academy of Pediatrics in November 2014 for the treatment of young children with bronchiolitis, Dr. Shawn L. Ralston and her colleagues assessed various treatment modalities, found many to be ineffective, and recommended discontinuing their routine use (Pediatrics 2014;134:e1474-e1502). Beta-agonists were at the forefront of this. Was the new guideline based on new data? For the most part, no. In my reading, itmostly reiterated the concerns about effectiveness that were expressed at the time of the prior guidelines from 2006, but removed the weasel words. I admire the dedication of this committee to evidence-based medicine. But will this revised clinical practice guideline actually change practice?
The saying is, “Build a better mousetrap and the world will beat a path to your door.” That quote has been attributed (without adequate documentation) to Ralph Waldo Emerson. He was a great poet, but not a scientist.
During the same month that the new bronchiolitis guidelinewas being released, America held some elections. In the post mortem, President Obama said, “There is a tendency sometimes for me to start thinking: As long as I get the policy right, then that’s what should matter.” He elaborated that “one thing that I do need to constantly remind myself and my team of is it’s not enough just to build the better mousetrap. People don’t automatically come beating to your door. We’ve got to sell it; we’ve got to reach out to the other side and where possible, persuade” (The Wall Street Journal, Nov. 10, 2014).
That isn’t poetry, but the President’s idea is probably more accurate than Emerson’s.
The bronchiolitis clinical practice guidelinewas written in a standardized fashion with 14 key action statements and 242 references. That makes for a good evidence-based medicine document, but is not the best sales pitch.
What will it take to translate these new guidelines into practice? One option is to teach new residents the new guidelines and expect dinosaurs such as myself to retire. If the average pediatrician works for about 34 years, then over a period of 17 years, we will have replaced half the miscreants simply by attrition.
A program of reaching out to the other side and persuading them to change is a better option.
In discussions about this topic on a listserv for pediatric hospitalists, I focused on my concerns. We need to clarify the harms associated with therapies such as beta-agonists, deep nasal suctioning, and continuous pulse oximetry. We need to clarify the goals of treatment, which might include a shorter length of stay, patient comfort, meeting parents’ expectations that we will do something, and/or explaining why we are contradicting any previous recommendations made to the parents. We need to mesh these bronchiolitis guidelines with the asthma action plans and medication lists advocated for wheezing children who are 24 months of age. My colleagues pointed out that all of that is just continuing to refine the policy.
Getting the policy right is necessary but insufficient. What we are really missing is a campaign strategy to sell it.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He is also listserv moderator for the American Academy of Pediatrics Section on Hospital Medicine.
Despite all the hoopla about Ebola and measles this winter, the most common reason for admitting an infant or young child to the hospital continues to be bronchiolitis. Yet clinical practice guidelines for diagnosing and treating this common infection have not been incorporated into clinical practice.
The use of over-the-counter cold medications to treat upper respiratory infections in young children was shown by meta-analysis in the mid-1990’s to be ineffective, but that use continued until the Food and Drug Administration mandated revisions to packaging in 2008. Antibiotics have been commonly prescribed to treat the ear infections and sinusitis that frequently occur with bronchiolitis. But over the past 20 years, the use of antibiotics has become less prevalent. I date that trend to the work of Dr. Jack Paradise, professor emeritus of pediatrics at the University of Pittsburgh, and Dr. Ellen Wald, now chair of pediatrics at the University of Wisconsin, Madison, in the mid-1990’s. RespiGam was approved in 1996, then supplanted with palivizumab, as a medication to reduce the burden of respiratory syncytial virus disease. In the summer of 2014, an updated analysis of the costs, risks, and benefits of RSV prophylaxis led to new recommendations that curtailed the indications for that treatment (Pediatrics 2014:134;415-20). What do these trends have in common? The time frame.
It is often cited that it takes 17 years for new evidence to be assimilated into clinical practice (J.R. Soc. Med. 2011;104:510-20). An Institute of Medicine report in 2001, “Crossing the Quality Chasm,” emphasized the importance of becoming more efficient at making progress. Those recommendations themselves are now 14 years old, and I’m not expecting a revolution in human behavior within the next 3 years.
In the new clinical practice guideline issued by the American Academy of Pediatrics in November 2014 for the treatment of young children with bronchiolitis, Dr. Shawn L. Ralston and her colleagues assessed various treatment modalities, found many to be ineffective, and recommended discontinuing their routine use (Pediatrics 2014;134:e1474-e1502). Beta-agonists were at the forefront of this. Was the new guideline based on new data? For the most part, no. In my reading, itmostly reiterated the concerns about effectiveness that were expressed at the time of the prior guidelines from 2006, but removed the weasel words. I admire the dedication of this committee to evidence-based medicine. But will this revised clinical practice guideline actually change practice?
The saying is, “Build a better mousetrap and the world will beat a path to your door.” That quote has been attributed (without adequate documentation) to Ralph Waldo Emerson. He was a great poet, but not a scientist.
During the same month that the new bronchiolitis guidelinewas being released, America held some elections. In the post mortem, President Obama said, “There is a tendency sometimes for me to start thinking: As long as I get the policy right, then that’s what should matter.” He elaborated that “one thing that I do need to constantly remind myself and my team of is it’s not enough just to build the better mousetrap. People don’t automatically come beating to your door. We’ve got to sell it; we’ve got to reach out to the other side and where possible, persuade” (The Wall Street Journal, Nov. 10, 2014).
That isn’t poetry, but the President’s idea is probably more accurate than Emerson’s.
The bronchiolitis clinical practice guidelinewas written in a standardized fashion with 14 key action statements and 242 references. That makes for a good evidence-based medicine document, but is not the best sales pitch.
What will it take to translate these new guidelines into practice? One option is to teach new residents the new guidelines and expect dinosaurs such as myself to retire. If the average pediatrician works for about 34 years, then over a period of 17 years, we will have replaced half the miscreants simply by attrition.
A program of reaching out to the other side and persuading them to change is a better option.
In discussions about this topic on a listserv for pediatric hospitalists, I focused on my concerns. We need to clarify the harms associated with therapies such as beta-agonists, deep nasal suctioning, and continuous pulse oximetry. We need to clarify the goals of treatment, which might include a shorter length of stay, patient comfort, meeting parents’ expectations that we will do something, and/or explaining why we are contradicting any previous recommendations made to the parents. We need to mesh these bronchiolitis guidelines with the asthma action plans and medication lists advocated for wheezing children who are 24 months of age. My colleagues pointed out that all of that is just continuing to refine the policy.
Getting the policy right is necessary but insufficient. What we are really missing is a campaign strategy to sell it.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He is also listserv moderator for the American Academy of Pediatrics Section on Hospital Medicine.
Despite all the hoopla about Ebola and measles this winter, the most common reason for admitting an infant or young child to the hospital continues to be bronchiolitis. Yet clinical practice guidelines for diagnosing and treating this common infection have not been incorporated into clinical practice.
The use of over-the-counter cold medications to treat upper respiratory infections in young children was shown by meta-analysis in the mid-1990’s to be ineffective, but that use continued until the Food and Drug Administration mandated revisions to packaging in 2008. Antibiotics have been commonly prescribed to treat the ear infections and sinusitis that frequently occur with bronchiolitis. But over the past 20 years, the use of antibiotics has become less prevalent. I date that trend to the work of Dr. Jack Paradise, professor emeritus of pediatrics at the University of Pittsburgh, and Dr. Ellen Wald, now chair of pediatrics at the University of Wisconsin, Madison, in the mid-1990’s. RespiGam was approved in 1996, then supplanted with palivizumab, as a medication to reduce the burden of respiratory syncytial virus disease. In the summer of 2014, an updated analysis of the costs, risks, and benefits of RSV prophylaxis led to new recommendations that curtailed the indications for that treatment (Pediatrics 2014:134;415-20). What do these trends have in common? The time frame.
It is often cited that it takes 17 years for new evidence to be assimilated into clinical practice (J.R. Soc. Med. 2011;104:510-20). An Institute of Medicine report in 2001, “Crossing the Quality Chasm,” emphasized the importance of becoming more efficient at making progress. Those recommendations themselves are now 14 years old, and I’m not expecting a revolution in human behavior within the next 3 years.
In the new clinical practice guideline issued by the American Academy of Pediatrics in November 2014 for the treatment of young children with bronchiolitis, Dr. Shawn L. Ralston and her colleagues assessed various treatment modalities, found many to be ineffective, and recommended discontinuing their routine use (Pediatrics 2014;134:e1474-e1502). Beta-agonists were at the forefront of this. Was the new guideline based on new data? For the most part, no. In my reading, itmostly reiterated the concerns about effectiveness that were expressed at the time of the prior guidelines from 2006, but removed the weasel words. I admire the dedication of this committee to evidence-based medicine. But will this revised clinical practice guideline actually change practice?
The saying is, “Build a better mousetrap and the world will beat a path to your door.” That quote has been attributed (without adequate documentation) to Ralph Waldo Emerson. He was a great poet, but not a scientist.
During the same month that the new bronchiolitis guidelinewas being released, America held some elections. In the post mortem, President Obama said, “There is a tendency sometimes for me to start thinking: As long as I get the policy right, then that’s what should matter.” He elaborated that “one thing that I do need to constantly remind myself and my team of is it’s not enough just to build the better mousetrap. People don’t automatically come beating to your door. We’ve got to sell it; we’ve got to reach out to the other side and where possible, persuade” (The Wall Street Journal, Nov. 10, 2014).
That isn’t poetry, but the President’s idea is probably more accurate than Emerson’s.
The bronchiolitis clinical practice guidelinewas written in a standardized fashion with 14 key action statements and 242 references. That makes for a good evidence-based medicine document, but is not the best sales pitch.
What will it take to translate these new guidelines into practice? One option is to teach new residents the new guidelines and expect dinosaurs such as myself to retire. If the average pediatrician works for about 34 years, then over a period of 17 years, we will have replaced half the miscreants simply by attrition.
A program of reaching out to the other side and persuading them to change is a better option.
