User login
Medicare Advantage: The good, the bad, and the ugly
As of 2023, most people eligible for Medicare are enrolled in Medicare Advantage plans administered by commercial insurers, rather than traditional Medicare plans sponsored by the federal government. The Kaiser Family Foundation reports that 31 million people are now enrolled in a Medicare Advantage plan, with almost half of them (47%) served by United Healthcare or Humana.
This is 51% of all people eligible for Medicare, compared with 19% in 2007. The Congressional Budget Office projects that 62% of Medicare participants will be in Medicare Advantage plans by 2033.
Given the explosive growth in Medicare Advantage participation, many readers have likely seen patients served by Medicare Advantage or will soon. Below is information about the program’s purpose, strengths, limitations, and effect on physicians.
How does Medicare Advantage differ from traditional Medicare?
A Medicare Advantage plan is approved by the U.S. Centers for Medicare & Medicaid Services and competes for customers by offering lower premiums and/or more benefits. Traditional Medicare plans are unified contracts across the country, with the same fees for the same services paid nationwide.
CMS pays Medicare Advantage plans a per-member rate, which can be increased for people who seem sicker than other plan participants – for example, someone with uncontrolled diabetes and multiple comorbidities. This so-called “risk adjustment” doesn’t exist in traditional Medicare.
CMS also gives incentive payments to Medicare Advantage plans whose members receive better care, measured by such metrics as lower unnecessary hospital admissions. There are some analogues to this in traditional Medicare, such as the Making Care Primary program, but value-based care is a larger component of Medicare Advantage.
“Being paid for outcomes is what we as physicians always thought we went into medicine to do,” said Sarah Candler, MD, an internist in Houston. She most recently worked for One Medical and her experiences with Veterans Affairs and One Medical focused on value-based contracts, including for Medicare Advantage plans.
How do patients benefit from Medicare Advantage?
“Honestly the financial benefits to patients are what’s really driving the rise in Medicare Advantage,” said
Claire Ankuda, MD, MPH, is a geriatrician at the Icahn School of Medicine at Mt. Sinai in New York who has published extensively about Medicare Advantage.
A spokesperson for Highmark Health, which provides Medicare Advantage plans, told us that “Medicare Advantage plans are offered by private health insurers, such as Highmark, and typically offer benefits that support members’ total health, such as low-cost access to doctors and preventive care, and cover things like prescription drugs, vision, and hearing services, dental, and chiropractic care. Medicare Advantage plans also protect members from unforeseen costs like hospitalizations, surgery, or an expensive drug. And unlike traditional Medicare, Medicare Advantage plans can offer set copays for doctor’s visits (rather than coinsurance) to help members budget for their costs.”
Dr. Ankuda said hospitalization costs are sometimes higher for Medicare Advantage but agreed that costs for doctor visits are often lower with Medicare Advantage plans than with traditional Medicare.
So while the overarching goal of Medicare Advantage makes sense, Dr. Candler said, the actual physician experience of working with Medicare Advantage can be challenging.
What challenges might physicians experience when treating patients in a Medicare Advantage plan?
“The plan itself has control over what it will pay for and they’re much more aggressive about it than traditional Medicare,” Dr. Candler said. Medicare Advantage plans are often structured as health maintenance organizations, with narrow provider networks and extensive prior authorization requirements.
Dr. Candler gave an example of a plan that offers transportation to medical appointments – a seemingly great benefit. But what if someone needs to see a cardiologist and the only cardiologist within the plan is 100 miles away? That’s too far for the transportation benefit, it turns out.
Or a Medicare Advantage plan requires a physician to first do a physical exam before ordering an MRI, even though in the physician’s judgment only the MRI will have diagnostic value. Or the plan denies coverage for a service that’s already occurred. These practices aim to weed out unnecessary care but at the cost of patient confusion or physician time in arguing why something should be covered.
“The argument from us as physicians would be, ‘Just trust us to practice good medicine,’” Dr. Candler said.
Beside these concerns at the physician level, the regulations surrounding Medicare Advantage plans may open the door to billing fraud.
How do Medicare Advantage Plans interact with diagnosis codes?
“I just can’t stand when I see fraud in the health care system,” said Nancy Keating, MD, MPH, an internist at Brigham and Women’s Hospital and a health policy professor at Harvard Medical School, both in Boston.
This July, Dr. Keating published a report in the Annals of Internal Medicine about a patient of hers whose health insurer – a Medicare Advantage plan – claimed had diabetes with comorbidities and was morbidly obese. None of this was true. But submitting such diagnoses to CMS would suggest that Dr. Keating’s patient was especially ill, leading to greater reimbursements from CMS for covering her care.
“I’m not averse to paying plans that are taking care of sicker patients more, but we need to figure out who those patients truly are,” Dr. Keating said.
“It is absolutely true and widely proven that Medicare Advantage plans do a lot of clever things” to inflate diagnoses, added Dr. Ankuda. Also, she said that Medicare Advantage plan representatives would say this is legitimate work, as the entire point of Medicare Advantage is to pay more for caring for sicker patients.
“I don’t think anyone here is acting in bad faith. It’s just that [there are] very different incentives,” Dr. Ankuda said.
How can Medicare Advantage be improved?
Dr. Keating believes that CMS should reduce the number of diagnosis codes allowed within Medicare Advantage to thwart the potential for upcoding. Dr. Ankuda thinks the biggest problem is that there is no good way for patients to choose among Medicare Advantage plans.
“I don’t see it as Medicare Advantage is bad or Medicare Advantage is good. MA plans are incredibly diverse,” Dr. Ankuda said. The problem is that it’s very hard for patients to tell which plans are delivering the best care, what their out-of-pocket costs will actually be, or how often a plan denies payment.
Dr. Ankuda argues for much more data transparency around such plan factors.
Dr. Candler and Dr. Ankuda had no relevant conflicts. Dr. Keating is a consultant to the Research Triangle Institute, which advises CMS about Medicare Advantage billing codes.
As of 2023, most people eligible for Medicare are enrolled in Medicare Advantage plans administered by commercial insurers, rather than traditional Medicare plans sponsored by the federal government. The Kaiser Family Foundation reports that 31 million people are now enrolled in a Medicare Advantage plan, with almost half of them (47%) served by United Healthcare or Humana.
This is 51% of all people eligible for Medicare, compared with 19% in 2007. The Congressional Budget Office projects that 62% of Medicare participants will be in Medicare Advantage plans by 2033.
Given the explosive growth in Medicare Advantage participation, many readers have likely seen patients served by Medicare Advantage or will soon. Below is information about the program’s purpose, strengths, limitations, and effect on physicians.
How does Medicare Advantage differ from traditional Medicare?
A Medicare Advantage plan is approved by the U.S. Centers for Medicare & Medicaid Services and competes for customers by offering lower premiums and/or more benefits. Traditional Medicare plans are unified contracts across the country, with the same fees for the same services paid nationwide.
CMS pays Medicare Advantage plans a per-member rate, which can be increased for people who seem sicker than other plan participants – for example, someone with uncontrolled diabetes and multiple comorbidities. This so-called “risk adjustment” doesn’t exist in traditional Medicare.
CMS also gives incentive payments to Medicare Advantage plans whose members receive better care, measured by such metrics as lower unnecessary hospital admissions. There are some analogues to this in traditional Medicare, such as the Making Care Primary program, but value-based care is a larger component of Medicare Advantage.
“Being paid for outcomes is what we as physicians always thought we went into medicine to do,” said Sarah Candler, MD, an internist in Houston. She most recently worked for One Medical and her experiences with Veterans Affairs and One Medical focused on value-based contracts, including for Medicare Advantage plans.
How do patients benefit from Medicare Advantage?
“Honestly the financial benefits to patients are what’s really driving the rise in Medicare Advantage,” said
Claire Ankuda, MD, MPH, is a geriatrician at the Icahn School of Medicine at Mt. Sinai in New York who has published extensively about Medicare Advantage.
A spokesperson for Highmark Health, which provides Medicare Advantage plans, told us that “Medicare Advantage plans are offered by private health insurers, such as Highmark, and typically offer benefits that support members’ total health, such as low-cost access to doctors and preventive care, and cover things like prescription drugs, vision, and hearing services, dental, and chiropractic care. Medicare Advantage plans also protect members from unforeseen costs like hospitalizations, surgery, or an expensive drug. And unlike traditional Medicare, Medicare Advantage plans can offer set copays for doctor’s visits (rather than coinsurance) to help members budget for their costs.”
Dr. Ankuda said hospitalization costs are sometimes higher for Medicare Advantage but agreed that costs for doctor visits are often lower with Medicare Advantage plans than with traditional Medicare.
So while the overarching goal of Medicare Advantage makes sense, Dr. Candler said, the actual physician experience of working with Medicare Advantage can be challenging.
What challenges might physicians experience when treating patients in a Medicare Advantage plan?
“The plan itself has control over what it will pay for and they’re much more aggressive about it than traditional Medicare,” Dr. Candler said. Medicare Advantage plans are often structured as health maintenance organizations, with narrow provider networks and extensive prior authorization requirements.
Dr. Candler gave an example of a plan that offers transportation to medical appointments – a seemingly great benefit. But what if someone needs to see a cardiologist and the only cardiologist within the plan is 100 miles away? That’s too far for the transportation benefit, it turns out.
Or a Medicare Advantage plan requires a physician to first do a physical exam before ordering an MRI, even though in the physician’s judgment only the MRI will have diagnostic value. Or the plan denies coverage for a service that’s already occurred. These practices aim to weed out unnecessary care but at the cost of patient confusion or physician time in arguing why something should be covered.
“The argument from us as physicians would be, ‘Just trust us to practice good medicine,’” Dr. Candler said.
Beside these concerns at the physician level, the regulations surrounding Medicare Advantage plans may open the door to billing fraud.
How do Medicare Advantage Plans interact with diagnosis codes?
“I just can’t stand when I see fraud in the health care system,” said Nancy Keating, MD, MPH, an internist at Brigham and Women’s Hospital and a health policy professor at Harvard Medical School, both in Boston.
This July, Dr. Keating published a report in the Annals of Internal Medicine about a patient of hers whose health insurer – a Medicare Advantage plan – claimed had diabetes with comorbidities and was morbidly obese. None of this was true. But submitting such diagnoses to CMS would suggest that Dr. Keating’s patient was especially ill, leading to greater reimbursements from CMS for covering her care.
“I’m not averse to paying plans that are taking care of sicker patients more, but we need to figure out who those patients truly are,” Dr. Keating said.
“It is absolutely true and widely proven that Medicare Advantage plans do a lot of clever things” to inflate diagnoses, added Dr. Ankuda. Also, she said that Medicare Advantage plan representatives would say this is legitimate work, as the entire point of Medicare Advantage is to pay more for caring for sicker patients.
“I don’t think anyone here is acting in bad faith. It’s just that [there are] very different incentives,” Dr. Ankuda said.
How can Medicare Advantage be improved?
Dr. Keating believes that CMS should reduce the number of diagnosis codes allowed within Medicare Advantage to thwart the potential for upcoding. Dr. Ankuda thinks the biggest problem is that there is no good way for patients to choose among Medicare Advantage plans.
“I don’t see it as Medicare Advantage is bad or Medicare Advantage is good. MA plans are incredibly diverse,” Dr. Ankuda said. The problem is that it’s very hard for patients to tell which plans are delivering the best care, what their out-of-pocket costs will actually be, or how often a plan denies payment.
Dr. Ankuda argues for much more data transparency around such plan factors.
Dr. Candler and Dr. Ankuda had no relevant conflicts. Dr. Keating is a consultant to the Research Triangle Institute, which advises CMS about Medicare Advantage billing codes.
As of 2023, most people eligible for Medicare are enrolled in Medicare Advantage plans administered by commercial insurers, rather than traditional Medicare plans sponsored by the federal government. The Kaiser Family Foundation reports that 31 million people are now enrolled in a Medicare Advantage plan, with almost half of them (47%) served by United Healthcare or Humana.
This is 51% of all people eligible for Medicare, compared with 19% in 2007. The Congressional Budget Office projects that 62% of Medicare participants will be in Medicare Advantage plans by 2033.
Given the explosive growth in Medicare Advantage participation, many readers have likely seen patients served by Medicare Advantage or will soon. Below is information about the program’s purpose, strengths, limitations, and effect on physicians.
How does Medicare Advantage differ from traditional Medicare?
A Medicare Advantage plan is approved by the U.S. Centers for Medicare & Medicaid Services and competes for customers by offering lower premiums and/or more benefits. Traditional Medicare plans are unified contracts across the country, with the same fees for the same services paid nationwide.
CMS pays Medicare Advantage plans a per-member rate, which can be increased for people who seem sicker than other plan participants – for example, someone with uncontrolled diabetes and multiple comorbidities. This so-called “risk adjustment” doesn’t exist in traditional Medicare.
CMS also gives incentive payments to Medicare Advantage plans whose members receive better care, measured by such metrics as lower unnecessary hospital admissions. There are some analogues to this in traditional Medicare, such as the Making Care Primary program, but value-based care is a larger component of Medicare Advantage.
“Being paid for outcomes is what we as physicians always thought we went into medicine to do,” said Sarah Candler, MD, an internist in Houston. She most recently worked for One Medical and her experiences with Veterans Affairs and One Medical focused on value-based contracts, including for Medicare Advantage plans.
How do patients benefit from Medicare Advantage?
“Honestly the financial benefits to patients are what’s really driving the rise in Medicare Advantage,” said
Claire Ankuda, MD, MPH, is a geriatrician at the Icahn School of Medicine at Mt. Sinai in New York who has published extensively about Medicare Advantage.
A spokesperson for Highmark Health, which provides Medicare Advantage plans, told us that “Medicare Advantage plans are offered by private health insurers, such as Highmark, and typically offer benefits that support members’ total health, such as low-cost access to doctors and preventive care, and cover things like prescription drugs, vision, and hearing services, dental, and chiropractic care. Medicare Advantage plans also protect members from unforeseen costs like hospitalizations, surgery, or an expensive drug. And unlike traditional Medicare, Medicare Advantage plans can offer set copays for doctor’s visits (rather than coinsurance) to help members budget for their costs.”
Dr. Ankuda said hospitalization costs are sometimes higher for Medicare Advantage but agreed that costs for doctor visits are often lower with Medicare Advantage plans than with traditional Medicare.
So while the overarching goal of Medicare Advantage makes sense, Dr. Candler said, the actual physician experience of working with Medicare Advantage can be challenging.
What challenges might physicians experience when treating patients in a Medicare Advantage plan?
“The plan itself has control over what it will pay for and they’re much more aggressive about it than traditional Medicare,” Dr. Candler said. Medicare Advantage plans are often structured as health maintenance organizations, with narrow provider networks and extensive prior authorization requirements.
Dr. Candler gave an example of a plan that offers transportation to medical appointments – a seemingly great benefit. But what if someone needs to see a cardiologist and the only cardiologist within the plan is 100 miles away? That’s too far for the transportation benefit, it turns out.
Or a Medicare Advantage plan requires a physician to first do a physical exam before ordering an MRI, even though in the physician’s judgment only the MRI will have diagnostic value. Or the plan denies coverage for a service that’s already occurred. These practices aim to weed out unnecessary care but at the cost of patient confusion or physician time in arguing why something should be covered.
“The argument from us as physicians would be, ‘Just trust us to practice good medicine,’” Dr. Candler said.
Beside these concerns at the physician level, the regulations surrounding Medicare Advantage plans may open the door to billing fraud.
How do Medicare Advantage Plans interact with diagnosis codes?
“I just can’t stand when I see fraud in the health care system,” said Nancy Keating, MD, MPH, an internist at Brigham and Women’s Hospital and a health policy professor at Harvard Medical School, both in Boston.
This July, Dr. Keating published a report in the Annals of Internal Medicine about a patient of hers whose health insurer – a Medicare Advantage plan – claimed had diabetes with comorbidities and was morbidly obese. None of this was true. But submitting such diagnoses to CMS would suggest that Dr. Keating’s patient was especially ill, leading to greater reimbursements from CMS for covering her care.
“I’m not averse to paying plans that are taking care of sicker patients more, but we need to figure out who those patients truly are,” Dr. Keating said.
“It is absolutely true and widely proven that Medicare Advantage plans do a lot of clever things” to inflate diagnoses, added Dr. Ankuda. Also, she said that Medicare Advantage plan representatives would say this is legitimate work, as the entire point of Medicare Advantage is to pay more for caring for sicker patients.
“I don’t think anyone here is acting in bad faith. It’s just that [there are] very different incentives,” Dr. Ankuda said.
How can Medicare Advantage be improved?
Dr. Keating believes that CMS should reduce the number of diagnosis codes allowed within Medicare Advantage to thwart the potential for upcoding. Dr. Ankuda thinks the biggest problem is that there is no good way for patients to choose among Medicare Advantage plans.
“I don’t see it as Medicare Advantage is bad or Medicare Advantage is good. MA plans are incredibly diverse,” Dr. Ankuda said. The problem is that it’s very hard for patients to tell which plans are delivering the best care, what their out-of-pocket costs will actually be, or how often a plan denies payment.
Dr. Ankuda argues for much more data transparency around such plan factors.
Dr. Candler and Dr. Ankuda had no relevant conflicts. Dr. Keating is a consultant to the Research Triangle Institute, which advises CMS about Medicare Advantage billing codes.
Novel PET tracer for perfusion imaging: What’s the potential?
The emerging advantages of PET myocardial perfusion imaging (MPI) for coronary artery disease (CAD) diagnosis and assessment of cardiovascular event risk has prompted growing use of this technology as an alternative to the more commonly used single photon–emission CT (SPECT) MPI.
The advantages of PET MPI include better diagnostic performance and shorter acquisition times. , including consistent, high-quality images and low radiation exposure. It also allows quantification of myocardial blood flow, and it has “strong prognostic power.”
Tracer availability
Despite these advantages, that position paper and subsequent studies note that PET MPI has been underutilized in the United States, largely owing to issues with the available tracers, which have characteristics that limit widespread use in the clinic.
Rubidium, arguably the most commonly used tracer for PET MPI, is not available in unit dosing and so can be expensive for low-volume centers, plus it also requires an on-site generator, Michael Salerno, MD, PhD, a member of the American College of Cardiology’s Imaging Council and section chief of cardiovascular imaging, Stanford (Calif.) University, told this news organization.
N-ammonia, the other U.S. Food and Drug Administration–approved tracer, is available in unit dosing, but its short half-life means that centers need an onsite cyclotron, Dr. Salerno said.
For cardiac perfusion imaging and myocardial blood flow (MBF) quantification, 15O-water is considered the gold standard, although it’s not approved by the FDA. This tracer also requires an on-site cyclotron and “is challenging to use,” Dr. Salerno said. Use has been largely restricted to research purposes, though efforts are underway to widen its availability.
Enter flurpiridaz F-18 (GE Healthcare), a novel PET MPI tracer labeled with fluorine-18. Its longer half-life – similar to that of fluorodeoxyglucose, a tracer used to detect various cancers – could broaden the number of sites that could perform perfusion PET studies, Dr. Salerno said.
“Flurpiridaz also is supposed to have a more linear relationship between flow and tracer uptake, which could improve the ability to perform quantification of perfusion,” he noted. “It also offers the ability to do exercise PET, which is impossible for rubidium and challenging for ammonia, given its 11-minute half-life.”
Flurpiridaz status
The FDA requires two phase-3 studies that show safety and sufficient diagnostic performance before it will approve a new tracer. The first required study, published in the Journal of the American College of Cardiology, showed that the tracer’s sensitivity for detection of greater than or equal to 50% stenosis by ICA was significantly higher than SPECT; however, the specificity did not meet the prespecified noninferiority criterion.
The second FDA-required study, published online recently, also in the Journal of the American College of Cardiology, was designed differently from the first in that only patients with suspected – not known – CAD were enrolled. The primary efficacy endpoint was sensitivity and specificity of flurpiridaz PET for overall detection of CAD, rather than comparing it to SPECT MPI (which became a secondary endpoint). PET and SPECT studies were both performed before invasive coronary angiography to minimize referral bias; SPECT studies included cadmium zinc telluride cameras.
In that study, which included 578 patients (mean age, 64; 32.5% women) from 48 centers in the United States, Canada, and Europe, flurpiridaz met the efficacy endpoints: Its sensitivity and specificity were significantly higher than the prespecified threshold value by two of the three readers; its sensitivity was higher than SPECT (80.3% vs. 68.7%); and its specificity was noninferior (63.8% vs. 61.7%).
PET areas under the receiver-operating characteristic curves were higher than SPECT in the overall population and in women and obese patients, at half the radiation dose of SPECT.
“Cardiac PET MPI is positioned to serve as the leading modality for the functional evaluation of suspected and known CAD,” Jamieson M. Bourque, MD, MHS, medical director of nuclear cardiology, echocardiography, and the Stress Laboratory, University of Virginia, Charlottesville, wrote in an editorial accompanying the second study . “18F-flurpiridaz will facilitate this upward progression with beneficial tracer characteristics that will increase access and availability, enable exercise stress, and optimize MBF quantification.”
At this point, FDA approval of flurpiridaz is expected sometime in 2024, said James E. Udelson, MD, principal investigator of the recent study, chief of the division of cardiology, and director of the Nuclear Cardiology Laboratory at Tufts University School of Medicine, Boston.
Learning curve
Flurpiridaz comes with “a really interesting and important” learning curve, Dr. Udelson said. “The images are really crisp, and they look very different from what most people are used to. The GE folks are going to have to make sure that the American Society of Nuclear Cardiology and other professional societies are tuned in to help in the education part, because it’s not an easy, automatic switch. Very good image readers can adapt, but it’s not just one day you do one, then switch to the other.”
A “somewhat apt” analogy would be the difference between an echocardiogram and an MRI, he explained. “The MRI is much crisper. You’re seeing edges more crisply. You’re seeing the difference between a thicker and a thinner segment of the wall more crisply, and that’s actually real. You can’t say the thinner segment is abnormal; it’s just that you’re seeing it better. So, with this tracer, normal differences in the thickness of a wall can almost look like a defect if you’re not used to knowing that’s the new normal.”
The expected approval of flurpiridaz “will be a win for cardiac PET, broadening the range of sites that could perform PET,” Dr. Salerno commented. “However, it is worth cautioning that all of the prior data with PET using different agents does not necessarily equate to the same performance with the new agent, given that the performance seems to be lower than that shown in prior PET studies using other agents.”
Dr. Salerno would like to see additional studies comparing flurpiridaz with rubidium or ammonia, as well as studies performing quantification with flurpiridaz, “which theoretically should have some advantages,” he said.
Dr. Udelson noted that MedTrace, a company in Denmark, is working on a radiolabeled water tracer based on 15-O-water that is just starting a pivotal trial. Dr. Udelson is a consultant to the company and is a steering committee member for the pivotal trial.
For now, “the big take-home is that there are a lot of ways these days to test people for CAD,” he said. “As the types of things we can do to test people expand, individuals and centers need to make sure they focus on providing any new service, however they do it, with really superb quality and experience.”
“You don’t just do something new because it’s new,” he added. “It has to be done really well. If you do the new thing badly, you’re not going to get better information.”
Dr. Udelson is a consultant and advisory board member for GE Healthcare, a consultant to MedTrace, and a steering committee member for MedTrace’s pivotal trial. Dr. Bourque has served on a GE Healthcare advisory board for amyloid imaging. Dr. Salerno reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The emerging advantages of PET myocardial perfusion imaging (MPI) for coronary artery disease (CAD) diagnosis and assessment of cardiovascular event risk has prompted growing use of this technology as an alternative to the more commonly used single photon–emission CT (SPECT) MPI.
The advantages of PET MPI include better diagnostic performance and shorter acquisition times. , including consistent, high-quality images and low radiation exposure. It also allows quantification of myocardial blood flow, and it has “strong prognostic power.”
