User login
Long-term use of prescription sleep meds unsupported by new research
a new study shows.
“While there are good data from [randomized, controlled trials] that these medications improve sleep disturbances in the short term,” few studies have examined whether they provide long-term benefits, stated the authors of the paper, which was published in BMJ Open.
“The current observational study does not support use of sleep medications over the long term, as there were no self-reported differences at 1 or 2 years of follow-up comparing sleep medication users with nonusers,” author Daniel H. Solomon, MD, MPH, from Brigham and Women’s Hospital, Boston, and colleagues wrote.
Women included in the analysis were drawn from the Study of Women’s Health Across the Nation (SWAN), an ongoing multicenter, longitudinal study examining women during the menopausal transition. The average age of the women included in the cohort was 49.5 years and approximately half were White. All women reported a sleep disturbance on at least 3 nights per week during a 2-week interval. At follow up, women were asked to use a Likert scale to rate three aspects of sleep: difficulty initiating sleep, frequent awakening, and waking up early. On the scale, 1 represented having no difficulties on any nights, 3 represented having difficulties 1-2 nights per week, and 5 represented having difficulty 5-7 nights per week.
Women already using prescription sleep medication at their baseline visit were excluded from the study. Medications used included benzodiazepines, selective BZD receptor agonists, and other hypnotics.
Over the 21 years of follow-up in the SWAN study (1995-2016), Dr. Solomon and colleagues identified 238 women using sleep medication and these were compared with a cohort of 447 propensity score–matched non–sleep medication uses. Overall, the 685 women included were similar in characteristics to each other as well as to the other potentially eligible women not included in the analysis.
Sleep disturbance patterns compared
At baseline, sleep disturbance patterns were similar between the two groups. Among medication users, the mean score for difficulty initiating sleep was 2.7 (95% confidence interval, 2.5-2.9), waking frequently 3.8 (95% CI, 3.6-3.9), and waking early 2.9 (95% CI, 2.7-3.1). Among the nonusers, the baseline scores were 2.6 (95% CI, 2.5-2.7), 3.7 (95% CI, 3.6-3.8), and 2.7 (95% CI, 2.5-2.8), respectively. After 1 year, there was no statistically significant difference in scores between the two groups. The average ratings for medication users were 2.6 (95% CI, 2.3-2.8) for difficulty initiating sleep, 3.8 (95% CI, 3.6-4.0) for waking frequently, and 2.8 (95% CI, 2.6-3.0) for waking early.
Average ratings among nonusers were 2.3 (95% CI, 2.2-2.4), 3.5 (95% CI, 3.3-3.6), and 2.5 (95% CI, 2.3-2.6), respectively.
After 2 years, there were still no statistically significant reductions in sleep disturbances among those taking prescription sleep medications, compared with those not taking medication.
The researchers noted that approximately half of the women in this cohort were current or past tobacco users and that 20% were moderate to heavy alcohol users.
More work-up, not more medication, needed
The study authors acknowledged the limitations of an observational study and noted that, since participants only reported medication use and sleep disturbances at annual visits, they did not know whether patients’ medication use was intermittent or of any interim outcomes. Additionally, the authors pointed out that those classified as “nonusers” may have been using over-the-counter medication.
“Investigations should look at detailed-use patterns, on a daily or weekly basis, with frequent outcomes data,” Dr. Solomon said in an interview. “While our data shed new light on chronic use, we only had data collected on an annual basis; daily or weekly data would provide more granular information.”
Regarding clinician prescribing practices, Dr. Solomon said, “short-term, intermittent use can be helpful, but use these agents sparingly” and “educate patients that chronic regular use of medications for sleep is not associated with improvement in sleep disturbances.”
Commenting on the study, Andrea Matsumura, MD, a sleep specialist at the Oregon Clinic in Portland, echoed this sentiment: “When someone says they are having trouble sleeping this is the tip of the iceberg and it warrants an evaluation to determine if someone has a breathing disorder, a circadian disorder, a life situation, or a type of insomnia that is driving the sleeplessness.”
“I think this study supports what we all should know,” Dr. Matsumura concluded. “Sleep aids are not meant to be used long term” and should not be used for longer than 2 weeks without further work-up.
Funding for this study was provided through a grant from the National Institutes of Health. Dr. Solomon has received salary support from research grants to Brigham and Women’s Hospital for unrelated work from AbbVie, Amgen, Corrona, Genentech and Pfizer. The other authors and Dr. Matsumura have reported no relevant financial relationships.
a new study shows.
“While there are good data from [randomized, controlled trials] that these medications improve sleep disturbances in the short term,” few studies have examined whether they provide long-term benefits, stated the authors of the paper, which was published in BMJ Open.
“The current observational study does not support use of sleep medications over the long term, as there were no self-reported differences at 1 or 2 years of follow-up comparing sleep medication users with nonusers,” author Daniel H. Solomon, MD, MPH, from Brigham and Women’s Hospital, Boston, and colleagues wrote.
Women included in the analysis were drawn from the Study of Women’s Health Across the Nation (SWAN), an ongoing multicenter, longitudinal study examining women during the menopausal transition. The average age of the women included in the cohort was 49.5 years and approximately half were White. All women reported a sleep disturbance on at least 3 nights per week during a 2-week interval. At follow up, women were asked to use a Likert scale to rate three aspects of sleep: difficulty initiating sleep, frequent awakening, and waking up early. On the scale, 1 represented having no difficulties on any nights, 3 represented having difficulties 1-2 nights per week, and 5 represented having difficulty 5-7 nights per week.
Women already using prescription sleep medication at their baseline visit were excluded from the study. Medications used included benzodiazepines, selective BZD receptor agonists, and other hypnotics.
Over the 21 years of follow-up in the SWAN study (1995-2016), Dr. Solomon and colleagues identified 238 women using sleep medication and these were compared with a cohort of 447 propensity score–matched non–sleep medication uses. Overall, the 685 women included were similar in characteristics to each other as well as to the other potentially eligible women not included in the analysis.
Sleep disturbance patterns compared
At baseline, sleep disturbance patterns were similar between the two groups. Among medication users, the mean score for difficulty initiating sleep was 2.7 (95% confidence interval, 2.5-2.9), waking frequently 3.8 (95% CI, 3.6-3.9), and waking early 2.9 (95% CI, 2.7-3.1). Among the nonusers, the baseline scores were 2.6 (95% CI, 2.5-2.7), 3.7 (95% CI, 3.6-3.8), and 2.7 (95% CI, 2.5-2.8), respectively. After 1 year, there was no statistically significant difference in scores between the two groups. The average ratings for medication users were 2.6 (95% CI, 2.3-2.8) for difficulty initiating sleep, 3.8 (95% CI, 3.6-4.0) for waking frequently, and 2.8 (95% CI, 2.6-3.0) for waking early.
Average ratings among nonusers were 2.3 (95% CI, 2.2-2.4), 3.5 (95% CI, 3.3-3.6), and 2.5 (95% CI, 2.3-2.6), respectively.
After 2 years, there were still no statistically significant reductions in sleep disturbances among those taking prescription sleep medications, compared with those not taking medication.
The researchers noted that approximately half of the women in this cohort were current or past tobacco users and that 20% were moderate to heavy alcohol users.
More work-up, not more medication, needed
The study authors acknowledged the limitations of an observational study and noted that, since participants only reported medication use and sleep disturbances at annual visits, they did not know whether patients’ medication use was intermittent or of any interim outcomes. Additionally, the authors pointed out that those classified as “nonusers” may have been using over-the-counter medication.
“Investigations should look at detailed-use patterns, on a daily or weekly basis, with frequent outcomes data,” Dr. Solomon said in an interview. “While our data shed new light on chronic use, we only had data collected on an annual basis; daily or weekly data would provide more granular information.”
Regarding clinician prescribing practices, Dr. Solomon said, “short-term, intermittent use can be helpful, but use these agents sparingly” and “educate patients that chronic regular use of medications for sleep is not associated with improvement in sleep disturbances.”
Commenting on the study, Andrea Matsumura, MD, a sleep specialist at the Oregon Clinic in Portland, echoed this sentiment: “When someone says they are having trouble sleeping this is the tip of the iceberg and it warrants an evaluation to determine if someone has a breathing disorder, a circadian disorder, a life situation, or a type of insomnia that is driving the sleeplessness.”
“I think this study supports what we all should know,” Dr. Matsumura concluded. “Sleep aids are not meant to be used long term” and should not be used for longer than 2 weeks without further work-up.
Funding for this study was provided through a grant from the National Institutes of Health. Dr. Solomon has received salary support from research grants to Brigham and Women’s Hospital for unrelated work from AbbVie, Amgen, Corrona, Genentech and Pfizer. The other authors and Dr. Matsumura have reported no relevant financial relationships.
a new study shows.
“While there are good data from [randomized, controlled trials] that these medications improve sleep disturbances in the short term,” few studies have examined whether they provide long-term benefits, stated the authors of the paper, which was published in BMJ Open.
“The current observational study does not support use of sleep medications over the long term, as there were no self-reported differences at 1 or 2 years of follow-up comparing sleep medication users with nonusers,” author Daniel H. Solomon, MD, MPH, from Brigham and Women’s Hospital, Boston, and colleagues wrote.
Women included in the analysis were drawn from the Study of Women’s Health Across the Nation (SWAN), an ongoing multicenter, longitudinal study examining women during the menopausal transition. The average age of the women included in the cohort was 49.5 years and approximately half were White. All women reported a sleep disturbance on at least 3 nights per week during a 2-week interval. At follow up, women were asked to use a Likert scale to rate three aspects of sleep: difficulty initiating sleep, frequent awakening, and waking up early. On the scale, 1 represented having no difficulties on any nights, 3 represented having difficulties 1-2 nights per week, and 5 represented having difficulty 5-7 nights per week.
Women already using prescription sleep medication at their baseline visit were excluded from the study. Medications used included benzodiazepines, selective BZD receptor agonists, and other hypnotics.
Over the 21 years of follow-up in the SWAN study (1995-2016), Dr. Solomon and colleagues identified 238 women using sleep medication and these were compared with a cohort of 447 propensity score–matched non–sleep medication uses. Overall, the 685 women included were similar in characteristics to each other as well as to the other potentially eligible women not included in the analysis.
Sleep disturbance patterns compared
At baseline, sleep disturbance patterns were similar between the two groups. Among medication users, the mean score for difficulty initiating sleep was 2.7 (95% confidence interval, 2.5-2.9), waking frequently 3.8 (95% CI, 3.6-3.9), and waking early 2.9 (95% CI, 2.7-3.1). Among the nonusers, the baseline scores were 2.6 (95% CI, 2.5-2.7), 3.7 (95% CI, 3.6-3.8), and 2.7 (95% CI, 2.5-2.8), respectively. After 1 year, there was no statistically significant difference in scores between the two groups. The average ratings for medication users were 2.6 (95% CI, 2.3-2.8) for difficulty initiating sleep, 3.8 (95% CI, 3.6-4.0) for waking frequently, and 2.8 (95% CI, 2.6-3.0) for waking early.
Average ratings among nonusers were 2.3 (95% CI, 2.2-2.4), 3.5 (95% CI, 3.3-3.6), and 2.5 (95% CI, 2.3-2.6), respectively.
After 2 years, there were still no statistically significant reductions in sleep disturbances among those taking prescription sleep medications, compared with those not taking medication.
The researchers noted that approximately half of the women in this cohort were current or past tobacco users and that 20% were moderate to heavy alcohol users.
More work-up, not more medication, needed
The study authors acknowledged the limitations of an observational study and noted that, since participants only reported medication use and sleep disturbances at annual visits, they did not know whether patients’ medication use was intermittent or of any interim outcomes. Additionally, the authors pointed out that those classified as “nonusers” may have been using over-the-counter medication.
“Investigations should look at detailed-use patterns, on a daily or weekly basis, with frequent outcomes data,” Dr. Solomon said in an interview. “While our data shed new light on chronic use, we only had data collected on an annual basis; daily or weekly data would provide more granular information.”
Regarding clinician prescribing practices, Dr. Solomon said, “short-term, intermittent use can be helpful, but use these agents sparingly” and “educate patients that chronic regular use of medications for sleep is not associated with improvement in sleep disturbances.”
Commenting on the study, Andrea Matsumura, MD, a sleep specialist at the Oregon Clinic in Portland, echoed this sentiment: “When someone says they are having trouble sleeping this is the tip of the iceberg and it warrants an evaluation to determine if someone has a breathing disorder, a circadian disorder, a life situation, or a type of insomnia that is driving the sleeplessness.”
“I think this study supports what we all should know,” Dr. Matsumura concluded. “Sleep aids are not meant to be used long term” and should not be used for longer than 2 weeks without further work-up.
Funding for this study was provided through a grant from the National Institutes of Health. Dr. Solomon has received salary support from research grants to Brigham and Women’s Hospital for unrelated work from AbbVie, Amgen, Corrona, Genentech and Pfizer. The other authors and Dr. Matsumura have reported no relevant financial relationships.
FROM BMJ OPEN
Study points to best treatments for depression in primary care
according to a network meta-analysis (NMA) comparing either and both approaches with control conditions in the primary care setting.
The findings are important, since the majority of depressed patients are treated by primary care physicians, yet relatively few randomized trials of treatment have focused on this setting, noted senior study author Pim Cuijpers, PhD, from Vrije Universiteit Amsterdam, and colleagues, in the paper, which was published in Annals of Family Medicine.
“The main message is that clinicians should certainly consider psychotherapy instead of pharmacotherapy, because this is preferred by most patients, and when possible, combined treatments should be the preferred choice because the outcomes are considerably better,” he said in an interview. Either way, he emphasized that “preference of patients is very important and all three treatments are better than usual care.”
The NMA included studies comparing psychotherapy, antidepressant medication, or a combination of both, with control conditions (defined as usual care, wait list, or pill placebo) in adult primary care patients with depression.
Patients could have major depression, persistent mood disorders (dysthymia), both, or high scores on self-rating depression scales. The primary outcome of the NMA was response, defined as a 50% improvement in the Hamilton Depression Rating scores (HAM-D).
A total of 58 studies met inclusion criteria, involving 9,301 patients.
Treatment options compared
Compared with usual care, both psychotherapy alone and pharmacotherapy alone had significantly better response rates, with no significant difference between them (relative risk, 1.60 and RR, 1.65, respectively). The combination of psychotherapy and pharmacotherapy was even better (RR, 2.15), whereas the wait list was less effective (RR, 0.68).
When comparing combined therapy with psychotherapy or pharmacotherapy, the superiority of combination therapy over psychotherapy was only slightly statistically significant (RR, 1.35; 95% confidence interval, 1.00-1.81), while pharmacotherapy was only slightly inferior (RR, 1.30; 95% CI, 0.98-1.73).
“The significance level is not very high, which is related to statistical power,” said Dr. Cuijpers. “But the mean benefit is quite substantial in my opinion, with a 35% higher chance of response in the combined treatment, compared to psychotherapy alone.”
Looking at the outcome of remission, (normally defined as a score of 7 or less on the HAM-D), the outcomes were “comparable to those for response, with the exception that combined treatment was not significantly different from psychotherapy,” they wrote.
One important caveat is that several studies included in the NMA included patients with moderate to severe depression, a population that is different from the usual primary care population of depressed patients who have mild to moderate symptoms. Antidepressant medications are also assumed to work better against more severe symptoms, added the authors. “The inclusion of these studies might therefore have resulted in an overestimation of the effects of pharmacotherapy in the present NMA.”
Among other limitations, the authors noted that studies included mixed populations of patients with dysthymia and major depression; they also made no distinction between different types of antidepressants.
Psychotherapies unknown, but meta-analysis is still useful
Commenting on these findings, Neil Skolnik, MD, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, said this is “an important study, confirming and extending the conclusions” of a systematic review published in 2016 as a Clinical Practice Guideline from the American College of Physicians.
“Unfortunately, the authors did not specify what type of psychotherapy was studied in the meta-analysis, so we have to look elsewhere if we want to advise our patients on what type of psychotherapy to seek, since there are important differences between different types of therapy,” he said.
Still, he described the study as providing “helpful information for the practicing clinician, as it gives us solid information with which to engage and advise patients in a shared decision-making process for effective treatment of depression.”
“Some patients will choose psychotherapy, some will choose medications. They can make either choice with the confidence that both approaches are effective,” Dr. Skolnik elaborated. “In addition, if psychotherapy does not seem to be sufficiently helping we are on solid ground adding an antidepressant medication to psychotherapy, with this data showing that the combined treatment works better than psychotherapy alone.”
Dr. Cuijpers receives allowances for his memberships on the board of directors of Mind, Fonds Psychische Gezondheid, and Korrelatie, and for being chair of the PACO committee of the Raad voor Civiel-militaire Zorg en Onderzoek of the Dutch Ministry of Defense. He also serves as deputy editor of Depression and Anxiety and associate editor of Psychological Bulletin, and he receives royalties for books he has authored or coauthored. He received grants from the European Union, ZonMw, and PFGV. Another study author reported receiving personal fees from Mitsubishi-Tanabe, MSD, and Shionogi and a grant from Mitsubishi-Tanabe outside the submitted work. One author has received research and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation, and Angelini Pharmam, while another reported receiving personal fees from Boehringer Ingelheim, Kyowa Kirin, ASKA Pharmaceutical, and Toyota Motor Corporation outside the submitted work. The other authors and Dr. Skolnik reported no conflicts.
according to a network meta-analysis (NMA) comparing either and both approaches with control conditions in the primary care setting.
The findings are important, since the majority of depressed patients are treated by primary care physicians, yet relatively few randomized trials of treatment have focused on this setting, noted senior study author Pim Cuijpers, PhD, from Vrije Universiteit Amsterdam, and colleagues, in the paper, which was published in Annals of Family Medicine.
“The main message is that clinicians should certainly consider psychotherapy instead of pharmacotherapy, because this is preferred by most patients, and when possible, combined treatments should be the preferred choice because the outcomes are considerably better,” he said in an interview. Either way, he emphasized that “preference of patients is very important and all three treatments are better than usual care.”
The NMA included studies comparing psychotherapy, antidepressant medication, or a combination of both, with control conditions (defined as usual care, wait list, or pill placebo) in adult primary care patients with depression.
Patients could have major depression, persistent mood disorders (dysthymia), both, or high scores on self-rating depression scales. The primary outcome of the NMA was response, defined as a 50% improvement in the Hamilton Depression Rating scores (HAM-D).
A total of 58 studies met inclusion criteria, involving 9,301 patients.
Treatment options compared
Compared with usual care, both psychotherapy alone and pharmacotherapy alone had significantly better response rates, with no significant difference between them (relative risk, 1.60 and RR, 1.65, respectively). The combination of psychotherapy and pharmacotherapy was even better (RR, 2.15), whereas the wait list was less effective (RR, 0.68).
When comparing combined therapy with psychotherapy or pharmacotherapy, the superiority of combination therapy over psychotherapy was only slightly statistically significant (RR, 1.35; 95% confidence interval, 1.00-1.81), while pharmacotherapy was only slightly inferior (RR, 1.30; 95% CI, 0.98-1.73).
“The significance level is not very high, which is related to statistical power,” said Dr. Cuijpers. “But the mean benefit is quite substantial in my opinion, with a 35% higher chance of response in the combined treatment, compared to psychotherapy alone.”
Looking at the outcome of remission, (normally defined as a score of 7 or less on the HAM-D), the outcomes were “comparable to those for response, with the exception that combined treatment was not significantly different from psychotherapy,” they wrote.
One important caveat is that several studies included in the NMA included patients with moderate to severe depression, a population that is different from the usual primary care population of depressed patients who have mild to moderate symptoms. Antidepressant medications are also assumed to work better against more severe symptoms, added the authors. “The inclusion of these studies might therefore have resulted in an overestimation of the effects of pharmacotherapy in the present NMA.”
Among other limitations, the authors noted that studies included mixed populations of patients with dysthymia and major depression; they also made no distinction between different types of antidepressants.
Psychotherapies unknown, but meta-analysis is still useful
Commenting on these findings, Neil Skolnik, MD, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, said this is “an important study, confirming and extending the conclusions” of a systematic review published in 2016 as a Clinical Practice Guideline from the American College of Physicians.
“Unfortunately, the authors did not specify what type of psychotherapy was studied in the meta-analysis, so we have to look elsewhere if we want to advise our patients on what type of psychotherapy to seek, since there are important differences between different types of therapy,” he said.
Still, he described the study as providing “helpful information for the practicing clinician, as it gives us solid information with which to engage and advise patients in a shared decision-making process for effective treatment of depression.”
“Some patients will choose psychotherapy, some will choose medications. They can make either choice with the confidence that both approaches are effective,” Dr. Skolnik elaborated. “In addition, if psychotherapy does not seem to be sufficiently helping we are on solid ground adding an antidepressant medication to psychotherapy, with this data showing that the combined treatment works better than psychotherapy alone.”
Dr. Cuijpers receives allowances for his memberships on the board of directors of Mind, Fonds Psychische Gezondheid, and Korrelatie, and for being chair of the PACO committee of the Raad voor Civiel-militaire Zorg en Onderzoek of the Dutch Ministry of Defense. He also serves as deputy editor of Depression and Anxiety and associate editor of Psychological Bulletin, and he receives royalties for books he has authored or coauthored. He received grants from the European Union, ZonMw, and PFGV. Another study author reported receiving personal fees from Mitsubishi-Tanabe, MSD, and Shionogi and a grant from Mitsubishi-Tanabe outside the submitted work. One author has received research and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation, and Angelini Pharmam, while another reported receiving personal fees from Boehringer Ingelheim, Kyowa Kirin, ASKA Pharmaceutical, and Toyota Motor Corporation outside the submitted work. The other authors and Dr. Skolnik reported no conflicts.
according to a network meta-analysis (NMA) comparing either and both approaches with control conditions in the primary care setting.
The findings are important, since the majority of depressed patients are treated by primary care physicians, yet relatively few randomized trials of treatment have focused on this setting, noted senior study author Pim Cuijpers, PhD, from Vrije Universiteit Amsterdam, and colleagues, in the paper, which was published in Annals of Family Medicine.
“The main message is that clinicians should certainly consider psychotherapy instead of pharmacotherapy, because this is preferred by most patients, and when possible, combined treatments should be the preferred choice because the outcomes are considerably better,” he said in an interview. Either way, he emphasized that “preference of patients is very important and all three treatments are better than usual care.”
The NMA included studies comparing psychotherapy, antidepressant medication, or a combination of both, with control conditions (defined as usual care, wait list, or pill placebo) in adult primary care patients with depression.
Patients could have major depression, persistent mood disorders (dysthymia), both, or high scores on self-rating depression scales. The primary outcome of the NMA was response, defined as a 50% improvement in the Hamilton Depression Rating scores (HAM-D).
A total of 58 studies met inclusion criteria, involving 9,301 patients.
Treatment options compared
Compared with usual care, both psychotherapy alone and pharmacotherapy alone had significantly better response rates, with no significant difference between them (relative risk, 1.60 and RR, 1.65, respectively). The combination of psychotherapy and pharmacotherapy was even better (RR, 2.15), whereas the wait list was less effective (RR, 0.68).
When comparing combined therapy with psychotherapy or pharmacotherapy, the superiority of combination therapy over psychotherapy was only slightly statistically significant (RR, 1.35; 95% confidence interval, 1.00-1.81), while pharmacotherapy was only slightly inferior (RR, 1.30; 95% CI, 0.98-1.73).
“The significance level is not very high, which is related to statistical power,” said Dr. Cuijpers. “But the mean benefit is quite substantial in my opinion, with a 35% higher chance of response in the combined treatment, compared to psychotherapy alone.”
Looking at the outcome of remission, (normally defined as a score of 7 or less on the HAM-D), the outcomes were “comparable to those for response, with the exception that combined treatment was not significantly different from psychotherapy,” they wrote.
One important caveat is that several studies included in the NMA included patients with moderate to severe depression, a population that is different from the usual primary care population of depressed patients who have mild to moderate symptoms. Antidepressant medications are also assumed to work better against more severe symptoms, added the authors. “The inclusion of these studies might therefore have resulted in an overestimation of the effects of pharmacotherapy in the present NMA.”
Among other limitations, the authors noted that studies included mixed populations of patients with dysthymia and major depression; they also made no distinction between different types of antidepressants.
Psychotherapies unknown, but meta-analysis is still useful
Commenting on these findings, Neil Skolnik, MD, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, said this is “an important study, confirming and extending the conclusions” of a systematic review published in 2016 as a Clinical Practice Guideline from the American College of Physicians.
“Unfortunately, the authors did not specify what type of psychotherapy was studied in the meta-analysis, so we have to look elsewhere if we want to advise our patients on what type of psychotherapy to seek, since there are important differences between different types of therapy,” he said.
Still, he described the study as providing “helpful information for the practicing clinician, as it gives us solid information with which to engage and advise patients in a shared decision-making process for effective treatment of depression.”
“Some patients will choose psychotherapy, some will choose medications. They can make either choice with the confidence that both approaches are effective,” Dr. Skolnik elaborated. “In addition, if psychotherapy does not seem to be sufficiently helping we are on solid ground adding an antidepressant medication to psychotherapy, with this data showing that the combined treatment works better than psychotherapy alone.”
Dr. Cuijpers receives allowances for his memberships on the board of directors of Mind, Fonds Psychische Gezondheid, and Korrelatie, and for being chair of the PACO committee of the Raad voor Civiel-militaire Zorg en Onderzoek of the Dutch Ministry of Defense. He also serves as deputy editor of Depression and Anxiety and associate editor of Psychological Bulletin, and he receives royalties for books he has authored or coauthored. He received grants from the European Union, ZonMw, and PFGV. Another study author reported receiving personal fees from Mitsubishi-Tanabe, MSD, and Shionogi and a grant from Mitsubishi-Tanabe outside the submitted work. One author has received research and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation, and Angelini Pharmam, while another reported receiving personal fees from Boehringer Ingelheim, Kyowa Kirin, ASKA Pharmaceutical, and Toyota Motor Corporation outside the submitted work. The other authors and Dr. Skolnik reported no conflicts.
