User login
Geriatrics update 2015: Vaccination, frailty, chronic disease guidelines, and cognition
Guidelines for the management of chronic disease are starting to recognize vulnerable elderly patients. The topics in this review, culled from recent studies and recommendations, were chosen because they may change geriatric care. They include the newest influenza and pneumococcal vaccines; recommendations for managing chronic heart failure, cholesterol, and blood pressure; preventing frailty; drug treatments for dementia; and the impact of cognitive impairment on health outcomes.
INFLUENZA VACCINATION: HIGH-DOSE SUPERIOR BUT COSTLIER
Three classes of influenza vaccines have been available for some time:
- The standard-dose, trivalent inactivated injectable vaccine (IIV3-SD) contains H1N1, H3N2, and influenza B strains and is approved for all ages over 6 months.
- The quadrivalent vaccine (available mostly for nasal administration) has the same strains as the trivalent vaccine plus a second, different influenza B strain. The inactivated vaccine is injectable for all persons over the age of 6 months; the live-attenuated vaccine is available as a nasal spray only for ages 2 through 49.
- The high-dose injectable vaccine (IIV3-HD) contains the same strains as the trivalent vaccine plus four times as much hemagglutinin—the influenza virus antigen that stimulates immunity.
Although IIV3-HD has been available since 2010, no clinical data existed until 2014 showing it to be superior to standard-dose vaccine.
DiazGranados et al1 randomized nearly 32,000 adults age 65 and older to receive either the standard-dose or the high-dose vaccine. The primary end point was laboratory-confirmed influenza caused by any influenza viral type or subtype, in association with a protocol-defined influenzalike illness.
The primary end point was reached in 1.4% of those with the high-dose vaccine and 1.9% of those with the standard-dose vaccine (relative efficacy 24.2%, 95% confidence interval 9.7–36.5). There was also a 26% reduction in respiratory illness regardless of laboratory confirmation. Mortality rates were identical and low (0.5%) in both groups. In those without laboratory confirmation of respiratory illness, there was a 26% lower rate of pneumonia but no statistical difference in rates of hospitalization, medication use, routine office visits, and emergency department visits.
These results can be interpreted as meaning that the high-dose vaccine prevented about a quarter of the laboratory-confirmed influenza cases that would have occurred with the standard-dose vaccine. However, due to the low rate of disease in those given the standard-dose vaccine, the number needed to treat to prevent one influenza infection was about 200 with the high-dose vs the standard-dose vaccine; to prevent one case of pneumonia, more than 270 would need to be treated.
The current price differential as well as the high number needed to treat to prevent one infection may discourage the use of the high-dose vaccine. Medicare Part B pays for one dose of either influenza vaccine per season. For patients who paid out of pocket, the 2014–2015 season cost at a typical pharmacy was about $32 for the standard-dose vaccine and $55 for the high-dose.
PNEUMOCOCCAL VACCINATION
Conjugate vaccine now recommended for seniors
The 23-valent polysaccharide vaccine (Pneumovax) has been available since 1983 and is recommended in the United States for all adults age 65 and over. A 13-valent pneumococcal diphtheria conjugate vaccine (Prevnar 13) has been available since 2010. Until recently, the conjugate vaccine was recommended for children; the only adults for whom it was recommended were those age 19 and over who either were immunocompromised or had a cochlear implant, asplenia, a cerebral spinal fluid leak, or renal failure.
The CAPITA trial2 (Community-Acquired Pneumonia Immunisation Trial in Adults) randomized nearly 85,000 people (most 65 and older, and some children) in the Netherlands to receive either the conjugate vaccine or placebo. It found a 46% reduction in community-acquired pneumonia (P = .0006), a 45% reduction in nonbacteremic nonvaccine-type community-acquired pneumonia (P = .0067), and a 75% reduction in vaccine-type invasive pneumococcal disease (P = .0005). Common side effects included pain, swelling at the injection site, limitation of arm movement, fatigue, headache, decreased appetite, chills, and rash.
Based on this one study, the Advisory Committee on Immunization Practices3 recommended that all adults 65 and older receive the conjugate vaccine.
The recommendations for the conjugate vaccine for all ages are complicated. Limited to those age 65 and older, current recommendations are:
- For those who have already received the polysaccharide vaccine: get the conjugated vaccine at least 1 year later
- For those who have never received the polysaccharide vaccine: get the conjugate vaccine now, then the polysaccharide vaccine 6 to 12 months later.
Whether the Netherlands findings fully apply to the United States is under question. At the time of the study, Dutch infants but not adults had received pneumonia conjugate vaccinations since 2002 with a high compliance rate. Unlike in the United States, the polysaccharide pneumococcal vaccine had not been routinely recommended in the Netherlands. There may have been some indirect immunity due to the “herd” effect, but no direct immunity. With a likely higher background immunity to pneumonia in the United States, the dramatic reduction in infection noted in the Netherlands may not be duplicated here.
For those without Medicare coverage, the 2014–2015 winter season cost at a pharmacy was about $95 for the polysaccharide vaccine and about $200 for the conjugate vaccine. As of February 2, 2015, the Centers for Medicare and Medicaid Services are implementing Medicare Part B coverage to allow initial pneumococcal vaccine for Medicare patients who never received a pneumococcal vaccine under Medicare Part B, and then a different, second pneumococcal vaccine, 1 year after the first vaccine was administered.
HEART FAILURE
Eplerenone’s new role in mild heart failure
Aldosterone antagonists have been recommended for moderate to severe heart failure (New York Heart Association [NYHA] classes III and IV) for some time. The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines also recommend them for mild heart failure (NYHA II).4
The EMPHASIS trial5 (Eplerenone in Patients With Systolic Heart Failure and Mild Symptoms) randomized 2,737 patients, median age 69, with NYHA class II heart failure and an ejection fraction of no more than 35% to receive the aldosterone antagonist eplerenone (up to 50 mg daily) or placebo, in addition to recommended therapy. The trial was stopped early, after a median follow-up of 21 months, when the treatment group was found to have a significantly lower risk of cardiovascular death or hospitalization for heart failure or for any cause.
Of note: hyperkalemia occurred in 11.8% of the eplerenone group vs 7.2% in the placebo group (P < .001). The high frequency of hyperkalemia in the placebo group may have been due to concomitant use of angiotensin-converting enzyme (ACE) inhibitors.
Sodium restriction reasonable
Although sodium restriction has been standard practice in heart failure for decades, restricting sodium in the elderly was given only a IIa (“reasonable”) classification, based on level C (very limited) evidence.4
Strong evidence exists that middle-aged and young older adults with heart failure (with preserved or reduced ejection fraction) should reduce their sodium intake by about 1 g per day or aim for a mean 24-hour urinary sodium excretion of about 2.3 g per day. However, little evidence exists to support a specific long-term target intake, and no evidence exists for “old-old” patients (loosely defined as older than 75 or 80).
Caution with digoxin
Use of digoxin has been recommended in patients with heart failure with reduced ejection fraction to reduce hospitalizations,4 but more recent publications have raised questions regarding its safety and efficacy.
Freeman et al,6 in a prospective study, followed 2,891 patients with newly diagnosed systolic heart failure over 2.5 years, of whom 529 were prescribed digoxin. The digoxin group had a higher rate of death (14.2 vs 11.2 per 100 patient-years) and heart failure-related hospitalization (28.2 vs 24.4 per 100 person-years).
The study was unable to determine if the digoxin level influenced the results, since about 30% of patients had no digoxin level drawn, and an additional 27% had only one level drawn during the study. For those with measured blood levels, the mean digoxin level for men was 0.83 ng/mL and 1.12 ng/mL for women. Risks and benefits of this medication should be weighed carefully.
Simultaneous interventions beneficial
The following evidence-based interventions are recommended for patients with heart failure with reduced ejection fraction:
- Heart failure education
- A beta-blocker
- An ACE inhibitor
- An aldosterone antagonist for NYHA class II–IV symptoms
- Anticoagulation for atrial fibrillation in patients with added risks (eg, hypertension, diabetes, prior transient ischemic attack or cerebrovascular accident, age at least 75)
- An implantable cardioverter-defibrillator and cardiac resynchronization therapy for select patients with symptoms, increased QRS duration, and left bundle branch block.
Fonarow et al7 studied these interventions in an analysis of a prospective study of outpatients with diagnosed heart failure or myocardial infarction and reduced left ventricular ejection fraction. Their nested case-control study compared 1,376 patients, mean age 72, who had died within 24 months and 2,752 propensity-matched controls who survived to 24 months. The survival rate was 37% higher with two simultaneous interventions than with one, and 70% higher with four simultaneous interventions than with one. Benefits plateaued with four to five interventions.
LIPID-LOWERING THERAPY FOR SENIORS
The 2013 ACC/AHA cholesterol guideline8 included new recommendations specifically relevant to the elderly. It advocates using a new cardiovascular disease risk calculator that provides an estimate of 10-year risk of atherosclerotic cardiovascular disease (ASCVD), based on data from multiple community-based populations and applicable to African American and non-Hispanic white men and women ages 40 through 79. Primary prevention with a statin is encouraged for those with a 10-year risk of 7.5% or higher. The tool generated controversy from the moment it was announced and may overestimate ASCVD risk by 67% in women and 86% in men.9
Emphasis on tolerability
The guideline focuses on statins as the main treatment and de-emphasizes the adjunctive use of other drugs to further lower lipids such as niacin, ezetimibe, and fenofibrate.
Statin tolerability is now stressed rather than specific lipid level targets. The guideline recommends reassessing statin choice and intensity according to pain, tenderness, stiffness, cramping, weakness, and fatigue (class IIa recommendation [“reasonable”], level of evidence B [“limited”]). Also recommended is reassessment of statin choice and intensity for patients older than 75 or for those taking multiple medications, drugs that alter metabolism, and conditions requiring complex medications (class IIa, level of evidence C [“very limited”]). For patients with confusion, statin and nonstatin causes should be considered as the source of the problem (class IIb [“consider”], level of evidence C).
Initiating high-intensity statin therapy is not recommended after age 75. However, continuing such treatment is reasonable for patients already receiving and tolerating the therapy for an appropriate indication. Initiation of moderate-intensity statin therapy in this age group is recommended for those with either clinical atherosclerotic cardiovascular disease or a low-density lipoprotein cholesterol (LDL-C) level of at least 190 mg/dL.
No specific guidance is provided for patients older than age 75 without ASCVD, with LDL-C less than 190 mg/dL, or with diabetes. In these groups, statin therapy may be initiated, continued, or intensified (class IIb, level of evidence C).
HYPERTENSION: LESS AGGRESSIVE GOALS FOR ELDERLY
The eighth Joint National Committee (JNC 8)10 made nine recommendations for managing high blood pressure, only one of which specifically addresses people 60 and older.
Drug therapy should be initiated if the blood pressure is 150/90 mm Hg or higher, and the blood pressure should be treated to less than that level (grade A recommendation, ie, strong). If treated systolic blood pressure is less than 140 mm Hg without adverse effects, it should be sustained (grade E recommendation, ie, based on expert opinion).
Tension between guidelines
The higher threshold for hypertension treatment and the lower threshold for statin therapy create tension between guidelines, and between guidelines and epidemiologic data.
For example, in a 67-year-old woman without diabetes and with a favorable lipid profile (eg, total cholesterol 130 mg/dL, high-density lipoprotein cholesterol 55 mg/dL), the ACC/AHA ASCVD risk calculator predicts a 10-year risk of less than 7.5% if her systolic blood pressure is 147 mm Hg. If the patient’s blood pressure were 148 or 149 mm Hg and all the other variables were the same, the JNC 8 would not recommend treatment with antihypertensive medication, but the ACC/AHA guidelines would recommend preventive statin therapy.
Another example is the relationship between heart failure and antihypertensive drugs. Multiple studies11,12 demonstrate a reduction in heart failure incidence with hypertension treatment. A 70-year-old man whose systolic blood pressure is 140 mm Hg has about a 15% lifetime risk of heart failure. If his systolic pressure were 160 mm Hg, his lifetime heart failure risk would be more than 50%.13 If his systolic pressure were 149, his lifetime risk of heart failure would be between 15% and 50%, but the JNC 8 criteria do not recommend antihypertensive therapy.
EXERCISE SLOWS PROGRESSION TO FRAILTY
In the absence of a gold standard, frailty has been operationally defined as meeting three out of five phenotypic criteria: diminished grip strength, low energy, slow gait, low physical activity, and unintentional weight loss. A “prefrail” stage, in which one or two criteria are present, identifies a vulnerable subset at high risk of progression to frailty.
About 42% of older adults in the community are considered vulnerable, or prefrail, and about 11% are frail.14 Interventions at the prefrail stage may prevent progression to frailty, but it is rare, without intervention, for a person to re-achieve the stronger stage once diagnosed with frailty.
Pahor et al15 randomized 1,635 sedentary adults ages 70 to 89 who met the criteria of prefrailty to either a moderate-intensity exercise program (consisting of aerobic, resistance, and flexibility exercises for 150 minutes per week, performed in a center and at home) or to a health education program with workshops on topics relevant to older adults and upper-extremity stretching exercises. Adherence to the exercise program was verified by questionnaire and an accelerometer device. Participants were assessed every 6 months for an average of 2.6 years.
The primary outcome measure was the development of major mobility disability as defined by the loss of ability to walk 400 m without assistance (a cane was acceptable, but not a walker). The primary outcome occurred in 30.1% of those in the exercise group and 35.5% of the health education group (hazard ratio 0.82, P = .03). Those in the exercise group also had one third fewer falls. No differences were found in death rates. The number needed to treat was about 19 to prevent one person from developing major disability. Those most likely to benefit were those who walked slowly at baseline (< 1.8 mph), were more mobility-impaired, and were more cognitively healthy.
SLOWING DEMENTIA IS STILL AN ELUSIVE GOAL
Vitamin E modestly improves cognitive function but may have a cost
Before new information emerged in 2014 regarding vitamin E and dementia, the best data were from a 1997 study16 that randomized patients with moderate dementia to either daily vitamin E 2,000 IU, the monoamine oxidase inhibitor selegiline 10 mg, both, or placebo for 2 years. No benefit of treatment for cognitive function was found. However, after adjusting for the baseline Mini-Mental State Examination score, the investigators found that either treatment was associated with a delay of about 7 months in the primary outcome (death, institutionalization, loss of activities of daily living, or severe dementia).
Unfortunately, selegiline is often poorly tolerated, causing dyskinesia in more than 10% of patients, nausea in 20%, and confusion, hallucinations, and syncope. Although vitamin E is better tolerated, in high doses it can cause fatigue, headache, and bleeding, with increased risk of hemorrhagic stroke. Studies conflict as to whether it increases the risk of death from any cause.17,18
Dysken et al,19 in a study reported in 2014, randomized 613 patients with mild to moderate Alzheimer disease, all of whom were taking an acetylcholinesterase inhibitor, to either daily vitamin E 2,000 IU, memantine 20 mg, both, or placebo. The primary outcome measure was an activities of daily living score (0–78, higher being better), which included the ability to perform such tasks as dressing oneself. Each task was scored from 0 (totally dependent on help) to 4 (able to perform completely independently).
Scores fell in both groups over the mean 2.7 years of the study, but the decrease was slightly slower in the vitamin E group: 3 points less at the end of the study compared with placebo. The groups taking memantine, vitamin E, or both did not differ significantly from one another. No significant differences were found in the secondary outcome of cognitive, neuropsychiatric, functional, and caregiver measures.
Based on the 1997 study, vitamin E may defer the time to important clinical outcomes by 7.5 months over a 2-year period in patients with moderate dementia. Based on the 2014 study, vitamin E may preserve half an activity of daily living over 2.7 years in patients with mild to moderate dementia. On the other hand, high doses of vitamin E may increase the risk of bleeding and falling, and whether they increase the risk of death is unclear.
Antidepressants for behavior issues
Other common problems in patients with major neurocognitive disorders include disturbed perception, thought content, mood, and behavior, collectively called behavioral and psychological symptoms of dementia. No known nondrug intervention is consistently effective for these problems, and no drug approved by the US Food and Drug Administration (FDA), except for a fixed-dose combination of dextromethorphan and quinidine, addresses any specific symptom.
Porsteinsson et al20 randomized 186 patients with Alzheimer disease and agitation to a psychological intervention plus either the selective serotonin reuptake inhibitor citalopram (titrated from 10 to 30 mg per day based on response and tolerability) or placebo. Agitation was reduced with citalopram compared with placebo, based on the agitation subscale of the Neurobehavioral Rating Scale.
Of those taking citalopram, 40% were much or very much improved, compared with 26% of those taking placebo. These results are comparable to or better than those with antipsychotic drugs, which should be avoided for treating dementia-related psychosis in elderly patients because of black-box warnings.
No differences were found in activities of daily living. An interesting finding, not seen in other studies, is that the Mini-Mental Status Examination score declined by 1 point in the treatment group vs no change in the placebo group (P = .03).
Prolonged QTc was found in 12.5% in the citalopram group vs 4.3% in the placebo group (P = .01). The FDA issued a warning in 2012 of a dose-dependent effect of citalopram on QTc and recommended a maximum dose of 20 mg for those over age 60; for “poor metabolizers” of cytochrome P450 2C19 (CYP 2C19); and for those taking medications that inhibit CYP 2C19, including proton pump inhibitors, cimetidine, fluvoxamine, fluoxetine, indomethacin, ketoconazole, modafinil, and probenecid. The United Kingdom has extended this warning to escitalopram. Unfortunately, in the Porsteinsson study, nearly 80% of the treatment group received the 30-mg dose and only 15% received the 20-mg dose, which provided insufficient data for independent analysis.
Possibly, citalopram cannot be administered in a dosage sufficient to produce the benefits seen in the study. Using escitalopram may also be risky. Based on this study, it would be prudent to monitor QTc when using these drugs.
Dextromethorphan and quinidine
A fixed-dose combination of dextromethorphan and quinidine (Nuedexta) was recently approved by the FDA for treatment of pseudobulbar affect in individuals with stroke, traumatic brain injury, or dementia. Pseudobulbar affect has been defined as a condition of contextually inappropriate or exaggerated emotional expression that often occurs in adults with neurologic damage.
Using a 20/10-mg dose combination, a small 12-week noncomparative trial demonstrated measurable improvement in pseudobulbar symptoms after 30 days (as measured by the Center for Neurologic Study-Lability Scale).21 Though individuals enrolled in this trial appeared to tolerate this dose, additional trials still need to be conducted to more clearly determine its long-term safety and efficacy. It is not approved for dementia with agitation, but a phase 2 trial suggests a benefit compared with placebo in reducing agitation and caregiver burden.22
RISKS OF MILD COGNITIVE IMPAIRMENT
The spectrum of cognitive impairment ranges from mild cognitive impairment (MCI), in which deficits are evident on neuropsychological testing but the person maintains overall function, to the different stages of dementia (mild, moderate, and severe). MCI was documented in the Cardiovascular Health Study in 22% of adults 75 and older.23
Despite presenting with apparently normal function, elderly people with MCI have a higher risk of falls, rehospitalization, and delirium. Screening is not typically performed for MCI in primary care. No study has compared clinical outcomes after screening vs not screening for cognitive impairment (whether MCI or dementia), and the US Preventive Services Task Force maintains that there is insufficient evidence for screening.24
Unrecognized cognitive impairment affects discharge outcomes
Nazir et al,25 in a 1-year longitudinal study, compared 976 patients age 65 and older who upon admission to a public hospital were either diagnosed with cognitive impairment (defined as scoring 7 or less on the 10-question Short Portable Mental Status Questionnaire) or not. They found that 42.5% were cognitively impaired on admission. Overall, 36.5% of patients were discharged to a facility rather than home; those who were cognitively impaired, older, and sicker were more likely to be discharged to a facility.
Interestingly, among those discharged to a facility, patients with cognitive impairment were less likely to be subsequently rehospitalized or die within 30 days of hospital discharge than those without cognitive impairment. Whether this can be explained by differences in comorbidities between the groups was not explored. Those discharged home had similar rates of death and rehospitalization whether or not they were cognitively impaired.
Patel et al26 screened 720 older patients upon discharge after hospitalization for heart failure with the Mini-Cog (a 3-minute test that consists of recall of three words and the ability to draw a clock face). About a quarter of patients were diagnosed with cognitive impairment based on this test.
Among those discharged home (about two-thirds of the group overall), patients were much more likely to be rehospitalized or die within 30 days if they were cognitively impaired. Among those discharged to a facility, the rates between the two groups were similar for the first 20 days; after that, people in the cognitively impaired group were much more likely to die or be readmitted to a hospital.
Dodson et al,27 in a study of 282 hospitalized patients with heart failure (mean age 80), identified 47% as having cognitive impairment at the time of hospitalization based on a score of less than 25 on the Mini-Mental State Examination. Of those found to have mild cognitive impairment (score 21–24), only 11% had documentation of a cognitive deficit in the medical record, and 39% of those found to have moderate to severe cognitive impairment (score < 21) had documentation of it in the medical record. Those with unrecognized impairment were 1.5 times more likely to die or be rehospitalized within 6 months than those with documented impairment.
Do interventions help?
It is unclear whether developing specific interventions tailored to cognitive impairment improves outcomes.
Davis et al28 studied 125 patients hospitalized for heart failure who were identified as having mild cognitive impairment based on a Montreal Cognitive Assessment score of 17 to 25 (out of 30) points. Patients were randomly assigned to either a targeted self-care teaching intervention or usual discharge care. The intervention included education and customized instruction on self-care tasks such as managing symptoms, organizing medications, and measuring fluid and sodium intake.
Thirty days after discharge, the intervention group had greater knowledge about heart failure than the control group, but no significant difference was found in ability to care for themselves or in readmission rates.
Interventions that target the patient-caregiver dyad may have more success. A pilot project in Indiana29 that developed an integrative care model for older people with mild cognitive impairment, dementia, or depression that targeted patients as well as their caregivers found that compared with patients from area primary care clinics, their patients had lower rehospitalization rates within 30 days of discharge (11% vs 20%) and higher rates of achieving a hemoglobin A1c of less than 8% (78% vs 51%). Results of an expanded innovations demonstration project awarded by the Centers for Medicare and Medicaid Services are pending.
The following more recently published data show promise for prevention of dementia through nonpharmacologic interventions.
The FINGER trial30 (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) screened 2,654 Finnish individuals ages 60 to 77 using the Cardiovascular Risk Factors, Aging and Dementia risk tool, identifying 1,260 individuals with higher levels of cognitive impairment and randomizing them to a 2-year intervention consisting of exercise, cognitive training, and vascular risk monitoring (n = 631), or a control group provided with general health advice only (n = 629). Neuropsychological testing was conducted to measure differences between the groups, and at the end of the study, the mean Z-score difference in the total testing score between the intervention and control group was 0.22 (P = .30). This trial demonstrated that if cognitive impairment were identified, a multimodal intervention could improve or maintain cognitive function in at-risk elderly individuals.
- DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–645.
- Bonten M, Bolkenbaas M, Huijts S, et al. Community acquired pneumonia immunisation trial in adults (CAPITA) (abstract). Pneumonia 2014; 3:95. Presented at 9th International Symposium on Pneumococci and Pneumococcal Diseases, 2014. Abstract 0541.
- Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2014; 63:822–825.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF-AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Freeman JV, Yang J, Sung SH, Hlatky MA, Go AS. Effectiveness and safety of digoxin among contemporary adults with incident systolic heart failure. Circ Cardiovasc Qual Outcomes 2013; 6:525–533.
- Fonarow GC, Albert NM, Curtis AB, et al. Incremental reduction in risk of death associated with use of guideline-recommended therapies in patients with heart failure: a nested case-control analysis of IMPROVE-HF. J Am Heart Assoc 2012; 1:16–26.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S1–S45.
- DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015; 162:266–275.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Kostis JB, Davis BR, Cutler J, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 1997; 278:212–216.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Lloyd-Jones DM, Larson MG, Leip EP, et al; Framingham Heart Study. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002; 106;3068–3072.
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60:1487–1492.
- Pahor M, Guralnik JM, Ambrosius WT, et al; LIFE study investigators. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014; 311:2387–2396.
- Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med 1997; 336:1216–1222.
- Miller ER 3rd, Pastor-Barriuso R, Dala D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142:37–46.
- Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci 2011; 4:158–170.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014; 311:682–691.
- Yang LP, Deeks ED. Dextromethorphan/quinidine: a review of its use in adults with pseudobulbar affect. Drugs 2015; 75:83–90.
- Cummings J, Lyketsos C, Tariot P, et al. Dextromethorphan/quinidine (AVP-923) efficacy and safety for treatment of agitation in persons with Alzheimer’s disease: results from a phase 2 study (NCT01584440) (S16.007). Neurology 2015; 84:S16.007.
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003; 60:1385–1389.
- Moyer VA; US Preventive Services Task Force. Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:791–797.
- Nazir A, LaMantia M, Chodosh J, et al. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc 2013; 61:1958–1963.
- Patel A, Parikh R, Howell E, Hsich E, Gorodeski E. Mini-Cog performance: a novel marker of risk among patients hospitalized for heart failure. J Am Coll Cardiol 4014; 63:A755.
- Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med 2013; 126:120–126.
- Davis KK, Mintzer M, Dennison Himmelfarb CR, Hayat MJ, Rotman S, Allen J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. Eur J Heart Fail 2012; 14:1041–1049.
- Boustani MA, Sachs GA, Alder CA, et al. Implementing innovative models of dementia care: The Healthy Aging Brain Center. Aging Ment Health 2011; 15:13–22.
- Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385:2255–2263.
Guidelines for the management of chronic disease are starting to recognize vulnerable elderly patients. The topics in this review, culled from recent studies and recommendations, were chosen because they may change geriatric care. They include the newest influenza and pneumococcal vaccines; recommendations for managing chronic heart failure, cholesterol, and blood pressure; preventing frailty; drug treatments for dementia; and the impact of cognitive impairment on health outcomes.
INFLUENZA VACCINATION: HIGH-DOSE SUPERIOR BUT COSTLIER
Three classes of influenza vaccines have been available for some time:
- The standard-dose, trivalent inactivated injectable vaccine (IIV3-SD) contains H1N1, H3N2, and influenza B strains and is approved for all ages over 6 months.
- The quadrivalent vaccine (available mostly for nasal administration) has the same strains as the trivalent vaccine plus a second, different influenza B strain. The inactivated vaccine is injectable for all persons over the age of 6 months; the live-attenuated vaccine is available as a nasal spray only for ages 2 through 49.
- The high-dose injectable vaccine (IIV3-HD) contains the same strains as the trivalent vaccine plus four times as much hemagglutinin—the influenza virus antigen that stimulates immunity.
Although IIV3-HD has been available since 2010, no clinical data existed until 2014 showing it to be superior to standard-dose vaccine.
DiazGranados et al1 randomized nearly 32,000 adults age 65 and older to receive either the standard-dose or the high-dose vaccine. The primary end point was laboratory-confirmed influenza caused by any influenza viral type or subtype, in association with a protocol-defined influenzalike illness.
The primary end point was reached in 1.4% of those with the high-dose vaccine and 1.9% of those with the standard-dose vaccine (relative efficacy 24.2%, 95% confidence interval 9.7–36.5). There was also a 26% reduction in respiratory illness regardless of laboratory confirmation. Mortality rates were identical and low (0.5%) in both groups. In those without laboratory confirmation of respiratory illness, there was a 26% lower rate of pneumonia but no statistical difference in rates of hospitalization, medication use, routine office visits, and emergency department visits.
These results can be interpreted as meaning that the high-dose vaccine prevented about a quarter of the laboratory-confirmed influenza cases that would have occurred with the standard-dose vaccine. However, due to the low rate of disease in those given the standard-dose vaccine, the number needed to treat to prevent one influenza infection was about 200 with the high-dose vs the standard-dose vaccine; to prevent one case of pneumonia, more than 270 would need to be treated.
The current price differential as well as the high number needed to treat to prevent one infection may discourage the use of the high-dose vaccine. Medicare Part B pays for one dose of either influenza vaccine per season. For patients who paid out of pocket, the 2014–2015 season cost at a typical pharmacy was about $32 for the standard-dose vaccine and $55 for the high-dose.
PNEUMOCOCCAL VACCINATION
Conjugate vaccine now recommended for seniors
The 23-valent polysaccharide vaccine (Pneumovax) has been available since 1983 and is recommended in the United States for all adults age 65 and over. A 13-valent pneumococcal diphtheria conjugate vaccine (Prevnar 13) has been available since 2010. Until recently, the conjugate vaccine was recommended for children; the only adults for whom it was recommended were those age 19 and over who either were immunocompromised or had a cochlear implant, asplenia, a cerebral spinal fluid leak, or renal failure.
The CAPITA trial2 (Community-Acquired Pneumonia Immunisation Trial in Adults) randomized nearly 85,000 people (most 65 and older, and some children) in the Netherlands to receive either the conjugate vaccine or placebo. It found a 46% reduction in community-acquired pneumonia (P = .0006), a 45% reduction in nonbacteremic nonvaccine-type community-acquired pneumonia (P = .0067), and a 75% reduction in vaccine-type invasive pneumococcal disease (P = .0005). Common side effects included pain, swelling at the injection site, limitation of arm movement, fatigue, headache, decreased appetite, chills, and rash.
Based on this one study, the Advisory Committee on Immunization Practices3 recommended that all adults 65 and older receive the conjugate vaccine.
The recommendations for the conjugate vaccine for all ages are complicated. Limited to those age 65 and older, current recommendations are:
- For those who have already received the polysaccharide vaccine: get the conjugated vaccine at least 1 year later
- For those who have never received the polysaccharide vaccine: get the conjugate vaccine now, then the polysaccharide vaccine 6 to 12 months later.
Whether the Netherlands findings fully apply to the United States is under question. At the time of the study, Dutch infants but not adults had received pneumonia conjugate vaccinations since 2002 with a high compliance rate. Unlike in the United States, the polysaccharide pneumococcal vaccine had not been routinely recommended in the Netherlands. There may have been some indirect immunity due to the “herd” effect, but no direct immunity. With a likely higher background immunity to pneumonia in the United States, the dramatic reduction in infection noted in the Netherlands may not be duplicated here.
For those without Medicare coverage, the 2014–2015 winter season cost at a pharmacy was about $95 for the polysaccharide vaccine and about $200 for the conjugate vaccine. As of February 2, 2015, the Centers for Medicare and Medicaid Services are implementing Medicare Part B coverage to allow initial pneumococcal vaccine for Medicare patients who never received a pneumococcal vaccine under Medicare Part B, and then a different, second pneumococcal vaccine, 1 year after the first vaccine was administered.
HEART FAILURE
Eplerenone’s new role in mild heart failure
Aldosterone antagonists have been recommended for moderate to severe heart failure (New York Heart Association [NYHA] classes III and IV) for some time. The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines also recommend them for mild heart failure (NYHA II).4
The EMPHASIS trial5 (Eplerenone in Patients With Systolic Heart Failure and Mild Symptoms) randomized 2,737 patients, median age 69, with NYHA class II heart failure and an ejection fraction of no more than 35% to receive the aldosterone antagonist eplerenone (up to 50 mg daily) or placebo, in addition to recommended therapy. The trial was stopped early, after a median follow-up of 21 months, when the treatment group was found to have a significantly lower risk of cardiovascular death or hospitalization for heart failure or for any cause.
Of note: hyperkalemia occurred in 11.8% of the eplerenone group vs 7.2% in the placebo group (P < .001). The high frequency of hyperkalemia in the placebo group may have been due to concomitant use of angiotensin-converting enzyme (ACE) inhibitors.
Sodium restriction reasonable
Although sodium restriction has been standard practice in heart failure for decades, restricting sodium in the elderly was given only a IIa (“reasonable”) classification, based on level C (very limited) evidence.4
Strong evidence exists that middle-aged and young older adults with heart failure (with preserved or reduced ejection fraction) should reduce their sodium intake by about 1 g per day or aim for a mean 24-hour urinary sodium excretion of about 2.3 g per day. However, little evidence exists to support a specific long-term target intake, and no evidence exists for “old-old” patients (loosely defined as older than 75 or 80).
Caution with digoxin
Use of digoxin has been recommended in patients with heart failure with reduced ejection fraction to reduce hospitalizations,4 but more recent publications have raised questions regarding its safety and efficacy.
Freeman et al,6 in a prospective study, followed 2,891 patients with newly diagnosed systolic heart failure over 2.5 years, of whom 529 were prescribed digoxin. The digoxin group had a higher rate of death (14.2 vs 11.2 per 100 patient-years) and heart failure-related hospitalization (28.2 vs 24.4 per 100 person-years).
The study was unable to determine if the digoxin level influenced the results, since about 30% of patients had no digoxin level drawn, and an additional 27% had only one level drawn during the study. For those with measured blood levels, the mean digoxin level for men was 0.83 ng/mL and 1.12 ng/mL for women. Risks and benefits of this medication should be weighed carefully.
Simultaneous interventions beneficial
The following evidence-based interventions are recommended for patients with heart failure with reduced ejection fraction:
- Heart failure education
- A beta-blocker
- An ACE inhibitor
- An aldosterone antagonist for NYHA class II–IV symptoms
- Anticoagulation for atrial fibrillation in patients with added risks (eg, hypertension, diabetes, prior transient ischemic attack or cerebrovascular accident, age at least 75)
- An implantable cardioverter-defibrillator and cardiac resynchronization therapy for select patients with symptoms, increased QRS duration, and left bundle branch block.
Fonarow et al7 studied these interventions in an analysis of a prospective study of outpatients with diagnosed heart failure or myocardial infarction and reduced left ventricular ejection fraction. Their nested case-control study compared 1,376 patients, mean age 72, who had died within 24 months and 2,752 propensity-matched controls who survived to 24 months. The survival rate was 37% higher with two simultaneous interventions than with one, and 70% higher with four simultaneous interventions than with one. Benefits plateaued with four to five interventions.
LIPID-LOWERING THERAPY FOR SENIORS
The 2013 ACC/AHA cholesterol guideline8 included new recommendations specifically relevant to the elderly. It advocates using a new cardiovascular disease risk calculator that provides an estimate of 10-year risk of atherosclerotic cardiovascular disease (ASCVD), based on data from multiple community-based populations and applicable to African American and non-Hispanic white men and women ages 40 through 79. Primary prevention with a statin is encouraged for those with a 10-year risk of 7.5% or higher. The tool generated controversy from the moment it was announced and may overestimate ASCVD risk by 67% in women and 86% in men.9
Emphasis on tolerability
The guideline focuses on statins as the main treatment and de-emphasizes the adjunctive use of other drugs to further lower lipids such as niacin, ezetimibe, and fenofibrate.
Statin tolerability is now stressed rather than specific lipid level targets. The guideline recommends reassessing statin choice and intensity according to pain, tenderness, stiffness, cramping, weakness, and fatigue (class IIa recommendation [“reasonable”], level of evidence B [“limited”]). Also recommended is reassessment of statin choice and intensity for patients older than 75 or for those taking multiple medications, drugs that alter metabolism, and conditions requiring complex medications (class IIa, level of evidence C [“very limited”]). For patients with confusion, statin and nonstatin causes should be considered as the source of the problem (class IIb [“consider”], level of evidence C).
Initiating high-intensity statin therapy is not recommended after age 75. However, continuing such treatment is reasonable for patients already receiving and tolerating the therapy for an appropriate indication. Initiation of moderate-intensity statin therapy in this age group is recommended for those with either clinical atherosclerotic cardiovascular disease or a low-density lipoprotein cholesterol (LDL-C) level of at least 190 mg/dL.
No specific guidance is provided for patients older than age 75 without ASCVD, with LDL-C less than 190 mg/dL, or with diabetes. In these groups, statin therapy may be initiated, continued, or intensified (class IIb, level of evidence C).
HYPERTENSION: LESS AGGRESSIVE GOALS FOR ELDERLY
The eighth Joint National Committee (JNC 8)10 made nine recommendations for managing high blood pressure, only one of which specifically addresses people 60 and older.
Drug therapy should be initiated if the blood pressure is 150/90 mm Hg or higher, and the blood pressure should be treated to less than that level (grade A recommendation, ie, strong). If treated systolic blood pressure is less than 140 mm Hg without adverse effects, it should be sustained (grade E recommendation, ie, based on expert opinion).
Tension between guidelines
The higher threshold for hypertension treatment and the lower threshold for statin therapy create tension between guidelines, and between guidelines and epidemiologic data.
For example, in a 67-year-old woman without diabetes and with a favorable lipid profile (eg, total cholesterol 130 mg/dL, high-density lipoprotein cholesterol 55 mg/dL), the ACC/AHA ASCVD risk calculator predicts a 10-year risk of less than 7.5% if her systolic blood pressure is 147 mm Hg. If the patient’s blood pressure were 148 or 149 mm Hg and all the other variables were the same, the JNC 8 would not recommend treatment with antihypertensive medication, but the ACC/AHA guidelines would recommend preventive statin therapy.
Another example is the relationship between heart failure and antihypertensive drugs. Multiple studies11,12 demonstrate a reduction in heart failure incidence with hypertension treatment. A 70-year-old man whose systolic blood pressure is 140 mm Hg has about a 15% lifetime risk of heart failure. If his systolic pressure were 160 mm Hg, his lifetime heart failure risk would be more than 50%.13 If his systolic pressure were 149, his lifetime risk of heart failure would be between 15% and 50%, but the JNC 8 criteria do not recommend antihypertensive therapy.
EXERCISE SLOWS PROGRESSION TO FRAILTY
In the absence of a gold standard, frailty has been operationally defined as meeting three out of five phenotypic criteria: diminished grip strength, low energy, slow gait, low physical activity, and unintentional weight loss. A “prefrail” stage, in which one or two criteria are present, identifies a vulnerable subset at high risk of progression to frailty.
About 42% of older adults in the community are considered vulnerable, or prefrail, and about 11% are frail.14 Interventions at the prefrail stage may prevent progression to frailty, but it is rare, without intervention, for a person to re-achieve the stronger stage once diagnosed with frailty.
Pahor et al15 randomized 1,635 sedentary adults ages 70 to 89 who met the criteria of prefrailty to either a moderate-intensity exercise program (consisting of aerobic, resistance, and flexibility exercises for 150 minutes per week, performed in a center and at home) or to a health education program with workshops on topics relevant to older adults and upper-extremity stretching exercises. Adherence to the exercise program was verified by questionnaire and an accelerometer device. Participants were assessed every 6 months for an average of 2.6 years.
The primary outcome measure was the development of major mobility disability as defined by the loss of ability to walk 400 m without assistance (a cane was acceptable, but not a walker). The primary outcome occurred in 30.1% of those in the exercise group and 35.5% of the health education group (hazard ratio 0.82, P = .03). Those in the exercise group also had one third fewer falls. No differences were found in death rates. The number needed to treat was about 19 to prevent one person from developing major disability. Those most likely to benefit were those who walked slowly at baseline (< 1.8 mph), were more mobility-impaired, and were more cognitively healthy.
SLOWING DEMENTIA IS STILL AN ELUSIVE GOAL
Vitamin E modestly improves cognitive function but may have a cost
Before new information emerged in 2014 regarding vitamin E and dementia, the best data were from a 1997 study16 that randomized patients with moderate dementia to either daily vitamin E 2,000 IU, the monoamine oxidase inhibitor selegiline 10 mg, both, or placebo for 2 years. No benefit of treatment for cognitive function was found. However, after adjusting for the baseline Mini-Mental State Examination score, the investigators found that either treatment was associated with a delay of about 7 months in the primary outcome (death, institutionalization, loss of activities of daily living, or severe dementia).
Unfortunately, selegiline is often poorly tolerated, causing dyskinesia in more than 10% of patients, nausea in 20%, and confusion, hallucinations, and syncope. Although vitamin E is better tolerated, in high doses it can cause fatigue, headache, and bleeding, with increased risk of hemorrhagic stroke. Studies conflict as to whether it increases the risk of death from any cause.17,18
Dysken et al,19 in a study reported in 2014, randomized 613 patients with mild to moderate Alzheimer disease, all of whom were taking an acetylcholinesterase inhibitor, to either daily vitamin E 2,000 IU, memantine 20 mg, both, or placebo. The primary outcome measure was an activities of daily living score (0–78, higher being better), which included the ability to perform such tasks as dressing oneself. Each task was scored from 0 (totally dependent on help) to 4 (able to perform completely independently).
Scores fell in both groups over the mean 2.7 years of the study, but the decrease was slightly slower in the vitamin E group: 3 points less at the end of the study compared with placebo. The groups taking memantine, vitamin E, or both did not differ significantly from one another. No significant differences were found in the secondary outcome of cognitive, neuropsychiatric, functional, and caregiver measures.
Based on the 1997 study, vitamin E may defer the time to important clinical outcomes by 7.5 months over a 2-year period in patients with moderate dementia. Based on the 2014 study, vitamin E may preserve half an activity of daily living over 2.7 years in patients with mild to moderate dementia. On the other hand, high doses of vitamin E may increase the risk of bleeding and falling, and whether they increase the risk of death is unclear.
Antidepressants for behavior issues
Other common problems in patients with major neurocognitive disorders include disturbed perception, thought content, mood, and behavior, collectively called behavioral and psychological symptoms of dementia. No known nondrug intervention is consistently effective for these problems, and no drug approved by the US Food and Drug Administration (FDA), except for a fixed-dose combination of dextromethorphan and quinidine, addresses any specific symptom.
Porsteinsson et al20 randomized 186 patients with Alzheimer disease and agitation to a psychological intervention plus either the selective serotonin reuptake inhibitor citalopram (titrated from 10 to 30 mg per day based on response and tolerability) or placebo. Agitation was reduced with citalopram compared with placebo, based on the agitation subscale of the Neurobehavioral Rating Scale.
Of those taking citalopram, 40% were much or very much improved, compared with 26% of those taking placebo. These results are comparable to or better than those with antipsychotic drugs, which should be avoided for treating dementia-related psychosis in elderly patients because of black-box warnings.
No differences were found in activities of daily living. An interesting finding, not seen in other studies, is that the Mini-Mental Status Examination score declined by 1 point in the treatment group vs no change in the placebo group (P = .03).
Prolonged QTc was found in 12.5% in the citalopram group vs 4.3% in the placebo group (P = .01). The FDA issued a warning in 2012 of a dose-dependent effect of citalopram on QTc and recommended a maximum dose of 20 mg for those over age 60; for “poor metabolizers” of cytochrome P450 2C19 (CYP 2C19); and for those taking medications that inhibit CYP 2C19, including proton pump inhibitors, cimetidine, fluvoxamine, fluoxetine, indomethacin, ketoconazole, modafinil, and probenecid. The United Kingdom has extended this warning to escitalopram. Unfortunately, in the Porsteinsson study, nearly 80% of the treatment group received the 30-mg dose and only 15% received the 20-mg dose, which provided insufficient data for independent analysis.
Possibly, citalopram cannot be administered in a dosage sufficient to produce the benefits seen in the study. Using escitalopram may also be risky. Based on this study, it would be prudent to monitor QTc when using these drugs.
Dextromethorphan and quinidine
A fixed-dose combination of dextromethorphan and quinidine (Nuedexta) was recently approved by the FDA for treatment of pseudobulbar affect in individuals with stroke, traumatic brain injury, or dementia. Pseudobulbar affect has been defined as a condition of contextually inappropriate or exaggerated emotional expression that often occurs in adults with neurologic damage.
Using a 20/10-mg dose combination, a small 12-week noncomparative trial demonstrated measurable improvement in pseudobulbar symptoms after 30 days (as measured by the Center for Neurologic Study-Lability Scale).21 Though individuals enrolled in this trial appeared to tolerate this dose, additional trials still need to be conducted to more clearly determine its long-term safety and efficacy. It is not approved for dementia with agitation, but a phase 2 trial suggests a benefit compared with placebo in reducing agitation and caregiver burden.22
RISKS OF MILD COGNITIVE IMPAIRMENT
The spectrum of cognitive impairment ranges from mild cognitive impairment (MCI), in which deficits are evident on neuropsychological testing but the person maintains overall function, to the different stages of dementia (mild, moderate, and severe). MCI was documented in the Cardiovascular Health Study in 22% of adults 75 and older.23
Despite presenting with apparently normal function, elderly people with MCI have a higher risk of falls, rehospitalization, and delirium. Screening is not typically performed for MCI in primary care. No study has compared clinical outcomes after screening vs not screening for cognitive impairment (whether MCI or dementia), and the US Preventive Services Task Force maintains that there is insufficient evidence for screening.24
Unrecognized cognitive impairment affects discharge outcomes
Nazir et al,25 in a 1-year longitudinal study, compared 976 patients age 65 and older who upon admission to a public hospital were either diagnosed with cognitive impairment (defined as scoring 7 or less on the 10-question Short Portable Mental Status Questionnaire) or not. They found that 42.5% were cognitively impaired on admission. Overall, 36.5% of patients were discharged to a facility rather than home; those who were cognitively impaired, older, and sicker were more likely to be discharged to a facility.
Interestingly, among those discharged to a facility, patients with cognitive impairment were less likely to be subsequently rehospitalized or die within 30 days of hospital discharge than those without cognitive impairment. Whether this can be explained by differences in comorbidities between the groups was not explored. Those discharged home had similar rates of death and rehospitalization whether or not they were cognitively impaired.
Patel et al26 screened 720 older patients upon discharge after hospitalization for heart failure with the Mini-Cog (a 3-minute test that consists of recall of three words and the ability to draw a clock face). About a quarter of patients were diagnosed with cognitive impairment based on this test.
Among those discharged home (about two-thirds of the group overall), patients were much more likely to be rehospitalized or die within 30 days if they were cognitively impaired. Among those discharged to a facility, the rates between the two groups were similar for the first 20 days; after that, people in the cognitively impaired group were much more likely to die or be readmitted to a hospital.
Dodson et al,27 in a study of 282 hospitalized patients with heart failure (mean age 80), identified 47% as having cognitive impairment at the time of hospitalization based on a score of less than 25 on the Mini-Mental State Examination. Of those found to have mild cognitive impairment (score 21–24), only 11% had documentation of a cognitive deficit in the medical record, and 39% of those found to have moderate to severe cognitive impairment (score < 21) had documentation of it in the medical record. Those with unrecognized impairment were 1.5 times more likely to die or be rehospitalized within 6 months than those with documented impairment.
Do interventions help?
It is unclear whether developing specific interventions tailored to cognitive impairment improves outcomes.
Davis et al28 studied 125 patients hospitalized for heart failure who were identified as having mild cognitive impairment based on a Montreal Cognitive Assessment score of 17 to 25 (out of 30) points. Patients were randomly assigned to either a targeted self-care teaching intervention or usual discharge care. The intervention included education and customized instruction on self-care tasks such as managing symptoms, organizing medications, and measuring fluid and sodium intake.
Thirty days after discharge, the intervention group had greater knowledge about heart failure than the control group, but no significant difference was found in ability to care for themselves or in readmission rates.
Interventions that target the patient-caregiver dyad may have more success. A pilot project in Indiana29 that developed an integrative care model for older people with mild cognitive impairment, dementia, or depression that targeted patients as well as their caregivers found that compared with patients from area primary care clinics, their patients had lower rehospitalization rates within 30 days of discharge (11% vs 20%) and higher rates of achieving a hemoglobin A1c of less than 8% (78% vs 51%). Results of an expanded innovations demonstration project awarded by the Centers for Medicare and Medicaid Services are pending.
The following more recently published data show promise for prevention of dementia through nonpharmacologic interventions.
The FINGER trial30 (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) screened 2,654 Finnish individuals ages 60 to 77 using the Cardiovascular Risk Factors, Aging and Dementia risk tool, identifying 1,260 individuals with higher levels of cognitive impairment and randomizing them to a 2-year intervention consisting of exercise, cognitive training, and vascular risk monitoring (n = 631), or a control group provided with general health advice only (n = 629). Neuropsychological testing was conducted to measure differences between the groups, and at the end of the study, the mean Z-score difference in the total testing score between the intervention and control group was 0.22 (P = .30). This trial demonstrated that if cognitive impairment were identified, a multimodal intervention could improve or maintain cognitive function in at-risk elderly individuals.
Guidelines for the management of chronic disease are starting to recognize vulnerable elderly patients. The topics in this review, culled from recent studies and recommendations, were chosen because they may change geriatric care. They include the newest influenza and pneumococcal vaccines; recommendations for managing chronic heart failure, cholesterol, and blood pressure; preventing frailty; drug treatments for dementia; and the impact of cognitive impairment on health outcomes.
INFLUENZA VACCINATION: HIGH-DOSE SUPERIOR BUT COSTLIER
Three classes of influenza vaccines have been available for some time:
- The standard-dose, trivalent inactivated injectable vaccine (IIV3-SD) contains H1N1, H3N2, and influenza B strains and is approved for all ages over 6 months.
- The quadrivalent vaccine (available mostly for nasal administration) has the same strains as the trivalent vaccine plus a second, different influenza B strain. The inactivated vaccine is injectable for all persons over the age of 6 months; the live-attenuated vaccine is available as a nasal spray only for ages 2 through 49.
- The high-dose injectable vaccine (IIV3-HD) contains the same strains as the trivalent vaccine plus four times as much hemagglutinin—the influenza virus antigen that stimulates immunity.
Although IIV3-HD has been available since 2010, no clinical data existed until 2014 showing it to be superior to standard-dose vaccine.
DiazGranados et al1 randomized nearly 32,000 adults age 65 and older to receive either the standard-dose or the high-dose vaccine. The primary end point was laboratory-confirmed influenza caused by any influenza viral type or subtype, in association with a protocol-defined influenzalike illness.
The primary end point was reached in 1.4% of those with the high-dose vaccine and 1.9% of those with the standard-dose vaccine (relative efficacy 24.2%, 95% confidence interval 9.7–36.5). There was also a 26% reduction in respiratory illness regardless of laboratory confirmation. Mortality rates were identical and low (0.5%) in both groups. In those without laboratory confirmation of respiratory illness, there was a 26% lower rate of pneumonia but no statistical difference in rates of hospitalization, medication use, routine office visits, and emergency department visits.
These results can be interpreted as meaning that the high-dose vaccine prevented about a quarter of the laboratory-confirmed influenza cases that would have occurred with the standard-dose vaccine. However, due to the low rate of disease in those given the standard-dose vaccine, the number needed to treat to prevent one influenza infection was about 200 with the high-dose vs the standard-dose vaccine; to prevent one case of pneumonia, more than 270 would need to be treated.
The current price differential as well as the high number needed to treat to prevent one infection may discourage the use of the high-dose vaccine. Medicare Part B pays for one dose of either influenza vaccine per season. For patients who paid out of pocket, the 2014–2015 season cost at a typical pharmacy was about $32 for the standard-dose vaccine and $55 for the high-dose.
PNEUMOCOCCAL VACCINATION
Conjugate vaccine now recommended for seniors
The 23-valent polysaccharide vaccine (Pneumovax) has been available since 1983 and is recommended in the United States for all adults age 65 and over. A 13-valent pneumococcal diphtheria conjugate vaccine (Prevnar 13) has been available since 2010. Until recently, the conjugate vaccine was recommended for children; the only adults for whom it was recommended were those age 19 and over who either were immunocompromised or had a cochlear implant, asplenia, a cerebral spinal fluid leak, or renal failure.
The CAPITA trial2 (Community-Acquired Pneumonia Immunisation Trial in Adults) randomized nearly 85,000 people (most 65 and older, and some children) in the Netherlands to receive either the conjugate vaccine or placebo. It found a 46% reduction in community-acquired pneumonia (P = .0006), a 45% reduction in nonbacteremic nonvaccine-type community-acquired pneumonia (P = .0067), and a 75% reduction in vaccine-type invasive pneumococcal disease (P = .0005). Common side effects included pain, swelling at the injection site, limitation of arm movement, fatigue, headache, decreased appetite, chills, and rash.
Based on this one study, the Advisory Committee on Immunization Practices3 recommended that all adults 65 and older receive the conjugate vaccine.
The recommendations for the conjugate vaccine for all ages are complicated. Limited to those age 65 and older, current recommendations are:
- For those who have already received the polysaccharide vaccine: get the conjugated vaccine at least 1 year later
- For those who have never received the polysaccharide vaccine: get the conjugate vaccine now, then the polysaccharide vaccine 6 to 12 months later.
Whether the Netherlands findings fully apply to the United States is under question. At the time of the study, Dutch infants but not adults had received pneumonia conjugate vaccinations since 2002 with a high compliance rate. Unlike in the United States, the polysaccharide pneumococcal vaccine had not been routinely recommended in the Netherlands. There may have been some indirect immunity due to the “herd” effect, but no direct immunity. With a likely higher background immunity to pneumonia in the United States, the dramatic reduction in infection noted in the Netherlands may not be duplicated here.
For those without Medicare coverage, the 2014–2015 winter season cost at a pharmacy was about $95 for the polysaccharide vaccine and about $200 for the conjugate vaccine. As of February 2, 2015, the Centers for Medicare and Medicaid Services are implementing Medicare Part B coverage to allow initial pneumococcal vaccine for Medicare patients who never received a pneumococcal vaccine under Medicare Part B, and then a different, second pneumococcal vaccine, 1 year after the first vaccine was administered.
HEART FAILURE
Eplerenone’s new role in mild heart failure
Aldosterone antagonists have been recommended for moderate to severe heart failure (New York Heart Association [NYHA] classes III and IV) for some time. The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines also recommend them for mild heart failure (NYHA II).4
The EMPHASIS trial5 (Eplerenone in Patients With Systolic Heart Failure and Mild Symptoms) randomized 2,737 patients, median age 69, with NYHA class II heart failure and an ejection fraction of no more than 35% to receive the aldosterone antagonist eplerenone (up to 50 mg daily) or placebo, in addition to recommended therapy. The trial was stopped early, after a median follow-up of 21 months, when the treatment group was found to have a significantly lower risk of cardiovascular death or hospitalization for heart failure or for any cause.
Of note: hyperkalemia occurred in 11.8% of the eplerenone group vs 7.2% in the placebo group (P < .001). The high frequency of hyperkalemia in the placebo group may have been due to concomitant use of angiotensin-converting enzyme (ACE) inhibitors.
Sodium restriction reasonable
Although sodium restriction has been standard practice in heart failure for decades, restricting sodium in the elderly was given only a IIa (“reasonable”) classification, based on level C (very limited) evidence.4
Strong evidence exists that middle-aged and young older adults with heart failure (with preserved or reduced ejection fraction) should reduce their sodium intake by about 1 g per day or aim for a mean 24-hour urinary sodium excretion of about 2.3 g per day. However, little evidence exists to support a specific long-term target intake, and no evidence exists for “old-old” patients (loosely defined as older than 75 or 80).
Caution with digoxin
Use of digoxin has been recommended in patients with heart failure with reduced ejection fraction to reduce hospitalizations,4 but more recent publications have raised questions regarding its safety and efficacy.
Freeman et al,6 in a prospective study, followed 2,891 patients with newly diagnosed systolic heart failure over 2.5 years, of whom 529 were prescribed digoxin. The digoxin group had a higher rate of death (14.2 vs 11.2 per 100 patient-years) and heart failure-related hospitalization (28.2 vs 24.4 per 100 person-years).
The study was unable to determine if the digoxin level influenced the results, since about 30% of patients had no digoxin level drawn, and an additional 27% had only one level drawn during the study. For those with measured blood levels, the mean digoxin level for men was 0.83 ng/mL and 1.12 ng/mL for women. Risks and benefits of this medication should be weighed carefully.
Simultaneous interventions beneficial
The following evidence-based interventions are recommended for patients with heart failure with reduced ejection fraction:
- Heart failure education
- A beta-blocker
- An ACE inhibitor
- An aldosterone antagonist for NYHA class II–IV symptoms
- Anticoagulation for atrial fibrillation in patients with added risks (eg, hypertension, diabetes, prior transient ischemic attack or cerebrovascular accident, age at least 75)
- An implantable cardioverter-defibrillator and cardiac resynchronization therapy for select patients with symptoms, increased QRS duration, and left bundle branch block.
Fonarow et al7 studied these interventions in an analysis of a prospective study of outpatients with diagnosed heart failure or myocardial infarction and reduced left ventricular ejection fraction. Their nested case-control study compared 1,376 patients, mean age 72, who had died within 24 months and 2,752 propensity-matched controls who survived to 24 months. The survival rate was 37% higher with two simultaneous interventions than with one, and 70% higher with four simultaneous interventions than with one. Benefits plateaued with four to five interventions.
LIPID-LOWERING THERAPY FOR SENIORS
The 2013 ACC/AHA cholesterol guideline8 included new recommendations specifically relevant to the elderly. It advocates using a new cardiovascular disease risk calculator that provides an estimate of 10-year risk of atherosclerotic cardiovascular disease (ASCVD), based on data from multiple community-based populations and applicable to African American and non-Hispanic white men and women ages 40 through 79. Primary prevention with a statin is encouraged for those with a 10-year risk of 7.5% or higher. The tool generated controversy from the moment it was announced and may overestimate ASCVD risk by 67% in women and 86% in men.9
Emphasis on tolerability
The guideline focuses on statins as the main treatment and de-emphasizes the adjunctive use of other drugs to further lower lipids such as niacin, ezetimibe, and fenofibrate.
Statin tolerability is now stressed rather than specific lipid level targets. The guideline recommends reassessing statin choice and intensity according to pain, tenderness, stiffness, cramping, weakness, and fatigue (class IIa recommendation [“reasonable”], level of evidence B [“limited”]). Also recommended is reassessment of statin choice and intensity for patients older than 75 or for those taking multiple medications, drugs that alter metabolism, and conditions requiring complex medications (class IIa, level of evidence C [“very limited”]). For patients with confusion, statin and nonstatin causes should be considered as the source of the problem (class IIb [“consider”], level of evidence C).
Initiating high-intensity statin therapy is not recommended after age 75. However, continuing such treatment is reasonable for patients already receiving and tolerating the therapy for an appropriate indication. Initiation of moderate-intensity statin therapy in this age group is recommended for those with either clinical atherosclerotic cardiovascular disease or a low-density lipoprotein cholesterol (LDL-C) level of at least 190 mg/dL.
No specific guidance is provided for patients older than age 75 without ASCVD, with LDL-C less than 190 mg/dL, or with diabetes. In these groups, statin therapy may be initiated, continued, or intensified (class IIb, level of evidence C).
HYPERTENSION: LESS AGGRESSIVE GOALS FOR ELDERLY
The eighth Joint National Committee (JNC 8)10 made nine recommendations for managing high blood pressure, only one of which specifically addresses people 60 and older.
Drug therapy should be initiated if the blood pressure is 150/90 mm Hg or higher, and the blood pressure should be treated to less than that level (grade A recommendation, ie, strong). If treated systolic blood pressure is less than 140 mm Hg without adverse effects, it should be sustained (grade E recommendation, ie, based on expert opinion).
Tension between guidelines
The higher threshold for hypertension treatment and the lower threshold for statin therapy create tension between guidelines, and between guidelines and epidemiologic data.
For example, in a 67-year-old woman without diabetes and with a favorable lipid profile (eg, total cholesterol 130 mg/dL, high-density lipoprotein cholesterol 55 mg/dL), the ACC/AHA ASCVD risk calculator predicts a 10-year risk of less than 7.5% if her systolic blood pressure is 147 mm Hg. If the patient’s blood pressure were 148 or 149 mm Hg and all the other variables were the same, the JNC 8 would not recommend treatment with antihypertensive medication, but the ACC/AHA guidelines would recommend preventive statin therapy.
Another example is the relationship between heart failure and antihypertensive drugs. Multiple studies11,12 demonstrate a reduction in heart failure incidence with hypertension treatment. A 70-year-old man whose systolic blood pressure is 140 mm Hg has about a 15% lifetime risk of heart failure. If his systolic pressure were 160 mm Hg, his lifetime heart failure risk would be more than 50%.13 If his systolic pressure were 149, his lifetime risk of heart failure would be between 15% and 50%, but the JNC 8 criteria do not recommend antihypertensive therapy.
EXERCISE SLOWS PROGRESSION TO FRAILTY
In the absence of a gold standard, frailty has been operationally defined as meeting three out of five phenotypic criteria: diminished grip strength, low energy, slow gait, low physical activity, and unintentional weight loss. A “prefrail” stage, in which one or two criteria are present, identifies a vulnerable subset at high risk of progression to frailty.
About 42% of older adults in the community are considered vulnerable, or prefrail, and about 11% are frail.14 Interventions at the prefrail stage may prevent progression to frailty, but it is rare, without intervention, for a person to re-achieve the stronger stage once diagnosed with frailty.
Pahor et al15 randomized 1,635 sedentary adults ages 70 to 89 who met the criteria of prefrailty to either a moderate-intensity exercise program (consisting of aerobic, resistance, and flexibility exercises for 150 minutes per week, performed in a center and at home) or to a health education program with workshops on topics relevant to older adults and upper-extremity stretching exercises. Adherence to the exercise program was verified by questionnaire and an accelerometer device. Participants were assessed every 6 months for an average of 2.6 years.
The primary outcome measure was the development of major mobility disability as defined by the loss of ability to walk 400 m without assistance (a cane was acceptable, but not a walker). The primary outcome occurred in 30.1% of those in the exercise group and 35.5% of the health education group (hazard ratio 0.82, P = .03). Those in the exercise group also had one third fewer falls. No differences were found in death rates. The number needed to treat was about 19 to prevent one person from developing major disability. Those most likely to benefit were those who walked slowly at baseline (< 1.8 mph), were more mobility-impaired, and were more cognitively healthy.
SLOWING DEMENTIA IS STILL AN ELUSIVE GOAL
Vitamin E modestly improves cognitive function but may have a cost
Before new information emerged in 2014 regarding vitamin E and dementia, the best data were from a 1997 study16 that randomized patients with moderate dementia to either daily vitamin E 2,000 IU, the monoamine oxidase inhibitor selegiline 10 mg, both, or placebo for 2 years. No benefit of treatment for cognitive function was found. However, after adjusting for the baseline Mini-Mental State Examination score, the investigators found that either treatment was associated with a delay of about 7 months in the primary outcome (death, institutionalization, loss of activities of daily living, or severe dementia).
Unfortunately, selegiline is often poorly tolerated, causing dyskinesia in more than 10% of patients, nausea in 20%, and confusion, hallucinations, and syncope. Although vitamin E is better tolerated, in high doses it can cause fatigue, headache, and bleeding, with increased risk of hemorrhagic stroke. Studies conflict as to whether it increases the risk of death from any cause.17,18
Dysken et al,19 in a study reported in 2014, randomized 613 patients with mild to moderate Alzheimer disease, all of whom were taking an acetylcholinesterase inhibitor, to either daily vitamin E 2,000 IU, memantine 20 mg, both, or placebo. The primary outcome measure was an activities of daily living score (0–78, higher being better), which included the ability to perform such tasks as dressing oneself. Each task was scored from 0 (totally dependent on help) to 4 (able to perform completely independently).
Scores fell in both groups over the mean 2.7 years of the study, but the decrease was slightly slower in the vitamin E group: 3 points less at the end of the study compared with placebo. The groups taking memantine, vitamin E, or both did not differ significantly from one another. No significant differences were found in the secondary outcome of cognitive, neuropsychiatric, functional, and caregiver measures.
Based on the 1997 study, vitamin E may defer the time to important clinical outcomes by 7.5 months over a 2-year period in patients with moderate dementia. Based on the 2014 study, vitamin E may preserve half an activity of daily living over 2.7 years in patients with mild to moderate dementia. On the other hand, high doses of vitamin E may increase the risk of bleeding and falling, and whether they increase the risk of death is unclear.
Antidepressants for behavior issues
Other common problems in patients with major neurocognitive disorders include disturbed perception, thought content, mood, and behavior, collectively called behavioral and psychological symptoms of dementia. No known nondrug intervention is consistently effective for these problems, and no drug approved by the US Food and Drug Administration (FDA), except for a fixed-dose combination of dextromethorphan and quinidine, addresses any specific symptom.
Porsteinsson et al20 randomized 186 patients with Alzheimer disease and agitation to a psychological intervention plus either the selective serotonin reuptake inhibitor citalopram (titrated from 10 to 30 mg per day based on response and tolerability) or placebo. Agitation was reduced with citalopram compared with placebo, based on the agitation subscale of the Neurobehavioral Rating Scale.
Of those taking citalopram, 40% were much or very much improved, compared with 26% of those taking placebo. These results are comparable to or better than those with antipsychotic drugs, which should be avoided for treating dementia-related psychosis in elderly patients because of black-box warnings.
No differences were found in activities of daily living. An interesting finding, not seen in other studies, is that the Mini-Mental Status Examination score declined by 1 point in the treatment group vs no change in the placebo group (P = .03).
Prolonged QTc was found in 12.5% in the citalopram group vs 4.3% in the placebo group (P = .01). The FDA issued a warning in 2012 of a dose-dependent effect of citalopram on QTc and recommended a maximum dose of 20 mg for those over age 60; for “poor metabolizers” of cytochrome P450 2C19 (CYP 2C19); and for those taking medications that inhibit CYP 2C19, including proton pump inhibitors, cimetidine, fluvoxamine, fluoxetine, indomethacin, ketoconazole, modafinil, and probenecid. The United Kingdom has extended this warning to escitalopram. Unfortunately, in the Porsteinsson study, nearly 80% of the treatment group received the 30-mg dose and only 15% received the 20-mg dose, which provided insufficient data for independent analysis.
Possibly, citalopram cannot be administered in a dosage sufficient to produce the benefits seen in the study. Using escitalopram may also be risky. Based on this study, it would be prudent to monitor QTc when using these drugs.
Dextromethorphan and quinidine
A fixed-dose combination of dextromethorphan and quinidine (Nuedexta) was recently approved by the FDA for treatment of pseudobulbar affect in individuals with stroke, traumatic brain injury, or dementia. Pseudobulbar affect has been defined as a condition of contextually inappropriate or exaggerated emotional expression that often occurs in adults with neurologic damage.
Using a 20/10-mg dose combination, a small 12-week noncomparative trial demonstrated measurable improvement in pseudobulbar symptoms after 30 days (as measured by the Center for Neurologic Study-Lability Scale).21 Though individuals enrolled in this trial appeared to tolerate this dose, additional trials still need to be conducted to more clearly determine its long-term safety and efficacy. It is not approved for dementia with agitation, but a phase 2 trial suggests a benefit compared with placebo in reducing agitation and caregiver burden.22
RISKS OF MILD COGNITIVE IMPAIRMENT
The spectrum of cognitive impairment ranges from mild cognitive impairment (MCI), in which deficits are evident on neuropsychological testing but the person maintains overall function, to the different stages of dementia (mild, moderate, and severe). MCI was documented in the Cardiovascular Health Study in 22% of adults 75 and older.23
Despite presenting with apparently normal function, elderly people with MCI have a higher risk of falls, rehospitalization, and delirium. Screening is not typically performed for MCI in primary care. No study has compared clinical outcomes after screening vs not screening for cognitive impairment (whether MCI or dementia), and the US Preventive Services Task Force maintains that there is insufficient evidence for screening.24
Unrecognized cognitive impairment affects discharge outcomes
Nazir et al,25 in a 1-year longitudinal study, compared 976 patients age 65 and older who upon admission to a public hospital were either diagnosed with cognitive impairment (defined as scoring 7 or less on the 10-question Short Portable Mental Status Questionnaire) or not. They found that 42.5% were cognitively impaired on admission. Overall, 36.5% of patients were discharged to a facility rather than home; those who were cognitively impaired, older, and sicker were more likely to be discharged to a facility.
Interestingly, among those discharged to a facility, patients with cognitive impairment were less likely to be subsequently rehospitalized or die within 30 days of hospital discharge than those without cognitive impairment. Whether this can be explained by differences in comorbidities between the groups was not explored. Those discharged home had similar rates of death and rehospitalization whether or not they were cognitively impaired.
Patel et al26 screened 720 older patients upon discharge after hospitalization for heart failure with the Mini-Cog (a 3-minute test that consists of recall of three words and the ability to draw a clock face). About a quarter of patients were diagnosed with cognitive impairment based on this test.
Among those discharged home (about two-thirds of the group overall), patients were much more likely to be rehospitalized or die within 30 days if they were cognitively impaired. Among those discharged to a facility, the rates between the two groups were similar for the first 20 days; after that, people in the cognitively impaired group were much more likely to die or be readmitted to a hospital.
Dodson et al,27 in a study of 282 hospitalized patients with heart failure (mean age 80), identified 47% as having cognitive impairment at the time of hospitalization based on a score of less than 25 on the Mini-Mental State Examination. Of those found to have mild cognitive impairment (score 21–24), only 11% had documentation of a cognitive deficit in the medical record, and 39% of those found to have moderate to severe cognitive impairment (score < 21) had documentation of it in the medical record. Those with unrecognized impairment were 1.5 times more likely to die or be rehospitalized within 6 months than those with documented impairment.
Do interventions help?
It is unclear whether developing specific interventions tailored to cognitive impairment improves outcomes.
Davis et al28 studied 125 patients hospitalized for heart failure who were identified as having mild cognitive impairment based on a Montreal Cognitive Assessment score of 17 to 25 (out of 30) points. Patients were randomly assigned to either a targeted self-care teaching intervention or usual discharge care. The intervention included education and customized instruction on self-care tasks such as managing symptoms, organizing medications, and measuring fluid and sodium intake.
Thirty days after discharge, the intervention group had greater knowledge about heart failure than the control group, but no significant difference was found in ability to care for themselves or in readmission rates.
Interventions that target the patient-caregiver dyad may have more success. A pilot project in Indiana29 that developed an integrative care model for older people with mild cognitive impairment, dementia, or depression that targeted patients as well as their caregivers found that compared with patients from area primary care clinics, their patients had lower rehospitalization rates within 30 days of discharge (11% vs 20%) and higher rates of achieving a hemoglobin A1c of less than 8% (78% vs 51%). Results of an expanded innovations demonstration project awarded by the Centers for Medicare and Medicaid Services are pending.
The following more recently published data show promise for prevention of dementia through nonpharmacologic interventions.
The FINGER trial30 (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) screened 2,654 Finnish individuals ages 60 to 77 using the Cardiovascular Risk Factors, Aging and Dementia risk tool, identifying 1,260 individuals with higher levels of cognitive impairment and randomizing them to a 2-year intervention consisting of exercise, cognitive training, and vascular risk monitoring (n = 631), or a control group provided with general health advice only (n = 629). Neuropsychological testing was conducted to measure differences between the groups, and at the end of the study, the mean Z-score difference in the total testing score between the intervention and control group was 0.22 (P = .30). This trial demonstrated that if cognitive impairment were identified, a multimodal intervention could improve or maintain cognitive function in at-risk elderly individuals.
- DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–645.
- Bonten M, Bolkenbaas M, Huijts S, et al. Community acquired pneumonia immunisation trial in adults (CAPITA) (abstract). Pneumonia 2014; 3:95. Presented at 9th International Symposium on Pneumococci and Pneumococcal Diseases, 2014. Abstract 0541.
- Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2014; 63:822–825.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF-AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Freeman JV, Yang J, Sung SH, Hlatky MA, Go AS. Effectiveness and safety of digoxin among contemporary adults with incident systolic heart failure. Circ Cardiovasc Qual Outcomes 2013; 6:525–533.
- Fonarow GC, Albert NM, Curtis AB, et al. Incremental reduction in risk of death associated with use of guideline-recommended therapies in patients with heart failure: a nested case-control analysis of IMPROVE-HF. J Am Heart Assoc 2012; 1:16–26.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S1–S45.
- DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015; 162:266–275.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Kostis JB, Davis BR, Cutler J, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 1997; 278:212–216.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Lloyd-Jones DM, Larson MG, Leip EP, et al; Framingham Heart Study. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002; 106;3068–3072.
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60:1487–1492.
- Pahor M, Guralnik JM, Ambrosius WT, et al; LIFE study investigators. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014; 311:2387–2396.
- Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med 1997; 336:1216–1222.
- Miller ER 3rd, Pastor-Barriuso R, Dala D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142:37–46.
- Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci 2011; 4:158–170.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014; 311:682–691.
- Yang LP, Deeks ED. Dextromethorphan/quinidine: a review of its use in adults with pseudobulbar affect. Drugs 2015; 75:83–90.
- Cummings J, Lyketsos C, Tariot P, et al. Dextromethorphan/quinidine (AVP-923) efficacy and safety for treatment of agitation in persons with Alzheimer’s disease: results from a phase 2 study (NCT01584440) (S16.007). Neurology 2015; 84:S16.007.
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003; 60:1385–1389.
- Moyer VA; US Preventive Services Task Force. Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:791–797.
- Nazir A, LaMantia M, Chodosh J, et al. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc 2013; 61:1958–1963.
- Patel A, Parikh R, Howell E, Hsich E, Gorodeski E. Mini-Cog performance: a novel marker of risk among patients hospitalized for heart failure. J Am Coll Cardiol 4014; 63:A755.
- Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med 2013; 126:120–126.
- Davis KK, Mintzer M, Dennison Himmelfarb CR, Hayat MJ, Rotman S, Allen J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. Eur J Heart Fail 2012; 14:1041–1049.
- Boustani MA, Sachs GA, Alder CA, et al. Implementing innovative models of dementia care: The Healthy Aging Brain Center. Aging Ment Health 2011; 15:13–22.
- Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385:2255–2263.
- DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–645.
- Bonten M, Bolkenbaas M, Huijts S, et al. Community acquired pneumonia immunisation trial in adults (CAPITA) (abstract). Pneumonia 2014; 3:95. Presented at 9th International Symposium on Pneumococci and Pneumococcal Diseases, 2014. Abstract 0541.
- Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2014; 63:822–825.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF-AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Freeman JV, Yang J, Sung SH, Hlatky MA, Go AS. Effectiveness and safety of digoxin among contemporary adults with incident systolic heart failure. Circ Cardiovasc Qual Outcomes 2013; 6:525–533.
- Fonarow GC, Albert NM, Curtis AB, et al. Incremental reduction in risk of death associated with use of guideline-recommended therapies in patients with heart failure: a nested case-control analysis of IMPROVE-HF. J Am Heart Assoc 2012; 1:16–26.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S1–S45.
- DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015; 162:266–275.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Kostis JB, Davis BR, Cutler J, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 1997; 278:212–216.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Lloyd-Jones DM, Larson MG, Leip EP, et al; Framingham Heart Study. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002; 106;3068–3072.
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60:1487–1492.
- Pahor M, Guralnik JM, Ambrosius WT, et al; LIFE study investigators. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014; 311:2387–2396.
- Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med 1997; 336:1216–1222.
- Miller ER 3rd, Pastor-Barriuso R, Dala D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142:37–46.
- Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci 2011; 4:158–170.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014; 311:682–691.
- Yang LP, Deeks ED. Dextromethorphan/quinidine: a review of its use in adults with pseudobulbar affect. Drugs 2015; 75:83–90.
- Cummings J, Lyketsos C, Tariot P, et al. Dextromethorphan/quinidine (AVP-923) efficacy and safety for treatment of agitation in persons with Alzheimer’s disease: results from a phase 2 study (NCT01584440) (S16.007). Neurology 2015; 84:S16.007.
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003; 60:1385–1389.
- Moyer VA; US Preventive Services Task Force. Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:791–797.
- Nazir A, LaMantia M, Chodosh J, et al. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc 2013; 61:1958–1963.
- Patel A, Parikh R, Howell E, Hsich E, Gorodeski E. Mini-Cog performance: a novel marker of risk among patients hospitalized for heart failure. J Am Coll Cardiol 4014; 63:A755.
- Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med 2013; 126:120–126.
- Davis KK, Mintzer M, Dennison Himmelfarb CR, Hayat MJ, Rotman S, Allen J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. Eur J Heart Fail 2012; 14:1041–1049.
- Boustani MA, Sachs GA, Alder CA, et al. Implementing innovative models of dementia care: The Healthy Aging Brain Center. Aging Ment Health 2011; 15:13–22.
- Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385:2255–2263.
KEY POINTS
- Vaccination costs will increase—with unclear added value—with new guidelines for influenza and pneumococcal vaccines.
- Multiple simultaneous interventions for heart failure have additive value. These are education, a beta-blocker, an angiotensin-converting enzyme inhibitor, and, in some, an aldosterone antagonist, anticoagulation for atrial fibrillation, and an implantable cardioverter-defibrillator or cardiac resynchronization therapy.
- Statin therapy should be intensified with an eye to goals of care and tolerability rather than a specific lipid goal.
- Exercise improves physical and mental health in all, including the elderly.
- Dementia still has no magic bullet. Selective serotonin reuptake inhibitors might help behavior issues, and vitamin E might bring modest cognitive improvement but with possible risk.
Dermatology update: The dawn of targeted treatment
New targeted therapies are changing the way patients with advanced dermatologic diseases are treated. Innovative molecular biology techniques developed as far back as the 1970s have engendered tremendous insight into the cellular and molecular pathogenesis of numerous diseases. Novel medications based on these insights are now bearing fruit, as directed biologic therapies that are revolutionizing clinical practice are increasingly becoming available.
This article reviews advances in targeted therapies for advanced basal cell carcinoma, psoriasis, and metastatic melanoma.
TARGETED THERAPY FOR BASAL CELL CARCINOMA
Case 1. A 56-year-old man presents with a progressively enlarging leg ulcer. Although it has been treated empirically for years as a venous stasis ulcer, biopsy reveals that it is basal cell carcinoma. Imaging shows muscle and tendon invasion, making surgical intervention short of amputation challenging (Figure 1). What are his options?
Basal cell carcinoma is the most common cancer in humans, accounting for 25% of all cancers and more than 2 million cases in the United States every year. In most cases, surgical excision is curative, but a subset of patients have inoperable, locally advanced, or metastatic disease that drastically limits treatment options. The median survival in metastatic basal cell carcinoma is 24 months, and conventional chemotherapy has not been shown to improve the prognosis.1,2
In addition to the burden of sporadic basal cell carcinoma, patients with the rare autosomal-dominant genetic disorder basal cell nevus syndrome (Gorlin syndrome) develop multiple basal cell lesions over their lifetime. The syndrome may also involve abnormalities of the skeletal system, genitourinary tract, and central nervous system, including development of medulloblastoma.
In Gorlin syndrome, basal cell carcinomas occur often and early; about half of white patients with the syndrome develop their first lesions by age 21, and 90% by age 35. The lesions occur in multiple numbers and can develop anywhere on the body, including on non–sun-exposed areas. Patients who have Gorlin syndrome need meticulous monitoring every 2 to 3 months so that basal cell lesions can be recognized early and treated before they become locally advanced. Keeping up with the numerous medical appointments and invasive treatments can be physically and mentally taxing for patients.
Specific pathway and mutations identified
In 1996, Gorlin syndrome was found to be caused by mutations of the human homolog of the PATCHED gene, which codes for a receptor in the “hedgehog” pathway.3 Two years later, the same mutations were found to be involved in many sporadic basal cell carcinomas, and we now believe that at least 85% of basal cell carcinomas involve abnormal activation of hedgehog pathway signaling.4,5
Vismodegib developed as targeted therapy
In 2009, Robarge et al6 described a potent inhibitor of the hedgehog pathway that was later optimized for potency and desirable pharmacologic traits, resulting in the drug vismodegib.7,8
Two phase 2 multicenter clinical trials9,10 of vismodegib were published in 2012. In the first, which was not randomized,9 33 patients with metastatic basal cell carcinoma and 63 patients with locally advanced disease were treated with vismodegib. Of those with metastatic disease, 30% achieved an objective response. Of those with locally advanced disease, 43% achieved an objective response and 21% achieved a complete response.
In the second trial,10 patients with Gorlin syndrome were randomized to either vismodegib (26 patients) or placebo (16 patients). After 8 months, the vismodegib group had developed significantly fewer new surgically eligible tumors (2 vs 29 per year), their tumors were smaller (change from baseline of the sum of the longest diameters –65% vs –11%), and they needed fewer surgeries (mean 0.31 vs 4.4 per patient). No tumors progressed in the treatment group. Results in some patients were dramatic, with complete healing of large ulcerative tumors. The trial was ended early in view of significant efficacy in the treatment group.
Based on these trials, the US Food and Drug Administration (FDA) approved vismodegib for treating metastatic and locally advanced basal cell carcinoma.
Resistance and adverse effects common
Unfortunately, vismodegib has significant drawbacks. About 20% of patients develop resistance, with tumors recurring after several months of therapy.11 Adverse effects most commonly reported were muscle spasms (68%), alopecia (63%), taste distortion (51%), weight loss (46%), and fatigue (36%). Although these effects were considered mild or moderate, they tended to persist, and almost every patient developed at least one. In the nonrandomized trial,9 more than 25% of patients discontinued treatment because of adverse effects, and more than half of patients did the same in the basal cell nevus syndrome trial.10
New uses may reduce shortcomings
Studies are under way to determine how best to use vismodegib.
One possibility is to use the drug for a few months to shrink tumors to the point that they become eligible for surgery. This is especially important for high-risk tumors, such as those near the eye or other vital structures. In 11 patients, Ally et al12 found that the surgical defect area was reduced by 27% from baseline after 4 months of treatment with vismodegib, allowing for curative surgery in some.
Another option is to combine vismodegib with other agents—either new ones on the horizon or existing nonspecific medications. For example, the antifungal itraconazole has been shown to inhibit hedgehog signaling and perhaps could be combined with vismodegib to increase response and reduce resistance.
Finally, a topical or intralesional form of vismodegib would be useful not only to reduce systemic toxicity, but also to increase efficacy when combined with other topical or systemic medications.
TARGETED THERAPY FOR PSORIASIS VULGARIS
Case 2. A 28-year-old woman presents with worsening psoriasis. About 35% of her body surface is involved, including the palms and soles, making it difficult for her to perform activities of daily living (Figure 2). What are her options?
Psoriasis is a chronic immune-mediated disease that affects up to 3% of people worldwide. In its moderate to severe forms, we recognize psoriasis as a systemic inflammatory disease that may adversely affect organ systems other than the skin. Commonly associated comorbid diseases include inflammatory (psoriatic) arthritis, cardiovascular disease, malignancies (eg, lymphoma), and inflammatory bowel disease. In addition, patients are well known to have significantly impaired quality of life because of low self-esteem, stigmatization affecting their social and work relationships, and, in up to 60%, clinical depression.13,14 The onset of psoriatic arthritis, particularly of erosive disease, is an important decision point for starting aggressive treatment, as joint destruction is irreversible.
Early targeted therapy aimed at TNF alpha, IL-12, and IL-23
Histologically, psoriasis involves thickening of the epidermis caused by hyperproliferation of keratinocytes. Based on this, prior to the 1980s, the dominant hypothesis concerning its pathogenesis was that it was caused by an inherent defect of keratinocytes. In the 1980s and 1990s, however, molecular research revealed that psoriasis was an immune-mediated disease caused by immunologic dysregulation predominantly involving T-helper 1 (Th-1) cells, with the inflammatory cytokines tumor necrosis factor (TNF) alpha, interferon gamma, interleukin (IL) 12, and IL-23 playing prominent roles.15 These findings led to the development and FDA approval of the first effective, targeted, psoriasis treatments, TNF-alpha inhibitors and the IL-12/23 inhibitor ustekinumab.
Etanercept, the first TNF-alpha inhibitor to become available, was approved in 2004 for moderate to severe psoriasis. In 2008, the IL-12/23 inhibitor ustekinumab was approved for the same indication. These drugs are efficacious, are generally safe, and have revolutionized the treatment of psoriasis and psoriatic arthritis, and they are now prescribed on a daily basis.16,17
In the clinical trials that led to approval of these drugs, the main outcome measure was the Psoriasis Area and Severity Index (PASI), a clinical scoring tool that assesses clinical aspects of psoriatic disease including body surface area involvement, degree of thickness, erythema, and scaling of psoriatic plaques. PASI scores range from 0 (no psoriasis) to 72 (most severe psoriasis). Achieving “PASI 75” indicates at least 75% improvement from the baseline score and represents the most common primary outcome measure in clinical trials assessing efficacy of new treatments. Up to 80% of patients who received currently available TNF-alpha inhibitors and ustekinumab in pivotal clinical trials achieved PASI 75 when assessed at 12 to 16 weeks after starting treatment. A moderate percentage of patients (19%–57%, depending on the trial) achieved 90% improvement (PASI 90), and a minority (up to 18%) achieved PASI 100, indicating complete clearing of their psoriasis.18–22
Newly developed therapies target IL-17A
In the mid-2000s, Th-17 cells were discovered, a new lineage of T cells distinct from Th-1 and Th-2 cells. Th-17 cells are characterized by their production of IL-17, a pro-inflammatory cytokine with six family members (IL-17A through IL-17F). Over the next few years, experiments revealed that Th-17 cells and IL-17A play key roles in psoriasis immunologic dysregulation.15 These findings led to a paradigm shift in hypotheses concerning psoriasis pathogenesis, with Th-17 cells and IL-17 replacing Th-1 cells and associated cytokines as dominant mediators of tissue damage.
Additionally, these findings led to new ideas for treatment. Three monoclonal antibodies that target IL-17 inhibition are currently under investigation. Secukinumab and ixekizumab bind to IL-17A and inhibit it from downstream signaling, whereas brodalumab binds to the IL-17A receptor, blocking all six IL-17 cytokines (IL-17A to IL-17F).23
Clinical trials of IL-17 inhibitors show excellent skin improvement
Secukinumab. In 2014, the results of two phase 3 trials of secukinumab were published.24
In the Efficacy and Safety of Subcutaneous Secukinumab for Moderate to Severe Chronic Plaque-type Psoriasis for up to 1 Year trial,24 patients were given either secukinumab 300 mg or 150 mg subcutaneously at defined time points; 82% and 72%, respectively, attained PASI 75 at 12 weeks.
Similar results were seen in the Safety and Efficacy of Secukinumab Compared to Etanercept in Subjects With Moderate to Severe, Chronic Plaque-Type Psoriasis study,24 in which PASI 75 was achieved by 77% of patients receiving secukinumab 300 mg, 67% of those receiving secukinumab 150 mg, and only 44% of those receiving etanercept 50 mg twice weekly at 12 weeks. Rates of infection with secukinumab and etanercept were similar.
The most striking results of these trials were that more than half of patients receiving the 300-mg dose achieved at least 90% improvement in their PASI score (PASI 90) by week 12, and in more than a quarter of patients the psoriasis completely cleared (PASI 100). These results were dramatically better than for etanercept (PASI 90 21%; PASI 100 4%).
Additionally, secukinumab worked fast. The median time to PASI 50 with secukinumab 300 mg was less than half that seen with etanercept (3 weeks vs 7 weeks).
Ixekizumab. In 2012, a phase 2 trial evaluated subcutaneous injections of ixekizumab in dosages ranging from 10 to 150 mg at defined intervals for 16 weeks.25 Of those receiving the highest dosage, 82% attained PASI 75 at 12 weeks, on par with what is noted in patients receiving TNF-alpha inhibitors and IL-12/23 inhibitors. Remarkably, however, almost three-quarters of patients (71%) achieved PASI 90, and 39% achieved PASI 100. Improvement in psoriasis was apparent as early as 1 week after injection.
Brodalumab. A 2012 phase 2 trial of various dosages of the IL-17 receptor inhibitor brodalumab26 also showed excellent PASI 75 achievement with the highest dosage (82%). Astonishingly, though, PASI 90 was achieved by 75% of patients, and PASI 100 by 62%.
Overall, although the percentages of patients achieving PASI 75 with the new IL-17 inhibitor drugs are comparable to those seen with TNF-alpha inhibitors and IL-12/23 inhibitors, the extraordinarily high percentages of patients who achieved PASI 90 and PASI 100 are unprecedented.18–22
Arthritis improvement not shown
Where the IL-17 inhibitors eventually settle within algorithms of psoriasis treatment largely depends on their efficacy in treating psoriatic arthritis compared with TNF-alpha inhibitors and IL-12/23 inhibitors. Joint inflammation is typically evaluated with the American College of Rheumatology (ACR) scoring tool, which in simple terms can be thought of as analogous to the PASI scoring tool for the skin. Although the ACR scoring tool was developed to assess joint inflammation in clinical trials for patients with rheumatoid arthritis, it is commonly used to assess improvement of psoriatic arthritis in clinical trials. The ACR tool involves assessing and scoring the number of swollen and tender joints, but also incorporates serologic assessment of acute-phase reactants (erythrocyte sedimentation rate or C-reactive protein level), patient and physician global assessment, pain, and function. ACR 20 implies roughly a 20% improvement in these criteria, whereas ACR 50 indicates 50% improvement, and so on.
Two phase 2 trials of IL-17 inhibitors for psoriatic arthritis have been published, one with secukinumab27 and one with brodalumab.28 Neither had impressive improvement in the ACR score vs TNF inhibitors—39% for ACR 20 at week 12 and less than 10% for ACR 70. Clinical trial design may have played a role, and phase 3 trials are under way for all three IL-17 inhibitors.
Adverse effects of IL-17 inhibitors
For the most part, adverse effects reported with the IL-17 inhibitors have been mild and similar to those reported with available biologic treatments for psoriasis. Adverse effects most commonly reported have been nasopharyngitis, upper respiratory infection, arthralgia, and mild injection-site reactions. In the future, attention will be paid to the rate of infections known to be associated with IL-17, mainly localized infections with Staphylococcus aureus and Candida species. Some patients have developed Candida esophagitis, but this appears to resolve with discontinuation of the drugs. Neutropenia has occurred, but very few patients have developed grade 3 (500–1,000 cells/mm3) or worse. All adverse effects were reversible with discontinuation of treatment.
Approval of secukinumab, and current studies of IL-17 inhibitors
On January 21, 2015, secukinumab was approved by the FDA for treatment of moderate to severe psoriasis vulgaris in adult patients and is now available by prescription.
More trials of IL-17 inhibitors for the treatment of psoriasis and psoriatic arthritis are under way and are at various phases at the time of this writing.23
TARGETED THERAPY FOR ADVANCED MELANOMA
Case 3. A 58-year-old man presents with an irregular pigmented lesion on his back. Biopsy shows malignant melanoma with an intense, chronic inflammatory infiltrate surrounding the tumor (Figure 3). The tumor was surgically excised with standard margins. Two years later, the patient developed multiple pigmented lesions on the face and complained of headache. Magnetic resonance imaging of the brain revealed multiple enhancing lesions consistent with metastatic melanoma (Figure 3). What are this patient’s options?
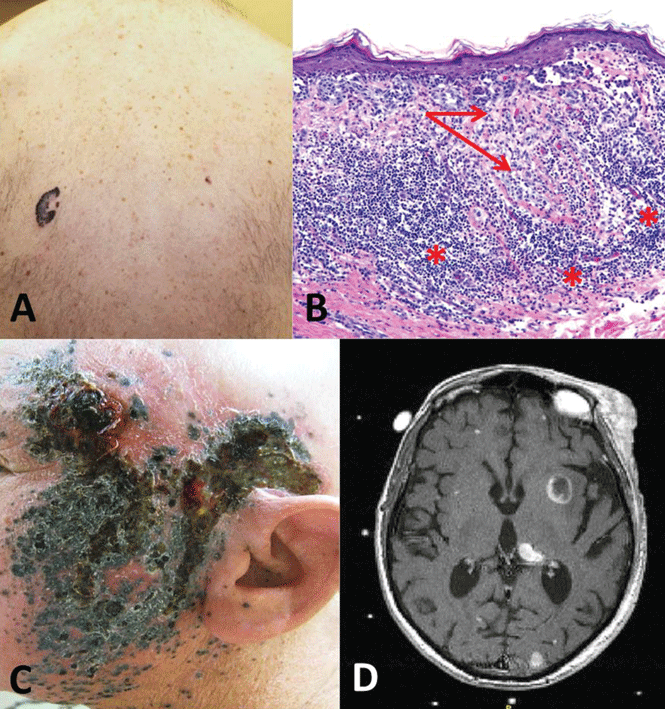
Melanoma is the fifth most common cancer in humans, with about 132,000 new cases diagnosed worldwide each year and 48,000 deaths from advanced disease. Its incidence has risen rapidly over the last few decades. Advanced disease has a poor prognosis, with the median overall survival less than 1 year and 5-year survival less than 10%.
Despite decades of research, a paucity of FDA-approved medications were available to treat advanced melanoma until recently. The alkylating agent dacarbazine was approved in 1975, interferon alpha in 1995, and high-dose IL-2 in 1998. Although some patients respond, studies have not shown significant improvement in survival with any of these medications.29–31
In 2002, Davies et al32 found that 50% to 65% of metastatic cutaneous melanomas have a mutation in the BRAF gene. Interestingly, 80% of these patients share a single specific mutation: substitution of glutamic acid for valine in codon 600 (BRAF V600E). The second most common mutation is a single substitution of a lysine for that same valine (BRAF V600K). Additionally, NRAS is mutated in about 20% of melanomas. These discoveries implicated a mitogen-activated protein kinase (MAPK) pathway (Figure 4) as playing a critical role in metastatic melanoma for a large percentage of patients.29
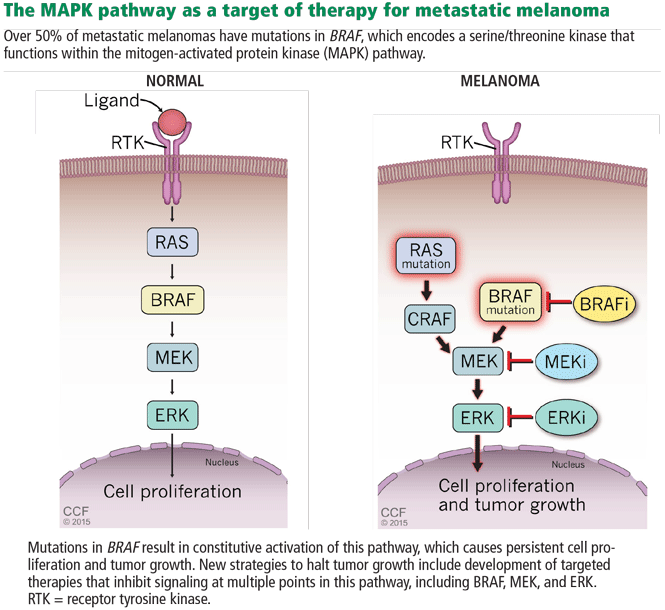
Based on this knowledge, several targeted therapies for melanoma have been developed, and some have been approved.
BRAF inhibitors—first success against melanoma
Vemurafenib. In 2010, Flaherty et al33 reported on a phase 1 and phase 2 clinical trial of vemurafenib (960 mg orally twice daily), a potent inhibitor of BRAF with the V600E mutation. They demonstrated a clinical benefit in 80% of patients with stage IV BRAF-mutant melanoma, an unprecedented response that opened the door to changes in the treatment of metastatic melanoma.
The phase 3 BRAF Inhibitor in Melanoma (BRIM)-3 clinical trial,34 published in 2011, randomized 675 previously untreated patients with advanced melanoma to either vemurafenib 960 mg orally twice daily or dacarbazine, the standard of care. The trial was terminated early when an interim analysis showed a significant overall advantage for vemurafenib (median progression-free survival 5.3 months vs 1.6 months for dacarbazine). Based on these results, vemurafenib was FDA-approved in August 2011 for use in patients with BRAF-mutant melanoma.
Dabrafenib. In a phase 3 clinical trial in 2012, Hauschild et al35 randomized 250 patients with BRAF (V600E)-mutated melanoma in a 3:1 ratio to receive either dabrafenib, a more potent second-generation BRAF inhibitor, or dacarbazine. Half of patients responded to dabrafenib, with a significantly improved progression-free survival rate (5.1 vs 2.7 months respectively), leading to FDA approval for its use in BRAF-mutant melanoma in May 2013.
Adverse effects common to vemurafenib and dabrafenib include rash, fatigue, fever, and joint pain. In addition, up to 25% of patients develop multiple secondary cutaneous squamous cell carcinomas and keratoacanthomas, usually within the first few months of therapy, which are believed to be caused by paradoxical activation of the MAPK pathway.
A more important problem with these medications is the development of resistance. Tumors typically progress again after a median progression-free survival of 6 to 7 months.
MEK inhibitors—another line of defense
Inhibitors of MEK—a serine-threonine kinase that is part of the same MAPK pathway involving BRAF—have been developed as well.
Trametinib. In 2012, trametinib, an allosteric MEK inhibitor, was used in an open-label phase 3 trial in 322 patients with advanced melanoma. Progression-free survival was 4.8 months for trametinib-treated patients compared with 1.5 months for the standard chemotherapy group (dacarbazine or paclitaxel).36 These results led to FDA approval of trametinib in May 2013 for treating BRAF-mutant melanoma.29
Cobimetinib is a second MEK inhibitor being evaluated alone and in combination with other targeted treatments for advanced melanoma.
Both MEK inhibitors have adverse effects similar to those seen with the BRAF inhibitors, including diarrhea, rash, fatigue, and edema. They also tend to cause asymptomatic elevated creatine kinase and transient retinopathy, reduced ejection fraction, and ventricular dysfunction. Unlike BRAF inhibitors, they are not associated with development of secondary cutaneous squamous cell carcinomas or keratoacanthomas. However, as with BRAF inhibitors, resistance is a problem with MEK inhibitors, with most patients relapsing less than a year after starting therapy.
Combination therapy improves outcomes
Possible mechanisms underlying resistance to these medications are being studied. A number of important factors appear to drive resistance, including expression of truncated BRAF proteins that do not bind the BRAF inhibitors and still activate downstream signaling, and amplification of BRAF to such a degree that it overwhelms the medications. This has led to the idea of combining BRAF inhibitors and MEK inhibitors to block the MAPK pathway at two points, potentially increasing the response and decreasing resistance.
Two trials have evaluated combinations of BRAF and MEK inhibitors in patients with advanced melanoma. Larkin et al37 conducted a phase 3 study evaluating combined vemurafenib (a BRAF inhibitor) and cobimetinib (a MEK inhibitor) vs combined vemurafenib and placebo. Survival with the combination therapy was 9.9 months vs 6.2 months with the single treatment.
The incidence of serious adverse effects was not significantly increased with the combination therapy, and keratoacanthomas, cutaneous squamous cell carcinomas, alopecia, and arthralgias were reduced compared with the vemurafenib and placebo group.
Another trial38 evaluating combined dabrafenib (a BRAF inhibitor) and trametinib (a MEK inhibitor) vs combined dabrafenib and placebo had similar findings: increased survival in the combined therapy group (9.3 months vs 8.8 months) and lower rates of squamous cell carcinoma (2% vs 9%).
In January 2014, the FDA approved the combination of BRAF and MEK inhibitors for the treatment of BRAF-mutant metastatic melanoma based on improved survival and generally reduced adverse effects.
IMMUNOTHERAPIES FOR NON-BRAF MELANOMA
Although BRAF and MEK inhibitors represent tremendous advances, their use is limited to the approximately 50% to 65% of patients with advanced melanoma who have BRAF V600 mutations. For others, only the traditional standard medications have been available until recently.
Two of those standard FDA-approved medications, interferon alpha-2b and IL-2, represent immunotherapies. Interferon alpha-2b up-regulates antigen presentation and increases antigen recognition by T cells. Overall, about 20% of patients in clinical trials have achieved responses.
IL-2 is a cytokine that increases T-cell proliferation and maturation into effector T cells. High-dose IL-2 has produced responses in 15% of patients, with a durable complete response in a small proportion.
Though success with these medications was modest, the fact that some patients responded to them indicates that immunotherapy could be a viable strategy for treating metastatic melanoma.30 This is underscored by the fact that some patients can mount an adaptive immune response specifically directed against antigenic proteins expressed in their tumors, resulting in expansion of cytotoxic T cells and control or even elimination of the malignancy.30
Tumors manipulate host immune checkpoints
Molecular biology has provided tremendous insight into tumor immunology over the past several decades, and we now recognize that a hallmark of cancer is escape from immune control.
Cancer cells contain a multitude of mutations that produce proteins that should be recognized by the immune system as foreign but in most individuals are not. This is because T-cell activity is down-regulated in cancer due to cancer cells’ ability to manipulate the host’s normal immunologic inhibitory pathways critical for maintaining self-tolerance.
In general, T-cell activation is initiated when an antigen-presenting cell presents an antigen to a T cell in a major histocompatibility complex-restricted manner. To prevent T cells from being activated by self-antigens and initiating autoimmunity, the interaction between antigen-presenting cells and T cells is regulated by checkpoints (Figure 5). First, for an antigen-presenting cell/T-cell interaction to result in T-cell activation, the T-cell receptor CD28 must bind CD80 on the antigen-presenting cell to drive a “positive” signal. Early in the interaction, the T-cell receptor CTLA-4 is up-regulated and competes with CD28 for binding of CD80. If CTLA-4, and not CD28, binds CD80, a “negative” signal is sent to the T cell and down-regulates it, making the interaction unproductive. Importantly, it is the CTLA-4:CD80 interaction that appears to be crucial for the ability of tumors to dampen T-cell responses to cancer cells.
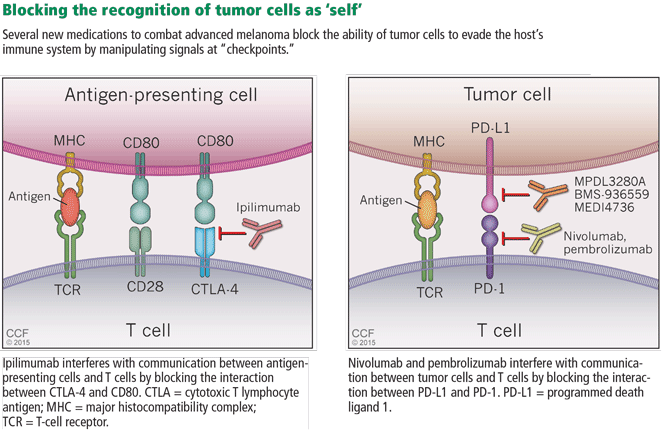
Ipilimumab is a fully humanized monoclonal antibody that binds to CTLA-4, blocking its ability to bind to CD80 and thereby enhancing T-cell activation. In a phase 3 trial, Hodi et al39 evaluated its use in treating advanced melanoma, with some enrolled patients having failed IL-2 treatment. Patients receiving ipilimumab with or without a glycoprotein-100 peptide vaccine (gp100) had an overall survival benefit of 10.1 months compared with 6.4 months for patients treated with gp100 alone. At 24 months, the survival rate with ipilimumab alone was 23.5%, almost double that of patients receiving gp100 alone.
Ipilimumab received FDA approval for treatment of metastatic melanoma in March 2011. This, and the BRAF inhibitors, were the first drugs approved by the FDA for the treatment of advanced melanoma in more than a decade.
Common adverse effects of ipilimumab include fatigue, diarrhea, rash, and pruritus. As expected, given its mechanism of action, up to about 25% of patients experience severe autoimmune-related events that may variably manifest as colitis, rash, hepatitis, neuritis, hypothyroidism, hypopituitarism, and hypophysitis. Another problem with this medication is that a subset of patients do not respond.
Cancer cells disguised as normal cells
Cancer cells can also manipulate another immunologic checkpoint to evade attack by the host immune system (Figure 5). Cytotoxic T cells may recognize antigens on tumor cells and become activated and primed to directly destroy them. However, tumor cells, like normal cells express the programmed death ligands RTK-L1 and PD-L2. These ligands function to bind to the PD-1 receptor on activated T cells to indicate they are “self” and inhibit the cytotoxic T cells from destroying them.
Evasion of immune system attack by manipulating checkpoints involving CTLA-4 and PD-1 helps explain why malignancies can seemingly be associated with brisk inflammatory responses, such as the tumor in Case 3, yet progress and eventually metastasize (Figure 3).
Two medications—nivolumab and pembrolizumab—have been developed in an attempt to disrupt the ability of tumor cells to trick the immune system into accepting them as “self” by manipulating the PD-L1/PD-L2: PD-1 interaction. Both drugs are monoclonal antibodies that bind to PD-1 and, thus, effectively block the ability of PD-L1 or PD-L2 on tumor cells to bind these ligands and signal to activated T cells that they are “self.” This blocking allows T cells to then carry out their killing of tumor cells they initially recognize as foreign.
Nivolumab. In 2014, a phase 3 trial40 compared nivolumab and dacarbazine in patients with untreated advanced melanoma without a BRAF mutation. Objective response rates were 40.0% in the nivolumab group vs 13.9% in the dacarbazine group. This trial was stopped early because of significantly better survival rates in patients taking nivolumab compared with standard chemotherapy.
Interestingly, only 35% of patients who responded to nivolumab had evidence of PD-L1 expression on the surface of their tumor cells as assessed by immunohistochemical assay. Regardless of PD-L1 status, nivolumab-treated patients had improved overall survival compared with those treated with dacarbazine. The response rate with nivolumab was only slightly better in the subgroup of patients whose tumors expressed PD-L1 than in the subgroup without PD-L1.
On December 22, 2014, the FDA granted accelerated approval to nivolumab for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab treatment and, if BRAF V600 mutation-positive, a BRAF inhibitor.
Pembrolizumab. Also in 2014, an open-label, randomized, phase 1b trial of pembrolizumab treatment at two different dosage schedules was conducted in patients with advanced melanoma that had become refractory either to ipilimumab or a BRAF inhibitor.41 Treatment with pembrolizumab had an objective response rate of 26% at both doses.
In September 2014, the FDA granted accelerated approval for the use of pembrolizumab to treat patients with unresectable or metastatic melanoma and disease progression following treatment with ipilimumab or a BRAF inhibitor.
Adverse effects of PD-1 inhibitors are similar to those seen with ipilimumab, the most common (occurring in at least 20%) being fatigue, cough, nausea, pruritus, rash, decreased appetite, constipation, muscle pain, and diarrhea. Serious effects from pembrolizumab (occurring in at least 2%) were kidney failure, dyspnea, pneumonia, and cellulitis. As seen with ipilimumab, clinically significant autoimmune adverse reactions occur with PD-1 inhibitors, including pneumonitis, colitis, hypophysitis, nephritis, and hepatitis.
Combination therapy under investigation
A phase 1 trial using combination therapy with both immune checkpoint inhibitors—nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4)—in patients with treatment-resistant metastatic melanoma was published in 2013.42 More than half of patients achieved objective responses, with tumor regression of at least 80% in those who had a response. Tumor response was evident in all subgroups of patients studied—those with pretreatment elevated lactate dehydrogenase levels (one of the strongest prognostic factors in metastatic melanoma), metastases to distant sites, and bulky, multifocal tumor burden. Based on these results, a phase 3 trial is now under way looking at the combination of these two medications vs either one alone.
In summary, targeted treatments are changing the paradigm of how common dermatologic conditions associated with significant morbidity and mortality are treated. Although implementation of the above treatments into everyday clinical practice is exciting, future studies surrounding each are needed to address unanswered issues, such as the optimal dosing and treatment schedules in terms of both disease response and inhibition of resistance, optimal patient/disease characteristics for use, and optimal drug treatment combinations. In the meantime, basic research still utilizing classic molecular biology techniques to uncover pathogenic disease mechanisms in even more detail is ongoing and hopefully will lead to development of even better targeted treatments or even cures for these diseases.
- Lyons TG, O’Kane GM, Kelly CM. Efficacy and safety of vismodegib: a new therapeutic agent in the treatment of basal cell carcinoma. Expert Opin Drug Saf 2014; 13:1125–1132.
- McCusker M, Basset-Sequin N, Dummer R, et al. Metastatic basal cell carcinoma: prognosis dependent on anatomic site and spread of disease. Eur J Cancer 2014; 50:774–783.
- Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 1996; 85:841–851.
- Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol 1998; 110:885–888.
- Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nat Rev Genet 2006; 7:841–850.
- Robarge KD, Brunton SA, Castanedo GM, et al. GDC-0449-a potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett 2009; 19:5576–5581.
- Proctor AE, Thompson LA, O’Bryant CL. Vismodegib: an inhibitor of the Hedgehog signaling pathway in the treatment of basal cell carcinoma. Ann Pharmacother 2014; 48:99–106.
- Dessinioti C, Plaka M, Stratigos AJ. Vismodegib for the treatment of basal cell carcinoma: results and implications of the ERIVANCE BCC trial. Future Oncol 2014; 10:927–936.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 2012; 366:2171–2179.
- Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with basal-cell nevus syndrome. N Engl J Med 2012; 366:2180–2188.
- Brinkhuizen T, Reinders MG, van Geel M, et al. Acquired resistance to the Hedgehog pathway inhibitor vismodegib due to smoothened mutations in treatment of locally advanced basal cell carcinoma. J Am Acad Dermatol 2014; 71:1005–1008.
- Ally MS, Aasi S, Wysong A, et al. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol 2014; 71:904–911.
- Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41:401–407.
- Gelfand JM, Niemann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006; 296:1735–1741.
- Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol 2014; 71:141–150.
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008; 117:244–279.
- Nestle FL, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361:496–509.
- Mentor A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2007; 58:106–115.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003; 349:2014–2022.
- Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005; 366:1367–1374.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371:1665–1674.
- Papp KA, Langley RG, Lebwohl M, et al; PHOENIX 2 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371:1675–1684.
- Leonardi CL, Gordon KB. New and emerging therapies in psoriasis. Semin Cut Med Surg 2014; 33(suppl 2):S37–S41.
- Langley RG, Elewski BE, Lebwohl, et al for the ERASURE and FIXTURE Study Groups. Secukinumab in plaque psorisis—results of two phase 3 trials. N Engl J Med 2014; 371:326–338.
- Leonardi C, Matheson R, Zachariae C. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 2012; 366:1190–1199.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 2012; 366:1181–1189.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis 2014; 73:349–356.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014; 370:2295–2306.
- Girotti MR, Saturno G, Lorigan P, Marais R. No longer an untreatable disease: how targeted and immunotherapies have changed the management of melanoma patients. Molec Oncol 2014, 8:1140–1158.
- Saranga-Perry V, Ambe C, Zager JS, Kudchadkar RR. Recent developments in the medical and surgical treatment of melanoma. CA Canc J Clin 2014; 64:171–185.
- Shah DJ, Dronca RS. Latest advances in chemotherapeutic, targeted, and immune approaches in the treatment of metastatic melanoma. Mayo Clin Proc 2014; 89:504–519.
- Davies H, Ignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417:949–954.
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010; 363:809–819.
- Chapman PB, Hauschild A, Robert C. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507–2516.
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380:358–365.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012; 367:107–114.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371:1867–1876.
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Eng J Med 2014; 371:1877–1888.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–723.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320–330.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384:1109–1117.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122–133.
New targeted therapies are changing the way patients with advanced dermatologic diseases are treated. Innovative molecular biology techniques developed as far back as the 1970s have engendered tremendous insight into the cellular and molecular pathogenesis of numerous diseases. Novel medications based on these insights are now bearing fruit, as directed biologic therapies that are revolutionizing clinical practice are increasingly becoming available.
This article reviews advances in targeted therapies for advanced basal cell carcinoma, psoriasis, and metastatic melanoma.
TARGETED THERAPY FOR BASAL CELL CARCINOMA
Case 1. A 56-year-old man presents with a progressively enlarging leg ulcer. Although it has been treated empirically for years as a venous stasis ulcer, biopsy reveals that it is basal cell carcinoma. Imaging shows muscle and tendon invasion, making surgical intervention short of amputation challenging (Figure 1). What are his options?

Basal cell carcinoma is the most common cancer in humans, accounting for 25% of all cancers and more than 2 million cases in the United States every year. In most cases, surgical excision is curative, but a subset of patients have inoperable, locally advanced, or metastatic disease that drastically limits treatment options. The median survival in metastatic basal cell carcinoma is 24 months, and conventional chemotherapy has not been shown to improve the prognosis.1,2
In addition to the burden of sporadic basal cell carcinoma, patients with the rare autosomal-dominant genetic disorder basal cell nevus syndrome (Gorlin syndrome) develop multiple basal cell lesions over their lifetime. The syndrome may also involve abnormalities of the skeletal system, genitourinary tract, and central nervous system, including development of medulloblastoma.
In Gorlin syndrome, basal cell carcinomas occur often and early; about half of white patients with the syndrome develop their first lesions by age 21, and 90% by age 35. The lesions occur in multiple numbers and can develop anywhere on the body, including on non–sun-exposed areas. Patients who have Gorlin syndrome need meticulous monitoring every 2 to 3 months so that basal cell lesions can be recognized early and treated before they become locally advanced. Keeping up with the numerous medical appointments and invasive treatments can be physically and mentally taxing for patients.
Specific pathway and mutations identified
In 1996, Gorlin syndrome was found to be caused by mutations of the human homolog of the PATCHED gene, which codes for a receptor in the “hedgehog” pathway.3 Two years later, the same mutations were found to be involved in many sporadic basal cell carcinomas, and we now believe that at least 85% of basal cell carcinomas involve abnormal activation of hedgehog pathway signaling.4,5
Vismodegib developed as targeted therapy
In 2009, Robarge et al6 described a potent inhibitor of the hedgehog pathway that was later optimized for potency and desirable pharmacologic traits, resulting in the drug vismodegib.7,8
Two phase 2 multicenter clinical trials9,10 of vismodegib were published in 2012. In the first, which was not randomized,9 33 patients with metastatic basal cell carcinoma and 63 patients with locally advanced disease were treated with vismodegib. Of those with metastatic disease, 30% achieved an objective response. Of those with locally advanced disease, 43% achieved an objective response and 21% achieved a complete response.
In the second trial,10 patients with Gorlin syndrome were randomized to either vismodegib (26 patients) or placebo (16 patients). After 8 months, the vismodegib group had developed significantly fewer new surgically eligible tumors (2 vs 29 per year), their tumors were smaller (change from baseline of the sum of the longest diameters –65% vs –11%), and they needed fewer surgeries (mean 0.31 vs 4.4 per patient). No tumors progressed in the treatment group. Results in some patients were dramatic, with complete healing of large ulcerative tumors. The trial was ended early in view of significant efficacy in the treatment group.
Based on these trials, the US Food and Drug Administration (FDA) approved vismodegib for treating metastatic and locally advanced basal cell carcinoma.
Resistance and adverse effects common
Unfortunately, vismodegib has significant drawbacks. About 20% of patients develop resistance, with tumors recurring after several months of therapy.11 Adverse effects most commonly reported were muscle spasms (68%), alopecia (63%), taste distortion (51%), weight loss (46%), and fatigue (36%). Although these effects were considered mild or moderate, they tended to persist, and almost every patient developed at least one. In the nonrandomized trial,9 more than 25% of patients discontinued treatment because of adverse effects, and more than half of patients did the same in the basal cell nevus syndrome trial.10
New uses may reduce shortcomings
Studies are under way to determine how best to use vismodegib.
One possibility is to use the drug for a few months to shrink tumors to the point that they become eligible for surgery. This is especially important for high-risk tumors, such as those near the eye or other vital structures. In 11 patients, Ally et al12 found that the surgical defect area was reduced by 27% from baseline after 4 months of treatment with vismodegib, allowing for curative surgery in some.
Another option is to combine vismodegib with other agents—either new ones on the horizon or existing nonspecific medications. For example, the antifungal itraconazole has been shown to inhibit hedgehog signaling and perhaps could be combined with vismodegib to increase response and reduce resistance.
Finally, a topical or intralesional form of vismodegib would be useful not only to reduce systemic toxicity, but also to increase efficacy when combined with other topical or systemic medications.
TARGETED THERAPY FOR PSORIASIS VULGARIS
Case 2. A 28-year-old woman presents with worsening psoriasis. About 35% of her body surface is involved, including the palms and soles, making it difficult for her to perform activities of daily living (Figure 2). What are her options?

Psoriasis is a chronic immune-mediated disease that affects up to 3% of people worldwide. In its moderate to severe forms, we recognize psoriasis as a systemic inflammatory disease that may adversely affect organ systems other than the skin. Commonly associated comorbid diseases include inflammatory (psoriatic) arthritis, cardiovascular disease, malignancies (eg, lymphoma), and inflammatory bowel disease. In addition, patients are well known to have significantly impaired quality of life because of low self-esteem, stigmatization affecting their social and work relationships, and, in up to 60%, clinical depression.13,14 The onset of psoriatic arthritis, particularly of erosive disease, is an important decision point for starting aggressive treatment, as joint destruction is irreversible.
Early targeted therapy aimed at TNF alpha, IL-12, and IL-23
Histologically, psoriasis involves thickening of the epidermis caused by hyperproliferation of keratinocytes. Based on this, prior to the 1980s, the dominant hypothesis concerning its pathogenesis was that it was caused by an inherent defect of keratinocytes. In the 1980s and 1990s, however, molecular research revealed that psoriasis was an immune-mediated disease caused by immunologic dysregulation predominantly involving T-helper 1 (Th-1) cells, with the inflammatory cytokines tumor necrosis factor (TNF) alpha, interferon gamma, interleukin (IL) 12, and IL-23 playing prominent roles.15 These findings led to the development and FDA approval of the first effective, targeted, psoriasis treatments, TNF-alpha inhibitors and the IL-12/23 inhibitor ustekinumab.
Etanercept, the first TNF-alpha inhibitor to become available, was approved in 2004 for moderate to severe psoriasis. In 2008, the IL-12/23 inhibitor ustekinumab was approved for the same indication. These drugs are efficacious, are generally safe, and have revolutionized the treatment of psoriasis and psoriatic arthritis, and they are now prescribed on a daily basis.16,17
In the clinical trials that led to approval of these drugs, the main outcome measure was the Psoriasis Area and Severity Index (PASI), a clinical scoring tool that assesses clinical aspects of psoriatic disease including body surface area involvement, degree of thickness, erythema, and scaling of psoriatic plaques. PASI scores range from 0 (no psoriasis) to 72 (most severe psoriasis). Achieving “PASI 75” indicates at least 75% improvement from the baseline score and represents the most common primary outcome measure in clinical trials assessing efficacy of new treatments. Up to 80% of patients who received currently available TNF-alpha inhibitors and ustekinumab in pivotal clinical trials achieved PASI 75 when assessed at 12 to 16 weeks after starting treatment. A moderate percentage of patients (19%–57%, depending on the trial) achieved 90% improvement (PASI 90), and a minority (up to 18%) achieved PASI 100, indicating complete clearing of their psoriasis.18–22
Newly developed therapies target IL-17A
In the mid-2000s, Th-17 cells were discovered, a new lineage of T cells distinct from Th-1 and Th-2 cells. Th-17 cells are characterized by their production of IL-17, a pro-inflammatory cytokine with six family members (IL-17A through IL-17F). Over the next few years, experiments revealed that Th-17 cells and IL-17A play key roles in psoriasis immunologic dysregulation.15 These findings led to a paradigm shift in hypotheses concerning psoriasis pathogenesis, with Th-17 cells and IL-17 replacing Th-1 cells and associated cytokines as dominant mediators of tissue damage.
Additionally, these findings led to new ideas for treatment. Three monoclonal antibodies that target IL-17 inhibition are currently under investigation. Secukinumab and ixekizumab bind to IL-17A and inhibit it from downstream signaling, whereas brodalumab binds to the IL-17A receptor, blocking all six IL-17 cytokines (IL-17A to IL-17F).23
Clinical trials of IL-17 inhibitors show excellent skin improvement
Secukinumab. In 2014, the results of two phase 3 trials of secukinumab were published.24
In the Efficacy and Safety of Subcutaneous Secukinumab for Moderate to Severe Chronic Plaque-type Psoriasis for up to 1 Year trial,24 patients were given either secukinumab 300 mg or 150 mg subcutaneously at defined time points; 82% and 72%, respectively, attained PASI 75 at 12 weeks.
Similar results were seen in the Safety and Efficacy of Secukinumab Compared to Etanercept in Subjects With Moderate to Severe, Chronic Plaque-Type Psoriasis study,24 in which PASI 75 was achieved by 77% of patients receiving secukinumab 300 mg, 67% of those receiving secukinumab 150 mg, and only 44% of those receiving etanercept 50 mg twice weekly at 12 weeks. Rates of infection with secukinumab and etanercept were similar.
The most striking results of these trials were that more than half of patients receiving the 300-mg dose achieved at least 90% improvement in their PASI score (PASI 90) by week 12, and in more than a quarter of patients the psoriasis completely cleared (PASI 100). These results were dramatically better than for etanercept (PASI 90 21%; PASI 100 4%).
Additionally, secukinumab worked fast. The median time to PASI 50 with secukinumab 300 mg was less than half that seen with etanercept (3 weeks vs 7 weeks).
Ixekizumab. In 2012, a phase 2 trial evaluated subcutaneous injections of ixekizumab in dosages ranging from 10 to 150 mg at defined intervals for 16 weeks.25 Of those receiving the highest dosage, 82% attained PASI 75 at 12 weeks, on par with what is noted in patients receiving TNF-alpha inhibitors and IL-12/23 inhibitors. Remarkably, however, almost three-quarters of patients (71%) achieved PASI 90, and 39% achieved PASI 100. Improvement in psoriasis was apparent as early as 1 week after injection.
Brodalumab. A 2012 phase 2 trial of various dosages of the IL-17 receptor inhibitor brodalumab26 also showed excellent PASI 75 achievement with the highest dosage (82%). Astonishingly, though, PASI 90 was achieved by 75% of patients, and PASI 100 by 62%.
Overall, although the percentages of patients achieving PASI 75 with the new IL-17 inhibitor drugs are comparable to those seen with TNF-alpha inhibitors and IL-12/23 inhibitors, the extraordinarily high percentages of patients who achieved PASI 90 and PASI 100 are unprecedented.18–22
Arthritis improvement not shown
Where the IL-17 inhibitors eventually settle within algorithms of psoriasis treatment largely depends on their efficacy in treating psoriatic arthritis compared with TNF-alpha inhibitors and IL-12/23 inhibitors. Joint inflammation is typically evaluated with the American College of Rheumatology (ACR) scoring tool, which in simple terms can be thought of as analogous to the PASI scoring tool for the skin. Although the ACR scoring tool was developed to assess joint inflammation in clinical trials for patients with rheumatoid arthritis, it is commonly used to assess improvement of psoriatic arthritis in clinical trials. The ACR tool involves assessing and scoring the number of swollen and tender joints, but also incorporates serologic assessment of acute-phase reactants (erythrocyte sedimentation rate or C-reactive protein level), patient and physician global assessment, pain, and function. ACR 20 implies roughly a 20% improvement in these criteria, whereas ACR 50 indicates 50% improvement, and so on.
Two phase 2 trials of IL-17 inhibitors for psoriatic arthritis have been published, one with secukinumab27 and one with brodalumab.28 Neither had impressive improvement in the ACR score vs TNF inhibitors—39% for ACR 20 at week 12 and less than 10% for ACR 70. Clinical trial design may have played a role, and phase 3 trials are under way for all three IL-17 inhibitors.
Adverse effects of IL-17 inhibitors
For the most part, adverse effects reported with the IL-17 inhibitors have been mild and similar to those reported with available biologic treatments for psoriasis. Adverse effects most commonly reported have been nasopharyngitis, upper respiratory infection, arthralgia, and mild injection-site reactions. In the future, attention will be paid to the rate of infections known to be associated with IL-17, mainly localized infections with Staphylococcus aureus and Candida species. Some patients have developed Candida esophagitis, but this appears to resolve with discontinuation of the drugs. Neutropenia has occurred, but very few patients have developed grade 3 (500–1,000 cells/mm3) or worse. All adverse effects were reversible with discontinuation of treatment.
Approval of secukinumab, and current studies of IL-17 inhibitors
On January 21, 2015, secukinumab was approved by the FDA for treatment of moderate to severe psoriasis vulgaris in adult patients and is now available by prescription.
More trials of IL-17 inhibitors for the treatment of psoriasis and psoriatic arthritis are under way and are at various phases at the time of this writing.23
TARGETED THERAPY FOR ADVANCED MELANOMA
Case 3. A 58-year-old man presents with an irregular pigmented lesion on his back. Biopsy shows malignant melanoma with an intense, chronic inflammatory infiltrate surrounding the tumor (Figure 3). The tumor was surgically excised with standard margins. Two years later, the patient developed multiple pigmented lesions on the face and complained of headache. Magnetic resonance imaging of the brain revealed multiple enhancing lesions consistent with metastatic melanoma (Figure 3). What are this patient’s options?

Melanoma is the fifth most common cancer in humans, with about 132,000 new cases diagnosed worldwide each year and 48,000 deaths from advanced disease. Its incidence has risen rapidly over the last few decades. Advanced disease has a poor prognosis, with the median overall survival less than 1 year and 5-year survival less than 10%.
Despite decades of research, a paucity of FDA-approved medications were available to treat advanced melanoma until recently. The alkylating agent dacarbazine was approved in 1975, interferon alpha in 1995, and high-dose IL-2 in 1998. Although some patients respond, studies have not shown significant improvement in survival with any of these medications.29–31
In 2002, Davies et al32 found that 50% to 65% of metastatic cutaneous melanomas have a mutation in the BRAF gene. Interestingly, 80% of these patients share a single specific mutation: substitution of glutamic acid for valine in codon 600 (BRAF V600E). The second most common mutation is a single substitution of a lysine for that same valine (BRAF V600K). Additionally, NRAS is mutated in about 20% of melanomas. These discoveries implicated a mitogen-activated protein kinase (MAPK) pathway (Figure 4) as playing a critical role in metastatic melanoma for a large percentage of patients.29

Based on this knowledge, several targeted therapies for melanoma have been developed, and some have been approved.
BRAF inhibitors—first success against melanoma
Vemurafenib. In 2010, Flaherty et al33 reported on a phase 1 and phase 2 clinical trial of vemurafenib (960 mg orally twice daily), a potent inhibitor of BRAF with the V600E mutation. They demonstrated a clinical benefit in 80% of patients with stage IV BRAF-mutant melanoma, an unprecedented response that opened the door to changes in the treatment of metastatic melanoma.
The phase 3 BRAF Inhibitor in Melanoma (BRIM)-3 clinical trial,34 published in 2011, randomized 675 previously untreated patients with advanced melanoma to either vemurafenib 960 mg orally twice daily or dacarbazine, the standard of care. The trial was terminated early when an interim analysis showed a significant overall advantage for vemurafenib (median progression-free survival 5.3 months vs 1.6 months for dacarbazine). Based on these results, vemurafenib was FDA-approved in August 2011 for use in patients with BRAF-mutant melanoma.
Dabrafenib. In a phase 3 clinical trial in 2012, Hauschild et al35 randomized 250 patients with BRAF (V600E)-mutated melanoma in a 3:1 ratio to receive either dabrafenib, a more potent second-generation BRAF inhibitor, or dacarbazine. Half of patients responded to dabrafenib, with a significantly improved progression-free survival rate (5.1 vs 2.7 months respectively), leading to FDA approval for its use in BRAF-mutant melanoma in May 2013.
Adverse effects common to vemurafenib and dabrafenib include rash, fatigue, fever, and joint pain. In addition, up to 25% of patients develop multiple secondary cutaneous squamous cell carcinomas and keratoacanthomas, usually within the first few months of therapy, which are believed to be caused by paradoxical activation of the MAPK pathway.
A more important problem with these medications is the development of resistance. Tumors typically progress again after a median progression-free survival of 6 to 7 months.
MEK inhibitors—another line of defense
Inhibitors of MEK—a serine-threonine kinase that is part of the same MAPK pathway involving BRAF—have been developed as well.
Trametinib. In 2012, trametinib, an allosteric MEK inhibitor, was used in an open-label phase 3 trial in 322 patients with advanced melanoma. Progression-free survival was 4.8 months for trametinib-treated patients compared with 1.5 months for the standard chemotherapy group (dacarbazine or paclitaxel).36 These results led to FDA approval of trametinib in May 2013 for treating BRAF-mutant melanoma.29
Cobimetinib is a second MEK inhibitor being evaluated alone and in combination with other targeted treatments for advanced melanoma.
Both MEK inhibitors have adverse effects similar to those seen with the BRAF inhibitors, including diarrhea, rash, fatigue, and edema. They also tend to cause asymptomatic elevated creatine kinase and transient retinopathy, reduced ejection fraction, and ventricular dysfunction. Unlike BRAF inhibitors, they are not associated with development of secondary cutaneous squamous cell carcinomas or keratoacanthomas. However, as with BRAF inhibitors, resistance is a problem with MEK inhibitors, with most patients relapsing less than a year after starting therapy.
Combination therapy improves outcomes
Possible mechanisms underlying resistance to these medications are being studied. A number of important factors appear to drive resistance, including expression of truncated BRAF proteins that do not bind the BRAF inhibitors and still activate downstream signaling, and amplification of BRAF to such a degree that it overwhelms the medications. This has led to the idea of combining BRAF inhibitors and MEK inhibitors to block the MAPK pathway at two points, potentially increasing the response and decreasing resistance.
Two trials have evaluated combinations of BRAF and MEK inhibitors in patients with advanced melanoma. Larkin et al37 conducted a phase 3 study evaluating combined vemurafenib (a BRAF inhibitor) and cobimetinib (a MEK inhibitor) vs combined vemurafenib and placebo. Survival with the combination therapy was 9.9 months vs 6.2 months with the single treatment.
The incidence of serious adverse effects was not significantly increased with the combination therapy, and keratoacanthomas, cutaneous squamous cell carcinomas, alopecia, and arthralgias were reduced compared with the vemurafenib and placebo group.
Another trial38 evaluating combined dabrafenib (a BRAF inhibitor) and trametinib (a MEK inhibitor) vs combined dabrafenib and placebo had similar findings: increased survival in the combined therapy group (9.3 months vs 8.8 months) and lower rates of squamous cell carcinoma (2% vs 9%).
In January 2014, the FDA approved the combination of BRAF and MEK inhibitors for the treatment of BRAF-mutant metastatic melanoma based on improved survival and generally reduced adverse effects.
IMMUNOTHERAPIES FOR NON-BRAF MELANOMA
Although BRAF and MEK inhibitors represent tremendous advances, their use is limited to the approximately 50% to 65% of patients with advanced melanoma who have BRAF V600 mutations. For others, only the traditional standard medications have been available until recently.
Two of those standard FDA-approved medications, interferon alpha-2b and IL-2, represent immunotherapies. Interferon alpha-2b up-regulates antigen presentation and increases antigen recognition by T cells. Overall, about 20% of patients in clinical trials have achieved responses.
IL-2 is a cytokine that increases T-cell proliferation and maturation into effector T cells. High-dose IL-2 has produced responses in 15% of patients, with a durable complete response in a small proportion.
Though success with these medications was modest, the fact that some patients responded to them indicates that immunotherapy could be a viable strategy for treating metastatic melanoma.30 This is underscored by the fact that some patients can mount an adaptive immune response specifically directed against antigenic proteins expressed in their tumors, resulting in expansion of cytotoxic T cells and control or even elimination of the malignancy.30
Tumors manipulate host immune checkpoints
Molecular biology has provided tremendous insight into tumor immunology over the past several decades, and we now recognize that a hallmark of cancer is escape from immune control.
Cancer cells contain a multitude of mutations that produce proteins that should be recognized by the immune system as foreign but in most individuals are not. This is because T-cell activity is down-regulated in cancer due to cancer cells’ ability to manipulate the host’s normal immunologic inhibitory pathways critical for maintaining self-tolerance.
In general, T-cell activation is initiated when an antigen-presenting cell presents an antigen to a T cell in a major histocompatibility complex-restricted manner. To prevent T cells from being activated by self-antigens and initiating autoimmunity, the interaction between antigen-presenting cells and T cells is regulated by checkpoints (Figure 5). First, for an antigen-presenting cell/T-cell interaction to result in T-cell activation, the T-cell receptor CD28 must bind CD80 on the antigen-presenting cell to drive a “positive” signal. Early in the interaction, the T-cell receptor CTLA-4 is up-regulated and competes with CD28 for binding of CD80. If CTLA-4, and not CD28, binds CD80, a “negative” signal is sent to the T cell and down-regulates it, making the interaction unproductive. Importantly, it is the CTLA-4:CD80 interaction that appears to be crucial for the ability of tumors to dampen T-cell responses to cancer cells.

Ipilimumab is a fully humanized monoclonal antibody that binds to CTLA-4, blocking its ability to bind to CD80 and thereby enhancing T-cell activation. In a phase 3 trial, Hodi et al39 evaluated its use in treating advanced melanoma, with some enrolled patients having failed IL-2 treatment. Patients receiving ipilimumab with or without a glycoprotein-100 peptide vaccine (gp100) had an overall survival benefit of 10.1 months compared with 6.4 months for patients treated with gp100 alone. At 24 months, the survival rate with ipilimumab alone was 23.5%, almost double that of patients receiving gp100 alone.
Ipilimumab received FDA approval for treatment of metastatic melanoma in March 2011. This, and the BRAF inhibitors, were the first drugs approved by the FDA for the treatment of advanced melanoma in more than a decade.
Common adverse effects of ipilimumab include fatigue, diarrhea, rash, and pruritus. As expected, given its mechanism of action, up to about 25% of patients experience severe autoimmune-related events that may variably manifest as colitis, rash, hepatitis, neuritis, hypothyroidism, hypopituitarism, and hypophysitis. Another problem with this medication is that a subset of patients do not respond.
Cancer cells disguised as normal cells
Cancer cells can also manipulate another immunologic checkpoint to evade attack by the host immune system (Figure 5). Cytotoxic T cells may recognize antigens on tumor cells and become activated and primed to directly destroy them. However, tumor cells, like normal cells express the programmed death ligands RTK-L1 and PD-L2. These ligands function to bind to the PD-1 receptor on activated T cells to indicate they are “self” and inhibit the cytotoxic T cells from destroying them.
Evasion of immune system attack by manipulating checkpoints involving CTLA-4 and PD-1 helps explain why malignancies can seemingly be associated with brisk inflammatory responses, such as the tumor in Case 3, yet progress and eventually metastasize (Figure 3).
Two medications—nivolumab and pembrolizumab—have been developed in an attempt to disrupt the ability of tumor cells to trick the immune system into accepting them as “self” by manipulating the PD-L1/PD-L2: PD-1 interaction. Both drugs are monoclonal antibodies that bind to PD-1 and, thus, effectively block the ability of PD-L1 or PD-L2 on tumor cells to bind these ligands and signal to activated T cells that they are “self.” This blocking allows T cells to then carry out their killing of tumor cells they initially recognize as foreign.
Nivolumab. In 2014, a phase 3 trial40 compared nivolumab and dacarbazine in patients with untreated advanced melanoma without a BRAF mutation. Objective response rates were 40.0% in the nivolumab group vs 13.9% in the dacarbazine group. This trial was stopped early because of significantly better survival rates in patients taking nivolumab compared with standard chemotherapy.
Interestingly, only 35% of patients who responded to nivolumab had evidence of PD-L1 expression on the surface of their tumor cells as assessed by immunohistochemical assay. Regardless of PD-L1 status, nivolumab-treated patients had improved overall survival compared with those treated with dacarbazine. The response rate with nivolumab was only slightly better in the subgroup of patients whose tumors expressed PD-L1 than in the subgroup without PD-L1.
On December 22, 2014, the FDA granted accelerated approval to nivolumab for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab treatment and, if BRAF V600 mutation-positive, a BRAF inhibitor.
Pembrolizumab. Also in 2014, an open-label, randomized, phase 1b trial of pembrolizumab treatment at two different dosage schedules was conducted in patients with advanced melanoma that had become refractory either to ipilimumab or a BRAF inhibitor.41 Treatment with pembrolizumab had an objective response rate of 26% at both doses.
In September 2014, the FDA granted accelerated approval for the use of pembrolizumab to treat patients with unresectable or metastatic melanoma and disease progression following treatment with ipilimumab or a BRAF inhibitor.
Adverse effects of PD-1 inhibitors are similar to those seen with ipilimumab, the most common (occurring in at least 20%) being fatigue, cough, nausea, pruritus, rash, decreased appetite, constipation, muscle pain, and diarrhea. Serious effects from pembrolizumab (occurring in at least 2%) were kidney failure, dyspnea, pneumonia, and cellulitis. As seen with ipilimumab, clinically significant autoimmune adverse reactions occur with PD-1 inhibitors, including pneumonitis, colitis, hypophysitis, nephritis, and hepatitis.
Combination therapy under investigation
A phase 1 trial using combination therapy with both immune checkpoint inhibitors—nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4)—in patients with treatment-resistant metastatic melanoma was published in 2013.42 More than half of patients achieved objective responses, with tumor regression of at least 80% in those who had a response. Tumor response was evident in all subgroups of patients studied—those with pretreatment elevated lactate dehydrogenase levels (one of the strongest prognostic factors in metastatic melanoma), metastases to distant sites, and bulky, multifocal tumor burden. Based on these results, a phase 3 trial is now under way looking at the combination of these two medications vs either one alone.
In summary, targeted treatments are changing the paradigm of how common dermatologic conditions associated with significant morbidity and mortality are treated. Although implementation of the above treatments into everyday clinical practice is exciting, future studies surrounding each are needed to address unanswered issues, such as the optimal dosing and treatment schedules in terms of both disease response and inhibition of resistance, optimal patient/disease characteristics for use, and optimal drug treatment combinations. In the meantime, basic research still utilizing classic molecular biology techniques to uncover pathogenic disease mechanisms in even more detail is ongoing and hopefully will lead to development of even better targeted treatments or even cures for these diseases.
New targeted therapies are changing the way patients with advanced dermatologic diseases are treated. Innovative molecular biology techniques developed as far back as the 1970s have engendered tremendous insight into the cellular and molecular pathogenesis of numerous diseases. Novel medications based on these insights are now bearing fruit, as directed biologic therapies that are revolutionizing clinical practice are increasingly becoming available.
This article reviews advances in targeted therapies for advanced basal cell carcinoma, psoriasis, and metastatic melanoma.
TARGETED THERAPY FOR BASAL CELL CARCINOMA
Case 1. A 56-year-old man presents with a progressively enlarging leg ulcer. Although it has been treated empirically for years as a venous stasis ulcer, biopsy reveals that it is basal cell carcinoma. Imaging shows muscle and tendon invasion, making surgical intervention short of amputation challenging (Figure 1). What are his options?

Basal cell carcinoma is the most common cancer in humans, accounting for 25% of all cancers and more than 2 million cases in the United States every year. In most cases, surgical excision is curative, but a subset of patients have inoperable, locally advanced, or metastatic disease that drastically limits treatment options. The median survival in metastatic basal cell carcinoma is 24 months, and conventional chemotherapy has not been shown to improve the prognosis.1,2
In addition to the burden of sporadic basal cell carcinoma, patients with the rare autosomal-dominant genetic disorder basal cell nevus syndrome (Gorlin syndrome) develop multiple basal cell lesions over their lifetime. The syndrome may also involve abnormalities of the skeletal system, genitourinary tract, and central nervous system, including development of medulloblastoma.
In Gorlin syndrome, basal cell carcinomas occur often and early; about half of white patients with the syndrome develop their first lesions by age 21, and 90% by age 35. The lesions occur in multiple numbers and can develop anywhere on the body, including on non–sun-exposed areas. Patients who have Gorlin syndrome need meticulous monitoring every 2 to 3 months so that basal cell lesions can be recognized early and treated before they become locally advanced. Keeping up with the numerous medical appointments and invasive treatments can be physically and mentally taxing for patients.
Specific pathway and mutations identified
In 1996, Gorlin syndrome was found to be caused by mutations of the human homolog of the PATCHED gene, which codes for a receptor in the “hedgehog” pathway.3 Two years later, the same mutations were found to be involved in many sporadic basal cell carcinomas, and we now believe that at least 85% of basal cell carcinomas involve abnormal activation of hedgehog pathway signaling.4,5
Vismodegib developed as targeted therapy
In 2009, Robarge et al6 described a potent inhibitor of the hedgehog pathway that was later optimized for potency and desirable pharmacologic traits, resulting in the drug vismodegib.7,8
Two phase 2 multicenter clinical trials9,10 of vismodegib were published in 2012. In the first, which was not randomized,9 33 patients with metastatic basal cell carcinoma and 63 patients with locally advanced disease were treated with vismodegib. Of those with metastatic disease, 30% achieved an objective response. Of those with locally advanced disease, 43% achieved an objective response and 21% achieved a complete response.
In the second trial,10 patients with Gorlin syndrome were randomized to either vismodegib (26 patients) or placebo (16 patients). After 8 months, the vismodegib group had developed significantly fewer new surgically eligible tumors (2 vs 29 per year), their tumors were smaller (change from baseline of the sum of the longest diameters –65% vs –11%), and they needed fewer surgeries (mean 0.31 vs 4.4 per patient). No tumors progressed in the treatment group. Results in some patients were dramatic, with complete healing of large ulcerative tumors. The trial was ended early in view of significant efficacy in the treatment group.
Based on these trials, the US Food and Drug Administration (FDA) approved vismodegib for treating metastatic and locally advanced basal cell carcinoma.
