User login
Characterizing Hospitalizations for Pediatric Concussion and Trends in Care
Approximately 14% of children who sustain a concussion are admitted to the hospital,1 although admission rates reportedly vary substantially among pediatric hospitals.2 Children hospitalized for concussion may be at a higher risk for persistent postconcussive symptoms,3,4 yet little is known about this subset of children and how they are managed while in the hospital. Characterizing children hospitalized for concussion and describing the inpatient care they received will promote hypothesis generation for further inquiry into indications for admission, as well as the relationship between inpatient management and concussion recovery.
We described a cohort of children admitted to 40 pediatric hospitals primarily for concussion and detailed care delivered during hospitalization. We explored individual-level factors and their association with prolonged length of stay (LOS) and emergency department (ED) readmission. Finally, we evaluated if there had been changes in inpatient care over the 8-year study period.
PATIENTS AND METHODS
Study Design
The Institutional Review Board determined that this retrospective cohort study was exempt from review.
Data Source
The Children’s Hospital Association’s Pediatric Health Information System (PHIS) is an administrative database from pediatric hospitals located within 17 major metropolitan areas in the United States. Data include: service dates, patient demographics, payer type, diagnosis codes, resource utilization information (eg, medications), and hospital characteristics.1,5 De-identified data undergo reliability and validity checks prior to inclusion.1,5 We analyzed data from 40 of 43 hospitals that contributed inpatient data during our study period. 2 hospitals were excluded due to inconsistent data submission, and 1 removed their data.
Study Population
Data were extracted for children 0 to 17 years old who were admitted to an inpatient or observational unit between January 1, 2007 and December 31, 2014 for traumatic brain injury (TBI). Children were identified using International Classification of Diseases, Clinical Modification, Ninth Revision (ICD-9-CM) diagnosis codes that denote TBI per the Centers for Disease Control (CDC): 800.0–801.9, 803.0–804.9, 850–854.1, and 959.01.6–8 To examine inpatient care for concussion, we only retained children with a primary (ie, first) concussion-related diagnosis code (850.0–850.99) for analyses. For patients with multiple visits during our study period, only the index admission was analyzed. We refined our cohort using 2 injury scores calculated from ICD-9-CM diagnosis codes using validated ICDMAP-90 injury coding software.6,10–12 The Abbreviated Injury Scale (AIS) ranges from 1 (minor injury) to 6 (not survivable). The total Injury Severity Score (ISS) is based on 6 body regions (head/neck, face, chest, abdomen, extremity, and external) and calculated by summing the squares of the 3 worst AIS scores.13 A concussion receives a head AIS score of 2 if there is an associated loss of consciousness or a score of 1 if there is not; therefore, children were excluded if the head AIS score was >2. We also excluded children with the following features, as they may be indicative of more severe injuries that were likely the cause of admission: ISS > 6, secondary diagnosis code of skull fracture or intracranial injury, intensive care unit (ICU) or operating room (OR) charges, or a LOS > 7 days. Because some children are hospitalized for potentially abusive minor head trauma pending a safe discharge plan, we excluded children 0 to 4 years of age with child abuse, which was determined using a specific set of diagnosis codes (E960-E96820, 995.54, and 995.55) similar to previous research.14
Data Elements and Outcomes
Outcomes
Based on previous reports,1,15 a LOS ≥ 2 days distinguished a typical hospitalization from a prolonged one. ED revisit was identified when a child had a visit with a TBI-related primary diagnosis code at a PHIS hospital within 30 days of initial admission and was discharged home. We limited analyses to children discharged, as children readmitted may have had an initially missed intracranial injury.
Patient Characteristics
We examined the following patient variables: age, race, sex, presence of chronic medical condition, payer type, household income, area of residence (eg, rural versus urban), and mechanism of injury. Age was categorized to represent early childhood (0 to 4 years), school age (5 to 12 years), and adolescence (12 to 17 years). Race was grouped as white, black, or other (Asian, Pacific Islander, American Indian, and “other” per PHIS). Ethnicity was described as Hispanic/Latino or not Hispanic/Latino. Children with medical conditions lasting at least 12 months and comorbidities that may impact TBI recovery were identified using a subgrouping of ICD-9-CM codes for children with “complex chronic conditions”.16 Payer type was categorized as government, private, and self-pay. We extracted a PHIS variable representing the 2010 median household income for the child’s home zip code and categorized it into quartiles based on the Federal Poverty Level for a family of 4.17,18 Area of residence was defined using a Rural–Urban Commuting Area (RUCA) classification system19 and grouped into large urban core, suburban area, large rural town, or small rural town/isolated rural area.17 Mechanism of injury was determined using E-codes and categorized using the CDC injury framework,20 with sports-related injuries identified using a previously described set of E-codes.1 Mechanisms of injury included fall, motor vehicle collision, other motorized transport (eg, all-terrain vehicles), sports-related, struck by or against (ie, objects), and all others (eg, cyclists).
Hospital Characteristics
Hospitals were characterized by region (Northeast, Central, South, and West) and size (small <200, medium 200–400, and large >400 beds). The trauma-level accreditation was identified with Level 1 reflecting the highest possible trauma resources.
Medical Care Variables
Care variables included medications, neuroimaging, and cost of stay. Medication classes included oral non-narcotic analgesics [acetaminophen, ibuprofen, and others (aspirin, tramadol, and naproxen)], oral narcotics (codeine, oxycodone, and narcotic–non-narcotic combinations), intravenous (IV) non-narcotics (ketorolac), IV narcotics (morphine, fentanyl, and hydromorphone), antiemetics [ondansetron, metoclopramide, and phenothiazines (prochlorperazine, chlorpromazine, and promethazine)], maintenance IV fluids (dextrose with electrolytes or 0.45% sodium chloride), and resuscitation IV fluids (0.9% sodium chloride or lactated Ringer’s solution). Receipt of neuroimaging was determined if head computed tomography (CT) had been conducted at the admitting hospital. Adjusted cost of stay was calculated using a hospital-specific cost-to-charge ratio with additional adjustments using the Center for Medicare & Medicaid’s Wage Index.
Statistical Analyses
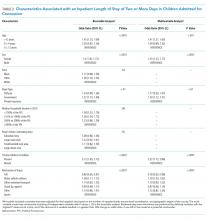
Descriptive statistics were calculated for individual, injury, and hospital, and care data elements, LOS, and ED readmissions. The number of children admitted with TBI was used as the denominator to assess the proportion of pediatric TBI admissions that were due to concussions. To identify factors associated with prolonged LOS (ie, ≥2 days) and ED readmission, we employed a mixed models approach that accounted for clustering of observations within hospitals. Independent variables included age, sex, race, ethnicity, payer type, household income, RUCA code, chronic medical condition, and injury mechanism. Models were adjusted for hospital location, size, and trauma-level accreditation. The binary distribution was specified along with a logit link function. A 2-phase process determined factors associated with each outcome. First, bivariable models were developed, followed by multivariable models that included independent variables with P values < .25 in the bivariable analysis. Backward step-wise elimination was performed, deleting variables with the highest P value one at a time. After each deletion, the percentage change in odds ratios was examined; if variable removal resulted in >10% change, the variable was retained as a potential confounder. This process was repeated until all remaining variables were significant (P < .05) with the exception of potential confounders. Finally, we examined the proportion of children receiving selected care practices annually. Descriptive and trend analyses were used to analyze adjusted median cost of stay. Analyses were performed using SAS software (Version 9.3, SAS Institute Inc., Cary, North Carolina).
RESULTS
Over 8 years, 88,526 children were admitted to 40 PHIS hospitals with a TBI-related diagnosis, among whom 13,708 had a primary diagnosis of concussion. We excluded 2,973 children with 1 or more of the following characteristics: a secondary diagnosis of intracranial injury (n = 58), head AIS score > 2 (n = 218), LOS > 7 days (n = 50), OR charges (n = 132), ICU charges (n = 1947), and ISS > 6 (n = 568). Six additional children aging 0 to 4 years were excluded due to child abuse. The remaining 10,729 children, averaging 1300 hospitalizations annually, were identified as being hospitalized primarily for concussion.
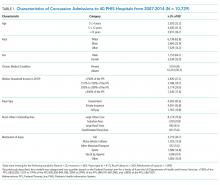
Table 1 summarizes the individual characteristics for this cohort. The average (standard deviation) age was 9.5 (5.1) years. Ethnicity was missing for 25.3% and therefore excluded from the multivariable models. Almost all children had a head AIS score of 2 (99.2%), and the majority had a total ISS ≤ 4 (73.4%). The majority of admissions were admitted to Level 1 trauma-accredited hospitals (78.7%) and medium-sized hospitals (63.9%).
The most commonly delivered medication classes were non-narcotic oral analgesics (53.7%), dextrose-containing IV fluids (45.0%), and antiemetic medications (34.1%). IV and oral narcotic use occurred in 19.7% and 10.2% of the children, respectively. Among our cohort, 16.7% received none of these medication classes. Of the 8,940 receiving medication, 32.6% received a single medication class, 29.5% received 2 classes, 20.5% 3 classes, 11.9% 4 classes, and 5.5% received 5 or more medication classes. Approximately 15% (n = 1597) received only oral medications, among whom 91.2% (n = 1457) received only non-narcotic analgesics and 3.9% (n = 63) received only oral narcotic analgesics. The majority (69.5%) received a head CT.
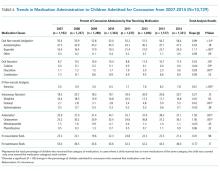
Table 4 summarizes medication administration trends over time. Oral non-narcotic administration increased significantly (slope = 0.99, P < .01) with the most pronounced change occurring in ibuprofen use (slope = 1.11, P < .001). Use of the IV non-narcotic ketorolac (slope = 0.61, P < .001) also increased significantly, as did the proportion of children receiving antiemetics (slope = 1.59, P = .001), with a substantial increase in ondansetron use (slope = 1.56, P = .001). The proportion of children receiving head CTs decreased linearly over time (slope= −1.75, P < .001), from 76.1% in 2007 to 63.7% in 2014. Median cost, adjusted for inflation, increased during our study period (P < .001) by approximately $353 each year, reaching $11,249 by 2014.
DISCUSSION
From 2007 to 2014, approximately 15% of children admitted to PHIS hospitals for TBI were admitted primarily for concussion. Since almost all children had a head AIS score of 2 and an ISS ≤ 4, our data suggest that most children had an associated loss of consciousness and that concussion was the only injury sustained, respectively. This study identified important subgroups that necessitated inpatient care but are rarely the focus of concussion research (eg, toddlers and those injured due to a motor vehicle collision). Most children (83.3%) received medications to treat common postconcussive symptoms (eg, pain and nausea), with almost half receiving 3 or more medication classes. Factors associated with the development of postconcussive syndrome (eg, female sex and adolescent age)4,21 were significantly associated with hospitalization of 2 or more days and ED revisit within 30 days of admission. In the absence of evidenced-based guidelines for inpatient concussion management, we identified significant trends in care, including increased use of specific pain [ie, oral and IV nonsteroidal anti-inflammatory drugs (NSAIDs)] and antiemetic (ie, ondansetron) medications and decreased use of head CT. Given the number of children admitted and receiving treatment for concussion symptomatology, influences on the decision to deliver specific care practices, as well as the impact and benefit of hospitalization, require closer examination.
Our study extends previous reports from the PHIS database by characterizing children admitted for concussion.1 We found that children admitted for concussion had similar characteristics to the broader population of children who sustain concussion (eg, school-aged children, male, and injured due to a fall or during sports).1,3,22 However, approximately 20% of the cohort were less than 5 years old, and less is known regarding appropriate treatment and outcomes of concussion in this age group.23 Uncertainty regarding optimal management and a young child’s inability to articulate symptoms may contribute to a physician’s decision to admit for close observation. Similar to Blinman et al., we found that a substantial proportion of children admitted with concussion were injured due to a motor vehicle collision,3 suggesting that although sports-related injuries are responsible for a significant proportion of pediatric concussions, children injured by other preventable mechanisms may also be incurring significant concussive injuries. Finally, the majority of our cohort was from an urban core, relative to a rural area, which is likely a reflection of the regionalization of trauma care, as well as variations in access to health care.
Although most children recover fully from concussion without specific interventions, 20%-30% may remain symptomatic at 1 month,3,4,21,24 and children who are hospitalized with concussion may be at higher risk for protracted symptoms. While specific individual or injury-related factors (eg, female sex, adolescent age, and injury due to motor vehicle collision) may contribute to more significant postconcussive symptoms, it is unclear how inpatient management affects recovery trajectory. Frequent sleep disruptions associated with inpatient care25 contradict current acute concussion management recommendations for physical and cognitive rest26 and could potentially impair symptom recovery. Additionally, we found widespread use of NSAIDs, although there is evidence suggesting that NSAIDs may potentially worsen concussive symptoms.26 We identified an increase in medication usage over time despite limited evidence of their effectiveness for pediatric concussion.27–29 This change may reflect improved symptom screening4,30 and/or increased awareness of specific medication safety profiles in pediatric trauma patients, especially for NSAIDs and ondansetron. Although we saw an increase in NSAID use, we did not see a proportional decrease in narcotic use. Similarly, while two-thirds of our cohort received IV medications, there is controversy about the need for IV fluids and medications for other pediatric illnesses, with research demonstrating that IV treatment may not reduce recovery time and may contribute to prolonged hospitalization and phlebitis.31,32 Thus, there is a need to understand the therapeutic effectiveness and benefits of medications and fluids on postconcussion recovery.
Neuroimaging rates for children receiving ED evaluation for concussion have been reported to be up to 60%-70%,1,22 although a more recent study spanning 2006 to 2011 found a 35-%–40% head CT rate in pediatric patients by hospital-based EDs in the United States.33 Our results appear to support decreasing head CT use over time in pediatric hospitals. Hospitalization for observation is costly1 but could decrease a child’s risk of malignancy from radiation exposure. Further work on balancing cost, risk, and shared decision-making with parents could guide decisions regarding emergent neuroimaging versus admission.
This study has limitations inherent to the use of an administrative dataset, including lack of information regarding why the child was admitted. Since the focus was to describe inpatient care of children with concussion, those discharged home from the ED were not included in this dataset. Consequently, we could not contrast the ED care of those discharged home with those who were admitted or assess trends in admission rates for concussion. Although the overall number of concussion admissions has continued to remain stable over time,1 due to a lack of prospectively collected clinical information, we are unable to determine whether observed trends in care are secondary to changes in practice or changes in concussion severity. However, there has been no research to date supporting the latter. Ethnicity was excluded due to high levels of missing data. Cost of stay was not extensively analyzed given hospital variation in designation of observational or inpatient status, which subsequently affects billing.34 Rates of neuroimaging and ED revisit may have been underestimated since children could have received care at a non-PHIS hospital. Similarly, the decrease in the proportion of children receiving neuroimaging over time may have been associated with an increase in children being transferred from a non-PHIS hospital for admission, although with increased regionalization in trauma care, we would not expect transfers of children with only concussion to have significantly increased. Finally, data were limited to the pediatric tertiary care centers participating in PHIS, thereby reducing generalizability and introducing selection bias by only including children who were able to access care at PHIS hospitals. Although the care practices we evaluated (eg, NSAIDs and head CT) are available at all hospitals, our analyses only reflect care delivered within the PHIS.
Concussion accounted for 15% of all pediatric TBI admissions during our study period. Further investigation of potential factors associated with admission and protracted recovery (eg, adolescent females needing treatment for severe symptomatology) could facilitate better understanding of how hospitalization affects recovery. Additionally, research on acute pharmacotherapies (eg, IV therapies and/or inpatient treatment until symptoms resolve) is needed to fully elucidate the acute and long-term benefits of interventions delivered to children.
ACKNOWLEDGMENTS
Colleen Mangeot: Biostatistician with extensive PHIS knowledge who contributed to database creation and statistical analysis. Yanhong (Amy) Liu: Research database programmer who developed the database, ran quality assurance measures, and cleaned all study data.
Disclosures
The authors have nothing to disclose.
Funding
This study was supported by grant R40 MC 268060102 from the Maternal and Child Health Research Program, Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services. The funding source was not involved in development of the study design; in the collection, analysis and interpretation of data; or in the writing of this report.
1. Colvin JD, Thurm C, Pate BM, Newland JG, Hall M, Meehan WP. Diagnosis and acute management of patients with concussion at children’s hospitals. Arch Dis Child. 2013;98(12):934-938. PubMed
2. Bourgeois FT, Monuteaux MC, Stack AM, Neuman MI. Variation in emergency department admission rates in US children’s hospitals. Pediatrics. 2014;134(3):539-545. PubMed
3. Blinman TA, Houseknecht E, Snyder C, Wiebe DJ, Nance ML. Postconcussive symptoms in hospitalized pediatric patients after mild traumatic brain injury. J Pediatr Surg. 2009;44(6):1223-1228. PubMed
4. Babcock L, Byczkowski T, Wade SL, Ho M, Mookerjee S, Bazarian JJ. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA pediatrics. 2013;167(2):156-161. PubMed
5. Conway PH, Keren R. Factors associated with variability in outcomes for children hospitalized with urinary tract infection. The Journal of pediatrics. 2009;154(6):789-796. PubMed
6. Services UDoHaH. International classification of diseases, 9th Revision, Clinical modification (ICD-9CM). Washington, DC: US Department of Health and Human Services. Public Health Service, Health Care Financing Administration 1989.
7. Marr AL, Coronado VG. Annual data submission standards. Central nervous system injury surveillance. In: US Department of Health and Human Services PHS, CDC, ed. Atlanta, GA 2001.
8. Organization WH. International classification of diseases: manual on the international statistical classification of diseases, injuries, and cause of death. In: Organization WH, ed. 9th rev. ed. Geneva, Switerland 1977.
9. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003.
10. Mackenzie E, Sacco WJ. ICDMAP-90 software: user’s guide. Baltimore, Maryland: Johns Hopkins University and Tri-Analytics. 1997:1-25.
11. MacKenzie EJ, Steinwachs DM, Shankar B. Classifying trauma severity based on hospital discharge diagnoses. Validation of an ICD-9CM to AIS-85 conversion table. Med Care. 1989;27(4):412-422. PubMed
12. Fleischman RJ, Mann NC, Dai M, et al. Validating the use of ICD-9 code mapping to generate injury severity scores. J Trauma Nurs. 2017;24(1):4-14. PubMed
13. Baker SP, O’Neill B, Haddon W, Jr., Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. The Journal of trauma. 1974;14(3):187-196. PubMed
14. Wood JN, Feudtner C, Medina SP, Luan X, Localio R, Rubin DM. Variation in occult injury screening for children with suspected abuse in selected US children’s hospitals. Pediatrics
15. Yang J, Phillips G, Xiang H, Allareddy V, Heiden E, Peek-Asa C. Hospitalisations for sport-related concussions in US children aged 5 to 18 years during 2000-2004. Br J Sports Med. 2008;42(8):664-669. PubMed
16. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106(1):205-209. PubMed
17. Peltz A, Wu CL, White ML, et al. Characteristics of rural children admitted to pediatric hospitals. Pediatrics. 2016;137(5): e20153156. PubMed
18. Services UDoHaH. Annual update of the HHS Poverty Guidelines. Federal Register; 2016-03-14 2011.
19. Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95(7):1149-1155. PubMed
20. Proposed Matrix of E-code Groupings| WISQARS | Injury Center | CDC. 2016; http://www.cdc.gov/injury/wisqars/ecode_matrix.html.
21. Zemek RL, Farion KJ, Sampson M, McGahern C. Prognosticators of persistent symptoms following pediatric concussion: A systematic review. JAMA Pediatr. 2013;167(3):259-265. PubMed
22. Meehan WP, Mannix R. Pediatric concussions in United States emergency departments in the years 2002 to 2006. J Pediatr. 2010;157(6):889-893. PubMed
23. Davis GA, Purcell LK. The evaluation and management of acute concussion differs in young children. Br J Sports Med. 2014;48(2):98-101. PubMed
24. Zemek R, Barrowman N, Freedman SB, et al. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315(10):1014-1025. PubMed
25. Hinds PS, Hockenberry M, Rai SN, et al. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncol Nurs Forum. 2007;34(2):393-402. PubMed
26. Patterson ZR, Holahan MR. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front Cell Neurosci. 2012;6:58. PubMed
27. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124, ix. PubMed
28. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533. PubMed
29. Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24):2250-2257. PubMed
30. Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126(2):e374-e381. PubMed
31. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. PubMed
32. Hartling L, Bellemare S, Wiebe N, Russell K, Klassen TP, Craig W. Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children. Cochrane Database Syst Rev. 2006(3):CD004390. PubMed
34. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-1058. PubMed
33. Zonfrillo MR, Kim KH, Arbogast KB. Emergency Department Visits and Head Computed Tomography Utilization for Concussion Patients From 2006 to 2011. Acad Emerg Med. 2015;22(7):872-877. PubMed
Approximately 14% of children who sustain a concussion are admitted to the hospital,1 although admission rates reportedly vary substantially among pediatric hospitals.2 Children hospitalized for concussion may be at a higher risk for persistent postconcussive symptoms,3,4 yet little is known about this subset of children and how they are managed while in the hospital. Characterizing children hospitalized for concussion and describing the inpatient care they received will promote hypothesis generation for further inquiry into indications for admission, as well as the relationship between inpatient management and concussion recovery.
We described a cohort of children admitted to 40 pediatric hospitals primarily for concussion and detailed care delivered during hospitalization. We explored individual-level factors and their association with prolonged length of stay (LOS) and emergency department (ED) readmission. Finally, we evaluated if there had been changes in inpatient care over the 8-year study period.
PATIENTS AND METHODS
Study Design
The Institutional Review Board determined that this retrospective cohort study was exempt from review.
Data Source
The Children’s Hospital Association’s Pediatric Health Information System (PHIS) is an administrative database from pediatric hospitals located within 17 major metropolitan areas in the United States. Data include: service dates, patient demographics, payer type, diagnosis codes, resource utilization information (eg, medications), and hospital characteristics.1,5 De-identified data undergo reliability and validity checks prior to inclusion.1,5 We analyzed data from 40 of 43 hospitals that contributed inpatient data during our study period. 2 hospitals were excluded due to inconsistent data submission, and 1 removed their data.
Study Population
Data were extracted for children 0 to 17 years old who were admitted to an inpatient or observational unit between January 1, 2007 and December 31, 2014 for traumatic brain injury (TBI). Children were identified using International Classification of Diseases, Clinical Modification, Ninth Revision (ICD-9-CM) diagnosis codes that denote TBI per the Centers for Disease Control (CDC): 800.0–801.9, 803.0–804.9, 850–854.1, and 959.01.6–8 To examine inpatient care for concussion, we only retained children with a primary (ie, first) concussion-related diagnosis code (850.0–850.99) for analyses. For patients with multiple visits during our study period, only the index admission was analyzed. We refined our cohort using 2 injury scores calculated from ICD-9-CM diagnosis codes using validated ICDMAP-90 injury coding software.6,10–12 The Abbreviated Injury Scale (AIS) ranges from 1 (minor injury) to 6 (not survivable). The total Injury Severity Score (ISS) is based on 6 body regions (head/neck, face, chest, abdomen, extremity, and external) and calculated by summing the squares of the 3 worst AIS scores.13 A concussion receives a head AIS score of 2 if there is an associated loss of consciousness or a score of 1 if there is not; therefore, children were excluded if the head AIS score was >2. We also excluded children with the following features, as they may be indicative of more severe injuries that were likely the cause of admission: ISS > 6, secondary diagnosis code of skull fracture or intracranial injury, intensive care unit (ICU) or operating room (OR) charges, or a LOS > 7 days. Because some children are hospitalized for potentially abusive minor head trauma pending a safe discharge plan, we excluded children 0 to 4 years of age with child abuse, which was determined using a specific set of diagnosis codes (E960-E96820, 995.54, and 995.55) similar to previous research.14
Data Elements and Outcomes
Outcomes
Based on previous reports,1,15 a LOS ≥ 2 days distinguished a typical hospitalization from a prolonged one. ED revisit was identified when a child had a visit with a TBI-related primary diagnosis code at a PHIS hospital within 30 days of initial admission and was discharged home. We limited analyses to children discharged, as children readmitted may have had an initially missed intracranial injury.
Patient Characteristics
We examined the following patient variables: age, race, sex, presence of chronic medical condition, payer type, household income, area of residence (eg, rural versus urban), and mechanism of injury. Age was categorized to represent early childhood (0 to 4 years), school age (5 to 12 years), and adolescence (12 to 17 years). Race was grouped as white, black, or other (Asian, Pacific Islander, American Indian, and “other” per PHIS). Ethnicity was described as Hispanic/Latino or not Hispanic/Latino. Children with medical conditions lasting at least 12 months and comorbidities that may impact TBI recovery were identified using a subgrouping of ICD-9-CM codes for children with “complex chronic conditions”.16 Payer type was categorized as government, private, and self-pay. We extracted a PHIS variable representing the 2010 median household income for the child’s home zip code and categorized it into quartiles based on the Federal Poverty Level for a family of 4.17,18 Area of residence was defined using a Rural–Urban Commuting Area (RUCA) classification system19 and grouped into large urban core, suburban area, large rural town, or small rural town/isolated rural area.17 Mechanism of injury was determined using E-codes and categorized using the CDC injury framework,20 with sports-related injuries identified using a previously described set of E-codes.1 Mechanisms of injury included fall, motor vehicle collision, other motorized transport (eg, all-terrain vehicles), sports-related, struck by or against (ie, objects), and all others (eg, cyclists).
Hospital Characteristics
Hospitals were characterized by region (Northeast, Central, South, and West) and size (small <200, medium 200–400, and large >400 beds). The trauma-level accreditation was identified with Level 1 reflecting the highest possible trauma resources.
Medical Care Variables
Care variables included medications, neuroimaging, and cost of stay. Medication classes included oral non-narcotic analgesics [acetaminophen, ibuprofen, and others (aspirin, tramadol, and naproxen)], oral narcotics (codeine, oxycodone, and narcotic–non-narcotic combinations), intravenous (IV) non-narcotics (ketorolac), IV narcotics (morphine, fentanyl, and hydromorphone), antiemetics [ondansetron, metoclopramide, and phenothiazines (prochlorperazine, chlorpromazine, and promethazine)], maintenance IV fluids (dextrose with electrolytes or 0.45% sodium chloride), and resuscitation IV fluids (0.9% sodium chloride or lactated Ringer’s solution). Receipt of neuroimaging was determined if head computed tomography (CT) had been conducted at the admitting hospital. Adjusted cost of stay was calculated using a hospital-specific cost-to-charge ratio with additional adjustments using the Center for Medicare & Medicaid’s Wage Index.
Statistical Analyses
Descriptive statistics were calculated for individual, injury, and hospital, and care data elements, LOS, and ED readmissions. The number of children admitted with TBI was used as the denominator to assess the proportion of pediatric TBI admissions that were due to concussions. To identify factors associated with prolonged LOS (ie, ≥2 days) and ED readmission, we employed a mixed models approach that accounted for clustering of observations within hospitals. Independent variables included age, sex, race, ethnicity, payer type, household income, RUCA code, chronic medical condition, and injury mechanism. Models were adjusted for hospital location, size, and trauma-level accreditation. The binary distribution was specified along with a logit link function. A 2-phase process determined factors associated with each outcome. First, bivariable models were developed, followed by multivariable models that included independent variables with P values < .25 in the bivariable analysis. Backward step-wise elimination was performed, deleting variables with the highest P value one at a time. After each deletion, the percentage change in odds ratios was examined; if variable removal resulted in >10% change, the variable was retained as a potential confounder. This process was repeated until all remaining variables were significant (P < .05) with the exception of potential confounders. Finally, we examined the proportion of children receiving selected care practices annually. Descriptive and trend analyses were used to analyze adjusted median cost of stay. Analyses were performed using SAS software (Version 9.3, SAS Institute Inc., Cary, North Carolina).
RESULTS
Over 8 years, 88,526 children were admitted to 40 PHIS hospitals with a TBI-related diagnosis, among whom 13,708 had a primary diagnosis of concussion. We excluded 2,973 children with 1 or more of the following characteristics: a secondary diagnosis of intracranial injury (n = 58), head AIS score > 2 (n = 218), LOS > 7 days (n = 50), OR charges (n = 132), ICU charges (n = 1947), and ISS > 6 (n = 568). Six additional children aging 0 to 4 years were excluded due to child abuse. The remaining 10,729 children, averaging 1300 hospitalizations annually, were identified as being hospitalized primarily for concussion.
Table 1 summarizes the individual characteristics for this cohort. The average (standard deviation) age was 9.5 (5.1) years. Ethnicity was missing for 25.3% and therefore excluded from the multivariable models. Almost all children had a head AIS score of 2 (99.2%), and the majority had a total ISS ≤ 4 (73.4%). The majority of admissions were admitted to Level 1 trauma-accredited hospitals (78.7%) and medium-sized hospitals (63.9%).
The most commonly delivered medication classes were non-narcotic oral analgesics (53.7%), dextrose-containing IV fluids (45.0%), and antiemetic medications (34.1%). IV and oral narcotic use occurred in 19.7% and 10.2% of the children, respectively. Among our cohort, 16.7% received none of these medication classes. Of the 8,940 receiving medication, 32.6% received a single medication class, 29.5% received 2 classes, 20.5% 3 classes, 11.9% 4 classes, and 5.5% received 5 or more medication classes. Approximately 15% (n = 1597) received only oral medications, among whom 91.2% (n = 1457) received only non-narcotic analgesics and 3.9% (n = 63) received only oral narcotic analgesics. The majority (69.5%) received a head CT.
Table 4 summarizes medication administration trends over time. Oral non-narcotic administration increased significantly (slope = 0.99, P < .01) with the most pronounced change occurring in ibuprofen use (slope = 1.11, P < .001). Use of the IV non-narcotic ketorolac (slope = 0.61, P < .001) also increased significantly, as did the proportion of children receiving antiemetics (slope = 1.59, P = .001), with a substantial increase in ondansetron use (slope = 1.56, P = .001). The proportion of children receiving head CTs decreased linearly over time (slope= −1.75, P < .001), from 76.1% in 2007 to 63.7% in 2014. Median cost, adjusted for inflation, increased during our study period (P < .001) by approximately $353 each year, reaching $11,249 by 2014.
DISCUSSION
From 2007 to 2014, approximately 15% of children admitted to PHIS hospitals for TBI were admitted primarily for concussion. Since almost all children had a head AIS score of 2 and an ISS ≤ 4, our data suggest that most children had an associated loss of consciousness and that concussion was the only injury sustained, respectively. This study identified important subgroups that necessitated inpatient care but are rarely the focus of concussion research (eg, toddlers and those injured due to a motor vehicle collision). Most children (83.3%) received medications to treat common postconcussive symptoms (eg, pain and nausea), with almost half receiving 3 or more medication classes. Factors associated with the development of postconcussive syndrome (eg, female sex and adolescent age)4,21 were significantly associated with hospitalization of 2 or more days and ED revisit within 30 days of admission. In the absence of evidenced-based guidelines for inpatient concussion management, we identified significant trends in care, including increased use of specific pain [ie, oral and IV nonsteroidal anti-inflammatory drugs (NSAIDs)] and antiemetic (ie, ondansetron) medications and decreased use of head CT. Given the number of children admitted and receiving treatment for concussion symptomatology, influences on the decision to deliver specific care practices, as well as the impact and benefit of hospitalization, require closer examination.
Our study extends previous reports from the PHIS database by characterizing children admitted for concussion.1 We found that children admitted for concussion had similar characteristics to the broader population of children who sustain concussion (eg, school-aged children, male, and injured due to a fall or during sports).1,3,22 However, approximately 20% of the cohort were less than 5 years old, and less is known regarding appropriate treatment and outcomes of concussion in this age group.23 Uncertainty regarding optimal management and a young child’s inability to articulate symptoms may contribute to a physician’s decision to admit for close observation. Similar to Blinman et al., we found that a substantial proportion of children admitted with concussion were injured due to a motor vehicle collision,3 suggesting that although sports-related injuries are responsible for a significant proportion of pediatric concussions, children injured by other preventable mechanisms may also be incurring significant concussive injuries. Finally, the majority of our cohort was from an urban core, relative to a rural area, which is likely a reflection of the regionalization of trauma care, as well as variations in access to health care.
Although most children recover fully from concussion without specific interventions, 20%-30% may remain symptomatic at 1 month,3,4,21,24 and children who are hospitalized with concussion may be at higher risk for protracted symptoms. While specific individual or injury-related factors (eg, female sex, adolescent age, and injury due to motor vehicle collision) may contribute to more significant postconcussive symptoms, it is unclear how inpatient management affects recovery trajectory. Frequent sleep disruptions associated with inpatient care25 contradict current acute concussion management recommendations for physical and cognitive rest26 and could potentially impair symptom recovery. Additionally, we found widespread use of NSAIDs, although there is evidence suggesting that NSAIDs may potentially worsen concussive symptoms.26 We identified an increase in medication usage over time despite limited evidence of their effectiveness for pediatric concussion.27–29 This change may reflect improved symptom screening4,30 and/or increased awareness of specific medication safety profiles in pediatric trauma patients, especially for NSAIDs and ondansetron. Although we saw an increase in NSAID use, we did not see a proportional decrease in narcotic use. Similarly, while two-thirds of our cohort received IV medications, there is controversy about the need for IV fluids and medications for other pediatric illnesses, with research demonstrating that IV treatment may not reduce recovery time and may contribute to prolonged hospitalization and phlebitis.31,32 Thus, there is a need to understand the therapeutic effectiveness and benefits of medications and fluids on postconcussion recovery.
Neuroimaging rates for children receiving ED evaluation for concussion have been reported to be up to 60%-70%,1,22 although a more recent study spanning 2006 to 2011 found a 35-%–40% head CT rate in pediatric patients by hospital-based EDs in the United States.33 Our results appear to support decreasing head CT use over time in pediatric hospitals. Hospitalization for observation is costly1 but could decrease a child’s risk of malignancy from radiation exposure. Further work on balancing cost, risk, and shared decision-making with parents could guide decisions regarding emergent neuroimaging versus admission.
This study has limitations inherent to the use of an administrative dataset, including lack of information regarding why the child was admitted. Since the focus was to describe inpatient care of children with concussion, those discharged home from the ED were not included in this dataset. Consequently, we could not contrast the ED care of those discharged home with those who were admitted or assess trends in admission rates for concussion. Although the overall number of concussion admissions has continued to remain stable over time,1 due to a lack of prospectively collected clinical information, we are unable to determine whether observed trends in care are secondary to changes in practice or changes in concussion severity. However, there has been no research to date supporting the latter. Ethnicity was excluded due to high levels of missing data. Cost of stay was not extensively analyzed given hospital variation in designation of observational or inpatient status, which subsequently affects billing.34 Rates of neuroimaging and ED revisit may have been underestimated since children could have received care at a non-PHIS hospital. Similarly, the decrease in the proportion of children receiving neuroimaging over time may have been associated with an increase in children being transferred from a non-PHIS hospital for admission, although with increased regionalization in trauma care, we would not expect transfers of children with only concussion to have significantly increased. Finally, data were limited to the pediatric tertiary care centers participating in PHIS, thereby reducing generalizability and introducing selection bias by only including children who were able to access care at PHIS hospitals. Although the care practices we evaluated (eg, NSAIDs and head CT) are available at all hospitals, our analyses only reflect care delivered within the PHIS.
Concussion accounted for 15% of all pediatric TBI admissions during our study period. Further investigation of potential factors associated with admission and protracted recovery (eg, adolescent females needing treatment for severe symptomatology) could facilitate better understanding of how hospitalization affects recovery. Additionally, research on acute pharmacotherapies (eg, IV therapies and/or inpatient treatment until symptoms resolve) is needed to fully elucidate the acute and long-term benefits of interventions delivered to children.
ACKNOWLEDGMENTS
Colleen Mangeot: Biostatistician with extensive PHIS knowledge who contributed to database creation and statistical analysis. Yanhong (Amy) Liu: Research database programmer who developed the database, ran quality assurance measures, and cleaned all study data.
Disclosures
The authors have nothing to disclose.
Funding
This study was supported by grant R40 MC 268060102 from the Maternal and Child Health Research Program, Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services. The funding source was not involved in development of the study design; in the collection, analysis and interpretation of data; or in the writing of this report.
Approximately 14% of children who sustain a concussion are admitted to the hospital,1 although admission rates reportedly vary substantially among pediatric hospitals.2 Children hospitalized for concussion may be at a higher risk for persistent postconcussive symptoms,3,4 yet little is known about this subset of children and how they are managed while in the hospital. Characterizing children hospitalized for concussion and describing the inpatient care they received will promote hypothesis generation for further inquiry into indications for admission, as well as the relationship between inpatient management and concussion recovery.
We described a cohort of children admitted to 40 pediatric hospitals primarily for concussion and detailed care delivered during hospitalization. We explored individual-level factors and their association with prolonged length of stay (LOS) and emergency department (ED) readmission. Finally, we evaluated if there had been changes in inpatient care over the 8-year study period.
PATIENTS AND METHODS
Study Design
The Institutional Review Board determined that this retrospective cohort study was exempt from review.
Data Source
The Children’s Hospital Association’s Pediatric Health Information System (PHIS) is an administrative database from pediatric hospitals located within 17 major metropolitan areas in the United States. Data include: service dates, patient demographics, payer type, diagnosis codes, resource utilization information (eg, medications), and hospital characteristics.1,5 De-identified data undergo reliability and validity checks prior to inclusion.1,5 We analyzed data from 40 of 43 hospitals that contributed inpatient data during our study period. 2 hospitals were excluded due to inconsistent data submission, and 1 removed their data.
Study Population
Data were extracted for children 0 to 17 years old who were admitted to an inpatient or observational unit between January 1, 2007 and December 31, 2014 for traumatic brain injury (TBI). Children were identified using International Classification of Diseases, Clinical Modification, Ninth Revision (ICD-9-CM) diagnosis codes that denote TBI per the Centers for Disease Control (CDC): 800.0–801.9, 803.0–804.9, 850–854.1, and 959.01.6–8 To examine inpatient care for concussion, we only retained children with a primary (ie, first) concussion-related diagnosis code (850.0–850.99) for analyses. For patients with multiple visits during our study period, only the index admission was analyzed. We refined our cohort using 2 injury scores calculated from ICD-9-CM diagnosis codes using validated ICDMAP-90 injury coding software.6,10–12 The Abbreviated Injury Scale (AIS) ranges from 1 (minor injury) to 6 (not survivable). The total Injury Severity Score (ISS) is based on 6 body regions (head/neck, face, chest, abdomen, extremity, and external) and calculated by summing the squares of the 3 worst AIS scores.13 A concussion receives a head AIS score of 2 if there is an associated loss of consciousness or a score of 1 if there is not; therefore, children were excluded if the head AIS score was >2. We also excluded children with the following features, as they may be indicative of more severe injuries that were likely the cause of admission: ISS > 6, secondary diagnosis code of skull fracture or intracranial injury, intensive care unit (ICU) or operating room (OR) charges, or a LOS > 7 days. Because some children are hospitalized for potentially abusive minor head trauma pending a safe discharge plan, we excluded children 0 to 4 years of age with child abuse, which was determined using a specific set of diagnosis codes (E960-E96820, 995.54, and 995.55) similar to previous research.14
Data Elements and Outcomes
Outcomes
Based on previous reports,1,15 a LOS ≥ 2 days distinguished a typical hospitalization from a prolonged one. ED revisit was identified when a child had a visit with a TBI-related primary diagnosis code at a PHIS hospital within 30 days of initial admission and was discharged home. We limited analyses to children discharged, as children readmitted may have had an initially missed intracranial injury.
Patient Characteristics
We examined the following patient variables: age, race, sex, presence of chronic medical condition, payer type, household income, area of residence (eg, rural versus urban), and mechanism of injury. Age was categorized to represent early childhood (0 to 4 years), school age (5 to 12 years), and adolescence (12 to 17 years). Race was grouped as white, black, or other (Asian, Pacific Islander, American Indian, and “other” per PHIS). Ethnicity was described as Hispanic/Latino or not Hispanic/Latino. Children with medical conditions lasting at least 12 months and comorbidities that may impact TBI recovery were identified using a subgrouping of ICD-9-CM codes for children with “complex chronic conditions”.16 Payer type was categorized as government, private, and self-pay. We extracted a PHIS variable representing the 2010 median household income for the child’s home zip code and categorized it into quartiles based on the Federal Poverty Level for a family of 4.17,18 Area of residence was defined using a Rural–Urban Commuting Area (RUCA) classification system19 and grouped into large urban core, suburban area, large rural town, or small rural town/isolated rural area.17 Mechanism of injury was determined using E-codes and categorized using the CDC injury framework,20 with sports-related injuries identified using a previously described set of E-codes.1 Mechanisms of injury included fall, motor vehicle collision, other motorized transport (eg, all-terrain vehicles), sports-related, struck by or against (ie, objects), and all others (eg, cyclists).
Hospital Characteristics
Hospitals were characterized by region (Northeast, Central, South, and West) and size (small <200, medium 200–400, and large >400 beds). The trauma-level accreditation was identified with Level 1 reflecting the highest possible trauma resources.
Medical Care Variables
Care variables included medications, neuroimaging, and cost of stay. Medication classes included oral non-narcotic analgesics [acetaminophen, ibuprofen, and others (aspirin, tramadol, and naproxen)], oral narcotics (codeine, oxycodone, and narcotic–non-narcotic combinations), intravenous (IV) non-narcotics (ketorolac), IV narcotics (morphine, fentanyl, and hydromorphone), antiemetics [ondansetron, metoclopramide, and phenothiazines (prochlorperazine, chlorpromazine, and promethazine)], maintenance IV fluids (dextrose with electrolytes or 0.45% sodium chloride), and resuscitation IV fluids (0.9% sodium chloride or lactated Ringer’s solution). Receipt of neuroimaging was determined if head computed tomography (CT) had been conducted at the admitting hospital. Adjusted cost of stay was calculated using a hospital-specific cost-to-charge ratio with additional adjustments using the Center for Medicare & Medicaid’s Wage Index.
Statistical Analyses
Descriptive statistics were calculated for individual, injury, and hospital, and care data elements, LOS, and ED readmissions. The number of children admitted with TBI was used as the denominator to assess the proportion of pediatric TBI admissions that were due to concussions. To identify factors associated with prolonged LOS (ie, ≥2 days) and ED readmission, we employed a mixed models approach that accounted for clustering of observations within hospitals. Independent variables included age, sex, race, ethnicity, payer type, household income, RUCA code, chronic medical condition, and injury mechanism. Models were adjusted for hospital location, size, and trauma-level accreditation. The binary distribution was specified along with a logit link function. A 2-phase process determined factors associated with each outcome. First, bivariable models were developed, followed by multivariable models that included independent variables with P values < .25 in the bivariable analysis. Backward step-wise elimination was performed, deleting variables with the highest P value one at a time. After each deletion, the percentage change in odds ratios was examined; if variable removal resulted in >10% change, the variable was retained as a potential confounder. This process was repeated until all remaining variables were significant (P < .05) with the exception of potential confounders. Finally, we examined the proportion of children receiving selected care practices annually. Descriptive and trend analyses were used to analyze adjusted median cost of stay. Analyses were performed using SAS software (Version 9.3, SAS Institute Inc., Cary, North Carolina).
RESULTS
Over 8 years, 88,526 children were admitted to 40 PHIS hospitals with a TBI-related diagnosis, among whom 13,708 had a primary diagnosis of concussion. We excluded 2,973 children with 1 or more of the following characteristics: a secondary diagnosis of intracranial injury (n = 58), head AIS score > 2 (n = 218), LOS > 7 days (n = 50), OR charges (n = 132), ICU charges (n = 1947), and ISS > 6 (n = 568). Six additional children aging 0 to 4 years were excluded due to child abuse. The remaining 10,729 children, averaging 1300 hospitalizations annually, were identified as being hospitalized primarily for concussion.
Table 1 summarizes the individual characteristics for this cohort. The average (standard deviation) age was 9.5 (5.1) years. Ethnicity was missing for 25.3% and therefore excluded from the multivariable models. Almost all children had a head AIS score of 2 (99.2%), and the majority had a total ISS ≤ 4 (73.4%). The majority of admissions were admitted to Level 1 trauma-accredited hospitals (78.7%) and medium-sized hospitals (63.9%).
The most commonly delivered medication classes were non-narcotic oral analgesics (53.7%), dextrose-containing IV fluids (45.0%), and antiemetic medications (34.1%). IV and oral narcotic use occurred in 19.7% and 10.2% of the children, respectively. Among our cohort, 16.7% received none of these medication classes. Of the 8,940 receiving medication, 32.6% received a single medication class, 29.5% received 2 classes, 20.5% 3 classes, 11.9% 4 classes, and 5.5% received 5 or more medication classes. Approximately 15% (n = 1597) received only oral medications, among whom 91.2% (n = 1457) received only non-narcotic analgesics and 3.9% (n = 63) received only oral narcotic analgesics. The majority (69.5%) received a head CT.
Table 4 summarizes medication administration trends over time. Oral non-narcotic administration increased significantly (slope = 0.99, P < .01) with the most pronounced change occurring in ibuprofen use (slope = 1.11, P < .001). Use of the IV non-narcotic ketorolac (slope = 0.61, P < .001) also increased significantly, as did the proportion of children receiving antiemetics (slope = 1.59, P = .001), with a substantial increase in ondansetron use (slope = 1.56, P = .001). The proportion of children receiving head CTs decreased linearly over time (slope= −1.75, P < .001), from 76.1% in 2007 to 63.7% in 2014. Median cost, adjusted for inflation, increased during our study period (P < .001) by approximately $353 each year, reaching $11,249 by 2014.
DISCUSSION
From 2007 to 2014, approximately 15% of children admitted to PHIS hospitals for TBI were admitted primarily for concussion. Since almost all children had a head AIS score of 2 and an ISS ≤ 4, our data suggest that most children had an associated loss of consciousness and that concussion was the only injury sustained, respectively. This study identified important subgroups that necessitated inpatient care but are rarely the focus of concussion research (eg, toddlers and those injured due to a motor vehicle collision). Most children (83.3%) received medications to treat common postconcussive symptoms (eg, pain and nausea), with almost half receiving 3 or more medication classes. Factors associated with the development of postconcussive syndrome (eg, female sex and adolescent age)4,21 were significantly associated with hospitalization of 2 or more days and ED revisit within 30 days of admission. In the absence of evidenced-based guidelines for inpatient concussion management, we identified significant trends in care, including increased use of specific pain [ie, oral and IV nonsteroidal anti-inflammatory drugs (NSAIDs)] and antiemetic (ie, ondansetron) medications and decreased use of head CT. Given the number of children admitted and receiving treatment for concussion symptomatology, influences on the decision to deliver specific care practices, as well as the impact and benefit of hospitalization, require closer examination.
Our study extends previous reports from the PHIS database by characterizing children admitted for concussion.1 We found that children admitted for concussion had similar characteristics to the broader population of children who sustain concussion (eg, school-aged children, male, and injured due to a fall or during sports).1,3,22 However, approximately 20% of the cohort were less than 5 years old, and less is known regarding appropriate treatment and outcomes of concussion in this age group.23 Uncertainty regarding optimal management and a young child’s inability to articulate symptoms may contribute to a physician’s decision to admit for close observation. Similar to Blinman et al., we found that a substantial proportion of children admitted with concussion were injured due to a motor vehicle collision,3 suggesting that although sports-related injuries are responsible for a significant proportion of pediatric concussions, children injured by other preventable mechanisms may also be incurring significant concussive injuries. Finally, the majority of our cohort was from an urban core, relative to a rural area, which is likely a reflection of the regionalization of trauma care, as well as variations in access to health care.
Although most children recover fully from concussion without specific interventions, 20%-30% may remain symptomatic at 1 month,3,4,21,24 and children who are hospitalized with concussion may be at higher risk for protracted symptoms. While specific individual or injury-related factors (eg, female sex, adolescent age, and injury due to motor vehicle collision) may contribute to more significant postconcussive symptoms, it is unclear how inpatient management affects recovery trajectory. Frequent sleep disruptions associated with inpatient care25 contradict current acute concussion management recommendations for physical and cognitive rest26 and could potentially impair symptom recovery. Additionally, we found widespread use of NSAIDs, although there is evidence suggesting that NSAIDs may potentially worsen concussive symptoms.26 We identified an increase in medication usage over time despite limited evidence of their effectiveness for pediatric concussion.27–29 This change may reflect improved symptom screening4,30 and/or increased awareness of specific medication safety profiles in pediatric trauma patients, especially for NSAIDs and ondansetron. Although we saw an increase in NSAID use, we did not see a proportional decrease in narcotic use. Similarly, while two-thirds of our cohort received IV medications, there is controversy about the need for IV fluids and medications for other pediatric illnesses, with research demonstrating that IV treatment may not reduce recovery time and may contribute to prolonged hospitalization and phlebitis.31,32 Thus, there is a need to understand the therapeutic effectiveness and benefits of medications and fluids on postconcussion recovery.
Neuroimaging rates for children receiving ED evaluation for concussion have been reported to be up to 60%-70%,1,22 although a more recent study spanning 2006 to 2011 found a 35-%–40% head CT rate in pediatric patients by hospital-based EDs in the United States.33 Our results appear to support decreasing head CT use over time in pediatric hospitals. Hospitalization for observation is costly1 but could decrease a child’s risk of malignancy from radiation exposure. Further work on balancing cost, risk, and shared decision-making with parents could guide decisions regarding emergent neuroimaging versus admission.