FROM ANNALS OF FAMILY MEDICINE
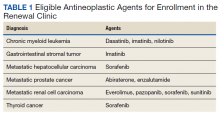
Outcomes Associated With Pharmacist- Led Consult Service for Opioid Tapering and Pharmacotherapy
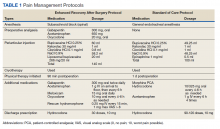
In the late 1980s and early 1990s, an emphasis on better pain management led health care professionals (HCPs) to increase prescribing of opioids to better manage patient’s pain. In 1991, 76 million prescriptions were written for opioids in the United States, and by 2011, the number had nearly tripled to 219 million.1 Overdose rates increased as well, nearly tripling from 1999 to 2014.2 Of the 52,404 US deaths from drug overdoses in the in 2015, 63% involved an opioid.2
Opioid Safety Initiative
In response to the growing opioid epidemic, the US Department of Veterans Affairs (VA) created the Opioid Safety Initiative in 2014.3 This comprehensive, multifaceted initiative was designed to improve the care and safety of veterans managed with opioid therapy and promote rational opioid prescribing and monitoring. In 2016 the Centers for Disease Control and Prevention (CDC) issued guidelines for opioid prescriptions, and the following year the VA and the US Department of Defense (DoD) updated the VA/DoD Clinical Practice Guidelines for Opioid Therapy for Chronic Pain (VA/DoD guidelines).4,5 After the release of these guidelines, the use of opioid tapers expanded. However, due to public outcry of forced opioid tapering in 2019, the US Food and Drug Administration updated its opioid labeling requirements to provide clearer guidance on opioid tapers for tolerant patients.6,7
As a result, HCPs began to develop various strategies to balance the safety and efficacy of opioid use in patients with chronic pain. The West Palm Beach VA Medical Center (WPBVAMC) in Florida has a Pain Clinic that includes 2 pain management clinical pharmacy specialists (CPSs) with specialized training in pain management, who are uniquely qualified to assess and evaluate medication therapy in complex pain patient cases. These CPSs were involved in the face-to-face management of patients requiring specialized pain care and participated in a pain pharmacy electronic consult (eConsult) service to document pain management consultative recommendations for patients appropriate for management at the primary care level. This formalized process increased specialty pain care access for veterans whose pain was managed by primary care providers (PCPs).
The pain pharmacy eConsult service was initiated at the WPBVAMC in June 2013 to assist PCPs in the management of outpatients with chronic pain. The eConsult service includes evaluation of a patient’s electronic health records (EHRs) by CPSs. The eConsult service also provided PCPs with the option to engage a pharmacist who could provide recommendations for opioid dosing conversion, opioid tapering, pain pharmacotherapy, or drug screen interpretation, without the necessity for an additional patient visit.
Subsequent to the release of the 2016 CDC (and later the 2017 VA/DoD) guidelines recommending reducing morphine equivalent daily dose (MEDD) levels, the WPBVAMC had a large increase in pain eConsult requests for opioid tapering and opioid pharmacotherapy. A 3.4-fold increase in requests occurred in March, April, and May vs the following 9 months, and a nearly 4-fold increase in requests for opioid tapers during the same period. However, the impact of the completed eConsults was unclear. Therefore, the primary objective of this study was to assess the effect of CPS services for opioid tapering and opioid pharmacotherapy by quantifying the number of recommendations accepted/implemented by PCPs. The secondary objectives included evaluating harms associated with the recommendations (eg, increase in visits to the emergency department [ED], hospitalizations, suicide attempts, or PCP visits) and provider satisfaction.
Methods
A retrospective chart review was completed to assess data of patients from the WPBVAMC and its associated community-based outpatient clinics (CBOCs). The project was approved by the WPBVAMC Scientific Advisory Committee as part of the facility’s performance improvement efforts.
Included patients had a pain pharmacy eConsult placed between April 1, 2016 and March 31, 2017. EHRs were reviewed and only eConsults for opioid pharmacotherapy recommendation or opioid tapers were evaluated. eConsults were excluded if the request was discontinued, completed by a HCP other than the pain CPS, or placed for an opioid dose conversion, nonopioid pharmacotherapy, or drug screen interpretation.
Data for analyses were entered into Microsoft Excel 2016 and were securely saved and accessible to relevant researchers. Patient protected health information used during patient care remained confidential.
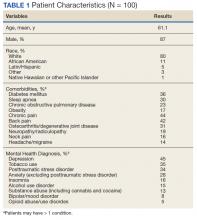
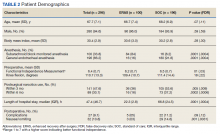
Demographic data were collected, including age, gender, race, pertinent medical comorbidities (eg, diabetes mellitus, sleep apnea), and mental health comorbidities. Pain scores were collected at baseline and 6-months postconsult. Pain medications used by patients were noted at baseline and 6 months postconsult, including concomitant opioid and benzodiazepine use, MEDD, and other pain medication. The duration of time needed by pain CPS to complete each eConsult and total time from eConsult entered to HCP implementation of the initial recommendation was collected. The number of actionable recommendations (eg, changes in drug therapy, urine drug screens [UDSs], and referrals to other services also were recorded and reviewed 6 months postconsult to determine the number and percentage of recommendations implemented by the HCP. The EHR was examined to determine adverse events (AEs) (eg, any documentation of suicide attempt, calls to the Veterans Crisis Line, or death 6 month postconsult). Collected data also included new eConsults, the reason for opioid tapering either by HCP or patient, and assessment of economic harms (count of the number of visits to ED, hospitalizations, or unscheduled PCP visits with uncontrolled pain as chief reason within 6 months postconsult). Last, PCPs were sent a survey to assess their satisfaction with the pain eConsult service.
Results
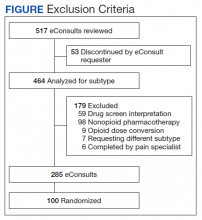
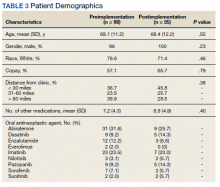
Of 517 eConsults received from April 1, 2016 to March 31, 2017, 285 (55.1%) met inclusion criteria (Figure). Using a random number generator, 100 eConsults were further reviewed for outcomes of interest.
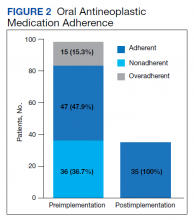
In this cohort, the mean age was 61 years, 87% were male, and 80% were White individuals. Most patients (83%) had ≥ 1 mental health comorbidity, and 53% had ≥ 2, with depressive symptoms, tobacco use, and/or posttraumatic stress disorder the most common diagnoses (Table 1). Eighty-seven percent of eConsults were for opioid tapers and the remaining 13% were for opioid pharmacotherapy.
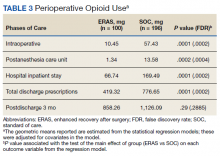
The median pain score at time of consult was 6 on a 10-point scale, with no change at 6 months postconsult. However, 41% of patients overall had a median 3.3-point drop in pain score, 17% had no change in pain score, and 42% had a median 2.6-point increase in pain score.
At time of consult, 24% of patients had an opioid and benzodiazepine prescribed concurrently. At the time of the initial request, the mean MEDD was 177.5 mg (median, 165; range, 0-577.5). At 6 months postconsult, the average MEDD was 71 mg (median, 90; range, 0-450) for a mean 44% MEDD decrease. Eighteen percent of patients had no change in MEDD, and 5% had an increase.
One concern was the number of patients whose pain management regimen consisted of either opioids as monotherapy or a combination of opioids and skeletal muscle relaxants (SMRs), which can increase the opioid overdose risk and are not indicated for long-term use (except for baclofen for spasticity). Thirty-five percent of patients were taking either opioid monotherapy or opioids and SMRs for chronic pain management at time of consult and 28% were taking opioid monotherapy or opioids and SMRs 6 months postconsult.
Electronic Consults
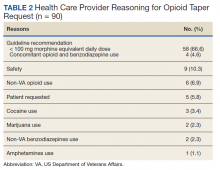
Table 2 describes the reasons eConsults were requested. The most common reason was to taper the dose to be in compliance with the CDC 2016 guideline recommendation of MEDD < 90 mg, which was later increased to 100 mg by the VA/DoD guideline.
On average, eConsults were completed within a mean of 11.5 days of the PCP request, including nights and weekends. The CPS spent a mean 66.8 minutes to complete each eConsult. Once the eConsult was completed, PCPs took a mean of 9 days to initiate the primary recommendation. This 9-day average does not include 11 eConsults with no accepted recommendations and 11 eConsults for which the PCP implemented the primary recommendation before the CPS completed the consult, most likely due to a phone call or direct contact with the CPS at the time the eConsult was ordered.
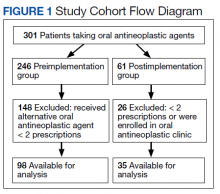
A mean 3.5 actionable recommendations were made by the CPS and a mean 1.6 recommendations were implemented within 6 months by the PCP. At least 1 recommendation was accepted/implemented for 89% of patients, with a mean 55% recommendations that were accepted/implemented. Eleven percent of the eConsult final recommendations were not accepted by PCPs and clear documentation of the reasons were not provided.
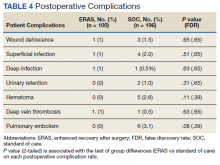
Adverse Outcomes
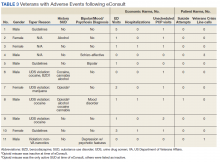
In the 6 months postconsult, 11 patients (7 men and 4 women) experienced 32 AEs (Table 3). Eight patients had 15 ED visits, with 3 of the visits resulting in hospitalizations, 8 patients had 9 unscheduled PCP visits, 1 patient reported suicidal ideation and 2 patients made a total of 4 calls to the Veterans Crisis Line. There were also 2 deaths; however, both were due to end-stage disease (cirrhosis and amyotrophic lateral sclerosis) and not believed to be related to eConsult recommendations.
Eight patients had a history of substance use disorders (SUDs) and 8 had a history of a mood disorder or psychosis. One patient had both SUD and a mood/psychosis-related mental health disorder, including a reported suicidal attempt/ideation at an ED visit and a subsequent hospitalization. A similar number of AEs occurred in patients with decreases in MEDD of 0 to 24% compared with those that received more aggressive tapers of 75 to 100% (Table 4).
Nine patients were reconsulted, with only 1 secondary to the PCP not implementing recommendations from the initial consult. No factors were found that correlated with likelihood of a patient being reconsulted.
Surveys on PCP satisfaction with the eConsult service were completed by 29 of the 55 PCPs. PCP feedback was generally positive with nearly 90% of PCPs planning to use the service in the future as well as recommending use to other providers.
PCPs also were given the option to indicate the most important factor for overall satisfaction with eConsult service (time, access, safety, expectations or confidence). Safety was provider’s top choice with time being a close second.
Discussion
Most (89%) PCPs accepted at least 1 recommendation from the completed eConsult, and MEDDs decreased by 60%, likely reducing the patient’s risk of overdose or other AEs from opioids. There also was a slight reduction in patient’s mean pain scores; however, 41% had a decrease and 42% had an increase in pain scores. There was no clear relationship when pain scores were compared with MEDDs, likely giving credence to the idea that pain scores are largely subjective and an unreliable surrogate marker for assessing effectiveness of analgesic regimens.
Eleven patients experienced AEs, including 1 patient for whom the recommendations were not implemented by the PCP. Eight of the 11 had multiple AEs. One interesting finding was that 7 of the 11 patients with an AE tested positive for unexpected substances on routine UDS or were arrested for driving while intoxicated (DWI). However, only 3 of the 7 had an active SUD diagnosis. With 25% of the AEs coming from patients with a history of SUD, it is important that any history of SUD be documented in the EHR. Maintaining this documentation can be especially difficult if patients switch VA medical centers or receive services outside the VA. Thorough and accurate history and chart review should ideally be completed before prescribing opioids.
Guidelines
While the PCPs were following VA/DoD and CDC recommendations for opioid tapering to < 100 or 90 mg MEDD, respectively, there is weak evidence in these guidelines to support specific MEDD cutoffs. The CDC guidelines even state, “a single dosage threshold for safe opioid use could not be identified.”5 One of the largest issues when using MEDD as a cutoff is the lack of agreement on its calculation. In 2014, Nuckols and colleagues al conducted a study to compare the existing guidelines on the use of opioids for chronic pain. While 13 guidelines were considered eligible, most recommendations were supported only by observational data or expert recommendations, and there was no consensus on what constitutes a “morphine equivalent.”8 Currently there is no universally accepted opioid-conversion method, resulting in a substantial problem when calculating a MEDD.9 A survey of 8 online opioid dose conversion tools found a -55% to +242% variation.10 As Fudin and colleagues concluded in response to the large variations found in these various analyses, the studies “unequivocally disqualify the validity of embracing MEDD to assess risk in any meaningful statistical way.”11 Pharmacogenetics, drug tolerance, drug-drug interactions, body surface area, and organ function are patient- specific factors that are not taken into consideration when relying solely on a MEDD calculation. Tapering to lowest functional dose rather than a specific number or cutoff may be a more effective way to treat patients, and providers should use the guidelines as recommendations and not a hardline mandate.
At 6 months, 6 patients were receiving no pain medications from the VA, and 24 of the patients were tapered from their opiate to discontinuation. It is unclear whether patients are no longer taking opioids or switched their care to non-VA providers to receive medications, including opioids, privately. This is difficult to verify, though a prescription drug monitoring program (PDMP) could be used to assess patient adherence. As many of the patients that were tapered due to identification of aberrant behaviors, lack of continuity of care across health care systems may result in future patient harm.
The results of this analysis highlight the importance of checking PDMP databases and routine UDSs when prescribing opioids—there can be serious safety concerns if patients are taking other prescribed or illicit medications. However, care must be taken; there were 2 instances of patients’ chronic opioid prescriptions discontinued by their VA provider after a review of the PDMP showed they had received non-VA opioids. In both cases, the quantity and doses received were small (counts of ≤ 12) and were received more than 6 months prior to the check of the PDMP. While this constitutes a breach of the Informed Consent for long-term opioid use, if there are no other concerning behaviors, it may be more prudent to review the informed consent with the patient and discuss why the behavior is a breach to ensure that patients and PCPs continue to work as a team to manage chronic pain.
Limitations
The study population was one limitation of this project. While data suggest that chronic pain affects women more than men, this study’s population was only 13% female. Thirty percent of the women in this study had an AE compared with only 8% of the men. Additional limitations included use of problem list for comorbidities, as lists may be inaccurate or outdated, and limiting the monitoring of AE to only 6 months. As some tapers were not initiated immediately and some taper schedules can last several months to years; therefor, outcomes may have been higher if patients were followed longer. Many of the patients with AEs had increased ED visits or unscheduled primary care visits as the tapers went on and their pain worsened, but the visits were outside the 6-month time frame for data collection. An additional weakness of this review included assessing a pain score, but not functional status, which may be a better predictor of the effectiveness of a patient’s pain management regimen. This assessment is needed in future studies for more reliable data. Finally, PCP survey results also should be viewed with caution. The current survey had only 29 respondents, and the 2014 survey had only 10 respondents and did not include CBOC providers.
Conclusion
A pain eConsult service managed by CPSs specializing in pain management can assist patients and PCPs with opioid therapy recommendations in a safe and timely manner, reducing risk of overdose secondary to high dose opioid therapy and with limited harm to patients.
1. National Institute on Drug Abuse. Increased drug availability is associated with increased use and overdose. Published June 9, 2020. Accessed February 19, 2021. https://www.drugabuse.gov/publications/research-reports/prescription-opioids-heroin/increased-drug-availability-associated-increased-use-overdose
2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30.doi:10.15585/mmwr.mm655051e1
3. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection – VA patterns of dispensing take-home opioids and monitoring patients on opioid therapy. Report 14-00895-163. Published May 14, 2014. Accessed February 2, 2021. https://www.va.gov/oig/pubs/VAOIG-14-00895-163.pdf
4. US Department of Veterans Affairs, US Department of Defense, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guidelines for opioid therapy for chronic pain. Version 3.0. Published December 2017. Accessed February 2, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016 [published correction appears in MMWR Recomm Rep. 2016;65(11):295]. MMWR Recomm Rep. 2016;65(1):1-49. Published 2016 Mar 18. doi:10.15585/mmwr.rr6501e1.
6. US Food and Drug Administration. (2019). FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. Updated April 17, 2019. Accessed February 2, 2021. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes
7. Dowell D, Haegerich T, Chou R. No Shortcuts to Safer Opioid Prescribing. N Engl J Med. 2019;380(24):2285-2287. doi:10.1056/NEJMp1904190
8. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160(1):38-47. doi:10.7326/0003-4819-160-1-201401070-00732
9. Rennick A, Atkinson T, Cimino NM, Strassels SA, McPherson ML, Fudin J. Variability in Opioid Equivalence Calculations. Pain Med. 2016;17(5):892-898. doi:10.1111/pme.12920
10. Shaw K, Fudin J. Evaluation and comparison of online equianalgesic opioid dose conversion calculators. Pract Pain Manag. 2013;13(7):61-66.
11. Fudin J, Pratt Cleary J, Schatman ME. The MEDD myth: the impact of pseudoscience on pain research and prescribing-guideline development. J Pain Res. 2016;9:153-156. Published 2016 Mar 23. doi:10.2147/JPR.S107794
In the late 1980s and early 1990s, an emphasis on better pain management led health care professionals (HCPs) to increase prescribing of opioids to better manage patient’s pain. In 1991, 76 million prescriptions were written for opioids in the United States, and by 2011, the number had nearly tripled to 219 million.1 Overdose rates increased as well, nearly tripling from 1999 to 2014.2 Of the 52,404 US deaths from drug overdoses in the in 2015, 63% involved an opioid.2
Opioid Safety Initiative
In response to the growing opioid epidemic, the US Department of Veterans Affairs (VA) created the Opioid Safety Initiative in 2014.3 This comprehensive, multifaceted initiative was designed to improve the care and safety of veterans managed with opioid therapy and promote rational opioid prescribing and monitoring. In 2016 the Centers for Disease Control and Prevention (CDC) issued guidelines for opioid prescriptions, and the following year the VA and the US Department of Defense (DoD) updated the VA/DoD Clinical Practice Guidelines for Opioid Therapy for Chronic Pain (VA/DoD guidelines).4,5 After the release of these guidelines, the use of opioid tapers expanded. However, due to public outcry of forced opioid tapering in 2019, the US Food and Drug Administration updated its opioid labeling requirements to provide clearer guidance on opioid tapers for tolerant patients.6,7
As a result, HCPs began to develop various strategies to balance the safety and efficacy of opioid use in patients with chronic pain. The West Palm Beach VA Medical Center (WPBVAMC) in Florida has a Pain Clinic that includes 2 pain management clinical pharmacy specialists (CPSs) with specialized training in pain management, who are uniquely qualified to assess and evaluate medication therapy in complex pain patient cases. These CPSs were involved in the face-to-face management of patients requiring specialized pain care and participated in a pain pharmacy electronic consult (eConsult) service to document pain management consultative recommendations for patients appropriate for management at the primary care level. This formalized process increased specialty pain care access for veterans whose pain was managed by primary care providers (PCPs).
The pain pharmacy eConsult service was initiated at the WPBVAMC in June 2013 to assist PCPs in the management of outpatients with chronic pain. The eConsult service includes evaluation of a patient’s electronic health records (EHRs) by CPSs. The eConsult service also provided PCPs with the option to engage a pharmacist who could provide recommendations for opioid dosing conversion, opioid tapering, pain pharmacotherapy, or drug screen interpretation, without the necessity for an additional patient visit.
Subsequent to the release of the 2016 CDC (and later the 2017 VA/DoD) guidelines recommending reducing morphine equivalent daily dose (MEDD) levels, the WPBVAMC had a large increase in pain eConsult requests for opioid tapering and opioid pharmacotherapy. A 3.4-fold increase in requests occurred in March, April, and May vs the following 9 months, and a nearly 4-fold increase in requests for opioid tapers during the same period. However, the impact of the completed eConsults was unclear. Therefore, the primary objective of this study was to assess the effect of CPS services for opioid tapering and opioid pharmacotherapy by quantifying the number of recommendations accepted/implemented by PCPs. The secondary objectives included evaluating harms associated with the recommendations (eg, increase in visits to the emergency department [ED], hospitalizations, suicide attempts, or PCP visits) and provider satisfaction.
Methods
A retrospective chart review was completed to assess data of patients from the WPBVAMC and its associated community-based outpatient clinics (CBOCs). The project was approved by the WPBVAMC Scientific Advisory Committee as part of the facility’s performance improvement efforts.
Included patients had a pain pharmacy eConsult placed between April 1, 2016 and March 31, 2017. EHRs were reviewed and only eConsults for opioid pharmacotherapy recommendation or opioid tapers were evaluated. eConsults were excluded if the request was discontinued, completed by a HCP other than the pain CPS, or placed for an opioid dose conversion, nonopioid pharmacotherapy, or drug screen interpretation.
Data for analyses were entered into Microsoft Excel 2016 and were securely saved and accessible to relevant researchers. Patient protected health information used during patient care remained confidential.
Demographic data were collected, including age, gender, race, pertinent medical comorbidities (eg, diabetes mellitus, sleep apnea), and mental health comorbidities. Pain scores were collected at baseline and 6-months postconsult. Pain medications used by patients were noted at baseline and 6 months postconsult, including concomitant opioid and benzodiazepine use, MEDD, and other pain medication. The duration of time needed by pain CPS to complete each eConsult and total time from eConsult entered to HCP implementation of the initial recommendation was collected. The number of actionable recommendations (eg, changes in drug therapy, urine drug screens [UDSs], and referrals to other services also were recorded and reviewed 6 months postconsult to determine the number and percentage of recommendations implemented by the HCP. The EHR was examined to determine adverse events (AEs) (eg, any documentation of suicide attempt, calls to the Veterans Crisis Line, or death 6 month postconsult). Collected data also included new eConsults, the reason for opioid tapering either by HCP or patient, and assessment of economic harms (count of the number of visits to ED, hospitalizations, or unscheduled PCP visits with uncontrolled pain as chief reason within 6 months postconsult). Last, PCPs were sent a survey to assess their satisfaction with the pain eConsult service.
Results
Of 517 eConsults received from April 1, 2016 to March 31, 2017, 285 (55.1%) met inclusion criteria (Figure). Using a random number generator, 100 eConsults were further reviewed for outcomes of interest.
In this cohort, the mean age was 61 years, 87% were male, and 80% were White individuals. Most patients (83%) had ≥ 1 mental health comorbidity, and 53% had ≥ 2, with depressive symptoms, tobacco use, and/or posttraumatic stress disorder the most common diagnoses (Table 1). Eighty-seven percent of eConsults were for opioid tapers and the remaining 13% were for opioid pharmacotherapy.
The median pain score at time of consult was 6 on a 10-point scale, with no change at 6 months postconsult. However, 41% of patients overall had a median 3.3-point drop in pain score, 17% had no change in pain score, and 42% had a median 2.6-point increase in pain score.
At time of consult, 24% of patients had an opioid and benzodiazepine prescribed concurrently. At the time of the initial request, the mean MEDD was 177.5 mg (median, 165; range, 0-577.5). At 6 months postconsult, the average MEDD was 71 mg (median, 90; range, 0-450) for a mean 44% MEDD decrease. Eighteen percent of patients had no change in MEDD, and 5% had an increase.
One concern was the number of patients whose pain management regimen consisted of either opioids as monotherapy or a combination of opioids and skeletal muscle relaxants (SMRs), which can increase the opioid overdose risk and are not indicated for long-term use (except for baclofen for spasticity). Thirty-five percent of patients were taking either opioid monotherapy or opioids and SMRs for chronic pain management at time of consult and 28% were taking opioid monotherapy or opioids and SMRs 6 months postconsult.
Electronic Consults
Table 2 describes the reasons eConsults were requested. The most common reason was to taper the dose to be in compliance with the CDC 2016 guideline recommendation of MEDD < 90 mg, which was later increased to 100 mg by the VA/DoD guideline.
On average, eConsults were completed within a mean of 11.5 days of the PCP request, including nights and weekends. The CPS spent a mean 66.8 minutes to complete each eConsult. Once the eConsult was completed, PCPs took a mean of 9 days to initiate the primary recommendation. This 9-day average does not include 11 eConsults with no accepted recommendations and 11 eConsults for which the PCP implemented the primary recommendation before the CPS completed the consult, most likely due to a phone call or direct contact with the CPS at the time the eConsult was ordered.
A mean 3.5 actionable recommendations were made by the CPS and a mean 1.6 recommendations were implemented within 6 months by the PCP. At least 1 recommendation was accepted/implemented for 89% of patients, with a mean 55% recommendations that were accepted/implemented. Eleven percent of the eConsult final recommendations were not accepted by PCPs and clear documentation of the reasons were not provided.
Adverse Outcomes
In the 6 months postconsult, 11 patients (7 men and 4 women) experienced 32 AEs (Table 3). Eight patients had 15 ED visits, with 3 of the visits resulting in hospitalizations, 8 patients had 9 unscheduled PCP visits, 1 patient reported suicidal ideation and 2 patients made a total of 4 calls to the Veterans Crisis Line. There were also 2 deaths; however, both were due to end-stage disease (cirrhosis and amyotrophic lateral sclerosis) and not believed to be related to eConsult recommendations.
Eight patients had a history of substance use disorders (SUDs) and 8 had a history of a mood disorder or psychosis. One patient had both SUD and a mood/psychosis-related mental health disorder, including a reported suicidal attempt/ideation at an ED visit and a subsequent hospitalization. A similar number of AEs occurred in patients with decreases in MEDD of 0 to 24% compared with those that received more aggressive tapers of 75 to 100% (Table 4).
Nine patients were reconsulted, with only 1 secondary to the PCP not implementing recommendations from the initial consult. No factors were found that correlated with likelihood of a patient being reconsulted.
Surveys on PCP satisfaction with the eConsult service were completed by 29 of the 55 PCPs. PCP feedback was generally positive with nearly 90% of PCPs planning to use the service in the future as well as recommending use to other providers.
PCPs also were given the option to indicate the most important factor for overall satisfaction with eConsult service (time, access, safety, expectations or confidence). Safety was provider’s top choice with time being a close second.