Resistance and adverse effects common
Unfortunately, vismodegib has significant drawbacks. About 20% of patients develop resistance, with tumors recurring after several months of therapy.11 Adverse effects most commonly reported were muscle spasms (68%), alopecia (63%), taste distortion (51%), weight loss (46%), and fatigue (36%). Although these effects were considered mild or moderate, they tended to persist, and almost every patient developed at least one. In the nonrandomized trial,9 more than 25% of patients discontinued treatment because of adverse effects, and more than half of patients did the same in the basal cell nevus syndrome trial.10
New uses may reduce shortcomings
Studies are under way to determine how best to use vismodegib.
One possibility is to use the drug for a few months to shrink tumors to the point that they become eligible for surgery. This is especially important for high-risk tumors, such as those near the eye or other vital structures. In 11 patients, Ally et al12 found that the surgical defect area was reduced by 27% from baseline after 4 months of treatment with vismodegib, allowing for curative surgery in some.
Another option is to combine vismodegib with other agents—either new ones on the horizon or existing nonspecific medications. For example, the antifungal itraconazole has been shown to inhibit hedgehog signaling and perhaps could be combined with vismodegib to increase response and reduce resistance.
Finally, a topical or intralesional form of vismodegib would be useful not only to reduce systemic toxicity, but also to increase efficacy when combined with other topical or systemic medications.
TARGETED THERAPY FOR PSORIASIS VULGARIS
Case 2. A 28-year-old woman presents with worsening psoriasis. About 35% of her body surface is involved, including the palms and soles, making it difficult for her to perform activities of daily living (Figure 2). What are her options?

Psoriasis is a chronic immune-mediated disease that affects up to 3% of people worldwide. In its moderate to severe forms, we recognize psoriasis as a systemic inflammatory disease that may adversely affect organ systems other than the skin. Commonly associated comorbid diseases include inflammatory (psoriatic) arthritis, cardiovascular disease, malignancies (eg, lymphoma), and inflammatory bowel disease. In addition, patients are well known to have significantly impaired quality of life because of low self-esteem, stigmatization affecting their social and work relationships, and, in up to 60%, clinical depression.13,14 The onset of psoriatic arthritis, particularly of erosive disease, is an important decision point for starting aggressive treatment, as joint destruction is irreversible.
Early targeted therapy aimed at TNF alpha, IL-12, and IL-23
Histologically, psoriasis involves thickening of the epidermis caused by hyperproliferation of keratinocytes. Based on this, prior to the 1980s, the dominant hypothesis concerning its pathogenesis was that it was caused by an inherent defect of keratinocytes. In the 1980s and 1990s, however, molecular research revealed that psoriasis was an immune-mediated disease caused by immunologic dysregulation predominantly involving T-helper 1 (Th-1) cells, with the inflammatory cytokines tumor necrosis factor (TNF) alpha, interferon gamma, interleukin (IL) 12, and IL-23 playing prominent roles.15 These findings led to the development and FDA approval of the first effective, targeted, psoriasis treatments, TNF-alpha inhibitors and the IL-12/23 inhibitor ustekinumab.
Etanercept, the first TNF-alpha inhibitor to become available, was approved in 2004 for moderate to severe psoriasis. In 2008, the IL-12/23 inhibitor ustekinumab was approved for the same indication. These drugs are efficacious, are generally safe, and have revolutionized the treatment of psoriasis and psoriatic arthritis, and they are now prescribed on a daily basis.16,17
In the clinical trials that led to approval of these drugs, the main outcome measure was the Psoriasis Area and Severity Index (PASI), a clinical scoring tool that assesses clinical aspects of psoriatic disease including body surface area involvement, degree of thickness, erythema, and scaling of psoriatic plaques. PASI scores range from 0 (no psoriasis) to 72 (most severe psoriasis). Achieving “PASI 75” indicates at least 75% improvement from the baseline score and represents the most common primary outcome measure in clinical trials assessing efficacy of new treatments. Up to 80% of patients who received currently available TNF-alpha inhibitors and ustekinumab in pivotal clinical trials achieved PASI 75 when assessed at 12 to 16 weeks after starting treatment. A moderate percentage of patients (19%–57%, depending on the trial) achieved 90% improvement (PASI 90), and a minority (up to 18%) achieved PASI 100, indicating complete clearing of their psoriasis.18–22
Newly developed therapies target IL-17A
In the mid-2000s, Th-17 cells were discovered, a new lineage of T cells distinct from Th-1 and Th-2 cells. Th-17 cells are characterized by their production of IL-17, a pro-inflammatory cytokine with six family members (IL-17A through IL-17F). Over the next few years, experiments revealed that Th-17 cells and IL-17A play key roles in psoriasis immunologic dysregulation.15 These findings led to a paradigm shift in hypotheses concerning psoriasis pathogenesis, with Th-17 cells and IL-17 replacing Th-1 cells and associated cytokines as dominant mediators of tissue damage.
Additionally, these findings led to new ideas for treatment. Three monoclonal antibodies that target IL-17 inhibition are currently under investigation. Secukinumab and ixekizumab bind to IL-17A and inhibit it from downstream signaling, whereas brodalumab binds to the IL-17A receptor, blocking all six IL-17 cytokines (IL-17A to IL-17F).23
Clinical trials of IL-17 inhibitors show excellent skin improvement
Secukinumab. In 2014, the results of two phase 3 trials of secukinumab were published.24
In the Efficacy and Safety of Subcutaneous Secukinumab for Moderate to Severe Chronic Plaque-type Psoriasis for up to 1 Year trial,24 patients were given either secukinumab 300 mg or 150 mg subcutaneously at defined time points; 82% and 72%, respectively, attained PASI 75 at 12 weeks.
Similar results were seen in the Safety and Efficacy of Secukinumab Compared to Etanercept in Subjects With Moderate to Severe, Chronic Plaque-Type Psoriasis study,24 in which PASI 75 was achieved by 77% of patients receiving secukinumab 300 mg, 67% of those receiving secukinumab 150 mg, and only 44% of those receiving etanercept 50 mg twice weekly at 12 weeks. Rates of infection with secukinumab and etanercept were similar.
The most striking results of these trials were that more than half of patients receiving the 300-mg dose achieved at least 90% improvement in their PASI score (PASI 90) by week 12, and in more than a quarter of patients the psoriasis completely cleared (PASI 100). These results were dramatically better than for etanercept (PASI 90 21%; PASI 100 4%).
Additionally, secukinumab worked fast. The median time to PASI 50 with secukinumab 300 mg was less than half that seen with etanercept (3 weeks vs 7 weeks).
Ixekizumab. In 2012, a phase 2 trial evaluated subcutaneous injections of ixekizumab in dosages ranging from 10 to 150 mg at defined intervals for 16 weeks.25 Of those receiving the highest dosage, 82% attained PASI 75 at 12 weeks, on par with what is noted in patients receiving TNF-alpha inhibitors and IL-12/23 inhibitors. Remarkably, however, almost three-quarters of patients (71%) achieved PASI 90, and 39% achieved PASI 100. Improvement in psoriasis was apparent as early as 1 week after injection.
Brodalumab. A 2012 phase 2 trial of various dosages of the IL-17 receptor inhibitor brodalumab26 also showed excellent PASI 75 achievement with the highest dosage (82%). Astonishingly, though, PASI 90 was achieved by 75% of patients, and PASI 100 by 62%.
Overall, although the percentages of patients achieving PASI 75 with the new IL-17 inhibitor drugs are comparable to those seen with TNF-alpha inhibitors and IL-12/23 inhibitors, the extraordinarily high percentages of patients who achieved PASI 90 and PASI 100 are unprecedented.18–22
Arthritis improvement not shown
Where the IL-17 inhibitors eventually settle within algorithms of psoriasis treatment largely depends on their efficacy in treating psoriatic arthritis compared with TNF-alpha inhibitors and IL-12/23 inhibitors. Joint inflammation is typically evaluated with the American College of Rheumatology (ACR) scoring tool, which in simple terms can be thought of as analogous to the PASI scoring tool for the skin. Although the ACR scoring tool was developed to assess joint inflammation in clinical trials for patients with rheumatoid arthritis, it is commonly used to assess improvement of psoriatic arthritis in clinical trials. The ACR tool involves assessing and scoring the number of swollen and tender joints, but also incorporates serologic assessment of acute-phase reactants (erythrocyte sedimentation rate or C-reactive protein level), patient and physician global assessment, pain, and function. ACR 20 implies roughly a 20% improvement in these criteria, whereas ACR 50 indicates 50% improvement, and so on.
Two phase 2 trials of IL-17 inhibitors for psoriatic arthritis have been published, one with secukinumab27 and one with brodalumab.28 Neither had impressive improvement in the ACR score vs TNF inhibitors—39% for ACR 20 at week 12 and less than 10% for ACR 70. Clinical trial design may have played a role, and phase 3 trials are under way for all three IL-17 inhibitors.
Adverse effects of IL-17 inhibitors
For the most part, adverse effects reported with the IL-17 inhibitors have been mild and similar to those reported with available biologic treatments for psoriasis. Adverse effects most commonly reported have been nasopharyngitis, upper respiratory infection, arthralgia, and mild injection-site reactions. In the future, attention will be paid to the rate of infections known to be associated with IL-17, mainly localized infections with Staphylococcus aureus and Candida species. Some patients have developed Candida esophagitis, but this appears to resolve with discontinuation of the drugs. Neutropenia has occurred, but very few patients have developed grade 3 (500–1,000 cells/mm3) or worse. All adverse effects were reversible with discontinuation of treatment.
Approval of secukinumab, and current studies of IL-17 inhibitors
On January 21, 2015, secukinumab was approved by the FDA for treatment of moderate to severe psoriasis vulgaris in adult patients and is now available by prescription.
More trials of IL-17 inhibitors for the treatment of psoriasis and psoriatic arthritis are under way and are at various phases at the time of this writing.23
TARGETED THERAPY FOR ADVANCED MELANOMA
Case 3. A 58-year-old man presents with an irregular pigmented lesion on his back. Biopsy shows malignant melanoma with an intense, chronic inflammatory infiltrate surrounding the tumor (Figure 3). The tumor was surgically excised with standard margins. Two years later, the patient developed multiple pigmented lesions on the face and complained of headache. Magnetic resonance imaging of the brain revealed multiple enhancing lesions consistent with metastatic melanoma (Figure 3). What are this patient’s options?

Melanoma is the fifth most common cancer in humans, with about 132,000 new cases diagnosed worldwide each year and 48,000 deaths from advanced disease. Its incidence has risen rapidly over the last few decades. Advanced disease has a poor prognosis, with the median overall survival less than 1 year and 5-year survival less than 10%.
Despite decades of research, a paucity of FDA-approved medications were available to treat advanced melanoma until recently. The alkylating agent dacarbazine was approved in 1975, interferon alpha in 1995, and high-dose IL-2 in 1998. Although some patients respond, studies have not shown significant improvement in survival with any of these medications.29–31
In 2002, Davies et al32 found that 50% to 65% of metastatic cutaneous melanomas have a mutation in the BRAF gene. Interestingly, 80% of these patients share a single specific mutation: substitution of glutamic acid for valine in codon 600 (BRAF V600E). The second most common mutation is a single substitution of a lysine for that same valine (BRAF V600K). Additionally, NRAS is mutated in about 20% of melanomas. These discoveries implicated a mitogen-activated protein kinase (MAPK) pathway (Figure 4) as playing a critical role in metastatic melanoma for a large percentage of patients.29

Based on this knowledge, several targeted therapies for melanoma have been developed, and some have been approved.
BRAF inhibitors—first success against melanoma
Vemurafenib. In 2010, Flaherty et al33 reported on a phase 1 and phase 2 clinical trial of vemurafenib (960 mg orally twice daily), a potent inhibitor of BRAF with the V600E mutation. They demonstrated a clinical benefit in 80% of patients with stage IV BRAF-mutant melanoma, an unprecedented response that opened the door to changes in the treatment of metastatic melanoma.
The phase 3 BRAF Inhibitor in Melanoma (BRIM)-3 clinical trial,34 published in 2011, randomized 675 previously untreated patients with advanced melanoma to either vemurafenib 960 mg orally twice daily or dacarbazine, the standard of care. The trial was terminated early when an interim analysis showed a significant overall advantage for vemurafenib (median progression-free survival 5.3 months vs 1.6 months for dacarbazine). Based on these results, vemurafenib was FDA-approved in August 2011 for use in patients with BRAF-mutant melanoma.
Dabrafenib. In a phase 3 clinical trial in 2012, Hauschild et al35 randomized 250 patients with BRAF (V600E)-mutated melanoma in a 3:1 ratio to receive either dabrafenib, a more potent second-generation BRAF inhibitor, or dacarbazine. Half of patients responded to dabrafenib, with a significantly improved progression-free survival rate (5.1 vs 2.7 months respectively), leading to FDA approval for its use in BRAF-mutant melanoma in May 2013.
Adverse effects common to vemurafenib and dabrafenib include rash, fatigue, fever, and joint pain. In addition, up to 25% of patients develop multiple secondary cutaneous squamous cell carcinomas and keratoacanthomas, usually within the first few months of therapy, which are believed to be caused by paradoxical activation of the MAPK pathway.
A more important problem with these medications is the development of resistance. Tumors typically progress again after a median progression-free survival of 6 to 7 months.
MEK inhibitors—another line of defense
Inhibitors of MEK—a serine-threonine kinase that is part of the same MAPK pathway involving BRAF—have been developed as well.
Trametinib. In 2012, trametinib, an allosteric MEK inhibitor, was used in an open-label phase 3 trial in 322 patients with advanced melanoma. Progression-free survival was 4.8 months for trametinib-treated patients compared with 1.5 months for the standard chemotherapy group (dacarbazine or paclitaxel).36 These results led to FDA approval of trametinib in May 2013 for treating BRAF-mutant melanoma.29
Cobimetinib is a second MEK inhibitor being evaluated alone and in combination with other targeted treatments for advanced melanoma.
Both MEK inhibitors have adverse effects similar to those seen with the BRAF inhibitors, including diarrhea, rash, fatigue, and edema. They also tend to cause asymptomatic elevated creatine kinase and transient retinopathy, reduced ejection fraction, and ventricular dysfunction. Unlike BRAF inhibitors, they are not associated with development of secondary cutaneous squamous cell carcinomas or keratoacanthomas. However, as with BRAF inhibitors, resistance is a problem with MEK inhibitors, with most patients relapsing less than a year after starting therapy.
Combination therapy improves outcomes
Possible mechanisms underlying resistance to these medications are being studied. A number of important factors appear to drive resistance, including expression of truncated BRAF proteins that do not bind the BRAF inhibitors and still activate downstream signaling, and amplification of BRAF to such a degree that it overwhelms the medications. This has led to the idea of combining BRAF inhibitors and MEK inhibitors to block the MAPK pathway at two points, potentially increasing the response and decreasing resistance.
Two trials have evaluated combinations of BRAF and MEK inhibitors in patients with advanced melanoma. Larkin et al37 conducted a phase 3 study evaluating combined vemurafenib (a BRAF inhibitor) and cobimetinib (a MEK inhibitor) vs combined vemurafenib and placebo. Survival with the combination therapy was 9.9 months vs 6.2 months with the single treatment.
The incidence of serious adverse effects was not significantly increased with the combination therapy, and keratoacanthomas, cutaneous squamous cell carcinomas, alopecia, and arthralgias were reduced compared with the vemurafenib and placebo group.
Another trial38 evaluating combined dabrafenib (a BRAF inhibitor) and trametinib (a MEK inhibitor) vs combined dabrafenib and placebo had similar findings: increased survival in the combined therapy group (9.3 months vs 8.8 months) and lower rates of squamous cell carcinoma (2% vs 9%).
In January 2014, the FDA approved the combination of BRAF and MEK inhibitors for the treatment of BRAF-mutant metastatic melanoma based on improved survival and generally reduced adverse effects.
IMMUNOTHERAPIES FOR NON-BRAF MELANOMA
Although BRAF and MEK inhibitors represent tremendous advances, their use is limited to the approximately 50% to 65% of patients with advanced melanoma who have BRAF V600 mutations. For others, only the traditional standard medications have been available until recently.
Two of those standard FDA-approved medications, interferon alpha-2b and IL-2, represent immunotherapies. Interferon alpha-2b up-regulates antigen presentation and increases antigen recognition by T cells. Overall, about 20% of patients in clinical trials have achieved responses.
IL-2 is a cytokine that increases T-cell proliferation and maturation into effector T cells. High-dose IL-2 has produced responses in 15% of patients, with a durable complete response in a small proportion.
Though success with these medications was modest, the fact that some patients responded to them indicates that immunotherapy could be a viable strategy for treating metastatic melanoma.30 This is underscored by the fact that some patients can mount an adaptive immune response specifically directed against antigenic proteins expressed in their tumors, resulting in expansion of cytotoxic T cells and control or even elimination of the malignancy.30
Tumors manipulate host immune checkpoints
Molecular biology has provided tremendous insight into tumor immunology over the past several decades, and we now recognize that a hallmark of cancer is escape from immune control.
Cancer cells contain a multitude of mutations that produce proteins that should be recognized by the immune system as foreign but in most individuals are not. This is because T-cell activity is down-regulated in cancer due to cancer cells’ ability to manipulate the host’s normal immunologic inhibitory pathways critical for maintaining self-tolerance.
In general, T-cell activation is initiated when an antigen-presenting cell presents an antigen to a T cell in a major histocompatibility complex-restricted manner. To prevent T cells from being activated by self-antigens and initiating autoimmunity, the interaction between antigen-presenting cells and T cells is regulated by checkpoints (Figure 5). First, for an antigen-presenting cell/T-cell interaction to result in T-cell activation, the T-cell receptor CD28 must bind CD80 on the antigen-presenting cell to drive a “positive” signal. Early in the interaction, the T-cell receptor CTLA-4 is up-regulated and competes with CD28 for binding of CD80. If CTLA-4, and not CD28, binds CD80, a “negative” signal is sent to the T cell and down-regulates it, making the interaction unproductive. Importantly, it is the CTLA-4:CD80 interaction that appears to be crucial for the ability of tumors to dampen T-cell responses to cancer cells.

Ipilimumab is a fully humanized monoclonal antibody that binds to CTLA-4, blocking its ability to bind to CD80 and thereby enhancing T-cell activation. In a phase 3 trial, Hodi et al39 evaluated its use in treating advanced melanoma, with some enrolled patients having failed IL-2 treatment. Patients receiving ipilimumab with or without a glycoprotein-100 peptide vaccine (gp100) had an overall survival benefit of 10.1 months compared with 6.4 months for patients treated with gp100 alone. At 24 months, the survival rate with ipilimumab alone was 23.5%, almost double that of patients receiving gp100 alone.
Ipilimumab received FDA approval for treatment of metastatic melanoma in March 2011. This, and the BRAF inhibitors, were the first drugs approved by the FDA for the treatment of advanced melanoma in more than a decade.
Common adverse effects of ipilimumab include fatigue, diarrhea, rash, and pruritus. As expected, given its mechanism of action, up to about 25% of patients experience severe autoimmune-related events that may variably manifest as colitis, rash, hepatitis, neuritis, hypothyroidism, hypopituitarism, and hypophysitis. Another problem with this medication is that a subset of patients do not respond.
Cancer cells disguised as normal cells
Cancer cells can also manipulate another immunologic checkpoint to evade attack by the host immune system (Figure 5). Cytotoxic T cells may recognize antigens on tumor cells and become activated and primed to directly destroy them. However, tumor cells, like normal cells express the programmed death ligands RTK-L1 and PD-L2. These ligands function to bind to the PD-1 receptor on activated T cells to indicate they are “self” and inhibit the cytotoxic T cells from destroying them.
Evasion of immune system attack by manipulating checkpoints involving CTLA-4 and PD-1 helps explain why malignancies can seemingly be associated with brisk inflammatory responses, such as the tumor in Case 3, yet progress and eventually metastasize (Figure 3).
Two medications—nivolumab and pembrolizumab—have been developed in an attempt to disrupt the ability of tumor cells to trick the immune system into accepting them as “self” by manipulating the PD-L1/PD-L2: PD-1 interaction. Both drugs are monoclonal antibodies that bind to PD-1 and, thus, effectively block the ability of PD-L1 or PD-L2 on tumor cells to bind these ligands and signal to activated T cells that they are “self.” This blocking allows T cells to then carry out their killing of tumor cells they initially recognize as foreign.
Nivolumab. In 2014, a phase 3 trial40 compared nivolumab and dacarbazine in patients with untreated advanced melanoma without a BRAF mutation. Objective response rates were 40.0% in the nivolumab group vs 13.9% in the dacarbazine group. This trial was stopped early because of significantly better survival rates in patients taking nivolumab compared with standard chemotherapy.
Interestingly, only 35% of patients who responded to nivolumab had evidence of PD-L1 expression on the surface of their tumor cells as assessed by immunohistochemical assay. Regardless of PD-L1 status, nivolumab-treated patients had improved overall survival compared with those treated with dacarbazine. The response rate with nivolumab was only slightly better in the subgroup of patients whose tumors expressed PD-L1 than in the subgroup without PD-L1.
On December 22, 2014, the FDA granted accelerated approval to nivolumab for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab treatment and, if BRAF V600 mutation-positive, a BRAF inhibitor.
Pembrolizumab. Also in 2014, an open-label, randomized, phase 1b trial of pembrolizumab treatment at two different dosage schedules was conducted in patients with advanced melanoma that had become refractory either to ipilimumab or a BRAF inhibitor.41 Treatment with pembrolizumab had an objective response rate of 26% at both doses.
In September 2014, the FDA granted accelerated approval for the use of pembrolizumab to treat patients with unresectable or metastatic melanoma and disease progression following treatment with ipilimumab or a BRAF inhibitor.
Adverse effects of PD-1 inhibitors are similar to those seen with ipilimumab, the most common (occurring in at least 20%) being fatigue, cough, nausea, pruritus, rash, decreased appetite, constipation, muscle pain, and diarrhea. Serious effects from pembrolizumab (occurring in at least 2%) were kidney failure, dyspnea, pneumonia, and cellulitis. As seen with ipilimumab, clinically significant autoimmune adverse reactions occur with PD-1 inhibitors, including pneumonitis, colitis, hypophysitis, nephritis, and hepatitis.
Combination therapy under investigation
A phase 1 trial using combination therapy with both immune checkpoint inhibitors—nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4)—in patients with treatment-resistant metastatic melanoma was published in 2013.42 More than half of patients achieved objective responses, with tumor regression of at least 80% in those who had a response. Tumor response was evident in all subgroups of patients studied—those with pretreatment elevated lactate dehydrogenase levels (one of the strongest prognostic factors in metastatic melanoma), metastases to distant sites, and bulky, multifocal tumor burden. Based on these results, a phase 3 trial is now under way looking at the combination of these two medications vs either one alone.
In summary, targeted treatments are changing the paradigm of how common dermatologic conditions associated with significant morbidity and mortality are treated. Although implementation of the above treatments into everyday clinical practice is exciting, future studies surrounding each are needed to address unanswered issues, such as the optimal dosing and treatment schedules in terms of both disease response and inhibition of resistance, optimal patient/disease characteristics for use, and optimal drug treatment combinations. In the meantime, basic research still utilizing classic molecular biology techniques to uncover pathogenic disease mechanisms in even more detail is ongoing and hopefully will lead to development of even better targeted treatments or even cures for these diseases.
- Lyons TG, O’Kane GM, Kelly CM. Efficacy and safety of vismodegib: a new therapeutic agent in the treatment of basal cell carcinoma. Expert Opin Drug Saf 2014; 13:1125–1132.
- McCusker M, Basset-Sequin N, Dummer R, et al. Metastatic basal cell carcinoma: prognosis dependent on anatomic site and spread of disease. Eur J Cancer 2014; 50:774–783.
- Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 1996; 85:841–851.
- Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol 1998; 110:885–888.
- Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nat Rev Genet 2006; 7:841–850.
- Robarge KD, Brunton SA, Castanedo GM, et al. GDC-0449-a potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett 2009; 19:5576–5581.
- Proctor AE, Thompson LA, O’Bryant CL. Vismodegib: an inhibitor of the Hedgehog signaling pathway in the treatment of basal cell carcinoma. Ann Pharmacother 2014; 48:99–106.
- Dessinioti C, Plaka M, Stratigos AJ. Vismodegib for the treatment of basal cell carcinoma: results and implications of the ERIVANCE BCC trial. Future Oncol 2014; 10:927–936.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 2012; 366:2171–2179.
- Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with basal-cell nevus syndrome. N Engl J Med 2012; 366:2180–2188.
- Brinkhuizen T, Reinders MG, van Geel M, et al. Acquired resistance to the Hedgehog pathway inhibitor vismodegib due to smoothened mutations in treatment of locally advanced basal cell carcinoma. J Am Acad Dermatol 2014; 71:1005–1008.
- Ally MS, Aasi S, Wysong A, et al. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol 2014; 71:904–911.
- Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41:401–407.
- Gelfand JM, Niemann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006; 296:1735–1741.
- Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol 2014; 71:141–150.
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008; 117:244–279.
- Nestle FL, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361:496–509.
- Mentor A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2007; 58:106–115.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003; 349:2014–2022.
- Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005; 366:1367–1374.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371:1665–1674.
- Papp KA, Langley RG, Lebwohl M, et al; PHOENIX 2 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371:1675–1684.
- Leonardi CL, Gordon KB. New and emerging therapies in psoriasis. Semin Cut Med Surg 2014; 33(suppl 2):S37–S41.
- Langley RG, Elewski BE, Lebwohl, et al for the ERASURE and FIXTURE Study Groups. Secukinumab in plaque psorisis—results of two phase 3 trials. N Engl J Med 2014; 371:326–338.
- Leonardi C, Matheson R, Zachariae C. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 2012; 366:1190–1199.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 2012; 366:1181–1189.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis 2014; 73:349–356.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014; 370:2295–2306.
- Girotti MR, Saturno G, Lorigan P, Marais R. No longer an untreatable disease: how targeted and immunotherapies have changed the management of melanoma patients. Molec Oncol 2014, 8:1140–1158.
- Saranga-Perry V, Ambe C, Zager JS, Kudchadkar RR. Recent developments in the medical and surgical treatment of melanoma. CA Canc J Clin 2014; 64:171–185.
- Shah DJ, Dronca RS. Latest advances in chemotherapeutic, targeted, and immune approaches in the treatment of metastatic melanoma. Mayo Clin Proc 2014; 89:504–519.
- Davies H, Ignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417:949–954.
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010; 363:809–819.
- Chapman PB, Hauschild A, Robert C. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507–2516.
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380:358–365.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012; 367:107–114.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371:1867–1876.
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Eng J Med 2014; 371:1877–1888.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–723.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320–330.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384:1109–1117.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122–133.
- Lyons TG, O’Kane GM, Kelly CM. Efficacy and safety of vismodegib: a new therapeutic agent in the treatment of basal cell carcinoma. Expert Opin Drug Saf 2014; 13:1125–1132.
- McCusker M, Basset-Sequin N, Dummer R, et al. Metastatic basal cell carcinoma: prognosis dependent on anatomic site and spread of disease. Eur J Cancer 2014; 50:774–783.
- Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 1996; 85:841–851.
- Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol 1998; 110:885–888.
- Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nat Rev Genet 2006; 7:841–850.
- Robarge KD, Brunton SA, Castanedo GM, et al. GDC-0449-a potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett 2009; 19:5576–5581.
- Proctor AE, Thompson LA, O’Bryant CL. Vismodegib: an inhibitor of the Hedgehog signaling pathway in the treatment of basal cell carcinoma. Ann Pharmacother 2014; 48:99–106.
- Dessinioti C, Plaka M, Stratigos AJ. Vismodegib for the treatment of basal cell carcinoma: results and implications of the ERIVANCE BCC trial. Future Oncol 2014; 10:927–936.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 2012; 366:2171–2179.
- Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with basal-cell nevus syndrome. N Engl J Med 2012; 366:2180–2188.
- Brinkhuizen T, Reinders MG, van Geel M, et al. Acquired resistance to the Hedgehog pathway inhibitor vismodegib due to smoothened mutations in treatment of locally advanced basal cell carcinoma. J Am Acad Dermatol 2014; 71:1005–1008.
- Ally MS, Aasi S, Wysong A, et al. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol 2014; 71:904–911.
- Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41:401–407.
- Gelfand JM, Niemann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006; 296:1735–1741.
- Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol 2014; 71:141–150.
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008; 117:244–279.
- Nestle FL, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361:496–509.
- Mentor A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2007; 58:106–115.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003; 349:2014–2022.
- Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005; 366:1367–1374.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371:1665–1674.
- Papp KA, Langley RG, Lebwohl M, et al; PHOENIX 2 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371:1675–1684.
- Leonardi CL, Gordon KB. New and emerging therapies in psoriasis. Semin Cut Med Surg 2014; 33(suppl 2):S37–S41.
- Langley RG, Elewski BE, Lebwohl, et al for the ERASURE and FIXTURE Study Groups. Secukinumab in plaque psorisis—results of two phase 3 trials. N Engl J Med 2014; 371:326–338.
- Leonardi C, Matheson R, Zachariae C. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 2012; 366:1190–1199.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 2012; 366:1181–1189.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis 2014; 73:349–356.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014; 370:2295–2306.
- Girotti MR, Saturno G, Lorigan P, Marais R. No longer an untreatable disease: how targeted and immunotherapies have changed the management of melanoma patients. Molec Oncol 2014, 8:1140–1158.
- Saranga-Perry V, Ambe C, Zager JS, Kudchadkar RR. Recent developments in the medical and surgical treatment of melanoma. CA Canc J Clin 2014; 64:171–185.
- Shah DJ, Dronca RS. Latest advances in chemotherapeutic, targeted, and immune approaches in the treatment of metastatic melanoma. Mayo Clin Proc 2014; 89:504–519.
- Davies H, Ignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417:949–954.
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010; 363:809–819.
- Chapman PB, Hauschild A, Robert C. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507–2516.
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380:358–365.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012; 367:107–114.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371:1867–1876.
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Eng J Med 2014; 371:1877–1888.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–723.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320–330.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384:1109–1117.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122–133.
KEY POINTS
- Vismodegib, an inhibitor of the “hedgehog” pathway, dramatically shrinks basal cell carcinomas, but resistance and adverse effects remain troublesome. Using it to shrink tumors to operable size may be its best future role.
- Th-17 cells and interleukin 17 are now thought to play central roles in the pathogenesis of psoriasis. Clinical trials of new drugs that block interleukin 17 show striking improvement in skin manifestations with few side effects. Benefits in psoriatic arthritis have not yet been shown.
- About half of patients with melanoma harbor BRAF mutations, and new treatments that target this pathway have improved survival rates. For melanoma not involving BRAF mutations, a better understanding of how tumors evade immune control has led to improved immunotherapies. These targeted medications mark the first major advancements in metastatic melanoma treatment in decades.
The Surviving Sepsis Campaign: Where have we been and where are we going?
Sepsis is familiar to most physicians in clinical practice, but guidance from the medical literature on how best to manage it has traditionally been confusing.
Starting in 2002, the Surviving Sepsis Campaign has worked to reduce worldwide mortality from severe sepsis and septic shock by developing and publicizing guidelines of best practices based on evidence from the literature. The campaign published its first management guidelines in 2004.
In this article, I review the most recent guidelines1,2 (published in 2013) and discuss the campaign’s ongoing performance-improvement program.
DEFINING SEPSIS
Sepsis is a known or suspected infection plus systemic manifestations of infection. This includes the sepsis inflammatory response syndrome. Criteria include:
- Tachycardia (heart rate > 90 beats per minute)
- Tachypnea (> 20 breaths/minute or Paco2 < 32 mm Hg)
- Fever (temperature > 38.3°C [100.9°F]) or hypothermia (core temperature < 36°C [96.8°F])
- High or low white blood cell count (> 12.0 × 109/L or < 4.0 × 109/L), or a normal count with more than 10% immature cells.
The definition of sepsis was broadened in 2002 to include other systemic manifestations of infection, such as changes in blood glucose level and organ dysfunction.
Severe sepsis is defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion due to infection, with tissue hypoperfusion defined as:
- Hypotension (systolic blood pressure < 90 mm Hg, or a drop in systolic blood pressure of > 40 mm Hg)
- Elevated lactate
- Low urine output
- Altered mental status.
In severe sepsis, organ dysfunction is caused by blood-borne toxins and involves acute lung and kidney injury, coagulopathy (thrombocytopenia and increased international normalized ratio), and liver dysfunction.
Septic shock is present when a patient requires vasopressors after adequate intravascular volume repletion.
SEPSIS IS DEADLY AND COSTLY
Severe sepsis is the leading cause of hospital death. Patients admitted with severe sepsis are eight times more likely to die than those admitted with other conditions.3 The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare.
THE SURVIVING SEPSIS CAMPAIGN
The Surviving Sepsis Campaign is a global effort to reduce the rate of death from severe sepsis. The campaign’s methods include:
- Educating physicians, the public, the media, and government about the high rates of morbidity and death in severe sepsis
- Creating evidence-based guidelines for managing sepsis and establishing global best-practice standards
- Facilitating the transfer of knowledge by developing performance-improvement programs to change bedside practice.
The campaign is funded with a grant from the Gordon and Betty Moore Foundation. The campaign’s guidelines are not associated with any direct or indirect industry support. The 2013 guidelines were backed by 30 international organizations.1,2
All recommendations are ranked with numerical and letter scores, according to the GRADE system: 1 indicates a strong recommendation and 2 a weak one. The letters A through D reflect the quality of evidence, ranging from high (A) to very low (D).
GIVING ANTIBIOTICS EARLY IMPROVES OUTCOMES
A number of studies have suggested that starting appropriate antibiotics early improves outcomes in severe sepsis and septic shock. The death rate increases with each hour of delay.4
Recommendation. Intravenous antibiotic therapy should be started as soon as possible, and within the first hour after recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C).
The feasibility of achieving this goal has not been scientifically validated, and the recommendation should not be misinterpreted as the current standard of care. Even hospitals that participate in performance-improvement programs often struggle to start antibiotics, even within 6 hours of recognition. Nevertheless, the goal is a good one.
Some have questioned the early antibiotic recommendation because of concerns about antibiotic overuse and resistance. For a patient with some manifestation of systemic inflammation, such as organ dysfunction or hypotension with no clear cause, the campaign’s position is to provide empiric antibiotics early and then, if a noninfectious cause is found, to stop the antibiotics. Moreover, as soon as a causative pathogen has been identified, the regimen should be switched to the most appropriate antimicrobial that covers the pathogen and is safe and cost-effective. Collaboration with an antimicrobial stewardship program, if available, is encouraged.
FIND THE INFECTION SOURCE PROMPTLY: SOURCE CONTROL MAY BE REQUIRED
Recommendation. A specific anatomic diagnosis of infection (eg, necrotizing soft-tissue infection, peritonitis complicated by intra-abdominal infection, cholangitis, intestinal infarction) requiring consideration of emergency source control should be confirmed or excluded as soon as possible. If needed, surgical drainage should be undertaken for source control within the first 12 hours after a diagnosis is made (grade 1C).
FLUID THERAPY: CRYSTALLOIDS FIRST
Recommendation. In fluid resuscitation of severe sepsis, use crystalloids first (grade 1B).
No head-to-head trial has shown albumin to be superior to crystalloids, and crystalloids are less expensive. However, normal saline has a higher chloride content than plasma, which leads to non-anion-gap metabolic acidosis. It is called an unbalanced crystalloid, having a high chloride content and no buffer. There is concern that this reduces renal blood flow and the glomerular filtration rate, creating the potential for acute kidney injury. Although no high-level evidence supports this concern, some animal studies and historical control studies suggest that a balanced crystalloid such as Ringer’s lactate, Ringer’s acetate, or PlasmaLyte (having a chloride content close to that of plasma and the buffers acetate or lactate) may be associated with better outcome in resuscitation of severe sepsis.
Use albumin solution if necessary
Recommendation. Albumin should be used in the fluid resuscitation of severe sepsis and septic shock for patients who require substantial amounts of crystalloids (grade 2C).
Finfer et al5 compared the effect of fluid resuscitation with either an albumin or saline solution in nearly 7,000 patients in intensive care and found that death rates over 28 days were nearly identical between the two groups. Although this study was not designed to measure an effect in subsets of patients, the subgroup with severe sepsis had a lower mortality rate with albumin (relative risk 0.87, 95% confidence interval 0.74–1.02). In a meta-analysis of 17 studies of albumin vs crystalloids or albumin vs saline, Delaney et al6 found a significant survival advantage with an albumin solution in patients with sepsis and severe septic shock.
Sometimes, in patients admitted to intensive care with septic shock and receiving two or three vasopressors and large amounts of a crystalloid solution, vasopressors can be reduced when fluid is being given, but as soon as the fluid infusion rate is decreased, the need for increasing vasopressors returns. This scenario is an indication for changing to an albumin solution.
Recommendation. Initial fluid challenge in sepsis-induced tissue hypoperfusion (as evidenced by hypotension or elevated lactate) with suspicion of hypovolemia should be a minimum of 30 mL/kg of crystalloids, a portion of which can be an albumin equivalent. Some patients require more rapid administration and greater amounts of fluid (grade 1B).
Other fluid resuscitation considerations
Recommendation. Hydroxyethyl starch (hetastarch) should not be used for fluid resuscitation of severe sepsis and septic shock (grade 1B).
Five large clinical trials7–11 compared hetastarch with crystalloids in the resuscitation of severe sepsis or septic shock. None found an advantage to using hetastarch, and three found it to be associated with higher rates of acute kidney injury and renal-replacement therapy.
Blood is not considered a resuscitation fluid.
Full fluid replacement is still needed in heart or kidney disease
Often, doctors hesitate to administer full fluid resuscitation to patients with septic shock or sepsis-induced hypotension who have baseline cardiomyopathy with a low ejection fraction or who have end-stage renal disease and are anuric. However, these patients’ baseline intravascular volume status has changed because of venodilation and capillary leak leading to reduced blood return to the heart. They require the same amount of fluids as other patients to return to their baseline state.
To avoid fluid overload in these patients, however, we recommend providing fluid in smaller boluses. For a young, previously healthy patient, 2 L of crystalloid should be provided as quickly as possible. Patients with heart or kidney disease should receive smaller (250- or 500-mL) boluses, with oxygen saturation checked after each dose, as hypoxemia is one of only two potential downsides of aggressive fluid resuscitation (the other being the further raising of intra-abdominal pressure in the intra-abdominal compartment syndrome).
WHAT DRIVES HYPOTENSION IN SEPTIC SHOCK?
In septic shock, mechanisms that can lower the blood pressure include capillary leakage (loss of intravascular volume), decreased arteriolar resistance, decreased cardiac contractility, increased ventricular compliance, and increased venous capacitance (loss of intra-arterial volume).
Capillary leakage ranges from moderate to severe, and it is difficult to know the severity early on during resuscitation. The extent of capillary leakage is often apparent only after 24 hours of fluid resuscitation, when the large amount of fluid needed to maintain intravascular volume produces significant tissue edema. Within the first 24 hours of resuscitation of a patient with septic shock or in the presence of ongoing inflammation, one cannot use intake and output to judge the adequacy of fluid resuscitation.
Reduced arteriolar resistance may be an advantage in the nonhypotensive severely septic patient, compensating for the decreased ejection fraction, but it becomes problematic in the presence of hypotension. In addition, venodilation increases venous capacitance, producing a “sink” for blood and inadequate return of blood volume to the heart.
Decreased contractility of the left and right ventricles leads to compensatory sinus tachycardia.12 Reduced heart contractility can be seen by radionuclide angiography: little difference in chamber size is apparent in systole (immediately before contraction) vs diastole (immediately after contraction) (Figure 1).
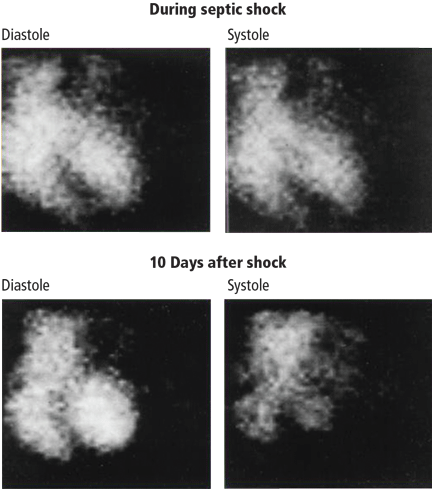
NOREPINEPHRINE IS THE FIRST-CHOICE VASOPRESSOR
If a patient remains hypotensive after replacement of intravascular volume, the hypotension is due to a combination of vasodilation and reduced contractility, and a combined inotrope-vasopressor is an appropriate drug to raise blood pressure. Therefore, the drug of first choice for raising blood pressure should be a combined inotrope-vasopressor.
There are three combined inotrope-vasopressors: dopamine, norepinephrine, and epinephrine. Head-to-head comparisons of norepinephrine and dopamine have supported a survival advantage with norepinephrine in patients with shock, including septic shock.13 A meta-analysis of six randomized trials totaling 2,768 patients also supports norepinephrine over dopamine in septic shock. Dopamine has been associated with a higher incidence of tachyarrhythmic events.14
Recommendations. Norepinephrine is the first choice for vasopressor therapy (grade 1B). If an additional agent is needed to maintain blood pressure, epinephrine should be added to norepinephrine (grade 2B). Alternatively, vasopressin (0.03 U/minute) can be added to norepinephrine to raise mean arterial pressure to target or to decrease the norepinephrine dose (ungraded recommendation).
Dopamine is not recommended as empiric or additive therapy for septic shock. It may be considered, however, in the presence of septic shock with sinus bradycardia.
Phenylephrine for special cases
Phenylephrine is a pure vasopressor: it decreases stroke volume and is particularly disadvantageous in patients with low cardiac output.
Recommendation. Phenylephrine is not recommended as empiric or additive therapy in the treatment of septic shock, with these exceptions (grade 1C):
- In unusual cases in which norepinephrine is associated with serious tachyarrhythmia, phenylephrine would be the least likely vasopressor to exacerbate arrhythmia
- If cardiac output is known to be high and blood pressure is persistently low
- If it is used as salvage therapy when combined inotrope-vasopressor drugs and low-dose vasopressin have failed to achieve the mean arterial pressure target.
RESUSCITATION OF SEPSIS-INDUCED TISSUE HYPOPERFUSION
A more severe form of sepsis-induced tissue hypoperfusion occurs in patients with severe sepsis, who require vasopressors after fluid challenge or have a lactate level of at least 4 mmol/L (36 mg/dL). Initial resuscitation is of utmost importance in these patients and often is done in the emergency department or regular hospital unit. These patients are targeted for “quantitative resuscitation,” ie, a protocol of fluid therapy and vasoactive agent support to achieve predefined end points.
Rivers et al15 published a landmark study of “early goal-directed therapy” targeting the early management of sepsis-induced tissue hypoperfusion (vasopressor requirement after fluid resuscitation or lactate > 4 mmol/L) and reported significant improvement in the survival rate when resuscitation was targeted to a superior vena cava oxygen saturation of 70%. Both control-group and active-treatment-group patients had central venous pressure targets of 8 mm Hg or greater. The Surviving Sepsis Campaign adopted these targets as recommendations in the original 2004 guidelines and continued through the 2013 guidelines, although the campaign’s sepsis management “bundles” that had originally included specific targets for central venous pressure and central venous oxygen saturation as above were changed in the 2013 guidelines to only measuring these variables (see discussion below).
Jones et al16 analyzed studies that involved early (within 24 hours of presentation) vs late (after 24 hours or unknown) quantitative resuscitation for sepsis-induced tissue hypoperfusion and found a significant reduction in the rate of death with early resuscitation but no difference with late resuscitation compared with standard therapy.
ALTERNATIVES TO MEASURING PRESSURE TO PREDICT RESPONSE TO FLUID
The campaign recognizes the limitation of pressure measurements to predict the response to fluid resuscitation. Some clinicians have objected to the guidelines, arguing that new bedside technology provides better information than central venous pressure or superior vena cava oxygen saturation.
It is useful to recall the Starling principle, which is based on the behavior of isolated myocardial fibrils that are put under the strain of graduated weights and then are stimulated to contract, modeling the contractility of the heart. The more the fibril is stretched, the more intense the contraction. Increased contractility explains why fluid resuscitation increases cardiac output; it is not simply a matter of increasing fluid volume in the veins. Increased volume in the left ventricle increases stretch, causing more intense contractility and higher stroke-volume cardiac output.
The guidelines are based on pressure measurements, but volume is the important measure that drives contractility. For this reason, the 2013 guidelines encourage the use of alternative measures if a hospital has the capability to assess and use them. These alternative measures include changes in pulse pressure, systolic pressure, and stroke volume during the respiratory cycle or with fluid bolus. The greater the variation in these measures, the more likely the patient will respond to additional fluid therapy.17 Normal values:
- Pulse pressure variation: < 13%
- Systolic pressure variation: < 10 mm Hg
- Stroke volume variation: < 10%.
The problem with the more sophisticated technologies is that they tend to be available only in academic centers and not at hospitals doing the critical early resuscitation of septic shock.
The serum lactate level
Measuring serum lactate levels is an alternative method for monitoring resuscitation of early septic shock. This method is widely available even with point-of-care testing. If the lactate level is elevated, quantitative resuscitation, fluids, inotropes, and oxygen delivery can be targeted to lactate clearance.
Recommendation. In patients in whom elevated lactate levels are used as a marker of tissue hypoperfusion, resuscitation should be targeted to normalize lactate as rapidly as possible (grade 2C).
STEROID THERAPY IS CONTROVERSIAL
Corticosteroid therapy for septic shock remains controversial. Although it has been deemphasized, it likely has a role in select patients.
Recommendation. Intravenous corticosteroids should not be used in adults with septic shock if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability (grade 2C). However, a patient on high doses of multiple vasopressors after adequate fluid resuscitation would likely benefit.
Recommendation. If corticosteroid therapy is used, hydrocortisone 200 mg should be given over 24 hours, preferentially by continuous intravenous infusion but alternatively 50 mg every 6 hours (grade 2D). This regimen can be continued for up to 7 days or tapered when shock resolves.
SURVIVING SEPSIS CAMPAIGN PERFORMANCE-IMPROVEMENT PROGRAM
By themselves, guidelines change bedside care very slowly. To effect change, protocols must be put in place and quality indicators must be measured. Beginning in 2005, the Surviving Sepsis Campaign converted its guidelines to selected sets of quality indicators, ie, severe sepsis bundles. The campaign published tools that hospitals could use to initiate performance improvement programs for diagnosis and management of severe sepsis and septic shock. The information was disseminated worldwide with a free software program. The program allowed data collection at the bedside to record performance with quality indicators.
In addition, the campaign requested user data so that performance could be tracked over time. In 2010, data on more than 10,000 patients in participating hospitals showed improved ability to achieve quality indicators. The longer a hospital continued the program, the better its compliance with management bundles; in addition, there was a concomitant reduction in hospital mortality rates.18
At this time, the database holds records for more than 30,000 patients. Mortality rates among campaign participants decreased from 37% in the first quarter to 26% in the 16th quarter worldwide, with a reduced relative risk of mortality of 28%.19 To assess whether background factors unrelated to campaign participation were contributing to the reduced rates, mortality rates of long-term participants were compared with those of new program participants; the finding supported the association with program participation.
Bundles revised
The campaign published updated performance bundles in the 2013 guidelines.
The 3-hour bundle remains the same. Within the first 3 hours of presentation with sepsis:
- Measure the serum lactate level.
- Obtain blood cultures before starting antibiotics.
- Start broad-spectrum antibiotics.
- Give a crystalloid (30 mL/kg) for hypotension or for lactate ≥ 4 mmol/L.
The 6-hour bundle has changed somewhat. Within 6 hours of presentation:
- If hypotension does not respond to initial fluid resuscitation, apply vasopressors to maintain mean arterial pressure ≥ 65 mm Hg.
- In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥ 4 mmol/L, measure central venous pressure and central venous oxygen saturation.
- Remeasure lactate if the initial lactate level was elevated.
In light of the campaign’s recognition of alternatives to central venous pressure and central venous oxygen saturation for quantitative resuscitation targets, specific targets for these measures were not defined, allowing institutions the flexibility to base decisions on other technologies, such as inferior vena cava ultrasonography, systolic pressure variation, and changes in flow measures or estimates with fluid boluses if they have the capability.
Moreover, the second point in the 6-hour bundle is being further revised. The Protocolized Care for Early Septic Shock (ProCESS) trial20 and the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,21 both published in 2013, demonstrated that measuring central venous pressure and central venous oxygen saturation, although safe, is not necessary for successful resuscitation of patients with septic shock. Therefore, newer versions of the 6-hour bundle propose that physicians reassess intravascular volume status and tissue perfusion, after initial 30 mL/kg crystalloid administration, in the event of persistent hypotension (mean arterial pressure < 65 mm Hg, ie, vasopressor requirement) or an initial lactate level of 4 mmol/L or higher, and then document the findings. To meet the requirements, one must document either a repeat focused examination by a licensed independent practitioner (to include vital signs, cardiopulmonary, capillary refill, pulse, and skin findings) or two alternative items from the following options: central venous pressure, central venous oxygen saturation, bedside cardiovascular ultrasonography, and dynamic assessment of fluid responsiveness with passive leg-raising or fluid challenge.
Of interest, the ProCESS20 and ARISE21 trials supported early identification of septic shock, early use of antibiotics, and early aggressive fluid resuscitation as the likely reasons for the reduced mortality rates across all treatment groups in these studies.
REDUCING HOSPITAL MORTALITY RATES
Phase 3 of the campaign involves data from 30,000 patients with severe sepsis or septic shock in emergency departments (52%), medical and surgical units (35%), and critical care units (13%).
Hospital mortality rates were 28% for those who presented to the emergency department with sepsis vs 47% for those who developed it in the hospital.22 The reason for the substantial difference is unclear; possibly, diagnosis takes longer in medical and surgical units because of a lower nurse-to-patient ratio, leading to delay in diagnosis and treatment.
The finding of the greater risk of dying from sepsis in those who develop severe sepsis on medical and surgical floors has led to initiation of phase 4 of the campaign, conducted in four US-based collaborative groups in California, Illinois, New Jersey, and Florida, with 12 to 20 sites per collaborative. The collaborative is funded by the Moore Foundation and sponsored by the Society of Critical Care Medicine and the Society of Hospital Medicine. The purpose is to improve early recognition of severe sepsis through nurse screening of every patient during every shift of every day of hospitalization. The program empowers nurses to recognize and report sepsis, severe sepsis, and septic shock. The response differs depending on the hospital: some employ a rapid response or “sepsis alert,” others have a designated hospitalist on each shift who is informed, and hospitals that use private doctors may have a call-in system.
MUCH REMAINS TO BE DONE
The Surviving Sepsis Campaign has come far since the initial guidelines published in 2004. Thirty international organizations now sponsor and support the evidence-based guidelines. The sepsis performance improvement program deployed internationally has been associated with significant improvement in outcome in patients with severe sepsis.
How much of this is related to the campaign as opposed to other changes in health care cannot be clearly ascertained. In addition, how much of the Surviving Sepsis Campaign’s performance-improvement program effect is from attention to this patient group or from precise indicators is difficult to deduce. However, most experts in the field believe the Surviving Sepsis Campaign has significantly improved outcomes since its inception in 2002. Much still needs to be done as new evidence evolves.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. HCHS Data Brief No. 62, June 2011. https://www.cdc.gov/nchs/products/databriefs/db62.htm.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Finfer S, Bellomo R, Boyce N, Frency J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39:389–391.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Guidet B, Martinet O, Boulain T, et al. Assessment of haemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94.
- Perner A, Haase N, Guttormsen AB, et al; the 6S Trial Group and the Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130.0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Annane D, Siami S, Jaber S, et al; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817.
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31:946–955.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012; 40:725–730.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Jones AE, Brown MD, Trzeciak S, et al; Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734–2739.
- Parry-Jones AJD, Pittman JAL. Arterial pressure and stroke volume variability as measurements for cardiovascular optimisation. Int J Intensive Care 2003; 2:67–72.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374.
- Levy M, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12:919–924.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693.
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506.
- Levy MM, Dellinger RP, Townsend SA, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222-231.
Sepsis is familiar to most physicians in clinical practice, but guidance from the medical literature on how best to manage it has traditionally been confusing.
Starting in 2002, the Surviving Sepsis Campaign has worked to reduce worldwide mortality from severe sepsis and septic shock by developing and publicizing guidelines of best practices based on evidence from the literature. The campaign published its first management guidelines in 2004.
In this article, I review the most recent guidelines1,2 (published in 2013) and discuss the campaign’s ongoing performance-improvement program.
DEFINING SEPSIS
Sepsis is a known or suspected infection plus systemic manifestations of infection. This includes the sepsis inflammatory response syndrome. Criteria include:
- Tachycardia (heart rate > 90 beats per minute)
- Tachypnea (> 20 breaths/minute or Paco2 < 32 mm Hg)
- Fever (temperature > 38.3°C [100.9°F]) or hypothermia (core temperature < 36°C [96.8°F])
- High or low white blood cell count (> 12.0 × 109/L or < 4.0 × 109/L), or a normal count with more than 10% immature cells.
The definition of sepsis was broadened in 2002 to include other systemic manifestations of infection, such as changes in blood glucose level and organ dysfunction.
Severe sepsis is defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion due to infection, with tissue hypoperfusion defined as:
- Hypotension (systolic blood pressure < 90 mm Hg, or a drop in systolic blood pressure of > 40 mm Hg)
- Elevated lactate
- Low urine output
- Altered mental status.
In severe sepsis, organ dysfunction is caused by blood-borne toxins and involves acute lung and kidney injury, coagulopathy (thrombocytopenia and increased international normalized ratio), and liver dysfunction.
Septic shock is present when a patient requires vasopressors after adequate intravascular volume repletion.
SEPSIS IS DEADLY AND COSTLY
Severe sepsis is the leading cause of hospital death. Patients admitted with severe sepsis are eight times more likely to die than those admitted with other conditions.3 The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare.
THE SURVIVING SEPSIS CAMPAIGN
The Surviving Sepsis Campaign is a global effort to reduce the rate of death from severe sepsis. The campaign’s methods include:
- Educating physicians, the public, the media, and government about the high rates of morbidity and death in severe sepsis
- Creating evidence-based guidelines for managing sepsis and establishing global best-practice standards
- Facilitating the transfer of knowledge by developing performance-improvement programs to change bedside practice.
The campaign is funded with a grant from the Gordon and Betty Moore Foundation. The campaign’s guidelines are not associated with any direct or indirect industry support. The 2013 guidelines were backed by 30 international organizations.1,2
All recommendations are ranked with numerical and letter scores, according to the GRADE system: 1 indicates a strong recommendation and 2 a weak one. The letters A through D reflect the quality of evidence, ranging from high (A) to very low (D).
GIVING ANTIBIOTICS EARLY IMPROVES OUTCOMES
A number of studies have suggested that starting appropriate antibiotics early improves outcomes in severe sepsis and septic shock. The death rate increases with each hour of delay.4
Recommendation. Intravenous antibiotic therapy should be started as soon as possible, and within the first hour after recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C).
The feasibility of achieving this goal has not been scientifically validated, and the recommendation should not be misinterpreted as the current standard of care. Even hospitals that participate in performance-improvement programs often struggle to start antibiotics, even within 6 hours of recognition. Nevertheless, the goal is a good one.
Some have questioned the early antibiotic recommendation because of concerns about antibiotic overuse and resistance. For a patient with some manifestation of systemic inflammation, such as organ dysfunction or hypotension with no clear cause, the campaign’s position is to provide empiric antibiotics early and then, if a noninfectious cause is found, to stop the antibiotics. Moreover, as soon as a causative pathogen has been identified, the regimen should be switched to the most appropriate antimicrobial that covers the pathogen and is safe and cost-effective. Collaboration with an antimicrobial stewardship program, if available, is encouraged.
FIND THE INFECTION SOURCE PROMPTLY: SOURCE CONTROL MAY BE REQUIRED
Recommendation. A specific anatomic diagnosis of infection (eg, necrotizing soft-tissue infection, peritonitis complicated by intra-abdominal infection, cholangitis, intestinal infarction) requiring consideration of emergency source control should be confirmed or excluded as soon as possible. If needed, surgical drainage should be undertaken for source control within the first 12 hours after a diagnosis is made (grade 1C).
FLUID THERAPY: CRYSTALLOIDS FIRST
Recommendation. In fluid resuscitation of severe sepsis, use crystalloids first (grade 1B).
No head-to-head trial has shown albumin to be superior to crystalloids, and crystalloids are less expensive. However, normal saline has a higher chloride content than plasma, which leads to non-anion-gap metabolic acidosis. It is called an unbalanced crystalloid, having a high chloride content and no buffer. There is concern that this reduces renal blood flow and the glomerular filtration rate, creating the potential for acute kidney injury. Although no high-level evidence supports this concern, some animal studies and historical control studies suggest that a balanced crystalloid such as Ringer’s lactate, Ringer’s acetate, or PlasmaLyte (having a chloride content close to that of plasma and the buffers acetate or lactate) may be associated with better outcome in resuscitation of severe sepsis.
Use albumin solution if necessary
Recommendation. Albumin should be used in the fluid resuscitation of severe sepsis and septic shock for patients who require substantial amounts of crystalloids (grade 2C).
Finfer et al5 compared the effect of fluid resuscitation with either an albumin or saline solution in nearly 7,000 patients in intensive care and found that death rates over 28 days were nearly identical between the two groups. Although this study was not designed to measure an effect in subsets of patients, the subgroup with severe sepsis had a lower mortality rate with albumin (relative risk 0.87, 95% confidence interval 0.74–1.02). In a meta-analysis of 17 studies of albumin vs crystalloids or albumin vs saline, Delaney et al6 found a significant survival advantage with an albumin solution in patients with sepsis and severe septic shock.
Sometimes, in patients admitted to intensive care with septic shock and receiving two or three vasopressors and large amounts of a crystalloid solution, vasopressors can be reduced when fluid is being given, but as soon as the fluid infusion rate is decreased, the need for increasing vasopressors returns. This scenario is an indication for changing to an albumin solution.
Recommendation. Initial fluid challenge in sepsis-induced tissue hypoperfusion (as evidenced by hypotension or elevated lactate) with suspicion of hypovolemia should be a minimum of 30 mL/kg of crystalloids, a portion of which can be an albumin equivalent. Some patients require more rapid administration and greater amounts of fluid (grade 1B).
Other fluid resuscitation considerations
Recommendation. Hydroxyethyl starch (hetastarch) should not be used for fluid resuscitation of severe sepsis and septic shock (grade 1B).
Five large clinical trials7–11 compared hetastarch with crystalloids in the resuscitation of severe sepsis or septic shock. None found an advantage to using hetastarch, and three found it to be associated with higher rates of acute kidney injury and renal-replacement therapy.
Blood is not considered a resuscitation fluid.
Full fluid replacement is still needed in heart or kidney disease
Often, doctors hesitate to administer full fluid resuscitation to patients with septic shock or sepsis-induced hypotension who have baseline cardiomyopathy with a low ejection fraction or who have end-stage renal disease and are anuric. However, these patients’ baseline intravascular volume status has changed because of venodilation and capillary leak leading to reduced blood return to the heart. They require the same amount of fluids as other patients to return to their baseline state.
To avoid fluid overload in these patients, however, we recommend providing fluid in smaller boluses. For a young, previously healthy patient, 2 L of crystalloid should be provided as quickly as possible. Patients with heart or kidney disease should receive smaller (250- or 500-mL) boluses, with oxygen saturation checked after each dose, as hypoxemia is one of only two potential downsides of aggressive fluid resuscitation (the other being the further raising of intra-abdominal pressure in the intra-abdominal compartment syndrome).
WHAT DRIVES HYPOTENSION IN SEPTIC SHOCK?
In septic shock, mechanisms that can lower the blood pressure include capillary leakage (loss of intravascular volume), decreased arteriolar resistance, decreased cardiac contractility, increased ventricular compliance, and increased venous capacitance (loss of intra-arterial volume).
Capillary leakage ranges from moderate to severe, and it is difficult to know the severity early on during resuscitation. The extent of capillary leakage is often apparent only after 24 hours of fluid resuscitation, when the large amount of fluid needed to maintain intravascular volume produces significant tissue edema. Within the first 24 hours of resuscitation of a patient with septic shock or in the presence of ongoing inflammation, one cannot use intake and output to judge the adequacy of fluid resuscitation.
Reduced arteriolar resistance may be an advantage in the nonhypotensive severely septic patient, compensating for the decreased ejection fraction, but it becomes problematic in the presence of hypotension. In addition, venodilation increases venous capacitance, producing a “sink” for blood and inadequate return of blood volume to the heart.
Decreased contractility of the left and right ventricles leads to compensatory sinus tachycardia.12 Reduced heart contractility can be seen by radionuclide angiography: little difference in chamber size is apparent in systole (immediately before contraction) vs diastole (immediately after contraction) (Figure 1).

NOREPINEPHRINE IS THE FIRST-CHOICE VASOPRESSOR
If a patient remains hypotensive after replacement of intravascular volume, the hypotension is due to a combination of vasodilation and reduced contractility, and a combined inotrope-vasopressor is an appropriate drug to raise blood pressure. Therefore, the drug of first choice for raising blood pressure should be a combined inotrope-vasopressor.
There are three combined inotrope-vasopressors: dopamine, norepinephrine, and epinephrine. Head-to-head comparisons of norepinephrine and dopamine have supported a survival advantage with norepinephrine in patients with shock, including septic shock.13 A meta-analysis of six randomized trials totaling 2,768 patients also supports norepinephrine over dopamine in septic shock. Dopamine has been associated with a higher incidence of tachyarrhythmic events.14
Recommendations. Norepinephrine is the first choice for vasopressor therapy (grade 1B). If an additional agent is needed to maintain blood pressure, epinephrine should be added to norepinephrine (grade 2B). Alternatively, vasopressin (0.03 U/minute) can be added to norepinephrine to raise mean arterial pressure to target or to decrease the norepinephrine dose (ungraded recommendation).
Dopamine is not recommended as empiric or additive therapy for septic shock. It may be considered, however, in the presence of septic shock with sinus bradycardia.
Phenylephrine for special cases
Phenylephrine is a pure vasopressor: it decreases stroke volume and is particularly disadvantageous in patients with low cardiac output.
Recommendation. Phenylephrine is not recommended as empiric or additive therapy in the treatment of septic shock, with these exceptions (grade 1C):
- In unusual cases in which norepinephrine is associated with serious tachyarrhythmia, phenylephrine would be the least likely vasopressor to exacerbate arrhythmia
- If cardiac output is known to be high and blood pressure is persistently low
- If it is used as salvage therapy when combined inotrope-vasopressor drugs and low-dose vasopressin have failed to achieve the mean arterial pressure target.
RESUSCITATION OF SEPSIS-INDUCED TISSUE HYPOPERFUSION
A more severe form of sepsis-induced tissue hypoperfusion occurs in patients with severe sepsis, who require vasopressors after fluid challenge or have a lactate level of at least 4 mmol/L (36 mg/dL). Initial resuscitation is of utmost importance in these patients and often is done in the emergency department or regular hospital unit. These patients are targeted for “quantitative resuscitation,” ie, a protocol of fluid therapy and vasoactive agent support to achieve predefined end points.
Rivers et al15 published a landmark study of “early goal-directed therapy” targeting the early management of sepsis-induced tissue hypoperfusion (vasopressor requirement after fluid resuscitation or lactate > 4 mmol/L) and reported significant improvement in the survival rate when resuscitation was targeted to a superior vena cava oxygen saturation of 70%. Both control-group and active-treatment-group patients had central venous pressure targets of 8 mm Hg or greater. The Surviving Sepsis Campaign adopted these targets as recommendations in the original 2004 guidelines and continued through the 2013 guidelines, although the campaign’s sepsis management “bundles” that had originally included specific targets for central venous pressure and central venous oxygen saturation as above were changed in the 2013 guidelines to only measuring these variables (see discussion below).
Jones et al16 analyzed studies that involved early (within 24 hours of presentation) vs late (after 24 hours or unknown) quantitative resuscitation for sepsis-induced tissue hypoperfusion and found a significant reduction in the rate of death with early resuscitation but no difference with late resuscitation compared with standard therapy.
ALTERNATIVES TO MEASURING PRESSURE TO PREDICT RESPONSE TO FLUID
The campaign recognizes the limitation of pressure measurements to predict the response to fluid resuscitation. Some clinicians have objected to the guidelines, arguing that new bedside technology provides better information than central venous pressure or superior vena cava oxygen saturation.
It is useful to recall the Starling principle, which is based on the behavior of isolated myocardial fibrils that are put under the strain of graduated weights and then are stimulated to contract, modeling the contractility of the heart. The more the fibril is stretched, the more intense the contraction. Increased contractility explains why fluid resuscitation increases cardiac output; it is not simply a matter of increasing fluid volume in the veins. Increased volume in the left ventricle increases stretch, causing more intense contractility and higher stroke-volume cardiac output.
The guidelines are based on pressure measurements, but volume is the important measure that drives contractility. For this reason, the 2013 guidelines encourage the use of alternative measures if a hospital has the capability to assess and use them. These alternative measures include changes in pulse pressure, systolic pressure, and stroke volume during the respiratory cycle or with fluid bolus. The greater the variation in these measures, the more likely the patient will respond to additional fluid therapy.17 Normal values:
- Pulse pressure variation: < 13%
- Systolic pressure variation: < 10 mm Hg
- Stroke volume variation: < 10%.
The problem with the more sophisticated technologies is that they tend to be available only in academic centers and not at hospitals doing the critical early resuscitation of septic shock.
The serum lactate level
Measuring serum lactate levels is an alternative method for monitoring resuscitation of early septic shock. This method is widely available even with point-of-care testing. If the lactate level is elevated, quantitative resuscitation, fluids, inotropes, and oxygen delivery can be targeted to lactate clearance.
Recommendation. In patients in whom elevated lactate levels are used as a marker of tissue hypoperfusion, resuscitation should be targeted to normalize lactate as rapidly as possible (grade 2C).
STEROID THERAPY IS CONTROVERSIAL
Corticosteroid therapy for septic shock remains controversial. Although it has been deemphasized, it likely has a role in select patients.
Recommendation. Intravenous corticosteroids should not be used in adults with septic shock if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability (grade 2C). However, a patient on high doses of multiple vasopressors after adequate fluid resuscitation would likely benefit.
Recommendation. If corticosteroid therapy is used, hydrocortisone 200 mg should be given over 24 hours, preferentially by continuous intravenous infusion but alternatively 50 mg every 6 hours (grade 2D). This regimen can be continued for up to 7 days or tapered when shock resolves.
SURVIVING SEPSIS CAMPAIGN PERFORMANCE-IMPROVEMENT PROGRAM
By themselves, guidelines change bedside care very slowly. To effect change, protocols must be put in place and quality indicators must be measured. Beginning in 2005, the Surviving Sepsis Campaign converted its guidelines to selected sets of quality indicators, ie, severe sepsis bundles. The campaign published tools that hospitals could use to initiate performance improvement programs for diagnosis and management of severe sepsis and septic shock. The information was disseminated worldwide with a free software program. The program allowed data collection at the bedside to record performance with quality indicators.
In addition, the campaign requested user data so that performance could be tracked over time. In 2010, data on more than 10,000 patients in participating hospitals showed improved ability to achieve quality indicators. The longer a hospital continued the program, the better its compliance with management bundles; in addition, there was a concomitant reduction in hospital mortality rates.18
At this time, the database holds records for more than 30,000 patients. Mortality rates among campaign participants decreased from 37% in the first quarter to 26% in the 16th quarter worldwide, with a reduced relative risk of mortality of 28%.19 To assess whether background factors unrelated to campaign participation were contributing to the reduced rates, mortality rates of long-term participants were compared with those of new program participants; the finding supported the association with program participation.
Bundles revised
The campaign published updated performance bundles in the 2013 guidelines.
The 3-hour bundle remains the same. Within the first 3 hours of presentation with sepsis:
- Measure the serum lactate level.
- Obtain blood cultures before starting antibiotics.
- Start broad-spectrum antibiotics.
- Give a crystalloid (30 mL/kg) for hypotension or for lactate ≥ 4 mmol/L.
The 6-hour bundle has changed somewhat. Within 6 hours of presentation:
- If hypotension does not respond to initial fluid resuscitation, apply vasopressors to maintain mean arterial pressure ≥ 65 mm Hg.
- In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥ 4 mmol/L, measure central venous pressure and central venous oxygen saturation.
- Remeasure lactate if the initial lactate level was elevated.
In light of the campaign’s recognition of alternatives to central venous pressure and central venous oxygen saturation for quantitative resuscitation targets, specific targets for these measures were not defined, allowing institutions the flexibility to base decisions on other technologies, such as inferior vena cava ultrasonography, systolic pressure variation, and changes in flow measures or estimates with fluid boluses if they have the capability.
Moreover, the second point in the 6-hour bundle is being further revised. The Protocolized Care for Early Septic Shock (ProCESS) trial20 and the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,21 both published in 2013, demonstrated that measuring central venous pressure and central venous oxygen saturation, although safe, is not necessary for successful resuscitation of patients with septic shock. Therefore, newer versions of the 6-hour bundle propose that physicians reassess intravascular volume status and tissue perfusion, after initial 30 mL/kg crystalloid administration, in the event of persistent hypotension (mean arterial pressure < 65 mm Hg, ie, vasopressor requirement) or an initial lactate level of 4 mmol/L or higher, and then document the findings. To meet the requirements, one must document either a repeat focused examination by a licensed independent practitioner (to include vital signs, cardiopulmonary, capillary refill, pulse, and skin findings) or two alternative items from the following options: central venous pressure, central venous oxygen saturation, bedside cardiovascular ultrasonography, and dynamic assessment of fluid responsiveness with passive leg-raising or fluid challenge.
Of interest, the ProCESS20 and ARISE21 trials supported early identification of septic shock, early use of antibiotics, and early aggressive fluid resuscitation as the likely reasons for the reduced mortality rates across all treatment groups in these studies.
REDUCING HOSPITAL MORTALITY RATES
Phase 3 of the campaign involves data from 30,000 patients with severe sepsis or septic shock in emergency departments (52%), medical and surgical units (35%), and critical care units (13%).
