User login
Engaging Veterans With Serious Mental Illness in Primary Care
People with serious mental illness (SMI) are at substantial risk for premature mortality, dying on average 10 to 20 years earlier than others.1 The reasons for this disparity are complex; however, the high prevalence of chronic disease and physical comorbidities in the SMI population have been identified as prominent factors.2 Engagement and reengagement in care, including primary care for medical comorbidities, can mitigate these mortality risks.2-4 Among veterans with SMI lost to follow-up care for more than 12 months, those not successfully reengaged in care were more likely to die compared with those reengaged in care.2,3
Given this evidence, health care systems, including the US Department of Veterans Affairs (VA), have looked to better engage these patients in care. These efforts have included mental health population health management, colocation of mental health with primary care, designation of primary care teams specializing in SMI, and integration of mental health and primary care services for patients experiencing homelessness.5-8
As part of a national approach to encourage locally driven quality improvement (QI), the VA compiles performance metrics for each facility, across a gamut of care settings, conditions, and veteran populations.9 Quarterly facility report cards, with longitudinal data and cross-facility comparisons, enable facilities to identify targets for QI and track improvement progress. One metric reports on the proportion of enrolled veterans with SMI who have primary care engagement, defined as having an assigned primary care practitioner (PCP) and a primary care visit in the prior 12 months.
In support of a QI initiative at the VA Greater Los Angeles Healthcare System (VAGLAHS), we sought to describe promising practices being utilized by VA facilities with higher levels of primary care engagement among their veterans with SMI populations.
Methods
We conducted semistructured telephone interviews with a purposeful sample of key informants at VA facilities with high levels of engagement in primary care among veterans with SMI. All project components were conducted by an interdisciplinary team, which included a medical anthropologist (JM), a mental health physician (PR), an internal medicine physician (KC), and other health services researchers (JB, AG). Because the primary objective of the project was QI, this project was designated as nonresearch by the VAGLAHS Institutional Review Board.
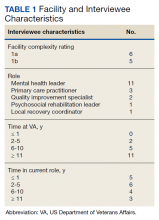
The VA Facility Complexity Model classifies facilities into 5 tiers: 1a (most complex), 1b, 1c, 2, and 3 (least complex), based on patient care volume, patient risk, complexity of clinical programs, and size of research and teaching programs. We sampled informants at VA facilities with complexity ratings of 1a or 1b with better than median scores for primary care engagement of veterans with SMI based on report cards from January 2019 to March 2019. To increase the likelihood of identifying lessons that can generalize to the VAGLAHS with its large population of veterans experiencing homelessness, we selected facilities serving populations consisting of more than 1000 veterans experiencing homelessness.
At each selected facility, we first aimed to interview mental health leaders responsible for quality measurement and improvement identified from a national VA database. We then used snowball sampling to identify other informants at these VA facilities who were knowledgeable about relevant processes. Potential interviewees were contacted via email.
Interviews
The interview guide was developed by the interdisciplinary team and based on published literature about strategies for engaging patients with SMI in care. Interview guide questions focused on local practice arrangements, panel management, population health practices, and quality measurement and improvement efforts for engaging veterans with SMI in primary care (Appendix). Interviews were conducted by telephone, from May 2019 through July 2019, by experienced qualitative interviewers (JM, JB). Interviewees were assured confidentiality of their responses.
Interview audio recordings were used to generate detailed notes (AG). Structured summaries were prepared from these notes, using a template based on the interview guide. We organized these summaries into matrices for analysis, grouping summarized points by interview domains to facilitate comparison across interviews.10-11 Our team reviewed and discussed the matrices, and iteratively identified and defined themes to identify the common engagement approaches and the nature of the connections between mental health and primary care. To ensure rigor, findings were checked by the senior qualitative lead (JM).
Results
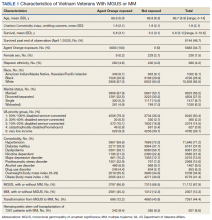
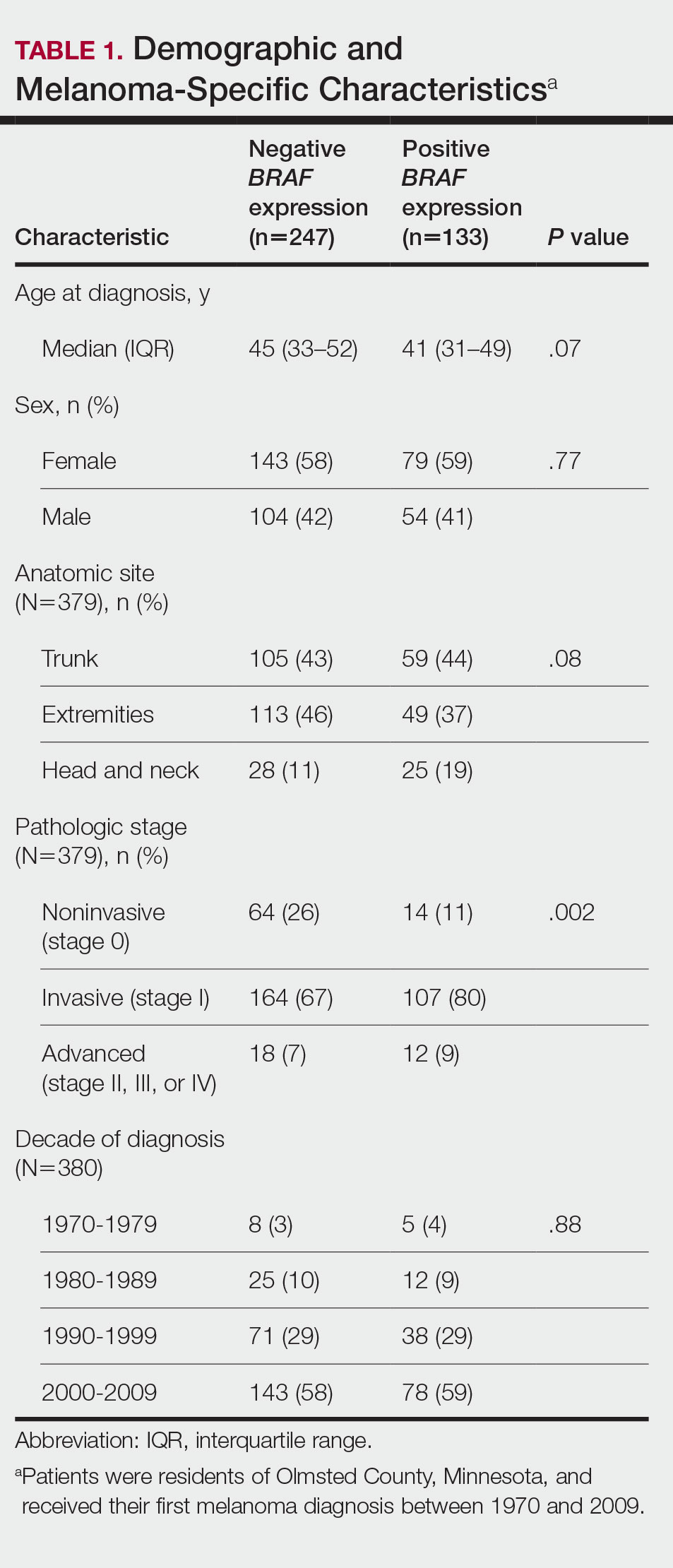
The median SMI engagement score—defined as the proportion of veterans with SMI who have had a primary care visit in the prior 12 months and who have an assigned PCP—was 75.6% across 1a and 1b VA facilities. We identified 16 VA facilities that had a median or higher score and more than 1000 enrolled veterans experiencing homelessness. From these16 facilities, we emailed 31 potential interviewees, 14 of whom were identified from a VA database and 17 referred by other interviewees. In total, we interviewed 18 key informants across 11 (69%) facilities, including chiefs of psychology and mental health services, PCPs with mental health expertise, QI specialists, a psychosocial rehabilitation leader, and a local recovery coordinator, who helps veterans with SMI access recovery-oriented services. Characteristics of the facilities and interviewees are shown in Table 1. Interviews lasted a mean 35 (range, 26-50) minutes.
Engagement Approaches
The strategies used to engage veterans with SMI were heterogenous, with no single strategy common across all facilities. However, we identified 2 categories of engagement approaches: targeted outreach and routine practices.
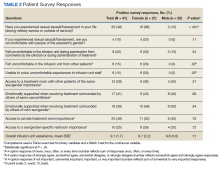
Targeted outreach strategies included deliberate, systematic approaches to reach veterans with SMI outside of regularly scheduled visits. These strategies were designed to be proactive, often prioritizing veterans at risk of disengaging from care. Designated VA care team members identified and reached out to veterans well before 12 months had passed since their prior visit (the VA definition of disengagement from care); visits included any care at VA, including, but not exclusively, primary care. Table 2 describes the key components of targeted outreach strategies: (1) identifying veterans’ last visit; (2) prioritizing which veterans to outreach to; and (3) assigning responsibility and reaching out. A key defining feature of targeted outreach is that veterans were identified and prioritized for outreach independent from any visits with mental health or other VA services.
In identifying veterans at risk for disengagement, a designated employee in mental health or primary care (eg, local recovery coordinator) reviewed a VA dashboard or locally developed report that identified veterans who have not engaged in care for several months. This process was repeated regularly. The designated employee either contacted those veterans directly or coordinated with other clinicians and support staff. When possible, a clinician or nurse with an existing relationship with the veteran would call them. If no such relationship existed, an administrative staff member made a cold call, sometimes accompanied by mailed outreach materials.
Routine practices were business-as-usual activities embedded in regular clinical workflows that facilitated engagement or reengagement of veterans with SMI in care. Of note, and in contrast to targeted outreach, these activities were tied to veteran visits with mental health practitioners. These practices were typically described as being at least as important as targeted outreach efforts. For example, during mental health visits, clinicians routinely checked the VA electronic health record to assess whether veterans had an assigned primary care team. If not, they would contact the primary care service to refer the patient for a primary care visit and assignment. If the patient already had a primary care team assigned, the mental health practitioner checked for recent primary care visits. If none were evident, the mental health practitioner might email the assigned PCP or contact them via instant message.
At some facilities, mental health support staff were able to directly schedule primary care appointments, which was identified as an important enabling factor in promoting mental health patient engagement in primary care. Some interviewees seemed to take for granted the idea that mental health practitioners would help engage patients in primary care—suggesting that these practices had perhaps become a cultural norm within their facility. However, some interviewees identified clear strategies for making these practices a consistent part of care—for example, by designing a protocol for initial mental health assessments to include a routine check for primary care engagement.
Mental Health/Primary Care Connections
Interviewees characterized the nature of the connections between mental health and primary care at their facilities. Nearly all interviewees described that their medical centers had extensive ties, formal and informal, between mental health and primary care.
Formal ties may include the reverse integration care model, in which primary care services are embedded in mental health settings. Interviewees at sites with programs based on this model noted that these programs enabled warm hand-offs from mental health to primary care and suggested that it can foster integration between primary care and mental health care for patients with SMI. However, the size, scope, and structure of these programs varied, sometimes serving a small proportion of a facility’s population of SMI patients. Other examples of formal ties included written agreements, establishing frequent, regular meetings between mental health and primary care leadership and front-line staff, and giving mental health clerks the ability to directly schedule primary care appointments.
Informal ties between mental health and primary care included communication and personal working relationships between mental health and PCPs, facilitated by mental health and primary care leaders working together in workgroups and other administrative activities. Some participants described a history of collaboration between mental health and primary care leaders yielding productive and trusting working relationships. Some interviewees described frequent direct communication between individual mental health practitioners and PCPs—either face-to-face or via secure messaging.
Discussion
VA facilities with high levels of primary care engagement among veterans with SMI used extensive engagement strategies, including a diverse array of targeted outreach and routine practices. In both approaches, intentional organizational structural and process decisions, as well as formal and informal ties between mental health and primary care, established and supported them. In addition, organizational cultural factors were especially relevant to routine practice strategies.
To enable targeted outreach, a bevy of organizational resources, both local and national were required. Large accountable care organizations and integrated delivery systems, like the VA, are often better able to create dashboards and other informational resources for population health management compared with smaller, less integrated health care systems. Though these resources are difficult to create in fragmented systems, comparable tools have been explored by multiple state health departments.12 Our findings suggest that these data tools, though resource intensive to develop, may enable facilities to be more methodical and reliable in conducting outreach to vulnerable patients.
In contrast to targeted outreach, routine practices depend less on population health management resources and more on cultural norms. Such norms are notoriously difficult to change, but intentional structural decisions like embedding primary care engagement in mental health protocols may signal that primary care engagement is an important and legitimate consideration for mental health care.13
We identified extensive and heterogenous connections between mental health and primary care in our sample of VA facilities with high engagement of patients with SMI in primary care. A growing body of literature on relational coordination studies the factors that contribute to organizational siloing and mechanisms for breaking down those silos so work can be coordinated across boundaries (eg, the organizational boundary between mental health and primary care).14 Coordinating care across these boundaries, through good relational coordination practices has been shown to improve outcomes in health care and other sectors. Notably, VA facilities in our sample had several of the defining characteristics of good relational coordination: relationships between mental health and primary care that include shared goals, shared knowledge, and mutual respect, all reinforced by frequent communication structured around problem solving.15 The relational coordination literature also offers a way to identify evidence-based interventions for facilitating relational coordination in places where it is lacking, for example, with information systems, boundary-spanning individuals, facility design, and formal conflict resolution.15 Future work might explore how relational coordination can be further used to optimize mental health and primary care connections to keep veterans with SMI engaged in care.
Our approach of interviewing informants in higher-performing facilities draws heavily on the idea of positive deviance, which holds that information on what works in health care is available from organizations that already are demonstrating “consistently exceptional performance.”16 This approach works best when high performance and organizational characteristics are observable for a large number of facilities, and when high-performing facilities are willing to share their strategies. These features allow investigators to identify promising practices and hypotheses that can then be empirically tested and compared. Such testing, including assessing for unintended consequences, is needed for the approaches we identified. Research is also needed to assess for factors that would promote the implementation of effective strategies.
Limitations
As a QI project seeking to identify promising practices, our interviews were limited to 18 key informants across 11 VA facilities with high engagement of care among veterans with SMI. No inferences can be made that these practices are directly related to this high level of engagement, nor the differential impact of different practices. Future work is needed to assess for these relationships. We also did not interview veterans to understand their perspectives on these strategies, which is an additional important topic for future work. In addition, these interviews were performed before the start of the COVID-19 pandemic. Further work is needed to understand how these strategies may have been modified in response to changes in practice. The shift to care from in-person to virtual services may have impacted both clinical interactions with veterans, as well as between clinicians.
Conclusions
Interviews with key informants demonstrate that while engaging and retaining veterans with SMI in primary care is vital, it also requires intentional and potentially resource-intensive practices, including targeted outreach and routine engagement strategies embedded into mental health visits. These promising practices can provide valuable insights for both VA and community health care systems providing care to patients with SMI.
Acknowledgments
We thank Gracielle J. Tan, MD for administrative assistance in preparing this manuscript.
1. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30-40. doi:10.1002/wps.20384
2. Bowersox NW, Kilbourne AM, Abraham KM, et al. Cause-specific mortality among veterans with serious mental illness lost to follow-up. Gen Hosp Psychiatry. 2012;34(6):651-653. doi:10.1016/j.genhosppsych.2012.05.014
3. Davis CL, Kilbourne AM, Blow FC, et al. Reduced mortality among Department of Veterans Affairs patients with schizophrenia or bipolar disorder lost to follow-up and engaged in active outreach to return for care. Am J Public Health. 2012;102(suppl 1):S74-S79. doi:10.2105/AJPH.2011.300502
4. Copeland LA, Zeber JE, Wang CP, et al. Patterns of primary care and mortality among patients with schizophrenia or diabetes: a cluster analysis approach to the retrospective study of healthcare utilization. BMC Health Serv Res. 2009;9:127. doi:10.1186/1472-6963-9-127
5. Abraham KM, Mach J, Visnic S, McCarthy JF. Enhancing treatment reengagement for veterans with serious mental illness: evaluating the effectiveness of SMI re-engage. Psychiatr Serv. 2018;69(8):887-895. doi:10.1176/appi.ps.201700407
6. Ward MC, Druss BG. Reverse integration initiatives for individuals with serious mental illness. Focus (Am Psychiatr Publ). 2017;15(3):271-278. doi:10.1176/appi.focus.20170011
7. Chang ET, Vinzon M, Cohen AN, Young AS. Effective models urgently needed to improve physical care for people with serious mental illnesses. Health Serv Insights. 2019;12:1178632919837628. Published 2019 Apr 2. doi:10.1177/1178632919837628
8. Gabrielian S, Gordon AJ, Gelberg L, et al. Primary care medical services for homeless veterans. Fed Pract. 2014;31(10):10-19.
9. Lemke S, Boden MT, Kearney LK, et al. Measurement-based management of mental health quality and access in VHA: SAIL mental health domain. Psychol Serv. 2017;14(1):1-12. doi:10.1037/ser0000097
10. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866. doi:10.1177/104973230201200611
11. Zuchowski JL, Chrystal JG, Hamilton AB, et al. Coordinating care across health care systems for Veterans with gynecologic malignancies: a qualitative analysis. Med Care. 2017;55(suppl 1):S53-S60. doi:10.1097/MLR.0000000000000737
12. Daumit GL, Stone EM, Kennedy-Hendricks A, Choksy S, Marsteller JA, McGinty EE. Care coordination and population health management strategies and challenges in a behavioral health home model. Med Care. 2019;57(1):79-84. doi:10.1097/MLR.0000000000001023
13. Parmelli E, Flodgren G, Beyer F, et al. The effectiveness of strategies to change organisational culture to improve healthcare performance: a systematic review. Implement Sci. 2011;6(33):1-8. doi:10.1186/1748-5908-6-33
14. Bolton R, Logan C, Gittell JH. Revisiting relational coordination: a systematic review. J Appl Behav Sci. 2021;57(3):290-322. doi:10.1177/0021886321991597
15. Gittell JH, Godfrey M, Thistlethwaite J. Interprofessional collaborative practice and relational coordination: improving healthcare through relationships. J Interprof Care. 2013;27(3):210-13. doi:10.3109/13561820.2012.730564
16. Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci. 2009;4:25. Published 2009 May 8. doi:10.1186/1748-5908-4-25
People with serious mental illness (SMI) are at substantial risk for premature mortality, dying on average 10 to 20 years earlier than others.1 The reasons for this disparity are complex; however, the high prevalence of chronic disease and physical comorbidities in the SMI population have been identified as prominent factors.2 Engagement and reengagement in care, including primary care for medical comorbidities, can mitigate these mortality risks.2-4 Among veterans with SMI lost to follow-up care for more than 12 months, those not successfully reengaged in care were more likely to die compared with those reengaged in care.2,3
Given this evidence, health care systems, including the US Department of Veterans Affairs (VA), have looked to better engage these patients in care. These efforts have included mental health population health management, colocation of mental health with primary care, designation of primary care teams specializing in SMI, and integration of mental health and primary care services for patients experiencing homelessness.5-8
As part of a national approach to encourage locally driven quality improvement (QI), the VA compiles performance metrics for each facility, across a gamut of care settings, conditions, and veteran populations.9 Quarterly facility report cards, with longitudinal data and cross-facility comparisons, enable facilities to identify targets for QI and track improvement progress. One metric reports on the proportion of enrolled veterans with SMI who have primary care engagement, defined as having an assigned primary care practitioner (PCP) and a primary care visit in the prior 12 months.
In support of a QI initiative at the VA Greater Los Angeles Healthcare System (VAGLAHS), we sought to describe promising practices being utilized by VA facilities with higher levels of primary care engagement among their veterans with SMI populations.
Methods
We conducted semistructured telephone interviews with a purposeful sample of key informants at VA facilities with high levels of engagement in primary care among veterans with SMI. All project components were conducted by an interdisciplinary team, which included a medical anthropologist (JM), a mental health physician (PR), an internal medicine physician (KC), and other health services researchers (JB, AG). Because the primary objective of the project was QI, this project was designated as nonresearch by the VAGLAHS Institutional Review Board.
The VA Facility Complexity Model classifies facilities into 5 tiers: 1a (most complex), 1b, 1c, 2, and 3 (least complex), based on patient care volume, patient risk, complexity of clinical programs, and size of research and teaching programs. We sampled informants at VA facilities with complexity ratings of 1a or 1b with better than median scores for primary care engagement of veterans with SMI based on report cards from January 2019 to March 2019. To increase the likelihood of identifying lessons that can generalize to the VAGLAHS with its large population of veterans experiencing homelessness, we selected facilities serving populations consisting of more than 1000 veterans experiencing homelessness.
At each selected facility, we first aimed to interview mental health leaders responsible for quality measurement and improvement identified from a national VA database. We then used snowball sampling to identify other informants at these VA facilities who were knowledgeable about relevant processes. Potential interviewees were contacted via email.
Interviews
The interview guide was developed by the interdisciplinary team and based on published literature about strategies for engaging patients with SMI in care. Interview guide questions focused on local practice arrangements, panel management, population health practices, and quality measurement and improvement efforts for engaging veterans with SMI in primary care (Appendix). Interviews were conducted by telephone, from May 2019 through July 2019, by experienced qualitative interviewers (JM, JB). Interviewees were assured confidentiality of their responses.
Interview audio recordings were used to generate detailed notes (AG). Structured summaries were prepared from these notes, using a template based on the interview guide. We organized these summaries into matrices for analysis, grouping summarized points by interview domains to facilitate comparison across interviews.10-11 Our team reviewed and discussed the matrices, and iteratively identified and defined themes to identify the common engagement approaches and the nature of the connections between mental health and primary care. To ensure rigor, findings were checked by the senior qualitative lead (JM).
Results
The median SMI engagement score—defined as the proportion of veterans with SMI who have had a primary care visit in the prior 12 months and who have an assigned PCP—was 75.6% across 1a and 1b VA facilities. We identified 16 VA facilities that had a median or higher score and more than 1000 enrolled veterans experiencing homelessness. From these16 facilities, we emailed 31 potential interviewees, 14 of whom were identified from a VA database and 17 referred by other interviewees. In total, we interviewed 18 key informants across 11 (69%) facilities, including chiefs of psychology and mental health services, PCPs with mental health expertise, QI specialists, a psychosocial rehabilitation leader, and a local recovery coordinator, who helps veterans with SMI access recovery-oriented services. Characteristics of the facilities and interviewees are shown in Table 1. Interviews lasted a mean 35 (range, 26-50) minutes.
Engagement Approaches
The strategies used to engage veterans with SMI were heterogenous, with no single strategy common across all facilities. However, we identified 2 categories of engagement approaches: targeted outreach and routine practices.
Targeted outreach strategies included deliberate, systematic approaches to reach veterans with SMI outside of regularly scheduled visits. These strategies were designed to be proactive, often prioritizing veterans at risk of disengaging from care. Designated VA care team members identified and reached out to veterans well before 12 months had passed since their prior visit (the VA definition of disengagement from care); visits included any care at VA, including, but not exclusively, primary care. Table 2 describes the key components of targeted outreach strategies: (1) identifying veterans’ last visit; (2) prioritizing which veterans to outreach to; and (3) assigning responsibility and reaching out. A key defining feature of targeted outreach is that veterans were identified and prioritized for outreach independent from any visits with mental health or other VA services.
In identifying veterans at risk for disengagement, a designated employee in mental health or primary care (eg, local recovery coordinator) reviewed a VA dashboard or locally developed report that identified veterans who have not engaged in care for several months. This process was repeated regularly. The designated employee either contacted those veterans directly or coordinated with other clinicians and support staff. When possible, a clinician or nurse with an existing relationship with the veteran would call them. If no such relationship existed, an administrative staff member made a cold call, sometimes accompanied by mailed outreach materials.
Routine practices were business-as-usual activities embedded in regular clinical workflows that facilitated engagement or reengagement of veterans with SMI in care. Of note, and in contrast to targeted outreach, these activities were tied to veteran visits with mental health practitioners. These practices were typically described as being at least as important as targeted outreach efforts. For example, during mental health visits, clinicians routinely checked the VA electronic health record to assess whether veterans had an assigned primary care team. If not, they would contact the primary care service to refer the patient for a primary care visit and assignment. If the patient already had a primary care team assigned, the mental health practitioner checked for recent primary care visits. If none were evident, the mental health practitioner might email the assigned PCP or contact them via instant message.
At some facilities, mental health support staff were able to directly schedule primary care appointments, which was identified as an important enabling factor in promoting mental health patient engagement in primary care. Some interviewees seemed to take for granted the idea that mental health practitioners would help engage patients in primary care—suggesting that these practices had perhaps become a cultural norm within their facility. However, some interviewees identified clear strategies for making these practices a consistent part of care—for example, by designing a protocol for initial mental health assessments to include a routine check for primary care engagement.
Mental Health/Primary Care Connections
Interviewees characterized the nature of the connections between mental health and primary care at their facilities. Nearly all interviewees described that their medical centers had extensive ties, formal and informal, between mental health and primary care.
Formal ties may include the reverse integration care model, in which primary care services are embedded in mental health settings. Interviewees at sites with programs based on this model noted that these programs enabled warm hand-offs from mental health to primary care and suggested that it can foster integration between primary care and mental health care for patients with SMI. However, the size, scope, and structure of these programs varied, sometimes serving a small proportion of a facility’s population of SMI patients. Other examples of formal ties included written agreements, establishing frequent, regular meetings between mental health and primary care leadership and front-line staff, and giving mental health clerks the ability to directly schedule primary care appointments.
Informal ties between mental health and primary care included communication and personal working relationships between mental health and PCPs, facilitated by mental health and primary care leaders working together in workgroups and other administrative activities. Some participants described a history of collaboration between mental health and primary care leaders yielding productive and trusting working relationships. Some interviewees described frequent direct communication between individual mental health practitioners and PCPs—either face-to-face or via secure messaging.
Discussion
VA facilities with high levels of primary care engagement among veterans with SMI used extensive engagement strategies, including a diverse array of targeted outreach and routine practices. In both approaches, intentional organizational structural and process decisions, as well as formal and informal ties between mental health and primary care, established and supported them. In addition, organizational cultural factors were especially relevant to routine practice strategies.
To enable targeted outreach, a bevy of organizational resources, both local and national were required. Large accountable care organizations and integrated delivery systems, like the VA, are often better able to create dashboards and other informational resources for population health management compared with smaller, less integrated health care systems. Though these resources are difficult to create in fragmented systems, comparable tools have been explored by multiple state health departments.12 Our findings suggest that these data tools, though resource intensive to develop, may enable facilities to be more methodical and reliable in conducting outreach to vulnerable patients.
In contrast to targeted outreach, routine practices depend less on population health management resources and more on cultural norms. Such norms are notoriously difficult to change, but intentional structural decisions like embedding primary care engagement in mental health protocols may signal that primary care engagement is an important and legitimate consideration for mental health care.13
We identified extensive and heterogenous connections between mental health and primary care in our sample of VA facilities with high engagement of patients with SMI in primary care. A growing body of literature on relational coordination studies the factors that contribute to organizational siloing and mechanisms for breaking down those silos so work can be coordinated across boundaries (eg, the organizational boundary between mental health and primary care).14 Coordinating care across these boundaries, through good relational coordination practices has been shown to improve outcomes in health care and other sectors. Notably, VA facilities in our sample had several of the defining characteristics of good relational coordination: relationships between mental health and primary care that include shared goals, shared knowledge, and mutual respect, all reinforced by frequent communication structured around problem solving.15 The relational coordination literature also offers a way to identify evidence-based interventions for facilitating relational coordination in places where it is lacking, for example, with information systems, boundary-spanning individuals, facility design, and formal conflict resolution.15 Future work might explore how relational coordination can be further used to optimize mental health and primary care connections to keep veterans with SMI engaged in care.
Our approach of interviewing informants in higher-performing facilities draws heavily on the idea of positive deviance, which holds that information on what works in health care is available from organizations that already are demonstrating “consistently exceptional performance.”16 This approach works best when high performance and organizational characteristics are observable for a large number of facilities, and when high-performing facilities are willing to share their strategies. These features allow investigators to identify promising practices and hypotheses that can then be empirically tested and compared. Such testing, including assessing for unintended consequences, is needed for the approaches we identified. Research is also needed to assess for factors that would promote the implementation of effective strategies.
Limitations
As a QI project seeking to identify promising practices, our interviews were limited to 18 key informants across 11 VA facilities with high engagement of care among veterans with SMI. No inferences can be made that these practices are directly related to this high level of engagement, nor the differential impact of different practices. Future work is needed to assess for these relationships. We also did not interview veterans to understand their perspectives on these strategies, which is an additional important topic for future work. In addition, these interviews were performed before the start of the COVID-19 pandemic. Further work is needed to understand how these strategies may have been modified in response to changes in practice. The shift to care from in-person to virtual services may have impacted both clinical interactions with veterans, as well as between clinicians.
Conclusions
Interviews with key informants demonstrate that while engaging and retaining veterans with SMI in primary care is vital, it also requires intentional and potentially resource-intensive practices, including targeted outreach and routine engagement strategies embedded into mental health visits. These promising practices can provide valuable insights for both VA and community health care systems providing care to patients with SMI.
Acknowledgments
We thank Gracielle J. Tan, MD for administrative assistance in preparing this manuscript.
People with serious mental illness (SMI) are at substantial risk for premature mortality, dying on average 10 to 20 years earlier than others.1 The reasons for this disparity are complex; however, the high prevalence of chronic disease and physical comorbidities in the SMI population have been identified as prominent factors.2 Engagement and reengagement in care, including primary care for medical comorbidities, can mitigate these mortality risks.2-4 Among veterans with SMI lost to follow-up care for more than 12 months, those not successfully reengaged in care were more likely to die compared with those reengaged in care.2,3
Given this evidence, health care systems, including the US Department of Veterans Affairs (VA), have looked to better engage these patients in care. These efforts have included mental health population health management, colocation of mental health with primary care, designation of primary care teams specializing in SMI, and integration of mental health and primary care services for patients experiencing homelessness.5-8
As part of a national approach to encourage locally driven quality improvement (QI), the VA compiles performance metrics for each facility, across a gamut of care settings, conditions, and veteran populations.9 Quarterly facility report cards, with longitudinal data and cross-facility comparisons, enable facilities to identify targets for QI and track improvement progress. One metric reports on the proportion of enrolled veterans with SMI who have primary care engagement, defined as having an assigned primary care practitioner (PCP) and a primary care visit in the prior 12 months.
In support of a QI initiative at the VA Greater Los Angeles Healthcare System (VAGLAHS), we sought to describe promising practices being utilized by VA facilities with higher levels of primary care engagement among their veterans with SMI populations.
Methods
We conducted semistructured telephone interviews with a purposeful sample of key informants at VA facilities with high levels of engagement in primary care among veterans with SMI. All project components were conducted by an interdisciplinary team, which included a medical anthropologist (JM), a mental health physician (PR), an internal medicine physician (KC), and other health services researchers (JB, AG). Because the primary objective of the project was QI, this project was designated as nonresearch by the VAGLAHS Institutional Review Board.
The VA Facility Complexity Model classifies facilities into 5 tiers: 1a (most complex), 1b, 1c, 2, and 3 (least complex), based on patient care volume, patient risk, complexity of clinical programs, and size of research and teaching programs. We sampled informants at VA facilities with complexity ratings of 1a or 1b with better than median scores for primary care engagement of veterans with SMI based on report cards from January 2019 to March 2019. To increase the likelihood of identifying lessons that can generalize to the VAGLAHS with its large population of veterans experiencing homelessness, we selected facilities serving populations consisting of more than 1000 veterans experiencing homelessness.
At each selected facility, we first aimed to interview mental health leaders responsible for quality measurement and improvement identified from a national VA database. We then used snowball sampling to identify other informants at these VA facilities who were knowledgeable about relevant processes. Potential interviewees were contacted via email.
Interviews
The interview guide was developed by the interdisciplinary team and based on published literature about strategies for engaging patients with SMI in care. Interview guide questions focused on local practice arrangements, panel management, population health practices, and quality measurement and improvement efforts for engaging veterans with SMI in primary care (Appendix). Interviews were conducted by telephone, from May 2019 through July 2019, by experienced qualitative interviewers (JM, JB). Interviewees were assured confidentiality of their responses.
Interview audio recordings were used to generate detailed notes (AG). Structured summaries were prepared from these notes, using a template based on the interview guide. We organized these summaries into matrices for analysis, grouping summarized points by interview domains to facilitate comparison across interviews.10-11 Our team reviewed and discussed the matrices, and iteratively identified and defined themes to identify the common engagement approaches and the nature of the connections between mental health and primary care. To ensure rigor, findings were checked by the senior qualitative lead (JM).
Results
The median SMI engagement score—defined as the proportion of veterans with SMI who have had a primary care visit in the prior 12 months and who have an assigned PCP—was 75.6% across 1a and 1b VA facilities. We identified 16 VA facilities that had a median or higher score and more than 1000 enrolled veterans experiencing homelessness. From these16 facilities, we emailed 31 potential interviewees, 14 of whom were identified from a VA database and 17 referred by other interviewees. In total, we interviewed 18 key informants across 11 (69%) facilities, including chiefs of psychology and mental health services, PCPs with mental health expertise, QI specialists, a psychosocial rehabilitation leader, and a local recovery coordinator, who helps veterans with SMI access recovery-oriented services. Characteristics of the facilities and interviewees are shown in Table 1. Interviews lasted a mean 35 (range, 26-50) minutes.
Engagement Approaches
The strategies used to engage veterans with SMI were heterogenous, with no single strategy common across all facilities. However, we identified 2 categories of engagement approaches: targeted outreach and routine practices.
Targeted outreach strategies included deliberate, systematic approaches to reach veterans with SMI outside of regularly scheduled visits. These strategies were designed to be proactive, often prioritizing veterans at risk of disengaging from care. Designated VA care team members identified and reached out to veterans well before 12 months had passed since their prior visit (the VA definition of disengagement from care); visits included any care at VA, including, but not exclusively, primary care. Table 2 describes the key components of targeted outreach strategies: (1) identifying veterans’ last visit; (2) prioritizing which veterans to outreach to; and (3) assigning responsibility and reaching out. A key defining feature of targeted outreach is that veterans were identified and prioritized for outreach independent from any visits with mental health or other VA services.
In identifying veterans at risk for disengagement, a designated employee in mental health or primary care (eg, local recovery coordinator) reviewed a VA dashboard or locally developed report that identified veterans who have not engaged in care for several months. This process was repeated regularly. The designated employee either contacted those veterans directly or coordinated with other clinicians and support staff. When possible, a clinician or nurse with an existing relationship with the veteran would call them. If no such relationship existed, an administrative staff member made a cold call, sometimes accompanied by mailed outreach materials.
Routine practices were business-as-usual activities embedded in regular clinical workflows that facilitated engagement or reengagement of veterans with SMI in care. Of note, and in contrast to targeted outreach, these activities were tied to veteran visits with mental health practitioners. These practices were typically described as being at least as important as targeted outreach efforts. For example, during mental health visits, clinicians routinely checked the VA electronic health record to assess whether veterans had an assigned primary care team. If not, they would contact the primary care service to refer the patient for a primary care visit and assignment. If the patient already had a primary care team assigned, the mental health practitioner checked for recent primary care visits. If none were evident, the mental health practitioner might email the assigned PCP or contact them via instant message.
At some facilities, mental health support staff were able to directly schedule primary care appointments, which was identified as an important enabling factor in promoting mental health patient engagement in primary care. Some interviewees seemed to take for granted the idea that mental health practitioners would help engage patients in primary care—suggesting that these practices had perhaps become a cultural norm within their facility. However, some interviewees identified clear strategies for making these practices a consistent part of care—for example, by designing a protocol for initial mental health assessments to include a routine check for primary care engagement.
Mental Health/Primary Care Connections
Interviewees characterized the nature of the connections between mental health and primary care at their facilities. Nearly all interviewees described that their medical centers had extensive ties, formal and informal, between mental health and primary care.
Formal ties may include the reverse integration care model, in which primary care services are embedded in mental health settings. Interviewees at sites with programs based on this model noted that these programs enabled warm hand-offs from mental health to primary care and suggested that it can foster integration between primary care and mental health care for patients with SMI. However, the size, scope, and structure of these programs varied, sometimes serving a small proportion of a facility’s population of SMI patients. Other examples of formal ties included written agreements, establishing frequent, regular meetings between mental health and primary care leadership and front-line staff, and giving mental health clerks the ability to directly schedule primary care appointments.
Informal ties between mental health and primary care included communication and personal working relationships between mental health and PCPs, facilitated by mental health and primary care leaders working together in workgroups and other administrative activities. Some participants described a history of collaboration between mental health and primary care leaders yielding productive and trusting working relationships. Some interviewees described frequent direct communication between individual mental health practitioners and PCPs—either face-to-face or via secure messaging.
Discussion
VA facilities with high levels of primary care engagement among veterans with SMI used extensive engagement strategies, including a diverse array of targeted outreach and routine practices. In both approaches, intentional organizational structural and process decisions, as well as formal and informal ties between mental health and primary care, established and supported them. In addition, organizational cultural factors were especially relevant to routine practice strategies.
To enable targeted outreach, a bevy of organizational resources, both local and national were required. Large accountable care organizations and integrated delivery systems, like the VA, are often better able to create dashboards and other informational resources for population health management compared with smaller, less integrated health care systems. Though these resources are difficult to create in fragmented systems, comparable tools have been explored by multiple state health departments.12 Our findings suggest that these data tools, though resource intensive to develop, may enable facilities to be more methodical and reliable in conducting outreach to vulnerable patients.
In contrast to targeted outreach, routine practices depend less on population health management resources and more on cultural norms. Such norms are notoriously difficult to change, but intentional structural decisions like embedding primary care engagement in mental health protocols may signal that primary care engagement is an important and legitimate consideration for mental health care.13
We identified extensive and heterogenous connections between mental health and primary care in our sample of VA facilities with high engagement of patients with SMI in primary care. A growing body of literature on relational coordination studies the factors that contribute to organizational siloing and mechanisms for breaking down those silos so work can be coordinated across boundaries (eg, the organizational boundary between mental health and primary care).14 Coordinating care across these boundaries, through good relational coordination practices has been shown to improve outcomes in health care and other sectors. Notably, VA facilities in our sample had several of the defining characteristics of good relational coordination: relationships between mental health and primary care that include shared goals, shared knowledge, and mutual respect, all reinforced by frequent communication structured around problem solving.15 The relational coordination literature also offers a way to identify evidence-based interventions for facilitating relational coordination in places where it is lacking, for example, with information systems, boundary-spanning individuals, facility design, and formal conflict resolution.15 Future work might explore how relational coordination can be further used to optimize mental health and primary care connections to keep veterans with SMI engaged in care.
Our approach of interviewing informants in higher-performing facilities draws heavily on the idea of positive deviance, which holds that information on what works in health care is available from organizations that already are demonstrating “consistently exceptional performance.”16 This approach works best when high performance and organizational characteristics are observable for a large number of facilities, and when high-performing facilities are willing to share their strategies. These features allow investigators to identify promising practices and hypotheses that can then be empirically tested and compared. Such testing, including assessing for unintended consequences, is needed for the approaches we identified. Research is also needed to assess for factors that would promote the implementation of effective strategies.
Limitations
As a QI project seeking to identify promising practices, our interviews were limited to 18 key informants across 11 VA facilities with high engagement of care among veterans with SMI. No inferences can be made that these practices are directly related to this high level of engagement, nor the differential impact of different practices. Future work is needed to assess for these relationships. We also did not interview veterans to understand their perspectives on these strategies, which is an additional important topic for future work. In addition, these interviews were performed before the start of the COVID-19 pandemic. Further work is needed to understand how these strategies may have been modified in response to changes in practice. The shift to care from in-person to virtual services may have impacted both clinical interactions with veterans, as well as between clinicians.
Conclusions
Interviews with key informants demonstrate that while engaging and retaining veterans with SMI in primary care is vital, it also requires intentional and potentially resource-intensive practices, including targeted outreach and routine engagement strategies embedded into mental health visits. These promising practices can provide valuable insights for both VA and community health care systems providing care to patients with SMI.
Acknowledgments
We thank Gracielle J. Tan, MD for administrative assistance in preparing this manuscript.
1. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30-40. doi:10.1002/wps.20384
2. Bowersox NW, Kilbourne AM, Abraham KM, et al. Cause-specific mortality among veterans with serious mental illness lost to follow-up. Gen Hosp Psychiatry. 2012;34(6):651-653. doi:10.1016/j.genhosppsych.2012.05.014
3. Davis CL, Kilbourne AM, Blow FC, et al. Reduced mortality among Department of Veterans Affairs patients with schizophrenia or bipolar disorder lost to follow-up and engaged in active outreach to return for care. Am J Public Health. 2012;102(suppl 1):S74-S79. doi:10.2105/AJPH.2011.300502
4. Copeland LA, Zeber JE, Wang CP, et al. Patterns of primary care and mortality among patients with schizophrenia or diabetes: a cluster analysis approach to the retrospective study of healthcare utilization. BMC Health Serv Res. 2009;9:127. doi:10.1186/1472-6963-9-127
5. Abraham KM, Mach J, Visnic S, McCarthy JF. Enhancing treatment reengagement for veterans with serious mental illness: evaluating the effectiveness of SMI re-engage. Psychiatr Serv. 2018;69(8):887-895. doi:10.1176/appi.ps.201700407
6. Ward MC, Druss BG. Reverse integration initiatives for individuals with serious mental illness. Focus (Am Psychiatr Publ). 2017;15(3):271-278. doi:10.1176/appi.focus.20170011
7. Chang ET, Vinzon M, Cohen AN, Young AS. Effective models urgently needed to improve physical care for people with serious mental illnesses. Health Serv Insights. 2019;12:1178632919837628. Published 2019 Apr 2. doi:10.1177/1178632919837628
8. Gabrielian S, Gordon AJ, Gelberg L, et al. Primary care medical services for homeless veterans. Fed Pract. 2014;31(10):10-19.
9. Lemke S, Boden MT, Kearney LK, et al. Measurement-based management of mental health quality and access in VHA: SAIL mental health domain. Psychol Serv. 2017;14(1):1-12. doi:10.1037/ser0000097
10. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866. doi:10.1177/104973230201200611
11. Zuchowski JL, Chrystal JG, Hamilton AB, et al. Coordinating care across health care systems for Veterans with gynecologic malignancies: a qualitative analysis. Med Care. 2017;55(suppl 1):S53-S60. doi:10.1097/MLR.0000000000000737
12. Daumit GL, Stone EM, Kennedy-Hendricks A, Choksy S, Marsteller JA, McGinty EE. Care coordination and population health management strategies and challenges in a behavioral health home model. Med Care. 2019;57(1):79-84. doi:10.1097/MLR.0000000000001023
13. Parmelli E, Flodgren G, Beyer F, et al. The effectiveness of strategies to change organisational culture to improve healthcare performance: a systematic review. Implement Sci. 2011;6(33):1-8. doi:10.1186/1748-5908-6-33
14. Bolton R, Logan C, Gittell JH. Revisiting relational coordination: a systematic review. J Appl Behav Sci. 2021;57(3):290-322. doi:10.1177/0021886321991597
15. Gittell JH, Godfrey M, Thistlethwaite J. Interprofessional collaborative practice and relational coordination: improving healthcare through relationships. J Interprof Care. 2013;27(3):210-13. doi:10.3109/13561820.2012.730564
16. Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci. 2009;4:25. Published 2009 May 8. doi:10.1186/1748-5908-4-25
1. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30-40. doi:10.1002/wps.20384
2. Bowersox NW, Kilbourne AM, Abraham KM, et al. Cause-specific mortality among veterans with serious mental illness lost to follow-up. Gen Hosp Psychiatry. 2012;34(6):651-653. doi:10.1016/j.genhosppsych.2012.05.014
3. Davis CL, Kilbourne AM, Blow FC, et al. Reduced mortality among Department of Veterans Affairs patients with schizophrenia or bipolar disorder lost to follow-up and engaged in active outreach to return for care. Am J Public Health. 2012;102(suppl 1):S74-S79. doi:10.2105/AJPH.2011.300502
4. Copeland LA, Zeber JE, Wang CP, et al. Patterns of primary care and mortality among patients with schizophrenia or diabetes: a cluster analysis approach to the retrospective study of healthcare utilization. BMC Health Serv Res. 2009;9:127. doi:10.1186/1472-6963-9-127
5. Abraham KM, Mach J, Visnic S, McCarthy JF. Enhancing treatment reengagement for veterans with serious mental illness: evaluating the effectiveness of SMI re-engage. Psychiatr Serv. 2018;69(8):887-895. doi:10.1176/appi.ps.201700407
6. Ward MC, Druss BG. Reverse integration initiatives for individuals with serious mental illness. Focus (Am Psychiatr Publ). 2017;15(3):271-278. doi:10.1176/appi.focus.20170011
7. Chang ET, Vinzon M, Cohen AN, Young AS. Effective models urgently needed to improve physical care for people with serious mental illnesses. Health Serv Insights. 2019;12:1178632919837628. Published 2019 Apr 2. doi:10.1177/1178632919837628
8. Gabrielian S, Gordon AJ, Gelberg L, et al. Primary care medical services for homeless veterans. Fed Pract. 2014;31(10):10-19.
9. Lemke S, Boden MT, Kearney LK, et al. Measurement-based management of mental health quality and access in VHA: SAIL mental health domain. Psychol Serv. 2017;14(1):1-12. doi:10.1037/ser0000097
10. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866. doi:10.1177/104973230201200611
11. Zuchowski JL, Chrystal JG, Hamilton AB, et al. Coordinating care across health care systems for Veterans with gynecologic malignancies: a qualitative analysis. Med Care. 2017;55(suppl 1):S53-S60. doi:10.1097/MLR.0000000000000737
12. Daumit GL, Stone EM, Kennedy-Hendricks A, Choksy S, Marsteller JA, McGinty EE. Care coordination and population health management strategies and challenges in a behavioral health home model. Med Care. 2019;57(1):79-84. doi:10.1097/MLR.0000000000001023
13. Parmelli E, Flodgren G, Beyer F, et al. The effectiveness of strategies to change organisational culture to improve healthcare performance: a systematic review. Implement Sci. 2011;6(33):1-8. doi:10.1186/1748-5908-6-33
14. Bolton R, Logan C, Gittell JH. Revisiting relational coordination: a systematic review. J Appl Behav Sci. 2021;57(3):290-322. doi:10.1177/0021886321991597
15. Gittell JH, Godfrey M, Thistlethwaite J. Interprofessional collaborative practice and relational coordination: improving healthcare through relationships. J Interprof Care. 2013;27(3):210-13. doi:10.3109/13561820.2012.730564
16. Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci. 2009;4:25. Published 2009 May 8. doi:10.1186/1748-5908-4-25
Association of BRAF V600E Status of Incident Melanoma and Risk for a Second Primary Malignancy: A Population-Based Study
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
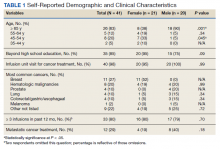
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results
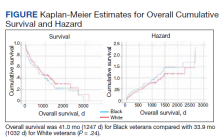
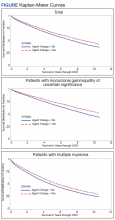
Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.
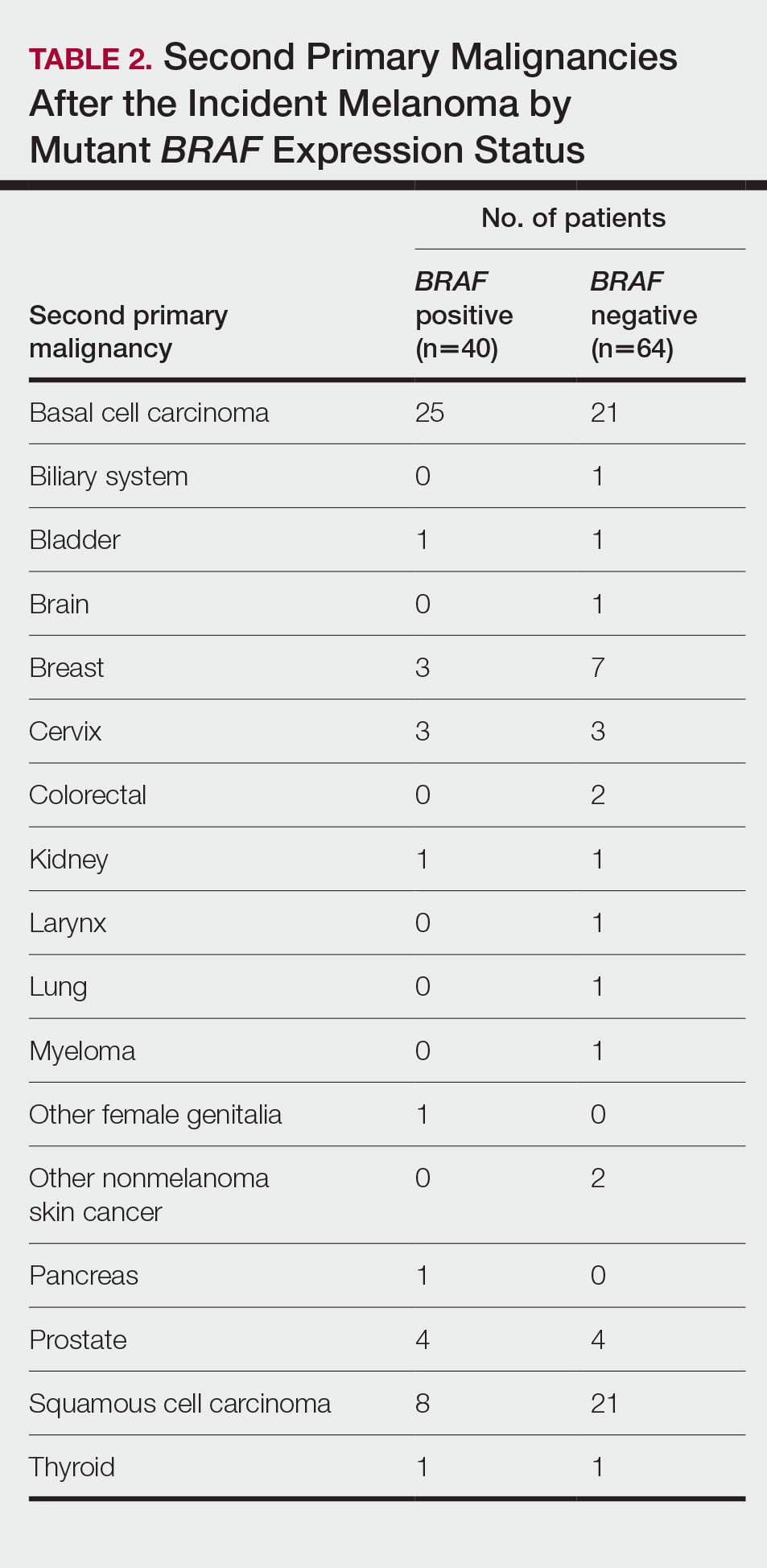
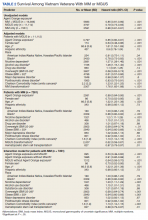
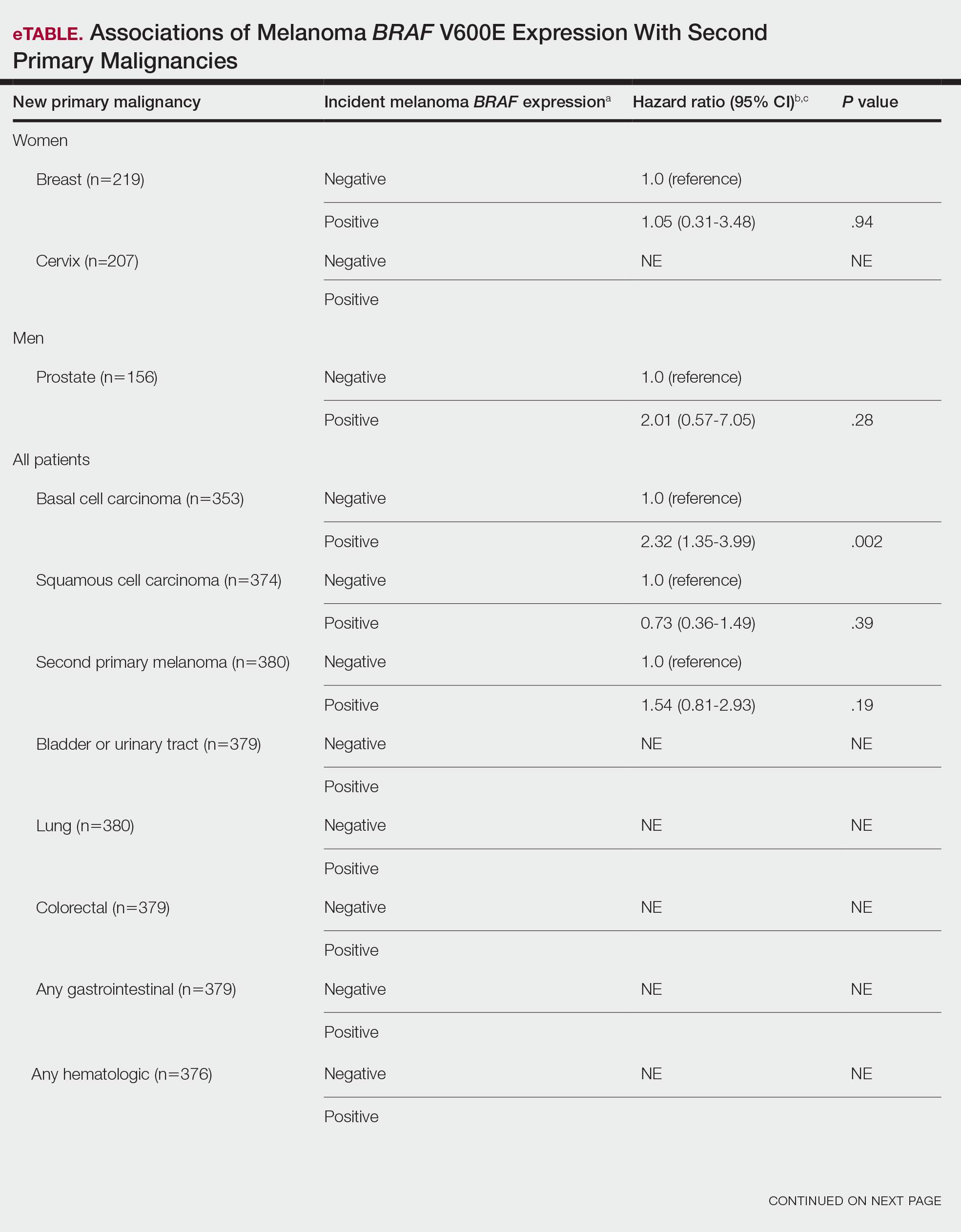
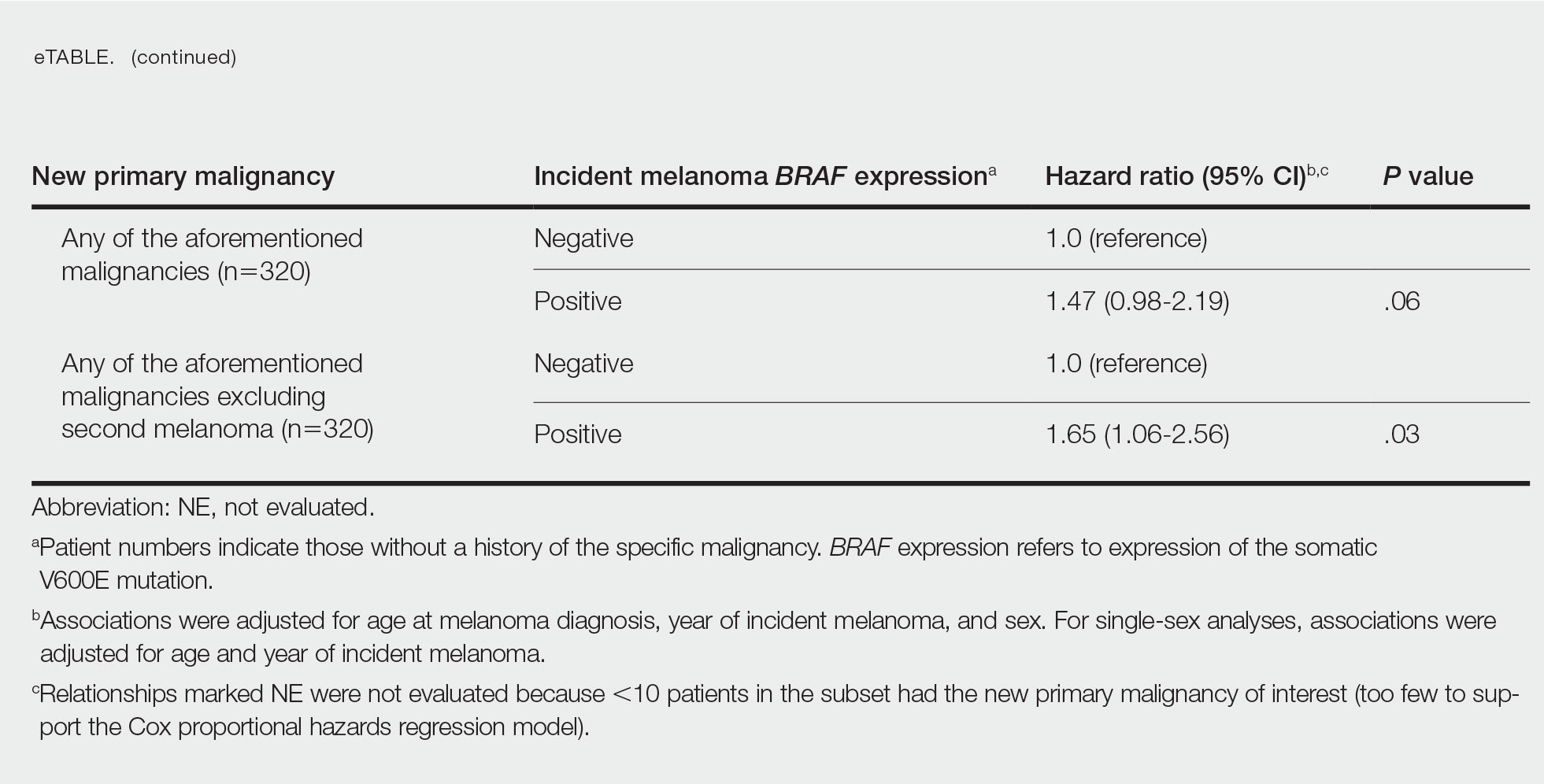
BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.
Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.
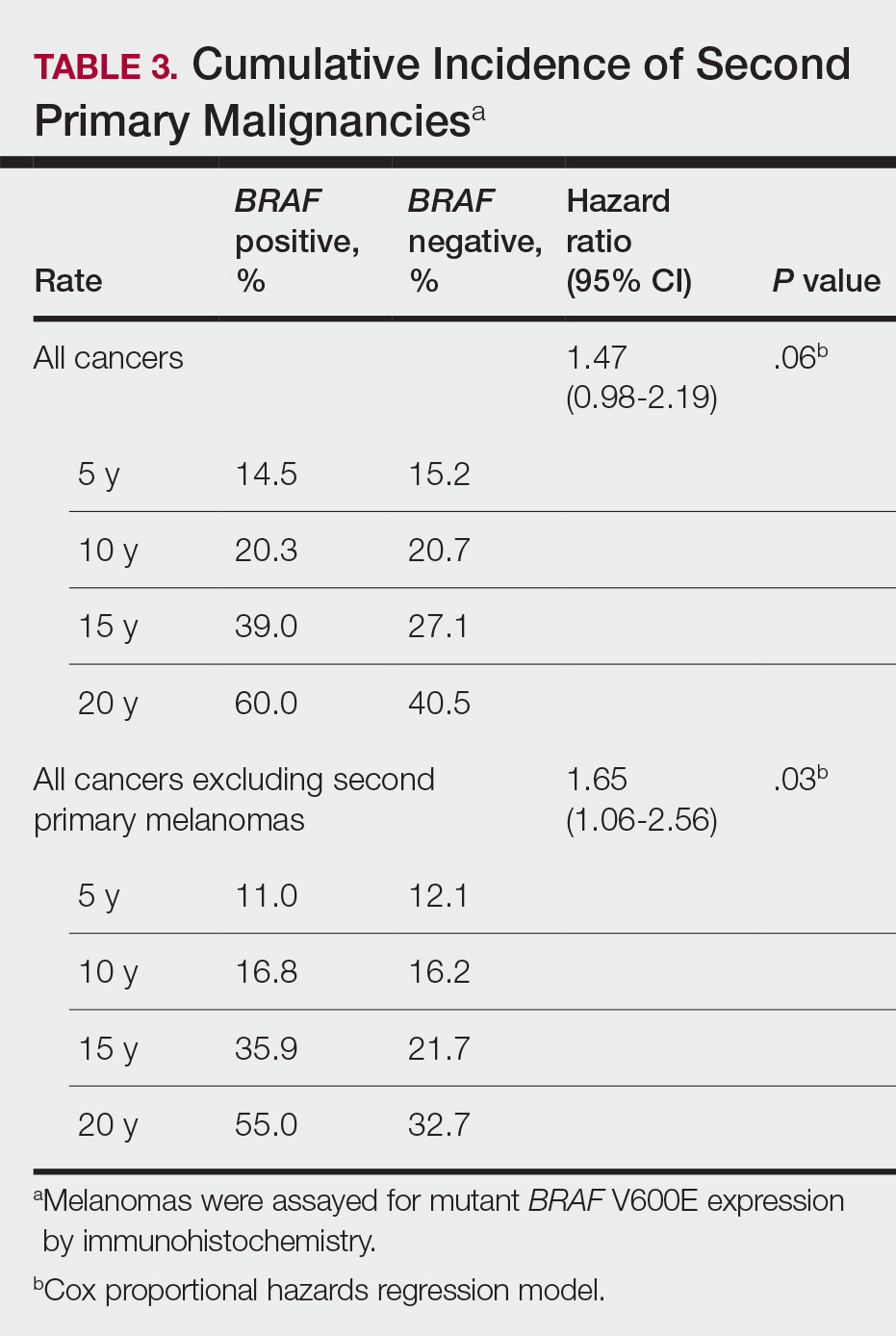
The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results

Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.

BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.


Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.

The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results

Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.

BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.


Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.

The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
Practice Points
- Dermatologists should be aware of the long-term risk of second primary malignancies after an incident melanoma.
- BRAF mutations occur in melanomas and several other cancers. Our study found that melanoma BRAF V600E expression is associated with an increased risk for basal cell carcinomas.
Gender and Patient Satisfaction in a Veterans Health Administration Outpatient Chemotherapy Unit
Gender differences in patient satisfaction with medical care have been evaluated in multiple settings; however, studies specific to the unique population of women veterans with cancer are lacking. Women are reported to value privacy, psychosocial support, and communication to a higher degree compared with men.1 Factors affecting satisfaction include the following: discomfort in sharing treatment rooms with the opposite gender, a desire for privacy with treatment and restroom use, anatomic or illness differences, and a personal history of abuse.2-4 Regrettably, up to 1 in 3 women in the United States are victims of sexual trauma in their lifetimes, and up to 1 in 4 women in the military are victims of military sexual trauma. Incidence in both settings is suspected to be higher due to underreporting.5,6
Chemotherapy treatment units are often uniquely designed as an open space, with several patients sharing a treatment area. The design reduces isolation and facilitates quick nurse-patient access during potentially toxic treatments known to have frequent adverse effects. Data suggest that nursing staff prefer open models to facilitate quick patient assessments and interventions as needed; however, patients and families prefer private treatment rooms, especially among women patients or those receiving longer infusions.7
The Veterans Health Administration (VHA) patient population is male predominant, comprised only of 10% female patients.8 Although the proportion of female patients in the VHA is expected to rise annually to about 16% by 2043, the low percentage of female veterans will persist for the foreseeable future.8 This low percentage of female veterans is reflected in the Veterans Affairs Portland Health Care System (VAPHCS) cancer patient population and in the use of the chemotherapy infusion unit, which is used for the ambulatory treatment of veterans undergoing cancer therapy.
The VHA has previously explored gender differences in health care, such as with cardiovascular disease, transgender care, and access to mental health.9-11 However, to the best of our knowledge, no analysis has explored gender differences within the outpatient cancer treatment experience. Patient satisfaction with outpatient cancer care may be magnified in the VHA setting due to the uniquely unequal gender populations, shared treatment space design, and high incidence of sexual abuse among women veterans. Given this, we aimed to identify gender-related preferences in outpatient cancer care in our chemotherapy infusion unit.
In our study, we used the terms male and female to reflect statistical data from the literature or labeled data from the electronic health record (EHR); whereas the terms men and women were used to describe and encompass the cultural implications and context of gender.12
Methods
This study was designated as a quality improvement (QI) project by the VAPHCS research office and Institutional Review Board in accordance with VHA policies.
The VAPHCS outpatient chemotherapy infusion unit is designed with 6 rooms for chemotherapy administration. One room is a large open space with 6 chairs for patients. The other rooms are smaller with glass dividers between the rooms, and 3 chairs inside each for patients. There are 2 private bathrooms, each gender neutral. Direct patient care is provided by physicians, nurse practitioners (NPs), infusion unit nurses, and nurse coordinators. Men represent the majority of hematology and oncology physicians (13 of 20 total: 5 women fellow physicians and 2 women attending physicians), and 2 of 4 NPs. Women represent 10 of 12 infusion unit and cancer coordinator nurses. We used the VHA Computerized Patient Record System (CPRS) EHR, to create a list of veterans treated at the VAPHCS outpatient chemotherapy infusion unit for a 2-year period (January 1, 2018, to December 31, 2020).
Male and female patient lists were first generated based on CPRS categorization. We identified all female veterans treated in the ambulatory infusion unit during the study period. Male patients were then chosen at random, recording the most recent names for each year until a matched number per year compared with the female cohort was reached. Patients were recorded only once even though they had multiple infusion unit visits. Patients were excluded who were deceased, on hospice care, lost to follow-up, could not be reached by phone, refused to take the survey, had undeliverable email addresses, or lacked internet or email access.
After filing the appropriate request through the VAPHCS Institutional Review Board committee in January 2021, patient records were reviewed for demographics data, contact information, and infusion treatment history. The survey was then conducted over a 2-week period during January and February 2021. Each patient was invited by phone to complete a 25-question anonymous online survey. The survey questions were created from patient-relayed experiences, then modeled into survey questions in a format similar to other patient satisfaction questionnaires described in cancer care and gender differences.2,13,14 The survey included self-identification of gender and was multiple choice for all except 2 questions, which allowed an open-ended response (Appendix). Only 1 answer per question was permitted. Only 1 survey link was sent to each veteran who gave permission for the survey. To protect anonymity for the small patient population, we excluded those identifying as gender nonbinary or transgender.
Statistical Analysis
Patient, disease, and treatment features are separated by male and female cohorts to reflect information from the EHR (Table 1). Survey percentages were calculated to reflect the affirmative response of the question asked (Table 2). Questions with answer options of not important, minimally important, important, or very important were calculated to reflect the sum of any importance in both cohorts. Questions with answer options of never, once, often, or every time were calculated to reflect any occurrence (sum of once, often, or every time) in both patient groups. Questions with answer options of strongly agree, somewhat agree, somewhat disagree, and strongly disagree were calculated to reflect any agreement (somewhat agree and strongly agree summed together) for both groups. Comparisons between cohorts were then conducted using a Fisher exact test. A Welch t test was used to calculate the significance of the continuous variable and overall ranking of the infusion unit experience between groups.
Results
In 2020, 414 individual patients were treated at the VAPAHCS outpatient infusion unit. Of these, 23 (5.6%) were female, and 18 agreed to take the survey. After deceased and duplicate names from 2020 were removed, another 14 eligible 2019 female patients were invited and 6 agreed to participate; 6 eligible 2018 female patients were invited and 4 agreed to take the survey (Figure). Thirty female veterans were sent a survey link and 21 (70%) responses were collected. Twenty-one male 2020 patients were contacted and 18 agreed to take the survey. After removing duplicate names and deceased individuals, 17 of 21 eligible 2019 male patients and 4 of 6 eligible 2018 patients agreed to take the survey. Five additional male veterans declined the online-based survey method. In total, 39 male veterans were reached who agreed to have the survey link emailed, and 20 (51%) total responses were collected.
Most respondents answered all questions in the survey. The most frequently skipped questions included 3 questions that were contingent on a yes answer to a prior question, and 2 openended questions asking for a write-in response. Percentages for female and male respondents were adjusted for number of responses when applicable.
Thirteen (62%) female patients were aged < 65 years, while 18 (90%) of male patients were aged ≥ 65 years. Education beyond high school was reported in 20 female and 15 male respondents. Almost all treatment administered in the infusion unit was for cancer-directed treatment, with only 1 reporting a noncancer treatment (IV iron). The most common malignancy among female patients was breast cancer (n = 11, 52%); for male patients prostate cancer (n = 4, 20%) and hematologic malignancy (n = 4, 20%) were most common. Four (19%) female and 8 (40%) male respondents reported having a metastatic diagnosis. Overall patient satisfaction ranked high with an average score of 9.1 on a 10-point scale. The mean (SD) satisfaction score for female respondents was 1 point lower than that for men: 8.7 (2.2) vs 9.6 (0.6) in men (P = .11).
Eighteen (86%) women reported a history of sexual abuse or harassment compared with 2 (10%) men (P < .001). The sexual abuse assailant was a different gender for 17 of 18 female respondents and of the same gender for both male respondents. Of those with sexual abuse history, 4 women reported feeling uncomfortable around their assailant’s gender vs no men (P = .11), but this difference was not statistically significant. Six women (29%) and 2 (10%) men reported feeling uncomfortable during clinical examinations from comments made by the clinician or during treatment administration (P = .24). Six (29%) women and no men reported that they “felt uncomfortable in the infusion unit by other patients” (P = .02). Six (29%) women and no men reported feeling unable to “voice uncomfortable experiences” to the infusion unit clinician (P = .02).
Ten (48%) women and 6 (30%) men reported emotional support when receiving treatments provided by staff of the same gender (P = .34). Eight (38%) women and 4 (20%) men noted that access to treatment with the same gender was important (P = .31). Six (29%) women and 4 (20%) men indicated that access to a sex or gender-specific restroom was important (P = .72). No gender preferences were identified in the survey questions regarding importance of private treatment room access and level of emotional support when receiving treatment with others of the same malignancy. These relationships were not statistically significant.
In addition, 2 open-ended questions were asked. Seventeen women and 14 men responded. Contact the corresponding author for more information on the questions and responses.
Discussion
Overall patient satisfaction was high among the men and women veterans with cancer who received treatment in our outpatient infusion unit; however, notable gender differences existed. Three items in the survey revealed statistically significant differences in the patient experience between men and women veterans: history of sexual abuse or harassment, uncomfortable feelings among other patients, and discomfort in relaying uncomfortable feelings to a clinician. Other items in the survey did not reach statistical significance; however, we have included discussion of the findings as they may highlight important trends and be of clinical significance.
We suspect differences among genders in patient satisfaction to be related to the high incidence of sexual abuse or harassment history reported by women, much higher at 86% than the one-third to one-fourth incidence rates estimated by the existing literature for civilian or military sexual abuse in women.5,6 These high sexual abuse or harassment rates are present in a majority of women who receive cancer-directed treatment toward a gender-specific breast malignancy, surrounded predominantly among men in a shared treatment space. Together, these factors are likely key reasons behind the differences in satisfaction observed. This sentiment is expressed in our cohort, where one-fifth of women with a sexual abuse or harassment history continue to remain uncomfortable around men, and 29% of women reporting some uncomfortable feelings during their treatment experience compared with none of the men. Additionally, 6 (29%) women vs no men felt uncomfortable in reporting an uncomfortable experience with a clinician; this represents a significant barrier in providing care for these patients.
A key gender preference among women included access to shared treatment rooms with other women and that sharing a treatment space with other women resulted in feeling more emotional support during treatments. Access to gender-specific restrooms was also preferred by women more than men. Key findings in both genders were that about half of men and women valued access to a private treatment room and would derive more emotional support when surrounded by others with the same cancer.
Prior studies on gender and patient satisfaction in general medical care and cancer care have found women value privacy more than men.1-3 Wessels and colleagues performed an analysis of 386 patients with cancer in Europe and found gender to be the strongest influence in patient preferences within cancer care. Specifically, the highest statically significant association in care preferences among women included privacy, support/counseling/rehabilitation access, and decreased wait times.2 These findings were most pronounced in those with breast cancer compared with other malignancy type and highlights that malignancy type and gender predominance impact care satisfaction.
Traditionally a shared treatment space design has been used in outpatient chemotherapy units, similar to the design of the VAPHCS. However, recent data report on the patient preference for a private treatment space, which was especially prominent among women and those receiving longer infusions.7 In another study that evaluated 225 patients with cancer preferences in sharing a treatment space with those of a different sexual orientation or gender identify, differences were found. Both men and women had a similar level of comfort in sharing a treatment room with someone of a different sexual orientation; however, more women reported discomfort in sharing a treatment space with a transgender woman compared with men who felt more comfortable sharing a space with a transgender man.4 We noted a gender preference may be present to explain the difference. Within our cohort, women valued access to treatment with other women and derived more emotional support when with other women; however, we did not inquire about feelings in sharing a treatment space among transgender individuals or differing sexual orientation.
Gender differences for privacy and in shared room preferences may result from the lasting impacts of prior sexual abuse or harassment. A history of sexual abuse negatively impacts later medical care access and use.15 Those veterans who experienced sexual abuse/harrassment reported higher feelings of lack of control, vulnerability, depression, and pursued less medical care.15,16 Within cancer care, these feelings are most pronounced among women with gender-specific malignancies, such as gynecologic cancers or breast cancer. Treatment, screening, and physical examinations by clinicians who are of the same gender as the sexual abuse/harassment assailant can recreate traumatic feelings.15,16
A majority of women (n = 18, 86%) in our cohort reported a history of sexual abuse or harassment and breast malignancy was the most common cancer among women. However women represent just 5.6% of the VAPHCS infusion unit treatment population. This combination of factors may explain the reasons for women veterans’ preference for privacy during treatments, access to gender-specific restrooms, and feeling more emotional support when surrounded by other women. Strategies to help patients with a history of abuse have been described and include discussions from the clinician asking about abuse history, allowing time for the patient to express fears with an examination or test, and training on how to deliver sensitive care for those with trauma.17,18
Quality Improvement
Project In the VAPHCS infusion unit, several low-cost interventions have been undertaken as a result of our survey findings. We presented our survey data to the VAPHCS Cancer Committee, accredited through the national American College of Surgeons Commission on Cancer. The committee awarded support for a yearlong QI project, including a formal framework of quarterly multidisciplinary meetings to discuss project updates, challenges, and resources. The QI project centers on education to raise awareness of survey results as well as specific interventions for improvement.
Education efforts have been applied through multiple department-wide emails, in-person education to our chemotherapy unit staff, abstract submission to national oncology conferences, and grand rounds department presentations at VAPHCS and at other VHA-affiliated university programs. Additionally, education to clinicians with specific contact information for psychology and women’s health to support mental health, trauma, and sexual abuse histories has been given to each clinician who cares for veterans in the chemotherapy unit.
We also have implemented a mandatory cancer care navigation consultation for all women veterans who have a new cancer or infusion need. The cancer care navigator has received specialized training in sensitive history-taking and provides women veterans with a direct number to reach the cancer care navigation nurse. Cancer care navigation also provides a continuum of support and referral access for psychosocial needs as indicated between infusion or health care visits. Our hope is that these resources may help offset the sentiment reflected in our cohort of women feeling unable to voice concerns to a clinician.
Other interventions underway include offering designated scheduling time each week to women so they can receive infusions in an area with other women. This may help mitigate the finding that women veterans felt more uncomfortable around other patients during infusion treatments compared with how men felt in the chemotherapy unit. We also have implemented gender-specific restrooms labeled with a sign on each bathroom door so men and women can have access to a designated restroom. Offering private or semiprivate treatment rooms is currently limited by space and capacity; however, these may offer the greatest opportunity to improve patient satisfaction, especially among women veterans. Working with the support of the VAPHCS Cancer Committee, we aim to reevaluate the impact of the education and QI efforts on gender differences and patient satisfaction at completion of the 1-year award.
Limitations
Limitations to our study include the overall small sample size. This is due to the combination of the low number of women treated at VAPHCS and many with advanced cancer who, unfortunately, have a limited overall survival and hinders accrual of a larger sample size. Other limitations included age as a possible confounder in our findings, with women representing a younger demographic compared with men. We did not collect responses on duration of infusion time, which also may impact overall satisfaction and patient experience. We also acknowledge that biologic male or female sex may not correspond to a specific individual’s gender. Use of CPRS to obtain a matched number of male and female patients through random selection relied on labeled data from the EHR. This potentially may have excluded male patients who identify as another gender that would have been captured on the anonymous survey.
Last, we restricted survey responses to online only, which excluded a small percentage who declined this approach.
Conclusions
Our findings may have broad applications to other VHA facilities and other cancer-directed treatment centers where the patient demographic and open shared infusion unit design may be similar. The study also may serve as a model of survey design and implementation from which other centers may consider improving patient satisfaction. We hope these survey results and interventions can provide insight and be used to improve patient satisfaction among all cancer patients at infusion units serving veterans and nonveterans.
Acknowledgments
We are very thankful to our cancer patients who took the time to take the survey. We also are very grateful to the VHA infusion unit nurses, staff, nurse practitioners, and physicians who have embraced this project and welcomed any changes that may positively impact treatment of veterans. Also, thank you to Tia Kohs for statistical support and Sophie West for gender discussions. Last, we specifically thank Barbara, for her pursuit of better care for women and for all veterans.
1. Clarke SA, Booth L, Velikova G, Hewison J. Social support: gender differences in cancer patients in the United Kingdom. Cancer Nurs. 2006;29(1):66-72. doi:10.1097/00002820-200601000-00012
2. Wessels H, de Graeff A, Wynia K, et al. Gender-related needs and preferences in cancer care indicate the need for an individualized approach to cancer patients. Oncologist. 2010;15(6):648-655. doi:10.1634/theoncologist.2009-0337
3. Hartigan SM, Bonnet K, Chisholm L, et al. Why do women not use the bathroom? Women’s attitudes and beliefs on using public restrooms. Int J Environ Res Public Health. 2020;17(6):2053. doi:10.3390/ijerph17062053
4. Alexander K, Walters CB, Banerjee SC. Oncology patients’ preferences regarding sexual orientation and gender identity (SOGI) disclosure and room sharing sharing. Patient Educ Couns. 2020;103(5):1041-1048. doi:10.1016/j.pec.2019.12.006
5. Centers for Disease Control and Prevention. Facts about sexual violence. Updated July 5, 2022. Accessed July 13, 2022. https://www.cdc.gov/injury/features /sexual-violence/index.html
6. US Department of Veterans Affairs. Military sexual trauma. Updated May 16, 2022. Accessed July 13, 2022. https:// www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
7. Wang Z, Pukszta M. Private Rooms, Semi-open areas, or open areas for chemotherapy care: perspectives of cancer patients, families, and nursing staff. HERD. 2018;11(3):94- 108. doi:10.1177/1937586718758445
8. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Accessed July 13, 2022. https://www.va.gov/vetdata /docs/specialreports/women_veterans_2015_final.pdf
9. Driscoll MA, Higgins DM, Seng EK, et al. Trauma, social support, family conflict, and chronic pain in recent service veterans: does gender matter? Pain Med. 2015;16(6):1101- 1111. doi:10.1111/pme.12744
10. Fox AB, Meyer EC, Vogt D. Attitudes about the VA healthcare setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58. doi:10.1037/a0038269
11. Virani SS, Woodard LD, Ramsey DJ, et al. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115(1):21-26. doi:10.1016/j.amjcard.2014.09.041
12. Tseng J. Sex, gender, and why the differences matter. Virtual Mentor. 2008;10(7):427-428. doi:10.1001/virtualmentor.2008.10.7.fred1-0807
13. Booij JC, Zegers M, Evers PMPJ, Hendricks M, Delnoij DMJ, Rademakers JJDJM. Improving cancer patient care: development of a generic cancer consumer quality index questionnaire for cancer patients. BMC Cancer. 2013;13(203). doi:10.1186/1471-2407-13-203
14. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113(12):3459-3466. doi:10.1002/cncr.23968 1
5. Schnur JB, Dillon MJ, Goldsmith RE, Montgomery GH. Cancer treatment experiences among survivors of childhood sexual abuse: a qualitative investigation of triggers and reactions to cumulative trauma. Palliat Support Care. 2018;16(6):767-776. doi:10.1017/S147895151700075X
16. Cadman L, Waller J, Ashdown-Barr L, Szarewski A. Barriers to cervical screening in women who have experienced sexual abuse: an exploratory study. J Fam Plann Reprod Health Care. 2012;38(4):214-220. doi:10.1136/jfprhc-2012-100378
17. Kelly S. The effects of childhood sexual abuse on women’s lives and their attitudes to cervical screening. J Fam Plann Reprod Health Care. 2012;38(4):212-213. doi:10.1136/jfprhc-2012-100418
18. McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep. 2006;121(4):435-444. doi:10.1177/003335490612100412
Gender differences in patient satisfaction with medical care have been evaluated in multiple settings; however, studies specific to the unique population of women veterans with cancer are lacking. Women are reported to value privacy, psychosocial support, and communication to a higher degree compared with men.1 Factors affecting satisfaction include the following: discomfort in sharing treatment rooms with the opposite gender, a desire for privacy with treatment and restroom use, anatomic or illness differences, and a personal history of abuse.2-4 Regrettably, up to 1 in 3 women in the United States are victims of sexual trauma in their lifetimes, and up to 1 in 4 women in the military are victims of military sexual trauma. Incidence in both settings is suspected to be higher due to underreporting.5,6
Chemotherapy treatment units are often uniquely designed as an open space, with several patients sharing a treatment area. The design reduces isolation and facilitates quick nurse-patient access during potentially toxic treatments known to have frequent adverse effects. Data suggest that nursing staff prefer open models to facilitate quick patient assessments and interventions as needed; however, patients and families prefer private treatment rooms, especially among women patients or those receiving longer infusions.7
The Veterans Health Administration (VHA) patient population is male predominant, comprised only of 10% female patients.8 Although the proportion of female patients in the VHA is expected to rise annually to about 16% by 2043, the low percentage of female veterans will persist for the foreseeable future.8 This low percentage of female veterans is reflected in the Veterans Affairs Portland Health Care System (VAPHCS) cancer patient population and in the use of the chemotherapy infusion unit, which is used for the ambulatory treatment of veterans undergoing cancer therapy.
The VHA has previously explored gender differences in health care, such as with cardiovascular disease, transgender care, and access to mental health.9-11 However, to the best of our knowledge, no analysis has explored gender differences within the outpatient cancer treatment experience. Patient satisfaction with outpatient cancer care may be magnified in the VHA setting due to the uniquely unequal gender populations, shared treatment space design, and high incidence of sexual abuse among women veterans. Given this, we aimed to identify gender-related preferences in outpatient cancer care in our chemotherapy infusion unit.
In our study, we used the terms male and female to reflect statistical data from the literature or labeled data from the electronic health record (EHR); whereas the terms men and women were used to describe and encompass the cultural implications and context of gender.12
Methods
This study was designated as a quality improvement (QI) project by the VAPHCS research office and Institutional Review Board in accordance with VHA policies.
The VAPHCS outpatient chemotherapy infusion unit is designed with 6 rooms for chemotherapy administration. One room is a large open space with 6 chairs for patients. The other rooms are smaller with glass dividers between the rooms, and 3 chairs inside each for patients. There are 2 private bathrooms, each gender neutral. Direct patient care is provided by physicians, nurse practitioners (NPs), infusion unit nurses, and nurse coordinators. Men represent the majority of hematology and oncology physicians (13 of 20 total: 5 women fellow physicians and 2 women attending physicians), and 2 of 4 NPs. Women represent 10 of 12 infusion unit and cancer coordinator nurses. We used the VHA Computerized Patient Record System (CPRS) EHR, to create a list of veterans treated at the VAPHCS outpatient chemotherapy infusion unit for a 2-year period (January 1, 2018, to December 31, 2020).
Male and female patient lists were first generated based on CPRS categorization. We identified all female veterans treated in the ambulatory infusion unit during the study period. Male patients were then chosen at random, recording the most recent names for each year until a matched number per year compared with the female cohort was reached. Patients were recorded only once even though they had multiple infusion unit visits. Patients were excluded who were deceased, on hospice care, lost to follow-up, could not be reached by phone, refused to take the survey, had undeliverable email addresses, or lacked internet or email access.
After filing the appropriate request through the VAPHCS Institutional Review Board committee in January 2021, patient records were reviewed for demographics data, contact information, and infusion treatment history. The survey was then conducted over a 2-week period during January and February 2021. Each patient was invited by phone to complete a 25-question anonymous online survey. The survey questions were created from patient-relayed experiences, then modeled into survey questions in a format similar to other patient satisfaction questionnaires described in cancer care and gender differences.2,13,14 The survey included self-identification of gender and was multiple choice for all except 2 questions, which allowed an open-ended response (Appendix). Only 1 answer per question was permitted. Only 1 survey link was sent to each veteran who gave permission for the survey. To protect anonymity for the small patient population, we excluded those identifying as gender nonbinary or transgender.
Statistical Analysis
Patient, disease, and treatment features are separated by male and female cohorts to reflect information from the EHR (Table 1). Survey percentages were calculated to reflect the affirmative response of the question asked (Table 2). Questions with answer options of not important, minimally important, important, or very important were calculated to reflect the sum of any importance in both cohorts. Questions with answer options of never, once, often, or every time were calculated to reflect any occurrence (sum of once, often, or every time) in both patient groups. Questions with answer options of strongly agree, somewhat agree, somewhat disagree, and strongly disagree were calculated to reflect any agreement (somewhat agree and strongly agree summed together) for both groups. Comparisons between cohorts were then conducted using a Fisher exact test. A Welch t test was used to calculate the significance of the continuous variable and overall ranking of the infusion unit experience between groups.
Results
In 2020, 414 individual patients were treated at the VAPAHCS outpatient infusion unit. Of these, 23 (5.6%) were female, and 18 agreed to take the survey. After deceased and duplicate names from 2020 were removed, another 14 eligible 2019 female patients were invited and 6 agreed to participate; 6 eligible 2018 female patients were invited and 4 agreed to take the survey (Figure). Thirty female veterans were sent a survey link and 21 (70%) responses were collected. Twenty-one male 2020 patients were contacted and 18 agreed to take the survey. After removing duplicate names and deceased individuals, 17 of 21 eligible 2019 male patients and 4 of 6 eligible 2018 patients agreed to take the survey. Five additional male veterans declined the online-based survey method. In total, 39 male veterans were reached who agreed to have the survey link emailed, and 20 (51%) total responses were collected.
Most respondents answered all questions in the survey. The most frequently skipped questions included 3 questions that were contingent on a yes answer to a prior question, and 2 openended questions asking for a write-in response. Percentages for female and male respondents were adjusted for number of responses when applicable.
Thirteen (62%) female patients were aged < 65 years, while 18 (90%) of male patients were aged ≥ 65 years. Education beyond high school was reported in 20 female and 15 male respondents. Almost all treatment administered in the infusion unit was for cancer-directed treatment, with only 1 reporting a noncancer treatment (IV iron). The most common malignancy among female patients was breast cancer (n = 11, 52%); for male patients prostate cancer (n = 4, 20%) and hematologic malignancy (n = 4, 20%) were most common. Four (19%) female and 8 (40%) male respondents reported having a metastatic diagnosis. Overall patient satisfaction ranked high with an average score of 9.1 on a 10-point scale. The mean (SD) satisfaction score for female respondents was 1 point lower than that for men: 8.7 (2.2) vs 9.6 (0.6) in men (P = .11).
Eighteen (86%) women reported a history of sexual abuse or harassment compared with 2 (10%) men (P < .001). The sexual abuse assailant was a different gender for 17 of 18 female respondents and of the same gender for both male respondents. Of those with sexual abuse history, 4 women reported feeling uncomfortable around their assailant’s gender vs no men (P = .11), but this difference was not statistically significant. Six women (29%) and 2 (10%) men reported feeling uncomfortable during clinical examinations from comments made by the clinician or during treatment administration (P = .24). Six (29%) women and no men reported that they “felt uncomfortable in the infusion unit by other patients” (P = .02). Six (29%) women and no men reported feeling unable to “voice uncomfortable experiences” to the infusion unit clinician (P = .02).
Ten (48%) women and 6 (30%) men reported emotional support when receiving treatments provided by staff of the same gender (P = .34). Eight (38%) women and 4 (20%) men noted that access to treatment with the same gender was important (P = .31). Six (29%) women and 4 (20%) men indicated that access to a sex or gender-specific restroom was important (P = .72). No gender preferences were identified in the survey questions regarding importance of private treatment room access and level of emotional support when receiving treatment with others of the same malignancy. These relationships were not statistically significant.
In addition, 2 open-ended questions were asked. Seventeen women and 14 men responded. Contact the corresponding author for more information on the questions and responses.
Discussion
Overall patient satisfaction was high among the men and women veterans with cancer who received treatment in our outpatient infusion unit; however, notable gender differences existed. Three items in the survey revealed statistically significant differences in the patient experience between men and women veterans: history of sexual abuse or harassment, uncomfortable feelings among other patients, and discomfort in relaying uncomfortable feelings to a clinician. Other items in the survey did not reach statistical significance; however, we have included discussion of the findings as they may highlight important trends and be of clinical significance.
We suspect differences among genders in patient satisfaction to be related to the high incidence of sexual abuse or harassment history reported by women, much higher at 86% than the one-third to one-fourth incidence rates estimated by the existing literature for civilian or military sexual abuse in women.5,6 These high sexual abuse or harassment rates are present in a majority of women who receive cancer-directed treatment toward a gender-specific breast malignancy, surrounded predominantly among men in a shared treatment space. Together, these factors are likely key reasons behind the differences in satisfaction observed. This sentiment is expressed in our cohort, where one-fifth of women with a sexual abuse or harassment history continue to remain uncomfortable around men, and 29% of women reporting some uncomfortable feelings during their treatment experience compared with none of the men. Additionally, 6 (29%) women vs no men felt uncomfortable in reporting an uncomfortable experience with a clinician; this represents a significant barrier in providing care for these patients.
A key gender preference among women included access to shared treatment rooms with other women and that sharing a treatment space with other women resulted in feeling more emotional support during treatments. Access to gender-specific restrooms was also preferred by women more than men. Key findings in both genders were that about half of men and women valued access to a private treatment room and would derive more emotional support when surrounded by others with the same cancer.
Prior studies on gender and patient satisfaction in general medical care and cancer care have found women value privacy more than men.1-3 Wessels and colleagues performed an analysis of 386 patients with cancer in Europe and found gender to be the strongest influence in patient preferences within cancer care. Specifically, the highest statically significant association in care preferences among women included privacy, support/counseling/rehabilitation access, and decreased wait times.2 These findings were most pronounced in those with breast cancer compared with other malignancy type and highlights that malignancy type and gender predominance impact care satisfaction.
Traditionally a shared treatment space design has been used in outpatient chemotherapy units, similar to the design of the VAPHCS. However, recent data report on the patient preference for a private treatment space, which was especially prominent among women and those receiving longer infusions.7 In another study that evaluated 225 patients with cancer preferences in sharing a treatment space with those of a different sexual orientation or gender identify, differences were found. Both men and women had a similar level of comfort in sharing a treatment room with someone of a different sexual orientation; however, more women reported discomfort in sharing a treatment space with a transgender woman compared with men who felt more comfortable sharing a space with a transgender man.4 We noted a gender preference may be present to explain the difference. Within our cohort, women valued access to treatment with other women and derived more emotional support when with other women; however, we did not inquire about feelings in sharing a treatment space among transgender individuals or differing sexual orientation.
Gender differences for privacy and in shared room preferences may result from the lasting impacts of prior sexual abuse or harassment. A history of sexual abuse negatively impacts later medical care access and use.15 Those veterans who experienced sexual abuse/harrassment reported higher feelings of lack of control, vulnerability, depression, and pursued less medical care.15,16 Within cancer care, these feelings are most pronounced among women with gender-specific malignancies, such as gynecologic cancers or breast cancer. Treatment, screening, and physical examinations by clinicians who are of the same gender as the sexual abuse/harassment assailant can recreate traumatic feelings.15,16
A majority of women (n = 18, 86%) in our cohort reported a history of sexual abuse or harassment and breast malignancy was the most common cancer among women. However women represent just 5.6% of the VAPHCS infusion unit treatment population. This combination of factors may explain the reasons for women veterans’ preference for privacy during treatments, access to gender-specific restrooms, and feeling more emotional support when surrounded by other women. Strategies to help patients with a history of abuse have been described and include discussions from the clinician asking about abuse history, allowing time for the patient to express fears with an examination or test, and training on how to deliver sensitive care for those with trauma.17,18
Quality Improvement
Project In the VAPHCS infusion unit, several low-cost interventions have been undertaken as a result of our survey findings. We presented our survey data to the VAPHCS Cancer Committee, accredited through the national American College of Surgeons Commission on Cancer. The committee awarded support for a yearlong QI project, including a formal framework of quarterly multidisciplinary meetings to discuss project updates, challenges, and resources. The QI project centers on education to raise awareness of survey results as well as specific interventions for improvement.
Education efforts have been applied through multiple department-wide emails, in-person education to our chemotherapy unit staff, abstract submission to national oncology conferences, and grand rounds department presentations at VAPHCS and at other VHA-affiliated university programs. Additionally, education to clinicians with specific contact information for psychology and women’s health to support mental health, trauma, and sexual abuse histories has been given to each clinician who cares for veterans in the chemotherapy unit.
We also have implemented a mandatory cancer care navigation consultation for all women veterans who have a new cancer or infusion need. The cancer care navigator has received specialized training in sensitive history-taking and provides women veterans with a direct number to reach the cancer care navigation nurse. Cancer care navigation also provides a continuum of support and referral access for psychosocial needs as indicated between infusion or health care visits. Our hope is that these resources may help offset the sentiment reflected in our cohort of women feeling unable to voice concerns to a clinician.
Other interventions underway include offering designated scheduling time each week to women so they can receive infusions in an area with other women. This may help mitigate the finding that women veterans felt more uncomfortable around other patients during infusion treatments compared with how men felt in the chemotherapy unit. We also have implemented gender-specific restrooms labeled with a sign on each bathroom door so men and women can have access to a designated restroom. Offering private or semiprivate treatment rooms is currently limited by space and capacity; however, these may offer the greatest opportunity to improve patient satisfaction, especially among women veterans. Working with the support of the VAPHCS Cancer Committee, we aim to reevaluate the impact of the education and QI efforts on gender differences and patient satisfaction at completion of the 1-year award.
Limitations
Limitations to our study include the overall small sample size. This is due to the combination of the low number of women treated at VAPHCS and many with advanced cancer who, unfortunately, have a limited overall survival and hinders accrual of a larger sample size. Other limitations included age as a possible confounder in our findings, with women representing a younger demographic compared with men. We did not collect responses on duration of infusion time, which also may impact overall satisfaction and patient experience. We also acknowledge that biologic male or female sex may not correspond to a specific individual’s gender. Use of CPRS to obtain a matched number of male and female patients through random selection relied on labeled data from the EHR. This potentially may have excluded male patients who identify as another gender that would have been captured on the anonymous survey.
Last, we restricted survey responses to online only, which excluded a small percentage who declined this approach.
Conclusions
Our findings may have broad applications to other VHA facilities and other cancer-directed treatment centers where the patient demographic and open shared infusion unit design may be similar. The study also may serve as a model of survey design and implementation from which other centers may consider improving patient satisfaction. We hope these survey results and interventions can provide insight and be used to improve patient satisfaction among all cancer patients at infusion units serving veterans and nonveterans.
Acknowledgments
We are very thankful to our cancer patients who took the time to take the survey. We also are very grateful to the VHA infusion unit nurses, staff, nurse practitioners, and physicians who have embraced this project and welcomed any changes that may positively impact treatment of veterans. Also, thank you to Tia Kohs for statistical support and Sophie West for gender discussions. Last, we specifically thank Barbara, for her pursuit of better care for women and for all veterans.
Gender differences in patient satisfaction with medical care have been evaluated in multiple settings; however, studies specific to the unique population of women veterans with cancer are lacking. Women are reported to value privacy, psychosocial support, and communication to a higher degree compared with men.1 Factors affecting satisfaction include the following: discomfort in sharing treatment rooms with the opposite gender, a desire for privacy with treatment and restroom use, anatomic or illness differences, and a personal history of abuse.2-4 Regrettably, up to 1 in 3 women in the United States are victims of sexual trauma in their lifetimes, and up to 1 in 4 women in the military are victims of military sexual trauma. Incidence in both settings is suspected to be higher due to underreporting.5,6
Chemotherapy treatment units are often uniquely designed as an open space, with several patients sharing a treatment area. The design reduces isolation and facilitates quick nurse-patient access during potentially toxic treatments known to have frequent adverse effects. Data suggest that nursing staff prefer open models to facilitate quick patient assessments and interventions as needed; however, patients and families prefer private treatment rooms, especially among women patients or those receiving longer infusions.7
The Veterans Health Administration (VHA) patient population is male predominant, comprised only of 10% female patients.8 Although the proportion of female patients in the VHA is expected to rise annually to about 16% by 2043, the low percentage of female veterans will persist for the foreseeable future.8 This low percentage of female veterans is reflected in the Veterans Affairs Portland Health Care System (VAPHCS) cancer patient population and in the use of the chemotherapy infusion unit, which is used for the ambulatory treatment of veterans undergoing cancer therapy.
The VHA has previously explored gender differences in health care, such as with cardiovascular disease, transgender care, and access to mental health.9-11 However, to the best of our knowledge, no analysis has explored gender differences within the outpatient cancer treatment experience. Patient satisfaction with outpatient cancer care may be magnified in the VHA setting due to the uniquely unequal gender populations, shared treatment space design, and high incidence of sexual abuse among women veterans. Given this, we aimed to identify gender-related preferences in outpatient cancer care in our chemotherapy infusion unit.
In our study, we used the terms male and female to reflect statistical data from the literature or labeled data from the electronic health record (EHR); whereas the terms men and women were used to describe and encompass the cultural implications and context of gender.12
Methods
This study was designated as a quality improvement (QI) project by the VAPHCS research office and Institutional Review Board in accordance with VHA policies.
The VAPHCS outpatient chemotherapy infusion unit is designed with 6 rooms for chemotherapy administration. One room is a large open space with 6 chairs for patients. The other rooms are smaller with glass dividers between the rooms, and 3 chairs inside each for patients. There are 2 private bathrooms, each gender neutral. Direct patient care is provided by physicians, nurse practitioners (NPs), infusion unit nurses, and nurse coordinators. Men represent the majority of hematology and oncology physicians (13 of 20 total: 5 women fellow physicians and 2 women attending physicians), and 2 of 4 NPs. Women represent 10 of 12 infusion unit and cancer coordinator nurses. We used the VHA Computerized Patient Record System (CPRS) EHR, to create a list of veterans treated at the VAPHCS outpatient chemotherapy infusion unit for a 2-year period (January 1, 2018, to December 31, 2020).
Male and female patient lists were first generated based on CPRS categorization. We identified all female veterans treated in the ambulatory infusion unit during the study period. Male patients were then chosen at random, recording the most recent names for each year until a matched number per year compared with the female cohort was reached. Patients were recorded only once even though they had multiple infusion unit visits. Patients were excluded who were deceased, on hospice care, lost to follow-up, could not be reached by phone, refused to take the survey, had undeliverable email addresses, or lacked internet or email access.
After filing the appropriate request through the VAPHCS Institutional Review Board committee in January 2021, patient records were reviewed for demographics data, contact information, and infusion treatment history. The survey was then conducted over a 2-week period during January and February 2021. Each patient was invited by phone to complete a 25-question anonymous online survey. The survey questions were created from patient-relayed experiences, then modeled into survey questions in a format similar to other patient satisfaction questionnaires described in cancer care and gender differences.2,13,14 The survey included self-identification of gender and was multiple choice for all except 2 questions, which allowed an open-ended response (Appendix). Only 1 answer per question was permitted. Only 1 survey link was sent to each veteran who gave permission for the survey. To protect anonymity for the small patient population, we excluded those identifying as gender nonbinary or transgender.
Statistical Analysis
Patient, disease, and treatment features are separated by male and female cohorts to reflect information from the EHR (Table 1). Survey percentages were calculated to reflect the affirmative response of the question asked (Table 2). Questions with answer options of not important, minimally important, important, or very important were calculated to reflect the sum of any importance in both cohorts. Questions with answer options of never, once, often, or every time were calculated to reflect any occurrence (sum of once, often, or every time) in both patient groups. Questions with answer options of strongly agree, somewhat agree, somewhat disagree, and strongly disagree were calculated to reflect any agreement (somewhat agree and strongly agree summed together) for both groups. Comparisons between cohorts were then conducted using a Fisher exact test. A Welch t test was used to calculate the significance of the continuous variable and overall ranking of the infusion unit experience between groups.
Results
In 2020, 414 individual patients were treated at the VAPAHCS outpatient infusion unit. Of these, 23 (5.6%) were female, and 18 agreed to take the survey. After deceased and duplicate names from 2020 were removed, another 14 eligible 2019 female patients were invited and 6 agreed to participate; 6 eligible 2018 female patients were invited and 4 agreed to take the survey (Figure). Thirty female veterans were sent a survey link and 21 (70%) responses were collected. Twenty-one male 2020 patients were contacted and 18 agreed to take the survey. After removing duplicate names and deceased individuals, 17 of 21 eligible 2019 male patients and 4 of 6 eligible 2018 patients agreed to take the survey. Five additional male veterans declined the online-based survey method. In total, 39 male veterans were reached who agreed to have the survey link emailed, and 20 (51%) total responses were collected.
Most respondents answered all questions in the survey. The most frequently skipped questions included 3 questions that were contingent on a yes answer to a prior question, and 2 openended questions asking for a write-in response. Percentages for female and male respondents were adjusted for number of responses when applicable.
Thirteen (62%) female patients were aged < 65 years, while 18 (90%) of male patients were aged ≥ 65 years. Education beyond high school was reported in 20 female and 15 male respondents. Almost all treatment administered in the infusion unit was for cancer-directed treatment, with only 1 reporting a noncancer treatment (IV iron). The most common malignancy among female patients was breast cancer (n = 11, 52%); for male patients prostate cancer (n = 4, 20%) and hematologic malignancy (n = 4, 20%) were most common. Four (19%) female and 8 (40%) male respondents reported having a metastatic diagnosis. Overall patient satisfaction ranked high with an average score of 9.1 on a 10-point scale. The mean (SD) satisfaction score for female respondents was 1 point lower than that for men: 8.7 (2.2) vs 9.6 (0.6) in men (P = .11).
Eighteen (86%) women reported a history of sexual abuse or harassment compared with 2 (10%) men (P < .001). The sexual abuse assailant was a different gender for 17 of 18 female respondents and of the same gender for both male respondents. Of those with sexual abuse history, 4 women reported feeling uncomfortable around their assailant’s gender vs no men (P = .11), but this difference was not statistically significant. Six women (29%) and 2 (10%) men reported feeling uncomfortable during clinical examinations from comments made by the clinician or during treatment administration (P = .24). Six (29%) women and no men reported that they “felt uncomfortable in the infusion unit by other patients” (P = .02). Six (29%) women and no men reported feeling unable to “voice uncomfortable experiences” to the infusion unit clinician (P = .02).
Ten (48%) women and 6 (30%) men reported emotional support when receiving treatments provided by staff of the same gender (P = .34). Eight (38%) women and 4 (20%) men noted that access to treatment with the same gender was important (P = .31). Six (29%) women and 4 (20%) men indicated that access to a sex or gender-specific restroom was important (P = .72). No gender preferences were identified in the survey questions regarding importance of private treatment room access and level of emotional support when receiving treatment with others of the same malignancy. These relationships were not statistically significant.
In addition, 2 open-ended questions were asked. Seventeen women and 14 men responded. Contact the corresponding author for more information on the questions and responses.
Discussion
Overall patient satisfaction was high among the men and women veterans with cancer who received treatment in our outpatient infusion unit; however, notable gender differences existed. Three items in the survey revealed statistically significant differences in the patient experience between men and women veterans: history of sexual abuse or harassment, uncomfortable feelings among other patients, and discomfort in relaying uncomfortable feelings to a clinician. Other items in the survey did not reach statistical significance; however, we have included discussion of the findings as they may highlight important trends and be of clinical significance.
We suspect differences among genders in patient satisfaction to be related to the high incidence of sexual abuse or harassment history reported by women, much higher at 86% than the one-third to one-fourth incidence rates estimated by the existing literature for civilian or military sexual abuse in women.5,6 These high sexual abuse or harassment rates are present in a majority of women who receive cancer-directed treatment toward a gender-specific breast malignancy, surrounded predominantly among men in a shared treatment space. Together, these factors are likely key reasons behind the differences in satisfaction observed. This sentiment is expressed in our cohort, where one-fifth of women with a sexual abuse or harassment history continue to remain uncomfortable around men, and 29% of women reporting some uncomfortable feelings during their treatment experience compared with none of the men. Additionally, 6 (29%) women vs no men felt uncomfortable in reporting an uncomfortable experience with a clinician; this represents a significant barrier in providing care for these patients.
A key gender preference among women included access to shared treatment rooms with other women and that sharing a treatment space with other women resulted in feeling more emotional support during treatments. Access to gender-specific restrooms was also preferred by women more than men. Key findings in both genders were that about half of men and women valued access to a private treatment room and would derive more emotional support when surrounded by others with the same cancer.
Prior studies on gender and patient satisfaction in general medical care and cancer care have found women value privacy more than men.1-3 Wessels and colleagues performed an analysis of 386 patients with cancer in Europe and found gender to be the strongest influence in patient preferences within cancer care. Specifically, the highest statically significant association in care preferences among women included privacy, support/counseling/rehabilitation access, and decreased wait times.2 These findings were most pronounced in those with breast cancer compared with other malignancy type and highlights that malignancy type and gender predominance impact care satisfaction.
Traditionally a shared treatment space design has been used in outpatient chemotherapy units, similar to the design of the VAPHCS. However, recent data report on the patient preference for a private treatment space, which was especially prominent among women and those receiving longer infusions.7 In another study that evaluated 225 patients with cancer preferences in sharing a treatment space with those of a different sexual orientation or gender identify, differences were found. Both men and women had a similar level of comfort in sharing a treatment room with someone of a different sexual orientation; however, more women reported discomfort in sharing a treatment space with a transgender woman compared with men who felt more comfortable sharing a space with a transgender man.4 We noted a gender preference may be present to explain the difference. Within our cohort, women valued access to treatment with other women and derived more emotional support when with other women; however, we did not inquire about feelings in sharing a treatment space among transgender individuals or differing sexual orientation.
Gender differences for privacy and in shared room preferences may result from the lasting impacts of prior sexual abuse or harassment. A history of sexual abuse negatively impacts later medical care access and use.15 Those veterans who experienced sexual abuse/harrassment reported higher feelings of lack of control, vulnerability, depression, and pursued less medical care.15,16 Within cancer care, these feelings are most pronounced among women with gender-specific malignancies, such as gynecologic cancers or breast cancer. Treatment, screening, and physical examinations by clinicians who are of the same gender as the sexual abuse/harassment assailant can recreate traumatic feelings.15,16
A majority of women (n = 18, 86%) in our cohort reported a history of sexual abuse or harassment and breast malignancy was the most common cancer among women. However women represent just 5.6% of the VAPHCS infusion unit treatment population. This combination of factors may explain the reasons for women veterans’ preference for privacy during treatments, access to gender-specific restrooms, and feeling more emotional support when surrounded by other women. Strategies to help patients with a history of abuse have been described and include discussions from the clinician asking about abuse history, allowing time for the patient to express fears with an examination or test, and training on how to deliver sensitive care for those with trauma.17,18
Quality Improvement
