User login
How to minimize the pain of local anesthetic administration
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
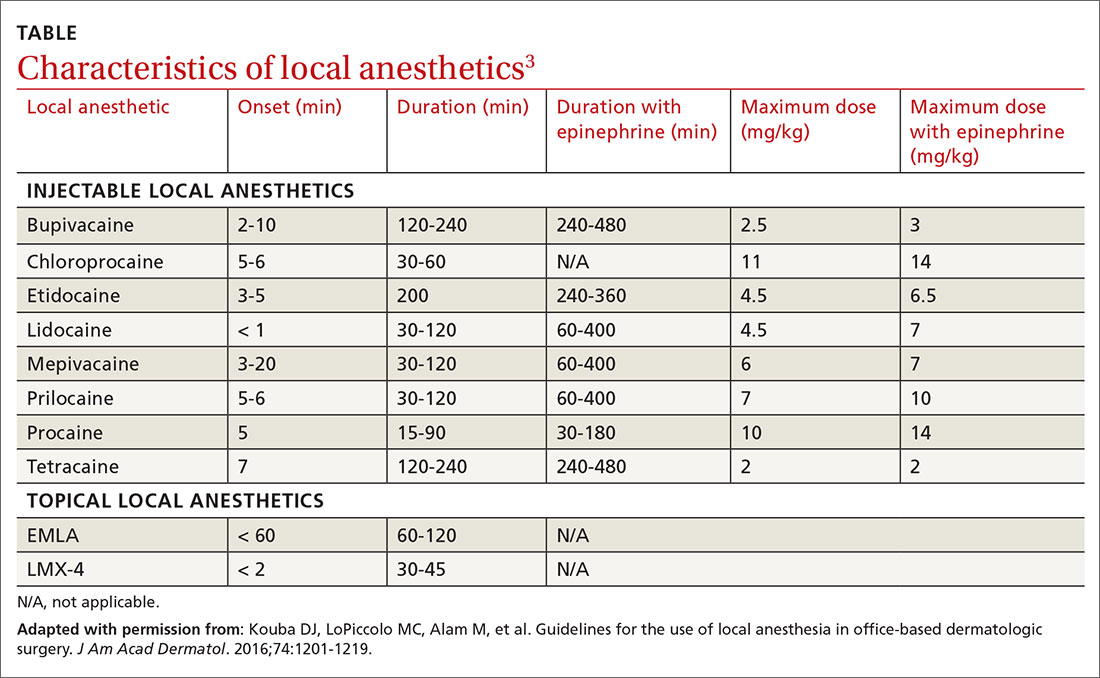
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.
Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
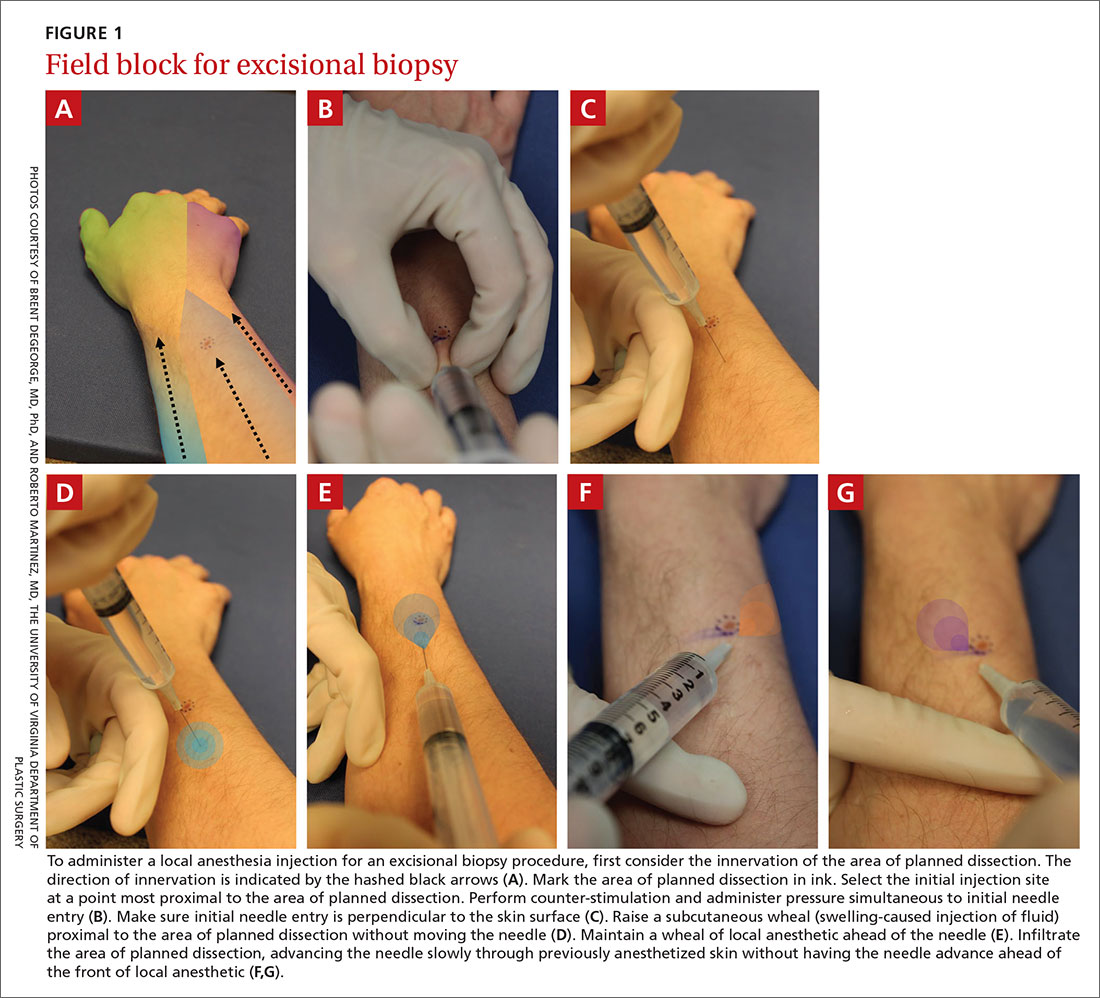
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42
Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
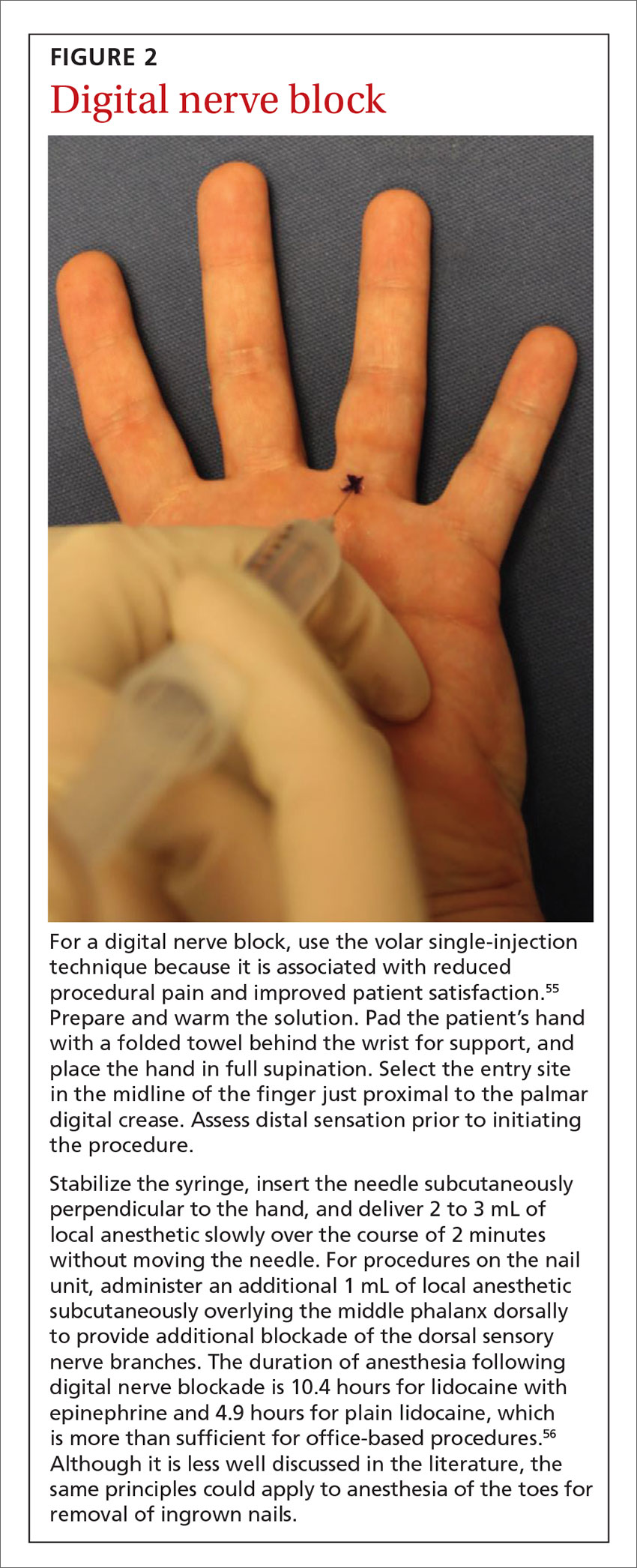
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; [email protected].
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.
Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42
Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; [email protected].
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.
Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42
Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; [email protected].
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
PRACTICE RECOMMENDATIONS
› Add epinephrine and sodium bicarbonate buffer to local anesthetic solution to reduce pain and procedural blood loss. A
› Use such techniques as counter-stimulation, a perpendicular angle of injection, a subcutaneous depth of injection, and a slow rate of injection to minimize patient discomfort. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
More on How to Decrease Dermatology Interview Costs
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP ([email protected]).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP ([email protected]).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP ([email protected]).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
COVID-19: A psychiatry resident’s perspective
During these unprecedented times, venturing into the unknown of the coronavirus disease 2019 (COVID-19) pandemic, a feeling of impending doom prevails. Almost all of us have been restricted to our homes. Although the physical dimensions of what we call home may vary, the meaning of this restriction is fairly universal. No matter how our sociodemographics differ, with no guidance for this situation from anything even remotely comparable in the past, our lives have been transformed into a work in progress.
During this pandemic, I have observed a wide range of human emotions and behavior—many of them familiar and predictable, some abysmal, and some inspiring.
’Why should I care?’
On December 31, 2019, health officials in China informed the World Health Organization about a pneumonia-like presentation in a group of people in Wuhan. On January 7, 2020, a novel coronavirus was identified as the cause, and the first death was reported a few days later. In the following days and weeks the disease rapidly spread, as did the growing sense that this was not a typical virus.
While these events occurred, the rest of the world was in what I call a ”Why should I care?” mode. Most humans tend to suffer from this indifference. This has been observed repeatedly through the years, such as when the Ebola outbreak occurred in Africa in 2014-2016. It was only when cases started to develop in Europe and the United States that other countries started to pay attention. A similar phenomenon has been observed every time we’ve faced a global outbreak (avian influenza, Middle East respiratory syndrome, etc.).
When are we going to learn? It is time to realize that global borders are more porous than we think, and human interactions cannot be blocked by any wall. When a catastrophic event, outbreak, or disaster starts in any part of the world, it is naive to assume that we will not be affected. We will eventually be affected—the only question is how, when, and to what extent? We are always all in this together.
An abundance of ignorance and stupidity
Within a few weeks of the first reports from China, cases of COVID-19 were reported in South Korea, Italy, Spain, Germany, and many other countries. Slowly, COVID-19 reached the United States, which as of mid-April had the highest number of cases worldwide. When COVID-19 hit the United States, the response was that of shock and anger. How could this happen to us? Why is the government not doing anything?
Amidst this pandemonium, ignorance and stupidity of the highest degree were commonplace. This was not restricted to any particular country or region. Almost 2 months into the pandemic, the Ministry of Tourism in my home country of Nepal declared Nepal a ”coronavirus-free zone” and took measures to bring in tourists, focusing specifically on China, where COVID-19 had already killed hundreds. In India, some people were drinking cow urine in hopes of warding off the virus. In the United Sates, thousands of young people flocked to beaches for Spring Break, disregarding measures for social distancing. ”If I get corona, I get corona,” one young man said in an interview that went viral. Personally, I have encountered people who responded to this pandemic by saying the disease was ”cooties” or ”just a flu,” and dismissing it with ”If I die from this, I die.”
Continue to: Rising panic and fear
Rising panic and fear
For most people, seeing COVID-19 at their doorstep triggered a panic, and sent many into a frenzy of buying and hoarding. Once again, we proved that people everywhere are equally stupid, as toilet paper began to vanish from stores across the globe. And yet, this again was a moment when some people began to experience a false sense of immunity: ”I have enough food, money, and toilet paper to last me for 2 years. Why should I be worried?”
When the numbers of COVID-19 deaths in Europe were first reported, the fear became palpable. In Italy and Spain, towns were locked down, and tens of thousands of people (mostly older adults) have died. It was truly heartbreaking to see people alone and at their weakest with no family members allowed to be by their side.
A glimmer of hope
Despite all of this, there were superheroes—the nurses, physicians, allied health professionals, first responders, store workers, restaurant workers, delivery personnel, and others who didn’t have the option of staying home, or who volunteered to help people in need. In moments like this, the actions of these individuals give us hope, reminding us that the human spirit is resilient, and that we will get through this.
A rotation in the emergency department during COVID-19
As a psychiatry resident, it is unlikely that my peers and I face the same risks as our colleagues in other medical specialities. But those of us who happened to be in medical rotations during this time have had the chance to experience this very closely. My personal experience, albeit a brief one, of working in an emergency department with suspected COVID-19 patients has been sobering. Watching nurses and physicians walk into a room wearing personal protective equipment, fearful inside but with a reassuring smile for a scared patient, definitely was one of the most compelling moments of my life. Living in a distant land, with my daughter, wife, parents, and extended family back home in Nepal, has made this even more challenging.
We will overcome this as we have overcome previous challenges in the past. There will be death and chaos, but we will prevail. The only thing is to ask ourselves: How do we want to continue living when this is over?
During these unprecedented times, venturing into the unknown of the coronavirus disease 2019 (COVID-19) pandemic, a feeling of impending doom prevails. Almost all of us have been restricted to our homes. Although the physical dimensions of what we call home may vary, the meaning of this restriction is fairly universal. No matter how our sociodemographics differ, with no guidance for this situation from anything even remotely comparable in the past, our lives have been transformed into a work in progress.
During this pandemic, I have observed a wide range of human emotions and behavior—many of them familiar and predictable, some abysmal, and some inspiring.
’Why should I care?’
On December 31, 2019, health officials in China informed the World Health Organization about a pneumonia-like presentation in a group of people in Wuhan. On January 7, 2020, a novel coronavirus was identified as the cause, and the first death was reported a few days later. In the following days and weeks the disease rapidly spread, as did the growing sense that this was not a typical virus.