Discussion
Most (89%) PCPs accepted at least 1 recommendation from the completed eConsult, and MEDDs decreased by 60%, likely reducing the patient’s risk of overdose or other AEs from opioids. There also was a slight reduction in patient’s mean pain scores; however, 41% had a decrease and 42% had an increase in pain scores. There was no clear relationship when pain scores were compared with MEDDs, likely giving credence to the idea that pain scores are largely subjective and an unreliable surrogate marker for assessing effectiveness of analgesic regimens.
Eleven patients experienced AEs, including 1 patient for whom the recommendations were not implemented by the PCP. Eight of the 11 had multiple AEs. One interesting finding was that 7 of the 11 patients with an AE tested positive for unexpected substances on routine UDS or were arrested for driving while intoxicated (DWI). However, only 3 of the 7 had an active SUD diagnosis. With 25% of the AEs coming from patients with a history of SUD, it is important that any history of SUD be documented in the EHR. Maintaining this documentation can be especially difficult if patients switch VA medical centers or receive services outside the VA. Thorough and accurate history and chart review should ideally be completed before prescribing opioids.
Guidelines
While the PCPs were following VA/DoD and CDC recommendations for opioid tapering to < 100 or 90 mg MEDD, respectively, there is weak evidence in these guidelines to support specific MEDD cutoffs. The CDC guidelines even state, “a single dosage threshold for safe opioid use could not be identified.”5 One of the largest issues when using MEDD as a cutoff is the lack of agreement on its calculation. In 2014, Nuckols and colleagues al conducted a study to compare the existing guidelines on the use of opioids for chronic pain. While 13 guidelines were considered eligible, most recommendations were supported only by observational data or expert recommendations, and there was no consensus on what constitutes a “morphine equivalent.”8 Currently there is no universally accepted opioid-conversion method, resulting in a substantial problem when calculating a MEDD.9 A survey of 8 online opioid dose conversion tools found a -55% to +242% variation.10 As Fudin and colleagues concluded in response to the large variations found in these various analyses, the studies “unequivocally disqualify the validity of embracing MEDD to assess risk in any meaningful statistical way.”11 Pharmacogenetics, drug tolerance, drug-drug interactions, body surface area, and organ function are patient- specific factors that are not taken into consideration when relying solely on a MEDD calculation. Tapering to lowest functional dose rather than a specific number or cutoff may be a more effective way to treat patients, and providers should use the guidelines as recommendations and not a hardline mandate.
At 6 months, 6 patients were receiving no pain medications from the VA, and 24 of the patients were tapered from their opiate to discontinuation. It is unclear whether patients are no longer taking opioids or switched their care to non-VA providers to receive medications, including opioids, privately. This is difficult to verify, though a prescription drug monitoring program (PDMP) could be used to assess patient adherence. As many of the patients that were tapered due to identification of aberrant behaviors, lack of continuity of care across health care systems may result in future patient harm.
The results of this analysis highlight the importance of checking PDMP databases and routine UDSs when prescribing opioids—there can be serious safety concerns if patients are taking other prescribed or illicit medications. However, care must be taken; there were 2 instances of patients’ chronic opioid prescriptions discontinued by their VA provider after a review of the PDMP showed they had received non-VA opioids. In both cases, the quantity and doses received were small (counts of ≤ 12) and were received more than 6 months prior to the check of the PDMP. While this constitutes a breach of the Informed Consent for long-term opioid use, if there are no other concerning behaviors, it may be more prudent to review the informed consent with the patient and discuss why the behavior is a breach to ensure that patients and PCPs continue to work as a team to manage chronic pain.
Limitations
The study population was one limitation of this project. While data suggest that chronic pain affects women more than men, this study’s population was only 13% female. Thirty percent of the women in this study had an AE compared with only 8% of the men. Additional limitations included use of problem list for comorbidities, as lists may be inaccurate or outdated, and limiting the monitoring of AE to only 6 months. As some tapers were not initiated immediately and some taper schedules can last several months to years; therefor, outcomes may have been higher if patients were followed longer. Many of the patients with AEs had increased ED visits or unscheduled primary care visits as the tapers went on and their pain worsened, but the visits were outside the 6-month time frame for data collection. An additional weakness of this review included assessing a pain score, but not functional status, which may be a better predictor of the effectiveness of a patient’s pain management regimen. This assessment is needed in future studies for more reliable data. Finally, PCP survey results also should be viewed with caution. The current survey had only 29 respondents, and the 2014 survey had only 10 respondents and did not include CBOC providers.
Conclusion
A pain eConsult service managed by CPSs specializing in pain management can assist patients and PCPs with opioid therapy recommendations in a safe and timely manner, reducing risk of overdose secondary to high dose opioid therapy and with limited harm to patients.
In the late 1980s and early 1990s, an emphasis on better pain management led health care professionals (HCPs) to increase prescribing of opioids to better manage patient’s pain. In 1991, 76 million prescriptions were written for opioids in the United States, and by 2011, the number had nearly tripled to 219 million.1 Overdose rates increased as well, nearly tripling from 1999 to 2014.2 Of the 52,404 US deaths from drug overdoses in the in 2015, 63% involved an opioid.2
Opioid Safety Initiative
In response to the growing opioid epidemic, the US Department of Veterans Affairs (VA) created the Opioid Safety Initiative in 2014.3 This comprehensive, multifaceted initiative was designed to improve the care and safety of veterans managed with opioid therapy and promote rational opioid prescribing and monitoring. In 2016 the Centers for Disease Control and Prevention (CDC) issued guidelines for opioid prescriptions, and the following year the VA and the US Department of Defense (DoD) updated the VA/DoD Clinical Practice Guidelines for Opioid Therapy for Chronic Pain (VA/DoD guidelines).4,5 After the release of these guidelines, the use of opioid tapers expanded. However, due to public outcry of forced opioid tapering in 2019, the US Food and Drug Administration updated its opioid labeling requirements to provide clearer guidance on opioid tapers for tolerant patients.6,7
As a result, HCPs began to develop various strategies to balance the safety and efficacy of opioid use in patients with chronic pain. The West Palm Beach VA Medical Center (WPBVAMC) in Florida has a Pain Clinic that includes 2 pain management clinical pharmacy specialists (CPSs) with specialized training in pain management, who are uniquely qualified to assess and evaluate medication therapy in complex pain patient cases. These CPSs were involved in the face-to-face management of patients requiring specialized pain care and participated in a pain pharmacy electronic consult (eConsult) service to document pain management consultative recommendations for patients appropriate for management at the primary care level. This formalized process increased specialty pain care access for veterans whose pain was managed by primary care providers (PCPs).
The pain pharmacy eConsult service was initiated at the WPBVAMC in June 2013 to assist PCPs in the management of outpatients with chronic pain. The eConsult service includes evaluation of a patient’s electronic health records (EHRs) by CPSs. The eConsult service also provided PCPs with the option to engage a pharmacist who could provide recommendations for opioid dosing conversion, opioid tapering, pain pharmacotherapy, or drug screen interpretation, without the necessity for an additional patient visit.
Subsequent to the release of the 2016 CDC (and later the 2017 VA/DoD) guidelines recommending reducing morphine equivalent daily dose (MEDD) levels, the WPBVAMC had a large increase in pain eConsult requests for opioid tapering and opioid pharmacotherapy. A 3.4-fold increase in requests occurred in March, April, and May vs the following 9 months, and a nearly 4-fold increase in requests for opioid tapers during the same period. However, the impact of the completed eConsults was unclear. Therefore, the primary objective of this study was to assess the effect of CPS services for opioid tapering and opioid pharmacotherapy by quantifying the number of recommendations accepted/implemented by PCPs. The secondary objectives included evaluating harms associated with the recommendations (eg, increase in visits to the emergency department [ED], hospitalizations, suicide attempts, or PCP visits) and provider satisfaction.
Methods
A retrospective chart review was completed to assess data of patients from the WPBVAMC and its associated community-based outpatient clinics (CBOCs). The project was approved by the WPBVAMC Scientific Advisory Committee as part of the facility’s performance improvement efforts.
Included patients had a pain pharmacy eConsult placed between April 1, 2016 and March 31, 2017. EHRs were reviewed and only eConsults for opioid pharmacotherapy recommendation or opioid tapers were evaluated. eConsults were excluded if the request was discontinued, completed by a HCP other than the pain CPS, or placed for an opioid dose conversion, nonopioid pharmacotherapy, or drug screen interpretation.
Data for analyses were entered into Microsoft Excel 2016 and were securely saved and accessible to relevant researchers. Patient protected health information used during patient care remained confidential.
Demographic data were collected, including age, gender, race, pertinent medical comorbidities (eg, diabetes mellitus, sleep apnea), and mental health comorbidities. Pain scores were collected at baseline and 6-months postconsult. Pain medications used by patients were noted at baseline and 6 months postconsult, including concomitant opioid and benzodiazepine use, MEDD, and other pain medication. The duration of time needed by pain CPS to complete each eConsult and total time from eConsult entered to HCP implementation of the initial recommendation was collected. The number of actionable recommendations (eg, changes in drug therapy, urine drug screens [UDSs], and referrals to other services also were recorded and reviewed 6 months postconsult to determine the number and percentage of recommendations implemented by the HCP. The EHR was examined to determine adverse events (AEs) (eg, any documentation of suicide attempt, calls to the Veterans Crisis Line, or death 6 month postconsult). Collected data also included new eConsults, the reason for opioid tapering either by HCP or patient, and assessment of economic harms (count of the number of visits to ED, hospitalizations, or unscheduled PCP visits with uncontrolled pain as chief reason within 6 months postconsult). Last, PCPs were sent a survey to assess their satisfaction with the pain eConsult service.
Results
Of 517 eConsults received from April 1, 2016 to March 31, 2017, 285 (55.1%) met inclusion criteria (Figure). Using a random number generator, 100 eConsults were further reviewed for outcomes of interest.
In this cohort, the mean age was 61 years, 87% were male, and 80% were White individuals. Most patients (83%) had ≥ 1 mental health comorbidity, and 53% had ≥ 2, with depressive symptoms, tobacco use, and/or posttraumatic stress disorder the most common diagnoses (Table 1). Eighty-seven percent of eConsults were for opioid tapers and the remaining 13% were for opioid pharmacotherapy.
The median pain score at time of consult was 6 on a 10-point scale, with no change at 6 months postconsult. However, 41% of patients overall had a median 3.3-point drop in pain score, 17% had no change in pain score, and 42% had a median 2.6-point increase in pain score.
At time of consult, 24% of patients had an opioid and benzodiazepine prescribed concurrently. At the time of the initial request, the mean MEDD was 177.5 mg (median, 165; range, 0-577.5). At 6 months postconsult, the average MEDD was 71 mg (median, 90; range, 0-450) for a mean 44% MEDD decrease. Eighteen percent of patients had no change in MEDD, and 5% had an increase.
One concern was the number of patients whose pain management regimen consisted of either opioids as monotherapy or a combination of opioids and skeletal muscle relaxants (SMRs), which can increase the opioid overdose risk and are not indicated for long-term use (except for baclofen for spasticity). Thirty-five percent of patients were taking either opioid monotherapy or opioids and SMRs for chronic pain management at time of consult and 28% were taking opioid monotherapy or opioids and SMRs 6 months postconsult.
Electronic Consults
Table 2 describes the reasons eConsults were requested. The most common reason was to taper the dose to be in compliance with the CDC 2016 guideline recommendation of MEDD < 90 mg, which was later increased to 100 mg by the VA/DoD guideline.
On average, eConsults were completed within a mean of 11.5 days of the PCP request, including nights and weekends. The CPS spent a mean 66.8 minutes to complete each eConsult. Once the eConsult was completed, PCPs took a mean of 9 days to initiate the primary recommendation. This 9-day average does not include 11 eConsults with no accepted recommendations and 11 eConsults for which the PCP implemented the primary recommendation before the CPS completed the consult, most likely due to a phone call or direct contact with the CPS at the time the eConsult was ordered.
A mean 3.5 actionable recommendations were made by the CPS and a mean 1.6 recommendations were implemented within 6 months by the PCP. At least 1 recommendation was accepted/implemented for 89% of patients, with a mean 55% recommendations that were accepted/implemented. Eleven percent of the eConsult final recommendations were not accepted by PCPs and clear documentation of the reasons were not provided.
Adverse Outcomes
In the 6 months postconsult, 11 patients (7 men and 4 women) experienced 32 AEs (Table 3). Eight patients had 15 ED visits, with 3 of the visits resulting in hospitalizations, 8 patients had 9 unscheduled PCP visits, 1 patient reported suicidal ideation and 2 patients made a total of 4 calls to the Veterans Crisis Line. There were also 2 deaths; however, both were due to end-stage disease (cirrhosis and amyotrophic lateral sclerosis) and not believed to be related to eConsult recommendations.
Eight patients had a history of substance use disorders (SUDs) and 8 had a history of a mood disorder or psychosis. One patient had both SUD and a mood/psychosis-related mental health disorder, including a reported suicidal attempt/ideation at an ED visit and a subsequent hospitalization. A similar number of AEs occurred in patients with decreases in MEDD of 0 to 24% compared with those that received more aggressive tapers of 75 to 100% (Table 4).
Nine patients were reconsulted, with only 1 secondary to the PCP not implementing recommendations from the initial consult. No factors were found that correlated with likelihood of a patient being reconsulted.
Surveys on PCP satisfaction with the eConsult service were completed by 29 of the 55 PCPs. PCP feedback was generally positive with nearly 90% of PCPs planning to use the service in the future as well as recommending use to other providers.
PCPs also were given the option to indicate the most important factor for overall satisfaction with eConsult service (time, access, safety, expectations or confidence). Safety was provider’s top choice with time being a close second.
Discussion
Most (89%) PCPs accepted at least 1 recommendation from the completed eConsult, and MEDDs decreased by 60%, likely reducing the patient’s risk of overdose or other AEs from opioids. There also was a slight reduction in patient’s mean pain scores; however, 41% had a decrease and 42% had an increase in pain scores. There was no clear relationship when pain scores were compared with MEDDs, likely giving credence to the idea that pain scores are largely subjective and an unreliable surrogate marker for assessing effectiveness of analgesic regimens.
Eleven patients experienced AEs, including 1 patient for whom the recommendations were not implemented by the PCP. Eight of the 11 had multiple AEs. One interesting finding was that 7 of the 11 patients with an AE tested positive for unexpected substances on routine UDS or were arrested for driving while intoxicated (DWI). However, only 3 of the 7 had an active SUD diagnosis. With 25% of the AEs coming from patients with a history of SUD, it is important that any history of SUD be documented in the EHR. Maintaining this documentation can be especially difficult if patients switch VA medical centers or receive services outside the VA. Thorough and accurate history and chart review should ideally be completed before prescribing opioids.
Guidelines
While the PCPs were following VA/DoD and CDC recommendations for opioid tapering to < 100 or 90 mg MEDD, respectively, there is weak evidence in these guidelines to support specific MEDD cutoffs. The CDC guidelines even state, “a single dosage threshold for safe opioid use could not be identified.”5 One of the largest issues when using MEDD as a cutoff is the lack of agreement on its calculation. In 2014, Nuckols and colleagues al conducted a study to compare the existing guidelines on the use of opioids for chronic pain. While 13 guidelines were considered eligible, most recommendations were supported only by observational data or expert recommendations, and there was no consensus on what constitutes a “morphine equivalent.”8 Currently there is no universally accepted opioid-conversion method, resulting in a substantial problem when calculating a MEDD.9 A survey of 8 online opioid dose conversion tools found a -55% to +242% variation.10 As Fudin and colleagues concluded in response to the large variations found in these various analyses, the studies “unequivocally disqualify the validity of embracing MEDD to assess risk in any meaningful statistical way.”11 Pharmacogenetics, drug tolerance, drug-drug interactions, body surface area, and organ function are patient- specific factors that are not taken into consideration when relying solely on a MEDD calculation. Tapering to lowest functional dose rather than a specific number or cutoff may be a more effective way to treat patients, and providers should use the guidelines as recommendations and not a hardline mandate.
At 6 months, 6 patients were receiving no pain medications from the VA, and 24 of the patients were tapered from their opiate to discontinuation. It is unclear whether patients are no longer taking opioids or switched their care to non-VA providers to receive medications, including opioids, privately. This is difficult to verify, though a prescription drug monitoring program (PDMP) could be used to assess patient adherence. As many of the patients that were tapered due to identification of aberrant behaviors, lack of continuity of care across health care systems may result in future patient harm.
The results of this analysis highlight the importance of checking PDMP databases and routine UDSs when prescribing opioids—there can be serious safety concerns if patients are taking other prescribed or illicit medications. However, care must be taken; there were 2 instances of patients’ chronic opioid prescriptions discontinued by their VA provider after a review of the PDMP showed they had received non-VA opioids. In both cases, the quantity and doses received were small (counts of ≤ 12) and were received more than 6 months prior to the check of the PDMP. While this constitutes a breach of the Informed Consent for long-term opioid use, if there are no other concerning behaviors, it may be more prudent to review the informed consent with the patient and discuss why the behavior is a breach to ensure that patients and PCPs continue to work as a team to manage chronic pain.
Limitations
The study population was one limitation of this project. While data suggest that chronic pain affects women more than men, this study’s population was only 13% female. Thirty percent of the women in this study had an AE compared with only 8% of the men. Additional limitations included use of problem list for comorbidities, as lists may be inaccurate or outdated, and limiting the monitoring of AE to only 6 months. As some tapers were not initiated immediately and some taper schedules can last several months to years; therefor, outcomes may have been higher if patients were followed longer. Many of the patients with AEs had increased ED visits or unscheduled primary care visits as the tapers went on and their pain worsened, but the visits were outside the 6-month time frame for data collection. An additional weakness of this review included assessing a pain score, but not functional status, which may be a better predictor of the effectiveness of a patient’s pain management regimen. This assessment is needed in future studies for more reliable data. Finally, PCP survey results also should be viewed with caution. The current survey had only 29 respondents, and the 2014 survey had only 10 respondents and did not include CBOC providers.
Conclusion
A pain eConsult service managed by CPSs specializing in pain management can assist patients and PCPs with opioid therapy recommendations in a safe and timely manner, reducing risk of overdose secondary to high dose opioid therapy and with limited harm to patients.
1. National Institute on Drug Abuse. Increased drug availability is associated with increased use and overdose. Published June 9, 2020. Accessed February 19, 2021. https://www.drugabuse.gov/publications/research-reports/prescription-opioids-heroin/increased-drug-availability-associated-increased-use-overdose
2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30.doi:10.15585/mmwr.mm655051e1
3. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection – VA patterns of dispensing take-home opioids and monitoring patients on opioid therapy. Report 14-00895-163. Published May 14, 2014. Accessed February 2, 2021. https://www.va.gov/oig/pubs/VAOIG-14-00895-163.pdf
4. US Department of Veterans Affairs, US Department of Defense, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guidelines for opioid therapy for chronic pain. Version 3.0. Published December 2017. Accessed February 2, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016 [published correction appears in MMWR Recomm Rep. 2016;65(11):295]. MMWR Recomm Rep. 2016;65(1):1-49. Published 2016 Mar 18. doi:10.15585/mmwr.rr6501e1.
6. US Food and Drug Administration. (2019). FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. Updated April 17, 2019. Accessed February 2, 2021. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes
7. Dowell D, Haegerich T, Chou R. No Shortcuts to Safer Opioid Prescribing. N Engl J Med. 2019;380(24):2285-2287. doi:10.1056/NEJMp1904190
8. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160(1):38-47. doi:10.7326/0003-4819-160-1-201401070-00732
9. Rennick A, Atkinson T, Cimino NM, Strassels SA, McPherson ML, Fudin J. Variability in Opioid Equivalence Calculations. Pain Med. 2016;17(5):892-898. doi:10.1111/pme.12920
10. Shaw K, Fudin J. Evaluation and comparison of online equianalgesic opioid dose conversion calculators. Pract Pain Manag. 2013;13(7):61-66.
11. Fudin J, Pratt Cleary J, Schatman ME. The MEDD myth: the impact of pseudoscience on pain research and prescribing-guideline development. J Pain Res. 2016;9:153-156. Published 2016 Mar 23. doi:10.2147/JPR.S107794
1. National Institute on Drug Abuse. Increased drug availability is associated with increased use and overdose. Published June 9, 2020. Accessed February 19, 2021. https://www.drugabuse.gov/publications/research-reports/prescription-opioids-heroin/increased-drug-availability-associated-increased-use-overdose
2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30.doi:10.15585/mmwr.mm655051e1
3. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection – VA patterns of dispensing take-home opioids and monitoring patients on opioid therapy. Report 14-00895-163. Published May 14, 2014. Accessed February 2, 2021. https://www.va.gov/oig/pubs/VAOIG-14-00895-163.pdf
4. US Department of Veterans Affairs, US Department of Defense, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guidelines for opioid therapy for chronic pain. Version 3.0. Published December 2017. Accessed February 2, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016 [published correction appears in MMWR Recomm Rep. 2016;65(11):295]. MMWR Recomm Rep. 2016;65(1):1-49. Published 2016 Mar 18. doi:10.15585/mmwr.rr6501e1.
6. US Food and Drug Administration. (2019). FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. Updated April 17, 2019. Accessed February 2, 2021. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes
7. Dowell D, Haegerich T, Chou R. No Shortcuts to Safer Opioid Prescribing. N Engl J Med. 2019;380(24):2285-2287. doi:10.1056/NEJMp1904190
8. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160(1):38-47. doi:10.7326/0003-4819-160-1-201401070-00732
9. Rennick A, Atkinson T, Cimino NM, Strassels SA, McPherson ML, Fudin J. Variability in Opioid Equivalence Calculations. Pain Med. 2016;17(5):892-898. doi:10.1111/pme.12920
10. Shaw K, Fudin J. Evaluation and comparison of online equianalgesic opioid dose conversion calculators. Pract Pain Manag. 2013;13(7):61-66.
11. Fudin J, Pratt Cleary J, Schatman ME. The MEDD myth: the impact of pseudoscience on pain research and prescribing-guideline development. J Pain Res. 2016;9:153-156. Published 2016 Mar 23. doi:10.2147/JPR.S107794
Impact of an Oral Antineoplastic Renewal Clinic on Medication Possession Ratio and Cost-Savings
Evaluation of oral antineoplastic agent (OAN) adherence patterns have identified correlations between nonadherence or over-adherence and poorer disease-related outcomes. Multiple studies have focused on imatinib use in chronic myeloid leukemia (CML) due to its continuous, long-term use. A study by Ganesan and colleagues found that nonadherence to imatinib showed a significant decrease in 5-year event-free survival between 76.7% of adherent participants compared with 59.8% of nonadherent participants.1 This study found that 44% of patients who were adherent to imatinib achieved complete cytogenetic response vs only 26% of patients who were nonadherent. In another study of imatinib for CML, major molecular response (MMR) was strongly correlated with adherence and no patients with adherence < 80% were able to achieve MMR.2 Similarly, in studies of tamoxifen for breast cancer, < 80% adherence resulted in a 10% decrease in survival when compared to those who were more adherent.3,4
In addition to the clinical implications of nonadherence, there can be a significant cost associated with suboptimal use of these medications. The price of a single dose of OAN medication may cost as much as $440.5
The benefits of multidisciplinary care teams have been identified in many studies.6,7 While studies are limited in oncology, pharmacists provide vital contributions to the oncology multidisciplinary team when managing OANs as these health care professionals have expert knowledge of the medications, potential adverse events (AEs), and necessary monitoring parameters.8 In one study, patients seen by the pharmacist-led oral chemotherapy management program experienced improved clinical outcomes and response to therapy when compared with preintervention patients (early molecular response, 88.9% vs 54.8%, P = .01; major molecular response, 83.3% vs 57.6%, P = .06).9 During the study, 318 AEs were reported, leading to 235 pharmacist interventions to ameliorate AEs and improve adherence.
The primary objective of this study was to measure the impact of a pharmacist-driven OAN renewal clinic on medication adherence. The secondary objective was to estimate cost-savings of this new service.
Methods
Prior to July 2014, several limitations were identified related to OAN prescribing and monitoring at the Richard L. Roudebush Veterans Affairs Medical Center in Indianapolis, Indiana (RLRVAMC). The prescription ordering process relied primarily on the patient to initiate refills, rather than the prescriber OAN prescriptions also lacked consistency for number of refills or quantities dispensed. Furthermore, ordering of antineoplastic products was not limited to hematology/oncology providers. Patients were identified with significant supply on hand at the time of medication discontinuation, creating concerns for medication waste, tolerability, and nonadherence.
As a result, opportunities were identified to improve the prescribing process, recommended monitoring, toxicity and tolerability evaluation, medication reconciliation, and medication adherence. In July of 2014, the RLRVAMC adopted a new chemotherapy order entry system capable of restricting prescriptions to hematology/oncology providers and limiting dispensed quantities and refill amounts. A comprehensive pharmacist driven OAN renewal clinic was implemented on September 1, 2014 with the goal of improving long-term adherence and tolerability, in addition to minimizing medication waste.
Patients were eligible for enrollment in the clinic if they had a cancer diagnosis and were concomitantly prescribed an OAN outlined in Table 1. All eligible patients were automatically enrolled in the clinic when they were deemed stable on their OAN by a hematology/oncology pharmacy specialist. Stability was defined as ≤ Grade 1 symptoms associated with the toxicities of OAN therapy managed with or without intervention as defined by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Once enrolled in the renewal clinic, patients were called by an oncology pharmacy resident (PGY2) 1 week prior to any OAN refill due date. Patients were asked a series of 5 adherence and tolerability questions (Table 2) to evaluate renewal criteria for approval or need for further evaluation. These questions were developed based on targeted information and published reports on monitoring adherence.10,11 Criteria for renewal included: < 10% self-reported missed doses of the OAN during the previous dispensing period, no hospitalizations or emergency department visits since most recent hematology/oncology provider appointment, no changes to concomitant medication therapies, and no new or worsening medication-related AEs. Patients meeting all criteria were given a 30-day supply of OAN. Prescribing, dispensing, and delivery of OAN were facilitated by the pharmacist. Patient cases that did not meet criteria for renewal were escalated to the hematology/oncology provider or oncology clinical pharmacy specialist for further evaluation.
Study Design and Setting
This was a pre/post retrospective cohort, quality improvement study of patients enrolled in the RLRVAMC OAN pharmacist renewal clinic. The study was deemed exempt from institutional review board (IRB) by the US Department of Veterans Affairs (VA) Research and Development Department.