Project In the VAPHCS infusion unit, several low-cost interventions have been undertaken as a result of our survey findings. We presented our survey data to the VAPHCS Cancer Committee, accredited through the national American College of Surgeons Commission on Cancer. The committee awarded support for a yearlong QI project, including a formal framework of quarterly multidisciplinary meetings to discuss project updates, challenges, and resources. The QI project centers on education to raise awareness of survey results as well as specific interventions for improvement.
Education efforts have been applied through multiple department-wide emails, in-person education to our chemotherapy unit staff, abstract submission to national oncology conferences, and grand rounds department presentations at VAPHCS and at other VHA-affiliated university programs. Additionally, education to clinicians with specific contact information for psychology and women’s health to support mental health, trauma, and sexual abuse histories has been given to each clinician who cares for veterans in the chemotherapy unit.
We also have implemented a mandatory cancer care navigation consultation for all women veterans who have a new cancer or infusion need. The cancer care navigator has received specialized training in sensitive history-taking and provides women veterans with a direct number to reach the cancer care navigation nurse. Cancer care navigation also provides a continuum of support and referral access for psychosocial needs as indicated between infusion or health care visits. Our hope is that these resources may help offset the sentiment reflected in our cohort of women feeling unable to voice concerns to a clinician.
Other interventions underway include offering designated scheduling time each week to women so they can receive infusions in an area with other women. This may help mitigate the finding that women veterans felt more uncomfortable around other patients during infusion treatments compared with how men felt in the chemotherapy unit. We also have implemented gender-specific restrooms labeled with a sign on each bathroom door so men and women can have access to a designated restroom. Offering private or semiprivate treatment rooms is currently limited by space and capacity; however, these may offer the greatest opportunity to improve patient satisfaction, especially among women veterans. Working with the support of the VAPHCS Cancer Committee, we aim to reevaluate the impact of the education and QI efforts on gender differences and patient satisfaction at completion of the 1-year award.
Limitations
Limitations to our study include the overall small sample size. This is due to the combination of the low number of women treated at VAPHCS and many with advanced cancer who, unfortunately, have a limited overall survival and hinders accrual of a larger sample size. Other limitations included age as a possible confounder in our findings, with women representing a younger demographic compared with men. We did not collect responses on duration of infusion time, which also may impact overall satisfaction and patient experience. We also acknowledge that biologic male or female sex may not correspond to a specific individual’s gender. Use of CPRS to obtain a matched number of male and female patients through random selection relied on labeled data from the EHR. This potentially may have excluded male patients who identify as another gender that would have been captured on the anonymous survey.
Last, we restricted survey responses to online only, which excluded a small percentage who declined this approach.
Conclusions
Our findings may have broad applications to other VHA facilities and other cancer-directed treatment centers where the patient demographic and open shared infusion unit design may be similar. The study also may serve as a model of survey design and implementation from which other centers may consider improving patient satisfaction. We hope these survey results and interventions can provide insight and be used to improve patient satisfaction among all cancer patients at infusion units serving veterans and nonveterans.
Acknowledgments
We are very thankful to our cancer patients who took the time to take the survey. We also are very grateful to the VHA infusion unit nurses, staff, nurse practitioners, and physicians who have embraced this project and welcomed any changes that may positively impact treatment of veterans. Also, thank you to Tia Kohs for statistical support and Sophie West for gender discussions. Last, we specifically thank Barbara, for her pursuit of better care for women and for all veterans.
1. Clarke SA, Booth L, Velikova G, Hewison J. Social support: gender differences in cancer patients in the United Kingdom. Cancer Nurs. 2006;29(1):66-72. doi:10.1097/00002820-200601000-00012
2. Wessels H, de Graeff A, Wynia K, et al. Gender-related needs and preferences in cancer care indicate the need for an individualized approach to cancer patients. Oncologist. 2010;15(6):648-655. doi:10.1634/theoncologist.2009-0337
3. Hartigan SM, Bonnet K, Chisholm L, et al. Why do women not use the bathroom? Women’s attitudes and beliefs on using public restrooms. Int J Environ Res Public Health. 2020;17(6):2053. doi:10.3390/ijerph17062053
4. Alexander K, Walters CB, Banerjee SC. Oncology patients’ preferences regarding sexual orientation and gender identity (SOGI) disclosure and room sharing sharing. Patient Educ Couns. 2020;103(5):1041-1048. doi:10.1016/j.pec.2019.12.006
5. Centers for Disease Control and Prevention. Facts about sexual violence. Updated July 5, 2022. Accessed July 13, 2022. https://www.cdc.gov/injury/features /sexual-violence/index.html
6. US Department of Veterans Affairs. Military sexual trauma. Updated May 16, 2022. Accessed July 13, 2022. https:// www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
7. Wang Z, Pukszta M. Private Rooms, Semi-open areas, or open areas for chemotherapy care: perspectives of cancer patients, families, and nursing staff. HERD. 2018;11(3):94- 108. doi:10.1177/1937586718758445
8. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Accessed July 13, 2022. https://www.va.gov/vetdata /docs/specialreports/women_veterans_2015_final.pdf
9. Driscoll MA, Higgins DM, Seng EK, et al. Trauma, social support, family conflict, and chronic pain in recent service veterans: does gender matter? Pain Med. 2015;16(6):1101- 1111. doi:10.1111/pme.12744
10. Fox AB, Meyer EC, Vogt D. Attitudes about the VA healthcare setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58. doi:10.1037/a0038269
11. Virani SS, Woodard LD, Ramsey DJ, et al. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115(1):21-26. doi:10.1016/j.amjcard.2014.09.041
12. Tseng J. Sex, gender, and why the differences matter. Virtual Mentor. 2008;10(7):427-428. doi:10.1001/virtualmentor.2008.10.7.fred1-0807
13. Booij JC, Zegers M, Evers PMPJ, Hendricks M, Delnoij DMJ, Rademakers JJDJM. Improving cancer patient care: development of a generic cancer consumer quality index questionnaire for cancer patients. BMC Cancer. 2013;13(203). doi:10.1186/1471-2407-13-203
14. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113(12):3459-3466. doi:10.1002/cncr.23968 1
5. Schnur JB, Dillon MJ, Goldsmith RE, Montgomery GH. Cancer treatment experiences among survivors of childhood sexual abuse: a qualitative investigation of triggers and reactions to cumulative trauma. Palliat Support Care. 2018;16(6):767-776. doi:10.1017/S147895151700075X
16. Cadman L, Waller J, Ashdown-Barr L, Szarewski A. Barriers to cervical screening in women who have experienced sexual abuse: an exploratory study. J Fam Plann Reprod Health Care. 2012;38(4):214-220. doi:10.1136/jfprhc-2012-100378
17. Kelly S. The effects of childhood sexual abuse on women’s lives and their attitudes to cervical screening. J Fam Plann Reprod Health Care. 2012;38(4):212-213. doi:10.1136/jfprhc-2012-100418
18. McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep. 2006;121(4):435-444. doi:10.1177/003335490612100412
1. Clarke SA, Booth L, Velikova G, Hewison J. Social support: gender differences in cancer patients in the United Kingdom. Cancer Nurs. 2006;29(1):66-72. doi:10.1097/00002820-200601000-00012
2. Wessels H, de Graeff A, Wynia K, et al. Gender-related needs and preferences in cancer care indicate the need for an individualized approach to cancer patients. Oncologist. 2010;15(6):648-655. doi:10.1634/theoncologist.2009-0337
3. Hartigan SM, Bonnet K, Chisholm L, et al. Why do women not use the bathroom? Women’s attitudes and beliefs on using public restrooms. Int J Environ Res Public Health. 2020;17(6):2053. doi:10.3390/ijerph17062053
4. Alexander K, Walters CB, Banerjee SC. Oncology patients’ preferences regarding sexual orientation and gender identity (SOGI) disclosure and room sharing sharing. Patient Educ Couns. 2020;103(5):1041-1048. doi:10.1016/j.pec.2019.12.006
5. Centers for Disease Control and Prevention. Facts about sexual violence. Updated July 5, 2022. Accessed July 13, 2022. https://www.cdc.gov/injury/features /sexual-violence/index.html
6. US Department of Veterans Affairs. Military sexual trauma. Updated May 16, 2022. Accessed July 13, 2022. https:// www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
7. Wang Z, Pukszta M. Private Rooms, Semi-open areas, or open areas for chemotherapy care: perspectives of cancer patients, families, and nursing staff. HERD. 2018;11(3):94- 108. doi:10.1177/1937586718758445
8. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Accessed July 13, 2022. https://www.va.gov/vetdata /docs/specialreports/women_veterans_2015_final.pdf
9. Driscoll MA, Higgins DM, Seng EK, et al. Trauma, social support, family conflict, and chronic pain in recent service veterans: does gender matter? Pain Med. 2015;16(6):1101- 1111. doi:10.1111/pme.12744
10. Fox AB, Meyer EC, Vogt D. Attitudes about the VA healthcare setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58. doi:10.1037/a0038269
11. Virani SS, Woodard LD, Ramsey DJ, et al. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115(1):21-26. doi:10.1016/j.amjcard.2014.09.041
12. Tseng J. Sex, gender, and why the differences matter. Virtual Mentor. 2008;10(7):427-428. doi:10.1001/virtualmentor.2008.10.7.fred1-0807
13. Booij JC, Zegers M, Evers PMPJ, Hendricks M, Delnoij DMJ, Rademakers JJDJM. Improving cancer patient care: development of a generic cancer consumer quality index questionnaire for cancer patients. BMC Cancer. 2013;13(203). doi:10.1186/1471-2407-13-203
14. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113(12):3459-3466. doi:10.1002/cncr.23968 1
5. Schnur JB, Dillon MJ, Goldsmith RE, Montgomery GH. Cancer treatment experiences among survivors of childhood sexual abuse: a qualitative investigation of triggers and reactions to cumulative trauma. Palliat Support Care. 2018;16(6):767-776. doi:10.1017/S147895151700075X
16. Cadman L, Waller J, Ashdown-Barr L, Szarewski A. Barriers to cervical screening in women who have experienced sexual abuse: an exploratory study. J Fam Plann Reprod Health Care. 2012;38(4):214-220. doi:10.1136/jfprhc-2012-100378
17. Kelly S. The effects of childhood sexual abuse on women’s lives and their attitudes to cervical screening. J Fam Plann Reprod Health Care. 2012;38(4):212-213. doi:10.1136/jfprhc-2012-100418
18. McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep. 2006;121(4):435-444. doi:10.1177/003335490612100412
The Effect of Race on Outcomes in Veterans With Hepatocellular Carcinoma at a Single Center
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
Value of a Pharmacy-Adjudicated Community Care Prior Authorization Drug Request Service
Veterans’ access to medical care was expanded outside of US Department of Veterans Affairs (VA) facilities with the inception of the 2014 Veterans Access, Choice, and Accountability Act (Choice Act).1 This legislation aimed to remove barriers some veterans were experiencing, specifically access to health care. In subsequent years, approximately 17% of veterans receiving care from the VA did so under the Choice Act.2 The Choice Act positively impacted medical care access for veterans but presented new challenges for VA pharmacies processing community care (CC) prescriptions, including limited access to outside health records, lack of interface between CC prescribers and the VA order entry system, and limited awareness of the VA national formulary.3,4 These factors made it difficult for VA pharmacies to assess prescriptions for clinical appropriateness, evaluate patient safety parameters, and manage expenditures.
In 2019, the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act, which expanded CC support and better defined which veterans are able to receive care outside the VA, updated the Choice Act.4,5 However, VA pharmacies faced challenges in managing pharmacy drug costs and ensuring clinical appropriateness of prescription drug therapy. As a result, VA pharmacy departments have adjusted how they allocate workload, time, and funds.5
Pharmacists improve clinical outcomes and reduce health care costs by decreasing medication errors, unnecessary prescribing, and adverse drug events.6-12 Pharmacist-driven formulary management through evaluation of prior authorization drug requests (PADRs) has shown economic value.13,14 VA pharmacy review of community care PADRs is important because outside health care professionals (HCPs) might not be familiar with the VA formulary. This could lead to high volume of PADRs that do not meet criteria and could result in increased potential for medication misuse, adverse drug events, medication errors, and cost to the health system. It is imperative that CC orders are evaluated as critically as traditional orders.
The value of a centralized CC pharmacy team has not been assessed in the literature. The primary objective of this study was to assess the direct cost savings achieved through a centralized CC PADR process. Secondary objectives were to characterize the CC PADRs submitted to the site, including approval rate, reason for nonapproval, which medications were requested and by whom, and to compare CC prescriptions with other high-complexity (1a) VA facilities.
Community Care Pharmacy
VA health systems are stratified according to complexity, which reflects size, patient population, and services offered. This study was conducted at the Durham Veterans Affairs Health Care System (DVAHCS), North Carolina, a high-complexity, 251-bed, tertiary care referral, teaching, and research system. DVAHCS provides general and specialty medical, surgical, inpatient psychiatric, and ambulatory services, and serves as a major referral center.
DVAHCS created a centralized pharmacy team for processing CC prescriptions and managing customer service. This team’s goal is to increase CC prescription processing efficiency and transparency, ensure accountability of the health care team, and promote veteran-centric customer service. The pharmacy team includes a pharmacist program manager and a dedicated CC pharmacist with administrative support from a health benefits assistant and 4 pharmacy technicians. The CC pharmacy team assesses every new prescription to ensure the veteran is authorized to receive care in the community. Once eligibility is verified, a pharmacy technician or pharmacist evaluates the prescription to ensure it contains all required information, then contacts the prescriber for any missing data. If clinically appropriate, the pharmacist processes the prescription.
In 2020, the CC pharmacy team implemented a new process for reviewing and documenting CC prescriptions that require a PADR. The closed national VA formulary is set up so that all nonformulary medications and some formulary medications, including those that are restricted because of safety and/or cost, require a PADR.15 After a CC pharmacy technician confirms a veteran’s eligibility, the technician assesses whether the requested medication requires submitting a PADR to the VA internal electronic health record. The PADR is then adjudicated by a formulary management pharmacist, CC program manager, or CC pharmacist who reviews health records to determine whether the CC prescription meets VA medication use policy requirements.
If additional information is needed or an alternate medication is suggested, the pharmacist comments back on the PADR and a CC pharmacy technician contacts the prescriber. The PADR is canceled administratively then resubmitted once all information is obtained. While waiting for a response from the prescriber, the CC pharmacy technician contacts that veteran to give an update on the prescription status, as appropriate. Once there is sufficient information to adjudicate the PADR, the outcome is documented, and if approved, the order is processed.
Methods
The DVAHCS Institutional Review Board approved this retrospective review of CC PADRs submitted from June 1, 2020, through November 30, 2020. CC PADRs were excluded if they were duplicates or were reactivated administratively but had an initial submission date before the study period. Local data were collected for nonapproved CC PADRs including drug requested, dosage and directions, medication specialty, alternative drug recommended, drug acquisition cost, PADR submission date, PADR completion date, PADR nonapproval rationale, and documented time spent per PADR. Additional data was obtained for CC prescriptions at all 42 high-complexity VA facilities from the VA national CC prescription database for the study time interval and included total PADRs, PADR approval status, total CC prescription cost, and total CC fills.
Direct cost savings were calculated by assessing the cost of requested therapy that was not approved minus the cost of recommended therapy and cost to review all PADRs, as described by Britt and colleagues.13 The cost of the requested and recommended therapy was calculated based on VA drug acquisition cost at time of data collection and multiplied by the expected duration of therapy up to 1 year. For each CC prescription, duration of therapy was based on the duration limit in the prescription or annualized if no duration limit was documented. Cost of PADR review was calculated based on the total time pharmacists and pharmacy technicians documented for each step of the review process for a representative sample of 100 nonapproved PADRs and then multiplied by the salary plus benefits of an entry-level pharmacist and pharmacy technician.16 The eAppendix describes specific equations used for determining direct cost savings. Descriptive statistics were used to evaluate study results.
Results
During the 6-month study period, 611 CC PADRs were submitted to the pharmacy and 526 met inclusion criteria (Figure 1). Of those, 243 (46.2%) were approved and 283 (53.8%) were not approved. The cost of requested therapies for nonapproved CC PADRs totaled $584,565.48 and the cost of all recommended therapies was $57,473.59. The mean time per CC PADR was 24 minutes; 16 minutes for pharmacists and 8 minutes for pharmacy technicians. Given an hourly wage (plus benefits) of $67.25 for a pharmacist and $25.53 for a pharmacy technician, the total cost of review per CC PADR was $21.33. After subtracting the costs of all recommended therapies and review of all included CC PADRs, the process generated $515,872.31 in direct cost savings. After factoring in administrative lag time, such as HCP communication, an average of 8 calendar days was needed to complete a nonapproved PADR.
The most common rationale for PADR nonapproval was that the formulary alternative was not exhausted. Ondansetron orally disintegrating tablets was the most commonly nonapproved medication and azelastine was the most commonly approved medication. Dulaglutide was the most expensive nonapproved and tafamidis was the most expensive approved PADR (Table 1). Gastroenterology, endocrinology, and neurology were the top specialties for nonapproved PADRs while neurology, pulmonology, and endocrinology were the top specialties for approved PADRs (Table 2).
Several high-complexity VA facilities had no reported data; we used the median for the analysis to account for these outliers (Figure 2). The median (IQR) adjudicated CC PADRs for all facilities was 97 (20-175), median (IQR) CC PADR approval rate was 80.9% (63.7%-96.8%), median (IQR) total CC prescriptions was 8440 (2464-14,466), and median (IQR) cost per fill was $136.05 ($76.27-$221.28).
Discussion
This study demonstrated direct cost savings of $515,872.31 over 6 months with theadjudication of CC PADRs by a centralized CC pharmacy team. This could result in > $1,000,000 of cost savings per fiscal year.
The CC PADRs observed at DVAHCS had a 46.2% approval rate; almost one-half the approval rate of 84.1% of all PADRs submitted to the study site by VA HCPs captured by Britt and colleagues.13 Results from this study showed that coordination of care for nonapproved CC PADRs between the VA pharmacy and non-VA prescriber took an average of 8 calendar days. The noted CC PADR approval rate and administrative burden might be because of lack of familiarity of non-VA providers regarding the VA national formulary. The National VA Pharmacy Benefits Management determines the formulary using cost-effectiveness criteria that considers the medical literature and VA-specific contract pricing and prepares extensive guidance for restricted medications via relevant criteria for use.15 HCPs outside the VA might not know this information is available online. Because gastroenterology, endocrinology, and neurology specialty medications were among the most frequently nonapproved PADRs, VA formulary education could begin with CC HCPs in these practice areas.
This study showed that the CC PADR process was not solely driven by cost, but also included patient safety. Nonapproval rationale for some requests included submission without an indication, submission by a prescriber that did not have the authority to prescribe a type of medication, or contraindication based on patient-specific factors.
Compared with other VA high-complexity facilities, DVAHCS was among the top health care systems for total volume of CC prescriptions (n = 16,096) and among the lowest for cost/fill ($75.74). Similarly, DVAHCS was among the top sites for total adjudicated CC PADRs within the 6-month study period (n = 611) and the lowest approval rate (44.2%). This study shows that despite high volumes of overall CC prescriptions and CC PADRs, it is possible to maintain a low overall CC prescription cost/fill compared with other similarly complex sites across the country. Wide variance in reported results exists across high-complexity VA facilities because some sites had low to no CC fills and/or CC PADRs. This is likely a result of administrative differences when handling CC prescriptions and presents an opportunity to standardize this process nationally.
Limitations
CC PADRs were assessed during the COVID-19 pandemic, which might have resulted in lower-than-normal CC prescription and PADR volumes, therefore underestimating the potential for direct cost savings. Entry-level salary was used to demonstrate cost savings potential from the perspective of a newly hired CC team; however, the cost savings might have been less if the actual salaries of site personnel were higher. National contract pricing data were gathered at the time of data collection and might have been different than at the time of PADR submission. Chronic medication prescriptions were annualized, which could overestimate cost savings if the medication was discontinued or changed to an alternative therapy within that time period.
The study’s exclusion criteria could only be applied locally and did not include data received from the VA CC prescription database. This can be seen by the discrepancy in CC PADR approval rates from the local and national data (46.2% vs 44.2%, respectively) and CC PADR volume. High-complexity VA facility data were captured without assessing the CC prescription process at each site. As a result, definitive conclusions cannot be made regarding the impact of a centralized CC pharmacy team compared with other facilities.
Conclusions
Adjudication of CC PADRs by a centralized CC pharmacy team over a 6-month period provided > $500,000 in direct cost savings to a VA health care system. Considering the CC PADR approval rate seen in this study, the VA could allocate resources to educate CC providers about the VA formulary to increase the PADR approval rate and reduce administrative burden for VA pharmacies and prescribers. Future research should evaluate CC prescription handling practices at other VA facilities to compare the effectiveness among varying approaches and develop recommendations for a nationally standardized process.
Acknowledgments
Concept and design (AJJ, JNB, RBB, LAM, MD, MGH); acquisition of data (AJJ, MGH); analysis and interpretation of data (AJJ, JNB, RBB, LAM, MD, MGH); drafting of the manuscript (AJJ); critical revision of the manuscript for important intellectual content (AJJ, JNB, RBB, LAM, MD, MGH); statistical analysis (AJJ); administrative, technical, or logistic support (LAM, MGH); and supervision (MGH).
1. Gellad WF, Cunningham FE, Good CB, et al. Pharmacy use in the first year of the Veterans Choice Program: a mixed-methods evaluation. Med Care. 2017(7 suppl 1);55:S26. doi:10.1097/MLR.0000000000000661
2. Mattocks KM, Yehia B. Evaluating the veterans choice program: lessons for developing a high-performing integrated network. Med Care. 2017(7 suppl 1);55:S1-S3. doi:10.1097/MLR.0000000000000743.
3. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(7 suppl 1):S71-S75. doi:10.1097/MLR.0000000000000667
4. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.08: VHA formulary management process. November 2, 2016. Accessed June 9, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3291
5. Massarweh NN, Itani KMF, Morris MS. The VA MISSION act and the future of veterans’ access to quality health care. JAMA. 2020;324:343-344. doi:10.1001/jama.2020.4505
6. Jourdan JP, Muzard A, Goyer I, et al. Impact of pharmacist interventions on clinical outcome and cost avoidance in a university teaching hospital. Int J Clin Pharm. 2018;40(6):1474-1481. doi:10.1007/s11096-018-0733-6
7. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077. doi:10.1093/ajhp/59.21.2070
8. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
9. De Rijdt T, Willems L, Simoens S. Economic effects of clinical pharmacy interventions: a literature review. Am J Health Syst Pharm. 2008;65(12):1161-1172. doi:10.2146/ajhp070506
10. Perez A, Doloresco F, Hoffman J, et al. Economic evaluation of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2009;29(1):128. doi:10.1592/phco.29.1.128
11. Nesbit TW, Shermock KM, Bobek MB, et al. Implementation and pharmacoeconomic analysis of a clinical staff pharmacist practice model. Am J Health Syst Pharm. 2001;58(9):784-790. doi:10.1093/ajhp/58.9.784
12. Yang S, Britt RB, Hashem MG, Brown JN. Outcomes of pharmacy-led hepatitis C direct-acting antiviral utilization management at a Veterans Affairs medical center. J Manag Care Pharm. 2017;23(3):364-369. doi:10.18553/jmcp.2017.23.3.364
13. Britt RB, Hashem MG, Bryan WE III, Kothapalli R, Brown JN. Economic outcomes associated with a pharmacist-adjudicated formulary consult service in a Veterans Affairs medical center. J Manag Care Pharm. 2016;22(9):1051-1061. doi:10.18553/jmcp.2016.22.9.1051
14. Jacob S, Britt RB, Bryan WE, Hashem MG, Hale JC, Brown JN. Economic outcomes associated with safety interventions by a pharmacist-adjudicated prior authorization consult service. J Manag Care Pharm. 2019;25(3):411-416. doi:10.18553/jmcp.2019.25.3.411
15. Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements, 2004-2014. J Manag Care Spec Pharm. 2016;22(9):1058-1063. doi:10.18553/jmcp.2016.22.9.1058
16. US Department of Veterans Affairs, Office of the Chief Human Capital Officer. Title 38 Pay Schedules. Updated January 26, 2022. Accessed June 9, 2022. https://www.va.gov/ohrm/pay
Veterans’ access to medical care was expanded outside of US Department of Veterans Affairs (VA) facilities with the inception of the 2014 Veterans Access, Choice, and Accountability Act (Choice Act).1 This legislation aimed to remove barriers some veterans were experiencing, specifically access to health care. In subsequent years, approximately 17% of veterans receiving care from the VA did so under the Choice Act.2 The Choice Act positively impacted medical care access for veterans but presented new challenges for VA pharmacies processing community care (CC) prescriptions, including limited access to outside health records, lack of interface between CC prescribers and the VA order entry system, and limited awareness of the VA national formulary.3,4 These factors made it difficult for VA pharmacies to assess prescriptions for clinical appropriateness, evaluate patient safety parameters, and manage expenditures.
In 2019, the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act, which expanded CC support and better defined which veterans are able to receive care outside the VA, updated the Choice Act.4,5 However, VA pharmacies faced challenges in managing pharmacy drug costs and ensuring clinical appropriateness of prescription drug therapy. As a result, VA pharmacy departments have adjusted how they allocate workload, time, and funds.5
Pharmacists improve clinical outcomes and reduce health care costs by decreasing medication errors, unnecessary prescribing, and adverse drug events.6-12 Pharmacist-driven formulary management through evaluation of prior authorization drug requests (PADRs) has shown economic value.13,14 VA pharmacy review of community care PADRs is important because outside health care professionals (HCPs) might not be familiar with the VA formulary. This could lead to high volume of PADRs that do not meet criteria and could result in increased potential for medication misuse, adverse drug events, medication errors, and cost to the health system. It is imperative that CC orders are evaluated as critically as traditional orders.
The value of a centralized CC pharmacy team has not been assessed in the literature. The primary objective of this study was to assess the direct cost savings achieved through a centralized CC PADR process. Secondary objectives were to characterize the CC PADRs submitted to the site, including approval rate, reason for nonapproval, which medications were requested and by whom, and to compare CC prescriptions with other high-complexity (1a) VA facilities.
Community Care Pharmacy
VA health systems are stratified according to complexity, which reflects size, patient population, and services offered. This study was conducted at the Durham Veterans Affairs Health Care System (DVAHCS), North Carolina, a high-complexity, 251-bed, tertiary care referral, teaching, and research system. DVAHCS provides general and specialty medical, surgical, inpatient psychiatric, and ambulatory services, and serves as a major referral center.
DVAHCS created a centralized pharmacy team for processing CC prescriptions and managing customer service. This team’s goal is to increase CC prescription processing efficiency and transparency, ensure accountability of the health care team, and promote veteran-centric customer service. The pharmacy team includes a pharmacist program manager and a dedicated CC pharmacist with administrative support from a health benefits assistant and 4 pharmacy technicians. The CC pharmacy team assesses every new prescription to ensure the veteran is authorized to receive care in the community. Once eligibility is verified, a pharmacy technician or pharmacist evaluates the prescription to ensure it contains all required information, then contacts the prescriber for any missing data. If clinically appropriate, the pharmacist processes the prescription.
In 2020, the CC pharmacy team implemented a new process for reviewing and documenting CC prescriptions that require a PADR. The closed national VA formulary is set up so that all nonformulary medications and some formulary medications, including those that are restricted because of safety and/or cost, require a PADR.15 After a CC pharmacy technician confirms a veteran’s eligibility, the technician assesses whether the requested medication requires submitting a PADR to the VA internal electronic health record. The PADR is then adjudicated by a formulary management pharmacist, CC program manager, or CC pharmacist who reviews health records to determine whether the CC prescription meets VA medication use policy requirements.
If additional information is needed or an alternate medication is suggested, the pharmacist comments back on the PADR and a CC pharmacy technician contacts the prescriber. The PADR is canceled administratively then resubmitted once all information is obtained. While waiting for a response from the prescriber, the CC pharmacy technician contacts that veteran to give an update on the prescription status, as appropriate. Once there is sufficient information to adjudicate the PADR, the outcome is documented, and if approved, the order is processed.
Methods
The DVAHCS Institutional Review Board approved this retrospective review of CC PADRs submitted from June 1, 2020, through November 30, 2020. CC PADRs were excluded if they were duplicates or were reactivated administratively but had an initial submission date before the study period. Local data were collected for nonapproved CC PADRs including drug requested, dosage and directions, medication specialty, alternative drug recommended, drug acquisition cost, PADR submission date, PADR completion date, PADR nonapproval rationale, and documented time spent per PADR. Additional data was obtained for CC prescriptions at all 42 high-complexity VA facilities from the VA national CC prescription database for the study time interval and included total PADRs, PADR approval status, total CC prescription cost, and total CC fills.
Direct cost savings were calculated by assessing the cost of requested therapy that was not approved minus the cost of recommended therapy and cost to review all PADRs, as described by Britt and colleagues.13 The cost of the requested and recommended therapy was calculated based on VA drug acquisition cost at time of data collection and multiplied by the expected duration of therapy up to 1 year. For each CC prescription, duration of therapy was based on the duration limit in the prescription or annualized if no duration limit was documented. Cost of PADR review was calculated based on the total time pharmacists and pharmacy technicians documented for each step of the review process for a representative sample of 100 nonapproved PADRs and then multiplied by the salary plus benefits of an entry-level pharmacist and pharmacy technician.16 The eAppendix describes specific equations used for determining direct cost savings. Descriptive statistics were used to evaluate study results.
Results
During the 6-month study period, 611 CC PADRs were submitted to the pharmacy and 526 met inclusion criteria (Figure 1). Of those, 243 (46.2%) were approved and 283 (53.8%) were not approved. The cost of requested therapies for nonapproved CC PADRs totaled $584,565.48 and the cost of all recommended therapies was $57,473.59. The mean time per CC PADR was 24 minutes; 16 minutes for pharmacists and 8 minutes for pharmacy technicians. Given an hourly wage (plus benefits) of $67.25 for a pharmacist and $25.53 for a pharmacy technician, the total cost of review per CC PADR was $21.33. After subtracting the costs of all recommended therapies and review of all included CC PADRs, the process generated $515,872.31 in direct cost savings. After factoring in administrative lag time, such as HCP communication, an average of 8 calendar days was needed to complete a nonapproved PADR.
The most common rationale for PADR nonapproval was that the formulary alternative was not exhausted. Ondansetron orally disintegrating tablets was the most commonly nonapproved medication and azelastine was the most commonly approved medication. Dulaglutide was the most expensive nonapproved and tafamidis was the most expensive approved PADR (Table 1). Gastroenterology, endocrinology, and neurology were the top specialties for nonapproved PADRs while neurology, pulmonology, and endocrinology were the top specialties for approved PADRs (Table 2).
Several high-complexity VA facilities had no reported data; we used the median for the analysis to account for these outliers (Figure 2). The median (IQR) adjudicated CC PADRs for all facilities was 97 (20-175), median (IQR) CC PADR approval rate was 80.9% (63.7%-96.8%), median (IQR) total CC prescriptions was 8440 (2464-14,466), and median (IQR) cost per fill was $136.05 ($76.27-$221.28).
Discussion
This study demonstrated direct cost savings of $515,872.31 over 6 months with theadjudication of CC PADRs by a centralized CC pharmacy team. This could result in > $1,000,000 of cost savings per fiscal year.
The CC PADRs observed at DVAHCS had a 46.2% approval rate; almost one-half the approval rate of 84.1% of all PADRs submitted to the study site by VA HCPs captured by Britt and colleagues.13 Results from this study showed that coordination of care for nonapproved CC PADRs between the VA pharmacy and non-VA prescriber took an average of 8 calendar days. The noted CC PADR approval rate and administrative burden might be because of lack of familiarity of non-VA providers regarding the VA national formulary. The National VA Pharmacy Benefits Management determines the formulary using cost-effectiveness criteria that considers the medical literature and VA-specific contract pricing and prepares extensive guidance for restricted medications via relevant criteria for use.15 HCPs outside the VA might not know this information is available online. Because gastroenterology, endocrinology, and neurology specialty medications were among the most frequently nonapproved PADRs, VA formulary education could begin with CC HCPs in these practice areas.
This study showed that the CC PADR process was not solely driven by cost, but also included patient safety. Nonapproval rationale for some requests included submission without an indication, submission by a prescriber that did not have the authority to prescribe a type of medication, or contraindication based on patient-specific factors.
Compared with other VA high-complexity facilities, DVAHCS was among the top health care systems for total volume of CC prescriptions (n = 16,096) and among the lowest for cost/fill ($75.74). Similarly, DVAHCS was among the top sites for total adjudicated CC PADRs within the 6-month study period (n = 611) and the lowest approval rate (44.2%). This study shows that despite high volumes of overall CC prescriptions and CC PADRs, it is possible to maintain a low overall CC prescription cost/fill compared with other similarly complex sites across the country. Wide variance in reported results exists across high-complexity VA facilities because some sites had low to no CC fills and/or CC PADRs. This is likely a result of administrative differences when handling CC prescriptions and presents an opportunity to standardize this process nationally.
Limitations
CC PADRs were assessed during the COVID-19 pandemic, which might have resulted in lower-than-normal CC prescription and PADR volumes, therefore underestimating the potential for direct cost savings. Entry-level salary was used to demonstrate cost savings potential from the perspective of a newly hired CC team; however, the cost savings might have been less if the actual salaries of site personnel were higher. National contract pricing data were gathered at the time of data collection and might have been different than at the time of PADR submission. Chronic medication prescriptions were annualized, which could overestimate cost savings if the medication was discontinued or changed to an alternative therapy within that time period.
The study’s exclusion criteria could only be applied locally and did not include data received from the VA CC prescription database. This can be seen by the discrepancy in CC PADR approval rates from the local and national data (46.2% vs 44.2%, respectively) and CC PADR volume. High-complexity VA facility data were captured without assessing the CC prescription process at each site. As a result, definitive conclusions cannot be made regarding the impact of a centralized CC pharmacy team compared with other facilities.
Conclusions
Adjudication of CC PADRs by a centralized CC pharmacy team over a 6-month period provided > $500,000 in direct cost savings to a VA health care system. Considering the CC PADR approval rate seen in this study, the VA could allocate resources to educate CC providers about the VA formulary to increase the PADR approval rate and reduce administrative burden for VA pharmacies and prescribers. Future research should evaluate CC prescription handling practices at other VA facilities to compare the effectiveness among varying approaches and develop recommendations for a nationally standardized process.
Acknowledgments
Concept and design (AJJ, JNB, RBB, LAM, MD, MGH); acquisition of data (AJJ, MGH); analysis and interpretation of data (AJJ, JNB, RBB, LAM, MD, MGH); drafting of the manuscript (AJJ); critical revision of the manuscript for important intellectual content (AJJ, JNB, RBB, LAM, MD, MGH); statistical analysis (AJJ); administrative, technical, or logistic support (LAM, MGH); and supervision (MGH).
Veterans’ access to medical care was expanded outside of US Department of Veterans Affairs (VA) facilities with the inception of the 2014 Veterans Access, Choice, and Accountability Act (Choice Act).1 This legislation aimed to remove barriers some veterans were experiencing, specifically access to health care. In subsequent years, approximately 17% of veterans receiving care from the VA did so under the Choice Act.2 The Choice Act positively impacted medical care access for veterans but presented new challenges for VA pharmacies processing community care (CC) prescriptions, including limited access to outside health records, lack of interface between CC prescribers and the VA order entry system, and limited awareness of the VA national formulary.3,4 These factors made it difficult for VA pharmacies to assess prescriptions for clinical appropriateness, evaluate patient safety parameters, and manage expenditures.
In 2019, the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act, which expanded CC support and better defined which veterans are able to receive care outside the VA, updated the Choice Act.4,5 However, VA pharmacies faced challenges in managing pharmacy drug costs and ensuring clinical appropriateness of prescription drug therapy. As a result, VA pharmacy departments have adjusted how they allocate workload, time, and funds.5
Pharmacists improve clinical outcomes and reduce health care costs by decreasing medication errors, unnecessary prescribing, and adverse drug events.6-12 Pharmacist-driven formulary management through evaluation of prior authorization drug requests (PADRs) has shown economic value.13,14 VA pharmacy review of community care PADRs is important because outside health care professionals (HCPs) might not be familiar with the VA formulary. This could lead to high volume of PADRs that do not meet criteria and could result in increased potential for medication misuse, adverse drug events, medication errors, and cost to the health system. It is imperative that CC orders are evaluated as critically as traditional orders.
The value of a centralized CC pharmacy team has not been assessed in the literature. The primary objective of this study was to assess the direct cost savings achieved through a centralized CC PADR process. Secondary objectives were to characterize the CC PADRs submitted to the site, including approval rate, reason for nonapproval, which medications were requested and by whom, and to compare CC prescriptions with other high-complexity (1a) VA facilities.
Community Care Pharmacy
VA health systems are stratified according to complexity, which reflects size, patient population, and services offered. This study was conducted at the Durham Veterans Affairs Health Care System (DVAHCS), North Carolina, a high-complexity, 251-bed, tertiary care referral, teaching, and research system. DVAHCS provides general and specialty medical, surgical, inpatient psychiatric, and ambulatory services, and serves as a major referral center.
DVAHCS created a centralized pharmacy team for processing CC prescriptions and managing customer service. This team’s goal is to increase CC prescription processing efficiency and transparency, ensure accountability of the health care team, and promote veteran-centric customer service. The pharmacy team includes a pharmacist program manager and a dedicated CC pharmacist with administrative support from a health benefits assistant and 4 pharmacy technicians. The CC pharmacy team assesses every new prescription to ensure the veteran is authorized to receive care in the community. Once eligibility is verified, a pharmacy technician or pharmacist evaluates the prescription to ensure it contains all required information, then contacts the prescriber for any missing data. If clinically appropriate, the pharmacist processes the prescription.
In 2020, the CC pharmacy team implemented a new process for reviewing and documenting CC prescriptions that require a PADR. The closed national VA formulary is set up so that all nonformulary medications and some formulary medications, including those that are restricted because of safety and/or cost, require a PADR.15 After a CC pharmacy technician confirms a veteran’s eligibility, the technician assesses whether the requested medication requires submitting a PADR to the VA internal electronic health record. The PADR is then adjudicated by a formulary management pharmacist, CC program manager, or CC pharmacist who reviews health records to determine whether the CC prescription meets VA medication use policy requirements.
If additional information is needed or an alternate medication is suggested, the pharmacist comments back on the PADR and a CC pharmacy technician contacts the prescriber. The PADR is canceled administratively then resubmitted once all information is obtained. While waiting for a response from the prescriber, the CC pharmacy technician contacts that veteran to give an update on the prescription status, as appropriate. Once there is sufficient information to adjudicate the PADR, the outcome is documented, and if approved, the order is processed.
Methods
The DVAHCS Institutional Review Board approved this retrospective review of CC PADRs submitted from June 1, 2020, through November 30, 2020. CC PADRs were excluded if they were duplicates or were reactivated administratively but had an initial submission date before the study period. Local data were collected for nonapproved CC PADRs including drug requested, dosage and directions, medication specialty, alternative drug recommended, drug acquisition cost, PADR submission date, PADR completion date, PADR nonapproval rationale, and documented time spent per PADR. Additional data was obtained for CC prescriptions at all 42 high-complexity VA facilities from the VA national CC prescription database for the study time interval and included total PADRs, PADR approval status, total CC prescription cost, and total CC fills.
Direct cost savings were calculated by assessing the cost of requested therapy that was not approved minus the cost of recommended therapy and cost to review all PADRs, as described by Britt and colleagues.13 The cost of the requested and recommended therapy was calculated based on VA drug acquisition cost at time of data collection and multiplied by the expected duration of therapy up to 1 year. For each CC prescription, duration of therapy was based on the duration limit in the prescription or annualized if no duration limit was documented. Cost of PADR review was calculated based on the total time pharmacists and pharmacy technicians documented for each step of the review process for a representative sample of 100 nonapproved PADRs and then multiplied by the salary plus benefits of an entry-level pharmacist and pharmacy technician.16 The eAppendix describes specific equations used for determining direct cost savings. Descriptive statistics were used to evaluate study results.
Results
During the 6-month study period, 611 CC PADRs were submitted to the pharmacy and 526 met inclusion criteria (Figure 1). Of those, 243 (46.2%) were approved and 283 (53.8%) were not approved. The cost of requested therapies for nonapproved CC PADRs totaled $584,565.48 and the cost of all recommended therapies was $57,473.59. The mean time per CC PADR was 24 minutes; 16 minutes for pharmacists and 8 minutes for pharmacy technicians. Given an hourly wage (plus benefits) of $67.25 for a pharmacist and $25.53 for a pharmacy technician, the total cost of review per CC PADR was $21.33. After subtracting the costs of all recommended therapies and review of all included CC PADRs, the process generated $515,872.31 in direct cost savings. After factoring in administrative lag time, such as HCP communication, an average of 8 calendar days was needed to complete a nonapproved PADR.
The most common rationale for PADR nonapproval was that the formulary alternative was not exhausted. Ondansetron orally disintegrating tablets was the most commonly nonapproved medication and azelastine was the most commonly approved medication. Dulaglutide was the most expensive nonapproved and tafamidis was the most expensive approved PADR (Table 1). Gastroenterology, endocrinology, and neurology were the top specialties for nonapproved PADRs while neurology, pulmonology, and endocrinology were the top specialties for approved PADRs (Table 2).
Several high-complexity VA facilities had no reported data; we used the median for the analysis to account for these outliers (Figure 2). The median (IQR) adjudicated CC PADRs for all facilities was 97 (20-175), median (IQR) CC PADR approval rate was 80.9% (63.7%-96.8%), median (IQR) total CC prescriptions was 8440 (2464-14,466), and median (IQR) cost per fill was $136.05 ($76.27-$221.28).
Discussion
This study demonstrated direct cost savings of $515,872.31 over 6 months with theadjudication of CC PADRs by a centralized CC pharmacy team. This could result in > $1,000,000 of cost savings per fiscal year.
The CC PADRs observed at DVAHCS had a 46.2% approval rate; almost one-half the approval rate of 84.1% of all PADRs submitted to the study site by VA HCPs captured by Britt and colleagues.13 Results from this study showed that coordination of care for nonapproved CC PADRs between the VA pharmacy and non-VA prescriber took an average of 8 calendar days. The noted CC PADR approval rate and administrative burden might be because of lack of familiarity of non-VA providers regarding the VA national formulary. The National VA Pharmacy Benefits Management determines the formulary using cost-effectiveness criteria that considers the medical literature and VA-specific contract pricing and prepares extensive guidance for restricted medications via relevant criteria for use.15 HCPs outside the VA might not know this information is available online. Because gastroenterology, endocrinology, and neurology specialty medications were among the most frequently nonapproved PADRs, VA formulary education could begin with CC HCPs in these practice areas.
This study showed that the CC PADR process was not solely driven by cost, but also included patient safety. Nonapproval rationale for some requests included submission without an indication, submission by a prescriber that did not have the authority to prescribe a type of medication, or contraindication based on patient-specific factors.
Compared with other VA high-complexity facilities, DVAHCS was among the top health care systems for total volume of CC prescriptions (n = 16,096) and among the lowest for cost/fill ($75.74). Similarly, DVAHCS was among the top sites for total adjudicated CC PADRs within the 6-month study period (n = 611) and the lowest approval rate (44.2%). This study shows that despite high volumes of overall CC prescriptions and CC PADRs, it is possible to maintain a low overall CC prescription cost/fill compared with other similarly complex sites across the country. Wide variance in reported results exists across high-complexity VA facilities because some sites had low to no CC fills and/or CC PADRs. This is likely a result of administrative differences when handling CC prescriptions and presents an opportunity to standardize this process nationally.
Limitations
CC PADRs were assessed during the COVID-19 pandemic, which might have resulted in lower-than-normal CC prescription and PADR volumes, therefore underestimating the potential for direct cost savings. Entry-level salary was used to demonstrate cost savings potential from the perspective of a newly hired CC team; however, the cost savings might have been less if the actual salaries of site personnel were higher. National contract pricing data were gathered at the time of data collection and might have been different than at the time of PADR submission. Chronic medication prescriptions were annualized, which could overestimate cost savings if the medication was discontinued or changed to an alternative therapy within that time period.
The study’s exclusion criteria could only be applied locally and did not include data received from the VA CC prescription database. This can be seen by the discrepancy in CC PADR approval rates from the local and national data (46.2% vs 44.2%, respectively) and CC PADR volume. High-complexity VA facility data were captured without assessing the CC prescription process at each site. As a result, definitive conclusions cannot be made regarding the impact of a centralized CC pharmacy team compared with other facilities.
Conclusions
Adjudication of CC PADRs by a centralized CC pharmacy team over a 6-month period provided > $500,000 in direct cost savings to a VA health care system. Considering the CC PADR approval rate seen in this study, the VA could allocate resources to educate CC providers about the VA formulary to increase the PADR approval rate and reduce administrative burden for VA pharmacies and prescribers. Future research should evaluate CC prescription handling practices at other VA facilities to compare the effectiveness among varying approaches and develop recommendations for a nationally standardized process.
Acknowledgments
Concept and design (AJJ, JNB, RBB, LAM, MD, MGH); acquisition of data (AJJ, MGH); analysis and interpretation of data (AJJ, JNB, RBB, LAM, MD, MGH); drafting of the manuscript (AJJ); critical revision of the manuscript for important intellectual content (AJJ, JNB, RBB, LAM, MD, MGH); statistical analysis (AJJ); administrative, technical, or logistic support (LAM, MGH); and supervision (MGH).
1. Gellad WF, Cunningham FE, Good CB, et al. Pharmacy use in the first year of the Veterans Choice Program: a mixed-methods evaluation. Med Care. 2017(7 suppl 1);55:S26. doi:10.1097/MLR.0000000000000661
2. Mattocks KM, Yehia B. Evaluating the veterans choice program: lessons for developing a high-performing integrated network. Med Care. 2017(7 suppl 1);55:S1-S3. doi:10.1097/MLR.0000000000000743.
3. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(7 suppl 1):S71-S75. doi:10.1097/MLR.0000000000000667
4. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.08: VHA formulary management process. November 2, 2016. Accessed June 9, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3291
5. Massarweh NN, Itani KMF, Morris MS. The VA MISSION act and the future of veterans’ access to quality health care. JAMA. 2020;324:343-344. doi:10.1001/jama.2020.4505
6. Jourdan JP, Muzard A, Goyer I, et al. Impact of pharmacist interventions on clinical outcome and cost avoidance in a university teaching hospital. Int J Clin Pharm. 2018;40(6):1474-1481. doi:10.1007/s11096-018-0733-6
7. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077. doi:10.1093/ajhp/59.21.2070
8. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
9. De Rijdt T, Willems L, Simoens S. Economic effects of clinical pharmacy interventions: a literature review. Am J Health Syst Pharm. 2008;65(12):1161-1172. doi:10.2146/ajhp070506
10. Perez A, Doloresco F, Hoffman J, et al. Economic evaluation of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2009;29(1):128. doi:10.1592/phco.29.1.128
11. Nesbit TW, Shermock KM, Bobek MB, et al. Implementation and pharmacoeconomic analysis of a clinical staff pharmacist practice model. Am J Health Syst Pharm. 2001;58(9):784-790. doi:10.1093/ajhp/58.9.784
12. Yang S, Britt RB, Hashem MG, Brown JN. Outcomes of pharmacy-led hepatitis C direct-acting antiviral utilization management at a Veterans Affairs medical center. J Manag Care Pharm. 2017;23(3):364-369. doi:10.18553/jmcp.2017.23.3.364
13. Britt RB, Hashem MG, Bryan WE III, Kothapalli R, Brown JN. Economic outcomes associated with a pharmacist-adjudicated formulary consult service in a Veterans Affairs medical center. J Manag Care Pharm. 2016;22(9):1051-1061. doi:10.18553/jmcp.2016.22.9.1051
14. Jacob S, Britt RB, Bryan WE, Hashem MG, Hale JC, Brown JN. Economic outcomes associated with safety interventions by a pharmacist-adjudicated prior authorization consult service. J Manag Care Pharm. 2019;25(3):411-416. doi:10.18553/jmcp.2019.25.3.411
15. Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements, 2004-2014. J Manag Care Spec Pharm. 2016;22(9):1058-1063. doi:10.18553/jmcp.2016.22.9.1058
16. US Department of Veterans Affairs, Office of the Chief Human Capital Officer. Title 38 Pay Schedules. Updated January 26, 2022. Accessed June 9, 2022. https://www.va.gov/ohrm/pay
1. Gellad WF, Cunningham FE, Good CB, et al. Pharmacy use in the first year of the Veterans Choice Program: a mixed-methods evaluation. Med Care. 2017(7 suppl 1);55:S26. doi:10.1097/MLR.0000000000000661
2. Mattocks KM, Yehia B. Evaluating the veterans choice program: lessons for developing a high-performing integrated network. Med Care. 2017(7 suppl 1);55:S1-S3. doi:10.1097/MLR.0000000000000743.
3. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(7 suppl 1):S71-S75. doi:10.1097/MLR.0000000000000667
4. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.08: VHA formulary management process. November 2, 2016. Accessed June 9, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3291
5. Massarweh NN, Itani KMF, Morris MS. The VA MISSION act and the future of veterans’ access to quality health care. JAMA. 2020;324:343-344. doi:10.1001/jama.2020.4505
6. Jourdan JP, Muzard A, Goyer I, et al. Impact of pharmacist interventions on clinical outcome and cost avoidance in a university teaching hospital. Int J Clin Pharm. 2018;40(6):1474-1481. doi:10.1007/s11096-018-0733-6
7. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077. doi:10.1093/ajhp/59.21.2070
8. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
9. De Rijdt T, Willems L, Simoens S. Economic effects of clinical pharmacy interventions: a literature review. Am J Health Syst Pharm. 2008;65(12):1161-1172. doi:10.2146/ajhp070506
10. Perez A, Doloresco F, Hoffman J, et al. Economic evaluation of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2009;29(1):128. doi:10.1592/phco.29.1.128
11. Nesbit TW, Shermock KM, Bobek MB, et al. Implementation and pharmacoeconomic analysis of a clinical staff pharmacist practice model. Am J Health Syst Pharm. 2001;58(9):784-790. doi:10.1093/ajhp/58.9.784
12. Yang S, Britt RB, Hashem MG, Brown JN. Outcomes of pharmacy-led hepatitis C direct-acting antiviral utilization management at a Veterans Affairs medical center. J Manag Care Pharm. 2017;23(3):364-369. doi:10.18553/jmcp.2017.23.3.364
13. Britt RB, Hashem MG, Bryan WE III, Kothapalli R, Brown JN. Economic outcomes associated with a pharmacist-adjudicated formulary consult service in a Veterans Affairs medical center. J Manag Care Pharm. 2016;22(9):1051-1061. doi:10.18553/jmcp.2016.22.9.1051
14. Jacob S, Britt RB, Bryan WE, Hashem MG, Hale JC, Brown JN. Economic outcomes associated with safety interventions by a pharmacist-adjudicated prior authorization consult service. J Manag Care Pharm. 2019;25(3):411-416. doi:10.18553/jmcp.2019.25.3.411
15. Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements, 2004-2014. J Manag Care Spec Pharm. 2016;22(9):1058-1063. doi:10.18553/jmcp.2016.22.9.1058
16. US Department of Veterans Affairs, Office of the Chief Human Capital Officer. Title 38 Pay Schedules. Updated January 26, 2022. Accessed June 9, 2022. https://www.va.gov/ohrm/pay
Impact of Race on Outcomes of High-Risk Patients With Prostate Cancer Treated With Moderately Hypofractionated Radiotherapy in an Equal Access Setting
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
Racial Disparities in the Diagnosis of Psoriasis
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.
Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).
Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.

Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).

Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.

Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).

Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
Practice Points
- Skin of color (SOC) patients can wait 3 times longer to receive a diagnosis of psoriasis than non-SOC patients.
- Patients with SOC more often present with severe forms of psoriasis and are more likely to have palmoplantar psoriasis.
- Skin of color patients can be 4 times as likely to require a biopsy to confirm psoriasis diagnosis compared to non-SOC patients.
Discrepancies in Skin Cancer Screening Reporting Among Patients, Primary Care Physicians, and Patient Medical Records
Keratinocyte carcinoma (KC), or nonmelanoma skin cancer, is the most commonly diagnosed cancer in the United States.1 Basal cell carcinoma comprises the majority of all KCs.2,3 Squamous cell carcinoma is the second most common skin cancer, representing approximately 20% of KCs and accounting for the majority of KC-related deaths.4-7 Malignant melanoma represents the majority of all skin cancer–related deaths.8 The incidence of basal cell carcinoma, squamous cell carcinoma, and malignant melanoma in the United States is on the rise and carries substantial morbidity and mortality with notable social and economic burdens.1,8-10
Prevention is necessary to reduce skin cancer morbidity and mortality as well as rising treatment costs. The most commonly used skin cancer screening method among dermatologists is the visual full-body skin examination (FBSE), which is a noninvasive, safe, quick, and cost-effective method of early detection and prevention.11 To effectively confront the growing incidence and health care burden of skin cancer, primary care providers (PCPs) must join dermatologists in conducting FBSEs.12,13
Despite being the predominant means of secondary skin cancer prevention, the US Preventive Services Task Force (USPSTF) issued an I rating for insufficient evidence to assess the benefits vs harms of screening the adult general population by PCPs.14,15 A major barrier to studying screening is the lack of a standardized method for conducting and reporting FBSEs.13 Systematic thorough skin examination generally is not performed in the primary care setting.16-18
We aimed to investigate what occurs during an FBSE in the primary care setting and how often they are performed. We examined whether there was potential variation in the execution of the examination, what was perceived by the patient vs reported by the physician, and what was ultimately included in the medical record. Miscommunication between patient and provider regarding performance of FBSEs has previously been noted,17-19 and we sought to characterize and quantify that miscommunication. We hypothesized that there would be lower patient-reported FBSEs compared to physicians and patient medical records. We also hypothesized that there would be variability in how physicians screened for skin cancer.
METHODS
This study was cross-sectional and was conducted based on interviews and a review of medical records at secondary- and tertiary-level units (clinics and hospitals) across the United States. We examined baseline data from a randomized controlled trial of a Web-based skin cancer early detection continuing education course—the Basic Skin Cancer Triage curriculum. Complete details have been described elsewhere.12 This study was approved by the institutional review boards of the Providence Veterans Affairs Medical Center, Rhode Island Hospital, and Brown University (all in Providence, Rhode Island), as well as those of all recruitment sites.
Data were collected from 2005 to 2008 and included physician online surveys, patient telephone interviews, and patient medical record data abstracted by research assistants. Primary care providers included in the study were general internists, family physicians, or medicine-pediatrics practitioners who were recruited from 4 collaborating centers across the United States in the mid-Atlantic region, Ohio, Kansas, and southern California, and who had been in practice for at least a year. Patients were recruited from participating physician practices and selected by research assistants who traveled to each clinic for coordination, recruitment, and performance of medical record reviews. Patients were selected as having minimal risk of melanoma (eg, no signs of severe photodamage to the skin). Patients completed structured telephone surveys within 1 to 2 weeks of the office visit regarding the practices observed and clinical questions asked during their recent clinical encounter with their PCP.
Measures
Demographics—Demographic variables asked of physicians included age, sex, ethnicity, academic degree (MD vs DO), years in practice, training, and prior dermatology training. Demographic information asked of patients included age, sex, ethnicity, education, and household income.
Physician-Reported Examination and Counseling Variables—Physicians were asked to characterize their clinical practices, prompted by questions regarding performance of FBSEs: “Please think of a typical month and using the scale below, indicate how frequently you perform a total body skin exam during an annual exam (eg, periodic follow-up exam).” Physicians responded to 3 questions on a 5-point scale (1=never, 2=sometimes, 3=about half, 4=often, 5=almost always).
Patient-Reported Examination Variables—Patients also were asked to characterize the skin examination experienced in their clinical encounter with their PCP, including: “During your last visit, as far as you could tell, did your physician: (1) look at the skin on your back? (2) look at the skin on your belly area? (3) look at the skin on the back of your legs?” Patient responses were coded as yes, no, don’t know, or refused. Participants who refused were excluded from analysis; participants who responded are detailed in Table 1. In addition, patients also reported the level of undress with their physician by answering the following question: “During your last medical exam, did you: 1=keep your clothes on; 2=partially undress; 3=totally undress except for undergarments; 4=totally undress, including all undergarments?”
Patient Medical Record–Extracted Data—Research assistants used a structured abstract form to extract the information from the patient’s medical record and graded it as 0 (absence) or 1 (presence) from the medical record.
Statistical Analysis
Descriptive statistics included mean and standard deviation (SD) for continuous variables as well as frequency and percentage for categorical variables. Logit/logistic regression analysis was used to predict the odds of patient-reported outcomes that were binary with physician-reported variables as the predictor. Linear regression analysis was used to assess the association between 2 continuous variables. All analyses were conducted using SPSS version 24 (IBM).20 Significance criterion was set at α of .05.
RESULTS Demographics
The final sample included data from 53 physicians and 3343 patients. The study sample mean age (SD) was 50.3 (9.9) years for PCPs (n=53) and 59.8 (16.9) years for patients (n=3343). The physician sample was 36% female and predominantly White (83%). Ninety-one percent of the PCPs had an MD (the remaining had a DO degree), and the mean (SD) years practicing was 21.8 (10.6) years. Seventeen percent of PCPs were trained in internal medicine, 4% in internal medicine and pediatrics, and 79% family medicine; 79% of PCPs had received prior training in dermatology. The patient sample was 58% female, predominantly White (84%), non-Hispanic/Latinx (95%), had completed high school (94%), and earned more than $40,000 annually (66%).
Physician- and Patient-Reported FBSEs
Physicians reported performing FBSEs with variable frequency. Among PCPs who conducted FBSEs with greater frequency, there was a modest increase in the odds that patients reported a particular body part was examined (back: odds ratio [OR], 24.5% [95% CI, 1.18-1.31; P<.001]; abdomen: OR, 23.3% [95% CI, 1.17-1.30; P<.001]; backs of legs: OR, 20.4% [95% CI, 1.13-1.28; P<.001])(Table 1). The patient-reported level of undress during examination was significantly associated with physician-reported FBSE (β=0.16 [95% CI, 0.13-0.18; P<.001])(Table 2).
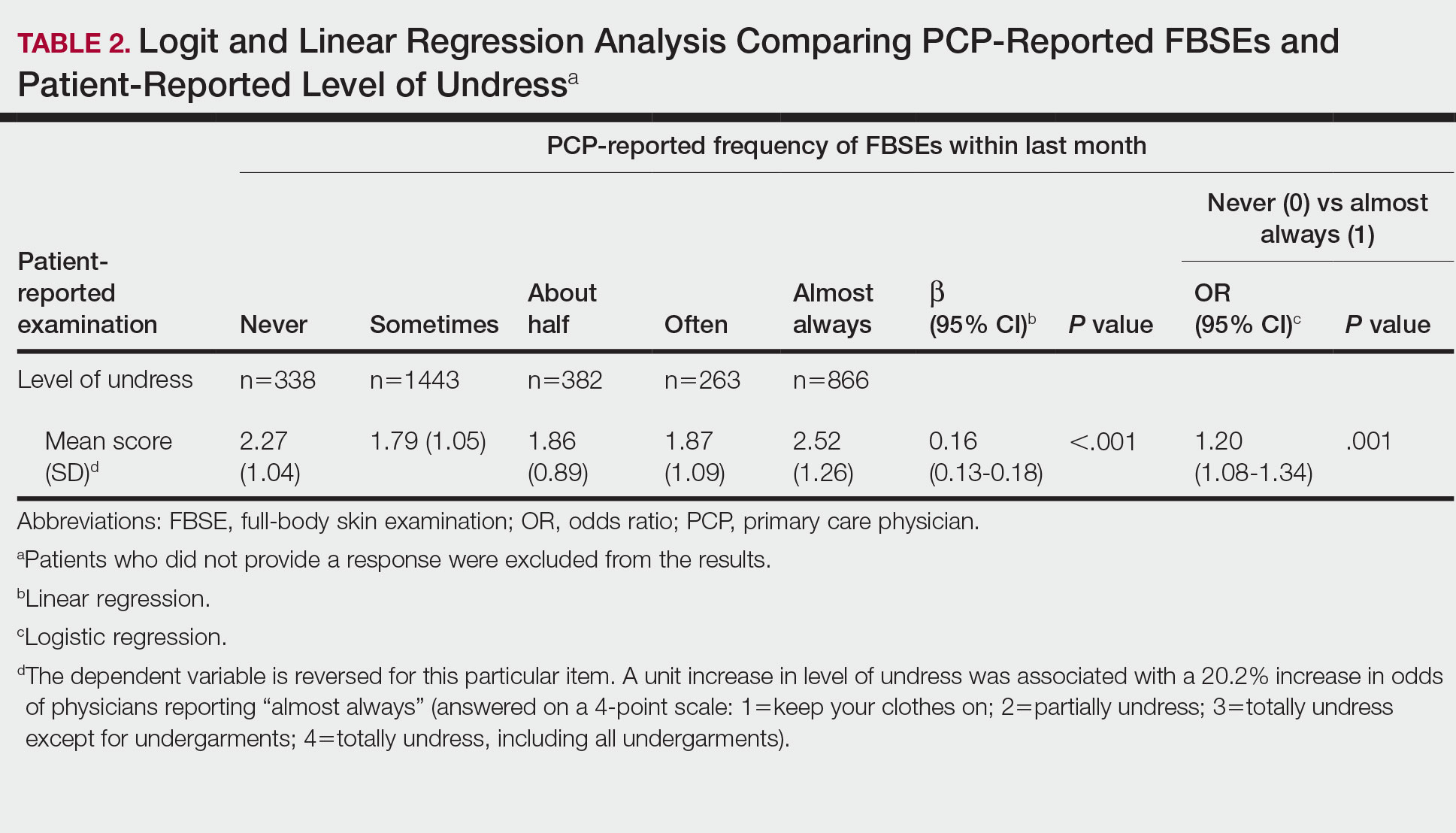
Because of the bimodal distribution of scores in the physician-reported frequency of FBSEs, particularly pertaining to the extreme points of the scale, we further repeated analysis with only the never and almost always groups (Table 1). Primary care providers who reported almost always for FBSE had 29.6% increased odds of patient-reported back examination (95% CI, 1.00-1.68; P=.048) and 59.3% increased odds of patient-reported abdomen examination (95% CI, 1.23-2.06; P<.001). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having never conducted an FBSE were 56%, 40%, and 26%, respectively. The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having almost always conducted an FBSE were 52%, 51%, and 30%, respectively. Raw percentages were calculated by dividing the number of "yes" responses by participants for each body part examined by thetotal number of participant responses (“yes” and “no”) for each respective body part. There was no significant change in odds of patient-reported backs of legs examined with PCP-reported never vs almost always conducting an FBSE. In addition, a greater patient-reported level of undress was associated with 20.2% increased odds of PCPs reporting almost always conducting an FBSE (95% CI, 1.08-1.34; P=.001).
FBSEs in Patient Medical Records
When comparing PCP-reported FBSE and report of FBSE in patient medical records, there was a 39.0% increased odds of the patient medical record indicating FBSE when physicians reported conducting an FBSE with greater frequency (95% CI, 1.30-1.48; P<.001)(eTable 1). When examining PCP-reported never vs almost always conducting an FBSE, a report of almost always was associated with 79.0% increased odds of the patient medical record indicating that an FBSE was conducted (95% CI, 1.28-2.49; P=.001). The raw percentage of the patient medical record indicating an FBSE was conducted when the PCP reported having never conducted an FBSE was 17% and 26% when the PCP reported having almost always conducted an FBSE.
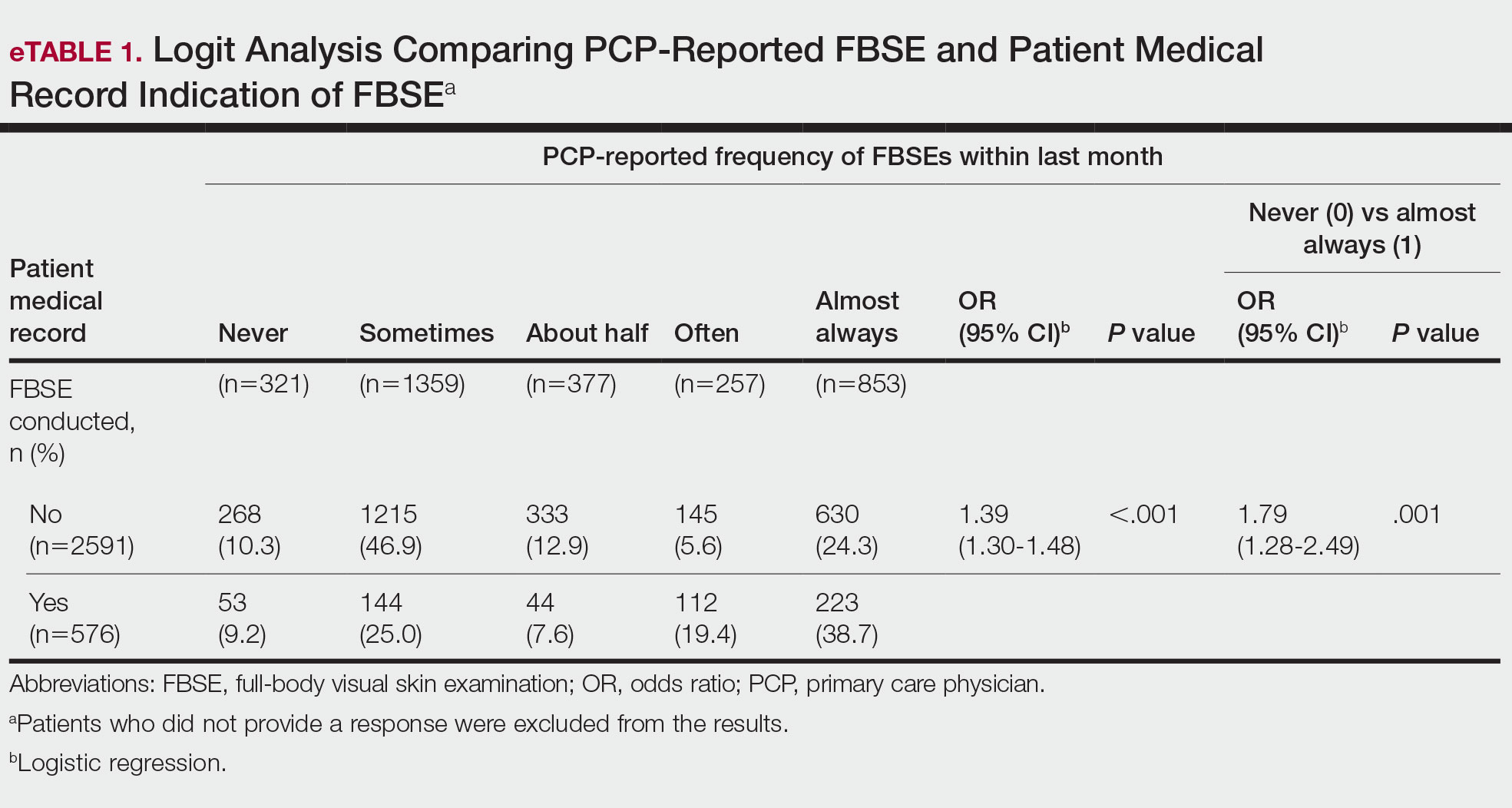
When comparing the patient-reported body part examined with patient FBSE medical record documentation, an indication of yes for FBSE on the patient medical record was associated with a considerable increase in odds that patients reported a particular body part was examined (back: 91.4% [95% CI, 1.59-2.31; P<.001]; abdomen: 75.0% [95% CI, 1.45-2.11; P<.001]; backs of legs: 91.6% [95% CI, 1.56-2.36; P<.001])(eTable 2). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined vs not examined when the patient medical record indicated an FBSE was completed were 24% vs 14%, 23% vs 15%, and 26% vs 16%, respectively. An increase in patient-reported level of undress was associated with a 57.0% increased odds of their medical record indicating an FBSE was conducted (95% CI, 1.45-1.70; P<.001).
COMMENT How PCPs Perform FBSEs Varies
We found that PCPs performed FBSEs with variable frequency, and among those who did, the patient report of their examination varied considerably (Table 1). There appears to be considerable ambiguity in each of these means of determining the extent to which the skin was inspected for skin cancer, which may render the task of improving such inspection more difficult. We asked patients whether their back, abdomen, and backs of legs were examined as an assessment of some of the variety of areas inspected during an FBSE. During a general well-visit appointment, a patient’s back and abdomen may be examined for multiple reasons. Patients may have misinterpreted elements of the pulmonary, cardiac, abdominal, or musculoskeletal examinations as being part of the FBSE. The back and abdomen—the least specific features of the FBSE—were reported by patients to be the most often examined. Conversely, the backs of the legs—the most specific feature of the FBSE—had the lowest odds of being examined (Table 1).
In addition to the potential limitations of patient awareness of physician activity, our results also could be explained by differences among PCPs in how they performed FBSEs. There is no standardized method of conducting an FBSE. Furthermore, not all medical students and residents are exposed to dermatology training. In our sample of 53 physicians, 79% had reported receiving dermatology training; however, we did not assess the extent to which they had been trained in conducting an FBSE and/or identifying malignant lesions. In an American survey of 659 medical students, more than two-thirds of students had never been trained or never examined a patient for skin cancer.21 In another American survey of 342 internal medicine, family medicine, pediatrics, and obstetrics/gynecology residents across 7 medical schools and 4 residency programs, more than three-quarters of residents had never been trained in skin cancer screening.22 Our findings reflect insufficient and inconsistent training in skin cancer screening and underscore the need for mandatory education to ensure quality FBSEs are performed in the primary care setting.
Frequency of PCPs Performing FBSEs
Similar to prior studies analyzing the frequency of FBSE performance in the primary care setting,16,19,23,24 more than half of our PCP sample reported sometimes to never conducting FBSEs. The percentage of physicians who reported conducting FBSEs in our sample was greater than the proportion reported by the National Health Interview Survey, in which only 8% of patients received an FBSE in the prior year by a PCP or obstetrician/gynecologist,16 but similar to a smaller patient study.19 In that study, 87% of patients, regardless of their skin cancer history, also reported that they would like their PCP to perform an FBSE regularly.19 Although some of our patient participants may have declined an FBSE, it is unlikely that that would have entirely accounted for the relatively low number of PCPs who reported frequently performing FBSEs.
Documentation in Medical Records of FBSEs
Compared to PCP self-reported performance of FBSEs, considerably fewer PCPs marked the patient medical record as having completed an FBSE. Among patients with medical records that indicated an FBSE had been conducted, they reported higher odds of all 3 body parts being examined, the highest being the backs of the legs. Also, when the patient medical record indicated an FBSE had been completed, the odds that the PCP reported an FBSE also were higher. The relatively low medical record documentation of FBSEs highlights the need for more rigorous enforcement of accurate documentation. However, among the cases that were recorded, it appeared that the content of the examinations was more consistent.
Benefits of PCP-Led FBSEs
Although the USPSTF issued an I rating for PCP-led FBSEs,14 multiple national medical societies, including the American Cancer Society,25 American Academy of Dermatology,26 and Skin Cancer Foundation,27 as well as international guidelines in Germany,28 Australia,29,30 and New Zealand,31 recommend regular FBSEs among the general or at-risk population; New Zealand and Australia have the highest incidence and prevalence of melanoma in the world.8 The benefits of physician-led FBSEs on detection of early-stage skin cancer, and in particular, melanoma detection, have been documented in numerous studies.30,32-38 However, the variability and often poor quality of skin screening may contribute in part to the just as numerous null results from prior skin screening studies,15 perpetuating the insufficient status of skin examinations by USPSTF standards.14 Our study underscores both the variability in frequency and content of PCP-administered FBSEs. It also highlights the need for standardization of screening examinations at the medical student, trainee, and physician level.
Study Limitations
The present study has several limitations. First, there was an unknown time lag between the FBSEs and physician self-reported surveys. Similarly, there was a variable time lag between the patient examination encounter and subsequent telephone survey. Both the physician and patient survey data may have been affected by recall bias. Second, patients were not asked directly whether an FBSE had been conducted. Furthermore, patients may not have appreciated whether the body part examined was part of the FBSE or another examination. Also, screenings often were not recorded in the medical record, assuming that the patient report and/or physician report was more accurate than the medical record.
Our study also was limited by demographics; our patient sample was largely comprised of White, educated, US adults, potentially limiting the generalizability of our findings. Conversely, a notable strength of our study was that our participants were recruited from 4 geographically diverse centers. Furthermore, we had a comparatively large sample size of patients and physicians. Also, the independent assessment of provider-reported examinations, objective assessment of medical records, and patient reports of their encounters provides a strong foundation for assessing the independent contributions of each data source.
CONCLUSION
Our study highlights the challenges future studies face in promoting skin cancer screening in the primary care setting. Our findings underscore the need for a standardized FBSE as well as clear clinical expectations regarding skin cancer screening that is expected of PCPs.
As long as skin cancer screening rates remain low in the United States, patients will be subject to potential delays and missed diagnoses, impacting morbidity and mortality.8 There are burgeoning resources and efforts in place to increase skin cancer screening. For example, free validated online training is available for early detection of melanoma and other skin cancers (https://www.visualdx.com/skin-cancer-education/).39-42 Future directions for bolstering screening numbers must focus on educating PCPs about skin cancer prevention and perhaps narrowing the screening population by age-appropriate risk assessments.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Dourmishev LA, Rusinova D, Botev I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J. 2013;4:12-17.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma outcomes: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309:243-251.
- Weinstock MA, Bogaars HA, Ashley M, et al. Nonmelanoma skin cancer mortality. a population-based study. Arch Dermatol. 1991;127:1194-1197.
- Matthews NH, Li W-Q, Qureshi AA, et al. Epidemiology of melanoma. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017:3-22.
- Cakir BO, Adamson P, Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2012;20:419-422.
- Guy GP, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol. 2007;143:21-28.
- Markova A, Weinstock MA, Risica P, et al. Effect of a web-based curriculum on primary care practice: basic skin cancer triage trial. Fam Med. 2013;45:558-568.
- Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
- Agency for Healthcare Research and Quality. Screening for skin cancer in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force. November 30, 2015. Accessed July 25, 2022. http://uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review159/skin-cancer-screening2
- Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for skin cancer in adults: updated evidence report and systematic review forthe US Preventive Services Task Force. JAMA. 2016;316:436-447.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Federman DG, Concato J, Caralis PV, et al. Screening for skin cancer in primary care settings. Arch Dermatol. 1997;133:1423-1425.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Federman DG, Kravetz JD, Tobin DG, et al. Full-body skin examinations: the patient’s perspective. Arch Dermatol. 2004;140:530-534.
- IBM. IBM SPSS Statistics for Windows. IBM Corp; 2015.
- Moore MM, Geller AC, Zhang Z, et al. Skin cancer examination teaching in US medical education. Arch Dermatol. 2006;142:439-444.
- Wise E, Singh D, Moore M, et al. Rates of skin cancer screening and prevention counseling by US medical residents. Arch Dermatol. 2009;145:1131-1136.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Coups EJ, Geller AC, Weinstock MA, et al. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123:439-445.
- American Cancer Society. Cancer facts & figures 2016. Accessed March 13, 2022. https://cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- American Academy of Dermatology. Skin cancer incidence rates. Updated April 22, 2022. Accessed August 1, 2022. https://www.aad.org/media/stats-skin-cancer
- Skin Cancer Foundation. Skin cancer prevention. Accessed July 25, 2022. http://skincancer.org/prevention/sun-protection/prevention-guidelines
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- Cancer Council Australia. Position statement: screening and early detection of skin cancer. Published July 2014. Accessed July 25, 2022. https://dermcoll.edu.au/wp-content/uploads/2014/05/PosStatEarlyDetectSkinCa.pdf
- Royal Australian College of General Practitioners. Guidelines for Preventive Activities in General Practice. 9th ed. The Royal Australian College of General Practitioners; 2016. Accessed July 27, 2022. https://www.racgp.org.au/download/Documents/Guidelines/Redbook9/17048-Red-Book-9th-Edition.pdf
- Cancer Council Australia and Australian Cancer Network and New Zealand Guidelines Group. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. The Cancer Council Australia and Australian Cancer Network, Sydney and New Zealand Guidelines Group, Wellington; 2008. Accessed July 27, 2022. https://www.health.govt.nz/system/files/documents/publications/melanoma-guideline-nov08-v2.pdf
- Swetter SM, Pollitt RA, Johnson TM, et al. Behavioral determinants of successful early melanoma detection: role of self and physician skin examination. Cancer. 2012;118:3725-3734.
- Terushkin V, Halpern AC. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481-500, viii.
- Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-458.
- Aitken JF, Elwood JM, Lowe JB, et al. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33-37.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Janda M, Lowe JB, Elwood M, et al. Do centralised skin screening clinics increase participation in melanoma screening (Australia)? Cancer Causes Control. 2006;17:161-168.
- Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54:105-114.
- Eide MJ, Asgari MM, Fletcher SW, et al. Effects on skills and practice from a web-based skin cancer course for primary care providers. J Am Board Fam Med. 2013;26:648-657.
- Weinstock MA, Ferris LK, Saul MI, et al. Downstream consequences of melanoma screening in a community practice setting: first results. Cancer. 2016;122:3152-3156.
- Matthews NH, Risica PM, Ferris LK, et al. Psychosocial impact of skin biopsies in the setting of melanoma screening: a cross-sectional survey. Br J Dermatol. 2019;180:664-665.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
Keratinocyte carcinoma (KC), or nonmelanoma skin cancer, is the most commonly diagnosed cancer in the United States.1 Basal cell carcinoma comprises the majority of all KCs.2,3 Squamous cell carcinoma is the second most common skin cancer, representing approximately 20% of KCs and accounting for the majority of KC-related deaths.4-7 Malignant melanoma represents the majority of all skin cancer–related deaths.8 The incidence of basal cell carcinoma, squamous cell carcinoma, and malignant melanoma in the United States is on the rise and carries substantial morbidity and mortality with notable social and economic burdens.1,8-10
Prevention is necessary to reduce skin cancer morbidity and mortality as well as rising treatment costs. The most commonly used skin cancer screening method among dermatologists is the visual full-body skin examination (FBSE), which is a noninvasive, safe, quick, and cost-effective method of early detection and prevention.11 To effectively confront the growing incidence and health care burden of skin cancer, primary care providers (PCPs) must join dermatologists in conducting FBSEs.12,13
Despite being the predominant means of secondary skin cancer prevention, the US Preventive Services Task Force (USPSTF) issued an I rating for insufficient evidence to assess the benefits vs harms of screening the adult general population by PCPs.14,15 A major barrier to studying screening is the lack of a standardized method for conducting and reporting FBSEs.13 Systematic thorough skin examination generally is not performed in the primary care setting.16-18
We aimed to investigate what occurs during an FBSE in the primary care setting and how often they are performed. We examined whether there was potential variation in the execution of the examination, what was perceived by the patient vs reported by the physician, and what was ultimately included in the medical record. Miscommunication between patient and provider regarding performance of FBSEs has previously been noted,17-19 and we sought to characterize and quantify that miscommunication. We hypothesized that there would be lower patient-reported FBSEs compared to physicians and patient medical records. We also hypothesized that there would be variability in how physicians screened for skin cancer.
METHODS
This study was cross-sectional and was conducted based on interviews and a review of medical records at secondary- and tertiary-level units (clinics and hospitals) across the United States. We examined baseline data from a randomized controlled trial of a Web-based skin cancer early detection continuing education course—the Basic Skin Cancer Triage curriculum. Complete details have been described elsewhere.12 This study was approved by the institutional review boards of the Providence Veterans Affairs Medical Center, Rhode Island Hospital, and Brown University (all in Providence, Rhode Island), as well as those of all recruitment sites.
Data were collected from 2005 to 2008 and included physician online surveys, patient telephone interviews, and patient medical record data abstracted by research assistants. Primary care providers included in the study were general internists, family physicians, or medicine-pediatrics practitioners who were recruited from 4 collaborating centers across the United States in the mid-Atlantic region, Ohio, Kansas, and southern California, and who had been in practice for at least a year. Patients were recruited from participating physician practices and selected by research assistants who traveled to each clinic for coordination, recruitment, and performance of medical record reviews. Patients were selected as having minimal risk of melanoma (eg, no signs of severe photodamage to the skin). Patients completed structured telephone surveys within 1 to 2 weeks of the office visit regarding the practices observed and clinical questions asked during their recent clinical encounter with their PCP.
Measures
Demographics—Demographic variables asked of physicians included age, sex, ethnicity, academic degree (MD vs DO), years in practice, training, and prior dermatology training. Demographic information asked of patients included age, sex, ethnicity, education, and household income.
Physician-Reported Examination and Counseling Variables—Physicians were asked to characterize their clinical practices, prompted by questions regarding performance of FBSEs: “Please think of a typical month and using the scale below, indicate how frequently you perform a total body skin exam during an annual exam (eg, periodic follow-up exam).” Physicians responded to 3 questions on a 5-point scale (1=never, 2=sometimes, 3=about half, 4=often, 5=almost always).
Patient-Reported Examination Variables—Patients also were asked to characterize the skin examination experienced in their clinical encounter with their PCP, including: “During your last visit, as far as you could tell, did your physician: (1) look at the skin on your back? (2) look at the skin on your belly area? (3) look at the skin on the back of your legs?” Patient responses were coded as yes, no, don’t know, or refused. Participants who refused were excluded from analysis; participants who responded are detailed in Table 1. In addition, patients also reported the level of undress with their physician by answering the following question: “During your last medical exam, did you: 1=keep your clothes on; 2=partially undress; 3=totally undress except for undergarments; 4=totally undress, including all undergarments?”