While these events occurred, the rest of the world was in what I call a ”Why should I care?” mode. Most humans tend to suffer from this indifference. This has been observed repeatedly through the years, such as when the Ebola outbreak occurred in Africa in 2014-2016. It was only when cases started to develop in Europe and the United States that other countries started to pay attention. A similar phenomenon has been observed every time we’ve faced a global outbreak (avian influenza, Middle East respiratory syndrome, etc.).
When are we going to learn? It is time to realize that global borders are more porous than we think, and human interactions cannot be blocked by any wall. When a catastrophic event, outbreak, or disaster starts in any part of the world, it is naive to assume that we will not be affected. We will eventually be affected—the only question is how, when, and to what extent? We are always all in this together.
An abundance of ignorance and stupidity
Within a few weeks of the first reports from China, cases of COVID-19 were reported in South Korea, Italy, Spain, Germany, and many other countries. Slowly, COVID-19 reached the United States, which as of mid-April had the highest number of cases worldwide. When COVID-19 hit the United States, the response was that of shock and anger. How could this happen to us? Why is the government not doing anything?
Amidst this pandemonium, ignorance and stupidity of the highest degree were commonplace. This was not restricted to any particular country or region. Almost 2 months into the pandemic, the Ministry of Tourism in my home country of Nepal declared Nepal a ”coronavirus-free zone” and took measures to bring in tourists, focusing specifically on China, where COVID-19 had already killed hundreds. In India, some people were drinking cow urine in hopes of warding off the virus. In the United Sates, thousands of young people flocked to beaches for Spring Break, disregarding measures for social distancing. ”If I get corona, I get corona,” one young man said in an interview that went viral. Personally, I have encountered people who responded to this pandemic by saying the disease was ”cooties” or ”just a flu,” and dismissing it with ”If I die from this, I die.”
Continue to: Rising panic and fear
Rising panic and fear
For most people, seeing COVID-19 at their doorstep triggered a panic, and sent many into a frenzy of buying and hoarding. Once again, we proved that people everywhere are equally stupid, as toilet paper began to vanish from stores across the globe. And yet, this again was a moment when some people began to experience a false sense of immunity: ”I have enough food, money, and toilet paper to last me for 2 years. Why should I be worried?”
When the numbers of COVID-19 deaths in Europe were first reported, the fear became palpable. In Italy and Spain, towns were locked down, and tens of thousands of people (mostly older adults) have died. It was truly heartbreaking to see people alone and at their weakest with no family members allowed to be by their side.
A glimmer of hope
Despite all of this, there were superheroes—the nurses, physicians, allied health professionals, first responders, store workers, restaurant workers, delivery personnel, and others who didn’t have the option of staying home, or who volunteered to help people in need. In moments like this, the actions of these individuals give us hope, reminding us that the human spirit is resilient, and that we will get through this.
A rotation in the emergency department during COVID-19
As a psychiatry resident, it is unlikely that my peers and I face the same risks as our colleagues in other medical specialities. But those of us who happened to be in medical rotations during this time have had the chance to experience this very closely. My personal experience, albeit a brief one, of working in an emergency department with suspected COVID-19 patients has been sobering. Watching nurses and physicians walk into a room wearing personal protective equipment, fearful inside but with a reassuring smile for a scared patient, definitely was one of the most compelling moments of my life. Living in a distant land, with my daughter, wife, parents, and extended family back home in Nepal, has made this even more challenging.
We will overcome this as we have overcome previous challenges in the past. There will be death and chaos, but we will prevail. The only thing is to ask ourselves: How do we want to continue living when this is over?
During these unprecedented times, venturing into the unknown of the coronavirus disease 2019 (COVID-19) pandemic, a feeling of impending doom prevails. Almost all of us have been restricted to our homes. Although the physical dimensions of what we call home may vary, the meaning of this restriction is fairly universal. No matter how our sociodemographics differ, with no guidance for this situation from anything even remotely comparable in the past, our lives have been transformed into a work in progress.
During this pandemic, I have observed a wide range of human emotions and behavior—many of them familiar and predictable, some abysmal, and some inspiring.
’Why should I care?’
On December 31, 2019, health officials in China informed the World Health Organization about a pneumonia-like presentation in a group of people in Wuhan. On January 7, 2020, a novel coronavirus was identified as the cause, and the first death was reported a few days later. In the following days and weeks the disease rapidly spread, as did the growing sense that this was not a typical virus.
While these events occurred, the rest of the world was in what I call a ”Why should I care?” mode. Most humans tend to suffer from this indifference. This has been observed repeatedly through the years, such as when the Ebola outbreak occurred in Africa in 2014-2016. It was only when cases started to develop in Europe and the United States that other countries started to pay attention. A similar phenomenon has been observed every time we’ve faced a global outbreak (avian influenza, Middle East respiratory syndrome, etc.).
When are we going to learn? It is time to realize that global borders are more porous than we think, and human interactions cannot be blocked by any wall. When a catastrophic event, outbreak, or disaster starts in any part of the world, it is naive to assume that we will not be affected. We will eventually be affected—the only question is how, when, and to what extent? We are always all in this together.
An abundance of ignorance and stupidity
Within a few weeks of the first reports from China, cases of COVID-19 were reported in South Korea, Italy, Spain, Germany, and many other countries. Slowly, COVID-19 reached the United States, which as of mid-April had the highest number of cases worldwide. When COVID-19 hit the United States, the response was that of shock and anger. How could this happen to us? Why is the government not doing anything?
Amidst this pandemonium, ignorance and stupidity of the highest degree were commonplace. This was not restricted to any particular country or region. Almost 2 months into the pandemic, the Ministry of Tourism in my home country of Nepal declared Nepal a ”coronavirus-free zone” and took measures to bring in tourists, focusing specifically on China, where COVID-19 had already killed hundreds. In India, some people were drinking cow urine in hopes of warding off the virus. In the United Sates, thousands of young people flocked to beaches for Spring Break, disregarding measures for social distancing. ”If I get corona, I get corona,” one young man said in an interview that went viral. Personally, I have encountered people who responded to this pandemic by saying the disease was ”cooties” or ”just a flu,” and dismissing it with ”If I die from this, I die.”
Continue to: Rising panic and fear
Rising panic and fear
For most people, seeing COVID-19 at their doorstep triggered a panic, and sent many into a frenzy of buying and hoarding. Once again, we proved that people everywhere are equally stupid, as toilet paper began to vanish from stores across the globe. And yet, this again was a moment when some people began to experience a false sense of immunity: ”I have enough food, money, and toilet paper to last me for 2 years. Why should I be worried?”
When the numbers of COVID-19 deaths in Europe were first reported, the fear became palpable. In Italy and Spain, towns were locked down, and tens of thousands of people (mostly older adults) have died. It was truly heartbreaking to see people alone and at their weakest with no family members allowed to be by their side.
A glimmer of hope
Despite all of this, there were superheroes—the nurses, physicians, allied health professionals, first responders, store workers, restaurant workers, delivery personnel, and others who didn’t have the option of staying home, or who volunteered to help people in need. In moments like this, the actions of these individuals give us hope, reminding us that the human spirit is resilient, and that we will get through this.
A rotation in the emergency department during COVID-19
As a psychiatry resident, it is unlikely that my peers and I face the same risks as our colleagues in other medical specialities. But those of us who happened to be in medical rotations during this time have had the chance to experience this very closely. My personal experience, albeit a brief one, of working in an emergency department with suspected COVID-19 patients has been sobering. Watching nurses and physicians walk into a room wearing personal protective equipment, fearful inside but with a reassuring smile for a scared patient, definitely was one of the most compelling moments of my life. Living in a distant land, with my daughter, wife, parents, and extended family back home in Nepal, has made this even more challenging.
We will overcome this as we have overcome previous challenges in the past. There will be death and chaos, but we will prevail. The only thing is to ask ourselves: How do we want to continue living when this is over?
Love in the time of coronavirus
Several months ago, I sat with a woman just a few days after the emergent Cesarean section delivery of her first child. She cried as she told me about her entire life—childhood trauma, a pattern of difficult relationships, several miscarriages, and now, finally, a baby—delivered under circumstances so scary, all she remembered was overwhelming fear. Now, she had returned to the hospital with severe postpartum depression, layered with struggles that are common during the first days with a newborn—little sleep, loss of autonomy, guilt, and loneliness. It was hard to listen to it all, but I encouraged her to express her pain, believing that burdens are lighter when shared.
Words often fail us in times of desperation. Much of my education has involved borrowing words, phrases, or ideas from my experienced attendings and mentors, applying them like a salve when I don’t know what else to say. Sitting with another person in silence is often powerful enough, but when something needs to be said, I fall back on these inherited ideas. One of the mantras I often use, and what I said to my patient that day, is about hope: “When you’re down in this depression, you feel hopeless, and you can’t see the hope. It doesn’t mean there isn’t hope; just that you can’t see it.” I’ve watched that idea take root in patients who—despite their own beliefs in the moment—do get better, thus proving the point. Another favorite phrase: “With any luck at all, tomorrow will be better than today.” When you talk to someone on the worst day of their life, what else is there to say?
Today, my conversation with that woman seems like an eternity ago. Public discourse has been overtaken by coronavirus disease 2019 (COVID-19)—the journalism, reflections on the journalism, medical advice, debate about the medical advice, and the innumerable ways in which this worldwide strife has created pain: celebrations and long-awaited plans cancelled, weddings and funerals put on hold, isolation, loneliness, death, and, of course, the fear of death. Those feelings and any other permutations are valid; another phrase, “It’s OK to feel what you are feeling,” carries weight for me these days. I work in a hospital, so I add to the list the breathless fears about what’s going to happen in our local environment. The chronic uncertainty was wearing us thin even before we had begun to do here in Ohio what was already being done elsewhere: working extra shifts, intubating new patients, praying we don’t get sick ourselves.
Our work during COVID-19
Amidst this, my colleagues and I continue our work as psychiatrists, sitting with humans experiencing complex grief (a man whose wife died alone in a nursing home, because of visitor restrictions), confusion (delirium resulting from respiratory failure), and even psychosis (inability to access stabilizing medications coupled with crippling paranoia). These remain just as real and debilitating in a pandemic as they do in other times. In addition to pre-existing mental illnesses, for some individuals, the shared anxiety will progress to clinically significant disorders that may last even longer than the effects of the virus. The resulting complex symptoms could affect everything from home lives to interpersonal relationships to our local and global economies. These are not minor issues. Although often triaged aside in a disaster, our collective mental health remains in some ways more central than ever.
Modern psychiatry would not often use the word “love,” but that’s what I am trying to do—show love to the people who need it the most right now (which is all of us, really). This love takes strange shapes, and sometimes new forms, but it’s just about all I have to give. Like everyone else, I don’t have concrete answers for the grief and fear and panic. But I’m content to share the burden of pain, believing that burdens are lighter when shared. And I have a few words that, however little comfort they offer in the moment, are eventually proven true: Just because you can’t see the hope doesn’t mean it isn’t there. It’s OK to feel what you are feeling. With any luck at all, tomorrow will be better than today.
Several months ago, I sat with a woman just a few days after the emergent Cesarean section delivery of her first child. She cried as she told me about her entire life—childhood trauma, a pattern of difficult relationships, several miscarriages, and now, finally, a baby—delivered under circumstances so scary, all she remembered was overwhelming fear. Now, she had returned to the hospital with severe postpartum depression, layered with struggles that are common during the first days with a newborn—little sleep, loss of autonomy, guilt, and loneliness. It was hard to listen to it all, but I encouraged her to express her pain, believing that burdens are lighter when shared.
Words often fail us in times of desperation. Much of my education has involved borrowing words, phrases, or ideas from my experienced attendings and mentors, applying them like a salve when I don’t know what else to say. Sitting with another person in silence is often powerful enough, but when something needs to be said, I fall back on these inherited ideas. One of the mantras I often use, and what I said to my patient that day, is about hope: “When you’re down in this depression, you feel hopeless, and you can’t see the hope. It doesn’t mean there isn’t hope; just that you can’t see it.” I’ve watched that idea take root in patients who—despite their own beliefs in the moment—do get better, thus proving the point. Another favorite phrase: “With any luck at all, tomorrow will be better than today.” When you talk to someone on the worst day of their life, what else is there to say?
Today, my conversation with that woman seems like an eternity ago. Public discourse has been overtaken by coronavirus disease 2019 (COVID-19)—the journalism, reflections on the journalism, medical advice, debate about the medical advice, and the innumerable ways in which this worldwide strife has created pain: celebrations and long-awaited plans cancelled, weddings and funerals put on hold, isolation, loneliness, death, and, of course, the fear of death. Those feelings and any other permutations are valid; another phrase, “It’s OK to feel what you are feeling,” carries weight for me these days. I work in a hospital, so I add to the list the breathless fears about what’s going to happen in our local environment. The chronic uncertainty was wearing us thin even before we had begun to do here in Ohio what was already being done elsewhere: working extra shifts, intubating new patients, praying we don’t get sick ourselves.
Our work during COVID-19
Amidst this, my colleagues and I continue our work as psychiatrists, sitting with humans experiencing complex grief (a man whose wife died alone in a nursing home, because of visitor restrictions), confusion (delirium resulting from respiratory failure), and even psychosis (inability to access stabilizing medications coupled with crippling paranoia). These remain just as real and debilitating in a pandemic as they do in other times. In addition to pre-existing mental illnesses, for some individuals, the shared anxiety will progress to clinically significant disorders that may last even longer than the effects of the virus. The resulting complex symptoms could affect everything from home lives to interpersonal relationships to our local and global economies. These are not minor issues. Although often triaged aside in a disaster, our collective mental health remains in some ways more central than ever.
Modern psychiatry would not often use the word “love,” but that’s what I am trying to do—show love to the people who need it the most right now (which is all of us, really). This love takes strange shapes, and sometimes new forms, but it’s just about all I have to give. Like everyone else, I don’t have concrete answers for the grief and fear and panic. But I’m content to share the burden of pain, believing that burdens are lighter when shared. And I have a few words that, however little comfort they offer in the moment, are eventually proven true: Just because you can’t see the hope doesn’t mean it isn’t there. It’s OK to feel what you are feeling. With any luck at all, tomorrow will be better than today.
Several months ago, I sat with a woman just a few days after the emergent Cesarean section delivery of her first child. She cried as she told me about her entire life—childhood trauma, a pattern of difficult relationships, several miscarriages, and now, finally, a baby—delivered under circumstances so scary, all she remembered was overwhelming fear. Now, she had returned to the hospital with severe postpartum depression, layered with struggles that are common during the first days with a newborn—little sleep, loss of autonomy, guilt, and loneliness. It was hard to listen to it all, but I encouraged her to express her pain, believing that burdens are lighter when shared.
Words often fail us in times of desperation. Much of my education has involved borrowing words, phrases, or ideas from my experienced attendings and mentors, applying them like a salve when I don’t know what else to say. Sitting with another person in silence is often powerful enough, but when something needs to be said, I fall back on these inherited ideas. One of the mantras I often use, and what I said to my patient that day, is about hope: “When you’re down in this depression, you feel hopeless, and you can’t see the hope. It doesn’t mean there isn’t hope; just that you can’t see it.” I’ve watched that idea take root in patients who—despite their own beliefs in the moment—do get better, thus proving the point. Another favorite phrase: “With any luck at all, tomorrow will be better than today.” When you talk to someone on the worst day of their life, what else is there to say?