Tracer availability
Despite these advantages, that position paper and subsequent studies note that PET MPI has been underutilized in the United States, largely owing to issues with the available tracers, which have characteristics that limit widespread use in the clinic.
Rubidium, arguably the most commonly used tracer for PET MPI, is not available in unit dosing and so can be expensive for low-volume centers, plus it also requires an on-site generator, Michael Salerno, MD, PhD, a member of the American College of Cardiology’s Imaging Council and section chief of cardiovascular imaging, Stanford (Calif.) University, told this news organization.
N-ammonia, the other U.S. Food and Drug Administration–approved tracer, is available in unit dosing, but its short half-life means that centers need an onsite cyclotron, Dr. Salerno said.
For cardiac perfusion imaging and myocardial blood flow (MBF) quantification, 15O-water is considered the gold standard, although it’s not approved by the FDA. This tracer also requires an on-site cyclotron and “is challenging to use,” Dr. Salerno said. Use has been largely restricted to research purposes, though efforts are underway to widen its availability.
Enter flurpiridaz F-18 (GE Healthcare), a novel PET MPI tracer labeled with fluorine-18. Its longer half-life – similar to that of fluorodeoxyglucose, a tracer used to detect various cancers – could broaden the number of sites that could perform perfusion PET studies, Dr. Salerno said.
“Flurpiridaz also is supposed to have a more linear relationship between flow and tracer uptake, which could improve the ability to perform quantification of perfusion,” he noted. “It also offers the ability to do exercise PET, which is impossible for rubidium and challenging for ammonia, given its 11-minute half-life.”
Flurpiridaz status
The FDA requires two phase-3 studies that show safety and sufficient diagnostic performance before it will approve a new tracer. The first required study, published in the Journal of the American College of Cardiology, showed that the tracer’s sensitivity for detection of greater than or equal to 50% stenosis by ICA was significantly higher than SPECT; however, the specificity did not meet the prespecified noninferiority criterion.
The second FDA-required study, published online recently, also in the Journal of the American College of Cardiology, was designed differently from the first in that only patients with suspected – not known – CAD were enrolled. The primary efficacy endpoint was sensitivity and specificity of flurpiridaz PET for overall detection of CAD, rather than comparing it to SPECT MPI (which became a secondary endpoint). PET and SPECT studies were both performed before invasive coronary angiography to minimize referral bias; SPECT studies included cadmium zinc telluride cameras.
In that study, which included 578 patients (mean age, 64; 32.5% women) from 48 centers in the United States, Canada, and Europe, flurpiridaz met the efficacy endpoints: Its sensitivity and specificity were significantly higher than the prespecified threshold value by two of the three readers; its sensitivity was higher than SPECT (80.3% vs. 68.7%); and its specificity was noninferior (63.8% vs. 61.7%).
PET areas under the receiver-operating characteristic curves were higher than SPECT in the overall population and in women and obese patients, at half the radiation dose of SPECT.
“Cardiac PET MPI is positioned to serve as the leading modality for the functional evaluation of suspected and known CAD,” Jamieson M. Bourque, MD, MHS, medical director of nuclear cardiology, echocardiography, and the Stress Laboratory, University of Virginia, Charlottesville, wrote in an editorial accompanying the second study . “18F-flurpiridaz will facilitate this upward progression with beneficial tracer characteristics that will increase access and availability, enable exercise stress, and optimize MBF quantification.”
At this point, FDA approval of flurpiridaz is expected sometime in 2024, said James E. Udelson, MD, principal investigator of the recent study, chief of the division of cardiology, and director of the Nuclear Cardiology Laboratory at Tufts University School of Medicine, Boston.
Learning curve
Flurpiridaz comes with “a really interesting and important” learning curve, Dr. Udelson said. “The images are really crisp, and they look very different from what most people are used to. The GE folks are going to have to make sure that the American Society of Nuclear Cardiology and other professional societies are tuned in to help in the education part, because it’s not an easy, automatic switch. Very good image readers can adapt, but it’s not just one day you do one, then switch to the other.”
A “somewhat apt” analogy would be the difference between an echocardiogram and an MRI, he explained. “The MRI is much crisper. You’re seeing edges more crisply. You’re seeing the difference between a thicker and a thinner segment of the wall more crisply, and that’s actually real. You can’t say the thinner segment is abnormal; it’s just that you’re seeing it better. So, with this tracer, normal differences in the thickness of a wall can almost look like a defect if you’re not used to knowing that’s the new normal.”
The expected approval of flurpiridaz “will be a win for cardiac PET, broadening the range of sites that could perform PET,” Dr. Salerno commented. “However, it is worth cautioning that all of the prior data with PET using different agents does not necessarily equate to the same performance with the new agent, given that the performance seems to be lower than that shown in prior PET studies using other agents.”
Dr. Salerno would like to see additional studies comparing flurpiridaz with rubidium or ammonia, as well as studies performing quantification with flurpiridaz, “which theoretically should have some advantages,” he said.
Dr. Udelson noted that MedTrace, a company in Denmark, is working on a radiolabeled water tracer based on 15-O-water that is just starting a pivotal trial. Dr. Udelson is a consultant to the company and is a steering committee member for the pivotal trial.
For now, “the big take-home is that there are a lot of ways these days to test people for CAD,” he said. “As the types of things we can do to test people expand, individuals and centers need to make sure they focus on providing any new service, however they do it, with really superb quality and experience.”
“You don’t just do something new because it’s new,” he added. “It has to be done really well. If you do the new thing badly, you’re not going to get better information.”
Dr. Udelson is a consultant and advisory board member for GE Healthcare, a consultant to MedTrace, and a steering committee member for MedTrace’s pivotal trial. Dr. Bourque has served on a GE Healthcare advisory board for amyloid imaging. Dr. Salerno reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The emerging advantages of PET myocardial perfusion imaging (MPI) for coronary artery disease (CAD) diagnosis and assessment of cardiovascular event risk has prompted growing use of this technology as an alternative to the more commonly used single photon–emission CT (SPECT) MPI.
The advantages of PET MPI include better diagnostic performance and shorter acquisition times. , including consistent, high-quality images and low radiation exposure. It also allows quantification of myocardial blood flow, and it has “strong prognostic power.”
Tracer availability
Despite these advantages, that position paper and subsequent studies note that PET MPI has been underutilized in the United States, largely owing to issues with the available tracers, which have characteristics that limit widespread use in the clinic.
Rubidium, arguably the most commonly used tracer for PET MPI, is not available in unit dosing and so can be expensive for low-volume centers, plus it also requires an on-site generator, Michael Salerno, MD, PhD, a member of the American College of Cardiology’s Imaging Council and section chief of cardiovascular imaging, Stanford (Calif.) University, told this news organization.
N-ammonia, the other U.S. Food and Drug Administration–approved tracer, is available in unit dosing, but its short half-life means that centers need an onsite cyclotron, Dr. Salerno said.
For cardiac perfusion imaging and myocardial blood flow (MBF) quantification, 15O-water is considered the gold standard, although it’s not approved by the FDA. This tracer also requires an on-site cyclotron and “is challenging to use,” Dr. Salerno said. Use has been largely restricted to research purposes, though efforts are underway to widen its availability.
Enter flurpiridaz F-18 (GE Healthcare), a novel PET MPI tracer labeled with fluorine-18. Its longer half-life – similar to that of fluorodeoxyglucose, a tracer used to detect various cancers – could broaden the number of sites that could perform perfusion PET studies, Dr. Salerno said.
“Flurpiridaz also is supposed to have a more linear relationship between flow and tracer uptake, which could improve the ability to perform quantification of perfusion,” he noted. “It also offers the ability to do exercise PET, which is impossible for rubidium and challenging for ammonia, given its 11-minute half-life.”
Flurpiridaz status
The FDA requires two phase-3 studies that show safety and sufficient diagnostic performance before it will approve a new tracer. The first required study, published in the Journal of the American College of Cardiology, showed that the tracer’s sensitivity for detection of greater than or equal to 50% stenosis by ICA was significantly higher than SPECT; however, the specificity did not meet the prespecified noninferiority criterion.
The second FDA-required study, published online recently, also in the Journal of the American College of Cardiology, was designed differently from the first in that only patients with suspected – not known – CAD were enrolled. The primary efficacy endpoint was sensitivity and specificity of flurpiridaz PET for overall detection of CAD, rather than comparing it to SPECT MPI (which became a secondary endpoint). PET and SPECT studies were both performed before invasive coronary angiography to minimize referral bias; SPECT studies included cadmium zinc telluride cameras.
In that study, which included 578 patients (mean age, 64; 32.5% women) from 48 centers in the United States, Canada, and Europe, flurpiridaz met the efficacy endpoints: Its sensitivity and specificity were significantly higher than the prespecified threshold value by two of the three readers; its sensitivity was higher than SPECT (80.3% vs. 68.7%); and its specificity was noninferior (63.8% vs. 61.7%).
PET areas under the receiver-operating characteristic curves were higher than SPECT in the overall population and in women and obese patients, at half the radiation dose of SPECT.
“Cardiac PET MPI is positioned to serve as the leading modality for the functional evaluation of suspected and known CAD,” Jamieson M. Bourque, MD, MHS, medical director of nuclear cardiology, echocardiography, and the Stress Laboratory, University of Virginia, Charlottesville, wrote in an editorial accompanying the second study . “18F-flurpiridaz will facilitate this upward progression with beneficial tracer characteristics that will increase access and availability, enable exercise stress, and optimize MBF quantification.”
At this point, FDA approval of flurpiridaz is expected sometime in 2024, said James E. Udelson, MD, principal investigator of the recent study, chief of the division of cardiology, and director of the Nuclear Cardiology Laboratory at Tufts University School of Medicine, Boston.
Learning curve
Flurpiridaz comes with “a really interesting and important” learning curve, Dr. Udelson said. “The images are really crisp, and they look very different from what most people are used to. The GE folks are going to have to make sure that the American Society of Nuclear Cardiology and other professional societies are tuned in to help in the education part, because it’s not an easy, automatic switch. Very good image readers can adapt, but it’s not just one day you do one, then switch to the other.”
A “somewhat apt” analogy would be the difference between an echocardiogram and an MRI, he explained. “The MRI is much crisper. You’re seeing edges more crisply. You’re seeing the difference between a thicker and a thinner segment of the wall more crisply, and that’s actually real. You can’t say the thinner segment is abnormal; it’s just that you’re seeing it better. So, with this tracer, normal differences in the thickness of a wall can almost look like a defect if you’re not used to knowing that’s the new normal.”
The expected approval of flurpiridaz “will be a win for cardiac PET, broadening the range of sites that could perform PET,” Dr. Salerno commented. “However, it is worth cautioning that all of the prior data with PET using different agents does not necessarily equate to the same performance with the new agent, given that the performance seems to be lower than that shown in prior PET studies using other agents.”
Dr. Salerno would like to see additional studies comparing flurpiridaz with rubidium or ammonia, as well as studies performing quantification with flurpiridaz, “which theoretically should have some advantages,” he said.
Dr. Udelson noted that MedTrace, a company in Denmark, is working on a radiolabeled water tracer based on 15-O-water that is just starting a pivotal trial. Dr. Udelson is a consultant to the company and is a steering committee member for the pivotal trial.
For now, “the big take-home is that there are a lot of ways these days to test people for CAD,” he said. “As the types of things we can do to test people expand, individuals and centers need to make sure they focus on providing any new service, however they do it, with really superb quality and experience.”
“You don’t just do something new because it’s new,” he added. “It has to be done really well. If you do the new thing badly, you’re not going to get better information.”
Dr. Udelson is a consultant and advisory board member for GE Healthcare, a consultant to MedTrace, and a steering committee member for MedTrace’s pivotal trial. Dr. Bourque has served on a GE Healthcare advisory board for amyloid imaging. Dr. Salerno reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
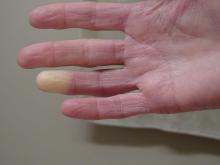
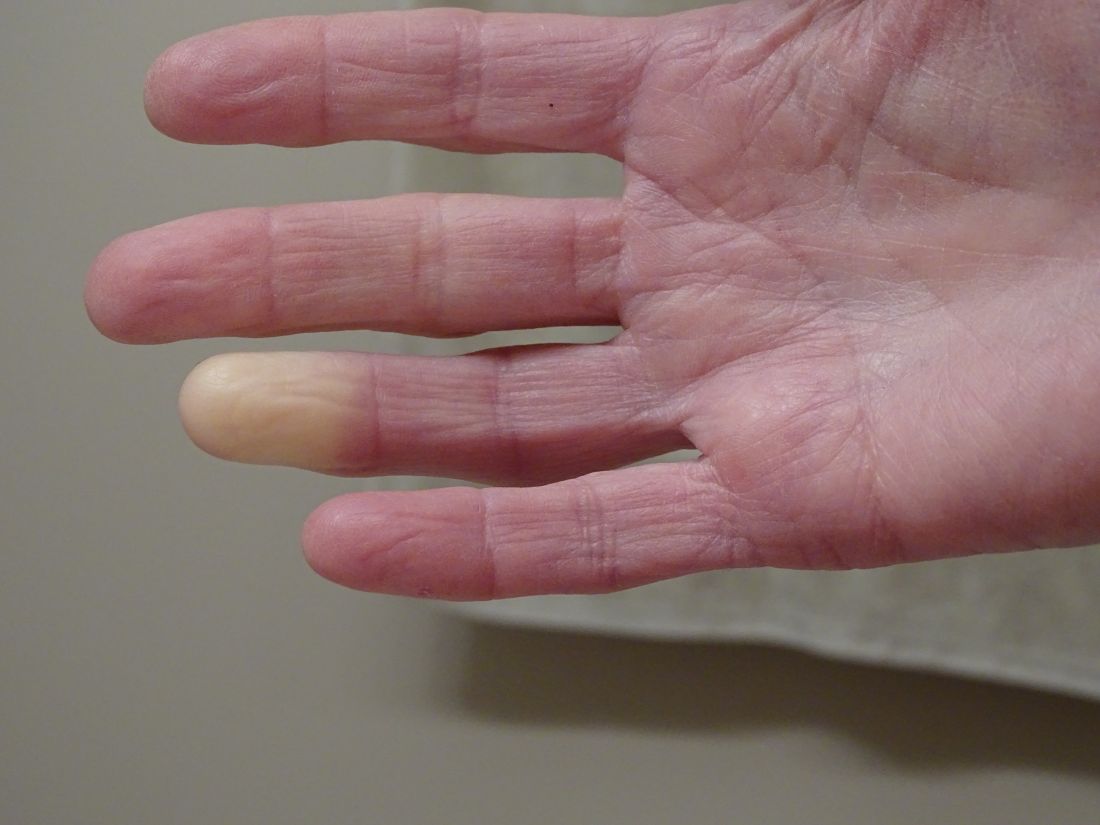
Researchers link two genes to Raynaud’s disease
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Prior authorization software: Saves time but hurdles remain
New England Baptist Hospital has been grappling with a serious problem facing health care today: insurers demanding prior authorizations for services ordered by physicians. Meeting payers’ requirements eats up time, delays treatment, and can be a costly drain on doctors’ practices.
To deal with this problem, the Boston orthopedic hospital has opted to automate submission of prior authorization requests on behalf of more than 100 mostly orthopedic surgeons on staff.
After 5 years using this system, “we can say that automation definitely works,” said Lidiya Hadzhieva, director of patient access at the hospital. The software has reduced write-offs by 30% and staff costs by 25%. Prior authorization gets approved 3 days after scheduling, compared with 11 days previously, she said.
“This software not only saves staff time, but it can also more accurately predict when prior authorization is needed,” she added.
For practices deluged with required prior authorizations by insurers, automation is emerging as a way for practices to make the process less time-consuming and save money. However, the software can be costly and may not be adoptable to many practices, and many physicians are not even aware it exists.
So far, the software is mainly used at large organizations like hospital systems. But as word gets out and the software becomes easier to use, private practices and other smaller entities may join the automation trend.
There is definitely a need to automate prior authorization. The American Medical Association reports that physicians spend 16 hours per week on prior authorizations. In a recent AMA survey, more than 60% of physicians indicated that it’s difficult to know when prior authorization is needed. And 93% of physicians reported care delays while waiting for authorization, the AMA said.
Experts estimate that 80% of prior authorization work could be automated, but most practices still use the phone or fax, even as numbers of prior authorizations continue to increase.
How it works
Automation software connects directly to the practice’s electronic health record (EHR). “When the doctor places an order in the EHR, the process starts automatically,” Ms. Hadzhieva said. “The doctor may not even notice it.”
In addition to using an EHR connection, many software products can communicate with the payer through its portal or by fax or phone, while still automating other parts of the process.
The software’s first step is to decide whether prior authorization is needed. This requires having an updated list of the rules that each payer uses for prior authorization. Manually keeping track of payer rules is very time-consuming, but automation uses bots to visit each payer site to look for rules changes. One vendor, Infinitus, uses a voice-based bot called Eva that calls up each payer and speaks with a representative.
“Automatically updating payer rules is not a new technology,” said YiDing Yu, MD, chief product officer at Olive, the automation vendor for New England Baptist. “What is new in the last 5 years is extracting the information needed for the prior authorization out of the clinical notes.”
This is challenging because each doctor has different ways to describe each step of clinical work. To identify this shorthand, Dr. Yu said Olive uses natural language processing, which is a form of artificial intelligence that learns how each doctor describes things.
Dr. Yu asserts that Olive is actually better than a practice’s staff at digging out clinical information. She said staff without much clinical training may miss terms that the software can catch, and they don’t have the time to go back many months into the record to find valuable information. But automation can do that.
In some instances, however, the software may not be able to find the information, in which case it alerts staff through a prompt in the EHR and the information is retrieved manually, Dr. Yu said.
Next, the Olive software puts the information it found into the request form and sends it to the payer. After submission, the software constantly checks on the status of each request, again visiting payer sites with a bot.
At New England Baptist, the software is used mainly by physicians in fairly small private practices who are on staff. They are using the software on the hospital’s dime, but it only works inside the hospital, Ms. Hadzhieva said. For their work outside of the hospital, they would have to purchase the Olive software on their own, she said.
Automation hasn’t spread to practices yet
Despite the promising outcomes for products like Olive, automation software is still primarily used by large organizations. Vendors say very few private practices have bought it yet. “The technology works, but it is still in the early-adopter phase,” Dr. Yu said.
For one thing, the software can be expensive. Very few vendors reveal their prices, but Dr. Yu did so. She said Olive normally costs about $50,000 a year for even a small organization. She insisted, however, that the savings from avoiding just one denial each month for a hip surgery would justify the expense.
On the other hand, some automation software is free, such as the Surescripts product for prior authorization of prescriptions. But it is unclear whether Surescripts does as much as Olive. Vendors’ descriptions of their products tend to be vague.
Also, Surescripts and Olive have entirely separate functions. Dr. Yu said Olive is limited to procedures, so it benefits specialties like oncology, neurosurgery, colorectal surgery, vascular surgery, and cardiology. Olive does not cover prescriptions, because they operate on a different technology.
Dr. Yu said another hurdle for adopting the software is the kind of EHR systems that doctors use. At this point, only a few EHR systems – such as Epic, Cerner, and Athena – are compatible with Olive. Large organizations tend to use Epic and Cerner, while many practices often use Athena or a variety of other systems, she said.
Despite stunted demand, there is no shortage of companies offering automation software for medical (that is, non-prescription) prior authorization. One compilation lists 25 such vendors, including companies like Myndshft, Rhyme, Infinitus, Infinx, and Waystar. As with any start-up technology, companies occasionally buy each other out.
In addition to issues like cost, specialty, and EHR compatibility, another hurdle is that few doctors even know the technology exists. Vendors say marketing focuses on larger provider organizations, not smaller practices.
Even many tech-savvy doctors, like Adam Bruggeman, MD, an orthopedist and CEO of Texas Spine Care Center in San Antonio, say they know little about the technology. “There is definitely a need to automate prior authorization,” he said. “But I don’t know of any colleagues who use it.” He has only just begun to explore vendors, he said.
Many medical practice consultants also have not yet explored the technology. “Automation makes a lot of sense, because there are a lot of repetitive tasks in prior authorization,” said Jill Arena, CEO of Portland, Ore.–based Health e Practices. “But I haven’t looked into it yet, and none of my clients has even asked about it.”
“I could see how it could be an easier sell for large organizations,” she added. “They have an IT person and a CFO who can explore the issue. Smaller practices usually don’t have that kind of expertise.”
Where does automation go from here?
Until now, clinicians who want to fully automate prior authorizations would have to buy two products – one for medical procedures and one for prescriptions. This has to do with incompatible electronic transmission standards, which are used to digitize information, said Susan Lawson-Dawson, content marketing strategist for the vendor Myndshft Health.
Myndshft has long been selling automation software for medical prior authorizations, but now it is introducing a product for prescriptions, Ms. Lawson-Dawson said. She said Myndshft will then be the only vendor to automate both kinds of prior authorizations.
Ms. Lawson-Dawson said Myndshft has 685 customers to date and is looking for more business. Recently the company entered the Google Cloud Marketplace. Google Cloud customers can now direct their committed spend with Google to purchasing Myndshft, meaning they could get it at a discount.
Software like Olive and Myndshft can operate independently of payers, but a vendor called Rhyme depends on payers for its software to function, said Rhyme CEO Joe Anstine. He said more than 300 payers have agreed to install the Rhyme system, and Rhyme has signed up a number of large health systems to use the product. Initially, he said, clinicians paid for the service, but now Rhyme is beginning to find payers to foot the costs and to let clinicians use it for free, which would open Rhyme up to smaller practices.
EHR companies themselves are beginning to offer automation, too. Epic, for example, has created a tool for prior authorization as part of its Epic Payer Platform. Like Rhyme, it requires payer cooperation, because information goes back and forth between clinician and payer in what is called bi-directional exchange.
The Epic product is still in its pilot phase. Epic reported that several large health systems were using its product in conjunction with a specific payer – for instance, Mayo Clinic with Blue Cross and Blue Shield of Minnesota and Ochsner Health with Humana. According to Epic, the arrangement reduced Mayo’s denials due to additional documentation requests by 63% for professional billing.
Automating with just one payer still means the clinician has to deal with manual processes at other payers, but a large clinician could have sufficient volume with that one payer to make the arrangement useful.
Will payers automate prior authorization?
Ultimately, payers may take the automation business away from vendors, offering a free product to all clinicians. But don’t hold your breath. Payers first have to rebuild their electronic systems to accommodate an electronic connection with providers. Even then, some payers might hold back from automating, forcing practices to continue manually processing some prior authorizations.
Efforts are underway, however, to mandate payers to support prior authorization automation. For this to happen, payers would have to revamp their data so that it could be easily read by practices’ EHRs. This would mean adopting a specific interoperability standard called Health Level 7 Fast Healthcare Interoperability Resources (FHIR).
Toward this goal, the Centers for Medicare & Medicaid Services proposes to require payers to adopt FHIR by January 2026. (CMS still has to finalize the rule.) Experts say the two-year ramp-up time is needed because it takes extensive work for payers to translate their data into FHIR.
The only payer so far to switch to FHIR for prior authorization is Regence in Washington state. In a pilot project, it has automated prior authorization with just one provider, MultiCare Connected Care, an accountable care organization (ACO), also in Washington state.
Anna Taylor, associate vice president of population health and value-based care at MultiCare, explained how the arrangement works. “Two separate entities are sharing one operational process,” she told this news organization. “That means they can have a digital conversation back and forth, so it is much easier to resolve prior authorization issues.”
Unlike many vendor products, the Regence service is free. And while the vendors market only to large organizations, most doctors in the MultiCare arrangement are in independent practices. Ms. Taylor said these doctors have been “enthusiastic” about the arrangement.