Patient Medical Record–Extracted Data—Research assistants used a structured abstract form to extract the information from the patient’s medical record and graded it as 0 (absence) or 1 (presence) from the medical record.
Statistical Analysis
Descriptive statistics included mean and standard deviation (SD) for continuous variables as well as frequency and percentage for categorical variables. Logit/logistic regression analysis was used to predict the odds of patient-reported outcomes that were binary with physician-reported variables as the predictor. Linear regression analysis was used to assess the association between 2 continuous variables. All analyses were conducted using SPSS version 24 (IBM).20 Significance criterion was set at α of .05.
RESULTS Demographics
The final sample included data from 53 physicians and 3343 patients. The study sample mean age (SD) was 50.3 (9.9) years for PCPs (n=53) and 59.8 (16.9) years for patients (n=3343). The physician sample was 36% female and predominantly White (83%). Ninety-one percent of the PCPs had an MD (the remaining had a DO degree), and the mean (SD) years practicing was 21.8 (10.6) years. Seventeen percent of PCPs were trained in internal medicine, 4% in internal medicine and pediatrics, and 79% family medicine; 79% of PCPs had received prior training in dermatology. The patient sample was 58% female, predominantly White (84%), non-Hispanic/Latinx (95%), had completed high school (94%), and earned more than $40,000 annually (66%).
Physician- and Patient-Reported FBSEs
Physicians reported performing FBSEs with variable frequency. Among PCPs who conducted FBSEs with greater frequency, there was a modest increase in the odds that patients reported a particular body part was examined (back: odds ratio [OR], 24.5% [95% CI, 1.18-1.31; P<.001]; abdomen: OR, 23.3% [95% CI, 1.17-1.30; P<.001]; backs of legs: OR, 20.4% [95% CI, 1.13-1.28; P<.001])(Table 1). The patient-reported level of undress during examination was significantly associated with physician-reported FBSE (β=0.16 [95% CI, 0.13-0.18; P<.001])(Table 2).

Because of the bimodal distribution of scores in the physician-reported frequency of FBSEs, particularly pertaining to the extreme points of the scale, we further repeated analysis with only the never and almost always groups (Table 1). Primary care providers who reported almost always for FBSE had 29.6% increased odds of patient-reported back examination (95% CI, 1.00-1.68; P=.048) and 59.3% increased odds of patient-reported abdomen examination (95% CI, 1.23-2.06; P<.001). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having never conducted an FBSE were 56%, 40%, and 26%, respectively. The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having almost always conducted an FBSE were 52%, 51%, and 30%, respectively. Raw percentages were calculated by dividing the number of "yes" responses by participants for each body part examined by thetotal number of participant responses (“yes” and “no”) for each respective body part. There was no significant change in odds of patient-reported backs of legs examined with PCP-reported never vs almost always conducting an FBSE. In addition, a greater patient-reported level of undress was associated with 20.2% increased odds of PCPs reporting almost always conducting an FBSE (95% CI, 1.08-1.34; P=.001).
FBSEs in Patient Medical Records
When comparing PCP-reported FBSE and report of FBSE in patient medical records, there was a 39.0% increased odds of the patient medical record indicating FBSE when physicians reported conducting an FBSE with greater frequency (95% CI, 1.30-1.48; P<.001)(eTable 1). When examining PCP-reported never vs almost always conducting an FBSE, a report of almost always was associated with 79.0% increased odds of the patient medical record indicating that an FBSE was conducted (95% CI, 1.28-2.49; P=.001). The raw percentage of the patient medical record indicating an FBSE was conducted when the PCP reported having never conducted an FBSE was 17% and 26% when the PCP reported having almost always conducted an FBSE.

When comparing the patient-reported body part examined with patient FBSE medical record documentation, an indication of yes for FBSE on the patient medical record was associated with a considerable increase in odds that patients reported a particular body part was examined (back: 91.4% [95% CI, 1.59-2.31; P<.001]; abdomen: 75.0% [95% CI, 1.45-2.11; P<.001]; backs of legs: 91.6% [95% CI, 1.56-2.36; P<.001])(eTable 2). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined vs not examined when the patient medical record indicated an FBSE was completed were 24% vs 14%, 23% vs 15%, and 26% vs 16%, respectively. An increase in patient-reported level of undress was associated with a 57.0% increased odds of their medical record indicating an FBSE was conducted (95% CI, 1.45-1.70; P<.001).

COMMENT How PCPs Perform FBSEs Varies
We found that PCPs performed FBSEs with variable frequency, and among those who did, the patient report of their examination varied considerably (Table 1). There appears to be considerable ambiguity in each of these means of determining the extent to which the skin was inspected for skin cancer, which may render the task of improving such inspection more difficult. We asked patients whether their back, abdomen, and backs of legs were examined as an assessment of some of the variety of areas inspected during an FBSE. During a general well-visit appointment, a patient’s back and abdomen may be examined for multiple reasons. Patients may have misinterpreted elements of the pulmonary, cardiac, abdominal, or musculoskeletal examinations as being part of the FBSE. The back and abdomen—the least specific features of the FBSE—were reported by patients to be the most often examined. Conversely, the backs of the legs—the most specific feature of the FBSE—had the lowest odds of being examined (Table 1).
In addition to the potential limitations of patient awareness of physician activity, our results also could be explained by differences among PCPs in how they performed FBSEs. There is no standardized method of conducting an FBSE. Furthermore, not all medical students and residents are exposed to dermatology training. In our sample of 53 physicians, 79% had reported receiving dermatology training; however, we did not assess the extent to which they had been trained in conducting an FBSE and/or identifying malignant lesions. In an American survey of 659 medical students, more than two-thirds of students had never been trained or never examined a patient for skin cancer.21 In another American survey of 342 internal medicine, family medicine, pediatrics, and obstetrics/gynecology residents across 7 medical schools and 4 residency programs, more than three-quarters of residents had never been trained in skin cancer screening.22 Our findings reflect insufficient and inconsistent training in skin cancer screening and underscore the need for mandatory education to ensure quality FBSEs are performed in the primary care setting.
Frequency of PCPs Performing FBSEs
Similar to prior studies analyzing the frequency of FBSE performance in the primary care setting,16,19,23,24 more than half of our PCP sample reported sometimes to never conducting FBSEs. The percentage of physicians who reported conducting FBSEs in our sample was greater than the proportion reported by the National Health Interview Survey, in which only 8% of patients received an FBSE in the prior year by a PCP or obstetrician/gynecologist,16 but similar to a smaller patient study.19 In that study, 87% of patients, regardless of their skin cancer history, also reported that they would like their PCP to perform an FBSE regularly.19 Although some of our patient participants may have declined an FBSE, it is unlikely that that would have entirely accounted for the relatively low number of PCPs who reported frequently performing FBSEs.
Documentation in Medical Records of FBSEs
Compared to PCP self-reported performance of FBSEs, considerably fewer PCPs marked the patient medical record as having completed an FBSE. Among patients with medical records that indicated an FBSE had been conducted, they reported higher odds of all 3 body parts being examined, the highest being the backs of the legs. Also, when the patient medical record indicated an FBSE had been completed, the odds that the PCP reported an FBSE also were higher. The relatively low medical record documentation of FBSEs highlights the need for more rigorous enforcement of accurate documentation. However, among the cases that were recorded, it appeared that the content of the examinations was more consistent.
Benefits of PCP-Led FBSEs
Although the USPSTF issued an I rating for PCP-led FBSEs,14 multiple national medical societies, including the American Cancer Society,25 American Academy of Dermatology,26 and Skin Cancer Foundation,27 as well as international guidelines in Germany,28 Australia,29,30 and New Zealand,31 recommend regular FBSEs among the general or at-risk population; New Zealand and Australia have the highest incidence and prevalence of melanoma in the world.8 The benefits of physician-led FBSEs on detection of early-stage skin cancer, and in particular, melanoma detection, have been documented in numerous studies.30,32-38 However, the variability and often poor quality of skin screening may contribute in part to the just as numerous null results from prior skin screening studies,15 perpetuating the insufficient status of skin examinations by USPSTF standards.14 Our study underscores both the variability in frequency and content of PCP-administered FBSEs. It also highlights the need for standardization of screening examinations at the medical student, trainee, and physician level.
Study Limitations
The present study has several limitations. First, there was an unknown time lag between the FBSEs and physician self-reported surveys. Similarly, there was a variable time lag between the patient examination encounter and subsequent telephone survey. Both the physician and patient survey data may have been affected by recall bias. Second, patients were not asked directly whether an FBSE had been conducted. Furthermore, patients may not have appreciated whether the body part examined was part of the FBSE or another examination. Also, screenings often were not recorded in the medical record, assuming that the patient report and/or physician report was more accurate than the medical record.
Our study also was limited by demographics; our patient sample was largely comprised of White, educated, US adults, potentially limiting the generalizability of our findings. Conversely, a notable strength of our study was that our participants were recruited from 4 geographically diverse centers. Furthermore, we had a comparatively large sample size of patients and physicians. Also, the independent assessment of provider-reported examinations, objective assessment of medical records, and patient reports of their encounters provides a strong foundation for assessing the independent contributions of each data source.
CONCLUSION
Our study highlights the challenges future studies face in promoting skin cancer screening in the primary care setting. Our findings underscore the need for a standardized FBSE as well as clear clinical expectations regarding skin cancer screening that is expected of PCPs.
As long as skin cancer screening rates remain low in the United States, patients will be subject to potential delays and missed diagnoses, impacting morbidity and mortality.8 There are burgeoning resources and efforts in place to increase skin cancer screening. For example, free validated online training is available for early detection of melanoma and other skin cancers (https://www.visualdx.com/skin-cancer-education/).39-42 Future directions for bolstering screening numbers must focus on educating PCPs about skin cancer prevention and perhaps narrowing the screening population by age-appropriate risk assessments.
Keratinocyte carcinoma (KC), or nonmelanoma skin cancer, is the most commonly diagnosed cancer in the United States.1 Basal cell carcinoma comprises the majority of all KCs.2,3 Squamous cell carcinoma is the second most common skin cancer, representing approximately 20% of KCs and accounting for the majority of KC-related deaths.4-7 Malignant melanoma represents the majority of all skin cancer–related deaths.8 The incidence of basal cell carcinoma, squamous cell carcinoma, and malignant melanoma in the United States is on the rise and carries substantial morbidity and mortality with notable social and economic burdens.1,8-10
Prevention is necessary to reduce skin cancer morbidity and mortality as well as rising treatment costs. The most commonly used skin cancer screening method among dermatologists is the visual full-body skin examination (FBSE), which is a noninvasive, safe, quick, and cost-effective method of early detection and prevention.11 To effectively confront the growing incidence and health care burden of skin cancer, primary care providers (PCPs) must join dermatologists in conducting FBSEs.12,13
Despite being the predominant means of secondary skin cancer prevention, the US Preventive Services Task Force (USPSTF) issued an I rating for insufficient evidence to assess the benefits vs harms of screening the adult general population by PCPs.14,15 A major barrier to studying screening is the lack of a standardized method for conducting and reporting FBSEs.13 Systematic thorough skin examination generally is not performed in the primary care setting.16-18
We aimed to investigate what occurs during an FBSE in the primary care setting and how often they are performed. We examined whether there was potential variation in the execution of the examination, what was perceived by the patient vs reported by the physician, and what was ultimately included in the medical record. Miscommunication between patient and provider regarding performance of FBSEs has previously been noted,17-19 and we sought to characterize and quantify that miscommunication. We hypothesized that there would be lower patient-reported FBSEs compared to physicians and patient medical records. We also hypothesized that there would be variability in how physicians screened for skin cancer.
METHODS
This study was cross-sectional and was conducted based on interviews and a review of medical records at secondary- and tertiary-level units (clinics and hospitals) across the United States. We examined baseline data from a randomized controlled trial of a Web-based skin cancer early detection continuing education course—the Basic Skin Cancer Triage curriculum. Complete details have been described elsewhere.12 This study was approved by the institutional review boards of the Providence Veterans Affairs Medical Center, Rhode Island Hospital, and Brown University (all in Providence, Rhode Island), as well as those of all recruitment sites.
Data were collected from 2005 to 2008 and included physician online surveys, patient telephone interviews, and patient medical record data abstracted by research assistants. Primary care providers included in the study were general internists, family physicians, or medicine-pediatrics practitioners who were recruited from 4 collaborating centers across the United States in the mid-Atlantic region, Ohio, Kansas, and southern California, and who had been in practice for at least a year. Patients were recruited from participating physician practices and selected by research assistants who traveled to each clinic for coordination, recruitment, and performance of medical record reviews. Patients were selected as having minimal risk of melanoma (eg, no signs of severe photodamage to the skin). Patients completed structured telephone surveys within 1 to 2 weeks of the office visit regarding the practices observed and clinical questions asked during their recent clinical encounter with their PCP.
Measures
Demographics—Demographic variables asked of physicians included age, sex, ethnicity, academic degree (MD vs DO), years in practice, training, and prior dermatology training. Demographic information asked of patients included age, sex, ethnicity, education, and household income.
Physician-Reported Examination and Counseling Variables—Physicians were asked to characterize their clinical practices, prompted by questions regarding performance of FBSEs: “Please think of a typical month and using the scale below, indicate how frequently you perform a total body skin exam during an annual exam (eg, periodic follow-up exam).” Physicians responded to 3 questions on a 5-point scale (1=never, 2=sometimes, 3=about half, 4=often, 5=almost always).
Patient-Reported Examination Variables—Patients also were asked to characterize the skin examination experienced in their clinical encounter with their PCP, including: “During your last visit, as far as you could tell, did your physician: (1) look at the skin on your back? (2) look at the skin on your belly area? (3) look at the skin on the back of your legs?” Patient responses were coded as yes, no, don’t know, or refused. Participants who refused were excluded from analysis; participants who responded are detailed in Table 1. In addition, patients also reported the level of undress with their physician by answering the following question: “During your last medical exam, did you: 1=keep your clothes on; 2=partially undress; 3=totally undress except for undergarments; 4=totally undress, including all undergarments?”

Patient Medical Record–Extracted Data—Research assistants used a structured abstract form to extract the information from the patient’s medical record and graded it as 0 (absence) or 1 (presence) from the medical record.
Statistical Analysis
Descriptive statistics included mean and standard deviation (SD) for continuous variables as well as frequency and percentage for categorical variables. Logit/logistic regression analysis was used to predict the odds of patient-reported outcomes that were binary with physician-reported variables as the predictor. Linear regression analysis was used to assess the association between 2 continuous variables. All analyses were conducted using SPSS version 24 (IBM).20 Significance criterion was set at α of .05.
RESULTS Demographics
The final sample included data from 53 physicians and 3343 patients. The study sample mean age (SD) was 50.3 (9.9) years for PCPs (n=53) and 59.8 (16.9) years for patients (n=3343). The physician sample was 36% female and predominantly White (83%). Ninety-one percent of the PCPs had an MD (the remaining had a DO degree), and the mean (SD) years practicing was 21.8 (10.6) years. Seventeen percent of PCPs were trained in internal medicine, 4% in internal medicine and pediatrics, and 79% family medicine; 79% of PCPs had received prior training in dermatology. The patient sample was 58% female, predominantly White (84%), non-Hispanic/Latinx (95%), had completed high school (94%), and earned more than $40,000 annually (66%).
Physician- and Patient-Reported FBSEs
Physicians reported performing FBSEs with variable frequency. Among PCPs who conducted FBSEs with greater frequency, there was a modest increase in the odds that patients reported a particular body part was examined (back: odds ratio [OR], 24.5% [95% CI, 1.18-1.31; P<.001]; abdomen: OR, 23.3% [95% CI, 1.17-1.30; P<.001]; backs of legs: OR, 20.4% [95% CI, 1.13-1.28; P<.001])(Table 1). The patient-reported level of undress during examination was significantly associated with physician-reported FBSE (β=0.16 [95% CI, 0.13-0.18; P<.001])(Table 2).

Because of the bimodal distribution of scores in the physician-reported frequency of FBSEs, particularly pertaining to the extreme points of the scale, we further repeated analysis with only the never and almost always groups (Table 1). Primary care providers who reported almost always for FBSE had 29.6% increased odds of patient-reported back examination (95% CI, 1.00-1.68; P=.048) and 59.3% increased odds of patient-reported abdomen examination (95% CI, 1.23-2.06; P<.001). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having never conducted an FBSE were 56%, 40%, and 26%, respectively. The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having almost always conducted an FBSE were 52%, 51%, and 30%, respectively. Raw percentages were calculated by dividing the number of "yes" responses by participants for each body part examined by thetotal number of participant responses (“yes” and “no”) for each respective body part. There was no significant change in odds of patient-reported backs of legs examined with PCP-reported never vs almost always conducting an FBSE. In addition, a greater patient-reported level of undress was associated with 20.2% increased odds of PCPs reporting almost always conducting an FBSE (95% CI, 1.08-1.34; P=.001).
FBSEs in Patient Medical Records
When comparing PCP-reported FBSE and report of FBSE in patient medical records, there was a 39.0% increased odds of the patient medical record indicating FBSE when physicians reported conducting an FBSE with greater frequency (95% CI, 1.30-1.48; P<.001)(eTable 1). When examining PCP-reported never vs almost always conducting an FBSE, a report of almost always was associated with 79.0% increased odds of the patient medical record indicating that an FBSE was conducted (95% CI, 1.28-2.49; P=.001). The raw percentage of the patient medical record indicating an FBSE was conducted when the PCP reported having never conducted an FBSE was 17% and 26% when the PCP reported having almost always conducted an FBSE.

When comparing the patient-reported body part examined with patient FBSE medical record documentation, an indication of yes for FBSE on the patient medical record was associated with a considerable increase in odds that patients reported a particular body part was examined (back: 91.4% [95% CI, 1.59-2.31; P<.001]; abdomen: 75.0% [95% CI, 1.45-2.11; P<.001]; backs of legs: 91.6% [95% CI, 1.56-2.36; P<.001])(eTable 2). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined vs not examined when the patient medical record indicated an FBSE was completed were 24% vs 14%, 23% vs 15%, and 26% vs 16%, respectively. An increase in patient-reported level of undress was associated with a 57.0% increased odds of their medical record indicating an FBSE was conducted (95% CI, 1.45-1.70; P<.001).