Hospital mortality rates were 28% for those who presented to the emergency department with sepsis vs 47% for those who developed it in the hospital.22 The reason for the substantial difference is unclear; possibly, diagnosis takes longer in medical and surgical units because of a lower nurse-to-patient ratio, leading to delay in diagnosis and treatment.
The finding of the greater risk of dying from sepsis in those who develop severe sepsis on medical and surgical floors has led to initiation of phase 4 of the campaign, conducted in four US-based collaborative groups in California, Illinois, New Jersey, and Florida, with 12 to 20 sites per collaborative. The collaborative is funded by the Moore Foundation and sponsored by the Society of Critical Care Medicine and the Society of Hospital Medicine. The purpose is to improve early recognition of severe sepsis through nurse screening of every patient during every shift of every day of hospitalization. The program empowers nurses to recognize and report sepsis, severe sepsis, and septic shock. The response differs depending on the hospital: some employ a rapid response or “sepsis alert,” others have a designated hospitalist on each shift who is informed, and hospitals that use private doctors may have a call-in system.
MUCH REMAINS TO BE DONE
The Surviving Sepsis Campaign has come far since the initial guidelines published in 2004. Thirty international organizations now sponsor and support the evidence-based guidelines. The sepsis performance improvement program deployed internationally has been associated with significant improvement in outcome in patients with severe sepsis.
How much of this is related to the campaign as opposed to other changes in health care cannot be clearly ascertained. In addition, how much of the Surviving Sepsis Campaign’s performance-improvement program effect is from attention to this patient group or from precise indicators is difficult to deduce. However, most experts in the field believe the Surviving Sepsis Campaign has significantly improved outcomes since its inception in 2002. Much still needs to be done as new evidence evolves.
Sepsis is familiar to most physicians in clinical practice, but guidance from the medical literature on how best to manage it has traditionally been confusing.
Starting in 2002, the Surviving Sepsis Campaign has worked to reduce worldwide mortality from severe sepsis and septic shock by developing and publicizing guidelines of best practices based on evidence from the literature. The campaign published its first management guidelines in 2004.
In this article, I review the most recent guidelines1,2 (published in 2013) and discuss the campaign’s ongoing performance-improvement program.
DEFINING SEPSIS
Sepsis is a known or suspected infection plus systemic manifestations of infection. This includes the sepsis inflammatory response syndrome. Criteria include:
- Tachycardia (heart rate > 90 beats per minute)
- Tachypnea (> 20 breaths/minute or Paco2 < 32 mm Hg)
- Fever (temperature > 38.3°C [100.9°F]) or hypothermia (core temperature < 36°C [96.8°F])
- High or low white blood cell count (> 12.0 × 109/L or < 4.0 × 109/L), or a normal count with more than 10% immature cells.
The definition of sepsis was broadened in 2002 to include other systemic manifestations of infection, such as changes in blood glucose level and organ dysfunction.
Severe sepsis is defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion due to infection, with tissue hypoperfusion defined as:
- Hypotension (systolic blood pressure < 90 mm Hg, or a drop in systolic blood pressure of > 40 mm Hg)
- Elevated lactate
- Low urine output
- Altered mental status.
In severe sepsis, organ dysfunction is caused by blood-borne toxins and involves acute lung and kidney injury, coagulopathy (thrombocytopenia and increased international normalized ratio), and liver dysfunction.
Septic shock is present when a patient requires vasopressors after adequate intravascular volume repletion.
SEPSIS IS DEADLY AND COSTLY
Severe sepsis is the leading cause of hospital death. Patients admitted with severe sepsis are eight times more likely to die than those admitted with other conditions.3 The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare.
THE SURVIVING SEPSIS CAMPAIGN
The Surviving Sepsis Campaign is a global effort to reduce the rate of death from severe sepsis. The campaign’s methods include:
- Educating physicians, the public, the media, and government about the high rates of morbidity and death in severe sepsis
- Creating evidence-based guidelines for managing sepsis and establishing global best-practice standards
- Facilitating the transfer of knowledge by developing performance-improvement programs to change bedside practice.
The campaign is funded with a grant from the Gordon and Betty Moore Foundation. The campaign’s guidelines are not associated with any direct or indirect industry support. The 2013 guidelines were backed by 30 international organizations.1,2
All recommendations are ranked with numerical and letter scores, according to the GRADE system: 1 indicates a strong recommendation and 2 a weak one. The letters A through D reflect the quality of evidence, ranging from high (A) to very low (D).
GIVING ANTIBIOTICS EARLY IMPROVES OUTCOMES
A number of studies have suggested that starting appropriate antibiotics early improves outcomes in severe sepsis and septic shock. The death rate increases with each hour of delay.4
Recommendation. Intravenous antibiotic therapy should be started as soon as possible, and within the first hour after recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C).
The feasibility of achieving this goal has not been scientifically validated, and the recommendation should not be misinterpreted as the current standard of care. Even hospitals that participate in performance-improvement programs often struggle to start antibiotics, even within 6 hours of recognition. Nevertheless, the goal is a good one.
Some have questioned the early antibiotic recommendation because of concerns about antibiotic overuse and resistance. For a patient with some manifestation of systemic inflammation, such as organ dysfunction or hypotension with no clear cause, the campaign’s position is to provide empiric antibiotics early and then, if a noninfectious cause is found, to stop the antibiotics. Moreover, as soon as a causative pathogen has been identified, the regimen should be switched to the most appropriate antimicrobial that covers the pathogen and is safe and cost-effective. Collaboration with an antimicrobial stewardship program, if available, is encouraged.
FIND THE INFECTION SOURCE PROMPTLY: SOURCE CONTROL MAY BE REQUIRED
Recommendation. A specific anatomic diagnosis of infection (eg, necrotizing soft-tissue infection, peritonitis complicated by intra-abdominal infection, cholangitis, intestinal infarction) requiring consideration of emergency source control should be confirmed or excluded as soon as possible. If needed, surgical drainage should be undertaken for source control within the first 12 hours after a diagnosis is made (grade 1C).
FLUID THERAPY: CRYSTALLOIDS FIRST
Recommendation. In fluid resuscitation of severe sepsis, use crystalloids first (grade 1B).
No head-to-head trial has shown albumin to be superior to crystalloids, and crystalloids are less expensive. However, normal saline has a higher chloride content than plasma, which leads to non-anion-gap metabolic acidosis. It is called an unbalanced crystalloid, having a high chloride content and no buffer. There is concern that this reduces renal blood flow and the glomerular filtration rate, creating the potential for acute kidney injury. Although no high-level evidence supports this concern, some animal studies and historical control studies suggest that a balanced crystalloid such as Ringer’s lactate, Ringer’s acetate, or PlasmaLyte (having a chloride content close to that of plasma and the buffers acetate or lactate) may be associated with better outcome in resuscitation of severe sepsis.
Use albumin solution if necessary
Recommendation. Albumin should be used in the fluid resuscitation of severe sepsis and septic shock for patients who require substantial amounts of crystalloids (grade 2C).
Finfer et al5 compared the effect of fluid resuscitation with either an albumin or saline solution in nearly 7,000 patients in intensive care and found that death rates over 28 days were nearly identical between the two groups. Although this study was not designed to measure an effect in subsets of patients, the subgroup with severe sepsis had a lower mortality rate with albumin (relative risk 0.87, 95% confidence interval 0.74–1.02). In a meta-analysis of 17 studies of albumin vs crystalloids or albumin vs saline, Delaney et al6 found a significant survival advantage with an albumin solution in patients with sepsis and severe septic shock.
Sometimes, in patients admitted to intensive care with septic shock and receiving two or three vasopressors and large amounts of a crystalloid solution, vasopressors can be reduced when fluid is being given, but as soon as the fluid infusion rate is decreased, the need for increasing vasopressors returns. This scenario is an indication for changing to an albumin solution.
Recommendation. Initial fluid challenge in sepsis-induced tissue hypoperfusion (as evidenced by hypotension or elevated lactate) with suspicion of hypovolemia should be a minimum of 30 mL/kg of crystalloids, a portion of which can be an albumin equivalent. Some patients require more rapid administration and greater amounts of fluid (grade 1B).
Other fluid resuscitation considerations
Recommendation. Hydroxyethyl starch (hetastarch) should not be used for fluid resuscitation of severe sepsis and septic shock (grade 1B).
Five large clinical trials7–11 compared hetastarch with crystalloids in the resuscitation of severe sepsis or septic shock. None found an advantage to using hetastarch, and three found it to be associated with higher rates of acute kidney injury and renal-replacement therapy.
Blood is not considered a resuscitation fluid.
Full fluid replacement is still needed in heart or kidney disease
Often, doctors hesitate to administer full fluid resuscitation to patients with septic shock or sepsis-induced hypotension who have baseline cardiomyopathy with a low ejection fraction or who have end-stage renal disease and are anuric. However, these patients’ baseline intravascular volume status has changed because of venodilation and capillary leak leading to reduced blood return to the heart. They require the same amount of fluids as other patients to return to their baseline state.
To avoid fluid overload in these patients, however, we recommend providing fluid in smaller boluses. For a young, previously healthy patient, 2 L of crystalloid should be provided as quickly as possible. Patients with heart or kidney disease should receive smaller (250- or 500-mL) boluses, with oxygen saturation checked after each dose, as hypoxemia is one of only two potential downsides of aggressive fluid resuscitation (the other being the further raising of intra-abdominal pressure in the intra-abdominal compartment syndrome).
WHAT DRIVES HYPOTENSION IN SEPTIC SHOCK?
In septic shock, mechanisms that can lower the blood pressure include capillary leakage (loss of intravascular volume), decreased arteriolar resistance, decreased cardiac contractility, increased ventricular compliance, and increased venous capacitance (loss of intra-arterial volume).
Capillary leakage ranges from moderate to severe, and it is difficult to know the severity early on during resuscitation. The extent of capillary leakage is often apparent only after 24 hours of fluid resuscitation, when the large amount of fluid needed to maintain intravascular volume produces significant tissue edema. Within the first 24 hours of resuscitation of a patient with septic shock or in the presence of ongoing inflammation, one cannot use intake and output to judge the adequacy of fluid resuscitation.
Reduced arteriolar resistance may be an advantage in the nonhypotensive severely septic patient, compensating for the decreased ejection fraction, but it becomes problematic in the presence of hypotension. In addition, venodilation increases venous capacitance, producing a “sink” for blood and inadequate return of blood volume to the heart.
Decreased contractility of the left and right ventricles leads to compensatory sinus tachycardia.12 Reduced heart contractility can be seen by radionuclide angiography: little difference in chamber size is apparent in systole (immediately before contraction) vs diastole (immediately after contraction) (Figure 1).

NOREPINEPHRINE IS THE FIRST-CHOICE VASOPRESSOR
If a patient remains hypotensive after replacement of intravascular volume, the hypotension is due to a combination of vasodilation and reduced contractility, and a combined inotrope-vasopressor is an appropriate drug to raise blood pressure. Therefore, the drug of first choice for raising blood pressure should be a combined inotrope-vasopressor.
There are three combined inotrope-vasopressors: dopamine, norepinephrine, and epinephrine. Head-to-head comparisons of norepinephrine and dopamine have supported a survival advantage with norepinephrine in patients with shock, including septic shock.13 A meta-analysis of six randomized trials totaling 2,768 patients also supports norepinephrine over dopamine in septic shock. Dopamine has been associated with a higher incidence of tachyarrhythmic events.14
Recommendations. Norepinephrine is the first choice for vasopressor therapy (grade 1B). If an additional agent is needed to maintain blood pressure, epinephrine should be added to norepinephrine (grade 2B). Alternatively, vasopressin (0.03 U/minute) can be added to norepinephrine to raise mean arterial pressure to target or to decrease the norepinephrine dose (ungraded recommendation).
Dopamine is not recommended as empiric or additive therapy for septic shock. It may be considered, however, in the presence of septic shock with sinus bradycardia.
Phenylephrine for special cases
Phenylephrine is a pure vasopressor: it decreases stroke volume and is particularly disadvantageous in patients with low cardiac output.
Recommendation. Phenylephrine is not recommended as empiric or additive therapy in the treatment of septic shock, with these exceptions (grade 1C):
- In unusual cases in which norepinephrine is associated with serious tachyarrhythmia, phenylephrine would be the least likely vasopressor to exacerbate arrhythmia
- If cardiac output is known to be high and blood pressure is persistently low
- If it is used as salvage therapy when combined inotrope-vasopressor drugs and low-dose vasopressin have failed to achieve the mean arterial pressure target.
RESUSCITATION OF SEPSIS-INDUCED TISSUE HYPOPERFUSION
A more severe form of sepsis-induced tissue hypoperfusion occurs in patients with severe sepsis, who require vasopressors after fluid challenge or have a lactate level of at least 4 mmol/L (36 mg/dL). Initial resuscitation is of utmost importance in these patients and often is done in the emergency department or regular hospital unit. These patients are targeted for “quantitative resuscitation,” ie, a protocol of fluid therapy and vasoactive agent support to achieve predefined end points.
Rivers et al15 published a landmark study of “early goal-directed therapy” targeting the early management of sepsis-induced tissue hypoperfusion (vasopressor requirement after fluid resuscitation or lactate > 4 mmol/L) and reported significant improvement in the survival rate when resuscitation was targeted to a superior vena cava oxygen saturation of 70%. Both control-group and active-treatment-group patients had central venous pressure targets of 8 mm Hg or greater. The Surviving Sepsis Campaign adopted these targets as recommendations in the original 2004 guidelines and continued through the 2013 guidelines, although the campaign’s sepsis management “bundles” that had originally included specific targets for central venous pressure and central venous oxygen saturation as above were changed in the 2013 guidelines to only measuring these variables (see discussion below).
Jones et al16 analyzed studies that involved early (within 24 hours of presentation) vs late (after 24 hours or unknown) quantitative resuscitation for sepsis-induced tissue hypoperfusion and found a significant reduction in the rate of death with early resuscitation but no difference with late resuscitation compared with standard therapy.
ALTERNATIVES TO MEASURING PRESSURE TO PREDICT RESPONSE TO FLUID
The campaign recognizes the limitation of pressure measurements to predict the response to fluid resuscitation. Some clinicians have objected to the guidelines, arguing that new bedside technology provides better information than central venous pressure or superior vena cava oxygen saturation.
It is useful to recall the Starling principle, which is based on the behavior of isolated myocardial fibrils that are put under the strain of graduated weights and then are stimulated to contract, modeling the contractility of the heart. The more the fibril is stretched, the more intense the contraction. Increased contractility explains why fluid resuscitation increases cardiac output; it is not simply a matter of increasing fluid volume in the veins. Increased volume in the left ventricle increases stretch, causing more intense contractility and higher stroke-volume cardiac output.
The guidelines are based on pressure measurements, but volume is the important measure that drives contractility. For this reason, the 2013 guidelines encourage the use of alternative measures if a hospital has the capability to assess and use them. These alternative measures include changes in pulse pressure, systolic pressure, and stroke volume during the respiratory cycle or with fluid bolus. The greater the variation in these measures, the more likely the patient will respond to additional fluid therapy.17 Normal values:
- Pulse pressure variation: < 13%
- Systolic pressure variation: < 10 mm Hg
- Stroke volume variation: < 10%.
The problem with the more sophisticated technologies is that they tend to be available only in academic centers and not at hospitals doing the critical early resuscitation of septic shock.
The serum lactate level
Measuring serum lactate levels is an alternative method for monitoring resuscitation of early septic shock. This method is widely available even with point-of-care testing. If the lactate level is elevated, quantitative resuscitation, fluids, inotropes, and oxygen delivery can be targeted to lactate clearance.
Recommendation. In patients in whom elevated lactate levels are used as a marker of tissue hypoperfusion, resuscitation should be targeted to normalize lactate as rapidly as possible (grade 2C).
STEROID THERAPY IS CONTROVERSIAL
Corticosteroid therapy for septic shock remains controversial. Although it has been deemphasized, it likely has a role in select patients.
Recommendation. Intravenous corticosteroids should not be used in adults with septic shock if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability (grade 2C). However, a patient on high doses of multiple vasopressors after adequate fluid resuscitation would likely benefit.
Recommendation. If corticosteroid therapy is used, hydrocortisone 200 mg should be given over 24 hours, preferentially by continuous intravenous infusion but alternatively 50 mg every 6 hours (grade 2D). This regimen can be continued for up to 7 days or tapered when shock resolves.
SURVIVING SEPSIS CAMPAIGN PERFORMANCE-IMPROVEMENT PROGRAM
By themselves, guidelines change bedside care very slowly. To effect change, protocols must be put in place and quality indicators must be measured. Beginning in 2005, the Surviving Sepsis Campaign converted its guidelines to selected sets of quality indicators, ie, severe sepsis bundles. The campaign published tools that hospitals could use to initiate performance improvement programs for diagnosis and management of severe sepsis and septic shock. The information was disseminated worldwide with a free software program. The program allowed data collection at the bedside to record performance with quality indicators.
In addition, the campaign requested user data so that performance could be tracked over time. In 2010, data on more than 10,000 patients in participating hospitals showed improved ability to achieve quality indicators. The longer a hospital continued the program, the better its compliance with management bundles; in addition, there was a concomitant reduction in hospital mortality rates.18
At this time, the database holds records for more than 30,000 patients. Mortality rates among campaign participants decreased from 37% in the first quarter to 26% in the 16th quarter worldwide, with a reduced relative risk of mortality of 28%.19 To assess whether background factors unrelated to campaign participation were contributing to the reduced rates, mortality rates of long-term participants were compared with those of new program participants; the finding supported the association with program participation.
Bundles revised
The campaign published updated performance bundles in the 2013 guidelines.
The 3-hour bundle remains the same. Within the first 3 hours of presentation with sepsis:
- Measure the serum lactate level.
- Obtain blood cultures before starting antibiotics.
- Start broad-spectrum antibiotics.
- Give a crystalloid (30 mL/kg) for hypotension or for lactate ≥ 4 mmol/L.
The 6-hour bundle has changed somewhat. Within 6 hours of presentation:
- If hypotension does not respond to initial fluid resuscitation, apply vasopressors to maintain mean arterial pressure ≥ 65 mm Hg.
- In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥ 4 mmol/L, measure central venous pressure and central venous oxygen saturation.
- Remeasure lactate if the initial lactate level was elevated.
In light of the campaign’s recognition of alternatives to central venous pressure and central venous oxygen saturation for quantitative resuscitation targets, specific targets for these measures were not defined, allowing institutions the flexibility to base decisions on other technologies, such as inferior vena cava ultrasonography, systolic pressure variation, and changes in flow measures or estimates with fluid boluses if they have the capability.
Moreover, the second point in the 6-hour bundle is being further revised. The Protocolized Care for Early Septic Shock (ProCESS) trial20 and the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,21 both published in 2013, demonstrated that measuring central venous pressure and central venous oxygen saturation, although safe, is not necessary for successful resuscitation of patients with septic shock. Therefore, newer versions of the 6-hour bundle propose that physicians reassess intravascular volume status and tissue perfusion, after initial 30 mL/kg crystalloid administration, in the event of persistent hypotension (mean arterial pressure < 65 mm Hg, ie, vasopressor requirement) or an initial lactate level of 4 mmol/L or higher, and then document the findings. To meet the requirements, one must document either a repeat focused examination by a licensed independent practitioner (to include vital signs, cardiopulmonary, capillary refill, pulse, and skin findings) or two alternative items from the following options: central venous pressure, central venous oxygen saturation, bedside cardiovascular ultrasonography, and dynamic assessment of fluid responsiveness with passive leg-raising or fluid challenge.
Of interest, the ProCESS20 and ARISE21 trials supported early identification of septic shock, early use of antibiotics, and early aggressive fluid resuscitation as the likely reasons for the reduced mortality rates across all treatment groups in these studies.
REDUCING HOSPITAL MORTALITY RATES
Phase 3 of the campaign involves data from 30,000 patients with severe sepsis or septic shock in emergency departments (52%), medical and surgical units (35%), and critical care units (13%).
Hospital mortality rates were 28% for those who presented to the emergency department with sepsis vs 47% for those who developed it in the hospital.22 The reason for the substantial difference is unclear; possibly, diagnosis takes longer in medical and surgical units because of a lower nurse-to-patient ratio, leading to delay in diagnosis and treatment.
The finding of the greater risk of dying from sepsis in those who develop severe sepsis on medical and surgical floors has led to initiation of phase 4 of the campaign, conducted in four US-based collaborative groups in California, Illinois, New Jersey, and Florida, with 12 to 20 sites per collaborative. The collaborative is funded by the Moore Foundation and sponsored by the Society of Critical Care Medicine and the Society of Hospital Medicine. The purpose is to improve early recognition of severe sepsis through nurse screening of every patient during every shift of every day of hospitalization. The program empowers nurses to recognize and report sepsis, severe sepsis, and septic shock. The response differs depending on the hospital: some employ a rapid response or “sepsis alert,” others have a designated hospitalist on each shift who is informed, and hospitals that use private doctors may have a call-in system.
MUCH REMAINS TO BE DONE
The Surviving Sepsis Campaign has come far since the initial guidelines published in 2004. Thirty international organizations now sponsor and support the evidence-based guidelines. The sepsis performance improvement program deployed internationally has been associated with significant improvement in outcome in patients with severe sepsis.
How much of this is related to the campaign as opposed to other changes in health care cannot be clearly ascertained. In addition, how much of the Surviving Sepsis Campaign’s performance-improvement program effect is from attention to this patient group or from precise indicators is difficult to deduce. However, most experts in the field believe the Surviving Sepsis Campaign has significantly improved outcomes since its inception in 2002. Much still needs to be done as new evidence evolves.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. HCHS Data Brief No. 62, June 2011. https://www.cdc.gov/nchs/products/databriefs/db62.htm.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Finfer S, Bellomo R, Boyce N, Frency J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39:389–391.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Guidet B, Martinet O, Boulain T, et al. Assessment of haemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94.
- Perner A, Haase N, Guttormsen AB, et al; the 6S Trial Group and the Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130.0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Annane D, Siami S, Jaber S, et al; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817.
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31:946–955.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012; 40:725–730.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Jones AE, Brown MD, Trzeciak S, et al; Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734–2739.
- Parry-Jones AJD, Pittman JAL. Arterial pressure and stroke volume variability as measurements for cardiovascular optimisation. Int J Intensive Care 2003; 2:67–72.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374.
- Levy M, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12:919–924.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693.
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506.
- Levy MM, Dellinger RP, Townsend SA, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222-231.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. HCHS Data Brief No. 62, June 2011. https://www.cdc.gov/nchs/products/databriefs/db62.htm.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Finfer S, Bellomo R, Boyce N, Frency J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39:389–391.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Guidet B, Martinet O, Boulain T, et al. Assessment of haemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94.
- Perner A, Haase N, Guttormsen AB, et al; the 6S Trial Group and the Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130.0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Annane D, Siami S, Jaber S, et al; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817.
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31:946–955.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012; 40:725–730.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Jones AE, Brown MD, Trzeciak S, et al; Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734–2739.
- Parry-Jones AJD, Pittman JAL. Arterial pressure and stroke volume variability as measurements for cardiovascular optimisation. Int J Intensive Care 2003; 2:67–72.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374.
- Levy M, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12:919–924.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693.
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506.
- Levy MM, Dellinger RP, Townsend SA, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222-231.
KEY POINTS
- Ideally, intravenous antibiotic therapy should start within the first hour after sepsis is recognized; performance improvement protocols set a target of within 3 hours.
- A specific source of infection that requires source control measures should be sought, diagnosed or excluded, and if located, treated as rapidly as possible.
- Crystalloids should be used for initial fluid resuscitation. Adding an albumin-based solution is suggested for patients who require substantial amounts of crystalloids.
- Vasopressors are indicated for those who remain hypotensive despite fluid resuscitation. Norepinephrine should be used initially, and if the target mean arterial pressure cannot be achieved, then epinephrine or low-dose vasopressin is added.
- Corticosteroids should be considered only for patients who remain unstable despite adequate fluid resuscitation and vasopressor therapy.
The ‘skinny’ on eosinophilic esophagitis
Eosinophilic esophagitis is a new disease defined by specific criteria that include a constellation of symptoms. Consensus guidelines define it as a chronic antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.1
Ten years ago, a biopsy that revealed eosinophils in the esophagus was diagnostic, because normally eosinophils are not seen in the esophagus. The current definition has evolved to become more comprehensive and includes clinical, demographic, and radiographic criteria.
This article presents an overview of eosinophilic esophagitis—its pathogenesis, epidemiology, clinical presentation, diagnosis, and management.
ALLERGIC ORIGIN
Eosinophilic esophagitis is best regarded as a systemic rather than a single-organ disease, although current treatments are mostly directed specifically at esophageal inflammation. Evidence is clear that eosinophilic esophagitis is allergy-mediated.
The current “two-hit” etiologic model involves exposure first to aeroallergens that prime the esophagus, followed by food allergens that cause an eosinophilic response with antigen recognition and stimulation of immune cells from the bone marrow. Other allergic avenues may also be present, including those involved with atopy, asthma, eczema, and food allergies, which stimulate the Th2 pathway and lead to esophageal eosinophilia and inflammation.2
The two-hit model is supported experimentally: the disease can be induced in mice by injecting ovalbumin under the skin as a sensitizing agent, then exposing the airway to an aerosol of Aspergillus fumigatus, producing an allergic reaction involving classic Th2 allergy pathways.3 Further evidence is that many patients report that asthma or rhinitis developed years before esophageal disease began.
Patients with eosinophilic esophagitis and their family members have a high prevalence of allergies, and the disease frequently flares up during allergy season. Endoscopic biopsy specimens from patients often reveal increased T cells, mast cells, interleukin (IL)-5, and tumor necrosis factor alpha, all of which stimulate eotaxin and are essential to an allergic reaction. They also have high levels of CD3, CDA, and CD1A antigen-presenting lymphocytes, which are all associated with allergy.
Eosinophilic esophagitis responds to allergy medications, including corticosteroids and IL-5 or IL-13 mast-cell inhibitors. The strongest evidence for an allergic etiology is that withdrawing culpable food allergens leads to resolution of the disease. Peterson et al4 gave 18 adults with eosinophilic esophagitis an elemental diet (ie, a pure amino acid, carbohydrate-based diet in which all suspected allergens have been removed), and in 2 to 4 weeks, the mean number of eosinophils seen histologically fell from 54 to 10 cells per high-power field. The response was nearly complete (≤ 10 eosinophils per high-power field) in 72% of patients. When patients resumed a normal diet, the eosinophil content increased substantially within a few days.
Role of leaky tight junctions
Normally, the junctions between epithelial cells are tight, but many conditions, including allergic and autoimmune diseases, are now believed to involve altered permeability of this tissue. Tight-junction proteins play an important role in regulating antigen delivery and are modulated by cytokines. Activation of cytokines causes the membrane to become more permeable, allowing antigens to get through, leading to an enhanced reaction. In eosinophilic esophagitis, it is postulated that food antigens that pass through the leaky membrane activate CD1-antigen-presenting cells, which then initiate an allergic reaction.5–9
PREVALENCE IS INCREASING
Eosinophilic esophagitis was first described in 1993 with a report of 12 patients who had dysphagia, normal endoscopy, no acid reflux, and intraepithelial eosinophilia.10 The authors recognized that these patients had a distinct disease.
Since then, the disease has increased in prevalence. Kapel et al11 reviewed more than 74,000 endoscopy slides from a national pathology database and found 363 cases, with increasing prevalence during the study period from 2002 to 2005. Looking back further in a similar study, Whitney-Miller et al12 found a 0.3% prevalence from the years 1992 to 2000 vs 3.8% from 2001 to 2004.
Sealock et al13 reviewed the literature to assess the prevalence of eosinophilic esophagitis and found considerable variation depending on the populations sampled. One study from Sweden14 found a prevalence of 0.4% by performing endoscopy in 1,000 randomly selected people from nearly 3,000 responders to a questionnaire on abdominal symptoms. A study based on a Swiss database15 found only a 0.02% prevalence. Other studies show higher rates: a study from Florida that examined biopsy specimens from patients who underwent endoscopy for any reason found a prevalence of 1%.16 Another US study found a 15% prevalence in patients with dysphagia.17 Since these studies were done nearly a decade ago, we can expect the prevalence to be higher today.
Celiac disease has also been increasing in recent decades, as has gluten sensitivity. Allergies in general are on the rise worldwide, including asthma and atopic dermatitis. Theories as to the cause of these increases have focused on ambient antigens, food additives, proton pump inhibitors (PPIs), and the microbiome.18,19
DIAGNOSING EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis is diagnosed with a combination of symptomatic, histologic, and radiographic findings (Table 1). The classic patient is a white male—a child, teenager, or young adult—with dysphagia.
A case series of 23 adult patients20 found a mean age of 35 (age range 18 to 57), with a male preponderance (14:9). There is commonly a history of other allergies, including asthma, allergic rhinitis, and atopic dermatitis. Patients more commonly present with dysphagia than heartburn or other esophageal symptoms.11
Endoscopic findings—eosinophils, later fibrosis
Finding eosinophils in the esophagus is nonspecific and is not sufficient to make the diagnosis. Other systemic diseases can involve esophageal eosinophilia, including Churg-Strauss syndrome, Crohn disease, and helminthic diseases. Whether some are related to eosinophilic esophagitis or are independent is not well understood.
Characteristic findings on endoscopy include a corrugated or ringed appearance and linear furrows, resulting from fibrosis and scarring. “Micro-tears” may also be visible projecting linearly up the esophagus. Multiple white specks are signs of conglomerations of eosinophils and are easily confused with yeast infection. Strictures from scar tissue cause the mucosa to be tight and fragile, making the esophagus very susceptible to tearing during endoscopy.
After years of untreated disease, the esophagus becomes increasingly inflamed and fibrotic. Adult patients with eosinophilic esophagitis who were followed for a decade were found to develop increasing collagen deposition in which the submucosa or even the entire esophageal wall was diffusely fibrotic.21
Radiographic findings—a narrow esophagus
On radiography, the esophagus may appear narrow—not uncommonly one-third to one-quarter the caliber of a normal esophagus. As the esophagus progressively narrows, both eating and treatment become extremely difficult.
Symptoms are different in children and adults
Symptoms reflect the endoscopic changes over time. In children, the condition manifests with feeding difficulties, vomiting, symptoms of gastroesophageal reflux, and abdominal pain as signs of inflammation. As the esophagus becomes fibrotic, teenagers and young adults tend to present with strictures, dysphagia, and food impaction. Of patients who present to an emergency department with food impaction, the major cause is now eosinophilic esophagitis.22
It is important to pay attention to symptoms in children to diagnose the condition and start treatment early to prevent or postpone disease advancement. Medical therapy does not clearly reverse the fibrosis.
As in many chronic benign diseases, patients learn to compensate, so a careful history is essential. Many deny having a swallowing problem, but questioning may reveal that they have always been slow, picky eaters, consuming mostly soft foods and drinking fluids with every bite.
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease can be a challenge, as signs and symptoms overlap.
Veerappan et al23 looked for predictors of eosinophilic esophagitis in 400 adults who underwent routine upper endoscopy, 6.5% of whom had eosinophilic esophagitis. They found significant overlap in medical history for patients with and without the disease; while a higher proportion of patients with eosinophilic esophagitis had a history of asthma, dysphagia, food impactions, dermatitis, and food allergies, these conditions also occurred in other patients.
Similarly, the classic endoscopic findings of eosinophilic esophagitis—rings, furrows, strictures, and plaques—also occur in other conditions.23 Reflux disease can cause scarring from excess acid and may even be associated with eosinophils in the esophagus, indicative of a combination of allergy and reflux. A small-caliber esophagus is also occasionally present in patients with reflux disease.
Ambulatory pH monitoring has been recommended to help determine if gastroesophageal reflux is the cause of esophageal eosinophilia and to guide therapy. However, in a prospective study of 51 patients,24 neither positive nor negative results of initial pH monitoring accurately predicted response to PPIs or steroid therapy. Another study found that half of patients with an eosinophilic esophagitis profile without evidence of acid reflux by pH monitoring responded to treatment with a PPI.25
This raises the question of whether some patients with eosinophilic esophagitis have more acid reflux than is detected by pH monitoring, or alternatively, whether PPIs have other, less-recognized effects besides reducing acidity. Investigators are now ascribing a host of anti-inflammatory actions to PPIs, including effects on antioxidants, inflammatory cells, endothelial cells, and the gut microflora.26 And PPIs may alleviate eosinophilic esophagitis through anti-inflammatory effects rather than by inhibiting secretion of gastric acid.
THREE TYPES OF THERAPY
In general, three types of therapy are available for patients with eosinophilic esophagitis: medications, allergen avoidance, and esophageal dilation (Table 2).
Medications: Try a PPI first, then a corticosteroid
A PPI should be tried even for patients with a classic presentation of eosinophilic esophagitis because some will respond, and long-term PPI therapy is preferable to long-term steroid treatment. Patients should be put on a 2-month course and should then undergo repeat biopsy.
For patients who do not respond to a PPI, a corticosteroid or montelukast can be tried. Topical therapy is showing promise as both a short- and long-term option to bring about remission.27 For administration, a corticosteroid (budesonide or fluticasone) is mixed with a viscous solution, such as water with honey or chocolate syrup, making it thick so it better coats the esophagus. The therapy can be very effective: in up to 8 weeks some patients have a 90% resolution of esophageal eosinophilia. However, about 5% of patients develop a yeast infection, and adrenal suppression is a concern but appears to be uncommon.
Avoidance of allergens
Because eosinophilic esophagitis is an allergic disease, eliminating allergens should be an effective treatment. Unfortunately, from a practical standpoint, elimination is very difficult. The elemental diet formula is expensive and unpalatable, making it impractical for a prolonged period.
Gonsalves et al28 put 50 adult patients with eosinophilic esophagitis on a diet eliminating the six most common foods believed to trigger the disease—wheat, milk, nuts, eggs, soy, and seafood—and found a marked reduction in eosinophils in the proximal and distal esophagus after 6 weeks. Additional triggers that have been identified include rice, corn, and legumes.29
Unfortunately, maintaining a diet without the most commonly identified allergens is not easy. Although some very motivated patients can do it, it is especially hard for teens and young adults. Variations of the diet, such as eliminating just two foods, make following a plan easier. Omitting milk alone would benefit an estimated 20% of patients with eosinophilic esophagitis.
Identifying food triggers is a challenge in itself as there is no good noninvasive method of identifying the allergens. The radioallergosorbent test measures immunoglobulin (Ig) E, and the skin-prick test measures acute hypersensitivity, but neither is very sensitive for the Th2-mediated reaction involved in eosinophilic esophagitis. In early trials, endoscopy and biopsy were painstakingly performed with the removal and reintroduction of every suspected food allergen, requiring multiple biopsies weekly, which is impractical for safety and economic reasons.
Attempts are being made to devise less invasive methods of sampling the esophageal mucosa. Transnasal endoscopy—done as an outpatient procedure with topical anesthesia—is a possibility. Another possibility is the esophageal string test,30 which involves swalling a weighted capsule on a string and then, after an hour, pulling it up again and testing the tissue on the string.
The “cytosponge,” a new device currently under investigation, also uses a string delivery system. The patient swallows a sponge contained in a gelatin capsule and attached to a string. When the capsule dissolves in the stomach—a process that takes only a few minutes—the sponge expands. The string is then pulled up, causing the sponge to sample the esophageal mucosa and thus obtaining a histologic specimen. This method shows promise as an inexpensive and noninvasive way to monitor the disease, although larger studies are needed to establish efficacy.31
Dilation—proceed with caution
Dilation can be an important therapy, especially in teenagers and adults with a fibrotic, narrowed esophagus.
Early on, the procedure often resulted in complications such as deep mucosal tears and perforations. Jung et al32 retrospectively analyzed 293 dilations in 161 patients with eosinophilic esophagitis and found a deep mucosal tear in 27 patients (9%), three perforations, and one incidence of major bleeding. All complications resolved without surgery. Factors associated with increased risk of complications were luminal narrowing in the upper and middle third of the esophagus, a luminal stricture that could not be traversed with a standard upper endoscope, and use of a Savary dilator.
It is critical that dilation be done slowly—a few millimeters at a time. Several sessions may be needed.
TREATMENT DURING REMISSION IS CONTROVERSIAL
Unless the patient with eosinophilic esophagitis can consistently control the disease by avoiding allergens, the question arises of whether to continue treating a patient who is in remission.
On the one hand, there is no known risk of Barrett esophagus or malignancy when the condition is not treated, and weight loss is uncommon because patients tend to accommodate to the condition. However, the long-term consequences are uncertain. Allergies are chronic, and disease progression with more fibrosis should be prevented. Also, food impaction commonly occurs and this requires aggressive dilation, which is risky.
On the other hand, chronic steroid therapy involves risk. The optimum steroid dosage during remission and whether alternate-day dosing is adequate have yet to be determined.
Long-term trials are needed to answer these questions. In the meantime, most physicians tend to aggressively treat this disease, if not with specific food avoidance, then with steroid maintenance therapy.
MONITORING THE DISEASE
Monitoring eosinophilic esophagitis by clinical indicators is difficult. Once fibrosis develops, symptoms often do not reflect underlying pathology. It may turn out that, as in Crohn disease, monitoring mucosal healing rather than symptoms may be best.
Until we know more about this condition, careful monitoring of patients is important. However, it is too early to give specific guidance, such as endoscopy every 2 months or annually. Whether the eosinophil count should be the critical consideration is also unknown.
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128:3–20.
- Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009; 137:1238–1249.
- Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 2001; 107:83–90.
- Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013; 108:759–766.
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 2010; 20:142–149.
- Chang F, Anderson S. Clinical and pathological features of eosinophilic oesophagitis: a review. Pathology 2008; 40:3–8.
- Orlando LA, Orlando RC. Dilated intercellular spaces as a marker of GERD. Curr Gastroenterol Rep 2009; 11:190–194.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet 2010; 42:289–291.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38:109–116.
- Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008; 134:1316–1321.
- Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol 2009; 131:788–792.
- Sealock RJ, Rendon G, El-Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther 2010; 32:712–719.
- Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 2007; 56:615–620.
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115:418–419.
- Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104:828–833.
- Prasad GA, Talley NJ, Romero Y, et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol 2007; 102:2627–2632.
- Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology 2011; 141:1586–1592.
- Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy 1999; 29:342–346.
- Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2008; 6:531–535.
- Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125:1660–1669.
- Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophiic inflammation with esophageal food impaction in adults. Gastrointest Endosc 2005; 61:795–801.
- Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009; 7:420–426.
- Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2012; 35:300–307.
- Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9:110–117.
- Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci 2009; 54:2312–2317.
- Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139:1526–1537.
- Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142:1451–1459.
- Lucendo AJ, Arias Á, González-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013; 131:797–804.
- Fillon SA, Harris JK, Wagner BD, et al. Novel device to sample the esophageal microbiome—the esophageal string test. PLoS One 2012; 7:e42938.
- Katzka DA, Geno DM, Ravi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014. pii: S1542-3565(14)00933-1. doi: 10.1016/j.cgh.2014.06.026. [Epub ahead of print]
- Jung KW, Gundersen N, Kopacova J, et al. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc 2011; 73:15–21.
Eosinophilic esophagitis is a new disease defined by specific criteria that include a constellation of symptoms. Consensus guidelines define it as a chronic antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.1
Ten years ago, a biopsy that revealed eosinophils in the esophagus was diagnostic, because normally eosinophils are not seen in the esophagus. The current definition has evolved to become more comprehensive and includes clinical, demographic, and radiographic criteria.
This article presents an overview of eosinophilic esophagitis—its pathogenesis, epidemiology, clinical presentation, diagnosis, and management.
ALLERGIC ORIGIN
Eosinophilic esophagitis is best regarded as a systemic rather than a single-organ disease, although current treatments are mostly directed specifically at esophageal inflammation. Evidence is clear that eosinophilic esophagitis is allergy-mediated.
The current “two-hit” etiologic model involves exposure first to aeroallergens that prime the esophagus, followed by food allergens that cause an eosinophilic response with antigen recognition and stimulation of immune cells from the bone marrow. Other allergic avenues may also be present, including those involved with atopy, asthma, eczema, and food allergies, which stimulate the Th2 pathway and lead to esophageal eosinophilia and inflammation.2
The two-hit model is supported experimentally: the disease can be induced in mice by injecting ovalbumin under the skin as a sensitizing agent, then exposing the airway to an aerosol of Aspergillus fumigatus, producing an allergic reaction involving classic Th2 allergy pathways.3 Further evidence is that many patients report that asthma or rhinitis developed years before esophageal disease began.
Patients with eosinophilic esophagitis and their family members have a high prevalence of allergies, and the disease frequently flares up during allergy season. Endoscopic biopsy specimens from patients often reveal increased T cells, mast cells, interleukin (IL)-5, and tumor necrosis factor alpha, all of which stimulate eotaxin and are essential to an allergic reaction. They also have high levels of CD3, CDA, and CD1A antigen-presenting lymphocytes, which are all associated with allergy.
Eosinophilic esophagitis responds to allergy medications, including corticosteroids and IL-5 or IL-13 mast-cell inhibitors. The strongest evidence for an allergic etiology is that withdrawing culpable food allergens leads to resolution of the disease. Peterson et al4 gave 18 adults with eosinophilic esophagitis an elemental diet (ie, a pure amino acid, carbohydrate-based diet in which all suspected allergens have been removed), and in 2 to 4 weeks, the mean number of eosinophils seen histologically fell from 54 to 10 cells per high-power field. The response was nearly complete (≤ 10 eosinophils per high-power field) in 72% of patients. When patients resumed a normal diet, the eosinophil content increased substantially within a few days.
Role of leaky tight junctions
Normally, the junctions between epithelial cells are tight, but many conditions, including allergic and autoimmune diseases, are now believed to involve altered permeability of this tissue. Tight-junction proteins play an important role in regulating antigen delivery and are modulated by cytokines. Activation of cytokines causes the membrane to become more permeable, allowing antigens to get through, leading to an enhanced reaction. In eosinophilic esophagitis, it is postulated that food antigens that pass through the leaky membrane activate CD1-antigen-presenting cells, which then initiate an allergic reaction.5–9
PREVALENCE IS INCREASING
Eosinophilic esophagitis was first described in 1993 with a report of 12 patients who had dysphagia, normal endoscopy, no acid reflux, and intraepithelial eosinophilia.10 The authors recognized that these patients had a distinct disease.
Since then, the disease has increased in prevalence. Kapel et al11 reviewed more than 74,000 endoscopy slides from a national pathology database and found 363 cases, with increasing prevalence during the study period from 2002 to 2005. Looking back further in a similar study, Whitney-Miller et al12 found a 0.3% prevalence from the years 1992 to 2000 vs 3.8% from 2001 to 2004.
Sealock et al13 reviewed the literature to assess the prevalence of eosinophilic esophagitis and found considerable variation depending on the populations sampled. One study from Sweden14 found a prevalence of 0.4% by performing endoscopy in 1,000 randomly selected people from nearly 3,000 responders to a questionnaire on abdominal symptoms. A study based on a Swiss database15 found only a 0.02% prevalence. Other studies show higher rates: a study from Florida that examined biopsy specimens from patients who underwent endoscopy for any reason found a prevalence of 1%.16 Another US study found a 15% prevalence in patients with dysphagia.17 Since these studies were done nearly a decade ago, we can expect the prevalence to be higher today.
Celiac disease has also been increasing in recent decades, as has gluten sensitivity. Allergies in general are on the rise worldwide, including asthma and atopic dermatitis. Theories as to the cause of these increases have focused on ambient antigens, food additives, proton pump inhibitors (PPIs), and the microbiome.18,19
DIAGNOSING EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis is diagnosed with a combination of symptomatic, histologic, and radiographic findings (Table 1). The classic patient is a white male—a child, teenager, or young adult—with dysphagia.
A case series of 23 adult patients20 found a mean age of 35 (age range 18 to 57), with a male preponderance (14:9). There is commonly a history of other allergies, including asthma, allergic rhinitis, and atopic dermatitis. Patients more commonly present with dysphagia than heartburn or other esophageal symptoms.11
Endoscopic findings—eosinophils, later fibrosis
Finding eosinophils in the esophagus is nonspecific and is not sufficient to make the diagnosis. Other systemic diseases can involve esophageal eosinophilia, including Churg-Strauss syndrome, Crohn disease, and helminthic diseases. Whether some are related to eosinophilic esophagitis or are independent is not well understood.
Characteristic findings on endoscopy include a corrugated or ringed appearance and linear furrows, resulting from fibrosis and scarring. “Micro-tears” may also be visible projecting linearly up the esophagus. Multiple white specks are signs of conglomerations of eosinophils and are easily confused with yeast infection. Strictures from scar tissue cause the mucosa to be tight and fragile, making the esophagus very susceptible to tearing during endoscopy.
After years of untreated disease, the esophagus becomes increasingly inflamed and fibrotic. Adult patients with eosinophilic esophagitis who were followed for a decade were found to develop increasing collagen deposition in which the submucosa or even the entire esophageal wall was diffusely fibrotic.21
Radiographic findings—a narrow esophagus
On radiography, the esophagus may appear narrow—not uncommonly one-third to one-quarter the caliber of a normal esophagus. As the esophagus progressively narrows, both eating and treatment become extremely difficult.
Symptoms are different in children and adults
Symptoms reflect the endoscopic changes over time. In children, the condition manifests with feeding difficulties, vomiting, symptoms of gastroesophageal reflux, and abdominal pain as signs of inflammation. As the esophagus becomes fibrotic, teenagers and young adults tend to present with strictures, dysphagia, and food impaction. Of patients who present to an emergency department with food impaction, the major cause is now eosinophilic esophagitis.22
It is important to pay attention to symptoms in children to diagnose the condition and start treatment early to prevent or postpone disease advancement. Medical therapy does not clearly reverse the fibrosis.
As in many chronic benign diseases, patients learn to compensate, so a careful history is essential. Many deny having a swallowing problem, but questioning may reveal that they have always been slow, picky eaters, consuming mostly soft foods and drinking fluids with every bite.
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease can be a challenge, as signs and symptoms overlap.
Veerappan et al23 looked for predictors of eosinophilic esophagitis in 400 adults who underwent routine upper endoscopy, 6.5% of whom had eosinophilic esophagitis. They found significant overlap in medical history for patients with and without the disease; while a higher proportion of patients with eosinophilic esophagitis had a history of asthma, dysphagia, food impactions, dermatitis, and food allergies, these conditions also occurred in other patients.
Similarly, the classic endoscopic findings of eosinophilic esophagitis—rings, furrows, strictures, and plaques—also occur in other conditions.23 Reflux disease can cause scarring from excess acid and may even be associated with eosinophils in the esophagus, indicative of a combination of allergy and reflux. A small-caliber esophagus is also occasionally present in patients with reflux disease.
Ambulatory pH monitoring has been recommended to help determine if gastroesophageal reflux is the cause of esophageal eosinophilia and to guide therapy. However, in a prospective study of 51 patients,24 neither positive nor negative results of initial pH monitoring accurately predicted response to PPIs or steroid therapy. Another study found that half of patients with an eosinophilic esophagitis profile without evidence of acid reflux by pH monitoring responded to treatment with a PPI.25
This raises the question of whether some patients with eosinophilic esophagitis have more acid reflux than is detected by pH monitoring, or alternatively, whether PPIs have other, less-recognized effects besides reducing acidity. Investigators are now ascribing a host of anti-inflammatory actions to PPIs, including effects on antioxidants, inflammatory cells, endothelial cells, and the gut microflora.26 And PPIs may alleviate eosinophilic esophagitis through anti-inflammatory effects rather than by inhibiting secretion of gastric acid.
THREE TYPES OF THERAPY
In general, three types of therapy are available for patients with eosinophilic esophagitis: medications, allergen avoidance, and esophageal dilation (Table 2).
Medications: Try a PPI first, then a corticosteroid
A PPI should be tried even for patients with a classic presentation of eosinophilic esophagitis because some will respond, and long-term PPI therapy is preferable to long-term steroid treatment. Patients should be put on a 2-month course and should then undergo repeat biopsy.
For patients who do not respond to a PPI, a corticosteroid or montelukast can be tried. Topical therapy is showing promise as both a short- and long-term option to bring about remission.27 For administration, a corticosteroid (budesonide or fluticasone) is mixed with a viscous solution, such as water with honey or chocolate syrup, making it thick so it better coats the esophagus. The therapy can be very effective: in up to 8 weeks some patients have a 90% resolution of esophageal eosinophilia. However, about 5% of patients develop a yeast infection, and adrenal suppression is a concern but appears to be uncommon.
Avoidance of allergens
Because eosinophilic esophagitis is an allergic disease, eliminating allergens should be an effective treatment. Unfortunately, from a practical standpoint, elimination is very difficult. The elemental diet formula is expensive and unpalatable, making it impractical for a prolonged period.
Gonsalves et al28 put 50 adult patients with eosinophilic esophagitis on a diet eliminating the six most common foods believed to trigger the disease—wheat, milk, nuts, eggs, soy, and seafood—and found a marked reduction in eosinophils in the proximal and distal esophagus after 6 weeks. Additional triggers that have been identified include rice, corn, and legumes.29
Unfortunately, maintaining a diet without the most commonly identified allergens is not easy. Although some very motivated patients can do it, it is especially hard for teens and young adults. Variations of the diet, such as eliminating just two foods, make following a plan easier. Omitting milk alone would benefit an estimated 20% of patients with eosinophilic esophagitis.
Identifying food triggers is a challenge in itself as there is no good noninvasive method of identifying the allergens. The radioallergosorbent test measures immunoglobulin (Ig) E, and the skin-prick test measures acute hypersensitivity, but neither is very sensitive for the Th2-mediated reaction involved in eosinophilic esophagitis. In early trials, endoscopy and biopsy were painstakingly performed with the removal and reintroduction of every suspected food allergen, requiring multiple biopsies weekly, which is impractical for safety and economic reasons.
Attempts are being made to devise less invasive methods of sampling the esophageal mucosa. Transnasal endoscopy—done as an outpatient procedure with topical anesthesia—is a possibility. Another possibility is the esophageal string test,30 which involves swalling a weighted capsule on a string and then, after an hour, pulling it up again and testing the tissue on the string.
The “cytosponge,” a new device currently under investigation, also uses a string delivery system. The patient swallows a sponge contained in a gelatin capsule and attached to a string. When the capsule dissolves in the stomach—a process that takes only a few minutes—the sponge expands. The string is then pulled up, causing the sponge to sample the esophageal mucosa and thus obtaining a histologic specimen. This method shows promise as an inexpensive and noninvasive way to monitor the disease, although larger studies are needed to establish efficacy.31
Dilation—proceed with caution
Dilation can be an important therapy, especially in teenagers and adults with a fibrotic, narrowed esophagus.
Early on, the procedure often resulted in complications such as deep mucosal tears and perforations. Jung et al32 retrospectively analyzed 293 dilations in 161 patients with eosinophilic esophagitis and found a deep mucosal tear in 27 patients (9%), three perforations, and one incidence of major bleeding. All complications resolved without surgery. Factors associated with increased risk of complications were luminal narrowing in the upper and middle third of the esophagus, a luminal stricture that could not be traversed with a standard upper endoscope, and use of a Savary dilator.
It is critical that dilation be done slowly—a few millimeters at a time. Several sessions may be needed.
TREATMENT DURING REMISSION IS CONTROVERSIAL
Unless the patient with eosinophilic esophagitis can consistently control the disease by avoiding allergens, the question arises of whether to continue treating a patient who is in remission.
On the one hand, there is no known risk of Barrett esophagus or malignancy when the condition is not treated, and weight loss is uncommon because patients tend to accommodate to the condition. However, the long-term consequences are uncertain. Allergies are chronic, and disease progression with more fibrosis should be prevented. Also, food impaction commonly occurs and this requires aggressive dilation, which is risky.
On the other hand, chronic steroid therapy involves risk. The optimum steroid dosage during remission and whether alternate-day dosing is adequate have yet to be determined.
Long-term trials are needed to answer these questions. In the meantime, most physicians tend to aggressively treat this disease, if not with specific food avoidance, then with steroid maintenance therapy.
MONITORING THE DISEASE
Monitoring eosinophilic esophagitis by clinical indicators is difficult. Once fibrosis develops, symptoms often do not reflect underlying pathology. It may turn out that, as in Crohn disease, monitoring mucosal healing rather than symptoms may be best.
Until we know more about this condition, careful monitoring of patients is important. However, it is too early to give specific guidance, such as endoscopy every 2 months or annually. Whether the eosinophil count should be the critical consideration is also unknown.
Eosinophilic esophagitis is a new disease defined by specific criteria that include a constellation of symptoms. Consensus guidelines define it as a chronic antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.1
Ten years ago, a biopsy that revealed eosinophils in the esophagus was diagnostic, because normally eosinophils are not seen in the esophagus. The current definition has evolved to become more comprehensive and includes clinical, demographic, and radiographic criteria.
This article presents an overview of eosinophilic esophagitis—its pathogenesis, epidemiology, clinical presentation, diagnosis, and management.
ALLERGIC ORIGIN
Eosinophilic esophagitis is best regarded as a systemic rather than a single-organ disease, although current treatments are mostly directed specifically at esophageal inflammation. Evidence is clear that eosinophilic esophagitis is allergy-mediated.
The current “two-hit” etiologic model involves exposure first to aeroallergens that prime the esophagus, followed by food allergens that cause an eosinophilic response with antigen recognition and stimulation of immune cells from the bone marrow. Other allergic avenues may also be present, including those involved with atopy, asthma, eczema, and food allergies, which stimulate the Th2 pathway and lead to esophageal eosinophilia and inflammation.2
The two-hit model is supported experimentally: the disease can be induced in mice by injecting ovalbumin under the skin as a sensitizing agent, then exposing the airway to an aerosol of Aspergillus fumigatus, producing an allergic reaction involving classic Th2 allergy pathways.3 Further evidence is that many patients report that asthma or rhinitis developed years before esophageal disease began.
Patients with eosinophilic esophagitis and their family members have a high prevalence of allergies, and the disease frequently flares up during allergy season. Endoscopic biopsy specimens from patients often reveal increased T cells, mast cells, interleukin (IL)-5, and tumor necrosis factor alpha, all of which stimulate eotaxin and are essential to an allergic reaction. They also have high levels of CD3, CDA, and CD1A antigen-presenting lymphocytes, which are all associated with allergy.
Eosinophilic esophagitis responds to allergy medications, including corticosteroids and IL-5 or IL-13 mast-cell inhibitors. The strongest evidence for an allergic etiology is that withdrawing culpable food allergens leads to resolution of the disease. Peterson et al4 gave 18 adults with eosinophilic esophagitis an elemental diet (ie, a pure amino acid, carbohydrate-based diet in which all suspected allergens have been removed), and in 2 to 4 weeks, the mean number of eosinophils seen histologically fell from 54 to 10 cells per high-power field. The response was nearly complete (≤ 10 eosinophils per high-power field) in 72% of patients. When patients resumed a normal diet, the eosinophil content increased substantially within a few days.
Role of leaky tight junctions
Normally, the junctions between epithelial cells are tight, but many conditions, including allergic and autoimmune diseases, are now believed to involve altered permeability of this tissue. Tight-junction proteins play an important role in regulating antigen delivery and are modulated by cytokines. Activation of cytokines causes the membrane to become more permeable, allowing antigens to get through, leading to an enhanced reaction. In eosinophilic esophagitis, it is postulated that food antigens that pass through the leaky membrane activate CD1-antigen-presenting cells, which then initiate an allergic reaction.5–9
PREVALENCE IS INCREASING
Eosinophilic esophagitis was first described in 1993 with a report of 12 patients who had dysphagia, normal endoscopy, no acid reflux, and intraepithelial eosinophilia.10 The authors recognized that these patients had a distinct disease.
Since then, the disease has increased in prevalence. Kapel et al11 reviewed more than 74,000 endoscopy slides from a national pathology database and found 363 cases, with increasing prevalence during the study period from 2002 to 2005. Looking back further in a similar study, Whitney-Miller et al12 found a 0.3% prevalence from the years 1992 to 2000 vs 3.8% from 2001 to 2004.
Sealock et al13 reviewed the literature to assess the prevalence of eosinophilic esophagitis and found considerable variation depending on the populations sampled. One study from Sweden14 found a prevalence of 0.4% by performing endoscopy in 1,000 randomly selected people from nearly 3,000 responders to a questionnaire on abdominal symptoms. A study based on a Swiss database15 found only a 0.02% prevalence. Other studies show higher rates: a study from Florida that examined biopsy specimens from patients who underwent endoscopy for any reason found a prevalence of 1%.16 Another US study found a 15% prevalence in patients with dysphagia.17 Since these studies were done nearly a decade ago, we can expect the prevalence to be higher today.
Celiac disease has also been increasing in recent decades, as has gluten sensitivity. Allergies in general are on the rise worldwide, including asthma and atopic dermatitis. Theories as to the cause of these increases have focused on ambient antigens, food additives, proton pump inhibitors (PPIs), and the microbiome.18,19
DIAGNOSING EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis is diagnosed with a combination of symptomatic, histologic, and radiographic findings (Table 1). The classic patient is a white male—a child, teenager, or young adult—with dysphagia.
A case series of 23 adult patients20 found a mean age of 35 (age range 18 to 57), with a male preponderance (14:9). There is commonly a history of other allergies, including asthma, allergic rhinitis, and atopic dermatitis. Patients more commonly present with dysphagia than heartburn or other esophageal symptoms.11
Endoscopic findings—eosinophils, later fibrosis
Finding eosinophils in the esophagus is nonspecific and is not sufficient to make the diagnosis. Other systemic diseases can involve esophageal eosinophilia, including Churg-Strauss syndrome, Crohn disease, and helminthic diseases. Whether some are related to eosinophilic esophagitis or are independent is not well understood.
Characteristic findings on endoscopy include a corrugated or ringed appearance and linear furrows, resulting from fibrosis and scarring. “Micro-tears” may also be visible projecting linearly up the esophagus. Multiple white specks are signs of conglomerations of eosinophils and are easily confused with yeast infection. Strictures from scar tissue cause the mucosa to be tight and fragile, making the esophagus very susceptible to tearing during endoscopy.
After years of untreated disease, the esophagus becomes increasingly inflamed and fibrotic. Adult patients with eosinophilic esophagitis who were followed for a decade were found to develop increasing collagen deposition in which the submucosa or even the entire esophageal wall was diffusely fibrotic.21
Radiographic findings—a narrow esophagus
On radiography, the esophagus may appear narrow—not uncommonly one-third to one-quarter the caliber of a normal esophagus. As the esophagus progressively narrows, both eating and treatment become extremely difficult.
Symptoms are different in children and adults
Symptoms reflect the endoscopic changes over time. In children, the condition manifests with feeding difficulties, vomiting, symptoms of gastroesophageal reflux, and abdominal pain as signs of inflammation. As the esophagus becomes fibrotic, teenagers and young adults tend to present with strictures, dysphagia, and food impaction. Of patients who present to an emergency department with food impaction, the major cause is now eosinophilic esophagitis.22
It is important to pay attention to symptoms in children to diagnose the condition and start treatment early to prevent or postpone disease advancement. Medical therapy does not clearly reverse the fibrosis.
As in many chronic benign diseases, patients learn to compensate, so a careful history is essential. Many deny having a swallowing problem, but questioning may reveal that they have always been slow, picky eaters, consuming mostly soft foods and drinking fluids with every bite.
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease can be a challenge, as signs and symptoms overlap.
Veerappan et al23 looked for predictors of eosinophilic esophagitis in 400 adults who underwent routine upper endoscopy, 6.5% of whom had eosinophilic esophagitis. They found significant overlap in medical history for patients with and without the disease; while a higher proportion of patients with eosinophilic esophagitis had a history of asthma, dysphagia, food impactions, dermatitis, and food allergies, these conditions also occurred in other patients.
Similarly, the classic endoscopic findings of eosinophilic esophagitis—rings, furrows, strictures, and plaques—also occur in other conditions.23 Reflux disease can cause scarring from excess acid and may even be associated with eosinophils in the esophagus, indicative of a combination of allergy and reflux. A small-caliber esophagus is also occasionally present in patients with reflux disease.
Ambulatory pH monitoring has been recommended to help determine if gastroesophageal reflux is the cause of esophageal eosinophilia and to guide therapy. However, in a prospective study of 51 patients,24 neither positive nor negative results of initial pH monitoring accurately predicted response to PPIs or steroid therapy. Another study found that half of patients with an eosinophilic esophagitis profile without evidence of acid reflux by pH monitoring responded to treatment with a PPI.25
This raises the question of whether some patients with eosinophilic esophagitis have more acid reflux than is detected by pH monitoring, or alternatively, whether PPIs have other, less-recognized effects besides reducing acidity. Investigators are now ascribing a host of anti-inflammatory actions to PPIs, including effects on antioxidants, inflammatory cells, endothelial cells, and the gut microflora.26 And PPIs may alleviate eosinophilic esophagitis through anti-inflammatory effects rather than by inhibiting secretion of gastric acid.
THREE TYPES OF THERAPY
In general, three types of therapy are available for patients with eosinophilic esophagitis: medications, allergen avoidance, and esophageal dilation (Table 2).
Medications: Try a PPI first, then a corticosteroid
A PPI should be tried even for patients with a classic presentation of eosinophilic esophagitis because some will respond, and long-term PPI therapy is preferable to long-term steroid treatment. Patients should be put on a 2-month course and should then undergo repeat biopsy.
For patients who do not respond to a PPI, a corticosteroid or montelukast can be tried. Topical therapy is showing promise as both a short- and long-term option to bring about remission.27 For administration, a corticosteroid (budesonide or fluticasone) is mixed with a viscous solution, such as water with honey or chocolate syrup, making it thick so it better coats the esophagus. The therapy can be very effective: in up to 8 weeks some patients have a 90% resolution of esophageal eosinophilia. However, about 5% of patients develop a yeast infection, and adrenal suppression is a concern but appears to be uncommon.
Avoidance of allergens
Because eosinophilic esophagitis is an allergic disease, eliminating allergens should be an effective treatment. Unfortunately, from a practical standpoint, elimination is very difficult. The elemental diet formula is expensive and unpalatable, making it impractical for a prolonged period.
Gonsalves et al28 put 50 adult patients with eosinophilic esophagitis on a diet eliminating the six most common foods believed to trigger the disease—wheat, milk, nuts, eggs, soy, and seafood—and found a marked reduction in eosinophils in the proximal and distal esophagus after 6 weeks. Additional triggers that have been identified include rice, corn, and legumes.29
Unfortunately, maintaining a diet without the most commonly identified allergens is not easy. Although some very motivated patients can do it, it is especially hard for teens and young adults. Variations of the diet, such as eliminating just two foods, make following a plan easier. Omitting milk alone would benefit an estimated 20% of patients with eosinophilic esophagitis.
Identifying food triggers is a challenge in itself as there is no good noninvasive method of identifying the allergens. The radioallergosorbent test measures immunoglobulin (Ig) E, and the skin-prick test measures acute hypersensitivity, but neither is very sensitive for the Th2-mediated reaction involved in eosinophilic esophagitis. In early trials, endoscopy and biopsy were painstakingly performed with the removal and reintroduction of every suspected food allergen, requiring multiple biopsies weekly, which is impractical for safety and economic reasons.
Attempts are being made to devise less invasive methods of sampling the esophageal mucosa. Transnasal endoscopy—done as an outpatient procedure with topical anesthesia—is a possibility. Another possibility is the esophageal string test,30 which involves swalling a weighted capsule on a string and then, after an hour, pulling it up again and testing the tissue on the string.
The “cytosponge,” a new device currently under investigation, also uses a string delivery system. The patient swallows a sponge contained in a gelatin capsule and attached to a string. When the capsule dissolves in the stomach—a process that takes only a few minutes—the sponge expands. The string is then pulled up, causing the sponge to sample the esophageal mucosa and thus obtaining a histologic specimen. This method shows promise as an inexpensive and noninvasive way to monitor the disease, although larger studies are needed to establish efficacy.31
Dilation—proceed with caution
Dilation can be an important therapy, especially in teenagers and adults with a fibrotic, narrowed esophagus.
Early on, the procedure often resulted in complications such as deep mucosal tears and perforations. Jung et al32 retrospectively analyzed 293 dilations in 161 patients with eosinophilic esophagitis and found a deep mucosal tear in 27 patients (9%), three perforations, and one incidence of major bleeding. All complications resolved without surgery. Factors associated with increased risk of complications were luminal narrowing in the upper and middle third of the esophagus, a luminal stricture that could not be traversed with a standard upper endoscope, and use of a Savary dilator.
It is critical that dilation be done slowly—a few millimeters at a time. Several sessions may be needed.
TREATMENT DURING REMISSION IS CONTROVERSIAL
Unless the patient with eosinophilic esophagitis can consistently control the disease by avoiding allergens, the question arises of whether to continue treating a patient who is in remission.
On the one hand, there is no known risk of Barrett esophagus or malignancy when the condition is not treated, and weight loss is uncommon because patients tend to accommodate to the condition. However, the long-term consequences are uncertain. Allergies are chronic, and disease progression with more fibrosis should be prevented. Also, food impaction commonly occurs and this requires aggressive dilation, which is risky.
On the other hand, chronic steroid therapy involves risk. The optimum steroid dosage during remission and whether alternate-day dosing is adequate have yet to be determined.
Long-term trials are needed to answer these questions. In the meantime, most physicians tend to aggressively treat this disease, if not with specific food avoidance, then with steroid maintenance therapy.
MONITORING THE DISEASE
Monitoring eosinophilic esophagitis by clinical indicators is difficult. Once fibrosis develops, symptoms often do not reflect underlying pathology. It may turn out that, as in Crohn disease, monitoring mucosal healing rather than symptoms may be best.
Until we know more about this condition, careful monitoring of patients is important. However, it is too early to give specific guidance, such as endoscopy every 2 months or annually. Whether the eosinophil count should be the critical consideration is also unknown.
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128:3–20.
- Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009; 137:1238–1249.
- Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 2001; 107:83–90.
- Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013; 108:759–766.
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 2010; 20:142–149.
- Chang F, Anderson S. Clinical and pathological features of eosinophilic oesophagitis: a review. Pathology 2008; 40:3–8.
- Orlando LA, Orlando RC. Dilated intercellular spaces as a marker of GERD. Curr Gastroenterol Rep 2009; 11:190–194.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet 2010; 42:289–291.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38:109–116.
- Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008; 134:1316–1321.
- Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol 2009; 131:788–792.
- Sealock RJ, Rendon G, El-Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther 2010; 32:712–719.
- Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 2007; 56:615–620.
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115:418–419.
- Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104:828–833.
- Prasad GA, Talley NJ, Romero Y, et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol 2007; 102:2627–2632.
- Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology 2011; 141:1586–1592.
- Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy 1999; 29:342–346.
- Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2008; 6:531–535.
- Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125:1660–1669.
- Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophiic inflammation with esophageal food impaction in adults. Gastrointest Endosc 2005; 61:795–801.
- Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009; 7:420–426.
- Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2012; 35:300–307.
- Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9:110–117.
- Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci 2009; 54:2312–2317.
- Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139:1526–1537.
- Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142:1451–1459.
- Lucendo AJ, Arias Á, González-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013; 131:797–804.
- Fillon SA, Harris JK, Wagner BD, et al. Novel device to sample the esophageal microbiome—the esophageal string test. PLoS One 2012; 7:e42938.
- Katzka DA, Geno DM, Ravi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014. pii: S1542-3565(14)00933-1. doi: 10.1016/j.cgh.2014.06.026. [Epub ahead of print]
- Jung KW, Gundersen N, Kopacova J, et al. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc 2011; 73:15–21.
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128:3–20.
- Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009; 137:1238–1249.
- Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 2001; 107:83–90.
- Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013; 108:759–766.
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 2010; 20:142–149.
- Chang F, Anderson S. Clinical and pathological features of eosinophilic oesophagitis: a review. Pathology 2008; 40:3–8.
- Orlando LA, Orlando RC. Dilated intercellular spaces as a marker of GERD. Curr Gastroenterol Rep 2009; 11:190–194.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet 2010; 42:289–291.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38:109–116.
- Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008; 134:1316–1321.
- Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol 2009; 131:788–792.
- Sealock RJ, Rendon G, El-Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther 2010; 32:712–719.
- Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 2007; 56:615–620.
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115:418–419.
- Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104:828–833.
- Prasad GA, Talley NJ, Romero Y, et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol 2007; 102:2627–2632.
- Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology 2011; 141:1586–1592.
- Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy 1999; 29:342–346.
- Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2008; 6:531–535.
- Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125:1660–1669.
- Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophiic inflammation with esophageal food impaction in adults. Gastrointest Endosc 2005; 61:795–801.
- Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009; 7:420–426.
- Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2012; 35:300–307.
- Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9:110–117.
- Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci 2009; 54:2312–2317.
- Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139:1526–1537.
- Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142:1451–1459.
- Lucendo AJ, Arias Á, González-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013; 131:797–804.
- Fillon SA, Harris JK, Wagner BD, et al. Novel device to sample the esophageal microbiome—the esophageal string test. PLoS One 2012; 7:e42938.
- Katzka DA, Geno DM, Ravi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014. pii: S1542-3565(14)00933-1. doi: 10.1016/j.cgh.2014.06.026. [Epub ahead of print]
- Jung KW, Gundersen N, Kopacova J, et al. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc 2011; 73:15–21.
KEY POINTS
- Eosinophilic esophagitis is an allergy-mediated, systemic disease.
- It is diagnosed by characteristic symptoms, esophageal biopsy (peak value ≥ 15 eosinophils per high-power field), and response to allergen avoidance or treatment with steroids.
- Therapy with a proton pump inhibitor should be tried even for patients with a classic presentation.
- Strict dietary avoidance of allergens has been shown to resolve the disease but is often impractical.
- Dilation is indicated for a narrowed esophagus but must be done cautiously because of the risk of tearing.
- How best to monitor the disease (eg, by annual endoscopy) is still uncertain.
Ebola virus: Questions, answers, and more questions
A 50-year-old man who returned from a business trip to Nigeria 24 days ago presents with complaints of the sudden onset of fever, diarrhea, myalgia, and headache. He reports 10 bowel movements per day and has seen bloody stools.
During his trip he flew in to Murtala Muhammed International Airport in Lagos, ate meals only in his hotel, and attended meetings in Lagos central business district. He had no exposure to animals, mosquitoes, ticks, or sick people, and no sexual activity. After returning home, he felt well for the first 3 weeks.
The patient has a history of hypertension. He does not smoke, drink alcohol, or use injection drugs. He is married, works with commercial banks and financial institutions, and lives in Cleveland, OH.