Today, my conversation with that woman seems like an eternity ago. Public discourse has been overtaken by coronavirus disease 2019 (COVID-19)—the journalism, reflections on the journalism, medical advice, debate about the medical advice, and the innumerable ways in which this worldwide strife has created pain: celebrations and long-awaited plans cancelled, weddings and funerals put on hold, isolation, loneliness, death, and, of course, the fear of death. Those feelings and any other permutations are valid; another phrase, “It’s OK to feel what you are feeling,” carries weight for me these days. I work in a hospital, so I add to the list the breathless fears about what’s going to happen in our local environment. The chronic uncertainty was wearing us thin even before we had begun to do here in Ohio what was already being done elsewhere: working extra shifts, intubating new patients, praying we don’t get sick ourselves.
Our work during COVID-19
Amidst this, my colleagues and I continue our work as psychiatrists, sitting with humans experiencing complex grief (a man whose wife died alone in a nursing home, because of visitor restrictions), confusion (delirium resulting from respiratory failure), and even psychosis (inability to access stabilizing medications coupled with crippling paranoia). These remain just as real and debilitating in a pandemic as they do in other times. In addition to pre-existing mental illnesses, for some individuals, the shared anxiety will progress to clinically significant disorders that may last even longer than the effects of the virus. The resulting complex symptoms could affect everything from home lives to interpersonal relationships to our local and global economies. These are not minor issues. Although often triaged aside in a disaster, our collective mental health remains in some ways more central than ever.
Modern psychiatry would not often use the word “love,” but that’s what I am trying to do—show love to the people who need it the most right now (which is all of us, really). This love takes strange shapes, and sometimes new forms, but it’s just about all I have to give. Like everyone else, I don’t have concrete answers for the grief and fear and panic. But I’m content to share the burden of pain, believing that burdens are lighter when shared. And I have a few words that, however little comfort they offer in the moment, are eventually proven true: Just because you can’t see the hope doesn’t mean it isn’t there. It’s OK to feel what you are feeling. With any luck at all, tomorrow will be better than today.
Double Masking and Decontamination: A Doctor's COVID-19 Routine

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about the PPE and decontamination processes she's using. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
PPE Routine at the Hospital
Dr Gary Ferenchick: You've developed your own PPE and decontamination routines. It's about protecting yourself at work but also about protecting your loved ones by not carrying the virus home. Could you walk us through it? I'll show it on the screen.
Dr Hannah Ferenchick: At work I wear scrubs, and I try to minimize any additional clothing. I don't wear a jacket over my scrubs, and I don't wear any T-shirts under my scrubs. If I'm going to be in a situation that might involve exposure to patient secretions or bodily fluids, then I also wear shoe covers.
Because so many of our patients are infected and we may be called upon at any time to do an aerosol-generating procedure, in the ED we have all taken to wearing N95 masks for our entire shift. I wear a fitted N95 mask. I cover that with a surgical mask.
We are anticipating N95 shortages because our use of the masks has increased exponentially. Every hospital has to think about how to protect their healthcare workers while conserving PPE. We cover the N95 mask with a surgical mask, so that if there is any soiling or droplets reaching the mask, we are able to change the surgical mask and continue to use the same N95.
In addition, eye protection is important. Generally throughout the shift I wear my own goggles. If I'm going to be involved in any procedure with the potential for aerosolization (intubation, performing CPR, bronchoscopy) then I wear a creation of my own, which is a welder's shield.
Many of our providers have chosen to use their own equipment, although we are still able to use hospital-provided equipment. There is probably no difference in effectiveness between these devices.
Cell Phones and Stethoscopes
I carry a personal cell phone at work (which I often use to look things up, use the calculator, and for other purposes), and I'm cognizant that when I touch it, I am potentially transmitting pathogens to my phone or its cover. So I've taken to keeping my phone in a plastic sandwich bag, which I disinfect a couple of times throughout the shift. The phone still works normally.
After my shift, in my "decontamination phase," I remove the phone from the plastic bag and disinfect the phone again.
I try to avoid bringing objects into the vicinity of the patient. That's different from my normal routine—I usually like to write down what the patient has told me—but unfortunately, carrying pen and paper or a clipboard into a patient's room is not feasible at this point. During this time, I've also avoided using my personal stethoscope.
There's also transmission risk associated with shared equipment. We share hospital-provided phones and they must be disinfected. We are each disinfecting our own workspaces: computer, keyboard, mouse, and countertop.
Obviously you are trying to minimize any contact with your mouth or face. You don't want to rub your eyes, touch your nose, or eat anything with your hands while you are at work. The assumption is that you are doing very frequent hand hygiene.
Decontamination Routine
One of our concerns as healthcare providers is the possibility that we could, either asymptomatically or through the objects that we use at work, be bringing the disease home. We want to protect the people who may be at higher risk just because they live with a healthcare provider. These are the decontamination practices I've developed for my own situation, taken from best practices and suggestions from others.
I remove my dirty scrubs and leave them at work, and I change into a clean pair of scrubs or clean clothes. I disinfect any inanimate objects that my hands may have touched during the shift using alcohol, sanitizer wipes, bleach wipes, or hospital-grade chemical wipes.
To keep those objects clean after disinfecting, I place them in clean plastic bags away from other objects (eg, a wallet or purse) that may not be easy to disinfect. Then I store those bags in the trunk of my car for my next shift, so I'm not taking them into my home.
I also change my shoes, leaving my work shoes in the trunk of my car, and wear another pair of shoes into the house.
When I get home, I basically do everything again. I disinfect my phone, I wash my hands, and I shower immediately. At that point, I consider myself sufficiently "disinfected."
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about the PPE and decontamination processes she's using. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
PPE Routine at the Hospital
Dr Gary Ferenchick: You've developed your own PPE and decontamination routines. It's about protecting yourself at work but also about protecting your loved ones by not carrying the virus home. Could you walk us through it? I'll show it on the screen.
Dr Hannah Ferenchick: At work I wear scrubs, and I try to minimize any additional clothing. I don't wear a jacket over my scrubs, and I don't wear any T-shirts under my scrubs. If I'm going to be in a situation that might involve exposure to patient secretions or bodily fluids, then I also wear shoe covers.
Because so many of our patients are infected and we may be called upon at any time to do an aerosol-generating procedure, in the ED we have all taken to wearing N95 masks for our entire shift. I wear a fitted N95 mask. I cover that with a surgical mask.
We are anticipating N95 shortages because our use of the masks has increased exponentially. Every hospital has to think about how to protect their healthcare workers while conserving PPE. We cover the N95 mask with a surgical mask, so that if there is any soiling or droplets reaching the mask, we are able to change the surgical mask and continue to use the same N95.
In addition, eye protection is important. Generally throughout the shift I wear my own goggles. If I'm going to be involved in any procedure with the potential for aerosolization (intubation, performing CPR, bronchoscopy) then I wear a creation of my own, which is a welder's shield.
Many of our providers have chosen to use their own equipment, although we are still able to use hospital-provided equipment. There is probably no difference in effectiveness between these devices.
Cell Phones and Stethoscopes
I carry a personal cell phone at work (which I often use to look things up, use the calculator, and for other purposes), and I'm cognizant that when I touch it, I am potentially transmitting pathogens to my phone or its cover. So I've taken to keeping my phone in a plastic sandwich bag, which I disinfect a couple of times throughout the shift. The phone still works normally.
After my shift, in my "decontamination phase," I remove the phone from the plastic bag and disinfect the phone again.
I try to avoid bringing objects into the vicinity of the patient. That's different from my normal routine—I usually like to write down what the patient has told me—but unfortunately, carrying pen and paper or a clipboard into a patient's room is not feasible at this point. During this time, I've also avoided using my personal stethoscope.
There's also transmission risk associated with shared equipment. We share hospital-provided phones and they must be disinfected. We are each disinfecting our own workspaces: computer, keyboard, mouse, and countertop.
Obviously you are trying to minimize any contact with your mouth or face. You don't want to rub your eyes, touch your nose, or eat anything with your hands while you are at work. The assumption is that you are doing very frequent hand hygiene.
Decontamination Routine
One of our concerns as healthcare providers is the possibility that we could, either asymptomatically or through the objects that we use at work, be bringing the disease home. We want to protect the people who may be at higher risk just because they live with a healthcare provider. These are the decontamination practices I've developed for my own situation, taken from best practices and suggestions from others.
I remove my dirty scrubs and leave them at work, and I change into a clean pair of scrubs or clean clothes. I disinfect any inanimate objects that my hands may have touched during the shift using alcohol, sanitizer wipes, bleach wipes, or hospital-grade chemical wipes.
To keep those objects clean after disinfecting, I place them in clean plastic bags away from other objects (eg, a wallet or purse) that may not be easy to disinfect. Then I store those bags in the trunk of my car for my next shift, so I'm not taking them into my home.
I also change my shoes, leaving my work shoes in the trunk of my car, and wear another pair of shoes into the house.
When I get home, I basically do everything again. I disinfect my phone, I wash my hands, and I shower immediately. At that point, I consider myself sufficiently "disinfected."
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about the PPE and decontamination processes she's using. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
PPE Routine at the Hospital
Dr Gary Ferenchick: You've developed your own PPE and decontamination routines. It's about protecting yourself at work but also about protecting your loved ones by not carrying the virus home. Could you walk us through it? I'll show it on the screen.
Dr Hannah Ferenchick: At work I wear scrubs, and I try to minimize any additional clothing. I don't wear a jacket over my scrubs, and I don't wear any T-shirts under my scrubs. If I'm going to be in a situation that might involve exposure to patient secretions or bodily fluids, then I also wear shoe covers.
Because so many of our patients are infected and we may be called upon at any time to do an aerosol-generating procedure, in the ED we have all taken to wearing N95 masks for our entire shift. I wear a fitted N95 mask. I cover that with a surgical mask.
We are anticipating N95 shortages because our use of the masks has increased exponentially. Every hospital has to think about how to protect their healthcare workers while conserving PPE. We cover the N95 mask with a surgical mask, so that if there is any soiling or droplets reaching the mask, we are able to change the surgical mask and continue to use the same N95.
In addition, eye protection is important. Generally throughout the shift I wear my own goggles. If I'm going to be involved in any procedure with the potential for aerosolization (intubation, performing CPR, bronchoscopy) then I wear a creation of my own, which is a welder's shield.
Many of our providers have chosen to use their own equipment, although we are still able to use hospital-provided equipment. There is probably no difference in effectiveness between these devices.
Cell Phones and Stethoscopes
I carry a personal cell phone at work (which I often use to look things up, use the calculator, and for other purposes), and I'm cognizant that when I touch it, I am potentially transmitting pathogens to my phone or its cover. So I've taken to keeping my phone in a plastic sandwich bag, which I disinfect a couple of times throughout the shift. The phone still works normally.
After my shift, in my "decontamination phase," I remove the phone from the plastic bag and disinfect the phone again.
I try to avoid bringing objects into the vicinity of the patient. That's different from my normal routine—I usually like to write down what the patient has told me—but unfortunately, carrying pen and paper or a clipboard into a patient's room is not feasible at this point. During this time, I've also avoided using my personal stethoscope.
There's also transmission risk associated with shared equipment. We share hospital-provided phones and they must be disinfected. We are each disinfecting our own workspaces: computer, keyboard, mouse, and countertop.
Obviously you are trying to minimize any contact with your mouth or face. You don't want to rub your eyes, touch your nose, or eat anything with your hands while you are at work. The assumption is that you are doing very frequent hand hygiene.
Decontamination Routine
One of our concerns as healthcare providers is the possibility that we could, either asymptomatically or through the objects that we use at work, be bringing the disease home. We want to protect the people who may be at higher risk just because they live with a healthcare provider. These are the decontamination practices I've developed for my own situation, taken from best practices and suggestions from others.
I remove my dirty scrubs and leave them at work, and I change into a clean pair of scrubs or clean clothes. I disinfect any inanimate objects that my hands may have touched during the shift using alcohol, sanitizer wipes, bleach wipes, or hospital-grade chemical wipes.
To keep those objects clean after disinfecting, I place them in clean plastic bags away from other objects (eg, a wallet or purse) that may not be easy to disinfect. Then I store those bags in the trunk of my car for my next shift, so I'm not taking them into my home.
I also change my shoes, leaving my work shoes in the trunk of my car, and wear another pair of shoes into the house.
When I get home, I basically do everything again. I disinfect my phone, I wash my hands, and I shower immediately. At that point, I consider myself sufficiently "disinfected."
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.
The DNA Mismatch Repair System in Sebaceous Tumors: An Update on the Genetics and Workup of Muir-Torre Syndrome
It is well known by now that tumor formation is driven by accumulation of numerous genetic and epigenetic mutations. Human cells are equipped with an apparatus called the DNA mismatch repair (MMR) system that corrects errors during replication.1 If these genes are themselves mutated, cells then start accumulating mutations in other genes, including oncogenes and tumor suppressor genes, which results in the development of sustained proliferative signaling pathways, evasion of growth suppression, resistance to cell death, and the potential for invasion and metastasis.2
Gene mutations in DNA MMR have been detected in several tumors, such as sebaceous tumors,3 colorectal adenocarcinomas,4 keratoacanthomas,5 and other visceral malignancies.6 Sebaceous tumors are rare in the general population; however, they are common in patients with inherited or acquired mutations in MMR genes.5 These patients also have been found to have other visceral malignancies such as colorectal adenocarcinomas and breast, lung, and central nervous system (CNS) tumors.7 This observation was made in the 1960s, and patients were referred to as having Muir-Torre syndrome (MTS).8 This article serves to briefly describe the DNA MMR system and its implication in sebaceous tumors as well as discuss the recent recommendations for screening for MTS in patients presenting with sebaceous tumors.
The DNA MMR System
Mismatch repair proteins are responsible for detecting and repairing errors during cell division, especially in microsatellite regions.9 Microsatellites are common and widely distributed DNA motifs consisting of repeated nucleotide sequences that normally account for 3% of the genome.10 Mutations in MMR result in insertion or deletion of nucleotides in these DNA motifs, making them either abnormally long or short, referred to as microsatellite instability (MSI), which results in downstream cumulative accumulation of mutations in oncogenes and tumor suppressor genes, and thus carcinogenesis.9
There are 7 human MMR proteins: MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2. These proteins are highly conserved across different living species.11 Loss of MMR proteins can be due to a mutation in the coding sequence of the gene or due to epigenetic hypermethylation of the gene promoter.12 These alterations can be inherited or acquired and in most cases result in MSI.