COMMENT How PCPs Perform FBSEs Varies
We found that PCPs performed FBSEs with variable frequency, and among those who did, the patient report of their examination varied considerably (Table 1). There appears to be considerable ambiguity in each of these means of determining the extent to which the skin was inspected for skin cancer, which may render the task of improving such inspection more difficult. We asked patients whether their back, abdomen, and backs of legs were examined as an assessment of some of the variety of areas inspected during an FBSE. During a general well-visit appointment, a patient’s back and abdomen may be examined for multiple reasons. Patients may have misinterpreted elements of the pulmonary, cardiac, abdominal, or musculoskeletal examinations as being part of the FBSE. The back and abdomen—the least specific features of the FBSE—were reported by patients to be the most often examined. Conversely, the backs of the legs—the most specific feature of the FBSE—had the lowest odds of being examined (Table 1).
In addition to the potential limitations of patient awareness of physician activity, our results also could be explained by differences among PCPs in how they performed FBSEs. There is no standardized method of conducting an FBSE. Furthermore, not all medical students and residents are exposed to dermatology training. In our sample of 53 physicians, 79% had reported receiving dermatology training; however, we did not assess the extent to which they had been trained in conducting an FBSE and/or identifying malignant lesions. In an American survey of 659 medical students, more than two-thirds of students had never been trained or never examined a patient for skin cancer.21 In another American survey of 342 internal medicine, family medicine, pediatrics, and obstetrics/gynecology residents across 7 medical schools and 4 residency programs, more than three-quarters of residents had never been trained in skin cancer screening.22 Our findings reflect insufficient and inconsistent training in skin cancer screening and underscore the need for mandatory education to ensure quality FBSEs are performed in the primary care setting.
Frequency of PCPs Performing FBSEs
Similar to prior studies analyzing the frequency of FBSE performance in the primary care setting,16,19,23,24 more than half of our PCP sample reported sometimes to never conducting FBSEs. The percentage of physicians who reported conducting FBSEs in our sample was greater than the proportion reported by the National Health Interview Survey, in which only 8% of patients received an FBSE in the prior year by a PCP or obstetrician/gynecologist,16 but similar to a smaller patient study.19 In that study, 87% of patients, regardless of their skin cancer history, also reported that they would like their PCP to perform an FBSE regularly.19 Although some of our patient participants may have declined an FBSE, it is unlikely that that would have entirely accounted for the relatively low number of PCPs who reported frequently performing FBSEs.
Documentation in Medical Records of FBSEs
Compared to PCP self-reported performance of FBSEs, considerably fewer PCPs marked the patient medical record as having completed an FBSE. Among patients with medical records that indicated an FBSE had been conducted, they reported higher odds of all 3 body parts being examined, the highest being the backs of the legs. Also, when the patient medical record indicated an FBSE had been completed, the odds that the PCP reported an FBSE also were higher. The relatively low medical record documentation of FBSEs highlights the need for more rigorous enforcement of accurate documentation. However, among the cases that were recorded, it appeared that the content of the examinations was more consistent.
Benefits of PCP-Led FBSEs
Although the USPSTF issued an I rating for PCP-led FBSEs,14 multiple national medical societies, including the American Cancer Society,25 American Academy of Dermatology,26 and Skin Cancer Foundation,27 as well as international guidelines in Germany,28 Australia,29,30 and New Zealand,31 recommend regular FBSEs among the general or at-risk population; New Zealand and Australia have the highest incidence and prevalence of melanoma in the world.8 The benefits of physician-led FBSEs on detection of early-stage skin cancer, and in particular, melanoma detection, have been documented in numerous studies.30,32-38 However, the variability and often poor quality of skin screening may contribute in part to the just as numerous null results from prior skin screening studies,15 perpetuating the insufficient status of skin examinations by USPSTF standards.14 Our study underscores both the variability in frequency and content of PCP-administered FBSEs. It also highlights the need for standardization of screening examinations at the medical student, trainee, and physician level.
Study Limitations
The present study has several limitations. First, there was an unknown time lag between the FBSEs and physician self-reported surveys. Similarly, there was a variable time lag between the patient examination encounter and subsequent telephone survey. Both the physician and patient survey data may have been affected by recall bias. Second, patients were not asked directly whether an FBSE had been conducted. Furthermore, patients may not have appreciated whether the body part examined was part of the FBSE or another examination. Also, screenings often were not recorded in the medical record, assuming that the patient report and/or physician report was more accurate than the medical record.
Our study also was limited by demographics; our patient sample was largely comprised of White, educated, US adults, potentially limiting the generalizability of our findings. Conversely, a notable strength of our study was that our participants were recruited from 4 geographically diverse centers. Furthermore, we had a comparatively large sample size of patients and physicians. Also, the independent assessment of provider-reported examinations, objective assessment of medical records, and patient reports of their encounters provides a strong foundation for assessing the independent contributions of each data source.
CONCLUSION
Our study highlights the challenges future studies face in promoting skin cancer screening in the primary care setting. Our findings underscore the need for a standardized FBSE as well as clear clinical expectations regarding skin cancer screening that is expected of PCPs.
As long as skin cancer screening rates remain low in the United States, patients will be subject to potential delays and missed diagnoses, impacting morbidity and mortality.8 There are burgeoning resources and efforts in place to increase skin cancer screening. For example, free validated online training is available for early detection of melanoma and other skin cancers (https://www.visualdx.com/skin-cancer-education/).39-42 Future directions for bolstering screening numbers must focus on educating PCPs about skin cancer prevention and perhaps narrowing the screening population by age-appropriate risk assessments.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Dourmishev LA, Rusinova D, Botev I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J. 2013;4:12-17.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma outcomes: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309:243-251.
- Weinstock MA, Bogaars HA, Ashley M, et al. Nonmelanoma skin cancer mortality. a population-based study. Arch Dermatol. 1991;127:1194-1197.
- Matthews NH, Li W-Q, Qureshi AA, et al. Epidemiology of melanoma. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017:3-22.
- Cakir BO, Adamson P, Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2012;20:419-422.
- Guy GP, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol. 2007;143:21-28.
- Markova A, Weinstock MA, Risica P, et al. Effect of a web-based curriculum on primary care practice: basic skin cancer triage trial. Fam Med. 2013;45:558-568.
- Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
- Agency for Healthcare Research and Quality. Screening for skin cancer in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force. November 30, 2015. Accessed July 25, 2022. http://uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review159/skin-cancer-screening2
- Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for skin cancer in adults: updated evidence report and systematic review forthe US Preventive Services Task Force. JAMA. 2016;316:436-447.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Federman DG, Concato J, Caralis PV, et al. Screening for skin cancer in primary care settings. Arch Dermatol. 1997;133:1423-1425.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Federman DG, Kravetz JD, Tobin DG, et al. Full-body skin examinations: the patient’s perspective. Arch Dermatol. 2004;140:530-534.
- IBM. IBM SPSS Statistics for Windows. IBM Corp; 2015.
- Moore MM, Geller AC, Zhang Z, et al. Skin cancer examination teaching in US medical education. Arch Dermatol. 2006;142:439-444.
- Wise E, Singh D, Moore M, et al. Rates of skin cancer screening and prevention counseling by US medical residents. Arch Dermatol. 2009;145:1131-1136.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Coups EJ, Geller AC, Weinstock MA, et al. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123:439-445.
- American Cancer Society. Cancer facts & figures 2016. Accessed March 13, 2022. https://cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- American Academy of Dermatology. Skin cancer incidence rates. Updated April 22, 2022. Accessed August 1, 2022. https://www.aad.org/media/stats-skin-cancer
- Skin Cancer Foundation. Skin cancer prevention. Accessed July 25, 2022. http://skincancer.org/prevention/sun-protection/prevention-guidelines
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- Cancer Council Australia. Position statement: screening and early detection of skin cancer. Published July 2014. Accessed July 25, 2022. https://dermcoll.edu.au/wp-content/uploads/2014/05/PosStatEarlyDetectSkinCa.pdf
- Royal Australian College of General Practitioners. Guidelines for Preventive Activities in General Practice. 9th ed. The Royal Australian College of General Practitioners; 2016. Accessed July 27, 2022. https://www.racgp.org.au/download/Documents/Guidelines/Redbook9/17048-Red-Book-9th-Edition.pdf
- Cancer Council Australia and Australian Cancer Network and New Zealand Guidelines Group. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. The Cancer Council Australia and Australian Cancer Network, Sydney and New Zealand Guidelines Group, Wellington; 2008. Accessed July 27, 2022. https://www.health.govt.nz/system/files/documents/publications/melanoma-guideline-nov08-v2.pdf
- Swetter SM, Pollitt RA, Johnson TM, et al. Behavioral determinants of successful early melanoma detection: role of self and physician skin examination. Cancer. 2012;118:3725-3734.
- Terushkin V, Halpern AC. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481-500, viii.
- Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-458.
- Aitken JF, Elwood JM, Lowe JB, et al. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33-37.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Janda M, Lowe JB, Elwood M, et al. Do centralised skin screening clinics increase participation in melanoma screening (Australia)? Cancer Causes Control. 2006;17:161-168.
- Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54:105-114.
- Eide MJ, Asgari MM, Fletcher SW, et al. Effects on skills and practice from a web-based skin cancer course for primary care providers. J Am Board Fam Med. 2013;26:648-657.
- Weinstock MA, Ferris LK, Saul MI, et al. Downstream consequences of melanoma screening in a community practice setting: first results. Cancer. 2016;122:3152-3156.
- Matthews NH, Risica PM, Ferris LK, et al. Psychosocial impact of skin biopsies in the setting of melanoma screening: a cross-sectional survey. Br J Dermatol. 2019;180:664-665.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Dourmishev LA, Rusinova D, Botev I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J. 2013;4:12-17.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma outcomes: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309:243-251.
- Weinstock MA, Bogaars HA, Ashley M, et al. Nonmelanoma skin cancer mortality. a population-based study. Arch Dermatol. 1991;127:1194-1197.
- Matthews NH, Li W-Q, Qureshi AA, et al. Epidemiology of melanoma. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017:3-22.
- Cakir BO, Adamson P, Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2012;20:419-422.
- Guy GP, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol. 2007;143:21-28.
- Markova A, Weinstock MA, Risica P, et al. Effect of a web-based curriculum on primary care practice: basic skin cancer triage trial. Fam Med. 2013;45:558-568.
- Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
- Agency for Healthcare Research and Quality. Screening for skin cancer in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force. November 30, 2015. Accessed July 25, 2022. http://uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review159/skin-cancer-screening2
- Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for skin cancer in adults: updated evidence report and systematic review forthe US Preventive Services Task Force. JAMA. 2016;316:436-447.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Federman DG, Concato J, Caralis PV, et al. Screening for skin cancer in primary care settings. Arch Dermatol. 1997;133:1423-1425.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Federman DG, Kravetz JD, Tobin DG, et al. Full-body skin examinations: the patient’s perspective. Arch Dermatol. 2004;140:530-534.
- IBM. IBM SPSS Statistics for Windows. IBM Corp; 2015.
- Moore MM, Geller AC, Zhang Z, et al. Skin cancer examination teaching in US medical education. Arch Dermatol. 2006;142:439-444.
- Wise E, Singh D, Moore M, et al. Rates of skin cancer screening and prevention counseling by US medical residents. Arch Dermatol. 2009;145:1131-1136.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Coups EJ, Geller AC, Weinstock MA, et al. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123:439-445.
- American Cancer Society. Cancer facts & figures 2016. Accessed March 13, 2022. https://cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- American Academy of Dermatology. Skin cancer incidence rates. Updated April 22, 2022. Accessed August 1, 2022. https://www.aad.org/media/stats-skin-cancer
- Skin Cancer Foundation. Skin cancer prevention. Accessed July 25, 2022. http://skincancer.org/prevention/sun-protection/prevention-guidelines
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- Cancer Council Australia. Position statement: screening and early detection of skin cancer. Published July 2014. Accessed July 25, 2022. https://dermcoll.edu.au/wp-content/uploads/2014/05/PosStatEarlyDetectSkinCa.pdf
- Royal Australian College of General Practitioners. Guidelines for Preventive Activities in General Practice. 9th ed. The Royal Australian College of General Practitioners; 2016. Accessed July 27, 2022. https://www.racgp.org.au/download/Documents/Guidelines/Redbook9/17048-Red-Book-9th-Edition.pdf
- Cancer Council Australia and Australian Cancer Network and New Zealand Guidelines Group. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. The Cancer Council Australia and Australian Cancer Network, Sydney and New Zealand Guidelines Group, Wellington; 2008. Accessed July 27, 2022. https://www.health.govt.nz/system/files/documents/publications/melanoma-guideline-nov08-v2.pdf
- Swetter SM, Pollitt RA, Johnson TM, et al. Behavioral determinants of successful early melanoma detection: role of self and physician skin examination. Cancer. 2012;118:3725-3734.
- Terushkin V, Halpern AC. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481-500, viii.
- Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-458.
- Aitken JF, Elwood JM, Lowe JB, et al. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33-37.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Janda M, Lowe JB, Elwood M, et al. Do centralised skin screening clinics increase participation in melanoma screening (Australia)? Cancer Causes Control. 2006;17:161-168.
- Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54:105-114.
- Eide MJ, Asgari MM, Fletcher SW, et al. Effects on skills and practice from a web-based skin cancer course for primary care providers. J Am Board Fam Med. 2013;26:648-657.
- Weinstock MA, Ferris LK, Saul MI, et al. Downstream consequences of melanoma screening in a community practice setting: first results. Cancer. 2016;122:3152-3156.
- Matthews NH, Risica PM, Ferris LK, et al. Psychosocial impact of skin biopsies in the setting of melanoma screening: a cross-sectional survey. Br J Dermatol. 2019;180:664-665.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
PRACTICE POINTS
- Dermatologists should be aware of the variability in practice and execution of full-body skin examinations (FBSEs) among primary care providers and offer comprehensive examinations for every patient.
- Variability in reporting and execution of FBSEs may impact the continued US Preventive Services Task Force I rating in their guidelines and promotion of skin cancer screening in the primary care setting.
Agent Orange Exposure, Transformation From MGUS to Multiple Myeloma, and Outcomes in Veterans
Multiple myeloma (MM) accounts for 1% to 2% of all cancers and slightly more than 17% of hematologic malignancies in the United States.1 MM is characterized by the neoplastic proliferation of immunoglobulin (Ig)-producing plasma cells with ≥ 10% clonal plasma cells in the bone marrow or biopsy-proven bony or soft tissue plasmacytoma, plus presence of related organ or tissue impairment or presence of a biomarker associated with near-inevitable progression to end-organ damage.2
Background
Up to 97% of patients with MM will have a monoclonal (M) protein produced and secreted by the malignant plasma cells, which can be detected by protein electrophoresis of the serum and an aliquot of urine from a 24-hour collection combined with immunofixation of the serum and urine. The M protein in MM usually consists of IgG 50% of the time and light chains 16% of the time. Patients who lack detectable M protein are considered to have nonsecretory myeloma. MM presents with end-organ damage, which includes hypercalcemia, renal dysfunction, anemia, or lytic bone lesions. Patients with MM frequently present with renal insufficiency due to cast nephropathy or light chain deposition disease.3
MM is thought to evolve from monoclonal gammopathy of uncertain significance (MGUS), an asymptomatic premalignant stage of clonal plasma cell proliferation with a risk of progression to active myeloma at 1% per year.4,5 Epidemiologic data suggest that people who develop MM have a genetic predisposition, but risk factors may develop or be acquired, such as age, immunosuppression, and environmental exposures. To better assess what causes transformation from MGUS to MM, it is important to identify agents that may cause this second hit.6
In November 1961, President John F. Kennedy authorized the start of Operation Ranch Hand, the US Air Force’s herbicide program during the Vietnam War. Twenty million gallons of various chemicals were sprayed in Vietnam, eastern Laos, and parts of Cambodia to defoliate rural land, depriving guerillas of their support base. Agent Orange (AO) was one of these chemicals; it is a mixed herbicide with traces of dioxin, a compound that has been associated with major health problems among exposed individuals.7 Several studies have evaluated exposure to AO and its potential harmful repercussions. Studies have assessed the link between AO and MGUS as well as AO to various leukemias, such as chronic lymphocytic leukemia.8,9 Other studies have shown the relationship between AO exposure and worse outcomes in persons with MM.10 To date, only a single abstract from a US Department of Veterans Affairs (VA) medical center has investigated the relationships between AO exposure and MGUS, MM, and the rate of transformation. The VA study of patients seen from 2005 to 2015 in Detroit, Michigan, found that AO exposure led to an increase in cumulative incidence rate of MGUS/MM, suggesting possible changes in disease biology and genetics.11
In this study, we aimed to determine the incidence of transformation of MGUS to MM in patients with and without exposure to AO. We then analyzed survival as a function of AO exposure, transformation, and clinical and sociodemographic variables. We also explored the impact of psychosocial variables and hematopoietic stem cell transplantation (HSCT), a standard of treatment for MM.
Methods
This retrospective cohort study assembled electronic health record (EHR) data from the Veterans Health Administration Corporate Data Warehouse (CDW). The VA Central Texas Veterans Healthcare System Institutional Review Board granted a waiver of consent for this record review. Eligible patients were Vietnam-era veterans who were in the military during the time that AO was used (1961-1971). Veterans were included if they were being cared for and received a diagnosis for MGUS or MM between October 1, 2009, and September 30, 2015 (all prevalent cases fiscal years 2010-2015). Cases were excluded if there was illogical death data or if age, race, ethnicity, body mass index (BMI), or prior-year diagnostic data were missing.
Measures
Patients were followed through April 2020. Presence of MGUS was defined by the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 273.1. MM was identified by ICD-9 diagnosis codes 203.00, 203.01, and 203.02. The study index date was the earliest date of diagnosis of MGUS or MM in fiscal years 2010-2015. It was suspected that some patients with MM may have had a history of MGUS prior to this period. Therefore, for patients with MM, historical diagnosis of MGUS was extracted going back through the earliest data in the CDW (October 1999). Patients diagnosed with both MGUS and MM were considered transformation patients.
Other measures included age at index date, sex, race, ethnicity, VA priority status (a value 1 to 8 summarizing why the veteran qualified for VA care, such as military service-connected disability or very low income), and AO exposure authenticated per VA enrollment files and disability records. Service years were separated into 1961 to 1968 and 1969 to 1971 to match a change in the formulation of AO associated with decreased carcinogenic effect. Comorbidity data from the year prior to first MGUS/MM diagnosis in the observation period were extracted. Lifestyle factors associated with development of MGUS/MM were determined using the following codes: obesity per BMI calculation or diagnosis (ICD-9, 278.0), tobacco use per diagnosis (ICD-9, 305.1, V15.82), and survival from MGUS/MM diagnosis index date to date of death from any cause. Comorbidity was assessed using ICD-9 diagnosis codes to calculate the Charlson Comorbidity Index (CCI), which includes cardiovascular diseases, diabetes mellitus, liver and kidney diseases, cancers, and metastatic solid tumors. Cancers were omitted from our adapted CCI to avoid collinearity in the multivariable models. The theoretical maximum CCI score in this study was 25.12,13 Additional conditions known to be associated with variation in outcomes among veterans using the VA were indicated, including major depressive disorder, posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), substance use disorder (SUD), and common chronic disease (hypertension, lipid disorders).14
Treatment with autologous HSCT was defined by Current Procedural Terminology and ICD-9 Clinical Modification procedure codes for bone marrow and autologous HSCT occurring at any time in the CDW (eAppendix). Days elapsed from MM diagnosis to HSCT were calculated.
Statistical Analysis
Sample characteristics were represented by frequencies and percentages for categorical variables and means and SDs (or medians and ranges where appropriate) for continuous variables. A χ2 test (or Fisher exact test when cell counts were low) assessed associations in bivariate comparisons. A 2-sample t test (or Wilcoxon rank sum test as appropriate) assessed differences in continuous variables between 2 groups. Kaplan-Meier curves depicted the unadjusted relationship of AO exposure to survival. Cox proportional hazards survival models examined an unadjusted model containing only the AO exposure indicator as a predictor and adjusted models were used for demographic and clinical factors for MGUS and patients with MM separately.
Predictors were age in decades, sex, Hispanic ethnicity, race, nicotine dependence, obesity, overweight, AUD, SUD, major depressive disorder, PTSD, and the adapted CCI. When modeling patients with MM, MGUS was added to the model to identify the transformation group. The interaction of AO with transformation was also analyzed for patients with MM. Results were reported as hazard ratios (HR) with their 95% CI.
Results
We identified 18,215 veterans diagnosed with either MGUS or MM during fiscal years 2010-2015 with 16,366 meeting inclusion criteria. Patients were excluded for missing data on exposure (n = 334), age (n = 12), race (n = 1058), ethnicity (n = 164), diagnosis (n = 47), treatment (n = 56), and BMI (n = 178). All were Vietnam War era veterans; 14 also served in other eras.
The cohort was 98.5% male (Table 1). Twenty-nine percent were Black veterans, 65% were White veterans, and 4% of individuals reported Hispanic ethnicity. Patients had a mean (SD) age of 66.7 (5.9) years (range, 52-96). Most patients were married (58%) or divorced/separated (27%). All were VA priority 1 to 5 (no 6, 7, or 8); 50% were priority 1 with 50% to 100% service-connected disability. Another 29% were eligible for VA care by reason of low income, 17% had 10% to 40% service-connected disability, and 4% were otherwise disabled.
During fiscal years 2010 to 2015, 68% of our cohort had a diagnosis of MGUS (n = 11,112; 9105 had MGUS only), 44% had MM (n = 7261; 5254 had MM only), and 12% of these were transformation patients (n = 2007). AO exposure characterized 3102 MGUS-only patients (34%), 1886 MM-only patients (36%), and 695 transformation patients (35%) (χ2 = 4.92, P = .09). Among 5683 AO-exposed patients, 695 (12.2%) underwent MGUS-to-MM transformation. Among 10,683 nonexposed veterans, 1312 (12.3%) experienced transformation.
Comorbidity in the year leading up to the index MGUS/MM date determined using CCI was a mean (SD) of 1.9 (2.1) (range, 0-14). Among disorders not included in the CCI, 71% were diagnosed with hypertension, 57% with lipid disorders, 22% with nicotine dependence, 14% with major depressive disorder, 13% with PTSD, and 9% with AUD. Overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) were common (35% and 41%, respectively). For 98% of patients, weight was measured within 90 days of their index MGUS/MM date. Most of the cohort (70%) were in Vietnam in 1961 to 1968.
HSCT was provided to 632 patients with MM (8.7%), including 441 patients who were treated after their index date and 219 patients treated before their index date. From fiscal years 2010 to 2015, the median (IQR) number of days from MM index date to HSCT receipt was 349 (243-650) days. Historical HSCT occurred a median (IQR) of 857 (353-1592) days before the index date, per data available back to October 1999; this median suggests long histories of MM in this cohort.
The unadjusted survival model found a very small inverse association of mortality with AO exposure in the total sample, meaning patients with documented AO exposure lived longer (HR, 0.85; 95% CI, 0.81-0.89; Table 2; Figure). Among 11,112 MGUS patients, AO was similarly associated with mortality (HR, 0.79; 95% CI, 0.74-0.84). The effect was also seen among 7269 patients with MM (HR, 0.86; 95% CI, 0.81-0.91).
In the adjusted model of the total sample, the mortality hazard was greater for veterans who were older, with AUD and nicotine dependence, greater comorbidity per the CCI, diagnosis of MM, and transformation from MGUS to MM. Protective effects were noted for AO exposure, female sex, Black race, obesity, overweight, PTSD, and HSCT.
After adjusting for covariates, AO exposure was still associated with lower mortality among 11,112 patients with MGUS (HR, 0.85; 95% CI, 0.80-0.91). Risk factors were older age, nicotine dependence, AUD, the adapted CCI score (HR, 1.23 per point increase in the index; 95% CI, 1.22-1.25), and transformation to MM (HR, 1.76; 95% CI, 1.65-1.88). Additional protective factors were female sex, Black race, obesity, overweight, and PTSD.
After adjusting for covariates and limiting the analytic cohort to MM patients, the effect of AO exposure persisted (HR, 0.89; 95% CI, 0.84-0.95). Mortality risk factors were older age, nicotine dependence, AUD, and higher CCI score. Also protective were female sex, Black race, obesity, overweight, diagnosis of MGUS (transformation), and HSCT.
In the final model on patients with MM, the interaction term of AO exposure with transformation was significant. The combination of AO exposure with MGUS transformation had a greater protective effect than either AO exposure alone or MGUS without prior AO exposure. Additional protective factors were female sex, Black race, obesity, overweight, and HSCT. Older age, AUD, nicotine dependence, and greater comorbidity increased mortality risk.
Disscussion
Elucidating the pathophysiology and risk of transformation from MGUS to MM is an ongoing endeavor, even 35 years after the end of US involvement in the Vietnam War. Our study sought to understand a relationship between AO exposure, risk of MGUS transforming to MM, and associated mortality in US Vietnam War veterans. The rate of transformation (MGUS progressing to active MM) is well cited at 1% per year.15 Here, we found 12% of our cohort had undergone this transformation over 10 years.
Vietnam War era veterans who were exposed to AO during the Operation Ranch Hand period had 2.4 times greater risk of developing MGUS compared with veterans not exposed to AO.8 Our study was not designed to look at this association of AO exposure and MGUS/MM as this was a retrospective review to assess the difference in outcomes based on AO exposure. We found that AO exposure is associated with a decrease in mortality in contrast to a prior study showing worse survival with individuals with AO exposure.10 Another single center study found no association between AO exposure and overall survival, but it did identify an increased risk of progression from MGUS to MM.11 Our study did not show increased risk of transformation but did show positive effect on survival.
Black individuals have twice the risk of developing MM compared with White individuals and are diagnosed at a younger age (66 vs 70 years, respectively).16 Interestingly, Black race was a protective factor in our study. Given the length of time (35 years) elapsed since the Vietnam War ended, it is likely that most vulnerable Black veterans did not survive until our observation period.
HSCT, as expected, was a protective factor for veterans undergoing this treatment modality, but it is unclear why such a small number (8%) underwent HSCT as this is a standard of care in the management of MM. Obesity was also found to be a protective factor in a prior study, which was also seen in our study cohort.8
Limitations
This study was limited by its retrospective review of survivors among the Vietnam-era cohort several decades after the exposure of concern. Clinician notes and full historical data, such as date of onset for any disorder, were unavailable. These data also relied on the practitioners caring for the veterans to make the correct diagnosis with the associated code so that the data could be captured. Neither AO exposure nor diagnoses codes were verified against other sources of data; however, validation studies over the years have supported the accuracy of the diagnosis codes recorded in the VA EHR.
Conclusions
Because AO exposure is a nonmodifiable risk factor, focus should be placed on modifiable risk factors (eg, nicotine dependence, alcohol and substance use disorders, underlying comorbid conditions) as these were associated with worse outcomes. Future studies will look at the correlation of AO exposure, cytogenetics, and clinical outcomes in these veterans to learn how best to identify their disease course and optimize their care in the latter part of their life.
Acknowledgments
This research was supported by the Central Texas Veterans Health Care System and Baylor Scott and White Health, both in Temple and Veterans Affairs Central Western Massachusetts Healthcare System, Leeds.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi:10.3322/caac.21442
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi:10.1016/S1470-2045(14)70442-5
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. doi:10.4065/78.1.21
4. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564- 569. doi:10.1056/NEJMoa01133202
5. International Myeloma Foundation. What Are MGUS, smoldering and active myeloma? Updated June 6, 2021. Accessed June 20, 2022. https://www.myeloma .org/what-are-mgus-smm-mm
6. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225-247. doi:10.1016/S0889-8588(18)30341-1
7. Buckingham Jr WA. Operation Ranch Hand: The Air Force and herbicides in southeast Asia, 1961-1971. Washington, DC: Office of Air Force History, United States Air Force; 1982. Accessed June 20, 2022. https://apps.dtic.mil/sti /pdfs/ADA121709.pdf
8. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal gammopathy of undetermined significance: an Operation Ranch Hand veteran cohort study. JAMA Oncol. 2015;1(8):1061-1068. doi:10.1001/jamaoncol.2015.2938
9. Mescher C, Gilbertson D, Randall NM, et al. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leuk Lymphoma. 2018;59(6):1348-1355. doi:10.1080/10428194.2017.1375109
10. Callander NS, Freytes CO, Luo S, Carson KR. Previous Agent Orange exposure is correlated with worse outcome in patients with multiple myeloma (MM) [abstract]. Blood. 2015;126(23):4194. doi:10.1182/blood.V126.23.4194.4194
11. Bumma N, Nagasaka M, Kim S, Vankayala HM, Ahmed S, Jasti P. Incidence of monoclonal gammopathy of undetermined significance (MGUS) and subsequent transformation to multiple myeloma (MM) and effect of exposure to Agent Orange (AO): a single center experience from VA Detroit [abstract]. Blood. 2017;130(suppl 1):5383. doi:10.1182/blood.V130.Suppl_1.5383.5383
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
14. Copeland LA, Zeber JE, Sako EY, et al. Serious mental illnesses associated with receipt of surgery in retrospective analysis of patients in the Veterans Health Administration. BMC Surg. 2015;15:74. doi:10.1186/s12893-015-0064-7
15. Younes MA, Perez JD, Alirhayim Z, Ochoa C, Patel R, Dabak VS. MGUS Transformation into multiple myeloma in patients with solid organ transplantation [Abstract presented at American Society of Hematology Annual Meeting, November 15, 2013]. Blood. 2013;122(21):5325. doi:10.1182/blood.V122.21.5325.5325
16. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population- based study. Blood. 2010 Dec 16;116(25):5501-5506. doi:10.1182/blood-2010-07-298760
Multiple myeloma (MM) accounts for 1% to 2% of all cancers and slightly more than 17% of hematologic malignancies in the United States.1 MM is characterized by the neoplastic proliferation of immunoglobulin (Ig)-producing plasma cells with ≥ 10% clonal plasma cells in the bone marrow or biopsy-proven bony or soft tissue plasmacytoma, plus presence of related organ or tissue impairment or presence of a biomarker associated with near-inevitable progression to end-organ damage.2
Background
Up to 97% of patients with MM will have a monoclonal (M) protein produced and secreted by the malignant plasma cells, which can be detected by protein electrophoresis of the serum and an aliquot of urine from a 24-hour collection combined with immunofixation of the serum and urine. The M protein in MM usually consists of IgG 50% of the time and light chains 16% of the time. Patients who lack detectable M protein are considered to have nonsecretory myeloma. MM presents with end-organ damage, which includes hypercalcemia, renal dysfunction, anemia, or lytic bone lesions. Patients with MM frequently present with renal insufficiency due to cast nephropathy or light chain deposition disease.3
MM is thought to evolve from monoclonal gammopathy of uncertain significance (MGUS), an asymptomatic premalignant stage of clonal plasma cell proliferation with a risk of progression to active myeloma at 1% per year.4,5 Epidemiologic data suggest that people who develop MM have a genetic predisposition, but risk factors may develop or be acquired, such as age, immunosuppression, and environmental exposures. To better assess what causes transformation from MGUS to MM, it is important to identify agents that may cause this second hit.6