Study Population
Patients were included in the preimplementation group if they had received at least 2 prescriptions of an eligible OAN. Therapy for the preimplementation group was required to be a monthly duration > 21 days and between the dates of September 1, 2013 and August 31, 2014. Patients were included in the postimplementation group if they had received at least 2 prescriptions of the studied OANs between September 1, 2014 and January 31, 2015. Patients were excluded if they had filled < 2 prescriptions of OAN; were managed by a non-VA oncologist or hematologist; or received an OAN other than those listed in Table 1.
Data Collection
For all patients in both the pre- and postimplementation cohorts, a standardized data collection tool was used to collect the following via electronic health record review by a PGY2 oncology resident: age, race, gender, oral antineoplastic agent, refill dates, days’ supply, estimated unit cost per dose cancer diagnosis, distance from the RLRVAMC, copay status, presence of hospitalizations/ED visits/dosage reductions, discontinuation rates, reasons for discontinuation, and total number of current prescriptions. The presence or absence of dosage reductions were collected to identify concerns for tolerability, but only the original dose for the preimplementation group and dosage at time of clinic enrollment for the postimplementation group was included in the analysis.
Outcomes and Statistical Analyses
The primary outcome was medication adherence defined as the median medication possession ratio (MPR) before and after implementation of the clinic. Secondary outcomes included the proportion of patients who were adherent from before implementation to after implementation and estimated cost-savings of this clinic after implementation. MPR was used to estimate medication adherence by taking the cumulative day supply of medication on hand divided by the number of days on therapy.12 Number of days on therapy was determined by taking the difference on the start date of the new medication regimen and the discontinuation date of the same regimen. Patients were grouped by adherence into one of the following categories: < 0.8, 0.8 to 0.89, 0.9 to 1, and > 1.1. Patients were considered adherent if they reported taking ≥ 90% (MPR ≥ 0.9) of prescribed doses, adopted from the study by Anderson and colleagues.12 A patient with an MPR > 1, likely due to filling prior to the anticipated refill date, was considered 100% adherent (MPR = 1). If a patient switched OAN during the study, both agents were included as separate entities.
A conservative estimate of cost-savings was made by multiplying the RLRVAMC cost per unit of medication at time of initial prescription fill by the number of units taken each day multiplied by the total days’ supply on hand at time of therapy discontinuation. Patients with an MPR < 1 at time of therapy discontinuation were assumed to have zero remaining units on hand and zero cost savings was estimated. Waste, for purposes of cost-savings, was calculated for all MPR values > 1. Additional supply anticipated to be on hand from dose reductions was not included in the estimated cost of unused medication.
Descriptive statistics compared demographic characteristics between the pre- and postimplementation groups. MPR data were not normally distributed, which required the use of nonparametric Mann-Whitney U tests to compare pre- and postMPRs. Pearson χ2 compared the proportion of adherent patients between groups while descriptive statistics were used to estimate cost savings. Significance was determined based on a P value < .05. IBM SPSS Statistics software was used for all statistical analyses. As this was a complete sample of all eligible subjects, no sample size calculation was performed.
Results
In the preimplementation period, 246 patients received an OAN and 61 patients received an OAN in the postimplementation period (Figure 1). Of the 246 patients in the preimplementation period, 98 were eligible and included in the preimplementation group. Similarly, of the 61 patients in the postimplementation period, 35 patients met inclusion criteria for the postimplementation group. The study population was predominantly male with an average age of approximately 70 years in both groups (Table 3). More than 70% of the population in each group was White. No statistically significant differences between groups were identified. The most commonly prescribed OAN in the preimplementation group were abiraterone, imatinib, and enzalutamide (Table 3). In the postimplementation group, the most commonly prescribed agents were abiraterone, imatinib, pazopanib, and dasatinib. No significant differences were observed in prescribing of individual agents between the pre- and postimplementation groups or other characteristics that may affect adherence including patient copay status, number of concomitant medications, and driving distance from the RLRVAMC.
Thirty-six (36.7%) patients in the preimplementation group were considered nonadherent (MPR < 0.9) and 18 (18.4%) had an MPR < 0.8. Fifteen (15.3%) patients in the preimplementation clinic were considered overadherent (MPR > 1.1). Forty-seven (47.9%) patients in the preimplementation group were considered adherent (MPR 0.9 - 1.1) while all 35 (100%) patients in the postimplementation group were considered adherent (MPR 0.9 - 1.1). No non- or overadherent patients were identified in the postimplementation group (Figure 2). The median MPR for all patients in the preimplementation group was 0.94 compared with 1.06 (P < .001) in the postimplementation group.
Thirty-five (35.7%) patients had therapy discontinued or held in the preimplementation group compared with 2 (5.7%) patients in the postimplementation group (P < .001). Reasons for discontinuation in the preimplementation group included disease progression (n = 27), death (n = 3), lost to follow up (n = 2), and intolerability of therapy (n = 3). Both patients that discontinued therapy in the postimplementation group did so due to disease progression. Of the 35 patients who had their OAN discontinued or held in the preimplementation group, 14 patients had excess supply on hand at time of discontinuation. The estimated value of the unused medication was $37,890. Nine (25%) of the 35 patients who discontinued therapy had a dosage reduction during the course of therapy and the additional supply was not included in the cost estimate. Similarly, 1 of the 2 patients in the postimplementation group had their OAN discontinued during study. The cost of oversupply of medication at the time of therapy discontinuation was estimated at $1,555. No patients in the postimplementation group had dose reductions. After implementation of the OAN renewal clinic, the total cost savings between pre ($37,890) and postimplementation ($1,555) groups was $36,355.
Discussion
OANs are widely used therapies, with more than 25 million doses administered per year in the United States alone.12 The use of these agents will continue to grow as more targeted agents become available and patients request more convenient treatment options. The role for hematology/oncology clinical pharmacy services must adapt to this increased usage of OANs, including increasing pharmacist involvement in medication education, adherence and tolerability assessments, and proactive drug interaction monitoring.However, additional research is needed to determine optimal management strategies.
Our study aimed to compare OAN adherence among patients at a tertiary care VA hospital before and after implementation of a renewal clinic. The preimplementation population had a median MPR of 0.94 compared with 1.06 in the postimplementation group (P < .001). Although an ideal MPR is 1.0, we aimed for a slightly higher MPR to allow a supply buffer in the event of prescription delivery delays, as more than 90% of prescriptions are mailed to patients from a regional mail-order pharmacy. Importantly, the median MPRs do not adequately convey the impact from this clinic. The proportion of patients who were considered adherent to OANs increased from 47.9% in the preimplementation to 100% in the postimplementation period. These finding suggest that the clinical pharmacist role to assess and encourage adherence through monitoring tolerability of these OANs improved the overall medication taking experience of these patients.
Upon initial evaluation of adherence pre- and postimplementation, median adherence rates in both groups appeared to be above goal at 0.94 and 1.06 respectively. Patients in the postimplementation group intentionally received a 5- to 7-day supply buffer to account for potential prescription delivery delays due to holidays and inclement weather. This would indicate that the patients in the postimplementation group would have 15% oversupply due to the 5-day supply buffer. After correcting for patients with confounding reasons for excess (dose reductions, breaks in treatment, etc.), the median MPR in the prerefill clinic group decreased to 0.9 and the MPR in the postrefill clinic group increased slightly to 1.08. Although the median adherence rate in both the pre- and postimplementation groups were above goal of 0.90, 36% of the patients in the preimplementation group were considered nonadherent (MPR < 0.9) compared with no patients in the postimplementation group. Therefore, our intervention to improve patient adherence appeared to be beneficial at our institution.
In addition to improving adherence, one of the goals of the renewal clinic was to minimize excess supply at the time of therapy discontinuation. This was accomplished by aligning medication fills with medical visits and objective monitoring, as well as limiting supply to no more than 30 days. Of the patients in the postimplementation group, only 1 patient had remaining medication at the time of therapy discontinuation compared with 14 patients in the preimplementation group. The estimated cost savings from excess supply was $36,335. Limiting the amount of unused supply not only saves money for the patient and the institution, but also decreases opportunity for improper hazardous waste disposal and unnecessary exposure of hazardous materials to others.
Our results show the pharmacist intervention in the coordination of renewals improved adherence, minimized medication waste, and saved money. The cost of pharmacist time participating in the refill clinic was not calculated. Each visit was completed in approximately 5 minutes, with subsequent documentation and coordination taking an additional 5 to 10 minutes. During the launch of this service, the oncology pharmacy resident provided all coverage of the clinic. Oversite of the resident was provided by hematology/oncology clinical pharmacy specialists. We have continued to utilize pharmacy resident coverage since that time to meet education needs and keep the estimated cost per visit low. Another option in the case that pharmacy residents are not available would be utilization of a pharmacy technician, intern, or professional student to conduct the adherence and tolerability phone assessments. Our escalation protocol allows intervention by clinical pharmacy specialist and/or other health care providers when necessary. Trainees have only required basic training on how to use the protocol.
Limitations
Due to this study’s retrospective design, an inherent limitation is dependence on prescriber and refill records for documentation of initiation and discontinuation dates. Therefore, only the association of impact of pharmacist intervention on medication adherence can be determined as opposed to causation. We did not take into account discrepancies in day supply secondary to ‘held’ therapies, dose reductions, or doses supplied during an inpatient admission, which may alter estimates of MPR and cost-savings data. Patients in the postimplementation group intentionally received a 5 to 7-day supply buffer to account for potential prescription delivery delays due to holidays and inclement weather. This would indicate that the patients in the postimplementation group would have 15% oversupply due to the 5-day supply buffer, thereby skewing MPR values. This study did not account for cost avoidance resulting from early identification and management of toxicity. Finally, the postimplementation data only spans 4 months and a longer duration of time is needed to more accurately determine sustainability of renewal clinic interventions and provide comprehensive evaluation of cost-avoidance.
Conclusion
Implementation of an OAN renewal clinic was associated with an increase in MPR, improved proportion of patients considered adherent, and an estimated $36,335 cost-savings. However, prospective evaluation and a longer study duration are needed to determine causality of improved adherence and cost-savings associated with a pharmacist-driven OAN renewal clinic.
1. Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 2011; 86: 471-474. doi:10.1002/ajh.22019
2. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010; 28: 2381-2388. doi:10.1200/JCO.2009.26.3087
3. McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 2008; 99: 1763-1768. doi:10.1038/sj.bjc.6604758
4. Lexicomp Online. Sunitinib. Hudson, Ohio: Lexi-Comp, Inc; August 20, 2019.
5. Babiker A, El Husseini M, Al Nemri A, et al. Health care professional development: Working as a team to improve patient care. Sudan J Paediatr. 2014;14(2):9-16.
6. Spence MM, Makarem AF, Reyes SL, et al. Evaluation of an outpatient pharmacy clinical services program on adherence and clinical outcomes among patients with diabetes and/or coronary artery disease. J Manag Care Spec Pharm. 2014;20(10):1036-1045. doi:10.18553/jmcp.2014.20.10.1036
7. Holle LM, Puri S, Clement JM. Physician-pharmacist collaboration for oral chemotherapy monitoring: Insights from an academic genitourinary oncology practice. J Oncol Pharm Pract 2015; doi:10.1177/1078155215581524
8. Muluneh B, Schneider M, Faso A, et al. Improved Adherence Rates and Clinical Outcomes of an Integrated, Closed-Loop, Pharmacist-Led Oral Chemotherapy Management Program. Journal of Oncology Practice. 2018;14(6):371-333. doi:10.1200/JOP.17.00039.
9. Font R, Espinas JA, Gil-Gil M, et al. Prescription refill, patient self-report and physician report in assessing adherence to oral endocrine therapy in early breast cancer patients: a retrospective cohort study in Catalonia, Spain. British Journal of Cancer. 2012 ;107(8):1249-1256. doi:10.1038/bjc.2012.389.
10. Anderson KR, Chambers CR, Lam N, et al. Medication adherence among adults prescribed imatinib, dasatinib, or nilotinib for the treatment of chronic myeloid leukemia. J Oncol Pharm Practice. 2015;21(1):19–25. doi:10.1177/1078155213520261
11. Weingart SN, Brown E, Bach PB, et al. NCCN Task Force Report: oral chemotherapy. J Natl Compr Canc Netw. 2008;6(3): S1-S14.
Evaluation of oral antineoplastic agent (OAN) adherence patterns have identified correlations between nonadherence or over-adherence and poorer disease-related outcomes. Multiple studies have focused on imatinib use in chronic myeloid leukemia (CML) due to its continuous, long-term use. A study by Ganesan and colleagues found that nonadherence to imatinib showed a significant decrease in 5-year event-free survival between 76.7% of adherent participants compared with 59.8% of nonadherent participants.1 This study found that 44% of patients who were adherent to imatinib achieved complete cytogenetic response vs only 26% of patients who were nonadherent. In another study of imatinib for CML, major molecular response (MMR) was strongly correlated with adherence and no patients with adherence < 80% were able to achieve MMR.2 Similarly, in studies of tamoxifen for breast cancer, < 80% adherence resulted in a 10% decrease in survival when compared to those who were more adherent.3,4
In addition to the clinical implications of nonadherence, there can be a significant cost associated with suboptimal use of these medications. The price of a single dose of OAN medication may cost as much as $440.5
The benefits of multidisciplinary care teams have been identified in many studies.6,7 While studies are limited in oncology, pharmacists provide vital contributions to the oncology multidisciplinary team when managing OANs as these health care professionals have expert knowledge of the medications, potential adverse events (AEs), and necessary monitoring parameters.8 In one study, patients seen by the pharmacist-led oral chemotherapy management program experienced improved clinical outcomes and response to therapy when compared with preintervention patients (early molecular response, 88.9% vs 54.8%, P = .01; major molecular response, 83.3% vs 57.6%, P = .06).9 During the study, 318 AEs were reported, leading to 235 pharmacist interventions to ameliorate AEs and improve adherence.
The primary objective of this study was to measure the impact of a pharmacist-driven OAN renewal clinic on medication adherence. The secondary objective was to estimate cost-savings of this new service.
Methods
Prior to July 2014, several limitations were identified related to OAN prescribing and monitoring at the Richard L. Roudebush Veterans Affairs Medical Center in Indianapolis, Indiana (RLRVAMC). The prescription ordering process relied primarily on the patient to initiate refills, rather than the prescriber OAN prescriptions also lacked consistency for number of refills or quantities dispensed. Furthermore, ordering of antineoplastic products was not limited to hematology/oncology providers. Patients were identified with significant supply on hand at the time of medication discontinuation, creating concerns for medication waste, tolerability, and nonadherence.
As a result, opportunities were identified to improve the prescribing process, recommended monitoring, toxicity and tolerability evaluation, medication reconciliation, and medication adherence. In July of 2014, the RLRVAMC adopted a new chemotherapy order entry system capable of restricting prescriptions to hematology/oncology providers and limiting dispensed quantities and refill amounts. A comprehensive pharmacist driven OAN renewal clinic was implemented on September 1, 2014 with the goal of improving long-term adherence and tolerability, in addition to minimizing medication waste.
Patients were eligible for enrollment in the clinic if they had a cancer diagnosis and were concomitantly prescribed an OAN outlined in Table 1. All eligible patients were automatically enrolled in the clinic when they were deemed stable on their OAN by a hematology/oncology pharmacy specialist. Stability was defined as ≤ Grade 1 symptoms associated with the toxicities of OAN therapy managed with or without intervention as defined by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Once enrolled in the renewal clinic, patients were called by an oncology pharmacy resident (PGY2) 1 week prior to any OAN refill due date. Patients were asked a series of 5 adherence and tolerability questions (Table 2) to evaluate renewal criteria for approval or need for further evaluation. These questions were developed based on targeted information and published reports on monitoring adherence.10,11 Criteria for renewal included: < 10% self-reported missed doses of the OAN during the previous dispensing period, no hospitalizations or emergency department visits since most recent hematology/oncology provider appointment, no changes to concomitant medication therapies, and no new or worsening medication-related AEs. Patients meeting all criteria were given a 30-day supply of OAN. Prescribing, dispensing, and delivery of OAN were facilitated by the pharmacist. Patient cases that did not meet criteria for renewal were escalated to the hematology/oncology provider or oncology clinical pharmacy specialist for further evaluation.
Study Design and Setting
This was a pre/post retrospective cohort, quality improvement study of patients enrolled in the RLRVAMC OAN pharmacist renewal clinic. The study was deemed exempt from institutional review board (IRB) by the US Department of Veterans Affairs (VA) Research and Development Department.
Study Population
Patients were included in the preimplementation group if they had received at least 2 prescriptions of an eligible OAN. Therapy for the preimplementation group was required to be a monthly duration > 21 days and between the dates of September 1, 2013 and August 31, 2014. Patients were included in the postimplementation group if they had received at least 2 prescriptions of the studied OANs between September 1, 2014 and January 31, 2015. Patients were excluded if they had filled < 2 prescriptions of OAN; were managed by a non-VA oncologist or hematologist; or received an OAN other than those listed in Table 1.
Data Collection
For all patients in both the pre- and postimplementation cohorts, a standardized data collection tool was used to collect the following via electronic health record review by a PGY2 oncology resident: age, race, gender, oral antineoplastic agent, refill dates, days’ supply, estimated unit cost per dose cancer diagnosis, distance from the RLRVAMC, copay status, presence of hospitalizations/ED visits/dosage reductions, discontinuation rates, reasons for discontinuation, and total number of current prescriptions. The presence or absence of dosage reductions were collected to identify concerns for tolerability, but only the original dose for the preimplementation group and dosage at time of clinic enrollment for the postimplementation group was included in the analysis.
Outcomes and Statistical Analyses
The primary outcome was medication adherence defined as the median medication possession ratio (MPR) before and after implementation of the clinic. Secondary outcomes included the proportion of patients who were adherent from before implementation to after implementation and estimated cost-savings of this clinic after implementation. MPR was used to estimate medication adherence by taking the cumulative day supply of medication on hand divided by the number of days on therapy.12 Number of days on therapy was determined by taking the difference on the start date of the new medication regimen and the discontinuation date of the same regimen. Patients were grouped by adherence into one of the following categories: < 0.8, 0.8 to 0.89, 0.9 to 1, and > 1.1. Patients were considered adherent if they reported taking ≥ 90% (MPR ≥ 0.9) of prescribed doses, adopted from the study by Anderson and colleagues.12 A patient with an MPR > 1, likely due to filling prior to the anticipated refill date, was considered 100% adherent (MPR = 1). If a patient switched OAN during the study, both agents were included as separate entities.
A conservative estimate of cost-savings was made by multiplying the RLRVAMC cost per unit of medication at time of initial prescription fill by the number of units taken each day multiplied by the total days’ supply on hand at time of therapy discontinuation. Patients with an MPR < 1 at time of therapy discontinuation were assumed to have zero remaining units on hand and zero cost savings was estimated. Waste, for purposes of cost-savings, was calculated for all MPR values > 1. Additional supply anticipated to be on hand from dose reductions was not included in the estimated cost of unused medication.
Descriptive statistics compared demographic characteristics between the pre- and postimplementation groups. MPR data were not normally distributed, which required the use of nonparametric Mann-Whitney U tests to compare pre- and postMPRs. Pearson χ2 compared the proportion of adherent patients between groups while descriptive statistics were used to estimate cost savings. Significance was determined based on a P value < .05. IBM SPSS Statistics software was used for all statistical analyses. As this was a complete sample of all eligible subjects, no sample size calculation was performed.
Results
In the preimplementation period, 246 patients received an OAN and 61 patients received an OAN in the postimplementation period (Figure 1). Of the 246 patients in the preimplementation period, 98 were eligible and included in the preimplementation group. Similarly, of the 61 patients in the postimplementation period, 35 patients met inclusion criteria for the postimplementation group. The study population was predominantly male with an average age of approximately 70 years in both groups (Table 3). More than 70% of the population in each group was White. No statistically significant differences between groups were identified. The most commonly prescribed OAN in the preimplementation group were abiraterone, imatinib, and enzalutamide (Table 3). In the postimplementation group, the most commonly prescribed agents were abiraterone, imatinib, pazopanib, and dasatinib. No significant differences were observed in prescribing of individual agents between the pre- and postimplementation groups or other characteristics that may affect adherence including patient copay status, number of concomitant medications, and driving distance from the RLRVAMC.
Thirty-six (36.7%) patients in the preimplementation group were considered nonadherent (MPR < 0.9) and 18 (18.4%) had an MPR < 0.8. Fifteen (15.3%) patients in the preimplementation clinic were considered overadherent (MPR > 1.1). Forty-seven (47.9%) patients in the preimplementation group were considered adherent (MPR 0.9 - 1.1) while all 35 (100%) patients in the postimplementation group were considered adherent (MPR 0.9 - 1.1). No non- or overadherent patients were identified in the postimplementation group (Figure 2). The median MPR for all patients in the preimplementation group was 0.94 compared with 1.06 (P < .001) in the postimplementation group.
Thirty-five (35.7%) patients had therapy discontinued or held in the preimplementation group compared with 2 (5.7%) patients in the postimplementation group (P < .001). Reasons for discontinuation in the preimplementation group included disease progression (n = 27), death (n = 3), lost to follow up (n = 2), and intolerability of therapy (n = 3). Both patients that discontinued therapy in the postimplementation group did so due to disease progression. Of the 35 patients who had their OAN discontinued or held in the preimplementation group, 14 patients had excess supply on hand at time of discontinuation. The estimated value of the unused medication was $37,890. Nine (25%) of the 35 patients who discontinued therapy had a dosage reduction during the course of therapy and the additional supply was not included in the cost estimate. Similarly, 1 of the 2 patients in the postimplementation group had their OAN discontinued during study. The cost of oversupply of medication at the time of therapy discontinuation was estimated at $1,555. No patients in the postimplementation group had dose reductions. After implementation of the OAN renewal clinic, the total cost savings between pre ($37,890) and postimplementation ($1,555) groups was $36,355.
Discussion
OANs are widely used therapies, with more than 25 million doses administered per year in the United States alone.12 The use of these agents will continue to grow as more targeted agents become available and patients request more convenient treatment options. The role for hematology/oncology clinical pharmacy services must adapt to this increased usage of OANs, including increasing pharmacist involvement in medication education, adherence and tolerability assessments, and proactive drug interaction monitoring.However, additional research is needed to determine optimal management strategies.
Our study aimed to compare OAN adherence among patients at a tertiary care VA hospital before and after implementation of a renewal clinic. The preimplementation population had a median MPR of 0.94 compared with 1.06 in the postimplementation group (P < .001). Although an ideal MPR is 1.0, we aimed for a slightly higher MPR to allow a supply buffer in the event of prescription delivery delays, as more than 90% of prescriptions are mailed to patients from a regional mail-order pharmacy. Importantly, the median MPRs do not adequately convey the impact from this clinic. The proportion of patients who were considered adherent to OANs increased from 47.9% in the preimplementation to 100% in the postimplementation period. These finding suggest that the clinical pharmacist role to assess and encourage adherence through monitoring tolerability of these OANs improved the overall medication taking experience of these patients.
Upon initial evaluation of adherence pre- and postimplementation, median adherence rates in both groups appeared to be above goal at 0.94 and 1.06 respectively. Patients in the postimplementation group intentionally received a 5- to 7-day supply buffer to account for potential prescription delivery delays due to holidays and inclement weather. This would indicate that the patients in the postimplementation group would have 15% oversupply due to the 5-day supply buffer. After correcting for patients with confounding reasons for excess (dose reductions, breaks in treatment, etc.), the median MPR in the prerefill clinic group decreased to 0.9 and the MPR in the postrefill clinic group increased slightly to 1.08. Although the median adherence rate in both the pre- and postimplementation groups were above goal of 0.90, 36% of the patients in the preimplementation group were considered nonadherent (MPR < 0.9) compared with no patients in the postimplementation group. Therefore, our intervention to improve patient adherence appeared to be beneficial at our institution.
In addition to improving adherence, one of the goals of the renewal clinic was to minimize excess supply at the time of therapy discontinuation. This was accomplished by aligning medication fills with medical visits and objective monitoring, as well as limiting supply to no more than 30 days. Of the patients in the postimplementation group, only 1 patient had remaining medication at the time of therapy discontinuation compared with 14 patients in the preimplementation group. The estimated cost savings from excess supply was $36,335. Limiting the amount of unused supply not only saves money for the patient and the institution, but also decreases opportunity for improper hazardous waste disposal and unnecessary exposure of hazardous materials to others.
Our results show the pharmacist intervention in the coordination of renewals improved adherence, minimized medication waste, and saved money. The cost of pharmacist time participating in the refill clinic was not calculated. Each visit was completed in approximately 5 minutes, with subsequent documentation and coordination taking an additional 5 to 10 minutes. During the launch of this service, the oncology pharmacy resident provided all coverage of the clinic. Oversite of the resident was provided by hematology/oncology clinical pharmacy specialists. We have continued to utilize pharmacy resident coverage since that time to meet education needs and keep the estimated cost per visit low. Another option in the case that pharmacy residents are not available would be utilization of a pharmacy technician, intern, or professional student to conduct the adherence and tolerability phone assessments. Our escalation protocol allows intervention by clinical pharmacy specialist and/or other health care providers when necessary. Trainees have only required basic training on how to use the protocol.
Limitations
Due to this study’s retrospective design, an inherent limitation is dependence on prescriber and refill records for documentation of initiation and discontinuation dates. Therefore, only the association of impact of pharmacist intervention on medication adherence can be determined as opposed to causation. We did not take into account discrepancies in day supply secondary to ‘held’ therapies, dose reductions, or doses supplied during an inpatient admission, which may alter estimates of MPR and cost-savings data. Patients in the postimplementation group intentionally received a 5 to 7-day supply buffer to account for potential prescription delivery delays due to holidays and inclement weather. This would indicate that the patients in the postimplementation group would have 15% oversupply due to the 5-day supply buffer, thereby skewing MPR values. This study did not account for cost avoidance resulting from early identification and management of toxicity. Finally, the postimplementation data only spans 4 months and a longer duration of time is needed to more accurately determine sustainability of renewal clinic interventions and provide comprehensive evaluation of cost-avoidance.