In discussions about this topic on a listserv for pediatric hospitalists, I focused on my concerns. We need to clarify the harms associated with therapies such as beta-agonists, deep nasal suctioning, and continuous pulse oximetry. We need to clarify the goals of treatment, which might include a shorter length of stay, patient comfort, meeting parents’ expectations that we will do something, and/or explaining why we are contradicting any previous recommendations made to the parents. We need to mesh these bronchiolitis guidelines with the asthma action plans and medication lists advocated for wheezing children who are 24 months of age. My colleagues pointed out that all of that is just continuing to refine the policy.
Getting the policy right is necessary but insufficient. What we are really missing is a campaign strategy to sell it.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He is also listserv moderator for the American Academy of Pediatrics Section on Hospital Medicine.
Too sick to work?
By yesterday at lunch time, you knew you were sick. The sniffly nose and scratchy throat of the previous 2 days were maturing into a full-blown cold or worse. You woke this morning feeling achy and a bit feverish. The only thermometer in the house is a scary looking thing in the cutlery drawer next to the kitchen stove. There have been no cases of influenza reported in the country or even the state.
It is Thursday, and it is your partner’s traditional day off. You think you remember him saying that he was planning on driving out of state to visit his daughter who was struggling in her freshman year in college. Your new associate is in St. Louis taking her boards. The questions that need to be answered by 7:30 this morning are: Do I see if I can reach my partner before he leaves town and ask him if can work for me? If he is already on the road, do I call the office and tell them to cancel the day’s schedule because I am too sick to work?
This is the kind of scenario that most have us have faced more than once in our working lives. Who will I be putting at risk by going to work when I am sick? Of course, there are my patients. Is my patient population particularly fragile because of their age or immunological vulnerabilities? And there are my coworkers. Last of all, will going to work make me even sicker so that I will miss more work?
Where do you go for help in answering the question of whether you are too sick to go to work? Should you try to find a thermometer at an all-night convenience store? If you find one, exactly what temperature is the threshold that will prompt you to call in sick? How many sneezes per hour will render you too contagious to work? How many coughs? If your illness is primarily gastrointestinal, are you still a threat to your patients if your trips to the bathroom are spaced far enough apart to allow you to spend 15 minutes trapped in an examining room?
Would wearing a mask be of any benefit? My sense is that it wouldn’t help and may make you more of a threat if you keeping fiddling with it to readjust it for comfort. And a mask will certainly alarm some parents.
There are situations in which you look or sound worse than you are. I seem to develop laryngitis several days after the worst of my cold has passed. Unfortunately, this scenario is not one of those situations. If you show up in the office, you are going to sound and maybe look like you are as sick as you feel.
What are you going to do? I am embarrassed to admit that I was one of those masochists who would have gone to work regardless of my state of health. You would have had to tether me to an IV bottle to keep me at home. As a recovering workaholic, I have had to accept the fact that I may have jeopardized the health of some of my patients by my pigheaded and at times selfish devotion to showing up in the office come hell or high fever. But, on days when I was the only show in town, it was easy to fall into the trap of believing that I was indispensable. Although full-time emergency room physicians and hospitalists hadn’t been invented yet, there were a few other primary care physicians. I guess it was pride that prevented me from admitting that I could have called on them for help, even though they weren’t board certified pediatricians.
On the other hand, I still wonder how much harm I did by dragging myself to work when I was sick. Because Brunswick is a small town, I know my overly intense devotion to work didn’t result in any deaths. But how great was the collateral damage in the form of lost days from school and work for my patients, their parents, and my coworkers? There is no way to know, but I am sure there was some.
It is unreasonable to say, “I won’t ever go to work if I am ill.” I may have set the bar too high, but I am interested to hear how you decide when you are too sick to go to work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
By yesterday at lunch time, you knew you were sick. The sniffly nose and scratchy throat of the previous 2 days were maturing into a full-blown cold or worse. You woke this morning feeling achy and a bit feverish. The only thermometer in the house is a scary looking thing in the cutlery drawer next to the kitchen stove. There have been no cases of influenza reported in the country or even the state.
It is Thursday, and it is your partner’s traditional day off. You think you remember him saying that he was planning on driving out of state to visit his daughter who was struggling in her freshman year in college. Your new associate is in St. Louis taking her boards. The questions that need to be answered by 7:30 this morning are: Do I see if I can reach my partner before he leaves town and ask him if can work for me? If he is already on the road, do I call the office and tell them to cancel the day’s schedule because I am too sick to work?
This is the kind of scenario that most have us have faced more than once in our working lives. Who will I be putting at risk by going to work when I am sick? Of course, there are my patients. Is my patient population particularly fragile because of their age or immunological vulnerabilities? And there are my coworkers. Last of all, will going to work make me even sicker so that I will miss more work?
Where do you go for help in answering the question of whether you are too sick to go to work? Should you try to find a thermometer at an all-night convenience store? If you find one, exactly what temperature is the threshold that will prompt you to call in sick? How many sneezes per hour will render you too contagious to work? How many coughs? If your illness is primarily gastrointestinal, are you still a threat to your patients if your trips to the bathroom are spaced far enough apart to allow you to spend 15 minutes trapped in an examining room?
Would wearing a mask be of any benefit? My sense is that it wouldn’t help and may make you more of a threat if you keeping fiddling with it to readjust it for comfort. And a mask will certainly alarm some parents.
There are situations in which you look or sound worse than you are. I seem to develop laryngitis several days after the worst of my cold has passed. Unfortunately, this scenario is not one of those situations. If you show up in the office, you are going to sound and maybe look like you are as sick as you feel.
What are you going to do? I am embarrassed to admit that I was one of those masochists who would have gone to work regardless of my state of health. You would have had to tether me to an IV bottle to keep me at home. As a recovering workaholic, I have had to accept the fact that I may have jeopardized the health of some of my patients by my pigheaded and at times selfish devotion to showing up in the office come hell or high fever. But, on days when I was the only show in town, it was easy to fall into the trap of believing that I was indispensable. Although full-time emergency room physicians and hospitalists hadn’t been invented yet, there were a few other primary care physicians. I guess it was pride that prevented me from admitting that I could have called on them for help, even though they weren’t board certified pediatricians.
On the other hand, I still wonder how much harm I did by dragging myself to work when I was sick. Because Brunswick is a small town, I know my overly intense devotion to work didn’t result in any deaths. But how great was the collateral damage in the form of lost days from school and work for my patients, their parents, and my coworkers? There is no way to know, but I am sure there was some.
It is unreasonable to say, “I won’t ever go to work if I am ill.” I may have set the bar too high, but I am interested to hear how you decide when you are too sick to go to work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
By yesterday at lunch time, you knew you were sick. The sniffly nose and scratchy throat of the previous 2 days were maturing into a full-blown cold or worse. You woke this morning feeling achy and a bit feverish. The only thermometer in the house is a scary looking thing in the cutlery drawer next to the kitchen stove. There have been no cases of influenza reported in the country or even the state.
It is Thursday, and it is your partner’s traditional day off. You think you remember him saying that he was planning on driving out of state to visit his daughter who was struggling in her freshman year in college. Your new associate is in St. Louis taking her boards. The questions that need to be answered by 7:30 this morning are: Do I see if I can reach my partner before he leaves town and ask him if can work for me? If he is already on the road, do I call the office and tell them to cancel the day’s schedule because I am too sick to work?
This is the kind of scenario that most have us have faced more than once in our working lives. Who will I be putting at risk by going to work when I am sick? Of course, there are my patients. Is my patient population particularly fragile because of their age or immunological vulnerabilities? And there are my coworkers. Last of all, will going to work make me even sicker so that I will miss more work?
Where do you go for help in answering the question of whether you are too sick to go to work? Should you try to find a thermometer at an all-night convenience store? If you find one, exactly what temperature is the threshold that will prompt you to call in sick? How many sneezes per hour will render you too contagious to work? How many coughs? If your illness is primarily gastrointestinal, are you still a threat to your patients if your trips to the bathroom are spaced far enough apart to allow you to spend 15 minutes trapped in an examining room?
Would wearing a mask be of any benefit? My sense is that it wouldn’t help and may make you more of a threat if you keeping fiddling with it to readjust it for comfort. And a mask will certainly alarm some parents.
There are situations in which you look or sound worse than you are. I seem to develop laryngitis several days after the worst of my cold has passed. Unfortunately, this scenario is not one of those situations. If you show up in the office, you are going to sound and maybe look like you are as sick as you feel.
What are you going to do? I am embarrassed to admit that I was one of those masochists who would have gone to work regardless of my state of health. You would have had to tether me to an IV bottle to keep me at home. As a recovering workaholic, I have had to accept the fact that I may have jeopardized the health of some of my patients by my pigheaded and at times selfish devotion to showing up in the office come hell or high fever. But, on days when I was the only show in town, it was easy to fall into the trap of believing that I was indispensable. Although full-time emergency room physicians and hospitalists hadn’t been invented yet, there were a few other primary care physicians. I guess it was pride that prevented me from admitting that I could have called on them for help, even though they weren’t board certified pediatricians.
On the other hand, I still wonder how much harm I did by dragging myself to work when I was sick. Because Brunswick is a small town, I know my overly intense devotion to work didn’t result in any deaths. But how great was the collateral damage in the form of lost days from school and work for my patients, their parents, and my coworkers? There is no way to know, but I am sure there was some.
It is unreasonable to say, “I won’t ever go to work if I am ill.” I may have set the bar too high, but I am interested to hear how you decide when you are too sick to go to work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
The 2014 AAP COID update on palivizumab
The American Academy of Pediatrics Committee on Infectious Diseases released a 2014 policy statement (Pediatrics 2014:134;415-20) updating guidance on use of palivizumab in high-risk patients infected with respiratory syncytial virus (RSV). The policy statement was accompanied by a detailed technical report to explain the committee’s rationale for the changes made since the previous update in 2012.
Since 2012, several observational studies were published related to risk factors associated with the need for hospitalization for RSV infection. The Committee on Infectious Diseases (COID) referred to these observational studies, along with other historical studies, in its 2014 technical report.
Some aspects of the updated guidance have met with controversy because the new advice limits palivizumab use among premature infants. The 2014 COID policy asserts that preterm infants born at greater than 29 weeks’ gestational age (GA) do not benefit substantially from palivizumab dosing during RSV season. This assertion has met with controversy because the justification detailed in the technical report cites several observational studies that don’t appear to support that conclusion. What percentage of a cohort would need to be hospitalized for RSV infection to identify them as high risk? COID doesn’t draw that line for us. Because approximately 3% of the term birth cohort is hospitalized with RSV, should we use this as the “baseline” and predetermine a percentage of hospitalization beyond baseline to define “at increased risk”?