The results of the pilot are impressive. Ms. Taylor said automation has resulted in a 233% productivity gain for MultiCare clinicians, and 89% of submissions to Regence get an immediate response.
There is a potential downside, however, to working directly with payers. A direct connection to clinicians allows payers to access the doctor’s clinical notes, which could make many doctors uneasy. But Ms. Taylor said Regence only has access to the “discrete data fields” on MultiCare’s EHR dashboard, not to the notes themselves.
The ultimate goal of the Regence-Multicare project is to include more payers and clinicians. Ms. Taylor said two of the 27 other payers that MultiCare works with are “highly interested,” but it would take a lot of work for them to get connected with practices and other clinicians.
Ultimately, payers could offer automation and third-party vendors might then fade away. However, physicians may resist working directly with payers if the arrangement requires full access to their medical records.
A version of this article first appeared on Medscape.com.
New England Baptist Hospital has been grappling with a serious problem facing health care today: insurers demanding prior authorizations for services ordered by physicians. Meeting payers’ requirements eats up time, delays treatment, and can be a costly drain on doctors’ practices.
To deal with this problem, the Boston orthopedic hospital has opted to automate submission of prior authorization requests on behalf of more than 100 mostly orthopedic surgeons on staff.
After 5 years using this system, “we can say that automation definitely works,” said Lidiya Hadzhieva, director of patient access at the hospital. The software has reduced write-offs by 30% and staff costs by 25%. Prior authorization gets approved 3 days after scheduling, compared with 11 days previously, she said.
“This software not only saves staff time, but it can also more accurately predict when prior authorization is needed,” she added.
For practices deluged with required prior authorizations by insurers, automation is emerging as a way for practices to make the process less time-consuming and save money. However, the software can be costly and may not be adoptable to many practices, and many physicians are not even aware it exists.
So far, the software is mainly used at large organizations like hospital systems. But as word gets out and the software becomes easier to use, private practices and other smaller entities may join the automation trend.
There is definitely a need to automate prior authorization. The American Medical Association reports that physicians spend 16 hours per week on prior authorizations. In a recent AMA survey, more than 60% of physicians indicated that it’s difficult to know when prior authorization is needed. And 93% of physicians reported care delays while waiting for authorization, the AMA said.
Experts estimate that 80% of prior authorization work could be automated, but most practices still use the phone or fax, even as numbers of prior authorizations continue to increase.
How it works
Automation software connects directly to the practice’s electronic health record (EHR). “When the doctor places an order in the EHR, the process starts automatically,” Ms. Hadzhieva said. “The doctor may not even notice it.”
In addition to using an EHR connection, many software products can communicate with the payer through its portal or by fax or phone, while still automating other parts of the process.
The software’s first step is to decide whether prior authorization is needed. This requires having an updated list of the rules that each payer uses for prior authorization. Manually keeping track of payer rules is very time-consuming, but automation uses bots to visit each payer site to look for rules changes. One vendor, Infinitus, uses a voice-based bot called Eva that calls up each payer and speaks with a representative.
“Automatically updating payer rules is not a new technology,” said YiDing Yu, MD, chief product officer at Olive, the automation vendor for New England Baptist. “What is new in the last 5 years is extracting the information needed for the prior authorization out of the clinical notes.”
This is challenging because each doctor has different ways to describe each step of clinical work. To identify this shorthand, Dr. Yu said Olive uses natural language processing, which is a form of artificial intelligence that learns how each doctor describes things.
Dr. Yu asserts that Olive is actually better than a practice’s staff at digging out clinical information. She said staff without much clinical training may miss terms that the software can catch, and they don’t have the time to go back many months into the record to find valuable information. But automation can do that.
In some instances, however, the software may not be able to find the information, in which case it alerts staff through a prompt in the EHR and the information is retrieved manually, Dr. Yu said.
Next, the Olive software puts the information it found into the request form and sends it to the payer. After submission, the software constantly checks on the status of each request, again visiting payer sites with a bot.
At New England Baptist, the software is used mainly by physicians in fairly small private practices who are on staff. They are using the software on the hospital’s dime, but it only works inside the hospital, Ms. Hadzhieva said. For their work outside of the hospital, they would have to purchase the Olive software on their own, she said.
Automation hasn’t spread to practices yet
Despite the promising outcomes for products like Olive, automation software is still primarily used by large organizations. Vendors say very few private practices have bought it yet. “The technology works, but it is still in the early-adopter phase,” Dr. Yu said.
For one thing, the software can be expensive. Very few vendors reveal their prices, but Dr. Yu did so. She said Olive normally costs about $50,000 a year for even a small organization. She insisted, however, that the savings from avoiding just one denial each month for a hip surgery would justify the expense.
On the other hand, some automation software is free, such as the Surescripts product for prior authorization of prescriptions. But it is unclear whether Surescripts does as much as Olive. Vendors’ descriptions of their products tend to be vague.
Also, Surescripts and Olive have entirely separate functions. Dr. Yu said Olive is limited to procedures, so it benefits specialties like oncology, neurosurgery, colorectal surgery, vascular surgery, and cardiology. Olive does not cover prescriptions, because they operate on a different technology.
Dr. Yu said another hurdle for adopting the software is the kind of EHR systems that doctors use. At this point, only a few EHR systems – such as Epic, Cerner, and Athena – are compatible with Olive. Large organizations tend to use Epic and Cerner, while many practices often use Athena or a variety of other systems, she said.
Despite stunted demand, there is no shortage of companies offering automation software for medical (that is, non-prescription) prior authorization. One compilation lists 25 such vendors, including companies like Myndshft, Rhyme, Infinitus, Infinx, and Waystar. As with any start-up technology, companies occasionally buy each other out.
In addition to issues like cost, specialty, and EHR compatibility, another hurdle is that few doctors even know the technology exists. Vendors say marketing focuses on larger provider organizations, not smaller practices.
Even many tech-savvy doctors, like Adam Bruggeman, MD, an orthopedist and CEO of Texas Spine Care Center in San Antonio, say they know little about the technology. “There is definitely a need to automate prior authorization,” he said. “But I don’t know of any colleagues who use it.” He has only just begun to explore vendors, he said.
Many medical practice consultants also have not yet explored the technology. “Automation makes a lot of sense, because there are a lot of repetitive tasks in prior authorization,” said Jill Arena, CEO of Portland, Ore.–based Health e Practices. “But I haven’t looked into it yet, and none of my clients has even asked about it.”
“I could see how it could be an easier sell for large organizations,” she added. “They have an IT person and a CFO who can explore the issue. Smaller practices usually don’t have that kind of expertise.”
Where does automation go from here?
Until now, clinicians who want to fully automate prior authorizations would have to buy two products – one for medical procedures and one for prescriptions. This has to do with incompatible electronic transmission standards, which are used to digitize information, said Susan Lawson-Dawson, content marketing strategist for the vendor Myndshft Health.
Myndshft has long been selling automation software for medical prior authorizations, but now it is introducing a product for prescriptions, Ms. Lawson-Dawson said. She said Myndshft will then be the only vendor to automate both kinds of prior authorizations.
Ms. Lawson-Dawson said Myndshft has 685 customers to date and is looking for more business. Recently the company entered the Google Cloud Marketplace. Google Cloud customers can now direct their committed spend with Google to purchasing Myndshft, meaning they could get it at a discount.
Software like Olive and Myndshft can operate independently of payers, but a vendor called Rhyme depends on payers for its software to function, said Rhyme CEO Joe Anstine. He said more than 300 payers have agreed to install the Rhyme system, and Rhyme has signed up a number of large health systems to use the product. Initially, he said, clinicians paid for the service, but now Rhyme is beginning to find payers to foot the costs and to let clinicians use it for free, which would open Rhyme up to smaller practices.
EHR companies themselves are beginning to offer automation, too. Epic, for example, has created a tool for prior authorization as part of its Epic Payer Platform. Like Rhyme, it requires payer cooperation, because information goes back and forth between clinician and payer in what is called bi-directional exchange.
The Epic product is still in its pilot phase. Epic reported that several large health systems were using its product in conjunction with a specific payer – for instance, Mayo Clinic with Blue Cross and Blue Shield of Minnesota and Ochsner Health with Humana. According to Epic, the arrangement reduced Mayo’s denials due to additional documentation requests by 63% for professional billing.
Automating with just one payer still means the clinician has to deal with manual processes at other payers, but a large clinician could have sufficient volume with that one payer to make the arrangement useful.
Will payers automate prior authorization?
Ultimately, payers may take the automation business away from vendors, offering a free product to all clinicians. But don’t hold your breath. Payers first have to rebuild their electronic systems to accommodate an electronic connection with providers. Even then, some payers might hold back from automating, forcing practices to continue manually processing some prior authorizations.
Efforts are underway, however, to mandate payers to support prior authorization automation. For this to happen, payers would have to revamp their data so that it could be easily read by practices’ EHRs. This would mean adopting a specific interoperability standard called Health Level 7 Fast Healthcare Interoperability Resources (FHIR).
Toward this goal, the Centers for Medicare & Medicaid Services proposes to require payers to adopt FHIR by January 2026. (CMS still has to finalize the rule.) Experts say the two-year ramp-up time is needed because it takes extensive work for payers to translate their data into FHIR.
The only payer so far to switch to FHIR for prior authorization is Regence in Washington state. In a pilot project, it has automated prior authorization with just one provider, MultiCare Connected Care, an accountable care organization (ACO), also in Washington state.
Anna Taylor, associate vice president of population health and value-based care at MultiCare, explained how the arrangement works. “Two separate entities are sharing one operational process,” she told this news organization. “That means they can have a digital conversation back and forth, so it is much easier to resolve prior authorization issues.”
Unlike many vendor products, the Regence service is free. And while the vendors market only to large organizations, most doctors in the MultiCare arrangement are in independent practices. Ms. Taylor said these doctors have been “enthusiastic” about the arrangement.
The results of the pilot are impressive. Ms. Taylor said automation has resulted in a 233% productivity gain for MultiCare clinicians, and 89% of submissions to Regence get an immediate response.
There is a potential downside, however, to working directly with payers. A direct connection to clinicians allows payers to access the doctor’s clinical notes, which could make many doctors uneasy. But Ms. Taylor said Regence only has access to the “discrete data fields” on MultiCare’s EHR dashboard, not to the notes themselves.
The ultimate goal of the Regence-Multicare project is to include more payers and clinicians. Ms. Taylor said two of the 27 other payers that MultiCare works with are “highly interested,” but it would take a lot of work for them to get connected with practices and other clinicians.
Ultimately, payers could offer automation and third-party vendors might then fade away. However, physicians may resist working directly with payers if the arrangement requires full access to their medical records.
A version of this article first appeared on Medscape.com.
New England Baptist Hospital has been grappling with a serious problem facing health care today: insurers demanding prior authorizations for services ordered by physicians. Meeting payers’ requirements eats up time, delays treatment, and can be a costly drain on doctors’ practices.
To deal with this problem, the Boston orthopedic hospital has opted to automate submission of prior authorization requests on behalf of more than 100 mostly orthopedic surgeons on staff.
After 5 years using this system, “we can say that automation definitely works,” said Lidiya Hadzhieva, director of patient access at the hospital. The software has reduced write-offs by 30% and staff costs by 25%. Prior authorization gets approved 3 days after scheduling, compared with 11 days previously, she said.
“This software not only saves staff time, but it can also more accurately predict when prior authorization is needed,” she added.
For practices deluged with required prior authorizations by insurers, automation is emerging as a way for practices to make the process less time-consuming and save money. However, the software can be costly and may not be adoptable to many practices, and many physicians are not even aware it exists.
So far, the software is mainly used at large organizations like hospital systems. But as word gets out and the software becomes easier to use, private practices and other smaller entities may join the automation trend.
There is definitely a need to automate prior authorization. The American Medical Association reports that physicians spend 16 hours per week on prior authorizations. In a recent AMA survey, more than 60% of physicians indicated that it’s difficult to know when prior authorization is needed. And 93% of physicians reported care delays while waiting for authorization, the AMA said.
Experts estimate that 80% of prior authorization work could be automated, but most practices still use the phone or fax, even as numbers of prior authorizations continue to increase.
How it works
Automation software connects directly to the practice’s electronic health record (EHR). “When the doctor places an order in the EHR, the process starts automatically,” Ms. Hadzhieva said. “The doctor may not even notice it.”
In addition to using an EHR connection, many software products can communicate with the payer through its portal or by fax or phone, while still automating other parts of the process.
The software’s first step is to decide whether prior authorization is needed. This requires having an updated list of the rules that each payer uses for prior authorization. Manually keeping track of payer rules is very time-consuming, but automation uses bots to visit each payer site to look for rules changes. One vendor, Infinitus, uses a voice-based bot called Eva that calls up each payer and speaks with a representative.
“Automatically updating payer rules is not a new technology,” said YiDing Yu, MD, chief product officer at Olive, the automation vendor for New England Baptist. “What is new in the last 5 years is extracting the information needed for the prior authorization out of the clinical notes.”
This is challenging because each doctor has different ways to describe each step of clinical work. To identify this shorthand, Dr. Yu said Olive uses natural language processing, which is a form of artificial intelligence that learns how each doctor describes things.
Dr. Yu asserts that Olive is actually better than a practice’s staff at digging out clinical information. She said staff without much clinical training may miss terms that the software can catch, and they don’t have the time to go back many months into the record to find valuable information. But automation can do that.
In some instances, however, the software may not be able to find the information, in which case it alerts staff through a prompt in the EHR and the information is retrieved manually, Dr. Yu said.
Next, the Olive software puts the information it found into the request form and sends it to the payer. After submission, the software constantly checks on the status of each request, again visiting payer sites with a bot.
At New England Baptist, the software is used mainly by physicians in fairly small private practices who are on staff. They are using the software on the hospital’s dime, but it only works inside the hospital, Ms. Hadzhieva said. For their work outside of the hospital, they would have to purchase the Olive software on their own, she said.
Automation hasn’t spread to practices yet
Despite the promising outcomes for products like Olive, automation software is still primarily used by large organizations. Vendors say very few private practices have bought it yet. “The technology works, but it is still in the early-adopter phase,” Dr. Yu said.
For one thing, the software can be expensive. Very few vendors reveal their prices, but Dr. Yu did so. She said Olive normally costs about $50,000 a year for even a small organization. She insisted, however, that the savings from avoiding just one denial each month for a hip surgery would justify the expense.
On the other hand, some automation software is free, such as the Surescripts product for prior authorization of prescriptions. But it is unclear whether Surescripts does as much as Olive. Vendors’ descriptions of their products tend to be vague.
Also, Surescripts and Olive have entirely separate functions. Dr. Yu said Olive is limited to procedures, so it benefits specialties like oncology, neurosurgery, colorectal surgery, vascular surgery, and cardiology. Olive does not cover prescriptions, because they operate on a different technology.
Dr. Yu said another hurdle for adopting the software is the kind of EHR systems that doctors use. At this point, only a few EHR systems – such as Epic, Cerner, and Athena – are compatible with Olive. Large organizations tend to use Epic and Cerner, while many practices often use Athena or a variety of other systems, she said.
Despite stunted demand, there is no shortage of companies offering automation software for medical (that is, non-prescription) prior authorization. One compilation lists 25 such vendors, including companies like Myndshft, Rhyme, Infinitus, Infinx, and Waystar. As with any start-up technology, companies occasionally buy each other out.
In addition to issues like cost, specialty, and EHR compatibility, another hurdle is that few doctors even know the technology exists. Vendors say marketing focuses on larger provider organizations, not smaller practices.
Even many tech-savvy doctors, like Adam Bruggeman, MD, an orthopedist and CEO of Texas Spine Care Center in San Antonio, say they know little about the technology. “There is definitely a need to automate prior authorization,” he said. “But I don’t know of any colleagues who use it.” He has only just begun to explore vendors, he said.
Many medical practice consultants also have not yet explored the technology. “Automation makes a lot of sense, because there are a lot of repetitive tasks in prior authorization,” said Jill Arena, CEO of Portland, Ore.–based Health e Practices. “But I haven’t looked into it yet, and none of my clients has even asked about it.”
“I could see how it could be an easier sell for large organizations,” she added. “They have an IT person and a CFO who can explore the issue. Smaller practices usually don’t have that kind of expertise.”
Where does automation go from here?
Until now, clinicians who want to fully automate prior authorizations would have to buy two products – one for medical procedures and one for prescriptions. This has to do with incompatible electronic transmission standards, which are used to digitize information, said Susan Lawson-Dawson, content marketing strategist for the vendor Myndshft Health.
Myndshft has long been selling automation software for medical prior authorizations, but now it is introducing a product for prescriptions, Ms. Lawson-Dawson said. She said Myndshft will then be the only vendor to automate both kinds of prior authorizations.
Ms. Lawson-Dawson said Myndshft has 685 customers to date and is looking for more business. Recently the company entered the Google Cloud Marketplace. Google Cloud customers can now direct their committed spend with Google to purchasing Myndshft, meaning they could get it at a discount.
Software like Olive and Myndshft can operate independently of payers, but a vendor called Rhyme depends on payers for its software to function, said Rhyme CEO Joe Anstine. He said more than 300 payers have agreed to install the Rhyme system, and Rhyme has signed up a number of large health systems to use the product. Initially, he said, clinicians paid for the service, but now Rhyme is beginning to find payers to foot the costs and to let clinicians use it for free, which would open Rhyme up to smaller practices.
EHR companies themselves are beginning to offer automation, too. Epic, for example, has created a tool for prior authorization as part of its Epic Payer Platform. Like Rhyme, it requires payer cooperation, because information goes back and forth between clinician and payer in what is called bi-directional exchange.
The Epic product is still in its pilot phase. Epic reported that several large health systems were using its product in conjunction with a specific payer – for instance, Mayo Clinic with Blue Cross and Blue Shield of Minnesota and Ochsner Health with Humana. According to Epic, the arrangement reduced Mayo’s denials due to additional documentation requests by 63% for professional billing.
Automating with just one payer still means the clinician has to deal with manual processes at other payers, but a large clinician could have sufficient volume with that one payer to make the arrangement useful.
Will payers automate prior authorization?
Ultimately, payers may take the automation business away from vendors, offering a free product to all clinicians. But don’t hold your breath. Payers first have to rebuild their electronic systems to accommodate an electronic connection with providers. Even then, some payers might hold back from automating, forcing practices to continue manually processing some prior authorizations.
Efforts are underway, however, to mandate payers to support prior authorization automation. For this to happen, payers would have to revamp their data so that it could be easily read by practices’ EHRs. This would mean adopting a specific interoperability standard called Health Level 7 Fast Healthcare Interoperability Resources (FHIR).
Toward this goal, the Centers for Medicare & Medicaid Services proposes to require payers to adopt FHIR by January 2026. (CMS still has to finalize the rule.) Experts say the two-year ramp-up time is needed because it takes extensive work for payers to translate their data into FHIR.
The only payer so far to switch to FHIR for prior authorization is Regence in Washington state. In a pilot project, it has automated prior authorization with just one provider, MultiCare Connected Care, an accountable care organization (ACO), also in Washington state.
Anna Taylor, associate vice president of population health and value-based care at MultiCare, explained how the arrangement works. “Two separate entities are sharing one operational process,” she told this news organization. “That means they can have a digital conversation back and forth, so it is much easier to resolve prior authorization issues.”
Unlike many vendor products, the Regence service is free. And while the vendors market only to large organizations, most doctors in the MultiCare arrangement are in independent practices. Ms. Taylor said these doctors have been “enthusiastic” about the arrangement.
The results of the pilot are impressive. Ms. Taylor said automation has resulted in a 233% productivity gain for MultiCare clinicians, and 89% of submissions to Regence get an immediate response.
There is a potential downside, however, to working directly with payers. A direct connection to clinicians allows payers to access the doctor’s clinical notes, which could make many doctors uneasy. But Ms. Taylor said Regence only has access to the “discrete data fields” on MultiCare’s EHR dashboard, not to the notes themselves.
The ultimate goal of the Regence-Multicare project is to include more payers and clinicians. Ms. Taylor said two of the 27 other payers that MultiCare works with are “highly interested,” but it would take a lot of work for them to get connected with practices and other clinicians.
Ultimately, payers could offer automation and third-party vendors might then fade away. However, physicians may resist working directly with payers if the arrangement requires full access to their medical records.
A version of this article first appeared on Medscape.com.
Docs weigh in on insurance coverage for obesity medications
You can’t argue with success, unless you are an insurance company faced with covering medications shown to improve obesity.
The ability of drugs originally designed for diabetes management to reduce body weight has spiked demand and taxed supplies, according to the U.S. Food and Drug Administration, which included semaglutide (both Wegovy and Ozempic) on its Drug Shortages List as of May 31, 2023.
Meanwhile, clinicians and patients report that insurance companies are pushing back against coverage of these medications that mimic glucagon-like peptide 1 (GLP-1) because of the costs. A recent study conducted by Prime Therapeutics, a pharmacy benefit management organization, showed that individuals who started GLP-1 drugs for weight loss and who were adherent to the treatment averaged a 59% increase in health care costs after 1 year; for those in a subgroup analysis who were treatment adherent, the increase in health care costs was 98%.
“Insurance coverage for obesity treatment is challenging, particularly regarding medications,” said Scott Kahan, MD, director of the National Center for Weight and Wellness at George Washington University, Washington, in an interview. Employers must opt in for patients to have coverage for these medications; therefore, relatively few patients have had access at reasonable out-of-pocket costs, he said.
For example, the University of Texas stated on its website that its prescription drug plans will no longer cover drugs with the active ingredients semaglutide (Wegovy) or liraglutide (Saxenda) for weight loss as of Sept. 1, 2023. Both products are FDA-approved for weight management, whereas the equally popular Ozempic is currently approved only as a treatment for diabetes. The school’s website noted that the current price of the drugs, which cost the plan more than $5 million per month as of May 2023, outstrips the most expensive cancer agents.
The University of Texas also found that among its patients, the compliance rate for those who began Wegovy or Saxenda for weight loss was only 46%, which was not enough to justify continued coverage. The plan advised patients to approach their insurers directly.
Eventually, more information may prompt more support from insurance across a range of medications, Dr. Kahan noted. “Most insurers are wanting cost-effectiveness data in order to support their investments in broader coverage,” he said.
However, costs do vary with and without insurance; some medications are less expensive than others without significant differences in outcomes, so encourage patients to explore all the options and not just one brand, Dr. Kahan said.
Educate patients on plan details
Clinicians can’t guarantee coverage, but they can offer guidance to their patients, according to said Andrew Kraftson, MD, an endocrinologist and internal medicine physician at the University of Michigan, Ann Arbor, who specializes in the care of people with obesity.
Unfortunately, some of the challenges to obtaining insurance coverage for weight-loss medications lie in the plan details because some insurers have a blanket prohibition against the use of weight-loss medications, he said.
If patients did not look for this particular aspect of coverage at the time of enrollment in their chosen plan, they may not have known about this exclusion, and they are disappointed to find that they are ineligible for weight-loss medications despite medical circumstances, Dr. Kraftson said in an interview.
If weight-loss medications are covered, prior authorization often is required, Dr. Kraftson added.
“Unfortunately, the requirements vary from insurer to insurer, and this can present challenges for the busy clinicians who may not have dedicated staff to assist with these authorizations. Sometimes, the requirements are exactingly particular, and denials can commonly occur,” he said.
Some insurers will cover weight-loss medications for an initial period then require a certain degree of weight loss before renewing the approval, Dr. Kraftson said.
“While this is reasonable, sometimes it is necessary to titrate a medication more slowly to help a patient get used to the medicine, so they may not reach the required weight loss in the time required by the insurer,” he said. “As such, the medical professional is ‘punished’ for trying to be safe and patient-sensitive, and the patient may lose coverage of the medicine.”