When assessing for MSI, tumor genomes can be divided into 3 subtypes: high-level and low-level MSI and stable microsatellites.13 Tumors with high-level MSI respond better to treatment and show a better prognosis than those with low-level MSI or stable microsatellites,14 which is thought to be due to tumor-induced immune activation. Microsatellite instability results in the generation of frameshift peptides that are immunogenic and induce tumor-specific immune responses.15 Several research laboratories have artificially synthesized frameshift peptides as vaccines and have successfully used them as targets for immune therapy as a way for preventing and treating malignancies.16
Sebaceous Tumors in MTS
A typical example of tumors that arise from mutations in the DNA MMR system is seen in MTS,a rare inherited genetic syndrome that predisposes patients to sebaceous neoplasms, keratoacanthomas, and visceral malignancies.17 It was first described as an autosomal-dominant condition in patients who have at least 1 sebaceous tumor and 1 visceral malignancy, with or without keratoacanthomas. It was then later characterized as a skin variant of Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer syndrome.18
Sebaceous tumors are the hallmark of MTS. Although sebaceous hyperplasia is common in the general population, sebaceous tumors are rare outside the context of MTS. There are 3 types of sebaceous tumors with distinct pathologic features: adenoma, epithelioma, and carcinoma.19 Sebaceous adenomas and epitheliomas are benign growths; however, sebaceous carcinomas can be aggressive and have metastatic potential.20 Because it is difficult to clinically distinguish carcinomas from the benign sebaceous growths, biopsy of a large, changing, or ulcerated lesion is important in these patients to rule out a sebaceous carcinoma. Other aggressive skin tumors can develop in MTS, such as rapidly growing keratoacanthomas and basal cell carcinomas with sebaceous differentiation.21
Types of MTS
For most cases, MTS is characterized by germline mutations in DNA MMR genes. The most common mutation involves MSH2 (MutS Homolog 2)—found in approximately 90% of patients—followed by MLH1 (MutL Homolog 1)—found in approximately 10% of patients.22 Other MMR genes such as MSH6 (MutS Homolog 6), PMS2 (PMS1 homolog 2, mismatch repair system component), and MLH3 (MutL Homolog 3) less commonly are reported in MTS. There is a subset of patients who lose MSH2 or MLH1 expression due to promoter hypermethylation rather than a germline mutation. Methylation results in biallelic inactivation of the gene and loss of expression.23
A new subtype of MTS has been identified that demonstrates an autosomal-recessive pattern of inheritance and is referred to as MTS type 2 (autosomal-recessive colorectal adenomatous polyposis).24 In contrast to the classic MTS type 1, MTS type 2 exhibits microsatellite stability. Recent molecular analyses revealed that type 2 is due to a mutation in a base excision repair gene called MUTYH (mutY DNA glycosylase).25 These patients are likely to develop hundreds of polyps at an early age.
Muir-Torre syndrome also can occur sporadically without inheriting a germline mutation, which has been reported in a transplant patient from de novo somatic mutations or promoter hypermethylation.26 A case report of a renal transplant patient showed that switching from tacrolimus to sirolimus halted the appearance of new sebaceous neoplasms, which suggests that patients with MTS who undergo organ transplantation should potentially avoid tacrolimus and be put on sirolimus instead.27
Visceral Malignancies in MTS
Apart from frequent skin examinations, MTS patients should have frequent and rigorous visceral malignancy screening. Patients most commonly develop colorectal adenocarcinoma, especially in the proximal parts of the colon.28 In addition, they can develop numerous premalignant tumors, especially in MTS type 2. Other common tumors include endometrial, ovarian, genitourinary, hepatobiliary, breast, lung, hematopoietic, and CNS malignancies.29
Studies showed that specific loss of certain MMR proteins predispose patients to different types of visceral malignancies.30-32 For example, loss of MSH2 predisposes patients to development of extracolonic tumors, while loss of MLH1 more strongly is associated with development of colorectal adenocarcinoma.30 Patients with MSH2 also are at risk for development of CNS tumors, while patients with MLH1 mutations have never been reported to develop CNS tumors.31 Patients with loss of PMS2 have the lowest risk for development of any visceral malignancy.32
Diagnosing MTS
Let us consider a scenario whereby a dermatologist biopsied a solitary lesion and it came back as a sebaceous tumor. What would be the next step to establish a diagnosis of MTS?
Sebaceous tumors are rare outside the context of MTS. Therefore, patients presenting with a solitary sebaceous tumor should be worked up for MTS, as there are implications for further cancer screening. One helpful clue that can affect the pretest probability for MTS diagnosis is location of the tumor. A sebaceous tumor inferior to the neck most likely is associated with MTS. On the other hand, tumors on the head and neck can be spontaneous or associated with MTS.33 Another helpful tool is the Mayo score, a risk score for MTS in patients with sebaceous tumors.34 The score is established by adding up points, with 1 point given to each of the following: age of onset of a sebaceous tumor less than 60 years, personal history of visceral malignancy, and family history of Lynch syndrome–related visceral malignancy. Two points are given if the patient has 2 or more sebaceous tumors. The score ranges from 0 to 5. A risk score of 2 or more has a sensitivity of 100% and specificity of 81% for predicting a germline mutation in MMR genes.34
Testing for loss of MMR proteins is performed using immunohistochemistry (IHC) as well as microsatellite gene analysis on the biopsied tumor. There is no need to perform another biopsy, as these tests can be performed on the paraffin-embedded formalin fixed tissue. Immunohistochemistry testing looks for loss of expression of one of the MMR proteins. Staining usually is performed for MSH2, MSH6, and MLH1, as the combination offers a sensitivity of 81% and a positive predictive value of 100%.23,35,36
If IHC shows loss of MMR proteins, then MSI gene analysis should be performed as a confirmatory test by using MSI gene locus assays, which utilize 5 markers of mononucleotide and dinucleotide repeats. If the genome is positive for 2 of 5 of these markers, then the patient most likely has MTS.13
One caveat for IHC analysis is that there is a subset of patients who develop a solitary sebaceous tumor due to a sporadic loss of MMR protein without having MTS. These tumors also exhibit BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations or loss of p16, features that distinguish these tumors from those developed in MTS.37 As such, in a patient with a low Mayo score who developed a solitary sebaceous tumor that showed loss of MMR protein on IHC without evidence of MSI, it is reasonable to perform IHC for BRAF and p16 to avoid inaccurate diagnosis of MTS.
Another caveat is that standard MSI analysis will not detect MSI in tumors with loss of MSH6 because the markers used in the MSI analysis do not detect MSI caused by MSH6 loss. For these patients, MSI analysis using a panel composed of mononucleotides alone (pentaplex assay) should be performed in lieu of the standard panel.38
It is important to note that these molecular tests are not helpful for patients with MTS type 2, as the sebaceous tumors maintain MMR proteins and have microsatellite stability. As such, if MTS is highly suspected based on the Mayo score (either personal history of malignancy or strong family history) but the IHC and MSI analysis are negative, then referral to a geneticist for identification for MUTYH gene mutation is a reasonable next step. These patients with high Mayo scores should still be managed as MTS patients and should be screened for visceral malignancies despite lack of confirmatory tests.
Final Thoughts
Dermatologists should be highly suspicious of MTS when they diagnose sebaceous tumors. Making a diagnosis of MTS notably affects patients’ primary care. Patients with MTS should have annual skin examinations, neurologic examinations, colonoscopies starting at the age of 18 years, and surveillance for breast and pelvic cancers in women (by annual transvaginal ultrasound and endometrial aspirations) or for prostate and testicular cancers in men.17,39,40 Other tests to be ordered annually include complete blood cell count with differential and urinalysis.19
- Yamamoto H, Imai K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin Oncol. 2019;46:261-270.
- Tamura K, Kaneda M, Futagawa M, et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol. 2019;24:999-1011.
- Shiki M, Hida T, Sugano K, et al. Muir-Torre syndrome caused by exonic deletion of MLH1 due to homologous recombination. Eur J Dermatol. 2017;27:54-58.
- Büttner R, Friedrichs N. Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany. Pathologe. 2019;40:584-591.
- Kuwabara K, Suzuki O, Chika N, et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn J Clin Oncol. 2018;48:514-521.
- Burris CKH, Rodriguez ME, Raven ML, et al. Muir-torre syndrome: the importance of a detailed family history. Case Rep Ophthalmol. 2019;10:180-185.
- Walsh MD, Jayasekara H, Huang A, et al. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: a large case series from a single Australian private pathology service. Australas J Dermatol. 2019;60:126-133.
- Georgeson P, Walsh MD, Clendenning M, et al. Tumor mutational signatures in sebaceous skin lesions from individuals with Lynch syndrome. Mol Genet Genomic Med. 2019;7:E00781.
- Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391-407.
- Li YC, Korol AB, Fahima T, et al. Microsatellites within genes: structure, function, and evolution [published online February 12, 2004]. Mol Biol Evol. 2004;21:991-1007.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435-445.
- Everett JN, Raymond VM, Dandapani M, et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014;150:1315-1321.
- Nojadeh JN, Sharif SB, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159-168.
- Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145:2891-2899.
- Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:E26517.
- Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Rubay D, Ohanisian L, Bank MP, et al. Muir-Torre syndrome, a rare phenotype of hereditary nonpolyposis colorectal cancer with cutaneous manifestations. ACG Case Reports J. 2019;6:E00188.
- Velter C, Caussade P, Fricker JP, et al. Muir-Torre syndrome and Turcot syndrome [in French]. Ann Dermatol Venereol. 2017;144:525-529.
- John AM, Schwartz RA. Muir-Torre syndrome (MTS): an update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558-566.
- Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312:25-31.
- Marcoval J, Talavera-Belmonte A, Fornons-Servent R, et al. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44:506-511.
- Roth RM, Haraldsdottir S, Hampel H, et al. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol. 2016;146:50-56.
- Westwood A, Glover A, Hutchins G, et al. Additional loss of MSH2 and MSH6 expression in sporadic deficient mismatch repair colorectal cancer due to MLH1 promoter hypermethylation. J Clin Pathol. 2019;72:443-447.
- Claes K, Dahan K, Tejpar S, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP). Acta Gastroenterol Belg. 2011;74:421-426.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39-41.
- Tomonari M, Shimada M, Nakada Y, et al. Muir-Torre syndrome: sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol. 2019;20:394
- Levi Z, Hazazi R, Kedar-Barnes I, et al. Switching from tacrolimus to sirolimus halts the appearance of new sebaceous neoplasms in Muir-Torre syndrome. Am J Transplant. 2007;7:476-479.
- Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16:357-361.
- Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24.
- Bansidhar BJ. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103-110.
- Kato A, Sato N, Sugawara T, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770-776.
- Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936-942.
- Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome [published online March 6, 2014]. Genet Med. 2014;16:711-716.
- Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21:159-164.
- Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient’s age or other clinical characteristics. Am J Surg Pathol. 2009;33:934-944.
- Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338-343.
- Campanella NC, Berardinelli GN, Scapulatempo-Neto C, et al. Optimization of a pentaplex panel for MSI analysis without control DNA in a Brazilian population: correlation with ancestry markers. Eur J Hum Genet. 2014;22:875-880.
- Ponti G, Manfredini M, Tomasi A, et al. Muir-Torre Syndrome and founder mismatch repair gene mutations: a long gone historical genetic challenge. Gene. 2016;589:127-132.
- Ferreira I, Wiedemeyer K, Demetter P, et al. Update on the pathology, genetics and somatic landscape of sebaceous tumours [published online December 10, 2019]. Histopathology. doi:10.1111/his.14044
It is well known by now that tumor formation is driven by accumulation of numerous genetic and epigenetic mutations. Human cells are equipped with an apparatus called the DNA mismatch repair (MMR) system that corrects errors during replication.1 If these genes are themselves mutated, cells then start accumulating mutations in other genes, including oncogenes and tumor suppressor genes, which results in the development of sustained proliferative signaling pathways, evasion of growth suppression, resistance to cell death, and the potential for invasion and metastasis.2
Gene mutations in DNA MMR have been detected in several tumors, such as sebaceous tumors,3 colorectal adenocarcinomas,4 keratoacanthomas,5 and other visceral malignancies.6 Sebaceous tumors are rare in the general population; however, they are common in patients with inherited or acquired mutations in MMR genes.5 These patients also have been found to have other visceral malignancies such as colorectal adenocarcinomas and breast, lung, and central nervous system (CNS) tumors.7 This observation was made in the 1960s, and patients were referred to as having Muir-Torre syndrome (MTS).8 This article serves to briefly describe the DNA MMR system and its implication in sebaceous tumors as well as discuss the recent recommendations for screening for MTS in patients presenting with sebaceous tumors.
The DNA MMR System
Mismatch repair proteins are responsible for detecting and repairing errors during cell division, especially in microsatellite regions.9 Microsatellites are common and widely distributed DNA motifs consisting of repeated nucleotide sequences that normally account for 3% of the genome.10 Mutations in MMR result in insertion or deletion of nucleotides in these DNA motifs, making them either abnormally long or short, referred to as microsatellite instability (MSI), which results in downstream cumulative accumulation of mutations in oncogenes and tumor suppressor genes, and thus carcinogenesis.9
There are 7 human MMR proteins: MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2. These proteins are highly conserved across different living species.11 Loss of MMR proteins can be due to a mutation in the coding sequence of the gene or due to epigenetic hypermethylation of the gene promoter.12 These alterations can be inherited or acquired and in most cases result in MSI.
When assessing for MSI, tumor genomes can be divided into 3 subtypes: high-level and low-level MSI and stable microsatellites.13 Tumors with high-level MSI respond better to treatment and show a better prognosis than those with low-level MSI or stable microsatellites,14 which is thought to be due to tumor-induced immune activation. Microsatellite instability results in the generation of frameshift peptides that are immunogenic and induce tumor-specific immune responses.15 Several research laboratories have artificially synthesized frameshift peptides as vaccines and have successfully used them as targets for immune therapy as a way for preventing and treating malignancies.16
Sebaceous Tumors in MTS
A typical example of tumors that arise from mutations in the DNA MMR system is seen in MTS,a rare inherited genetic syndrome that predisposes patients to sebaceous neoplasms, keratoacanthomas, and visceral malignancies.17 It was first described as an autosomal-dominant condition in patients who have at least 1 sebaceous tumor and 1 visceral malignancy, with or without keratoacanthomas. It was then later characterized as a skin variant of Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer syndrome.18
Sebaceous tumors are the hallmark of MTS. Although sebaceous hyperplasia is common in the general population, sebaceous tumors are rare outside the context of MTS. There are 3 types of sebaceous tumors with distinct pathologic features: adenoma, epithelioma, and carcinoma.19 Sebaceous adenomas and epitheliomas are benign growths; however, sebaceous carcinomas can be aggressive and have metastatic potential.20 Because it is difficult to clinically distinguish carcinomas from the benign sebaceous growths, biopsy of a large, changing, or ulcerated lesion is important in these patients to rule out a sebaceous carcinoma. Other aggressive skin tumors can develop in MTS, such as rapidly growing keratoacanthomas and basal cell carcinomas with sebaceous differentiation.21
Types of MTS
For most cases, MTS is characterized by germline mutations in DNA MMR genes. The most common mutation involves MSH2 (MutS Homolog 2)—found in approximately 90% of patients—followed by MLH1 (MutL Homolog 1)—found in approximately 10% of patients.22 Other MMR genes such as MSH6 (MutS Homolog 6), PMS2 (PMS1 homolog 2, mismatch repair system component), and MLH3 (MutL Homolog 3) less commonly are reported in MTS. There is a subset of patients who lose MSH2 or MLH1 expression due to promoter hypermethylation rather than a germline mutation. Methylation results in biallelic inactivation of the gene and loss of expression.23
A new subtype of MTS has been identified that demonstrates an autosomal-recessive pattern of inheritance and is referred to as MTS type 2 (autosomal-recessive colorectal adenomatous polyposis).24 In contrast to the classic MTS type 1, MTS type 2 exhibits microsatellite stability. Recent molecular analyses revealed that type 2 is due to a mutation in a base excision repair gene called MUTYH (mutY DNA glycosylase).25 These patients are likely to develop hundreds of polyps at an early age.