The 2014 technical report states that RSV hospitalization rates from Stevens et al. (Arch. Pediatr. Adolesc. Med. 2000;154:55-61) were 7.5% and 4.4% for infants 28-30 weeks’ gestational age (GA) and 30-32 weeks’ GA, respectively. However, the rates quoted by COID were not the generalizable rates reported by Dr. T. P. Stevens and his team in their study. The authors calculated community-wide hospitalization rates for these groups to be 10% and 6.4%, respectively. These author-reported community-wide rates closely mirror rates reported in several other large observational studies, and still likely underestimate the true burden of RSV hospitalization based on the lower sensitivity of RSV detection testing done 15 years ago when that study was performed.
Similarly, the COID states that Dr. A.G. Winterstein’s study (JAMA Pediatrics 2013;167:1118-24) shows hospitalization rates among 32-34 weeks’ GA infants of 3.1% and 4.5% in two states based on health care insurance claims. Because fewer than half of infants are ever tested for RSV as the possible cause of their lower respiratory tract infection, these claims data are most certainly an underestimate of true rates. When active testing for RSV is done among hospitalized infants in this same GA category, using sensitive and specific PCR-based technology, hospitalization rates are 9.1%. Dr. Winterstein and associates also reported that RSV hospitalization rates increase as gestational age decreases.
In Dr. Caroline Breese Hall’s 2013 observational study (Pediatrics 2013;132:341-8) she and her associates recognized that hospitalization rates in children under 2 years of age were similar among term and preterm infants. The COID used these data in its 2014 technical report to suggest that preterm infants are no longer at high risk. The detail omitted from the report was that approximately 70% of eligible preterm infants in Dr. Hall’s study (according to the 2012 COID guidelines) had received palivizumab prophylaxis. This suggests that maintaining RSV prophylaxis according to the 2012 COID policy statement (and not the 2014 policy statement) can be successful in reducing the rate of RSV hospitalizations in preterm infants to that observed in term infants.
Finally, the technical report cites “overall declining incidence of hospitalizations for bronchiolitis in the United States” from the 2013 publication by Hasegawa et al. (Pediatrics 2013:132:28-36) without defining this observation for the AAP readers. While Hasegawa did report such a decline, the decline was specific to term infants, as no such decline was observed in the higher-risk preterm infants.
Based on careful review of several of the manuscripts referenced by COID, it’s difficult to determine why the committee’s recommendations changed so dramatically in the most recent iteration. It appears that the data need another look.
Dr. Domachowske is professor of pediatrics and professor of microbiology and immunology at the State University of New York Upstate Medical University in Syracuse. He serves on the New York State American Academy of Pediatrics Chapter 1 executive committee, volunteers as his district’s immunization champion, and is an appointed member of the New York State Immunization Advisory Council. He is a Pediatric News Editorial Advisory Board member. Dr. Domachowske disclosed he does consulting for the vaccine divisions of GlaxoSmithKline, Medimmune, Pfizer, Merck, Sanofi Pasteur, and Novartis; he performs clinical trials with GlaxoSmithKline, Medimmune, Merck, and Novartis; and he does basic and translational research with GlaxoSmithKline. E-mail him at [email protected].
The American Academy of Pediatrics Committee on Infectious Diseases released a 2014 policy statement (Pediatrics 2014:134;415-20) updating guidance on use of palivizumab in high-risk patients infected with respiratory syncytial virus (RSV). The policy statement was accompanied by a detailed technical report to explain the committee’s rationale for the changes made since the previous update in 2012.
Since 2012, several observational studies were published related to risk factors associated with the need for hospitalization for RSV infection. The Committee on Infectious Diseases (COID) referred to these observational studies, along with other historical studies, in its 2014 technical report.
Some aspects of the updated guidance have met with controversy because the new advice limits palivizumab use among premature infants. The 2014 COID policy asserts that preterm infants born at greater than 29 weeks’ gestational age (GA) do not benefit substantially from palivizumab dosing during RSV season. This assertion has met with controversy because the justification detailed in the technical report cites several observational studies that don’t appear to support that conclusion. What percentage of a cohort would need to be hospitalized for RSV infection to identify them as high risk? COID doesn’t draw that line for us. Because approximately 3% of the term birth cohort is hospitalized with RSV, should we use this as the “baseline” and predetermine a percentage of hospitalization beyond baseline to define “at increased risk”?
The 2014 technical report states that RSV hospitalization rates from Stevens et al. (Arch. Pediatr. Adolesc. Med. 2000;154:55-61) were 7.5% and 4.4% for infants 28-30 weeks’ gestational age (GA) and 30-32 weeks’ GA, respectively. However, the rates quoted by COID were not the generalizable rates reported by Dr. T. P. Stevens and his team in their study. The authors calculated community-wide hospitalization rates for these groups to be 10% and 6.4%, respectively. These author-reported community-wide rates closely mirror rates reported in several other large observational studies, and still likely underestimate the true burden of RSV hospitalization based on the lower sensitivity of RSV detection testing done 15 years ago when that study was performed.
Similarly, the COID states that Dr. A.G. Winterstein’s study (JAMA Pediatrics 2013;167:1118-24) shows hospitalization rates among 32-34 weeks’ GA infants of 3.1% and 4.5% in two states based on health care insurance claims. Because fewer than half of infants are ever tested for RSV as the possible cause of their lower respiratory tract infection, these claims data are most certainly an underestimate of true rates. When active testing for RSV is done among hospitalized infants in this same GA category, using sensitive and specific PCR-based technology, hospitalization rates are 9.1%. Dr. Winterstein and associates also reported that RSV hospitalization rates increase as gestational age decreases.
In Dr. Caroline Breese Hall’s 2013 observational study (Pediatrics 2013;132:341-8) she and her associates recognized that hospitalization rates in children under 2 years of age were similar among term and preterm infants. The COID used these data in its 2014 technical report to suggest that preterm infants are no longer at high risk. The detail omitted from the report was that approximately 70% of eligible preterm infants in Dr. Hall’s study (according to the 2012 COID guidelines) had received palivizumab prophylaxis. This suggests that maintaining RSV prophylaxis according to the 2012 COID policy statement (and not the 2014 policy statement) can be successful in reducing the rate of RSV hospitalizations in preterm infants to that observed in term infants.
Finally, the technical report cites “overall declining incidence of hospitalizations for bronchiolitis in the United States” from the 2013 publication by Hasegawa et al. (Pediatrics 2013:132:28-36) without defining this observation for the AAP readers. While Hasegawa did report such a decline, the decline was specific to term infants, as no such decline was observed in the higher-risk preterm infants.
Based on careful review of several of the manuscripts referenced by COID, it’s difficult to determine why the committee’s recommendations changed so dramatically in the most recent iteration. It appears that the data need another look.
Dr. Domachowske is professor of pediatrics and professor of microbiology and immunology at the State University of New York Upstate Medical University in Syracuse. He serves on the New York State American Academy of Pediatrics Chapter 1 executive committee, volunteers as his district’s immunization champion, and is an appointed member of the New York State Immunization Advisory Council. He is a Pediatric News Editorial Advisory Board member. Dr. Domachowske disclosed he does consulting for the vaccine divisions of GlaxoSmithKline, Medimmune, Pfizer, Merck, Sanofi Pasteur, and Novartis; he performs clinical trials with GlaxoSmithKline, Medimmune, Merck, and Novartis; and he does basic and translational research with GlaxoSmithKline. E-mail him at [email protected].
The American Academy of Pediatrics Committee on Infectious Diseases released a 2014 policy statement (Pediatrics 2014:134;415-20) updating guidance on use of palivizumab in high-risk patients infected with respiratory syncytial virus (RSV). The policy statement was accompanied by a detailed technical report to explain the committee’s rationale for the changes made since the previous update in 2012.
Since 2012, several observational studies were published related to risk factors associated with the need for hospitalization for RSV infection. The Committee on Infectious Diseases (COID) referred to these observational studies, along with other historical studies, in its 2014 technical report.
Some aspects of the updated guidance have met with controversy because the new advice limits palivizumab use among premature infants. The 2014 COID policy asserts that preterm infants born at greater than 29 weeks’ gestational age (GA) do not benefit substantially from palivizumab dosing during RSV season. This assertion has met with controversy because the justification detailed in the technical report cites several observational studies that don’t appear to support that conclusion. What percentage of a cohort would need to be hospitalized for RSV infection to identify them as high risk? COID doesn’t draw that line for us. Because approximately 3% of the term birth cohort is hospitalized with RSV, should we use this as the “baseline” and predetermine a percentage of hospitalization beyond baseline to define “at increased risk”?
The 2014 technical report states that RSV hospitalization rates from Stevens et al. (Arch. Pediatr. Adolesc. Med. 2000;154:55-61) were 7.5% and 4.4% for infants 28-30 weeks’ gestational age (GA) and 30-32 weeks’ GA, respectively. However, the rates quoted by COID were not the generalizable rates reported by Dr. T. P. Stevens and his team in their study. The authors calculated community-wide hospitalization rates for these groups to be 10% and 6.4%, respectively. These author-reported community-wide rates closely mirror rates reported in several other large observational studies, and still likely underestimate the true burden of RSV hospitalization based on the lower sensitivity of RSV detection testing done 15 years ago when that study was performed.
Similarly, the COID states that Dr. A.G. Winterstein’s study (JAMA Pediatrics 2013;167:1118-24) shows hospitalization rates among 32-34 weeks’ GA infants of 3.1% and 4.5% in two states based on health care insurance claims. Because fewer than half of infants are ever tested for RSV as the possible cause of their lower respiratory tract infection, these claims data are most certainly an underestimate of true rates. When active testing for RSV is done among hospitalized infants in this same GA category, using sensitive and specific PCR-based technology, hospitalization rates are 9.1%. Dr. Winterstein and associates also reported that RSV hospitalization rates increase as gestational age decreases.
