User login
Surgical Treatment of Nonmelanoma Skin Cancer in Older Adult Veterans
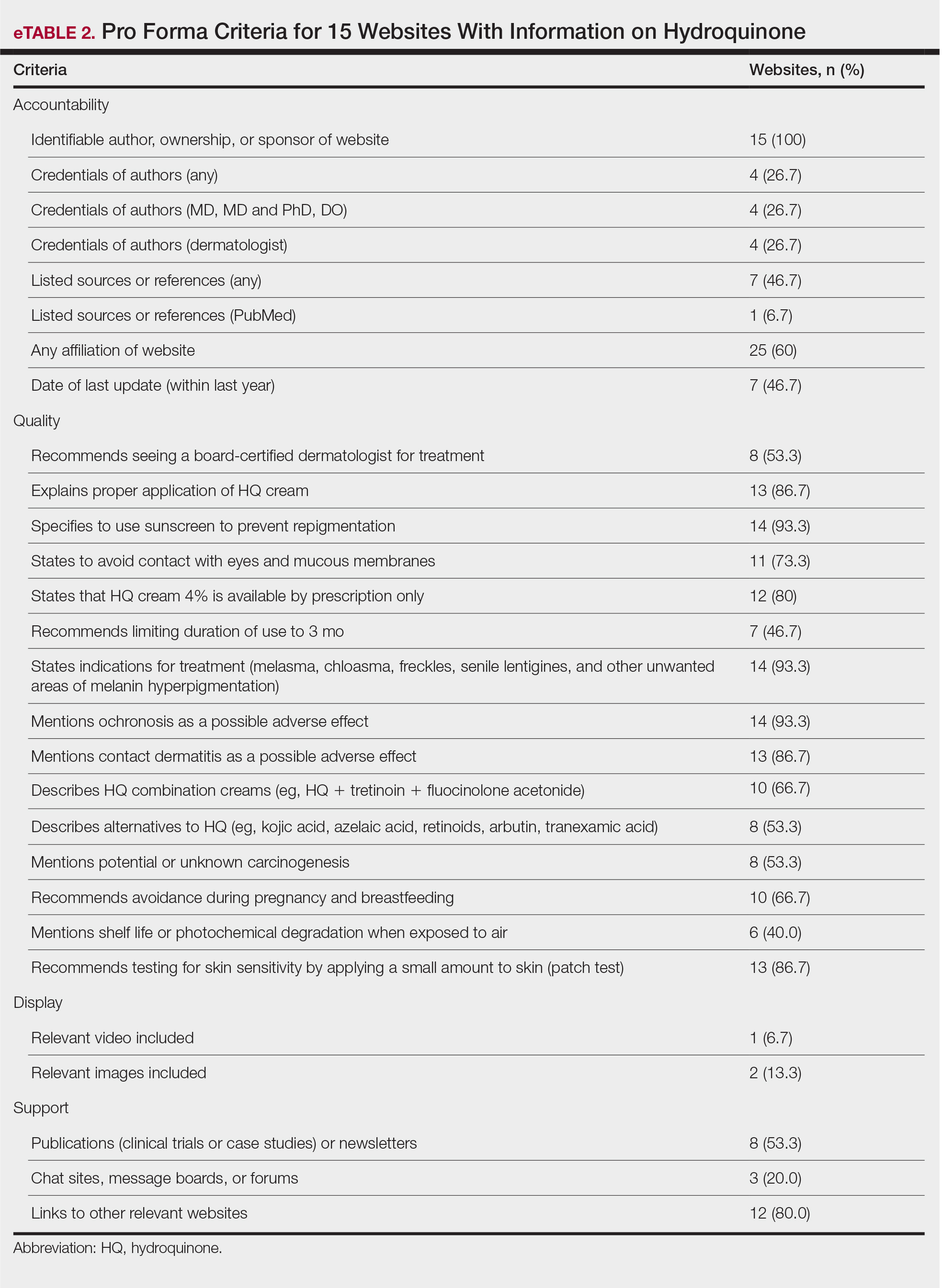
Skin cancer is the most diagnosed cancer in the United States. Nonmelanoma skin cancers (NMSC), which include basal cell carcinoma and squamous cell carcinoma, are usually cured with removal.1 The incidence of NMSC increases with age and is commonly found in nursing homes and geriatric units. These cancers are not usually metastatic or fatal but can cause local destruction and disfigurement if neglected.2 The current standard of care is to treat diagnosed NMSC; however, the dermatology and geriatric care literature have questioned the logic of treating asymptomatic skin cancers that will not affect a patient’s life expectancy.2-4
Forty-seven percent of the current living veteran population is aged ≥ 65 years.5 Older adult patients are frequently referred to the US Department of Veterans Affairs (VA) surgical service for the treatment of NMSC. The veteran population includes a higher percentage of individuals at an elevated risk of skin cancers (older, White, and male) compared with the general population.6 World War II veterans deployed in regions closer to the equator have been found to have an elevated risk of melanoma and nonmelanoma skin carcinomas.7 A retrospective study of Vietnam veterans exposed to Agent Orange (2,3,7,8-tetrachlorodibenzodioxin) found a significantly higher risk of invasive NMSC in Fitzpatrick skin types I-IV compared with an age-matched subset of the general population.8 Younger veterans who were deployed in Afghanistan and Iraq for Operation Enduring Freedom/Operation Iraqi Freedom worked at more equatorial latitudes than the rest of the US population and may be at increased risk of NMSC. Inadequate sunscreen access, immediate safety concerns, outdoor recreational activities, harsh weather, and insufficient emphasis on sun protection have created a multifactorial challenge for the military population. Riemenschneider and colleagues recommended targeted screening for at-risk veteran patients and prioritizing annual skin cancer screenings during medical mission physical examinations for active military.7
The plastic surgery service regularly receives consults from dermatology, general surgery, and primary care to remove skin cancers on the face, scalp, hands, and forearms. Skin cancer treatment can create serious hardships for older adult patients and their families with multiple appointments for the consult, procedure, and follow-up. Patients are often told to hold their anticoagulant medications when the surgery will be performed on a highly vascular region, such as the scalp or face. This can create wide swings in their laboratory test values and result in life-threatening complications from either bleeding or clotting. The appropriateness of offering surgery to patients with serious comorbidities and a limited life expectancy has been questioned.2-4 The purpose of this study was to measure the morbidity and unrelated 5-year mortality for patients with skin cancer referred to the plastic surgery service to help patients and families make a more informed treatment decision, particularly when the patients are aged > 80 years and have significant life-threatening comorbidities.
Methods
The University of Florida and Malcom Randall VA Medical Center Institutional review board in Gainesville, approved a retrospective review of all consults completed by the plastic surgery service for the treatment of NMSC performed from July 1, 2011 to June 30, 2015. Data collected included age and common life-limiting comorbidities at the time of referral. Morbidities were found on the electronic health record, including coronary artery disease (CAD), congestive heart failure (CHF), cerebral vascular disease (CVD), peripheral vascular disease, dementia, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), tobacco use, diabetes mellitus (DM), liver disease, alcohol use, and obstructive sleep apnea.
Treatment, complications, and 5-year mortality were recorded. A χ2 analysis with P value < .05 was used to determine statistical significance between individual risk factors and 5-year mortality. The relative risk of 5-year mortality was calculated by combining advanced age (aged > 80 years) with the individual comorbidities.
Results
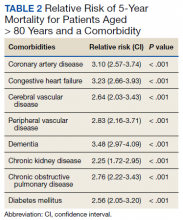
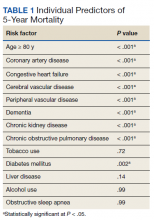
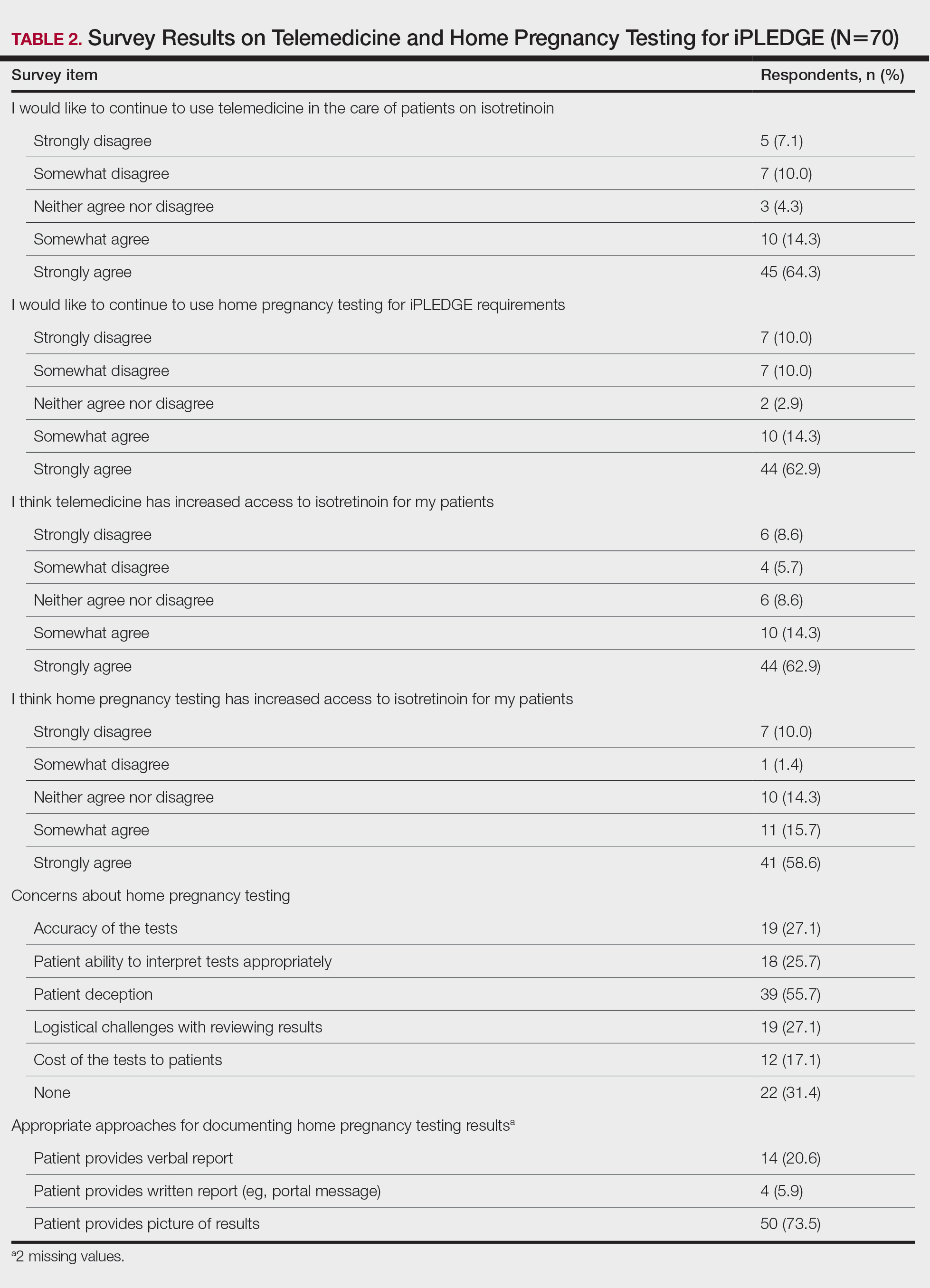
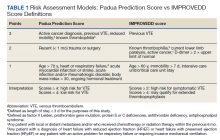
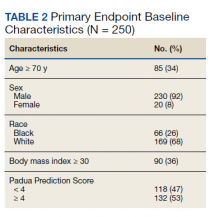
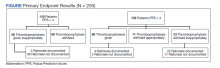
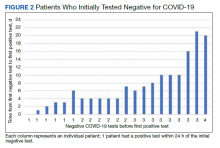
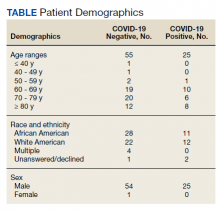
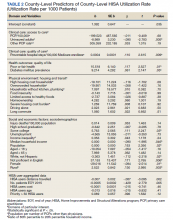
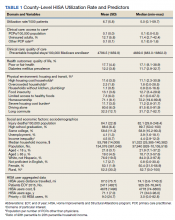
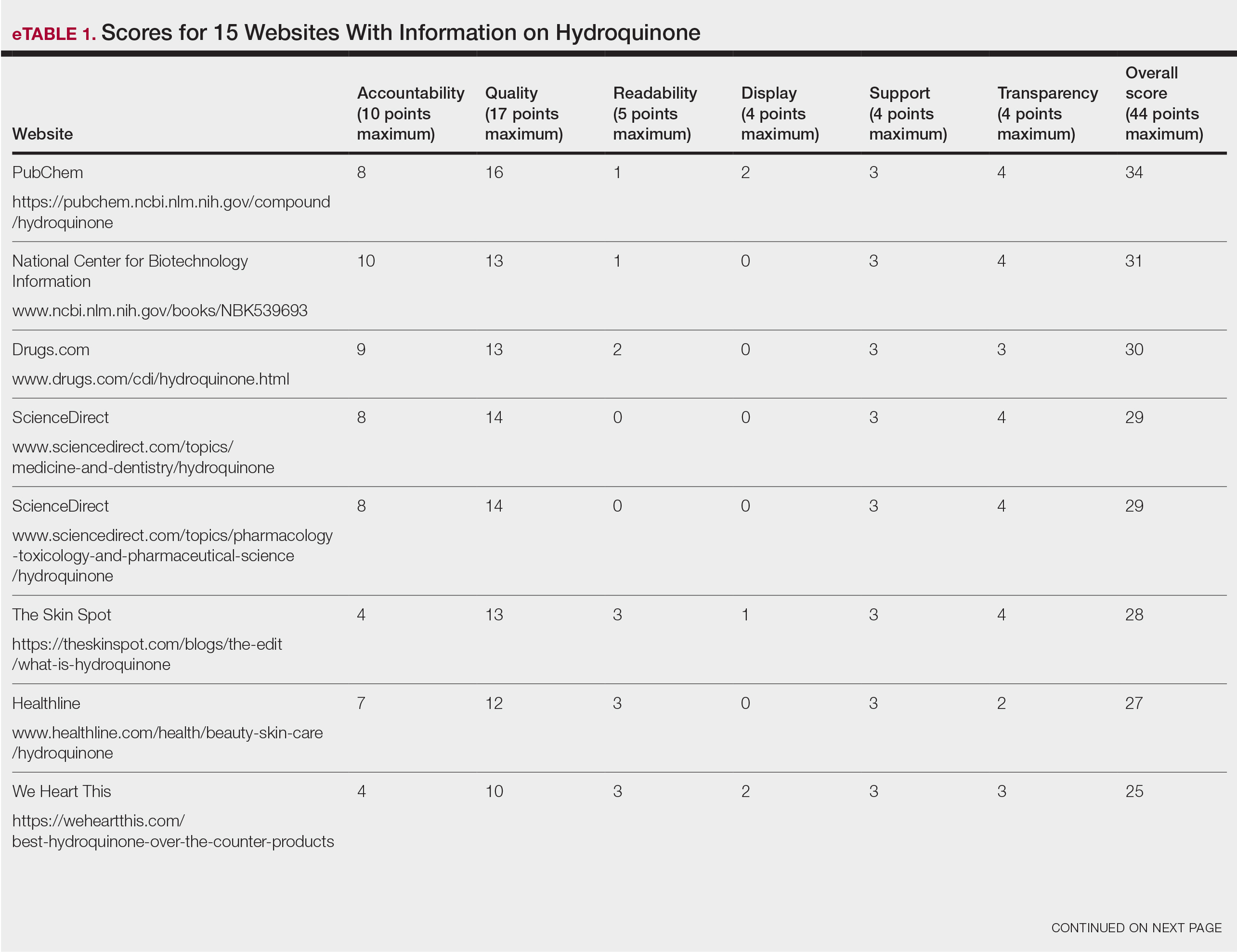
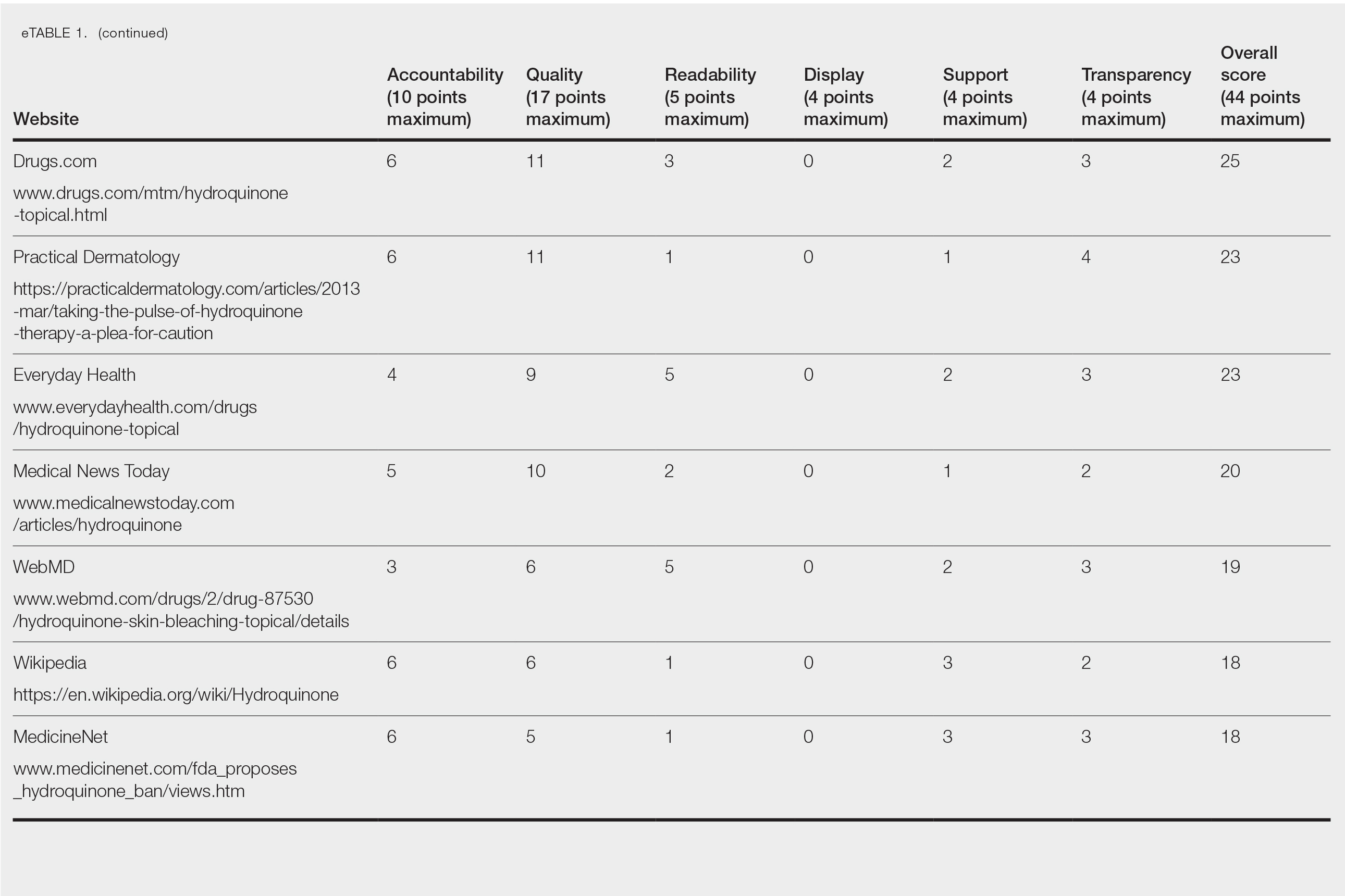
Over 4 years, 800 consults for NMSC were completed by the plastic surgery service. Treatment decisions included 210 excisions (with or without reconstruction) in the operating room, 402 excisions (with or without reconstruction) under local anesthesia in clinic, 55 Mohs surgical dermatology referrals, 21 other service or hospital referrals, and 112 patient who were observed, declined intervention, or died prior to intervention. Five-year mortality was 28.6%. No patients died of NMSC. The median age at consult submission for patients deceased 5 years later was 78 years. Complication rate was 5% and included wound infection, dehiscence, bleeding, or graft loss. Two patients, both deceased within 5 years, had unplanned admissions due to bleeding from either a skin graft donor site or recipient bleeding. Aged ≥ 80 years, CAD, CHF, CVD, peripheral vascular disease, dementia, CKD, COPD, and DM were all found individually to be statistically significant predictors of 5-year mortality (Table 1). Combining aged ≥ 80 years plus CAD, CHF, or dementia all increased the 5-year mortality by a relative risk of > 3 (Table 2).
Discussion
The standard of care is to treat NMSC. Most NMSCs are treated surgically without consideration of patient age or life expectancy.2,4,9,10 A prospective cohort study involving a university-based private practice and a VA medical center in San Francisco found a 22.6% overall 5-year mortality and a 43.3% mortality in the group defined as limited life expectancy (LLE) based on age (≥ 85 years) and medical comorbidities. None died due to the NMSC. Leading cause of death was cardiac, cerebrovascular, and respiratory disease, lung and prostate cancer, and Alzheimer disease. The authors suggested the LLE group may be exposed to wound complications without benefiting from the treatment.4
Another study of 440 patients receiving excision for biopsy-proven facial NMSC at the Roudebush VA Medical Center in Indianapolis, Indiana, found no residual carcinoma in 35.3% of excisions, and in patients aged > 90 years, more than half of the excisions had no residual carcinoma. More than half of the patients aged > 90 years died within 1 year, not as a result of the NMSC. The authors argued for watchful waiting in select patients to maximize comfort and outcomes.10
NMSCs are often asymptomatic and not immediately life threatening. Although NMSCs tend to have a favorable prognosis, studies have found that NMSC may be a marker for other poor health outcomes. A significant increased risk for all-cause mortality was found for patients with a history of SCC, which may be attributed to immune status.11 The aging veteran population has more complex health care needs to be considered when developing surgical treatment plans. These medical problems may limit their life expectancy much sooner than the skin cancer will become symptomatic. We found that individuals aged ≥ 80 years who had CAD, CHF, or dementia had a relative risk of 3 or higher for 5-year mortality. The leading cause of death in the United States in years 2011 to 2015 was heart disease. Alzheimer disease was the sixth leading cause of death in those same years.12-14
Skin cancer excisions do not typically require general anesthesia, deep sedation, or large fluid shifts; however, studies have found that when frail patients undergo low-risk procedures, they tend to have a higher mortality rate than their healthier counterparts.15 Frailty is a concept that identifies patients who are at increased risk of dying in 6 to 60 months due to a decline in their physical reserve. Frail patients have increased rates of perioperative mortality and complications. Various tools have been used to assess the components of physical performance, speed, mobility, nutrition status, mental health, and cognition.16 Frailty screening has been initiated in several VA hospitals, including our own in Gainesville, Florida, with the goal of decreasing postoperative morbidity and mortality in older adult patients.17 The patients are given a 1-page screening assessment that asks about their living situation, medical conditions, nutrition status, cognition, and activities of daily living. The results can trigger the clinician to rethink the surgical plan and mobilize more resources to optimize the patient’s health. This study period precedes the initiative at our institution.
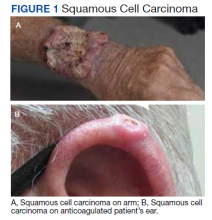
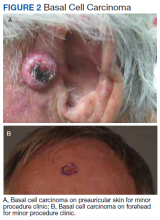
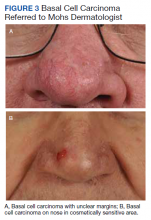
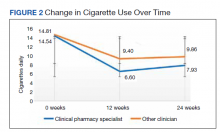
The plastic surgery service’s routine practice is to excise skin cancers in the operating room if sedation or general anesthesia will be needed (Figure 1A), for optimal control of bleeding (Figure 1B) in a patient who cannot safely stop blood thinners, or for excision of a highly vascularized area such as the scalp. Surgery is offered in an office-based setting if the area can be closed primarily, left open to close secondarily, or closed with a small skin graft under local anesthesia only (Figure 2). We prefer treating frail patients in the minor procedure clinic, when possible, to avoid the risks of sedation and the additional preoperative visits and transportation requirements. NMSC with unclear margins (Figure 3A) or in cosmetically sensitive areas where tissue needs to be preserved (Figure 3B) are referred to the Mohs dermatologist. The skin cancers in this study were most frequently found on the face, scalp, hands, and forearms based on referral patterns.
Other treatment options for NMSC include curettage and electrodessication, cryotherapy, and radiation; however, ours is a surgical service and patients are typically referred to us by primary care or dermatology when those are not reasonable or desirable options.18 Published complication rates of patients having skin cancer surgery without age restriction have a rate of 3% to 6%, which is consistent with our study of 5%.19-21 Two bleeding complications that needed to be admitted did not require more than a bedside procedure and neither required transfusions. One patient had been instructed to continue taking coumadin during the perioperative office-based procedure due to a recent carotid stent placement in the setting of a rapidly growing basal cell on an easily accessible location.
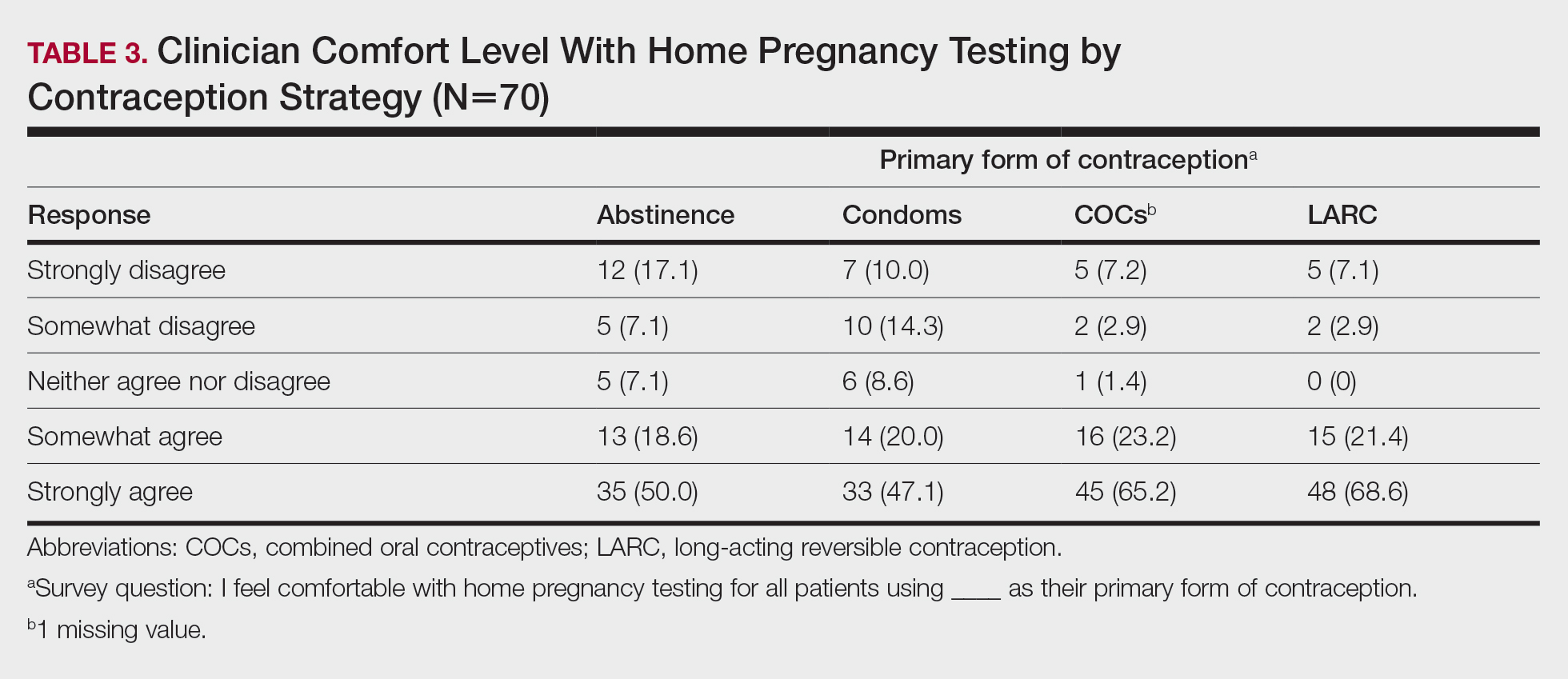
The most noted comorbidity in patients with wound complications was found to be DM; however, this was not found to be a statistically significant risk factor for wound complications (P = .10). We do not have a set rule for advising for or against NMSC surgery. We do counsel frail patients and their families that not all cancer is immediately life threatening and will work with them to do whatever makes the most sense to achieve their goals, occasionally accepting positive margins in order to debulk a symptomatic growth. The objective of this paper is to contribute to the discussion of performing invasive procedures on older adult veterans with life-limiting comorbidities. Patients and their families will have different thresholds for what they feel needs intervention, especially if other medical problems are consuming much of their time. We also have the community care referral option for patients whose treatment decisions are being dictated by travel hardships.
Strengths and Limitations
A strength of this study is that the data were obtained from a closed system. Patients tend to stay long-term within the VA and their health record is accessible throughout the country as long as they are seen at a VA facility. Complications, therefore, return to the treating service or primary care, who would route the patient back to the surgeon.
One limitation of the study is that this is a retrospective review from 2011. The authors are limited to data that are recorded in the patient record. Multiple health care professionals saw the patients and notes lack consistency in detail. Size of the lesions were not consistently recorded and did not get logged into our database for that reason.
Conclusions
Treatment of NMSC in older adult patients has a low morbidity but needs to be balanced against a patient and family’s goals when the patient presents with life-limiting comorbidities. An elevated 5-year mortality in patients aged > 80 years with serious unrelated medical conditions is intuitive, but this study may help put treatment plans into perspective for families and health care professionals who want to provide an indicated service while maximizing patient quality of life.
Acknowledgments
This manuscript is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. American Cancer Society. Cancer Facts & Figures 2021. Accessed May 26, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
2. Albert A, Knoll MA, Conti JA, Zbar RIS. Non-melanoma skin cancers in the older patient. Curr Oncol Rep. 2019;21(9):79. Published 2019 Jul 29. doi:10.1007/s11912-019-0828-9
3. Linos E, Chren MM, Stijacic Cenzer I, Covinsky KE. Skin cancer in U.S. elderly adults: does life expectancy play a role in treatment decisions? J Am Geriatr Soc. 2016;64(8):1610-1615. doi:10.1111/jgs.14202
4. Linos E, Parvataneni R, Stuart SE, Boscardin WJ, Landefeld CS, Chren MM. Treatment of nonfatal conditions at the end of life: nonmelanoma skin cancer. JAMA Intern Med. 2013;173(11):1006-1012. doi:10.1001/jamainternmed.2013.639
5. O’Malley KA, Vinson L, Kaiser AP, Sager Z, Hinrichs K. Mental health and aging veterans: how the Veterans Health Administration meets the needs of aging veterans. Public Policy Aging Rep. 2020;30(1):19-23. doi:10.1093/ppar/prz027
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2017. Accessed May 26, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2017.pdf 7. Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78(6):1185-1192. doi:10.1016/j.jaad.2017.11.062
8. Clemens MW, Kochuba AL, Carter ME, Han K, Liu J, Evans K. Association between Agent Orange exposure and nonmelanotic invasive skin cancer: a pilot study. Plast Reconstr Surg. 2014;133(2):432-437. doi:10.1097/01.prs.0000436859.40151.cf
9. Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303-317. doi:10.1016/j.jaad.2018.03.060
10. Chauhan R, Munger BN, Chu MW, et al. Age at diagnosis as a relative contraindication for intervention in facial nonmelanoma skin cancer. JAMA Surg. 2018;153(4):390-392. doi:10.1001/jamasurg.2017.5073
11. Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309(4):243-251. doi:10.1007/s00403-017-1724-5
12. Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2013. NCHS Data Brief 178. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db178.htm
13. Xu JQ, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS Data Brief 168. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db168.htm
14. Xu JQ, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief 267. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db267.htm
15. Varley PR , Borrebach JD, Arya S, et al. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. 2021;274(6):e1230-e1237. doi:10.1097/SLA.0000000000003808
16. Hall DE, Arya S , Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175-182. doi:10.1001/jamasurg.2016.4202
17. US Department of Veterans Affairs, Health Services Research & Development. Improving healthcare for aging veterans. Updated August 30, 2017. Accessed May 26, 2022. https://www.hsrd.research.va.gov/news/feature/aging0917.cfm
18. Leus AJG, Frie M, Haisma MS, et al. Treatment of keratinocyte carcinoma in elderly patients – a review of the current literature. J Eur Acad Dermatol Venereol. 2020;34(9):1932-1943. doi:10.1111/jdv.16268
19. Amici JM, Rogues AM, Lasheras A, et al. A prospective study of the incidence of complications associated with dermatological surgery. Br J Dermatol. 2005;153(5):967-971. doi:10.1111/j.1365-2133.2005.06861.x
20. Arguello-Guerra L, Vargas-Chandomid E, Díaz-González JM, Méndez-Flores S, Ruelas-Villavicencio A, Domínguez-Cherit J. Incidence of complications in dermatological surgery of melanoma and non-melanoma skin cancer in patients with multiple comorbidity and/or antiplatelet-anticoagulants. Five-year experience in our hospital. Cir Cir. 2019;86(1):15-23. doi:10.24875/CIRUE.M18000003
21. Keith DJ, de Berker DA, Bray AP, Cheung ST, Brain A, Mohd Mustapa MF. British Association of Dermatologists’ national audit on nonmelanoma skin cancer excision, 2014. Clin Exp Dermatol. 2017;42(1):46-53. doi:10.1111/ced.12990
Skin cancer is the most diagnosed cancer in the United States. Nonmelanoma skin cancers (NMSC), which include basal cell carcinoma and squamous cell carcinoma, are usually cured with removal.1 The incidence of NMSC increases with age and is commonly found in nursing homes and geriatric units. These cancers are not usually metastatic or fatal but can cause local destruction and disfigurement if neglected.2 The current standard of care is to treat diagnosed NMSC; however, the dermatology and geriatric care literature have questioned the logic of treating asymptomatic skin cancers that will not affect a patient’s life expectancy.2-4
Forty-seven percent of the current living veteran population is aged ≥ 65 years.5 Older adult patients are frequently referred to the US Department of Veterans Affairs (VA) surgical service for the treatment of NMSC. The veteran population includes a higher percentage of individuals at an elevated risk of skin cancers (older, White, and male) compared with the general population.6 World War II veterans deployed in regions closer to the equator have been found to have an elevated risk of melanoma and nonmelanoma skin carcinomas.7 A retrospective study of Vietnam veterans exposed to Agent Orange (2,3,7,8-tetrachlorodibenzodioxin) found a significantly higher risk of invasive NMSC in Fitzpatrick skin types I-IV compared with an age-matched subset of the general population.8 Younger veterans who were deployed in Afghanistan and Iraq for Operation Enduring Freedom/Operation Iraqi Freedom worked at more equatorial latitudes than the rest of the US population and may be at increased risk of NMSC. Inadequate sunscreen access, immediate safety concerns, outdoor recreational activities, harsh weather, and insufficient emphasis on sun protection have created a multifactorial challenge for the military population. Riemenschneider and colleagues recommended targeted screening for at-risk veteran patients and prioritizing annual skin cancer screenings during medical mission physical examinations for active military.7
The plastic surgery service regularly receives consults from dermatology, general surgery, and primary care to remove skin cancers on the face, scalp, hands, and forearms. Skin cancer treatment can create serious hardships for older adult patients and their families with multiple appointments for the consult, procedure, and follow-up. Patients are often told to hold their anticoagulant medications when the surgery will be performed on a highly vascular region, such as the scalp or face. This can create wide swings in their laboratory test values and result in life-threatening complications from either bleeding or clotting. The appropriateness of offering surgery to patients with serious comorbidities and a limited life expectancy has been questioned.2-4 The purpose of this study was to measure the morbidity and unrelated 5-year mortality for patients with skin cancer referred to the plastic surgery service to help patients and families make a more informed treatment decision, particularly when the patients are aged > 80 years and have significant life-threatening comorbidities.
Methods
The University of Florida and Malcom Randall VA Medical Center Institutional review board in Gainesville, approved a retrospective review of all consults completed by the plastic surgery service for the treatment of NMSC performed from July 1, 2011 to June 30, 2015. Data collected included age and common life-limiting comorbidities at the time of referral. Morbidities were found on the electronic health record, including coronary artery disease (CAD), congestive heart failure (CHF), cerebral vascular disease (CVD), peripheral vascular disease, dementia, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), tobacco use, diabetes mellitus (DM), liver disease, alcohol use, and obstructive sleep apnea.
Treatment, complications, and 5-year mortality were recorded. A χ2 analysis with P value < .05 was used to determine statistical significance between individual risk factors and 5-year mortality. The relative risk of 5-year mortality was calculated by combining advanced age (aged > 80 years) with the individual comorbidities.
Results
Over 4 years, 800 consults for NMSC were completed by the plastic surgery service. Treatment decisions included 210 excisions (with or without reconstruction) in the operating room, 402 excisions (with or without reconstruction) under local anesthesia in clinic, 55 Mohs surgical dermatology referrals, 21 other service or hospital referrals, and 112 patient who were observed, declined intervention, or died prior to intervention. Five-year mortality was 28.6%. No patients died of NMSC. The median age at consult submission for patients deceased 5 years later was 78 years. Complication rate was 5% and included wound infection, dehiscence, bleeding, or graft loss. Two patients, both deceased within 5 years, had unplanned admissions due to bleeding from either a skin graft donor site or recipient bleeding. Aged ≥ 80 years, CAD, CHF, CVD, peripheral vascular disease, dementia, CKD, COPD, and DM were all found individually to be statistically significant predictors of 5-year mortality (Table 1). Combining aged ≥ 80 years plus CAD, CHF, or dementia all increased the 5-year mortality by a relative risk of > 3 (Table 2).
Discussion
The standard of care is to treat NMSC. Most NMSCs are treated surgically without consideration of patient age or life expectancy.2,4,9,10 A prospective cohort study involving a university-based private practice and a VA medical center in San Francisco found a 22.6% overall 5-year mortality and a 43.3% mortality in the group defined as limited life expectancy (LLE) based on age (≥ 85 years) and medical comorbidities. None died due to the NMSC. Leading cause of death was cardiac, cerebrovascular, and respiratory disease, lung and prostate cancer, and Alzheimer disease. The authors suggested the LLE group may be exposed to wound complications without benefiting from the treatment.4
Another study of 440 patients receiving excision for biopsy-proven facial NMSC at the Roudebush VA Medical Center in Indianapolis, Indiana, found no residual carcinoma in 35.3% of excisions, and in patients aged > 90 years, more than half of the excisions had no residual carcinoma. More than half of the patients aged > 90 years died within 1 year, not as a result of the NMSC. The authors argued for watchful waiting in select patients to maximize comfort and outcomes.10
NMSCs are often asymptomatic and not immediately life threatening. Although NMSCs tend to have a favorable prognosis, studies have found that NMSC may be a marker for other poor health outcomes. A significant increased risk for all-cause mortality was found for patients with a history of SCC, which may be attributed to immune status.11 The aging veteran population has more complex health care needs to be considered when developing surgical treatment plans. These medical problems may limit their life expectancy much sooner than the skin cancer will become symptomatic. We found that individuals aged ≥ 80 years who had CAD, CHF, or dementia had a relative risk of 3 or higher for 5-year mortality. The leading cause of death in the United States in years 2011 to 2015 was heart disease. Alzheimer disease was the sixth leading cause of death in those same years.12-14
Skin cancer excisions do not typically require general anesthesia, deep sedation, or large fluid shifts; however, studies have found that when frail patients undergo low-risk procedures, they tend to have a higher mortality rate than their healthier counterparts.15 Frailty is a concept that identifies patients who are at increased risk of dying in 6 to 60 months due to a decline in their physical reserve. Frail patients have increased rates of perioperative mortality and complications. Various tools have been used to assess the components of physical performance, speed, mobility, nutrition status, mental health, and cognition.16 Frailty screening has been initiated in several VA hospitals, including our own in Gainesville, Florida, with the goal of decreasing postoperative morbidity and mortality in older adult patients.17 The patients are given a 1-page screening assessment that asks about their living situation, medical conditions, nutrition status, cognition, and activities of daily living. The results can trigger the clinician to rethink the surgical plan and mobilize more resources to optimize the patient’s health. This study period precedes the initiative at our institution.
The plastic surgery service’s routine practice is to excise skin cancers in the operating room if sedation or general anesthesia will be needed (Figure 1A), for optimal control of bleeding (Figure 1B) in a patient who cannot safely stop blood thinners, or for excision of a highly vascularized area such as the scalp. Surgery is offered in an office-based setting if the area can be closed primarily, left open to close secondarily, or closed with a small skin graft under local anesthesia only (Figure 2). We prefer treating frail patients in the minor procedure clinic, when possible, to avoid the risks of sedation and the additional preoperative visits and transportation requirements. NMSC with unclear margins (Figure 3A) or in cosmetically sensitive areas where tissue needs to be preserved (Figure 3B) are referred to the Mohs dermatologist. The skin cancers in this study were most frequently found on the face, scalp, hands, and forearms based on referral patterns.
Other treatment options for NMSC include curettage and electrodessication, cryotherapy, and radiation; however, ours is a surgical service and patients are typically referred to us by primary care or dermatology when those are not reasonable or desirable options.18 Published complication rates of patients having skin cancer surgery without age restriction have a rate of 3% to 6%, which is consistent with our study of 5%.19-21 Two bleeding complications that needed to be admitted did not require more than a bedside procedure and neither required transfusions. One patient had been instructed to continue taking coumadin during the perioperative office-based procedure due to a recent carotid stent placement in the setting of a rapidly growing basal cell on an easily accessible location.