This study has limitations inherent to the use of an administrative dataset, including lack of information regarding why the child was admitted. Since the focus was to describe inpatient care of children with concussion, those discharged home from the ED were not included in this dataset. Consequently, we could not contrast the ED care of those discharged home with those who were admitted or assess trends in admission rates for concussion. Although the overall number of concussion admissions has continued to remain stable over time,1 due to a lack of prospectively collected clinical information, we are unable to determine whether observed trends in care are secondary to changes in practice or changes in concussion severity. However, there has been no research to date supporting the latter. Ethnicity was excluded due to high levels of missing data. Cost of stay was not extensively analyzed given hospital variation in designation of observational or inpatient status, which subsequently affects billing.34 Rates of neuroimaging and ED revisit may have been underestimated since children could have received care at a non-PHIS hospital. Similarly, the decrease in the proportion of children receiving neuroimaging over time may have been associated with an increase in children being transferred from a non-PHIS hospital for admission, although with increased regionalization in trauma care, we would not expect transfers of children with only concussion to have significantly increased. Finally, data were limited to the pediatric tertiary care centers participating in PHIS, thereby reducing generalizability and introducing selection bias by only including children who were able to access care at PHIS hospitals. Although the care practices we evaluated (eg, NSAIDs and head CT) are available at all hospitals, our analyses only reflect care delivered within the PHIS.
Concussion accounted for 15% of all pediatric TBI admissions during our study period. Further investigation of potential factors associated with admission and protracted recovery (eg, adolescent females needing treatment for severe symptomatology) could facilitate better understanding of how hospitalization affects recovery. Additionally, research on acute pharmacotherapies (eg, IV therapies and/or inpatient treatment until symptoms resolve) is needed to fully elucidate the acute and long-term benefits of interventions delivered to children.
ACKNOWLEDGMENTS
Colleen Mangeot: Biostatistician with extensive PHIS knowledge who contributed to database creation and statistical analysis. Yanhong (Amy) Liu: Research database programmer who developed the database, ran quality assurance measures, and cleaned all study data.
Disclosures
The authors have nothing to disclose.
Funding
This study was supported by grant R40 MC 268060102 from the Maternal and Child Health Research Program, Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services. The funding source was not involved in development of the study design; in the collection, analysis and interpretation of data; or in the writing of this report.
1. Colvin JD, Thurm C, Pate BM, Newland JG, Hall M, Meehan WP. Diagnosis and acute management of patients with concussion at children’s hospitals. Arch Dis Child. 2013;98(12):934-938. PubMed
2. Bourgeois FT, Monuteaux MC, Stack AM, Neuman MI. Variation in emergency department admission rates in US children’s hospitals. Pediatrics. 2014;134(3):539-545. PubMed
3. Blinman TA, Houseknecht E, Snyder C, Wiebe DJ, Nance ML. Postconcussive symptoms in hospitalized pediatric patients after mild traumatic brain injury. J Pediatr Surg. 2009;44(6):1223-1228. PubMed
4. Babcock L, Byczkowski T, Wade SL, Ho M, Mookerjee S, Bazarian JJ. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA pediatrics. 2013;167(2):156-161. PubMed
5. Conway PH, Keren R. Factors associated with variability in outcomes for children hospitalized with urinary tract infection. The Journal of pediatrics. 2009;154(6):789-796. PubMed
6. Services UDoHaH. International classification of diseases, 9th Revision, Clinical modification (ICD-9CM). Washington, DC: US Department of Health and Human Services. Public Health Service, Health Care Financing Administration 1989.
7. Marr AL, Coronado VG. Annual data submission standards. Central nervous system injury surveillance. In: US Department of Health and Human Services PHS, CDC, ed. Atlanta, GA 2001.
8. Organization WH. International classification of diseases: manual on the international statistical classification of diseases, injuries, and cause of death. In: Organization WH, ed. 9th rev. ed. Geneva, Switerland 1977.
9. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003.
10. Mackenzie E, Sacco WJ. ICDMAP-90 software: user’s guide. Baltimore, Maryland: Johns Hopkins University and Tri-Analytics. 1997:1-25.
11. MacKenzie EJ, Steinwachs DM, Shankar B. Classifying trauma severity based on hospital discharge diagnoses. Validation of an ICD-9CM to AIS-85 conversion table. Med Care. 1989;27(4):412-422. PubMed
12. Fleischman RJ, Mann NC, Dai M, et al. Validating the use of ICD-9 code mapping to generate injury severity scores. J Trauma Nurs. 2017;24(1):4-14. PubMed
13. Baker SP, O’Neill B, Haddon W, Jr., Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. The Journal of trauma. 1974;14(3):187-196. PubMed
14. Wood JN, Feudtner C, Medina SP, Luan X, Localio R, Rubin DM. Variation in occult injury screening for children with suspected abuse in selected US children’s hospitals. Pediatrics
15. Yang J, Phillips G, Xiang H, Allareddy V, Heiden E, Peek-Asa C. Hospitalisations for sport-related concussions in US children aged 5 to 18 years during 2000-2004. Br J Sports Med. 2008;42(8):664-669. PubMed
16. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106(1):205-209. PubMed
17. Peltz A, Wu CL, White ML, et al. Characteristics of rural children admitted to pediatric hospitals. Pediatrics. 2016;137(5): e20153156. PubMed
18. Services UDoHaH. Annual update of the HHS Poverty Guidelines. Federal Register; 2016-03-14 2011.
19. Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95(7):1149-1155. PubMed
20. Proposed Matrix of E-code Groupings| WISQARS | Injury Center | CDC. 2016; http://www.cdc.gov/injury/wisqars/ecode_matrix.html.
21. Zemek RL, Farion KJ, Sampson M, McGahern C. Prognosticators of persistent symptoms following pediatric concussion: A systematic review. JAMA Pediatr. 2013;167(3):259-265. PubMed
22. Meehan WP, Mannix R. Pediatric concussions in United States emergency departments in the years 2002 to 2006. J Pediatr. 2010;157(6):889-893. PubMed
23. Davis GA, Purcell LK. The evaluation and management of acute concussion differs in young children. Br J Sports Med. 2014;48(2):98-101. PubMed
24. Zemek R, Barrowman N, Freedman SB, et al. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315(10):1014-1025. PubMed
25. Hinds PS, Hockenberry M, Rai SN, et al. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncol Nurs Forum. 2007;34(2):393-402. PubMed
26. Patterson ZR, Holahan MR. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front Cell Neurosci. 2012;6:58. PubMed
27. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124, ix. PubMed
28. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533. PubMed
29. Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24):2250-2257. PubMed
30. Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126(2):e374-e381. PubMed
31. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. PubMed
32. Hartling L, Bellemare S, Wiebe N, Russell K, Klassen TP, Craig W. Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children. Cochrane Database Syst Rev. 2006(3):CD004390. PubMed
34. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-1058. PubMed
33. Zonfrillo MR, Kim KH, Arbogast KB. Emergency Department Visits and Head Computed Tomography Utilization for Concussion Patients From 2006 to 2011. Acad Emerg Med. 2015;22(7):872-877. PubMed
1. Colvin JD, Thurm C, Pate BM, Newland JG, Hall M, Meehan WP. Diagnosis and acute management of patients with concussion at children’s hospitals. Arch Dis Child. 2013;98(12):934-938. PubMed
2. Bourgeois FT, Monuteaux MC, Stack AM, Neuman MI. Variation in emergency department admission rates in US children’s hospitals. Pediatrics. 2014;134(3):539-545. PubMed
3. Blinman TA, Houseknecht E, Snyder C, Wiebe DJ, Nance ML. Postconcussive symptoms in hospitalized pediatric patients after mild traumatic brain injury. J Pediatr Surg. 2009;44(6):1223-1228. PubMed
4. Babcock L, Byczkowski T, Wade SL, Ho M, Mookerjee S, Bazarian JJ. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA pediatrics. 2013;167(2):156-161. PubMed
5. Conway PH, Keren R. Factors associated with variability in outcomes for children hospitalized with urinary tract infection. The Journal of pediatrics. 2009;154(6):789-796. PubMed
6. Services UDoHaH. International classification of diseases, 9th Revision, Clinical modification (ICD-9CM). Washington, DC: US Department of Health and Human Services. Public Health Service, Health Care Financing Administration 1989.
7. Marr AL, Coronado VG. Annual data submission standards. Central nervous system injury surveillance. In: US Department of Health and Human Services PHS, CDC, ed. Atlanta, GA 2001.
8. Organization WH. International classification of diseases: manual on the international statistical classification of diseases, injuries, and cause of death. In: Organization WH, ed. 9th rev. ed. Geneva, Switerland 1977.
9. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003.
10. Mackenzie E, Sacco WJ. ICDMAP-90 software: user’s guide. Baltimore, Maryland: Johns Hopkins University and Tri-Analytics. 1997:1-25.
11. MacKenzie EJ, Steinwachs DM, Shankar B. Classifying trauma severity based on hospital discharge diagnoses. Validation of an ICD-9CM to AIS-85 conversion table. Med Care. 1989;27(4):412-422. PubMed
12. Fleischman RJ, Mann NC, Dai M, et al. Validating the use of ICD-9 code mapping to generate injury severity scores. J Trauma Nurs. 2017;24(1):4-14. PubMed
13. Baker SP, O’Neill B, Haddon W, Jr., Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. The Journal of trauma. 1974;14(3):187-196. PubMed
14. Wood JN, Feudtner C, Medina SP, Luan X, Localio R, Rubin DM. Variation in occult injury screening for children with suspected abuse in selected US children’s hospitals. Pediatrics
15. Yang J, Phillips G, Xiang H, Allareddy V, Heiden E, Peek-Asa C. Hospitalisations for sport-related concussions in US children aged 5 to 18 years during 2000-2004. Br J Sports Med. 2008;42(8):664-669. PubMed
16. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106(1):205-209. PubMed
17. Peltz A, Wu CL, White ML, et al. Characteristics of rural children admitted to pediatric hospitals. Pediatrics. 2016;137(5): e20153156. PubMed
18. Services UDoHaH. Annual update of the HHS Poverty Guidelines. Federal Register; 2016-03-14 2011.
19. Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95(7):1149-1155. PubMed
20. Proposed Matrix of E-code Groupings| WISQARS | Injury Center | CDC. 2016; http://www.cdc.gov/injury/wisqars/ecode_matrix.html.
21. Zemek RL, Farion KJ, Sampson M, McGahern C. Prognosticators of persistent symptoms following pediatric concussion: A systematic review. JAMA Pediatr. 2013;167(3):259-265. PubMed
22. Meehan WP, Mannix R. Pediatric concussions in United States emergency departments in the years 2002 to 2006. J Pediatr. 2010;157(6):889-893. PubMed
23. Davis GA, Purcell LK. The evaluation and management of acute concussion differs in young children. Br J Sports Med. 2014;48(2):98-101. PubMed
24. Zemek R, Barrowman N, Freedman SB, et al. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315(10):1014-1025. PubMed
25. Hinds PS, Hockenberry M, Rai SN, et al. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncol Nurs Forum. 2007;34(2):393-402. PubMed
26. Patterson ZR, Holahan MR. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front Cell Neurosci. 2012;6:58. PubMed
27. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124, ix. PubMed
28. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533. PubMed
29. Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24):2250-2257. PubMed
30. Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126(2):e374-e381. PubMed
31. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. PubMed
32. Hartling L, Bellemare S, Wiebe N, Russell K, Klassen TP, Craig W. Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children. Cochrane Database Syst Rev. 2006(3):CD004390. PubMed
34. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-1058. PubMed
33. Zonfrillo MR, Kim KH, Arbogast KB. Emergency Department Visits and Head Computed Tomography Utilization for Concussion Patients From 2006 to 2011. Acad Emerg Med. 2015;22(7):872-877. PubMed
© 2018 Society of Hospital Medicine
Focused Ethnography of Diagnosis in Academic Medical Centers
Diagnostic error—defined as a failure to establish an accurate and timely explanation of the patient’s health problem—is an important source of patient harm.1 Data suggest that all patients will experience at least 1 diagnostic error in their lifetime.2-4 Not surprisingly, diagnostic errors are among the leading categories of paid malpractice claims in the United States.5
Despite diagnostic errors being morbid and sometimes deadly in the hospital,6,7 little is known about how residents and learners approach diagnostic decision making. Errors in diagnosis are believed to stem from cognitive or system failures,8 with errors in cognition believed to occur due to rapid, reflexive thinking operating in the absence of a more analytical, deliberate process. System-based problems (eg, lack of expert availability, technology barriers, and access to data) have also been cited as contributors.9 However, whether and how these apply to trainees is not known.
Therefore, we conducted a focused ethnography of inpatient medicine teams (ie, attendings, residents, interns, and medical students) in 2 affiliated teaching hospitals, aiming to (a) observe the process of diagnosis by trainees and (b) identify methods to improve the diagnostic process and prevent errors.
METHODS
We designed a multimethod, focused ethnographic study to examine diagnostic decision making in hospital settings.10,11 In contrast to anthropologic ethnographies that study entire fields using open-ended questions, our study was designed to examine the process of diagnosis from the perspective of clinicians engaged in this activity.11 This approach allowed us to capture diagnostic decisions and cognitive and system-based factors in a manner currently lacking in the literature.12
Setting and Participants
Between January 2016 and May 2016, we observed the members of four inpatient internal medicine teaching teams at 2 affiliated teaching hospitals. We purposefully selected teaching teams for observation because they are the primary model of care in academic settings and we have expertise in carrying out similar studies.13,14 Teaching teams typically consisted of a medical attending (senior-level physician), 1 senior resident (a second- or third-year postgraduate trainee), two interns (a trainee in their first postgraduate year), and two to four medical students. Teams were selected at random using existing schedules and followed Monday to Friday so as to permit observation of work on call and noncall days. Owing to manpower limitations, weekend and night shifts were not observed. However, overnight events were captured during morning rounds.
Most of the teams began rounds at 8:30 AM. Typically, rounds lasted for 90–120 min and concluded with a recap (ie, “running the list”) with a review of explicit plans for patients after they had been evaluated by the attending. This discussion often occurred in the team rooms, with the attending leading the discussion with the trainees.
Data Collection
A multidisciplinary team, including clinicians (eg, physicians, nurses), nonclinicians (eg, qualitative researchers, social scientists), and healthcare engineers, conducted the observations. We observed preround activities of interns and residents before arrival of the attending (7:00 AM - 8:30 AM), followed by morning rounds with the entire team, and afternoon work that included senior residents, interns, and students.
To capture multiple aspects of the diagnostic process, we collected data using field notes modeled on components of the National Academy of Science model for diagnosis (Appendix).1,15 This model encompasses phases of the diagnostic process (eg, data gathering, integration, formulation of a working diagnosis, treatment delivery, and outcomes) and the work system (team members, organization, technology and tools, physical environment, tasks).
Focus Groups and Interviews
At the end of weekly observations, we conducted focus groups with the residents and one-on- one interviews with the attendings. Focus groups with the residents were conducted to encourage a group discussion about the diagnostic process. Separate interviews with the attendings were performed to ensure that power differentials did not influence discussions. During focus groups, we specifically asked about challenges and possible solutions to improve diagnosis. Experienced qualitative methodologists (J.F., M.H., M.Q.) used semistructured interview guides for discussions (Appendix).
Data Analysis
After aggregating and reading the data, three reviewers (V.C., S.K., S.S.) began inductive analysis by handwriting notes and initial reflective thoughts to create preliminary codes. Multiple team members then reread the original field notes and the focus group/interview data to refine the preliminary codes and develop additional codes. Next, relationships between codes were identified and used to develop key themes. Triangulation of data collected from observations and interview/focus group sessions was carried out to compare data that we surmised with data that were verbalized by the team. The developed themes were discussed as a group to ensure consistency of major findings.
Ethical and Regulatory Oversight
This study was reviewed and approved by the Institutional Review Boards at the University of Michigan Health System (HUM-00106657) and the VA Ann Arbor Healthcare System (1-2016-010040).
RESULTS
Four teaching teams (4 attendings, 4 senior residents, 9 interns, and 14 medical students) were observed over 33 distinct shifts and 168 hours. Observations included morning rounds (96 h), postround call days (52 h), and postround non-call days (20 h). Morning rounds lasted an average of 127 min (range: 48-232 min) and included an average of 9 patients (range: 4-16 patients).
Themes Regarding the Diagnostic Process
We identified the following 4 primary themes related to the diagnostic process in teaching hospitals: (1) diagnosis is a social phenomenon; (2) data necessary to make diagnoses are fragmented; (3) distractions undermine the diagnostic process; and (4) time pressures interfere with diagnostic decision making (Appendix Table 1).
(1) Diagnosis is a Social Phenomenon.
Team members viewed the process of diagnosis as a social exchange of facts, findings, and strategies within a defined structure. The opportunity to discuss impressions with others was valued as a means to share, test, and process assumptions.
“Rounds are the most important part of the process. That is where we make most decisions in a collective, collaborative way with the attending present. We bounce ideas off each other.” (Intern)
Typical of social processes, variations based on time of day and schedule were observed. For instance, during call days, learners gathered data and formed working diagnosis and treatment plans with minimal attending interaction. This separation of roles and responsibilities introduced a hierarchy within diagnosis as follows:
“The interns would not call me first; they would talk to the senior resident and then if the senior thought he should chat with me, then they would call. But for the most part, they gather information and come up with the plan.” (Attending).
The work system was suited to facilitate social interactions. For instance, designated rooms (with team members informally assigned to a computer) provided physical proximity of the resident to interns and medical students. In this space, numerous informal discussions between team members (eg, “What do you think about this test?” “I’m not sure what to do about this finding.” “Should I call a [consult] on this patient?”) were observed. Although proximity to each other was viewed as beneficial, dangers to the social nature of diagnosis in the form of anchoring (ie, a cognitive bias where emphasis is placed on the first piece of data)16 were also mentioned. Similarly, the paradox associated with social proof (ie, the pressure to assume conformity within a group) was also observed as disagreement between team members and attendings rarely occurred during observations.
“I mean, they’re the attending, right? It’s hard to argue with them when they want a test or something done. When I do push back, it’s rare that others will support me–so it’s usually me and the attending.” (Resident)
“I would push back if I think it’s really bad for the patient or could cause harm–but the truth is, it doesn’t happen much.” (Intern)
(2) Data Necessary to Make Diagnoses are Fragmented
Team members universally cited fragmentation in data delivery, retrieval, and processing as a barrier to diagnosis. Team members indicated that test results might not be looked at or acted upon in a timely manner, and participants pointed to the electronic medical record as a source of this challenge.
“Before I knew about [the app for Epic], I would literally sit on the computer to get all the information we would need on rounds. Its key to making decisions. We often say we will do something, only to find the test result doesn’t support it–and then we’re back to square 1.” (Intern)
Information used by teams came from myriad sources (eg, patients, family members, electronic records) and from various settings (eg, emergency department, patient rooms, discussions with consultants). Additionally, test results often appeared without warning. Thus, availability of information was poorly aligned with clinical duties.
“They (the lab) will call us when a blood culture is positive or something is off. That is very helpful but it often comes later in the day, when we’re done with rounds.” (Resident)
The work system was highlighted as a key contributor to data fragmentation. Peculiarities of our electronic medical record (EMR) and how data were collected, stored, or presented were described as “frustrating,” and “unsafe,” by team members. Correspondingly, we frequently observed interns asking for assistance for tasks such as ordering tests or finding information despite being “trained” to use the EMR.
“People have to learn how to filter, how to recognize the most important points and link data streams together in terms of causality. But we assume they know where to find that information. It’s actually a very hard thing to do, for both the house staff and me.” (Attending)
(3) Distractions Undermine the Diagnostic Process
Distractions often created cognitive difficulties. For example, ambient noise and interruptions from neighbors working on other teams were cited as barriers to diagnosis. In addition, we observed several team members using headphones to drown out ambient noise while working on the computer.
“I know I shouldn’t do it (wear headphones), but I have no other way of turning down the noise so I can concentrate.” (Intern)
Similarly, the unpredictable nature and the volume of pages often interrupted thinking about diagnosis.
“Sometimes the pager just goes off all the time and (after making sure its not an urgent issue), I will just ignore it for a bit, especially if I am in the middle of something. It would be great if I could finish my thought process knowing I would not be interrupted.” (Resident)
To mitigate this problem, 1 attending described how he would proactively seek out nurses caring for his patients to “head off” questions (eg, “I will renew the restraints and medications this morning,” and “Is there anything you need in terms of orders for this patient that I can take care of now?”) that might lead to pages. Another resident described his approach as follows:
“I make it a point to tell the nurses where I will be hanging out and where they can find me if they have any questions. I tell them to come talk to me rather than page me since that will be less distracting.” (Resident).
Most of the interns described documentation work such as writing admission and progress notes in negative terms (“an academic exercise,” “part of the billing activity”). However, in the context of interruptions, some described this as helpful.
“The most valuable part of the thinking process was writing the assessment and plan because that’s actually my schema for all problems. It literally is the only time where I can sit and collect my thoughts to formulate a diagnosis and plan.” (Intern)
(4) Time Pressures Interfere With Diagnostic Decision Making
All team members spoke about the challenge of finding time for diagnosis during the workday. Often, they had to skip learning sessions for this purpose.
“They tell us we should go to morning report or noon conference but when I’m running around trying to get things done. I hate having to choose between my education and doing what’s best for the patient–but that’s often what it comes down to.” (Intern)
When specifically asked whether setting aside dedicated time to specifically review and formulate diagnoses would be valuable, respondents were uniformly enthusiastic. Team members described attentional conflicts as being the worst when “cross covering” other teams on call days, as their patient load effectively doubled during this time. Of note, cross-covering occurred when teams were also on call—and thus took them away from important diagnostic activities such as data gathering or synthesis for patients they were admitting.
“If you were to ever design a system where errors were likely–this is how you would design it: take a team with little supervision, double their patient load, keep them busy with new challenging cases and then ask questions about patients they know little about.” (Resident)
DISCUSSION
Although diagnostic errors have been called “the next frontier for patient safety,”17 little is known about the process, barriers, and facilitators to diagnosis in teaching hospitals. In this focused ethnography conducted at 2 academic medical centers, we identified multiple cognitive and system-level challenges and potential strategies to improve diagnosis from trainees engaged in this activity. Key themes identified by those we observed included the social nature of diagnosis, fragmented information delivery, constant distractions and interruptions, and time pressures. In turn, these insights allow us to generate strategies that can be applied to improve the diagnostic process in teaching hospitals.
Our study underscores the importance of social interactions in diagnosis. In contrast, most of the interventions to prevent diagnostic errors target individual providers through practices such as metacognition and “thinking about thinking.”18-20 These interventions are based on Daniel Kahnemann’s work on dual thought process. Type 1 thought processes are fast, subconscious, reflexive, largely intuitive, and more vulnerable to error. In contrast, Type 2 processes are slower, deliberate, analytic, and less prone to error.21 Although an individual’s Type 2 thought capacity is limited, a major goal of cognitive interventions is to encourage Type 2 over Type 1 thinking, an approach termed “de-biasing.”22-24 Unfortunately, cognitive interventions testing such approaches have suffered mixed results–perhaps because of lack of focus on collective wisdom or group thinking, which may be key to diagnosis from our findings.9,25 In this sense, morning rounds were a social gathering used to strategize and develop care plans, but with limited time to think about diagnosis.26 Introduction of defined periods for individuals to engage in diagnostic activities such as de-biasing (ie, asking “what else could this be)27 before or after rounds may provide an opportunity for reflection and improving diagnosis. In addition, embedding tools such as diagnosis expanders and checklists within these defined time slots28,29 may prove to be useful in reflecting on diagnosis and preventing diagnostic errors.
An unexpected yet important finding from this study were the challenges posed by distractions and the physical environment. Potentially maladaptive workarounds to these interruptions included use of headphones; more productive strategies included updating nurses with plans to avert pages and creating a list of activities to ensure that key tasks were not forgotten.30,31 Applying lessons from aviation, a focused effort to limit distractions during key portions of the day, might be worth considering for diagnostic safety.32 Similarly, improving the environment in which diagnosis occurs—including creating spaces that are quiet, orderly, and optimized for thinking—may be valuable.33Our study has limitations. First, our findings are limited to direct observations; we are thus unable to comment on how unobserved aspects of care (eg, cognitive processes) might have influenced our findings. Our observations of clinical care might also have introduced a Hawthorne effect. However, because we were closely integrated with teams and conducted focus groups to corroborate our assessments, we believe that this was not the case. Second, we did not identify diagnostic errors or link processes we observed to errors. Third, our approach is limited to 2 teaching centers, thereby limiting the generalizability of findings. Relatedly, we were only able to conduct observations during weekdays; differences in weekend and night resources might affect our insights.
The cognitive and system-based barriers faced by clinicians in teaching hospitals suggest that new methods to improve diagnosis are needed. Future interventions such as defined “time-outs” for diagnosis, strategies focused on limiting distractions, and methods to improve communication between team members are novel and have parallels in other industries. As challenges to quantify diagnostic errors abound,34 improving cognitive- and system-based factors via reflection through communication, concentration, and organization is necessary to improve medical decision making in academic medical centers.
Disclosures
None declared for all coauthors.
Funding
This project was supported by grant number P30HS024385 from the Agency for Healthcare Research and Quality. The funding source played no role in study design, data acquisition, analysis or decision to report these data. Dr. Chopra is supported by a career development award from the Agency of Healthcare Research and Quality (1-K08-HS022835-01). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). Dr. Singh is partially supported by Houston VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the Department of Veterans Affairs.
1. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press. http://www.nap.edu/21794. Accessed November 1; 2016:2015. https://doi.org/10.17226/21794.
2. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. http://dx.doi.org/10.1001/archinternmed.2009.333. PubMed
3. Sonderegger-Iseli K, Burger S, Muntwyler J, Salomon F. Diagnostic errors in three medical eras: A necropsy study. Lancet. 2000;355(9220):2027-2031. http://dx.doi.org/10.1016/S0140-6736(00)02349-7. PubMed
4. Winters B, Custer J, Galvagno SM Jr, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. http://dx.doi.org/10.1136/bmjqs-2012-000803. PubMed
5. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-Year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. http://dx.doi.org/10.1136/bmjqs-2012-001550. PubMed
6. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77(10):981-992. http://dx.doi.org/10.1097/00001888-200210000-00009. PubMed
7. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2018;27(1):53-60. 10.1136/bmjqs-2017-006774. PubMed
8. van Noord I, Eikens MP, Hamersma AM, de Bruijne MC. Application of root cause analysis on malpractice claim files related to diagnostic failures. Qual Saf Health Care. 2010;19(6):e21. http://dx.doi.org/10.1136/qshc.2008.029801. PubMed
9. Croskerry P, Petrie DA, Reilly JB, Tait G. Deciding about fast and slow decisions. Acad Med. 2014;89(2):197-200. 10.1097/ACM.0000000000000121. PubMed
10. Higginbottom GM, Pillay JJ, Boadu NY. Guidance on performing focused ethnographies with an emphasis on healthcare research. Qual Rep. 2013;18(9):1-6. https://doi.org/10.7939/R35M6287P.
11. Savage J. Participative observation: standing in the shoes of others? Qual Health Res. 2000;10(3):324-339. http://dx.doi.org/10.1177/104973200129118471. PubMed
12. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Thousand Oaks, CA: SAGE Publications; 2002.
13. Harrod M, Weston LE, Robinson C, Tremblay A, Greenstone CL, Forman J. “It goes beyond good camaraderie”: A qualitative study of the process of becoming an interprofessional healthcare “teamlet.” J Interprof Care. 2016;30(3):295-300. http://dx.doi.org/10.3109/13561820.2015.1130028. PubMed
14. Houchens N, Harrod M, Moody S, Fowler KE, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. http://dx.doi.org/10.12788/jhm.2763. PubMed
15. Mulhall A. In the field: notes on observation in qualitative research. J Adv Nurs. 2003;41(3):306-313. http://dx.doi.org/10.1046/j.1365-2648.2003.02514.x. PubMed
16. Zwaan L, Monteiro S, Sherbino J, Ilgen J, Howey B, Norman G. Is bias in the eye of the beholder? A vignette study to assess recognition of cognitive biases in clinical case workups. BMJ Qual Saf. 2017;26(2):104-110. http://dx.doi.org/10.1136/bmjqs-2015-005014. PubMed
17. Singh H, Graber ML. Improving diagnosis in health care--the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. http://dx.doi.org/10.1056/NEJMp1512241. PubMed
18. Croskerry P. From mindless to mindful practice--cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448. http://dx.doi.org/10.1056/NEJMp1303712. PubMed
19. van den Berge K, Mamede S. Cognitive diagnostic error in internal medicine. Eur J Intern Med. 2013;24(6):525-529. http://dx.doi.org/10.1016/j.ejim.2013.03.006. PubMed
20. Norman G, Sherbino J, Dore K, et al. The etiology of diagnostic errors: A controlled trial of system 1 versus system 2 reasoning. Acad Med. 2014;89(2):277-284. 10.1097/ACM.0000000000000105 PubMed
21. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf. 2017;26(2):87-89. http://dx.doi.org/10.1136/bmjqs-2016-005267. PubMed
22. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: Origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58-iiii64. http://dx.doi.org/10.1136/bmjqs-2012-001712. PubMed
23. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: Impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-iiii72. http://dx.doi.org/10.1136/bmjqs-2012-001713. PubMed
24. Reilly JB, Ogdie AR, Von Feldt JM, Myers JS. Teaching about how doctors think: a longitudinal curriculum in cognitive bias and diagnostic error for residents. BMJ Qual Saf. 2013;22(12):1044-1050. http://dx.doi.org/10.1136/bmjqs-2013-001987. PubMed
25. Schmidt HG, Mamede S, van den Berge K, van Gog T, van Saase JL, Rikers RM. Exposure to media information about a disease can cause doctors to misdiagnose similar-looking clinical cases. Acad Med. 2014;89(2):285-291. http://dx.doi.org/10.1097/ACM.0000000000000107. PubMed
26. Hess BJ, Lipner RS, Thompson V, Holmboe ES, Graber ML. Blink or think: can further reflection improve initial diagnostic impressions? Acad Med. 2015;90(1):112-118. http://dx.doi.org/10.1097/ACM.0000000000000550. PubMed
27. Lambe KA, O’Reilly G, Kelly BD, Curristan S. Dual-process cognitive interventions to enhance diagnostic reasoning: A systematic review. BMJ Qual Saf. 2016;25(10):808-820. http://dx.doi.org/10.1136/bmjqs-2015-004417. PubMed
28. Graber ML, Kissam S, Payne VL, et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21(7):535-557. http://dx.doi.org/10.1136/bmjqs-2011-000149. PubMed
29. McDonald KM, Matesic B, Contopoulos-Ioannidis DG, et al. Patient safety strategies targeted at diagnostic errors: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):381-389. http://dx.doi.org/10.7326/0003-4819-158-5-201303051-00004. PubMed
30. Wray CM, Chaudhry S, Pincavage A, et al. Resident shift handoff strategies in US internal medicine residency programs. JAMA. 2016;316(21):2273-2275. http://dx.doi.org/10.1001/jama.2016.17786. PubMed
31. Choo KJ, Arora VM, Barach P, Johnson JK, Farnan JM. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014;9(3):169-175. http://dx.doi.org/10.1002/jhm.2150. PubMed
32. Carayon P, Wood KE. Patient safety - the role of human factors and systems engineering. Stud Health Technol Inform. 2010;153:23-46.
.http://dx.doi.org/10.1001/jama.2015.13453 PubMed
34. McGlynn EA, McDonald KM, Cassel CK. Measurement is essential for improving diagnosis and reducing diagnostic error: A report from the Institute of Medicine. JAMA. 2015;314(23):2501-2502.
.http://dx.doi.org/10.1136/bmjqs-2013-001812 PubMed
33. Carayon P, Xie A, Kianfar S. Human factors and ergonomics as a patient safety practice. BMJ Qual Saf. 2014;23(3):196-205. PubMed
Diagnostic error—defined as a failure to establish an accurate and timely explanation of the patient’s health problem—is an important source of patient harm.1 Data suggest that all patients will experience at least 1 diagnostic error in their lifetime.2-4 Not surprisingly, diagnostic errors are among the leading categories of paid malpractice claims in the United States.5
Despite diagnostic errors being morbid and sometimes deadly in the hospital,6,7 little is known about how residents and learners approach diagnostic decision making. Errors in diagnosis are believed to stem from cognitive or system failures,8 with errors in cognition believed to occur due to rapid, reflexive thinking operating in the absence of a more analytical, deliberate process. System-based problems (eg, lack of expert availability, technology barriers, and access to data) have also been cited as contributors.9 However, whether and how these apply to trainees is not known.
Therefore, we conducted a focused ethnography of inpatient medicine teams (ie, attendings, residents, interns, and medical students) in 2 affiliated teaching hospitals, aiming to (a) observe the process of diagnosis by trainees and (b) identify methods to improve the diagnostic process and prevent errors.
METHODS
We designed a multimethod, focused ethnographic study to examine diagnostic decision making in hospital settings.10,11 In contrast to anthropologic ethnographies that study entire fields using open-ended questions, our study was designed to examine the process of diagnosis from the perspective of clinicians engaged in this activity.11 This approach allowed us to capture diagnostic decisions and cognitive and system-based factors in a manner currently lacking in the literature.12
Setting and Participants
Between January 2016 and May 2016, we observed the members of four inpatient internal medicine teaching teams at 2 affiliated teaching hospitals. We purposefully selected teaching teams for observation because they are the primary model of care in academic settings and we have expertise in carrying out similar studies.13,14 Teaching teams typically consisted of a medical attending (senior-level physician), 1 senior resident (a second- or third-year postgraduate trainee), two interns (a trainee in their first postgraduate year), and two to four medical students. Teams were selected at random using existing schedules and followed Monday to Friday so as to permit observation of work on call and noncall days. Owing to manpower limitations, weekend and night shifts were not observed. However, overnight events were captured during morning rounds.
Most of the teams began rounds at 8:30 AM. Typically, rounds lasted for 90–120 min and concluded with a recap (ie, “running the list”) with a review of explicit plans for patients after they had been evaluated by the attending. This discussion often occurred in the team rooms, with the attending leading the discussion with the trainees.
Data Collection
A multidisciplinary team, including clinicians (eg, physicians, nurses), nonclinicians (eg, qualitative researchers, social scientists), and healthcare engineers, conducted the observations. We observed preround activities of interns and residents before arrival of the attending (7:00 AM - 8:30 AM), followed by morning rounds with the entire team, and afternoon work that included senior residents, interns, and students.
To capture multiple aspects of the diagnostic process, we collected data using field notes modeled on components of the National Academy of Science model for diagnosis (Appendix).1,15 This model encompasses phases of the diagnostic process (eg, data gathering, integration, formulation of a working diagnosis, treatment delivery, and outcomes) and the work system (team members, organization, technology and tools, physical environment, tasks).
Focus Groups and Interviews
At the end of weekly observations, we conducted focus groups with the residents and one-on- one interviews with the attendings. Focus groups with the residents were conducted to encourage a group discussion about the diagnostic process. Separate interviews with the attendings were performed to ensure that power differentials did not influence discussions. During focus groups, we specifically asked about challenges and possible solutions to improve diagnosis. Experienced qualitative methodologists (J.F., M.H., M.Q.) used semistructured interview guides for discussions (Appendix).
Data Analysis
After aggregating and reading the data, three reviewers (V.C., S.K., S.S.) began inductive analysis by handwriting notes and initial reflective thoughts to create preliminary codes. Multiple team members then reread the original field notes and the focus group/interview data to refine the preliminary codes and develop additional codes. Next, relationships between codes were identified and used to develop key themes. Triangulation of data collected from observations and interview/focus group sessions was carried out to compare data that we surmised with data that were verbalized by the team. The developed themes were discussed as a group to ensure consistency of major findings.
Ethical and Regulatory Oversight
This study was reviewed and approved by the Institutional Review Boards at the University of Michigan Health System (HUM-00106657) and the VA Ann Arbor Healthcare System (1-2016-010040).
RESULTS
Four teaching teams (4 attendings, 4 senior residents, 9 interns, and 14 medical students) were observed over 33 distinct shifts and 168 hours. Observations included morning rounds (96 h), postround call days (52 h), and postround non-call days (20 h). Morning rounds lasted an average of 127 min (range: 48-232 min) and included an average of 9 patients (range: 4-16 patients).
Themes Regarding the Diagnostic Process
We identified the following 4 primary themes related to the diagnostic process in teaching hospitals: (1) diagnosis is a social phenomenon; (2) data necessary to make diagnoses are fragmented; (3) distractions undermine the diagnostic process; and (4) time pressures interfere with diagnostic decision making (Appendix Table 1).
(1) Diagnosis is a Social Phenomenon.
Team members viewed the process of diagnosis as a social exchange of facts, findings, and strategies within a defined structure. The opportunity to discuss impressions with others was valued as a means to share, test, and process assumptions.
“Rounds are the most important part of the process. That is where we make most decisions in a collective, collaborative way with the attending present. We bounce ideas off each other.” (Intern)
Typical of social processes, variations based on time of day and schedule were observed. For instance, during call days, learners gathered data and formed working diagnosis and treatment plans with minimal attending interaction. This separation of roles and responsibilities introduced a hierarchy within diagnosis as follows:
“The interns would not call me first; they would talk to the senior resident and then if the senior thought he should chat with me, then they would call. But for the most part, they gather information and come up with the plan.” (Attending).
The work system was suited to facilitate social interactions. For instance, designated rooms (with team members informally assigned to a computer) provided physical proximity of the resident to interns and medical students. In this space, numerous informal discussions between team members (eg, “What do you think about this test?” “I’m not sure what to do about this finding.” “Should I call a [consult] on this patient?”) were observed. Although proximity to each other was viewed as beneficial, dangers to the social nature of diagnosis in the form of anchoring (ie, a cognitive bias where emphasis is placed on the first piece of data)16 were also mentioned. Similarly, the paradox associated with social proof (ie, the pressure to assume conformity within a group) was also observed as disagreement between team members and attendings rarely occurred during observations.
“I mean, they’re the attending, right? It’s hard to argue with them when they want a test or something done. When I do push back, it’s rare that others will support me–so it’s usually me and the attending.” (Resident)
“I would push back if I think it’s really bad for the patient or could cause harm–but the truth is, it doesn’t happen much.” (Intern)
(2) Data Necessary to Make Diagnoses are Fragmented
Team members universally cited fragmentation in data delivery, retrieval, and processing as a barrier to diagnosis. Team members indicated that test results might not be looked at or acted upon in a timely manner, and participants pointed to the electronic medical record as a source of this challenge.
“Before I knew about [the app for Epic], I would literally sit on the computer to get all the information we would need on rounds. Its key to making decisions. We often say we will do something, only to find the test result doesn’t support it–and then we’re back to square 1.” (Intern)
Information used by teams came from myriad sources (eg, patients, family members, electronic records) and from various settings (eg, emergency department, patient rooms, discussions with consultants). Additionally, test results often appeared without warning. Thus, availability of information was poorly aligned with clinical duties.
“They (the lab) will call us when a blood culture is positive or something is off. That is very helpful but it often comes later in the day, when we’re done with rounds.” (Resident)
The work system was highlighted as a key contributor to data fragmentation. Peculiarities of our electronic medical record (EMR) and how data were collected, stored, or presented were described as “frustrating,” and “unsafe,” by team members. Correspondingly, we frequently observed interns asking for assistance for tasks such as ordering tests or finding information despite being “trained” to use the EMR.
“People have to learn how to filter, how to recognize the most important points and link data streams together in terms of causality. But we assume they know where to find that information. It’s actually a very hard thing to do, for both the house staff and me.” (Attending)
(3) Distractions Undermine the Diagnostic Process
Distractions often created cognitive difficulties. For example, ambient noise and interruptions from neighbors working on other teams were cited as barriers to diagnosis. In addition, we observed several team members using headphones to drown out ambient noise while working on the computer.
“I know I shouldn’t do it (wear headphones), but I have no other way of turning down the noise so I can concentrate.” (Intern)
Similarly, the unpredictable nature and the volume of pages often interrupted thinking about diagnosis.
“Sometimes the pager just goes off all the time and (after making sure its not an urgent issue), I will just ignore it for a bit, especially if I am in the middle of something. It would be great if I could finish my thought process knowing I would not be interrupted.” (Resident)
To mitigate this problem, 1 attending described how he would proactively seek out nurses caring for his patients to “head off” questions (eg, “I will renew the restraints and medications this morning,” and “Is there anything you need in terms of orders for this patient that I can take care of now?”) that might lead to pages. Another resident described his approach as follows:
“I make it a point to tell the nurses where I will be hanging out and where they can find me if they have any questions. I tell them to come talk to me rather than page me since that will be less distracting.” (Resident).
Most of the interns described documentation work such as writing admission and progress notes in negative terms (“an academic exercise,” “part of the billing activity”). However, in the context of interruptions, some described this as helpful.
“The most valuable part of the thinking process was writing the assessment and plan because that’s actually my schema for all problems. It literally is the only time where I can sit and collect my thoughts to formulate a diagnosis and plan.” (Intern)
(4) Time Pressures Interfere With Diagnostic Decision Making
All team members spoke about the challenge of finding time for diagnosis during the workday. Often, they had to skip learning sessions for this purpose.
“They tell us we should go to morning report or noon conference but when I’m running around trying to get things done. I hate having to choose between my education and doing what’s best for the patient–but that’s often what it comes down to.” (Intern)
When specifically asked whether setting aside dedicated time to specifically review and formulate diagnoses would be valuable, respondents were uniformly enthusiastic. Team members described attentional conflicts as being the worst when “cross covering” other teams on call days, as their patient load effectively doubled during this time. Of note, cross-covering occurred when teams were also on call—and thus took them away from important diagnostic activities such as data gathering or synthesis for patients they were admitting.
“If you were to ever design a system where errors were likely–this is how you would design it: take a team with little supervision, double their patient load, keep them busy with new challenging cases and then ask questions about patients they know little about.” (Resident)
DISCUSSION
Although diagnostic errors have been called “the next frontier for patient safety,”17 little is known about the process, barriers, and facilitators to diagnosis in teaching hospitals. In this focused ethnography conducted at 2 academic medical centers, we identified multiple cognitive and system-level challenges and potential strategies to improve diagnosis from trainees engaged in this activity. Key themes identified by those we observed included the social nature of diagnosis, fragmented information delivery, constant distractions and interruptions, and time pressures. In turn, these insights allow us to generate strategies that can be applied to improve the diagnostic process in teaching hospitals.
Our study underscores the importance of social interactions in diagnosis. In contrast, most of the interventions to prevent diagnostic errors target individual providers through practices such as metacognition and “thinking about thinking.”18-20 These interventions are based on Daniel Kahnemann’s work on dual thought process. Type 1 thought processes are fast, subconscious, reflexive, largely intuitive, and more vulnerable to error. In contrast, Type 2 processes are slower, deliberate, analytic, and less prone to error.21 Although an individual’s Type 2 thought capacity is limited, a major goal of cognitive interventions is to encourage Type 2 over Type 1 thinking, an approach termed “de-biasing.”22-24 Unfortunately, cognitive interventions testing such approaches have suffered mixed results–perhaps because of lack of focus on collective wisdom or group thinking, which may be key to diagnosis from our findings.9,25 In this sense, morning rounds were a social gathering used to strategize and develop care plans, but with limited time to think about diagnosis.26 Introduction of defined periods for individuals to engage in diagnostic activities such as de-biasing (ie, asking “what else could this be)27 before or after rounds may provide an opportunity for reflection and improving diagnosis. In addition, embedding tools such as diagnosis expanders and checklists within these defined time slots28,29 may prove to be useful in reflecting on diagnosis and preventing diagnostic errors.
An unexpected yet important finding from this study were the challenges posed by distractions and the physical environment. Potentially maladaptive workarounds to these interruptions included use of headphones; more productive strategies included updating nurses with plans to avert pages and creating a list of activities to ensure that key tasks were not forgotten.30,31 Applying lessons from aviation, a focused effort to limit distractions during key portions of the day, might be worth considering for diagnostic safety.32 Similarly, improving the environment in which diagnosis occurs—including creating spaces that are quiet, orderly, and optimized for thinking—may be valuable.33Our study has limitations. First, our findings are limited to direct observations; we are thus unable to comment on how unobserved aspects of care (eg, cognitive processes) might have influenced our findings. Our observations of clinical care might also have introduced a Hawthorne effect. However, because we were closely integrated with teams and conducted focus groups to corroborate our assessments, we believe that this was not the case. Second, we did not identify diagnostic errors or link processes we observed to errors. Third, our approach is limited to 2 teaching centers, thereby limiting the generalizability of findings. Relatedly, we were only able to conduct observations during weekdays; differences in weekend and night resources might affect our insights.
The cognitive and system-based barriers faced by clinicians in teaching hospitals suggest that new methods to improve diagnosis are needed. Future interventions such as defined “time-outs” for diagnosis, strategies focused on limiting distractions, and methods to improve communication between team members are novel and have parallels in other industries. As challenges to quantify diagnostic errors abound,34 improving cognitive- and system-based factors via reflection through communication, concentration, and organization is necessary to improve medical decision making in academic medical centers.
Disclosures
None declared for all coauthors.
Funding
This project was supported by grant number P30HS024385 from the Agency for Healthcare Research and Quality. The funding source played no role in study design, data acquisition, analysis or decision to report these data. Dr. Chopra is supported by a career development award from the Agency of Healthcare Research and Quality (1-K08-HS022835-01). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). Dr. Singh is partially supported by Houston VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the Department of Veterans Affairs.
Diagnostic error—defined as a failure to establish an accurate and timely explanation of the patient’s health problem—is an important source of patient harm.1 Data suggest that all patients will experience at least 1 diagnostic error in their lifetime.2-4 Not surprisingly, diagnostic errors are among the leading categories of paid malpractice claims in the United States.5
Despite diagnostic errors being morbid and sometimes deadly in the hospital,6,7 little is known about how residents and learners approach diagnostic decision making. Errors in diagnosis are believed to stem from cognitive or system failures,8 with errors in cognition believed to occur due to rapid, reflexive thinking operating in the absence of a more analytical, deliberate process. System-based problems (eg, lack of expert availability, technology barriers, and access to data) have also been cited as contributors.9 However, whether and how these apply to trainees is not known.
Therefore, we conducted a focused ethnography of inpatient medicine teams (ie, attendings, residents, interns, and medical students) in 2 affiliated teaching hospitals, aiming to (a) observe the process of diagnosis by trainees and (b) identify methods to improve the diagnostic process and prevent errors.
METHODS
We designed a multimethod, focused ethnographic study to examine diagnostic decision making in hospital settings.10,11 In contrast to anthropologic ethnographies that study entire fields using open-ended questions, our study was designed to examine the process of diagnosis from the perspective of clinicians engaged in this activity.11 This approach allowed us to capture diagnostic decisions and cognitive and system-based factors in a manner currently lacking in the literature.12
Setting and Participants
Between January 2016 and May 2016, we observed the members of four inpatient internal medicine teaching teams at 2 affiliated teaching hospitals. We purposefully selected teaching teams for observation because they are the primary model of care in academic settings and we have expertise in carrying out similar studies.13,14 Teaching teams typically consisted of a medical attending (senior-level physician), 1 senior resident (a second- or third-year postgraduate trainee), two interns (a trainee in their first postgraduate year), and two to four medical students. Teams were selected at random using existing schedules and followed Monday to Friday so as to permit observation of work on call and noncall days. Owing to manpower limitations, weekend and night shifts were not observed. However, overnight events were captured during morning rounds.
Most of the teams began rounds at 8:30 AM. Typically, rounds lasted for 90–120 min and concluded with a recap (ie, “running the list”) with a review of explicit plans for patients after they had been evaluated by the attending. This discussion often occurred in the team rooms, with the attending leading the discussion with the trainees.
Data Collection
A multidisciplinary team, including clinicians (eg, physicians, nurses), nonclinicians (eg, qualitative researchers, social scientists), and healthcare engineers, conducted the observations. We observed preround activities of interns and residents before arrival of the attending (7:00 AM - 8:30 AM), followed by morning rounds with the entire team, and afternoon work that included senior residents, interns, and students.
To capture multiple aspects of the diagnostic process, we collected data using field notes modeled on components of the National Academy of Science model for diagnosis (Appendix).1,15 This model encompasses phases of the diagnostic process (eg, data gathering, integration, formulation of a working diagnosis, treatment delivery, and outcomes) and the work system (team members, organization, technology and tools, physical environment, tasks).
Focus Groups and Interviews
At the end of weekly observations, we conducted focus groups with the residents and one-on- one interviews with the attendings. Focus groups with the residents were conducted to encourage a group discussion about the diagnostic process. Separate interviews with the attendings were performed to ensure that power differentials did not influence discussions. During focus groups, we specifically asked about challenges and possible solutions to improve diagnosis. Experienced qualitative methodologists (J.F., M.H., M.Q.) used semistructured interview guides for discussions (Appendix).
Data Analysis
After aggregating and reading the data, three reviewers (V.C., S.K., S.S.) began inductive analysis by handwriting notes and initial reflective thoughts to create preliminary codes. Multiple team members then reread the original field notes and the focus group/interview data to refine the preliminary codes and develop additional codes. Next, relationships between codes were identified and used to develop key themes. Triangulation of data collected from observations and interview/focus group sessions was carried out to compare data that we surmised with data that were verbalized by the team. The developed themes were discussed as a group to ensure consistency of major findings.
Ethical and Regulatory Oversight
This study was reviewed and approved by the Institutional Review Boards at the University of Michigan Health System (HUM-00106657) and the VA Ann Arbor Healthcare System (1-2016-010040).
RESULTS
Four teaching teams (4 attendings, 4 senior residents, 9 interns, and 14 medical students) were observed over 33 distinct shifts and 168 hours. Observations included morning rounds (96 h), postround call days (52 h), and postround non-call days (20 h). Morning rounds lasted an average of 127 min (range: 48-232 min) and included an average of 9 patients (range: 4-16 patients).