In Dr. Caroline Breese Hall’s 2013 observational study (Pediatrics 2013;132:341-8) she and her associates recognized that hospitalization rates in children under 2 years of age were similar among term and preterm infants. The COID used these data in its 2014 technical report to suggest that preterm infants are no longer at high risk. The detail omitted from the report was that approximately 70% of eligible preterm infants in Dr. Hall’s study (according to the 2012 COID guidelines) had received palivizumab prophylaxis. This suggests that maintaining RSV prophylaxis according to the 2012 COID policy statement (and not the 2014 policy statement) can be successful in reducing the rate of RSV hospitalizations in preterm infants to that observed in term infants.
Finally, the technical report cites “overall declining incidence of hospitalizations for bronchiolitis in the United States” from the 2013 publication by Hasegawa et al. (Pediatrics 2013:132:28-36) without defining this observation for the AAP readers. While Hasegawa did report such a decline, the decline was specific to term infants, as no such decline was observed in the higher-risk preterm infants.
Based on careful review of several of the manuscripts referenced by COID, it’s difficult to determine why the committee’s recommendations changed so dramatically in the most recent iteration. It appears that the data need another look.
Dr. Domachowske is professor of pediatrics and professor of microbiology and immunology at the State University of New York Upstate Medical University in Syracuse. He serves on the New York State American Academy of Pediatrics Chapter 1 executive committee, volunteers as his district’s immunization champion, and is an appointed member of the New York State Immunization Advisory Council. He is a Pediatric News Editorial Advisory Board member. Dr. Domachowske disclosed he does consulting for the vaccine divisions of GlaxoSmithKline, Medimmune, Pfizer, Merck, Sanofi Pasteur, and Novartis; he performs clinical trials with GlaxoSmithKline, Medimmune, Merck, and Novartis; and he does basic and translational research with GlaxoSmithKline. E-mail him at [email protected].
Telemedicine and the potential for liability
The development of an electronic health care system has revolutionized the way in which patients receive medical treatment. Every physician in practice is familiar with how EHRs have changed the way we record and retrieve information and even interact with patients.
Many of us are just beginning to become aware of a new trend: telemedicine. Proponents of telemedicine believe that it will give patients increased ease of access to trained health care professionals at low cost and skeptics feel that the quality of care will be low without either a physical exam or an ongoing relationship to help guide care. One thing, unfortunately, is for certain, with the evolution of health care comes the evolution of health care litigation. Physicians who are considering seeing patients through a telemedicine service should be aware of liability issues that may arise when seeing patients without actually seeing patients.
In 2009, Congress enacted the Health Information Technology for Economic and Clinical Health (HITECH) Act to develop a national health care infrastructure fueled by modern technology. In addition to offering health care providers financial incentives to replace patient medical charts with EHRs, leading over 400,000 health care providers to participate in the Medicaid and Medicare incentive programs, the HITECH Act also has encouraged the advancement of telemedicine through the use of virtual visits and electronic doctor/patient communication.
Telemedicine allows physicians and patients to exchange health information through telecommunication technologies while in different geographic locations (Model policy for appropriate use of telemedicine technologies in the practice of medicine, Federation of State Medical Boards, April 2014). Telemedicine emerged in the 1960s after NASA funded early research to allow astronauts to communicate with physicians while traveling in space. In recent years, telemedical practice has evolved into a much more practical and convenient method of practicing medicine.
Virtual visits now allow a patient to use a computer or other videoconference device to have a virtual visit with a doctor in lieu of having an in-person physical examination. Jason Gorevic, CEO of Teladoc, a leading telemedicine company, expects Teladoc to conduct a half a million telemedicine visits in 2015 (“Video visits, telemedicine today are like retail clinics were in the 1990s,” mobihealthnews, Nov. 4, 2014). The increase in the use of virtual visits comes largely from the high use of telemedicine in rural areas, where long distances exists between a patient and his/her physician.
Telemedicine has expanded rapidly, and state licensure requirements and regulation of telemedicine have not caught up with its use, leading regulations to vary from state to state. In an attempt to eliminate the state regulation disparities, the Federation of State Medical Boards (FSMB) has provided a framework for standards of care in regulating the practice of telemedicine, but each state still has its own requirements for telemedical practice (50 State Telemedicine Gaps Analysis: Physician Practice Standards and Licensure, American Telemedicine Association, September 2014).
Only nine states offer a conditional or telemedicine license that allows out-of-state physicians to practice telemedicine, and Maryland, New York, and Virginia are the only states that offer reciprocity to licensed physicians in bordering states. In California, a Colorado doctor was criminally prosecuted for prescribing medication via the web to a patient in California (Hageseth v. Superior Court). The California Court of Appeals found that jurisdiction was proper in California because the doctor willfully practiced medicine in that state by agreeing to prescribe medication to a California resident online.
Physicians also should be aware of the need to obtain informed consent from the patient before conducting any telemedicine visit or examination. Most states require a physician to obtain written or verbal informed consent from a telehealth patient. Although research is slowly establishing the reliability of telemedicine, there are inherent risks associated with receiving a consultation via telecommunication of which a patient should be aware, such as the limits of the evaluation given that a complete physical exam cannot be performed. The physician should inform the patient when the visit is being recorded and receive his/her consent before proceeding.
The physician also should be very careful when selecting telemedical technology to ensure that the technology is HIPAA/HITECH certified and permitted under the state regulations in the state in which the doctor is practicing. In Oklahoma, a physician was sanctioned by the state’s medical board for conducting a virtual visit via Skype. (“Oklahoma state medical board adopts policy following Skype case,” The Oklahoman, Sept. 25, 2013). Although Oklahoma permits the use of virtual visits, the state prohibits a physician from conducting these visits via Skype. The Oklahoma doctor turned out to not be subject to a HIPAA violation because the Skype website is encrypted.
Given telemedicine’s rapid expansion, experience and litigation in this area are limited. The one thing that the current litigation trend shows is that physicians must be particularly careful when treating patients outside of the state in which they practice, both with regard to entering into a patient-physician relationship, making a diagnosis, and particularly when prescribing medication. As patients and physicians enjoy the benefits of this new method of providing medical care, they must be careful to follow the evolving telemedicine litigation and legislative landscape.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Mr. Jones and Ms. Dandy are both attorneys at Marks, O’Neill, O’Brien, Doherty & Kelly, P.C. in Philadelphia.
Note: An earlier version of this article incorrectly stated that the FSMB had recommended a national licensure and practice standard.
The development of an electronic health care system has revolutionized the way in which patients receive medical treatment. Every physician in practice is familiar with how EHRs have changed the way we record and retrieve information and even interact with patients.
Many of us are just beginning to become aware of a new trend: telemedicine. Proponents of telemedicine believe that it will give patients increased ease of access to trained health care professionals at low cost and skeptics feel that the quality of care will be low without either a physical exam or an ongoing relationship to help guide care. One thing, unfortunately, is for certain, with the evolution of health care comes the evolution of health care litigation. Physicians who are considering seeing patients through a telemedicine service should be aware of liability issues that may arise when seeing patients without actually seeing patients.
In 2009, Congress enacted the Health Information Technology for Economic and Clinical Health (HITECH) Act to develop a national health care infrastructure fueled by modern technology. In addition to offering health care providers financial incentives to replace patient medical charts with EHRs, leading over 400,000 health care providers to participate in the Medicaid and Medicare incentive programs, the HITECH Act also has encouraged the advancement of telemedicine through the use of virtual visits and electronic doctor/patient communication.
Telemedicine allows physicians and patients to exchange health information through telecommunication technologies while in different geographic locations (Model policy for appropriate use of telemedicine technologies in the practice of medicine, Federation of State Medical Boards, April 2014). Telemedicine emerged in the 1960s after NASA funded early research to allow astronauts to communicate with physicians while traveling in space. In recent years, telemedical practice has evolved into a much more practical and convenient method of practicing medicine.
Virtual visits now allow a patient to use a computer or other videoconference device to have a virtual visit with a doctor in lieu of having an in-person physical examination. Jason Gorevic, CEO of Teladoc, a leading telemedicine company, expects Teladoc to conduct a half a million telemedicine visits in 2015 (“Video visits, telemedicine today are like retail clinics were in the 1990s,” mobihealthnews, Nov. 4, 2014). The increase in the use of virtual visits comes largely from the high use of telemedicine in rural areas, where long distances exists between a patient and his/her physician.
Telemedicine has expanded rapidly, and state licensure requirements and regulation of telemedicine have not caught up with its use, leading regulations to vary from state to state. In an attempt to eliminate the state regulation disparities, the Federation of State Medical Boards (FSMB) has provided a framework for standards of care in regulating the practice of telemedicine, but each state still has its own requirements for telemedical practice (50 State Telemedicine Gaps Analysis: Physician Practice Standards and Licensure, American Telemedicine Association, September 2014).
Only nine states offer a conditional or telemedicine license that allows out-of-state physicians to practice telemedicine, and Maryland, New York, and Virginia are the only states that offer reciprocity to licensed physicians in bordering states. In California, a Colorado doctor was criminally prosecuted for prescribing medication via the web to a patient in California (Hageseth v. Superior Court). The California Court of Appeals found that jurisdiction was proper in California because the doctor willfully practiced medicine in that state by agreeing to prescribe medication to a California resident online.
Physicians also should be aware of the need to obtain informed consent from the patient before conducting any telemedicine visit or examination. Most states require a physician to obtain written or verbal informed consent from a telehealth patient. Although research is slowly establishing the reliability of telemedicine, there are inherent risks associated with receiving a consultation via telecommunication of which a patient should be aware, such as the limits of the evaluation given that a complete physical exam cannot be performed. The physician should inform the patient when the visit is being recorded and receive his/her consent before proceeding.
The physician also should be very careful when selecting telemedical technology to ensure that the technology is HIPAA/HITECH certified and permitted under the state regulations in the state in which the doctor is practicing. In Oklahoma, a physician was sanctioned by the state’s medical board for conducting a virtual visit via Skype. (“Oklahoma state medical board adopts policy following Skype case,” The Oklahoman, Sept. 25, 2013). Although Oklahoma permits the use of virtual visits, the state prohibits a physician from conducting these visits via Skype. The Oklahoma doctor turned out to not be subject to a HIPAA violation because the Skype website is encrypted.