The most noted comorbidity in patients with wound complications was found to be DM; however, this was not found to be a statistically significant risk factor for wound complications (P = .10). We do not have a set rule for advising for or against NMSC surgery. We do counsel frail patients and their families that not all cancer is immediately life threatening and will work with them to do whatever makes the most sense to achieve their goals, occasionally accepting positive margins in order to debulk a symptomatic growth. The objective of this paper is to contribute to the discussion of performing invasive procedures on older adult veterans with life-limiting comorbidities. Patients and their families will have different thresholds for what they feel needs intervention, especially if other medical problems are consuming much of their time. We also have the community care referral option for patients whose treatment decisions are being dictated by travel hardships.
Strengths and Limitations
A strength of this study is that the data were obtained from a closed system. Patients tend to stay long-term within the VA and their health record is accessible throughout the country as long as they are seen at a VA facility. Complications, therefore, return to the treating service or primary care, who would route the patient back to the surgeon.
One limitation of the study is that this is a retrospective review from 2011. The authors are limited to data that are recorded in the patient record. Multiple health care professionals saw the patients and notes lack consistency in detail. Size of the lesions were not consistently recorded and did not get logged into our database for that reason.
Conclusions
Treatment of NMSC in older adult patients has a low morbidity but needs to be balanced against a patient and family’s goals when the patient presents with life-limiting comorbidities. An elevated 5-year mortality in patients aged > 80 years with serious unrelated medical conditions is intuitive, but this study may help put treatment plans into perspective for families and health care professionals who want to provide an indicated service while maximizing patient quality of life.
Acknowledgments
This manuscript is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
Skin cancer is the most diagnosed cancer in the United States. Nonmelanoma skin cancers (NMSC), which include basal cell carcinoma and squamous cell carcinoma, are usually cured with removal.1 The incidence of NMSC increases with age and is commonly found in nursing homes and geriatric units. These cancers are not usually metastatic or fatal but can cause local destruction and disfigurement if neglected.2 The current standard of care is to treat diagnosed NMSC; however, the dermatology and geriatric care literature have questioned the logic of treating asymptomatic skin cancers that will not affect a patient’s life expectancy.2-4
Forty-seven percent of the current living veteran population is aged ≥ 65 years.5 Older adult patients are frequently referred to the US Department of Veterans Affairs (VA) surgical service for the treatment of NMSC. The veteran population includes a higher percentage of individuals at an elevated risk of skin cancers (older, White, and male) compared with the general population.6 World War II veterans deployed in regions closer to the equator have been found to have an elevated risk of melanoma and nonmelanoma skin carcinomas.7 A retrospective study of Vietnam veterans exposed to Agent Orange (2,3,7,8-tetrachlorodibenzodioxin) found a significantly higher risk of invasive NMSC in Fitzpatrick skin types I-IV compared with an age-matched subset of the general population.8 Younger veterans who were deployed in Afghanistan and Iraq for Operation Enduring Freedom/Operation Iraqi Freedom worked at more equatorial latitudes than the rest of the US population and may be at increased risk of NMSC. Inadequate sunscreen access, immediate safety concerns, outdoor recreational activities, harsh weather, and insufficient emphasis on sun protection have created a multifactorial challenge for the military population. Riemenschneider and colleagues recommended targeted screening for at-risk veteran patients and prioritizing annual skin cancer screenings during medical mission physical examinations for active military.7
The plastic surgery service regularly receives consults from dermatology, general surgery, and primary care to remove skin cancers on the face, scalp, hands, and forearms. Skin cancer treatment can create serious hardships for older adult patients and their families with multiple appointments for the consult, procedure, and follow-up. Patients are often told to hold their anticoagulant medications when the surgery will be performed on a highly vascular region, such as the scalp or face. This can create wide swings in their laboratory test values and result in life-threatening complications from either bleeding or clotting. The appropriateness of offering surgery to patients with serious comorbidities and a limited life expectancy has been questioned.2-4 The purpose of this study was to measure the morbidity and unrelated 5-year mortality for patients with skin cancer referred to the plastic surgery service to help patients and families make a more informed treatment decision, particularly when the patients are aged > 80 years and have significant life-threatening comorbidities.
Methods
The University of Florida and Malcom Randall VA Medical Center Institutional review board in Gainesville, approved a retrospective review of all consults completed by the plastic surgery service for the treatment of NMSC performed from July 1, 2011 to June 30, 2015. Data collected included age and common life-limiting comorbidities at the time of referral. Morbidities were found on the electronic health record, including coronary artery disease (CAD), congestive heart failure (CHF), cerebral vascular disease (CVD), peripheral vascular disease, dementia, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), tobacco use, diabetes mellitus (DM), liver disease, alcohol use, and obstructive sleep apnea.
Treatment, complications, and 5-year mortality were recorded. A χ2 analysis with P value < .05 was used to determine statistical significance between individual risk factors and 5-year mortality. The relative risk of 5-year mortality was calculated by combining advanced age (aged > 80 years) with the individual comorbidities.
Results
Over 4 years, 800 consults for NMSC were completed by the plastic surgery service. Treatment decisions included 210 excisions (with or without reconstruction) in the operating room, 402 excisions (with or without reconstruction) under local anesthesia in clinic, 55 Mohs surgical dermatology referrals, 21 other service or hospital referrals, and 112 patient who were observed, declined intervention, or died prior to intervention. Five-year mortality was 28.6%. No patients died of NMSC. The median age at consult submission for patients deceased 5 years later was 78 years. Complication rate was 5% and included wound infection, dehiscence, bleeding, or graft loss. Two patients, both deceased within 5 years, had unplanned admissions due to bleeding from either a skin graft donor site or recipient bleeding. Aged ≥ 80 years, CAD, CHF, CVD, peripheral vascular disease, dementia, CKD, COPD, and DM were all found individually to be statistically significant predictors of 5-year mortality (Table 1). Combining aged ≥ 80 years plus CAD, CHF, or dementia all increased the 5-year mortality by a relative risk of > 3 (Table 2).
Discussion
The standard of care is to treat NMSC. Most NMSCs are treated surgically without consideration of patient age or life expectancy.2,4,9,10 A prospective cohort study involving a university-based private practice and a VA medical center in San Francisco found a 22.6% overall 5-year mortality and a 43.3% mortality in the group defined as limited life expectancy (LLE) based on age (≥ 85 years) and medical comorbidities. None died due to the NMSC. Leading cause of death was cardiac, cerebrovascular, and respiratory disease, lung and prostate cancer, and Alzheimer disease. The authors suggested the LLE group may be exposed to wound complications without benefiting from the treatment.4
Another study of 440 patients receiving excision for biopsy-proven facial NMSC at the Roudebush VA Medical Center in Indianapolis, Indiana, found no residual carcinoma in 35.3% of excisions, and in patients aged > 90 years, more than half of the excisions had no residual carcinoma. More than half of the patients aged > 90 years died within 1 year, not as a result of the NMSC. The authors argued for watchful waiting in select patients to maximize comfort and outcomes.10
NMSCs are often asymptomatic and not immediately life threatening. Although NMSCs tend to have a favorable prognosis, studies have found that NMSC may be a marker for other poor health outcomes. A significant increased risk for all-cause mortality was found for patients with a history of SCC, which may be attributed to immune status.11 The aging veteran population has more complex health care needs to be considered when developing surgical treatment plans. These medical problems may limit their life expectancy much sooner than the skin cancer will become symptomatic. We found that individuals aged ≥ 80 years who had CAD, CHF, or dementia had a relative risk of 3 or higher for 5-year mortality. The leading cause of death in the United States in years 2011 to 2015 was heart disease. Alzheimer disease was the sixth leading cause of death in those same years.12-14
Skin cancer excisions do not typically require general anesthesia, deep sedation, or large fluid shifts; however, studies have found that when frail patients undergo low-risk procedures, they tend to have a higher mortality rate than their healthier counterparts.15 Frailty is a concept that identifies patients who are at increased risk of dying in 6 to 60 months due to a decline in their physical reserve. Frail patients have increased rates of perioperative mortality and complications. Various tools have been used to assess the components of physical performance, speed, mobility, nutrition status, mental health, and cognition.16 Frailty screening has been initiated in several VA hospitals, including our own in Gainesville, Florida, with the goal of decreasing postoperative morbidity and mortality in older adult patients.17 The patients are given a 1-page screening assessment that asks about their living situation, medical conditions, nutrition status, cognition, and activities of daily living. The results can trigger the clinician to rethink the surgical plan and mobilize more resources to optimize the patient’s health. This study period precedes the initiative at our institution.
The plastic surgery service’s routine practice is to excise skin cancers in the operating room if sedation or general anesthesia will be needed (Figure 1A), for optimal control of bleeding (Figure 1B) in a patient who cannot safely stop blood thinners, or for excision of a highly vascularized area such as the scalp. Surgery is offered in an office-based setting if the area can be closed primarily, left open to close secondarily, or closed with a small skin graft under local anesthesia only (Figure 2). We prefer treating frail patients in the minor procedure clinic, when possible, to avoid the risks of sedation and the additional preoperative visits and transportation requirements. NMSC with unclear margins (Figure 3A) or in cosmetically sensitive areas where tissue needs to be preserved (Figure 3B) are referred to the Mohs dermatologist. The skin cancers in this study were most frequently found on the face, scalp, hands, and forearms based on referral patterns.
Other treatment options for NMSC include curettage and electrodessication, cryotherapy, and radiation; however, ours is a surgical service and patients are typically referred to us by primary care or dermatology when those are not reasonable or desirable options.18 Published complication rates of patients having skin cancer surgery without age restriction have a rate of 3% to 6%, which is consistent with our study of 5%.19-21 Two bleeding complications that needed to be admitted did not require more than a bedside procedure and neither required transfusions. One patient had been instructed to continue taking coumadin during the perioperative office-based procedure due to a recent carotid stent placement in the setting of a rapidly growing basal cell on an easily accessible location.
The most noted comorbidity in patients with wound complications was found to be DM; however, this was not found to be a statistically significant risk factor for wound complications (P = .10). We do not have a set rule for advising for or against NMSC surgery. We do counsel frail patients and their families that not all cancer is immediately life threatening and will work with them to do whatever makes the most sense to achieve their goals, occasionally accepting positive margins in order to debulk a symptomatic growth. The objective of this paper is to contribute to the discussion of performing invasive procedures on older adult veterans with life-limiting comorbidities. Patients and their families will have different thresholds for what they feel needs intervention, especially if other medical problems are consuming much of their time. We also have the community care referral option for patients whose treatment decisions are being dictated by travel hardships.
Strengths and Limitations
A strength of this study is that the data were obtained from a closed system. Patients tend to stay long-term within the VA and their health record is accessible throughout the country as long as they are seen at a VA facility. Complications, therefore, return to the treating service or primary care, who would route the patient back to the surgeon.
One limitation of the study is that this is a retrospective review from 2011. The authors are limited to data that are recorded in the patient record. Multiple health care professionals saw the patients and notes lack consistency in detail. Size of the lesions were not consistently recorded and did not get logged into our database for that reason.
Conclusions
Treatment of NMSC in older adult patients has a low morbidity but needs to be balanced against a patient and family’s goals when the patient presents with life-limiting comorbidities. An elevated 5-year mortality in patients aged > 80 years with serious unrelated medical conditions is intuitive, but this study may help put treatment plans into perspective for families and health care professionals who want to provide an indicated service while maximizing patient quality of life.
Acknowledgments
This manuscript is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. American Cancer Society. Cancer Facts & Figures 2021. Accessed May 26, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
2. Albert A, Knoll MA, Conti JA, Zbar RIS. Non-melanoma skin cancers in the older patient. Curr Oncol Rep. 2019;21(9):79. Published 2019 Jul 29. doi:10.1007/s11912-019-0828-9
3. Linos E, Chren MM, Stijacic Cenzer I, Covinsky KE. Skin cancer in U.S. elderly adults: does life expectancy play a role in treatment decisions? J Am Geriatr Soc. 2016;64(8):1610-1615. doi:10.1111/jgs.14202
4. Linos E, Parvataneni R, Stuart SE, Boscardin WJ, Landefeld CS, Chren MM. Treatment of nonfatal conditions at the end of life: nonmelanoma skin cancer. JAMA Intern Med. 2013;173(11):1006-1012. doi:10.1001/jamainternmed.2013.639
5. O’Malley KA, Vinson L, Kaiser AP, Sager Z, Hinrichs K. Mental health and aging veterans: how the Veterans Health Administration meets the needs of aging veterans. Public Policy Aging Rep. 2020;30(1):19-23. doi:10.1093/ppar/prz027
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2017. Accessed May 26, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2017.pdf 7. Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78(6):1185-1192. doi:10.1016/j.jaad.2017.11.062
8. Clemens MW, Kochuba AL, Carter ME, Han K, Liu J, Evans K. Association between Agent Orange exposure and nonmelanotic invasive skin cancer: a pilot study. Plast Reconstr Surg. 2014;133(2):432-437. doi:10.1097/01.prs.0000436859.40151.cf
9. Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303-317. doi:10.1016/j.jaad.2018.03.060
10. Chauhan R, Munger BN, Chu MW, et al. Age at diagnosis as a relative contraindication for intervention in facial nonmelanoma skin cancer. JAMA Surg. 2018;153(4):390-392. doi:10.1001/jamasurg.2017.5073
11. Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309(4):243-251. doi:10.1007/s00403-017-1724-5
12. Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2013. NCHS Data Brief 178. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db178.htm
13. Xu JQ, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS Data Brief 168. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db168.htm
14. Xu JQ, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief 267. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db267.htm
15. Varley PR , Borrebach JD, Arya S, et al. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. 2021;274(6):e1230-e1237. doi:10.1097/SLA.0000000000003808
16. Hall DE, Arya S , Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175-182. doi:10.1001/jamasurg.2016.4202
17. US Department of Veterans Affairs, Health Services Research & Development. Improving healthcare for aging veterans. Updated August 30, 2017. Accessed May 26, 2022. https://www.hsrd.research.va.gov/news/feature/aging0917.cfm
18. Leus AJG, Frie M, Haisma MS, et al. Treatment of keratinocyte carcinoma in elderly patients – a review of the current literature. J Eur Acad Dermatol Venereol. 2020;34(9):1932-1943. doi:10.1111/jdv.16268
19. Amici JM, Rogues AM, Lasheras A, et al. A prospective study of the incidence of complications associated with dermatological surgery. Br J Dermatol. 2005;153(5):967-971. doi:10.1111/j.1365-2133.2005.06861.x
20. Arguello-Guerra L, Vargas-Chandomid E, Díaz-González JM, Méndez-Flores S, Ruelas-Villavicencio A, Domínguez-Cherit J. Incidence of complications in dermatological surgery of melanoma and non-melanoma skin cancer in patients with multiple comorbidity and/or antiplatelet-anticoagulants. Five-year experience in our hospital. Cir Cir. 2019;86(1):15-23. doi:10.24875/CIRUE.M18000003
21. Keith DJ, de Berker DA, Bray AP, Cheung ST, Brain A, Mohd Mustapa MF. British Association of Dermatologists’ national audit on nonmelanoma skin cancer excision, 2014. Clin Exp Dermatol. 2017;42(1):46-53. doi:10.1111/ced.12990
1. American Cancer Society. Cancer Facts & Figures 2021. Accessed May 26, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
2. Albert A, Knoll MA, Conti JA, Zbar RIS. Non-melanoma skin cancers in the older patient. Curr Oncol Rep. 2019;21(9):79. Published 2019 Jul 29. doi:10.1007/s11912-019-0828-9
3. Linos E, Chren MM, Stijacic Cenzer I, Covinsky KE. Skin cancer in U.S. elderly adults: does life expectancy play a role in treatment decisions? J Am Geriatr Soc. 2016;64(8):1610-1615. doi:10.1111/jgs.14202
4. Linos E, Parvataneni R, Stuart SE, Boscardin WJ, Landefeld CS, Chren MM. Treatment of nonfatal conditions at the end of life: nonmelanoma skin cancer. JAMA Intern Med. 2013;173(11):1006-1012. doi:10.1001/jamainternmed.2013.639
5. O’Malley KA, Vinson L, Kaiser AP, Sager Z, Hinrichs K. Mental health and aging veterans: how the Veterans Health Administration meets the needs of aging veterans. Public Policy Aging Rep. 2020;30(1):19-23. doi:10.1093/ppar/prz027
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2017. Accessed May 26, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2017.pdf 7. Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78(6):1185-1192. doi:10.1016/j.jaad.2017.11.062
8. Clemens MW, Kochuba AL, Carter ME, Han K, Liu J, Evans K. Association between Agent Orange exposure and nonmelanotic invasive skin cancer: a pilot study. Plast Reconstr Surg. 2014;133(2):432-437. doi:10.1097/01.prs.0000436859.40151.cf
9. Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303-317. doi:10.1016/j.jaad.2018.03.060
10. Chauhan R, Munger BN, Chu MW, et al. Age at diagnosis as a relative contraindication for intervention in facial nonmelanoma skin cancer. JAMA Surg. 2018;153(4):390-392. doi:10.1001/jamasurg.2017.5073
11. Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309(4):243-251. doi:10.1007/s00403-017-1724-5
12. Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2013. NCHS Data Brief 178. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db178.htm
13. Xu JQ, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS Data Brief 168. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db168.htm
14. Xu JQ, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief 267. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db267.htm
15. Varley PR , Borrebach JD, Arya S, et al. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. 2021;274(6):e1230-e1237. doi:10.1097/SLA.0000000000003808
16. Hall DE, Arya S , Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175-182. doi:10.1001/jamasurg.2016.4202
17. US Department of Veterans Affairs, Health Services Research & Development. Improving healthcare for aging veterans. Updated August 30, 2017. Accessed May 26, 2022. https://www.hsrd.research.va.gov/news/feature/aging0917.cfm
18. Leus AJG, Frie M, Haisma MS, et al. Treatment of keratinocyte carcinoma in elderly patients – a review of the current literature. J Eur Acad Dermatol Venereol. 2020;34(9):1932-1943. doi:10.1111/jdv.16268
19. Amici JM, Rogues AM, Lasheras A, et al. A prospective study of the incidence of complications associated with dermatological surgery. Br J Dermatol. 2005;153(5):967-971. doi:10.1111/j.1365-2133.2005.06861.x
20. Arguello-Guerra L, Vargas-Chandomid E, Díaz-González JM, Méndez-Flores S, Ruelas-Villavicencio A, Domínguez-Cherit J. Incidence of complications in dermatological surgery of melanoma and non-melanoma skin cancer in patients with multiple comorbidity and/or antiplatelet-anticoagulants. Five-year experience in our hospital. Cir Cir. 2019;86(1):15-23. doi:10.24875/CIRUE.M18000003
21. Keith DJ, de Berker DA, Bray AP, Cheung ST, Brain A, Mohd Mustapa MF. British Association of Dermatologists’ national audit on nonmelanoma skin cancer excision, 2014. Clin Exp Dermatol. 2017;42(1):46-53. doi:10.1111/ced.12990
Insulin Injection-Site Acanthosis Nigricans: Skin Reactions and Clinical Implications
Insulin injection therapy is one of the most widely used health care interventions to manage both type 1 and type 2 diabetes mellitus (T1DM/T2DM). Globally, more than 150 to 200 million people inject insulin into their upper posterior arms, buttocks, anterior and lateral thighs, or abdomen.1,2 In an ideal world, every patient would be using the correct site and rotating their insulin injection sites in accordance with health care professional (HCP) recommendations—systematic injections in one general body location, at least 1 cm away from the previous injection.2 Unfortunately, same-site insulin injection (repeatedly in the same region within 1 cm of previous injections) is a common mistake made by patients with DM—in one study, 63% of participants either did not rotate sites correctly or failed to do so at all.
Insulin-resistant cutaneous complications may occur as a result of same-site insulin injections. The most common is lipohypertrophy, reported in some studies in nearly 50% of patients with DM on insulin therapy.4 Other common cutaneous complications include lipoatrophy and amyloidosis. Injection-site acanthosis nigricans, although uncommon, has been reported in 18 cases in the literature.
Most articles suggest that same-site insulin injections decrease local insulin sensitivity and result in tissue hypertrophy because of the anabolic properties of insulin and increase in insulin binding to insulin-like growth factor-1 (IGF-1) receptor.5-20 The hyperkeratotic growth and varying insulin absorption rates associated with these cutaneous complications increase chances of either hyper- or hypoglycemic episodes in patients.10,11,13 It is the responsibility of the DM care professional to provide proper insulin-injection technique education and perform routine inspection of injection sites to reduce cutaneous complications of insulin therapy. The purpose of this article is to (1) describe a case of acanthosis nigricans resulting from insulin injection at the same site; (2) review case reports
Case Presentation
A 75-year-old patient with an 8-year history of T2DM, as well as stable coronary artery disease, atrial fibrillation, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and stage 3 chronic kidney disease, presented with 2 discrete abdominal hyperpigmented plaques. At the time of the initial clinic visit, the patient was taking metformin 1000 mg twice daily and insulin glargine 40 units once daily. When insulin was initiated 7 years prior, the patient received
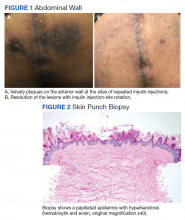
The patient reported 5 years of progressive, asymptomatic hyperpigmentation of the skin surrounding his insulin glargine injection sites and injecting in these same sites daily without rotation. He reported no additional skin changes or symptoms. He had noticed no skin changes while using NPH insulin during his first year of insulin therapy. On examination, the abdominal wall skin demonstrated 2 well-demarcated, nearly black, soft, velvety plaques, measuring 9 × 8 cm on the left side and 4 × 3.5 cm on the right, suggesting acanthosis nigricans (Figure 1A). The remainder of the skin examination, including the flexures, was normal. Of note, the patient received biweekly intramuscular testosterone injections in the gluteal region for secondary hypogonadism with no adverse dermatologic effects. A skin punch biopsy was performed and revealed epidermal papillomatosis and hyperkeratosis, confirming the clinical diagnosis of acanthosis nigricans (Figure 2).
After a review of insulin-injection technique at his clinic visit, the patient started rotating insulin injection sites over his entire abdomen, and the acanthosis nigricans partially improved. A few months later, the patient stopped rotating the insulin injection site, and the acanthosis nigricans worsened again. Because of worsening glycemic control, the patient was then started on insulin aspart. He did not develop any skin changes at the insulin aspart injection site, although he was not rotating its site of injection.
Subsequently, with reeducation and proper injection-site rotation, the patient had resolution of his acanthosis nigricans (Figure 1b).
Discussion
A review of the literature revealed 18 reported cases of acanthosis nigricans at sites of repeated insulin injection (Table).5-20 Acanthosis nigricans at the site of insulin injection afflicts patients of any age, with cases observed in patients aged 14 to 75 years. Sixteen (84%) of 19 cases were male. Fourteen cases (73%) had T2DM; the rest of the patients had T1DM. The duration of insulin injection therapy prior to onset ranged from immediate to 13 years (median 4 years). Fourteen cases (73%) were reported on the abdomen; however, other sites, such as thighs and upper arm, also were reported. Lesions size varied from 12 to 360 cm2. Two cases had associated amyloidosis. The average HbA1c reported at presentation was 10%. Following insulin injection-site rotation, most of the cases reported improvement of both glycemic control and acanthosis nigricans appearance.
In the case described by Kudo and colleagues, a 59-year-old male patient with T2DM had been injecting insulin into the same spot on his abdomen for 10 years. He developed acanthosis nigricans and an amyloidoma so large and firm that it bent the needle when he injected insulin.11
Most of the cases we found in the literature were after 2005 and associated with the use of human or analog insulin. These cases may be related to a bias, as cases may be easier to find in digital archives in the later years, when human or analog insulins have been in common use. Also noteworthy, in cases that reported dosage, most were not very high, and the highest daily dose was 240 IU/d. Ten reports of injection-site acanthosis nigricans were in dermatology journals; only 5 reports were in endocrinology journals and 3 in general medical journals, indicating possible less awareness of this phenomenon in other HCPs who care for patients with DM.
Complications of Same-Site Injections
Acanthosis nigricans. Commonly found in the armpits, neck folds, and groin, acanthosis nigricans is known as one of the calling cards for insulin resistance, obesity, and hyperinsulinemia.21 Acanthosis nigricans can be seen in people with or without DM and is not limited to those on insulin therapy. However, same-site insulin injections for 4 to 6 years also may result in injection-site acanthosis nigricans–like lesions because of factors such as insulin exposure at the local tissue level.16
Acanthosis nigricans development is characterized by hyperpigmented, hyperkeratotic, velvety, and sometimes verrucous plaques.6 Acanthosis nigricans surrounding repeated injection sites is hypothesized to develop as a result of localized hyperinsulinemia secondary to insulin resistance, which increases the stimulation of IGF, thereby causing epidermal hypertrophy.5-20 If insulin injection therapy continues to be administered through the acanthosis nigricans lesion, it results in decreased insulin absorption, leading to poor glycemic control.13
Acanthosis nigricans associated with insulin injection is reversible. After rotation of injection sites, lesions either decrease in size or severity of appearance.5-8,11 Also, by avoiding injection into the hyperkeratotic plaques and using normal subcutaneous tissue for injection, patients’ response to insulin improves, as measured by HbA1c and by decreased daily insulin requirement.6-8,10,12,18-20
Lipohypertrophy. This is characterized by an increase in localized adipose tissue and is the most common cutaneous complication of insulin therapy.2 Lipohypertrophy presents as a firm, rubbery mass in the location of same-site insulin injections.22 Development of lipohypertrophy is suspected to be the result of either (1) anabolic effect of insulin on local adipocytes, promoting fat and protein synthesis; (2) an autoimmune response by immunoglobulin (Ig) G or IgE antibodies to insulin, immune response to insulin of different species, or to insulin injection techniques; or (3) repeated trauma to the injection site from repeated needle usage.4,23
In a study assessing the prevalence of lipohypertrophy and its relation to insulin technique, 49.1% of participants with
Primary prevention measures include injection site inspection and patient education about rotation and abstaining from needle reuse.22 If a patient already has signs of lipohypertrophy, data supports education and insulin injection technique practice as simple and effective means to reduce insulin action variability and increase glycemic control.24
Lipoatrophy. Lipoatrophy is described as a local loss of subcutaneous adipose tissue often in the face, buttocks, legs and arm regions and can be rooted in genetic, immune, or drug-associated etiologies.25 Insulin-induced lipoatrophy is suspected to be the result of tumor necrosis factor-α hyperproduction in reaction to insulin crystal presence at the injection site.26,27 Overall, lipoatrophy development has decreased since the use of recombinant human insulin and analog insulin therapy.28 The decrease is hypothesized to be due to increased subcutaneous tissue absorption rate of human insulin and its analog, decreasing overall adipocyte exposure to localized high insulin concentration.27 Treatments for same-site insulin-derived lipoatrophy include changing injection sites and preparation of insulin.26 When injection into the lipoatrophic site was avoided, glycemic control and lipoatrophy appearance improved.26
Amyloidosis. Amyloidosis indicates the presence of an extracellular bundle of insoluble polymeric protein fibrils in tissues and organs.29 Insulin-induced amyloidosis presents as a hard mass or ball near the injection site.29 Insulin is one of many hormones that can form amyloid fibrils, and there have been several dozen cases reported of amyloid formation at the site of insulin injection.29-31 Although insulin-derived amyloidosis is rare, it may be misdiagnosed as lipohypertrophy due to a lack of histopathologic testing or general awareness of the complication.29
In a case series of 7 patients with amyloidosis, all patients had a mean HbA1c of 9.3% (range, 8.5-10.2%) and averaged 1 IU/kg bodyweight before intervention.30 After the discovery of the mass, participants were instructed to avoid injection into the amyloidoma, and average insulin requirements decreased to 0.48 IU/kg body weight (P = .40).30 Patients with amyloidosis who rotated their injection sites experienced better glycemic control and decreased insulin requirements.30
Pathophysiology of Localized Insulin Resistance
Insulin regulates glucose homeostasis in skeletal muscle and adipose tissue, increases hepatic and adipocyte lipid synthesis, and decreases adipocyte fatty acid release.32 Generalized insulin resistance occurs when target tissues have decreased glucose uptake in response to circulating insulin.32 Insulin resistance increases the amount of free insulin in surrounding tissues. At high concentrations, insulin fosters tissue growth by binding to IGF-1 receptors, stimulating hypertrophy and reproduction of keratinocytes and fibroblasts.33 This pathophysiology helps explain the origin of localized acanthosis nigricans at same-site insulin injections.
Conclusions
Cutaneous complications are a local adverse effect of long-term failure to rotate insulin injection sites. Our case serves as a call to action for HCPs to improve education regarding insulin injection-site rotation, conduct routine injection-site inspection, and actively document cases as they occur to increase public awareness of these important complications.
If a patient with DM presents with unexplained poor glycemic control, consider questioning the patient about injection-site location and how often they are rotating the insulin injection site. Inspect the site for cutaneous complications. Of note, if a patient has a cutaneous complication due to insulin injection, adjust or decrease the insulin dosage when rotating sites to mitigate the risk of hypoglycemic episodes.
Improvement of glycemic control, cosmetic appearance of injection site, and insulin use all begin with skin inspection, injection technique education, and periodic review by a HCP.
1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi:10.1089/dia.2018.0384
2. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231-1255. doi:10.1016/j.mayocp.2016.06.010
3. Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445-453. doi:10.1016/j.diabet.2013.05.006
4. Johansson UB, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28(8):2025-2027. doi:10.2337/diacare.28.8.2025
5. Erickson L, Lipschutz DE, Wrigley W, Kearse WO. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209(6):934-935. doi:10.1001/jama.1969.03160190056019
6. Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122(9):1054-1056. doi:10.1001/archderm.1986.01660210104028 7. Gannon D, Ross MW, Mahajan T. Acanthosis nigricans-like plaque and lipohypertrophy in type 1 diabetes. Pract Diabetes International. 2005;22(6).
8. Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144(1):126-127. doi:10.1001/archdermatol.2007.27
9. Pachón Burgos A, Chan Aguilar MP. Visual vignette. Hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14(4):514. doi:10.4158/EP.14.4.514
10. Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94(2):e34-e36. doi:10.1016/j.diabres.2011.07.023
11. Kudo-Watanuki S, Kurihara E, Yamamoto K, Mukai K, Chen KR. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2012;38(1):25-29. doi:10.1111/j.1365-2230.2012.04373.x
12. Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11(12):e85-e87.
13. Kanwar A, Sawatkar G, Dogra S, Bhadada S. Acanthosis nigricans—an uncommon cutaneous adverse effect of a common medication: report of two cases. Indian J Dermatol Venereol Leprol. 2013;79(4):553. doi:10.4103/0378-6323.113112
14. Dhingra M, Garg G, Gupta M, Khurana U, Thami GP. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19(1):9. Published 2013 Jan 15.
15. Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57(1):127-129. doi:10.4103/0377-4929.130920
16. Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39(1):5-9.
17. Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39(12):e163. doi:10.1097/DAD.0000000000000659
18. Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190(47):E1390. doi:10.1503/cmaj.180705
19. Pal R, Bhattacharjee R, Chatterjee D, Bhadada SK, Bhansali A, Dutta P. Exogenous insulin-induced localized acanthosis nigricans: a rare injection site complication. Can J Diabetes. 2020;44(3):219-221. doi:10.1016/j.jcjd.2019.08.010
20. Bomar L, Lewallen R, Jorizzo J. Localized acanthosis nigricans at the site of repetitive insulin injections. Cutis. 2020;105(2);E20-E22.
21. Karadağ AS, You Y, Danarti R, Al-Khuzaei S, Chen W. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36(1):48-53. doi:10.1016/j.clindermatol.2017.09.008
22. Kalra S, Kumar A, Gupta Y. Prevention of lipohypertrophy. J Pak Med Assoc. 2016;66(7):910-911.
23. Singha A, Bhattarcharjee R, Ghosh S, Chakrabarti SK, Baidya A, Chowdhury S. Concurrence of lipoatrophy and lipohypertrophy in children with type 1 diabetes using recombinant human insulin: two case reports. Clin Diabetes. 2016;34(1):51-53. doi:10.2337/diaclin.34.1.51
24. Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486-1492. doi:10.2337/dc16-0610.
25. Reitman ML, Arioglu E, Gavrilova O, Taylor SI. Lipoatrophy revisited. Trends Endocrinol Metab. 2000;11(10):410-416. doi:10.1016/s1043-2760(00)00309-x
26. Kondo A, Nakamura A, Takeuchi J, Miyoshi H, Atsumi T. Insulin-Induced Distant Site Lipoatrophy. Diabetes Care. 2017;40(6):e67-e68. doi:10.2337/dc16-2385
27. Jermendy G, Nádas J, Sápi Z. “Lipoblastoma-like” lipoatrophy induced by human insulin: morphological evidence for local dedifferentiation of adipocytes?. Diabetologia. 2000;43(7):955-956. doi:10.1007/s001250051476
28. Mokta JK, Mokta KK, Panda P. Insulin lipodystrophy and lipohypertrophy. Indian J Endocrinol Metab. 2013;17(4):773-774. doi:10.4103/2230-8210.113788
29. Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19(1):174-177. doi:10.4103/2230-8210.146879
30. Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127(5):450-454. doi:10.1016/j.amjmed.2013.10.029
31. Swift B. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabet Med. 2002;19(10):881-882. doi:10.1046/j.1464-5491.2002.07581.x
32. Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20(4):665-679. doi:10.1016/j.beem.2006.09.007
33. Phiske MM. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014;5(3):239-249. doi:10.4103/2229-5178.137765
Insulin injection therapy is one of the most widely used health care interventions to manage both type 1 and type 2 diabetes mellitus (T1DM/T2DM). Globally, more than 150 to 200 million people inject insulin into their upper posterior arms, buttocks, anterior and lateral thighs, or abdomen.1,2 In an ideal world, every patient would be using the correct site and rotating their insulin injection sites in accordance with health care professional (HCP) recommendations—systematic injections in one general body location, at least 1 cm away from the previous injection.2 Unfortunately, same-site insulin injection (repeatedly in the same region within 1 cm of previous injections) is a common mistake made by patients with DM—in one study, 63% of participants either did not rotate sites correctly or failed to do so at all.
Insulin-resistant cutaneous complications may occur as a result of same-site insulin injections. The most common is lipohypertrophy, reported in some studies in nearly 50% of patients with DM on insulin therapy.4 Other common cutaneous complications include lipoatrophy and amyloidosis. Injection-site acanthosis nigricans, although uncommon, has been reported in 18 cases in the literature.
Most articles suggest that same-site insulin injections decrease local insulin sensitivity and result in tissue hypertrophy because of the anabolic properties of insulin and increase in insulin binding to insulin-like growth factor-1 (IGF-1) receptor.5-20 The hyperkeratotic growth and varying insulin absorption rates associated with these cutaneous complications increase chances of either hyper- or hypoglycemic episodes in patients.10,11,13 It is the responsibility of the DM care professional to provide proper insulin-injection technique education and perform routine inspection of injection sites to reduce cutaneous complications of insulin therapy. The purpose of this article is to (1) describe a case of acanthosis nigricans resulting from insulin injection at the same site; (2) review case reports
Case Presentation
A 75-year-old patient with an 8-year history of T2DM, as well as stable coronary artery disease, atrial fibrillation, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and stage 3 chronic kidney disease, presented with 2 discrete abdominal hyperpigmented plaques. At the time of the initial clinic visit, the patient was taking metformin 1000 mg twice daily and insulin glargine 40 units once daily. When insulin was initiated 7 years prior, the patient received
The patient reported 5 years of progressive, asymptomatic hyperpigmentation of the skin surrounding his insulin glargine injection sites and injecting in these same sites daily without rotation. He reported no additional skin changes or symptoms. He had noticed no skin changes while using NPH insulin during his first year of insulin therapy. On examination, the abdominal wall skin demonstrated 2 well-demarcated, nearly black, soft, velvety plaques, measuring 9 × 8 cm on the left side and 4 × 3.5 cm on the right, suggesting acanthosis nigricans (Figure 1A). The remainder of the skin examination, including the flexures, was normal. Of note, the patient received biweekly intramuscular testosterone injections in the gluteal region for secondary hypogonadism with no adverse dermatologic effects. A skin punch biopsy was performed and revealed epidermal papillomatosis and hyperkeratosis, confirming the clinical diagnosis of acanthosis nigricans (Figure 2).
After a review of insulin-injection technique at his clinic visit, the patient started rotating insulin injection sites over his entire abdomen, and the acanthosis nigricans partially improved. A few months later, the patient stopped rotating the insulin injection site, and the acanthosis nigricans worsened again. Because of worsening glycemic control, the patient was then started on insulin aspart. He did not develop any skin changes at the insulin aspart injection site, although he was not rotating its site of injection.
Subsequently, with reeducation and proper injection-site rotation, the patient had resolution of his acanthosis nigricans (Figure 1b).
Discussion
A review of the literature revealed 18 reported cases of acanthosis nigricans at sites of repeated insulin injection (Table).5-20 Acanthosis nigricans at the site of insulin injection afflicts patients of any age, with cases observed in patients aged 14 to 75 years. Sixteen (84%) of 19 cases were male. Fourteen cases (73%) had T2DM; the rest of the patients had T1DM. The duration of insulin injection therapy prior to onset ranged from immediate to 13 years (median 4 years). Fourteen cases (73%) were reported on the abdomen; however, other sites, such as thighs and upper arm, also were reported. Lesions size varied from 12 to 360 cm2. Two cases had associated amyloidosis. The average HbA1c reported at presentation was 10%. Following insulin injection-site rotation, most of the cases reported improvement of both glycemic control and acanthosis nigricans appearance.
In the case described by Kudo and colleagues, a 59-year-old male patient with T2DM had been injecting insulin into the same spot on his abdomen for 10 years. He developed acanthosis nigricans and an amyloidoma so large and firm that it bent the needle when he injected insulin.11
Most of the cases we found in the literature were after 2005 and associated with the use of human or analog insulin. These cases may be related to a bias, as cases may be easier to find in digital archives in the later years, when human or analog insulins have been in common use. Also noteworthy, in cases that reported dosage, most were not very high, and the highest daily dose was 240 IU/d. Ten reports of injection-site acanthosis nigricans were in dermatology journals; only 5 reports were in endocrinology journals and 3 in general medical journals, indicating possible less awareness of this phenomenon in other HCPs who care for patients with DM.
Complications of Same-Site Injections
Acanthosis nigricans. Commonly found in the armpits, neck folds, and groin, acanthosis nigricans is known as one of the calling cards for insulin resistance, obesity, and hyperinsulinemia.21 Acanthosis nigricans can be seen in people with or without DM and is not limited to those on insulin therapy. However, same-site insulin injections for 4 to 6 years also may result in injection-site acanthosis nigricans–like lesions because of factors such as insulin exposure at the local tissue level.16
Acanthosis nigricans development is characterized by hyperpigmented, hyperkeratotic, velvety, and sometimes verrucous plaques.6 Acanthosis nigricans surrounding repeated injection sites is hypothesized to develop as a result of localized hyperinsulinemia secondary to insulin resistance, which increases the stimulation of IGF, thereby causing epidermal hypertrophy.5-20 If insulin injection therapy continues to be administered through the acanthosis nigricans lesion, it results in decreased insulin absorption, leading to poor glycemic control.13
Acanthosis nigricans associated with insulin injection is reversible. After rotation of injection sites, lesions either decrease in size or severity of appearance.5-8,11 Also, by avoiding injection into the hyperkeratotic plaques and using normal subcutaneous tissue for injection, patients’ response to insulin improves, as measured by HbA1c and by decreased daily insulin requirement.6-8,10,12,18-20
Lipohypertrophy. This is characterized by an increase in localized adipose tissue and is the most common cutaneous complication of insulin therapy.2 Lipohypertrophy presents as a firm, rubbery mass in the location of same-site insulin injections.22 Development of lipohypertrophy is suspected to be the result of either (1) anabolic effect of insulin on local adipocytes, promoting fat and protein synthesis; (2) an autoimmune response by immunoglobulin (Ig) G or IgE antibodies to insulin, immune response to insulin of different species, or to insulin injection techniques; or (3) repeated trauma to the injection site from repeated needle usage.4,23
In a study assessing the prevalence of lipohypertrophy and its relation to insulin technique, 49.1% of participants with
Primary prevention measures include injection site inspection and patient education about rotation and abstaining from needle reuse.22 If a patient already has signs of lipohypertrophy, data supports education and insulin injection technique practice as simple and effective means to reduce insulin action variability and increase glycemic control.24
Lipoatrophy. Lipoatrophy is described as a local loss of subcutaneous adipose tissue often in the face, buttocks, legs and arm regions and can be rooted in genetic, immune, or drug-associated etiologies.25 Insulin-induced lipoatrophy is suspected to be the result of tumor necrosis factor-α hyperproduction in reaction to insulin crystal presence at the injection site.26,27 Overall, lipoatrophy development has decreased since the use of recombinant human insulin and analog insulin therapy.28 The decrease is hypothesized to be due to increased subcutaneous tissue absorption rate of human insulin and its analog, decreasing overall adipocyte exposure to localized high insulin concentration.27 Treatments for same-site insulin-derived lipoatrophy include changing injection sites and preparation of insulin.26 When injection into the lipoatrophic site was avoided, glycemic control and lipoatrophy appearance improved.26
Amyloidosis. Amyloidosis indicates the presence of an extracellular bundle of insoluble polymeric protein fibrils in tissues and organs.29 Insulin-induced amyloidosis presents as a hard mass or ball near the injection site.29 Insulin is one of many hormones that can form amyloid fibrils, and there have been several dozen cases reported of amyloid formation at the site of insulin injection.29-31 Although insulin-derived amyloidosis is rare, it may be misdiagnosed as lipohypertrophy due to a lack of histopathologic testing or general awareness of the complication.29
In a case series of 7 patients with amyloidosis, all patients had a mean HbA1c of 9.3% (range, 8.5-10.2%) and averaged 1 IU/kg bodyweight before intervention.30 After the discovery of the mass, participants were instructed to avoid injection into the amyloidoma, and average insulin requirements decreased to 0.48 IU/kg body weight (P = .40).30 Patients with amyloidosis who rotated their injection sites experienced better glycemic control and decreased insulin requirements.30
Pathophysiology of Localized Insulin Resistance
Insulin regulates glucose homeostasis in skeletal muscle and adipose tissue, increases hepatic and adipocyte lipid synthesis, and decreases adipocyte fatty acid release.32 Generalized insulin resistance occurs when target tissues have decreased glucose uptake in response to circulating insulin.32 Insulin resistance increases the amount of free insulin in surrounding tissues. At high concentrations, insulin fosters tissue growth by binding to IGF-1 receptors, stimulating hypertrophy and reproduction of keratinocytes and fibroblasts.33 This pathophysiology helps explain the origin of localized acanthosis nigricans at same-site insulin injections.