On physical examination his temperature is 100.0˚F (37.8˚C), pulse 98, respirations 15, blood pressure 105/70 mm Hg, and weight 78 kg (172 lb). He appears comfortable but is a little diaphoretic. His abdomen is tender to palpation in the epigastrium and slightly to the right; he has no signs of peritonitis. His skin is without rash, bleeding, or bruising. The remainder of the examination is normal.
His white blood cell count is 17 × 109/L, hemoglobin 15 g/dL, hematocrit 41%, and platelet count 172 × 109/L. His sodium level is 126 mmol/L, potassium 3.8 mmol/L, chloride 95 mmol/L, carbon dioxide 20 mmol/L, blood urea nitrogen 11 mg/dL, creatinine 0.7 mg/dL, and glucose 130 mg/dL. His aminotransferase and alkaline phosphatase levels are normal.
Could this patient have Ebola virus disease?
With Ebola virus disease on the rise in West Africa, physicians who encounter patients like this one need to include it in the differential diagnosis. Because the disease is new, many questions are raised for which we as yet have no answers. Here, I will review what we know and do not know in an effort to remove some of the fear and uncertainty.
A NEW DISEASE
Ebola virus disease is a severe hemorrhagic fever caused by negative-sense single-stranded RNA viruses classified by the International Committee on Taxonomy of Viruses as belonging to the genus Ebolavirus in the family Filoviridae. Filoviruses get their name from the Latin filum, or thread-like structure.
The family Filoviridae was discovered in 1967 after inadvertent importation of infected monkeys from Uganda into Yugoslavia and Marburg, Germany. Outbreaks of severe illness occurred in workers at a vaccine plant who came into direct contact with the animals by killing them, removing their kidneys, or preparing primary cell cultures for polio vaccine production.
Ebola virus was discovered in 1976 by Peter Piot, who was working at the Institute of Tropical Medicine in Antwerp, Belgium. The blood of a Belgian woman who had been working in what is now the Democratic Republic of the Congo (formerly Zaire) had been sent to the institute; she and Mabalo Lokela, a school headmaster and the first recorded victim of Ebola virus, had been working near Yambuku, about 96 km from the Ebola River.
Before the 2014 outbreak, all known outbreaks had caused fewer than 2,400 cases across a dozen African countries over 3 decades.
Five species of Ebola virus
The genus Ebolavirus contains five species, each associated with a consistent case-fatality rate and a more or less well-identified endemic area.1
Zaire ebolavirus was recognized in 1976; it has caused multiple outbreaks, with high case-fatality rates.
Sudan ebolavirus was seen first in the 1970s; it has a 50% case-fatality rate.
Tai Forest ebolavirus has been found in only one person, an ethologist working with deceased chimpanzees.
Bundibugyo ebolavirus emerged in 2007 and has a 30% case-fatality rate.
Reston ebolavirus is maintained in an animal reservoir in the Philippines and is not found in Africa. It caused an outbreak of lethal infection in macaques imported into the United States in 1989. There is evidence that Reston ebolavirus can cause asymptomatic infection in humans. None of the caretakers of the macaques became ill, nor did farmers working with infected pigs, although both groups seroconverted.
A reservoir in bats?
A reservoir in nonhuman primates was initially suspected. However, studies subsequently showed that monkeys are susceptible to rapidly lethal filoviral disease, precluding any role as a host for persistent viral infection. It is likely that Ebola virus is maintained in small animals that serve as a source of infection for both humans and wild primates. A prominent suspect is fruit bats, which are consumed in soup in West Africa.
Transmission is person-to-person or nosocomial
Ebola virus is transmitted by direct contact with body fluids such as blood, urine, sweat, vomitus, semen, and breast milk. Filoviruses can initiate infection via ingestion, inhalation (although probably not Ebola), or passage through breaks in the skin. Droplet inoculation into the mouth or eyes has been shown to result from inadvertent transfer of virus from contaminated hands. Patients transmit the virus while febrile and through later stages of disease, as well as postmortem through contact with the body during funeral preparations. The virus has been isolated in semen for as many as 61 days after illness onset.
Ebola virus can also be spread nosocomially. In 1976, a 44-year-old teacher sought care for fever at the Yambuku Mission Hospital. He was given parenteral chloroquine as empiric treatment for presumed malaria, which was routine for all febrile patients. However, he had unrecognized Ebola virus infection. Moreover, syringes were rinsed in the same pan of water and reused, which spread the infection to nearly 100 people, all of whom developed fulminant Ebola virus disease and died. Infection then spread to family caregivers, the hospital staff, and those who prepared the bodies for burial.
Nosocomial transmission was also responsible for an outbreak of Lake Victoria Marburg virus in Uige Province in northern Angola in 2005, with 374 putative cases and 329 deaths. When teams from Médecins Sans Frontières started setting up the Marburg ward, there were five patients with hemorrhagic fever in a makeshift isolation room in the hospital, together with corpses that the hospital staff had been too afraid to remove. Healers found in many rural African communities were administering injections in homes or in makeshift clinics with reused needles or syringes.2
There is no evidence that filoviruses are carried by mosquitoes or other biting arthropods. Also, the risk of transmission via fomites appears to be low when currently recommended infection-control guidelines for the viral hemorrhagic fevers are followed.3 One primary human case generates only one to three secondary cases on average.
EBOLA IS AN IMMUNODEFICIENCY VIRUS
The main targets of infection are endothelial cells, mononuclear phagocytes, and hepatocytes. Ebola virus replicates at an unusually high rate. Macrophages infected with Zaire ebolavirus produce tumor necrosis factor alpha, interleukin (IL) 1 beta, IL-6, macrophage chemotactic protein 1, and nitric oxide. Virus-infected macrophages synthesize cell-surface tissue factor, triggering the extrinsic coagulation pathway.
Ebola is an immunodeficiency virus. Dendritic cells, which initiate adaptive immune responses, are a major site of filoviral replication. Infected cells cannot present antigens to naïve lymphocytes. Patients who die of Ebola virus disease do not develop antibodies to the virus. Lymphocytes remain uninfected, but undergo “bystander” apoptosis induced by inflammatory mediators.
CLINICAL MANIFESTATIONS
The incubation period is generally 5 to 7 days (range 2 to 28 days), during which the patient is not infectious. Symptoms begin abruptly, with fever, chills, general malaise, weakness, severe headache, and myalgia. By the time of case detection in West Africa, most patients also had nausea, vomiting, diarrhea, and abdominal pain. Once symptoms arise, patients have high levels of the virus in their blood and fluids and are infectious. Hemorrhagic symptoms have apparently been uncommon in West Africa, occurring in 1.0% to 5.7%, but “unexplained bleeding” has been documented in 18% of cases.4 Among those in whom the disease enters its hemorrhagic terminal phase, there is characteristic internal and subcutaneous bleeding, vomiting of blood, and subconjunctival hemorrhage.4
Laboratory findings include lymphocytopenia (often with counts as low as 1.0 × 109/L), thrombocytopenia (with counts in the range of 50 to 100 × 109/L), elevated aminotransferase levels (including aspartate aminotransferase levels 7 to 12 times higher than alanine aminotransferase in fatal cases), low total protein (due to capillary leak), and disseminated intravascular coagulation. Those who survive begin to improve in the second week, during which viremia resolves in association with the appearance of virus-specific antibodies.4
DIAGNOSIS
In symptomatic patients, Ebola virus infection is diagnosed by detection in blood or body fluids of viral antigens by enzyme-linked immunosorbent assay, or RNA sequences by reverse transcriptase polymerase chain reaction. The diagnosis is confirmed with cell culture (in a BSL-4 containment laboratory) showing characteristic viral particles by electron microscopy.
CARING FOR PATIENTS
The most detailed descriptions of the care of patients with Ebola virus disease have come from Dr. Bruce Ribner, of Emory University Hospital, in an October 2014 report of his experience caring for Ebola-infected patients at Emory University Hospital in Atlanta, GA.5 He described fluid losses of 5 to 10 L/day, profound hyponatremia, hypokalemia, and hypocalcemia, which were associated with cardiac arrhythmias and the need for intravenous and oral electrolyte repletion and hemodialysis. Intensive one-to-one nursing was critical, as was the coordination of many medical subspecialties. The Emory team arranged point-of-care testing near the unit and generally kept laboratory testing to a minimum. The team was surprised to learn that commercial carriers refused to transport specimens even when they were licensed for category A agents. Difficulties with the local water authority and waste disposal contractor required the hospital to dedicate an autoclave to process all materials used in clinical care.
TREATMENT: SUPPORTIVE AND EXPERIMENTAL
Treatment is supportive to maintain circulatory function and blood pressure and to correct coagulopathy. However, a variety of vaccines, antibodies, small-molecule agents, and antiviral agents are undergoing testing, mostly in animals at this point.
Vaccines. A therapeutic vaccine that worked only slightly was a live-attenuated recombinant vesicular stomatitis virus expressing Ebola virus transmembrane glycoproteins, which was tested in mice, guinea pigs, and rhesus macaques who had been exposed to Ebola virus.6
A preventive vaccine worked better. Stanley et al7 evaluated a replication-defective chimpanzee adenovirus 3-vectored vaccine that also contained Ebola virus glycoprotein. They gave macaques a single injection of this vaccine, and then 5 weeks later gave them a lethal dose of Ebola virus. All the vaccinated animals survived the infection, and half (2 of 4) survived when challenged 10 months later. With a prime-boost strategy (modified vaccinia virus Ankara, a poxvirus), all survived when challenged 10 months later.
KZ52, a neutralizing antibody, did not work. Oswald et al8 gave a human IgG monoclonal antibody against Zaire Ebola virus, designated KZ52, to four rhesus macaques, challenged them with the virus 24 hours later, and administered a second shot of KZ52 on day 4. All of them died.
ZMAb is a combination of three murine monoclonal antibodies, designated 1H3, 2G4, and 4G7. Ad-IFN is a human adenovirus, serotype 5, that expresses human interferon alpha. Qui et al9 gave ZMAb and Ad-IFN to macaques in several experiments. In experiment 1, eight macaques were infected and then were given ZMAb and Ad-IFN 3 days later, and ZMAb again on days 6 and 9. Seven of the eight survived. In a second experiment, Ad-IFN was given first, when the viral load was still less than the limit of detection of known assays, and then ZMAb was given upon detection of viremia and fever. Two of four macaques survived. Control animals had undetectable levels of IgG, whereas Ebola virus GP–specific IgG levels were detected in all survivors. IFN-gamma ELISpots showed high EBOV-GP–specific T-cell response in all survivors.
ZMapp is another cocktail of monoclonal antibodies, containing two from ZMab (2G4 and 4G7), plus a third, c13C6. In experiments in rhesus macaques, three groups of six animals each received three doses of ZMapp at varying times after being infected with Ebola virus: at 3, 6, and 9 days; at 4, 7, and 10 days, and at 5, 8, and 11 days. All 18 macaques treated with ZMapp survived. Thus, Zmapp extended the treatment window to 5 days postexposure.10 One of the American health care workers who contracted Ebola virus in Liberia received this medication.
HSPA5-PMO. Endoplasmic reticulum chaperone heat shock 70 kDa protein 5 (HSPA5) is instrumental in the maturation of envelope proteins in hepatitis C and influenza A virus. It plays a role in viral entry for coxsackievirus A9 and dengue virus serotype 2, and it may be involved in Ebola viral budding. Phosphorodiamidate morpholino oligomers (PMOs) are a class of antisense DNA nucleotide analogs.
Reid et al11 reported that mice treated with HSPA5–PMO were completely protected from lethal Ebola challenge. Therefore, HSPA5 appears to be a promising target for the development of antifilovirus countermeasures.
Favipiravir, an antiviral agent also known as T-705, is a pyrazinecarboxamide derivative. Invented in 2002 by Toyama Chemicals as an inhibitor of influenza virus replication, it acts as a nucleotide analog, selectively inhibiting the viral RNA-dependent RNA polymerase, or causes lethal mutagenesis upon incorporation into the virus RNA. Favipiravir suppresses Ebola virus replication by 4 log10 units in cell culture.12
Mice were challenged with intranasal inoculation of 1,000 focus-forming units of Ebola virus diluted in phosphate-buffered saline. Until the first day of treatment (postinfection day 6), all mice in the T-705 group lost weight similarly to control mice, developed viremia, and showed elevated serum levels of aspartate aminotransferase and alanine aminotransferase. Within 4 days of T-705 treatment (post-infection day 10), the animals had cleared the virus from blood. Surviving mice developed Ebola virus-specific antibodies and CD8+ T cells specific for the viral nucleoprotein.12
The authors hypothesized that suppression of virus replication by T-705 allowed the host to mount a virus-specific adaptive immune response, and concluded that T-705 was 100% effective in the treatment of Zaire Ebola virus infection up to postinfection day 6 but was hardly beneficial at the terminal stage of disease.12 Of note, favipiravir is undergoing phase 2 and phase 3 trials as an anti-influenza agent in Japan.
THE CURRENT OUTBREAK
The current outbreak is with Zaire ebolavirus. It seems to have started in a 2-year-old child who died in Meliandou in Guéckédou Prefecture, Guinea, on December 6, 2013. On March 21, 2014, the Guinea Ministry of Health reported the outbreak of an illness characterized by fever, severe diarrhea, vomiting, and a high case-fatality rate (59%) in 49 persons. On May 25, 2014, Kenema Government Hospital confirmed the first case of Ebola virus disease in Sierra Leone, probably brought there by a traditional healer who had treated Ebola patients from Guinea. Tracing led to 13 additional cases—all women who attended the burial.13
The Center for Systems Biology at Harvard University and the Broad Institute of Massachusetts Institute of Technology generated 99 Ebola virus genome sequences from 78 patients with confirmed disease, representing more than 70% of the patients diagnosed with the disease in Sierra Leone from May to mid-June 2014. They found genetic similarity across the sequenced 2014 samples, suggesting a single transmission from the natural reservoir, followed by human-to-human transmission during the outbreak. Continued human-reservoir exposure is unlikely to have contributed to the growth of this epidemic.14
As of October 14, 2014, there were 8,914 suspected and confirmed cases of Ebola virus infection, and 4,477 deaths.15
But how did Zaire Ebola virus make the 2,000-mile trek from Central Africa to Guinea in West Africa? There are two possibilities: it has always been present in the region but we just never noticed, or it was recently introduced. Bayesian phylogenetic analyses and sequence divergence studies suggest the virus has been present in bat populations in Guinea without previously infecting humans.
Why Guinea and why Guéckédou? Guinea is one of the poorest countries in the world, ranking 178th of 187 countries on the Human Development Index of the United Nations Development Programme, just behind Liberia (174th) and Sierra Leone (177th). In Guinea, the life expectancy is 56 years and the gross national income per capita is $440. The region has been systematically plundered and the forest decimated by clear-cut logging, leaving the Guinea Forest Region largely deforested, resulting in increased contact between humans and the small animals that serve as the source of infection.1
LIMITED CAPACITY, EVEN IN THE UNITED STATES
A few hospitals in the United States have dedicated units to handle serious infectious diseases such as Ebola: Emory University Hospital; Nebraska Medicine in Omaha; Providence St. Patrick Hospital in Missoula, MT; and the National Institutes of Health in Bethesda, MD. However, in total they have only 19 beds.
QUESTIONS, ANSWERS—AND MORE QUESTIONS
(The following is from a question-and-answer discussion that followed Dr. Brizendine’s Grand Rounds presentation.)
Q: Are there any differences between survivors and those who die of the disease? A: We do not know. Patient survival depends on early recognition and supportive care. There are disparities in the care of patients. Schieffelin et al16 analyzed the characteristics of patients who died or who survived in Sierra Leone and found that the mortality rate was higher in older patients and those with a higher viral load on presentation.
Q: Does the virus block production or release of interferon early in infection? A: Yes, it has been shown17 that Ebola virus protein VP24 inhibits signaling downstream of both interferon alpha/beta and interferon gamma by indirectly impairing the transport of a transcription factor termed STAT1. VP24 is also able to bind STAT1 directly. The resulting suppression of host interferon very early on in the incubation phase is key to the virulence of the virus.
Q: Does infection with one of the viral species confer immunity from other species? A: No, there is no cross-immunity.
Q: How soon do patients test positive? A: About 5 days after exposure, when they develop a fever. At this time patients are highly viremic, which PCR can detect.
Q: Before the virus is detectable in the blood, where is it? A: The liver, endothelial cells, antigen-presenting cells, and adrenal glands.
Q: Do we really need to quarantine ill patients and health care workers returning from Africa, per CDC recommendations? A: We don’t know everything, and some people do make bad decisions, such as traveling while symptomatic. I support a period of observation, although confinement is not reasonable, as it may pose a disincentive to cooperation.
Q: What is the role of giving plasma from survivors? A: Dr. Kent Brantly (see American citizens infected with Ebola) received the blood of a 14-year-old who survived. We don’t know. It is not proved. It did not result in improvement in animal models.
Q: Is the bleeding caused by a mechanism similar to that in enterohemorrhagic Escherichia coli infection? A: No. That is a bacterial toxin, whereas this is more like disseminated intravascular coagulation, with an intrinsic pathway anticoagulation cascade.
Q: How long does the virus remain viable outside the body? A: In one study,18 Ebola virus could not be recovered from experimentally contaminated surfaces (plastic, metal or glass) at room temperature. In another in which it was dried onto a surface,19 Ebola virus survived in the dark for several hours between 20 and 25°C. When dried in tissue culture media onto glass and stored at 4°C, it has survived for over 50 days.
Q: How long does the virus remain in breast milk? A: We know it has been detected 15 days after disease onset and think possibly as late as 28 days from symptom onset.3
Q: How are people actually infected? A: I believe people get the virus on their hands and then touch their face, eyes, or mouth. If you are wearing personal protective equipment, it must occur while doffing the equipment.
Q: Could we increase the sensitivity of the test so that we could detect the virus before the onset of symptoms? A: In theory it may be possible. The virus is somewhere in the body during the incubation period. Perhaps we could sample the right compartment in an enriched mononuclear cell line.
Q: When can patients who recover resume their normal activities? A: After their viral load returns to 0, I would still advise abstaining from unprotected sex and from breastfeeding for a few months. but as for other activities, no special precautions are needed.
Q: Does the virus appear to be mutating at a high rate? A: Looking back to 2004, mutations are occurring, but there is no sign that any of these mutations has contributed to the size of the outbreak by changing the characteristics of the Ebola virus. Can it become aerosolized? It has been suggested that the virus that caused the outbreak separated from those that caused past Ebola outbreaks but does not seem to be affecting the spread or efficacy of experimental drugs and vaccines. So, even though it is an RNA virus and mutations are occurring, no serious changes have emerged.14
BACK TO OUR PATIENT
The differential diagnosis for the patient described at the beginning of this paper includes travelers’ diarrhea, malaria, typhoid fever, yellow fever, meningococcal disease … and Ebola virus disease, although this is much less likely in view of the epidemiology and incubation period of this disease. When his stool was tested by enzyme immunoassay and culture, it was found to be positive for Campylobacter. He recovered with oral rehydration.
- Bausch DG, Schwarz L. Outbreak of ebola virus disease in Guinea: where ecology meets economy. PLoS Negl Trop Dis 2014; 8:e3056.
- Roddy P, Thomas SL, Jeffs B, et al. Factors associated with Marburg hemorrhagic fever: analysis of patient data from Uige, Angola. J Infect Dis 2010; 201:1909–1918.
- Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196(suppl 2):S142–S147.
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–1495.
- Ribner BS. Treating patients with Ebola virus infections in the US: lessons learned. Presented at IDWeek, October 8, 2014. Philadelphia PA.
- Feldman H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3:e2.
- Stanley DA, Honko AN, Asiedu C, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126–1129.
- Oswald WB, Geisbert TW, Davis KJ, et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLos Pathog 2007; 3:e9.
- Qui X, Wong G, Fernando L, et al. mAbs and Ad-vectored IFN-a therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med 2013; 5:207ra143.
- Qui X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47–53.
- Reid SP, Shurtleff AC, Costantino JA, et al. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 2014; 109:171–174.
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 2014; 105:17–21.
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus dsease in Guinea. N Engl J Med 2014; 371:1418–1425.
- Gire SK, Goba A, Andersen KG, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014; 345:1369–1372.
- Chamary JV. 4000 deaths and counting: the Ebola epidemic in 4 charts. Forbes. http://www.forbes.com/sites/jvchamary/2014/10/13/ebola-trends. Accessed November 5, 2014.
- Schieffelin JS, Shaffer JG, Goba A, et al, for the KGH Lassa Fever Program, the Viral Hemorrhagic Fever Consortium, and the WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014 Oct 29 [Epub ahead of print]. DOI: 10.1056/NEJMoa1411680.
- Zhang AP, Bornholdt ZA, Liu T, et al. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog 2012; 8:e1002550.
- Piercy TJ, Smither SJ, Steward JA, Eastaugh L, Lever MS. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol 2010; 109:1531–1539.
- Sagripanti JL, Rom AM, Holland LE. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch Virol 2010; 155:2035–2039.
A 50-year-old man who returned from a business trip to Nigeria 24 days ago presents with complaints of the sudden onset of fever, diarrhea, myalgia, and headache. He reports 10 bowel movements per day and has seen bloody stools.
During his trip he flew in to Murtala Muhammed International Airport in Lagos, ate meals only in his hotel, and attended meetings in Lagos central business district. He had no exposure to animals, mosquitoes, ticks, or sick people, and no sexual activity. After returning home, he felt well for the first 3 weeks.
The patient has a history of hypertension. He does not smoke, drink alcohol, or use injection drugs. He is married, works with commercial banks and financial institutions, and lives in Cleveland, OH.
On physical examination his temperature is 100.0˚F (37.8˚C), pulse 98, respirations 15, blood pressure 105/70 mm Hg, and weight 78 kg (172 lb). He appears comfortable but is a little diaphoretic. His abdomen is tender to palpation in the epigastrium and slightly to the right; he has no signs of peritonitis. His skin is without rash, bleeding, or bruising. The remainder of the examination is normal.
His white blood cell count is 17 × 109/L, hemoglobin 15 g/dL, hematocrit 41%, and platelet count 172 × 109/L. His sodium level is 126 mmol/L, potassium 3.8 mmol/L, chloride 95 mmol/L, carbon dioxide 20 mmol/L, blood urea nitrogen 11 mg/dL, creatinine 0.7 mg/dL, and glucose 130 mg/dL. His aminotransferase and alkaline phosphatase levels are normal.
Could this patient have Ebola virus disease?
With Ebola virus disease on the rise in West Africa, physicians who encounter patients like this one need to include it in the differential diagnosis. Because the disease is new, many questions are raised for which we as yet have no answers. Here, I will review what we know and do not know in an effort to remove some of the fear and uncertainty.
A NEW DISEASE
Ebola virus disease is a severe hemorrhagic fever caused by negative-sense single-stranded RNA viruses classified by the International Committee on Taxonomy of Viruses as belonging to the genus Ebolavirus in the family Filoviridae. Filoviruses get their name from the Latin filum, or thread-like structure.
The family Filoviridae was discovered in 1967 after inadvertent importation of infected monkeys from Uganda into Yugoslavia and Marburg, Germany. Outbreaks of severe illness occurred in workers at a vaccine plant who came into direct contact with the animals by killing them, removing their kidneys, or preparing primary cell cultures for polio vaccine production.
Ebola virus was discovered in 1976 by Peter Piot, who was working at the Institute of Tropical Medicine in Antwerp, Belgium. The blood of a Belgian woman who had been working in what is now the Democratic Republic of the Congo (formerly Zaire) had been sent to the institute; she and Mabalo Lokela, a school headmaster and the first recorded victim of Ebola virus, had been working near Yambuku, about 96 km from the Ebola River.
Before the 2014 outbreak, all known outbreaks had caused fewer than 2,400 cases across a dozen African countries over 3 decades.
Five species of Ebola virus
The genus Ebolavirus contains five species, each associated with a consistent case-fatality rate and a more or less well-identified endemic area.1
Zaire ebolavirus was recognized in 1976; it has caused multiple outbreaks, with high case-fatality rates.
Sudan ebolavirus was seen first in the 1970s; it has a 50% case-fatality rate.
Tai Forest ebolavirus has been found in only one person, an ethologist working with deceased chimpanzees.
Bundibugyo ebolavirus emerged in 2007 and has a 30% case-fatality rate.
Reston ebolavirus is maintained in an animal reservoir in the Philippines and is not found in Africa. It caused an outbreak of lethal infection in macaques imported into the United States in 1989. There is evidence that Reston ebolavirus can cause asymptomatic infection in humans. None of the caretakers of the macaques became ill, nor did farmers working with infected pigs, although both groups seroconverted.
A reservoir in bats?
A reservoir in nonhuman primates was initially suspected. However, studies subsequently showed that monkeys are susceptible to rapidly lethal filoviral disease, precluding any role as a host for persistent viral infection. It is likely that Ebola virus is maintained in small animals that serve as a source of infection for both humans and wild primates. A prominent suspect is fruit bats, which are consumed in soup in West Africa.
Transmission is person-to-person or nosocomial
Ebola virus is transmitted by direct contact with body fluids such as blood, urine, sweat, vomitus, semen, and breast milk. Filoviruses can initiate infection via ingestion, inhalation (although probably not Ebola), or passage through breaks in the skin. Droplet inoculation into the mouth or eyes has been shown to result from inadvertent transfer of virus from contaminated hands. Patients transmit the virus while febrile and through later stages of disease, as well as postmortem through contact with the body during funeral preparations. The virus has been isolated in semen for as many as 61 days after illness onset.
Ebola virus can also be spread nosocomially. In 1976, a 44-year-old teacher sought care for fever at the Yambuku Mission Hospital. He was given parenteral chloroquine as empiric treatment for presumed malaria, which was routine for all febrile patients. However, he had unrecognized Ebola virus infection. Moreover, syringes were rinsed in the same pan of water and reused, which spread the infection to nearly 100 people, all of whom developed fulminant Ebola virus disease and died. Infection then spread to family caregivers, the hospital staff, and those who prepared the bodies for burial.
Nosocomial transmission was also responsible for an outbreak of Lake Victoria Marburg virus in Uige Province in northern Angola in 2005, with 374 putative cases and 329 deaths. When teams from Médecins Sans Frontières started setting up the Marburg ward, there were five patients with hemorrhagic fever in a makeshift isolation room in the hospital, together with corpses that the hospital staff had been too afraid to remove. Healers found in many rural African communities were administering injections in homes or in makeshift clinics with reused needles or syringes.2
There is no evidence that filoviruses are carried by mosquitoes or other biting arthropods. Also, the risk of transmission via fomites appears to be low when currently recommended infection-control guidelines for the viral hemorrhagic fevers are followed.3 One primary human case generates only one to three secondary cases on average.
EBOLA IS AN IMMUNODEFICIENCY VIRUS
The main targets of infection are endothelial cells, mononuclear phagocytes, and hepatocytes. Ebola virus replicates at an unusually high rate. Macrophages infected with Zaire ebolavirus produce tumor necrosis factor alpha, interleukin (IL) 1 beta, IL-6, macrophage chemotactic protein 1, and nitric oxide. Virus-infected macrophages synthesize cell-surface tissue factor, triggering the extrinsic coagulation pathway.
Ebola is an immunodeficiency virus. Dendritic cells, which initiate adaptive immune responses, are a major site of filoviral replication. Infected cells cannot present antigens to naïve lymphocytes. Patients who die of Ebola virus disease do not develop antibodies to the virus. Lymphocytes remain uninfected, but undergo “bystander” apoptosis induced by inflammatory mediators.
CLINICAL MANIFESTATIONS
The incubation period is generally 5 to 7 days (range 2 to 28 days), during which the patient is not infectious. Symptoms begin abruptly, with fever, chills, general malaise, weakness, severe headache, and myalgia. By the time of case detection in West Africa, most patients also had nausea, vomiting, diarrhea, and abdominal pain. Once symptoms arise, patients have high levels of the virus in their blood and fluids and are infectious. Hemorrhagic symptoms have apparently been uncommon in West Africa, occurring in 1.0% to 5.7%, but “unexplained bleeding” has been documented in 18% of cases.4 Among those in whom the disease enters its hemorrhagic terminal phase, there is characteristic internal and subcutaneous bleeding, vomiting of blood, and subconjunctival hemorrhage.4
Laboratory findings include lymphocytopenia (often with counts as low as 1.0 × 109/L), thrombocytopenia (with counts in the range of 50 to 100 × 109/L), elevated aminotransferase levels (including aspartate aminotransferase levels 7 to 12 times higher than alanine aminotransferase in fatal cases), low total protein (due to capillary leak), and disseminated intravascular coagulation. Those who survive begin to improve in the second week, during which viremia resolves in association with the appearance of virus-specific antibodies.4
DIAGNOSIS
In symptomatic patients, Ebola virus infection is diagnosed by detection in blood or body fluids of viral antigens by enzyme-linked immunosorbent assay, or RNA sequences by reverse transcriptase polymerase chain reaction. The diagnosis is confirmed with cell culture (in a BSL-4 containment laboratory) showing characteristic viral particles by electron microscopy.
CARING FOR PATIENTS
The most detailed descriptions of the care of patients with Ebola virus disease have come from Dr. Bruce Ribner, of Emory University Hospital, in an October 2014 report of his experience caring for Ebola-infected patients at Emory University Hospital in Atlanta, GA.5 He described fluid losses of 5 to 10 L/day, profound hyponatremia, hypokalemia, and hypocalcemia, which were associated with cardiac arrhythmias and the need for intravenous and oral electrolyte repletion and hemodialysis. Intensive one-to-one nursing was critical, as was the coordination of many medical subspecialties. The Emory team arranged point-of-care testing near the unit and generally kept laboratory testing to a minimum. The team was surprised to learn that commercial carriers refused to transport specimens even when they were licensed for category A agents. Difficulties with the local water authority and waste disposal contractor required the hospital to dedicate an autoclave to process all materials used in clinical care.
TREATMENT: SUPPORTIVE AND EXPERIMENTAL
Treatment is supportive to maintain circulatory function and blood pressure and to correct coagulopathy. However, a variety of vaccines, antibodies, small-molecule agents, and antiviral agents are undergoing testing, mostly in animals at this point.
Vaccines. A therapeutic vaccine that worked only slightly was a live-attenuated recombinant vesicular stomatitis virus expressing Ebola virus transmembrane glycoproteins, which was tested in mice, guinea pigs, and rhesus macaques who had been exposed to Ebola virus.6
A preventive vaccine worked better. Stanley et al7 evaluated a replication-defective chimpanzee adenovirus 3-vectored vaccine that also contained Ebola virus glycoprotein. They gave macaques a single injection of this vaccine, and then 5 weeks later gave them a lethal dose of Ebola virus. All the vaccinated animals survived the infection, and half (2 of 4) survived when challenged 10 months later. With a prime-boost strategy (modified vaccinia virus Ankara, a poxvirus), all survived when challenged 10 months later.
KZ52, a neutralizing antibody, did not work. Oswald et al8 gave a human IgG monoclonal antibody against Zaire Ebola virus, designated KZ52, to four rhesus macaques, challenged them with the virus 24 hours later, and administered a second shot of KZ52 on day 4. All of them died.
ZMAb is a combination of three murine monoclonal antibodies, designated 1H3, 2G4, and 4G7. Ad-IFN is a human adenovirus, serotype 5, that expresses human interferon alpha. Qui et al9 gave ZMAb and Ad-IFN to macaques in several experiments. In experiment 1, eight macaques were infected and then were given ZMAb and Ad-IFN 3 days later, and ZMAb again on days 6 and 9. Seven of the eight survived. In a second experiment, Ad-IFN was given first, when the viral load was still less than the limit of detection of known assays, and then ZMAb was given upon detection of viremia and fever. Two of four macaques survived. Control animals had undetectable levels of IgG, whereas Ebola virus GP–specific IgG levels were detected in all survivors. IFN-gamma ELISpots showed high EBOV-GP–specific T-cell response in all survivors.
ZMapp is another cocktail of monoclonal antibodies, containing two from ZMab (2G4 and 4G7), plus a third, c13C6. In experiments in rhesus macaques, three groups of six animals each received three doses of ZMapp at varying times after being infected with Ebola virus: at 3, 6, and 9 days; at 4, 7, and 10 days, and at 5, 8, and 11 days. All 18 macaques treated with ZMapp survived. Thus, Zmapp extended the treatment window to 5 days postexposure.10 One of the American health care workers who contracted Ebola virus in Liberia received this medication.
HSPA5-PMO. Endoplasmic reticulum chaperone heat shock 70 kDa protein 5 (HSPA5) is instrumental in the maturation of envelope proteins in hepatitis C and influenza A virus. It plays a role in viral entry for coxsackievirus A9 and dengue virus serotype 2, and it may be involved in Ebola viral budding. Phosphorodiamidate morpholino oligomers (PMOs) are a class of antisense DNA nucleotide analogs.
Reid et al11 reported that mice treated with HSPA5–PMO were completely protected from lethal Ebola challenge. Therefore, HSPA5 appears to be a promising target for the development of antifilovirus countermeasures.
Favipiravir, an antiviral agent also known as T-705, is a pyrazinecarboxamide derivative. Invented in 2002 by Toyama Chemicals as an inhibitor of influenza virus replication, it acts as a nucleotide analog, selectively inhibiting the viral RNA-dependent RNA polymerase, or causes lethal mutagenesis upon incorporation into the virus RNA. Favipiravir suppresses Ebola virus replication by 4 log10 units in cell culture.12
Mice were challenged with intranasal inoculation of 1,000 focus-forming units of Ebola virus diluted in phosphate-buffered saline. Until the first day of treatment (postinfection day 6), all mice in the T-705 group lost weight similarly to control mice, developed viremia, and showed elevated serum levels of aspartate aminotransferase and alanine aminotransferase. Within 4 days of T-705 treatment (post-infection day 10), the animals had cleared the virus from blood. Surviving mice developed Ebola virus-specific antibodies and CD8+ T cells specific for the viral nucleoprotein.12
The authors hypothesized that suppression of virus replication by T-705 allowed the host to mount a virus-specific adaptive immune response, and concluded that T-705 was 100% effective in the treatment of Zaire Ebola virus infection up to postinfection day 6 but was hardly beneficial at the terminal stage of disease.12 Of note, favipiravir is undergoing phase 2 and phase 3 trials as an anti-influenza agent in Japan.
THE CURRENT OUTBREAK
The current outbreak is with Zaire ebolavirus. It seems to have started in a 2-year-old child who died in Meliandou in Guéckédou Prefecture, Guinea, on December 6, 2013. On March 21, 2014, the Guinea Ministry of Health reported the outbreak of an illness characterized by fever, severe diarrhea, vomiting, and a high case-fatality rate (59%) in 49 persons. On May 25, 2014, Kenema Government Hospital confirmed the first case of Ebola virus disease in Sierra Leone, probably brought there by a traditional healer who had treated Ebola patients from Guinea. Tracing led to 13 additional cases—all women who attended the burial.13
The Center for Systems Biology at Harvard University and the Broad Institute of Massachusetts Institute of Technology generated 99 Ebola virus genome sequences from 78 patients with confirmed disease, representing more than 70% of the patients diagnosed with the disease in Sierra Leone from May to mid-June 2014. They found genetic similarity across the sequenced 2014 samples, suggesting a single transmission from the natural reservoir, followed by human-to-human transmission during the outbreak. Continued human-reservoir exposure is unlikely to have contributed to the growth of this epidemic.14
As of October 14, 2014, there were 8,914 suspected and confirmed cases of Ebola virus infection, and 4,477 deaths.15
But how did Zaire Ebola virus make the 2,000-mile trek from Central Africa to Guinea in West Africa? There are two possibilities: it has always been present in the region but we just never noticed, or it was recently introduced. Bayesian phylogenetic analyses and sequence divergence studies suggest the virus has been present in bat populations in Guinea without previously infecting humans.
Why Guinea and why Guéckédou? Guinea is one of the poorest countries in the world, ranking 178th of 187 countries on the Human Development Index of the United Nations Development Programme, just behind Liberia (174th) and Sierra Leone (177th). In Guinea, the life expectancy is 56 years and the gross national income per capita is $440. The region has been systematically plundered and the forest decimated by clear-cut logging, leaving the Guinea Forest Region largely deforested, resulting in increased contact between humans and the small animals that serve as the source of infection.1
LIMITED CAPACITY, EVEN IN THE UNITED STATES
A few hospitals in the United States have dedicated units to handle serious infectious diseases such as Ebola: Emory University Hospital; Nebraska Medicine in Omaha; Providence St. Patrick Hospital in Missoula, MT; and the National Institutes of Health in Bethesda, MD. However, in total they have only 19 beds.
QUESTIONS, ANSWERS—AND MORE QUESTIONS
(The following is from a question-and-answer discussion that followed Dr. Brizendine’s Grand Rounds presentation.)
Q: Are there any differences between survivors and those who die of the disease? A: We do not know. Patient survival depends on early recognition and supportive care. There are disparities in the care of patients. Schieffelin et al16 analyzed the characteristics of patients who died or who survived in Sierra Leone and found that the mortality rate was higher in older patients and those with a higher viral load on presentation.
Q: Does the virus block production or release of interferon early in infection? A: Yes, it has been shown17 that Ebola virus protein VP24 inhibits signaling downstream of both interferon alpha/beta and interferon gamma by indirectly impairing the transport of a transcription factor termed STAT1. VP24 is also able to bind STAT1 directly. The resulting suppression of host interferon very early on in the incubation phase is key to the virulence of the virus.
Q: Does infection with one of the viral species confer immunity from other species? A: No, there is no cross-immunity.
Q: How soon do patients test positive? A: About 5 days after exposure, when they develop a fever. At this time patients are highly viremic, which PCR can detect.
Q: Before the virus is detectable in the blood, where is it? A: The liver, endothelial cells, antigen-presenting cells, and adrenal glands.
Q: Do we really need to quarantine ill patients and health care workers returning from Africa, per CDC recommendations? A: We don’t know everything, and some people do make bad decisions, such as traveling while symptomatic. I support a period of observation, although confinement is not reasonable, as it may pose a disincentive to cooperation.
Q: What is the role of giving plasma from survivors? A: Dr. Kent Brantly (see American citizens infected with Ebola) received the blood of a 14-year-old who survived. We don’t know. It is not proved. It did not result in improvement in animal models.
Q: Is the bleeding caused by a mechanism similar to that in enterohemorrhagic Escherichia coli infection? A: No. That is a bacterial toxin, whereas this is more like disseminated intravascular coagulation, with an intrinsic pathway anticoagulation cascade.
Q: How long does the virus remain viable outside the body? A: In one study,18 Ebola virus could not be recovered from experimentally contaminated surfaces (plastic, metal or glass) at room temperature. In another in which it was dried onto a surface,19 Ebola virus survived in the dark for several hours between 20 and 25°C. When dried in tissue culture media onto glass and stored at 4°C, it has survived for over 50 days.
Q: How long does the virus remain in breast milk? A: We know it has been detected 15 days after disease onset and think possibly as late as 28 days from symptom onset.3
Q: How are people actually infected? A: I believe people get the virus on their hands and then touch their face, eyes, or mouth. If you are wearing personal protective equipment, it must occur while doffing the equipment.
Q: Could we increase the sensitivity of the test so that we could detect the virus before the onset of symptoms? A: In theory it may be possible. The virus is somewhere in the body during the incubation period. Perhaps we could sample the right compartment in an enriched mononuclear cell line.
Q: When can patients who recover resume their normal activities? A: After their viral load returns to 0, I would still advise abstaining from unprotected sex and from breastfeeding for a few months. but as for other activities, no special precautions are needed.
Q: Does the virus appear to be mutating at a high rate? A: Looking back to 2004, mutations are occurring, but there is no sign that any of these mutations has contributed to the size of the outbreak by changing the characteristics of the Ebola virus. Can it become aerosolized? It has been suggested that the virus that caused the outbreak separated from those that caused past Ebola outbreaks but does not seem to be affecting the spread or efficacy of experimental drugs and vaccines. So, even though it is an RNA virus and mutations are occurring, no serious changes have emerged.14
BACK TO OUR PATIENT
The differential diagnosis for the patient described at the beginning of this paper includes travelers’ diarrhea, malaria, typhoid fever, yellow fever, meningococcal disease … and Ebola virus disease, although this is much less likely in view of the epidemiology and incubation period of this disease. When his stool was tested by enzyme immunoassay and culture, it was found to be positive for Campylobacter. He recovered with oral rehydration.
A 50-year-old man who returned from a business trip to Nigeria 24 days ago presents with complaints of the sudden onset of fever, diarrhea, myalgia, and headache. He reports 10 bowel movements per day and has seen bloody stools.
During his trip he flew in to Murtala Muhammed International Airport in Lagos, ate meals only in his hotel, and attended meetings in Lagos central business district. He had no exposure to animals, mosquitoes, ticks, or sick people, and no sexual activity. After returning home, he felt well for the first 3 weeks.
The patient has a history of hypertension. He does not smoke, drink alcohol, or use injection drugs. He is married, works with commercial banks and financial institutions, and lives in Cleveland, OH.
On physical examination his temperature is 100.0˚F (37.8˚C), pulse 98, respirations 15, blood pressure 105/70 mm Hg, and weight 78 kg (172 lb). He appears comfortable but is a little diaphoretic. His abdomen is tender to palpation in the epigastrium and slightly to the right; he has no signs of peritonitis. His skin is without rash, bleeding, or bruising. The remainder of the examination is normal.
His white blood cell count is 17 × 109/L, hemoglobin 15 g/dL, hematocrit 41%, and platelet count 172 × 109/L. His sodium level is 126 mmol/L, potassium 3.8 mmol/L, chloride 95 mmol/L, carbon dioxide 20 mmol/L, blood urea nitrogen 11 mg/dL, creatinine 0.7 mg/dL, and glucose 130 mg/dL. His aminotransferase and alkaline phosphatase levels are normal.
Could this patient have Ebola virus disease?
With Ebola virus disease on the rise in West Africa, physicians who encounter patients like this one need to include it in the differential diagnosis. Because the disease is new, many questions are raised for which we as yet have no answers. Here, I will review what we know and do not know in an effort to remove some of the fear and uncertainty.
A NEW DISEASE
Ebola virus disease is a severe hemorrhagic fever caused by negative-sense single-stranded RNA viruses classified by the International Committee on Taxonomy of Viruses as belonging to the genus Ebolavirus in the family Filoviridae. Filoviruses get their name from the Latin filum, or thread-like structure.
The family Filoviridae was discovered in 1967 after inadvertent importation of infected monkeys from Uganda into Yugoslavia and Marburg, Germany. Outbreaks of severe illness occurred in workers at a vaccine plant who came into direct contact with the animals by killing them, removing their kidneys, or preparing primary cell cultures for polio vaccine production.
Ebola virus was discovered in 1976 by Peter Piot, who was working at the Institute of Tropical Medicine in Antwerp, Belgium. The blood of a Belgian woman who had been working in what is now the Democratic Republic of the Congo (formerly Zaire) had been sent to the institute; she and Mabalo Lokela, a school headmaster and the first recorded victim of Ebola virus, had been working near Yambuku, about 96 km from the Ebola River.
Before the 2014 outbreak, all known outbreaks had caused fewer than 2,400 cases across a dozen African countries over 3 decades.
Five species of Ebola virus
The genus Ebolavirus contains five species, each associated with a consistent case-fatality rate and a more or less well-identified endemic area.1
Zaire ebolavirus was recognized in 1976; it has caused multiple outbreaks, with high case-fatality rates.
Sudan ebolavirus was seen first in the 1970s; it has a 50% case-fatality rate.
Tai Forest ebolavirus has been found in only one person, an ethologist working with deceased chimpanzees.
Bundibugyo ebolavirus emerged in 2007 and has a 30% case-fatality rate.
Reston ebolavirus is maintained in an animal reservoir in the Philippines and is not found in Africa. It caused an outbreak of lethal infection in macaques imported into the United States in 1989. There is evidence that Reston ebolavirus can cause asymptomatic infection in humans. None of the caretakers of the macaques became ill, nor did farmers working with infected pigs, although both groups seroconverted.
A reservoir in bats?
A reservoir in nonhuman primates was initially suspected. However, studies subsequently showed that monkeys are susceptible to rapidly lethal filoviral disease, precluding any role as a host for persistent viral infection. It is likely that Ebola virus is maintained in small animals that serve as a source of infection for both humans and wild primates. A prominent suspect is fruit bats, which are consumed in soup in West Africa.
Transmission is person-to-person or nosocomial
Ebola virus is transmitted by direct contact with body fluids such as blood, urine, sweat, vomitus, semen, and breast milk. Filoviruses can initiate infection via ingestion, inhalation (although probably not Ebola), or passage through breaks in the skin. Droplet inoculation into the mouth or eyes has been shown to result from inadvertent transfer of virus from contaminated hands. Patients transmit the virus while febrile and through later stages of disease, as well as postmortem through contact with the body during funeral preparations. The virus has been isolated in semen for as many as 61 days after illness onset.
Ebola virus can also be spread nosocomially. In 1976, a 44-year-old teacher sought care for fever at the Yambuku Mission Hospital. He was given parenteral chloroquine as empiric treatment for presumed malaria, which was routine for all febrile patients. However, he had unrecognized Ebola virus infection. Moreover, syringes were rinsed in the same pan of water and reused, which spread the infection to nearly 100 people, all of whom developed fulminant Ebola virus disease and died. Infection then spread to family caregivers, the hospital staff, and those who prepared the bodies for burial.
Nosocomial transmission was also responsible for an outbreak of Lake Victoria Marburg virus in Uige Province in northern Angola in 2005, with 374 putative cases and 329 deaths. When teams from Médecins Sans Frontières started setting up the Marburg ward, there were five patients with hemorrhagic fever in a makeshift isolation room in the hospital, together with corpses that the hospital staff had been too afraid to remove. Healers found in many rural African communities were administering injections in homes or in makeshift clinics with reused needles or syringes.2
There is no evidence that filoviruses are carried by mosquitoes or other biting arthropods. Also, the risk of transmission via fomites appears to be low when currently recommended infection-control guidelines for the viral hemorrhagic fevers are followed.3 One primary human case generates only one to three secondary cases on average.
EBOLA IS AN IMMUNODEFICIENCY VIRUS
The main targets of infection are endothelial cells, mononuclear phagocytes, and hepatocytes. Ebola virus replicates at an unusually high rate. Macrophages infected with Zaire ebolavirus produce tumor necrosis factor alpha, interleukin (IL) 1 beta, IL-6, macrophage chemotactic protein 1, and nitric oxide. Virus-infected macrophages synthesize cell-surface tissue factor, triggering the extrinsic coagulation pathway.
Ebola is an immunodeficiency virus. Dendritic cells, which initiate adaptive immune responses, are a major site of filoviral replication. Infected cells cannot present antigens to naïve lymphocytes. Patients who die of Ebola virus disease do not develop antibodies to the virus. Lymphocytes remain uninfected, but undergo “bystander” apoptosis induced by inflammatory mediators.
CLINICAL MANIFESTATIONS
The incubation period is generally 5 to 7 days (range 2 to 28 days), during which the patient is not infectious. Symptoms begin abruptly, with fever, chills, general malaise, weakness, severe headache, and myalgia. By the time of case detection in West Africa, most patients also had nausea, vomiting, diarrhea, and abdominal pain. Once symptoms arise, patients have high levels of the virus in their blood and fluids and are infectious. Hemorrhagic symptoms have apparently been uncommon in West Africa, occurring in 1.0% to 5.7%, but “unexplained bleeding” has been documented in 18% of cases.4 Among those in whom the disease enters its hemorrhagic terminal phase, there is characteristic internal and subcutaneous bleeding, vomiting of blood, and subconjunctival hemorrhage.4
Laboratory findings include lymphocytopenia (often with counts as low as 1.0 × 109/L), thrombocytopenia (with counts in the range of 50 to 100 × 109/L), elevated aminotransferase levels (including aspartate aminotransferase levels 7 to 12 times higher than alanine aminotransferase in fatal cases), low total protein (due to capillary leak), and disseminated intravascular coagulation. Those who survive begin to improve in the second week, during which viremia resolves in association with the appearance of virus-specific antibodies.4
DIAGNOSIS
In symptomatic patients, Ebola virus infection is diagnosed by detection in blood or body fluids of viral antigens by enzyme-linked immunosorbent assay, or RNA sequences by reverse transcriptase polymerase chain reaction. The diagnosis is confirmed with cell culture (in a BSL-4 containment laboratory) showing characteristic viral particles by electron microscopy.
CARING FOR PATIENTS
The most detailed descriptions of the care of patients with Ebola virus disease have come from Dr. Bruce Ribner, of Emory University Hospital, in an October 2014 report of his experience caring for Ebola-infected patients at Emory University Hospital in Atlanta, GA.5 He described fluid losses of 5 to 10 L/day, profound hyponatremia, hypokalemia, and hypocalcemia, which were associated with cardiac arrhythmias and the need for intravenous and oral electrolyte repletion and hemodialysis. Intensive one-to-one nursing was critical, as was the coordination of many medical subspecialties. The Emory team arranged point-of-care testing near the unit and generally kept laboratory testing to a minimum. The team was surprised to learn that commercial carriers refused to transport specimens even when they were licensed for category A agents. Difficulties with the local water authority and waste disposal contractor required the hospital to dedicate an autoclave to process all materials used in clinical care.
TREATMENT: SUPPORTIVE AND EXPERIMENTAL
Treatment is supportive to maintain circulatory function and blood pressure and to correct coagulopathy. However, a variety of vaccines, antibodies, small-molecule agents, and antiviral agents are undergoing testing, mostly in animals at this point.
Vaccines. A therapeutic vaccine that worked only slightly was a live-attenuated recombinant vesicular stomatitis virus expressing Ebola virus transmembrane glycoproteins, which was tested in mice, guinea pigs, and rhesus macaques who had been exposed to Ebola virus.6
A preventive vaccine worked better. Stanley et al7 evaluated a replication-defective chimpanzee adenovirus 3-vectored vaccine that also contained Ebola virus glycoprotein. They gave macaques a single injection of this vaccine, and then 5 weeks later gave them a lethal dose of Ebola virus. All the vaccinated animals survived the infection, and half (2 of 4) survived when challenged 10 months later. With a prime-boost strategy (modified vaccinia virus Ankara, a poxvirus), all survived when challenged 10 months later.
KZ52, a neutralizing antibody, did not work. Oswald et al8 gave a human IgG monoclonal antibody against Zaire Ebola virus, designated KZ52, to four rhesus macaques, challenged them with the virus 24 hours later, and administered a second shot of KZ52 on day 4. All of them died.
ZMAb is a combination of three murine monoclonal antibodies, designated 1H3, 2G4, and 4G7. Ad-IFN is a human adenovirus, serotype 5, that expresses human interferon alpha. Qui et al9 gave ZMAb and Ad-IFN to macaques in several experiments. In experiment 1, eight macaques were infected and then were given ZMAb and Ad-IFN 3 days later, and ZMAb again on days 6 and 9. Seven of the eight survived. In a second experiment, Ad-IFN was given first, when the viral load was still less than the limit of detection of known assays, and then ZMAb was given upon detection of viremia and fever. Two of four macaques survived. Control animals had undetectable levels of IgG, whereas Ebola virus GP–specific IgG levels were detected in all survivors. IFN-gamma ELISpots showed high EBOV-GP–specific T-cell response in all survivors.
ZMapp is another cocktail of monoclonal antibodies, containing two from ZMab (2G4 and 4G7), plus a third, c13C6. In experiments in rhesus macaques, three groups of six animals each received three doses of ZMapp at varying times after being infected with Ebola virus: at 3, 6, and 9 days; at 4, 7, and 10 days, and at 5, 8, and 11 days. All 18 macaques treated with ZMapp survived. Thus, Zmapp extended the treatment window to 5 days postexposure.10 One of the American health care workers who contracted Ebola virus in Liberia received this medication.
HSPA5-PMO. Endoplasmic reticulum chaperone heat shock 70 kDa protein 5 (HSPA5) is instrumental in the maturation of envelope proteins in hepatitis C and influenza A virus. It plays a role in viral entry for coxsackievirus A9 and dengue virus serotype 2, and it may be involved in Ebola viral budding. Phosphorodiamidate morpholino oligomers (PMOs) are a class of antisense DNA nucleotide analogs.
Reid et al11 reported that mice treated with HSPA5–PMO were completely protected from lethal Ebola challenge. Therefore, HSPA5 appears to be a promising target for the development of antifilovirus countermeasures.
Favipiravir, an antiviral agent also known as T-705, is a pyrazinecarboxamide derivative. Invented in 2002 by Toyama Chemicals as an inhibitor of influenza virus replication, it acts as a nucleotide analog, selectively inhibiting the viral RNA-dependent RNA polymerase, or causes lethal mutagenesis upon incorporation into the virus RNA. Favipiravir suppresses Ebola virus replication by 4 log10 units in cell culture.12
Mice were challenged with intranasal inoculation of 1,000 focus-forming units of Ebola virus diluted in phosphate-buffered saline. Until the first day of treatment (postinfection day 6), all mice in the T-705 group lost weight similarly to control mice, developed viremia, and showed elevated serum levels of aspartate aminotransferase and alanine aminotransferase. Within 4 days of T-705 treatment (post-infection day 10), the animals had cleared the virus from blood. Surviving mice developed Ebola virus-specific antibodies and CD8+ T cells specific for the viral nucleoprotein.12
The authors hypothesized that suppression of virus replication by T-705 allowed the host to mount a virus-specific adaptive immune response, and concluded that T-705 was 100% effective in the treatment of Zaire Ebola virus infection up to postinfection day 6 but was hardly beneficial at the terminal stage of disease.12 Of note, favipiravir is undergoing phase 2 and phase 3 trials as an anti-influenza agent in Japan.
THE CURRENT OUTBREAK
The current outbreak is with Zaire ebolavirus. It seems to have started in a 2-year-old child who died in Meliandou in Guéckédou Prefecture, Guinea, on December 6, 2013. On March 21, 2014, the Guinea Ministry of Health reported the outbreak of an illness characterized by fever, severe diarrhea, vomiting, and a high case-fatality rate (59%) in 49 persons. On May 25, 2014, Kenema Government Hospital confirmed the first case of Ebola virus disease in Sierra Leone, probably brought there by a traditional healer who had treated Ebola patients from Guinea. Tracing led to 13 additional cases—all women who attended the burial.13
The Center for Systems Biology at Harvard University and the Broad Institute of Massachusetts Institute of Technology generated 99 Ebola virus genome sequences from 78 patients with confirmed disease, representing more than 70% of the patients diagnosed with the disease in Sierra Leone from May to mid-June 2014. They found genetic similarity across the sequenced 2014 samples, suggesting a single transmission from the natural reservoir, followed by human-to-human transmission during the outbreak. Continued human-reservoir exposure is unlikely to have contributed to the growth of this epidemic.14
As of October 14, 2014, there were 8,914 suspected and confirmed cases of Ebola virus infection, and 4,477 deaths.15
But how did Zaire Ebola virus make the 2,000-mile trek from Central Africa to Guinea in West Africa? There are two possibilities: it has always been present in the region but we just never noticed, or it was recently introduced. Bayesian phylogenetic analyses and sequence divergence studies suggest the virus has been present in bat populations in Guinea without previously infecting humans.
Why Guinea and why Guéckédou? Guinea is one of the poorest countries in the world, ranking 178th of 187 countries on the Human Development Index of the United Nations Development Programme, just behind Liberia (174th) and Sierra Leone (177th). In Guinea, the life expectancy is 56 years and the gross national income per capita is $440. The region has been systematically plundered and the forest decimated by clear-cut logging, leaving the Guinea Forest Region largely deforested, resulting in increased contact between humans and the small animals that serve as the source of infection.1
LIMITED CAPACITY, EVEN IN THE UNITED STATES
A few hospitals in the United States have dedicated units to handle serious infectious diseases such as Ebola: Emory University Hospital; Nebraska Medicine in Omaha; Providence St. Patrick Hospital in Missoula, MT; and the National Institutes of Health in Bethesda, MD. However, in total they have only 19 beds.
QUESTIONS, ANSWERS—AND MORE QUESTIONS
(The following is from a question-and-answer discussion that followed Dr. Brizendine’s Grand Rounds presentation.)
Q: Are there any differences between survivors and those who die of the disease? A: We do not know. Patient survival depends on early recognition and supportive care. There are disparities in the care of patients. Schieffelin et al16 analyzed the characteristics of patients who died or who survived in Sierra Leone and found that the mortality rate was higher in older patients and those with a higher viral load on presentation.
Q: Does the virus block production or release of interferon early in infection? A: Yes, it has been shown17 that Ebola virus protein VP24 inhibits signaling downstream of both interferon alpha/beta and interferon gamma by indirectly impairing the transport of a transcription factor termed STAT1. VP24 is also able to bind STAT1 directly. The resulting suppression of host interferon very early on in the incubation phase is key to the virulence of the virus.
Q: Does infection with one of the viral species confer immunity from other species? A: No, there is no cross-immunity.
Q: How soon do patients test positive? A: About 5 days after exposure, when they develop a fever. At this time patients are highly viremic, which PCR can detect.
Q: Before the virus is detectable in the blood, where is it? A: The liver, endothelial cells, antigen-presenting cells, and adrenal glands.
Q: Do we really need to quarantine ill patients and health care workers returning from Africa, per CDC recommendations? A: We don’t know everything, and some people do make bad decisions, such as traveling while symptomatic. I support a period of observation, although confinement is not reasonable, as it may pose a disincentive to cooperation.
Q: What is the role of giving plasma from survivors? A: Dr. Kent Brantly (see American citizens infected with Ebola) received the blood of a 14-year-old who survived. We don’t know. It is not proved. It did not result in improvement in animal models.
Q: Is the bleeding caused by a mechanism similar to that in enterohemorrhagic Escherichia coli infection? A: No. That is a bacterial toxin, whereas this is more like disseminated intravascular coagulation, with an intrinsic pathway anticoagulation cascade.
Q: How long does the virus remain viable outside the body? A: In one study,18 Ebola virus could not be recovered from experimentally contaminated surfaces (plastic, metal or glass) at room temperature. In another in which it was dried onto a surface,19 Ebola virus survived in the dark for several hours between 20 and 25°C. When dried in tissue culture media onto glass and stored at 4°C, it has survived for over 50 days.
Q: How long does the virus remain in breast milk? A: We know it has been detected 15 days after disease onset and think possibly as late as 28 days from symptom onset.3
Q: How are people actually infected? A: I believe people get the virus on their hands and then touch their face, eyes, or mouth. If you are wearing personal protective equipment, it must occur while doffing the equipment.
Q: Could we increase the sensitivity of the test so that we could detect the virus before the onset of symptoms? A: In theory it may be possible. The virus is somewhere in the body during the incubation period. Perhaps we could sample the right compartment in an enriched mononuclear cell line.
Q: When can patients who recover resume their normal activities? A: After their viral load returns to 0, I would still advise abstaining from unprotected sex and from breastfeeding for a few months. but as for other activities, no special precautions are needed.
Q: Does the virus appear to be mutating at a high rate? A: Looking back to 2004, mutations are occurring, but there is no sign that any of these mutations has contributed to the size of the outbreak by changing the characteristics of the Ebola virus. Can it become aerosolized? It has been suggested that the virus that caused the outbreak separated from those that caused past Ebola outbreaks but does not seem to be affecting the spread or efficacy of experimental drugs and vaccines. So, even though it is an RNA virus and mutations are occurring, no serious changes have emerged.14
BACK TO OUR PATIENT
The differential diagnosis for the patient described at the beginning of this paper includes travelers’ diarrhea, malaria, typhoid fever, yellow fever, meningococcal disease … and Ebola virus disease, although this is much less likely in view of the epidemiology and incubation period of this disease. When his stool was tested by enzyme immunoassay and culture, it was found to be positive for Campylobacter. He recovered with oral rehydration.
- Bausch DG, Schwarz L. Outbreak of ebola virus disease in Guinea: where ecology meets economy. PLoS Negl Trop Dis 2014; 8:e3056.
- Roddy P, Thomas SL, Jeffs B, et al. Factors associated with Marburg hemorrhagic fever: analysis of patient data from Uige, Angola. J Infect Dis 2010; 201:1909–1918.
- Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196(suppl 2):S142–S147.
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–1495.
- Ribner BS. Treating patients with Ebola virus infections in the US: lessons learned. Presented at IDWeek, October 8, 2014. Philadelphia PA.
- Feldman H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3:e2.
- Stanley DA, Honko AN, Asiedu C, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126–1129.
- Oswald WB, Geisbert TW, Davis KJ, et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLos Pathog 2007; 3:e9.
- Qui X, Wong G, Fernando L, et al. mAbs and Ad-vectored IFN-a therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med 2013; 5:207ra143.
- Qui X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47–53.
- Reid SP, Shurtleff AC, Costantino JA, et al. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 2014; 109:171–174.
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 2014; 105:17–21.
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus dsease in Guinea. N Engl J Med 2014; 371:1418–1425.
- Gire SK, Goba A, Andersen KG, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014; 345:1369–1372.
- Chamary JV. 4000 deaths and counting: the Ebola epidemic in 4 charts. Forbes. http://www.forbes.com/sites/jvchamary/2014/10/13/ebola-trends. Accessed November 5, 2014.
- Schieffelin JS, Shaffer JG, Goba A, et al, for the KGH Lassa Fever Program, the Viral Hemorrhagic Fever Consortium, and the WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014 Oct 29 [Epub ahead of print]. DOI: 10.1056/NEJMoa1411680.
- Zhang AP, Bornholdt ZA, Liu T, et al. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog 2012; 8:e1002550.
- Piercy TJ, Smither SJ, Steward JA, Eastaugh L, Lever MS. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol 2010; 109:1531–1539.
- Sagripanti JL, Rom AM, Holland LE. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch Virol 2010; 155:2035–2039.
- Bausch DG, Schwarz L. Outbreak of ebola virus disease in Guinea: where ecology meets economy. PLoS Negl Trop Dis 2014; 8:e3056.
- Roddy P, Thomas SL, Jeffs B, et al. Factors associated with Marburg hemorrhagic fever: analysis of patient data from Uige, Angola. J Infect Dis 2010; 201:1909–1918.
- Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196(suppl 2):S142–S147.
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–1495.
- Ribner BS. Treating patients with Ebola virus infections in the US: lessons learned. Presented at IDWeek, October 8, 2014. Philadelphia PA.
- Feldman H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3:e2.
- Stanley DA, Honko AN, Asiedu C, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126–1129.
- Oswald WB, Geisbert TW, Davis KJ, et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLos Pathog 2007; 3:e9.
- Qui X, Wong G, Fernando L, et al. mAbs and Ad-vectored IFN-a therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med 2013; 5:207ra143.
- Qui X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47–53.
- Reid SP, Shurtleff AC, Costantino JA, et al. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 2014; 109:171–174.
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 2014; 105:17–21.
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus dsease in Guinea. N Engl J Med 2014; 371:1418–1425.
- Gire SK, Goba A, Andersen KG, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014; 345:1369–1372.
- Chamary JV. 4000 deaths and counting: the Ebola epidemic in 4 charts. Forbes. http://www.forbes.com/sites/jvchamary/2014/10/13/ebola-trends. Accessed November 5, 2014.
- Schieffelin JS, Shaffer JG, Goba A, et al, for the KGH Lassa Fever Program, the Viral Hemorrhagic Fever Consortium, and the WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014 Oct 29 [Epub ahead of print]. DOI: 10.1056/NEJMoa1411680.
- Zhang AP, Bornholdt ZA, Liu T, et al. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog 2012; 8:e1002550.
- Piercy TJ, Smither SJ, Steward JA, Eastaugh L, Lever MS. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol 2010; 109:1531–1539.
- Sagripanti JL, Rom AM, Holland LE. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch Virol 2010; 155:2035–2039.
KEY POINTS
- Ebola virus is spread by contact with body fluids, with no evidence to date that it is airborne.
- Ebola virus is likely maintained in a reservoir of small animals, possibly bats.
- The incubation period is about 5 to 7 days, during which the patient is not infectious.
- Symptoms begin abruptly, with fever, chills, and general malaise, which in some patients leads to weakness, severe headache, myalgia, nausea, vomiting, diarrhea, and abdominal pain.
- Once the disease is symptomatic, patients have high levels of virus in the blood and other body fluids and are therefore infectious.
- Survivors show improvement in the second week of illness, during which viremia resolves and virus-specific antibodies appear.
Why are we doing cardiovascular outcome trials in type 2 diabetes?
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
KEY POINTS
- The worldwide burden of type 2 diabetes is increasing dramatically as obesity rates increase, populations age, and people around the world adopt a Western diet.
- Diabetes increases the risk of atherosclerotic cardiovascular disease, which remains the leading cause of death in diabetic patients.
- Lowering blood glucose alone may not necessarily amount to reduction in adverse cardiovascular events.
- Clinical trials of new drugs for type 2 diabetes must prove cardiovascular safety in addition to glucose-lowering potential before the drugs gain final regulatory approval.
- Aggressive risk factor modification (smoking cessation, control of hypertension, and treatment of hyperlipidemia with statins) remains paramount in reducing cardiovascular risk in people with diabetes.
Six screening tests for adults: What’s recommended? What’s controversial?
A 68-year-old man with a history of hyperlipidemia is evaluated during a routine examination. He has a 25-pack-year cigarette smoking history but quit 12 years ago. He has no history of hypertension, diabetes mellitus, or stroke. A review of systems is unremarkable, and he has no family history of heart disease or cancer. He has noted no change in his bowel movements, and his most recent screening colonoscopy, done at age 60, was normal. His only current medication is lovastatin.
Physical examination reveals no abnormalities. His blood pressure is 130/82 mm Hg, and his body mass index is 24 kg/m2. His total cholesterol level is 213 mg/dL, and his high-density lipoprotein level is 48 mg/dL.
Which screening tests, if any, would be appropriate for this patient?
The advent in recent years of several new screening tests, along with changing and conflicting screening recommendations, has made it a challenge to manage this aspect of patient care. This article reviews six common screening tests and presents the current recommendations for their use (Table 1).
SCREENING CAN HARM
Screening is used to detect a disease in people who have no signs or symptoms of that disease; if signs or symptoms are present, diagnostic testing is indicated instead. Ideally, screening allows for early treatment to reduce the risk of illness and death associated with a disease.
Problems with screening relate to lead-time bias (detection of disease earlier in its course without actually affecting survival time), length-time bias (detection of indolent and benign cancers rather than aggressive ones), and overdiagnosis (detection of abnormalities that would not cause a problem in the patient’s lifetime, causing unnecessary concern, cost, or treatment).
The leading advisory groups on screening are the US Preventive Services Task Force (USPSTF),1 which is stringently evidence-based in its recommendations, and subspecialty societies, which often rely on expert opinion.2,3
ULTRASONOGRAPHY FOR ABDOMINAL AORTIC ANEURYSM
In 2005, the USPSTF gave a grade-B recommendation (recommended; benefit outweighs harm) for one-time ultrasonographic screening for abdominal aortic aneurysm in men ages 65 to 75 who have ever smoked at least 100 cigarettes over a lifetime. For men in the same age range who have never smoked, they gave a grade-C recommendation (no recommendation; small net benefit). The USPSTF updated its recommendation in 2014. For women ages 65 to 75 who smoke, the USPSTF thinks the evidence is insufficient to recommend for or against screening (grade-I recommendation).
Our patient described above—male, age 68, and with a 25 pack-year smoking history—is a candidate for screening for abdominal aortic aneurysm.
CT SCREENING FOR LUNG CANCER
In December 2013, the USPSTF gave a B-grade recommendation for annual screening for lung cancer with low-dose computed tomography (CT) for adults ages 55 to 80 who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that limits life expectancy or the ability to undergo curative lung surgery.
These recommendations were based on the outcomes of the National Lung Screening Trial.4 However, whereas this trial was in people ages 55 to 74, the USPSTF boosted the upper age limit to 80 based on computer modeling, a decision that was somewhat controversial.
Patz et al5 analyzed data from the National Lung Screening Trial and found that about 18% of lung cancers detected by low-dose CT appeared to be indolent and were unlikely to become clinically apparent during the patient’s lifetime. The authors concluded that overdiagnosis should be considered when guidelines for mass screening programs are developed.
Our 68-year-old patient would not qualify for CT screening for lung cancer, since his smoking history is less than 30 pack-years.
COLORECTAL CANCER SCREENING AND PREVENTION
Unlike other cancer screening tests, colorectal cancer screening can also be a preventive measure; removing polyps found during screening with colonoscopy or sigmoidoscopy is an effective strategy in preventing colon cancer.
The USPSTF last updated its colorectal screening recommendations in 2008, giving a grade-A recommendation (strongly recommended; benefit far outweighs harm) to screening using fecal occult blood testing, sigmoidoscopy, or colonoscopy for adults ages 50 to 75. The risks and benefits of these screening methods vary. For adults ages 76 to 85, the task force recommends against routine screening but gives a grade-C recommendation for screening in that age group in some circumstances. They give a grade-D recommendation for screening after age 85.
The USPSTF concluded that the evidence is insufficient to assess the benefits and harms of CT colonography and fecal DNA testing for colorectal cancer screening.
The American Cancer Society issued similar guidelines in 2013, recommending that starting at age 50, men and women at low risk of colorectal cancer should be screened using one of the following schedules (the first four methods help detect both polyps and cancers, and the others detect only cancer)6:
- Colonoscopy every 10 years
- Flexible sigmoidoscopy every 5 years
- A double-contrast barium enema every 5 years
- CT colonography (“virtual colonoscopy”) every 5 years
- A guaiac-based fecal occult blood test annually
- A fecal immunochemical test annually.
Those at moderate or high risk of colorectal cancer are advised to talk with a doctor about a different testing schedule. (eg, colonoscopy every 5 years in patients with a significant family history of colon cancer).
Our patient last underwent colonoscopy 8 years ago and so does not need to be screened again for another 2 years.
CERVICAL CANCER SCREENING: MOVING TOWARD HPV TESTING FIRST?
Cervical cancer screening recommendations are fairly uniform across the major guideline-setting organizations.7 In general, they are:
- Ages 21–29: Check cytology every 3 years
- Ages 30–65: Cytology plus human papillomavirus (HPV) testing every 5 years (or cytology alone every 3 years)
- After age 65: Stop screening if prior screenings have been adequate and negative over the past 20 years.