Given telemedicine’s rapid expansion, experience and litigation in this area are limited. The one thing that the current litigation trend shows is that physicians must be particularly careful when treating patients outside of the state in which they practice, both with regard to entering into a patient-physician relationship, making a diagnosis, and particularly when prescribing medication. As patients and physicians enjoy the benefits of this new method of providing medical care, they must be careful to follow the evolving telemedicine litigation and legislative landscape.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Mr. Jones and Ms. Dandy are both attorneys at Marks, O’Neill, O’Brien, Doherty & Kelly, P.C. in Philadelphia.
Note: An earlier version of this article incorrectly stated that the FSMB had recommended a national licensure and practice standard.
The development of an electronic health care system has revolutionized the way in which patients receive medical treatment. Every physician in practice is familiar with how EHRs have changed the way we record and retrieve information and even interact with patients.
Many of us are just beginning to become aware of a new trend: telemedicine. Proponents of telemedicine believe that it will give patients increased ease of access to trained health care professionals at low cost and skeptics feel that the quality of care will be low without either a physical exam or an ongoing relationship to help guide care. One thing, unfortunately, is for certain, with the evolution of health care comes the evolution of health care litigation. Physicians who are considering seeing patients through a telemedicine service should be aware of liability issues that may arise when seeing patients without actually seeing patients.
In 2009, Congress enacted the Health Information Technology for Economic and Clinical Health (HITECH) Act to develop a national health care infrastructure fueled by modern technology. In addition to offering health care providers financial incentives to replace patient medical charts with EHRs, leading over 400,000 health care providers to participate in the Medicaid and Medicare incentive programs, the HITECH Act also has encouraged the advancement of telemedicine through the use of virtual visits and electronic doctor/patient communication.
Telemedicine allows physicians and patients to exchange health information through telecommunication technologies while in different geographic locations (Model policy for appropriate use of telemedicine technologies in the practice of medicine, Federation of State Medical Boards, April 2014). Telemedicine emerged in the 1960s after NASA funded early research to allow astronauts to communicate with physicians while traveling in space. In recent years, telemedical practice has evolved into a much more practical and convenient method of practicing medicine.
Virtual visits now allow a patient to use a computer or other videoconference device to have a virtual visit with a doctor in lieu of having an in-person physical examination. Jason Gorevic, CEO of Teladoc, a leading telemedicine company, expects Teladoc to conduct a half a million telemedicine visits in 2015 (“Video visits, telemedicine today are like retail clinics were in the 1990s,” mobihealthnews, Nov. 4, 2014). The increase in the use of virtual visits comes largely from the high use of telemedicine in rural areas, where long distances exists between a patient and his/her physician.
Telemedicine has expanded rapidly, and state licensure requirements and regulation of telemedicine have not caught up with its use, leading regulations to vary from state to state. In an attempt to eliminate the state regulation disparities, the Federation of State Medical Boards (FSMB) has provided a framework for standards of care in regulating the practice of telemedicine, but each state still has its own requirements for telemedical practice (50 State Telemedicine Gaps Analysis: Physician Practice Standards and Licensure, American Telemedicine Association, September 2014).
Only nine states offer a conditional or telemedicine license that allows out-of-state physicians to practice telemedicine, and Maryland, New York, and Virginia are the only states that offer reciprocity to licensed physicians in bordering states. In California, a Colorado doctor was criminally prosecuted for prescribing medication via the web to a patient in California (Hageseth v. Superior Court). The California Court of Appeals found that jurisdiction was proper in California because the doctor willfully practiced medicine in that state by agreeing to prescribe medication to a California resident online.
Physicians also should be aware of the need to obtain informed consent from the patient before conducting any telemedicine visit or examination. Most states require a physician to obtain written or verbal informed consent from a telehealth patient. Although research is slowly establishing the reliability of telemedicine, there are inherent risks associated with receiving a consultation via telecommunication of which a patient should be aware, such as the limits of the evaluation given that a complete physical exam cannot be performed. The physician should inform the patient when the visit is being recorded and receive his/her consent before proceeding.
The physician also should be very careful when selecting telemedical technology to ensure that the technology is HIPAA/HITECH certified and permitted under the state regulations in the state in which the doctor is practicing. In Oklahoma, a physician was sanctioned by the state’s medical board for conducting a virtual visit via Skype. (“Oklahoma state medical board adopts policy following Skype case,” The Oklahoman, Sept. 25, 2013). Although Oklahoma permits the use of virtual visits, the state prohibits a physician from conducting these visits via Skype. The Oklahoma doctor turned out to not be subject to a HIPAA violation because the Skype website is encrypted.
Given telemedicine’s rapid expansion, experience and litigation in this area are limited. The one thing that the current litigation trend shows is that physicians must be particularly careful when treating patients outside of the state in which they practice, both with regard to entering into a patient-physician relationship, making a diagnosis, and particularly when prescribing medication. As patients and physicians enjoy the benefits of this new method of providing medical care, they must be careful to follow the evolving telemedicine litigation and legislative landscape.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Mr. Jones and Ms. Dandy are both attorneys at Marks, O’Neill, O’Brien, Doherty & Kelly, P.C. in Philadelphia.
Note: An earlier version of this article incorrectly stated that the FSMB had recommended a national licensure and practice standard.
5 reasons why EHRs can’t be called failures
Much has been said about the failures of electronic health records. The shortcomings discussed have ranged from lack of cost benefits to interoperability with medical devices and security of interoperability with medical devices. We use IT via computers or smartphones daily for social, financial, or consumer aspects of lives. Health care has lagged behind other sectors of society in the adoption of digital technology because of regulatory issues, cost, and resistance to change. There are many positive aspects of EHRs, some more obvious than others.
They are what patients expect.
Patients live in the digital world. Seventy-eight percent of office-based physicians use an EHR, according to a study in the journal Health Affairs. Patients expect that their test results and records are easily accessible by all their providers. The promise of interoperability – the easy digital transfer of data from one data source or EHR system to another – has yet to be realized. This is one of the fundamental potential benefits of digital health technology. HIMSS, an advocacy organization focused on better health with information technology, has sent to Congress its recommendations on achieving interoperability within the next 3 years. This is a pivotal issue in creating the EHR envisioned by both patients and physicians.
They can be used to mitigate risk management.
Adoption of any significant change in health care practice presents challenges specifically with regards to risk management. HIPAA privacy regulations and security are of paramount importance. Most risk managers deal with legal issues after an incident has occurred. Digital health technologies can also potentially mitigate risk.
They can (Yes!) enhance the patient encounter.
While many physicians believe that EHRs destroy the patient encounter, there is another way of viewing the interaction. It all depends upon how it is presented in the office. The computer screen may impede the all-important eye contact between the physician and patient (either because of the physical presence of the screen or the physician’s persistent gaze at it). This is a surefire recipe for disengagement and subsequent destruction of the patient-physician relationship. However, the introduction of the computer (asking permission to use it) with physician and patient triangulated with the screen produces a care team atmosphere. Demonstrating the EHR’s functionality while highlighting pertinent clinical information provides a positive experience for both participants.
They brought health care into the digital age.
EHRs are not the face of all of digital health technologies. They do represent the hub around which other technologies need to flow, because this is where the patient interfaces (pun intended) with the physician. Digital technologies will enhance patient engagement. EHRs are the first experience many physicians have with digital health technologies, and they have yet to fulfill their intended goals. They are in their first iteration. Physician groups and health care enterprises have made themselves heard to the EHR vendors and change is coming. Other digital health technologies are here and will improve health care on many fronts. They themselves will transform the EHR into a more useful clinical tool, which will increase patient education, engagement, and connectivity.
They will be much different and better in the near future.
The American Medical Association got it right, in my opinion, with respect to its recommendations for design overhaul of EHRs. The organization outlined an extension of its study with the Rand Corp. and listed priorities of what should constitute design overhaul of the EHR. These include the incorporation of tools that support team-based care, promotion of care coordination among providers, product modularity and ability for configuration, the reduction of cognitive workload, the promotion of data liquidity, the facilitation of digital and mobile patient engagement, and the ability to expedite user input into design and postimplementation feedback.
As digital technology becomes a more substantive part of health care, there will be a need for physician IT champions who can make this process easier and more fulfilling for others. I look forward to seeing this happen.
Dr. Scher is an electrophysiologist with the Heart Group of Lancaster (Pa.) General Health. He is also director of DLS Healthcare Consulting, Harrisburg, Pa., and clinical associate professor of medicine at the Pennsylvania State University, Hershey.
Much has been said about the failures of electronic health records. The shortcomings discussed have ranged from lack of cost benefits to interoperability with medical devices and security of interoperability with medical devices. We use IT via computers or smartphones daily for social, financial, or consumer aspects of lives. Health care has lagged behind other sectors of society in the adoption of digital technology because of regulatory issues, cost, and resistance to change. There are many positive aspects of EHRs, some more obvious than others.
They are what patients expect.
Patients live in the digital world. Seventy-eight percent of office-based physicians use an EHR, according to a study in the journal Health Affairs. Patients expect that their test results and records are easily accessible by all their providers. The promise of interoperability – the easy digital transfer of data from one data source or EHR system to another – has yet to be realized. This is one of the fundamental potential benefits of digital health technology. HIMSS, an advocacy organization focused on better health with information technology, has sent to Congress its recommendations on achieving interoperability within the next 3 years. This is a pivotal issue in creating the EHR envisioned by both patients and physicians.
They can be used to mitigate risk management.
Adoption of any significant change in health care practice presents challenges specifically with regards to risk management. HIPAA privacy regulations and security are of paramount importance. Most risk managers deal with legal issues after an incident has occurred. Digital health technologies can also potentially mitigate risk.
They can (Yes!) enhance the patient encounter.
While many physicians believe that EHRs destroy the patient encounter, there is another way of viewing the interaction. It all depends upon how it is presented in the office. The computer screen may impede the all-important eye contact between the physician and patient (either because of the physical presence of the screen or the physician’s persistent gaze at it). This is a surefire recipe for disengagement and subsequent destruction of the patient-physician relationship. However, the introduction of the computer (asking permission to use it) with physician and patient triangulated with the screen produces a care team atmosphere. Demonstrating the EHR’s functionality while highlighting pertinent clinical information provides a positive experience for both participants.
They brought health care into the digital age.