Conclusions
Cutaneous complications are a local adverse effect of long-term failure to rotate insulin injection sites. Our case serves as a call to action for HCPs to improve education regarding insulin injection-site rotation, conduct routine injection-site inspection, and actively document cases as they occur to increase public awareness of these important complications.
If a patient with DM presents with unexplained poor glycemic control, consider questioning the patient about injection-site location and how often they are rotating the insulin injection site. Inspect the site for cutaneous complications. Of note, if a patient has a cutaneous complication due to insulin injection, adjust or decrease the insulin dosage when rotating sites to mitigate the risk of hypoglycemic episodes.
Improvement of glycemic control, cosmetic appearance of injection site, and insulin use all begin with skin inspection, injection technique education, and periodic review by a HCP.
Insulin injection therapy is one of the most widely used health care interventions to manage both type 1 and type 2 diabetes mellitus (T1DM/T2DM). Globally, more than 150 to 200 million people inject insulin into their upper posterior arms, buttocks, anterior and lateral thighs, or abdomen.1,2 In an ideal world, every patient would be using the correct site and rotating their insulin injection sites in accordance with health care professional (HCP) recommendations—systematic injections in one general body location, at least 1 cm away from the previous injection.2 Unfortunately, same-site insulin injection (repeatedly in the same region within 1 cm of previous injections) is a common mistake made by patients with DM—in one study, 63% of participants either did not rotate sites correctly or failed to do so at all.
Insulin-resistant cutaneous complications may occur as a result of same-site insulin injections. The most common is lipohypertrophy, reported in some studies in nearly 50% of patients with DM on insulin therapy.4 Other common cutaneous complications include lipoatrophy and amyloidosis. Injection-site acanthosis nigricans, although uncommon, has been reported in 18 cases in the literature.
Most articles suggest that same-site insulin injections decrease local insulin sensitivity and result in tissue hypertrophy because of the anabolic properties of insulin and increase in insulin binding to insulin-like growth factor-1 (IGF-1) receptor.5-20 The hyperkeratotic growth and varying insulin absorption rates associated with these cutaneous complications increase chances of either hyper- or hypoglycemic episodes in patients.10,11,13 It is the responsibility of the DM care professional to provide proper insulin-injection technique education and perform routine inspection of injection sites to reduce cutaneous complications of insulin therapy. The purpose of this article is to (1) describe a case of acanthosis nigricans resulting from insulin injection at the same site; (2) review case reports
Case Presentation
A 75-year-old patient with an 8-year history of T2DM, as well as stable coronary artery disease, atrial fibrillation, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and stage 3 chronic kidney disease, presented with 2 discrete abdominal hyperpigmented plaques. At the time of the initial clinic visit, the patient was taking metformin 1000 mg twice daily and insulin glargine 40 units once daily. When insulin was initiated 7 years prior, the patient received
The patient reported 5 years of progressive, asymptomatic hyperpigmentation of the skin surrounding his insulin glargine injection sites and injecting in these same sites daily without rotation. He reported no additional skin changes or symptoms. He had noticed no skin changes while using NPH insulin during his first year of insulin therapy. On examination, the abdominal wall skin demonstrated 2 well-demarcated, nearly black, soft, velvety plaques, measuring 9 × 8 cm on the left side and 4 × 3.5 cm on the right, suggesting acanthosis nigricans (Figure 1A). The remainder of the skin examination, including the flexures, was normal. Of note, the patient received biweekly intramuscular testosterone injections in the gluteal region for secondary hypogonadism with no adverse dermatologic effects. A skin punch biopsy was performed and revealed epidermal papillomatosis and hyperkeratosis, confirming the clinical diagnosis of acanthosis nigricans (Figure 2).
After a review of insulin-injection technique at his clinic visit, the patient started rotating insulin injection sites over his entire abdomen, and the acanthosis nigricans partially improved. A few months later, the patient stopped rotating the insulin injection site, and the acanthosis nigricans worsened again. Because of worsening glycemic control, the patient was then started on insulin aspart. He did not develop any skin changes at the insulin aspart injection site, although he was not rotating its site of injection.
Subsequently, with reeducation and proper injection-site rotation, the patient had resolution of his acanthosis nigricans (Figure 1b).
Discussion
A review of the literature revealed 18 reported cases of acanthosis nigricans at sites of repeated insulin injection (Table).5-20 Acanthosis nigricans at the site of insulin injection afflicts patients of any age, with cases observed in patients aged 14 to 75 years. Sixteen (84%) of 19 cases were male. Fourteen cases (73%) had T2DM; the rest of the patients had T1DM. The duration of insulin injection therapy prior to onset ranged from immediate to 13 years (median 4 years). Fourteen cases (73%) were reported on the abdomen; however, other sites, such as thighs and upper arm, also were reported. Lesions size varied from 12 to 360 cm2. Two cases had associated amyloidosis. The average HbA1c reported at presentation was 10%. Following insulin injection-site rotation, most of the cases reported improvement of both glycemic control and acanthosis nigricans appearance.
In the case described by Kudo and colleagues, a 59-year-old male patient with T2DM had been injecting insulin into the same spot on his abdomen for 10 years. He developed acanthosis nigricans and an amyloidoma so large and firm that it bent the needle when he injected insulin.11
Most of the cases we found in the literature were after 2005 and associated with the use of human or analog insulin. These cases may be related to a bias, as cases may be easier to find in digital archives in the later years, when human or analog insulins have been in common use. Also noteworthy, in cases that reported dosage, most were not very high, and the highest daily dose was 240 IU/d. Ten reports of injection-site acanthosis nigricans were in dermatology journals; only 5 reports were in endocrinology journals and 3 in general medical journals, indicating possible less awareness of this phenomenon in other HCPs who care for patients with DM.
Complications of Same-Site Injections
Acanthosis nigricans. Commonly found in the armpits, neck folds, and groin, acanthosis nigricans is known as one of the calling cards for insulin resistance, obesity, and hyperinsulinemia.21 Acanthosis nigricans can be seen in people with or without DM and is not limited to those on insulin therapy. However, same-site insulin injections for 4 to 6 years also may result in injection-site acanthosis nigricans–like lesions because of factors such as insulin exposure at the local tissue level.16
Acanthosis nigricans development is characterized by hyperpigmented, hyperkeratotic, velvety, and sometimes verrucous plaques.6 Acanthosis nigricans surrounding repeated injection sites is hypothesized to develop as a result of localized hyperinsulinemia secondary to insulin resistance, which increases the stimulation of IGF, thereby causing epidermal hypertrophy.5-20 If insulin injection therapy continues to be administered through the acanthosis nigricans lesion, it results in decreased insulin absorption, leading to poor glycemic control.13
Acanthosis nigricans associated with insulin injection is reversible. After rotation of injection sites, lesions either decrease in size or severity of appearance.5-8,11 Also, by avoiding injection into the hyperkeratotic plaques and using normal subcutaneous tissue for injection, patients’ response to insulin improves, as measured by HbA1c and by decreased daily insulin requirement.6-8,10,12,18-20
Lipohypertrophy. This is characterized by an increase in localized adipose tissue and is the most common cutaneous complication of insulin therapy.2 Lipohypertrophy presents as a firm, rubbery mass in the location of same-site insulin injections.22 Development of lipohypertrophy is suspected to be the result of either (1) anabolic effect of insulin on local adipocytes, promoting fat and protein synthesis; (2) an autoimmune response by immunoglobulin (Ig) G or IgE antibodies to insulin, immune response to insulin of different species, or to insulin injection techniques; or (3) repeated trauma to the injection site from repeated needle usage.4,23
In a study assessing the prevalence of lipohypertrophy and its relation to insulin technique, 49.1% of participants with
Primary prevention measures include injection site inspection and patient education about rotation and abstaining from needle reuse.22 If a patient already has signs of lipohypertrophy, data supports education and insulin injection technique practice as simple and effective means to reduce insulin action variability and increase glycemic control.24
Lipoatrophy. Lipoatrophy is described as a local loss of subcutaneous adipose tissue often in the face, buttocks, legs and arm regions and can be rooted in genetic, immune, or drug-associated etiologies.25 Insulin-induced lipoatrophy is suspected to be the result of tumor necrosis factor-α hyperproduction in reaction to insulin crystal presence at the injection site.26,27 Overall, lipoatrophy development has decreased since the use of recombinant human insulin and analog insulin therapy.28 The decrease is hypothesized to be due to increased subcutaneous tissue absorption rate of human insulin and its analog, decreasing overall adipocyte exposure to localized high insulin concentration.27 Treatments for same-site insulin-derived lipoatrophy include changing injection sites and preparation of insulin.26 When injection into the lipoatrophic site was avoided, glycemic control and lipoatrophy appearance improved.26
Amyloidosis. Amyloidosis indicates the presence of an extracellular bundle of insoluble polymeric protein fibrils in tissues and organs.29 Insulin-induced amyloidosis presents as a hard mass or ball near the injection site.29 Insulin is one of many hormones that can form amyloid fibrils, and there have been several dozen cases reported of amyloid formation at the site of insulin injection.29-31 Although insulin-derived amyloidosis is rare, it may be misdiagnosed as lipohypertrophy due to a lack of histopathologic testing or general awareness of the complication.29
In a case series of 7 patients with amyloidosis, all patients had a mean HbA1c of 9.3% (range, 8.5-10.2%) and averaged 1 IU/kg bodyweight before intervention.30 After the discovery of the mass, participants were instructed to avoid injection into the amyloidoma, and average insulin requirements decreased to 0.48 IU/kg body weight (P = .40).30 Patients with amyloidosis who rotated their injection sites experienced better glycemic control and decreased insulin requirements.30
Pathophysiology of Localized Insulin Resistance
Insulin regulates glucose homeostasis in skeletal muscle and adipose tissue, increases hepatic and adipocyte lipid synthesis, and decreases adipocyte fatty acid release.32 Generalized insulin resistance occurs when target tissues have decreased glucose uptake in response to circulating insulin.32 Insulin resistance increases the amount of free insulin in surrounding tissues. At high concentrations, insulin fosters tissue growth by binding to IGF-1 receptors, stimulating hypertrophy and reproduction of keratinocytes and fibroblasts.33 This pathophysiology helps explain the origin of localized acanthosis nigricans at same-site insulin injections.
Conclusions
Cutaneous complications are a local adverse effect of long-term failure to rotate insulin injection sites. Our case serves as a call to action for HCPs to improve education regarding insulin injection-site rotation, conduct routine injection-site inspection, and actively document cases as they occur to increase public awareness of these important complications.
If a patient with DM presents with unexplained poor glycemic control, consider questioning the patient about injection-site location and how often they are rotating the insulin injection site. Inspect the site for cutaneous complications. Of note, if a patient has a cutaneous complication due to insulin injection, adjust or decrease the insulin dosage when rotating sites to mitigate the risk of hypoglycemic episodes.
Improvement of glycemic control, cosmetic appearance of injection site, and insulin use all begin with skin inspection, injection technique education, and periodic review by a HCP.
1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi:10.1089/dia.2018.0384
2. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231-1255. doi:10.1016/j.mayocp.2016.06.010
3. Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445-453. doi:10.1016/j.diabet.2013.05.006
4. Johansson UB, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28(8):2025-2027. doi:10.2337/diacare.28.8.2025
5. Erickson L, Lipschutz DE, Wrigley W, Kearse WO. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209(6):934-935. doi:10.1001/jama.1969.03160190056019
6. Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122(9):1054-1056. doi:10.1001/archderm.1986.01660210104028 7. Gannon D, Ross MW, Mahajan T. Acanthosis nigricans-like plaque and lipohypertrophy in type 1 diabetes. Pract Diabetes International. 2005;22(6).
8. Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144(1):126-127. doi:10.1001/archdermatol.2007.27
9. Pachón Burgos A, Chan Aguilar MP. Visual vignette. Hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14(4):514. doi:10.4158/EP.14.4.514
10. Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94(2):e34-e36. doi:10.1016/j.diabres.2011.07.023
11. Kudo-Watanuki S, Kurihara E, Yamamoto K, Mukai K, Chen KR. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2012;38(1):25-29. doi:10.1111/j.1365-2230.2012.04373.x
12. Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11(12):e85-e87.
13. Kanwar A, Sawatkar G, Dogra S, Bhadada S. Acanthosis nigricans—an uncommon cutaneous adverse effect of a common medication: report of two cases. Indian J Dermatol Venereol Leprol. 2013;79(4):553. doi:10.4103/0378-6323.113112
14. Dhingra M, Garg G, Gupta M, Khurana U, Thami GP. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19(1):9. Published 2013 Jan 15.
15. Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57(1):127-129. doi:10.4103/0377-4929.130920
16. Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39(1):5-9.
17. Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39(12):e163. doi:10.1097/DAD.0000000000000659
18. Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190(47):E1390. doi:10.1503/cmaj.180705
19. Pal R, Bhattacharjee R, Chatterjee D, Bhadada SK, Bhansali A, Dutta P. Exogenous insulin-induced localized acanthosis nigricans: a rare injection site complication. Can J Diabetes. 2020;44(3):219-221. doi:10.1016/j.jcjd.2019.08.010
20. Bomar L, Lewallen R, Jorizzo J. Localized acanthosis nigricans at the site of repetitive insulin injections. Cutis. 2020;105(2);E20-E22.
21. Karadağ AS, You Y, Danarti R, Al-Khuzaei S, Chen W. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36(1):48-53. doi:10.1016/j.clindermatol.2017.09.008
22. Kalra S, Kumar A, Gupta Y. Prevention of lipohypertrophy. J Pak Med Assoc. 2016;66(7):910-911.
23. Singha A, Bhattarcharjee R, Ghosh S, Chakrabarti SK, Baidya A, Chowdhury S. Concurrence of lipoatrophy and lipohypertrophy in children with type 1 diabetes using recombinant human insulin: two case reports. Clin Diabetes. 2016;34(1):51-53. doi:10.2337/diaclin.34.1.51
24. Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486-1492. doi:10.2337/dc16-0610.
25. Reitman ML, Arioglu E, Gavrilova O, Taylor SI. Lipoatrophy revisited. Trends Endocrinol Metab. 2000;11(10):410-416. doi:10.1016/s1043-2760(00)00309-x
26. Kondo A, Nakamura A, Takeuchi J, Miyoshi H, Atsumi T. Insulin-Induced Distant Site Lipoatrophy. Diabetes Care. 2017;40(6):e67-e68. doi:10.2337/dc16-2385
27. Jermendy G, Nádas J, Sápi Z. “Lipoblastoma-like” lipoatrophy induced by human insulin: morphological evidence for local dedifferentiation of adipocytes?. Diabetologia. 2000;43(7):955-956. doi:10.1007/s001250051476
28. Mokta JK, Mokta KK, Panda P. Insulin lipodystrophy and lipohypertrophy. Indian J Endocrinol Metab. 2013;17(4):773-774. doi:10.4103/2230-8210.113788
29. Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19(1):174-177. doi:10.4103/2230-8210.146879
30. Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127(5):450-454. doi:10.1016/j.amjmed.2013.10.029
31. Swift B. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabet Med. 2002;19(10):881-882. doi:10.1046/j.1464-5491.2002.07581.x
32. Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20(4):665-679. doi:10.1016/j.beem.2006.09.007
33. Phiske MM. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014;5(3):239-249. doi:10.4103/2229-5178.137765
1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi:10.1089/dia.2018.0384
2. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231-1255. doi:10.1016/j.mayocp.2016.06.010
3. Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445-453. doi:10.1016/j.diabet.2013.05.006
4. Johansson UB, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28(8):2025-2027. doi:10.2337/diacare.28.8.2025
5. Erickson L, Lipschutz DE, Wrigley W, Kearse WO. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209(6):934-935. doi:10.1001/jama.1969.03160190056019
6. Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122(9):1054-1056. doi:10.1001/archderm.1986.01660210104028 7. Gannon D, Ross MW, Mahajan T. Acanthosis nigricans-like plaque and lipohypertrophy in type 1 diabetes. Pract Diabetes International. 2005;22(6).
8. Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144(1):126-127. doi:10.1001/archdermatol.2007.27
9. Pachón Burgos A, Chan Aguilar MP. Visual vignette. Hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14(4):514. doi:10.4158/EP.14.4.514
10. Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94(2):e34-e36. doi:10.1016/j.diabres.2011.07.023
11. Kudo-Watanuki S, Kurihara E, Yamamoto K, Mukai K, Chen KR. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2012;38(1):25-29. doi:10.1111/j.1365-2230.2012.04373.x
12. Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11(12):e85-e87.
13. Kanwar A, Sawatkar G, Dogra S, Bhadada S. Acanthosis nigricans—an uncommon cutaneous adverse effect of a common medication: report of two cases. Indian J Dermatol Venereol Leprol. 2013;79(4):553. doi:10.4103/0378-6323.113112
14. Dhingra M, Garg G, Gupta M, Khurana U, Thami GP. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19(1):9. Published 2013 Jan 15.
15. Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57(1):127-129. doi:10.4103/0377-4929.130920
16. Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39(1):5-9.
17. Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39(12):e163. doi:10.1097/DAD.0000000000000659
18. Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190(47):E1390. doi:10.1503/cmaj.180705
19. Pal R, Bhattacharjee R, Chatterjee D, Bhadada SK, Bhansali A, Dutta P. Exogenous insulin-induced localized acanthosis nigricans: a rare injection site complication. Can J Diabetes. 2020;44(3):219-221. doi:10.1016/j.jcjd.2019.08.010
20. Bomar L, Lewallen R, Jorizzo J. Localized acanthosis nigricans at the site of repetitive insulin injections. Cutis. 2020;105(2);E20-E22.
21. Karadağ AS, You Y, Danarti R, Al-Khuzaei S, Chen W. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36(1):48-53. doi:10.1016/j.clindermatol.2017.09.008
22. Kalra S, Kumar A, Gupta Y. Prevention of lipohypertrophy. J Pak Med Assoc. 2016;66(7):910-911.
23. Singha A, Bhattarcharjee R, Ghosh S, Chakrabarti SK, Baidya A, Chowdhury S. Concurrence of lipoatrophy and lipohypertrophy in children with type 1 diabetes using recombinant human insulin: two case reports. Clin Diabetes. 2016;34(1):51-53. doi:10.2337/diaclin.34.1.51
24. Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486-1492. doi:10.2337/dc16-0610.
25. Reitman ML, Arioglu E, Gavrilova O, Taylor SI. Lipoatrophy revisited. Trends Endocrinol Metab. 2000;11(10):410-416. doi:10.1016/s1043-2760(00)00309-x
26. Kondo A, Nakamura A, Takeuchi J, Miyoshi H, Atsumi T. Insulin-Induced Distant Site Lipoatrophy. Diabetes Care. 2017;40(6):e67-e68. doi:10.2337/dc16-2385
27. Jermendy G, Nádas J, Sápi Z. “Lipoblastoma-like” lipoatrophy induced by human insulin: morphological evidence for local dedifferentiation of adipocytes?. Diabetologia. 2000;43(7):955-956. doi:10.1007/s001250051476
28. Mokta JK, Mokta KK, Panda P. Insulin lipodystrophy and lipohypertrophy. Indian J Endocrinol Metab. 2013;17(4):773-774. doi:10.4103/2230-8210.113788
29. Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19(1):174-177. doi:10.4103/2230-8210.146879
30. Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127(5):450-454. doi:10.1016/j.amjmed.2013.10.029
31. Swift B. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabet Med. 2002;19(10):881-882. doi:10.1046/j.1464-5491.2002.07581.x
32. Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20(4):665-679. doi:10.1016/j.beem.2006.09.007
33. Phiske MM. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014;5(3):239-249. doi:10.4103/2229-5178.137765
Telemedicine and Home Pregnancy Testing for iPLEDGE: A Survey of Clinician Perspectives
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
PRACTICE POINTS
- The majority of clinicians report that the use of telemedicine and home pregnancy testing for iPLEDGE has improved access to care and that they would like to continue these practices.
- Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care for patients treated with isotretinoin.
Administrative Burden of iPLEDGE Deters Isotretinoin Prescriptions: Results From a Survey of Dermatologists
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.
Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.
Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.
Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.
Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.
Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.
Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
Practice Points
- Of clinicians who regularly prescribe isotretinoin, approximately 30% have at times chosen not to prescribe isotretinoin to patients with severe acne because of the burden of the iPLEDGE program.
- The US Food and Drug Administration should consider further streamlining the iPLEDGE program, as it is causing physician burden and therefore suboptimal treatment plans for patients.
Pharmacist-Assisted Varenicline Tobacco Cessation Treatment for Veterans
Tobacco smoking remains the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths annually.1 An estimated 50.6 million US adults (20.8%) identify as tobacco users, with even higher rates among veterans (29.2%).2,3 Tobacco use is estimated to cost the US more than $300 billion annually in direct and indirect medical costs.4 According to a 2015 report, more than two-thirds of adult smokers reported a desire to quit, while only 7.5% reported successfully quitting in the past year.5 According to that same report, only 57.2% of smokers who had seen a health professional in the past year reported receiving advice to quit.5 This statistic is unfortunate, as interventions that combine behavioral and pharmacologic support can drastically increase tobacco cessation rates compared with self-help materials or no treatment.6
Currently, 7 first-line medications (5 nicotine, 2 nonnicotine) have been shown to increase long-term smoking abstinence rates. Varenicline was approved by the US Food and Drug Administration (FDA) in 2006 for use in adults as an aid to smoking cessation treatment. As a partial agonist of the α4β2 nicotinic acetylcholine receptor, varenicline’s mechanism of action is believed to involve reduction of nicotine’s rewarding capacity.7 Varenicline not only aids in complete tobacco cessation but also has been found to be effective for reducing cigarette consumption among smokers not yet willing or able to make a quit attempt.8 Furthermore, varenicline has demonstrated efficacy among users of smokeless tobacco in achieving continuous abstinence.9
Widespread adoption of varenicline into clinical practice was perhaps slowed by early concerns of psychiatric complications, prompting the FDA to issue a boxed warning for risk of serious neuropsychiatric events. This boxed warning was removed in 2016 in response to publication of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES). In this randomized controlled trial of more than 8000 participants, among whom 50.5% had a psychiatric disorder determined to be stable, varenicline significantly increased rates of continuous tobacco cessation compared with bupropion or the nicotine patch without an increased risk of neuropsychiatric events.10 This study underscored not only the safety of varenicline, but also its superiority over other first-line cessation products. The most recently published clinical practice guidelines recommend varenicline as a first-line agent for helping patients achieve long-term smoking cessation.11,12
Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. Indeed, multiple studies have demonstrated the effectiveness of pharmacist-led interventions on tobacco cessation.13-15 As of 2019, only 12 states had statutes or regulations addressing pharmacist prescribing of tobacco cessation aids without a collaborative practice agreement or local standing order.16 Until recently, most of these states limited pharmacists’ prescriptive authority to
Within the US Department of Veterans Affairs (VA), the clinical pharmacy specialist (CPS) is credentialed as an advanced practitioner with authority to independently manage patient medication therapy for a variety of diseases specified under a scope of practice. Although CPSs have provided tobacco cessation services for years, expansion of their scope to include varenicline did not occur until June 26, 2019, at the Southern Arizona VA Health Care System (SAVAHCS). All VA prescribers must follow the same criteria for prescribing varenicline. Unless previously trialed on varenicline, patients must have failed an appropriate trial of first-line agents (NRT, bupropion, or combination therapy) or have a contraindication to use of these first-line therapies before varenicline can be considered. Exclusions to therapy would include history of serious hypersensitivity to varenicline; suicidal intent, plan, or attempt within the past 12 months; current substance use disorder other than nicotine (unless varenicline recommended or prescribed by mental health professional); or unstable mental health disorder.18
The purpose of this study was to evaluate the efficacy and safety of CPS management of varenicline compared with other clinicians. We hope that this study provides insight regarding how the expansion of CPS scope to include prescriptive authority for varenicline has affected patient outcomes.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.
Discussion
The results of this single center study suggest that management of varenicline by CPSs is associated with similar reductions in tobacco use and abstinence rates compared with management by other clinicians. These results provide evidence that CPS management of varenicline may be as safe and effective as management by other clinicians.
Adherence rates (reported as proportion of days covered when assessing varenicline refill data) were higher on average among patients managed by a CPS compared with patients managed by other clinicians. However, this outcome may not be as reflective of adherence as initially intended, given delays in follow-up (see limitations section). Time to first follow-up was drastically different between the groups, with much sooner follow-up by CPSs compared with other clinicians. Despite similar tobacco cessation rates between groups, more frequent follow-up by CPSs helps to assess patient barriers to cessation, adherence to therapy, and AEs with varenicline. A higher percentage of AEs were documented within the CPS group that could be attributed to disparities in documentation rather than true rates of AEs. While rates of AEs were initially intended to serve as the primary safety outcome, they may instead reflect pharmacists’ diligence in monitoring and documenting tolerability of medication therapy.
Limitations
Several limitations to this study should be noted. First, the data collected were only as detailed as the extent to which prescribers documented tobacco use, previous cessation trials, and AEs; thus, various data points are likely missing within this study that could impact the results presented. In line with lack of documentation, delays in follow-up (ie, annual primary care visits) sorely undermined proportion of days covered, making these data less indicative of true medication adherence. Furthermore, this study did not account for concurrent therapies, such as combination varenicline and nicotine gum/lozenges, or behavioral treatment strategies like cessation classes.
Another limitation was that some primary care practitioners prescribed varenicline but then referred these patients to a CPS for tobacco cessation follow-up. Per the study’s protocol, these patients were included within the other clinician group, which could have brought results closer to the null. Finally, the timing of this chart review (July 1, 2019, to July 31, 2020) intersects with the start of the COVID-19 pandemic, presenting a possible confounding factor if patients’ quit attempts were hindered by the stress and isolation of the pandemic.19 All pharmacist visits during the pandemic were conducted by telephone, which may have affected results.
Conclusions
In this study of veterans receiving varenicline, management by CPSs resulted in similar reductions of tobacco use and rates of complete abstinence compared with management by other clinicians. Pharmacist management was associated with greater adherence and shorter time to first follow-up compared with other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of clinical pharmacist scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
It would be interesting to see more studies outside of the VA system to determine the impact of pharmacist management of varenicline for a more heterogenous patient population. At some point, a prospective controlled trial should be conducted to overcome the various confounding factors that limit the results of retrospective chart reviews
Acknowledgments
This article was prepared, and research was conducted with resources and the use of facilities at the Southern Arizona Veterans Affairs Health Care System in Tucson.
1. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Updated March 17, 2022. Accessed May 31, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736-1742. doi:10.15585/mmwr.mm6946a4
3. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
4. Hall W, Doran C. How much can the USA reduce health care costs by reducing smoking? PLoS Med. 2016;13(5):e1002021. doi:10.1371/journal.pmed.1002021.
5. Centers for Disease Control and Prevention. Smoking cessation: fast facts. Updated March 21, 2022. Accessed June 1, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
6. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Chapter 6, Interventions for smoking cessation and treatments for nicotine dependence. In: Smoking Cessation: A Report of the Surgeon General [Internet]. Washington, DC: US Department of Health and Human Services; 2020. Accessed June 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555596
7. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. doi:10.1016/j.neuropharm.2006.10.016
8. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi:10.1001/jama.2015.280
9. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi:10.1136/bmj.c6549
10. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0
11. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
12. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
13. Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247. doi:10.1111/jcpt.12131
14. Augustine JM, Taylor AM, Pelger M, Schiefer D, Warholak TL. Smoking quit rates among patients receiving pharmacist-provided pharmacotherapy and telephonic smoking cessation counseling. J Am Pharm Assoc. 2016;56(2):129-136. doi:10.1016/j.japh.2016.02.001
15. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040-1051. doi:10.1592/phco.27.7.1040
16. National Alliance of State Pharmacy Associations. Pharmacist prescribing: tobacco cessation aids. February 10, 2021. Accessed June 1, 2022. https://naspa.us/resource/tobacco-cessation
17. Shen X, Bachyrycz A, Anderson JR, Tinker D, Raisch DW. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20(6):579-587. doi:10.18553/jmcp.2014.20.6.579
18. VA Center for Medication Safety, Tobacco Use Cessation Technical Advisory Group, Public Health Strategic Healthcare Group, VA Pharmacy Benefits Management Services, VISN Pharmacist Executives, and Medical Advisory Panel. Varenicline criteria for prescribing. 2008. Updated July 2011. Accessed June 9, 2022. https://www.healthquality.va.gov/tuc/VareniclineCriteriaforPrescribing.pdf
19. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929-1930. doi:10.1001/jama.2021.5601
Tobacco smoking remains the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths annually.1 An estimated 50.6 million US adults (20.8%) identify as tobacco users, with even higher rates among veterans (29.2%).2,3 Tobacco use is estimated to cost the US more than $300 billion annually in direct and indirect medical costs.4 According to a 2015 report, more than two-thirds of adult smokers reported a desire to quit, while only 7.5% reported successfully quitting in the past year.5 According to that same report, only 57.2% of smokers who had seen a health professional in the past year reported receiving advice to quit.5 This statistic is unfortunate, as interventions that combine behavioral and pharmacologic support can drastically increase tobacco cessation rates compared with self-help materials or no treatment.6
Currently, 7 first-line medications (5 nicotine, 2 nonnicotine) have been shown to increase long-term smoking abstinence rates. Varenicline was approved by the US Food and Drug Administration (FDA) in 2006 for use in adults as an aid to smoking cessation treatment. As a partial agonist of the α4β2 nicotinic acetylcholine receptor, varenicline’s mechanism of action is believed to involve reduction of nicotine’s rewarding capacity.7 Varenicline not only aids in complete tobacco cessation but also has been found to be effective for reducing cigarette consumption among smokers not yet willing or able to make a quit attempt.8 Furthermore, varenicline has demonstrated efficacy among users of smokeless tobacco in achieving continuous abstinence.9
Widespread adoption of varenicline into clinical practice was perhaps slowed by early concerns of psychiatric complications, prompting the FDA to issue a boxed warning for risk of serious neuropsychiatric events. This boxed warning was removed in 2016 in response to publication of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES). In this randomized controlled trial of more than 8000 participants, among whom 50.5% had a psychiatric disorder determined to be stable, varenicline significantly increased rates of continuous tobacco cessation compared with bupropion or the nicotine patch without an increased risk of neuropsychiatric events.10 This study underscored not only the safety of varenicline, but also its superiority over other first-line cessation products. The most recently published clinical practice guidelines recommend varenicline as a first-line agent for helping patients achieve long-term smoking cessation.11,12
Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. Indeed, multiple studies have demonstrated the effectiveness of pharmacist-led interventions on tobacco cessation.13-15 As of 2019, only 12 states had statutes or regulations addressing pharmacist prescribing of tobacco cessation aids without a collaborative practice agreement or local standing order.16 Until recently, most of these states limited pharmacists’ prescriptive authority to
Within the US Department of Veterans Affairs (VA), the clinical pharmacy specialist (CPS) is credentialed as an advanced practitioner with authority to independently manage patient medication therapy for a variety of diseases specified under a scope of practice. Although CPSs have provided tobacco cessation services for years, expansion of their scope to include varenicline did not occur until June 26, 2019, at the Southern Arizona VA Health Care System (SAVAHCS). All VA prescribers must follow the same criteria for prescribing varenicline. Unless previously trialed on varenicline, patients must have failed an appropriate trial of first-line agents (NRT, bupropion, or combination therapy) or have a contraindication to use of these first-line therapies before varenicline can be considered. Exclusions to therapy would include history of serious hypersensitivity to varenicline; suicidal intent, plan, or attempt within the past 12 months; current substance use disorder other than nicotine (unless varenicline recommended or prescribed by mental health professional); or unstable mental health disorder.18
The purpose of this study was to evaluate the efficacy and safety of CPS management of varenicline compared with other clinicians. We hope that this study provides insight regarding how the expansion of CPS scope to include prescriptive authority for varenicline has affected patient outcomes.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.
Discussion
The results of this single center study suggest that management of varenicline by CPSs is associated with similar reductions in tobacco use and abstinence rates compared with management by other clinicians. These results provide evidence that CPS management of varenicline may be as safe and effective as management by other clinicians.
Adherence rates (reported as proportion of days covered when assessing varenicline refill data) were higher on average among patients managed by a CPS compared with patients managed by other clinicians. However, this outcome may not be as reflective of adherence as initially intended, given delays in follow-up (see limitations section). Time to first follow-up was drastically different between the groups, with much sooner follow-up by CPSs compared with other clinicians. Despite similar tobacco cessation rates between groups, more frequent follow-up by CPSs helps to assess patient barriers to cessation, adherence to therapy, and AEs with varenicline. A higher percentage of AEs were documented within the CPS group that could be attributed to disparities in documentation rather than true rates of AEs. While rates of AEs were initially intended to serve as the primary safety outcome, they may instead reflect pharmacists’ diligence in monitoring and documenting tolerability of medication therapy.
Limitations
Several limitations to this study should be noted. First, the data collected were only as detailed as the extent to which prescribers documented tobacco use, previous cessation trials, and AEs; thus, various data points are likely missing within this study that could impact the results presented. In line with lack of documentation, delays in follow-up (ie, annual primary care visits) sorely undermined proportion of days covered, making these data less indicative of true medication adherence. Furthermore, this study did not account for concurrent therapies, such as combination varenicline and nicotine gum/lozenges, or behavioral treatment strategies like cessation classes.
Another limitation was that some primary care practitioners prescribed varenicline but then referred these patients to a CPS for tobacco cessation follow-up. Per the study’s protocol, these patients were included within the other clinician group, which could have brought results closer to the null. Finally, the timing of this chart review (July 1, 2019, to July 31, 2020) intersects with the start of the COVID-19 pandemic, presenting a possible confounding factor if patients’ quit attempts were hindered by the stress and isolation of the pandemic.19 All pharmacist visits during the pandemic were conducted by telephone, which may have affected results.
Conclusions
In this study of veterans receiving varenicline, management by CPSs resulted in similar reductions of tobacco use and rates of complete abstinence compared with management by other clinicians. Pharmacist management was associated with greater adherence and shorter time to first follow-up compared with other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of clinical pharmacist scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
It would be interesting to see more studies outside of the VA system to determine the impact of pharmacist management of varenicline for a more heterogenous patient population. At some point, a prospective controlled trial should be conducted to overcome the various confounding factors that limit the results of retrospective chart reviews
Acknowledgments
This article was prepared, and research was conducted with resources and the use of facilities at the Southern Arizona Veterans Affairs Health Care System in Tucson.
Tobacco smoking remains the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths annually.1 An estimated 50.6 million US adults (20.8%) identify as tobacco users, with even higher rates among veterans (29.2%).2,3 Tobacco use is estimated to cost the US more than $300 billion annually in direct and indirect medical costs.4 According to a 2015 report, more than two-thirds of adult smokers reported a desire to quit, while only 7.5% reported successfully quitting in the past year.5 According to that same report, only 57.2% of smokers who had seen a health professional in the past year reported receiving advice to quit.5 This statistic is unfortunate, as interventions that combine behavioral and pharmacologic support can drastically increase tobacco cessation rates compared with self-help materials or no treatment.6
Currently, 7 first-line medications (5 nicotine, 2 nonnicotine) have been shown to increase long-term smoking abstinence rates. Varenicline was approved by the US Food and Drug Administration (FDA) in 2006 for use in adults as an aid to smoking cessation treatment. As a partial agonist of the α4β2 nicotinic acetylcholine receptor, varenicline’s mechanism of action is believed to involve reduction of nicotine’s rewarding capacity.7 Varenicline not only aids in complete tobacco cessation but also has been found to be effective for reducing cigarette consumption among smokers not yet willing or able to make a quit attempt.8 Furthermore, varenicline has demonstrated efficacy among users of smokeless tobacco in achieving continuous abstinence.9
Widespread adoption of varenicline into clinical practice was perhaps slowed by early concerns of psychiatric complications, prompting the FDA to issue a boxed warning for risk of serious neuropsychiatric events. This boxed warning was removed in 2016 in response to publication of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES). In this randomized controlled trial of more than 8000 participants, among whom 50.5% had a psychiatric disorder determined to be stable, varenicline significantly increased rates of continuous tobacco cessation compared with bupropion or the nicotine patch without an increased risk of neuropsychiatric events.10 This study underscored not only the safety of varenicline, but also its superiority over other first-line cessation products. The most recently published clinical practice guidelines recommend varenicline as a first-line agent for helping patients achieve long-term smoking cessation.11,12
Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. Indeed, multiple studies have demonstrated the effectiveness of pharmacist-led interventions on tobacco cessation.13-15 As of 2019, only 12 states had statutes or regulations addressing pharmacist prescribing of tobacco cessation aids without a collaborative practice agreement or local standing order.16 Until recently, most of these states limited pharmacists’ prescriptive authority to
Within the US Department of Veterans Affairs (VA), the clinical pharmacy specialist (CPS) is credentialed as an advanced practitioner with authority to independently manage patient medication therapy for a variety of diseases specified under a scope of practice. Although CPSs have provided tobacco cessation services for years, expansion of their scope to include varenicline did not occur until June 26, 2019, at the Southern Arizona VA Health Care System (SAVAHCS). All VA prescribers must follow the same criteria for prescribing varenicline. Unless previously trialed on varenicline, patients must have failed an appropriate trial of first-line agents (NRT, bupropion, or combination therapy) or have a contraindication to use of these first-line therapies before varenicline can be considered. Exclusions to therapy would include history of serious hypersensitivity to varenicline; suicidal intent, plan, or attempt within the past 12 months; current substance use disorder other than nicotine (unless varenicline recommended or prescribed by mental health professional); or unstable mental health disorder.18
The purpose of this study was to evaluate the efficacy and safety of CPS management of varenicline compared with other clinicians. We hope that this study provides insight regarding how the expansion of CPS scope to include prescriptive authority for varenicline has affected patient outcomes.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.
Discussion
The results of this single center study suggest that management of varenicline by CPSs is associated with similar reductions in tobacco use and abstinence rates compared with management by other clinicians. These results provide evidence that CPS management of varenicline may be as safe and effective as management by other clinicians.
Adherence rates (reported as proportion of days covered when assessing varenicline refill data) were higher on average among patients managed by a CPS compared with patients managed by other clinicians. However, this outcome may not be as reflective of adherence as initially intended, given delays in follow-up (see limitations section). Time to first follow-up was drastically different between the groups, with much sooner follow-up by CPSs compared with other clinicians. Despite similar tobacco cessation rates between groups, more frequent follow-up by CPSs helps to assess patient barriers to cessation, adherence to therapy, and AEs with varenicline. A higher percentage of AEs were documented within the CPS group that could be attributed to disparities in documentation rather than true rates of AEs. While rates of AEs were initially intended to serve as the primary safety outcome, they may instead reflect pharmacists’ diligence in monitoring and documenting tolerability of medication therapy.
Limitations
Several limitations to this study should be noted. First, the data collected were only as detailed as the extent to which prescribers documented tobacco use, previous cessation trials, and AEs; thus, various data points are likely missing within this study that could impact the results presented. In line with lack of documentation, delays in follow-up (ie, annual primary care visits) sorely undermined proportion of days covered, making these data less indicative of true medication adherence. Furthermore, this study did not account for concurrent therapies, such as combination varenicline and nicotine gum/lozenges, or behavioral treatment strategies like cessation classes.
Another limitation was that some primary care practitioners prescribed varenicline but then referred these patients to a CPS for tobacco cessation follow-up. Per the study’s protocol, these patients were included within the other clinician group, which could have brought results closer to the null. Finally, the timing of this chart review (July 1, 2019, to July 31, 2020) intersects with the start of the COVID-19 pandemic, presenting a possible confounding factor if patients’ quit attempts were hindered by the stress and isolation of the pandemic.19 All pharmacist visits during the pandemic were conducted by telephone, which may have affected results.
Conclusions
In this study of veterans receiving varenicline, management by CPSs resulted in similar reductions of tobacco use and rates of complete abstinence compared with management by other clinicians. Pharmacist management was associated with greater adherence and shorter time to first follow-up compared with other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of clinical pharmacist scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
It would be interesting to see more studies outside of the VA system to determine the impact of pharmacist management of varenicline for a more heterogenous patient population. At some point, a prospective controlled trial should be conducted to overcome the various confounding factors that limit the results of retrospective chart reviews
Acknowledgments
This article was prepared, and research was conducted with resources and the use of facilities at the Southern Arizona Veterans Affairs Health Care System in Tucson.
1. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Updated March 17, 2022. Accessed May 31, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736-1742. doi:10.15585/mmwr.mm6946a4
3. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
4. Hall W, Doran C. How much can the USA reduce health care costs by reducing smoking? PLoS Med. 2016;13(5):e1002021. doi:10.1371/journal.pmed.1002021.
5. Centers for Disease Control and Prevention. Smoking cessation: fast facts. Updated March 21, 2022. Accessed June 1, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
6. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Chapter 6, Interventions for smoking cessation and treatments for nicotine dependence. In: Smoking Cessation: A Report of the Surgeon General [Internet]. Washington, DC: US Department of Health and Human Services; 2020. Accessed June 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555596
7. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. doi:10.1016/j.neuropharm.2006.10.016
8. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi:10.1001/jama.2015.280
9. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi:10.1136/bmj.c6549
10. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0
11. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
12. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
13. Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247. doi:10.1111/jcpt.12131
14. Augustine JM, Taylor AM, Pelger M, Schiefer D, Warholak TL. Smoking quit rates among patients receiving pharmacist-provided pharmacotherapy and telephonic smoking cessation counseling. J Am Pharm Assoc. 2016;56(2):129-136. doi:10.1016/j.japh.2016.02.001
15. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040-1051. doi:10.1592/phco.27.7.1040
16. National Alliance of State Pharmacy Associations. Pharmacist prescribing: tobacco cessation aids. February 10, 2021. Accessed June 1, 2022. https://naspa.us/resource/tobacco-cessation
17. Shen X, Bachyrycz A, Anderson JR, Tinker D, Raisch DW. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20(6):579-587. doi:10.18553/jmcp.2014.20.6.579
18. VA Center for Medication Safety, Tobacco Use Cessation Technical Advisory Group, Public Health Strategic Healthcare Group, VA Pharmacy Benefits Management Services, VISN Pharmacist Executives, and Medical Advisory Panel. Varenicline criteria for prescribing. 2008. Updated July 2011. Accessed June 9, 2022. https://www.healthquality.va.gov/tuc/VareniclineCriteriaforPrescribing.pdf
19. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929-1930. doi:10.1001/jama.2021.5601
1. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Updated March 17, 2022. Accessed May 31, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736-1742. doi:10.15585/mmwr.mm6946a4
3. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
4. Hall W, Doran C. How much can the USA reduce health care costs by reducing smoking? PLoS Med. 2016;13(5):e1002021. doi:10.1371/journal.pmed.1002021.
5. Centers for Disease Control and Prevention. Smoking cessation: fast facts. Updated March 21, 2022. Accessed June 1, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
6. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Chapter 6, Interventions for smoking cessation and treatments for nicotine dependence. In: Smoking Cessation: A Report of the Surgeon General [Internet]. Washington, DC: US Department of Health and Human Services; 2020. Accessed June 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555596
7. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. doi:10.1016/j.neuropharm.2006.10.016
8. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi:10.1001/jama.2015.280
9. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi:10.1136/bmj.c6549
10. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0
11. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
12. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
13. Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247. doi:10.1111/jcpt.12131
14. Augustine JM, Taylor AM, Pelger M, Schiefer D, Warholak TL. Smoking quit rates among patients receiving pharmacist-provided pharmacotherapy and telephonic smoking cessation counseling. J Am Pharm Assoc. 2016;56(2):129-136. doi:10.1016/j.japh.2016.02.001
15. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040-1051. doi:10.1592/phco.27.7.1040
16. National Alliance of State Pharmacy Associations. Pharmacist prescribing: tobacco cessation aids. February 10, 2021. Accessed June 1, 2022. https://naspa.us/resource/tobacco-cessation
17. Shen X, Bachyrycz A, Anderson JR, Tinker D, Raisch DW. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20(6):579-587. doi:10.18553/jmcp.2014.20.6.579
18. VA Center for Medication Safety, Tobacco Use Cessation Technical Advisory Group, Public Health Strategic Healthcare Group, VA Pharmacy Benefits Management Services, VISN Pharmacist Executives, and Medical Advisory Panel. Varenicline criteria for prescribing. 2008. Updated July 2011. Accessed June 9, 2022. https://www.healthquality.va.gov/tuc/VareniclineCriteriaforPrescribing.pdf
19. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929-1930. doi:10.1001/jama.2021.5601
Appropriateness of Pharmacologic Thromboprophylaxis Prescribing Based on Padua Score Among Inpatient Veterans
Venous thromboembolism (VTE) presents as deep venous thromboembolism (DVT) or pulmonary embolism (PE). VTE is the third most common vascular disease and a leading cardiovascular complication.1,2 Hospitalized patients are at increased risk of developing VTE due to multiple factors such as inflammatory processes from acute illness, recent surgery or trauma leading to hypercoagulable states, and prolonged periods of immobilization.3 Additional risk factors for complications include presence of malignancy, obesity, and prior history of VTE. About half of VTE cases in the community setting occur as a result of a hospital admission for recent or ongoing acute illness or surgery.1 Hospitalized patients are often categorized as high risk for VTE, and this risk may persist postdischarge.4
The risk of hospital-associated VTE may be mitigated with either mechanical or pharmacologic thromboprophylaxis.5 Risk assessment models (RAMs), such as Padua Prediction Score (PPS) and IMPROVEDD, have been developed to assist in evaluating hospitalized patients’ risk of VTE and need for pharmacologic thromboprophylaxis (Table 1).1,5 The PPS is externally validated and can assist clinicians in VTE risk assessment when integrated into clinical decision making.6 Patients with a PPS ≥ 4 are deemed high risk for VTE, and pharmacologic thromboprophylaxis is indicated as long as the patient is not at high risk for bleeding. IMPROVEDD added D-dimer as an additional risk factor to IMPROVE and was validated in 2017 to help predict the risk of symptomatic VTE in acutely ill patients hospitalized for up to 77 days.7 IMPROVEDD scores ≥ 2 identify patients at high risk for symptomatic VTE through 77 days hospitalization, while scores ≥ 4 identify patients who may qualify for extended thromboprophylaxis.7 Despite their utility, RAMs may not be used appropriately within clinical practice, and whether patients should receive extended-duration thromboprophylaxis postdischarge and for how long is debatable.5
VTE events contribute to increased health care spending, morbidity, and mortality, thus it is imperative to evaluate current hospital practices with respect to appropriate prescribing of pharmacologic thromboprophylaxis.8 Appropriately identifying high-risk patients and prescribing pharmacologic thromboprophylaxis to limit preventable VTEs is essential. Conversely, it is important to withhold pharmacologic thromboprophylaxis from those deemed low risk to limit bleeding complications.9 Health care professionals must be good stewards of anticoagulant prescribing when implementing these tools along with clinical knowledge to weigh the risks vs benefits to promote medication safety and prevent further complications.10This quality improvement project aimed to evaluate if VTE thromboprophylaxis was appropriately given or withheld in hospitalized medical patients based on PPS calculated upon admission using a link to an online calculator embedded within an admission order set. Additionally, this study aimed to characterize patients readmitted for VTE within 45 days postdischarge to generate hypotheses for future stu
Methods
This was an observational, retrospective cohort study that took place at the US Department of Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS). TVHS is a multisite health care system with campuses in Nashville and Murfreesboro. Clinical pharmacists employed at the study site and the primary research investigators designed this study and oversaw its execution. The study was reviewed and deemed exempt as a quality improvement study by the TVHS Institutional Review Board.
This study included adult veterans aged ≥ 18 years admitted to a general medicine floor or the medical intensive care unit between June 1, 2017, and June 30, 2020. Patients were excluded if they were on chronic therapeutic anticoagulation prior to their index hospitalization, required therapeutic anticoagulation on admission for index hospitalization (ie, acute coronary syndrome requiring a heparin drip), or were bedded within the surgical intensive care unit. All patients admitted to the TVHS within the prespecified date range were extracted from the electronic health record. A second subset of patients meeting inclusion criteria and readmitted for VTE within 45 days of index hospitalization with International Classification of Diseases, Tenth Revision (ICD-10) descriptions including thrombosis or embolism were extracted for review of a secondary endpoint. Patients with preexisting clots, history of prior DVT or PE, or history of portal vein thrombosis were not reviewed.
The primary endpoint was the percentage of patients for whom pharmacologic thromboprophylaxis was appropriately initiated or withheld based on a PPS calculated upon admission (Table 2). PPS was chosen for review as it is the only RAM currently used at TVHS. Secondary endpoints were the percentage of patients with documented rationale for ordering thromboprophylaxis when not indicated, based on PPS, or withholding despite indication as well as the number of patients readmitted to TVHS for VTE within 45 days of discharge with IMPROVEDD scores ≥ 4 and < 4 (eAppendix available at doi:10.12788/fp.0291). The primary investigators performed a manual health record review of all patients meeting inclusion criteria. Descriptive statistics were used given this was a quality improvement study, therefore, sample size and power calculations were not necessary. Data were stored in Microsoft Excel spreadsheets that were encrypted and password protected. To maintain security of personal health information, all study files were kept on the TVHS internal network, and access was limited to the research investigators.
Results
Two hundred fifty patients meeting inclusion criteria were randomly selected for review for the primary endpoint. Of the patients reviewed for the primary endpoint, 118 had a PPS < 4 and 132 a PPS ≥ 4 (Figure). Pharmacologic thromboprophylaxis was inappropriately given or withheld based on their PPS for 91 (36.4%) patients. This included 58 (49.2%) patients in the low-risk group (PPS < 4) who had thromboprophylaxis inappropriately given and 33 (25.0%) patients in the high-risk group (PPS ≥ 4) who had thromboprophylaxis inappropriately withheld. Of the 58 patients with a PPS < 4 who were given prophylaxis, only 2 (3.4%) patients had documented rationale as to why anticoagulation was administered. Of the 132 patients with a PPS ≥ 4, 44 patients had thromboprophylaxis withheld. Eleven (8.3%) patients had thromboprophylaxis appropriately withheld due to presence or concern for bleeding. Commonly documented rationale for inappropriately withholding thromboprophylaxis when indicated included use of sequential compression devices (40.9%), pancytopenia (18.2%), dual antiplatelet therapy (9.1%), or patient was ambulatory (4.5%).
A secondary endpoint characterized patients at highest risk for developing a VTE after hospitalization for an acute illness. Seventy patients were readmitted within 45 days of discharge from the index hospitalization with ICD descriptions for embolism or thrombosis. Only 15 of those patients were readmitted with a newly diagnosed VTE not previously identified; 14 (93.3%) had a PPS ≥ 4 upon index admission and 10 (66.7%) appropriately received pharmacologic prophylaxis within 24 hours of admission. Of the 15 patients, 3 (20.0%) did not receive pharmacologic thromboprophylaxis within 24 hours of admission and 1 (6.7%) received thromboprophylaxis despite having a PPS < 4.
Looking at IMPROVEDD scores for the 15 patients at the index hospitalization discharge, 1 (6.7%) patient had an IMPROVEDD score < 2, 11 (73.3%) patients had IMPROVEDD scores ≥ 2, and 3 (20.0%) patients had IMPROVEDD scores ≥ 4. Two of the patients with IMPROVEDD scores ≥ 4 had a history of VTE and were aged > 60 years. Of the 15 patients reviewed, 7 had a diagnosis of cancer, and 3 were actively undergoing chemotherapy.
Discussion
PPS is the RAM embedded in our system’s order set, which identifies hospitalized medical patients at risk for VTE.6 In the original study that validated PPS, the results suggested that implementation of preventive measures during hospitalization in patients labeled as having high thrombotic risk confers longstanding protection against thromboembolic complications in comparison with untreated patients.6 However, PPS must be used consistently and appropriately to realize this benefit. Our results showed that pharmacologic thromboprophylaxis is frequently inappropriately given or withheld despite the incorporation of a RAM in an admission order set, suggesting there is a significant gap between written policy and actual practice. More than one-third of patients had thromboprophylaxis given or withheld inappropriately according to the PPS calculated manually on review. With this, there is concern for over- and underprescribing of thromboprophylaxis, which increases the risk of adverse events. Overprescribing can lead to unnecessary bleeding complications, whereas underprescribing can lead to preventable VTE.
One issue identified during this study was the need for a user-friendly interface. The PPS calculator currently embedded in our admission order set is a hyperlink to an online calculator. This is time consuming and cumbersome for clinicians tending to a high volume of patients, which may cause them to overlook the calculator and estimate risk based on clinician judgement. Noted areas for improvement regarding thromboprophylaxis during inpatient admissions include the failure to implement or adhere to risk stratification protocols, lack of appropriate assessment for thromboprophylaxis, and the overutilization of pharmacologic thromboprophylaxis in low-risk patients.11
Certain patients develop a VTE postdischarge despite efforts at prevention during their index hospitalization, which led us to explore our secondary endpoint looking at readmissions. Regarding thromboprophylaxis postdischarge, the duration of therapy is an area of current debate.5 Extended-duration thromboprophylaxis is defined as anticoagulation prescribed beyond hospitalization for up to 42 days total.1,12 To date, there have been 5 clinical trials to evaluate the utility of extended-duration thromboprophylaxis in hospitalized medically ill patients. While routine use is not recommended by the 2018 American Society of Hematology guidelines for management of VTE, more recent data suggest certain medically ill patients may derive benefit from extended-duration thromboprophylaxis.4 The IMPROVEDD score aimed to address this need, which is why it was calculated on index discharge for our patients readmitted within 45 days. Research is still needed to identify such patients and RAMs for capturing these subpopulations.1,11
Our secondary endpoint sought to characterize patients at highest risk for developing a VTE postdischarge. Of the 15 patients reviewed, 7 had a diagnosis of cancer and 3 were actively undergoing chemotherapy. With that, the Khorana Risk Score may have been a more appropriate RAM for some given the Khorana score is validated in ambulatory patients undergoing chemotherapy. D-dimer was only collected for 1 of the 15 patients, therefore, VTE risk could have been underestimated with the IMPROVEDD scores calculated. More than 75% of patients readmitted for VTE appropriately received thromboprophylaxis on index admission yet still went on to develop a VTE. It is essential to increase clinician awareness about hospital-acquired and postdischarge VTE. In line with guidance from the North American Thrombosis Forum, extended-duration thromboprophylaxis should be thoughtfully considered in high-risk patients.5 Pathways, including follow-up, are needed to implement postdischarge thromboprophylaxis when appropriate
Limitations
There were some inherent limitations to this study with its retrospective nature and small sample size. Data extraction was limited to health records within the VA, so there is a chance relevant history could be missed via incomplete documentation. Thus, our results could be an underestimation of postdischarge VTE prevalence if patients sought medical attention outside of the VA. Given this study was a retrospective chart review, data collection was limited to what was explicitly documented in the chart. Rationale for giving thromboprophylaxis when not indicated or holding when indicated may have been underestimated if clinicians did not document thoroughly in the electronic health record. Last, for the secondary endpoint reviewing the IMPROVEDD score, a D-dimer was not consistently obtained on admission, which could lead to underestimation of risk.
Conclusions
The results of this study showed that more than one-third of patients admitted to our facility within the prespecified timeframe had pharmacologic thromboprophylaxis inappropriately given or withheld according to a PPS manually calculated on admission. The PPS calculator currently embedded within our admission order set is not being utilized appropriately or consistently in clinical practice. Additionally, results from the secondary endpoint looking at IMPROVEDD scores highlight an unmet need for thromboprophylaxis at discharge. Pathways are needed to implement postdischarge thromboprophylaxis when appropriate for patients at highest thromboembolic risk.
1. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. doi:10.1182/bloodadvances.2018022954
2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi:10.1038/nrcardio.2015.83
3. Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ. 2002;325(7369):887-890. doi:10.1136/bmj.325.7369.887
4. Bajaj NS, Vaduganathan M, Qamar A, et al. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: A trial sequential and cumulative meta-analysis. Cannegieter SC, ed. PLoS Med. 2019;16(4):e1002797. doi:10.1371/journal.pmed.1002797
5. Barkoudah E, Piazza G, Hecht TEH, et al. Extended venous thromboembolism prophylaxis in medically ill patients: an NATF anticoagulation action initiative. Am J Med. 2020;133 (suppl 1):1-27. doi:10.1016/j.amjmed.2019.12.001
6. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450-7. doi:10.1111/j.1538-7836.2010.04044.x
7. Gibson CM, Spyropoulos AC, Cohen AT, et al. The IMPROVEDD VTE risk score: incorporation of D-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open. 2017;1(1):e56-e65. doi:10.1055/s-0037-1603929
8. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134(5):931-938. doi:10.1016/j.thromres.2014.08.014
9. Pavon JM, Sloane RJ, Pieper CF, et al. Poor adherence to risk stratification guidelines results in overuse of venous thromboembolism prophylaxis in hospitalized older adults. J Hosp Med. 2018;13(6):403-404. doi:10.12788/jhm.2916
10. Core elements of anticoagulation stewardship programs. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum-excellence.org/Resource-Center/resource_files/-2019-09-18-110254.pdf
11. Core elements of anticoagulation stewardship programs administrative oversight gap analysis: hospital and skilled nursing facilities. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum.org/web/downloads/ACF%20Gap%20Analysis%20Report.pdf
12. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e278S-e325S. doi:10.1378/chest.11-2404
Venous thromboembolism (VTE) presents as deep venous thromboembolism (DVT) or pulmonary embolism (PE). VTE is the third most common vascular disease and a leading cardiovascular complication.1,2 Hospitalized patients are at increased risk of developing VTE due to multiple factors such as inflammatory processes from acute illness, recent surgery or trauma leading to hypercoagulable states, and prolonged periods of immobilization.3 Additional risk factors for complications include presence of malignancy, obesity, and prior history of VTE. About half of VTE cases in the community setting occur as a result of a hospital admission for recent or ongoing acute illness or surgery.1 Hospitalized patients are often categorized as high risk for VTE, and this risk may persist postdischarge.4
The risk of hospital-associated VTE may be mitigated with either mechanical or pharmacologic thromboprophylaxis.5 Risk assessment models (RAMs), such as Padua Prediction Score (PPS) and IMPROVEDD, have been developed to assist in evaluating hospitalized patients’ risk of VTE and need for pharmacologic thromboprophylaxis (Table 1).1,5 The PPS is externally validated and can assist clinicians in VTE risk assessment when integrated into clinical decision making.6 Patients with a PPS ≥ 4 are deemed high risk for VTE, and pharmacologic thromboprophylaxis is indicated as long as the patient is not at high risk for bleeding. IMPROVEDD added D-dimer as an additional risk factor to IMPROVE and was validated in 2017 to help predict the risk of symptomatic VTE in acutely ill patients hospitalized for up to 77 days.7 IMPROVEDD scores ≥ 2 identify patients at high risk for symptomatic VTE through 77 days hospitalization, while scores ≥ 4 identify patients who may qualify for extended thromboprophylaxis.7 Despite their utility, RAMs may not be used appropriately within clinical practice, and whether patients should receive extended-duration thromboprophylaxis postdischarge and for how long is debatable.5
VTE events contribute to increased health care spending, morbidity, and mortality, thus it is imperative to evaluate current hospital practices with respect to appropriate prescribing of pharmacologic thromboprophylaxis.8 Appropriately identifying high-risk patients and prescribing pharmacologic thromboprophylaxis to limit preventable VTEs is essential. Conversely, it is important to withhold pharmacologic thromboprophylaxis from those deemed low risk to limit bleeding complications.9 Health care professionals must be good stewards of anticoagulant prescribing when implementing these tools along with clinical knowledge to weigh the risks vs benefits to promote medication safety and prevent further complications.10This quality improvement project aimed to evaluate if VTE thromboprophylaxis was appropriately given or withheld in hospitalized medical patients based on PPS calculated upon admission using a link to an online calculator embedded within an admission order set. Additionally, this study aimed to characterize patients readmitted for VTE within 45 days postdischarge to generate hypotheses for future stu
Methods
This was an observational, retrospective cohort study that took place at the US Department of Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS). TVHS is a multisite health care system with campuses in Nashville and Murfreesboro. Clinical pharmacists employed at the study site and the primary research investigators designed this study and oversaw its execution. The study was reviewed and deemed exempt as a quality improvement study by the TVHS Institutional Review Board.
This study included adult veterans aged ≥ 18 years admitted to a general medicine floor or the medical intensive care unit between June 1, 2017, and June 30, 2020. Patients were excluded if they were on chronic therapeutic anticoagulation prior to their index hospitalization, required therapeutic anticoagulation on admission for index hospitalization (ie, acute coronary syndrome requiring a heparin drip), or were bedded within the surgical intensive care unit. All patients admitted to the TVHS within the prespecified date range were extracted from the electronic health record. A second subset of patients meeting inclusion criteria and readmitted for VTE within 45 days of index hospitalization with International Classification of Diseases, Tenth Revision (ICD-10) descriptions including thrombosis or embolism were extracted for review of a secondary endpoint. Patients with preexisting clots, history of prior DVT or PE, or history of portal vein thrombosis were not reviewed.
The primary endpoint was the percentage of patients for whom pharmacologic thromboprophylaxis was appropriately initiated or withheld based on a PPS calculated upon admission (Table 2). PPS was chosen for review as it is the only RAM currently used at TVHS. Secondary endpoints were the percentage of patients with documented rationale for ordering thromboprophylaxis when not indicated, based on PPS, or withholding despite indication as well as the number of patients readmitted to TVHS for VTE within 45 days of discharge with IMPROVEDD scores ≥ 4 and < 4 (eAppendix available at doi:10.12788/fp.0291). The primary investigators performed a manual health record review of all patients meeting inclusion criteria. Descriptive statistics were used given this was a quality improvement study, therefore, sample size and power calculations were not necessary. Data were stored in Microsoft Excel spreadsheets that were encrypted and password protected. To maintain security of personal health information, all study files were kept on the TVHS internal network, and access was limited to the research investigators.
Results
Two hundred fifty patients meeting inclusion criteria were randomly selected for review for the primary endpoint. Of the patients reviewed for the primary endpoint, 118 had a PPS < 4 and 132 a PPS ≥ 4 (Figure). Pharmacologic thromboprophylaxis was inappropriately given or withheld based on their PPS for 91 (36.4%) patients. This included 58 (49.2%) patients in the low-risk group (PPS < 4) who had thromboprophylaxis inappropriately given and 33 (25.0%) patients in the high-risk group (PPS ≥ 4) who had thromboprophylaxis inappropriately withheld. Of the 58 patients with a PPS < 4 who were given prophylaxis, only 2 (3.4%) patients had documented rationale as to why anticoagulation was administered. Of the 132 patients with a PPS ≥ 4, 44 patients had thromboprophylaxis withheld. Eleven (8.3%) patients had thromboprophylaxis appropriately withheld due to presence or concern for bleeding. Commonly documented rationale for inappropriately withholding thromboprophylaxis when indicated included use of sequential compression devices (40.9%), pancytopenia (18.2%), dual antiplatelet therapy (9.1%), or patient was ambulatory (4.5%).
A secondary endpoint characterized patients at highest risk for developing a VTE after hospitalization for an acute illness. Seventy patients were readmitted within 45 days of discharge from the index hospitalization with ICD descriptions for embolism or thrombosis. Only 15 of those patients were readmitted with a newly diagnosed VTE not previously identified; 14 (93.3%) had a PPS ≥ 4 upon index admission and 10 (66.7%) appropriately received pharmacologic prophylaxis within 24 hours of admission. Of the 15 patients, 3 (20.0%) did not receive pharmacologic thromboprophylaxis within 24 hours of admission and 1 (6.7%) received thromboprophylaxis despite having a PPS < 4.
Looking at IMPROVEDD scores for the 15 patients at the index hospitalization discharge, 1 (6.7%) patient had an IMPROVEDD score < 2, 11 (73.3%) patients had IMPROVEDD scores ≥ 2, and 3 (20.0%) patients had IMPROVEDD scores ≥ 4. Two of the patients with IMPROVEDD scores ≥ 4 had a history of VTE and were aged > 60 years. Of the 15 patients reviewed, 7 had a diagnosis of cancer, and 3 were actively undergoing chemotherapy.
Discussion
PPS is the RAM embedded in our system’s order set, which identifies hospitalized medical patients at risk for VTE.6 In the original study that validated PPS, the results suggested that implementation of preventive measures during hospitalization in patients labeled as having high thrombotic risk confers longstanding protection against thromboembolic complications in comparison with untreated patients.6 However, PPS must be used consistently and appropriately to realize this benefit. Our results showed that pharmacologic thromboprophylaxis is frequently inappropriately given or withheld despite the incorporation of a RAM in an admission order set, suggesting there is a significant gap between written policy and actual practice. More than one-third of patients had thromboprophylaxis given or withheld inappropriately according to the PPS calculated manually on review. With this, there is concern for over- and underprescribing of thromboprophylaxis, which increases the risk of adverse events. Overprescribing can lead to unnecessary bleeding complications, whereas underprescribing can lead to preventable VTE.
One issue identified during this study was the need for a user-friendly interface. The PPS calculator currently embedded in our admission order set is a hyperlink to an online calculator. This is time consuming and cumbersome for clinicians tending to a high volume of patients, which may cause them to overlook the calculator and estimate risk based on clinician judgement. Noted areas for improvement regarding thromboprophylaxis during inpatient admissions include the failure to implement or adhere to risk stratification protocols, lack of appropriate assessment for thromboprophylaxis, and the overutilization of pharmacologic thromboprophylaxis in low-risk patients.11
Certain patients develop a VTE postdischarge despite efforts at prevention during their index hospitalization, which led us to explore our secondary endpoint looking at readmissions. Regarding thromboprophylaxis postdischarge, the duration of therapy is an area of current debate.5 Extended-duration thromboprophylaxis is defined as anticoagulation prescribed beyond hospitalization for up to 42 days total.1,12 To date, there have been 5 clinical trials to evaluate the utility of extended-duration thromboprophylaxis in hospitalized medically ill patients. While routine use is not recommended by the 2018 American Society of Hematology guidelines for management of VTE, more recent data suggest certain medically ill patients may derive benefit from extended-duration thromboprophylaxis.4 The IMPROVEDD score aimed to address this need, which is why it was calculated on index discharge for our patients readmitted within 45 days. Research is still needed to identify such patients and RAMs for capturing these subpopulations.1,11
Our secondary endpoint sought to characterize patients at highest risk for developing a VTE postdischarge. Of the 15 patients reviewed, 7 had a diagnosis of cancer and 3 were actively undergoing chemotherapy. With that, the Khorana Risk Score may have been a more appropriate RAM for some given the Khorana score is validated in ambulatory patients undergoing chemotherapy. D-dimer was only collected for 1 of the 15 patients, therefore, VTE risk could have been underestimated with the IMPROVEDD scores calculated. More than 75% of patients readmitted for VTE appropriately received thromboprophylaxis on index admission yet still went on to develop a VTE. It is essential to increase clinician awareness about hospital-acquired and postdischarge VTE. In line with guidance from the North American Thrombosis Forum, extended-duration thromboprophylaxis should be thoughtfully considered in high-risk patients.5 Pathways, including follow-up, are needed to implement postdischarge thromboprophylaxis when appropriate
Limitations
There were some inherent limitations to this study with its retrospective nature and small sample size. Data extraction was limited to health records within the VA, so there is a chance relevant history could be missed via incomplete documentation. Thus, our results could be an underestimation of postdischarge VTE prevalence if patients sought medical attention outside of the VA. Given this study was a retrospective chart review, data collection was limited to what was explicitly documented in the chart. Rationale for giving thromboprophylaxis when not indicated or holding when indicated may have been underestimated if clinicians did not document thoroughly in the electronic health record. Last, for the secondary endpoint reviewing the IMPROVEDD score, a D-dimer was not consistently obtained on admission, which could lead to underestimation of risk.
Conclusions
The results of this study showed that more than one-third of patients admitted to our facility within the prespecified timeframe had pharmacologic thromboprophylaxis inappropriately given or withheld according to a PPS manually calculated on admission. The PPS calculator currently embedded within our admission order set is not being utilized appropriately or consistently in clinical practice. Additionally, results from the secondary endpoint looking at IMPROVEDD scores highlight an unmet need for thromboprophylaxis at discharge. Pathways are needed to implement postdischarge thromboprophylaxis when appropriate for patients at highest thromboembolic risk.
Venous thromboembolism (VTE) presents as deep venous thromboembolism (DVT) or pulmonary embolism (PE). VTE is the third most common vascular disease and a leading cardiovascular complication.1,2 Hospitalized patients are at increased risk of developing VTE due to multiple factors such as inflammatory processes from acute illness, recent surgery or trauma leading to hypercoagulable states, and prolonged periods of immobilization.3 Additional risk factors for complications include presence of malignancy, obesity, and prior history of VTE. About half of VTE cases in the community setting occur as a result of a hospital admission for recent or ongoing acute illness or surgery.1 Hospitalized patients are often categorized as high risk for VTE, and this risk may persist postdischarge.4
The risk of hospital-associated VTE may be mitigated with either mechanical or pharmacologic thromboprophylaxis.5 Risk assessment models (RAMs), such as Padua Prediction Score (PPS) and IMPROVEDD, have been developed to assist in evaluating hospitalized patients’ risk of VTE and need for pharmacologic thromboprophylaxis (Table 1).1,5 The PPS is externally validated and can assist clinicians in VTE risk assessment when integrated into clinical decision making.6 Patients with a PPS ≥ 4 are deemed high risk for VTE, and pharmacologic thromboprophylaxis is indicated as long as the patient is not at high risk for bleeding. IMPROVEDD added D-dimer as an additional risk factor to IMPROVE and was validated in 2017 to help predict the risk of symptomatic VTE in acutely ill patients hospitalized for up to 77 days.7 IMPROVEDD scores ≥ 2 identify patients at high risk for symptomatic VTE through 77 days hospitalization, while scores ≥ 4 identify patients who may qualify for extended thromboprophylaxis.7 Despite their utility, RAMs may not be used appropriately within clinical practice, and whether patients should receive extended-duration thromboprophylaxis postdischarge and for how long is debatable.5
VTE events contribute to increased health care spending, morbidity, and mortality, thus it is imperative to evaluate current hospital practices with respect to appropriate prescribing of pharmacologic thromboprophylaxis.8 Appropriately identifying high-risk patients and prescribing pharmacologic thromboprophylaxis to limit preventable VTEs is essential. Conversely, it is important to withhold pharmacologic thromboprophylaxis from those deemed low risk to limit bleeding complications.9 Health care professionals must be good stewards of anticoagulant prescribing when implementing these tools along with clinical knowledge to weigh the risks vs benefits to promote medication safety and prevent further complications.10This quality improvement project aimed to evaluate if VTE thromboprophylaxis was appropriately given or withheld in hospitalized medical patients based on PPS calculated upon admission using a link to an online calculator embedded within an admission order set. Additionally, this study aimed to characterize patients readmitted for VTE within 45 days postdischarge to generate hypotheses for future stu
Methods
This was an observational, retrospective cohort study that took place at the US Department of Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS). TVHS is a multisite health care system with campuses in Nashville and Murfreesboro. Clinical pharmacists employed at the study site and the primary research investigators designed this study and oversaw its execution. The study was reviewed and deemed exempt as a quality improvement study by the TVHS Institutional Review Board.
This study included adult veterans aged ≥ 18 years admitted to a general medicine floor or the medical intensive care unit between June 1, 2017, and June 30, 2020. Patients were excluded if they were on chronic therapeutic anticoagulation prior to their index hospitalization, required therapeutic anticoagulation on admission for index hospitalization (ie, acute coronary syndrome requiring a heparin drip), or were bedded within the surgical intensive care unit. All patients admitted to the TVHS within the prespecified date range were extracted from the electronic health record. A second subset of patients meeting inclusion criteria and readmitted for VTE within 45 days of index hospitalization with International Classification of Diseases, Tenth Revision (ICD-10) descriptions including thrombosis or embolism were extracted for review of a secondary endpoint. Patients with preexisting clots, history of prior DVT or PE, or history of portal vein thrombosis were not reviewed.
The primary endpoint was the percentage of patients for whom pharmacologic thromboprophylaxis was appropriately initiated or withheld based on a PPS calculated upon admission (Table 2). PPS was chosen for review as it is the only RAM currently used at TVHS. Secondary endpoints were the percentage of patients with documented rationale for ordering thromboprophylaxis when not indicated, based on PPS, or withholding despite indication as well as the number of patients readmitted to TVHS for VTE within 45 days of discharge with IMPROVEDD scores ≥ 4 and < 4 (eAppendix available at doi:10.12788/fp.0291). The primary investigators performed a manual health record review of all patients meeting inclusion criteria. Descriptive statistics were used given this was a quality improvement study, therefore, sample size and power calculations were not necessary. Data were stored in Microsoft Excel spreadsheets that were encrypted and password protected. To maintain security of personal health information, all study files were kept on the TVHS internal network, and access was limited to the research investigators.
Results
Two hundred fifty patients meeting inclusion criteria were randomly selected for review for the primary endpoint. Of the patients reviewed for the primary endpoint, 118 had a PPS < 4 and 132 a PPS ≥ 4 (Figure). Pharmacologic thromboprophylaxis was inappropriately given or withheld based on their PPS for 91 (36.4%) patients. This included 58 (49.2%) patients in the low-risk group (PPS < 4) who had thromboprophylaxis inappropriately given and 33 (25.0%) patients in the high-risk group (PPS ≥ 4) who had thromboprophylaxis inappropriately withheld. Of the 58 patients with a PPS < 4 who were given prophylaxis, only 2 (3.4%) patients had documented rationale as to why anticoagulation was administered. Of the 132 patients with a PPS ≥ 4, 44 patients had thromboprophylaxis withheld. Eleven (8.3%) patients had thromboprophylaxis appropriately withheld due to presence or concern for bleeding. Commonly documented rationale for inappropriately withholding thromboprophylaxis when indicated included use of sequential compression devices (40.9%), pancytopenia (18.2%), dual antiplatelet therapy (9.1%), or patient was ambulatory (4.5%).
A secondary endpoint characterized patients at highest risk for developing a VTE after hospitalization for an acute illness. Seventy patients were readmitted within 45 days of discharge from the index hospitalization with ICD descriptions for embolism or thrombosis. Only 15 of those patients were readmitted with a newly diagnosed VTE not previously identified; 14 (93.3%) had a PPS ≥ 4 upon index admission and 10 (66.7%) appropriately received pharmacologic prophylaxis within 24 hours of admission. Of the 15 patients, 3 (20.0%) did not receive pharmacologic thromboprophylaxis within 24 hours of admission and 1 (6.7%) received thromboprophylaxis despite having a PPS < 4.
Looking at IMPROVEDD scores for the 15 patients at the index hospitalization discharge, 1 (6.7%) patient had an IMPROVEDD score < 2, 11 (73.3%) patients had IMPROVEDD scores ≥ 2, and 3 (20.0%) patients had IMPROVEDD scores ≥ 4. Two of the patients with IMPROVEDD scores ≥ 4 had a history of VTE and were aged > 60 years. Of the 15 patients reviewed, 7 had a diagnosis of cancer, and 3 were actively undergoing chemotherapy.
Discussion
PPS is the RAM embedded in our system’s order set, which identifies hospitalized medical patients at risk for VTE.6 In the original study that validated PPS, the results suggested that implementation of preventive measures during hospitalization in patients labeled as having high thrombotic risk confers longstanding protection against thromboembolic complications in comparison with untreated patients.6 However, PPS must be used consistently and appropriately to realize this benefit. Our results showed that pharmacologic thromboprophylaxis is frequently inappropriately given or withheld despite the incorporation of a RAM in an admission order set, suggesting there is a significant gap between written policy and actual practice. More than one-third of patients had thromboprophylaxis given or withheld inappropriately according to the PPS calculated manually on review. With this, there is concern for over- and underprescribing of thromboprophylaxis, which increases the risk of adverse events. Overprescribing can lead to unnecessary bleeding complications, whereas underprescribing can lead to preventable VTE.
One issue identified during this study was the need for a user-friendly interface. The PPS calculator currently embedded in our admission order set is a hyperlink to an online calculator. This is time consuming and cumbersome for clinicians tending to a high volume of patients, which may cause them to overlook the calculator and estimate risk based on clinician judgement. Noted areas for improvement regarding thromboprophylaxis during inpatient admissions include the failure to implement or adhere to risk stratification protocols, lack of appropriate assessment for thromboprophylaxis, and the overutilization of pharmacologic thromboprophylaxis in low-risk patients.11
Certain patients develop a VTE postdischarge despite efforts at prevention during their index hospitalization, which led us to explore our secondary endpoint looking at readmissions. Regarding thromboprophylaxis postdischarge, the duration of therapy is an area of current debate.5 Extended-duration thromboprophylaxis is defined as anticoagulation prescribed beyond hospitalization for up to 42 days total.1,12 To date, there have been 5 clinical trials to evaluate the utility of extended-duration thromboprophylaxis in hospitalized medically ill patients. While routine use is not recommended by the 2018 American Society of Hematology guidelines for management of VTE, more recent data suggest certain medically ill patients may derive benefit from extended-duration thromboprophylaxis.4 The IMPROVEDD score aimed to address this need, which is why it was calculated on index discharge for our patients readmitted within 45 days. Research is still needed to identify such patients and RAMs for capturing these subpopulations.1,11
Our secondary endpoint sought to characterize patients at highest risk for developing a VTE postdischarge. Of the 15 patients reviewed, 7 had a diagnosis of cancer and 3 were actively undergoing chemotherapy. With that, the Khorana Risk Score may have been a more appropriate RAM for some given the Khorana score is validated in ambulatory patients undergoing chemotherapy. D-dimer was only collected for 1 of the 15 patients, therefore, VTE risk could have been underestimated with the IMPROVEDD scores calculated. More than 75% of patients readmitted for VTE appropriately received thromboprophylaxis on index admission yet still went on to develop a VTE. It is essential to increase clinician awareness about hospital-acquired and postdischarge VTE. In line with guidance from the North American Thrombosis Forum, extended-duration thromboprophylaxis should be thoughtfully considered in high-risk patients.5 Pathways, including follow-up, are needed to implement postdischarge thromboprophylaxis when appropriate
Limitations
There were some inherent limitations to this study with its retrospective nature and small sample size. Data extraction was limited to health records within the VA, so there is a chance relevant history could be missed via incomplete documentation. Thus, our results could be an underestimation of postdischarge VTE prevalence if patients sought medical attention outside of the VA. Given this study was a retrospective chart review, data collection was limited to what was explicitly documented in the chart. Rationale for giving thromboprophylaxis when not indicated or holding when indicated may have been underestimated if clinicians did not document thoroughly in the electronic health record. Last, for the secondary endpoint reviewing the IMPROVEDD score, a D-dimer was not consistently obtained on admission, which could lead to underestimation of risk.
Conclusions
The results of this study showed that more than one-third of patients admitted to our facility within the prespecified timeframe had pharmacologic thromboprophylaxis inappropriately given or withheld according to a PPS manually calculated on admission. The PPS calculator currently embedded within our admission order set is not being utilized appropriately or consistently in clinical practice. Additionally, results from the secondary endpoint looking at IMPROVEDD scores highlight an unmet need for thromboprophylaxis at discharge. Pathways are needed to implement postdischarge thromboprophylaxis when appropriate for patients at highest thromboembolic risk.
1. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. doi:10.1182/bloodadvances.2018022954
2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi:10.1038/nrcardio.2015.83
3. Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ. 2002;325(7369):887-890. doi:10.1136/bmj.325.7369.887
4. Bajaj NS, Vaduganathan M, Qamar A, et al. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: A trial sequential and cumulative meta-analysis. Cannegieter SC, ed. PLoS Med. 2019;16(4):e1002797. doi:10.1371/journal.pmed.1002797
5. Barkoudah E, Piazza G, Hecht TEH, et al. Extended venous thromboembolism prophylaxis in medically ill patients: an NATF anticoagulation action initiative. Am J Med. 2020;133 (suppl 1):1-27. doi:10.1016/j.amjmed.2019.12.001
6. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450-7. doi:10.1111/j.1538-7836.2010.04044.x
7. Gibson CM, Spyropoulos AC, Cohen AT, et al. The IMPROVEDD VTE risk score: incorporation of D-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open. 2017;1(1):e56-e65. doi:10.1055/s-0037-1603929
8. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134(5):931-938. doi:10.1016/j.thromres.2014.08.014
9. Pavon JM, Sloane RJ, Pieper CF, et al. Poor adherence to risk stratification guidelines results in overuse of venous thromboembolism prophylaxis in hospitalized older adults. J Hosp Med. 2018;13(6):403-404. doi:10.12788/jhm.2916
10. Core elements of anticoagulation stewardship programs. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum-excellence.org/Resource-Center/resource_files/-2019-09-18-110254.pdf
11. Core elements of anticoagulation stewardship programs administrative oversight gap analysis: hospital and skilled nursing facilities. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum.org/web/downloads/ACF%20Gap%20Analysis%20Report.pdf
12. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e278S-e325S. doi:10.1378/chest.11-2404
1. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. doi:10.1182/bloodadvances.2018022954
2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi:10.1038/nrcardio.2015.83
3. Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ. 2002;325(7369):887-890. doi:10.1136/bmj.325.7369.887
4. Bajaj NS, Vaduganathan M, Qamar A, et al. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: A trial sequential and cumulative meta-analysis. Cannegieter SC, ed. PLoS Med. 2019;16(4):e1002797. doi:10.1371/journal.pmed.1002797
5. Barkoudah E, Piazza G, Hecht TEH, et al. Extended venous thromboembolism prophylaxis in medically ill patients: an NATF anticoagulation action initiative. Am J Med. 2020;133 (suppl 1):1-27. doi:10.1016/j.amjmed.2019.12.001
6. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450-7. doi:10.1111/j.1538-7836.2010.04044.x
7. Gibson CM, Spyropoulos AC, Cohen AT, et al. The IMPROVEDD VTE risk score: incorporation of D-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open. 2017;1(1):e56-e65. doi:10.1055/s-0037-1603929
8. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134(5):931-938. doi:10.1016/j.thromres.2014.08.014
9. Pavon JM, Sloane RJ, Pieper CF, et al. Poor adherence to risk stratification guidelines results in overuse of venous thromboembolism prophylaxis in hospitalized older adults. J Hosp Med. 2018;13(6):403-404. doi:10.12788/jhm.2916
10. Core elements of anticoagulation stewardship programs. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum-excellence.org/Resource-Center/resource_files/-2019-09-18-110254.pdf
11. Core elements of anticoagulation stewardship programs administrative oversight gap analysis: hospital and skilled nursing facilities. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum.org/web/downloads/ACF%20Gap%20Analysis%20Report.pdf
12. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e278S-e325S. doi:10.1378/chest.11-2404
COVID-19 Cycle Threshold/Cycle Number Testing at a Community Living Center
COVID-19, caused by SARS-CoV-2, is more severe in individuals with underlying illnesses. Because complete social distancing might be more difficult in nursing homes and community living centers (CLCs), public health leaders and clinicians have been concerned about the epidemiology and disease course in nursing homes even before the COVID-19 pandemic.1-7 A report of a COVID-19 outbreak in a nursing home facility in King County, Washington, documented a 33.7% overall fatality rate for residents and 52.4% among the most critically ill.4,5 The experience at King County, Washington, shows that proactive steps to identify, monitor, and apply preventive control measures is important for future outbreaks.5
Reverse transcriptase polymerase chain reaction (RT-PCR) testing produces a cycle threshold (CT) or cycle number (CN) that correlates with viral load and infectiousness. 8-14 CT/CN represents the number of RT-PCR cycles required for the fluorescent signal to cross the detection threshold (exceed background level) and is inversely proportional to the viral load. Effectively, the higher the viral load, the lower the CT/ CN value (Figure 1). Tracking CT/CN values was not documented in the Washington nursing home outbreak. Reports of COVID- 19 testing in CLCs during outbreaks are sparse, and CT/CN values and demographic distribution of these veterans has not been reported.15 The CLC veteran population, with known higher vulnerability to infection and chronic diseases, is epidemiologically different from the general nursing home population.15-18 To address these literature gaps, we present the first report of COVID- 19 testing with CT/CN value correlations in the high-risk veteran CLC population.