Clinicians can help patients increase their chances for insurance coverage by providing a patient instruction guide to walk them through the steps that allow the patient to make inquiries with their own insurer, Dr. Kraftson said.
This guide should instruct patients on how to read their prescription coverage card to correctly contact their insurer, along with a guide to medical coverage terminology.
Lauren Oshman, MD, also of the University of Michigan, heads a collaborative quality initiative in the state known as Michigan Collaborative for Type 2 Diabetes (MCT2D). Dr. Oshman and her colleagues created a user-friendly list of terms to help patients understand their plans and better advocate for coverage (see below). The list was designed to guide patients with diabetes but applies to any medication.
Learn the lingo (common insurance terms and definitions)
- Deductible: Predetermined amount that must be paid annually before insurance pays for anything.
- Copayment: Set amount paid for a prescription.
- Coinsurance: Amount you pay after your deductible is met. Your insurance pays their portion. Coinsurance only applies to prescriptions and services covered under your health plan.
- Medication tier: Levels of insurance medication coverage; you play a smaller amount for a lower tier and a higher amount for a higher tier.
- Out-of-pocket max: Annual limit on what you pay before insurance covers 100% of covered services. Deductibles, copayment, and coinsurance all apply toward your out-of-pocket maximum.
- Prior authorization: Request made by your doctor to insurance company for coverage of a medication.
- Quantity limit: Limitation on the number of pills covered for a period of time.
- Step therapy: Medication you must have tried prior to approval of a nonpreferred medication, typically prior to trying a more expensive medication.
(Source: Learn the Lingo: A Guide to Common Insurance Terms and Definitions, courtesy of Lauren Oshman, MD, and MCT2D)
Also, make sure patients understand that they need to find out whether they have a deductible and if so, how much it is, Dr. Kraftson said.
Pros and cons of compounding
Compounded drugs are not approved by the FDA; however, that does not mean they are not available, and patients may pursue them as an option for weight-loss drugs.
In a statement issued on May 31, 2023, the FDA cited reports of adverse events associated with the use of compounded weight-loss drugs as a lower-cost alternative to the approved product. The FDA emphasized that the agency does not review compounded versions of weight-loss drugs for safety, efficacy, or quality.
Dr. Kraftson cited the lack of quality control, transparency, and safety data as reasons to discourage his patients from pursuing compounded medications.
“If a patient insists on pursuing it, then I review the position statement from the Obesity Medicine Association,” he said. The OMA statement recommends that anti-obesity medications undergo clinical trials and noted the lack of FDA oversight on these products. The OMA statement also advises compounded peptides to be “legally produced by companies whose identities are readily disclosed, and who have documented manufacturing processes compliant with oversight by applicable regulatory agencies.”
Tracking outcomes might boost coverage
Robust data on the long-term cost-effectiveness of weight-loss medications are lacking, although this is changing, Dr. Kraftson said. A 2022 study published last year in the Journal of Managed Care and Specialty Pharmacy showed that a 2.4-mg dose of semaglutide was cost-effective, compared with no treatment, diet and exercise, and other anti-obesity medications based on gains in quality of life.
“Regardless, insurers are not as motivated by long-term cost effectiveness,” Dr. Kraftson said. Insurers are accustomed to employee turnover and are more likely to be motivated by short-term costs and benefits, he said. “Obesity treatment provides some short-term benefit, but the majority of the benefit can be experienced when we look at the long-term horizon,” he said.
Looking ahead, “We need better ways to account for the myriad benefits experienced by patients with successful weight control beyond what is currently measured as metrics of success, including better ways to qualify and quantify quality-of-life benefits,” Dr. Kraftson said.
Also, clinicians should address the stigma associated with obesity, Dr. Kraftson said.
“We would not see the spate of coverage restrictions if we were talking about heart disease or cancer; insurers can get away with this because obesity is held to a different standard and patients with obesity are used to being undertreated and mistreated by the medical community and society,” he said. “We need to better account for the true costs of excess weight/obesity beyond what is traditionally accepted. This would help make the case for the cost-effective nature of treatment.”
Dr. Kraftson and Dr. Oshman disclosed no relevant financial relationships. Dr. Kahan had no financial conflicts and serves on the Medscape Editorial Advisory Board.
You can’t argue with success, unless you are an insurance company faced with covering medications shown to improve obesity.
The ability of drugs originally designed for diabetes management to reduce body weight has spiked demand and taxed supplies, according to the U.S. Food and Drug Administration, which included semaglutide (both Wegovy and Ozempic) on its Drug Shortages List as of May 31, 2023.
Meanwhile, clinicians and patients report that insurance companies are pushing back against coverage of these medications that mimic glucagon-like peptide 1 (GLP-1) because of the costs. A recent study conducted by Prime Therapeutics, a pharmacy benefit management organization, showed that individuals who started GLP-1 drugs for weight loss and who were adherent to the treatment averaged a 59% increase in health care costs after 1 year; for those in a subgroup analysis who were treatment adherent, the increase in health care costs was 98%.
“Insurance coverage for obesity treatment is challenging, particularly regarding medications,” said Scott Kahan, MD, director of the National Center for Weight and Wellness at George Washington University, Washington, in an interview. Employers must opt in for patients to have coverage for these medications; therefore, relatively few patients have had access at reasonable out-of-pocket costs, he said.
For example, the University of Texas stated on its website that its prescription drug plans will no longer cover drugs with the active ingredients semaglutide (Wegovy) or liraglutide (Saxenda) for weight loss as of Sept. 1, 2023. Both products are FDA-approved for weight management, whereas the equally popular Ozempic is currently approved only as a treatment for diabetes. The school’s website noted that the current price of the drugs, which cost the plan more than $5 million per month as of May 2023, outstrips the most expensive cancer agents.
The University of Texas also found that among its patients, the compliance rate for those who began Wegovy or Saxenda for weight loss was only 46%, which was not enough to justify continued coverage. The plan advised patients to approach their insurers directly.
Eventually, more information may prompt more support from insurance across a range of medications, Dr. Kahan noted. “Most insurers are wanting cost-effectiveness data in order to support their investments in broader coverage,” he said.
However, costs do vary with and without insurance; some medications are less expensive than others without significant differences in outcomes, so encourage patients to explore all the options and not just one brand, Dr. Kahan said.
Educate patients on plan details
Clinicians can’t guarantee coverage, but they can offer guidance to their patients, according to said Andrew Kraftson, MD, an endocrinologist and internal medicine physician at the University of Michigan, Ann Arbor, who specializes in the care of people with obesity.
Unfortunately, some of the challenges to obtaining insurance coverage for weight-loss medications lie in the plan details because some insurers have a blanket prohibition against the use of weight-loss medications, he said.
If patients did not look for this particular aspect of coverage at the time of enrollment in their chosen plan, they may not have known about this exclusion, and they are disappointed to find that they are ineligible for weight-loss medications despite medical circumstances, Dr. Kraftson said in an interview.
If weight-loss medications are covered, prior authorization often is required, Dr. Kraftson added.
“Unfortunately, the requirements vary from insurer to insurer, and this can present challenges for the busy clinicians who may not have dedicated staff to assist with these authorizations. Sometimes, the requirements are exactingly particular, and denials can commonly occur,” he said.
Some insurers will cover weight-loss medications for an initial period then require a certain degree of weight loss before renewing the approval, Dr. Kraftson said.
“While this is reasonable, sometimes it is necessary to titrate a medication more slowly to help a patient get used to the medicine, so they may not reach the required weight loss in the time required by the insurer,” he said. “As such, the medical professional is ‘punished’ for trying to be safe and patient-sensitive, and the patient may lose coverage of the medicine.”
Clinicians can help patients increase their chances for insurance coverage by providing a patient instruction guide to walk them through the steps that allow the patient to make inquiries with their own insurer, Dr. Kraftson said.
This guide should instruct patients on how to read their prescription coverage card to correctly contact their insurer, along with a guide to medical coverage terminology.
Lauren Oshman, MD, also of the University of Michigan, heads a collaborative quality initiative in the state known as Michigan Collaborative for Type 2 Diabetes (MCT2D). Dr. Oshman and her colleagues created a user-friendly list of terms to help patients understand their plans and better advocate for coverage (see below). The list was designed to guide patients with diabetes but applies to any medication.
Learn the lingo (common insurance terms and definitions)
- Deductible: Predetermined amount that must be paid annually before insurance pays for anything.
- Copayment: Set amount paid for a prescription.
- Coinsurance: Amount you pay after your deductible is met. Your insurance pays their portion. Coinsurance only applies to prescriptions and services covered under your health plan.
- Medication tier: Levels of insurance medication coverage; you play a smaller amount for a lower tier and a higher amount for a higher tier.
- Out-of-pocket max: Annual limit on what you pay before insurance covers 100% of covered services. Deductibles, copayment, and coinsurance all apply toward your out-of-pocket maximum.
- Prior authorization: Request made by your doctor to insurance company for coverage of a medication.
- Quantity limit: Limitation on the number of pills covered for a period of time.
- Step therapy: Medication you must have tried prior to approval of a nonpreferred medication, typically prior to trying a more expensive medication.
(Source: Learn the Lingo: A Guide to Common Insurance Terms and Definitions, courtesy of Lauren Oshman, MD, and MCT2D)
Also, make sure patients understand that they need to find out whether they have a deductible and if so, how much it is, Dr. Kraftson said.
Pros and cons of compounding
Compounded drugs are not approved by the FDA; however, that does not mean they are not available, and patients may pursue them as an option for weight-loss drugs.
In a statement issued on May 31, 2023, the FDA cited reports of adverse events associated with the use of compounded weight-loss drugs as a lower-cost alternative to the approved product. The FDA emphasized that the agency does not review compounded versions of weight-loss drugs for safety, efficacy, or quality.
Dr. Kraftson cited the lack of quality control, transparency, and safety data as reasons to discourage his patients from pursuing compounded medications.
“If a patient insists on pursuing it, then I review the position statement from the Obesity Medicine Association,” he said. The OMA statement recommends that anti-obesity medications undergo clinical trials and noted the lack of FDA oversight on these products. The OMA statement also advises compounded peptides to be “legally produced by companies whose identities are readily disclosed, and who have documented manufacturing processes compliant with oversight by applicable regulatory agencies.”
Tracking outcomes might boost coverage
Robust data on the long-term cost-effectiveness of weight-loss medications are lacking, although this is changing, Dr. Kraftson said. A 2022 study published last year in the Journal of Managed Care and Specialty Pharmacy showed that a 2.4-mg dose of semaglutide was cost-effective, compared with no treatment, diet and exercise, and other anti-obesity medications based on gains in quality of life.
“Regardless, insurers are not as motivated by long-term cost effectiveness,” Dr. Kraftson said. Insurers are accustomed to employee turnover and are more likely to be motivated by short-term costs and benefits, he said. “Obesity treatment provides some short-term benefit, but the majority of the benefit can be experienced when we look at the long-term horizon,” he said.
Looking ahead, “We need better ways to account for the myriad benefits experienced by patients with successful weight control beyond what is currently measured as metrics of success, including better ways to qualify and quantify quality-of-life benefits,” Dr. Kraftson said.
Also, clinicians should address the stigma associated with obesity, Dr. Kraftson said.
“We would not see the spate of coverage restrictions if we were talking about heart disease or cancer; insurers can get away with this because obesity is held to a different standard and patients with obesity are used to being undertreated and mistreated by the medical community and society,” he said. “We need to better account for the true costs of excess weight/obesity beyond what is traditionally accepted. This would help make the case for the cost-effective nature of treatment.”
Dr. Kraftson and Dr. Oshman disclosed no relevant financial relationships. Dr. Kahan had no financial conflicts and serves on the Medscape Editorial Advisory Board.
You can’t argue with success, unless you are an insurance company faced with covering medications shown to improve obesity.
The ability of drugs originally designed for diabetes management to reduce body weight has spiked demand and taxed supplies, according to the U.S. Food and Drug Administration, which included semaglutide (both Wegovy and Ozempic) on its Drug Shortages List as of May 31, 2023.
Meanwhile, clinicians and patients report that insurance companies are pushing back against coverage of these medications that mimic glucagon-like peptide 1 (GLP-1) because of the costs. A recent study conducted by Prime Therapeutics, a pharmacy benefit management organization, showed that individuals who started GLP-1 drugs for weight loss and who were adherent to the treatment averaged a 59% increase in health care costs after 1 year; for those in a subgroup analysis who were treatment adherent, the increase in health care costs was 98%.
“Insurance coverage for obesity treatment is challenging, particularly regarding medications,” said Scott Kahan, MD, director of the National Center for Weight and Wellness at George Washington University, Washington, in an interview. Employers must opt in for patients to have coverage for these medications; therefore, relatively few patients have had access at reasonable out-of-pocket costs, he said.
For example, the University of Texas stated on its website that its prescription drug plans will no longer cover drugs with the active ingredients semaglutide (Wegovy) or liraglutide (Saxenda) for weight loss as of Sept. 1, 2023. Both products are FDA-approved for weight management, whereas the equally popular Ozempic is currently approved only as a treatment for diabetes. The school’s website noted that the current price of the drugs, which cost the plan more than $5 million per month as of May 2023, outstrips the most expensive cancer agents.
The University of Texas also found that among its patients, the compliance rate for those who began Wegovy or Saxenda for weight loss was only 46%, which was not enough to justify continued coverage. The plan advised patients to approach their insurers directly.
Eventually, more information may prompt more support from insurance across a range of medications, Dr. Kahan noted. “Most insurers are wanting cost-effectiveness data in order to support their investments in broader coverage,” he said.
However, costs do vary with and without insurance; some medications are less expensive than others without significant differences in outcomes, so encourage patients to explore all the options and not just one brand, Dr. Kahan said.
Educate patients on plan details
Clinicians can’t guarantee coverage, but they can offer guidance to their patients, according to said Andrew Kraftson, MD, an endocrinologist and internal medicine physician at the University of Michigan, Ann Arbor, who specializes in the care of people with obesity.
Unfortunately, some of the challenges to obtaining insurance coverage for weight-loss medications lie in the plan details because some insurers have a blanket prohibition against the use of weight-loss medications, he said.
If patients did not look for this particular aspect of coverage at the time of enrollment in their chosen plan, they may not have known about this exclusion, and they are disappointed to find that they are ineligible for weight-loss medications despite medical circumstances, Dr. Kraftson said in an interview.
If weight-loss medications are covered, prior authorization often is required, Dr. Kraftson added.
“Unfortunately, the requirements vary from insurer to insurer, and this can present challenges for the busy clinicians who may not have dedicated staff to assist with these authorizations. Sometimes, the requirements are exactingly particular, and denials can commonly occur,” he said.
Some insurers will cover weight-loss medications for an initial period then require a certain degree of weight loss before renewing the approval, Dr. Kraftson said.
“While this is reasonable, sometimes it is necessary to titrate a medication more slowly to help a patient get used to the medicine, so they may not reach the required weight loss in the time required by the insurer,” he said. “As such, the medical professional is ‘punished’ for trying to be safe and patient-sensitive, and the patient may lose coverage of the medicine.”
Clinicians can help patients increase their chances for insurance coverage by providing a patient instruction guide to walk them through the steps that allow the patient to make inquiries with their own insurer, Dr. Kraftson said.
This guide should instruct patients on how to read their prescription coverage card to correctly contact their insurer, along with a guide to medical coverage terminology.
Lauren Oshman, MD, also of the University of Michigan, heads a collaborative quality initiative in the state known as Michigan Collaborative for Type 2 Diabetes (MCT2D). Dr. Oshman and her colleagues created a user-friendly list of terms to help patients understand their plans and better advocate for coverage (see below). The list was designed to guide patients with diabetes but applies to any medication.
Learn the lingo (common insurance terms and definitions)
- Deductible: Predetermined amount that must be paid annually before insurance pays for anything.
- Copayment: Set amount paid for a prescription.
- Coinsurance: Amount you pay after your deductible is met. Your insurance pays their portion. Coinsurance only applies to prescriptions and services covered under your health plan.
- Medication tier: Levels of insurance medication coverage; you play a smaller amount for a lower tier and a higher amount for a higher tier.
- Out-of-pocket max: Annual limit on what you pay before insurance covers 100% of covered services. Deductibles, copayment, and coinsurance all apply toward your out-of-pocket maximum.
- Prior authorization: Request made by your doctor to insurance company for coverage of a medication.
- Quantity limit: Limitation on the number of pills covered for a period of time.
- Step therapy: Medication you must have tried prior to approval of a nonpreferred medication, typically prior to trying a more expensive medication.
(Source: Learn the Lingo: A Guide to Common Insurance Terms and Definitions, courtesy of Lauren Oshman, MD, and MCT2D)
Also, make sure patients understand that they need to find out whether they have a deductible and if so, how much it is, Dr. Kraftson said.
Pros and cons of compounding
Compounded drugs are not approved by the FDA; however, that does not mean they are not available, and patients may pursue them as an option for weight-loss drugs.
In a statement issued on May 31, 2023, the FDA cited reports of adverse events associated with the use of compounded weight-loss drugs as a lower-cost alternative to the approved product. The FDA emphasized that the agency does not review compounded versions of weight-loss drugs for safety, efficacy, or quality.
Dr. Kraftson cited the lack of quality control, transparency, and safety data as reasons to discourage his patients from pursuing compounded medications.
“If a patient insists on pursuing it, then I review the position statement from the Obesity Medicine Association,” he said. The OMA statement recommends that anti-obesity medications undergo clinical trials and noted the lack of FDA oversight on these products. The OMA statement also advises compounded peptides to be “legally produced by companies whose identities are readily disclosed, and who have documented manufacturing processes compliant with oversight by applicable regulatory agencies.”
Tracking outcomes might boost coverage
Robust data on the long-term cost-effectiveness of weight-loss medications are lacking, although this is changing, Dr. Kraftson said. A 2022 study published last year in the Journal of Managed Care and Specialty Pharmacy showed that a 2.4-mg dose of semaglutide was cost-effective, compared with no treatment, diet and exercise, and other anti-obesity medications based on gains in quality of life.
“Regardless, insurers are not as motivated by long-term cost effectiveness,” Dr. Kraftson said. Insurers are accustomed to employee turnover and are more likely to be motivated by short-term costs and benefits, he said. “Obesity treatment provides some short-term benefit, but the majority of the benefit can be experienced when we look at the long-term horizon,” he said.
Looking ahead, “We need better ways to account for the myriad benefits experienced by patients with successful weight control beyond what is currently measured as metrics of success, including better ways to qualify and quantify quality-of-life benefits,” Dr. Kraftson said.
Also, clinicians should address the stigma associated with obesity, Dr. Kraftson said.
“We would not see the spate of coverage restrictions if we were talking about heart disease or cancer; insurers can get away with this because obesity is held to a different standard and patients with obesity are used to being undertreated and mistreated by the medical community and society,” he said. “We need to better account for the true costs of excess weight/obesity beyond what is traditionally accepted. This would help make the case for the cost-effective nature of treatment.”
Dr. Kraftson and Dr. Oshman disclosed no relevant financial relationships. Dr. Kahan had no financial conflicts and serves on the Medscape Editorial Advisory Board.
Mixed CRC screening messaging. Confusing? Some docs think so
Recently updated colorectal cancer (CRC) screening guidance from the American College of Physicians is raising concerns among some specialists.
The ACP’s clinical guidance, published in Annals of Internal Medicine, called for CRC screenings to start at age 50 in average-risk individuals who are asymptomatic. This recommendation, however, conflicts with guidelines from the American Cancer Society and the U.S. Preventive Services Task Force, which, in 2021, officially lowered the recommended initial age of screening to 45.
Following the ACP’s announcement, several professional organizations, such as the American College of Radiology, criticized the new guidelines, calling them “a step backward” and warning they may hinder recent gains against CRC.
Some physicians believe the discordance will confuse patients and lead to varying referral practices among primary care physicians. And while insurers will likely continue to pay for screening procedures based on the USPSTF guidelines, which dictate insurance coverage, some physicians worry that insurers could create additional roadblocks for CRC screening coverage, such as requiring prior authorization.
“We’re in a conflicted space on this issue as a country,” said John L. Marshall, MD, a GI oncologist and director of The Ruesch Center for the Cure of GI Cancers at Georgetown University, Washington.
Ultimately, the physician community wants an inexpensive screening test that’s effective at preventing cancer and deaths, but the evidence thus far doesn’t necessarily support colonoscopy as that test, said Dr. Marshall, also chief medical officer for Lombardi Comprehensive Cancer Center.
Although colonoscopy can prevent CRC by removing precancerous polyps and can reduce deaths from cancer, it has not been shown to lower all-cause mortality, Dr. Marshall explained. A recent meta-analysis, for example, found that, aside from sigmoidoscopy for colon cancer screening, no other cancer screening modalities meaningfully changed life expectancy.
“That’s why we’re struggling,” Dr. Marshall said. “We’re emotionally invested in having screening available to younger people because we’re seeing colon cancer in younger people. So, we want it to move earlier, but it’s expensive and it’s invasive.”
Docs debate differing guidance
The new ACP guidance, based on a critical review of existing guidelines, evidence, and modeling studies, argues that the potential harms of screening average-risk individuals under age 50 may outweigh the potential benefits.
The benefits of screening, of course, include identifying and removing precancerous lesions or localized cancer, while the potential harms include false positives that may lead to unnecessary additional tests, treatments, and costs. More invasive screening procedures, such as colonoscopy, can also come with their own risks, including serious bleeding and perforation.
For colonoscopy, for instance, the ACP team determined that starting screening at age 45 vs. 50 could prevent three additional CRC cases per 1,000 individuals screened (58 vs. 61) and one CRC death (27 vs. 28) over the recommended screening time frame. On the flip side, screening starting at age 45 could increase the incidence of gastrointestinal or cardiovascular events (14 vs. 16).
“Even if we assumed the modeling study had no limitations and accepted the results at face value, we would conclude that the small estimated benefits and harms roughly balance each other out, resulting in an inadequate net benefit to warrant CRC screening in average-risk adults aged 45 to 49 years,” Amir Qaseem, MD, PhD, and ACP coauthors write.
Family physician Kenny Lin, MD, MPH, believes the updated ACP guidelines are reasonable, and points out the ACP is not the first group to disagree with the USPSTF’s recommendations.
“I think the [ACP] guidelines make a lot of sense,” said Dr. Lin, who practices in Lancaster, Pa. The American Academy of Family Physicians “also did not endorse the recommendations to start screenings at 45.” In its 2021 updated guidance, the AAFP recommended screening for CRC starting at age 50, concluding there was “insufficient evidence to assess the benefits and harms of screening” in the 45 to 49 population.
However, Jason R. Woloski, MD, a family physician based in Wilkes-Barre, Pa., expressed concern that the differing guidelines will confuse patients as well as present challenges for primary care physicians.
“I feel like we took the last couple of years convincing people that earlier is better,” said Dr. Woloski, an associate professor of family medicine at Geisinger Commonwealth School of Medicine, Scranton, Pa. “It can send a mixed message to a patient after we’ve been stressing the importance of earlier [screening], and then saying, ‘Maybe we got it wrong; maybe we were okay the first time.’ ”
Mark A. Lewis, MD, a GI oncologist, had a similar initial reaction upon hearing about the updated guidelines: “The lack of synchronization across groups is going to create confusion among patients.”
Although he could not say definitively whether the recommendations will affect GI oncologists, because he only sees patients with advanced CRC, he does see the demands in primary care and gastroenterology shifting.
“I think the much bigger impact will be on primary care physicians and gastroenterologists,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “My best guess is that the procedural burden on the latter will be mitigated by more stool testing ordered by primary care physicians. Patients may understandably prefer the convenience and lack of invasiveness of home-based fecal testing, but a positive FIT [fecal immunochemical test] without a follow-up scope is an incomplete screening.”