Muir-Torre syndrome also can occur sporadically without inheriting a germline mutation, which has been reported in a transplant patient from de novo somatic mutations or promoter hypermethylation.26 A case report of a renal transplant patient showed that switching from tacrolimus to sirolimus halted the appearance of new sebaceous neoplasms, which suggests that patients with MTS who undergo organ transplantation should potentially avoid tacrolimus and be put on sirolimus instead.27
Visceral Malignancies in MTS
Apart from frequent skin examinations, MTS patients should have frequent and rigorous visceral malignancy screening. Patients most commonly develop colorectal adenocarcinoma, especially in the proximal parts of the colon.28 In addition, they can develop numerous premalignant tumors, especially in MTS type 2. Other common tumors include endometrial, ovarian, genitourinary, hepatobiliary, breast, lung, hematopoietic, and CNS malignancies.29
Studies showed that specific loss of certain MMR proteins predispose patients to different types of visceral malignancies.30-32 For example, loss of MSH2 predisposes patients to development of extracolonic tumors, while loss of MLH1 more strongly is associated with development of colorectal adenocarcinoma.30 Patients with MSH2 also are at risk for development of CNS tumors, while patients with MLH1 mutations have never been reported to develop CNS tumors.31 Patients with loss of PMS2 have the lowest risk for development of any visceral malignancy.32
Diagnosing MTS
Let us consider a scenario whereby a dermatologist biopsied a solitary lesion and it came back as a sebaceous tumor. What would be the next step to establish a diagnosis of MTS?
Sebaceous tumors are rare outside the context of MTS. Therefore, patients presenting with a solitary sebaceous tumor should be worked up for MTS, as there are implications for further cancer screening. One helpful clue that can affect the pretest probability for MTS diagnosis is location of the tumor. A sebaceous tumor inferior to the neck most likely is associated with MTS. On the other hand, tumors on the head and neck can be spontaneous or associated with MTS.33 Another helpful tool is the Mayo score, a risk score for MTS in patients with sebaceous tumors.34 The score is established by adding up points, with 1 point given to each of the following: age of onset of a sebaceous tumor less than 60 years, personal history of visceral malignancy, and family history of Lynch syndrome–related visceral malignancy. Two points are given if the patient has 2 or more sebaceous tumors. The score ranges from 0 to 5. A risk score of 2 or more has a sensitivity of 100% and specificity of 81% for predicting a germline mutation in MMR genes.34
Testing for loss of MMR proteins is performed using immunohistochemistry (IHC) as well as microsatellite gene analysis on the biopsied tumor. There is no need to perform another biopsy, as these tests can be performed on the paraffin-embedded formalin fixed tissue. Immunohistochemistry testing looks for loss of expression of one of the MMR proteins. Staining usually is performed for MSH2, MSH6, and MLH1, as the combination offers a sensitivity of 81% and a positive predictive value of 100%.23,35,36
If IHC shows loss of MMR proteins, then MSI gene analysis should be performed as a confirmatory test by using MSI gene locus assays, which utilize 5 markers of mononucleotide and dinucleotide repeats. If the genome is positive for 2 of 5 of these markers, then the patient most likely has MTS.13
One caveat for IHC analysis is that there is a subset of patients who develop a solitary sebaceous tumor due to a sporadic loss of MMR protein without having MTS. These tumors also exhibit BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations or loss of p16, features that distinguish these tumors from those developed in MTS.37 As such, in a patient with a low Mayo score who developed a solitary sebaceous tumor that showed loss of MMR protein on IHC without evidence of MSI, it is reasonable to perform IHC for BRAF and p16 to avoid inaccurate diagnosis of MTS.
Another caveat is that standard MSI analysis will not detect MSI in tumors with loss of MSH6 because the markers used in the MSI analysis do not detect MSI caused by MSH6 loss. For these patients, MSI analysis using a panel composed of mononucleotides alone (pentaplex assay) should be performed in lieu of the standard panel.38
It is important to note that these molecular tests are not helpful for patients with MTS type 2, as the sebaceous tumors maintain MMR proteins and have microsatellite stability. As such, if MTS is highly suspected based on the Mayo score (either personal history of malignancy or strong family history) but the IHC and MSI analysis are negative, then referral to a geneticist for identification for MUTYH gene mutation is a reasonable next step. These patients with high Mayo scores should still be managed as MTS patients and should be screened for visceral malignancies despite lack of confirmatory tests.
Final Thoughts
Dermatologists should be highly suspicious of MTS when they diagnose sebaceous tumors. Making a diagnosis of MTS notably affects patients’ primary care. Patients with MTS should have annual skin examinations, neurologic examinations, colonoscopies starting at the age of 18 years, and surveillance for breast and pelvic cancers in women (by annual transvaginal ultrasound and endometrial aspirations) or for prostate and testicular cancers in men.17,39,40 Other tests to be ordered annually include complete blood cell count with differential and urinalysis.19
It is well known by now that tumor formation is driven by accumulation of numerous genetic and epigenetic mutations. Human cells are equipped with an apparatus called the DNA mismatch repair (MMR) system that corrects errors during replication.1 If these genes are themselves mutated, cells then start accumulating mutations in other genes, including oncogenes and tumor suppressor genes, which results in the development of sustained proliferative signaling pathways, evasion of growth suppression, resistance to cell death, and the potential for invasion and metastasis.2
Gene mutations in DNA MMR have been detected in several tumors, such as sebaceous tumors,3 colorectal adenocarcinomas,4 keratoacanthomas,5 and other visceral malignancies.6 Sebaceous tumors are rare in the general population; however, they are common in patients with inherited or acquired mutations in MMR genes.5 These patients also have been found to have other visceral malignancies such as colorectal adenocarcinomas and breast, lung, and central nervous system (CNS) tumors.7 This observation was made in the 1960s, and patients were referred to as having Muir-Torre syndrome (MTS).8 This article serves to briefly describe the DNA MMR system and its implication in sebaceous tumors as well as discuss the recent recommendations for screening for MTS in patients presenting with sebaceous tumors.
The DNA MMR System
Mismatch repair proteins are responsible for detecting and repairing errors during cell division, especially in microsatellite regions.9 Microsatellites are common and widely distributed DNA motifs consisting of repeated nucleotide sequences that normally account for 3% of the genome.10 Mutations in MMR result in insertion or deletion of nucleotides in these DNA motifs, making them either abnormally long or short, referred to as microsatellite instability (MSI), which results in downstream cumulative accumulation of mutations in oncogenes and tumor suppressor genes, and thus carcinogenesis.9
There are 7 human MMR proteins: MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2. These proteins are highly conserved across different living species.11 Loss of MMR proteins can be due to a mutation in the coding sequence of the gene or due to epigenetic hypermethylation of the gene promoter.12 These alterations can be inherited or acquired and in most cases result in MSI.
When assessing for MSI, tumor genomes can be divided into 3 subtypes: high-level and low-level MSI and stable microsatellites.13 Tumors with high-level MSI respond better to treatment and show a better prognosis than those with low-level MSI or stable microsatellites,14 which is thought to be due to tumor-induced immune activation. Microsatellite instability results in the generation of frameshift peptides that are immunogenic and induce tumor-specific immune responses.15 Several research laboratories have artificially synthesized frameshift peptides as vaccines and have successfully used them as targets for immune therapy as a way for preventing and treating malignancies.16
Sebaceous Tumors in MTS
A typical example of tumors that arise from mutations in the DNA MMR system is seen in MTS,a rare inherited genetic syndrome that predisposes patients to sebaceous neoplasms, keratoacanthomas, and visceral malignancies.17 It was first described as an autosomal-dominant condition in patients who have at least 1 sebaceous tumor and 1 visceral malignancy, with or without keratoacanthomas. It was then later characterized as a skin variant of Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer syndrome.18
Sebaceous tumors are the hallmark of MTS. Although sebaceous hyperplasia is common in the general population, sebaceous tumors are rare outside the context of MTS. There are 3 types of sebaceous tumors with distinct pathologic features: adenoma, epithelioma, and carcinoma.19 Sebaceous adenomas and epitheliomas are benign growths; however, sebaceous carcinomas can be aggressive and have metastatic potential.20 Because it is difficult to clinically distinguish carcinomas from the benign sebaceous growths, biopsy of a large, changing, or ulcerated lesion is important in these patients to rule out a sebaceous carcinoma. Other aggressive skin tumors can develop in MTS, such as rapidly growing keratoacanthomas and basal cell carcinomas with sebaceous differentiation.21
Types of MTS
For most cases, MTS is characterized by germline mutations in DNA MMR genes. The most common mutation involves MSH2 (MutS Homolog 2)—found in approximately 90% of patients—followed by MLH1 (MutL Homolog 1)—found in approximately 10% of patients.22 Other MMR genes such as MSH6 (MutS Homolog 6), PMS2 (PMS1 homolog 2, mismatch repair system component), and MLH3 (MutL Homolog 3) less commonly are reported in MTS. There is a subset of patients who lose MSH2 or MLH1 expression due to promoter hypermethylation rather than a germline mutation. Methylation results in biallelic inactivation of the gene and loss of expression.23
A new subtype of MTS has been identified that demonstrates an autosomal-recessive pattern of inheritance and is referred to as MTS type 2 (autosomal-recessive colorectal adenomatous polyposis).24 In contrast to the classic MTS type 1, MTS type 2 exhibits microsatellite stability. Recent molecular analyses revealed that type 2 is due to a mutation in a base excision repair gene called MUTYH (mutY DNA glycosylase).25 These patients are likely to develop hundreds of polyps at an early age.
Muir-Torre syndrome also can occur sporadically without inheriting a germline mutation, which has been reported in a transplant patient from de novo somatic mutations or promoter hypermethylation.26 A case report of a renal transplant patient showed that switching from tacrolimus to sirolimus halted the appearance of new sebaceous neoplasms, which suggests that patients with MTS who undergo organ transplantation should potentially avoid tacrolimus and be put on sirolimus instead.27
Visceral Malignancies in MTS
Apart from frequent skin examinations, MTS patients should have frequent and rigorous visceral malignancy screening. Patients most commonly develop colorectal adenocarcinoma, especially in the proximal parts of the colon.28 In addition, they can develop numerous premalignant tumors, especially in MTS type 2. Other common tumors include endometrial, ovarian, genitourinary, hepatobiliary, breast, lung, hematopoietic, and CNS malignancies.29
Studies showed that specific loss of certain MMR proteins predispose patients to different types of visceral malignancies.30-32 For example, loss of MSH2 predisposes patients to development of extracolonic tumors, while loss of MLH1 more strongly is associated with development of colorectal adenocarcinoma.30 Patients with MSH2 also are at risk for development of CNS tumors, while patients with MLH1 mutations have never been reported to develop CNS tumors.31 Patients with loss of PMS2 have the lowest risk for development of any visceral malignancy.32
Diagnosing MTS
Let us consider a scenario whereby a dermatologist biopsied a solitary lesion and it came back as a sebaceous tumor. What would be the next step to establish a diagnosis of MTS?
Sebaceous tumors are rare outside the context of MTS. Therefore, patients presenting with a solitary sebaceous tumor should be worked up for MTS, as there are implications for further cancer screening. One helpful clue that can affect the pretest probability for MTS diagnosis is location of the tumor. A sebaceous tumor inferior to the neck most likely is associated with MTS. On the other hand, tumors on the head and neck can be spontaneous or associated with MTS.33 Another helpful tool is the Mayo score, a risk score for MTS in patients with sebaceous tumors.34 The score is established by adding up points, with 1 point given to each of the following: age of onset of a sebaceous tumor less than 60 years, personal history of visceral malignancy, and family history of Lynch syndrome–related visceral malignancy. Two points are given if the patient has 2 or more sebaceous tumors. The score ranges from 0 to 5. A risk score of 2 or more has a sensitivity of 100% and specificity of 81% for predicting a germline mutation in MMR genes.34
Testing for loss of MMR proteins is performed using immunohistochemistry (IHC) as well as microsatellite gene analysis on the biopsied tumor. There is no need to perform another biopsy, as these tests can be performed on the paraffin-embedded formalin fixed tissue. Immunohistochemistry testing looks for loss of expression of one of the MMR proteins. Staining usually is performed for MSH2, MSH6, and MLH1, as the combination offers a sensitivity of 81% and a positive predictive value of 100%.23,35,36
If IHC shows loss of MMR proteins, then MSI gene analysis should be performed as a confirmatory test by using MSI gene locus assays, which utilize 5 markers of mononucleotide and dinucleotide repeats. If the genome is positive for 2 of 5 of these markers, then the patient most likely has MTS.13
One caveat for IHC analysis is that there is a subset of patients who develop a solitary sebaceous tumor due to a sporadic loss of MMR protein without having MTS. These tumors also exhibit BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations or loss of p16, features that distinguish these tumors from those developed in MTS.37 As such, in a patient with a low Mayo score who developed a solitary sebaceous tumor that showed loss of MMR protein on IHC without evidence of MSI, it is reasonable to perform IHC for BRAF and p16 to avoid inaccurate diagnosis of MTS.
Another caveat is that standard MSI analysis will not detect MSI in tumors with loss of MSH6 because the markers used in the MSI analysis do not detect MSI caused by MSH6 loss. For these patients, MSI analysis using a panel composed of mononucleotides alone (pentaplex assay) should be performed in lieu of the standard panel.38
It is important to note that these molecular tests are not helpful for patients with MTS type 2, as the sebaceous tumors maintain MMR proteins and have microsatellite stability. As such, if MTS is highly suspected based on the Mayo score (either personal history of malignancy or strong family history) but the IHC and MSI analysis are negative, then referral to a geneticist for identification for MUTYH gene mutation is a reasonable next step. These patients with high Mayo scores should still be managed as MTS patients and should be screened for visceral malignancies despite lack of confirmatory tests.