Methods
A retrospective review of all COVID-19 CT/CN testing at the Corporal Michael J. Crescenz Veterans Affairs Medical Center (VAMC) CLC in Philadelphia, Pennsylvania, from March 28, 2020, to April 24, 2020, was performed with a US Department of Veterans Affairs (VA) Veterans Health Information System Architecture VistA/FileMan search. Only veteran residents were included in this review. Data collected included initial and serial test results, CT/CN on positive test results, test dates, testing platform used, demographic information (age, self-reported ethnicity, and sex), and clinical follow-up information. Health records were reviewed retrospectively to identify death, the first day after diagnosis with no documented symptoms, or hospitalization status.
RT-PCR testing was performed with the Abbott RealTime SARS-CoV-2 assay on the Abbott m2000 platform and the Xpert Xpress SARS-CoV-2 assay on the Cepheid Infinity platform. The Xpert Xpress assay gave 2 CT values for the E and N2 targets on positive samples.19 For this assay to indicate a positive specimen, amplification by RT-PCR of the N2 target or both the N2 and E target is required. The Xpert Xpress assay results as presumptive positive if only the E target amplified. This assay counts a maximum of 45 cycles. The Abbott RealTime SARS-CoV-2 assay gave 1 CN derived from the RNA-dependent RNA polymerase and N targets on positive samples.20 The Abbott assay on the m2000 counts a maximum of 37 cycles. The CT/CN value is the number of cycles required by RT-PCR for the fluorescence signal to cross a threshold value exceeding background level.19,20
Samples that are negative for COVID-19 by RT-PCR do not produce a CT/CN value. Although both instruments were used for RT-PCR, the precise CT/CN values are not interchangeable and CT/CN observations over time between the 2 instruments during the disease course would be based on CT/CN value movement (general upward or downward trend) rather than absolute CT/CN differences. Both assays have been approved by emergency use authorization as qualitative tests for the presence/absence of COVID-19. Although the CT/CN value is available to laboratory staff after test completion, the CT/CN value is not reported routinely in the patient health record. All veteran patients identified on the initial review from March 28, 2020, to April 24, 2020, had all serial COVID-19 testing recorded until November 10, 2020. The CN values at the limit of detection (LOD) for the Abbott m2000 platform from the initial validation study were reviewed for reference.21
Results
Of 80 patients, 25 (31%) were COVID-19 positive over the course of testing. The study population had a mean age of 73.5 years; 92% were aged > 60 years. The group was predominantly male (79 male vs 1 female). Among the 77 patients with a stated ethnicity, 39 (51%) were African American. In comparison, 43% of residents in Philadelphia County are African American (Table).22,23 Additionally, a previously published total COVID-19 tested population by ethnicity at the same regional VAMC revealed 46.8% of tested veteran patients were African American. 24 Three patients had no stated ethnicity. Among those who tested positive, 11 were African American patients, 12 were White patients, and 2 had no stated ethnicity. Four patients tested positive on their first test. The other 21 patients were positive on repeat testing. Interestingly, 6 patients had 1 initial negative test before a positive test, 6 patients had 2, 8 patients had 3, and 1 patient had 4 initial negative tests before a positive test result. Among the 25 positive patients, 22 were either positive within 10 days of the initial negative test result or initially positive (Figure 2). Three patients who tested positive after 10 days did so at 16, 20, and 21 days after the initial negative test result. Among the 25 positive patients, 23 had initial and serial testing from both the Abbott and Xpert Xpress assays. The remaining 2 positive patients had initial and serial testing from the Abbott assay exclusively.
Only positive COVID-19 results by RTPCR produced a CT/CN value. After disease resolution with a negative test, no CT/CN value was produced with the negative test result on either testing platform. Because repeat testing after the initial positive result took place no sooner than 10 days, we observed that the CT/CN value increased after the initial positive result until the disease resolved, and a negative result was obtained (eAppendix 1, available online at doi:10.12788/fp.0276). A t test comparing the initial CT/CN value to the value more than 10 days after the initial positive showed the CT/CN was statistically significantly higher (P < .05).
Prompt repeat testing after the initial test can show a decrease in the CT/CN value because of increasing viral load before the expected increase until disease resolution if the initial test caught the infection early. Twelve patients had a negative test result between 2 serial positive results. These negative test results occurred later, near the end of the disease course. Among the 12 patients with this positive-negativepositive CT/CN pattern, 7 were symptomatic and no longer had documented symptoms or hospitalization around the time of this positive-negative-positive pattern. Four of these individuals were asymptomatic during the entire infection course. One of the 12 patients with this pattern expired with the negative result occurring on day 27 of the disease in the context of rising CT/CN. One of these 12 patients only had a presumptive positive test result on the Cepheid because it detected only the E target with a CT value of 38.7. In 1 of the 12 patients, the negative test result occurred between 2 positive test results with CT/CN values < 20 (12.05 and 19.05 for the positive tests before and after the negative result, respectively). When the initial CT/CN values was separated based on ethnicity, the average CT/CN value for African Americans (23.3) was higher than for other ethnicities (19.9), although it did not reach statistical significance (P = .35).
Ten of the 25 patients testing positive were admitted to the hospital, including 1 admitted 15 days before diagnosis (patient 20) and 1 admitted 80 days after diagnosis (patient 7). Among these 10 patients, 6 were admitted to the intensive care unit, including patient 7. None of the patients were intubated. Three of the 10 admitted patients died (patients 7, 20, and 24). Patient 7 was a 79-year-old male with a history of dementia, cerebrovascular accident, hypertension, hyperlipidemia, and chronic kidney disease with symptoms of lethargy and refusal of oral intake when he was diagnosed with COVID-19. He was admitted 80 days after diagnosis for hyponatremia and acute renal failure, with death on day 87 recorded as complications from the earlier COVID-19 infection. Patient 20, an 89-year-old male with a history of dementia, chronic kidney disease, and hyperlipidemia, had been admitted with fever, cough, and leukocytosis 17 days before COVID-19 diagnosis. He continued to be symptomatic after diagnosis with development of hypotension, dehydration, and refusal of oral intake while on comfort measures/endof- life care and died 15 days after COVID- 19 infection diagnosis. Patient 24 was a 96-year-old male with history of heart failure, hypertension, coronary artery disease, prostate carcinoma, and dementia who developed a cough at the time of diagnosis; because of his underlying condition, he remained in the CLC on comfort care. His symptoms, including hypoxia, worsened until he died 7 days after diagnosis.
Among the 25 patients, 17 were symptomatic at the time of diagnosis; the 14 initially symptomatic patients who survived improved clinically and returned to baseline. Eight of the 25 patients were asymptomatic initially and 3 developed symptoms 2 to 5 days after diagnosis. Only 1 patient who remained asymptomatic was admitted for inability to adhere to quarantine at the CLC. Review of the health records of all surviving symptomatic patients showed symptom resolution with return to baseline that corresponds to an increasing CT/CN value. A 1-tailed t test comparing the initial CT/ CN at the time of diagnosis to the last CT/CN value for symptomatic patients who recovered revealed a statistically significant increase (P < .05). For the symptomatic, symptom resolution and hospital discharge took (if required) a mean 20 days (range, 7-46). Among those who were not hospitalized, symptoms resolved in 7 to 36 days (18 days). Among those requiring hospitalization at any time (excluding patients who died or were asymptomatic), symptom and hospitalization resolution took a mean 22 days (range, 10-46). Asymptomatic patients (patients 8, 10, 15, 16, and 25) also showed increasing CT/CN value during the infection course, although there was no correlation with the continued lack of symptoms.
During the initial validation of the Abbott m2000 instrument, an LOD study included concentrations of 1000, 500, 250, 100, 70, 60, and 50 virus copies/mL (eAppendix 2, available online at doi:10.12788/fp.0276).21 The average CN at 100 virus copies/mL—the manufacturer provided LOD in the instructions for use—was 25.74.20 At a concentration of one-half that (50 virus copies/mL), the average CN was 28.39.
Discussion
This is the first study in the English literature to track CT/CN values as part of serial testing of a veteran CLC. Widescale testing and repeat screening in the absence of symptoms of nursing home residents would identify those who are infected and allow providers to track viral load clearance.9-14 CT/CN values, when serially tracked during the infection course, appear to increase with illness resolution, consistent with earlier reports that CT/CN correlates with viral load.8-14 Serial CT/CN values that are high (> 25) and continue to increase with each test suggest progression toward disease resolution or viral RNA clearance.8-14 After symptom resolution, patients can have a persistent low level of viral shedding (corresponding to a high CT/CN value).10-14,25 Near the end of disease resolution, a negative serial RT-PCR sample test before a subsequent positive might be a promising clinical sign of near disease recovery. Once the viral load is low with a CT/CN significantly higher than 25, some specimens might result as negative but turn up positive on subsequent sampling with a high CT/CN value. This pattern, with attendant high CT/CN values for the positive results, are consistent with the known effect of viral load (ie, a low viral load correlates to a high CT/CN) and adequacy of specimen collection on CT/CN values.25 If the patient’s viral load is low, the sample collected might have a viral load at or near the testing platform’s LOD.
For Abbott m2000, the manufacturer provided LOD is 100 virus copies/mL, although the instrument was able to detect virus concentrations below that level during the initial validation.20 The actual LOD of the instrument at our institution is < 100 virus copies/mL. For the Cepheid Xpert Xpress SARS-CoV-2 assay, the manufacturer-provided LOD is 250 virus copies/mL.19 An LOD study including samples below the manufacturer-provided LOD was not part of the initial validation study for the Xpert Xpress assay. Nonetheless, the virus concentration of samples with very high CT values at or near the maximum CT value of 45 is expected to be at or near the platform’s actual LOD.
If the samples collected near the end of the patient’s disease course have viral loads near these low concentrations, the encouraging positive-negative-positive pattern with high CT/CN values might be a promising sign for viral clearance. On the other hand, a positive-negative-positive pattern in the setting of low CT/CN values before and after the negative test might indicate poor sampling for the negative specimen. The back-and-forth or positive-negative-positive pattern generally appears to indicate near resolution of the infection course, although clinical correlation is necessary to rule out inadequate sampling earlier in the disease course or prolonged viral RNA shedding.9-14 In all of the surviving symptomatic patients who showed the positive-negative-positive pattern, this sign occurred around or after symptom resolution. It also is important to consider that in some patients, SARS-CoV-2 RNA might remain detectable with increasing CT/CN after symptom resolution, and samples from these patients might not result positive. Therefore, CT/CN values cannot be interpreted without considering the clinical picture.25
Studies on infectiousness and virus culture from COVID-19 samples with CT/ CN correlation have shown that patients with high CT/CN at the end of their disease course might not be as infectious.9-14,25 Because 1 patient had a presumptive positive result after the negative result, this study shows that this positive-negative-positive pattern could include presumptive positive results. Also, in the setting of a recent positive result on the same testing platform, a patient with this pattern is presumed to be positive for COVID-19 RNA because of scant viral material.
Taiwan’s public health response to the outbreak illustrates the ability to mitigate an outbreak throughout a society.26 These actions could help blunt an outbreak within a civilian nursing home population.5 Mitigation within a veteran CLC population has been documented, but the study, which focused on mitigation, did not consider CT/CN values, demographic distribution, testing access of the studied population, or laboratory findings related to disease pathophysiology.15 A key ingredient in widescale, serial testing is the availability of a rapid turnaround from testing in-house that allowed identification within 24 hours instead of several days at a reference laboratory. 15 Rapid widescale testing would allow clinical teams to optimize the Triangle of Benefit of Widescale Timely Tests for CLC (Figure 3).15 Timely laboratory testing remains pivotal for CLC veteran residents to aid successful clinical triage and management. Reporting serial CT/CN values can provide additional information to clinicians about the disease course because CT/ CN correlates with viral load, which varies based on where the patient is in the disease course.9-14 CT/CN values carry significant prognostic value, particularly with respect to intubation and mortality.8
Limitations
Important limitations to our study include the use of 2 separate RT-PCR platforms. Using different RT-PCR platforms is common in clinical laboratories trying to take advantage of the unique characteristics of different platforms—for example, turnaround time vs high throughput— to manage COVID-19 testing workflow.25 However, the exact CT/CN values obtained from each platform might not translate to the other, and the general trend (CT/CN values are rising or falling across serial tests) rather than a single value could be useful for clinical correlation. Even when the same platform is used for the serial testing, CT/CN values can be affected by adequacy of specimen collection; therefore, clinical correlation and considering the trend in CT/CN values is necessary for interpretation.10-14,25 Because of the known trend in viral dynamics, a positive specimen collected with a high CT/CN followed by a subsequent (within 2 days) positive specimen collected with a low CT/CN might be compatible with early detection of COVID- 19 infection in the appropriate clinical context. 10-14 However, detection late in the infection course or even after the symptomatic disease resolved with prolonged viral shedding might show serial positive samples with increasing CT/CN values.10-14
Patients with prolonged viral shedding might not be infectious.27 Because of the clinical correlation required for interpretation and the other factors that might affect CT/CN values, recommendations advise against using CT/CN values in clinical practice at this time, although these recommendations could change with future research.25 Serial CT/CN values have the potential, if appropriately correlated with the clinical picture, to provide useful information, such as whether the viral load of the sample is relatively high or low and increasing or decreasing.
Veterans, as a population, are more susceptible to poor health outcomes and morbidity compared with similar civilian counterparts.2,14-16 Veteran CLC patients likely would experience worse outcomes with COVID-19, including more infections, expiration, and morbidity compared with similar general population nursing homes. Similar to what had been reported for the civilian population, a trend (high CT/CN values early in the disease course with repeat testing needed to detect all positives followed by lower CT/CN value to correlate with increased viral load and then increased CT/CN value as the infection resolved) also was observed in this veteran population.
It has been extensively documented that minority groups experience decreased health care access and worse health outcomes. 28-30 Considering the critical medical supply shortages, including personal protective equipment, ventilators, and even testing supplies, there is the potential for a resource access disparity by ethnicity.28-31 Because the VA does not depend on measures of wealth and privilege such as health insurance, there was no disparity noted in access to testing by race or ethnicity at the VAMC CLC. When considering the health outcome of viral load from the measured CT/CN value, the viral loads of African American patients and those of other ethnicities was not significantly different in this study.
Conclusions
This is the first study to bring up critical points including serial CT/CN value correlation in RT-PCR tests, demographic distributions demonstrating easy and equal access in a veteran nursing home to COVID-19 testing, and clinical laboratory signs related to disease pathophysiology. Unlike other populations who have undergone serial CT/CN monitoring, nursing homes represent a particularly vulnerable population who require measures to prevent the spread and mitigate outbreaks of COVID-19.2,4,5 Test measurements obtained such as the CT/CN value during routine clinical care can provide useful information for public health, epidemiologic, or clinical purposes with appropriate correlation to clinical and other laboratory parameters. This study demonstrates early intervention of serial testing of an outbreak in a veterans nursing home with CT/CN value correlation.
1. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi:10.1136/bmj.m1091
2. Tsan L, Davis C, Langberg R, et al. Prevalence of nursing home-associated infections in the Department of Veterans Affairs nursing home care units. Am J Infect Control. 2008;36(3):173-179. doi:10.1016/j.ajic.2007.06.008
3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
4. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612-1614. doi:10.1001/jama.2020.4326
5. McMichael TM, Currie DW, Clark S, et al. Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. doi:10.1056/NEJMoa2005412
6. Childs A, Zullo AR, Joyce NR, et al. The burden of respiratory infections among older adults in long-term care: a systematic review. BMC Geriatr. 2019;19(1):210. doi:10.1186/s12877-019-1236-6
7. Eriksen HM, Iversen BG, Aavitsland PJ. Prevalence of nosocomial infections and use of antibiotics in long-term care facilities in Norway, 2002 and 2003. Hosp Infect. 2004;57(4):316-320. doi:10.1016/j.jhin.2004.03.028
8. Magleby R, Westblade LF, Trzebucki A, et al. Impact Severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis. 2021;73(11):e4197-e4205. doi:10.1093/cid/ciaa851
9. Buchan B, Hoff J, Gmehlin C, et al. Distribution of SARSCoV- 2 PCR cycle threshold values provide practical insight into overall and target-specific sensitivity among symptomatic patients. Am Clin Pathol. 2020;154:479-485. doi:10.1093/ajcp/aqaa133
10. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672-675. doi:10.1038/s41591-020-0869-5
11. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 Viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177-1179. doi:10.1056/NEJMc2001737
12. Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25(32):2001483. doi:10.2807/1560-7917.ES.2020.25.32.2001483
13. Salvatore P, Dawson P, Wadhwa A, et al. Epidemiological correlates of PCR cycles threshold values in the detection of SARS-CoV-2. Clin Infect Dis. 2021;72(11):e761-e767. doi:10.1093/cid/ciaa1469
14. Kissler S, Fauver J, Mack C, et al. Viral dynamics of SARS-CoV-2 infection and the predictive value of repeat testing. medRxiv. 2020;10.21.20217042. doi:10.1101/2020.10.21.20217042 1
5. Escobar DJ, Lanzi M, Saberi P, et al. Mitigation of a COVID-19 outbreak in a nursing home through serial testing of residents and staff. Clin Infect Dis. 2021;72(9):e394- e396. doi:10.1093/cid/ciaa1021
16. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13.
17. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
18. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5 Pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448.x 1
9. Xpert Xpress SARS-CoV-2. Instructions for use. Cepheid. 302-2562, Rev. C April 2020. Accessed January 7, 2021. https://www.fda.gov/media/136314/download
20. Abbott RealTime SARS-CoV-2. Instructions for use Abbott. 09N77-95. July 2020. Accessed January 7, 2021. https:// www.fda.gov/media/136258/download
21. Petersen JM, Dalal S, Jhala D. Successful implementation of SARS-CoV-2 testing in midst of pandemic with emphasis on all phases of testing. J Clin Pathol. 2021;74:273- 278. doi:10.1136/jclinpath-2020-207175
22. United States Census Bureau. Quick Facts: Philadelphia County, Pennsylvania. Accessed April 16, 2020. https://www .census.gov/quickfacts/philadelphiacountypennsylvania
23. Centers for Disease Control and Prevention. United States COVID-19 cases, deaths, and laboratory testing (NAATS) by state, territory, and jurisdiction. Accessed April 26, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases -updates/cases-in-us.html 2
4. Petersen J, Jhala D. Ethnicity, comorbid medical conditions, and SARS-CoV-2 test cycle thresholds in the veteran population [published online ahead of print, 2021 Jul 28]. J Racial Ethn Health Disparities. 2021;1-8. doi:10.1007/s40615-021-01114-4
25. Infectious Diseases Society of America, Association for Molecular Pathology. IDSA and AMP joint statement on the use of SARS-CoV-2 PCR cycle threshold (Ct) values for clinical decision-making. Accessed August 28, 2021. https://www.idsociety.org/globalassets/idsa/public-health /covid-19/idsa-amp-statement.pdf
26. Wang J, Ng CY, Brook RH. Response to COVID-19 in Taiwan: big data analysis, new technology, and proactive testing. JAMA. 2020;323(14):1341-1342. doi:10.1001/jama.2020.3151
27. Centers for Disease Control and Prevention. Overview of testing for SARS-CoV-2, the virus that causes COVID- 19. Accessed July 28, 2021. https://www.cdc.gov /coronavirus/2019-ncov/hcp/testing-overview.html
28. Zuvekas SH, Taliaferro GS. Pathways to access: health insurance, the health care delivery system, and racial/ethnic disparities, 1996-1999. Health Aff. 2003;22(2):139-153. doi:10.1377/hlthaff.22.2.139
29. Egede LE. Race, ethnicity, culture, and disparities in health care. J Gen Intern Med. 2006;21(6):667-669. doi:10.1111/j.1525-1497.2006.0512.x
30. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, eds. Unequal treatment: confronting racial and ethnic disparities in health care. National Academies Press; 2003. doi:10.17226/12875
31. Ranney ML, Griffeth V, Jha AK. Critical supply shortages – the need for ventilators and personal protective equipment during the Covid-19 Pandemic. N Engl J Med. 2020;382(18):e41. doi:10.1056/NEJMp2006141
COVID-19, caused by SARS-CoV-2, is more severe in individuals with underlying illnesses. Because complete social distancing might be more difficult in nursing homes and community living centers (CLCs), public health leaders and clinicians have been concerned about the epidemiology and disease course in nursing homes even before the COVID-19 pandemic.1-7 A report of a COVID-19 outbreak in a nursing home facility in King County, Washington, documented a 33.7% overall fatality rate for residents and 52.4% among the most critically ill.4,5 The experience at King County, Washington, shows that proactive steps to identify, monitor, and apply preventive control measures is important for future outbreaks.5
Reverse transcriptase polymerase chain reaction (RT-PCR) testing produces a cycle threshold (CT) or cycle number (CN) that correlates with viral load and infectiousness. 8-14 CT/CN represents the number of RT-PCR cycles required for the fluorescent signal to cross the detection threshold (exceed background level) and is inversely proportional to the viral load. Effectively, the higher the viral load, the lower the CT/ CN value (Figure 1). Tracking CT/CN values was not documented in the Washington nursing home outbreak. Reports of COVID- 19 testing in CLCs during outbreaks are sparse, and CT/CN values and demographic distribution of these veterans has not been reported.15 The CLC veteran population, with known higher vulnerability to infection and chronic diseases, is epidemiologically different from the general nursing home population.15-18 To address these literature gaps, we present the first report of COVID- 19 testing with CT/CN value correlations in the high-risk veteran CLC population.
Methods
A retrospective review of all COVID-19 CT/CN testing at the Corporal Michael J. Crescenz Veterans Affairs Medical Center (VAMC) CLC in Philadelphia, Pennsylvania, from March 28, 2020, to April 24, 2020, was performed with a US Department of Veterans Affairs (VA) Veterans Health Information System Architecture VistA/FileMan search. Only veteran residents were included in this review. Data collected included initial and serial test results, CT/CN on positive test results, test dates, testing platform used, demographic information (age, self-reported ethnicity, and sex), and clinical follow-up information. Health records were reviewed retrospectively to identify death, the first day after diagnosis with no documented symptoms, or hospitalization status.
RT-PCR testing was performed with the Abbott RealTime SARS-CoV-2 assay on the Abbott m2000 platform and the Xpert Xpress SARS-CoV-2 assay on the Cepheid Infinity platform. The Xpert Xpress assay gave 2 CT values for the E and N2 targets on positive samples.19 For this assay to indicate a positive specimen, amplification by RT-PCR of the N2 target or both the N2 and E target is required. The Xpert Xpress assay results as presumptive positive if only the E target amplified. This assay counts a maximum of 45 cycles. The Abbott RealTime SARS-CoV-2 assay gave 1 CN derived from the RNA-dependent RNA polymerase and N targets on positive samples.20 The Abbott assay on the m2000 counts a maximum of 37 cycles. The CT/CN value is the number of cycles required by RT-PCR for the fluorescence signal to cross a threshold value exceeding background level.19,20
Samples that are negative for COVID-19 by RT-PCR do not produce a CT/CN value. Although both instruments were used for RT-PCR, the precise CT/CN values are not interchangeable and CT/CN observations over time between the 2 instruments during the disease course would be based on CT/CN value movement (general upward or downward trend) rather than absolute CT/CN differences. Both assays have been approved by emergency use authorization as qualitative tests for the presence/absence of COVID-19. Although the CT/CN value is available to laboratory staff after test completion, the CT/CN value is not reported routinely in the patient health record. All veteran patients identified on the initial review from March 28, 2020, to April 24, 2020, had all serial COVID-19 testing recorded until November 10, 2020. The CN values at the limit of detection (LOD) for the Abbott m2000 platform from the initial validation study were reviewed for reference.21
Results
Of 80 patients, 25 (31%) were COVID-19 positive over the course of testing. The study population had a mean age of 73.5 years; 92% were aged > 60 years. The group was predominantly male (79 male vs 1 female). Among the 77 patients with a stated ethnicity, 39 (51%) were African American. In comparison, 43% of residents in Philadelphia County are African American (Table).22,23 Additionally, a previously published total COVID-19 tested population by ethnicity at the same regional VAMC revealed 46.8% of tested veteran patients were African American. 24 Three patients had no stated ethnicity. Among those who tested positive, 11 were African American patients, 12 were White patients, and 2 had no stated ethnicity. Four patients tested positive on their first test. The other 21 patients were positive on repeat testing. Interestingly, 6 patients had 1 initial negative test before a positive test, 6 patients had 2, 8 patients had 3, and 1 patient had 4 initial negative tests before a positive test result. Among the 25 positive patients, 22 were either positive within 10 days of the initial negative test result or initially positive (Figure 2). Three patients who tested positive after 10 days did so at 16, 20, and 21 days after the initial negative test result. Among the 25 positive patients, 23 had initial and serial testing from both the Abbott and Xpert Xpress assays. The remaining 2 positive patients had initial and serial testing from the Abbott assay exclusively.
Only positive COVID-19 results by RTPCR produced a CT/CN value. After disease resolution with a negative test, no CT/CN value was produced with the negative test result on either testing platform. Because repeat testing after the initial positive result took place no sooner than 10 days, we observed that the CT/CN value increased after the initial positive result until the disease resolved, and a negative result was obtained (eAppendix 1, available online at doi:10.12788/fp.0276). A t test comparing the initial CT/CN value to the value more than 10 days after the initial positive showed the CT/CN was statistically significantly higher (P < .05).
Prompt repeat testing after the initial test can show a decrease in the CT/CN value because of increasing viral load before the expected increase until disease resolution if the initial test caught the infection early. Twelve patients had a negative test result between 2 serial positive results. These negative test results occurred later, near the end of the disease course. Among the 12 patients with this positive-negativepositive CT/CN pattern, 7 were symptomatic and no longer had documented symptoms or hospitalization around the time of this positive-negative-positive pattern. Four of these individuals were asymptomatic during the entire infection course. One of the 12 patients with this pattern expired with the negative result occurring on day 27 of the disease in the context of rising CT/CN. One of these 12 patients only had a presumptive positive test result on the Cepheid because it detected only the E target with a CT value of 38.7. In 1 of the 12 patients, the negative test result occurred between 2 positive test results with CT/CN values < 20 (12.05 and 19.05 for the positive tests before and after the negative result, respectively). When the initial CT/CN values was separated based on ethnicity, the average CT/CN value for African Americans (23.3) was higher than for other ethnicities (19.9), although it did not reach statistical significance (P = .35).
Ten of the 25 patients testing positive were admitted to the hospital, including 1 admitted 15 days before diagnosis (patient 20) and 1 admitted 80 days after diagnosis (patient 7). Among these 10 patients, 6 were admitted to the intensive care unit, including patient 7. None of the patients were intubated. Three of the 10 admitted patients died (patients 7, 20, and 24). Patient 7 was a 79-year-old male with a history of dementia, cerebrovascular accident, hypertension, hyperlipidemia, and chronic kidney disease with symptoms of lethargy and refusal of oral intake when he was diagnosed with COVID-19. He was admitted 80 days after diagnosis for hyponatremia and acute renal failure, with death on day 87 recorded as complications from the earlier COVID-19 infection. Patient 20, an 89-year-old male with a history of dementia, chronic kidney disease, and hyperlipidemia, had been admitted with fever, cough, and leukocytosis 17 days before COVID-19 diagnosis. He continued to be symptomatic after diagnosis with development of hypotension, dehydration, and refusal of oral intake while on comfort measures/endof- life care and died 15 days after COVID- 19 infection diagnosis. Patient 24 was a 96-year-old male with history of heart failure, hypertension, coronary artery disease, prostate carcinoma, and dementia who developed a cough at the time of diagnosis; because of his underlying condition, he remained in the CLC on comfort care. His symptoms, including hypoxia, worsened until he died 7 days after diagnosis.
Among the 25 patients, 17 were symptomatic at the time of diagnosis; the 14 initially symptomatic patients who survived improved clinically and returned to baseline. Eight of the 25 patients were asymptomatic initially and 3 developed symptoms 2 to 5 days after diagnosis. Only 1 patient who remained asymptomatic was admitted for inability to adhere to quarantine at the CLC. Review of the health records of all surviving symptomatic patients showed symptom resolution with return to baseline that corresponds to an increasing CT/CN value. A 1-tailed t test comparing the initial CT/ CN at the time of diagnosis to the last CT/CN value for symptomatic patients who recovered revealed a statistically significant increase (P < .05). For the symptomatic, symptom resolution and hospital discharge took (if required) a mean 20 days (range, 7-46). Among those who were not hospitalized, symptoms resolved in 7 to 36 days (18 days). Among those requiring hospitalization at any time (excluding patients who died or were asymptomatic), symptom and hospitalization resolution took a mean 22 days (range, 10-46). Asymptomatic patients (patients 8, 10, 15, 16, and 25) also showed increasing CT/CN value during the infection course, although there was no correlation with the continued lack of symptoms.
During the initial validation of the Abbott m2000 instrument, an LOD study included concentrations of 1000, 500, 250, 100, 70, 60, and 50 virus copies/mL (eAppendix 2, available online at doi:10.12788/fp.0276).21 The average CN at 100 virus copies/mL—the manufacturer provided LOD in the instructions for use—was 25.74.20 At a concentration of one-half that (50 virus copies/mL), the average CN was 28.39.
Discussion
This is the first study in the English literature to track CT/CN values as part of serial testing of a veteran CLC. Widescale testing and repeat screening in the absence of symptoms of nursing home residents would identify those who are infected and allow providers to track viral load clearance.9-14 CT/CN values, when serially tracked during the infection course, appear to increase with illness resolution, consistent with earlier reports that CT/CN correlates with viral load.8-14 Serial CT/CN values that are high (> 25) and continue to increase with each test suggest progression toward disease resolution or viral RNA clearance.8-14 After symptom resolution, patients can have a persistent low level of viral shedding (corresponding to a high CT/CN value).10-14,25 Near the end of disease resolution, a negative serial RT-PCR sample test before a subsequent positive might be a promising clinical sign of near disease recovery. Once the viral load is low with a CT/CN significantly higher than 25, some specimens might result as negative but turn up positive on subsequent sampling with a high CT/CN value. This pattern, with attendant high CT/CN values for the positive results, are consistent with the known effect of viral load (ie, a low viral load correlates to a high CT/CN) and adequacy of specimen collection on CT/CN values.25 If the patient’s viral load is low, the sample collected might have a viral load at or near the testing platform’s LOD.
For Abbott m2000, the manufacturer provided LOD is 100 virus copies/mL, although the instrument was able to detect virus concentrations below that level during the initial validation.20 The actual LOD of the instrument at our institution is < 100 virus copies/mL. For the Cepheid Xpert Xpress SARS-CoV-2 assay, the manufacturer-provided LOD is 250 virus copies/mL.19 An LOD study including samples below the manufacturer-provided LOD was not part of the initial validation study for the Xpert Xpress assay. Nonetheless, the virus concentration of samples with very high CT values at or near the maximum CT value of 45 is expected to be at or near the platform’s actual LOD.
If the samples collected near the end of the patient’s disease course have viral loads near these low concentrations, the encouraging positive-negative-positive pattern with high CT/CN values might be a promising sign for viral clearance. On the other hand, a positive-negative-positive pattern in the setting of low CT/CN values before and after the negative test might indicate poor sampling for the negative specimen. The back-and-forth or positive-negative-positive pattern generally appears to indicate near resolution of the infection course, although clinical correlation is necessary to rule out inadequate sampling earlier in the disease course or prolonged viral RNA shedding.9-14 In all of the surviving symptomatic patients who showed the positive-negative-positive pattern, this sign occurred around or after symptom resolution. It also is important to consider that in some patients, SARS-CoV-2 RNA might remain detectable with increasing CT/CN after symptom resolution, and samples from these patients might not result positive. Therefore, CT/CN values cannot be interpreted without considering the clinical picture.25
Studies on infectiousness and virus culture from COVID-19 samples with CT/ CN correlation have shown that patients with high CT/CN at the end of their disease course might not be as infectious.9-14,25 Because 1 patient had a presumptive positive result after the negative result, this study shows that this positive-negative-positive pattern could include presumptive positive results. Also, in the setting of a recent positive result on the same testing platform, a patient with this pattern is presumed to be positive for COVID-19 RNA because of scant viral material.
Taiwan’s public health response to the outbreak illustrates the ability to mitigate an outbreak throughout a society.26 These actions could help blunt an outbreak within a civilian nursing home population.5 Mitigation within a veteran CLC population has been documented, but the study, which focused on mitigation, did not consider CT/CN values, demographic distribution, testing access of the studied population, or laboratory findings related to disease pathophysiology.15 A key ingredient in widescale, serial testing is the availability of a rapid turnaround from testing in-house that allowed identification within 24 hours instead of several days at a reference laboratory. 15 Rapid widescale testing would allow clinical teams to optimize the Triangle of Benefit of Widescale Timely Tests for CLC (Figure 3).15 Timely laboratory testing remains pivotal for CLC veteran residents to aid successful clinical triage and management. Reporting serial CT/CN values can provide additional information to clinicians about the disease course because CT/ CN correlates with viral load, which varies based on where the patient is in the disease course.9-14 CT/CN values carry significant prognostic value, particularly with respect to intubation and mortality.8
Limitations
Important limitations to our study include the use of 2 separate RT-PCR platforms. Using different RT-PCR platforms is common in clinical laboratories trying to take advantage of the unique characteristics of different platforms—for example, turnaround time vs high throughput— to manage COVID-19 testing workflow.25 However, the exact CT/CN values obtained from each platform might not translate to the other, and the general trend (CT/CN values are rising or falling across serial tests) rather than a single value could be useful for clinical correlation. Even when the same platform is used for the serial testing, CT/CN values can be affected by adequacy of specimen collection; therefore, clinical correlation and considering the trend in CT/CN values is necessary for interpretation.10-14,25 Because of the known trend in viral dynamics, a positive specimen collected with a high CT/CN followed by a subsequent (within 2 days) positive specimen collected with a low CT/CN might be compatible with early detection of COVID- 19 infection in the appropriate clinical context. 10-14 However, detection late in the infection course or even after the symptomatic disease resolved with prolonged viral shedding might show serial positive samples with increasing CT/CN values.10-14
Patients with prolonged viral shedding might not be infectious.27 Because of the clinical correlation required for interpretation and the other factors that might affect CT/CN values, recommendations advise against using CT/CN values in clinical practice at this time, although these recommendations could change with future research.25 Serial CT/CN values have the potential, if appropriately correlated with the clinical picture, to provide useful information, such as whether the viral load of the sample is relatively high or low and increasing or decreasing.
Veterans, as a population, are more susceptible to poor health outcomes and morbidity compared with similar civilian counterparts.2,14-16 Veteran CLC patients likely would experience worse outcomes with COVID-19, including more infections, expiration, and morbidity compared with similar general population nursing homes. Similar to what had been reported for the civilian population, a trend (high CT/CN values early in the disease course with repeat testing needed to detect all positives followed by lower CT/CN value to correlate with increased viral load and then increased CT/CN value as the infection resolved) also was observed in this veteran population.
It has been extensively documented that minority groups experience decreased health care access and worse health outcomes. 28-30 Considering the critical medical supply shortages, including personal protective equipment, ventilators, and even testing supplies, there is the potential for a resource access disparity by ethnicity.28-31 Because the VA does not depend on measures of wealth and privilege such as health insurance, there was no disparity noted in access to testing by race or ethnicity at the VAMC CLC. When considering the health outcome of viral load from the measured CT/CN value, the viral loads of African American patients and those of other ethnicities was not significantly different in this study.
Conclusions
This is the first study to bring up critical points including serial CT/CN value correlation in RT-PCR tests, demographic distributions demonstrating easy and equal access in a veteran nursing home to COVID-19 testing, and clinical laboratory signs related to disease pathophysiology. Unlike other populations who have undergone serial CT/CN monitoring, nursing homes represent a particularly vulnerable population who require measures to prevent the spread and mitigate outbreaks of COVID-19.2,4,5 Test measurements obtained such as the CT/CN value during routine clinical care can provide useful information for public health, epidemiologic, or clinical purposes with appropriate correlation to clinical and other laboratory parameters. This study demonstrates early intervention of serial testing of an outbreak in a veterans nursing home with CT/CN value correlation.
COVID-19, caused by SARS-CoV-2, is more severe in individuals with underlying illnesses. Because complete social distancing might be more difficult in nursing homes and community living centers (CLCs), public health leaders and clinicians have been concerned about the epidemiology and disease course in nursing homes even before the COVID-19 pandemic.1-7 A report of a COVID-19 outbreak in a nursing home facility in King County, Washington, documented a 33.7% overall fatality rate for residents and 52.4% among the most critically ill.4,5 The experience at King County, Washington, shows that proactive steps to identify, monitor, and apply preventive control measures is important for future outbreaks.5
Reverse transcriptase polymerase chain reaction (RT-PCR) testing produces a cycle threshold (CT) or cycle number (CN) that correlates with viral load and infectiousness. 8-14 CT/CN represents the number of RT-PCR cycles required for the fluorescent signal to cross the detection threshold (exceed background level) and is inversely proportional to the viral load. Effectively, the higher the viral load, the lower the CT/ CN value (Figure 1). Tracking CT/CN values was not documented in the Washington nursing home outbreak. Reports of COVID- 19 testing in CLCs during outbreaks are sparse, and CT/CN values and demographic distribution of these veterans has not been reported.15 The CLC veteran population, with known higher vulnerability to infection and chronic diseases, is epidemiologically different from the general nursing home population.15-18 To address these literature gaps, we present the first report of COVID- 19 testing with CT/CN value correlations in the high-risk veteran CLC population.
Methods
A retrospective review of all COVID-19 CT/CN testing at the Corporal Michael J. Crescenz Veterans Affairs Medical Center (VAMC) CLC in Philadelphia, Pennsylvania, from March 28, 2020, to April 24, 2020, was performed with a US Department of Veterans Affairs (VA) Veterans Health Information System Architecture VistA/FileMan search. Only veteran residents were included in this review. Data collected included initial and serial test results, CT/CN on positive test results, test dates, testing platform used, demographic information (age, self-reported ethnicity, and sex), and clinical follow-up information. Health records were reviewed retrospectively to identify death, the first day after diagnosis with no documented symptoms, or hospitalization status.