Conclusion
Implementation of an OAN renewal clinic was associated with an increase in MPR, improved proportion of patients considered adherent, and an estimated $36,335 cost-savings. However, prospective evaluation and a longer study duration are needed to determine causality of improved adherence and cost-savings associated with a pharmacist-driven OAN renewal clinic.
Evaluation of oral antineoplastic agent (OAN) adherence patterns have identified correlations between nonadherence or over-adherence and poorer disease-related outcomes. Multiple studies have focused on imatinib use in chronic myeloid leukemia (CML) due to its continuous, long-term use. A study by Ganesan and colleagues found that nonadherence to imatinib showed a significant decrease in 5-year event-free survival between 76.7% of adherent participants compared with 59.8% of nonadherent participants.1 This study found that 44% of patients who were adherent to imatinib achieved complete cytogenetic response vs only 26% of patients who were nonadherent. In another study of imatinib for CML, major molecular response (MMR) was strongly correlated with adherence and no patients with adherence < 80% were able to achieve MMR.2 Similarly, in studies of tamoxifen for breast cancer, < 80% adherence resulted in a 10% decrease in survival when compared to those who were more adherent.3,4
In addition to the clinical implications of nonadherence, there can be a significant cost associated with suboptimal use of these medications. The price of a single dose of OAN medication may cost as much as $440.5
The benefits of multidisciplinary care teams have been identified in many studies.6,7 While studies are limited in oncology, pharmacists provide vital contributions to the oncology multidisciplinary team when managing OANs as these health care professionals have expert knowledge of the medications, potential adverse events (AEs), and necessary monitoring parameters.8 In one study, patients seen by the pharmacist-led oral chemotherapy management program experienced improved clinical outcomes and response to therapy when compared with preintervention patients (early molecular response, 88.9% vs 54.8%, P = .01; major molecular response, 83.3% vs 57.6%, P = .06).9 During the study, 318 AEs were reported, leading to 235 pharmacist interventions to ameliorate AEs and improve adherence.
The primary objective of this study was to measure the impact of a pharmacist-driven OAN renewal clinic on medication adherence. The secondary objective was to estimate cost-savings of this new service.
Methods
Prior to July 2014, several limitations were identified related to OAN prescribing and monitoring at the Richard L. Roudebush Veterans Affairs Medical Center in Indianapolis, Indiana (RLRVAMC). The prescription ordering process relied primarily on the patient to initiate refills, rather than the prescriber OAN prescriptions also lacked consistency for number of refills or quantities dispensed. Furthermore, ordering of antineoplastic products was not limited to hematology/oncology providers. Patients were identified with significant supply on hand at the time of medication discontinuation, creating concerns for medication waste, tolerability, and nonadherence.
As a result, opportunities were identified to improve the prescribing process, recommended monitoring, toxicity and tolerability evaluation, medication reconciliation, and medication adherence. In July of 2014, the RLRVAMC adopted a new chemotherapy order entry system capable of restricting prescriptions to hematology/oncology providers and limiting dispensed quantities and refill amounts. A comprehensive pharmacist driven OAN renewal clinic was implemented on September 1, 2014 with the goal of improving long-term adherence and tolerability, in addition to minimizing medication waste.
Patients were eligible for enrollment in the clinic if they had a cancer diagnosis and were concomitantly prescribed an OAN outlined in Table 1. All eligible patients were automatically enrolled in the clinic when they were deemed stable on their OAN by a hematology/oncology pharmacy specialist. Stability was defined as ≤ Grade 1 symptoms associated with the toxicities of OAN therapy managed with or without intervention as defined by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Once enrolled in the renewal clinic, patients were called by an oncology pharmacy resident (PGY2) 1 week prior to any OAN refill due date. Patients were asked a series of 5 adherence and tolerability questions (Table 2) to evaluate renewal criteria for approval or need for further evaluation. These questions were developed based on targeted information and published reports on monitoring adherence.10,11 Criteria for renewal included: < 10% self-reported missed doses of the OAN during the previous dispensing period, no hospitalizations or emergency department visits since most recent hematology/oncology provider appointment, no changes to concomitant medication therapies, and no new or worsening medication-related AEs. Patients meeting all criteria were given a 30-day supply of OAN. Prescribing, dispensing, and delivery of OAN were facilitated by the pharmacist. Patient cases that did not meet criteria for renewal were escalated to the hematology/oncology provider or oncology clinical pharmacy specialist for further evaluation.
Study Design and Setting
This was a pre/post retrospective cohort, quality improvement study of patients enrolled in the RLRVAMC OAN pharmacist renewal clinic. The study was deemed exempt from institutional review board (IRB) by the US Department of Veterans Affairs (VA) Research and Development Department.
Study Population
Patients were included in the preimplementation group if they had received at least 2 prescriptions of an eligible OAN. Therapy for the preimplementation group was required to be a monthly duration > 21 days and between the dates of September 1, 2013 and August 31, 2014. Patients were included in the postimplementation group if they had received at least 2 prescriptions of the studied OANs between September 1, 2014 and January 31, 2015. Patients were excluded if they had filled < 2 prescriptions of OAN; were managed by a non-VA oncologist or hematologist; or received an OAN other than those listed in Table 1.
Data Collection
For all patients in both the pre- and postimplementation cohorts, a standardized data collection tool was used to collect the following via electronic health record review by a PGY2 oncology resident: age, race, gender, oral antineoplastic agent, refill dates, days’ supply, estimated unit cost per dose cancer diagnosis, distance from the RLRVAMC, copay status, presence of hospitalizations/ED visits/dosage reductions, discontinuation rates, reasons for discontinuation, and total number of current prescriptions. The presence or absence of dosage reductions were collected to identify concerns for tolerability, but only the original dose for the preimplementation group and dosage at time of clinic enrollment for the postimplementation group was included in the analysis.
Outcomes and Statistical Analyses
The primary outcome was medication adherence defined as the median medication possession ratio (MPR) before and after implementation of the clinic. Secondary outcomes included the proportion of patients who were adherent from before implementation to after implementation and estimated cost-savings of this clinic after implementation. MPR was used to estimate medication adherence by taking the cumulative day supply of medication on hand divided by the number of days on therapy.12 Number of days on therapy was determined by taking the difference on the start date of the new medication regimen and the discontinuation date of the same regimen. Patients were grouped by adherence into one of the following categories: < 0.8, 0.8 to 0.89, 0.9 to 1, and > 1.1. Patients were considered adherent if they reported taking ≥ 90% (MPR ≥ 0.9) of prescribed doses, adopted from the study by Anderson and colleagues.12 A patient with an MPR > 1, likely due to filling prior to the anticipated refill date, was considered 100% adherent (MPR = 1). If a patient switched OAN during the study, both agents were included as separate entities.
A conservative estimate of cost-savings was made by multiplying the RLRVAMC cost per unit of medication at time of initial prescription fill by the number of units taken each day multiplied by the total days’ supply on hand at time of therapy discontinuation. Patients with an MPR < 1 at time of therapy discontinuation were assumed to have zero remaining units on hand and zero cost savings was estimated. Waste, for purposes of cost-savings, was calculated for all MPR values > 1. Additional supply anticipated to be on hand from dose reductions was not included in the estimated cost of unused medication.
Descriptive statistics compared demographic characteristics between the pre- and postimplementation groups. MPR data were not normally distributed, which required the use of nonparametric Mann-Whitney U tests to compare pre- and postMPRs. Pearson χ2 compared the proportion of adherent patients between groups while descriptive statistics were used to estimate cost savings. Significance was determined based on a P value < .05. IBM SPSS Statistics software was used for all statistical analyses. As this was a complete sample of all eligible subjects, no sample size calculation was performed.
Results
In the preimplementation period, 246 patients received an OAN and 61 patients received an OAN in the postimplementation period (Figure 1). Of the 246 patients in the preimplementation period, 98 were eligible and included in the preimplementation group. Similarly, of the 61 patients in the postimplementation period, 35 patients met inclusion criteria for the postimplementation group. The study population was predominantly male with an average age of approximately 70 years in both groups (Table 3). More than 70% of the population in each group was White. No statistically significant differences between groups were identified. The most commonly prescribed OAN in the preimplementation group were abiraterone, imatinib, and enzalutamide (Table 3). In the postimplementation group, the most commonly prescribed agents were abiraterone, imatinib, pazopanib, and dasatinib. No significant differences were observed in prescribing of individual agents between the pre- and postimplementation groups or other characteristics that may affect adherence including patient copay status, number of concomitant medications, and driving distance from the RLRVAMC.
Thirty-six (36.7%) patients in the preimplementation group were considered nonadherent (MPR < 0.9) and 18 (18.4%) had an MPR < 0.8. Fifteen (15.3%) patients in the preimplementation clinic were considered overadherent (MPR > 1.1). Forty-seven (47.9%) patients in the preimplementation group were considered adherent (MPR 0.9 - 1.1) while all 35 (100%) patients in the postimplementation group were considered adherent (MPR 0.9 - 1.1). No non- or overadherent patients were identified in the postimplementation group (Figure 2). The median MPR for all patients in the preimplementation group was 0.94 compared with 1.06 (P < .001) in the postimplementation group.
Thirty-five (35.7%) patients had therapy discontinued or held in the preimplementation group compared with 2 (5.7%) patients in the postimplementation group (P < .001). Reasons for discontinuation in the preimplementation group included disease progression (n = 27), death (n = 3), lost to follow up (n = 2), and intolerability of therapy (n = 3). Both patients that discontinued therapy in the postimplementation group did so due to disease progression. Of the 35 patients who had their OAN discontinued or held in the preimplementation group, 14 patients had excess supply on hand at time of discontinuation. The estimated value of the unused medication was $37,890. Nine (25%) of the 35 patients who discontinued therapy had a dosage reduction during the course of therapy and the additional supply was not included in the cost estimate. Similarly, 1 of the 2 patients in the postimplementation group had their OAN discontinued during study. The cost of oversupply of medication at the time of therapy discontinuation was estimated at $1,555. No patients in the postimplementation group had dose reductions. After implementation of the OAN renewal clinic, the total cost savings between pre ($37,890) and postimplementation ($1,555) groups was $36,355.
Discussion
OANs are widely used therapies, with more than 25 million doses administered per year in the United States alone.12 The use of these agents will continue to grow as more targeted agents become available and patients request more convenient treatment options. The role for hematology/oncology clinical pharmacy services must adapt to this increased usage of OANs, including increasing pharmacist involvement in medication education, adherence and tolerability assessments, and proactive drug interaction monitoring.However, additional research is needed to determine optimal management strategies.
Our study aimed to compare OAN adherence among patients at a tertiary care VA hospital before and after implementation of a renewal clinic. The preimplementation population had a median MPR of 0.94 compared with 1.06 in the postimplementation group (P < .001). Although an ideal MPR is 1.0, we aimed for a slightly higher MPR to allow a supply buffer in the event of prescription delivery delays, as more than 90% of prescriptions are mailed to patients from a regional mail-order pharmacy. Importantly, the median MPRs do not adequately convey the impact from this clinic. The proportion of patients who were considered adherent to OANs increased from 47.9% in the preimplementation to 100% in the postimplementation period. These finding suggest that the clinical pharmacist role to assess and encourage adherence through monitoring tolerability of these OANs improved the overall medication taking experience of these patients.
Upon initial evaluation of adherence pre- and postimplementation, median adherence rates in both groups appeared to be above goal at 0.94 and 1.06 respectively. Patients in the postimplementation group intentionally received a 5- to 7-day supply buffer to account for potential prescription delivery delays due to holidays and inclement weather. This would indicate that the patients in the postimplementation group would have 15% oversupply due to the 5-day supply buffer. After correcting for patients with confounding reasons for excess (dose reductions, breaks in treatment, etc.), the median MPR in the prerefill clinic group decreased to 0.9 and the MPR in the postrefill clinic group increased slightly to 1.08. Although the median adherence rate in both the pre- and postimplementation groups were above goal of 0.90, 36% of the patients in the preimplementation group were considered nonadherent (MPR < 0.9) compared with no patients in the postimplementation group. Therefore, our intervention to improve patient adherence appeared to be beneficial at our institution.
In addition to improving adherence, one of the goals of the renewal clinic was to minimize excess supply at the time of therapy discontinuation. This was accomplished by aligning medication fills with medical visits and objective monitoring, as well as limiting supply to no more than 30 days. Of the patients in the postimplementation group, only 1 patient had remaining medication at the time of therapy discontinuation compared with 14 patients in the preimplementation group. The estimated cost savings from excess supply was $36,335. Limiting the amount of unused supply not only saves money for the patient and the institution, but also decreases opportunity for improper hazardous waste disposal and unnecessary exposure of hazardous materials to others.
Our results show the pharmacist intervention in the coordination of renewals improved adherence, minimized medication waste, and saved money. The cost of pharmacist time participating in the refill clinic was not calculated. Each visit was completed in approximately 5 minutes, with subsequent documentation and coordination taking an additional 5 to 10 minutes. During the launch of this service, the oncology pharmacy resident provided all coverage of the clinic. Oversite of the resident was provided by hematology/oncology clinical pharmacy specialists. We have continued to utilize pharmacy resident coverage since that time to meet education needs and keep the estimated cost per visit low. Another option in the case that pharmacy residents are not available would be utilization of a pharmacy technician, intern, or professional student to conduct the adherence and tolerability phone assessments. Our escalation protocol allows intervention by clinical pharmacy specialist and/or other health care providers when necessary. Trainees have only required basic training on how to use the protocol.
Limitations
Due to this study’s retrospective design, an inherent limitation is dependence on prescriber and refill records for documentation of initiation and discontinuation dates. Therefore, only the association of impact of pharmacist intervention on medication adherence can be determined as opposed to causation. We did not take into account discrepancies in day supply secondary to ‘held’ therapies, dose reductions, or doses supplied during an inpatient admission, which may alter estimates of MPR and cost-savings data. Patients in the postimplementation group intentionally received a 5 to 7-day supply buffer to account for potential prescription delivery delays due to holidays and inclement weather. This would indicate that the patients in the postimplementation group would have 15% oversupply due to the 5-day supply buffer, thereby skewing MPR values. This study did not account for cost avoidance resulting from early identification and management of toxicity. Finally, the postimplementation data only spans 4 months and a longer duration of time is needed to more accurately determine sustainability of renewal clinic interventions and provide comprehensive evaluation of cost-avoidance.
Conclusion
Implementation of an OAN renewal clinic was associated with an increase in MPR, improved proportion of patients considered adherent, and an estimated $36,335 cost-savings. However, prospective evaluation and a longer study duration are needed to determine causality of improved adherence and cost-savings associated with a pharmacist-driven OAN renewal clinic.
1. Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 2011; 86: 471-474. doi:10.1002/ajh.22019
2. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010; 28: 2381-2388. doi:10.1200/JCO.2009.26.3087
3. McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 2008; 99: 1763-1768. doi:10.1038/sj.bjc.6604758
4. Lexicomp Online. Sunitinib. Hudson, Ohio: Lexi-Comp, Inc; August 20, 2019.
5. Babiker A, El Husseini M, Al Nemri A, et al. Health care professional development: Working as a team to improve patient care. Sudan J Paediatr. 2014;14(2):9-16.
6. Spence MM, Makarem AF, Reyes SL, et al. Evaluation of an outpatient pharmacy clinical services program on adherence and clinical outcomes among patients with diabetes and/or coronary artery disease. J Manag Care Spec Pharm. 2014;20(10):1036-1045. doi:10.18553/jmcp.2014.20.10.1036
7. Holle LM, Puri S, Clement JM. Physician-pharmacist collaboration for oral chemotherapy monitoring: Insights from an academic genitourinary oncology practice. J Oncol Pharm Pract 2015; doi:10.1177/1078155215581524
8. Muluneh B, Schneider M, Faso A, et al. Improved Adherence Rates and Clinical Outcomes of an Integrated, Closed-Loop, Pharmacist-Led Oral Chemotherapy Management Program. Journal of Oncology Practice. 2018;14(6):371-333. doi:10.1200/JOP.17.00039.
9. Font R, Espinas JA, Gil-Gil M, et al. Prescription refill, patient self-report and physician report in assessing adherence to oral endocrine therapy in early breast cancer patients: a retrospective cohort study in Catalonia, Spain. British Journal of Cancer. 2012 ;107(8):1249-1256. doi:10.1038/bjc.2012.389.
10. Anderson KR, Chambers CR, Lam N, et al. Medication adherence among adults prescribed imatinib, dasatinib, or nilotinib for the treatment of chronic myeloid leukemia. J Oncol Pharm Practice. 2015;21(1):19–25. doi:10.1177/1078155213520261
11. Weingart SN, Brown E, Bach PB, et al. NCCN Task Force Report: oral chemotherapy. J Natl Compr Canc Netw. 2008;6(3): S1-S14.
1. Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 2011; 86: 471-474. doi:10.1002/ajh.22019
2. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010; 28: 2381-2388. doi:10.1200/JCO.2009.26.3087
3. McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 2008; 99: 1763-1768. doi:10.1038/sj.bjc.6604758
4. Lexicomp Online. Sunitinib. Hudson, Ohio: Lexi-Comp, Inc; August 20, 2019.
5. Babiker A, El Husseini M, Al Nemri A, et al. Health care professional development: Working as a team to improve patient care. Sudan J Paediatr. 2014;14(2):9-16.
6. Spence MM, Makarem AF, Reyes SL, et al. Evaluation of an outpatient pharmacy clinical services program on adherence and clinical outcomes among patients with diabetes and/or coronary artery disease. J Manag Care Spec Pharm. 2014;20(10):1036-1045. doi:10.18553/jmcp.2014.20.10.1036
7. Holle LM, Puri S, Clement JM. Physician-pharmacist collaboration for oral chemotherapy monitoring: Insights from an academic genitourinary oncology practice. J Oncol Pharm Pract 2015; doi:10.1177/1078155215581524
8. Muluneh B, Schneider M, Faso A, et al. Improved Adherence Rates and Clinical Outcomes of an Integrated, Closed-Loop, Pharmacist-Led Oral Chemotherapy Management Program. Journal of Oncology Practice. 2018;14(6):371-333. doi:10.1200/JOP.17.00039.
9. Font R, Espinas JA, Gil-Gil M, et al. Prescription refill, patient self-report and physician report in assessing adherence to oral endocrine therapy in early breast cancer patients: a retrospective cohort study in Catalonia, Spain. British Journal of Cancer. 2012 ;107(8):1249-1256. doi:10.1038/bjc.2012.389.
10. Anderson KR, Chambers CR, Lam N, et al. Medication adherence among adults prescribed imatinib, dasatinib, or nilotinib for the treatment of chronic myeloid leukemia. J Oncol Pharm Practice. 2015;21(1):19–25. doi:10.1177/1078155213520261
11. Weingart SN, Brown E, Bach PB, et al. NCCN Task Force Report: oral chemotherapy. J Natl Compr Canc Netw. 2008;6(3): S1-S14.
Reduction of Opioid Use With Enhanced Recovery Program for Total Knee Arthroplasty
Total knee arthroplasty (TKA) is one of the most common surgical procedures in the United States. The volume of TKAs is projected to substantially increase over the next 30 years.1 Adequate pain control after TKA is critically important to achieve early mobilization, shorten the length of hospital stay, and reduce postoperative complications. The evolution and inclusion of multimodal pain-management protocols have had a major impact on the clinical outcomes for TKA patients.2,3
Pain-management protocols typically use several modalities to control pain throughout the perioperative period. Multimodal opioid and nonopioid oral medications are administered during the pre- and postoperative periods and often involve a combination of acetaminophen, gabapentinoids, and cyclooxygenase-2 inhibitors.4 Peripheral nerve blocks and central neuraxial blockades are widely used and have been shown to be effective in reducing postoperative pain as well as overall opioid consumption.5,6 Finally, intraoperative periarticular injections have been shown to reduce postoperative pain and opioid consumption as well as improve patient satisfaction scores.7-9 These strategies are routinely used in TKA with the goal of minimizing overall opioid consumption and adverse events, reducing perioperative complications, and improving patient satisfaction.
Periarticular injections during surgery are an integral part of the multimodal pain-management protocols, though no consensus has been reached on proper injection formulation or technique. Liposomal bupivacaine is a local anesthetic depot formulation approved by the US Food and Drug Administration for surgical patients. The reported results have been discrepant regarding the efficacy of using liposomal bupivacaine injection in patients with TKA. Several studies have reported no added benefit of liposomal bupivacaine in contrast to a mixture of local anesthetics.10,11 Other studies have demonstrated superior pain relief.12 Many factors may contribute to the discrepant data, such as injection techniques, infiltration volume, and the assessment tools used to measure efficacy and safety.13
The US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care to a large patient population. Many of the patients in that system have high-risk profiles, including medical comorbidities; exposure to chronic pain and opioid use; and psychological and central nervous system injuries, including posttraumatic stress disorder and traumatic brain injury. Hadlandsmyth and colleagues reported increased risk of prolonged opioid use in VA patients after TKA surgery.14 They found that 20% of the patients were still on long-term opioids more than 90 days after TKA.
The purpose of this study was to evaluate the efficacy of the implementation of a comprehensive enhanced recovery after surgery (ERAS) protocol at a regional VA medical center. We hypothesize that the addition of liposomal bupivacaine in a multidisciplinary ERAS protocol would reduce the length of hospital stay and opioid consumption without any deleterious effects on postoperative outcomes.
Methods
A postoperative recovery protocol was implemented in 2013 at VA North Texas Health Care System (VANTHCS) in Dallas, and many of the patients continued to have issues with satisfactory pain control, prolonged length of stay, and extended opioid consumption postoperatively. A multimodal pain-management protocol and multidisciplinary perioperative case-management protocol were implemented in 2016 to further improve the clinical outcomes of patients undergoing TKA surgery. The senior surgeon (JM) organized a multidisciplinary team of health care providers to identify and implement potential solutions. This task force met weekly and consisted of surgeons, anesthesiologists, certified registered nurse anesthetists, orthopedic physician assistants, a nurse coordinator, a physical therapist, and an occupational therapist, as well as operating room, postanesthesia care unit (PACU), and surgical ward nurses. In addition, the staff from the home health agencies and social services attended the weekly meetings.
We conducted a retrospective review of all patients who had undergone unilateral TKA from 2013 to 2018 at VANTHCS. This was a consecutive, unselected cohort. All patients were under the care of a single surgeon using identical implant systems and identical surgical techniques. This study was approved by the institutional review board at VANTHCS. Patients were divided into 2 distinct and consecutive cohorts. The standard of care (SOC) group included all patients from 2013 to 2016. The ERAS group included all patients after the institution of the standardized protocol until the end of the study period.
Data on patient demographics, the American Society of Anesthesiologists risk classification, and preoperative functional status were extracted. Anesthesia techniques included either general endotracheal anesthesia or subarachnoid block with monitored anesthesia care. The quantity of the opioids given during surgery, in the PACU, during the inpatient stay, as discharge prescriptions, and as refills of the narcotic prescriptions up to 3 months postsurgery were recorded. All opioids were converted into morphine equivalent dosages (MED) in order to be properly analyzed using the statistical methodologies described in the statistical section.15 The VHA is a closed health care delivery system; therefore, all of the prescriptions ordered by surgery providers were recorded in the electronic health record.
ERAS Protocol
The SOC cohort was predominantly managed with general endotracheal anesthesia. The ERAS group was predominantly managed with subarachnoid blocks (Table 1). For the ERAS protocol preoperatively, the patients were administered oral gabapentin 300 mg, acetaminophen 650 mg, and oxycodone 20 mg, and IV ondansetron 4 mg. Intraoperatively, minimal opioids were used. In the PACU, the patients received dilaudid 0.25 mg IV as needed every 15 minutes for up to 1 mg/h. The nursing staff was trained to use the visual analog pain scale scores to titrate the medication. During the inpatient stay, patients received 1 g IV acetaminophen every 6 hours for 3 doses. The patients thereafter received oral acetaminophen as needed. Other medications in the multimodal pain-management protocol included gabapentin 300 mg twice daily, meloxicam 15 mg daily, and oxycodone 10 mg every 4 hours as needed. Rescue medication for insufficient pain relief was dilaudid 0.25 mg IV every 15 minutes for visual analog pain scale > 8. On discharge, the patients received a prescription of 30 tablets of hydrocodone 10 mg.
Periarticular Injections
Intraoperatively, all patients in the SOC and ERAS groups received periarticular injections. The liposomal bupivacaine injection was added to the standard injection mixture for the ERAS group. For the SOC group, the total volume of 100 ml was divided into 10 separate 10 cc syringes, and for the ERAS group, the total volume of 140 ml was divided into 14 separate 10 cc syringes. The SOC group injections were performed with an 18-gauge needle and the periarticular soft tissues grossly infiltrated. The ERAS group injections were done with more attention to anatomical detail. Injection sites for the ERAS group included the posterior joint capsule, the medial compartment, the lateral compartment, the tibial fat pad, the quadriceps and the patellar tendon, the femoral and tibial periosteum circumferentially, and the anterior joint capsule. Each needle-stick in the ERAS group delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee.
Outcome Variable
The primary outcome measure was total oral MED intraoperatively, in the PACU, during the hospital inpatient stay, in the hospital discharge prescription, and during the 3-month period after hospital discharge. Incidence of nausea and vomiting during the inpatient stay and any narcotic use at 6 months postsurgery were secondary binary outcomes.
Statistical Analysis
Demographic data and the clinical characteristics for the entire group were described using the sample mean and SD for continuous variables and the frequency and percentage for categorical variables. Differences between the 2 cohorts were analyzed using a 2-independent-sample t test and Fisher exact test.
The estimation of the total oral MED throughout all phases of care was done using a separate Poisson model due to the data being not normally distributed. A log-linear regression model was used to evaluate the main effect of ERAS vs the SOC cohort on the total oral MED used. Finally, a separate multiple logistic regression model was used to estimate the odds of postoperative nausea and vomiting and narcotic use at 6 months postsurgery between the cohorts. The adjusted odds ratio (OR) was estimated from the logistic model. Age, sex, body mass index, preoperative functional independence score, narcotic use within 3 months prior to surgery, anesthesia type used (subarachnoid block with monitored anesthesia care vs general endotracheal anesthesia), and postoperative complications (yes/no) were included as covariates in each model. The length of hospital stay and the above-mentioned factors were also included as covariates in the model estimating the total oral MED during the hospital stay, on hospital discharge, during the 3-month period after hospital discharge, and at 6 months following hospital discharge.