Dr. Marshall, however, had a different take. He does not envision the updated guidelines having much of a practical impact on physician practice. Most of the country is already not receiving proper colon cancer screenings, he said. Research shows more than 40% of Americans skip standard CRC screenings. Even anecdotally, he noted, friends in their 60s come to him and admit they haven’t had a colonoscopy yet.
Potential impact on patient outcomes, costs
Beyond mixed messaging, some experts worry that pushing CRC screening later could mean cancers are caught later, when they’re more advanced.
Finding cancers earlier, when they are easier and less expensive to treat, make earlier CRC screenings worthwhile, Dr. Woloski explained.
Dr. Lewis sees earlier screening as a way to stop a tumor from progressing before it can really pick up steam.
“To me the biggest advantage of colonoscopy is the interruption of the adenoma-to-carcinoma sequence, whereby a polyp that is completely removed cannot become an invasive adenocarcinoma,” Dr. Lewis said. “We’ve also had evidence for well over a decade that flexible sigmoidoscopy, which doesn’t come close to visualizing the entire colon, can confer a survival benefit.”
Another concern is the potential effect on insurance coverage.
Medicare and other insurers use USPSTF guidelines to make coverage decisions. However, because of this mixed message, Dr. Woloski questioned whether there would be more challenges regarding insurance coverage. “Does it mean primary care doctors are going to have to preauthorize a lot of these screenings even if you have shared decision-making with the patient?” he asked.
When it comes to screening referrals, Douglas A. Corley, MD, PhD, a gastroenterologist at Kaiser Permanente in northern California, said it’s critical for primary care physicians to educate patients about the differing views on screening benefits and harms as well as the different screening options.
“Given the different opinions, it is important to let people in this age group know that screening is an option recommended by some groups,” Dr. Corley said. “Colorectal cancer screening is very effective for decreasing the risk for death from colorectal cancer, which is the second leading cause of cancer death in the United States. Making sure all eligible people know this is an option provides the best way for patients to have an informed choice.”
Dr. Lin has already begun talking with patients about the differing recommendations. He said it’s helpful to simplify the issue and focus the conversation on what patients value most. For more assertive patients whose priority is finding every possible cancer early, starting screenings at age 45 may be reasonable, he said, whereas other patients may not find the process or possible side effects worth it.
“And then you have the middle group that decides, ‘Yes, I want to start at 45, but I want the fecal test. I don’t want to just jump into colonoscopy.’ ” Dr. Lin said. “That would be kind of a compromise where you’d be starting screening earlier, but not subjecting yourself to something that has more potential for harms.”
Dr. Woloski said he plans to continue making referrals based on the USPSTF recommendations.
“With every screening, it is about informed decision-making with the patient, but I think for now, since USPSTF still supports the earlier screening, I will probably stick with offering it earlier,” he said.
But when deciding on the appropriate timing for evaluating CRC, the most important distinction is between screening and diagnosis, Dr. Lewis added.
“The former is only appropriate in patients who are truly asymptomatic and who are truly average-risk,” he said. “The latter is critical in any patient with symptoms. I cannot count the number of times I have seen blood in the stool discounted as hemorrhoids without even an exam, digital rectal, or scope, to demonstrate that hemorrhoids are present and the culprit for blood loss.”
A version of this article first appeared on Medscape.com.
Recently updated colorectal cancer (CRC) screening guidance from the American College of Physicians is raising concerns among some specialists.
The ACP’s clinical guidance, published in Annals of Internal Medicine, called for CRC screenings to start at age 50 in average-risk individuals who are asymptomatic. This recommendation, however, conflicts with guidelines from the American Cancer Society and the U.S. Preventive Services Task Force, which, in 2021, officially lowered the recommended initial age of screening to 45.
Following the ACP’s announcement, several professional organizations, such as the American College of Radiology, criticized the new guidelines, calling them “a step backward” and warning they may hinder recent gains against CRC.
Some physicians believe the discordance will confuse patients and lead to varying referral practices among primary care physicians. And while insurers will likely continue to pay for screening procedures based on the USPSTF guidelines, which dictate insurance coverage, some physicians worry that insurers could create additional roadblocks for CRC screening coverage, such as requiring prior authorization.
“We’re in a conflicted space on this issue as a country,” said John L. Marshall, MD, a GI oncologist and director of The Ruesch Center for the Cure of GI Cancers at Georgetown University, Washington.
Ultimately, the physician community wants an inexpensive screening test that’s effective at preventing cancer and deaths, but the evidence thus far doesn’t necessarily support colonoscopy as that test, said Dr. Marshall, also chief medical officer for Lombardi Comprehensive Cancer Center.
Although colonoscopy can prevent CRC by removing precancerous polyps and can reduce deaths from cancer, it has not been shown to lower all-cause mortality, Dr. Marshall explained. A recent meta-analysis, for example, found that, aside from sigmoidoscopy for colon cancer screening, no other cancer screening modalities meaningfully changed life expectancy.
“That’s why we’re struggling,” Dr. Marshall said. “We’re emotionally invested in having screening available to younger people because we’re seeing colon cancer in younger people. So, we want it to move earlier, but it’s expensive and it’s invasive.”
Docs debate differing guidance
The new ACP guidance, based on a critical review of existing guidelines, evidence, and modeling studies, argues that the potential harms of screening average-risk individuals under age 50 may outweigh the potential benefits.
The benefits of screening, of course, include identifying and removing precancerous lesions or localized cancer, while the potential harms include false positives that may lead to unnecessary additional tests, treatments, and costs. More invasive screening procedures, such as colonoscopy, can also come with their own risks, including serious bleeding and perforation.
For colonoscopy, for instance, the ACP team determined that starting screening at age 45 vs. 50 could prevent three additional CRC cases per 1,000 individuals screened (58 vs. 61) and one CRC death (27 vs. 28) over the recommended screening time frame. On the flip side, screening starting at age 45 could increase the incidence of gastrointestinal or cardiovascular events (14 vs. 16).
“Even if we assumed the modeling study had no limitations and accepted the results at face value, we would conclude that the small estimated benefits and harms roughly balance each other out, resulting in an inadequate net benefit to warrant CRC screening in average-risk adults aged 45 to 49 years,” Amir Qaseem, MD, PhD, and ACP coauthors write.
Family physician Kenny Lin, MD, MPH, believes the updated ACP guidelines are reasonable, and points out the ACP is not the first group to disagree with the USPSTF’s recommendations.
“I think the [ACP] guidelines make a lot of sense,” said Dr. Lin, who practices in Lancaster, Pa. The American Academy of Family Physicians “also did not endorse the recommendations to start screenings at 45.” In its 2021 updated guidance, the AAFP recommended screening for CRC starting at age 50, concluding there was “insufficient evidence to assess the benefits and harms of screening” in the 45 to 49 population.
However, Jason R. Woloski, MD, a family physician based in Wilkes-Barre, Pa., expressed concern that the differing guidelines will confuse patients as well as present challenges for primary care physicians.
“I feel like we took the last couple of years convincing people that earlier is better,” said Dr. Woloski, an associate professor of family medicine at Geisinger Commonwealth School of Medicine, Scranton, Pa. “It can send a mixed message to a patient after we’ve been stressing the importance of earlier [screening], and then saying, ‘Maybe we got it wrong; maybe we were okay the first time.’ ”
Mark A. Lewis, MD, a GI oncologist, had a similar initial reaction upon hearing about the updated guidelines: “The lack of synchronization across groups is going to create confusion among patients.”
Although he could not say definitively whether the recommendations will affect GI oncologists, because he only sees patients with advanced CRC, he does see the demands in primary care and gastroenterology shifting.
“I think the much bigger impact will be on primary care physicians and gastroenterologists,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “My best guess is that the procedural burden on the latter will be mitigated by more stool testing ordered by primary care physicians. Patients may understandably prefer the convenience and lack of invasiveness of home-based fecal testing, but a positive FIT [fecal immunochemical test] without a follow-up scope is an incomplete screening.”
Dr. Marshall, however, had a different take. He does not envision the updated guidelines having much of a practical impact on physician practice. Most of the country is already not receiving proper colon cancer screenings, he said. Research shows more than 40% of Americans skip standard CRC screenings. Even anecdotally, he noted, friends in their 60s come to him and admit they haven’t had a colonoscopy yet.
Potential impact on patient outcomes, costs
Beyond mixed messaging, some experts worry that pushing CRC screening later could mean cancers are caught later, when they’re more advanced.
Finding cancers earlier, when they are easier and less expensive to treat, make earlier CRC screenings worthwhile, Dr. Woloski explained.
Dr. Lewis sees earlier screening as a way to stop a tumor from progressing before it can really pick up steam.
“To me the biggest advantage of colonoscopy is the interruption of the adenoma-to-carcinoma sequence, whereby a polyp that is completely removed cannot become an invasive adenocarcinoma,” Dr. Lewis said. “We’ve also had evidence for well over a decade that flexible sigmoidoscopy, which doesn’t come close to visualizing the entire colon, can confer a survival benefit.”
Another concern is the potential effect on insurance coverage.
Medicare and other insurers use USPSTF guidelines to make coverage decisions. However, because of this mixed message, Dr. Woloski questioned whether there would be more challenges regarding insurance coverage. “Does it mean primary care doctors are going to have to preauthorize a lot of these screenings even if you have shared decision-making with the patient?” he asked.
When it comes to screening referrals, Douglas A. Corley, MD, PhD, a gastroenterologist at Kaiser Permanente in northern California, said it’s critical for primary care physicians to educate patients about the differing views on screening benefits and harms as well as the different screening options.
“Given the different opinions, it is important to let people in this age group know that screening is an option recommended by some groups,” Dr. Corley said. “Colorectal cancer screening is very effective for decreasing the risk for death from colorectal cancer, which is the second leading cause of cancer death in the United States. Making sure all eligible people know this is an option provides the best way for patients to have an informed choice.”
Dr. Lin has already begun talking with patients about the differing recommendations. He said it’s helpful to simplify the issue and focus the conversation on what patients value most. For more assertive patients whose priority is finding every possible cancer early, starting screenings at age 45 may be reasonable, he said, whereas other patients may not find the process or possible side effects worth it.
“And then you have the middle group that decides, ‘Yes, I want to start at 45, but I want the fecal test. I don’t want to just jump into colonoscopy.’ ” Dr. Lin said. “That would be kind of a compromise where you’d be starting screening earlier, but not subjecting yourself to something that has more potential for harms.”
Dr. Woloski said he plans to continue making referrals based on the USPSTF recommendations.
“With every screening, it is about informed decision-making with the patient, but I think for now, since USPSTF still supports the earlier screening, I will probably stick with offering it earlier,” he said.
But when deciding on the appropriate timing for evaluating CRC, the most important distinction is between screening and diagnosis, Dr. Lewis added.
“The former is only appropriate in patients who are truly asymptomatic and who are truly average-risk,” he said. “The latter is critical in any patient with symptoms. I cannot count the number of times I have seen blood in the stool discounted as hemorrhoids without even an exam, digital rectal, or scope, to demonstrate that hemorrhoids are present and the culprit for blood loss.”
A version of this article first appeared on Medscape.com.
Recently updated colorectal cancer (CRC) screening guidance from the American College of Physicians is raising concerns among some specialists.
The ACP’s clinical guidance, published in Annals of Internal Medicine, called for CRC screenings to start at age 50 in average-risk individuals who are asymptomatic. This recommendation, however, conflicts with guidelines from the American Cancer Society and the U.S. Preventive Services Task Force, which, in 2021, officially lowered the recommended initial age of screening to 45.
Following the ACP’s announcement, several professional organizations, such as the American College of Radiology, criticized the new guidelines, calling them “a step backward” and warning they may hinder recent gains against CRC.
Some physicians believe the discordance will confuse patients and lead to varying referral practices among primary care physicians. And while insurers will likely continue to pay for screening procedures based on the USPSTF guidelines, which dictate insurance coverage, some physicians worry that insurers could create additional roadblocks for CRC screening coverage, such as requiring prior authorization.
“We’re in a conflicted space on this issue as a country,” said John L. Marshall, MD, a GI oncologist and director of The Ruesch Center for the Cure of GI Cancers at Georgetown University, Washington.
Ultimately, the physician community wants an inexpensive screening test that’s effective at preventing cancer and deaths, but the evidence thus far doesn’t necessarily support colonoscopy as that test, said Dr. Marshall, also chief medical officer for Lombardi Comprehensive Cancer Center.
Although colonoscopy can prevent CRC by removing precancerous polyps and can reduce deaths from cancer, it has not been shown to lower all-cause mortality, Dr. Marshall explained. A recent meta-analysis, for example, found that, aside from sigmoidoscopy for colon cancer screening, no other cancer screening modalities meaningfully changed life expectancy.
“That’s why we’re struggling,” Dr. Marshall said. “We’re emotionally invested in having screening available to younger people because we’re seeing colon cancer in younger people. So, we want it to move earlier, but it’s expensive and it’s invasive.”
Docs debate differing guidance
The new ACP guidance, based on a critical review of existing guidelines, evidence, and modeling studies, argues that the potential harms of screening average-risk individuals under age 50 may outweigh the potential benefits.
The benefits of screening, of course, include identifying and removing precancerous lesions or localized cancer, while the potential harms include false positives that may lead to unnecessary additional tests, treatments, and costs. More invasive screening procedures, such as colonoscopy, can also come with their own risks, including serious bleeding and perforation.
For colonoscopy, for instance, the ACP team determined that starting screening at age 45 vs. 50 could prevent three additional CRC cases per 1,000 individuals screened (58 vs. 61) and one CRC death (27 vs. 28) over the recommended screening time frame. On the flip side, screening starting at age 45 could increase the incidence of gastrointestinal or cardiovascular events (14 vs. 16).
“Even if we assumed the modeling study had no limitations and accepted the results at face value, we would conclude that the small estimated benefits and harms roughly balance each other out, resulting in an inadequate net benefit to warrant CRC screening in average-risk adults aged 45 to 49 years,” Amir Qaseem, MD, PhD, and ACP coauthors write.
Family physician Kenny Lin, MD, MPH, believes the updated ACP guidelines are reasonable, and points out the ACP is not the first group to disagree with the USPSTF’s recommendations.
“I think the [ACP] guidelines make a lot of sense,” said Dr. Lin, who practices in Lancaster, Pa. The American Academy of Family Physicians “also did not endorse the recommendations to start screenings at 45.” In its 2021 updated guidance, the AAFP recommended screening for CRC starting at age 50, concluding there was “insufficient evidence to assess the benefits and harms of screening” in the 45 to 49 population.
However, Jason R. Woloski, MD, a family physician based in Wilkes-Barre, Pa., expressed concern that the differing guidelines will confuse patients as well as present challenges for primary care physicians.
“I feel like we took the last couple of years convincing people that earlier is better,” said Dr. Woloski, an associate professor of family medicine at Geisinger Commonwealth School of Medicine, Scranton, Pa. “It can send a mixed message to a patient after we’ve been stressing the importance of earlier [screening], and then saying, ‘Maybe we got it wrong; maybe we were okay the first time.’ ”
Mark A. Lewis, MD, a GI oncologist, had a similar initial reaction upon hearing about the updated guidelines: “The lack of synchronization across groups is going to create confusion among patients.”
Although he could not say definitively whether the recommendations will affect GI oncologists, because he only sees patients with advanced CRC, he does see the demands in primary care and gastroenterology shifting.
“I think the much bigger impact will be on primary care physicians and gastroenterologists,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “My best guess is that the procedural burden on the latter will be mitigated by more stool testing ordered by primary care physicians. Patients may understandably prefer the convenience and lack of invasiveness of home-based fecal testing, but a positive FIT [fecal immunochemical test] without a follow-up scope is an incomplete screening.”
Dr. Marshall, however, had a different take. He does not envision the updated guidelines having much of a practical impact on physician practice. Most of the country is already not receiving proper colon cancer screenings, he said. Research shows more than 40% of Americans skip standard CRC screenings. Even anecdotally, he noted, friends in their 60s come to him and admit they haven’t had a colonoscopy yet.
Potential impact on patient outcomes, costs
Beyond mixed messaging, some experts worry that pushing CRC screening later could mean cancers are caught later, when they’re more advanced.
Finding cancers earlier, when they are easier and less expensive to treat, make earlier CRC screenings worthwhile, Dr. Woloski explained.
Dr. Lewis sees earlier screening as a way to stop a tumor from progressing before it can really pick up steam.
“To me the biggest advantage of colonoscopy is the interruption of the adenoma-to-carcinoma sequence, whereby a polyp that is completely removed cannot become an invasive adenocarcinoma,” Dr. Lewis said. “We’ve also had evidence for well over a decade that flexible sigmoidoscopy, which doesn’t come close to visualizing the entire colon, can confer a survival benefit.”
Another concern is the potential effect on insurance coverage.
Medicare and other insurers use USPSTF guidelines to make coverage decisions. However, because of this mixed message, Dr. Woloski questioned whether there would be more challenges regarding insurance coverage. “Does it mean primary care doctors are going to have to preauthorize a lot of these screenings even if you have shared decision-making with the patient?” he asked.
When it comes to screening referrals, Douglas A. Corley, MD, PhD, a gastroenterologist at Kaiser Permanente in northern California, said it’s critical for primary care physicians to educate patients about the differing views on screening benefits and harms as well as the different screening options.
“Given the different opinions, it is important to let people in this age group know that screening is an option recommended by some groups,” Dr. Corley said. “Colorectal cancer screening is very effective for decreasing the risk for death from colorectal cancer, which is the second leading cause of cancer death in the United States. Making sure all eligible people know this is an option provides the best way for patients to have an informed choice.”
Dr. Lin has already begun talking with patients about the differing recommendations. He said it’s helpful to simplify the issue and focus the conversation on what patients value most. For more assertive patients whose priority is finding every possible cancer early, starting screenings at age 45 may be reasonable, he said, whereas other patients may not find the process or possible side effects worth it.
“And then you have the middle group that decides, ‘Yes, I want to start at 45, but I want the fecal test. I don’t want to just jump into colonoscopy.’ ” Dr. Lin said. “That would be kind of a compromise where you’d be starting screening earlier, but not subjecting yourself to something that has more potential for harms.”
Dr. Woloski said he plans to continue making referrals based on the USPSTF recommendations.
“With every screening, it is about informed decision-making with the patient, but I think for now, since USPSTF still supports the earlier screening, I will probably stick with offering it earlier,” he said.
But when deciding on the appropriate timing for evaluating CRC, the most important distinction is between screening and diagnosis, Dr. Lewis added.
“The former is only appropriate in patients who are truly asymptomatic and who are truly average-risk,” he said. “The latter is critical in any patient with symptoms. I cannot count the number of times I have seen blood in the stool discounted as hemorrhoids without even an exam, digital rectal, or scope, to demonstrate that hemorrhoids are present and the culprit for blood loss.”
A version of this article first appeared on Medscape.com.
Survival on the upswing in myeloma
Back then, the treatments for MM were chemotherapy and steroids. Stem-cell transplants were on the horizon, as was a most unexpected therapy: the infamous drug thalidomide.
But in the wake of the rib facture, the health of Stanley Katz, MD, worsened and he died after 25 weeks, Dr. Berenson recalled in an interview. At that time, Dr. Katz’s horrifically shortened lifespan following diagnosis was not unusual.
About 4 decades later, hematologists like Dr. Berenson are heralding a new era in MM, a sharp reversal of the previous eras of grim prognoses.
In a new study, Dr. Berenson tracked 161 patients with MM treated at his West Hollywood, Calif., private clinic from 2006 to 2023 and found that their median survival was 136.2 months – more than 11 years. “The OS reported in this study ... is the longest reported to date in an unselected, newly diagnosed MM population,” the study authors write.
Dr. Berenson’s patients are unique: They’re largely White, and they didn’t undergo stem-cell transplants. But other recent studies also suggest that lifespans of more than 10 years are increasingly possible after MM diagnosis. Former TV news anchor Tom Brokaw, for one, has reached that point.
In fact, a pair of other hematologists say the overall survival in Dr. Berenson’s report is hardly out of the question. And, they say, patients diagnosed today could potentially live even longer, because treatments continue to improve.
“With data that’s 10 years old, we expect the median overall survival to be 10 years,” hematologist Sagar Lonial, MD, who’s been tracking survival data in MM, said in an interview. “When patients ask about my outlook, I say it’s a constantly evolving field. Things are changing fast enough that I use 10 years as a floor.”
Dr. Lonial is chair of the department of hematology and medical oncology and chief medical officer at Emory University, Atlanta, Winship Cancer Institute.
Hematologist Rafael Fonseca, MD, chief innovation officer at Mayo Clinic–Arizona, put it this way in an interview: Dr. Berenson’s results “are probably in sync with what we would anticipate with similar cohorts of patients. The reality is that we’ve seen a huge improvement in the life expectancy of patients who were diagnosed with multiple myeloma. It’s not unusual to see patients in the clinic now that are 15 or 20 years out from their diagnosis.”
According to Yale Medicine, MM accounts for 10% of blood cancers and 1%-2% of all cancers and is more common in men vs. women and Blacks vs. Whites. It’s most frequently diagnosed between the ages of 65 and 74, according to the National Cancer Institute, and the median age at diagnosis is 69.
Among the most famous American people currently battling MM are newsman Mr. Brokaw, the former NBC News anchor, and Republican Congressman Steve Scalise, majority leader of the U.S. House of Representatives and a candidate for House speaker.
Mr. Brokaw was diagnosed in 2013 while in his early 70s and has talked about his intense struggle with the disease: the infections, operations, infusions, and daily regimens of 24 pills.
“I didn’t want to be Tom Brokaw, cancer victim,” he said in 2018 at the annual meeting of the American Society of Hematology. But he opened up about his illness, and became “the multiple myeloma poster boy.”
Rep. Scalise, who’s in his late 50s, is undergoing chemotherapy. He survived being gravely wounded in an assassination attempt in 2017.
Dr. Berenson’s new study, published in Targeted Oncology, tracked 161 patients (89 women, 72 men; median age, 65.4; 125 White, 3 Black, 10 Hispanic, 15 Asian, and 8 multi-ethnic).
All started frontline treatment at Dr. Berenson’s clinic and were included if they could read consent forms and gave permission for blood draws. None underwent stem-cell transplants as part of initial therapy. Another 1,036 patients had been treated elsewhere and were not included in the study.
Over a median of 42.7 months (range, 1.9-195.1), the 1-, 3-, and 5-year survival rates were 97.5%, 85.3%, and 76.2%, respectively.
The study claims “these results are considerably better than those reported from patients enrolled in clinical trials and those from countries with national registries.”
In the interview, Dr. Berenson said the study is unique because it’s not limited like many studies to younger, healthier patients. Nor does it include those treated at other facilities, he said.
The study is unusual in other ways. Dr. Berenson said his drug regimens aren’t necessarily standard, and he doesn’t treat patients with stem-cell transplants. “I stopped transplanting in about 2000 because clearly it was not improving the length of life,” he said.
Dr. Berenson said colleagues can learn from his insistence on sensitively treating the quality of life of patients, his embrace of clinical trials with novel combinations, and his close monitoring of myeloma proteins to gauge whether patients need to rapidly switch therapies.
He noted that his drug regimens are typically off-label and vary by patient. “We’re not using as high doses of drugs like Velcade [bortezomib] or Revlimid [lenalidomide] as my colleagues. We’re not necessarily giving as many doses. Also, we’re not adding as many drugs in many cases as they are. We’re taking it slower.”
The National Comprehensive Cancer Network recommends bortezomib and lenalidomide as standard induction treatments in patients with MM who are candidates for stem-cell transplantation, a procedure it considers the “preferred approach in transplant-eligible patients.”