Themes Regarding the Diagnostic Process
We identified the following 4 primary themes related to the diagnostic process in teaching hospitals: (1) diagnosis is a social phenomenon; (2) data necessary to make diagnoses are fragmented; (3) distractions undermine the diagnostic process; and (4) time pressures interfere with diagnostic decision making (Appendix Table 1).
(1) Diagnosis is a Social Phenomenon.
Team members viewed the process of diagnosis as a social exchange of facts, findings, and strategies within a defined structure. The opportunity to discuss impressions with others was valued as a means to share, test, and process assumptions.
“Rounds are the most important part of the process. That is where we make most decisions in a collective, collaborative way with the attending present. We bounce ideas off each other.” (Intern)
Typical of social processes, variations based on time of day and schedule were observed. For instance, during call days, learners gathered data and formed working diagnosis and treatment plans with minimal attending interaction. This separation of roles and responsibilities introduced a hierarchy within diagnosis as follows:
“The interns would not call me first; they would talk to the senior resident and then if the senior thought he should chat with me, then they would call. But for the most part, they gather information and come up with the plan.” (Attending).
The work system was suited to facilitate social interactions. For instance, designated rooms (with team members informally assigned to a computer) provided physical proximity of the resident to interns and medical students. In this space, numerous informal discussions between team members (eg, “What do you think about this test?” “I’m not sure what to do about this finding.” “Should I call a [consult] on this patient?”) were observed. Although proximity to each other was viewed as beneficial, dangers to the social nature of diagnosis in the form of anchoring (ie, a cognitive bias where emphasis is placed on the first piece of data)16 were also mentioned. Similarly, the paradox associated with social proof (ie, the pressure to assume conformity within a group) was also observed as disagreement between team members and attendings rarely occurred during observations.
“I mean, they’re the attending, right? It’s hard to argue with them when they want a test or something done. When I do push back, it’s rare that others will support me–so it’s usually me and the attending.” (Resident)
“I would push back if I think it’s really bad for the patient or could cause harm–but the truth is, it doesn’t happen much.” (Intern)
(2) Data Necessary to Make Diagnoses are Fragmented
Team members universally cited fragmentation in data delivery, retrieval, and processing as a barrier to diagnosis. Team members indicated that test results might not be looked at or acted upon in a timely manner, and participants pointed to the electronic medical record as a source of this challenge.
“Before I knew about [the app for Epic], I would literally sit on the computer to get all the information we would need on rounds. Its key to making decisions. We often say we will do something, only to find the test result doesn’t support it–and then we’re back to square 1.” (Intern)
Information used by teams came from myriad sources (eg, patients, family members, electronic records) and from various settings (eg, emergency department, patient rooms, discussions with consultants). Additionally, test results often appeared without warning. Thus, availability of information was poorly aligned with clinical duties.
“They (the lab) will call us when a blood culture is positive or something is off. That is very helpful but it often comes later in the day, when we’re done with rounds.” (Resident)
The work system was highlighted as a key contributor to data fragmentation. Peculiarities of our electronic medical record (EMR) and how data were collected, stored, or presented were described as “frustrating,” and “unsafe,” by team members. Correspondingly, we frequently observed interns asking for assistance for tasks such as ordering tests or finding information despite being “trained” to use the EMR.
“People have to learn how to filter, how to recognize the most important points and link data streams together in terms of causality. But we assume they know where to find that information. It’s actually a very hard thing to do, for both the house staff and me.” (Attending)
(3) Distractions Undermine the Diagnostic Process
Distractions often created cognitive difficulties. For example, ambient noise and interruptions from neighbors working on other teams were cited as barriers to diagnosis. In addition, we observed several team members using headphones to drown out ambient noise while working on the computer.
“I know I shouldn’t do it (wear headphones), but I have no other way of turning down the noise so I can concentrate.” (Intern)
Similarly, the unpredictable nature and the volume of pages often interrupted thinking about diagnosis.
“Sometimes the pager just goes off all the time and (after making sure its not an urgent issue), I will just ignore it for a bit, especially if I am in the middle of something. It would be great if I could finish my thought process knowing I would not be interrupted.” (Resident)
To mitigate this problem, 1 attending described how he would proactively seek out nurses caring for his patients to “head off” questions (eg, “I will renew the restraints and medications this morning,” and “Is there anything you need in terms of orders for this patient that I can take care of now?”) that might lead to pages. Another resident described his approach as follows:
“I make it a point to tell the nurses where I will be hanging out and where they can find me if they have any questions. I tell them to come talk to me rather than page me since that will be less distracting.” (Resident).
Most of the interns described documentation work such as writing admission and progress notes in negative terms (“an academic exercise,” “part of the billing activity”). However, in the context of interruptions, some described this as helpful.
“The most valuable part of the thinking process was writing the assessment and plan because that’s actually my schema for all problems. It literally is the only time where I can sit and collect my thoughts to formulate a diagnosis and plan.” (Intern)
(4) Time Pressures Interfere With Diagnostic Decision Making
All team members spoke about the challenge of finding time for diagnosis during the workday. Often, they had to skip learning sessions for this purpose.
“They tell us we should go to morning report or noon conference but when I’m running around trying to get things done. I hate having to choose between my education and doing what’s best for the patient–but that’s often what it comes down to.” (Intern)
When specifically asked whether setting aside dedicated time to specifically review and formulate diagnoses would be valuable, respondents were uniformly enthusiastic. Team members described attentional conflicts as being the worst when “cross covering” other teams on call days, as their patient load effectively doubled during this time. Of note, cross-covering occurred when teams were also on call—and thus took them away from important diagnostic activities such as data gathering or synthesis for patients they were admitting.
“If you were to ever design a system where errors were likely–this is how you would design it: take a team with little supervision, double their patient load, keep them busy with new challenging cases and then ask questions about patients they know little about.” (Resident)
DISCUSSION
Although diagnostic errors have been called “the next frontier for patient safety,”17 little is known about the process, barriers, and facilitators to diagnosis in teaching hospitals. In this focused ethnography conducted at 2 academic medical centers, we identified multiple cognitive and system-level challenges and potential strategies to improve diagnosis from trainees engaged in this activity. Key themes identified by those we observed included the social nature of diagnosis, fragmented information delivery, constant distractions and interruptions, and time pressures. In turn, these insights allow us to generate strategies that can be applied to improve the diagnostic process in teaching hospitals.
Our study underscores the importance of social interactions in diagnosis. In contrast, most of the interventions to prevent diagnostic errors target individual providers through practices such as metacognition and “thinking about thinking.”18-20 These interventions are based on Daniel Kahnemann’s work on dual thought process. Type 1 thought processes are fast, subconscious, reflexive, largely intuitive, and more vulnerable to error. In contrast, Type 2 processes are slower, deliberate, analytic, and less prone to error.21 Although an individual’s Type 2 thought capacity is limited, a major goal of cognitive interventions is to encourage Type 2 over Type 1 thinking, an approach termed “de-biasing.”22-24 Unfortunately, cognitive interventions testing such approaches have suffered mixed results–perhaps because of lack of focus on collective wisdom or group thinking, which may be key to diagnosis from our findings.9,25 In this sense, morning rounds were a social gathering used to strategize and develop care plans, but with limited time to think about diagnosis.26 Introduction of defined periods for individuals to engage in diagnostic activities such as de-biasing (ie, asking “what else could this be)27 before or after rounds may provide an opportunity for reflection and improving diagnosis. In addition, embedding tools such as diagnosis expanders and checklists within these defined time slots28,29 may prove to be useful in reflecting on diagnosis and preventing diagnostic errors.
An unexpected yet important finding from this study were the challenges posed by distractions and the physical environment. Potentially maladaptive workarounds to these interruptions included use of headphones; more productive strategies included updating nurses with plans to avert pages and creating a list of activities to ensure that key tasks were not forgotten.30,31 Applying lessons from aviation, a focused effort to limit distractions during key portions of the day, might be worth considering for diagnostic safety.32 Similarly, improving the environment in which diagnosis occurs—including creating spaces that are quiet, orderly, and optimized for thinking—may be valuable.33Our study has limitations. First, our findings are limited to direct observations; we are thus unable to comment on how unobserved aspects of care (eg, cognitive processes) might have influenced our findings. Our observations of clinical care might also have introduced a Hawthorne effect. However, because we were closely integrated with teams and conducted focus groups to corroborate our assessments, we believe that this was not the case. Second, we did not identify diagnostic errors or link processes we observed to errors. Third, our approach is limited to 2 teaching centers, thereby limiting the generalizability of findings. Relatedly, we were only able to conduct observations during weekdays; differences in weekend and night resources might affect our insights.
The cognitive and system-based barriers faced by clinicians in teaching hospitals suggest that new methods to improve diagnosis are needed. Future interventions such as defined “time-outs” for diagnosis, strategies focused on limiting distractions, and methods to improve communication between team members are novel and have parallels in other industries. As challenges to quantify diagnostic errors abound,34 improving cognitive- and system-based factors via reflection through communication, concentration, and organization is necessary to improve medical decision making in academic medical centers.
Disclosures
None declared for all coauthors.
Funding
This project was supported by grant number P30HS024385 from the Agency for Healthcare Research and Quality. The funding source played no role in study design, data acquisition, analysis or decision to report these data. Dr. Chopra is supported by a career development award from the Agency of Healthcare Research and Quality (1-K08-HS022835-01). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). Dr. Singh is partially supported by Houston VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the Department of Veterans Affairs.
1. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press. http://www.nap.edu/21794. Accessed November 1; 2016:2015. https://doi.org/10.17226/21794.
2. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. http://dx.doi.org/10.1001/archinternmed.2009.333. PubMed
3. Sonderegger-Iseli K, Burger S, Muntwyler J, Salomon F. Diagnostic errors in three medical eras: A necropsy study. Lancet. 2000;355(9220):2027-2031. http://dx.doi.org/10.1016/S0140-6736(00)02349-7. PubMed
4. Winters B, Custer J, Galvagno SM Jr, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. http://dx.doi.org/10.1136/bmjqs-2012-000803. PubMed
5. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-Year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. http://dx.doi.org/10.1136/bmjqs-2012-001550. PubMed
6. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77(10):981-992. http://dx.doi.org/10.1097/00001888-200210000-00009. PubMed
7. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2018;27(1):53-60. 10.1136/bmjqs-2017-006774. PubMed
8. van Noord I, Eikens MP, Hamersma AM, de Bruijne MC. Application of root cause analysis on malpractice claim files related to diagnostic failures. Qual Saf Health Care. 2010;19(6):e21. http://dx.doi.org/10.1136/qshc.2008.029801. PubMed
9. Croskerry P, Petrie DA, Reilly JB, Tait G. Deciding about fast and slow decisions. Acad Med. 2014;89(2):197-200. 10.1097/ACM.0000000000000121. PubMed
10. Higginbottom GM, Pillay JJ, Boadu NY. Guidance on performing focused ethnographies with an emphasis on healthcare research. Qual Rep. 2013;18(9):1-6. https://doi.org/10.7939/R35M6287P.
11. Savage J. Participative observation: standing in the shoes of others? Qual Health Res. 2000;10(3):324-339. http://dx.doi.org/10.1177/104973200129118471. PubMed
12. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Thousand Oaks, CA: SAGE Publications; 2002.
13. Harrod M, Weston LE, Robinson C, Tremblay A, Greenstone CL, Forman J. “It goes beyond good camaraderie”: A qualitative study of the process of becoming an interprofessional healthcare “teamlet.” J Interprof Care. 2016;30(3):295-300. http://dx.doi.org/10.3109/13561820.2015.1130028. PubMed
14. Houchens N, Harrod M, Moody S, Fowler KE, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. http://dx.doi.org/10.12788/jhm.2763. PubMed
15. Mulhall A. In the field: notes on observation in qualitative research. J Adv Nurs. 2003;41(3):306-313. http://dx.doi.org/10.1046/j.1365-2648.2003.02514.x. PubMed
16. Zwaan L, Monteiro S, Sherbino J, Ilgen J, Howey B, Norman G. Is bias in the eye of the beholder? A vignette study to assess recognition of cognitive biases in clinical case workups. BMJ Qual Saf. 2017;26(2):104-110. http://dx.doi.org/10.1136/bmjqs-2015-005014. PubMed
17. Singh H, Graber ML. Improving diagnosis in health care--the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. http://dx.doi.org/10.1056/NEJMp1512241. PubMed
18. Croskerry P. From mindless to mindful practice--cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448. http://dx.doi.org/10.1056/NEJMp1303712. PubMed
19. van den Berge K, Mamede S. Cognitive diagnostic error in internal medicine. Eur J Intern Med. 2013;24(6):525-529. http://dx.doi.org/10.1016/j.ejim.2013.03.006. PubMed
20. Norman G, Sherbino J, Dore K, et al. The etiology of diagnostic errors: A controlled trial of system 1 versus system 2 reasoning. Acad Med. 2014;89(2):277-284. 10.1097/ACM.0000000000000105 PubMed
21. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf. 2017;26(2):87-89. http://dx.doi.org/10.1136/bmjqs-2016-005267. PubMed
22. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: Origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58-iiii64. http://dx.doi.org/10.1136/bmjqs-2012-001712. PubMed
23. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: Impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-iiii72. http://dx.doi.org/10.1136/bmjqs-2012-001713. PubMed
24. Reilly JB, Ogdie AR, Von Feldt JM, Myers JS. Teaching about how doctors think: a longitudinal curriculum in cognitive bias and diagnostic error for residents. BMJ Qual Saf. 2013;22(12):1044-1050. http://dx.doi.org/10.1136/bmjqs-2013-001987. PubMed
25. Schmidt HG, Mamede S, van den Berge K, van Gog T, van Saase JL, Rikers RM. Exposure to media information about a disease can cause doctors to misdiagnose similar-looking clinical cases. Acad Med. 2014;89(2):285-291. http://dx.doi.org/10.1097/ACM.0000000000000107. PubMed
26. Hess BJ, Lipner RS, Thompson V, Holmboe ES, Graber ML. Blink or think: can further reflection improve initial diagnostic impressions? Acad Med. 2015;90(1):112-118. http://dx.doi.org/10.1097/ACM.0000000000000550. PubMed
27. Lambe KA, O’Reilly G, Kelly BD, Curristan S. Dual-process cognitive interventions to enhance diagnostic reasoning: A systematic review. BMJ Qual Saf. 2016;25(10):808-820. http://dx.doi.org/10.1136/bmjqs-2015-004417. PubMed
28. Graber ML, Kissam S, Payne VL, et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21(7):535-557. http://dx.doi.org/10.1136/bmjqs-2011-000149. PubMed
29. McDonald KM, Matesic B, Contopoulos-Ioannidis DG, et al. Patient safety strategies targeted at diagnostic errors: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):381-389. http://dx.doi.org/10.7326/0003-4819-158-5-201303051-00004. PubMed
30. Wray CM, Chaudhry S, Pincavage A, et al. Resident shift handoff strategies in US internal medicine residency programs. JAMA. 2016;316(21):2273-2275. http://dx.doi.org/10.1001/jama.2016.17786. PubMed
31. Choo KJ, Arora VM, Barach P, Johnson JK, Farnan JM. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014;9(3):169-175. http://dx.doi.org/10.1002/jhm.2150. PubMed
32. Carayon P, Wood KE. Patient safety - the role of human factors and systems engineering. Stud Health Technol Inform. 2010;153:23-46.
.http://dx.doi.org/10.1001/jama.2015.13453 PubMed
34. McGlynn EA, McDonald KM, Cassel CK. Measurement is essential for improving diagnosis and reducing diagnostic error: A report from the Institute of Medicine. JAMA. 2015;314(23):2501-2502.
.http://dx.doi.org/10.1136/bmjqs-2013-001812 PubMed
33. Carayon P, Xie A, Kianfar S. Human factors and ergonomics as a patient safety practice. BMJ Qual Saf. 2014;23(3):196-205. PubMed
1. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press. http://www.nap.edu/21794. Accessed November 1; 2016:2015. https://doi.org/10.17226/21794.
2. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. http://dx.doi.org/10.1001/archinternmed.2009.333. PubMed
3. Sonderegger-Iseli K, Burger S, Muntwyler J, Salomon F. Diagnostic errors in three medical eras: A necropsy study. Lancet. 2000;355(9220):2027-2031. http://dx.doi.org/10.1016/S0140-6736(00)02349-7. PubMed
4. Winters B, Custer J, Galvagno SM Jr, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. http://dx.doi.org/10.1136/bmjqs-2012-000803. PubMed
5. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-Year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. http://dx.doi.org/10.1136/bmjqs-2012-001550. PubMed
6. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77(10):981-992. http://dx.doi.org/10.1097/00001888-200210000-00009. PubMed
7. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2018;27(1):53-60. 10.1136/bmjqs-2017-006774. PubMed
8. van Noord I, Eikens MP, Hamersma AM, de Bruijne MC. Application of root cause analysis on malpractice claim files related to diagnostic failures. Qual Saf Health Care. 2010;19(6):e21. http://dx.doi.org/10.1136/qshc.2008.029801. PubMed
9. Croskerry P, Petrie DA, Reilly JB, Tait G. Deciding about fast and slow decisions. Acad Med. 2014;89(2):197-200. 10.1097/ACM.0000000000000121. PubMed
10. Higginbottom GM, Pillay JJ, Boadu NY. Guidance on performing focused ethnographies with an emphasis on healthcare research. Qual Rep. 2013;18(9):1-6. https://doi.org/10.7939/R35M6287P.
11. Savage J. Participative observation: standing in the shoes of others? Qual Health Res. 2000;10(3):324-339. http://dx.doi.org/10.1177/104973200129118471. PubMed
12. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Thousand Oaks, CA: SAGE Publications; 2002.
13. Harrod M, Weston LE, Robinson C, Tremblay A, Greenstone CL, Forman J. “It goes beyond good camaraderie”: A qualitative study of the process of becoming an interprofessional healthcare “teamlet.” J Interprof Care. 2016;30(3):295-300. http://dx.doi.org/10.3109/13561820.2015.1130028. PubMed
14. Houchens N, Harrod M, Moody S, Fowler KE, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. http://dx.doi.org/10.12788/jhm.2763. PubMed
15. Mulhall A. In the field: notes on observation in qualitative research. J Adv Nurs. 2003;41(3):306-313. http://dx.doi.org/10.1046/j.1365-2648.2003.02514.x. PubMed
16. Zwaan L, Monteiro S, Sherbino J, Ilgen J, Howey B, Norman G. Is bias in the eye of the beholder? A vignette study to assess recognition of cognitive biases in clinical case workups. BMJ Qual Saf. 2017;26(2):104-110. http://dx.doi.org/10.1136/bmjqs-2015-005014. PubMed
17. Singh H, Graber ML. Improving diagnosis in health care--the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. http://dx.doi.org/10.1056/NEJMp1512241. PubMed
18. Croskerry P. From mindless to mindful practice--cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448. http://dx.doi.org/10.1056/NEJMp1303712. PubMed
19. van den Berge K, Mamede S. Cognitive diagnostic error in internal medicine. Eur J Intern Med. 2013;24(6):525-529. http://dx.doi.org/10.1016/j.ejim.2013.03.006. PubMed
20. Norman G, Sherbino J, Dore K, et al. The etiology of diagnostic errors: A controlled trial of system 1 versus system 2 reasoning. Acad Med. 2014;89(2):277-284. 10.1097/ACM.0000000000000105 PubMed
21. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf. 2017;26(2):87-89. http://dx.doi.org/10.1136/bmjqs-2016-005267. PubMed
22. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: Origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58-iiii64. http://dx.doi.org/10.1136/bmjqs-2012-001712. PubMed
23. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: Impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-iiii72. http://dx.doi.org/10.1136/bmjqs-2012-001713. PubMed
24. Reilly JB, Ogdie AR, Von Feldt JM, Myers JS. Teaching about how doctors think: a longitudinal curriculum in cognitive bias and diagnostic error for residents. BMJ Qual Saf. 2013;22(12):1044-1050. http://dx.doi.org/10.1136/bmjqs-2013-001987. PubMed
25. Schmidt HG, Mamede S, van den Berge K, van Gog T, van Saase JL, Rikers RM. Exposure to media information about a disease can cause doctors to misdiagnose similar-looking clinical cases. Acad Med. 2014;89(2):285-291. http://dx.doi.org/10.1097/ACM.0000000000000107. PubMed
26. Hess BJ, Lipner RS, Thompson V, Holmboe ES, Graber ML. Blink or think: can further reflection improve initial diagnostic impressions? Acad Med. 2015;90(1):112-118. http://dx.doi.org/10.1097/ACM.0000000000000550. PubMed
27. Lambe KA, O’Reilly G, Kelly BD, Curristan S. Dual-process cognitive interventions to enhance diagnostic reasoning: A systematic review. BMJ Qual Saf. 2016;25(10):808-820. http://dx.doi.org/10.1136/bmjqs-2015-004417. PubMed
28. Graber ML, Kissam S, Payne VL, et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21(7):535-557. http://dx.doi.org/10.1136/bmjqs-2011-000149. PubMed
29. McDonald KM, Matesic B, Contopoulos-Ioannidis DG, et al. Patient safety strategies targeted at diagnostic errors: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):381-389. http://dx.doi.org/10.7326/0003-4819-158-5-201303051-00004. PubMed
30. Wray CM, Chaudhry S, Pincavage A, et al. Resident shift handoff strategies in US internal medicine residency programs. JAMA. 2016;316(21):2273-2275. http://dx.doi.org/10.1001/jama.2016.17786. PubMed
31. Choo KJ, Arora VM, Barach P, Johnson JK, Farnan JM. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014;9(3):169-175. http://dx.doi.org/10.1002/jhm.2150. PubMed
32. Carayon P, Wood KE. Patient safety - the role of human factors and systems engineering. Stud Health Technol Inform. 2010;153:23-46.
.http://dx.doi.org/10.1001/jama.2015.13453 PubMed
34. McGlynn EA, McDonald KM, Cassel CK. Measurement is essential for improving diagnosis and reducing diagnostic error: A report from the Institute of Medicine. JAMA. 2015;314(23):2501-2502.
.http://dx.doi.org/10.1136/bmjqs-2013-001812 PubMed
33. Carayon P, Xie A, Kianfar S. Human factors and ergonomics as a patient safety practice. BMJ Qual Saf. 2014;23(3):196-205. PubMed
© 2018 Society of Hospital Medicine
Stroke Increases the Risk of All-Cause Dementia
Protecting the blood supply to the brain could reduce the risk of incident dementia.
Stroke is a strong independent risk factor for all-cause dementia, according to research published online ahead of print August 25 in Alzheimer’s & Dementia. Clinicians should incorporate stroke-prevention strategies into their health interventions to reduce patients’ risk of dementia, said the authors.
“Around a third of dementia cases are thought to be potentially preventable, though this estimate does not take into account the risk associated with stroke,” said David Llewellyn, PhD, Senior Research Fellow at University of Exeter Medical School in the United Kingdom. “Our findings indicate that this figure could be even higher and reinforce the importance of protecting the blood supply to the brain when attempting to reduce the global burden of dementia.”
Meta-Analysis of Previous Research
Stroke is a recognized risk factor for all-cause dementia, but no researchers had previously performed a meta-analysis to quantify the risk. Dr. Llewellyn and colleagues searched Medline, PsycINFO, and Embase databases for prospective studies that investigated the association between prevalent or incident stroke and incident all-cause dementia. They excluded studies that lacked a comparison group or that had a comparison group other than a stroke-free group. The investigators pooled adjusted estimates across studies using random effects meta-analysis and evaluated potential effect modifiers with meta-regression.
Dr. Llewellyn and colleagues identified 11,129 articles, 26 of which were eligible for analysis. They also included 16 studies from a previous systematic review and four studies identified through backward and forward citation searches. In all, 36 studies examined prevalent stroke (1.9 million participants), and 12 studies examined incident stroke (1.3 million participants). The studies were conducted in America, Europe, Asia, and Australia and included more than three million participants. Follow-up periods ranged from nine months to 25 years.
Stroke Affected Dementia Risk
When the researchers pooled results from 22 cohorts of participants who were cognitively normal at baseline, they found that those with prevalent stroke had a higher adjusted risk of incident dementia, compared with those without stroke (hazard ratio [HR], 1.69). Sensitivity analyses did not change the results significantly. Prevalent stroke was associated with a higher risk of incident dementia among men than among women. Sex explained 50.2% of heterogeneity between studies for prevalent stroke.
After combining the adjusted results from eight studies, Dr. Llewellyn and colleagues found that incident stroke more than doubled the risk of incident all-cause dementia, compared with no incident stroke (risk ratio [RR], 2.18). For a sensitivity analysis, the investigators excluded three studies that combined stroke with transient ischemic attack; this adjustment strengthened the association.
The study’s strengths include the investigators’ search of several major databases and their contacts with authors who provided relevant data. The analysis reflects the limitations of the original studies, however. These limitations include selective samples and differences in stroke assessment and dementia diagnosis criteria. In addition, dementia may develop years before it is diagnosed. “More detailed reporting of the interval between stroke occurrence and dementia diagnosis in future studies will help to better characterize the role of time since stroke in the risk of dementia,” said Dr. Llewellyn.
—Erik Greb
Kuz´ma E, Lourida I, Moore SF, et al. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018 Aug 25 [Epub ahead of print].
Protecting the blood supply to the brain could reduce the risk of incident dementia.
Protecting the blood supply to the brain could reduce the risk of incident dementia.
Stroke is a strong independent risk factor for all-cause dementia, according to research published online ahead of print August 25 in Alzheimer’s & Dementia. Clinicians should incorporate stroke-prevention strategies into their health interventions to reduce patients’ risk of dementia, said the authors.
“Around a third of dementia cases are thought to be potentially preventable, though this estimate does not take into account the risk associated with stroke,” said David Llewellyn, PhD, Senior Research Fellow at University of Exeter Medical School in the United Kingdom. “Our findings indicate that this figure could be even higher and reinforce the importance of protecting the blood supply to the brain when attempting to reduce the global burden of dementia.”
Meta-Analysis of Previous Research
Stroke is a recognized risk factor for all-cause dementia, but no researchers had previously performed a meta-analysis to quantify the risk. Dr. Llewellyn and colleagues searched Medline, PsycINFO, and Embase databases for prospective studies that investigated the association between prevalent or incident stroke and incident all-cause dementia. They excluded studies that lacked a comparison group or that had a comparison group other than a stroke-free group. The investigators pooled adjusted estimates across studies using random effects meta-analysis and evaluated potential effect modifiers with meta-regression.
Dr. Llewellyn and colleagues identified 11,129 articles, 26 of which were eligible for analysis. They also included 16 studies from a previous systematic review and four studies identified through backward and forward citation searches. In all, 36 studies examined prevalent stroke (1.9 million participants), and 12 studies examined incident stroke (1.3 million participants). The studies were conducted in America, Europe, Asia, and Australia and included more than three million participants. Follow-up periods ranged from nine months to 25 years.
Stroke Affected Dementia Risk
When the researchers pooled results from 22 cohorts of participants who were cognitively normal at baseline, they found that those with prevalent stroke had a higher adjusted risk of incident dementia, compared with those without stroke (hazard ratio [HR], 1.69). Sensitivity analyses did not change the results significantly. Prevalent stroke was associated with a higher risk of incident dementia among men than among women. Sex explained 50.2% of heterogeneity between studies for prevalent stroke.
After combining the adjusted results from eight studies, Dr. Llewellyn and colleagues found that incident stroke more than doubled the risk of incident all-cause dementia, compared with no incident stroke (risk ratio [RR], 2.18). For a sensitivity analysis, the investigators excluded three studies that combined stroke with transient ischemic attack; this adjustment strengthened the association.
The study’s strengths include the investigators’ search of several major databases and their contacts with authors who provided relevant data. The analysis reflects the limitations of the original studies, however. These limitations include selective samples and differences in stroke assessment and dementia diagnosis criteria. In addition, dementia may develop years before it is diagnosed. “More detailed reporting of the interval between stroke occurrence and dementia diagnosis in future studies will help to better characterize the role of time since stroke in the risk of dementia,” said Dr. Llewellyn.
—Erik Greb
Kuz´ma E, Lourida I, Moore SF, et al. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018 Aug 25 [Epub ahead of print].
Stroke is a strong independent risk factor for all-cause dementia, according to research published online ahead of print August 25 in Alzheimer’s & Dementia. Clinicians should incorporate stroke-prevention strategies into their health interventions to reduce patients’ risk of dementia, said the authors.
“Around a third of dementia cases are thought to be potentially preventable, though this estimate does not take into account the risk associated with stroke,” said David Llewellyn, PhD, Senior Research Fellow at University of Exeter Medical School in the United Kingdom. “Our findings indicate that this figure could be even higher and reinforce the importance of protecting the blood supply to the brain when attempting to reduce the global burden of dementia.”
Meta-Analysis of Previous Research
Stroke is a recognized risk factor for all-cause dementia, but no researchers had previously performed a meta-analysis to quantify the risk. Dr. Llewellyn and colleagues searched Medline, PsycINFO, and Embase databases for prospective studies that investigated the association between prevalent or incident stroke and incident all-cause dementia. They excluded studies that lacked a comparison group or that had a comparison group other than a stroke-free group. The investigators pooled adjusted estimates across studies using random effects meta-analysis and evaluated potential effect modifiers with meta-regression.
Dr. Llewellyn and colleagues identified 11,129 articles, 26 of which were eligible for analysis. They also included 16 studies from a previous systematic review and four studies identified through backward and forward citation searches. In all, 36 studies examined prevalent stroke (1.9 million participants), and 12 studies examined incident stroke (1.3 million participants). The studies were conducted in America, Europe, Asia, and Australia and included more than three million participants. Follow-up periods ranged from nine months to 25 years.
Stroke Affected Dementia Risk
When the researchers pooled results from 22 cohorts of participants who were cognitively normal at baseline, they found that those with prevalent stroke had a higher adjusted risk of incident dementia, compared with those without stroke (hazard ratio [HR], 1.69). Sensitivity analyses did not change the results significantly. Prevalent stroke was associated with a higher risk of incident dementia among men than among women. Sex explained 50.2% of heterogeneity between studies for prevalent stroke.
After combining the adjusted results from eight studies, Dr. Llewellyn and colleagues found that incident stroke more than doubled the risk of incident all-cause dementia, compared with no incident stroke (risk ratio [RR], 2.18). For a sensitivity analysis, the investigators excluded three studies that combined stroke with transient ischemic attack; this adjustment strengthened the association.
The study’s strengths include the investigators’ search of several major databases and their contacts with authors who provided relevant data. The analysis reflects the limitations of the original studies, however. These limitations include selective samples and differences in stroke assessment and dementia diagnosis criteria. In addition, dementia may develop years before it is diagnosed. “More detailed reporting of the interval between stroke occurrence and dementia diagnosis in future studies will help to better characterize the role of time since stroke in the risk of dementia,” said Dr. Llewellyn.
—Erik Greb
Kuz´ma E, Lourida I, Moore SF, et al. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018 Aug 25 [Epub ahead of print].
Timing of Adverse Events Following Geriatric Hip Fracture Surgery: A Study of 19,873 Patients in the American College of Surgeons National Surgical Quality Improvement Program
ABSTRACT
This study uses a prospective surgical registry to characterize the timing of 10 postoperative adverse events following geriatric hip fracture surgery. There were 19,873 patients identified who were ≥70 years undergoing surgery for hip fracture as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). The median postoperative day of diagnosis (and interquartile range) for myocardial infarction was 3 (1-5), cardiac arrest requiring cardiopulmonary resuscitation 3 (0-8), stroke 3 (1-10), pneumonia 4 (2-10), pulmonary embolism 4 (2-11), urinary tract infection 7 (2-13), deep vein thrombosis 9 (4-16), sepsis 9 (4-18), mortality 11 (6-19), and surgical site infection 16 (11-22). For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30. Findings help to enable more targeted clinical surveillance, inform patient counseling, and determine the duration of follow-up required to study specific adverse events effectively. Orthopedic surgeons should have the lowest threshold for testing for each adverse event during the time period of greatest risk.
Continue to: Geriatric hip fracture surgery is associated with...
Geriatric hip fracture surgery is associated with a higher rate of occurrence of postoperative adverse events than any other commonly performed orthopedic procedure.1-4 Indeed, the 90-day mortality rate following a geriatric hip fracture surgery may be as high as 15%2 and the 30-day morbidity rate as high as 30%.3 Furthermore, more than half of postoperative mortalities following orthopedic procedures occur after surgery for hip fracture.4 Therefore, extensive research has been conducted regarding interventions to reduce the rates of adverse events following a hip fracture surgery.5-12 For example, randomized trials have been conducted involving venous thromboembolism prophylaxis,5,6nutritional supplementation,7 delirium prevention,8-10 anemia correction,11 geriatrics consultation,9 and anesthetic technique.12
Despite these extensive research efforts, there is currently little information in the literature regarding when postoperative adverse events occur. A clear depiction of the timing of adverse events could help target clinical surveillance, inform patient counseling, and determine the duration of follow-up required for studies. The reason that the timing of adverse events has not been previously characterized may be that the sample sizes available through standard single- or multi-institutional studies may be insufficient to accurately characterize the timing of rare adverse events (eg, myocardial infarction, stroke, etc.). Moreover, although administrative datasets have become common data sources for investigation of rare postoperative adverse events,13-16 such data sources often do not contain data on the timing of diagnosis.
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) is a relatively new and growing surgical registry.1,3,13-22 The registry follows up patients undergoing surgical procedures at several hundred community and academic institutions nationwide. Unlike the administrative datasets discussed above, the ACS-NSQIP characterizes the postoperative day of diagnosis of well-defined adverse events during the first 30 postoperative days.22
In this study, data collected by the ACS-NSQIP are used to characterize the timing of 10 specific postoperative adverse events following a geriatric hip fracture surgery.
Continue to: METHODS...
METHODS
A retrospective analysis of data collected prospectively through the ACS-NSQIP was conducted. Geriatric patients who underwent hip fracture surgery during 2010 to 2013 were identified. Specific inclusion criteria were (1) International Classification of Diseases, Ninth Revision, diagnosis code 820, (2) primary Current Procedural Terminology codes 27125, 27130, 27235, 27236, 27244, or 27245, and (3) age ≥70 years.
The ACS-NSQIP captures patient demographic, comorbidity, and procedural characteristics at baseline.22 At the end of the 30-day follow-up period, the ACS-NSQIP personnel review both inpatient and outpatient charts to characterize the occurrence vs nonoccurrence of specific postoperative adverse events.22-25 When an adverse event does occur, the postoperative day of diagnosis is recorded.
For this study, the following adverse event categories were investigated: myocardial infarction, cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, pulmonary embolism, urinary tract infection, deep vein thrombosis, sepsis (either with or without shock), mortality, and surgical site infection (including superficial surgical site infection, deep surgical site infection, and organ or space surgical site infection). Detailed definitions of each adverse event are provided in ACS-NSQIP materials.22
First, the 30-day incidence (and the associated 95% confidence interval) was determined for each adverse event. Second, the median postoperative day of diagnosis (and the associated interquartile range) was determined for each adverse event. Third, the postoperative length of stay was used to estimate the proportion of diagnoses occurring prior to vs following discharge for each adverse event. Finally, multivariate Cox proportional hazards models were used to identify independent risk factors for earlier occurrence of postoperative adverse events. The final models were selected using a backward stepwise process that sequentially eliminated variables with the weakest associations until all variables had P < .05.
Because the ACS-NSQIP reports timing data in calendar days, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, it was not possible to ascertain whether the diagnosis occurred prior to or following discharge. For this study, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, the adverse event was considered to have been diagnosed following discharge. The rationale for this is that for most of the adverse events, it was thought to be unlikely that an inpatient would be discharged before the end of the same day as an inpatient diagnosis. However, there was one exception to this rule; when the postoperative day of discharge, the postoperative length of stay, and the postoperative day of death were all equivalent, the adverse event was considered to have occurred prior to discharge. This is because when a patient dies during the initial inpatient stay, the ACS-NSQIP considers the postoperative length of stay to be equivalent to the postoperative day of death. This makes it much more likely that a diagnosis on the final hospital day had occurred in a patient who had not been discharged.
The mandatory ACS-NSQIP statement is “The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.”26
Continue to: RESULTS...
RESULTS
In total, 19,873 geriatric patients undergoing a hip fracture surgery were identified (Table 1). The rates of adverse events ranged from 6.7% for urinary tract infection to 0.6% for pulmonary embolism (Table 2).
Table 1. Patient Population
| Number | Percent |
Total | 19,873 | 100.0% |
Age |
|
|
70-74 years | 1852 | 9.3% |
75-79 years | 2764 | 13.9% |
80-84 years | 4328 | 21.8% |
85-89 years | 5525 | 27.8% |
≥90 years | 5404 | 27.2% |
Sex |
|
|
Male | 5359 | 27.0% |
Female | 14,514 | 73.0% |
Body mass index |
|
|
<30 kg/m2 | 17,733 | 89.2% |
≥30 kg/m2 | 2140 | 10.8% |
Functional status |
|
|
Independent | 14,348 | 72.2% |
Dependent | 5525 | 27.8% |
Diabetes | 3321 | 16.7% |
Congestive heart failure | 738 | 3.7% |
Dyspnea on exertion | 1542 | 7.8% |
Hypertension | 14,265 | 71.8% |
End-stage renal disease | 322 | 1.6% |
COPD | 2239 | 11.3% |
Current smoker | 1506 | 7.6% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 2. Patients with Adverse Events Diagnosed During the First 30 postoperative days (N = 19,873)
Adverse Event | Number | Percent | 95% CI |
Urinary tract infection | 1321 | 6.7% | 6.3%-7.0% |
Mortality | 1240 | 6.2% | 5.9%-6.6% |
Pneumonia | 771 | 3.9% | 3.6%-4.2% |
Sepsis | 428 | 2.2% | 2.0%-2.4% |
Myocardial infarction | 347 | 1.8% | 1.6%-1.9% |
Surgical site infection | 247 | 1.2% | 1.1%-1.4% |
Deep vein thrombosis | 199 | 1.0% | 0.9%-1.1% |
Stroke | 144 | 0.7% | 0.6%-0.8% |
Cardiac arrest | 136 | 0.7% | 0.6%-0.8% |
Pulmonary embolism | 126 | 0.6% | 0.5%-0.7% |
Abbreviation: CI, confidence interval.
Figure 1 depicts the timing of postoperative adverse events in detail in histograms and timing curves. For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30.
Figure 2 provides the summary statistics for adverse events diagnosed in the first 30 postoperative days. The median postoperative day of diagnosis (and the interquartile range) was 3 (1-5) for myocardial infarction, 3 (0-8) for cardiac arrest requiring cardiopulmonary resuscitation, 3 (1-10) for stroke, 4 (2-10) for pneumonia, 4 (2-11) for pulmonary embolism, 7 (2-13) for urinary tract infection, 9 (4-16) for deep vein thrombosis, 9 (4-18) for sepsis, 11 (6-19) for mortality, and 16 (11-22) for surgical site infection.
Figure 3 depicts the timing of adverse events relative to discharge. The proportions of adverse events diagnosed prior to discharge were 81.0% for myocardial infarction, 77.8% for stroke, 76.1% for cardiac arrest requiring cardiopulmonary resuscitation, 71.9% for pulmonary embolism, 71.1% for pneumonia, 58.0% for urinary tract infection, 52.1% for sepsis, 46.9% for deep vein thrombosis, 44.3% for mortality, and 27.6% for surgical site infection.
Table 3 shows the independent risk factors for earlier occurrence of adverse events. Following multivariate stepwise selection of final models, at least 1 patient characteristic was independently associated with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death. In contrast, no patient characteristics were independently associated with the timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, and surgical site infection.
Table 3. Timing of Diagnosis of Adverse Eventsa
Adverse events and associated baseline characteristic(s) | Median postoperative day of diagnosis with vs without baseline characteristic | P-valueb |
Cardiac arrest |
|
|
End-stage renal disease | 1 vs 3 | .005 |
Stroke |
|
|
Hypertension | 4 vs 2 | .025 |
Dependent functional status | 2 vs 4 | .027 |
Urinary tract infection |
|
|
Female sex | 6 vs 8 | .009 |
Deep vein thrombosis |
|
|
Body mass index ≥30 kg/m2 | 5 vs 10 | .015 |
Death |
|
|
End-stage renal disease | 10 vs 11 | .031 |
aBaseline characteristics that were independently associated with the timing of each adverse event were identified through a backwards stepwise selection process initially including all characteristics listed in Table 1, and sequentially excluding characteristics with the weakest associations until only characteristics with P < .05 remained. Independent associations with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death are shown. There were no characteristics independently associated with timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, or surgical site infection; hence, these adverse events are not listed in the table.
bFrom final Cox proportional hazards models identified through multivariate stepwise selection.
Continue to: DISCUSSION...
DISCUSSION
Adverse events are extremely common following a geriatric hip fracture surgery.1-4 Despite extensive investigation regarding methods to prevent these events,5-12 there is limited published description of the timing at which such events occur. This study used a large prospectively followed up cohort of geriatric patients undergoing a hip fracture surgery to deliver a better description of the timing of adverse events than was previously available. The findings of this study should enable more targeted clinical surveillance, inform patient counseling, and help determine the duration of follow-up required for studies on adverse events.
There was wide variability in the timing at which the different postoperative adverse events were diagnosed (Figures 1, 2). Myocardial infarction was diagnosed the earliest, with more than three-fourth of diagnoses in the first postoperative week. Other relatively early-diagnosed adverse events included cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, and pulmonary embolism.
The latest-diagnosed adverse event was surgical site infection (Figures 1, 2). Surgical site infection was actually the only adverse event with a rate of diagnosis during the first week that was lower than the rate of diagnosis later in the month (as can be seen by the inflection in the timing curve for surgical site infection in Figure 1). Mortality showed a relatively consistent rate of diagnosis throughout the entire first postoperative month. Other relatively late-diagnosed postoperative events, including sepsis, deep vein thrombosis, and urinary tract infection, showed varying degrees of decreased rate of diagnosis near the end of the first postoperative month. Of note, for the later-diagnosed adverse events, the estimated median and interquartile ranges (Figure 2) were presumably quite biased toward earlier diagnosis, as the 30-day follow-up period clearly failed to capture a large proportion of later-occurring adverse events (Figure 1).
Certain risk factors were independently associated with earlier occurrence of adverse events. Perhaps most strikingly, body mass index in the obese range was associated with substantially earlier occurrence of deep vein thrombosis (median of 5 vs 10 days). This finding suggests that clinical monitoring for deep vein thrombosis should be performed earlier in patients with greater body mass index. Also notable is the earlier occurrence of cardiac arrest and death among patients with end-stage renal disease than among those without. Patients with end-stage renal disease may have a greater risk for these adverse events immediately following the cardiac stresses of surgery.27 Similarly, such patients may be more prone to early electrolyte abnormalities and arrhythmia.
Continue to: In addition to its clinical implications, this study...
In addition to its clinical implications, this study informs about the interpretation of the many studies of adverse events following hip fracture procedures that have been conducted using retrospective data. Several such studies have relied on inpatient-only administrative databases.4,13,14,28-35 As clearly demonstrated in Figure 3, for most of the commonly studied adverse events, inpatient-only databases failed to capture a large proportion of adverse events occurring in the first postoperative month. This highlights a substantial limitation of this commonly published type of study that is often not emphasized in the literature.
There has also been an increase in the publication of studies of adverse events following a hip fracture surgery using the ACS-NSQIP data.3,13,14,17,18,21 As discussed, the ACS-NSQIP provides data on 30-days of follow-up. This relatively extended follow-up is often touted as a distinct advantage. However, this study demonstrates that even the 30-day follow-up afforded by the ACS-NSQIP is limited in its ability to enable investigation of the later-occurring adverse events (Figure 1). In particular, the rate of surgical site infection shows little sign of slowing by postoperative day 30. Similarly, the rates of mortality, sepsis, deep vein thrombosis, and urinary tract infection remain substantial.
This study does have limitations. First, as discussed, the duration of follow-up is a limitation of any ACS-NSQIP-based investigation, including this study. Second, the ACS-NSQIP does not capture relevant orthopedic-specific outcomes (eg, screw cutout). In addition, it could not be determined with certainty whether adverse events occurring on the final hospital day occurred prior to or following discharge. However, only a small proportion of most of the adverse events was diagnosed on the final hospital day. Finally, the ACS-NSQIP reports on days from the operation until diagnosis of the adverse event. Although some adverse events are probably diagnosed quickly after they have occurred (eg, myocardial infarction and cardiac arrest), other adverse events may have a delayed diagnosis (eg, surgical site infection may be identified days after its initial occurrence during a follow-up examination). Therefore, it is important to note the subtle distinction between occurrence and diagnosis throughout the article. This article reports on the timing of diagnosis, not actual occurrence.
CONCLUSION
The timing of postoperative adverse events has been understudied in the past. This may be due to an inability of standard single- or multi-institutional investigations to achieve sample sizes adequate to study the less commonly occurring adverse events. Using a relatively new prospective surgical registry, this study provides a far more detailed description of the timing of adverse events following surgery than was previously available. The authors anticipate that these data can be used to inform patient counseling, target clinical surveillance, and direct clinical research. The authors chose to study the timing of postoperative adverse events following geriatric hip fracture surgery because of the high rate of adverse events associated with the procedure. However, future ACS-NSQIP studies may involve characterization of the timing of adverse events following other orthopedic and non-orthopedic procedures.
This paper will be judged for the Resident Writer’s Award.
1. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/jbjs.i.00735.
2. Forte ML, Virnig BA, Swiontkowski MF, et al. Ninety-day mortality after intertrochanteric hip fracture: does provider volume matter? J Bone Joint Surg Am. 2010;92(4):799-806. doi:10.2106/jbjs.h.01204.
3. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma.2014;28(2):63-69. doi:10.1097/BOT.0b013e3182a22744.
4. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84-a(4):562-572.
5. Eriksson BI, Lassen MR. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2003;163(11):1337-1342. doi:10.1001/archinte.163.11.1337.
6. Handoll HH, Farrar MJ, McBirnie J, Tytherleigh-Strong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev.2002;(4):Cd000305. doi:10.1002/14651858.cd000305.
7. Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in the elderly. Cochrane Database Syst Rev. 2004;(1):Cd001880. doi:10.1002/14651858.CD001880.pub2.
8. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. doi:10.1046/j.1532-5415.2001.49108.x.
9. Deschodt M, Braes T, Flamaing J, et al. Preventing delirium in older adults with recent hip fracture through multidisciplinary geriatric consultation. J Am Geriatr Soc. 2012;60(4):733-739. doi:10.1111/j.1532-5415.2012.03899.x.
10. Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59 Suppl 2:S282-S288. doi:10.1111/j.1532-5415.2011.03691.x.
11. Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010;92(2):265-269. doi:10.2106/jbjs.i.00883.
12. Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455. doi:10.1093/oxfordjournals.bja.a013468.
13. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
14. Bohl DD, Grauer JN, Leopold SS. Editor's spotlight/Take 5: nationwide inpatient sample and national surgical quality improvement program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671. doi:10.1007/s11999-014-3595-9.
15. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/jbjs.m.01490.
16. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: "Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198. doi:10.2106/jbjs.n.00890.
17. Basques BA, Bohl DD, Golinvaux NS, Leslie MP, Baumgaertner MR, Grauer JN. Postoperative length of stay and thirty-day readmission following geriatric hip fracture: an analysis of 8,434 patients. J Orthop Trauma. 2015;29(3):e115-e120. doi:10.1097/bot.0000000000000222.
18. Golinvaux NS, Bohl DD, Basques BA, Baumgaertner MR, Grauer JN. Diabetes confers little to no increased risk of postoperative complications after hip fracture surgery in geriatric patients. Clin Orthop Relat Res. 2015;473(3):1043-1051. doi:10.1007/s11999-014-3945-7.
19. Maciejewski ML, Radcliff TA, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276. doi:10.1682/jrrd.2013.02.0041.
20. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res.2015;473(5):1574-1581. doi:10.1007/s11999-014-3597-7.
21. Bohl DD, Basques BA, Golinvaux NS, Miller CP, Baumgaertner MR, Grauer JN. Extramedullary compared with intramedullary implants for intertrochanteric hip fractures: thirty-day outcomes of 4432 procedures from the ACS NSQIP database. J Bone Joint Surg Am. 2014;96(22):1871-1877. doi:10.2106/jbjs.n.00041.
22. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine (Phila Pa 1976). 2009;34(18):1956-1962. doi:10.1097/BRS.0b013e3181ab930e.
23. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA.2009;302(1):58-66. doi:10.1001/jama.2009.956.
24. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267. doi:10.1016/j.yasu.2010.05.003.
25. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
26. ACS-NSQIP. Data Use Agreement. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip/participant-use/puf-form. Accessed September 20, 2018.
27. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938-942. doi:10.1161/hy1001.096358.
28. Browne JA, Cook C, Olson SA, Bolognesi MP. Resident duty-hour reform associated with increased morbidity following hip fracture. J Bone Joint Surg Am. 2009;91(9):2079-2085. doi:10.2106/jbjs.h.01240.
29. Browne JA, Pietrobon R, Olson SA. Hip fracture outcomes: does surgeon or hospital volume really matter? J Trauma. 2009;66(3):809-814. doi:10.1097/TA.0b013e31816166bb.
30. Menendez ME, Ring D. Failure to rescue after proximal femur fracture surgery. J Orthop Trauma. 2015;29(3):e96-e102. doi:10.1097/bot.0000000000000234.
31. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9-17. doi:10.2106/jbjs.j.01077.
32. Anderson KL, Koval KJ, Spratt KF. Hip fracture outcome: is there a “July effect”? Am J Orthop. 2009;38(12):606-611.
33. Koval KJ, Rust CL, Spratt KF. The effect of hospital setting and teaching status on outcomes after hip fracture. Am J Orthop. 2011;40(1):19-28.
34. Bacon WE. Secular trends in hip fracture occurrence and survival: age and sex differences. J Aging Health. 1996;8(4):538-553. doi:10.1177/089826439600800404.