Women who have been vaccinated against HPV have the same screening recommendations as above. Women who have had a hysterectomy for benign reasons do not need further screening.
The future of cervical cancer screening may be “reflex testing.” Rather than checking cervical samples for cytologic study (Papanicolaou smear) and HPV status together, we may one day screen samples first for HPV and, if that is positive, follow up with cytologic study. Easy-to-use home tests for HPV will likely be developed and should increase screening rates.
PROSTATE CANCER SCREENING: A SHARED DECISION
Prostate cancer screening remains controversial. Different guideline-setting bodies have different recommendations, creating confusion for patients. Physicians must follow what fits their own practice and beliefs.
The USPSTF in 2012 gave a grade-D recommendation to prostate-specific antigen (PSA) testing to screen for prostate cancer, stating that it did more harm than good. However, some men continue to be screened for PSA.
The American Cancer Society in 2013 recommended against routine testing for prostate cancer without a full discussion between physician and patient of the pros and cons of testing.8 If screening is decided upon, it should be done with annual PSA measurement or digital rectal examination, or both, starting at age 50. Men at high risk (ie, African American men, and men with a first-degree relative diagnosed with prostate cancer before age 65) should begin screening at age 45.
The American College of Physicians in 2013 issued a statement that clinicians should inform men between the ages of 50 and 69 about the limited potential benefits and substantial harms of prostate cancer screening.9 They recommended against PSA screening in men of average risk who are younger than age 50 or older than age 69, or those whose life expectancy is less than 10 to 15 years.
The American Urological Association in 2013 advised that10:
- PSA screening is not recommended in men younger than 40.
- Routine screening is not recommended in men between ages 40 and 54 at average risk.
- In men ages 55 to 69, decisions about PSA screening should be shared and based on each patient’s values and preferences. The decision to undergo PSA screening involves weighing the benefits of preventing death from prostate cancer in 1 man for every 1,000 men screened over a decade against the known potential harms associated with screening and treatment.
- To reduce the harm of screening, a routine interval of 2 years may be chosen over annual screening; such a schedule may preserve most benefits and reduce overdiagnosis and false-positive results.
- Routine PSA screening is not recommended in men ages 70 and older or with less than a 10- to 15-year life expectancy.
Shared decision-making. Many of the guidelines for prostate cancer screening are based on the concept of shared decision-making. However, studies indicate that many patients do not receive a full discussion of the issue,11 and in any event, patient education may make little difference in PSA testing rates.12,13
On the horizon for prostate cancer screening is the hope of finding a more predictable test. There is also discussion of using the PSA test earlier: some evidence shows that a very low result at age 45 predicts a less than 1% chance of developing metastatic prostate cancer by age 75, so it is possible that screening could stop in that population.
BREAST CANCER SCREENING: DIVERGENT RECOMMENDATIONS
The USPSTF created considerable controversy a few years ago when it recommended screening mammography from ages 50 to 74, and then only every 2 years—a departure from the traditional practice of starting screening at age 40. Few doctors heed the USPSTF guideline: most of the other guideline-setting organizations (eg, the American Cancer Society, the American Congress of Obstetricians and Gynecologists) recommend annual mammography for women starting at age 40.
Overdiagnosis is an especially pertinent issue with screening mammography for breast cancer because some cancers are indolent and will not cause a problem during a lifetime. Falk et al14 analyzed a Norwegian breast cancer screening program and found that overdiagnosis occurred in 10% to 20% of cases. Welch and Passow15 quantified the benefits and harms of screening mammography in 50-year-old women in the United States and found that of 1,000 women screened annually for a decade, 0.3 to 3.2 will avoid a breast cancer death, 490 to 670 will have at least one false alarm, and 3 to 14 will be overdiagnosed and treated needlessly.
Mammography screening for breast cancer will likely stay controversial for some time as we await additional data.
OTHER CANCERS: SCREENING NOT RECOMMENDED
The USPSTF currently does not recommend screening for ovarian cancer (guideline issued in 2012), pancreatic cancer (2004), or testicular cancer (2011), giving each a grade-D recommendation, indicating that screening does more harm than good. It also stated that there is insufficient evidence to recommend screening for oral cancer (2013), skin cancer (2009), and bladder cancer (2011).
- US Preventive Services Task Force. www.uspreventiveservicestask-force.org. Accessed August 11, 2014.
- Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831–841. Erratum in: JAMA 2009; 301:1544.
- Lee DH, Vielemeyer O. Analysis of overall level of evidence behind Infectious Diseases Society of America practice guidelines. Arch Intern Med 2011; 171:18–22.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Patz EF, Pinsky P, Gatsonis C, et al; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014; 174:269–274.
- American Cancer Society. Colorectal cancer screening and surveillance guidelines. www.cancer.org/healthy/informationforhealth-careprofessionals/colonmdclinicansinformationsource/colorec-talcancerscreeningandsurveillanceguidelines/index. Accessed August 11, 2014.
- Jin XW, Lipold L, McKenzie M, Sikon A. Cervical cancer screening: what’s new and what’s coming? Cleve Clin J Med 2013; 80:153–160.
- American Cancer Society. Prostate cancer screening guidelines. www.cancer.org/healthy/informationforhealthcareprofessionals/pros-tatemdcliniciansinformationsource/prostatecancerscreeningguide-lines/index. Accessed August 11, 2014.
- Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2013; 158:761–769.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed September 5, 2014.
- Han PK, Kobrin S, Breen N, et al. National evidence on the use of shared decision making in prostate-specific antigen screening. Ann Fam Med 2013; 11:306–314.
- Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med 2013; 173:1704–1712.
- Landrey AR, Matlock DD, Andrews L, Bronsert M, Denberg T. Shared decision making in prostate-specific antigen testing: the effect of a mailed patient flyer prior to an annual exam. J Prim Care Community Health 2013; 4:67–74.
- Falk RS, Hofvind S, Skaane P, Haldorsen T. Overdiagnosis among women attending a population-based mammography screening program. Int J Cancer 2013; 133:705–712.
- Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA Intern Med 2014; 174:448–454.
A 68-year-old man with a history of hyperlipidemia is evaluated during a routine examination. He has a 25-pack-year cigarette smoking history but quit 12 years ago. He has no history of hypertension, diabetes mellitus, or stroke. A review of systems is unremarkable, and he has no family history of heart disease or cancer. He has noted no change in his bowel movements, and his most recent screening colonoscopy, done at age 60, was normal. His only current medication is lovastatin.
Physical examination reveals no abnormalities. His blood pressure is 130/82 mm Hg, and his body mass index is 24 kg/m2. His total cholesterol level is 213 mg/dL, and his high-density lipoprotein level is 48 mg/dL.
Which screening tests, if any, would be appropriate for this patient?
The advent in recent years of several new screening tests, along with changing and conflicting screening recommendations, has made it a challenge to manage this aspect of patient care. This article reviews six common screening tests and presents the current recommendations for their use (Table 1).
SCREENING CAN HARM
Screening is used to detect a disease in people who have no signs or symptoms of that disease; if signs or symptoms are present, diagnostic testing is indicated instead. Ideally, screening allows for early treatment to reduce the risk of illness and death associated with a disease.
Problems with screening relate to lead-time bias (detection of disease earlier in its course without actually affecting survival time), length-time bias (detection of indolent and benign cancers rather than aggressive ones), and overdiagnosis (detection of abnormalities that would not cause a problem in the patient’s lifetime, causing unnecessary concern, cost, or treatment).
The leading advisory groups on screening are the US Preventive Services Task Force (USPSTF),1 which is stringently evidence-based in its recommendations, and subspecialty societies, which often rely on expert opinion.2,3
ULTRASONOGRAPHY FOR ABDOMINAL AORTIC ANEURYSM
In 2005, the USPSTF gave a grade-B recommendation (recommended; benefit outweighs harm) for one-time ultrasonographic screening for abdominal aortic aneurysm in men ages 65 to 75 who have ever smoked at least 100 cigarettes over a lifetime. For men in the same age range who have never smoked, they gave a grade-C recommendation (no recommendation; small net benefit). The USPSTF updated its recommendation in 2014. For women ages 65 to 75 who smoke, the USPSTF thinks the evidence is insufficient to recommend for or against screening (grade-I recommendation).
Our patient described above—male, age 68, and with a 25 pack-year smoking history—is a candidate for screening for abdominal aortic aneurysm.
CT SCREENING FOR LUNG CANCER
In December 2013, the USPSTF gave a B-grade recommendation for annual screening for lung cancer with low-dose computed tomography (CT) for adults ages 55 to 80 who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that limits life expectancy or the ability to undergo curative lung surgery.
These recommendations were based on the outcomes of the National Lung Screening Trial.4 However, whereas this trial was in people ages 55 to 74, the USPSTF boosted the upper age limit to 80 based on computer modeling, a decision that was somewhat controversial.
Patz et al5 analyzed data from the National Lung Screening Trial and found that about 18% of lung cancers detected by low-dose CT appeared to be indolent and were unlikely to become clinically apparent during the patient’s lifetime. The authors concluded that overdiagnosis should be considered when guidelines for mass screening programs are developed.
Our 68-year-old patient would not qualify for CT screening for lung cancer, since his smoking history is less than 30 pack-years.
COLORECTAL CANCER SCREENING AND PREVENTION
Unlike other cancer screening tests, colorectal cancer screening can also be a preventive measure; removing polyps found during screening with colonoscopy or sigmoidoscopy is an effective strategy in preventing colon cancer.
The USPSTF last updated its colorectal screening recommendations in 2008, giving a grade-A recommendation (strongly recommended; benefit far outweighs harm) to screening using fecal occult blood testing, sigmoidoscopy, or colonoscopy for adults ages 50 to 75. The risks and benefits of these screening methods vary. For adults ages 76 to 85, the task force recommends against routine screening but gives a grade-C recommendation for screening in that age group in some circumstances. They give a grade-D recommendation for screening after age 85.
The USPSTF concluded that the evidence is insufficient to assess the benefits and harms of CT colonography and fecal DNA testing for colorectal cancer screening.
The American Cancer Society issued similar guidelines in 2013, recommending that starting at age 50, men and women at low risk of colorectal cancer should be screened using one of the following schedules (the first four methods help detect both polyps and cancers, and the others detect only cancer)6:
- Colonoscopy every 10 years
- Flexible sigmoidoscopy every 5 years
- A double-contrast barium enema every 5 years
- CT colonography (“virtual colonoscopy”) every 5 years
- A guaiac-based fecal occult blood test annually
- A fecal immunochemical test annually.
Those at moderate or high risk of colorectal cancer are advised to talk with a doctor about a different testing schedule. (eg, colonoscopy every 5 years in patients with a significant family history of colon cancer).
Our patient last underwent colonoscopy 8 years ago and so does not need to be screened again for another 2 years.
CERVICAL CANCER SCREENING: MOVING TOWARD HPV TESTING FIRST?
Cervical cancer screening recommendations are fairly uniform across the major guideline-setting organizations.7 In general, they are:
- Ages 21–29: Check cytology every 3 years
- Ages 30–65: Cytology plus human papillomavirus (HPV) testing every 5 years (or cytology alone every 3 years)
- After age 65: Stop screening if prior screenings have been adequate and negative over the past 20 years.
Women who have been vaccinated against HPV have the same screening recommendations as above. Women who have had a hysterectomy for benign reasons do not need further screening.
The future of cervical cancer screening may be “reflex testing.” Rather than checking cervical samples for cytologic study (Papanicolaou smear) and HPV status together, we may one day screen samples first for HPV and, if that is positive, follow up with cytologic study. Easy-to-use home tests for HPV will likely be developed and should increase screening rates.
PROSTATE CANCER SCREENING: A SHARED DECISION
Prostate cancer screening remains controversial. Different guideline-setting bodies have different recommendations, creating confusion for patients. Physicians must follow what fits their own practice and beliefs.
The USPSTF in 2012 gave a grade-D recommendation to prostate-specific antigen (PSA) testing to screen for prostate cancer, stating that it did more harm than good. However, some men continue to be screened for PSA.
The American Cancer Society in 2013 recommended against routine testing for prostate cancer without a full discussion between physician and patient of the pros and cons of testing.8 If screening is decided upon, it should be done with annual PSA measurement or digital rectal examination, or both, starting at age 50. Men at high risk (ie, African American men, and men with a first-degree relative diagnosed with prostate cancer before age 65) should begin screening at age 45.
The American College of Physicians in 2013 issued a statement that clinicians should inform men between the ages of 50 and 69 about the limited potential benefits and substantial harms of prostate cancer screening.9 They recommended against PSA screening in men of average risk who are younger than age 50 or older than age 69, or those whose life expectancy is less than 10 to 15 years.
The American Urological Association in 2013 advised that10:
- PSA screening is not recommended in men younger than 40.
- Routine screening is not recommended in men between ages 40 and 54 at average risk.
- In men ages 55 to 69, decisions about PSA screening should be shared and based on each patient’s values and preferences. The decision to undergo PSA screening involves weighing the benefits of preventing death from prostate cancer in 1 man for every 1,000 men screened over a decade against the known potential harms associated with screening and treatment.
- To reduce the harm of screening, a routine interval of 2 years may be chosen over annual screening; such a schedule may preserve most benefits and reduce overdiagnosis and false-positive results.
- Routine PSA screening is not recommended in men ages 70 and older or with less than a 10- to 15-year life expectancy.
Shared decision-making. Many of the guidelines for prostate cancer screening are based on the concept of shared decision-making. However, studies indicate that many patients do not receive a full discussion of the issue,11 and in any event, patient education may make little difference in PSA testing rates.12,13
On the horizon for prostate cancer screening is the hope of finding a more predictable test. There is also discussion of using the PSA test earlier: some evidence shows that a very low result at age 45 predicts a less than 1% chance of developing metastatic prostate cancer by age 75, so it is possible that screening could stop in that population.
BREAST CANCER SCREENING: DIVERGENT RECOMMENDATIONS
The USPSTF created considerable controversy a few years ago when it recommended screening mammography from ages 50 to 74, and then only every 2 years—a departure from the traditional practice of starting screening at age 40. Few doctors heed the USPSTF guideline: most of the other guideline-setting organizations (eg, the American Cancer Society, the American Congress of Obstetricians and Gynecologists) recommend annual mammography for women starting at age 40.
Overdiagnosis is an especially pertinent issue with screening mammography for breast cancer because some cancers are indolent and will not cause a problem during a lifetime. Falk et al14 analyzed a Norwegian breast cancer screening program and found that overdiagnosis occurred in 10% to 20% of cases. Welch and Passow15 quantified the benefits and harms of screening mammography in 50-year-old women in the United States and found that of 1,000 women screened annually for a decade, 0.3 to 3.2 will avoid a breast cancer death, 490 to 670 will have at least one false alarm, and 3 to 14 will be overdiagnosed and treated needlessly.
Mammography screening for breast cancer will likely stay controversial for some time as we await additional data.
OTHER CANCERS: SCREENING NOT RECOMMENDED
The USPSTF currently does not recommend screening for ovarian cancer (guideline issued in 2012), pancreatic cancer (2004), or testicular cancer (2011), giving each a grade-D recommendation, indicating that screening does more harm than good. It also stated that there is insufficient evidence to recommend screening for oral cancer (2013), skin cancer (2009), and bladder cancer (2011).
A 68-year-old man with a history of hyperlipidemia is evaluated during a routine examination. He has a 25-pack-year cigarette smoking history but quit 12 years ago. He has no history of hypertension, diabetes mellitus, or stroke. A review of systems is unremarkable, and he has no family history of heart disease or cancer. He has noted no change in his bowel movements, and his most recent screening colonoscopy, done at age 60, was normal. His only current medication is lovastatin.
Physical examination reveals no abnormalities. His blood pressure is 130/82 mm Hg, and his body mass index is 24 kg/m2. His total cholesterol level is 213 mg/dL, and his high-density lipoprotein level is 48 mg/dL.
Which screening tests, if any, would be appropriate for this patient?
The advent in recent years of several new screening tests, along with changing and conflicting screening recommendations, has made it a challenge to manage this aspect of patient care. This article reviews six common screening tests and presents the current recommendations for their use (Table 1).
SCREENING CAN HARM
Screening is used to detect a disease in people who have no signs or symptoms of that disease; if signs or symptoms are present, diagnostic testing is indicated instead. Ideally, screening allows for early treatment to reduce the risk of illness and death associated with a disease.
Problems with screening relate to lead-time bias (detection of disease earlier in its course without actually affecting survival time), length-time bias (detection of indolent and benign cancers rather than aggressive ones), and overdiagnosis (detection of abnormalities that would not cause a problem in the patient’s lifetime, causing unnecessary concern, cost, or treatment).
The leading advisory groups on screening are the US Preventive Services Task Force (USPSTF),1 which is stringently evidence-based in its recommendations, and subspecialty societies, which often rely on expert opinion.2,3
ULTRASONOGRAPHY FOR ABDOMINAL AORTIC ANEURYSM
In 2005, the USPSTF gave a grade-B recommendation (recommended; benefit outweighs harm) for one-time ultrasonographic screening for abdominal aortic aneurysm in men ages 65 to 75 who have ever smoked at least 100 cigarettes over a lifetime. For men in the same age range who have never smoked, they gave a grade-C recommendation (no recommendation; small net benefit). The USPSTF updated its recommendation in 2014. For women ages 65 to 75 who smoke, the USPSTF thinks the evidence is insufficient to recommend for or against screening (grade-I recommendation).
Our patient described above—male, age 68, and with a 25 pack-year smoking history—is a candidate for screening for abdominal aortic aneurysm.
CT SCREENING FOR LUNG CANCER
In December 2013, the USPSTF gave a B-grade recommendation for annual screening for lung cancer with low-dose computed tomography (CT) for adults ages 55 to 80 who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that limits life expectancy or the ability to undergo curative lung surgery.
These recommendations were based on the outcomes of the National Lung Screening Trial.4 However, whereas this trial was in people ages 55 to 74, the USPSTF boosted the upper age limit to 80 based on computer modeling, a decision that was somewhat controversial.
Patz et al5 analyzed data from the National Lung Screening Trial and found that about 18% of lung cancers detected by low-dose CT appeared to be indolent and were unlikely to become clinically apparent during the patient’s lifetime. The authors concluded that overdiagnosis should be considered when guidelines for mass screening programs are developed.
Our 68-year-old patient would not qualify for CT screening for lung cancer, since his smoking history is less than 30 pack-years.
COLORECTAL CANCER SCREENING AND PREVENTION
Unlike other cancer screening tests, colorectal cancer screening can also be a preventive measure; removing polyps found during screening with colonoscopy or sigmoidoscopy is an effective strategy in preventing colon cancer.
The USPSTF last updated its colorectal screening recommendations in 2008, giving a grade-A recommendation (strongly recommended; benefit far outweighs harm) to screening using fecal occult blood testing, sigmoidoscopy, or colonoscopy for adults ages 50 to 75. The risks and benefits of these screening methods vary. For adults ages 76 to 85, the task force recommends against routine screening but gives a grade-C recommendation for screening in that age group in some circumstances. They give a grade-D recommendation for screening after age 85.
The USPSTF concluded that the evidence is insufficient to assess the benefits and harms of CT colonography and fecal DNA testing for colorectal cancer screening.
The American Cancer Society issued similar guidelines in 2013, recommending that starting at age 50, men and women at low risk of colorectal cancer should be screened using one of the following schedules (the first four methods help detect both polyps and cancers, and the others detect only cancer)6:
- Colonoscopy every 10 years
- Flexible sigmoidoscopy every 5 years
- A double-contrast barium enema every 5 years
- CT colonography (“virtual colonoscopy”) every 5 years
- A guaiac-based fecal occult blood test annually
- A fecal immunochemical test annually.
Those at moderate or high risk of colorectal cancer are advised to talk with a doctor about a different testing schedule. (eg, colonoscopy every 5 years in patients with a significant family history of colon cancer).
Our patient last underwent colonoscopy 8 years ago and so does not need to be screened again for another 2 years.
CERVICAL CANCER SCREENING: MOVING TOWARD HPV TESTING FIRST?
Cervical cancer screening recommendations are fairly uniform across the major guideline-setting organizations.7 In general, they are:
- Ages 21–29: Check cytology every 3 years
- Ages 30–65: Cytology plus human papillomavirus (HPV) testing every 5 years (or cytology alone every 3 years)
- After age 65: Stop screening if prior screenings have been adequate and negative over the past 20 years.
Women who have been vaccinated against HPV have the same screening recommendations as above. Women who have had a hysterectomy for benign reasons do not need further screening.
The future of cervical cancer screening may be “reflex testing.” Rather than checking cervical samples for cytologic study (Papanicolaou smear) and HPV status together, we may one day screen samples first for HPV and, if that is positive, follow up with cytologic study. Easy-to-use home tests for HPV will likely be developed and should increase screening rates.
PROSTATE CANCER SCREENING: A SHARED DECISION
Prostate cancer screening remains controversial. Different guideline-setting bodies have different recommendations, creating confusion for patients. Physicians must follow what fits their own practice and beliefs.
The USPSTF in 2012 gave a grade-D recommendation to prostate-specific antigen (PSA) testing to screen for prostate cancer, stating that it did more harm than good. However, some men continue to be screened for PSA.
The American Cancer Society in 2013 recommended against routine testing for prostate cancer without a full discussion between physician and patient of the pros and cons of testing.8 If screening is decided upon, it should be done with annual PSA measurement or digital rectal examination, or both, starting at age 50. Men at high risk (ie, African American men, and men with a first-degree relative diagnosed with prostate cancer before age 65) should begin screening at age 45.
The American College of Physicians in 2013 issued a statement that clinicians should inform men between the ages of 50 and 69 about the limited potential benefits and substantial harms of prostate cancer screening.9 They recommended against PSA screening in men of average risk who are younger than age 50 or older than age 69, or those whose life expectancy is less than 10 to 15 years.
The American Urological Association in 2013 advised that10:
- PSA screening is not recommended in men younger than 40.
- Routine screening is not recommended in men between ages 40 and 54 at average risk.
- In men ages 55 to 69, decisions about PSA screening should be shared and based on each patient’s values and preferences. The decision to undergo PSA screening involves weighing the benefits of preventing death from prostate cancer in 1 man for every 1,000 men screened over a decade against the known potential harms associated with screening and treatment.
- To reduce the harm of screening, a routine interval of 2 years may be chosen over annual screening; such a schedule may preserve most benefits and reduce overdiagnosis and false-positive results.
- Routine PSA screening is not recommended in men ages 70 and older or with less than a 10- to 15-year life expectancy.
Shared decision-making. Many of the guidelines for prostate cancer screening are based on the concept of shared decision-making. However, studies indicate that many patients do not receive a full discussion of the issue,11 and in any event, patient education may make little difference in PSA testing rates.12,13
On the horizon for prostate cancer screening is the hope of finding a more predictable test. There is also discussion of using the PSA test earlier: some evidence shows that a very low result at age 45 predicts a less than 1% chance of developing metastatic prostate cancer by age 75, so it is possible that screening could stop in that population.
BREAST CANCER SCREENING: DIVERGENT RECOMMENDATIONS
The USPSTF created considerable controversy a few years ago when it recommended screening mammography from ages 50 to 74, and then only every 2 years—a departure from the traditional practice of starting screening at age 40. Few doctors heed the USPSTF guideline: most of the other guideline-setting organizations (eg, the American Cancer Society, the American Congress of Obstetricians and Gynecologists) recommend annual mammography for women starting at age 40.
Overdiagnosis is an especially pertinent issue with screening mammography for breast cancer because some cancers are indolent and will not cause a problem during a lifetime. Falk et al14 analyzed a Norwegian breast cancer screening program and found that overdiagnosis occurred in 10% to 20% of cases. Welch and Passow15 quantified the benefits and harms of screening mammography in 50-year-old women in the United States and found that of 1,000 women screened annually for a decade, 0.3 to 3.2 will avoid a breast cancer death, 490 to 670 will have at least one false alarm, and 3 to 14 will be overdiagnosed and treated needlessly.
Mammography screening for breast cancer will likely stay controversial for some time as we await additional data.
OTHER CANCERS: SCREENING NOT RECOMMENDED
The USPSTF currently does not recommend screening for ovarian cancer (guideline issued in 2012), pancreatic cancer (2004), or testicular cancer (2011), giving each a grade-D recommendation, indicating that screening does more harm than good. It also stated that there is insufficient evidence to recommend screening for oral cancer (2013), skin cancer (2009), and bladder cancer (2011).
- US Preventive Services Task Force. www.uspreventiveservicestask-force.org. Accessed August 11, 2014.
- Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831–841. Erratum in: JAMA 2009; 301:1544.
- Lee DH, Vielemeyer O. Analysis of overall level of evidence behind Infectious Diseases Society of America practice guidelines. Arch Intern Med 2011; 171:18–22.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Patz EF, Pinsky P, Gatsonis C, et al; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014; 174:269–274.
- American Cancer Society. Colorectal cancer screening and surveillance guidelines. www.cancer.org/healthy/informationforhealth-careprofessionals/colonmdclinicansinformationsource/colorec-talcancerscreeningandsurveillanceguidelines/index. Accessed August 11, 2014.
- Jin XW, Lipold L, McKenzie M, Sikon A. Cervical cancer screening: what’s new and what’s coming? Cleve Clin J Med 2013; 80:153–160.
- American Cancer Society. Prostate cancer screening guidelines. www.cancer.org/healthy/informationforhealthcareprofessionals/pros-tatemdcliniciansinformationsource/prostatecancerscreeningguide-lines/index. Accessed August 11, 2014.
- Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2013; 158:761–769.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed September 5, 2014.
- Han PK, Kobrin S, Breen N, et al. National evidence on the use of shared decision making in prostate-specific antigen screening. Ann Fam Med 2013; 11:306–314.
- Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med 2013; 173:1704–1712.
- Landrey AR, Matlock DD, Andrews L, Bronsert M, Denberg T. Shared decision making in prostate-specific antigen testing: the effect of a mailed patient flyer prior to an annual exam. J Prim Care Community Health 2013; 4:67–74.
- Falk RS, Hofvind S, Skaane P, Haldorsen T. Overdiagnosis among women attending a population-based mammography screening program. Int J Cancer 2013; 133:705–712.
- Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA Intern Med 2014; 174:448–454.
- US Preventive Services Task Force. www.uspreventiveservicestask-force.org. Accessed August 11, 2014.
- Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831–841. Erratum in: JAMA 2009; 301:1544.
- Lee DH, Vielemeyer O. Analysis of overall level of evidence behind Infectious Diseases Society of America practice guidelines. Arch Intern Med 2011; 171:18–22.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Patz EF, Pinsky P, Gatsonis C, et al; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014; 174:269–274.
- American Cancer Society. Colorectal cancer screening and surveillance guidelines. www.cancer.org/healthy/informationforhealth-careprofessionals/colonmdclinicansinformationsource/colorec-talcancerscreeningandsurveillanceguidelines/index. Accessed August 11, 2014.
- Jin XW, Lipold L, McKenzie M, Sikon A. Cervical cancer screening: what’s new and what’s coming? Cleve Clin J Med 2013; 80:153–160.
- American Cancer Society. Prostate cancer screening guidelines. www.cancer.org/healthy/informationforhealthcareprofessionals/pros-tatemdcliniciansinformationsource/prostatecancerscreeningguide-lines/index. Accessed August 11, 2014.
- Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2013; 158:761–769.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed September 5, 2014.
- Han PK, Kobrin S, Breen N, et al. National evidence on the use of shared decision making in prostate-specific antigen screening. Ann Fam Med 2013; 11:306–314.
- Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med 2013; 173:1704–1712.
- Landrey AR, Matlock DD, Andrews L, Bronsert M, Denberg T. Shared decision making in prostate-specific antigen testing: the effect of a mailed patient flyer prior to an annual exam. J Prim Care Community Health 2013; 4:67–74.
- Falk RS, Hofvind S, Skaane P, Haldorsen T. Overdiagnosis among women attending a population-based mammography screening program. Int J Cancer 2013; 133:705–712.
- Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA Intern Med 2014; 174:448–454.
KEY POINTS
- The USPSTF has stringent standards of evidence and therefore its recommendations tend to be more conservative than those of other organizations that issue guidelines. Recommendations are available at www.uspreventiveservicestaskforce.org.
- Because screening can result in harm as well as benefit, screening should be done after shared decision-making with the patient, especially if the screening is controversial, as is the case with mammography for breast cancer and prostate-specific antigen testing for prostate cancer.
- Screening for lung cancer using low-dose computed tomography is recommended yearly beginning at age 55 for people who have at least a 30-pack-year smoking history.
- In women over age 30, cervical cancer screening with Papanicolaou (Pap) and human papillomavirus (HPV) testing is now recommended every 5 years rather than every 3 years. Testing for HPV infection may soon become the first-line screening test, with Pap testing reserved for patients who have a positive HPV result.
- Although the USPSTF no longer recommends mammography for women ages 40 to 49, other organizations continue to do so.
Keeping up with immunizations for adults
A 58-year-old man with a history of irritable bowel syndrome and diabetes presents for an evaluation in early November. He is taking metformin and insulin glargine 10 units. He smokes 1 pack per day. He believes that his childhood immunizations were completed, but he has no records. He thinks his last “shot” was 15 years ago when he cut his hand on some wood.
Which immunizations, if any, would be most appropriate for this patient?
The explosion of new vaccines, new formulations, and new combinations made available in recent years makes managing immunizations a challenge. This article reviews common immunizations and current recommendations for their appropriate use.
Immunization recommendations (Table 1) are made predominantly by the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC). The last 15 years have seen the arrival of new vaccines (eg, varicella, hepatitis A, pneumococcal, and human papillomavirus), new formulations (eg, intranasal influenza), and new combinations.
To keep clinicians abreast of new indications, the ACIP issues immunization schedules annually for children and adults, available online and downloadable for easy reference.1 For adults, the ACIP provides schedules based on age and medical condition. The schedule for medical conditions offers specific information regarding immunization and pregnancy, human immunodeficiency virus (HIV) infection, kidney failure, heart disease, asplenia, and other conditions. The ACIP also provides guidance on contraindications; for example, pregnant and immunocompromised patients should not receive the live-attenuated vaccines, ie, zoster, varicella, and combined measles, mumps, and rubella [MMR]).
Adult awareness of vaccines is low, as are vaccination rates: in people older than 60, the vaccination rate is about 70% for influenza, 60% for pneumococcus, 50% for tetanus, and 15% for zoster. The lack of vaccine awareness and the availability of new vaccines and indications have made it difficult to manage immunizations in the primary care setting. The electronic medical record is useful for tracking patient vaccine needs. Ideally, keeping up with immunizations should be a routine part of visits provided by a physician’s care team and does not always require direct physician coordination.
TETANUS, DIPHTHERIA, PERTUSSIS EVERY 10 YEARS
Tetanus (also called “lockjaw”) is a nervous system disorder characterized by muscle spasms. Caused by infection with Clostridium tetani, it is a rare disease in the United States thanks to widespread immunization, and it causes fewer than 50 cases annually. Worldwide, the incidence is about 1 million cases a year with 200,000 to 300,000 deaths.
Diphtheria (formerly sometimes called “throat distemper”) is caused by the gram-positive bacillus Corynebacterium diphtheriae and can occur as a respiratory illness or as a milder cutaneous form. The last outbreak in the United States was in Seattle in the 1970s, with the last reported case in 2003. The ACIP recommends booster shots for tetanus and diphtheria every 10 years following completion of the primary series.
Pertussis or whooping cough, caused by Bordetella pertussis infection, is a highly contagious disease increasingly seen in adults in the United States. It causes few deaths but high morbidity, with coughing that can persist up to 3 months. Coughing can be severe enough to cause vomiting, a characteristic sign.
In July 2012, the CDC reported that the United States was at a 50-year high for pertussis, with 18,000 cases reported and 8 deaths.2 In Washington State alone, more than 2,520 cases had been seen through June 16 of that year, a 1,300% increase over the previous year. Rates were high in older children and adolescents despite previous vaccination, suggesting an early waning of immunity.
The ACIP recommends a single dose of the combination of high-dose tetanus and low-dose diphtheria and pertussis vaccines (Tdap) for all adults regardless of age and for all pregnant women with each pregnancy between 27 and 36 weeks of gestation. A dose of Tdap counts as the tetanus-diphtheria booster shot that is recommended every 10 years.
The patient described above is due for his tetanus-diphtheria booster and so should be given Tdap.
MEASLES, MUMPS, RUBELLA FOR THOSE BORN AFTER 1957
Measles remains a problem in the developing world, with an estimated average of 330 deaths daily. The number of cases fell 99% in the United States following the vaccination program that started in the early 1960s. Before the measles vaccine was available, an estimated 90% of children acquired measles by age 15.
The clinical syndrome consists of fever, conjunctivitis, cough, rash, and the characteristic Koplik spots—small white spots occurring on the inside of cheeks early in the disease course.
During the first 5 months of 2014, the CDC reported 334 cases of measles in the United States in 18 states, with most people affected being unvaccinated.3 In comparison, from 2001 to 2008, the number of cases averaged 56 annually.
Many of the recent cases were associated with infections brought from the Philippines. The increased number of measles cases underscores the need for vaccination to prevent measles and its complications.
Mumps is an acute, self-limited viral syndrome, and parotitis is the hallmark. Vaccination led to a 99% decline in cases in the United States. Although complications are rare, they can be serious and include orchitis (with risk of sterility), meningoencephalitis, and deafness.
Mumps outbreaks still occur, especially in close-contact settings such as schools, colleges, and camps. During the first half of 2014, central Ohio had more than 400 cases linked to The Ohio State University.
Rubella, also known as German measles, is a generally mild infection but is associated with congenital rubella syndrome. If a woman is infected with rubella in the first trimester of pregnancy, the risk of miscarriage is greater than 80%, as is the risk of birth defects, including hearing loss, developmental delay, growth retardation, and cardiac and eye defects.
Recommendations for MMR vaccination. People born before 1957 are considered immune to measles and usually to mumps. Health care workers should document immunity before assuming no vaccination is needed.
People born in 1957 or after should have one dose of MMR vaccine unless immunity is documented or unless there is a contraindication such as immunosuppression. A second dose is recommended for those born in or after 1957 who are considered to be at high risk: eg, health care workers, students entering college, and international travelers. The second dose should be given 4 weeks after the first.
Women of childbearing age should be screened for immunity to rubella. Susceptible women should receive MMR, although not during pregnancy and not if they may get pregnant within 4 weeks.
The patient described above was born before 1957, and so he is probably immune to measles and mumps.
HEPATITIS B FOR THOSE AT RISK
Hepatitis B vaccination is recommended for all adolescents and adults at increased risk: eg, men who have sex with men, intravenous drug users, people with multiple sexual partners, health care workers, patients with end-stage renal disease on hemodialysis, patients with chronic liver disease, and those with diabetes (age < 60).
Immunization consists of a series of three shots (at 0, 1–2, and 4–6 months). Booster doses are not recommended. Postvaccination testing for immunity is available and is recommended for health care workers, patients on hemodialysis, patients with HIV infection or who are otherwise immunocompromised, and sexual partners of people who are positive for hepatitis B surface antigen. Nonresponders should be revaccinated with the entire three-shot schedule. Hepatitis B vaccination is safe in pregnancy.
The patient described above has diabetes and so is a candidate for vaccination.
HEPATITIS A: A SLIGHTLY DIFFERENT RISK GROUP
Hepatitis A vaccination is recommended only for at-risk populations: international travelers; intravenous drug users; men who have sex with men; patients with clotting disorders, chronic liver disease, or hepatitis C infection; international adoptees; and laboratory personnel working with hepatitis A virus. The vaccination is given in two doses with a minimum interval of 6 months between doses.
A hepatitis A and hepatitis B combination vaccine (Twinrix) is also available. It is given in three doses, at 0, 1, and 6 months.
ANNUAL INFLUENZA VACCINE FOR ALL
In 2010, the ACIP recommended a policy of universal annual vaccination for everyone age 6 months and older. Some patients are at especially high risk themselves or are at high risk of exposing others and so are given higher priority during vaccine shortages—ie, patients who are immunosuppressed or have other medical risk factors, health care workers, household members of at-risk patients, and pregnant women after 13 weeks of gestation.
There are few contraindications, so almost everyone should be encouraged to receive the influenza vaccine. The flu shot does not cause the flu, but it may cause soreness at the injection site. Those with severe egg allergy should not receive the standard flu shot; a recombinant vaccine that does not use egg culture is available.
The standard flu shot is an inactivated influenza vaccine. In the past, most formulations were trivalent, but quadrivalent formulations are becoming more common. Special high-dose formulations are believed to elicit a better immune response and can be recommended for people over age 65. Intradermal and intramuscular formulations are available.
An intranasal live-attenuated influenza vaccine is also available and may be used for people ages 2 through 49. It should not be given to immunosuppressed people or to pregnant women.
Our patient should get a flu shot.
PNEUMOCOCCAL VACCINE FOR THOSE AGE 65 AND OLDER OR AT RISK
Two formulations are now available for pneumococcal immunization. The standard is a 23-valent polysaccharide vaccine (PPSV23; Pneumovax) indicated for people age 65 and older.
Patients under age 65 can receive PPSV23 if they have chronic lung disease, chronic cardiovascular disease, diabetes, chronic liver disease, or alcoholism or are a resident of a nursing home or an active smoker.
Our patient is a candidate for PPSV23 since he smokes and has diabetes.
The other formulation is a conjugate 13-valent vaccine (PCV13; Prevnar 13). Patients over age 19 at high risk should be given PCV13 plus the PPSV23 8 weeks later. Those who already received PPSV23 should be given PCV13 vaccine more than 1 year later. Candidates for PCV13 are those with immunocompromising conditions (including chronic renal failure and nephrotic syndrome), functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants.
The current revaccination schedule for PPSV23 is as follows:
- One-time revaccination 5 years after the first dose in patients with chronic renal failure, nephrotic syndrome, asplenia, or an immunosuppressive condition
- One-time revaccination for patients age 65 or older if they were younger than 65 when first immunized (with one or two doses of PPSV23) and 5 years have passed
- No revaccination is needed for people vaccinated with PPSV23 after age 65.
HUMAN PAPILLOMAVIRUS VACCINE
Human papillomavirus is the most common sexually transmitted infection in the United States and is strongly associated with cervical cancer. Immunization is now indicated for both sexes, generally between the ages of 9 and 26. Two vaccines are available: the quadrivalent formulation (Gardasil) for males or females and the bivalent formulation (Cervarix) for females only.
Immunization should be given in three doses: at 0, 1 to 2 months, and 6 months. It can be given to patients who are immunocompromised as a result of infection (including HIV infection), disease, or medications, or who have a history of genital warts, an abnormal Papanicolaou test, or a positive human papillomavirus DNA test.
It is hoped that immunization will lead to a significant decrease in cervical cancer rates. Eradication is unlikely because other papillomavirus strains also can lead to cancer, so cancer screening is still warranted. For men who have sex with men, it is hoped that immunization will prevent condyloma and anal cancer.
CHICKENPOX AND SHINGLES VACCINES
Varicella vaccine (Varivax) contains a live-attenuated virus to protect against chickenpox. It is recommended for all adults who have no evidence of immunity. Immunity is assumed with a history of chickenpox, being born before 1980, or having positive titers. Vaccination should be emphasized for those who come in contact with patients at high risk of severe disease (eg, health care workers, family contacts of immunocompromised patients) and in individuals with a high risk of personal exposure (eg, teachers, day care workers).
The vaccine is given in two doses, 4 to 8 weeks apart. Women who are pregnant or who may get pregnant within 4 weeks should not be vaccinated.
The shingles vaccine (Zostavax) is a larger dose of the varicella vaccine and reduces the incidence of shingles by 50% and postherpetic neuralgia by 66%.4 It was approved by the US Food and Drug Administration in May 2006 for people starting at age 50, but was recommended by ACIP in October 2006 for people age 60 and older; as a result, some insurance companies deny coverage for patients ages 50 through 59.
The shingles vaccine can be given to patients who have already had shingles. Pregnancy and severe immunodeficiency are contraindications.
Our patient, 58 years old, could be considered for shingles vaccine if covered by his insurance company or if he wishes to pay for it.
MENINGOCOCCUS VACCINE
Meningococcal immunization is recommended for people at high risk: college students who plan to live in dormitories, adults without a spleen or with complement deficiencies or HIV infection, or travelers to the “meningitis belt” of sub-Saharan Africa.
Two types of meningococcal vaccine are available: the conjugate quadrivalent vaccine (MCV4) for people age 55 and younger, and the polysaccharide quadrivalent vaccine (MPSV4) for people over age 56.
- US Centers for Disease Control and Prevention (CDC). Adult immunization schedules. www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed August 21, 2014.
- US Centers for Disease Control and Prevention (CDC). Pertussis epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:517–522.
- US Centers for Disease Control and Prevention (CDC). Measles cases and outbreaks, January 1 to August 15, 2014. www.cdc.gov/measles/cases-outbreaks.html. Accessed August 21, 2014.
- Oxman MN, Levin MJ, Johnson MS, et al; for the Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
A 58-year-old man with a history of irritable bowel syndrome and diabetes presents for an evaluation in early November. He is taking metformin and insulin glargine 10 units. He smokes 1 pack per day. He believes that his childhood immunizations were completed, but he has no records. He thinks his last “shot” was 15 years ago when he cut his hand on some wood.
Which immunizations, if any, would be most appropriate for this patient?
The explosion of new vaccines, new formulations, and new combinations made available in recent years makes managing immunizations a challenge. This article reviews common immunizations and current recommendations for their appropriate use.
Immunization recommendations (Table 1) are made predominantly by the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC). The last 15 years have seen the arrival of new vaccines (eg, varicella, hepatitis A, pneumococcal, and human papillomavirus), new formulations (eg, intranasal influenza), and new combinations.
To keep clinicians abreast of new indications, the ACIP issues immunization schedules annually for children and adults, available online and downloadable for easy reference.1 For adults, the ACIP provides schedules based on age and medical condition. The schedule for medical conditions offers specific information regarding immunization and pregnancy, human immunodeficiency virus (HIV) infection, kidney failure, heart disease, asplenia, and other conditions. The ACIP also provides guidance on contraindications; for example, pregnant and immunocompromised patients should not receive the live-attenuated vaccines, ie, zoster, varicella, and combined measles, mumps, and rubella [MMR]).
Adult awareness of vaccines is low, as are vaccination rates: in people older than 60, the vaccination rate is about 70% for influenza, 60% for pneumococcus, 50% for tetanus, and 15% for zoster. The lack of vaccine awareness and the availability of new vaccines and indications have made it difficult to manage immunizations in the primary care setting. The electronic medical record is useful for tracking patient vaccine needs. Ideally, keeping up with immunizations should be a routine part of visits provided by a physician’s care team and does not always require direct physician coordination.
TETANUS, DIPHTHERIA, PERTUSSIS EVERY 10 YEARS
Tetanus (also called “lockjaw”) is a nervous system disorder characterized by muscle spasms. Caused by infection with Clostridium tetani, it is a rare disease in the United States thanks to widespread immunization, and it causes fewer than 50 cases annually. Worldwide, the incidence is about 1 million cases a year with 200,000 to 300,000 deaths.
Diphtheria (formerly sometimes called “throat distemper”) is caused by the gram-positive bacillus Corynebacterium diphtheriae and can occur as a respiratory illness or as a milder cutaneous form. The last outbreak in the United States was in Seattle in the 1970s, with the last reported case in 2003. The ACIP recommends booster shots for tetanus and diphtheria every 10 years following completion of the primary series.
Pertussis or whooping cough, caused by Bordetella pertussis infection, is a highly contagious disease increasingly seen in adults in the United States. It causes few deaths but high morbidity, with coughing that can persist up to 3 months. Coughing can be severe enough to cause vomiting, a characteristic sign.
In July 2012, the CDC reported that the United States was at a 50-year high for pertussis, with 18,000 cases reported and 8 deaths.2 In Washington State alone, more than 2,520 cases had been seen through June 16 of that year, a 1,300% increase over the previous year. Rates were high in older children and adolescents despite previous vaccination, suggesting an early waning of immunity.
The ACIP recommends a single dose of the combination of high-dose tetanus and low-dose diphtheria and pertussis vaccines (Tdap) for all adults regardless of age and for all pregnant women with each pregnancy between 27 and 36 weeks of gestation. A dose of Tdap counts as the tetanus-diphtheria booster shot that is recommended every 10 years.
The patient described above is due for his tetanus-diphtheria booster and so should be given Tdap.
MEASLES, MUMPS, RUBELLA FOR THOSE BORN AFTER 1957
Measles remains a problem in the developing world, with an estimated average of 330 deaths daily. The number of cases fell 99% in the United States following the vaccination program that started in the early 1960s. Before the measles vaccine was available, an estimated 90% of children acquired measles by age 15.
The clinical syndrome consists of fever, conjunctivitis, cough, rash, and the characteristic Koplik spots—small white spots occurring on the inside of cheeks early in the disease course.
During the first 5 months of 2014, the CDC reported 334 cases of measles in the United States in 18 states, with most people affected being unvaccinated.3 In comparison, from 2001 to 2008, the number of cases averaged 56 annually.
Many of the recent cases were associated with infections brought from the Philippines. The increased number of measles cases underscores the need for vaccination to prevent measles and its complications.
Mumps is an acute, self-limited viral syndrome, and parotitis is the hallmark. Vaccination led to a 99% decline in cases in the United States. Although complications are rare, they can be serious and include orchitis (with risk of sterility), meningoencephalitis, and deafness.
Mumps outbreaks still occur, especially in close-contact settings such as schools, colleges, and camps. During the first half of 2014, central Ohio had more than 400 cases linked to The Ohio State University.
Rubella, also known as German measles, is a generally mild infection but is associated with congenital rubella syndrome. If a woman is infected with rubella in the first trimester of pregnancy, the risk of miscarriage is greater than 80%, as is the risk of birth defects, including hearing loss, developmental delay, growth retardation, and cardiac and eye defects.
Recommendations for MMR vaccination. People born before 1957 are considered immune to measles and usually to mumps. Health care workers should document immunity before assuming no vaccination is needed.
People born in 1957 or after should have one dose of MMR vaccine unless immunity is documented or unless there is a contraindication such as immunosuppression. A second dose is recommended for those born in or after 1957 who are considered to be at high risk: eg, health care workers, students entering college, and international travelers. The second dose should be given 4 weeks after the first.
Women of childbearing age should be screened for immunity to rubella. Susceptible women should receive MMR, although not during pregnancy and not if they may get pregnant within 4 weeks.
The patient described above was born before 1957, and so he is probably immune to measles and mumps.
HEPATITIS B FOR THOSE AT RISK
Hepatitis B vaccination is recommended for all adolescents and adults at increased risk: eg, men who have sex with men, intravenous drug users, people with multiple sexual partners, health care workers, patients with end-stage renal disease on hemodialysis, patients with chronic liver disease, and those with diabetes (age < 60).
Immunization consists of a series of three shots (at 0, 1–2, and 4–6 months). Booster doses are not recommended. Postvaccination testing for immunity is available and is recommended for health care workers, patients on hemodialysis, patients with HIV infection or who are otherwise immunocompromised, and sexual partners of people who are positive for hepatitis B surface antigen. Nonresponders should be revaccinated with the entire three-shot schedule. Hepatitis B vaccination is safe in pregnancy.
The patient described above has diabetes and so is a candidate for vaccination.
HEPATITIS A: A SLIGHTLY DIFFERENT RISK GROUP
Hepatitis A vaccination is recommended only for at-risk populations: international travelers; intravenous drug users; men who have sex with men; patients with clotting disorders, chronic liver disease, or hepatitis C infection; international adoptees; and laboratory personnel working with hepatitis A virus. The vaccination is given in two doses with a minimum interval of 6 months between doses.
A hepatitis A and hepatitis B combination vaccine (Twinrix) is also available. It is given in three doses, at 0, 1, and 6 months.
ANNUAL INFLUENZA VACCINE FOR ALL
In 2010, the ACIP recommended a policy of universal annual vaccination for everyone age 6 months and older. Some patients are at especially high risk themselves or are at high risk of exposing others and so are given higher priority during vaccine shortages—ie, patients who are immunosuppressed or have other medical risk factors, health care workers, household members of at-risk patients, and pregnant women after 13 weeks of gestation.
There are few contraindications, so almost everyone should be encouraged to receive the influenza vaccine. The flu shot does not cause the flu, but it may cause soreness at the injection site. Those with severe egg allergy should not receive the standard flu shot; a recombinant vaccine that does not use egg culture is available.
The standard flu shot is an inactivated influenza vaccine. In the past, most formulations were trivalent, but quadrivalent formulations are becoming more common. Special high-dose formulations are believed to elicit a better immune response and can be recommended for people over age 65. Intradermal and intramuscular formulations are available.
An intranasal live-attenuated influenza vaccine is also available and may be used for people ages 2 through 49. It should not be given to immunosuppressed people or to pregnant women.
Our patient should get a flu shot.
PNEUMOCOCCAL VACCINE FOR THOSE AGE 65 AND OLDER OR AT RISK
Two formulations are now available for pneumococcal immunization. The standard is a 23-valent polysaccharide vaccine (PPSV23; Pneumovax) indicated for people age 65 and older.
Patients under age 65 can receive PPSV23 if they have chronic lung disease, chronic cardiovascular disease, diabetes, chronic liver disease, or alcoholism or are a resident of a nursing home or an active smoker.
Our patient is a candidate for PPSV23 since he smokes and has diabetes.
The other formulation is a conjugate 13-valent vaccine (PCV13; Prevnar 13). Patients over age 19 at high risk should be given PCV13 plus the PPSV23 8 weeks later. Those who already received PPSV23 should be given PCV13 vaccine more than 1 year later. Candidates for PCV13 are those with immunocompromising conditions (including chronic renal failure and nephrotic syndrome), functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants.
The current revaccination schedule for PPSV23 is as follows:
- One-time revaccination 5 years after the first dose in patients with chronic renal failure, nephrotic syndrome, asplenia, or an immunosuppressive condition
- One-time revaccination for patients age 65 or older if they were younger than 65 when first immunized (with one or two doses of PPSV23) and 5 years have passed
- No revaccination is needed for people vaccinated with PPSV23 after age 65.
HUMAN PAPILLOMAVIRUS VACCINE
Human papillomavirus is the most common sexually transmitted infection in the United States and is strongly associated with cervical cancer. Immunization is now indicated for both sexes, generally between the ages of 9 and 26. Two vaccines are available: the quadrivalent formulation (Gardasil) for males or females and the bivalent formulation (Cervarix) for females only.
Immunization should be given in three doses: at 0, 1 to 2 months, and 6 months. It can be given to patients who are immunocompromised as a result of infection (including HIV infection), disease, or medications, or who have a history of genital warts, an abnormal Papanicolaou test, or a positive human papillomavirus DNA test.
It is hoped that immunization will lead to a significant decrease in cervical cancer rates. Eradication is unlikely because other papillomavirus strains also can lead to cancer, so cancer screening is still warranted. For men who have sex with men, it is hoped that immunization will prevent condyloma and anal cancer.
CHICKENPOX AND SHINGLES VACCINES
Varicella vaccine (Varivax) contains a live-attenuated virus to protect against chickenpox. It is recommended for all adults who have no evidence of immunity. Immunity is assumed with a history of chickenpox, being born before 1980, or having positive titers. Vaccination should be emphasized for those who come in contact with patients at high risk of severe disease (eg, health care workers, family contacts of immunocompromised patients) and in individuals with a high risk of personal exposure (eg, teachers, day care workers).
The vaccine is given in two doses, 4 to 8 weeks apart. Women who are pregnant or who may get pregnant within 4 weeks should not be vaccinated.
The shingles vaccine (Zostavax) is a larger dose of the varicella vaccine and reduces the incidence of shingles by 50% and postherpetic neuralgia by 66%.4 It was approved by the US Food and Drug Administration in May 2006 for people starting at age 50, but was recommended by ACIP in October 2006 for people age 60 and older; as a result, some insurance companies deny coverage for patients ages 50 through 59.
The shingles vaccine can be given to patients who have already had shingles. Pregnancy and severe immunodeficiency are contraindications.
Our patient, 58 years old, could be considered for shingles vaccine if covered by his insurance company or if he wishes to pay for it.
MENINGOCOCCUS VACCINE
Meningococcal immunization is recommended for people at high risk: college students who plan to live in dormitories, adults without a spleen or with complement deficiencies or HIV infection, or travelers to the “meningitis belt” of sub-Saharan Africa.
Two types of meningococcal vaccine are available: the conjugate quadrivalent vaccine (MCV4) for people age 55 and younger, and the polysaccharide quadrivalent vaccine (MPSV4) for people over age 56.
A 58-year-old man with a history of irritable bowel syndrome and diabetes presents for an evaluation in early November. He is taking metformin and insulin glargine 10 units. He smokes 1 pack per day. He believes that his childhood immunizations were completed, but he has no records. He thinks his last “shot” was 15 years ago when he cut his hand on some wood.
Which immunizations, if any, would be most appropriate for this patient?
The explosion of new vaccines, new formulations, and new combinations made available in recent years makes managing immunizations a challenge. This article reviews common immunizations and current recommendations for their appropriate use.
Immunization recommendations (Table 1) are made predominantly by the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC). The last 15 years have seen the arrival of new vaccines (eg, varicella, hepatitis A, pneumococcal, and human papillomavirus), new formulations (eg, intranasal influenza), and new combinations.
To keep clinicians abreast of new indications, the ACIP issues immunization schedules annually for children and adults, available online and downloadable for easy reference.1 For adults, the ACIP provides schedules based on age and medical condition. The schedule for medical conditions offers specific information regarding immunization and pregnancy, human immunodeficiency virus (HIV) infection, kidney failure, heart disease, asplenia, and other conditions. The ACIP also provides guidance on contraindications; for example, pregnant and immunocompromised patients should not receive the live-attenuated vaccines, ie, zoster, varicella, and combined measles, mumps, and rubella [MMR]).
Adult awareness of vaccines is low, as are vaccination rates: in people older than 60, the vaccination rate is about 70% for influenza, 60% for pneumococcus, 50% for tetanus, and 15% for zoster. The lack of vaccine awareness and the availability of new vaccines and indications have made it difficult to manage immunizations in the primary care setting. The electronic medical record is useful for tracking patient vaccine needs. Ideally, keeping up with immunizations should be a routine part of visits provided by a physician’s care team and does not always require direct physician coordination.
TETANUS, DIPHTHERIA, PERTUSSIS EVERY 10 YEARS
Tetanus (also called “lockjaw”) is a nervous system disorder characterized by muscle spasms. Caused by infection with Clostridium tetani, it is a rare disease in the United States thanks to widespread immunization, and it causes fewer than 50 cases annually. Worldwide, the incidence is about 1 million cases a year with 200,000 to 300,000 deaths.
Diphtheria (formerly sometimes called “throat distemper”) is caused by the gram-positive bacillus Corynebacterium diphtheriae and can occur as a respiratory illness or as a milder cutaneous form. The last outbreak in the United States was in Seattle in the 1970s, with the last reported case in 2003. The ACIP recommends booster shots for tetanus and diphtheria every 10 years following completion of the primary series.
Pertussis or whooping cough, caused by Bordetella pertussis infection, is a highly contagious disease increasingly seen in adults in the United States. It causes few deaths but high morbidity, with coughing that can persist up to 3 months. Coughing can be severe enough to cause vomiting, a characteristic sign.
In July 2012, the CDC reported that the United States was at a 50-year high for pertussis, with 18,000 cases reported and 8 deaths.2 In Washington State alone, more than 2,520 cases had been seen through June 16 of that year, a 1,300% increase over the previous year. Rates were high in older children and adolescents despite previous vaccination, suggesting an early waning of immunity.
The ACIP recommends a single dose of the combination of high-dose tetanus and low-dose diphtheria and pertussis vaccines (Tdap) for all adults regardless of age and for all pregnant women with each pregnancy between 27 and 36 weeks of gestation. A dose of Tdap counts as the tetanus-diphtheria booster shot that is recommended every 10 years.
The patient described above is due for his tetanus-diphtheria booster and so should be given Tdap.
MEASLES, MUMPS, RUBELLA FOR THOSE BORN AFTER 1957
Measles remains a problem in the developing world, with an estimated average of 330 deaths daily. The number of cases fell 99% in the United States following the vaccination program that started in the early 1960s. Before the measles vaccine was available, an estimated 90% of children acquired measles by age 15.
The clinical syndrome consists of fever, conjunctivitis, cough, rash, and the characteristic Koplik spots—small white spots occurring on the inside of cheeks early in the disease course.
During the first 5 months of 2014, the CDC reported 334 cases of measles in the United States in 18 states, with most people affected being unvaccinated.3 In comparison, from 2001 to 2008, the number of cases averaged 56 annually.
Many of the recent cases were associated with infections brought from the Philippines. The increased number of measles cases underscores the need for vaccination to prevent measles and its complications.
Mumps is an acute, self-limited viral syndrome, and parotitis is the hallmark. Vaccination led to a 99% decline in cases in the United States. Although complications are rare, they can be serious and include orchitis (with risk of sterility), meningoencephalitis, and deafness.
Mumps outbreaks still occur, especially in close-contact settings such as schools, colleges, and camps. During the first half of 2014, central Ohio had more than 400 cases linked to The Ohio State University.
Rubella, also known as German measles, is a generally mild infection but is associated with congenital rubella syndrome. If a woman is infected with rubella in the first trimester of pregnancy, the risk of miscarriage is greater than 80%, as is the risk of birth defects, including hearing loss, developmental delay, growth retardation, and cardiac and eye defects.
Recommendations for MMR vaccination. People born before 1957 are considered immune to measles and usually to mumps. Health care workers should document immunity before assuming no vaccination is needed.
People born in 1957 or after should have one dose of MMR vaccine unless immunity is documented or unless there is a contraindication such as immunosuppression. A second dose is recommended for those born in or after 1957 who are considered to be at high risk: eg, health care workers, students entering college, and international travelers. The second dose should be given 4 weeks after the first.
Women of childbearing age should be screened for immunity to rubella. Susceptible women should receive MMR, although not during pregnancy and not if they may get pregnant within 4 weeks.
The patient described above was born before 1957, and so he is probably immune to measles and mumps.
HEPATITIS B FOR THOSE AT RISK
Hepatitis B vaccination is recommended for all adolescents and adults at increased risk: eg, men who have sex with men, intravenous drug users, people with multiple sexual partners, health care workers, patients with end-stage renal disease on hemodialysis, patients with chronic liver disease, and those with diabetes (age < 60).
Immunization consists of a series of three shots (at 0, 1–2, and 4–6 months). Booster doses are not recommended. Postvaccination testing for immunity is available and is recommended for health care workers, patients on hemodialysis, patients with HIV infection or who are otherwise immunocompromised, and sexual partners of people who are positive for hepatitis B surface antigen. Nonresponders should be revaccinated with the entire three-shot schedule. Hepatitis B vaccination is safe in pregnancy.
The patient described above has diabetes and so is a candidate for vaccination.
HEPATITIS A: A SLIGHTLY DIFFERENT RISK GROUP
Hepatitis A vaccination is recommended only for at-risk populations: international travelers; intravenous drug users; men who have sex with men; patients with clotting disorders, chronic liver disease, or hepatitis C infection; international adoptees; and laboratory personnel working with hepatitis A virus. The vaccination is given in two doses with a minimum interval of 6 months between doses.
A hepatitis A and hepatitis B combination vaccine (Twinrix) is also available. It is given in three doses, at 0, 1, and 6 months.
ANNUAL INFLUENZA VACCINE FOR ALL
In 2010, the ACIP recommended a policy of universal annual vaccination for everyone age 6 months and older. Some patients are at especially high risk themselves or are at high risk of exposing others and so are given higher priority during vaccine shortages—ie, patients who are immunosuppressed or have other medical risk factors, health care workers, household members of at-risk patients, and pregnant women after 13 weeks of gestation.
There are few contraindications, so almost everyone should be encouraged to receive the influenza vaccine. The flu shot does not cause the flu, but it may cause soreness at the injection site. Those with severe egg allergy should not receive the standard flu shot; a recombinant vaccine that does not use egg culture is available.
The standard flu shot is an inactivated influenza vaccine. In the past, most formulations were trivalent, but quadrivalent formulations are becoming more common. Special high-dose formulations are believed to elicit a better immune response and can be recommended for people over age 65. Intradermal and intramuscular formulations are available.
An intranasal live-attenuated influenza vaccine is also available and may be used for people ages 2 through 49. It should not be given to immunosuppressed people or to pregnant women.
Our patient should get a flu shot.
PNEUMOCOCCAL VACCINE FOR THOSE AGE 65 AND OLDER OR AT RISK
Two formulations are now available for pneumococcal immunization. The standard is a 23-valent polysaccharide vaccine (PPSV23; Pneumovax) indicated for people age 65 and older.
Patients under age 65 can receive PPSV23 if they have chronic lung disease, chronic cardiovascular disease, diabetes, chronic liver disease, or alcoholism or are a resident of a nursing home or an active smoker.
Our patient is a candidate for PPSV23 since he smokes and has diabetes.
The other formulation is a conjugate 13-valent vaccine (PCV13; Prevnar 13). Patients over age 19 at high risk should be given PCV13 plus the PPSV23 8 weeks later. Those who already received PPSV23 should be given PCV13 vaccine more than 1 year later. Candidates for PCV13 are those with immunocompromising conditions (including chronic renal failure and nephrotic syndrome), functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants.
The current revaccination schedule for PPSV23 is as follows:
- One-time revaccination 5 years after the first dose in patients with chronic renal failure, nephrotic syndrome, asplenia, or an immunosuppressive condition
- One-time revaccination for patients age 65 or older if they were younger than 65 when first immunized (with one or two doses of PPSV23) and 5 years have passed
- No revaccination is needed for people vaccinated with PPSV23 after age 65.
HUMAN PAPILLOMAVIRUS VACCINE
Human papillomavirus is the most common sexually transmitted infection in the United States and is strongly associated with cervical cancer. Immunization is now indicated for both sexes, generally between the ages of 9 and 26. Two vaccines are available: the quadrivalent formulation (Gardasil) for males or females and the bivalent formulation (Cervarix) for females only.
Immunization should be given in three doses: at 0, 1 to 2 months, and 6 months. It can be given to patients who are immunocompromised as a result of infection (including HIV infection), disease, or medications, or who have a history of genital warts, an abnormal Papanicolaou test, or a positive human papillomavirus DNA test.
It is hoped that immunization will lead to a significant decrease in cervical cancer rates. Eradication is unlikely because other papillomavirus strains also can lead to cancer, so cancer screening is still warranted. For men who have sex with men, it is hoped that immunization will prevent condyloma and anal cancer.
CHICKENPOX AND SHINGLES VACCINES
Varicella vaccine (Varivax) contains a live-attenuated virus to protect against chickenpox. It is recommended for all adults who have no evidence of immunity. Immunity is assumed with a history of chickenpox, being born before 1980, or having positive titers. Vaccination should be emphasized for those who come in contact with patients at high risk of severe disease (eg, health care workers, family contacts of immunocompromised patients) and in individuals with a high risk of personal exposure (eg, teachers, day care workers).
The vaccine is given in two doses, 4 to 8 weeks apart. Women who are pregnant or who may get pregnant within 4 weeks should not be vaccinated.
The shingles vaccine (Zostavax) is a larger dose of the varicella vaccine and reduces the incidence of shingles by 50% and postherpetic neuralgia by 66%.4 It was approved by the US Food and Drug Administration in May 2006 for people starting at age 50, but was recommended by ACIP in October 2006 for people age 60 and older; as a result, some insurance companies deny coverage for patients ages 50 through 59.
The shingles vaccine can be given to patients who have already had shingles. Pregnancy and severe immunodeficiency are contraindications.
Our patient, 58 years old, could be considered for shingles vaccine if covered by his insurance company or if he wishes to pay for it.
MENINGOCOCCUS VACCINE
Meningococcal immunization is recommended for people at high risk: college students who plan to live in dormitories, adults without a spleen or with complement deficiencies or HIV infection, or travelers to the “meningitis belt” of sub-Saharan Africa.
Two types of meningococcal vaccine are available: the conjugate quadrivalent vaccine (MCV4) for people age 55 and younger, and the polysaccharide quadrivalent vaccine (MPSV4) for people over age 56.
- US Centers for Disease Control and Prevention (CDC). Adult immunization schedules. www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed August 21, 2014.
- US Centers for Disease Control and Prevention (CDC). Pertussis epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:517–522.
- US Centers for Disease Control and Prevention (CDC). Measles cases and outbreaks, January 1 to August 15, 2014. www.cdc.gov/measles/cases-outbreaks.html. Accessed August 21, 2014.
- Oxman MN, Levin MJ, Johnson MS, et al; for the Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
- US Centers for Disease Control and Prevention (CDC). Adult immunization schedules. www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed August 21, 2014.
- US Centers for Disease Control and Prevention (CDC). Pertussis epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:517–522.
- US Centers for Disease Control and Prevention (CDC). Measles cases and outbreaks, January 1 to August 15, 2014. www.cdc.gov/measles/cases-outbreaks.html. Accessed August 21, 2014.
- Oxman MN, Levin MJ, Johnson MS, et al; for the Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
KEY POINTS
- Information on immunization schedules, including an app for mobile devices, is available at www.cdc.gov/vaccines/schedules/hcp/adult.html.
- Vaccination rates in adults are low, and appropriate vaccinations should be encouraged. The electronic medical record can help remind us when vaccinations are due.
- The live-attenuated vaccines, ie, zoster, varicella, and combined measles, mumps, and rubella, are contraindicated during pregnancy and in immunocompromised patients.
Bench-to-bedside challenges in developing immune protection against breast cancer
The most proven, effective way to control disease is through prophylactic vaccination. The childhood vaccination program is a testament to this disease prevention approach, and in its current form protects us from diseases caused by 16 different pathogens.1
Childhood immunization ends in the teen years with recommended vaccination against multiple strains of human papillomavirus that are associated with several cancers, most notably cervical carcinoma.2 However, even though we have known for over 30 years that the immune system can provide considerable vaccine-induced protection against the development of cancer,3 we have not produced any vaccines that prevent cancers that commonly occur with age, such as breast and prostate cancer, which afflict 1 of 8 women and 1 of 6 men, respectively.4,5
The lack of an adult vaccine program that provides protection against such commonly occurring adult-onset cancers represents a glaring health care deficiency and a challenge for this current generation to protect coming generations.
THE ‘RETIRED’ PROTEIN HYPOTHESIS
Given that most cancers are not associated with any disease-inducing pathogens, at what targets can we aim our immune system to induce safe and effective protection against these commonly occurring adult-onset cancers?
Perhaps an understanding of the natural aging process may provide us with insights regarding possible vaccine targets. As we age, there is a decline in expression of many tissue-specific proteins, often to the point where they may be considered “retired” and no longer found at detectable or immunogenic levels in normal cells. Examples of this natural aging process include the pigment proteins as our hair whitens, certain lactation proteins when breastfeeding ceases, and some ovarian proteins as menopause begins and production of mature egg follicles ceases. If these retired proteins are expressed in invigorated emerging tumors, then preemptive immunity directed against these retired proteins would attack and destroy the emerging tumors and ignore normal tissues, thereby avoiding any complicating collateral autoimmune damage.
Thus, we propose that retired tissue-specific self-proteins may substitute for unavailable pathogens as targets for mediating safe and effective immune protection against adult-onset cancers such as breast cancer.
SAFE AND EFFECTIVE PREVENTION OF BREAST CANCER IN MICE
To test this retired-protein hypothesis for immunoprevention of breast cancer, we selected alpha-lactalbumin as our vaccine target, for two reasons:
- Alpha-lactalbumin is a protein expressed exclusively in lactating breast tissue and is not expressed at immunogenic levels in either normal nonlactating breast tissues or in any of 78 other normal human tissues examined.6–8
- Alpha-lactalbumin is expressed in most human triple-negative breast cancers (TNBC),9,10 the most aggressive and lethal form of breast cancer, and the predominant form that occurs in women with mutations in the breast cancer 1, early-onset gene (BRCA1).11,12
We found that alpha-lactalbumin vaccination consistently inhibited the formation and growth of breast tumors in three different mouse models commonly used in breast cancer research.13 More importantly, the observed immune protection against the development of breast cancer in mice occurred in the absence of any detectable autoimmune inflammatory damage in any normal tissues examined. Thus, we concluded that alpha-lactalbumin vaccination could provide healthy women with safe and effective immune protection against the more malignant forms of breast cancer.
FROM BENCH TO BEDSIDE
How then do we determine whether alpha-lactalbumin vaccination prevents the development of TNBC in otherwise healthy cancer-free women, and whether it prevents recurrence of TNBC in women already diagnosed with TNBC? Our initial approach will involve two phase 1 clinical trials designed to determine the safety of the vaccine as well as the dose and number of vaccinations needed to induce optimum tumor immunity.
The first (phase 1a) trial will involve vaccination of women recently diagnosed with TNBC who have recovered with the current standard of care. These women will be vaccinated in groups receiving various doses of both recombinant human alpha-lactalbumin and an appropriate immune adjuvant that activates the immune system so it responds aggressively to the alpha-lactalbumin and creates the proinflammatory T-cell response needed for effective tumor immunity. This trial will simply provide dosage and safety profiles of the vaccine and will thereby lay the groundwork for subsequent (phase 2 and 3) trials designed to determine whether alpha-lactalbumin vaccination is effective in preventing recurrence of TNBC in women already diagnosed with this disease.
The dosage and number of immunizations shown to provide optimum immunity in the phase 1a trial will be used in a second (phase 1b) trial designed primarily to determine the safety of alpha-lactalbumin vaccination in healthy cancer-free women who have elected to undergo voluntary prophylactic mastectomy to reduce their breast cancer risk. Most of the women who elect to have this surgery have an established family history of breast cancer or a known BRCA1 mutation associated with high breast cancer risk, or both.11,12 Consenting women will be vaccinated against alpha-lactalbumin several months before their mastectomy, and their surgically removed breast tissues will be examined extensively for signs of vaccine-induced autoimmune damage. Thus, this trial will determine the safety of alpha-lactalbumin vaccination in healthy cancer-free women and will lay the groundwork for subsequent phase 2 and 3 trials designed to determine whether alpha-lactalbumin vaccination is effective in preventing TNBC in women at high risk of developing this form of breast cancer.