Statistical analysis was done using SAS version 9.4. The level of significance was set at α = 0.05 (2 tailed), and we implemented the false discovery rate (FDR) procedure to control false positives over multiple tests.16
Results
Two hundred forty-nine patients had 296 elective unilateral TKAs in this study from 2013 through 2018. Thirty-one patients had both unilateral TKAs under the SOC protocol; 5 patients had both unilateral TKAs under the ERAS protocol. Eleven of the patients who eventually had both knees replaced had 1 operation under each protocol The SOC group included 196 TKAs and the ERAS group included 100 TKAs. Of the 196 SOC patients, 94% were male. The mean age was 68.2 years (range, 48-86). The length of hospital stay ranged from 36.6 to 664.3 hours. Of the 100 ERAS patients, 96% were male (Table 2). The mean age was 66.7 years (range, 48-85). The length of hospital stay ranged from 12.5 to 45 hours.
Perioperative Opioid Use
Of the SOC patients, 99.0% received narcotics intraoperatively (range, 0-198 mg MED), and 74.5% received narcotics during PACU recovery (range, 0-141 mg MED). The total oral MED during the hospital stay for the SOC patients ranged from 10 to 2,946 mg. Of the ERAS patients, 86% received no narcotics during surgery (range, 0-110 mg MED), and 98% received no narcotics during PACU recovery (range, 0-65 mg MED). The total oral MED during the hospital stay for the ERAS patients ranged from 10 to 240 mg.
The MED used was significantly lower for the ERAS patients than it was for the SOC patients during surgery (10.5 mg vs 57.4 mg, P = .0001, FDR = .0002) and in the PACU (1.3 mg vs 13.6 mg, P = .0002, FDR = .0004), during the inpatient stay (66.7 mg vs 169.5 mg, P = .0001, FDR = .0002), and on hospital discharge (419.3 mg vs 776.7 mg, P = .0001, FDR = .0002). However, there was no significant difference in the total MED prescriptions filled between patients on the ERAS protocol vs those who received SOC during the 3-month period after hospital discharge (858.3 mg vs 1126.1 mg, P = .29, FDR = .29)(Table 3).
Finally, the logistic regression analysis, adjusting for the covariates demonstrated that the ERAS patients were less likely to take narcotics at 6 months following hospital discharge (OR, 0.23; P = .013; FDR = .018) and less likely to have postoperative nausea and vomiting (OR, 0.18; P = .019; FDR = .02) than SOC patients. There was no statistically significant difference between complication rates for the SOC and ERAS groups, which were 11.2% and 5.0%, respectively, with an overall complication rate of 9.1% (P = .09)(Table 4).
Discussion
Orthopedic surgery has been associated with long-term opioid use and misuse. Orthopedic surgeons are frequently among the highest prescribers of narcotics. According to Volkow and colleagues, orthopedic surgeons were the fourth largest prescribers of opioids in 2009, behind primary care physicians, internists, and dentists.17 The opioid crisis in the United States is well recognized. In 2017, > 70,000 deaths occurred due to drug overdoses, with 68% involving a prescription or illicit opioid. The Centers for Disease Control and Prevention has estimated a total economic burden of $78.5 billion per year as a direct result of misused prescribed opioids.18 This includes the cost of health care, lost productivity, addiction treatment, and the impact on the criminal justice system.
The current opioid crisis places further emphasis on opioid-reducing or sparing techniques in patients undergoing TKA. The use of liposomal bupivacaine for intraoperative periarticular injection is debated in the literature regarding its efficacy and whether it should be included in multimodal protocols. Researchers have argued that liposomal bupivacaine is not superior to regular bupivacaine and because of its increased cost is not justified.19,20 A meta-analysis from Zhao and colleagues showed no difference in pain control and functional recovery when comparing liposomal bupivacaine and control.21 In a randomized clinical trial, Schroer and colleagues matched liposomal bupivacaine against regular bupivacaine and found no difference in pain scores and similar narcotic use during hospitalization.22
Studies evaluating liposomal bupivacaine have demonstrated postoperative benefits in pain relief and potential opioid consumption.23 In a multicenter randomized controlled trial, Barrington and colleagues noted improved pain control at 6 and 12 hours after surgery with liposomal bupivacaine as a periarticular injection vs ropivacaine, though results were similar when compared with intrathecal morphine.24 Snyder and colleagues reported higher patient satisfaction in pain control and overall experience as well as decreased MED consumption in the PACU and on postoperative days 0 to 2 when using liposomal bupivacaine vs a multidrug cocktail for periarticular injection.25
The PILLAR trial, an industry-sponsored study, was designed to compare the effects of local infiltration anesthesia with and without liposomal bupivacaine with emphasis on a meticulous standardized infiltration technique. In our study, we used a similar technique with an expanded volume of injection solution to 140 ml that was delivered throughout the knee in a series of 14 syringes. Each needle-stick delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee. Infiltration technique has varied among the literature focused on periarticular injections.
In our experience, a standard infiltration technique is critical to the effective delivery of liposomal bupivacaine throughout all compartments of the knee and to obtaining reproducible pain control. The importance of injection technique cannot be overemphasized, and variations can be seen in studies published to date.26 Well-designed trials are needed to address this key component.
There have been limited data focused on the veteran population regarding postoperative pain-management strategies and recovery pathways either with or without liposomal bupivacaine. In a retrospective review, Sakamoto and colleagues found VA patients undergoing TKA had reduced opioid use in the first 24 hours after primary TKA with the use of intraoperative liposomal bupivacaine.27 The VA population has been shown to be at high risk for opioid misuse. The prevalence of comorbidities such as traumatic brain injury, posttraumatic stress disorder, and depression in the VA population also places them at risk for polypharmacy of central nervous system–acting medications.28 This emphasizes the importance of multimodal strategies, which can limit or eliminate narcotics in the perioperative period. The implementation of our ERAS protocol reduced opioid use during intraoperative, PACU, and inpatient hospital stay.
While the financial implications of our recovery protocol were not a primary focus of this study, there are many notable benefits on the overall inpatient cost to the VHA. According to the Health Economics Resource Center, the average daily cost of stay while under VA care for an inpatient surgical bed increased from $4,831 in 2013 to $6,220 in 2018.29 Our reduction in length of stay between our cohorts is 44.5 hours, which translates to a substantial financial savings per patient after protocol implementation. A more detailed look at the financial aspect of our protocol would need to be performed to evaluate the financial impact of other aspects of our protocol, such as the elimination of patient-controlled anesthesia and the reduction in total narcotics prescribed in the postoperative global period.
Limitations
The limitations of this study include its retrospective study design. With the VHA patient population, it may be subject to selection bias, as the population is mostly older and predominantly male compared with that of the general population. This could potentially influence the efficacy of our protocol on a population of patients with more women. In a recent study by Perruccio and colleagues, sex was found to moderate the effects of comorbidities, low back pain, and depressive symptoms on postoperative pain in patients undergoing TKA.30
With regard to outpatient narcotic prescriptions, although we cannot fully know whether these filled prescriptions were used for pain control, it is a reasonable assumption that patients who are dealing with continued postoperative or chronic pain issues will fill these prescriptions or seek refills. It is important to note that the data on prescriptions and refills in the 3-month postoperative period include all narcotic prescriptions filled by any VHA prescriber and are not specifically limited to our orthopedic team. For outpatient narcotic use, we were not able to access accurate pill counts for any discharge prescriptions or subsequent refills that were given throughout the VA system. We were able to report on total prescriptions filled in the first 3 months following TKA.
We calculated total oral MEDs to better understand the amount of narcotics being distributed throughout our population of patients. We believe this provides important information about the overall narcotic burden in the veteran population. There was no significant difference between the SOC and ERAS groups regarding oral MED prescribed in the 3-month postoperative period; however, at the 6-month follow-up visit, only 16% of patients in the ERAS group were taking any type of narcotic vs 37.2% in the SOC group (P = .0002).
Conclusions
A multidisciplinary ERAS protocol implemented at VANTHCS was effective in reducing length of stay and opioid burden throughout all phases of surgical care in our patients undergoing primary TKA. Patient and nursing education seem to be critical components to the implementation of a successful multimodal pain protocol. Reducing the narcotic burden has valuable financial and medical benefits in this at-risk population.
1. Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25(11):1797-1803. doi:10.1016/j.joca.2017.07.022
2. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council [published correction appears in J Pain. 2016 Apr;17(4):508-10. Dosage error in article text]. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
3. Moucha CS, Weiser MC, Levin EJ. Current Strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
4. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084. doi:10.2106/JBJS.J.01095
5. Jenstrup MT, Jæger P, Lund J, et al. Effects of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand. 2012;56(3):357-364. doi:10.1111/j.1399-6576.2011.02621.x
6. Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anesthesia improve outcome after total knee arthroplasty?. Clin Orthop Relat Res. 2009;467(9):2379-2402. doi:10.1007/s11999-008-0666-9
7. Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6)(suppl 2):33-38. doi:10.1016/j.arth.2007.03.034
8. Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88(5):959-963. doi:10.2106/JBJS.E.00344
9. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
10. Hyland SJ, Deliberato DG, Fada RA, Romanelli MJ, Collins CL, Wasielewski RC. Liposomal bupivacaine versus standard periarticular injection in total knee arthroplasty with regional anesthesia: a prospective randomized controlled trial. J Arthroplasty. 2019;34(3):488-494. doi:10.1016/j.arth.2018.11.026
11. Barrington JW, Lovald ST, Ong KL, Watson HN, Emerson RH Jr. Postoperative pain after primary total knee arthroplasty: comparison of local injection analgesic cocktails and the role of demographic and surgical factors. J Arthroplasty. 2016;31(9) (suppl):288-292. doi:10.1016/j.arth.2016.05.002
12. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536. doi:10.1016/j.knee.2011.12.004
13. Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo D. Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty. 2018;33(1):90-96. doi:10.1016/j.arth.2017.07.024
14. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
15. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737. doi:10.1002/pds.3945
16. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289-300. doi:10.1111/j.2517-6161.1995.tb02031.x
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301. doi:10.1001/jama.2011.401
18. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
19. Pichler L, Poeran J, Zubizarreta N, et al. Liposomal bupivacaine does not reduce inpatient opioid prescription or related complications after knee arthroplasty: a database analysis. Anesthesiology. 2018;129(4):689-699. doi:10.1097/ALN.0000000000002267
20. Jain RK, Porat MD, Klingenstein GG, Reid JJ, Post RE, Schoifet SD. The AAHKS Clinical Research Award: liposomal bupivacaine and periarticular injection are not superior to single-shot intra-articular injection for pain control in total knee arthroplasty. J Arthroplasty. 2016;31(9)(suppl):22-25. doi:10.1016/j.arth.2016.03.036
21. Zhao B, Ma X, Zhang J, Ma J, Cao Q. The efficacy of local liposomal bupivacaine infiltration on pain and recovery after total joint arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(3):e14092. doi:10.1097/MD.0000000000014092
22. Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME. Does extended-release liposomal bupivacaine better control pain than bupivacaine after total knee arthroplasty (TKA)? A prospective, randomized clinical trial. J Arthroplasty. 2015;30(9)(suppl):64-67. doi:10.1016/j.arth.2015.01.059
23. Ma J, Zhang W, Yao S. Liposomal bupivacaine infiltration versus femoral nerve block for pain control in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2016;36(Pt A): 44-55. doi:10.1016/j.ijsu.2016.10.007
24. Barrington JW, Emerson RH, Lovald ST, Lombardi AV, Berend KR. No difference in early analgesia between liposomal bupivacaine injection and intrathecal morphine after TKA. Clin Orthop Relat Res. 2017;475(1):94-105. doi:10.1007/s11999-016-4931-z
25. Snyder MA, Scheuerman CM, Gregg JL, Ruhnke CJ, Eten K. Improving total knee arthroplasty perioperative pain management using a periarticular injection with bupivacaine liposomal suspension. Arthroplast Today. 2016;2(1):37-42. doi:10.1016/j.artd.2015.05.005
26. Kuang MJ,Du Y, Ma JX, He W, Fu L, Ma XL. The efficacy of liposomal bupivacaine using periarticular injection in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2017;32(4):1395-1402. doi:10.1016/j.arth.2016.12.025
27. Sakamoto B, Keiser S, Meldrum R, Harker G, Freese A. Efficacy of liposomal bupivacaine infiltration on the management of total knee arthroplasty. JAMA Surg. 2017;152(1):90-95. doi:10.1001/jamasurg.2016.3474
28. Collett GA, Song K, Jaramillo CA, Potter JS, Finley EP, Pugh MJ. Prevalence of central nervous system polypharmacy and associations with overdose and suicide-related behaviors in Iraq and Afghanistan war veterans in VA care 2010-2011. Drugs Real World Outcomes. 2016;3(1):45-52. doi:10.1007/s40801-015-0055-0
29. US Department of Veterans Affairs. HERC inpatient average cost data. Updated April 2, 2021. Accessed April 16, 2021. https://www.herc.research.va.gov/include/page.asp?id=inpatient#herc-inpat-avg-cost
30. Perruccio AV, Fitzpatrick J, Power JD, et al. Sex-modified effects of depression, low back pain, and comorbidities on pain after total knee arthroplasty for osteoarthritis. Arthritis Care Res (Hoboken). 2020;72(8):1074-1080. doi:10.1002/acr.24002
Total knee arthroplasty (TKA) is one of the most common surgical procedures in the United States. The volume of TKAs is projected to substantially increase over the next 30 years.1 Adequate pain control after TKA is critically important to achieve early mobilization, shorten the length of hospital stay, and reduce postoperative complications. The evolution and inclusion of multimodal pain-management protocols have had a major impact on the clinical outcomes for TKA patients.2,3
Pain-management protocols typically use several modalities to control pain throughout the perioperative period. Multimodal opioid and nonopioid oral medications are administered during the pre- and postoperative periods and often involve a combination of acetaminophen, gabapentinoids, and cyclooxygenase-2 inhibitors.4 Peripheral nerve blocks and central neuraxial blockades are widely used and have been shown to be effective in reducing postoperative pain as well as overall opioid consumption.5,6 Finally, intraoperative periarticular injections have been shown to reduce postoperative pain and opioid consumption as well as improve patient satisfaction scores.7-9 These strategies are routinely used in TKA with the goal of minimizing overall opioid consumption and adverse events, reducing perioperative complications, and improving patient satisfaction.
Periarticular injections during surgery are an integral part of the multimodal pain-management protocols, though no consensus has been reached on proper injection formulation or technique. Liposomal bupivacaine is a local anesthetic depot formulation approved by the US Food and Drug Administration for surgical patients. The reported results have been discrepant regarding the efficacy of using liposomal bupivacaine injection in patients with TKA. Several studies have reported no added benefit of liposomal bupivacaine in contrast to a mixture of local anesthetics.10,11 Other studies have demonstrated superior pain relief.12 Many factors may contribute to the discrepant data, such as injection techniques, infiltration volume, and the assessment tools used to measure efficacy and safety.13
The US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care to a large patient population. Many of the patients in that system have high-risk profiles, including medical comorbidities; exposure to chronic pain and opioid use; and psychological and central nervous system injuries, including posttraumatic stress disorder and traumatic brain injury. Hadlandsmyth and colleagues reported increased risk of prolonged opioid use in VA patients after TKA surgery.14 They found that 20% of the patients were still on long-term opioids more than 90 days after TKA.
The purpose of this study was to evaluate the efficacy of the implementation of a comprehensive enhanced recovery after surgery (ERAS) protocol at a regional VA medical center. We hypothesize that the addition of liposomal bupivacaine in a multidisciplinary ERAS protocol would reduce the length of hospital stay and opioid consumption without any deleterious effects on postoperative outcomes.
Methods
A postoperative recovery protocol was implemented in 2013 at VA North Texas Health Care System (VANTHCS) in Dallas, and many of the patients continued to have issues with satisfactory pain control, prolonged length of stay, and extended opioid consumption postoperatively. A multimodal pain-management protocol and multidisciplinary perioperative case-management protocol were implemented in 2016 to further improve the clinical outcomes of patients undergoing TKA surgery. The senior surgeon (JM) organized a multidisciplinary team of health care providers to identify and implement potential solutions. This task force met weekly and consisted of surgeons, anesthesiologists, certified registered nurse anesthetists, orthopedic physician assistants, a nurse coordinator, a physical therapist, and an occupational therapist, as well as operating room, postanesthesia care unit (PACU), and surgical ward nurses. In addition, the staff from the home health agencies and social services attended the weekly meetings.
We conducted a retrospective review of all patients who had undergone unilateral TKA from 2013 to 2018 at VANTHCS. This was a consecutive, unselected cohort. All patients were under the care of a single surgeon using identical implant systems and identical surgical techniques. This study was approved by the institutional review board at VANTHCS. Patients were divided into 2 distinct and consecutive cohorts. The standard of care (SOC) group included all patients from 2013 to 2016. The ERAS group included all patients after the institution of the standardized protocol until the end of the study period.
Data on patient demographics, the American Society of Anesthesiologists risk classification, and preoperative functional status were extracted. Anesthesia techniques included either general endotracheal anesthesia or subarachnoid block with monitored anesthesia care. The quantity of the opioids given during surgery, in the PACU, during the inpatient stay, as discharge prescriptions, and as refills of the narcotic prescriptions up to 3 months postsurgery were recorded. All opioids were converted into morphine equivalent dosages (MED) in order to be properly analyzed using the statistical methodologies described in the statistical section.15 The VHA is a closed health care delivery system; therefore, all of the prescriptions ordered by surgery providers were recorded in the electronic health record.
ERAS Protocol
The SOC cohort was predominantly managed with general endotracheal anesthesia. The ERAS group was predominantly managed with subarachnoid blocks (Table 1). For the ERAS protocol preoperatively, the patients were administered oral gabapentin 300 mg, acetaminophen 650 mg, and oxycodone 20 mg, and IV ondansetron 4 mg. Intraoperatively, minimal opioids were used. In the PACU, the patients received dilaudid 0.25 mg IV as needed every 15 minutes for up to 1 mg/h. The nursing staff was trained to use the visual analog pain scale scores to titrate the medication. During the inpatient stay, patients received 1 g IV acetaminophen every 6 hours for 3 doses. The patients thereafter received oral acetaminophen as needed. Other medications in the multimodal pain-management protocol included gabapentin 300 mg twice daily, meloxicam 15 mg daily, and oxycodone 10 mg every 4 hours as needed. Rescue medication for insufficient pain relief was dilaudid 0.25 mg IV every 15 minutes for visual analog pain scale > 8. On discharge, the patients received a prescription of 30 tablets of hydrocodone 10 mg.
Periarticular Injections
Intraoperatively, all patients in the SOC and ERAS groups received periarticular injections. The liposomal bupivacaine injection was added to the standard injection mixture for the ERAS group. For the SOC group, the total volume of 100 ml was divided into 10 separate 10 cc syringes, and for the ERAS group, the total volume of 140 ml was divided into 14 separate 10 cc syringes. The SOC group injections were performed with an 18-gauge needle and the periarticular soft tissues grossly infiltrated. The ERAS group injections were done with more attention to anatomical detail. Injection sites for the ERAS group included the posterior joint capsule, the medial compartment, the lateral compartment, the tibial fat pad, the quadriceps and the patellar tendon, the femoral and tibial periosteum circumferentially, and the anterior joint capsule. Each needle-stick in the ERAS group delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee.
Outcome Variable
The primary outcome measure was total oral MED intraoperatively, in the PACU, during the hospital inpatient stay, in the hospital discharge prescription, and during the 3-month period after hospital discharge. Incidence of nausea and vomiting during the inpatient stay and any narcotic use at 6 months postsurgery were secondary binary outcomes.
Statistical Analysis
Demographic data and the clinical characteristics for the entire group were described using the sample mean and SD for continuous variables and the frequency and percentage for categorical variables. Differences between the 2 cohorts were analyzed using a 2-independent-sample t test and Fisher exact test.
The estimation of the total oral MED throughout all phases of care was done using a separate Poisson model due to the data being not normally distributed. A log-linear regression model was used to evaluate the main effect of ERAS vs the SOC cohort on the total oral MED used. Finally, a separate multiple logistic regression model was used to estimate the odds of postoperative nausea and vomiting and narcotic use at 6 months postsurgery between the cohorts. The adjusted odds ratio (OR) was estimated from the logistic model. Age, sex, body mass index, preoperative functional independence score, narcotic use within 3 months prior to surgery, anesthesia type used (subarachnoid block with monitored anesthesia care vs general endotracheal anesthesia), and postoperative complications (yes/no) were included as covariates in each model. The length of hospital stay and the above-mentioned factors were also included as covariates in the model estimating the total oral MED during the hospital stay, on hospital discharge, during the 3-month period after hospital discharge, and at 6 months following hospital discharge.
Statistical analysis was done using SAS version 9.4. The level of significance was set at α = 0.05 (2 tailed), and we implemented the false discovery rate (FDR) procedure to control false positives over multiple tests.16
Results
Two hundred forty-nine patients had 296 elective unilateral TKAs in this study from 2013 through 2018. Thirty-one patients had both unilateral TKAs under the SOC protocol; 5 patients had both unilateral TKAs under the ERAS protocol. Eleven of the patients who eventually had both knees replaced had 1 operation under each protocol The SOC group included 196 TKAs and the ERAS group included 100 TKAs. Of the 196 SOC patients, 94% were male. The mean age was 68.2 years (range, 48-86). The length of hospital stay ranged from 36.6 to 664.3 hours. Of the 100 ERAS patients, 96% were male (Table 2). The mean age was 66.7 years (range, 48-85). The length of hospital stay ranged from 12.5 to 45 hours.
Perioperative Opioid Use
Of the SOC patients, 99.0% received narcotics intraoperatively (range, 0-198 mg MED), and 74.5% received narcotics during PACU recovery (range, 0-141 mg MED). The total oral MED during the hospital stay for the SOC patients ranged from 10 to 2,946 mg. Of the ERAS patients, 86% received no narcotics during surgery (range, 0-110 mg MED), and 98% received no narcotics during PACU recovery (range, 0-65 mg MED). The total oral MED during the hospital stay for the ERAS patients ranged from 10 to 240 mg.
The MED used was significantly lower for the ERAS patients than it was for the SOC patients during surgery (10.5 mg vs 57.4 mg, P = .0001, FDR = .0002) and in the PACU (1.3 mg vs 13.6 mg, P = .0002, FDR = .0004), during the inpatient stay (66.7 mg vs 169.5 mg, P = .0001, FDR = .0002), and on hospital discharge (419.3 mg vs 776.7 mg, P = .0001, FDR = .0002). However, there was no significant difference in the total MED prescriptions filled between patients on the ERAS protocol vs those who received SOC during the 3-month period after hospital discharge (858.3 mg vs 1126.1 mg, P = .29, FDR = .29)(Table 3).
Finally, the logistic regression analysis, adjusting for the covariates demonstrated that the ERAS patients were less likely to take narcotics at 6 months following hospital discharge (OR, 0.23; P = .013; FDR = .018) and less likely to have postoperative nausea and vomiting (OR, 0.18; P = .019; FDR = .02) than SOC patients. There was no statistically significant difference between complication rates for the SOC and ERAS groups, which were 11.2% and 5.0%, respectively, with an overall complication rate of 9.1% (P = .09)(Table 4).
Discussion
Orthopedic surgery has been associated with long-term opioid use and misuse. Orthopedic surgeons are frequently among the highest prescribers of narcotics. According to Volkow and colleagues, orthopedic surgeons were the fourth largest prescribers of opioids in 2009, behind primary care physicians, internists, and dentists.17 The opioid crisis in the United States is well recognized. In 2017, > 70,000 deaths occurred due to drug overdoses, with 68% involving a prescription or illicit opioid. The Centers for Disease Control and Prevention has estimated a total economic burden of $78.5 billion per year as a direct result of misused prescribed opioids.18 This includes the cost of health care, lost productivity, addiction treatment, and the impact on the criminal justice system.
The current opioid crisis places further emphasis on opioid-reducing or sparing techniques in patients undergoing TKA. The use of liposomal bupivacaine for intraoperative periarticular injection is debated in the literature regarding its efficacy and whether it should be included in multimodal protocols. Researchers have argued that liposomal bupivacaine is not superior to regular bupivacaine and because of its increased cost is not justified.19,20 A meta-analysis from Zhao and colleagues showed no difference in pain control and functional recovery when comparing liposomal bupivacaine and control.21 In a randomized clinical trial, Schroer and colleagues matched liposomal bupivacaine against regular bupivacaine and found no difference in pain scores and similar narcotic use during hospitalization.22
Studies evaluating liposomal bupivacaine have demonstrated postoperative benefits in pain relief and potential opioid consumption.23 In a multicenter randomized controlled trial, Barrington and colleagues noted improved pain control at 6 and 12 hours after surgery with liposomal bupivacaine as a periarticular injection vs ropivacaine, though results were similar when compared with intrathecal morphine.24 Snyder and colleagues reported higher patient satisfaction in pain control and overall experience as well as decreased MED consumption in the PACU and on postoperative days 0 to 2 when using liposomal bupivacaine vs a multidrug cocktail for periarticular injection.25
The PILLAR trial, an industry-sponsored study, was designed to compare the effects of local infiltration anesthesia with and without liposomal bupivacaine with emphasis on a meticulous standardized infiltration technique. In our study, we used a similar technique with an expanded volume of injection solution to 140 ml that was delivered throughout the knee in a series of 14 syringes. Each needle-stick delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee. Infiltration technique has varied among the literature focused on periarticular injections.
In our experience, a standard infiltration technique is critical to the effective delivery of liposomal bupivacaine throughout all compartments of the knee and to obtaining reproducible pain control. The importance of injection technique cannot be overemphasized, and variations can be seen in studies published to date.26 Well-designed trials are needed to address this key component.
There have been limited data focused on the veteran population regarding postoperative pain-management strategies and recovery pathways either with or without liposomal bupivacaine. In a retrospective review, Sakamoto and colleagues found VA patients undergoing TKA had reduced opioid use in the first 24 hours after primary TKA with the use of intraoperative liposomal bupivacaine.27 The VA population has been shown to be at high risk for opioid misuse. The prevalence of comorbidities such as traumatic brain injury, posttraumatic stress disorder, and depression in the VA population also places them at risk for polypharmacy of central nervous system–acting medications.28 This emphasizes the importance of multimodal strategies, which can limit or eliminate narcotics in the perioperative period. The implementation of our ERAS protocol reduced opioid use during intraoperative, PACU, and inpatient hospital stay.