35. Orces CH. In-hospital hip fracture mortality trends in older adults: the National Hospital Discharge Survey, 1988-2007. J Am Geriatr Soc. 2013;61(12):2248-2249. doi:10.1111/jgs.12567.
ABSTRACT
This study uses a prospective surgical registry to characterize the timing of 10 postoperative adverse events following geriatric hip fracture surgery. There were 19,873 patients identified who were ≥70 years undergoing surgery for hip fracture as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). The median postoperative day of diagnosis (and interquartile range) for myocardial infarction was 3 (1-5), cardiac arrest requiring cardiopulmonary resuscitation 3 (0-8), stroke 3 (1-10), pneumonia 4 (2-10), pulmonary embolism 4 (2-11), urinary tract infection 7 (2-13), deep vein thrombosis 9 (4-16), sepsis 9 (4-18), mortality 11 (6-19), and surgical site infection 16 (11-22). For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30. Findings help to enable more targeted clinical surveillance, inform patient counseling, and determine the duration of follow-up required to study specific adverse events effectively. Orthopedic surgeons should have the lowest threshold for testing for each adverse event during the time period of greatest risk.
Continue to: Geriatric hip fracture surgery is associated with...
Geriatric hip fracture surgery is associated with a higher rate of occurrence of postoperative adverse events than any other commonly performed orthopedic procedure.1-4 Indeed, the 90-day mortality rate following a geriatric hip fracture surgery may be as high as 15%2 and the 30-day morbidity rate as high as 30%.3 Furthermore, more than half of postoperative mortalities following orthopedic procedures occur after surgery for hip fracture.4 Therefore, extensive research has been conducted regarding interventions to reduce the rates of adverse events following a hip fracture surgery.5-12 For example, randomized trials have been conducted involving venous thromboembolism prophylaxis,5,6nutritional supplementation,7 delirium prevention,8-10 anemia correction,11 geriatrics consultation,9 and anesthetic technique.12
Despite these extensive research efforts, there is currently little information in the literature regarding when postoperative adverse events occur. A clear depiction of the timing of adverse events could help target clinical surveillance, inform patient counseling, and determine the duration of follow-up required for studies. The reason that the timing of adverse events has not been previously characterized may be that the sample sizes available through standard single- or multi-institutional studies may be insufficient to accurately characterize the timing of rare adverse events (eg, myocardial infarction, stroke, etc.). Moreover, although administrative datasets have become common data sources for investigation of rare postoperative adverse events,13-16 such data sources often do not contain data on the timing of diagnosis.
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) is a relatively new and growing surgical registry.1,3,13-22 The registry follows up patients undergoing surgical procedures at several hundred community and academic institutions nationwide. Unlike the administrative datasets discussed above, the ACS-NSQIP characterizes the postoperative day of diagnosis of well-defined adverse events during the first 30 postoperative days.22
In this study, data collected by the ACS-NSQIP are used to characterize the timing of 10 specific postoperative adverse events following a geriatric hip fracture surgery.
Continue to: METHODS...
METHODS
A retrospective analysis of data collected prospectively through the ACS-NSQIP was conducted. Geriatric patients who underwent hip fracture surgery during 2010 to 2013 were identified. Specific inclusion criteria were (1) International Classification of Diseases, Ninth Revision, diagnosis code 820, (2) primary Current Procedural Terminology codes 27125, 27130, 27235, 27236, 27244, or 27245, and (3) age ≥70 years.
The ACS-NSQIP captures patient demographic, comorbidity, and procedural characteristics at baseline.22 At the end of the 30-day follow-up period, the ACS-NSQIP personnel review both inpatient and outpatient charts to characterize the occurrence vs nonoccurrence of specific postoperative adverse events.22-25 When an adverse event does occur, the postoperative day of diagnosis is recorded.
For this study, the following adverse event categories were investigated: myocardial infarction, cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, pulmonary embolism, urinary tract infection, deep vein thrombosis, sepsis (either with or without shock), mortality, and surgical site infection (including superficial surgical site infection, deep surgical site infection, and organ or space surgical site infection). Detailed definitions of each adverse event are provided in ACS-NSQIP materials.22
First, the 30-day incidence (and the associated 95% confidence interval) was determined for each adverse event. Second, the median postoperative day of diagnosis (and the associated interquartile range) was determined for each adverse event. Third, the postoperative length of stay was used to estimate the proportion of diagnoses occurring prior to vs following discharge for each adverse event. Finally, multivariate Cox proportional hazards models were used to identify independent risk factors for earlier occurrence of postoperative adverse events. The final models were selected using a backward stepwise process that sequentially eliminated variables with the weakest associations until all variables had P < .05.
Because the ACS-NSQIP reports timing data in calendar days, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, it was not possible to ascertain whether the diagnosis occurred prior to or following discharge. For this study, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, the adverse event was considered to have been diagnosed following discharge. The rationale for this is that for most of the adverse events, it was thought to be unlikely that an inpatient would be discharged before the end of the same day as an inpatient diagnosis. However, there was one exception to this rule; when the postoperative day of discharge, the postoperative length of stay, and the postoperative day of death were all equivalent, the adverse event was considered to have occurred prior to discharge. This is because when a patient dies during the initial inpatient stay, the ACS-NSQIP considers the postoperative length of stay to be equivalent to the postoperative day of death. This makes it much more likely that a diagnosis on the final hospital day had occurred in a patient who had not been discharged.
The mandatory ACS-NSQIP statement is “The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.”26
Continue to: RESULTS...
RESULTS
In total, 19,873 geriatric patients undergoing a hip fracture surgery were identified (Table 1). The rates of adverse events ranged from 6.7% for urinary tract infection to 0.6% for pulmonary embolism (Table 2).
Table 1. Patient Population
| Number | Percent |
Total | 19,873 | 100.0% |
Age |
|
|
70-74 years | 1852 | 9.3% |
75-79 years | 2764 | 13.9% |
80-84 years | 4328 | 21.8% |
85-89 years | 5525 | 27.8% |
≥90 years | 5404 | 27.2% |
Sex |
|
|
Male | 5359 | 27.0% |
Female | 14,514 | 73.0% |
Body mass index |
|
|
<30 kg/m2 | 17,733 | 89.2% |
≥30 kg/m2 | 2140 | 10.8% |
Functional status |
|
|
Independent | 14,348 | 72.2% |
Dependent | 5525 | 27.8% |
Diabetes | 3321 | 16.7% |
Congestive heart failure | 738 | 3.7% |
Dyspnea on exertion | 1542 | 7.8% |
Hypertension | 14,265 | 71.8% |
End-stage renal disease | 322 | 1.6% |
COPD | 2239 | 11.3% |
Current smoker | 1506 | 7.6% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 2. Patients with Adverse Events Diagnosed During the First 30 postoperative days (N = 19,873)
Adverse Event | Number | Percent | 95% CI |
Urinary tract infection | 1321 | 6.7% | 6.3%-7.0% |
Mortality | 1240 | 6.2% | 5.9%-6.6% |
Pneumonia | 771 | 3.9% | 3.6%-4.2% |
Sepsis | 428 | 2.2% | 2.0%-2.4% |
Myocardial infarction | 347 | 1.8% | 1.6%-1.9% |
Surgical site infection | 247 | 1.2% | 1.1%-1.4% |
Deep vein thrombosis | 199 | 1.0% | 0.9%-1.1% |
Stroke | 144 | 0.7% | 0.6%-0.8% |
Cardiac arrest | 136 | 0.7% | 0.6%-0.8% |
Pulmonary embolism | 126 | 0.6% | 0.5%-0.7% |
Abbreviation: CI, confidence interval.
Figure 1 depicts the timing of postoperative adverse events in detail in histograms and timing curves. For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30.
Figure 2 provides the summary statistics for adverse events diagnosed in the first 30 postoperative days. The median postoperative day of diagnosis (and the interquartile range) was 3 (1-5) for myocardial infarction, 3 (0-8) for cardiac arrest requiring cardiopulmonary resuscitation, 3 (1-10) for stroke, 4 (2-10) for pneumonia, 4 (2-11) for pulmonary embolism, 7 (2-13) for urinary tract infection, 9 (4-16) for deep vein thrombosis, 9 (4-18) for sepsis, 11 (6-19) for mortality, and 16 (11-22) for surgical site infection.
Figure 3 depicts the timing of adverse events relative to discharge. The proportions of adverse events diagnosed prior to discharge were 81.0% for myocardial infarction, 77.8% for stroke, 76.1% for cardiac arrest requiring cardiopulmonary resuscitation, 71.9% for pulmonary embolism, 71.1% for pneumonia, 58.0% for urinary tract infection, 52.1% for sepsis, 46.9% for deep vein thrombosis, 44.3% for mortality, and 27.6% for surgical site infection.
Table 3 shows the independent risk factors for earlier occurrence of adverse events. Following multivariate stepwise selection of final models, at least 1 patient characteristic was independently associated with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death. In contrast, no patient characteristics were independently associated with the timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, and surgical site infection.
Table 3. Timing of Diagnosis of Adverse Eventsa
Adverse events and associated baseline characteristic(s) | Median postoperative day of diagnosis with vs without baseline characteristic | P-valueb |
Cardiac arrest |
|
|
End-stage renal disease | 1 vs 3 | .005 |
Stroke |
|
|
Hypertension | 4 vs 2 | .025 |
Dependent functional status | 2 vs 4 | .027 |
Urinary tract infection |
|
|
Female sex | 6 vs 8 | .009 |
Deep vein thrombosis |
|
|
Body mass index ≥30 kg/m2 | 5 vs 10 | .015 |
Death |
|
|
End-stage renal disease | 10 vs 11 | .031 |
aBaseline characteristics that were independently associated with the timing of each adverse event were identified through a backwards stepwise selection process initially including all characteristics listed in Table 1, and sequentially excluding characteristics with the weakest associations until only characteristics with P < .05 remained. Independent associations with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death are shown. There were no characteristics independently associated with timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, or surgical site infection; hence, these adverse events are not listed in the table.
bFrom final Cox proportional hazards models identified through multivariate stepwise selection.
Continue to: DISCUSSION...
DISCUSSION
Adverse events are extremely common following a geriatric hip fracture surgery.1-4 Despite extensive investigation regarding methods to prevent these events,5-12 there is limited published description of the timing at which such events occur. This study used a large prospectively followed up cohort of geriatric patients undergoing a hip fracture surgery to deliver a better description of the timing of adverse events than was previously available. The findings of this study should enable more targeted clinical surveillance, inform patient counseling, and help determine the duration of follow-up required for studies on adverse events.
There was wide variability in the timing at which the different postoperative adverse events were diagnosed (Figures 1, 2). Myocardial infarction was diagnosed the earliest, with more than three-fourth of diagnoses in the first postoperative week. Other relatively early-diagnosed adverse events included cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, and pulmonary embolism.
The latest-diagnosed adverse event was surgical site infection (Figures 1, 2). Surgical site infection was actually the only adverse event with a rate of diagnosis during the first week that was lower than the rate of diagnosis later in the month (as can be seen by the inflection in the timing curve for surgical site infection in Figure 1). Mortality showed a relatively consistent rate of diagnosis throughout the entire first postoperative month. Other relatively late-diagnosed postoperative events, including sepsis, deep vein thrombosis, and urinary tract infection, showed varying degrees of decreased rate of diagnosis near the end of the first postoperative month. Of note, for the later-diagnosed adverse events, the estimated median and interquartile ranges (Figure 2) were presumably quite biased toward earlier diagnosis, as the 30-day follow-up period clearly failed to capture a large proportion of later-occurring adverse events (Figure 1).
Certain risk factors were independently associated with earlier occurrence of adverse events. Perhaps most strikingly, body mass index in the obese range was associated with substantially earlier occurrence of deep vein thrombosis (median of 5 vs 10 days). This finding suggests that clinical monitoring for deep vein thrombosis should be performed earlier in patients with greater body mass index. Also notable is the earlier occurrence of cardiac arrest and death among patients with end-stage renal disease than among those without. Patients with end-stage renal disease may have a greater risk for these adverse events immediately following the cardiac stresses of surgery.27 Similarly, such patients may be more prone to early electrolyte abnormalities and arrhythmia.
Continue to: In addition to its clinical implications, this study...
In addition to its clinical implications, this study informs about the interpretation of the many studies of adverse events following hip fracture procedures that have been conducted using retrospective data. Several such studies have relied on inpatient-only administrative databases.4,13,14,28-35 As clearly demonstrated in Figure 3, for most of the commonly studied adverse events, inpatient-only databases failed to capture a large proportion of adverse events occurring in the first postoperative month. This highlights a substantial limitation of this commonly published type of study that is often not emphasized in the literature.
There has also been an increase in the publication of studies of adverse events following a hip fracture surgery using the ACS-NSQIP data.3,13,14,17,18,21 As discussed, the ACS-NSQIP provides data on 30-days of follow-up. This relatively extended follow-up is often touted as a distinct advantage. However, this study demonstrates that even the 30-day follow-up afforded by the ACS-NSQIP is limited in its ability to enable investigation of the later-occurring adverse events (Figure 1). In particular, the rate of surgical site infection shows little sign of slowing by postoperative day 30. Similarly, the rates of mortality, sepsis, deep vein thrombosis, and urinary tract infection remain substantial.
This study does have limitations. First, as discussed, the duration of follow-up is a limitation of any ACS-NSQIP-based investigation, including this study. Second, the ACS-NSQIP does not capture relevant orthopedic-specific outcomes (eg, screw cutout). In addition, it could not be determined with certainty whether adverse events occurring on the final hospital day occurred prior to or following discharge. However, only a small proportion of most of the adverse events was diagnosed on the final hospital day. Finally, the ACS-NSQIP reports on days from the operation until diagnosis of the adverse event. Although some adverse events are probably diagnosed quickly after they have occurred (eg, myocardial infarction and cardiac arrest), other adverse events may have a delayed diagnosis (eg, surgical site infection may be identified days after its initial occurrence during a follow-up examination). Therefore, it is important to note the subtle distinction between occurrence and diagnosis throughout the article. This article reports on the timing of diagnosis, not actual occurrence.
CONCLUSION
The timing of postoperative adverse events has been understudied in the past. This may be due to an inability of standard single- or multi-institutional investigations to achieve sample sizes adequate to study the less commonly occurring adverse events. Using a relatively new prospective surgical registry, this study provides a far more detailed description of the timing of adverse events following surgery than was previously available. The authors anticipate that these data can be used to inform patient counseling, target clinical surveillance, and direct clinical research. The authors chose to study the timing of postoperative adverse events following geriatric hip fracture surgery because of the high rate of adverse events associated with the procedure. However, future ACS-NSQIP studies may involve characterization of the timing of adverse events following other orthopedic and non-orthopedic procedures.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
This study uses a prospective surgical registry to characterize the timing of 10 postoperative adverse events following geriatric hip fracture surgery. There were 19,873 patients identified who were ≥70 years undergoing surgery for hip fracture as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). The median postoperative day of diagnosis (and interquartile range) for myocardial infarction was 3 (1-5), cardiac arrest requiring cardiopulmonary resuscitation 3 (0-8), stroke 3 (1-10), pneumonia 4 (2-10), pulmonary embolism 4 (2-11), urinary tract infection 7 (2-13), deep vein thrombosis 9 (4-16), sepsis 9 (4-18), mortality 11 (6-19), and surgical site infection 16 (11-22). For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30. Findings help to enable more targeted clinical surveillance, inform patient counseling, and determine the duration of follow-up required to study specific adverse events effectively. Orthopedic surgeons should have the lowest threshold for testing for each adverse event during the time period of greatest risk.
Continue to: Geriatric hip fracture surgery is associated with...
Geriatric hip fracture surgery is associated with a higher rate of occurrence of postoperative adverse events than any other commonly performed orthopedic procedure.1-4 Indeed, the 90-day mortality rate following a geriatric hip fracture surgery may be as high as 15%2 and the 30-day morbidity rate as high as 30%.3 Furthermore, more than half of postoperative mortalities following orthopedic procedures occur after surgery for hip fracture.4 Therefore, extensive research has been conducted regarding interventions to reduce the rates of adverse events following a hip fracture surgery.5-12 For example, randomized trials have been conducted involving venous thromboembolism prophylaxis,5,6nutritional supplementation,7 delirium prevention,8-10 anemia correction,11 geriatrics consultation,9 and anesthetic technique.12
Despite these extensive research efforts, there is currently little information in the literature regarding when postoperative adverse events occur. A clear depiction of the timing of adverse events could help target clinical surveillance, inform patient counseling, and determine the duration of follow-up required for studies. The reason that the timing of adverse events has not been previously characterized may be that the sample sizes available through standard single- or multi-institutional studies may be insufficient to accurately characterize the timing of rare adverse events (eg, myocardial infarction, stroke, etc.). Moreover, although administrative datasets have become common data sources for investigation of rare postoperative adverse events,13-16 such data sources often do not contain data on the timing of diagnosis.
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) is a relatively new and growing surgical registry.1,3,13-22 The registry follows up patients undergoing surgical procedures at several hundred community and academic institutions nationwide. Unlike the administrative datasets discussed above, the ACS-NSQIP characterizes the postoperative day of diagnosis of well-defined adverse events during the first 30 postoperative days.22
In this study, data collected by the ACS-NSQIP are used to characterize the timing of 10 specific postoperative adverse events following a geriatric hip fracture surgery.
Continue to: METHODS...
METHODS
A retrospective analysis of data collected prospectively through the ACS-NSQIP was conducted. Geriatric patients who underwent hip fracture surgery during 2010 to 2013 were identified. Specific inclusion criteria were (1) International Classification of Diseases, Ninth Revision, diagnosis code 820, (2) primary Current Procedural Terminology codes 27125, 27130, 27235, 27236, 27244, or 27245, and (3) age ≥70 years.
The ACS-NSQIP captures patient demographic, comorbidity, and procedural characteristics at baseline.22 At the end of the 30-day follow-up period, the ACS-NSQIP personnel review both inpatient and outpatient charts to characterize the occurrence vs nonoccurrence of specific postoperative adverse events.22-25 When an adverse event does occur, the postoperative day of diagnosis is recorded.
For this study, the following adverse event categories were investigated: myocardial infarction, cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, pulmonary embolism, urinary tract infection, deep vein thrombosis, sepsis (either with or without shock), mortality, and surgical site infection (including superficial surgical site infection, deep surgical site infection, and organ or space surgical site infection). Detailed definitions of each adverse event are provided in ACS-NSQIP materials.22
First, the 30-day incidence (and the associated 95% confidence interval) was determined for each adverse event. Second, the median postoperative day of diagnosis (and the associated interquartile range) was determined for each adverse event. Third, the postoperative length of stay was used to estimate the proportion of diagnoses occurring prior to vs following discharge for each adverse event. Finally, multivariate Cox proportional hazards models were used to identify independent risk factors for earlier occurrence of postoperative adverse events. The final models were selected using a backward stepwise process that sequentially eliminated variables with the weakest associations until all variables had P < .05.
Because the ACS-NSQIP reports timing data in calendar days, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, it was not possible to ascertain whether the diagnosis occurred prior to or following discharge. For this study, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, the adverse event was considered to have been diagnosed following discharge. The rationale for this is that for most of the adverse events, it was thought to be unlikely that an inpatient would be discharged before the end of the same day as an inpatient diagnosis. However, there was one exception to this rule; when the postoperative day of discharge, the postoperative length of stay, and the postoperative day of death were all equivalent, the adverse event was considered to have occurred prior to discharge. This is because when a patient dies during the initial inpatient stay, the ACS-NSQIP considers the postoperative length of stay to be equivalent to the postoperative day of death. This makes it much more likely that a diagnosis on the final hospital day had occurred in a patient who had not been discharged.
The mandatory ACS-NSQIP statement is “The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.”26
Continue to: RESULTS...
RESULTS
In total, 19,873 geriatric patients undergoing a hip fracture surgery were identified (Table 1). The rates of adverse events ranged from 6.7% for urinary tract infection to 0.6% for pulmonary embolism (Table 2).
Table 1. Patient Population
| Number | Percent |
Total | 19,873 | 100.0% |
Age |
|
|
70-74 years | 1852 | 9.3% |
75-79 years | 2764 | 13.9% |
80-84 years | 4328 | 21.8% |
85-89 years | 5525 | 27.8% |
≥90 years | 5404 | 27.2% |
Sex |
|
|
Male | 5359 | 27.0% |
Female | 14,514 | 73.0% |
Body mass index |
|
|
<30 kg/m2 | 17,733 | 89.2% |
≥30 kg/m2 | 2140 | 10.8% |
Functional status |
|
|
Independent | 14,348 | 72.2% |
Dependent | 5525 | 27.8% |
Diabetes | 3321 | 16.7% |
Congestive heart failure | 738 | 3.7% |
Dyspnea on exertion | 1542 | 7.8% |
Hypertension | 14,265 | 71.8% |
End-stage renal disease | 322 | 1.6% |
COPD | 2239 | 11.3% |
Current smoker | 1506 | 7.6% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 2. Patients with Adverse Events Diagnosed During the First 30 postoperative days (N = 19,873)
Adverse Event | Number | Percent | 95% CI |
Urinary tract infection | 1321 | 6.7% | 6.3%-7.0% |
Mortality | 1240 | 6.2% | 5.9%-6.6% |
Pneumonia | 771 | 3.9% | 3.6%-4.2% |
Sepsis | 428 | 2.2% | 2.0%-2.4% |
Myocardial infarction | 347 | 1.8% | 1.6%-1.9% |
Surgical site infection | 247 | 1.2% | 1.1%-1.4% |
Deep vein thrombosis | 199 | 1.0% | 0.9%-1.1% |
Stroke | 144 | 0.7% | 0.6%-0.8% |
Cardiac arrest | 136 | 0.7% | 0.6%-0.8% |
Pulmonary embolism | 126 | 0.6% | 0.5%-0.7% |
Abbreviation: CI, confidence interval.
Figure 1 depicts the timing of postoperative adverse events in detail in histograms and timing curves. For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30.
Figure 2 provides the summary statistics for adverse events diagnosed in the first 30 postoperative days. The median postoperative day of diagnosis (and the interquartile range) was 3 (1-5) for myocardial infarction, 3 (0-8) for cardiac arrest requiring cardiopulmonary resuscitation, 3 (1-10) for stroke, 4 (2-10) for pneumonia, 4 (2-11) for pulmonary embolism, 7 (2-13) for urinary tract infection, 9 (4-16) for deep vein thrombosis, 9 (4-18) for sepsis, 11 (6-19) for mortality, and 16 (11-22) for surgical site infection.
Figure 3 depicts the timing of adverse events relative to discharge. The proportions of adverse events diagnosed prior to discharge were 81.0% for myocardial infarction, 77.8% for stroke, 76.1% for cardiac arrest requiring cardiopulmonary resuscitation, 71.9% for pulmonary embolism, 71.1% for pneumonia, 58.0% for urinary tract infection, 52.1% for sepsis, 46.9% for deep vein thrombosis, 44.3% for mortality, and 27.6% for surgical site infection.
Table 3 shows the independent risk factors for earlier occurrence of adverse events. Following multivariate stepwise selection of final models, at least 1 patient characteristic was independently associated with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death. In contrast, no patient characteristics were independently associated with the timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, and surgical site infection.
Table 3. Timing of Diagnosis of Adverse Eventsa
Adverse events and associated baseline characteristic(s) | Median postoperative day of diagnosis with vs without baseline characteristic | P-valueb |
Cardiac arrest |
|
|
End-stage renal disease | 1 vs 3 | .005 |
Stroke |
|
|
Hypertension | 4 vs 2 | .025 |
Dependent functional status | 2 vs 4 | .027 |
Urinary tract infection |
|
|
Female sex | 6 vs 8 | .009 |
Deep vein thrombosis |
|
|
Body mass index ≥30 kg/m2 | 5 vs 10 | .015 |
Death |
|
|
End-stage renal disease | 10 vs 11 | .031 |
aBaseline characteristics that were independently associated with the timing of each adverse event were identified through a backwards stepwise selection process initially including all characteristics listed in Table 1, and sequentially excluding characteristics with the weakest associations until only characteristics with P < .05 remained. Independent associations with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death are shown. There were no characteristics independently associated with timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, or surgical site infection; hence, these adverse events are not listed in the table.
bFrom final Cox proportional hazards models identified through multivariate stepwise selection.
Continue to: DISCUSSION...
DISCUSSION
Adverse events are extremely common following a geriatric hip fracture surgery.1-4 Despite extensive investigation regarding methods to prevent these events,5-12 there is limited published description of the timing at which such events occur. This study used a large prospectively followed up cohort of geriatric patients undergoing a hip fracture surgery to deliver a better description of the timing of adverse events than was previously available. The findings of this study should enable more targeted clinical surveillance, inform patient counseling, and help determine the duration of follow-up required for studies on adverse events.
There was wide variability in the timing at which the different postoperative adverse events were diagnosed (Figures 1, 2). Myocardial infarction was diagnosed the earliest, with more than three-fourth of diagnoses in the first postoperative week. Other relatively early-diagnosed adverse events included cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, and pulmonary embolism.
The latest-diagnosed adverse event was surgical site infection (Figures 1, 2). Surgical site infection was actually the only adverse event with a rate of diagnosis during the first week that was lower than the rate of diagnosis later in the month (as can be seen by the inflection in the timing curve for surgical site infection in Figure 1). Mortality showed a relatively consistent rate of diagnosis throughout the entire first postoperative month. Other relatively late-diagnosed postoperative events, including sepsis, deep vein thrombosis, and urinary tract infection, showed varying degrees of decreased rate of diagnosis near the end of the first postoperative month. Of note, for the later-diagnosed adverse events, the estimated median and interquartile ranges (Figure 2) were presumably quite biased toward earlier diagnosis, as the 30-day follow-up period clearly failed to capture a large proportion of later-occurring adverse events (Figure 1).
Certain risk factors were independently associated with earlier occurrence of adverse events. Perhaps most strikingly, body mass index in the obese range was associated with substantially earlier occurrence of deep vein thrombosis (median of 5 vs 10 days). This finding suggests that clinical monitoring for deep vein thrombosis should be performed earlier in patients with greater body mass index. Also notable is the earlier occurrence of cardiac arrest and death among patients with end-stage renal disease than among those without. Patients with end-stage renal disease may have a greater risk for these adverse events immediately following the cardiac stresses of surgery.27 Similarly, such patients may be more prone to early electrolyte abnormalities and arrhythmia.
Continue to: In addition to its clinical implications, this study...
In addition to its clinical implications, this study informs about the interpretation of the many studies of adverse events following hip fracture procedures that have been conducted using retrospective data. Several such studies have relied on inpatient-only administrative databases.4,13,14,28-35 As clearly demonstrated in Figure 3, for most of the commonly studied adverse events, inpatient-only databases failed to capture a large proportion of adverse events occurring in the first postoperative month. This highlights a substantial limitation of this commonly published type of study that is often not emphasized in the literature.
There has also been an increase in the publication of studies of adverse events following a hip fracture surgery using the ACS-NSQIP data.3,13,14,17,18,21 As discussed, the ACS-NSQIP provides data on 30-days of follow-up. This relatively extended follow-up is often touted as a distinct advantage. However, this study demonstrates that even the 30-day follow-up afforded by the ACS-NSQIP is limited in its ability to enable investigation of the later-occurring adverse events (Figure 1). In particular, the rate of surgical site infection shows little sign of slowing by postoperative day 30. Similarly, the rates of mortality, sepsis, deep vein thrombosis, and urinary tract infection remain substantial.
This study does have limitations. First, as discussed, the duration of follow-up is a limitation of any ACS-NSQIP-based investigation, including this study. Second, the ACS-NSQIP does not capture relevant orthopedic-specific outcomes (eg, screw cutout). In addition, it could not be determined with certainty whether adverse events occurring on the final hospital day occurred prior to or following discharge. However, only a small proportion of most of the adverse events was diagnosed on the final hospital day. Finally, the ACS-NSQIP reports on days from the operation until diagnosis of the adverse event. Although some adverse events are probably diagnosed quickly after they have occurred (eg, myocardial infarction and cardiac arrest), other adverse events may have a delayed diagnosis (eg, surgical site infection may be identified days after its initial occurrence during a follow-up examination). Therefore, it is important to note the subtle distinction between occurrence and diagnosis throughout the article. This article reports on the timing of diagnosis, not actual occurrence.
CONCLUSION
The timing of postoperative adverse events has been understudied in the past. This may be due to an inability of standard single- or multi-institutional investigations to achieve sample sizes adequate to study the less commonly occurring adverse events. Using a relatively new prospective surgical registry, this study provides a far more detailed description of the timing of adverse events following surgery than was previously available. The authors anticipate that these data can be used to inform patient counseling, target clinical surveillance, and direct clinical research. The authors chose to study the timing of postoperative adverse events following geriatric hip fracture surgery because of the high rate of adverse events associated with the procedure. However, future ACS-NSQIP studies may involve characterization of the timing of adverse events following other orthopedic and non-orthopedic procedures.
This paper will be judged for the Resident Writer’s Award.
1. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/jbjs.i.00735.
2. Forte ML, Virnig BA, Swiontkowski MF, et al. Ninety-day mortality after intertrochanteric hip fracture: does provider volume matter? J Bone Joint Surg Am. 2010;92(4):799-806. doi:10.2106/jbjs.h.01204.
3. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma.2014;28(2):63-69. doi:10.1097/BOT.0b013e3182a22744.
4. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84-a(4):562-572.
5. Eriksson BI, Lassen MR. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2003;163(11):1337-1342. doi:10.1001/archinte.163.11.1337.
6. Handoll HH, Farrar MJ, McBirnie J, Tytherleigh-Strong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev.2002;(4):Cd000305. doi:10.1002/14651858.cd000305.
7. Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in the elderly. Cochrane Database Syst Rev. 2004;(1):Cd001880. doi:10.1002/14651858.CD001880.pub2.
8. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. doi:10.1046/j.1532-5415.2001.49108.x.
9. Deschodt M, Braes T, Flamaing J, et al. Preventing delirium in older adults with recent hip fracture through multidisciplinary geriatric consultation. J Am Geriatr Soc. 2012;60(4):733-739. doi:10.1111/j.1532-5415.2012.03899.x.
10. Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59 Suppl 2:S282-S288. doi:10.1111/j.1532-5415.2011.03691.x.
11. Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010;92(2):265-269. doi:10.2106/jbjs.i.00883.
12. Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455. doi:10.1093/oxfordjournals.bja.a013468.
13. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
14. Bohl DD, Grauer JN, Leopold SS. Editor's spotlight/Take 5: nationwide inpatient sample and national surgical quality improvement program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671. doi:10.1007/s11999-014-3595-9.
15. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/jbjs.m.01490.
16. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: "Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198. doi:10.2106/jbjs.n.00890.
17. Basques BA, Bohl DD, Golinvaux NS, Leslie MP, Baumgaertner MR, Grauer JN. Postoperative length of stay and thirty-day readmission following geriatric hip fracture: an analysis of 8,434 patients. J Orthop Trauma. 2015;29(3):e115-e120. doi:10.1097/bot.0000000000000222.
18. Golinvaux NS, Bohl DD, Basques BA, Baumgaertner MR, Grauer JN. Diabetes confers little to no increased risk of postoperative complications after hip fracture surgery in geriatric patients. Clin Orthop Relat Res. 2015;473(3):1043-1051. doi:10.1007/s11999-014-3945-7.
19. Maciejewski ML, Radcliff TA, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276. doi:10.1682/jrrd.2013.02.0041.
20. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res.2015;473(5):1574-1581. doi:10.1007/s11999-014-3597-7.
21. Bohl DD, Basques BA, Golinvaux NS, Miller CP, Baumgaertner MR, Grauer JN. Extramedullary compared with intramedullary implants for intertrochanteric hip fractures: thirty-day outcomes of 4432 procedures from the ACS NSQIP database. J Bone Joint Surg Am. 2014;96(22):1871-1877. doi:10.2106/jbjs.n.00041.
22. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine (Phila Pa 1976). 2009;34(18):1956-1962. doi:10.1097/BRS.0b013e3181ab930e.
23. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA.2009;302(1):58-66. doi:10.1001/jama.2009.956.
24. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267. doi:10.1016/j.yasu.2010.05.003.
25. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
26. ACS-NSQIP. Data Use Agreement. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip/participant-use/puf-form. Accessed September 20, 2018.
27. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938-942. doi:10.1161/hy1001.096358.
28. Browne JA, Cook C, Olson SA, Bolognesi MP. Resident duty-hour reform associated with increased morbidity following hip fracture. J Bone Joint Surg Am. 2009;91(9):2079-2085. doi:10.2106/jbjs.h.01240.
29. Browne JA, Pietrobon R, Olson SA. Hip fracture outcomes: does surgeon or hospital volume really matter? J Trauma. 2009;66(3):809-814. doi:10.1097/TA.0b013e31816166bb.
30. Menendez ME, Ring D. Failure to rescue after proximal femur fracture surgery. J Orthop Trauma. 2015;29(3):e96-e102. doi:10.1097/bot.0000000000000234.
31. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9-17. doi:10.2106/jbjs.j.01077.
32. Anderson KL, Koval KJ, Spratt KF. Hip fracture outcome: is there a “July effect”? Am J Orthop. 2009;38(12):606-611.
33. Koval KJ, Rust CL, Spratt KF. The effect of hospital setting and teaching status on outcomes after hip fracture. Am J Orthop. 2011;40(1):19-28.
34. Bacon WE. Secular trends in hip fracture occurrence and survival: age and sex differences. J Aging Health. 1996;8(4):538-553. doi:10.1177/089826439600800404.
35. Orces CH. In-hospital hip fracture mortality trends in older adults: the National Hospital Discharge Survey, 1988-2007. J Am Geriatr Soc. 2013;61(12):2248-2249. doi:10.1111/jgs.12567.
1. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/jbjs.i.00735.
2. Forte ML, Virnig BA, Swiontkowski MF, et al. Ninety-day mortality after intertrochanteric hip fracture: does provider volume matter? J Bone Joint Surg Am. 2010;92(4):799-806. doi:10.2106/jbjs.h.01204.
3. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma.2014;28(2):63-69. doi:10.1097/BOT.0b013e3182a22744.
4. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84-a(4):562-572.
5. Eriksson BI, Lassen MR. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2003;163(11):1337-1342. doi:10.1001/archinte.163.11.1337.
6. Handoll HH, Farrar MJ, McBirnie J, Tytherleigh-Strong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev.2002;(4):Cd000305. doi:10.1002/14651858.cd000305.
7. Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in the elderly. Cochrane Database Syst Rev. 2004;(1):Cd001880. doi:10.1002/14651858.CD001880.pub2.
8. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. doi:10.1046/j.1532-5415.2001.49108.x.
9. Deschodt M, Braes T, Flamaing J, et al. Preventing delirium in older adults with recent hip fracture through multidisciplinary geriatric consultation. J Am Geriatr Soc. 2012;60(4):733-739. doi:10.1111/j.1532-5415.2012.03899.x.
10. Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59 Suppl 2:S282-S288. doi:10.1111/j.1532-5415.2011.03691.x.
11. Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010;92(2):265-269. doi:10.2106/jbjs.i.00883.
12. Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455. doi:10.1093/oxfordjournals.bja.a013468.
13. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
14. Bohl DD, Grauer JN, Leopold SS. Editor's spotlight/Take 5: nationwide inpatient sample and national surgical quality improvement program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671. doi:10.1007/s11999-014-3595-9.
15. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/jbjs.m.01490.
16. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: "Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198. doi:10.2106/jbjs.n.00890.
17. Basques BA, Bohl DD, Golinvaux NS, Leslie MP, Baumgaertner MR, Grauer JN. Postoperative length of stay and thirty-day readmission following geriatric hip fracture: an analysis of 8,434 patients. J Orthop Trauma. 2015;29(3):e115-e120. doi:10.1097/bot.0000000000000222.
18. Golinvaux NS, Bohl DD, Basques BA, Baumgaertner MR, Grauer JN. Diabetes confers little to no increased risk of postoperative complications after hip fracture surgery in geriatric patients. Clin Orthop Relat Res. 2015;473(3):1043-1051. doi:10.1007/s11999-014-3945-7.
19. Maciejewski ML, Radcliff TA, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276. doi:10.1682/jrrd.2013.02.0041.
20. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res.2015;473(5):1574-1581. doi:10.1007/s11999-014-3597-7.
21. Bohl DD, Basques BA, Golinvaux NS, Miller CP, Baumgaertner MR, Grauer JN. Extramedullary compared with intramedullary implants for intertrochanteric hip fractures: thirty-day outcomes of 4432 procedures from the ACS NSQIP database. J Bone Joint Surg Am. 2014;96(22):1871-1877. doi:10.2106/jbjs.n.00041.
22. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine (Phila Pa 1976). 2009;34(18):1956-1962. doi:10.1097/BRS.0b013e3181ab930e.
23. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA.2009;302(1):58-66. doi:10.1001/jama.2009.956.
24. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267. doi:10.1016/j.yasu.2010.05.003.
25. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
26. ACS-NSQIP. Data Use Agreement. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip/participant-use/puf-form. Accessed September 20, 2018.
27. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938-942. doi:10.1161/hy1001.096358.
28. Browne JA, Cook C, Olson SA, Bolognesi MP. Resident duty-hour reform associated with increased morbidity following hip fracture. J Bone Joint Surg Am. 2009;91(9):2079-2085. doi:10.2106/jbjs.h.01240.
29. Browne JA, Pietrobon R, Olson SA. Hip fracture outcomes: does surgeon or hospital volume really matter? J Trauma. 2009;66(3):809-814. doi:10.1097/TA.0b013e31816166bb.
30. Menendez ME, Ring D. Failure to rescue after proximal femur fracture surgery. J Orthop Trauma. 2015;29(3):e96-e102. doi:10.1097/bot.0000000000000234.
31. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9-17. doi:10.2106/jbjs.j.01077.
32. Anderson KL, Koval KJ, Spratt KF. Hip fracture outcome: is there a “July effect”? Am J Orthop. 2009;38(12):606-611.
33. Koval KJ, Rust CL, Spratt KF. The effect of hospital setting and teaching status on outcomes after hip fracture. Am J Orthop. 2011;40(1):19-28.
34. Bacon WE. Secular trends in hip fracture occurrence and survival: age and sex differences. J Aging Health. 1996;8(4):538-553. doi:10.1177/089826439600800404.
35. Orces CH. In-hospital hip fracture mortality trends in older adults: the National Hospital Discharge Survey, 1988-2007. J Am Geriatr Soc. 2013;61(12):2248-2249. doi:10.1111/jgs.12567.
TAKE-HOME POINTS
- The median postoperative day of diagnosis for myocardial infarction was 3, 3 for cardiac arrest requiring cardiopulmonary resuscitation, 3 for stroke, 4 for pneumonia, 4 for pulmonary embolism, 7 for urinary tract infection, 9 for deep vein thrombosis, 9 for sepsis, 11 for mortality, and 16 for surgical site infection.
- For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30; however, for the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30.
- The proportions of adverse events diagnosed prior to discharge were 81.0% for myocardial infarction, 77.8% for stroke, 76.1% for cardiac arrest requiring cardiopulmonary resuscitation, 71.9% for pulmonary embolism, 71.1% for pneumonia, 58.0% for urinary tract infection, 52.1% for sepsis, 46.9% for deep vein thrombosis, 44.3% for mortality, and 27.6% for surgical site infection.
- These results facilitate targeted clinical surveillance, guide patient counseling, and inform the duration of follow-up required in research studies.
- Clinicians should have the lowest threshold for testing for each adverse event during the time period of greatest risk.
Utilization of Primary Care Physicians by Medical Residents: A Survey-Based Study
From the University of Michigan Medical School, Ann Arbor, MI.
Abstract
- Objective: Existing research has demonstrated overall low rates of residents establishing care with a primary care physician (PCP). We conducted a survey-based study to better understand chronic illness, PCP utilization, and prescription medication use patterns in resident physician populations.
- Methods: In 2017, we invited internal and family medicine trainees from a convenience sample of U.S. residency programs to participate in a survey. We compared the characteristics of residents who had established care with a PCP to those who had not.
- Results: The response rate was 45% (348/766 residents). The majority (n = 205, 59%) of respondents stated they had established care with a PCP primarily for routine preventative care (n = 159, 79%) and access in the event of an emergency (n = 132, 66%). However, 31% (n = 103) denied having had a wellness visit in over 3 years. Nearly a quarter of residents (n = 77, 23%) reported a chronic medical illness and 14% (n = 45) reported a preexisting mental health condition prior to residency. One-third (n = 111, 33%) reported taking a long-term prescription medication. Compared to residents who had not established care, those with a PCP (n = 205) more often reported a chronic condition (P < 0.001), seeing a subspecialist (P = 0.01), or taking long-term prescription medications (P < 0.001). One in 5 (n = 62,19%) respondents reported receiving prescriptions for an acute illness from an individual with whom they did not have a doctor-patient relationship.
- Conclusion: Medical residents have a substantial burden of chronic illness that may not be met through interactions with PCPs. Further understanding their medical needs and barriers to accessing care is necessary to ensure trainee well-being.
Keywords: Medical education-graduate, physician behavior, survey research, access to care.
Although internal medicine (IM) and family medicine (FM) residents must learn to provide high-quality primary care to their patients, little is known about whether they appropriately access such care themselves. Resident burnout and resilience has received attention [1,2], but there has been limited focus on understanding the burden of chronic medical and mental illness among residents. In particular, little is known about whether residents access primary care physicians (PCPs)—for either acute or chronic medical needs—and about resident self-medication practices.
Residency is often characterized by a life-changing geographic relocation. Even residents who do not relocate may still need to establish care with a new PCP due to health insurance or loss of access to a student clinic [3]. Establishing primary care with a new doctor typically requires scheduling a new patient visit, often with a wait time of several days to weeks [4,5]. Furthermore, lack of time, erratic schedules, and concerns about privacy and the stigma of being ill as a physician are barriers to establishing care [6-8]. Individuals who have not established primary care may experience delays in routine preventative health services, screening for chronic medical and mental health conditions, as well as access to care during acute illnesses [9,10]. Worse, they may engage in potentially unsafe practices, such as having colleagues write prescriptions for them, or even self-prescribing [8,11,12].
Existing research has demonstrated overall low rates of residents establishing care with a PCP [6–8,13]. However, these studies have either been limited to large academic centers or conducted outside the United States. Improving resident well-being may prove challenging without a clear understanding of current primary care utilization practices, the burden of chronic illness among residents, and patterns of prescription medication use and needs. Therefore, we conducted a survey-based study to understand primary care utilization and the burden of chronic illness among residents. We also assessed whether lack of primary care is associated with potentially risky behaviors, such as self-prescribing of medications.
Methods
Study Setting and Participants
The survey was distributed to current residents at IM and FM programs within the United States in 2017. Individual programs were recruited by directly contacting program directors or chief medical residents via email. Rather than contacting sites directly through standard templated emails, we identified programs both through personal contacts as well as the Electronic Residency Application Service list of accredited IM training programs. We elected to use this approach in order to increase response rates and to ensure that a sample representative of the trainee population was constructed. Programs were located in the Northeast, Midwest, South, and Pacific regions, and included small community-based programs and large academic centers.
Development of the Survey
The survey instrument was developed by the authors and reviewed by residents and PCPs at the University of Michigan to ensure relevance and comprehension of questions (The survey is available in the Appendix.). Once finalized, the survey was programmed into an online survey tool (Qualtrics, Provo, UT) and pilot-tested before being disseminated to the sampling frame. Data collected in the survey included: respondent’s utilization of a PCP, burden of chronic illness, long-term prescription medications, prescribing source, and demographic characteristics.
Each participating program distributed the survey to their residents through an email containing an anonymous hyperlink. The survey was available for completion for 4 weeks. We asked participating programs to send email reminders to encourage participation. Participants were given the option of receiving a $10 Amazon gift card after completion. All responses were recorded anonymously. The study received a “not regulated” status by the University of Michigan Institutional Review Board (HUM 00123888).
Statistical Analysis
Descriptive statistics were used to tabulate results. Respondents were encouraged, but not required, to answer all questions. Therefore, the response rate for each question was calculated using the total number of responses for that question as the denominator. Bivariable comparisons were made using Chi-squared or Fisher’s exact tests, as appropriate, for categorical data. A P value < 0.05, with 2-sided alpha, was considered statistically significant. All statistical analyses were conducted using Stata 13 SE (StataCorp, College Station, TX).
Results
Respondent Characteristics
Of the 29 programs contacted, 10 agreed to participate within the study timeframe. Of 766 potential respondents, 348 (45%) residents answered the survey (Table 1). The majority of respondents (n = 276, 82%) were from IM programs. Respondents were from all training years as follows: postgraduate year 1 residents (PGY-1, or interns; n = 130, 39%), PGY-2 residents (n = 98, 29%), PGY-3 residents (n = 93, 28%), and PGY-4 residents (n = 12, 4%). Most respondents were from the South (n = 130, 39%) and Midwest (n = 123, 37%) regions, and over half (n = 179, 54%) were female. Most respondents (n = 285, 86%) stated that they did not have children. The majority (n = 236, 71%) were completing residency in an area where they had not previously lived for more than 1 year.
Primary Care Utilization
Among the 348 respondents, 59% (n = 205) reported having established care with a PCP. An additional 6% (n = 21) had established care with an obstetrician/gynecologist for routine needs (Table 2). The 2 most common reasons for establishing care with a PCP were routine primary care needs, including contraception (n = 159, 79%), and access to a physician in the event of an acute medical need (n = 132, 66%).
Among respondents who had established care with a PCP, most (n = 188, 94%) had completed at least 1 appointment. However, among these 188 respondents, 68% (n = 127) stated that they had not made an acute visit in more than 12 months. When asked about wellness visits, almost one third of respondents (n = 103, 31%) stated that they had not been seen for a wellness visit in the past 3 years.
Burden of Chronic Illness
Most respondents (n = 223, 67%) stated that they did not have a chronic medical or mental health condition prior to residency (Table 3). However, 23% (n = 77) of respondents stated that they had been diagnosed with a chronic medical illness prior to residency, and 14% (n = 45) indicated they had been diagnosed with a mental health condition prior to residency. Almost one fifth of respondents (n = 60, 18%) reported seeing a subspecialist for a medical illness, and 33% (n = 111) reported taking a long-term prescription medication. With respect to major medical issues, the majority of residents (n = 239, 72%) denied experiencing events such as pregnancy, hospitalization, surgery, or an emergency department (ED) visit during training.
[polldaddy:10116940]
Inappropriate Prescriptions
While the majority of respondents denied writing a prescription for themselves for an acute or chronic medical condition, almost one fifth (n = 62, 19%) had received a prescription for an acute medical need from a provider outside of a clinical relationship (ie, from someone other than their PCP or specialty provider). Notably, 5% (n = 15) reported that this had occurred at least 2 or 3 times in the past 12 months (Table 4). Compared to respondents not taking long-term prescription medications, respondents who were already taking long-term prescription medications more frequently reported inappropriately receiving chronic prescriptions outside of an established clinical relationship (n = 14, 13% vs. n = 14, 6%; P = 0.05) and more often self-prescribed medications for acute needs (n = 12, 11% vs. n = 7, 3%; P = 0.005).
Comparison of Residents With and Without a PCP
Important differences were noted between residents who had a PCP versus those who did not (Table 5). For example, a higher percentage of residents with a PCP indicated they had been diagnosed with a chronic medical illness (n = 55, 28% vs. n = 22, 16%; P = 0.01) or a chronic mental health condition (n = 34, 17% vs. n = 11, 8%; P = 0.02) before residency. Additionally, a higher percentage of residents with a PCP (n = 70, 35% vs. n = 25, 18%; P = 0.001) reported experiencing medical events such as pregnancy, hospitalization, surgery, ED visit, or new diagnosis of a chronic medical illness during residency. Finally, a higher percentage of respondents with a PCP stated that they had visited a subspecialist for a medical illness (n = 44, 22% vs. n = 16,12%; P = 0.01) or were taking long-term prescription medications (n = 86, 43% vs. n = 25; 18%; P < 0.001). When comparing PGY-1 to PGY-2–PGY-4 residents, the former reported having established a medical relationship with a PCP significantly less frequently (n = 56, 43% vs. n = 142, 70%; P < 0.001).
Discussion
This survey-based study of medical residents across the United States suggests that a substantial proportion do not establish relationships with PCPs. Additionally, our data suggest that despite establishing care, few residents subsequently visited their PCP during training for wellness visits or routine care. Self-reported rates of chronic medical and mental health conditions were substantial in our sample. Furthermore, inappropriate self-prescription and the receipt of prescriptions outside of a medical relationship were also reported. These findings suggest that future studies that focus on the unique medical and mental health needs of physicians in training, as well as interventions to encourage care in this vulnerable period, are necessary.
We observed that most respondents that established primary care were female trainees. Although it is impossible to know with certainty, one hypothesis behind this discrepancy is that women routinely need to access preventative care for gynecologic needs such as pap smears, contraception, and potentially pregnancy and preconception counseling [14,15]. Similarly, residents with a chronic medical or mental health condition prior to residency established care with a local PCP at a significantly greater frequency than those without such diagnoses. While selection bias cannot be excluded, this finding suggests that illness is a driving factor in establishing care. There also appears to be an association between accessing the medical system (either for prescription medications or subspecialist care) and having established care with a PCP. Collectively, these data suggest that individuals without a compelling reason to access medical services might have barriers to accessing care in the event of medical needs or may not receive routine preventative care [9,10].