There are limitations to Dr. Berenson’s new study. The patients aren’t representative of people with MM as a whole: His cohort is overwhelmingly White (78%) and just 2% Black, while an estimated one-fifth of patients with MM in the United States are Black and have poorer outcomes.
Dr. Berenson also acknowledged that his patients are most likely a wealthier group, although he said it’s not feasible to ask them about income. The study provides no information about socioeconomics.
Dr. Lonial said survival of 10-11 years is fairly typical in MM, with standard-risk patients reaching 14 years.
He highlighted a 2021 Canadian study that tracked 3,030 patients with newly diagnosed MM from 2007 to 2018 (average age, 64; 58.6% men). Those who received an upfront autologous stem-cell transplant had a median overall survival of 122.0 months (95% confidence interval, 115.0-135.0 months) vs. 54.3 months (95% CI, 50.8-58.8 months) for those who didn’t get the transplants. Not surprisingly, survival dipped with each subsequent treatment regimen.
Dr. Lonial is coauthor of a 2020 study that tracked 1,000 consecutive patients (mean age, 61; 35.2% Black) with newly diagnosed myeloma who were treated with RVD (lenalidomide, bortezomib, and dexamethasone) induction therapy from 2007 to 2016. The median overall survival was 126.6 months (95% CI, 113.3-139.8 months).
Dr. Fonseca noted that the news about MM survival rates is not entirely positive. Patients with high-risk disease often die early on in their disease course, he said.
Research suggests even the youngest patients with MM may die within years of diagnosis. A 2021 French study tracked 214 patients in the 18-40 age group for 15 years (2000-2015). At 5 years, “relative survival compared with same age- and sex-matched individuals was 83.5%,” and estimated overall survival was 14.5 years.
Still, a “very, very fertile environment for the development of drugs” has made a huge difference, Dr. Fonseca said. “We’ve had about 19 FDA approvals in the last 15 or 20 years.”
He urged colleagues to keep in mind that survival drops as patients decline in a line of therapy and need to switch to another one. “It might make intuitive sense to say ‘I’m gonna save something for later. I want to keep my powder dry.’ But put your best foot forward. Always go with your best treatments first.”
This approach can play out in a decision, say, to start with a four-drug initial regimen instead of a weaker two-drug regimen, he said. “Be mindful of managing toxicities, but hit harder.”
As he noted, side effects were worse with older generations of drugs. In regard to cost, multidrug treatments can cost hundreds of thousands of dollars. Dr. Fonseca said insurance tends to cover drugs that are approved by guidelines.
Dr. Fonseca discloses relationships with AbbVie, Adaptive Biotechnologies, Amgen, AstraZeneca, Bayer, Binding Site, BMS (Celgene), Millennium Takeda, Janssen, Juno, Kite, Merck, Pfizer, Pharmacyclics, Regeneron, Sanofi, Adaptive Biotechnologies, Caris Life Sciences, Oncotracker, Antegene, and AZBio, and a patent in MM. Dr. Berenson discloses ties with Janssen, Amgen, Sanofi, BMS, Karyopharm, and Incyte. Dr. Lonial reports ties with TG Therapeutics, Celgene, BMS, Janssen, Novartis, GlaxoSmithKline, AbbVie, Takeda, Merck, Sanofi, Pfizer, Regeneron, and Novartis.
Back then, the treatments for MM were chemotherapy and steroids. Stem-cell transplants were on the horizon, as was a most unexpected therapy: the infamous drug thalidomide.
But in the wake of the rib facture, the health of Stanley Katz, MD, worsened and he died after 25 weeks, Dr. Berenson recalled in an interview. At that time, Dr. Katz’s horrifically shortened lifespan following diagnosis was not unusual.
About 4 decades later, hematologists like Dr. Berenson are heralding a new era in MM, a sharp reversal of the previous eras of grim prognoses.
In a new study, Dr. Berenson tracked 161 patients with MM treated at his West Hollywood, Calif., private clinic from 2006 to 2023 and found that their median survival was 136.2 months – more than 11 years. “The OS reported in this study ... is the longest reported to date in an unselected, newly diagnosed MM population,” the study authors write.
Dr. Berenson’s patients are unique: They’re largely White, and they didn’t undergo stem-cell transplants. But other recent studies also suggest that lifespans of more than 10 years are increasingly possible after MM diagnosis. Former TV news anchor Tom Brokaw, for one, has reached that point.
In fact, a pair of other hematologists say the overall survival in Dr. Berenson’s report is hardly out of the question. And, they say, patients diagnosed today could potentially live even longer, because treatments continue to improve.
“With data that’s 10 years old, we expect the median overall survival to be 10 years,” hematologist Sagar Lonial, MD, who’s been tracking survival data in MM, said in an interview. “When patients ask about my outlook, I say it’s a constantly evolving field. Things are changing fast enough that I use 10 years as a floor.”
Dr. Lonial is chair of the department of hematology and medical oncology and chief medical officer at Emory University, Atlanta, Winship Cancer Institute.
Hematologist Rafael Fonseca, MD, chief innovation officer at Mayo Clinic–Arizona, put it this way in an interview: Dr. Berenson’s results “are probably in sync with what we would anticipate with similar cohorts of patients. The reality is that we’ve seen a huge improvement in the life expectancy of patients who were diagnosed with multiple myeloma. It’s not unusual to see patients in the clinic now that are 15 or 20 years out from their diagnosis.”
According to Yale Medicine, MM accounts for 10% of blood cancers and 1%-2% of all cancers and is more common in men vs. women and Blacks vs. Whites. It’s most frequently diagnosed between the ages of 65 and 74, according to the National Cancer Institute, and the median age at diagnosis is 69.
Among the most famous American people currently battling MM are newsman Mr. Brokaw, the former NBC News anchor, and Republican Congressman Steve Scalise, majority leader of the U.S. House of Representatives and a candidate for House speaker.
Mr. Brokaw was diagnosed in 2013 while in his early 70s and has talked about his intense struggle with the disease: the infections, operations, infusions, and daily regimens of 24 pills.
“I didn’t want to be Tom Brokaw, cancer victim,” he said in 2018 at the annual meeting of the American Society of Hematology. But he opened up about his illness, and became “the multiple myeloma poster boy.”
Rep. Scalise, who’s in his late 50s, is undergoing chemotherapy. He survived being gravely wounded in an assassination attempt in 2017.
Dr. Berenson’s new study, published in Targeted Oncology, tracked 161 patients (89 women, 72 men; median age, 65.4; 125 White, 3 Black, 10 Hispanic, 15 Asian, and 8 multi-ethnic).
All started frontline treatment at Dr. Berenson’s clinic and were included if they could read consent forms and gave permission for blood draws. None underwent stem-cell transplants as part of initial therapy. Another 1,036 patients had been treated elsewhere and were not included in the study.
Over a median of 42.7 months (range, 1.9-195.1), the 1-, 3-, and 5-year survival rates were 97.5%, 85.3%, and 76.2%, respectively.
The study claims “these results are considerably better than those reported from patients enrolled in clinical trials and those from countries with national registries.”
In the interview, Dr. Berenson said the study is unique because it’s not limited like many studies to younger, healthier patients. Nor does it include those treated at other facilities, he said.
The study is unusual in other ways. Dr. Berenson said his drug regimens aren’t necessarily standard, and he doesn’t treat patients with stem-cell transplants. “I stopped transplanting in about 2000 because clearly it was not improving the length of life,” he said.
Dr. Berenson said colleagues can learn from his insistence on sensitively treating the quality of life of patients, his embrace of clinical trials with novel combinations, and his close monitoring of myeloma proteins to gauge whether patients need to rapidly switch therapies.
He noted that his drug regimens are typically off-label and vary by patient. “We’re not using as high doses of drugs like Velcade [bortezomib] or Revlimid [lenalidomide] as my colleagues. We’re not necessarily giving as many doses. Also, we’re not adding as many drugs in many cases as they are. We’re taking it slower.”
The National Comprehensive Cancer Network recommends bortezomib and lenalidomide as standard induction treatments in patients with MM who are candidates for stem-cell transplantation, a procedure it considers the “preferred approach in transplant-eligible patients.”
There are limitations to Dr. Berenson’s new study. The patients aren’t representative of people with MM as a whole: His cohort is overwhelmingly White (78%) and just 2% Black, while an estimated one-fifth of patients with MM in the United States are Black and have poorer outcomes.
Dr. Berenson also acknowledged that his patients are most likely a wealthier group, although he said it’s not feasible to ask them about income. The study provides no information about socioeconomics.
Dr. Lonial said survival of 10-11 years is fairly typical in MM, with standard-risk patients reaching 14 years.
He highlighted a 2021 Canadian study that tracked 3,030 patients with newly diagnosed MM from 2007 to 2018 (average age, 64; 58.6% men). Those who received an upfront autologous stem-cell transplant had a median overall survival of 122.0 months (95% confidence interval, 115.0-135.0 months) vs. 54.3 months (95% CI, 50.8-58.8 months) for those who didn’t get the transplants. Not surprisingly, survival dipped with each subsequent treatment regimen.
Dr. Lonial is coauthor of a 2020 study that tracked 1,000 consecutive patients (mean age, 61; 35.2% Black) with newly diagnosed myeloma who were treated with RVD (lenalidomide, bortezomib, and dexamethasone) induction therapy from 2007 to 2016. The median overall survival was 126.6 months (95% CI, 113.3-139.8 months).
Dr. Fonseca noted that the news about MM survival rates is not entirely positive. Patients with high-risk disease often die early on in their disease course, he said.
Research suggests even the youngest patients with MM may die within years of diagnosis. A 2021 French study tracked 214 patients in the 18-40 age group for 15 years (2000-2015). At 5 years, “relative survival compared with same age- and sex-matched individuals was 83.5%,” and estimated overall survival was 14.5 years.
Still, a “very, very fertile environment for the development of drugs” has made a huge difference, Dr. Fonseca said. “We’ve had about 19 FDA approvals in the last 15 or 20 years.”
He urged colleagues to keep in mind that survival drops as patients decline in a line of therapy and need to switch to another one. “It might make intuitive sense to say ‘I’m gonna save something for later. I want to keep my powder dry.’ But put your best foot forward. Always go with your best treatments first.”
This approach can play out in a decision, say, to start with a four-drug initial regimen instead of a weaker two-drug regimen, he said. “Be mindful of managing toxicities, but hit harder.”
As he noted, side effects were worse with older generations of drugs. In regard to cost, multidrug treatments can cost hundreds of thousands of dollars. Dr. Fonseca said insurance tends to cover drugs that are approved by guidelines.
Dr. Fonseca discloses relationships with AbbVie, Adaptive Biotechnologies, Amgen, AstraZeneca, Bayer, Binding Site, BMS (Celgene), Millennium Takeda, Janssen, Juno, Kite, Merck, Pfizer, Pharmacyclics, Regeneron, Sanofi, Adaptive Biotechnologies, Caris Life Sciences, Oncotracker, Antegene, and AZBio, and a patent in MM. Dr. Berenson discloses ties with Janssen, Amgen, Sanofi, BMS, Karyopharm, and Incyte. Dr. Lonial reports ties with TG Therapeutics, Celgene, BMS, Janssen, Novartis, GlaxoSmithKline, AbbVie, Takeda, Merck, Sanofi, Pfizer, Regeneron, and Novartis.
Back then, the treatments for MM were chemotherapy and steroids. Stem-cell transplants were on the horizon, as was a most unexpected therapy: the infamous drug thalidomide.
But in the wake of the rib facture, the health of Stanley Katz, MD, worsened and he died after 25 weeks, Dr. Berenson recalled in an interview. At that time, Dr. Katz’s horrifically shortened lifespan following diagnosis was not unusual.
About 4 decades later, hematologists like Dr. Berenson are heralding a new era in MM, a sharp reversal of the previous eras of grim prognoses.
In a new study, Dr. Berenson tracked 161 patients with MM treated at his West Hollywood, Calif., private clinic from 2006 to 2023 and found that their median survival was 136.2 months – more than 11 years. “The OS reported in this study ... is the longest reported to date in an unselected, newly diagnosed MM population,” the study authors write.
Dr. Berenson’s patients are unique: They’re largely White, and they didn’t undergo stem-cell transplants. But other recent studies also suggest that lifespans of more than 10 years are increasingly possible after MM diagnosis. Former TV news anchor Tom Brokaw, for one, has reached that point.
In fact, a pair of other hematologists say the overall survival in Dr. Berenson’s report is hardly out of the question. And, they say, patients diagnosed today could potentially live even longer, because treatments continue to improve.
“With data that’s 10 years old, we expect the median overall survival to be 10 years,” hematologist Sagar Lonial, MD, who’s been tracking survival data in MM, said in an interview. “When patients ask about my outlook, I say it’s a constantly evolving field. Things are changing fast enough that I use 10 years as a floor.”
Dr. Lonial is chair of the department of hematology and medical oncology and chief medical officer at Emory University, Atlanta, Winship Cancer Institute.
Hematologist Rafael Fonseca, MD, chief innovation officer at Mayo Clinic–Arizona, put it this way in an interview: Dr. Berenson’s results “are probably in sync with what we would anticipate with similar cohorts of patients. The reality is that we’ve seen a huge improvement in the life expectancy of patients who were diagnosed with multiple myeloma. It’s not unusual to see patients in the clinic now that are 15 or 20 years out from their diagnosis.”
According to Yale Medicine, MM accounts for 10% of blood cancers and 1%-2% of all cancers and is more common in men vs. women and Blacks vs. Whites. It’s most frequently diagnosed between the ages of 65 and 74, according to the National Cancer Institute, and the median age at diagnosis is 69.
Among the most famous American people currently battling MM are newsman Mr. Brokaw, the former NBC News anchor, and Republican Congressman Steve Scalise, majority leader of the U.S. House of Representatives and a candidate for House speaker.
Mr. Brokaw was diagnosed in 2013 while in his early 70s and has talked about his intense struggle with the disease: the infections, operations, infusions, and daily regimens of 24 pills.
“I didn’t want to be Tom Brokaw, cancer victim,” he said in 2018 at the annual meeting of the American Society of Hematology. But he opened up about his illness, and became “the multiple myeloma poster boy.”
Rep. Scalise, who’s in his late 50s, is undergoing chemotherapy. He survived being gravely wounded in an assassination attempt in 2017.
Dr. Berenson’s new study, published in Targeted Oncology, tracked 161 patients (89 women, 72 men; median age, 65.4; 125 White, 3 Black, 10 Hispanic, 15 Asian, and 8 multi-ethnic).
All started frontline treatment at Dr. Berenson’s clinic and were included if they could read consent forms and gave permission for blood draws. None underwent stem-cell transplants as part of initial therapy. Another 1,036 patients had been treated elsewhere and were not included in the study.
Over a median of 42.7 months (range, 1.9-195.1), the 1-, 3-, and 5-year survival rates were 97.5%, 85.3%, and 76.2%, respectively.
The study claims “these results are considerably better than those reported from patients enrolled in clinical trials and those from countries with national registries.”
In the interview, Dr. Berenson said the study is unique because it’s not limited like many studies to younger, healthier patients. Nor does it include those treated at other facilities, he said.
The study is unusual in other ways. Dr. Berenson said his drug regimens aren’t necessarily standard, and he doesn’t treat patients with stem-cell transplants. “I stopped transplanting in about 2000 because clearly it was not improving the length of life,” he said.
Dr. Berenson said colleagues can learn from his insistence on sensitively treating the quality of life of patients, his embrace of clinical trials with novel combinations, and his close monitoring of myeloma proteins to gauge whether patients need to rapidly switch therapies.
He noted that his drug regimens are typically off-label and vary by patient. “We’re not using as high doses of drugs like Velcade [bortezomib] or Revlimid [lenalidomide] as my colleagues. We’re not necessarily giving as many doses. Also, we’re not adding as many drugs in many cases as they are. We’re taking it slower.”
The National Comprehensive Cancer Network recommends bortezomib and lenalidomide as standard induction treatments in patients with MM who are candidates for stem-cell transplantation, a procedure it considers the “preferred approach in transplant-eligible patients.”
There are limitations to Dr. Berenson’s new study. The patients aren’t representative of people with MM as a whole: His cohort is overwhelmingly White (78%) and just 2% Black, while an estimated one-fifth of patients with MM in the United States are Black and have poorer outcomes.
Dr. Berenson also acknowledged that his patients are most likely a wealthier group, although he said it’s not feasible to ask them about income. The study provides no information about socioeconomics.
Dr. Lonial said survival of 10-11 years is fairly typical in MM, with standard-risk patients reaching 14 years.
He highlighted a 2021 Canadian study that tracked 3,030 patients with newly diagnosed MM from 2007 to 2018 (average age, 64; 58.6% men). Those who received an upfront autologous stem-cell transplant had a median overall survival of 122.0 months (95% confidence interval, 115.0-135.0 months) vs. 54.3 months (95% CI, 50.8-58.8 months) for those who didn’t get the transplants. Not surprisingly, survival dipped with each subsequent treatment regimen.
Dr. Lonial is coauthor of a 2020 study that tracked 1,000 consecutive patients (mean age, 61; 35.2% Black) with newly diagnosed myeloma who were treated with RVD (lenalidomide, bortezomib, and dexamethasone) induction therapy from 2007 to 2016. The median overall survival was 126.6 months (95% CI, 113.3-139.8 months).
Dr. Fonseca noted that the news about MM survival rates is not entirely positive. Patients with high-risk disease often die early on in their disease course, he said.
Research suggests even the youngest patients with MM may die within years of diagnosis. A 2021 French study tracked 214 patients in the 18-40 age group for 15 years (2000-2015). At 5 years, “relative survival compared with same age- and sex-matched individuals was 83.5%,” and estimated overall survival was 14.5 years.
Still, a “very, very fertile environment for the development of drugs” has made a huge difference, Dr. Fonseca said. “We’ve had about 19 FDA approvals in the last 15 or 20 years.”
He urged colleagues to keep in mind that survival drops as patients decline in a line of therapy and need to switch to another one. “It might make intuitive sense to say ‘I’m gonna save something for later. I want to keep my powder dry.’ But put your best foot forward. Always go with your best treatments first.”
This approach can play out in a decision, say, to start with a four-drug initial regimen instead of a weaker two-drug regimen, he said. “Be mindful of managing toxicities, but hit harder.”
As he noted, side effects were worse with older generations of drugs. In regard to cost, multidrug treatments can cost hundreds of thousands of dollars. Dr. Fonseca said insurance tends to cover drugs that are approved by guidelines.
Dr. Fonseca discloses relationships with AbbVie, Adaptive Biotechnologies, Amgen, AstraZeneca, Bayer, Binding Site, BMS (Celgene), Millennium Takeda, Janssen, Juno, Kite, Merck, Pfizer, Pharmacyclics, Regeneron, Sanofi, Adaptive Biotechnologies, Caris Life Sciences, Oncotracker, Antegene, and AZBio, and a patent in MM. Dr. Berenson discloses ties with Janssen, Amgen, Sanofi, BMS, Karyopharm, and Incyte. Dr. Lonial reports ties with TG Therapeutics, Celgene, BMS, Janssen, Novartis, GlaxoSmithKline, AbbVie, Takeda, Merck, Sanofi, Pfizer, Regeneron, and Novartis.
Illicit steroids: If MDs don’t ask, patients won’t tell
Before he attended medical school, Thomas O’Connor, MD, had a not-very-well-kept secret: As a competitive powerlifter, he had used steroids to build strength.
Now an internist and clinical instructor of medicine at the University of Connecticut, Farmington, Dr. O’Connor’s practice focuses on the needs of men taking testosterone and other anabolic steroids – a group he feels is poorly understood and largely neglected by conventional medical care, perceptions borne out by a 2020 study of steroid users he helped conduct.
“They felt discriminated against, they did not feel comfortable working with their physicians, and they felt that the doctors did not know what they were doing,” Dr. O’Connor said in an interview. His patients often express anger and frustration with doctors they had seen previously.
Patients turning to home tests
Clients can order a panel of labs designed to screen for health conditions commonly associated with use of AAS, such as dyslipidemia, renal and hepatic dysfunction, polycythemia, thrombosis, and insulin resistance. The panels also include tests for levels of different hormones.
Sales of direct-to-consumer tests topped $3.6 billion in the United States in 2022 and are predicted to grow. Some of that spending is coming from people, mostly men, using illegally obtained steroids to build muscle. Although published data on the size of the bodybuilder market are unavailable, the Internet is a ready source of relatively inexpensive tests aimed at helping individuals monitor their health.
While clinicians may have their doubts about allowing patients to pick and choose tests and interpret their results, proponents claim they empower consumers to take control of their health – and save themselves money in the process.
But a test panel designed to help the user monitor the effects of banned substances is a bit unnerving to many clinicians, including Dr. O’Connor.
“People using anabolic steroids should be aware of the health risks associated with such use and that laboratory analysis is an important step toward improving health outcomes,” he said, “I’m all about open education, but not self-diagnosis and treatment.”
Testosterone and other AAS such as nandrolone, trenbolone, and boldenone are Schedule III controlled substances that have been banned by numerous athletic governing bodies. Yet recreational users can easily obtain them from online international pharmacies without a prescription. Should they be monitoring themselves for side effects?
A basic problem is that few primary care clinicians routinely ask their patients about the use of AAS or feel competent to manage the complications or withdrawal symptoms associated with the agents. And they may have no idea what the average AAS user looks like.
The American College of Sports Medicine updated its statement on the use of AAS in 2021. The statement warned of a growing new segment of users – up to 70% of people who take the drugs do so recreationally in pursuit of a more muscular appearance, rather than competitive athletes seeking enhanced performance.
The ACSM highlighted the syndrome of muscle dysmorphia, also known as “megarexia” or “bigorexia” (think of it as “reverse anorexia”), as a major risk factor for illicit use of AAS.
Stuart Phillips, PhD, professor and director of the department of kinesiology at McMaster University, Hamilton, Ont., coauthored the ACSM guidelines.
“The prince in Snow White circa 1950s was a guy with nice hair,” said Dr. Phillips, pointing to a change in cultural expectations for the male body. “But then fast forward to the prince or hero in any other Disney movie recently – and the guy is jacked.”
Since the last guidelines were published in 1987, Dr. Phillips has seen some cultural shifts. Testosterone has gone from a banned substance no one talked about to a mainstream medical therapy for men with low androgen levels, as any television viewer of primetime sports can attest. “But the other thing that’s changed,” he added, “is that we’ve seen the proliferation of illicit anabolic steroid use solely for the purpose of aesthetics.”
As an adolescent medicine physician who specializes in eating disorders, Jason Nagata, MD, MSc, sees many young men in his practice who engage in different behaviors to increase their muscle mass – from exercising, consuming high protein diets or taking protein supplements, even injecting AAS.
“A third of teenage boys across the U.S. report they’re trying to gain weight to bulk up and gain muscle,” said Dr. Nagata, an associate professor of pediatrics at the University of California, San Francisco.
In a 2020 study published in JAMA Pediatrics, Dr. Nagata and colleagues found that use of legal performance-enhancing substances in young men aged 18-26 years was associated with a higher odds of using AAS 7 years later (adjusted odds ratio, 3.18; 95% confidence interval, 1.90-5.32). “Some of the legal performance-enhancing substances like the protein powders or creatine may serve as a gateway to use of AAS,” Dr. Nagata said.
Another important factor is exposure to AAS use on social media, where muscular influencers gain huge followings. Dr. Nagata said most of the research on eating disorders and social media has examined the role of media on weight loss in girls.
“Although there’s less research on the social media impact on boys and men, a few studies have shown links between more Instagram use and muscle dissatisfaction, as well as thinking about using steroids,” he said.