EHRs are not the face of all of digital health technologies. They do represent the hub around which other technologies need to flow, because this is where the patient interfaces (pun intended) with the physician. Digital technologies will enhance patient engagement. EHRs are the first experience many physicians have with digital health technologies, and they have yet to fulfill their intended goals. They are in their first iteration. Physician groups and health care enterprises have made themselves heard to the EHR vendors and change is coming. Other digital health technologies are here and will improve health care on many fronts. They themselves will transform the EHR into a more useful clinical tool, which will increase patient education, engagement, and connectivity.
They will be much different and better in the near future.
The American Medical Association got it right, in my opinion, with respect to its recommendations for design overhaul of EHRs. The organization outlined an extension of its study with the Rand Corp. and listed priorities of what should constitute design overhaul of the EHR. These include the incorporation of tools that support team-based care, promotion of care coordination among providers, product modularity and ability for configuration, the reduction of cognitive workload, the promotion of data liquidity, the facilitation of digital and mobile patient engagement, and the ability to expedite user input into design and postimplementation feedback.
As digital technology becomes a more substantive part of health care, there will be a need for physician IT champions who can make this process easier and more fulfilling for others. I look forward to seeing this happen.
Dr. Scher is an electrophysiologist with the Heart Group of Lancaster (Pa.) General Health. He is also director of DLS Healthcare Consulting, Harrisburg, Pa., and clinical associate professor of medicine at the Pennsylvania State University, Hershey.
Much has been said about the failures of electronic health records. The shortcomings discussed have ranged from lack of cost benefits to interoperability with medical devices and security of interoperability with medical devices. We use IT via computers or smartphones daily for social, financial, or consumer aspects of lives. Health care has lagged behind other sectors of society in the adoption of digital technology because of regulatory issues, cost, and resistance to change. There are many positive aspects of EHRs, some more obvious than others.
They are what patients expect.
Patients live in the digital world. Seventy-eight percent of office-based physicians use an EHR, according to a study in the journal Health Affairs. Patients expect that their test results and records are easily accessible by all their providers. The promise of interoperability – the easy digital transfer of data from one data source or EHR system to another – has yet to be realized. This is one of the fundamental potential benefits of digital health technology. HIMSS, an advocacy organization focused on better health with information technology, has sent to Congress its recommendations on achieving interoperability within the next 3 years. This is a pivotal issue in creating the EHR envisioned by both patients and physicians.
They can be used to mitigate risk management.
Adoption of any significant change in health care practice presents challenges specifically with regards to risk management. HIPAA privacy regulations and security are of paramount importance. Most risk managers deal with legal issues after an incident has occurred. Digital health technologies can also potentially mitigate risk.
They can (Yes!) enhance the patient encounter.
While many physicians believe that EHRs destroy the patient encounter, there is another way of viewing the interaction. It all depends upon how it is presented in the office. The computer screen may impede the all-important eye contact between the physician and patient (either because of the physical presence of the screen or the physician’s persistent gaze at it). This is a surefire recipe for disengagement and subsequent destruction of the patient-physician relationship. However, the introduction of the computer (asking permission to use it) with physician and patient triangulated with the screen produces a care team atmosphere. Demonstrating the EHR’s functionality while highlighting pertinent clinical information provides a positive experience for both participants.
They brought health care into the digital age.
EHRs are not the face of all of digital health technologies. They do represent the hub around which other technologies need to flow, because this is where the patient interfaces (pun intended) with the physician. Digital technologies will enhance patient engagement. EHRs are the first experience many physicians have with digital health technologies, and they have yet to fulfill their intended goals. They are in their first iteration. Physician groups and health care enterprises have made themselves heard to the EHR vendors and change is coming. Other digital health technologies are here and will improve health care on many fronts. They themselves will transform the EHR into a more useful clinical tool, which will increase patient education, engagement, and connectivity.
They will be much different and better in the near future.
The American Medical Association got it right, in my opinion, with respect to its recommendations for design overhaul of EHRs. The organization outlined an extension of its study with the Rand Corp. and listed priorities of what should constitute design overhaul of the EHR. These include the incorporation of tools that support team-based care, promotion of care coordination among providers, product modularity and ability for configuration, the reduction of cognitive workload, the promotion of data liquidity, the facilitation of digital and mobile patient engagement, and the ability to expedite user input into design and postimplementation feedback.
As digital technology becomes a more substantive part of health care, there will be a need for physician IT champions who can make this process easier and more fulfilling for others. I look forward to seeing this happen.
Dr. Scher is an electrophysiologist with the Heart Group of Lancaster (Pa.) General Health. He is also director of DLS Healthcare Consulting, Harrisburg, Pa., and clinical associate professor of medicine at the Pennsylvania State University, Hershey.
Outcomes: Getting to the patient’s bottom line
It’s easy to get so caught up in our day-to-day routines. At the hospital, we’re meeting core measures, documenting correctly, following clinical guidelines, and simply striving to stay up to date with the literature. At home, there are soccer games, recitals, and homework – and some rare personal time. We often shift to automatic pilot in a desperate attempt to balance the seemingly never-ending demands.
But does the very nature of our hectic lives sometimes prevent us from seeing the bigger picture, especially when it comes to the things that are really important to our patients? Yes, we know what lab values automatically trigger an order for a statin, and what ejection fraction on the echocardiogram warrants an ACE inhibitor, but how often do we really take the time to find out about the outcomes that are important to our patients? Sometimes they aren’t the evidence-based clinical outcomes we are trying to reproduce with our treatments.
For many patients, the desired outcome is to feel better, plain and simple. All the fancy lingo and drugs with unpronounceable names and unintelligible indications can be overwhelming. They make some patients shut down, and ultimately shut us out. We may not even realize it until our patients are readmitted as a result of noncompliance with our well-thought-out treatment plans.
There are our male patients who rarely take their blood pressure medicine because of the side effect of sexual dysfunction. And then there are those patients who don’t take their medications or see their doctors regularly because they just cannot afford it. While they seem to be in agreement with the follow-up plan for medical visits and testing, patients may be ashamed to admit they are uninsured or underinsured. They know they will never be adherent because they just cannot afford the costs of our treatment plan.
Instead of getting frustrated with our noncompliant patients, we could better serve them by getting more personal – gaining their trust as we carefully and respectfully uncover the layers of the limitations they face and the outcomes that matter to them. We need to aim to be viewed as our patients’ caring advocates and not just aloof professionals with no clue about their daily struggles.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
It’s easy to get so caught up in our day-to-day routines. At the hospital, we’re meeting core measures, documenting correctly, following clinical guidelines, and simply striving to stay up to date with the literature. At home, there are soccer games, recitals, and homework – and some rare personal time. We often shift to automatic pilot in a desperate attempt to balance the seemingly never-ending demands.
But does the very nature of our hectic lives sometimes prevent us from seeing the bigger picture, especially when it comes to the things that are really important to our patients? Yes, we know what lab values automatically trigger an order for a statin, and what ejection fraction on the echocardiogram warrants an ACE inhibitor, but how often do we really take the time to find out about the outcomes that are important to our patients? Sometimes they aren’t the evidence-based clinical outcomes we are trying to reproduce with our treatments.
For many patients, the desired outcome is to feel better, plain and simple. All the fancy lingo and drugs with unpronounceable names and unintelligible indications can be overwhelming. They make some patients shut down, and ultimately shut us out. We may not even realize it until our patients are readmitted as a result of noncompliance with our well-thought-out treatment plans.
There are our male patients who rarely take their blood pressure medicine because of the side effect of sexual dysfunction. And then there are those patients who don’t take their medications or see their doctors regularly because they just cannot afford it. While they seem to be in agreement with the follow-up plan for medical visits and testing, patients may be ashamed to admit they are uninsured or underinsured. They know they will never be adherent because they just cannot afford the costs of our treatment plan.
Instead of getting frustrated with our noncompliant patients, we could better serve them by getting more personal – gaining their trust as we carefully and respectfully uncover the layers of the limitations they face and the outcomes that matter to them. We need to aim to be viewed as our patients’ caring advocates and not just aloof professionals with no clue about their daily struggles.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
It’s easy to get so caught up in our day-to-day routines. At the hospital, we’re meeting core measures, documenting correctly, following clinical guidelines, and simply striving to stay up to date with the literature. At home, there are soccer games, recitals, and homework – and some rare personal time. We often shift to automatic pilot in a desperate attempt to balance the seemingly never-ending demands.
But does the very nature of our hectic lives sometimes prevent us from seeing the bigger picture, especially when it comes to the things that are really important to our patients? Yes, we know what lab values automatically trigger an order for a statin, and what ejection fraction on the echocardiogram warrants an ACE inhibitor, but how often do we really take the time to find out about the outcomes that are important to our patients? Sometimes they aren’t the evidence-based clinical outcomes we are trying to reproduce with our treatments.
For many patients, the desired outcome is to feel better, plain and simple. All the fancy lingo and drugs with unpronounceable names and unintelligible indications can be overwhelming. They make some patients shut down, and ultimately shut us out. We may not even realize it until our patients are readmitted as a result of noncompliance with our well-thought-out treatment plans.
There are our male patients who rarely take their blood pressure medicine because of the side effect of sexual dysfunction. And then there are those patients who don’t take their medications or see their doctors regularly because they just cannot afford it. While they seem to be in agreement with the follow-up plan for medical visits and testing, patients may be ashamed to admit they are uninsured or underinsured. They know they will never be adherent because they just cannot afford the costs of our treatment plan.
Instead of getting frustrated with our noncompliant patients, we could better serve them by getting more personal – gaining their trust as we carefully and respectfully uncover the layers of the limitations they face and the outcomes that matter to them. We need to aim to be viewed as our patients’ caring advocates and not just aloof professionals with no clue about their daily struggles.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
When your diagnosis is questioned
When patients question your diagnosis, how do you react?
As physicians, we take great pride in our ability to diagnose and treat disease, and as hospitalists, our patients are sicker, so we need to make the right diagnosis and make it fast. A diagnostic delay of even a few days can sometimes cost a patient his life.
So when the patient or a family member disagrees with your diagnosis – especially when they have no remote understanding of the condition – it can be easy to dismiss their concerns. And then there are the times when you’ve missed something and they are right.