While the financial implications of our recovery protocol were not a primary focus of this study, there are many notable benefits on the overall inpatient cost to the VHA. According to the Health Economics Resource Center, the average daily cost of stay while under VA care for an inpatient surgical bed increased from $4,831 in 2013 to $6,220 in 2018.29 Our reduction in length of stay between our cohorts is 44.5 hours, which translates to a substantial financial savings per patient after protocol implementation. A more detailed look at the financial aspect of our protocol would need to be performed to evaluate the financial impact of other aspects of our protocol, such as the elimination of patient-controlled anesthesia and the reduction in total narcotics prescribed in the postoperative global period.
Limitations
The limitations of this study include its retrospective study design. With the VHA patient population, it may be subject to selection bias, as the population is mostly older and predominantly male compared with that of the general population. This could potentially influence the efficacy of our protocol on a population of patients with more women. In a recent study by Perruccio and colleagues, sex was found to moderate the effects of comorbidities, low back pain, and depressive symptoms on postoperative pain in patients undergoing TKA.30
With regard to outpatient narcotic prescriptions, although we cannot fully know whether these filled prescriptions were used for pain control, it is a reasonable assumption that patients who are dealing with continued postoperative or chronic pain issues will fill these prescriptions or seek refills. It is important to note that the data on prescriptions and refills in the 3-month postoperative period include all narcotic prescriptions filled by any VHA prescriber and are not specifically limited to our orthopedic team. For outpatient narcotic use, we were not able to access accurate pill counts for any discharge prescriptions or subsequent refills that were given throughout the VA system. We were able to report on total prescriptions filled in the first 3 months following TKA.
We calculated total oral MEDs to better understand the amount of narcotics being distributed throughout our population of patients. We believe this provides important information about the overall narcotic burden in the veteran population. There was no significant difference between the SOC and ERAS groups regarding oral MED prescribed in the 3-month postoperative period; however, at the 6-month follow-up visit, only 16% of patients in the ERAS group were taking any type of narcotic vs 37.2% in the SOC group (P = .0002).
Conclusions
A multidisciplinary ERAS protocol implemented at VANTHCS was effective in reducing length of stay and opioid burden throughout all phases of surgical care in our patients undergoing primary TKA. Patient and nursing education seem to be critical components to the implementation of a successful multimodal pain protocol. Reducing the narcotic burden has valuable financial and medical benefits in this at-risk population.
Total knee arthroplasty (TKA) is one of the most common surgical procedures in the United States. The volume of TKAs is projected to substantially increase over the next 30 years.1 Adequate pain control after TKA is critically important to achieve early mobilization, shorten the length of hospital stay, and reduce postoperative complications. The evolution and inclusion of multimodal pain-management protocols have had a major impact on the clinical outcomes for TKA patients.2,3
Pain-management protocols typically use several modalities to control pain throughout the perioperative period. Multimodal opioid and nonopioid oral medications are administered during the pre- and postoperative periods and often involve a combination of acetaminophen, gabapentinoids, and cyclooxygenase-2 inhibitors.4 Peripheral nerve blocks and central neuraxial blockades are widely used and have been shown to be effective in reducing postoperative pain as well as overall opioid consumption.5,6 Finally, intraoperative periarticular injections have been shown to reduce postoperative pain and opioid consumption as well as improve patient satisfaction scores.7-9 These strategies are routinely used in TKA with the goal of minimizing overall opioid consumption and adverse events, reducing perioperative complications, and improving patient satisfaction.
Periarticular injections during surgery are an integral part of the multimodal pain-management protocols, though no consensus has been reached on proper injection formulation or technique. Liposomal bupivacaine is a local anesthetic depot formulation approved by the US Food and Drug Administration for surgical patients. The reported results have been discrepant regarding the efficacy of using liposomal bupivacaine injection in patients with TKA. Several studies have reported no added benefit of liposomal bupivacaine in contrast to a mixture of local anesthetics.10,11 Other studies have demonstrated superior pain relief.12 Many factors may contribute to the discrepant data, such as injection techniques, infiltration volume, and the assessment tools used to measure efficacy and safety.13
The US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care to a large patient population. Many of the patients in that system have high-risk profiles, including medical comorbidities; exposure to chronic pain and opioid use; and psychological and central nervous system injuries, including posttraumatic stress disorder and traumatic brain injury. Hadlandsmyth and colleagues reported increased risk of prolonged opioid use in VA patients after TKA surgery.14 They found that 20% of the patients were still on long-term opioids more than 90 days after TKA.
The purpose of this study was to evaluate the efficacy of the implementation of a comprehensive enhanced recovery after surgery (ERAS) protocol at a regional VA medical center. We hypothesize that the addition of liposomal bupivacaine in a multidisciplinary ERAS protocol would reduce the length of hospital stay and opioid consumption without any deleterious effects on postoperative outcomes.
Methods
A postoperative recovery protocol was implemented in 2013 at VA North Texas Health Care System (VANTHCS) in Dallas, and many of the patients continued to have issues with satisfactory pain control, prolonged length of stay, and extended opioid consumption postoperatively. A multimodal pain-management protocol and multidisciplinary perioperative case-management protocol were implemented in 2016 to further improve the clinical outcomes of patients undergoing TKA surgery. The senior surgeon (JM) organized a multidisciplinary team of health care providers to identify and implement potential solutions. This task force met weekly and consisted of surgeons, anesthesiologists, certified registered nurse anesthetists, orthopedic physician assistants, a nurse coordinator, a physical therapist, and an occupational therapist, as well as operating room, postanesthesia care unit (PACU), and surgical ward nurses. In addition, the staff from the home health agencies and social services attended the weekly meetings.
We conducted a retrospective review of all patients who had undergone unilateral TKA from 2013 to 2018 at VANTHCS. This was a consecutive, unselected cohort. All patients were under the care of a single surgeon using identical implant systems and identical surgical techniques. This study was approved by the institutional review board at VANTHCS. Patients were divided into 2 distinct and consecutive cohorts. The standard of care (SOC) group included all patients from 2013 to 2016. The ERAS group included all patients after the institution of the standardized protocol until the end of the study period.
Data on patient demographics, the American Society of Anesthesiologists risk classification, and preoperative functional status were extracted. Anesthesia techniques included either general endotracheal anesthesia or subarachnoid block with monitored anesthesia care. The quantity of the opioids given during surgery, in the PACU, during the inpatient stay, as discharge prescriptions, and as refills of the narcotic prescriptions up to 3 months postsurgery were recorded. All opioids were converted into morphine equivalent dosages (MED) in order to be properly analyzed using the statistical methodologies described in the statistical section.15 The VHA is a closed health care delivery system; therefore, all of the prescriptions ordered by surgery providers were recorded in the electronic health record.
ERAS Protocol
The SOC cohort was predominantly managed with general endotracheal anesthesia. The ERAS group was predominantly managed with subarachnoid blocks (Table 1). For the ERAS protocol preoperatively, the patients were administered oral gabapentin 300 mg, acetaminophen 650 mg, and oxycodone 20 mg, and IV ondansetron 4 mg. Intraoperatively, minimal opioids were used. In the PACU, the patients received dilaudid 0.25 mg IV as needed every 15 minutes for up to 1 mg/h. The nursing staff was trained to use the visual analog pain scale scores to titrate the medication. During the inpatient stay, patients received 1 g IV acetaminophen every 6 hours for 3 doses. The patients thereafter received oral acetaminophen as needed. Other medications in the multimodal pain-management protocol included gabapentin 300 mg twice daily, meloxicam 15 mg daily, and oxycodone 10 mg every 4 hours as needed. Rescue medication for insufficient pain relief was dilaudid 0.25 mg IV every 15 minutes for visual analog pain scale > 8. On discharge, the patients received a prescription of 30 tablets of hydrocodone 10 mg.
Periarticular Injections
Intraoperatively, all patients in the SOC and ERAS groups received periarticular injections. The liposomal bupivacaine injection was added to the standard injection mixture for the ERAS group. For the SOC group, the total volume of 100 ml was divided into 10 separate 10 cc syringes, and for the ERAS group, the total volume of 140 ml was divided into 14 separate 10 cc syringes. The SOC group injections were performed with an 18-gauge needle and the periarticular soft tissues grossly infiltrated. The ERAS group injections were done with more attention to anatomical detail. Injection sites for the ERAS group included the posterior joint capsule, the medial compartment, the lateral compartment, the tibial fat pad, the quadriceps and the patellar tendon, the femoral and tibial periosteum circumferentially, and the anterior joint capsule. Each needle-stick in the ERAS group delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee.
Outcome Variable
The primary outcome measure was total oral MED intraoperatively, in the PACU, during the hospital inpatient stay, in the hospital discharge prescription, and during the 3-month period after hospital discharge. Incidence of nausea and vomiting during the inpatient stay and any narcotic use at 6 months postsurgery were secondary binary outcomes.
Statistical Analysis
Demographic data and the clinical characteristics for the entire group were described using the sample mean and SD for continuous variables and the frequency and percentage for categorical variables. Differences between the 2 cohorts were analyzed using a 2-independent-sample t test and Fisher exact test.
The estimation of the total oral MED throughout all phases of care was done using a separate Poisson model due to the data being not normally distributed. A log-linear regression model was used to evaluate the main effect of ERAS vs the SOC cohort on the total oral MED used. Finally, a separate multiple logistic regression model was used to estimate the odds of postoperative nausea and vomiting and narcotic use at 6 months postsurgery between the cohorts. The adjusted odds ratio (OR) was estimated from the logistic model. Age, sex, body mass index, preoperative functional independence score, narcotic use within 3 months prior to surgery, anesthesia type used (subarachnoid block with monitored anesthesia care vs general endotracheal anesthesia), and postoperative complications (yes/no) were included as covariates in each model. The length of hospital stay and the above-mentioned factors were also included as covariates in the model estimating the total oral MED during the hospital stay, on hospital discharge, during the 3-month period after hospital discharge, and at 6 months following hospital discharge.
Statistical analysis was done using SAS version 9.4. The level of significance was set at α = 0.05 (2 tailed), and we implemented the false discovery rate (FDR) procedure to control false positives over multiple tests.16
Results
Two hundred forty-nine patients had 296 elective unilateral TKAs in this study from 2013 through 2018. Thirty-one patients had both unilateral TKAs under the SOC protocol; 5 patients had both unilateral TKAs under the ERAS protocol. Eleven of the patients who eventually had both knees replaced had 1 operation under each protocol The SOC group included 196 TKAs and the ERAS group included 100 TKAs. Of the 196 SOC patients, 94% were male. The mean age was 68.2 years (range, 48-86). The length of hospital stay ranged from 36.6 to 664.3 hours. Of the 100 ERAS patients, 96% were male (Table 2). The mean age was 66.7 years (range, 48-85). The length of hospital stay ranged from 12.5 to 45 hours.
Perioperative Opioid Use
Of the SOC patients, 99.0% received narcotics intraoperatively (range, 0-198 mg MED), and 74.5% received narcotics during PACU recovery (range, 0-141 mg MED). The total oral MED during the hospital stay for the SOC patients ranged from 10 to 2,946 mg. Of the ERAS patients, 86% received no narcotics during surgery (range, 0-110 mg MED), and 98% received no narcotics during PACU recovery (range, 0-65 mg MED). The total oral MED during the hospital stay for the ERAS patients ranged from 10 to 240 mg.
The MED used was significantly lower for the ERAS patients than it was for the SOC patients during surgery (10.5 mg vs 57.4 mg, P = .0001, FDR = .0002) and in the PACU (1.3 mg vs 13.6 mg, P = .0002, FDR = .0004), during the inpatient stay (66.7 mg vs 169.5 mg, P = .0001, FDR = .0002), and on hospital discharge (419.3 mg vs 776.7 mg, P = .0001, FDR = .0002). However, there was no significant difference in the total MED prescriptions filled between patients on the ERAS protocol vs those who received SOC during the 3-month period after hospital discharge (858.3 mg vs 1126.1 mg, P = .29, FDR = .29)(Table 3).
Finally, the logistic regression analysis, adjusting for the covariates demonstrated that the ERAS patients were less likely to take narcotics at 6 months following hospital discharge (OR, 0.23; P = .013; FDR = .018) and less likely to have postoperative nausea and vomiting (OR, 0.18; P = .019; FDR = .02) than SOC patients. There was no statistically significant difference between complication rates for the SOC and ERAS groups, which were 11.2% and 5.0%, respectively, with an overall complication rate of 9.1% (P = .09)(Table 4).
Discussion
Orthopedic surgery has been associated with long-term opioid use and misuse. Orthopedic surgeons are frequently among the highest prescribers of narcotics. According to Volkow and colleagues, orthopedic surgeons were the fourth largest prescribers of opioids in 2009, behind primary care physicians, internists, and dentists.17 The opioid crisis in the United States is well recognized. In 2017, > 70,000 deaths occurred due to drug overdoses, with 68% involving a prescription or illicit opioid. The Centers for Disease Control and Prevention has estimated a total economic burden of $78.5 billion per year as a direct result of misused prescribed opioids.18 This includes the cost of health care, lost productivity, addiction treatment, and the impact on the criminal justice system.
The current opioid crisis places further emphasis on opioid-reducing or sparing techniques in patients undergoing TKA. The use of liposomal bupivacaine for intraoperative periarticular injection is debated in the literature regarding its efficacy and whether it should be included in multimodal protocols. Researchers have argued that liposomal bupivacaine is not superior to regular bupivacaine and because of its increased cost is not justified.19,20 A meta-analysis from Zhao and colleagues showed no difference in pain control and functional recovery when comparing liposomal bupivacaine and control.21 In a randomized clinical trial, Schroer and colleagues matched liposomal bupivacaine against regular bupivacaine and found no difference in pain scores and similar narcotic use during hospitalization.22
Studies evaluating liposomal bupivacaine have demonstrated postoperative benefits in pain relief and potential opioid consumption.23 In a multicenter randomized controlled trial, Barrington and colleagues noted improved pain control at 6 and 12 hours after surgery with liposomal bupivacaine as a periarticular injection vs ropivacaine, though results were similar when compared with intrathecal morphine.24 Snyder and colleagues reported higher patient satisfaction in pain control and overall experience as well as decreased MED consumption in the PACU and on postoperative days 0 to 2 when using liposomal bupivacaine vs a multidrug cocktail for periarticular injection.25
The PILLAR trial, an industry-sponsored study, was designed to compare the effects of local infiltration anesthesia with and without liposomal bupivacaine with emphasis on a meticulous standardized infiltration technique. In our study, we used a similar technique with an expanded volume of injection solution to 140 ml that was delivered throughout the knee in a series of 14 syringes. Each needle-stick delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee. Infiltration technique has varied among the literature focused on periarticular injections.
In our experience, a standard infiltration technique is critical to the effective delivery of liposomal bupivacaine throughout all compartments of the knee and to obtaining reproducible pain control. The importance of injection technique cannot be overemphasized, and variations can be seen in studies published to date.26 Well-designed trials are needed to address this key component.
There have been limited data focused on the veteran population regarding postoperative pain-management strategies and recovery pathways either with or without liposomal bupivacaine. In a retrospective review, Sakamoto and colleagues found VA patients undergoing TKA had reduced opioid use in the first 24 hours after primary TKA with the use of intraoperative liposomal bupivacaine.27 The VA population has been shown to be at high risk for opioid misuse. The prevalence of comorbidities such as traumatic brain injury, posttraumatic stress disorder, and depression in the VA population also places them at risk for polypharmacy of central nervous system–acting medications.28 This emphasizes the importance of multimodal strategies, which can limit or eliminate narcotics in the perioperative period. The implementation of our ERAS protocol reduced opioid use during intraoperative, PACU, and inpatient hospital stay.
While the financial implications of our recovery protocol were not a primary focus of this study, there are many notable benefits on the overall inpatient cost to the VHA. According to the Health Economics Resource Center, the average daily cost of stay while under VA care for an inpatient surgical bed increased from $4,831 in 2013 to $6,220 in 2018.29 Our reduction in length of stay between our cohorts is 44.5 hours, which translates to a substantial financial savings per patient after protocol implementation. A more detailed look at the financial aspect of our protocol would need to be performed to evaluate the financial impact of other aspects of our protocol, such as the elimination of patient-controlled anesthesia and the reduction in total narcotics prescribed in the postoperative global period.
Limitations
The limitations of this study include its retrospective study design. With the VHA patient population, it may be subject to selection bias, as the population is mostly older and predominantly male compared with that of the general population. This could potentially influence the efficacy of our protocol on a population of patients with more women. In a recent study by Perruccio and colleagues, sex was found to moderate the effects of comorbidities, low back pain, and depressive symptoms on postoperative pain in patients undergoing TKA.30
With regard to outpatient narcotic prescriptions, although we cannot fully know whether these filled prescriptions were used for pain control, it is a reasonable assumption that patients who are dealing with continued postoperative or chronic pain issues will fill these prescriptions or seek refills. It is important to note that the data on prescriptions and refills in the 3-month postoperative period include all narcotic prescriptions filled by any VHA prescriber and are not specifically limited to our orthopedic team. For outpatient narcotic use, we were not able to access accurate pill counts for any discharge prescriptions or subsequent refills that were given throughout the VA system. We were able to report on total prescriptions filled in the first 3 months following TKA.
We calculated total oral MEDs to better understand the amount of narcotics being distributed throughout our population of patients. We believe this provides important information about the overall narcotic burden in the veteran population. There was no significant difference between the SOC and ERAS groups regarding oral MED prescribed in the 3-month postoperative period; however, at the 6-month follow-up visit, only 16% of patients in the ERAS group were taking any type of narcotic vs 37.2% in the SOC group (P = .0002).
Conclusions
A multidisciplinary ERAS protocol implemented at VANTHCS was effective in reducing length of stay and opioid burden throughout all phases of surgical care in our patients undergoing primary TKA. Patient and nursing education seem to be critical components to the implementation of a successful multimodal pain protocol. Reducing the narcotic burden has valuable financial and medical benefits in this at-risk population.
1. Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25(11):1797-1803. doi:10.1016/j.joca.2017.07.022
2. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council [published correction appears in J Pain. 2016 Apr;17(4):508-10. Dosage error in article text]. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
3. Moucha CS, Weiser MC, Levin EJ. Current Strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
4. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084. doi:10.2106/JBJS.J.01095
5. Jenstrup MT, Jæger P, Lund J, et al. Effects of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand. 2012;56(3):357-364. doi:10.1111/j.1399-6576.2011.02621.x
6. Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anesthesia improve outcome after total knee arthroplasty?. Clin Orthop Relat Res. 2009;467(9):2379-2402. doi:10.1007/s11999-008-0666-9
7. Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6)(suppl 2):33-38. doi:10.1016/j.arth.2007.03.034
8. Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88(5):959-963. doi:10.2106/JBJS.E.00344
9. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
10. Hyland SJ, Deliberato DG, Fada RA, Romanelli MJ, Collins CL, Wasielewski RC. Liposomal bupivacaine versus standard periarticular injection in total knee arthroplasty with regional anesthesia: a prospective randomized controlled trial. J Arthroplasty. 2019;34(3):488-494. doi:10.1016/j.arth.2018.11.026
11. Barrington JW, Lovald ST, Ong KL, Watson HN, Emerson RH Jr. Postoperative pain after primary total knee arthroplasty: comparison of local injection analgesic cocktails and the role of demographic and surgical factors. J Arthroplasty. 2016;31(9) (suppl):288-292. doi:10.1016/j.arth.2016.05.002
12. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536. doi:10.1016/j.knee.2011.12.004
13. Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo D. Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty. 2018;33(1):90-96. doi:10.1016/j.arth.2017.07.024
14. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
15. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737. doi:10.1002/pds.3945
16. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289-300. doi:10.1111/j.2517-6161.1995.tb02031.x
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301. doi:10.1001/jama.2011.401
18. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
19. Pichler L, Poeran J, Zubizarreta N, et al. Liposomal bupivacaine does not reduce inpatient opioid prescription or related complications after knee arthroplasty: a database analysis. Anesthesiology. 2018;129(4):689-699. doi:10.1097/ALN.0000000000002267
20. Jain RK, Porat MD, Klingenstein GG, Reid JJ, Post RE, Schoifet SD. The AAHKS Clinical Research Award: liposomal bupivacaine and periarticular injection are not superior to single-shot intra-articular injection for pain control in total knee arthroplasty. J Arthroplasty. 2016;31(9)(suppl):22-25. doi:10.1016/j.arth.2016.03.036
21. Zhao B, Ma X, Zhang J, Ma J, Cao Q. The efficacy of local liposomal bupivacaine infiltration on pain and recovery after total joint arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(3):e14092. doi:10.1097/MD.0000000000014092
22. Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME. Does extended-release liposomal bupivacaine better control pain than bupivacaine after total knee arthroplasty (TKA)? A prospective, randomized clinical trial. J Arthroplasty. 2015;30(9)(suppl):64-67. doi:10.1016/j.arth.2015.01.059
23. Ma J, Zhang W, Yao S. Liposomal bupivacaine infiltration versus femoral nerve block for pain control in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2016;36(Pt A): 44-55. doi:10.1016/j.ijsu.2016.10.007
24. Barrington JW, Emerson RH, Lovald ST, Lombardi AV, Berend KR. No difference in early analgesia between liposomal bupivacaine injection and intrathecal morphine after TKA. Clin Orthop Relat Res. 2017;475(1):94-105. doi:10.1007/s11999-016-4931-z
25. Snyder MA, Scheuerman CM, Gregg JL, Ruhnke CJ, Eten K. Improving total knee arthroplasty perioperative pain management using a periarticular injection with bupivacaine liposomal suspension. Arthroplast Today. 2016;2(1):37-42. doi:10.1016/j.artd.2015.05.005
26. Kuang MJ,Du Y, Ma JX, He W, Fu L, Ma XL. The efficacy of liposomal bupivacaine using periarticular injection in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2017;32(4):1395-1402. doi:10.1016/j.arth.2016.12.025
27. Sakamoto B, Keiser S, Meldrum R, Harker G, Freese A. Efficacy of liposomal bupivacaine infiltration on the management of total knee arthroplasty. JAMA Surg. 2017;152(1):90-95. doi:10.1001/jamasurg.2016.3474
28. Collett GA, Song K, Jaramillo CA, Potter JS, Finley EP, Pugh MJ. Prevalence of central nervous system polypharmacy and associations with overdose and suicide-related behaviors in Iraq and Afghanistan war veterans in VA care 2010-2011. Drugs Real World Outcomes. 2016;3(1):45-52. doi:10.1007/s40801-015-0055-0
29. US Department of Veterans Affairs. HERC inpatient average cost data. Updated April 2, 2021. Accessed April 16, 2021. https://www.herc.research.va.gov/include/page.asp?id=inpatient#herc-inpat-avg-cost
30. Perruccio AV, Fitzpatrick J, Power JD, et al. Sex-modified effects of depression, low back pain, and comorbidities on pain after total knee arthroplasty for osteoarthritis. Arthritis Care Res (Hoboken). 2020;72(8):1074-1080. doi:10.1002/acr.24002
1. Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25(11):1797-1803. doi:10.1016/j.joca.2017.07.022
2. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council [published correction appears in J Pain. 2016 Apr;17(4):508-10. Dosage error in article text]. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
3. Moucha CS, Weiser MC, Levin EJ. Current Strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
4. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084. doi:10.2106/JBJS.J.01095
5. Jenstrup MT, Jæger P, Lund J, et al. Effects of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand. 2012;56(3):357-364. doi:10.1111/j.1399-6576.2011.02621.x
6. Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anesthesia improve outcome after total knee arthroplasty?. Clin Orthop Relat Res. 2009;467(9):2379-2402. doi:10.1007/s11999-008-0666-9
7. Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6)(suppl 2):33-38. doi:10.1016/j.arth.2007.03.034
8. Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88(5):959-963. doi:10.2106/JBJS.E.00344
9. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
10. Hyland SJ, Deliberato DG, Fada RA, Romanelli MJ, Collins CL, Wasielewski RC. Liposomal bupivacaine versus standard periarticular injection in total knee arthroplasty with regional anesthesia: a prospective randomized controlled trial. J Arthroplasty. 2019;34(3):488-494. doi:10.1016/j.arth.2018.11.026
11. Barrington JW, Lovald ST, Ong KL, Watson HN, Emerson RH Jr. Postoperative pain after primary total knee arthroplasty: comparison of local injection analgesic cocktails and the role of demographic and surgical factors. J Arthroplasty. 2016;31(9) (suppl):288-292. doi:10.1016/j.arth.2016.05.002
12. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536. doi:10.1016/j.knee.2011.12.004
13. Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo D. Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty. 2018;33(1):90-96. doi:10.1016/j.arth.2017.07.024
14. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
15. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737. doi:10.1002/pds.3945
16. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289-300. doi:10.1111/j.2517-6161.1995.tb02031.x
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301. doi:10.1001/jama.2011.401
18. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
19. Pichler L, Poeran J, Zubizarreta N, et al. Liposomal bupivacaine does not reduce inpatient opioid prescription or related complications after knee arthroplasty: a database analysis. Anesthesiology. 2018;129(4):689-699. doi:10.1097/ALN.0000000000002267
20. Jain RK, Porat MD, Klingenstein GG, Reid JJ, Post RE, Schoifet SD. The AAHKS Clinical Research Award: liposomal bupivacaine and periarticular injection are not superior to single-shot intra-articular injection for pain control in total knee arthroplasty. J Arthroplasty. 2016;31(9)(suppl):22-25. doi:10.1016/j.arth.2016.03.036
21. Zhao B, Ma X, Zhang J, Ma J, Cao Q. The efficacy of local liposomal bupivacaine infiltration on pain and recovery after total joint arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(3):e14092. doi:10.1097/MD.0000000000014092
22. Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME. Does extended-release liposomal bupivacaine better control pain than bupivacaine after total knee arthroplasty (TKA)? A prospective, randomized clinical trial. J Arthroplasty. 2015;30(9)(suppl):64-67. doi:10.1016/j.arth.2015.01.059
23. Ma J, Zhang W, Yao S. Liposomal bupivacaine infiltration versus femoral nerve block for pain control in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2016;36(Pt A): 44-55. doi:10.1016/j.ijsu.2016.10.007
24. Barrington JW, Emerson RH, Lovald ST, Lombardi AV, Berend KR. No difference in early analgesia between liposomal bupivacaine injection and intrathecal morphine after TKA. Clin Orthop Relat Res. 2017;475(1):94-105. doi:10.1007/s11999-016-4931-z
25. Snyder MA, Scheuerman CM, Gregg JL, Ruhnke CJ, Eten K. Improving total knee arthroplasty perioperative pain management using a periarticular injection with bupivacaine liposomal suspension. Arthroplast Today. 2016;2(1):37-42. doi:10.1016/j.artd.2015.05.005
26. Kuang MJ,Du Y, Ma JX, He W, Fu L, Ma XL. The efficacy of liposomal bupivacaine using periarticular injection in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2017;32(4):1395-1402. doi:10.1016/j.arth.2016.12.025
27. Sakamoto B, Keiser S, Meldrum R, Harker G, Freese A. Efficacy of liposomal bupivacaine infiltration on the management of total knee arthroplasty. JAMA Surg. 2017;152(1):90-95. doi:10.1001/jamasurg.2016.3474
28. Collett GA, Song K, Jaramillo CA, Potter JS, Finley EP, Pugh MJ. Prevalence of central nervous system polypharmacy and associations with overdose and suicide-related behaviors in Iraq and Afghanistan war veterans in VA care 2010-2011. Drugs Real World Outcomes. 2016;3(1):45-52. doi:10.1007/s40801-015-0055-0
29. US Department of Veterans Affairs. HERC inpatient average cost data. Updated April 2, 2021. Accessed April 16, 2021. https://www.herc.research.va.gov/include/page.asp?id=inpatient#herc-inpat-avg-cost
30. Perruccio AV, Fitzpatrick J, Power JD, et al. Sex-modified effects of depression, low back pain, and comorbidities on pain after total knee arthroplasty for osteoarthritis. Arthritis Care Res (Hoboken). 2020;72(8):1074-1080. doi:10.1002/acr.24002
Keep antibiotics unchanged in breakthrough UTIs
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
FROM PAS 2021
Quinolones and tendon health: Third-generation drugs may be safer
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
FROM ANNALS OF FAMILY MEDICINE
Intramuscular glucocorticoid injections seen as noninferior to intra-articular in knee OA
Intramuscular injections of glucocorticoids have efficacy similar to that of intra-articular injections in reducing pain in knee osteoarthritis but without the concerns about joint infection and the challenges of administration, according to results from a randomized, controlled trial reported at the OARSI 2021 World Congress.