The number of people using AAS is not trivial. In a longitudinal study (led by Nagata) of young U.S. adults surveyed multiple times between 1994 and 2002, a total of 2.7% of 18- to 26-year-old men and 0.4% of women reported using AAS. In a more recent cohort of adolescents in Minnesota aged 14-22 years followed between 2010 and 2018, a total of 2.2% of males and 1% of females initiated AAS use.
The Endocrine Society has estimated that between 2.9 and 4 million Americans have used an AAS at some point in their lives. Given that use is illegal without a prescription, a limitation of any survey is that participants may not be willing to disclose their AAS habit, leading to an underestimate of the actual number.
Nor are the complications of AAS use negligible. The drugs can have wide-ranging effects on the body, potentially affecting the brain, heart, liver, kidneys, musculoskeletal system, immune system, and reproductive systems. And individuals might unknowingly expose themselves to AAS: A recent literature review found that over a quarter of dietary supplements tested were found to contain undeclared substances that are on the World Anti-Doping Agency’s list of banned agents.
Alarming mistrust of MDs
Dr. O’Connor’s study shed some light on why AAS users might resort to surfing the Internet looking for a way to diagnose their own complications from steroid use. The web-based survey of nearly 2,400 men who said they took the drugs found that participants considered physicians to be the worst source of information, ranking them below coaches, online bodybuilding forums and sites, other AAS users, and bodybuilding books or magazines. The majority (56%) did not reveal AAS use to their clinicians. Of those who did, 55% reported feeling discriminated against for the admission.
Dr. O’Connor said physicians receive scant education on the many different drugs and regimens used by bodybuilders and have no idea how to a manage withdrawal syndrome for people trying to get off steroids. He urged the medical community to develop an educational campaign for clinicians, similar to those from public health officials aimed at combating the opioid epidemic: “Let’s educate med students and the residents. Let’s put [steroid use] on our agenda.”
Consumer testing evangelist ... or physician nemesis?
Nelson Vergel, BSChE, MBA, is on a mission to make medical lab testing affordable and accessible to everyone. The chemical engineer founded Discounted Labs 8 years ago, offering commonly ordered tests, such as complete blood counts, liver function tests, and cholesterol levels.
Mr. Vergel has advocated for the use of hormones to treat HIV-wasting disease for nearly 40 years, after his own diagnosis of the infection in 1986. After losing 40 pounds, steroids saved his life, he said.
Mr. Vergel said he was shocked to learn about the lack of continuing medical education on AAS for physicians and agreed with Dr. O’Connor that more training is needed for the medical profession. He also recognized that stigma on the part of clinicians is a huge barrier for many AAS users.
“We have to accept the fact that people are using them instead of demonizing them,” Mr. Vergel said. “What I was seeing is that there was so much stigma – and patients would not even talk to their doctors about their use.”
After reviewing Google analytics for his lab’s website and seeing how often “bodybuilder” came up as a search term, he added a panel of labs a year ago that allows AAS users to monitor themselves for adverse events.
Although he doesn’t condone the use of AAS without a medical indication and advises customers to discuss their results with a doctor, “we have to make sure people are reducing their harm or risk,” he said. “That’s really my goal.”
Many health care professionals would disagree with that statement. Dr. Nagata said he was concerned that management of side effects is too complicated. “There are a lot of nuances in the interpretation of these tests.” Arriving at the correct interpretation of the results requires a clinician’s thorough review of each patient’s health history, family history, and mental health history along with lab results.
‘I’m concerned’
In a second article outlining harm-reduction strategies designed to improve care for patients using AAS, Dr. O’Connor and colleagues outlined an approach for talking with patients who are concerned about their health and are seeking guidance from a clinician.
The first step is to work on developing a rapport, and not to demand that patients stop their use of AAS. His recommended opening line is: “I want to be honest with you – I’m concerned.”
The initial interaction is an opportunity to find out why the person uses AAS, what health concerns they have at present, and why they are seeking care. Open-ended questions may reveal concerns that the patient has about fertility or side effects.
Consistent with harm-reduction approaches used for other public health epidemics – such as opioid abuse and blood-borne pathogens among people who inject drugs – follow-up visits can include nonjudgmental discussions about decreasing or stopping their use.
Ultimately, minimizing the harms of AAS use can serve as a bridge to their cessation, but the medical community needs to build up trust with a community of users who currently rely more on each other and the Internet for guidance than their primary care physicians. “We need more education. We’re going to need resources to do it,” Dr. O’Connell said. “And we’re going to have to do it.”
Dr. Phillips and Dr. Nagata have no financial disclosures. Mr. Vergel is the owner and founder of Discounted Labs but reported no other financial conflicts. Dr. O’Connor owns Anabolic Doc but has no additional financial disclosures.
A version of this article first appeared on Medscape.com.
Before he attended medical school, Thomas O’Connor, MD, had a not-very-well-kept secret: As a competitive powerlifter, he had used steroids to build strength.
Now an internist and clinical instructor of medicine at the University of Connecticut, Farmington, Dr. O’Connor’s practice focuses on the needs of men taking testosterone and other anabolic steroids – a group he feels is poorly understood and largely neglected by conventional medical care, perceptions borne out by a 2020 study of steroid users he helped conduct.
“They felt discriminated against, they did not feel comfortable working with their physicians, and they felt that the doctors did not know what they were doing,” Dr. O’Connor said in an interview. His patients often express anger and frustration with doctors they had seen previously.
Patients turning to home tests
Clients can order a panel of labs designed to screen for health conditions commonly associated with use of AAS, such as dyslipidemia, renal and hepatic dysfunction, polycythemia, thrombosis, and insulin resistance. The panels also include tests for levels of different hormones.
Sales of direct-to-consumer tests topped $3.6 billion in the United States in 2022 and are predicted to grow. Some of that spending is coming from people, mostly men, using illegally obtained steroids to build muscle. Although published data on the size of the bodybuilder market are unavailable, the Internet is a ready source of relatively inexpensive tests aimed at helping individuals monitor their health.
While clinicians may have their doubts about allowing patients to pick and choose tests and interpret their results, proponents claim they empower consumers to take control of their health – and save themselves money in the process.
But a test panel designed to help the user monitor the effects of banned substances is a bit unnerving to many clinicians, including Dr. O’Connor.
“People using anabolic steroids should be aware of the health risks associated with such use and that laboratory analysis is an important step toward improving health outcomes,” he said, “I’m all about open education, but not self-diagnosis and treatment.”
Testosterone and other AAS such as nandrolone, trenbolone, and boldenone are Schedule III controlled substances that have been banned by numerous athletic governing bodies. Yet recreational users can easily obtain them from online international pharmacies without a prescription. Should they be monitoring themselves for side effects?
A basic problem is that few primary care clinicians routinely ask their patients about the use of AAS or feel competent to manage the complications or withdrawal symptoms associated with the agents. And they may have no idea what the average AAS user looks like.
The American College of Sports Medicine updated its statement on the use of AAS in 2021. The statement warned of a growing new segment of users – up to 70% of people who take the drugs do so recreationally in pursuit of a more muscular appearance, rather than competitive athletes seeking enhanced performance.
The ACSM highlighted the syndrome of muscle dysmorphia, also known as “megarexia” or “bigorexia” (think of it as “reverse anorexia”), as a major risk factor for illicit use of AAS.
Stuart Phillips, PhD, professor and director of the department of kinesiology at McMaster University, Hamilton, Ont., coauthored the ACSM guidelines.
“The prince in Snow White circa 1950s was a guy with nice hair,” said Dr. Phillips, pointing to a change in cultural expectations for the male body. “But then fast forward to the prince or hero in any other Disney movie recently – and the guy is jacked.”
Since the last guidelines were published in 1987, Dr. Phillips has seen some cultural shifts. Testosterone has gone from a banned substance no one talked about to a mainstream medical therapy for men with low androgen levels, as any television viewer of primetime sports can attest. “But the other thing that’s changed,” he added, “is that we’ve seen the proliferation of illicit anabolic steroid use solely for the purpose of aesthetics.”
As an adolescent medicine physician who specializes in eating disorders, Jason Nagata, MD, MSc, sees many young men in his practice who engage in different behaviors to increase their muscle mass – from exercising, consuming high protein diets or taking protein supplements, even injecting AAS.
“A third of teenage boys across the U.S. report they’re trying to gain weight to bulk up and gain muscle,” said Dr. Nagata, an associate professor of pediatrics at the University of California, San Francisco.
In a 2020 study published in JAMA Pediatrics, Dr. Nagata and colleagues found that use of legal performance-enhancing substances in young men aged 18-26 years was associated with a higher odds of using AAS 7 years later (adjusted odds ratio, 3.18; 95% confidence interval, 1.90-5.32). “Some of the legal performance-enhancing substances like the protein powders or creatine may serve as a gateway to use of AAS,” Dr. Nagata said.
Another important factor is exposure to AAS use on social media, where muscular influencers gain huge followings. Dr. Nagata said most of the research on eating disorders and social media has examined the role of media on weight loss in girls.
“Although there’s less research on the social media impact on boys and men, a few studies have shown links between more Instagram use and muscle dissatisfaction, as well as thinking about using steroids,” he said.
The number of people using AAS is not trivial. In a longitudinal study (led by Nagata) of young U.S. adults surveyed multiple times between 1994 and 2002, a total of 2.7% of 18- to 26-year-old men and 0.4% of women reported using AAS. In a more recent cohort of adolescents in Minnesota aged 14-22 years followed between 2010 and 2018, a total of 2.2% of males and 1% of females initiated AAS use.
The Endocrine Society has estimated that between 2.9 and 4 million Americans have used an AAS at some point in their lives. Given that use is illegal without a prescription, a limitation of any survey is that participants may not be willing to disclose their AAS habit, leading to an underestimate of the actual number.
Nor are the complications of AAS use negligible. The drugs can have wide-ranging effects on the body, potentially affecting the brain, heart, liver, kidneys, musculoskeletal system, immune system, and reproductive systems. And individuals might unknowingly expose themselves to AAS: A recent literature review found that over a quarter of dietary supplements tested were found to contain undeclared substances that are on the World Anti-Doping Agency’s list of banned agents.
Alarming mistrust of MDs
Dr. O’Connor’s study shed some light on why AAS users might resort to surfing the Internet looking for a way to diagnose their own complications from steroid use. The web-based survey of nearly 2,400 men who said they took the drugs found that participants considered physicians to be the worst source of information, ranking them below coaches, online bodybuilding forums and sites, other AAS users, and bodybuilding books or magazines. The majority (56%) did not reveal AAS use to their clinicians. Of those who did, 55% reported feeling discriminated against for the admission.
Dr. O’Connor said physicians receive scant education on the many different drugs and regimens used by bodybuilders and have no idea how to a manage withdrawal syndrome for people trying to get off steroids. He urged the medical community to develop an educational campaign for clinicians, similar to those from public health officials aimed at combating the opioid epidemic: “Let’s educate med students and the residents. Let’s put [steroid use] on our agenda.”
Consumer testing evangelist ... or physician nemesis?
Nelson Vergel, BSChE, MBA, is on a mission to make medical lab testing affordable and accessible to everyone. The chemical engineer founded Discounted Labs 8 years ago, offering commonly ordered tests, such as complete blood counts, liver function tests, and cholesterol levels.
Mr. Vergel has advocated for the use of hormones to treat HIV-wasting disease for nearly 40 years, after his own diagnosis of the infection in 1986. After losing 40 pounds, steroids saved his life, he said.
Mr. Vergel said he was shocked to learn about the lack of continuing medical education on AAS for physicians and agreed with Dr. O’Connor that more training is needed for the medical profession. He also recognized that stigma on the part of clinicians is a huge barrier for many AAS users.
“We have to accept the fact that people are using them instead of demonizing them,” Mr. Vergel said. “What I was seeing is that there was so much stigma – and patients would not even talk to their doctors about their use.”
After reviewing Google analytics for his lab’s website and seeing how often “bodybuilder” came up as a search term, he added a panel of labs a year ago that allows AAS users to monitor themselves for adverse events.
Although he doesn’t condone the use of AAS without a medical indication and advises customers to discuss their results with a doctor, “we have to make sure people are reducing their harm or risk,” he said. “That’s really my goal.”
Many health care professionals would disagree with that statement. Dr. Nagata said he was concerned that management of side effects is too complicated. “There are a lot of nuances in the interpretation of these tests.” Arriving at the correct interpretation of the results requires a clinician’s thorough review of each patient’s health history, family history, and mental health history along with lab results.
‘I’m concerned’
In a second article outlining harm-reduction strategies designed to improve care for patients using AAS, Dr. O’Connor and colleagues outlined an approach for talking with patients who are concerned about their health and are seeking guidance from a clinician.
The first step is to work on developing a rapport, and not to demand that patients stop their use of AAS. His recommended opening line is: “I want to be honest with you – I’m concerned.”
The initial interaction is an opportunity to find out why the person uses AAS, what health concerns they have at present, and why they are seeking care. Open-ended questions may reveal concerns that the patient has about fertility or side effects.
Consistent with harm-reduction approaches used for other public health epidemics – such as opioid abuse and blood-borne pathogens among people who inject drugs – follow-up visits can include nonjudgmental discussions about decreasing or stopping their use.
Ultimately, minimizing the harms of AAS use can serve as a bridge to their cessation, but the medical community needs to build up trust with a community of users who currently rely more on each other and the Internet for guidance than their primary care physicians. “We need more education. We’re going to need resources to do it,” Dr. O’Connell said. “And we’re going to have to do it.”
Dr. Phillips and Dr. Nagata have no financial disclosures. Mr. Vergel is the owner and founder of Discounted Labs but reported no other financial conflicts. Dr. O’Connor owns Anabolic Doc but has no additional financial disclosures.
A version of this article first appeared on Medscape.com.
Before he attended medical school, Thomas O’Connor, MD, had a not-very-well-kept secret: As a competitive powerlifter, he had used steroids to build strength.
Now an internist and clinical instructor of medicine at the University of Connecticut, Farmington, Dr. O’Connor’s practice focuses on the needs of men taking testosterone and other anabolic steroids – a group he feels is poorly understood and largely neglected by conventional medical care, perceptions borne out by a 2020 study of steroid users he helped conduct.
“They felt discriminated against, they did not feel comfortable working with their physicians, and they felt that the doctors did not know what they were doing,” Dr. O’Connor said in an interview. His patients often express anger and frustration with doctors they had seen previously.
Patients turning to home tests
Clients can order a panel of labs designed to screen for health conditions commonly associated with use of AAS, such as dyslipidemia, renal and hepatic dysfunction, polycythemia, thrombosis, and insulin resistance. The panels also include tests for levels of different hormones.
Sales of direct-to-consumer tests topped $3.6 billion in the United States in 2022 and are predicted to grow. Some of that spending is coming from people, mostly men, using illegally obtained steroids to build muscle. Although published data on the size of the bodybuilder market are unavailable, the Internet is a ready source of relatively inexpensive tests aimed at helping individuals monitor their health.
While clinicians may have their doubts about allowing patients to pick and choose tests and interpret their results, proponents claim they empower consumers to take control of their health – and save themselves money in the process.
But a test panel designed to help the user monitor the effects of banned substances is a bit unnerving to many clinicians, including Dr. O’Connor.
“People using anabolic steroids should be aware of the health risks associated with such use and that laboratory analysis is an important step toward improving health outcomes,” he said, “I’m all about open education, but not self-diagnosis and treatment.”
Testosterone and other AAS such as nandrolone, trenbolone, and boldenone are Schedule III controlled substances that have been banned by numerous athletic governing bodies. Yet recreational users can easily obtain them from online international pharmacies without a prescription. Should they be monitoring themselves for side effects?
A basic problem is that few primary care clinicians routinely ask their patients about the use of AAS or feel competent to manage the complications or withdrawal symptoms associated with the agents. And they may have no idea what the average AAS user looks like.
The American College of Sports Medicine updated its statement on the use of AAS in 2021. The statement warned of a growing new segment of users – up to 70% of people who take the drugs do so recreationally in pursuit of a more muscular appearance, rather than competitive athletes seeking enhanced performance.
The ACSM highlighted the syndrome of muscle dysmorphia, also known as “megarexia” or “bigorexia” (think of it as “reverse anorexia”), as a major risk factor for illicit use of AAS.
Stuart Phillips, PhD, professor and director of the department of kinesiology at McMaster University, Hamilton, Ont., coauthored the ACSM guidelines.
“The prince in Snow White circa 1950s was a guy with nice hair,” said Dr. Phillips, pointing to a change in cultural expectations for the male body. “But then fast forward to the prince or hero in any other Disney movie recently – and the guy is jacked.”
Since the last guidelines were published in 1987, Dr. Phillips has seen some cultural shifts. Testosterone has gone from a banned substance no one talked about to a mainstream medical therapy for men with low androgen levels, as any television viewer of primetime sports can attest. “But the other thing that’s changed,” he added, “is that we’ve seen the proliferation of illicit anabolic steroid use solely for the purpose of aesthetics.”
As an adolescent medicine physician who specializes in eating disorders, Jason Nagata, MD, MSc, sees many young men in his practice who engage in different behaviors to increase their muscle mass – from exercising, consuming high protein diets or taking protein supplements, even injecting AAS.
“A third of teenage boys across the U.S. report they’re trying to gain weight to bulk up and gain muscle,” said Dr. Nagata, an associate professor of pediatrics at the University of California, San Francisco.
In a 2020 study published in JAMA Pediatrics, Dr. Nagata and colleagues found that use of legal performance-enhancing substances in young men aged 18-26 years was associated with a higher odds of using AAS 7 years later (adjusted odds ratio, 3.18; 95% confidence interval, 1.90-5.32). “Some of the legal performance-enhancing substances like the protein powders or creatine may serve as a gateway to use of AAS,” Dr. Nagata said.
Another important factor is exposure to AAS use on social media, where muscular influencers gain huge followings. Dr. Nagata said most of the research on eating disorders and social media has examined the role of media on weight loss in girls.
“Although there’s less research on the social media impact on boys and men, a few studies have shown links between more Instagram use and muscle dissatisfaction, as well as thinking about using steroids,” he said.
The number of people using AAS is not trivial. In a longitudinal study (led by Nagata) of young U.S. adults surveyed multiple times between 1994 and 2002, a total of 2.7% of 18- to 26-year-old men and 0.4% of women reported using AAS. In a more recent cohort of adolescents in Minnesota aged 14-22 years followed between 2010 and 2018, a total of 2.2% of males and 1% of females initiated AAS use.
The Endocrine Society has estimated that between 2.9 and 4 million Americans have used an AAS at some point in their lives. Given that use is illegal without a prescription, a limitation of any survey is that participants may not be willing to disclose their AAS habit, leading to an underestimate of the actual number.
Nor are the complications of AAS use negligible. The drugs can have wide-ranging effects on the body, potentially affecting the brain, heart, liver, kidneys, musculoskeletal system, immune system, and reproductive systems. And individuals might unknowingly expose themselves to AAS: A recent literature review found that over a quarter of dietary supplements tested were found to contain undeclared substances that are on the World Anti-Doping Agency’s list of banned agents.
Alarming mistrust of MDs
Dr. O’Connor’s study shed some light on why AAS users might resort to surfing the Internet looking for a way to diagnose their own complications from steroid use. The web-based survey of nearly 2,400 men who said they took the drugs found that participants considered physicians to be the worst source of information, ranking them below coaches, online bodybuilding forums and sites, other AAS users, and bodybuilding books or magazines. The majority (56%) did not reveal AAS use to their clinicians. Of those who did, 55% reported feeling discriminated against for the admission.
Dr. O’Connor said physicians receive scant education on the many different drugs and regimens used by bodybuilders and have no idea how to a manage withdrawal syndrome for people trying to get off steroids. He urged the medical community to develop an educational campaign for clinicians, similar to those from public health officials aimed at combating the opioid epidemic: “Let’s educate med students and the residents. Let’s put [steroid use] on our agenda.”
Consumer testing evangelist ... or physician nemesis?
Nelson Vergel, BSChE, MBA, is on a mission to make medical lab testing affordable and accessible to everyone. The chemical engineer founded Discounted Labs 8 years ago, offering commonly ordered tests, such as complete blood counts, liver function tests, and cholesterol levels.
Mr. Vergel has advocated for the use of hormones to treat HIV-wasting disease for nearly 40 years, after his own diagnosis of the infection in 1986. After losing 40 pounds, steroids saved his life, he said.
Mr. Vergel said he was shocked to learn about the lack of continuing medical education on AAS for physicians and agreed with Dr. O’Connor that more training is needed for the medical profession. He also recognized that stigma on the part of clinicians is a huge barrier for many AAS users.
“We have to accept the fact that people are using them instead of demonizing them,” Mr. Vergel said. “What I was seeing is that there was so much stigma – and patients would not even talk to their doctors about their use.”
After reviewing Google analytics for his lab’s website and seeing how often “bodybuilder” came up as a search term, he added a panel of labs a year ago that allows AAS users to monitor themselves for adverse events.
Although he doesn’t condone the use of AAS without a medical indication and advises customers to discuss their results with a doctor, “we have to make sure people are reducing their harm or risk,” he said. “That’s really my goal.”
Many health care professionals would disagree with that statement. Dr. Nagata said he was concerned that management of side effects is too complicated. “There are a lot of nuances in the interpretation of these tests.” Arriving at the correct interpretation of the results requires a clinician’s thorough review of each patient’s health history, family history, and mental health history along with lab results.
‘I’m concerned’
In a second article outlining harm-reduction strategies designed to improve care for patients using AAS, Dr. O’Connor and colleagues outlined an approach for talking with patients who are concerned about their health and are seeking guidance from a clinician.
The first step is to work on developing a rapport, and not to demand that patients stop their use of AAS. His recommended opening line is: “I want to be honest with you – I’m concerned.”
The initial interaction is an opportunity to find out why the person uses AAS, what health concerns they have at present, and why they are seeking care. Open-ended questions may reveal concerns that the patient has about fertility or side effects.
Consistent with harm-reduction approaches used for other public health epidemics – such as opioid abuse and blood-borne pathogens among people who inject drugs – follow-up visits can include nonjudgmental discussions about decreasing or stopping their use.
Ultimately, minimizing the harms of AAS use can serve as a bridge to their cessation, but the medical community needs to build up trust with a community of users who currently rely more on each other and the Internet for guidance than their primary care physicians. “We need more education. We’re going to need resources to do it,” Dr. O’Connell said. “And we’re going to have to do it.”
Dr. Phillips and Dr. Nagata have no financial disclosures. Mr. Vergel is the owner and founder of Discounted Labs but reported no other financial conflicts. Dr. O’Connor owns Anabolic Doc but has no additional financial disclosures.
A version of this article first appeared on Medscape.com.
Another day in the ED: Walking the line between empathy and desensitization
Patient after patient, emergency medicine physicians experience highs and lows, sometimes minutes apart. “It might be another Tuesday for us, but for the patient in that dramatic life moment on that day, it’s everything,” said Charissa Pacella, MD, chief of emergency medicine at UPMC Presbyterian in Pittsburgh.
Emergency department (ED) physicians frequently encounter fatal situations, feel frustration when they can’t save a person, and constantly see patients in distress. How do physicians weather the emotional storm of life in the ED with both their mental health and empathy intact?
Reserve time for emotions
Dr. Pacella, who has been practicing emergency medicine for 22 years, also serves in a leadership role for Physicians for Physicians, a confidential peer support program at UPMC for doctors struggling with the impact of adverse events and the stress they face. She said it’s essential to know how to compartmentalize and focus on the task at hand, but later revisit emotions from a personal perspective.
“We all separate our cognitive and leadership roles from our emotional response in the moment,” she said. “Everybody is just focused on doing the next right thing. And often it’s not until sometime later when you sit down or go home or maybe even going in for your next shift that it really hits you in a more emotional way.”