Final Thoughts
Dermatologists should be highly suspicious of MTS when they diagnose sebaceous tumors. Making a diagnosis of MTS notably affects patients’ primary care. Patients with MTS should have annual skin examinations, neurologic examinations, colonoscopies starting at the age of 18 years, and surveillance for breast and pelvic cancers in women (by annual transvaginal ultrasound and endometrial aspirations) or for prostate and testicular cancers in men.17,39,40 Other tests to be ordered annually include complete blood cell count with differential and urinalysis.19
- Yamamoto H, Imai K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin Oncol. 2019;46:261-270.
- Tamura K, Kaneda M, Futagawa M, et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol. 2019;24:999-1011.
- Shiki M, Hida T, Sugano K, et al. Muir-Torre syndrome caused by exonic deletion of MLH1 due to homologous recombination. Eur J Dermatol. 2017;27:54-58.
- Büttner R, Friedrichs N. Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany. Pathologe. 2019;40:584-591.
- Kuwabara K, Suzuki O, Chika N, et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn J Clin Oncol. 2018;48:514-521.
- Burris CKH, Rodriguez ME, Raven ML, et al. Muir-torre syndrome: the importance of a detailed family history. Case Rep Ophthalmol. 2019;10:180-185.
- Walsh MD, Jayasekara H, Huang A, et al. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: a large case series from a single Australian private pathology service. Australas J Dermatol. 2019;60:126-133.
- Georgeson P, Walsh MD, Clendenning M, et al. Tumor mutational signatures in sebaceous skin lesions from individuals with Lynch syndrome. Mol Genet Genomic Med. 2019;7:E00781.
- Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391-407.
- Li YC, Korol AB, Fahima T, et al. Microsatellites within genes: structure, function, and evolution [published online February 12, 2004]. Mol Biol Evol. 2004;21:991-1007.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435-445.
- Everett JN, Raymond VM, Dandapani M, et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014;150:1315-1321.
- Nojadeh JN, Sharif SB, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159-168.
- Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145:2891-2899.
- Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:E26517.
- Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Rubay D, Ohanisian L, Bank MP, et al. Muir-Torre syndrome, a rare phenotype of hereditary nonpolyposis colorectal cancer with cutaneous manifestations. ACG Case Reports J. 2019;6:E00188.
- Velter C, Caussade P, Fricker JP, et al. Muir-Torre syndrome and Turcot syndrome [in French]. Ann Dermatol Venereol. 2017;144:525-529.
- John AM, Schwartz RA. Muir-Torre syndrome (MTS): an update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558-566.
- Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312:25-31.
- Marcoval J, Talavera-Belmonte A, Fornons-Servent R, et al. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44:506-511.
- Roth RM, Haraldsdottir S, Hampel H, et al. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol. 2016;146:50-56.
- Westwood A, Glover A, Hutchins G, et al. Additional loss of MSH2 and MSH6 expression in sporadic deficient mismatch repair colorectal cancer due to MLH1 promoter hypermethylation. J Clin Pathol. 2019;72:443-447.
- Claes K, Dahan K, Tejpar S, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP). Acta Gastroenterol Belg. 2011;74:421-426.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39-41.
- Tomonari M, Shimada M, Nakada Y, et al. Muir-Torre syndrome: sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol. 2019;20:394
- Levi Z, Hazazi R, Kedar-Barnes I, et al. Switching from tacrolimus to sirolimus halts the appearance of new sebaceous neoplasms in Muir-Torre syndrome. Am J Transplant. 2007;7:476-479.
- Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16:357-361.
- Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24.
- Bansidhar BJ. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103-110.
- Kato A, Sato N, Sugawara T, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770-776.
- Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936-942.
- Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome [published online March 6, 2014]. Genet Med. 2014;16:711-716.
- Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21:159-164.
- Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient’s age or other clinical characteristics. Am J Surg Pathol. 2009;33:934-944.
- Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338-343.
- Campanella NC, Berardinelli GN, Scapulatempo-Neto C, et al. Optimization of a pentaplex panel for MSI analysis without control DNA in a Brazilian population: correlation with ancestry markers. Eur J Hum Genet. 2014;22:875-880.
- Ponti G, Manfredini M, Tomasi A, et al. Muir-Torre Syndrome and founder mismatch repair gene mutations: a long gone historical genetic challenge. Gene. 2016;589:127-132.
- Ferreira I, Wiedemeyer K, Demetter P, et al. Update on the pathology, genetics and somatic landscape of sebaceous tumours [published online December 10, 2019]. Histopathology. doi:10.1111/his.14044
- Yamamoto H, Imai K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin Oncol. 2019;46:261-270.
- Tamura K, Kaneda M, Futagawa M, et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol. 2019;24:999-1011.
- Shiki M, Hida T, Sugano K, et al. Muir-Torre syndrome caused by exonic deletion of MLH1 due to homologous recombination. Eur J Dermatol. 2017;27:54-58.
- Büttner R, Friedrichs N. Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany. Pathologe. 2019;40:584-591.
- Kuwabara K, Suzuki O, Chika N, et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn J Clin Oncol. 2018;48:514-521.
- Burris CKH, Rodriguez ME, Raven ML, et al. Muir-torre syndrome: the importance of a detailed family history. Case Rep Ophthalmol. 2019;10:180-185.
- Walsh MD, Jayasekara H, Huang A, et al. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: a large case series from a single Australian private pathology service. Australas J Dermatol. 2019;60:126-133.
- Georgeson P, Walsh MD, Clendenning M, et al. Tumor mutational signatures in sebaceous skin lesions from individuals with Lynch syndrome. Mol Genet Genomic Med. 2019;7:E00781.
- Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391-407.
- Li YC, Korol AB, Fahima T, et al. Microsatellites within genes: structure, function, and evolution [published online February 12, 2004]. Mol Biol Evol. 2004;21:991-1007.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435-445.
- Everett JN, Raymond VM, Dandapani M, et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014;150:1315-1321.
- Nojadeh JN, Sharif SB, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159-168.
- Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145:2891-2899.
- Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:E26517.
- Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Rubay D, Ohanisian L, Bank MP, et al. Muir-Torre syndrome, a rare phenotype of hereditary nonpolyposis colorectal cancer with cutaneous manifestations. ACG Case Reports J. 2019;6:E00188.
- Velter C, Caussade P, Fricker JP, et al. Muir-Torre syndrome and Turcot syndrome [in French]. Ann Dermatol Venereol. 2017;144:525-529.
- John AM, Schwartz RA. Muir-Torre syndrome (MTS): an update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558-566.
- Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312:25-31.
- Marcoval J, Talavera-Belmonte A, Fornons-Servent R, et al. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44:506-511.
- Roth RM, Haraldsdottir S, Hampel H, et al. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol. 2016;146:50-56.
- Westwood A, Glover A, Hutchins G, et al. Additional loss of MSH2 and MSH6 expression in sporadic deficient mismatch repair colorectal cancer due to MLH1 promoter hypermethylation. J Clin Pathol. 2019;72:443-447.
- Claes K, Dahan K, Tejpar S, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP). Acta Gastroenterol Belg. 2011;74:421-426.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39-41.
- Tomonari M, Shimada M, Nakada Y, et al. Muir-Torre syndrome: sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol. 2019;20:394
- Levi Z, Hazazi R, Kedar-Barnes I, et al. Switching from tacrolimus to sirolimus halts the appearance of new sebaceous neoplasms in Muir-Torre syndrome. Am J Transplant. 2007;7:476-479.
- Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16:357-361.
- Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24.
- Bansidhar BJ. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103-110.
- Kato A, Sato N, Sugawara T, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770-776.
- Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936-942.
- Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome [published online March 6, 2014]. Genet Med. 2014;16:711-716.
- Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21:159-164.
- Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient’s age or other clinical characteristics. Am J Surg Pathol. 2009;33:934-944.
- Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338-343.
- Campanella NC, Berardinelli GN, Scapulatempo-Neto C, et al. Optimization of a pentaplex panel for MSI analysis without control DNA in a Brazilian population: correlation with ancestry markers. Eur J Hum Genet. 2014;22:875-880.
- Ponti G, Manfredini M, Tomasi A, et al. Muir-Torre Syndrome and founder mismatch repair gene mutations: a long gone historical genetic challenge. Gene. 2016;589:127-132.
- Ferreira I, Wiedemeyer K, Demetter P, et al. Update on the pathology, genetics and somatic landscape of sebaceous tumours [published online December 10, 2019]. Histopathology. doi:10.1111/his.14044
Resident Pearls
- When patients present with a solitary sebaceous tumor, there is a high likelihood they have Muir-Torre syndrome (MTS) and thus are at a high risk to develop visceral malignancies.
- It is important to perform further testing using immunohistochemistry for DNA mismatch repair proteins and microsatellite instability gene analysis in some cases to confirm the diagnosis of MTS and to perform the appropriate cancer screening tests.
'Silent Hypoxemia' and Other Curious Clinical Observations in COVID-19

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about what's going on in Detroit, and also about PPE and decontamination processes. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
Dr Gary Ferenchick: We were talking earlier about some of the not-well-described clinical scenarios that patients with definitive COVID might present with. One of these was the idea of "silent hypoxemia." Could you describe that?
Dr Hannah Ferenchick: Silent hypoxemia is being described in many of these COVID patients. That means the patient is very hypoxemic—they may have an oxygen saturation of about 85% on room air, but clinically they look very comfortable—they are not dyspneic or tachypneic and may not even verbalize a significant sense of shortness of breath. It's not every patient, but it has been interesting to see patients sitting there looking fairly normal, with a resting oxygen saturation much lower than you would expect for someone who doesn't have underlying pulmonary disease or other symptoms.
Dr Gary Ferenchick: What abnormalities are you seeing on standard or not-so-standard lab tests?
Dr Hannah Ferenchick: Some of the characteristic lab findings we are seeing are lymphopenia and elevated inflammatory markers (eg, CRP). A couple of other atypical findings seem to be specific for COVID—elevated LDH, ferritin, CPK, and procalcitonin levels. Some of the hematologic markers that we look at—the coagulation profile studies—are also abnormal, showing thrombocytopenia and elevated D-dimer levels.
That constellation of symptoms represents more of a clinical picture. A lot of times we have only a very high clinical suspicion, because in many parts of the country it still takes days to get back a confirmatory PCR test.
Much like we do for the flu, the confirmatory test is a nasopharyngeal swab that is run for COVID/coronavirus PCR. Unfortunately the sensitivity of that test is not great. Some studies have quoted 75%-80%, so even a negative PCR does not necessarily rule out the disease, especially if you have a high clinical suspicion. A clinical suspicion is based on the typical symptoms. Many patients, although not all, will have symptoms of lower respiratory tract infection.
Dr Gary Ferenchick: So the right clinical scenario with the right hematologic/biochemical findings dramatically raises the chance that the patient has COVID?
Dr Hannah Ferenchick: Yes, and one thing that we have all been astonished by is how terrible some of these x-rays can look. There are a lot of typical findings on x-ray. Some describe them as looking like pulmonary edema, but the patient has no history of heart failure. Peripheral consolidation and ground-glass opacities are classically described. If you saw one of these x-rays from a patient with bacterial pneumonia, you would expect that patient to be very ill-appearing. Sometimes we get x-rays on patients who are sitting there, maybe mildly symptomatic on room air, and we are astonished by how terrible their x-rays look.
Unfortunately, imaging studies are something we haven't been able to rely on too much for diagnosis. Part of that is to maintain hospital safety, because to take a patient to CT scan, you have to consider the turnaround time for cleaning the CT scanner and the exposure of additional staff to a possibly infected patient. Some of those logistical considerations have limited the availability of radiography.
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine, at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about what's going on in Detroit, and also about PPE and decontamination processes. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
Dr Gary Ferenchick: We were talking earlier about some of the not-well-described clinical scenarios that patients with definitive COVID might present with. One of these was the idea of "silent hypoxemia." Could you describe that?
Dr Hannah Ferenchick: Silent hypoxemia is being described in many of these COVID patients. That means the patient is very hypoxemic—they may have an oxygen saturation of about 85% on room air, but clinically they look very comfortable—they are not dyspneic or tachypneic and may not even verbalize a significant sense of shortness of breath. It's not every patient, but it has been interesting to see patients sitting there looking fairly normal, with a resting oxygen saturation much lower than you would expect for someone who doesn't have underlying pulmonary disease or other symptoms.
Dr Gary Ferenchick: What abnormalities are you seeing on standard or not-so-standard lab tests?
Dr Hannah Ferenchick: Some of the characteristic lab findings we are seeing are lymphopenia and elevated inflammatory markers (eg, CRP). A couple of other atypical findings seem to be specific for COVID—elevated LDH, ferritin, CPK, and procalcitonin levels. Some of the hematologic markers that we look at—the coagulation profile studies—are also abnormal, showing thrombocytopenia and elevated D-dimer levels.
That constellation of symptoms represents more of a clinical picture. A lot of times we have only a very high clinical suspicion, because in many parts of the country it still takes days to get back a confirmatory PCR test.
Much like we do for the flu, the confirmatory test is a nasopharyngeal swab that is run for COVID/coronavirus PCR. Unfortunately the sensitivity of that test is not great. Some studies have quoted 75%-80%, so even a negative PCR does not necessarily rule out the disease, especially if you have a high clinical suspicion. A clinical suspicion is based on the typical symptoms. Many patients, although not all, will have symptoms of lower respiratory tract infection.
Dr Gary Ferenchick: So the right clinical scenario with the right hematologic/biochemical findings dramatically raises the chance that the patient has COVID?
Dr Hannah Ferenchick: Yes, and one thing that we have all been astonished by is how terrible some of these x-rays can look. There are a lot of typical findings on x-ray. Some describe them as looking like pulmonary edema, but the patient has no history of heart failure. Peripheral consolidation and ground-glass opacities are classically described. If you saw one of these x-rays from a patient with bacterial pneumonia, you would expect that patient to be very ill-appearing. Sometimes we get x-rays on patients who are sitting there, maybe mildly symptomatic on room air, and we are astonished by how terrible their x-rays look.
Unfortunately, imaging studies are something we haven't been able to rely on too much for diagnosis. Part of that is to maintain hospital safety, because to take a patient to CT scan, you have to consider the turnaround time for cleaning the CT scanner and the exposure of additional staff to a possibly infected patient. Some of those logistical considerations have limited the availability of radiography.
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine, at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about what's going on in Detroit, and also about PPE and decontamination processes. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
Dr Gary Ferenchick: We were talking earlier about some of the not-well-described clinical scenarios that patients with definitive COVID might present with. One of these was the idea of "silent hypoxemia." Could you describe that?
Dr Hannah Ferenchick: Silent hypoxemia is being described in many of these COVID patients. That means the patient is very hypoxemic—they may have an oxygen saturation of about 85% on room air, but clinically they look very comfortable—they are not dyspneic or tachypneic and may not even verbalize a significant sense of shortness of breath. It's not every patient, but it has been interesting to see patients sitting there looking fairly normal, with a resting oxygen saturation much lower than you would expect for someone who doesn't have underlying pulmonary disease or other symptoms.
Dr Gary Ferenchick: What abnormalities are you seeing on standard or not-so-standard lab tests?