In general, we found that rates of reported inappropriate prescriptions were lower than those reported in prior studies where a comparable resident population was surveyed [8,12,16]. Inclusion of multiple institutions, differences in temporality, social desirability bias, and reporting bias might have influenced our findings in this regard. Surprisingly, we found that having a PCP did not influence likelihood of inappropriate prescription receipt, perhaps suggesting that this behavior reflects some degree of universal difficulty in accessing care. Alternatively, this finding might relate to a cultural tendency to self-prescribe among resident physicians. The fact that individuals on chronic medications more often both received and wrote inappropriate prescriptions suggests this problem might be more pronounced in individuals who take medications more often, as these residents have specific needs [12]. Future studies targeting these individuals thus appear warranted.
Our study has several limitations. First, our sample size was modest and the response rate of 45% was low. However, to our knowledge, this remains among the largest survey on this topic, and our response rate is comparable to similar trainee studies [8,11,13]. Second, we designed and created a novel survey for this study. While the questions were pilot-tested with users prior to dissemination, validation of the instrument was not performed. Third, since the study population was restricted to residents in fields that participate in primary care, our findings may not be generalizable to patterns of PCP use in other specialties [6].
These limitations aside, our study has important strengths. This is the first national study of its kind with specific questions addressing primary care access and utilization, prescription medication use and related practices, and the prevalence of medical conditions among trainees. Important differences in the rates of establishing primary care between male and female respondents, first- year and senior residents, and those with and without chronic disease suggest a need to target specific resident groups (males, interns, those without pre-existing conditions) for wellness-related interventions. Such interventions could include distribution of a list of local providers to first year residents, advanced protected time for doctor’s appointments, and safeguards to ensure health information is protected from potential supervisors. Future studies should also include residents from non-primary care oriented specialties such as surgery, emergency medicine, and anesthesiology to obtain results that are more generalizable to the resident population as a whole. Additionally, the rates of inappropriate prescriptions were not insignificant and warrant further evaluation of the driving forces behind these behaviors.
Conclusion
Medical residents have a substantial burden of chronic illness that may not be met through interactions with PCPs. More research into barriers that residents face while accessing care and an assessment of interventions to facilitate their access to care is important to promote trainee well-being. Without such direction and initiative, it may prove harder for physicians to heal themselves or those for whom they provide care.
Acknowledgments: We thank Suzanne Winter, the study coordinator, for her support with editing and formatting the manuscript, Latoya Kuhn for performing the statistical analysis and creating data tables, and Dr. Namita Sachdev and Dr. Renuka Tipirneni for providing feedback on the survey instrument. We also thank the involved programs for their participation.
Corresponding author: Vineet Chopra, NCRC 2800 Plymouth Rd., Bldg 16, 432, Ann Arbor, MI 48109, [email protected].
Financial disclosures: None.
Previous presentations: Results were presented at the Annual Michigan Medicine 2017 Internal Medicine Research Symposium.
1. Kassam A, Horton J, Shoimer I, Patten S. Predictors of well-being in resident physicians: a descriptive and psychometric study. J Grad Med Educ 2015;7:70–4.
2. Shanafelt TD, Bradley KA, Wipf JE, Back AL. Burnout and self-reported patient care in an internal medicine residency program. Ann Intern Med 2002;136:358–67.
3. Burstin HR, Swartz K, O’Neil AC, et al. The effect of change of health insurance on access to care. Inquiry 1998;35:389–97.
4. Rhodes KV, Basseyn S, Friedman AB, et al. Access to primary care appointments following 2014 insurance expansions. Ann Fam Med 2017;15:107–12.
5. Polsky D, Richards M, Basseyn S, et al. Appointment availability after increases in Medicaid payments for primary care. N Engl J Med 2015;372:537–45.
6. Gupta G, Schleinitz MD, Reinert SE, McGarry KA. Resident physician preventive health behaviors and perspectives on primary care. R I Med J (2013) 2013;96:43–7.
7. Rosen IM, Christine JD, Bellini LM, Asch DA. Health and health care among housestaff in four U.S. internal medicine residency programs. J Gen Intern Med 2000;15:116-21.
8. Campbell S, Delva D. Physician do not heal thyself. Survey of personal health practices among medical residents. Can Fam Physician 2003;49:1121–7.
9. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005;83(3):457-502.
10. Weissman JS, Stern R, Fielding SL, et al. Delayed access to health care: risk factors, reasons, and consequences. Ann Intern Med 1991;114:325–31.
11. Guille C, Sen S. Prescription drug use and self-prescription among training physicians. Arch Intern Med 2012;172:371–2.
12. Roberts LW, Kim JP. Informal health care practices of residents: “curbside” consultation and self-diagnosis and treatment. Acad Psychiatry 2015;39:22-30.
13. Cohen JS, Patten S. Well-being in residency training: a survey examining resident physician satisfaction both within and outside of residency training and mental health in Alberta. BMC Med Educ 2005;5:21.
14. U.S. Preventive Services Task Force. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Published March 2012. Accessed August 21, 2018.
15. Health Resources and Services Administration. Women’s preventative services guidelines. https://www.hrsa.gov/womensguidelines2016/index.html. Updated October 2017. Accessed August 21, 2018.
16. Christie JD, Rosen IM, Bellini LM, et al. Prescription drug use and self-prescription among resident physicians. JAMA 1998;280(14):1253–5.
From the University of Michigan Medical School, Ann Arbor, MI.
Abstract
- Objective: Existing research has demonstrated overall low rates of residents establishing care with a primary care physician (PCP). We conducted a survey-based study to better understand chronic illness, PCP utilization, and prescription medication use patterns in resident physician populations.
- Methods: In 2017, we invited internal and family medicine trainees from a convenience sample of U.S. residency programs to participate in a survey. We compared the characteristics of residents who had established care with a PCP to those who had not.
- Results: The response rate was 45% (348/766 residents). The majority (n = 205, 59%) of respondents stated they had established care with a PCP primarily for routine preventative care (n = 159, 79%) and access in the event of an emergency (n = 132, 66%). However, 31% (n = 103) denied having had a wellness visit in over 3 years. Nearly a quarter of residents (n = 77, 23%) reported a chronic medical illness and 14% (n = 45) reported a preexisting mental health condition prior to residency. One-third (n = 111, 33%) reported taking a long-term prescription medication. Compared to residents who had not established care, those with a PCP (n = 205) more often reported a chronic condition (P < 0.001), seeing a subspecialist (P = 0.01), or taking long-term prescription medications (P < 0.001). One in 5 (n = 62,19%) respondents reported receiving prescriptions for an acute illness from an individual with whom they did not have a doctor-patient relationship.
- Conclusion: Medical residents have a substantial burden of chronic illness that may not be met through interactions with PCPs. Further understanding their medical needs and barriers to accessing care is necessary to ensure trainee well-being.
Keywords: Medical education-graduate, physician behavior, survey research, access to care.
Although internal medicine (IM) and family medicine (FM) residents must learn to provide high-quality primary care to their patients, little is known about whether they appropriately access such care themselves. Resident burnout and resilience has received attention [1,2], but there has been limited focus on understanding the burden of chronic medical and mental illness among residents. In particular, little is known about whether residents access primary care physicians (PCPs)—for either acute or chronic medical needs—and about resident self-medication practices.
Residency is often characterized by a life-changing geographic relocation. Even residents who do not relocate may still need to establish care with a new PCP due to health insurance or loss of access to a student clinic [3]. Establishing primary care with a new doctor typically requires scheduling a new patient visit, often with a wait time of several days to weeks [4,5]. Furthermore, lack of time, erratic schedules, and concerns about privacy and the stigma of being ill as a physician are barriers to establishing care [6-8]. Individuals who have not established primary care may experience delays in routine preventative health services, screening for chronic medical and mental health conditions, as well as access to care during acute illnesses [9,10]. Worse, they may engage in potentially unsafe practices, such as having colleagues write prescriptions for them, or even self-prescribing [8,11,12].
Existing research has demonstrated overall low rates of residents establishing care with a PCP [6–8,13]. However, these studies have either been limited to large academic centers or conducted outside the United States. Improving resident well-being may prove challenging without a clear understanding of current primary care utilization practices, the burden of chronic illness among residents, and patterns of prescription medication use and needs. Therefore, we conducted a survey-based study to understand primary care utilization and the burden of chronic illness among residents. We also assessed whether lack of primary care is associated with potentially risky behaviors, such as self-prescribing of medications.
Methods
Study Setting and Participants
The survey was distributed to current residents at IM and FM programs within the United States in 2017. Individual programs were recruited by directly contacting program directors or chief medical residents via email. Rather than contacting sites directly through standard templated emails, we identified programs both through personal contacts as well as the Electronic Residency Application Service list of accredited IM training programs. We elected to use this approach in order to increase response rates and to ensure that a sample representative of the trainee population was constructed. Programs were located in the Northeast, Midwest, South, and Pacific regions, and included small community-based programs and large academic centers.
Development of the Survey
The survey instrument was developed by the authors and reviewed by residents and PCPs at the University of Michigan to ensure relevance and comprehension of questions (The survey is available in the Appendix.). Once finalized, the survey was programmed into an online survey tool (Qualtrics, Provo, UT) and pilot-tested before being disseminated to the sampling frame. Data collected in the survey included: respondent’s utilization of a PCP, burden of chronic illness, long-term prescription medications, prescribing source, and demographic characteristics.
Each participating program distributed the survey to their residents through an email containing an anonymous hyperlink. The survey was available for completion for 4 weeks. We asked participating programs to send email reminders to encourage participation. Participants were given the option of receiving a $10 Amazon gift card after completion. All responses were recorded anonymously. The study received a “not regulated” status by the University of Michigan Institutional Review Board (HUM 00123888).
Statistical Analysis
Descriptive statistics were used to tabulate results. Respondents were encouraged, but not required, to answer all questions. Therefore, the response rate for each question was calculated using the total number of responses for that question as the denominator. Bivariable comparisons were made using Chi-squared or Fisher’s exact tests, as appropriate, for categorical data. A P value < 0.05, with 2-sided alpha, was considered statistically significant. All statistical analyses were conducted using Stata 13 SE (StataCorp, College Station, TX).
Results
Respondent Characteristics
Of the 29 programs contacted, 10 agreed to participate within the study timeframe. Of 766 potential respondents, 348 (45%) residents answered the survey (Table 1). The majority of respondents (n = 276, 82%) were from IM programs. Respondents were from all training years as follows: postgraduate year 1 residents (PGY-1, or interns; n = 130, 39%), PGY-2 residents (n = 98, 29%), PGY-3 residents (n = 93, 28%), and PGY-4 residents (n = 12, 4%). Most respondents were from the South (n = 130, 39%) and Midwest (n = 123, 37%) regions, and over half (n = 179, 54%) were female. Most respondents (n = 285, 86%) stated that they did not have children. The majority (n = 236, 71%) were completing residency in an area where they had not previously lived for more than 1 year.
Primary Care Utilization
Among the 348 respondents, 59% (n = 205) reported having established care with a PCP. An additional 6% (n = 21) had established care with an obstetrician/gynecologist for routine needs (Table 2). The 2 most common reasons for establishing care with a PCP were routine primary care needs, including contraception (n = 159, 79%), and access to a physician in the event of an acute medical need (n = 132, 66%).
Among respondents who had established care with a PCP, most (n = 188, 94%) had completed at least 1 appointment. However, among these 188 respondents, 68% (n = 127) stated that they had not made an acute visit in more than 12 months. When asked about wellness visits, almost one third of respondents (n = 103, 31%) stated that they had not been seen for a wellness visit in the past 3 years.
Burden of Chronic Illness
Most respondents (n = 223, 67%) stated that they did not have a chronic medical or mental health condition prior to residency (Table 3). However, 23% (n = 77) of respondents stated that they had been diagnosed with a chronic medical illness prior to residency, and 14% (n = 45) indicated they had been diagnosed with a mental health condition prior to residency. Almost one fifth of respondents (n = 60, 18%) reported seeing a subspecialist for a medical illness, and 33% (n = 111) reported taking a long-term prescription medication. With respect to major medical issues, the majority of residents (n = 239, 72%) denied experiencing events such as pregnancy, hospitalization, surgery, or an emergency department (ED) visit during training.
[polldaddy:10116940]
Inappropriate Prescriptions
While the majority of respondents denied writing a prescription for themselves for an acute or chronic medical condition, almost one fifth (n = 62, 19%) had received a prescription for an acute medical need from a provider outside of a clinical relationship (ie, from someone other than their PCP or specialty provider). Notably, 5% (n = 15) reported that this had occurred at least 2 or 3 times in the past 12 months (Table 4). Compared to respondents not taking long-term prescription medications, respondents who were already taking long-term prescription medications more frequently reported inappropriately receiving chronic prescriptions outside of an established clinical relationship (n = 14, 13% vs. n = 14, 6%; P = 0.05) and more often self-prescribed medications for acute needs (n = 12, 11% vs. n = 7, 3%; P = 0.005).
Comparison of Residents With and Without a PCP
Important differences were noted between residents who had a PCP versus those who did not (Table 5). For example, a higher percentage of residents with a PCP indicated they had been diagnosed with a chronic medical illness (n = 55, 28% vs. n = 22, 16%; P = 0.01) or a chronic mental health condition (n = 34, 17% vs. n = 11, 8%; P = 0.02) before residency. Additionally, a higher percentage of residents with a PCP (n = 70, 35% vs. n = 25, 18%; P = 0.001) reported experiencing medical events such as pregnancy, hospitalization, surgery, ED visit, or new diagnosis of a chronic medical illness during residency. Finally, a higher percentage of respondents with a PCP stated that they had visited a subspecialist for a medical illness (n = 44, 22% vs. n = 16,12%; P = 0.01) or were taking long-term prescription medications (n = 86, 43% vs. n = 25; 18%; P < 0.001). When comparing PGY-1 to PGY-2–PGY-4 residents, the former reported having established a medical relationship with a PCP significantly less frequently (n = 56, 43% vs. n = 142, 70%; P < 0.001).
Discussion
This survey-based study of medical residents across the United States suggests that a substantial proportion do not establish relationships with PCPs. Additionally, our data suggest that despite establishing care, few residents subsequently visited their PCP during training for wellness visits or routine care. Self-reported rates of chronic medical and mental health conditions were substantial in our sample. Furthermore, inappropriate self-prescription and the receipt of prescriptions outside of a medical relationship were also reported. These findings suggest that future studies that focus on the unique medical and mental health needs of physicians in training, as well as interventions to encourage care in this vulnerable period, are necessary.
We observed that most respondents that established primary care were female trainees. Although it is impossible to know with certainty, one hypothesis behind this discrepancy is that women routinely need to access preventative care for gynecologic needs such as pap smears, contraception, and potentially pregnancy and preconception counseling [14,15]. Similarly, residents with a chronic medical or mental health condition prior to residency established care with a local PCP at a significantly greater frequency than those without such diagnoses. While selection bias cannot be excluded, this finding suggests that illness is a driving factor in establishing care. There also appears to be an association between accessing the medical system (either for prescription medications or subspecialist care) and having established care with a PCP. Collectively, these data suggest that individuals without a compelling reason to access medical services might have barriers to accessing care in the event of medical needs or may not receive routine preventative care [9,10].
In general, we found that rates of reported inappropriate prescriptions were lower than those reported in prior studies where a comparable resident population was surveyed [8,12,16]. Inclusion of multiple institutions, differences in temporality, social desirability bias, and reporting bias might have influenced our findings in this regard. Surprisingly, we found that having a PCP did not influence likelihood of inappropriate prescription receipt, perhaps suggesting that this behavior reflects some degree of universal difficulty in accessing care. Alternatively, this finding might relate to a cultural tendency to self-prescribe among resident physicians. The fact that individuals on chronic medications more often both received and wrote inappropriate prescriptions suggests this problem might be more pronounced in individuals who take medications more often, as these residents have specific needs [12]. Future studies targeting these individuals thus appear warranted.
Our study has several limitations. First, our sample size was modest and the response rate of 45% was low. However, to our knowledge, this remains among the largest survey on this topic, and our response rate is comparable to similar trainee studies [8,11,13]. Second, we designed and created a novel survey for this study. While the questions were pilot-tested with users prior to dissemination, validation of the instrument was not performed. Third, since the study population was restricted to residents in fields that participate in primary care, our findings may not be generalizable to patterns of PCP use in other specialties [6].
These limitations aside, our study has important strengths. This is the first national study of its kind with specific questions addressing primary care access and utilization, prescription medication use and related practices, and the prevalence of medical conditions among trainees. Important differences in the rates of establishing primary care between male and female respondents, first- year and senior residents, and those with and without chronic disease suggest a need to target specific resident groups (males, interns, those without pre-existing conditions) for wellness-related interventions. Such interventions could include distribution of a list of local providers to first year residents, advanced protected time for doctor’s appointments, and safeguards to ensure health information is protected from potential supervisors. Future studies should also include residents from non-primary care oriented specialties such as surgery, emergency medicine, and anesthesiology to obtain results that are more generalizable to the resident population as a whole. Additionally, the rates of inappropriate prescriptions were not insignificant and warrant further evaluation of the driving forces behind these behaviors.
Conclusion
Medical residents have a substantial burden of chronic illness that may not be met through interactions with PCPs. More research into barriers that residents face while accessing care and an assessment of interventions to facilitate their access to care is important to promote trainee well-being. Without such direction and initiative, it may prove harder for physicians to heal themselves or those for whom they provide care.
Acknowledgments: We thank Suzanne Winter, the study coordinator, for her support with editing and formatting the manuscript, Latoya Kuhn for performing the statistical analysis and creating data tables, and Dr. Namita Sachdev and Dr. Renuka Tipirneni for providing feedback on the survey instrument. We also thank the involved programs for their participation.
Corresponding author: Vineet Chopra, NCRC 2800 Plymouth Rd., Bldg 16, 432, Ann Arbor, MI 48109, [email protected].
Financial disclosures: None.
Previous presentations: Results were presented at the Annual Michigan Medicine 2017 Internal Medicine Research Symposium.
From the University of Michigan Medical School, Ann Arbor, MI.
Abstract
- Objective: Existing research has demonstrated overall low rates of residents establishing care with a primary care physician (PCP). We conducted a survey-based study to better understand chronic illness, PCP utilization, and prescription medication use patterns in resident physician populations.
- Methods: In 2017, we invited internal and family medicine trainees from a convenience sample of U.S. residency programs to participate in a survey. We compared the characteristics of residents who had established care with a PCP to those who had not.
- Results: The response rate was 45% (348/766 residents). The majority (n = 205, 59%) of respondents stated they had established care with a PCP primarily for routine preventative care (n = 159, 79%) and access in the event of an emergency (n = 132, 66%). However, 31% (n = 103) denied having had a wellness visit in over 3 years. Nearly a quarter of residents (n = 77, 23%) reported a chronic medical illness and 14% (n = 45) reported a preexisting mental health condition prior to residency. One-third (n = 111, 33%) reported taking a long-term prescription medication. Compared to residents who had not established care, those with a PCP (n = 205) more often reported a chronic condition (P < 0.001), seeing a subspecialist (P = 0.01), or taking long-term prescription medications (P < 0.001). One in 5 (n = 62,19%) respondents reported receiving prescriptions for an acute illness from an individual with whom they did not have a doctor-patient relationship.
- Conclusion: Medical residents have a substantial burden of chronic illness that may not be met through interactions with PCPs. Further understanding their medical needs and barriers to accessing care is necessary to ensure trainee well-being.
Keywords: Medical education-graduate, physician behavior, survey research, access to care.
Although internal medicine (IM) and family medicine (FM) residents must learn to provide high-quality primary care to their patients, little is known about whether they appropriately access such care themselves. Resident burnout and resilience has received attention [1,2], but there has been limited focus on understanding the burden of chronic medical and mental illness among residents. In particular, little is known about whether residents access primary care physicians (PCPs)—for either acute or chronic medical needs—and about resident self-medication practices.
Residency is often characterized by a life-changing geographic relocation. Even residents who do not relocate may still need to establish care with a new PCP due to health insurance or loss of access to a student clinic [3]. Establishing primary care with a new doctor typically requires scheduling a new patient visit, often with a wait time of several days to weeks [4,5]. Furthermore, lack of time, erratic schedules, and concerns about privacy and the stigma of being ill as a physician are barriers to establishing care [6-8]. Individuals who have not established primary care may experience delays in routine preventative health services, screening for chronic medical and mental health conditions, as well as access to care during acute illnesses [9,10]. Worse, they may engage in potentially unsafe practices, such as having colleagues write prescriptions for them, or even self-prescribing [8,11,12].
Existing research has demonstrated overall low rates of residents establishing care with a PCP [6–8,13]. However, these studies have either been limited to large academic centers or conducted outside the United States. Improving resident well-being may prove challenging without a clear understanding of current primary care utilization practices, the burden of chronic illness among residents, and patterns of prescription medication use and needs. Therefore, we conducted a survey-based study to understand primary care utilization and the burden of chronic illness among residents. We also assessed whether lack of primary care is associated with potentially risky behaviors, such as self-prescribing of medications.
Methods
Study Setting and Participants
The survey was distributed to current residents at IM and FM programs within the United States in 2017. Individual programs were recruited by directly contacting program directors or chief medical residents via email. Rather than contacting sites directly through standard templated emails, we identified programs both through personal contacts as well as the Electronic Residency Application Service list of accredited IM training programs. We elected to use this approach in order to increase response rates and to ensure that a sample representative of the trainee population was constructed. Programs were located in the Northeast, Midwest, South, and Pacific regions, and included small community-based programs and large academic centers.
Development of the Survey
The survey instrument was developed by the authors and reviewed by residents and PCPs at the University of Michigan to ensure relevance and comprehension of questions (The survey is available in the Appendix.). Once finalized, the survey was programmed into an online survey tool (Qualtrics, Provo, UT) and pilot-tested before being disseminated to the sampling frame. Data collected in the survey included: respondent’s utilization of a PCP, burden of chronic illness, long-term prescription medications, prescribing source, and demographic characteristics.
Each participating program distributed the survey to their residents through an email containing an anonymous hyperlink. The survey was available for completion for 4 weeks. We asked participating programs to send email reminders to encourage participation. Participants were given the option of receiving a $10 Amazon gift card after completion. All responses were recorded anonymously. The study received a “not regulated” status by the University of Michigan Institutional Review Board (HUM 00123888).
Statistical Analysis
Descriptive statistics were used to tabulate results. Respondents were encouraged, but not required, to answer all questions. Therefore, the response rate for each question was calculated using the total number of responses for that question as the denominator. Bivariable comparisons were made using Chi-squared or Fisher’s exact tests, as appropriate, for categorical data. A P value < 0.05, with 2-sided alpha, was considered statistically significant. All statistical analyses were conducted using Stata 13 SE (StataCorp, College Station, TX).
Results
Respondent Characteristics
Of the 29 programs contacted, 10 agreed to participate within the study timeframe. Of 766 potential respondents, 348 (45%) residents answered the survey (Table 1). The majority of respondents (n = 276, 82%) were from IM programs. Respondents were from all training years as follows: postgraduate year 1 residents (PGY-1, or interns; n = 130, 39%), PGY-2 residents (n = 98, 29%), PGY-3 residents (n = 93, 28%), and PGY-4 residents (n = 12, 4%). Most respondents were from the South (n = 130, 39%) and Midwest (n = 123, 37%) regions, and over half (n = 179, 54%) were female. Most respondents (n = 285, 86%) stated that they did not have children. The majority (n = 236, 71%) were completing residency in an area where they had not previously lived for more than 1 year.
Primary Care Utilization
Among the 348 respondents, 59% (n = 205) reported having established care with a PCP. An additional 6% (n = 21) had established care with an obstetrician/gynecologist for routine needs (Table 2). The 2 most common reasons for establishing care with a PCP were routine primary care needs, including contraception (n = 159, 79%), and access to a physician in the event of an acute medical need (n = 132, 66%).
Among respondents who had established care with a PCP, most (n = 188, 94%) had completed at least 1 appointment. However, among these 188 respondents, 68% (n = 127) stated that they had not made an acute visit in more than 12 months. When asked about wellness visits, almost one third of respondents (n = 103, 31%) stated that they had not been seen for a wellness visit in the past 3 years.
Burden of Chronic Illness
Most respondents (n = 223, 67%) stated that they did not have a chronic medical or mental health condition prior to residency (Table 3). However, 23% (n = 77) of respondents stated that they had been diagnosed with a chronic medical illness prior to residency, and 14% (n = 45) indicated they had been diagnosed with a mental health condition prior to residency. Almost one fifth of respondents (n = 60, 18%) reported seeing a subspecialist for a medical illness, and 33% (n = 111) reported taking a long-term prescription medication. With respect to major medical issues, the majority of residents (n = 239, 72%) denied experiencing events such as pregnancy, hospitalization, surgery, or an emergency department (ED) visit during training.
[polldaddy:10116940]
Inappropriate Prescriptions
While the majority of respondents denied writing a prescription for themselves for an acute or chronic medical condition, almost one fifth (n = 62, 19%) had received a prescription for an acute medical need from a provider outside of a clinical relationship (ie, from someone other than their PCP or specialty provider). Notably, 5% (n = 15) reported that this had occurred at least 2 or 3 times in the past 12 months (Table 4). Compared to respondents not taking long-term prescription medications, respondents who were already taking long-term prescription medications more frequently reported inappropriately receiving chronic prescriptions outside of an established clinical relationship (n = 14, 13% vs. n = 14, 6%; P = 0.05) and more often self-prescribed medications for acute needs (n = 12, 11% vs. n = 7, 3%; P = 0.005).
Comparison of Residents With and Without a PCP
Important differences were noted between residents who had a PCP versus those who did not (Table 5). For example, a higher percentage of residents with a PCP indicated they had been diagnosed with a chronic medical illness (n = 55, 28% vs. n = 22, 16%; P = 0.01) or a chronic mental health condition (n = 34, 17% vs. n = 11, 8%; P = 0.02) before residency. Additionally, a higher percentage of residents with a PCP (n = 70, 35% vs. n = 25, 18%; P = 0.001) reported experiencing medical events such as pregnancy, hospitalization, surgery, ED visit, or new diagnosis of a chronic medical illness during residency. Finally, a higher percentage of respondents with a PCP stated that they had visited a subspecialist for a medical illness (n = 44, 22% vs. n = 16,12%; P = 0.01) or were taking long-term prescription medications (n = 86, 43% vs. n = 25; 18%; P < 0.001). When comparing PGY-1 to PGY-2–PGY-4 residents, the former reported having established a medical relationship with a PCP significantly less frequently (n = 56, 43% vs. n = 142, 70%; P < 0.001).
Discussion
This survey-based study of medical residents across the United States suggests that a substantial proportion do not establish relationships with PCPs. Additionally, our data suggest that despite establishing care, few residents subsequently visited their PCP during training for wellness visits or routine care. Self-reported rates of chronic medical and mental health conditions were substantial in our sample. Furthermore, inappropriate self-prescription and the receipt of prescriptions outside of a medical relationship were also reported. These findings suggest that future studies that focus on the unique medical and mental health needs of physicians in training, as well as interventions to encourage care in this vulnerable period, are necessary.
We observed that most respondents that established primary care were female trainees. Although it is impossible to know with certainty, one hypothesis behind this discrepancy is that women routinely need to access preventative care for gynecologic needs such as pap smears, contraception, and potentially pregnancy and preconception counseling [14,15]. Similarly, residents with a chronic medical or mental health condition prior to residency established care with a local PCP at a significantly greater frequency than those without such diagnoses. While selection bias cannot be excluded, this finding suggests that illness is a driving factor in establishing care. There also appears to be an association between accessing the medical system (either for prescription medications or subspecialist care) and having established care with a PCP. Collectively, these data suggest that individuals without a compelling reason to access medical services might have barriers to accessing care in the event of medical needs or may not receive routine preventative care [9,10].
In general, we found that rates of reported inappropriate prescriptions were lower than those reported in prior studies where a comparable resident population was surveyed [8,12,16]. Inclusion of multiple institutions, differences in temporality, social desirability bias, and reporting bias might have influenced our findings in this regard. Surprisingly, we found that having a PCP did not influence likelihood of inappropriate prescription receipt, perhaps suggesting that this behavior reflects some degree of universal difficulty in accessing care. Alternatively, this finding might relate to a cultural tendency to self-prescribe among resident physicians. The fact that individuals on chronic medications more often both received and wrote inappropriate prescriptions suggests this problem might be more pronounced in individuals who take medications more often, as these residents have specific needs [12]. Future studies targeting these individuals thus appear warranted.
Our study has several limitations. First, our sample size was modest and the response rate of 45% was low. However, to our knowledge, this remains among the largest survey on this topic, and our response rate is comparable to similar trainee studies [8,11,13]. Second, we designed and created a novel survey for this study. While the questions were pilot-tested with users prior to dissemination, validation of the instrument was not performed. Third, since the study population was restricted to residents in fields that participate in primary care, our findings may not be generalizable to patterns of PCP use in other specialties [6].
These limitations aside, our study has important strengths. This is the first national study of its kind with specific questions addressing primary care access and utilization, prescription medication use and related practices, and the prevalence of medical conditions among trainees. Important differences in the rates of establishing primary care between male and female respondents, first- year and senior residents, and those with and without chronic disease suggest a need to target specific resident groups (males, interns, those without pre-existing conditions) for wellness-related interventions. Such interventions could include distribution of a list of local providers to first year residents, advanced protected time for doctor’s appointments, and safeguards to ensure health information is protected from potential supervisors. Future studies should also include residents from non-primary care oriented specialties such as surgery, emergency medicine, and anesthesiology to obtain results that are more generalizable to the resident population as a whole. Additionally, the rates of inappropriate prescriptions were not insignificant and warrant further evaluation of the driving forces behind these behaviors.
Conclusion
Medical residents have a substantial burden of chronic illness that may not be met through interactions with PCPs. More research into barriers that residents face while accessing care and an assessment of interventions to facilitate their access to care is important to promote trainee well-being. Without such direction and initiative, it may prove harder for physicians to heal themselves or those for whom they provide care.
Acknowledgments: We thank Suzanne Winter, the study coordinator, for her support with editing and formatting the manuscript, Latoya Kuhn for performing the statistical analysis and creating data tables, and Dr. Namita Sachdev and Dr. Renuka Tipirneni for providing feedback on the survey instrument. We also thank the involved programs for their participation.
Corresponding author: Vineet Chopra, NCRC 2800 Plymouth Rd., Bldg 16, 432, Ann Arbor, MI 48109, [email protected].
Financial disclosures: None.
Previous presentations: Results were presented at the Annual Michigan Medicine 2017 Internal Medicine Research Symposium.
1. Kassam A, Horton J, Shoimer I, Patten S. Predictors of well-being in resident physicians: a descriptive and psychometric study. J Grad Med Educ 2015;7:70–4.
2. Shanafelt TD, Bradley KA, Wipf JE, Back AL. Burnout and self-reported patient care in an internal medicine residency program. Ann Intern Med 2002;136:358–67.
3. Burstin HR, Swartz K, O’Neil AC, et al. The effect of change of health insurance on access to care. Inquiry 1998;35:389–97.
4. Rhodes KV, Basseyn S, Friedman AB, et al. Access to primary care appointments following 2014 insurance expansions. Ann Fam Med 2017;15:107–12.
5. Polsky D, Richards M, Basseyn S, et al. Appointment availability after increases in Medicaid payments for primary care. N Engl J Med 2015;372:537–45.
6. Gupta G, Schleinitz MD, Reinert SE, McGarry KA. Resident physician preventive health behaviors and perspectives on primary care. R I Med J (2013) 2013;96:43–7.
7. Rosen IM, Christine JD, Bellini LM, Asch DA. Health and health care among housestaff in four U.S. internal medicine residency programs. J Gen Intern Med 2000;15:116-21.
8. Campbell S, Delva D. Physician do not heal thyself. Survey of personal health practices among medical residents. Can Fam Physician 2003;49:1121–7.
9. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005;83(3):457-502.
10. Weissman JS, Stern R, Fielding SL, et al. Delayed access to health care: risk factors, reasons, and consequences. Ann Intern Med 1991;114:325–31.
11. Guille C, Sen S. Prescription drug use and self-prescription among training physicians. Arch Intern Med 2012;172:371–2.
12. Roberts LW, Kim JP. Informal health care practices of residents: “curbside” consultation and self-diagnosis and treatment. Acad Psychiatry 2015;39:22-30.
13. Cohen JS, Patten S. Well-being in residency training: a survey examining resident physician satisfaction both within and outside of residency training and mental health in Alberta. BMC Med Educ 2005;5:21.
14. U.S. Preventive Services Task Force. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Published March 2012. Accessed August 21, 2018.
15. Health Resources and Services Administration. Women’s preventative services guidelines. https://www.hrsa.gov/womensguidelines2016/index.html. Updated October 2017. Accessed August 21, 2018.
16. Christie JD, Rosen IM, Bellini LM, et al. Prescription drug use and self-prescription among resident physicians. JAMA 1998;280(14):1253–5.
1. Kassam A, Horton J, Shoimer I, Patten S. Predictors of well-being in resident physicians: a descriptive and psychometric study. J Grad Med Educ 2015;7:70–4.
2. Shanafelt TD, Bradley KA, Wipf JE, Back AL. Burnout and self-reported patient care in an internal medicine residency program. Ann Intern Med 2002;136:358–67.
3. Burstin HR, Swartz K, O’Neil AC, et al. The effect of change of health insurance on access to care. Inquiry 1998;35:389–97.
4. Rhodes KV, Basseyn S, Friedman AB, et al. Access to primary care appointments following 2014 insurance expansions. Ann Fam Med 2017;15:107–12.
5. Polsky D, Richards M, Basseyn S, et al. Appointment availability after increases in Medicaid payments for primary care. N Engl J Med 2015;372:537–45.
6. Gupta G, Schleinitz MD, Reinert SE, McGarry KA. Resident physician preventive health behaviors and perspectives on primary care. R I Med J (2013) 2013;96:43–7.
7. Rosen IM, Christine JD, Bellini LM, Asch DA. Health and health care among housestaff in four U.S. internal medicine residency programs. J Gen Intern Med 2000;15:116-21.
8. Campbell S, Delva D. Physician do not heal thyself. Survey of personal health practices among medical residents. Can Fam Physician 2003;49:1121–7.
9. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005;83(3):457-502.
10. Weissman JS, Stern R, Fielding SL, et al. Delayed access to health care: risk factors, reasons, and consequences. Ann Intern Med 1991;114:325–31.
11. Guille C, Sen S. Prescription drug use and self-prescription among training physicians. Arch Intern Med 2012;172:371–2.
12. Roberts LW, Kim JP. Informal health care practices of residents: “curbside” consultation and self-diagnosis and treatment. Acad Psychiatry 2015;39:22-30.
13. Cohen JS, Patten S. Well-being in residency training: a survey examining resident physician satisfaction both within and outside of residency training and mental health in Alberta. BMC Med Educ 2005;5:21.
14. U.S. Preventive Services Task Force. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Published March 2012. Accessed August 21, 2018.
15. Health Resources and Services Administration. Women’s preventative services guidelines. https://www.hrsa.gov/womensguidelines2016/index.html. Updated October 2017. Accessed August 21, 2018.
16. Christie JD, Rosen IM, Bellini LM, et al. Prescription drug use and self-prescription among resident physicians. JAMA 1998;280(14):1253–5.
The Effect of Age on the Benefits of Early Decompression for Cervical Spondylotic Myelopathy
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
TAKE-HOME POINTS
- Decompression of cervical myelopathy within 24 months of symptom onset results in greater functional improvement compared to delayed decompression.
- The improvement with respect to time is more significant for patients older than 65 years compared to younger patients.
- Duration of symptoms does not seem to influence the severity of the preoperative Nurick score.
- Preoperative severity of symptoms is related to postoperative outcomes.
- Other significant predictors of worse outcomes include tobacco use, diabetes, and number of levels fused.
The Effect of Insurance Type on Patient Access to Ankle Fracture Care Under the Affordable Care Act
ABSTRACT
The purpose of this study is to assess the effect of insurance type (Medicaid, Medicare, private insurance) on the ability for patients with operative ankle fractures to access orthopedic traumatologists. The research team called 245 board-certified orthopedic surgeons specializing in orthopedic trauma within 8 representative states. The caller requested an appointment for their fictitious mother in order to be evaluated for an ankle fracture which was previously evaluated by her primary care physician and believed to require surgery. Each office was called 3 times to assess the response for each insurance type. For each call, information was documented regarding whether the patient was able to receive an appointment and the barriers the patient confronted to receive an appointment. Overall, 35.7% of offices scheduled an appointment for a patient with Medicaid, in comparison to 81.4%and 88.6% for Medicare and BlueCross, respectively (P < .0001). Medicaid patients confronted more barriers for receiving appointments. There was no statistically significant difference in access for Medicaid patients in states that had expanded Medicaid eligibility vs states that had not expanded Medicaid. Medicaid reimbursement for open reduction and internal fixation of an ankle fracture did not significantly correlate with appointment success rates or wait times. Despite the passage of the Affordable Care Act, patients with Medicaid have reduced access to orthopedic surgeons and more complex barriers to receiving appointments. A more robust strategy for increasing care-access for patients with Medicaid would be more equitable.
Continue to: In 2010, the Patient Protection and Affordable Care Act...
In 2010, the Patient Protection and Affordable Care Act (PPACA) expanded the eligibility criteria for Medicaid to all individuals with an income up to 138% of the poverty level.1 A Supreme Court ruling stated that the decision to expand Medicaid was to be decided by individual states.2 Currently, 31 states have chosen to expand Medicaid eligibility to their residents.2 This expansion has allowed an additional 11.7 million people to enroll in Medicaid and the Children’s Health Insurance Program by May 2015.3-5
Even with the passage of the PPACA, Medicaid patients seeking specialty orthopedic care have experienced more barriers to accessing care than Medicare or commercially-insured patients.2,6-10 One major cited reason is Medicaid’s low reimbursement, which may discourage physicians from open panel participation in Medicaid.11,12
A common fundamental teaching for orthopedic traumatologists is the notion that they should be available to treat all injuries regardless of the patient’s ability to pay.13 This has resulted in both trauma centers and trauma surgeons becoming financially challenged due to the higher proportion of Medicaid and uninsured trauma patients and lower Medicaid reimbursement levels.14,15
This study focuses on the effect of different types of insurance (Medicaid, Medicare, or commercial insurance) on the ability of patients to obtain care for operative ankle fractures. The purpose of this study is to evaluate, in the context of the PPACA, patient access to orthopedic surgeons for operative ankle fractures based on insurance-type. We hypothesized that patients with Medicaid would face a greater volume of obstacles when seeking appointments for an ankle fracture, even after the PPACA.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
The study population included board-certified orthopedic surgeons who belonged to the Orthopaedic Trauma Association (OTA) from 8 representative states; 4 states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and 4 states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas). These states were selected due to their ability to represent diverse healthcare marketplaces throughout the country. Using the OTA website’s “Find a Surgeon” search tool,16 we created a list of surgeons for each state and matched each surgeon with a random number. The list of surgeons was ordered according to the value of the surgeon’s associated random number, and surgeons were called in ascending order. We excluded disconnected or inaccurate numbers from the calling list. Surgeons who did not manage ankle fractures were removed from the dataset. Approximately 30 orthopedic trauma surgeons per state were contacted.
Each office was called to make an appointment for the caller’s mother. Every surgeon’s office was specifically asked if the surgeon would accept the patient to be evaluated for an ankle fracture that occurred out-of-state. The caller had a standardized protocol to limit intra- and inter-office variations (Appendix). The scenario involved a request to be evaluated for an unstable ankle fracture, with the patient having Medicaid, Medicare, or BlueCross insurance. The scenario required 3 separate calls to the same surgeon in order to obtain data regarding each insurance-type. The calls were separated by at least 1 week to avoid caller recognition by the surgeon’s office.
Appendix
Scenario
1. Date of Birth: Medicaid–2/07/55; BlueCross PPO–2/09/55; Medicare–7/31/45.
2. Ankle fracture evaluated by primary care physician 1 or 2 days ago
3. Not seen previously by your clinic or hospital, she would be a new patient
4. Asked how early she could be scheduled for an appointment
5. Script:
“I’m calling for my mother who injured her ankle a few days ago. Her family doctor took an X-ray and believes she has a fracture and needs surgery. Is Dr. X accepting new patients for evaluation and treatment of ankle fractures?” If YES →
“I was wondering if you take Medicaid/Medicare/BlueCross plan?” If YES →
“When is your soonest available appointment?”
The date of each phone call and date of appointment, if provided, were recorded. If the office did not give an appointment, we asked for reasons why. If an appointment was denied for a patient with Medicaid, we asked for a referral to another office that accepted Medicaid. We considered barriers to obtaining an initial appointment, such as requiring a referral from a primary care physician (PCP), as an unsuccessful attempt at making an appointment. We determined the waiting period for an appointment by calculating the time between the date of the call and the date of the appointment. Appointments were not scheduled to ensure that actual patients were not disadvantaged. For both appointment success rates and waiting periods, we stratified the data into 2 groups: states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas).
We obtained Medicaid reimbursement rates for open reduction and internal fixation of an ankle fracture by querying each state’s reimbursement rate using Current Procedural Terminology code 27822.
Chi-square test or Fisher’s exact test was used to analyze acceptance rate differences based on the patient’s type of insurance. To compare the waiting periods for an appointment, we used an independent samples t-test after applying natural log-transformation, as the data was not normally distributed. We performed logistic regression analysis to detect whether reimbursement was a significant predictor of successfully making an appointment for patients, and a linear regression analysis was used to evaluate whether reimbursement predicted waiting periods. Unless otherwise stated, all statistical testing was performed two-tailed at an alpha-level of 0.05.
This study was approved by the Institutional Review Board of Yale University School of Medicine (HIC No. 1363).
Continue to: RESULTS...
RESULTS
In total, 350 offices were contacted across 8 states (4 states with and 4 states without expanded Medicaid eligibility) of which we identified 245 orthopedic surgeons who would surgically treat ankle fractures. The 245 surgeons’ offices were called 3 times for each separate insurance-type.
Table 1. Appointment Success Rate
| Medicaid | Medicare | Private |
All states |
|
| |
Yes (%) | 100 (35.7) | 228 (81.4) | 248 (88.6) |
No (%) | 180 (64.3) | 52 (18.60 | 32 (11.4) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 55 (39.6) | 116 (83.5) | 124 (89.2) |
No (%) | 84 (60.4) | 23 (16.5) | 15 (10.8) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 45 (31.9) | 112 (79.4) | 124 (87.9) |
No (%) | 96 (68.1) | 29 (20.6) | 17 (12.1) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross (Table 1). For states with expanded Medicaid eligibility, the success rate for obtaining an appointment was 39.6%, 83.5%, and 89.2% for Medicaid, Medicare, and BlueCross, respectively. For states without expanded Medicaid eligibility, the success rate for obtaining an appointment was 31.9% for Medicaid, 79.4% for Medicare, and 87.9% for BlueCross. In all cases, the success rate for obtaining an appointment was significantly lower for Medicaid, compared to Medicare (P < .0001) or BlueCross (P < .0001). Medicaid appointment success rate was 39.6% in expanded states vs 31.9% in non-expanded states, however, the difference was not statistically significant (Table 2).
Table 2. Medicaid Appointment Success Rate in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 55 (39.6) | 45 (31.9) | .181 |
No (%) | 84 (60.4) | 96 (68.1) |
|
In 43.7% of occasions, patients with Medicaid did not have their insurance accepted, compared to 7.3% for Medicare and 0% for BlueCross. The majority of offices which did not accept Medicaid were not able to refer patients to another surgeon who would accept Medicaid. The requirement to have a primary care referral was the second most common reason for Medicaid patients not obtaining an appointment. No Medicare (10.4% vs 0.0%, P < .0001) or BlueCross (10.4% vs 0.0%, P < .0001) patients experienced this requirement (Table 3). There was no difference found between the percent of Medicaid patients who were required to have referrals in states with and without expanded Medicaid eligibility (Table 4).
Table 3. Referral Rate
| Medicaid | Medicare | Private |
All states |
|
|
|
Yes (%) | 29 (10.4) | 0 (0) | 0 (0) |
No (%) | 251 (89.6) | 280 (100) | 280 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 12 (8.6) | 0 (0) | 0 (0) |
No (%) | 127 (91.4) | 139 (100) | 139 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 17 (12.1) | 0 (0) | 0 (0) |
No (%) | 124 (87.9) | 141 (100) | 141 (100) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
Table 4. Medicaid Referral Rates in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 12 (9.7) | 17 (14.0) | .35 |
No (%) | 127 (91.4) | 124 (87.9) |
|
Reimbursements for ankle fracture varied across states (Table 5). For Medicaid, Georgia paid the highest reimbursement ($1049.95) and Florida paid the lowest ($469.44). Logistic and linear regression analysis did not demonstrate a significant relationship between reimbursement and appointment success rate or waiting periods.
Table 5. Medicaid Reimbursements for Ankle Fracture Repair (CPT and HCPCS 27822) in 2014
State | Medicaid reimbursement |
Californiaa | $785.55 |
Texas | $678.95 |
Florida | $469.44 |
Ohioa | $617.08 |
New Yorka | $500.02 |
North Carolina | $621.63 |
Massachusettsa | $627.94 |
Georgia | $1,049.95 |
Average | $668.82 |
aStates with expanded Medicaid eligibility.
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
Waiting periods (Table 6) varied significantly by the type of insurance (7.3 days for Medicaid, 6.0 days for Medicare, and 6.0 days for BlueCross; P = .002). For states with expanded Medicaid eligibility, waiting periods varied significantly by insurance (7.7 days for Medicaid, 6.2 days for Medicare, P = .003; and 6.1 days for BlueCross, P = .01). Waiting periods did not vary significantly for states without expanded Medicaid. Additionally, waiting periods did not differ significantly when comparing between states with and without Medicaid expansion.
Table 6. Waiting Period (Days) by Insurance Type.
| Medicaid | Medicare | Private |
Comparison by Insurance Type |
|
|
|
All states |
|
|
|
Waiting period | 7.3 | 6.0 | 6.0 |
P-value |
| 0.002 | 0.002 |
States with expanded Medicaid eligibility |
|
|
|
Waiting period | 7.7 | 6.2 | 6.1 |
P-value |
| 0.003 | 0.01 |
States without expanded Medicaid eligibility |
|
|
|
Waiting period | 6.9 | 5.9 | 5.9 |
P-value |
| 0.15 | 0.15 |
Comparison by Medicaid Expansion |
|
|
|
States with expanded Medicaid eligibility | 7.7 | 6.2 | 6.1 |
States without expanded Medicaid eligibility | 6.9 | 5.9 | 5.9 |
P-value | 0.17 | 0.13 | 0.07 |
Continue to: DISCUSSION...
DISCUSSION
This study assessed how insurance type (Medicaid, Medicare, and BlueCross) affects patient access to orthopedic trauma surgeons in 8 geographically representative states. We selected unstable ankle fractures as they are basic fractures treated by nearly all trauma surgeons and should often be surgically treated to prevent serious long-term consequences. Our hypothesis stated that despite the passage of the PPACA, patients with Medicaid would have reduced access to care. As the PPACA has changed the healthcare marketplace by increasing the number of Medicaid enrollees, it is important to ensure that patient access to care improves.
This nationwide survey of orthopedic trauma surgeons demonstrates that Medicaid patients experience added barriers to care that ultimately results in lower rates of successfully obtaining care. This is consistent with other investigations which have assessed Medicaid patient healthcare access.6,8,10,17-19 This study did not demonstrate a statistically significant difference between Medicaid patients’ ability to obtain appointments in states with expanded Medicaid eligibility vs in states without expanded Medicaid eligibility (39.6% vs 31.9%, P < .18); this has been demonstrated in the literature.6
A barrier that was unique to Medicaid patients was the requirement to have a PCP referral (Table 3). A PCP referral was not a barrier to receiving an appointment for patients with Medicare or BlueCross. One reason to explain why Medicaid patients may be required to have PCP referrals is due to their increased medical complexity, extra documentation requirements, and low reimbursement.4 Patients who have obtained a PCP referral may be characterized as being more medically compliant.
It is important to note that the Medicaid policies for 4 states included in this study (Massachusetts, North Carolina, Texas, and New York) required a PCP referral in order to see a specialist. However, we found that many orthopedic trauma practices in these states scheduled appointments for Medicaid patients without a PCP referral, suggesting that the decision depended on individual policy. In addition, the majority of offices within these states cited that they simply did not accept Medicaid as an insurance policy, and not that they required a referral.
Our regression analysis did not find a significant relationship between being able to successfully obtain an appointment to be evaluated for an ankle fracture and reimbursement rates for Medicaid. Although studies have stressed the importance of Medicaid reimbursements on physician participation, this result is consistent with previous studies regarding carpal tunnel release and total ankle replacements.17,19 Long20 suggested that although reimbursements may help, additional strategies for promoting Medicaid acceptance may be needed, including: lowering the costs of participating in Medicaid by simplifying administrative processes, speeding up reimbursement, and reducing the costs associated with caring for those patients.
Continue to: Previous studies have demonstrated...
Previous studies have demonstrated that more physicians may accept Medicaid if reimbursements increased.4,12 Given the high percentage of trauma patients with Medicaid as their primary insurance or whom are emergently enrolled in Medicaid by hospital systems, it is concerning that the PPACA is reducing payments under the Medicare and Medicaid Disproportionate Share Hospital programs which provide hospitals for uncompensated care given to low-income and uninsured patients.21 Trauma centers generally operate at a deficit due to the higher proportion of Medicaid and uninsured patients.14 This is currently worsened by additional federal funding cuts for supporting trauma service’s humane mission.21
This study has several limitations. While the study evaluated access to care in 8 representative states, a thorough nationwide survey would be more representative. Some results may have become statistically significant if we had performed the study with a larger sample size. In addition, we were unable to control for many factors which could impact appointment wait times, such as physician call schedules and vacations. Socioeconomic factors can influence a patient’s ability to attend an appointment, such as transportation costs, time off from work, and childcare availability. In addition, this study did not assess access for the uninsured, who are predominantly the working poor who cannot afford health insurance, even with federal and state subsidies.