I will never forget a 60-year-old male patient I encountered early in my career as a hospitalist. He had presented with diffuse abdominal pain which later localized to both lower quadrants, diarrhea, and CT scan evidence of gastroenteritis. Multiple doctors who saw the patient before me all had the same diagnosis, a simple case of gastroenteritis. By day 2, he was afebrile, had a normal white blood cell count, was eating, and was ambulating down the hallway with his large family, seemingly in no distress.
He related that he still had abdominal pain, but felt comfortable with his diagnosis and was amenable to being discharged to follow-up with the gastroenterologist who had consulted on him during his stay in the hospital. His niece, on the other hand, was not happy with the diagnosis. The look on her face was intense, not disrespectful, as she related her conviction that her uncle had something more going on than a bout of gastroenteritis. She knew her uncle far better than I did, and his pain was concerning to her.
So I went back to the drawing board to make sure nothing had been missed, and there, hidden in plain sight, was a vital piece of information that we had all overlooked. The CT scan report that showed signs consistent with gastroenteritis made no mention whatsoever of his appendix.
Not satisfied with simply having another radiologist read the film, I insisted that a surgeon see the patient. To the surgeon’s great surprise, and mine, he found evidence of appendicitis. By 10 a.m. the next morning, the patient was in the OR having a now-perforated appendix removed. After numerous apologies to the family and patient, he was discharged home on postop day 2, doing well.
That very scary near miss taught me a valuable lesson: Sometimes the gut instinct of patients and their family members is just as accurate as the gut instinct of a physician, and we need to fully respect their input, whether or not we agree with them.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
When patients question your diagnosis, how do you react?
As physicians, we take great pride in our ability to diagnose and treat disease, and as hospitalists, our patients are sicker, so we need to make the right diagnosis and make it fast. A diagnostic delay of even a few days can sometimes cost a patient his life.
So when the patient or a family member disagrees with your diagnosis – especially when they have no remote understanding of the condition – it can be easy to dismiss their concerns. And then there are the times when you’ve missed something and they are right.
I will never forget a 60-year-old male patient I encountered early in my career as a hospitalist. He had presented with diffuse abdominal pain which later localized to both lower quadrants, diarrhea, and CT scan evidence of gastroenteritis. Multiple doctors who saw the patient before me all had the same diagnosis, a simple case of gastroenteritis. By day 2, he was afebrile, had a normal white blood cell count, was eating, and was ambulating down the hallway with his large family, seemingly in no distress.
He related that he still had abdominal pain, but felt comfortable with his diagnosis and was amenable to being discharged to follow-up with the gastroenterologist who had consulted on him during his stay in the hospital. His niece, on the other hand, was not happy with the diagnosis. The look on her face was intense, not disrespectful, as she related her conviction that her uncle had something more going on than a bout of gastroenteritis. She knew her uncle far better than I did, and his pain was concerning to her.
So I went back to the drawing board to make sure nothing had been missed, and there, hidden in plain sight, was a vital piece of information that we had all overlooked. The CT scan report that showed signs consistent with gastroenteritis made no mention whatsoever of his appendix.
Not satisfied with simply having another radiologist read the film, I insisted that a surgeon see the patient. To the surgeon’s great surprise, and mine, he found evidence of appendicitis. By 10 a.m. the next morning, the patient was in the OR having a now-perforated appendix removed. After numerous apologies to the family and patient, he was discharged home on postop day 2, doing well.
That very scary near miss taught me a valuable lesson: Sometimes the gut instinct of patients and their family members is just as accurate as the gut instinct of a physician, and we need to fully respect their input, whether or not we agree with them.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
When patients question your diagnosis, how do you react?
As physicians, we take great pride in our ability to diagnose and treat disease, and as hospitalists, our patients are sicker, so we need to make the right diagnosis and make it fast. A diagnostic delay of even a few days can sometimes cost a patient his life.
So when the patient or a family member disagrees with your diagnosis – especially when they have no remote understanding of the condition – it can be easy to dismiss their concerns. And then there are the times when you’ve missed something and they are right.
I will never forget a 60-year-old male patient I encountered early in my career as a hospitalist. He had presented with diffuse abdominal pain which later localized to both lower quadrants, diarrhea, and CT scan evidence of gastroenteritis. Multiple doctors who saw the patient before me all had the same diagnosis, a simple case of gastroenteritis. By day 2, he was afebrile, had a normal white blood cell count, was eating, and was ambulating down the hallway with his large family, seemingly in no distress.
He related that he still had abdominal pain, but felt comfortable with his diagnosis and was amenable to being discharged to follow-up with the gastroenterologist who had consulted on him during his stay in the hospital. His niece, on the other hand, was not happy with the diagnosis. The look on her face was intense, not disrespectful, as she related her conviction that her uncle had something more going on than a bout of gastroenteritis. She knew her uncle far better than I did, and his pain was concerning to her.
So I went back to the drawing board to make sure nothing had been missed, and there, hidden in plain sight, was a vital piece of information that we had all overlooked. The CT scan report that showed signs consistent with gastroenteritis made no mention whatsoever of his appendix.
Not satisfied with simply having another radiologist read the film, I insisted that a surgeon see the patient. To the surgeon’s great surprise, and mine, he found evidence of appendicitis. By 10 a.m. the next morning, the patient was in the OR having a now-perforated appendix removed. After numerous apologies to the family and patient, he was discharged home on postop day 2, doing well.
That very scary near miss taught me a valuable lesson: Sometimes the gut instinct of patients and their family members is just as accurate as the gut instinct of a physician, and we need to fully respect their input, whether or not we agree with them.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
DEA Schedule Change Inhibits Practice and Patient Care
Note from NP Editor-in-Chief Marie-Eileen Onieal, PhD, CPNP, FAANP: Recently, the authors of this column and I started discussing the decision by the Drug Enforcement Administration (DEA) to reschedule hydrocodone and the resulting barriers to care. By the end of the conversation, it was evident that my colleagues needed to “get the word out” to our readers—so I afforded them this opportunity to do so.
On October 6, 2014, hydrocodone combination products were reclassified from Schedule III to Schedule II of the Controlled Substances Act, per a final ruling issued by the DEA’s Office of Diversion Control. The DEA’s ruling was the result of an evaluation of scientific and medical evidence supplied by multiple agencies, as well as considerations related to the FDA Safety and Innovation Act of 2012.1 The rationale for rescheduling hydrocodone preparations was based on evidence of high potential for abuse, high rates of dependency, and epidemic levels of drug diversion related to these products.
Our purpose in this editorial is not to debate the DEA’s decision to reschedule hydrocodone preparations but rather to demonstrate that all policy changes have consequences. In this case, these include substantial limitations on the ability of NPs in several states to adequately manage acute and chronic pain for their patients. As a result, NPs may be prevented from delivering comprehensive care to patients with certain conditions. (We are aware that our PA colleagues may be similarly affected by this ruling but will restrict our commentary to NPs, as we are most familiar with our profession’s circumstances.)
Each state grants specific prescriptive privileges to advanced practice registered nurses (APRNs), resulting in wide variation across the country. Therefore, the effect of this ruling on patients’ access to care and providers’ ability to treat certain conditions differs by state. The states in which APRNs have prescriptive privileges for Schedule III but not Schedule II medications—those most impacted by this rule change—include Arkansas, Georgia, Missouri, Nebraska, Oklahoma, South Carolina, Texas, and West Virginia.2 Prior to the effective date of this ruling, NPs in these states had hydrocodone and codeine preparations, as well as the nonnarcotic tramadol, in their armamentaria to treat pain.
The DEA ruling—coupled with restrictive state regulations—reduces the options for pain management in these states. The only other Schedule III narcotic treatment options are codeine preparations; for patients with codeine sensitivities or allergies, NPs are now unable to adequately manage acute or chronic pain. Besides inconvenience, this change results in additional costs, since patients will also need to be assessed by a provider with Schedule II prescriptive privileges if they hope to achieve adequate pain management. This is particularly burdensome in underserved or rural populations, which many of the affected states have.
In response to this (presumably) unintended consequence, legislatures need to consider the impact this ruling will have on patient care and move to modernize prescriptive authority for APRNs, especially in the most affected states. The Future of Nursing report3 from the Institute of Medicine (IOM) recommends that APRNs’ scope of practice be reformed to conform with the model rules and regulations established by the National Council of State Boards of Nursing (NCSBN). The NCSBN’s consensus model report supports full scope of practice for all APRNs, as well as collaboration among all health care disciplines as a professional norm, instead of the current restrictive practices.4
Continued on next page >>
Historically, APRNs have struggled to gain total support from state legislators in the quest for full practice authority, including prescriptive privileges. Our professional organizations, with support from the IOM, the National Governors Association, and the Federal Trade Commission, must be ready to provide accurate data on safety and outcomes to state and federal legislators. This evidence would support efforts to modernize NP scope of practice acts, including prescribing regulations, that are currently outdated and prevent APRNs from providing optimal care.5
This year (2015) marks the 50th anniversary of the NP role. Thus, it is the proper time for state legislatures to recognize and support the role of the NP in the delivery of comprehensive, cost-effective health care in the United States. With millions of previously uninsured Americans seeking primary care and the increasing shortage of primary care physicians, NPs are part of the answer to the problem of access to acute and chronic care in both urban and rural communities. With NPs available to close this gap, it is imperative that both state and federal legislatures support initiatives that will eliminate barriers to care and promote legislation that offers full scope of practice to NPs.
We welcome your feedback on this topic. Please send your comments to NPEditor@frontline medcom.com.
REFERENCES
1. Drug Enforcement Administration. Schedules of controlled substances: rescheduling of hydrocodone combination products from schedule III to schedule II (document no: 2014-19922). Federal Register. https://federalregister.gov/a/2014-19922. Accessed February 13, 2015.
2. Drug Enforcement Administration Office of Diversion. Mid-level practitioners authorization by state. www.deadiversion.usdoj.gov/drugreg/practioners. Accessed February 13, 2015.
3. Institute of Medicine. The Future of Nursing: Leading Change, Advancing Health. Washington, DC: The National Academies Press; 2011.
4. National Council of State Boards of Nursing. The Consensus Model for APRN Regulation, Licensure, Accreditation, Certification and Education (2008). www.ncsbn.org/Consen sus_Model_for_APRN_Regulation_July_2008.pdf. Accessed February 13, 2015.