In November 1961, President John F. Kennedy authorized the start of Operation Ranch Hand, the US Air Force’s herbicide program during the Vietnam War. Twenty million gallons of various chemicals were sprayed in Vietnam, eastern Laos, and parts of Cambodia to defoliate rural land, depriving guerillas of their support base. Agent Orange (AO) was one of these chemicals; it is a mixed herbicide with traces of dioxin, a compound that has been associated with major health problems among exposed individuals.7 Several studies have evaluated exposure to AO and its potential harmful repercussions. Studies have assessed the link between AO and MGUS as well as AO to various leukemias, such as chronic lymphocytic leukemia.8,9 Other studies have shown the relationship between AO exposure and worse outcomes in persons with MM.10 To date, only a single abstract from a US Department of Veterans Affairs (VA) medical center has investigated the relationships between AO exposure and MGUS, MM, and the rate of transformation. The VA study of patients seen from 2005 to 2015 in Detroit, Michigan, found that AO exposure led to an increase in cumulative incidence rate of MGUS/MM, suggesting possible changes in disease biology and genetics.11
In this study, we aimed to determine the incidence of transformation of MGUS to MM in patients with and without exposure to AO. We then analyzed survival as a function of AO exposure, transformation, and clinical and sociodemographic variables. We also explored the impact of psychosocial variables and hematopoietic stem cell transplantation (HSCT), a standard of treatment for MM.
Methods
This retrospective cohort study assembled electronic health record (EHR) data from the Veterans Health Administration Corporate Data Warehouse (CDW). The VA Central Texas Veterans Healthcare System Institutional Review Board granted a waiver of consent for this record review. Eligible patients were Vietnam-era veterans who were in the military during the time that AO was used (1961-1971). Veterans were included if they were being cared for and received a diagnosis for MGUS or MM between October 1, 2009, and September 30, 2015 (all prevalent cases fiscal years 2010-2015). Cases were excluded if there was illogical death data or if age, race, ethnicity, body mass index (BMI), or prior-year diagnostic data were missing.
Measures
Patients were followed through April 2020. Presence of MGUS was defined by the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 273.1. MM was identified by ICD-9 diagnosis codes 203.00, 203.01, and 203.02. The study index date was the earliest date of diagnosis of MGUS or MM in fiscal years 2010-2015. It was suspected that some patients with MM may have had a history of MGUS prior to this period. Therefore, for patients with MM, historical diagnosis of MGUS was extracted going back through the earliest data in the CDW (October 1999). Patients diagnosed with both MGUS and MM were considered transformation patients.
Other measures included age at index date, sex, race, ethnicity, VA priority status (a value 1 to 8 summarizing why the veteran qualified for VA care, such as military service-connected disability or very low income), and AO exposure authenticated per VA enrollment files and disability records. Service years were separated into 1961 to 1968 and 1969 to 1971 to match a change in the formulation of AO associated with decreased carcinogenic effect. Comorbidity data from the year prior to first MGUS/MM diagnosis in the observation period were extracted. Lifestyle factors associated with development of MGUS/MM were determined using the following codes: obesity per BMI calculation or diagnosis (ICD-9, 278.0), tobacco use per diagnosis (ICD-9, 305.1, V15.82), and survival from MGUS/MM diagnosis index date to date of death from any cause. Comorbidity was assessed using ICD-9 diagnosis codes to calculate the Charlson Comorbidity Index (CCI), which includes cardiovascular diseases, diabetes mellitus, liver and kidney diseases, cancers, and metastatic solid tumors. Cancers were omitted from our adapted CCI to avoid collinearity in the multivariable models. The theoretical maximum CCI score in this study was 25.12,13 Additional conditions known to be associated with variation in outcomes among veterans using the VA were indicated, including major depressive disorder, posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), substance use disorder (SUD), and common chronic disease (hypertension, lipid disorders).14
Treatment with autologous HSCT was defined by Current Procedural Terminology and ICD-9 Clinical Modification procedure codes for bone marrow and autologous HSCT occurring at any time in the CDW (eAppendix). Days elapsed from MM diagnosis to HSCT were calculated.
Statistical Analysis
Sample characteristics were represented by frequencies and percentages for categorical variables and means and SDs (or medians and ranges where appropriate) for continuous variables. A χ2 test (or Fisher exact test when cell counts were low) assessed associations in bivariate comparisons. A 2-sample t test (or Wilcoxon rank sum test as appropriate) assessed differences in continuous variables between 2 groups. Kaplan-Meier curves depicted the unadjusted relationship of AO exposure to survival. Cox proportional hazards survival models examined an unadjusted model containing only the AO exposure indicator as a predictor and adjusted models were used for demographic and clinical factors for MGUS and patients with MM separately.
Predictors were age in decades, sex, Hispanic ethnicity, race, nicotine dependence, obesity, overweight, AUD, SUD, major depressive disorder, PTSD, and the adapted CCI. When modeling patients with MM, MGUS was added to the model to identify the transformation group. The interaction of AO with transformation was also analyzed for patients with MM. Results were reported as hazard ratios (HR) with their 95% CI.
Results
We identified 18,215 veterans diagnosed with either MGUS or MM during fiscal years 2010-2015 with 16,366 meeting inclusion criteria. Patients were excluded for missing data on exposure (n = 334), age (n = 12), race (n = 1058), ethnicity (n = 164), diagnosis (n = 47), treatment (n = 56), and BMI (n = 178). All were Vietnam War era veterans; 14 also served in other eras.
The cohort was 98.5% male (Table 1). Twenty-nine percent were Black veterans, 65% were White veterans, and 4% of individuals reported Hispanic ethnicity. Patients had a mean (SD) age of 66.7 (5.9) years (range, 52-96). Most patients were married (58%) or divorced/separated (27%). All were VA priority 1 to 5 (no 6, 7, or 8); 50% were priority 1 with 50% to 100% service-connected disability. Another 29% were eligible for VA care by reason of low income, 17% had 10% to 40% service-connected disability, and 4% were otherwise disabled.
During fiscal years 2010 to 2015, 68% of our cohort had a diagnosis of MGUS (n = 11,112; 9105 had MGUS only), 44% had MM (n = 7261; 5254 had MM only), and 12% of these were transformation patients (n = 2007). AO exposure characterized 3102 MGUS-only patients (34%), 1886 MM-only patients (36%), and 695 transformation patients (35%) (χ2 = 4.92, P = .09). Among 5683 AO-exposed patients, 695 (12.2%) underwent MGUS-to-MM transformation. Among 10,683 nonexposed veterans, 1312 (12.3%) experienced transformation.
Comorbidity in the year leading up to the index MGUS/MM date determined using CCI was a mean (SD) of 1.9 (2.1) (range, 0-14). Among disorders not included in the CCI, 71% were diagnosed with hypertension, 57% with lipid disorders, 22% with nicotine dependence, 14% with major depressive disorder, 13% with PTSD, and 9% with AUD. Overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) were common (35% and 41%, respectively). For 98% of patients, weight was measured within 90 days of their index MGUS/MM date. Most of the cohort (70%) were in Vietnam in 1961 to 1968.
HSCT was provided to 632 patients with MM (8.7%), including 441 patients who were treated after their index date and 219 patients treated before their index date. From fiscal years 2010 to 2015, the median (IQR) number of days from MM index date to HSCT receipt was 349 (243-650) days. Historical HSCT occurred a median (IQR) of 857 (353-1592) days before the index date, per data available back to October 1999; this median suggests long histories of MM in this cohort.
The unadjusted survival model found a very small inverse association of mortality with AO exposure in the total sample, meaning patients with documented AO exposure lived longer (HR, 0.85; 95% CI, 0.81-0.89; Table 2; Figure). Among 11,112 MGUS patients, AO was similarly associated with mortality (HR, 0.79; 95% CI, 0.74-0.84). The effect was also seen among 7269 patients with MM (HR, 0.86; 95% CI, 0.81-0.91).
In the adjusted model of the total sample, the mortality hazard was greater for veterans who were older, with AUD and nicotine dependence, greater comorbidity per the CCI, diagnosis of MM, and transformation from MGUS to MM. Protective effects were noted for AO exposure, female sex, Black race, obesity, overweight, PTSD, and HSCT.
After adjusting for covariates, AO exposure was still associated with lower mortality among 11,112 patients with MGUS (HR, 0.85; 95% CI, 0.80-0.91). Risk factors were older age, nicotine dependence, AUD, the adapted CCI score (HR, 1.23 per point increase in the index; 95% CI, 1.22-1.25), and transformation to MM (HR, 1.76; 95% CI, 1.65-1.88). Additional protective factors were female sex, Black race, obesity, overweight, and PTSD.
After adjusting for covariates and limiting the analytic cohort to MM patients, the effect of AO exposure persisted (HR, 0.89; 95% CI, 0.84-0.95). Mortality risk factors were older age, nicotine dependence, AUD, and higher CCI score. Also protective were female sex, Black race, obesity, overweight, diagnosis of MGUS (transformation), and HSCT.
In the final model on patients with MM, the interaction term of AO exposure with transformation was significant. The combination of AO exposure with MGUS transformation had a greater protective effect than either AO exposure alone or MGUS without prior AO exposure. Additional protective factors were female sex, Black race, obesity, overweight, and HSCT. Older age, AUD, nicotine dependence, and greater comorbidity increased mortality risk.
Disscussion
Elucidating the pathophysiology and risk of transformation from MGUS to MM is an ongoing endeavor, even 35 years after the end of US involvement in the Vietnam War. Our study sought to understand a relationship between AO exposure, risk of MGUS transforming to MM, and associated mortality in US Vietnam War veterans. The rate of transformation (MGUS progressing to active MM) is well cited at 1% per year.15 Here, we found 12% of our cohort had undergone this transformation over 10 years.
Vietnam War era veterans who were exposed to AO during the Operation Ranch Hand period had 2.4 times greater risk of developing MGUS compared with veterans not exposed to AO.8 Our study was not designed to look at this association of AO exposure and MGUS/MM as this was a retrospective review to assess the difference in outcomes based on AO exposure. We found that AO exposure is associated with a decrease in mortality in contrast to a prior study showing worse survival with individuals with AO exposure.10 Another single center study found no association between AO exposure and overall survival, but it did identify an increased risk of progression from MGUS to MM.11 Our study did not show increased risk of transformation but did show positive effect on survival.
Black individuals have twice the risk of developing MM compared with White individuals and are diagnosed at a younger age (66 vs 70 years, respectively).16 Interestingly, Black race was a protective factor in our study. Given the length of time (35 years) elapsed since the Vietnam War ended, it is likely that most vulnerable Black veterans did not survive until our observation period.
HSCT, as expected, was a protective factor for veterans undergoing this treatment modality, but it is unclear why such a small number (8%) underwent HSCT as this is a standard of care in the management of MM. Obesity was also found to be a protective factor in a prior study, which was also seen in our study cohort.8
Limitations
This study was limited by its retrospective review of survivors among the Vietnam-era cohort several decades after the exposure of concern. Clinician notes and full historical data, such as date of onset for any disorder, were unavailable. These data also relied on the practitioners caring for the veterans to make the correct diagnosis with the associated code so that the data could be captured. Neither AO exposure nor diagnoses codes were verified against other sources of data; however, validation studies over the years have supported the accuracy of the diagnosis codes recorded in the VA EHR.
Conclusions
Because AO exposure is a nonmodifiable risk factor, focus should be placed on modifiable risk factors (eg, nicotine dependence, alcohol and substance use disorders, underlying comorbid conditions) as these were associated with worse outcomes. Future studies will look at the correlation of AO exposure, cytogenetics, and clinical outcomes in these veterans to learn how best to identify their disease course and optimize their care in the latter part of their life.
Acknowledgments
This research was supported by the Central Texas Veterans Health Care System and Baylor Scott and White Health, both in Temple and Veterans Affairs Central Western Massachusetts Healthcare System, Leeds.
Multiple myeloma (MM) accounts for 1% to 2% of all cancers and slightly more than 17% of hematologic malignancies in the United States.1 MM is characterized by the neoplastic proliferation of immunoglobulin (Ig)-producing plasma cells with ≥ 10% clonal plasma cells in the bone marrow or biopsy-proven bony or soft tissue plasmacytoma, plus presence of related organ or tissue impairment or presence of a biomarker associated with near-inevitable progression to end-organ damage.2
Background
Up to 97% of patients with MM will have a monoclonal (M) protein produced and secreted by the malignant plasma cells, which can be detected by protein electrophoresis of the serum and an aliquot of urine from a 24-hour collection combined with immunofixation of the serum and urine. The M protein in MM usually consists of IgG 50% of the time and light chains 16% of the time. Patients who lack detectable M protein are considered to have nonsecretory myeloma. MM presents with end-organ damage, which includes hypercalcemia, renal dysfunction, anemia, or lytic bone lesions. Patients with MM frequently present with renal insufficiency due to cast nephropathy or light chain deposition disease.3
MM is thought to evolve from monoclonal gammopathy of uncertain significance (MGUS), an asymptomatic premalignant stage of clonal plasma cell proliferation with a risk of progression to active myeloma at 1% per year.4,5 Epidemiologic data suggest that people who develop MM have a genetic predisposition, but risk factors may develop or be acquired, such as age, immunosuppression, and environmental exposures. To better assess what causes transformation from MGUS to MM, it is important to identify agents that may cause this second hit.6
In November 1961, President John F. Kennedy authorized the start of Operation Ranch Hand, the US Air Force’s herbicide program during the Vietnam War. Twenty million gallons of various chemicals were sprayed in Vietnam, eastern Laos, and parts of Cambodia to defoliate rural land, depriving guerillas of their support base. Agent Orange (AO) was one of these chemicals; it is a mixed herbicide with traces of dioxin, a compound that has been associated with major health problems among exposed individuals.7 Several studies have evaluated exposure to AO and its potential harmful repercussions. Studies have assessed the link between AO and MGUS as well as AO to various leukemias, such as chronic lymphocytic leukemia.8,9 Other studies have shown the relationship between AO exposure and worse outcomes in persons with MM.10 To date, only a single abstract from a US Department of Veterans Affairs (VA) medical center has investigated the relationships between AO exposure and MGUS, MM, and the rate of transformation. The VA study of patients seen from 2005 to 2015 in Detroit, Michigan, found that AO exposure led to an increase in cumulative incidence rate of MGUS/MM, suggesting possible changes in disease biology and genetics.11
In this study, we aimed to determine the incidence of transformation of MGUS to MM in patients with and without exposure to AO. We then analyzed survival as a function of AO exposure, transformation, and clinical and sociodemographic variables. We also explored the impact of psychosocial variables and hematopoietic stem cell transplantation (HSCT), a standard of treatment for MM.
Methods
This retrospective cohort study assembled electronic health record (EHR) data from the Veterans Health Administration Corporate Data Warehouse (CDW). The VA Central Texas Veterans Healthcare System Institutional Review Board granted a waiver of consent for this record review. Eligible patients were Vietnam-era veterans who were in the military during the time that AO was used (1961-1971). Veterans were included if they were being cared for and received a diagnosis for MGUS or MM between October 1, 2009, and September 30, 2015 (all prevalent cases fiscal years 2010-2015). Cases were excluded if there was illogical death data or if age, race, ethnicity, body mass index (BMI), or prior-year diagnostic data were missing.
Measures
Patients were followed through April 2020. Presence of MGUS was defined by the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 273.1. MM was identified by ICD-9 diagnosis codes 203.00, 203.01, and 203.02. The study index date was the earliest date of diagnosis of MGUS or MM in fiscal years 2010-2015. It was suspected that some patients with MM may have had a history of MGUS prior to this period. Therefore, for patients with MM, historical diagnosis of MGUS was extracted going back through the earliest data in the CDW (October 1999). Patients diagnosed with both MGUS and MM were considered transformation patients.
Other measures included age at index date, sex, race, ethnicity, VA priority status (a value 1 to 8 summarizing why the veteran qualified for VA care, such as military service-connected disability or very low income), and AO exposure authenticated per VA enrollment files and disability records. Service years were separated into 1961 to 1968 and 1969 to 1971 to match a change in the formulation of AO associated with decreased carcinogenic effect. Comorbidity data from the year prior to first MGUS/MM diagnosis in the observation period were extracted. Lifestyle factors associated with development of MGUS/MM were determined using the following codes: obesity per BMI calculation or diagnosis (ICD-9, 278.0), tobacco use per diagnosis (ICD-9, 305.1, V15.82), and survival from MGUS/MM diagnosis index date to date of death from any cause. Comorbidity was assessed using ICD-9 diagnosis codes to calculate the Charlson Comorbidity Index (CCI), which includes cardiovascular diseases, diabetes mellitus, liver and kidney diseases, cancers, and metastatic solid tumors. Cancers were omitted from our adapted CCI to avoid collinearity in the multivariable models. The theoretical maximum CCI score in this study was 25.12,13 Additional conditions known to be associated with variation in outcomes among veterans using the VA were indicated, including major depressive disorder, posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), substance use disorder (SUD), and common chronic disease (hypertension, lipid disorders).14
Treatment with autologous HSCT was defined by Current Procedural Terminology and ICD-9 Clinical Modification procedure codes for bone marrow and autologous HSCT occurring at any time in the CDW (eAppendix). Days elapsed from MM diagnosis to HSCT were calculated.
Statistical Analysis
Sample characteristics were represented by frequencies and percentages for categorical variables and means and SDs (or medians and ranges where appropriate) for continuous variables. A χ2 test (or Fisher exact test when cell counts were low) assessed associations in bivariate comparisons. A 2-sample t test (or Wilcoxon rank sum test as appropriate) assessed differences in continuous variables between 2 groups. Kaplan-Meier curves depicted the unadjusted relationship of AO exposure to survival. Cox proportional hazards survival models examined an unadjusted model containing only the AO exposure indicator as a predictor and adjusted models were used for demographic and clinical factors for MGUS and patients with MM separately.
Predictors were age in decades, sex, Hispanic ethnicity, race, nicotine dependence, obesity, overweight, AUD, SUD, major depressive disorder, PTSD, and the adapted CCI. When modeling patients with MM, MGUS was added to the model to identify the transformation group. The interaction of AO with transformation was also analyzed for patients with MM. Results were reported as hazard ratios (HR) with their 95% CI.
Results
We identified 18,215 veterans diagnosed with either MGUS or MM during fiscal years 2010-2015 with 16,366 meeting inclusion criteria. Patients were excluded for missing data on exposure (n = 334), age (n = 12), race (n = 1058), ethnicity (n = 164), diagnosis (n = 47), treatment (n = 56), and BMI (n = 178). All were Vietnam War era veterans; 14 also served in other eras.
The cohort was 98.5% male (Table 1). Twenty-nine percent were Black veterans, 65% were White veterans, and 4% of individuals reported Hispanic ethnicity. Patients had a mean (SD) age of 66.7 (5.9) years (range, 52-96). Most patients were married (58%) or divorced/separated (27%). All were VA priority 1 to 5 (no 6, 7, or 8); 50% were priority 1 with 50% to 100% service-connected disability. Another 29% were eligible for VA care by reason of low income, 17% had 10% to 40% service-connected disability, and 4% were otherwise disabled.
During fiscal years 2010 to 2015, 68% of our cohort had a diagnosis of MGUS (n = 11,112; 9105 had MGUS only), 44% had MM (n = 7261; 5254 had MM only), and 12% of these were transformation patients (n = 2007). AO exposure characterized 3102 MGUS-only patients (34%), 1886 MM-only patients (36%), and 695 transformation patients (35%) (χ2 = 4.92, P = .09). Among 5683 AO-exposed patients, 695 (12.2%) underwent MGUS-to-MM transformation. Among 10,683 nonexposed veterans, 1312 (12.3%) experienced transformation.
Comorbidity in the year leading up to the index MGUS/MM date determined using CCI was a mean (SD) of 1.9 (2.1) (range, 0-14). Among disorders not included in the CCI, 71% were diagnosed with hypertension, 57% with lipid disorders, 22% with nicotine dependence, 14% with major depressive disorder, 13% with PTSD, and 9% with AUD. Overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) were common (35% and 41%, respectively). For 98% of patients, weight was measured within 90 days of their index MGUS/MM date. Most of the cohort (70%) were in Vietnam in 1961 to 1968.
HSCT was provided to 632 patients with MM (8.7%), including 441 patients who were treated after their index date and 219 patients treated before their index date. From fiscal years 2010 to 2015, the median (IQR) number of days from MM index date to HSCT receipt was 349 (243-650) days. Historical HSCT occurred a median (IQR) of 857 (353-1592) days before the index date, per data available back to October 1999; this median suggests long histories of MM in this cohort.
The unadjusted survival model found a very small inverse association of mortality with AO exposure in the total sample, meaning patients with documented AO exposure lived longer (HR, 0.85; 95% CI, 0.81-0.89; Table 2; Figure). Among 11,112 MGUS patients, AO was similarly associated with mortality (HR, 0.79; 95% CI, 0.74-0.84). The effect was also seen among 7269 patients with MM (HR, 0.86; 95% CI, 0.81-0.91).
In the adjusted model of the total sample, the mortality hazard was greater for veterans who were older, with AUD and nicotine dependence, greater comorbidity per the CCI, diagnosis of MM, and transformation from MGUS to MM. Protective effects were noted for AO exposure, female sex, Black race, obesity, overweight, PTSD, and HSCT.
After adjusting for covariates, AO exposure was still associated with lower mortality among 11,112 patients with MGUS (HR, 0.85; 95% CI, 0.80-0.91). Risk factors were older age, nicotine dependence, AUD, the adapted CCI score (HR, 1.23 per point increase in the index; 95% CI, 1.22-1.25), and transformation to MM (HR, 1.76; 95% CI, 1.65-1.88). Additional protective factors were female sex, Black race, obesity, overweight, and PTSD.
After adjusting for covariates and limiting the analytic cohort to MM patients, the effect of AO exposure persisted (HR, 0.89; 95% CI, 0.84-0.95). Mortality risk factors were older age, nicotine dependence, AUD, and higher CCI score. Also protective were female sex, Black race, obesity, overweight, diagnosis of MGUS (transformation), and HSCT.
In the final model on patients with MM, the interaction term of AO exposure with transformation was significant. The combination of AO exposure with MGUS transformation had a greater protective effect than either AO exposure alone or MGUS without prior AO exposure. Additional protective factors were female sex, Black race, obesity, overweight, and HSCT. Older age, AUD, nicotine dependence, and greater comorbidity increased mortality risk.
Disscussion
Elucidating the pathophysiology and risk of transformation from MGUS to MM is an ongoing endeavor, even 35 years after the end of US involvement in the Vietnam War. Our study sought to understand a relationship between AO exposure, risk of MGUS transforming to MM, and associated mortality in US Vietnam War veterans. The rate of transformation (MGUS progressing to active MM) is well cited at 1% per year.15 Here, we found 12% of our cohort had undergone this transformation over 10 years.
Vietnam War era veterans who were exposed to AO during the Operation Ranch Hand period had 2.4 times greater risk of developing MGUS compared with veterans not exposed to AO.8 Our study was not designed to look at this association of AO exposure and MGUS/MM as this was a retrospective review to assess the difference in outcomes based on AO exposure. We found that AO exposure is associated with a decrease in mortality in contrast to a prior study showing worse survival with individuals with AO exposure.10 Another single center study found no association between AO exposure and overall survival, but it did identify an increased risk of progression from MGUS to MM.11 Our study did not show increased risk of transformation but did show positive effect on survival.
Black individuals have twice the risk of developing MM compared with White individuals and are diagnosed at a younger age (66 vs 70 years, respectively).16 Interestingly, Black race was a protective factor in our study. Given the length of time (35 years) elapsed since the Vietnam War ended, it is likely that most vulnerable Black veterans did not survive until our observation period.
HSCT, as expected, was a protective factor for veterans undergoing this treatment modality, but it is unclear why such a small number (8%) underwent HSCT as this is a standard of care in the management of MM. Obesity was also found to be a protective factor in a prior study, which was also seen in our study cohort.8
Limitations
This study was limited by its retrospective review of survivors among the Vietnam-era cohort several decades after the exposure of concern. Clinician notes and full historical data, such as date of onset for any disorder, were unavailable. These data also relied on the practitioners caring for the veterans to make the correct diagnosis with the associated code so that the data could be captured. Neither AO exposure nor diagnoses codes were verified against other sources of data; however, validation studies over the years have supported the accuracy of the diagnosis codes recorded in the VA EHR.
Conclusions
Because AO exposure is a nonmodifiable risk factor, focus should be placed on modifiable risk factors (eg, nicotine dependence, alcohol and substance use disorders, underlying comorbid conditions) as these were associated with worse outcomes. Future studies will look at the correlation of AO exposure, cytogenetics, and clinical outcomes in these veterans to learn how best to identify their disease course and optimize their care in the latter part of their life.
Acknowledgments
This research was supported by the Central Texas Veterans Health Care System and Baylor Scott and White Health, both in Temple and Veterans Affairs Central Western Massachusetts Healthcare System, Leeds.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi:10.3322/caac.21442
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi:10.1016/S1470-2045(14)70442-5
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. doi:10.4065/78.1.21
4. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564- 569. doi:10.1056/NEJMoa01133202
5. International Myeloma Foundation. What Are MGUS, smoldering and active myeloma? Updated June 6, 2021. Accessed June 20, 2022. https://www.myeloma .org/what-are-mgus-smm-mm
6. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225-247. doi:10.1016/S0889-8588(18)30341-1
7. Buckingham Jr WA. Operation Ranch Hand: The Air Force and herbicides in southeast Asia, 1961-1971. Washington, DC: Office of Air Force History, United States Air Force; 1982. Accessed June 20, 2022. https://apps.dtic.mil/sti /pdfs/ADA121709.pdf
8. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal gammopathy of undetermined significance: an Operation Ranch Hand veteran cohort study. JAMA Oncol. 2015;1(8):1061-1068. doi:10.1001/jamaoncol.2015.2938
9. Mescher C, Gilbertson D, Randall NM, et al. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leuk Lymphoma. 2018;59(6):1348-1355. doi:10.1080/10428194.2017.1375109
10. Callander NS, Freytes CO, Luo S, Carson KR. Previous Agent Orange exposure is correlated with worse outcome in patients with multiple myeloma (MM) [abstract]. Blood. 2015;126(23):4194. doi:10.1182/blood.V126.23.4194.4194
11. Bumma N, Nagasaka M, Kim S, Vankayala HM, Ahmed S, Jasti P. Incidence of monoclonal gammopathy of undetermined significance (MGUS) and subsequent transformation to multiple myeloma (MM) and effect of exposure to Agent Orange (AO): a single center experience from VA Detroit [abstract]. Blood. 2017;130(suppl 1):5383. doi:10.1182/blood.V130.Suppl_1.5383.5383
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
14. Copeland LA, Zeber JE, Sako EY, et al. Serious mental illnesses associated with receipt of surgery in retrospective analysis of patients in the Veterans Health Administration. BMC Surg. 2015;15:74. doi:10.1186/s12893-015-0064-7
15. Younes MA, Perez JD, Alirhayim Z, Ochoa C, Patel R, Dabak VS. MGUS Transformation into multiple myeloma in patients with solid organ transplantation [Abstract presented at American Society of Hematology Annual Meeting, November 15, 2013]. Blood. 2013;122(21):5325. doi:10.1182/blood.V122.21.5325.5325
16. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population- based study. Blood. 2010 Dec 16;116(25):5501-5506. doi:10.1182/blood-2010-07-298760
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi:10.3322/caac.21442
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi:10.1016/S1470-2045(14)70442-5
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. doi:10.4065/78.1.21
4. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564- 569. doi:10.1056/NEJMoa01133202
5. International Myeloma Foundation. What Are MGUS, smoldering and active myeloma? Updated June 6, 2021. Accessed June 20, 2022. https://www.myeloma .org/what-are-mgus-smm-mm
6. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225-247. doi:10.1016/S0889-8588(18)30341-1
7. Buckingham Jr WA. Operation Ranch Hand: The Air Force and herbicides in southeast Asia, 1961-1971. Washington, DC: Office of Air Force History, United States Air Force; 1982. Accessed June 20, 2022. https://apps.dtic.mil/sti /pdfs/ADA121709.pdf
8. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal gammopathy of undetermined significance: an Operation Ranch Hand veteran cohort study. JAMA Oncol. 2015;1(8):1061-1068. doi:10.1001/jamaoncol.2015.2938
9. Mescher C, Gilbertson D, Randall NM, et al. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leuk Lymphoma. 2018;59(6):1348-1355. doi:10.1080/10428194.2017.1375109
10. Callander NS, Freytes CO, Luo S, Carson KR. Previous Agent Orange exposure is correlated with worse outcome in patients with multiple myeloma (MM) [abstract]. Blood. 2015;126(23):4194. doi:10.1182/blood.V126.23.4194.4194
11. Bumma N, Nagasaka M, Kim S, Vankayala HM, Ahmed S, Jasti P. Incidence of monoclonal gammopathy of undetermined significance (MGUS) and subsequent transformation to multiple myeloma (MM) and effect of exposure to Agent Orange (AO): a single center experience from VA Detroit [abstract]. Blood. 2017;130(suppl 1):5383. doi:10.1182/blood.V130.Suppl_1.5383.5383
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
14. Copeland LA, Zeber JE, Sako EY, et al. Serious mental illnesses associated with receipt of surgery in retrospective analysis of patients in the Veterans Health Administration. BMC Surg. 2015;15:74. doi:10.1186/s12893-015-0064-7
15. Younes MA, Perez JD, Alirhayim Z, Ochoa C, Patel R, Dabak VS. MGUS Transformation into multiple myeloma in patients with solid organ transplantation [Abstract presented at American Society of Hematology Annual Meeting, November 15, 2013]. Blood. 2013;122(21):5325. doi:10.1182/blood.V122.21.5325.5325
16. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population- based study. Blood. 2010 Dec 16;116(25):5501-5506. doi:10.1182/blood-2010-07-298760
Nonphysician Clinicians in Dermatology Residencies: Cross-sectional Survey on Residency Education
To the Editor:
There is increasing demand for medical care in the United States due to expanded health care coverage; an aging population; and advancements in diagnostics, treatment, and technology.1 It is predicted that by 2050 the number of dermatologists will be 24.4% short of the expected estimate of demand.2
Accordingly, dermatologists are increasingly practicing in team-based care delivery models that incorporate nonphysician clinicians (NPCs), including nurse practitioners and physician assistants.1 Despite recognition that NPCs are taking a larger role in medical teams, there is, to our knowledge, limited training for dermatologists and dermatologists in-training to optimize this professional alliance.
The objectives of this study included (1) determining whether residency programs adequately prepare residents to work with or supervise NPCs and (2) understanding the relationship between NPCs and dermatology residents across residency programs in the United States.
An anonymous cross-sectional, Internet-based survey designed using Google Forms survey creation and administration software was distributed to 117 dermatology residency program directors through email, with a request for further dissemination to residents through self-maintained listserves. Four email reminders about completing and disseminating the survey were sent to program directors between August and November 2020. The study was approved by the Emory University institutional review board. All respondents consented to participate in this survey prior to completing it.
The survey included questions pertaining to demographic information, residents’ experiences working with NPCs, residency program training specific to working with NPCs, and residents’ and residency program directors’ opinions on NPCs’ impact on education and patient care. Program directors were asked to respond N/A to 6 questions on the survey because data from those questions represented residents’ opinions only. Questions relating to residents’ and residency program directors’ opinions were based on a 5-point scale of impact (1=strongly impact in a negative way; 5=strongly impact in a positive way) or importance (1=not at all important; 5=extremely important). The survey was not previously validated.
Descriptive analysis and a paired t test were conducted when appropriate. Missing data were excluded.
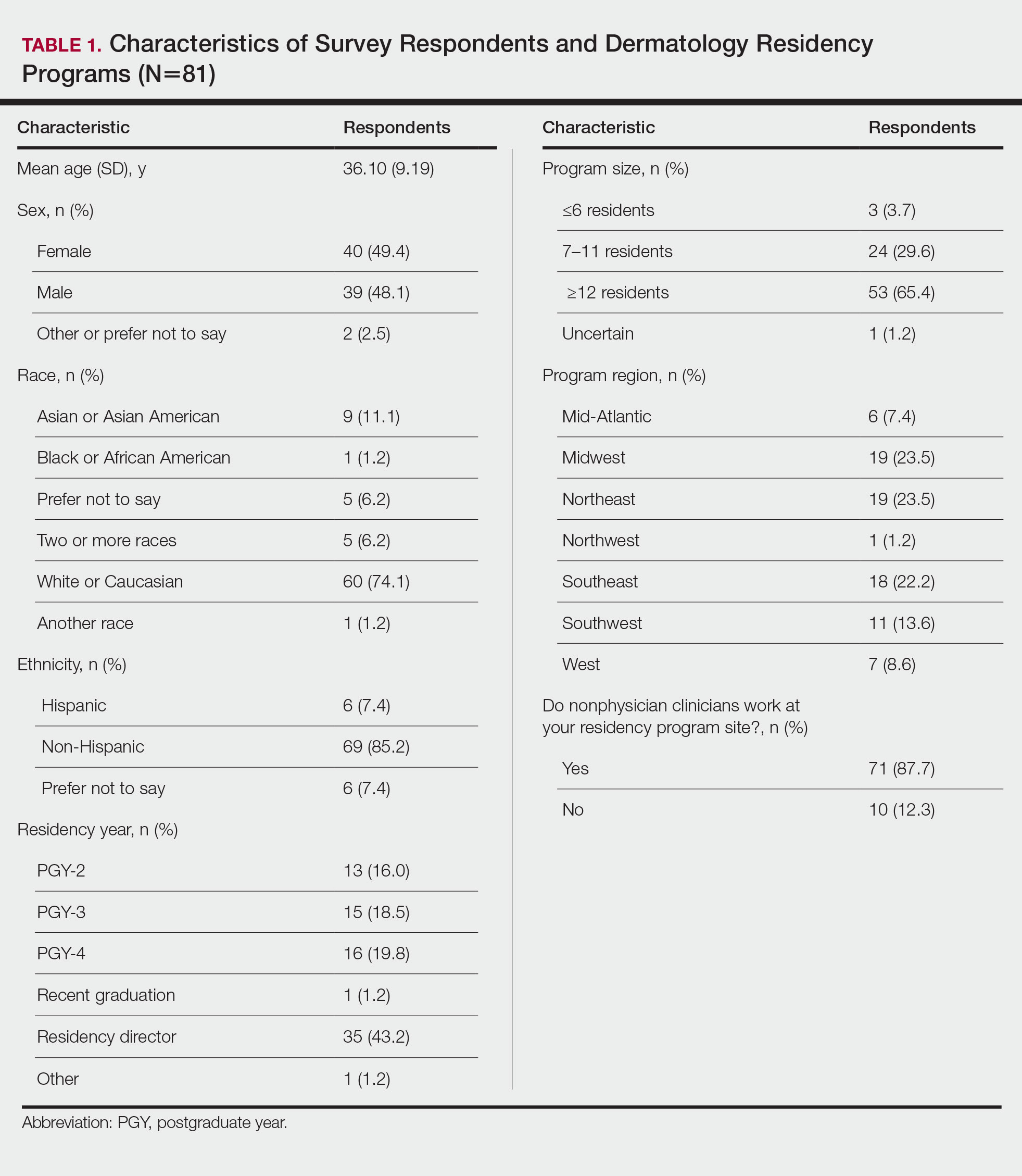
There were 81 respondents to the survey. Demographic information is shown Table 1. Thirty-five dermatology residency program directors (29.9% of 117 programs) responded. Of the 45 residents or recent graduates, 29 (64.4%) reported that they foresaw the need to work with or supervise NPCs in the future (Table 2). Currently, 29 (64.4%) residents also reported that (1) they do not feel adequately trained to provide supervision of or to work with NPCs or (2) were uncertain whether they could do so. Sixty-five (80.2%) respondents stated that there was no formalized training in their program for supervising or working with NPCs; 45 (55.6%) respondents noted that they do not think that their program provided adequate training in supervising NPCs.