Dr Hannah Ferenchick: Some of the characteristic lab findings we are seeing are lymphopenia and elevated inflammatory markers (eg, CRP). A couple of other atypical findings seem to be specific for COVID—elevated LDH, ferritin, CPK, and procalcitonin levels. Some of the hematologic markers that we look at—the coagulation profile studies—are also abnormal, showing thrombocytopenia and elevated D-dimer levels.
That constellation of symptoms represents more of a clinical picture. A lot of times we have only a very high clinical suspicion, because in many parts of the country it still takes days to get back a confirmatory PCR test.
Much like we do for the flu, the confirmatory test is a nasopharyngeal swab that is run for COVID/coronavirus PCR. Unfortunately the sensitivity of that test is not great. Some studies have quoted 75%-80%, so even a negative PCR does not necessarily rule out the disease, especially if you have a high clinical suspicion. A clinical suspicion is based on the typical symptoms. Many patients, although not all, will have symptoms of lower respiratory tract infection.
Dr Gary Ferenchick: So the right clinical scenario with the right hematologic/biochemical findings dramatically raises the chance that the patient has COVID?
Dr Hannah Ferenchick: Yes, and one thing that we have all been astonished by is how terrible some of these x-rays can look. There are a lot of typical findings on x-ray. Some describe them as looking like pulmonary edema, but the patient has no history of heart failure. Peripheral consolidation and ground-glass opacities are classically described. If you saw one of these x-rays from a patient with bacterial pneumonia, you would expect that patient to be very ill-appearing. Sometimes we get x-rays on patients who are sitting there, maybe mildly symptomatic on room air, and we are astonished by how terrible their x-rays look.
Unfortunately, imaging studies are something we haven't been able to rely on too much for diagnosis. Part of that is to maintain hospital safety, because to take a patient to CT scan, you have to consider the turnaround time for cleaning the CT scanner and the exposure of additional staff to a possibly infected patient. Some of those logistical considerations have limited the availability of radiography.
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine, at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.
Clinical Case-Viewing Sessions in Dermatology: The Patient Perspective
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.
The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.
The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.
The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
Practice Points
- Patient willingness to attend dermatology clinical case-viewing (CCV) sessions is relatively unchanged before and after the session.
- Participants generally consider CCV sessions to be a positive experience.
NYU med student joins COVID fight: ‘Time to step up’
On the evening of March 24, I got the email. When the bolded letters “We ask for your help” flashed across my screen, I knew exactly what was being asked of me: to graduate early and join the fight against COVID-19.
For the 120 fourth-year medical students in my class at NYU Grossman School of Medicine, the arrival of that email was always more a question of when than if. Similar moves had already been made in Italy as well as the United Kingdom, where the surge in patients with COVID-19 has devastated hospitals and left healthcare workers dead or drained. The New York hospitals where I’ve trained, places I have grown to love over the past 4 years, are now experiencing similar horrors. Residents and attending doctors – mentors and teachers – are burned out and exhausted. They need help.
Like most medical students, I chose to pursue medicine out of a desire to help. On both my medical school and residency applications, I spoke about my resolve to bear witness to and provide support to those suffering. Yet, being recruited to the front lines of a global pandemic felt deeply unsettling. Is this how I want to finally enter the world of medicine? The scope of what is actually being asked of me was immense.
Given the onslaught of bad news coming in on every device I had cozied up to during my social distancing, how could I want to do this? I’ve seen the death toll climb in Italy, with dozens of doctors dead. I’ve seen the photos of faces marred by masks worn for 12-16 hours at a time. I’ve been repeatedly reminded that we are just behind Italy. Things are certainly going to get worse.
It sounds selfish and petty, but I feel like COVID-19 has already robbed me of so much. Yet that was my first thought when I received the email. The end of fourth year in medical school is supposed to be a joyous, celebratory time. We have worked years for this moment. So many of us have fought burnout to reach this time, a brief moment of rest between being a medical student and becoming a full-fledged physician.
I matched into residency just 4 days before being asked to join the front lines of the pandemic. I found out my match results without the usual fanfare, sitting on a bench in Madison Square Park, FaceTiming my dad and safely social-distanced from my mom. They both cried tears of joy. Like so many people around the world right now, I couldn’t even embrace my parents. Would they want me to volunteer?
I reached out to my classmates. I thought that some of them would certainly share my worries. I thought they also had to be carrying this uncomfortable kind of grief, a heavy and acidic feeling of dreams collapsing into a moral duty. I received a unanimous reply: “We are needed. It’s our time to step up.” No matter how many “what ifs” I voiced, they wouldn’t crack or waver. Still, even if they never admitted it to me, I wondered whether they privately shared some of my concerns and fears.
Everyone knows information is shared instantly in our Twitter-centric world, but I was still shocked and unprepared for how quickly I was at the center of a major news story. Within an hour of that email, I was contacted by an old acquaintance from elementary school, now a journalist. He had found me through Facebook and asked, “Will you be one of the NYU students graduating early? Would love to get a comment.” Another friend texted me a photo of the leaked email, quipping, “Are you going to save us from the pandemic, Dr. Gabe?!” “It’s not a small decision!” I snapped back.
I went through something like the seven stages of grief in rapid succession. I found that with each excuse I made why I shouldn’t volunteer, I somehow became increasingly more anxious. To my surprise, when I decided I would join 50 of my peers at NYU, graduate early, and volunteer, my mind settled. The more I thought about it, the more I was overtaken by the selfless beauty of the profession I’m entering. This is what it means to be a doctor. I recalled a key part of the Hippocratic Oath: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.”
I am going to fulfill my special obligations.
The fear is still there. I’m scared of COVID. I’m scared to infect others. I’m scared of winding up paralyzed and intubated. But I have also realized that all we have is each other. Healthcare workers supporting healthcare workers. New Yorkers supporting New Yorkers. Citizens of the world supporting citizens of the world. This is my time to be there for others, unwaveringly.
Logistical details continue to roll in, although they feel trivial in relation to the decision I have already made. The paperwork tells me that I will be onboarded to NYU’s internal medicine residency program. I will be compensated and protected under a similar contract to what current NYU residents sign. I have been promised that I will remain insured until I start my official residency program in July. My student loans won’t begin accruing interest until my normally planned graduation date. I am told that I will have personal protective equipment in line with the Centers for Disease Control and Prevention recommendations.
Questions still linger. Is it safe for me and my newly minted physician peers to continue living with our spouses, children, and friends? How long will I need to quarantine after my contract ends? Will there be a virtual graduation ceremony for my parents and loved ones to enjoy? In these challenging times, each day gives me a little more clarity about what exactly I am signing up for, but there are still so many uncertainties.
Am I naive to say that I do feel prepared? Or at least as prepared as anyone can be. With respect to my training, I have completed the requirements to graduate, which is why I am being permitted to graduate early in the first place. Our faculty points to our professionalism as the most promising indicator of our preparedness. They are heartened that we have embraced this truest test: our duty to others.
There is an eerie calm to New York City that contradicts what is shown on the news. With stores closed and streets quiet, it almost feels like Christmas morning here. Yet, inside the hospital, a fire rages. All the metaphors being used right now speak about violence, devastation, and immeasurable human suffering. “A war is being fought.” Or so I have heard. I guess I am about to find out.
Gabriel Redel-Traub is a fourth-year medical student at NYU Grossman School of Medicine. He will be starting residency in internal medicine at Columbia Presbyterian this summer. He is the former editor-in-chief of Dartmouth College’s Mouth Magazine, an editor of NYU’s LitMed Database, and has published most recently in the Hasting’s Center Magazine. Gabriel Redel-Traub has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
On the evening of March 24, I got the email. When the bolded letters “We ask for your help” flashed across my screen, I knew exactly what was being asked of me: to graduate early and join the fight against COVID-19.
For the 120 fourth-year medical students in my class at NYU Grossman School of Medicine, the arrival of that email was always more a question of when than if. Similar moves had already been made in Italy as well as the United Kingdom, where the surge in patients with COVID-19 has devastated hospitals and left healthcare workers dead or drained. The New York hospitals where I’ve trained, places I have grown to love over the past 4 years, are now experiencing similar horrors. Residents and attending doctors – mentors and teachers – are burned out and exhausted. They need help.
Like most medical students, I chose to pursue medicine out of a desire to help. On both my medical school and residency applications, I spoke about my resolve to bear witness to and provide support to those suffering. Yet, being recruited to the front lines of a global pandemic felt deeply unsettling. Is this how I want to finally enter the world of medicine? The scope of what is actually being asked of me was immense.
Given the onslaught of bad news coming in on every device I had cozied up to during my social distancing, how could I want to do this? I’ve seen the death toll climb in Italy, with dozens of doctors dead. I’ve seen the photos of faces marred by masks worn for 12-16 hours at a time. I’ve been repeatedly reminded that we are just behind Italy. Things are certainly going to get worse.
It sounds selfish and petty, but I feel like COVID-19 has already robbed me of so much. Yet that was my first thought when I received the email. The end of fourth year in medical school is supposed to be a joyous, celebratory time. We have worked years for this moment. So many of us have fought burnout to reach this time, a brief moment of rest between being a medical student and becoming a full-fledged physician.
I matched into residency just 4 days before being asked to join the front lines of the pandemic. I found out my match results without the usual fanfare, sitting on a bench in Madison Square Park, FaceTiming my dad and safely social-distanced from my mom. They both cried tears of joy. Like so many people around the world right now, I couldn’t even embrace my parents. Would they want me to volunteer?
I reached out to my classmates. I thought that some of them would certainly share my worries. I thought they also had to be carrying this uncomfortable kind of grief, a heavy and acidic feeling of dreams collapsing into a moral duty. I received a unanimous reply: “We are needed. It’s our time to step up.” No matter how many “what ifs” I voiced, they wouldn’t crack or waver. Still, even if they never admitted it to me, I wondered whether they privately shared some of my concerns and fears.
Everyone knows information is shared instantly in our Twitter-centric world, but I was still shocked and unprepared for how quickly I was at the center of a major news story. Within an hour of that email, I was contacted by an old acquaintance from elementary school, now a journalist. He had found me through Facebook and asked, “Will you be one of the NYU students graduating early? Would love to get a comment.” Another friend texted me a photo of the leaked email, quipping, “Are you going to save us from the pandemic, Dr. Gabe?!” “It’s not a small decision!” I snapped back.
I went through something like the seven stages of grief in rapid succession. I found that with each excuse I made why I shouldn’t volunteer, I somehow became increasingly more anxious. To my surprise, when I decided I would join 50 of my peers at NYU, graduate early, and volunteer, my mind settled. The more I thought about it, the more I was overtaken by the selfless beauty of the profession I’m entering. This is what it means to be a doctor. I recalled a key part of the Hippocratic Oath: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.”
I am going to fulfill my special obligations.
The fear is still there. I’m scared of COVID. I’m scared to infect others. I’m scared of winding up paralyzed and intubated. But I have also realized that all we have is each other. Healthcare workers supporting healthcare workers. New Yorkers supporting New Yorkers. Citizens of the world supporting citizens of the world. This is my time to be there for others, unwaveringly.
Logistical details continue to roll in, although they feel trivial in relation to the decision I have already made. The paperwork tells me that I will be onboarded to NYU’s internal medicine residency program. I will be compensated and protected under a similar contract to what current NYU residents sign. I have been promised that I will remain insured until I start my official residency program in July. My student loans won’t begin accruing interest until my normally planned graduation date. I am told that I will have personal protective equipment in line with the Centers for Disease Control and Prevention recommendations.
Questions still linger. Is it safe for me and my newly minted physician peers to continue living with our spouses, children, and friends? How long will I need to quarantine after my contract ends? Will there be a virtual graduation ceremony for my parents and loved ones to enjoy? In these challenging times, each day gives me a little more clarity about what exactly I am signing up for, but there are still so many uncertainties.
Am I naive to say that I do feel prepared? Or at least as prepared as anyone can be. With respect to my training, I have completed the requirements to graduate, which is why I am being permitted to graduate early in the first place. Our faculty points to our professionalism as the most promising indicator of our preparedness. They are heartened that we have embraced this truest test: our duty to others.
There is an eerie calm to New York City that contradicts what is shown on the news. With stores closed and streets quiet, it almost feels like Christmas morning here. Yet, inside the hospital, a fire rages. All the metaphors being used right now speak about violence, devastation, and immeasurable human suffering. “A war is being fought.” Or so I have heard. I guess I am about to find out.
Gabriel Redel-Traub is a fourth-year medical student at NYU Grossman School of Medicine. He will be starting residency in internal medicine at Columbia Presbyterian this summer. He is the former editor-in-chief of Dartmouth College’s Mouth Magazine, an editor of NYU’s LitMed Database, and has published most recently in the Hasting’s Center Magazine. Gabriel Redel-Traub has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
On the evening of March 24, I got the email. When the bolded letters “We ask for your help” flashed across my screen, I knew exactly what was being asked of me: to graduate early and join the fight against COVID-19.
For the 120 fourth-year medical students in my class at NYU Grossman School of Medicine, the arrival of that email was always more a question of when than if. Similar moves had already been made in Italy as well as the United Kingdom, where the surge in patients with COVID-19 has devastated hospitals and left healthcare workers dead or drained. The New York hospitals where I’ve trained, places I have grown to love over the past 4 years, are now experiencing similar horrors. Residents and attending doctors – mentors and teachers – are burned out and exhausted. They need help.
Like most medical students, I chose to pursue medicine out of a desire to help. On both my medical school and residency applications, I spoke about my resolve to bear witness to and provide support to those suffering. Yet, being recruited to the front lines of a global pandemic felt deeply unsettling. Is this how I want to finally enter the world of medicine? The scope of what is actually being asked of me was immense.
Given the onslaught of bad news coming in on every device I had cozied up to during my social distancing, how could I want to do this? I’ve seen the death toll climb in Italy, with dozens of doctors dead. I’ve seen the photos of faces marred by masks worn for 12-16 hours at a time. I’ve been repeatedly reminded that we are just behind Italy. Things are certainly going to get worse.
It sounds selfish and petty, but I feel like COVID-19 has already robbed me of so much. Yet that was my first thought when I received the email. The end of fourth year in medical school is supposed to be a joyous, celebratory time. We have worked years for this moment. So many of us have fought burnout to reach this time, a brief moment of rest between being a medical student and becoming a full-fledged physician.
I matched into residency just 4 days before being asked to join the front lines of the pandemic. I found out my match results without the usual fanfare, sitting on a bench in Madison Square Park, FaceTiming my dad and safely social-distanced from my mom. They both cried tears of joy. Like so many people around the world right now, I couldn’t even embrace my parents. Would they want me to volunteer?
I reached out to my classmates. I thought that some of them would certainly share my worries. I thought they also had to be carrying this uncomfortable kind of grief, a heavy and acidic feeling of dreams collapsing into a moral duty. I received a unanimous reply: “We are needed. It’s our time to step up.” No matter how many “what ifs” I voiced, they wouldn’t crack or waver. Still, even if they never admitted it to me, I wondered whether they privately shared some of my concerns and fears.