If you try to avoid or skip over this part of the process by shoving the emotions down and ignoring them, Dr. Pacella said, you leave out a crucial part of the care process. And over the course of a career, you’ll risk losing empathy and the human connection that most doctors went into medicine for, she told this news organization.
Connect with your colleagues
Physicians supporting each other is crucial, said Dr Pacella. And luckily, she added, connection tends to be a strength of the specialty.
“As emergency medicine physicians, we share a lot in common, and part of it is what brought us to choose this specialty in the first place. You picked it because there’s something appealing to you about the unknown. It’s a unique role, a unique environment, and a unique relationship you have with patients and being able to connect with colleagues,” she said.
Lisa Williford, MD, emergency medicine specialist at Texas Health Harris Methodist Hospital in Fort Worth, said her 14-year career has taught her that no matter how focused a medical professional can stay in the moment, emotions are happening at some level.
“During a level 1 trauma, there are a lot of people in the room – trauma surgeons, residents, nurses, x-ray techs, respiratory therapy, anesthesia – and every one of us is having emotions. It’s not realistic to think anyone is avoiding it.”
But beyond simply recognizing a shared experience, it’s important to talk to each other. It’s not just about how you’re feeling, but also what you do to help manage that emotional load.
“I’d say that more of us, especially since COVID, are learning that actually getting a therapist is a good thing, having a life coach is a good thing,” said Dr. Williford. Accepting mental health care and learning how to manage it is also a good thing.
Accept unpredictability
You may think you know how a difficult situation will affect you, but that assumption can put you in a vulnerable position. Dr. Pacella said she’s learned that for most physicians, a stress response to a critical incident often has less to do with the type of event and more about who is involved or your past experiences.
“I have reacted in a very emotional way at moments that I would never have expected or predicted,” said Dr. Pacella. “And it’s not always because of some awful event. It’s usually because of some emotional connection or trigger embedded in that encounter.”
For example, she said, you may have had a past case as an emergency physician where the outcome was not favorable, or the patient involved may remind you of yourself or someone you love.
“It might not necessarily be a horrible thing happening to a young, healthy person that triggers someone; it might be a minor problem involving a patient you, for whatever reason, identify with,” she said. “Or you may have had a similar patient where things didn’t go well for them. It’s just highly variable, even for an individual.”
Just as you can’t know what medical issues you’ll face in a day, you can’t predict how you’ll react. Approach each scenario with the knowledge that you may veer off emotional course – and prepare accordingly.
Bring mental wellness to the forefront of training
Dr. Williford, who also serves as regional director for ScribeNest, a doctor-operated company that trains medical scribes who are on the path to becoming medical professionals, said she feels strongly about bringing this conversation to the younger generation.
“For me, nobody at the med school level or residency level taught or talked about how to compartmentalize and cope with the traumatic experiences that we saw,” she said. “Only in the last decade have we started teaching our residents and medical students about burnout and resilience.
“I say things like, ‘Hey, we just witnessed an 18-year-old in cardiac arrest. We did CPR for an hour and didn’t get him back. And then you witnessed me tell his mom, who wailed. And then we turned around and treated an ankle sprain. Let’s sit down and talk about how jarring that all is and how nobody else experiences these things.’
“We have this expectation that our physicians know how to move on and connect with each new patient with care and empathy, but we have to tell our future doctors the actual steps we take to be able to do that.”
Seasoned physicians can lead the way for the next generation and turn the tide toward the normalization of talking about these struggles. By making it part of training, it becomes part of a physician’s skill set.
“With a happy, healthy career, we can pay it forward to the next generation and teach them how to be better than we were,” said Dr. Williford.
A version of this article appeared on Medscape.com.
Patient after patient, emergency medicine physicians experience highs and lows, sometimes minutes apart. “It might be another Tuesday for us, but for the patient in that dramatic life moment on that day, it’s everything,” said Charissa Pacella, MD, chief of emergency medicine at UPMC Presbyterian in Pittsburgh.
Emergency department (ED) physicians frequently encounter fatal situations, feel frustration when they can’t save a person, and constantly see patients in distress. How do physicians weather the emotional storm of life in the ED with both their mental health and empathy intact?
Reserve time for emotions
Dr. Pacella, who has been practicing emergency medicine for 22 years, also serves in a leadership role for Physicians for Physicians, a confidential peer support program at UPMC for doctors struggling with the impact of adverse events and the stress they face. She said it’s essential to know how to compartmentalize and focus on the task at hand, but later revisit emotions from a personal perspective.
“We all separate our cognitive and leadership roles from our emotional response in the moment,” she said. “Everybody is just focused on doing the next right thing. And often it’s not until sometime later when you sit down or go home or maybe even going in for your next shift that it really hits you in a more emotional way.”
If you try to avoid or skip over this part of the process by shoving the emotions down and ignoring them, Dr. Pacella said, you leave out a crucial part of the care process. And over the course of a career, you’ll risk losing empathy and the human connection that most doctors went into medicine for, she told this news organization.
Connect with your colleagues
Physicians supporting each other is crucial, said Dr Pacella. And luckily, she added, connection tends to be a strength of the specialty.
“As emergency medicine physicians, we share a lot in common, and part of it is what brought us to choose this specialty in the first place. You picked it because there’s something appealing to you about the unknown. It’s a unique role, a unique environment, and a unique relationship you have with patients and being able to connect with colleagues,” she said.
Lisa Williford, MD, emergency medicine specialist at Texas Health Harris Methodist Hospital in Fort Worth, said her 14-year career has taught her that no matter how focused a medical professional can stay in the moment, emotions are happening at some level.
“During a level 1 trauma, there are a lot of people in the room – trauma surgeons, residents, nurses, x-ray techs, respiratory therapy, anesthesia – and every one of us is having emotions. It’s not realistic to think anyone is avoiding it.”
But beyond simply recognizing a shared experience, it’s important to talk to each other. It’s not just about how you’re feeling, but also what you do to help manage that emotional load.
“I’d say that more of us, especially since COVID, are learning that actually getting a therapist is a good thing, having a life coach is a good thing,” said Dr. Williford. Accepting mental health care and learning how to manage it is also a good thing.
Accept unpredictability
You may think you know how a difficult situation will affect you, but that assumption can put you in a vulnerable position. Dr. Pacella said she’s learned that for most physicians, a stress response to a critical incident often has less to do with the type of event and more about who is involved or your past experiences.
“I have reacted in a very emotional way at moments that I would never have expected or predicted,” said Dr. Pacella. “And it’s not always because of some awful event. It’s usually because of some emotional connection or trigger embedded in that encounter.”
For example, she said, you may have had a past case as an emergency physician where the outcome was not favorable, or the patient involved may remind you of yourself or someone you love.
“It might not necessarily be a horrible thing happening to a young, healthy person that triggers someone; it might be a minor problem involving a patient you, for whatever reason, identify with,” she said. “Or you may have had a similar patient where things didn’t go well for them. It’s just highly variable, even for an individual.”
Just as you can’t know what medical issues you’ll face in a day, you can’t predict how you’ll react. Approach each scenario with the knowledge that you may veer off emotional course – and prepare accordingly.
Bring mental wellness to the forefront of training
Dr. Williford, who also serves as regional director for ScribeNest, a doctor-operated company that trains medical scribes who are on the path to becoming medical professionals, said she feels strongly about bringing this conversation to the younger generation.
“For me, nobody at the med school level or residency level taught or talked about how to compartmentalize and cope with the traumatic experiences that we saw,” she said. “Only in the last decade have we started teaching our residents and medical students about burnout and resilience.
“I say things like, ‘Hey, we just witnessed an 18-year-old in cardiac arrest. We did CPR for an hour and didn’t get him back. And then you witnessed me tell his mom, who wailed. And then we turned around and treated an ankle sprain. Let’s sit down and talk about how jarring that all is and how nobody else experiences these things.’
“We have this expectation that our physicians know how to move on and connect with each new patient with care and empathy, but we have to tell our future doctors the actual steps we take to be able to do that.”
Seasoned physicians can lead the way for the next generation and turn the tide toward the normalization of talking about these struggles. By making it part of training, it becomes part of a physician’s skill set.
“With a happy, healthy career, we can pay it forward to the next generation and teach them how to be better than we were,” said Dr. Williford.
A version of this article appeared on Medscape.com.
Patient after patient, emergency medicine physicians experience highs and lows, sometimes minutes apart. “It might be another Tuesday for us, but for the patient in that dramatic life moment on that day, it’s everything,” said Charissa Pacella, MD, chief of emergency medicine at UPMC Presbyterian in Pittsburgh.
Emergency department (ED) physicians frequently encounter fatal situations, feel frustration when they can’t save a person, and constantly see patients in distress. How do physicians weather the emotional storm of life in the ED with both their mental health and empathy intact?
Reserve time for emotions
Dr. Pacella, who has been practicing emergency medicine for 22 years, also serves in a leadership role for Physicians for Physicians, a confidential peer support program at UPMC for doctors struggling with the impact of adverse events and the stress they face. She said it’s essential to know how to compartmentalize and focus on the task at hand, but later revisit emotions from a personal perspective.
“We all separate our cognitive and leadership roles from our emotional response in the moment,” she said. “Everybody is just focused on doing the next right thing. And often it’s not until sometime later when you sit down or go home or maybe even going in for your next shift that it really hits you in a more emotional way.”
If you try to avoid or skip over this part of the process by shoving the emotions down and ignoring them, Dr. Pacella said, you leave out a crucial part of the care process. And over the course of a career, you’ll risk losing empathy and the human connection that most doctors went into medicine for, she told this news organization.
Connect with your colleagues
Physicians supporting each other is crucial, said Dr Pacella. And luckily, she added, connection tends to be a strength of the specialty.
“As emergency medicine physicians, we share a lot in common, and part of it is what brought us to choose this specialty in the first place. You picked it because there’s something appealing to you about the unknown. It’s a unique role, a unique environment, and a unique relationship you have with patients and being able to connect with colleagues,” she said.
Lisa Williford, MD, emergency medicine specialist at Texas Health Harris Methodist Hospital in Fort Worth, said her 14-year career has taught her that no matter how focused a medical professional can stay in the moment, emotions are happening at some level.
“During a level 1 trauma, there are a lot of people in the room – trauma surgeons, residents, nurses, x-ray techs, respiratory therapy, anesthesia – and every one of us is having emotions. It’s not realistic to think anyone is avoiding it.”
But beyond simply recognizing a shared experience, it’s important to talk to each other. It’s not just about how you’re feeling, but also what you do to help manage that emotional load.
“I’d say that more of us, especially since COVID, are learning that actually getting a therapist is a good thing, having a life coach is a good thing,” said Dr. Williford. Accepting mental health care and learning how to manage it is also a good thing.
Accept unpredictability
You may think you know how a difficult situation will affect you, but that assumption can put you in a vulnerable position. Dr. Pacella said she’s learned that for most physicians, a stress response to a critical incident often has less to do with the type of event and more about who is involved or your past experiences.
“I have reacted in a very emotional way at moments that I would never have expected or predicted,” said Dr. Pacella. “And it’s not always because of some awful event. It’s usually because of some emotional connection or trigger embedded in that encounter.”
For example, she said, you may have had a past case as an emergency physician where the outcome was not favorable, or the patient involved may remind you of yourself or someone you love.
“It might not necessarily be a horrible thing happening to a young, healthy person that triggers someone; it might be a minor problem involving a patient you, for whatever reason, identify with,” she said. “Or you may have had a similar patient where things didn’t go well for them. It’s just highly variable, even for an individual.”
Just as you can’t know what medical issues you’ll face in a day, you can’t predict how you’ll react. Approach each scenario with the knowledge that you may veer off emotional course – and prepare accordingly.
Bring mental wellness to the forefront of training
Dr. Williford, who also serves as regional director for ScribeNest, a doctor-operated company that trains medical scribes who are on the path to becoming medical professionals, said she feels strongly about bringing this conversation to the younger generation.
“For me, nobody at the med school level or residency level taught or talked about how to compartmentalize and cope with the traumatic experiences that we saw,” she said. “Only in the last decade have we started teaching our residents and medical students about burnout and resilience.
“I say things like, ‘Hey, we just witnessed an 18-year-old in cardiac arrest. We did CPR for an hour and didn’t get him back. And then you witnessed me tell his mom, who wailed. And then we turned around and treated an ankle sprain. Let’s sit down and talk about how jarring that all is and how nobody else experiences these things.’
“We have this expectation that our physicians know how to move on and connect with each new patient with care and empathy, but we have to tell our future doctors the actual steps we take to be able to do that.”
Seasoned physicians can lead the way for the next generation and turn the tide toward the normalization of talking about these struggles. By making it part of training, it becomes part of a physician’s skill set.
“With a happy, healthy career, we can pay it forward to the next generation and teach them how to be better than we were,” said Dr. Williford.
A version of this article appeared on Medscape.com.
Growing ‘tranq’ threat poses challenges for PCPs
The widening threat of the animal tranquilizer xylazine, otherwise known as tranq, which has been found in illegally manufactured fentanyl, necessitates wider testing, a better understanding of its effects, and more research on treatment options, according to a narrative review published in the Annals of Internal Medicine.
“A lot of doctors and providers are asking about this drug,” said Joseph D’Orazio, MD, an addiction medicine specialist and medical toxicologist at Cooper University Healthcare, Camden, N.J., who led the review.
Xylazine is believed to prolong or intensify the effects of opioids, making it a popular additive to illegally produced opioids, particularly fentanyl, according to the Drug Enforcement Administration. Users end up in a zombie-like state with slowed breathing, and they sometimes develop skin ulcers. Because xylazine is not an opioid, common antidotes such as naloxone are ineffective. The White House has called the fentanyl-xylazine combo an “emerging threat.”
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA administrator Anne Milgram, in a statement on the agency’s website. “DEA has seized xylazine and fentanyl mixtures in 48 of 50 States. The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Dr. D’Orazio paired clinical experience with available research to provide guidance on the care of patients exposed to xylazine.
He and his team issued a call for more research on the drug’s effects, including more details on dependency and withdrawal.
Testing a patient who may have been exposed to xylazine requires forensic lab capabilities, which makes testing complicated and costly. The review found no evidence of the origin of the drug or why it causes open sores.
The review calls for more education of providers, including primary care physicians, on the treatment and care of patients who have used xylazine and fentanyl. The authors also call for expanding standard urine analysis to test for xylazine and for intensifying surveillance of the drug supply and distribution of xylazine test strips.
The authors of an editorial that accompanied the journal article urged the health care community to get ahead of xylazine before the crisis worsens.
“Not testing for xylazine in current unaffected areas and populations may lead to delays in responding if and when the drug becomes prevalent in the drug supply,” the authors wrote.
Xylazine was detected in 90% of street opioid samples tested in Philadelphia in 2021, and a toxic surveillance study of drug paraphernalia in Maryland found xylazine in 80% of samples tested between 2021 and 2022.
Dr. D’Orazio stressed that although Narcan is ineffective in treating xylazine, because the sedative is almost always mixed with fentanyl or another opiate, the opioid antagonist should still be used in emergencies.
Angelique Campen, MD, an emergency medicine physician at Providence St. Joseph Medical Center, Burbank, Calif., said she has seen an increase in patients entering the emergency department under the influence of what seems like fentanyl or heroin, but standard treatments such as Narcan have a limited effect. These patients remain in a prolonged period of sedation.
Recently, she admitted to her hospital’s intensive care unit a patient suspected of a xylazine overdose who was not responding to treatment.
Dr. Campen said that patients are screened for fentanyl, but because no test is available for xylazine, she presumed xylazine was causing the complication.
“It makes perfect medical sense to me that that’s what was going on,” Dr. Campen, who has worked at St. Joseph’s for 25 years, said. “I’m hoping with physicians being more aware of it that we can have that part of our regular urine drug screen.”
Dr. Campen also said she hopes an antidote is soon developed.
“If we can just keep delivering that message, hopefully, [to] more and more people, it will get through to them,” she said. “Every time you’re taking this, even though you may have taken it a week before and been fine, you never know: The next dose you take may be the lethal dose.”
A review author reports being awarded $1,000 to cover travel cost for Best Overall Abstract at the American Society of Addiction Medicine 2023 Annual Meeting. Another author reports receiving payments for training conducted as part of a NJDMAHS training grant to educate on substance use disorders. Dr. D’Orazio reports a $500 honorarium for a one-time lecture on xylazine at Yale; and a $500 honorarium for speaking one to three times per year on various topics regarding opioid use disorder at the Health Federation of Philadelphia. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
The widening threat of the animal tranquilizer xylazine, otherwise known as tranq, which has been found in illegally manufactured fentanyl, necessitates wider testing, a better understanding of its effects, and more research on treatment options, according to a narrative review published in the Annals of Internal Medicine.
“A lot of doctors and providers are asking about this drug,” said Joseph D’Orazio, MD, an addiction medicine specialist and medical toxicologist at Cooper University Healthcare, Camden, N.J., who led the review.
Xylazine is believed to prolong or intensify the effects of opioids, making it a popular additive to illegally produced opioids, particularly fentanyl, according to the Drug Enforcement Administration. Users end up in a zombie-like state with slowed breathing, and they sometimes develop skin ulcers. Because xylazine is not an opioid, common antidotes such as naloxone are ineffective. The White House has called the fentanyl-xylazine combo an “emerging threat.”
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA administrator Anne Milgram, in a statement on the agency’s website. “DEA has seized xylazine and fentanyl mixtures in 48 of 50 States. The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Dr. D’Orazio paired clinical experience with available research to provide guidance on the care of patients exposed to xylazine.
He and his team issued a call for more research on the drug’s effects, including more details on dependency and withdrawal.
Testing a patient who may have been exposed to xylazine requires forensic lab capabilities, which makes testing complicated and costly. The review found no evidence of the origin of the drug or why it causes open sores.
The review calls for more education of providers, including primary care physicians, on the treatment and care of patients who have used xylazine and fentanyl. The authors also call for expanding standard urine analysis to test for xylazine and for intensifying surveillance of the drug supply and distribution of xylazine test strips.
The authors of an editorial that accompanied the journal article urged the health care community to get ahead of xylazine before the crisis worsens.
“Not testing for xylazine in current unaffected areas and populations may lead to delays in responding if and when the drug becomes prevalent in the drug supply,” the authors wrote.
Xylazine was detected in 90% of street opioid samples tested in Philadelphia in 2021, and a toxic surveillance study of drug paraphernalia in Maryland found xylazine in 80% of samples tested between 2021 and 2022.
Dr. D’Orazio stressed that although Narcan is ineffective in treating xylazine, because the sedative is almost always mixed with fentanyl or another opiate, the opioid antagonist should still be used in emergencies.
Angelique Campen, MD, an emergency medicine physician at Providence St. Joseph Medical Center, Burbank, Calif., said she has seen an increase in patients entering the emergency department under the influence of what seems like fentanyl or heroin, but standard treatments such as Narcan have a limited effect. These patients remain in a prolonged period of sedation.
Recently, she admitted to her hospital’s intensive care unit a patient suspected of a xylazine overdose who was not responding to treatment.
Dr. Campen said that patients are screened for fentanyl, but because no test is available for xylazine, she presumed xylazine was causing the complication.
“It makes perfect medical sense to me that that’s what was going on,” Dr. Campen, who has worked at St. Joseph’s for 25 years, said. “I’m hoping with physicians being more aware of it that we can have that part of our regular urine drug screen.”
Dr. Campen also said she hopes an antidote is soon developed.
“If we can just keep delivering that message, hopefully, [to] more and more people, it will get through to them,” she said. “Every time you’re taking this, even though you may have taken it a week before and been fine, you never know: The next dose you take may be the lethal dose.”
A review author reports being awarded $1,000 to cover travel cost for Best Overall Abstract at the American Society of Addiction Medicine 2023 Annual Meeting. Another author reports receiving payments for training conducted as part of a NJDMAHS training grant to educate on substance use disorders. Dr. D’Orazio reports a $500 honorarium for a one-time lecture on xylazine at Yale; and a $500 honorarium for speaking one to three times per year on various topics regarding opioid use disorder at the Health Federation of Philadelphia. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
The widening threat of the animal tranquilizer xylazine, otherwise known as tranq, which has been found in illegally manufactured fentanyl, necessitates wider testing, a better understanding of its effects, and more research on treatment options, according to a narrative review published in the Annals of Internal Medicine.
“A lot of doctors and providers are asking about this drug,” said Joseph D’Orazio, MD, an addiction medicine specialist and medical toxicologist at Cooper University Healthcare, Camden, N.J., who led the review.
Xylazine is believed to prolong or intensify the effects of opioids, making it a popular additive to illegally produced opioids, particularly fentanyl, according to the Drug Enforcement Administration. Users end up in a zombie-like state with slowed breathing, and they sometimes develop skin ulcers. Because xylazine is not an opioid, common antidotes such as naloxone are ineffective. The White House has called the fentanyl-xylazine combo an “emerging threat.”
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA administrator Anne Milgram, in a statement on the agency’s website. “DEA has seized xylazine and fentanyl mixtures in 48 of 50 States. The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Dr. D’Orazio paired clinical experience with available research to provide guidance on the care of patients exposed to xylazine.
He and his team issued a call for more research on the drug’s effects, including more details on dependency and withdrawal.
Testing a patient who may have been exposed to xylazine requires forensic lab capabilities, which makes testing complicated and costly. The review found no evidence of the origin of the drug or why it causes open sores.
The review calls for more education of providers, including primary care physicians, on the treatment and care of patients who have used xylazine and fentanyl. The authors also call for expanding standard urine analysis to test for xylazine and for intensifying surveillance of the drug supply and distribution of xylazine test strips.
The authors of an editorial that accompanied the journal article urged the health care community to get ahead of xylazine before the crisis worsens.
“Not testing for xylazine in current unaffected areas and populations may lead to delays in responding if and when the drug becomes prevalent in the drug supply,” the authors wrote.
Xylazine was detected in 90% of street opioid samples tested in Philadelphia in 2021, and a toxic surveillance study of drug paraphernalia in Maryland found xylazine in 80% of samples tested between 2021 and 2022.
Dr. D’Orazio stressed that although Narcan is ineffective in treating xylazine, because the sedative is almost always mixed with fentanyl or another opiate, the opioid antagonist should still be used in emergencies.
Angelique Campen, MD, an emergency medicine physician at Providence St. Joseph Medical Center, Burbank, Calif., said she has seen an increase in patients entering the emergency department under the influence of what seems like fentanyl or heroin, but standard treatments such as Narcan have a limited effect. These patients remain in a prolonged period of sedation.
Recently, she admitted to her hospital’s intensive care unit a patient suspected of a xylazine overdose who was not responding to treatment.
Dr. Campen said that patients are screened for fentanyl, but because no test is available for xylazine, she presumed xylazine was causing the complication.
“It makes perfect medical sense to me that that’s what was going on,” Dr. Campen, who has worked at St. Joseph’s for 25 years, said. “I’m hoping with physicians being more aware of it that we can have that part of our regular urine drug screen.”
Dr. Campen also said she hopes an antidote is soon developed.
“If we can just keep delivering that message, hopefully, [to] more and more people, it will get through to them,” she said. “Every time you’re taking this, even though you may have taken it a week before and been fine, you never know: The next dose you take may be the lethal dose.”
A review author reports being awarded $1,000 to cover travel cost for Best Overall Abstract at the American Society of Addiction Medicine 2023 Annual Meeting. Another author reports receiving payments for training conducted as part of a NJDMAHS training grant to educate on substance use disorders. Dr. D’Orazio reports a $500 honorarium for a one-time lecture on xylazine at Yale; and a $500 honorarium for speaking one to three times per year on various topics regarding opioid use disorder at the Health Federation of Philadelphia. No other disclosures were reported.
A version of this article first appeared on Medscape.com.