The authors apologize for inconveniencing these offices, however, data collection could not be achieved in a better manner. We hope that the value of this study compensates any inconvenience.
CONCLUSION
Overall, our results demonstrate that despite the ratification of the PPACA, Medicaid patients are confronted with more barriers to accessing care by comparison to patients with Medicare and BlueCross insurance. Medicaid patients have worse baseline health22 and are at an increased risk of complications. These disparities are thought to be due to decreased healthcare access,23,24 as well as socioeconomic challenges. Interventions, such as increasing Medicaid’s reimbursement levels, reducing burdensome administrative responsibilities, and establishing partnerships between trauma centers and trauma surgeons, may enable underinsured patients to be appropriately cared for.
This paper will be judged for the Resident Writer’s Award.
1. Blumenthal D, Collins SR. Health care coverage under the affordable care act--a progress report. N Engl J Med. 2014;371(3):275-281. doi:10.1056/NEJMhpr1405667.
2. Sommers BD. Health care reform's unfinished work--remaining barriers to coverage and access. N Engl J Med. 2015;373(25):2395-2397. doi:10.1056/NEJMp1509462.
3. US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicaid & CHIP: February 2015 monthly applications, eligibility determinations and enrollment report. https://www.medicaid.gov/medicaid/program-information/downloads/medicaid-and-chip-february-2015-application-eligibility-and-enrollment-data.pdf. Published May 1, 2015. Accessed May 2015.
4. Iglehart JK, Sommers BD. Medicaid at 50--from welfare program to nation's largest health insurer. N Engl J Med. 2015;372(22):2152-2159. doi:10.1056/NEJMhpr1500791.
5. Kaiser Family Foundation. Medicaid moving forward. http://kff.org/medicaid/fact-sheet/the-medicaid-program-at-a-glance-update/. Updated 2014. Accessed October 10, 2014.
6. Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the affordable care act. J Arthroplasty. 2015;30(9):1498-1501. doi:10.1016/j.arth.2015.03.015.
7. Draeger RW, Patterson BM, Olsson EC, Schaffer A, Patterson JM. The influence of patient insurance status on access to outpatient orthopedic care for flexor tendon lacerations. J Hand Surg Am. 2014;39(3):527-533. doi:10.1016/j.jhsa.2013.10.031.
8. Patterson BM, Spang JT, Draeger RW, Olsson EC, Creighton RA, Kamath GV. Access to outpatient care for adult rotator cuff patients with private insurance versus Medicaid in North Carolina. J Shoulder Elbow Surg. 2013;22(12):1623-1627. doi:10.1016/j.jse.2013.07.051.
9. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
10. Schwarzkopf R, Phan D, Hoang M, Ross S, Mukamel D. Do patients with income-based insurance have access to total joint arthroplasty? J Arthroplasty. 2014;29(6):1083-1086. doi:10.1016/j.arth.2013.11.022.
11. Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff (Millwood). 2012;31(8):1673-1679 doi:10.1377/hlthaff.2012.0294.
12. Perloff JD, Kletke P, Fossett JW. Which physicians limit their Medicaid participation, and why. Health Serv Res. 1995;30(1):7-26.
13. Althausen PL. Building a successful trauma practice in a community setting. J Orthop Trauma. 2011;25 Suppl 3:S113-S117. doi:10.1097/BOT.0b013e318237bcce.
14. Greenberg S, Mir HR, Jahangir AA, Mehta S, Sethi MK. Impacting policy change for orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S14-S16. doi:10.1097/BOT.0000000000000216.
15. Wiznia DH, Averbukh L, Kim CY, Goel A, Leslie MP. Motorcycle helmets: The economic burden of an incomplete helmet law to medical care in the state of Connecticut. Conn Med. 2015;79(8):453-459.
16. Orthopaedic Trauma Association. Find a surgeon. https://online.ota.org/otassa/otacenssafindasurgeon.query_page. Updated 2015. Accessed July, 2015.
17. Kim CY, Wiznia DH, Roth AS, Walls RJ, Pelker RR. Survey of patient insurance status on access to specialty foot and ankle care under the affordable care act. Foot Ankle Int. 2016;37(7):776-781. doi:1071100716642015.
18. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of Medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
19. Kim CY, Wiznia DH, Wang Y, et al. The effect of insurance type on patient access to carpal tunnel release under the affordable care act. J Hand Surg Am. 2016;41(4):503-509.e1. doi:S0363-5023(16)00104-0.
20. Long SK. Physicians may need more than higher reimbursements to expand Medicaid participation: findings from Washington state. Health Aff (Millwood). 2013;32(9):1560-1567. doi:10.1377/hlthaff.2012.1010.
21. Issar NM, Jahangir AA. The affordable care act and orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S5-S7. doi:10.1097/BOT.0000000000000211.
22. Hahn B, Flood AB. No insurance, public insurance, and private insurance: do these options contribute to differences in general health? J Health Care Poor Underserved. 1995;6(1):41-59.
23. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):9-14. doi:10.1016/j.arth.2008.05.010.
24. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi:10.1097/MLR.0000000000000177.
ABSTRACT
The purpose of this study is to assess the effect of insurance type (Medicaid, Medicare, private insurance) on the ability for patients with operative ankle fractures to access orthopedic traumatologists. The research team called 245 board-certified orthopedic surgeons specializing in orthopedic trauma within 8 representative states. The caller requested an appointment for their fictitious mother in order to be evaluated for an ankle fracture which was previously evaluated by her primary care physician and believed to require surgery. Each office was called 3 times to assess the response for each insurance type. For each call, information was documented regarding whether the patient was able to receive an appointment and the barriers the patient confronted to receive an appointment. Overall, 35.7% of offices scheduled an appointment for a patient with Medicaid, in comparison to 81.4%and 88.6% for Medicare and BlueCross, respectively (P < .0001). Medicaid patients confronted more barriers for receiving appointments. There was no statistically significant difference in access for Medicaid patients in states that had expanded Medicaid eligibility vs states that had not expanded Medicaid. Medicaid reimbursement for open reduction and internal fixation of an ankle fracture did not significantly correlate with appointment success rates or wait times. Despite the passage of the Affordable Care Act, patients with Medicaid have reduced access to orthopedic surgeons and more complex barriers to receiving appointments. A more robust strategy for increasing care-access for patients with Medicaid would be more equitable.
Continue to: In 2010, the Patient Protection and Affordable Care Act...
In 2010, the Patient Protection and Affordable Care Act (PPACA) expanded the eligibility criteria for Medicaid to all individuals with an income up to 138% of the poverty level.1 A Supreme Court ruling stated that the decision to expand Medicaid was to be decided by individual states.2 Currently, 31 states have chosen to expand Medicaid eligibility to their residents.2 This expansion has allowed an additional 11.7 million people to enroll in Medicaid and the Children’s Health Insurance Program by May 2015.3-5
Even with the passage of the PPACA, Medicaid patients seeking specialty orthopedic care have experienced more barriers to accessing care than Medicare or commercially-insured patients.2,6-10 One major cited reason is Medicaid’s low reimbursement, which may discourage physicians from open panel participation in Medicaid.11,12
A common fundamental teaching for orthopedic traumatologists is the notion that they should be available to treat all injuries regardless of the patient’s ability to pay.13 This has resulted in both trauma centers and trauma surgeons becoming financially challenged due to the higher proportion of Medicaid and uninsured trauma patients and lower Medicaid reimbursement levels.14,15
This study focuses on the effect of different types of insurance (Medicaid, Medicare, or commercial insurance) on the ability of patients to obtain care for operative ankle fractures. The purpose of this study is to evaluate, in the context of the PPACA, patient access to orthopedic surgeons for operative ankle fractures based on insurance-type. We hypothesized that patients with Medicaid would face a greater volume of obstacles when seeking appointments for an ankle fracture, even after the PPACA.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
The study population included board-certified orthopedic surgeons who belonged to the Orthopaedic Trauma Association (OTA) from 8 representative states; 4 states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and 4 states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas). These states were selected due to their ability to represent diverse healthcare marketplaces throughout the country. Using the OTA website’s “Find a Surgeon” search tool,16 we created a list of surgeons for each state and matched each surgeon with a random number. The list of surgeons was ordered according to the value of the surgeon’s associated random number, and surgeons were called in ascending order. We excluded disconnected or inaccurate numbers from the calling list. Surgeons who did not manage ankle fractures were removed from the dataset. Approximately 30 orthopedic trauma surgeons per state were contacted.
Each office was called to make an appointment for the caller’s mother. Every surgeon’s office was specifically asked if the surgeon would accept the patient to be evaluated for an ankle fracture that occurred out-of-state. The caller had a standardized protocol to limit intra- and inter-office variations (Appendix). The scenario involved a request to be evaluated for an unstable ankle fracture, with the patient having Medicaid, Medicare, or BlueCross insurance. The scenario required 3 separate calls to the same surgeon in order to obtain data regarding each insurance-type. The calls were separated by at least 1 week to avoid caller recognition by the surgeon’s office.
Appendix
Scenario
1. Date of Birth: Medicaid–2/07/55; BlueCross PPO–2/09/55; Medicare–7/31/45.
2. Ankle fracture evaluated by primary care physician 1 or 2 days ago
3. Not seen previously by your clinic or hospital, she would be a new patient
4. Asked how early she could be scheduled for an appointment
5. Script:
“I’m calling for my mother who injured her ankle a few days ago. Her family doctor took an X-ray and believes she has a fracture and needs surgery. Is Dr. X accepting new patients for evaluation and treatment of ankle fractures?” If YES →
“I was wondering if you take Medicaid/Medicare/BlueCross plan?” If YES →
“When is your soonest available appointment?”
The date of each phone call and date of appointment, if provided, were recorded. If the office did not give an appointment, we asked for reasons why. If an appointment was denied for a patient with Medicaid, we asked for a referral to another office that accepted Medicaid. We considered barriers to obtaining an initial appointment, such as requiring a referral from a primary care physician (PCP), as an unsuccessful attempt at making an appointment. We determined the waiting period for an appointment by calculating the time between the date of the call and the date of the appointment. Appointments were not scheduled to ensure that actual patients were not disadvantaged. For both appointment success rates and waiting periods, we stratified the data into 2 groups: states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas).
We obtained Medicaid reimbursement rates for open reduction and internal fixation of an ankle fracture by querying each state’s reimbursement rate using Current Procedural Terminology code 27822.
Chi-square test or Fisher’s exact test was used to analyze acceptance rate differences based on the patient’s type of insurance. To compare the waiting periods for an appointment, we used an independent samples t-test after applying natural log-transformation, as the data was not normally distributed. We performed logistic regression analysis to detect whether reimbursement was a significant predictor of successfully making an appointment for patients, and a linear regression analysis was used to evaluate whether reimbursement predicted waiting periods. Unless otherwise stated, all statistical testing was performed two-tailed at an alpha-level of 0.05.
This study was approved by the Institutional Review Board of Yale University School of Medicine (HIC No. 1363).
Continue to: RESULTS...
RESULTS
In total, 350 offices were contacted across 8 states (4 states with and 4 states without expanded Medicaid eligibility) of which we identified 245 orthopedic surgeons who would surgically treat ankle fractures. The 245 surgeons’ offices were called 3 times for each separate insurance-type.
Table 1. Appointment Success Rate
| Medicaid | Medicare | Private |
All states |
|
| |
Yes (%) | 100 (35.7) | 228 (81.4) | 248 (88.6) |
No (%) | 180 (64.3) | 52 (18.60 | 32 (11.4) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 55 (39.6) | 116 (83.5) | 124 (89.2) |
No (%) | 84 (60.4) | 23 (16.5) | 15 (10.8) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 45 (31.9) | 112 (79.4) | 124 (87.9) |
No (%) | 96 (68.1) | 29 (20.6) | 17 (12.1) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross (Table 1). For states with expanded Medicaid eligibility, the success rate for obtaining an appointment was 39.6%, 83.5%, and 89.2% for Medicaid, Medicare, and BlueCross, respectively. For states without expanded Medicaid eligibility, the success rate for obtaining an appointment was 31.9% for Medicaid, 79.4% for Medicare, and 87.9% for BlueCross. In all cases, the success rate for obtaining an appointment was significantly lower for Medicaid, compared to Medicare (P < .0001) or BlueCross (P < .0001). Medicaid appointment success rate was 39.6% in expanded states vs 31.9% in non-expanded states, however, the difference was not statistically significant (Table 2).
Table 2. Medicaid Appointment Success Rate in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 55 (39.6) | 45 (31.9) | .181 |
No (%) | 84 (60.4) | 96 (68.1) |
|
In 43.7% of occasions, patients with Medicaid did not have their insurance accepted, compared to 7.3% for Medicare and 0% for BlueCross. The majority of offices which did not accept Medicaid were not able to refer patients to another surgeon who would accept Medicaid. The requirement to have a primary care referral was the second most common reason for Medicaid patients not obtaining an appointment. No Medicare (10.4% vs 0.0%, P < .0001) or BlueCross (10.4% vs 0.0%, P < .0001) patients experienced this requirement (Table 3). There was no difference found between the percent of Medicaid patients who were required to have referrals in states with and without expanded Medicaid eligibility (Table 4).
Table 3. Referral Rate
| Medicaid | Medicare | Private |
All states |
|
|
|
Yes (%) | 29 (10.4) | 0 (0) | 0 (0) |
No (%) | 251 (89.6) | 280 (100) | 280 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 12 (8.6) | 0 (0) | 0 (0) |
No (%) | 127 (91.4) | 139 (100) | 139 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 17 (12.1) | 0 (0) | 0 (0) |
No (%) | 124 (87.9) | 141 (100) | 141 (100) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
Table 4. Medicaid Referral Rates in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 12 (9.7) | 17 (14.0) | .35 |
No (%) | 127 (91.4) | 124 (87.9) |
|
Reimbursements for ankle fracture varied across states (Table 5). For Medicaid, Georgia paid the highest reimbursement ($1049.95) and Florida paid the lowest ($469.44). Logistic and linear regression analysis did not demonstrate a significant relationship between reimbursement and appointment success rate or waiting periods.
Table 5. Medicaid Reimbursements for Ankle Fracture Repair (CPT and HCPCS 27822) in 2014
State | Medicaid reimbursement |
Californiaa | $785.55 |
Texas | $678.95 |
Florida | $469.44 |
Ohioa | $617.08 |
New Yorka | $500.02 |
North Carolina | $621.63 |
Massachusettsa | $627.94 |
Georgia | $1,049.95 |
Average | $668.82 |
aStates with expanded Medicaid eligibility.
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
Waiting periods (Table 6) varied significantly by the type of insurance (7.3 days for Medicaid, 6.0 days for Medicare, and 6.0 days for BlueCross; P = .002). For states with expanded Medicaid eligibility, waiting periods varied significantly by insurance (7.7 days for Medicaid, 6.2 days for Medicare, P = .003; and 6.1 days for BlueCross, P = .01). Waiting periods did not vary significantly for states without expanded Medicaid. Additionally, waiting periods did not differ significantly when comparing between states with and without Medicaid expansion.
Table 6. Waiting Period (Days) by Insurance Type.
| Medicaid | Medicare | Private |
Comparison by Insurance Type |
|
|
|
All states |
|
|
|
Waiting period | 7.3 | 6.0 | 6.0 |
P-value |
| 0.002 | 0.002 |
States with expanded Medicaid eligibility |
|
|
|
Waiting period | 7.7 | 6.2 | 6.1 |
P-value |
| 0.003 | 0.01 |
States without expanded Medicaid eligibility |
|
|
|
Waiting period | 6.9 | 5.9 | 5.9 |
P-value |
| 0.15 | 0.15 |
Comparison by Medicaid Expansion |
|
|
|
States with expanded Medicaid eligibility | 7.7 | 6.2 | 6.1 |
States without expanded Medicaid eligibility | 6.9 | 5.9 | 5.9 |
P-value | 0.17 | 0.13 | 0.07 |
Continue to: DISCUSSION...
DISCUSSION
This study assessed how insurance type (Medicaid, Medicare, and BlueCross) affects patient access to orthopedic trauma surgeons in 8 geographically representative states. We selected unstable ankle fractures as they are basic fractures treated by nearly all trauma surgeons and should often be surgically treated to prevent serious long-term consequences. Our hypothesis stated that despite the passage of the PPACA, patients with Medicaid would have reduced access to care. As the PPACA has changed the healthcare marketplace by increasing the number of Medicaid enrollees, it is important to ensure that patient access to care improves.
This nationwide survey of orthopedic trauma surgeons demonstrates that Medicaid patients experience added barriers to care that ultimately results in lower rates of successfully obtaining care. This is consistent with other investigations which have assessed Medicaid patient healthcare access.6,8,10,17-19 This study did not demonstrate a statistically significant difference between Medicaid patients’ ability to obtain appointments in states with expanded Medicaid eligibility vs in states without expanded Medicaid eligibility (39.6% vs 31.9%, P < .18); this has been demonstrated in the literature.6
A barrier that was unique to Medicaid patients was the requirement to have a PCP referral (Table 3). A PCP referral was not a barrier to receiving an appointment for patients with Medicare or BlueCross. One reason to explain why Medicaid patients may be required to have PCP referrals is due to their increased medical complexity, extra documentation requirements, and low reimbursement.4 Patients who have obtained a PCP referral may be characterized as being more medically compliant.
It is important to note that the Medicaid policies for 4 states included in this study (Massachusetts, North Carolina, Texas, and New York) required a PCP referral in order to see a specialist. However, we found that many orthopedic trauma practices in these states scheduled appointments for Medicaid patients without a PCP referral, suggesting that the decision depended on individual policy. In addition, the majority of offices within these states cited that they simply did not accept Medicaid as an insurance policy, and not that they required a referral.
Our regression analysis did not find a significant relationship between being able to successfully obtain an appointment to be evaluated for an ankle fracture and reimbursement rates for Medicaid. Although studies have stressed the importance of Medicaid reimbursements on physician participation, this result is consistent with previous studies regarding carpal tunnel release and total ankle replacements.17,19 Long20 suggested that although reimbursements may help, additional strategies for promoting Medicaid acceptance may be needed, including: lowering the costs of participating in Medicaid by simplifying administrative processes, speeding up reimbursement, and reducing the costs associated with caring for those patients.
Continue to: Previous studies have demonstrated...
Previous studies have demonstrated that more physicians may accept Medicaid if reimbursements increased.4,12 Given the high percentage of trauma patients with Medicaid as their primary insurance or whom are emergently enrolled in Medicaid by hospital systems, it is concerning that the PPACA is reducing payments under the Medicare and Medicaid Disproportionate Share Hospital programs which provide hospitals for uncompensated care given to low-income and uninsured patients.21 Trauma centers generally operate at a deficit due to the higher proportion of Medicaid and uninsured patients.14 This is currently worsened by additional federal funding cuts for supporting trauma service’s humane mission.21
This study has several limitations. While the study evaluated access to care in 8 representative states, a thorough nationwide survey would be more representative. Some results may have become statistically significant if we had performed the study with a larger sample size. In addition, we were unable to control for many factors which could impact appointment wait times, such as physician call schedules and vacations. Socioeconomic factors can influence a patient’s ability to attend an appointment, such as transportation costs, time off from work, and childcare availability. In addition, this study did not assess access for the uninsured, who are predominantly the working poor who cannot afford health insurance, even with federal and state subsidies.
The authors apologize for inconveniencing these offices, however, data collection could not be achieved in a better manner. We hope that the value of this study compensates any inconvenience.
CONCLUSION
Overall, our results demonstrate that despite the ratification of the PPACA, Medicaid patients are confronted with more barriers to accessing care by comparison to patients with Medicare and BlueCross insurance. Medicaid patients have worse baseline health22 and are at an increased risk of complications. These disparities are thought to be due to decreased healthcare access,23,24 as well as socioeconomic challenges. Interventions, such as increasing Medicaid’s reimbursement levels, reducing burdensome administrative responsibilities, and establishing partnerships between trauma centers and trauma surgeons, may enable underinsured patients to be appropriately cared for.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The purpose of this study is to assess the effect of insurance type (Medicaid, Medicare, private insurance) on the ability for patients with operative ankle fractures to access orthopedic traumatologists. The research team called 245 board-certified orthopedic surgeons specializing in orthopedic trauma within 8 representative states. The caller requested an appointment for their fictitious mother in order to be evaluated for an ankle fracture which was previously evaluated by her primary care physician and believed to require surgery. Each office was called 3 times to assess the response for each insurance type. For each call, information was documented regarding whether the patient was able to receive an appointment and the barriers the patient confronted to receive an appointment. Overall, 35.7% of offices scheduled an appointment for a patient with Medicaid, in comparison to 81.4%and 88.6% for Medicare and BlueCross, respectively (P < .0001). Medicaid patients confronted more barriers for receiving appointments. There was no statistically significant difference in access for Medicaid patients in states that had expanded Medicaid eligibility vs states that had not expanded Medicaid. Medicaid reimbursement for open reduction and internal fixation of an ankle fracture did not significantly correlate with appointment success rates or wait times. Despite the passage of the Affordable Care Act, patients with Medicaid have reduced access to orthopedic surgeons and more complex barriers to receiving appointments. A more robust strategy for increasing care-access for patients with Medicaid would be more equitable.
Continue to: In 2010, the Patient Protection and Affordable Care Act...
In 2010, the Patient Protection and Affordable Care Act (PPACA) expanded the eligibility criteria for Medicaid to all individuals with an income up to 138% of the poverty level.1 A Supreme Court ruling stated that the decision to expand Medicaid was to be decided by individual states.2 Currently, 31 states have chosen to expand Medicaid eligibility to their residents.2 This expansion has allowed an additional 11.7 million people to enroll in Medicaid and the Children’s Health Insurance Program by May 2015.3-5
Even with the passage of the PPACA, Medicaid patients seeking specialty orthopedic care have experienced more barriers to accessing care than Medicare or commercially-insured patients.2,6-10 One major cited reason is Medicaid’s low reimbursement, which may discourage physicians from open panel participation in Medicaid.11,12
A common fundamental teaching for orthopedic traumatologists is the notion that they should be available to treat all injuries regardless of the patient’s ability to pay.13 This has resulted in both trauma centers and trauma surgeons becoming financially challenged due to the higher proportion of Medicaid and uninsured trauma patients and lower Medicaid reimbursement levels.14,15
This study focuses on the effect of different types of insurance (Medicaid, Medicare, or commercial insurance) on the ability of patients to obtain care for operative ankle fractures. The purpose of this study is to evaluate, in the context of the PPACA, patient access to orthopedic surgeons for operative ankle fractures based on insurance-type. We hypothesized that patients with Medicaid would face a greater volume of obstacles when seeking appointments for an ankle fracture, even after the PPACA.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
The study population included board-certified orthopedic surgeons who belonged to the Orthopaedic Trauma Association (OTA) from 8 representative states; 4 states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and 4 states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas). These states were selected due to their ability to represent diverse healthcare marketplaces throughout the country. Using the OTA website’s “Find a Surgeon” search tool,16 we created a list of surgeons for each state and matched each surgeon with a random number. The list of surgeons was ordered according to the value of the surgeon’s associated random number, and surgeons were called in ascending order. We excluded disconnected or inaccurate numbers from the calling list. Surgeons who did not manage ankle fractures were removed from the dataset. Approximately 30 orthopedic trauma surgeons per state were contacted.
Each office was called to make an appointment for the caller’s mother. Every surgeon’s office was specifically asked if the surgeon would accept the patient to be evaluated for an ankle fracture that occurred out-of-state. The caller had a standardized protocol to limit intra- and inter-office variations (Appendix). The scenario involved a request to be evaluated for an unstable ankle fracture, with the patient having Medicaid, Medicare, or BlueCross insurance. The scenario required 3 separate calls to the same surgeon in order to obtain data regarding each insurance-type. The calls were separated by at least 1 week to avoid caller recognition by the surgeon’s office.
Appendix
Scenario
1. Date of Birth: Medicaid–2/07/55; BlueCross PPO–2/09/55; Medicare–7/31/45.
2. Ankle fracture evaluated by primary care physician 1 or 2 days ago
3. Not seen previously by your clinic or hospital, she would be a new patient
4. Asked how early she could be scheduled for an appointment
5. Script:
“I’m calling for my mother who injured her ankle a few days ago. Her family doctor took an X-ray and believes she has a fracture and needs surgery. Is Dr. X accepting new patients for evaluation and treatment of ankle fractures?” If YES →
“I was wondering if you take Medicaid/Medicare/BlueCross plan?” If YES →
“When is your soonest available appointment?”
The date of each phone call and date of appointment, if provided, were recorded. If the office did not give an appointment, we asked for reasons why. If an appointment was denied for a patient with Medicaid, we asked for a referral to another office that accepted Medicaid. We considered barriers to obtaining an initial appointment, such as requiring a referral from a primary care physician (PCP), as an unsuccessful attempt at making an appointment. We determined the waiting period for an appointment by calculating the time between the date of the call and the date of the appointment. Appointments were not scheduled to ensure that actual patients were not disadvantaged. For both appointment success rates and waiting periods, we stratified the data into 2 groups: states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas).
We obtained Medicaid reimbursement rates for open reduction and internal fixation of an ankle fracture by querying each state’s reimbursement rate using Current Procedural Terminology code 27822.
Chi-square test or Fisher’s exact test was used to analyze acceptance rate differences based on the patient’s type of insurance. To compare the waiting periods for an appointment, we used an independent samples t-test after applying natural log-transformation, as the data was not normally distributed. We performed logistic regression analysis to detect whether reimbursement was a significant predictor of successfully making an appointment for patients, and a linear regression analysis was used to evaluate whether reimbursement predicted waiting periods. Unless otherwise stated, all statistical testing was performed two-tailed at an alpha-level of 0.05.
This study was approved by the Institutional Review Board of Yale University School of Medicine (HIC No. 1363).
Continue to: RESULTS...
RESULTS
In total, 350 offices were contacted across 8 states (4 states with and 4 states without expanded Medicaid eligibility) of which we identified 245 orthopedic surgeons who would surgically treat ankle fractures. The 245 surgeons’ offices were called 3 times for each separate insurance-type.
Table 1. Appointment Success Rate
| Medicaid | Medicare | Private |
All states |
|
| |
Yes (%) | 100 (35.7) | 228 (81.4) | 248 (88.6) |
No (%) | 180 (64.3) | 52 (18.60 | 32 (11.4) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 55 (39.6) | 116 (83.5) | 124 (89.2) |
No (%) | 84 (60.4) | 23 (16.5) | 15 (10.8) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 45 (31.9) | 112 (79.4) | 124 (87.9) |
No (%) | 96 (68.1) | 29 (20.6) | 17 (12.1) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross (Table 1). For states with expanded Medicaid eligibility, the success rate for obtaining an appointment was 39.6%, 83.5%, and 89.2% for Medicaid, Medicare, and BlueCross, respectively. For states without expanded Medicaid eligibility, the success rate for obtaining an appointment was 31.9% for Medicaid, 79.4% for Medicare, and 87.9% for BlueCross. In all cases, the success rate for obtaining an appointment was significantly lower for Medicaid, compared to Medicare (P < .0001) or BlueCross (P < .0001). Medicaid appointment success rate was 39.6% in expanded states vs 31.9% in non-expanded states, however, the difference was not statistically significant (Table 2).
Table 2. Medicaid Appointment Success Rate in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 55 (39.6) | 45 (31.9) | .181 |
No (%) | 84 (60.4) | 96 (68.1) |
|
In 43.7% of occasions, patients with Medicaid did not have their insurance accepted, compared to 7.3% for Medicare and 0% for BlueCross. The majority of offices which did not accept Medicaid were not able to refer patients to another surgeon who would accept Medicaid. The requirement to have a primary care referral was the second most common reason for Medicaid patients not obtaining an appointment. No Medicare (10.4% vs 0.0%, P < .0001) or BlueCross (10.4% vs 0.0%, P < .0001) patients experienced this requirement (Table 3). There was no difference found between the percent of Medicaid patients who were required to have referrals in states with and without expanded Medicaid eligibility (Table 4).
Table 3. Referral Rate
| Medicaid | Medicare | Private |
All states |
|
|
|
Yes (%) | 29 (10.4) | 0 (0) | 0 (0) |
No (%) | 251 (89.6) | 280 (100) | 280 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 12 (8.6) | 0 (0) | 0 (0) |
No (%) | 127 (91.4) | 139 (100) | 139 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 17 (12.1) | 0 (0) | 0 (0) |
No (%) | 124 (87.9) | 141 (100) | 141 (100) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
Table 4. Medicaid Referral Rates in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 12 (9.7) | 17 (14.0) | .35 |
No (%) | 127 (91.4) | 124 (87.9) |
|
Reimbursements for ankle fracture varied across states (Table 5). For Medicaid, Georgia paid the highest reimbursement ($1049.95) and Florida paid the lowest ($469.44). Logistic and linear regression analysis did not demonstrate a significant relationship between reimbursement and appointment success rate or waiting periods.
Table 5. Medicaid Reimbursements for Ankle Fracture Repair (CPT and HCPCS 27822) in 2014
State | Medicaid reimbursement |
Californiaa | $785.55 |
Texas | $678.95 |
Florida | $469.44 |
Ohioa | $617.08 |
New Yorka | $500.02 |
North Carolina | $621.63 |
Massachusettsa | $627.94 |
Georgia | $1,049.95 |
Average | $668.82 |
aStates with expanded Medicaid eligibility.
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
Waiting periods (Table 6) varied significantly by the type of insurance (7.3 days for Medicaid, 6.0 days for Medicare, and 6.0 days for BlueCross; P = .002). For states with expanded Medicaid eligibility, waiting periods varied significantly by insurance (7.7 days for Medicaid, 6.2 days for Medicare, P = .003; and 6.1 days for BlueCross, P = .01). Waiting periods did not vary significantly for states without expanded Medicaid. Additionally, waiting periods did not differ significantly when comparing between states with and without Medicaid expansion.
Table 6. Waiting Period (Days) by Insurance Type.
| Medicaid | Medicare | Private |
Comparison by Insurance Type |
|
|
|
All states |
|
|
|
Waiting period | 7.3 | 6.0 | 6.0 |
P-value |
| 0.002 | 0.002 |
States with expanded Medicaid eligibility |
|
|
|
Waiting period | 7.7 | 6.2 | 6.1 |
P-value |
| 0.003 | 0.01 |
States without expanded Medicaid eligibility |
|
|
|
Waiting period | 6.9 | 5.9 | 5.9 |
P-value |
| 0.15 | 0.15 |
Comparison by Medicaid Expansion |
|
|
|
States with expanded Medicaid eligibility | 7.7 | 6.2 | 6.1 |
States without expanded Medicaid eligibility | 6.9 | 5.9 | 5.9 |
P-value | 0.17 | 0.13 | 0.07 |
Continue to: DISCUSSION...
DISCUSSION
This study assessed how insurance type (Medicaid, Medicare, and BlueCross) affects patient access to orthopedic trauma surgeons in 8 geographically representative states. We selected unstable ankle fractures as they are basic fractures treated by nearly all trauma surgeons and should often be surgically treated to prevent serious long-term consequences. Our hypothesis stated that despite the passage of the PPACA, patients with Medicaid would have reduced access to care. As the PPACA has changed the healthcare marketplace by increasing the number of Medicaid enrollees, it is important to ensure that patient access to care improves.
This nationwide survey of orthopedic trauma surgeons demonstrates that Medicaid patients experience added barriers to care that ultimately results in lower rates of successfully obtaining care. This is consistent with other investigations which have assessed Medicaid patient healthcare access.6,8,10,17-19 This study did not demonstrate a statistically significant difference between Medicaid patients’ ability to obtain appointments in states with expanded Medicaid eligibility vs in states without expanded Medicaid eligibility (39.6% vs 31.9%, P < .18); this has been demonstrated in the literature.6
A barrier that was unique to Medicaid patients was the requirement to have a PCP referral (Table 3). A PCP referral was not a barrier to receiving an appointment for patients with Medicare or BlueCross. One reason to explain why Medicaid patients may be required to have PCP referrals is due to their increased medical complexity, extra documentation requirements, and low reimbursement.4 Patients who have obtained a PCP referral may be characterized as being more medically compliant.
It is important to note that the Medicaid policies for 4 states included in this study (Massachusetts, North Carolina, Texas, and New York) required a PCP referral in order to see a specialist. However, we found that many orthopedic trauma practices in these states scheduled appointments for Medicaid patients without a PCP referral, suggesting that the decision depended on individual policy. In addition, the majority of offices within these states cited that they simply did not accept Medicaid as an insurance policy, and not that they required a referral.
Our regression analysis did not find a significant relationship between being able to successfully obtain an appointment to be evaluated for an ankle fracture and reimbursement rates for Medicaid. Although studies have stressed the importance of Medicaid reimbursements on physician participation, this result is consistent with previous studies regarding carpal tunnel release and total ankle replacements.17,19 Long20 suggested that although reimbursements may help, additional strategies for promoting Medicaid acceptance may be needed, including: lowering the costs of participating in Medicaid by simplifying administrative processes, speeding up reimbursement, and reducing the costs associated with caring for those patients.
Continue to: Previous studies have demonstrated...
Previous studies have demonstrated that more physicians may accept Medicaid if reimbursements increased.4,12 Given the high percentage of trauma patients with Medicaid as their primary insurance or whom are emergently enrolled in Medicaid by hospital systems, it is concerning that the PPACA is reducing payments under the Medicare and Medicaid Disproportionate Share Hospital programs which provide hospitals for uncompensated care given to low-income and uninsured patients.21 Trauma centers generally operate at a deficit due to the higher proportion of Medicaid and uninsured patients.14 This is currently worsened by additional federal funding cuts for supporting trauma service’s humane mission.21
This study has several limitations. While the study evaluated access to care in 8 representative states, a thorough nationwide survey would be more representative. Some results may have become statistically significant if we had performed the study with a larger sample size. In addition, we were unable to control for many factors which could impact appointment wait times, such as physician call schedules and vacations. Socioeconomic factors can influence a patient’s ability to attend an appointment, such as transportation costs, time off from work, and childcare availability. In addition, this study did not assess access for the uninsured, who are predominantly the working poor who cannot afford health insurance, even with federal and state subsidies.
The authors apologize for inconveniencing these offices, however, data collection could not be achieved in a better manner. We hope that the value of this study compensates any inconvenience.
CONCLUSION
Overall, our results demonstrate that despite the ratification of the PPACA, Medicaid patients are confronted with more barriers to accessing care by comparison to patients with Medicare and BlueCross insurance. Medicaid patients have worse baseline health22 and are at an increased risk of complications. These disparities are thought to be due to decreased healthcare access,23,24 as well as socioeconomic challenges. Interventions, such as increasing Medicaid’s reimbursement levels, reducing burdensome administrative responsibilities, and establishing partnerships between trauma centers and trauma surgeons, may enable underinsured patients to be appropriately cared for.
This paper will be judged for the Resident Writer’s Award.
1. Blumenthal D, Collins SR. Health care coverage under the affordable care act--a progress report. N Engl J Med. 2014;371(3):275-281. doi:10.1056/NEJMhpr1405667.
2. Sommers BD. Health care reform's unfinished work--remaining barriers to coverage and access. N Engl J Med. 2015;373(25):2395-2397. doi:10.1056/NEJMp1509462.
3. US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicaid & CHIP: February 2015 monthly applications, eligibility determinations and enrollment report. https://www.medicaid.gov/medicaid/program-information/downloads/medicaid-and-chip-february-2015-application-eligibility-and-enrollment-data.pdf. Published May 1, 2015. Accessed May 2015.
4. Iglehart JK, Sommers BD. Medicaid at 50--from welfare program to nation's largest health insurer. N Engl J Med. 2015;372(22):2152-2159. doi:10.1056/NEJMhpr1500791.
5. Kaiser Family Foundation. Medicaid moving forward. http://kff.org/medicaid/fact-sheet/the-medicaid-program-at-a-glance-update/. Updated 2014. Accessed October 10, 2014.
6. Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the affordable care act. J Arthroplasty. 2015;30(9):1498-1501. doi:10.1016/j.arth.2015.03.015.
7. Draeger RW, Patterson BM, Olsson EC, Schaffer A, Patterson JM. The influence of patient insurance status on access to outpatient orthopedic care for flexor tendon lacerations. J Hand Surg Am. 2014;39(3):527-533. doi:10.1016/j.jhsa.2013.10.031.
8. Patterson BM, Spang JT, Draeger RW, Olsson EC, Creighton RA, Kamath GV. Access to outpatient care for adult rotator cuff patients with private insurance versus Medicaid in North Carolina. J Shoulder Elbow Surg. 2013;22(12):1623-1627. doi:10.1016/j.jse.2013.07.051.
9. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
10. Schwarzkopf R, Phan D, Hoang M, Ross S, Mukamel D. Do patients with income-based insurance have access to total joint arthroplasty? J Arthroplasty. 2014;29(6):1083-1086. doi:10.1016/j.arth.2013.11.022.
11. Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff (Millwood). 2012;31(8):1673-1679 doi:10.1377/hlthaff.2012.0294.
12. Perloff JD, Kletke P, Fossett JW. Which physicians limit their Medicaid participation, and why. Health Serv Res. 1995;30(1):7-26.
13. Althausen PL. Building a successful trauma practice in a community setting. J Orthop Trauma. 2011;25 Suppl 3:S113-S117. doi:10.1097/BOT.0b013e318237bcce.
14. Greenberg S, Mir HR, Jahangir AA, Mehta S, Sethi MK. Impacting policy change for orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S14-S16. doi:10.1097/BOT.0000000000000216.
15. Wiznia DH, Averbukh L, Kim CY, Goel A, Leslie MP. Motorcycle helmets: The economic burden of an incomplete helmet law to medical care in the state of Connecticut. Conn Med. 2015;79(8):453-459.
16. Orthopaedic Trauma Association. Find a surgeon. https://online.ota.org/otassa/otacenssafindasurgeon.query_page. Updated 2015. Accessed July, 2015.
17. Kim CY, Wiznia DH, Roth AS, Walls RJ, Pelker RR. Survey of patient insurance status on access to specialty foot and ankle care under the affordable care act. Foot Ankle Int. 2016;37(7):776-781. doi:1071100716642015.
18. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of Medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
19. Kim CY, Wiznia DH, Wang Y, et al. The effect of insurance type on patient access to carpal tunnel release under the affordable care act. J Hand Surg Am. 2016;41(4):503-509.e1. doi:S0363-5023(16)00104-0.
20. Long SK. Physicians may need more than higher reimbursements to expand Medicaid participation: findings from Washington state. Health Aff (Millwood). 2013;32(9):1560-1567. doi:10.1377/hlthaff.2012.1010.
21. Issar NM, Jahangir AA. The affordable care act and orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S5-S7. doi:10.1097/BOT.0000000000000211.
22. Hahn B, Flood AB. No insurance, public insurance, and private insurance: do these options contribute to differences in general health? J Health Care Poor Underserved. 1995;6(1):41-59.
23. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):9-14. doi:10.1016/j.arth.2008.05.010.
24. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi:10.1097/MLR.0000000000000177.
1. Blumenthal D, Collins SR. Health care coverage under the affordable care act--a progress report. N Engl J Med. 2014;371(3):275-281. doi:10.1056/NEJMhpr1405667.
2. Sommers BD. Health care reform's unfinished work--remaining barriers to coverage and access. N Engl J Med. 2015;373(25):2395-2397. doi:10.1056/NEJMp1509462.
3. US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicaid & CHIP: February 2015 monthly applications, eligibility determinations and enrollment report. https://www.medicaid.gov/medicaid/program-information/downloads/medicaid-and-chip-february-2015-application-eligibility-and-enrollment-data.pdf. Published May 1, 2015. Accessed May 2015.
4. Iglehart JK, Sommers BD. Medicaid at 50--from welfare program to nation's largest health insurer. N Engl J Med. 2015;372(22):2152-2159. doi:10.1056/NEJMhpr1500791.
5. Kaiser Family Foundation. Medicaid moving forward. http://kff.org/medicaid/fact-sheet/the-medicaid-program-at-a-glance-update/. Updated 2014. Accessed October 10, 2014.
6. Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the affordable care act. J Arthroplasty. 2015;30(9):1498-1501. doi:10.1016/j.arth.2015.03.015.
7. Draeger RW, Patterson BM, Olsson EC, Schaffer A, Patterson JM. The influence of patient insurance status on access to outpatient orthopedic care for flexor tendon lacerations. J Hand Surg Am. 2014;39(3):527-533. doi:10.1016/j.jhsa.2013.10.031.
8. Patterson BM, Spang JT, Draeger RW, Olsson EC, Creighton RA, Kamath GV. Access to outpatient care for adult rotator cuff patients with private insurance versus Medicaid in North Carolina. J Shoulder Elbow Surg. 2013;22(12):1623-1627. doi:10.1016/j.jse.2013.07.051.
9. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
10. Schwarzkopf R, Phan D, Hoang M, Ross S, Mukamel D. Do patients with income-based insurance have access to total joint arthroplasty? J Arthroplasty. 2014;29(6):1083-1086. doi:10.1016/j.arth.2013.11.022.
11. Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff (Millwood). 2012;31(8):1673-1679 doi:10.1377/hlthaff.2012.0294.
12. Perloff JD, Kletke P, Fossett JW. Which physicians limit their Medicaid participation, and why. Health Serv Res. 1995;30(1):7-26.
13. Althausen PL. Building a successful trauma practice in a community setting. J Orthop Trauma. 2011;25 Suppl 3:S113-S117. doi:10.1097/BOT.0b013e318237bcce.
14. Greenberg S, Mir HR, Jahangir AA, Mehta S, Sethi MK. Impacting policy change for orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S14-S16. doi:10.1097/BOT.0000000000000216.
15. Wiznia DH, Averbukh L, Kim CY, Goel A, Leslie MP. Motorcycle helmets: The economic burden of an incomplete helmet law to medical care in the state of Connecticut. Conn Med. 2015;79(8):453-459.
16. Orthopaedic Trauma Association. Find a surgeon. https://online.ota.org/otassa/otacenssafindasurgeon.query_page. Updated 2015. Accessed July, 2015.
17. Kim CY, Wiznia DH, Roth AS, Walls RJ, Pelker RR. Survey of patient insurance status on access to specialty foot and ankle care under the affordable care act. Foot Ankle Int. 2016;37(7):776-781. doi:1071100716642015.
18. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of Medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
19. Kim CY, Wiznia DH, Wang Y, et al. The effect of insurance type on patient access to carpal tunnel release under the affordable care act. J Hand Surg Am. 2016;41(4):503-509.e1. doi:S0363-5023(16)00104-0.
20. Long SK. Physicians may need more than higher reimbursements to expand Medicaid participation: findings from Washington state. Health Aff (Millwood). 2013;32(9):1560-1567. doi:10.1377/hlthaff.2012.1010.
21. Issar NM, Jahangir AA. The affordable care act and orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S5-S7. doi:10.1097/BOT.0000000000000211.
22. Hahn B, Flood AB. No insurance, public insurance, and private insurance: do these options contribute to differences in general health? J Health Care Poor Underserved. 1995;6(1):41-59.
23. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):9-14. doi:10.1016/j.arth.2008.05.010.
24. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi:10.1097/MLR.0000000000000177.
TAKE-HOME POINTS
- One method in which the PPACA increased the number of individuals with health insurance coverage was by expanding Medicaid eligibility requirements.
- Despite this, Medicaid patients confronted more barriers to accessing care.
- The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross. Patients with Medicaid also confronted longer appointment wait times.
- The disparity in access for this operative trauma scenario suggests that patients with Medicaid are likely to be excluded from the practice of their choice and may need to make considerably more effort to secure an appointment.
- Ultimately, Medicaid patients may have access to care through federally funded community health centers and public and non-profit safety net hospitals, which generally care for more uninsured and Medicaid patient populations.
Improved Transitional Care Through an Innovative Hospitalist Model: Expanding Clinician Practice From Acute to Subacute Care
Hospitalist physician rotations between acute inpatient hospitals and subacute care facilities with dedicated time in each environment may foster quality improvement and educational opportunities.
Care transitions between hospitals and skilled nursing facilities (SNFs) are a vulnerable time for patients. The current health care climate of decreasing hospital length of stay, readmission penalties, and increasing patient complexity has made hospital care transitions an important safety concern. Suboptimal transitions across clinical settings can result in adverse events, inadequately controlled comorbidities, deficient patient and caregiver preparation for discharge, medication errors, relocation stress, and overall increased morbidity and mortality.1,2 Such care transitions also may generate unnecessary spending, including avoidable readmissions, emergency department utilization, and duplicative laboratory and imaging studies. Approximately 23% of patients admitted to SNFs are readmitted to acute care hospitals within 30 days, and these patients have increased mortality rates in risk-adjusted analyses. 3,4
Compounding the magnitude of this risk and vulnerability is the significant growth in the number of patients discharged to SNFs over the past 30 years. In 2013, more than 20% of Medicare patients discharged from acute care hospitals were destined for SNFs.5,6 Paradoxically, despite the increasing need for SNF providers, there is a shortage of clinicians with training in geriatrics or nursing home care.7 The result is a growing need to identify organizational systems to optimize physician practice in these settings, enhance quality of care, especially around transitions, and increase educational training opportunities in SNFs for future practitioners.
Many SNFs today are staffed by physicians and other licensed clinicians whose exclusive practice location is the nursing facility or possibly several such facilities. This prevailing model of care can isolate the physicians, depriving them of interaction with clinicians in other specialties, and can contribute to burnout.8 This model does not lend itself to academic scholarship, quality improvement (QI), and student or resident training, as each of these endeavors depends on interprofessional collaboration as well as access to an academic medical center with additional resources.9
Few studies have described innovative hospitalist rotation models from acute to subacute care. The Cleveland Clinic implemented the Connected Care model where hospital-employed physicians and advanced practice professionals integrated into postacute care and reduced the 30-day hospital readmission rate from SNFs from 28% to 22%.10 Goth and colleagues performed a comparative effectiveness trial between a postacute care hospitalist (PACH) model and a community-based physician model of nursing home care. They found that the institution of a PACH model in a nursing home was associated with a significant increase in laboratory costs, nonsignificant reduction in medication errors and pharmacy costs, and no improvement in fall rates.11 The conclusion was that the PACH model may lead to greater clinician involvement and that the potential decrease in pharmacy costs and medications errors may offset the costs associated with additional laboratory testing. Overall, there has been a lack of studies on the impact of these hospitalist rotation models from acute to subacute care on educational programs, QI activities, and the interprofessional environment.
To achieve a system in which physicians in a SNF can excel in these areas, Veterans Affairs Boston Healthcare System (VABHS) adopted a staffing model in which academic hospitalist physicians rotate between the inpatient hospital and subacute settings. This report describes the model structure, the varying roles of the physicians, and early indicators of its positive effects on educational programs, QI activities, and the interprofessional environment.
Methods
The VABHS consists of a 159-bed acute care hospital in West Roxbury, Massachusetts; and a 110-bed SNF in Brockton, Massachusetts, with 3 units: a 65-bed transitional care unit (TCU), a 30-bed long-term care unit, and a 15-bed palliative care/hospice unit. The majority of patients admitted to the SNF are transferred from the acute care hospital in West Roxbury and other regional hospitals. Prior to 2015, the TCU was staffed with full-time clinicians who exclusively practiced in the SNF.
In the new staffing model, 6 hospitalist physicians divide their clinical time between the acute care hospital’s inpatient medical service and the TCU. The hospitalists come from varied backgrounds in terms of years in practice and advanced training (Table 1).
The amount of nonclinical (protected) time and clinical time on the acute inpatient service and the TCU varies for each physician. For example, a physician serves as principal investigator for several major research grants and has a hospital-wide administrative leadership role; as a result, the principal investigator has fewer months of clinical responsibility. Physicians are expected to use the protected time for scholarship, educational program development and teaching, QI, and administrative responsibilities. The VABHS leadership determines the amount of protected time based on individualized benchmarks for research, education, and administrative responsibilities that follow VA national and local institutional guidelines. These metrics and time allocations are negotiated at the time of recruitment and then are reviewed annually.
The TCU also is staffed with 4 full-time clinicians (2 physicians and 2 physician assistants) who provide additional continuity of care. The new hospitalist staffing model only required an approximate 10% increase in TCU clinical staffing full-time equivalents. Patients and admissions are divided equally among clinicians on service (census per clinician 12-15 patients), with redistribution of patients at times of transition from clinical to nonclinical time. Blocks of clinical time are scheduled for greater than 2 weeks at a time to preserve continuity. In addition, the new staffing model allocates assignment of clinical responsibilities that allows for clinicians to take leave without resultant shortages in clinical coverage.
To facilitate communication among physicians serving in the acute inpatient facility and the TCU, leaders of both of these programs meet monthly and ad hoc to review the transitions of care between the 2 settings. The description of this model and its assessment have been reviewed and deemed exempt from oversight by the VA Boston Healthcare System Research and Development Committee.
Results
Since the implementation of this staffing model in 2015, the system has grown considerably in the breadth and depth of educational programming, QI, and systems redesign in the TCU and, more broadly, in the SNF. The TCU, which previously had limited training opportunities, has experienced marked expansion of educational offerings. It is now a site for core general medicine rotations for first-year psychiatry residents and physician assistant students. The TCU also has expanded as a clinical site for transitions-in-care internal medicine resident curricula and electives, as well as a clinical site for a geriatrics fellowship.
A hospitalist developed and implemented a 4-week interprofessional curriculum for all clinical trainees and students, which occurs continuously. The curriculum includes a monthly academic conference and 12 didactic lectures and is taught by 16 interprofessional faculty from the TCU and the Palliative Care/Hospice Unit, including medicine, geriatric and palliative care physicians, physician assistants, social workers, physical and occupational therapists, pharmacists, and a geriatric psychologist. The goal of the curriculum is to provide learners the knowledge, attitudes, and skills necessary to perform effective, efficient, and safe transfers between clinical settings as well as education in transitional care. In addition, using a team of interprofessional faculty, the curriculum develops the interprofessional competencies of teamwork and communication. The curriculum also has provided a significant opportunity for interprofessional collaboration among faculty who have volunteered their teaching time in the development and teaching of the curriculum, with potential for improved clinical staff knowledge of other disciplines.