RT-PCR testing was performed with the Abbott RealTime SARS-CoV-2 assay on the Abbott m2000 platform and the Xpert Xpress SARS-CoV-2 assay on the Cepheid Infinity platform. The Xpert Xpress assay gave 2 CT values for the E and N2 targets on positive samples.19 For this assay to indicate a positive specimen, amplification by RT-PCR of the N2 target or both the N2 and E target is required. The Xpert Xpress assay results as presumptive positive if only the E target amplified. This assay counts a maximum of 45 cycles. The Abbott RealTime SARS-CoV-2 assay gave 1 CN derived from the RNA-dependent RNA polymerase and N targets on positive samples.20 The Abbott assay on the m2000 counts a maximum of 37 cycles. The CT/CN value is the number of cycles required by RT-PCR for the fluorescence signal to cross a threshold value exceeding background level.19,20
Samples that are negative for COVID-19 by RT-PCR do not produce a CT/CN value. Although both instruments were used for RT-PCR, the precise CT/CN values are not interchangeable and CT/CN observations over time between the 2 instruments during the disease course would be based on CT/CN value movement (general upward or downward trend) rather than absolute CT/CN differences. Both assays have been approved by emergency use authorization as qualitative tests for the presence/absence of COVID-19. Although the CT/CN value is available to laboratory staff after test completion, the CT/CN value is not reported routinely in the patient health record. All veteran patients identified on the initial review from March 28, 2020, to April 24, 2020, had all serial COVID-19 testing recorded until November 10, 2020. The CN values at the limit of detection (LOD) for the Abbott m2000 platform from the initial validation study were reviewed for reference.21
Results
Of 80 patients, 25 (31%) were COVID-19 positive over the course of testing. The study population had a mean age of 73.5 years; 92% were aged > 60 years. The group was predominantly male (79 male vs 1 female). Among the 77 patients with a stated ethnicity, 39 (51%) were African American. In comparison, 43% of residents in Philadelphia County are African American (Table).22,23 Additionally, a previously published total COVID-19 tested population by ethnicity at the same regional VAMC revealed 46.8% of tested veteran patients were African American. 24 Three patients had no stated ethnicity. Among those who tested positive, 11 were African American patients, 12 were White patients, and 2 had no stated ethnicity. Four patients tested positive on their first test. The other 21 patients were positive on repeat testing. Interestingly, 6 patients had 1 initial negative test before a positive test, 6 patients had 2, 8 patients had 3, and 1 patient had 4 initial negative tests before a positive test result. Among the 25 positive patients, 22 were either positive within 10 days of the initial negative test result or initially positive (Figure 2). Three patients who tested positive after 10 days did so at 16, 20, and 21 days after the initial negative test result. Among the 25 positive patients, 23 had initial and serial testing from both the Abbott and Xpert Xpress assays. The remaining 2 positive patients had initial and serial testing from the Abbott assay exclusively.
Only positive COVID-19 results by RTPCR produced a CT/CN value. After disease resolution with a negative test, no CT/CN value was produced with the negative test result on either testing platform. Because repeat testing after the initial positive result took place no sooner than 10 days, we observed that the CT/CN value increased after the initial positive result until the disease resolved, and a negative result was obtained (eAppendix 1, available online at doi:10.12788/fp.0276). A t test comparing the initial CT/CN value to the value more than 10 days after the initial positive showed the CT/CN was statistically significantly higher (P < .05).
Prompt repeat testing after the initial test can show a decrease in the CT/CN value because of increasing viral load before the expected increase until disease resolution if the initial test caught the infection early. Twelve patients had a negative test result between 2 serial positive results. These negative test results occurred later, near the end of the disease course. Among the 12 patients with this positive-negativepositive CT/CN pattern, 7 were symptomatic and no longer had documented symptoms or hospitalization around the time of this positive-negative-positive pattern. Four of these individuals were asymptomatic during the entire infection course. One of the 12 patients with this pattern expired with the negative result occurring on day 27 of the disease in the context of rising CT/CN. One of these 12 patients only had a presumptive positive test result on the Cepheid because it detected only the E target with a CT value of 38.7. In 1 of the 12 patients, the negative test result occurred between 2 positive test results with CT/CN values < 20 (12.05 and 19.05 for the positive tests before and after the negative result, respectively). When the initial CT/CN values was separated based on ethnicity, the average CT/CN value for African Americans (23.3) was higher than for other ethnicities (19.9), although it did not reach statistical significance (P = .35).
Ten of the 25 patients testing positive were admitted to the hospital, including 1 admitted 15 days before diagnosis (patient 20) and 1 admitted 80 days after diagnosis (patient 7). Among these 10 patients, 6 were admitted to the intensive care unit, including patient 7. None of the patients were intubated. Three of the 10 admitted patients died (patients 7, 20, and 24). Patient 7 was a 79-year-old male with a history of dementia, cerebrovascular accident, hypertension, hyperlipidemia, and chronic kidney disease with symptoms of lethargy and refusal of oral intake when he was diagnosed with COVID-19. He was admitted 80 days after diagnosis for hyponatremia and acute renal failure, with death on day 87 recorded as complications from the earlier COVID-19 infection. Patient 20, an 89-year-old male with a history of dementia, chronic kidney disease, and hyperlipidemia, had been admitted with fever, cough, and leukocytosis 17 days before COVID-19 diagnosis. He continued to be symptomatic after diagnosis with development of hypotension, dehydration, and refusal of oral intake while on comfort measures/endof- life care and died 15 days after COVID- 19 infection diagnosis. Patient 24 was a 96-year-old male with history of heart failure, hypertension, coronary artery disease, prostate carcinoma, and dementia who developed a cough at the time of diagnosis; because of his underlying condition, he remained in the CLC on comfort care. His symptoms, including hypoxia, worsened until he died 7 days after diagnosis.
Among the 25 patients, 17 were symptomatic at the time of diagnosis; the 14 initially symptomatic patients who survived improved clinically and returned to baseline. Eight of the 25 patients were asymptomatic initially and 3 developed symptoms 2 to 5 days after diagnosis. Only 1 patient who remained asymptomatic was admitted for inability to adhere to quarantine at the CLC. Review of the health records of all surviving symptomatic patients showed symptom resolution with return to baseline that corresponds to an increasing CT/CN value. A 1-tailed t test comparing the initial CT/ CN at the time of diagnosis to the last CT/CN value for symptomatic patients who recovered revealed a statistically significant increase (P < .05). For the symptomatic, symptom resolution and hospital discharge took (if required) a mean 20 days (range, 7-46). Among those who were not hospitalized, symptoms resolved in 7 to 36 days (18 days). Among those requiring hospitalization at any time (excluding patients who died or were asymptomatic), symptom and hospitalization resolution took a mean 22 days (range, 10-46). Asymptomatic patients (patients 8, 10, 15, 16, and 25) also showed increasing CT/CN value during the infection course, although there was no correlation with the continued lack of symptoms.
During the initial validation of the Abbott m2000 instrument, an LOD study included concentrations of 1000, 500, 250, 100, 70, 60, and 50 virus copies/mL (eAppendix 2, available online at doi:10.12788/fp.0276).21 The average CN at 100 virus copies/mL—the manufacturer provided LOD in the instructions for use—was 25.74.20 At a concentration of one-half that (50 virus copies/mL), the average CN was 28.39.
Discussion
This is the first study in the English literature to track CT/CN values as part of serial testing of a veteran CLC. Widescale testing and repeat screening in the absence of symptoms of nursing home residents would identify those who are infected and allow providers to track viral load clearance.9-14 CT/CN values, when serially tracked during the infection course, appear to increase with illness resolution, consistent with earlier reports that CT/CN correlates with viral load.8-14 Serial CT/CN values that are high (> 25) and continue to increase with each test suggest progression toward disease resolution or viral RNA clearance.8-14 After symptom resolution, patients can have a persistent low level of viral shedding (corresponding to a high CT/CN value).10-14,25 Near the end of disease resolution, a negative serial RT-PCR sample test before a subsequent positive might be a promising clinical sign of near disease recovery. Once the viral load is low with a CT/CN significantly higher than 25, some specimens might result as negative but turn up positive on subsequent sampling with a high CT/CN value. This pattern, with attendant high CT/CN values for the positive results, are consistent with the known effect of viral load (ie, a low viral load correlates to a high CT/CN) and adequacy of specimen collection on CT/CN values.25 If the patient’s viral load is low, the sample collected might have a viral load at or near the testing platform’s LOD.
For Abbott m2000, the manufacturer provided LOD is 100 virus copies/mL, although the instrument was able to detect virus concentrations below that level during the initial validation.20 The actual LOD of the instrument at our institution is < 100 virus copies/mL. For the Cepheid Xpert Xpress SARS-CoV-2 assay, the manufacturer-provided LOD is 250 virus copies/mL.19 An LOD study including samples below the manufacturer-provided LOD was not part of the initial validation study for the Xpert Xpress assay. Nonetheless, the virus concentration of samples with very high CT values at or near the maximum CT value of 45 is expected to be at or near the platform’s actual LOD.
If the samples collected near the end of the patient’s disease course have viral loads near these low concentrations, the encouraging positive-negative-positive pattern with high CT/CN values might be a promising sign for viral clearance. On the other hand, a positive-negative-positive pattern in the setting of low CT/CN values before and after the negative test might indicate poor sampling for the negative specimen. The back-and-forth or positive-negative-positive pattern generally appears to indicate near resolution of the infection course, although clinical correlation is necessary to rule out inadequate sampling earlier in the disease course or prolonged viral RNA shedding.9-14 In all of the surviving symptomatic patients who showed the positive-negative-positive pattern, this sign occurred around or after symptom resolution. It also is important to consider that in some patients, SARS-CoV-2 RNA might remain detectable with increasing CT/CN after symptom resolution, and samples from these patients might not result positive. Therefore, CT/CN values cannot be interpreted without considering the clinical picture.25
Studies on infectiousness and virus culture from COVID-19 samples with CT/ CN correlation have shown that patients with high CT/CN at the end of their disease course might not be as infectious.9-14,25 Because 1 patient had a presumptive positive result after the negative result, this study shows that this positive-negative-positive pattern could include presumptive positive results. Also, in the setting of a recent positive result on the same testing platform, a patient with this pattern is presumed to be positive for COVID-19 RNA because of scant viral material.
Taiwan’s public health response to the outbreak illustrates the ability to mitigate an outbreak throughout a society.26 These actions could help blunt an outbreak within a civilian nursing home population.5 Mitigation within a veteran CLC population has been documented, but the study, which focused on mitigation, did not consider CT/CN values, demographic distribution, testing access of the studied population, or laboratory findings related to disease pathophysiology.15 A key ingredient in widescale, serial testing is the availability of a rapid turnaround from testing in-house that allowed identification within 24 hours instead of several days at a reference laboratory. 15 Rapid widescale testing would allow clinical teams to optimize the Triangle of Benefit of Widescale Timely Tests for CLC (Figure 3).15 Timely laboratory testing remains pivotal for CLC veteran residents to aid successful clinical triage and management. Reporting serial CT/CN values can provide additional information to clinicians about the disease course because CT/ CN correlates with viral load, which varies based on where the patient is in the disease course.9-14 CT/CN values carry significant prognostic value, particularly with respect to intubation and mortality.8
Limitations
Important limitations to our study include the use of 2 separate RT-PCR platforms. Using different RT-PCR platforms is common in clinical laboratories trying to take advantage of the unique characteristics of different platforms—for example, turnaround time vs high throughput— to manage COVID-19 testing workflow.25 However, the exact CT/CN values obtained from each platform might not translate to the other, and the general trend (CT/CN values are rising or falling across serial tests) rather than a single value could be useful for clinical correlation. Even when the same platform is used for the serial testing, CT/CN values can be affected by adequacy of specimen collection; therefore, clinical correlation and considering the trend in CT/CN values is necessary for interpretation.10-14,25 Because of the known trend in viral dynamics, a positive specimen collected with a high CT/CN followed by a subsequent (within 2 days) positive specimen collected with a low CT/CN might be compatible with early detection of COVID- 19 infection in the appropriate clinical context. 10-14 However, detection late in the infection course or even after the symptomatic disease resolved with prolonged viral shedding might show serial positive samples with increasing CT/CN values.10-14
Patients with prolonged viral shedding might not be infectious.27 Because of the clinical correlation required for interpretation and the other factors that might affect CT/CN values, recommendations advise against using CT/CN values in clinical practice at this time, although these recommendations could change with future research.25 Serial CT/CN values have the potential, if appropriately correlated with the clinical picture, to provide useful information, such as whether the viral load of the sample is relatively high or low and increasing or decreasing.
Veterans, as a population, are more susceptible to poor health outcomes and morbidity compared with similar civilian counterparts.2,14-16 Veteran CLC patients likely would experience worse outcomes with COVID-19, including more infections, expiration, and morbidity compared with similar general population nursing homes. Similar to what had been reported for the civilian population, a trend (high CT/CN values early in the disease course with repeat testing needed to detect all positives followed by lower CT/CN value to correlate with increased viral load and then increased CT/CN value as the infection resolved) also was observed in this veteran population.
It has been extensively documented that minority groups experience decreased health care access and worse health outcomes. 28-30 Considering the critical medical supply shortages, including personal protective equipment, ventilators, and even testing supplies, there is the potential for a resource access disparity by ethnicity.28-31 Because the VA does not depend on measures of wealth and privilege such as health insurance, there was no disparity noted in access to testing by race or ethnicity at the VAMC CLC. When considering the health outcome of viral load from the measured CT/CN value, the viral loads of African American patients and those of other ethnicities was not significantly different in this study.
Conclusions
This is the first study to bring up critical points including serial CT/CN value correlation in RT-PCR tests, demographic distributions demonstrating easy and equal access in a veteran nursing home to COVID-19 testing, and clinical laboratory signs related to disease pathophysiology. Unlike other populations who have undergone serial CT/CN monitoring, nursing homes represent a particularly vulnerable population who require measures to prevent the spread and mitigate outbreaks of COVID-19.2,4,5 Test measurements obtained such as the CT/CN value during routine clinical care can provide useful information for public health, epidemiologic, or clinical purposes with appropriate correlation to clinical and other laboratory parameters. This study demonstrates early intervention of serial testing of an outbreak in a veterans nursing home with CT/CN value correlation.
1. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi:10.1136/bmj.m1091
2. Tsan L, Davis C, Langberg R, et al. Prevalence of nursing home-associated infections in the Department of Veterans Affairs nursing home care units. Am J Infect Control. 2008;36(3):173-179. doi:10.1016/j.ajic.2007.06.008
3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
4. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612-1614. doi:10.1001/jama.2020.4326
5. McMichael TM, Currie DW, Clark S, et al. Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. doi:10.1056/NEJMoa2005412
6. Childs A, Zullo AR, Joyce NR, et al. The burden of respiratory infections among older adults in long-term care: a systematic review. BMC Geriatr. 2019;19(1):210. doi:10.1186/s12877-019-1236-6
7. Eriksen HM, Iversen BG, Aavitsland PJ. Prevalence of nosocomial infections and use of antibiotics in long-term care facilities in Norway, 2002 and 2003. Hosp Infect. 2004;57(4):316-320. doi:10.1016/j.jhin.2004.03.028
8. Magleby R, Westblade LF, Trzebucki A, et al. Impact Severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis. 2021;73(11):e4197-e4205. doi:10.1093/cid/ciaa851
9. Buchan B, Hoff J, Gmehlin C, et al. Distribution of SARSCoV- 2 PCR cycle threshold values provide practical insight into overall and target-specific sensitivity among symptomatic patients. Am Clin Pathol. 2020;154:479-485. doi:10.1093/ajcp/aqaa133
10. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672-675. doi:10.1038/s41591-020-0869-5
11. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 Viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177-1179. doi:10.1056/NEJMc2001737
12. Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25(32):2001483. doi:10.2807/1560-7917.ES.2020.25.32.2001483
13. Salvatore P, Dawson P, Wadhwa A, et al. Epidemiological correlates of PCR cycles threshold values in the detection of SARS-CoV-2. Clin Infect Dis. 2021;72(11):e761-e767. doi:10.1093/cid/ciaa1469
14. Kissler S, Fauver J, Mack C, et al. Viral dynamics of SARS-CoV-2 infection and the predictive value of repeat testing. medRxiv. 2020;10.21.20217042. doi:10.1101/2020.10.21.20217042 1
5. Escobar DJ, Lanzi M, Saberi P, et al. Mitigation of a COVID-19 outbreak in a nursing home through serial testing of residents and staff. Clin Infect Dis. 2021;72(9):e394- e396. doi:10.1093/cid/ciaa1021
16. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13.
17. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
18. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5 Pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448.x 1
9. Xpert Xpress SARS-CoV-2. Instructions for use. Cepheid. 302-2562, Rev. C April 2020. Accessed January 7, 2021. https://www.fda.gov/media/136314/download
20. Abbott RealTime SARS-CoV-2. Instructions for use Abbott. 09N77-95. July 2020. Accessed January 7, 2021. https:// www.fda.gov/media/136258/download
21. Petersen JM, Dalal S, Jhala D. Successful implementation of SARS-CoV-2 testing in midst of pandemic with emphasis on all phases of testing. J Clin Pathol. 2021;74:273- 278. doi:10.1136/jclinpath-2020-207175
22. United States Census Bureau. Quick Facts: Philadelphia County, Pennsylvania. Accessed April 16, 2020. https://www .census.gov/quickfacts/philadelphiacountypennsylvania
23. Centers for Disease Control and Prevention. United States COVID-19 cases, deaths, and laboratory testing (NAATS) by state, territory, and jurisdiction. Accessed April 26, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases -updates/cases-in-us.html 2
4. Petersen J, Jhala D. Ethnicity, comorbid medical conditions, and SARS-CoV-2 test cycle thresholds in the veteran population [published online ahead of print, 2021 Jul 28]. J Racial Ethn Health Disparities. 2021;1-8. doi:10.1007/s40615-021-01114-4
25. Infectious Diseases Society of America, Association for Molecular Pathology. IDSA and AMP joint statement on the use of SARS-CoV-2 PCR cycle threshold (Ct) values for clinical decision-making. Accessed August 28, 2021. https://www.idsociety.org/globalassets/idsa/public-health /covid-19/idsa-amp-statement.pdf
26. Wang J, Ng CY, Brook RH. Response to COVID-19 in Taiwan: big data analysis, new technology, and proactive testing. JAMA. 2020;323(14):1341-1342. doi:10.1001/jama.2020.3151
27. Centers for Disease Control and Prevention. Overview of testing for SARS-CoV-2, the virus that causes COVID- 19. Accessed July 28, 2021. https://www.cdc.gov /coronavirus/2019-ncov/hcp/testing-overview.html
28. Zuvekas SH, Taliaferro GS. Pathways to access: health insurance, the health care delivery system, and racial/ethnic disparities, 1996-1999. Health Aff. 2003;22(2):139-153. doi:10.1377/hlthaff.22.2.139
29. Egede LE. Race, ethnicity, culture, and disparities in health care. J Gen Intern Med. 2006;21(6):667-669. doi:10.1111/j.1525-1497.2006.0512.x
30. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, eds. Unequal treatment: confronting racial and ethnic disparities in health care. National Academies Press; 2003. doi:10.17226/12875
31. Ranney ML, Griffeth V, Jha AK. Critical supply shortages – the need for ventilators and personal protective equipment during the Covid-19 Pandemic. N Engl J Med. 2020;382(18):e41. doi:10.1056/NEJMp2006141
1. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi:10.1136/bmj.m1091
2. Tsan L, Davis C, Langberg R, et al. Prevalence of nursing home-associated infections in the Department of Veterans Affairs nursing home care units. Am J Infect Control. 2008;36(3):173-179. doi:10.1016/j.ajic.2007.06.008
3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
4. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612-1614. doi:10.1001/jama.2020.4326
5. McMichael TM, Currie DW, Clark S, et al. Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. doi:10.1056/NEJMoa2005412
6. Childs A, Zullo AR, Joyce NR, et al. The burden of respiratory infections among older adults in long-term care: a systematic review. BMC Geriatr. 2019;19(1):210. doi:10.1186/s12877-019-1236-6
7. Eriksen HM, Iversen BG, Aavitsland PJ. Prevalence of nosocomial infections and use of antibiotics in long-term care facilities in Norway, 2002 and 2003. Hosp Infect. 2004;57(4):316-320. doi:10.1016/j.jhin.2004.03.028
8. Magleby R, Westblade LF, Trzebucki A, et al. Impact Severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis. 2021;73(11):e4197-e4205. doi:10.1093/cid/ciaa851
9. Buchan B, Hoff J, Gmehlin C, et al. Distribution of SARSCoV- 2 PCR cycle threshold values provide practical insight into overall and target-specific sensitivity among symptomatic patients. Am Clin Pathol. 2020;154:479-485. doi:10.1093/ajcp/aqaa133
10. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672-675. doi:10.1038/s41591-020-0869-5
11. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 Viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177-1179. doi:10.1056/NEJMc2001737
12. Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25(32):2001483. doi:10.2807/1560-7917.ES.2020.25.32.2001483
13. Salvatore P, Dawson P, Wadhwa A, et al. Epidemiological correlates of PCR cycles threshold values in the detection of SARS-CoV-2. Clin Infect Dis. 2021;72(11):e761-e767. doi:10.1093/cid/ciaa1469
14. Kissler S, Fauver J, Mack C, et al. Viral dynamics of SARS-CoV-2 infection and the predictive value of repeat testing. medRxiv. 2020;10.21.20217042. doi:10.1101/2020.10.21.20217042 1
5. Escobar DJ, Lanzi M, Saberi P, et al. Mitigation of a COVID-19 outbreak in a nursing home through serial testing of residents and staff. Clin Infect Dis. 2021;72(9):e394- e396. doi:10.1093/cid/ciaa1021
16. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13.
17. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
18. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5 Pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448.x 1
9. Xpert Xpress SARS-CoV-2. Instructions for use. Cepheid. 302-2562, Rev. C April 2020. Accessed January 7, 2021. https://www.fda.gov/media/136314/download
20. Abbott RealTime SARS-CoV-2. Instructions for use Abbott. 09N77-95. July 2020. Accessed January 7, 2021. https:// www.fda.gov/media/136258/download
21. Petersen JM, Dalal S, Jhala D. Successful implementation of SARS-CoV-2 testing in midst of pandemic with emphasis on all phases of testing. J Clin Pathol. 2021;74:273- 278. doi:10.1136/jclinpath-2020-207175
22. United States Census Bureau. Quick Facts: Philadelphia County, Pennsylvania. Accessed April 16, 2020. https://www .census.gov/quickfacts/philadelphiacountypennsylvania
23. Centers for Disease Control and Prevention. United States COVID-19 cases, deaths, and laboratory testing (NAATS) by state, territory, and jurisdiction. Accessed April 26, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases -updates/cases-in-us.html 2
4. Petersen J, Jhala D. Ethnicity, comorbid medical conditions, and SARS-CoV-2 test cycle thresholds in the veteran population [published online ahead of print, 2021 Jul 28]. J Racial Ethn Health Disparities. 2021;1-8. doi:10.1007/s40615-021-01114-4
25. Infectious Diseases Society of America, Association for Molecular Pathology. IDSA and AMP joint statement on the use of SARS-CoV-2 PCR cycle threshold (Ct) values for clinical decision-making. Accessed August 28, 2021. https://www.idsociety.org/globalassets/idsa/public-health /covid-19/idsa-amp-statement.pdf
26. Wang J, Ng CY, Brook RH. Response to COVID-19 in Taiwan: big data analysis, new technology, and proactive testing. JAMA. 2020;323(14):1341-1342. doi:10.1001/jama.2020.3151
27. Centers for Disease Control and Prevention. Overview of testing for SARS-CoV-2, the virus that causes COVID- 19. Accessed July 28, 2021. https://www.cdc.gov /coronavirus/2019-ncov/hcp/testing-overview.html
28. Zuvekas SH, Taliaferro GS. Pathways to access: health insurance, the health care delivery system, and racial/ethnic disparities, 1996-1999. Health Aff. 2003;22(2):139-153. doi:10.1377/hlthaff.22.2.139
29. Egede LE. Race, ethnicity, culture, and disparities in health care. J Gen Intern Med. 2006;21(6):667-669. doi:10.1111/j.1525-1497.2006.0512.x
30. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, eds. Unequal treatment: confronting racial and ethnic disparities in health care. National Academies Press; 2003. doi:10.17226/12875
31. Ranney ML, Griffeth V, Jha AK. Critical supply shortages – the need for ventilators and personal protective equipment during the Covid-19 Pandemic. N Engl J Med. 2020;382(18):e41. doi:10.1056/NEJMp2006141
Health Systems Education Leadership: Learning From the VA Designated Education Officer Role
The US Department of Veterans Affairs (VA) operates the largest integrated health care system in the United States, providing physical and mental health care to more than 9 million veterans enrolled each year through a national system of inpatient, outpatient, and long-term care settings.1 As 1 of 4 statutory missions, the VA conducts the largest training effort for health professionals in cooperation with affiliated academic institutions. From 2016 through 2020, an average of 123,000 trainees from various professions received training at the VA.2 Physician residents comprised the largest trainee group (37%), followed by associated health students and residents (20%), and nursing professionals (21%).2 In VA, associated health professions include all health care disciplines other than allopathic and osteopathic medicine, dentistry, and nursing. The associated health professions encompass about 40 specialties, including audiology, dietetics, physical and occupational therapy, optometry, pharmacy, podiatry, psychology, and social work.
The VA also trains a smaller number of advanced fellows to address specialties important to the nation and veterans health that are not sufficiently addressed by standard accredited professional training.3 The VA Advanced Fellowship programs include 22 postresidency, postdoctoral, and postmasters fellowships to physicians and dentists, and associated health professions, including psychologists, social workers, and pharmacists. 3 From 2015 to 2019, 57 to 61% of medical school students reported having a VA clinical training experience during medical school.4 Of current VA employees, 20% of registered nurses, 64% of physicians, 73% of podiatrists and optometrists, and 81% of psychologists reported VA training prior to employment.5
Health professions education is led by the designated education officer (DEO) at each VA facility.6 Also known as the associate chief of staff for education (ACOS/E), the DEO is a leadership position that is accountable to local VA facility executive leadership as well as the national Office of Academic Affiliations (OAA), which directs all VA health professions training across the US.6 At most VA facilities, the DEO oversees clinical training and education reporting directly to the facility chief of staff. At the same time, the ACOS/E is accountable to the OAA to ensure adherence with national education directives and policy. The DEO oversees trainee programs through collaboration with training program directors, faculty, academic affiliates, and accreditation agencies across > 40 health professions.
The DEO is expected to possess expertise in leadership attributes identified by the US Office of Personnel Management as essential to build a federal corporate culture that drives results, serves customers, and builds successful teams and coalitions within and outside the VA.7 These leadership attributes include leading change, leading people, driving results, business acumen, and building coalitions.7 They are operationalized by OAA as 4 domains of expertise required to lead education across multiple professions, including: (1) creating and sustaining an organizational work environment that supports learning, discovery, and continuous improvement; (2) aligning and managing fiscal, human, and capital resources to meet organizational learning needs; (3) driving learning and performance results to impact organizational success; and (4) leading change and transformation through positioning and implementing innovative learning and education strategies (Table 1).6
In this article we describe the VA DEO leadership role and the tasks required to lead education across multiple professions within the VA health care system. Given the broad scope of leading educational programs across multiple clinical professions and the interprofessional backgrounds of DEOs across the VA, we evaluated DEO self-perceived effectiveness to impact educational decisions and behavior by professional discipline. Our evaluation question is: Are different professional education and practice backgrounds functionally capable of providing leadership over all education of health professions training programs? Finally, we describe DEOs perceptions of facilitators and barriers to performing their DEO role within the VA.
Methods
We conducted a mixed methods analysis of data collected by OAA to assess DEO needs within a multiprofessional clinical learning environment. The needs assessment was conducted by an OAA evaluator (NH) with input on instrument development and data analysis from OAA leadership (KS, MB). This evaluation is categorized as an operations activity based on VA Handbook 1200 where information generated is used for business operations and quality improvement. 8 The overall project was subject to administrative rather than institutional review board oversight.
A needs assessment tool was developed based on the OAA domains of expertise.6 Prior to its administration, the tool was piloted with 8 DEOs in the field and the survey shortened based on their feedback. DEOs were asked about individual professional characteristics (eg, clinical profession, academic appointment, type of health professions training programs at the VA site) and their self-perceived effectiveness in impacting educational decisions and behaviors on general and profession-specific tasks within each of the 4 domains of expertise on a 5-point Likert scale (1, not effective; 5, very effective). 6,9 The needs assessment also included an open-ended question asking respondents to comment on any issues they felt important to understanding DEO role effectiveness.
The needs assessment was administered online via SurveyMonkey to 132 DEOs via email in September and October 2019. The DEOs represented 148 of 160 VA facilities with health professions education; 14 DEOs covered > 1 VA facility, and 12 positions were vacant. Email reminders were sent to nonresponders after 1 week. At 2 weeks, nonresponders received telephone reminders and personalized follow-up emails from OAA staff. The response rate at the end of 3 weeks was 96%.
Data Analysis
Mixed methods analyses included quantitative analyses to identify differences in general and profession-specific self-ratings of effectiveness in influencing educational decisions and behaviors by DEO profession, and qualitative analyses to further understand DEO’s perceptions of facilitators and barriers to DEO task effectiveness.10,11 Quantitative analyses included descriptive statistics for all variables followed by nonparametric tests including χ2 and Mann- Whitney U tests to assess differences between physician and other professional DEOs in descriptive characteristics and selfperceived effectiveness on general and profession- specific tasks. Quantitative analyses were conducted using SPSS software, version 26. Qualitative analyses consisted of rapid assessment procedures to identify facilitators and barriers to DEO effectiveness by profession using Atlas.ti version 8, which involved reviewing responses to the open-ended question and assigning each response to predetermined categories based on the organizational level it applied to (eg, individual DEO, VA facility, or external to the organization).12,13 Responses within categories were then summarized to identify the main themes.
Results
Completed surveys were received from 127 respondents representing 139 VA facilities. Eighty percent were physicians and 20% were other professionals, including psychologists, pharmacists, dentists, dieticians, nurses, and nonclinicians. There were no statistically significant differences between physician and other professional DEOs in the percent working full time or length of time spent working in the position. About one-third of the sample had been in the position for < 2 years, one-third had been in the position for 2 to < 5 years, and one-third had been in the role for ≥ 5 years. Eighty percent reported having a faculty appointment with an academic affiliate. While 92% of physician DEOs had a faculty appointment, only 40% of other professional DEOs did (P < .001). Most faculty appointments for both groups were with a school of medicine. More physician DEOs than other professionals had training programs at their site for physicians (P = .003) and dentists (P < .001), but there were no statistically significant differences for having associated health, nursing, or advanced fellowship training programs at their sites. Across all DEOs, 98% reported training programs at their site for associated health professions, 95% for physician training, 93% for nursing training, 59% for dental training, and 48% for advanced fellowships.
Self-Perceived Effectiveness
There were no statistically significant differences between physician and other professional DEOs on self-perceived effectiveness in impacting educational decisions or behaviors for general tasks applicable across professions (Table 2). This result held even after controlling for length of time in the position and whether the DEO had an academic appointment. Generally, both groups reported being effective on tasks in the enabling learning domain, including applying policies and procedures related to trainees who rotate through the VA and maintaining adherence with accreditation agency standards across health professions. Mean score ranges for both physician and other professional DEOs reported moderate effectiveness in aligning resources effectiveness questions (2.45-3.72 vs 2.75-3.76), driving results questions (3.02-3.60 vs 3.39-3.48), and leading change questions (3.12-3.50 vs 3.42-3.80).
For profession-specific tasks, effectiveness ratings between the 2 groups were generally not statistically significant for medical, dental, and advanced fellowship training programs (Table 3). There was a pattern of statistically significant differences between physician and other professional DEOs for associated health and nursing training programs on tasks across the 4 domains of expertise with physicians having lower mean ratings compared with other professionals. Generally, physician DEOs had higher task effectiveness when compared with other professionals for medical training programs, and other professionals had higher task effectiveness ratings than did physicians for associated health or nursing training programs.
Facilitators and Barriers
Seventy responses related to facilitators and barriers to DEO effectiveness were received (59 from physicians and 11 from other professionals). Most responses were categorized as individual level facilitators or barriers (53% for physician and 64% for other professionals). Only 3% of comments were categorized as external to the organization (all made by physicians). The themes were similar for both groups and were aggregated in Table 4. Facilitators included continuing education, having a mentor who works at a similar type of facility, maintaining balance and time management when working with different training programs, learning to work and develop relationships with training program directors, developing an overall picture of each type of health professions training program, holding regular meetings with all health training programs and academic affiliates, having a formal education service line with budget and staffing, facility executive leadership who are knowledgeable of the education mission and DEO role, having a national oversight body, and the DEO’s relationships with academic affiliates.
Barriers to role effectiveness at the individual DEO level included assignment of multiple roles and a focus on regulation and monitoring with little time for development of new programs and strategic planning. The organizational level barriers included difficulty getting core services to engage with health professions trainees and siloed education leadership.
Discussion
DEOs oversee multiple health professions training programs within local facilities. The DEO is accountable to local VA facility leadership and a national education office to lead local health professions education at local facilities and integrate these educational activities across the national VA system.
The VA DEO role is similar to the Accreditation Council for Graduate Medical Education designated institutional official (DIO) except that the VA DEO provides oversight of > 40 health professions training programs.14,15 The VA DEO, therefore, has broader oversight than the DIO role that focuses only on graduate physician education. Similar to the DIO, the VA DEO role initially emphasized the enabling learning and aligning resources domains to provide oversight and administration of health professions training programs. Over time, both roles have expanded to include defining and ensuring healthy clinical learning environments, aligning educational resources and training with the institutional mission, workforce, and societal needs, and creating continuous educational improvement models.6,16,17 To accomplish these expanded goals, both the DEO and the DIO work closely with other educational leaders at the academic affiliate and the VA facility. As health professions education advances, there will be increased emphasis placed on delivering educational programs to improve clinical practice and health care outcomes.18
Our findings that DEO profession did not influence self-ratings of effectiveness to influence educational decisions or behaviors on general tasks applicable across health professions suggest that education and practice background are not factors influencing selfratings. Nor were self-ratings influenced by other factors. Since the DEO is a senior leadership position, candidates for the position already may possess managerial and leadership skills. In our sample, several individuals commented that they had prior education leadership positions, eg, training program director or had years of experience working in the VA. Similarly, having an academic appointment may not be important for the performance of general administrative tasks. However, an academic appointment may be important for effective performance of educational tasks, such as clinical teaching, didactic training, and curriculum development, which were not measured in this study.
The finding of differences in self-ratings between physicians and other professionals on profession-specific tasks for associated health and nursing suggests that physicians may require additional curriculum to enhance their knowledge in managing other professional educational programs. For nursing specifically, this finding could also reflect substantial input from the lead nurse executive in the facility. DEOs also identified practical ways to facilitate their work with multiple health professions that could immediately be put into practice, including developing relationships and enhancing communication with training program directors, faculty, and academic affiliates of each profession.
Taken together, the quantitative and qualitative findings indicate that despite differences in professional backgrounds, DEOs have high self-ratings of their own effectiveness to influence educational decisions and behaviors on general tasks they are expected to accomplish. There are some professionspecific tasks where professional background does influence self-perceived effectiveness, ie, physicians have higher self-ratings on physician-specific tasks and other professionals have higher self-ratings on associated health or nursing tasks. These perceived differences may be mitigated by increasing facilitators and decreasing barriers identified for the individual DEO, within the organization, and external to the organization.
Limitations Our findings should be interpreted with the following limitations in mind. The selfreport nature of the data opens the possibility of self-report bias or Dunning-Kruger effects where effectiveness ratings could have been overestimated by respondents.21 Although respondents were assured of their anonymity and that results would only be reported in the aggregate, there is potential for providing more positive responses on a needs assessment administered by the national education program office. We recommend further work be conducted to validate the needs assessment tool against other data collection methods, such as actual outcomes of educational effectiveness. Our study did not incorporate measures of educational effectiveness to determine whether self-perceived DEO effectiveness is translated to better trainee or learning outcomes. Before this can happen, educational policymakers must identify the most important facility-level learning outcomes. Since the DEO is a facility level educational administrator, learning efeffectiveness must be defined at the facility level. The qualitative findings could also be expanded through the application of more detailed qualitative methods, such as indepth interviews. The tasks rated by DEOs were based on OAA’s current definition of the DEO role.6 As the field advances, DEO tasks will also evolve.22-24
Conclusions
The DEO is a senior educational leadership role that oversees all health professions training in the VA. Our findings are supportive of individuals from various health disciplines serving in the VA DEO role with responsibilities that span multiple health profession training programs. We recommend further work to validate the instrument used in this study, as well as the application of qualitative methods like indepth interviews to further our understanding of the DEO role.
1. US Department of Veterans Affairs, Veterans Health Administration. Updated April 18, 2022. Accessed May 6, 2022. https://www.va.gov/health/aboutvha.asp
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education: academic Year 2019-2020. Published 2020. Accessed May 6, 2022. https://www.va.gov/OAA/docs /OAA_Statistics_2020.pdf
3. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Advanced Fellowships and Professional Development. Updated November 26, 2021. Accessed May 6, 2022. https://www.va.gov/oaa /advancedfellowships/advanced-fellowships.asp
4. Association of American Medical Colleges. Medical school graduation questionnaire, 2019 all schools summary report. Published July 2019. Accessed May 6, 2022. https://www.aamc.org/system/files/2019-08/2019-gq-all-schools -summary-report.pdf
5. US Department of Veterans Affairs, National Center for Organization Development. VA all employee survey. Published 2019. Accessed May 6, 2022. https://www.va.gov /NCOD/VAworkforcesurveys.asp
6. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Education leaders in the VA: the role of the designated education officer (DEO). Published December 2019. Accessed May 6, 2022. https://www.va.gov/OAA/docs/DEO_Learning _Leader_2019.pdf
7. US Office of Personnel Management. Policy, data oversight: guide to senior executive service qualifications. Published 2010. Accessed May 6, 2022. https://www.opm .gov/policy-data-oversight/senior-executive-service /executive-core-qualifications/
8. US Department of Veterans Affairs, Office of Research and Development. Program guide: 1200.21 VHA operations activities that may constitute research. Published January 9, 2019. Accessed May 6, 2022. https://www.research .va.gov/resources/policies/ProgramGuide-1200-21-VHA -Operations-Activities.pdf
9. Riesenberg LA, Rosenbaum PF, Stick SL. Competencies, essential training, and resources viewed by designated institutional officials as important to the position in graduate medical education [published correction appears in Acad Med. 2006 Dec;81(12):1025]. Acad Med. 2006;81(5):426- 431. doi:10.1097/01.ACM.0000222279.28824.f5
10. Palinkas LA, Mendon SJ, Hamilton AB. Inn o v a t i o n s i n M i x e d M e t h o d s E v a l u a - tions. Annu Rev Public Health. 2019;40:423-442. doi:10.1146/annurev-publhealth-040218-044215
11. Tashakkori A, Creswell JW. Exploring the nature of research questions in mixed methods research. J Mix Methods Res. 2007;1(3):207-211. doi:10.1177/1558689807302814
12. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866. doi:10.1177/104973230201200611
13. Hamilton AB, Finley EP. Qualitative methods in implementation research: An introduction. Psychiatry Res. 2019;280:112516.
14. Bellini L, Hartmann D, Opas L. Beyond must: supporting the evolving role of the designated institutional official. J Grad Med Educ. 2010;2(2):147-150. doi:10.4300/JGME-D-10-00073.1
15. Riesenberg LA, Rosenbaum P, Stick SL. Characteristics, roles, and responsibilities of the Designated Institutional Official (DIO) position in graduate medical education education [published correction appears in Acad Med. 2006 Dec;81(12):1025] [published correction appears in Acad Med. 2006 Mar;81(3):274]. Acad Med. 2006;81(1):8-19. doi:10.1097/00001888-200601000-00005
16. Group on Resident Affairs Core Competency Task Force. Institutional GME leadership competencies. 2015. Accessed May 6, 2022. https://www.aamc.org/system /files/c/2/441248-institutionalgmeleadershipcompetencies .pdf
17. Weiss KB, Bagian JP, Nasca TJ. The clinical learning environment: the foundation of graduate medical education. JAMA. 2013;309(16):1687-1688. doi:10.1001/jama.2013.1931
18. Beliveau ME, Warnes CA, Harrington RA, et al. Organizational change, leadership, and the transformation of continuing professional development: lessons learned from the American College of Cardiology. J Contin Educ Health Prof. 2015;35(3):201-210. doi:10.1002/chp.21301
19. World Health Organization. Framework for Action on Interprofessional Education and Collaborative Practice. Published September 1, 2020. Accessed May 10, 2022. https://www.who.int/publications/i/item/framework -for-action-on-interprofessional-education-collaborative -practice
20. Weiss K, Passiment M, Riordan L, Wagner R for the National Collaborative for Improving the Clinical Learning Environment IP-CLE Report Work Group. Achieving the optimal interprofessional clinical learning environment: proceedings from an NCICLE symposium. Published January 18, 2019. Accessed May 6, 2022. doi:10.33385/NCICLE.0002
21. Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211-217. Published 2016 May 4. doi:10.2147/JMDH.S104807
22. Gilman SC, Chokshi DA, Bowen JL, Rugen KW, Cox M. Connecting the dots: interprofessional health education and delivery system redesign at the Veterans Health Administration. Acad Med. 2014;89(8):1113-1116. doi:10.1097/ACM.0000000000000312
23. Health Professions Accreditors Collaborative. Guidance on developing quality interprofessional education for the health professions. Published February 1, 2019. Accessed May 6, 2022. https://healthprofessionsaccreditors.org/wp -content/uploads/2019/02/HPACGuidance02-01-19.pdf
24. Watts BV, Paull DE, Williams LC, Neily J, Hemphill RR, Brannen JL. Department of Veterans Affairs Chief Resident in Quality and Patient Safety Program: a model to spread change. Am J Med Qual. 2016;31(6):598-600. doi:10.1177/1062860616643403
The US Department of Veterans Affairs (VA) operates the largest integrated health care system in the United States, providing physical and mental health care to more than 9 million veterans enrolled each year through a national system of inpatient, outpatient, and long-term care settings.1 As 1 of 4 statutory missions, the VA conducts the largest training effort for health professionals in cooperation with affiliated academic institutions. From 2016 through 2020, an average of 123,000 trainees from various professions received training at the VA.2 Physician residents comprised the largest trainee group (37%), followed by associated health students and residents (20%), and nursing professionals (21%).2 In VA, associated health professions include all health care disciplines other than allopathic and osteopathic medicine, dentistry, and nursing. The associated health professions encompass about 40 specialties, including audiology, dietetics, physical and occupational therapy, optometry, pharmacy, podiatry, psychology, and social work.