Everyone knows information is shared instantly in our Twitter-centric world, but I was still shocked and unprepared for how quickly I was at the center of a major news story. Within an hour of that email, I was contacted by an old acquaintance from elementary school, now a journalist. He had found me through Facebook and asked, “Will you be one of the NYU students graduating early? Would love to get a comment.” Another friend texted me a photo of the leaked email, quipping, “Are you going to save us from the pandemic, Dr. Gabe?!” “It’s not a small decision!” I snapped back.
I went through something like the seven stages of grief in rapid succession. I found that with each excuse I made why I shouldn’t volunteer, I somehow became increasingly more anxious. To my surprise, when I decided I would join 50 of my peers at NYU, graduate early, and volunteer, my mind settled. The more I thought about it, the more I was overtaken by the selfless beauty of the profession I’m entering. This is what it means to be a doctor. I recalled a key part of the Hippocratic Oath: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.”
I am going to fulfill my special obligations.
The fear is still there. I’m scared of COVID. I’m scared to infect others. I’m scared of winding up paralyzed and intubated. But I have also realized that all we have is each other. Healthcare workers supporting healthcare workers. New Yorkers supporting New Yorkers. Citizens of the world supporting citizens of the world. This is my time to be there for others, unwaveringly.
Logistical details continue to roll in, although they feel trivial in relation to the decision I have already made. The paperwork tells me that I will be onboarded to NYU’s internal medicine residency program. I will be compensated and protected under a similar contract to what current NYU residents sign. I have been promised that I will remain insured until I start my official residency program in July. My student loans won’t begin accruing interest until my normally planned graduation date. I am told that I will have personal protective equipment in line with the Centers for Disease Control and Prevention recommendations.
Questions still linger. Is it safe for me and my newly minted physician peers to continue living with our spouses, children, and friends? How long will I need to quarantine after my contract ends? Will there be a virtual graduation ceremony for my parents and loved ones to enjoy? In these challenging times, each day gives me a little more clarity about what exactly I am signing up for, but there are still so many uncertainties.
Am I naive to say that I do feel prepared? Or at least as prepared as anyone can be. With respect to my training, I have completed the requirements to graduate, which is why I am being permitted to graduate early in the first place. Our faculty points to our professionalism as the most promising indicator of our preparedness. They are heartened that we have embraced this truest test: our duty to others.
There is an eerie calm to New York City that contradicts what is shown on the news. With stores closed and streets quiet, it almost feels like Christmas morning here. Yet, inside the hospital, a fire rages. All the metaphors being used right now speak about violence, devastation, and immeasurable human suffering. “A war is being fought.” Or so I have heard. I guess I am about to find out.
Gabriel Redel-Traub is a fourth-year medical student at NYU Grossman School of Medicine. He will be starting residency in internal medicine at Columbia Presbyterian this summer. He is the former editor-in-chief of Dartmouth College’s Mouth Magazine, an editor of NYU’s LitMed Database, and has published most recently in the Hasting’s Center Magazine. Gabriel Redel-Traub has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Missing pieces
On the first day of my third postgraduate year, I sat at a table with my entire PGY-3 class and our attending physician. This was my first case discussion of the new academic year, and the attending was someone I hadn’t worked with previously. He was an older gentleman who primarily worked in private practice, but enjoyed teaching and maintained his academic affiliations. He started the discussion with a simple question: “Does anyone have a case they would like to discuss?”
The silence we were accustomed to as new interns on the first day of service fell over the group. Everyone seemed a bit apprehensive, as this attending was somewhat intimidating. He was educated at Hahnemann University Hospital, and classically trained in psychoanalysis. He had a wealth of research knowledge, and continued to publish in academic journals on a regular basis.
Finally, someone volunteered to present a case. The case involved a 45-year-old woman with a long history of depression. She had received multiple medication trials that did not result in remission. In fact, she had never experienced significant relief of any of her depressive symptoms. The case was clearly shaping up to look like treatment-resistant depression. The resident continued with the case and discussed the differential diagnosis and treatment plan. The treatment plan involved a combination of pharmacotherapy and psychotherapy—not much different from the previous treatments the patient had tried. I anxiously ant
After listening attentively and taking a moment to gather his thoughts, the attending responded with one word: “Egregious.” He was blunt, and clearly viewed the case formulation and management of this patient as “basic.” It was clear to me that I, and the rest of my class, were missing something. It was something that was not going to come from a textbook or treatment algorithm. He was the first attending in some time who was challenging us to truly think.
A profound point
I ruminated on his surprising response for a moment, as the treatment plan presented was commonly seen on the inpatient unit. It was not an unreasonable approach, but it lacked depth and sophistication. However, no attending I worked with in the past ever called it “egregious.” Now I was intrigued, and honestly, it had been some time since I felt excited about a case discussion. The attending’s point was simple: our patients are suffering, and they are coming to us in their most vulnerable state seeking answers. When we make decisions based on FDA approvals and blindly follow treatment algorithms, we fail to see the vast untapped potential to help patients that resides outside of these strict guidelines. This is not to say there is no place for algorithm-based psychiatry and FDA-approved medications; in fact, many times these will be the cornerstones of treatment.
During the discussion, this attending proceeded to make another profound statement that I continue to remind myself of each day. He said, “What would be the point of these patients coming to see you if you are going to practice psychiatry like a primary care provider?” I had to agree with him on many levels, because these patients are suffering, and they are looking for hope. If we simply offer them the same standard treatments, they are likely to get the same poor results. Our patients are coming to us because we are experts in the field of psychiatry; we owe them the respect to think outside the box. As specialists, the most complicated and difficult-to-treat cases will be referred to us. We need to possess a deep understanding of all treatment options, and know where to go when your first, second, and third options fail to produce the desired result.
The attending offered his thoughts on the case, and discussed his approach to treating this patient. He explained the importance of not being afraid to try medications in doses above the FDA-approved maximums in select cases. He explained the robust research behind monoamine oxidase inhibitors (MAOIs), and how to safely prescribe them. He explained why tricyclic antidepressants may be a more effective choice for some patients.
Continue to: These were discussions...
These were discussions I never had the opportunity to have in the past. In many instances, the possibility of using an MAOI would be quickly dismissed by my attendings as “too dangerous” or “better options are available.” In this attending’s view, it wasn’t the danger of an adverse outcome we are facing, but the danger of missing potentially life-changing treatments for our patients. The attending concluded with, “It’s sad that many of you will graduate without starting a patient on an MAOI, without titrating a tricyclic antidepressant and monitoring blood levels, and without ever really thinking for yourself.” These were powerful words, and he was speaking a truth that deep down I already knew.
When I reflect on this discussion and my first 2 years of training, I realize the value in learning structured methods of treating patients. I am aware of the need to practice in a safe manner that does not put the patient at unnecessary risk. However, I also realize I am going to face difficult cases where many smart and capable clinicians have attempted treatment and failed to get the desired outcome. It’s essential that as specialists we learn to use all the tools available to us to treat patients. If we limit ourselves out of fear, or blindly follow algorithms, we miss important opportunities to act boldly to help patients in their darkest moments.
On the first day of my third postgraduate year, I sat at a table with my entire PGY-3 class and our attending physician. This was my first case discussion of the new academic year, and the attending was someone I hadn’t worked with previously. He was an older gentleman who primarily worked in private practice, but enjoyed teaching and maintained his academic affiliations. He started the discussion with a simple question: “Does anyone have a case they would like to discuss?”
The silence we were accustomed to as new interns on the first day of service fell over the group. Everyone seemed a bit apprehensive, as this attending was somewhat intimidating. He was educated at Hahnemann University Hospital, and classically trained in psychoanalysis. He had a wealth of research knowledge, and continued to publish in academic journals on a regular basis.
Finally, someone volunteered to present a case. The case involved a 45-year-old woman with a long history of depression. She had received multiple medication trials that did not result in remission. In fact, she had never experienced significant relief of any of her depressive symptoms. The case was clearly shaping up to look like treatment-resistant depression. The resident continued with the case and discussed the differential diagnosis and treatment plan. The treatment plan involved a combination of pharmacotherapy and psychotherapy—not much different from the previous treatments the patient had tried. I anxiously ant
After listening attentively and taking a moment to gather his thoughts, the attending responded with one word: “Egregious.” He was blunt, and clearly viewed the case formulation and management of this patient as “basic.” It was clear to me that I, and the rest of my class, were missing something. It was something that was not going to come from a textbook or treatment algorithm. He was the first attending in some time who was challenging us to truly think.
A profound point
I ruminated on his surprising response for a moment, as the treatment plan presented was commonly seen on the inpatient unit. It was not an unreasonable approach, but it lacked depth and sophistication. However, no attending I worked with in the past ever called it “egregious.” Now I was intrigued, and honestly, it had been some time since I felt excited about a case discussion. The attending’s point was simple: our patients are suffering, and they are coming to us in their most vulnerable state seeking answers. When we make decisions based on FDA approvals and blindly follow treatment algorithms, we fail to see the vast untapped potential to help patients that resides outside of these strict guidelines. This is not to say there is no place for algorithm-based psychiatry and FDA-approved medications; in fact, many times these will be the cornerstones of treatment.
During the discussion, this attending proceeded to make another profound statement that I continue to remind myself of each day. He said, “What would be the point of these patients coming to see you if you are going to practice psychiatry like a primary care provider?” I had to agree with him on many levels, because these patients are suffering, and they are looking for hope. If we simply offer them the same standard treatments, they are likely to get the same poor results. Our patients are coming to us because we are experts in the field of psychiatry; we owe them the respect to think outside the box. As specialists, the most complicated and difficult-to-treat cases will be referred to us. We need to possess a deep understanding of all treatment options, and know where to go when your first, second, and third options fail to produce the desired result.
The attending offered his thoughts on the case, and discussed his approach to treating this patient. He explained the importance of not being afraid to try medications in doses above the FDA-approved maximums in select cases. He explained the robust research behind monoamine oxidase inhibitors (MAOIs), and how to safely prescribe them. He explained why tricyclic antidepressants may be a more effective choice for some patients.
Continue to: These were discussions...
These were discussions I never had the opportunity to have in the past. In many instances, the possibility of using an MAOI would be quickly dismissed by my attendings as “too dangerous” or “better options are available.” In this attending’s view, it wasn’t the danger of an adverse outcome we are facing, but the danger of missing potentially life-changing treatments for our patients. The attending concluded with, “It’s sad that many of you will graduate without starting a patient on an MAOI, without titrating a tricyclic antidepressant and monitoring blood levels, and without ever really thinking for yourself.” These were powerful words, and he was speaking a truth that deep down I already knew.
When I reflect on this discussion and my first 2 years of training, I realize the value in learning structured methods of treating patients. I am aware of the need to practice in a safe manner that does not put the patient at unnecessary risk. However, I also realize I am going to face difficult cases where many smart and capable clinicians have attempted treatment and failed to get the desired outcome. It’s essential that as specialists we learn to use all the tools available to us to treat patients. If we limit ourselves out of fear, or blindly follow algorithms, we miss important opportunities to act boldly to help patients in their darkest moments.
On the first day of my third postgraduate year, I sat at a table with my entire PGY-3 class and our attending physician. This was my first case discussion of the new academic year, and the attending was someone I hadn’t worked with previously. He was an older gentleman who primarily worked in private practice, but enjoyed teaching and maintained his academic affiliations. He started the discussion with a simple question: “Does anyone have a case they would like to discuss?”
The silence we were accustomed to as new interns on the first day of service fell over the group. Everyone seemed a bit apprehensive, as this attending was somewhat intimidating. He was educated at Hahnemann University Hospital, and classically trained in psychoanalysis. He had a wealth of research knowledge, and continued to publish in academic journals on a regular basis.
Finally, someone volunteered to present a case. The case involved a 45-year-old woman with a long history of depression. She had received multiple medication trials that did not result in remission. In fact, she had never experienced significant relief of any of her depressive symptoms. The case was clearly shaping up to look like treatment-resistant depression. The resident continued with the case and discussed the differential diagnosis and treatment plan. The treatment plan involved a combination of pharmacotherapy and psychotherapy—not much different from the previous treatments the patient had tried. I anxiously ant
After listening attentively and taking a moment to gather his thoughts, the attending responded with one word: “Egregious.” He was blunt, and clearly viewed the case formulation and management of this patient as “basic.” It was clear to me that I, and the rest of my class, were missing something. It was something that was not going to come from a textbook or treatment algorithm. He was the first attending in some time who was challenging us to truly think.
A profound point
I ruminated on his surprising response for a moment, as the treatment plan presented was commonly seen on the inpatient unit. It was not an unreasonable approach, but it lacked depth and sophistication. However, no attending I worked with in the past ever called it “egregious.” Now I was intrigued, and honestly, it had been some time since I felt excited about a case discussion. The attending’s point was simple: our patients are suffering, and they are coming to us in their most vulnerable state seeking answers. When we make decisions based on FDA approvals and blindly follow treatment algorithms, we fail to see the vast untapped potential to help patients that resides outside of these strict guidelines. This is not to say there is no place for algorithm-based psychiatry and FDA-approved medications; in fact, many times these will be the cornerstones of treatment.
During the discussion, this attending proceeded to make another profound statement that I continue to remind myself of each day. He said, “What would be the point of these patients coming to see you if you are going to practice psychiatry like a primary care provider?” I had to agree with him on many levels, because these patients are suffering, and they are looking for hope. If we simply offer them the same standard treatments, they are likely to get the same poor results. Our patients are coming to us because we are experts in the field of psychiatry; we owe them the respect to think outside the box. As specialists, the most complicated and difficult-to-treat cases will be referred to us. We need to possess a deep understanding of all treatment options, and know where to go when your first, second, and third options fail to produce the desired result.
The attending offered his thoughts on the case, and discussed his approach to treating this patient. He explained the importance of not being afraid to try medications in doses above the FDA-approved maximums in select cases. He explained the robust research behind monoamine oxidase inhibitors (MAOIs), and how to safely prescribe them. He explained why tricyclic antidepressants may be a more effective choice for some patients.
Continue to: These were discussions...
These were discussions I never had the opportunity to have in the past. In many instances, the possibility of using an MAOI would be quickly dismissed by my attendings as “too dangerous” or “better options are available.” In this attending’s view, it wasn’t the danger of an adverse outcome we are facing, but the danger of missing potentially life-changing treatments for our patients. The attending concluded with, “It’s sad that many of you will graduate without starting a patient on an MAOI, without titrating a tricyclic antidepressant and monitoring blood levels, and without ever really thinking for yourself.” These were powerful words, and he was speaking a truth that deep down I already knew.
When I reflect on this discussion and my first 2 years of training, I realize the value in learning structured methods of treating patients. I am aware of the need to practice in a safe manner that does not put the patient at unnecessary risk. However, I also realize I am going to face difficult cases where many smart and capable clinicians have attempted treatment and failed to get the desired outcome. It’s essential that as specialists we learn to use all the tools available to us to treat patients. If we limit ourselves out of fear, or blindly follow algorithms, we miss important opportunities to act boldly to help patients in their darkest moments.