Intra-articular injections of glucocorticoids are commonly used to relieve OA pain, but some general practitioners have difficulty administering them to patients, said Qiuke Wang, a PhD candidate at Erasmus University Medical Center in Rotterdam, the Netherlands. There are also concerns about whether intra-articular injections may cause damage to knee cartilage, Mr. Wang said at the conference, which is sponsored by the Osteoarthritis Research Society International.
Mr. Wang and colleagues conducted a randomized, controlled trial in which 145 patients with symptomatic knee OA received either an intramuscular or intra-articular injection of 40 mg triamcinolone acetonide, and then followed up at regular intervals for 24 weeks.
The study showed that Knee Injury and Osteoarthritis Outcome Scores for pain improved in both the intra-articular and intramuscular groups. Improvements in pain scores peaked in the intra-articular injection group at the 4-week mark, when the difference with intramuscular injections was statistically significant. However, the two groups showed no significant differences in pain improvement at the 8-, 12-, and 24-week follow-up points.
“Intra-articular injection can act immediately on inhibiting joint inflammation after injection,” Mr. Wang said in an interview. “In contrast, for intramuscular injection, glucocorticoid needs firstly to be absorbed by muscle into blood and then travel into the knee via the circulatory system.”
The study also showed no significant differences between the two groups in the secondary outcomes of patient symptoms, stiffness, function, and sport and quality of life scores. There were more adverse events in the intra-articular injection group: 42% of patients reported an adverse event, compared to 33% in the intramuscular group, and the adverse events reported in the intramuscular group were nonserious events, such as headache and flushing.
Mr. Wang told the conference that while the intramuscular injection was inferior to intra-articular injections at 4 weeks, it was noninferior at 8 and 24 weeks and should be considered an effective way to reduce pain in patients with knee OA.
“This trial provides evidence for shared decision making because in some cases a patient may have a preference for specific injection and the GP may feel incompetent to administer the intra-articular injection,” he said.
An audience member pointed out that there was now a growing body of evidence suggesting that intra-articular injections may contribute to faster progression of knee OA because of effects on knee cartilage.
Mr. Wang acknowledged that their own research had shown these side effects of intra-articular injections, which was why the trial was intended to examine whether intramuscular injections might achieve the same pain relief.
“In the real practice, I would say that both injections are effective, but the intra-articular injection may provide a slightly [better] effect in the short term,” he said.
Commenting on the findings, Martin van der Esch, PhD, of Amsterdam University of Applied Sciences, said there were no guidelines as to whether intra-articular or intramuscular injections were the best option, so it really came down to the clinician’s decision.
“Therefore this is really an interesting study, because it gives some light – not the answer – but some light in what direction it could go for specific groups of patients,” Dr. van der Esch said in an interview.
Dr. van der Esch suggested that intramuscular injections might be more appropriate for patients with more systemic disease affecting multiple joints, but intra-articular injections might offer greater benefits in a patient with severe and long-lasting disease in a single joint.
No conflicts of interest were declared.
Intramuscular injections of glucocorticoids have efficacy similar to that of intra-articular injections in reducing pain in knee osteoarthritis but without the concerns about joint infection and the challenges of administration, according to results from a randomized, controlled trial reported at the OARSI 2021 World Congress.
Intra-articular injections of glucocorticoids are commonly used to relieve OA pain, but some general practitioners have difficulty administering them to patients, said Qiuke Wang, a PhD candidate at Erasmus University Medical Center in Rotterdam, the Netherlands. There are also concerns about whether intra-articular injections may cause damage to knee cartilage, Mr. Wang said at the conference, which is sponsored by the Osteoarthritis Research Society International.
Mr. Wang and colleagues conducted a randomized, controlled trial in which 145 patients with symptomatic knee OA received either an intramuscular or intra-articular injection of 40 mg triamcinolone acetonide, and then followed up at regular intervals for 24 weeks.
The study showed that Knee Injury and Osteoarthritis Outcome Scores for pain improved in both the intra-articular and intramuscular groups. Improvements in pain scores peaked in the intra-articular injection group at the 4-week mark, when the difference with intramuscular injections was statistically significant. However, the two groups showed no significant differences in pain improvement at the 8-, 12-, and 24-week follow-up points.
“Intra-articular injection can act immediately on inhibiting joint inflammation after injection,” Mr. Wang said in an interview. “In contrast, for intramuscular injection, glucocorticoid needs firstly to be absorbed by muscle into blood and then travel into the knee via the circulatory system.”
The study also showed no significant differences between the two groups in the secondary outcomes of patient symptoms, stiffness, function, and sport and quality of life scores. There were more adverse events in the intra-articular injection group: 42% of patients reported an adverse event, compared to 33% in the intramuscular group, and the adverse events reported in the intramuscular group were nonserious events, such as headache and flushing.
Mr. Wang told the conference that while the intramuscular injection was inferior to intra-articular injections at 4 weeks, it was noninferior at 8 and 24 weeks and should be considered an effective way to reduce pain in patients with knee OA.
“This trial provides evidence for shared decision making because in some cases a patient may have a preference for specific injection and the GP may feel incompetent to administer the intra-articular injection,” he said.
An audience member pointed out that there was now a growing body of evidence suggesting that intra-articular injections may contribute to faster progression of knee OA because of effects on knee cartilage.
Mr. Wang acknowledged that their own research had shown these side effects of intra-articular injections, which was why the trial was intended to examine whether intramuscular injections might achieve the same pain relief.
“In the real practice, I would say that both injections are effective, but the intra-articular injection may provide a slightly [better] effect in the short term,” he said.
Commenting on the findings, Martin van der Esch, PhD, of Amsterdam University of Applied Sciences, said there were no guidelines as to whether intra-articular or intramuscular injections were the best option, so it really came down to the clinician’s decision.
“Therefore this is really an interesting study, because it gives some light – not the answer – but some light in what direction it could go for specific groups of patients,” Dr. van der Esch said in an interview.
Dr. van der Esch suggested that intramuscular injections might be more appropriate for patients with more systemic disease affecting multiple joints, but intra-articular injections might offer greater benefits in a patient with severe and long-lasting disease in a single joint.
No conflicts of interest were declared.
Intramuscular injections of glucocorticoids have efficacy similar to that of intra-articular injections in reducing pain in knee osteoarthritis but without the concerns about joint infection and the challenges of administration, according to results from a randomized, controlled trial reported at the OARSI 2021 World Congress.
Intra-articular injections of glucocorticoids are commonly used to relieve OA pain, but some general practitioners have difficulty administering them to patients, said Qiuke Wang, a PhD candidate at Erasmus University Medical Center in Rotterdam, the Netherlands. There are also concerns about whether intra-articular injections may cause damage to knee cartilage, Mr. Wang said at the conference, which is sponsored by the Osteoarthritis Research Society International.
Mr. Wang and colleagues conducted a randomized, controlled trial in which 145 patients with symptomatic knee OA received either an intramuscular or intra-articular injection of 40 mg triamcinolone acetonide, and then followed up at regular intervals for 24 weeks.
The study showed that Knee Injury and Osteoarthritis Outcome Scores for pain improved in both the intra-articular and intramuscular groups. Improvements in pain scores peaked in the intra-articular injection group at the 4-week mark, when the difference with intramuscular injections was statistically significant. However, the two groups showed no significant differences in pain improvement at the 8-, 12-, and 24-week follow-up points.
“Intra-articular injection can act immediately on inhibiting joint inflammation after injection,” Mr. Wang said in an interview. “In contrast, for intramuscular injection, glucocorticoid needs firstly to be absorbed by muscle into blood and then travel into the knee via the circulatory system.”
The study also showed no significant differences between the two groups in the secondary outcomes of patient symptoms, stiffness, function, and sport and quality of life scores. There were more adverse events in the intra-articular injection group: 42% of patients reported an adverse event, compared to 33% in the intramuscular group, and the adverse events reported in the intramuscular group were nonserious events, such as headache and flushing.
Mr. Wang told the conference that while the intramuscular injection was inferior to intra-articular injections at 4 weeks, it was noninferior at 8 and 24 weeks and should be considered an effective way to reduce pain in patients with knee OA.
“This trial provides evidence for shared decision making because in some cases a patient may have a preference for specific injection and the GP may feel incompetent to administer the intra-articular injection,” he said.
An audience member pointed out that there was now a growing body of evidence suggesting that intra-articular injections may contribute to faster progression of knee OA because of effects on knee cartilage.
Mr. Wang acknowledged that their own research had shown these side effects of intra-articular injections, which was why the trial was intended to examine whether intramuscular injections might achieve the same pain relief.
“In the real practice, I would say that both injections are effective, but the intra-articular injection may provide a slightly [better] effect in the short term,” he said.
Commenting on the findings, Martin van der Esch, PhD, of Amsterdam University of Applied Sciences, said there were no guidelines as to whether intra-articular or intramuscular injections were the best option, so it really came down to the clinician’s decision.
“Therefore this is really an interesting study, because it gives some light – not the answer – but some light in what direction it could go for specific groups of patients,” Dr. van der Esch said in an interview.
Dr. van der Esch suggested that intramuscular injections might be more appropriate for patients with more systemic disease affecting multiple joints, but intra-articular injections might offer greater benefits in a patient with severe and long-lasting disease in a single joint.
No conflicts of interest were declared.
FROM OARSI 2021
FDA okays upfront pembro for advanced HER2+ gastric cancer
The checkpoint inhibitor is to be used in conjunction with trastuzumab (Herceptin) and fluoropyrimidine- and platinum-containing chemotherapy.
Previously, pembrolizumab was approved as a single agent for these cancers for patients whose tumors express PD-L1 and whose disease progressed after two or more lines of treatment that included chemotherapy and HER2-targeted therapy.
The new approval comes about a year after the FDA’s first-ever approval of a checkpoint inhibitor (nivolumab [Opdivo] in combination with chemotherapies) for the frontline treatment of gastric cancers, as reported by this news organization.
The new approval is based on interim data from the first 264 patients of the ongoing KEYNOTE-811 trial, a randomized, double-blind, placebo-controlled trial involving patients with HER2-positive advanced gastric or GEJ adenocarcinoma who had not previously received systemic therapy for their metastatic disease.
Patients were randomly assigned (1:1) to receive either pembrolizumab at 200 mg or placebo every 3 weeks in combination with trastuzumab and either fluorouracil plus cisplatin or capecitabine plus oxaliplatin.
The overall response rate, which is the primary outcome, was 74% in the pembrolizumab arm and 52% in the placebo arm (one-sided P < .0001).
The median duration of response was 10.6 months in the pembrolizumab arm and 9.5 months in the placebo arm.
The adverse-reaction profile for patients receiving pembrolizumab is consistent with the known pembrolizumab safety profile, the FDA said in a statement.
The recommended pembrolizumab dose in this setting is 200 mg every 3 weeks or 400 mg every 6 weeks.
The FDA’s review, which was granted priority status, used the Real-Time Oncology Review pilot program, which allows streamlined data submission prior to the filing of the full clinical application, and Assessment Aid, a voluntary submission that facilitates the FDA’s assessment.
A version of this article first appeared on Medscape.com.
The checkpoint inhibitor is to be used in conjunction with trastuzumab (Herceptin) and fluoropyrimidine- and platinum-containing chemotherapy.
Previously, pembrolizumab was approved as a single agent for these cancers for patients whose tumors express PD-L1 and whose disease progressed after two or more lines of treatment that included chemotherapy and HER2-targeted therapy.
The new approval comes about a year after the FDA’s first-ever approval of a checkpoint inhibitor (nivolumab [Opdivo] in combination with chemotherapies) for the frontline treatment of gastric cancers, as reported by this news organization.
The new approval is based on interim data from the first 264 patients of the ongoing KEYNOTE-811 trial, a randomized, double-blind, placebo-controlled trial involving patients with HER2-positive advanced gastric or GEJ adenocarcinoma who had not previously received systemic therapy for their metastatic disease.
Patients were randomly assigned (1:1) to receive either pembrolizumab at 200 mg or placebo every 3 weeks in combination with trastuzumab and either fluorouracil plus cisplatin or capecitabine plus oxaliplatin.
The overall response rate, which is the primary outcome, was 74% in the pembrolizumab arm and 52% in the placebo arm (one-sided P < .0001).
The median duration of response was 10.6 months in the pembrolizumab arm and 9.5 months in the placebo arm.
The adverse-reaction profile for patients receiving pembrolizumab is consistent with the known pembrolizumab safety profile, the FDA said in a statement.
The recommended pembrolizumab dose in this setting is 200 mg every 3 weeks or 400 mg every 6 weeks.
The FDA’s review, which was granted priority status, used the Real-Time Oncology Review pilot program, which allows streamlined data submission prior to the filing of the full clinical application, and Assessment Aid, a voluntary submission that facilitates the FDA’s assessment.
A version of this article first appeared on Medscape.com.
The checkpoint inhibitor is to be used in conjunction with trastuzumab (Herceptin) and fluoropyrimidine- and platinum-containing chemotherapy.
Previously, pembrolizumab was approved as a single agent for these cancers for patients whose tumors express PD-L1 and whose disease progressed after two or more lines of treatment that included chemotherapy and HER2-targeted therapy.
The new approval comes about a year after the FDA’s first-ever approval of a checkpoint inhibitor (nivolumab [Opdivo] in combination with chemotherapies) for the frontline treatment of gastric cancers, as reported by this news organization.
The new approval is based on interim data from the first 264 patients of the ongoing KEYNOTE-811 trial, a randomized, double-blind, placebo-controlled trial involving patients with HER2-positive advanced gastric or GEJ adenocarcinoma who had not previously received systemic therapy for their metastatic disease.
Patients were randomly assigned (1:1) to receive either pembrolizumab at 200 mg or placebo every 3 weeks in combination with trastuzumab and either fluorouracil plus cisplatin or capecitabine plus oxaliplatin.
The overall response rate, which is the primary outcome, was 74% in the pembrolizumab arm and 52% in the placebo arm (one-sided P < .0001).
The median duration of response was 10.6 months in the pembrolizumab arm and 9.5 months in the placebo arm.
The adverse-reaction profile for patients receiving pembrolizumab is consistent with the known pembrolizumab safety profile, the FDA said in a statement.
The recommended pembrolizumab dose in this setting is 200 mg every 3 weeks or 400 mg every 6 weeks.
The FDA’s review, which was granted priority status, used the Real-Time Oncology Review pilot program, which allows streamlined data submission prior to the filing of the full clinical application, and Assessment Aid, a voluntary submission that facilitates the FDA’s assessment.
A version of this article first appeared on Medscape.com.
COVID-19 impact on breast cancer: Upfront endocrine Rx increased
The use of neoadjuvant endocrine therapy (NET) increased significantly during the first 8 months of the COVID-19 pandemic for women with estrogen receptor–positive (ER+) breast cancer. These patients would normally undergo surgery first, but because of operating room restrictions, those surgeries were delayed because of the pandemic, according to a new study.
“We hypothesized that by offering a nontoxic therapy, we would be able to ‘hold over’ patients until such time when personal protective equipment supplies were renewed and we could get into the operating room,” lead author Lee Wilke, MD, professor of surgery, University of Wisconsin, Madison, said in an interview.
“And while a small number of women with ER+ tumors get NET anyway, we found over one-third of patients with ER+ breast cancer were treated with NET due to COVID-19 during the first 8 months of last year,” she said.
“One year later, 31% of the same patient population is still getting NET,” she added.
The study was presented during the online annual meeting of the American Society of Breast Surgeons (ASBrS).
COVID-specific registry
Dr. Wilke believes that this study presents an accurate snapshot of changes in treatment caused by the pandemic.
She and her colleagues compared data collected in the ASBrS Mastery Program registry to data collected in an embedded but separate COVID-19 segment. The data were for the period from March 1 to Oct. 28, 2020.
Almost three-quarters of the surgeons who entered patients into the COVID-19 segment were from urban areas; 95% reported stopping mammographic screening during part of this period.
The preliminary analysis focused on data collected from 2,476 patients in the COVID-19 segment and 2,303 patients within the Mastery registry.
For patients with ER+/HER2- breast cancer, NET was described as a usual approach in 6.5% of patients in the COVID-19 registry. In the Mastery registry, 7.8% of patients received NET.
Compared with surgery first/usual practice, which served as the reference, older patients were more likely to receive NET first because of the COVID-19 pandemic than younger patients, and they were more likely to receive NET first if they lived in the Northeast or the Southeast compared to other regions of the United States. Dr. Wilke pointed out that the Northeast and the Southeast were hardest hit by COVID-19 early on in the pandemic.
Genomic testing was carried out in a small subgroup of patients; 24% of those patients underwent testing on the core biopsy specimen because of COVID-19, the investigators noted. Genomic testing on a core biopsy specimen helps determine whether it’s feasible to forgo chemotherapy and use NET instead or whether the patient should proceed directly to surgery. The authors noted that almost 11% of patients required a change in the usual surgical approach because of COVID-19. Such changes were made primarily to avoid hospitalizations during the early phase of the pandemic for patients who were to undergo mastectomy or reconstruction.
“Patients who needed standard approaches still got them,” Dr. Wilke emphasized in a statement. For example, women with aggressive triple-negative and HER2+ tumors were treated with neoadjuvant chemotherapy, she added. “However, NET is a very good approach for a moderate subset of patients, and we think we will see it being used more often in the U.S. now,” Dr. Wilke observed.
“But especially early during the pandemic, these revised treatments were necessary because access to hospital ORs was limited or unavailable, so our algorithmic-based treatment guidelines allowed us to offer high-quality, evidence-based care fine-tuned for a patient’s specific cancer profile,” she affirmed.
Dr. Wilke has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The use of neoadjuvant endocrine therapy (NET) increased significantly during the first 8 months of the COVID-19 pandemic for women with estrogen receptor–positive (ER+) breast cancer. These patients would normally undergo surgery first, but because of operating room restrictions, those surgeries were delayed because of the pandemic, according to a new study.
“We hypothesized that by offering a nontoxic therapy, we would be able to ‘hold over’ patients until such time when personal protective equipment supplies were renewed and we could get into the operating room,” lead author Lee Wilke, MD, professor of surgery, University of Wisconsin, Madison, said in an interview.
“And while a small number of women with ER+ tumors get NET anyway, we found over one-third of patients with ER+ breast cancer were treated with NET due to COVID-19 during the first 8 months of last year,” she said.
“One year later, 31% of the same patient population is still getting NET,” she added.
The study was presented during the online annual meeting of the American Society of Breast Surgeons (ASBrS).
COVID-specific registry
Dr. Wilke believes that this study presents an accurate snapshot of changes in treatment caused by the pandemic.
She and her colleagues compared data collected in the ASBrS Mastery Program registry to data collected in an embedded but separate COVID-19 segment. The data were for the period from March 1 to Oct. 28, 2020.
Almost three-quarters of the surgeons who entered patients into the COVID-19 segment were from urban areas; 95% reported stopping mammographic screening during part of this period.
The preliminary analysis focused on data collected from 2,476 patients in the COVID-19 segment and 2,303 patients within the Mastery registry.
For patients with ER+/HER2- breast cancer, NET was described as a usual approach in 6.5% of patients in the COVID-19 registry. In the Mastery registry, 7.8% of patients received NET.
Compared with surgery first/usual practice, which served as the reference, older patients were more likely to receive NET first because of the COVID-19 pandemic than younger patients, and they were more likely to receive NET first if they lived in the Northeast or the Southeast compared to other regions of the United States. Dr. Wilke pointed out that the Northeast and the Southeast were hardest hit by COVID-19 early on in the pandemic.
Genomic testing was carried out in a small subgroup of patients; 24% of those patients underwent testing on the core biopsy specimen because of COVID-19, the investigators noted. Genomic testing on a core biopsy specimen helps determine whether it’s feasible to forgo chemotherapy and use NET instead or whether the patient should proceed directly to surgery. The authors noted that almost 11% of patients required a change in the usual surgical approach because of COVID-19. Such changes were made primarily to avoid hospitalizations during the early phase of the pandemic for patients who were to undergo mastectomy or reconstruction.
“Patients who needed standard approaches still got them,” Dr. Wilke emphasized in a statement. For example, women with aggressive triple-negative and HER2+ tumors were treated with neoadjuvant chemotherapy, she added. “However, NET is a very good approach for a moderate subset of patients, and we think we will see it being used more often in the U.S. now,” Dr. Wilke observed.
“But especially early during the pandemic, these revised treatments were necessary because access to hospital ORs was limited or unavailable, so our algorithmic-based treatment guidelines allowed us to offer high-quality, evidence-based care fine-tuned for a patient’s specific cancer profile,” she affirmed.
Dr. Wilke has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The use of neoadjuvant endocrine therapy (NET) increased significantly during the first 8 months of the COVID-19 pandemic for women with estrogen receptor–positive (ER+) breast cancer. These patients would normally undergo surgery first, but because of operating room restrictions, those surgeries were delayed because of the pandemic, according to a new study.
“We hypothesized that by offering a nontoxic therapy, we would be able to ‘hold over’ patients until such time when personal protective equipment supplies were renewed and we could get into the operating room,” lead author Lee Wilke, MD, professor of surgery, University of Wisconsin, Madison, said in an interview.
“And while a small number of women with ER+ tumors get NET anyway, we found over one-third of patients with ER+ breast cancer were treated with NET due to COVID-19 during the first 8 months of last year,” she said.
“One year later, 31% of the same patient population is still getting NET,” she added.
The study was presented during the online annual meeting of the American Society of Breast Surgeons (ASBrS).
COVID-specific registry
Dr. Wilke believes that this study presents an accurate snapshot of changes in treatment caused by the pandemic.
She and her colleagues compared data collected in the ASBrS Mastery Program registry to data collected in an embedded but separate COVID-19 segment. The data were for the period from March 1 to Oct. 28, 2020.
Almost three-quarters of the surgeons who entered patients into the COVID-19 segment were from urban areas; 95% reported stopping mammographic screening during part of this period.
The preliminary analysis focused on data collected from 2,476 patients in the COVID-19 segment and 2,303 patients within the Mastery registry.
For patients with ER+/HER2- breast cancer, NET was described as a usual approach in 6.5% of patients in the COVID-19 registry. In the Mastery registry, 7.8% of patients received NET.
Compared with surgery first/usual practice, which served as the reference, older patients were more likely to receive NET first because of the COVID-19 pandemic than younger patients, and they were more likely to receive NET first if they lived in the Northeast or the Southeast compared to other regions of the United States. Dr. Wilke pointed out that the Northeast and the Southeast were hardest hit by COVID-19 early on in the pandemic.
Genomic testing was carried out in a small subgroup of patients; 24% of those patients underwent testing on the core biopsy specimen because of COVID-19, the investigators noted. Genomic testing on a core biopsy specimen helps determine whether it’s feasible to forgo chemotherapy and use NET instead or whether the patient should proceed directly to surgery. The authors noted that almost 11% of patients required a change in the usual surgical approach because of COVID-19. Such changes were made primarily to avoid hospitalizations during the early phase of the pandemic for patients who were to undergo mastectomy or reconstruction.
“Patients who needed standard approaches still got them,” Dr. Wilke emphasized in a statement. For example, women with aggressive triple-negative and HER2+ tumors were treated with neoadjuvant chemotherapy, she added. “However, NET is a very good approach for a moderate subset of patients, and we think we will see it being used more often in the U.S. now,” Dr. Wilke observed.
“But especially early during the pandemic, these revised treatments were necessary because access to hospital ORs was limited or unavailable, so our algorithmic-based treatment guidelines allowed us to offer high-quality, evidence-based care fine-tuned for a patient’s specific cancer profile,” she affirmed.
Dr. Wilke has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.