The VA also trains a smaller number of advanced fellows to address specialties important to the nation and veterans health that are not sufficiently addressed by standard accredited professional training.3 The VA Advanced Fellowship programs include 22 postresidency, postdoctoral, and postmasters fellowships to physicians and dentists, and associated health professions, including psychologists, social workers, and pharmacists. 3 From 2015 to 2019, 57 to 61% of medical school students reported having a VA clinical training experience during medical school.4 Of current VA employees, 20% of registered nurses, 64% of physicians, 73% of podiatrists and optometrists, and 81% of psychologists reported VA training prior to employment.5
Health professions education is led by the designated education officer (DEO) at each VA facility.6 Also known as the associate chief of staff for education (ACOS/E), the DEO is a leadership position that is accountable to local VA facility executive leadership as well as the national Office of Academic Affiliations (OAA), which directs all VA health professions training across the US.6 At most VA facilities, the DEO oversees clinical training and education reporting directly to the facility chief of staff. At the same time, the ACOS/E is accountable to the OAA to ensure adherence with national education directives and policy. The DEO oversees trainee programs through collaboration with training program directors, faculty, academic affiliates, and accreditation agencies across > 40 health professions.
The DEO is expected to possess expertise in leadership attributes identified by the US Office of Personnel Management as essential to build a federal corporate culture that drives results, serves customers, and builds successful teams and coalitions within and outside the VA.7 These leadership attributes include leading change, leading people, driving results, business acumen, and building coalitions.7 They are operationalized by OAA as 4 domains of expertise required to lead education across multiple professions, including: (1) creating and sustaining an organizational work environment that supports learning, discovery, and continuous improvement; (2) aligning and managing fiscal, human, and capital resources to meet organizational learning needs; (3) driving learning and performance results to impact organizational success; and (4) leading change and transformation through positioning and implementing innovative learning and education strategies (Table 1).6
In this article we describe the VA DEO leadership role and the tasks required to lead education across multiple professions within the VA health care system. Given the broad scope of leading educational programs across multiple clinical professions and the interprofessional backgrounds of DEOs across the VA, we evaluated DEO self-perceived effectiveness to impact educational decisions and behavior by professional discipline. Our evaluation question is: Are different professional education and practice backgrounds functionally capable of providing leadership over all education of health professions training programs? Finally, we describe DEOs perceptions of facilitators and barriers to performing their DEO role within the VA.
Methods
We conducted a mixed methods analysis of data collected by OAA to assess DEO needs within a multiprofessional clinical learning environment. The needs assessment was conducted by an OAA evaluator (NH) with input on instrument development and data analysis from OAA leadership (KS, MB). This evaluation is categorized as an operations activity based on VA Handbook 1200 where information generated is used for business operations and quality improvement. 8 The overall project was subject to administrative rather than institutional review board oversight.
A needs assessment tool was developed based on the OAA domains of expertise.6 Prior to its administration, the tool was piloted with 8 DEOs in the field and the survey shortened based on their feedback. DEOs were asked about individual professional characteristics (eg, clinical profession, academic appointment, type of health professions training programs at the VA site) and their self-perceived effectiveness in impacting educational decisions and behaviors on general and profession-specific tasks within each of the 4 domains of expertise on a 5-point Likert scale (1, not effective; 5, very effective). 6,9 The needs assessment also included an open-ended question asking respondents to comment on any issues they felt important to understanding DEO role effectiveness.
The needs assessment was administered online via SurveyMonkey to 132 DEOs via email in September and October 2019. The DEOs represented 148 of 160 VA facilities with health professions education; 14 DEOs covered > 1 VA facility, and 12 positions were vacant. Email reminders were sent to nonresponders after 1 week. At 2 weeks, nonresponders received telephone reminders and personalized follow-up emails from OAA staff. The response rate at the end of 3 weeks was 96%.
Data Analysis
Mixed methods analyses included quantitative analyses to identify differences in general and profession-specific self-ratings of effectiveness in influencing educational decisions and behaviors by DEO profession, and qualitative analyses to further understand DEO’s perceptions of facilitators and barriers to DEO task effectiveness.10,11 Quantitative analyses included descriptive statistics for all variables followed by nonparametric tests including χ2 and Mann- Whitney U tests to assess differences between physician and other professional DEOs in descriptive characteristics and selfperceived effectiveness on general and profession- specific tasks. Quantitative analyses were conducted using SPSS software, version 26. Qualitative analyses consisted of rapid assessment procedures to identify facilitators and barriers to DEO effectiveness by profession using Atlas.ti version 8, which involved reviewing responses to the open-ended question and assigning each response to predetermined categories based on the organizational level it applied to (eg, individual DEO, VA facility, or external to the organization).12,13 Responses within categories were then summarized to identify the main themes.
Results
Completed surveys were received from 127 respondents representing 139 VA facilities. Eighty percent were physicians and 20% were other professionals, including psychologists, pharmacists, dentists, dieticians, nurses, and nonclinicians. There were no statistically significant differences between physician and other professional DEOs in the percent working full time or length of time spent working in the position. About one-third of the sample had been in the position for < 2 years, one-third had been in the position for 2 to < 5 years, and one-third had been in the role for ≥ 5 years. Eighty percent reported having a faculty appointment with an academic affiliate. While 92% of physician DEOs had a faculty appointment, only 40% of other professional DEOs did (P < .001). Most faculty appointments for both groups were with a school of medicine. More physician DEOs than other professionals had training programs at their site for physicians (P = .003) and dentists (P < .001), but there were no statistically significant differences for having associated health, nursing, or advanced fellowship training programs at their sites. Across all DEOs, 98% reported training programs at their site for associated health professions, 95% for physician training, 93% for nursing training, 59% for dental training, and 48% for advanced fellowships.
Self-Perceived Effectiveness
There were no statistically significant differences between physician and other professional DEOs on self-perceived effectiveness in impacting educational decisions or behaviors for general tasks applicable across professions (Table 2). This result held even after controlling for length of time in the position and whether the DEO had an academic appointment. Generally, both groups reported being effective on tasks in the enabling learning domain, including applying policies and procedures related to trainees who rotate through the VA and maintaining adherence with accreditation agency standards across health professions. Mean score ranges for both physician and other professional DEOs reported moderate effectiveness in aligning resources effectiveness questions (2.45-3.72 vs 2.75-3.76), driving results questions (3.02-3.60 vs 3.39-3.48), and leading change questions (3.12-3.50 vs 3.42-3.80).
For profession-specific tasks, effectiveness ratings between the 2 groups were generally not statistically significant for medical, dental, and advanced fellowship training programs (Table 3). There was a pattern of statistically significant differences between physician and other professional DEOs for associated health and nursing training programs on tasks across the 4 domains of expertise with physicians having lower mean ratings compared with other professionals. Generally, physician DEOs had higher task effectiveness when compared with other professionals for medical training programs, and other professionals had higher task effectiveness ratings than did physicians for associated health or nursing training programs.
Facilitators and Barriers
Seventy responses related to facilitators and barriers to DEO effectiveness were received (59 from physicians and 11 from other professionals). Most responses were categorized as individual level facilitators or barriers (53% for physician and 64% for other professionals). Only 3% of comments were categorized as external to the organization (all made by physicians). The themes were similar for both groups and were aggregated in Table 4. Facilitators included continuing education, having a mentor who works at a similar type of facility, maintaining balance and time management when working with different training programs, learning to work and develop relationships with training program directors, developing an overall picture of each type of health professions training program, holding regular meetings with all health training programs and academic affiliates, having a formal education service line with budget and staffing, facility executive leadership who are knowledgeable of the education mission and DEO role, having a national oversight body, and the DEO’s relationships with academic affiliates.
Barriers to role effectiveness at the individual DEO level included assignment of multiple roles and a focus on regulation and monitoring with little time for development of new programs and strategic planning. The organizational level barriers included difficulty getting core services to engage with health professions trainees and siloed education leadership.
Discussion
DEOs oversee multiple health professions training programs within local facilities. The DEO is accountable to local VA facility leadership and a national education office to lead local health professions education at local facilities and integrate these educational activities across the national VA system.
The VA DEO role is similar to the Accreditation Council for Graduate Medical Education designated institutional official (DIO) except that the VA DEO provides oversight of > 40 health professions training programs.14,15 The VA DEO, therefore, has broader oversight than the DIO role that focuses only on graduate physician education. Similar to the DIO, the VA DEO role initially emphasized the enabling learning and aligning resources domains to provide oversight and administration of health professions training programs. Over time, both roles have expanded to include defining and ensuring healthy clinical learning environments, aligning educational resources and training with the institutional mission, workforce, and societal needs, and creating continuous educational improvement models.6,16,17 To accomplish these expanded goals, both the DEO and the DIO work closely with other educational leaders at the academic affiliate and the VA facility. As health professions education advances, there will be increased emphasis placed on delivering educational programs to improve clinical practice and health care outcomes.18
Our findings that DEO profession did not influence self-ratings of effectiveness to influence educational decisions or behaviors on general tasks applicable across health professions suggest that education and practice background are not factors influencing selfratings. Nor were self-ratings influenced by other factors. Since the DEO is a senior leadership position, candidates for the position already may possess managerial and leadership skills. In our sample, several individuals commented that they had prior education leadership positions, eg, training program director or had years of experience working in the VA. Similarly, having an academic appointment may not be important for the performance of general administrative tasks. However, an academic appointment may be important for effective performance of educational tasks, such as clinical teaching, didactic training, and curriculum development, which were not measured in this study.
The finding of differences in self-ratings between physicians and other professionals on profession-specific tasks for associated health and nursing suggests that physicians may require additional curriculum to enhance their knowledge in managing other professional educational programs. For nursing specifically, this finding could also reflect substantial input from the lead nurse executive in the facility. DEOs also identified practical ways to facilitate their work with multiple health professions that could immediately be put into practice, including developing relationships and enhancing communication with training program directors, faculty, and academic affiliates of each profession.
Taken together, the quantitative and qualitative findings indicate that despite differences in professional backgrounds, DEOs have high self-ratings of their own effectiveness to influence educational decisions and behaviors on general tasks they are expected to accomplish. There are some professionspecific tasks where professional background does influence self-perceived effectiveness, ie, physicians have higher self-ratings on physician-specific tasks and other professionals have higher self-ratings on associated health or nursing tasks. These perceived differences may be mitigated by increasing facilitators and decreasing barriers identified for the individual DEO, within the organization, and external to the organization.
Limitations Our findings should be interpreted with the following limitations in mind. The selfreport nature of the data opens the possibility of self-report bias or Dunning-Kruger effects where effectiveness ratings could have been overestimated by respondents.21 Although respondents were assured of their anonymity and that results would only be reported in the aggregate, there is potential for providing more positive responses on a needs assessment administered by the national education program office. We recommend further work be conducted to validate the needs assessment tool against other data collection methods, such as actual outcomes of educational effectiveness. Our study did not incorporate measures of educational effectiveness to determine whether self-perceived DEO effectiveness is translated to better trainee or learning outcomes. Before this can happen, educational policymakers must identify the most important facility-level learning outcomes. Since the DEO is a facility level educational administrator, learning efeffectiveness must be defined at the facility level. The qualitative findings could also be expanded through the application of more detailed qualitative methods, such as indepth interviews. The tasks rated by DEOs were based on OAA’s current definition of the DEO role.6 As the field advances, DEO tasks will also evolve.22-24
Conclusions
The DEO is a senior educational leadership role that oversees all health professions training in the VA. Our findings are supportive of individuals from various health disciplines serving in the VA DEO role with responsibilities that span multiple health profession training programs. We recommend further work to validate the instrument used in this study, as well as the application of qualitative methods like indepth interviews to further our understanding of the DEO role.
The US Department of Veterans Affairs (VA) operates the largest integrated health care system in the United States, providing physical and mental health care to more than 9 million veterans enrolled each year through a national system of inpatient, outpatient, and long-term care settings.1 As 1 of 4 statutory missions, the VA conducts the largest training effort for health professionals in cooperation with affiliated academic institutions. From 2016 through 2020, an average of 123,000 trainees from various professions received training at the VA.2 Physician residents comprised the largest trainee group (37%), followed by associated health students and residents (20%), and nursing professionals (21%).2 In VA, associated health professions include all health care disciplines other than allopathic and osteopathic medicine, dentistry, and nursing. The associated health professions encompass about 40 specialties, including audiology, dietetics, physical and occupational therapy, optometry, pharmacy, podiatry, psychology, and social work.
The VA also trains a smaller number of advanced fellows to address specialties important to the nation and veterans health that are not sufficiently addressed by standard accredited professional training.3 The VA Advanced Fellowship programs include 22 postresidency, postdoctoral, and postmasters fellowships to physicians and dentists, and associated health professions, including psychologists, social workers, and pharmacists. 3 From 2015 to 2019, 57 to 61% of medical school students reported having a VA clinical training experience during medical school.4 Of current VA employees, 20% of registered nurses, 64% of physicians, 73% of podiatrists and optometrists, and 81% of psychologists reported VA training prior to employment.5
Health professions education is led by the designated education officer (DEO) at each VA facility.6 Also known as the associate chief of staff for education (ACOS/E), the DEO is a leadership position that is accountable to local VA facility executive leadership as well as the national Office of Academic Affiliations (OAA), which directs all VA health professions training across the US.6 At most VA facilities, the DEO oversees clinical training and education reporting directly to the facility chief of staff. At the same time, the ACOS/E is accountable to the OAA to ensure adherence with national education directives and policy. The DEO oversees trainee programs through collaboration with training program directors, faculty, academic affiliates, and accreditation agencies across > 40 health professions.
The DEO is expected to possess expertise in leadership attributes identified by the US Office of Personnel Management as essential to build a federal corporate culture that drives results, serves customers, and builds successful teams and coalitions within and outside the VA.7 These leadership attributes include leading change, leading people, driving results, business acumen, and building coalitions.7 They are operationalized by OAA as 4 domains of expertise required to lead education across multiple professions, including: (1) creating and sustaining an organizational work environment that supports learning, discovery, and continuous improvement; (2) aligning and managing fiscal, human, and capital resources to meet organizational learning needs; (3) driving learning and performance results to impact organizational success; and (4) leading change and transformation through positioning and implementing innovative learning and education strategies (Table 1).6
In this article we describe the VA DEO leadership role and the tasks required to lead education across multiple professions within the VA health care system. Given the broad scope of leading educational programs across multiple clinical professions and the interprofessional backgrounds of DEOs across the VA, we evaluated DEO self-perceived effectiveness to impact educational decisions and behavior by professional discipline. Our evaluation question is: Are different professional education and practice backgrounds functionally capable of providing leadership over all education of health professions training programs? Finally, we describe DEOs perceptions of facilitators and barriers to performing their DEO role within the VA.
Methods
We conducted a mixed methods analysis of data collected by OAA to assess DEO needs within a multiprofessional clinical learning environment. The needs assessment was conducted by an OAA evaluator (NH) with input on instrument development and data analysis from OAA leadership (KS, MB). This evaluation is categorized as an operations activity based on VA Handbook 1200 where information generated is used for business operations and quality improvement. 8 The overall project was subject to administrative rather than institutional review board oversight.
A needs assessment tool was developed based on the OAA domains of expertise.6 Prior to its administration, the tool was piloted with 8 DEOs in the field and the survey shortened based on their feedback. DEOs were asked about individual professional characteristics (eg, clinical profession, academic appointment, type of health professions training programs at the VA site) and their self-perceived effectiveness in impacting educational decisions and behaviors on general and profession-specific tasks within each of the 4 domains of expertise on a 5-point Likert scale (1, not effective; 5, very effective). 6,9 The needs assessment also included an open-ended question asking respondents to comment on any issues they felt important to understanding DEO role effectiveness.
The needs assessment was administered online via SurveyMonkey to 132 DEOs via email in September and October 2019. The DEOs represented 148 of 160 VA facilities with health professions education; 14 DEOs covered > 1 VA facility, and 12 positions were vacant. Email reminders were sent to nonresponders after 1 week. At 2 weeks, nonresponders received telephone reminders and personalized follow-up emails from OAA staff. The response rate at the end of 3 weeks was 96%.
Data Analysis
Mixed methods analyses included quantitative analyses to identify differences in general and profession-specific self-ratings of effectiveness in influencing educational decisions and behaviors by DEO profession, and qualitative analyses to further understand DEO’s perceptions of facilitators and barriers to DEO task effectiveness.10,11 Quantitative analyses included descriptive statistics for all variables followed by nonparametric tests including χ2 and Mann- Whitney U tests to assess differences between physician and other professional DEOs in descriptive characteristics and selfperceived effectiveness on general and profession- specific tasks. Quantitative analyses were conducted using SPSS software, version 26. Qualitative analyses consisted of rapid assessment procedures to identify facilitators and barriers to DEO effectiveness by profession using Atlas.ti version 8, which involved reviewing responses to the open-ended question and assigning each response to predetermined categories based on the organizational level it applied to (eg, individual DEO, VA facility, or external to the organization).12,13 Responses within categories were then summarized to identify the main themes.
Results
Completed surveys were received from 127 respondents representing 139 VA facilities. Eighty percent were physicians and 20% were other professionals, including psychologists, pharmacists, dentists, dieticians, nurses, and nonclinicians. There were no statistically significant differences between physician and other professional DEOs in the percent working full time or length of time spent working in the position. About one-third of the sample had been in the position for < 2 years, one-third had been in the position for 2 to < 5 years, and one-third had been in the role for ≥ 5 years. Eighty percent reported having a faculty appointment with an academic affiliate. While 92% of physician DEOs had a faculty appointment, only 40% of other professional DEOs did (P < .001). Most faculty appointments for both groups were with a school of medicine. More physician DEOs than other professionals had training programs at their site for physicians (P = .003) and dentists (P < .001), but there were no statistically significant differences for having associated health, nursing, or advanced fellowship training programs at their sites. Across all DEOs, 98% reported training programs at their site for associated health professions, 95% for physician training, 93% for nursing training, 59% for dental training, and 48% for advanced fellowships.
Self-Perceived Effectiveness
There were no statistically significant differences between physician and other professional DEOs on self-perceived effectiveness in impacting educational decisions or behaviors for general tasks applicable across professions (Table 2). This result held even after controlling for length of time in the position and whether the DEO had an academic appointment. Generally, both groups reported being effective on tasks in the enabling learning domain, including applying policies and procedures related to trainees who rotate through the VA and maintaining adherence with accreditation agency standards across health professions. Mean score ranges for both physician and other professional DEOs reported moderate effectiveness in aligning resources effectiveness questions (2.45-3.72 vs 2.75-3.76), driving results questions (3.02-3.60 vs 3.39-3.48), and leading change questions (3.12-3.50 vs 3.42-3.80).
For profession-specific tasks, effectiveness ratings between the 2 groups were generally not statistically significant for medical, dental, and advanced fellowship training programs (Table 3). There was a pattern of statistically significant differences between physician and other professional DEOs for associated health and nursing training programs on tasks across the 4 domains of expertise with physicians having lower mean ratings compared with other professionals. Generally, physician DEOs had higher task effectiveness when compared with other professionals for medical training programs, and other professionals had higher task effectiveness ratings than did physicians for associated health or nursing training programs.
Facilitators and Barriers
Seventy responses related to facilitators and barriers to DEO effectiveness were received (59 from physicians and 11 from other professionals). Most responses were categorized as individual level facilitators or barriers (53% for physician and 64% for other professionals). Only 3% of comments were categorized as external to the organization (all made by physicians). The themes were similar for both groups and were aggregated in Table 4. Facilitators included continuing education, having a mentor who works at a similar type of facility, maintaining balance and time management when working with different training programs, learning to work and develop relationships with training program directors, developing an overall picture of each type of health professions training program, holding regular meetings with all health training programs and academic affiliates, having a formal education service line with budget and staffing, facility executive leadership who are knowledgeable of the education mission and DEO role, having a national oversight body, and the DEO’s relationships with academic affiliates.
Barriers to role effectiveness at the individual DEO level included assignment of multiple roles and a focus on regulation and monitoring with little time for development of new programs and strategic planning. The organizational level barriers included difficulty getting core services to engage with health professions trainees and siloed education leadership.
Discussion
DEOs oversee multiple health professions training programs within local facilities. The DEO is accountable to local VA facility leadership and a national education office to lead local health professions education at local facilities and integrate these educational activities across the national VA system.
The VA DEO role is similar to the Accreditation Council for Graduate Medical Education designated institutional official (DIO) except that the VA DEO provides oversight of > 40 health professions training programs.14,15 The VA DEO, therefore, has broader oversight than the DIO role that focuses only on graduate physician education. Similar to the DIO, the VA DEO role initially emphasized the enabling learning and aligning resources domains to provide oversight and administration of health professions training programs. Over time, both roles have expanded to include defining and ensuring healthy clinical learning environments, aligning educational resources and training with the institutional mission, workforce, and societal needs, and creating continuous educational improvement models.6,16,17 To accomplish these expanded goals, both the DEO and the DIO work closely with other educational leaders at the academic affiliate and the VA facility. As health professions education advances, there will be increased emphasis placed on delivering educational programs to improve clinical practice and health care outcomes.18
Our findings that DEO profession did not influence self-ratings of effectiveness to influence educational decisions or behaviors on general tasks applicable across health professions suggest that education and practice background are not factors influencing selfratings. Nor were self-ratings influenced by other factors. Since the DEO is a senior leadership position, candidates for the position already may possess managerial and leadership skills. In our sample, several individuals commented that they had prior education leadership positions, eg, training program director or had years of experience working in the VA. Similarly, having an academic appointment may not be important for the performance of general administrative tasks. However, an academic appointment may be important for effective performance of educational tasks, such as clinical teaching, didactic training, and curriculum development, which were not measured in this study.
The finding of differences in self-ratings between physicians and other professionals on profession-specific tasks for associated health and nursing suggests that physicians may require additional curriculum to enhance their knowledge in managing other professional educational programs. For nursing specifically, this finding could also reflect substantial input from the lead nurse executive in the facility. DEOs also identified practical ways to facilitate their work with multiple health professions that could immediately be put into practice, including developing relationships and enhancing communication with training program directors, faculty, and academic affiliates of each profession.
Taken together, the quantitative and qualitative findings indicate that despite differences in professional backgrounds, DEOs have high self-ratings of their own effectiveness to influence educational decisions and behaviors on general tasks they are expected to accomplish. There are some professionspecific tasks where professional background does influence self-perceived effectiveness, ie, physicians have higher self-ratings on physician-specific tasks and other professionals have higher self-ratings on associated health or nursing tasks. These perceived differences may be mitigated by increasing facilitators and decreasing barriers identified for the individual DEO, within the organization, and external to the organization.
Limitations Our findings should be interpreted with the following limitations in mind. The selfreport nature of the data opens the possibility of self-report bias or Dunning-Kruger effects where effectiveness ratings could have been overestimated by respondents.21 Although respondents were assured of their anonymity and that results would only be reported in the aggregate, there is potential for providing more positive responses on a needs assessment administered by the national education program office. We recommend further work be conducted to validate the needs assessment tool against other data collection methods, such as actual outcomes of educational effectiveness. Our study did not incorporate measures of educational effectiveness to determine whether self-perceived DEO effectiveness is translated to better trainee or learning outcomes. Before this can happen, educational policymakers must identify the most important facility-level learning outcomes. Since the DEO is a facility level educational administrator, learning efeffectiveness must be defined at the facility level. The qualitative findings could also be expanded through the application of more detailed qualitative methods, such as indepth interviews. The tasks rated by DEOs were based on OAA’s current definition of the DEO role.6 As the field advances, DEO tasks will also evolve.22-24
Conclusions
The DEO is a senior educational leadership role that oversees all health professions training in the VA. Our findings are supportive of individuals from various health disciplines serving in the VA DEO role with responsibilities that span multiple health profession training programs. We recommend further work to validate the instrument used in this study, as well as the application of qualitative methods like indepth interviews to further our understanding of the DEO role.
1. US Department of Veterans Affairs, Veterans Health Administration. Updated April 18, 2022. Accessed May 6, 2022. https://www.va.gov/health/aboutvha.asp
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education: academic Year 2019-2020. Published 2020. Accessed May 6, 2022. https://www.va.gov/OAA/docs /OAA_Statistics_2020.pdf
3. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Advanced Fellowships and Professional Development. Updated November 26, 2021. Accessed May 6, 2022. https://www.va.gov/oaa /advancedfellowships/advanced-fellowships.asp
4. Association of American Medical Colleges. Medical school graduation questionnaire, 2019 all schools summary report. Published July 2019. Accessed May 6, 2022. https://www.aamc.org/system/files/2019-08/2019-gq-all-schools -summary-report.pdf
5. US Department of Veterans Affairs, National Center for Organization Development. VA all employee survey. Published 2019. Accessed May 6, 2022. https://www.va.gov /NCOD/VAworkforcesurveys.asp
6. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Education leaders in the VA: the role of the designated education officer (DEO). Published December 2019. Accessed May 6, 2022. https://www.va.gov/OAA/docs/DEO_Learning _Leader_2019.pdf
7. US Office of Personnel Management. Policy, data oversight: guide to senior executive service qualifications. Published 2010. Accessed May 6, 2022. https://www.opm .gov/policy-data-oversight/senior-executive-service /executive-core-qualifications/
8. US Department of Veterans Affairs, Office of Research and Development. Program guide: 1200.21 VHA operations activities that may constitute research. Published January 9, 2019. Accessed May 6, 2022. https://www.research .va.gov/resources/policies/ProgramGuide-1200-21-VHA -Operations-Activities.pdf
9. Riesenberg LA, Rosenbaum PF, Stick SL. Competencies, essential training, and resources viewed by designated institutional officials as important to the position in graduate medical education [published correction appears in Acad Med. 2006 Dec;81(12):1025]. Acad Med. 2006;81(5):426- 431. doi:10.1097/01.ACM.0000222279.28824.f5
10. Palinkas LA, Mendon SJ, Hamilton AB. Inn o v a t i o n s i n M i x e d M e t h o d s E v a l u a - tions. Annu Rev Public Health. 2019;40:423-442. doi:10.1146/annurev-publhealth-040218-044215
11. Tashakkori A, Creswell JW. Exploring the nature of research questions in mixed methods research. J Mix Methods Res. 2007;1(3):207-211. doi:10.1177/1558689807302814
12. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866. doi:10.1177/104973230201200611
13. Hamilton AB, Finley EP. Qualitative methods in implementation research: An introduction. Psychiatry Res. 2019;280:112516.
14. Bellini L, Hartmann D, Opas L. Beyond must: supporting the evolving role of the designated institutional official. J Grad Med Educ. 2010;2(2):147-150. doi:10.4300/JGME-D-10-00073.1
15. Riesenberg LA, Rosenbaum P, Stick SL. Characteristics, roles, and responsibilities of the Designated Institutional Official (DIO) position in graduate medical education education [published correction appears in Acad Med. 2006 Dec;81(12):1025] [published correction appears in Acad Med. 2006 Mar;81(3):274]. Acad Med. 2006;81(1):8-19. doi:10.1097/00001888-200601000-00005
16. Group on Resident Affairs Core Competency Task Force. Institutional GME leadership competencies. 2015. Accessed May 6, 2022. https://www.aamc.org/system /files/c/2/441248-institutionalgmeleadershipcompetencies .pdf
17. Weiss KB, Bagian JP, Nasca TJ. The clinical learning environment: the foundation of graduate medical education. JAMA. 2013;309(16):1687-1688. doi:10.1001/jama.2013.1931
18. Beliveau ME, Warnes CA, Harrington RA, et al. Organizational change, leadership, and the transformation of continuing professional development: lessons learned from the American College of Cardiology. J Contin Educ Health Prof. 2015;35(3):201-210. doi:10.1002/chp.21301
19. World Health Organization. Framework for Action on Interprofessional Education and Collaborative Practice. Published September 1, 2020. Accessed May 10, 2022. https://www.who.int/publications/i/item/framework -for-action-on-interprofessional-education-collaborative -practice
20. Weiss K, Passiment M, Riordan L, Wagner R for the National Collaborative for Improving the Clinical Learning Environment IP-CLE Report Work Group. Achieving the optimal interprofessional clinical learning environment: proceedings from an NCICLE symposium. Published January 18, 2019. Accessed May 6, 2022. doi:10.33385/NCICLE.0002
21. Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211-217. Published 2016 May 4. doi:10.2147/JMDH.S104807
22. Gilman SC, Chokshi DA, Bowen JL, Rugen KW, Cox M. Connecting the dots: interprofessional health education and delivery system redesign at the Veterans Health Administration. Acad Med. 2014;89(8):1113-1116. doi:10.1097/ACM.0000000000000312
23. Health Professions Accreditors Collaborative. Guidance on developing quality interprofessional education for the health professions. Published February 1, 2019. Accessed May 6, 2022. https://healthprofessionsaccreditors.org/wp -content/uploads/2019/02/HPACGuidance02-01-19.pdf
24. Watts BV, Paull DE, Williams LC, Neily J, Hemphill RR, Brannen JL. Department of Veterans Affairs Chief Resident in Quality and Patient Safety Program: a model to spread change. Am J Med Qual. 2016;31(6):598-600. doi:10.1177/1062860616643403
1. US Department of Veterans Affairs, Veterans Health Administration. Updated April 18, 2022. Accessed May 6, 2022. https://www.va.gov/health/aboutvha.asp
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education: academic Year 2019-2020. Published 2020. Accessed May 6, 2022. https://www.va.gov/OAA/docs /OAA_Statistics_2020.pdf
3. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Advanced Fellowships and Professional Development. Updated November 26, 2021. Accessed May 6, 2022. https://www.va.gov/oaa /advancedfellowships/advanced-fellowships.asp
4. Association of American Medical Colleges. Medical school graduation questionnaire, 2019 all schools summary report. Published July 2019. Accessed May 6, 2022. https://www.aamc.org/system/files/2019-08/2019-gq-all-schools -summary-report.pdf
5. US Department of Veterans Affairs, National Center for Organization Development. VA all employee survey. Published 2019. Accessed May 6, 2022. https://www.va.gov /NCOD/VAworkforcesurveys.asp
6. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Education leaders in the VA: the role of the designated education officer (DEO). Published December 2019. Accessed May 6, 2022. https://www.va.gov/OAA/docs/DEO_Learning _Leader_2019.pdf
7. US Office of Personnel Management. Policy, data oversight: guide to senior executive service qualifications. Published 2010. Accessed May 6, 2022. https://www.opm .gov/policy-data-oversight/senior-executive-service /executive-core-qualifications/
8. US Department of Veterans Affairs, Office of Research and Development. Program guide: 1200.21 VHA operations activities that may constitute research. Published January 9, 2019. Accessed May 6, 2022. https://www.research .va.gov/resources/policies/ProgramGuide-1200-21-VHA -Operations-Activities.pdf
9. Riesenberg LA, Rosenbaum PF, Stick SL. Competencies, essential training, and resources viewed by designated institutional officials as important to the position in graduate medical education [published correction appears in Acad Med. 2006 Dec;81(12):1025]. Acad Med. 2006;81(5):426- 431. doi:10.1097/01.ACM.0000222279.28824.f5
10. Palinkas LA, Mendon SJ, Hamilton AB. Inn o v a t i o n s i n M i x e d M e t h o d s E v a l u a - tions. Annu Rev Public Health. 2019;40:423-442. doi:10.1146/annurev-publhealth-040218-044215
11. Tashakkori A, Creswell JW. Exploring the nature of research questions in mixed methods research. J Mix Methods Res. 2007;1(3):207-211. doi:10.1177/1558689807302814
12. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866. doi:10.1177/104973230201200611
13. Hamilton AB, Finley EP. Qualitative methods in implementation research: An introduction. Psychiatry Res. 2019;280:112516.
14. Bellini L, Hartmann D, Opas L. Beyond must: supporting the evolving role of the designated institutional official. J Grad Med Educ. 2010;2(2):147-150. doi:10.4300/JGME-D-10-00073.1
15. Riesenberg LA, Rosenbaum P, Stick SL. Characteristics, roles, and responsibilities of the Designated Institutional Official (DIO) position in graduate medical education education [published correction appears in Acad Med. 2006 Dec;81(12):1025] [published correction appears in Acad Med. 2006 Mar;81(3):274]. Acad Med. 2006;81(1):8-19. doi:10.1097/00001888-200601000-00005
16. Group on Resident Affairs Core Competency Task Force. Institutional GME leadership competencies. 2015. Accessed May 6, 2022. https://www.aamc.org/system /files/c/2/441248-institutionalgmeleadershipcompetencies .pdf
17. Weiss KB, Bagian JP, Nasca TJ. The clinical learning environment: the foundation of graduate medical education. JAMA. 2013;309(16):1687-1688. doi:10.1001/jama.2013.1931
18. Beliveau ME, Warnes CA, Harrington RA, et al. Organizational change, leadership, and the transformation of continuing professional development: lessons learned from the American College of Cardiology. J Contin Educ Health Prof. 2015;35(3):201-210. doi:10.1002/chp.21301
19. World Health Organization. Framework for Action on Interprofessional Education and Collaborative Practice. Published September 1, 2020. Accessed May 10, 2022. https://www.who.int/publications/i/item/framework -for-action-on-interprofessional-education-collaborative -practice
20. Weiss K, Passiment M, Riordan L, Wagner R for the National Collaborative for Improving the Clinical Learning Environment IP-CLE Report Work Group. Achieving the optimal interprofessional clinical learning environment: proceedings from an NCICLE symposium. Published January 18, 2019. Accessed May 6, 2022. doi:10.33385/NCICLE.0002
21. Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211-217. Published 2016 May 4. doi:10.2147/JMDH.S104807
22. Gilman SC, Chokshi DA, Bowen JL, Rugen KW, Cox M. Connecting the dots: interprofessional health education and delivery system redesign at the Veterans Health Administration. Acad Med. 2014;89(8):1113-1116. doi:10.1097/ACM.0000000000000312
23. Health Professions Accreditors Collaborative. Guidance on developing quality interprofessional education for the health professions. Published February 1, 2019. Accessed May 6, 2022. https://healthprofessionsaccreditors.org/wp -content/uploads/2019/02/HPACGuidance02-01-19.pdf
24. Watts BV, Paull DE, Williams LC, Neily J, Hemphill RR, Brannen JL. Department of Veterans Affairs Chief Resident in Quality and Patient Safety Program: a model to spread change. Am J Med Qual. 2016;31(6):598-600. doi:10.1177/1062860616643403
Predictors of County-Level Home Modification Use Across the US
This article is part of a series of articles on the Home Improvements and Structural Alterations program (HISA), a home modification (HM) program within the Veterans Health Administration (VHA). HISA is a benefit awarded to veterans with disabilities (VWDs) and is instrumental in affording physical accessibility and structural alterations to veterans’ homes.1 The overarching goals of this project are to describe and understand HISA use by VWDs. Previous work has shown geographical variation in the number of HISA prescriptions across counties in the US (Figure 1).1 The current work seeks to describe and predict the county-level rates of HISA use. Information about what predicts HISA utilization at the county level is important because it enhances understanding of program utilization at a national level. The long-term goal of the series is to provide knowledge about HM services within VHA to improve community-based independent living of VWDs by increasing awareness and utilization of HM services.
Background
A health care professional (HCP) approves a HM support award by evaluating the practicality of the support to improve the built environment of a given veteran’s disability.1,2 Previously we detailed some of the preliminary research into the HISA program, including HISA user demographic and clinical characteristics, types of HMs received, user suggestions for improvement, and geospatial analysis of HISA prescriptions concentration.1-4
The geospatial analyses of HISA prescriptions revealed clusters of high numbers of HISA users (hot spots) and low numbers of HISA users (cold spots), indicating that HISA is either not prescribed or uniformly used across the US. The previous research prompted investigation into county-level variables that may impact HISA utilization rates. This inquiry focuses on county characteristics associated with HISA use rates, such as measures of clinical care and quality of care (eg, access to health services variables, lack of insurance, preventable hospital stays), physical environment, and sociodemographic characteristics. Clinical care and quality of care measures promote the interaction with HCPs. Moreover, access to health care is an important indicator of health outcomes.5,6 An individual’s capacity to access health services, such as a HM program, greatly impacts well-being, safety, independence, and health.2,4 Well-being, safety, independence, and health become compromised if individuals cannot access care, if needed care is lacking in their area, if HCPs are not available, or are unwilling to provide care due to lack of insurance coverage.7-12 In locations where health care services are minimal due to lack of specialists or health care facilities, the quality of (or access to) care may be compromised, resulting in preventable conditions becoming problematic.13,14 These conditions may result in unnecessary hospitalizations for conditions that could have been treated during routine care. Financial barriers to care particularly among low-income people and the uninsured have proven detrimental to health.15,16 On the other hand, preventable hospital stays are a quality of care measure (ie, a proxy for poor quality of care). HISA operates within a health care system; thus, it is imperative to include these measures impacting health.
In this study, we sought to identify county-level predictors—in particular, county-level proxies for access to care—that may be associated with county-level HISA use. We define HISA utilization rate as the percentage of a county’s VHA patients who have received a HISA award.
Methods
This study used data from the National Prosthetics Patient Database (NPPD), US Department of Veterans Affairs (VA) medical database inpatient and outpatient datasets, VHA Support Service Center (VSSC) data cubes, and the County Health Rankings database (CHRD). First, the study cohort was identified from NPPD users who have obtained a HISA award from fiscal years (FY) 2015 to 2018. Analysis started with FY 2015 following new regulations (38 CFR § 17) governing the operations of the HISA program.2 The study cohort was matched with records from NPPD and VA inpatient and outpatient datasets to obtain information about the veterans’ demographic characteristics and their HM characteristics and costs. The number of VHA end-of-year (EOY) patients per county was extracted from the VSSC Current Enrollment Cube, which was used in calculation of the county-level HISA utilization rate.17 Finally, zip code–based locational data were used to calculate approximate drive time and distance from the HISA user’s approximate location to the facility where they received their HM prescription. Drive times and drive distances were calculated with Esri ArcGIS Pro (v2.6.3) by placing zip code centroid and VHA facilities on a nationwide road network that contains both road speeds and distances.
Calculations
Patient-level data were aggregated up to county-level variables by calculating the sum, mean, or percent per county. HISA user sample characteristics, including sex, race, rurality (urban, rural), marital status, and Class 1 vs Class 2 disability-related eligibility groups, were aggregated to the county level by calculating percentages of HISA users of the given characteristics out of total HISA users in the county. Disability-related eligibility groups (Class 1 vs Class 2 HISA users) determines the maximum lifetime award dollar amount. Specifically, those with service-connected disabilities or those with a ≥ 50% disability rating (regardless of whether or not their disability is service connected) are classified as Class 1 HISA users and are eligible to receive a maximum lifetime award of $6800. Those with a recorded disability that is not connected to their military service, and who have a disability rating of < 50% are classified as Class 2 HISA users and are eligible to receive a lifetime maximum award of $2000.
The county-level number of HISA users was used as the numerator for calculation of county-level HISA utilization rate. Counties with zero HISA users were excluded. The number of EOY VHA patients per county in FY 2018 was divided by 1000 and used as the denominator in the calculation of county-level HISA utilization rate. Thus, the outcome variable is HISA utilization rate per 1000 VHA patients in FY 2018 (HISA utilization rate).
County-Level Variables
County-level variables were downloaded from the 2020 CHRD.5,6 An explanation of the CHRD model and the factors used in this study are shown in the eAppendix (available at doi: 10.12788/fp.0279).6 County-level aggregated HISA user data and the CHRD data were matched using county Federal Information Processing Standards codes. Access to care measures collected from CHRD included percentages uninsured and ratios of population to primary care physicians, dentists, mental health professionals, and other primary care professionals. Other CHRD measures included those for quality of care (rate of preventable hospital stay) and housing quality (percent of households with high housing costs, percent of households with overcrowding, percent of households with lack of kitchen or plumbing, percent of households with severe housing cost burden, percent of homeownership). Of secondary interest was county population rurality, as previous research findings showed the annual average of HISA users who are from rural areas ranged from 30 to 35%.
Analysis Methods
SAS (v9.4), R (v4.0.2), and RStudio (v1.3.1093) were used for data preparation and analysis.18 Multiple regression analysis was used to predict county-level utilization rate from county-level variables. Sociodemographic characteristics from CHRD and HISA data were included as important control predictors in the regression model, though our focus for this paper are the access to care and housing quality predictors.
Model diagnostics (examination of model residuals, Breusch-Godfrey test, Breusch-Pagan test) revealed significant heteroskedasticity of the model; thus, robust standard errors and associated P values were computed using the R estimatr package (v0.30.2).19 Some predictor variables of interest (eg, ratio of mental health professionals) were removed during the model building process either due to problems of multicollinearity or excessive missingness that would have resulted in listwise deletion.
Results
County-level HISA utilization rate per 1000 EOY VHA patients ranged from 0.09 to 59.7%, with a 6.6% mean and 5% median (Figure 2). The data were highly positively skewed. The final model included 33 total predictor variables (Table 1). The final regression model was a significantly better predictor of county-level HISA utilization rate than a null model (F[33-2184], 10.18; P < .001). The adjusted model R2 showed that the overall model accounted for approximately 23% of variance in county-level HISA utilization rate (Table 2).
Among the primary variables of interest, percent uninsured adults and rate of preventable hospital stays emerged as significant predictors of county-level HISA utilization rate. Specifically, county percentage of uninsured adults was negatively related to county-level HISA utilization rate (b = -8.99, P = .005), indicating that the higher the proportion of uninsured adults—with all other predictors held constant—the lower the HISA utilization rate. Percent uninsured adults ranged from 2.7 to 42.4% across counties, with a mean (SD) of 12.7% (5.8%) and 11.4% median.