Quality improvement and system redesign projects in care transitions also have expanded (Table 2).
Early assessment indicates that the new staffing model is having positive effects on the clinical environment of the TCU. A survey was conducted of a convenience sample of all physicians, nurse managers, social workers, and other members of the clinical team in the TCU (N=24)(Table 3), with response categories ranging on a Likert scale from 1 (very negative) to 5 (very positive).
Although not rigorously analyzed using qualitative research methods, comments from respondents have consistently indicated that this staffing model increases the transfer of clinical and logistical knowledge among staff members working in the acute inpatient facility and the TCU.
Discussion
With greater numbers of increasingly complex patients transitioning from the hospital to SNF, health care systems need to expand the capacity of their skilled nursing systems, not only to provide clinical care, but also to support QI and medical education. The VABHS developed a physician staffing model with the goal of enriching physician practice and enhancing QI and educational opportunities in its SNF. The model offers an opportunity to improve transitions in care as physicians gain a greater knowledge of both the hospital and subacute clinical settings. This hospitalist rotation model may improve the knowledge necessary for caring for patients moving across care settings, as well as improve communication between settings. It also has served as a foundation for systematic innovation in QI and education at this institution. Clinical staff in the transitional care setting have reported positive effects of this model on clinical skills and patient care, educational opportunities, as well as a desire for replication in other health care systems.
The potential generalizability of this model requires careful consideration. The VABHS is a tertiary care integrated health care system, enabling physicians to work in multiple clinical settings. Other settings may not have the staffing or clinical volume to sustain such a model. In addition, this model may increase discontinuity in patient care as hospitalists move between acute and subacute settings and nonclinical roles. This loss of continuity may be a greater concern in the SNF setting, as the inpatient hospitalist model generally involves high provider turnover as shift work. Our survey included nurse managers, and not floor nurses due to survey administration limitations, and feedback may not have captured a comprehensive view from CLC staff. Moreover, some of the perceived positive impacts also may be related to professional and personal attributes of the physicians rather than the actual model of care. In addition, the survey response rate was 86%. However, the nature of the improvement work (focused on care transitions) and educational opportunities (interprofessional care) would likely not occur had the physicians been based in one clinical setting.
Other new physician staffing models have been designed to improve the continuity between the hospital, subacute, and outpatient settings. For example, the University of Chicago Comprehensive Care model pairs patients with trained hospitalists who provide both inpatient and outpatient care, thereby optimizing continuity between these settings.14 At CareMore Health System, high-risk patients also are paired with hospitalists, referred to as “extensivists,” who lead care teams that follow patients between settings and provide acute, postacute, and outpatient care.15 In these models, a single physician takes responsibility for the patient throughout transitions of care and through various care settings. Both models have shown reduction in hospital readmissions. One concern with such models is that the treatment teams need to coexist in the various settings of care, and the ability to impact and create systematic change within each environment is limited. This may limit QI, educational opportunities, and system level impact within each environment of care.
In comparison, the “transitionalist” model proposed here features hospitalist physicians rotating between the acute inpatient hospital and subacute care with dedicated time in each environment. This innovative organizational structure may enhance physician practice and enrich QI and educational opportunities in SNFs. Further evaluation will include the impact on quality metrics of patient care and patient satisfaction, as this model has the potential to influence quality, cost, and overall health outcomes.
Acknowledgments
We would like to thank Shivani Jindal, Matthew Russell, Matthew Ronan, Juman Hijab, Wei Shen, Sandra Vilbrun-Bruno, and Jack Earnshaw for their significant contributions to this staffing model. We would also like to thank Paul Conlin, Jay Orlander, and the leadership team of Veterans Affairs Boston Healthcare System for supporting this staffing model.
1. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20(4):317-323.
2. Murtaugh CM, Litke A. Transitions through postacute and long-term care settings: patterns of use and outcomes for a national cohort of elders. Med Care. 2002;40(3):227-236.
3. Burke RE, Whitfield EA, Hittle D, et al. Hospital readmission from post-acute care facilities: risk factors, timing, and outcomes. J Am Med Dir Assoc. 2016;17(3):249-255.
4. Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57-64.
5. Tian W. An all-payer view of hospital discharge to postacute care, 2013: Statistical Brief #205. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.jsp. Published May 2016. Accessed August 13, 2018.
6. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time–measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6.
7. Golden AG, Silverman MA, Mintzer MJ. Is geriatric medicine terminally ill? Ann Intern Med. 2012;156(9):654-656.
8. Nazir A, Smalbrugge M, Moser A, et al. The prevalence of burnout among nursing home physicians: an international perspective. J Am Med Dir Assoc. 2018;19(1):86-88.
9. Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141(7):533-536.
10. Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244.
11. Gloth MF, Gloth MJ. A comparative effectiveness trial between a post-acute care hospitalist model and a community-based physician model of nursing home care. J Am Med Dir Assoc. 2011;12(5):384-386.
12. Baughman AW, Cain G, Ruopp MD, et al. Improving access to care by admission process redesign in a veterans affairs skilled nursing facility. Jt Comm J Qual Patient Saf. 2018;44(8):454-462.
13. Mixon A, Smith GR, Dalal A et al. The Multi-Center Medication Reconciliation Quality Improvement Study 2 (MARQUIS2): methods and implementation. Abstract 248. Present at: Society of Hospital Medicine Annual Meeting; 2018 Apr 8 – 11, 2018; Orlando, FL. https://www.shmabstracts.com/abstract/the-multi-center-medication-reconciliation-quality-improvement-study-2-marquis2-methods-and-implementation. Accessed August 13, 2018.
14. Meltzer DO, Ruhnke GW. Redesigning care for patients at increased hospitalization risk: the comprehensive care physician model. Health Aff (Millwood). 2014;33(5):770-777.
15. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: back to the future? JAMA. 2016;315(1):23-24.
Hospitalist physician rotations between acute inpatient hospitals and subacute care facilities with dedicated time in each environment may foster quality improvement and educational opportunities.
Hospitalist physician rotations between acute inpatient hospitals and subacute care facilities with dedicated time in each environment may foster quality improvement and educational opportunities.
Care transitions between hospitals and skilled nursing facilities (SNFs) are a vulnerable time for patients. The current health care climate of decreasing hospital length of stay, readmission penalties, and increasing patient complexity has made hospital care transitions an important safety concern. Suboptimal transitions across clinical settings can result in adverse events, inadequately controlled comorbidities, deficient patient and caregiver preparation for discharge, medication errors, relocation stress, and overall increased morbidity and mortality.1,2 Such care transitions also may generate unnecessary spending, including avoidable readmissions, emergency department utilization, and duplicative laboratory and imaging studies. Approximately 23% of patients admitted to SNFs are readmitted to acute care hospitals within 30 days, and these patients have increased mortality rates in risk-adjusted analyses. 3,4
Compounding the magnitude of this risk and vulnerability is the significant growth in the number of patients discharged to SNFs over the past 30 years. In 2013, more than 20% of Medicare patients discharged from acute care hospitals were destined for SNFs.5,6 Paradoxically, despite the increasing need for SNF providers, there is a shortage of clinicians with training in geriatrics or nursing home care.7 The result is a growing need to identify organizational systems to optimize physician practice in these settings, enhance quality of care, especially around transitions, and increase educational training opportunities in SNFs for future practitioners.
Many SNFs today are staffed by physicians and other licensed clinicians whose exclusive practice location is the nursing facility or possibly several such facilities. This prevailing model of care can isolate the physicians, depriving them of interaction with clinicians in other specialties, and can contribute to burnout.8 This model does not lend itself to academic scholarship, quality improvement (QI), and student or resident training, as each of these endeavors depends on interprofessional collaboration as well as access to an academic medical center with additional resources.9
Few studies have described innovative hospitalist rotation models from acute to subacute care. The Cleveland Clinic implemented the Connected Care model where hospital-employed physicians and advanced practice professionals integrated into postacute care and reduced the 30-day hospital readmission rate from SNFs from 28% to 22%.10 Goth and colleagues performed a comparative effectiveness trial between a postacute care hospitalist (PACH) model and a community-based physician model of nursing home care. They found that the institution of a PACH model in a nursing home was associated with a significant increase in laboratory costs, nonsignificant reduction in medication errors and pharmacy costs, and no improvement in fall rates.11 The conclusion was that the PACH model may lead to greater clinician involvement and that the potential decrease in pharmacy costs and medications errors may offset the costs associated with additional laboratory testing. Overall, there has been a lack of studies on the impact of these hospitalist rotation models from acute to subacute care on educational programs, QI activities, and the interprofessional environment.
To achieve a system in which physicians in a SNF can excel in these areas, Veterans Affairs Boston Healthcare System (VABHS) adopted a staffing model in which academic hospitalist physicians rotate between the inpatient hospital and subacute settings. This report describes the model structure, the varying roles of the physicians, and early indicators of its positive effects on educational programs, QI activities, and the interprofessional environment.
Methods
The VABHS consists of a 159-bed acute care hospital in West Roxbury, Massachusetts; and a 110-bed SNF in Brockton, Massachusetts, with 3 units: a 65-bed transitional care unit (TCU), a 30-bed long-term care unit, and a 15-bed palliative care/hospice unit. The majority of patients admitted to the SNF are transferred from the acute care hospital in West Roxbury and other regional hospitals. Prior to 2015, the TCU was staffed with full-time clinicians who exclusively practiced in the SNF.
In the new staffing model, 6 hospitalist physicians divide their clinical time between the acute care hospital’s inpatient medical service and the TCU. The hospitalists come from varied backgrounds in terms of years in practice and advanced training (Table 1).
The amount of nonclinical (protected) time and clinical time on the acute inpatient service and the TCU varies for each physician. For example, a physician serves as principal investigator for several major research grants and has a hospital-wide administrative leadership role; as a result, the principal investigator has fewer months of clinical responsibility. Physicians are expected to use the protected time for scholarship, educational program development and teaching, QI, and administrative responsibilities. The VABHS leadership determines the amount of protected time based on individualized benchmarks for research, education, and administrative responsibilities that follow VA national and local institutional guidelines. These metrics and time allocations are negotiated at the time of recruitment and then are reviewed annually.
The TCU also is staffed with 4 full-time clinicians (2 physicians and 2 physician assistants) who provide additional continuity of care. The new hospitalist staffing model only required an approximate 10% increase in TCU clinical staffing full-time equivalents. Patients and admissions are divided equally among clinicians on service (census per clinician 12-15 patients), with redistribution of patients at times of transition from clinical to nonclinical time. Blocks of clinical time are scheduled for greater than 2 weeks at a time to preserve continuity. In addition, the new staffing model allocates assignment of clinical responsibilities that allows for clinicians to take leave without resultant shortages in clinical coverage.
To facilitate communication among physicians serving in the acute inpatient facility and the TCU, leaders of both of these programs meet monthly and ad hoc to review the transitions of care between the 2 settings. The description of this model and its assessment have been reviewed and deemed exempt from oversight by the VA Boston Healthcare System Research and Development Committee.
Results
Since the implementation of this staffing model in 2015, the system has grown considerably in the breadth and depth of educational programming, QI, and systems redesign in the TCU and, more broadly, in the SNF. The TCU, which previously had limited training opportunities, has experienced marked expansion of educational offerings. It is now a site for core general medicine rotations for first-year psychiatry residents and physician assistant students. The TCU also has expanded as a clinical site for transitions-in-care internal medicine resident curricula and electives, as well as a clinical site for a geriatrics fellowship.
A hospitalist developed and implemented a 4-week interprofessional curriculum for all clinical trainees and students, which occurs continuously. The curriculum includes a monthly academic conference and 12 didactic lectures and is taught by 16 interprofessional faculty from the TCU and the Palliative Care/Hospice Unit, including medicine, geriatric and palliative care physicians, physician assistants, social workers, physical and occupational therapists, pharmacists, and a geriatric psychologist. The goal of the curriculum is to provide learners the knowledge, attitudes, and skills necessary to perform effective, efficient, and safe transfers between clinical settings as well as education in transitional care. In addition, using a team of interprofessional faculty, the curriculum develops the interprofessional competencies of teamwork and communication. The curriculum also has provided a significant opportunity for interprofessional collaboration among faculty who have volunteered their teaching time in the development and teaching of the curriculum, with potential for improved clinical staff knowledge of other disciplines.
Quality improvement and system redesign projects in care transitions also have expanded (Table 2).
Early assessment indicates that the new staffing model is having positive effects on the clinical environment of the TCU. A survey was conducted of a convenience sample of all physicians, nurse managers, social workers, and other members of the clinical team in the TCU (N=24)(Table 3), with response categories ranging on a Likert scale from 1 (very negative) to 5 (very positive).
Although not rigorously analyzed using qualitative research methods, comments from respondents have consistently indicated that this staffing model increases the transfer of clinical and logistical knowledge among staff members working in the acute inpatient facility and the TCU.
Discussion
With greater numbers of increasingly complex patients transitioning from the hospital to SNF, health care systems need to expand the capacity of their skilled nursing systems, not only to provide clinical care, but also to support QI and medical education. The VABHS developed a physician staffing model with the goal of enriching physician practice and enhancing QI and educational opportunities in its SNF. The model offers an opportunity to improve transitions in care as physicians gain a greater knowledge of both the hospital and subacute clinical settings. This hospitalist rotation model may improve the knowledge necessary for caring for patients moving across care settings, as well as improve communication between settings. It also has served as a foundation for systematic innovation in QI and education at this institution. Clinical staff in the transitional care setting have reported positive effects of this model on clinical skills and patient care, educational opportunities, as well as a desire for replication in other health care systems.
The potential generalizability of this model requires careful consideration. The VABHS is a tertiary care integrated health care system, enabling physicians to work in multiple clinical settings. Other settings may not have the staffing or clinical volume to sustain such a model. In addition, this model may increase discontinuity in patient care as hospitalists move between acute and subacute settings and nonclinical roles. This loss of continuity may be a greater concern in the SNF setting, as the inpatient hospitalist model generally involves high provider turnover as shift work. Our survey included nurse managers, and not floor nurses due to survey administration limitations, and feedback may not have captured a comprehensive view from CLC staff. Moreover, some of the perceived positive impacts also may be related to professional and personal attributes of the physicians rather than the actual model of care. In addition, the survey response rate was 86%. However, the nature of the improvement work (focused on care transitions) and educational opportunities (interprofessional care) would likely not occur had the physicians been based in one clinical setting.
Other new physician staffing models have been designed to improve the continuity between the hospital, subacute, and outpatient settings. For example, the University of Chicago Comprehensive Care model pairs patients with trained hospitalists who provide both inpatient and outpatient care, thereby optimizing continuity between these settings.14 At CareMore Health System, high-risk patients also are paired with hospitalists, referred to as “extensivists,” who lead care teams that follow patients between settings and provide acute, postacute, and outpatient care.15 In these models, a single physician takes responsibility for the patient throughout transitions of care and through various care settings. Both models have shown reduction in hospital readmissions. One concern with such models is that the treatment teams need to coexist in the various settings of care, and the ability to impact and create systematic change within each environment is limited. This may limit QI, educational opportunities, and system level impact within each environment of care.
In comparison, the “transitionalist” model proposed here features hospitalist physicians rotating between the acute inpatient hospital and subacute care with dedicated time in each environment. This innovative organizational structure may enhance physician practice and enrich QI and educational opportunities in SNFs. Further evaluation will include the impact on quality metrics of patient care and patient satisfaction, as this model has the potential to influence quality, cost, and overall health outcomes.
Acknowledgments
We would like to thank Shivani Jindal, Matthew Russell, Matthew Ronan, Juman Hijab, Wei Shen, Sandra Vilbrun-Bruno, and Jack Earnshaw for their significant contributions to this staffing model. We would also like to thank Paul Conlin, Jay Orlander, and the leadership team of Veterans Affairs Boston Healthcare System for supporting this staffing model.
Care transitions between hospitals and skilled nursing facilities (SNFs) are a vulnerable time for patients. The current health care climate of decreasing hospital length of stay, readmission penalties, and increasing patient complexity has made hospital care transitions an important safety concern. Suboptimal transitions across clinical settings can result in adverse events, inadequately controlled comorbidities, deficient patient and caregiver preparation for discharge, medication errors, relocation stress, and overall increased morbidity and mortality.1,2 Such care transitions also may generate unnecessary spending, including avoidable readmissions, emergency department utilization, and duplicative laboratory and imaging studies. Approximately 23% of patients admitted to SNFs are readmitted to acute care hospitals within 30 days, and these patients have increased mortality rates in risk-adjusted analyses. 3,4
Compounding the magnitude of this risk and vulnerability is the significant growth in the number of patients discharged to SNFs over the past 30 years. In 2013, more than 20% of Medicare patients discharged from acute care hospitals were destined for SNFs.5,6 Paradoxically, despite the increasing need for SNF providers, there is a shortage of clinicians with training in geriatrics or nursing home care.7 The result is a growing need to identify organizational systems to optimize physician practice in these settings, enhance quality of care, especially around transitions, and increase educational training opportunities in SNFs for future practitioners.
Many SNFs today are staffed by physicians and other licensed clinicians whose exclusive practice location is the nursing facility or possibly several such facilities. This prevailing model of care can isolate the physicians, depriving them of interaction with clinicians in other specialties, and can contribute to burnout.8 This model does not lend itself to academic scholarship, quality improvement (QI), and student or resident training, as each of these endeavors depends on interprofessional collaboration as well as access to an academic medical center with additional resources.9
Few studies have described innovative hospitalist rotation models from acute to subacute care. The Cleveland Clinic implemented the Connected Care model where hospital-employed physicians and advanced practice professionals integrated into postacute care and reduced the 30-day hospital readmission rate from SNFs from 28% to 22%.10 Goth and colleagues performed a comparative effectiveness trial between a postacute care hospitalist (PACH) model and a community-based physician model of nursing home care. They found that the institution of a PACH model in a nursing home was associated with a significant increase in laboratory costs, nonsignificant reduction in medication errors and pharmacy costs, and no improvement in fall rates.11 The conclusion was that the PACH model may lead to greater clinician involvement and that the potential decrease in pharmacy costs and medications errors may offset the costs associated with additional laboratory testing. Overall, there has been a lack of studies on the impact of these hospitalist rotation models from acute to subacute care on educational programs, QI activities, and the interprofessional environment.
To achieve a system in which physicians in a SNF can excel in these areas, Veterans Affairs Boston Healthcare System (VABHS) adopted a staffing model in which academic hospitalist physicians rotate between the inpatient hospital and subacute settings. This report describes the model structure, the varying roles of the physicians, and early indicators of its positive effects on educational programs, QI activities, and the interprofessional environment.
Methods
The VABHS consists of a 159-bed acute care hospital in West Roxbury, Massachusetts; and a 110-bed SNF in Brockton, Massachusetts, with 3 units: a 65-bed transitional care unit (TCU), a 30-bed long-term care unit, and a 15-bed palliative care/hospice unit. The majority of patients admitted to the SNF are transferred from the acute care hospital in West Roxbury and other regional hospitals. Prior to 2015, the TCU was staffed with full-time clinicians who exclusively practiced in the SNF.
In the new staffing model, 6 hospitalist physicians divide their clinical time between the acute care hospital’s inpatient medical service and the TCU. The hospitalists come from varied backgrounds in terms of years in practice and advanced training (Table 1).
The amount of nonclinical (protected) time and clinical time on the acute inpatient service and the TCU varies for each physician. For example, a physician serves as principal investigator for several major research grants and has a hospital-wide administrative leadership role; as a result, the principal investigator has fewer months of clinical responsibility. Physicians are expected to use the protected time for scholarship, educational program development and teaching, QI, and administrative responsibilities. The VABHS leadership determines the amount of protected time based on individualized benchmarks for research, education, and administrative responsibilities that follow VA national and local institutional guidelines. These metrics and time allocations are negotiated at the time of recruitment and then are reviewed annually.
The TCU also is staffed with 4 full-time clinicians (2 physicians and 2 physician assistants) who provide additional continuity of care. The new hospitalist staffing model only required an approximate 10% increase in TCU clinical staffing full-time equivalents. Patients and admissions are divided equally among clinicians on service (census per clinician 12-15 patients), with redistribution of patients at times of transition from clinical to nonclinical time. Blocks of clinical time are scheduled for greater than 2 weeks at a time to preserve continuity. In addition, the new staffing model allocates assignment of clinical responsibilities that allows for clinicians to take leave without resultant shortages in clinical coverage.
To facilitate communication among physicians serving in the acute inpatient facility and the TCU, leaders of both of these programs meet monthly and ad hoc to review the transitions of care between the 2 settings. The description of this model and its assessment have been reviewed and deemed exempt from oversight by the VA Boston Healthcare System Research and Development Committee.
Results
Since the implementation of this staffing model in 2015, the system has grown considerably in the breadth and depth of educational programming, QI, and systems redesign in the TCU and, more broadly, in the SNF. The TCU, which previously had limited training opportunities, has experienced marked expansion of educational offerings. It is now a site for core general medicine rotations for first-year psychiatry residents and physician assistant students. The TCU also has expanded as a clinical site for transitions-in-care internal medicine resident curricula and electives, as well as a clinical site for a geriatrics fellowship.
A hospitalist developed and implemented a 4-week interprofessional curriculum for all clinical trainees and students, which occurs continuously. The curriculum includes a monthly academic conference and 12 didactic lectures and is taught by 16 interprofessional faculty from the TCU and the Palliative Care/Hospice Unit, including medicine, geriatric and palliative care physicians, physician assistants, social workers, physical and occupational therapists, pharmacists, and a geriatric psychologist. The goal of the curriculum is to provide learners the knowledge, attitudes, and skills necessary to perform effective, efficient, and safe transfers between clinical settings as well as education in transitional care. In addition, using a team of interprofessional faculty, the curriculum develops the interprofessional competencies of teamwork and communication. The curriculum also has provided a significant opportunity for interprofessional collaboration among faculty who have volunteered their teaching time in the development and teaching of the curriculum, with potential for improved clinical staff knowledge of other disciplines.
Quality improvement and system redesign projects in care transitions also have expanded (Table 2).
Early assessment indicates that the new staffing model is having positive effects on the clinical environment of the TCU. A survey was conducted of a convenience sample of all physicians, nurse managers, social workers, and other members of the clinical team in the TCU (N=24)(Table 3), with response categories ranging on a Likert scale from 1 (very negative) to 5 (very positive).
Although not rigorously analyzed using qualitative research methods, comments from respondents have consistently indicated that this staffing model increases the transfer of clinical and logistical knowledge among staff members working in the acute inpatient facility and the TCU.
Discussion
With greater numbers of increasingly complex patients transitioning from the hospital to SNF, health care systems need to expand the capacity of their skilled nursing systems, not only to provide clinical care, but also to support QI and medical education. The VABHS developed a physician staffing model with the goal of enriching physician practice and enhancing QI and educational opportunities in its SNF. The model offers an opportunity to improve transitions in care as physicians gain a greater knowledge of both the hospital and subacute clinical settings. This hospitalist rotation model may improve the knowledge necessary for caring for patients moving across care settings, as well as improve communication between settings. It also has served as a foundation for systematic innovation in QI and education at this institution. Clinical staff in the transitional care setting have reported positive effects of this model on clinical skills and patient care, educational opportunities, as well as a desire for replication in other health care systems.
The potential generalizability of this model requires careful consideration. The VABHS is a tertiary care integrated health care system, enabling physicians to work in multiple clinical settings. Other settings may not have the staffing or clinical volume to sustain such a model. In addition, this model may increase discontinuity in patient care as hospitalists move between acute and subacute settings and nonclinical roles. This loss of continuity may be a greater concern in the SNF setting, as the inpatient hospitalist model generally involves high provider turnover as shift work. Our survey included nurse managers, and not floor nurses due to survey administration limitations, and feedback may not have captured a comprehensive view from CLC staff. Moreover, some of the perceived positive impacts also may be related to professional and personal attributes of the physicians rather than the actual model of care. In addition, the survey response rate was 86%. However, the nature of the improvement work (focused on care transitions) and educational opportunities (interprofessional care) would likely not occur had the physicians been based in one clinical setting.
Other new physician staffing models have been designed to improve the continuity between the hospital, subacute, and outpatient settings. For example, the University of Chicago Comprehensive Care model pairs patients with trained hospitalists who provide both inpatient and outpatient care, thereby optimizing continuity between these settings.14 At CareMore Health System, high-risk patients also are paired with hospitalists, referred to as “extensivists,” who lead care teams that follow patients between settings and provide acute, postacute, and outpatient care.15 In these models, a single physician takes responsibility for the patient throughout transitions of care and through various care settings. Both models have shown reduction in hospital readmissions. One concern with such models is that the treatment teams need to coexist in the various settings of care, and the ability to impact and create systematic change within each environment is limited. This may limit QI, educational opportunities, and system level impact within each environment of care.
In comparison, the “transitionalist” model proposed here features hospitalist physicians rotating between the acute inpatient hospital and subacute care with dedicated time in each environment. This innovative organizational structure may enhance physician practice and enrich QI and educational opportunities in SNFs. Further evaluation will include the impact on quality metrics of patient care and patient satisfaction, as this model has the potential to influence quality, cost, and overall health outcomes.
Acknowledgments
We would like to thank Shivani Jindal, Matthew Russell, Matthew Ronan, Juman Hijab, Wei Shen, Sandra Vilbrun-Bruno, and Jack Earnshaw for their significant contributions to this staffing model. We would also like to thank Paul Conlin, Jay Orlander, and the leadership team of Veterans Affairs Boston Healthcare System for supporting this staffing model.
1. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20(4):317-323.
2. Murtaugh CM, Litke A. Transitions through postacute and long-term care settings: patterns of use and outcomes for a national cohort of elders. Med Care. 2002;40(3):227-236.
3. Burke RE, Whitfield EA, Hittle D, et al. Hospital readmission from post-acute care facilities: risk factors, timing, and outcomes. J Am Med Dir Assoc. 2016;17(3):249-255.
4. Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57-64.
5. Tian W. An all-payer view of hospital discharge to postacute care, 2013: Statistical Brief #205. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.jsp. Published May 2016. Accessed August 13, 2018.
6. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time–measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6.
7. Golden AG, Silverman MA, Mintzer MJ. Is geriatric medicine terminally ill? Ann Intern Med. 2012;156(9):654-656.
8. Nazir A, Smalbrugge M, Moser A, et al. The prevalence of burnout among nursing home physicians: an international perspective. J Am Med Dir Assoc. 2018;19(1):86-88.
9. Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141(7):533-536.
10. Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244.
11. Gloth MF, Gloth MJ. A comparative effectiveness trial between a post-acute care hospitalist model and a community-based physician model of nursing home care. J Am Med Dir Assoc. 2011;12(5):384-386.
12. Baughman AW, Cain G, Ruopp MD, et al. Improving access to care by admission process redesign in a veterans affairs skilled nursing facility. Jt Comm J Qual Patient Saf. 2018;44(8):454-462.
13. Mixon A, Smith GR, Dalal A et al. The Multi-Center Medication Reconciliation Quality Improvement Study 2 (MARQUIS2): methods and implementation. Abstract 248. Present at: Society of Hospital Medicine Annual Meeting; 2018 Apr 8 – 11, 2018; Orlando, FL. https://www.shmabstracts.com/abstract/the-multi-center-medication-reconciliation-quality-improvement-study-2-marquis2-methods-and-implementation. Accessed August 13, 2018.
14. Meltzer DO, Ruhnke GW. Redesigning care for patients at increased hospitalization risk: the comprehensive care physician model. Health Aff (Millwood). 2014;33(5):770-777.
15. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: back to the future? JAMA. 2016;315(1):23-24.
1. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20(4):317-323.
2. Murtaugh CM, Litke A. Transitions through postacute and long-term care settings: patterns of use and outcomes for a national cohort of elders. Med Care. 2002;40(3):227-236.
3. Burke RE, Whitfield EA, Hittle D, et al. Hospital readmission from post-acute care facilities: risk factors, timing, and outcomes. J Am Med Dir Assoc. 2016;17(3):249-255.
4. Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57-64.
5. Tian W. An all-payer view of hospital discharge to postacute care, 2013: Statistical Brief #205. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.jsp. Published May 2016. Accessed August 13, 2018.
6. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time–measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6.
7. Golden AG, Silverman MA, Mintzer MJ. Is geriatric medicine terminally ill? Ann Intern Med. 2012;156(9):654-656.
8. Nazir A, Smalbrugge M, Moser A, et al. The prevalence of burnout among nursing home physicians: an international perspective. J Am Med Dir Assoc. 2018;19(1):86-88.
9. Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141(7):533-536.
10. Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244.
11. Gloth MF, Gloth MJ. A comparative effectiveness trial between a post-acute care hospitalist model and a community-based physician model of nursing home care. J Am Med Dir Assoc. 2011;12(5):384-386.
12. Baughman AW, Cain G, Ruopp MD, et al. Improving access to care by admission process redesign in a veterans affairs skilled nursing facility. Jt Comm J Qual Patient Saf. 2018;44(8):454-462.
13. Mixon A, Smith GR, Dalal A et al. The Multi-Center Medication Reconciliation Quality Improvement Study 2 (MARQUIS2): methods and implementation. Abstract 248. Present at: Society of Hospital Medicine Annual Meeting; 2018 Apr 8 – 11, 2018; Orlando, FL. https://www.shmabstracts.com/abstract/the-multi-center-medication-reconciliation-quality-improvement-study-2-marquis2-methods-and-implementation. Accessed August 13, 2018.
14. Meltzer DO, Ruhnke GW. Redesigning care for patients at increased hospitalization risk: the comprehensive care physician model. Health Aff (Millwood). 2014;33(5):770-777.
15. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: back to the future? JAMA. 2016;315(1):23-24.
Time-to-Surgery for Definitive Fixation of Hip Fractures: A Look at Outcomes Based Upon Delay
ABSTRACT
The morbidity and mortality after hip fracture in the elderly are influenced by non-modifiable comorbidities. Time-to-surgery is a modifiable factor that may play a role in postoperative morbidity. This study investigates the outcomes and complications in the elderly hip fracture surgery as a function of time-to-surgery.
Using the American College of Surgeons-National Surgical Quality Improvement Program data from 2011 to 2012, a study population was generated using the Current Procedural Terminology codes for percutaneous or open treatment of femoral neck fractures (27235, 27236) and fixation with a screw and side plate or intramedullary fixation (27244, 27245) for peritrochanteric fractures. Three time-to-surgery groups (<24 hours to surgical intervention, 24-48 hours, and >48 hours) were created and matched for surgery type, sex, age, and American Society of Anesthesiologists class. Time-to-surgery was then studied for its effect on the post-surgical outcomes using the adjusted regression modeling.
A study population of 6036 hip fractures was created, and 2012 patients were assigned to each matched time-to-surgery group. The unadjusted models showed that the earlier surgical intervention groups (<24 hours and 24-48 hours) exhibited a lower overall complication rate (P = .034) compared with the group waiting for surgery >48 hours. The unadjusted mortality rates increased with delay to surgical intervention (P = .039). Time-to-surgery caused no effect on the return to the operating room rate (P = .554) nor readmission rate (P = .285). Compared with other time-to-surgeries, the time-to-surgery of >48 hours was associated with prolonged total hospital length of stay (10.9 days) (P < .001) and a longer surgery-to-discharge time (hazard ratio, 95% confidence interval: 0.74, 0.69-0.79) (P < .001). Adjusted analyses showed no time-to-surgery related difference in complications (P = .143) but presented an increase in the total length of stay (P < .001) and surgery-to-discharge time (P < .001).
Timeliness of surgical intervention in a comorbidity-adjusted population of elderly hip fracture patients causes no effect on the overall complications, readmissions, nor 30-day mortality. However, time-to-surgery of >48 hours is associated with costly increase in the total length of stay, including an increased post-surgery-to-discharge time.
Continue to: Despite the best efforts to optimize surgical care...
Despite the best efforts to optimize surgical care and postoperative rehabilitation following hip fracture, elderly patients feature alarmingly high in-hospital and 1-year mortality rates of 4.35% to 9.2%1-4 and 36%,5 respectively. Those who survive are unlikely to return to independent living, with only 17% of the patients following hip fracture being able to walk independently 6 months postoperatively, and 12% being able to climb stairs6. Possibly, these poor outcomes reflect a preoperative medical comorbidity burden rather than a measure of medical or surgical quality. Given the absence of consensus regarding optimal time-to-surgery, treating physicians often opt to delay surgical intervention for the purposes of medically optimizing highly comorbid patients without significant data to suggest clinical benefit of such practice.
Numerous investigators have attempted to identify the modifiable risk factors for complication after surgical care of elderly hip fracture patients. However, consensus guidelines of care are missing. This condition is largely due to the difficulties in effectively modifying preoperative demographic and medical comorbidities on a semi-urgent basis. However, timeliness to surgery is one area for study that the care team can affect. Although time-to-surgery is dependent on multiple factors, including time of presentation, day of week of admission, difficulties with scheduling, and administrative delays, the care team plays a role in hastening or retarding time-to-surgery. Several studies have considered various time cut-offs (24, 48, 72, and 120 hours) to define early intervention, but none have defined a specific role for early or delayed surgery. Several investigators have discovered a positive association between delayed time-to-surgery and mortality;4,8-14 however, the most rigorously conducted studies that stringently control for preoperative comorbidities and demographics conclude that variance in time-to-surgery causes no effect on the in-hospital or 1-year mortality risk.1-3,15-18
Other investigators have shown that with early surgical intervention for hip fracture, patients experience shorter hospital stays,1,3,16,17,19-22 less days in pain,19 decreased risk of decubitus ulcers,15,17,19,22 and an increased likelihood of independence following fracture,22-25 regardless of preoperative medical status. Despite this evidence of improved outcomes with early surgery, 40% to 54% of hip fracture patients in the United States experience surgical delays of more than 24 to 48 hours. Additionally, with the recent (2013) national estimates of cost per day spent in the hospital falling between $1791 to $2289,26 minimizing the days spent in the hospital would likely lead to significant cost-savings, presuming no adverse effect on health-related outcomes. To this end, we hypothesize that the value (outcomes per associated cost)7 of hip fracture surgical care can be positively influenced by minimizing surgical wait-times. We assessed the effect of early surgical intervention, within 24 or 48 hours of presentation, on 30-day mortality, postoperative morbidity, hospital length of stay, and readmission rates in a comorbidity-adjusted population from a nationally representative cohort.
Continue to: METHODS AND MATERIALS...
METHODS AND MATERIALS
This study used the data from the American College of Surgeon-National Surgical Quality Improvement Program (ACS-NSQIP) database. With over 258 participating hospitals, this database has been widely used to identify national trends in various surgical specialties.27-34 The database includes information from participants in 43 states with hospitals ranging from rural community hospitals to large academic centers. Each site employs surgical clinical reviewers who are rigorously trained to collect data through chart review and discussion with the treating surgeon and/or patient,35 allowing for the use of robust and quality data with proven inter-rater reliability.36,37
Using the 2011 to 2012 NSQIP database, we used primary Current Procedural Terminology codes to identify all patients who underwent percutaneous (27235) or open (27236) fixation of femoral neck fractures; and fixation with a screw and side plate (27244) or intramedullary fixation (27245) for peritrochanteric fractures. The sample was divided into 3 time-to-surgery groups (<24 hours from presentation to surgery, 24-48 hours, and >48 hours) which were matched for fracture type (femoral neck or peritrochanteric), sex, age (under 75 years or ≥75 years), and American Society of Anesthesiologists (ASA) class used as a surrogate for severity of medical infirmary. The subjects were randomly matched 1:1:1 to create 3 statistically equivalent time-to-surgery groups using Proc SurveySelect (SAS version 9.2, SAS Institute).
Generalized linear models using logit link function for binary variables and identity link function for normally distributed characteristics were used to compare the 3 time-to-surgery groups. Descriptive statistics are presented as counts and percentages or least-square means with standard deviations. Preoperative lab values that were not normally distributed were log transformed and presented in their original scales with median values and 25th to 75th percentiles. Outcomes were similarly modeled.
Total hospital stay was modeled with a negative binomial distribution. Proportional hazards models were used to model the time from operating room (OR) to discharge, censoring patients who died before discharge, with results presented as hazard ratios (HR) and 95% confidence intervals (CI) (Figure). The assumption of the proportional hazards was tested using a Wald test. Using this model, a HR of <1 denotes a longer postoperative hospital stay, as a longer hospital stay decreases the “risk” for discharge.
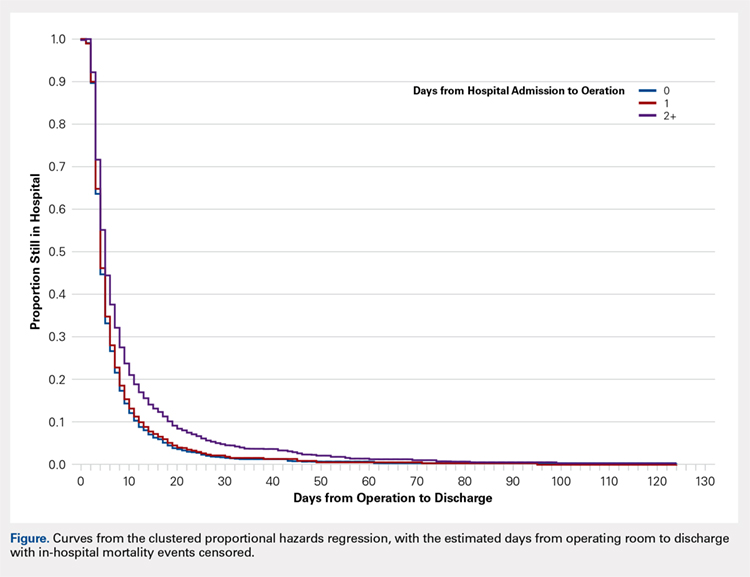
All models were adjusted for confounders, including race, body mass index (BMI), hypertension, chronic obstructive pulmonary disease, cancer, bleeding disorders, transfusion within 72 hours before surgery, preoperative levels of creatinine, platelet count, white blood cells (WBCs), hematocrit anesthesia type, and wound infection. These covariates were selected based upon their observed relationship to the studied outcomes and time-to-surgery groups, and were evaluated across the models for all outcomes for consistency and clarity. All statistical analyses were run at a type I error rate of 5% and performed in SAS version 9.2 software.
Continue to: RESULTS...
RESULTS
A study population of 6036 hip fractures was identified and divided into 3 groups of 2012 subjects each based upon time-to-surgery. The groups were successfully matched for surgery type, age (≥75 years old), gender, and ASA class. In each group, 594 of the 2012 (29.5%) patients were male, 1525 (75.8%) were ≥75 years of age, 9 (.5%) were ASA Class I, 269 (13.4%) were ASA Class II, 1424 (70.8%) were ASA class III, and 309 (15.4%) were ASA class IV.
Significant differences in preoperative comorbidity burden and preoperative lab values were identified between the 3 cohorts. Increased time-to-surgery was associated with differences in race (P < .001), elevated BMI (P = .010), higher rates of congestive heart failure (P < .001), hypertension medication (P = .020), bleeding disorders (P < .001), blood transfusion within 72 hours of surgery (P < .001), and systemic sepsis (P = .001). Delay to surgery was also associated with lower preoperative sodium (P = .005), blood urea nitrogen (P = .013), serum WBC (P < .001), hematocrit (P < .001), and platelets (P < .001) (Table 1).
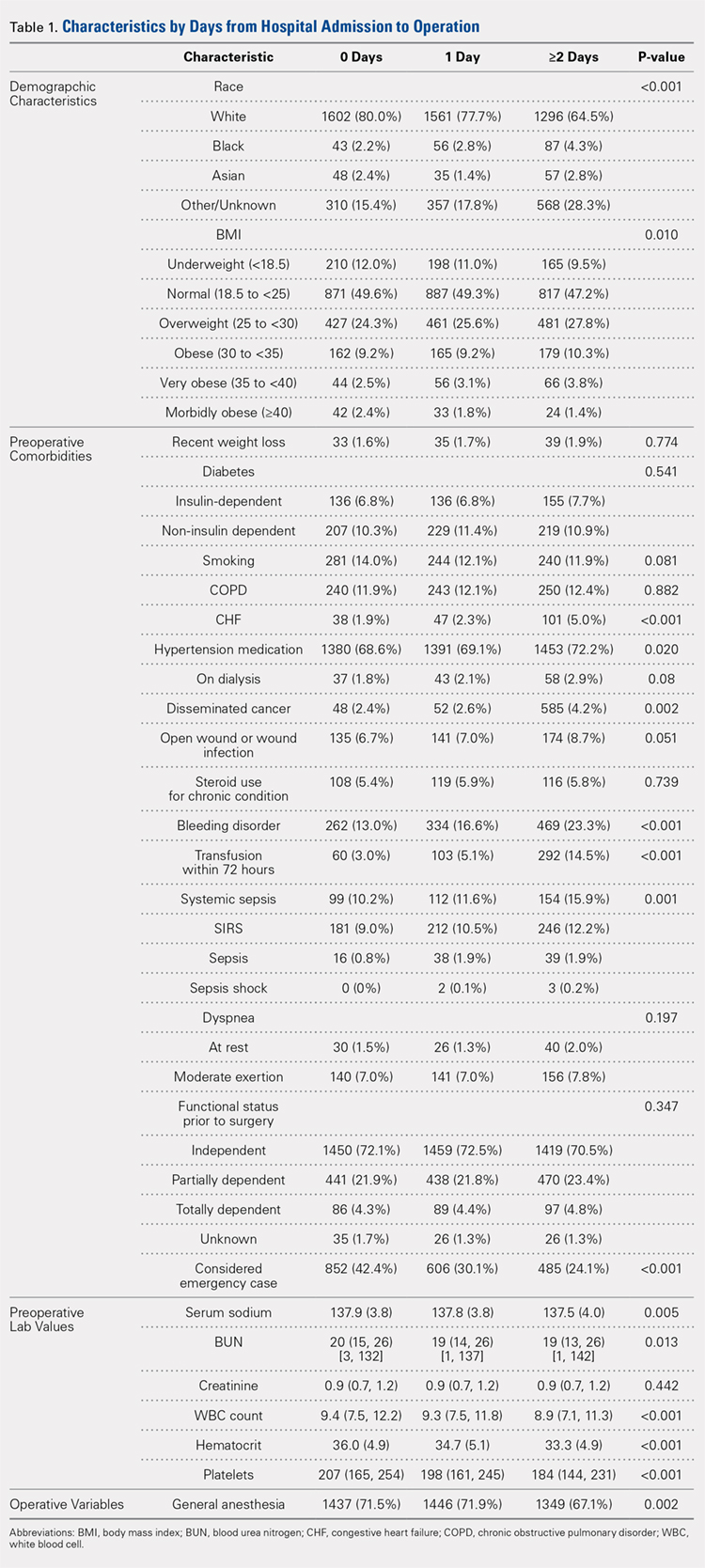
The unadjusted analyses revealed no association between time-to-surgery and return to OR (P = .554) nor readmission (P = .285). However, increasing time-to-surgery was associated with an increase in overall complications (P = .034), total length of hospital stay (P < .001), and 30-day mortality (P = .039) (Table 2).
Table 2. Estimated Event Rates from Matched Cohorts (Unadjusted)
| Time From Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 15.30% | 15.30% | 17.90% | 0.034 |
Total length of stay | 5.4 | 6.7 | 10.9 | <0.001 |
(mean days, 95% confidence interval) | (5.2, 5.7) | (6.5, 7.0) | (10.3, 11.5) | |
Time from OR to discharge | -ref- | 0.96 | 0.74 | <0.001 |
(Hazard ratio) | (0.90,1.02) | (0.69, 0.79) | ||
Return to OR | 2.40% | 2.40% | 2.00% | 0.554 |
Readmission | 9.60% | 8.40% | 8.30% | 0.285 |
30-day mortality rate | 5.80% | 5.30% | 7.20% | 0.039 |
Abbreviation: OR, operating room.
The adjusted analysis controlling for preoperative demographic and comorbidity variables revealed trends toward the increased overall complications and 30-day mortality with increased time-to-surgery; these trends showed no statistical significance (P = .143 and P = .08). No statistical relationship was observed between return to OR nor readmission and time-to-surgery. Increasing time-to-surgery remained significantly associated with the increased total length of hospital stay (P < .001). The adjusted analysis also revealed that the delay of >48 hours in time-to-surgery resulted in a longer surgery-to-discharge time (P < .001) (Table 3). No evidence of violation of the proportional hazards assumption was observed in the unadjusted nor adjusted clustered proportional hazards models (Wald test, P = .27 and P = .25, respectively).
Table 3. Estimated Event Rates from Matched Cohorts (Adjusteda)
| Time from Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 11.70% | 10.70% | 12.60% | 0.143 |
Total length of stay | 4.2 | 5.1 | 7.6 | <0.001 |
(mean days, 95% confidence interval) | (4.0, 4.5) | (4.8, 5.5) | (7.1, 8.3) | |
Time from OR to discharge | -ref- | 1.03 | 0.87 | <0.001 |
(Hazard ratio) | (0.97, 1.09) | (0.81, 0.92) | ||
Return to OR | 2.10% | 2.10% | 1.60% | 0.541 |
Readmission | 7.20% | 6.40% | 6.00% | 0.304 |
30-day mortality rate | 4.20% | 3.70% | 5.20% | 0.08 |
aModel adjusted for race, hypertension medication, cancer, bleeding disorders, transfusion within 72 hours before surgery, emergency status, wound infection, anesthesia type (general), body mass index (18.5-25), history of chronic obstructive pulmonary disease, and preoperative levels of creatinine, platelet count, white blood cell count, and hematocrit.
Continue to: DISCUSSION...
DISCUSSION
Previous research has demonstrated an association between age,3,4,25 comorbidity burden,1,3,25 gender,3,4 and ASA class4,18,21 with outcomes following hip fractures and serves as the basis of our matched analysis statistical methodology in assessing the effect of time-to-surgery on the outcome following hip fracture surgery. Prior investigators have also established the positive correlation between increased preoperative comorbidity burden and delay in time-to-surgery.10,15 This finding was confirmed in our unadjusted comparison of 3 time-to-surgery groups. However, prior investigations have not established a clear association between time-to-surgical intervention and postoperative morbidity and mortality.1,15,16,18,20,38 This study utilized a nationally representative dataset known for its data integrity and from which 6036 patients with surgically treated hip fractures, matched for surgery type, age, gender, and ASA class (a surrogate for severity of medical infirmary), were studied using adjusted regression modeling to afford an isolated statistical assessment of the effect of time-to-surgery on outcomes following hip fracture surgery.
Despite a large sample size and rigorous statistical methodology, for many outcome measures, our results show no support for the early or late operative intervention following hip fracture. We found no difference in 30-day mortality, readmission rate, nor total complication rate between the 3 time-to-surgery cohorts. This result indicates that the care of elderly patients following hip fracture is inherently complicated and that perioperative complication risk is probably only modestly modifiable by best medical practices, including optimizing time from clinical presentation to surgery.
As expected, patients who experienced longer delays from presentation to surgery were on average, more comorbid and more likely to yield abnormal preoperative lab values. However, in the adjusted analysis, delay in time-to-surgery, presumably for medical management, was not found to be associated with improved outcomes. In the same adjusted analysis, we uniquely identified that in the patients whose surgeries were delayed for more than 48 hours, the time from surgery-to-discharge was significantly increased. As a result, these patients spent extra days in the hospital both preoperatively and postoperatively, but without any corollary improvement in the outcomes.
Continue to: Recent estimates of the cost of hospital admission...
Recent estimates of the cost of hospital admission is approximated nationally at $2000/day.26 Although our data fail to support the formal cost-analysis of the effect of time-to-surgery in hip fracture care, a simple value-based analysis indicates that quality is preserved (no difference in outcome), whereas costly hospital days are eliminated with earlier surgery. The value in elderly hip fracture care. defined as the outcomes relative to the costs,7 is ultimately optimized by earlier time-to-surgery.
Although using a large, multi-institutional database is advantageous for finding population-based trends that are representative of a large cohort, using the ACS-NSQIP database features its limitations. Our analysis was limited to the defined scope of NSQIP and nature of the injury, whereas root cause for delay was not available for study. We were unable to identify which patients were delayed for administrative reasons or surgical convenience and which were delayed for medical optimization. Participation in the ACS-NSQIP database is voluntary, and no randomized hospital sampling was conducted. Participating hospitals were de-identified in the database. As expected, we were unable to identify the specific institution-based hip fracture protocols that may affect the outcomes following treatment for these fractures. Further, socioeconomic information and payer-status are unavailable for the study. Additionally, observations are limited to 30 days postoperative, and we cannot comment on longer-term outcomes. Finally, discharge disposition and functional outcome data are not represented, and we were unable to correlate time-to-surgery and functional recovery. However, previous studies have established that delay in time-to-surgery following hip fractures is negatively correlated with functional outcomes.22-25
Nevertheless, the ACS-NSQIP database remains one of the largest American surgical databases available, and includes care centers from nearly every state with variable demographics including rural, urban, and academic centers. The ACS performs broad-based inter-rater reliability audits on every participating site and has found an overall disagreement rate of only 1.8%. As such, although discrepancies exist between the complete patient chart and the data entered, the data found in the ACS-NSQIP database are reliable and considered a valid source of study.34,35 The large sample size, quality of data collection, wide geographic representation, and varied hospital types within the dataset possibly make our findings relevant in the majority of American healthcare settings.