5. National Council of State Boards of Nursing. Changes In Healthcare Professions’ Scope of Practice: Legislative Considerations (2009). www.ncsbn.org/ScopeofPractice_09.pdf. Accessed February 13, 2015.
Note from NP Editor-in-Chief Marie-Eileen Onieal, PhD, CPNP, FAANP: Recently, the authors of this column and I started discussing the decision by the Drug Enforcement Administration (DEA) to reschedule hydrocodone and the resulting barriers to care. By the end of the conversation, it was evident that my colleagues needed to “get the word out” to our readers—so I afforded them this opportunity to do so.
On October 6, 2014, hydrocodone combination products were reclassified from Schedule III to Schedule II of the Controlled Substances Act, per a final ruling issued by the DEA’s Office of Diversion Control. The DEA’s ruling was the result of an evaluation of scientific and medical evidence supplied by multiple agencies, as well as considerations related to the FDA Safety and Innovation Act of 2012.1 The rationale for rescheduling hydrocodone preparations was based on evidence of high potential for abuse, high rates of dependency, and epidemic levels of drug diversion related to these products.
Our purpose in this editorial is not to debate the DEA’s decision to reschedule hydrocodone preparations but rather to demonstrate that all policy changes have consequences. In this case, these include substantial limitations on the ability of NPs in several states to adequately manage acute and chronic pain for their patients. As a result, NPs may be prevented from delivering comprehensive care to patients with certain conditions. (We are aware that our PA colleagues may be similarly affected by this ruling but will restrict our commentary to NPs, as we are most familiar with our profession’s circumstances.)
Each state grants specific prescriptive privileges to advanced practice registered nurses (APRNs), resulting in wide variation across the country. Therefore, the effect of this ruling on patients’ access to care and providers’ ability to treat certain conditions differs by state. The states in which APRNs have prescriptive privileges for Schedule III but not Schedule II medications—those most impacted by this rule change—include Arkansas, Georgia, Missouri, Nebraska, Oklahoma, South Carolina, Texas, and West Virginia.2 Prior to the effective date of this ruling, NPs in these states had hydrocodone and codeine preparations, as well as the nonnarcotic tramadol, in their armamentaria to treat pain.
The DEA ruling—coupled with restrictive state regulations—reduces the options for pain management in these states. The only other Schedule III narcotic treatment options are codeine preparations; for patients with codeine sensitivities or allergies, NPs are now unable to adequately manage acute or chronic pain. Besides inconvenience, this change results in additional costs, since patients will also need to be assessed by a provider with Schedule II prescriptive privileges if they hope to achieve adequate pain management. This is particularly burdensome in underserved or rural populations, which many of the affected states have.
In response to this (presumably) unintended consequence, legislatures need to consider the impact this ruling will have on patient care and move to modernize prescriptive authority for APRNs, especially in the most affected states. The Future of Nursing report3 from the Institute of Medicine (IOM) recommends that APRNs’ scope of practice be reformed to conform with the model rules and regulations established by the National Council of State Boards of Nursing (NCSBN). The NCSBN’s consensus model report supports full scope of practice for all APRNs, as well as collaboration among all health care disciplines as a professional norm, instead of the current restrictive practices.4
Continued on next page >>
Historically, APRNs have struggled to gain total support from state legislators in the quest for full practice authority, including prescriptive privileges. Our professional organizations, with support from the IOM, the National Governors Association, and the Federal Trade Commission, must be ready to provide accurate data on safety and outcomes to state and federal legislators. This evidence would support efforts to modernize NP scope of practice acts, including prescribing regulations, that are currently outdated and prevent APRNs from providing optimal care.5
This year (2015) marks the 50th anniversary of the NP role. Thus, it is the proper time for state legislatures to recognize and support the role of the NP in the delivery of comprehensive, cost-effective health care in the United States. With millions of previously uninsured Americans seeking primary care and the increasing shortage of primary care physicians, NPs are part of the answer to the problem of access to acute and chronic care in both urban and rural communities. With NPs available to close this gap, it is imperative that both state and federal legislatures support initiatives that will eliminate barriers to care and promote legislation that offers full scope of practice to NPs.
We welcome your feedback on this topic. Please send your comments to NPEditor@frontline medcom.com.
REFERENCES
1. Drug Enforcement Administration. Schedules of controlled substances: rescheduling of hydrocodone combination products from schedule III to schedule II (document no: 2014-19922). Federal Register. https://federalregister.gov/a/2014-19922. Accessed February 13, 2015.
2. Drug Enforcement Administration Office of Diversion. Mid-level practitioners authorization by state. www.deadiversion.usdoj.gov/drugreg/practioners. Accessed February 13, 2015.
3. Institute of Medicine. The Future of Nursing: Leading Change, Advancing Health. Washington, DC: The National Academies Press; 2011.
4. National Council of State Boards of Nursing. The Consensus Model for APRN Regulation, Licensure, Accreditation, Certification and Education (2008). www.ncsbn.org/Consen sus_Model_for_APRN_Regulation_July_2008.pdf. Accessed February 13, 2015.
5. National Council of State Boards of Nursing. Changes In Healthcare Professions’ Scope of Practice: Legislative Considerations (2009). www.ncsbn.org/ScopeofPractice_09.pdf. Accessed February 13, 2015.
Note from NP Editor-in-Chief Marie-Eileen Onieal, PhD, CPNP, FAANP: Recently, the authors of this column and I started discussing the decision by the Drug Enforcement Administration (DEA) to reschedule hydrocodone and the resulting barriers to care. By the end of the conversation, it was evident that my colleagues needed to “get the word out” to our readers—so I afforded them this opportunity to do so.
On October 6, 2014, hydrocodone combination products were reclassified from Schedule III to Schedule II of the Controlled Substances Act, per a final ruling issued by the DEA’s Office of Diversion Control. The DEA’s ruling was the result of an evaluation of scientific and medical evidence supplied by multiple agencies, as well as considerations related to the FDA Safety and Innovation Act of 2012.1 The rationale for rescheduling hydrocodone preparations was based on evidence of high potential for abuse, high rates of dependency, and epidemic levels of drug diversion related to these products.
Our purpose in this editorial is not to debate the DEA’s decision to reschedule hydrocodone preparations but rather to demonstrate that all policy changes have consequences. In this case, these include substantial limitations on the ability of NPs in several states to adequately manage acute and chronic pain for their patients. As a result, NPs may be prevented from delivering comprehensive care to patients with certain conditions. (We are aware that our PA colleagues may be similarly affected by this ruling but will restrict our commentary to NPs, as we are most familiar with our profession’s circumstances.)
Each state grants specific prescriptive privileges to advanced practice registered nurses (APRNs), resulting in wide variation across the country. Therefore, the effect of this ruling on patients’ access to care and providers’ ability to treat certain conditions differs by state. The states in which APRNs have prescriptive privileges for Schedule III but not Schedule II medications—those most impacted by this rule change—include Arkansas, Georgia, Missouri, Nebraska, Oklahoma, South Carolina, Texas, and West Virginia.2 Prior to the effective date of this ruling, NPs in these states had hydrocodone and codeine preparations, as well as the nonnarcotic tramadol, in their armamentaria to treat pain.
The DEA ruling—coupled with restrictive state regulations—reduces the options for pain management in these states. The only other Schedule III narcotic treatment options are codeine preparations; for patients with codeine sensitivities or allergies, NPs are now unable to adequately manage acute or chronic pain. Besides inconvenience, this change results in additional costs, since patients will also need to be assessed by a provider with Schedule II prescriptive privileges if they hope to achieve adequate pain management. This is particularly burdensome in underserved or rural populations, which many of the affected states have.
In response to this (presumably) unintended consequence, legislatures need to consider the impact this ruling will have on patient care and move to modernize prescriptive authority for APRNs, especially in the most affected states. The Future of Nursing report3 from the Institute of Medicine (IOM) recommends that APRNs’ scope of practice be reformed to conform with the model rules and regulations established by the National Council of State Boards of Nursing (NCSBN). The NCSBN’s consensus model report supports full scope of practice for all APRNs, as well as collaboration among all health care disciplines as a professional norm, instead of the current restrictive practices.4
Continued on next page >>
Historically, APRNs have struggled to gain total support from state legislators in the quest for full practice authority, including prescriptive privileges. Our professional organizations, with support from the IOM, the National Governors Association, and the Federal Trade Commission, must be ready to provide accurate data on safety and outcomes to state and federal legislators. This evidence would support efforts to modernize NP scope of practice acts, including prescribing regulations, that are currently outdated and prevent APRNs from providing optimal care.5
This year (2015) marks the 50th anniversary of the NP role. Thus, it is the proper time for state legislatures to recognize and support the role of the NP in the delivery of comprehensive, cost-effective health care in the United States. With millions of previously uninsured Americans seeking primary care and the increasing shortage of primary care physicians, NPs are part of the answer to the problem of access to acute and chronic care in both urban and rural communities. With NPs available to close this gap, it is imperative that both state and federal legislatures support initiatives that will eliminate barriers to care and promote legislation that offers full scope of practice to NPs.
We welcome your feedback on this topic. Please send your comments to NPEditor@frontline medcom.com.
REFERENCES
1. Drug Enforcement Administration. Schedules of controlled substances: rescheduling of hydrocodone combination products from schedule III to schedule II (document no: 2014-19922). Federal Register. https://federalregister.gov/a/2014-19922. Accessed February 13, 2015.
2. Drug Enforcement Administration Office of Diversion. Mid-level practitioners authorization by state. www.deadiversion.usdoj.gov/drugreg/practioners. Accessed February 13, 2015.
3. Institute of Medicine. The Future of Nursing: Leading Change, Advancing Health. Washington, DC: The National Academies Press; 2011.
4. National Council of State Boards of Nursing. The Consensus Model for APRN Regulation, Licensure, Accreditation, Certification and Education (2008). www.ncsbn.org/Consen sus_Model_for_APRN_Regulation_July_2008.pdf. Accessed February 13, 2015.
5. National Council of State Boards of Nursing. Changes In Healthcare Professions’ Scope of Practice: Legislative Considerations (2009). www.ncsbn.org/ScopeofPractice_09.pdf. Accessed February 13, 2015.
