We estimate that completing our preclinical studies, obtaining permission from the US Food and Drug Administration to test our investigational new drug, and completing both phase 1 clinical trials will require about 5 years. Thereafter, completion of phase 2 and 3 trials designed to prevent both recurrence of TNBC in women already diagnosed with this disease and occurrence of TNBC in otherwise healthy, cancer-free women will likely take at least another 5 years, so that this vaccine will likely not be available to the general public before 2024.
TO SUM UP
Although our immune system is potentially capable of protecting us from some cancers, we currently have no immune protection against cancers we commonly confront as we age. We propose that tissue-specific self proteins that are retired from expression with age in normal tissues but are expressed at immunogenic levels in emerging tumors may substitute for unavailable pathogens as targets for immunoprevention of adult-onset cancers that commonly occur with age. We know that the retired breast-specific protein, alpha-lactalbumin, is overexpressed in TNBC and that vaccination with alpha-lactalbumin provides safe and effective protection from breast cancer in preclinical mouse studies. Clinical trials are planned to ultimately determine whether alpha-lactalbumin vaccination can prevent recurrence of TNBC in women already diagnosed with this disease and prevent the initiation of TNBC in women at high risk of developing this most aggressive and lethal form of breast cancer.
Acknowledgment: This work was supported by a grant from Shield Biotech, Inc., Cleveland, OH. In addition, the author wishes to recognize and express his sincere gratitude for the support and encouragement received from numerous organizations that have been instrumental in making this work possible, including November Philanthropy, Brakes for Breasts, the Breast Health and Healing Foundation, the Toni Turchi Foundation, the Coalition of Women Who Care About Breast Cancer, the Sisters for Prevention, the Previvors and Survivors, the Champions of the Pink Vaccine, the Race at Legacy Village, the National Greek Orthodox Ladies Philopto-chos Society, the Daughters of Penelope Icarus Chapter 321, Can’t Stop Won’t Stop, the Babylon Breast Cancer Coalition, and Walk With A Doc.
- Centers for Disease Control and Prevention. Immunization schedules. www.cdc.gov/vaccines/schedules/. Accessed September 4, 2014.
- Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol 2012; 10:681–692.
- Van Pel A, Boon T. Protection against a nonimmunogenic mouse leukemia by an immunogenic variant obtained by mutagenesis. Proc Natl Acad Sci USA 1982; 79:4718–4722.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30.
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Previous version: SEER cancer statistics review 1975–2010. http://seer.cancer.gov/csr/1975_2010/. Accessed September 4, 2014.
- Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based human protein atlas. Nat Biotechnol 2010; 28:1248–1250.
- Pontén F, Gry M, Fagerberg L, et al. A global view of protein expression in human cells, tissues, and organs. Mol Syst Biol 2009; 5:337.
- The Human Protein Atlas. www.proteinatlas.org. Accessed September 4, 2014.
- Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 2004; 6:1–6.
- ONCOMINEdatabase. www.oncomine.org/resource/login.html. Accessed September 4, 2014.
- Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol 2008; 26:4282–4288.
- Comen E, Davids M, Kirchhoff T, Hudis C, Offit K, Robson M. Relative contributions of BRCA1 and BRCA2 mutations to “triple-negative” breast cancer in Ashkenazi women. Breast Cancer Res Treat 2011; 129:185–190.
- Jaini R, Kesaraju P, Johnson JM, Altuntas CZ, Jane-Wit D, Tuohy VK. An autoimmune-mediated strategy for prophylactic breast cancer vaccination. Nat Med 2010; 16:799–803.
The most proven, effective way to control disease is through prophylactic vaccination. The childhood vaccination program is a testament to this disease prevention approach, and in its current form protects us from diseases caused by 16 different pathogens.1
Childhood immunization ends in the teen years with recommended vaccination against multiple strains of human papillomavirus that are associated with several cancers, most notably cervical carcinoma.2 However, even though we have known for over 30 years that the immune system can provide considerable vaccine-induced protection against the development of cancer,3 we have not produced any vaccines that prevent cancers that commonly occur with age, such as breast and prostate cancer, which afflict 1 of 8 women and 1 of 6 men, respectively.4,5
The lack of an adult vaccine program that provides protection against such commonly occurring adult-onset cancers represents a glaring health care deficiency and a challenge for this current generation to protect coming generations.
THE ‘RETIRED’ PROTEIN HYPOTHESIS
Given that most cancers are not associated with any disease-inducing pathogens, at what targets can we aim our immune system to induce safe and effective protection against these commonly occurring adult-onset cancers?
Perhaps an understanding of the natural aging process may provide us with insights regarding possible vaccine targets. As we age, there is a decline in expression of many tissue-specific proteins, often to the point where they may be considered “retired” and no longer found at detectable or immunogenic levels in normal cells. Examples of this natural aging process include the pigment proteins as our hair whitens, certain lactation proteins when breastfeeding ceases, and some ovarian proteins as menopause begins and production of mature egg follicles ceases. If these retired proteins are expressed in invigorated emerging tumors, then preemptive immunity directed against these retired proteins would attack and destroy the emerging tumors and ignore normal tissues, thereby avoiding any complicating collateral autoimmune damage.
Thus, we propose that retired tissue-specific self-proteins may substitute for unavailable pathogens as targets for mediating safe and effective immune protection against adult-onset cancers such as breast cancer.
SAFE AND EFFECTIVE PREVENTION OF BREAST CANCER IN MICE
To test this retired-protein hypothesis for immunoprevention of breast cancer, we selected alpha-lactalbumin as our vaccine target, for two reasons:
- Alpha-lactalbumin is a protein expressed exclusively in lactating breast tissue and is not expressed at immunogenic levels in either normal nonlactating breast tissues or in any of 78 other normal human tissues examined.6–8
- Alpha-lactalbumin is expressed in most human triple-negative breast cancers (TNBC),9,10 the most aggressive and lethal form of breast cancer, and the predominant form that occurs in women with mutations in the breast cancer 1, early-onset gene (BRCA1).11,12
We found that alpha-lactalbumin vaccination consistently inhibited the formation and growth of breast tumors in three different mouse models commonly used in breast cancer research.13 More importantly, the observed immune protection against the development of breast cancer in mice occurred in the absence of any detectable autoimmune inflammatory damage in any normal tissues examined. Thus, we concluded that alpha-lactalbumin vaccination could provide healthy women with safe and effective immune protection against the more malignant forms of breast cancer.
FROM BENCH TO BEDSIDE
How then do we determine whether alpha-lactalbumin vaccination prevents the development of TNBC in otherwise healthy cancer-free women, and whether it prevents recurrence of TNBC in women already diagnosed with TNBC? Our initial approach will involve two phase 1 clinical trials designed to determine the safety of the vaccine as well as the dose and number of vaccinations needed to induce optimum tumor immunity.
The first (phase 1a) trial will involve vaccination of women recently diagnosed with TNBC who have recovered with the current standard of care. These women will be vaccinated in groups receiving various doses of both recombinant human alpha-lactalbumin and an appropriate immune adjuvant that activates the immune system so it responds aggressively to the alpha-lactalbumin and creates the proinflammatory T-cell response needed for effective tumor immunity. This trial will simply provide dosage and safety profiles of the vaccine and will thereby lay the groundwork for subsequent (phase 2 and 3) trials designed to determine whether alpha-lactalbumin vaccination is effective in preventing recurrence of TNBC in women already diagnosed with this disease.
The dosage and number of immunizations shown to provide optimum immunity in the phase 1a trial will be used in a second (phase 1b) trial designed primarily to determine the safety of alpha-lactalbumin vaccination in healthy cancer-free women who have elected to undergo voluntary prophylactic mastectomy to reduce their breast cancer risk. Most of the women who elect to have this surgery have an established family history of breast cancer or a known BRCA1 mutation associated with high breast cancer risk, or both.11,12 Consenting women will be vaccinated against alpha-lactalbumin several months before their mastectomy, and their surgically removed breast tissues will be examined extensively for signs of vaccine-induced autoimmune damage. Thus, this trial will determine the safety of alpha-lactalbumin vaccination in healthy cancer-free women and will lay the groundwork for subsequent phase 2 and 3 trials designed to determine whether alpha-lactalbumin vaccination is effective in preventing TNBC in women at high risk of developing this form of breast cancer.
We estimate that completing our preclinical studies, obtaining permission from the US Food and Drug Administration to test our investigational new drug, and completing both phase 1 clinical trials will require about 5 years. Thereafter, completion of phase 2 and 3 trials designed to prevent both recurrence of TNBC in women already diagnosed with this disease and occurrence of TNBC in otherwise healthy, cancer-free women will likely take at least another 5 years, so that this vaccine will likely not be available to the general public before 2024.
TO SUM UP
Although our immune system is potentially capable of protecting us from some cancers, we currently have no immune protection against cancers we commonly confront as we age. We propose that tissue-specific self proteins that are retired from expression with age in normal tissues but are expressed at immunogenic levels in emerging tumors may substitute for unavailable pathogens as targets for immunoprevention of adult-onset cancers that commonly occur with age. We know that the retired breast-specific protein, alpha-lactalbumin, is overexpressed in TNBC and that vaccination with alpha-lactalbumin provides safe and effective protection from breast cancer in preclinical mouse studies. Clinical trials are planned to ultimately determine whether alpha-lactalbumin vaccination can prevent recurrence of TNBC in women already diagnosed with this disease and prevent the initiation of TNBC in women at high risk of developing this most aggressive and lethal form of breast cancer.
Acknowledgment: This work was supported by a grant from Shield Biotech, Inc., Cleveland, OH. In addition, the author wishes to recognize and express his sincere gratitude for the support and encouragement received from numerous organizations that have been instrumental in making this work possible, including November Philanthropy, Brakes for Breasts, the Breast Health and Healing Foundation, the Toni Turchi Foundation, the Coalition of Women Who Care About Breast Cancer, the Sisters for Prevention, the Previvors and Survivors, the Champions of the Pink Vaccine, the Race at Legacy Village, the National Greek Orthodox Ladies Philopto-chos Society, the Daughters of Penelope Icarus Chapter 321, Can’t Stop Won’t Stop, the Babylon Breast Cancer Coalition, and Walk With A Doc.
The most proven, effective way to control disease is through prophylactic vaccination. The childhood vaccination program is a testament to this disease prevention approach, and in its current form protects us from diseases caused by 16 different pathogens.1
Childhood immunization ends in the teen years with recommended vaccination against multiple strains of human papillomavirus that are associated with several cancers, most notably cervical carcinoma.2 However, even though we have known for over 30 years that the immune system can provide considerable vaccine-induced protection against the development of cancer,3 we have not produced any vaccines that prevent cancers that commonly occur with age, such as breast and prostate cancer, which afflict 1 of 8 women and 1 of 6 men, respectively.4,5
The lack of an adult vaccine program that provides protection against such commonly occurring adult-onset cancers represents a glaring health care deficiency and a challenge for this current generation to protect coming generations.
THE ‘RETIRED’ PROTEIN HYPOTHESIS
Given that most cancers are not associated with any disease-inducing pathogens, at what targets can we aim our immune system to induce safe and effective protection against these commonly occurring adult-onset cancers?
Perhaps an understanding of the natural aging process may provide us with insights regarding possible vaccine targets. As we age, there is a decline in expression of many tissue-specific proteins, often to the point where they may be considered “retired” and no longer found at detectable or immunogenic levels in normal cells. Examples of this natural aging process include the pigment proteins as our hair whitens, certain lactation proteins when breastfeeding ceases, and some ovarian proteins as menopause begins and production of mature egg follicles ceases. If these retired proteins are expressed in invigorated emerging tumors, then preemptive immunity directed against these retired proteins would attack and destroy the emerging tumors and ignore normal tissues, thereby avoiding any complicating collateral autoimmune damage.
Thus, we propose that retired tissue-specific self-proteins may substitute for unavailable pathogens as targets for mediating safe and effective immune protection against adult-onset cancers such as breast cancer.
SAFE AND EFFECTIVE PREVENTION OF BREAST CANCER IN MICE
To test this retired-protein hypothesis for immunoprevention of breast cancer, we selected alpha-lactalbumin as our vaccine target, for two reasons:
- Alpha-lactalbumin is a protein expressed exclusively in lactating breast tissue and is not expressed at immunogenic levels in either normal nonlactating breast tissues or in any of 78 other normal human tissues examined.6–8
- Alpha-lactalbumin is expressed in most human triple-negative breast cancers (TNBC),9,10 the most aggressive and lethal form of breast cancer, and the predominant form that occurs in women with mutations in the breast cancer 1, early-onset gene (BRCA1).11,12
We found that alpha-lactalbumin vaccination consistently inhibited the formation and growth of breast tumors in three different mouse models commonly used in breast cancer research.13 More importantly, the observed immune protection against the development of breast cancer in mice occurred in the absence of any detectable autoimmune inflammatory damage in any normal tissues examined. Thus, we concluded that alpha-lactalbumin vaccination could provide healthy women with safe and effective immune protection against the more malignant forms of breast cancer.
FROM BENCH TO BEDSIDE
How then do we determine whether alpha-lactalbumin vaccination prevents the development of TNBC in otherwise healthy cancer-free women, and whether it prevents recurrence of TNBC in women already diagnosed with TNBC? Our initial approach will involve two phase 1 clinical trials designed to determine the safety of the vaccine as well as the dose and number of vaccinations needed to induce optimum tumor immunity.
The first (phase 1a) trial will involve vaccination of women recently diagnosed with TNBC who have recovered with the current standard of care. These women will be vaccinated in groups receiving various doses of both recombinant human alpha-lactalbumin and an appropriate immune adjuvant that activates the immune system so it responds aggressively to the alpha-lactalbumin and creates the proinflammatory T-cell response needed for effective tumor immunity. This trial will simply provide dosage and safety profiles of the vaccine and will thereby lay the groundwork for subsequent (phase 2 and 3) trials designed to determine whether alpha-lactalbumin vaccination is effective in preventing recurrence of TNBC in women already diagnosed with this disease.
The dosage and number of immunizations shown to provide optimum immunity in the phase 1a trial will be used in a second (phase 1b) trial designed primarily to determine the safety of alpha-lactalbumin vaccination in healthy cancer-free women who have elected to undergo voluntary prophylactic mastectomy to reduce their breast cancer risk. Most of the women who elect to have this surgery have an established family history of breast cancer or a known BRCA1 mutation associated with high breast cancer risk, or both.11,12 Consenting women will be vaccinated against alpha-lactalbumin several months before their mastectomy, and their surgically removed breast tissues will be examined extensively for signs of vaccine-induced autoimmune damage. Thus, this trial will determine the safety of alpha-lactalbumin vaccination in healthy cancer-free women and will lay the groundwork for subsequent phase 2 and 3 trials designed to determine whether alpha-lactalbumin vaccination is effective in preventing TNBC in women at high risk of developing this form of breast cancer.
We estimate that completing our preclinical studies, obtaining permission from the US Food and Drug Administration to test our investigational new drug, and completing both phase 1 clinical trials will require about 5 years. Thereafter, completion of phase 2 and 3 trials designed to prevent both recurrence of TNBC in women already diagnosed with this disease and occurrence of TNBC in otherwise healthy, cancer-free women will likely take at least another 5 years, so that this vaccine will likely not be available to the general public before 2024.
TO SUM UP
Although our immune system is potentially capable of protecting us from some cancers, we currently have no immune protection against cancers we commonly confront as we age. We propose that tissue-specific self proteins that are retired from expression with age in normal tissues but are expressed at immunogenic levels in emerging tumors may substitute for unavailable pathogens as targets for immunoprevention of adult-onset cancers that commonly occur with age. We know that the retired breast-specific protein, alpha-lactalbumin, is overexpressed in TNBC and that vaccination with alpha-lactalbumin provides safe and effective protection from breast cancer in preclinical mouse studies. Clinical trials are planned to ultimately determine whether alpha-lactalbumin vaccination can prevent recurrence of TNBC in women already diagnosed with this disease and prevent the initiation of TNBC in women at high risk of developing this most aggressive and lethal form of breast cancer.
Acknowledgment: This work was supported by a grant from Shield Biotech, Inc., Cleveland, OH. In addition, the author wishes to recognize and express his sincere gratitude for the support and encouragement received from numerous organizations that have been instrumental in making this work possible, including November Philanthropy, Brakes for Breasts, the Breast Health and Healing Foundation, the Toni Turchi Foundation, the Coalition of Women Who Care About Breast Cancer, the Sisters for Prevention, the Previvors and Survivors, the Champions of the Pink Vaccine, the Race at Legacy Village, the National Greek Orthodox Ladies Philopto-chos Society, the Daughters of Penelope Icarus Chapter 321, Can’t Stop Won’t Stop, the Babylon Breast Cancer Coalition, and Walk With A Doc.
- Centers for Disease Control and Prevention. Immunization schedules. www.cdc.gov/vaccines/schedules/. Accessed September 4, 2014.
- Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol 2012; 10:681–692.
- Van Pel A, Boon T. Protection against a nonimmunogenic mouse leukemia by an immunogenic variant obtained by mutagenesis. Proc Natl Acad Sci USA 1982; 79:4718–4722.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30.
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Previous version: SEER cancer statistics review 1975–2010. http://seer.cancer.gov/csr/1975_2010/. Accessed September 4, 2014.
- Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based human protein atlas. Nat Biotechnol 2010; 28:1248–1250.
- Pontén F, Gry M, Fagerberg L, et al. A global view of protein expression in human cells, tissues, and organs. Mol Syst Biol 2009; 5:337.
- The Human Protein Atlas. www.proteinatlas.org. Accessed September 4, 2014.
- Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 2004; 6:1–6.
- ONCOMINEdatabase. www.oncomine.org/resource/login.html. Accessed September 4, 2014.
- Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol 2008; 26:4282–4288.
- Comen E, Davids M, Kirchhoff T, Hudis C, Offit K, Robson M. Relative contributions of BRCA1 and BRCA2 mutations to “triple-negative” breast cancer in Ashkenazi women. Breast Cancer Res Treat 2011; 129:185–190.
- Jaini R, Kesaraju P, Johnson JM, Altuntas CZ, Jane-Wit D, Tuohy VK. An autoimmune-mediated strategy for prophylactic breast cancer vaccination. Nat Med 2010; 16:799–803.
- Centers for Disease Control and Prevention. Immunization schedules. www.cdc.gov/vaccines/schedules/. Accessed September 4, 2014.
- Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol 2012; 10:681–692.
- Van Pel A, Boon T. Protection against a nonimmunogenic mouse leukemia by an immunogenic variant obtained by mutagenesis. Proc Natl Acad Sci USA 1982; 79:4718–4722.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30.
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Previous version: SEER cancer statistics review 1975–2010. http://seer.cancer.gov/csr/1975_2010/. Accessed September 4, 2014.
- Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based human protein atlas. Nat Biotechnol 2010; 28:1248–1250.
- Pontén F, Gry M, Fagerberg L, et al. A global view of protein expression in human cells, tissues, and organs. Mol Syst Biol 2009; 5:337.
- The Human Protein Atlas. www.proteinatlas.org. Accessed September 4, 2014.
- Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 2004; 6:1–6.
- ONCOMINEdatabase. www.oncomine.org/resource/login.html. Accessed September 4, 2014.
- Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol 2008; 26:4282–4288.
- Comen E, Davids M, Kirchhoff T, Hudis C, Offit K, Robson M. Relative contributions of BRCA1 and BRCA2 mutations to “triple-negative” breast cancer in Ashkenazi women. Breast Cancer Res Treat 2011; 129:185–190.
- Jaini R, Kesaraju P, Johnson JM, Altuntas CZ, Jane-Wit D, Tuohy VK. An autoimmune-mediated strategy for prophylactic breast cancer vaccination. Nat Med 2010; 16:799–803.
KEY POINTS
- “Retired” tissue-specific self proteins may substitute for unavailable pathogens as vaccine targets for mediating immune prevention of adult-onset cancers.
- Vaccination against the retired breast-specific protein alpha-lactalbumin provides safe and effective immune protection against the development of breast tumors in several mouse models.
- Alpha-lactalbumin is overexpressed in most human triple-negative breast cancers (TNBC), the most aggressive and lethal form of human breast cancer.
- Forthcoming are clinical trials designed to prevent the initiation of TNBC in otherwise healthy cancer-free women, as well as trials designed to prevent recurrence of TNBC in women already diagnosed with this disease.
Advances in autosomal dominant polycystic kidney disease—2014 and beyond
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disease, has an estimated prevalence of 1:400 to 1:1,000 live births in the United States, and occurs worldwide.1,2 There are about 700,000 people living with it in the United States, and about 6,000 new cases arise annually. It accounts for nearly 5% of all patients with end-stage renal disease in the United States.3
This paper will offer an overview of the pathogenesis of renal cysts, review some of the clinical aspects of ADPKD including diagnosis and management of complications, and discuss recent drug trials and current management.
TWO TYPES—PKD1 IS MORE COMMON AND PROGRESSES MORE RAPIDLY
Two major forms of ADPKD are recognized and can usually be determined by genetic testing: PKD1, accounting for about 85% of cases, and PKD2, accounting for 15%.
The gene locus for PKD1 is on the short arm of the 16th chromosome (16p13.3), and its glycoprotein gene product is polycystin 1 (PC1), a large molecule with 4,303 amino acids.2 PC1 has a long N-terminal extracellular tail that can function as a mechanosensor. Disease progression is much faster with PKD1, and end-stage renal disease usually occurs before age 56.4
In PKD2, the gene locus is on the long arm of the fourth chromosome (4q21–23), and has a smaller glycoprotein gene product, polycystin 2 (PC2), that plays a role in calcium transport. The disease course of PKD2 tends to be slower. End-stage renal disease might not develop in the patient’s lifetime, since it typically develops when the patient is more than 70 years old.4
Although the growth rate of renal cysts is similar between the two types, patients with PKD1 develop about twice as many cysts as those with PDK2, and their cyst development starts at a younger age.5
Typically, patients have a clear phenotype and a positive family history, but in about 10% of possible ADPKD cases, there is no family history of ADPKD. Genetic variations such as incompletely penetrant PKD1 alleles,6 hypomorphic alleles,7 and trans-heterozygous mutations8 account for at least some of these cases.
IMAGING CRITERIA HAVE BROADENED
Ultrasonographic criteria for the diagnosis of ADPKD that were published in 1994 were based on patients who had a family history of PKD1.9 The criteria have since been modified (the “unified criteria”) to include patients with a family history of PKD2 who begin cyst development at a later age and with lower numbers.10 For patients ages 30 to 39, a previously difficult diagnostic group, the criterion for the minimum number of cysts visible on ultrasonography changed from four to three, improving the sensitivity of detecting disease from approximately 76% to approximately 95% (Table 1).9,10 It is important to note that these criteria apply only to patients “at risk,” ie, with a positive family history of ADPKD.
Computed tomography (CT) and magnetic resonance imaging (MRI) classically show bilaterally enlarged multicystic kidneys, though variations can be seen.
DISEASE CAN PRESENT IN MYRIAD WAYS
Although cystic kidney disease is the basic underlying problem, undiagnosed patients may present with a variety of symptoms caused by other manifestations of ADPKD (Table 2).
Hypertension is the most common presentation, occurring in about 50% of patients ages 20 to 34, and essentially 100% of those with end-stage renal disease.11 It is associated with up-regulation of the renin-angiotensin-aldosterone system.
Pain is typically located in the abdomen, flank, or back and can occur in a localized or diffuse manner. Early abdominal distress is often simply described as “fullness.” Localized pain is usually caused by bleeding into or rupture of a cyst, renal stones, or infection.12 Because renal cysts are noncommunicating, bleeding can occur into a cyst and cause pain without gross hematuria. Compression by greatly enlarged kidneys, liver, or both can cause a variety of gastrointestinal symptoms such as reflux esophagitis and varying degrees of constipation. Diffuse pain is often musculoskeletal and related to exaggerated lordosis from increasing abdominal size due to enlarging cystic kidneys and sometimes liver.12 In carefully selected cases, cyst aspiration may be helpful.11
Although renal carcinomas are rare and not more frequent than in the general population, they can occur at an earlier age and with constitutional symptoms.11
Urinary tract infections are increased in frequency. A patient may have a simple urinary tract infection that is cured with the appropriate antibiotic. However, a urinary tract infection repeatedly recurring with the same organism is a strong clue that an infected cyst is the source and requires more intensive treatment with the appropriate cyst-penetrating antibiotic. On the other hand, because cysts are noncommunicating, an infected cyst might be present despite a negative urine culture.
Identifying infected cysts can be a challenge with conventional imaging techniques, but combined positron emission tomography and CT (PET/CT) can be a valuable though expensive diagnostic tool to identify an infected kidney or liver cyst, or to identify an unsuspected source of the pain and infection.13
Jouret et al13 evaluated 27 PET/CT scans performed in 24 patients with ADPKD and suspicion of an abdominal infection. Patients were deemed to have probable cyst infection if they met all of the following criteria: temperature more than 38°C for longer than 3 days, loin or liver tenderness, plasma C-reactive protein level greater than 5 mg/dL, and no evidence of intracystic bleeding on CT. Patients with only two or three of these criteria were classified as having fever of unknown origin. Diagnosis of cyst infection was confirmed by cyst fluid analysis.
PET/CT identified a kidney or liver cyst infection in 85% of 13 infectious events in 11 patients who met all the criteria for probable cyst infection; CT alone contributed to the diagnosis in only one patient.13 In those with fever of unknown origin, PET/CT identified a source of infection in 64% of 14 events in 13 patients: two infected renal cysts, as well as one patient each with other infections that would be difficult to diagnose clinically, ie, small bowel diverticulitis, psoas abscess, diverticulitis of the right colon, pyelonephritis in a transplanted kidney, infected abdominal aortic aneurysm, prostatitis, colitis, and Helicobacter pylori gastritis. Results of PET/CT were negative in five patients with intracystic bleeding.
Kidney stones occur in 20% to 36% of patients.11,14 Uric acid stones occur at almost the same frequency as calcium oxalate stones.
Chronic kidney disease not previously diagnosed may be the presenting condition in a small percentage of patients, sometimes those in whom much earlier hypertension was not fully evaluated. ADPKD is typically not associated with significant proteinuria (eg, nephrotic range), and the presence of heavy proteinuria usually indicates the presence of a superimposed primary glomerulopathy.15
Cysts in other locations. By MRI, liver cysts are present in 58% of patients ages 15 to 24, rising to 94% in those ages 35 to 46.11 Because liver cysts are estrogen-dependent, they are more prominent in women. A small percentage of patients develop cysts in the pancreas (5%), arachnoid membranes (8%), and seminal vesicles (40% of men with ADPKD).11
Cardiovascular abnormalities occur in almost one-third of patients with ADPKD, usually as mitral and aortic valve abnormalities.16 Aneurysms of the aortic root and abdominal aorta can also occur, in addition to intracranial aneurysms (see below).17
Intracranial aneurysms are not uncommon, and size usually determines their risk.
Intracranial aneurysms are strongly influenced by family history: 16% of ADPKD patients with a family history of intracranial aneurysm also develop them, compared with 5% to 6% of patients with no family history.11 The anterior cerebral circulation is involved in about 80% of cases. A sentinel or sudden “thunderclap” headache is a classic presentation that may precede full-blown rupture in about 17% of cases.18 Patients who rupture an intracranial aneurysm have a mean age of 39, usually have normal renal function, and can be normotensive.11
For patients with no history of subarachnoid hemorrhage, the 5-year cumulative rupture rates for patients with aneurysms located in the internal carotid artery, anterior communicating or anterior cerebral artery, or middle cerebral artery were 0% for aneurysms less than 7 mm, 2.6% for those 7 to 12 mm, 14.5% for those 13 to 24 mm, and 40% for those 25 mm or larger, with higher rates for the same sizes in the posterior circulation.11
In patients without symptoms, size is correlated with risk of rupture: less than 4 mm is usually associated with very low risk, 4 to less than 7 mm with moderate risk, and 7 mm or more with increasing risk. An aneurysm larger than 10 mm is associated with roughly a 1% risk of rupture per year.19
Irazabal et al20 retrospectively studied 407 patients with ADPKD who were screened for intracranial aneurysm. Saccular aneurysms were detected in 45 patients; most were small (median diameter 3.5 mm). During cumulative imaging follow-up of 243 years, only one new intracranial aneurysm was detected (increasing from 2 to 4.4 mm over 144 months) and two previously identified aneurysms grew (one increasing 4.5 to 5.9 mm over 69 months and the other 4.7 to 6.2 mm over 184 months). No change occurred in 28 patients. Seven patients were lost to follow-up, however. During cumulative clinical follow-up of 316 years, no aneurysm ruptured. Two patients were lost to follow-up, three had surgical clipping, and five died of unrelated causes. The authors concluded that presymptomatic intracranial aneurysms are usually small, and that growth and rupture risks are no higher than for unruptured intracranial aneurysms in the general population. A 2014 study also suggests a conservative approach for managing intracranial aneurysm in the general population.21
In asymptomatic ADPKD patients, it is reasonable to reserve screening for those with a positive family history of intracranial aneurysm or subarachnoid hemorrhage, those with a previous ruptured aneurysm, those in high-risk professions (eg, pilots), and for patients prior to anticoagulant therapy or major surgery possibly associated with hemodynamic instability.11,22 Certain extremely anxious patients might also need to be studied. Screening can be performed with magnetic resonance angiography without gadolinium contrast. It is prudent to have patients with an intracranial aneurysm thoroughly evaluated by an experienced neurosurgeon with continued follow-up.
PROGRESSION OF ADPKD
The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) study23 evaluated 241 patients with ADPKD (ages 15 to 46) by measuring the annual rate of change in total kidney volume, total cyst volume, and iothalamate glomerular filtration rate (GFR) over 3 years. The annual increase in total kidney volume averaged 5.3%,23 though the reported range with various imaging techniques is from 4% to 12.8% in adults.24 This study focused on macrocystic disease, ie, cysts that are visible by MRI and measurably increase total kidney volume. Although larger total kidney volume at baseline generally predicted a more rapid decline in GFR, there were wide and overlapping variations in yearly GFR declines within and among different total-kidney-volume groups.23
SPECIAL CLINICAL PROBLEMS IN ADPKD
Case 1: A man with ADPKD develops new and increasing proteinuria
A 55-year-old man with ADPKD and stage 3 chronic kidney disease developed new and increasing proteinuria, rising to 5,500 mg per 24 hours. What is the most likely explanation?
- Rapidly progressive renal failure with increasing proteinuria in ADPKD
- Bilateral renal vein thromboses because of cyst compression
- Malignant hypertension with bilateral renal artery compression
- Superimposed primary glomerulopathy
- Multiple infected renal cysts with pyonephrosis
Answer: Superimposed primary glomerulopathy.
ADPKD (similar to uncomplicated obstructive uropathy, pyelonephritis, main renal artery disease, and often cases of interstitial nephritis without secondary glomerular changes) typically does not result in nephrotic-range proteinuria. A superimposed primary glomerulopathy, focal segmental glomerulosclerosis, was the biopsy-proved diagnosis.
At least 21 cases have been reported of AD-PKD with nephrotic-range proteinuria and a renal biopsy showing a primary glomerulopathy, including focal segmental glomerulosclerosis (5 cases), minimal-change disease (5), membranous nephropathy (3), IgA nephropathy (2), and one each of crescentic glomerulonephropathy, diabetic nephropathy, membranoproliferative glomerulonephritis, postinfectious glomerulonephropathy, amyloid glomerulopathy, and mesangioproliferative glomerulopathy.15 Treatment was directed at the primary glomerulopathy, and the outcomes corresponded to the primary diagnosis (eg, with appropriate treatment, three of the five patients with focal segmental glomerulosclerosis progressed to end-stage renal disease, all of the patients with minimal-change disease went into remission, and one of the two cases with IgA nephropathy improved).15
Case 2: A woman with ADPKD and advanced renal failure develops shortness of breath
A 47-year-old woman with very large polycystic kidneys (total kidney volume 7,500 mL; normal range for a single kidney approximately 136–295 mL, mean 196)25 and estimated GFR of 25 mL/min developed new-onset shortness of breath while climbing steps and later even when making a bed. She had no chest pain, cough, or edema. She was sent directly to the emergency department and was admitted and treated; her condition improved, and she was discharged after 6 days. What did she have?
- Presentation of rare cystic pulmonary disease in ADPKD
- Onset of pneumonia with early bacteremia
- Progressive reduction in ventilatory capacity from massive polycystic kidneys and liver elevating both sides of the diaphragm
- Pulmonary emboli from an iliac vein or inferior vena cava source
- Progressive anemia accompanying rapidly worsening stage 4 chronic kidney disease
Answer: She had pulmonary emboli from an iliac vein (right) or inferior vena cava source.
Pulmonary emboli in ADPKD can be caused by thrombi in the inferior vena cava or the iliac or femoral vein because of compression by a massive right polycystic kidney. Four cases were reported at Mayo Clinic,26 three diagnosed by MRI and one with CT. One additional case occurred at Cleveland Clinic. All patients survived after treatment with anticoagulation therapy; early nephrectomy was required in two cases.
Interestingly, following kidney transplantation, the patients at greatest risk for pulmonary emboli are those with ADPKD as their original disease.27
RENAL CYSTS RESULT FROM COMBINED MUTATIONS, INJURY
The germline ADPKD mutation that occurs in one allele of all renal tubular epithelial cells is necessary but not sufficient for cystogenesis.28 One or more additional somatic mutations of the normal allele—the “second hit”—also develop within individual tubular epithelial cells.28,29 These epithelial cells undergo clonal proliferation, resulting in tubular dilatation and cyst formation. Monoclonality of cells in cysts has been documented.
Ischemia-reperfusion injury can be viewed as a “third hit.”30 In PKD1 knockout mice, which at 5 weeks of age normally develop only mild cystic kidney disease, the superimposition of unilateral ischemia-reperfusion injury at 8 weeks caused widespread and rapid cyst formation. It is believed that acute renal injury reactivates developmental signaling pathways within 48 hours that trigger epithelial cell proliferation and then cyst development detectable by MRI 2 weeks later. Although this phenomenon has not been documented in humans, it is a cautionary tale.
CYSTOGENESIS INVOLVES MULTIPLE PATHWAYS
A comprehensive description of pathways leading to renal cyst formation is beyond the scope of this article, and the reader is referred to much more detailed and extensive reviews.2,31 Disturbances in at least three major interconnected pathways promote cystogenesis in renal tubular epithelial cells:
- Normal calcium transport into the endoplasmic reticulum is disrupted by abnormal polycystins on the surface of the primary cilium
- Vasopressin and other stimuli increase the production of cyclic adenosine monophosphate (cAMP)
- The mammalian target of rapamycin (mTOR) proliferative pathway is up-regulated.
DISRUPTION OF CALCIUM TRANSPORT IN THE PRIMARY CILIUM
Primary cilia are nonmotile cellular organelles of varying size, from about 0.25 μm up to about 1 μm.32 Each primary cilium has nine peripheral pairs of microtubules but lacks a centrally located pair that is present in motile cilia. Primary cilia are ubiquitous and have been highly conserved throughout evolution. A single cilium is present on almost all vertebral cells.33
Cilial defects have been identified in autosomal dominant as well as recessive diseases and are known as ciliopathies.33 Although rare in humans, they can affect a broad spectrum of organs other than the kidney, including the eye, liver, and brain.33
Urine flow in a renal tubule is believed to exert mechanical stimulation on the extracellular flagellum-like N-terminal tail of PC1 that extends from a primary cilium into the urinary space. PC1 in concert with PC2 opens PC2 calcium channels, allowing calcium ions to flow down the microtubules to ryanodine receptors and the basal body.32,33 This leads to local release of calcium ions that regulate cell proliferation.32,34 However, in ADPKD kidneys, PC1 and PC2 molecules are sparse or mutated, resulting in defective calcium transport, increased and unregulated tubular epithelial cell proliferation, and cyst formation.
In a totally different clinical setting, biopsies of human renal transplants that sustained acute tubular necrosis during transplantation reveal that a cilium dramatically elongates in response to injury,35 possibly as a compensatory mechanism to maintain calcium transport in the presence of meager urine flow and to restore the proliferation of tubular epithelial cells in a regulated repair process.
THE ROLE OF VASOPRESSIN AND ACTIVATION OF cAMP
In classic experiments, Wang et al36 cross-bred rats having genetically inherited polycystic kidney disease (actually, autosomal recessive polycystic kidney disease) with Brattleboro rats that completely lack vasopressin. At 10 and 20 weeks of age, the offspring had virtually complete inhibition of cystogenesis because of the absence of vasopressin. However, when vasopressin was restored by exogenous administration continuously for 8 weeks, the animals formed massive renal cysts.
Vasopressin activates cAMP, which then functions as a second messenger in cell signaling. cAMP increases the activation of the protein kinase A (PKA) pathway, which in turn increases downstream activity of the B-raf/ERK pathway. Up-regulation of cAMP and PKA appears to perpetuate activation of canonical Wnt signaling, down-regulate non-canonical Wnt/planar cell polarity signaling, and lead to loss of tubular diameter control, resulting in cyst formation.31 Normally, cAMP is degraded by phosphodiesterase. However, because of the primary cilium calcium transport defect in ADPKD, phosphodiesterase is reduced and cAMP persists.37 In conjunction with the defective primary cilial calcium transport, cAMP exerts a proliferative effect on renal tubular epithelial cells that is opposite to its effect in normal kidneys.31,32 cAMP also up-regulates the cystic fibrosis transmembrane conductance regulator (CFTR) that promotes chloride ion transport. Sodium ions follow the chloride ions, leading to fluid accumulation and cyst enlargement.31
Inhibiting vasopressin by increasing water intake
A simple key mechanism for limiting vasopressin secretion is by sufficient water ingestion. Nagao et al38 found that rats with polycystic kidney disease given water with 5% glucose (resulting in 3.5-fold increased fluid intake compared with rats given tap water) had a 68% reduction in urinary vasopressin and a urine osmolality less than 290 mOsm/kg. The high-water-intake rats had dramatically reduced cystic areas in the kidney and a 28% reduction of kidney-to-body weight ratio vs controls.
In an obvious oversimplification, these findings raised the question of whether a sufficient increase in water intake could be an effective therapy for polycystic kidney disease.39 A pilot clinical study evaluated changes in urine osmolality in eight patients with ADPKD who had normal renal function.40 At baseline, 24-hour urine osmolality was typically elevated to approximately 753 mOsm/kg compared to the plasma at 285 mOsm/kg, indicating that antidiuresis is the usual state. During the 2-week study, urine volume and osmolality were measured, and additional water intake was adjusted in order to achieve a urine osmolality goal of 285 ± 45 mOsm/kg. These adjustments resulted in water intake that appeared to be in the range of 2,400 to 3,000 mL per 24 hours. The major limitations of the study were that it was very short term, and there was no opportunity to measure changes in total kidney volume or estimated GFR.
In a recent preliminary report from Japan, high water intake (2,500–3,000 mL daily) in 18 ADPKD patients was compared over 12 months with ad libitum water intake in 14 ADPKD controls (clinicaltrials.gov NCT 01348505). There was no statistically significant change in total kidney volume or cystatin-estimated GFR in those on high water intake, but serious defects in study design (patients in the high water intake group were allowed to decrease their intake if it was causing them difficulty, and patients in the ad libitum water intake group had no measurement of their actual water intake) prevent any conclusions because there was no evidence that the groups were different from one another with respect to the key element of the study, namely, water intake.
Blocking the vasopressin receptor slows disease progression
Using another approach, Gattone et al41 inhibited the effect of vasopressin by blocking the vasopressin 2 receptor (V2R) in mouse and rat models of polycystic kidney disease, using an experimental drug, OPC31260. The drug halted disease progression and, in one situation, appeared to cause regression of established disease. As noted by Torres and Harris,31 even though both increased water intake and V2R antagonists decrease cAMP in the distal tubules and collecting ducts, circulating levels of vasopressin are decreased by increased water intake but increased by V2R antagonists.
After these remarkable results in animal models, clinical trials of the V2R antagonist tolvaptan were conducted in patients with ADPKD. In the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4 study,42 1,445 adults (ages 18 to 50) with ADPKD in 133 centers worldwide were randomized to receive either tolvaptan or placebo for 3 years. Key inclusion criteria included good renal function (estimated GFR ≥ 60 mL/min) and total kidney volume of at least 750 mL (mean 1,700 mL) as measured by MRI. Tolvaptan was titrated to the highest tolerated twice-daily dose (average total of 95 mg/day). All patients were advised to maintain good hydration and to avoid thirst by drinking a glass of water after each urination. Unfortunately, neither water intake nor urine output was measured.
The primary end point was the annual rate of change in total kidney volume, with secondary end points of clinical progression (worsening kidney function, pain, hypertension, albuminuria), and rate of decline in kidney function as measured by the slope of the reciprocal of serum creatinine.42
Patients in the tolvaptan arm had a slower annual increase in total kidney volume than controls (2.8% vs 5.5%, respectively, P < .001) and a slower annual decline in renal function (−2.61 vs −3.81 mg/mL−1, respectively, P < .001).42 More participants in the treatment group withdrew than in the placebo group (23% vs 14%, respectively).
Adverse events occurred more frequently with tolvaptan.42 Liver enzyme elevations of greater than three times the upper limit of normal occurred in 4.4% of patients in the treatment group, leading to a drug warning issued in January 2013. As expected, side effects related to diuresis (urinary frequency, nocturia, polyuria, and thirst) were more frequent in the treatment group, occurring in up to 55% of participants.
The authors noted, “Although maintaining hydration helped ensure that the blinding in the study was maintained, the suppression of vasopressin release in the placebo group may have led to an underestimation of the beneficial effect of tolvaptan and may account for the lower rates of kidney growth observed in the placebo group.”42
In 2013, the US Food and Drug Administration (FDA) denied a new drug application for tolvaptan as a treatment for ADPKD.
THE mTOR PATHWAY IS UP-REGULATED
The mTOR pathway that plays a major role in cell growth and proliferation includes interaction of the cytoplasmic tail of polycystin 1 with tuberin.43 Activation products of mTOR, including phospho-S6K, have been found in tubular epithelial cells lining cysts of ADPKD kidneys but not in normal kidneys.43 Mutant mice with polycystic disease had phospho-S6K in tubular epithelial cells of cysts, whereas those treated with the mTOR inhibitor rapamycin did not.43 But subsequent studies have shown that only a low level of mTOR activation is present in 65% to 70% of ADPKD cysts.44
Two major studies of the treatment of ADPKD with rapamycin that were published contemporaneously in 2010 failed to demonstrate any significant benefit with mTOR inhibitor treatment.45,46
Serra et al45 conducted an 18-month, open-label trial of 100 ADPKD patients ages 18 to 40 with an estimated GFR (eGFR) of at least 70 mL/min. Patients were randomized to receive rapamycin, given as sirolimus 2 mg per day, or standard care. The primary end point was the reduction in the growth rate of total kidney volume, measured by MRI. Secondary end points were eGFR and protein excretion (albumin-creatinine ratio). No significant difference was found in total kidney volume, but a nonsignificant stabilization of eGFR was noted.
Walz et al46 in a 2-year, multicenter, double-blind trial, randomized 433 patients (mean age 44; mean eGFR 54.5 mL/min) to treatment with either the short-acting mTOR inhibitor everolimus (2.5 mg twice daily) or placebo. Although patients in the treatment group had less of an increase in total kidney volume (significant at 1 year but not at 2 years), they tended to show a decline in eGFR. But further analysis showed that the only patients who had a reduction in eGFR were males who already had impaired kidney function at baseline.47
In a pilot study, 30 patients with ADPKD (mean age 49) were randomized to one of three therapies:
- Low-dose rapamycin (trough blood level 2–5 ng/mL)
- Standard-dose rapamycin (trough blood level > 5–8 ng/mL)
- Standard care without rapamycin.48
In contrast to other studies, the primary end point was the change in iothalamate GFR at 12 months, with change in total kidney volume being a secondary end point.
At 12 months, with 26 patients completing the study, the low-dose rapamycin group (n = 9) had a significant increase in iothalamate GFR of 7.7 ± 12.5 mL/min/1.73 m2, whereas the standard-dose rapamycin group (n = 8) had a nonsignificant increase of 1.6 ± 12.1 mL/min/1.73 m2, and the no-rapamycin group (n = 9) had a fall in iothalamate GFR of 11.2 ± 9.1 mL/min/1.73 m2 (P = .005 for low-dose vs no rapamycin; P = .07 for standard-dose vs no rapamycin; P = .52 for low-dose vs standard-dose rapamycin; and P = .002 for combined low-dose and standard-dose rapamycin vs no rapamycin.).48 These differences were observed despite there being no significant change in total kidney volume in any of the groups. Patients on low-dose rapamycin had fewer adverse effects than those on standard dose and were more often able to continue therapy for the entire study. This, and the use of iothalamate GFR rather than eGFR to measure GFR, are believed to be the main reasons that low-dose effects were more pronounced than those with standard doses. One may speculate that rapamycin may have its effect on microcysts and cystogenic cells, resulting in stabilization of or improvement in renal function without detectable slowing in total kidney volume enlargement. Mechanisms for this possibility involve new concepts, as discussed below.
NEW CONCEPTS
Specialized cells also promote renal cyst formation
Specialized cells that promote cyst formation have been identified by Karihaloo et al49 in a mouse model of polycystic kidney disease. In this model, alternatively activated macrophages homed to cystic areas and promoted cyst growth. These findings suggested that interrupting the homing and proliferative signals of macrophages could be a therapeutic target for ADPKD. Although rapamycin can suppress macrophage proliferation by macrophage colony-stimulating factor and inhibit macrophage function,50 alternatively activated macrophages have not been specifically studied for rapamycin responsiveness.
More promising is evidence that CD133+ progenitor cells from human ADPKD kidneys—but not from normal human kidneys—form cysts in vitro and in severe combined immunodeficient mouse models.51 Treatment with rapamycin decreased proliferation of the de-differentiated CD133+ cells from ADPKD patients and reduced cystogenesis.51
Visible cysts are the tip of the iceberg
Using ADPKD nephrectomy specimens from eight patients, Grantham et al52 compared cyst counts by MRI and by histology and found that for every renal cyst detected by MRI, about 62 smaller cysts (< 0.9 mm) are present in the kidney. For a typical patient having an average of 587 cysts in both kidneys that are detectable by MRI, this means that more than 36,000 cysts are actually present, and MRI detects less than 2% of the total cysts present.
Although microcysts are too small to contribute much to total kidney volume, they can interfere with kidney function. Microcysts can reduce GFR in two major ways: by compressing microvasculature, tubules, and glomeruli in the cortex; or by blocking the drainage of multiple upstream nephrons when they form in or block medullary collecting ducts.52 Although the growth rates of microcysts less than 1 mm in size have not yet been measured, the adult combined growth rates of the renal cyst component is approximately 12% per year, with yearly individual cyst growth rates up to 71%, and with fetal cyst growth rates even higher for cysts larger than 7.0 mm.53 Before and during an accelerated growth period, microcysts may be susceptible to certain therapies that could first improve GFR and only later change measurable total kidney volume by slowing microcyst progression to macrocysts either directly or through specialized cells that may be sensitive to rapamycin.
CURRENT MANAGEMENT OF ADPKD
Blood pressure control is essential—but too low is not good. For adult patients with hypertension caused by ADPKD, an acceptable blood pressure range is 120–130/70–80 mm Hg. However, further information from recently published blood pressure guidelines54 and the results of the Halt Progression of Polycystic Kidney Disease (HALT-PKD) study to be reported in late 201455 may provide more precise ranges for blood pressure control in ADPKD.
Although substantial experimental evidence exists for the benefits of inhibiting the up-regulation of the renin-angiotensin-aldosterone system in ADPKD, clinical proof is not yet available to confirm that angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) are preferred therapy.55 This may be determined by results of the HALT-PKD study, due for release in late 2014.55
Controlling blood pressure should be done with caution. Patients with low GFRs whose blood pressure is too low tend to have a more rapid decline of GFR, as suggested in the Modification of Diet in Renal Disease (MDRD) study in 1995.56
Experimental evidence suggests that avoiding calcium channel blockers may be advisable. Yamaguchi et al34 found that calcium channel blockers worsen the calcium transport defect and convert tubular epithelial cells to a proliferative phenotype.34
High fluid intake (2,500–3,000 mL/day), because it suppresses vasopressin, may be useful if permitted by several factors such as the patient’s cardiopulmonary and renal and electrolyte status, other medications, and diet.31 The reader is referred to a detailed description of the precautions necessary when prescribing high water intake.31 Patients should have their fluid intake managed by a physician and their renal function and serum sodium and electrolytes monitored regularly in order to avoid hyponatremia. Severe hyponatremia has occurred in patients with ADPKD and impaired kidney function who drank excessive quantities of water. Cardiac and pulmonary complications from excessive fluid intake are also possible, especially with a low GFR and compromised cardiac function.
A low-sodium diet, if not a contributing factor in hyponatremia, can be used under physician direction in the management of hypertension as well as in the prevention of calcium oxalate kidney stones.
Caffeine should be avoided because it may interfere with the activity of the phosphodiesterase that is necessary for the catabolism of cAMP to 5′AMP.
A low-protein diet is of unproven benefit,56 but it is prudent to avoid high protein intake.57
Complications such as bleeding (into or from cysts), infection (urinary tract, kidney cysts, and liver cysts), kidney stones, and urinary tract obstruction should be treated promptly and may require hospitalization.
Regular symptom reviews and physical examinations need to be performed with nonrenal concerns also in mind, such as intracranial aneurysms and cardiac valve lesions.11,58
Formal genetic counseling and molecular testing are becoming more frequently indicated as more complex inheritance patterns arise.6–8,59
Renal replacement therapy in the form of dialysis or transplantation is usually available for the patient when end-stage renal disease occurs. In the largest study thus far, ADPKD patient survival with a kidney transplant was similar to that of patients without ADPKD (about 93% at 5 years), and from 5 years to 15 years death-censored graft survival was actually better.60 Thromboembolic events are more frequent after transplantation,27,60 but they may also occur before transplantation from a massive right kidney compressing the iliac vein or the inferior vena cava, or both, leading to thrombus formation.26
Investigational as well as standard drug studies have intensified. Results from a large randomized study in approximately 1,000 adult ADPKD patients that evaluated over 6 to 8 years the effects of ACE inhibition with or without ARB treatment of hypertension, at both usual and lower blood pressure ranges in those with good renal function, are expected in late 2014.55 Outcomes from a few small clinical studies, eg, one with long-acting somatostatin31,61 and one using low-dose rapamycin48 in adults with ADPKD, will require confirmation in large randomized placebo-controlled clinical studies. In a new 3-year randomized placebo-controlled study of 91 children and young adults (ages 8 to 22) with ADPKD and essentially normal renal function who continued treatment with lisinopril, the addition of pravastatin (20 mg or 40 mg daily based on age) resulted in a significant reduction in the number of patients (46% vs 68%, respectively, P = .03) experiencing a greater than 20% change (increase) in height-adjusted total kidney volume.62 Change in GFR was not reported,62 but an earlier 4-week study in 10 patients treated with simvastatin did show an increase in renal blood flow and GFR.63 Numerous other agents that lack human studies include some described in older experimental work (eg, amiloride,31,64 citrate31,65) and many others from a growing list of potential therapeutic targets.31,66–73 It must be emphasized that there is no FDA-approved medication specifically for the treatment of ADPKD.
Future specific treatments of ADPKD may also involve minimally toxic doses of combination or sequential therapy directed at precystic and then both micro- and macrocystic/cystic fluid expansion aspects of ADPKD.48,74 Unfortunately, at the present time there is no specific FDA-approved therapy for ADPKD.
- Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2006; 2:40–55.
- Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int 2009; 76:149–168.
- United States Renal Data System. 2013 atlas of CKD & ESRD. Volume 2 - atlas ESRD:172. www.usrds.org/atlas.aspx. Accessed June 4, 2014.
- Barua M, Cil O, Paerson AD, et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol 2009, 20:1833–1838.
- Harris PC, Bae KT, Rossetti S, et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2006; 17:3013–3019.
- Vujic M, Heyer CM, Ars E, et al. Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol 2010; 21:1097–1102.
- Harris PC. What is the role of somatic mutation in autosomal dominant polycystic kidney disease? J Am Soc Nephrol 2010; 21:1073–1076.
- Watnick T, He N, Wang K, et al. Mutations of PKD1 in ADPKD2 cysts suggest a pathogenic effect of trans-heterozygous mutations. Nat Genet 2000; 25:143–144.
- Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 1994; 343:824–827.
- Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 2009; 20:205–212.
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007; 369:1287–1301.
- Bajwa ZH, Sial KA, Malik AB, Steinman TI. Pain patterns in patients with polycystic kidney disease. Kidney Int 2004; 66:1561–1569.
- Jouret F, Lhommel R, Beguin C, et al. Positron-emission computed tomography in cyst infection diagnosis in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6:1644–1650.
- Nishiura JL, Neves RF, Eloi SR, Cintra SM, Ajzen SA, Heilberg IP. Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephrol 2009; 4:838–844.
- Hiura T, Yamazaki H, Saeki T, et al. Nephrotic syndrome and IgA nephropathy in polycystic kidney disease. Clin Exp Nephrol 2006; 10:136–139.
- Hossack KF, Leddy CL, Johnson AM, Schrier RW, Gabow PA. Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med 1988; 319:907–912.
- Rossetti S, Chauveau D, Kubly V, et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet 2003; 361:2196–2201.
- Linn FH, Wijdicks EF, van der Graaf Y, Weerdesteyn-van Vliet FA, Bartelds AI, van Gijn J. Prospective study of sentinel headache in aneurismal subarachnoid haemorrhage. Lancet 1994; 344:590–593.
- Belz MM, Fick-Brosnahan GM, Hughes RL, et al. Recurrence of intracranial aneurysms in autosomal-dominant polycystic kidney disease. Kidney Int 2003; 63:1824–1830.
- Irazabal MV, Huston J, Kubly V, et al. Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6:1274–1285.
- Salman A-S, White PM, Counsell CE, et al; Scottish Audit of Intracranial Vascular Malformations Collaborators. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA 2014; 311:1661–1669.
- Vijay A, Vijay A, Pankaj P. Autosomal dominant polycystic kidney disease: a comprehensive review. Nephrourol Mon 2010; 2:172–192.
- Grantham JJ, Torres VE, Chapman AB, et al; CRISP Investigators. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354:2122–2130.
- Bae KT, Grantham JJ. Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6:96–106.
- van den Dool SW, Wasser NM, de Fijter JW, Hoekstra J, van der Geest RJ. Functional renal volume: quantitative analysis at gadolinium-enhanced MR angiography—feasibility study in healthy potential kidney donors. Radiology 2005; 236:189–195.
- O’Sullivan DA, Torres VE, Heit JA, Liggett S, King BF. Compression of the inferior vena cava by right renal cysts: an unusual cause of IVC and/or iliofemoral thrombosis with pulmonary embolism in autosomal dominant polycystic kidney disease. Clin Nephrol 1998; 49:332–334.
- Tveit DP, Hypolite I, Bucci J, et al. Risk factors for hospitalizations resulting from pulmonary embolism after renal transplantation in the United States. J Nephrol 2001; 14:361–368.
- Pei Y. A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med 2001; 7:151–156.
- Qian F, Germino GG. “Mistakes happen”: somatic mutation and disease. Am J Hum Genet 1997; 61:1000–1005.
- Takakura A, Contrino L, Zhou X, et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 2009; 18:2523–2531.
- Torres VE, Harris PC. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 2014; 25:18–32.
- Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 2003; 33:129–137.
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med 2011; 364:1533–1543.
- Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 2004; 279:40419–40430.
- Verghese E, Ricardo SD, Weidenfeld R, et al. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol 2009; 20:2147–2153.
- Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 2008; 19:102–108.
- Torres VE. Cyclic AMP, at the hub of the cystic cycle. Kidney Int 2004; 66:1283–1285.
- Nagao S, Nishii K, Katsuyama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 2006; 17:2220–2227.
- Grantham JJ. Therapy for polycystic kidney disease? It’s water, stupid! J Am Soc Nephrol 2008; 19:1–7.
- Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol 2011; 6:192–197.
- Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 2003; 9:1323–1326.
- Torres VE, Chapman AB, Devuyst O, et al; TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012; 367:2407–2418.
- Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 2006; 103:5466–5471.
- Hartman TR, Liu D, Zilfou JT, et al. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet 2009; 18:161–163.
- Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363:820–829.
- Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363:830–840. Errata in: N Engl J Med 2010; 363:1190 and N Engl J Med 2010; 363:1977.
- Walz G, Budde K, Eckardt K-U. mTOR inhibitors and autosomal dominant polycystic kidney disease (correspondence). N Engl J Med 2011; 364:287–288.
- Braun WE, Schold JD, Stephany BR, Spinko RA, Herfs BR. Low dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized control pilot study. Clin J Am Soc Nephrol 2014; 9:881–888.
- Karihaloo A, Koraishy F, Huen SC, et al. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 2011; 22:1809–1814.
- Fox R, Nhan TQ, Law GL, Morris DR, Liles WC, Schwartz SM. PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. EMBO J 2007; 26:505–515. Erratum in: EMBO J 2007; 26:2605.
- Carvalhosa R, Deambrosis I, Carrera P, et al. Cystogenic potential of CD133+ progenitor cells of human polycystic kidneys. J Pathol 2011; 225:129–141.
- Grantham JJ, Mulamalla S, Grantham CJ, et al. Detected renal cysts are tips of the iceberg in adults with ADPKD. Clin J Am Soc Nephrol 2012; 7:1087–1093.
- Grantham JJ, Cook LT, Wetzel LH, Cadnapaphornchai MA, Bae KT. Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol 2010; 5:889–896.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol 2010; 5:102–109.
- Klahr S, Breyer JA, Beck GJ, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J Am Soc Nephrol 1995; 5:2037–2047.
- Thilly N. Low-protein diet in chronic kidney disease: from questions of effectiveness to those of feasibility. Nephrol Dial Transplant 2013; 28:2203–2205.
- Luciano RL, Dahl NK. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol Dial Transplant 2014; 29:247–254.
- Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6:197–206.
- Jacquet A, Pallet N, Kessler M, et al. Outcomes of renal transplantation in patients with autosomal dominant polycystic kidney disease: a nationwide longitudinal study. Transpl Int 2011; 24:582–587.
- Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int 2005; 68:206–216.
- Cadnapaphornchai MA, George DM, McFann K, et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2014; 9:889–896.
- van Dijk MA, Kamper AM, van Veen S, Souverjin JH, Blauw GJ. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2001; 16:2152–2157.
- Grantham JJ, Uchich M, Cragoe EL, et al. Chemical modification of cell proliferation and fluid secretion in renal cysts. Kidney Int 1989; 35:1379–1389.
- Tanner GA. Potassium citrate/citric acid intake improves renal function in rats with polycystic kidney disease. J Am Soc Nephrol 1998; 9:1242–1248.
- Belibi FA, Edelstein CL. Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Investig Drugs 2010; 19:315–328.
- Tao Y, Kim J, Yin Y, et al. VEGF receptor inhibition slows the progression of polycystic kidney disease. Kidney Int 2007; 72:1358–1366.
- Terryn S, Ho A, Beauwens R, Devuyst O. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta 2011; 1812:1314–1321.
- Thiagarajah JR, Verkman AS. CFTR inhibitors for treating diarrheal disease. Clin Pharmacol Ther 2012; 92:287–290.
- Boehn SN, Spahn S, Neudecker S, et al. Inhibition of Comt with tolcapone slows proression of polycystic kidney disease in the more severely affected PKD/Mhm (cy/+) substrain of the Hannover Sprague-Dawley rat. Nephrol Dial Transplant 2013; 28:2045–2058.
- Rees S, Kittikulsuth W, Roos K, Strait KA, Van Hoek A, Kohan DE. Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J Am Soc Nephrol 2014; 25:232–237.
- Buchholz B, Schley G, Faria D, et al. Hypoxia-inducible factor-1a causes renal cyst expansion through calcium-activated chloride secretion. J Am Soc Nephrol 2014; 25:465–474.
- Wallace DP, White C, Savinkova L, et al. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int 2014; 85:845–854.
- Leuenroth SJ, Crews CM. Targeting cyst initiation in ADPKD. J Am Soc Nephrol 2009; 20:1–3.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disease, has an estimated prevalence of 1:400 to 1:1,000 live births in the United States, and occurs worldwide.1,2 There are about 700,000 people living with it in the United States, and about 6,000 new cases arise annually. It accounts for nearly 5% of all patients with end-stage renal disease in the United States.3
This paper will offer an overview of the pathogenesis of renal cysts, review some of the clinical aspects of ADPKD including diagnosis and management of complications, and discuss recent drug trials and current management.
TWO TYPES—PKD1 IS MORE COMMON AND PROGRESSES MORE RAPIDLY
Two major forms of ADPKD are recognized and can usually be determined by genetic testing: PKD1, accounting for about 85% of cases, and PKD2, accounting for 15%.
The gene locus for PKD1 is on the short arm of the 16th chromosome (16p13.3), and its glycoprotein gene product is polycystin 1 (PC1), a large molecule with 4,303 amino acids.2 PC1 has a long N-terminal extracellular tail that can function as a mechanosensor. Disease progression is much faster with PKD1, and end-stage renal disease usually occurs before age 56.4
In PKD2, the gene locus is on the long arm of the fourth chromosome (4q21–23), and has a smaller glycoprotein gene product, polycystin 2 (PC2), that plays a role in calcium transport. The disease course of PKD2 tends to be slower. End-stage renal disease might not develop in the patient’s lifetime, since it typically develops when the patient is more than 70 years old.4
Although the growth rate of renal cysts is similar between the two types, patients with PKD1 develop about twice as many cysts as those with PDK2, and their cyst development starts at a younger age.5
Typically, patients have a clear phenotype and a positive family history, but in about 10% of possible ADPKD cases, there is no family history of ADPKD. Genetic variations such as incompletely penetrant PKD1 alleles,6 hypomorphic alleles,7 and trans-heterozygous mutations8 account for at least some of these cases.
IMAGING CRITERIA HAVE BROADENED
Ultrasonographic criteria for the diagnosis of ADPKD that were published in 1994 were based on patients who had a family history of PKD1.9 The criteria have since been modified (the “unified criteria”) to include patients with a family history of PKD2 who begin cyst development at a later age and with lower numbers.10 For patients ages 30 to 39, a previously difficult diagnostic group, the criterion for the minimum number of cysts visible on ultrasonography changed from four to three, improving the sensitivity of detecting disease from approximately 76% to approximately 95% (Table 1).9,10 It is important to note that these criteria apply only to patients “at risk,” ie, with a positive family history of ADPKD.
Computed tomography (CT) and magnetic resonance imaging (MRI) classically show bilaterally enlarged multicystic kidneys, though variations can be seen.
DISEASE CAN PRESENT IN MYRIAD WAYS
Although cystic kidney disease is the basic underlying problem, undiagnosed patients may present with a variety of symptoms caused by other manifestations of ADPKD (Table 2).
Hypertension is the most common presentation, occurring in about 50% of patients ages 20 to 34, and essentially 100% of those with end-stage renal disease.11 It is associated with up-regulation of the renin-angiotensin-aldosterone system.
Pain is typically located in the abdomen, flank, or back and can occur in a localized or diffuse manner. Early abdominal distress is often simply described as “fullness.” Localized pain is usually caused by bleeding into or rupture of a cyst, renal stones, or infection.12 Because renal cysts are noncommunicating, bleeding can occur into a cyst and cause pain without gross hematuria. Compression by greatly enlarged kidneys, liver, or both can cause a variety of gastrointestinal symptoms such as reflux esophagitis and varying degrees of constipation. Diffuse pain is often musculoskeletal and related to exaggerated lordosis from increasing abdominal size due to enlarging cystic kidneys and sometimes liver.12 In carefully selected cases, cyst aspiration may be helpful.11
Although renal carcinomas are rare and not more frequent than in the general population, they can occur at an earlier age and with constitutional symptoms.11
Urinary tract infections are increased in frequency. A patient may have a simple urinary tract infection that is cured with the appropriate antibiotic. However, a urinary tract infection repeatedly recurring with the same organism is a strong clue that an infected cyst is the source and requires more intensive treatment with the appropriate cyst-penetrating antibiotic. On the other hand, because cysts are noncommunicating, an infected cyst might be present despite a negative urine culture.
Identifying infected cysts can be a challenge with conventional imaging techniques, but combined positron emission tomography and CT (PET/CT) can be a valuable though expensive diagnostic tool to identify an infected kidney or liver cyst, or to identify an unsuspected source of the pain and infection.13
Jouret et al13 evaluated 27 PET/CT scans performed in 24 patients with ADPKD and suspicion of an abdominal infection. Patients were deemed to have probable cyst infection if they met all of the following criteria: temperature more than 38°C for longer than 3 days, loin or liver tenderness, plasma C-reactive protein level greater than 5 mg/dL, and no evidence of intracystic bleeding on CT. Patients with only two or three of these criteria were classified as having fever of unknown origin. Diagnosis of cyst infection was confirmed by cyst fluid analysis.
PET/CT identified a kidney or liver cyst infection in 85% of 13 infectious events in 11 patients who met all the criteria for probable cyst infection; CT alone contributed to the diagnosis in only one patient.13 In those with fever of unknown origin, PET/CT identified a source of infection in 64% of 14 events in 13 patients: two infected renal cysts, as well as one patient each with other infections that would be difficult to diagnose clinically, ie, small bowel diverticulitis, psoas abscess, diverticulitis of the right colon, pyelonephritis in a transplanted kidney, infected abdominal aortic aneurysm, prostatitis, colitis, and Helicobacter pylori gastritis. Results of PET/CT were negative in five patients with intracystic bleeding.
Kidney stones occur in 20% to 36% of patients.11,14 Uric acid stones occur at almost the same frequency as calcium oxalate stones.
Chronic kidney disease not previously diagnosed may be the presenting condition in a small percentage of patients, sometimes those in whom much earlier hypertension was not fully evaluated. ADPKD is typically not associated with significant proteinuria (eg, nephrotic range), and the presence of heavy proteinuria usually indicates the presence of a superimposed primary glomerulopathy.15
Cysts in other locations. By MRI, liver cysts are present in 58% of patients ages 15 to 24, rising to 94% in those ages 35 to 46.11 Because liver cysts are estrogen-dependent, they are more prominent in women. A small percentage of patients develop cysts in the pancreas (5%), arachnoid membranes (8%), and seminal vesicles (40% of men with ADPKD).11
Cardiovascular abnormalities occur in almost one-third of patients with ADPKD, usually as mitral and aortic valve abnormalities.16 Aneurysms of the aortic root and abdominal aorta can also occur, in addition to intracranial aneurysms (see below).17
Intracranial aneurysms are not uncommon, and size usually determines their risk.
Intracranial aneurysms are strongly influenced by family history: 16% of ADPKD patients with a family history of intracranial aneurysm also develop them, compared with 5% to 6% of patients with no family history.11 The anterior cerebral circulation is involved in about 80% of cases. A sentinel or sudden “thunderclap” headache is a classic presentation that may precede full-blown rupture in about 17% of cases.18 Patients who rupture an intracranial aneurysm have a mean age of 39, usually have normal renal function, and can be normotensive.11
For patients with no history of subarachnoid hemorrhage, the 5-year cumulative rupture rates for patients with aneurysms located in the internal carotid artery, anterior communicating or anterior cerebral artery, or middle cerebral artery were 0% for aneurysms less than 7 mm, 2.6% for those 7 to 12 mm, 14.5% for those 13 to 24 mm, and 40% for those 25 mm or larger, with higher rates for the same sizes in the posterior circulation.11
In patients without symptoms, size is correlated with risk of rupture: less than 4 mm is usually associated with very low risk, 4 to less than 7 mm with moderate risk, and 7 mm or more with increasing risk. An aneurysm larger than 10 mm is associated with roughly a 1% risk of rupture per year.19
Irazabal et al20 retrospectively studied 407 patients with ADPKD who were screened for intracranial aneurysm. Saccular aneurysms were detected in 45 patients; most were small (median diameter 3.5 mm). During cumulative imaging follow-up of 243 years, only one new intracranial aneurysm was detected (increasing from 2 to 4.4 mm over 144 months) and two previously identified aneurysms grew (one increasing 4.5 to 5.9 mm over 69 months and the other 4.7 to 6.2 mm over 184 months). No change occurred in 28 patients. Seven patients were lost to follow-up, however. During cumulative clinical follow-up of 316 years, no aneurysm ruptured. Two patients were lost to follow-up, three had surgical clipping, and five died of unrelated causes. The authors concluded that presymptomatic intracranial aneurysms are usually small, and that growth and rupture risks are no higher than for unruptured intracranial aneurysms in the general population. A 2014 study also suggests a conservative approach for managing intracranial aneurysm in the general population.21
In asymptomatic ADPKD patients, it is reasonable to reserve screening for those with a positive family history of intracranial aneurysm or subarachnoid hemorrhage, those with a previous ruptured aneurysm, those in high-risk professions (eg, pilots), and for patients prior to anticoagulant therapy or major surgery possibly associated with hemodynamic instability.11,22 Certain extremely anxious patients might also need to be studied. Screening can be performed with magnetic resonance angiography without gadolinium contrast. It is prudent to have patients with an intracranial aneurysm thoroughly evaluated by an experienced neurosurgeon with continued follow-up.
PROGRESSION OF ADPKD
The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) study23 evaluated 241 patients with ADPKD (ages 15 to 46) by measuring the annual rate of change in total kidney volume, total cyst volume, and iothalamate glomerular filtration rate (GFR) over 3 years. The annual increase in total kidney volume averaged 5.3%,23 though the reported range with various imaging techniques is from 4% to 12.8% in adults.24 This study focused on macrocystic disease, ie, cysts that are visible by MRI and measurably increase total kidney volume. Although larger total kidney volume at baseline generally predicted a more rapid decline in GFR, there were wide and overlapping variations in yearly GFR declines within and among different total-kidney-volume groups.23
SPECIAL CLINICAL PROBLEMS IN ADPKD
Case 1: A man with ADPKD develops new and increasing proteinuria
A 55-year-old man with ADPKD and stage 3 chronic kidney disease developed new and increasing proteinuria, rising to 5,500 mg per 24 hours. What is the most likely explanation?
- Rapidly progressive renal failure with increasing proteinuria in ADPKD
- Bilateral renal vein thromboses because of cyst compression
- Malignant hypertension with bilateral renal artery compression
- Superimposed primary glomerulopathy
- Multiple infected renal cysts with pyonephrosis
Answer: Superimposed primary glomerulopathy.