CONCLUSION
This study demonstrates an associated increased length of hospital stay, including the increased time from surgery-to-discharge, in patients with hip fractures whose surgical intervention is delayed for >48 hours after presentation. Given the prior evidence that early surgical intervention improves the functional outcomes and the current evidence that surgical delay for any cause increases costly hospital length of stay without corollary improvement in the outcomes, a value-based assessment of hip fracture care argues for early surgical intervention whenever possible. Our findings should inform physician, institution, and policy maker value-based decision making regarding the best practices in geriatric hip fracture care.
1. Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233. doi:10.7326/0003-4819-155-4-201108160-00006.
2. Verbeek DO, Ponsen KJ, Goslings JC, Heetveld MJ. Effect of surgical delay on outcome in hip fracture patients: a retrospective multivariate analysis of 192 patients. Int Orthop. 2008;32(1):13-18. doi:10.1007/s00264-006-0290-9.
3. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927. doi:10.1302/0301-620X.91B7.22446.
4. Uzoigwe CE, Burnand HG, Cheesman CL, Aghedo DO, Faizi M, Middleton RG. Early and ultra-early surgery in hip fracture patients improves survival. Injury. 2013;44(6):726-729. doi:10.1016/j.injury.2012.08.025.
5. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525. doi:10.1056/NEJM199606063342307.
6. Marottoli RA, Berkman LF, Cooney LM Jr. Decline in physical function following hip fracture. J Am Geriatr Soc. 1992;40(9):861-866. doi:10.1111/j.1532-5415.1992.tb01980.x.
7. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481. doi:10.1056/NEJMp1011024.
8. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42. doi:10.2106/JBJS.G.00065.
9. Novack V, Jotkowitz A, Etzion O, Porath A. Does delay in surgery after hip fracture lead to worse outcomes? A multicenter survey. Int J Qual Health Care. 2007;19(3):170-176. doi:10.1093/intqhc/mzm003.
10. Weller I, Wai EK, Jaglal S, Kreder HJ. The effect of hospital type and surgical delay on mortality after surgery for hip fracture. J Bone Joint Surg Br. 2005;87(3):361-366. doi:10.1302/0301-620X.87B3.15300.
11. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489. doi:10.2106/JBJS.D.01796.
12. Holt G, Smith R, Duncan K, McKeown DW. Does delay to theatre for medical reasons affect the peri-operative mortality in patients with a fracture of the hip? J Bone Joint Surg Br. 2010;92(6):835-841. doi:10.1302/0301-620X.92B6.24463.
13. Pioli G, Lauretani F, Davoli ML, et al. Older people with hip fracture and IADL disability require earlier surgery. J Gerontol A Biol Sci Med Sci. 2012;67(11):1272-1277. doi:10.1093/gerona/gls097.
14. Mackenzie DG, Wild S, Muir R. Mortality associated with delay in operation after hip fracture: Scottish data provide additional information. BMJ. 2006;332(7549):1093. doi:10.1136/bmj.332.7549.1093.
15. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709. doi:10.1016/S0002-9343(02)01119-1.
16. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559. doi:10.1097/01.mlr.0000215812.13720.2e.
17. Hommel A, Ulander K, Bjorkelund KB, Norrman PO, Wingstrand H, Thorngren KG. Influence of optimised treatment of people with hip fracture on time to operation, length of hospital stay, reoperations and mortality within 1 year. Injury. 2008;39(10):1164-1174. doi:10.1016/j.injury.2008.01.048.
18. Rae HC, Harris IA, McEvoy L, Todorova T. Delay to surgery and mortality after hip fracture. ANZ J Surg. 2007;77(10):889-891. doi:10.1111/j.1445-2197.2007.04267.x.
19. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743. doi:10.1001/jama.291.14.1738.
20. Bergeron E, Lavoie A, Moore L, et al. Is the delay to surgery for isolated hip fracture predictive of outcome in efficient systems? J Trauma. 2006;60(4):753-757. doi:10.1097/01.ta.0000214649.53190.2a.
21. Siegmeth AW, Gurusamy K, Parker MJ. Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur. J Bone Joint Surg Br. 2005;87(8):1123-1126. doi:10.1302/0301-620X.87B8.16357.
22. Al-Ani AN, Samuelsson B, Tidermark J, et al. Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. J Bone Joint Surg Am. 2008;90(7):1436-1442. doi:10.2106/JBJS.G.00890.
23. Hoenig H, Rubenstein LV, Sloane R, Horner R, Kahn K. What is the role of timing in the surgical and rehabilitative care of community-dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157(5):513-520.
24. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. doi:10.1016/j.archger.2004.03.004.
25. Yonezawa T, Yamazaki K, Atsumi T, Obara S. Influence of the timing of surgery on mortality and activity of hip fracture in elderly patients. J Orthop Sci Off J Jpn Orthop Assoc. 2009;14(5):566-573. doi:10.1007/s00776-009-1380-5.
26. Henry J Kaiser Family Foundation. Hospital adjusted expenses per inpatient day by ownership. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 14, 2013.
27. Belmont PJ Jr, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg. 2011;213(3):370-378. doi:10.1016/j.jamcollsurg.2011.05.019.
28. Davis SS Jr, Husain FA, Lin E, Nandipati KC, Perez S, Sweeney JF. Resident participation in index laparoscopic general surgical cases: impact of the learning environment on surgical outcomes. J Am Coll Surg. 2013;216(1):96-104. doi:10.1016/j.jamcollsurg.2012.08.014.
29. Gart MS, Smetona JT, Hanwright PJ, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2013;216(2):229-238. doi:10.1016/j.jamcollsurg.2012.11.003.
30. Greenblatt DY, Rajamanickam V, Pugely AJ, Heise CP, Foley EF, Kennedy GD. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg. 2011;212(5):844-854. doi:10.1016/j.jamcollsurg.2011.01.005.
31. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199. doi:10.2106/JBJS.K.01682.
32. Rao RD. Risk factors for complications and mortality after spine surgery assessed with the NSQIP database: where do we go from here? Commentary on an article by Andrew J Schoenfeld, MD, et al.: "Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program". J Bone Joint Surg Am. 2011;93(17):e101:(101-102). doi:10.2106/JBJS.K.00786.
33. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/JBJS.I.00735.
34. Tsilimparis N, Perez S, Dayama A, Ricotta JJ 2nd. Age-stratified results from 20,095 aortoiliac aneurysm repairs: should we approach octogenarians and nonagenarians differently? J Am Coll Surg. 2012;215(5):690-701. doi:10.1016/j.jamcollsurg.2012.06.411.
35. ACS National Surgical Quality Improvement Program. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip?. Accessed March 14, 2013.
36. Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg. 2009;198(5 Suppl):S19-S27. doi:10.1016/j.amjsurg.2009.07.025.
37. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
38. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697. doi:10.1016/j.injury.2009.01.010.
ABSTRACT
The morbidity and mortality after hip fracture in the elderly are influenced by non-modifiable comorbidities. Time-to-surgery is a modifiable factor that may play a role in postoperative morbidity. This study investigates the outcomes and complications in the elderly hip fracture surgery as a function of time-to-surgery.
Using the American College of Surgeons-National Surgical Quality Improvement Program data from 2011 to 2012, a study population was generated using the Current Procedural Terminology codes for percutaneous or open treatment of femoral neck fractures (27235, 27236) and fixation with a screw and side plate or intramedullary fixation (27244, 27245) for peritrochanteric fractures. Three time-to-surgery groups (<24 hours to surgical intervention, 24-48 hours, and >48 hours) were created and matched for surgery type, sex, age, and American Society of Anesthesiologists class. Time-to-surgery was then studied for its effect on the post-surgical outcomes using the adjusted regression modeling.
A study population of 6036 hip fractures was created, and 2012 patients were assigned to each matched time-to-surgery group. The unadjusted models showed that the earlier surgical intervention groups (<24 hours and 24-48 hours) exhibited a lower overall complication rate (P = .034) compared with the group waiting for surgery >48 hours. The unadjusted mortality rates increased with delay to surgical intervention (P = .039). Time-to-surgery caused no effect on the return to the operating room rate (P = .554) nor readmission rate (P = .285). Compared with other time-to-surgeries, the time-to-surgery of >48 hours was associated with prolonged total hospital length of stay (10.9 days) (P < .001) and a longer surgery-to-discharge time (hazard ratio, 95% confidence interval: 0.74, 0.69-0.79) (P < .001). Adjusted analyses showed no time-to-surgery related difference in complications (P = .143) but presented an increase in the total length of stay (P < .001) and surgery-to-discharge time (P < .001).
Timeliness of surgical intervention in a comorbidity-adjusted population of elderly hip fracture patients causes no effect on the overall complications, readmissions, nor 30-day mortality. However, time-to-surgery of >48 hours is associated with costly increase in the total length of stay, including an increased post-surgery-to-discharge time.
Continue to: Despite the best efforts to optimize surgical care...
Despite the best efforts to optimize surgical care and postoperative rehabilitation following hip fracture, elderly patients feature alarmingly high in-hospital and 1-year mortality rates of 4.35% to 9.2%1-4 and 36%,5 respectively. Those who survive are unlikely to return to independent living, with only 17% of the patients following hip fracture being able to walk independently 6 months postoperatively, and 12% being able to climb stairs6. Possibly, these poor outcomes reflect a preoperative medical comorbidity burden rather than a measure of medical or surgical quality. Given the absence of consensus regarding optimal time-to-surgery, treating physicians often opt to delay surgical intervention for the purposes of medically optimizing highly comorbid patients without significant data to suggest clinical benefit of such practice.
Numerous investigators have attempted to identify the modifiable risk factors for complication after surgical care of elderly hip fracture patients. However, consensus guidelines of care are missing. This condition is largely due to the difficulties in effectively modifying preoperative demographic and medical comorbidities on a semi-urgent basis. However, timeliness to surgery is one area for study that the care team can affect. Although time-to-surgery is dependent on multiple factors, including time of presentation, day of week of admission, difficulties with scheduling, and administrative delays, the care team plays a role in hastening or retarding time-to-surgery. Several studies have considered various time cut-offs (24, 48, 72, and 120 hours) to define early intervention, but none have defined a specific role for early or delayed surgery. Several investigators have discovered a positive association between delayed time-to-surgery and mortality;4,8-14 however, the most rigorously conducted studies that stringently control for preoperative comorbidities and demographics conclude that variance in time-to-surgery causes no effect on the in-hospital or 1-year mortality risk.1-3,15-18
Other investigators have shown that with early surgical intervention for hip fracture, patients experience shorter hospital stays,1,3,16,17,19-22 less days in pain,19 decreased risk of decubitus ulcers,15,17,19,22 and an increased likelihood of independence following fracture,22-25 regardless of preoperative medical status. Despite this evidence of improved outcomes with early surgery, 40% to 54% of hip fracture patients in the United States experience surgical delays of more than 24 to 48 hours. Additionally, with the recent (2013) national estimates of cost per day spent in the hospital falling between $1791 to $2289,26 minimizing the days spent in the hospital would likely lead to significant cost-savings, presuming no adverse effect on health-related outcomes. To this end, we hypothesize that the value (outcomes per associated cost)7 of hip fracture surgical care can be positively influenced by minimizing surgical wait-times. We assessed the effect of early surgical intervention, within 24 or 48 hours of presentation, on 30-day mortality, postoperative morbidity, hospital length of stay, and readmission rates in a comorbidity-adjusted population from a nationally representative cohort.
Continue to: METHODS AND MATERIALS...
METHODS AND MATERIALS
This study used the data from the American College of Surgeon-National Surgical Quality Improvement Program (ACS-NSQIP) database. With over 258 participating hospitals, this database has been widely used to identify national trends in various surgical specialties.27-34 The database includes information from participants in 43 states with hospitals ranging from rural community hospitals to large academic centers. Each site employs surgical clinical reviewers who are rigorously trained to collect data through chart review and discussion with the treating surgeon and/or patient,35 allowing for the use of robust and quality data with proven inter-rater reliability.36,37
Using the 2011 to 2012 NSQIP database, we used primary Current Procedural Terminology codes to identify all patients who underwent percutaneous (27235) or open (27236) fixation of femoral neck fractures; and fixation with a screw and side plate (27244) or intramedullary fixation (27245) for peritrochanteric fractures. The sample was divided into 3 time-to-surgery groups (<24 hours from presentation to surgery, 24-48 hours, and >48 hours) which were matched for fracture type (femoral neck or peritrochanteric), sex, age (under 75 years or ≥75 years), and American Society of Anesthesiologists (ASA) class used as a surrogate for severity of medical infirmary. The subjects were randomly matched 1:1:1 to create 3 statistically equivalent time-to-surgery groups using Proc SurveySelect (SAS version 9.2, SAS Institute).
Generalized linear models using logit link function for binary variables and identity link function for normally distributed characteristics were used to compare the 3 time-to-surgery groups. Descriptive statistics are presented as counts and percentages or least-square means with standard deviations. Preoperative lab values that were not normally distributed were log transformed and presented in their original scales with median values and 25th to 75th percentiles. Outcomes were similarly modeled.
Total hospital stay was modeled with a negative binomial distribution. Proportional hazards models were used to model the time from operating room (OR) to discharge, censoring patients who died before discharge, with results presented as hazard ratios (HR) and 95% confidence intervals (CI) (Figure). The assumption of the proportional hazards was tested using a Wald test. Using this model, a HR of <1 denotes a longer postoperative hospital stay, as a longer hospital stay decreases the “risk” for discharge.

All models were adjusted for confounders, including race, body mass index (BMI), hypertension, chronic obstructive pulmonary disease, cancer, bleeding disorders, transfusion within 72 hours before surgery, preoperative levels of creatinine, platelet count, white blood cells (WBCs), hematocrit anesthesia type, and wound infection. These covariates were selected based upon their observed relationship to the studied outcomes and time-to-surgery groups, and were evaluated across the models for all outcomes for consistency and clarity. All statistical analyses were run at a type I error rate of 5% and performed in SAS version 9.2 software.
Continue to: RESULTS...
RESULTS
A study population of 6036 hip fractures was identified and divided into 3 groups of 2012 subjects each based upon time-to-surgery. The groups were successfully matched for surgery type, age (≥75 years old), gender, and ASA class. In each group, 594 of the 2012 (29.5%) patients were male, 1525 (75.8%) were ≥75 years of age, 9 (.5%) were ASA Class I, 269 (13.4%) were ASA Class II, 1424 (70.8%) were ASA class III, and 309 (15.4%) were ASA class IV.
Significant differences in preoperative comorbidity burden and preoperative lab values were identified between the 3 cohorts. Increased time-to-surgery was associated with differences in race (P < .001), elevated BMI (P = .010), higher rates of congestive heart failure (P < .001), hypertension medication (P = .020), bleeding disorders (P < .001), blood transfusion within 72 hours of surgery (P < .001), and systemic sepsis (P = .001). Delay to surgery was also associated with lower preoperative sodium (P = .005), blood urea nitrogen (P = .013), serum WBC (P < .001), hematocrit (P < .001), and platelets (P < .001) (Table 1).

The unadjusted analyses revealed no association between time-to-surgery and return to OR (P = .554) nor readmission (P = .285). However, increasing time-to-surgery was associated with an increase in overall complications (P = .034), total length of hospital stay (P < .001), and 30-day mortality (P = .039) (Table 2).
Table 2. Estimated Event Rates from Matched Cohorts (Unadjusted)
| Time From Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 15.30% | 15.30% | 17.90% | 0.034 |
Total length of stay | 5.4 | 6.7 | 10.9 | <0.001 |
(mean days, 95% confidence interval) | (5.2, 5.7) | (6.5, 7.0) | (10.3, 11.5) | |
Time from OR to discharge | -ref- | 0.96 | 0.74 | <0.001 |
(Hazard ratio) | (0.90,1.02) | (0.69, 0.79) | ||
Return to OR | 2.40% | 2.40% | 2.00% | 0.554 |
Readmission | 9.60% | 8.40% | 8.30% | 0.285 |
30-day mortality rate | 5.80% | 5.30% | 7.20% | 0.039 |
Abbreviation: OR, operating room.
The adjusted analysis controlling for preoperative demographic and comorbidity variables revealed trends toward the increased overall complications and 30-day mortality with increased time-to-surgery; these trends showed no statistical significance (P = .143 and P = .08). No statistical relationship was observed between return to OR nor readmission and time-to-surgery. Increasing time-to-surgery remained significantly associated with the increased total length of hospital stay (P < .001). The adjusted analysis also revealed that the delay of >48 hours in time-to-surgery resulted in a longer surgery-to-discharge time (P < .001) (Table 3). No evidence of violation of the proportional hazards assumption was observed in the unadjusted nor adjusted clustered proportional hazards models (Wald test, P = .27 and P = .25, respectively).
Table 3. Estimated Event Rates from Matched Cohorts (Adjusteda)
| Time from Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 11.70% | 10.70% | 12.60% | 0.143 |
Total length of stay | 4.2 | 5.1 | 7.6 | <0.001 |
(mean days, 95% confidence interval) | (4.0, 4.5) | (4.8, 5.5) | (7.1, 8.3) | |
Time from OR to discharge | -ref- | 1.03 | 0.87 | <0.001 |
(Hazard ratio) | (0.97, 1.09) | (0.81, 0.92) | ||
Return to OR | 2.10% | 2.10% | 1.60% | 0.541 |
Readmission | 7.20% | 6.40% | 6.00% | 0.304 |
30-day mortality rate | 4.20% | 3.70% | 5.20% | 0.08 |
aModel adjusted for race, hypertension medication, cancer, bleeding disorders, transfusion within 72 hours before surgery, emergency status, wound infection, anesthesia type (general), body mass index (18.5-25), history of chronic obstructive pulmonary disease, and preoperative levels of creatinine, platelet count, white blood cell count, and hematocrit.
Continue to: DISCUSSION...
DISCUSSION
Previous research has demonstrated an association between age,3,4,25 comorbidity burden,1,3,25 gender,3,4 and ASA class4,18,21 with outcomes following hip fractures and serves as the basis of our matched analysis statistical methodology in assessing the effect of time-to-surgery on the outcome following hip fracture surgery. Prior investigators have also established the positive correlation between increased preoperative comorbidity burden and delay in time-to-surgery.10,15 This finding was confirmed in our unadjusted comparison of 3 time-to-surgery groups. However, prior investigations have not established a clear association between time-to-surgical intervention and postoperative morbidity and mortality.1,15,16,18,20,38 This study utilized a nationally representative dataset known for its data integrity and from which 6036 patients with surgically treated hip fractures, matched for surgery type, age, gender, and ASA class (a surrogate for severity of medical infirmary), were studied using adjusted regression modeling to afford an isolated statistical assessment of the effect of time-to-surgery on outcomes following hip fracture surgery.
Despite a large sample size and rigorous statistical methodology, for many outcome measures, our results show no support for the early or late operative intervention following hip fracture. We found no difference in 30-day mortality, readmission rate, nor total complication rate between the 3 time-to-surgery cohorts. This result indicates that the care of elderly patients following hip fracture is inherently complicated and that perioperative complication risk is probably only modestly modifiable by best medical practices, including optimizing time from clinical presentation to surgery.
As expected, patients who experienced longer delays from presentation to surgery were on average, more comorbid and more likely to yield abnormal preoperative lab values. However, in the adjusted analysis, delay in time-to-surgery, presumably for medical management, was not found to be associated with improved outcomes. In the same adjusted analysis, we uniquely identified that in the patients whose surgeries were delayed for more than 48 hours, the time from surgery-to-discharge was significantly increased. As a result, these patients spent extra days in the hospital both preoperatively and postoperatively, but without any corollary improvement in the outcomes.
Continue to: Recent estimates of the cost of hospital admission...
Recent estimates of the cost of hospital admission is approximated nationally at $2000/day.26 Although our data fail to support the formal cost-analysis of the effect of time-to-surgery in hip fracture care, a simple value-based analysis indicates that quality is preserved (no difference in outcome), whereas costly hospital days are eliminated with earlier surgery. The value in elderly hip fracture care. defined as the outcomes relative to the costs,7 is ultimately optimized by earlier time-to-surgery.
Although using a large, multi-institutional database is advantageous for finding population-based trends that are representative of a large cohort, using the ACS-NSQIP database features its limitations. Our analysis was limited to the defined scope of NSQIP and nature of the injury, whereas root cause for delay was not available for study. We were unable to identify which patients were delayed for administrative reasons or surgical convenience and which were delayed for medical optimization. Participation in the ACS-NSQIP database is voluntary, and no randomized hospital sampling was conducted. Participating hospitals were de-identified in the database. As expected, we were unable to identify the specific institution-based hip fracture protocols that may affect the outcomes following treatment for these fractures. Further, socioeconomic information and payer-status are unavailable for the study. Additionally, observations are limited to 30 days postoperative, and we cannot comment on longer-term outcomes. Finally, discharge disposition and functional outcome data are not represented, and we were unable to correlate time-to-surgery and functional recovery. However, previous studies have established that delay in time-to-surgery following hip fractures is negatively correlated with functional outcomes.22-25
Nevertheless, the ACS-NSQIP database remains one of the largest American surgical databases available, and includes care centers from nearly every state with variable demographics including rural, urban, and academic centers. The ACS performs broad-based inter-rater reliability audits on every participating site and has found an overall disagreement rate of only 1.8%. As such, although discrepancies exist between the complete patient chart and the data entered, the data found in the ACS-NSQIP database are reliable and considered a valid source of study.34,35 The large sample size, quality of data collection, wide geographic representation, and varied hospital types within the dataset possibly make our findings relevant in the majority of American healthcare settings.
CONCLUSION
This study demonstrates an associated increased length of hospital stay, including the increased time from surgery-to-discharge, in patients with hip fractures whose surgical intervention is delayed for >48 hours after presentation. Given the prior evidence that early surgical intervention improves the functional outcomes and the current evidence that surgical delay for any cause increases costly hospital length of stay without corollary improvement in the outcomes, a value-based assessment of hip fracture care argues for early surgical intervention whenever possible. Our findings should inform physician, institution, and policy maker value-based decision making regarding the best practices in geriatric hip fracture care.
ABSTRACT
The morbidity and mortality after hip fracture in the elderly are influenced by non-modifiable comorbidities. Time-to-surgery is a modifiable factor that may play a role in postoperative morbidity. This study investigates the outcomes and complications in the elderly hip fracture surgery as a function of time-to-surgery.
Using the American College of Surgeons-National Surgical Quality Improvement Program data from 2011 to 2012, a study population was generated using the Current Procedural Terminology codes for percutaneous or open treatment of femoral neck fractures (27235, 27236) and fixation with a screw and side plate or intramedullary fixation (27244, 27245) for peritrochanteric fractures. Three time-to-surgery groups (<24 hours to surgical intervention, 24-48 hours, and >48 hours) were created and matched for surgery type, sex, age, and American Society of Anesthesiologists class. Time-to-surgery was then studied for its effect on the post-surgical outcomes using the adjusted regression modeling.
A study population of 6036 hip fractures was created, and 2012 patients were assigned to each matched time-to-surgery group. The unadjusted models showed that the earlier surgical intervention groups (<24 hours and 24-48 hours) exhibited a lower overall complication rate (P = .034) compared with the group waiting for surgery >48 hours. The unadjusted mortality rates increased with delay to surgical intervention (P = .039). Time-to-surgery caused no effect on the return to the operating room rate (P = .554) nor readmission rate (P = .285). Compared with other time-to-surgeries, the time-to-surgery of >48 hours was associated with prolonged total hospital length of stay (10.9 days) (P < .001) and a longer surgery-to-discharge time (hazard ratio, 95% confidence interval: 0.74, 0.69-0.79) (P < .001). Adjusted analyses showed no time-to-surgery related difference in complications (P = .143) but presented an increase in the total length of stay (P < .001) and surgery-to-discharge time (P < .001).
Timeliness of surgical intervention in a comorbidity-adjusted population of elderly hip fracture patients causes no effect on the overall complications, readmissions, nor 30-day mortality. However, time-to-surgery of >48 hours is associated with costly increase in the total length of stay, including an increased post-surgery-to-discharge time.
Continue to: Despite the best efforts to optimize surgical care...
Despite the best efforts to optimize surgical care and postoperative rehabilitation following hip fracture, elderly patients feature alarmingly high in-hospital and 1-year mortality rates of 4.35% to 9.2%1-4 and 36%,5 respectively. Those who survive are unlikely to return to independent living, with only 17% of the patients following hip fracture being able to walk independently 6 months postoperatively, and 12% being able to climb stairs6. Possibly, these poor outcomes reflect a preoperative medical comorbidity burden rather than a measure of medical or surgical quality. Given the absence of consensus regarding optimal time-to-surgery, treating physicians often opt to delay surgical intervention for the purposes of medically optimizing highly comorbid patients without significant data to suggest clinical benefit of such practice.
Numerous investigators have attempted to identify the modifiable risk factors for complication after surgical care of elderly hip fracture patients. However, consensus guidelines of care are missing. This condition is largely due to the difficulties in effectively modifying preoperative demographic and medical comorbidities on a semi-urgent basis. However, timeliness to surgery is one area for study that the care team can affect. Although time-to-surgery is dependent on multiple factors, including time of presentation, day of week of admission, difficulties with scheduling, and administrative delays, the care team plays a role in hastening or retarding time-to-surgery. Several studies have considered various time cut-offs (24, 48, 72, and 120 hours) to define early intervention, but none have defined a specific role for early or delayed surgery. Several investigators have discovered a positive association between delayed time-to-surgery and mortality;4,8-14 however, the most rigorously conducted studies that stringently control for preoperative comorbidities and demographics conclude that variance in time-to-surgery causes no effect on the in-hospital or 1-year mortality risk.1-3,15-18
Other investigators have shown that with early surgical intervention for hip fracture, patients experience shorter hospital stays,1,3,16,17,19-22 less days in pain,19 decreased risk of decubitus ulcers,15,17,19,22 and an increased likelihood of independence following fracture,22-25 regardless of preoperative medical status. Despite this evidence of improved outcomes with early surgery, 40% to 54% of hip fracture patients in the United States experience surgical delays of more than 24 to 48 hours. Additionally, with the recent (2013) national estimates of cost per day spent in the hospital falling between $1791 to $2289,26 minimizing the days spent in the hospital would likely lead to significant cost-savings, presuming no adverse effect on health-related outcomes. To this end, we hypothesize that the value (outcomes per associated cost)7 of hip fracture surgical care can be positively influenced by minimizing surgical wait-times. We assessed the effect of early surgical intervention, within 24 or 48 hours of presentation, on 30-day mortality, postoperative morbidity, hospital length of stay, and readmission rates in a comorbidity-adjusted population from a nationally representative cohort.
Continue to: METHODS AND MATERIALS...
METHODS AND MATERIALS
This study used the data from the American College of Surgeon-National Surgical Quality Improvement Program (ACS-NSQIP) database. With over 258 participating hospitals, this database has been widely used to identify national trends in various surgical specialties.27-34 The database includes information from participants in 43 states with hospitals ranging from rural community hospitals to large academic centers. Each site employs surgical clinical reviewers who are rigorously trained to collect data through chart review and discussion with the treating surgeon and/or patient,35 allowing for the use of robust and quality data with proven inter-rater reliability.36,37
Using the 2011 to 2012 NSQIP database, we used primary Current Procedural Terminology codes to identify all patients who underwent percutaneous (27235) or open (27236) fixation of femoral neck fractures; and fixation with a screw and side plate (27244) or intramedullary fixation (27245) for peritrochanteric fractures. The sample was divided into 3 time-to-surgery groups (<24 hours from presentation to surgery, 24-48 hours, and >48 hours) which were matched for fracture type (femoral neck or peritrochanteric), sex, age (under 75 years or ≥75 years), and American Society of Anesthesiologists (ASA) class used as a surrogate for severity of medical infirmary. The subjects were randomly matched 1:1:1 to create 3 statistically equivalent time-to-surgery groups using Proc SurveySelect (SAS version 9.2, SAS Institute).
Generalized linear models using logit link function for binary variables and identity link function for normally distributed characteristics were used to compare the 3 time-to-surgery groups. Descriptive statistics are presented as counts and percentages or least-square means with standard deviations. Preoperative lab values that were not normally distributed were log transformed and presented in their original scales with median values and 25th to 75th percentiles. Outcomes were similarly modeled.
Total hospital stay was modeled with a negative binomial distribution. Proportional hazards models were used to model the time from operating room (OR) to discharge, censoring patients who died before discharge, with results presented as hazard ratios (HR) and 95% confidence intervals (CI) (Figure). The assumption of the proportional hazards was tested using a Wald test. Using this model, a HR of <1 denotes a longer postoperative hospital stay, as a longer hospital stay decreases the “risk” for discharge.

All models were adjusted for confounders, including race, body mass index (BMI), hypertension, chronic obstructive pulmonary disease, cancer, bleeding disorders, transfusion within 72 hours before surgery, preoperative levels of creatinine, platelet count, white blood cells (WBCs), hematocrit anesthesia type, and wound infection. These covariates were selected based upon their observed relationship to the studied outcomes and time-to-surgery groups, and were evaluated across the models for all outcomes for consistency and clarity. All statistical analyses were run at a type I error rate of 5% and performed in SAS version 9.2 software.
Continue to: RESULTS...
RESULTS
A study population of 6036 hip fractures was identified and divided into 3 groups of 2012 subjects each based upon time-to-surgery. The groups were successfully matched for surgery type, age (≥75 years old), gender, and ASA class. In each group, 594 of the 2012 (29.5%) patients were male, 1525 (75.8%) were ≥75 years of age, 9 (.5%) were ASA Class I, 269 (13.4%) were ASA Class II, 1424 (70.8%) were ASA class III, and 309 (15.4%) were ASA class IV.
Significant differences in preoperative comorbidity burden and preoperative lab values were identified between the 3 cohorts. Increased time-to-surgery was associated with differences in race (P < .001), elevated BMI (P = .010), higher rates of congestive heart failure (P < .001), hypertension medication (P = .020), bleeding disorders (P < .001), blood transfusion within 72 hours of surgery (P < .001), and systemic sepsis (P = .001). Delay to surgery was also associated with lower preoperative sodium (P = .005), blood urea nitrogen (P = .013), serum WBC (P < .001), hematocrit (P < .001), and platelets (P < .001) (Table 1).

The unadjusted analyses revealed no association between time-to-surgery and return to OR (P = .554) nor readmission (P = .285). However, increasing time-to-surgery was associated with an increase in overall complications (P = .034), total length of hospital stay (P < .001), and 30-day mortality (P = .039) (Table 2).
Table 2. Estimated Event Rates from Matched Cohorts (Unadjusted)
| Time From Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 15.30% | 15.30% | 17.90% | 0.034 |
Total length of stay | 5.4 | 6.7 | 10.9 | <0.001 |
(mean days, 95% confidence interval) | (5.2, 5.7) | (6.5, 7.0) | (10.3, 11.5) | |
Time from OR to discharge | -ref- | 0.96 | 0.74 | <0.001 |
(Hazard ratio) | (0.90,1.02) | (0.69, 0.79) | ||
Return to OR | 2.40% | 2.40% | 2.00% | 0.554 |
Readmission | 9.60% | 8.40% | 8.30% | 0.285 |
30-day mortality rate | 5.80% | 5.30% | 7.20% | 0.039 |
Abbreviation: OR, operating room.
The adjusted analysis controlling for preoperative demographic and comorbidity variables revealed trends toward the increased overall complications and 30-day mortality with increased time-to-surgery; these trends showed no statistical significance (P = .143 and P = .08). No statistical relationship was observed between return to OR nor readmission and time-to-surgery. Increasing time-to-surgery remained significantly associated with the increased total length of hospital stay (P < .001). The adjusted analysis also revealed that the delay of >48 hours in time-to-surgery resulted in a longer surgery-to-discharge time (P < .001) (Table 3). No evidence of violation of the proportional hazards assumption was observed in the unadjusted nor adjusted clustered proportional hazards models (Wald test, P = .27 and P = .25, respectively).
Table 3. Estimated Event Rates from Matched Cohorts (Adjusteda)
| Time from Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 11.70% | 10.70% | 12.60% | 0.143 |
Total length of stay | 4.2 | 5.1 | 7.6 | <0.001 |
(mean days, 95% confidence interval) | (4.0, 4.5) | (4.8, 5.5) | (7.1, 8.3) | |
Time from OR to discharge | -ref- | 1.03 | 0.87 | <0.001 |
(Hazard ratio) | (0.97, 1.09) | (0.81, 0.92) | ||
Return to OR | 2.10% | 2.10% | 1.60% | 0.541 |
Readmission | 7.20% | 6.40% | 6.00% | 0.304 |
30-day mortality rate | 4.20% | 3.70% | 5.20% | 0.08 |
aModel adjusted for race, hypertension medication, cancer, bleeding disorders, transfusion within 72 hours before surgery, emergency status, wound infection, anesthesia type (general), body mass index (18.5-25), history of chronic obstructive pulmonary disease, and preoperative levels of creatinine, platelet count, white blood cell count, and hematocrit.
Continue to: DISCUSSION...
DISCUSSION
Previous research has demonstrated an association between age,3,4,25 comorbidity burden,1,3,25 gender,3,4 and ASA class4,18,21 with outcomes following hip fractures and serves as the basis of our matched analysis statistical methodology in assessing the effect of time-to-surgery on the outcome following hip fracture surgery. Prior investigators have also established the positive correlation between increased preoperative comorbidity burden and delay in time-to-surgery.10,15 This finding was confirmed in our unadjusted comparison of 3 time-to-surgery groups. However, prior investigations have not established a clear association between time-to-surgical intervention and postoperative morbidity and mortality.1,15,16,18,20,38 This study utilized a nationally representative dataset known for its data integrity and from which 6036 patients with surgically treated hip fractures, matched for surgery type, age, gender, and ASA class (a surrogate for severity of medical infirmary), were studied using adjusted regression modeling to afford an isolated statistical assessment of the effect of time-to-surgery on outcomes following hip fracture surgery.
Despite a large sample size and rigorous statistical methodology, for many outcome measures, our results show no support for the early or late operative intervention following hip fracture. We found no difference in 30-day mortality, readmission rate, nor total complication rate between the 3 time-to-surgery cohorts. This result indicates that the care of elderly patients following hip fracture is inherently complicated and that perioperative complication risk is probably only modestly modifiable by best medical practices, including optimizing time from clinical presentation to surgery.
As expected, patients who experienced longer delays from presentation to surgery were on average, more comorbid and more likely to yield abnormal preoperative lab values. However, in the adjusted analysis, delay in time-to-surgery, presumably for medical management, was not found to be associated with improved outcomes. In the same adjusted analysis, we uniquely identified that in the patients whose surgeries were delayed for more than 48 hours, the time from surgery-to-discharge was significantly increased. As a result, these patients spent extra days in the hospital both preoperatively and postoperatively, but without any corollary improvement in the outcomes.
Continue to: Recent estimates of the cost of hospital admission...
Recent estimates of the cost of hospital admission is approximated nationally at $2000/day.26 Although our data fail to support the formal cost-analysis of the effect of time-to-surgery in hip fracture care, a simple value-based analysis indicates that quality is preserved (no difference in outcome), whereas costly hospital days are eliminated with earlier surgery. The value in elderly hip fracture care. defined as the outcomes relative to the costs,7 is ultimately optimized by earlier time-to-surgery.
Although using a large, multi-institutional database is advantageous for finding population-based trends that are representative of a large cohort, using the ACS-NSQIP database features its limitations. Our analysis was limited to the defined scope of NSQIP and nature of the injury, whereas root cause for delay was not available for study. We were unable to identify which patients were delayed for administrative reasons or surgical convenience and which were delayed for medical optimization. Participation in the ACS-NSQIP database is voluntary, and no randomized hospital sampling was conducted. Participating hospitals were de-identified in the database. As expected, we were unable to identify the specific institution-based hip fracture protocols that may affect the outcomes following treatment for these fractures. Further, socioeconomic information and payer-status are unavailable for the study. Additionally, observations are limited to 30 days postoperative, and we cannot comment on longer-term outcomes. Finally, discharge disposition and functional outcome data are not represented, and we were unable to correlate time-to-surgery and functional recovery. However, previous studies have established that delay in time-to-surgery following hip fractures is negatively correlated with functional outcomes.22-25
Nevertheless, the ACS-NSQIP database remains one of the largest American surgical databases available, and includes care centers from nearly every state with variable demographics including rural, urban, and academic centers. The ACS performs broad-based inter-rater reliability audits on every participating site and has found an overall disagreement rate of only 1.8%. As such, although discrepancies exist between the complete patient chart and the data entered, the data found in the ACS-NSQIP database are reliable and considered a valid source of study.34,35 The large sample size, quality of data collection, wide geographic representation, and varied hospital types within the dataset possibly make our findings relevant in the majority of American healthcare settings.
CONCLUSION
This study demonstrates an associated increased length of hospital stay, including the increased time from surgery-to-discharge, in patients with hip fractures whose surgical intervention is delayed for >48 hours after presentation. Given the prior evidence that early surgical intervention improves the functional outcomes and the current evidence that surgical delay for any cause increases costly hospital length of stay without corollary improvement in the outcomes, a value-based assessment of hip fracture care argues for early surgical intervention whenever possible. Our findings should inform physician, institution, and policy maker value-based decision making regarding the best practices in geriatric hip fracture care.
1. Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233. doi:10.7326/0003-4819-155-4-201108160-00006.
2. Verbeek DO, Ponsen KJ, Goslings JC, Heetveld MJ. Effect of surgical delay on outcome in hip fracture patients: a retrospective multivariate analysis of 192 patients. Int Orthop. 2008;32(1):13-18. doi:10.1007/s00264-006-0290-9.
3. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927. doi:10.1302/0301-620X.91B7.22446.
4. Uzoigwe CE, Burnand HG, Cheesman CL, Aghedo DO, Faizi M, Middleton RG. Early and ultra-early surgery in hip fracture patients improves survival. Injury. 2013;44(6):726-729. doi:10.1016/j.injury.2012.08.025.
5. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525. doi:10.1056/NEJM199606063342307.
6. Marottoli RA, Berkman LF, Cooney LM Jr. Decline in physical function following hip fracture. J Am Geriatr Soc. 1992;40(9):861-866. doi:10.1111/j.1532-5415.1992.tb01980.x.
7. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481. doi:10.1056/NEJMp1011024.
8. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42. doi:10.2106/JBJS.G.00065.
9. Novack V, Jotkowitz A, Etzion O, Porath A. Does delay in surgery after hip fracture lead to worse outcomes? A multicenter survey. Int J Qual Health Care. 2007;19(3):170-176. doi:10.1093/intqhc/mzm003.
10. Weller I, Wai EK, Jaglal S, Kreder HJ. The effect of hospital type and surgical delay on mortality after surgery for hip fracture. J Bone Joint Surg Br. 2005;87(3):361-366. doi:10.1302/0301-620X.87B3.15300.
11. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489. doi:10.2106/JBJS.D.01796.
12. Holt G, Smith R, Duncan K, McKeown DW. Does delay to theatre for medical reasons affect the peri-operative mortality in patients with a fracture of the hip? J Bone Joint Surg Br. 2010;92(6):835-841. doi:10.1302/0301-620X.92B6.24463.
13. Pioli G, Lauretani F, Davoli ML, et al. Older people with hip fracture and IADL disability require earlier surgery. J Gerontol A Biol Sci Med Sci. 2012;67(11):1272-1277. doi:10.1093/gerona/gls097.
14. Mackenzie DG, Wild S, Muir R. Mortality associated with delay in operation after hip fracture: Scottish data provide additional information. BMJ. 2006;332(7549):1093. doi:10.1136/bmj.332.7549.1093.
15. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709. doi:10.1016/S0002-9343(02)01119-1.
16. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559. doi:10.1097/01.mlr.0000215812.13720.2e.
17. Hommel A, Ulander K, Bjorkelund KB, Norrman PO, Wingstrand H, Thorngren KG. Influence of optimised treatment of people with hip fracture on time to operation, length of hospital stay, reoperations and mortality within 1 year. Injury. 2008;39(10):1164-1174. doi:10.1016/j.injury.2008.01.048.
18. Rae HC, Harris IA, McEvoy L, Todorova T. Delay to surgery and mortality after hip fracture. ANZ J Surg. 2007;77(10):889-891. doi:10.1111/j.1445-2197.2007.04267.x.
19. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743. doi:10.1001/jama.291.14.1738.
20. Bergeron E, Lavoie A, Moore L, et al. Is the delay to surgery for isolated hip fracture predictive of outcome in efficient systems? J Trauma. 2006;60(4):753-757. doi:10.1097/01.ta.0000214649.53190.2a.
21. Siegmeth AW, Gurusamy K, Parker MJ. Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur. J Bone Joint Surg Br. 2005;87(8):1123-1126. doi:10.1302/0301-620X.87B8.16357.
22. Al-Ani AN, Samuelsson B, Tidermark J, et al. Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. J Bone Joint Surg Am. 2008;90(7):1436-1442. doi:10.2106/JBJS.G.00890.
23. Hoenig H, Rubenstein LV, Sloane R, Horner R, Kahn K. What is the role of timing in the surgical and rehabilitative care of community-dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157(5):513-520.
24. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. doi:10.1016/j.archger.2004.03.004.
25. Yonezawa T, Yamazaki K, Atsumi T, Obara S. Influence of the timing of surgery on mortality and activity of hip fracture in elderly patients. J Orthop Sci Off J Jpn Orthop Assoc. 2009;14(5):566-573. doi:10.1007/s00776-009-1380-5.
26. Henry J Kaiser Family Foundation. Hospital adjusted expenses per inpatient day by ownership. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 14, 2013.
27. Belmont PJ Jr, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg. 2011;213(3):370-378. doi:10.1016/j.jamcollsurg.2011.05.019.
28. Davis SS Jr, Husain FA, Lin E, Nandipati KC, Perez S, Sweeney JF. Resident participation in index laparoscopic general surgical cases: impact of the learning environment on surgical outcomes. J Am Coll Surg. 2013;216(1):96-104. doi:10.1016/j.jamcollsurg.2012.08.014.
29. Gart MS, Smetona JT, Hanwright PJ, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2013;216(2):229-238. doi:10.1016/j.jamcollsurg.2012.11.003.
30. Greenblatt DY, Rajamanickam V, Pugely AJ, Heise CP, Foley EF, Kennedy GD. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg. 2011;212(5):844-854. doi:10.1016/j.jamcollsurg.2011.01.005.
31. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199. doi:10.2106/JBJS.K.01682.
32. Rao RD. Risk factors for complications and mortality after spine surgery assessed with the NSQIP database: where do we go from here? Commentary on an article by Andrew J Schoenfeld, MD, et al.: "Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program". J Bone Joint Surg Am. 2011;93(17):e101:(101-102). doi:10.2106/JBJS.K.00786.
33. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/JBJS.I.00735.
34. Tsilimparis N, Perez S, Dayama A, Ricotta JJ 2nd. Age-stratified results from 20,095 aortoiliac aneurysm repairs: should we approach octogenarians and nonagenarians differently? J Am Coll Surg. 2012;215(5):690-701. doi:10.1016/j.jamcollsurg.2012.06.411.
35. ACS National Surgical Quality Improvement Program. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip?. Accessed March 14, 2013.
36. Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg. 2009;198(5 Suppl):S19-S27. doi:10.1016/j.amjsurg.2009.07.025.
37. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
38. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697. doi:10.1016/j.injury.2009.01.010.
1. Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233. doi:10.7326/0003-4819-155-4-201108160-00006.
2. Verbeek DO, Ponsen KJ, Goslings JC, Heetveld MJ. Effect of surgical delay on outcome in hip fracture patients: a retrospective multivariate analysis of 192 patients. Int Orthop. 2008;32(1):13-18. doi:10.1007/s00264-006-0290-9.
3. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927. doi:10.1302/0301-620X.91B7.22446.
4. Uzoigwe CE, Burnand HG, Cheesman CL, Aghedo DO, Faizi M, Middleton RG. Early and ultra-early surgery in hip fracture patients improves survival. Injury. 2013;44(6):726-729. doi:10.1016/j.injury.2012.08.025.
5. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525. doi:10.1056/NEJM199606063342307.
6. Marottoli RA, Berkman LF, Cooney LM Jr. Decline in physical function following hip fracture. J Am Geriatr Soc. 1992;40(9):861-866. doi:10.1111/j.1532-5415.1992.tb01980.x.
7. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481. doi:10.1056/NEJMp1011024.
8. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42. doi:10.2106/JBJS.G.00065.
9. Novack V, Jotkowitz A, Etzion O, Porath A. Does delay in surgery after hip fracture lead to worse outcomes? A multicenter survey. Int J Qual Health Care. 2007;19(3):170-176. doi:10.1093/intqhc/mzm003.
10. Weller I, Wai EK, Jaglal S, Kreder HJ. The effect of hospital type and surgical delay on mortality after surgery for hip fracture. J Bone Joint Surg Br. 2005;87(3):361-366. doi:10.1302/0301-620X.87B3.15300.
11. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489. doi:10.2106/JBJS.D.01796.
12. Holt G, Smith R, Duncan K, McKeown DW. Does delay to theatre for medical reasons affect the peri-operative mortality in patients with a fracture of the hip? J Bone Joint Surg Br. 2010;92(6):835-841. doi:10.1302/0301-620X.92B6.24463.
13. Pioli G, Lauretani F, Davoli ML, et al. Older people with hip fracture and IADL disability require earlier surgery. J Gerontol A Biol Sci Med Sci. 2012;67(11):1272-1277. doi:10.1093/gerona/gls097.
14. Mackenzie DG, Wild S, Muir R. Mortality associated with delay in operation after hip fracture: Scottish data provide additional information. BMJ. 2006;332(7549):1093. doi:10.1136/bmj.332.7549.1093.
15. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709. doi:10.1016/S0002-9343(02)01119-1.
16. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559. doi:10.1097/01.mlr.0000215812.13720.2e.
17. Hommel A, Ulander K, Bjorkelund KB, Norrman PO, Wingstrand H, Thorngren KG. Influence of optimised treatment of people with hip fracture on time to operation, length of hospital stay, reoperations and mortality within 1 year. Injury. 2008;39(10):1164-1174. doi:10.1016/j.injury.2008.01.048.
18. Rae HC, Harris IA, McEvoy L, Todorova T. Delay to surgery and mortality after hip fracture. ANZ J Surg. 2007;77(10):889-891. doi:10.1111/j.1445-2197.2007.04267.x.
19. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743. doi:10.1001/jama.291.14.1738.
20. Bergeron E, Lavoie A, Moore L, et al. Is the delay to surgery for isolated hip fracture predictive of outcome in efficient systems? J Trauma. 2006;60(4):753-757. doi:10.1097/01.ta.0000214649.53190.2a.
21. Siegmeth AW, Gurusamy K, Parker MJ. Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur. J Bone Joint Surg Br. 2005;87(8):1123-1126. doi:10.1302/0301-620X.87B8.16357.
22. Al-Ani AN, Samuelsson B, Tidermark J, et al. Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. J Bone Joint Surg Am. 2008;90(7):1436-1442. doi:10.2106/JBJS.G.00890.
23. Hoenig H, Rubenstein LV, Sloane R, Horner R, Kahn K. What is the role of timing in the surgical and rehabilitative care of community-dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157(5):513-520.
24. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. doi:10.1016/j.archger.2004.03.004.
25. Yonezawa T, Yamazaki K, Atsumi T, Obara S. Influence of the timing of surgery on mortality and activity of hip fracture in elderly patients. J Orthop Sci Off J Jpn Orthop Assoc. 2009;14(5):566-573. doi:10.1007/s00776-009-1380-5.
26. Henry J Kaiser Family Foundation. Hospital adjusted expenses per inpatient day by ownership. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 14, 2013.
27. Belmont PJ Jr, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg. 2011;213(3):370-378. doi:10.1016/j.jamcollsurg.2011.05.019.
28. Davis SS Jr, Husain FA, Lin E, Nandipati KC, Perez S, Sweeney JF. Resident participation in index laparoscopic general surgical cases: impact of the learning environment on surgical outcomes. J Am Coll Surg. 2013;216(1):96-104. doi:10.1016/j.jamcollsurg.2012.08.014.
29. Gart MS, Smetona JT, Hanwright PJ, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2013;216(2):229-238. doi:10.1016/j.jamcollsurg.2012.11.003.
30. Greenblatt DY, Rajamanickam V, Pugely AJ, Heise CP, Foley EF, Kennedy GD. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg. 2011;212(5):844-854. doi:10.1016/j.jamcollsurg.2011.01.005.
31. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199. doi:10.2106/JBJS.K.01682.
32. Rao RD. Risk factors for complications and mortality after spine surgery assessed with the NSQIP database: where do we go from here? Commentary on an article by Andrew J Schoenfeld, MD, et al.: "Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program". J Bone Joint Surg Am. 2011;93(17):e101:(101-102). doi:10.2106/JBJS.K.00786.
33. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/JBJS.I.00735.
34. Tsilimparis N, Perez S, Dayama A, Ricotta JJ 2nd. Age-stratified results from 20,095 aortoiliac aneurysm repairs: should we approach octogenarians and nonagenarians differently? J Am Coll Surg. 2012;215(5):690-701. doi:10.1016/j.jamcollsurg.2012.06.411.
35. ACS National Surgical Quality Improvement Program. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip?. Accessed March 14, 2013.
36. Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg. 2009;198(5 Suppl):S19-S27. doi:10.1016/j.amjsurg.2009.07.025.
37. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
38. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697. doi:10.1016/j.injury.2009.01.010.
TAKE-HOME POINTS
- Time-to-surgery for definitive fixation of hip fractures is a modifiable risk factor.
- This study fails to demonstrate a benefit in delaying surgery for medical optimization as there were no time-to-surgery related differences in complications (P = 1.43).
- Delay in definitive surgery results in an increase in the total length of stay (P < .001) and surgery-to-discharge time (P < .001) without an improvement in overall complications, readmission or 30-day mortality rates.
- Despite numerous investigations, there are no consensus guidelines to decrease complications and mortality rates following hip fracture surgery.
- ACS-NSQIP database is a reliable and validated database.