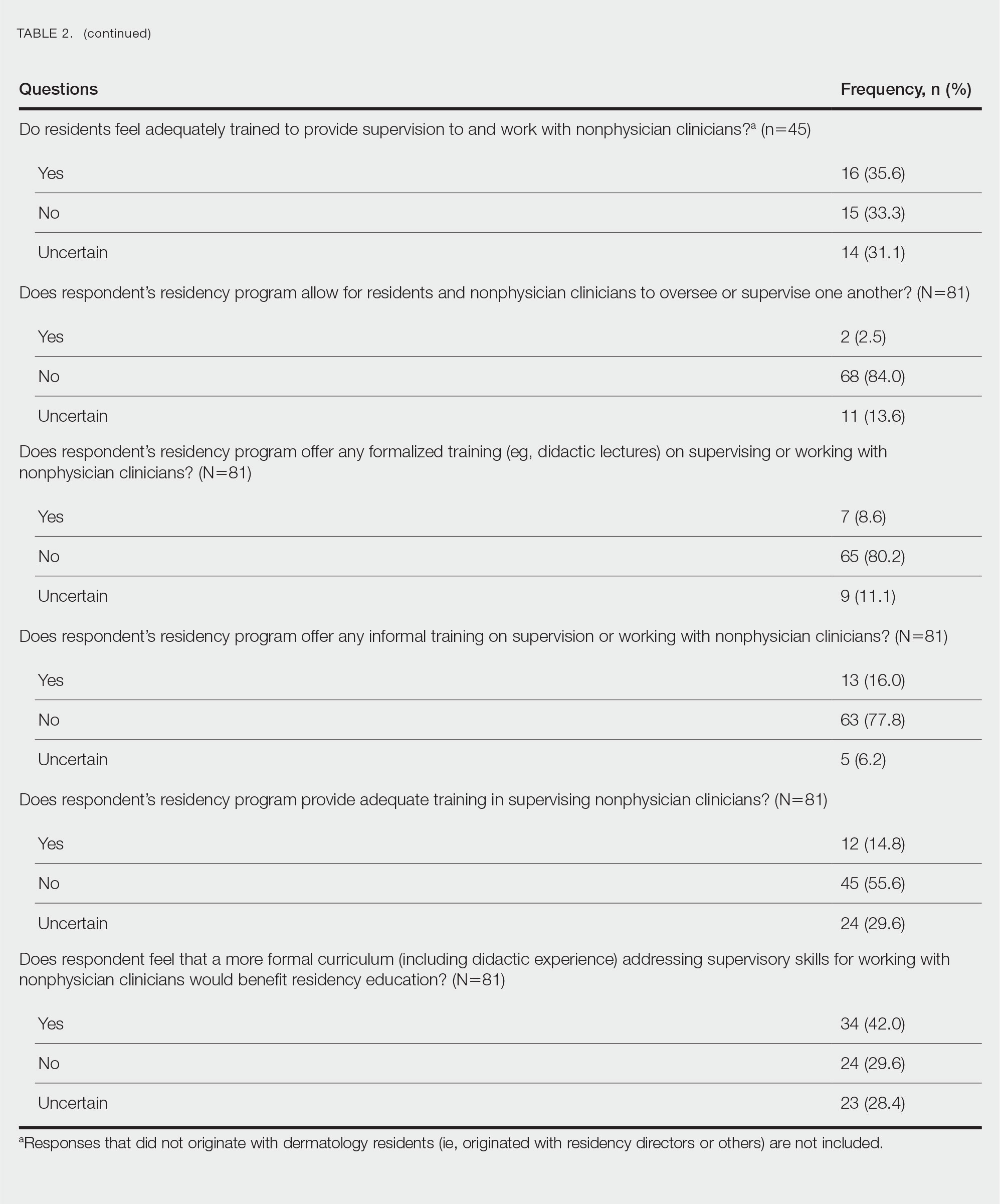
Regarding NPCs impact on care, residency program directors who completed the survey were more likely to rank NPCs as having a more significant positive impact on patient care than residents (mean score, 3.43 vs 2.78; P=.043; 95% CI, –1.28 to –0.20)(Table 3).
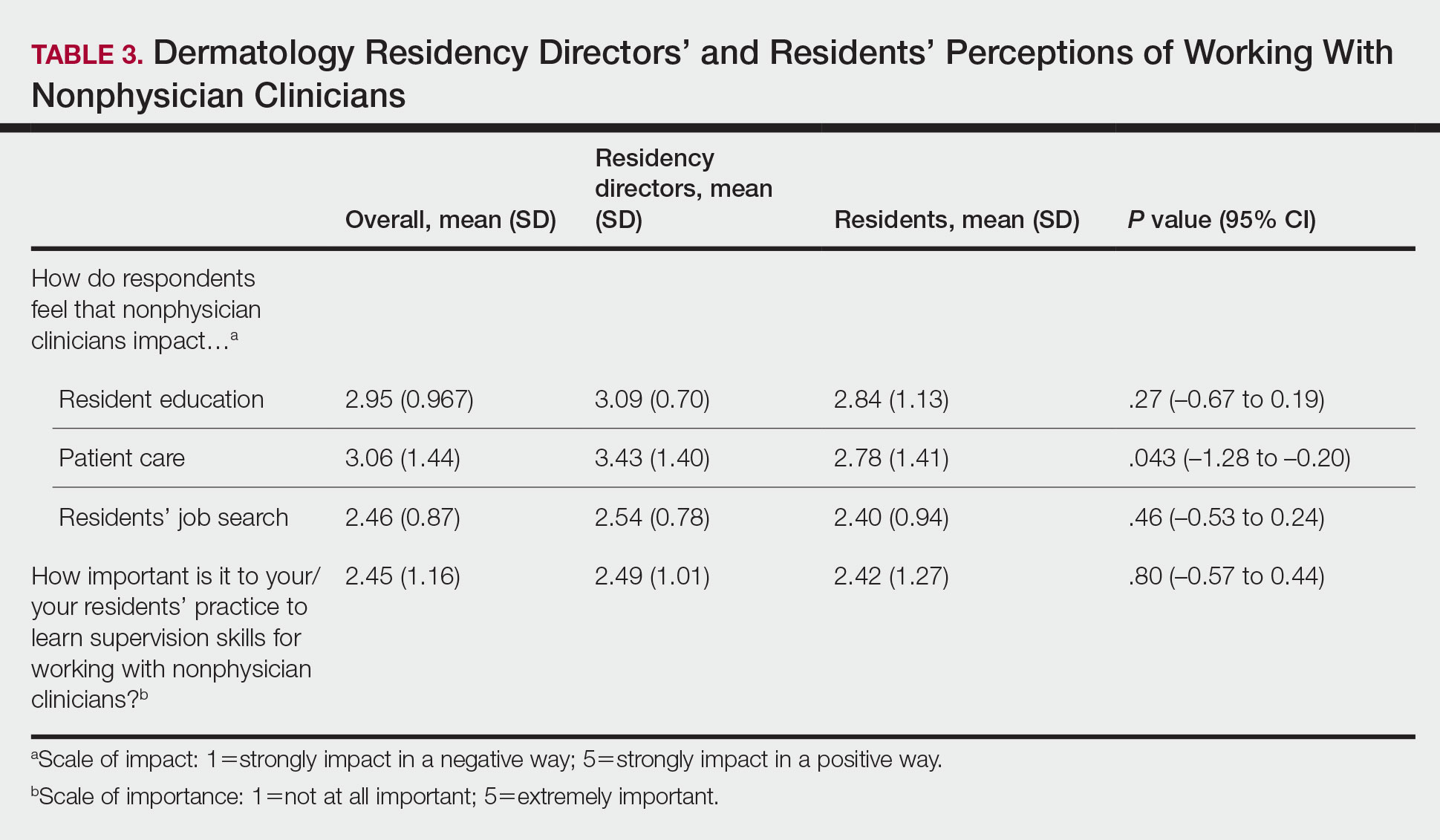
This study demonstrated a lack of dermatology training related to working with NPCs in a professional setting and highlighted residents’ perception that formal education in working with and supervising NPCs could be of benefit to their education. Furthermore, residency directors perceived NPCs as having a greater positive impact on patient care than residents did, underscoring the importance of the continued need to educate residents on working synergistically with NPCs to optimize patient care. Ultimately, these results suggest a potential area for further development of residency curricula.
There are approximately 360,000 NPCs serving as integral members of interdisciplinary medical teams across the United States.3,4 In a 2014 survey, 46% of 2001 dermatologists noted that they already employed 1 or more NPCs, a number that has increased over time and is likely to continue to do so.5 Although the number of NPCs in dermatology has increased, there remain limited formal training and certificate programs for these providers.1,6
Furthermore, the American Academy of Dermatology recommends that “[w]hen practicing in a dermatological setting, non-dermatologist physicians and non-physician clinicians . . . should be directly supervised by a board-certified dermatologist.”7 Therefore, the responsibility for a dermatology-specific education can fall on the dermatologist, necessitating adequate supervision and training of NPCs.
The findings of this study were limited by a small sample size; response bias because distribution of the survey relied on program directors disseminating the instrument to their residents, thereby limiting generalizability; and a lack of predissemination validation of the survey. Additional research in this area should focus on survey validation and distribution directly to dermatology residents, instead of relying on dermatology program directors to disseminate the survey.
- Sargen MR, Shi L, Hooker RS, et al. Future growth of physicians and non-physician providers within the U.S. Dermatology workforce. Dermatol Online J. 2017;23:13030/qt840223q6
- The current and projected dermatology workforce in the United States. J Am Acad Dermatol. 2016;74(suppl 1):AB122. doi:10.1016/j.jaad.2016.02.478
- Nurse anesthetists, nurse midwives, and nurse practitioners.Occupational Outlook Handbook. Washington, DC: US Department of Labor. Updated April 18, 2022. Accessed July 14, 2022. https://www.bls.gov/ooh/health care/nurse-anesthetists-nurse-midwives-and-nurse-practitioners.htm
- Physician assistants. Occupational Outlook Handbook. Washington, DC: US Department of Labor. Updated April 18, 2022. Accessed July 14, 2022. https://www.bls.gov/ooh/healthcare/physician-assistants.htm
- Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol. 2017;77:746-752. doi:10.1016/j.jaad.2017.06.030
- Anderson AM, Matsumoto M, Saul MI, et al. Accuracy of skin cancer diagnosis by physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154:569-573. doi:10.1001/jamadermatol.2018.0212s
- American Academy of Dermatology Association. Position statement on the practice of dermatology: protecting and preserving patient safety and quality care. Revised May 21, 2016. Accessed July 14, 2022. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Practice of Dermatology-Protecting Preserving Patient Safety Quality Care.pdf?
To the Editor:
There is increasing demand for medical care in the United States due to expanded health care coverage; an aging population; and advancements in diagnostics, treatment, and technology.1 It is predicted that by 2050 the number of dermatologists will be 24.4% short of the expected estimate of demand.2
Accordingly, dermatologists are increasingly practicing in team-based care delivery models that incorporate nonphysician clinicians (NPCs), including nurse practitioners and physician assistants.1 Despite recognition that NPCs are taking a larger role in medical teams, there is, to our knowledge, limited training for dermatologists and dermatologists in-training to optimize this professional alliance.
The objectives of this study included (1) determining whether residency programs adequately prepare residents to work with or supervise NPCs and (2) understanding the relationship between NPCs and dermatology residents across residency programs in the United States.
An anonymous cross-sectional, Internet-based survey designed using Google Forms survey creation and administration software was distributed to 117 dermatology residency program directors through email, with a request for further dissemination to residents through self-maintained listserves. Four email reminders about completing and disseminating the survey were sent to program directors between August and November 2020. The study was approved by the Emory University institutional review board. All respondents consented to participate in this survey prior to completing it.
The survey included questions pertaining to demographic information, residents’ experiences working with NPCs, residency program training specific to working with NPCs, and residents’ and residency program directors’ opinions on NPCs’ impact on education and patient care. Program directors were asked to respond N/A to 6 questions on the survey because data from those questions represented residents’ opinions only. Questions relating to residents’ and residency program directors’ opinions were based on a 5-point scale of impact (1=strongly impact in a negative way; 5=strongly impact in a positive way) or importance (1=not at all important; 5=extremely important). The survey was not previously validated.
Descriptive analysis and a paired t test were conducted when appropriate. Missing data were excluded.

There were 81 respondents to the survey. Demographic information is shown Table 1. Thirty-five dermatology residency program directors (29.9% of 117 programs) responded. Of the 45 residents or recent graduates, 29 (64.4%) reported that they foresaw the need to work with or supervise NPCs in the future (Table 2). Currently, 29 (64.4%) residents also reported that (1) they do not feel adequately trained to provide supervision of or to work with NPCs or (2) were uncertain whether they could do so. Sixty-five (80.2%) respondents stated that there was no formalized training in their program for supervising or working with NPCs; 45 (55.6%) respondents noted that they do not think that their program provided adequate training in supervising NPCs.


Regarding NPCs impact on care, residency program directors who completed the survey were more likely to rank NPCs as having a more significant positive impact on patient care than residents (mean score, 3.43 vs 2.78; P=.043; 95% CI, –1.28 to –0.20)(Table 3).

This study demonstrated a lack of dermatology training related to working with NPCs in a professional setting and highlighted residents’ perception that formal education in working with and supervising NPCs could be of benefit to their education. Furthermore, residency directors perceived NPCs as having a greater positive impact on patient care than residents did, underscoring the importance of the continued need to educate residents on working synergistically with NPCs to optimize patient care. Ultimately, these results suggest a potential area for further development of residency curricula.
There are approximately 360,000 NPCs serving as integral members of interdisciplinary medical teams across the United States.3,4 In a 2014 survey, 46% of 2001 dermatologists noted that they already employed 1 or more NPCs, a number that has increased over time and is likely to continue to do so.5 Although the number of NPCs in dermatology has increased, there remain limited formal training and certificate programs for these providers.1,6
Furthermore, the American Academy of Dermatology recommends that “[w]hen practicing in a dermatological setting, non-dermatologist physicians and non-physician clinicians . . . should be directly supervised by a board-certified dermatologist.”7 Therefore, the responsibility for a dermatology-specific education can fall on the dermatologist, necessitating adequate supervision and training of NPCs.
The findings of this study were limited by a small sample size; response bias because distribution of the survey relied on program directors disseminating the instrument to their residents, thereby limiting generalizability; and a lack of predissemination validation of the survey. Additional research in this area should focus on survey validation and distribution directly to dermatology residents, instead of relying on dermatology program directors to disseminate the survey.
To the Editor:
There is increasing demand for medical care in the United States due to expanded health care coverage; an aging population; and advancements in diagnostics, treatment, and technology.1 It is predicted that by 2050 the number of dermatologists will be 24.4% short of the expected estimate of demand.2
Accordingly, dermatologists are increasingly practicing in team-based care delivery models that incorporate nonphysician clinicians (NPCs), including nurse practitioners and physician assistants.1 Despite recognition that NPCs are taking a larger role in medical teams, there is, to our knowledge, limited training for dermatologists and dermatologists in-training to optimize this professional alliance.
The objectives of this study included (1) determining whether residency programs adequately prepare residents to work with or supervise NPCs and (2) understanding the relationship between NPCs and dermatology residents across residency programs in the United States.
An anonymous cross-sectional, Internet-based survey designed using Google Forms survey creation and administration software was distributed to 117 dermatology residency program directors through email, with a request for further dissemination to residents through self-maintained listserves. Four email reminders about completing and disseminating the survey were sent to program directors between August and November 2020. The study was approved by the Emory University institutional review board. All respondents consented to participate in this survey prior to completing it.
The survey included questions pertaining to demographic information, residents’ experiences working with NPCs, residency program training specific to working with NPCs, and residents’ and residency program directors’ opinions on NPCs’ impact on education and patient care. Program directors were asked to respond N/A to 6 questions on the survey because data from those questions represented residents’ opinions only. Questions relating to residents’ and residency program directors’ opinions were based on a 5-point scale of impact (1=strongly impact in a negative way; 5=strongly impact in a positive way) or importance (1=not at all important; 5=extremely important). The survey was not previously validated.
Descriptive analysis and a paired t test were conducted when appropriate. Missing data were excluded.

There were 81 respondents to the survey. Demographic information is shown Table 1. Thirty-five dermatology residency program directors (29.9% of 117 programs) responded. Of the 45 residents or recent graduates, 29 (64.4%) reported that they foresaw the need to work with or supervise NPCs in the future (Table 2). Currently, 29 (64.4%) residents also reported that (1) they do not feel adequately trained to provide supervision of or to work with NPCs or (2) were uncertain whether they could do so. Sixty-five (80.2%) respondents stated that there was no formalized training in their program for supervising or working with NPCs; 45 (55.6%) respondents noted that they do not think that their program provided adequate training in supervising NPCs.


Regarding NPCs impact on care, residency program directors who completed the survey were more likely to rank NPCs as having a more significant positive impact on patient care than residents (mean score, 3.43 vs 2.78; P=.043; 95% CI, –1.28 to –0.20)(Table 3).

This study demonstrated a lack of dermatology training related to working with NPCs in a professional setting and highlighted residents’ perception that formal education in working with and supervising NPCs could be of benefit to their education. Furthermore, residency directors perceived NPCs as having a greater positive impact on patient care than residents did, underscoring the importance of the continued need to educate residents on working synergistically with NPCs to optimize patient care. Ultimately, these results suggest a potential area for further development of residency curricula.
There are approximately 360,000 NPCs serving as integral members of interdisciplinary medical teams across the United States.3,4 In a 2014 survey, 46% of 2001 dermatologists noted that they already employed 1 or more NPCs, a number that has increased over time and is likely to continue to do so.5 Although the number of NPCs in dermatology has increased, there remain limited formal training and certificate programs for these providers.1,6
Furthermore, the American Academy of Dermatology recommends that “[w]hen practicing in a dermatological setting, non-dermatologist physicians and non-physician clinicians . . . should be directly supervised by a board-certified dermatologist.”7 Therefore, the responsibility for a dermatology-specific education can fall on the dermatologist, necessitating adequate supervision and training of NPCs.
The findings of this study were limited by a small sample size; response bias because distribution of the survey relied on program directors disseminating the instrument to their residents, thereby limiting generalizability; and a lack of predissemination validation of the survey. Additional research in this area should focus on survey validation and distribution directly to dermatology residents, instead of relying on dermatology program directors to disseminate the survey.
- Sargen MR, Shi L, Hooker RS, et al. Future growth of physicians and non-physician providers within the U.S. Dermatology workforce. Dermatol Online J. 2017;23:13030/qt840223q6
- The current and projected dermatology workforce in the United States. J Am Acad Dermatol. 2016;74(suppl 1):AB122. doi:10.1016/j.jaad.2016.02.478
- Nurse anesthetists, nurse midwives, and nurse practitioners.Occupational Outlook Handbook. Washington, DC: US Department of Labor. Updated April 18, 2022. Accessed July 14, 2022. https://www.bls.gov/ooh/health care/nurse-anesthetists-nurse-midwives-and-nurse-practitioners.htm
- Physician assistants. Occupational Outlook Handbook. Washington, DC: US Department of Labor. Updated April 18, 2022. Accessed July 14, 2022. https://www.bls.gov/ooh/healthcare/physician-assistants.htm
- Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol. 2017;77:746-752. doi:10.1016/j.jaad.2017.06.030
- Anderson AM, Matsumoto M, Saul MI, et al. Accuracy of skin cancer diagnosis by physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154:569-573. doi:10.1001/jamadermatol.2018.0212s
- American Academy of Dermatology Association. Position statement on the practice of dermatology: protecting and preserving patient safety and quality care. Revised May 21, 2016. Accessed July 14, 2022. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Practice of Dermatology-Protecting Preserving Patient Safety Quality Care.pdf?
- Sargen MR, Shi L, Hooker RS, et al. Future growth of physicians and non-physician providers within the U.S. Dermatology workforce. Dermatol Online J. 2017;23:13030/qt840223q6
- The current and projected dermatology workforce in the United States. J Am Acad Dermatol. 2016;74(suppl 1):AB122. doi:10.1016/j.jaad.2016.02.478
- Nurse anesthetists, nurse midwives, and nurse practitioners.Occupational Outlook Handbook. Washington, DC: US Department of Labor. Updated April 18, 2022. Accessed July 14, 2022. https://www.bls.gov/ooh/health care/nurse-anesthetists-nurse-midwives-and-nurse-practitioners.htm
- Physician assistants. Occupational Outlook Handbook. Washington, DC: US Department of Labor. Updated April 18, 2022. Accessed July 14, 2022. https://www.bls.gov/ooh/healthcare/physician-assistants.htm
- Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol. 2017;77:746-752. doi:10.1016/j.jaad.2017.06.030
- Anderson AM, Matsumoto M, Saul MI, et al. Accuracy of skin cancer diagnosis by physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154:569-573. doi:10.1001/jamadermatol.2018.0212s
- American Academy of Dermatology Association. Position statement on the practice of dermatology: protecting and preserving patient safety and quality care. Revised May 21, 2016. Accessed July 14, 2022. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Practice of Dermatology-Protecting Preserving Patient Safety Quality Care.pdf?
Practice Points
- Most dermatology residency programs do not offer training on working with and supervising nonphysician clinicians.
- Dermatology residents think that formal training in supervising nonphysician clinicians would be a beneficial addition to the residency curriculum.