ADPKD (similar to uncomplicated obstructive uropathy, pyelonephritis, main renal artery disease, and often cases of interstitial nephritis without secondary glomerular changes) typically does not result in nephrotic-range proteinuria. A superimposed primary glomerulopathy, focal segmental glomerulosclerosis, was the biopsy-proved diagnosis.
At least 21 cases have been reported of AD-PKD with nephrotic-range proteinuria and a renal biopsy showing a primary glomerulopathy, including focal segmental glomerulosclerosis (5 cases), minimal-change disease (5), membranous nephropathy (3), IgA nephropathy (2), and one each of crescentic glomerulonephropathy, diabetic nephropathy, membranoproliferative glomerulonephritis, postinfectious glomerulonephropathy, amyloid glomerulopathy, and mesangioproliferative glomerulopathy.15 Treatment was directed at the primary glomerulopathy, and the outcomes corresponded to the primary diagnosis (eg, with appropriate treatment, three of the five patients with focal segmental glomerulosclerosis progressed to end-stage renal disease, all of the patients with minimal-change disease went into remission, and one of the two cases with IgA nephropathy improved).15
Case 2: A woman with ADPKD and advanced renal failure develops shortness of breath
A 47-year-old woman with very large polycystic kidneys (total kidney volume 7,500 mL; normal range for a single kidney approximately 136–295 mL, mean 196)25 and estimated GFR of 25 mL/min developed new-onset shortness of breath while climbing steps and later even when making a bed. She had no chest pain, cough, or edema. She was sent directly to the emergency department and was admitted and treated; her condition improved, and she was discharged after 6 days. What did she have?
- Presentation of rare cystic pulmonary disease in ADPKD
- Onset of pneumonia with early bacteremia
- Progressive reduction in ventilatory capacity from massive polycystic kidneys and liver elevating both sides of the diaphragm
- Pulmonary emboli from an iliac vein or inferior vena cava source
- Progressive anemia accompanying rapidly worsening stage 4 chronic kidney disease
Answer: She had pulmonary emboli from an iliac vein (right) or inferior vena cava source.
Pulmonary emboli in ADPKD can be caused by thrombi in the inferior vena cava or the iliac or femoral vein because of compression by a massive right polycystic kidney. Four cases were reported at Mayo Clinic,26 three diagnosed by MRI and one with CT. One additional case occurred at Cleveland Clinic. All patients survived after treatment with anticoagulation therapy; early nephrectomy was required in two cases.
Interestingly, following kidney transplantation, the patients at greatest risk for pulmonary emboli are those with ADPKD as their original disease.27
RENAL CYSTS RESULT FROM COMBINED MUTATIONS, INJURY
The germline ADPKD mutation that occurs in one allele of all renal tubular epithelial cells is necessary but not sufficient for cystogenesis.28 One or more additional somatic mutations of the normal allele—the “second hit”—also develop within individual tubular epithelial cells.28,29 These epithelial cells undergo clonal proliferation, resulting in tubular dilatation and cyst formation. Monoclonality of cells in cysts has been documented.
Ischemia-reperfusion injury can be viewed as a “third hit.”30 In PKD1 knockout mice, which at 5 weeks of age normally develop only mild cystic kidney disease, the superimposition of unilateral ischemia-reperfusion injury at 8 weeks caused widespread and rapid cyst formation. It is believed that acute renal injury reactivates developmental signaling pathways within 48 hours that trigger epithelial cell proliferation and then cyst development detectable by MRI 2 weeks later. Although this phenomenon has not been documented in humans, it is a cautionary tale.
CYSTOGENESIS INVOLVES MULTIPLE PATHWAYS
A comprehensive description of pathways leading to renal cyst formation is beyond the scope of this article, and the reader is referred to much more detailed and extensive reviews.2,31 Disturbances in at least three major interconnected pathways promote cystogenesis in renal tubular epithelial cells:
- Normal calcium transport into the endoplasmic reticulum is disrupted by abnormal polycystins on the surface of the primary cilium
- Vasopressin and other stimuli increase the production of cyclic adenosine monophosphate (cAMP)
- The mammalian target of rapamycin (mTOR) proliferative pathway is up-regulated.
DISRUPTION OF CALCIUM TRANSPORT IN THE PRIMARY CILIUM
Primary cilia are nonmotile cellular organelles of varying size, from about 0.25 μm up to about 1 μm.32 Each primary cilium has nine peripheral pairs of microtubules but lacks a centrally located pair that is present in motile cilia. Primary cilia are ubiquitous and have been highly conserved throughout evolution. A single cilium is present on almost all vertebral cells.33
Cilial defects have been identified in autosomal dominant as well as recessive diseases and are known as ciliopathies.33 Although rare in humans, they can affect a broad spectrum of organs other than the kidney, including the eye, liver, and brain.33
Urine flow in a renal tubule is believed to exert mechanical stimulation on the extracellular flagellum-like N-terminal tail of PC1 that extends from a primary cilium into the urinary space. PC1 in concert with PC2 opens PC2 calcium channels, allowing calcium ions to flow down the microtubules to ryanodine receptors and the basal body.32,33 This leads to local release of calcium ions that regulate cell proliferation.32,34 However, in ADPKD kidneys, PC1 and PC2 molecules are sparse or mutated, resulting in defective calcium transport, increased and unregulated tubular epithelial cell proliferation, and cyst formation.
In a totally different clinical setting, biopsies of human renal transplants that sustained acute tubular necrosis during transplantation reveal that a cilium dramatically elongates in response to injury,35 possibly as a compensatory mechanism to maintain calcium transport in the presence of meager urine flow and to restore the proliferation of tubular epithelial cells in a regulated repair process.
THE ROLE OF VASOPRESSIN AND ACTIVATION OF cAMP
In classic experiments, Wang et al36 cross-bred rats having genetically inherited polycystic kidney disease (actually, autosomal recessive polycystic kidney disease) with Brattleboro rats that completely lack vasopressin. At 10 and 20 weeks of age, the offspring had virtually complete inhibition of cystogenesis because of the absence of vasopressin. However, when vasopressin was restored by exogenous administration continuously for 8 weeks, the animals formed massive renal cysts.
Vasopressin activates cAMP, which then functions as a second messenger in cell signaling. cAMP increases the activation of the protein kinase A (PKA) pathway, which in turn increases downstream activity of the B-raf/ERK pathway. Up-regulation of cAMP and PKA appears to perpetuate activation of canonical Wnt signaling, down-regulate non-canonical Wnt/planar cell polarity signaling, and lead to loss of tubular diameter control, resulting in cyst formation.31 Normally, cAMP is degraded by phosphodiesterase. However, because of the primary cilium calcium transport defect in ADPKD, phosphodiesterase is reduced and cAMP persists.37 In conjunction with the defective primary cilial calcium transport, cAMP exerts a proliferative effect on renal tubular epithelial cells that is opposite to its effect in normal kidneys.31,32 cAMP also up-regulates the cystic fibrosis transmembrane conductance regulator (CFTR) that promotes chloride ion transport. Sodium ions follow the chloride ions, leading to fluid accumulation and cyst enlargement.31
Inhibiting vasopressin by increasing water intake
A simple key mechanism for limiting vasopressin secretion is by sufficient water ingestion. Nagao et al38 found that rats with polycystic kidney disease given water with 5% glucose (resulting in 3.5-fold increased fluid intake compared with rats given tap water) had a 68% reduction in urinary vasopressin and a urine osmolality less than 290 mOsm/kg. The high-water-intake rats had dramatically reduced cystic areas in the kidney and a 28% reduction of kidney-to-body weight ratio vs controls.
In an obvious oversimplification, these findings raised the question of whether a sufficient increase in water intake could be an effective therapy for polycystic kidney disease.39 A pilot clinical study evaluated changes in urine osmolality in eight patients with ADPKD who had normal renal function.40 At baseline, 24-hour urine osmolality was typically elevated to approximately 753 mOsm/kg compared to the plasma at 285 mOsm/kg, indicating that antidiuresis is the usual state. During the 2-week study, urine volume and osmolality were measured, and additional water intake was adjusted in order to achieve a urine osmolality goal of 285 ± 45 mOsm/kg. These adjustments resulted in water intake that appeared to be in the range of 2,400 to 3,000 mL per 24 hours. The major limitations of the study were that it was very short term, and there was no opportunity to measure changes in total kidney volume or estimated GFR.
In a recent preliminary report from Japan, high water intake (2,500–3,000 mL daily) in 18 ADPKD patients was compared over 12 months with ad libitum water intake in 14 ADPKD controls (clinicaltrials.gov NCT 01348505). There was no statistically significant change in total kidney volume or cystatin-estimated GFR in those on high water intake, but serious defects in study design (patients in the high water intake group were allowed to decrease their intake if it was causing them difficulty, and patients in the ad libitum water intake group had no measurement of their actual water intake) prevent any conclusions because there was no evidence that the groups were different from one another with respect to the key element of the study, namely, water intake.
Blocking the vasopressin receptor slows disease progression
Using another approach, Gattone et al41 inhibited the effect of vasopressin by blocking the vasopressin 2 receptor (V2R) in mouse and rat models of polycystic kidney disease, using an experimental drug, OPC31260. The drug halted disease progression and, in one situation, appeared to cause regression of established disease. As noted by Torres and Harris,31 even though both increased water intake and V2R antagonists decrease cAMP in the distal tubules and collecting ducts, circulating levels of vasopressin are decreased by increased water intake but increased by V2R antagonists.
After these remarkable results in animal models, clinical trials of the V2R antagonist tolvaptan were conducted in patients with ADPKD. In the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4 study,42 1,445 adults (ages 18 to 50) with ADPKD in 133 centers worldwide were randomized to receive either tolvaptan or placebo for 3 years. Key inclusion criteria included good renal function (estimated GFR ≥ 60 mL/min) and total kidney volume of at least 750 mL (mean 1,700 mL) as measured by MRI. Tolvaptan was titrated to the highest tolerated twice-daily dose (average total of 95 mg/day). All patients were advised to maintain good hydration and to avoid thirst by drinking a glass of water after each urination. Unfortunately, neither water intake nor urine output was measured.
The primary end point was the annual rate of change in total kidney volume, with secondary end points of clinical progression (worsening kidney function, pain, hypertension, albuminuria), and rate of decline in kidney function as measured by the slope of the reciprocal of serum creatinine.42
Patients in the tolvaptan arm had a slower annual increase in total kidney volume than controls (2.8% vs 5.5%, respectively, P < .001) and a slower annual decline in renal function (−2.61 vs −3.81 mg/mL−1, respectively, P < .001).42 More participants in the treatment group withdrew than in the placebo group (23% vs 14%, respectively).
Adverse events occurred more frequently with tolvaptan.42 Liver enzyme elevations of greater than three times the upper limit of normal occurred in 4.4% of patients in the treatment group, leading to a drug warning issued in January 2013. As expected, side effects related to diuresis (urinary frequency, nocturia, polyuria, and thirst) were more frequent in the treatment group, occurring in up to 55% of participants.
The authors noted, “Although maintaining hydration helped ensure that the blinding in the study was maintained, the suppression of vasopressin release in the placebo group may have led to an underestimation of the beneficial effect of tolvaptan and may account for the lower rates of kidney growth observed in the placebo group.”42
In 2013, the US Food and Drug Administration (FDA) denied a new drug application for tolvaptan as a treatment for ADPKD.
THE mTOR PATHWAY IS UP-REGULATED
The mTOR pathway that plays a major role in cell growth and proliferation includes interaction of the cytoplasmic tail of polycystin 1 with tuberin.43 Activation products of mTOR, including phospho-S6K, have been found in tubular epithelial cells lining cysts of ADPKD kidneys but not in normal kidneys.43 Mutant mice with polycystic disease had phospho-S6K in tubular epithelial cells of cysts, whereas those treated with the mTOR inhibitor rapamycin did not.43 But subsequent studies have shown that only a low level of mTOR activation is present in 65% to 70% of ADPKD cysts.44
Two major studies of the treatment of ADPKD with rapamycin that were published contemporaneously in 2010 failed to demonstrate any significant benefit with mTOR inhibitor treatment.45,46
Serra et al45 conducted an 18-month, open-label trial of 100 ADPKD patients ages 18 to 40 with an estimated GFR (eGFR) of at least 70 mL/min. Patients were randomized to receive rapamycin, given as sirolimus 2 mg per day, or standard care. The primary end point was the reduction in the growth rate of total kidney volume, measured by MRI. Secondary end points were eGFR and protein excretion (albumin-creatinine ratio). No significant difference was found in total kidney volume, but a nonsignificant stabilization of eGFR was noted.
Walz et al46 in a 2-year, multicenter, double-blind trial, randomized 433 patients (mean age 44; mean eGFR 54.5 mL/min) to treatment with either the short-acting mTOR inhibitor everolimus (2.5 mg twice daily) or placebo. Although patients in the treatment group had less of an increase in total kidney volume (significant at 1 year but not at 2 years), they tended to show a decline in eGFR. But further analysis showed that the only patients who had a reduction in eGFR were males who already had impaired kidney function at baseline.47
In a pilot study, 30 patients with ADPKD (mean age 49) were randomized to one of three therapies:
- Low-dose rapamycin (trough blood level 2–5 ng/mL)
- Standard-dose rapamycin (trough blood level > 5–8 ng/mL)
- Standard care without rapamycin.48
In contrast to other studies, the primary end point was the change in iothalamate GFR at 12 months, with change in total kidney volume being a secondary end point.
At 12 months, with 26 patients completing the study, the low-dose rapamycin group (n = 9) had a significant increase in iothalamate GFR of 7.7 ± 12.5 mL/min/1.73 m2, whereas the standard-dose rapamycin group (n = 8) had a nonsignificant increase of 1.6 ± 12.1 mL/min/1.73 m2, and the no-rapamycin group (n = 9) had a fall in iothalamate GFR of 11.2 ± 9.1 mL/min/1.73 m2 (P = .005 for low-dose vs no rapamycin; P = .07 for standard-dose vs no rapamycin; P = .52 for low-dose vs standard-dose rapamycin; and P = .002 for combined low-dose and standard-dose rapamycin vs no rapamycin.).48 These differences were observed despite there being no significant change in total kidney volume in any of the groups. Patients on low-dose rapamycin had fewer adverse effects than those on standard dose and were more often able to continue therapy for the entire study. This, and the use of iothalamate GFR rather than eGFR to measure GFR, are believed to be the main reasons that low-dose effects were more pronounced than those with standard doses. One may speculate that rapamycin may have its effect on microcysts and cystogenic cells, resulting in stabilization of or improvement in renal function without detectable slowing in total kidney volume enlargement. Mechanisms for this possibility involve new concepts, as discussed below.
NEW CONCEPTS
Specialized cells also promote renal cyst formation
Specialized cells that promote cyst formation have been identified by Karihaloo et al49 in a mouse model of polycystic kidney disease. In this model, alternatively activated macrophages homed to cystic areas and promoted cyst growth. These findings suggested that interrupting the homing and proliferative signals of macrophages could be a therapeutic target for ADPKD. Although rapamycin can suppress macrophage proliferation by macrophage colony-stimulating factor and inhibit macrophage function,50 alternatively activated macrophages have not been specifically studied for rapamycin responsiveness.
More promising is evidence that CD133+ progenitor cells from human ADPKD kidneys—but not from normal human kidneys—form cysts in vitro and in severe combined immunodeficient mouse models.51 Treatment with rapamycin decreased proliferation of the de-differentiated CD133+ cells from ADPKD patients and reduced cystogenesis.51
Visible cysts are the tip of the iceberg
Using ADPKD nephrectomy specimens from eight patients, Grantham et al52 compared cyst counts by MRI and by histology and found that for every renal cyst detected by MRI, about 62 smaller cysts (< 0.9 mm) are present in the kidney. For a typical patient having an average of 587 cysts in both kidneys that are detectable by MRI, this means that more than 36,000 cysts are actually present, and MRI detects less than 2% of the total cysts present.
Although microcysts are too small to contribute much to total kidney volume, they can interfere with kidney function. Microcysts can reduce GFR in two major ways: by compressing microvasculature, tubules, and glomeruli in the cortex; or by blocking the drainage of multiple upstream nephrons when they form in or block medullary collecting ducts.52 Although the growth rates of microcysts less than 1 mm in size have not yet been measured, the adult combined growth rates of the renal cyst component is approximately 12% per year, with yearly individual cyst growth rates up to 71%, and with fetal cyst growth rates even higher for cysts larger than 7.0 mm.53 Before and during an accelerated growth period, microcysts may be susceptible to certain therapies that could first improve GFR and only later change measurable total kidney volume by slowing microcyst progression to macrocysts either directly or through specialized cells that may be sensitive to rapamycin.
CURRENT MANAGEMENT OF ADPKD
Blood pressure control is essential—but too low is not good. For adult patients with hypertension caused by ADPKD, an acceptable blood pressure range is 120–130/70–80 mm Hg. However, further information from recently published blood pressure guidelines54 and the results of the Halt Progression of Polycystic Kidney Disease (HALT-PKD) study to be reported in late 201455 may provide more precise ranges for blood pressure control in ADPKD.
Although substantial experimental evidence exists for the benefits of inhibiting the up-regulation of the renin-angiotensin-aldosterone system in ADPKD, clinical proof is not yet available to confirm that angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) are preferred therapy.55 This may be determined by results of the HALT-PKD study, due for release in late 2014.55
Controlling blood pressure should be done with caution. Patients with low GFRs whose blood pressure is too low tend to have a more rapid decline of GFR, as suggested in the Modification of Diet in Renal Disease (MDRD) study in 1995.56
Experimental evidence suggests that avoiding calcium channel blockers may be advisable. Yamaguchi et al34 found that calcium channel blockers worsen the calcium transport defect and convert tubular epithelial cells to a proliferative phenotype.34
High fluid intake (2,500–3,000 mL/day), because it suppresses vasopressin, may be useful if permitted by several factors such as the patient’s cardiopulmonary and renal and electrolyte status, other medications, and diet.31 The reader is referred to a detailed description of the precautions necessary when prescribing high water intake.31 Patients should have their fluid intake managed by a physician and their renal function and serum sodium and electrolytes monitored regularly in order to avoid hyponatremia. Severe hyponatremia has occurred in patients with ADPKD and impaired kidney function who drank excessive quantities of water. Cardiac and pulmonary complications from excessive fluid intake are also possible, especially with a low GFR and compromised cardiac function.
A low-sodium diet, if not a contributing factor in hyponatremia, can be used under physician direction in the management of hypertension as well as in the prevention of calcium oxalate kidney stones.
Caffeine should be avoided because it may interfere with the activity of the phosphodiesterase that is necessary for the catabolism of cAMP to 5′AMP.
A low-protein diet is of unproven benefit,56 but it is prudent to avoid high protein intake.57
Complications such as bleeding (into or from cysts), infection (urinary tract, kidney cysts, and liver cysts), kidney stones, and urinary tract obstruction should be treated promptly and may require hospitalization.
Regular symptom reviews and physical examinations need to be performed with nonrenal concerns also in mind, such as intracranial aneurysms and cardiac valve lesions.11,58
Formal genetic counseling and molecular testing are becoming more frequently indicated as more complex inheritance patterns arise.6–8,59
Renal replacement therapy in the form of dialysis or transplantation is usually available for the patient when end-stage renal disease occurs. In the largest study thus far, ADPKD patient survival with a kidney transplant was similar to that of patients without ADPKD (about 93% at 5 years), and from 5 years to 15 years death-censored graft survival was actually better.60 Thromboembolic events are more frequent after transplantation,27,60 but they may also occur before transplantation from a massive right kidney compressing the iliac vein or the inferior vena cava, or both, leading to thrombus formation.26
Investigational as well as standard drug studies have intensified. Results from a large randomized study in approximately 1,000 adult ADPKD patients that evaluated over 6 to 8 years the effects of ACE inhibition with or without ARB treatment of hypertension, at both usual and lower blood pressure ranges in those with good renal function, are expected in late 2014.55 Outcomes from a few small clinical studies, eg, one with long-acting somatostatin31,61 and one using low-dose rapamycin48 in adults with ADPKD, will require confirmation in large randomized placebo-controlled clinical studies. In a new 3-year randomized placebo-controlled study of 91 children and young adults (ages 8 to 22) with ADPKD and essentially normal renal function who continued treatment with lisinopril, the addition of pravastatin (20 mg or 40 mg daily based on age) resulted in a significant reduction in the number of patients (46% vs 68%, respectively, P = .03) experiencing a greater than 20% change (increase) in height-adjusted total kidney volume.62 Change in GFR was not reported,62 but an earlier 4-week study in 10 patients treated with simvastatin did show an increase in renal blood flow and GFR.63 Numerous other agents that lack human studies include some described in older experimental work (eg, amiloride,31,64 citrate31,65) and many others from a growing list of potential therapeutic targets.31,66–73 It must be emphasized that there is no FDA-approved medication specifically for the treatment of ADPKD.
Future specific treatments of ADPKD may also involve minimally toxic doses of combination or sequential therapy directed at precystic and then both micro- and macrocystic/cystic fluid expansion aspects of ADPKD.48,74 Unfortunately, at the present time there is no specific FDA-approved therapy for ADPKD.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disease, has an estimated prevalence of 1:400 to 1:1,000 live births in the United States, and occurs worldwide.1,2 There are about 700,000 people living with it in the United States, and about 6,000 new cases arise annually. It accounts for nearly 5% of all patients with end-stage renal disease in the United States.3
This paper will offer an overview of the pathogenesis of renal cysts, review some of the clinical aspects of ADPKD including diagnosis and management of complications, and discuss recent drug trials and current management.
TWO TYPES—PKD1 IS MORE COMMON AND PROGRESSES MORE RAPIDLY
Two major forms of ADPKD are recognized and can usually be determined by genetic testing: PKD1, accounting for about 85% of cases, and PKD2, accounting for 15%.
The gene locus for PKD1 is on the short arm of the 16th chromosome (16p13.3), and its glycoprotein gene product is polycystin 1 (PC1), a large molecule with 4,303 amino acids.2 PC1 has a long N-terminal extracellular tail that can function as a mechanosensor. Disease progression is much faster with PKD1, and end-stage renal disease usually occurs before age 56.4
In PKD2, the gene locus is on the long arm of the fourth chromosome (4q21–23), and has a smaller glycoprotein gene product, polycystin 2 (PC2), that plays a role in calcium transport. The disease course of PKD2 tends to be slower. End-stage renal disease might not develop in the patient’s lifetime, since it typically develops when the patient is more than 70 years old.4
Although the growth rate of renal cysts is similar between the two types, patients with PKD1 develop about twice as many cysts as those with PDK2, and their cyst development starts at a younger age.5
Typically, patients have a clear phenotype and a positive family history, but in about 10% of possible ADPKD cases, there is no family history of ADPKD. Genetic variations such as incompletely penetrant PKD1 alleles,6 hypomorphic alleles,7 and trans-heterozygous mutations8 account for at least some of these cases.
IMAGING CRITERIA HAVE BROADENED
Ultrasonographic criteria for the diagnosis of ADPKD that were published in 1994 were based on patients who had a family history of PKD1.9 The criteria have since been modified (the “unified criteria”) to include patients with a family history of PKD2 who begin cyst development at a later age and with lower numbers.10 For patients ages 30 to 39, a previously difficult diagnostic group, the criterion for the minimum number of cysts visible on ultrasonography changed from four to three, improving the sensitivity of detecting disease from approximately 76% to approximately 95% (Table 1).9,10 It is important to note that these criteria apply only to patients “at risk,” ie, with a positive family history of ADPKD.
Computed tomography (CT) and magnetic resonance imaging (MRI) classically show bilaterally enlarged multicystic kidneys, though variations can be seen.
DISEASE CAN PRESENT IN MYRIAD WAYS
Although cystic kidney disease is the basic underlying problem, undiagnosed patients may present with a variety of symptoms caused by other manifestations of ADPKD (Table 2).
Hypertension is the most common presentation, occurring in about 50% of patients ages 20 to 34, and essentially 100% of those with end-stage renal disease.11 It is associated with up-regulation of the renin-angiotensin-aldosterone system.
Pain is typically located in the abdomen, flank, or back and can occur in a localized or diffuse manner. Early abdominal distress is often simply described as “fullness.” Localized pain is usually caused by bleeding into or rupture of a cyst, renal stones, or infection.12 Because renal cysts are noncommunicating, bleeding can occur into a cyst and cause pain without gross hematuria. Compression by greatly enlarged kidneys, liver, or both can cause a variety of gastrointestinal symptoms such as reflux esophagitis and varying degrees of constipation. Diffuse pain is often musculoskeletal and related to exaggerated lordosis from increasing abdominal size due to enlarging cystic kidneys and sometimes liver.12 In carefully selected cases, cyst aspiration may be helpful.11
Although renal carcinomas are rare and not more frequent than in the general population, they can occur at an earlier age and with constitutional symptoms.11
Urinary tract infections are increased in frequency. A patient may have a simple urinary tract infection that is cured with the appropriate antibiotic. However, a urinary tract infection repeatedly recurring with the same organism is a strong clue that an infected cyst is the source and requires more intensive treatment with the appropriate cyst-penetrating antibiotic. On the other hand, because cysts are noncommunicating, an infected cyst might be present despite a negative urine culture.
Identifying infected cysts can be a challenge with conventional imaging techniques, but combined positron emission tomography and CT (PET/CT) can be a valuable though expensive diagnostic tool to identify an infected kidney or liver cyst, or to identify an unsuspected source of the pain and infection.13
Jouret et al13 evaluated 27 PET/CT scans performed in 24 patients with ADPKD and suspicion of an abdominal infection. Patients were deemed to have probable cyst infection if they met all of the following criteria: temperature more than 38°C for longer than 3 days, loin or liver tenderness, plasma C-reactive protein level greater than 5 mg/dL, and no evidence of intracystic bleeding on CT. Patients with only two or three of these criteria were classified as having fever of unknown origin. Diagnosis of cyst infection was confirmed by cyst fluid analysis.
PET/CT identified a kidney or liver cyst infection in 85% of 13 infectious events in 11 patients who met all the criteria for probable cyst infection; CT alone contributed to the diagnosis in only one patient.13 In those with fever of unknown origin, PET/CT identified a source of infection in 64% of 14 events in 13 patients: two infected renal cysts, as well as one patient each with other infections that would be difficult to diagnose clinically, ie, small bowel diverticulitis, psoas abscess, diverticulitis of the right colon, pyelonephritis in a transplanted kidney, infected abdominal aortic aneurysm, prostatitis, colitis, and Helicobacter pylori gastritis. Results of PET/CT were negative in five patients with intracystic bleeding.
Kidney stones occur in 20% to 36% of patients.11,14 Uric acid stones occur at almost the same frequency as calcium oxalate stones.
Chronic kidney disease not previously diagnosed may be the presenting condition in a small percentage of patients, sometimes those in whom much earlier hypertension was not fully evaluated. ADPKD is typically not associated with significant proteinuria (eg, nephrotic range), and the presence of heavy proteinuria usually indicates the presence of a superimposed primary glomerulopathy.15
Cysts in other locations. By MRI, liver cysts are present in 58% of patients ages 15 to 24, rising to 94% in those ages 35 to 46.11 Because liver cysts are estrogen-dependent, they are more prominent in women. A small percentage of patients develop cysts in the pancreas (5%), arachnoid membranes (8%), and seminal vesicles (40% of men with ADPKD).11
Cardiovascular abnormalities occur in almost one-third of patients with ADPKD, usually as mitral and aortic valve abnormalities.16 Aneurysms of the aortic root and abdominal aorta can also occur, in addition to intracranial aneurysms (see below).17
Intracranial aneurysms are not uncommon, and size usually determines their risk.
Intracranial aneurysms are strongly influenced by family history: 16% of ADPKD patients with a family history of intracranial aneurysm also develop them, compared with 5% to 6% of patients with no family history.11 The anterior cerebral circulation is involved in about 80% of cases. A sentinel or sudden “thunderclap” headache is a classic presentation that may precede full-blown rupture in about 17% of cases.18 Patients who rupture an intracranial aneurysm have a mean age of 39, usually have normal renal function, and can be normotensive.11
For patients with no history of subarachnoid hemorrhage, the 5-year cumulative rupture rates for patients with aneurysms located in the internal carotid artery, anterior communicating or anterior cerebral artery, or middle cerebral artery were 0% for aneurysms less than 7 mm, 2.6% for those 7 to 12 mm, 14.5% for those 13 to 24 mm, and 40% for those 25 mm or larger, with higher rates for the same sizes in the posterior circulation.11
In patients without symptoms, size is correlated with risk of rupture: less than 4 mm is usually associated with very low risk, 4 to less than 7 mm with moderate risk, and 7 mm or more with increasing risk. An aneurysm larger than 10 mm is associated with roughly a 1% risk of rupture per year.19
Irazabal et al20 retrospectively studied 407 patients with ADPKD who were screened for intracranial aneurysm. Saccular aneurysms were detected in 45 patients; most were small (median diameter 3.5 mm). During cumulative imaging follow-up of 243 years, only one new intracranial aneurysm was detected (increasing from 2 to 4.4 mm over 144 months) and two previously identified aneurysms grew (one increasing 4.5 to 5.9 mm over 69 months and the other 4.7 to 6.2 mm over 184 months). No change occurred in 28 patients. Seven patients were lost to follow-up, however. During cumulative clinical follow-up of 316 years, no aneurysm ruptured. Two patients were lost to follow-up, three had surgical clipping, and five died of unrelated causes. The authors concluded that presymptomatic intracranial aneurysms are usually small, and that growth and rupture risks are no higher than for unruptured intracranial aneurysms in the general population. A 2014 study also suggests a conservative approach for managing intracranial aneurysm in the general population.21
In asymptomatic ADPKD patients, it is reasonable to reserve screening for those with a positive family history of intracranial aneurysm or subarachnoid hemorrhage, those with a previous ruptured aneurysm, those in high-risk professions (eg, pilots), and for patients prior to anticoagulant therapy or major surgery possibly associated with hemodynamic instability.11,22 Certain extremely anxious patients might also need to be studied. Screening can be performed with magnetic resonance angiography without gadolinium contrast. It is prudent to have patients with an intracranial aneurysm thoroughly evaluated by an experienced neurosurgeon with continued follow-up.
PROGRESSION OF ADPKD
The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) study23 evaluated 241 patients with ADPKD (ages 15 to 46) by measuring the annual rate of change in total kidney volume, total cyst volume, and iothalamate glomerular filtration rate (GFR) over 3 years. The annual increase in total kidney volume averaged 5.3%,23 though the reported range with various imaging techniques is from 4% to 12.8% in adults.24 This study focused on macrocystic disease, ie, cysts that are visible by MRI and measurably increase total kidney volume. Although larger total kidney volume at baseline generally predicted a more rapid decline in GFR, there were wide and overlapping variations in yearly GFR declines within and among different total-kidney-volume groups.23
SPECIAL CLINICAL PROBLEMS IN ADPKD
Case 1: A man with ADPKD develops new and increasing proteinuria
A 55-year-old man with ADPKD and stage 3 chronic kidney disease developed new and increasing proteinuria, rising to 5,500 mg per 24 hours. What is the most likely explanation?
- Rapidly progressive renal failure with increasing proteinuria in ADPKD
- Bilateral renal vein thromboses because of cyst compression
- Malignant hypertension with bilateral renal artery compression
- Superimposed primary glomerulopathy
- Multiple infected renal cysts with pyonephrosis
Answer: Superimposed primary glomerulopathy.
ADPKD (similar to uncomplicated obstructive uropathy, pyelonephritis, main renal artery disease, and often cases of interstitial nephritis without secondary glomerular changes) typically does not result in nephrotic-range proteinuria. A superimposed primary glomerulopathy, focal segmental glomerulosclerosis, was the biopsy-proved diagnosis.
At least 21 cases have been reported of AD-PKD with nephrotic-range proteinuria and a renal biopsy showing a primary glomerulopathy, including focal segmental glomerulosclerosis (5 cases), minimal-change disease (5), membranous nephropathy (3), IgA nephropathy (2), and one each of crescentic glomerulonephropathy, diabetic nephropathy, membranoproliferative glomerulonephritis, postinfectious glomerulonephropathy, amyloid glomerulopathy, and mesangioproliferative glomerulopathy.15 Treatment was directed at the primary glomerulopathy, and the outcomes corresponded to the primary diagnosis (eg, with appropriate treatment, three of the five patients with focal segmental glomerulosclerosis progressed to end-stage renal disease, all of the patients with minimal-change disease went into remission, and one of the two cases with IgA nephropathy improved).15
Case 2: A woman with ADPKD and advanced renal failure develops shortness of breath
A 47-year-old woman with very large polycystic kidneys (total kidney volume 7,500 mL; normal range for a single kidney approximately 136–295 mL, mean 196)25 and estimated GFR of 25 mL/min developed new-onset shortness of breath while climbing steps and later even when making a bed. She had no chest pain, cough, or edema. She was sent directly to the emergency department and was admitted and treated; her condition improved, and she was discharged after 6 days. What did she have?
- Presentation of rare cystic pulmonary disease in ADPKD
- Onset of pneumonia with early bacteremia
- Progressive reduction in ventilatory capacity from massive polycystic kidneys and liver elevating both sides of the diaphragm
- Pulmonary emboli from an iliac vein or inferior vena cava source
- Progressive anemia accompanying rapidly worsening stage 4 chronic kidney disease
Answer: She had pulmonary emboli from an iliac vein (right) or inferior vena cava source.
Pulmonary emboli in ADPKD can be caused by thrombi in the inferior vena cava or the iliac or femoral vein because of compression by a massive right polycystic kidney. Four cases were reported at Mayo Clinic,26 three diagnosed by MRI and one with CT. One additional case occurred at Cleveland Clinic. All patients survived after treatment with anticoagulation therapy; early nephrectomy was required in two cases.
Interestingly, following kidney transplantation, the patients at greatest risk for pulmonary emboli are those with ADPKD as their original disease.27
RENAL CYSTS RESULT FROM COMBINED MUTATIONS, INJURY
The germline ADPKD mutation that occurs in one allele of all renal tubular epithelial cells is necessary but not sufficient for cystogenesis.28 One or more additional somatic mutations of the normal allele—the “second hit”—also develop within individual tubular epithelial cells.28,29 These epithelial cells undergo clonal proliferation, resulting in tubular dilatation and cyst formation. Monoclonality of cells in cysts has been documented.
Ischemia-reperfusion injury can be viewed as a “third hit.”30 In PKD1 knockout mice, which at 5 weeks of age normally develop only mild cystic kidney disease, the superimposition of unilateral ischemia-reperfusion injury at 8 weeks caused widespread and rapid cyst formation. It is believed that acute renal injury reactivates developmental signaling pathways within 48 hours that trigger epithelial cell proliferation and then cyst development detectable by MRI 2 weeks later. Although this phenomenon has not been documented in humans, it is a cautionary tale.
CYSTOGENESIS INVOLVES MULTIPLE PATHWAYS
A comprehensive description of pathways leading to renal cyst formation is beyond the scope of this article, and the reader is referred to much more detailed and extensive reviews.2,31 Disturbances in at least three major interconnected pathways promote cystogenesis in renal tubular epithelial cells:
- Normal calcium transport into the endoplasmic reticulum is disrupted by abnormal polycystins on the surface of the primary cilium
- Vasopressin and other stimuli increase the production of cyclic adenosine monophosphate (cAMP)
- The mammalian target of rapamycin (mTOR) proliferative pathway is up-regulated.
DISRUPTION OF CALCIUM TRANSPORT IN THE PRIMARY CILIUM
Primary cilia are nonmotile cellular organelles of varying size, from about 0.25 μm up to about 1 μm.32 Each primary cilium has nine peripheral pairs of microtubules but lacks a centrally located pair that is present in motile cilia. Primary cilia are ubiquitous and have been highly conserved throughout evolution. A single cilium is present on almost all vertebral cells.33
Cilial defects have been identified in autosomal dominant as well as recessive diseases and are known as ciliopathies.33 Although rare in humans, they can affect a broad spectrum of organs other than the kidney, including the eye, liver, and brain.33
Urine flow in a renal tubule is believed to exert mechanical stimulation on the extracellular flagellum-like N-terminal tail of PC1 that extends from a primary cilium into the urinary space. PC1 in concert with PC2 opens PC2 calcium channels, allowing calcium ions to flow down the microtubules to ryanodine receptors and the basal body.32,33 This leads to local release of calcium ions that regulate cell proliferation.32,34 However, in ADPKD kidneys, PC1 and PC2 molecules are sparse or mutated, resulting in defective calcium transport, increased and unregulated tubular epithelial cell proliferation, and cyst formation.
In a totally different clinical setting, biopsies of human renal transplants that sustained acute tubular necrosis during transplantation reveal that a cilium dramatically elongates in response to injury,35 possibly as a compensatory mechanism to maintain calcium transport in the presence of meager urine flow and to restore the proliferation of tubular epithelial cells in a regulated repair process.
THE ROLE OF VASOPRESSIN AND ACTIVATION OF cAMP
In classic experiments, Wang et al36 cross-bred rats having genetically inherited polycystic kidney disease (actually, autosomal recessive polycystic kidney disease) with Brattleboro rats that completely lack vasopressin. At 10 and 20 weeks of age, the offspring had virtually complete inhibition of cystogenesis because of the absence of vasopressin. However, when vasopressin was restored by exogenous administration continuously for 8 weeks, the animals formed massive renal cysts.
Vasopressin activates cAMP, which then functions as a second messenger in cell signaling. cAMP increases the activation of the protein kinase A (PKA) pathway, which in turn increases downstream activity of the B-raf/ERK pathway. Up-regulation of cAMP and PKA appears to perpetuate activation of canonical Wnt signaling, down-regulate non-canonical Wnt/planar cell polarity signaling, and lead to loss of tubular diameter control, resulting in cyst formation.31 Normally, cAMP is degraded by phosphodiesterase. However, because of the primary cilium calcium transport defect in ADPKD, phosphodiesterase is reduced and cAMP persists.37 In conjunction with the defective primary cilial calcium transport, cAMP exerts a proliferative effect on renal tubular epithelial cells that is opposite to its effect in normal kidneys.31,32 cAMP also up-regulates the cystic fibrosis transmembrane conductance regulator (CFTR) that promotes chloride ion transport. Sodium ions follow the chloride ions, leading to fluid accumulation and cyst enlargement.31
Inhibiting vasopressin by increasing water intake
A simple key mechanism for limiting vasopressin secretion is by sufficient water ingestion. Nagao et al38 found that rats with polycystic kidney disease given water with 5% glucose (resulting in 3.5-fold increased fluid intake compared with rats given tap water) had a 68% reduction in urinary vasopressin and a urine osmolality less than 290 mOsm/kg. The high-water-intake rats had dramatically reduced cystic areas in the kidney and a 28% reduction of kidney-to-body weight ratio vs controls.
In an obvious oversimplification, these findings raised the question of whether a sufficient increase in water intake could be an effective therapy for polycystic kidney disease.39 A pilot clinical study evaluated changes in urine osmolality in eight patients with ADPKD who had normal renal function.40 At baseline, 24-hour urine osmolality was typically elevated to approximately 753 mOsm/kg compared to the plasma at 285 mOsm/kg, indicating that antidiuresis is the usual state. During the 2-week study, urine volume and osmolality were measured, and additional water intake was adjusted in order to achieve a urine osmolality goal of 285 ± 45 mOsm/kg. These adjustments resulted in water intake that appeared to be in the range of 2,400 to 3,000 mL per 24 hours. The major limitations of the study were that it was very short term, and there was no opportunity to measure changes in total kidney volume or estimated GFR.
In a recent preliminary report from Japan, high water intake (2,500–3,000 mL daily) in 18 ADPKD patients was compared over 12 months with ad libitum water intake in 14 ADPKD controls (clinicaltrials.gov NCT 01348505). There was no statistically significant change in total kidney volume or cystatin-estimated GFR in those on high water intake, but serious defects in study design (patients in the high water intake group were allowed to decrease their intake if it was causing them difficulty, and patients in the ad libitum water intake group had no measurement of their actual water intake) prevent any conclusions because there was no evidence that the groups were different from one another with respect to the key element of the study, namely, water intake.
Blocking the vasopressin receptor slows disease progression
Using another approach, Gattone et al41 inhibited the effect of vasopressin by blocking the vasopressin 2 receptor (V2R) in mouse and rat models of polycystic kidney disease, using an experimental drug, OPC31260. The drug halted disease progression and, in one situation, appeared to cause regression of established disease. As noted by Torres and Harris,31 even though both increased water intake and V2R antagonists decrease cAMP in the distal tubules and collecting ducts, circulating levels of vasopressin are decreased by increased water intake but increased by V2R antagonists.
After these remarkable results in animal models, clinical trials of the V2R antagonist tolvaptan were conducted in patients with ADPKD. In the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4 study,42 1,445 adults (ages 18 to 50) with ADPKD in 133 centers worldwide were randomized to receive either tolvaptan or placebo for 3 years. Key inclusion criteria included good renal function (estimated GFR ≥ 60 mL/min) and total kidney volume of at least 750 mL (mean 1,700 mL) as measured by MRI. Tolvaptan was titrated to the highest tolerated twice-daily dose (average total of 95 mg/day). All patients were advised to maintain good hydration and to avoid thirst by drinking a glass of water after each urination. Unfortunately, neither water intake nor urine output was measured.
The primary end point was the annual rate of change in total kidney volume, with secondary end points of clinical progression (worsening kidney function, pain, hypertension, albuminuria), and rate of decline in kidney function as measured by the slope of the reciprocal of serum creatinine.42
Patients in the tolvaptan arm had a slower annual increase in total kidney volume than controls (2.8% vs 5.5%, respectively, P < .001) and a slower annual decline in renal function (−2.61 vs −3.81 mg/mL−1, respectively, P < .001).42 More participants in the treatment group withdrew than in the placebo group (23% vs 14%, respectively).
Adverse events occurred more frequently with tolvaptan.42 Liver enzyme elevations of greater than three times the upper limit of normal occurred in 4.4% of patients in the treatment group, leading to a drug warning issued in January 2013. As expected, side effects related to diuresis (urinary frequency, nocturia, polyuria, and thirst) were more frequent in the treatment group, occurring in up to 55% of participants.
The authors noted, “Although maintaining hydration helped ensure that the blinding in the study was maintained, the suppression of vasopressin release in the placebo group may have led to an underestimation of the beneficial effect of tolvaptan and may account for the lower rates of kidney growth observed in the placebo group.”42
In 2013, the US Food and Drug Administration (FDA) denied a new drug application for tolvaptan as a treatment for ADPKD.
THE mTOR PATHWAY IS UP-REGULATED
The mTOR pathway that plays a major role in cell growth and proliferation includes interaction of the cytoplasmic tail of polycystin 1 with tuberin.43 Activation products of mTOR, including phospho-S6K, have been found in tubular epithelial cells lining cysts of ADPKD kidneys but not in normal kidneys.43 Mutant mice with polycystic disease had phospho-S6K in tubular epithelial cells of cysts, whereas those treated with the mTOR inhibitor rapamycin did not.43 But subsequent studies have shown that only a low level of mTOR activation is present in 65% to 70% of ADPKD cysts.44
Two major studies of the treatment of ADPKD with rapamycin that were published contemporaneously in 2010 failed to demonstrate any significant benefit with mTOR inhibitor treatment.45,46
Serra et al45 conducted an 18-month, open-label trial of 100 ADPKD patients ages 18 to 40 with an estimated GFR (eGFR) of at least 70 mL/min. Patients were randomized to receive rapamycin, given as sirolimus 2 mg per day, or standard care. The primary end point was the reduction in the growth rate of total kidney volume, measured by MRI. Secondary end points were eGFR and protein excretion (albumin-creatinine ratio). No significant difference was found in total kidney volume, but a nonsignificant stabilization of eGFR was noted.
Walz et al46 in a 2-year, multicenter, double-blind trial, randomized 433 patients (mean age 44; mean eGFR 54.5 mL/min) to treatment with either the short-acting mTOR inhibitor everolimus (2.5 mg twice daily) or placebo. Although patients in the treatment group had less of an increase in total kidney volume (significant at 1 year but not at 2 years), they tended to show a decline in eGFR. But further analysis showed that the only patients who had a reduction in eGFR were males who already had impaired kidney function at baseline.47
In a pilot study, 30 patients with ADPKD (mean age 49) were randomized to one of three therapies:
- Low-dose rapamycin (trough blood level 2–5 ng/mL)
- Standard-dose rapamycin (trough blood level > 5–8 ng/mL)
- Standard care without rapamycin.48
In contrast to other studies, the primary end point was the change in iothalamate GFR at 12 months, with change in total kidney volume being a secondary end point.
At 12 months, with 26 patients completing the study, the low-dose rapamycin group (n = 9) had a significant increase in iothalamate GFR of 7.7 ± 12.5 mL/min/1.73 m2, whereas the standard-dose rapamycin group (n = 8) had a nonsignificant increase of 1.6 ± 12.1 mL/min/1.73 m2, and the no-rapamycin group (n = 9) had a fall in iothalamate GFR of 11.2 ± 9.1 mL/min/1.73 m2 (P = .005 for low-dose vs no rapamycin; P = .07 for standard-dose vs no rapamycin; P = .52 for low-dose vs standard-dose rapamycin; and P = .002 for combined low-dose and standard-dose rapamycin vs no rapamycin.).48 These differences were observed despite there being no significant change in total kidney volume in any of the groups. Patients on low-dose rapamycin had fewer adverse effects than those on standard dose and were more often able to continue therapy for the entire study. This, and the use of iothalamate GFR rather than eGFR to measure GFR, are believed to be the main reasons that low-dose effects were more pronounced than those with standard doses. One may speculate that rapamycin may have its effect on microcysts and cystogenic cells, resulting in stabilization of or improvement in renal function without detectable slowing in total kidney volume enlargement. Mechanisms for this possibility involve new concepts, as discussed below.
NEW CONCEPTS
Specialized cells also promote renal cyst formation
Specialized cells that promote cyst formation have been identified by Karihaloo et al49 in a mouse model of polycystic kidney disease. In this model, alternatively activated macrophages homed to cystic areas and promoted cyst growth. These findings suggested that interrupting the homing and proliferative signals of macrophages could be a therapeutic target for ADPKD. Although rapamycin can suppress macrophage proliferation by macrophage colony-stimulating factor and inhibit macrophage function,50 alternatively activated macrophages have not been specifically studied for rapamycin responsiveness.
More promising is evidence that CD133+ progenitor cells from human ADPKD kidneys—but not from normal human kidneys—form cysts in vitro and in severe combined immunodeficient mouse models.51 Treatment with rapamycin decreased proliferation of the de-differentiated CD133+ cells from ADPKD patients and reduced cystogenesis.51
Visible cysts are the tip of the iceberg
Using ADPKD nephrectomy specimens from eight patients, Grantham et al52 compared cyst counts by MRI and by histology and found that for every renal cyst detected by MRI, about 62 smaller cysts (< 0.9 mm) are present in the kidney. For a typical patient having an average of 587 cysts in both kidneys that are detectable by MRI, this means that more than 36,000 cysts are actually present, and MRI detects less than 2% of the total cysts present.
Although microcysts are too small to contribute much to total kidney volume, they can interfere with kidney function. Microcysts can reduce GFR in two major ways: by compressing microvasculature, tubules, and glomeruli in the cortex; or by blocking the drainage of multiple upstream nephrons when they form in or block medullary collecting ducts.52 Although the growth rates of microcysts less than 1 mm in size have not yet been measured, the adult combined growth rates of the renal cyst component is approximately 12% per year, with yearly individual cyst growth rates up to 71%, and with fetal cyst growth rates even higher for cysts larger than 7.0 mm.53 Before and during an accelerated growth period, microcysts may be susceptible to certain therapies that could first improve GFR and only later change measurable total kidney volume by slowing microcyst progression to macrocysts either directly or through specialized cells that may be sensitive to rapamycin.
CURRENT MANAGEMENT OF ADPKD
Blood pressure control is essential—but too low is not good. For adult patients with hypertension caused by ADPKD, an acceptable blood pressure range is 120–130/70–80 mm Hg. However, further information from recently published blood pressure guidelines54 and the results of the Halt Progression of Polycystic Kidney Disease (HALT-PKD) study to be reported in late 201455 may provide more precise ranges for blood pressure control in ADPKD.
Although substantial experimental evidence exists for the benefits of inhibiting the up-regulation of the renin-angiotensin-aldosterone system in ADPKD, clinical proof is not yet available to confirm that angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) are preferred therapy.55 This may be determined by results of the HALT-PKD study, due for release in late 2014.55
Controlling blood pressure should be done with caution. Patients with low GFRs whose blood pressure is too low tend to have a more rapid decline of GFR, as suggested in the Modification of Diet in Renal Disease (MDRD) study in 1995.56
Experimental evidence suggests that avoiding calcium channel blockers may be advisable. Yamaguchi et al34 found that calcium channel blockers worsen the calcium transport defect and convert tubular epithelial cells to a proliferative phenotype.34
High fluid intake (2,500–3,000 mL/day), because it suppresses vasopressin, may be useful if permitted by several factors such as the patient’s cardiopulmonary and renal and electrolyte status, other medications, and diet.31 The reader is referred to a detailed description of the precautions necessary when prescribing high water intake.31 Patients should have their fluid intake managed by a physician and their renal function and serum sodium and electrolytes monitored regularly in order to avoid hyponatremia. Severe hyponatremia has occurred in patients with ADPKD and impaired kidney function who drank excessive quantities of water. Cardiac and pulmonary complications from excessive fluid intake are also possible, especially with a low GFR and compromised cardiac function.
A low-sodium diet, if not a contributing factor in hyponatremia, can be used under physician direction in the management of hypertension as well as in the prevention of calcium oxalate kidney stones.
Caffeine should be avoided because it may interfere with the activity of the phosphodiesterase that is necessary for the catabolism of cAMP to 5′AMP.
A low-protein diet is of unproven benefit,56 but it is prudent to avoid high protein intake.57
Complications such as bleeding (into or from cysts), infection (urinary tract, kidney cysts, and liver cysts), kidney stones, and urinary tract obstruction should be treated promptly and may require hospitalization.
Regular symptom reviews and physical examinations need to be performed with nonrenal concerns also in mind, such as intracranial aneurysms and cardiac valve lesions.11,58
Formal genetic counseling and molecular testing are becoming more frequently indicated as more complex inheritance patterns arise.6–8,59
Renal replacement therapy in the form of dialysis or transplantation is usually available for the patient when end-stage renal disease occurs. In the largest study thus far, ADPKD patient survival with a kidney transplant was similar to that of patients without ADPKD (about 93% at 5 years), and from 5 years to 15 years death-censored graft survival was actually better.60 Thromboembolic events are more frequent after transplantation,27,60 but they may also occur before transplantation from a massive right kidney compressing the iliac vein or the inferior vena cava, or both, leading to thrombus formation.26
Investigational as well as standard drug studies have intensified. Results from a large randomized study in approximately 1,000 adult ADPKD patients that evaluated over 6 to 8 years the effects of ACE inhibition with or without ARB treatment of hypertension, at both usual and lower blood pressure ranges in those with good renal function, are expected in late 2014.55 Outcomes from a few small clinical studies, eg, one with long-acting somatostatin31,61 and one using low-dose rapamycin48 in adults with ADPKD, will require confirmation in large randomized placebo-controlled clinical studies. In a new 3-year randomized placebo-controlled study of 91 children and young adults (ages 8 to 22) with ADPKD and essentially normal renal function who continued treatment with lisinopril, the addition of pravastatin (20 mg or 40 mg daily based on age) resulted in a significant reduction in the number of patients (46% vs 68%, respectively, P = .03) experiencing a greater than 20% change (increase) in height-adjusted total kidney volume.62 Change in GFR was not reported,62 but an earlier 4-week study in 10 patients treated with simvastatin did show an increase in renal blood flow and GFR.63 Numerous other agents that lack human studies include some described in older experimental work (eg, amiloride,31,64 citrate31,65) and many others from a growing list of potential therapeutic targets.31,66–73 It must be emphasized that there is no FDA-approved medication specifically for the treatment of ADPKD.
Future specific treatments of ADPKD may also involve minimally toxic doses of combination or sequential therapy directed at precystic and then both micro- and macrocystic/cystic fluid expansion aspects of ADPKD.48,74 Unfortunately, at the present time there is no specific FDA-approved therapy for ADPKD.
- Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2006; 2:40–55.
- Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int 2009; 76:149–168.
- United States Renal Data System. 2013 atlas of CKD & ESRD. Volume 2 - atlas ESRD:172. www.usrds.org/atlas.aspx. Accessed June 4, 2014.
- Barua M, Cil O, Paerson AD, et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol 2009, 20:1833–1838.
- Harris PC, Bae KT, Rossetti S, et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2006; 17:3013–3019.
- Vujic M, Heyer CM, Ars E, et al. Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol 2010; 21:1097–1102.
- Harris PC. What is the role of somatic mutation in autosomal dominant polycystic kidney disease? J Am Soc Nephrol 2010; 21:1073–1076.
- Watnick T, He N, Wang K, et al. Mutations of PKD1 in ADPKD2 cysts suggest a pathogenic effect of trans-heterozygous mutations. Nat Genet 2000; 25:143–144.
- Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 1994; 343:824–827.
- Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 2009; 20:205–212.
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007; 369:1287–1301.
- Bajwa ZH, Sial KA, Malik AB, Steinman TI. Pain patterns in patients with polycystic kidney disease. Kidney Int 2004; 66:1561–1569.
- Jouret F, Lhommel R, Beguin C, et al. Positron-emission computed tomography in cyst infection diagnosis in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6:1644–1650.
- Nishiura JL, Neves RF, Eloi SR, Cintra SM, Ajzen SA, Heilberg IP. Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephrol 2009; 4:838–844.
- Hiura T, Yamazaki H, Saeki T, et al. Nephrotic syndrome and IgA nephropathy in polycystic kidney disease. Clin Exp Nephrol 2006; 10:136–139.
- Hossack KF, Leddy CL, Johnson AM, Schrier RW, Gabow PA. Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med 1988; 319:907–912.
- Rossetti S, Chauveau D, Kubly V, et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet 2003; 361:2196–2201.
- Linn FH, Wijdicks EF, van der Graaf Y, Weerdesteyn-van Vliet FA, Bartelds AI, van Gijn J. Prospective study of sentinel headache in aneurismal subarachnoid haemorrhage. Lancet 1994; 344:590–593.
- Belz MM, Fick-Brosnahan GM, Hughes RL, et al. Recurrence of intracranial aneurysms in autosomal-dominant polycystic kidney disease. Kidney Int 2003; 63:1824–1830.
- Irazabal MV, Huston J, Kubly V, et al. Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6:1274–1285.
- Salman A-S, White PM, Counsell CE, et al; Scottish Audit of Intracranial Vascular Malformations Collaborators. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA 2014; 311:1661–1669.
- Vijay A, Vijay A, Pankaj P. Autosomal dominant polycystic kidney disease: a comprehensive review. Nephrourol Mon 2010; 2:172–192.
- Grantham JJ, Torres VE, Chapman AB, et al; CRISP Investigators. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354:2122–2130.
- Bae KT, Grantham JJ. Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6:96–106.
- van den Dool SW, Wasser NM, de Fijter JW, Hoekstra J, van der Geest RJ. Functional renal volume: quantitative analysis at gadolinium-enhanced MR angiography—feasibility study in healthy potential kidney donors. Radiology 2005; 236:189–195.
- O’Sullivan DA, Torres VE, Heit JA, Liggett S, King BF. Compression of the inferior vena cava by right renal cysts: an unusual cause of IVC and/or iliofemoral thrombosis with pulmonary embolism in autosomal dominant polycystic kidney disease. Clin Nephrol 1998; 49:332–334.
- Tveit DP, Hypolite I, Bucci J, et al. Risk factors for hospitalizations resulting from pulmonary embolism after renal transplantation in the United States. J Nephrol 2001; 14:361–368.
- Pei Y. A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med 2001; 7:151–156.
- Qian F, Germino GG. “Mistakes happen”: somatic mutation and disease. Am J Hum Genet 1997; 61:1000–1005.
- Takakura A, Contrino L, Zhou X, et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 2009; 18:2523–2531.
- Torres VE, Harris PC. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 2014; 25:18–32.
- Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 2003; 33:129–137.
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med 2011; 364:1533–1543.
- Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 2004; 279:40419–40430.
- Verghese E, Ricardo SD, Weidenfeld R, et al. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol 2009; 20:2147–2153.
- Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 2008; 19:102–108.
- Torres VE. Cyclic AMP, at the hub of the cystic cycle. Kidney Int 2004; 66:1283–1285.
- Nagao S, Nishii K, Katsuyama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 2006; 17:2220–2227.
- Grantham JJ. Therapy for polycystic kidney disease? It’s water, stupid! J Am Soc Nephrol 2008; 19:1–7.
- Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol 2011; 6:192–197.
- Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 2003; 9:1323–1326.
- Torres VE, Chapman AB, Devuyst O, et al; TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012; 367:2407–2418.
- Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 2006; 103:5466–5471.
- Hartman TR, Liu D, Zilfou JT, et al. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet 2009; 18:161–163.
- Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363:820–829.
- Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363:830–840. Errata in: N Engl J Med 2010; 363:1190 and N Engl J Med 2010; 363:1977.
- Walz G, Budde K, Eckardt K-U. mTOR inhibitors and autosomal dominant polycystic kidney disease (correspondence). N Engl J Med 2011; 364:287–288.
- Braun WE, Schold JD, Stephany BR, Spinko RA, Herfs BR. Low dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized control pilot study. Clin J Am Soc Nephrol 2014; 9:881–888.
- Karihaloo A, Koraishy F, Huen SC, et al. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 2011; 22:1809–1814.
- Fox R, Nhan TQ, Law GL, Morris DR, Liles WC, Schwartz SM. PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. EMBO J 2007; 26:505–515. Erratum in: EMBO J 2007; 26:2605.
- Carvalhosa R, Deambrosis I, Carrera P, et al. Cystogenic potential of CD133+ progenitor cells of human polycystic kidneys. J Pathol 2011; 225:129–141.
- Grantham JJ, Mulamalla S, Grantham CJ, et al. Detected renal cysts are tips of the iceberg in adults with ADPKD. Clin J Am Soc Nephrol 2012; 7:1087–1093.
- Grantham JJ, Cook LT, Wetzel LH, Cadnapaphornchai MA, Bae KT. Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol 2010; 5:889–896.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol 2010; 5:102–109.
- Klahr S, Breyer JA, Beck GJ, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J Am Soc Nephrol 1995; 5:2037–2047.
- Thilly N. Low-protein diet in chronic kidney disease: from questions of effectiveness to those of feasibility. Nephrol Dial Transplant 2013; 28:2203–2205.
- Luciano RL, Dahl NK. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol Dial Transplant 2014; 29:247–254.
- Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6:197–206.
- Jacquet A, Pallet N, Kessler M, et al. Outcomes of renal transplantation in patients with autosomal dominant polycystic kidney disease: a nationwide longitudinal study. Transpl Int 2011; 24:582–587.
- Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int 2005; 68:206–216.
- Cadnapaphornchai MA, George DM, McFann K, et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2014; 9:889–896.
- van Dijk MA, Kamper AM, van Veen S, Souverjin JH, Blauw GJ. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2001; 16:2152–2157.
- Grantham JJ, Uchich M, Cragoe EL, et al. Chemical modification of cell proliferation and fluid secretion in renal cysts. Kidney Int 1989; 35:1379–1389.
- Tanner GA. Potassium citrate/citric acid intake improves renal function in rats with polycystic kidney disease. J Am Soc Nephrol 1998; 9:1242–1248.
- Belibi FA, Edelstein CL. Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Investig Drugs 2010; 19:315–328.
- Tao Y, Kim J, Yin Y, et al. VEGF receptor inhibition slows the progression of polycystic kidney disease. Kidney Int 2007; 72:1358–1366.
- Terryn S, Ho A, Beauwens R, Devuyst O. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta 2011; 1812:1314–1321.
- Thiagarajah JR, Verkman AS. CFTR inhibitors for treating diarrheal disease. Clin Pharmacol Ther 2012; 92:287–290.
- Boehn SN, Spahn S, Neudecker S, et al. Inhibition of Comt with tolcapone slows proression of polycystic kidney disease in the more severely affected PKD/Mhm (cy/+) substrain of the Hannover Sprague-Dawley rat. Nephrol Dial Transplant 2013; 28:2045–2058.
- Rees S, Kittikulsuth W, Roos K, Strait KA, Van Hoek A, Kohan DE. Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J Am Soc Nephrol 2014; 25:232–237.
- Buchholz B, Schley G, Faria D, et al. Hypoxia-inducible factor-1a causes renal cyst expansion through calcium-activated chloride secretion. J Am Soc Nephrol 2014; 25:465–474.
- Wallace DP, White C, Savinkova L, et al. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int 2014; 85:845–854.
- Leuenroth SJ, Crews CM. Targeting cyst initiation in ADPKD. J Am Soc Nephrol 2009; 20:1–3.
- Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2006; 2:40–55.
- Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int 2009; 76:149–168.
- United States Renal Data System. 2013 atlas of CKD & ESRD. Volume 2 - atlas ESRD:172. www.usrds.org/atlas.aspx. Accessed June 4, 2014.
- Barua M, Cil O, Paerson AD, et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol 2009, 20:1833–1838.
- Harris PC, Bae KT, Rossetti S, et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2006; 17:3013–3019.
- Vujic M, Heyer CM, Ars E, et al. Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol 2010; 21:1097–1102.
- Harris PC. What is the role of somatic mutation in autosomal dominant polycystic kidney disease? J Am Soc Nephrol 2010; 21:1073–1076.
- Watnick T, He N, Wang K, et al. Mutations of PKD1 in ADPKD2 cysts suggest a pathogenic effect of trans-heterozygous mutations. Nat Genet 2000; 25:143–144.
- Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 1994; 343:824–827.
- Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 2009; 20:205–212.
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007; 369:1287–1301.
- Bajwa ZH, Sial KA, Malik AB, Steinman TI. Pain patterns in patients with polycystic kidney disease. Kidney Int 2004; 66:1561–1569.
- Jouret F, Lhommel R, Beguin C, et al. Positron-emission computed tomography in cyst infection diagnosis in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6:1644–1650.
- Nishiura JL, Neves RF, Eloi SR, Cintra SM, Ajzen SA, Heilberg IP. Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephrol 2009; 4:838–844.
- Hiura T, Yamazaki H, Saeki T, et al. Nephrotic syndrome and IgA nephropathy in polycystic kidney disease. Clin Exp Nephrol 2006; 10:136–139.
- Hossack KF, Leddy CL, Johnson AM, Schrier RW, Gabow PA. Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med 1988; 319:907–912.
- Rossetti S, Chauveau D, Kubly V, et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet 2003; 361:2196–2201.
- Linn FH, Wijdicks EF, van der Graaf Y, Weerdesteyn-van Vliet FA, Bartelds AI, van Gijn J. Prospective study of sentinel headache in aneurismal subarachnoid haemorrhage. Lancet 1994; 344:590–593.
- Belz MM, Fick-Brosnahan GM, Hughes RL, et al. Recurrence of intracranial aneurysms in autosomal-dominant polycystic kidney disease. Kidney Int 2003; 63:1824–1830.
- Irazabal MV, Huston J, Kubly V, et al. Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6:1274–1285.
- Salman A-S, White PM, Counsell CE, et al; Scottish Audit of Intracranial Vascular Malformations Collaborators. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA 2014; 311:1661–1669.
- Vijay A, Vijay A, Pankaj P. Autosomal dominant polycystic kidney disease: a comprehensive review. Nephrourol Mon 2010; 2:172–192.
- Grantham JJ, Torres VE, Chapman AB, et al; CRISP Investigators. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354:2122–2130.
- Bae KT, Grantham JJ. Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6:96–106.
- van den Dool SW, Wasser NM, de Fijter JW, Hoekstra J, van der Geest RJ. Functional renal volume: quantitative analysis at gadolinium-enhanced MR angiography—feasibility study in healthy potential kidney donors. Radiology 2005; 236:189–195.
- O’Sullivan DA, Torres VE, Heit JA, Liggett S, King BF. Compression of the inferior vena cava by right renal cysts: an unusual cause of IVC and/or iliofemoral thrombosis with pulmonary embolism in autosomal dominant polycystic kidney disease. Clin Nephrol 1998; 49:332–334.
- Tveit DP, Hypolite I, Bucci J, et al. Risk factors for hospitalizations resulting from pulmonary embolism after renal transplantation in the United States. J Nephrol 2001; 14:361–368.
- Pei Y. A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med 2001; 7:151–156.
- Qian F, Germino GG. “Mistakes happen”: somatic mutation and disease. Am J Hum Genet 1997; 61:1000–1005.
- Takakura A, Contrino L, Zhou X, et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 2009; 18:2523–2531.
- Torres VE, Harris PC. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 2014; 25:18–32.
- Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 2003; 33:129–137.
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med 2011; 364:1533–1543.
- Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 2004; 279:40419–40430.
- Verghese E, Ricardo SD, Weidenfeld R, et al. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol 2009; 20:2147–2153.
- Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 2008; 19:102–108.
- Torres VE. Cyclic AMP, at the hub of the cystic cycle. Kidney Int 2004; 66:1283–1285.
- Nagao S, Nishii K, Katsuyama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 2006; 17:2220–2227.
- Grantham JJ. Therapy for polycystic kidney disease? It’s water, stupid! J Am Soc Nephrol 2008; 19:1–7.
- Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol 2011; 6:192–197.
- Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 2003; 9:1323–1326.
- Torres VE, Chapman AB, Devuyst O, et al; TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012; 367:2407–2418.
- Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 2006; 103:5466–5471.
- Hartman TR, Liu D, Zilfou JT, et al. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet 2009; 18:161–163.
- Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363:820–829.
- Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363:830–840. Errata in: N Engl J Med 2010; 363:1190 and N Engl J Med 2010; 363:1977.
- Walz G, Budde K, Eckardt K-U. mTOR inhibitors and autosomal dominant polycystic kidney disease (correspondence). N Engl J Med 2011; 364:287–288.
- Braun WE, Schold JD, Stephany BR, Spinko RA, Herfs BR. Low dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized control pilot study. Clin J Am Soc Nephrol 2014; 9:881–888.
- Karihaloo A, Koraishy F, Huen SC, et al. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 2011; 22:1809–1814.
- Fox R, Nhan TQ, Law GL, Morris DR, Liles WC, Schwartz SM. PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. EMBO J 2007; 26:505–515. Erratum in: EMBO J 2007; 26:2605.
- Carvalhosa R, Deambrosis I, Carrera P, et al. Cystogenic potential of CD133+ progenitor cells of human polycystic kidneys. J Pathol 2011; 225:129–141.
- Grantham JJ, Mulamalla S, Grantham CJ, et al. Detected renal cysts are tips of the iceberg in adults with ADPKD. Clin J Am Soc Nephrol 2012; 7:1087–1093.
- Grantham JJ, Cook LT, Wetzel LH, Cadnapaphornchai MA, Bae KT. Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol 2010; 5:889–896.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol 2010; 5:102–109.
- Klahr S, Breyer JA, Beck GJ, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J Am Soc Nephrol 1995; 5:2037–2047.
- Thilly N. Low-protein diet in chronic kidney disease: from questions of effectiveness to those of feasibility. Nephrol Dial Transplant 2013; 28:2203–2205.
- Luciano RL, Dahl NK. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol Dial Transplant 2014; 29:247–254.
- Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6:197–206.
- Jacquet A, Pallet N, Kessler M, et al. Outcomes of renal transplantation in patients with autosomal dominant polycystic kidney disease: a nationwide longitudinal study. Transpl Int 2011; 24:582–587.
- Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int 2005; 68:206–216.
- Cadnapaphornchai MA, George DM, McFann K, et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2014; 9:889–896.
- van Dijk MA, Kamper AM, van Veen S, Souverjin JH, Blauw GJ. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2001; 16:2152–2157.
- Grantham JJ, Uchich M, Cragoe EL, et al. Chemical modification of cell proliferation and fluid secretion in renal cysts. Kidney Int 1989; 35:1379–1389.
- Tanner GA. Potassium citrate/citric acid intake improves renal function in rats with polycystic kidney disease. J Am Soc Nephrol 1998; 9:1242–1248.
- Belibi FA, Edelstein CL. Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Investig Drugs 2010; 19:315–328.
- Tao Y, Kim J, Yin Y, et al. VEGF receptor inhibition slows the progression of polycystic kidney disease. Kidney Int 2007; 72:1358–1366.
- Terryn S, Ho A, Beauwens R, Devuyst O. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta 2011; 1812:1314–1321.
- Thiagarajah JR, Verkman AS. CFTR inhibitors for treating diarrheal disease. Clin Pharmacol Ther 2012; 92:287–290.
- Boehn SN, Spahn S, Neudecker S, et al. Inhibition of Comt with tolcapone slows proression of polycystic kidney disease in the more severely affected PKD/Mhm (cy/+) substrain of the Hannover Sprague-Dawley rat. Nephrol Dial Transplant 2013; 28:2045–2058.
- Rees S, Kittikulsuth W, Roos K, Strait KA, Van Hoek A, Kohan DE. Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J Am Soc Nephrol 2014; 25:232–237.
- Buchholz B, Schley G, Faria D, et al. Hypoxia-inducible factor-1a causes renal cyst expansion through calcium-activated chloride secretion. J Am Soc Nephrol 2014; 25:465–474.
- Wallace DP, White C, Savinkova L, et al. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int 2014; 85:845–854.
- Leuenroth SJ, Crews CM. Targeting cyst initiation in ADPKD. J Am Soc Nephrol 2009; 20:1–3.
KEY POINTS
- For at-risk patients in the previously difficult diagnostic group from 30 to 39 years of age, newer ultrasonographic criteria for diagnosing PKD1 and PKD2 now require a minimum total of three renal cysts.
- An intracranial aneurysm occurs in approximately 16% of ADPKD patients who have a family member with ADPKD plus an intracranial aneurysm or subarachnoid hemorrhage. Appropriate screening is warranted.
- Combined positron-emission and computed tomography helps identify infected renal or liver cysts and may uncover other unsuspected abdominal or pelvic infections.
- Cyst expansion and increasing total kidney volume might be slowed by increasing water intake to 2,500 to 3,000 mL per day, although formal documentation of this is not published. However, this must be done under a physician’s supervision because of possible adverse effects.
- Tolvaptan, a promising new drug for treating ADPKD, failed to receive US approval. Rapamycin is another potentially effective agent but has had mixed results in clinical trials.