County rate of preventable hospital stays, however, was significantly and positively related to county-level HISA utilization rate (b = .0004, P = .009), indicating that the higher the rate of preventable hospital stays—with all other predictors held constant—the higher the HISA utilization rate. The direction of this effect is the opposite of the direction of the effect of percent uninsured adults (positive rather than negative), even though both could be considered measures of access to care. The standardized β for these 2 predictors indicate that county rate of preventable hospital stays is a somewhat stronger predictor of county-level HISA utilization rate than is county percent of uninsured adults (β = .11 and β = -.09, respectively). Rate of preventable hospital stays ranged from 683 to 16,802 across counties included in this analysis, with a mean (SD) of 4,796.5 (1659.9) and a 4669 median.
Of secondary interest was county rurality. The county-level percentage of rural residents was significantly and positively related to county-level HISA utilization rate, indicating that the higher the proportion of individuals within county considered rural—all other predictors held constant—the higher the HISA utilization rate. The mean (SD) percentage of rural residents per county was 52.3% (30.2) and 52.7 % median.
Discussion
This study examined whether county-level characteristics, specifically variables for access to care, quality of care, and housing quality, were predictive of a county’s HISA utilization rate. Given that this series of work on the HISA program is (to our knowledge) the first of its kind, and given the exploratory nature of this analysis, we did not have specific predictions for the effects of any one given variable. Nevertheless, some of the results were surprising, and we believe they warrant additional study. In particular, the opposing direction of effects for access to care and quality of care variables were hard to reconcile.
The county percent of uninsured adults (an access to care variable, specifically, a proxy for poor access to care) was negatively associated with county-level HISA utilization rate, whereas the county rate of preventable hospital stays (a quality of care variable, but also potentially an access to care variable, and specifically, proxies for poor quality of care or poor access to care) was positively associated with county-level HISA utilization rate. To describe the relationships more generally, one coefficient in the regression model indicated that the poorer the access to care, the lower the HISA utilization rate (higher percent of uninsured adults predicts lower HISA utilization rate), while another coefficient in the regression model indicated the poorer the quality of and access to care, the higher the HISA utilization rate (higher rate of preventable hospital stays predicts higher HISA utilization rate). Future study is warranted to disentangle and reconcile the various community-level predictors of this service.
Housing quality measures (eg, percent of households with high housing costs, percent of households with overcrowding, percent of households with lack of kitchen or plumbing, percent of households with severe housing cost burden, and percent of homeownership) are important in the consideration of whether a HM will be performed or should be performed. For example, if a person is cost burdened by the amount of expenditure spent in housing there will be little discretionary funds to perform a HM. Individuals who do not own their home may experience complications in obtaining permission from landlords to perform a HM. County-level predictors of housing quality (percent of households with high housing costs, overcrowding, and lack of kitchen or plumbing) were not significantly associated with county-level HISA utilization rate but are also nevertheless relevant to the discussion of home modifications. Of particular interest is the percent of households with lack of kitchen or plumbing variable, which was positively related to county-level HISA utilization rate although not statistically significant. HM elements related to the kitchen (eg, heighten countertop) add to the accessibility of the home allowing for the performing of activities of daily living such as cooking. Between FY 2015 and FY 2018, we discovered 131 prescriptions for kitchen (n = 90) and plumbing (n = 41) HMs, which is a very small proportion of the 30,780 total HMs (there were 24,397 bathroom HMs). The nonsignificant coefficient for this variable may reflect the small number of veterans that obtained these HM.
Limitations
The potentially conflicting direction of effects for a significant access to care variable (percent uninsured adults) and a significant access to care and quality of care variable (preventable hospital stays) are interesting and warrant additional study, but the inability to interpret or explain this apparent inconsistency constitutes a limitation of the current data and analyses presented here. Another limitation is that this analysis uses county-level predictors for what is ultimately an individual-level outcome. It would have been ideal to have both individual- and county-level data to conduct a multilevel analysis; in particular, individual-level data within counties of individuals (both veterans and nonveterans) who did not receive a HISA award (including both those who applied and were denied, and who did not apply) would be highly valuable.
Conclusions
Our continuing research into veterans’ use of HM fills a gap in the literature about the characteristics of HISA users, the impact of county-level variables on the use of HISA, and the geographic distribution and use of HISA within the VHA. While it is important to examine the influence of broader systems on individual outcomes, there could be myriad other factors that are more proximal and more closely related to whether any one individual applies for, let alone receives, a HISA award. Indeed, a low overall adjusted model R2 indicates that there is considerable variability in county-level HISA utilization rate that was not accounted for by the current model; this further speaks to warranted additional study.
More research is needed to understand and account for geographical variation in HISA utilization rate across the US. However, this work serves as an exploratory first step at quantifying and predicting HISA utilization rate at a broad level, with the ultimate goal of increasing access to HMs for veterans with disabilities.
Acknowledgments
This research was supported by grant 15521 from the US Department of Veterans Affairs, Office of Rural Health. Furthermore, the research was supported in part by grant K12 HD055929 from the National Institutes of Health. We want to acknowledge Cheri E. Knecht, Project Coordinator, for her assistance throughout all aspects of our research study and for her thoughtful contributions during the writing of this manuscript.
1. Semeah LM, Ahrentzen S, Jia H, Cowper-Ripley DC, Levy CE, Mann WC. The home improvements and structural alterations benefits program: veterans with disabilities and home accessibility. J Disability Policy Studies. 2017;28(1):43-51. doi:10.1177/1044207317696275
2. Semeah LM, Wang X, Cowper Ripley DC, Lee MJ, Ahonle ZJ, Ganesh SP, et al. Improving health through a home modification service for veterans. In: Fiedler BA, ed. Three Facets of Public Health and Paths to Improvements. Academic Press; 2020:381-416.
3. Semeah LM, Ahrentzen S, Cowper-Ripley DC, Santos-Roman LM, Beamish JO, Farley K. Rental housing needs and barriers from the perspective of veterans with disabilities. Housing Policy Debate. 2019;29(4):542-558. doi:10.1080/10511482.2018.1543203
4. Semeah LM, Ganesh SP, Wang X, et al. Home modification and health services utilization by rural and urban veterans with disabilities. Housing Policy Debate. 2021;31(6):862-874.doi:10.1080/10511482.2020.1858923
5. University of Wisconsin Population Health Institute. County health rankings model. Accessed May 13, 2022. https://www.countyhealthrankings.org/about-us
6. Remington PL, Catlin BB, Gennuso KP. The County Health Rankings: rationale and methods. Popul Health Metr. 2015;13(11). doi:10.1186/s12963-015-0044-2
7. National Academies of Sciences, Engineering, and Medicine. Health-Care Utilization as a Proxy in Disability Determination. Washington, DC: The National Academies Press; 2018.
8. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129(6):611-20. doi:10.1016/j.puhe.2015.04.001
9. Medicaid and Chip Payment and Access Commission (MACPAC). Medicaid access in brief: adults’ experiences in obtaining medical care. November 2016. Accessed May 13, 2022. https://www.macpac.gov/publication/access-in-brief-adults-experiences-in-obtaining-medical-care
10. Tolbert J, Orgera, K, Damico A. Key facts about the uninsured population. November 6, 2020. Accessed May 13, 2022. https://www.kff.org/uninsured/issue-brief/key-facts-about-the-uninsured-population
11. Meit M, Knudson A, Gilbert T, et al. The 2014 update of the rural-urban chartbook, 2014. October 2014. Accessed May 13, 2022. http://www.ruralhealthresearch.org
12. National Center for Health Statistics (US). Report No.: 2016-1232. Health, United States, 2015: with special feature on racial and ethnic health disparities. Hyattsville, MD: National Center for Health Statistics.
13. Broussard DL, Mason KE, Carruth AR, Carton TW. Assessing potentially preventable hospitalizations at the county level: a comparison of measures using Medicare data and state hospital discharge data. Popul Health Manag. 2018;21(6):438-445. doi:10.1089/pop.2017.0141
14. Pezzin LE, Bogner HR, Kurichi JE, et al. Preventable hospitalizations, barriers to care, and disability. Medicine (Baltimore). 2018;97:e0691 doi:10.1097/MD.0000000000010691
15. Davis K, Ballreich J. Equitable access to care: how the United States ranks internationally. N Engl J Med. 2014;371(17):1567-70. doi:10.1056/NEJMp1406707
16. Squires D, Anderson C. U.S. health care from a global perspective: spending, use of services, prices, and health in 13 countries. Issue Brief (Commonw Fund). 2015;15:1-15.
17. VHA Service Support Center. Current enrollment cube (vssc.med.va.gov). Retrieved August 06, 2019. [Data not verified.]
18. Bunn A, Korpela M. R: A language and environment for statistical computing: an introduction to dplR. January 29, 2021. Accessed May 13, 2022. http://r.meteo.uni.wroc.pl/web/packages/dplR/vignettes/intro-dplR.pdf
19. Sheppard BH, Hartwick J, Warshaw PR. The theory of reasoned action: a meta-analysis of past research with recommendations for modifications and future research. J Consumer Research. 1988;15(3):325-343. doi:10.1086/209170
This article is part of a series of articles on the Home Improvements and Structural Alterations program (HISA), a home modification (HM) program within the Veterans Health Administration (VHA). HISA is a benefit awarded to veterans with disabilities (VWDs) and is instrumental in affording physical accessibility and structural alterations to veterans’ homes.1 The overarching goals of this project are to describe and understand HISA use by VWDs. Previous work has shown geographical variation in the number of HISA prescriptions across counties in the US (Figure 1).1 The current work seeks to describe and predict the county-level rates of HISA use. Information about what predicts HISA utilization at the county level is important because it enhances understanding of program utilization at a national level. The long-term goal of the series is to provide knowledge about HM services within VHA to improve community-based independent living of VWDs by increasing awareness and utilization of HM services.
Background
A health care professional (HCP) approves a HM support award by evaluating the practicality of the support to improve the built environment of a given veteran’s disability.1,2 Previously we detailed some of the preliminary research into the HISA program, including HISA user demographic and clinical characteristics, types of HMs received, user suggestions for improvement, and geospatial analysis of HISA prescriptions concentration.1-4
The geospatial analyses of HISA prescriptions revealed clusters of high numbers of HISA users (hot spots) and low numbers of HISA users (cold spots), indicating that HISA is either not prescribed or uniformly used across the US. The previous research prompted investigation into county-level variables that may impact HISA utilization rates. This inquiry focuses on county characteristics associated with HISA use rates, such as measures of clinical care and quality of care (eg, access to health services variables, lack of insurance, preventable hospital stays), physical environment, and sociodemographic characteristics. Clinical care and quality of care measures promote the interaction with HCPs. Moreover, access to health care is an important indicator of health outcomes.5,6 An individual’s capacity to access health services, such as a HM program, greatly impacts well-being, safety, independence, and health.2,4 Well-being, safety, independence, and health become compromised if individuals cannot access care, if needed care is lacking in their area, if HCPs are not available, or are unwilling to provide care due to lack of insurance coverage.7-12 In locations where health care services are minimal due to lack of specialists or health care facilities, the quality of (or access to) care may be compromised, resulting in preventable conditions becoming problematic.13,14 These conditions may result in unnecessary hospitalizations for conditions that could have been treated during routine care. Financial barriers to care particularly among low-income people and the uninsured have proven detrimental to health.15,16 On the other hand, preventable hospital stays are a quality of care measure (ie, a proxy for poor quality of care). HISA operates within a health care system; thus, it is imperative to include these measures impacting health.
In this study, we sought to identify county-level predictors—in particular, county-level proxies for access to care—that may be associated with county-level HISA use. We define HISA utilization rate as the percentage of a county’s VHA patients who have received a HISA award.
Methods
This study used data from the National Prosthetics Patient Database (NPPD), US Department of Veterans Affairs (VA) medical database inpatient and outpatient datasets, VHA Support Service Center (VSSC) data cubes, and the County Health Rankings database (CHRD). First, the study cohort was identified from NPPD users who have obtained a HISA award from fiscal years (FY) 2015 to 2018. Analysis started with FY 2015 following new regulations (38 CFR § 17) governing the operations of the HISA program.2 The study cohort was matched with records from NPPD and VA inpatient and outpatient datasets to obtain information about the veterans’ demographic characteristics and their HM characteristics and costs. The number of VHA end-of-year (EOY) patients per county was extracted from the VSSC Current Enrollment Cube, which was used in calculation of the county-level HISA utilization rate.17 Finally, zip code–based locational data were used to calculate approximate drive time and distance from the HISA user’s approximate location to the facility where they received their HM prescription. Drive times and drive distances were calculated with Esri ArcGIS Pro (v2.6.3) by placing zip code centroid and VHA facilities on a nationwide road network that contains both road speeds and distances.
Calculations
Patient-level data were aggregated up to county-level variables by calculating the sum, mean, or percent per county. HISA user sample characteristics, including sex, race, rurality (urban, rural), marital status, and Class 1 vs Class 2 disability-related eligibility groups, were aggregated to the county level by calculating percentages of HISA users of the given characteristics out of total HISA users in the county. Disability-related eligibility groups (Class 1 vs Class 2 HISA users) determines the maximum lifetime award dollar amount. Specifically, those with service-connected disabilities or those with a ≥ 50% disability rating (regardless of whether or not their disability is service connected) are classified as Class 1 HISA users and are eligible to receive a maximum lifetime award of $6800. Those with a recorded disability that is not connected to their military service, and who have a disability rating of < 50% are classified as Class 2 HISA users and are eligible to receive a lifetime maximum award of $2000.
The county-level number of HISA users was used as the numerator for calculation of county-level HISA utilization rate. Counties with zero HISA users were excluded. The number of EOY VHA patients per county in FY 2018 was divided by 1000 and used as the denominator in the calculation of county-level HISA utilization rate. Thus, the outcome variable is HISA utilization rate per 1000 VHA patients in FY 2018 (HISA utilization rate).
County-Level Variables
County-level variables were downloaded from the 2020 CHRD.5,6 An explanation of the CHRD model and the factors used in this study are shown in the eAppendix (available at doi: 10.12788/fp.0279).6 County-level aggregated HISA user data and the CHRD data were matched using county Federal Information Processing Standards codes. Access to care measures collected from CHRD included percentages uninsured and ratios of population to primary care physicians, dentists, mental health professionals, and other primary care professionals. Other CHRD measures included those for quality of care (rate of preventable hospital stay) and housing quality (percent of households with high housing costs, percent of households with overcrowding, percent of households with lack of kitchen or plumbing, percent of households with severe housing cost burden, percent of homeownership). Of secondary interest was county population rurality, as previous research findings showed the annual average of HISA users who are from rural areas ranged from 30 to 35%.
Analysis Methods
SAS (v9.4), R (v4.0.2), and RStudio (v1.3.1093) were used for data preparation and analysis.18 Multiple regression analysis was used to predict county-level utilization rate from county-level variables. Sociodemographic characteristics from CHRD and HISA data were included as important control predictors in the regression model, though our focus for this paper are the access to care and housing quality predictors.
Model diagnostics (examination of model residuals, Breusch-Godfrey test, Breusch-Pagan test) revealed significant heteroskedasticity of the model; thus, robust standard errors and associated P values were computed using the R estimatr package (v0.30.2).19 Some predictor variables of interest (eg, ratio of mental health professionals) were removed during the model building process either due to problems of multicollinearity or excessive missingness that would have resulted in listwise deletion.
Results
County-level HISA utilization rate per 1000 EOY VHA patients ranged from 0.09 to 59.7%, with a 6.6% mean and 5% median (Figure 2). The data were highly positively skewed. The final model included 33 total predictor variables (Table 1). The final regression model was a significantly better predictor of county-level HISA utilization rate than a null model (F[33-2184], 10.18; P < .001). The adjusted model R2 showed that the overall model accounted for approximately 23% of variance in county-level HISA utilization rate (Table 2).
Among the primary variables of interest, percent uninsured adults and rate of preventable hospital stays emerged as significant predictors of county-level HISA utilization rate. Specifically, county percentage of uninsured adults was negatively related to county-level HISA utilization rate (b = -8.99, P = .005), indicating that the higher the proportion of uninsured adults—with all other predictors held constant—the lower the HISA utilization rate. Percent uninsured adults ranged from 2.7 to 42.4% across counties, with a mean (SD) of 12.7% (5.8%) and 11.4% median.
County rate of preventable hospital stays, however, was significantly and positively related to county-level HISA utilization rate (b = .0004, P = .009), indicating that the higher the rate of preventable hospital stays—with all other predictors held constant—the higher the HISA utilization rate. The direction of this effect is the opposite of the direction of the effect of percent uninsured adults (positive rather than negative), even though both could be considered measures of access to care. The standardized β for these 2 predictors indicate that county rate of preventable hospital stays is a somewhat stronger predictor of county-level HISA utilization rate than is county percent of uninsured adults (β = .11 and β = -.09, respectively). Rate of preventable hospital stays ranged from 683 to 16,802 across counties included in this analysis, with a mean (SD) of 4,796.5 (1659.9) and a 4669 median.
Of secondary interest was county rurality. The county-level percentage of rural residents was significantly and positively related to county-level HISA utilization rate, indicating that the higher the proportion of individuals within county considered rural—all other predictors held constant—the higher the HISA utilization rate. The mean (SD) percentage of rural residents per county was 52.3% (30.2) and 52.7 % median.
Discussion
This study examined whether county-level characteristics, specifically variables for access to care, quality of care, and housing quality, were predictive of a county’s HISA utilization rate. Given that this series of work on the HISA program is (to our knowledge) the first of its kind, and given the exploratory nature of this analysis, we did not have specific predictions for the effects of any one given variable. Nevertheless, some of the results were surprising, and we believe they warrant additional study. In particular, the opposing direction of effects for access to care and quality of care variables were hard to reconcile.
The county percent of uninsured adults (an access to care variable, specifically, a proxy for poor access to care) was negatively associated with county-level HISA utilization rate, whereas the county rate of preventable hospital stays (a quality of care variable, but also potentially an access to care variable, and specifically, proxies for poor quality of care or poor access to care) was positively associated with county-level HISA utilization rate. To describe the relationships more generally, one coefficient in the regression model indicated that the poorer the access to care, the lower the HISA utilization rate (higher percent of uninsured adults predicts lower HISA utilization rate), while another coefficient in the regression model indicated the poorer the quality of and access to care, the higher the HISA utilization rate (higher rate of preventable hospital stays predicts higher HISA utilization rate). Future study is warranted to disentangle and reconcile the various community-level predictors of this service.
Housing quality measures (eg, percent of households with high housing costs, percent of households with overcrowding, percent of households with lack of kitchen or plumbing, percent of households with severe housing cost burden, and percent of homeownership) are important in the consideration of whether a HM will be performed or should be performed. For example, if a person is cost burdened by the amount of expenditure spent in housing there will be little discretionary funds to perform a HM. Individuals who do not own their home may experience complications in obtaining permission from landlords to perform a HM. County-level predictors of housing quality (percent of households with high housing costs, overcrowding, and lack of kitchen or plumbing) were not significantly associated with county-level HISA utilization rate but are also nevertheless relevant to the discussion of home modifications. Of particular interest is the percent of households with lack of kitchen or plumbing variable, which was positively related to county-level HISA utilization rate although not statistically significant. HM elements related to the kitchen (eg, heighten countertop) add to the accessibility of the home allowing for the performing of activities of daily living such as cooking. Between FY 2015 and FY 2018, we discovered 131 prescriptions for kitchen (n = 90) and plumbing (n = 41) HMs, which is a very small proportion of the 30,780 total HMs (there were 24,397 bathroom HMs). The nonsignificant coefficient for this variable may reflect the small number of veterans that obtained these HM.
Limitations
The potentially conflicting direction of effects for a significant access to care variable (percent uninsured adults) and a significant access to care and quality of care variable (preventable hospital stays) are interesting and warrant additional study, but the inability to interpret or explain this apparent inconsistency constitutes a limitation of the current data and analyses presented here. Another limitation is that this analysis uses county-level predictors for what is ultimately an individual-level outcome. It would have been ideal to have both individual- and county-level data to conduct a multilevel analysis; in particular, individual-level data within counties of individuals (both veterans and nonveterans) who did not receive a HISA award (including both those who applied and were denied, and who did not apply) would be highly valuable.
Conclusions
Our continuing research into veterans’ use of HM fills a gap in the literature about the characteristics of HISA users, the impact of county-level variables on the use of HISA, and the geographic distribution and use of HISA within the VHA. While it is important to examine the influence of broader systems on individual outcomes, there could be myriad other factors that are more proximal and more closely related to whether any one individual applies for, let alone receives, a HISA award. Indeed, a low overall adjusted model R2 indicates that there is considerable variability in county-level HISA utilization rate that was not accounted for by the current model; this further speaks to warranted additional study.
More research is needed to understand and account for geographical variation in HISA utilization rate across the US. However, this work serves as an exploratory first step at quantifying and predicting HISA utilization rate at a broad level, with the ultimate goal of increasing access to HMs for veterans with disabilities.
Acknowledgments
This research was supported by grant 15521 from the US Department of Veterans Affairs, Office of Rural Health. Furthermore, the research was supported in part by grant K12 HD055929 from the National Institutes of Health. We want to acknowledge Cheri E. Knecht, Project Coordinator, for her assistance throughout all aspects of our research study and for her thoughtful contributions during the writing of this manuscript.
This article is part of a series of articles on the Home Improvements and Structural Alterations program (HISA), a home modification (HM) program within the Veterans Health Administration (VHA). HISA is a benefit awarded to veterans with disabilities (VWDs) and is instrumental in affording physical accessibility and structural alterations to veterans’ homes.1 The overarching goals of this project are to describe and understand HISA use by VWDs. Previous work has shown geographical variation in the number of HISA prescriptions across counties in the US (Figure 1).1 The current work seeks to describe and predict the county-level rates of HISA use. Information about what predicts HISA utilization at the county level is important because it enhances understanding of program utilization at a national level. The long-term goal of the series is to provide knowledge about HM services within VHA to improve community-based independent living of VWDs by increasing awareness and utilization of HM services.
Background
A health care professional (HCP) approves a HM support award by evaluating the practicality of the support to improve the built environment of a given veteran’s disability.1,2 Previously we detailed some of the preliminary research into the HISA program, including HISA user demographic and clinical characteristics, types of HMs received, user suggestions for improvement, and geospatial analysis of HISA prescriptions concentration.1-4
The geospatial analyses of HISA prescriptions revealed clusters of high numbers of HISA users (hot spots) and low numbers of HISA users (cold spots), indicating that HISA is either not prescribed or uniformly used across the US. The previous research prompted investigation into county-level variables that may impact HISA utilization rates. This inquiry focuses on county characteristics associated with HISA use rates, such as measures of clinical care and quality of care (eg, access to health services variables, lack of insurance, preventable hospital stays), physical environment, and sociodemographic characteristics. Clinical care and quality of care measures promote the interaction with HCPs. Moreover, access to health care is an important indicator of health outcomes.5,6 An individual’s capacity to access health services, such as a HM program, greatly impacts well-being, safety, independence, and health.2,4 Well-being, safety, independence, and health become compromised if individuals cannot access care, if needed care is lacking in their area, if HCPs are not available, or are unwilling to provide care due to lack of insurance coverage.7-12 In locations where health care services are minimal due to lack of specialists or health care facilities, the quality of (or access to) care may be compromised, resulting in preventable conditions becoming problematic.13,14 These conditions may result in unnecessary hospitalizations for conditions that could have been treated during routine care. Financial barriers to care particularly among low-income people and the uninsured have proven detrimental to health.15,16 On the other hand, preventable hospital stays are a quality of care measure (ie, a proxy for poor quality of care). HISA operates within a health care system; thus, it is imperative to include these measures impacting health.
In this study, we sought to identify county-level predictors—in particular, county-level proxies for access to care—that may be associated with county-level HISA use. We define HISA utilization rate as the percentage of a county’s VHA patients who have received a HISA award.
Methods
This study used data from the National Prosthetics Patient Database (NPPD), US Department of Veterans Affairs (VA) medical database inpatient and outpatient datasets, VHA Support Service Center (VSSC) data cubes, and the County Health Rankings database (CHRD). First, the study cohort was identified from NPPD users who have obtained a HISA award from fiscal years (FY) 2015 to 2018. Analysis started with FY 2015 following new regulations (38 CFR § 17) governing the operations of the HISA program.2 The study cohort was matched with records from NPPD and VA inpatient and outpatient datasets to obtain information about the veterans’ demographic characteristics and their HM characteristics and costs. The number of VHA end-of-year (EOY) patients per county was extracted from the VSSC Current Enrollment Cube, which was used in calculation of the county-level HISA utilization rate.17 Finally, zip code–based locational data were used to calculate approximate drive time and distance from the HISA user’s approximate location to the facility where they received their HM prescription. Drive times and drive distances were calculated with Esri ArcGIS Pro (v2.6.3) by placing zip code centroid and VHA facilities on a nationwide road network that contains both road speeds and distances.
Calculations
Patient-level data were aggregated up to county-level variables by calculating the sum, mean, or percent per county. HISA user sample characteristics, including sex, race, rurality (urban, rural), marital status, and Class 1 vs Class 2 disability-related eligibility groups, were aggregated to the county level by calculating percentages of HISA users of the given characteristics out of total HISA users in the county. Disability-related eligibility groups (Class 1 vs Class 2 HISA users) determines the maximum lifetime award dollar amount. Specifically, those with service-connected disabilities or those with a ≥ 50% disability rating (regardless of whether or not their disability is service connected) are classified as Class 1 HISA users and are eligible to receive a maximum lifetime award of $6800. Those with a recorded disability that is not connected to their military service, and who have a disability rating of < 50% are classified as Class 2 HISA users and are eligible to receive a lifetime maximum award of $2000.
The county-level number of HISA users was used as the numerator for calculation of county-level HISA utilization rate. Counties with zero HISA users were excluded. The number of EOY VHA patients per county in FY 2018 was divided by 1000 and used as the denominator in the calculation of county-level HISA utilization rate. Thus, the outcome variable is HISA utilization rate per 1000 VHA patients in FY 2018 (HISA utilization rate).
County-Level Variables
County-level variables were downloaded from the 2020 CHRD.5,6 An explanation of the CHRD model and the factors used in this study are shown in the eAppendix (available at doi: 10.12788/fp.0279).6 County-level aggregated HISA user data and the CHRD data were matched using county Federal Information Processing Standards codes. Access to care measures collected from CHRD included percentages uninsured and ratios of population to primary care physicians, dentists, mental health professionals, and other primary care professionals. Other CHRD measures included those for quality of care (rate of preventable hospital stay) and housing quality (percent of households with high housing costs, percent of households with overcrowding, percent of households with lack of kitchen or plumbing, percent of households with severe housing cost burden, percent of homeownership). Of secondary interest was county population rurality, as previous research findings showed the annual average of HISA users who are from rural areas ranged from 30 to 35%.
Analysis Methods
SAS (v9.4), R (v4.0.2), and RStudio (v1.3.1093) were used for data preparation and analysis.18 Multiple regression analysis was used to predict county-level utilization rate from county-level variables. Sociodemographic characteristics from CHRD and HISA data were included as important control predictors in the regression model, though our focus for this paper are the access to care and housing quality predictors.
Model diagnostics (examination of model residuals, Breusch-Godfrey test, Breusch-Pagan test) revealed significant heteroskedasticity of the model; thus, robust standard errors and associated P values were computed using the R estimatr package (v0.30.2).19 Some predictor variables of interest (eg, ratio of mental health professionals) were removed during the model building process either due to problems of multicollinearity or excessive missingness that would have resulted in listwise deletion.
Results
County-level HISA utilization rate per 1000 EOY VHA patients ranged from 0.09 to 59.7%, with a 6.6% mean and 5% median (Figure 2). The data were highly positively skewed. The final model included 33 total predictor variables (Table 1). The final regression model was a significantly better predictor of county-level HISA utilization rate than a null model (F[33-2184], 10.18; P < .001). The adjusted model R2 showed that the overall model accounted for approximately 23% of variance in county-level HISA utilization rate (Table 2).
Among the primary variables of interest, percent uninsured adults and rate of preventable hospital stays emerged as significant predictors of county-level HISA utilization rate. Specifically, county percentage of uninsured adults was negatively related to county-level HISA utilization rate (b = -8.99, P = .005), indicating that the higher the proportion of uninsured adults—with all other predictors held constant—the lower the HISA utilization rate. Percent uninsured adults ranged from 2.7 to 42.4% across counties, with a mean (SD) of 12.7% (5.8%) and 11.4% median.
County rate of preventable hospital stays, however, was significantly and positively related to county-level HISA utilization rate (b = .0004, P = .009), indicating that the higher the rate of preventable hospital stays—with all other predictors held constant—the higher the HISA utilization rate. The direction of this effect is the opposite of the direction of the effect of percent uninsured adults (positive rather than negative), even though both could be considered measures of access to care. The standardized β for these 2 predictors indicate that county rate of preventable hospital stays is a somewhat stronger predictor of county-level HISA utilization rate than is county percent of uninsured adults (β = .11 and β = -.09, respectively). Rate of preventable hospital stays ranged from 683 to 16,802 across counties included in this analysis, with a mean (SD) of 4,796.5 (1659.9) and a 4669 median.
Of secondary interest was county rurality. The county-level percentage of rural residents was significantly and positively related to county-level HISA utilization rate, indicating that the higher the proportion of individuals within county considered rural—all other predictors held constant—the higher the HISA utilization rate. The mean (SD) percentage of rural residents per county was 52.3% (30.2) and 52.7 % median.
Discussion
This study examined whether county-level characteristics, specifically variables for access to care, quality of care, and housing quality, were predictive of a county’s HISA utilization rate. Given that this series of work on the HISA program is (to our knowledge) the first of its kind, and given the exploratory nature of this analysis, we did not have specific predictions for the effects of any one given variable. Nevertheless, some of the results were surprising, and we believe they warrant additional study. In particular, the opposing direction of effects for access to care and quality of care variables were hard to reconcile.
The county percent of uninsured adults (an access to care variable, specifically, a proxy for poor access to care) was negatively associated with county-level HISA utilization rate, whereas the county rate of preventable hospital stays (a quality of care variable, but also potentially an access to care variable, and specifically, proxies for poor quality of care or poor access to care) was positively associated with county-level HISA utilization rate. To describe the relationships more generally, one coefficient in the regression model indicated that the poorer the access to care, the lower the HISA utilization rate (higher percent of uninsured adults predicts lower HISA utilization rate), while another coefficient in the regression model indicated the poorer the quality of and access to care, the higher the HISA utilization rate (higher rate of preventable hospital stays predicts higher HISA utilization rate). Future study is warranted to disentangle and reconcile the various community-level predictors of this service.
Housing quality measures (eg, percent of households with high housing costs, percent of households with overcrowding, percent of households with lack of kitchen or plumbing, percent of households with severe housing cost burden, and percent of homeownership) are important in the consideration of whether a HM will be performed or should be performed. For example, if a person is cost burdened by the amount of expenditure spent in housing there will be little discretionary funds to perform a HM. Individuals who do not own their home may experience complications in obtaining permission from landlords to perform a HM. County-level predictors of housing quality (percent of households with high housing costs, overcrowding, and lack of kitchen or plumbing) were not significantly associated with county-level HISA utilization rate but are also nevertheless relevant to the discussion of home modifications. Of particular interest is the percent of households with lack of kitchen or plumbing variable, which was positively related to county-level HISA utilization rate although not statistically significant. HM elements related to the kitchen (eg, heighten countertop) add to the accessibility of the home allowing for the performing of activities of daily living such as cooking. Between FY 2015 and FY 2018, we discovered 131 prescriptions for kitchen (n = 90) and plumbing (n = 41) HMs, which is a very small proportion of the 30,780 total HMs (there were 24,397 bathroom HMs). The nonsignificant coefficient for this variable may reflect the small number of veterans that obtained these HM.
Limitations
The potentially conflicting direction of effects for a significant access to care variable (percent uninsured adults) and a significant access to care and quality of care variable (preventable hospital stays) are interesting and warrant additional study, but the inability to interpret or explain this apparent inconsistency constitutes a limitation of the current data and analyses presented here. Another limitation is that this analysis uses county-level predictors for what is ultimately an individual-level outcome. It would have been ideal to have both individual- and county-level data to conduct a multilevel analysis; in particular, individual-level data within counties of individuals (both veterans and nonveterans) who did not receive a HISA award (including both those who applied and were denied, and who did not apply) would be highly valuable.
Conclusions
Our continuing research into veterans’ use of HM fills a gap in the literature about the characteristics of HISA users, the impact of county-level variables on the use of HISA, and the geographic distribution and use of HISA within the VHA. While it is important to examine the influence of broader systems on individual outcomes, there could be myriad other factors that are more proximal and more closely related to whether any one individual applies for, let alone receives, a HISA award. Indeed, a low overall adjusted model R2 indicates that there is considerable variability in county-level HISA utilization rate that was not accounted for by the current model; this further speaks to warranted additional study.
More research is needed to understand and account for geographical variation in HISA utilization rate across the US. However, this work serves as an exploratory first step at quantifying and predicting HISA utilization rate at a broad level, with the ultimate goal of increasing access to HMs for veterans with disabilities.
Acknowledgments
This research was supported by grant 15521 from the US Department of Veterans Affairs, Office of Rural Health. Furthermore, the research was supported in part by grant K12 HD055929 from the National Institutes of Health. We want to acknowledge Cheri E. Knecht, Project Coordinator, for her assistance throughout all aspects of our research study and for her thoughtful contributions during the writing of this manuscript.
1. Semeah LM, Ahrentzen S, Jia H, Cowper-Ripley DC, Levy CE, Mann WC. The home improvements and structural alterations benefits program: veterans with disabilities and home accessibility. J Disability Policy Studies. 2017;28(1):43-51. doi:10.1177/1044207317696275
2. Semeah LM, Wang X, Cowper Ripley DC, Lee MJ, Ahonle ZJ, Ganesh SP, et al. Improving health through a home modification service for veterans. In: Fiedler BA, ed. Three Facets of Public Health and Paths to Improvements. Academic Press; 2020:381-416.
3. Semeah LM, Ahrentzen S, Cowper-Ripley DC, Santos-Roman LM, Beamish JO, Farley K. Rental housing needs and barriers from the perspective of veterans with disabilities. Housing Policy Debate. 2019;29(4):542-558. doi:10.1080/10511482.2018.1543203
4. Semeah LM, Ganesh SP, Wang X, et al. Home modification and health services utilization by rural and urban veterans with disabilities. Housing Policy Debate. 2021;31(6):862-874.doi:10.1080/10511482.2020.1858923
5. University of Wisconsin Population Health Institute. County health rankings model. Accessed May 13, 2022. https://www.countyhealthrankings.org/about-us
6. Remington PL, Catlin BB, Gennuso KP. The County Health Rankings: rationale and methods. Popul Health Metr. 2015;13(11). doi:10.1186/s12963-015-0044-2
7. National Academies of Sciences, Engineering, and Medicine. Health-Care Utilization as a Proxy in Disability Determination. Washington, DC: The National Academies Press; 2018.
8. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129(6):611-20. doi:10.1016/j.puhe.2015.04.001
9. Medicaid and Chip Payment and Access Commission (MACPAC). Medicaid access in brief: adults’ experiences in obtaining medical care. November 2016. Accessed May 13, 2022. https://www.macpac.gov/publication/access-in-brief-adults-experiences-in-obtaining-medical-care
10. Tolbert J, Orgera, K, Damico A. Key facts about the uninsured population. November 6, 2020. Accessed May 13, 2022. https://www.kff.org/uninsured/issue-brief/key-facts-about-the-uninsured-population
11. Meit M, Knudson A, Gilbert T, et al. The 2014 update of the rural-urban chartbook, 2014. October 2014. Accessed May 13, 2022. http://www.ruralhealthresearch.org
12. National Center for Health Statistics (US). Report No.: 2016-1232. Health, United States, 2015: with special feature on racial and ethnic health disparities. Hyattsville, MD: National Center for Health Statistics.
13. Broussard DL, Mason KE, Carruth AR, Carton TW. Assessing potentially preventable hospitalizations at the county level: a comparison of measures using Medicare data and state hospital discharge data. Popul Health Manag. 2018;21(6):438-445. doi:10.1089/pop.2017.0141
14. Pezzin LE, Bogner HR, Kurichi JE, et al. Preventable hospitalizations, barriers to care, and disability. Medicine (Baltimore). 2018;97:e0691 doi:10.1097/MD.0000000000010691
15. Davis K, Ballreich J. Equitable access to care: how the United States ranks internationally. N Engl J Med. 2014;371(17):1567-70. doi:10.1056/NEJMp1406707
16. Squires D, Anderson C. U.S. health care from a global perspective: spending, use of services, prices, and health in 13 countries. Issue Brief (Commonw Fund). 2015;15:1-15.
17. VHA Service Support Center. Current enrollment cube (vssc.med.va.gov). Retrieved August 06, 2019. [Data not verified.]
18. Bunn A, Korpela M. R: A language and environment for statistical computing: an introduction to dplR. January 29, 2021. Accessed May 13, 2022. http://r.meteo.uni.wroc.pl/web/packages/dplR/vignettes/intro-dplR.pdf
19. Sheppard BH, Hartwick J, Warshaw PR. The theory of reasoned action: a meta-analysis of past research with recommendations for modifications and future research. J Consumer Research. 1988;15(3):325-343. doi:10.1086/209170
1. Semeah LM, Ahrentzen S, Jia H, Cowper-Ripley DC, Levy CE, Mann WC. The home improvements and structural alterations benefits program: veterans with disabilities and home accessibility. J Disability Policy Studies. 2017;28(1):43-51. doi:10.1177/1044207317696275
2. Semeah LM, Wang X, Cowper Ripley DC, Lee MJ, Ahonle ZJ, Ganesh SP, et al. Improving health through a home modification service for veterans. In: Fiedler BA, ed. Three Facets of Public Health and Paths to Improvements. Academic Press; 2020:381-416.
3. Semeah LM, Ahrentzen S, Cowper-Ripley DC, Santos-Roman LM, Beamish JO, Farley K. Rental housing needs and barriers from the perspective of veterans with disabilities. Housing Policy Debate. 2019;29(4):542-558. doi:10.1080/10511482.2018.1543203
4. Semeah LM, Ganesh SP, Wang X, et al. Home modification and health services utilization by rural and urban veterans with disabilities. Housing Policy Debate. 2021;31(6):862-874.doi:10.1080/10511482.2020.1858923
5. University of Wisconsin Population Health Institute. County health rankings model. Accessed May 13, 2022. https://www.countyhealthrankings.org/about-us
6. Remington PL, Catlin BB, Gennuso KP. The County Health Rankings: rationale and methods. Popul Health Metr. 2015;13(11). doi:10.1186/s12963-015-0044-2
7. National Academies of Sciences, Engineering, and Medicine. Health-Care Utilization as a Proxy in Disability Determination. Washington, DC: The National Academies Press; 2018.
8. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129(6):611-20. doi:10.1016/j.puhe.2015.04.001
9. Medicaid and Chip Payment and Access Commission (MACPAC). Medicaid access in brief: adults’ experiences in obtaining medical care. November 2016. Accessed May 13, 2022. https://www.macpac.gov/publication/access-in-brief-adults-experiences-in-obtaining-medical-care
10. Tolbert J, Orgera, K, Damico A. Key facts about the uninsured population. November 6, 2020. Accessed May 13, 2022. https://www.kff.org/uninsured/issue-brief/key-facts-about-the-uninsured-population
11. Meit M, Knudson A, Gilbert T, et al. The 2014 update of the rural-urban chartbook, 2014. October 2014. Accessed May 13, 2022. http://www.ruralhealthresearch.org
12. National Center for Health Statistics (US). Report No.: 2016-1232. Health, United States, 2015: with special feature on racial and ethnic health disparities. Hyattsville, MD: National Center for Health Statistics.
13. Broussard DL, Mason KE, Carruth AR, Carton TW. Assessing potentially preventable hospitalizations at the county level: a comparison of measures using Medicare data and state hospital discharge data. Popul Health Manag. 2018;21(6):438-445. doi:10.1089/pop.2017.0141
14. Pezzin LE, Bogner HR, Kurichi JE, et al. Preventable hospitalizations, barriers to care, and disability. Medicine (Baltimore). 2018;97:e0691 doi:10.1097/MD.0000000000010691
15. Davis K, Ballreich J. Equitable access to care: how the United States ranks internationally. N Engl J Med. 2014;371(17):1567-70. doi:10.1056/NEJMp1406707
16. Squires D, Anderson C. U.S. health care from a global perspective: spending, use of services, prices, and health in 13 countries. Issue Brief (Commonw Fund). 2015;15:1-15.
17. VHA Service Support Center. Current enrollment cube (vssc.med.va.gov). Retrieved August 06, 2019. [Data not verified.]
18. Bunn A, Korpela M. R: A language and environment for statistical computing: an introduction to dplR. January 29, 2021. Accessed May 13, 2022. http://r.meteo.uni.wroc.pl/web/packages/dplR/vignettes/intro-dplR.pdf
19. Sheppard BH, Hartwick J, Warshaw PR. The theory of reasoned action: a meta-analysis of past research with recommendations for modifications and future research. J Consumer Research. 1988;15(3):325-343. doi:10.1086/209170
Online Information About Hydroquinone: An Assessment of Accuracy and Readability
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1
We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3
Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was
The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.
The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1
We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3
Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was
The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.
The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1
We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3
Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was
The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.
The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
Practice Points
- Hydroquinone (HQ) 4% is US Food and Drug Administration (FDA) approved for skin hyperpigmentation including melasma.
- In September 2020, the FDA enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform, announcing that HQ is not classified as Category II/not generally recognized as safe and effective, thus prohibiting the distribution of OTC HQ products.
- Exogenous ochronosis is a potential side effect associated with HQ.
- There is a need for dermatologists to develop online patient education materials on HQ that are characterized by high-quality and up-to-date medical information.